Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/171/01. The contractual start date was in January 2017. The draft report began editorial review in March 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Crawford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Diabetes-related foot ulcers give rise to considerable morbidity, generate a high monetary cost for health and care services and are known to precede the majority of diabetes-related lower extremity amputation (LEA). 1,2 Identifying those at risk of developing a foot ulcer and providing an effective intervention to prevent these wounds developing has been a long-time goal of many working in the field.
Routinely collected data from Scotland show that the incidence of diabetes-related foot ulceration (DFU) among people with diabetes mellitus was 4.9% in 2014. 3 An estimated prevalence of 2.5% across the whole of the UK diabetes mellitus population generates an annual economic burden of £300M to provide community and primary health care for those with the condition. 4 The additional cost of LEA more than doubles this cost to approximately £662M. 4 High levels of variation in diabetes-related LEA between primary care trusts in England have been reported, and one possible explanation for these differences in patient outcomes might be differences in the delivery of care. 5 For those who experience a DFU, the likelihood of 5-year survival is poor, with mortality estimates of between 25% and 50% consistently reported over a 20-year period in the UK and in other parts of Europe. 6
A reduction in all LEA in Scotland between 2004 and 2008 reached statistical significance only for those with diabetes mellitus, but the cause of this was unclear. 7 Improvements in the recording of cases of diabetes mellitus may have confounded the data analysis but the effects of large-scale public health interventions and trends in prescribing may also have contributed. Legislation to ban smoking in public places was introduced in Scotland in 2006 and led to reductions in the number of admissions for acute coronary syndrome and the incidence of cerebral infarctions. 8 A cohort study using data from 46,864 people with diabetes mellitus and without diabetes mellitus in Spain also found the prescribing of statins to significantly reduce atherosclerotic cardiovascular disease in those with diabetes mellitus, but not in the general population. 9 Given the direct association between smoking, cardiovascular disease and LEA, it is possible that amputation rates are influenced more by large-scale public health interventions and prescribing habits than by interventions focusing on the foot.
Many clinical prediction rules (CPRs) for the assessment of foot ulceration risk in people with diabetes mellitus are available, but few have been subject to validation. 10 In the UK, two clinical guidelines11,12 for diabetes mellitus make recommendations about the management of the foot, and recommend risk assessment procedures and preventative interventions for those found to be at risk. However, the recommendations in these influential documents are based predominantly on clinical consensus, and robust evidence to show that routine monitoring reduces the number of ulcers or LEA is scarce. 13
Current clinical guidelines for the management of the diabetic foot from the National Institute for Health and Care Excellence (NICE) recommend that people with diabetes mellitus undergo an annual foot examination, involving several elements, and a vascular assessment including measurement of ankle–brachial pressure index (ABI). For those judged to be at moderate or high risk, monitoring is escalated to 6-monthly intervals and up to a maximum frequency of once per week. 11 As peripheral neuropathy (the most common foot complication of diabetes mellitus) is irreversible, such frequent monitoring is unlikely to positively influence patient outcomes. The diabetes guideline from the Scottish Intercollegiate Guidelines Network (SIGN) [which is synonymous with the Scottish Care Information – Diabetes Collaboration (SCI-Diabetes), a computerised decision-support tool] recommends a foot examination assessing five risk factors and advocates the use of some expensive equipment not readily available outside specialist care settings. 12 The SIGN diabetes guideline states that monitoring should take place at least annually, but concedes that the optimal frequency is unknown, citing evidence from one cohort study in which low-risk patients had a 99.6% [95% confidence interval (CI) 99.5% to 99.7%] chance of being ulcer free at 1.7 years after testing. 14
A systematic review (SR) and meta-analysis of individual patient data (IPD) collected worldwide [Prediction Of Diabetic foot UlcerationS (PODUS)]15 enabled the external validation of a predictive model involving only three predictors: insensitivity to a 10-g monofilament, absent pedal pulses and a history of ulceration or LEA. All three predictors are easy and cheap to ascertain and, therefore, likely to be used in clinical practice. These three predictors also performed well in external validation using an independent data set; however, the results of the PODUS analyses were expressed as summary odds ratios (ORs) from a meta-analysis, which do not readily allow clinicians to assess the risk of ulceration for individual patients. The development of a CPR based on the PODUS analyses was needed and the development of a simple scoring system to identify patients at higher risk of ulceration is a key objective of this research. The majority of the 1221 foot ulcers experienced by a group of 16,385 patients occurred 2 years after risk assessment, supporting a recommendation for 2-year monitoring of those at low risk. What is not clear, however, is how often people who are at moderate or high risk should be tested.
It is reasonable to expect that, once a person is identified as being at moderate or high risk of foot ulcer, effective preventative measures will be available. Unfortunately, although both of the UK national diabetes guidelines advise that patients in the higher-risk categories be referred to a multidisciplinary foot clinic for specialist care, there is a lack of evidence to show whether or not these expensive teams of clinicians and resource-intense arrangements result in fewer lesions. 16 Furthermore, the nature and effect of the particular interventions they provide and the best composition of the specialist team are unclear. Routine risk assessments for bad outcomes without effective preventative interventions might result only in worried patients; however, an effective CPR might allow diabetic patients at high risk to be triaged into more effective but more expensive preventative regimens. High-performance monitoring is more costly; therefore, we need to evaluate the cost-effectiveness of different monitoring frequencies.
It has been suggested that a large, robust randomised controlled trial (RCT) to evaluate the effect of a CPR used at different monitoring frequencies to underpin a stratified approach is overdue, as is a thorough concurrent evaluation of the cost-effectiveness of this type of care pathway. 17,18 However, each of the necessary elements of the pathway remains in need of evaluation to ensure the creation of a truly evidence-based clinical approach. The purpose of this research is to create an evidence-based clinical pathway to identify those at risk of foot ulceration and provide effective interventions that are likely to reduce foot ulceration in people with diabetes mellitus.
Given the increased prevalence of diabetes mellitus, such an evidence-based approach could replace the frequent, detailed foot examinations people with diabetes mellitus currently receive, identify effective preventative interventions and reduce the large burden of costs on NHS services tasked with delivering foot care to people with diabetes mellitus.
Chapter 2 Overall research objectives
Aim
We aim to undertake an evidence-based evaluation of the clinical effectiveness and cost-effectiveness of foot ulcer risk assessments and structured care interventions for people with diabetes mellitus.
Research questions
-
What is the estimated clinical effectiveness and cost-effectiveness of a validated CPR as part of structured care to reduce the incidence of DFU?
-
What is the likely clinical effectiveness and cost-effectiveness of alternative strategies, including monitoring intervals?
-
Is there worth in undertaking further research, particularly a RCT?
Objectives
Our research objectives are to produce an evidence clinical pathway by:
-
extending (developing) our existing prognostic model into a CPR and conducting its external validation
-
undertaking a survival analysis of the time to ulceration to inform the economic model
-
conducting an overview of SRs to identify the effects and costs of available interventions (simple interventions such as pressure-relieving insoles and complex interventions such as specialist foot care teams)
-
combining the evidence from research questions (i), (ii) and (iii) in a cost-effectiveness decision model framework and analysing alternative clinical and cost-effective regimens at different monitoring intervals
-
carrying out a value-of-information analysis.
Chapter 3 Clinical prediction rule: PODUS data
Introduction
A CPR is a way of presenting a statistical model that facilitates predictions that inform clinical decision-making. Statistical models can be unwieldy; they may have many predictors or predictors requiring transformation from their original scale, which can be off-putting to end-users and increase the scope for human error. In addition, the type of statistical model that is used for prediction is generally either a logistic regression model or a Cox proportional hazards model. These two models can be used to investigate the relationship between predictors and a binary or a categorical outcome (logistic regression) or the time until a binary outcome occurs (Cox proportional hazards model). Both types of statistical model require the use of a calculator, or similar, to make a prediction for an individual patient, as the estimate requires taking an exponential.
This chapter describes how we developed a statistical model for the prediction of DFU, used this model to create a simple-to-use CPR and validated the CPR in a data set not used in the development phase. We used the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement as a framework for reporting (see Appendix 3, Table 41). 19
Clinical prediction rules can be presented simply as a regression equation, a nomogram or a scoring system; other formats are also possible. Whichever format is chosen, it should be remembered that the CPR is only as good as its underlying statistical model; therefore, the methodological requirements for good practice when building and validating a statistical model apply equally to CPRs. In addition, the presentation of a CPR can affect its acceptability to end-users. Our aim was to produce a CPR that does not require a calculator and is simple enough to be of very little burden in a busy clinic.
The benefit of a CPR is based not only on ease of use, but also, for example, on whether or not it provides useful information not otherwise available: will it improve patient outcomes and are there other ways to predict foot ulcer? The burden and sequelae of DFU to patients and the NHS are immense, so there is enormous interest in predicting which patients will develop ulceration. Therefore, it is unsurprising that we are not the first to attempt to make the prediction of ulcer easier for health professionals working directly with patients. This project, PODUS 2020, is a development of the work conducted in PODUS 2015, a SR and meta-analysis of IPD,15 in which we used the PODUS data sets to calculate ORs to quantify the association between risk categories, based on the recommendations of the International Working Group on Diabetic Foot (IWGDF), NICE and SIGN, and foot ulcer, as these are the guidelines likely to be used in the UK. The guidelines did not produce ORs that were significantly different from those obtained using insensitivity to monofilament only. Our final PODUS 2020 prediction model is simpler than current guidelines as it has only three predictors; it also includes insensitivity to monofilament. We knew, therefore, that we could use the PODUS data to develop and validate a simpler CPR that could perform at least as well as existing guidelines.
Methods
Source of data
The data for PODUS 2020 came from a previous research project, PODUS 2015 (see Appendix 3), published in the National Institute for Health Research (NIHR) Health Technology Assessment journal. 15 PODUS 2015 obtained eight studies and had access to another two identified from an IPD SR. Eight studies contributed data to PODUS 2015. 20–27 Access to a ninth study28 was available via a Safe Haven facility; a 10th study29 was not directly available but the PODUS 2015 team could request results of analyses from the data set. After the publication of PODUS 2015, we re-ran the searches to identify new studies and found only one that met the inclusion criteria. Unfortunately, the authors of that study did not respond to requests to share their data. 30 The search strategy to find studies was last run in June 2017 for MEDLINE and in August 2017 for EMBASE, and was published as appendix 3 of the PODUS 2015 Health Technology Assessment journal publication.
Inclusion criteria for development and validation studies
Studies could be included in PODUS 2015 if patients had diabetes mellitus, predictors had been assessed at recruitment, foot ulcer status was assessed at follow-up and the study had recruited at least 100 patients. In addition, for a study to be included in PODUS 2020 development data sets, we required that it collected data on insensitivity to a 10-g monofilament, presence/absence of pedal pulses, history of ulceration or amputation, and the time period in which the ulcer occurs. As we planned to conduct a one-step meta-analysis at the development stage, we needed to merge all of the development data sets, and so required them to be stored on the same server. These criteria reduced the number of eligible development studies to four. 20,21,24,25 Four studies did not provide data on sensitivity to monofilaments and/or the presence or absence of a pedal pulse,22,23,26,27 and the access arrangements for the Leese et al. 28 and Boyko et al. 29 data sets meant that they could not be stored on the same server as those of the other studies. The Boyko et al. 29 data set had been used for validation in PODUS 2015, but included a very small proportion of women (< 2%). We therefore decided to use the Leese et al. 28 data set for validation of the CPR.
We had no date restriction on studies. Recruitment dates ranged from 1 May 1995 to 10 November 2007 in the development data sets, and the final follow-up date was 5 December 2008. In the Leese et al. 28 validation data set, recruitment dates ranged from 28 January 2001 to 8 December 2006 and the final follow-up date was 2007.
Critical appraisal of contributing studies
We used the Prediction model Risk Of Bias ASsessment Tool (PROBAST) tool to critically appraise the four validation studies and the external validation study. 31 This was not used for PODUS 2015 because PODUS 2015 predated the publication of the PROBAST tool.
Participants
The four studies for development in PODUS 2020 comprised two studies set in the community in the UK and two hospital-based studies: one in mainland Europe and one in the USA. All studies recruited a consecutive sample. The Leese et al. 28 data set used for validation is from another community-set study in the UK. The inclusion criteria for the data to be collected from each patient were as described in Inclusion criteria for development and validation studies; however, we also stipulated for both PODUS 2015 and PODUS 2020 that patients had to be aged ≥ 18 years and ulcer free at the time of recruitment. This meant that we had to remove from the analysis data set a small proportion of patients in some studies who had an ulcer at the time of recruitment. All studies were observational, and patients received the standard care in that setting.
Outcome
In PODUS 2020 we defined a binary outcome of presence or absence of foot ulceration within 2 years. Ulceration status was assessed by podiatrists (persons who diagnose and treat foot ailments; also known as chiropodists) or self-report questionnaires. We chose 2 years as the time interval as it is sufficient for an at-risk patient to develop an ulcer, it is clinically meaningful and it allowed us to use the largest study20 (> 6000 patients) that had defined the outcome as development of an ulcer by 2 years. The other three development data sets21,24,25 included either date of ulceration or time to ulceration, and, therefore, the data could be recoded to match the largest data set. However, we note that the planned length of follow-up in the Crawford et al. 21 data set was only 1 year, and this was accounted for in our analyses. Assessment of outcome was, where possible, blinded to test results in three of the four development studies, but not in the Monteiro-Soares and Dinis-Ribeiro24 and Leese et al. 28 validation study. It is, of course, not possible to blind podiatrists to previous amputations. As time to ulceration is also of interest, we conducted a survival analyses with the three studies with time-to-event data and present the results in Appendix 3.
Selection of predictors in PODUS 2020
In PODUS 2015, six predictors were selected from a potential candidate list of 22: age, sex, body mass index, smoking, height, weight, alcohol intake, glycated haemoglobin (HbA1c), insulin regime, duration of diabetes mellitus, eye problems, kidney problems, insensitivity to a 10-g monofilament, absence of pedal pulses, tuning fork, biothesiometer, ankle reflexes, ABI, peak plantar pressure, prior ulcer, prior amputation and foot deformity. Predictors were chosen for clinical plausibility, availability in at least three studies and lack of clinical heterogeneity. Statistical criteria such as small p-values were not used. Six variables were chosen for inclusion in the primary model in PODUS 2015: age, sex, duration of diabetes mellitus, insensitivity to a 10-g monofilament, absence of pedal pulses and prior ulcer or amputation. The analysis was a two-step meta-analysis. In each data set we fitted a logistic regression model with the six predictors, which gave us adjusted estimates for each predictor. We conducted a meta-analysis for each predictor using the generic inverse method. 32 We had used a two-step method so that we could include, in the second stage, aggregate data (i.e. log-odds ratios and their variances) derived from the Leese et al. 28 data set with > 3000 patients. The Leese et al. 28 data set was housed on a different server and so could not be used in a one-step meta-analysis, although this is the preference of some methodologists. 33
We tested these six predictors in the 10th, externally held data set,29 which had 1489 people and 229 ulcer outcomes. We considered the PODUS 2015 results to be replicated in the external data set if the predictor achieved statistical significance, if its effect was in the same direction as the PODUS 2015 estimate and if its CIs overlapped. The predictors that survived this process were insensitivity to a 10-g monofilament, absence of pedal pulses and prior ulcer or amputation.
For the CPR, we decided not to use the three predictors that were not replicated in the Boyko et al. 29 data set: age, sex and duration of diabetes mellitus. Age and duration of diabetes mellitus are credible predictors of any diabetic complication, including foot ulcer. They are also continuous, which means that, in theory, they could be used to generate more precise risk estimates than categorical predictors; however, their inclusion in the CPR would require a calculator, or similar, to estimate risk. CPRs are a form of clinical decision support system that tend not to be used unless they are integrated into the existing workflow. 34 The project did not have access to resources to support a website, or similar, that would calculate risk for health professionals or embed the CPR into NHS information technology systems. However, we could use three binary predictors that were replicated to produce a simple CPR that can be paper based and does not require any calculation from the users to implement. Practicalities as well as the lack of replication in the Boyko et al. 29 data set were reasons to drop age and duration of diabetes mellitus from our CPR model; however, we understand that some individuals will be interested in the six predictor model, and the results from this model are in Appendix 3 and make direct comparisons with the three-predictor model. We also investigated possible reasons why age and duration of diabetes mellitus did not reach statistical significance or were not replicated predictors in the Boyko et al. 29 data set. For simplicity, we also chose not to use the category sex as a predictor. Discussion with potential users of the CPR showed that they were very much in favour of a simpler model.
Definition of the PODUS 2020 predictors
We decided to use the three replicated predictors only (i.e. monofilaments, pulses and history) in the CPR. These three binary predictors were measured at the initial assessment of each patient in each study. In detail, the predictors are:
-
Insensitivity to a 10-g monofilament at any site on either foot was defined as test positive. This test is carried out by podiatrists. The podiatrist touches the sole of the patient’s foot with a monofilament and the patient states whether or not he or she felt it.
-
In general, there are two pulses tested in each foot: the dorsalis pedis and the posterior tibial pulses. We defined absence of either pulse on either foot as test positive, although it is known that the dorsalis pedis pulse is missing in some healthy individuals. 35
-
History of ulceration or amputation was ascertained either at initial assessment or from patient records. Patients were considered test positive for history if they had experienced either ulcer or amputation.
As predictors were measured before outcome in three of the four development studies, the measurement of predictors was blind to outcome. However, assessment of predictors blind to other predictors generally did not occur and would not always be possible; for example, toe amputation would be apparent to any podiatrist assessing monofilaments or pulses.
As in PODUS 2015, we chose to use patient rather than foot as the unit of analysis. The three binary predictors are defined as above, as this was the only way to have a consistent definition in all four development data sets; for example, Crawford et al. 21 recorded the presence or absence of each of the four foot pulses, whereas Abbott et al. 20 recorded the number of foot pulses per person (0–4). The outcome was binary and was defined as the occurrence or not of ulcer by 2 years.
In the PODUS 2015 publication, there is an extensive examination of differences and similarities between the studies as sources of heterogeneity. 15 We repeat some of those analyses here to provide a description of the contributing data sets, with emphasis on the predictors chosen for PODUS 2020.
Sample size considerations
Sample size calculations are generally not carried out for meta-analyses conducted as part of a SR, as the aim is to use not an acceptable minimum but all of the available data. Post hoc sample size calculations are problematic and not recommended by statisticians, and so we did not conduct any. 36 The development data sets have a total of 8255 people with 430 ulcer outcomes, giving 143 events per variable. This is well above the often-cited rule of thumb of 10 events per variable. 37
Statistical models can often give overly optimistic results if the data from which they were derived come from small data sets, if data-driven methods are used to select variables or if too many variables are used. A way of compensating for optimistic results is to use a shrinkage factor:38 a number < 1 by which the coefficients are multiplied. All of the shrinkage factors that we calculated during the model development phase were > 0.9999, which would have resulted in negligible changes, so we did not use shrinkage factors. Shrinkage factors are affected by sample size and complexity of model but our model is simple and our sample size (events) is large relative to the number of included predictors.
The external validation data set had 3324 patients and 128 ulcer outcomes, meeting the recommendation of at least 100 events and 100 non-events to investigate model performance. 39
Missing data
To account for missing data, we would have considered multiple imputation if we thought that data were likely to be missing at random (MAR). 40 However, the proportion of missing data was very small (0–3% in the development data sets and < 2% in the largest data set of > 6000 patients) and so the results of any imputation exercise would not have made any notable difference to our results; therefore, we analysed the data using complete cases only, that is, patients for whom data on monofilaments, pulses, history and ulcer outcomes at 2 years were available.
One reason why outcome information at 2 years might be missing is death of the patient before 2 years. However, death was not consistently recorded across the data sets; for example, in the development studies, the largest study had recorded only one death in 2 years and another did not record deaths at all. The other two development studies were more systematic about including death data. Overall, the proportion of patients recorded as having died was 2%. If a patient had died, but had all information on predictors and outcome, that person was included in our analyses.
Some patients had missing data on previous amputation or ulcer history. However, the clinical context in which these data were collected means that it is very important to record when ulcers and amputations have occurred; therefore, the data were not MAR and are far more likely to be missing if the patients did not have previous ulcers and did not have amputations. Patients who were missing ulcer or amputation history were, therefore, recoded as test negative for these two items. The numbers of patients whose data were recoded are given in each study’s flow chart (Figures 1–4).
FIGURE 1.
Flow of patients in the Abbott et al. 20 data set. All patients had 2-year ulcer outcome recorded. Not all patients are shown at each stage.

FIGURE 2.
Flow of patients in the Crawford et al. 21 data set. Not all patients are shown at each stage.
Length of follow-up in the Crawford et al.21 data set
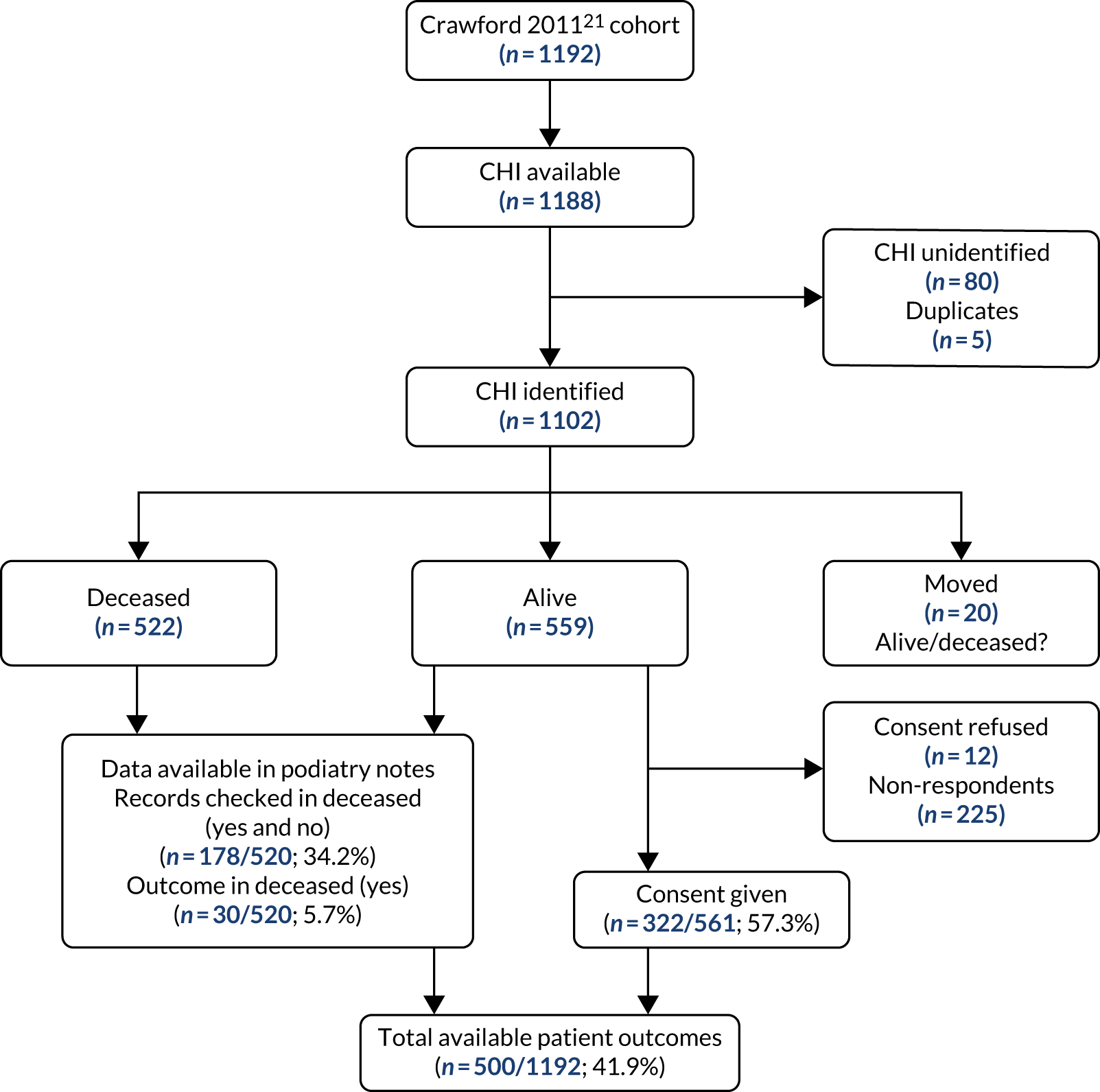
The PODUS 2020 outcome variable is ulcer occurrence by 2 years, and we knew that the Crawford et al. 21 study had prespecified the follow-up period to be 12 months. We received ethics approval from the Scotland A Research Ethics Committee (reference 16/SS/0213: Integrated Research Approval System project ID 97542), Caldicott approval from NHS Tayside (reference IGTCAL3842) and NHS Tayside approval (reference 2017DM03, NHS Research Scotland reference NRS17/9754) for permission to contact the participants in the study by Crawford et al. 21 to ask if they would consent to include longer-term data in PODUS 2020, some of which were stored on paper records. Despite all these efforts, follow-up data were obtained from only 42% of the original sample (see Appendix 3, Figure 33). Efforts were hampered by the non-retention of patient records for more than 8 years post death, patients being uncontactable and patients who did not consent. The PODUS 2020 Steering Committee discussed this issue and recommended that the data should not be used for the current project.
Analysis
Statistical analysis methods: choice of model
Given a binary outcome, the obvious method of analysis is logistic regression. Although other methods are available, we chose to base the CPR on a logistic regression model because this model is simple to implement and acceptable to the medical community, and the methods for assessing its performance are well developed. The selection of predictors was described in Definition of the PODUS 2020 predictors. We did not consider adding any interaction terms as these often do not improve the predictive ability of the model19 and would have made the CPR more complex.
As the data came from four studies, we used logistic regression with a separate intercept for each study to allow for clustering of participants within studies and to allow for between-study variation in baseline risk. This was especially important because of the inclusion of the Crawford et al. 21 study, which had a follow-up duration of only 1 year, compared with 2 years in the other three studies. Although ORs of included predictors were similar in studies with 1- and 2-year’ follow-up, the baseline risk was not comparable, as it was higher in those studies with 2-year’ follow-up because of the longer time period.
For defining the intercept for our final CPR based on this logistic regression model, we chose a weighted average of the intercept estimates from the three studies with 2-year follow-up. This weighted average was obtained by using a random-effects meta-analysis of the three intercepts, and fitting using the DerSimonian and Laird method, which allows for both within-study variability (i.e. variance of intercept estimates) and between-study heterogeneity (i.e. genuine differences in baseline risk across studies beyond chance) (see Appendix 3, Figure 42). Therefore, the intercept in our final CPR model was not based on the Crawford et al. 21 study (because of its 1-year follow-up), but predictor effects were based on the four developmental studies.
Statistical analysis methods: transformation of the logistic regression model into a clinical prediction rule
We adapted the method described by Steyerberg41 to generate a CPR from our logistic regression analyses. In brief, Steyerberg’s method is (1) multiply and round regression coefficients, (2) search scores for continuous predictors, (3) estimate the multiplication factor for the scores and (4) estimate the intercept and present a score chart. We omitted the second step because we had no continuous predictors and the third because our multiplication factor was 1. Steyerberg’s method could be applied to many different kinds of statistical model. We made a further modification to allow for the effect of the non-linear logit function used in logistic regression.
The outcome variable in binary logistic regression is the natural logarithm of the odds, or log-odds, of the binary event occurring:
where βs are log-odds ratios and the xs are the predictors. The intercept is the log-odds of the outcome occurring when all the predictors are zero. The probability of the outcome occurring can be calculated from the log-odds. For each unit change in x, the log-odds will increase by the corresponding β (a fixed amount), but the effect on the probability of outcome is not fixed because of the non-linear nature of the log-odds; for example, if the log-odds is 1.3, the corresponding probability is 78.6%. If the log-odds increases by 0.5 to 1.8, the probability becomes 85.8%, an increase in probability of 7.2%. If the log-odds is 2.3 and it is increased again by 0.5 to 2.8, the probability changes from 90.9% to 94.3%, an increase in probability of 3.4%, less than half the change before. The same change in log-odds does not mean the same change in probability given different values of initial log-odds; therefore, when considering the transformation of the logistic regression model into a simpler CPR, we also took account of the probabilities that would result from the scoring system as well as the size of the coefficients. This process was greatly simplified by having only three binary predictors. The number of possible predictor combinations is only eight, and it is not onerous to calculate the probability of ulcer for each combination.
To be explicit, our method was as follows:
-
Fit the logistic regression model with the three risk factor predictors (monofilament, pulses and history) and study. This gives coefficients showing the extent to which the log-odds change for patients who have a test-positive result for monofilaments, pulses or history in comparison with lower-risk test-negative patients. There are also individual estimates for the intercept for each study. The intercept is the baseline risk of ulcer on the log-odds scale. We used SAS® PROC LOGISTIC (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the US and other countries. ® indicates USA registration) with maximum likelihood estimation.
-
Conduct a random-effects meta-analysis of the three intercepts from the studies with 2-year follow-up to get a single average intercept.
-
Use this average intercept and the log-odds coefficients for the three predictors to calculate the probability of ulcer for each possible predictor combination; as there are three binary predictors, there are eight combinations.
-
Multiply and round the coefficients of the predictors to get a CPR scoring scheme, bearing in mind that we wanted predictor combinations with similar probabilities of ulcer to have the same score.
-
Repeat step 1 and step 2 using only the CPR score instead of monofilaments, pulses and history.
-
Calculate the probability of ulcer for each score using a population average method.
In the case of a patient who has already contributed to one of the four development data sets, the most accurate estimate of baseline risk will be the appropriate study-specific intercept. A common way to estimate baseline risk for patients not recruited to the development data sets is simply to use the average intercept; however, our preference is to use the population average intercept method described by Pavlou et al. 42 to get estimates of ulcer risk that are applicable to patients in new studies.
Validation of the clinical prediction rule
We assessed the internal validity of the CPR by examining its discrimination and calibration. Discrimination addresses how well the model’s predicted risks discriminate between those who will and those who will not develop an ulcer, and the calibration of how well the estimated risk matches the actual risk of ulceration. We used receiver operating characteristic (ROC) plots and the area under the ROC curve as a statistic of discrimination; the latter is also known as a c-statistic. We assessed calibration with calibration plots, estimation of the calibration slope, and calibration in the large. We assessed the external validity of the CPR in the Leese et al. 28 data set again by examining the discrimination and calibration in the same way. For the validity analyses, we used the probability of ulcer as estimated by the CPR score and compared it with actual ulcer outcome at 2 years.
Other methods of assessing model performance in terms of clinical benefit are available, such as net benefit and decision curves, but we also noted that the performance of the CPR would be addressed using a health economic model.
Using discrimination and calibration statistics in both the development data sets and the Leese et al. 28 validation data set aids comparison of the internal and external validity of the CPR. Exploratory analyses of all the data sets and investigation of heterogeneity was part of PODUS 2015. Hence we knew that the Leese et al. 28 data set was broadly similar to the other data sets. In fact, there was an overlap of patients recruited to the Crawford et al. 21 and Leese et al. 28 data sets, and so we had to remove some patients from the Leese data set to avoid duplication of data. Relevant tables are in Results.
We have also included a net benefit graph to assess potential clinical impact. 43 All analyses were conducted with SAS 9.4 [URL: www.sas.com (accessed 19 February 2019)] and R 3.4.2 [URL: https://cran.r-project.org/ (accessed 19 February 2019)]. The pROC,44 meta32 and rms45 packages in R statistical software (The R Foundation for Statistical Computing, Vienna, Austria) were used.
Results
Description of the individual studies
The quality of the cohort studies used to create the PODUS CPR is detailed in Table 1. 31
| First author and year of publication | Risk of bias | Applicability | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| Participants | Predictors | Outcome | Participants | Predictors | Outcome | Risk of bias | Applicability | |
| Abbott 200220 | + | + | + | + | + | + | + | + |
| Crawford 201121 | + | + | + | + | + | + | + | + |
| Monteiro-Soares 201024 | + | + | + | + | + | + | + | + |
| Pham 200025 | + | + | + | + | + | + | + | + |
| Leese 201128 | + | + | – | + | + | + | – | + |
The flow of patients in the Abbott et al. 20 data set is shown in Figure 1. The 38 patients with a history of amputation or ulcer and the 23 patients with a history of neither were coded accordingly. Thus, the number of complete cases was 6417 (97%) when recoded ulcer and amputation history was excluded and 6478 (98%) when it was included. One death was recorded in the study, but this patient was also missing pulses and so could not have been included in the development data set.
The number of complete cases in the Crawford et al. 21 data set was 1175 (98.5%), as 18 patients were dropped from the analysis because information on monofilament sensitivity was absent and a further five were dropped because no follow-up time was provided and so ulcer occurrence by 2 years could not be calculated. There were 59 deaths in total in the Crawford et al. 21 data set.
All of the variables required by the CPR were fully recorded in the Monteiro-Soares and Dinis-Ribeiro24 (n = 360) study and so we did not create a flow diagram. As the study setting was secondary care, these data are likely to be accurate. Some other data were missing in the Monteiro-Soares and Dinis-Ribeiro24 study, for example 189 (53%) patients were missing vibration perception threshold (VPT) data, but these were not required for the CPR. Deaths were not recorded.
In the Pham et al. 25 study, the number of complete cases was 242 (97.6%). Three patients were missing a monofilament measurement and three had no time to ulcer/end of follow-up. One patient with a negative amputation history but no ulcer history was coded as negative for history. There were 13 deaths in the Pham et al. 25 study.
The total number of patients in the development data sets was 8404 and the total number who contributed to the analyses was 8255, an overall rate of complete data of 98%.
Among the Leese et al. 28 data set, 295 patients were removed from the analysis as they were included in the Crawford et al. 21 data set. The Crawford et al. 21 and Leese et al. 28 studies recruited in a similar time period in overlapping geographical areas; however, we used the Scottish NHS patient identifier, the Community Health Index number46 (URL: www.ndc.scot.nhs.uk/Dictionary-A-Z/Definitions/index.asp?ID=128%26Title=CHI%20Number), to remove Crawford et al. 21 patients from the Leese et al. 28 data set. This reduced the size of the Leese et al. 28 data set from 3707 to 3412 patients.
The percentage of complete cases in the Leese et al. 28 data set was 97.4%; again, we considered this high enough not to require multiple imputation. During the follow-up period, 95 patients died.
We calculated summary statistics for all the predictors considered for the primary analysis of PODUS 2015, while noting that there is an extensive description of all the data sets in the PODUS 2015 publication. 15
Summary statistics for age, duration of diabetes mellitus, sex, length of follow-up, sensitivity to monofilaments, absent pulses, history of amputation or ulceration and the results of outcomes (ulcer) are in Tables 2–9.
| First author and year of publication | Recorded (n) | Missing (n) | Age (years) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum | Maximum | |||
| Abbott 200220 | 6572 | 31 | 61.3 | 13.2 | 63 | 18 | 95 |
| Crawford 201121 | 1193 | 0 | 70.5 | 10 | 72 | 22 | 94 |
| Monteiro-Soares 201024 | 360 | 0 | 64.3 | 10.4 | 65 | 22 | 90 |
| Pham 200025 | 247 | 1 | 58.3 | 12.5 | 58 | 20 | 83 |
| All development data sets | 8372 | 32 | 62.7 | 13.1 | 64 | 18 | 95 |
| Leese 201128 | 3412 | 0 | 65.1 | 13.1 | 67 | 19 | 101 |
| First author and year of publication | Recorded (n) | Missing (n) | Duration of diabetes (years) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum | Maximum | |||
| Abbott 200220 | 6570 | 33 | 8.2 | 8.2 | 5 | 0 | 60 |
| Crawford 201121 | 1191 | 2 | 8.8 | 8.4 | 6 | 0 | 63 |
| Monteiro-Soares 201024 | 360 | 0 | 15.8 | 10.4 | 15 | 1 | 45 |
| Pham 200025 | 247 | 1 | 13.9 | 10.8 | 12 | 0 | 54 |
| All development data sets | 8368 | 36 | 8.8 | 8.6 | 6 | 0 | 63 |
| Leese 201128 | 3402 | 10 | 6.8 | 7.8 | 4 | 0 | 58 |
| First author and year of publication | Missing | Men | Women | Total (N) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Abbott 200220 | 1 | 0.02 | 3515 | 35.2 | 3087 | 46.8 | 6603 |
| Crawford 201128 | 0 | 0 | 611 | 51.2 | 582 | 48.8 | 1193 |
| Monteiro-Soares 201024 | 0 | 0 | 164 | 45.6 | 196 | 54.4 | 360 |
| Pham 200025 | 0 | 0 | 124 | 50.0 | 124 | 50.0 | 248 |
| All development data sets | 1 | 0.0 | 4414 | 52.5 | 3989 | 47.5 | 8404 |
| Leese 201128 | 0 | 0 | 1931 | 56.6 | 1481 | 43.4 | 3412 |
| First author and year of publication | Recorded (n) | Missing (n) | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Abbott 200220 | 6603 | 0 | 24 | 0 | 24 | 24 | 24 |
| Crawford 201128 | 1188 | 5 | 11.2 | 2.8 | 12 | 0 | 29 |
| Monteiro-Soares 201024 | 360 | 0 | 30.8 | 22.2 | 25 | 3 | 86 |
| Pham 200025 | 244 | 4 | 24.4 | 11.2 | 24 | 0 | 40 |
| All development data sets | 8395 | 9 | 22.5 | 7 | 24 | 0 | 86 |
| First author and year of publication | Missing | Sensitive | Insensitive | Total (N) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Abbott 200220 | 125 | 1.89 | 5200 | 78.8 | 1278 | 19.4 | 6603 |
| Crawford 201128 | 13 | 1.09 | 914 | 76.6 | 266 | 22.3 | 1193 |
| Monteiro-Soares 201024 | 0 | 0 | 194 | 53.9 | 166 | 46.1 | 360 |
| Pham 200025 | 3 | 1.21 | 60 | 24.2 | 185 | 74.6 | 248 |
| All development data sets | 141 | 1.68 | 6368 | 75.8 | 1895 | 22.5 | 8404 |
| Leese 201128 | 2 | 0.06 | 2703 | 79.2 | 707 | 20.7 | 3412 |
| First author and year of publication | Missing | Present | Absent | Total (N) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Abbott 200220 | 3 | 0.05 | 4643 | 70.4 | 1957 | 29.7 | 6603 |
| Crawford 201128 | 0 | 0 | 969 | 81.2 | 224 | 18.8 | 1193 |
| Monteiro-Soares 201024 | 0 | 0 | 287 | 79.7 | 73 | 20.3 | 360 |
| Pham 200025 | 2 | 0.81 | 210 | 85.4 | 36 | 14.6 | 248 |
| All development data sets | 5 | 0.06 | 6109 | 72.7 | 2290 | 27.2 | 8404 |
| Leese 201128 | 76 | 2.23 | 2858 | 83.8 | 478 | 14.0 | 3412 |
| First author and year of publication | No history | History | Total (N) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Abbott 200220 | 6291 | 95.3 | 312 | 4.7 | 6603 |
| Crawford 201128 | 1107 | 92.8 | 86 | 7.2 | 1193 |
| Monteiro-Soares 201024 | 223 | 61.9 | 137 | 38.1 | 360 |
| Pham 200025 | 71 | 28.6 | 177 | 71.4 | 248 |
| All development data sets | 7692 | 91.5 | 712 | 8.5 | 8404 |
| Leese 201128 | 3216 | 94.3 | 196 | 5.7 | 3412 |
| First author and year of publication | Missing | No ulcer | Ulcer | Total (N) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Abbott 200220 | 0 | 0 | 6312 | 95.6 | 291 | 4.4 | 6603 |
| Crawford 201128 | 5 | 0.42 | 1165 | 97.7 | 23 | 1.9 | 1193 |
| Monteiro-Soares 201024 | 0 | 0 | 308 | 85.6 | 52 | 14.4 | 360 |
| Pham 200025 | 4 | 1.61 | 175 | 70.6 | 69 | 27.8 | 248 |
| All development data sets | 9 | 0.11 | 7960 | 94.7 | 435 | 5.2 | 8404 |
| Leese 201128 | 0 | 0 | 3279 | 96.1 | 133 | 3.9 | 3412 |
Although the Leese et al. 28 study recorded patients’ test dates, in the case of occurrence only the year was recorded. Therefore, for this data set, we recorded an ulcer as having occurred within 2 years if one was recorded within 2 years of the year that the patient was first seen. This is not a precise way of coding ulcer outcome by 2 years, but it allowed us to use the data set. Ulcer outcomes were recorded from 2001 to 2007; the median year of occurrence was 2005.
From the coding detailed above, the total number of patients from the development data sets used in the logistic regression model underlying the CPR was 8255 (98%), of whom 430 had ulcer-positive outcomes and 7825 had ulcer-negative outcomes at 2 years. In the Leese et al. 28 validation data set 3324 patients had suitable data, of whom 128 had an ulcer by 2 years and 3196 did not. We did not compute unadjusted ORs for the predictor and outcome, as this work had already been done as part of PODUS 2015. 15
Development and testing of the clinical prediction rule: initial logistic regression model and random-effects meta-analysis
As outlined in Statistical analysis methods: transformation of the logistic regression model into a clinical prediction rule, the results of steps 1 and 2 of building the CPR are presented here.
On the log-odds scale, the initial logistic regression model with original predictors (coded 0 if test negative and 1 if test positive) was:
The intercept of –3.81 was taken from a random-effects meta-analysis of the intercepts of the three studies with 2-year follow-up data.
Calculating probability of ulcer for each predictor combination
We used Equation 2 to carry out step 3 of the CPR building by first calculating the log-odds of ulcer for each prediction combination and then converting that log-odds to a probability.
Generating a scoring scheme
Part of step 4 was examining ulcer risk probabilities (Table 10). This showed that some different predictor combinations had similar risk. For example, we wanted the (0,0,1) predictor combination with a probability of 0.134 to have the same score as the (1,1,0) combination with a probability of 0.118. Using the probabilities and the method of multiplying and rounding the predictor coefficients described by Steyerberg,41 the CPR scoring method is:
-
score 1 if patient is insensitive to monofilaments
-
score 1 if patient is missing any pulse
-
score 2 if patient has a history of ulcer or amputation.
| Monofilament sensitive | Pulses present | No history of ulcer or amputation | Probability of ulcer at 2 years |
|---|---|---|---|
| 0 | 0 | 0 | 0.022 |
| 0 | 1 | 0 | 0.043 |
| 1 | 0 | 0 | 0.062 |
| 1 | 1 | 0 | 0.118 |
| 0 | 0 | 1 | 0.134 |
| 0 | 1 | 1 | 0.238 |
| 1 | 0 | 1 | 0.318 |
| 1 | 1 | 1 | 0.484 |
This results in a CPR that gives scores from 0 to 4. We calculated this score for each patient and refitted the logistic regression model using CPR score as the only predictor.
Refitting the logistic regression model with clinical prediction rule score as the only predictor
The resulting logistic regression model from steps 4 and 5 in Statistical analysis methods: transformation of the logistic regression model into a clinical prediction rule using CPR score is:
The intercept again was taken from a random-effects meta-analysis of the intercepts of the three studies with 2-year follow-up data. We did not use this formula to calculate the probability of an ulcer, but, if we had decided to, the corresponding formula for probability would be:
Using Equation 4 would be perfectly acceptable, but we could calculate population-averaged probabilities of ulcer, which should be generalisable to new studies. The formula for doing so is complex, and not something that can be done easily without statistical software. 42 We therefore calculated the probabilities for our end-users, as one of our aims is that our CPR be easy to use. This is the sixth and final step outlined in Statistical analysis methods: transformation of the logistic regression model into a clinical prediction rule.
Internal validity of the clinical prediction rule
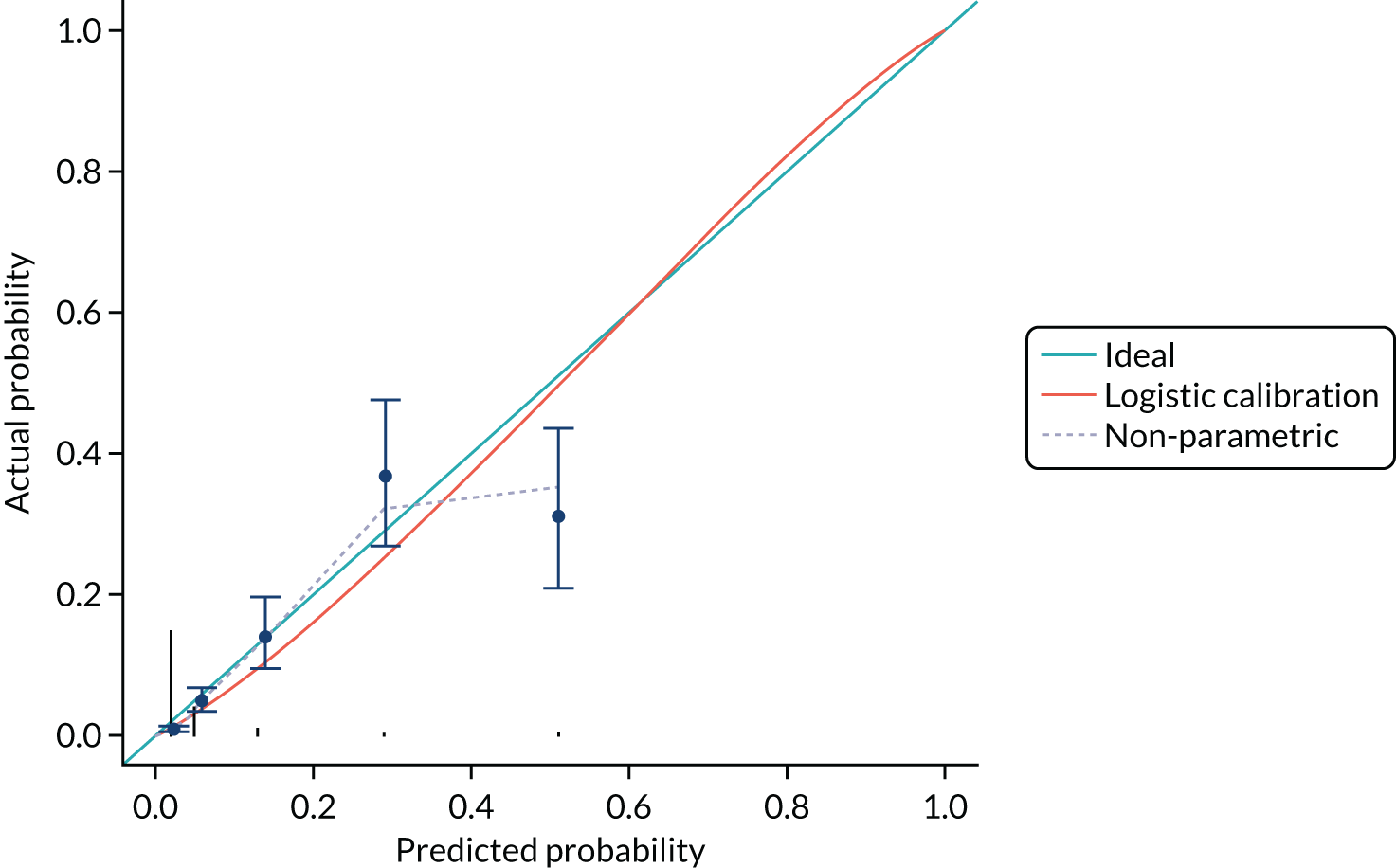
The calibration of the CPR is shown in Figure 5 (using the study-specific estimates) and in Figure 6 (using the population average estimates). The study-specific estimates, by definition, have a calibration slope of 1 and an intercept of 0, showing that the model has ideal calibration in the data set in which it was developed. The changes in slope and intercept for the population average estimates show that the CPR has been slightly recalibrated. We show these graphs for comparison with the calibration plot obtained with the Leese et al. 28 validation data set and because external calibration is a better guide of how a model will perform than internal calibration.
FIGURE 5.
Calibration plot for the CPR using study-specific estimates from the development data sets.

FIGURE 6.
Calibration plot for the CPR using population average estimates from the development studies.
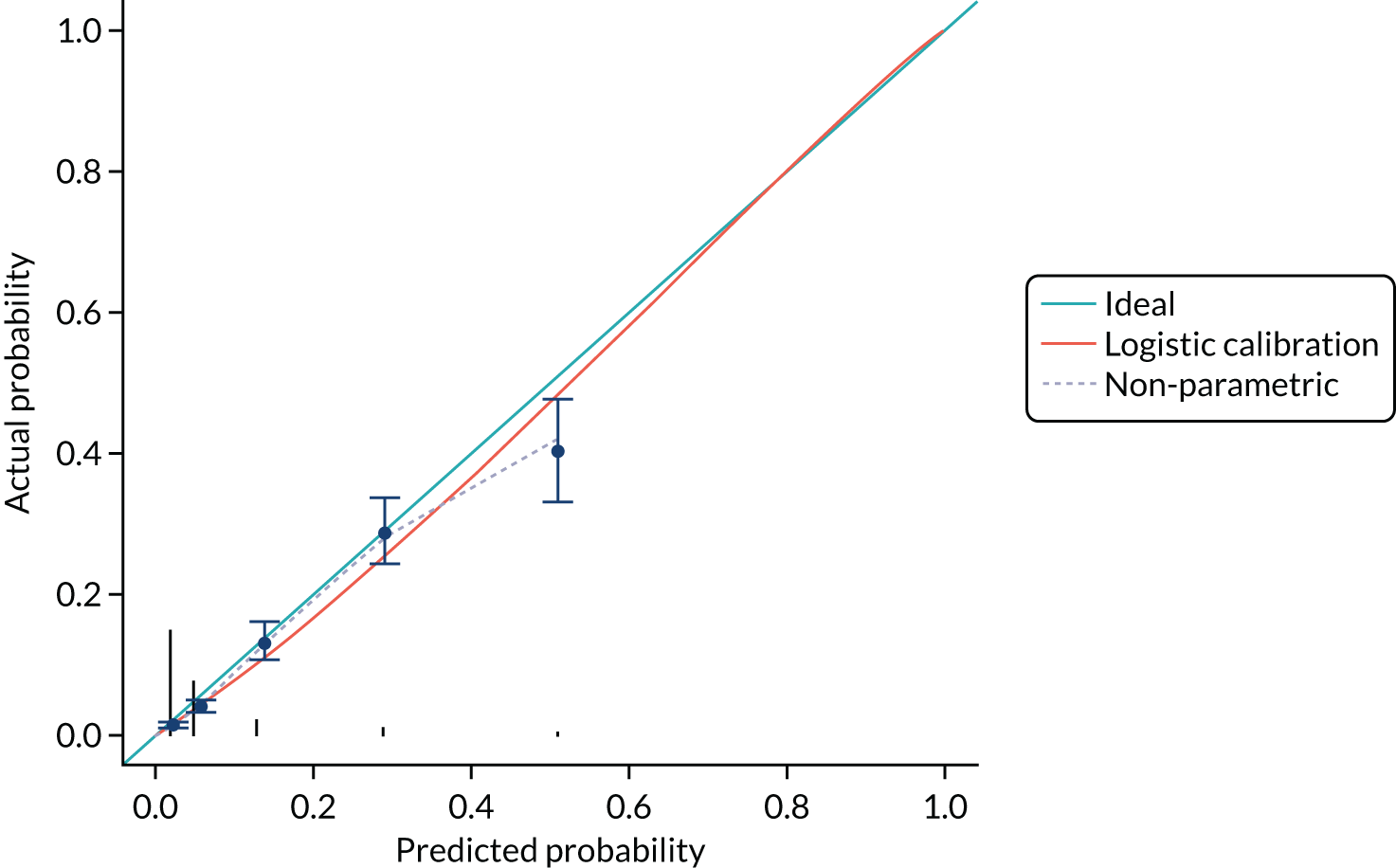
Discrimination of the CPR shown in Table 11 was assessed by calculating the area under the ROC curve (Figure 7). The c-statistic for the CPR is 0.796 (95% CI 0.772 to 0.820) and for the three-predictor model (monofilaments, pulses and history) is 0.802 (95% CI 0.778 to 0.825).
| CPR score | Patients (n) | Probability of ulcer at 2 years | 95% CI |
|---|---|---|---|
| 0 | 4646 | 0.024 | 0.014 to 0.03 |
| 1 | 2406 | 0.060 | 0.035 to 0.09 |
| 2 | 676 | 0.140 | 0.085 to 0.21 |
| 3 | 358 | 0.292 | 0.192 to 0.41 |
| 4 | 169 | 0.511 | 0.379 to 0.641 |
FIGURE 7.
The ROC curves for the CPR and three-predictor model for the prediction of ulcer at 2 years derived from the development data sets.

External validity of the clinical prediction rule
The discrimination and calibration plots generated by the CPR in the Leese et al. 28 data set show very similar results to those of the internal validation (Figures 8–10). Again, the calibration statistics suggest that the probability of ulcer at 2 years is underestimated by the CPR.
FIGURE 10.
The external validation calibration plot from the Leese et al. 28 data set for the three-predictor model.

We also compared the performance of the CPR with that of the original three-predictor model and found very little loss of accuracy with the CPR (Table 12). Appendix 3 gives a further comparison of the three-predictor and score models, using the development data sets.
The c-statistic for the CPR is 0.829 (95% CI 0.790 to 0.868). The c-statistic for the three-predictor model is 0.834 (95% CI 0.794 to 0.873).
| Model | Intercept (95% CI) | Slope (95% CI) |
|---|---|---|
| Three predictors | 0.046 (–0.336 to 0.428) | 1.133 (0.990 to 1.276) |
| CPR score | –0.059 (–0.431 to 0.314) | 1.139 (0.994 to 1.283) |
At a risk threshold of 6%, the net benefit is 0 for treat none and < 0 for treat all, but 0.015 for using the CPR (Figure 11). This can be interpreted as follows: if we choose to treat patients with CPR scores of ≥ 1, then, for every 1000 individuals, 15 additional cases of ulcer at 2 years would be correctly identified for treatment by the CPR, without increasing the number treated unnecessarily. At a risk threshold of 14%, the number of additional cases of ulcer at 2 years identified for treatment would be 10 per 1000 individuals.
FIGURE 11.
Net benefit plot for use of the CPR to identify patients who would benefit from an intervention to prevent foot ulcer, generated from the Leese et al. 28 validation data set.

