Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/12/04. The contractual start date was in April 2017. The draft report began editorial review in March 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Medina-Lara et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background and rationale
The rate of cancer survival in the UK is lower than the European average for most cancers: for example, the 5-year survival rate for stomach cancer is 17.2% in the UK, compared with the European average of 25.1%; for colon cancer, it is 51.8% in the UK, compared with the European average of 57%. 1 Efforts to reduce the time to making a cancer diagnosis have the potential to improve prognosis,2 because earlier diagnosis is associated with earlier cancer stage at diagnosis,3 and earlier treatment is associated with improved survival. 4 There is also the potential to reduce presentation via emergency admissions, and to prevent the poorer survival associated with that route of diagnosis. 5 National cancer screening programmes in the NHS (for breast, bowel and cervical cancer) and the National Awareness and Early Diagnosis Initiative (NAEDI) (to increase public awareness of the signs and symptoms of cancer4) are intended to improve early diagnosis. As many individuals go through primary care as a route for diagnosis,5 efforts there could improve cancer survival.
Cancer diagnosis in primary care is not straightforward. Symptoms of cancer are commonly seen, but mostly have non-cancer origins. 6 Of those individuals referred from primary care via the 2-week-wait (2WW) referrals for suspected head and neck cancer, approximately 9% were ultimately diagnosed with cancer. 7 The type and presence of symptoms can vary greatly,8 and it is not surprising that patients can have multiple general practitioner (GP) consultations before being referred, especially for those cancers that have less well-known signs and symptoms. 9 Thus, tools to help improve cancer diagnosis in primary care have great potential to affect diagnoses and subsequent treatment options, leading to better outcomes for patients.
Diagnostic prediction models combine multiple predictors, such as symptoms and patient characteristics, to obtain the risk of the presence or absence of a disease in an individual patient. 10,11 These prediction models can then be used to develop diagnostic tools (such as a website risk calculator or a mouse mat detailing estimates of risk depending on features) to assist doctors in estimating probabilities, and can potentially influence doctors’ decision-making. 11 To evaluate diagnostic prediction models, there are three important stages, or types, of studies: prediction model development, prediction model validation and assessment of the impact of prediction models in practice (generally implemented as diagnostic tools). The first two are often conducted as part of the same study, and are generally evaluated using a single cohort design. These types of studies are commonly found in the diagnostic prediction literature, with some studies also reporting results of an external validation. 12 To assess the impact of the prediction model (the third stage), comparative studies are required to evaluate the ability of the tool to guide patient management. In the literature on prediction models in general, very few diagnostic prediction models that are developed go on to be evaluated for their clinical impact. 12
Tools currently available to GPs to help cancer diagnosis, beyond the National Institute for Health and Care Excellence (NICE) guidelines for suspected cancer referral,6 are based on the following diagnostic prediction models:
-
the risk assessment tool (RAT) developed by Hamilton et al. ,13 which provides estimates of cancer risk for 17 cancers, based on symptoms alone
-
the QCancer® (ClinRisk Ltd, Leeds, UK) tool, which estimates the risk of 10 cancers, based on symptoms and patient characteristics, such as age, smoking status and body mass index.
There are clear differences in the derivation of the RAT and QCancer. The RAT used a case–control design to predict likely cancer diagnosis, whereas QCancer used a cohort design. Many of the QCancer prediction models have subsequently been externally validated and reported to have good diagnostic performance. 14,15 There has, however, been no comparison of the clinical effectiveness of these diagnostic tools in clinical practice, or research on whether or not GPs currently have access to and are using these tools.
In 2013, Hamilton et al. 13 reported an increase in cancer referrals and investigations associated with the introduction of RATs as mouse mats and desktop flip charts for lung cancer and colorectal cancer (CRC), and an increase in the awareness of GPs of cancer symptoms, especially those symptoms that are less known in those cancers. 16 A 2015 evaluation of an electronic version of RATs for lung cancer and CRC highlighted the potential issue of prompt overload from the system, cautioned on potential variation in data used by the tool, and the extent to which the aid might increase pressure on secondary care owing to increased referral (a finding that could be generalised to all such diagnostic tools). 17
An Australian study using simulated GP consultations explored the implementation of an aid based on QCancer. 18 The study found that GPs agreed that the diagnostic aid was potentially useful in practice, but noted that different GPs interpreted the same set of symptoms differently, leading to inconsistent estimates of risk from the QCancer aid. In collaboration with the NAEDI, Macmillan Cancer Support developed, with BMJ Informatica, and evaluated the introduction of an electronic clinical decision support (eCDS) containing the RAT and QCancer for colorectal, lung, oesophagogastric, pancreatic and ovarian cancers. It was found that the impact of eCDSs varied across practice, from no impact on referrals to increased referrals and investigations in other practices,19 with use of eCDSs leading to further investigation or referral of the patient that would not have occurred otherwise in 19% of cases.
However, there is very little evidence as to whether or not these tools have led to increased or quicker cancer diagnoses, and, ultimately, to impacts on patient quality of life or survival. A study protocol for a randomised controlled trial (RCT) to evaluate eCDSs for assessing symptoms indicative of stomach cancer was published in 2016. 20 Other diagnostic prediction models have been developed in the UK, such as that reported by Iyen-Omofoman et al. 21 for lung cancer and the Bristol–Birmingham (BB) equation for CRC,22 plus those developed outside the UK, such as Benign, Lonely, Irregular, Nervous, Change, Known (BLINCK) clues in Australia for skin cancer23 and that developed in the USA for ovarian cancer,24 which may have the potential to be useful in the NHS context. However, little is known about whether or not, and how, these diagnostic prediction models and tools affect patient outcomes, and would affect NHS resources.
Although we are unclear about the evidence on the clinical effectiveness of these diagnostic tools to affect patient quality of life and survival, a systematic review conducted by Neal et al. 25 found a large number of studies looking at the impact on patient outcomes of reducing diagnostic and/or treatment intervals for cancer. Only a small number of studies were found to be of high quality, and there was substantial variation in the type of intervals evaluated and the findings within and between cancer types. Compared with other cancer types, studies of colorectal, breast, head and neck, and testicular cancers and melanoma suggested that shorter time intervals were associated with improved patient outcomes. However, for each of these cancer types, there were also studies reporting no association between time interval and patient outcome.
The possible trade-offs between the costs and the harms, and the inherent uncertainty, of using these diagnostic tools in primary care are also unclear, as well as the extent to which reducing times to diagnosis and/or treatment could affect patient outcomes.
Aims and objectives
The aim of this project was to evaluate the evidence on the development, validation, clinical effectiveness and cost-effectiveness of cancer diagnostic tools in primary care, and to understand the extent to which existing tools are currently used in the primary care setting in the NHS.
The objectives were to:
-
identify evidence evaluating the clinical effectiveness of symptom-based diagnostic tools that that could be used to inform cancer diagnosis decision-making in primary care (see Chapters 2 and 3)
-
identify and summarise studies reporting the development, validation or accuracy of any diagnostic prediction model that could be used as a tool to aid cancer diagnosis in primary care (see Chapters 2 and 4)
-
update a previous systematic review25 assessing the association of the durations of different intervals in the diagnostic process to clinical outcomes, and conduct a more in-depth evaluation of CRC studies, focusing on methods, to identify studies that are likely to provide the best estimate of the impact of diagnostic intervals on patient outcomes for informing the decision-analytic mode (see Chapter 5)
-
use a decision-analytic model to explore uncertainties in the cost-effectiveness of using symptom-based diagnostic tools, including the impacts on health service resource use, costs and patient outcomes, using CRC as an example (see Chapters 6 and 7)
-
understand the extent to which GPs currently have access to cancer diagnostic tools and are using them in primary care to inform their decision-making (see Chapter 8).
Chapter 2 Systematic reviews 1 and 2: literature search strategy
Introduction
Two systematic reviews were conducted: systematic review (SR) 1 and SR2.
The research question (SR1) was ‘what evidence is there for the clinical effectiveness and cost-effectiveness of symptom-based diagnostic tools that could be used to inform cancer diagnosis decision-making in primary care?’. It was anticipated that limited evidence would be identified; therefore, a second review was planned (SR2) to identify studies reporting the development and validation of any diagnostic prediction model that could be used as a tool to help cancer decision-making in primary care, that is to provide a list of cancer models that might have the potential to be developed into diagnostic tools. This chapter clarifies the definitions of diagnostic tools and prediction models used and describes the search methods employed for SR1 and SR2. The results are presented separately in Chapters 3 (SR1) and 4 (SR2).
Diagnostic prediction models can be categorised according to the different stages of development and evaluation of the model (Table 1). However, a number of predictive research studies have differed in what they consider to fall under the ‘predictive model research’ header. This variation primarily related to whether or not they incorporated predictor finding studies as an initial stage and, at the other end of the spectrum, whether or not they incorporated impact or implementation studies. For the purpose of our reviews, we have differentiated between prediction models and prediction tools. Diagnostic prediction models are defined as multivariate statistical models that predict the probability or risk that a patient currently has cancer based on a combination of known features of that patient, such as symptoms, signs, test results and patient characteristics. 26 Symptoms could be self-reported by the patient, or prompted by a physician’s questioning. Signs and test results are identified in primary care via routine testing (e.g. full blood count, urine dipstick testing, clinical signs), and patient characteristics are also determined in primary care (e.g. sociodemographic variables, personal and family history). The prediction tools implement the models to provide a numerical risk of having cancer. As examples, prediction tools may be mouse mats or desktop flip charts, or may be integrated into information technology (IT) systems.
| Predictive research stage | Classification of stage used for coding studies | Description | Exclude or include |
|---|---|---|---|
| Identifying single predictors | Predictor identification | Studies that aim to explore which predictors out of a number of candidate predictors independently contribute to the prediction of (i.e. are associated with) a diagnostic (or prognostic) outcome26 | Exclude |
| Model development only | Development only (apparent performance) | Studies in which performance is directly evaluated using exactly the same data used to derive the model | Include in SR2 |
| Model development with internal validation | Internal validation I | Studies that use only the original study sample for both development and validation using resampling techniques (e.g. cross-validating, bootstrapping, jackknifing) | |
| Internal validation II | Studies that use only the original study sample for both development and validation using split sampling, whereby part of the sample is used for model derivation and the other part is used for validation | ||
| External validation | External validation | Studies that aim to assess and compare the predictive performance of an existing prediction model using new participant data that were not used in the development process | |
| Model updating | Model update | External validation studies in which the model is adjusted or updated in the case of poor performance, based on the validation | |
| Impact assessment | Impact assessment | Studies that aim to quantify the effect or impact of using a diagnostic tool (relative to not using the tool) on patient or physician behaviour and management, patient health outcomes, or cost-effectiveness of care.26 There are two types of analyses:
|
Include in SR1 |
As a 2009 study by Moons et al. 28 points out, studies assessing the impact of prediction tools need designs and outcome measures that are different from those used in studies that develop and evaluate prediction models. Studies of predictive research26,27,29,30 also differ slightly in the way they categorise studies, based on whether they considered the evaluation of internal validity to be part of the model development stage or a separate stage.
Table 1 summarises the spectrum of potential study types and clarifies the inclusion and exclusion criteria for SR1 and SR2.
Methods
The systematic reviews were conducted in accordance with good practice guidelines. 31 As the inclusion and exclusion criteria were very similar for SR1 and SR2, the same search strategy was used for both reviews; however, two separate protocols were developed for reviewing the evidence. Further details are presented in this chapter.
Search strategy
Bibliographic searches of relevant databases [MEDLINE (1946 to May week 1 2017), MEDLINE In-Process & Other Non-Indexed Citations, EMBASE (1974 to 10 May 2017), the Cochrane Library and Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA)] were conducted in May 2017, with updated searches conducted in November 2018. SR1 and SR2 were conducted in parallel using the same search strategy, but each review had different inclusion and exclusion criteria.
The search strategies were developed by an information specialist (SR) and comprised terms for cancer, terms for primary care, terms for decision support tools and terms for diagnosis (Table 2). No date, language, study design or other limits were used. Search filters for clinical prediction models were investigated but none was thought to be fully tested or reliable. A balance was sought between the sensitivity of the search results and the number of papers to be screened.
| 1. | exp Neoplasms/ |
| 2. | (cancer$ or neopla$).tw. |
| 3. | (tumour$ or tumor$).tw. |
| 4. | or/1-3 |
| 5. | Primary Health Care/ |
| 6. | exp General Practice/ |
| 7. | General Practitioners/ |
| 8. | (primary care or general practi$ or family practi$).tw. |
| 9. | (primary adj3 (healthcare or health care)).tw. |
| 10. | Or 5/9 |
| 11. | Decision Support Systems, Clinical/ |
| 12. | Decision Support Techniques/ |
| 13. | (tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw. |
| 14. | or/11-13 |
| 15. | “Early Detection of Cancer”/ |
| 16. | (predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw. |
| 17. | (2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw. |
| 18. | or/15-17 |
| 19. | 4 and 10 and 14 and 18 |
The search results were exported to EndNote X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and de-duplicated using automatic and manual checking.
