Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/196/08. The contractual start date was in August 2014. The draft report began editorial review in January 2020 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. This report has been published following a shortened production process and, therefore, did not undergo the usual number of proof stages and opportunities for correction. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Barker et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The COmmunity based Rehabilitation after Knee Arthroplasty (CORKA) trial was commissioned by the National Institute for Health Research Health Technology Assessment (HTA) programme to address the question ‘What is the clinical and cost-effectiveness of intensive rehabilitation programmes following knee joint replacement for chronic osteoarthritis?’. 1 The commissioning brief specified that the intervention should be intensive rehabilitation that is likely to bridge between hospital and home and should target older adults who have undergone elective knee arthroplasty (KA) who are considered to be at risk of poorer functional outcomes.
The CORKA trial compared the effect of two rehabilitation approaches on a range of functional outcomes: traditional clinic-based therapy and a rehabilitation package delivered by rehabilitation assistants supported by qualified therapists in participants’ homes. This chapter provides background information on KA and rehabilitation approaches.
Background to the problem
Knee osteoarthritis is a common musculoskeletal condition that causes pain and loss of function. It is the most common cause of disability in older people,2 with painful osteoarthritis affecting 18.2% of people over the age of 45 years in the UK. 3 The use of KA for end-stage knee osteoarthritis is an established and effective treatment for patients who have already completed all non-surgical options but continue to experience significant pain and decreased function.
The number of KA operations taking place in the UK is continuing to rise. The 15th Annual National Joint Registry Report (2018)4 showed that over 100,000 KA operations were performed in the UK in 2017. This is an increase of over 3% from 2010. Part of the reason for this increase is that KA is increasingly performed for patients who are older and who have other health conditions in addition to their osteoarthritis. A further change relates to the increasing use of unicompartmental knee arthroplasty (UKA). Approximately 90% of KAs are a total knee arthroplasty (TKA) and 10% are a UKA or partial KA. In TKA, the entire tibiofemoral joint is replaced, whereas in UKA the affected medial or lateral side of the tibiofemoral joint is replaced only. National outcome data have revealed that patients’ age and American Society of Anesthesiologists (ASA) classification grades are increasing. In the 15th National Joint Registry Report of 2018,4 15% of patients were aged 80–89 years and 1% were over 90 years of age. The average ASA classification grade is also increasing, with 19% reported as ASA classification grade 3 (severe systemic disease that limits activity). 4
Age should not be a barrier to a good KA outcome, with reports of successful outcomes in patients aged over 80 years. 5,6
Data from patient-reported outcome measures (PROMs) have shown that most patients achieve a satisfactory outcome following KA. However, between 15% and 30% of patients are unsatisfied or report little or no improvement after KA. 7 We do not yet know how to identify these patients or target rehabilitation to improve their outcomes.
Predicting which patients will have poor outcomes
The existing literature has demonstrated that it is difficult to predict who will do well after KA. Any prediction is complex and extends far beyond a simple linear relationship with factors such as age or presurgical function. A number of studies have explored the influence of pre-operative predictors on post-operative KA outcomes and have generally agreed that patients who have a higher pre-operative status, such as better pre-operative function or less pain, tend to have better post-operative outcomes. 8–12 However, no screening tool that can accurately identify and predict who is at risk of a poor post-operative outcome currently exists, despite much previous work exploring this subject. 13–15
Rehabilitation pathway
Many hospitals follow an accelerated or enhanced recovery protocol, mobilising patients for the first time in the first 4 hours after surgery. Bohl et al. 16 and Okamoto et al. 17 demonstrated that early mobilisation within the first 24 hours after surgery shortened length of stay, but did not affect longer-term outcomes. Most hospitals have discharge criteria governing when patients are discharged from hospital that are based on safe mobilisation, ability to perform self-care and attainment of a minimum amount of knee flexion (commonly set at 90 degrees). Patients will have reached a minimal level of function at discharge, but will continue to have rehabilitation needs to help restore muscle strength and endurance, range of motion, walking distance and performance of higher-level functional activities.
Patients are commonly referred for further physiotherapy in an outpatient setting to assist with these issues. The timing of this referral varies, with some hospitals advocating immediate commencement of therapy after discharge from hospital and others waiting until the first post-operative review at around 6 weeks before considering referral. The type of therapy also differs, with some sites using self-directed home exercise and others advocating for more formal outpatient treatment using manual therapy, individually progressed exercises and functional activities in an individual or group setting.
Outpatient rehabilitation approaches
We conducted a systematic review evaluating the effectiveness of exercise after KA. We found that functional physiotherapy exercise interventions following discharge after elective primary TKA offered short-term benefit. 18 We found a small to moderate standardised effect size [0.33, 95% confidence interval (CI) 0.08 to 0.58] in favour of functional exercise at 3–4 months post operatively, which included small to moderate weighted mean differences of 2.90° (95% CI 0.61° to 5.20°) for range of motion and 1.70 (95% CI –1.00 to 4.30) for quality of life, in favour of functional exercise. However, the post-treatment benefits faded and were not carried through to 1 year post operation.
The review revealed the complexity that is involved in deciding the best rehabilitation after arthroplasty. A growing number of studies have chosen to apply an intervention later in the rehabilitation pathway (often between 6 weeks and 6 months after surgery), which suggests that a delay in starting the intervention avoids the period of early post-operative pain, swelling and limited motion. 19–21 Similarly, assessing outcomes after arthroplasty is known to be complex and multifaceted. 22,23 The lack of certainty about what constitutes best physiotherapy practice has been recognised internationally, with considerable variation in the post-operative rehabilitation protocols used. A recent Australian survey of physiotherapy practice24 and an observational cohort study of US rehabilitation services25 have started to address this knowledge gap. However, there are no published randomised controlled trials (RCTs) of occupational therapy after KA. Other studies do not allow the occupational therapy input to be disaggregated from the overarching rehabilitation package to assess its contribution.
Patients in the UK usually receive a short course of between four and six sessions of post-operative physiotherapy after their surgery, usually in a physiotherapy outpatient clinic setting. Concern has been raised that many exercise programmes lack adequate intensity to lead to optimal recovery. 24,26 Internationally, where much longer courses of physiotherapy are often provided, research has indicated that 12–18 hours of physiotherapy21 or a mean of 17 visits27 may be needed to produce benefit. These levels of care are substantially higher than those provided in the UK and, in the current economic climate, may be more than the NHS can afford given the rising number of KA operations that are performed each year. Patients are likely to have an occupational therapy assessment before surgery to identify any potential issues on discharge, such as home layout. However, it is not usual for patients to have further input from occupational therapists unless they have particular problems.
Given the rising number of KA operations, the relatively limited therapy resources available and the increasing age and frailty of patients receiving this surgery, it is important that we concentrate our rehabilitation resources on those patients who need the most help to achieve a good outcome. It is important that exercise and functional rehabilitation is linked to demonstrable increases in function and participation levels. Not all patients need physiotherapy to help them recover, and some patients will recover fully using self-directed rehabilitation. 28–31 It is, thus, pertinent to focus our resources on those who are most likely to be at risk of a poor outcome, least likely to be able to engage with a self-management approach and able to benefit from rehabilitation input.
It is clear that current rehabilitation strategies do not meet the needs of all patients, particularly those who are socially isolated, do not have easy access to transport and are frail. This is a particular concern given both the projected increased need for joint arthroplasty over the next decade to accommodate an ageing population and the pressure of potential reductions in NHS funding. Evaluating the value of treatment modalities offered to these patients is crucial because many more patients are being discharged home earlier from the acute setting. There is less time available for acute physical recovery, rehabilitation and education in hospital, which increases the potential burden of care for these patients and their families.
Rationale for the CORKA trial
There is considerable variation in the quantity and content of rehabilitation received by patients after KA. Previous evidence has shown that some patients do well with minimal input or self-directed exercise programmes,30–33 but that many patients continue to experience pain and poor function after their surgery and report dissatisfaction with the rehabilitation that they have received.
In the CORKA trial, we aimed to evaluate a different approach designed to cater to the needs of an older, frailer population. We evaluated two strategies of rehabilitation after KA: a traditional outpatient clinic-based model and a rehabilitation programme delivered in participants’ homes by rehabilitation assistants supported by qualified therapists.
The programmes were designed to be acceptable to NHS physiotherapists and occupational therapists, based on current available evidence, and affordable to UK commissioners. A parallel economic study enabled conclusions to be made about cost-effectiveness. A nested qualitative study assessed the acceptability of the interventions to participants and the therapists who delivered them.
Research objectives
The CORKA research objectives were published in the protocol paper in Trials in 2016:34
-
to design a prognostic screening tool based on an analysis of factors associated with poor outcomes following KA to guide patient selection for the trial
-
to evaluate if a multicomponent rehabilitation programme delivered in patients’ homes could improve their outcomes compared with those receiving standard outpatient rehabilitation over 12 months
-
to undertake a nested qualitative study exploring patients’ and clinicians’ perceptions of the community-based rehabilitation programme
-
to undertake an economic analysis to compare the cost-effectiveness of the interventions.
Chapter 2 Methods
The protocol for the RCT was published in Trials in 2016. 34 The trial was delivered as published with no changes to the protocol.
Trial design
The CORKA trial was a prospective, individually randomised, two-arm controlled trial with blinded outcome assessment for the clinical outcomes at baseline, 6 months and 12 months. It aimed to determine if a multicomponent rehabilitation programme provided to participants who were deemed at risk of a poor outcome following KA by the CORKA screening tool (see Chapter 7) was better than usual care. The trial took place in 14 NHS hospitals across the UK. The design included a parallel health economic evaluation and a nested qualitative study. Figure 1 gives an overview of the flow of participants through the trial and the timing of assessment procedures.
FIGURE 1.
Flow of participants through the trial.
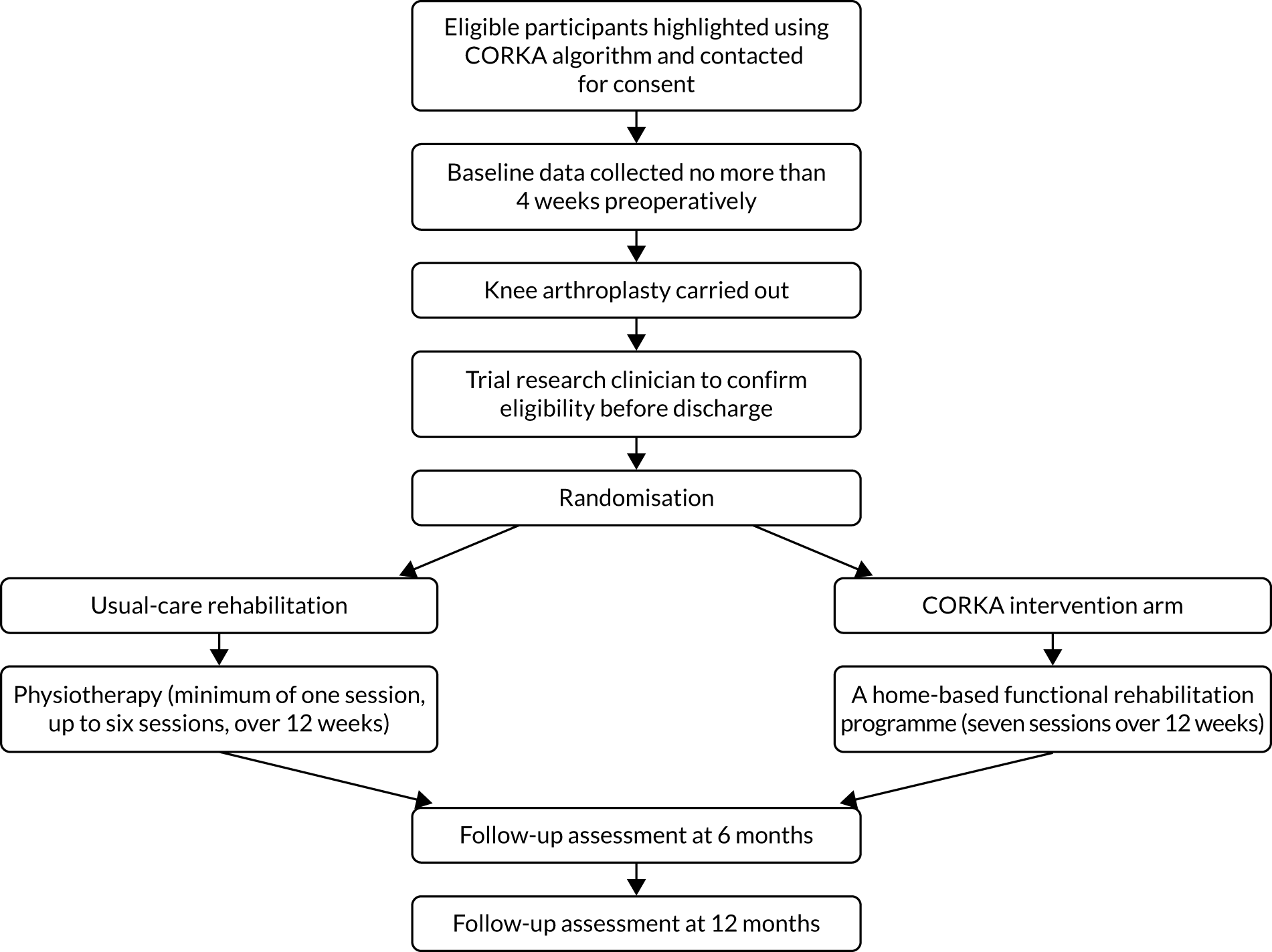
Randomised controlled trial
During the trial, people undergoing KA were screened for suitability during their pre-operative assessment clinic appointment using the screening tool that had been developed for the trial. Once informed consent was gained, participants were enrolled in the trial and baseline data were collected. After surgery, participants’ eligibility was confirmed and randomisation took place. Participants were allocated to receive one of two rehabilitation options: ‘usual care’ or the ‘home-based intervention’. Both participants and those delivering the rehabilitation were aware of the treatment allocation owing to the nature of the interventions.
Participants remained in the trial until data relating to their 12-month follow-up were collected. The trial had two follow-up time points: 6 and 12 months after randomisation. The physiotherapists carrying out the follow-ups remained blinded to the participants’ allocation.
Economic analysis
Once all participants completed their 12-month follow-up appointment, an economic analysis comparing the community-based rehabilitation programme with usual care was conducted (described in Chapter 5).
Qualitative study
As part of the main study, a nested qualitative study was conducted with 10 participants who undertook the community-based rehabilitation programme and 11 clinical staff who provided the treatment (five therapists and six rehabilitation assistants). We conducted one-to-one interviews with these participants to obtain their in-depth views about the intervention and how it was delivered (described in Chapter 6).
Participants
Inclusion criteria
Participants were included in the trial if they met all of the following inclusion criteria:
-
was willing and able to give informed consent for participation in the trial
-
was male or female, aged ≥ 55 years
-
was scheduled to have a primary UKA procedure
-
was deemed by the study screening tool to be at risk of a poor outcome
-
was willing to allow therapists to visit their home to deliver the community-based rehabilitation programme if randomised to the intervention arm.
Exclusion criteria
Participants were excluded from the study if they met any of the following exclusion criteria:
-
had any absolute contraindications to exercise
-
had severe cardiovascular or pulmonary disease (New York Heart Association III-IV)35
-
had severe dementia, assessed using the hospital dementia screening tool
-
had rheumatoid arthritis
-
had further lower limb surgery planned within 12 months
-
had serious perioperative complications.
Screening and recruitment
Patients were initially identified from clinic lists. Those who were scheduled to receive a KA were sent the CORKA participant invitation letter and participant information sheet. This pack was sent out by a member of the patient’s usual-care team before they attended their pre-operative assessment clinic appointment. Patients who had been sent the information were approached during their pre-operative assessment clinic appointment to determine if they were interested in the trial, and were given the opportunity to discuss the trial further and ask any questions. If the patient indicated that they were interested in taking part, the CORKA screening tool was used to identify if they were at risk of a poor outcome. They were also then checked for eligibility into the trial. Once they were deemed eligible, an appointment was made to gain informed consent and collect baseline outcome data with a member of the research team. This baseline appointment took place either in the hospital or in the patient’s own home no more than 4 weeks before the date of surgery.
Patients were not formally recruited into the trial or randomised until their eligibility had been re-checked on day three after surgery. Patients with serious peri-operative complications were excluded, as they would not be able to complete routine post-operative rehabilitation. Those participants who were still eligible were then asked to confirm their consent verbally to a member of the research team before being enrolled into the trial and randomised (described in Intervention monitoring and support).
Settings and locations
The trial was run in 14 NHS sites (names correct at time of participation):
-
Nuffield Orthopaedic Centre, Oxford University Hospitals NHS Foundation Trust
-
Horton Hospital – Banbury
-
The Royal Orthopaedic Hospital NHS Foundation Trust
-
Ashford and St Peter’s Hospitals NHS Foundation Trust
-
Northumbria Healthcare NHS Foundation Trust
-
Southern Health NHS Foundation Trust
-
Dorset County Hospital NHS Foundation Trust
-
Dorset Healthcare University NHS Foundation Trust
-
Royal United Hospitals Bath NHS Foundation Trust
-
Countess of Chester Hospital NHS Foundation Trust
-
Central Manchester NHS Foundation Trust
-
Medway NHS Foundation Trust
-
Medway Community Healthcare
-
Epsom and St Helier University Hospitals NHS Trust.
Interventions
Full details of the interventions are provided in Chapter 3, but are described briefly here. This trial tested physiotherapy and occupational therapy in a multicomponent package of rehabilitation that was delivered by a generic rehabilitation therapist. The occupational therapy element focused on assessment of and adaptations to participants’ homes to enable a safe environment for home exercise and everyday functional tasks. The physiotherapy element included a home-based intervention with an emphasis on functional, activity-based rehabilitation. We tested whether or not the intervention could be delivered by generic rehabilitation assistants rather than uni-professionally.
Usual care can vary geographically, including the number of sessions of physiotherapy given post discharge. 36 To standardise the usual-care arm without changing it significantly, we set a minimum and maximum number of sessions allowed for usual care. Participants were expected to attend at least one and a maximum of six sessions of outpatient physiotherapy.
Data collection
Baseline assessments: pre surgery
Baseline data were collected on paper questionnaires and most measures were participant reported. Baseline assessments were carried out either in the participant’s home or in the hospital. Wherever the baseline assessment took place, the 6- and 12-month follow-ups were conducted in the same location. All sites were given a research clinician manual that gave detailed instructions on how to complete all baseline and follow-up measures.
In addition to the battery of primary and secondary outcome measures, the following data were collected at baseline: name, contact details, age, surgery, ASA classification rating, review of medical notes and Functional Co-morbidities Index. The Functional Co-morbidities Index is a measure of comorbidity and was completed because comorbidities affecting physical outcome are likely to be present in this more frail population. 35 Table 1 shows the measures collected at each time point.
| Measure | Baseline | 6 months | 12 months |
|---|---|---|---|
| Demographics | ✗ | ||
| Medical history | ✗ | ||
| Late Life Function and Disability Instrument | ✗ | ✗ | ✗ |
| Oxford Knee Score | ✗ | ✗ | ✗ |
| Quality-of-life subscale of the Knee Osteoarthritis Outcome Score | ✗ | ✗ | |
| Physical Activity Scale for the Elderly | ✗ | ✗ | ✗ |
| Health economics using EQ-5D-5L | ✗ | ✗ | ✗ |
| 30-Second Chair Stand Test | ✗ | ✗ | ✗ |
| Figure-of-8 Walk Test | ✗ | ✗ | ✗ |
| Single Leg Stance test | ✗ | ✗ | ✗ |
| Participant diarya | ✗ | ✗ |
Follow-up visits
The trial had two follow-up time points at 6 and 12 months post randomisation. Local site staff organised follow-up and liaised directly with the participants to organise home-based follow-ups. In addition to the outcome measures, the following data were collected at the two follow-up time points:
-
complications/adverse events, including any apparent KA-related complications since discharge
-
falls, including time to first fall.
Outcomes
Follow-up data collection was carried out at face-to-face clinical assessments at 6 and 12 months following randomisation. Where face-to-face assessment was not possible, postal and telephone data collection methods were used to obtain self-reported core data. Table 1 shows the measures collected at each time point.
Primary outcome
The primary outcome was the Late Life Function and Disability Instrument (LLFDI) overall function score. This outcome instrument was developed specifically for community-dwelling older adults. It assesses and responds to meaningful change in two distinct outcomes: a person’s ability to do discrete actions or activities (function) and a person’s performance of socially-defined life tasks (disability). 37 The overall function score consists of 32 items. Scores range from 0 to 100, with higher scores indicating better function. The summary of the outcomes measured has previously been published in the protocol paper by the authors. 34
Secondary outcomes
-
LLFDI disability frequency and limitation total dimension scores. These scores each consist of 16 items. They have a total score ranging from 0 to 100, with higher scores indicating higher frequency and better capability of participation in life tasks.
-
Oxford Knee Score (OKS). The OKS is a disease-specific measure to assess function and allow comparison with data from large epidemiological cohort studies. It is a 12-item PROM that is designed to measure pain and function after KA surgery. 38 Each item is scored from 0 to 4, and total scores are calculated as a sum across the individual items. Scores range from 0 (least severe symptoms) to 48 (most severe symptoms).
-
Knee injury and Osteoarthritis Outcome Score (KOOS) – Quality of Life subscale. This is a specific instrument for knee osteoarthritis, which can also be analysed to calculate the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). It is a self-reported questionnaire consisting of five subscales: pain, other symptoms, function in daily living, function in sport and recreation, and knee-related quality of life (QoL). The quality-of-life subscale of KOOS consists of four self-reported questions. 39 Total scores range from 0 (extreme problems) to 100 (no problems).
-
Physical Activity Scale for the Elderly (PASE) questionnaire. This is a self-reported scale designed to measure the physical activity level of those aged ≥ 65 years. It consists of three subscales: leisure time activity, household activity and work-related activity. It is a short, self-administered questionnaire to assess activity in the past week. 40 Time spent participating in each activity is weighted by the difficulty of that activity to calculate a total score. Higher scores indicate greater levels of physical activity.
-
Health economics using EuroQol-5 Dimensions, five-level version (EQ-5D-5L). This is a self-reported outcome measure consisting of five dimensions: mobility, self-care, usual activity, pain and discomfort, and anxiety and depression. Each dimension has five categories of response. It is designed to provide a generic measure of health status for clinical and/or economic evaluation. 41 Responses are converted into multi-attribute utility scores, where 1 represents perfect health, 0 represents death and scores < 0 represent a QoL worse than death.
-
Physical measures. Physical measures included measures of balance, mobility and physical activity, which are all affected by KA. Each test is reliable and valid, has been used with older, community-dwelling adults and has been shown to be responsive in previous rehabilitation studies. Physical function was measured by three physical performance tasks:42–44
-
Figure of 8 Walk Test (F8WT). In the F8WT, participants are asked to walk in a figure of eight around two cones. Their walk is timed, the number of steps taken counted and their accuracy in performing the task (ability to stay within the test boundary) recorded.
-
30-Second Chair Stand Test (30SCST). The participant starts in a seated position on a chair with a seat height of 17 inches. They are asked to complete as many full stands in 30 seconds as possible, sitting down after each stand. The number of full stands they can complete is recorded.
-
Single Leg Stance (SLS). The participant is asked to stand and lift one leg off of the ground without using any other support. They are timed standing on one leg. The participant stands with a chair in front of them should they require support. They are timed three times on each leg, for a maximum of 45 seconds for each trial.
-
-
Health resource diary. On discharge, participants were given a diary to regularly record:
-
daily exercises undertaken (completed daily for 6 weeks and weekly thereafter)
-
medication taken, including dose and frequency
-
use of health-care services and personnel
-
falls
-
adverse events.
-
Intervention monitoring and support
Quality assurance checks took place at all CORKA research sites. They included fidelity checks, during which assessment and treatment delivery were observed. All aspects of the intervention were checked against a predefined fidelity checklist that was created in line with the study protocol. Following checks, feedback was given and any training needs or action points identified. The investigator site file was also reviewed and any necessary changes were recorded. Staff at research sites were assured that support could be provided as required, not just during a monitoring visit. Staff were encouraged to contact the central CORKA trial team in Oxford if they had any questions or concerns about any aspect of the trial.
Randomisation
Randomisation took place using the Registration/Randomisation and Management of Product website provided by the Oxford Clinical Trials Research Unit randomisation service.
Allocation
Participants were randomly allocated by a computer-generated system to either ‘usual care’ or ‘home-based exercise programme’ in a 1 : 1 ratio. Randomisation using permuted blocks of various sizes (2, 4 and 6) in a 1 : 2 : 1 ratio was stratified by recruitment site to account for any site effects.
Blinding
Participants and those delivering the rehabilitation were aware of the treatment allocation owing to the nature of the intervention. The therapists who carried out the follow-up visits remained blinded.
Sample size
We chose the LLFDI as the primary outcome using the overall function subscore at 1 year. The sample size calculation was based on a moderately small standardised effect size of 0.275. This standardised effect size is, for example, equivalent to detecting a three-point difference between treatment arms on the LLFDI overall function score, assuming a standard deviation (SD) of 10.91 and no clustering effect across sites. 45
A total of 558 participants (279 per arm) would be required to detect a standardised effect size of 0.275, with 90% power and 5% (two-sided) significance. A 10% loss to follow-up was chosen based on previous experience of trials in a similar population, which resulted in the planned sample size of 620 participants (310 per arm).
An internal pilot study was conducted at one site (Oxford) to review recruitment feasibility and confirm the intervention package. Fifteen participants were randomised during this pilot study. These participants are included in the final analysis, as the interventions were not changed between the pilot and the main trial.
Data analysis
General analysis principles
Two analysis populations were considered: the intention-to-treat (ITT) population and the per-protocol (PP) population. The ITT population, which was the primary analysis set, included all randomised participants who were analysed according to their allocated intervention. Analyses of the primary and key secondary outcomes were repeated for the PP population, which included participants who received at least one session of their allocated intervention, did not receive more treatment than intended (more than six sessions of usual care or seven sessions of home-based rehabilitation) and provided follow-up data. The statistical significance level used throughout was 0.05, and 95% CIs are reported. All analyses were undertaken using Stata® 15 (StataCorp LP, College Station, TX, USA).
The statistical analysis plan for this trial was prespecified and was approved by the trial Data Safety Monitoring Committee and the Trial Steering Committee in October 2017. The agreed statistical analysis plan was then published in Trials. 46
Descriptive analyses and availability of data
The flow of participants through the trial from screening, through randomisation, allocation and follow-up, to analysis was summarised using a Consolidated Standards of Reporting Trials (CONSORT) patient-reported outcomes extension flow chart (see Figure 4). 47 The baseline comparability of the two treatment groups was summarised using numbers with percentages for binary and categorical variables, and means and SDs or medians and interquartile ranges (IQRs) for continuous variables, as appropriate, in terms of (1) risk factors for a poor outcome after KA according to the CORKA screening tool,48 (2) baseline characteristics and (3) primary and secondary outcomes. Losses to follow-up and withdrawals before 6-month follow-up and 12-month follow-up were reported by intervention arm with reasons. Absolute risk differences were tested to ensure that there were no differential losses between the two groups. The availability of all primary and secondary outcomes from baseline to 12 months post randomisation was also summarised by intervention arm.
Treatment
Compliance with home-based rehabilitation was defined as completing at least four treatment sessions, and with usual care as completing at least one treatment session. The number and percentage of compliers in each treatment group was calculated. The average (median and IQR) number of treatment sessions received in each treatment group was summarised overall and by site. Details of what the treatment sessions involved were also summarised by treatment site for each intervention.
Analysis of the primary outcome
The LLFDI function scores at 6 and 12 months post randomisation were summarised by treatment group and were analysed using a linear mixed-effects model with repeated measures adjusted for baseline score and recruiting site (stratification factor). Time was treated as categorical and an interaction between the outcome measurement time point and the randomised group was included so that the treatment effect at each time point could be estimated, reported as the adjusted mean difference in LLFDI between groups with 95% CI and associated p-value. The underlying assumptions of this model were assessed. The primary time point was 12 months post randomisation. The primary analysis was performed for the ITT population using multiple imputation to impute missing data. The multiple imputation model included type of KA (TKA or UKA), gender, whether or not the participant had had previous lower limb surgery, Charnley Classification score49 (whether the participant had single knee arthropathy, bilateral knee arthropathy or multiple joint disease), whether or not a walking aid was currently used, recruiting site and baseline score. Imputation was performed separately for each treatment group, and 10 data sets were imputed (approximately equal to the percentage of missing data).
To examine the robustness of conclusions of different assumptions about missing data and departure from randomised allocation, this analysis was repeated for the ITT population using available cases only and for the PP population using available cases. A complier-average causal effect analysis was undertaken using an instrumental variable approach. 50,51
Analysis of secondary outcomes
Linear mixed-effects models with repeated measures, similar to those described for the primary outcome, were used to analyse each of the secondary PROMs (LLFDI disability limitation, LLFDI disability frequency, OKS, KOOS QoL, PASE, EQ-5D-5L utility and visual analogue scale), 30SCST (number of stands) and F8WT (time and steps). These analyses were performed for the ITT population using available cases only.
For the key secondary outcomes (LLFDI disability limitation, LLFDI disability frequency, OKS and KOOS QoL), these analyses were repeated for the ITT population using multiple imputation (imputation model as outlined for the primary outcome) and the PP population using available cases. A complier-average causal effect analysis was also undertaken.
Average SLS test times were summarised using medians and IQRs for each treatment group at each time point. Differences between the two groups were tested using a Wilcoxon rank-sum test, and p-values were reported owing to the substantial floor and ceiling effects observed in this outcome. These analyses were unadjusted and carried out separately at each time point.
Analysis of safety data
The number of adverse events and the number and percentage of participants experiencing adverse events up to 6 months post randomisation, and between 6 and 12 months post randomisation, were reported by treatment group. The number of participants with an adverse event was compared across treatment groups using risk differences. Similar methods were used to analyse serious adverse events.
Monitoring and approvals
Formal approvals
The CORKA trial was approved by the National Research Ethics Service Committee South Central – Oxford B in February 2015 (Research Ethics Committee reference 15/SC/0019) and by the Research and Development department of each participating site. The Clinical Trials and Research Governance office at the University of Oxford confirmed sponsorship in December 2014.
The first substantial amendment was submitted and approved in August 2015, in which the sample size was recalculated using a consistent description of the clinically important standardised mean difference, a 45-minute travel inclusion zone was added and the KOOS was added to the questionnaire pack. A second substantial amendment was granted in December 2015, which added the internal pilot participants to the sample size, increasing the sample size from 620 to 635, and sought approval to send appointment reminder letters to participants. A third substantial amendment was requested in February 2017 to reduce the sample size back to 620.
Trial Steering Committee
A Trial Steering Committee was responsible for monitoring and supervising the progress of the CORKA trial throughout its duration. The committee consisted of three independent experts, a lay member and leading members of the trial management group.
Data Safety Monitoring Committee
The Data Safety Monitoring Committee was independent of the trial and was tasked with monitoring progress, conduct, participant safety and data integrity. The trial statistician provided data and analyses requested by the committee at each meeting.
Trial management group
A trial management group was responsible for the day-to-day management of the trial, consisting of the chief investigator, research physiotherapist, statistician and trial manager. They ensured the overall integrity of the trial, compliance with the protocols, welfare of all participants and appropriate reporting of the trial.
Chapter 3 Intervention development
This chapter describes the steps taken by the CORKA trial team to produce the CORKA home-based intervention. It describes the development process and each component of the intervention. The CORKA home-based intervention was developed with consideration of the Medical Research Council’s guidelines for developing and evaluating complex interventions52 and the Template for Intervention Description and Replication checklist. 53 A recent systematic review54 of intervention development approaches has categorised differing approaches in a taxonomy. Although the CORKA home-based intervention was developed before this taxonomy was published, we can classify the development approach as a combined approach, with parts of the development aligning with the ‘target population centred’, ‘theory and evidence-based’, and ‘intervention-specific’ taxonomy categories.
Overview of the development process
The CORKA home-based intervention development drew on a number of sources, including reviewing the relevant evidence base on post-arthroplasty rehabilitation, identifying appropriate exercise guidelines and seeking the views of relevant stakeholders. An intervention development day was organised that was attended by research staff, physiotherapists and occupational therapists. During the day, potential intervention approaches were discussed and refined, and factors associated with the design, content and intervention delivery were discussed. A draft intervention was presented.
The draft intervention was reviewed and refined, then tested in a clinical NHS setting as part of a pilot phase, which included participant and clinician feedback.
The development steps are outlined below and are represented in Figure 2.
FIGURE 2.
Intervention development considerations.

Health Technology Assessment programme brief
The first step in developing the CORKA home-based intervention was considering the HTA commissioning brief. This brief called for an intensive and, if possible, multidisciplinary rehabilitation intervention in the community setting that was designed for older adults who were at risk of poorer functional outcomes following elective knee joint arthroplasty surgery that could be compared with usual care.