Table 13 shows the PODUS CPR that is designed to predict the risk of ulceration within 2 years of patients with diabetes mellitus who do not currently have a foot ulcer.
| PODUS CPR | Score |
|---|---|
|
Test with 10-g monofilament Insensitive at any site – score 1 point Sensitive at all sites – score 0 points |
|
|
Check pedal pulses Any pulse missing – score 1 point Four pulses present – score 0 points |
|
|
Has there been an ulcer or amputation previously? Any ulcer or amputation – score 2 points No ulcer or amputation – score 0 points |
|
| Total score out of 4 |
Discussion
The CPR is simple and can be used without a calculator. The elements of the scoring system comprise neurological damage, as assessed by sensitivity to a 10-g monofilament; vascular damage, as assessed by the presence or absence of pedal pulses; and propensity to ulcerate, as assessed by history. This will give the CPR face validity for end-users. Monofilament sensitivity and the presence of pulses are quick, simple and cheap to measure. History of ulcer or amputation should be noted in the patient’s records or identifiable from the patient’s presentation.
An important component in the development of complications in diabetes mellitus is self-care by patients. We have very few data on this in the PODUS data sets, and so the statistical model underlying our CPR is incomplete. This may be why the CPR underestimates risk as some of the risk of ulcer development will depend on the level of diabetic control achieved by the patient; however, how self-care should be measured is the subject of ongoing research. 47
The performance of the CPR in the Leese et al. 28 validation data set suggests that simplifying the three-predictor model into the CPR resulted in little loss of discrimination and calibration. The calibration graphs indicate that both the CPR and the three-predictor model are least accurate for high-risk patients: those with a history of ulceration or amputation and at least one other risk factor. However, the treatment pathway for these patients is the same, so the use of neither the CPR nor the three-predictor model would result in a change in their care.
Ideally, the CPR will be validated in a new, prospective study. A new study’s results would be applicable to patients living with diabetes mellitus today. Although we made every reasonable effort to gather all of the data that were available, the data sets are not very recent and the factors driving the development of foot ulcer may have changed.
A small number of patients developed ulcers despite exhibiting no neurological or vascular damage (< 5% of all ulcers), but amputation is rarely necessary in such cases. The proportion of patients with this predictor combination was 1.96% in the development data sets and 0.93% in the validation data set.
Chapter 4 Systematic review of preventative interventions for foot ulceration in diabetes mellitus: an overview
Introduction
Accurate prediction models are useful for informing treatment decisions, but their value is ultimately dependent on the availability of effective interventions to modify an individual’s probability of developing a condition without causing further harm. 41,48 The effect of interventions is most reliably assessed in RCTs, as this is the only method of clinical evaluation that controls for known and unknown confounding factors. We were aware of important SRs of RCTs published from 1998 onwards that had identified RCTs that evaluated interventions to prevent foot ulceration in diabetes mellitus. 16,18,49–51 The existence of these SRs with similar objectives to our own introduced the possibility that the most efficient way to obtain numerical summaries of data (evidence) could be from an overview of SRs.
Overviews are used to summarise the effect of multiple interventions for a single condition. 52 They share some of the characteristics of SRs: a planned methodological approach, a defined question, a search strategy, methods of data extraction and assessment of review quality. We sought to obtain estimates of effect for preventative interventions for foot ulceration in diabetes mellitus from SRs of RCTs sufficient to populate an economic model. 53,54 The protocol for the overview was registered with PROSPERO (CRD42016052324).
Overview question
How effective are preventative strategies for foot ulceration?
Aim
The aim was to produce an overview of the effects of interventions to prevent foot ulceration in diabetes mellitus.
Objectives
The objectives were to identify SRs of RCTs and to obtain data about their effect on the incidence of foot ulcers to calculate measures of effect from individual RCTs, or pool estimates from several trials with which to populate an economic model.
Method
Searches
We searched for SRs using electronic search strategies created for Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands) MEDLINE, Ovid EMBASE and the Cochrane Library without language restrictions from inception until February 2019 (see Appendix 4). Our approach to searching was informed by a search string created by staff at the Centre for Reviews and Dissemination at the University of York to identify SRs. SRs in progress were identified via PROSPERO [URL: www.crd.york.ac.uk/prospero/ (last accessed 1 May 2020)].
Eligibility criteria
We assessed the scope of each review to ensure that it matched our objectives.
Participants
The participants were people of any age with a diagnosis of diabetes mellitus, either type 1 or type 2.
Intervention(s)
Simple interventions (e.g. pressure-distributing insoles or bespoke footwear or education packages in relation to foot care or other aspects of self-management aimed at patients or health-care professionals) or complex interventions (e.g. care from a specialist multidisciplinary team in which several interacting interventions were evident) were considered for inclusion in the review. SRs of wound treatments, including trials that evaluated dressings for foot ulcers, were excluded.
Comparator(s)
We included SRs that reported standard care or active comparators, including simple and complex interventions.
Outcomes
Primary outcomes
Incident (new) and recurrent foot ulcers were reported as binary outcomes (present or absent). These were defined in various ways, including as ‘a full thickness skin defect that requires more than 14 days to heal’55 or using a classification system:56
-
absolute numbers of incident ulcers
-
absolute numbers of recurrent ulcers.
Secondary outcomes
-
Amputation [minor, intrinsic to the foot (i.e. below the ankle), or major, involving the foot and leg].
-
Mortality.
-
Gangrene.
-
Infection.
-
Adverse events.
-
Harms.
-
Time to ulceration.
-
Quality of life (QoL) [assessed using the EuroQol-5 Dimensions (EQ-5D), Short Form questionnaire-12 items (SF-12), or Short Form questionnaire-36 items (SF-36)].
-
Timing of screening.
-
Self-care.
-
Hospital admissions.
-
Psychological (knowledge/behaviour).
Study design
We sought to obtain evidence of the effectiveness of interventions from SRs of RCTs. Where we identified SRs that included randomised and non-randomised studies, we included the review but extracted data only from the RCTs.
Review selection and data extraction
One reviewer screened all titles and abstracts to identify potentially relevant SRs. A second reviewer screened a 10% random sample of the yield. Two reviewers (DN and AA) working independently screened the full texts of titles considered potentially relevant to determine whether or not the objectives of each review matched our own with regard to the population, interventions, comparisons, outcomes and study design. Disagreements were resolved by discussion with a third reviewer (FC). Data were extracted into a review-specific data extraction tool (see Appendix 5, Data extraction and quality assessment: systematic review of randomised controlled trials) by two reviewers working independently (DN and FC). Separate data extraction and quality assessment tools were designed and piloted for the SRs. The following data were extracted:
-
review author and funder details
-
review objectives
-
eligibility criteria for trials to be included in the review
-
populations (including risk of ulceration), interventions, comparisons, outcomes and method of synthesis (e.g. narrative synthesis or meta-analysis).
Risk-of-bias (quality) assessment
We undertook an assessment of the quality of reporting using the risk of bias in systematic reviews (ROBIS) tool. 57 This tool assesses bias in four domains that correspond to the main processes for conducting SRs: determination of study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis methods and findings (Tables 14 and 15).
| Review (first author and year of publication) | Domain | |||
|---|---|---|---|---|
| Study eligibility criteria | Study identification and selection | Data collection and study appraisal | Synthesis methods and findings | |
| Adiewere 201858 | Unclear | Unclear | Unclear | High |
| Arad 201159 | Unclear | High | Low | High |
| Binning 201960 | Low | Low | High | Low |
| Dorresteijn 201251 | Low | Low | Low | Low |
| He 201361 | Low | High | Unclear | High |
| Hoogeveen 201516 | Low | Low | Low | Low |
| Kaltenthaler 199862 | Unclear | High | Unclear | Low |
| Mason 199918 | Unclear | High | Low | Low |
| O’Meara 200049 | Low | Low | Low | Low |
| Spencer 200050 | Low | Low | Low | Low |
| Review (first author and year of publication) | Domain | |||
|---|---|---|---|---|
| Study eligibility criteria | Study identification and selection | Data collection and study appraisal | Synthesis methods and findings | |
| Buckley 201363 | Low | High | Unclear | Low |
| Bus 201664 and Bus 200865 | Low | Low | Low | High |
| Healy 201466 | Low | High | Low | Unclear |
| Heuch 201667 | Low | High | Low | Low |
| Maciejewski 200468 | Unclear | High | Low | Low |
| Mayfield 200069 | High | High | Unclear | Unclear |
| Paton 201170 | Low | Unclear | Low | High |
| Ahmad Sharoni 201671 | Low | High | Low | Unclear |
| van Netten 201672 | Low | Low | Low | High |
We distinguished between SRs that included study designs other than RCTs and those that included RCTs alone, and we tabulated the two groups separately. This is because the ROBIS tool contains an assessment of the appropriateness of the synthesis relating to the nature and similarity in the research questions, study designs and outcomes across included studies (ROBIS domain 4; item 4.3), and we anticipated that the syntheses of data in reviews that included both RCTs and observational studies might be based on all included study designs (randomised and observational) and, if reviewers failed to separate the data from different types of study designs, might not reflect the findings from the RCT data alone.
Plan for data analysis
From each included SR we extracted absolute numbers for the primary and secondary outcomes and measures of effect with associated 95% CIs as reported in the reviews. We also noted the reviews’ overall conclusions about the effects of preventative interventions.
Results
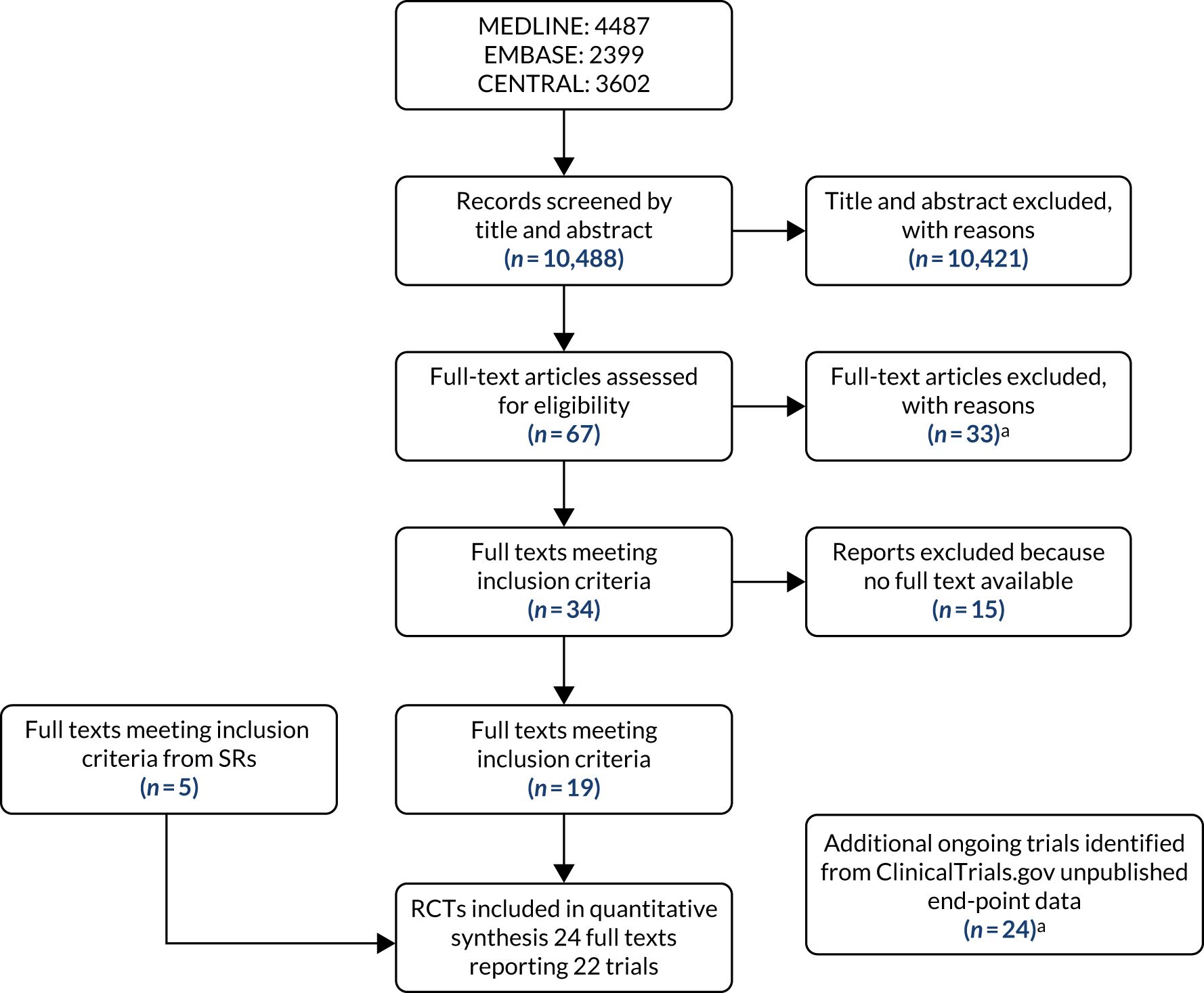
Our search, conducted up to February 2019, retrieved 7020 references. The level of agreement between the two reviewers (DN and AA) for selecting records by title and abstract was 58%, and disagreements were resolved by discussion with a third reviewer (FC). The flow diagram in Appendix 4, Figure 51, shows the number of articles at each stage of the process. We scrutinised 136 articles in a full-paper check, subsequently excluding 118 (see Appendix 3, Table 41). We added two reviews that were identified from searching the reference lists of the 18 reviews identified from the search of databases.
In total, we identified 20 SRs that aimed to evaluate interventions to prevent foot ulceration in diabetes mellitus. Ten included only RCTs, and 10 included RCTs and observational studies; among the latter, one SR was an updated version of another. 64,65 All reviews were published between 1998 and 2019. Tables 16 and 17 detail the scope of the reviews and Tables 18 and 19 detail the results of the overview.
| SR (first author and year of publication)/country/funder | Aim | Search strategy | Definition of ulcer | RCTs and total number of patients |
|---|---|---|---|---|
|
Adiewere 201858 UK The Independent Diabetes Trust |
To examine the effectiveness of patient education in preventing or reducing the incidence or recurrence of foot ulcers in adults with diabetes |
Six databases: MEDLINE, EMBASE, CINAHL, PsycINFO, the Cochrane Library and Evidence-based Nursing. Searched from inception until September 2017 Nursing portal, National Library for Health, Excerpta Medica (Excepta Medica BV, Amsterdam, the Netherlands) and Google Scholar (Google Inc., Mountain View, CA, USA), bibliographies of relevant textbooks Search strategy reported: not reported Excluded studies reported: no |
Not reported | Six RCTs; 1525 |
|
Arad 201159 USA NR |
To evaluate trials of interventions to prevent DFU and not methods that simply predict the likelihood of future ulcers or treat pre-existing foot ulcers |
Six databases: MEDLINE, PubMed, Clinical Trials section of the Cochrane Library, ClinicalTrials.gov, WHO’s International Clinical Trials Registry Platform Number registry and Google databases. Searched from 1 January 1960 to 30 April 2010 Search strategy reported: not reported/unclear Excluded studies reported: no |
Not reported | Eight RCTs; 3520 |
|
Binning 201960 UK NR |
To determine whether or not motivational interviewing is an effective intervention to improve adherence behaviours for the prevention of DFU |
Eleven databases: MEDLINE, CINAHL, ProQuest® (ProQuest LLC, Ann Arbor, MI, USA), Nursing & Allied Health Database, PsycINFO, CENTRAL, AMED, EMBASE, Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA) core collections and Science Direct® (Elsevier, Amsterdam, the Netherlands) Search strategy reported: yes Excluded studies reported: no |
Foot ulceration | One RCT; 131 |
|
Dorresteijn 201251 The Netherlands NR |
To assess the effects of patient education on the prevention of DFU |
Five databases: Cochrane Wounds – 3 September 2014; CENTRAL up to 2012 issue 7; MEDLINE, EMBASE, and CINAHL up to July 2012; Ovid MEDLINE 2009 to July week 3 2012; Ovid MEDLINE In-Process & Other Non-Indexed Citations, 31 July 2012; Ovid EMBASE (2009 to 2012 week 30) and CINAHL [via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA)] 2009 to 26 July 2012 Search strategy reported: yes Excluded studies reported: yes |
Foot ulcers are open sores | Two RCTs; 225 |
|
He 201361 China NR |
To assess the effectiveness of intensive vs. routine education on diabetes mellitus for preventing DFU |
Five databases: CENTRAL up to 2013 issue 1; PubMed and EMBASE (1978–2013); VIP (1989–2013); and Wang Fang Data (1980–2013) Search strategy reported: insufficient Excluded studies reported: no |
Not reported | Two RCTs; 231 |
|
Hoogeveen 201516 The Netherlands NR |
To determine the effectiveness of complex interventions against single interventions for the prevention of DFU. A complex intervention is defined as an integrated care approach, combining two or more prevention strategies on at least two different levels of care: the patient, the health-care provider and/or the structure of health care |
Nine databases: Cochrane Wounds up to 22 May 2015; CENTRAL, the DARE, the HTA database, the NHS EED via the Cochrane Library up to 2015, issue 4; MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations from 1946 to 21 May 2015; EMBASE from 1974 to 21 May 2015; and CINAHL from 1982 to 22 May 2015 Search strategy reported: yes Excluded studies reported: yes |
SR did not report, but this differed for each study | Three RCTs; 2458 |
|
Kaltenthaler 199862 UK NR |
To critically review evidence on the effectiveness of interventions for treating and preventing DFU |
Eight databases: CINAHL, the Cochrane Library, EMBASE, HealthSTAR, MEDLINE, PharmacoEconomics & Outcomes News, NHS EED and DataStar (Absolute Technology Ltd, Southampton, UK). Searched from 1986 to 1996 Search strategy reported: no Excluded studies reported: no |
Wagner Classification System divides DFUs into grades of severity 0–573 | Two RCTs; 464 |
|
Mason 199918 UK NHS Executive and British Diabetic Association |
To identify effective interventions for the management of the diabetic foot |
Eight databases: Cochrane Trials Register, MEDLINE, EMBASE, CINAHL, HealthSTAR, PsychLIT, Science Citation Index, and Social Science Citation Index. Searched from 1983 ‘onwards’ Search strategy reported: no Excluded studies reported: no |
At risk or damaged foot in diabetes defined not DFUs in particular74 | Three RCTs; 2465 |
|
O’Meara 200049 UK National Institute for Health Research, UK |
To examine the clinical effectiveness and cost-effectiveness of interventions for the prevention and treatment of DFU; to identify significant gaps in the research evidence; to outline the type of research needed to provide relevant information to the NHS |
Nineteen databases: MEDLINE from 1966 up to end of 1998; and Science Citation Index (Clarivate Analytics, Philadelphia, PA, USA), BIOSIS, British Diabetic Association Database, CINAHL, CISCOM, Cochrane Database of Systematic Reviews, Cochrane Wounds, Current Research in Britain, DARE, Dissertation Abstracts International, Department of Health and Social Security data, EconLit, EMBASE, Index to Scientific & Technical Proceedings, NHS EED (NHS Centre for Reviews and Dissemination), Royal College of Nursing Database, System for Information on Grey Literature in Europe (SIGLE – BLAISE-LINE) and National Research Register up to the end of 1998 Search strategy reported: yes Excluded studies reported: yes |
Author defined diabetic foot, using Wagner’s system75 for the classification of diabetic feet, but did not define DFU | Four RCTs; 2625 |
|
Spencer 200050 UK No external sources of support |
To assess the effectiveness of pressure-relieving interventions in the prevention and treatment of DFU |
Nineteen databases: in the paper reported only Cochrane Wounds Group methods used in search strategy. This uses the Cochrane Wounds and CENTRAL – both dates not reported; MEDLINE from 1946 onwards; EMBASE from 1974 onwards; EBSCOhost from 1982 onwards; CINAHL trial registries; ClinicalTrials.gov; the WHO’s International Clinical Trial Registry platform; and the European Union Clinical Trials Register (all dates not reported) Search strategy reported: insufficient Excluded studies reported: yes |
Not reported | One RCT; 69 |
| SR (first author and year of publication)/country/funder | Aim | Search strategy | Definition of ulcer | RCTs and total number of patients |
|---|---|---|---|---|
|
Buckley 201363 Ireland Health Research Board Ireland |
To determine the effect of contact with a podiatrist on the occurrence of LEAs in people with diabetes |
Four databases: PubMed from 1966 to 25 September 2011; CINAHL from 1981 to 25 September 2011; EMBASE from 1974 to 25 September 2011; and the Cochrane databases from 1991 to 25 September 2011 Search strategy reported: yes Excluded studies reported: yes |
Not reported | One RCT; 91 |
|
The Netherlands NR |
To assess the effectiveness of footwear and offloading interventions to prevent or heal foot ulcers or to reduce mechanical pressure |
Eight databases: PubMed, EMBASE, CINAHL, Cochrane Database of Systematic Reviews, CENTRAL, NHS Economic Evaluation Database, and Health Technology Assessment Database. Searched from May 2006 to July 2014 Search strategy reported: yes Excluded studies reported: yes |
Not reported | Seven RCTs; 1476 |
|
Healy 201466 UK NR |
To examine the quality and effectiveness of footwear to prevent DFU or to reduce biomechanical risk factors for ulceration |
Three databases: CINAHL, MEDLINE and Cochrane Register of Controlled Trials. Searched up to December 2012 Search strategy reported: insufficient Excluded studies reported: no |
Not reported | Two RCTs; 469 |
|
Heuch 201667 Australia NR |
To identify, critically appraise and synthesise the best available evidence on methods of offloading to prevent the development, and reduce the risk, of primary foot ulceration in adults with diabetes |
Thirteen databases: PubMed, Cochrane Database of Systematic Reviews, CINAHL, EMBASE, Scopus, Google Scholar, Cochrane – Protocols, Research and Trials Register, ClinicalTrials.gov, NHS Research Register, regard (database of Economic and Social Research Council), OpenSIGLE, MedNar, WorldWideScience. Searched up to November 2013 Search strategy reported: yes Excluded studies reported: yes |
Cochrane Wound Group:76 an area of skin loss resulting from poor blood supply and/or reduced nerve function in the lower limb caused by diabetes mellitus | 0 RCTs |
|
Maciejewski 200468 USA Department of Veterans Affairs |
To review the evidence for the effectiveness of therapeutic footwear in preventing re-ulceration in people with diabetes and to discuss factors influencing study findings |
One database: MEDLINE from 1980 to ‘present’ Search strategy reported: insufficient Excluded studies reported: no |
Not reported | Two RCTs; 469 |
|
Mayfield 200069 USA NR |
To evaluate Semmes–Weinstein monofilament and other threshold testing in preventing ulcers and amputation |
One database: MEDLINE from 1985 to 2000 Search strategy reported: insufficient Excluded studies reported: no |
Not reported | One RCT; 2001 |
|
Paton 201170 UK NR |
To evaluate the effectiveness of insoles used for the prevention of ulcer in neuropathic diabetic foot |
Two databases: MEDLINE and CINAHL. Searched up to 2008 Search strategy reported: insufficient Excluded studies reported: no |
Not reported | One RCT; 69 |
|
Ahmad Sharoni 201671 Malaysia NR |
To assess the effectiveness of health education programmes to improve foot self-care practices and foot problems among older people with diabetes |
Six databases: EBSCOhost medical collections (MEDLINE, CINAHL, Psychology and Behavioural Sciences Collection), SAGE, Wiley Online Library (John Wiley & Sons, Inc., Hoboken, NJ, USA), ScienceDirect, SpringerLink (Springer Nature Switzerland AG, Cham, Switzerland), and Web of Science. Searched from January 2000 to March 2015. Search strategy reported: yes Excluded studies reported: no |
Not reported | One RCT; 172 |
|
van Netten 201672 The Netherlands NR |
To determine the effectiveness of patient education to prevent foot ulceration in persons with diabetes who are at risk of foot ulceration and do not have a current foot ulcer |
Eight databases: PubMed, Excerpta Medica database (EMBASE) via OvidSP (Health First, Rockledge, FL, USA), CINAHL, Cochrane Database of Systematic Reviews, DARE, CENTRAL, WHO’s International Clinical Trials Registry Platform and ClinicalTrials.gov. Searched up to July 2014 Search strategy reported: yes Excluded studies reported: no |
A DFU was defined as a ‘full thickness lesion of the skin distal to the malleoli in a person with DM’77 | 17 RCTs; 3107 |
| SR (first author and year of publication) | Intervention/control/risk status | Outcomes | Synthesis of data |
|---|---|---|---|
| Adiewere 201858 |
Intervention: patient education Control: standard care Risk status: unclear |
Patient education Monami 1995. 78 DFU: RR 0.08 (95% CI 0.00 to 1.34). Knowledge improved; p = 0.001 Gershater 2011. 79 Ulcer recurrence: intervention group (n = 19) 48%, control group (n = 22) 38% (p > 0.05) Lincoln 2008. 80 Recurrent ulcers: foot care behaviour showed a significant improvement in intervention group (p = 0.03). No clinical benefits from education Rönnemaa 1997. 81 Foot care knowledge improved in the intervention group after 12 months. No effects of education on DFUs or amputation rate. Increase in foot care knowledge in the intervention group (p = 0.004) Malone 1989. 82 Marked reduction in ulceration incidence in the intervention group (n = 8) compared with the control group (n = 28): RR 0.31 (95% CI 0.14 to 0.66). Amputation: RR 0.33 (95% CI 0.15 to 0.76) Bloomgarden 1987. 83 Education had no significant effects on ulceration, amputation, callus formation, nail dystrophy or fungal infection |
Meta-analysis |
| Arad 201159 |
Interventions:Control: no information Risk status: high risk |
Patient education Litzelman 1993. 6 Number of participants not reported; outcomes unclear Lincoln 2008. 80 No difference in the rate of foot ulcers McCabe 1998. 84 Decrease in major amputations but not in minor amputations or ulcerations Therapeutic footwear Uccioli 1995. 85 At 1 year there was a significant difference (27.7% vs. 58.3%) but the direction of the effect is unclear Reiber 2002. 86 No differences in incidence of foot ulceration between the two groups Lavery et al. (unpublished: personal communication to Arad59). Patients with a history of foot ulceration showed a reduction of > 90% but patients without a history did not Plantar foot temperature-guided avoidance therapy Lavery 2004. 87 Patients (n = 85), 7% vs. 2% (the ulceration rate in the intervention and control groups); p = 0.01 Lavery 2007. 88 Ulceration rate was 30% in the intervention and control groups and 8.5% in the temperature-guided avoidance therapy group Armstrong 2007. 89 Ulcer rates for two groups unclear (12.2–4.7% in the temperature group) |
Narrative |
| Binning 201960 |
Intervention: motivational interviewing Control: not reported Risk status: ‘at risk of DFUs’ |
Gershater 2011.79 Incidence of ulceration as an outcome. The intervention did not improve ulceration rates compared with the control group | Narrative |
| Dorresteijn 201251 |
Intervention: education aimed at DFUs, diabetes in general, including foot care education, diabetic foot programme with patient education on foot care Control: all types of controls were considered for inclusion and so this varied between trials Risk status: varied |
Intensive compared with brief educational interventions Lincoln 2008. 80 Ulcer rate at 12 months: 36/87 vs. 35/85. Amputation at 12 months: 9/87 vs. 9/85 Cisneros 2010. 90 Foot ulcers were observed in 22/51 people. The accompanying survival curve in the trial report showed a trend towards longer event-free survival in intervention group participants, but this was not statistically significant (p = 0.362; hazard ratio not reported) |
Numerical summary, data plotted on a forest plot without summary statistic |
| He 201361 |
Intervention: intensive diabetic education. Unclear how provided Control: routine diabetes education Risk status: not reported |
Lincoln 2008. 80 Ulceration: RR 1.00 (95% CI 0.70 to 1.44) Amputation: RR 0.97 (95% CI 0.37 to 2.59) Cisneros 2010. 90 Ulcers: RR 0.53 (95% CI 0.28 to 1.02) |
Meta-analysis with summary statistic: RR, OR, mean differences, 95% CI |
| Hoogeveen 201516 |
Intervention: complex integrating care combining two or more prevention strategies on two or more different levels of care: patient, health-care provider and/or structure of health care. Differed for each study. Included education and footwear Control: differed for each study but included written foot care instructions only as a single intervention, with usual care or alternative complex intervention, which differed from the experiment on two different levels Risk status: varied |
More intensive and comprehensive complex interventions vs. usual care McCabe 1998. 84 Ulcers at 2-year follow-up: intervention group 24/1001 vs. control group 35/1000; RR 0.69 (95% CI 0.41 to 1.14). Amputation: intervention group 7/1001; control group 23/1000 (RR 0.30, 95% CI 0.13 to 0.71) Liang 2012. 91 Ulcers: intervention group, 0/31; control group, 7/31. Amputation: intervention group, 0/31; control group, 2/31 Educationally focused interventions vs. usual care or less intensive programmes Litzelman 1993. 92 Amputation: intervention group, 1/191; control group, 4/205 No ulcer data mentioned |
Meta-analysis without summary statistic; with summary statistic risk ratios and 95% CI |
| Kaltenthaler 199862 |
Intervention: health education, therapeutic footwear Control: no education, and patients wore their own shoes Risk status: not reported |
Health education Litzelman 1993. 92 Lower extremity abnormality: 59% reduction in risk in the intervention group Therapeutic shoes Uccioli 1995. 85 Ulcer relapse rate: 27.7% in the intervention group vs. 58.3% in the control group |
Narrative |
| Mason 199918 |
Intervention:Control: none or normal education, usual care, conventional podiatric care, patient’s own shoes Risk status: not reported |
Patient education Litzelman 1993. 92 Significant reduction in serious lesions (OR 0.41, 95% CI 0.16 to 1.00; p = 0.05). Amputation rate 1/191 in the intervention group and 4/205 in the control group Screening and interventions for patients with raised risk of ulceration McCabe 1998. 84 Ulcer rate: 24/1001 in the intervention group vs. 35/1000 in the control group Proportion of ulcers leading to amputations: intervention group, 7/24; control group, 23/35 Amputations (major): intervention group, 1/1001; control group, 6/1001 Footwear in patients with raised risk of ulceration Uccioli 1995. 85 Ulcer relapse rate: intervention group, 9/33; control group, 21/36 |
Narrative |
| O’Meara 200049 |
Intervention: orthotics, podiatry, therapeutic shoes with custom-moulded insoles, standard below-knee elastic stockings, education, insulin treatment, multifaceted health-care intervention, simple education and routine diabetic teaching, screening, prevention programme Control: traditional podiatrist treatment/routine patient care – written instructions, ordinary non-therapeutic shoes, elastic stockings vs. no stockings, usual care, no special foot care education Risk status: high risk |
Uccioli 1995. 85 Ulcer relapse at 1 year: intervention group, 9/33; control group, 21/36 (OR 0.29, 95% CI 0.11 to 0.74) Ulcer-free time: intervention group mean, 9.1 (SD 3.7) months, control group mean, 3.7 (SD 3.1) months Belcaro 1992. 93 Number of ulcerated limbs at year 4: intervention group, 3/148; control group, 10/150 (OR 0.33, 95% CI 0.11 to 1.00). Total number of ulcers: intervention group, 3/74; control group, 10/75 (OR 0.31, 95% CI 0.10 to 0.98) Litzelman 1993. 92 Serious foot lesions: intervention group, 7/176; control group, 16/175 (OR 0.32, 95% CI 0.19 to 1.00). Amputation rate (foot or limb): intervention group, 1/191; control group, 4/205 (OR 0.32, 95% CI 0.05 to 1.86) McCabe 1998. 84 Incidence of ulceration: intervention group, 24/1001; control group, 35/1000 Proportion of ulcers leading to amputations: intervention group, 29%; control group, 66% Number of amputations: intervention group, 7 (one major and six minor); control group; 25 (12 major and 13 minor) |
Narrative and meta-analysis with summary statistic ORs |
| Spencer 200050 |
Intervention: orthotics, podiatry, total contact casting, therapeutic shoes, education Control: not reported Risk status: varied |
Pressure-relieving devices vs. standard care Uccioli 1995. 85 Incidence of ulcer relapse: intervention group, 9/33; control group, 21/36 (OR 0.29, 95% CI 0.11 to 0.74) Mean ulcer-free time WMD: 5.40 (95% CI 3.78 to 7.02) |
Meta-analysis without pooled summary statistics |
| SR (first author and year of publication) | Intervention/control/risk status | Outcomes | Synthesis of data |
|---|---|---|---|
| Buckley 201363 |
Intervention: patient education, podiatry, chiropody Control: written information or chiropodist treatment, not specifically recommended Risk status: varied |
Chiropodist visit Plank 200394 Recurrence rate of ulcers: not reported in SR Amputation at 1 year: intervention group, 2; control group, 1 |
Meta-analysis with summary statistic: RR. Separate forest plots for RCT/cohort |
| Bus 201664 and Bus 200865 | Interventions:
|
Footwear and orthoses Lavery 2012. 95 Ulcer recurrence: intervention group, 2.0% (n = 3/149); control group, 6.7% (n = 10/150) (p = 0.08) Rizzo 2012. 96 Ulcer incidence at 1 year: intervention group, 12.8%; control group, 38.6% (p < 0.0001). At 3 years: intervention group, 17.6%; control group, 61.0% (p < 0.0001). At 5 years: intervention group, 23.5%; control group, 72.0% (p = 0.0001) Scirè 2009:97 Ulcer incidence: intervention group, 1.1% (1/89); control group, 15.4% (12/78) (p < 0.001) Uccioli 1995. 85 Ulcer recurrence: intervention group, 27.7%; control group, 58.3%; p = 0.009 Ulbrecht 2014. 98 Ulcer recurrence at 16.5 months: intervention group, 9.1%; control group 25.0% (p < 0.007; HR 3.4, 95% CI 1.3 to 8.7). All lesions at 6 months: in intervention group significantly less than in control group (p = 0.042). Ulcer recurrence at 6 months: in intervention group significantly less than in control group (p = 0.003) Bus 2013. 99 Ulcer recurrence: intervention group, 33 of 85 (38.8%); control group, 38 of 86 (44.2%) (p = 0.48; OR 0.80, 95% CI 0.44 to 1.47). Ulcer recurrence in 79 adherent patients (i.e. > 80% of steps in prescribed footwear): intervention group, 9 of 35 (25.7%); control group, 21 of 44 (47.8%) (p = 0.045; OR 0.38, 95% CI 0.15 to 0.99). All lesions at 12 months: not significantly different (p = 0.073). Ulcer recurrence at 12 months: in intervention group significantly less than in control group (p = 0.0041) Reiber 2002. 86 Recurrent ulcers in 2 years: intervention group 1, 26; intervention group 2, 31; control group, 38. Number of patients with an ulcer in 2 years: intervention group 1, 14.9% (18/121), intervention group 2, 14.3% (17/119), control group, 16.9% (27/160). Relative risk ratios: intervention group 1 vs. control group 0.88 (95% CI 0.51 to 1.52), intervention group 2 vs. control group 0.85 (95% CI 0.48 to 1.48) |
Not reported. Reported in discrete sections |
| Healy 201466 |
Intervention: footwear as a method for offloading to prevent DFU Control: usual footwear Risk status: high risk |
Reiber 2002. 86 Ulceration: intervention group 1.15%; intervention group 2, 14%; control group 7% Uccioli 1995. 85 Ulcer relapse: intervention group 2, 7.7%; control group 5, 8.3% (p = 0.009) (Absolute numbers unclear for both studies) |
Narrative |
| Heuch 201667 |
Intervention: customised rigid orthotic device Control: traditional treatment by podiatrist Risk status: low risk |
No RCTs were identified that met the criteria for our overview | Narrative reported in discrete sections |
| Maciejewski 200468 |
Intervention: therapeutic footwear, therapeutic shoes with insoles (cork/polyurethane inserts), slippers Control: unclear in reporting Risk status: high risk |
Uccioli 1995. 85 Re-ulceration: intervention group. 27.7%; control group, 58.3% (RR 0.47, 95% CI 0.23 to 0.90; OR 0.26) Reiber 2002:86 No significant difference in re-ulceration between cork and control groups (RR 0.88, 95% 0.51 to 1.52) or between polyurethane and control groups (RR 0.85, 95% 0.48 to 1.48) |
Narrative reported in discrete sections |
| Mayfield 200069 |
Intervention: screening Control: unclear in reporting Risk status: low risk |
Klenerman and McCabe 1998.84,100 Ulcers: intervention group 24 vs. control group 35. Minor amputations: intervention group 6 vs. control group 13. Major amputations: intervention group 6 vs. control group 12 | Narrative reported in discrete sections |
| Paton 201170 |
Intervention: therapeutic footwear with PPT and Plastazote®-casted insoles (Zotefoam, Croydon, UK), magnetic insoles Control: own footwear or sham Risk status: high |
Uccioli 1995.85 Ulcer relapses: intervention group, 27.7%; control group, 58.3%. Therapeutic insoles plus shoes not associated with ulceration: R = –0.315 (95% CI –0.54 to –0.08). Ulcer-free time: intervention group, 9.1 ± 3.7 months, control group, 3.7 ± 3.1 months | Narrative reported in discrete sections |
| Ahmad Sharoni 201671 |
Intervention: education programmes to improve foot self-care practices and foot problems Control: usual care Risk status: not reported |
Lincoln 2008.80 No significant difference was observed between groups in ulcer or amputation incidence at either 6 or 12 months | Narrative reported in discrete sections |
| van Netten 201672 |
Interventions:Control: usual care Risk status: ‘at risk’ |
Foot care programmes Liang 2012. 91 Ulcers: intervention group, 0%; control group, 24.1% (n = 7) (p = 0.0137). Minor amputation: intervention, group, 0% (n = 0); control group, 6.9% (n = 2) (p = 0.4569) Van Putten 2010. 101 Ulcer incidence: intervention group, 10% (n = 28); control group, 11% (n = 30) (p = 0.89). Severe ulcers: (infected or deep ulcers): intervention group, 11% (n = 3/28); control group, 37% (n = 11/30) (p = 0.03). Amputation: intervention group, 1% (n = 2); control group, 2% (n = 6) (p = 0.29) Cisneros 2010. 90 Ulcer intervention group, 38.1% (8/30); control group, 5.1% (8/23) (p = 0.29) Plank 2003. 94 Recurrence (per patient): intervention group, 38% (n = 18); control group, 57% (n = 25) (HR 0.60, 95% CI 0.32 to 1.09; p = 0.9). Ulcer recurrence (per foot): intervention group, 22% (n = 20); control group, 38% (n = 32) (RR 0.52, 95% CI 0.29 to 0.93; p = 0.03). Amputation: intervention group, 4% (n = 2); both minor); control group, 2% (n = 1, minor). Mortality: intervention group, 4% (n = 2); control group, 9% (n = 4). Aggregated DFUs, amputation and mortality: intervention group, 38% (n = 18); control group, 66% (n = 29) (HR 0.54, 95% CI 0.30 to 0.96; p = 0.03) Self-management Armstrong 2005. 102 Ulcer: intervention group, 5.9% (n = 2); control group, 5.6% (n = 2) (p = 0.9). No difference in unexpected visits or missed appointments Armstrong 2007. 89 Ulcer: intervention group, 4.7% (n = 5); control group, 12.2% (n = 14) (OR 3.0, 95% CI 1.0 to 8.5; p = 0.038) Lavery 2004. 87 Ulcer/Charcot fracture: intervention group, 2.4% (n = 1); control group, 20.0% (n = 9) (p = 0.0; RR 10.3, 95% CI 1.2 to 85.3). Ulcer: intervention group, 2.4% (n = 1); control group, 1.6% (n = 7) (p < 0.05). Amputation: intervention group, 0% (n = 0); control group, 2.3% (n = 1) Lavery 2007. 88 Ulcer/Charcot fracture: intervention group, 8.5% (n = 5); control group 1, 30.4% (n = 17); control group 2, 29.3% (n = 17). Intervention vs. control group 1; OR 4.71 (95% CI 1.60 to 13.85) (p = 0.0061). Control group 1 vs. control group 2: OR 4.48 (95% CI 1.53 to 13.14) (p = 0.008). Time to ulceration: intervention vs. control group 1 vs. control group 2; p = 0.011 Patient education Gershater 2011. 79 Ulcer recurrence: intervention group, 48% (n = 19); control group, 38% (n = 22) (p > 0.05). Time to recurrence not significantly different between intervention group and control group (no p-value reported) Lincoln 2008. 80 Recurrent ulcers: intervention group, 41.4% (n = 36); control group, 41.2% (n = 35) (RR 0.997, 95% CI 0.776 to 1.280). Amputation: intervention group, 10.3% (n = 9) (one major, eight minor) (RR 1.003, 95% CI 0.905 to 1.111). Recommended foot care behaviours were better in the intervention than in the control group at 12 months Footwear and orthoses Scirè 2009. 97 Ulcer incidence: intervention group, 1.1% (1/89); control group, 15.4% (12/78) (p < 0.001) Lavery 2012. 95 Ulcer recurrence: intervention group, 2.0% (n = 3); control group, 6.7% (n = 10) (p = 0.08) Rizzo 2012. 96 Ulcer incidence at 1 year: intervention group, 12.8%; control group, 38.6% (p = 0.0001). Ulcer incidence at 3 years: intervention group, 17.6%; control group, 61.0% (p = 0.0001). Ulcer incidence at 5 years: intervention group, 23.5%; control group, 72.0% (p = 0.0001) Ulbrecht 2014. Ulcer/Charcot fracture: intervention group, 2.4% (n = 1); control group, 20.0% (n = 9) (p = 0.01; RR 10.3, 95% CI 1.2 to 85.3). Ulcer: intervention group, 2.4% (n = 1); control group, 1.6% (n = 7) (p < 0.05). Amputation: intervention group, 0% (n = 0); control group, 2.3% (n = 1) Bus 2013. 99 Ulcer recurrence: intervention group, 33 of 85 (38.8%); control group, 38 of 86 (44.2%) (p = 0.48; OR 0.80, 95% CI 0.44 to 1.47). Ulcer recurrence in 79 adherent patients (i.e. > 80% of steps in prescribed footwear): intervention group, 9 of 35 (25.7%); control group, 21 of 44 (47.8%) (p = 0.045; OR 0.38, 95% CI 0.15 to 0.99) Reiber 2002. 86 Number of recurrent ulcers in 2 years: interventon group 1, 26; intervention group 2, 31, control group, 38 (not significant). Number of patients with an ulcer in 2 years: intervention group 1, 14.9% (18/121); intervention group 2, 14.3% (17/119); control group, 16.9% (27/160). RR ratio: intervention group 1 vs. control group 0.88 (95% CI 0.51 to 1.52); intervention group 2 vs. control group 0.85 (95% CI 0.48 to 1.48) Uccioli 1995. 85 Ulcer recurrence: intervention group, 27.7%; control group, 58.3% (p = 0.009) |
Narrative reported in discrete sections |
Interventions
Education alone
Researchers from Glasgow, UK, and Amsterdam, the Netherlands, conducted a SR to assess the effect of motivational interviewing on adherence to interventions to prevent diabetic foot ulceration. 60 A search of 11 databases for articles published until 2018 found one RCT79 that met our eligibility criteria. The reviewers used a 21-item checklist designed to identify bias in and quality of studies of the foot in diabetes mellitus. Only one of the included trials measured foot ulceration as an outcome. Other outcomes included behaviour and knowledge of foot care practices. A narrative synthesis found insufficient evidence about the value of motivational interviewing (or similar behavioural interventions) in preventing DFU.
Our assessment of bias in this review found weaknesses in the data collection, which involved only one reviewer.
Researchers from Utrech, the Netherlands, reported a SR that assessed the effect of educational interventions on the prevention of diabetic foot ulcerations, which was published in the Cochrane Library. 51 A search of five databases from inception until 2012 identified two RCTs that met our eligibility criteria (n = 231 people with diabetes mellitus who either had a history of foot ulceration or were at high risk of foot ulceration). A variety of outcomes were measured in the included trials: foot care knowledge, behaviour, self-confidence scores and common foot disorders including callus and nail dystrophies. With regard to foot ulceration and amputation, the authors concluded that only two sufficiently powered trials reported the effect of education and that there was insufficient robust evidence that it was effective. The risk of bias in the RCTs was assessed using the Cochrane risk-of-bias tool. Our assessment of review quality using the ROBIS tool found the review to be at low risk of bias.
A group of researchers from Ya’an, China, conducted a SR61 that was published in Chinese and translated for our team by a researcher fluent in Chinese (Xin Wang) and using the translate function of Google (Google, Inc., Mountain View, CA, USA). A search of five databases from inception to 2012 identified two RCTs (n = 231 people with diabetes mellitus). The participants’ risk of foot ulceration was not reported. The meta-analysis (n = 1189) showed that the incidence of foot ulcers was lower in the diabetes mellitus education group than in the control group (64/610 vs. 102/579), and the difference was statistically significant [relative risk (RR) 0.51, 95% CI 0.30 to 0.84]. The reviewers used the Cochrane risk-of-bias tool to assess the quality of the included trials and found them to be mainly at high risk of bias. The reviewers concluded that, compared with routine education, intensive education could reduce the incidence of DFUs but that this needed to be verified in high-quality studies.
We were unable to replicate this meta-analysis because we could not obtain three of the RCTs from the British Library. 104–106 In our assessment of the risk of bias of this SR, the search strategy was judged to be weak because of limited search terms and because restrictions on search dates may have led to trials being missed.
A team of researchers from Selangor, Malaysia,71 undertook a SR to assess the effectiveness of health education programmes in improving foot self-care practices and foot problems among older people with diabetes mellitus. 85 A search of six databases between January 2000 and March 2015 found one RCT that met our eligibility criteria (n = 172). The reviewers also included one RCT that explicitly excluded people with diabetes mellitus, despite the review title indicating that this was the population of interest. 107 The reviewers assessed study quality using the Cochrane risk-of-bias tool. 52 The reviewers found no statistical differences between the groups in terms of foot ulcer and amputation incidence at 6 or 12 months. The reviewers concluded that education programmes showed an improvement in self-care scores and foot problems but further evaluations are required.
In our assessment of the quality of this review, we found several threats to the validity of the findings: the approach to applying the review eligibility criteria was ambiguous, and the inclusion of a trial that excluded patients with diabetes mellitus appeared to contradict the eligibility criteria of the review.
A team of researchers from Nottingham, UK,66 undertook a SR to assess the effectiveness of education interventions in preventing or reducing the incidence or recurrence of foot ulcerations or amputations in adults with diabetes mellitus. A search of six databases from inception to September 2017 identified six RCTs, only three of which met our eligibility criteria (n = 423). The reviewers assessed study quality using the Cochrane risk-of-bias tool and a Critical Appraisal Skills Programme (CASP) tool. Three forest plots presented pooled outcomes. The first pooled RR of foot ulceration collected from people receiving education versus usual care (RR 0.52, 95% CI 0.23 to 1.15). The second pooled estimate evaluated the effect of education on foot ulcer and amputation rate combined (RR 0.37, 95% CI 0.14 to 1.01). The third assessed foot ulcer and amputation rates among people receiving intensive education compared with the rates among those receiving brief education interventions (RR 0.57, 95% CI 0.20 to 1.63). The reviewers concluded that the education interventions led to a statistically significant effect based on a p-value of 0.05.
In our assessment of the quality of this review, we found all three CIs from the meta-analyses included 1. Despite this, the researchers concluded that, overall, an intensive education approach offered a positive effect.
Footwear and offloading
A team of researchers from Amsterdam, the Netherlands, carried out a SR of footwear and offloading interventions to prevent or heal foot ulcers or to reduce mechanical pressure. 65 An updated search of eight databases of articles published between May 2006 and July 2014 identified a further seven RCTs (n = 1476). 64 The interventions were casting, footwear and surgical offloading. The reviewers used the Cochrane risk-of-bias tool to assess trial quality and found it to be variable. The reviewers concluded that therapeutic footwear leads to a reduction in plantar pressure, which they suggest will, in turn, prevent plantar foot ulcer recurrence. The reviewers also concluded that there is no evidence that therapeutic footwear prevents first foot ulcers, only recurrent ulcers, and that custom-made footwear results in fewer ulcers than no prescribed footwear among people with a history of foot ulceration.
Our risk-of-bias assessment judged this review to be at a low risk of bias.
A group of researchers from Staffordshire, UK, reported a SR that examined the quality and effectiveness of footwear to prevent DFU or to reduce biomechanical risk factors for ulceration. 66 A search of three databases identified two RCTs relevant to our overview (n = 469). Quality was assessed based on three criteria (sampling method, inclusion criteria and the approach to statistical analysis) and the two included RCTs were reported to be of poor quality. The authors’ conclusions were based on the findings from observational studies, but the authors did acknowledge the need for further randomised trials.
Our assessment of the bias of this trial found a risk of bias arising from study selection, which was carried out by one reviewer, and from the fact that the searches were limited to English-language studies.
Researchers in Adelaide, Australia, undertook a SR to identify, critically appraise and synthesise the best available evidence for offloading interventions to prevent the development and reduce the risk of primary foot ulceration in adults with diabetes mellitus who were at low risk of foot ulceration. The review identified no RCTs that were relevant to our review from a literature search of 14 databases (until November 2013), but the eligibility criteria did match that of our overview. 67 The reviewers used the Joanna Briggs Institute’s critical appraisal checklist108 to assess study quality.
The reviewers concluded that there is limited evidence that offloading prevents the development and reduces the risk of primary foot ulceration in adults with diabetes mellitus at low risk of foot ulceration.
A research team from Washington, USA, published a SR in 2004 that aimed to review the evidence of the effectiveness of therapeutic footwear in preventing re-ulceration in people with diabetes mellitus and to discuss factors influencing study findings. 68 A search of one database (1980 to ‘the present’) identified two RCTs relevant to our overview (n = 469) evaluating therapeutic footwear. There was an assessment of study validity. 109 The reviewers found no consistent evidence to support the use of therapeutic shoes and inserts to prevent DFU, owing to methodological weaknesses in the studies, and so concluded that there is no significant therapeutic benefit from therapeutic footwear. Nor did they identify evidence to support the practice of dispensing free therapeutic shoes with insoles to all patients with diabetes mellitus.
In a SR, a team from Plymouth, UK, evaluated the effectiveness of insoles in the prevention of ulcers in people with a history of foot ulceration. 70 A search of two databases (from inception to 2008) identified only one RCT that met our eligibility criteria (n = 69). This trial evaluated the effect of a magnetic insole constructed using Professional Protective Technology, Inc. (Deer Park, NY, USA). The trial investigators reported a reduction in foot ulcer relapses [27.7% vs. 58.3%; p = 0.009; OR 0.26 (95% CI 0.2 to 1.54)]. 85 The reviewers assessed the quality of studies using an assessment tool for use in randomised and non-randomised studies. 110 They concluded that insoles designed to prevent ulceration in the diabetic neuropathic foot appear to be of some value and should be considered as part of a prevention strategy. It was not possible for these reviewers to recommend any particular type or specification of insoles.
Complex interventions
A research group from Utrecht, the Netherlands, published a SR in the Cochrane Library. The review aimed to evaluate the effectiveness of complex interventions in comparison with a single intervention for the prevention of DFU. 16 The review included trials that involved people with diabetes mellitus with different levels of ulcer risk. A search of nine databases identified three RCTs relevant to our overview. The Cochrane risk-of-bias tool52 was used to assess trial quality.
The reviewers defined a complex intervention as an integrated care approach combining two or more prevention strategies on at least two different levels of care (patients, health-care providers and/or structure of health care). The review included three trials with DFUs as an outcome that compared the effect of educationally oriented complex interventions plus either screening tests or follow-up or more intensive complex interventions that included screening and multidisciplinary care for those at risk. 84,91,92 The review concludes that there is insufficient evidence to support the effectiveness of complex interventions, but this should be interpreted as a lack of evidence rather than as evidence of no effect. Our assessment of the risk of bias found the review to be at a low risk.
Screening
Reviewers in Washington, USA, conducted an evaluation of the effect of Semmes–Weinstein monofilament and other threshold testing in preventing foot ulcers and amputations. 69 Their search of one database from 1985 to 2000 found one RCT84,100 (n = 2001). The trial recruited and screened 2001 people with diabetes mellitus. Those at risk of ulceration were enrolled in a specialist foot care service. The quality assessment tool that the reviewers used was suitable for assessing the quality of studies of diagnostic tests. The authors concluded that the Semmes–Weinstein monofilament has excellent predictive ability for the risk of foot ulceration in diabetes mellitus but that the value of repeated tests for assessing established neuropathy is unknown.
Mixed interventions
An international group of researchers undertook a SR to determine the effectiveness of interventions to prevent foot ulceration in people at high risk of ulceration. 72 A search of eight databases from inception until 2014 without restrictions identified 17 RCTs that were relevant to our overview (n = 3107 people with diabetes mellitus). Randomised and non-randomised studies were eligible for inclusion. All trial participants were reported to be at risk of ulceration at the time of recruitment. The assessment of study quality was conducted using the Cochrane risk-of-bias tool. The included interventions were patient education, self-management, therapeutic footwear, surgical interventions and integrated foot care. A narrative synthesis was conducted and the authors concluded that there was no evidence of a preventative effect on the development of a first foot ulcer but that there was strong evidence that footwear interventions are effective in preventing recurrent foot ulcerations. This review presents data that appear to be amenable to meta-analysis, but no a priori plan for the analysis is included in the review methods and the reviewers present their results as a narrative.
Otherwise, this review was judged to be at a low risk of bias.
Researchers from Los Altos, USA, published a SR that evaluated trials of interventions to prevent foot ulcers in diabetes mellitus. 59 The reviewers searched six databases (search dates 1960 to April 2010) and found eight RCTs (n = 3520). The interventions included enhanced patient education and caretaker monitoring, therapeutic footwear and insoles, surgical interventions (debridement and surgical Achilles tendon lengthening) and plantar foot temperature-guided avoidance therapy. No information about the control interventions was provided. The assessment of trial quality was performed using the Amsterdam–Maastricht consensus list. 111 The reviewers concluded that the foot temperature-guided avoidance therapy was beneficial and was applicable to similar populations at risk of foot ulceration.
Our assessment of the risk of bias revealed a high risk as a result of restricted search dates, ambiguous eligibility criteria and the absence of a table of study characteristics.
A group of researchers from Sheffield, UK, conducted an overview to critically review evidence of the effectiveness of interventions for treating and preventing DFUs. 62 The reviewers stated that it was not their intention to conduct their review systematically, but because their approach meets the widely accepted definitions of a SR we included it in our overview. 52 The reviewers searched eight databases (from 1986 to 1996) and identified two RCTs that met our overview criteria (n = 464). The review focused on trials of education and therapeutic footwear. The assessment of study quality was conducted using the Jadad checklist. 112 The reviewers concluded that, currently, few interventions to prevent foot ulceration are supported by evidence, but therapeutic shoes appear potentially beneficial.
Our risk-of-bias assessment judged this review to be at a high risk because of the restrictions on search dates and language.
A SR by researchers based in York, UK, sought to identify effective interventions for the management of the diabetic foot. 18 The reviewers searched eight databases (from 1983 onwards) and identified three RCTs evaluating preventative strategies that met the eligibility criteria of our overview (n = 2465). The interventions were patient education, screening for risk assessment and footwear incorporating an orthotic device and custom-moulded insoles. 84,85,92 Quality was assessed using a checklist of four items: blinding level, baseline comparability, numbers randomised and loss to follow-up. The reviewers concluded that there is no evidence that foot risk assessment is of benefit and findings about the value of education are inconsistent.
Our risk-of-bias assessment found that the SR was at a high risk regarding eligibility criteria because there was insufficient information about the search strategy and the process for selecting studies.
Researchers in Leeds, UK,49 searched 19 databases for studies of preventative interventions for foot ulceration in diabetes mellitus and found four RCTs84,85,92,93 that met the eligibility criteria of our overview (n = 2625). The interventions studied were orthoses, education, footwear with custom-moulded insoles, below-knee elastic stockings and complex interventions including screening. Assessment of study quality was based on an assembled checklist of items including concealment of allocation, a priori sample size calculation, baseline comparability of groups, inclusion/exclusion criteria, adequate follow-up period, withdrawals and follow-up stated with reasons, and intention-to-treat analysis. The reviewers concluded that there is much uncertainty about the most clinically effective and cost-effective interventions for the prevention of DFU and that further and more rigorous evaluations are needed. The review strongly recommended more good-quality RCTs of interventions to prevent and treat foot ulcers in diabetes mellitus, with concurrent economic evaluations. We judged the risk of bias of this review to be low.
A team in Newcastle upon Tyne, UK, conducted a SR of the effectiveness of pressure-relieving interventions that was published in the Cochrane Library. 50 The reviewers searched 10 databases (search dates: MEDLINE, 1946 onwards; EMBASE, 1974 onwards; EBSCOhost, 1982 onwards; other databases, not reported). The searches identified one RCT that met our eligibility criteria (n = 69). 85 Study quality was assessed using a standard checklist that included allocation concealment, intention-to-treat analysis, loss to follow-up and blinding. The reviewers concluded that footwear and customised insoles provide some benefit but there was uncertainty about the most beneficial type of orthotic device.
A group of researchers in Cork, Ireland, conducted a SR of the effects of podiatric care on the incidence of LEAs. 63 The reviewers searched four databases (from inception to 2011) and included a single trial that reported the incidence of foot ulceration as an outcome. 94 The intervention was podiatric care at least once per month and the comparison was ad hoc podiatric. Quality was assessed using a modified version of the checklist created by Downs and Black. 110 The reviewers found insufficient evidence to determine whether or not contact with a podiatrist can be effective in reducing amputation rates. Our assessment of the risk of bias found that the exclusion of non-English-language studies was a weakness.
Discussion
A key step in the process of systematic reviewing is to establish whether a review with the same, or similar, objectives exists before embarking on a new review. 113 In undertaking an overview of SRs to prevent foot ulceration in diabetes mellitus, we found examples of SRs of varying quality. Those published in the Cochrane Library were considered to be at a low risk of bias, as was a previous Health Technology Assessment (HTA) programme-funded review.
Although the 20 reviews do not all share the same scope with regard to interventions or populations of people with diabetes mellitus, there is a great deal of overlap in the RCTs that they include, and the majority of reviewers concluded that more primary research is required. Although no robust pooled estimates of effect were identified, the majority of SRs by researchers globally to identify preventative interventions for DFUs reflects the high degree of clinical uncertainty among those delivering care and a clear desire to establish an evidence-based approach to the prevention of foot ulcers.
Many of these SRs were accepted for publication before the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was widely adopted. The older reviews lacked features, such as flow diagrams and search strategies, that are now demanded by journal editors,114 and other important items were also absent from many of the reports published since 2009.
Search strategies and date and language restrictions in the database searches plus our own lack of access to sources of biomedical information from China meant that we could not re-run the searches and update the reviews to the same point in time. This presented a major barrier to our conducting a thorough overview of SRs.
Systematic reviews require considerable resources and support from information specialists, statisticians, experienced reviewers and clinical experts. 113 The majority of reviews in this overview did not report any source of funding, and this may have had a detrimental effect on the reviewers’ ability to conduct the research to the highest methodological standards.
Although one review presented suitable data in a format amenable to recalculating estimates of effect,72 the scope of the review focused on those at high risk of foot ulceration, and we concluded that a new SR to obtain estimates of effect on a wide population of people with diabetes mellitus was justified.
Chapter 5 Preventative interventions for foot ulceration in diabetes mellitus: a systematic review
Background
Systematic reviews have played an important role in the creation of evidence-based health care,115 and there has been a proliferation in the number published in the biomedical literature since the early 1990s. In Chapter 4, we presented the results of an overview of 20 SRs of interventions to prevent foot ulceration in diabetes mellitus from which up-to-date and reliable estimates of effect could not easily be obtained; therefore, we undertook a new SR to produce RRs and 95% CIs with which to populate an economic model to assess the cost-effectiveness of preventative interventions.
Review question
Aim
The aim was to conduct a SR of the evidence of preventative effects of interventions for foot ulceration in diabetes mellitus that have been evaluated in RCTs.
Objective
The objective was to produce estimates of the effect of interventions with which to populate an economic model.
Method
Searches
We searched for eligible RCTs of interventions using search strategies created for Ovid MEDLINE (from inception to February 2019), Ovid EMBASE and Central Register of Controlled Trials (CENTRAL) without restrictions (from inception until October 2018). Randomised controlled trials in progress were identified via the International Standard Randomised Controlled Trial Number (ISRCTN) registry (searched to February 2019).
Eligibility criteria
Participants
The participants were people of any age with a diagnosis of diabetes mellitus, either type 1 or type 2, who had participated in RCTs of interventions to prevent foot ulceration in diabetes mellitus.
Interventions
Eligible interventions were considered either simple or, if several interacting components were evident, complex. 116 We defined a complex intervention as an integrated care approach combining two or more prevention strategies at least two different levels of care: the patient, the health-care provider and/or the structure of health care. Randomised controlled trials of interventions to manage established wounds (e.g. dressings) were excluded.
Comparators
We included RCTs that compared the effects of interventions with those of standard care or active comparators.
Outcomes
Primary outcomes
The primary outcomes were incident (new) and recurrent foot ulcers reported as binary outcomes (present or absent):
-
absolute numbers of incident ulcers
-
absolute numbers of recurrent ulcers.
We accepted a variety of definitions of foot ulceration, including ‘a full thickness skin defect that requires more than 14 days to heal’,117 or an objective scoring system such as an ulcer classification system. 56
Secondary outcomes
-
Amputation [minor, intrinsic to the foot (i.e. below the ankle), or major, involving the foot and leg].
-
Mortality.
-
Gangrene.
-
Infection.
-
Adverse events.
-
Harms.
-
Time to ulceration.
-
QoL (assessed using the EQ-5D, SF-12 or SF-36).
-
Timing of screening.
-
Self-care.
-
Hospital admissions.
-
Psychological (knowledge/behaviour).
Study selection and data extraction
One reviewer screened all RCT titles and abstracts to identify potentially relevant literature. A second reviewer screened a 10% random sample of the yield. Two reviewers scrutinised the full text of trials thought to meet the eligibility criteria. Disagreements were resolved by discussion with a third reviewer. Data were extracted into a review-specific data extraction tool by two reviewers working independently. The following data were extracted:
-
study authors and funders’ details
-
study objectives
-
eligibility criteria for trial participants
-
trial setting, the population, numbers randomised, a description of interventions and comparators, the included population’s level of risk of ulceration, absolute numbers for the primary outcome, number of foot ulcers, amputations and secondary outcomes.
Risk-of-bias (quality) assessment
For RCTs, we carried out an assessment of the risk of bias using the recommended items in the Cochrane Handbook for Systematic Reviews of Interventions. 52
Plan for data analysis
For each included trial, we calculated the pooled RRs of effects and 95% CIs using a frequentist meta-analytical approach with data analysed on an intention-to-treat basis. Trials were weighted in accordance with the inverse variance method for the dichotomous primary outcome of the review, namely foot ulceration. Heterogeneity was assessed using the I2 statistic.
To examine the effects of heterogeneity on patient characteristics, such as baseline risk of foot ulceration, and trial quality, we intended to use meta-regression techniques when sufficient data were available. 52
Results
Twenty-two RCTs met the review eligibility criteria; the characteristics of the included trials are in Table 20, the risk-of-bias assessments are in Table 21, the process of selection is presented in Appendix 4, Figure 51, and a flow diagram showing the flow of literature can be found in Appendix 5, Figure 52. A list of excluded RCTs is in Table 45 in Appendix 4.
| First author and year of publication, | Population characteristics | Details of experimental interventions and control interventions | Standard care | Outcome (unit of analysis) and length of follow-up |
|---|---|---|---|---|
| Antifungal nail lacquer | ||||
| Armstrong 2005102 |
n = 70 (intervention group, n = 34; control group, n = 36) Male: 97% Mean age: 70 years Previous ulcer: 57% T2DM: NR Mean diabetes duration: 12 years Ulcer risk: high (IWGDF risk group 2/3) |
Intervention: antifungal treatment (ciclopirox 8%) and self-management (daily inspection) Control: self-management (daily inspection) A staff podiatrist examined each patient recruited A clinician-to-staff 24-hour/day foot hotline with staff familiar with the care and status of these patients. But it was unclear who provided training regarding the intervention |
Preventative care programme and telephone support |
Ulcers (number of patients), one or more unexpected visits, missed appointments, tinea/HK at the start and end of the study All in (%) 12 months |
| Elastic compression stockings | ||||
| Belcaro 199293 |
n = 160 (intervention group, n = 80; control group, n = 80) Male: 50% Mean age: 53 years Previous ulcers: none T2DM: NR Mean diabetes duration: 15 years Ulcer risk: microangiopathy measured with laser Doppler, VPT also measured |
Intervention: knee elastic stockings with compression at the ankle of 25 mmHg worn at least 6 hours per day while active and/or working Control: no stockings (no other information) |
Not reported |
Number of ulcers (%), number of limbs (n) Deterioration of microcirculation RF (mean and SD) VAR (median and range) 48 months |
| Digital silicone device | ||||
| Scirè 200997 |
n = 167 (intervention group, n = 89; control group, n = 78) Male: NR Mean age: 56.5 years Previous ulcers: unclear T2DM: 88% Mean diabetes duration: 16 years Ulcer risk: high (VPT ≥ 25 V) |
Intervention: partial digital silicone orthoses (Podikon®, Saccolongo, Italy) and regular care at the diabetic foot clinic Control: no orthoses but regular care at the diabetic foot clinic |
Callus management. Soft insole and extra-deep shoe |
Ulcers (%) Hyperkeratosis [plantar, dorsal, interdigital (%)] Skin hardness (%) Stable deformities (%) Podobarometric evaluationa (pre and post evaluation, mean and SD) 3 months |
| Education alone | ||||
| Monami 201578 |
n = 121 (intervention group, n = 61; control group, n = 60) Male: 60% Mean age: 71 years Previous ulcers: 11% T2DM: 100% Mean diabetes duration: 15 years Ulcer risk: high Participants defined as ‘high risk’ if neuropathy diagnosed, previous DFUs or foot abnormalities |
Intervention: brief educational programme (2-hour programme provided to groups of 5–7 patients, 30-minute face-to-face lesson on risk factors for foot ulcers and 90-minute interactive session with practical exercises on behaviours to reduce risk) Control: brief leaflet and standard care Physician (for 15 minutes) and a nurse (for the remaining 105 minutes) provided this |
All patients had previously received standard multidisciplinary education for diabetes (with a structured group programme at diagnosis or first contact, and follow-up meetings every 2 years) |
Ulcers at 6 months, amputation, mortality, knowledge score (all absolulte numbers) Time spent for intervention and ulcer care in control (minutes per patient) 6 months |
| Gershater 201179 |
n = 131 (intervention group, n = 61; control group, n = 70) Male: 73% Mean age: 64 years Previous ulcers: 100% T2DM: 67% Mean diabetes duration: NR Ulcer risk: high (IWGDF) |
Intervention: group-session discussions Foot care education (60 minutes) from a registered nurse in the diabetes department including oral and written instructions based on International Consensus on the Diabetic Foot118 plus standard care. Provided by diabetes specialist nurse Control: standard information, oral and written instructions, on self-care based on the International Consensus on the Diabetic Foot118 |
Routine care from staff Adjusted shoes for indoor and outdoor use and individually fitted insoles |
New ulcers Cause of ulcers (stress, trauma, other) Location of ulcer (big toe or other, plantar, other including heel) All in (n) (%) 6 months |
| Lincoln 200880 |
n = 172 (intervention group, n = 87; control group, n = 85) Male: 67% Mean age: NR Previous ulcers: 100% T2DM: 77% Mean diabetes duration: NR Ulcer risk: high (10-g monofilament, Neurotip™ (Owen Mumford Ltd, Woodstock, UK), VPT ≥ 25 V) |
Intervention: structured foot care education session. Provided by general practitioner, specialist clinic or both – according to individual circumstances Control: standard care and the same foot care leaflets as the intervention group |
Regular podiatry and suitable orthoses when appropriate. Overall medical care followed national UK guidelines |
Incidence of ulcer (n) Incidence of amputation (n) QoL (DFS-SF) Mood (HADS), HAD-A, HAD-D Protective foot care behaviours (NAFF) 6 and 12 months |
| Podiatric care | ||||
| Plank 200394 |
n = 91 (intervention group, n = 47; control group, n = 44) Male: 56% Mean age: 65 years Previous ulcers: 100% T2DM: 93% Mean diabetes duration: 16 years Ulcer risk: high (reduced sensation assessed by 128-Hz tuning fork, 5.07-g monofilament) |
Intervention: chiropodist care and standard care Control: standard care and chiropodist care only if patient was interested |
Instructed on the possible benefits of regular chiropody care |
Ulcers: intention to treat, per protocol (feet, patients) Death and amputation (n) Aggregated end points for all above (HR, CI, p-value) 12 months |
| Complex interventions | ||||
| Cisneros 201090 |
n = 53 (intervention group, n = 30; control group, n = 23) Male: 62% Mean age: 62 years Previous ulcers: 28% T2DM: 96% Mean diabetes duration: 14.5 years Ulcer risk: IWGDF risk group (intervention/control) 1 (6/10), 2 (15/7), 3 (3/3) or 4 (6/3) |
Intervention: complex Four 90-minute sessions of therapeutic education in groups of eight, two pairs of protective shoes, testing for neuropathy Provided by researcher Control: participants received information on regular foot care and footwear use according to spontaneous demand during the individual consultations with the researcher |
Routine care from staff Instructions on foot care when requested Testing for neuropathy |
Occurrence (n) Recurrence (n) Time until foot ulceration (survival time) 24 months |
| LeMaster 2008119 |
n = 79 (intervention group, n = 41; control group, n = 38) Male: 51% Mean age: 66 years Previous ulcers: 42% T2DM: 94% Mean diabetes duration: 11 years Ulcer risk: moderate or high |
Intervention: complex Part 1 (1–3 months), physical therapist-led exercises to strengthen lower extremity muscles and promote balance over eight sessions. Part 2 (4–12 months) increased moderately intense activity by 50% over 12 months among community-dwelling. Provided by physical therapist and study nurse Control: standard care |
Foot-related self-care skills education, daily foot examination. Usual medical care from participants’ own health-care providers Participants referred to local orthotists or podiatrists to obtain therapeutic footwear at enrolment |
Foot ulcer rates (lesions/lesion episodes, full-thickness ulcer/ulcer episode, weight-bearing full-thickness plantar ulcer/ulcer episode) Step activity – person-year at risk (all means and 95% CIs) 12 months |
| Liang 201291 |
n = 62 (intervention group, n = 31; control group, n = 31) Male: 56% Mean age: 56 years Previous ulcers: 0% T2DM: 87% Mean diabetes duration: 11 years Ulcer risk: ADA risk category 1/2/3 High risk: 100% |
Intervention: session, foot care kit (foot care cream, 10-g monofilament, a thermometer for the temperature of the water for washing feet, alcohol cotton pieces and a mirror) Daily foot care, diabetes education classes. Provided by diabetes nurse-led multidisciplinary team – three endocrinologists, four nurses and one dietitian Control: standard care |
Conventional care alone according to ADA standards; medication adjustment, foot assessment, and 2 hours of education about diabetes foot care |
Incidence of foot ulcer (n, %) Incidence of amputation (n, %) HbA1c level (mmol, %) Diabetes knowledge Foot care behaviour (baseline, 1 year, 2 years; all means and SDs) 24 months |
| Litzelman 199392 |
n = 396 (intervention group, n = 191; control group, n = 205) Male: 19% Mean age: 60 years Previous ulcers: NR T2DM: 100% Mean diabetes duration: 10 years Ulcer risk: NR |
Intervention: patient education sessions, self-foot care, reinforced through telephone follow-up (2 weeks) and postcard reminder (1 month and 3 months) Informational flow sheets on foot-related risk factors for amputation in diabetic patients Prompts for health-care providers to:Provided by nurse clinicians Control: care as usual plus standard care |
1 year after the initial assessment, all patients underwent a repeated history and physical examination, performed by nurse clinicians blind to patients’ randomised allocation |
Patient outcomes: patient behaviour (5-point scale) Behaviour of health-care provider Physical findings (ulcers, physical examination, dry/cracked skin, corns, calluses, ingrown nail, fungal infections, improperly trimmed nails, foot/leg cellulitis, leg deformity, sensory examination) 12 months |
| McCabe 199884 |
n = 1997 (intervention group, n = 997; control group, n = 1000) Male: 53% Mean age: 60 years Previous ulcers: unclear T2DM: 80% Mean diabetes duration: NR Ulcer risk: low, moderate, high ABI ≤ 0.75, history of foot ulcers = high risk |
Intervention: primary foot screening examination, the biothesiometer and palpation of pedal pulses Foot pressures, subcutaneous oxygen levels, ABIs and radiography, and weekly diabetic foot clinic for high-risk patients. Provided by general diabetic outpatient clinic Control: patients were silently tagged and continued to attend the general outpatient clinic but received no special care |
Patients were advised to inspect and wash their feet daily, to avoid wearing constricting clothing and footwear, to wear prescribed footwear at all times and to contact the clinic whenever they felt it to be necessary |
Patient outcomes [ulcer (number of patients), ulcer progressing to amputation (%), amputation (%)], process outcomes [screening cost (£), compliance with follow-up/treatment (%)] 24 months |
| Digital infrared thermometry | ||||
| Armstrong 200789 |
n = 70 (intervention group, n = 34, control group, n = 36) Male: 97% Mean age: 70 years Previous ulcer: 57% T2DM: NR Mean diabetes duration: 13 years Ulcer risk: high (IWGDF risk group 2/3) |
Intervention: infrared dermal thermometry and a complex intervention. Attending physicians provided this Control: a complex intervention only Therapeutic footwear, diabetic foot education, regular foot care |
Footwear, education and professional foot care |
Ulcers (n, %) Rate of ulcer (HR) Temperature difference at ulcer site (survival curve) 18 months |
| Lavery 200487 |
n = 85 (intervention group, n = 41; control group, n = 44) Male: 49% Mean age: 55 years Previous ulcers: 41% T2DM: NR Mean diabetes duration: 14 years Ulcer risk: IWGDF risk group 2/3 |
Intervention: infrared skin thermometer and a complex intervention provided by treating physician – evaluation, nurse case manager – contact and podiatrist follow-up Control: a complex intervention – foot evaluation by a podiatrist every 10–12 weeks, therapeutic footwear, diabetic foot education |
Footwear, education and professional foot care |
Foot complication: ulcers, Charcot foot, infection and amputation (n) QoL [pre and post physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health (SF-36 scores)] 6 months |
| Lavery 200788 |
n = 173 (intervention group, n = 59; intervention group 2, n = 56, control group, n = 58) Male: 54% Mean age: 65 years Previous ulcers: 100% T2DM: 95% Mean diabetes duration: 13 years Ulcer risk: high (10-g monofilament, VPT ≥ 25 V, palpation of pulses, Doppler, ABI ≥ 0.07) |
Digital infrared skin thermometer and a complex intervention. Study nurse for contact, treating physician for foot evaluations, podiatrist for assessing shoes/insoles Intervention 1: enhanced care Intervention 2: structured care Control: standard care |
Lower extremity evaluation, education programme, therapeutic insoles and footwear. All participants received a pedometer to record their daily activity in a log book. Patients told to inspect their feet daily and to contact a nurse if need be |
Foot ulcers Foot trauma Fracture Death Osteomyelitis but no ulcer (all in n %) Time to ulcerate (mean and SD) 15 months |
| Skafjeld 2015120 |
n = 41 (intervention group, n = 21; control group, n = 20) Male: 56% Mean age: 58 years Previous ulcers: 100% T2DM: 71% Mean diabetes duration: 18 years Ulcer risk: IWGDF risk group 3 |
Intervention: foot skin temperature monitoring, theory-based counselling, contact study nurse if increase in temperature for more than 2 days. Study nurse provided this Control: standard care |
Foot care, daily recording of observations, customised footwear |
Incidence of foot ulcer Increased skin temperature Use of customised footwear > 12 hours/day Patients contacted study nurse (worried, ulcer, foot ulcer) (all n %) 12 months |
| Custom-made footwear and offloading | ||||
| Bus 201399 |
n = 171 (intervention group, n = 85; control group, n = 86) Male: 82.5% Mean age: 62 years Previous ulcers: 100% T2DM: 71% Mean diabetes duration: 17 years Ulcer risk: high (assessed with 10-g monofilament and vibration perception plus dorsalis pedis tests) |
Intervention: custom-made footwear of which the offloading properties were improved and subsequently preserved based on in-shoe plantar pressure measurement and analysis. Local specialist provided the footwear and local orthopaedic shoe technician manufactured the footwear Control: custom-made footwear that was not improved based on in-shoe pressure measurement (i.e. usual care) |
Each patient received written and verbal instructions on foot care and on proper use of footwear. All footwear in both study groups was evaluated at delivery and at 3-month follow-up visits (pressure measurements, temperature monitor and activity monitor) |
Ulcer recurrence (patients with ulcer, previous ulcer location, complicated foot ulcers) Ulcer recurrence according to adherence, non-ulcerative lesions (all in n %) In-shoe peak pressure, daily step count, adherence (mean and SD) 18 months |
| Reiber 200286 |
n = 400 (intervention group 1, n = 121; intervention group 2, n = 119; control group, n = 160 Male: 77% Mean age: 62 years Previous foot ulcers or infection requiring antibiotics: 100% T2DM: 93% Mean diabetes duration:Ulcer risk: high (assessed by 10-g monofilament and presence of foot deformity) |
Therapeutic shoes with two types of inserts and standard care. Study pedorthist provided this and panel of three foot care specialists evaluated Intervention 1: three pairs of therapeutic shoes and customised medium-density cork inserts with a neoprene closed cell cover Intervention 2: three pairs of therapeutic shoes and prefabricated, tapered polyurethane inserts with a brushed nylon cover Control: usual footwear and standard care |
Participants continued to receive regular health care and foot care from the VA or GHC. No participants received such education or care at the study site. A lightweight terry-cloth house slipper (Tru Stitch Footwear Inc., Malone, NY, USA) with no internal seam and textured sole was designed for all participants to use to minimise differences in out-of-shoe exposure |
Lesions and ulcers (ulcers, non-ulcerative, total, person-years of follow-up) Incidence per person (number of persons ≥ 1 ulcer, cumulative incidence per person, risk ratio) Incidence per person-year (ulcer and ulcer episode – total number, incidence rate, risk ratio) Pivotal events for ulcer episodes (shoe and non-shoe related). All in (n and 95% CI) 24 months |
| Rizzo 201296 |
n = 298 (intervention group, n = 148; control group, n = 150) Male: NR Mean age: 67 years Previous ulcers: 20% T2DM: 84% Mean diabetes duration: 18 years Ulcer risk: high (IWGDF risk group ≥ 2) |
Intervention: orthoses and shoes, in-depth education, daily feet checks, referral to clinic for urgent consultations and standard care. Screened by experienced podologist, evaluation of foot and current DFU risk: team of diabetologist, podologist and orthopaedic technician provided this Control: standard care |
Received an in-depth education on how to prevent ulceration, advice regarding footwear Urgent consultation within 24 hours if ulcers developed |
Foot ulcer (number of patients) New foot ulcers (n) Cumulative incidence of ulcers and recurrences (3 years, 5 years – χ2, % and p-value) DFUs due to trauma or hyperpressure (n %) VPT (mean and SD) and cost evaluation (euros) 12 months |
| Lavery 201295 |
n = 299 (intervention group, n = 149; control group, n = 150) Male: 67% Mean age: 70.5 years Previous ulcers: 26.95% T2DM: NR Mean diabetes duration: 12.5 years Ulcer risk: high (IWGDF risk group 2/3) |
Intervention: a shear-reducing insole and a complex intervention. Provided by study nurse for concerns and patient evaluation by a physician Control: standard care |
Foot and lower extremity evaluation by a physician every 10–12 weeks, an education programme that focused on foot complications, self-care practices Therapeutic shoes and standard insoles. Education, contact with study nurse if concerned |
Ulcers Footwear compliance (4, 4–8, 8–12, 12–16 hours/day) Time to ulcer (Cox proportional hazards regression) (all n %) 18 months |
| Uccioli 199585 |
n = 69 (intervention group, n = 33; control group, n = 36) Male: 62% Mean age: 60 years Previous ulcers: 100% T2DM: 75% Mean diabetes duration: 17 years Ulcer risk: high (mean VPT ≥ 25 V) |
Intervention: therapeutic shoes with custom insoles specially designed for diabetic patients (Podiabetes by Burrato, Italy) Control: participants were free to wear ordinary shoes/their own non-therapeutic shoes unless these were clearly dangerous |
All patients received the same educational guidelines on foot care and general information on the importance of appropriate footwear (i.e. proper size, durability and sole) |
Ulcer relapses (n %) Cumulative incidence of relapse (multiple regression analysis) Ulcer relapse between groups (χ2, % and p-value) Ulcer-free time, peripheral neuropathy – VPT, peripheral vascular disease – ABI (mean and SD) Use of therapeutic shoes (scale) 12 months |
| Ulbrecht 201498 |
n = 150 (intervention group n = 79; control group, n = 71) Male: 68% Mean age: 59.5 years Previous ulcers: 100% T2DM: NR Mean diabetes duration: NR Ulcer risk: high (inability to feel a 10-g monofilament, high plantar pressure, ABI) |
Intervention: bespoke orthoses with offloading properties. Study co-ordinator (clinicians) provided this Control: three different manufacturers’ orthoses along with three pairs of identical orthoses to be rotated while using the primary study footwear in accordance with a written rotation protocol, changing the numbered orthoses in a set rotation every month. Participants were also offered one of two types of footwear models |
Self-care behaviours with all participants with a focus on wearing the study shoes for all steps taken and on examining the feet daily to note and report problems An educational brochure to reinforce advice |
Primary end point occurrence, ulcers, (n %); peak barefoot plantar pressure vs. lesion (ulcer, non-ulcerative, no lesion) (kPa) Questionnaires for QoL (scaled to 100), foot self-care (0–1), fear of falling (scale to 100), subject satisfaction (five-level Likert scale) 16.5 months (follow-up at + 1 week, + 3 weeks, + 6 weeks, then every 3 months for another 15 months, potential 16.5 months) |
Risk of bias
| Trial (first author and year of publication) | Type of potential bias | |||
|---|---|---|---|---|
| Adequate sequence generation | Adequate allocation concealment | Adequate outcome assessor blinding | Incomplete outcome data | |
| Armstrong 2005102 | + | ? | ? | + |
| Armstrong 200789 | + | + | + | ? |
| Belcaro 199293 | ? | – | – | + |
| Bus 201399 | + | + | + | + |
| Cisneros 201090 | ? | ? | + | ? |
| Gershater 201179 | + | + | – | + |
| Lavery 200487 | ? | ? | + | + |
| Lavery 200788 | + | + | + | + |
| Lavery 201295 | ? | ? | + | + |
| LeMaster 2008119 | + | + | + | + |
| Liang 201291 | ? | ? | ? | ? |
| Lincoln 200880 | + | + | + | + |
| Litzelman 199392 | ? | ? | + | + |
| McCabe 199884 | ? | ? | ? | + |
| Monami 201578 | + | + | – | – |
| Plank 200394 | + | + | ? | + |
| Reiber 200286 | + | ? | + | + |
| Rizzo 201296 | + | ? | – | + |
| Scirè 200997 | + | ? | + | + |
| Skafjeld 2015120 | + | ? | + | + |
| Uccioli 199585 | ? | ? | ? | + |
| Ulbrecht 201498 | + | + | + | + |
We identified eight separate interventions:
-
antifungal treatment
-
elastic compression stockings
-
digital silicone device
-
education alone
-
podiatric care
-
digital thermometry
-
complex interventions
-
custom-made footwear and offloading.
Antifungal treatment
One trial evaluating the effect of antifungal treatment was identified by our searches. 102 Thirty-four participants in the intervention group received self-management advice (daily foot inspection) and antifungal nail lacquer (8% ciclopirax) for daily application, while 36 participants in the control group received only advice about foot self-inspection. Almost all patients (97%) were male; patients’ mean age was 70 years, and 57% had experienced previous foot ulcers. Their mean duration of diabetes mellitus was 12 years, but it was not reported how many had type 2 diabetes mellitus (T2DM). Standard care was reported to be a preventative care programme and telephone support, but the exact arrangements for this were unclear. At 12-month follow-up, there were two ulcerations in each group (RR 1.06, 95% CI 0.19 to 5.76). The concealment of the allocation and the blinding of the outcome assessor were unclear and the trial was rated as being at risk of selection and performance bias. No secondary outcomes were reported.
Elastic compression stockings
In one RCT evaluating the effect of elastic compression stockings,93 160 participants were randomised in equal numbers to the intervention or the control group for 48 months. Half of the trial participants were male; patients’ mean age was 53 years and none had a history of foot ulcers. Their mean duration of diabetes mellitus was 15 years, but the number with T2DM was not reported. The intervention group received knee-length elastic stockings with compression at the ankle of 25 mmHg, worn for at least 6 hours per day. There was a difference in the number of limbs that ulcerated in each group (three in the intervention group and 10 in the control group), but this did not reach statistical significance (RR 0.37, 95% CI 0.11 to 1.02). It was unclear how the random allocation was generated, and the nature of the intervention meant that it was not possible to blind patients to the allocation. The outcome assessment was not conducted by an investigator blinded to the random allocation, and the trial was judged to be at risk of selection bias and performance bias.
Secondary outcomes: elastic compression stockings
Thirteen amputations were reported during the 48-month trial: 3 out of 74 in the intervention arm and 10 out of 75 in the control arm.
Digital silicone device
One RCT evaluated the effect of digital silicone devices. 97 One hundred and sixty-seven participants with forefoot deformities who were considered to be at high risk of ulceration, as defined by a VPT of ≥ 25 V, were recruited into the trial. Their mean age was 56.5 years but gender was not reported. Eighty-eight per cent had T2DM and their mean duration of diabetes mellitus was 16 years. Participants in the intervention arm (n = 89) received a bespoke silicone digital orthotic with a variety of therapeutic intents and densities depending on the characteristics of the deformity. The patients in the intervention group received instructions about maintaining the orthotic and were advised to wear it until the end of the follow-up period. The 78 people in the control arm received standard therapy, which comprised the same examinations and procedures as the intervention group, but silicone orthotics were not provided. Both groups also received an accommodating soft insole and an extra-deep shoe. Outcomes collected at 3 months showed a statistically significant difference in the numbers of foot ulcers, with the digital orthotic group experiencing a beneficial effect (RR 0.07, 95% CI 0.01 to 0.55). Concealment of the allocation was not possible because of the nature of the intervention, and the trial was at risk of selection bias. No secondary outcomes were reported.
Podiatric care
Plank et al. 94 compared free chiropody care (n = 47) with no recommendation for chiropody care (n = 44) for 12 months. Fifty-six per cent of the 91 participants were male and their mean age was 65 years. All participants had a history of foot ulceration and were at high risk of another ulcer. Their mean duration of diabetes mellitus was 16 years, and 93% had T2DM. Those receiving free chiropody were recommended to seek care at least once per month. No recommendation was given to patients in the control group, but they could seek chiropody care if they were willing to pay for it. Standard care consisted of instructions on the possible benefits of regular chiropody care. It was unclear if both groups received standard care. There was no statistically significant difference in effect of number of foot ulcers (RR 0.67, 95% CI 0.43 to 1.05). In addition, there were fewer ulcerations in the intervention group (n =18) than in the control group (n = 25) but there were more amputations in the intervention group than in the control group (two vs. one, respectively). Fewer people died in the intervention group (n = 2) than in the control group (n = 4). It was not reported whether or not the outcome was assessed by an investigator who was blind to the allocation and, therefore, the trial data are at risk of performance bias.
Secondary outcomes: podiatric care
Two amputations occurred among 47 participants in the intervention arm and one amputation occurred among 44 participants in the control arm. Two people in the intervention arm died and four people in the control arm died. 33
Data on other secondary outcomes of interest, such as gangrene, self-care, hospital admissions, timing of screening and adverse events or harms, were absent from the trial reports.
Education alone
Three RCTs78–80 evaluated education alone.
One hundred and twenty-one people were followed up for 6 months. 78 Most of the individuals were male (60%); participants’ mean age was 71 years, and almost 11% had a history of foot ulceration. All had T2DM and their mean duration of diabetes mellitus was 15 years. The 60 people in the intervention group received a 2-hour group education programme (in groups of 5–7 patients). This comprised 30-minute face-to-face lessons on risk factors for foot ulcers, instructions on how to check their feet regularly, and information on ulcers and other foot conditions. In an additional 90-minute interactive session, health-care professionals demonstrated practical actions that would reduce the risk of foot ulcers. The 60 people in the control arm received a leaflet with recommendations for preventing foot ulcers. People in both arms received a standard multidisciplinary general education about diabetes mellitus at diagnosis, but they did not receive foot-specific education. At the 6-month follow-up, no statistically significant beneficial effect was observed in the intervention group (RR 0.08, 95% CI 0.00 to 1.31). The trial quality was compromised by a lack of concealment of the random allocation schedule, lack of collection of outcomes by an independent investigator and incomplete outcome data.
The RCT by Gershater79 comprised 131 people who had previously received treatment for a foot ulcer in a diabetes specialist foot clinic. They were recruited after the ulcers had healed and most of them were men (73%); their mean age was 64 years. The majority had T2DM, but the overall mean duration of diabetes mellitus was not reported. Those in the intervention group (n = 61) received foot care education led by a diabetes specialist nurse, designed to improve participants’ confidence in managing their foot health. This consisted of one 60-minute group session with 2–5 participants of the same gender in each group. The group discussions took place in the diabetes foot clinic conference room and were facilitated by a diabetes specialist nurse. Participants could choose to adopt a set of predefined actions/goals.
The 70 people in the control arm received standard, oral and written instructions on self-care based on the International Consensus on the Diabetic Foot. 118 Both arms received standard care, which comprised adjusted shoes and individually fitted insoles, and the recommendation for regular chiropody. All participants also received standard oral and written instructions on self-care provided by a diabetes specialist nurse. The trial quality was compromised by a lack of concealment of the random allocation schedule (selection bias) and lack of the collection of outcomes by an independent investigator (performance bias).
Lincoln et al. 80 randomised 172 people (two-thirds of whom were male) who had previously had ulcers to an intervention or a control group. Eighty-seven people in the intervention arm received a single 1-hour structured foot care education session from a researcher at home. The session included an explanation of the main causes of foot ulcers and a foot examination to identify risk factors (deformity, ischaemia or neuropathy), and advice was reinforced with written information. Shoes and insoles were examined for suitability and participants were advised to contact the clinic immediately if any new or recurrent foot problem emerged. The control group (n = 85) received standard care, consisting of unstructured and opportunistic foot care and the same written information as given to the intervention group. Standard care included podiatry and suitable orthoses when appropriate. At the 12-month follow-up, there was no statistically significant difference in the number of patients with foot ulceration between the two groups (RR 1.00, 95% CI 0.70 to 1.44). All quality assessment items were judged to be at a low risk of bias.
Education alone: meta-analysis
A pooled estimate of the effect of education on the incidence of foot ulceration at 6 months found that foot ulceration was not statistically significantly reduced in three trials of 423 people with diabetes mellitus (RR 1.04, 95% CI 0.55 to 1.97) (Figure 12). 78–80 The heterogeneity (I2) was 54%.
FIGURE 12.
Meta-analysis: education alone.
Secondary outcomes: education alone
Two trials of education interventions reported data on amputation,78,80 mortality,78 knowledge,78 behaviour80 and/or QoL. 80 One trial78 reported no amputations in either of its arms after 6 months’ follow-up. The other trial80 reported three amputations among 85 participants in the intervention arm, compared with no amputations among the 85 participants in the control arm at 6 months; by 12 months there was no difference between the arms (nine amputations in both).
One trial78 reported that two participants, one in each arm, had died by 6 months. In the same trial, a statistically significant difference in knowledge (as measured with the Patient Interpretation of Neuropathy knowledge score) was observed in the intervention arm. 78
One trial80 reported on QoL and found no differences between the two arms on the Diabetic Foot Scale, but scores on the Nottingham Assessment of Functional Footcare questionnaire, which assesses behaviour, were higher in the education arm than in the control arm.
Digital thermometry
Four RCTs87–89,120 involving 468 patients were identified by our searches.
Armstrong et al. 89 followed up 225 people (approximately 69 years of age) for 18 months. Their mean duration of diabetes mellitus was 13 years, but the authors did not report how many had T2DM. The number of participants randomised to the intervention group or the control group is not known. The intervention was an infrared skin thermometer to measure temperatures on six sites on the foot twice per day. Patients were told that, if they recorded a temperature difference of > 4 °F between feet at the same site, they should contact the study co-ordinator and reduce activity until their temperature normalised. Patients also received standard care consisting of footwear, education and professional foot care. The control group also received standard care and were advised to contact the study co-ordinator if they had any foot abnormalities. Participants’ feet were also checked for any signs of irritation from shoes or signs of impending ulcer. The number of ulcers in the intervention and control groups was reported to be 5 and 14, respectively, but the absence of the denominators for each group prevents the calculation of an effect. The quality of the trial is compromised because there was no allocation concealment, and because of the incompleteness of data reporting (selection and attrition bias).
A second RCT (i.e Lavery et al. 87) recruited 85 people (49% were male) with a mean age of 55 years. The mean duration of diabetes mellitus was 14 years, but it was not reported how many had T2DM. The participants were randomised to digital thermometry or standard care and followed up for 6 months. Forty-one participants received a hand-held infrared skin thermometer to measure the temperature at six predetermined sites on the sole of each foot in the morning and the evening. They received additional standard footwear, education and professional foot care, and were advised to contact a nurse case manager and to significantly reduce walking if they recorded a temperature difference of > 4 °F between feet at the same site. The control group received standard care footwear, education and professional foot care. No statistically significant difference was observed between the two groups (RR 0.15, 95% CI 0.02 to 1.19). There was a risk of bias from the generation and concealment of allocation being unclearly reported and there were incomplete outcome data. The method of generating the random sequence and the concealment of the allocation were both unclear and the trial data were at risk of selection bias.
The same authors (i.e. Lavery et al. 88) conducted a three-arm trial over 15 months. Of the 173 participants, 54% were male; participants’ mean age was 65 years and all had a history of foot ulceration. Approximately 95% of participants had T2DM, and their mean duration of diabetes mellitus was 13 years. The 59 participants in the first intervention arm received standard care plus a digital infrared skin thermometer and were asked to measure the temperature on the sole of their foot in the morning and the evening and record this information in a logbook. The participants were advised to contact a nurse manager if the difference in temperature between feet was > 4 °F and to reduce their activity until their temperature normalised. A video was used to teach participants how to use the infrared thermometer. In the second intervention arm, 56 participants received standard therapy plus training to conduct a structured foot examination twice daily using a mirror (to identify redness, swelling, inflammation, etc.). These participants could also could contact a nurse if they detected any abnormalities.
The 58 participants in the control received standard care (including lower extremity evaluation by a physician every 8 weeks, an education programme focused on foot complications and inspection, and therapeutic insoles and footwear). A podiatrist replaced or repaired insoles or footwear if needed. There was also an education component provided by video, and the participants were told to inspect their feet daily and to contact a nurse if necessary. There was statistically a significant difference between the digital thermometry group and the standard care group (RR 0.29, 95% CI 0.11 to 0.73). This trial was at a low risk of bias in all four quality assessment items.
Skafjeld et al. 120 followed up 41 participants (56% male; mean age of 58 years) for 12 months. All participants had previously had ulcers (71% had T2DM), and their mean duration of diabetes mellitus was 18 years. The 21 people randomised to the intervention group received a hand-held device with an infrared heat sensor to monitor their foot temperature. Participants recorded their daily physical activity using a step counter during the first week of the study, and throughout the study their recorded temperature at the same six places on the sole of each foot. Patients were advised that if the recorded skin temperature at the same spot differed by > 4 °F between their feet on 2 consecutive days they should contact the study nurse and reduce their physical activity until their temperature normalised. The control group of 20 participants received standard care, which involved inspecting their feet under the toes, below the toes and between the toes. Participants could contact the study nurse if they noticed changes in their feet, including a new ulcer. They were advised to always wear their customised footwear and consult their general practitioner if necessary. No statistically significant difference was observed in the number of ulcers between the intervention group and the control group (RR 0.67, 95% CI 0.32 to 1.41). Whether or not the allocation was concealed was not clear; in addition, the outcome data were incomplete and the trial data were at risk of selection and performance bias.
Digital thermometry: meta-analysis
A pooled analysis of data from three RCTs87,88,120 found that the use of digital infrared skin thermometry reduced the number of foot ulcers among 243 people with a history of foot ulceration (RR 0.41, 95% CI 0.19 to 0.86) (Figure 13). An I2 of 33% was observed.
FIGURE 13.
Meta-analysis: digital thermometry.
Secondary outcomes: digital thermometry
Trials of dermal thermometry variously reported on amputation following infection,89 QoL (assessed using the SF-36),37 adherence to therapy87,88 and time to ulceration. 39,40
In one trial, amputations following infections were required in 0 out of 41 participants in the intervention group and in 2 out of 44 in the comparator group. 38 In the same trial, there was no statistically significant difference in QoL measured using SF-36 in any category or in the overall score. 87
Two trials88,120 found no statistically significant difference between the dermal thermometry group and the comparator group in the time for which prescribed footwear and insoles were worn, as measured using a self-report questionnaire with an ordinal scale of < 4 hours to > 12 hours per day. The time to ulceration was statistically significantly longer in the dermal thermometry treatment group than in the standard care group in one trial88 but not in another. 120
Complex interventions
Five RCTs evaluated the effects of complex interventions on the development of foot ulcer. 84,90–92,119
Cisneros90 recruited 53 participants (62% were male) with a mean age of 62 years, of whom 28% had previously had ulcers and 96% had T2DM. They had a mean duration of diabetes mellitus of 14.5 years. The 24-month intervention (n = 30) was a researcher-led preventative programme comprising therapeutic education (weekly group meetings) and the provision of two pairs of special protective shoes. The education component was delivered over four meetings, each lasting 90 minutes, in groups of up to eight participants; this addressed, with discussion, issues around the prevention of foot ulcers, inspection and foot hygiene, and the choice and use of footwear. Bespoke games were used as teaching aids to prompt individual reflection about lifestyle and diabetes mellitus, and there was a discussion at the end of each meeting between health professionals and individual patients. The pedagogical technique was based on communications and learning. Footwear was provided after completion of the educational programme, at the beginning of the study and after the fourth re-evaluation.
The control group (n = 23) received information on regular foot care and footwear use in response to demand during the individual consultations with the researcher. Both groups received standard care monitoring of their feet to survey the incidence and recurrence of neuropathic injury. Monofilament testing was performed using the monofilament Semmes–Weinstein 5.07 (10 g) to identify risk at three sites on the foot the (digital pulp of the hallux and the first and fifth metatarsal heads). All items in the quality assessment were unclear and the trial was possibly at risk from selection, performance, attrition and selective reporting bias.
A second RCT119 followed 79 participants (51% were male) with a mean age of 66 years for 12 months. It was reported that 42% of participants had previously had ulcers. The majority (94%) had T2DM, and their mean duration of diabetes mellitus was 11 years. Forty-one patients in the intervention received care in two parts. For the first 3 months, they worked with a physical therapist in exercise sessions to strengthen their lower extremity muscles and promote balance. In the second part, over 4–12 months, they increased their exercise activity by 50%. In addition, they received training in self-care and foot examination skills. The 38 participants in the control group were taught foot-related self-care skills, including daily foot examination, and could access their usual medical care from their own health-care providers. Every participant was referred to a local orthotist or podiatrist at enrolment and received therapeutic footwear to wear when weight bearing inside and outside the home. There was no statistically significant difference in the number of foot ulcers between the two groups (RR 0.19, 95% CI 0.02 to 1.52). All quality assessment items were rated as being at a low risk of bias.
A third RCT91 evaluating a complex intervention comprised 62 participants (56% were male) with a mean age of 56 years. Twenty-five per cent of participants had previously experienced an ulcer, and 87% were classified as having T2DM. On average, they had had diabetes mellitus for 11 years. Thirty-one participants in the intervention group received a foot care kit that contained nail clippers, foot care cream, a monofilament with 10-g pressure, a thermometer to measure the temperature of water for foot washing, alcohol cotton pieces and a mirror. They were shown how to use the kit and asked to carry out daily foot care, checking their feet every day with a mirror. Participants could attend diabetes education classes every 3–6 months and were followed up every month for 2 years by a nurse and an endocrinologist performing a foot examination. Both the control group and the intervention group received standard care consisting of medication adjustment, foot assessment and 2 hours of diabetes education. No statistically significant effect in the number of foot ulcers was evident (RR 0.07, 95% CI 0.00 to 1.12). All items in the quality assessment were unclear and the trial may be at risk from selection, performance, attrition and selective reporting bias.
In a fourth RCT92 evaluating a complex intervention, 396 participants were followed up for 12 months. Most of the participants were women (81%) and they had a mean age of 60 years; it was unclear whether or not they had previously experienced foot ulcers. Every patient had T2DM and had received a diagnosis at least 10 years before. The 191 patients in the intervention group received nurse-led patient education sessions involving one to four patients and covering appropriate foot care behaviours and footwear. Patients entered into a behavioural contract for desired self-foot care. A systems intervention was designed to prevent patient-specific risks using information flow sheets on foot-related risk factors for amputation in diabetic patients. Health-care providers used prompts to ask patients to remove their footwear and perform foot examinations, and patients were also given foot care education. Both groups received standard care, which consisted of a physical examination performed by nurse clinicians who were blind to patients’ randomised treatment. There was no statistically significant difference in the number of foot ulcers between the two groups (RR 0.47, 95% CI 0.20 to 1.12). All items in the quality assessment were judged to be unclear and the trial was possibly at risk from selection, performance, attrition and selective reporting bias.
In a RCT evaluating a complex intervention,84 the majority of the 2001 participants were male (53%) and the participants’ mean age was 60 years. It was not reported how many had previously had foot ulcers. Eighty per cent had T2DM, but their mean duration of diabetes mellitus was not reported. The 1001 participants in the intervention group received a foot risk assessment comprising testing of sensitivity to Semmes–Weinstein monofilaments, measurement of VPT using a biothesiometer and the palpation of pedal pulses. Those deemed to be at high risk of foot ulceration were invited to attend a weekly diabetic foot clinic, where they were provided with self-care advice, chiropody care and support hosiery and/or protective shoes. The 1000 participants in the control group continued to attend the general outpatient clinic and had ulcer and amputation outcomes collected but received no special care. The diabetes outpatient service advised those in the control group about the importance of daily foot inspection and washing, appropriate hosiery and footwear and making contact with the clinic whenever they deemed it necessary. No statistically significantly different effect in the number of foot ulcers was observed between the two groups (RR 0.69, 95% CI 0.41 to 1.15). Generation and concealment of allocation and blinding were unclear. Three items in the quality assessment were rated as unclear (apart from incomplete outcome data, which was rated as high risk) and the trial was rated as being at possible risk of selection, performance, attrition and selective reporting bias.
Complex interventions: meta-analysis
A pooled analysis of data from the 2587 people with diabetes mellitus in five RCTs showed that complex interventions statistically significantly reduced the number of foot ulcers in the intervention groups (pooled RR 0.59, 95% CI 0.38 to 0.90) (Figure 14). The ulcer risk category of those who took part was not reported in three trials,84,92,119 but two trials included 44 patients who had not previously experienced a foot ulcer. 90,91 Heterogeneity was found to be I2 10%; however, with the exception of one trial,119 the validity of these data may be affected by bias.
FIGURE 14.
Meta-analysis: complex intervention.