Items included after full-text screening were forward and backward citation-chased using Scopus® (Elsevier, Amsterdam, the Netherlands) to identify additional relevant studies. Once relevant models and tools were identified from the initial searches, additional searches were conducted to identify names of tools [e.g. QCancer, RAT, Cancer Prediction in Exeter (CAPER), BB equation, GP Skin Cancer Toolkit], to ensure that search results were sufficiently comprehensive.
The full search strategies and results are in Appendix 1.
Inclusion and exclusion criteria
For SR1, diagnostic tools were considered initially as diagnostic prediction models that are used in clinical practice to assist doctors in estimating probabilities to aid decision-making. Pilot searches identified one study that assessed the impact of implementing diagnostic prediction models. Therefore, the definition of ‘diagnostic tool’ for SR1 was expanded to include any quantitative tool used to support a GP in deciding which patient warrants further investigation for cancer. Such investigation could be via referral to secondary care or involve further testing in primary care. In other words, the diagnostic tools may be based not only on diagnostic prediction models, but also on scoring systems/algorithms, etc. Studies were excluded that simply looked at ‘red-flag symptoms’ or symptom lists and (weighted) scores that did not provide a numerical risk of current cancer. Owing to the limited anticipated number of relevant studies, we sought any study reporting on impact, regardless of study design.
For SR2, diagnostic prediction models are defined as multivariate statistical models that predict the probability or risk that a patient currently has cancer based on a combination of known features of that patient, such as symptoms, signs, test results and patient characteristics. 26 Symptoms could be self-reported by the patient or prompted by a physician’s questioning. Signs and test results are identified in primary care via routine testing (e.g. full blood count, urine dipstick testing, clinical signs), and patient characteristics are also determined in primary care (e.g. sociodemographic variables, personal and family history). Studies that simply looked at ‘red-flag symptoms’ or symptom lists and (weighted) scores that did not provide a numerical risk of current cancer were excluded. Models developed with secondary care data (i.e. referred patients) were included only if an attempt was made to validate the models with primary care data.
Inclusion and exclusion criteria for SR1 and SR2 are presented in Table 3.
| Criterion | SR1 | SR2 |
|---|---|---|
| Population | Included: symptomatic patients (with symptoms being indicative of cancer) presenting at primary care or patients referred with symptoms indicative of cancer | |
| Excluded: asymptomatic patients (screening population) | ||
| Technology | Included: featurea-based diagnostic tools implemented/used in primary care to provide additional information on the risk of cancer. The tool may be used for the purpose of diagnosing cancer in primary care (leading to referral for treatment) or to inform decisions about referring for further tests (with possible diagnosis occurring in secondary care) | Included: diagnostic prediction models, based on two or more features,a that estimate the risk of prevalent but undiagnosed cancer |
| Excluded: prognostic or screening prediction models; statistical tools that estimate the probability of developing cancer over a defined period of time | ||
| Setting | Included: primary care | |
| Excluded: secondary care; online tools developed for use by the general population | Exclusion: models developed into tools for online use by the general population | |
| Study design | Included: comparative studies of diagnostic tools that assessed impact in clinical practice (RCTs, controlled before and after, and interrupted time series); studies analysing national trends in cancer diagnosis before and after diagnostic tools became available | Included: any design for the development, validation or accuracy of diagnostic prediction models (as defined in the ‘Technology’ row of this table) |
| Excluded: uncontrolled studies reporting qualitative data | ||
| Comparison | Usual care or the use of another diagnostic tool | N/A |
| Outcomes | Primary outcomes:
|
|
Secondary outcome:
|
Excluded: models that report the risk of survival (or stage at diagnosis, etc.) | |
| Publication type | Included: published in full and English-language publication | |
| Excluded: commentaries, letters | ||
Selection of studies
Although slightly different inclusion and exclusion criteria were used for SR1 and SR2, the screening of articles was conducted simultaneously by two reviewers (RL and BG). An algorithm was used whereby if studies met the mutual inclusion criteria for SR1 and SR2 (population, setting, publication type), they were then assessed further as to whether they were appropriate for SR1 or SR2 (or excluded).
Titles and abstracts were screened for relevance independently; any disagreements were resolved by consensus. Pilot screening was undertaken for the first 100 hits to ensure that both reviewers were interpreting the inclusion and exclusion criteria in the same way. Articles retained were obtained in full and further screened independently by the two reviewers (RL and BG). Disagreements were discussed between the two reviewers; if not resolved, a third reviewer (Christopher Hyde) made the final decision.
For SR2, multiple studies reported the development and validation aspects of particular prediction models (e.g. the development and internal validation of the prediction model by Hippisley-Cox et al. 32 in one paper, and the external validation in a separate paper. 14 All studies related to each specific prediction model were collated, regardless of whether they refer to the development or validation of that tool.
Results
Studies identified
Search phrases were finalised and searches were run in May 2017 (see Appendix 1). A total of 9352 records were obtained through database searching. Additional reference and citation searches on tool names resulted in another 4171 records. After de-duplication, 9780 records were obtained. The database searches were updated in January 2018, resulting in 631 additional new records (after de-duplication), and again in November 2018, when 702 hits were identifeid. Discussions with collaborators led to the identification of relevant grey literature, but no such studies were deemed eligible for inclusion.
As hits were screened simultaneously for inclusion in SR1 or SR2, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)33 flow diagram shows the results for both reviews: 260 full-text articles were screened: five studies met the inclusion criteria for SR1 and 41 studies met the inclusion criteria for SR2 (Figure 1).
FIGURE 1.
The PRISMA flow diagram of the included studies for SR1 and SR2.

Details on the methods for data extraction, on assessing the risk of bias of the included studies and on the findings of the SRs are presented in Chapters 3 (SR1) and 4 (SR2).
Chapter 3 Systematic review 1
Objective
The objective was to identify evidence evaluating the clinical effectiveness of symptom-based diagnostic tools that that could be used to inform cancer diagnosis decision-making in primary care.
Methods
Identification of studies
Information related to the search strategy, eligibility criteria and selection of studies is provided in Chapter 2.
Data extraction
To extract relevant data from each included study, standardised data extraction forms were used, which evolved following piloting and discussion among reviewers. One reviewer (BG) extracted the data, which were checked by a second reviewer (RL). Extracted data included cancer type(s); study design; country; sample size; patient recruitment (with inclusion and exclusion criteria); characteristics of the tool (including whether based on symptoms alone or other features in addition to symptoms); definition of outcomes (including the number of cancer diagnoses, time to cancer diagnosis, stage of cancer at diagnosis, resection rates, patient health-related quality of life, other patient-reported outcome measures); main results, including confidence intervals (CIs); and subgroup analyses, when available.
Critical appraisal
A risk-of-bias form based on the Cochrane Effective Practice and Organisation of Care group recommendations34 was used to assess potential features of different study designs that may lead to biased estimates of clinical effectiveness. This was conducted by one reviewer (BG) and checked by a second reviewer (RL).
Data synthesis
Owing to the heterogeneity between included studies, a narrative review of the studies was conducted.
Results
Studies identified
The impact of three ‘overarching’ diagnostic tools was assessed by five studies:13,35–38 an algorithm for differentiating malignant and benign skin lesions, a skin cancer toolkit and the RATs, which have been developed for various cancer sites. The data extracted from the five included studies are presented in Appendix 2.
Study design was heterogeneous, consisting of RCTs,35,36 one field trial,37 one cohort study13 and one case–control study. 38
Three of these studies36–38 assessed two different decision support tools for skin cancer (Table 4). Del Mar and Green37 and English et al. 36 describe two evaluations of an algorithm to improve the diagnosis of malignant melanocytic lesions and, consequently, reduce the proportion of total lesions excised that prove to be benign. Both studies were conducted in Australia. The Del Mar and Green37 study was designed as a field trial in two cities: the impact of using the algorithm in one city was compared with not using it in the second city, which acted as a control.
| Cancer type(s) | Prediction tool | Study | Country of tool development | Tool description |
|---|---|---|---|---|
| Skin cancer | Melanoma ‘algorithm’ (plus camera) | Del Mar 1995;37 English 200336 | International (meta-analysis), adapted to Australian guidelines | An algorithm for managing clinically suspicious naevi, aided by the use of a camera |
| Skin cancer | GP skin cancer toolkit web resource | Gulati 201538 | International (meta-analysis), adapted to Australian guidelines | The toolkit consisted of a referral decision aid (referral guidelines based on red flags), lesion recognition resource (a series of images), clinical cases and a quiz |
| Multiple (lung, colorectal) | RAT presented on a mouse mat and desktop flip chart | Hamilton 201313 | UK | RAT gives risk estimates for patients aged > 40 years presenting to primary care with symptoms of possible cancer, for single symptoms, pairs of symptoms and repeat attendances with the same symptom. The values are colour coded to aid interpretation |
| Multiple (breast, prostate, colorectal or lung) | Education resource card containing the RAT | Emery 201735 | UK (RAT), Australia (guidelines) | Resource card containing the RAT tables for colorectal, lung and prostate cancer, as well as the Australian National Breast and Ovarian Cancer Centre’s guidelines for investigating new breast symptoms |
Gulati et al. 38 evaluated the impact of a UK-wide, online, skin cancer recognition toolkit on GP confidence and knowledge in diagnosing skin cancers and referral behaviour. Additional skin cancer referral data were obtained to assess the appropriateness of referrals, and a survey was also conducted to investigate GP confidence in diagnosing skin cancer.
The other two included studies13,35 evaluated the impact of previously developed diagnostic prediction models in practice. Both evaluated the use of RATs. Hamilton et al. 13 investigated the number of times two RATs39 (one for lung cancer and one for CRC) were used, together with the number of subsequent referrals and investigations, before and 6 months after the introduction of the tools in general practice in the UK.
Emery et al. 35 evaluated the impact of two complex interventions in rural Australia, a GP intervention and a cancer awareness campaign, in a 2 × 2 design trial, compared with control groups. The GP intervention consisted of an ‘education resource card’ that included RATs for colorectal, lung and prostate cancer, together with summaries of relevant guidelines for colorectal, lung and prostate cancer, with the addition of guidelines for breast cancer and training on the use of these resources. The RATs were based on diagnostic prediction models developed using a patient cohort from the UK39 (further details are provided in Chapter 4). Emery et al. 35 used the total diagnostic interval (TDI), that is the time from first symptom to cancer diagnosis, as an outcome measure.
Critical appraisal
Studies were heterogeneous in how they addressed risk of bias (Table 5). Three of the studies were not randomised. Allocation blinding was another area of vulnerability for the majority of the studies, although this could not be assessed for one of them. 35 All studies managed to ensure reasonably similar baseline characteristics and measurements for the study groups. Among the studies included, Emery et al. 35 raised the fewest concerns for risk of bias.
| Study | Bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Baseline outcome measurements similar | Baseline characteristics similar | Incomplete outcome data | Knowledge of the allocated interventions adequately prevented during the study | Protection against contamination | Selective outcome reporting | Other risks of bias | |
| RCTs | |||||||||
| English 200336 | ✓ | ✓ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ? |
| Emery 201735 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Field trials | |||||||||
| Del Mar 199537 | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ? |
| Case–control study | |||||||||
| Gulati 201538 | N/A | N/A | ✓ | ✓ | ? | N/A | N/A | ? | ✗ |
| Pre–post study | |||||||||
| Hamilton 201313 | N/A | N/A | N/A | N/A | ? | N/A | N/A | ? | ✗ |
Study outcomes
The outcome that Del Mar and Green37 used was the percentage of lesions excised that were benign. The study37 observed that use of the algorithm seemed to reduce the ratio of excised benign lesions to melanomas (from 93.8% to 88.8%; p < 0.001), without reducing the number of melanomas diagnosed. English et al. 36 used a ‘slightly modified’ algorithm that was evaluated by randomising general practices in Perth, Australia, to use the algorithm, while others were used as controls. The outcome used was the ratio of excised benign lesions to excised melanomas. The study37 found no reduction in the ratio of benign to malignant lesions excised.