Evidence base
Before designing a draft intervention, we undertook a systematic search of the literature on rehabilitation post-KA, and a wider search on the field of rehabilitation following arthroplasty. This process identified a number of papers that were used in the design of the intervention. These papers are outlined in Table 2 and are described in more detail in the following section on the rationale underlying the CORKA home-based intervention.
| Authors and year of publication | Design | Conclusion or key considerations | Relevant intervention component |
|---|---|---|---|
| Bade et al. 201055 | Cohort | Impairments can persist 6 months after TKA. More intensive therapeutic approaches may be needed | Functional task practice |
| Bade and Stevens-Lapsley 201156 | Cohort | High-intensity early rehabilitation after TKA may lead to better functional performance | Strengthening |
| Bade and Stevens-Lapsley 201257 | Review | Higher-intensity rehabilitation programmes using progressive resistance strengthening produce long-term strength and functional gains | Functional task practice |
| Strengthening | |||
| Bhave et al. 200558 | Cohort | Functional limitations can be present after knee replacement. A structured programme may be needed | Range of movement exercises |
| Functional task practice | |||
| Bruun-Olsen et al. 201320 | RCT | Improvements in the 6-minute walk test were demonstrated after a walking skills programme | Gait skills |
| Graduated walking programme | |||
| Coulter et al. 200959 | Cohort | Strengthening exercises delivered during one-to-one physiotherapy or class-based physiotherapy after joint replacement showed no difference in WOMAC scores | Strengthening |
| Fitzsimmons et al. 201060 | Systematic review | Stiffness is a common problem after total knee replacement | Range of movement exercises |
| Harmer et al. 200961 | RCT | Similar outcomes were demonstrated with land-based and water-based rehabilitation after TKA | Graduated walking programme |
| Heiberg et al. 201062 | Cohort | Difficulty can be experienced with strenuous activities, including walking long distances, after TKA | Gait skill |
| Graduated walking programme | |||
| Kearns et al. 200863 | Cohort | Falling was reported by 78 out of 1341 consecutive patients after TKA | Balance |
| Knoop et al. 201164 | Narrative review | People with knee osteoarthritis have decreased knee proprioception | Balance |
| Lee et al. 201465 | Cross-section | Lower-limb strength and the Y-balance test were weakly correlated | Strength |
| Balance | |||
| Liao et al. 201366 | RCT | Significant changes in the 10-metre walk test and the Timed Up and Go Test were observed after balance training | Balance |
| Mandeville et al. 200867 | Cohort | Gait stability can be affected by total knee replacement surgery | Balance |
| Gait skills | |||
| McClelland et al. 200768 | Systematic review | Altered gait patterns are observed following TKA | Gait skills |
| Meier et al. 200869 | Review | Quadriceps muscle impairment can contribute to functional limitations after total knee replacement | Functional task practice |
| Strengthening | |||
| Minns Lowe et al. 200718 | Systematic review | Functional physiotherapy exercises resulted in short-term benefit after total knee replacement | Functional task practice |
| Mizner et al. 200570 | Cohort | Functional performance was highly correlated with quadriceps strength | Strengthening |
| Moffet et al. 200421 | RCT | Short- and mid-term functional ability was improved by intensive functional rehabilitation | Strengthening |
| Naylor and Ko 201271 | Mixed methods study (nested within a RCT) | Patients were able to exercise at moderately hard-intensity levels after total knee replacement | Graduated walking programme |
| Strengthening | |||
| Noble et al. 200572 | Cross-section | Significant functional impairments were experienced by TKA patients | Functional task practice |
| Pandy et al. 201073 | Review | The muscles of the hip and calf play a significant role in gait | Strengthening |
| Gait skills | |||
| Philbin et al. 199574 | Cross-section | Those with end-stage osteoarthritis of the lower extremity can be severely deconditioned | Graduated walking programme |
| Piva et al. 201019 | Pilot RCT | Balance training after TKA is feasible, as supported by high adherence and a low dropout rate | Balance |
| Piva et al. 201175 | Cross-section | Physical function after arthroplasty can be influenced by hip abduction strength | Strengthening |
| Pozzi et al. 201376 | Systematic review | Strengthening and intensive functional exercises should be included in optimal physiotherapy programmes | Strengthening |
| Functional task practice | |||
| Ries et al. 199677 | Cohort | There can be improvements in cardiovascular fitness at 1 or 2 years after TKA | Graduated walking programme |
| Rowe et al. 200078 | Cohort | A suitable goal for knee rehabilitation is to reach 110° of knee flexion | Range of movement exercises |
| Stratford et al. 201079 | Cohort | The first 12 weeks post arthroplasty is when the greatest range of movement improvements took place | Range of movement exercises |
| Stevens et al. 200380 | Cohort | Reduced quadriceps strength was observed in those with knee osteoarthritis. This weakness persisted post surgery | Strengthening |
| Su et al. 201081 | Review | Stiffness is a frequent complication after TKA | Range of movement exercises |
| Turcot et al. 201382 | Cohort | Gait parameters can affect patient satisfaction after TKA | Gait skills |
| Graduated walking programme | |||
| Walsh et al. 199883 | Cohort | Physical impairments and functional limitations may persist at 1 year after TKA | Strengthening |
| Wiik et al. 201384 | Cohort | Gait parameters were much closer to normal after unicompartmental knee replacement than total knee replacement patients, but were not as good as control participants | Gait skills |
| Graduated walking programme |
Rationale underlying the CORKA intervention
The different components of the CORKA home-based intervention were informed by a review of existing trials. The intervention primarily consists of a functional exercise programme. The rationale for the following sections of this exercise programme is given below:
-
range of movement exercises
-
strengthening exercises
-
balance exercises
-
gait and aerobic exercise
-
functional exercise.
Range of movement
Both flexion and extension can be limited after KA surgery. 58,79 Stiffness is a common complication after surgery,78 with as many as 60% of those undergoing KA experiencing stiffness. 73 Many functional activities require a knee joint range of 0–110°,80 and a significant proportion of this range is likely to be attained within the first 12 weeks after surgery. 79 A number of studies have, therefore, included range of movement exercises in their post-arthroplasty interventions. 56,66,75
Strengthening
Decreased quadricep strength is a common finding in people with knee osteoarthritis,80 and these strength deficits can persist for a long time after KA. 76 One year after arthroplasty, both knee flexor and extensor peak muscle torque are lower in those who have undergone surgery than age-matched controls. 83 As there is a close link between muscle strength and functional performance, strength is an important consideration for the population in question. For example, the ability to change position from sitting to standing and the ability climb stairs have been linked to quadriceps strength. 70 The ability to get up from a chair, climb-up stairs, walk and change direction have been linked with hip abduction strength. 75 Hip and calf strength also perform a significant role in gait and balance. 65,73 Strengthening exercises have formed part of previous interventions following lower limb arthroplasty, targeting muscle groups such as the quadriceps, hamstrings, hip abductors and calf muscles. 21,56,59,75
Balance
As KA surgery can affect gait, balance and stability,67 it is not surprising that falling has been reported as a problem after KA. 63 In a pilot study, Piva et al. 19 gave participants functional training or functional training enhanced by a balance training programme after KA. The pilot study’s design was underpowered to detect changes between groups but showed that the balance training group had high adherence, had a low dropout rate and reported no adverse events. A RCT66 that investigated the effect of adding a balance programme to functional training after KA reported that those in the balance training group had significantly better outcomes, including on the Timed Up and Go, 10-metre walk, 30SCST and Stair Climb tests.
Gait skills and aerobic exercise
McClelland et al. 68 conducted a systematic review that studied gait analysis following KA and concluded that patients exhibit altered gait patterns after surgery. Gait problems after KA include specific gait deficits linked to underlying problems, such as reduced range of movement or pain, and general problems, such as decreased walking speed and endurance. 62,68,83 Such gait problems may continue to persist 1 year after surgery. Patients undergoing UKA are more likely to exhibit a gait pattern closer to normal than patients undergoing TKA. 84 It is important to consider gait in patients who have undergone KA, as gait outcomes influence patient satisfaction. 82
Bruun-Olsen et al. 20 undertook a RCT comparing a walking skills intervention with usual physiotherapy. They reported that the walking skills group demonstrated better short-term and long-term functional mobility. Walking can also be considered from a wider cardiovascular perspective. People with end-stage lower limb osteoarthritis are likely to be physically deconditioned,74,77 which can be exacerbated immediately after surgery owing to reduced mobility. Naylor and Ko71 found that moderate-intensity exercise was tolerable and safe following KA. Later studies have included aerobic exercise using cycling on a static bike or walking on a treadmill. 59,61
Functional exercise
Functional limitations can persist after the immediate post-operative period following KA. 57,69 One year post KA surgery, it is common for patients to be significantly slower with activities, such as walking or stair climbing, and have more functional impairments with tasks that involve kneeling or squatting than matched controls. 72,83 Measurements of function, such as the Timed Up and Go, 6-minute walk, Stair Climb and SLS tests, have been found to be worse in participants following KA at 1, 3 and 6 months after surgery than in healthy participants. 55 A 2007 systematic review18 investigating the effectiveness of post-KA exercise advocated for the use of functional physiotherapy exercise interventions to achieve short-term benefits. It found that the interventions had a small to moderate effect on functional outcomes, QoL and joint range of movement at 3 to 4 months after surgery; however, these benefits were not found 1 year after surgery. A later study explored function after KA57 and a 2013 systematic review76 of exercise after KA recommended that physiotherapy interventions include strengthening and functional exercise.
Guidelines
Currently, there are no specific exercise guidelines for patients after KA. We considered two relevant guidelines for patients following KA when developing the CORKA home-based intervention:
-
Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. 85
-
Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. 86
These guidelines cover exercise guidelines for adults aged 65 years or older and aged 50–64 years with functional limitations or a chronic condition,85 and apparently healthy adults of all ages. 86 On the basis of these guidelines, it is recommended that participants accumulate five sessions of 30 minutes of moderate-intensity physical activity per week, which is accumulated in at least 10-minute bouts.
Intervention development day and workshop
An intervention development day was attended by researchers, physiotherapists and occupational therapists. A draft intervention developed using the evidence base and guidelines outlined above was presented at the workshop. The proposed intervention programme, strategies for exercise progression and patient materials were discussed and reviewed. Feedback was given on all aspects of the proposed intervention, leading to changes in the content of the intervention and patient materials.
Pilot phase
Following the workshop, a final version of the CORKA home-based intervention was compiled. It was then tested in a pilot phase with 15 participants. Three participants and two clinicians were interviewed after the participants had completed their treatment sessions to get feedback on the intervention content and the mode of delivery. As a result, changes were made to the participant materials and intervention procedures. These modifications included retaking some of the photographs used in the participant exercise sheets to make them brighter and more vibrant, and providing a space on the sheets for participants to make comments.
The CORKA intervention arms
The CORKA trial had two intervention arms:
-
usual care
-
the CORKA home-based intervention.
The intervention arms are described in the following sections.
Usual care
Those who were allocated to the usual-care arm received standard post-operative physiotherapy, as offered by their local physiotherapy department. It was recognised that usual care after KA could vary considerably across the trial’s UK locations. 36 However, there was a reasonable likelihood that it would include some of the following: one to six sessions of physiotherapy in an outpatient setting, class-based setting or hydrotherapy; written advice on home exercise at discharge from hospital; and an assessment of any potential home requirements and barriers to discharge by an occupational therapist. To standardise usual care as much as possible, participants were expected to attend at least one and no more than six sessions of usual-care physiotherapy.
The CORKA home-based intervention
The CORKA home-based intervention was an individually tailored, multicomponent rehabilitation programme that could be adapted for each participant. The intervention’s aim was to improve the function and participation of participants who were at risk of a poor outcome post KA. The primary component was an individually adapted exercise programme that was conducted in the participant’s own home, with additional components consisting of functional task practice, appropriate adherence approaches and, if required, the provision of appropriate aids and equipment.
The CORKA home-based intervention started within 4 weeks of surgery. It comprised an initial home assessment appointment, followed by up to six follow-up sessions. It was designed to be delivered by a mixture of qualified occupational therapy and physiotherapy staff, and rehabilitation assistants. The CORKA home-based intervention is outlined further in Figure 3.
FIGURE 3.
Components of the CORKA home-based intervention exercise programme. ROM, range of movement.

Home exercise programme
The CORKA home-based intervention exercise programme comprised groups of possible exercises that were arranged into the following categories:
-
knee flexion range of movement
-
knee extension range of movement
-
basic quadriceps strengthening
-
strengthening – quadriceps
-
strengthening – hamstrings
-
strengthening – hip abductors
-
strengthening – calf
-
balance
-
gait skills.
The programme included a range of exercises in each category, and at least one exercise from each category was to be selected for a participant’s exercise programme. This allowed the intervention to cover a range of exercises and be tailored to each participant.
Functional task practice
A functional task practice component was also included. During a participant’s first appointment, the therapist focused on tasks that the participant had identified as being potentially problematic. The exercise programme was, therefore, tailored to the specific needs, goals and functional problems of the individual. As necessary, different techniques were used as part of a problem-solving approach, including breaking the task down and identifying any specific components for practice. Each task could also be demonstrated and practised during the intervention sessions. To reinforce the importance of practising the selected tasks during their daily life, participants were encouraged to follow the written advice in the exercise diary.
Graduated walking programme
The graduated walking programme was included as it was deemed the most practical and relevant method to increase walking endurance and include moderate-intensity aerobic exercise within the exercise programme. It was considered likely that participants would have a wide range of mobility levels at entry into the trial. The aim of the graduated walking programme was to increase walking distance and/or time. During session 3 of the CORKA home-based intervention, participants were asked about their walking and how far or how long they were currently able to walk. They were then asked to gradually increase this distance and/or time, giving consideration to any pain or swelling. Walking was reviewed at subsequent follow-up sessions.
Prescription and progression
Therapists and assistants were asked to use treatment algorithms and decision aids to prescribe and progress participants’ exercises, which are outlined and described in Table 3. The Rating of Perceived Exertion scale87 that is mentioned is an 11-point scale that allows participants to rate how hard they feel they are working during exercise.
| Exercise type | Repetitions and sets | Decrease intensity/difficulty | Maintain intensity/difficulty | Increase intensity/difficulty |
|---|---|---|---|---|
| Strengthening exercises |
|
|
|
|
| Balance exercises |
|
|
|
|
| Range of movement exercises |
|
|
|
|
| Gait skills |
|
|
|
|
| Walking |
|
|
|
|
| Task practice |
|
|
|
|
Exercise duration and frequency
It was envisaged that the whole exercise programme would take 15–25 minutes to complete. Participants were advised that their exercise programme could be performed as one block or spread throughout the day. They were asked to perform the exercise programme daily, although it was recognised that this would not be possible for everyone.
Modifications
The therapists and rehabilitation assistants delivering the CORKA home-based intervention were encouraged to use their clinical reasoning skills when assessing and treating participants. If at any time they felt that it would be unsafe or inappropriate to give a participant an exercise from one or more of the intervention’s categories, then they were advised not to do so. If they decided that a participant needed additional exercises from an intervention category, for example if they felt that a participant had a particular problem with knee flexion and needed to be given more than one exercise from the knee flexion range of movement category, then they were encouraged to do so. Clinicians were asked to record any modifications that they made on the treatment logs that were returned to the central CORKA trial team.
Information booklet
Participants were given an information booklet that contained information about topics relevant to patients after KA, such as wound management, pain and swelling, expected symptoms, scar massage, walking, slips, trips and falls, kneeling, stairs, driving, returning to work, and returning to leisure activities or sports.
Adherence approaches
A number of adherence strategies were used within the intervention to encourage participants to adhere to their exercise programme, including an exercise diary, a behavioural contract and goal-setting. Participants recorded their exercises, repetitions and sets in an exercise diary so that they could monitor their own progress.
The CORKA trial participant materials included a behavioural contract. Participants were asked when they would be able to perform their exercises, whether or not there was a specific location where they could perform their exercises and if there was anyone who could help them undertake their exercise programme. The therapists and participant signed this contract to encourage adherence.
The therapist and participant took time to discuss the goals that were important to the participant. These goals were written in the appropriate section in the participant materials, along with the steps that the participant would follow to meet the goals: (1) practice tasks related to the goals, (2) undertake their tailored exercise programme and (3) record their exercise progress in the exercise diary provided. Each participant was given opportunities to review and set new goals as required in follow-up treatment sessions.
Intervention materials
Trial sites were given at least one copy of the treating therapists’ manual. This manual gave a detailed overview of the intervention and a point-by-point guide for each intervention session, including how to select the initial exercise level for each section of the exercise programme. It also included copies of all of the paperwork needed for the intervention and the contact details for the central CORKA team.
The CORKA website (https://corka.octru.ox.ac.uk/) was a further resource for staff at research sites. It contained a section for site staff that sites gained access to once they had signed up for the trial. This section included digital copies of all trial documents needed for that site.
Intervention providers and setting
The CORKA home-based intervention was undertaken using qualified therapists and rehabilitation assistants. The initial appointment consisted of a home assessment that was conducted by a therapist and a rehabilitation assistant. Subsequent rehabilitation sessions were undertaken by the rehabilitation assistant, except for one session midway through the treatment that was undertaken by the qualified therapist. Clear channels of communication were encouraged, and rehabilitation assistants were asked to feed back to the qualified staff member after all treatment sessions. All of the therapists that delivered the intervention were UK-registered physiotherapists or occupational therapists, with NHS banding ranging from 5 to 7. The rehabilitation assistants were NHS bands 3 or 4.
Training
All members of staff who were involved in delivering the CORKA home-based intervention received a 2- to 3-hour training session that was delivered by the central CORKA trial team. The training included instructions on how to assess and treat CORKA participants, prescribe and progress the different categories within the exercise programme, and complete the trial paperwork, in line with the trial protocol. None of the intervention components was beyond the normal scope of practice for the staff who delivered the intervention.
Safety and serious adverse events
The safety of participants and therapists was paramount and was considered as part of the CORKA home-based intervention. All trial site staff members delivering the intervention were encouraged to consider their and their patients’ safety. One of the significant considerations for a community-based trial like CORKA is that staff are by themselves when visiting participants in their homes. All staff members at all sites were, thus, asked to adhere to their organisation’s lone-working policy. They were also asked to report anything that they felt might constitute a serious adverse event. To make this process as easy as possible for clinicians involved in the treatment sessions, they were encouraged to discuss any questions or concerns with the central CORKA team in Oxford.
Chapter 4 Results
Study participants
The trial opened for recruitment on 17 March 2015, and the first participant was randomised on 27 March 2015. As recruitment was slower than predicted in the first year, the funder (HTA programme) requested a recovery plan be put in place. The recovery plan was reviewed by the Trial Steering Committee on 16 June 2016 and submitted to the HTA programme on 8 July 2016. In February 2017, a funding extension was agreed with the HTA programme with a revised trial end date of 31 December 2019.
The final randomisation took place on 26 January 2018. In total, 2788 patients were screened and 621 participants were recruited to the trial. The 12-month follow-up was completed in February 2019. The CONSORT flow chart (Figure 4) summarises the flow of participants through the trial, including details of the number of participants randomised and the numbers allocated to and receiving at least one session of each treatment. The number of participants followed up at 6 and 12 months and providing the primary outcome measure is summarised by treatment group. Reasons why participants were not followed up are also included. The numbers of participants included in the ITT and PP populations are summarised by treatment group.
FIGURE 4.
The CONSORT flow chart. LTFU, lost to follow-up. a, Clinical withdrawals include those who withdrew owing to an adverse event, serious adverse event or medical contraindication; b, missing follow-up data in the ITT population were imputed using multiple imputation for the primary outcome analysis; c, analysis of the PP population was based on available data only.
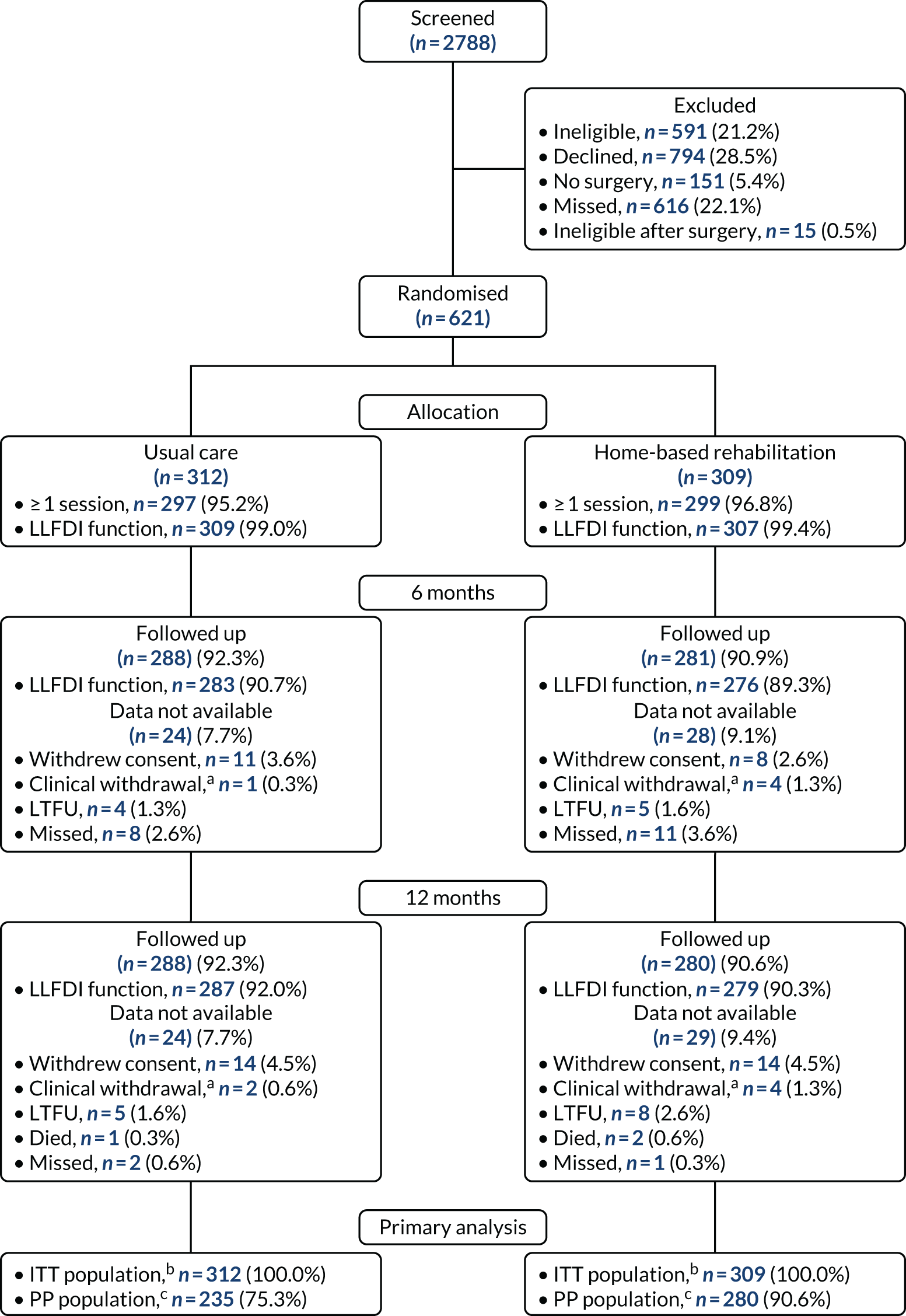
Available data
The data available at baseline and at each follow-up time point (6 and 12 months) are summarised by treatment group in Table 4. These data include which participants returned a case report form (CRF) at each time point and which participants returned a questionnaire. Reasons for data not being available are also summarised. The proportion of available data is high at both 6 and 12 months; approximately 90% for each treatment group. There were no significant differences in data availability between the two groups (Table 5).
| Data | Usual care (N = 312), n (%) | Home-based rehabilitation (N = 309), n (%) |
|---|---|---|
| Baseline | ||
| Data available | 312 (100.0) | 309 (100.0) |
| Completed CRF | 312 (100.0) | 309 (100.0) |
| Completed questionnaire | 312 (100.0) | 309 (100.0) |
| 6 months | ||
| Data available | 288 (92.3) | 281 (90.9) |
| Completed CRF | 280 (89.7) | 276 (89.3) |
| Completed questionnaire | 284 (91.0) | 278 (90.0) |
| Data not available | 24 (7.7) | 28 (9.1) |
| Withdrawna | 12 (2.8) | 12 (3.9) |
| Missingb | 12 (3.8) | 16 (5.2) |
| 12 months | ||
| Data available | 288 (92.3) | 280 (90.6) |
| Completed CRF | 282 (90.4) | 276 (89.3) |
| Completed questionnaire | 287 (92.0) | 279 (90.3) |
| Data not available | 24 (7.7) | 29 (9.4) |
| Withdrawna | 16 (5.1) | 18 (5.8) |
| Missingb | 7 (2.2) | 9 (2.9) |
| Died | 1 (0.3) | 2 (0.6) |
| Time point | Risk difference (95% CI) | p-value |
|---|---|---|
| 6 months | –0.01 (–0.06 to 0.03) | 0.54 |
| 12 months | –0.02 (–0.06 to 0.03) | 0.45 |
Withdrawals
Thirty-four participants (5.5%) withdrew from follow-up during the course of the trial. Most withdrawals were because of participants withdrawing their consent (n = 28), with the rest a result of a clinical decision (n = 6). Most of the withdrawals happened before the 6-month follow-up (n = 24) and the remainder happened between the 6-month and the 12-month follow-up (n = 10). Three participants died between the 6-month and the 12-month follow-up.
Baseline characteristics
Randomisation in the CORKA trial was stratified by recruiting site. The number and proportion of participants randomised to each treatment group at each site are summarised in Table 6. Participants were well balanced between the two groups at each site.
| Trial site | Usual care (N = 312), n (%) | Home-based rehabilitation (N = 309), n (%) | Total (N = 621), n (%) |
|---|---|---|---|
| Site 1 | 33 (10.6) | 34 (11.0) | 67 (10.8) |
| Site 2 | 38 (12.2) | 38 (12.3) | 76 (12.2) |
| Site 3 | 4 (1.3) | 4 (1.3) | 8 (1.3) |
| Site 4 | 8 (2.6) | 7 (2.3) | 15 (2.4) |
| Site 5 | 8 (2.6) | 7 (2.3) | 15 (2.4) |
| Site 6 | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Site 7 | 3 (1.0) | 2 (0.6) | 5 (0.8) |
| Site 8 | 1 (0.3) | 2 (0.6) | 3 (0.5) |
| Site 9 | 2 (0.6) | 2 (0.6) | 4 (0.6) |
| Site 10 | 15 (4.8) | 14 (4.5) | 29 (4.7) |
| Site 11 | 30 (9.6) | 30 (9.7) | 60 (9.7) |
| Site 12 | 99 (31.7) | 100 (32.4) | 199 (32.0) |
| Site 13 | 65 (20.8) | 63 (20.4) | 128 (20.6) |
| Site 14 | 5 (1.6) | 5 (1.6) | 10 (1.6) |
A screening tool was used to identify participants at higher risk of a poor outcome following KA for inclusion in the CORKA trial (see Chapter 7). The key features of the screening tool were body mass index (BMI), pain, health status and anxiety or depression.
Table 7 summarises the number and proportion of people satisfying each of the criteria, which combine to give the total screening tool score. These appear well balanced across the two treatment groups. The slight apparent imbalance in BMI may have been because this variable was treated as categorical (see Table 8). Table 7 also included the contribution of each characteristic to the total score, the number of participants with each score and the constituent parts that make up that score. All participants met the moderate to severe usual pain from the knee criteria, which was usually combined with a high BMI.
| Screening tool details | Usual care (N = 312), n (%) | Home-based rehabilitation (N = 309), n (%) | Total (N = 621), n (%) |
|---|---|---|---|
| BMI | |||
| Normal (0) | 11 (3.5) | 7 (2.3) | 18 (2.9) |
| Overweight (1) | 121 (38.8) | 138 (44.7) | 259 (41.7) |
| Obese (2) | 180 (57.7) | 164 (53.1) | 344 (55.4) |
| Usual pain from knee | |||
| Moderate or severe (4) | 312 (100.0) | 309 (100.0) | 621 (100.0) |
| Health status | |||
| Fit and healthy (0) | 296 (94.9) | 298 (96.4) | 594 (95.7) |
| Severe systemic disease (2) | 16 (5.1) | 11 (3.6) | 27 (4.3) |
| Limited | |||
| None, a little or some of the time (0) | 248 (79.5) | 248 (80.3) | 496 (79.9) |
| Most or all of the time (2) | 64 (20.5) | 61 (19.7) | 125 (20.1) |
| Screening tool scores and constituent parts | |||
| 5 – Overweight | 87 (27.9) | 104 (33.7) | 191 (30.8) |
| 6 – Obese | 150 (48.1) | 135 (43.7) | 285 (45.9) |
| 6 – Severe systemic disease | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| 6 – Limited most/all of the time | 10 (3.2) | 7 (2.3) | 17 (2.7) |
| 7 – Overweight and severe systemic disease | 4 (1.3) | 6 (1.9) | 10 (1.6) |
| 7 – Overweight and limited most/all of the time | 26 (8.3) | 27 (8.7) | 53 (8.5) |
| 8 – Obese and severe systemic disease | 6 (1.9) | 3 (1.0) | 9 (1.4) |
| 8 – Obese and limited most/all of the time | 23 (7.4) | 25 (8.1) | 48 (7.7) |
| 9 – Overweight and severe systemic disease and limited most/all of the time | 4 (1.3) | 1 (0.3) | 5 (0.8) |
| 10 – Obese and severe systemic disease and limited most/all of the time | 1 (0.3) | 1 (0.3) | 2 (0.3) |
The descriptive characteristics of all randomised participants are summarised by treatment group, and overall, in Table 8. These values are presented as numbers and percentages for binary and categorical factors, and as means and SDs or medians and IQRs, as appropriate, for continuous variables. These variables appear well balanced across the two treatment groups.
| Characteristic | Usual care (N = 312) | Home-based rehabilitation (N = 309) | Total (N = 621) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 125 (40.1) | 125 (40.5) | 250 (40.3) |
| Female | 187 (59.9) | 184 (59.5) | 371 (59.7) |
| Age (years), mean (SD) | 70.18 (8.14) | 70.67 (8.01) | 70.42 (8.07) |
| BMI (kg/m2), mean (SD) | 31.65 (4.99) | 31.34 (4.48) | 31.50 (4.74) |
| Side of operation, n (%) | |||
| Right | 169 (54.2) | 169 (54.7) | 338 (54.4) |
| Left | 142 (45.5) | 139 (45.0) | 281 (45.2) |
| Not recorded | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Knee arthroplasty type, n (%) | |||
| TKA | 229 (73.4) | 231 (74.8) | 460 (74.1) |
| UKA | 82 (26.3) | 77 (24.9) | 159 (25.6) |
| Not recorded | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| ASA classification grade, n (%) | |||
| Healthy | 38 (12.2) | 55 (17.8) | 93 (15.0) |
| Mild systemic disease | 218 (69.9) | 202 (65.4) | 420 (67.6) |
| Severe systemic disease | 43 (13.8) | 44 (14.2) | 87 (14.0) |
| Not recorded | 13 (4.2) | 8 (2.6) | 21 (3.4) |
| Falls in the last year | |||
| Yes, n (%) | 77 (24.7) | 89 (28.8) | 166 (26.7) |
| No, n (%) | 235 (75.3) | 220 (71.2) | 455 (73.3) |
| If yes, number of falls, median (IQR) | 1 (1, 3) | 2 (1, 3) | 2 (1, 3) |
| Previous lower limb surgery, n (%) | |||
| Yes | 200 (64.1) | 189 (61.2) | 389 (62.6) |
| No | 112 (35.9) | 120 (38.8) | 232 (37.4) |
| Screening tool score, median (IQR) | 6 (5, 6) | 6 (5, 6) | 6 (5, 6) |
| Charnley ABC, n (%) | |||
| A: single KA | 134 (42.9) | 138 (44.7) | 272 (43.8) |
| B: both knees affected | 140 (44.9) | 145 (46.9) | 285 (45.9) |
| C: multiple joint disease/other disability | 38 (12.2) | 26 (8.4) | 64 (10.3) |
| Stairs mobility, n (%) | |||
| Normal | 19 (6.1) | 19 (6.1) | 38 (6.1) |
| One step at a time | 34 (10.9) | 39 (12.6) | 73 (11.8) |
| Down with rail | 18 (5.8) | 19 (6.1) | 37 (6.0) |
| Up/down with rail | 225 (72.1) | 216 (69.9) | 441 (71.0) |
| Unable down | 2 (0.6) | 3 (1.0) | 5 (0.8) |
| Unable | 14 (4.5) | 12 (3.9) | 26 (4.2) |
| Missing | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Support mobility, n (%) | |||
| None | 178 (57.1) | 178 (57.6) | 356 (57.3) |
| Stick outdoors | 83 (26.6) | 78 (25.2) | 161 (25.9) |
| Stick always | 34 (10.9) | 31 (10.0) | 65 (10.5) |
| Two sticks | 6 (1.9) | 7 (2.3) | 13 (2.1) |
| Two crutches | 5 (1.6) | 7 (2.3) | 12 (1.9) |
| Walking frame | 6 (1.9) | 8 (2.6) | 14 (2.3) |
| Functional comorbidity index,a n (%) | |||
| 0 | 189 (60.6) | 176 (57.0) | 365 (58.8) |
| 1–3 | 112 (35.9) | 125 (40.5) | 237 (38.2) |
| 4–6 | 8 (2.6) | 6 (1.9) | 14 (2.3) |
| 7 or more | 2 (0.6) | 0 (0.0) | 2 (0.3) |
| Missing | 1 (0.3) | 2 (0.6) | 3 (0.5) |
The PROMs and physical measure baseline values are summarised by treatment group, and overall, in Table 9, and are all similar across the two treatment groups.
| Outcome | Usual care (N = 312) | Home-based rehabilitation (N = 309) | Total (N = 621) |
|---|---|---|---|
| LLFDI function, mean (SD) | 51.21 (7.09) | 51.68 (7.17) | 51.45 (7.13) |
| LLFDI disability (frequency), mean (SD) | 51.28 (7.25) | 51.55 (7.46) | 51.42 (7.35) |
| LLFDI disability (limitation), mean (SD) | 66.26 (11.96) | 66.25 (12.48) | 66.26 (12.21) |
| EQ-5D-5L utility, median (IQR) | 0.59 (0.39, 0.70) | 0.59 (0.39, 0.70) | 0.59 (0.39, 0.70) |
| EQ-5D-5L VAS, median (IQR) | 70.0 (58.0, 80.0) | 75.0 (55.0, 80.0) | 70.0 (55.0, 80.0) |
| OKS,a mean (SD) | 20.59 (7.50) | 20.81 (7.31) | 20.70 (7.40) |
| PASE, median (IQR) | 114.4 (62.2, 160.0) | 98.5 (62.2, 149.6) | 108.2 (62.2, 157.4) |
| KOOS, median (IQR) | 25.0 (12.5, 37.5) | 25.0 (12.5, 37.5) | 25.0 (12.5, 37.5) |
| 30SCST number of stands, median (IQR) | 8 (6, 10) | 8 (6, 11) | 8 (6, 10) |
| 30SCST adaptations, n (%) | |||
| None | 211 (67.6) | 214 (69.3) | 425 (68.4) |
| Uses hands on legs | 94 (30.1) | 90 (29.1) | 184 (29.6) |
| Uses walking aid | 2 (0.6) | 4 (1.3) | 6 (1.0) |
| Not tested: unable | 5 (1.6) | 1 (0.3) | 6 (1.0) |
| F8WT | |||
| Time (seconds), median (IQR) | 10.3 (8.6, 13.0) | 10.9 (9.0, 14.0) | 10.6 (8.8, 13.5) |
| Steps, median (IQR) | 16.0 (13.0, 19.0) | 16.0 (14.0, 19.0) | 16.0 (14.0, 19.0) |
| Stayed within cones, n (%) | 303 (97.1) | 304 (98.4) | 607 (97.7) |
| F8WT smoothness score, n (%) | |||
| 0 | 9 (2.9) | 11 (3.6) | 20 (3.2) |
| 1 | 73 (23.4) | 87 (28.2) | 160 (25.8) |
| 2 | 50 (16.0) | 56 (18.1) | 106 (17.1) |
| 3 | 180 (57.7) | 155 (50.2) | 335 (53.9) |
| SLS test average time (seconds), median (IQR) | |||
| KA side | 5.3 (2.6, 13.0) | 4.5 (1.9, 14.5) | 5.1 (2.2, 14.0) |
| Other side | 7.2 (3.6, 22.0) | 6.3 (2.6, 18.0) | 6.9 (3.2, 19.8) |
Treatment compliance
Participants were defined as complying with usual-care if they attended at least one treatment session. Compliance with home-based rehabilitation was defined as receiving at least four treatment sessions. Table 10 summarises the number and percentage of compliers in each treatment group, the number of participants who attended no sessions and the average number of treatment sessions received.
| Treatment details | Usual care (N = 312) | Home-based rehabilitation (N = 309) |
|---|---|---|
| Minimum number of sessions for compliance | 1 | 4 |
| Compliers, n (%) | 297 (95.2) | 269 (87.1) |
| Participants with no treatment logs, n (%) | 12 (3.8) | 10 (3.2) |
| Number of sessions, median (IQR) | 4 (2, 6) | 5 (4, 7) |
| Number of sessions (minimum, maximum) | (0, 27) | (0, 8) |
| Number of sessions by replacement type, median (IQR) | ||
| TKA | 4 (2, 6) | 5 (4, 7) |
| UKA | 3 (3, 4) | 5 (4, 6) |
| Time to first session, n (%) | ||
| ≤ 4 weeks | 273 (87.5) | 276 (89.3) |
| 4–8 weeks | 15 (4.8) | 20 (6.5) |
| > 8 weeks | 7 (2.2) | 2 (0.6) |
| Did not start | 13 (4.2) | 10 (3.2) |
| Missing | 4 (1.3) | 1 (0.3) |
Compliance was high in both groups. The median number of treatment sessions was higher in the home-based rehabilitation arm than in the usual-care arm. However, there was significant variability in the number of sessions of usual care received, ranging from 0 to 27 sessions. Participants who underwent UKA and TKA attended similar average (median, IQR) numbers of sessions. Figure 5 shows how many participants in each treatment group received each number of sessions.