Secondary outcomes: complex interventions
Amputation,84,91 time to ulceration90 and/or knowledge of foot care91 were reported in three trials. In one trial,91 amputations occurred only in the control arm (two among 31 participants, compared with none among the 31 participants in the intervention arm) and in a second trial84 there were fewer amputations in the intervention group (one major and six minor) than in the control group (12 major and 13 minor). 84 The time to ulceration was shorter in the control group than in the intervention group in one trial, but this finding did not reach statistical significance. 90
In one trial, participants’ knowledge of foot care (as measured using a diabetes knowledge questionnaire) was statistically significantly better in the intervention group than in the control group. 91
Custom-made footwear and offloading
We identified six RCTs evaluating custom-made footwear and offloading devices.
Bus et al. 99 recruited, and followed up for 18 months, 171 participants, most of whom were men (82.5%), with a mean age of 62 years. All had previously had ulcers. Nearly three-quarters (71%) of participants had T2DM, and their mean duration of diabetes mellitus was 17 years. Eighty-five people in the intervention arm received either custom-made footwear or semi-customised footwear with offloading properties improved, which was prescribed by a specialist in physical and rehabilitation medicine and manufactured by a local orthopaedic shoe technician. The custom-made shoes were created from a plaster-cast mould of the foot or from three-dimensional digital scans. Participants received orthoses made from either a cork base with added microcork and a mid-layer of ethylene vinyl acetate or a Plastazote leather. Participants were permitted to wear any additional custom-made footwear they owned at study entry or that was prescribed during follow-up. The 86 people in the control group wore footwear that was not improved based on in-shoe pressure measurement. Both groups received written and verbal instructions on foot care and on proper use of footwear as standard practice. No statistically significantly different effect was detected (RR 0.88, 95% CI 0.61 to 1.26). All four quality assessment items were judged to be at a low risk of bias.
A three-arm trial of footwear and orthoses86 recruited, and followed up for 24 months, 400 participants (77% were male) with a mean age of 62 years, all of whom had experienced a foot ulcer. The majority of participants (93%) had T2DM and the duration of diabetes mellitus was > 25 years in 56% of the trial population. Participants were randomly allocated to a group receiving three pairs of therapeutic shoes with medium-density cork inserts and a neoprene closed-cell cover or to a group receiving three pairs of therapeutic shoes with prefabricated, tapered polyurethane inserts and a brushed nylon cover or to the control group, who received usual footwear. All groups received standard professional foot care and a lightweight terry-cloth house slipper. There was no statistically significant difference in number of foot ulcers between those who received therapeutic shoes with either neoprene inserts or polyurethane inserts and the control group (RR 1.00, 95% CI 0.70 to 1.43). The reporting of concealment of allocation was unclear.
A third RCT, of footwear and pressure-relieving devices,96 included 298 participants, with a mean age of 67 years, of whom 20% had had a previous ulcer. The majority (84%) had T2DM, for a mean duration of 18 years. One hundred and forty-eight participants were randomised to the intervention group and received custom-made orthoses and shoes. Shoes were mostly semi-orthopaedic footwear, available on the open market, or craft-made orthopaedic shoes. Each patient also received additional education session about the need to wear the shoes and to inspect their feet daily. Patients could receive an urgent consultation within 24 hours if they developed a new foot ulcer. Follow-up took place every 3 months to assess the feet and the condition of the footwear. Standard care for 150 people in the control group comprised education to prevent foot ulcers and advice about footwear. A statistically significant difference between groups was observed (RR 0.30, 95% CI 0.18 to 0.49), with custom-made orthoses and shoes producing a beneficial effect. The concealment of allocation and outcome assessor blinding were unclear and the trial data are at risk of selection and performance bias.
A fourth RCT, of custom-made footwear and insoles,95 comprised 299 participants (67% were male and the mean age was 70.5 years). Twenty-five per cent had experienced a foot ulcer and, on average, they had had diabetes mellitus for 12.5 years, but the number with T2DM was not reported. The 149 participants in the intervention group received therapeutic shoes in which the standard insole had been replaced with an insole designed to reduce shear. The control group (n = 150) received the same brand of therapeutic shoes containing the standard insole. In both groups insoles were replaced every 4 months and the shoes were replaced once per year. Both groups also received standard care comprising a foot and lower extremity evaluation by a physician every 10–12 weeks and an educational video that focused on foot complications and self-care practices. The video addressed the aetiology of DFUs, risk factors, self-care practices and early warning signs of diabetic foot disease. Patients were to contact the study nurse if they identified an area of concern on their feet. After 18 months’ follow-up, no statistically significant difference in effect was detected (RR 0.30, 95% CI 0.08 to 1.08). The generation and concealment of allocation were unclearly reported, as was the completeness of the outcome data, and the validity of the trial data may be compromised by selection and reporting bias.
Uccioli et al. 85 randomised 69 participants (62% were male and the mean age was 60 years). All participants had experienced a foot ulcer, 75% had T2DM, and the mean duration of diabetes mellitus of those recruited was 17 years. The period of follow-up was 12 months. The intervention arm comprised 33 people who received therapeutic shoes made from soft thermoformable leather with semirocker soles and custom-moulded insoles (which were of extra deep fit customised insoles and accommodate toe deformities) plus standard care. The 36 people in the control group were free to wear ordinary shoes, unless it was clearly dangerous for them to do so, and received only standard care, which comprised educational guidelines on foot care and general information about the importance of appropriate footwear. A statistically significant effect was observed (RR 0.47, 95% CI 0.25 to 0.87). Generation of the random allocation was judged to be at a high risk of bias, and concealment of allocation and blinding and completeness of the outcome data were unclear; consequently, the trial data may be at risk of selection, performance, attrition and reporting bias.
The sixth RCT98 randomised 150 participants (68% were male) with foot ulcers who had a mean age of 59.5 years. The number of participants with T2DM and the duration of diabetes mellitus were not reported. All participants were advised to remain in their healing devices or removable cast walkers until their foot ulcers healed and before shoes were dispensed at ≈7 weeks after randomisation. Seventy-nine people in the intervention group were randomised to receive bespoke orthoses with offloading properties to reduce pressure on the metatarsal heads. Modified using a computer-aided design process, the orthoses were milled from a block of ethylene vinyl acetate foam and covered with a polyurethane foam top cover. Seventy-one people in the control group received standard orthoses from three different manufacturers. Both groups were provided with extra-depth shoes from PW Minor (Batavia, NY, USA) but a different specification was required to accommodate the bespoke orthoses (DX2 as opposed to the extra-depth specification that was received by the control group). Study co-ordinators discussed self-care behaviour with all patients, focusing on wearing the study shoes for all steps taken and examining the feet daily to note and report problems to the study team. There was a statistically significant difference in effect at 15 months (RR 0.34, 95% CI 0.14 to 0.81). The risk of bias was low for all four quality assessment items.
Secondary outcomes: custom-made footwear and offloading
Adherence95,96,99 and/or cost96 data were reported in four trials. One trial measured adherence using a temperature-based monitor placed inside the shoe, and found that 35 out of 85 participants in the intervention group and 42 out of 86 participants in the control group adhered to wearing their allocated footwear. 99 The trial authors conducted a subgroup analysis in participants who wore their allocated footwear, which showed a statistically greater reduction in ulcer recurrence in the intervention group; however, the analysis using data from the entire trial population failed to detect a beneficial association. A second trial of custom-made footwear and offloading insoles measured adherence using a self-reported physical activity questionnaire, and found that footwear and insole use was high in the groups that received cork inserts (83%) and prefabricated insoles (86%). 86 A third trial measured participant compliance with footwear using self-reports of the number of hours per day that the shoes were worn. There were no statistically significant differences between the groups in the number of people who wore the shoes for < 4 hours per day (23/149 vs. 16/150), 4–8 hours per day (77/149 vs. 83/150), 8–12 hours per day (38/149 vs. 46/150) or 12–16 hours per day (10/149 vs. 6/150). 95
Cost data collected in one trial published in 2012 showed that supplying footwear and insoles cost €675 per person per year. 96
Custom-made footwear and offloading: meta-analysis
A pooled estimate of data collected from trials evaluating custom-made footwear and offloading insoles showed a reduction in the number of foot ulcers (pooled RR 0.53, 95% CI 0.33 to 0.86) in 1387 people, of whom 464 had no history of foot ulceration; however, there was a high level of heterogeneity (I2 = 78%) possibly arising from variation in the construction of the shoes and insoles as well as the difference in risk (Figure 15).
FIGURE 15.
Meta-analysis: custom-made footwear and offloading.