Gulati et al. 38 showed no significant changes in the number of urgent GP referrals for suspected skin cancer, diagnoses of melanoma or diagnoses of non-melanoma skin cancer between the toolkit users and the non-users in the study periods, despite increased GP confidence in making skin cancer referrals. The proportion of appropriate referrals increased with the use of the toolkit; however, the differences between toolkit users and non-users did not reach statistical significance.
Hamilton et al. 13 reported on changes in investigations carried out and rapid referrals before and after the introduction of the tools. They found an increase of 31% in rapid referrals for lung cancer and a 4% increase in GP-mandated chest X-ray investigations, as well as a 26% increase in referrals for CRC and a 15% increase in GP requests for colonoscopies after introduction of the tools. However, only absolute numbers are reported, without data on total numbers of patients and GP visits, or the appropriateness of the referral.
Emery et al. 35 did not find significant differences in the median or log-transformed (ln) mean time to diagnosis at either intervention level (community intervention vs. control, GP intervention vs. control) or when analysed by factorial design, tumour group or subintervals of the TDI.
None of the included studies reported outcomes such as diagnoses made, quality of life, survival or NHS resource use.
Study results are summarised in Table 6.
| Cancer type(s) | Study | Prediction tool | Country | Study design | Intended purpose | Main results |
|---|---|---|---|---|---|---|
| Melanoma | Del Mar 199537 | Melanoma ‘algorithm’ (plus camera) | Australia | Field trial | To evaluate whether or not an algorithm can reduce the number of benign lesions being excised without reducing the excision of invasive lesions, by comparing numbers of excised lesions with and the number without algorithm use | Total number of excised lesions
|
Percentage of excised lesions that are neither invasive or potentially malignant
|
||||||
The median number of excisions per doctor (2.5% and 97.5% percentiles)
|
||||||
| There were significant differences in the percentages of benign lesions reported in the intervention and control cities (88.8% and 93.8%; p < 0.001) after the intervention | ||||||
| Melanoma | English 200336 | Melanoma ‘algorithm’ (plus camera) | Australia | RCT | To determine whether or not an aid to the diagnosis of pigmented skin lesions reduces the ratio of benign lesions to melanomas excised in general practice |
Number of excised skin lesions (including seborrheic keratoses) At baseline:Trial period: |
| Provision of the algorithm and camera did not decrease the ratio of benign pigmented skin lesions to melanomas excised by GPs (OR 1.03, 95% CI 0.71 to 1.50; p = 0.88) | ||||||
| Skin cancer | Gulati 201538 | GP Skin Cancer Toolkit | UK | Case–control | To assess the impact of the toolkit by comparing before-and-after national skin cancer referral data, data from cross-sectional questionnaires and data on urgent skin cancer referrals to two NHS trusts | 21,000 GPs were invited to use the tool; 8163 GPs accessed the tool during the 2012 period. There were no significant changes in the number of urgent GP referrals for suspected skin cancer (Spearman’s rank 0.20; p < 0.001), diagnoses of melanoma (Spearman’s rank 0.064; p < 0.001) or diagnoses of non-melanoma skin cancer (Spearman’s rank 0.068; p < 0.001) between the toolkit user and the non-user groups. The proportion of appropriate referrals increased from 21.37% in 2011 to 32.3% in 2012, giving an incidence rate ratio of 3.13 (95% CI 2.21 to 4.42, z-statistic 6.46; p < 0.0001) |
| The differences in numbers of appropriate referrals between toolkit users and non-toolkit users did not reach statistical significance by Spearman’s rank test and ANOVA | ||||||
| Multiple (lung, colorectal) | Hamilton 201313 | RAT for lung cancer and CRC in two formats: mouse mat and desktop flip chart | UK | Pre–post study | To compare referrals and investigations for colorectal and lung cancer before and after the implementation of RATs | Lung cancer: 31% increase in 2-week referrals (332 before, 436 after); 4% increase in related investigations (chest X-ray) (7431 before, 7723 after) |
| CRC: 26% increase in 2-week referrals (1173 before, 1477 after); 15% increase in colonoscopies (1762 before, 2032 after) | ||||||
| No conclusion possible on the clinical effectiveness of the intervention | ||||||
| Multiple (breast, prostate, colorectal or lung) | Emery 201735 | Education resource card including RAT for colorectal, lung and prostate cancers | Australia | Factorial cluster RCT | To measure the effect of community-based symptom awareness and GP-based educational interventions on the time to diagnosis (i.e. TDI) for patients presenting with breast, prostate, colorectal or lung cancer in rural Western Australia | No significant differences in the median or ln mean TDI at either intervention level:
|
Discussion
This review attempted to summarise existing evaluations of feature-based cancer diagnostic tools used in primary care. Our strategy was able to identify a limited number of heterogeneous studies that did not provide strong evidence of the impact of feature-based diagnostic tools on patient-related outcomes or referral patterns. The small number of studies (n = 5) and the heterogeneity in reported outcomes did not allow for a meta-analysis of the results. The included studies provided limited evidence of the clinical effectiveness of using diagnostic tools.
A few other reviews have looked at feature-based cancer diagnostic tools in primary care. Williams et al. 40 conducted a systematic review of studies that described, validated or assessed the impact of CRC diagnostic tools. However, they did not identify any studies that tested whether or not patients who were diagnosed with the aid of the tool fared better than those who were diagnosed without it. Schmidt-Hansen et al. 41 conducted a similar review of lung cancer tools and found limited evidence to support the recommendation of any of the identified risk prediction tools, owing to lack of external validation or cost impact assessment. Similarly, Usher-Smith et al. 42 concluded that, even though some of the prediction models had the potential for clinical application, there remains considerable uncertainty about their clinical utility.
Other reviews have looked at cancer RATs in primary care. 43,44 However, they differ from this review in that they reported on tools that estimate the risk of developing cancer in the future, rather than the risk of having an undiagnosed cancer based on current signs and symptoms.
Although the intention of our review was to explore tools based only on prediction models, it was not clear whether or not, in practice, the impact of such tools could be isolated from other decision-making tools available to practitioners, such as diagnostic algorithms45 or guidelines. 6 With limited evidence available on the impact of implemented diagnostic models, we decided to report on identified studies on the algorithm-based tools as well; however, the evidence was still sparse.
Among the limitations of the included studies were lack of randomisation, lack of patient-related outcomes and use of models developed on different populations. The outcome measures used by some of the studies make it difficult to interpret reports of an increase in referral rate without including reasonable assessment of the appropriateness of the referral or subsequent impact on cancer versus non-cancer diagnosis.
Furthermore, concerns on the quality of the studies make it unclear whether the lack of effect was due to poor implementation of the tools in practice, insufficient uptake by the GPs or limited marginal contribution of the tools in assessing the risk of cancer. The best-quality study35 also failed to show a significant effect; however, the composite intervention used, combining older versions of several instruments (developed on populations from a different country), could have limited the clinical effectiveness of the diagnostic tools. These findings could be further obfuscated by publication bias, whose magnitude on this topic remains unknown.
Conclusion
Current evaluations provide limited evidence of the impact on patient outcomes of using feature-based cancer diagnostic tools in primary care. Better research is needed to provide these data, possibly through better study design and choice of outcomes. However, identifying the ideal approach may not be straightforward. Practical reasons may highlight the potential need for a cluster and pragmatic trial design. Arguably, by comparing average times to diagnosis, patients not prioritised for quick referrals are less at risk of being missed. The debate, however, is ongoing on the most appropriate outcomes for evaluating interventions to improve cancer diagnosis and referral.
Chapter 4 Systematic review 2
Objective
Systematic review 2 was conducted as a complementary study to SR1, described in Chapter 3. The objective was to identify and summarise studies reporting the development, validation or accuracy of any diagnostic prediction model that could be used as a tool to aid cancer diagnosis in primary care.
Methods
Identification of studies
Information related to the search strategy, eligibility criteria and selection of studies is provided in Chapter 2.
Data extraction
An adaptation of the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS)30 was used to extract the following data from each included study: country, cancer type(s), study design, data source, sample size, number of participants with specific cancer, recruitment (including inclusion and exclusion criteria), participant characteristics, features of the model (what symptoms, test results, patient demographics, etc. are included), how features are defined and measured, definition of primary and secondary outcomes, how and when outcomes are assessed, main results (including model performance, validation and estimates of risk) and features included in final model.
Critical appraisal
Risk of bias was assessed with the use of a form based on the work of the Prediction model Risk Of Bias ASsessment Tool (PROBAST)46 group. Because a final version of this checklist was not publicly available at the time of the appraisal, we followed recent recommendations on reporting reviews of prediction models. 47–49 Like the PROBAST checklist, the derived checklist assesses the risk of bias and applicability of prediction-modelling studies on five domains: participant selection, predictors, outcome, sample size and missing data, and analysis (Table 7).
| Domain | Items |
|---|---|
| I. Participant selection | 1a. Were appropriate data sources used, for example cohort, controlled trial or nested case–control study data? |
| 1b. Were all inclusions and exclusions of participants appropriate? | |
| 1c. Were participants enrolled at a similar state of health, or were predictors considered to account for differences? | |
| II. Predictors | 2a. Were predictors defined and assessed in a similar way for all participants in the study? |
| 2b. Were predictor assessments made without knowledge of outcome data? | |
| 2c. Are all predictors available at the time the model is intended to be used? | |
| 2d. Were all relevant predictors analysed? | |
| III. Outcome | 3a. Was a prespecified outcome definition used? |
| 3b. Were predictors excluded from the outcome definition? | |
| 3c. Was the outcome defined and determined in a similar way for all participants? | |
| 3d. Was the outcome determined without knowledge of predictor information? | |
| IV. Sample size and missing data | 4a. Were there a reasonable number of outcome events? |
| 4b. Was the time interval between predictor assessment and outcome determination appropriate? | |
| 4c. Were all enrolled participants included in the analysis? | |
| 4d. Were participants with missing data handled appropriately? | |
| V. Analysis | 5a. Were non-binary predictors handled appropriately? |
| 5b. Was selection of predictors based on univariable analysis avoided? | |
| 5c. Was model overfitting (optimism in model performance) accounted for, for example using bootstrapping or shrinkage techniques? | |
| 5d. Were any complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | |
| 5e. Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | |
| 5f. For the model or any simplified score, were relevant performance measures evaluated, for example calibration, discrimination, (re)classification and net benefit? | |
| 5g. Was the model recalibrated or was it likely (based on the evidence presented, e.g. calibration plot) that recalibration was not needed? |
Data synthesis
Owing to the heterogeneity between included studies, a narrative synthesis of the studies was conducted.
Results
Studies identified
There were 41 included records from the searches, including two systematic reviews. A further two studies were identified from one of the reviews. The primary are summarised in Appendix 3.
Systematic reviews
Two included records41,50 are systematic reviews that had some overlap with the included studies described in this section (Table 8).
| Review | Aim | Overlap with included studies in SR2 |
|---|---|---|
| Schmidt-Hansen 201741 | To review the existing risk prediction tools for patients presenting in primary care with symptoms that may indicate lung cancer | Hamilton 2005,51 Hippisley-Cox 2011,52 Hippisley-Cox 2013,53 Hippisley-Cox 201354 and Iyen-Omofoman 201321 |
| Elias 201750 | To validate published diagnostic models for their ability to safely reduce unnecessary endoscopy referrals in primary care patients suspected of significant colorectal disease | Fijten 1995,55 Marshall 2011,22 Muris 199556 and Nørrelund 199657 |
Schmidt-Hansen et al. 41 conducted a systematic review of the literature to identify risk prediction tools to be used in primary care to aid diagnosis of lung cancer. Five separate tools were identified: RAT,51 QCancer,52–54 the equation from Iyen-Omofoman et al. ,21 and tables from Jones et al. 58 and Jordan et al. 59 Schmidt-Hansen et al. 41 concluded that, so far, none of the tools has been externally validated, yet there is a need to improve early diagnosis.
Elias et al. 50 aimed to identify and validate published diagnostic models to safely reduce unnecessary endoscopy referrals in CRC. A systematic review of the literature was undertaken and identified models were validated using a cross-sectional Dutch data set (n = 810). The definition of model used by Elias et al. 50 was very broad and included guidelines and weighted scores. Therefore, although Elias et al. 50 identified 18 models, only four are relevant to our review: Fijten et al. 55 and Marshall et al. 22 were previously identified from our searches, whereas Muris et al. 56 and Nørrelund et al. 57 are new inclusions. Because Elias et al. 50 attempted to validate the models they found, their validation of these four models is included in the results presented later in this chapter.