FIGURE 5.
Comparison of number of treatment sessions received in the two treatment groups.

The time from randomisation to first treatment session is also summarised in Table 10. Most participants received their first session within 4 weeks, as per the protocol. Similar numbers of participants received their first treatment within 4 weeks in the two treatment arms.
The average (median and IQR) and minimum and maximum number of treatment sessions received were calculated separately by treatment group for each recruiting site (Table 11). The ‘other’ category for recruiting sites includes all sites that recruited < 15 participants. Sites 4 and 5 provided follow-up care jointly and are, therefore, combined in this table.
| Site | Usual care | Home-based rehabilitation | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | (Min, max) | n | Median (IQR) | (Min, max) | n | |
| Site 1 | 6 (4, 8) | (0, 12) | 33 | 4 (4, 6) | (0, 7) | 34 |
| Site 2 | 4 (3, 6) | (1, 15) | 38 | 5 (4, 6) | (1, 8) | 38 |
| Sites 4 and 5a | 4 (3, 5.5) | (2, 9) | 16 | 7 (6, 7) | (3, 7) | 14 |
| Site 10 | 3 (0, 4) | (0, 7) | 15 | 7 (5, 7) | (0, 7) | 14 |
| Site 11 | 4 (4, 5) | (0, 8) | 30 | 4 (4, 6) | (0, 7) | 30 |
| Site 12 | 4 (3, 6) | (0, 23) | 99 | 5 (4, 6) | (0, 7) | 100 |
| Site 13 | 2 (2, 4) | (0, 12) | 65 | 6 (5, 7) | (0, 8) | 63 |
| Other sitesb | 3.5 (1, 7) | (0, 27) | 16 | 4 (2.5, 7) | (0, 8) | 16 |
Substantial variability by site in the number of treatment sessions received was identified. The median number of sessions of usual-care treatment ranged from just two sessions at site 13 to six sessions at site 1. Similarly, the median number of sessions of home-based rehabilitation varied from four sessions at site 1 to seven sessions at sites 4 and 5 and site 10. Site 1 participants who were allocated to usual care received more treatment sessions, on average, than those allocated to home-based rehabilitation.
Details of the usual care delivered by each site are summarised in Table 12. Again, the ‘other’ sites are grouped together and sites 4 and 5 are combined. The total number of sessions provided, the number of participants recruited and the average number of sessions are given for each site. The number and proportion of sessions taking place in each location (home and hospital) are summarised by site. Although > 80% of sessions took place in hospital at most sites, only 21.6% of sessions took place in hospital at site 13, with the rest of the usual-care patients seen in a community setting.
| Site | Sessions (n) | Participants (n) | Sessions/participant | Location, n (%) | Individual,a n (%) | Exercise therapy,b n (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Min, max | Hospital | Community | Home | |||||
| Site 1 | 186 | 33 | 6 (4, 8) | 0, 12 | 181 (97.3) | 0 (0.0) | 5 (2.7) | 108 (58.1) | 186 (100.0) |
| Site 2 | 182 | 38 | 4 (3, 6) | 1, 15 | 178 (97.8) | 4 (2.2) | 0 (0.0) | 173 (95.1) | 171 (94.0) |
| Sites 4 and 5c | 72 | 16 | 4 (3, 5.5) | 2, 9 | 67 (93.1) | 0 (0.0) | 5 (6.9) | 61 (84.7) | 69 (95.8) |
| Site 10 | 37 | 15 | 3 (0, 4) | 0, 7 | 31 (83.8) | 0 (0.0) | 5 (13.5) | 30 (81.1) | 33 (89.2) |
| Site 11 | 121 | 30 | 4 (4, 5) | 0, 8 | 114 (94.2) | 3 (2.5) | 4 (3.3) | 58 (47.9) | 119 (98.3) |
| Site 12 | 467 | 99 | 4 (3, 6) | 0, 23 | 466 (99.8) | 1 (0.2) | 0 (0.0) | 424 (90.8) | 438 (93.8) |
| Site 13 | 208 | 65 | 2 (2, 4) | 0, 12 | 45 (21.6) | 1 (0.5) | 162 (77.9) | 179 (86.1) | 201 (96.6) |
| Other sitesd | 89 | 16 | 3.5 (1, 7) | 0, 27 | 73 (82.0) | 12 (13.5) | 4 (4.5) | 50 (56.2) | 79 (88.8) |
The number and proportion of sessions of individual therapy provided at each site are summarised. The sites can be separated into two groups. At sites 1 and 11 and the ‘other’ sites, the sessions were split relatively evenly between individual therapy (40% to 60% of participants) and group therapy. At the remaining sites, > 80% of the sessions were individual therapy. Most sessions at all sites were exercise therapy (> 85%).
Details of the home-based rehabilitation treatment delivered by each site are summarised in Table 13. The number of participants and the total number of treatment sessions given are included in this table. The number and proportion of treatment sessions, including each type of exercise, appear similar across sites, with some notable exceptions. A smaller proportion of sessions delivered by site 1 than the rest of the sites included flexion, extension and basic quadriceps exercises, and a smaller proportion of sessions delivered by site 13 than the rest of the sites included static balance and gait skills exercises.
| Site | Sessions (n) | Participants (n) | Exercise, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flexion | Extension | Basic quadriceps | Quadriceps | Hamstrings | Hip abductor | Calf | Static balance | Gait skills | Additional exercises | |||
| Site 1 | 150 | 34 | 104 (69) | 80 (53) | 97 (65) | 145 (97) | 147 (98) | 140 (93) | 140 (93) | 130 (87) | 115 (77) | 27 (18) |
| Site 2 | 192 | 38 | 189 (98) | 190 (99) | 189 (98) | 186 (97) | 169 (88) | 169 (88) | 167 (87) | 150 (78) | 140 (73) | 49 (26) |
| Sites 4 and 5a | 90 | 14 | 82 (91) | 74 (82) | 71 (79) | 89 (99) | 88 (98) | 80 (89) | 81 (90) | 84 (93) | 81 (90) | 13 (14) |
| Site 10 | 80 | 14 | 79 (99) | 79 (99) | 79 (99) | 80 (100) | 79 (99) | 78 (98) | 78 (98) | 79 (99) | 75 (94) | 16 (20) |
| Site 11 | 134 | 30 | 133 (99) | 130 (97) | 125 (93) | 132 (99) | 128 (96) | 121 (90) | 129 (96) | 113 (84) | 124 (93) | 97 (72) |
| Site 12 | 523 | 100 | 511 (98) | 510 (98) | 510 (98) | 510 (98) | 493 (94) | 489 (93) | 491 (94) | 487 (93) | 472 (90) | 257 (49) |
| Site 13 | 355 | 63 | 330 (93) | 343 (97) | 315 (89) | 310 (87) | 314 (88) | 233 (66) | 292 (82) | 188 (53) | 204 (57) | 122 (34) |
| Other sitesb | 66 | 16 | 55 (83) | 60 (91) | 59 (89) | 64 (97) | 57 (86) | 53 (80) | 49 (74) | 54 (82) | 51 (77) | 15 (23) |
Primary outcome analyses
Trends in LLFDI function scores over time are plotted for each treatment group in Figure 6. There was a substantial improvement between baseline and 6 months in both groups, with minimal additional difference between 6 and 12 months post randomisation. No substantial differences between the two groups are visible at either time point.
FIGURE 6.
The LLFDI function score from baseline to 12 months post randomisation for each treatment group.

The primary analysis of the LLFDI function score was carried out for the ITT population using multiple imputation. There was no statistically significant difference between the two treatment groups at the primary time point of 12 months post randomisation (adjusted difference 0.49, 95% CI –0.89 to 1.88; p = 0.34) or at 6 months (Table 14).
| Time point | Usual care | Home-based rehabilitation | Adjusted difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Total | Mean (SD) | Total | |||
| ITT multiple imputation | ||||||
| Baseline | 51.20 (7.07) | 312 | 51.64 (7.19) | 309 | – | – |
| 6 months | 59.07 (8.58) | 312 | 59.73 (8.65) | 309 | 0.66 (–0.70 to 2.03) | 0.34 |
| 12 months | 59.97 (8.70) | 312 | 60.47 (8.60) | 309 | 0.49 (–0.89 to 1.88) | 0.48 |
| ITT available cases | ||||||
| Baseline | 51.21 (7.09) | 309 | 51.68 (7.17) | 307 | – | – |
| 6 months | 59.37 (8.22) | 282 | 60.01 (8.21) | 276 | 0.64 (–0.72 to 2.01) | 0.36 |
| 12 months | 60.29 (8.25) | 286 | 60.76 (8.24) | 279 | 0.47 (–0.89 to 1.83) | 0.50 |
| PP available cases | ||||||
| Baseline | 51.07 (7.10) | 242 | 51.81 (7.23) | 293 | – | – |
| 6 months | 59.85 (8.17) | 222 | 60.00 (8.19) | 270 | 0.15 (–1.31 to 1.60) | 0.84 |
| 12 months | 60.86 (8.22) | 228 | 60.74 (8.20) | 270 | –0.13 (–1.58 to 1.32) | 0.86 |
| CACE | ||||||
| Overall | – | – | – | – | 0.62 (–0.79 to 2.03) | 0.39 |
Sensitivity analyses were carried out considering the ITT population using available cases only, the PP population using available cases and a complier-average causal effects analysis. No significant differences between the two treatment groups at either time point were identified (see Table 14). Additional sensitivity analyses of the primary outcome included unadjusted models; models with no treatment-by-time interaction, including time as a continuous rather than categorical predictor; and models excluding data recorded outside the specified time windows. None led to a different conclusion.
Subgroup analyses
Consistency of the treatment effect across sites was explored by including an interaction between treatments and recruiting site in the model. A forest plot of treatment effects for each recruitment site is provided in Figure 7. The treatment effects varied across sites, from an effect of 9 points in favour of home-based rehabilitation at site 10 (95% CI 2.95 to 15.10 points) to 1 point in favour of usual care at site 1 (95% CI –4.80 to 2.86 points). This variability appears to be at least in part related to the amount of treatment received at each site, with participants in site 1 receiving more sessions of usual care and less sessions of home-based rehabilitation than those in site 10.
FIGURE 7.
Forest plot of treatment effects by recruiting site. Sites 4 and 5 provided follow-up jointly, so are reported together. ‘Other’ sites include all those that recruited fewer than 15 participants.
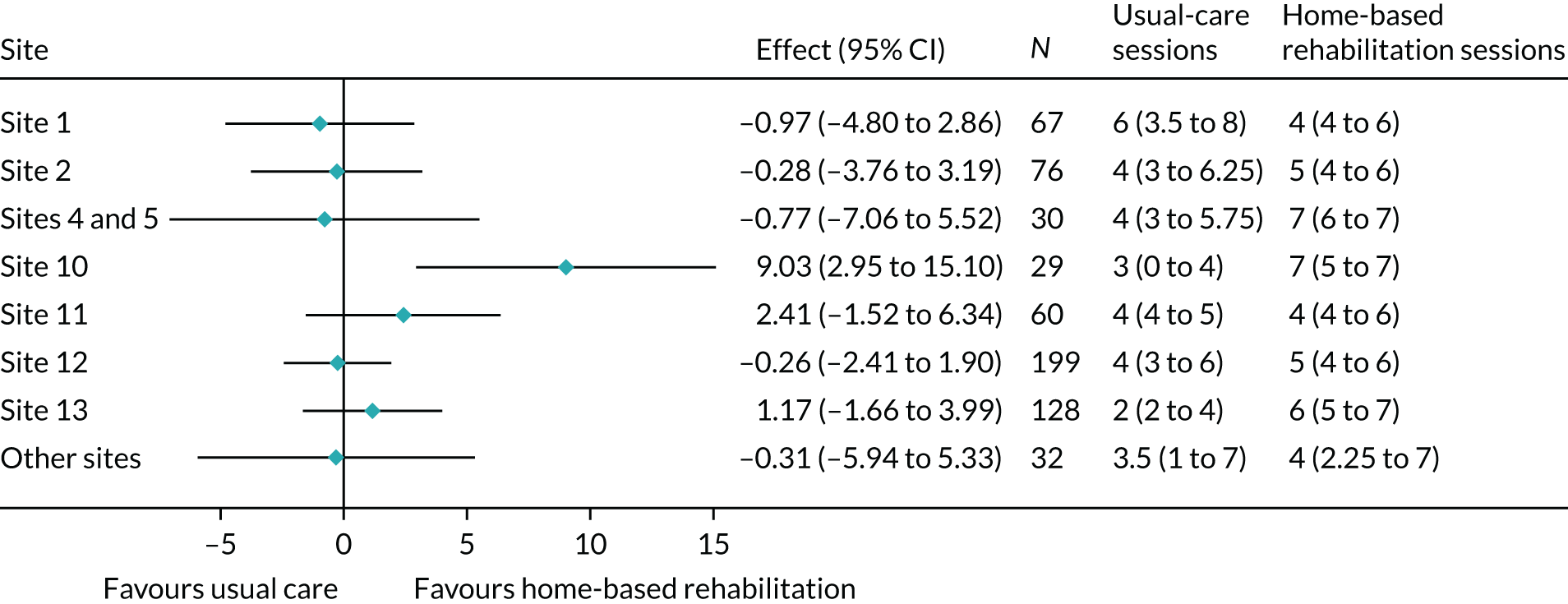
Similar analyses were used to explore the relationship between treatment effect and age, gender, number of falls, screening tool score and the constituent parts of the screening tool (BMI category, health status and anxiety/depression). No clear patterns were identified. A forest plot of treatment effects by screening tool score is provided in Appendix 1.
The Late Life Function and Disability Instrument function subscales
The LLFDI function component is made up of three subscales: upper extremity function, basic lower extremity function and advanced lower extremity function. The values of each subscale are summarised from baseline to 12-month follow-up by treatment group in Table 15. The trends in each subscale were similar for the two treatment groups, with most of the observed improvement seen by 6 months post randomisation. The improvements in upper extremity function were more modest than those in basic and advanced lower extremity function, as would be anticipated.
| LLFDI subscales | Usual care | Home-based rehabilitation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |||||||
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| Upper | 76.0 (15.0) | 311 | 82.8 (16.0) | 284 | 83.2 (15.7) | 287 | 76.5 (15.8) | 307 | 82.9 (16.2) | 277 | 83.8 (15.3) | 279 |
| Basic lower | 59.8 (10.8) | 312 | 71.2 (15.6) | 284 | 72.5 (15.9) | 287 | 60.9 (11.3) | 307 | 73.5 (15.2) | 277 | 73.9 (15.0) | 279 |
| Advanced lower | 32.8 (13.3) | 310 | 46.2 (17.3) | 283 | 47.8 (17.9) | 287 | 33.8 (13.3) | 307 | 47.9 (16.4) | 276 | 48.5 (18.1) | 279 |
Secondary outcome analyses
Patient-reported outcome measures
Trends over time for each of the key secondary PROMs are plotted in Figure 8. Although there was only a small improvement over time in the two LLFDI disability variables, the OKS and KOOS both showed similar substantial improvement between baseline and 6 months in both treatment groups. There was little additional improvement between 6 and 12 months for either variable.
FIGURE 8.
Key secondary participant-reported outcome measures from baseline to 12 months post randomisation by treatment group. (a) LLFDI disability frequency; (b) LLFDI disability limitation; (c) OKS; and (d) KOOS.

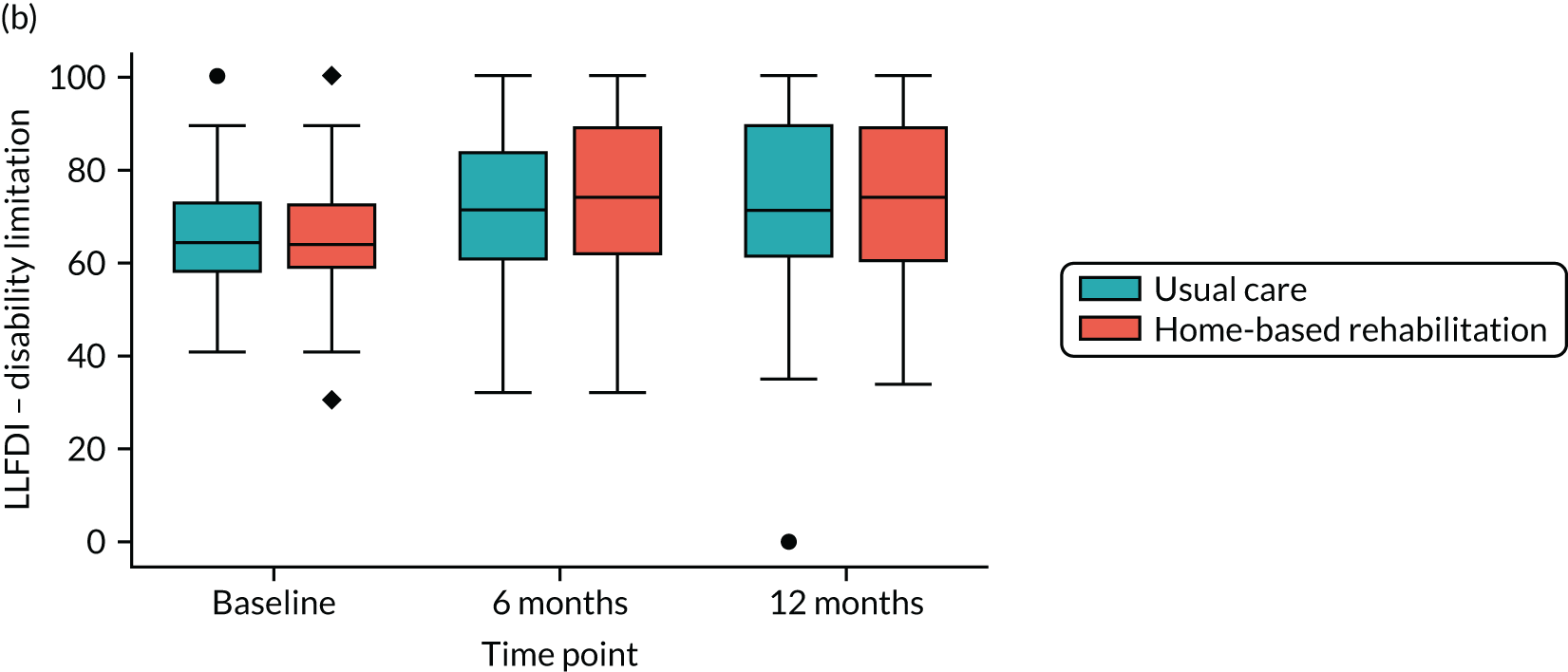
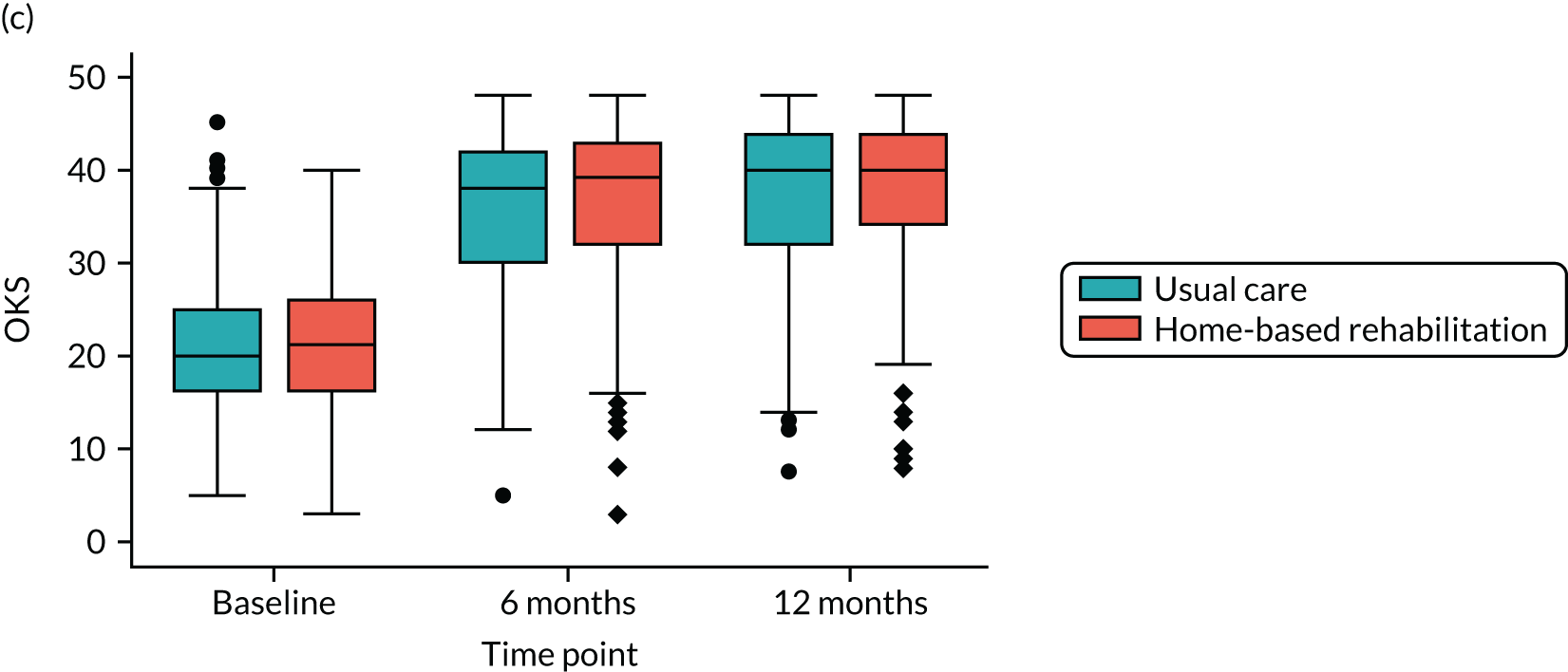
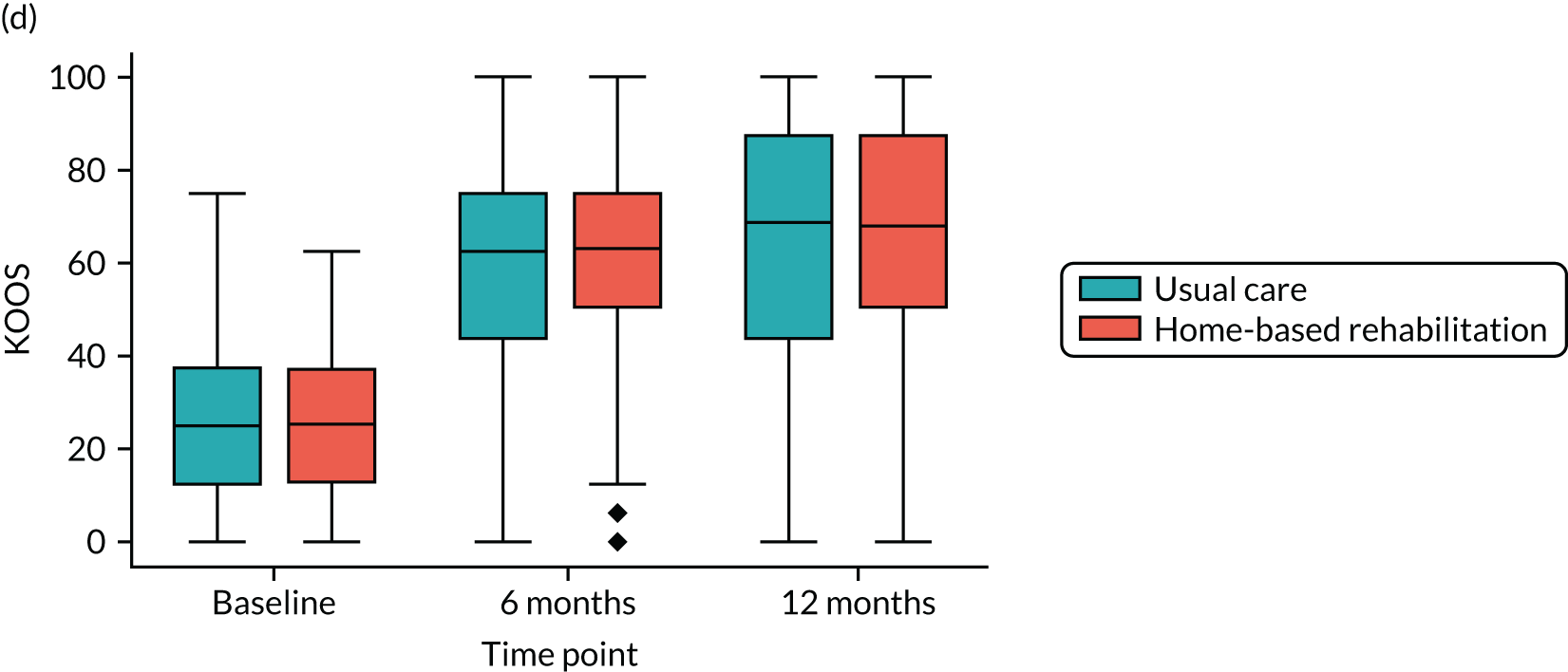
Trends over time are plotted for the PASE and EQ-5D-5L secondary outcomes in Figure 9, showing small improvements over time.
FIGURE 9.
Additional secondary participant-reported outcome measures from baseline to 12 months post randomisation by treatment group. (a) PASE; (b) EQ-5D-5L utility; and (c) EQ-5D-5L visual analogue scale.

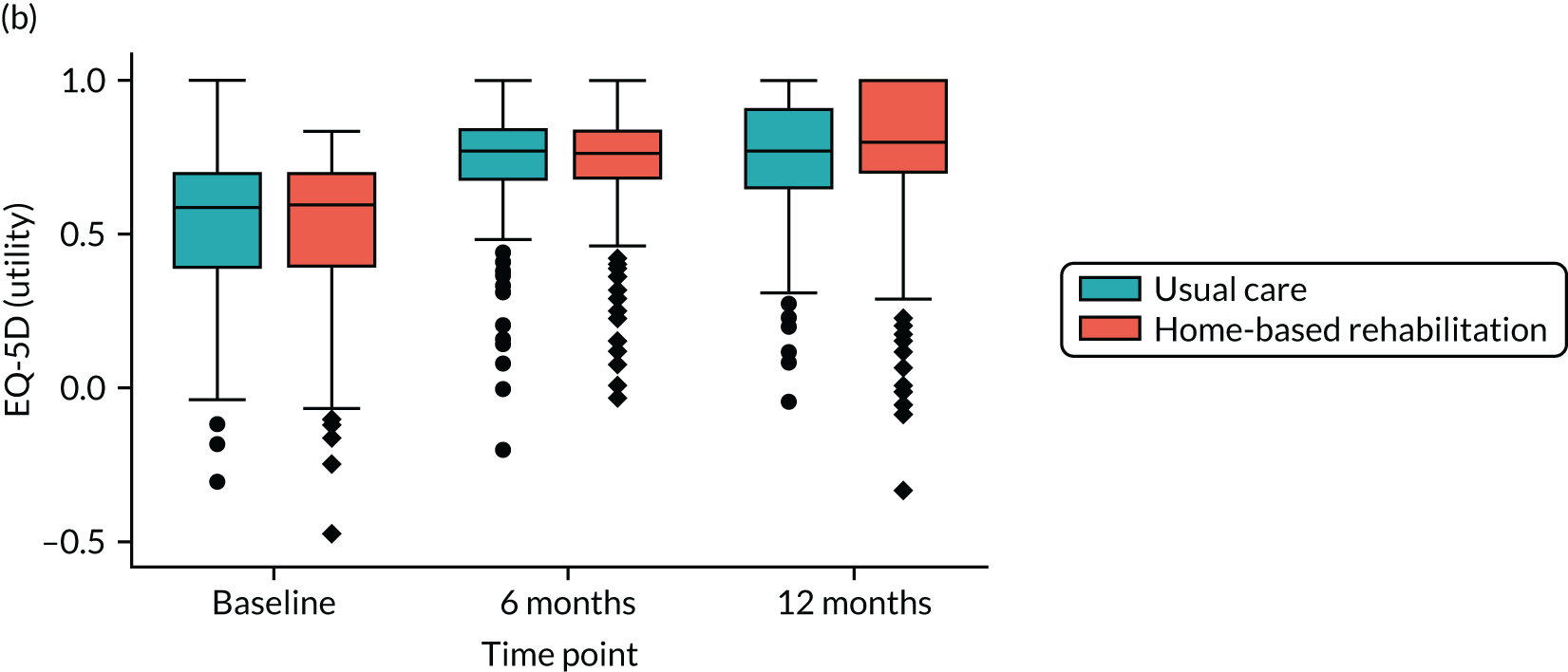

The primary analysis for each of the secondary PROMs was conducted for the ITT population using available cases only. No significant differences between the two treatment groups at either time point (6 and 12 months) were identified for any of the secondary outcomes (Table 16).
| Time point | Usual care | Home-based rehabilitation | Adjusted difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Total | Mean (SD) | Total | |||
| LLFDI disability (frequency) | ||||||
| Baseline | 51.28 (7.25) | 311 | 51.55 (7.46) | 308 | – | – |
| 6 months | 54.27 (6.86) | 283 | 54.91 (6.85) | 275 | 0.64 (–0.50 to 1.78) | 0.27 |
| 12 months | 54.13 (6.87) | 287 | 55.05 (6.87) | 279 | 0.93 (–0.21 to 2.06) | 0.11 |
| LLFDI disability (limitation) | ||||||
| Baseline | 66.26 (11.96) | 312 | 66.25 (12.48) | 305 | – | – |
| 6 months | 72.33 (15.25) | 284 | 74.72 (15.24) | 275 | 2.39 (–0.14 to 4.92) | 0.06 |
| 12 months | 74.10 (15.27) | 287 | 74.75 (15.28) | 277 | 0.65 (–1.87 to 3.18) | 0.61 |
| OKS | ||||||
| Baseline | 20.59 (7.50) | 312 | 20.81 (7.31) | 308 | – | – |
| 6 months | 35.69 (7.69) | 284 | 36.23 (7.69) | 277 | 0.54 (–0.73 to 1.81) | 0.40 |
| 12 months | 37.34 (7.71) | 287 | 37.80 (7.71) | 278 | 0.46 (–0.81 to 1.73) | 0.48 |
| KOOS – QoL subscale | ||||||
| Baseline | 25.50 (16.58) | 301 | 25.50 (15.89) | 300 | – | – |
| 6 months | 61.26 (22.31) | 273 | 62.47 (22.29) | 269 | 1.21 (–2.55 to 4.97) | 0.53 |
| 12 months | 65.53 (22.37) | 277 | 65.26 (22.36) | 271 | –0.27 (–4.02 to 3.47) | 0.89 |
| PASE | ||||||
| Baseline | 121.24 (73.90) | 302 | 115.11 (72.95) | 297 | – | – |
| 6 months | 150.62 (66.72) | 259 | 149.41 (66.46) | 245 | –1.22 (–12.86 to 10.42) | 0.84 |
| 12 months | 156.58 (66.82) | 263 | 154.63 (66.73) | 251 | –1.95 (–13.51 to 9.61) | 0.74 |
| EQ-5D-5L utility | ||||||
| Baseline | 0.52 (0.22) | 308 | 0.52 (0.23) | 308 | – | – |
| 6 months | 0.75 (0.18) | 277 | 0.75 (0.18) | 275 | 0.00 (–0.03 to 0.03) | 0.85 |
| 12 months | 0.76 (0.18) | 282 | 0.76 (0.18) | 278 | –0.00 (–0.03 to 0.03) | 0.99 |
| EQ-5D–5L VAS | ||||||
| Baseline | 68.53 (19.46) | 312 | 68.03 (19.13) | 308 | – | – |
| 6 months | 75.81 (14.55) | 283 | 75.51 (14.54) | 275 | –0.30 (–2.71 to 2.11) | 0.81 |
| 12 months | 76.80 (14.57) | 287 | 77.05 (14.58) | 278 | 0.24 (–2.16 to 2.65) | 0.84 |
For the key secondary PROMs (LLFDI disability frequency and limitation, OKS and KOOS QoL), sensitivity analyses were performed for the PP population using available cases and for the ITT population using multiple imputation, and complier-average causal effects were calculated. Only one significant difference was identified, which was for the LLFDI disability limitation at 6 months using the ITT population and multiple imputation (Table 17). This effect only just reached significance (adjusted difference 2.67, 95% CI 0.14 to 5.19; p = 0.04) and is probably a chance effect.