Custom-made footwear and offloading: subgroup analyses
A subgroup analysis of data collected only from patients with a history of foot ulceration (n = 424) did not find a statistically significantly different effect in these patients (RR 0.71, 95% 0.47 to 1.06), and the degree of heterogeneity was I2 of 61% (Figures 16 and 17). Conversely, the RR of pooled data from two RCTs that included patients with no history of foot ulceration (n = 297) was 0.30 (95% 0.19 to 0.47) with I2 of 0%. It should be noted that many of the data in these analyses may be affected by biases emanating from the conduct of the trials, as only two trials were judged to be completely free of bias. 98,99
FIGURE 16.
Subgroup analysis: custom-made footwear and offloading in patients with a history of foot ulceration.

FIGURE 17.
Subgroup analysis: custom-made footwear and offloading in patients with no history of foot ulceration.

Table 20 shows the outcomes from the quality assessment process. Only 5 of the 23 trials were judged to be free of bias.
Ongoing randomised controlled trials
The searches for ongoing trials to prevent foot ulceration in diabetes mellitus from the ClinicalTrials.gov website found 24 studies being conducted worldwide, the details of which are presented in Appendix 4.
Discussion
The purpose of this SR was to evaluate the evidence base and obtain summary statistics for preventative interventions for foot ulceration in diabetes mellitus to create a cost-effective, evidence-based care pathway. The meta-analyses of dermal infrared thermometry, complex interventions and therapeutic footwear with offloading insoles suggest that these interventions can help prevent foot ulceration in people with diabetes mellitus.
The meta-analysis of data from RCTs of dermal infrared thermometry in people with a history of foot ulceration and a moderate to high risk of ulceration indicates that this is a promising intervention deserving of further evaluation in randomised trials with larger participant samples, and we note from our search of the ClinicalTrials.gov trial registry that new trials are currently under way. If foot ulcer prevention can be confirmed in large, well-conducted trials, this form of self-monitoring could relieve pressure on health-care systems; however, advising individuals to abstain from all weight-bearing activities when the difference in foot temperature exceeds 4 °F may prove challenging, and poor adherence might diminish any benefit in a real-world context outside a trial setting.
Specialist foot care of the type evaluated in the included trials of complex interventions is considered a marker of good-quality diabetes service delivery, and it is intuitively correct to suppose that this leads to improved outcomes. Although a statistically significant reduction in foot ulcers was apparent in our meta-analysis, such an effect was not evident in any single trial. This does support the suggestion of others that very large sample sizes may be needed for trials of this nature. 13 Surprisingly, there was a low level of statistical heterogeneity in the pooled data, despite quite marked differences in the clinical care provided in the intervention arms of the trials and the inclusion of people with three different levels of ulcer risk.
Our review did not identify any trials of complex interventions that reflect the composition of multidisciplinary foot services as recommended in clinical guidelines. 11,12 These influential documents advise that the core team in a diabetes foot care service should include diabetologists, podiatrists, vascular surgeons, diabetes specialist nurses and orthotists, but patient outcomes from such health-care service arrangements have not been evaluated in RCTs. An evaluation of outcomes for people at different levels of ulceration risk who receive care in specialist foot care settings would be worthwhile.
The true value of therapeutic footwear and offloading insoles in preventing foot ulcers has been obscured by contradictory trial results and poor interpretation of data in SRs; two larger trials involving only those with a history of foot ulcers both failed to detect evidence of effectiveness,86,99 and visual inspection of our analyses of pooled data from all six trials found that the greatest beneficial effect was in trials in which the majority of participants were considered to be at high or moderate risk but had not experienced a foot ulcer,95,96 although the results reached statistical significance in only one trial. 18 Our subgroup analysis of data from four trials of participants with a history of foot ulceration found no statistically significant difference in the number of recurrent ulcers between the custom-made footwear groups and the control groups.
This observation calls into question the conclusions of several SRs evaluating footwear and insoles for the prevention of foot ulcers. 59,64,72 The most recent SR included randomised and non-randomised data and adopted a consensus approach to analysis. The reviewers concluded that there is strong evidence that footwear interventions prevent recurrent plantar foot ulcers but no evidence that they prevent a first foot ulcer. 72 An individual participant data analysis using data from these six trials together with data from the 10 ongoing studies of offloading insoles identified by our search of the ClinicalTrials.gov database could allow for subgroup analyses to explore the value of footwear and offloading insoles in people with different baseline risks and, potentially, resolve these ongoing uncertainties.
The marked reduction in ulcerations reported with the use of a dermal silicone device by individuals at high risk of ulceration is encouraging. 97 These devices are simple to make at the chair-side and easy for wearers to keep clean. Although they are a type of offloading intervention, we did not include these data in the meta-analysis of footwear and offloading insoles because these devices differ substantially in that they are worn only around the toes.
Three separate small trials evaluating the effects of daily application of an antifungal nail lacquer (ciclopirox 8%) plus daily foot inspections,89 the use of elastic compression stockings93 and podiatry94 all failed to show a reduction in foot ulcers, possibly as a result of small sample sizes.
The standard care arrangements in the control arms of the included trials trial varied greatly, and no coherent conclusions can be drawn about current clinical practice from the trial reports.
We have comprehensively reviewed a body of evidence from RCTs and made the fullest use of the data currently available to derive the best estimates of treatment effects to inform a wider piece of work. In so doing, we have highlighted uncertainties, gaps and limitations in the existing evidence base to inform practice, generated new research hypotheses and added value to this area of research.
The weaknesses of this review arise from the potential biases identified in many of the trial reports, especially for complex interventions, which may have produced unreliable results. Previous authors of SRs have cited a lack of similarity between studies,49 a lack of standardisation in terminology, prescription, manufacture and material properties of interventions,65 heterogeneity in study designs, methodology and participant populations,66 and differences in participant demographics70 as reasons for not conducting meta-analyses, and we are aware of the potential limitations in the pooled analyses that we present here in both the number and the quality of trials. We have tried to produce conservative, less biased summary measures by adopting an intention-to-treat approach and a random-effects model. We acknowledge criticisms about the use of the latter,52 but believe that the insights gleaned and the generation of new research hypotheses justify our decision to pool data. Our analyses found evidence of beneficial effects of four types of intervention to prevent foot ulcers in people with diabetes mellitus, but considerable uncertainty remains about what works and who is most likely to benefit. Attention should be given to recommendations for the conduct of trials of interventions for the foot in diabetes mellitus, and researchers conducting future trials should endeavour to complete the trial to target recruitment as informed by an a priori sample size calculation. 103
Chapter 6 Economic model: evidence-based pathway
Background
Diabetes-related foot ulcers give rise to considerable morbidity and generate a high monetary cost for health and social care services. They precede 80% of diabetes-related LEA; hence, the clinical need to identify patients at risk of DFUs as early as possible is clear.
We have developed and validated a CPR suitable for routine practice that can help to identify a patient’s risk status for future diabetic foot ulceration (see Chapter 4). In addition, our SR on the prevention of DFU (see Chapter 6) has identified three interventions that have the potential to reduce the risk of DFUs in patients at risk. Drawing together the CPR and the results of the SR allows us to develop an evidence-based care pathway for the treatment of patients at risk of DFUs.
In this chapter, we report an economic evaluation of this evidence-based care pathway. The purpose of our analysis was to provide an economic rationale for the choice of preventative treatment and optimal monitoring frequency for patients at risk of DFU. We then assess the value of further research in this area and identify particular areas of uncertainty around which future research should be focused.
Objectives
-
Question 1: what is the potential cost-effectiveness of the use of a validated CPR as part of structured care to reduce the incidence of DFU?
-
Question 2: what is the potential cost-effectiveness of alternative strategies, including monitoring intervals?
-
Question 3: is there potential worth in undertaking further research, particularly a RCT?
To address these questions, we first conducted a SR of published health economic models used to estimate the cost-effectiveness of DFU prevention. Informed by the review of the literature, we then developed a de novo health economic model capable of estimating the potential cost-effectiveness of alternative care pathways for the prevention of DFUs. This model was informed by epidemiological modelling of clinical data from a diabetes register in Fife (NHS Fife SCI-Diabetes), Scotland, between 2010 and 2018. Value-of-information analysis was then used to quantify the value of conducting further research and to suggest where such research should be focused.
Literature review of cost–utility analyses
Aim
The aim was to undertake a review of published cost–utility analyses of the prevention of DFUs.
Methods
We undertook a review to identify (1) costs and outcomes of relevant interventions for the prevention of diabetic foot ulceration and (2) published cost–utility analyses of such interventions. The review methods are provided in Appendix 5.
Results
Our search returned 11 relevant papers to be reviewed. Four other relevant papers were identified by ‘hand-searching’ the references of the 11 papers identified. Of these 15 papers, five were cost–utility analyses of the prevention of DFUs. 121–125 The remaining papers either were concerned with the cost–utility of treatments for patients who had already experienced DFUs or were papers relating to the cost burden of DFUs. 126–134 Further hand-searching did identify other studies in which an economic analysis was undertaken (i.e. some assessment of costs and outcomes); however, as these papers were not cost-effectiveness papers and were beyond the scope of this review, they were excluded.
We review here the five papers that were cost–utility analyses of the prevention of DFUs. Full details of the papers reviewed are given in Appendix 6, Table 52.
Quality of economic evaluations
All five cost–utility papers were quality assessed using the Drummond checklist135 for economic evaluations. All studies were deemed to be of high quality.
Review of papers
Eastman et al. 121 used a Markov model to simulate the natural history of diabetic patients and the rate of complications (including DFUs and LEA) and to evaluate the impact of preventative treatments. Information on age, sex, ethnicity and incidence and prevalence rates were based on US community and population studies. The authors then estimated the cost-effectiveness of glycaemic control for reducing diabetes-related complications. Costs and outcomes were estimated for patients aged between 25 and 74 years. The authors found that treatment that maintains an HbA1c value of 7.2% is associated with a reduction in LEA of 67%. They found the cost per additional quality-adjusted life-year (QALY) of treatment was £16,002.
Ragnarson Tennvall and Apelqvist122 used a Markov model to assess the cost–utility of an ‘optimal’ prevention programme for patients at risk of DFUs and amputation. The prevention programme included foot inspection, appropriate footwear and education. Patients were stratified in the model according to their risk of ulceration. The treatment effect of the preventative programme was based on RCTs and observational studies reported in the literature (see Table 21). Costs and outcomes were assessed over a 5-year time horizon. The study found that optimal preventative treatment of high-risk patients was cost saving based on the achievement of a 25% lower incidence of DFUs and extremity amputation than in baseline prevention scenarios. Similarly, Ortegon et al. 123 used a Markov model to assess the cost–utility of optimal prevention and treatment of diabetic foot. The preventative programme included protective foot care, education, regular inspection, risk identification and the involvement of a multidisciplinary team. Treatment effect was based on a study that reported a reduction in LEA of between 49% and 85%. 135 Patients were stratified in the model according to risk. Costs and outcomes were measured over the lifetime of a patient. The study found that, based on the achievement of a reduction in ulceration incidence of 10%, preventative treatment was cost-effective, with a cost per QALY gained of < US$25,000 (2003 prices).
Rauner et al. 124 used a Markov model to assess the cost–utility of preventative education programmes to reduce DFUs. Treatment effect was based on the assumption of a 25–50% reduction in DFUs, based on estimates obtained from the literature. 82,136 The authors’ model built on previous work by stratifying patients according to not only risk but also age, allowing for the potential for more targeted decision-making. Costs and outcomes were assessed over a 10-year period. Their results suggest that preventative programmes are highly cost-effective when targeted at patients at high risk of DFUs and LEAs.
Barshes et al. 125 used a Markov model to assess the cost–utility of primary prevention programmes aimed at reducing the incidence of DFUs. In contrast to the other papers under review, which took the cost and effectiveness of treatment as given and estimated the cost-effectiveness, Barshes et al. 125 sought to estimate the effectiveness target necessary for a treatment to be considered cost saving. Costs and outcomes were estimated over a 5-year period. The study found that preventative programmes had a > 90% probability of being cost-saving when the annual prevention costs were < US$50 per person and/or when DFUs are reduced by at least 25%. If patients were deemed to be at high risk, programmes were cost-saving when incidence of DFU reduced by 10%.
All studies, with the exception of Eastman et al. ,121 used a Markov model to simulate the clinically relevant states experienced by patients at risk of developing DFUs. All of these studies included clinical states representing pre-ulceration, ulceration and post-ulceration outcomes; however, they varied in terms of which other relevant clinical factors they included in the modelling. The studies by Ortegon et al. 123 and Rauner et al. 124 were the only ones to stratify patients according to risk state. Three studies123–125 included additional clinical states associated with diabetic foot, such as ischaemia (e.g. neuropathy, peripheral vascular disease). Eastman et al. 121 used a Monte Carlo simulation to estimate the rate of complications, including DFUs and LEAs, associated with all patients with T2DM. The disparity in modelling strategies among these studies is a natural consequence of the complexity of the disease and the range of possible complications. Furthermore, the choice of modelling strategy, and the clinical events included, is a function of the specific decision problem addressed in each study; however, the result is that uncertainty about the most appropriate choice of model for estimating the cost-effectiveness of strategies to prevent DFUs.
All of the papers reviewed found that preventative treatment for DFUs was cost-effective or, in some cases, cost saving. However, the evidence on effectiveness in these studies comes from research conducted some time ago and/or studies that have small sample sizes; therefore, significant uncertainty remains about the treatment efficacy of potential interventions.
Only the model of Ortegon et al. 123 estimated the lifetime costs and outcomes relating to DFUs. As the costs (in terms of the ongoing care required) and outcomes (in terms of fewer DFU and amputations), and the significant morbidity associated with these, will have an impact over a patient’s full lifespan, it is necessary to take a lifetime perspective when estimating cost-effectiveness.
All the studies reviewed suggest that the effectiveness (and cost-effectiveness) of treatments is likely to vary according to patient risk, and that treatment is most likely to be considered cost-effective in the subgroup of patients at high risk. However, none of the papers reviewed have modelled how often a patient’s risk should be assessed (risk monitoring frequency).
Summary
Our review highlights considerable heterogeneity in how the clinical and cost consequences of treatments to prevent DFUs have been modelled in the literature. Furthermore, at the time these studies were published, there was little consensus about the efficacy of treatments available for preventing DFUs; therefore, existing models of cost-effectiveness rely on assumptions about treatment effect. Another important limitation of current models is that risk monitoring frequency has not been considered.
Development of health economic model
Overview
There is a lack of consensus among clinical guidelines about the monitoring frequency required for patients who are at risk of developing DFUs. NICE currently recommends annual monitoring of patients at low risk, and monitoring as frequently as once every 1 or 2 weeks for patients at high risk. SIGN recommends that the monitoring take place at least annually, but concedes that the optimal monitoring frequency is unknown; hence, there is a need for new economic evaluations to consider the impact of alternative monitoring frequencies on DFU outcomes and the implications for cost-effectiveness. As discussed in Review of papers, the choice of modelling strategy will depend on the specific decision problem at hand and, thus, clinical input into the model choice is crucial from an early stage. The literature review of economic models has shown that interventions that are able to reduce the incidence of DFUs in at-risk patients have the potential to be cost-effective. Based on our review of the literature, and on advice from our clinical colleagues, we developed a de novo health economic model to answer the questions stated in our objectives.
Cost perspective
This economic evaluation was undertaken from the perspective of the UK NHS and Personal Social Services137 that is, the costs relevant to the economic analysis were those incurred by the NHS and Personal Social Services.
Time horizon
This economic evaluation investigated the costs and health outcomes associated with each clinical pathway over a 20-year time horizon.
Discount rate
Future costs and health outcomes were discounted at 3.5%, in line with recommended practice. 137
Conceptual model of disease and treatment pathways
Our task was to undertake an economic evaluation of an ‘evidence-based pathway’ for the prevention of DFUs. To do so, we had to understand the important clinical and economic events in the care pathway. The conceptual model in Figure 18 is a visual representation of the events we sought to capture in our model and how these events relate to costs and QALY outcomes.
FIGURE 18.
Conceptual model of decision problem.

Conceptual model implemented as a Markov model
The model was implemented as a semi-Markov model, as the survival analyses used to estimate transitions indicated that transition probabilities varied according to time in state. Submodels were developed to estimate total discounted costs and QALYs for ulceration and amputation events to simplify the implementation of the model. Figure 19 illustrates graphically the transitions we require to estimate this conceptual model as a Markov model.
FIGURE 19.
Schematic of semi-Markov model and submodel.
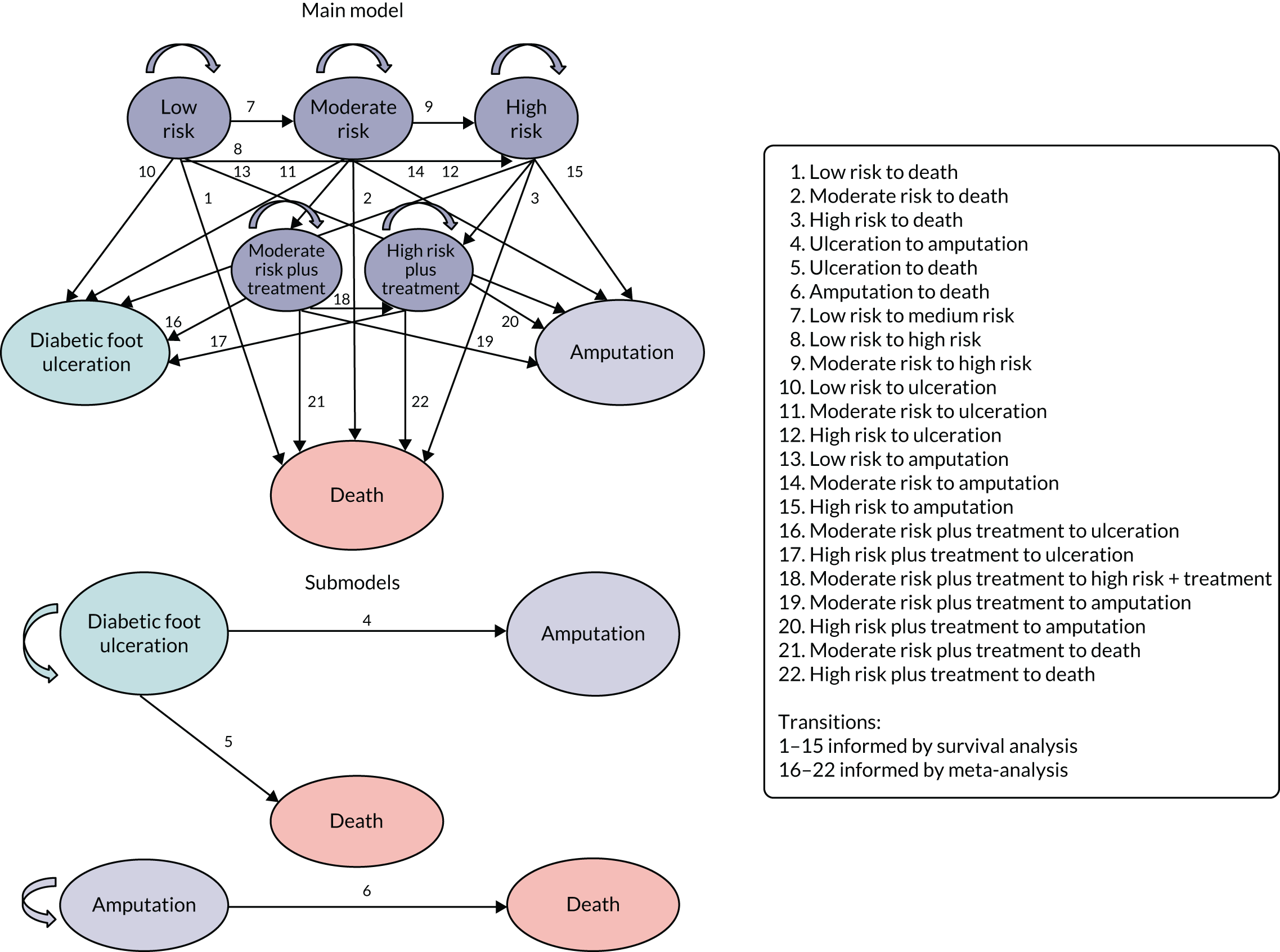
NHS Fife Scottish Care Information – Diabetes Collaboration data set
NHS Fife SCI-Diabetes is an electronic patient record used in the routine management of NHS patients with diabetes mellitus. The data set we obtained contained information about individual patients who received foot care in NHS Fife foot monitoring clinics over a 12-year period. The data contained the results of risk assessments of the risk factors recommended by the SIGN guideline,12 only three of which are required in our validated CPR as reported in Chapter 4: monofilament sensitivity, pedal pulses and history of ulceration (all binary variables). Data were available from the beginning of 2005 until the end of 2017. A separate data set from NHS Fife SCI-Diabetes provided death data that were merged with the SCI-Diabetes foot monitoring data. No death data were recorded before 1 March 2009 in the data set; therefore, this date was used as the entry point into the defined cohort as we would not know whether or not patients with records that stopped before that date had died. The use of the SCI-Diabetes anonymised data set was approved by the information governance department of NHS Fife.
Clinical prediction rule definition of risk status
The economic model includes the use of the CPR, which defines a patient’s DFU risk status as follows:
-
Low risk [CPR score of 0 or 1; probability of ulcer at 2 years ≤ 0.05 (see Table 10)] – negative for history and also negative for at least one of monofilament sensitivity and pedal pulses.
-
Moderate risk (CPR score of 2; probability of ulcer at 2 years = 0.12) – positive for history but negative for both monofilament sensitivity and pedal pulses, or negative for history but positive for both monofilament sensitivity and pedal pulses.
-
High risk (CPR score of 3 or 4; probability of ulcer at 2 years ≥ 0.25) – positive for history and also positive for at least one of monofilament sensitivity or pedal pulses.
This categorisation, although arbitrary, was chosen for ease of use in a clinical setting (just three categories of risk) and was decided on following group discussions at project team meetings.
NHS Fife Scottish Care Information – Diabetes Collaboration population
Patients who were recorded in the data before 1 March 2009 and were attending foot monitoring clinics on or after 1 March 2009 (prevalent patients) or who started to attend foot monitoring clinics after this date (incident patients) were defined as eligible for analysis. Having a mix of prevalent and incident patients is appropriate as any changes in the monitoring policy would apply to all patients. In the case of patients whose first recorded appointment was before 1 March 2009, the date of entry to the analysis cohort was considered to be 1 March 2009 and their CPR status was the last recorded CPR status before 1 March 2009. In the case of those patients whose first recorded appointment was on or after 1 March 2009, the date of entry to the cohort was taken as that date, alongside the patient’s CPR status at that date. Use of these criteria resulted in 26,154 patients being included in the cohort used to inform important parameters of the economic evaluation model. Table 22 summarises the key demographics of this cohort.
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| Mean (SD) | 68 (14) |
| Sex | |
| Male | 14,053 (54) |
| Female | 12,101 (46) |
| SIMD deciles (socioeconomic deprivation) | |
| 1 (most deprived) | 1628 (6) |
| 2 | 3324 (13) |
| 3 | 3009 (12) |
| 4 | 3017 (12) |
| 5 | 2959 (11) |
| 6 | 2633 (10) |
| 7 | 2069 (8) |
| 8 | 2539 (10) |
| 9 | 2415 (9) |
| 10 (least deprived) | 1488 (6) |
| Missing SIMD data | 1073 (4) |
| Total | 26,154 (100) |
Figure 20 shows the total number of clinical visits per patient in the cohort over the period of approximately 8 years. The follow-up time varied considerably between each patient depending on whether they were a prevalent or an incident patient (and when they became an incident patient) and also if the patient died. As can be seen from Figure 20, almost 80% of patients in the data set had more than one visit to the foot assessment clinic, and it was not uncommon for a patient to have more than 10 clinical encounters.
FIGURE 20.
Histogram of total number of clinical visits per patient in the cohort.

Table 23 presents the number of key events of interest for the economic model that occurred during the follow-up of the identified cohort. Among the 26,154 patients, approximately 4% developed an ulcer, 1% required an amputation and almost 24% died.
| Event | Number of events | % of population having event |
|---|---|---|
| Ulceration | 980 | 3.8 |
| Amputation | 286 | 1.1 |
| Death | 6213 | 23.8 |
If the only record for a patient in the data set was of their death (and hence there were no risk assessment data), we excluded that patient from the cohort used for analysis (analysis cohort, n = 26,086).
Missing data
There were significant numbers of missing data in the NHS Fife SCI-Diabetes data set. This was particularly an issue with regard to records for patients’ test results for the three CPR predictors (monofilament sensitivity, pedal pulses and history of ulceration) that were used to estimate risk status in the cohort. To determine the most likely mechanism for the occurrence of missing data, regression modelling was used to investigate the association between patient demographics and missingness. Patients’ characteristics were found to be associated with missingness, ruling out the possibility that the missing data mechanism was missing completely at random. 138 After consultations with clinicians and data controllers, we considered that MAR was also unlikely and, therefore, ruled out a multiple imputation approach to account for missing data. After consultating with NHS colleagues, we proceeded with the following rules.
When a patient had a missing record for one of their CPR predictor variables, the patient was recorded as not being at risk from this predictor (i.e. if the record for monofilaments was left blank, we assumed that the patient was sensitive to monofilaments). When a patient had a valid recording (positive or negative) followed by missing data in subsequent visits, we assumed that the patient did not change risk status for this variable unless indicated otherwise. Full details of the missing data approach are given in Appendix 6, Table 54.
Clinical prediction rule risk status over time
Table 24 shows that, at the first clinic appointment, 25,003 patients (96%) were classified as at low risk. This decreased to 23,867 patients (91%) by the final clinic appointment. Over time, there was an increase in patients classified as at moderate risk (first visit, 3%; final visit, 4%) and at high risk (first visit, 1%; final visit, 4%). Overall, 1397 (5%) of the cohort changed their risk status between their first visit and their final visit. The numbers in Table 24 that indicate a change in risk category are shown in italics.
| First visit | Final visit (n) | Total (N) | ||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Low | 23,867 | 639 | 497 | 25,003 |
| Moderate | 0 | 452 | 261 | 713 |
| High | 0 | 0 | 370 | 370 |
| Total | 23,867 | 1091 | 1128 | 26,086 |
| Transition required | Estimation procedure | Number of events | Survival probability at (95% CI) | ||
|---|---|---|---|---|---|
| 1 year | 2 years | 8 years | |||
| Transitions 1–3: time to death conditional on CPR state |
n = 26,086 Population: patients in low-, moderate- or high-risk states Event: death. t0 = time of entry into cohort Censoring events: ulceration, amputation Time-varying covariate: CPR state Accelerated failure effects: |
5877 | 0.973 (0.971 to 0.975) | 0.947 (0.944 to 0.950) | 0.767 (0.761 to 0.773) |
| Transition 4: time from ulceration to amputation |
n = 927 Population: patients in ulceration state Event: death. t0 = time of entry into ulceration state Censoring events: death |
100 | 0.94 (0.930 to 0.959) | 0.922 (0.903 to 0.938) | 0.880 (0.861 to 0.904) |
| Transition 5: time from ulceration to death |
n = 980 Population: patients in ulceration state Event: death. t0 = time of entry into ulceration state Censoring events: amputation |
517 | 0.983 (0.973 to 0.990) | 0.943 (0.927 to 0.956) | 0.494 (0.461 to 0.525) |
| Transition 6: time from amputation to death |
n = 286 Population: patients in amputation state Event: death. t0 = time of entry into amputation state Censoring events: none |
131 | 0.996 (0.975 to 0.999) | 0.975 (0.943 to 0.988) | 0.545 (0.484 to 0.602) |
| Transitions 7: time from low to moderate risk |
n = 25,000 Population: patients in low-risk state Event: change to moderate risk. t0 = time of entry into low-risk state Censoring events: ulceration, amputation, death, change to high risk |
564 | 0.995 (0.994 to 0.996) | 0.990 (0.989 to 0.996) | 0.973 (0.970 to 0.975) |
| Transitions 8: time from low to high risk |
n = 25,000 Population: patients in low-risk state Event: change to high risk. t0 = time of entry into low-risk state Censoring events: ulceration, amputation, death, change to moderate risk |
0 | 1 | 1 | 1 |
| Transitions 9: time from moderate to high risk |
n = 1041 Population: patients in moderate risk state Event: change to high risk. t0 = time of entry into low-risk state Censoring events: ulceration, amputation, death |
163 | 0.955 (0.939 to 0.967) | 0.913 (0.893 to 0.931) | 0.778 (0.745 to 0.807) |
| Transitions 10–12: time to ulceration conditional on CPR state |
n = 26,086 Population: patients in low-, moderate- or high-risk states Event: ulceration. t0 = time of entry into cohort Time-varying covariate: CPR state Censoring events: death, amputation Accelerated failure effects: |
666 | 0.992 (0.991 to 0.993) | 0.989 (0.988 to 0.991) | 0.974 (0.971 to 0.976) |
| Transitions 13–15: time to amputation conditional on CPR state |
n = 26,086 Population: patients in low-, moderate- or high-risk states Event: amputation. t0 = time of entry into cohort Time-varying covariate: CPR state Censoring events: death, ulceration Hazard ratios: |
169 | 0.999 (0.998 to 0.999) | 0.998 (0.997 to 0.998) | 0.994 (0.993 to 0.995) |
Estimation of transition probabilities
Transition probabilities required for the model were estimated based on a set of parametric survival models built on the aforementioned analysis cohort (Table 25). We tested the following parametric models: exponential, Weibull, Gompertz, log-logistic, log-normal and generalised gamma. The choices of distribution were determined by a visual examination of Kaplan–Meier plots and log-cumulative hazard plots and Akaike information criterion (AIC)/Bayesian information criterion (BIC) tests of model fit, as recommended by NICE Decision Support Unit Technical Support Document on survival analysis methodology (see Appendix 6). 139 Kaplan–Meier survival plots for the key transitions in the economic model are presented in Appendix 6.
Results
Estimating costs and utilities
Costs
We calculated total costs by attaching unit costs to resource use per patient with the relevant clinical event. Unit cost estimates were identified from the literature and from microcosting based on input from clinical experts. 131 The inclusion and exclusion of resource use items were validated with the project team and diabetic foot clinicians. All unit costs were presented in Great British pounds for the price year 2017. Table 26 provides a list of the unit cost estimates, unit of measurement and source for each resource included in the model. Further details regarding costs are given in Appendix 6.
| Cost item | Cost estimate (base year 2017) | Unit of measurement | Source |
|---|---|---|---|
| Ulceration | £3751 | Per patient, annually | Kerr 2017131 |
| Amputation | £8916 | Per patient, annually | Kerr 2017131 |
| Off-the-shelf footwear plus insole | £100 | Per patient, annually | Microcosting |
| Digital infrared thermometry | £26 | Per patient, annually | Microcosting |
| Complex intervention | £561 | Per patient, annually | Microcosting |
| Monitoring cost | £26 | Per patient, per visit | Microcosting |
Health utilities
Health utilities were identified from the SR of economic models of the prevention of DFU. Utility estimates from Redekop et al. 132 were obtained from the general population (the Netherlands), making them particularly suitable for economic evaluation, which requires the relative preferences for health states across multiple disease areas (Table 27).
Cost-effectiveness analysis
Costs and effects were measured for each potential treatment strategy. The cost-effectiveness of the alternative treatment pathways were evaluated based on their incremental cost-effectiveness ratio (ICER) and their net monetary benefit (NMB). ICERs are calculated as follows:
where ΔCosts is the difference in total costs between interventions and ΔQALYs is the difference in QALY gain between interventions.
If a new treatment is found to be more costly and less effective than the current treatment, the new treatment is said to be ‘dominated’. Conversely, if the new treatment is more effective and less costly than the current treatment, the new treatment is said to dominate the current treatment.
The NMB is a measure of the health benefit, expressed in monetary terms, which incorporates the cost of the new strategy, the health gain obtained and the societal willingness to pay for health gains. The NMB is expressed using the following formula:
where E is effectiveness, WTP is the willingness-to-pay threshold (£20,000 in the UK) and C is cost.
The NMB approach is recommended when comparing more than one intervention and provides a clear decision rule (i.e. if NMB > 0, the new strategy is cost-effective).
Comparators
The base-case treatment strategy in all scenarios is current practice. Current practice is defined as the natural history of the disease, with no CPR to monitor risk, and will involve whatever interventions are currently offered in practice and hence captured in the health outcomes of the current analysis cohort. Current practice is taken as the base case and is compared with the treatment strategies outlined in Table 28.
| Clinical pathway | Description |
|---|---|
| Current practice | Natural history of the disease, as observed in the cohort derived from NHS Fife NHS Fife SCI-Diabetes population (analysis cohort). No CPR to monitor risk. No additional intervention given |
| Monitoring every 2 years | Natural history of the disease, as observed in the analysis cohort. Plus use of the CPR to assess risk every 2 years. Interventiona is given to patients classified as at moderate or high risk |
| Monitoring annually | Natural history of the disease, as observed in the analysis cohort. Plus use of the CPR to assess risk annually. Intervention is given to patients classified as at moderate or high risk |
| Monitoring every 6 months | Natural history of the disease, as observed in the analysis cohort. Plus use of the CPR to assess risk every 6 months. Intervention is given to patients classified as at moderate or high risk |
| Treat all | Natural history of the disease, as observed in the analysis cohort. No CPR to monitor risk. Intervention is given to all patients attending foot-risk assessment clinics, regardless of CPR risk category |
This modelling strategy allows us to incorporate the recommendations of clinical guideline bodies such as NICE and SIGN. Our model included the use of a CPR that is based on a patient’s response to monofilaments, presence or absence of pedal pulses and history of ulceration. These risk factors are recommended, although not exclusively, by NICE and SIGN. There is no evidence-based consensus on the most appropriate strategy for managing patients at risk of DFUs. Although both NICE and SIGN recommend that specialist footwear be considered, only SIGN recommends the use of multidisciplinary care teams. The use of digital thermometry is not recommended in clinical guidelines and is currently the focus of RCTs. SIGN notes that there is no evidence on the ideal monitoring frequency for patients at risk of DFUs; however, it advises annual monitoring. NICE, on the other hand, recommends monitoring annually for low-risk patients, every 3–6 months for patients at moderate risk, every 1–2 months for patients at high risk and every 1–2 weeks for patients for whom there is an immediate, pressing risk of DFUs. By investigating the use of the CPR at 2 years, 1 year and 6 months, our model allows the costs and outcomes associated with a range of preventative strategies recommended by clinical guideline bodies to be explored.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was used to explore uncertainty in the model input parameters and to describe the impact that this uncertainty has on the model outcomes, namely costs and QALYs gained. A 1000-iteration Monte Carlo simulation was undertaken. Gamma distributions were used to represent uncertainty in the cost parameters; multivariate, normal distributions were used for (log-)survival regression parameters; beta distributions were used for health utility parameters; and a log-normal distribution was used to represent treatment effect parameters.
The distribution of ICERs produced by the Monte Carlo simulation was presented on the cost-effectiveness plane. Cost-effectiveness acceptability curves (CEACs) were used to present the uncertainty in the decision regarding the most cost-effective option, over a variety of monetary willingness-to-pay thresholds. 140
Value-of-information analysis
We conducted a value-of-information (VOI) analysis to determine the value to society of collecting further information on the effectiveness, costs and cost-effectiveness of alternative clinical pathway strategies.
To order to establish whether or not future research would be worthwhile, we calculated the expected value of perfect information (EVPI), which is the difference in net benefit of a decision based on our current information (evidence) and a decision with perfect information (i.e. no uncertainty). The situation of perfect information can be thought of as reducing the width of CIs around all of our model parameters to zero. We estimated the EVPI summed across the potential DFU population in NHS Fife and for a time period during which we expect this treatment strategy to remain the ‘gold standard’. In our analysis, we assumed that a reasonable time period for estimating the VOI would be 10 years. We also assumed that the number of patients in the NHS Fife SCI-Diabetes data set would be the number of eligible patients over a 10-year period (26,086 patients over 8 years in NHS Fife SCI-Diabetes, and hence 3261 patients per year). This equates to an ‘effective population’ (e.g. discounted population) of 15,239 patients, or 1524 patients per year, eligible in Fife. If the EVPI for the population is greater than the costs of carrying out the additional research, then carriyng out this research is potentially cost-effective. To determine what type of new evidence would be most valuable, we calculated the expected value of partial perfect information (EVPPI) for parameters of interest. Owing to the non-normal distribution of NMB, we estimated the VOI using a non-parametric simulation approach. 141
Results
Custom-made footwear and offloading
Table 29 presents the results of the economic model in which patients are monitored with the CPR and treated with custom-made footwear and offloading. The results suggest that, in this scenario, treating all patients with special footwear is the most cost-effective strategy.
| Model outcomes over 20 years | Expected cost | Expected QALYs | Incremental costs | Incremental QALYs | Incremental ICER | Incremental mean NMB (WTP = £20,000) |
|---|---|---|---|---|---|---|
| Current practice | £290 | 6.791 | – | – | – | – |
| Monitoring every 2 years | £423 | 6.804 | £133 | 0.013 | Extendedly dominated | £120.39 |
| Monitoring annually | £520 | 6.805 | £230 | 0.014 | Extendedly dominated | £43.16 |
| Monitoring every 6 months | £708 | 6.805 | £418 | 0.014 | Extendedly dominated | –£134.25 |
| All patients treated | £999 | 6.865 | £709 | 0.074 | £9615 | £765.91 |
Digital infrared thermometry
Table 30 presents the results of the economic model in which patients are monitored with the CPR and treated with digital infrared thermometry. The results suggest that, in this scenario, treating all patients with digital thermometry is the most cost-effective strategy.
| Model outcomes over 20 years | Expected cost | Expected QALYs | Incremental costs | Incremental QALYs | Incremental ICER | Incremental mean NMB (WTP = £20,000) |
|---|---|---|---|---|---|---|
| Current practice | £290 | 6.791 | – | – | – | – |
| Monitoring every 2 years | £386 | 6.807 | £97 | 0.016 | Extendedly dominated | £231.19 |
| Monitoring annually | £481 | 6.809 | £191 | 0.018 | Extendedly dominated | £161.29 |
| Monitoring every 6 months | £668 | 6.809 | £370 | 0.018 | Extendedly dominated | –£12.01 |
| All patients treated | £381 | 6.886 | £92 | 0.095 | £967.91 | £1801.86 |
Complex interventions
Table 31 presents the results of the economic model in which patients are monitored with the CPR and treated with complex interventions. It can be seen that the expected costs of the different strategies with complex interventions are higher than for the others, but with no advantage in QALYs. As a result, at a willingness to pay of £20,000, current practice is the preferred option.
| Model outcomes over 20 years | Expected cost | Expected QALYs | Incremental costs | Incremental QALYs | Incremental ICER | Incremental mean NMB (WTP = £20,000) |
|---|---|---|---|---|---|---|
| Current practice | £290 | – | – | – | – | – |
| Monitoring every 2 years | £624 | 6.801 | £334 | 0.011 | £29,618 | –£108.61 |
| Monitoring annually | £729 | 6.802 | £439 | 0.012 | Extendedly dominated | –£196.51 |
| Monitoring every 6 months | £922 | 6.802 | £632 | 0.013 | Extendedly dominated | –£379.45 |
| All patients treated | £4699 | 6.857 | £4410 | 0.066 | £74,805 | –£3094.17 |
Probabilistic sensitivity analysis
Cost-effectiveness planes
Figures 21–23 are graphical illustrations of the uncertainty surrounding the ICERs produced from a 1000-iteration of a Monte Carlo simulation. All three simulations suggest that there is considerable uncertainty surrounding the QALYs associated with each treatment strategy; however, there is comparatively less uncertainty around the total costs. Figure 23 shows that treating all with complex interventions is likely to have a significantly higher total cost than other treatment strategies.
FIGURE 21.
Distribution of incremental costs and QALYs associated with custom-made footwear and offloading.

FIGURE 22.
Distribution of incremental costs and QALYs associated with digital infrared thermometry.

FIGURE 23.
Distribution of incremental costs and QALYs associated with complex intervention.
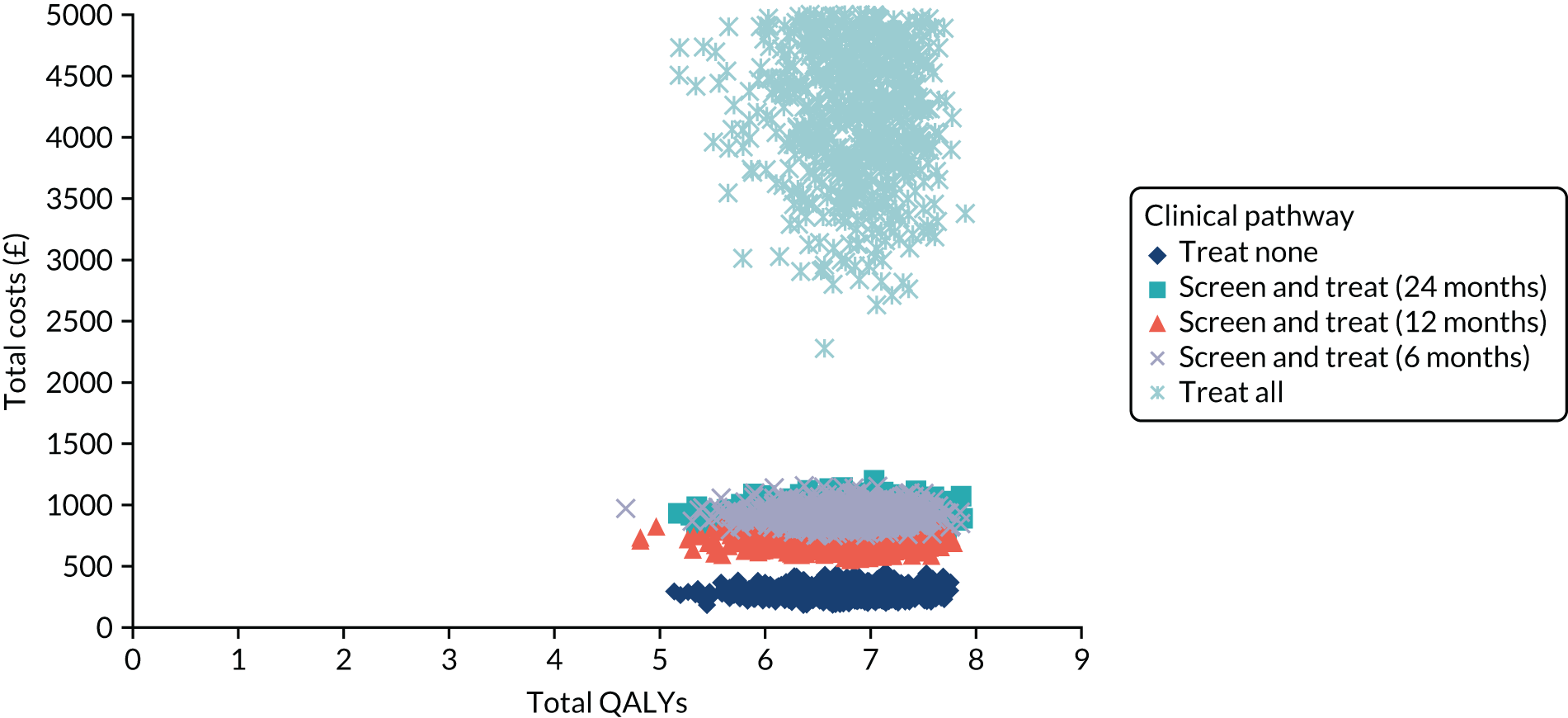
Cost-effectiveness acceptability curves
Figures 24–26 show the probability that each treatment strategy is considered cost-effective, compared with the alternatives, for a range of thresholds of willingness to pay for a single QALY gain. Figures 24 and 26 show considerable uncertainty surrounding which intervention is most likely to be deemed cost-effective, with no clear strategy producing the greatest probability at a willingness-to-pay threshold of £20,000 per QALY gained. Only in the case of digital infrared thermometry (see Figure 25) does the treat-all strategy emerge as having the greatest probability of being cost-effective, although, even for this intervention, the CEAC suggests a probability of just over 30% that this strategy is the most cost-effective at a willingness-to-pay threshold of £20,000 per QALY.
FIGURE 24.
The CEAC for custom-made footwear and offloading.
FIGURE 25.
The CEAC for digital infrared thermometry.

FIGURE 26.
The CEAC for complex intervention.

One-way sensitivity analysis
One-way sensitivity analysis assessed the impact on cost-effectiveness of varying our chosen willingness-to-pay threshold from £20,000 per QALY gained (as recommended by NICE) to £13,000 per QALY gained (Claxton et al. 142). This had the effect of reducing the cost-effectiveness of custom-made footwear and offloading (from an incremental NMB of £766 to £250) and of infrared thermometry (from an incremental NMB of £1802 to £1139). The use of complex interventions is still considered not to be cost-effective. There was no change in the relative position of the cost-effectiveness of screening intervals.
Value of information
The VOI analysis is conducted for the three potential interventions separately, as these are mutually exclusive. In all VOI analyses that follow, the most cost-effective option for each intervention is considered against current practice.
The EVPI per patient affected by the decision to recommend treatment based on a strategy of treating all with custom-made footwear and offloading is estimated to be £9226.
Based on our assumptions of an effective population of 1218 eligible patients per year in NHS Fife and at a willingness-to-pay threshold of £20,000 per QALY gained, this equates to an EVPI of £11.2M per year. (Figure 27 shows how this varies with willingness-to-pay threshold.) If we assume that the period of time for which this treatment strategy would be expected to be considered ‘gold standard’ is 10 years, then the estimated total population EVPI is £112.4M over a 10-year period for the NHS Fife diabetes mellitus population.
FIGURE 27.
The EVPI (population, £), based on custom-made footwear and offloading.
The EVPPI suggests that the majority of the value of reducing parameter uncertainty in our model would be in reducing uncertainty around the parameters obtained from our survival analysis of NHS Fife SCI-Diabetes data (Figure 28). This suggests that the uncertainty having the greatest impact on our decision problem is related to the number of events (ulceration, amputation, death) in the NHS Fife SCI-Diabetes data set.
FIGURE 28.
The EVPPI per patient (£), based on custom-made footwear and offloading.

Digital infrared thermometry
The EVPI per patient affected by the decision to recommend treatment based on a strategy of treating all with digital infrared thermometry is estimated to be £8533.
Based on the same assumptions as above with regard to eligible population, time horizon and willingness to pay for QALY gains, we estimate an EVPI of £10.4M per year and of £104M over 10 years. Figure 29 illustrates how this EVPI varies with willingness-to-pay threshold.
FIGURE 29.
The EVPI (population, £), based on infrared digital thermography.
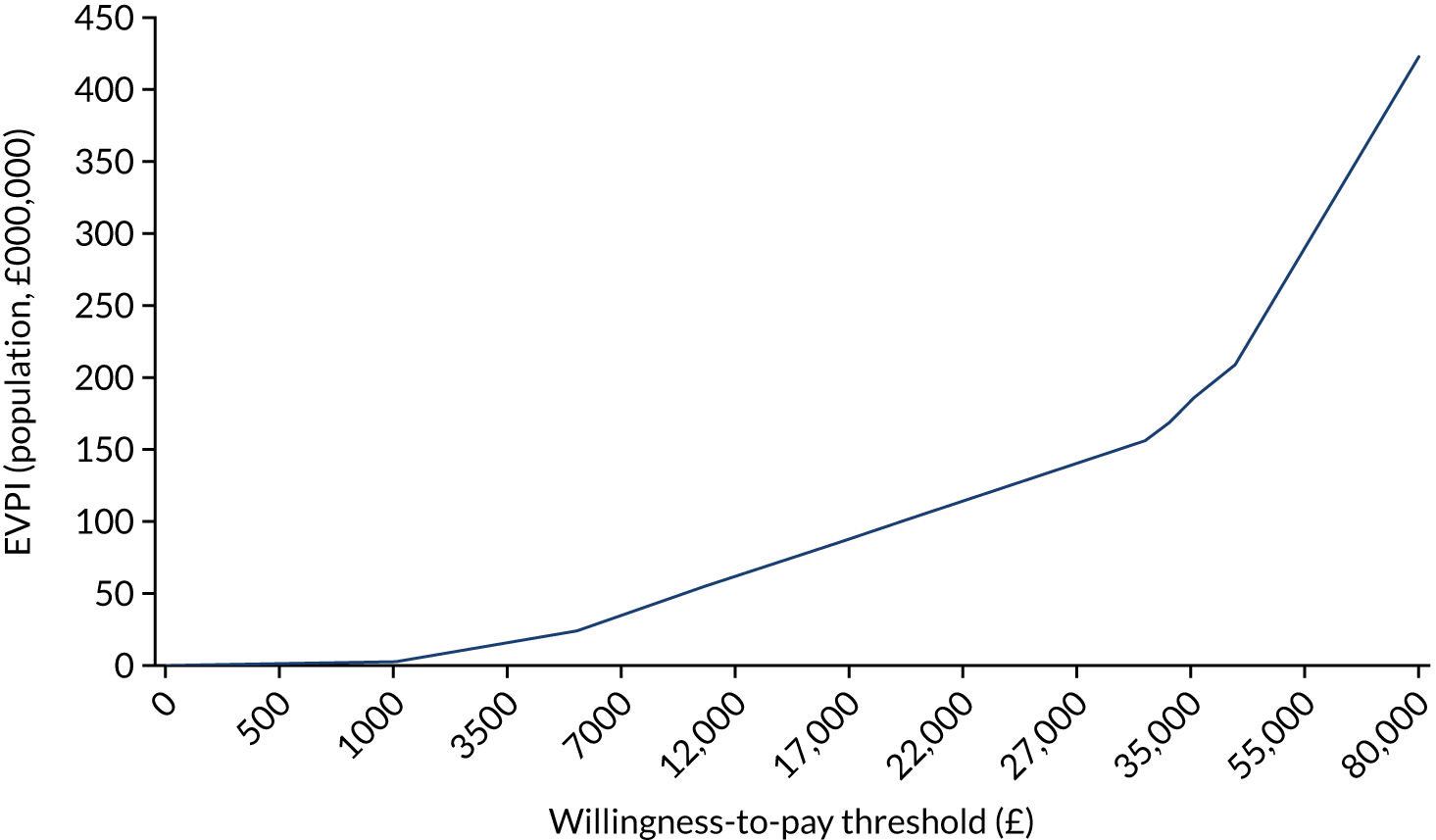
The EVPPI suggests that the majority of the value of reducing parameter uncertainty in our model would be generated from reducing uncertainty around the parameters obtained from our survival analysis of NHS Fife SCI-Diabetes data (Figure 30).
FIGURE 30.
The EVPPI per patient (£), based on digital infrared thermometry.