Prediction models
The 41 included studies (39 identified from the searches plus two identified from Elias et al. 50) reported on 12 different prediction models (which are briefly summarised in the following paragraphs): (1) a RAT for 15 different cancer sites; (2) QCancer for six cancer sites, plus male and female versions for all cancers; (3) a clinical prediction rule for breast cancer;60 (4) the BB equation for CRC;22 (5) the Netherlands model for CRC;55 (6) a machine-learning algorithm for CRC;61 (7) a Danish model for CRC;57 (8) a UK model for lung cancer;21 (9) a UK model for pancreatic cancer;62 (10) a European model for abdominal cancers;63 (11) a UK model for paediatric cancers;64 and (12) a Dutch model for multiple cancers. 56
The RATs were designed to be used with patients presenting to primary care with ‘low-risk-but-not-no-risk symptoms’. 65 Early versions of RATs were developed using case–control data from Devon, UK, as part of the CAPER studies. 39 Later models were derived using UK-wide primary care data – the Clinical Practice Research Datalink (CPRD) (formerly known as the General Practice Research Database),66–73 and The Health Improvement Network (THIN) database. 74,75 So far, models for 14 separate cancer sites have been published (colorectal, oesophageal, lung, ovarian, kidney, bladder, pancreas, breast, uterine, brain, prostate, Hodgkin lymphoma, non-Hodgkin lymphoma and multiple myeloma), plus one model for metastatic cancer. The RATs are available as prints on common office objects (e.g. mouse mats) and are integrated into GP software in the form of the electronic cancer decision support (eCDS). Regardless of the format, they provide risk estimates for patients with single symptoms of possible cancer, pairs of symptoms and repeat attendances with the same symptoms. Elias et al. 50 used a Dutch data set to externally validate the colorectal version of RATs.
The QCancer series of models can be used in both symptomatic (diagnostic models) and asymptomatic (prognostic models) patients. 42 QCancer was developed in the QResearch database, a large database comprising > 12 million anonymised health records from 602 general practices throughout the UK, using the EMIS Health (Leeds, UK) computer system. Initially, several models were developed for each cancer type in symptomatic populations (colorectal, gastro-oesophageal, lung, renal, pancreatic and ovarian cancer). An updated approach incorporates multiple risk factors and symptoms into one model to predict cancer risk. Most of these models have been externally validated in UK-wide populations (e.g. THIN database76). QCancer is available as an online calculator (www.qcancer.org), which provides estimates of the absolute risk of any cancer, with a breakdown of type of cancer based on both risk factors such as age, sex and family history, which increase the likelihood of cancer, and risk markers such as haemoptysis or features (usually symptoms, e.g. weight loss) suggesting that cancer is already present.
McCowan et al. 60 developed a clinical prediction model for breast cancer using secondary care data on symptomatic patients at one hospital in Scotland. The authors validated the model using data from 202 patients with symptomatic breast problems attending 11 general practices in Scotland.
Marshall et al. 22 used data from the THIN data set (> 40,000 participants) to construct a model for CRC, known as the BB equation, which they validated using the CAPER data set. Data from 290 patients presenting to GPs in the Netherlands with rectal bleeding (from 1988 to 1990) were used by Fijten et al. 55 to develop a prediction model for CRC. Two studies validated this model: Hodder et al. 77 used secondary care data from the UK, whereas Elias et al. 50 used a Dutch data set. Kop et al. 61 used a machine-learning algorithm to develop a prediction model for CRC using the electronic records of almost 220,000 patients from two general practices in the Netherlands. A Danish CRC model57 has also been developed for use in primary care; this was externally validated by Elias et al. 50 using a Dutch data set.
Iyen-Omofoman et al. 21 developed a prediction model for lung cancer using data on > 130,000 participants in the THIN data set. Keane et al. 62 also used the THIN data set and developed two prediction models: one for pancreatic ductal adenocarcinoma and one for biliary tract cancers.
Dommett et al. 64 is the only model our searches identified that considers paediatric cancers. The authors used the CPRD to develop prediction models of bone and soft tissue, central nervous system and abdominal cancers, and leukaemia and lymphoma. The authors also developed a model to consider all paediatric cancers.
Muris et al. 56 developed a model using data from the Netherlands to predict multiple cancers, and Elias et al. 50 externally validated it.
Holtedahl et al. 63 report details of the development of a prediction model for abdominal cancers. These are defined as all cancers of the digestive organs, female genital organs and urinary organs (including testis). Data on 61,802 patients, recorded during GP consultations in Norway, Denmark, Sweden, Scotland, Belgium and the Netherlands over a 10-day period, were used to develop the model. No validation of the model is reported.
Prediction model characteristics
Colorectal cancer was associated with the greatest number of models (six in total): (1) the BB equation,22 (2) the Netherlands model,55 (3) the machine-learning algorithm,61,78,79 (4) the Danish model,57 (5) QCancer80 and (6) the RAT. 72,74,81 We identified three models for lung cancer (UK model,21 QCancer52 and RAT51) and three models for pancreatic cancer (UK model,62 QCancer32 and RAT70). Only versions of QCancer and RAT were found for gastro-oesophageal cancer,71,82 ovarian cancer83,84 and renal cancer. 66,85 There are two RATs for blood cancers: one for leukaemia67 and one for myeloma. 68 For the other cancer sites, only one model for each was identified, and, apart from the breast cancer model,60 the metastatic cancer model86 and the abdominal model,63 they are versions of RATs. Two versions of the QCancer model (one for females53 and one for males54), a Dutch model56 and the UK paediatric cancers model64 were all developed to evaluate the risk prediction of multiple cancers.
The models are in various stages of development. A total of 18 models (or versions of models) have assessed only apparent performance, three models have been internally validated using a split-sampling technique21,52–54 and one model was updated as a result of using a different data source. 74 Five of the QCancer versions,32,80,82,84,85 one RAT version81 and five of the other prediction models22,55–57,60 have been externally validated, which is the highest level of evidence identified in this systematic review.
All but one of the models were developed in primary care settings. McCowan et al. 60 developed a clinical prediction rule model for breast cancer using secondary care data, but with the intention of the model being used in primary care. Only four models were developed outside the UK: those by Fijten et al. ,55 Kop et al. 61 and Muris et al. 56 were developed in the Netherlands, and the one by Nørrelund et al. 57 was developed in Denmark. For the models that were externally validated, most were validated in the country in which they were developed, except for the following: the validation77 of the Netherlands CRC model55 in a UK population, the validation of the Danish CRC model57 in a Dutch population,50 and the validation of the colorectal version of RATs (UK)81 in a Dutch population. 50
Table 9 provides a brief description of the models, their stages of development, the cancer sites covered and study designs. Owing to the heterogeneity in tools, cancer sites, outcomes measured and study design, a narrative review of the studies was conducted.
| Cancer site and prediction model | Number and categories of descriptors | Stage of development | Study design | Country | Source |
|---|---|---|---|---|---|
| Bladder | |||||
| RAT | 8; symptoms, medical history, test results | Apparent performance | Case–control | UK | Shephard 201269 |
| Blood | |||||
| RAT (leukaemia) | 10 (chronic leukaemia); symptoms | Apparent performance | Case–control | UK | Shephard 201667 |
| 13 (acute leukaemia); symptoms | |||||
| RAT (myeloma) | 16; symptoms, test results | Apparent performance | Case–control | UK | Shephard 201568 |
| Brain | |||||
| RAT | 8; symptoms | Apparent performance | Case–control | UK | Hamilton 200787 |
| Breast | |||||
| Clinical prediction rule | 5; patient demographics, symptoms | External validation | Prospective cohort | UK | McCowan 201160 |
| Colorectal | |||||
| BB equation | 8; symptoms, test results | External validation | Retrospective case–control | UK | Marshall 201122 |
| External validation | Prospective cohort | The Netherlands | Elias 201750 | ||
| The Netherlands model | 3; symptoms, patient demographics | Apparent performance | Prospective cohort | The Netherlands | Fijten 199555 |
| External validation | Prospective cohort | UK | Hodder 200577 | ||
| External validation | Prospective cohort | The Netherlands | Elias 201750 | ||
| Machine learning algorithm | Numerous models are reported; patient demographics, symptoms, medical history, test results | Apparent performance | Case–control | The Netherlands | Kop 2015;61 Kop 2016;79 and Hoogendoorn 201578 |
| Danish model | 2; patient demographics, symptoms | Apparent performance | Prospective cohort | Denmark | Nørrelund 199657 |
| External validation | Prospective cohort | The Netherlands | Elias 201750 | ||
| QCancer | 6 (females) and 7 (males); symptoms, medical history, test results | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201280 |
| External validation | Prospective cohort | UK | Collins 201215 | ||
| RAT | 10; symptoms, test results | Apparent performance | Case–control | UK | Hamilton 200581 |
| External validation | Prospective cohort | The Netherlands | Elias 201750 | ||
| RAT | 8; symptoms, test results | Apparent performance | Case–control | UK | Hamilton 200974 |
| RAT (bowel) | 10; symptoms, test results | Apparent performance | Case–control | UK | Stapley 201772 |
| Gastro-oesophageal | |||||
| QCancer | 7 (females) and 6 (males); symptoms, test results, patient demographics | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201182 |
| External validation | Retrospective cohort | UK | Collins 201388 | ||
| RAT | 16; symptoms, test results | Apparent performance | Case–control | UK | Stapley 201371 |
| Lung | |||||
| UK (Iyen-Omofoman et al.21) model | 15; patient demographics, symptoms | Internal validation II | Case–control | UK | Iyen-Omofoman 201321 |
| QCancer | 9 (females) and 8 (males); symptoms, patient demographics, test results | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201152 |
| RAT | 13; symptoms, patient demographics | Apparent performance | Case–control | UK | Hamilton 200551 |
| Ovarian | |||||
| QCancer | 8; medical history, symptoms, test results | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201184 |
| External validation | Retrospective cohort | UK | Collins 201389 | ||
| RAT | 7; symptoms | Apparent performance | Case–control | UK | Hamilton 200983 |
| Pancreas | |||||
| QCancer | 7 (females) and 8 (males); patient demographics, medical history, symptoms | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201232 |
| External validation | Retrospective cohort | UK | Collins 201314 | ||
| RAT | 9; medical history, symptoms | Apparent performance | Case–control | UK | Stapley 201270 |
| UK models for PDAC and BTC | 13 (PDAC) and 9 (BTC); symptoms, medical history, test results | Apparent performance | Case–control | UK | Keane 201462 |
| Prostate | |||||
| RAT | 9; symptom, test results | Apparent performance | Case–control | UK | Hamilton 200690 |
| Renal | |||||
| QCancer | 7 (females) and 5 (males); medical history (females), patient demographics, symptoms, test results | Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201285 |
| External validation | Retrospective cohort | UK | Collins 201391 | ||
| RAT | 15; symptoms, test results | Apparent performance | Case–control | UK | Shephard 201366 |
| Uterine | |||||
| RAT | 9; symptoms, test results | Apparent performance | Case–control | UK | Walker 201373 |
| Metastatic | |||||
| RAT | 7; symptoms, test results | Apparent performance | Case–control | UK | Hamilton 201586 |
| Multiple | |||||
| QCancer (female) |
|
Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201353 |
| QCancer (male) |
|
Internal validation II | Open prospective cohort | UK | Hippisley-Cox 201354 |
| UK paediatric model |
|
Apparent performance | Prospective case–control | UK | Dommett 201364 |
| Muris (the Netherlands) model | 5; symptoms, patient demographics, test results | Apparent performance | Prospective cohort | The Netherlands | Muris 199556 |
| External validation | Prospective cohort | The Netherlands | Elias 201750 | ||
| Abdominal model | 4; symptoms, patient demographics | Apparent performance | Prospective cohort | Belgium, Denmark, the Netherlands Norway, Scotland and Sweden | Holtedahl 201863 |
Critical appraisal
The assessment of risk of bias is summarised in Table 10, and is given in more detail in Appendix 3 (see Tables 41 and 42). Note that for the RATs and QCancer models, only one entry each is shown, as all versions of the RAT or QCancer model scored the same for each aspect of the risk-of-bias tool used. Most of the included models were judged as having low risk of bias for participant selection and aspects of the outcome definition. For a number of studies, there was a high risk of bias surrounding aspects of the analysis. Much of this stemmed from methods reported for the selection of model variables based on univariate analyses, a lack of evaluation of calibration or discrimination, or no accounting for overfitting. For features of the predictors and participant flow, there was much uncertainty as to what had been done in the studies, and so the risk of bias of the findings could not be determined.