| Time point | Usual care | Home-based rehabilitation | Adjusted difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Total | Mean (SD) | Total | |||
| Per-protocol population using available cases | ||||||
| LLFDI disability (frequency) | ||||||
| Baseline | 51.22 (7.41) | 243 | 51.74 (7.50) | 293 | – | – |
| 6 months | 54.39 (6.96) | 223 | 55.03 (6.96) | 269 | 0.63 (–0.60 to 1.87) | 0.32 |
| 12 months | 54.37 (6.97) | 229 | 55.09 (6.97) | 270 | 0.72 (–0.51 to 1.95) | 0.25 |
| LLFDI disability (limitation) | ||||||
| Baseline | 65.87 (11.25) | 244 | 66.30 (12.39) | 291 | – | – |
| 6 months | 73.12 (15.35) | 224 | 74.73 (15.37) | 269 | 1.60 (–1.13 to 4.33) | 0.25 |
| 12 months | 74.44 (15.38) | 229 | 74.99 (15.38) | 268 | 0.55 (–2.17 to 3.27) | 0.69 |
| OKS | ||||||
| Baseline | 20.57 (7.41) | 244 | 20.98 (7.32) | 293 | – | – |
| 6 months | 36.23 (7.53) | 224 | 36.34 (7.56) | 271 | 0.11 (–1.22 to 1.45) | 0.87 |
| 12 months | 37.96 (7.57) | 229 | 37.82 (7.56) | 269 | –0.14 (–1.48 to 1.19) | 0.83 |
| KOOS – QoL subscale | ||||||
| Baseline | 25.53 (16.67) | 235 | 25.66 (15.83) | 285 | – | – |
| 6 months | 63.14 (22.00) | 215 | 62.70 (22.05) | 263 | –0.44 (–4.41 to 3.54) | 0.83 |
| 12 months | 67.40 (22.08) | 220 | 65.76 (22.06) | 262 | –1.64 (–5.60 to 2.32) | 0.42 |
| Intent-to-treat population using multiple imputation | ||||||
| LLFDI disability (frequency) | ||||||
| Baseline | 51.28 (7.24) | 312 | 51.52 (7.46) | 309 | – | – |
| 6 months | 53.954 (7.284) | 312 | 54.58 (8.09) | 309 | 0.63 (–0.54 to 1.79) | 0.29 |
| 12 months | 53.81 (7.13) | 312 | 54.63 (7.73) | 309 | 0.82 (–0.32 to 1.96) | 0.16 |
| LLFDI disability (limitation) | ||||||
| Baseline | 66.26 (11.96) | 312 | 66.19 (12.49) | 309 | – | – |
| 6 months | 71.97 (15.88) | 312 | 74.51 (15.62) | 309 | 2.67 (0.14 to 5.19) | 0.04 |
| 12 months | 73.80 (16.63) | 312 | 74.51 (15.62) | 309 | 0.71 (–1.88 to 3.30) | 0.59 |
| OKS | ||||||
| Baseline | 20.59 (7.50) | 312 | 20.82 (7.30) | 309 | – | – |
| 6 months | 35.51 (8.69) | 312 | 36.05 (8.42) | 309 | 0.54 (–0.80 to 1.89) | 0.43 |
| 12 months | 37.18 (8.50) | 312 | 37.80 (8.04) | 309 | 0.62 (–0.70 to 1.94) | 0.35 |
| KOOS – QoL subscale | ||||||
| Baseline | 25.49 (16.33) | 312 | 25.66 (15.70) | 309 | – | – |
| 6 months | 61.09 (23.51) | 312 | 62.50 (24.60) | 309 | 1.41 (–2.36 to 5.18) | 0.46 |
| 12 months | 65.22 (23.64) | 312 | 65.24 (23.59) | 309 | 0.02 (–3.62 to 3.66) | 0.99 |
| Complier-average causal effect | ||||||
| LLFDI disability (frequency) | ||||||
| Overall | – | – | – | – | 0.87 (–0.22 to 1.97) | 0.12 |
| LLFDI disability (limitation) | ||||||
| Overall | – | – | – | – | 1.69 (–0.79 to 4.17) | 0.18 |
| OKS | ||||||
| Overall | – | – | – | – | 0.56 (–0.76 to 1.88) | 0.41 |
| KOOS – QoL subscale | ||||||
| Overall | – | – | – | – | 0.51 (–3.28 to 4.31) | 0.79 |
Physical measures
Trends over time in the physical measures are summarised in Figure 10. There were small improvements over time in each measure, but none was substantial.
FIGURE 10.
Physical measures from baseline to 12-month follow-up by treatment group. (a) 30SCST number of stands; (b) F8WT time in seconds; (c) F8WT number of steps; (d) arthroplasty side stance time; and (e) contralateral side stance time.




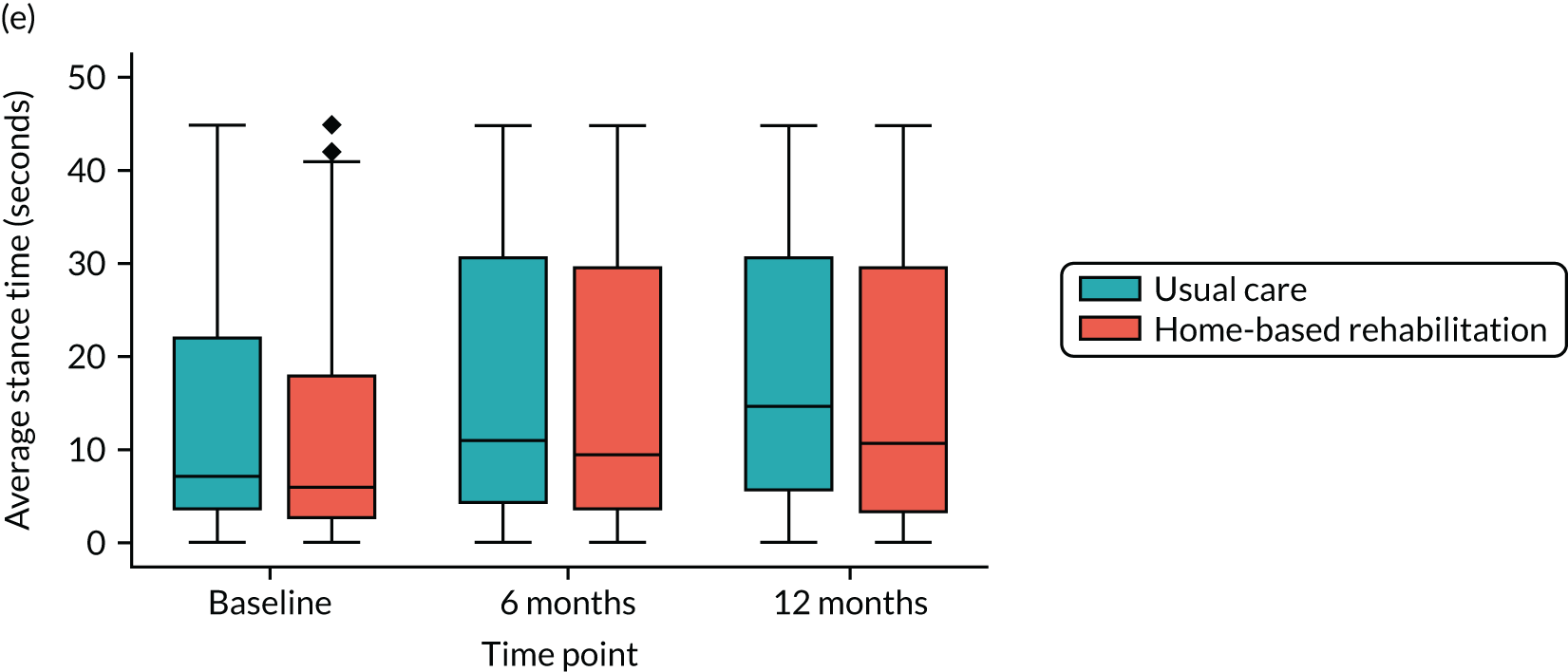
The physical measures are summarised by treatment group over time in Table 18. No statistically significant differences between the two groups were identified in the 30SCST or F8WT. There was a statistically significant difference in the SLS test between the stance time at baseline and the stance time at 12 months on the contralateral leg (p = 0.03). However, it is difficult to see how this change could be related to the treatment received, and is likely to have been because of improved overall fitness and strength.
| Time point | Usual care (N = 312) | Home-based rehabilitation (N = 309) | Adjusted difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Summary | n | Summary | n | |||
| 30SCST number of stands, mean (SD) | ||||||
| Baseline | 8.2 (3.5) | 312 | 8.4 (3.7) | 309 | – | – |
| 6 months | 11.1 (3.1) | 278 | 11.0 (3.1) | 275 | –0.06 (–0.58 to 0.46) | 0.82 |
| 12 months | 11.7 (3.1) | 279 | 11.5 (3.1) | 276 | –0.22 (–0.74 to 0.3) | 0.41 |
| F8WT time (seconds), mean (SD) | ||||||
| Baseline | 11.8 (5.4) | 312 | 12.1 (4.9) | 309 | – | – |
| 6 months | 10.2 (3.6) | 277 | 9.6 (3.6) | 275 | –0.57 (–1.17 to 0.04) | 0.07 |
| 12 months | 9.3 (3.6) | 280 | 9.1 (3.6) | 274 | –0.17 (–0.78 to 0.43) | 0.58 |
| F8WT steps, mean (SD) | ||||||
| Baseline | 16.4 (4.5) | 312 | 17.3 (5.0) | 309 | – | – |
| 6 months | 15.5 (3.2) | 277 | 15.2 (3.2) | 275 | –0.36 (–0.91 to 0.18) | 0.19 |
| 12 months | 15.2 (3.2) | 280 | 14.9 (3.3) | 274 | –0.23 (–0.77 to 0.31) | 0.40 |
| KA leg SLS average time (seconds), median (IQR) | ||||||
| Baseline | 5.3 (2.6, 13.0) | 310 | 4.5 (1.9, 14.5) | 308 | – | – |
| 6 months | 10.0 (3.9, 30.3) | 279 | 10.0 (3.6, 26.8) | 276 | – | 0.57 |
| 12 months | 13.3 (5.9, 31.1) | 280 | 11.7 (3.4, 31.1) | 276 | – | 0.14 |
| Contralateral leg SLS average time (seconds), median (IQR) | ||||||
| Baseline | 7.2 (3.6, 22.0) | 310 | 6.3 (2.6, 18.0) | 308 | – | – |
| 6 months | 10.9 (4.2, 30.8) | 279 | 9.8 (3.5, 29.7) | 276 | – | 0.43 |
| 12 months | 14.7 (5.6, 30.7) | 280 | 11.0 (3.2, 29.7) | 276 | – | 0.03 |
Details of adaptations used to perform the 30SCST are summarised by treatment group and follow-up time point in Table 19. Fewer participants required an adaptation as time went on. This table also summarises the proportion of participants who stayed within the cones in the F8WT (high throughout) and the F8WT smoothness scores, which improved over time.
| Usual care (N = 312), n (%) | Home-based rehabilitation (N = 309), n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| 30SCST adaptations | ||||||
| None | 211 (67.6) | 224 (80.6) | 231 (82.8) | 214 (69.3) | 229 (83.3) | 223 (80.8) |
| Uses hands on legs | 94 (30.1) | 50 (18.0) | 47 (16.8) | 90 (29.1) | 44 (16.0) | 49 (17.8) |
| Uses walking aid | 2 (0.6) | 2 (0.7) | 1 (0.4) | 4 (1.3) | 1 (0.4) | 1 (0.4) |
| Not tested: unable | 5 (1.6) | 1 (0.4) | 0 (0.0) | 1 (0.3) | 1 (0.4) | 3 (1.1) |
| Not tested: refused | – | 1 (0.4) | – | – | 0 (0.0) | – |
| F8WT | ||||||
| Stayed within cones? | 303 (97.1) | 274 (98.9) | 273 (97.8) | 304 (97.4) | 273 (98.6) | 270 (96.8) |
| F8WT smoothness score | ||||||
| 0 | 9 (2.9) | 5 (1.8) | 2 (0.7) | 11 (3.6) | 5 (1.8) | 4 (1.5) |
| 1 | 73 (23.4) | 26 (9.4) | 33 (11.8) | 87 (28.2) | 32 (11.6) | 26 (9.5) |
| 2 | 50 (16.0) | 35 (12.6) | 22 (7.9) | 56 (18.1) | 29 (10.5) | 27 (9.9) |
| 3 | 180 (57.7) | 211 (76.2) | 222 (79.6) | 155 (50.2) | 209 (76.0) | 217 (79.2) |
Safety
The number of adverse events and serious adverse events experienced are summarised by treatment group in Table 20. Overall, the number of such events was low, with 7.8% of participants experiencing an adverse event in the first 6 months post randomisation, 4% experiencing an adverse event between 6 and 12 months post randomisation and 5.2% of participants experiencing a serious adverse event. The System Organ Classes codes89 for the serious adverse events are also summarised by treatment group.
| Adverse events and serious adverse events | Usual care | Home-based rehabilitation | Risk difference (95% CI) | p-value |
|---|---|---|---|---|
| 0–6 months post randomisation | ||||
| Number of adverse events | 24 | 34 | – | – |
| Participants with adverse events, n (%) | 22 (7.1) | 27 (8.7) | 1.7% (–2.6% to 5.9%) | 0.44 |
| 6–12 months post randomisation | ||||
| Number of adverse events | 12 | 15 | – | – |
| Participants with adverse events, n (%) | 10 (3.2) | 15 (4.9) | 1.6% (–1.4% to 4.7%) | 0.30 |
| 0–12 months post randomisation | ||||
| Number of serious adverse events | 18 | 20 | – | – |
| Participants with serious adverse events, n (%) | 14 (4.5) | 18 (5.8) | 1.3% (–2.1% to 4.8%) | 0.45 |
| System Organ Classes codes, n | ||||
| Blood/lymphatic | 2 | 2 | – | – |
| Cardiac | 3 | 1 | – | – |
| Endocrine | 1 | 0 | – | – |
| Gastrointestinal | 2 | 3 | – | – |
| Immune system | 0 | 1 | – | – |
| Infections/infestations | 2 | 4 | – | – |
| Musculoskeletal | 2 | 6 | – | – |
| Nervous system | 0 | 1 | – | – |
| Renal/urinary | 3 | 0 | – | – |
| Respiratory/thoracic | 0 | 1 | – | – |
| Skin | 1 | 0 | – | – |
| Social circumstances | 1 | 0 | – | – |
| Vascular | 1 | 0 | – | – |
| Unknown | 0 | 1 | – | – |
Additional analyses
The Trial Steering Committee and Data Safety Monitoring Committee met jointly to review the results on 13 June 2019. At this meeting they requested that additional analyses be performed that explored further the definition of the PP population. The committees queried whether or not the key part of the new intervention might be the home-based nature of the treatment, rather than the exact number of sessions. They requested that the analysis of the primary outcome be repeated using two more definitions of the PP population:
-
excluding those who received a session of usual-care treatment at home
-
excluding all participants randomised at site 13, at which the delivery of usual care differed significantly from other sites.
These additional analyses were performed and the results are presented here.
Sensitivity to per-protocol population definition
In view of the recommendations made by the trial committees, the following definitions of the PP population were considered:
-
PP population – no home-based care: participants were excluded from this population if they had received a treatment session at home (usual-care group only), had not received any treatment sessions (either group) or had not provided any follow-up data (either group).
-
PP population – no site 13 participants: participants were excluded from this population if they were randomised at site 13, had not received any treatment sessions or had not provided any follow-up data.
The analysis of the primary outcome was repeated for each of these populations using a repeated measures mixed-effects model, as in the primary analysis. The results of these analyses were similar to those for the other populations considered for the primary outcome (Table 21). No significant differences between the two treatment groups were identified for either of the populations at either of the time points.
| PP population definition | Usual care | Home-based rehabilitation | Adjusted difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Total | Mean (SD) | Total | ||||
| PP – no home-based usual care | Baseline | 51.41 (7.00) | 226 | 51.75 (7.21) | 298 | – | – |
| 6 months | 59.61 (8.59) | 216 | 60.05 (8.51) | 274 | 0.43 (–1.12 to 1.99) | 0.58 | |
| 12 months | 60.92 (8.57) | 215 | 60.78 (8.51) | 274 | –0.14 (–1.70 to 1.42) | 0.86 | |
| PP – no site 13 participants | Baseline | 51.18 (7.11) | 231 | 51.56 (7.33) | 238 | – | – |
| 6 months | 59.30 (8.46) | 220 | 59.90 (8.42) | 222 | 0.60 (–0.98 to 2.17) | 0.46 | |
| 12 months | 60.64 (8.44) | 219 | 60.50 (8.42) | 221 | –0.15 (–1.73 to 1.43) | 0.85 | |
Chapter 5 Health economics
This chapter reports the methods and results of a within-trial cost-effectiveness analysis of the CORKA trial. The CORKA trial randomly allocated 621 adults aged ≥ 55 years with a primary unilateral KA scheduled who were at risk of a poor outcome following surgery to usual care (i.e. outpatient physiotherapy) or to a bespoke community-based multidisciplinary rehabilitation programme (the CORKA home-based intervention). Participants were followed up for 1 year after randomisation. Information on recruitment, including inclusion and exclusion criteria, is presented in more detail in Chapter 2. Participant characteristics at recruitment and clinical results are presented in Chapter 4.
We compared usual care with the CORKA home-based intervention in terms of quality-adjusted life-years (QALYs) gained, health and wider societal costs and calculated incremental cost-effectiveness ratios (ICERs).
Methods
Resource use and costing
Participants were asked to complete two diaries reporting their use of health-care services, their time off work and any informal care received because of their knee, between randomisation and 6 months post randomisation and from 6 months to 12 months post randomisation. Participants were asked to complete the diary daily at home for 6 weeks, and weekly thereafter. Data are reported on visits to health-care practitioners, admissions to hospital, medication use, equipment provided or purchased, informal care received and time away from paid employment. Each of these components of resource use is described in greater detail below. Participants’ receipt of the allocated intervention was also recorded in treatment logs. Participants were also asked to complete the EQ-5D-5L questionnaire at baseline and at 6 and 12 months post randomisation.
Unit costs were derived from national databases,90–96 reports,97,98 or websites99 (see Appendix 2, Tables 35–39). All unit costs are inflated, where necessary, to 2017–18 prices using the health-care and community health services inflation index. 91
The location of care (clinic, home or community) and type of contact (one to one or group) were recorded in the treatment log for those allocated usual care, and costs are attached accordingly. In cases where location or type of contact data were missing (n = 32), the most frequent response was used. No information about the staff member(s) delivering each treatment session was recorded for those allocated to the CORKA home-based intervention. In accordance with the protocol, these participants had seven home visits: two delivered by a qualified physiotherapist and five by a rehabilitation assistant. The average cost applied per home visit reflects this expected staff-mix (see Appendix 2, Table 35).
In question 1 of the resource use diary, participants were asked to record their attendances with a general practitioner (surgery, home or telephone), practice nurse (surgery), district nurse (home visit), physiotherapist (clinic or home), occupational therapist (clinic or home), hospital accident and emergency department, fracture clinic, outpatient clinic (not physiotherapy or occupational therapy), counsellor or psychologist, and were asked to record their receipt of hydrotherapy, social services home care, a falls prevention programme, acupuncture or other complimentary therapies. They were invited to report other types of health care received as free text, which were allocated to existing resource use categories or additional categories where possible. If participants provided any response to this question, we assumed that the diary was complete. We excluded physiotherapy visits that were part of usual care or the CORKA intervention up to the number of visits recorded in the treatment logs. Unit costs for each type of health-care visit are reported in Appendix 2, Table 36.
Participants were asked to report the duration of, and reason for, any hospital admissions. A cost per day in accordance with whether the patient was in an ordinary ward or critical care was applied to hospitalisations (see Appendix 2, Table 36). Participants were asked to record contacts with private physiotherapists, occupational therapists, chiropractors or osteopaths, and record the receipt of acupuncture or complimentary therapies. These were also valued using the unit costs reported in Appendix 2, Table 36.
Participants were asked to report any medications taken in relation to their knee, whether they were purchased or prescribed, and the dosage, duration and frequency of said medication. Data on dosage, duration and frequency were often missing. Each self-reported medication was categorised according to its chemical name where possible. Using all data from the 2018 Prescription Cost Analysis,92 the most frequent medication within each chemical name was identified and the cost per item prescribed extracted. Each medication was classified as likely to be a one-off or long-term prescription. For those drugs considered long term, we identified the typical number of prescriptions per year based on recommended use and standard pack sizes from the British National Formulary. 100 We assumed that drugs recorded in the 0- to 6-month diary were taken from baseline to 6 months, or up to exit from the trial (if this happened before 6 months). We assumed that drugs recorded in the 6- to 12-month diary were taken from 6 months to exit from the trial. Appendix 2, Table 37, lists medication costs. Where data on whether a drug was purchased or prescribed were missing, they were imputed using the more common of the two in fully reported entries with the same chemical.
Participants were asked to report details of any equipment purchased or provided after surgery. Self-reported equipment types were allocated to one of 43 categories, and costs were attached to each category (see Appendix 2, Table 38). The same unit costs were assumed regardless of whether the equipment was purchased or provided.
Participants were asked to report whether or not they had received unpaid care from family or friends, the number of weeks that any care was received and the number of hours of care provided per week. Unit costs per hour of unpaid care were attached to reported activity (see Appendix 2, Table 39). Where data on weeks or hours per week of care were missing but care was reported to have been received, mean imputation was used.
Participants were asked to report whether or not they had to take time off from paid employment and, if so, how many days. This was converted to weeks off work and costed using data on average gross wages and hours worked per week (see Appendix 2, Table 39).
Quality-adjusted life-years
Responses to EQ-5D-5L questionnaires were converted into utility scores using the cross-walk to the three-level version101 and valued using the UK set. 102 QALYs were calculated using the area under the curve approach, which involved estimating the average EQ-5D-5L utility between each follow-up time point and weighting it by survival time.
Methods for dealing with missing data
We followed best-practice methods for addressing missing data in cost-effectiveness studies. 103 Missing data on participant characteristics at baseline were imputed using unconditional mean imputation. As data on the receipt of allocated interventions and deaths were considered to be complete, no imputation was performed. For components of resource use where participants provided responses to any questions in the resource diary, we imputed missing values as zero.
We used multiple imputation by chained equations to impute missing data on EQ-5D-5L utility scores and cost components (except costs related to the allocated intervention) at each follow-up time point. Each missing value was imputed as a function of follow-up period, sex, age, BMI, recruitment site, LLFDI function score (baseline and 6 and 12 months), baseline EQ-5D-5L score, updated EQ-5D-5L score and components of costs. We used predictive mean matching to create 40 imputed data sets. We imputed annual costs and EQ-5D-5L utility scores in each period, adjusting if death was observed in that period. We assumed that costs were incurred linearly over time; if a participant died halfway through a period, they incurred half of the predicted costs. For QALYs, we assumed that the imputed utility score prevailed until the time of death. Imputation was performed separately in subgroups by treatment allocation and follow-up period (0–6 months and 6–12 months).
Analysis
We report descriptive statistics (means and SDs) for resource use, costs and EQ-5D-5L utilities at each follow-up time point using complete data only. Differences between arms were estimated using linear regression controlling for follow-up period, treatment allocation, an interaction between follow-up time and treatment allocation, recruitment site, and baseline utility score for EQ-5D-5L. Standard errors were adjusted for multiple observations within individuals.
Following multiple imputation, we estimated total costs and QALYs for all 621 participants in the CORKA trial from the date of trial recruitment to the earliest date of death or withdrawal from the trial, or the end of follow-up at 1 year by treatment allocation. On each imputed data set we estimated mean costs (by type) and QALYs using separate linear regression models controlling for treatment allocation, recruitment site and for QALYs baseline EQ-5D-5L utility. Estimates derived from each imputed data set were combined using Rubin’s rule to estimate the adjusted mean difference and standard error for each outcome. As a sensitivity analysis, we explored using a complete-case analysis that included only individuals who provided complete data over the 12-month trial duration. In the preliminary analysis, we also considered using linear regression in imputation models rather than predictive mean matching.
We calculated the ICER by dividing the mean cost differences (total NHS costs and total societal costs) between the CORKA home-based intervention and usual care by the mean QALY difference. We estimated the joint uncertainty around incremental total costs and QALYs (i.e. the difference between the CORKA home-based intervention and the usual-care arm) and in the cost-effectiveness by bootstrapping 200 times from each of our 40 imputed data sets (creating 8000 bootstraps), running the estimation model on each bootstrapped data set and extracting the estimated treatment effects. From these bootstrapped results, we calculated the probability that the CORKA home-based intervention was more cost-effective than usual care for different threshold values per QALY gained,104 by estimating the proportion of bootstrap replicates with a net monetary benefit of above zero for each threshold value. The net monetary benefit was given by the product of the mean difference in QALYs and the threshold value minus the mean difference in costs.
All analyses were conducted using R version 3.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study participant follow-up time
Out of the 621 trial participants, 3 died and 34 withdrew from the trial.
Table 22 presents the percentage of missing data observations for resource use and EQ-5D-5L utility at each follow-up point by treatment allocation. Overall, the number of missing data was fairly low. Missing data were more common for resource use than EQ-5D-5L, and marginally more common in those allocated to the CORKA home-based intervention than those allocated to usual care. Patterns of missing data were very similar for these two outcomes in both treatment arms and at each follow-up time point.
| Time point | Resource use data, n (%) | EQ-5D-5L data, n (%) | ||
|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| Baseline | – | – | 4 (1.3) | 1 (0.3) |
| 6 months | 48 (15.4) | 46 (14.9) | 31 (9.9) | 34 (11.0) |
| 12 months | 39 (12.5) | 36 (11.7) | 26 (8.3) | 31 (10.0) |
Resource use and costs during follow-up
Appendix 3, Tables 40–45, provides comprehensive data on resource use and responses to the EQ-5D-5L questionnaire by treatment allocation and follow-up period. Table 23 presents the mean costs for each cost type and totals by treatment allocation and follow-up period, and adjusted mean differences. Period costs for all cost types and differences between treatment groups were substantially greater between baseline and 6 months than between 6 months and 1 year. Average intervention costs were lower and subsequent NHS costs were higher (reflecting higher costs associated with hospital admissions) in those allocated to the CORKA home-based intervention than those allocated to usual care. Private health-care costs and differences by treatment allocation were substantially lower than those for NHS costs. Between 6 months and 1 year the average costs associated with informal care and time away from paid employment were substantially lower for the CORKA home-based intervention than for the usual-care arm, and were larger than the differences in health-care costs.
| Cost categories | Baseline to 6 months | 6 months to 12 months | ||||
|---|---|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Differencea (home-based rehabilitation vs. usual care) | Usual care | Home-based rehabilitation | Differencea (Home-based rehabilitation vs. usual care) | |
| Number of respondents, n | 264 | 263 | – | 273 | 273 | – |
| Total NHS costs | £823 (£898) | £776 (£1008) | £–43 (–£206 to £120) | £189 (£441) | £268 (£871) | £86 (–£31 to £204) |
| Intervention costsb | £250 (£183) | £184 (£60) | £–66 (–£87 to –£45) | – | – | £0 (–£3 to £3) |
| Total subsequent NHS costs | £563 (£868) | £587 (£1005) | £28 (–£133 to £188) | £189 (£441) | £268 (£871) | £85 (–£33 to £202) |
| Primary care | £67 (£83) | £81 (£132) | £14 (–£5 to £33) | £15 (£40) | £18 (£50) | £4 (–£4 to £12) |
| Physiotherapy | £87 (£136) | £46 (£128) | £–41 (–£63 to –£19) | £15 (£81) | £20 (£85) | £4 (–£10 to £18) |
| Outpatient care | £100 (£146) | £116 (£209) | £14 (–£14 to £43) | £36 (£124) | £33 (£123) | £–3 (–£24 to £18) |
| Medications | £26 (£68) | £24 (£57) | £–1 (–£10 to £8) | £23 (£75) | £19 (£54) | £–3 (–£13 to £7) |
| Hospital admissions | £172 (£772) | £204 (£850) | £36 (–£103 to £175) | £83 (£378) | £153 (£818) | £72 (–£36 to £181) |
| Equipment | £45 (£141) | £39 (£57) | £–6 (–£23 to £12) | £5 (£20) | £6 (£18) | £1 (–£3 to £5) |
| Other NHS care | £67 (£172) | £77 (£222) | £10 (–£22 to £43) | £13 (£62) | £19 (£90) | £8 (–£6 to £22) |
| Total private health care | £74 (£464) | £44 (£205) | £–30 (–£92 to £32) | £11 (£49) | £27 (£157) | £16 (–£4 to £35) |
| Physiotherapy | £14 (£69) | £18 (£89) | £4 (–£9 to £18) | £6 (£34) | £7 (£40) | £1 (–£5 to £7) |
| Equipment | £51 (£458) | £22 (£185) | £–29 (–£89 to £31) | £1 (£7) | £14 (£148) | £13 (–£5 to £31) |
| Other | £9 (£41) | £4 (£27) | £–5 (–£11 to £1) | £4 (£34) | £6 (£37) | £1 (–£5 to £8) |
| Informal care | £428 (£1332) | £347 (£1003) | £–79 (–£279 to £121) | £20 (£129) | £55 (£290) | £52 (£9 to £95) |
| Time off work | £980 (£2692) | £601 (£1916) | £–384 (–£786 to £17) | £100 (£987) | £109 (£1012) | £18 (–£148 to £185) |
| Total societal costs | £2305 (£3387) | £1767 (£2521) | £–537 (–£1049 to –£25) | £321 (£1167) | £459 (£1397) | £172 (–£46 to £390) |
EuroQol-5 Dimensions, five-level version, utility
Table 24 presents EQ-5D-5L utility scores and differences by treatment allocation at each time point. EQ-5D-5L scores were very similar in the two treatment groups at all follow-up time points, improving similarly between baseline and 6 months. The distribution of responses to each EQ-5D-5L domain at each follow-up time point is presented by treatment allocation in Appendix 3, Table 44.
| Time point | Usual care | Home-based rehabilitation | Differencea (95% CI) (home-based rehabilitation vs. usual care) | ||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| Baseline | 308 | 0.52 (0.22) | 308 | 0.5 (0.23) | –0.00 (–0.0 to 0.04) |
| 6 months | 281 | 0.75 (0.19) | 275 | 0.76 (0.19) | 0.00 (–0.03 to 0.03) |
| 12 months | 286 | 0.76 (0.20) | 278 | 0.768 (0.23) | 0.004 (–0.03 to 0.04) |
Main analysis
Table 25 shows the main analysis results at 1 year. Appendix 3, Table 45, shows descriptive statistics for the costs and QoL at each follow-up time point after multiple imputation. There was a small, non-significant difference in QALYs (0.003, 95% CI –0.017 to 0.023) favouring the CORKA home-based intervention.
| Usual care | Home-based rehabilitation | Differencea (home-based rehabilitation vs. usual care) | |
|---|---|---|---|
| N | 312 | 309 | – |
| Life-years | 1.000 (0.000) | 1.000 (0.053) | 0.000 (–0.001 to 0.000) |
| QALYs | 0.696 (0.402) | 0.698 (0.411) | 0.003 (–0.017 to 0.023) |
| Total NHS costs | £1015 (£38) | £1088 (£51) | £77 (–£138 to £291) |
| Intervention costsb | £250 (£14) | £184 (£8) | –£65 (–£86 to –£44) |
| Total subsequent NHS costs | £765 (£38) | £904 (£51) | £142 (–£70 to £354) |
| Total private health care | £103 (£22) | £88 (£18) | –£15 (–£76 to £46) |
| Informal care | £441 (£41) | £413 (£37) | –£23 (–£210 to £164) |
| Time off work | £1105 (£86) | £744 (£66) | –£355 (–£820 to £110) |
| Total societal costs | £2664 (£97) | £2332 (£82) | –£316 (–£892 to £260) |
| ICERb | |||
| NHS costs only | – | – | £28,372 |
| Total societal costs | – | – | Dominant (more effective and less costly) |
Post-operative physiotherapy (intervention) costs were lower on average in the CORKA trial arm than in usual care (–£65, 95% CI –£86 to –£44). However, as the CORKA trial arm reported higher subsequent health-care costs (£142, 95% CI –£70 to £354), the total NHS costs at 12 months were higher in CORKA (£77, 95% CI –£138 to £291). By contrast, costs associated with private health-care use (–£15, 95% CI –£76 to £46), informal care (–£23, 95% CI –£210 to £164) and time away from paid employment (–£355, 95% CI –£820 to £110) were lower in the CORKA trial arm than usual care at 12 months. As a result, total societal costs (adding health care and other costs) were lower for CORKA than for usual care (–£316, 95% CI –£892 to £260).