Complex intervention
The EVPI per patient affected by the decision to recommend treatment based on a strategy of treating all with complex interventions is estimated to be £8968.
Based on the same assumptions as above with regard to eligible population, time horizon and willingness to pay for QALY gains, we estimate an EVPI of £10.9M per year and of £109M over 10 years. Figure 31 illustrates how this EVPI varies by willingness-to-pay threshold.
FIGURE 31.
The EVPI (population, £), based on complex intervention.

The EVPPI suggests that the majority of the value of reducing parameter uncertainty in our model would be generated from reducing uncertainty around the parameters obtained from our survival analysis of NHS Fife SCI-Diabetes data (Figure 32).
FIGURE 32.
The EVPPI per patient (£), based on complex interventions.
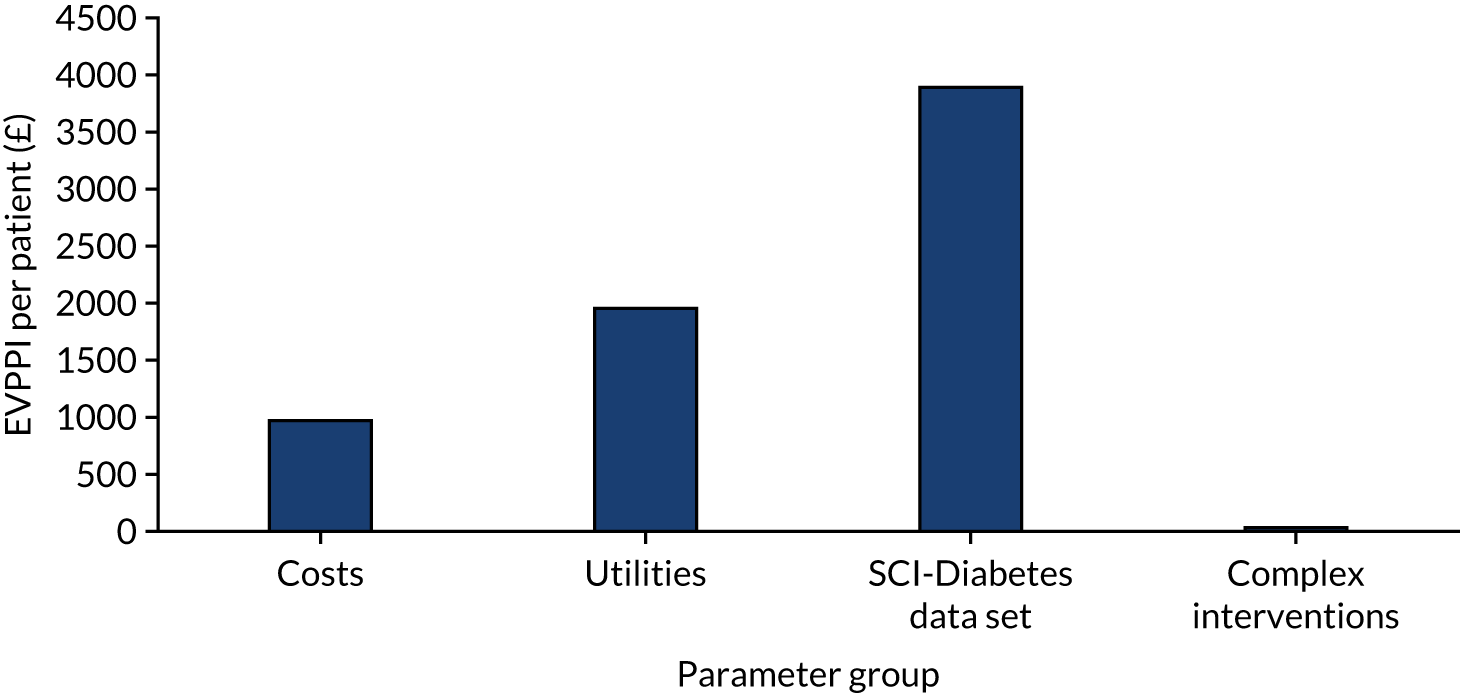
Across all three interventions available, the VOI analysis suggests that there is value in reducing the uncertainty related to the decision problem (i.e. whether or not it is cost-effective to use this treatment strategy). In terms of the specific groups of parameters we looked at, there was value in reducing the uncertainty relating to all of them, but reducing the uncertainty relating to the NHS Fife SCI-Diabetes data had the greatest value across all three analyses. This suggests that increasing our knowledge of the epidemiology of DFUs, in terms of how patients move between risk states and how often events occur will allow us to better capture the true cost and outcomes associated with our proposed treatment strategy, and allow us to minimise the health losses from choosing the wrong treatment strategy.
Discussion
Summary of principal findings
-
Our analysis of the NHS Fife SCI-Diabetes population that attend foot clinics suggests that patients’ DFU risk does not readily change over time.
-
Despite the significant uncertainty, our health economic model suggests that preventative DFU interventions have the potential to be considered cost-effective.
-
The CPR developed in this study can be used as a tool for identifying patients who are suitable for treatment.
In this chapter, we have (1) undertaken a review of published cost–utility models for the prevention of DFUs; (2) used this information, alongside input from clinical experts, to develop a health economic model capable of estimating the cost-effectiveness of DFU prevention treatments and alternative monitoring frequencies with a CPR; and (3) estimated the value of further research and suggested where this research should focus. Our results suggest that preventative treatments for DFUs have the potential to be considered cost-effective at a willingness-to-pay threshold £20,000 per QALY gained.
Our model included the use of annual monitoring with the CPR, as recommended by NICE and SIGN. We also looked at the impact of biannual monitoring and monitoring every 6 months; however, our model did not look at the strategy of monitoring only high-risk patients as often as every 1–2 months or monitoring patients of immediate concern every 1–2 weeks. As noted Chapter 3, Methods, our design of the economic model was influenced by the results of the survival analysis of the NHS Fife SCI-Diabetes data. Analysis of these data suggests that patients do not readily change risk status (i.e. move from low to moderate risk or from moderate to high risk). Indeed, over the 8 years for which we had data, just 5% of individuals changed risk status; therefore, it is unlikely that increasing the monitoring frequency from 6-monthly (which is included in the model) to, for example, every 1–2 months or every 1–2 weeks, will capture enough additional events to offset the additional monitoring costs.
Further research aimed at predicting which patients will change risk status is warranted, as these are the patients best placed to benefit from preventative treatment. Further research, for example in the form of developing a prognostic model capable of predicting the patients whose risk status is likely to change, rather than those who are likely to develop ulceration, would be welcome.
In conducting an economic evaluation of the introduction of the CPR, we needed to compare the effect of directing therapy with and without the CPR. In the model, we have assumed that if we do not have the CPR we have two options: give no-one the intervention(s) or give everyone the intervention(s). This is rather simplistic given that some kind of risk assessment currently takes place;28 however, given the multiple combinations of treatment strategies across both the NICE and the SIGN guidelines, in terms of risk factors, methods of assessment, and interventions and monitoring frequencies, it would have been challenging to define a specific strategy including a CPR that could constitute ‘current practice’. Hence, our comparison with ‘no CPR’ allows us to estimate the potential value of the new CPR and the value of further research into the new CPR.
In our analysis, we investigated the impact of introducing a CPR based on three risk factors. Current guidelines on how patients should be assessed for risk involve a greater range of potential risk factors (NICE: insensitivity to a 10-g monofilament, absence of pedal pulses, presence of significant structural abnormality, neuropathy disability score (> 5), history of ulceration, more formal assessments using a biothesiometer, Doppler ultrasound and/or an ABI test; SIGN: insensitivity to a 10-g monofilament, limb ischaemia, ulceration, callus, infection or inflammation, deformity, gangrene, amputation, Charcot arthropathy). Given the range of potential risk factors suggested by NICE and SIGN, and the lack of clarity about the adherence of clinicians to any particular strategy, it was not possible in our study to include the use of a current prognostic model to identify patients at risk of DFUs, with which we could compare the new CPR model developed in this study. Further research aimed at comparing different prognostic models would be welcome. Treating all patients attending a foot screening clinic is not currently recommended by the NICE or the SIGN guidelines; however, considering a ‘treat all patients attending foot screening’ strategy, and hence not using a CPR, allows us to distinguish between the value of introducing the CPR and the value of the interventions themselves.
The meta-analysis and economic modelling of potential preventative treatments suggests that custom-made footwear and offloading and digital thermometry have the potential to be cost-effective; however, given that patient adherence to both of these treatments is unknown and likely to be a critical factor, further research into patient acceptability of and adherence to these treatments would be welcome.
Our VOI analysis suggests that reducing the uncertainty about the epidemiology of DFUs is likely to produce the greatest value. This may be partly explained by the small number of ulceration and amputation events in this data set and, hence, the associated uncertainty. To reduce this uncertainty, a study similar to the one presented in this chapter could be run using diabetes mellitus register data for the whole of Scotland or the UK, and for longer follow-up periods, to reduce the uncertainty related to the number of events.
Our VOI analysis also suggested that there was little value in further research relating to evidence on the treatment effect of interventions; however, it should be stressed that only one form of uncertainty is captured by the VOI analysis, namely the uncertainty related to the parameters estimated and used in the health economic model (i.e. the CIs around the estimate). The analysis does not capture the uncertainty related to issues such as publication bias and heterogeneous populations in the original studies that informed the treatment effect estimates. Hence, the decision uncertainty relating to the treatment effect in our VOI analysis (and the value of reducing this) is likely to be an underestimate. It should also be noted that in our analysis the treatments were regarded as mutually exclusive; however, in reality a combination of these treatments could be offered to patients. Our analysis also assumed that treatment effect remained constant across level of patient risk, as the relative effectiveness of these treatments in patients with different levels of risk is unknown. In addition, the effectiveness of any of these treatments used at alternative monitoring frequencies is also unknown; hence, the effectiveness of combined treatments (for specific risk groups, and used at alternative frequencies) should be explored further. To address the uncertainty surrounding treatment efficacy, a RCT would be required; however, we found that, in an at-risk population of (approximately) 26,000, few patients changed risk status, or developed ulceration, or received amputations over an 8-year follow-up period. Given this, the size, duration and cost of a RCT in this area, and what it would add compared with a large observational study (based on routine data sources), would need to be considered carefully.
Strengths of this study
The NHS Fife SCI-Diabetes data set was obtained from monitoring patients in routine clinical practice. This has given us an insight into the treatment of patients in a ‘real-world’ setting. The data set contained > 26,000 patients followed up over an 8-year period. This allowed us to assess empirically how risk of ulceration changes over time.
As part of this study, we developed a de novo health economic model (guided by the advice of clinicians involved in the prevention of DFUs) capable of including both the use of a CPR and the costs and outcomes associated with alternative clinical pathways.
Limitations of this study
The NHS Fife SCI-Diabetes data set was obtained by monitoring patients in routine clinical practice (i.e. it was not designed to be used for research); therefore, considerable data cleaning and manipulation was required. Despite this, issues of missing data remained in the data set. In particular, patients’ results for the three predictive tests included in the CPR were frequently missing. Furthermore, the project team’s clinicians suggested that amputation events may be under-recorded. This is because patients who progress to the stage of requiring an amputation may no longer be seen by the foot care team and, as a result, their data are not captured by risk assessment monitoring. For each variable in our analysis, we have chosen what we believe to be the most appropriate form of imputation – that is, what we believe is most likely to produce a consistent and plausible record of patient monitoring, reflecting what is most likely to have happened in clinical practice.
Costs of ulceration were identified from the literature. 131 As patients may be treated either in an inpatient setting or in an outpatient setting, our cost of ulceration is a weighted average of the two costs, assuming that 90% of patients are treated in an outpatient setting (based on clinical expert opinion). Amputation costs were weighted according to which type of amputation was required. Based on the literature, we assumed that two-thirds of amputations that are required are minor and that one-third are major. 143 There was also some uncertainty about the costs of interventions. Digital infrared thermometry involves a relatively inexpensive device; however, because of the lack of data on its use in routine practice, it is not clear what other resource may be required to implement this treatment strategy (e.g. contacting nurses to report high temperatures and to seek further advice). The precise elements of a ‘complex intervention’ vary across the UK, with no single agreed-on approach or clarity about the best types of specialist care required; therefore, our estimate of resource use for complex intervention may differ from that delivered in practice across the UK.
The diabetes mellitus population used in our analysis was a mix of an incident and prevalent population. This was justified on the grounds that any new monitoring policy would probably be introduced for all patients, rather than only for new patients; however, it should be noted that we do not explicitly model the process of patient entry and we take the estimates from the survival analysis as representing a static cohort. Although this is not strictly technically correct, we believe that this is a reasonable compromise to simplify the modelling.
Our choice of low, moderate and high risk of DFUs was based on the predicted probabilities of DFUs from the CPR and was agreed in consultation with the wider project team; however, it should be noted that, based on our definitions of risk, the proportion of patients classified as high risk is lower than that found in other studies. This may have implications for our findings regarding the lack of movement between risk states over time. Further work on this model should include investigating the impact on cost-effectiveness of alternative definitions of risk according to the CPR; however, we do not believe that this is likely to significantly alter our overall results owing to the significant uncertainty in the epidemiology of the NHS Fife SCI-Diabetes data, the true costs of interventions and the effectiveness of potential interventions.
Implications of our findings
-
The finding that patients’ DFU risk status does not change readily suggests that a move towards less frequent risk monitoring of at-risk patients would be acceptable.
-
As the proportion of patients whose DFU risk status changes over time is low (5%), but the costs and health outcomes associated with this group are significant, further work to predict those patients whose risk score is most likely to change would be welcome.
-
There is a need for further research into the effectiveness and acceptability of and adherence to potential preventative DFU interventions.
-
There is a need to better understand what constitutes ‘current practice’ in DFU prevention across the UK, in terms of risk assessment methods (risk factors and how they are assessed), interventions offered and adherence to clinical guidelines.
Conclusion
Our analysis suggests that DFU treatments have the potential to be cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained. In the case of custom-made footwear and offloading and digital infrared thermometry, our results suggest that treating all patients attending routine foot monitoring is likely to be considered the most cost-effective strategy, with the additional cost of these strategies compensated for by the additional increase in QALYs. In the case of complex interventions, our results suggest that these treatments are not likely to be considered cost-effective, regardless of monitoring frequency. This is because of the significant cost associated with complex interventions.
Our probabilistic sensitivity analysis suggests that there is considerable uncertainty surrounding which treatment strategy is most likely to be considered cost-effective at our willingness-to-pay threshold. This is driven primarily by the uncertainty surrounding the QALY gain associated with each treatment strategy. Our VOI analysis suggests that there is value in conducting further research in this area and, specifically, in reducing the uncertainty about how many patients experience ulcers, require an amputation or die over a given time horizon.
Given our findings, we believe that preventative treatments for patients at risk of DFUs have the potential to be cost-effective, but that significant uncertainty remains surrounding these findings; therefore, further research would need to be undertaken before we could recommend an optimal treatment strategy.
Chapter 7 Overall discussions
It is widely believed that stratifying people with diabetes mellitus according to their risk of developing a foot ulcer and providing a suitable programme of care will reduce the incidence of foot ulcers and, ultimately, reduce diabetes-related LEA. The absence of robust empirical evidence of such a direct effect has overshadowed the considerable efforts of those providing foot-related health care worldwide.
For risk assessment programmes to be effective, simple clinical assessments that can be used by health-care staff with varying degrees of skill are needed. 103 The CPR developed and validated by our group is based on only three risk factors that can be assessed cheaply, easily and accurately and used to identify those at risk of ulceration, especially those at low risk (i.e. the vast majority of people with diabetes mellitus). The use of CPR in clinical practice could simplify current approaches to risk assessment, which could reduce the time spent testing, the costs associated with expensive tests and the time needed to train staff to perform more complex diagnostic procedures. 10–12,144
To our knowledge, the time between foot risk assessments has not been subject to evaluation before. By using data from the electronic health record (EHR) of people with diabetes mellitus in one health board area in Scotland, we are able to show that the foot ulcer risk status of most people with diabetes mellitus does not readily change over time, and a move towards less frequent risk assessment is indicated for the majority. Because the proportion of patients in the low-risk group whose ulcer risk status changed over time was small (5%), and because of the significant costs and health outcomes associated with this group, further work to improve the accuracy with which we can identify which patients’ risk score is most likely to change is merited. The use of data from EHRs to answer research questions is often compromised by missing data;45 therefore, improving the recording of patients’ test results and the number of important events in the SCI-Diabetes data set and EHR more generally would be of value.
Clinical prediction rules by themselves do not automatically produce improvements in patients’ outcomes, and risk assessment in the absence of effective treatments is not recommended. The large number of SRs aiming to identify effective interventions to prevent foot ulceration did not reach clear, reproducible conclusions about the effect of treatments. As most of the researchers undertaking these summaries lacked sources of funding, this is possibly unsurprising. The absence of meta-analyses of data in the SRs may also have contributed to the opacity. It was by pooling data that we were able to detect effective interventions for reducing the incidence of foot ulcers.
The trials of digital infrared thermometers showed that these are effective in reducing foot ulcers if activity is reduced when foot temperature increases. It is to be hoped that these effects can be replicated in currently ongoing trials. Assessing trial populations’ levels of compliance with advice to rest will also be important.
The marked difference in effect in the subgroup analysis between data from two trials of footwear and offloading devices that involved mostly people with no history of ulcers and data from another four trials that included people who had previously developed ulcers is interesting. It seems reasonable to suppose that the ability to influence patient outcomes will diminish as the risk of foot ulceration increases. During the process of developing the CPR we found that those with a history of foot ulceration are at much greater risk of ulceration than those either with a lack of sensation or in whom pedal pulses are absent. If an agreement to share data among the investigators of the trials of footwear and offloading could be reached, a comparison of outcomes from subgroups of people in these six trials in an IPD meta-analysis and in the 10 ongoing studies could bring further clarity without incurring the high cost of a new trial.
The fact that individual trials of complex interventions failed to show beneficial effects until data were pooled in a meta-analysis does support the opinion of others that trials of specialist foot care need to recruit very large samples of patients. 13 An IPD meta-analysis could also help to identify the benefits of specialist foot centres; however, the two largest trials are among the oldest that we reviewed, so it might not be possible to obtain the data.
There is a need to better understand what constitutes ‘current practice’ in foot care programmes across the UK in terms of risk assessment methods (risk factors and how they are assessed), interventions offered and level of adherence to clinical guidelines.
Education by itself appears to be ineffective in reducing foot ulcers, and the small trials of antifungal nail lacquer and elastic stockings not only lacked evidence of effectiveness but seemed to us to lack biological plausibility.
In considering whether or not there is potential worth in undertaking further research, particularly a RCT, for further research is needed into the effectiveness and acceptability of and adherence to potential preventative DFU interventions. However, it is clear that improving the recording of patient data in EHRs will bring benefits to researchers and, ultimately, the public.
The economic evaluation showed that the DFU treatments identified by the SR have the potential to be cost-effective, but uncertainty in model parameters and other elements (e.g. patient acceptability of and adherence to interventions) prohibits a strong conclusion.
Chapter 8 Overall conclusions
Strengths and weaknesses of our research
The strengths of this research arise mainly from our access two large data sets: the PODUS data set that was used to develop and validate the CPR and a SCI-Diabetes data set that was used to assess the transitional probabilities of people with diabetes mellitus moving from one risk state to another.
The weaknesses of the research relate to the large numbers of missing data in the SCI-Diabetes data set and the poor quality of some of the RCTs in the SR. It is also possible that the decision to separate the comparators by intervention may have modified our VOI analysis. An approach in which all interventions are considered together may well make RCTs more important in the VOI analysis, as they would inform the differences between preventative interventions and allow any interactions to be identified.
Overall conclusions
This research has led to development and validation of a simple CPR for use in clinical practice and an analysis of EHR data to show that the risk of foot ulceration does not change over a 10-year period for most people with diabetes mellitus. Our overview found that, although there are a large number of SRs of preventative interventions for foot ulceration, clear, reproducible conclusions were rare. Our new SR of trial data has allowed the identification of effective preventative interventions for foot ulceration in diabetes mellitus; digital thermometry and meta-analyses revealed the potentially beneficial effect of complex interventions and custom-made footwear and offloading. However, remains uncertainly remains about the clinical effectiveness and cost-effectiveness of interventions to prevent foot ulceration in diabetes mellitus.
We make the following recommendations for future research:
-
There is a need for further research into the effectiveness and acceptability of and adherence to potential preventative DFU interventions.
-
There is a need to better understand what constitutes ‘current practice’ in DFU prevention across the UK in terms of risk assessment methods (risk factors and how they are assessed), interventions offered and adherence to clinical guidelines.
-
There is a need for more complete EHR data to be collected, particularly on those parameters relating to foot disease in diabetes mellitus.
-
We recommend that researchers share their trial data for IPD analyses to explore subgroup effects for interventions.
-
Further research using the new CPR is merited, as is treating all patients attending a foot screening clinic with a ‘treat-all patients attending foot screening’ strategy compared with care using a CPR.
-
The effectiveness of combined treatments for specific risk groups and used at alternative frequencies should be explored further. To address the uncertainty surrounding treatment efficacy, a RCT would be required; however, the size, duration and cost of a RCT in this area require careful consideration.
-
Further research aimed at comparing different prognostic models would be welcome.
Acknowledgements
The authors thank the following members of the project Study Steering Committee for their time, advice and generously given expertise:
Dr Sara Twaddle, Director of Evidence, Healthcare Improvement Scotland, who expertly chaired our committee; Mr Bill Morrison, Patient and Public Representative; Professor Tom Fahey, Professor of Primary Care, Royal College of Surgeons in Ireland; Ms Genevieve Cezard, PhD Registrant in Statistics and Epidemiology, University of St Andrews; Dr Beth Woods, Health Economist, Centre for Health Economics, University of York; Dr Heather McIntosh, Health Services Researcher, Healthcare Improvement Scotland; Dr Hannah Robertson, Consultant Diabetologist, Aberdeen Royal Infirmary; Dr Farina Hashmi, Senior Lecturer in Podiatry, School of Health Sciences, University of Salford; Dr Purva Abhyankar, Lecturer in Decision-making, University of Stirling; and Mr Wesley Stuart, Consultant Vascular Surgeon, Queen Elizabeth University Hospital, Glasgow.
We extend our thanks to Ms Xin Wang, PhD Registrant in Population Health Sciences at the University of Edinburgh, for the translation work involved with the Chinese SR and trials. Ms Karen Baxter, Podiatry Head of Service (Management), NHS Fife, for providing data and costs pertaining to the provision of podiatry services. Dr Matilde Monteiro-Soares, Post-doctoral Fellow, Centre for Research in Health Technologies and Information Systems, University of Porto, Portugal; Professor Aristidis Veves, Professor of Surgery, Harvard Medical School, MA, USA; Dr Caroline A Abbott, Research Fellow, Manchester Metropolitan University, and Professor Andrew JM Boulton, University of Manchester, who all shared the data from their cohort studies of predictive factors for foot ulceration in diabetes mellitus to aid the development of the CPR in Chapter 3. Ms Kay Brown, Specialist Diabetes Podiatrist, NHS Tayside, who collected data for the long-term follow-up of patient outcomes for the Crawford et al. 21 study (see Chapter 3 and Appendix 3, Figure 33).
Contributions of authors
All authors contributed to the project.
Fay Crawford (https://orcid.org/0000-0002-0473-9959) (Chief Investigator) obtained all the data used in this project and was responsible for all aspects of the research. She designed the overview and the SR of preventative interventions for foot ulceration in diabetes mellitus as a reviewer, contributed to the meta-analyses, led the writing and contributed to the interpretation of results in all sections of this report.
Francesca M Chappell (https://orcid.org/0000-0002-7742-1757), Margaret Horne (https://orcid.org/0000-0001-7621-2462) and Richard D Riley (https://orcid.org/0000-0001-8699-0735) created the CPR from a subgroup of data from the original PODUS data set, conducted all the analyses and wrote Chapter 3. Francesca Chappell and Margaret Horne prepared the NHS Fife data set for analysis by the health economist collaborators. Francesca Chappell also performed the meta-analyses in Chapter 5.
Robert Heggie (https://orcid.org/0000-0001-7396-4773) had day-to-day responsibility for the economic evaluation, which was led by James Lewsey (https://orcid.org/0000-0002-3811-8165) with input from Neil Hawkins (https://orcid.org/0000-0003-3199-221X). In addition to the review of economic literature and the creation of a new economic model and a VOI analysis, they analysed the NHS Fife SCI-Diabetes data set and examined the effects of different foot-monitoring intervals. They authored Chapter 6.
Donald Nicolson (https://orcid.org/0000-0003-4190-6434) was the lead reviewer for the overview and SR and was responsible for the day-to-day research for the reviews.
Marie Smith (https://orcid.org/0000-0002-1572-0268) provided ongoing support for the literature searches and obtaining the reports for the review.
Aparna Amanna was the project research assistant/administrator. In addition to working on the reviews, she was responsible for formatting this report.
Graham Leese (https://orcid.org/0000-0003-0570-5678), David Weller (https://orcid.org/0000-0002-8112-718X) and Julie Brittenden (https://orcid.org/0000-0002-1441-6774) together with Saket Gupta (https://orcid.org/0000-0003-2643-626X), Angela Martin and Karen Gray (https://orcid.org/0000-0001-7091-7568) commented on various aspects of the research during the course of the project and brought content expertise and their various clinical perspectives to the project to ensure that it reflected current clinical practice in the NHS.
Publications
Chappell FM, Horne M, Crawford F. Surprising Results When Selecting Predictors for a Clinical Prediction Rule. Methods for Evaluation of Medical Prediction Models, Tests and Biomarkers (MEMTAB) 2018 Symposium, Utrecht, the Netherlands, 2–3 July 2018. URL: https://diagnprognres.biomedcentral.com/articles/10.1186/s41512-018-0036-3 (accessed 2 July 2018).
Crawford F, Nicolson D, Ammana, A, Green A, Gray K, Chalmers, J et al. Preventing Foot Ulceration in Diabetes: Evidence from an Overview of 17 Systematic Reviews. Poster presentation at the World Congress for the Prevention of Diabetes and its Complications, Edinburgh, UK, 15–18 July 2018. URL: https://confpartners.eventsair.com/QuickEventWebsitePortal/wcpdc2018/agenda/Agenda/AgendaItemDetail?id=f8a5ff9c-766b-4e72-9d4f-6cd34f09ea61 (accessed 2 July 2018).
Crawford F, Nicolson DJ, Amanna AE, Martin A, Gupta S, Leese GP, et al. Preventing foot ulceration in diabetes: a systematic review and meta-analyses of RCT data. Diabetologia 2020;63:49–64.
Crawford F, Nicolson DJ, Amanna AE, Martin A, Gupta S, Leese G, et al. Preventing foot ulceration in diabetes: systematic review and meta-analyses of RCT data. Diabetologia 2020;63:49.
Chappell FM, Crawford F, Horne M, Leese GP, Martin A, Weller D, et al. Development and validation of a clinical prediction rule for diabetic foot ulceration: an individual participant data meta-analysis. (Submitted August 2020.)
Heggie R, Chappell F, Crawford F, Martin A, Gupta S, Hawkins N, et al. Complication rate among people with diabetes at low risk of foot ulceration in Fife, UK: an analysis of routinely collected data. Diabetic Medicine 2020.
Data-sharing statement
All available data can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719-24. https://doi.org/10.1016/S0140-6736(05)67698-2.
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513-21. https://doi.org/10.2337/diacare.13.5.513.
- Scottish Diabetes Survey Monitoring Group . Scottish Diabetes Survey 2014 n.d. www.diabetesinscotland.org.uk/Publications.aspx (accessed 17 January 2016).
- Kerr M. Footcare For People With Diabetes. The Economic Case For Change. NHS Diabetes; 2012.
- Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 2012;55:1919-25. https://doi.org/10.1007/s00125-012-2468-6.
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33:1493-8. https://doi.org/10.1111/dme.13054.
- Kennon B, Leese GP, Cochrane L, Colhoun H, Wild S, Strang D, et al. Reduced incidence of lower-extremity amputations in people with diabetes in Scotland. A nationwide study. Diabetes Care 2012;35:2588-90. https://doi.org/10.2337/dc12-0511.
- Mackay DF, Haw S, Newby DE, Langhorne P, Lloyd SM, McConnachie A, et al. Impact of Scotland’s comprehensive, smoke-free legislation on stroke. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0062597.
- Ramos R, Comas-Cufí M, Martí-Lluch R, Balló E, Ponjoan A, Alves-Cabratosa L, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ 2018;362. https://doi.org/10.1136/bmj.k3359.
- Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Risk stratification systems for diabetic foot ulcers: a systematic review. Diabetologia 2011;54:1190-9. https://doi.org/10.1007/s00125-010-2030-3.
- NICE . Diabetic Foot Problems Prevention and Management. NICE Guideline (NG19) n.d. www.nice.org.uk/guidance/ng19 (accessed 17 January 2016).
- SIGN . Management of Diabetes: A National Clinical Guideline 116. 2010. www.sign.ac.uk/assets/sign116.pdf (accessed 17 January 2016).
- Jeffcoate WJ. Stratification of foot risk predicts the incidence of new foot disease, but do we yet know that the adoption of routine screening reduces it?. Diabetologia 2011;54:991-3. https://doi.org/10.1007/s00125-011-2075-y.
- Leese GP, Reid F, Green V, McAlpine R, Cunningham S, Emslie-Smith AM, et al. Stratification of foot ulcer risk in patients with diabetes: a population-based study. Int J Clin Pract 2006;60:541-5. https://doi.org/10.1111/j.1368-5031.2006.00899.x.
- Crawford F, Cezard G, Chappell FM, Murray GD, Price JF, Sheikh A, et al. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS). Health Technol Assess 2015;19. https://doi.org/10.3310/hta19570.
- Hoogeveen RC, Dorresteijn JA, Kriegsman DM, Valk GD. Complex interventions for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2015;8. https://doi.org/10.1002/14651858.CD007610.pub3.
- Leese GP, Stang D, Pearson DW. Scottish Diabetes Foot Action Group . A national approach to diabetes foot risk stratification and foot care. Scott Med J 2011;56:151-5. https://doi.org/10.1258/smj.2011.011113.
- Mason J, O’Keeffe C, McIntosh A, Hutchinson A, Booth A, Young RJ. A systematic review of foot ulcer in patients with type 2 diabetes mellitus. I: prevention. Diabet Med 1999;16:801-12. https://doi.org/10.1046/j.1464-5491.1999.00133.x.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. https://doi.org/10.7326/M14-0698.
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377-84. https://doi.org/10.1046/j.1464-5491.2002.00698.x.
- Crawford F, McCowan C, Dimitrov BD, Woodburn J, Wylie GH, Booth E, et al. The risk of foot ulceration in people with diabetes screened in community settings: findings from a cohort study. QJM 2011;104:403-10. https://doi.org/10.1093/qjmed/hcq227.
- Kästenbauer T, Sauseng S, Sokol G, Auinger M, Irsigler K. A prospective study of predictors for foot ulceration in type 2 diabetes. J Am Podiatr Med Assoc 2001;91:343-50. https://doi.org/10.7547/87507315-91-7-343.
- Monami M, Vivarelli M, Desideri CM, Colombi C, Marchionni N, Mannucci E. Pulse pressure and prediction of incident foot ulcers in type 2 diabetes. Diabetes Care 2009;32:897-9. https://doi.org/10.2337/dc08-1679.
- Monteiro-Soares M, Dinis-Ribeiro M. External validation and optimisation of a model for predicting foot ulcers in patients with diabetes. Diabetologia 2010;53:1525-33. https://doi.org/10.1007/s00125-010-1731-y.
- Pham H, Armstrong DG, Harvery C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration. Diabetes Care 2000;23:606-11. https://doi.org/10.2337/diacare.23.5.606.
- Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care 1992;15:1386-9. https://doi.org/10.2337/diacare.15.10.1386.
- Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994;17:557-60. https://doi.org/10.2337/diacare.17.6.557.
- Leese GP, Cochrane L, Mackie AD, Stang D, Brown K, Green V. Measuring the accuracy of different ways to identify the ‘at-risk’ foot in routine clinical practice. Diabet Med 2011;28:747-54. https://doi.org/10.1111/j.1464-5491.2011.03297.x.
- Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 2006;29:1202-7. https://doi.org/10.2337/dc05-2031.
- Hurley L, Kelly L, Garrow AP, Glynn LG, McIntosh C, Alvarez-Iglesias A, et al. A prospective study of risk factors for foot ulceration: the West of Ireland Diabetes Foot Study. QJM 2013;106:1103-10. https://doi.org/10.1093/qjmed/hct182.
- Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1-W33. https://doi.org/10.7326/M18-1377.
- Schwarzer G. Meta: an R package for meta-analysis. R News 2007;7:40-5.
- Mathew T, Nordström K. Comparison of one-step and two-step meta-analysis models using individual patient data. Biom J 2010;52:271-87. https://doi.org/10.1002/bimj.200900143.
- Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330. https://doi.org/10.1136/bmj.38398.500764.8F.
- Silverman JJ. The incidence of palpable dorsalis and pedis and posterior tibial pulsations in soldiers; an analysis of over 1,000 infantry soldiers. Am Heart J 1946;32:82-7. https://doi.org/10.1016/0002-8703(46)90228-1.
- Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 1994;121:200-6. https://doi.org/10.7326/0003-4819-121-3-199408010-00008.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. https://doi.org/10.1016/S0895-4356(96)00236-3.
- Van Houwelingen JC, Le Cessie S. Predictive value of statistical models. Stat Med 1990;9:1303-25. https://doi.org/10.1002/sim.4780091109.
- Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med 2016;35:214-26. https://doi.org/10.1002/sim.6787.
- Pedersen AB, Mikkelsen EM, Cronin-Fenton D, Kristensen NR, Pham TM, Pedersen L, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017;9:157-66. https://doi.org/10.2147/CLEP.S129785.
- Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009.
- Pavlou M, Ambler G, Seaman S, Omar RZ. A note on obtaining correct marginal predictions from a random intercepts model for binary outcomes. BMC Med Res Methodol 2015;15. https://doi.org/10.1186/s12874-015-0046-6.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. https://doi.org/10.1177/0272989X06295361.
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12. https://doi.org/10.1186/1471-2105-12-77.
- Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag; 2001.
- Information Services Division . Data Dictionary A–Z n.d. www.ndc.scot.nhs.uk/Dictionary-A-Z/Definitions/index.asp?ID=128%26Title=CHI%20Number (accessed 18 September 2020).
- Lu Y, Xu J, Zhao W, Han HR. Measuring self-care in persons with type 2 diabetes: a systematic review. Eval Health Prof 2016;39:131-84. https://doi.org/10.1177/0163278715588927.
- Riley RD, van der Windt DA, Croft P, Moons KGM. Prognosis Research in Health Care. Concept Methods and Impact. Oxford: Oxford University Press; 2019.
- O’Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol Assess 2000;4. https://doi.org/10.3310/hta4210.
- Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst Rev 2000;3. https://doi.org/10.1002/14651858.CD002302.
- Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD001488.pub4.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions 2019. www.training.cochrane.org/handbook.
- Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-15.
- Ballard M, Montgomery P. Risk of bias in overviews of reviews: a scoping review of methodological guidance and four-item checklist. Res Synth Methods 2017;8:92-108. https://doi.org/10.1002/jrsm.1229.
- Boyko EJ, Ahroni JH, Nelson KM. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: response to Leese and Morris. Diabetes Care 2006;29. https://doi.org/10.2337/dc06-1661.
- Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician 2002;66:1655-62.
- Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
- Adiewere P, Gillis RB, Imran Jirwani S, Meal A, Shaw L, Adams GG. A systematic review and meta-analysis of patient education in preventing and reducing the incidence or recurrence of adult diabetes foot ulcers. Heliyon 2018;4. https://doi.org/10.1016/j.heliyon.2018.e00614.
- Arad Y, Fonseca V, Peters A, Vinik A. Beyond the monofilament for the insensate diabetic foot: a systematic review of randomized trials to prevent the occurrence of plantar foot ulcers in patients with diabetes. Diabetes Care 2011;34:1041-6. https://doi.org/10.2337/dc10-1666.
- Binning J, Woodburn J, Bus SA, Barn R. Motivational interviewing to improve adherence behaviours for the prevention of diabetic foot ulceration. Diabetes Metab Res Rev 2019;35. https://doi.org/10.1002/dmrr.3105.
- He JD, Zhang L, LIU L, Zhu YJ. Intensive versus routine education on diabetes mellitus for prevention diabetic foot ulcer: a systematic review. Chin J Evid Based Med 2013;13:1470-4.
- Kaltenthaler E, Morrell CJ, Booth A, Akehurst RL. The prevention and treatment of diabetic foot ulcers: a review of clinical effectiveness studies. J Clin Eff 1998;3:99-104. https://doi.org/10.1108/eb020882.
- Buckley CM, Perry IJ, Bradley CP, Kearney PM. Does contact with a podiatrist prevent the occurrence of a lower extremity amputation in people with diabetes? A systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002331.
- Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR. International Working Group on the Diabetic Foot . Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev 2016;32:99-118. https://doi.org/10.1002/dmrr.2702.
- Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlavácek P, et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev 2008;24:162-80. https://doi.org/10.1002/dmrr.850.
- Healy A, Naemi R, Chockalingam N. The effectiveness of footwear and other removable off-loading devices in the treatment of diabetic foot ulcers: a systematic review. Curr Diabetes Rev 2014;10:215-30. https://doi.org/10.2174/1573399810666140918121438.
- Heuch L, Streak Gomersall J. Effectiveness of offloading methods in preventing primary diabetic foot ulcers in adults with diabetes: a systematic review. JBI Database System Rev Implement Rep 2016;14:236-65. https://doi.org/10.11124/JBISRIR-2016-003013.
- Maciejewski ML, Reiber GE, Smith DG, Wallace C, Hayes S, Boyko EJ. Effectiveness of diabetic therapeutic footwear in preventing reulceration. Diabetes Care 2004;27:1774-82. https://doi.org/10.2337/diacare.27.7.1774.
- Mayfield JA, Sugarman JR. The use of the Semmes-Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract 2000;49:17-29.
- Paton J, Bruce G, Jones R, Stenhouse E. Effectiveness of insoles used for the prevention of ulceration in the neuropathic diabetic foot: a systematic review. J Diabetes Complicat 2011;25:52-6. https://doi.org/10.1016/j.jdiacomp.2009.09.002.
- Ahmad Sharoni SK, Minhat HS, Mohd Zulkefli NA, Baharom A. Health education programmes to improve foot self-care practices and foot problems among older people with diabetes: a systematic review. Int J Older People Nurs 2016;11:214-39. https://doi.org/10.1111/opn.12112.
- van Netten JJ, Price PE, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al. International Working Group on the Diabetic Foot . Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev 2016;32:84-98. https://doi.org/10.1002/dmrr.2701.
- Shenaq SM, Klebuc MJ, Vargo D. How to help diabetic patients avoid amputation. Prevention and management of foot ulcers. Postgrad Med 1994;96. https://doi.org/10.1080/00325481.1994.11945916.
- Krans HMJ. Diabetes Care and Research in Europe the St. Vincent Declaration Action Programme; Implementation Document. Copenhagen: World Health Organization Regional Office for Europe; 1992.
- Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64-122. https://doi.org/10.1177/107110078100200202.
- Cochrane Wounds Group . Cochrane Wounds Glossary 2015 n.d. https://wounds.cochrane.org/cochrane-wounds-glossary (accessed 7 November 2015).
- Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K. International Working Group on the Diabetic Foot (IWGDF) . Prevention and management of foot problems in diabetes: a Summary Guidance for Daily Practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract 2017;124:84-92. https://doi.org/10.1016/j.diabres.2016.12.007.
- Monami M, Zannoni S, Gaias M, Nreu B, Marchionni N, Mannucci E. Effects of a short educational program for the prevention of foot ulcers in high-risk patients: a randomized controlled trial. Int J Endocrinol 2015;2015. https://doi.org/10.1155/2015/615680.
- Gershater AM, Pilhammar E, Apelqvist J, AlmRoijer C. Patient education for the prevention of diabetic foot ulcers: interim analysis of a randomised controlled trial due to morbidity and mortality of participants. Eur Diabetes Nurs 2011;8:102-7b. https://doi.org/10.1002/edn.189.
- Lincoln NB, Radford KA, Game FL, Jeffcoate WJ. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia 2008;51:1954-61. https://doi.org/10.1007/s00125-008-1110-0.
- Rönnemaa T, Hamalainen H, Toikka T, Liukkonen I. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care 1997;20:1833-7. https://doi.org/10.2337/diacare.20.12.1833.
- Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg 1989;158:520-3. https://doi.org/10.1016/0002-9610(89)90183-9.
- Bloomgarden ZT, Karmally W, Metzger MJ. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10:263-72. https://doi.org/10.2337/diacare.10.3.263.
- McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med 1998;15:80-4. https://doi.org/10.1002/(SICI)1096-9136(199801)15: 1<80::AID-DIA517>3.0.CO;2-K.
- Uccioli L, Faglia E, Monticone G, Favales F, Durola L, Aldeghi A, et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care 1995;18:1376-8. https://doi.org/10.2337/diacare.18.10.1376.
- Reiber GE, Smith DG, Wallace C, Sullivan K, Hayes S, Vath C, et al. Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. JAMA 2002;287:2552-8. https://doi.org/10.1001/jama.287.19.2552.
- Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004;27:2642-7. https://doi.org/10.2337/diacare.27.11.2642.
- Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care 2007;30:14-20. https://doi.org/10.2337/dc06-1600.
- Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med 2007;120:1042-6. https://doi.org/10.1016/j.amjmed.2007.06.028.
- Cisneros LL. Evaluation of a neuropathic ulcers prevention program for patients with diabetes. Rev Bras Fisioter 2010;14:31-7. https://doi.org/10.1590/S1413-35552010000100006.
- Liang R, Dai X, Zuojie L, Zhou A, Meijuan C. Two-year foot care program for minority patients with type 2 diabetes mellitus of Zhuang Tribe in Guangxi, China. Can J Diabetes 2012;36:15-8. https://doi.org/10.1016/j.jcjd.2011.08.002.
- Litzelman DK, Slemenda CW, Langefeld CD, Hays LM, Welch MA, Bild DE, et al. Reduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1993;119:36-41. https://doi.org/10.7326/0003-4819-119-1-199307010-00006.
- Belcaro G, Laurora G, Cesarone MR, Pomante P. Elastic stockings in diabetic microangiopathy. Long-term clinical and microcirculatory evaluation. VASA 1992;21:193-7.
- Plank J, Haas W, Rakovac I, Görzer E, Sommer R, Siebenhofer A, et al. Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects. Diabetes Care 2003;26:1691-5. https://doi.org/10.2337/diacare.26.6.1691.
- Lavery LA, LaFontaine J, Higgins KR, Lanctot DR, Constantinides G. Shear-reducing insoles to prevent foot ulceration in high-risk diabetic patients. Adv Skin Wound Care 2012;25:519-24. https://doi.org/10.1097/01.ASW.0000422625.17407.93.
- Rizzo L, Tedeschi A, Fallani E, Coppelli A, Vallini V, Iacopi E, et al. Custom-made orthesis and shoes in a structured follow-up program reduces the incidence of neuropathic ulcers in high-risk diabetic foot patients. Int J Low Extrem Wounds 2012;11:59-64. https://doi.org/10.1177/1534734612438729.
- Scirè V, Leporati E, Teobaldi I, Nobili LA, Rizzo L, Piaggesi A. Effectiveness and safety of using Podikon digital silicone padding in the primary prevention of neuropathic lesions in the forefoot of diabetic patients. J Am Podiatr Med Assoc 2009;99:28-34. https://doi.org/10.7547/0980028.
- Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care 2014;37:1982-9. https://doi.org/10.2337/dc13-2956.
- Bus SA, Waaijman R, Arts M, de Haart M, Busch-Westbroek T, van Baal J, et al. Effect of custom-made footwear on foot ulcer recurrence in diabetes: a multicenter randomized controlled trial. Diabetes Care 2013;36:4109-16. https://doi.org/10.2337/dc13-0996.
- Klenerman L, McCabe C, Cogley D, Crerand P, Laing P, White M. Screening for patients risk of diabetic foot ulceration in a general diabetic outpatient clinic. Diabetic Med 1996;13:561-3. https://doi.org/10.1002/(SICI)1096-9136(199606)13:6<561::AID-DIA112>3.0.CO;2-P.
- Van Putten M, Leffers P, Schaper NC. Podiatric Insoles Cause Foot Ulcers in Diabetic Patients n.d.
- Armstrong DG, Holtz K, Wu S. Can the use of a topical antifungal nail lacquer reduce risk for diabetic foot ulceration? Results from a randomised controlled pilot study. Int Wound J 2005;2:166-70. https://doi.org/10.1111/j.1742-4801.2005.00097.x.
- Jeffcoate WJ, Bus SA, Game FL, Hinchliffe RJ, Price PE, Schaper NC. International Working Group on the Diabetic Foot and the European Wound Management Association . Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabetes Endocrinol 2016;4:781-8. https://doi.org/10.1016/S2213-8587(16)30012-2.
- Zhengguang L, Xiaokui L, Yan L, Hulin C, Yucheng Y, Yuxiong C, et al. Evaluation of preventive effect of preventive health education on senile diabetic foot ulcer. Chin J Pract Intern Med 2008;28:68-9.
- Xue-hua H. Effect of diabetes education on prevention of diabetic foot. Chin Foreign Health Dig 2010;33.
- Xiaomin L, Jianning W. The significance of individualized educational intervention in preventing diabetic foot. J Med Sci 2010;20:212-13.
- Waxman R, Woodburn H, Powell M, Woodburn J, Blackburn S, Helliwell P. FOOTSTEP: a randomized controlled trial investigating the clinical and cost effectiveness of a patient self-management program for basic foot care in the elderly. J Clin Epidemiol 2003;56:1092-9. https://doi.org/10.1016/S0895-4356(03)00197-5.
- Joanna Briggs Institute . Joanna Briggs Institute Critical Appraisal Checklist for Randomized Control Pseudo-Randomized Trial 2017 n.d. https://joannabriggs.org/research/critical-appraisal-tools (accessed 18 September 2020).
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Methods Work Group, Third US Preventive Services Task Force . Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21-35. https://doi.org/10.1016/S0749-3797(01)00261-6.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. https://doi.org/10.1136/jech.52.6.377.
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. https://doi.org/10.1016/S0895-4356(98)00131-0.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials 1996;17:1-12. https://doi.org/10.1016/0197-2456(95)00134-4.
- Centre for Reviews and Dissemination . Guidance For Undertaking Reviews in Health Care 2008. www.york.ac.uk/inst/crd/index_guidance.htm (accessed 27 February 2019).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Sheldon T, Chalmers I. The UK Cochrane Centre and the NHS Centre for reviews and dissemination: respective roles within the information systems strategy of the NHS R.D programme, coordination and principles underlying collaboration. Health Econ 1994;3:201-3. https://doi.org/10.1002/hec.4730030308.
- Medical Research Council . The National Archives 2006. https://webarchive.nationalarchives.gov.uk/20140102233131/http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002452 (accessed 18 September 2020).
- Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999;22:1036-42. https://doi.org/10.2337/diacare.22.7.1036.
- International Working Group on the Diabetic Foot (IWGDF) . IWGDF Guidelines n.d. https://iwgdfguidelines.org/ (accessed 6 May 2020).
- LeMaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther 2008;88:1385-98. https://doi.org/10.2522/ptj.20080019.
- Skafjeld A, Iversen MM, Holme I, Ribu L, Hvaal K, Kilhovd BK. A pilot study testing the feasibility of skin temperature monitoring to reduce recurrent foot ulcers in patients with diabetes – a randomized controlled trial. BMC Endocr Disord 2015;15. https://doi.org/10.1186/s12902-015-0054-x.
- Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Zbrozek AS, Dong F, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care 1997;20:725-34. https://doi.org/10.2337/diacare.20.5.725.
- Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost–utility analysis based on Markov model simulations. Diabetologia 2001;44:2077-87. https://doi.org/10.1007/s001250100013.
- Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care 2004;27:901-7. https://doi.org/10.2337/diacare.27.4.901.
- Rauner MS, Heidenberger K, Pesendorfer EM. Model-based evaluation of diabetic foot prevention strategies in Austria. Health Care Manag Sci 2005;8:253-65. https://doi.org/10.1007/s10729-005-4136-6.
- Barshes NR, Saedi S, Wrobel J, Kougias P, Kundakcioglu OE, Armstrong DG. A model to estimate cost-savings in diabetic foot ulcer prevention efforts. J Diabetes Complicat 2017;31:700-7. https://doi.org/10.1016/j.jdiacomp.2016.12.017.
- Barshes NR, Belkin M. MOVIE Study Collaborators. J Am Coll Surg 2011;213:552-66.e5. https://doi.org/10.1016/j.jamcollsurg.2011.07.011.
- de Leon J, Miller E, Keith M. A cost-effectiveness evaluating of vacuum-assisted closure treatment for hospitalized diabetic foot ulcer wound patient. J Wound Ostomy Cont 2006;33.
- Ghatnekar O, Persson U, Willis M, Wright T, Odegaard K. The cost-effectiveness in the UK of treating diabetic lower extremity ulcers with becaplermin gel. J Med Econ 2000 n.d.;3:87-95.
- Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care 2002;11:70-4. https://doi.org/10.12968/jowc.2002.11.2.26675.
- Kantor J, Margolis D. Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis. Home Healthc Consult 2002;9:25-30.
- Kerr M. Diabetic Foot Care in England: An Economic Study. London: Insight Health Economics; 2017.
- Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab 2004;30:549-56. https://doi.org/10.1016/S1262-3636(07)70154-4.
- Sibbald RG, Torrance G, Hux M, Attard C, Milkovich N. Cost-effectiveness of becaplermin for nonhealing neuropathic diabetic foot ulcers. Ostomy Wound Manage 2003;49:76-84.
- Waycaster C, Gillingham AM. Comparative Cost-Effectiveness of Becaplermin Gel on Wound Healing in Patients With Diabetic Foot Ulcer: Changes in Wound Surface Area. Value in Health n.d.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programme. Oxford: Oxford University Press; 2005.
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes Metab Res Rev 2000;16:75-83. https://doi.org/10.1002/1520-7560(200009/10)16:1+<::AID-DMRR139>3.0.CO;2-8.
- NICE . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9 (accessed 5 April 2017).
- Saunders JA, Morrow-Howell N, Spitznagel E, Dore P, Proctor EK, Pescarino R. Imputing missing data: a comparison of methods for social work researchers. Soc Work Res 2006;30:19-31. https://doi.org/10.1093/swr/30.1.19.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. https://doi.org/10.1177/0272989X12472398.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Handbooks in Economic Evaluation. Oxford: Oxford University Press; 2011.
- Strong M, Oakley JE, Brennan A. Estimating multi-parameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a non-parametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Diabetes UK . Record Levels of Diabetes-Related Amputations 2017. www.diabetes.org.uk/about_us/news/record-levels-of-diabetes-related-amputations (accessed 20 February 2019).
- Crawford F, Welch K, Andras A, Chappell FM. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD010680.pub2.
- Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411-21. https://doi.org/10.1097/01.psy.0000127692.23278.a9.
- O’Riordan P, Stevens PE, Lamb EJ. Estimated glomerular filtration rate. BMJ 2014;348. https://doi.org/10.1136/bmj.g264.
- Resche-Rigon M, White IR, Bartlett JW, Peters SA, Thompson SG. PROG-IMT Study Group . Multiple imputation for handling systematically missing confounders in meta-analysis of individual participant data. Stat Med 2013;32:4890-905. https://doi.org/10.1002/sim.5894.
- Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press Books; 2011.
- Jackson C. Flexsurv: a platform for parametric survival modeling in R. J Stat Softw 2016;70. https://doi.org/10.18637/jss.v070.i08.
- Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance, and foot problems in type 2 diabetes. Diabetic Med 1991;8:111-17. https://doi.org/10.1111/j.1464-5491.1991.tb01555.x.
- Borges W. The Impact of a Brief Foot Care Intervention for Persons With Diabetes. Texas Medical Center Dissertations, University of San Francisco; 2004.
- Borges WJ, Ostwald SK. Improving foot self-care behaviors with Pies Sanos. West J Nurs Res 2008;30:325-41. https://doi.org/10.1177/0193945907303104.
- Colagiuri S, Marsden L, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract 1995;28:29-34. https://doi.org/10.1016/0168-8227(95)01050-N.
- Corbett CFC. A randomized pilot study of improving foot care in home health patients with diabetes. Diabetes Educ 2003;29:269-70. https://doi.org/10.1177/014572170302900218.
- Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT Programme makes a difference. Diabet Med 2006;23:944-54. https://doi.org/10.1111/j.1464-5491.2006.01906.x.
- Donohoe ME, Fletton JA, Hook A, Powell R, Robinson I, Stead JW, et al. Improving foot care for people with diabetes mellitus – a randomized controlled trial of an integrated care approach. Diabetic Med 2000;17:581-7. https://doi.org/10.1046/j.1464-5491.2000.00336.x.
- Frank KI. Self-management of foot care for patients 65 years of age or older with diabetes. Dissertation Abstracts International 2003;64.
- Frank KI, Martin J, Bennett SJ. Self management of foot care for patients 65 years of age or older with diabetes: D132. J Am Geriatr Soc 2005;53.
- Huang P, Huang JM, Li GR. The effect observations of the intensified education on diabetic knowledge for the prevention of diabetic foot. Modern Preventive Medicine 2009;15.
- Kruger S, Guthrie D. Foot care: knowledge retention and self-care practices. Diabetes Educ 1992;18:487-90. https://doi.org/10.1177/014572179201800606.
- Mazzuca SA, Moorman NH, Wheeler ML. The Diabetes Education Study: a controlled trial of the effects of diabetes patient education. Diabetes Care 1986;9:1-10. https://doi.org/10.2337/diacare.9.1.1.
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002;40:566-75. https://doi.org/10.1053/ajkd.2002.34915.
- Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP, Drury DA, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care 1989;12:384-8. https://doi.org/10.2337/diacare.12.6.384.
- Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am 2003;85:1436-45. https://doi.org/10.2106/00004623-200308000-00003.
- Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, et al. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabetic Med 1998;15:412-17. https://doi.org/10.1002/(SICI)1096-9136(199805)15:5<412::AID-DIA584>3.0.CO;2-1.
- Pieber TR, Holler A, Siebenhofer A, Brunner GA, Semlitsch B, Schattenberg S, et al. Evaluation of a structured teaching and treatment programme for type 2 diabetes in general practice in a rural area of Austria. Diabetic Med 1995;12:349-54. https://doi.org/10.1111/j.1464-5491.1995.tb00491.x.
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304-9. https://doi.org/10.1056/NEJM199307293290502.
- Rettig BA, Shrauger DG, Recker RR, Gallagher TF, Wiltse H. A randomized study of the effects of a home diabetes education program. Diabetes Care 1986;9:173-8. https://doi.org/10.2337/diacare.9.2.173.
- Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, et al. Magnetic Research Group. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil 2003;84:736-46. https://doi.org/10.1016/S0003-9993(03)00106-0.
- Wooldridge J, Moreno L. Evaluation of the costs to medicare of covering therapeutic shoes for diabetic patients. Diabetes Care 1994;17:541-7. https://doi.org/10.2337/diacare.17.6.541.
- Wooldridge J, Bergeron J, Thornton C. Preventing diabetic foot disease: lessons from the medicare therapeutic shoe demonstration. Am J Public Health 1996;86:935-8. https://doi.org/10.2105/AJPH.86.7.935.
- Tennvall RG, Apelqvist J, Eneroth M. The inpatient care of patients with diabetes mellitus and foot ulcers. A validation study of the correspondence between medical records and the Swedish Inpatient Registry with the consequences for cost estimations. J Intern Med 2000;248:397-405. https://doi.org/10.1046/j.1365-2796.2000.00748.x.
- Javitt JC, Aiello LP, Chiang Y, Ferris FL, Canner JK, Greenfield S. Preventive eye care in people with diabetes is cost-saving to the federal government. Implications for health-care reform. Diabetes Care 1994;17:909-17. https://doi.org/10.2337/diacare.17.8.909.
- Brechner RJ, Cowie CC, Howie LJ, Herman WH, Will JC, Harris MI. Ophthalmic examination among adults with diagnosed diabetes mellitus. JAMA 1993;270:1714-18. https://doi.org/10.1001/jama.1993.03510140074032.
- The Diabetes Control and Complications Trial Research Group . Resource utilization and costs of care in the diabetes control and complications trial. Diabetes Care 1995;18:1468-78. https://doi.org/10.2337/diacare.18.11.1468.
- Eckman MH, Greenfield S, Mackey WC, Wong JB, Kaplan S, Sullivan L, et al. Foot infections in diabetic patients. Decision and cost-effectiveness analyses. JAMA 1995;273:712-20. https://doi.org/10.1001/jama.1995.03520330042035.
- Apelqvist J, Ragnarson-Tennvall G, Persson U, Larsson J. Diabetic foot ulcers in a multidisciplinary setting. An economic analysis of primary healing and healing with amputation. J Intern Med 1994;235:463-71. https://doi.org/10.1111/j.1365-2796.1994.tb01104.x.
- Apelqvist J, Ragnarson-Tennvall G, Larsson J, Persson U. Long-term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int 1995;16:388-94. https://doi.org/10.1177/107110079501600702.
- UK Prospective Diabetes Study Group . Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care 1999;22:1125-36. https://doi.org/10.2337/diacare.22.7.1125.
- Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006;49:2819-23. https://doi.org/10.1007/s00125-006-0468-0.
- Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med 1998;15:508-14. https://doi.org/10.1002/(SICI)1096-9136(199806)15:6<508::AID-DIA613>3.0.CO;2-L.
- Barshes NR, Chambers JD, Cohen J, Belkin M. Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg 2012;56:1015-24.e1. https://doi.org/10.1016/j.jvs.2012.02.069.
Appendix 1 Study Steering Committee members
| Name | Designation |
|---|---|
| Dr Sara Twaddle (chairperson) | Director, Healthcare Improvement Scotland |
| Dr Beth Woods | Health Economist, University of York |
| Mr Bill Morrison | Public partner |
| Dr Farina Hashmi | Lecturer in Podiatry, University of Salford |
| Dr Fay Crawford | Senior Research Adviser, Research and Development, NHS Fife |
| Dr Francesca Chappell | Medical Statistician, University of Edinburgh |
| Ms Genevieve Cezard | PhD student in statistics and epidemiology |
| Dr Hannah Robertson | Consultant Diabetologist, Aberdeen Royal Infirmary |
| Dr Heather McIntosh | Senior Health Services Researcher, Healthcare Improvement Scotland |
| Professor Jim Lewsey | Professor, Health Economics and Health Technology Assessment, University of Glasgow |
| Dr Purva Abhyankar | Lecturer in Health Sciences, University of Stirling |
| Professor Tom Fahey | Professor, General Practice, Royal College of Surgeons in Ireland |
Appendix 2 Protocol changes (Scotland A – Research Ethics Committee)
Substantial amendments
Objective II: the optimal monitoring interval
We will also investigate how frequently patients should be monitored. Three of the PODUS data sets include time-to-ulceration, monofilament and pulses data. We can use these data to provide estimates for the health economic model regarding optimal frequency of modelling.
Substantial amendment 1 (in italics and specific request underlined)
We suspect that the very small proportion of ulcers found in the Crawford dataset (n = 23) was due to the short length of follow-up, which was only 1 year, whereas in the other datasets most ulcers develop more than 1 year post baseline tests. We are therefore planning to follow up this cohort of patients via their hand held podiatry records to increase the length of follow-up in the Crawford (10) dataset and estimate that another 100 patients with ulcer will be identified.
Our favourable opinion from Scotland A REC (reference 16/SS/0213) approved the follow up of people with diabetes who gave consent to their participation in the cohort study by Crawford (2011) using the routinely collected data on SCI Diabetes and hand-held podiatry notes. The REC A specified that the project researchers must first of all check the survival of those who gave consent in 2006/2007 in order to ensure that no deceased patients relatives were contacted about the follow up study. Only then could the surviving participants be contacted to obtain consent to follow up. After confirmation from the podiatry department that patients hand held records are archived for at least 7 years after death we now seek approval to collected foot-related follow up data from the podiatry notes of those participants who have deceased since being recruited to the original cohort study. This amendment to our protocol has been recommended by our Study Steering Committee on 15th March 2017 and is justified as a result of the known association between foot ulceration and death in those with diabetes. However, we have now had confirmation by the NHS Tayside MCN data facilitator that 45% of the original cohort of patients has died in the intervening period and this will greatly reduce the statistical power of our analysis. We now seek approval to collect foot ulcer data for those study patients who have deceased.
Substantial amendment 2 (in italics and specific request underlined)
In order to strengthen our analysis of the optimal risk assessment (monitoring) frequency we wish to obtain all NHS Fife patients’ foot screening data from SCI Diabetes over a 10 year period. These data will be anonymised by staff at the Health Informatics Centre at the University of Dundee (where NHS Fife routinely collected data is stored). We have discussed the need for Caldicott approval [Public Benefit Privacy Panel (PBPP)] with the NHS Fife Information Governance Advisor and attach her response to our enquiry for PBPP approval.
We can use these data to provide hazard ratios, estimates of the rates of ulceration, and estimates of sensitivity and specificity of the CPR and other risk assessment tools (e.g. NICE and SIGN guidelines) calculated for different time periods as required by the health economic model. The NHS Fife SCI Diabetes routinely collected data will be used by the project statisticians and health economists to calculate the transitional probability of a patient transitioning from one risk category to another and to a state of foot ulceration. We have been advised by NHS Fife data manager the numbers of people with diabetes who have received foot risk assessments are as follows:
-
2007 n = 10,405
-
2008 n = 10,829
-
2009 n = 12,413
-
2010 n = 10,949
-
2011 n = 12,635
-
2012 n = 13,003
-
2013 n = 13,832
-
2014 n = 14,496
-
2015 n = 13,549
-
2016 n = 4249
The identification of the optimal screening interval base case will require an estimate of the potential effectiveness and cost-effectiveness of the clinical risk score calculated during patients’ annual reviews based on the PODUS time-to-event data and the NHS Fife SCI-Diabetes data. This corresponds to the intent to develop a simple-to-use screen that can be used during routine assessments for people with diabetes mellitus. We will then estimate the rate at which the risk score varies and will try to estimate its effectiveness when applied more frequently at 6-monthly intervals and less frequently at biennial intervals using a hidden Markov chain analysis.
Approval granted 17 October 2017 (REC reference: 16/SS/0213/AM01).
Substantial amendment 2 (in italics and specific request underlined)
In accordance with our favourable opinion from Scotland A REC we have now contacted 649 participants by post and 243 gave written consent for the researchers to access their health records to obtain follow up data relating to foot ulcerations. We now seek another substantial amendment to send out a second and third invitation to participate in the follow up study for those who have not responded.
Approval granted 23 July 2018 (REC reference: 16/SS/0213/AM02).
Objective III: an overview of the evidence of the effectiveness of simple and complex interventions (structured care) to prevent foot ulceration in people with diabetes mellitus
Substantial amendment 3 (in italics and specific request underlined)
We also intend to conduct a search of electronic databases for individual randomised controlled trials which meet the following eligibility criteria.
Participants and target condition
People of any age with a diagnosis of diabetes either type 1 or type 2.
Types of interventions
Simple interventions such as insoles or bespoke footwear, education packages tailored for patients or health care professionals or complex interventions such as care from a specialist multidisciplinary team, used alone or in combination will be considered for inclusion in the review.
Types of comparisons
We will include simple or complex interventions used alone or in combination and standard care comparators.
Types of outcomes
A foot ulcer has been defined as a full thickness skin defect that requires more than 14 days to heal.
Data collection
Two reviewers will screen review titles and abstracts to identify potentially relevant literature. They will then screen the full text of reviews and RCTs deemed to be potentially relevant. Disagreement will be resolved by discussion with a third author. Data will be extracted into a review-specific data extraction tool by a lead reviewer and checked by a second who will be unaware of the findings of the lead reviewer. A second data extraction sheet and quality assessment tool will be created to capture data from RCTs identified by our search for primary studies and an assessment of bias will be performed using the following items:
-
Adequate sequence generation? (Yes/no.)
-
Allocation concealment? (Yes/no.)
-
Were participants and outcome assessors blind to the allocation? (Yes/no.)
-
Were incomplete outcome data adequately assessed? (Yes/no.)
-
Are reports of the study free of selective outcome reporting? (Yes/no.)
-
Free of any other bias? (Yes/describe the risk of bias.)
Amendment acknowledged by the CSO [Chief Scientific Officer] in feedback dated 30 January to the interim report of 1 January 2018.
Appendix 3 Clinical prediction rule
PODUS 1
PODUS 2015 was a NIHR HTA-funded project that aimed to identify the predictors of foot ulcer in people with diabetes mellitus and to develop and validate a predictive model. This was published as a NIHR monograph in the Health Technology Assessment journal. 15 Details of the methodology and results of that analysis that pertain to PODUS 2020 (a CPR based on the predictive model) are given here, with emphasis on the predictor selection process for PODUS 2015 and the consequent methodological choices made for PODUS 2020.
In PODUS 2015, we obtained IPD from eight studies. 20–27 Another data set (i.e. Leese et al. 28) was available via a Safe Haven facility. A 10th data set (i.e. Boyko et al. 29) could not be used directly by the PODUS 2015 researchers, although we could request the results of the analyses of this data set, and so we used it as an externally held validation data set. These 10 studies were identified through a SR encompassing the stages of literature searching and critical appraisal using a bespoke tool, as at the time no published critical appraisal tool for prognostic studies was available. 15
In PODUS 2015, we had a large number of potential predictors. We knew that using data-driven methods to select predictors can easily result in spurious findings that cannot be replicated in further studies. There is a notable body of research advising against such practices. 45,145 This ruled out methods such as stepwise selection of predictors, or selecting predictors with small p-values in univariate analyses for inclusion in multivariable models. We therefore needed our own criteria on which to base our choice of predictors.
To maximise the number of data we could use, we used only predictors that had been collected in at least three of the PODUS data sets. To aid interpretation, we required that predictors had been consistently defined across studies, or could be recoded as such. We also required that the extent of clinical heterogeneity did not rule out meta-analysis. Twenty-two predictors met the first two criteria at a first pass through the data sets: age, sex, body mass index, smoking, height, weight, alcohol intake, HbA1c level, insulin regime, duration of diabetes mellitus, eye problems, kidney problems, insensitivity to monofilament, absence of pedal pulses, tuning fork, biothesiometer, ankle reflexes, ABI, peak plantar pressure, prior ulcer, prior amputation and foot deformity.
The above predictors were presented as forest plots to the whole PODUS 2015 group, which comprised methodologists and clinicians. Many predictors were dropped at this stage. For example, kidney problems was removed from the primary analysis as in some studies it had been defined as nephropathy and in others we had used estimated glomerular filtration rate as a proxy, and this may or may not have been adequate. 146 We were also in favour of having fewer rather than more predictors so that we could use as many data as possible. In general, there was an inverse relationship between the number of predictors we chose and the number of studies we could include; if we added a predictor to the model, but some of the studies did not include that predictor, we had to remove those studies from the analysis. We were aware of emerging research on the handling of studies with predictors entirely missing in meta-analysis of IPD, but the methodology was new and is still largely untested. 147 Only four predictors had been collected in all 10 studies, namely age, sex, duration of diabetes mellitus and prior ulcer.
Six variables were chosen for inclusion in the primary model in PODUS 2015: age, sex, duration of diabetes mellitus, insensitivity to a 10-g monofilament, absent pedal pulses, and history of ulcer or amputation. These variables were chosen for clinical plausibility, availability in the data sets and consistency of definition. Another consideration was ease of collection, with little burden to either the patient or the podiatrist. Selection was not based on statistical significance; for example, age was retained despite not achieving statistical significance in four studies and achieving only borderline significance in another two studies when looking at forest plots of univariate ORs.
For PODUS 2015, we conducted a two-step meta-analysis, which meant fitting a logistic regression model in each data set with the six predictors and using the ORs from each study in a random-effects meta-analysis. We used a two-step method so that we could include, in the second stage, aggregate data (i.e. log-odds ratios and their variances) derived from the Leese et al. 28 data set with over 3000 patients. The Leese et al. 28 data set was housed on a different server and so could not be used in a one-step meta-analysis, although this is the preference of some methodologists. 33
Predictor replication in PODUS 2015
The Boyko et al. 29 data set was not supplied to the PODUS 2015 team, but a SAS® software program (SAS Institute Inc., Cary, NC, USA) was sent to the Boyko et al. 29 team to be run in the data, and the analysis results were sent back. The SAS program refitted the logistic regression model using the same six predictors. This method meant that the Boyko et al. 29 team were kept blind to the results of the PODUS 2015 analyses and the Boyko et al. 29 analysis was completely prespecified, and the predictors were tested in a new data set.
Predictors were considered both predictive and replicated if the Boyko et al. 29 ORs achieved statistical significance and had the same direction as the PODUS 2015 ORs and if the CIs of the PODUS 2015 and Boyko et al. 29 ORs overlapped.
We compared the summary ORs from the meta-analysis of estimates from multivariable logistic regression using the data sets held by the PODUS 2015 researchers with the results of a multivariate logistic regression conducted in the external Boyko et al. 29 data set. The Boyko et al. 29 analysis did not replicate the results of the PODUS 2015 team for three predictors: age, sex and duration of diabetes mellitus. The effect of age was small and not significant in both the PODUS 2015 and the Boyko et al. 29 data sets, and the estimates had different directions (increasing age in PODUS 2015, but decreasing age in Boyko et al. 29) and were associated with increased ulcer risk (PODUS 2015: OR 1.005, 95% CI 0.994 to 1.016; Boyko et al. :29 OR 0.993, 95% CI 0.977 to 1.009). In addition, age was not a statistically significant predictor of ulceration in six of the nine studies held by the PODUS team. The effect of duration of diabetes mellitus was also not replicated, being predictive in the PODUS analyses and protective in the Boyko et al. 29 analyses (PODUS 2015: OR 1.024, 95% CI 1.011 to 1.036; Boyko et al. :29 OR 0.981, 95% CI 0.968 to 0.994). However, it should be noted that the recorded duration of diabetes mellitus is simply the known duration, and people may have had diabetes mellitus for years before a diagnosis was made; in addition, the health-care systems in the Boyko et al. 29 and other PODUS data sets differed. The Boyko et al. 29 data set was not suitable for testing sex as a predictor, as the study recruited veterans and 98% of these veterans were male, which is not reflective of the wider population of people with diabetes mellitus. 15 All ORs taken from the Boyko et al. 29 data set and all ORs taken from the PODUS 2015 data sets were adjusted for the predictors specified for the primary PODUS 2015 model.
The Boyko et al. 29 analyses did replicate the PODUS 2015 results for three predictors: monofilament sensitivity (PODUS 2015: OR 3.184, 95% CI 2.654 to 3.820; Boyko et al. :29 OR 3.489, 95% CI 2.486 to 4.896), pulses (PODUS 2015: OR 1.968, 95% CI 1.624 to 2.386; Boyko et al. :29 OR 2.557, 95% CI 1.220 to 5.361) and history (PODUS 2015: OR 6.589, 95% CI 2.488 to 17.45; Boyko et al. :29 OR 2.979, 95% CI 2.146 to 4.135). 15
Therefore, at the end of the PODUS 2015 project, we knew that we had evidence of an association between foot ulcer outcome and three binary variables in the internal data sets, and that the results of these three binary variables were similar in external data.
Crawford et al.21 follow-up study
Comparison of the three-predictor model (monofilament sensitivity, pulses, history) with the six-predictor PODUS 2015 primary model (monofilament sensitivity, pulses, history, sex, age and duration of diabetes mellitus)
This appendix compares the PODUS 2015 six-predictor model with the PODUS 2020 three-predictor model by providing estimates for each study used in the development phase. We also investigated non-linearity in the two continuous variables from the PODUS 2015 model, namely age and known duration of diabetes mellitus.
Comparison of the three-predictor with the six-predictor PODUS model
Table 32 shows, for each study, the baseline risk of ulcer at 2 years predicted by the two models in the case of a hypothetical 65-year-old female patient who has tested negative for monofilament sensitivity, pulses and history and who was diagnosed with diabetes mellitus 1 month previously. The three-predictor model comprises monofilaments, pulses and history, whereas the six-predictor model also includes age, sex and duration of diabetes mellitus.
| First author and year of publication | Number of predictors | Baseline risk (%) | 95% CI |
|---|---|---|---|
| Abbott 200220 | 3 | 1.7 | 1.4 to 2.1 |
| Abbott 200220 | 6 | 1.3 | 1.0 to 1.7 |
| Crawford 201121 | 3 | 0.5 | 0.2 to 1.2 |
| Crawford 201121 | 6 | 0.3 | 0.1 to 0.9 |
| Monteiro-Soares 201024 | 3 | 5.9 | 3.3 to 10.3 |
| Monteiro-Soares 201024 | 6 | 4.7 | 2.0 to 10.5 |
| Pham 200025 | 3 | 9.6 | 4.2 to 20.5 |
| Pham 200025 | 6 | 5.1 | 1.8 to 13.7 |
Figure 34 shows the results for insensitivity to a 10-g monofilament and risk of developing an ulcer by 2 years, with the model fitted separately to each study. Estimates in the three-predictor group have been adjusted for pulses and history and estimates in the six-predictor group have been adjusted for pulses, history, sex, age and known duration of diabetes mellitus.
FIGURE 34.
Results for insensitivity to a 10-g monofilament.