| Model (first author of first version) | Stage of development covered | Risk-of-bias domain | ||||
|---|---|---|---|---|---|---|
| I. Participant selectiona | II. Predictorsa | III. Outcomea | IV. Sample size and participant flowa | V. Analysisa | ||
| RAT (Hamilton) series of models for multiple sites51,66–74,81,83,86,87,90 | Apparent performance | ✓ | ? | ✓ | ? | ✗ |
| External validation (colorectal)50 | ✓ | ✓ | ✓ | ? | ? | |
| QCancer (Hippisley-Cox) series of models for multiple sites and populations (female/male)14,15,32,52–54,80,82,84,85,88,89,91 | Internal validation II | ✓ | ✓ | ✓ | ✓ | ✓ |
| External validation (not for lung, ‘male’, or ‘female’) | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Clinical prediction rule (McCowan 201160) for breast cancer | External validation (developed in secondary care for use in primary care) | ✓ | ✓ | ✓ | ✗ | ✗ |
| BB (Marshall)22 model for CRC | External validation | ✓ | ? | ✓ | ? | ✓ |
| External validation (Elias 201750) | ✓ | ✓ | ✓ | ? | ? | |
| The Netherlands’ (Fitjen 199555) model for CRC | Apparent performance | ✗ | ✓ | ✓ | ? | ✗ |
| External validation (Hodder 200577) | ✗ | ? | ✗ | ✓ | ? | |
| External validation (Elias 201750) | ✓ | ✓ | ✓ | ? | ? | |
| The Netherlands’ (Kop)79 ‘machine learning’ for CRC | Apparent performance | ✓ | ? | ✓ | ? | ? |
| Danish (Norrelund 199657) model for CRC | Apparent performance | ✓ | ? | ✓ | ? | ✗ |
| External validation (Elias 201750) | ✓ | ✓ | ✓ | ? | ? | |
| The Netherlands’ (Muris 199556) model for CRC | Apparent performance | ? | ✓ | ✓ | ? | ✗ |
| External validation (Elias 201750) | ✓ | ✓ | ✓ | ? | ? | |
| UK (Iyen-Omofoman 201321) model for lung cancer | Internal validation II | ✓ | ? | ✓ | ? | ✗ |
| UK (Keane 201462) model for pancreatic cancer | Apparent performance | ✓ | ✓ | ✓ | ? | ✓ |
| UK (Dommett 201364) model for paediatric cancer | Apparent performance | ✓ | ✓ | ✓ | ✓ | ? |
| Prediction model for abdominal cancers (Holtedahl 201863) | Apparent performance | ? | ✓ | ? | ✗ | ? |
Discussion
Looking at symptom-based cancer diagnostic prediction models currently under development, we were able to identify 43 studies in total. The majority of these reported on various aspects of just two such modelling versions, QCancer and RAT, both developed in the UK. Most of the reported work also seemed to be located in Europe, with two of the models developed in the Netherlands.
The majority of the models included in this review were developed only with the sample used to derive the model. With the exception of the RAT (colorectal) model, there were no reports to suggest that models were being updated based on new available data. Some models were validated using split-sample techniques. A number of models, in particular the ones developed under the QCancer name, were externally validated in independent studies.
The two main models highlight important knowledge gaps; the development of the QCancer models was based on higher-quality data (cohort data) than that of the RATs, and were mostly externally validated, but lack impact assessment. By contrast, the RAT series has more evidence of impact on practice, but was developed from case–control studies and has limited external validation.
Our systematic review was limited to the inclusion of diagnostic prediction models; however, the search strategy also highlighted a number of studies concerned with the development of symptom-based decision tools that were not based on prediction models, such as scoring systems, as well as the development of diagnostic prediction models for secondary care. These are listed in Appendix 3 (see Tables 43 and 44) as a resource for further research.
Evidence suggested that there has been a great deal of external validation work for QCancer, whereas we found only one attempt at external validation of RATs. Ideally, this is an area for further development of the RATs and the four other models that have not yet been externally validated.
Conclusion
To our knowledge, this is the first systematic review of diagnostic prediction models for use in primary care to aid cancer diagnosis. We have identified models that have the potential to be developed into tools and be used by GPs. However, there are gaps in the literature that we have identified.
Currently, most research on developing symptom-based cancer risk prediction models is concentrated in Europe and, in particular, the UK. QCancer and RATs are the dominant prediction models. Although there has been a great deal of external validation work done for QCancer, only one study was identified that reports the external validation of RATs.
Chapter 5 Updated review
Introduction
An update of a previous systematic review by Neal et al. 25 (published in 2015) was undertaken to examine the association between different durations of time from first symptom to diagnosis or treatment and clinical outcomes across all major cancers for symptomatic presentations, to investigate whether or not a more timely cancer diagnosis is associated with more favourable outcomes. It had been anticipated that there would be very little evidence on whether or not diagnostic tools have led to increased or quicker cancer diagnoses and, ultimately, impacts on patient survival. This updated review would therefore provide important information to inform the proposed decision model of a cancer diagnostic pathway for assessing the impact of using diagnostic tools.
The cancer diagnostic pathway is complex, incorporating multiple key time points and intervals from the patient first experiencing symptoms to receiving a definitive diagnosis or treatment (Figure 2). An initiative to improve the design and reporting of early-cancer diagnosis research included a review of existing instruments used to measure time points and intervals was undertaken. The project also incorporated a consensus conference approach combined with nominal group techniques to develop a series of recommendations for definitions and methodological approaches, which culminated in the Aarhus consensus statement, published in 2012. 92 The Aarhus checklist is a resource for early-cancer diagnosis research that aims to promote greater precision and transparency in both definitions and methods used. 92
FIGURE 2.
Illustration of the different interval types. T1, time from symptom onset to first seen in primary care (‘patient interval’); T2, time from symptom onset to referral to specialist care; T3, time from symptom onset to first seen in specialist care; T4, time from symptom onset to diagnosis; T5, time from symptom onset to treatment; T6, time from first seen in primary care to referral to specialist care (‘referral interval’); T7, time from first seen in primary care to first seen in specialist care; T8, time from first seen in primary care to diagnosis (‘diagnostic interval’); T9, time from first seen in primary care to treatment; T10, time from referral to specialist care to first seen in specialist care; T11, time from referral to specialist care to diagnosis; T12, time from referral to specialist care to treatment; T13, time from first seen in specialist care to diagnosis; T14, time from first seen in specialist care to treatment; T15, time from diagnosis to treatment (treatment interval).
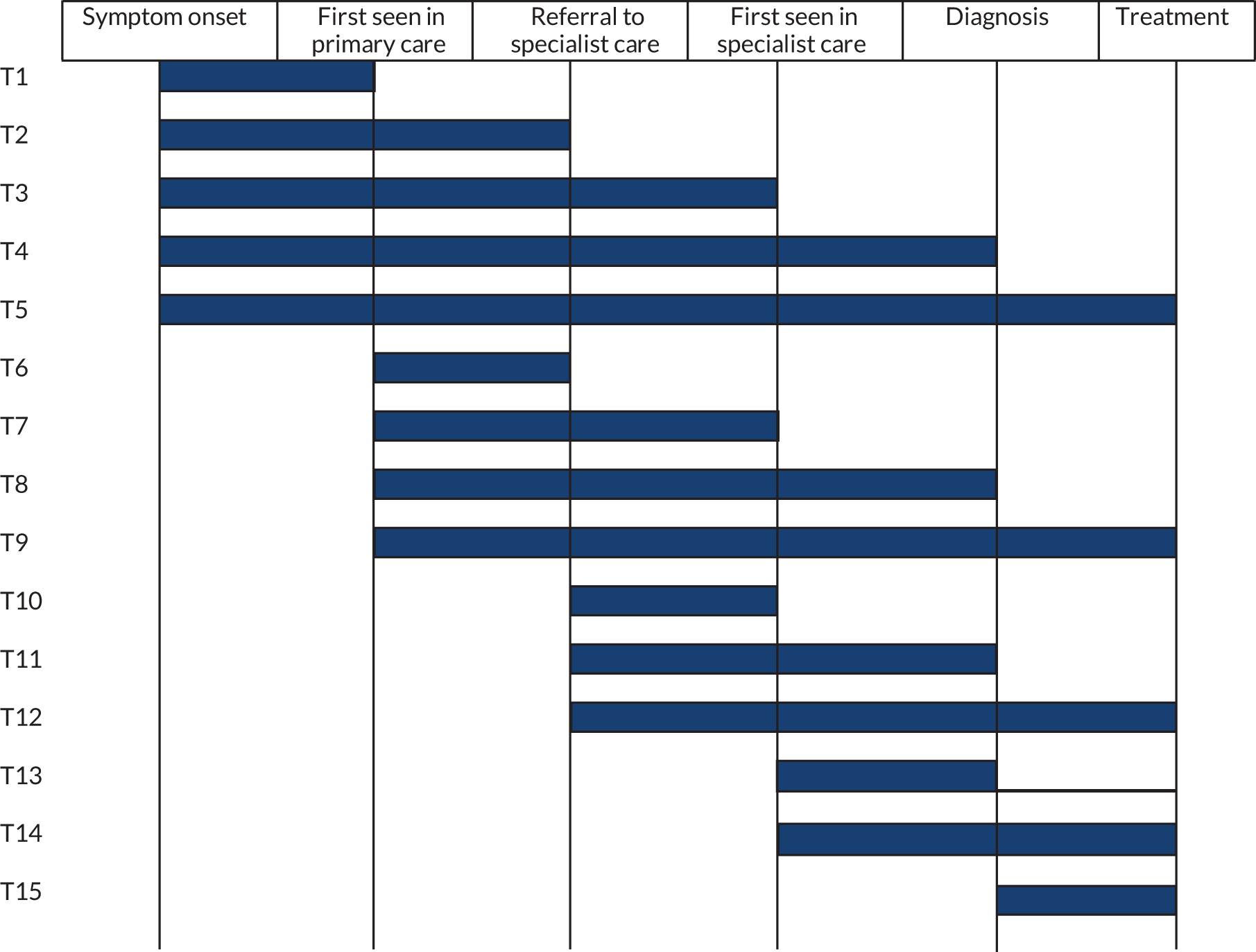
The broader evaluation of how reductions in time to diagnosis or treatment can affect patient outcomes with any cancer not only allows the consideration of the probable impact of using diagnostic tools in these cancers, but also provides a greater evidence base on the probable impact that could be expected from reducing time to diagnosis and/or treatment in general. A more in-depth evaluation of CRC studies was undertaken in this updated review, as this was the focus of our decision-analytic model. This in-depth evaluation focused on the methodology used in those studies. The decision-analytic model focused on the use of diagnostic tools in an adult population; therefore, unlike the previous review of diagnostic intervals by Neal et al. ,25 we did not consider childhood cancers as part of the updated review. The previous review and this updated review considered any type of interval along this pathway, which were grouped according to accepted definitions, relating to the patient, primary care, secondary care or combinations of any of those categories. 92
Aim
The aim of this review was to update a previous systematic review25 assessing the evidence linking the durations of different intervals in the diagnostic process to clinical outcomes. A more in-depth evaluation of CRC studies, focusing on methods, was conducted to identify studies that are likely to provide the most valid estimate of the impact of diagnostic intervals on patient outcomes for informing the decision-analytic model.
Methods
Identification of studies
Search strategy
Searches conducted for the previous review and developing the methods for the current update
The previous review25 was conducted in two phases. The original review was conducted in 2008–10, and then a subsequent review was conducted in 2013–14; both phases were published as a single review in 2015. The first phase did not include breast cancer or CRC, because of existing systematic reviews (e.g. Richards et al. ;93 Ramos et al. ;94 Ramos et al. ;95 and Thompson et al. 96). However, these cancer sites were included in the subsequent phase (in 2013–14).
The literature searches for the previous review, which covered multiple databases, were first conducted during Phase I in 2010 (covering the literature from inception of the databases to February 2010) and then during Phase II in 2013 (covering the literature from February 2010 to November 2013). Both the original and subsequent searches (Neal et al. ;25 Phases I and II) yielded a very high number of references to screen. Our estimate from scoping searches was that an update of the Neal et al. 25 review from 2013 to 2018 would yield some 25,000 studies to screen. We therefore adopted a pragmatic alternative method to update this review, using forward citation-chasing.