Adopting an NHS health and social care perspective, the ICER for the CORKA home-based intervention versus usual care was £28,372, which is close to the standard threshold for cost-effectiveness in the UK. Adopting a societal perspective, the CORKA home-based intervention was cost-saving and more effective than, and thus dominant over, usual care.
Figure 11 presents the cost-effectiveness scatterplot that illustrates differences in mean total costs and QALYs for the CORKA home-based intervention versus usual care, adopting the NHS health care and social care (blue dots) and societal (red dots) perspective. Figure 12 presents the cost-effectiveness acceptability curve that gives the probability that the CORKA home-based intervention is cost-effective compared with usual care for different threshold values for a QALY (from £0 to £50,000 per QALY) for each costing perspective. Adopting an NHS or a health-care cost perspective, the probability that the CORKA home-based intervention is cost-effective is around 25% when the willingness to pay for an additional QALY is £0. As the willingness to pay for an additional QALY increases the probability of cost-effective increases, reaching around 43% at a threshold value of £30,000 per QALY. Adopting a societal perspective, the probability that the CORKA home-based intervention is cost-effective decreases with a higher willingness-to-pay threshold. The probability of cost-effectiveness is 75% at a threshold of £30,000 per QALY.
FIGURE 11.
Cost-effective scatterplot for the base-case analysis, assuming equal implant device costs.
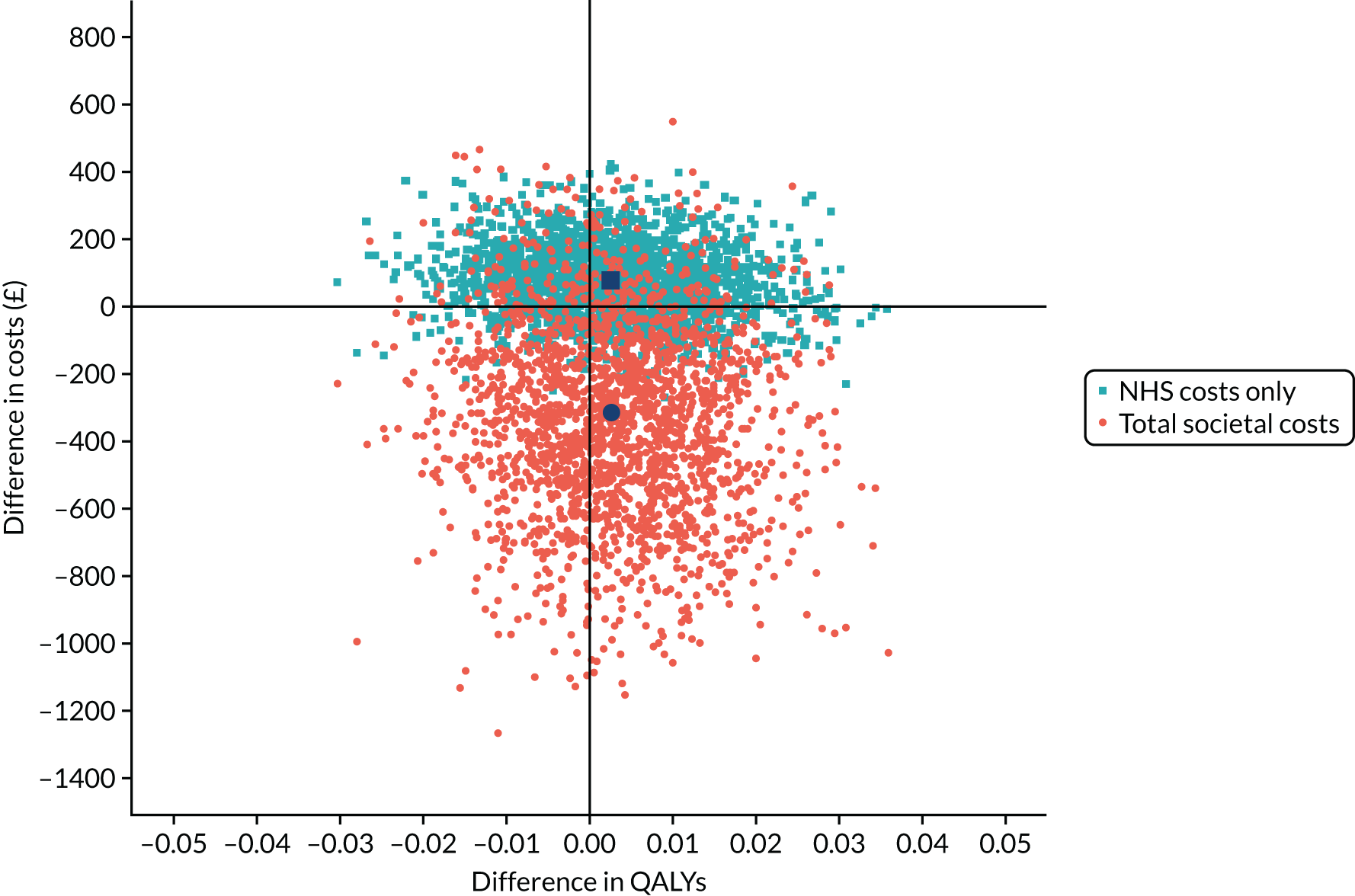
FIGURE 12.
Cost-effectiveness acceptability curves.

Sensitivity analysis
Table 26 presents the complete-case analysis results at 1 year. Data were contributed to the complete-case analysis by 489 (79%) participants (usual care, 78%; CORKA home-based intervention, 80%). The results of the complete-case analysis were similar to those from the multiple imputation analysis. There was no evidence of a difference in QALYs by treatment allocation. NHS costs were higher and societal costs lower, although not significantly so, for the CORKA home-based intervention versus usual care. Adopting an NHS health and personal social care perspective, the CORKA home-based intervention was more costly and less effective than usual care and, thus, dominated by usual care. Adopting a societal perspective, the ICER for the CORKA home-based intervention versus usual care was £224,210, which is considerably higher than the standard threshold for cost-effectiveness in the UK. As usual care was more effective, the CORKA home-based intervention was, therefore, cost-effective, as higher ICER values indicate greater cost-effectiveness for CORKA.
| Usual care | Home-based rehabilitation | Differencea (home-based rehabilitation vs. usual care) | |
|---|---|---|---|
| N | 243 | 246 | – |
| QALYs | 0.706 (0.155) | 0.704 (0.166) | –0.002 (–0.023 to 0.020) |
| Total NHS costs | £1034 (£1068) | £1016 (£1380) | £12 (–£208 to £231) |
| Intervention costsb | £262 (£186) | £189 (£50) | –£67 (–£91 to –£44) |
| Total subsequent NHS costs | £773 (£1040) | £827 (£1380) | £79 (–£139 to £297) |
| Total private health care | £92 (£491) | £74 (£327) | –£20 (–£94 to £55) |
| Informal care | £471 (£1385) | £400 (£1091) | –£26 (–£241 to £190) |
| Time off work | £1046 (£3131) | £717 (£2390) | –£308 (–£806 to £189) |
| Total societal costs | £2644 (£3911) | £2207 (£3156) | –£342 (–£972 to £288) |
| ICERb | |||
| NHS costs only | – | – | Dominated (less effective and more costly) |
| Total societal costs | – | – | £224,210 |
Chapter 6 Qualitative study
Introduction and objective of the study
This qualitative study was carried out to explore the experiences of the patients participating in the CORKA home-based intervention and the physiotherapists and rehabilitation assistants delivering the intervention. We aimed to explore barriers to and facilitators of adherence and gain insight into experiences that might influence trial outcomes.
Methods
Approaching and recruiting participants
Potential patients, physiotherapists and rehabilitation assistants were identified by the trial co-ordinator and were contacted by the qualitative researcher to arrange a convenient time and venue. All those who were approached were given a participant information sheet and given at least 24 hours to decide if they wanted to take part. Those who were willing to participate were given a consent form to complete and sign.
Sample
We anticipated that approximately 10 patients from the intervention group and 10 clinicians, around 20 people in total, would provide rich insight. Eventually, 10 participants, five physiotherapists and six rehabilitation assistants were recruited. Recruitment was halted when a wide range of views had been obtained and no new topic areas were being raised. Female participants (n = 4) ranged in age from 64 to 90 years, and male participants (n = 6) from 65 to 89 years. The participant screening tool score ranged from 6 to 7 out of 10. The physiotherapists had worked for a range of 1 to 32 years post qualification and the assistants for a range of 1 to 30 years.
Interviews
The same experienced qualitative researcher completed all of the interviews (FT). Written consent was obtained at the start of visits before interviews commenced. Although an interview guide was developed after discussion with the trial team, it was not followed rigidly. Follow-up questions were used to help the interview flow, which ensured that relevant areas were covered and allowed participants to introduce new relevant areas. Participant interviews took place in their own homes at a convenient time for them, usually at the weekend. Two rehabilitation assistant interviews took place on the telephone and were recorded. The rest of the physiotherapist and rehabilitation assistant interviews took place in a quiet room at work. All interviewees were encouraged to discuss any areas that they felt were relevant. Interviews were digitally audio-recorded and transcribed.
Data analyses
Audio-recordings were listened to and transcripts read. Transcript data were broken down into discrete units, making concerted efforts to remain close to the data and continually explore meaning. The first three transcripts were independently coded by two researchers (FT and JR). As similar coding units were identified by both researchers, FT coded subsequent transcripts. All transcripts were coded by one researcher who then grouped together units with a shared essence into categories (FT) using NVivo 11 (NVivo 11, QSR, Portsmouth, UK). Two researchers (JR and KB) checked each unit, the unit descriptor and the data supporting each unit to make sure that the category was grounded in the primary data. Data, codes and categories were constantly compared with each other, and the team met regularly to discuss the data and analysis. Rigour was, therefore, promoted through collaboration.
Findings
Physiotherapists and rehabilitation assistants
Using thematic analysis, we developed seven themes that cut across participants (Figure 13). We illustrate these ideas below with narrative exemplars.
FIGURE 13.
Physiotherapist and rehabilitation assistant themes.

Seeing the person in their world
This theme describes the value of seeing the person as a whole, not just a body part, in their own world. The home setting enhanced a holistic approach, rather than a biomedical approach:
I think that holistic approach is really, really important, so yes we’re there to get their knee bending . . . but in the bigger picture, I want them to be able to go outside and use their knee . . . to be able to go and see friends, or kind of do dancing and things like that . . . [In outpatients] it would have been very much focused on the knee.
Physiotherapist
Clinicians compared the clinical encounter, which tended to focus on the body part, with the more relational home encounter:
[Being at home] sort of takes it out of that context where the mindset of you’re just there just to fix you . . . you’re with that chance to be able to talk with people, they’re able to see you as being human . . . they were a lot more relaxed . . . They’re more open . . . they may well talk about and what their social lives are like . . . It’s a more open environment.
Assistant
In the home environment, the balance of power shifts. Entering a person’s home was described as a privilege in which you see the patient on their own terms: it is less paternalistic and the patient becomes a person:
It was very much on the patients’ terms whereas I can imagine kind of in a hospital setting, it’s a bit more kind of like on our terms.
Assistant
You’re in there and it’s a privilege to be in someone’s home. They’ve let you in. You’re not just seeing them; you’re seeing everything really . . . it’s quite invasive into their world.
Physiotherapist
Clinical staff reflected on the benefits of seeing the person in their environment and the changes that they would make to their future clinical practice to ‘see the person’:
I would say that I’d strive now to see the person as an individual . . . strive more to try and just understand where that person’s coming from, because even with something that you think is as simple as a knee replacement . . . in terms of the impact on that one person’s life . . . You don’t just know that stuff instinctively . . . you’ve gotta find out a bit about them, and so I think that’s hugely important.
Physiotherapist
Developing people skills
This theme describes the need to develop ‘people skills’ to effectively enter a person’s world. These skills are integral to effective treatment and develop with experience:
To be truthful they [clinicians] need to have good people skills to be able to talk to people and not talk down to them . . . don’t make ‘em feel like they’re on detention . . . it’s the way you go about it . . . personal skills count a lot . . . You’ve sort of got to win their confidence and once you do that . . . it’s amazing what you can get out of them once you get their confidence.
Assistant
Good ‘people skills’ meant that you had to get to know the person in their world and flexibly respond to their needs:
The reality is that things change quickly, you have to be pragmatic . . . real life is wonderfully, beautifully messy . . . you can’t know a thing that’s gonna come up.
Physiotherapist
Thinking outside the cubicle
This theme explores the way in which seeing a person in their own world fosters creative thinking and enhances positive outcomes. Clinical staff described the world in situ as the real world:
In a sort of sterile clinic or environment where the floor’s totally flat, there’s no obstacles . . . it doesn’t bear that much resemblance to somebody’s house . . . I think seeing people in their own home, it’s just different . . . being able to relate more to what the patient’s saying ‘cause you can see it, it’s not just a theoretical problem . . . they can show you.
Physiotherapist
Clinical staff enjoyed the freedom to be creative:
I enjoyed being able to give the people the realisation that you can do some exercise; you don’t need any fancy equipment . . . it’s sort of utilising the equipment that they’ve got, so their chairs, their stairs . . . to make use of, like, towels or, I don’t know, a bit of rope that the husband has got in the shed . . . It was taking it away from just ‘here’s a sheet with some exercises on it’.
Assistant
This creative approach was compared with the clinical space with its associated time constraints:
I think in the very time-pressured nature of outpatients, you probably can’t build up as much of a picture, and you can’t see the home environment that you’re working with, so it’s really great that we’re able to kind of walk through a day in the life of a, rather than just kind of talk through it . . . I think [we] probably get a bit trapped in the cubicle thinking.
Physiotherapist
I gained personally from doing that bit extra
This theme describes the personal gain from working holistically and recognising the impact that one can have:
I definitely gained a professional insight . . . but also personally as well: building those relationships, being able to gain more of an insight into human beings . . . this has been really, really important to me to be able to spend the time talking to people and understanding what makes them tick . . . I want to take on the stories; I want to learn more about people, (a) to help them, but (b) to kind of develop as well . . . personally and professionally . . . I take satisfaction from doing the little bit extra: from talking to the patient . . . I think if I wasn’t doing that, I wasn’t taking on the patients’ stories, I’d be less satisfied – and more likely to burn out . . . that’s the bit that I enjoy the, the above and beyond.
Physiotherapist
Clinical staff described the satisfaction that comes from having a positive impact on people’s lives:
I had this other chap, he lost his wife . . . I said to him, ‘What is your main goal?’ he said, ‘Walking to the grave’ . . . I said, ‘Well how far is it?’ he said, ‘Well I need to drive my car first’ . . . I said, ‘Well that’s not impossible . . . we’ll get there’ . . . I think it was about on the fifth time . . . he said, to me, ‘. . . I actually got to the cemetery . . . but I couldn’t quite do the walk.’. I said, ‘Well that’s an achievement, you must be really proud of yourself . . . and on the seventh time he said, ‘I’ve done it . . . I’ve actually gone to the grave’ . . . and I thought that was fantastic . . . it just feels such an achievement . . . It makes me feel so happy then.
Assistant
There is a fine line between patient and friend
This theme describes the challenge of managing the boundary between getting to know someone in their own world and remaining professional. These challenges were exacerbated in the home environment:
I think you always have to realise that the patient who you’re going out to see is a patient and not a friend . . . there is that fine line. I think it’s always being clear to them that you’re here to rehab them . . . I mean, some patients want to have a laugh and a joke with you but I wouldn’t say that’s unprofessional . . . that’s just making the patient feel comfortable . . . it’s polite to have that 5-minute conversation, how are you doing, how have you been, what have you been up to? And then once that’s out the way, get on with the treatment.
Assistant
Clinical staff described individual cases for which it was more challenging to remain professional. For example, there were those whom you ‘clicked with’:
They’re the patient and not friends. Some people you do sort of click with . . . so, I was almost like trying to be, sort of not friends, but be professional.
Assistant
I think the most tricky people are perhaps someone like, I’m treating a guy that’s a similar age to me . . . it’s just trying to maintain that you’re coming at things from an angle as a professional . . . maybe it’s more challenging when they’re, like, ‘Did you enjoy the pub the other night?’. [Laughter] . . . it’s again trusting [achieving] balance between the importance on knowing that person and building a relationship versus maintaining a professional identity.
Physiotherapist
Clinicians described a tension between having to develop good rapport to build their confidence and get the job complete and the need to maintain boundaries:
Building rapport is really, really important to get the patient engaged, but it’s managing the professional relationship is the difficult bit, I think . . . You do get to you know their family, you meet their kids, you know their dogs’ names . . . and you obviously share your own life stories as well . . . I found it kind of difficult to negotiate that barrier sometimes.
Physiotherapist
Clinicians described a ‘middle area of closeness’, in which you enter a person’s world far enough to be able to use your knowledge effectively, but not so far that you become ineffective:
There was one man [who] had other issues that he wanted to talk about . . . kind of brought [the assistant] into the drama of his neighbourhood. I think there is kind of a middle area of closeness . . . it’s not the more you get into their lives, the more help you can offer . . . there’s a threshold that helps, and then maybe as you get too far, you’re not objective . . .
Physiotherapist
Feeling outside my comfort zone
This theme describes times of discomfort for rehabilitation assistants working alone in a person’s home. Some described how they could feel vulnerable and on the spot as an unqualified health-care worker:
They were sort of under the impression that I was a physio[therapist], and then I say, “Well, I’m not a physio[therapist],” then they’re sort of, “What do you mean, you’re not a physio[therapist]?” . . . There was a couple of times where that’s sort of happened, and it just sort of made me feel a little bit, uncomfortable.
Assistant
Rehabilitation assistants described occasions where they asked for physiotherapist input, for example when patients exhibited severe pain, swelling or lack of expected progress, or when things just ‘didn’t look right’:
It was just mainly with the people who weren’t progressing as much . . . the lady who wasn’t getting beyond 30 degrees of movement . . . I felt I’d sort of done everything that I could. So, of course, I’d go back and touch base with [the physiotherapist] . . . even [the physiotherapist] was a bit baffled . . . he said, ‘Well . . . you’ve gone by the book . . . she’s doing what she can, she’s not progressing – she needs the MUA [manipulation under anaesthetic]’.
Assistant
Physiotherapists recognised that this sort of situation could be extremely challenging for rehabilitation assistants and emphasised the need for clear lines of communication:
I think the important thing would be just to emphasise openness and communication . . . ‘Don’t sit on something if you’re worried about something, tell me . . . I might be worried about it as well, and I’ll tell someone else’. [Laughter] . . . that wasn’t necessarily a formalised part of the training in the same way that we laid out how to deal with the exercises . . . but actually, probably just as important.
Physiotherapist
Needing a support network
This theme describes the need for peer support and effective two-way communication. Assistants described an effective support network in which someone had ‘tabs on them’; they did not feel abandoned:
I worked very closely with [the physiotherapist] . . . we did have a patient that was very tearful and rehab was quite difficult, and I called [the physiotherapist] as soon as I finished the appointment and we had a chat on the telephone and the next morning straightaway, we had a meeting to discuss how it was going on . . . which I think was really helpful because sometimes you just need that qualified [person] there just to cast their eye over it and just make sure everything’s going OK.
Assistant
This ongoing support was not only important on a personal level for the assistant, but also important in maintaining the patient’s confidence in the rehabilitation assistant:
If I ever [felt worried] I wouldn’t do it. Or I would get someone to come . . . I would always say, if you’re not sure of something just say . . . ‘I will find out’ . . . because it’s hard to change it when you go next time . . . then they could lose their confidence in you . . . Don’t put myself in a position where I say . . . ‘do that, do that’ and think, ‘oh my god no that could be wrong’.
Assistant
Physiotherapists described the challenge of balancing the need for support and fostering an environment of independence. Assistants described the need to feel trusted, yet also supported:
It’s quite nice from a physio[therapist] assistant point of view to feel like you’re making an impact independently and [the physiotherapist] trust[s] you . . . But at the same time, it’s nice to have that support . . . I didn’t feel at all like I was kind of abandoned or deserted . . . I think if the physio[therapist] was coming in every single time they’d be pressure . . . are they kind of judging [me]? . . . it was a really nice balance.
Assistant
This collaborative partnership between physiotherapist and rehabilitation assistant provided a safe place to learn new skills:
So, it is nice to have that bit of encouragement from a physio[therapist] . . . I took on a lot of skills and I learnt a lot about myself, I learnt a lot more about how to present myself to a patient because I was independent and I was learning from the [physiotherapists] . . . so I was pitching the best skills from everybody and putting them into, to what I want to become, so that was really good.
Assistant
Patients
We aimed to explore patients’ experience of being part of the CORKA trial. Using thematic analysis we developed themes that fell into three main categories:
-
the benefits of having treatment at home
-
the challenges of CORKA
-
factors that might have an impact on the treatment outcome.
The benefits of having treatment at home
Participants described five main benefits: it was a relief not travel; I got an hour’s work done in an hour; they can work with your surroundings; I wouldn’t have done it on my own; and there is nothing like company (Figure 14).
FIGURE 14.
Participant themes: the benefits of home therapy.

It was a relief not to travel
Participants described the difficulty that they would have in getting to the hospital and the great relief that it was to have been allocated to the home exercises. Often they would rely on other people to get them to the hospital:
I would have had to ask people to take me . . . we were having really bad traffic works at the time and it could take anything up to 2 or 3 hours to get in . . . you never knew how long it was gonna take you and the thought of having to make a physio[therapy] appointment . . . you’ve got somebody to take you and they’ve got to wait around and bring you back again . . . It’s exhausting doing those exercises . . . and then having to sit in the car and drive back . . . sitting in that car is exhausting when you got a bad knee, I can’t tell you, you’d never believe it, you really wouldn’t.
Female 29
It was a relief [to have treatment at home] . . . we’re fairly au fait with the bus services now but at the time I hadn’t been out so it was very much a sort of case of whenever I went anywhere it was calling a taxi to get there . . . because my partner has health problems as well, so what with that and the eye problems, and that we’re sort of permanently backwards and forwards.
Female 32
I got an hour’s work done in an hour
Participants felt that doing exercise at home made the best use of time, and described health-care experiences where their time had been wasted:
We did an hour’s work and we got on with it . . . it just worked all the time and that was good . . . I got an hour’s work done . . . [in hospital] I should think I got quarter of an hour’s work done in an hour . . . I prefer just to get on with the work and get it done with . . . it was just being kept waiting . . . it seemed to drag out a long time, over an hour, and you’d get very little done . . . you’re standing there thinking, ‘Come on, let’s get on with the next bit . . . I’m wasting my time here’.
Female 31
One woman described the need to consider the time cost to the patient and their employers (and family), not just the health-care costs:
I think sometimes it’s a matter of looking at the larger picture, because it’s not just the cost to the NHS it’s the cost to everybody isn’t it . . . you have to cost what it’s costing you for sending someone to the home, what it’s costing the individual, what it’s costing their employers . . . if you’re taking time off work to go to a hospital appointment and then you’re spending half the day there for what is essentially a 10-minute or a half-hour session . . . I know it’s expensive for the hospitals but it’s expensive for the patients as well . . . it is normally half a day isn’t it, instead of maybe half an hour of your appointment at home.
Female 32
They can work with your surroundings
Participants described the benefits of being in their own environment, as opposed to a ‘sterile room’. At home, the therapist can see what they have to work with and can improvise and adjust exercises; they can be creative and adaptive to individual goals in a real-life setting:
I have always maintained that physio[therapy] is best given in your own home because then people can see what you’re working with . . . [my] house is small . . . and that’s quite hard to explain when you say, ‘Oh, I live in a little house.’. People think, ‘Oh well, she can still walk around in a figure of 8,’ well you can’t, cos there isn’t enough room? . . . They can actually see what you’ve got and what you’re coping with . . . we went for a walk one day with the dog . . . huge confidence boost that was . . . they came here and looked at everything . . . I could see them looking . . . they’ve got to see what you’ve got and what you get round . . . you’re working with your surroundings, that’s why it worked so successful and it was such a good idea because . . . you were working with what that person has to live with every day.
Female 29
. . . knowing what your home environment is like and what you’re going to be able to do of those exercises . . . if they give you something sort of running for 10 yards or something inside, that’s not gonna work if you’re in a confined area, or if you can’t manage these stairs or you know if you’ve got nothing, no space, I mean we’re fairly compact here, there’s not a large area, just seeing that I think, and how you can manage the exercises in your home environment . . . Rather than . . . you get home and you think, ‘Well, how do I do that?’.
Female 32
I wouldn’t have done it on my own
This describes the confidence gained by being overseen by a health professional who tailors the exercises to the individual. Patients did not feel that they had the capability to progress in this way on their own:
They check you as to whether you were able to do this exercise or not and so . . . they were able to actually tailor the exercises to the way you were progressing. . . . now I wouldn’t have been able to do that on my own . . . [they] came in a couple of times as well and check that my knees were, my knee was flexible enough you know . . . without the contact there isn’t the progress.
Male 33
I found [exercises at home] very comforting because he was there and he was telling me what it’s for and what, what’s the best way of doing it . . . I sort of felt . . . confident because they, they were on-hand all the time.
Male 30
Participants described how knowing that someone would be coming to ‘check-up’ on them was a motivation to keep doing the exercises, and this motivation got them into the habit. There was a sense of duty towards the therapist who had come ‘all that way’; they did not want to let the therapist down:
It was, it was beneficial because I knew he was coming . . . which made me do the exercises . . . that was the main thing actually it kept me going . . . I feel that I’m so active because he came here . . . because he came, I felt I had to do them because I didn’t want to let him down coming all this way . . . I wouldn’t be as active. I am convinced that I would not be . . . ‘cos I am a lazy person . . . I probably wouldn’t be where I am now . . . whereas because I knew [name] was coming, it made me do them. You know, it’s as simple as that really.
Female 25
I think if he hadn’t have come I wouldn’t have bothered doing it . . . there was somebody there to make you do it too . . . which of course got me into the habit of doing it . . . I think if I’d just been given the sheets I would have thought, ‘Yeah, that’s alright, I’ll do that when I’ve got a spare minute,’ [laughs] . . . I mean I was a bit miffed at the time and thought, ‘Oh blow, I got to do that’ . . . once you get into a routine it’s not so bad, it’s just part of the day then, your ritual . . . I think we’re all people of habit aren’t we?
Female 31
There is nothing like company
Finally, participants describe the social benefits of a therapist coming to their own house. This social aspect facilitated exercise:
[I was] quite pleased to see her when she came . . . If you’re a bit younger and more active perhaps you might think differently, but in my position I was glad to see someone, I’m glad to see anyone that breaks the day up a little bit . . . for someone of my age and my way of life now, it was a welcome break to have someone come round and chat to you for 10 minutes, quarter of an hour. Umm, it’s got a bit of a social benefit I think.
Male 28
It did two things to me in my circumstances: it meant I had someone to talk to . . . So that was good, and not only that . . . I quite look forward to someone coming in . . . if you’ve got two people together one encourages the other . . . I mean you can go to these sports centres and . . . but I don’t think that’s the answer . . . it’s team sports you need isn’t it? . . . It’s also the social side that goes with it, isn’t it? . . . There’s nothing like company . . . I can vouch for that, there’s nothing like company.
Male 33
Challenges of having treatment at home
Participants also described two challenges of having treatment at home: they put me through my paces and feeling at sea now that it is over.
They put me through my paces
Participants felt a bit surprised about the pace and duration of exercises and felt exhausted:
Some days if I had gone through all the exercises I would have been doing them 24 hours a day . . . if I’d have been a sort of 40-year-old I would probably have coped with them, but being an 80-year-old was slightly different and I don’t know whether that was really taken into consideration.
Male 30
I was surprised how intensive it was . . . he did put me through my paces . . . pulling my leg up and stretching it out, that kind of thing . . . that really hurt and . . . the ones I’m doing is the lifting one which I find quite hard to do on this chair . . . and on the bed I can lift my leg up quite nicely and the bend of course, the bloody bend . . . which isn’t bad but it’s not brilliant, but it does hurt.
Female 25
I do feel a bit a sea now it’s over
Participants also described being worried about what would follow: they felt a bit abandoned. There was a sense that something should follow on, even if they had to organise it themselves:
I do feel a bit at sea, I’m thinking, ‘Hmm.’. And I know it’s gonna be up to me and sometimes I can think, ‘Oh’. But I think I need to see if I can find some . . . NHS physio[therapy] . . . Quite how I go about that I haven’t got a clue but I’ll think about it when I got my head . . . my head’s not quite in the right place yet . . . it’s something else you got to think about and organise.
Female 29
I done my 8 weeks or 6 weeks . . . and I think there’s a thing that, you can get a referral to the gym down in the town . . . I don’t know, I’ll have to make enquiries . . . who I’d have to get referred by . . . so, I don’t see them again then? . . . I want to know what’s going on . . . you get a lot of support in that first 6 months, but then there doesn’t seem to be anything . . . you feel as if right you’ve had your 6 months – bang that’s the end of it.
Male 30
Factors that might have effect outcome
Participants describe factors that might have an impact on their outcome from treatment: my get up and go has got up and gone; a variable social life; if you take to the person it goes a long way; and a lot would shirk from putting on a pair of running shoes.
If you take to the person it goes a long way
Participants described an effective health-care interaction as hinging on ‘taking to the person’. Personality factors were described as integral to being good health-care professionals and none of the participants was negative about being treated by an assistant therapist:
He’s [not a physiotherapist] bless him . . . but he’s also had injuries himself so he knows, he knows what it’s like to have a lot of pain . . . he’s an extraordinarily empathetic young man.
Female 29
A therapist should be kind, understanding, empathetic and be able to put people at ease. They should also be able to have a bit of fun and to know what ‘makes people tick’. They should not appear disinterested or ‘perfunctory’:
[Previous treatment] was perfunctory . . . it was done by the book . . . they’ve got to tick certain boxes . . . a lot of physio[therapy] workis personality . . . you can log-on to that personality with someone, you’ll get far more out of them . . . a lot of physio[therapy] is how you interact with people . . . it was a personal thing . . . interpersonal skills are just crucial. You’ve got to be able to talk to people on any level really . . . got to be able to relate to what that person is telling them . . . you have to have empathy . . . you have to be able to cotton on to what makes them tick really . . . the best ones put an element of fun, they get on your wave length you know, pretty quickly . . . It’s just building up a relationship . . . you have to do it very quickly don’t you . . . so you have to have the innate ability to build up a fairly good relationship with somebody.
Female 29
[He] was wonderful, he really was . . . everybody has been very, very kind and all gone over the top with me, let's put it that way if you like, with the helpfulness . . . I can’t fault anything. I really can’t. . . . I liked [name], you know, she kind of put me at my ease, never put any pressure on me at all . . . I think if you take to the person that’s talking to you it goes a long way to anything, you know.
Female 25
One woman made a comparison to palliative care therapists:
I used to listen to [palliative care] physio[therapist]s all day, getting people to go up and down these stairs, who didn’t want to . . . they were dying, they didn’t want to know about it but she was gonna get them up. And the way she did it was amazing, because, because of her personality . . . cos it’s not about dying, it’s about making the most of living really . . . I think they’re quite similar actually . . . the empathy and the relating to people . . . I mean, you’ve got to have all those qualities haven’t you? You’ve got to have the same qualities.
Female 29
My get up and go has got up and gone
Participants describe other health issues with advancing age that might have an impact on recovery, including high blood pressure, chronic arthritis, hip replacements, diabetes, heart attacks, itching, chronic cough, hearing loss, sight loss and incontinence. Some described this as ‘just old age’ or ‘my get up and go has got up and gone’:
I developed diabetes . . . then I got angina 3 years later, I had a heart attack . . . now I’ve got a terrible blasted itch I’ve had it for 4 years, no one knows what it is. . . . It makes my life an absolute misery. . . . Another thorn of mine: hearing, I’m completely deaf in this ear . . . and I’ve had this cough since about December I suppose but that’s the least of my problems. I think most of my troubles are just age . . . Lack of stamina compared to when I was young . . . put it another way: my get up and go has got up and gone . . . I mean age has to catch up with everyone doesn’t it eventually, unless you’re gonna die in your sleep at 50, that’s luck . . . I can’t die young can I now? I think that’s really the basic trouble: age. I think so.
Male 28
I do have other health problems . . . I used to enjoy swimming a lot and I’ve not been able to do that for about 5 years . . . that was partly my knee but . . . it’s the eyesight issue as much as anything . . . vision certainly affects what I decide to do.
Female 32
A variable social life
Some participants described a diminished social life that could have a profound impact on their motivation and recovery. Two men had quite recently lost their wives and described the impact of this loss:
I think my mental thing is, is more related to the loss of my wife in many ways . . . Because I’m in a house on my own . . . I don’t have anybody to talk to . . . I get up in the morning to have my breakfast . . . but some days I don’t bother to have any food at all, because I can’t be bothered. We always used to lay the table up here for lunch, you know, and we would sit down and have a chat; television was turned off, radio was turned off, we used to talk at lunchtimes always . . . that’s the big issue with me mentally . . . Now whether that’s had an impact on the way I’ve, I’ve recovered I don’t know . . . but I feel it has had an effect . . . I still talk to my wife [laughs].
Male 33
I don’t do much really. Umm, my daughter has every Friday off and does the housework and this that and the other, she looks after me quite a lot when she’s here, but she’s not here a great deal. Umm, I don’t get out a lot at all . . . I watch the television mostly in the evening; I haven’t succumbed to daytime television yet . . . all my friends have died really . . . And you do lose a lot of friends when you stop work. But once again they’ve all died off one by one; almost the longest survivor I think.
Male 28
Others described a more active participation in life with friends and family:
I play Bridge a lot . . . I teach Bridge . . . I enjoy doing that . . . they wanted to learn over at the club . . . So I said to the girls, ‘Do you want me to do it?’. ‘Oh yes please’ sort of thing, and so I, I started them from scratch and that was 14 years ago . . . and we’re still learning [laughs]. Yes, once a week. And then I’d go with one or two of them to different clubs, a couple of clubs to get them used to going out and joining clubs and that, yeah they enjoy it, it’s nice.