Figure 35 shows the results for absence of any pedal pulse and risk of developing an ulcer by 2 years, with the model fitted separately to each study. Estimates in the three-predictor group has been adjusted for monofilaments and history and estimates in the six-predictor group have been adjusted for monofilaments, history, sex, age and known duration of diabetes mellitus.
FIGURE 35.
Results for absence of any pedal pulse.

Figure 36 shows the results for history of ulcer or amputation and association with ulcer by 2 years, with model fitted separately to each study. Estimates in the three-predictor group have been adjusted for monofilaments and pulses; estimates in the six-predictor group have been adjusted for monofilaments, pulses, sex, age and known duration of diabetes mellitus.
FIGURE 36.
Results for history of ulcer or amputation.

Figures 34–36 suggest that the estimates of risk associated with positive results for monofilament sensitivity, pulses and history vary little between the three-predictor and six-predictor model in each study. There is, perhaps, greater variation in the estimate of baseline risk (the risk of ulcer at 2 years when all predictors are test negative, sex is female, duration of diabetes mellitus is ≤ 1 month and age is 65 years); however, all estimates are comparable.
Figure 37 shows the forest plot for age (per year increase) and association with ulcer by 2 years, with the model fitted separately to each study. Estimates have been adjusted for monofilament sensitivity, pulses, history of ulcer or amputation, sex and known duration of diabetes mellitus.
FIGURE 37.
Forest plot for age (per year increase).
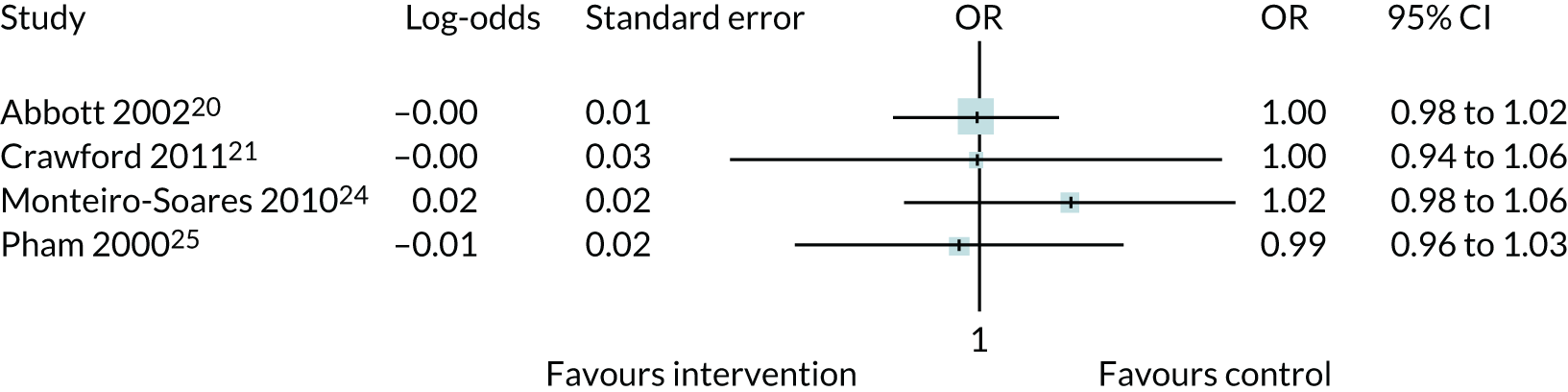
Figure 38 shows the forest plot for sex and association with ulcer by 2 years, with the model fitted separately to each study. Estimates have been adjusted for monofilament sensitivity, pulses, history of ulcer or amputation, age and known duration of diabetes mellitus.
FIGURE 38.
Forest plot for sex.
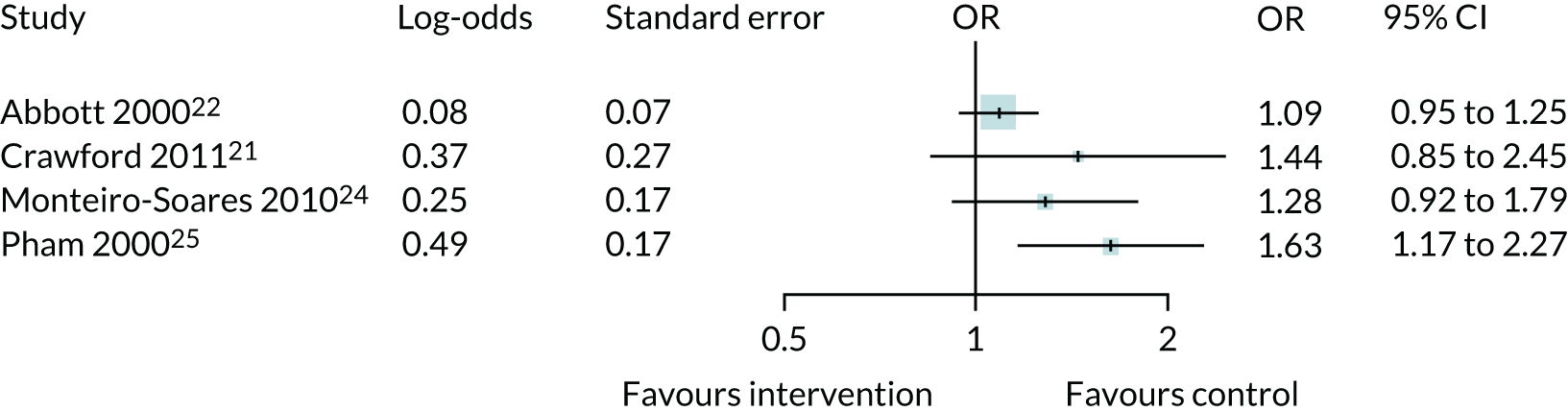
Figure 39 shows the forest plot for known duration of diabetes mellitus (per year increase) and association with ulcer by 2 years, with the model fitted separately to each study. Estimates have been adjusted for monofilament sensitivity, pulses, history of ulcer or amputation, age and sex.
FIGURE 39.
Forest plot for known duration of diabetes mellitus (per year increase).

Investigation of non-linearity
Non-linearity was investigated by using the Box–Tidwell method. 148 In brief, this method involves calculating a new variable from the variable thought to have a possibly non-linear effect. If this variable is x, the new variable is x × log(x), where the log is the natural logarithm. Both the new and the original variables are then included in the logistic regression model, and, if the new variable is statistically significant, then there is evidence of a curvilinear effect. Our two candidate predictors for curvilinear effects are age and duration of diabetes mellitus. Two models were fitted with all six predictors from the PODUS 2015 model (monofilament sensitivity, pulses, history, sex, age and duration of diabetes mellitus) and study as a fixed effect; one model had the Box–Tidwell age-transformed variable, and the other had the Box–Tidwell known duration of diabetes-transformed variable.
Our tentative conclusion from Tables 33 and 34 and forest plots and the results given Appendix 3 PODUS 2015 is that there is no strong evidence that age is a predictor of ulcer at 2 years. The evidence of an association with ulcer at 2 years is stronger for duration of diabetes mellitus, but, given the result in the Boyko et al. 29 study, that longer duration was protective against ulcer, this result may not be generalisable to different settings.
| Variable | OR | 95% CI |
|---|---|---|
| Age | 1.21 | 0.89 to 1.65 |
| Box–Tidwell age | 0.96 | 0.91 to 1.02 |
| Variable | OR | 95% CI |
|---|---|---|
| Duration of diabetes mellitus | 1.09 | 0.10 to 1.20 |
| Box–Tidwell duration | 0.98 | 0.96 to 1.01 |
Meta-analyses using three or six predictors
We also present results of meta-analyses using the method described in Chapter 3, where study is fitted as a fixed effect in a logistic regression, using the four development data sets and either three or six predictors (Tables 35 and 36).
| Predictor | OR | 95% CI |
|---|---|---|
| Abbott 200220 | 0.019 | 0.016 to 0.023 |
| Crawford 201121 | 0.007 | 0.004 to 0.011 |
| Monteiro-Soares 201024 | 0.023 | 0.015 to 0.034 |
| Pham 200025 | 0.029 | 0.019 to 0.043 |
| Monofilaments | 3.00 | 2.39 to 3.77 |
| Pulses | 2.01 | 1.62 to 2.51 |
| History | 7.02 | 5.39 to 9.14 |
| Predictor | OR | 95% CI |
|---|---|---|
| Abbott 200220 | 0.013 | 0.010 to 0.017 |
| Crawford 201121 | 0.005 | 0.003 to 0.008 |
| Monteiro-Soares 201024 | 0.015 | 0.010 to 0.024 |
| Pham 200025 | 0.021 | 0.013 to 0.033 |
| Monofilaments | 2.78 | 2.20 to 3.51 |
| Pulses | 1.98 | 1.58 to 2.48 |
| History | 6.23 | 4.76 to 8.16 |
| Sex | 1.43 | 1.15 to 1.79 |
| Age | 1.00 | 0.99 to 1.01 |
| Known duration of diabetes mellitus | 1.02 | 1.01 to 1.03 |
The three-predictor plus study meta-analysis had a c-statistic of 0.813 (95% CI 0.790 to 0.835). The six-predictor plus study meta-analysis had a c-statistic of 0.820 (95% CI 0.797 to 0.842). The performance of the six-predictor model is slightly better, but ease of use of the three-predictor model in a CPR is much greater. The results of the three- versus six-predictor analyses (see Figures 40–42) do not show a huge advantage of the six-predictor model; the discrimination and calibration results are similar.
FIGURE 40.
Calibration plot for the three-predictor plus study meta-analysis.

FIGURE 41.
Calibration plot for the six-predictor plus study meta-analysis.

FIGURE 42.
Discrimination plot for the three-predictor plus study meta-analysis vs. the six-predictor plus study meta-analysis.

Clinical prediction rule development: study estimates in the three-predictor and clinical prediction rule logistic regression models
The development of the CPR involved fitting two logistic regression models with study fitted as a fixed effect. The first logistic regression used monofilament sensitivity, pulses and history as predictors; the second used CPR score as a predictor. We conducted random-effects meta-analyses of the study estimates using the three studies with follow-up at 2 years to obtain an overall estimate of baseline risk. The Crawford study21 was not used as its follow-up period was 1 year. We tried to obtain longer-term follow-up (see Figure 33).
As the CPR score model is a simplified version of the three-predictor model, it was important to check that the study estimates did not vary much between the two models. Changes in study estimates between the CPR score model and the three-predictor model would suggest that the CPR score was not an adequate proxy for monofilament sensitivity, pulses and history; however, Figure 43 suggests that the study estimates are similar.
FIGURE 43.
Study estimates from the three-predictor logistic regression and the CPR score logistic regression.

Figure 44 suggests that the study estimates are similar; the corresponding ORs are identical to two decimal places. Meta-analysis of the Abbott et al. ,20 Monteiro-Soares et al. 24 and Pham et al. 25 estimates from the three-predictor model gives a summary estimate of –3.81 (95% CI –4.04 to –3.58) on the log-odds scale. The corresponding estimate from the CPR score model is –3.74 (95% CI –4.10 to –3.38) on the log-odds scale. These estimates give ORs of 0.022 and 0.024, respectively.
FIGURE 44.
Random-effects meta-analysis of the baseline risk in the three studies with 2-years’ follow-up.

Survival analysis
The CPR described in Chapter 3 is based on a logistic regression model. Logistic regression models can be used to model the probability of a binary or a categorical outcome. Survival analysis is a branch of statistics that can be used to model the time to a binary outcome. Both binary logistic regression and survival analysis require data on the occurrence or not of an event. Survival analysis also requires the time until the event occurred or the time until end of follow-up for each patient in whom the event did not occur.
If a data set has time-to-event data, it is generally preferable to use survival analysis. It is a more sophisticated model, uses more of the data and has greater statistical power. However, we used logistic regression for the CPR, as the largest data set,20 constituting 75% of the CPR development data with > 6000 patients, included outcome at 2 years but not when during that 2-year period the outcome had occurred, ruling out survival analysis. The other three development studies21,24,25 did include time-to-event data.
To allow comparison with the logistic regression model underlying the CPR, in particular the regression coefficients for the three predictors used in the development of the CPR, we present survival analyses here. We do not emphasise any differences in baseline risk estimates between the CPR logistic regression model and the survival models because the survival analyses did not include the Abbott et al. 20 data set. The Abbott et al. 20 data set was derived from a community-based low-risk patient sample. The Crawford data set, with > 1000 patients, came from a similar setting, but the Monteiro-Soares and Pham et al. 25 data sets came from relatively high-risk secondary care settings. We therefore would not expect the baseline risk estimates to be similar, given that the proportion of low-risk patients dropped from 92% in the CPR logistic regression analyses to 66% in the survival analyses.
Methods
In the time-to-event analyses undertaken, the outcome was the first foot ulcer that occurred during follow-up. Follow-up is defined as the time from the date of recruitment into a study until the date of the first foot ulcer. If no foot ulcer occurs, then follow-up is censored at the date of the last appointment or the end date of the study, whichever occurs first, or at the date of death of the patient.
Hazard ratios for the predictors were derived using two different statistical techniques for time-to-event analysis. Royston–Parmar flexible parametric survival analysis methods were used for the main analyses, because this statistical technique includes the baseline hazard and baseline cumulative hazard functions in the progression to ulceration during follow-up. 148,149 The more common Cox proportional hazards regression was used in sensitivity analyses; this modelling technique does not make any assumption about the shape of the underlying hazard function and, thus, leaves the baseline hazard rate unspecified. The proportional hazards assumption was checked for each predictor by using statistical tests and graphically examining log-minus-log plots and scaled Schoenfeld residuals on functions of time.
Baseline hazard functions were created for each study using splines with differing numbers of knots, and their shapes were examined. The three predictors (i.e. insensitivity to a 10-g monofilament, one or more absent pedal pulses, and history of ulceration or LEA) were included in the flexible parametric survival model, together with two dummy variables for each of two of the three studies, to take account of the different study populations. Similar models were developed with one, two and three internal knots to ascertain which model best fitted the data. The model with the lower AIC and lower log-likelihood value was preferred, unless the two AIC and log-likelihood values were very close in size, in which case the simpler model (with fewer degrees of freedom) was selected. Our final decision was to use the simplest one-knot model (Figure 45). For the Cox regression models, the three predictors were included first in a stratified model, to account for the differences between the studies, and then in a model that was adjusted for study. In each model, the Monteiro-Soares study was taken as the reference study, as the follow-up in this study was longer than those in the Crawford and Pham et al. 25 studies.
FIGURE 45.
Baseline hazard function for three studies with one internal knot (k = 1). The dashed lines represent the upper and lower confidence intervals for each of the baseline hazard functions for the individual studies.
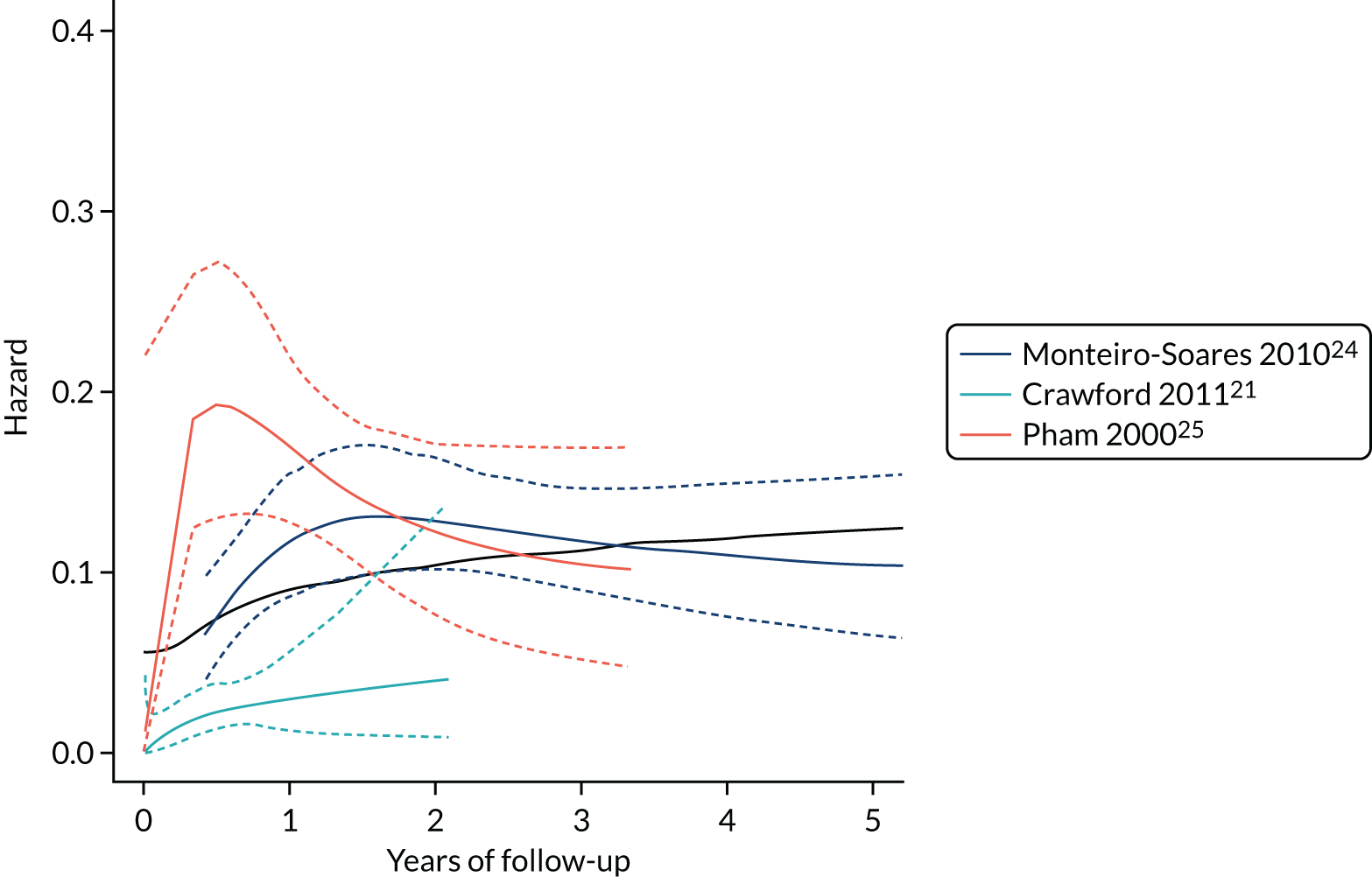
All analyses were performed with IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) (URL: www.ibm.com/products/spss-statistics) and RStudio version 1.0.143 (RStudio Inc., Boston, MA, USA) (URL: www.rstudio.com/), which is an integrated development environment for R (The R Foundation for Statistical Computing, Vienna, Austria) (URL: https://cran.r-project.org/).
Results
For basic demographic and predictor descriptive statistics for each study, see Tables 2–9 and Figures 1–4.
As expected, Figure 43 shows that the baseline hazard for the Crawford data set is lower than that found in either the Monteiro-Soares or the Pham et al. 25 data set. Univariate results for each of the predictors are shown in Figures 46–48.
FIGURE 46.
Kaplan–Meier plot for progression to foot ulceration, comparing adults who are sensitive with adults who are insensitive to a 10-g monofilament.
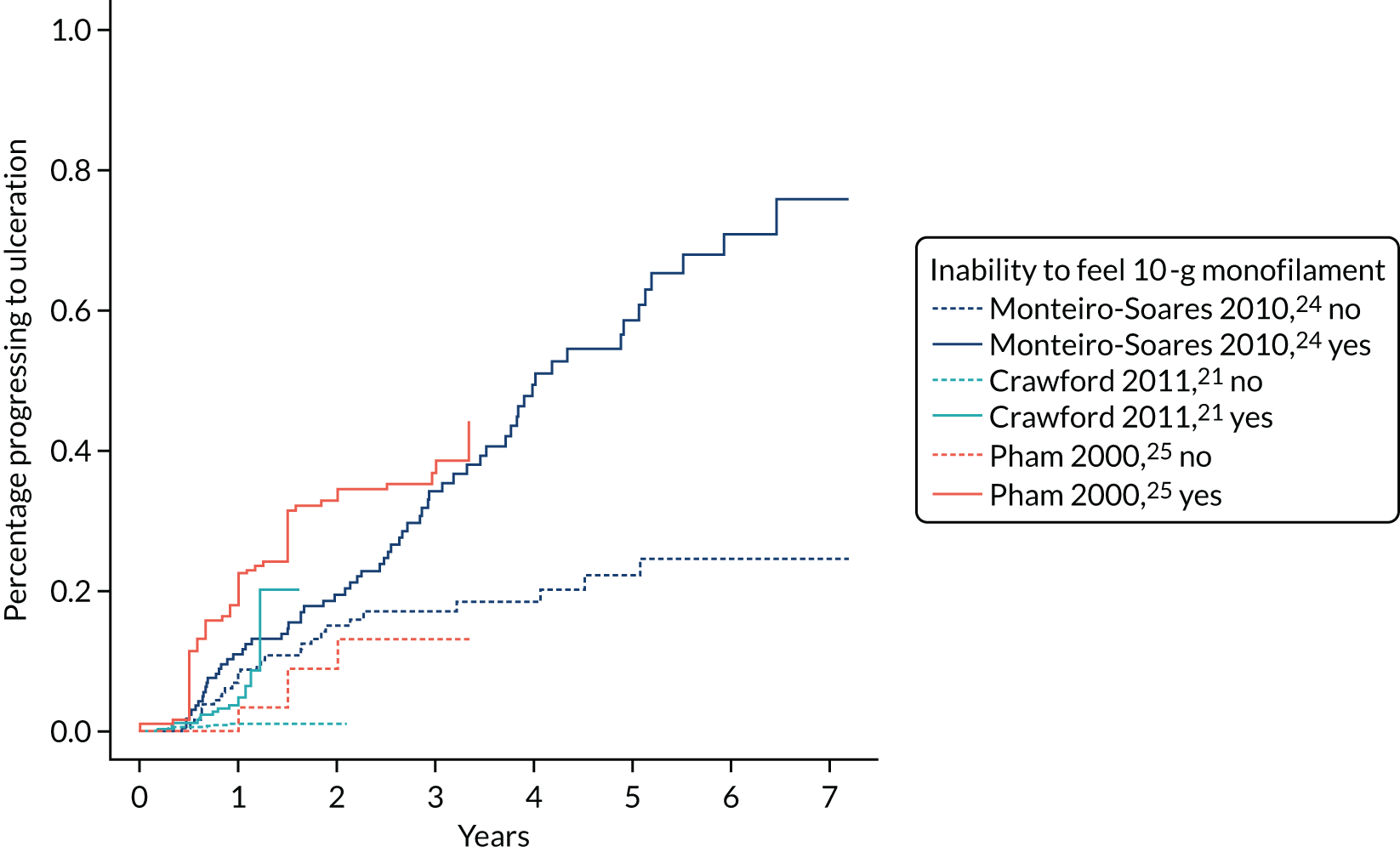
FIGURE 47.
Kaplan–Meier plot for progression to foot ulceration, comparing adults with at least one absent pedal pulse with adults with no absent pedal pulses.
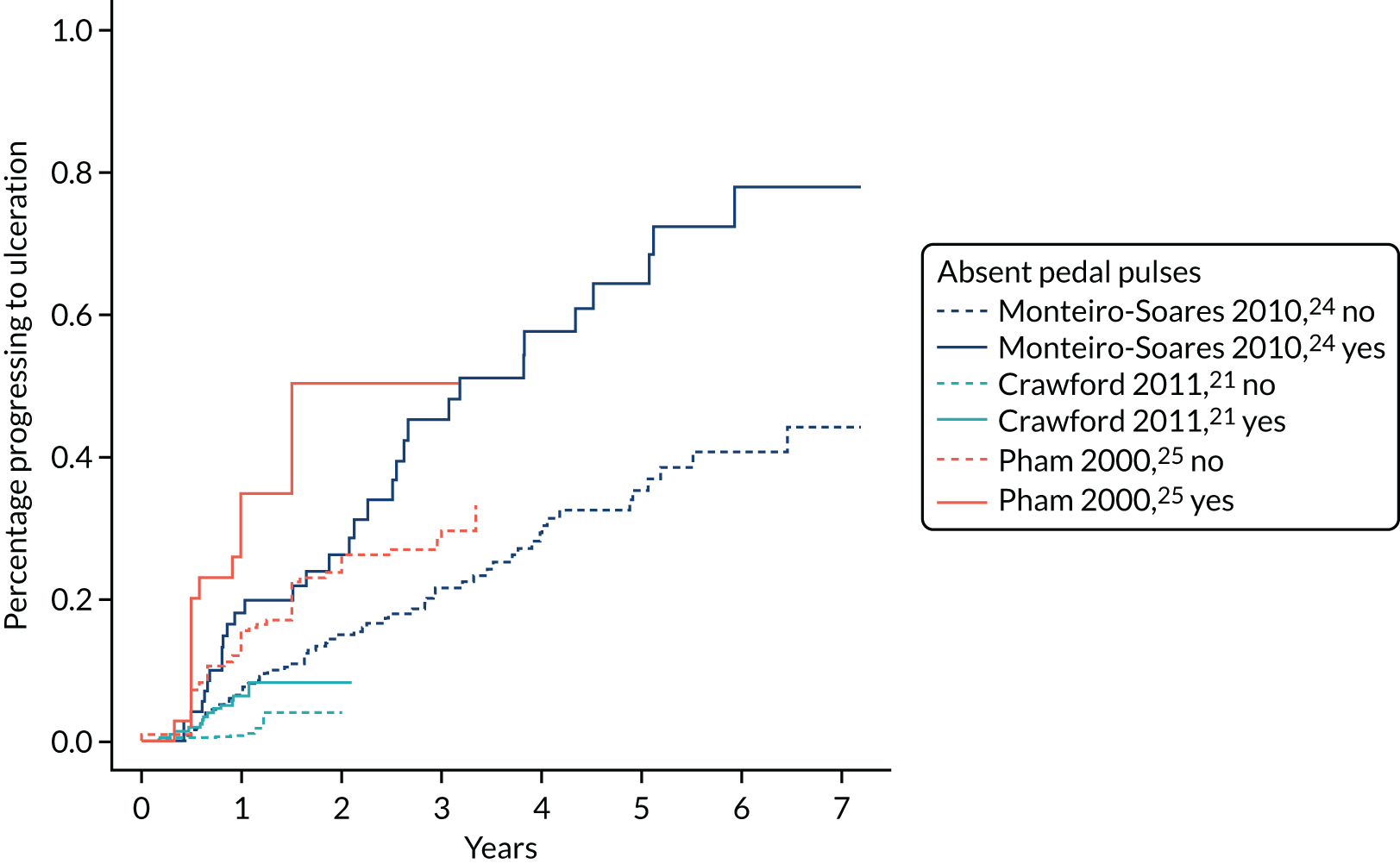
FIGURE 48.
Kaplan–Meier plot for progression to foot ulceration, comparing adults who have experienced a prior foot ulceration or LEA with adults who have not.
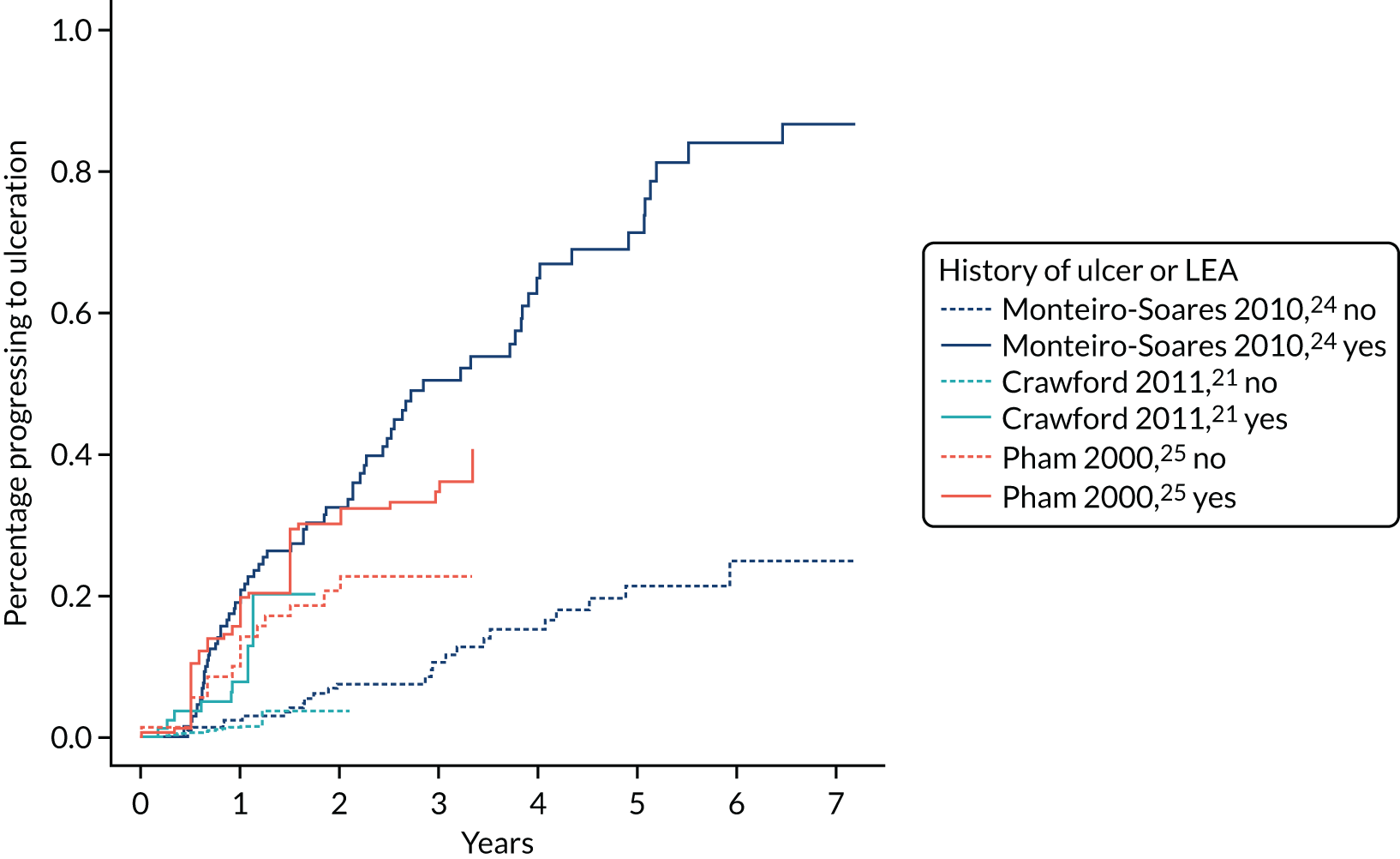
Flexible parametric model
The flexible parametric model comprises the three predictors and two dummy variables (labelled Crawford study and Pham et al. 25 study), and the results are given in Table 37. A pattern broadly similar to that of the CPR logistic regression results is shown, with the coefficient for history approximately twice the size of those for monofilament sensitivity or pulses.
| Predictor | β | Standard error (β) | Hazard ratio = exp(β) | 95% CI |
|---|---|---|---|---|
| Gamma 0 | –1.516 | 0.804 | – | – |
| Gamma 1 | 2.198 | 0.402 | – | – |
| Gamma 2 | 0.047 | 0.019 | – | – |
| Monofilament, insensitive | 0.703 | 0.190 | 2.020 | 1.393 to 2.929 |
| Pulses, absent pedal | 0.677 | 0.162 | 1.967 | 1.431 to 2.704 |
| History of ulcer/LEA | 1.159 | 0.193 | 3.188 | 2.185 to 4.651 |
| Crawford 201121 | –0.813 | 0.273 | 0.444 | 0.260 to 0.758 |
| Pham 200025 | –0.032 | 0.170 | 0.969 | 0.695 to 1.351 |
Cox proportional hazards model
Cox proportional hazards regression is most frequently used to create a time-to-event model, and two analyses were undertaken: one adjusting for the Crawford and Pham et al. 25 studies in a similar manner to the flexible parametric models and the other stratified by ‘study’ (Table 38). In the model in which the predictors were adjusted for study, the results for absent pedal pulses were identical to the results of the model that was stratified by study.
| Predictor | Final model: flexible parametric survival analysis with one internal knot | Final model: Cox regression adjusted for study | Final model: Cox regression stratified by study | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Monofilament | 2.02 | 1.39 to 2.93 | 2.05 | 1.41 to 2.97 | 1.98 | 1.36 to 2.88 |
| Pulses | 1.97 | 1.43 to 2.70 | 1.94 | 1.41 to 2.67 | 1.94 | 1.41 to 2.67 |
| History | 3.19 | 2.18 to 4.65 | 3.23 | 2.21 to 4.71 | 3.30 | 2.26 to 4.81 |
Checking the proportional hazards assumption
It is important to check the proportional hazards assumption when using Cox proportional hazards regression. Log-minus-log plots for the three pooled studies are in Figure 49. The results of additional statistical tests and graphs based on the scaled Schoenfeld residuals are also given in Figure 49.
As the Schoenfeld residuals are independent of time, the plots of residuals should be randomly distributed; if the plots of residuals are not randomly distributed, this would imply a violation of the proportional hazards assumption. In the final column of Table 39, the p-values for all three predictors and the global test are non-significant, which supports the proportional hazards assumption. We concluded from our checks that there is no strong evidence that the proportional hazard assumption has been violated (Table 39 and Figures 49 and 50).
FIGURE 49.
Log-minus-log (LML) plots for the three predictors to check that the proportional hazards assumption holds. (a) Sensitive vs. not sensitive; (b) pulses absent vs. pulses present; and (c) no ulcer vs. previous ulcer.
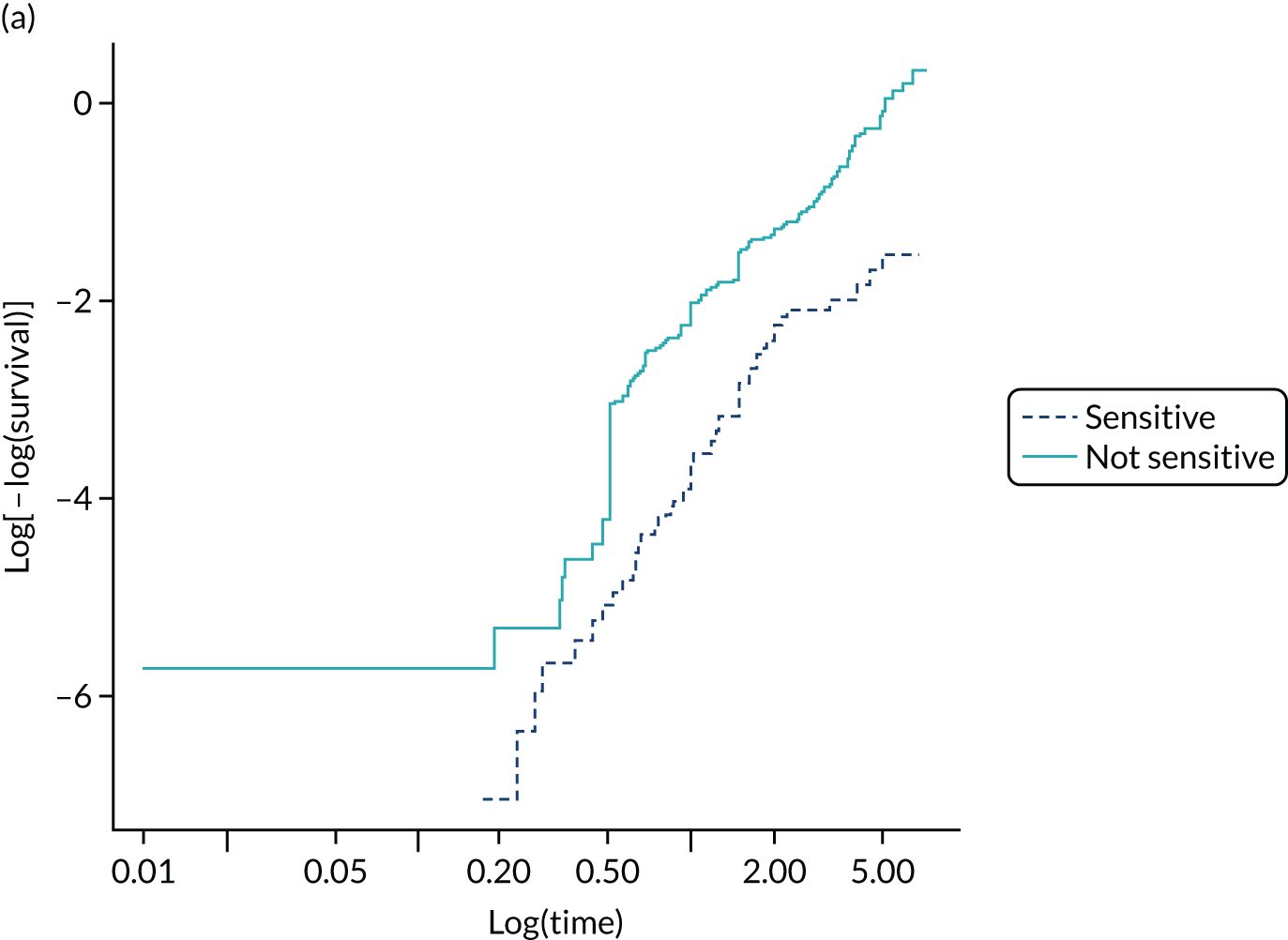