Search strategy for the current updated review
The forward citation searches for the current update were conducted in August 2017 and updated in February 2018. Forward citations of the original list of 177 studies included in Neal et al. 25 (from both the 2010 and the 2013 searches) were chased using Scopus and the Web of Science. The search results were exported to EndNote X7 and de-duplicated using automatic and manual checking, yielding 2769 studies for screening. The full text of included studies identified from screening were also forward citation-chased (second-order chasing) in the same way until the investigations were exhausted and all included studies had been citation-chased.
It is uncertain as to what is the best method for updating systematic reviews. 97 The value of alternative methods of searching is acknowledged, but more research is needed on the clinical effectiveness of different techniques. 98 To test the thoroughness of our novel approach, we sought forward citations of all the full-text references in the 2010 searches and compared the results with those of the full updated review carried out in 2013. The citation-chasing approach found 34 of the 71 full-text papers identified in the 2013 updated review. However, the 2013 review also incorporated some revisions to the inclusion criteria to make the review more focused, making results less directly comparable.
Selection of studies
Two independent reviewers (RL and BG) screened the titles and abstracts of all records identified by the searches for relevance, and then assessed the potentially relevant records subsequently retrieved as full texts for inclusion. Disagreements were resolved by discussion or, if necessary, taken to a third reviewer (JP).
Eligibility criteria
Studies were included if they fulfilled the following criteria:
-
They primarily set out to determine the association of at least one time interval to diagnosis or treatment with patient outcomes.
-
They included symptomatic adult patients with primary cancers (excluding screening- and biomarker-detected cancers).
-
They investigated at least one diagnostic interval (patient, primary care, or a combination), which could be assessed against accepted definitions (Aarhus statement92). Studies that investigated the impact of time from diagnosis to treatment were included only if they also reported data on pre-diagnostic time. (The diagnostic pathway begins at the time of symptom onset and ends at the point of definitive diagnosis or treatment, whereas the interval between diagnosis and treatment falls within the treatment pathway and generally relates to secondary care only.)
-
They reported data on survival, morbidity, or stage at diagnosis.
-
They were available as a full-text paper in English.
Studies comparing two or more diagnostic or referral pathways (e.g. NICE 2WW referrals vs. non-urgent referrals) were included only if (overall) group-level numeric values for each interval (see Figure 2) were reported, that is the study also assessed the impact of different intervals on patient outcomes, irrespective of the referral pathway.
Data extraction
Data extraction was undertaken by one reviewer (RL) and checked by another (JP). For studies of CRC, this included data on a study’s aims; design; population; location; setting; number of participants sampled and, subsequently, recruited and analysed; definitions of time duration; data collection methods; outcome measures used; and the authors’ conclusions. The main results were also extracted, including the methods used for assessing the association between interval and outcomes, statistical significance, CIs and any subgroup analysis. A more condensed summary was extracted for studies of other cancer sites.
Critical appraisal
The methodological quality of each CRC study included was assessed using the same bias assessment tool used in the previous review25 (see Appendix 4, Box 1). Bias assessment was undertaken by two independent reviewers (RL and JP); there were no disagreements that needed be taken to a third person.
Waiting-time paradox
The risk-of-bias assessment also included identifying studies that addressed the ‘waiting-time paradox’, as they are likely to be of better analytical quality.
The observed association between a short diagnostic interval and poor outcomes has been referred to as the ‘waiting-time paradox’. Two underlying theories have been proposed to explain this effect. One widely held belief is confounding by severity of disease, whereby high-risk precursors, such as aggressiveness of the tumour, act as unmeasured confounders that mask the effect of the exposure. 99 The underlying assumption here is that rapidly growing or more aggressive tumours are more likely to present with alarm symptoms that will make the patient or doctor think of cancer, whereas slow-growing or less well-differentiated tumours result in vague initial symptoms that are difficult to detect. 99 To help mitigate this bias, information is required on factors associated with the aggressiveness of the tumour, such as histology, grade, stage, tumour volume, genetic factors. The alternative assumption is that the paradoxical findings reflect confounding by indication, caused by differentiated clinical triage. 99 The assumption here is that GPs expedite patients presenting with high-risk symptoms, especially if they look ill, and at the same time are more reluctant to refer healthy-looking people with low-risk symptoms. 99 To help mitigate this bias, information is required on what triggers the GP to either refer immediately or adopt a watchful waiting approach. 99
In the previous systematic review, studies that addressed the waiting-time paradox were defined as:
. . . articles that undertake an analysis or sub-analysis that specifically includes or excludes patients who are either diagnosed very quickly (e.g., within 4–8 weeks, although this will vary between cancers), or have very poor outcomes (e.g., deaths within a short time after diagnosis, e.g., within 4–8 weeks).
Neal et al. 25
Papers that simply reported that the waiting-time paradox may have confounded their data were not classified as having addressed this potential source of bias. In the current updated review, we recorded whether or not studies had performed adjusted analyses for aspects such as emergency presentation, severity of presenting symptoms, tumour grade or aggressiveness. This is discussed in more detail in Colorectal cancer.
Data synthesis
A narrative synthesis was undertaken, which followed the same approach as that used in the previous review. 25 Overall findings were presented in a table format, with studies grouped by cancer location. Information reported included type of diagnostic interval, and whether the analysis considered patient outcomes, such as survival or stage of disease, or other outcomes. A key output was to identify the association between reduced diagnostic interval and improved patient outcome. This information fed directly into the decision-analytic model and permitted an exploration of the variation in findings from different studies. The previous review25 identified a large degree of variability between included studies.
In the table of overall findings, studies that reported ‘positive’ associations (i.e. there was evidence of shorter intervals being associated with more favourable outcomes) were presented first. Studies that reported no associations were reported next. Studies that reported ‘negative’ associations (i.e. there was evidence of shorter intervals being associated with less favourable outcomes) were reported at the end of the table. Within each grouping, studies were ordered by the patient outcomes: survival or mortality first (for simplicity, both are referred to as survival in the table), followed by stage and then ‘other’ outcomes.
Figure 2 summarises the definitions that were used to categorise the different time (T) durations evaluated by included studies. Each duration was categorised as one of 15 intervals.
Data synthesis
The results of the current updated review are first presented for all cancers using the same table format as in the previous review. 25 They are followed by more in-depth evaluation for CRC. No meta-analyses were planned or conducted as a result of the (anticipated) heterogeneity: the review of CRC therefore focuses on identifying studies that are likely to provide the best estimate of the impact of diagnostic intervals on patient outcomes for informing the decision-analytic model.
The CRC review incorporates studies identified during our updated review and better-quality studies identified during the previous review. We focus only on more recent or better-quality studies as they are considered to provide more valuable information for our decision model for the following reasons:
-
They are more likely to account for the non-linear association between time to diagnosis and stage or survival.
-
They are more likely to account for the waiting-time paradox, whereby patients with more advanced or aggressive disease are inclined to present earlier and have definitive symptoms, or are prone to be referred quickly and prioritised for treatment.
-
They use clearer definitions of diagnostic intervals, following the publication of the Aarhus consensus statement in 2012. 92
Results
Studies identified
Our searches identified 1810 references after de-duplication, of which 104 were considered relevant and retrieved as full-text studies. Thirty-five studies were identified that met the inclusion criteria (Figure 3). This includes one study for which the interlibrary loan was still outstanding at the time of the report; therefore, the data extraction is based on an abstract. This study122 included patients with well-differentiated neuroendocrine tumours.
FIGURE 3.
Updated review: the PRISMA flow diagram.
A summary of the included studies is provided in Appendix 4 (see Table 51). Two studies considered multiple cancer sites, whereas the remaining studies evaluated a sole cancer: bladder (n = 1), breast (n = 4), colorectal (n = 8), lung (n = 5), lymphoma (n = 1), myeloma (n = 1), neuroendocrine (n = 1), oral (n = 2), ovarian (n = 2), pancreatic (n = 2), penile (n = 1), prostate (n = 1), sarcoma (n = 3) and testicular (n = 1). The studies were from a wide range of countries. Eight studies were undertaken in the UK, five in Canada and four in Spain; two studies were undertaken in each of the following countries: the USA, the Netherlands, Mexico and Japan; and one study was carried out in each of the following countries: China, France, Islamic Republic of Iran, Israel, Italy, Montenegro, Poland, Rwanda, Sri Lanka and Uganda.
Findings of the previous review
The previous review25 identified a large number of relevant studies (n = 209), with only a small number considered to be of high quality. 25 There was substantial variation in the type of intervals evaluated and the findings within and between cancer types. Compared with other cancer types, studies of colorectal, breast, head and neck, and testicular cancers and melanoma suggested that shorter time intervals were associated with improved patient outcomes. However, for each of these cancer types, there were also studies reporting no association between time interval and patient outcome. Some of the included studies also showed that shorter intervals were associated with decreased survival. This important heterogeneity is likely to have been caused by confounding, as discussed in Waiting-time paradox. A summary table of the previous review findings is provided in Appendix 4 (see Table 58).
Findings of the updated review
A summary of the findings of the 35 new studies included in the updated review is presented in Table 11, using the same format as used in the original review. Some studies assessed the impact of longer intervals or perceived delay on patient outcomes, which we converted to represent the opposite effect associated with shorter intervals. The original interpretation of the results and how each was converted to show the corresponding impact of short intervals are presented in Appendix 4 (see Table 53). Some of the studies that evaluated the impact of an interval (e.g. T1) as a categorical variable (e.g. 1–2, > 2–3 and > 3–4 months) reported statistically significant findings for some intervals and not others, the results of which are also shown in Appendix 4 (see Table 53).