Female 31
I have two sons and a daughter and that family with the grandchildren and their partners and I’ve begged one of them to give me a wedding [laughter] . . . they’ve all got partners, two step grandchildren, great grandchildren . . . they’ve been together 10 years and they’re just expecting their first child together . . . I'm very proud of them all, they've all got homes, jobs and if they’re not buying a home they're renting one.
Female 25
A lot would shirk putting on a pair of running shoes
Personal motivation to exercise was described as a factor that might influence adherence and, therefore, outcome. Participants described the ‘exercise type’ or ‘not the exercise type’:
I do have to give myself a good talking to . . . you’re talking to the wrong person, cos I am literally the laziest person, I hate exercise. Yes. And you know I have a lovely place to walk in, you know, I used to say that to myself before, ‘I don’t live in a tower block where I’ve got to walk round and round the sofa’, you know I just have to step out the door and this time of year it’s beautiful, you know. And I don’t even have to go very far, I can just walk down the village and stop for a cup of tea down the other end and come back again. You know, I have no excuses other than the fact that I am just bone idle and I hate exercise.
Female 29
They’ve probably never exercised in the first place . . . a lot of individuals that I know would shirk putting on a pair of running shoes [laughs] . . . My wife was a townie . . . She’s never been interested in sport. She’s never done any sport at all in her life . . . When I was at school we had . . . form games . . . school games . . . and then you had the school represented teams . . . so I’ve always been physical as it were. But I mean my son he’s, he, he’s not physical, he doesn’t do exercises, he would blow a gasket if you asked him to do some exercises.
Male 33
Discussion
Implications for clinical practice and education
Physiotherapists and assistants
We found that both physiotherapists and rehabilitation assistants were positive about working with each other in the community and that themes cut across both groups.
Our findings indicate that there are personal skills that are integral to good clinical practice that extend beyond professional knowledge: seeing the person in their own world, developing people skills and thinking outside the cubicle. We found that the home therapy environment encouraged clinicians to develop these skills, and the clinicians indicated that they would transfer these skills to other clinical settings.
Our findings also suggest that there are barriers to developing personal skills that should be considered. It can be a challenge to tread the fine line between being a friend and being a patient, and there can be times that you feel outside your comfort zone. Physiotherapists and assistants described the need for a support network to help them to meet these challenges. Having successfully managed these challenges, clinical staff described the ‘above and beyond’ – getting to know the patient and, therefore, having an impact on their lives – that made their role rewarding in the long term.
Patients
We found that the interviewed participants were extremely positive about the CORKA home-based intervention. Benefits included good, effective use of their time (‘It was a relief not to travel’ and ‘I got an hour’s work done in an hour’), being able to tailor the intervention to their own environment and having someone to oversee exercise progression. Home therapy was described as a social benefit that contributed to exercise adherence and motivation.
The challenges of home therapy included the intense nature of home treatment (where you got an hour’s work done in an hour). For some, this intensity could become burdensome. Participants also described feeling abandoned after the intervention was over.
Our findings highlighted factors that might affect the outcome of trial interventions. These included personal circumstances, such as physical health, social life and personal feelings about exercise. They also included the personality and social skills of the therapist, which participants described as integral to adherence and motivation. This supports findings from the clinician interviews.
Strengths and limitations of the study
The sampling strategy successfully recruited participants with a range of ages and experience. Qualitative research is an interpretive methodology that does not aim to be statistically representative of the whole: it aims to distil ideas from the essence of collected data. Thus, the rigour of our qualitative study hinged on our collaborative approach to analysis and the experience of the qualitative researcher. It is not possible to precisely estimate an appropriate sample size for qualitative studies when projects require numbers of participants and resources to be estimated in advance to be granted funding, and the number of participants in qualitative studies ranges widely. Further research exploring the issue of saturation would be useful. Our study has distilled ideas that would be useful in developing clinical practice for frail older adults undergoing joint replacement and for clinical education.
Chapter 7 Screening tool development
Introduction
This chapter describes the development of the screening tool to identify participants at risk of a poor outcome after TKA, who would then be suitable for recruitment into the CORKA trial. During trial design, we identified that no existing screening tool existed to identify patients at risk of a poor outcome after KA. It is reported following the Transparent Reporting of Multivariable Prediction Models for Individual Prognosis or Diagnosis statement. 105,106
Study population
The data used to develop the screening tool came from the Knee Arthroplasty Trial (KAT) data set. 107 The KAT was a pragmatic, multicentre RCT designed to determine whether or not a metal-backed plate for the tibial component was more effective and cost-effective than a single high-density polyethylene component or resurfacing the patella, and whether or not a mobile bearing between the tibial and the femoral components was associated with better outcomes than standard designs without a mobile bearing. 107
The KAT involved 116 surgeons at 34 UK sites. All patients under the care of a collaborating surgeon were potentially eligible for inclusion in the trial if a decision had been made to have primary knee replacement surgery. A patient was ineligible for the trial if their surgeon considered that a particular type of operation was clearly indicated, for example if a patient required a highly constrained knee replacement to replace function of the collateral ligaments. A participant remained eligible only if their surgeon was convinced that there was no indication for one of the trial surgeries. For example, a patient with a very thin patella would not be eligible for the patellar resurfacing comparison because patellar resurfacing would not be suitable for them. 108
Knee Arthroplasty Trial data collection
The KAT data collection was carried out using standard forms to record pre-operative, peri-operative and post-operative information. Data describing functional status and QoL were collected in forms that were sent to the participants by mail. These follow-up forms were meant to be completed by participants around 3 months, 1 year and 2 years after the surgery.
If follow-up questionnaires were not returned after a mail reminder, the trial team gave a telephone reminder and offered participants the option of completing the form over the telephone. Further details of the KAT design and data collection can be found elsewhere. 107
The KAT time points and the data collected at each are described in Table 27.
| Time point | Information collected | Questionnaires |
|---|---|---|
| 1. First contact with patients (emergency department presentation) | Baseline characteristics, clinical assessment, health on the day of the interview, general health and health problems caused by the knee |
|
| 2. Follow-up at 3 months post surgery | Health on the day of the interview, general health, health problems caused by the knee and contact details for future contact |
|
| 3. Follow-up at 1 and 2 yearsa | Health on the day of the interview, general health and health problems caused by the knee |
|
Individual patient data used to develop the screening tool
The KAT data set contained individual participant information at baseline and 3-month, 1-year and 2-year follow-up, which was collected from 2318 participants randomised to receive one of the three KAT interventions.
To develop the CORKA screening tool, we excluded data from KAT participants aged ≤ 55 years (n = 115) to match the CORKA trial exclusion criteria. We also excluded data from KAT participants with a BMI of > 50kg/m2 (n = 9). The final development data set comprised information from 2194 KAT participants.
Available variables and initial selection of candidate predictors
Of the variables available in the KAT data set, those measuring sociodemographic indicators (e.g. age, gender and BMI), pre-surgery QoL, physical function, current mobility levels and pain status were considered potentially useful for identifying KA patients at risk of a poor surgery outcome. We selected eight of these variables as a priori candidate predictors for testing for inclusion in the screening tool. These candidate predictors were selected based on their clinical importance, existing statistical evidence and relevance to the patient. The initial selection was made internally by the research team, taking into account the literature and the team’s expertise.
Table 28 lists the pre-selected candidate predictor variables, what type of variable they are, how they were collected and the number of missing data. The table also includes information for height and weight, which were used to calculate the eighth variable, BMI.
| Type | Variable name | Categories/units | Questionnaire | Missing values, n (%) |
|---|---|---|---|---|
| Binary | Gender | Male, female | Participant details form | 0 (0) |
| Categorical or ordinal | ASA classification grade | Completely fit and healthy; some illness but has no effect on normal activity; symptomatic illness present, but minimal restriction; symptomatic illness causing severe restriction | Baseline questionnaire | 109 (5) |
| Have you accomplished less than you would like as a result of your physical health? (SF-12 question 6) | All of the time; most of the time; a good bit of the time; some of the time; a little of the time; none of the time | Baseline questionnaire | 148 (6) | |
| Have you felt downhearted and low? (SF-12 question 11) | All of the time; most of the time; a good bit of the time; some of the time; a little of the time; none of the time | Baseline questionnaire | 141 (6) | |
| Pain on the knee (OKS question 1) | None; very mild; mild; moderate; severe | Baseline questionnaire | 113 (5) | |
| Mobility (EQ-5D-5L question 1) | No problems in walking about; some problems in walking about; confined to bed | Baseline questionnaire | 94 (4) | |
| Continuous | Age | Years | Participant details form | 0 (0) |
| Height | Metres | Participant details form | 148 (6) | |
| Weight | Kilograms | Participant details form | 107 (5) | |
| BMI | kg/m2 | Not on questionnaire (calculated) | 157 (7) |
Outcome definition
The outcome of interest for the predictive tool was functional status 1 year after TKA, measured by the OKS. To identify the participants who would most benefit from the CORKA home-based intervention, we defined an OKS score of 26 or less as the threshold for a poor outcome.
The OKS was the primary outcome measure in KAT. Table 29 presents a summary of the available outcome data in the KAT data set, the number of participants with a good or poor outcome and the number of missing data.
| Outcome | Good outcome/no event (OKS of > 26) | Poor outcome/event (OKS of ≤ 26) | Missing data | Total |
|---|---|---|---|---|
| Functional status outcome 1 year after TKA | 1319 (56.9%) | 389 (16.8%) | 610 (26.3%) | 2318 |
Exploratory analysis and data transformation
The baseline characteristics of the candidate predictors in the KAT data set were summarised using means and SDs for continuous variables, and frequencies and percentages for binary and categorical variables.
Binary and categorical predictors were tabulated against the outcomes to check for empty or low cell counts (n < 5). Where empty or low counts were found, categorical variables were re-categorised by joining some of the categories together if it made clinical sense to do so.
Although most of the KAT participants had their Short Form questionnaire-12 items (SF-12) assessment performed with version 2.0 of the questionnaire, some were assessed with version 1.0. The two predictors that were based on questions from this instrument were re-categorised to ensure data consistency.
The list of manipulated variables and the changes made for each are presented in Table 30.
| Variable | In the original data set | After exploratory analysis/data manipulation | ||
|---|---|---|---|---|
| Type | Categories/units | Type | Categories/units | |
| Age | Continuous | Years | Binary |
|
| BMI | Continuous | kg/m2 | Categorical |
|
| ASA classification grade | Categorical |
|
Binary |
|
| Have you accomplished less than you would like as a result of your physical health? | Categorical |
|
Binary |
|
| Pain on the knee | Categorical |
|
Binary |
|
Handling missing data
The percentage of missing data in the CORKA screening tool development data set was presented for each candidate predictor variable in Table 28 and the outcome in Table 29. To conform to current guidelines, multiple imputation for all participants with at least one missing value was performed. 109 As several predictor variables of different types had missing data (binary, categorical and continuous), multiple imputation by chained equations was carried out using the mi impute chained function in Stata 15. We used the option logit for binary variables, mlogit for categorical variables and truncreg for continuous variables, setting the lower and upper limits for imputed values as the correspondent scale limits.
We assumed that all missing data were missing at random and that the imputation models included all available observed characteristics for the predictors of interest, predictors of predictors (e.g. weight and height for BMI) and outcomes, as recommended by White et al. 109 There were low rates of missing data, with no more than 6% missing for any one candidate predictor. Based on the number of missing data for the variable with the highest rate of missing observations, we produced 10 complete imputed data sets. We did not perform data transformations on continuous predictor variables before imputing missing observations.
Despite using the augmented-regression approach,110 some categorical predictors were excluded during imputation owing to perfect prediction. 111 Perfect prediction occurs when one of the categories in a categorical explanatory variable is always observed with one of the possible outcomes, for example if all women had a good outcome. Perfect prediction is a problem because it leads to infinite coefficients with infinite standard errors, causing instability during estimation and preventing the imputation model from achieving convergence. It is often resolved by discarding the observations corresponding to the offending covariate patterns or by discarding the independent variables that perfectly predict the outcome during estimation. We decided to keep all of the observations in the data set and discard the variables that caused perfect prediction from the multiple imputation model. As a result, we discarded two baseline variables: ‘mobility’ and ‘have you felt downhearted or low’.
Sample size considerations
Sample size requirements for logistic regression are based on the concept of events per variable (EPV). It is widely recommended that a data set contains a minimum of 10 EPV to avoid overfitting the model. 112–117
As the KAT database contained 389 events of a poor outcome 1 year after surgery (see Table 30), we could examine up to 39 (389/10) candidate predictor variables in the model. After excluding two of the preselected candidate predictors owing to perfect prediction, only six of our baseline candidate predictors remained for testing. We, therefore, had 65 EPV available, making overfitting unlikely.
Data modelling
As the outcome was binary (poor outcome after TKA: yes/no), the screening tool was developed using a logistic regression modelling framework with the logit probability of an adverse outcome as the response variable. We removed candidate predictors that were not statistically significantly associated with the outcome. The remaining candidate predictors were included in the final logistic regression. As we had 65 EPV, we did not shrink the regression coefficients.
To aid implementation of the screening tool, we simplified the final multivariable model by assigning integers to the predictors based on the final model. The integers can be summed to obtain a total to identify participants who are likely to have a poor outcome 1 year after TKA.
Predictive accuracy
The performance of the CORKA screening tool was characterised by evaluating its discrimination. Calibration was not assessed, as the aim of the screening tool was to generate a score for an individual and not predict the probability of the outcome.
Discrimination measures the screening tool’s ability to correctly rank individuals. The overall discriminatory ability was summarised by the area under the receiver operating characteristic (AUROC) curve (c-index), with 95% CIs. The AUROC was classified as follows: 0.5 to < 0.6 poor, 0.6 to < 0.7 fair, 0.7 to < 0.8 moderate, 0.8 to < 0.9 good and 0.9–1 excellent. Owing to the large number of EPV, we did not carry out a formal internal validation of the model using bootstrapping or cross-validation, for example.
Results
Table 31 summarises the candidate predictor data available for modelling in the KAT data set before and after exclusions and multiple imputation. Multiple imputation and excluding KAT participants with characteristics that would not meet the inclusion and exclusion criteria for the CORKA trial from the model development data set did not greatly alter the sample’s characteristics. For example, average age changed from approximately 69.99 ± 8.38 years before to 71.01 ± 7.22 years after exclusion and multiple imputation, and BMI changed from 29.70 ± 5.47 kg/m2 before to 29.53 ± 4.88 kg/m2 after exclusion and multiple imputation.
| Variable | Before multiple imputation | After multiple imputation |
|---|---|---|
| Age (years), mean (SD) | 69.99 (8.38) | 71.01 (7.22) |
| Height (m), mean (SD) | 1.65 (9.89) | 1.65 (9.85) |
| Weight (kg), mean (SD) | 81.00 (16.45) | 80.54 (15.57) |
| BMI (kg/m2), mean (SD) | 29.70 (5.47) | 29.53 (4.88) |
| Gender, n (%) | ||
| Male | 1014 (43.74) | 955 (43.53) |
| Female | 1304 (56.26) | 1239 (56.47) |
| Pain on the knee, n (%) | ||
| None | 5 (0.23) | 5 (0.23) |
| Very mild | 31 (1.41) | 33 (1.5) |
| Mild | 97 (4.42) | 94 (4.28) |
| Moderate | 953 (43.38) | 968 (44.12) |
| Severe | 1111 (50.57) | 1094 (49.86) |
| ASA classification grade, n (%) | ||
| Fit/healthy | 367 (17.10) | 367 (16.73) |
| Some illness | 1324 (61.70) | 1350 (61.53) |
| Symptomatic illness with minimal restriction | 440 (20.50) | 460 (20.97) |
| Symptomatic illness causing severe restriction | 15 (0.70) | 17 (0.77) |
| During the past 4 weeks, have you accomplished less than you would like as a result of your physical health? n (%) | ||
| None or very mild or mild | 1759 (81.06) | 1781 (81.18) |
| Moderate or severe | 411 (18.94) | 413 (18.82) |
We kept continuous variables in their original format without data transformation for multiple imputation of missing data.
A summary of the unadjusted and fully adjusted multivariable models’ estimates (odds ratios with 95% CIs and p-values) is presented in Table 32. In the unadjusted analysis, all remaining candidate predictors were significantly associated with the outcome, except for gender. In the fully adjusted multivariable model with the predictors, all variables remained statistically significant. Age was negatively associated with the likelihood of a poor outcome 1 year after TKA. The research team made the pragmatic decision to omit this variable from the final multivariable model, as we did not have a plausible clinical explanation for the observed direction of association.
| Variable | Unadjusted analysis | Full multivariable model | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age (56–65 years) | 1.55 (1.24 to 1.92) | < 0.001 | 1.55 (1.24 to 1.95) | < 0.001 |
| Gender (female) | 1.10 (0.88 to 1.37) | 0.399 | – | – |
| BMI | ||||
| Overweight (25–29.9 kg/m2) | 1.41 (1.03 to 1.94) | 1.39 (1.01 to 1.93) | ||
| Obesity (≥ 30 kg/m2) | 2.05 (1.50 to 2.79) | < 0.001 | 1.80 (1.31 to 2.48) | < 0.001 |
| OKS | ||||
| Moderate/severe pain on the knee | 2.8 (1.59 to 4.91) | < 0.001 | 3.61 (2.47 to 4.63) | 0.001 |
| ASA classification grade | ||||
| Symptomatic illness with minimal restriction/symptomatic illness causing severe restriction | 2.63 (2.10 to 3.28) | < 0.001 | 2.60 (2.07 to 3.26) | < 0.001 |
| SF-12 | ||||
| Accomplishing less than would like as a result of physical health all the time/most of the time | 2.19 (1.74 to 2.76) | < 0.001 | 2.04 (1.60 to 2.59) | < 0.001 |
A summary of the final multivariable logistic regression model (odds ratios, 95% CIs and p-values) is presented in Table 33.
| Variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| BMI | ||
| Overweight (25–29.9 kg/m2) | 1.44 (1.04 to 1.99) | < 0.001 |
| Obesity (≥ 30 kg/m2) | 1.93 (1.41 to 2.66) | |
| OKS (moderate/severe pain on the knee) | 3.63 (1.49 to 5.67) | < 0.001 |
| ASA classification grade (symptomatic illness with minimal restriction/symptomatic illness causing severe restriction) | 2.49 (1.99 to 3.13) | < 0.001 |
| SF-12 (accomplishing less than would like as a result of physical health all the time/most of the time) | 2.04 (1.61 to 2.59) | < 0.001 |
| Intercept | 0.05 (0.03 to 0.10) | < 0.001 |
As all of the odds ratios were greater than 1, the CORKA screening tool was developed by rounding the observed odds ratios for each predictor or predictor category. Any patient with a score of 5 or more would be classified as being at increased risk of a poor outcome after 1 year and likely to benefit from the CORKA home-based intervention. The threshold score of 5 was a pragmatic choice taken by the research team based on forecasted numbers of individuals declared as at high risk of a poor outcome. The final CORKA screening tool is presented in Table 34.
| Variable | Categories | Points |
|---|---|---|
| BMI | Normal weight (< 25 kg/m2) | 0 |
| Overweight (25–29.9 kg/m2) | 1 | |
| Obesity (≥ 30 kg/m2) | 2 | |
| OKS question 1:a during the past 4 weeks, how would you describe the pain you usually have in your knee? | None or very mild or mild | 0 |
| Moderate or severe | 4 | |
| ASA classification grade | Fit/healthy | 0 |
| Some illness or symptomatic illness with minimal restriction or symptomatic illness causing severe restriction | 2 | |
| SF-12 question 6:b in the past 4 weeks, have you been limited in the kind of work you do or other regular activities you carry out as a result of feeling anxious or depressed? | All the time or most of the time | 2 |
| Some of the time or a little of the time or none of the time | 0 |
The model performance was assessed in terms of discrimination. The model had a fair overall discriminatory ability to predict a poor outcome 1 year after TKA (apparent performance), as measured by the AUROC curve (0.66, 95% CI 0.64 to 0.69).
Discussion
The CORKA screening tool was developed to be a simple-to-use tool that identified participants likely to be at increased risk of a poor outcome 1 year after TKA and, therefore, most likely to benefit from the CORKA home-based intervention. As the screening tool was developed using a large existing data set with a small number of candidate predictors, we were not concerned about overfitting the model. The apparent performance of the model was fair, as measured by its discrimination.
The research team was confident that the developed screening tool would help to identify most patients at an increased risk of a poor outcome and most likely to benefit from the CORKA home-based intervention, owing to the quality of the development data set, data optimisation with missing data multiple imputation and model building strategy.
We did not conduct any cross-validation or internal validation because EPV is a marker of the potential for overfitting, with an EPV value of < 10 widely considered to be the level at which concerns may be raised.
Our EPV for model development was very large at an EPV of 65. Simulation studies118,119 illustrate that once EPV is > 20, the bias owing to any overfitting is negligible/eliminated, such that the apparent performance (reported in the monograph) converges to the true underlying large sample performance. 118,119
We chose to use the KAT data set and the OKS as an indicator of poor outcome because of the availability of a large data set. The use of clinical trial data is potentially challenging, particularly if there is a large treatment effect observed in the trial. The potential of using clinical trial data has recently been described by Pajouheshnia et al. 120 Options are to include treatment (if the effect is large) in the model or to use the control arm only (particularly if the arm was a placebo arm). The KAT observed no differences in OKS between the treatment arms, which reinforced our justification of using the entire trial data. Furthermore, available data following the natural course of patients are uncommon, individuals will usually have received specific treatments during follow-up, i.e. confounding by indication. 120
Chapter 8 Discussion
This discussion summarises the trial findings and the issues associated with its internal and external validity, and provides interpretation for clinical practice in the NHS.
Overview of the trial findings and key messages
Knee arthroplasty is one of the most commonly performed musculoskeletal operations. Although it effectively reduces pain and improves physical function for most patients and has good reported cost-effectiveness, around 15% of patients report dissatisfaction with their post-operative outcome. 7,121,122
In the short term, KA results in substantial loss of quadricep strength and decreased range of knee motion and function, compared with the pre-operative status. 123 Pain, reduced function, decreased mobility and resulting falls cause significant morbidity for those patients with a poor KA outcome. However, there is evidence to support the use of functional exercises by physiotherapists to improve the range of joint motion and QoL. 18 Draft clinical guidelines from the National Institute for Health and Care Excellence (NICE) recommended targeted physiotherapy interventions for patients at higher risk of a poor outcome for whom a self-managed exercise programme at home may not be suitable. 124
The CORKA trial was a large multicentre RCT that targeted patients who were at risk of poor outcomes after KA. We randomised 621 participants: 312 to usual care and 309 to home-based rehabilitation. Most participants had a screening tool score of 5 or 6, all met the criteria of moderate or severe knee pain and most were overweight or obese. The two treatment groups were well balanced at baseline. The average age of the trial participants was 70.5 years.
Approximately one-quarter of participants received a UKA. TKA has been the traditional treatment of choice for older patients with knee pain and limited activity because of arthritis. Undertaking UKA is possible for some of these patients, but the indications outlined by Kozinn and Scott125 in 1989 suggested that the procedure was not suitable for patients with higher degrees of comorbidity. The original indications for UKA included arthritis isolated to the medial compartment in patients under 60 years of age with low levels of physical activity and weighing under 82 kg. This was thought to be around 6–12% of patients undergoing knee replacement surgery. 126 However, more recently, Campi et al,126 reported that surgeons have widened the indications, especially for weight, age and activity, and up to 50% of patients may now be eligible for UKA.
The recent TOPKAT trial127 compared TKA with UKA, and demonstrated that both were effective options for patients with medial knee arthritis with similar clinical outcomes. However, participants receiving a UKA had slightly better outcomes in terms of lower surgery costs and follow-up health costs in the first 5 years. The lower costs of surgery for UKA may be a result of the increasing use of enhanced recovery pathways, which are having a positive effect on outcomes. 121 These pathways can adopt rehabilitation strategies that encourage early mobilisation and discharge. 128 Within the CORKA trial, the same post-operative intervention was used irrespective of implant. However, both treatment options may have been shorter and less intense for the UKA patients, who rarely struggle to regain their range of movement.
There was no difference in evidence of the benefit between usual care and home-based rehabilitation in terms of LLFDI function score at 12 months post randomisation (ITT multiple imputation difference 0.49, favouring CORKA; 95% CI –0.89 to 1.88; p = 0.48), 6 months post randomisation (ITT multiple imputation difference 0.66; 95% CI –0.70 to 2.03; p = 0.34) or based on any sensitivity analysis. As previously discussed, this may be in part because participants in the usual-care group received more treatment sessions than anticipated. Treatment effects varied substantially by site and showed some relation to the average number of sessions of usual care and home-based rehabilitation received at each site.
There were also no significant differences between the two groups in terms of any of the secondary PROMs or physical measures for the ITT population using available cases. Sensitivity analyses of the key secondary PROMs (LLFDI disability frequency and limitation, OKS and KOOS QoL subscale) identified one significant difference between the two groups, for LLFDI disability limitation using the ITT population and multiple imputation. However, we do not believe that this is a difference that is explicable by the package tested and think that it is more likely to arise from the number of tests performed than being a true finding when considered in the context of all the other tests, physical and self-reported, that were conducted with no statistically or clinically significant differences. The number of participants experiencing a serious adverse event was small in both the usual-care arm (14/312, 4.5%) and the home-based rehabilitation arm (18/309, 5.8%), with no significant difference between them (risk difference 1.3%, 95% CI –2.1% to 4.8%; p = 0.45).
Over the 1 year of the CORKA trial, we found a small non-significant difference in QALYs favouring the CORKA home-based intervention (0.003 QALYs, 95% CI –0.017 to 0.023 QALYs). The CORKA intervention itself was cheaper, but the participants in the CORKA arm reported higher use of other NHS resources in that year than participants in the usual-care arm. This cost was offset by lower societal costs, with more CORKA participants returning to the workplace and using less social services than usual-care participants.
The participant diaries that were used to capture health and societal costs showed that participants in the CORKA arm had less time off work and returned to work sooner than participants in the usual-care arm. We do not believe that this is a spurious finding. We can only speculate on the reason for this. The CORKA intervention was much more functionally focused, with individual goal-setting aiming to return to activities that the participants had selected as important to them. Conventional physiotherapy has a more biomechanical approach focusing on strength and mobility. We believe that the more individually tailored functional approach of the CORKA intervention may have been a factor in a larger number of people returning to work. Overall, however, because of the age of most participants, a relatively small number of participants in both groups returned to work.
Usual care used a traditional clinic-based or hospital-based model of outpatient physiotherapy delivered by qualified, registered physiotherapists. By contrast, the CORKA home-based intervention was multidisciplinary in content, was delivered in participants’ own homes and used a staffing model of rehabilitation assistants supervised by a qualified physiotherapist or occupational therapist. The trial data suggest that the two treatments were similarly effective and safe in this population.
Interviews with patients who received and therapists who delivered the CORKA home-based intervention showed that it was acceptable to both patients and clinicians. Being treated at home facilitated a holistic rather than biomedical approach that underpinned a collaborative partnership.
Internal validity and methodological limitations
The CORKA trial was pragmatic in the context of the funding envelope available for physiotherapy within the UK. It sought to estimate the effect of treatment strategies based on clinic-based or home-based treatment using different staffing models. It used a range of recognised measures with a 12-month follow-up, which is longer than many previous trials.
We recruited 621 participants. Based on our published statistical analysis plan,46 the sample size calculation required data from a minimum of 620 participants, assuming a moderately small standardised effect size of 0.275, or 3 points on the LLFDI function score, with a power of 90% and an alpha of 0.05. This allowed for a withdrawal rate of 10%.
The availability of follow-up data in this trial was very good. Primary outcome data were available for 92% of participants in the usual-care group and 89.4% of participants in the home-based rehabilitation group at 12-month follow-up. Rates of withdrawals (34/621, 5.5%) and deaths (3/621, 0.5%) were very low, resulting in a loss to follow-up rate lower than the 10% we had allowed for. We had primary outcome data at 12 months for 566 participants (91.1%), providing sufficient statistical power to detect a difference between the groups as originally specified.
Randomisation was conducted by a central facility at the Oxford Clinical Trials Research Unit and was stratified by site using variable block lengths, preventing research staff from anticipating or influencing treatment allocation for any given participant and resulting in two well-matched arms. Owing to stratification by recruiting site when allocating participants to the groups, the numbers recruited were not divided into exactly equally sized groups, but did not differ significantly. Demographic data, self-reported functional measures, clinical impairment measures and disease stability were all very similar.
Owing to the nature of physiotherapy interventions, it was not possible to blind participants to their treatment allocation. However, we endeavoured to ensure that outcome assessors remained blinded throughout the trial. This was hard to achieve, with a high risk of inadvertent unblinding by participants to research staff. We used a number of strategies to reduce the risk of unblinding, including explicitly instructing participants not to inform their research physiotherapist of their group allocation at every visit. 129 Barker et al. 130 have previously reported that using these strategies resulted in successfully maintaining blinding in 81–91% of the assessments, meeting the expected level for a successfully blinded trial, as defined by Minns Lowe et al. 129 and Boutron et al. 131 As the primary outcome measure was self-reported by the participants, it was unlikely to have been influenced by unblinding of the outcome assessors.
Compliance with home-based rehabilitation was prespecified as receipt of at least four treatment sessions, and the compliance rate was high (269/309, 87.1%). The average number of treatment sessions in each group was similar, with participants in the usual-care group receiving a median of four sessions (IQR two to six sessions) and participants in the home-based rehabilitation group receiving a median of five sessions (IQR four to seven sessions). The number of treatment sessions received in the usual-care group was larger than anticipated, which may have affected the observed treatment effect. There was also wide variability in the number of usual-care treatment sessions offered to participants (range 0–27). The median number of usual-care sessions differed substantially by site, ranging from two at site 13 to six at site 1. This suggests that there is a lack of standardisation in usual rehabilitation offered to these patients across the country.
The interventions in both of the treatment arms were well received by participants according to the qualitative study results.
Quality assurance checks and observation visits determined that the therapists delivered the content well, although there was variation in the number of sessions delivered.
The treatment logs and assurance visits demonstrated that exercise progression was delivered as planned. In designing the interventions, incremental progression of exercises and activity levels were considered important to achieve the underlying physiological changes required to improve strength and balance. There was good evidence from the content analysis of treatment logs that participants had progressed the quantity and difficulty of the programme content, incrementally increasing the amount of activity expected throughout the course of the treatment programme.
The trial team achieved a high level of data completeness for the primary outcome, with over 90% complete. The completeness for the physical measures was slightly lower at 89.2% for the F8WT, 89.4% for the 30SCST and 89.9% for the SLS, but was still close to the desired level of 90%, reflecting that the physical outcome tests required a visit to the site. The pragmatic approach to collecting outcome data by post or telephone when participants were unable or unwilling to attend face-to-face assessments resulted in a higher completion of the questionnaire data.
A small proportion of participants (5.8%) withdrew and were distributed evenly across the two treatment arms. There were no significant baseline differences between those retained and those lost to follow-up. Only three participants (0.5%) were lost to the trial because of death. This was lower than expected considering that, on average, participants were over 70 years of age and had a BMI of over 31, and 81% were categorised as being in an ASA classification category of mild or moderate systemic disease at baseline.
An internal pilot study was conducted at one site (Oxford) to review the recruitment feasibility and confirm the intervention package. The 15 participants randomised during this pilot study were used in the final analysis, as no changes were made to the interventions or assessment tools between the pilot and the main trial.
External validity/generalisability of findings
Overall, we believe that the generalisability of the findings of the trial is good. We recruited sites across a number of NHS trusts, resulting in variation in trust size and a range of site sizes and geographical locations. The training burden on qualified therapists and rehabilitation assistants was relatively small because the core principles underpinning the programme were already within the skill set and expertise of most NHS therapy staff. Thus, we believe that the intervention could be implemented in other NHS sites relatively easily, particularly where a model for community care already exists.
Post-operative physiotherapy: case of need
Although physiotherapy is generally regarded to be a key component in achieving optimal results following KA, there is considerable international variation in the rehabilitation provided. In the UK, inpatient rehabilitation is of short duration, with patients typically discharged 3–5 days following arthroplasty after an accelerated recovery pathway. The key criteria for discharge home from hospital are safe mobilisation and adequate knee flexion. Patients are usually referred to receive further physiotherapy input through individual or group-based outpatient physiotherapy.
It is assumed that increased physiotherapy provision correlates with better outcomes. However, the benefits of post-operative physiotherapeutic interventions are poorly established, with no consensus as to whether or not outpatient therapy provides superior outcomes over other service delivery models. The content, intensity and duration of existing physiotherapy programmes delivered in the outpatient setting have been shown to vary widely. 132–135 Rehabilitation provision following discharge varies widely and no definitive guidelines for rehabilitation post knee replacement exist. The generally held assumption is that increased therapist contact enhances the rehabilitation provision.
One of the interesting but potentially expected results from the CORKA trial was the variation seen in usual care. Participants in the usual-care arm received an average of four treatment sessions, with a range of 0 to 27 sessions. Provision of rehabilitation following KA varies in what is provided, where it is provided and for how long it lasts. 124 As the ultimate aim of rehabilitation is to improve patients’ outcomes, it is reasonable to consider how best to deliver rehabilitation after KA.
Previous studies33,136 have investigated how much supervision is required in the rehabilitation process following KA and whether or not self-management is sufficient. NICE recently reviewed the evidence comparing self-directed outpatient rehabilitation and supervised outpatient rehabilitation. 124 No clinically important differences were found in QoL, PROMs or functional outcomes between group-based or individually based supervision and self-directed rehabilitation. In the light of this evidence, we suggest that self-directed rehabilitation makes sense for many people, with some caveats. As some patients may benefit from supervised rehabilitation, a tool that identifies them from those who can rely on self-directed rehabilitation would be useful, similar to that developed in Chapter 7. There may also be benefits to supervised rehabilitation that are not captured by the current evidence or the outcome measures used in existing studies. For example, participants in our embedded qualitative study were very positive about the CORKA home-based intervention and the supervision provided by therapists and rehabilitation assistants (see Chapter 6).