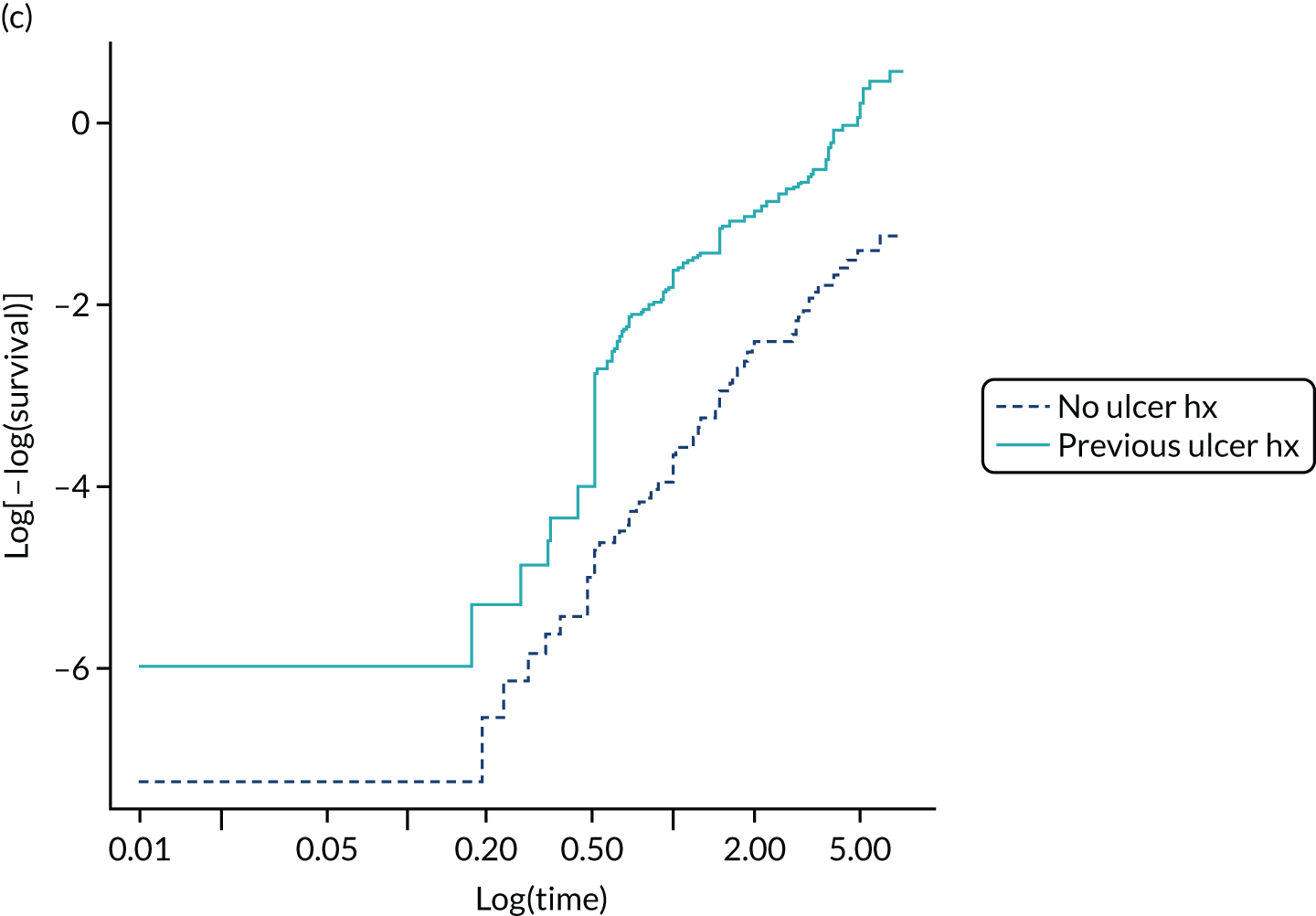
| Predictor | P | χ2 | p-value |
|---|---|---|---|
| Monofilament insensitivity | 0.053 | 0.687 | 0.407 |
| Absent pedal pulses | –0.056 | 0.622 | 0.430 |
| History of ulcer/LEA | –0.066 | 1.087 | 0.297 |
| Global | – | 1.991 | 0.574 |
FIGURE 50.
Plots of the scaled Schoenfeld residuals against transformed time to check that the proportional hazards assumption holds. (a) Schoenfeld individual test: p = 4072; (b) Schoenfeld individual test: p = 0.4304; and (c) Schoenfeld individual test: p = 0.2972. Global Schoenfeld test: p = 0.5743.
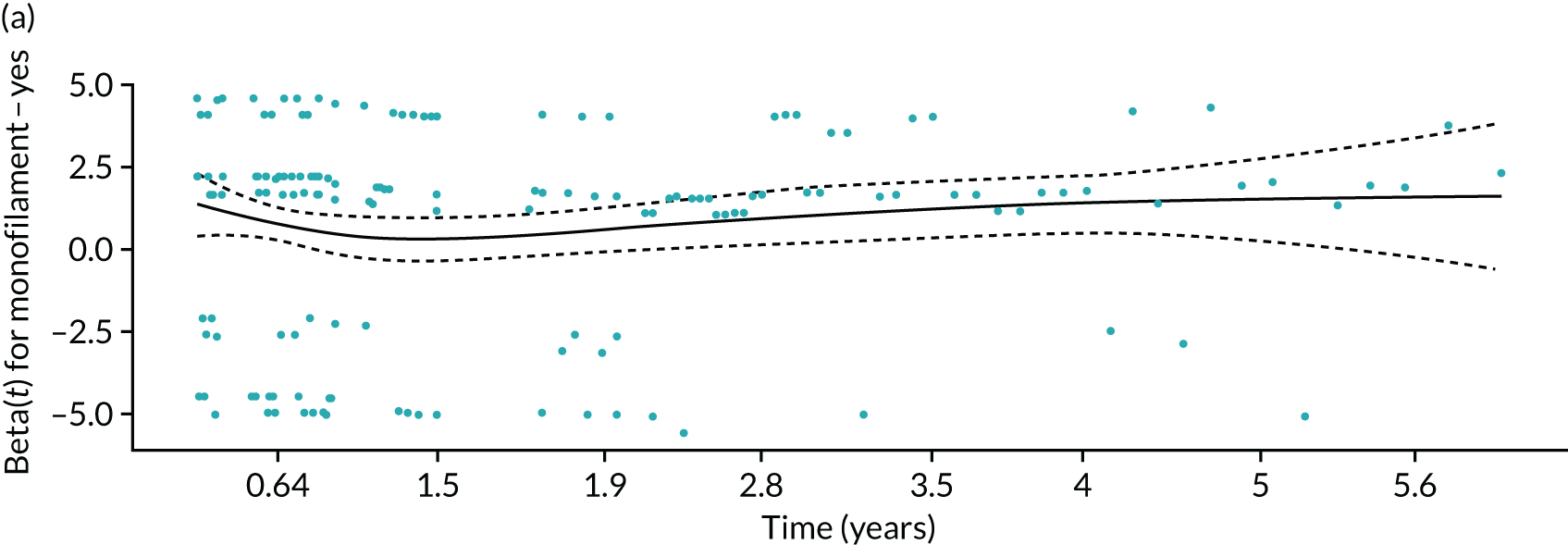
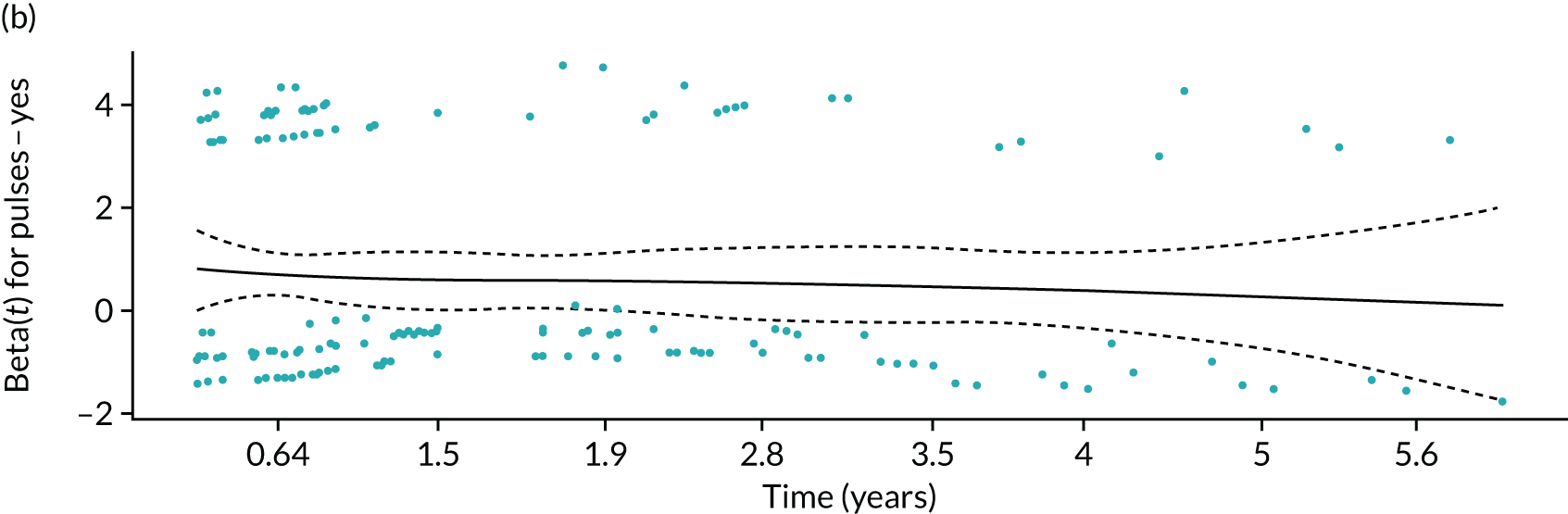
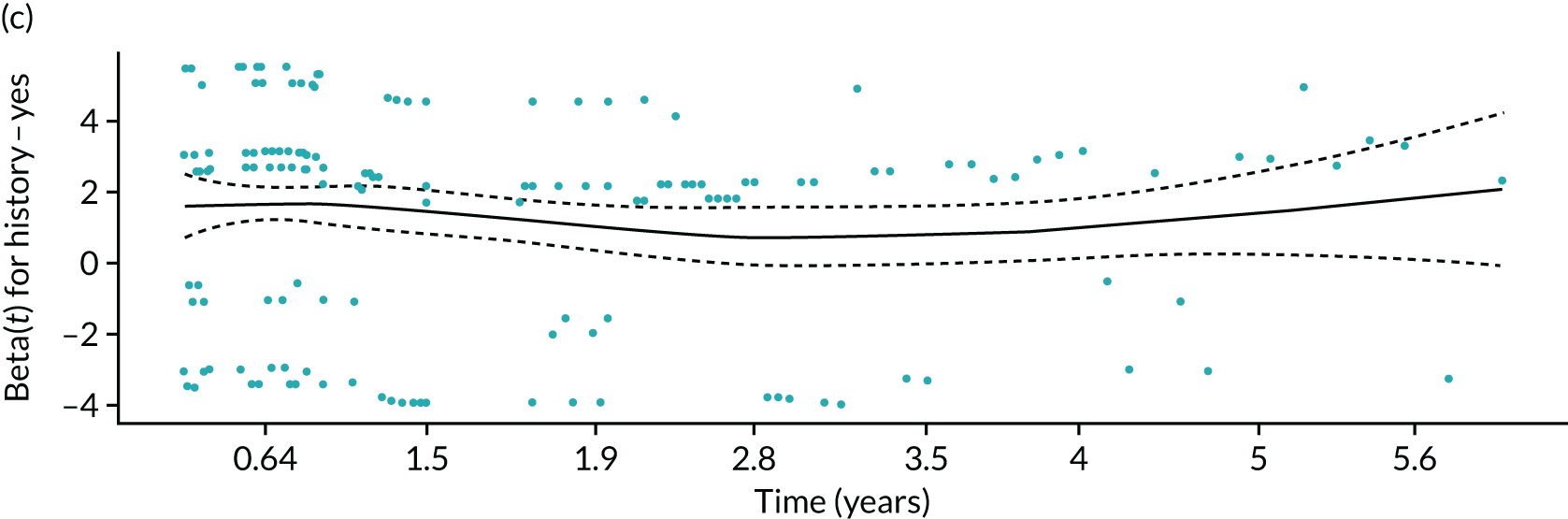
The health economic model has considered three risk groups: low-risk people with CPR scores of 0 or 1, medium-risk people with CPR scores of 2, and high-risk people with CPR scores of 3 or 4. We present the rates of ulcer per risk group in Table 40.
| Risk group | Length of follow-up (years) | New foot ulcer, n (%) | No new foot ulcer, n | Total, n (%) |
|---|---|---|---|---|
| Low | ≤ 1 | 20 (45) | 704 | 724 (56) |
| 1–2 | 15 (34) | 415 | 430 (33) | |
| 2–3 | 3 (7) | 67 | 70 (5) | |
| ≥ 3 | 6 (14) | 78 | 84 (6) | |
| Total | 44 (100) | 1264 | 1308 (100) | |
| Medium | ≤ 1 | 15 (52) | 76 | 91 (51) |
| 1–2 | 9 (31) | 44 | 53 (29) | |
| 2–3 | 1 (3) | 20 | 21 (12) | |
| ≥ 3 | 4 (14) | 10 | 14 (8) | |
| Total | 29 (100) | 150 | 179 (100) | |
| High | ≤ 1 | 57 (50) | 50 | 107 (36) |
| 1–2 | 25 (22) | 53 | 78 (26) | |
| 2–3 | 16 (14) | 60 | 76 (26) | |
| ≥ 3 | 17 (14) | 20 | 37 (12) | |
| Total | 115 (100) | 183 | 298 (100) |
The survival analyses suggest broad agreement with the logistic regression analyses in terms of the weighting of the coefficients of monofilament sensitivity, pulses and history. Across the entire follow-up period, the risk categories have an overall percentage of ulceration of 3.4%, 16.2% and 38.6% for low-, medium- and high-risk groups, respectively.
| Section/topic | Item | Development/validation | Checklist item | Section |
|---|---|---|---|---|
| Title and abstract | ||||
| Title | 1 | D; V | Identify the study as developing and/or validating a multivariable prediction model, the target population and the outcome to be predicted | Chapter 3, Introduction |
| Abstract | 2 | D; V | Provide a summary of objectives, study design, setting, participants, sample size, predictors, outcome, statistical analysis, results and conclusions | NA |
| Introduction | ||||
| Background and objectives | 3a | D; V | Explain the medical context (including whether diagnostic or prognostic) and rationale for developing or validating the multivariable prediction model, including references to existing models | Chapter 3, Introduction and Appendix 3 |
| 3b | D; V | Specify the objectives, including whether the study describes the development or validation of the model or both | Chapter 3, Introduction | |
| Methods | ||||
| Source of data | 4a | D; V | Describe the study design or source of data (e.g. randomised trial, cohort or registry data) separately for the development and validation data sets, if applicable | Chapter 3, Methods |
| 4b | D; V | Specify the key study dates, including start of accrual, end of accrual and, if applicable, end of follow-up | Chapter 3, Methods | |
| Participants | 5a | D; V | Specify key elements of the study setting (e.g. primary care, secondary care, general population) including number and location of centres | Chapter 3, Methods |
| 5b | D; V | Describe eligibility criteria for participants | Chapter 3, Methods | |
| 5c | D; V | Give details of treatments received, if relevant | Chapter 3, Methods | |
| Outcome | 6a | D; V | Clearly define the outcome that is predicted by the prediction model, including how and when assessed | Chapter 3, Methods |
| 6b | D; V | Report any actions to blind assessment of the outcome to be predicted | Chapter 3, Methods | |
| Predictors | 7a | D; V | Clearly define all predictors used in developing or validating the multivariable prediction model, including how and when they were measured | Chapter 3, Methods |
| 7b | D; V | Report any actions to blind assessment of predictors for the outcome and other predictors | Chapter 3, Methods | |
| Sample size | 8 | D; V | Explain how the study size was arrived at | Chapter 3, Methods |
| Missing data | 9 | D; V | Describe how missing data were handled (e.g. complete-case analysis, single imputation, multiple imputation) with details of any imputation method | Chapter 3, Methods |
| Statistical analysis methods | 10a | D | Describe how predictors were handled in the analyses | Chapter 3, Methods |
| 10b | D | Specify type of model, all model-building procedures (including any predictor selection) and method for internal validation | Chapter 3, Methods and Appendix 3 | |
| 10c | V | For validation, describe how the predictions were calculated | Chapter 3, Methods | |
| 10d | D; V | Specify all measures used to assess model performance and, if relevant, to compare multiple models | Chapter 3, Methods | |
| 10e | V | Describe any model updating (e.g. recalibration) arising from the validation, if done | NA | |
| Risk groups | 11 | D; V | Provide details on how risk groups were created, if done | Chapter 3, Methods |
| Development vs. validation | 12 | V | For validation, identify any differences from the development data in setting, eligibility criteria, outcome and predictors | Chapter 3, Methods |
| Results | ||||
| Participants | 13a | D; V | Describe the flow of participants through the study, including the number of participants with and without the outcome and, if applicable, a summary of the follow-up time. A diagram may be helpful | Chapter 3, Results |
| 13b | D; V | Describe the characteristics of the participants (basic demographics, clinical features, available predictors), including the number of participants with missing data for predictors and outcome | Chapter 3, Results | |
| 13c | V | For validation, show a comparison with the development data of the distribution of important variables (demographics, predictors and outcome) | Chapter 3, Results | |
| Model development | 14a | D | Specify the number of participants and outcome events in each analysis | Chapter 3, Results |
| 14b | D | If done, report the unadjusted association between each candidate predictor and outcome | NA | |
| Model specification | 15a | D | Present the full prediction model to allow predictions for individuals (i.e. all regression coefficients, and model intercept or baseline survival at a given time point) | Chapter 3, Results |
| 15b | D | Explain how to the use the prediction model | Chapter 3, Results and Appendix 3 | |
| Model performance | 16 | D; V | Report performance measures (with CIs) for the prediction model | Chapter 3, Results |
| Model updating | 17 | V | If done, report the results from any model updating (i.e. model specification, model performance) | NA |
| Discussion | ||||
| Limitations | 18 | D; V | Discuss any limitations of the study (such as non-representative sample, few events per predictor, missing data) | Chapter 3, Discussion |
| Interpretation | 19a | V | For validation, discuss the results with reference to performance in the development data, and any other validation data | Chapter 3, Discussion |
| 19b | D; V | Give an overall interpretation of the results, considering objectives, limitations, results from similar studies and other relevant evidence | Chapter 3, Discussion | |
| Implications | 20 | D; V | Discuss the potential clinical use of the model and implications for future research | Chapter 3, Discussion |
| Other information | ||||
| Supplementary information | 21 | D; V | Provide information about the availability of supplementary resources, such as study protocol, web calculator and data sets | Chapter 8 |
| Funding | 22 | D; V | Give the source of funding and the role of the funders for the present study | Chapter 8 |
Appendix 4 Chapter 4-related appendices
Search strategies
| # | Searches | Results | |
|---|---|---|---|
| 1 | exp Foot Orthoses/ | 597 | |
| 2 | exp Shoes/ | 5861 | |
| 3 | exp health education/ | 155,049 | |
| 4 | exp primary health care/ | 133,354 | |
| 5 | exp Emollients/ | 4590 | |
| 6 | insole*.mp. | 1429 | |
| 7 | footwear*.mp. | 2732 | |
| 8 | educat*.mp. | 808,235 | |
| 9 | specialist car*.mp. | 1843 | |
| 10 | multi disciplinary team*.mp. | 1041 | |
| 11 | multidisciplinary team*.mp | 13,050 | |
| 12 | routine podiatry car*.mp. | 4 | |
| 13 | exp general practice/ | 71,783 | |
| 14 | exp community health services/ | 280,617 | |
| 15 | off load*.mp. | 516 | |
| 16 | offload*.mp. | 618 | |
| 17 | emollient*.mp. | 2517 | |
| 18 | shoe*.mp. | 10,839 | |
| 19 | or/1-18 | ALL INTERVENTIONS | 1,211,803 |
| 20 | exp Foot/ | 47,255 | |
| 21 | exp Foot Diseases/ | 20,474 | |
| 22 | exp Diabetic Foot/ | 7470 | |
| 23 | exp Diabetic Neuropathies/ | 20,272 | |
| 24 | exp Diabetes Mellitus/ | 377,598 | |
| 25 | exp Diabetic Angiopathies/ | 44,655 | |
| 26 | exp Diabetes Complications/ | 119,833 | |
| 27 | exp Podiatry/ | 2110 | |
| 28 | exp Foot Ulcer/ | 8769 | |
| 29 | exp Skin Ulcer/ | 40,829 | |
| 30 | exp Ischemia/ | 55,699 | |
| 31 | exp Bacterial Infections/ | 830,709 | |
| 32 | (diabet* adj3 ulcer*).mp. | 4473 | |
| 33 | (diabet* adj3 (foot or feet)).mp. | 10,385 | |
| 34 | (diabet* adj3 wound*).mp. | 2418 | |
| 35 | (diabet* adj3 amputat*).mp. | 943 | |
| 36 | or/20-35 | ALL CONDITIONS | 1,341,638 |
| 37 | systematic* review*.mp. | 110,394 | |
| 38 | meta-analysis as topic/ | 16,124 | |
| 39 | (meta-analytic* or meta-analysis or metanalysis or metaanalysis or meta analysis or meta synthesis or meta-synthesis or metasynthesis or meta-regression or metaregression or meta regression).mp. | 135,296 | |
| 40 | (synthes* adj3 literature).mp. | 2302 | |
| 41 | (synthes* adj3 evidence).mp. | 6909 | |
| 42 | (integrative review or data synthesis).mp. | 11,729 | |
| 43 | (research synthesis or narrative synthesis).mp. | 1755 | |
| 44 | (systematic study or systematic studies).mp. | 10,041 | |
| 45 | (systematic comparison* or systematic overview*).mp. | 2670 | |
| 46 | ((evidence based or comprehensive or critical or quantitative or structured) adj review).mp. | 27,447 | |
| 47 | (realist adj (review or synthesis)).mp. | 287 | |
| 48 | or/37-47 | ALL METHODS | 251,135 |
| 49 | review.pt. | 2,316,960 | |
| 50 | (medline or pubmed or embase or cinahl or psyc?lit or psyc?info).ab. | 146,733 | |
| 51 | ((literature or database* or bibliographic or electronic or computeri?ed or internet) adj3 search*).mp. | 92,793 | |
| 52 | (electronic adj3 database*).mp. | 19,642 | |
| 53 | included studies.ab. | 14,433 | |
| 54 | (inclusion adj3 studies).ab. | 11,371 | |
| 55 | ((inclusion or selection or predefined or predetermined) adj criteria).ab. | 84,815 | |
| 56 | (assess* adj3 (quality or validity)).ab. | 60,800 | |
| 57 | (select* adj3 (study or studies)).ab. | 53,516 | |
| 58 | (data adj3 extract*).ab. | 45,707 | |
| 59 | extracted data.ab. | 10,556 | |
| 60 | (data adj3 abstraction).ab. | 1277 | |
| 61 | published intervention*.ab. | 144 | |
| 62 | ((study or studies) adj2 evaluat*).ab. | 149,931 | |
| 63 | (intervention* adj2 evaluat*).ab. | 8850 | |
| 64 | (confidence interval* or heterogeneity or pooled or pooling or odds ratio*).ab. | 583,558 | |
| 65 | (Jadad or coding).ab. | 153,954 | |
| 66 | or/50-65 | ALL ABSTRACTS | 1,141,840 |
| 67 | 49 and 66 | COMBINE REVIEW.pt AND ABSTRACTS | 186,735 |
| 68 | review.ti. | 365,140 | |
| 69 | 66 and 68 | COMBINE ABSTRACTS AND REVIEW TITLE | 90,484 |
| 70 | (review* adj4 (papers or trials or studies or evidence or intervention* or evaluation*)).mp. | 147,629 | |
| 71 | 48 or 67 or 68 or 70 | COMBINE METHODS, AND REVIEW.pt & ABSTRACTS, AND ABSTRACT AND REVIEW TITLE, AND REVIEW.TIABSTRACTS | 435,212 |
| 72 | letter.pt. | 975,446 | |
| 73 | editorial.pt. | 442,803 | |
| 74 | comment.pt. | 693,044 | |
| 75 | or/72-74 | ALL PUBLICATIONS | 1,592,398 |
| 76 | 71 not 75 | PUBLICATIONS REMOVED | 424,748 |
| 78 | exp animals/not humans/ | 4,419,620 | |
| 77 | 76 not 77 | ANIMALS REMOVED | 413,286 |
| 79 | 19 and 36 and 78 | GRAND COMBINE | 1545 |
| # | Searches | Results | |
|---|---|---|---|
| 1 | exp Foot Orthosis/ | 1783 | |
| 2 | exp Shoe/ | 8340 | |
| 3 | exp health education/ | 279,708 | |
| 4 | exp primary health care/ | 137,391 | |
| 5 | emollient agent/ | 4806 | |
| 6 | insole*.mp. | 1769 | |
| 7 | footwear*.mp. | 3499 | |
| 8 | educat*.mp. | 1,130,926 | |
| 9 | specialist car*.mp. | 2713 | |
| 10 | multi disciplinary team*.mp. | 2710 | |
| 11 | multidisciplinary team*.mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] | 22,086 | |
| 12 | routine podiatry car*.mp. | 4 | |
| 13 | exp general practice/ | 76,174 | |
| 14 | exp community care/ | 110,817 | |
| 15 | off load*.mp. | 614 | |
| 16 | offload*.mp. | 800 | |
| 17 | emollient*.mp. | 5569 | |
| 18 | shoe*.mp. | 14,386 | |
| 19 | or/1-18 | ALL INTERVENTIONS | 1,460,708 |
| 20 | exp Foot/ | 47,405 | |
| 21 | exp Foot Disease/ | 69,761 | |
| 22 | exp Diabetic Foot/ | 11,959 | |
| 23 | exp Diabetic Neuropathy/ | 20,680 | |
| 24 | exp Diabetes Mellitus/ | 783,108 | |
| 25 | exp Diabetic Angiopathy/ | 11,784 | |
| 26 | (diabet* adj3 complicat*).mp. | 39,093 | |
| 27 | exp Podiatry/ | 2293 | |
| 28 | exp Foot Ulcer/ | 4598 | |
| 29 | exp Skin Ulcer/ | 61,358 | |
| 30 | exp Ischemia/ | 683,484 | |
| 31 | exp Bacterial Infection/ | 815,279 | |
| 32 | (diabet* adj3 ulcer*).mp. | 6114 | |
| 33 | (diabet* adj3 (foot or feet)).mp. | 14,451 | |
| 34 | (diabet* adj3 wound*).mp. | 3250 | |
| 35 | (diabet* adj3 amputat*).mp. | 1231 | |
| 36 | or/20-35 | ALL CONDITIONS | 2,319,256 |
| 37 | systematic* review*.mp. | 198,683 | |
| 38 | meta analysis/ | 128,965 | |
| 39 | (meta-analytic* or meta-analysis or metanalysis or metaanalysis or meta analysis or meta synthesis or meta-synthesis or metasynthesis or meta-regression or metaregression or meta regression).mp. | 201,471 | |
| 40 | (synthes* adj3 literature).mp. | 2631 | |
| 41 | (synthes* adj3 evidence).mp. | 7723 | |
| 42 | (integrative review or data synthesis).mp. | 14,150 | |
| 43 | (research synthesis or narrative synthesis).mp. | 1823 | |
| 44 | (systematic study or systematic studies).mp. | 10,732 | |
| 45 | (systematic comparison* or systematic overview*).mp. | 2845 | |
| 46 | ((evidence based or comprehensive or critical or quantitative or structured) adj review).mp. | 30,357 | |
| 47 | (realist adj (review or synthesis)).mp. | 271 | |
| 48 | or/37-47 | ALL METHODS | 363,489 |
| 49 | review.pt. | 2,272,717 | |
| 50 | (medline or pubmed or embase or cinahl or psyc?lit or psyc?info).ab. | 171,015 | |
| 51 | ((literature or database* or bibliographic or electronic or computeri?ed or internet) adj3 search*).mp. | 112,943 | |
| 52 | (electronic adj3 database*).mp. | 25,563 | |
| 53 | included studies.ab. | 17,536 | |
| 54 | (inclusion adj3 studies).ab. | 13,486 | |
| 55 | ((inclusion or selection or predefined or predetermined) adj criteria).ab. | 125,186 | |
| 56 | (assess* adj3 (quality or validity)).ab. | 76,902 | |
| 57 | (select* adj3 (study or studies)).ab. | 67,123 | |
| 58 | (data adj3 extract*).ab. | 58,865 | |
| 59 | extracted data.ab. | 12,921 | |
| 60 | (data adj3 abstraction).ab. | 1795 | |
| 61 | published intervention*.ab. | 169 | |
| 62 | ((study or studies) adj2 evaluat*).ab. | 202,130 | |
| 63 | (intervention* adj2 evaluat*).ab. | 11,727 | |
| 64 | (confidence interval* or heterogeneity or pooled or pooling or odds ratio*).ab. | 698,437 | |
| 65 | (Jadad or coding).ab. | 175,200 | |
| 66 | or/50-65 | ALL ABSTRACTS | 1,416,110 |
| 67 | 49 and 66 | COMBINE REVIEW.pt AND ABSTRACTS | 162,318 |
| 68 | review.ti. | 410,964 | |
| 69 | 66 and 68 | COMBINE ABSTRACTS AND REVIEW TITLE | 105,269 |
| 70 | (review* adj4 (papers or trials or studies or evidence or intervention* or evaluation*)).mp. | 172,793 | |
| 71 | 48 or 67 or 69 or 70 | COMBINE METHODS, AND REVIEW.pt & ABSTRACTS, AND ABSTRACT AND REVIEW TITLE, AND REVIEW.TIABSTRACTS | 554,812 |
| 72 | letter.pt. | 978,666 | |
| 73 | editorial.pt. | 538,173 | |
| 74 | or/72-73 | ALL PUBLICATIONS | 1,516,839 |
| 75 | 71 not 74 | PUBLICATIONS REMOVED | 540,237 |
| 76 | exp animal/not human/ | 4,795,342 | |
| 77 | 75 not 76 | ANIMALS REMOVED | 527,970 |
| 78 | 19 and 36 and 77 | GRAND COMBINE | 4507 |
Cochrane
Medical subject heading (MeSH) search:
-
diabet* (56,868)
-
foot ulcer* (1411)
-
prevent* (176,372)
-
#1 and #2 (1218)
-
#4 and #3 (335).
Database of Abstracts of Reviews of Effects (DARE) search
Search in all Cochrane sites for ‘diabetic foot ulcer prevention’.
Health Technology Assessment database search
Search term: diabetic foot ulcer.
| PROSPERO number | Title | Authors |
|---|---|---|
| CRD42017072816 | Footwear and insole design features to prevent foot ulceration in people with diabetes: a systematic review protocol | Richard Collings, Jennifer Freeman, Jos Latour, Sam Glasser and Joanne Paton |
| CRD42018105681 | Effectiveness of offloading interventions to heal foot ulcers and reduce mechanical pressure in persons with diabetic foot ulcers: a systematic review | Peter Lazzarini, Sicco Bus, David Armstrong, Carlo Caravaggi, Vijay Vishwanathan, Gustav Jarl and Catherine Gooday |
| CRD42018105073 | Interventions to reduce modifiable risk factors for foot ulcers in at-risk patients with diabetes: a systematic review | Jaap van Netten, Sicco Bus, Matilde Monteiro-Soares, Larry Lavery, Anne Rasmussen, Anita Raspovic and Isabel Sacco |
Overview flow diagram
List of excluded studies from the overview of systematic reviews
| OVID results number | Author (year) | Reason for exclusion | |
|---|---|---|---|
| 1. | 3775 | Al-Saweer 2006 | Not an empirical paper. Provides an easy-access overview for patients |
| 2. | 2598 | Arad 2011 | A review of Arad (2011) paper, which is included. This is not primary research |
| 3. | 376 | Arsanjani Shirazi 2016 | Narrative review and not an actual SR |
| 4. | 1473 | Attridge 2014 | Assessed health-care advice and not prevention of DFUs |
| 5. | 486 | Baptista 2016 | Chronic care model developed to provide chronic disease patients with self-care and tracking systems and not preventing DFUs |
| 6. | 860 | Baradaran 2010 | Treating and not preventing DFUs |
| 7. | 1982 | Baptista 2013 | Conference abstract meets our inclusion criteria, but we do not have enough information for data extraction and quality assessment |
| 8. | 3890 | Bazian 2005 | Reanalysis of Valk (2015), not a SR |
| 9. | 424 | Behforootan 2017 | Overview of modelling techniques in the field of foot and footwear biomechanics and to investigate their applicability in a clinical setting, not prevention of DFUs |
| 10. | 3739 | Behrenberg 2006 | Patient knowledge and not preventing DFUs |
| 11. | 3649 | Beran 2006 | Diabetic care and not preventing DFUs |
| 12. | 4210 | Bowering 2001 | Treatment and not preventing DFUs |
| 13. | 4481 | Braid 1992 | Structured audit review and not a SR |
| 14. | 1429 | Braun 2014 | About new adjunctive management of DFUs and not preventing DFUs |
| 15. | 1666 | Brownrigg 2013 | Narrative review and not an SR |
| 16. | 1526 | Buggy 2017 | Management of the diabetic foot; not prevention of DFUs |
| 17. | 3345 | Bus 2008 | Clinical controlled trial is not an eligible study design for our SR |
| 18. | 21 | Bus 2016 | Literature review and not a SR |
| 19. | 120 | Bus 2016 | Integrated diabetes annual review and not a SR |
| 20. | 1756 | Bus 2013 | Pressure-time integral data analysed and reported next to peak pressure data: outcome not relevant to overview |
| 21. | 1595 | Caporale 2013 | Prevalence and disease burden and not preventing DFUs |
| 22. | 4046 | Cavanagh 2004 | Narrative and not an SR |
| 23. | 2448 | Cavanagh 2010 | Narrative and not an SR |
| 24. | 1544 | Cook 1997 | The focus is on the diabetic foot and not preventing DFUs |
| 25. | 2832 | Cooper 2009 | General diabetes and not prevention of DFUs |
| 26. | 3217 | Couch 2008 | General diabetes and not prevention of DFUs |
| 27. | HTA | Crawford 2015 | Prediction not prevention |
| 28. | 186 | Creamer 2016 | General diabetes and not prevention of DFUs |
| 29. | 619 | Crews 2016 | The focus is on risk of physical activity and not preventing DFUs |
| 30. | 1039 | de Oliveira 2015 | The focus is on treating and not preventing DFUs |
| 31. | 4165 | Echeverry 2003 | Foot examination and not preventing DFUs |
| 32. | 1241 | Eldor 2004 | The focus is on a range of new treatments and not preventing DFUs |
| 33. | 104 | Elraiyah 2016 | The focus is on offloading and not preventing DFUs |
| 34. | 105 | Elraiyah 2016b | Does not look at prevention. Includes one RCT that mentions prevention; incidental and therefore the SR is excluded |
| 35. | 1028 | Eneroth 2008 | Treatment and not prevention |
| 36. | 1069 | Farid 2015 | Treatment and not prevention |
| 37. | 3628 | Farrow 2005 | Rrheumatoid arthritis and not preventing DFUs |
| 38. | 413 | Formosa 2016 | The focus is on screening and not preventing DFUs |
| 39. | 297 | Francis 2016 | Protocol and not a SR |
| 40. | 1574 | Gemechu 2013 | Diabetic foot infection and not DFU prevention |
| 41. | 1402 | Griffin 2000 | General diabetes and not prevention of DFUs |
| 42. | 285 | Healy 201) | Alteration of biomechanical factors associated with ulcer healing and not preventing DFUs |
| 43. | 3285 | Herber 2007 | Examined QoL and not preventing DFUs |
| 44. | 2116 | Heuch 2012 | Protocol of Heuch (2016); not a SR |
| 45. | Hinchliffe 2016 | Not prevention: SR of treatments for DFUs | |
| 46. | 106 | Hingorani 2016 | Not a SR but a report of a clinical practice guideline based on five SRs |
| 47. | 3381 | Hume 2008 | Focused on lower limb injuries and not preventing DFUs |
| 48. | 928 | Hunt 2009 | Treatment of ulcers and not prevention |
| 49. | 226 | Janisse 2015 | Literature review and not a SR |
| 50. | 1534 | Jarl 2016 | Adherence to wearing therapeutic shoes and not preventing DFUs |
| 51. | 3401 | Jeffcoate 2008 | Foot management and not preventing DFUs |
| 52. | 1156 | La Fontaine 2014 | Narrative review and not an actual SR |
| 53. | 1832 | Lavery 2013 | Case–control study and not preventing DFUs |
| 54. | 2505 | Lefebvre 2011 | Not an intervention to prevent DFUs |
| 55. | 3260 | Leung 2007 | Literature review and not a SR |
| 56. | 473 | Lewis 2013 | Treatment and not preventing DFUs |
| 57. | 158 | Lipsky 2016 | Treatment and not preventing DFUs |
| 58. | 698 | Ma 2016 | Analysis microbiological profile and drug resistance of diabetic foot infections; not DFU prevention |
| 59. | 4324 | Margolis 2000 | Estimating risk factors and not preventing DFUs |
| 60. | 160 | Markakis 2016 | Overview and not a SR |
| 61. | 237 | Matricciani 2015 | Psychosocial barriers to and enablers of foot self-care practices; not prevention or a priori outcome |
| 62. | 2729 | Mills 2010 | Foot orthosis and gait, not prevention of DFUs |
| 63. | 944 | Morey-Vargas 2015 | Literature review and not a SR |
| 64. | 1646 | Morona 2013 | Adherence to wearing therapeutic shoes: not prevention or a priori outcome |
| 65. | 764 | Moxey 2011 | Quantify global variation in the incidence of LEA and not prevention of DFUs |
| 66. | 192 | Naidoo 2015 | Literature review and not a SR |
| 67. | 200 | Navarro-Flores 2015 | Meta-review. We checked the included relevant SRs and none meets our eligibility criteria |
| 68. | HTA | Nelson 2006 | Treatment and not preventing DFUs |
| 69. | 1528 | Noor 2017 | Management and not preventing DFUs |
| 70. | 273 | Nordheim 2014 | Clinical, behavioural organisational outcomes of leg and foot ulcers and not preventing DFUs |
| 71. | 4117 | O’Brien 2003 | Intervention study and not a SR |
| 72. | 1081 | Oosterveld 2015 | Peak pressure and not preventing DFUs |
| 73. | 1537 | Otter 2015 | Plantar pressure and not ulcer is the outcome |
| 74. | 424 | Patry 2013 | Pathogenesis of DFUs and not preventing DFUs |
| 75. | 1019 | Perrin 2008 | Behavioural outcome, not ulcer outcome |
| 76. | 1771 | Pinilla 2013 | Literature review and not a SR |
| 77. | 3538 | Pinzur 2007 | Charcot’s arthropathy, not prevention of DFUs and not a SR |
| 78. | 4406 | Pinzur 1997 | Narrative and not an actual SR |
| 79. | 3734 | Rakel 2006 | Literature review and not a SR |
| 80. | 2041 | Rawal 2012 | General diabetic outcome and not preventing DFUs |
| 81. | 4026 | Rheeder 2004 | Management and not preventing DFUs |
| 82. | 1888 | Rice 2013 | Audit and not a SR |
| 83. | 1529 | Rice 2014 | Audit and not a SR |
| 84. | 4323 | Rith-Najarian 2000 | Management post amputation and not preventing DFUs |
| 85. | 665 | Robineau 2016 | Treatments for diabetic foot infections and not preventing DFUs |
| 86. | 204 | Sanders Thompson 2015 | General diabetic outcome and not preventing DFUs |
| 87. | 3147 | Saunders 2009 | General diabetic outcome and not preventing DFUs |
| 88. | 45 | Schaper 2017 | Summary guidance and not a SR |
| 89. | 2389 | Schunk 2011 | Evaluates diabetes care and not a SR |
| 90. | 1292 | Seah 2014 | Reducing diabetic complications and not preventing DFUs |
| 91. | 3987 | Selwitz 2003 | Risk of diabetes development and not preventing DFUs |
| 92. | 3580 | Shank 2006 | Diabetes foot management and not preventing DFUs |
| 93. | 3011 | Sharma 2010 | General diabetic outcome and not preventing DFUs |
| 94. | 58 | Sherifali 2017 | Developing coaching model and not preventing DFUs |
| 95. | 796 | Shrivastava 2015 | Guideline development and not a SR |
| 96. | 198 | Shrivastava 2016 | Diabetes management and not preventing DFUs |
| 97. | 1234 | Singh 2005 | Clinical review and not a SR |
| 98. | 2754 | Spencer 2010 | Explores causal factors of deteriorating metabolic control and not preventing DFUs |
| 99. | 1088 | Srulovici 2015 | General diabetic outcome and not preventing DFUs |
| 100. | 3828 | Stengel 2005 | Looks at amputations but no ulcer outcome |
| 101. | 2719 | Stolt 2010 | Foot health care and not preventing DFUs |
| 102. | 3889 | Stuart 2005 | One-page commentary and not a SR |
| 103. | 3198 | Tabrizi 2008 | General diabetic outcome and not preventing DFUs |
| 104. | 299 | Tang 2014 | Risk of amputation and not preventing DFUs |
| 105. | 279 | Telfer 2014 | Finite element analysis-based computational simulations of diabetic foot and not preventing DFUs |
| 106. | 212 | Torsello 2015 | Overview of 2012–14 comments on DFU treatment clinical trials and meta-analyses; not a SR |
| 107. | 154 | Uckay 2015 | Diabetic foot infection and not DFU prevention |
| 108. | 3349 | Unwin 2008 | Narrative and not an actual SR |
| 109. | 346 | Valencia 2017 | Microvascular diabetes prevention and not preventing of DFUs |
| 110. | 1233 | Valk 2002 | Original manuscript published electronically in the Cochrane Library (Valk GD, Kriegsman DMW, Assendelft WJJ. Patient education for preventing diabetic foot ulceration. Oxford: Update Software Ltd; 2001). This has since been updated by Dorresteijn et al.,51 which we have included |
| 111. | 358 | van Acker 2014 | Assessed QoL and burden of DFUs, but not preventing DFUs |
| 112. | 3199 | van den Berg 2008 | General health promotion and not preventing DFUs |
| 113. | 3764 | Vijgen 2006 | General diabetes and not preventing DFUs |
| 114. | 4286 | Wheatley 2001 | Audit protocol and not a SR |
| 115. | 3600 | Younes 2006 | Reviews spectrum of foot problems in patients with diabetes, underlying aetiological factors; not prevention |
| 116. | 1539 | Ndip 2012 | Unit of analysis includes SRs; this is more of an umbrella review |
References of excluded studies (overview)
Al-Saweer A. Diabetic foot – evidence that counts. Bahrain Med Bull 2006;28:1–5.
Arad Y, Mize DLE, Gandhi GY. Review: evidence for the effectiveness of interventions to prevent foot ulcers in patients with diabetes is limited. Ann Intern Med 2011;155:JC4–08.
Arsanjani Shirazi A, Nasiri M, Yazdanpanah L. Dermatological and musculoskeletal assessment of diabetic foot: a narrative review. Diabetes Metab Syndr Clin Res Rev 2016;10:S158–64.
Attridge M, Creamer J, Ramsden M, Cannings John R, Hawthorne K. Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus. Cochrane Database Syst Rev 2014;9:CD006424.
Baptista DR, Wiens A, Pontarolo R, Regis L, Reis WCT, Correr CJ. The chronic care model for type 2 diabetes: a systematic review. Diabetol Metab Syndr 2016;8:7.
Baradaran HR, ShamsHosseini N, NooriHekmat S, TehraniBanihashemi A, Khamseh ME. Effectiveness of diabetes educational interventions in Iran: a systematic review. Diabetes Technol Ther 2010;12:317–31.
Batista M, Oliveira C. The impact of educational programs on the prevention of diabetic foot complications: a systematic review. Aten Primaria 2013;45:132–88.
Bazian, London UK. Systematic Review: education to prevent foot ulcers in diabetes. Evid Based Healthc Public Health 2005;9:351–8.
Behforootan S, Chatzistergos P, Naemi R, Chockalingam N. Finite element modelling of the foot for clinical application: a systematic review. Med Eng Phys 2017;39:1–11.
Behrenberg BL, Abholz HH. The influence of patient-education programmes on ‘knowledge’ and outcome of care in patients with type 1 diabetes. Z Allgemeinmed 2006;82:495–501.
Beran D and Yudkin JS. Diabetes care in sub-Saharan Africa. Lancet 2006;368:1689–95.
Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can Fam Physician 2001;47:1007–16.
Braid E, Campbell B, Curtis S, Eden G, Keast T, Hardcastle S, et al. The diabetes annual review as an educational tool: assessment and learning integrated with care, screening, and audit. Diabetic Med 1992;9:389–94.
Braun LR, Fisk WA, Lev-Tov H, Kirsner RS, Isseroff RR. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol 2014;15:267–81.
Brownrigg JRW, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg 2013;45:673–81.
Buggy A, Moore Z. The impact of the multidisciplinary team in the management of individuals with diabetic foot ulcers: a systematic review. J Wound Care 2017;26:324–39.
Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg 2016;138(Suppl. 3):179S–87S.
Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlaváček P, et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev 2008;24:S162–80.
Bus SA, van Deursen RW, Armstrong DG, Lewis JEA, Caravaggi CF, Cavanagh PR, et al. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev 2016;32(Suppl. 1):99–118.
Bus SA, Waaijman R. The value of reporting pressure-time integral data in addition to peak pressure data in studies on the diabetic foot: a systematic review. Clin Biomech 2013;28:117–21.
Caporale JE, Elgart JF, Gagliardino JJ. Diabetes in Argentina: cost and management of diabetes and its complications and challenges for health policy. Glob Health 2013;9:54.
Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg 2010;52(Suppl. 3):37S–43S.
Cavanagh PR. Therapeutic footwear for people with diabetes. Diabetes Metab Res Rev 2004;20(Suppl. 1):S51–5.
Cook K. Clinical implications of diabetes on the foot. J Athl Train 1997;32:55–8.
Cooper H, Cooper J, Milton B. Technology-based approaches to patient education for young people living with diabetes: a systematic literature review. Pediatr Diabetes 2009;10:474–83.
Couch R, Jetha M, Dryden DM, Hooten N, Liang Y, Durec T, et al. Diabetes education for children with type 1 diabetes mellitus and their families. Evid Rep Technol Assess 2008;166:1–144.
Crawford F, Cezard G, Chappell FM, Murray GD, Price JF, Sheikh A, et al. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS). Health Technol Assess 2015;19(57).
Creamer J, Attridge M, Ramsden M, Cannings John R, Hawthorne K. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: an updated Cochrane Review of randomized controlled trials. Diabetic Med 2016;33:169–83.
Crews RT, Schneider KL, Yalla SV, Reeves ND, Vileikyte L. Physiological and psychological challenges of increasing physical activity and exercise in patients at risk of diabetic foot ulcers: a critical review. Diabetes Metab Res 2016;32:791–804.
de Oliveira AL, Moore Z. Treatment of the diabetic foot by offloading: a systematic review. J Wound Care 2015;24:562–70.
Echeverry DM, Dike MR, Washington C, Davidson MB. The impact of using a low-literacy patient education tool on process measures of diabetes care in a minority population. J Natl Med Assoc 2003;95:1074–81.
Eldor R, Raz I, Ben Yehuda A, Boulton AJM. New and experimental approaches to treatment of diabetic foot ulcers: a comprehensive review of emerging treatment strategies. Diabetic Med 2004;21:1161–73.
Elraiyah T, Tsapas A, Prutsky G, Domecq JP, Hasan R, Firwana B. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg 2016;63(Suppl. 2):46S–58S.
Elraiyah T, Prutsky G, Domecq JP, Tsapas A, Nabhan M, Frykberg RG, et al. A systematic review and meta-analysis of off-loading methods for diabetic foot ulcers. J Vasc Surg 2016;63(Suppl. 2):59S–68S.e1–2.
Eneroth M, van Houtum WH. The value of debridement and Vacuum-Assisted Closure (V.A.C.) Therapy in diabetic foot ulcers. Diabetes Metab Res Rev 2008;24(Suppl. 1):S76–80.
Farid Y, Smaoui MR, Farid D. Prevention Strategies in Cardiomyopathy Diseases Related to Type 2 Diabetes in Children and Adolescents. Echocardiography. 21st World Congress of Echocardiography and Cardiology, ISCU (International Council for Science, formerly International Council of Scientific Unions), Istanbul, Turkey, 20–22 November 2015.
Farrow SJ, Kingsley GH, Scott DL. Interventions for foot disease in rheumatoid arthritis: a systematic review. Arthritis Rheum 2005;53:593–602.
Formosa C, Gatt A, Chockalingam N. A critical evaluation of existing diabetic foot screening guidelines. Rev Diabet Stud 2016;13:158–86.
Francis DK, Lazzarini PA, Ferguson TS, Jen SD, Cumberbatch C, Welch V. Education of health professionals for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2016;11:CD010433.
Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician 2013;88:177–84.
Griffin S, Kinmonth AL. Diabetes care: the effectiveness of systems for routine surveillance for people with diabetes. Cochrane Database Syst Rev 2000;2:CD00054.
Healy A, Naemi R, Chockalingam N. The effectiveness of footwear and other removable off-loading devices in the treatment of diabetic foot ulcers: a systematic review. Curr Diabetes Rev 2014;10:215–30.
Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007;5:44.
Heuch L, Tyndall J, CookJohnson R, Cowin A. The effectiveness of methods of off-loading to prevent diabetic foot ulcers in adults with diabetes: a systematic review. JBI Database Syst Rev 2012;10:S148–S161.
Hinchliffe RJ, Brownrigg JRW, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev 2016;32:37–44.
Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz, L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vas Surg 2016;63(Suppl.):3S–21S.
Hume P, Hopkins W, Rome K, Maulder P, Coyle G, Nigg B. Effectiveness of foot orthoses for treatment and prevention of lower limb injuries: a review. Sports Med 2008;38:759–79.
Hunt D. Diabetes: foot ulcers and amputations. Am Fam Physician 2009;80:789.
Janisse D, Janisse E. Pedorthic management of the diabetic foot. Prosthet Orthot Int 2015;39:40–7.
Jarl G, Lundqvist L. Adherence to wearing therapeutic shoes among people with diabetes: a systematic review and reflections. Patient Prefer Adherence 2016;10:1521–8.
Jeffcoate WJ, Lipsky BA, Berendt AR, Cavanagh PR, Bus SA, Peters EJG, et al. Unresolved issues in the management of ulcers of the foot in diabetes. Diabetic Med 2008;25:1380–9.
La Fontaine J, Lavery LA, Hunt NA, Murdoch DP. The role of surgical off-loading to prevent recurrent ulcerations. Int J Low Extrem Wounds 2014;13:320–34.
Lavery LA, La Fontaine J, Kim PJ. Preventing the first or recurrent ulcers. Med Clin North Am 2013;97:807–20.
Lefebvre KM, Lavery LA. Disparities in amputations in minorities. Clin Orthop Relat Res 2011;469:1941–50.
Leung PC. Diabetic foot ulcers – a comprehensive review. Surgeon 2007;5:219–31.
Lewis J, Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev 2013;1:CD002302.
Lipsky BA. Diabetic foot infections: current treatment and delaying the ‘post-antibiotic era’. Diabetes Metab Res Rev 2016;32(Suppl. 1):246–53.
Ma J, Gao Y, Wang C, Du L, Ran X. Microbiological Profile of Diabetic Foot Infections in China: A 20-year Systematic Review. 20th Scientific Meeting of the Chinese Diabetes Society, Xiamen, China, 16–19 November 2016.
Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol 2000;136:1531–5.
Markakis K, Bowling FL, Boulton AJM. The diabetic foot in 2015: an overview. Diabetes Metab Res Rev 2016;32(Suppl. 1):169–78.
Matricciani LBP, Jones S. who cares about foot care? barriers and enablers of foot self-care practices among non-institutionalised older adults diagnosed with diabetes: an integrative review. Diabetes Educ 2015;41:106–17.
Mills K, Blanch P, Chapman AR, McPoil TG, Vicenzino B. Foot orthoses and gait: a systematic review and meta-analysis of literature pertaining to potential mechanisms. Br J Sports Med 2010;44:1035–46.
Morey-Vargas OL, Smith SA. BE SMART: strategies for foot care and prevention of foot complications in patients with diabetes. Prosthet Orthot Int 2015;39:48–60.
Morona JK, Buckley ES, Jones S, Reddin EA, Merlin TL. Comparison of the clinical effectiveness of different off-loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2013;29:183–93.
Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, et al. Lower extremity amputations – a review of global variability in incidence. Diabetic Med 2011;28:1144–53.
Naidoo P, Liu VJ, Mautone M, Bergin S. Lower limb complications of diabetes mellitus: a comprehensive review with clinicopathological insights from a dedicated high-risk diabetic foot multidisciplinary team. Br J Radiol 2015;88:20150135.
Navarro-Flores E, Gijon-Nogueron G, Cervera-Marin JA, Labajos-Manzanares MT. Assessment of foot self-care in patients with diabetes: retrospective assessment (2008–2014). Foot Ankle Spec 2015;8:406–12.
Ndip A, Ebah L, Mbako A. Neuropathic diabetic foot ulcers – evidence-to-practice. Int J Gen Med 2012;5:129–34.
Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess 2006;10(12).
Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr 2017;11:149–56.
Nordheim LV, Marianne TH, Marjolein MI. Effect of telemedicine follow-up care of leg and foot ulcers: a systematic review. BMC Health Serv Res 2014;14:565.
O’Brien KE, Chandramohan V, Nelson DA, Fischer JRJ, Stevens G, Poremba JA. Effect of a physician-directed educational campaign on performance of proper diabetic foot exams in an outpatient setting. J Gen Intern Med 2003;18:258–65.
Oosterveld F, Klien Overmeen F, De Vries J, Bieleman A. Systematic Review and Meta-analysis of Diagnostic Foot Pressure Measurements. Physiotherapy (United Kingdom). World Confederation for Physical Therapy Congress 2015, Singapore, 1–4 May 2015.
Otter SJ, Rome K, Ihaka B, South A, Smith M, Gupta A, et al. Protective socks for people with diabetes: a systematic review and narrative analysis. J Foot Ankle Res 2015;8:9.
Patry J, Belley R, Cote M, Chateau-Degat M. Plantar pressures, plantar forces, and their influence on the pathogenesis of diabetic foot ulcers: a review. J Am Podiatr Med Assoc 2013;103:322–32.
Perrin B, Swerissen H. The behaviour and psychological functioning of people at high risk of diabetes-related foot complications. Diabetes Educ 2008;34:493–500.
Pinilla AE, Barrera MP, Sanchez AL, Mejia A. Risk factors of diabetes mellitus and diabetic foot: a primary approach to prevention. Revi Colomb Cardiol 2013;20:213–22.
Pinzur MS. Current concepts review: Charcot arthropathy of the foot and ankle. Foot Ankle Int 2007;28:952–9.
Pinzur MS. The diabetic foot. Curr Opin Orthop 1997;8:31–4.
Rakel A, Huot C, Ekoe JM. Canadian Diabetes Association technical review: the diabetic foot and hyperbaric oxygen therapy. Can J Diabetes 2006;30:411–21.
Rawal LB, Tapp RJ, Williams ED, Chan C, Yasin S, Oldenburg B. Prevention of type 2 diabetes and its complications in developing countries: a review. Int J Behav Med 2012;19:121–33.
Rheeder P. The diabetic foot. J Endocrinol Metabol Diabetes S Afr 2004;9:74–8.
Rice A, Gallagher A, Higgins KS. Audit of Inpatient Management of Diabetic Foot Problems in Patients Who Have Subsequently Undergone a Major Lower Limb Amputation: Implications for the Multidisciplinary Diabetic Foot Team. Diabetic Medicine. Diabetes UK Professional Conference, Manchester, UK, 13–15 March 2013.
Rice C, Higgins KS, Gallagher A. Follow-up Audit: Inpatient Management of Diabetic Foot Problems in Patients Who Have Subsequently Undergone a Major Lower Limb Amputation and Implications for the Multidisciplinary Diabetic Foot Team. Diabetic Medicine. Diabetes UK Professional Conference, Liverpool, UK, 5–7 March 2014.
Rith-Najarian SJ, Reiber GE. Prevention of foot problems in persons with diabetes. J Fam Pract 2000;49(Suppl.):S30–9.
Robineau O, Nguyen S, Senneville E. Optimising the quality and outcomes of treatments for diabetic foot infections. Expert Rev Anti Infect Ther 2016;14:817–27.
Sanders Thompson VL, Johnson-Jennings M, Bauman AA, Proctor E. Use of culturally focused theoretical frameworks for adapting diabetes prevention programs: a qualitative review. Prev Chronic Dis 2015;12:E60.
Saunders M. Assessing the Impact of Conducting an Essential Public Health Services Assessment in Diabetes Prevention and Control Programs. International Diabetes Federation 20th World Diabetes Congress, Montréal, QC, Canada, 18–22 October 2009.
Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K, International Working Group on the Diabetic Foot (IWGDF). Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract 2017;124:84–92.
Schunk M, Stark R, Reitmeir P, Rathmann W, Meisinger C, Holle R. [Improvements in type 2 diabetes care? Pooled analysis of survey data in southern Germany (KORA) from 1999–2008.] Bundesgesundheitsblatt, Gesundheitsforschung Gesundheitsschutz 2011;54:1187–96.
Seah JM, Yao H, MacIsaac RJ, Ekinci EI, Jerums G. Reducing the complications of type 2 diabetes: challenges in individualizing care. Med Today 2014;15:37–47.
Selwitz RH, Pihlstrom BL. How to lower risk of developing diabetes and its complications: recommendations for the patient. J Am Dent Assoc 2003;134:54S–58S.
Shank CF, Feibel JB. Osteomyelitis in the diabetic foot: diagnosis and management. Foot Ankle Clin 2006;11:775–89.
Sharma T, Cronkright P, Phillips L, Loftus,T. Residents Plan-Do Group Visits for Diabetes Management. 33rd Annual Meeting of the Society of General Internal Medicine, Minneapolis, MN, USA, 28 April–1 May 2010.
Sherifali D. Diabetes coaching for individuals with type 2 diabetes: a state-of-the-science review and rationale for a coaching model. J Diabetes 2017;9:547–54.
Shrivastava U, Misra A. Need for ethnic-specific guidelines for prevention, diagnosis, and management of type 2 diabetes in South Asians. Diabetes Technol 2015;17:435–39.
Shrivastava U, Misra A, Gupta R, Viswanathan V. Socioeconomic factors relating to diabetes and its management in India. J Diabetes 2016;8:12–23.
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28.
Spencer J, Cooper H, Milton B. Qualitative studies of type 1 diabetes in adolescence: a systematic literature review. Pediatr Diabetes 2010;11:364–75.
Srulovici E, Kay C, Rotem M, Golfenshtein N, Balicer R, Shadmi E. Diabetes Conversation Maps and Health Outcomes: a Systematic Literature Review. International Society for Pharmacoeconomics and Outcomes Research 18th Annual European Congress, Milan, Italy, 7–11 November 2015.
Stengel D, Graninger W. Antimicrobial treatment of diabetic foot infections. Effects of primary and secondary prevention measures on amputation rates. J Chemother 2005;14:191–7.
Stolt M, Suhonen R, Voutilainen P, LeinoKilpi H. Foot health in older people and the nurses’ role in foot health care-a review of literature. Scand J Caring Sci 2010;24:194–201.
Stuart L and Wiles P. The role of patient education in preventing foot ulcers: the elusive Pimpernel? Evid Based Healthc Public Health 2005;9:359–60.
Tabrizi JS, Wilson AJ, Coyne ET, O’Rourke PK. Review of patient-reported type 2 diabetes service quality. Aust Health Rev 2008;32:23–33.
Tang, ZQ, Chen HL, Zhao FF. Gender differences of lower extremity amputation risk in patients with diabetic foot: a meta-analysis. Int J Low Extrem Wounds 2014;13:197–204.
Telfer S, Erdemir A, Woodburn J, Cavanagh PR. What has finite element analysis taught us about diabetic foot disease and its management? A systematic review. PLOS ONE 2014;9:e109994.
Torsello G, Debus S, Meyer F, Grundmann RT. Vascular medicine needs more evidence: recent results and meta-analyses for the treatment of diabetic feet. Zentralbla Chir 2015;140:219–27.
Uckay I, Aragon Sanchez J, Lew D, Lipsky BA. Diabetic foot infections: what have we learned in the last 30 years?. Int J Infect Dis 2015;40:81–91.
Unwin N. The diabetic foot in the developing world. Diabetes Metab Res Rev 2008;24(Suppl. 1):S31–3.
Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017;356:i6505.
Valk GD, Kriegsman DMW, Assendelft WJJ. Patient education for preventing diabetic foot ulceration: a systematic review. Endocrinol Metab Clin North Am 2002;31:633–58.
van Acker K, Leger P, Hartemann A, Chawla A, Siddiqui MK. Burden of diabetic foot disorders, guidelines for management and disparities in implementation in Europe: a systematic literature review. Diabetes Metab Res 2014;30:635–45.
van den Berg M, de Wit GA, Vijgen SM, Busch MC, Schuit AJ. Cost-effectiveness of prevention: opportunities for public health policy in the Netherlands. Ned Tijdschr Geneeskd 2008;152:1329–34.
Vijgen SM, Hoogendoorn M, Baan CA, de Wit GA, Limburg W, Feenstra TL. Cost effectiveness of preventive interventions in type 2 diabetes mellitus: a systematic literature review. PharmacoEconomics 2006;24:425–41.
Wheatley C. Audit protocol: part one: prevention of diabetic foot ulcers – the non-complicated foot. J Clin Govern 2001;9:93–100.
Younes NA, Ahmad AT. Diabetic foot disease. Endocr Pract 2006;12:583–92.
Data Extraction and Quality Assessment Sheet (DEQA): overview
Appendix 5 Chapter 5-related appendices
Search strategies
| # | Searches |
|---|---|
| 1. | MeSH descriptor: [Foot Orthoses] explode all trees |
| 2. | MeSH descriptor: [Shoes] explode all trees |
| 3. | MeSH descriptor: [Health Education] explode all trees |
| 4. | MeSH descriptor: [Primary Health Care] explode all trees |
| 5. | MeSH descriptor: [Emollients] explode all trees |
| 6. | insole* |
| 7. | footwear* |
| 8. | educat* |
| 9. | specialist car* |
| 10. | multi disciplinary team* |
| 11. | multidisciplinary team* |
| 12. | routine podiatry car* |
| 13. | MeSH descriptor: [General Practice] explode all trees |
| 14. | MeSH descriptor: [Community Health Services] explode all trees |
| 15. | off load* |
| 16. | offload* |
| 17. | emollient* |
| 18. | shoe* |
| 19. | {or #1-#18} |
| 20. | MeSH descriptor: [Foot] explode all trees |
| 21. | MeSH descriptor: [Foot Diseases] explode all trees |
| 22. | MeSH descriptor: [Diabetic Foot] explode all trees |
| 23. | MeSH descriptor: [Diabetic Neuropathies] explode all trees |
| 24. | MeSH descriptor: [Diabetes Mellitus] explode all trees |
| 25. | MeSH descriptor: [Diabetic Angiopathies] explode all trees |
| 26. | MeSH descriptor: [Diabetes Complications] explode all trees |
| 27. | MeSH descriptor: [Podiatry] explode all trees |
| 28. | MeSH descriptor: [Foot Ulcer] explode all trees |
| 29. | MeSH descriptor: [Skin Ulcer] explode all trees |
| 30. | MeSH descriptor: [Ischemia] explode all trees |
| 31. | MeSH descriptor: [Bacterial Infections] explode all trees |
| 32. | diabet* near/3 ulcer* |
| 33. | diabet* near/3 (foot or feet) |
| 34. | diabet* near/3 wound* |
| 35. | diabet* near/3 amputat* |
| 36. | {or #20-#35} |
| 37. | #19 and #36 |
| # | Searches |
|---|---|
| 1. | exp Foot Orthosis/ |
| 2. | exp Shoe/ |
| 3. | exp health education/ |
| 4. | exp primary health care/ |
| 5. | emollient agent/ |
| 6. | insole*.mp. |
| 7. | footwear*.mp. |
| 8. | educat*.mp. |
| 9. | specialist car*.mp. |
| 10. | multi disciplinary team*.mp. |
| 11. | multidisciplinary team*.mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] |
| 12. | routine podiatry car*.mp. |
| 13. | exp general practice/ |
| 14. | exp community care/ |
| 15. | off load*.mp. |
| 16. | offload*.mp. |
| 17. | emollient*.mp. |
| 18. | shoe*.mp. |
| 19. | or/1-18 |
| 20. | exp Foot/ |
| 21. | exp Foot Disease/ |
| 22. | exp Diabetic Foot/ |
| 23. | exp Diabetic Neuropathy/ |
| 24. | exp Diabetes Mellitus/ |
| 25. | exp Diabetic Angiopathy/ |
| 26. | (diabet* adj3 complicat*).mp. |
| 27. | exp Podiatry/ |
| 28. | exp Foot Ulcer/ |
| 29. | exp Skin Ulcer/ |
| 30. | exp Ischemia/ |
| 31. | exp Bacterial Infection/ |
| 32. | (diabet* adj3 ulcer*).mp. |
| 33. | (diabet* adj3 (foot or feet)).mp. |
| 34. | (diabet* adj3 wound*).mp. |
| 35. | (diabet* adj3 amputat*).mp. |
| 36. | or/20-35 |
| 37. | systematic* review*.mp. |
| 38. | meta analysis/ |
| 39. | (meta-analytic* or meta-analysis or metanalysis or metaanalysis or meta analysis or meta synthesis or meta-synthesis or metasynthesis or meta-regression or metaregression or meta regression).mp. |
| 40. | (synthes* adj3 literature).mp. |
| 41. | (synthes* adj3 evidence).mp. |
| 42. | (integrative review or data synthesis).mp. |
| 43. | (research synthesis or narrative synthesis).mp. |
| 44. | (systematic study or systematic studies).mp. |
| 45. | (systematic comparison* or systematic overview*).mp. |
| 46. | ((evidence based or comprehensive or critical or quantitative or structured) adj review).mp. |
| 47. | (realist adj (review or synthesis)).mp. |
| 48. | or/37-47 |
| 49. | review.pt. |
| 50. | (medline or pubmed or embase or cinahl or psyc?lit or psyc?info).ab. |
| 51. | ((literature or database* or bibliographic or electronic or computeri?ed or internet) adj3 search*).mp. |
| 52. | (electronic adj3 database*).mp. |
| 53. | included studies.ab. |
| 54. | (inclusion adj3 studies).ab. |
| 55. | ((inclusion or selection or predefined or predetermined) adj criteria).ab. |
| 56. | (assess* adj3 (quality or validity)).ab. |
| 57. | (select* adj3 (study or studies)).ab. |
| 58. | (data adj3 extract*).ab. |
| 59. | extracted data.ab. |
| 60. | (data adj3 abstraction).ab. |
| 61. | published intervention*.ab. |
| 62. | ((study or studies) adj2 evaluat*).ab. |
| 63. | (intervention* adj2 evaluat*).ab. |
| 64. | (confidence interval* or heterogeneity or pooled or pooling or odds ratio*).ab. |
| 65. | (Jadad or coding).ab. |
| 66. | or/50-65 |
| 67. | 49 and 66 |
| 68. | review.ti. |
| 69. | 66 and 68 |
| 70. | (review* adj4 (papers or trials or studies or evidence or intervention* or evaluation*)).mp. |
| 71. | 48 or 67 or 69 or 70 |
| 72. | letter.pt. |
| 73. | editorial.pt. |
| 74. | or/72-73 |
| 75. | 71 not 74 |
| # | Searches |
|---|---|
| 1. | exp Foot Orthoses/ |
| 2. | exp Shoes/ |
| 3. | exp health education/ |
| 4. | exp primary health care/ |
| 5. | exp Emollients/ |
| 6. | insole*.mp. |
| 7. | footwear*.mp. |
| 8. | educat*.mp. |
| 9. | specialist car*.mp. |
| 10. | multi disciplinary team*.mp. |
| 11. | multidisciplinary team*.mp. |
| 12. | routine podiatry car*.mp. |
| 13. | exp general practice/ |
| 14. | exp community health services/ |
| 15. | off load*.mp. |
| 16. | offload*.mp. |
| 17. | emollient*.mp. |
| 18. | shoe*.mp. |
| 19. | or/1-18 |
| 20. | exp Foot/ |
| 21. | exp Foot Diseases/ |
| 22. | exp Diabetic Foot/ |
| 23. | exp Diabetic Neuropathies/ |
| 24. | exp Diabetes Mellitus/ |
| 25. | exp Diabetic Angiopathies/ |
| 26. | exp Diabetes Complications/ |
| 27. | exp Podiatry/ |
| 28. | exp Foot Ulcer/ |
| 29. | exp Skin Ulcer/ |
| 30. | exp Ischemia/ |
| 31. | exp Bacterial Infections/ |
| 32. | (diabet* adj3 ulcer*).mp. |
| 33. | (diabet* adj3 (foot or feet)).mp. |
| 34. | (diabet* adj3 wound*).mp. |
| 35. | (diabet* adj3 amputat*).mp. |
| 36. | or/20-35 |
| 37. | systematic* review*.mp. |
| 38. | meta-analysis as topic/ |
| 39. | (meta-analytic* or meta-analysis or metanalysis or metaanalysis or meta analysis or meta synthesis or meta-synthesis or metasynthesis or meta-regression or metaregression or meta regression).mp. |
| 40. | (synthes* adj3 literature).mp. |
| 41. | (synthes* adj3 evidence).mp. |
| 42. | (integrative review or data synthesis).mp. |
| 43. | (research synthesis or narrative synthesis).mp. |
| 44. | (systematic study or systematic studies).mp. |
| 45. | (systematic comparison* or systematic overview*).mp. |
| 46. | ((evidence based or comprehensive or critical or quantitative or structured) adj review).mp. |
| 47. | (realist adj (review or synthesis)).mp. |
| 48. | or/37-47 |
| 49. | review.pt. |
| 50. | (medline or pubmed or embase or cinahl or psyc?lit or psyc?info).ab. |
| 51. | ((literature or database* or bibliographic or electronic or computeri?ed or internet) adj3 search*).mp. |
| 52. | (electronic adj3 database*).mp. |
| 53. | included studies.ab. |
| 54. | (inclusion adj3 studies).ab. |
| 55. | ((inclusion or selection or predefined or predetermined) adj criteria).ab. |
| 56. | (assess* adj3 (quality or validity)).ab. |
| 57. | (select* adj3 (study or studies)).ab. |
| 58. | (data adj3 extract*).ab. |
| 59. | extracted data.ab. |
| 60. | (data adj3 abstraction).ab. |
| 61. | published intervention*.ab. |
| 62. | ((study or studies) adj2 evaluat*).ab. |
| 63. | (intervention* adj2 evaluat*).ab. |
| 64. | (confidence interval* or heterogeneity or pooled or pooling or odds ratio*).ab. |
| 65. | (Jadad or coding).ab. |
| 66. | or/50-65 |
| 67. | 49 and 66 |
| 68. | review.ti. |
| 69. | 66 and 68 |
| 70. | (review* adj4 (papers or trials or studies or evidence or intervention* or evaluation*)).mp. |
| 71. | 48 or 67 or 69 or 70 |
| 72. | letter.pt. |
| 73. | editorial.pt. |
| 74. | comment.pt. |
| 75. | or/72-74 |
| 76. | 71 not 75 |
| 77. | exp animals/not humans/ |
| 78. | 76 not 77 |
| 79. | 19 and 36 and 78 |
Search was carried out on 21 February 2019.
| Number | Title | Status | Study results | Conditions | Interventions | Locations |
|---|---|---|---|---|---|---|
| 1 | Patients with diabetic neuropathy who receive physiotherapy treatment will have a decrease in diabetic foot ulcers | Not yet recruiting | No results available | DFU, diabetic neuropathy peripheral | Other: physiotherapy protocol | |
| 2 | Foot intervention study utilizing commercially available infrared thermometers with individuals with diabetes | Recruiting | No results available |
Diabetic foot Device: education and thermometer group |
Other: education only group | Memorial University, St John’s, NL, Canada |
| 3 | Preventing diabetic foot ulcers through cleaner feet | Enrolling by invitation | No results available | DFU | Drug: chlorhexidine | Baltimore Veterans Affairs Medical Center Veterans Affairs Maryland Health Care System, Baltimore, MD, USA |
| 4 | Diabetic foot ulcer recurrence: pilot study | Recruiting | No results available | Diabetic foot, ulcer foot | Other: no interventions | Indiana University Health Methodist Hospital, Indianapolis, IN, USA |
| 5 | Abnormal plantar pressure in patients with diabetes | Completed | No results available | Diabetes complications | Other: retrospective observational study with no intervention | Department and Division of Medical Rehabilitation, Wroclaw, Lower Silesia, Poland |
| 6 | Reliability of a diabetic foot ulcer risk stratification and referral algorithm | Completed | No results available | Foot ulcer, diabetic | St Joseph’s Healthcare Primary Care Diabetes Support Program, St Joseph’s Healthcare Family Medical and Dental Centre, London, ON, Canada | |
| 7 | Predictors of skin temperature, plantar pressure and ulceration in diabetic foot patients | Enrolling by invitation | No results available | Diabetic foot, diabetes | ||
| 8 | Offloading interventions for diabetic foot problems in upper Egypt | Not yet recruiting | No results available | DFU |
Device: cast shoe Device: forefoot offloading shoe |
|
| 9 | Pressure and diabetic foot | Recruiting | No results available | Diabetes and risk of DFUs | Other: measure of cutaneous microcirculation | Service d’endocrinologie, Centre Hospitalier Lyon Sud, Pierre-Bénite, France |
| 10 | Foot assessment in people with diabetes: a quantitative diagnostic approach | Recruiting | No results available | Diabetes, diabetic foot, diabetes complications, diabetes; neuropathic (manifestation) | Diagnostic test: observation | Staffordshire University, Stoke-on-Trent, Staffordshire, UK |
| 11 | Novel offloading for diabetic foot ulcers with pulseflow: a prospective study | Recruiting | No results available | DFU, offloading | Device: offloading boot: pulse flow | Baylor College of Medicine, Houston, TX, USA |
| 12 | Prevention of amputation in diabetic foot ulcers using amniotic tissue | Active, not recruiting | No results available | DFU | Boise Veterans Affairs Medical Center, Boise, ID, USA | |
| 13 | Pressure-sensing insoles in the neuropathic ulcer treatment pathway | Recruiting | No results available | Diabetes, diabetes complications, neuropathy, peripheral neuropathy, DFU | Device: SurroSense Rx™ System (Orpyx Medical Technologies Inc., Calgary, AB, Canada) | Zivot Limb Preservation Centre – Peter Lougheed Centre, Calgary, AB, Canada |
| 14 | Diabetic Foot Ulcer Prevention System (DFUPS) – part 2 | Unknown status | No results available | Diabetic foot | Device: DFUPS |
King’s College Hospital, London, UK The Pennine Acute Hospitals NHS Trust, Manchester, UK The Newcastle upon Tyne Hospitals, Newcastle upon Tyne, UK |
| 15 | Implementation of foot thermometry and sms and voice messaging to prevent diabetic foot ulcer | Completed | No results available | Diabetic foot | Behavioural: SMS and voice messaging device: thermometry |
Hospital Cayetano Heredia, Lima, Peru Hospital Nacional Arzobispo Loayza, Lima, Peru |
| 16 | Diabetic foot ulcer prevention system | Completed | No results available | Diabetic foot | Device: DFUPS |
King’s College Hospital, London, UK The Pennine Acute Hospitals NHS Trust, Manchester, UK The Newcastle upon Tyne Hospitals, Newcastle upon Tyne, UK |
| 17 | Randomized, prospective evaluation of the toad brace in plantar ulcer off-loading and healing | Unknown status | No results available | Diabetic foot, pedal ulcers |
Device: optimal medical therapy Debridement + toad brace Other: optimal medical therapy – debridement |
University Hospitals Cleveland Medical Center, Cleveland, OH, USA |
| 18 | Off-loading shoe to improve healing and prevention of recurrence of neuropathic diabetic plantar foot ulcers | Completed | No results available | Diabetes mellitus, diabetic neuropathic foot ulcer |
Device: Sanidiab Device: Barouk |
Groupe hospitalier Pitié-Salpêtrière, Paris, France |
| 19 | Prevention of secondary foot ulcers in patients with diabetes using systematic measuring of skin temperature | Completed | No results available | Foot ulcer, diabetic |
Device: Temp Touch (Diabetica Solutions Inc., San Antonio, TX, USA) Other: Inspection |
Oslo University Hospital Ulleval, Oslo, Norway |
| 20 | Developing a diabetic foot ulcer protocol | Unknown status | No results available | DFU |
Other: type of footwear Other: collagen dressing with and without silver |
Harris County Hospital District Community Health, Houston, TX, USA |
| 21 | A comparison of insoles used to prevent neuropathic diabetic foot ulceration | Completed | No results available | Diabetes, neuropathic foot | Device: insole |
Liskeard Community Hospital, Liskeard, UK Mount Gould Local Care Centre, Plymouth, UK |
| 22 | Shear and pressure reducing insoles for the diabetic foot | Completed | No results available | Diabetes, ulceration, amputation, foot deformity, neuropathy |
Device: pressure-reducing insole Device: GlideSoft® |
Kevin R Higgins, Doctor of Podiatric Medicine, San Antonio, TX, USA |
| 23 | Evaluating the effects of foot orthotics on plantar pressures in people with diabetes | Completed | No results available | Diabetes | Other: no intervention |
Texas Diabetes Institute, San Antonio, TX, USA South Texas Veterans Healthcare System, San Antonio, TX, USA |
| 24 | FDG-PET imaging in complicated diabetic foot | Active, not recruiting | No results available | Diabetic foot disease | Procedure: FDG-PET imaging | Hospital of the University of Pennsylvania, PA, USA |
Systematic review: flow diagram
List of randomised controlled trials: full text unavailable
| 1. Higgins – unpublished |
| 2. Huang 2010 – sent request for full text to no avail |
| 3. Fan 2006 – sent request for full text to no avail |
| 4. Souellier 1986 – not available at The British Library |
| 5. Tyrell 1998 – not available at The British Library |
| 6. Van Putten 2010 – conference abstracts only |
| 7. Veitenhansl 2001 – conference abstracts only |
| 8. Veitenhansl 2003a – not available at The British Library |
| 9. Veitenhansl 2003b – not available at The British Library |
| 10. Veitenhansl 2004 – not available at The British Library |
| 11. Xiaomin 2010 – sent request for full text to no avail |
| 12. Xue-hua 2010 – sent request for full text to no avail |
| 13. Zhengguang 2008 – sent request for full text to no avail |
| 14. Zhenghua 2011 – Dorresteijn excluded because no full text |
-
Higgins KR, Lavery LA, Athanasiou KA, Lanctot DR, Costantinidis GP, Agrawal CM, et al. Personal communication with Arad Y.
-
Huang P. Effect of diabetes education on prevention of diabetic foot. Chinese journal of multiple organ diseases in the elderly 2010;33:362.
-
Lifeng F, Zheng L, Ju-ming L, Zheng Y. Strengthening the long-term effect of educational intervention on prevention of diabetic foot. Chin J Mul Organ Dis 2006;5:24–9.
-
Soulier SM. The use of running shoes in the prevention of plantar diabetic ulcers. J Am Podiatr Med Assoc 1986;76:395–400.
-
Tyrell W, Philips C, Gibby O, Price P. An Investigation into the Therapeutic Effectivness and Cost-Effectiveness of Othortic Therapy Provided for Those Attending the Diabetic Foot Clinicat Richmond House Diabetes Centre, Royal Gwent Hospital, Newport. Report prepared for the Wales Office of Research and Development for Health and Social Care 1998.
-
Van Putten M, Leffers P, Schaper NC. Podiatric Insoles Cause Foot Ulcers in Diabetic Patients. 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
-
Veitenhansl M, Stegner K, Hierl FX, Dieterle C, Feldmeier H, Gutt B, et al. Special pre-manufactured footwear with insoles can prevent ulceration in diabetic patients with diabetic foot syndrome by pressure reduction. A prospective randomised study. Diabetologia 2004;47:A1–A464.
-
Veitenhansl M, Hierl FX, Landgraf R. Pressure reduction through various premanufactured shoe models with insoles in diabetic foot syndrome to prevent ulceration: a prospective randomised study. Diabetologia 2003;46:A4–A5.
-
Veitenhansl M, Stegner K, Hierl EX, Dieterle C, Feldmeier H, Gutt B. Special pre-manufactured footwear can prevent ulceration in diabetic patients with diabetic foot syndrome. Diabetologia 2003;46:6.
-
Veitenhansl M, Stegner K, Hierl FX, Dieterle C, Feldmeier H, Gutt B, et al. Special pre-manufactured footwear with insoles can prevent ulceration in diabetic patients with diabetic foot syndrome by pressure reduction: a prospective randomised study. Diabetologia 2004;47:A3.
-
Xiaomin L, Jianning W. The significance of individualized educational intervention in preventing diabetic foot. J Med Sci 2010;20:212–13.
-
Xue-hua H. Effect of diabetes education on prevention of diabetic foot. Chin For Health Dig 2010;33:362.
-
Zhengguang L, Xiaokui L, Yan L, Hulin C, Yucheng Y, Yuxiong C, Shiwei Z. Evaluation of preventive effect of preventive health education on senile diabetic foot ulcer. Chin J Pract Intern Med 2008;28:68–9.
-
Zhenghua X, Dingyu C, Qiling Y, Qian Z, Jin X, Chunling H, et al. Individualised Diabetic Education Can Contribute to Decrease the Incidence of Diabetic Foot and Avoid Amputation: Results of a 9-year Prospective Study. 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
List of excluded studies, with reasons
| First author and year | Reason for exclusion | |
|---|---|---|
| 1. | Barth 1991150 | Not all participants ulcer free at baseline |
| 2. | Bloomgarden 198783 | Not all participants ulcer free at baseline |
| 3. | Borges 2004151 | No DFU outcome |
| 4. | Borges 2008152 | No DFU outcome |
| 5. | Colagiuri 1995153 | No DFU outcome |
| 6. | Corbett 2003154 | No DFU outcome |
| 7. | Deakin 2006155 | No DFU outcome |
| 8. | Donaghue 1996 | No DFU outcome |
| 9. | Donohoe 2000156 | No DFU outcome |
| 10. | Frank 2003157 | No DFU outcome |
| 11. | Frank 2005158 | No DFU outcome |
| 12. | Huang 2009159 | Not all participants ulcer free at baseline |
| 13. | Jeffcoate 2007 | Not all participants ulcer free at baseline |
| 14. | Kruger 1992160 | No DFU outcome |
| 15. | Malone 1989 | Not all participants ulcer free at baseline |
| 16. | Mazzuca 1986161 | No DFU outcome |
| 17. | McMurray 2002162 | No DFU outcome |
| 18. | Mueller 1989163 | Treatment study |
| 19. | Mueller 2003164 | Not all participants ulcer free at baseline |
| 20. | Piaggesi 1998165 | Treatment study |
| 21. | Pieber 1995166 | Not a RCT |
| 22. | Reiber 1997 | Not a RCT |
| 23. | Reichard 1993167 | Purpose of RCT was not to evaluate intervention to prevent DFUs |
| 24. | Rettig 1986168 | No DFU outcome |
| 25. | Rönnemaa 199781 | No DFU outcome |
| 26. | Spraul 2015 | Not a RCT |
| 27. | Tazi 2008 | Not a RCT |
| 28. | Viswanathan 2004 | Not a RCT |
| 29. | Waxman 2003107 | Not diabetic patients |
| 30. | Weintraub 2003169 | Treatment study |
| 31. | Westphal 2011 | Not all participants ulcer free at baseline |
| 32. | Wooldridge 1994170 | No DFU outcome |
| 33. | Wooldridge 1996171 | No DFU outcome |
-
Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance, and foot problems in type 2 diabetes. Diabetic Med 1991;8:111–17.
-
Bloomgarden ZT, Karmally W, Metzger MJ. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10:263–72.
-
Borges W. The Impact of a Brief Foot Care Intervention for Persons With Diabetes. Texas Medical Center Dissertations, University of San Francisco; 2004.
-
Borges WJ, Ostwald SK. Improving foot self-care behaviors with Pies Sanos. West J Nurs Res 2008;30:325–41.
-
Colagiuri S, Marsden L, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract 1995;28:29–34.
-
Corbett CFC. A randomized pilot study of improving foot care in home health patients with diabetes. Diabetes Educ 2003;29:269–70.
-
Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT Programme makes a difference. Diabet Med 2006;23:944–54.
-
Donaghue VM, Sarnow MR, Giurini JM, Chrzan JS, Habershaw GM, Veves A. Longitudinal in-shoe foot pressure relief achieved by specially designed footwear in high risk diabetic patients. Diabetes Res Clin Pract 1996;31:109–14.
-
Donohoe ME, Fletton JA, Hook A, Powell R, Robinson I, Stead JW, et al. Improving foot care for people with diabetes mellitus – a randomized controlled trial of an integrated care approach. Diabetic Med 2000;17:581–7.
-
Frank KI. Self-management of foot care for patients 65 years of age or older with diabetes. Dissertation Abstracts International 2003;64:4863.
-
Frank KI, Martin J, Bennett SJ. Self management of foot care for patients 65 years of age or older with diabetes: D132. J Am Geriatr Soc 2005;53(Suppl. 1):S215.
-
Huang P, Huang JM, Li GR. The effect observations of the intensified education on diabetic knowledge for the prevention of diabetic foot. Modern Preventive Medicine 2009;15:38.
-
Jeffcoate W, Radford K, Ince P, Smith M, Game F, Lincoln N. Randomised controlled trial of education in the prevention of foot ulcer recurrence in diabetes. Diabetologia 2007;50:S457–S458.
-
Kruger S, Guthrie D. Foot care: knowledge retention and self-care practices. Diabetes Educ 1992;18:487–90.
-
Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway Jr GA, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg 1989;158:520–4.
-
Mazzuca SA, Moorman NH, Wheeler ML. The Diabetes Education Study: a controlled trial of the effects of diabetes patient education. Diabetes Care 1986;9:1–10.
-
McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002;40:566–75.
-
Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair III VP, Drury DA, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care 1989;12:384–8.
-
Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am 2003;85:1436–45.
-
Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, et al. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabetic Med 1998;15:412–17.
-
Pieber TR, Holler A, Siebenhofer A, Brunner GA, Semlitsch B, Schattenberg S, et al. Evaluation of a structured teaching and treatment programme for type 2 diabetes in general practice in a rural area of Austria. Diabetic Med 1995;12:349–54.
-
Reiber GE, Smith DG, Boone DA, del Aguila M, Borchers RE, Mathews D, et al. Design and pilot testing of the DVA/Seattle Footwear System for diabetic patients with foot insensitivity. J Rehabil Res Dev 1997;34:1–8.
-
Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304–9.
-
Rettig BA, Shrauger DG, Recker RR, Gallagher TF, Wiltse H. A randomized study of the effects of a home diabetes education program. Diabetes Care 1986;9:173–8.
-
Rönnemaa T, Hamalainen H, Toikka T, Liukkonen I. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care 1997;20:1833–7.
-
Spraul S. Special foot padding to prevent recurrent ulcers. MMW Fortschr Med 2015;157:35.
-
Tazi O, Debure C. [Preventing high-risk diabetic foot ulceration by a new method of custom-made shoes in high-risk patients. Prospective study.] J Mal Vasc 2008;33:191–5.
-
Viswanathan V, Madhavan S, Gnanasundaram S, Gopalakrishna G, Nath Das B, Rajasekar S, et al. Effectiveness of different types of footwear insoles for the diabetic neuropathic foot: a follow-up study. Diabetes Care 2004;27:474–7.
-
Waxman R, Woodburn H, Powell M, Woodburn J, Blackburn S, Helliwell P. FOOTSTEP: a randomized controlled trial investigating the clinical and cost effectiveness of a patient self-management program for basic foot care in the elderly. J Clin Epidemiol 2003;56:1092–9.
-
Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, et al. Magnetic Research Group. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil 2003;84:736–46.
-
Westphal C, Neame IM, Harrison JC, Bower VM, Gurr JM. A diabetic foot ulcer pilot study: does silicone gel sheeting reduce the incidence of reulceration? J Am Podiatr Med Assoc 2011;101:116–23.
-
Wooldridge J, Bergeron J, Thornton C. Preventing diabetic foot disease: lessons from the medicare therapeutic shoe demonstration. Am J Public Health 1996;86:935–8.
-
Wooldridge J, Moreno L. Evaluation of the costs to medicare of covering therapeutic shoes for diabetic patients. Diabetes Care 1994;17:541–7.
Data extraction and quality assessment: systematic review of randomised controlled trials
Appendix 6 Chapter 6 health economics-related appendices
Literature review of cost–utility analyse
Aim
To undertake a review of published cost–utility analyses of the prevention of DFUs.
Population
Studies including patients with a diagnosis of diabetes mellitus who were at risk of DFU.
Intervention
Any intervention aimed at preventing DFUs.
Comparator
Any comparator.
Criterion for inclusion of studies
Any study that included both costs and a measure of health utility relating to an intervention aimed at preventing DFUs.
Search strategy
Our search strategy involved the combination of terms: (1) diabetic patients, (2) with foot/plantar ulcers/lesions and (3) cost-effectiveness/utility models. We searched MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Cochrane Economic Evaluation Database. Only studies in the English language were included owing to resource constraints. The databases were searched from inception to January 2018. No start date was chosen as we were not concerned with whether or not the treatments involved were still clinically relevant; rather, we were interested in understanding the different modelling approaches used, for which there was no specific timespan of interest.
Results
Our search returned 11 relevant papers to be reviewed. Four other relevant papers were identified by ‘hand-searching’ the references of the 11 papers identified. Of the 15 papers identified in our review, five were cost–utility analyses of the prevention of DFUs. The remaining papers were either concerned with the cost–utility of treatments for patients who had already experienced DFUs or related to the cost burden of DFUs.
Table 52 provides details of the economic study characteristics. Figure 53 is the PRISMA flow diagram of the literature selection process.
| Study (first author and year) | Type of analysis | Intervention | Measure of effectiveness | Evidence for effectiveness | Costs | Utilities | Results (ICERs) |
|---|---|---|---|---|---|---|---|
| Eastman 1997121 | Cost–utility, using Monte Carlo simulation | Glycaemic control (average HbA1c level of 7.2% maintained for life) | Reduction in LEAs | Based on US community and population data on incidence of NIDDM complications | Javitt et al.;173 Brechner et al.;174 and the DCCT Study Group.175 Reported in US dollars | Eckman et al.176 | US$16,003 per QALY gained |
| Tennvall 2000172 | Cost–utility, using a Markov model | Education, specialised footwear, multidisciplinary foot care team | Reduction in DFUs | Reduced by 25%, based on Malone et al.82 and McCabe et al.84 | Apelqvist et al.;177 Apelqvist et al.;178 and Tennvall.172 Reported in euros | UK Prospective Diabetes Study Group;179 and Tennvall et al.172 | Varied across risk groups, but all < €6000 per QALY gained |
| Ortegon 2004123 | Cost–utility, using a Markov model | Glycaemic control, specialised foot care, education, regular inspection, identification of risk, multidisciplinary team | Reduction in LEAs | Reduction of between 49% and 85%, based on Larsson et al.180 | Redekop et al.132 Reported in US dollars | Redekop et al.132 | < US$25,000 per QALY gained |
| Rauner 2005124 | Cost–utility, using a Markov model | Specialised footwear, patient education, chiropody, and regular checks by general practitioners and/or in hospital | Reduction in DFUs | Reduction of between 25% and 50%, based on Malone et al.82 and Boulton et al.181 | Costs were obtained from literature review and clinical expert (references not given). Reported in euros | Tennvall et al.172 | Varied across risk groups. Risk 1, not cost-effective; risk 2, cost-effective; risks 3 and 4, cost-effective and cost saving |
| Barshes 2017125 | Cost–utility, using a Markov model | Primary prevention (would care, digital substraction angiography, revascularisation) | Reduction in DFUs | N/A | Barshes et al.182 Reported in US dollars | Barshes et al.126 | Cost and effectiveness estimates required for cost saving |
FIGURE 53.
Flow diagram of search process.