| Interval and outcome measurea | Time | Study | ||
|---|---|---|---|---|
| Positive association | No association | Negative association | ||
| Breast cancer | ||||
| Survival | ||||
| Patient interval | T1 | Ángeles-Llerenas 2016100 | ||
| Symptom onset to treatment | T5 | Ángeles-Llerenas 2016100 | ||
| Diagnostic interval | T8 | bRedaniel 2015101 | ||
| Primary care to treatment | T9 | Murchie 2015102 | ||
| Treatment interval | T15 | Ángeles-Llerenas 2016100 | ||
| Stage | ||||
| Patient interval | T1 | Pace 2015103 | ||
| Symptom onset to specialist care | T3 | Unger-Saldaña 2015104 | ||
| Diagnostic interval | T8 | Pace 2015103 | ||
| Seen in primary care to treatment | T9 | Murchie 2015102 | ||
| From specialist care to treatment | T14 | Unger-Saldaña 2015104 | ||
| Bladder cancer | ||||
| Survival | ||||
| Symptom onset to referral | T2 | Bryan 2015105 | ||
| Symptom onset to treatment | T5 | Bryan 2015105 | ||
| Referral to first seen by specialist | T10 | Bryan 2015105 | ||
| Referral to treatment | T12 | Bryan 2015105 | ||
| Specialist care to treatment | T14 | Bryan 2015105 | ||
| Stage | ||||
| Symptom onset to referral | T2 | Bryan 2015105 | ||
| Symptom onset to treatment | T5 | Bryan 2015105 | ||
| Referral to seen by specialist | T10 | Bryan 2015105 | ||
| Referral to treatment | T12 | Bryan 2015105 | ||
| Specialist care to treatment | T14 | Bryan 2015105 | ||
| Other (tumour size) | ||||
| Referral to treatment | T12 | Bryan 2015105 | ||
| Specialist care to treatment | T14 | Bryan 2015105 | ||
| CRC | ||||
| Survival | ||||
| Primary care to treatment | T9 | Helewa 2013;106 Murchie 2014107 | ||
| Diagnostic interval | T8 | bRedaniel 2015;101 Dregan 2013108 | ||
| Symptom onset to diagnosis | T4 | Pita-Fernández 2016109 | ||
| Referral to treatment | T12 | Aslam 2017110 | Patel 2018111 | |
| Stage | ||||
| Patient interval | T1 | Chen 2017 (≥ 50 years)112 | Chen 2017 (< 50 years)112 | |
| Symptom onset to diagnosis | T4 | Chen 2017 (≥ 50 years);112 Leiva 2017113 | Chen 2017 (< 50 years)112 | |
| Diagnostic interval | T8 | Chen 2017;112 Leiva 2017 (GP records)113 | Leiva 2017 (hospital records)113 | |
| Primary care to treatment | T9 | Murchie 2014107 | ||
| Referral to treatment | T12 | Patel 2018111 | ||
| Other (metastases or nodal involvement) | ||||
| Referral to diagnosis | T11 | Janssen 2016114 | ||
| Gastro-oesophageal cancer | ||||
| Survival | ||||
| Diagnostic interval | T8 | bDregan 2013108 | ||
| Lung cancer | ||||
| Survival | ||||
| Patient interval | T1 | Radzikowska 2013 (SCLC);115 Živković 2014116 | ||
| Symptom onset to specialist care | T3 | Gonzalez-Barcala 2014117 | ||
| Symptom onset to diagnosis | T4 | Živković 2014116 | ||
| Diagnostic interval | T8 | bDregan 2013108 | bRadzikowska 2013 (SCLC);115 Redaniel 2015101 | |
| Referral to diagnosis | T11 | Gonzalez-Barcala 2014117 | ||
| Specialist care to treatment | T14 | Gonzalez-Barcala 2014117 | ||
| Treatment interval | T15 | Gonzalez-Barcala 2014117 | ||
| Stage | ||||
| Specialist care to diagnosis | T13 | Gildea 2017118 | Kim 2016 (NSCLC)119 | |
| Specialist care to treatment | T14 | Kim 2016 (NSCLC)119 | ||
| Treatment interval | T15 | Kim 2016 (NSCLC)119 | ||
| Lymphoma | ||||
| Survival | ||||
| Primary care to specialist | T7 | Nikonova 2015120 | ||
| Specialist care to treatment | T14 | Nikonova 2015120 | ||
| Myeloma | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Goldschmidt 2016121 | ||
| Stage | ||||
| Symptom onset to diagnosis | T4 | Goldschmidt 2016121 | ||
| Neuroendocrine tumours | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Keizer 2016122 | ||
| Stage | ||||
| Symptom onset to diagnosis | T4 | Keizer 2016122 | ||
| Oral cancer | ||||
| Stage | ||||
| Patient interval | T1 | Alahapperuma 2017123 | ||
| Referral to seen by specialist | T10 | Alahapperuma 2017123 | ||
| Symptom onset to treatment | T5 | Esmaelbeigi 2014124 | ||
| Ovarian cancer | ||||
| Stage | ||||
| Patient interval | T1 | Lim 2016125 | ||
| Diagnostic interval | T8 | Altman 2017126 | Lim 2016125 | |
| Pancreatic cancer | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Gobbi 2013127 | ||
| Symptom onset to treatment | T5 | Jooste 2016128 | ||
| Penile cancer | ||||
| Survival | ||||
| Patient interval | T1 | Gao 2016129 | ||
| Stage | ||||
| Patient interval | T1 | Gao 2016129 | ||
| Other (tumour size; metastases; lymph node involvement) | ||||
| Patient interval (tumour size) | T1 | Gao 2016129 | ||
| Patient interval (metastases) | T1 | Gao 2016129 | ||
| Patient interval (lymph node involvement) | T1 | Gao 2016129 | ||
| Prostate cancer | ||||
| Stage | ||||
| Diagnostic interval | T8 | Bonfill 2015130 | bRedaniel 2015101 | |
| Treatment interval | T15 | Bonfill 2015130 | ||
| Sarcoma | ||||
| Survival | ||||
| Symptom onset to specialist care | T3 | Urakawa 2015131 | ||
| Symptom onset to diagnosis | T4 | Goedhart 2016132 | ||
| Referral to diagnosis | T11 | Goedhart 2016132 | ||
| Stage | ||||
| Patient interval | T1 | De Boer 2014133 | ||
| Other (metastases) | ||||
| Symptom onset to diagnosis | T4 | Goedhart 2016132 | ||
| Testicular cancer | ||||
| Survival | ||||
| Patient interval | T1 | Kobayashi 2014134 | ||
| Stage | ||||
| Patient interval | T1 | Kobayashi 2014134 | ||
| Other (tumour size) | ||||
| Patient interval | T1 | Kobayashi 2014134 | ||
| Urinary tract cancer | ||||
| Survival | ||||
| Diagnostic interval | T8 | bDregan 2013108 | ||
A larger number of studies identified during the updated review reported non-statistically significant findings or negative association between shorter intervals and patient outcomes. The potential of a ‘U’-shaped association between time intervals and outcomes is explored in more detail in Colorectal cancer.
Colorectal cancer
The more in-depth summary for CRC presented here focuses on the 10 studies identified in our updated review (see Table 11 for overall findings; this included two studies that considered multiple cancer sites) and four good-quality studies (published between 2011 and 2013) identified in the previous review that addressed the waiting-time paradox. The broader findings presented in the previous review,25 and other previous systematic reviews by Ramos et al. 94,95 and Thompson et al. ,96 are considered to provide a good summary of the wider (prior) evidence base.
A more in-depth data extraction was conducted for the 14 included CRC studies. The results are provided in Appendix 4, presented over three tables summarising each study’s characteristics (see Table 55), critical appraisal (see Table 56) and results (see Table 57). These results tables include a summary of both the magnitude and the direction (and statistical significance) of the associations between intervals and patient outcomes for each included study.
The following section presents the overall findings for the CRC studies, and an evaluation of the included studies that was conducted to aid the selection of specific studies to inform the decision model.
The overall findings
Five studies were undertaken in the UK, three in Denmark and two in each of the USA, Canada and Spain. Six studies were based in primary care, six in secondary care (including specialist centres) and two in both primary and secondary care. Four of the studies undertaken in secondary care were of single sites (hospitals). The three studies from Demark were by the same authors (i.e. Tørring et al. 99,135,136) and included the same or overlapping study populations. Tørring et al. 136 focused solely on CRC, included 268 participants and data on estimated 3-year survival. Tørring et al. 135 analysed data from three population-based studies, one of which was Tørring et al. 136 Tørring et al. 99 evaluated the impact of diagnostic intervals in five common cancers, including CRC. The colorectal cohort for this study appears to be identical to that of Tørring et al. 136 (n = 268), but the analysis is based on 5-year survival. As our review is based on a narrative synthesis, all three studies99,135,136 are included and described below.
The only interval to be evaluated by more than two studies was T8, which is the ‘diagnostic interval’ (time from first seen in primary care to diagnosis). The impact of the diagnostic interval on survival was evaluated by six studies,99,101,108,135–137 one of which137 evaluated colon and rectal cancers separately, and another of which136 stratified analysis by whether the presenting symptoms were considered by the GP as alarm and/or serious versus vague. Four studies99,135–137 found that longer intervals were associated with statistically significant poorer survival outcomes: one137 in colon cancer only, and one136 for alarm and/or serious symptoms only. The overall effect sizes that were reported by the studies (see Appendix 4, Table 57) were small, except for Tørring et al. ,135 which reported a medium size effect (odds ratio of 4.74) for the association between a very short interval and survival (Tørring et al. 135,136 also reported wide CIs). The diagnostic interval was analysed as both a categorical and a continuous variable in all three studies by Tørring et al. ,99,135,136 whereas it was evaluated as a dichotomised variable by Pruitt et al. 137 The diagnostic interval, when analysed as multiple categorical variables, was divided into three groups, based on the interval quartiles. The middle-interval group, which included both the second and the third quartiles combined, was used as the reference. The findings of the categorical analysis were statistically significant for the very-short-interval group in both Tørring et al. 136 (alarm or serious symptoms only) and Tørring et al. ,99 whereas Tørring et al. 135 reported significant results for both the very short- and the long-interval groups (see Appendix 4, Table 57). Tørring et al. 135 specifically aimed to test the theory of a U-shaped association between the diagnostic interval and mortality after diagnosis using data from three population-based studies. 81,136,138 The analysis of the combined data, from all three studies,81,136,138 and the analysis of the data from the study based on GP data138 showed that both the shorter- and the longer-interval groups were associated with a statistically significant increased risk of mortality at 5 years. 135 In all three studies by Tørring et al. ,99,135,136 the analysis and the graphical display of the diagnostic interval assessed as a continuous variable had a convex (U-shaped) association with mortality (these trends were statistically significant in Tørring et al. 99,135). In other words, patients with very short or very long intervals had higher mortality than the rest. However, for patients presenting with vague symptoms,136 there was a trend towards a concave association between diagnostic interval and mortality, but the results were not statistically significant and lacked power.
Two studies112,113 assessed the impact of the diagnostic interval (T8) on stage at diagnosis. Leiva et al. 113 measured the interval using two separate data sources (GP and hospital records); Chen et al. 112 reported subgroup analysis according to age at diagnosis (< 50 and ≥ 50 years). Leiva et al. 113 found that shorter intervals were associated with more advanced cancer stage at diagnosis, based on data from the hospital records only. Chen et al. 112 and Leiva et al. 113 also assessed the association between other intervals and stage at diagnosis, including duration between symptom onset and diagnosis (T4) (both studies) and the ‘patient interval’ (T1) (Chen et al. 112 only). Among younger patients (aged < 50 years), those with stages III or IV cancer had statistically significantly shorter durations for both T4 and T1 than those with stages I or II disease. One study109 evaluated the association between T4 and survival, and found that longer symptoms-to-diagnosis intervals were not associated with poorer survival (or advanced stage) in CRC patients (see Appendix 4, Table 57).
Two further interval types were each investigated by two studies: T9106,107 and T12. 110,111 Only Patel et al. 111 found one of these intervals (T12) to be associated with statistically significant findings: long-term survival and the proportion of patients with early-stage cancer were greatest in patients who did not receive treatment within the recommended 62 days (see Appendix 4, Table 57).
The intervals T11 (between referral to a specialist care and diagnosis)114 and T15 (between definitive diagnosis and treatment)137 were both evaluated by a single study. Janssen et al. 114 did not find shorter intervals to be associated with an increased likelihood of having distant metastases or a node-positive tumour. Pruitt et al. 137 found that shorter treatment intervals were associated with better survival in colon, but not rectal, cancer.
Summary of the methods used
Non-linear association
Tørring et al. 99,135,136 showed that the association between diagnostic interval and patient outcomes for CRC is U-shaped, with both very short and long intervals being associated with poor outcomes. 99,135,136 Five included studies used spline regression analysis to account for this non-linear relationship. 99,107,109,135,136 In the Murchie et al. 107 study, the spline curves for the unadjusted analyses showed a statistically significant non-linear association between time from being seen in primary care and treatment (T9, which the authors refer to as ‘provider delay’107) and both stage and mortality, but these were no longer present after adjusting for confounders. Pita-Fernández et al. 109 presented spline regression analyses for colon and rectal cancers, which showed very short symptom-to-diagnosis (T4) intervals to be associated with higher mortality in those with rectal tumours. In colon cancer, no significant relationship was found between interval and survival. In addition to analysing the interval as a continuous variable, the study computed Kaplan–Meier curves for each interval quartile. The Cox regression model, adjusting for age and sex, showed that patients with cancer of the rectum in the first interval quartile had statistically significant lower survival than the rest, but this was no longer significant when controlling for stage; no significant difference was found for colon cancer.
Waiting-time paradox
Six studies99,101,107,108,135,136 collected data on patients seen in primary care. Four studies99,101,136,137 conducted a stratified analysis according to the GP’s perceived seriousness of the presenting symptoms. Two studies107,113 included the perceived seriousness (vague, serious, alarm) of the presenting symptom,113 and/or specific (high-risk) symptoms107,113 as covariates in their multivariate analyses. One study,108 which evaluated the time intervals between specific alarm symptoms and subsequent cancer diagnosis, also compared the survival of patients with alarm symptoms with that of patients without alarm symptoms. One study137 stratified diagnostic delay models by the four most common presenting symptom types as part of their sensitivity analysis, but did not present the results, noting only that these did not substantially change the findings.
A number of studies controlled for the confounding effect of tumour grade or aggressiveness. Potential important confounding factors include tumour stage, tumour grade, degree of differentiation, type of hospital admission (emergency or elective), symptoms and signs. Five studies101,106,109,113,137 used multivariate analysis that accounted for one or more of these factors. Six studies also conducted stratified analysis according to stage,137 emergency admission106,110,111 or presenting symptoms (either type of or perceived seriousness). 99,101,137 Emergency admission was also considered as an exclusion criterion in two studies. 101,114 However, some argue that stage at diagnosis can be conceived as a mediator or intermediate factor (longer delays cause more advanced disease, and more advanced stages are associated with poorer survival), which means that adjusting for tumour stage will introduce spurious confounding (see Pita-Fernandez et al. 109). However, Pruitt et al. 137 found that patients with longer diagnostic delays had earlier-stage disease, which they state is refuting the common assumption that stage is an intermediate factor in the causal chain between diagnostic delay and survival. A simpler explanation might be that the first symptom was not from the cancer, and the diagnostic interval has been artificially inflated. 139 However, the findings could also be a simple reflection of the fact that the association between diagnostic delay and survival is seriously confounded as an estimate of causal effect.