A significant amount of evidence suggests that patients benefit from rehabilitation after KA regardless of where it takes place, how it is delivered and who supervises it. There are many potential pathways for rehabilitation that seem to result in positive outcomes for patients. This could be positive for those who design, implement and commission rehabilitation services. As patients seem to do well regardless of how rehabilitation is delivered, rehabilitation pathways could be directed by local or subgroup needs. However, some meta-analyses have highlighted that post-operative physical therapy applied uniformly to all following TKA does not effectively improve patients’ outcomes after 1 year. 134 As most patients report a good result after TKA, some might benefit from a targeted intervention; for example, the CORKA trial focused on patients at risk of poor outcomes.
The CORKA home-based intervention could benefit patients who find it difficult to travel a significant distance to a larger hospital, for example owing to frailty. Using the CORKA trial combination of qualified therapists and rehabilitation assistants might make sense for certain areas of the UK or other countries because of the large geographical areas covered by therapists. It may be important to consider how we identify and focus resources on those subgroups that may benefit from supervised or more closely monitored rehabilitation.
Although many studies have compared outpatient physiotherapy with supervised home exercises, no RCTs have compared physiotherapy after KA with no treatment. Both patients and surgeons have concerns that the absence of physiotherapy would lead to poor outcomes and potentially further surgery for manipulation under anaesthesia.
The CORKA intervention
While developing the CORKA trial, in particular the CORKA home-based intervention, we considered the breadth of potential improvements that rehabilitation could facilitate after KA. One way to categorise how disability, or in this case KA, might affect people is to use the World Health Organization International Classification of Functioning, Disability and Health constructs of impairment, activity restriction and limited participation. 137 Impairment is related to body functions or structure, activity refers to difficulties in undertaking tasks or actions, and participation refers to difficulties experienced within life situations.
Knee arthroplasty rehabilitation tends to focus on physical impairment and activity,138 which the CORKA home-based intervention accounted for. However, the intervention was also designed to consider social participation, reflected by the CORKA primary outcome measure: the LLFDI. 37,139 The LLFDI assesses function, which records the participant’s ability to perform discrete tasks or activities, and disability, which measures the participant’s ability to undertake socially defined life tasks.
It was reported as early as 2011 that rehabilitation programmes after arthroplasty need to focus on more than just physical impairment. Davis et al. 140 investigated the trajectory of symptom improvement, daily activities and participation for 1 year after total hip or knee replacement. They concluded that KA rehabilitation programmes should target physical impairments, the ability to undertake activities and social participation.
Wylde et al. 138 recognised that outcome measures recorded post arthroplasty tended to focus on impairment or activity, but less so on limited participation. They aimed to investigate the importance of and difficulty in participating in leisure activities before and after knee or hip arthroplasty. They found that roughly one-quarter of all participants and almost one-third of KA participants were unable to carry out their leisure activities after surgery. The study concluded that leisure activities are important to people undergoing knee or hip arthroplasty and that limitations in participation should be measured after surgery. Maxwell et al. 141 explored the predictors and extent of participation restriction for patients after TKA. They also found that approximately 30% of study participants reported limitations in carrying out activities after TKA. They reported modifiable factors associated with participation restriction and suggested that these could be targeted in rehabilitation programmes.
Other evidence has suggested that patients can return to leisure pursuits after KA, referring to the activity and participation categories of the International Classification of Functioning, Disability and Health framework. For example, a systematic review suggested that patients are able to return to athletic activities after lower limb arthroplasty, although sometimes at a lower intensity than before surgery. 142 In the light of this body of evidence, it was important that the CORKA home-based intervention was developed considering impairment, activity and participation.
The CORKA trial was designed to include participants who were at risk of a poor outcome. One aspect of the intervention that could help to achieve a better outcome, particularly for those at risk of a poorer outcome, was that it was designed to be an individually tailored programme for each participant. This approach is in line with a recent study by Barker et al. ,143 which aimed to determine what functional or leisure activities were most important to those undergoing KA and to compare the actual time to return to these activities with patients’ expectations before surgery. The authors discussed the factors that could influence a return to activities valued by patients and recommended a tailored, personalised approach.
These considerations helped direct the development of the CORKA intervention. The full process is outlined in Chapter 3. The intervention was an individually tailored package consisting of range of movement, strengthening and balance exercises; gait skills; a graduated walking programme; practising functional tasks of relevance to the individual; information provision; adherence strategies; and the provision of appropriate aids and equipment. A breadth of components was included to target impairment, activity and participation.
The CORKA intervention took some steps to considering spousal support, such as asking participants who might be able to help them with achieving their exercise goal, as part of a behavioural contract. Future interventions should consider an ecological approach, considering not just the individual patient and clinical staff but other relevant agents in the environment, in particular the support of spouses or family. For example, it has been shown in physical activity promotion, that increases in an individual’s physical activity level positively affects a spouse’s physical activity engagement. 144 It may be that if the CORKA intervention targeted couples or families more specifically there may have been a difference in adherence with exercise programmes. 144
Workforce model: rehabilitation assistants
Our trial design used a workforce model based on experienced rehabilitation assistants supervised by a qualified therapist. This is an emerging service delivery model in the UK and elsewhere, where the need to provide greater levels of care to meet the needs of an ageing population is set against a backdrop of insufficient commissioned training places for students. 145 This model works well in the USA, where trials comparing the delivery of the Otago falls programme by qualified physical therapists and physical therapy assistants supervised by qualified physical therapists have demonstrated good outcomes. 146
Published guidance from professional bodies on competency training and roles that may be allocated to assistant staff can support advanced or experienced rehabilitation assistants to deliver effective protocol-driven care. 147 This will free up qualified staff for complex care and management of the overall patient pathway. This model is more advanced in speech and language therapy. Several RCTs have compared the roles of speech therapy assistants and qualified therapists undertaking speech therapy for children in schools and for swallowing practice. 148,149
In preparing the CORKA intervention, we were aware of the need to map the proposed tasks in the programme to the competencies and experience of advanced rehabilitation assistants. We drew on the work of Moran et al. 150 to prepare a programme that optimised the chances of effectiveness by establishing clear communication and supervision structures and resourcing training and pay to reflect the responsibility being undertaken.
Our findings showed that a model of supervision by a qualified therapist who acted as a consultant available to discuss and evaluate patients’ needs and who reviewed patients at two time points to ensure adequate progression was safe and as effective as a traditional model using qualified practitioners.
Qualitative study
Our qualitative findings indicate positive outcomes from CORKA that extended beyond the trial. Clinician interviews indicated that working with participants in their own homes facilitated a holistic rather than biomedical approach. It encouraged clinicians to develop people skills and flexibility when prescribing exercise and planning. These skills were transferable beyond the trial and into other clinical settings. Clinicians described the long-term personal rewards of going ‘above and beyond’, which may affect staff retention.
Our findings indicate areas of focus for clinical education. How do we help clinicians to balance the need to build rapport with the need to remain professional at all times, both of which are integral to effective outcomes? How do we help clinical line managers balance their need to provide support with assistants’ freedom to work independently and develop clinical skills?
In interviews, patient participants indicated that they did not object to having treatment delivered by physiotherapy assistants. Rather, our findings suggest that an individualised approach alongside a collaborative relationship enhances the effectiveness of exercise therapy. Our findings also highlight the social benefits of home therapy, particularly for those who are socially isolated. Future research should consider effects beyond physical intervention.
In a synthesis of 13 qualitative studies on patients’ perceptions of physical activity, Smith et al. 151 highlighted the facilitators of and barriers to physical activity before and after joint replacement. They found that the focus of physical activity after joint replacement is a desire for a new lease of life, being able to participate and enjoy life and being able to do ‘obligatory activities’ (such as personal care). Patients did not seem to be focused on public health or improving health. Smith et al. 151 suggested that external engagement and support to undertake exercise facilitated physical activity after joint replacement surgery, which resonates with our findings. They highlighted the importance of social contact, particularly postoperatively when social networks can be lost. Enjoying and taking part in physical exercise may supersede health as a global aim. Similar to our findings under the theme ‘I would not have done it on my own’, Smith et al. 151 suggested that a significant barrier to physical activity following TKA is fear of not ‘doing the right thing’. They highlighted a window of opportunity to encourage physical activity immediately following surgery as ‘motivating time for people to undergo rehabilitation and may therefore be a captive period to engagement in physical activity education’. 151 We have added that exercise should be individualised and relevant to the person and that exercise should be sociable and mutually supported.
Our qualitative findings also highlighted the cost benefits of the CORKA trial for patients, with regard to travel time and ‘getting an hour’s work done in an hour’. These costs should be considered in health-care provision and trials.
Critique of methods
We designed our trial to test interventions that could be delivered in routine NHS physiotherapy practice within the current commissioning constraints of the number of sessions remunerated by most care commissioning groups. The trial showed that it was practicable and successful to deliver the intervention content in both clinical and community settings.
Late Life Function and Disability Index
Our primary outcome measure was the function component of the LLFDI. This is a PROM designed to assess change, particularly in older adults. The scale is made up of an overall function domain and three subdomains: advanced lower extremity function (e.g. getting up from the floor), basic lower extremity function (e.g. walking within the home) and upper extremity function (e.g. putting on and taking off a coat). Each domain is calibrated on a 0–100 scale on which 0 indicates the worst functional level and 100 indicates the best functional level. It asks patients to report their current level of difficulty in performing 32 physical tasks on a typical day without assistance. It has good reported reliability and sensitivity to change. 152 We felt that the LLFDI encompassed the range of activities that were pertinent after arthroplasty, with a basic and advanced subsection that could capture changes in patients who achieved a good or poor outcome from surgery. We chose the function subscale over the disability subscale because self-reported functional difficulties in elderly patients are strongly predictive of disability. 153,154
Beauchamp et al. 155 reported the minimal clinically important difference for the function component of the LLFDI. They reported that substantial changes are reflected in a 5-point change in the overall function scale, a 6-point change in the basic lower extremity subscale and a 9-point change in the advanced lower extremity subscale. They reported that small but meaningful changes are reflected in a 2-point change in the overall function scale, a 3-point change in the basic lower extremity subscale and a 4-point change in the advanced lower extremity subscale.
We found substantial changes from baseline to 1 year in both groups. The CORKA home-based intervention resulted in an overall change of 8.9 points and usual care resulted in an average change of 8.7 points, indicating that the combined effect of surgery and rehabilitation was a highly significant improvement in function. Although most of this improvement was probably because of the surgical procedure, it may not have been so large without appropriate, effective rehabilitation. We cannot quantify the contribution of one without the other.
Secondary outcome measures
Despite recruiting only those at risk of poor outcomes to CORKA, the mean 52-week OKS achieved by both treatment arms was within the 4-point minimal clinically important difference of the wider UK average OKS following KA (35 points). The actual method of delivering physiotherapy may therefore be comparatively unimportant.
Study limitations
There are limitations to our work. We developed a screening tool to identify the patients in the worst half for predicted outcome. To make this pragmatic, we based the key questions on those already collected as standard practice in most pre-operative assessment clinics or collected as part of the standard national PROMs data set for KA. This may have resulted in a tool that was insufficiently sensitive to identify the patients most at risk of a poor outcome that could be addressed by rehabilitation after surgery. In developing our screening tool, we made an assumption that participants with an OKS of 26 or less would be more likely to benefit from the home intervention than from traditional physiotherapy. We recognise that this assumption was informed by the consensus opinion of groups of orthopaedic physiotherapy practitioners that we consulted in developing our research study and interventions and that it is not self-evident or based on hard evidence.
As we compared the multidisciplinary home-based intervention with the current UK best practice of outpatient physiotherapy, we cannot comment on the effect of the treatment arms compared with no treatment. The research available to date focuses on the efficacy of different types of physiotherapy following KA, with a lack of consensus. However, physiotherapy remains an accepted component of the treatment pathway for KA in the UK. A control group withholding all post-operative outpatient physiotherapy was not possible, as it was judged unacceptable to surgeons (who would not allow their patients to enter into such a trial) and unethical to deny physiotherapy to patients already highlighted as being at risk of poor outcomes.
We chose the LLFDI as the primary outcome measure, which is not an outcome tool specifically designed to capture change in outcome after KA. It was selected as it maps to the World Health Organization’s International Classification of Functioning, Disability and Health, and allows analysis of function, activity and participation. It has also been used in other rehabilitation trials of older adults. It is arguable that a more specific measure targeted at KA may have been a better choice. We included the OKS as a secondary measure to address this potential limitation. The results of the LLFDI and OKS were well-matched.
Owing to the nature of this therapy trial, it was not feasible to blind patients or therapists to treatment allocation. To ensure generalisability, we compared physiotherapy interventions that are deliverable within the current standard commissioning envelope for NHS physiotherapy, setting physiotherapy provision to approximately six sessions of individual physiotherapy.
Our cost-effectiveness analysis has several limitations, including missing data on resource use and EQ-5D-5L. We accounted for missing data using multiple imputation, which assumed that the data were missing at random conditional on modelled covariates. 103 We found no strong evidence to contradict this assumption and found that multiple imputation and complete cases analysis gave the same results about the cost-effectiveness of the CORKA intervention.
Implications for clinical practice and policy
The NHS 5 Year Forward Plan156 has identified the need for further productivity and efficiency in health-care delivery. It has also identified that the demands on health services are growing owing to an ageing population with more long-term conditions and greater patient expectations. Part of the NHS plan is to decentralise services, with greater community provision and less emphasis on care provided in large acute trusts where that is not clinically mandated.
We suggest that the CORKA home-based intervention is a model that meets this strategy as it targets care to those at higher risk who most need it, within participants’ own homes and communities. It addresses the issue of workforce shortage by using an innovative workforce model of advanced rehabilitation assistants, moving UK service provision closer to that which has been proven to be effective in North America, where the use of physical therapy assistant graded staff is well embedded.
Patient and public involvement
We benefited from the support of highly engaged patient and public involvement (PPI) representatives at various stages of the trial. Before the trial began we were supported by the Biomedical Research Unit PPI officer, who helped in the recruitment of a PPI representative as a co-applicant and fully voting member of the CORKA Trial Steering Committee. This PPI representative had previously undergone KA surgery with a relatively poor outcome. They, therefore, understood the rationale behind the CORKA trial, in addition to the aim of the intervention to help those at risk of a poor outcome. The trial team were able to update the PPI member throughout the study, with feedback welcomed.
Patient and public involvement engagement also occurred in the development of the intervention used for the CORKA trial. In the development and piloting phase, changes were made to participant materials and intervention procedures based on comments and advice from people who had undergone KA surgery; these are covered in more detail in Chapter 3. More informally, we were extensively supported by members of our local research engagement group and by former and current patients undergoing physiotherapy following KA. Their generous time and comments were hugely influential in shaping the trial, designing the intervention, and producing the patient information and documentation to support the trial. The PPI representative supported the write up of the CORKA monograph by reading the chapters that they were involved in and PPI groups will be used to help disseminate the results of the CORKA trial.
Further research
The findings from the cost-effectiveness analysis are important and consistent with the findings of the trial: there is no meaningful difference in costs and QoL between the CORKA intervention and the usual-care arm at the 12-month follow-up time point, although there is some evidence that CORKA may be cost-effective from an NHS and societal perspective. Further work to review the relative importance of NHS and societal costs in this population may be important, particularly if health care moves towards a more centrally funded health and social care integrated model.
It is suggested that further research should focus on developing a screening tool that is more sensitive in identifying those patients who will benefit from additional input, as recommended by the draft NICE guidance. 124 The NICE committee did not feel that self-directed rehabilitation without further supervision was adequate, and did not recommend supervised rehabilitation for all. However, they commented that ‘it would be very useful if a tool existed that indicated those who would benefit from supervised rehabilitation or required adaptations to self-directed rehabilitation’ [reproduced with permission from NICE124 © NICE 2020 Joint replacement (primary): hip, knee and shoulder. Available from www.nice.org.uk/guidance/ng157 All rights reserved. Subject to Notice of rights NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this publication].
The screening tool developed in the CORKA trial identified the population most at risk of a poor outcome after KA. It could be further developed to identify factors linked to engagement with rehabilitation.
The CORKA home-based intervention was delivered by rehabilitation assistants supervised by qualified therapists in a ratio of five sessions to two sessions. To the best of our knowledge, the use of different workforce models and particularly a hybrid model of assistants supervised at a distance by qualified therapists has not been researched in any depth in the UK. With projected deficits in health-care staffing from NHS England, modelling further work looking at different workforce models and interventions using rehabilitation assistants may form an important subject for further research. The use of a different staffing model is also worthy of exploration in terms of overall costs and affordability, making good quality rehabilitation affordable to all, particularly with the anticipated continued growth in the number of KA procedures performed each year.
Research to explore the relative benefits of alternative ways of delivering interventions would also be useful, particularly if this improved adherence. Similarly, it would be of interest to explore the reasons that older people do not engage fully with treatment interventions to improve the delivery of future programmes using strategies to enhance engagement.
Acknowledgements
The CORKA trial hospitals
Ashford and St. Peter’s Hospital NHS Foundation Trust (Ashford Hospital), Countess of Chester Hospital NHS Foundation Trust (Countess of Chester Hospital), Dorset County Hospital NHS Foundation Trust (Dorset County Hospital), Dorset Healthcare University NHS Foundation Trust (Dorset Healthcare Hospital), Epsom and St Helier University Hospitals NHS Trust (Epsom Hospital), Manchester University NHS Foundation Trust (Manchester Royal Infirmary and Trafford Hospital), Medway Community Healthcare (Medway Community Hospital), Medway NHS Foundation Trust (Medway Maritime Hospital), Northumbria Healthcare NHS Foundation Trust (North Tyneside General Hospital and Wansbeck Hospital), Oxford University Hospitals NHS Foundation Trust (Horton Hospital and Nuffield Orthopaedic Centre), Royal United Hospitals Bath NHS Foundation Trust (Bath Royal United Hospital), Southern Health NHS Foundation Trust (Moorgreen Hospital) and The Royal Orthopaedic Hospital NHS Foundation Trust (The Royal Orthopaedic Hospital).
The CORKA trial team
Chief investigator: Professor Karen L Barker.
Co-investigators: Professor David Beard, Professor Gary Collins, Professor Avril Drummond, Professor Sarah Lamb, Professor Andrew Price, Dr Helen Campbell, Dr Francine Toye, Professor Martin Underwood and Dr Ly-Mee Yu.
Trial manager: Nicola Kenealy.
Trial statisticians: Dr Ruth Knight and Susan Dutton.
Health economists: Dr Jose Leal.
Qualitative: Dr Francine Toye.
Site principal investigators and research clinicians
Ashford Hospital: Leon Palmer-Wilson and Ana Glennon.
Countess of Chester Hospital: Helen Wilson.
Dorset County Hospital: Christian Brookes and Denise Hill.
Dorset Healthcare Hospital: Susan Dowdle and Hazel Burt.
Epsom Hospital: Jane Harrison.
Manchester Royal Infirmary: Sarah Adcock and Justine Theaker.
Medway Community Hospital: Gladys Nadar Arulmani.
Medway Maritime Hospital: Sunil Jain.
North Tyneside General Hospital: Mike Reed.
Oxford University Hospitals: Jonathan Room.
Royal United Hospitals Bath: Genevieve Simpson and Gemma Knight.
Moorgreen Hospital: Tricia Monroe.
Royal Orthopaedic Hospital Birmingham: Gareth Stephens and Sarah Rich.
Trial Steering Committee
Professor Anne Forster (Chairperson), Dr Derek Kyte, Dr Mark Kelson, Dr Mindy Cairns, Ms Rachel Dalton, Professor Jenny Butler, Professor Martin Underwood and Professor Avril Drummond.
Data Monitoring Committee
Dr Karen Smith (Chairperson), Professor Ruth Pickering and Dr Toby Smith.
Other acknowledgements
Dr David Smith and Tamsin Hughes for covering maternity leave of trial manager. David Kerr for trial data entry.
Cathy Jenkins, Erin Hannink and Martha Batting from Physiotherapy Research Unit, Nuffield Orthopaedic Centre, Oxford.
Dr Jennifer de Beyer of the Centre for Statistics in Medicine, University of Oxford, Oxford, for English-language editing.
Contributions of authors
Karen L Barker (https://orcid.org/0000-0001-9363-0383) (Professor of Physiotherapy) was the chief investigator, and led the funding application, trial conception and design, development of interventions, supervision, and writing and reviewing of the report.
Jon Room (https://orcid.org/0000-0002-1257-834X) (Research Physiotherapist) was the principal investigator, was involved in the development of the intervention, and writing and reviewing the report.
Ruth Knight (https://orcid.org/0000-0001-6810-2845) (Statistician) was involved in conducting the statistical analysis, and writing and reviewing the report.
Susan J Dutton (https://orcid.org/0000-0003-4573-5257) (Statistician) was involved in designing and conducting the statistical analysis, and writing and reviewing the report.
Fran Toye (https://orcid.org/0000-0002-8144-6519) (Qualitative Researcher) was the qualitative lead and was involved in conducting interviews and analysis, and writing Chapter 6 for the report.
Jose Leal (https://orcid.org/0000-0001-7870-6730) (Health Economist) was involved in designing and conducting the economic analysis, supervision, and writing and reviewing the report.
Seamus Kent (https://orcid.org/0000-0001-7298-3163) (Health Economist) was involved in conducting the economic analysis, and writing and reviewing the report.
Nicola Kenealy (https://orcid.org/0000-0002-3113-0305) (Trial Manager) was involved in trial management and writing and reviewing the report.
Michael M Schussel (https://orcid.org/0000-0002-1711-9310) (Statistician) was involved in screening tool development and writing for the report.
Gary Collins (https://orcid.org/0000-0002-2772-2316) (Co-applicant) supervised screening tool development.
David J Beard (https://orcid.org/0000-0001-7884-6389) (Co-applicant) provided clinical trials expertise and contributed to the report.
Andrew Price (https://orcid.org/0000-0002-4258-5866) (Co-applicant) provided surgical expertise and contributed to the report.
Martin Underwood (https://orcid.org/0000-0002-0309-1708) (Co-applicant) provided primary care expertise and contributed to the report.
Avril Drummond (https://orcid.org/0000-0003-1220-8354) (Co-applicant) provided occupational therapy expertise and contributed to the report.
Elaine Cook (https://orcid.org/0000-0001-5038-6034) (Co-applicant) was the patient and public involvement lead.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Co-applicant) provided clinical trials expertise, was a key member of the trial management group, and was involved in writing and reviewing the report.
Publications
Barker KL, Beard D, Price A, Toye F, Underwood M, Drummond A, et al. COmmunity-based Rehabilitation after Knee Arthroplasty (CORKA): study protocol for a randomised controlled trial. Trials 2016;13:17.
Vadher K, Knight R, Barker KL, Dutton SJ. COmmunity-based Rehabilitation after Knee Arthroplasty (CORKA): statistical analysis plan for a randomised controlled trial. Trials 2018;19:19.
Barker KL, Batting M, Maia Schlussel M, Newman M. The reliability and validity of the Figure of 8 Walk test in older people with knee replacement: does the setting have an impact? Physiotherapy 2019;105:1.
Room J, Batting M, Barker KL. Development of a functional rehabilitation intervention for post knee arthroplasty patients: Community based Rehabilitation post Knee Arthroplasty (CORKA) trial. Physiotherapy 2020;106:52–64.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health Research . Funding Awards. COmmunity Based Rehabilitation After Knee Arthroplasty (CORKA) n.d. www.fundingawards.nihr.ac.uk/award/12/196/08 (accessed 22 April 2020).
- Martin J, Meltzer H, Elliot D. OPCS Surveys of Disability in Great Britain. Report 1. The Prevalence of Disability Among Adults 1988.
- Arthritis V. The State of Musculoskeletal Health 2019 n.d. www.versusarthritis.org/about-arthritis/data-and-statistics/state-of-musculoskeletal-health-2019/ (accessed 22 April 2020).
- National Joint Registry . 15th Annual Report 2018 n.d. www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2015th%20Annual%20R10eport%202018.pdf (accessed 22 April 2020).
- Clement ND, MacDonald D, Howie CR, Biant LC. The outcome of primary total hip and knee arthroplasty in patients aged 80 years or more. J Bone Joint Surg Br 2011;93:1265-70. https://doi.org/10.1302/0301-620X.93B9.25962.
- Brander VA, Malhotra S, Jet J, Heinemann AW, Stulberg SD. Outcome of hip and knee arthroplasty in persons aged 80 years and older. Clin Orthop Relat Res 1997;345:67-78. https://doi.org/10.1097/00003086-199712000-00011.
- Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME. Total joint arthroplasties: current concepts of patient outcomes after surgery. Rheum Dis Clin North Am 2007;33:71-86. https://doi.org/10.1016/j.rdc.2006.12.008.
- Desmeules F, Dionne CE, Belzile ÉL, Bourbonnais R, Champagne F, Frémont P. Determinants of pain, functional limitations and health-related quality of life six months after total knee arthroplasty: results from a prospective cohort study. BMC Sports Sci Med Rehabil 2013;5. https://doi.org/10.1186/2052-1847-5-2.
- Tilbury C, Haanstra TM, Verdegaal SHM, Nelissen RGHH, de Vet HCW, Vliet Vlieland TPM, et al. Patients’ pre-operative general and specific outcome expectations predict postoperative pain and function after total knee and total hip arthroplasties. Scand J Pain 2018;18:457-66. https://doi.org/10.1515/sjpain-2018-0022.
- Cooper NA, Rakel BA, Zimmerman B, Tonelli SM, Herr KA, Clark CR, et al. Predictors of multidimensional functional outcomes after total knee arthroplasty. J Orthop Res 2017;35:2790-8. https://doi.org/10.1002/jor.23596.
- Hawker GA, Badley EM, Borkhoff CM, Croxford R, Davis AM, Dunn S, et al. Which patients are most likely to benefit from total joint arthroplasty?. Arthritis Rheum 2013;65:1243-52. https://doi.org/10.1002/art.37901.
- Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology 2012;51:1804-13. https://doi.org/10.1093/rheumatology/kes075.
- Calkins TE, Culvern C, Nahhas CR, Della Valle CJ, Gerlinger TL, Levine BR, et al. External validity of a new prediction model for patient satisfaction after total knee arthroplasty. J Arthroplasty 2019;34:1677-81. https://doi.org/10.1016/j.arth.2019.04.021.
- Arden N, Altman D, Beard D, Carr A, Clarke N, Collins G, et al. Lower limb arthroplasty: can we produce a tool to predict outcome and failure, and is it cost-effective? An epidemiological study. Programme Grants Appl Res 2017;5. https://doi.org/10.3310/pgfar05120.
- Sanchez-Santos MT, Garriga C, Judge A, Batra RN, Price AJ, Liddle AD, et al. Development and validation of a clinical prediction model for patient-reported pain and function after primary total knee replacement surgery. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-21714-1.
- Bohl DD, Li J, Calkins TE, Darrith B, Edmiston TA, Nam D, et al. Physical therapy on postoperative day zero following total knee arthroplasty: a randomized, controlled trial of 394 patients. J Arthroplasty 2019;34:S173-S177.e1. https://doi.org/10.1016/j.arth.2019.02.010.
- Okamoto T, Ridley RJ, Edmondston SJ, Visser M, Headford J, Yates PJ. Day-of-surgery mobilization reduces the length of stay after elective hip arthroplasty. J Arthroplasty 2016;31:2227-30. https://doi.org/10.1016/j.arth.2016.03.066.
- Minns Lowe CJ, Barker KL, Dewey M, Sackley CM. Effectiveness of physiotherapy exercise after knee arthroplasty for osteoarthritis: systematic review and meta-analysis of randomised controlled trials. BMJ 2007;335. https://doi.org/10.1136/bmj.39311.460093.BE.
- Piva SR, Gil AB, Almeida GJ, DiGioia AM, Levison TJ, Fitzgerald GK. A balance exercise program appears to improve function for patients with total knee arthroplasty: a randomized clinical trial. Phys Ther 2010;90:880-94. https://doi.org/10.2522/ptj.20090150.
- Bruun-Olsen V, Heiberg KE, Wahl AK, Mengshoel AM. The immediate and long-term effects of a walking-skill program compared to usual physiotherapy care in patients who have undergone total knee arthroplasty (TKA): a randomized controlled trial. Disabil Rehabil 2013;35:2008-15. https://doi.org/10.3109/09638288.2013.770084.
- Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil 2004;85:546-56. https://doi.org/10.1016/j.apmr.2003.08.080.
- Woolhead GM, Donovan JL, Dieppe PA. Outcomes of total knee replacement: a qualitative study. Rheumatology 2005;44:1032-7. https://doi.org/10.1093/rheumatology/keh674.
- Street RL, Richardson MN, Cox V, Suarez-Almazor ME. (Mis)understanding in patient-health care provider communication about total knee replacement. Arthritis Rheum 2009;61:100-7. https://doi.org/10.1002/art.24371.
- Naylor J, Harmer A, Fransen M, Crosbie J, Innes L. Status of physiotherapy rehabilitation after total knee replacement in Australia. Physiother Res Int 2006;11:35-47. https://doi.org/10.1002/pri.40.
- DeJong G, Tian W, Smout RJ, Horn SD, Putman K, Smith P, et al. Use of rehabilitation and other health care services by patients with joint replacement after discharge from skilled nursing and inpatient rehabilitation facilities. Arch Phys Med Rehabil 2009;90:1297-305. https://doi.org/10.1016/j.apmr.2008.12.029.
- Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD007099.
- Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb W, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum 2009;61:174-83. https://doi.org/10.1002/art.24167.
- Rajan RA, Pack Y, Jackson H, Gillies C, Asirvatham R. No need for outpatient physiotherapy following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthop Scand 2004;75:71-3. https://doi.org/10.1080/00016470410001708140.
- Piva SR, Schneider MJ, Moore CG, Catelani MB, Gil AB, Klatt BA, et al. Effectiveness of later-stage exercise programs vs usual medical care on physical function and activity after total knee replacement: a randomized clinical trial. JAMA Netw Open 2019;2. https://doi.org/10.1001/jamanetworkopen.2019.0018.
- Artz N, Dixon S, Wylde V, Marques E, Beswick AD, Lenguerrand E, et al. Comparison of group-based outpatient physiotherapy with usual care after total knee replacement: a feasibility study for a randomized controlled trial. Clin Rehabil 2017;31:487-99. https://doi.org/10.1177/0269215516642503.
- Fillingham YA, Darrith B, Lonner JH, Culvern C, Crizer M, Della Valle CJ. Formal physical therapy may not be necessary after unicompartmental knee arthroplasty: a randomized clinical trial. J Arthroplasty 2018;33:S93-S9.e3. https://doi.org/10.1016/j.arth.2018.02.049.
- Florez-García M, García-Pérez F, Curbelo R, Pérez-Porta I, Nishishinya B, Rosario Lozano MP, et al. Efficacy and safety of home-based exercises versus individualized supervised outpatient physical therapy programs after total knee arthroplasty: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2017;25:3340-53. https://doi.org/10.1007/s00167-016-4231-x.
- Han AS, Nairn L, Harmer AR, Crosbie J, March L, Parker D, et al. Early rehabilitation after total knee replacement surgery: a multicenter, noninferiority, randomized clinical trial comparing a home exercise program with usual outpatient care. Arthritis Care Res 2015;67:196-202. https://doi.org/10.1002/acr.22457.
- Barker KL, Beard D, Price A, Toye F, Underwood M, Drummond A, et al. COmmunity-based Rehabilitation after Knee Arthroplasty (CORKA): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1629-1.
- American Heart Association . Classes of Heart Failure 2019. www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure (accessed 22 April 2020).
- Artz N, Dixon S, Wylde V, Beswick A, Blom A, Gooberman-Hill R. Physiotherapy provision following discharge after total hip and total knee replacement: a survey of current practice at high-volume NHS hospitals in England and Wales. Musculoskeletal Care 2013;11:31-8. https://doi.org/10.1002/msc.1027.
- Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci 2002;57:M217-22. https://doi.org/10.1093/gerona/57.4.m217.
- Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007;89:1010-14. https://doi.org/10.1302/0301-620X.89B8.19424.
- Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) – validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-17.
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. https://doi.org/10.1016/0895-4356(93)90053-4.
- EuroQol Group . EuroQol -- a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113-19. https://doi.org/10.1080/02701367.1999.10608028.
- Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther 2007;30:8-15. https://doi.org/10.1519/00139143-200704000-00003.
- Hess RJ, Brach JS, Piva SR, VanSwearingen JM. Walking skill can be assessed in older adults: validity of the Figure-of-8 Walk Test. Phys Ther 2010;90:89-9. https://doi.org/10.2522/ptj.20080121.
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. Hilsdale, MI: Lawrence Erlbaum Associates; 1988.
- Vadher K, Knight R, Barker KL, Dutton SJ. COmmunity-based Rehabilitation after Knee Arthroplasty (CORKA): statistical analysis plan for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-3031-7.
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814-22. https://doi.org/10.1001/jama.2013.879.
- Schlussel M, Collins G, Dutton S, Barker K. Development of the CORKA trial screening tool for identifying patients at increased risk of poor outcome following knee replacement. Trials 2017;18.
- Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol 2010;11:203-9. https://doi.org/10.1007/s10195-010-0115-x.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. https://doi.org/10.1191/0962280205sm403oa.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. https://doi.org/10.1177/096228029900800102.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- O’Cathain A, Croot L, Sworn K, Duncan E, Rousseau N, Turner K, et al. Taxonomy of approaches to developing interventions to improve health: a systematic methods overview. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0425-6.
- Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40:559-67. https://doi.org/10.2519/jospt.2010.3317.
- Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther 2011;41:932-41. https://doi.org/10.2519/jospt.2011.3734.
- Bade MJ, Stevens-Lapsley JE. Restoration of physical function in patients following total knee arthroplasty: an update on rehabilitation practices. Curr Opin Rheumatol 2012;24:208-14. https://doi.org/10.1097/BOR.0b013e32834ff26d.
- Bhave A, Mont M, Tennis S, Nickey M, Starr R, Etienne G. Functional problems and treatment solutions after total hip and knee joint arthroplasty. J Bone Joint Surg Am 2005;87:9-21. https://doi.org/10.2106/00004623-200511002-00002.
- Coulter CL, Weber JM, Scarvell JM. Group physiotherapy provides similar outcomes for participants after joint replacement surgery as 1-to-1 physiotherapy: a sequential cohort study. Arch Phys Med Rehabil 2009;90:1727-33. https://doi.org/10.1016/j.apmr.2009.04.019.