Survival analysis of Scottish Care Information – Diabetes Collaboration data
Table 53 provides our rationale about the extrapolation of ulceration, amputation and death event data from SCI-Diabetes.
| Transition(s) | Parametric model choice | Diagnostic tests used | Reasons |
|---|---|---|---|
| Transitions 1–3: time to death conditional on CPR state (Figure 54) | Log-logistic | AIC/BIC, KM plot, log-CM plot | Lowest AIC/BIC; does not appear contradicted by KM or log-CM plots |
| Transition 4: time from ulceration to amputation | Gompertz | AIC/BIC, KM plot, parametric model plot | Lowest AIC/BIC; does not appear contradicted by KM plot; visually fits well to Gompertz model |
| Transition 5: time from ulceration to death | Weibull | AIC/BIC, KM plot, parametric model plot | Lowest AIC/BIC; does not appear contradicted by KM plot; visually fits well to Weibull model |
| Transition 6: time from amputation to death | Log-normal | AIC/BIC, KM plot, parametric model plot | Lowest AIC/BIC; does not appear contradicted by KM plot; visually fits well to log-normal model |
| Transitions 7: time from low to moderate risk (Figure 55) | Gompertz | AIC/BIC, KM plot, parametric model plot | Gompertz has lowest AIC/BIC; does not appear contradicted by KM plot; visually fits very well to log-normal model |
| Transitions 8: time from low to high risk | N/A | 0 and 2 failures, respectively; do not think we can fit model | |
| Transitions 9: time from moderate to high risk (Figure 56) | Gompertz | AIC/BIC, KM plot, parametric model plot | Lowest AIC/BIC, does not appear contradicted by KM plot; visually fits well to Gompertz model |
| Transitions 10–12: time to ulceration conditional on CPR state (Figure 57) | Log-logistic | AIC/BIC, KM plot, log-CM plot | Lowest AIC/BIC; does not appear contradicted by KM or log-CM plot. Hazard appears to increase initially and then decease (hence require flexibility) |
| Transitions 13–15: time to amputation conditional on CPR state (Figure 58) | Gompertz | AIC/BIC, KM plot, log-CM plot | Lowest AIC/BIC; does not appear contradicted by KM or log-CM plots |
Kaplan–Meier plots for a selection of the key transitions required for the model are in Figures 54–58.
FIGURE 54.
Kaplan–Meier survival estimates. Time to death, stratified by risk status (CPR) (transitions 1–3).

FIGURE 55.
Kaplan–Meier survival estimates. Time to change from low to moderate risk (transition 7).
FIGURE 56.
Kaplan–Meier survival estimates. Time to change from moderate to high risk (transition 9).
FIGURE 57.
Kaplan–Meier survival estimates. Time to ulceration, stratified by risk status (CPR) (transitions 10–12).
FIGURE 58.
Kaplan–Meier survival estimates. Time to amputation, stratified by risk status (CPR) (transitions 13–15).
Scottish Care Information – Diabetes Collaboration missing data and assumptions
Table 54 presents the name, number of observations and proportion of missing data for each variable in the SCI-Diabetes data set, which we received from the Health Informatics Centre at the University of Dundee via the University of Edinburgh, where the data were cleaned. The fact that there is more than one observation per patient influences the interpretation of the proportion of missing data. Our discussions with clinicians who were involved in entering data into SCI-Diabetes suggest that some clinicians may input patient information only when a risk is present, for example highlighting active ulcer or amputation when it occurs, and not input this as negative at visits prior to that time. For example, in the case of variables such as active ulcer or amputation, a patient may have 10 visits and be negative for both conditions at the first nine and then develop one or the other (or both) by the final visit. This would result in nine missing observations and one observation recorded. Table 55 lists the variables that were created by the University of Glasgow (the SCI-Diabetes NHS Fife data dictionary).
| Variable name | Description | Observationsa (n) | Missing (%) |
|---|---|---|---|
| PROCHI (MISUMI Europa GmbH, Frankfurt am Main, Germany) | Patient ID | 231,846 | N/A |
| Date | Date of visit | 231,846 | 0 |
| DoDeath | Date of death | 57,289 | 0 |
| Death | Binary variable for death | 57,289 | 0 |
| PeripheralPulsesLeft | Peripheral pulses absent, left foot | 197,195 | 15 |
| PeripheralPulsesRight | Peripheral pulses absent, left foot | 197,543 | 15 |
| MonofilamentLeftSites | Insensitive to monofilament, left foot | 145,084 | 37 |
| MonofilamentRightSites | Insensitive to monofilament, right foot | 145,088 | 37 |
| PreviousUlcerLeft | Previous ulcer, left foot | 94,675 | 59 |
| PreviousUlcerRight | Previous ulcer, right foot | 94,661 | 59 |
| ActiveUlcerLeft | Ulcer, left foot | 96,406 | 58 |
| ActiveUlcerRight | Ulcer, right foot | 96,454 | 58 |
| UoE_LHS_amput | Amputation, left foot | 38,121 | 84 |
| UoE_RHS_amput | Amputation, right foot | 38,192 | 84 |
| Variable name | Description | Observations (n) | Positive (at risk) (n) |
|---|---|---|---|
| Pulses | 1, Pulses absent in either foot; 0, both present | 231,914 | 5255 |
| Monofilament sensitivity | 1, Absent for either foot; 0, both present | 231,914 | 24,600 |
| History | 1, Previous ulcer on either foot; 0, no history for either foot | 231,914 | 3536 |
| Active ulcer | 1, Active ulcer on either foot, 0, no active ulcer on either foot | 231,914 | 3131 |
| Amputation | 1, Amputation of either foot (or leg); 0, no amputation of either foot (leg) | 231,914 | 1316 |
| CPR low-risk status | Negative for history AND also negative for at least one of monofilaments or pulses | 26,086 | 25,003a |
| CPR moderate-risk status | Positive for history but negative for both monofilaments and pulses OR negative for history but positive for both monofilaments and pulses | 26,086 | 7,13a |
| CPR high-risk status | Positive for history and also positive for at least one of monofilaments and pulses | 26,086 | 370a |
| Survival timeb | Variable for time from time 0 to event of interest | N/A | N/A |
| Event/censoringb | Event/censoring variables, which take the value 1 if the event of interest occurs and 0 otherwise | N/A | N/A |
Assumptions regarding missing data
Owing to the type of missing data, and the lack of other clinical data relating to the patient from which to predict the missing data, imputation methods were not deemed to be appropriate. The following are the assumptions made with regard to missing data.
-
Dates: owing to a lack of death records prior to 2005, we removed patients with any dates before 1 January 2005.
-
CPR data: when no data existed for a patient predictor, we assumed that the patient did not have this risk factor (i.e. if pulses = ’missing’ then pulses = 0 = present). Once a patient is recorded as having a risk factor, then they will have the risk factor for all subsequent visits (i.e. if pulses = 1, then pulses always = 1).
-
Ulceration data: when no data existed for ulceration, we assume that the patient did not have an ulcer. Patients who were recorded as having an ‘active ulcer’ on their first visit were recoded as having a previous ulcer.
-
Amputation data: patients who were recorded as having an amputation on their first visit were recoded as having a previous amputation.
-
Patients with only one visit recorded, if this visit was recorded as ‘death’, were removed from the data set. As no other information is available for these patients, they cannot be included in the survival analysis.
Cost data
The costs of both ulceration and amputation were obtained from the literature. 131 The cost of ulceration was based on the cost of an ‘episode’, that is the mean cost of a single ulceration, weighted by duration of ulceration and the proportion of ulcer treatments that are carried out as an inpatient (10%) or outpatient procedure (90%) (proportions based on expert clinical opinion).
The cost of amputation was similarly estimated as the mean cost of an amputation, including acute and ongoing costs, weighted by the proportion that were major (33.3%) or minor (66.6%) amputations. 143
Footwear and insole costs were based on the advice of clinical experts involved in the treatment of patients at risk of DFUs. Off-the-shelf footwear was estimated at £80 per pair, with a cost of £20 for insoles. Cost estimates were obtained from the surgical appliances manager of NHS Fife, who is in charge of purchasing and negotiating with external providers.
Digital infrared thermometry costs are highly uncertain. We obtained the device cost from a clinical expert who was involved in a clinical trial of digital thermometry among DFU patients in the UK. 89 The cost was estimated at £10, plus a cost of 15 minutes’ face-to-face time with a diabetic foot clinician for advice on usage.
The cost of a complex intervention was based on the advice of clinical experts involved in the treatment of patients at risk of DFUs. We included the cost of footwear and insoles, foot screening with face-to-face education with (30-minute) consultation, consultation with complex intervention team (30 minutes) and a consultation with an Agenda for Change band 8 practitioner, representing vascular surgeons and/or diabetologists (15 minutes). These consultations are assumed to take place quarterly; hence, each consultation cost is multiplied by 4 to give the annual cost.
List of abbreviations
- ABI
- ankle–brachial index
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CPR
- clinical prediction rule
- DFU
- diabetes-related foot ulceration
- EHR
- electronic health record
- EQ-5D
- EuroQol-5 Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- HbA1c
- glycated haemoglobin
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- IWGDF
- International Working Group on Diabetic Foot
- LEA
- lower extremity amputation
- MAR
- missing at random
- MeSH
- medical subject heading
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OR
- odds ratio
- PODUS
- Prediction Of Diabetic foot UlcerationS
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST
- Prediction model Risk Of Bias ASsessment Tool
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROBIS
- risk of bias in systematic reviews
- ROC
- receiver operating characteristic
- RR
- relative risk
- SCI-Diabetes
- Scottish Care Information – Diabetes Collaboration
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SR
- systematic review
- T2DM
- type 2 diabetes mellitus
- VOI
- value of information
- VPT
- vibration perception threshold