- Fitzsimmons SE, Vazquez EA, Bronson MJ. How to treat the stiff total knee arthroplasty? A systematic review. Clin Orthop Relat Res 2010;468:1096-106. https://doi.org/10.1007/s11999-010-1230-y.
- Harmer AR, Naylor JM, Crosbie J, Russell T. Land-based versus water-based rehabilitation following total knee replacement: a randomized, single-blind trial. Arthritis Rheum 2009;61:184-91. https://doi.org/10.1002/art.24420.
- Heiberg K, Bruun-Olsen V, Mengshoel AM. Pain and recovery of physical functioning nine months after total knee arthroplasty. J Rehabil Med 2010;42:614-19. https://doi.org/10.2340/16501977-0568.
- Kearns RJ, O’Connor DP, Brinker MR. Management of falls after total knee arthroplasty. Orthopedics 2008;31. https://doi.org/10.3928/01477447-20080301-20.
- Knoop J, Steultjens MPM, van der Leeden M, van der Esch M, Thorstensson CA, Roorda LD, et al. Proprioception in knee osteoarthritis: a narrative review. Osteoarthr Cartil 2011;19:381-8. https://doi.org/10.1016/j.joca.2011.01.003.
- Lee DK, Kim GM, Ha SM, Oh JS. Correlation of the y-balance test with lower-limb strength of adult women. J Phys Ther Sci 2014;26:641-3. https://doi.org/10.1589/jpts.26.641.
- Liao CD, Liou TH, Huang YY, Huang YC. Effects of balance training on functional outcome after total knee replacement in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2013;27:697-709. https://doi.org/10.1177/0269215513476722.
- Mandeville D, Osternig LR, Chou LS. The effect of total knee replacement surgery on gait stability. Gait Posture 2008;27:103-9. https://doi.org/10.1016/j.gaitpost.2007.02.009.
- McClelland JA, Webster KE, Feller JA. Gait analysis of patients following total knee replacement: a systematic review. Knee 2007;14:253-63. https://doi.org/10.1016/j.knee.2007.04.003.
- Meier W, Mizner RL, Marcus RL, Dibble LE, Peters C, Lastayo PC. Total knee arthroplasty: muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther 2008;38:246-56. https://doi.org/10.2519/jospt.2008.2715.
- Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 2005;35:424-36. https://doi.org/10.2519/jospt.2005.35.7.424.
- Naylor JM, Ko V. Heart rate response and factors affecting exercise performance during home- or class-based rehabilitation for knee replacement recipients: lessons for clinical practice. J Eval Clin Pract 2012;18:449-58. https://doi.org/10.1111/j.1365-2753.2010.01596.x.
- Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function?. Clin Orthop Relat Res 2005;431:157-65. https://doi.org/10.1097/01.blo.0000150130.03519.fb.
- Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng 2010;12:401-33. https://doi.org/10.1146/annurev-bioeng-070909-105259.
- Philbin EF, Groff GD, Ries MD, Miller TE. Cardiovascular fitness and health in patients with end-stage osteoarthritis. Arthritis Rheum 1995;38:799-805. https://doi.org/10.1002/art.1780380613.
- Piva SR, Teixeira PE, Almeida GJ, Gil AB, DiGioia AM, Levison TJ, et al. Contribution of hip abductor strength to physical function in patients with total knee arthroplasty. Phys Ther 2011;91:225-33. https://doi.org/10.2522/ptj.20100122.
- Pozzi F, Snyder-Mackler L, Zeni J. Physical exercise after knee arthroplasty: a systematic review of controlled trials. Eur J Phys Rehabil Med 2013;49:877-92.
- Ries MD, Philbin EF, Groff GD, Sheesley KA, Richman JA, Lynch F. Improvement in cardiovascular fitness after total knee arthroplasty. J Bone Joint Surg Am 1996;78:1696-701. https://doi.org/10.2106/00004623-199611000-00009.
- Rowe PJ, Myles CM, Walker C, Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life?. Gait Posture 2000;12:143-55. https://doi.org/10.1016/S0966-6362(00)00060-6.
- Stratford PW, Kennedy DM, Robarts SF. Modelling knee range of motion post arthroplasty: clinical applications. Physiother Can 2010;62:378-87. https://doi.org/10.3138/physio.62.4.378.
- Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res 2003;21:775-9. https://doi.org/10.1016/S0736-0266(03)00052-4.
- Su EP, Su SL, Della Valle AG. Stiffness after TKR: how to avoid repeat surgery. Orthopedics 2010;33.
- Turcot K, Sagawa Y, Fritschy D, Hoffmeyer P, Suvà D, Armand S. How gait and clinical outcomes contribute to patients’ satisfaction three months following a total knee arthroplasty. J Arthroplasty 2013;28:1297-300. https://doi.org/10.1016/j.arth.2013.01.031.
- Walsh M, Woodhouse LJ, Thomas SG, Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther 1998;78:248-58. https://doi.org/10.1093/ptj/78.3.248.
- Wiik AV, Manning V, Strachan RK, Amis AA, Cobb JP. Unicompartmental knee arthroplasty enables near normal gait at higher speeds, unlike total knee arthroplasty. J Arthroplasty 2013;28:176-8. https://doi.org/10.1016/j.arth.2013.07.036.
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1094-105. https://doi.org/10.1161/CIRCULATIONAHA.107.185650.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. https://doi.org/10.1249/MSS.0b013e318213fefb.
- Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review?. Med Care 1996;34:73-84. https://doi.org/10.1097/00005650-199601000-00006.
- MedDRA Maintenance and Support Services Organization . Introductory Guide to MedDRA Version 14.0 2011. https://www.who.int/medical_devices/innovation/MedDRAintroguide_version14_0_March2011.pdf.
- Department of Health and Social Care . NHS Reference Costs 2017 to 2018 2018. https://improvement.nhs.uk/resources/reference-costs/#archive (accessed 22 April 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- NHS Business Services Authority . Prescription Cost Analysis (PCA) Data 2018. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data (accessed 22 April 2020).
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. National Institute for Health Research School for Primary Care Research . Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30. https://doi.org/10.1016/S0140-6736(16)00620-6.
- Department of Health: Leeds, UK . The Information Centre, 2006 07 UK General Practice Workload Survey, Primary Care Statistics 2007 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/gp-earnings-and-expenses-estimates/gp-workload-survey-results (accessed 22 April 2020).
- GOV.UK . National Minimum Wage and National Living Wage Rates 2019. www.gov.uk/national-minimum-wage-rates (accessed 22 April 2020).
- Office for National Statistics . Annual Survey of Hours and Earnings Time Series of Selected Estimates 2019. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/ashe1997to2015selectedestimates (accessed 22 April 2020).
- Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al. Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9390.
- National Institute for Health and Care Excellence (NICE) . Falls in Older People: Assessing Risk and Prevention. Clinical Guideline CG161 2013. www.nice.org.uk/guidance/CG161 (accessed 22 April 2020).
- Co-operative Mobility n.d. www.co-opmobility.co.uk/ (accessed 1 November 2019).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 1 November 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55-63. https://doi.org/10.7326/M14-0697.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. https://doi.org/10.7326/M14-0698.
- Johnston L, MacLennan G, McCormack K, Ramsay C, Walker A. KAT Trial Group . The Knee Arthroplasty Trial (KAT) design features, baseline characteristics, and two-year functional outcomes after alternative approaches to knee replacement. J Bone Joint Surg Am 2009;91:134-41. https://doi.org/10.2106/JBJS.G.01074.
- Murray DW, MacLennan GS, Breeman S, Dakin HA, Johnston L, Campbell MK, et al. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of different knee prostheses: the Knee Arthroplasty Trial (KAT). Health Technol Assess 2014;18. https://doi.org/10.3310/hta18190.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- White IR, Daniel R, Royston P. Avoiding bias due to perfect prediction in multiple imputation of incomplete categorical variables. Comput Stat Data Anal 2010;54:2267-75. https://doi.org/10.1016/j.csda.2010.04.005.
- Albert A, Anderson JA. On the existence of maximum likelihood estimates in logistic regression models. Biometrika 1984;71:1-10. https://doi.org/10.1093/biomet/71.1.1.
- Harrell FE, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med 1984;3:143-52. https://doi.org/10.1002/sim.4780030207.
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. https://doi.org/10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. https://doi.org/10.1016/S0895-4356(96)00236-3.
- Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001744.
- Pavlou M, Ambler G, Seaman SR, Guttmann O, Elliott P, King M, et al. How to develop a more accurate risk prediction model when there are few events. BMJ 2015;351. https://doi.org/10.1136/bmj.h3868.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-18. https://doi.org/10.1093/aje/kwk052.
- Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 2016;76:175-82. https://doi.org/10.1016/j.jclinepi.2016.02.031.
- Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54:774-81. https://doi.org/10.1016/S0895-4356(01)00341-9.
- Pajouheshnia R, Groenwold RHH, Peelen LM, Reitsma JB, Moons KGM. When and how to use data from randomised trials to develop or validate prognostic models. BMJ 2019;365. https://doi.org/10.1136/bmj.l2154.
- Price AJ, Alvand A, Troelsen A, Katz JN, Hooper G, Gray A, et al. Knee replacement. Lancet 2018;392:1672-82. https://doi.org/10.1016/S0140-6736(18)32344-4.
- Hamilton DF, Lane JV, Gaston P, Patton JT, Macdonald D, Simpson AH, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002525.
- Curry AL, Goehring MT, Bell J, Jette DU. Effect of physical therapy interventions in the acute care setting on function, activity, and participation after total knee arthroplasty: a systematic review. J Acute Care Phys Ther 2018;9:93-106. https://doi.org/10.1097/JAT.0000000000000079.
- National Institute for Health and Care Excellence (NICE) . Joint Replacement (Primary): Hip, Knee and Shoulder 2020.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am 1989;71:145-50.
- Campi S, Tibrewal S, Cuthbert R, Tibrewal SB. Unicompartmental knee replacement - Current perspectives. J Clin Orthop Trauma 2018;9:17-23. https://doi.org/10.1016/j.jcot.2017.11.013.
- Beard DJ, Davies LJ, Cook JA, MacLennan G, Price A, Kent S, et al. The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5-year outcomes of a randomised controlled trial. Lancet 2019;394:746-56. https://doi.org/10.1016/S0140-6736(19)31281-4.
- Jenkins C, Jackson W, Bottomley N, Price A, Murray D, Barker K. Introduction of an innovative day surgery pathway for unicompartmental knee replacement: no need for early knee flexion. Physiotherapy 2019;105:46-52. https://doi.org/10.1016/j.physio.2018.11.305.
- Minns Lowe CJ, Wilson MS, Sackley CM, Barker KL. Blind outcome assessment: the development and use of procedures to maintain and describe blinding in a pragmatic physiotherapy rehabilitation trial. Clin Rehabil 2011;25:264-74. https://doi.org/10.1177/0269215510380824.
- Barker KL, Newman M, Stallard N, Leal J, Minns Lowe C, Javaid MK, et al. Exercise or manual physiotherapy compared with a single session of physiotherapy for osteoporotic vertebral fracture: three-arm PROVE RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23440.
- Boutron I, Estellat C, Ravaud P. A review of blinding in randomized controlled trials found results inconsistent and questionable. J Clin Epidemiol 2005;58:1220-6. https://doi.org/10.1016/j.jclinepi.2005.04.006.
- Hamilton DF, Loth FC, MacDonald DJ, MacFarlane GJ, Beard DJ, Simpson AHR, et al. Exploring variation in patient access of post-discharge physiotherapy following total hip and knee arthroplasty under a choice based system in the UK: an observational cohort study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-021614.
- Khan F, Ng L, Gonzalez S, Hale T, Turner-Stokes L. Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD004957.pub3.
- Artz N, Elvers KT, Lowe CM, Sackley C, Jepson P, Beswick AD. Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0469-6.
- Roos EM. Effectiveness and practice variation of rehabilitation after joint replacement. Curr Opin Rheumatol 2003;15:160-2. https://doi.org/10.1097/00002281-200303000-00014.
- Madsen M, Larsen K, Madsen IK, Søe H, Hansen TB. Late group-based rehabilitation has no advantages compared with supervised home-exercises after total knee arthroplasty. Dan Med J 2013;60.
- World Health Organization (WHO) . How to Use the ICF: A Practical Manual for Using the International Classification of Functioning, Disability and Health (ICF) 2013.
- Wylde V, Livesey C, Blom AW. Restriction in participation in leisure activities after joint replacement: an exploratory study. Age Ageing 2012;41:246-9. https://doi.org/10.1093/ageing/afr180.
- Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci 2002;57:M209-16. https://doi.org/10.1093/gerona/57.4.m209.
- Davis AM, Perruccio AV, Ibrahim S, Hogg-Johnson S, Wong R, Streiner DL, et al. The trajectory of recovery and the inter-relationships of symptoms, activity and participation in the first year following total hip and knee replacement. Osteoarthr Cartil 2011;19:1413-21. https://doi.org/10.1016/j.joca.2011.08.007.
- Maxwell JL, Keysor JJ, Niu J, Singh JA, Wise BL, Frey-Law L, et al. Participation following knee replacement: the MOST cohort study. Phys Ther 2013;93:1467-74. https://doi.org/10.2522/ptj.20130109.
- Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med 2016;46:269-92. https://doi.org/10.1007/s40279-015-0421-9.
- Barker KL, Hannink E, Pemberton S, Jenkins C. Knee arthroplasty patients predicted versus actual recovery: what are their expectations about time of recovery after surgery and how long before they can do the tasks they want to do?. Arch Phys Med Rehabil 2018;99:2230-7. https://doi.org/10.1016/j.apmr.2018.03.022.
- Cobb LK, Godino JG, Selvin E, Kucharska-Newton A, Coresh J, Koton S. Spousal influence on physical activity in middle-aged and older adults: the ARIC study. Am J Epidemiol 2016;183:444-51. https://doi.org/10.1093/aje/kwv104.
- Sarigiovannis P, Cropper S. An audit of the utilization of physiotherapy assistants in the musculoskeletal outpatients setting within a primary care physiotherapy service. Musculoskeletal Care 2018;16:405-8. https://doi.org/10.1002/msc.1238.
- Shubert TE, Smith ML, Goto L, Jiang L, Ory MG. Otago exercise program in the United States: comparison of 2 implementation models. Phys Ther 2017;97:187-97. https://doi.org/10.2522/ptj.20160236.
- Charted Society of Physiotherapy . Supervision, Accountability &Amp; Delegation. 2017. www.csp.org.uk/system/files/supervision_accountability_delegation_final.pdf (accessed 22 April 2020).
- Boyle JM, McCartney E, O’Hare A, Forbes J. Direct versus indirect and individual versus group modes of language therapy for children with primary language impairment: principal outcomes from a randomized controlled trial and economic evaluation. Int J Lang Commun Disord 2009;44:826-46. https://doi.org/10.1080/13682820802371848.
- Schwarz M, Ward EC, Cornwell P, Coccetti A, Kalapac N. Evaluating the feasibility and validity of using trained allied health assistants to assist in mealtime monitoring of dysphagic patients. Dysphagia 2019;34:350-9. https://doi.org/10.1007/s00455-018-9947-y.
- Moran A, Nancarrow SA, Enderby P. Mechanisms to enhance the effectiveness of allied health and social care assistants in community-based rehabilitation services: a qualitative study. Health Soc Care Community 2015;23:389-98. https://doi.org/10.1111/hsc.12158.
- Smith TO, Latham S, Maskrey V, Blyth A. Patients’ perceptions of physical activity before and after joint replacement: a systematic review with meta-ethnographic analysis. Postgrad Med J 2015;91:483-91. https://doi.org/10.1136/postgradmedj-2015-133507.
- Beauchamp MK, Schmidt CT, Pedersen MM, Bean JF, Jette AM. Psychometric properties of the Late-Life Function and Disability Instrument: a systematic review. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-12.
- Beauchamp MK, Jette AM, Ward RE, Kurlinski LA, Kiely D, Latham NK, et al. Predictive validity and responsiveness of patient-reported and performance-based measures of function in the Boston RISE study. J Gerontol A Biol Sci Med Sci 2015;70:616-22. https://doi.org/10.1093/gerona/glu227.
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556-61. https://doi.org/10.1056/NEJM199503023320902.
- Beauchamp MK, Ward RE, Jette AM, Bean JF. Meaningful change estimates for the late-life function and disability instrument in older adults. J Gerontol A Biol Sci Med Sci 2019;74:556-9. https://doi.org/10.1093/gerona/gly230.
- NHS England . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 7 November 2020).
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU, University of Kent; 2009.
- NHS Digital . GP Workload Survey, Primary Care Statistics 2007.
Appendix 1 Late Life Function and Disability Instrument effect by screen score
For patients with a score of 5 or more, continue to screen for eligibility onto the study. For patients with a score below 5, stop the screening process and record this in the screening log.

Appendix 2 Health economics unit costs
| Treatment type | Unit cost (UK 2018) | Source/details |
|---|---|---|
| Usual care | ||
| Clinic, one to one | £55.90 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, service code 650 |
| Clinic, group | ||
| Home, one to one | £57.26 | NHS Reference Cost schedule 2017–18,90 community health services, currency code A08A1 |
| Community, one to one | ||
| Community, group | £48.48 | NHS Reference Cost schedule 2017–18,90 community health services, currency code A08AG |
| CORKA programme | £35.31 | Weighted average of costs for physiotherapist at home (£57.26; see above) and physiotherapy assistants (£26.53) with a ratio of 2 : 5. Physiotherapy assistant cost derived by applying NHS Agenda for Change grade 3 salary (£18,813) to band 4 community staff costs from section 9 of PSSRU 2018.91 Assume 1 hour per patient including travel time |
| Resource use type | Unit cost (UK 2018) | Source/details |
|---|---|---|
| GP: surgerya | £34.30 | Cost per minute of GP time: PSSRU 201891 (see table 10.3b) |
| GP: homea | £76.39 | Cost per minute of GP time: PSSRU 201891 (see table 10.3b). Average consultation length of 9.2 minutes;93 assume average 12 minutes travel time for home visits: PSSRU 2009157 |
| GP: telephonea | £15.10 | Cost per minute of GP time: PSSRU 201891 (see table 10.5) |
| Practice nurse: surgery | £14.90 | PSSRU 2018,91 table 10.6 (practice nurse). Mean hourly cost is £56 for face-to-face contact (£57.70 at current prices). Average consultation is 15.5 minutes: 2006–7 GP workload survey158 |
| District nurse: home | £38.45 | NHS Reference Cost schedule 2017–18,90 tab CHS, currency code N02AF |
| Physiotherapist: clinic | £55.90 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, service code 650 |
| Physiotherapist: home | £57.26 | NHS Reference Cost schedule 2017–18,90 tab CHS, currency code A08A1 |
| Occupational therapist: clinic | £73.25 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, service code 651 |
| Occupational therapist: home | £81.31 | NHS Reference Cost schedule 2017–18,90 tab CHS, currency code A06A1 |
| A&E | £160.32 | NHS Reference Cost schedule 2017–18,90 tab AE, weighted average of all service codes |
| Fracture clinic: hospital | £123.94 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, service code 110 (trauma and orthopaedics) |
| Outpatient clinic | £125.01 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, weighted average of all service codes |
| Outpatient, not face to face | £90.83 | NHS Reference Cost schedule 2017–18,90 tab total consultant led, non face-to-face, follow-up outpatient attendances, weighted average of all service codes |
| Counsellor/psychologist | £170.27 | NHS Reference Cost schedule 2017–18,90 tab total outpatient attendances, service code 656 (clinical psychology) |
| Hydrotherapy | £29.93 | Cost per session in UK 2000 prices is £17.19.97 Inflated to 2018 prices using HCHS index from PSSRU 201891 |
| Social services | £84.00 | PSSRU 2018,91 table 11.1 (social care worker, adult services). Use cost per hour of client-related work. |
| Falls prevention programme | £21.81 | NICE CG1613,98 appendix K. Unit cost per non-acute admitted patient |
| Acupuncture | £139.44 | NHS Reference Cost schedule 2017–18,90 tab OPROC, service code 191 (pain management), currency code AB23Z |
| Chiropractor | £55.90 | Assumed same as NHS physiotherapy clinic |
| Osteopath | £55.90 | Assumed same as NHS physiotherapy clinic |
| Private complimentary therapies | £55.90 | Assumed same as NHS physiotherapy clinic |
| Pharmacy | £6.75 | Cost per hour: PSSRU 2018,91 table 9 (band 66). Assume mean consultation same as for GP, i.e. 9.2 minutes93 |
| X-ray | £31.49 | NHS Reference Cost schedule 2017–18,90 directly accessed diagnostic services, direct access plain film (currency code DAPF) |
| Ultrasound | £51.38 | NHS Reference Cost schedule 2017–18,90 diagnostic imaging, direct access; weighted average cost of currency code RD4 |
| CAT scan | £88.87 | NHS Reference Cost schedule 2017–18,90 diagnostic imaging, direct access; weighted average cost of currency code RD2 |
| MRI scan | £132.81 | NHS Reference Cost schedule 2017–18,90 diagnostic imaging, direct access; weighted average cost of currency code RD0 |
| Echocardiogram | £76.65 | NHS Reference Cost schedule 2017–18,90 directly accessed diagnostic services, currency code EY50Z |
| Day surgery | £139.59 | NHS Reference Cost schedule 2017–18,90 weighted average of all outpatient procedures |
| Cost per bed-day | £345.76 | NHS Reference Cost schedule 2017–18,90 weighted average of all excess bed-day costs |
| Cost per day in critical care | £1049.23 | NHS Reference Cost schedule 2017–18,90 tab CC, weighted average of service code CCU03 |
| British National Formulary chemical name | Cost per item |
|---|---|
| Paracetamol | £1.77 |
| Codeine phosphate | £2.74 |
| Ibuprofen | £2.82 |
| Co-codamol (codeine phosphate/paracetamol) | £3.60 |
| Morphine sulfate | £5.19 |
| Tramadol hydrochloride | £2.47 |
| Oxycodone hydrochloride | £13.79 |
| Dihydrocodeine tartrate | £2.85 |
| Naproxen | £3.29 |
| Dalteparin sodiuma | £52.34 |
| Sennab | £1.25 |
| Enoxaparina | £56.33 |
| Gabapentina | £6.63 |
| Omeprazolea | £0.99 |
| Tinzaparin sodiuma | £73.48 |
| Docusate sodiumb | £4.32 |
| Lactuloseb | £2.57 |
| Lansoprazolea | £1.10 |
| Flucloxacillin sodium | £2.06 |
| Macrogol 3350b | £5.71 |
| Pregabalina | £4.24 |
| Cyclizine hydrochloride | £3.62 |
| Diclofenac sodium | £5.67 |
| Co-dydramol (dihydrocodeine/paracetamol) | £2.51 |
| Amitriptyline hydrochloridea | £1.86 |
| Rivaroxabana | £52.40 |
| Furosemidea | £1.01 |
| Nefopam hydrochloride | £15.01 |
| Aspirin | £0.51 |
| Ramiprila | £1.17 |
| Clarithromycin | £2.10 |
| Arnica montana | £6.38 |
| Other health supplement preps | £5.97 |
| Apixabana | £51.80 |
| Amoxicillin | £0.91 |
| Levothyroxine sodiuma | £1.39 |
| Bisacodylb | £3.75 |
| Amlodipinea | £1.35 |
| Rifampicin | £76.34 |
| Ciprofloxacin | £1.59 |
| Bendroflumethiazidea | £0.54 |
| Atorvastatina | £1.00 |
| Quinine sulfatea | £1.47 |
| Other toiletry preps | £8.80 |
| Zopiclone | £0.53 |
| Meloxicam | £1.07 |
| Cetirizine hydrochloride | £0.86 |
| Insulin asparta | £43.00 |
| Buprenorphinea | £9.95 |
| Phenoxymethylpenicillin (penicillin V) | £1.99 |
| Doxazosin mesilatea | £1.60 |
| Celecoxib | £1.90 |
| Tamsulosin hydrochloridea | £4.17 |
| Prednisolone | £1.03 |
| Ferrous sulfatea | £2.12 |
| Co-amoxiclav (amoxicillin/clavulanic acid) | £2.16 |
| Atenolola | £0.65 |
| Calcium carbonatea | £4.94 |
| Pravastatin sodiuma | £1.24 |
| Heparin flushesa | £21.73 |
| Prochlorperazine maleate | £1.11 |
| Potassium chloride | £7.03 |
| Loperamide hydrochloride | £1.99 |
| Diazepam | £0.49 |
| Glycerolb | £2.18 |
| Clindamycin hydrochloride | £10.02 |
| Colecalciferola | £3.40 |
| Ranitidine hydrochloridea | £0.81 |
| Finasteridea | £1.08 |
| Oxybutynina | £1.92 |
| Cod liver oil | £0.68 |
| Ferrous fumaratea | £2.41 |
| Ketoprofen | £2.54 |
| Indapamidea | £1.05 |
| Perindopril erbuminea | £5.55 |
| Ondansetron hydrochloride | £12.72 |
| Solifenacina | £28.30 |
| Promethazine teoclate | £3.42 |
| Magnesium hydroxide | £9.69 |
| Other emollient preparationsa | £6.87 |
| Paracetamol and ibuprofen | £14.38 |
| Doxycycline hyclate | £1.14 |
| Betamethasone valerate | £4.58 |
| Spironolactonea | £0.93 |
| Dorzolamidea | £2.66 |
| Fentanyla | £18.78 |
| Heparinoid | £8.61 |
| Alginic acid compound preparations | £5.97 |
| Clotrimazole | £1.30 |
| Nystatin | £2.07 |
| Lacidipine | £4.40 |
| Metformin hydrochloride | £2.86 |
| Lithium carbonate | £1.52 |
| Mirtazapine | £1.90 |
| Other tincture preps | £9.36 |
| Warfarin sodium | £1.03 |
| Nicorandil | £2.43 |
| Simvastatin | £0.89 |
| Haloperidol | £16.94 |
| Type of equipment | Unit cost (UK 2018) |
|---|---|
| Crutches | £10.99 |
| Walking stick | £4.99 |
| Toilet seat | £23.99 |
| Shoe horn | £3.49 |
| Toilet frame | £99.99 |
| Zimmer frame | £22.99 |
| Grab sticks | £4.49 |
| Stools | £35.99 |
| Chairs | £35.99 |
| Bathroom shower seat | £18.99 |
| Food trolley | £38.99 |
| Leg lifter | £4.49 |
| Bath seat | £16.99 |
| Commode | £23.99 |
| Ice pack | £1.89 |
| Cushion | £28.99 |
| Bath bench | £58.49 |
| Stair lift | £2046.00 |
| Wheelchair self-powered | £99.99 |
| Rails | £9.99 |
| Brace | £1.99 |
| Knee support | £15.99 |
| Step | £44.16 |
| Stockings | £16.99 |
| Walker (4 wheels) | £53.99 |
| Weights | £44.16 |
| Bath rail | £44.99 |
| Bed frame | £4.49 |
| Bottles | £25.99 |
| Leg cover | £4.59 |
| Sock aids | £1.89 |
| Shopping trolley | £40.99 |
| Walker (3 wheels) | £62.99 |
| New bath or shower | £5078.00 |
| Bath mat | £18.99 |
| Bed cradle | £8.99 |
| Bed pan | £7.49 |
| Bed tray | £23.99 |
| Car seat | £6.99 |
| Heat wrap | £6.49 |
| Portable toilet | £23.99 |
| Electric wheelchair | £1350.00 |
| Type of equipment | Unit cost (UK 2018) | Source/details |
|---|---|---|
| Cost per hour of informal care | £7.93 | UK national minimum wage 2 |
| Cost per week away from paid employment | £556.43 | The mean weekly gross wage of £16.76 multiplied by the average number of hours worked per week, i.e. 33.2 hours95 |
Appendix 3 Health economics resource use
| Treatment received | Mean number of contacts |
|---|---|
| Usual care (n = 312) | 4.46 |
| Clinic, one to one | 2.92 |
| Clinic, group | 0.88 |
| Home, one to one | 0.60 |
| Community, one to one | 0.05 |
| Community, group | 0.02 |
| CORKA intervention (n = 309) | 5.21 |
| Baseline to 6 months | 6 months to 12 months | |||
|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| Total, n | 264 | 263 | 273 | 273 |
| NHS health care | ||||
| GP | 1.35 | 1.42 | 0.38 | 0.46 |
| Nurse | 1.42 | 1.65 | 0.15 | 0.19 |
| Physiotherapist | 1.56 | 0.81 | 0.27 | 0.36 |
| Outpatient | 0.80 | 0.93 | 0.29 | 0.27 |
| A&E | 0.14 | 0.16 | 0.02 | 0.05 |
| Other | 0.74 | 0.65 | 0.20 | 0.17 |
| Medications | 2.42 | 2.22 | 0.90 | 0.85 |
| Equipment | 2.02 | 2.05 | 0.31 | 0.32 |
| Private health care | ||||
| Physiotherapist | 0.25 | 0.33 | 0.10 | 0.12 |
| Other | 0.13 | 0.04 | 0.05 | 0.07 |
| Medications | 0.25 | 0.24 | 0.13 | 0.10 |
| Equipment | 0.32 | 0.41 | 0.10 | 0.13 |
| Baseline to 6 months | 6 months to 12 months | |||
|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| Total, n | 264 | 263 | 273 | 273 |
| Number of hospital admissions | 0.11 | 0.10 | 0.08 | 0.11 |
| Days in hospital (excluding critical care) | 0.46 | 0.57 | 0.24 | 0.42 |
| Days in critical care | 0.02 | 0.01 | 0.00 | 0.01 |
| Baseline to 6 months | 6 months to 12 months | |||
|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| Informal care | ||||
| Any care (%) | 50.4 | 44.1 | 4.0 | 7.0 |
| Hours of care | 54.7 | 44.3 | 2.6 | 7.1 |
| Time away from paid employment | ||||
| Any time (%) | 17.8 | 12.9 | 2.2 | 1.8 |
| Weeks off | 1.8 | 1.1 | 0.2 | 0.2 |
| Baseline (%) | 6 months (%) | 12 months (%) | ||||
|---|---|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| Total, n | 308 | 308 | 281 | 275 | 286 | 278 |
| Mobility | ||||||
| No problems | 5 | 4 | 38 | 41 | 44 | 47 |
| Slight problems | 18 | 18 | 29 | 29 | 24 | 26 |
| Some problems | 52 | 51 | 21 | 15 | 19 | 11 |
| Severe problems | 25 | 27 | 3 | 4 | 4 | 6 |
| Extreme problems | 0 | 0 | 0 | 0 | 0 | 1 |
| Self-care | ||||||
| No problems | 54 | 58 | 73 | 72 | 71 | 74 |
| Slight problems | 31 | 27 | 12 | 13 | 14 | 11 |
| Some problems | 14 | 11 | 5 | 2 | 6 | 5 |
| Severe problems | 1 | 3 | 1 | 2 | 0 | 1 |
| Extreme problems | 0 | 0 | 0 | 0 | 0 | 0 |
| Usual activities | ||||||
| No problems | 15 | 17 | 49 | 50 | 50 | 56 |
| Slight problems | 30 | 31 | 24 | 26 | 25 | 21 |
| Some problems | 38 | 34 | 13 | 11 | 13 | 9 |
| Severe problems | 15 | 15 | 3 | 2 | 3 | 2 |
| Extreme problems | 2 | 3 | 0 | 0 | 1 | 1 |
| Pain | ||||||
| No problems | 1 | 1 | 24 | 23 | 31 | 32 |
| Slight problems | 11 | 11 | 42 | 45 | 38 | 40 |
| Some problems | 54 | 53 | 20 | 16 | 18 | 12 |
| Severe problems | 28 | 28 | 4 | 4 | 5 | 4 |
| Extreme problems | 7 | 6 | 1 | 1 | 0 | 2 |
| Anxiety/depression | ||||||
| No problems | 62 | 63 | 66 | 65 | 67 | 67 |
| Slight problems | 25 | 20 | 15 | 18 | 16 | 13 |
| Some problems | 9 | 13 | 7 | 4 | 6 | 9 |
| Severe problems | 3 | 2 | 1 | 1 | 1 | 1 |
| Extreme problems | 1 | 3 | 1 | 0 | 1 | 0 |
| Baseline, mean (SD) | 6 months, mean (SD) | 12 months, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | Usual care | Home-based rehabilitation | |
| EQ-5D-5L utility | 0.520 (0.220) | 0.518 (0.228) | 0.750 (0.436) | 0.754 (0.441) | 0.765 (0.445) | 0.767 (0.475) |
| Total NHS costs (£) | – | – | 794 (32) | 805 (44) | 221 (26) | 284 (33) |
| Intervention costsa | – | – | 250 (14) | 184 (8) | 0 (0) | 0 (0) |
| Total subsequent NHS costs | – | – | 544 (31) | 621 (44) | 221 (26) | 284 (33) |
| Total private health care (£) | – | – | 88 (21) | 57 (15) | 15 (7) | 31 (13) |
| Informal care (£) | – | – | 414 (40) | 352 (34) | 27 (13) | 61 (19) |
| Time off work (£) | – | – | 1015 (84) | 643 (63) | 90 (31) | 101 (31) |
| Total societal costs (£) | – | – | 2312 (94) | 1856 (75) | 353 (37) | 476 (42) |
List of abbreviations
- 30SCST
- 30-Second Chair Stand Test
- ASA
- American Society of Anesthesiologists
- AUROC
- area under receiver operating characteristic
- BMI
- body mass index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CORKA
- COmmunity based Rehabilitation after Knee Arthroplasty
- CRF
- case report form
- EPV
- events per variable
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- F8WT
- Figure-of-8 Walk Test
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- KA
- knee arthroplasty
- KAT
- Knee Arthroplasty Trial
- KOOS
- Knee injury and Osteoarthritis Outcome Score
- LLFDI
- Late Life Function and Disability Instrument
- NICE
- National Institute for Health and Care Excellence
- OKS
- Oxford Knee Score
- PASE
- Physical Activity Scale for the Elderly
- PP
- per protocol
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SLS
- Single Leg Stance
- TKA
- total knee arthroplasty
- UKA
- unicompartmental Knee Arthroplasty
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index