Clear definitions of diagnostic intervals
All included studies provided clear definitions of the intervals that they were evaluating. However, only one study99 defined its interval according to the Aarhus statement. 92 Two previous studies135,136 conducted by the same authors were published prior to the Aarhus statement. 92 Four studies101,107,109,137 used definitions similar to those reported in the Aarhus statement,92 and all but one study101 referred to the time period as provider, diagnostic, or treatment ‘delay’, rather than using the term ‘interval’, which the Aarhus statement92 recommends. Three studies110,111,114 used definitions that reflect a national guideline target. In one study,113 which compared the use of three different data sources, the defined TDI could have been interpreted differently according to whether the data were obtained from the hospital records, GP records or patient questionnaires.
Lead time bias or immortal time bias
The previous review25 recommended the use of survival as the ‘gold-standard’ outcome measure. Methodological issues to consider when interpreting the findings include the potential effect of either lead time bias or immortal time bias. Lead time bias may be present when early detection advances what would have been the original date of diagnosis to an earlier point in time, while not necessarily delaying a patient’s time of death. 99 It has been suggested that, when studying diagnostic delay, survival time should ideally be measured from the date of first symptom,93,94 rather than the date of diagnosis, to overcome lead time bias. 99 This was done by one study;107 however, it may not always be possible, particularly when using retrospective analyses of administrative data. 137 Furthermore, calculating survival time from the onset of symptoms includes a period before the occurrence of the defined exposure of interest (i.e. diagnosis), which may, in turn, create an immortal time bias. 99 One study135 used data obtained from three previous studies,81,136,138 one of which collected data on date of symptom onset using patient interview questionnaires. 138 To account for ‘immortal person-time’ in the patient-based study, Tørring et al. 135 specified delayed entry (left truncation) from the date of the interview. Pruitt et al. 137 used a case–control design to try to mitigate both these biases. Cases (deaths due to CRC) and controls (deaths due to other causes or censored) were matched on survival time, and the association between delay and death was examined using logistic regression. 137
Survival as a function of age
Tørring et al. 99 adjusted for age at diagnosis, which they state would ensure that any increase in mortality is not a natural function of becoming older. Most of the studies included age in their multivariate analysis, but this was incorporated as a simple binary measure in some studies (≤ 69 vs. > 69 years,108 ≥ 70 vs. < 70 years106 and < 65 vs. ≥ 65 years113). Four studies adjusted for age categorised as multiple groups: 18–59, 60–74 and ≥ 75 years;99 < 50, 50–60, 60–70, 70–80 and > 80 years;109 ≤ 65; 66–69, 70–74, 75–79, 80–84 and ≥ 85 years;137 and 15–44, 45–54, 55–64, 65–74 and ≥ 75 years. 101 One study107 included age as a continuous variable.
Further potential sources of bias or confounding
Most studies were retrospective and set in linked databases. Other data sources for interval data or timing of events were GP questionnaires, patient records and interviews. Potential limitations include recall bias and validity and accuracy of the data. Another consideration is the accuracy of the data collection conducted as part of the study. The use of multiple sources for the same data is one approach to augment or validate the data. Janssen et al. 114 reported that two investigators independently reviewed a subset of subject data to obtain a measure of interobserver agreement. No other study reported doing this. No other study reported using a process to account for the accuracy of interval classification. Some studies did report excluding cases with missing data or discrepant data for calculating the intervals.
The 2011 study by Tørring et al. 136 relied on the GPs retrospectively ascribing a date to relevant milestones on the diagnostic pathway of each patient. The authors acknowledge that these dates may have been affected by differential information bias, because of the GPs’ extensive knowledge of their patients. The subsequent (2012) study by Tørring et al. 135 was based on three cohort studies that used different sources to ascertain the date of first presentation: GP questionnaires (Tørring et al. 136), interviewer-administered patient questionnaires (Korsgaard et al. 138) and primary care records (Hamilton et al. 81). Leiva et al. 113 also compared data on intervals identified using three different sources of information (patient interviews, hospital records and GP records), but there was heterogeneity in the types of intervals studied.
Another potential issue that has implications for decision-analytic modelling is that some studies have shown that rectal and colon cancers have differing results, suggesting that these two should not be analysed together. 137 Only Tørring et al. 135 adjusted for the separate cancer sites.
Discussion
Summary of the findings
The current review updates the findings of a previous systematic review25 that examined the association between different durations of time from first symptom to diagnosis or treatment and clinical outcomes across all major cancers. Summary details of included studies are presented, along with an overall assessment of whether or not each study reported an association between shorter times to diagnosis and a more favourable outcome. A more in-depth evaluation was conducted of CRC studies to inform the decision-analytic model. Summary details of included studies, including their results, are presented in structured tables in Appendix 4. No meta-analyses were undertaken because of heterogeneity, which included variability and how intervals were assessed. The findings of some of the more recent studies indicate that the relationship between diagnostic interval and patient outcomes is likely to be U-shaped, with both very short and long intervals associated with poor outcomes. The review also identified important biases and other factors that may affect the findings of studies in this field.
Existing reviews of colorectal cancer
Previous systematic reviews conducted by Ramos et al. 94,95 found no association between delays and stage at diagnosis95 or survival94 for CRC. The initial review by Ramos et al. 94 included 26 studies, 20 of which showed no associations between delay and survival. In contrast, four studies showed that the delay was a factor contributing to better prognosis, and two showed that it contributed to poor prognosis. 94 The detailed literature review by Thompson et al. 96 also found no strong theoretical basis for a benefit from earlier diagnosis of symptomatic bowel cancer. Five studies were identified that demonstrated improvement in survival due to early diagnosis, and 16 studies, paradoxically, showed worse outcomes associated with prompt treatment. The authors also acknowledged that it was difficult to draw firm conclusions from observational studies because of the potential biases. The overall findings of these reviews may be due to the apparent U-shaped association between the interval from first symptom to diagnosis or treatment and patient outcomes in CRC.
Strengths and limitations of the updated review
The original review,25 which we have updated, was the first systematic review to evaluate the impact of any pre-diagnostic interval on patient outcomes in any cancer. This meant that the review included a very large number of heterogeneous studies. As a result, only a broad narrative synthesis was undertaken, with the overall findings for all cancer sites presented in a summary table, similar to Table 11. The current review focused on providing an update of the previous review’s overall findings, and also emphasised whether or not studies found statistically significant findings, and the direction of the effects of any such findings. The synthesis of both the previous and the current review essentially represents a ‘vote counting’ process, and does not take into account the magnitude of the effect (or any potential U-shaped association).
In view of the fact that only a narrative synthesis was feasible, and that the association between the interval T8 and the outcome survival was the only association to be evaluated by more than two studies, the current review of CRC studies focused on providing an assessment of the methodology of included studies; the summary results of individual studies are presented in Appendix 4.
One of the main limitations of relying on statistical significance to identify relevant associations is that it ignores studies that lack sufficient power to detect small differences. One of the main advantages of a meta-analysis is that it can improve power by synthesising the findings of multiple studies. Very few included CRC studies assessed the associations between the same intervals using the same outcome measure to allow any meta-analysis, and, when they did, they varied in the approach used to analyse the data; whether the interval was considered as a continuous, categorical or dichotomised variable; and the selected cut-off points. However, even if a sufficient number of homogeneous studies were available, accounting for the potential U-shaped association in a meta-analysis is not trivial. The intervals would need to be considered as a categorical or continuous variable, which may not be reported in the primary studies.
Another important limitation of the updated review is the likelihood of selective outcome reporting. Some studies reported evaluating multiple intervals but did not provide the outcome data for all of them. It is also likely that some studies did not report all the intervals they evaluated, and reported the findings for selected intervals only. Selective reporting may have been a particular issue with studies that incorporated a broader aim of evaluating more than just the association between timey diagnoses and favourable outcomes; for example, they may also have aimed to describe the diagnostic journey and identify factors associated with delay.
The literature searches for the original review identified > 193,077 references. Our updated review represents a supplementary review that was conducted to inform the decision model, and needed to be completed in a timely manner. The impracticality of screening thousands of references in a timely manner when updating any systematic reviews means that new efficient methods of undertaking this process are needed. Our updated review used an innovative and pragmatic method for searching the literature, which can contribute to the future development and availability of such methods. We tested the appropriateness of the new approach by applying it to the second phase of the previous review25 and comparing the findings with those achieved in the original literature searches. Our novel approach did not capture all the relevant studies in this test. However, some of this may be because of the refinement in the inclusion criteria that occurred between Phases I and II of the original review. It is therefore unclear if the current review has missed any important studies using these pragmatic search methods. Further work is needed to refine and test this new searching approach.
The original systematic review25 was undertaken in two phases: an initial review was conducted in 2010 and a subsequent review undertaken in 2013 covering the literature published since 2010. Both phases were published as a single review in 2015. 25 The original 2010 phase did not include a review of CRC studies, as there were existing systematic reviews by Ramos et al. 94,95 (one in 200794 for survival outcomes and another in 200895 for stage) for this cancer site. This means that the previous review25 included primary studies for CRC published only after 2010. The 2007 Ramos et al. 94 review identified 12 studies that they reported as having used multivariate analysis to evaluate the association between diagnostic or therapeutic intervals and survival. However, the authors did not report which studies these were; therefore, we were unable to consider these studies for inclusion in our review as studies addressing the waiting-time paradox.
Conclusion
The overall findings of the updated review highlight the uncertainty in the evidence base for the extent to which reducing times to diagnosis and/or treatment leads to improved patient outcomes. There is still a lack of firm evidence to demonstrate an association between timely diagnosis and favourable outcomes in those with CRC. The evidence indicates a U-shaped relationship, but also reflects considerable heterogeneity in terms of the methods and intervals evaluated, and highlights some important methodological challenges in evaluating whether or not timely diagnosis leads to better outcomes. The findings support the need to expedite the diagnosis of symptomatic patients and to work to prevent the preventable delays that some patients continue to experience.
Chapter 6 Data for informing the economic decision model
Objective
The economic decision-analytic model explored the uncertainties regarding the clinical effectiveness and cost-effectiveness of the tools to aid cancer diagnosis decision-making in primary care in the NHS. Given the scope of the current study, it was not feasible to model the impact of diagnostic tools for all common cancer types for which diagnostic tools exist. Instead, at the outset, we chose to model one common cancer, CRC, to illustrate a case for which we believe there are good a priori reasons why diagnostic tools in primary care could affect patient outcomes and may be effective and cost-effective. CRC is among the cancers with the shorter diagnostic intervals (31–60 days); cancers with longer diagnostic intervals, for example bladder cancer (61–91 days) and lung cancer (91–120 days), would have greater scope for diagnosis. If clinical effectiveness and cost-effectiveness results are favourable for CRC, the modelling approach could be extended to other cancers, but this is beyond the scope of the current study.
We will use the decision-analytic model to link empirically demonstrated impacts on short- and medium-term outcomes to the effects of treatment, to estimate the impact on longer-term outcomes. In particular, we will explore the potential impacts on health service resource use, costs and patient outcomes in relation to CRC. This chapter focuses on the sources of information from previous chapters, a methodological review of the decision-economic models on CRC that have been published and the data for populating the decision-analytic model.
Sources of information
The decision-analytic model is informed by the findings from SR1 (clinical effectiveness of the diagnostic tools), the accuracy of the diagnostic tools (identified in SR2), and by the updating of a previous systematic review25 which provides evidence on the impact of reducing time to cancer diagnosis and/or treatment on patient outcomes. Relevant studies were those that evaluated tools in UK primary care patients, and reported outcomes associated with the use of the tools in terms of referral, diagnostic or treatment intervals, stage at diagnosis, patient health-related quality of life or survival.
The model is also informed by a review of decision-analytic models that sought to identify the strengths and limitations of previous models and quality of evidence on parameter values for resource use, costs and utilities for populating a decision model of primary diagnostic strategies for suspected cancers. Because modelling the use of the diagnostic tools required a CRC disease model to translate diagnostic outcomes into clinical outcomes following treatment, and existing studies of CRC screening were known to us that included whole disease history model for the UK, we extended the scope of our review of economic studies to include the population of asymptomatic patients so that relevant disease models could be identified.
Summary of findings for the decision-analytic model
Systematic review 1
Two studies from SR1 were relevant for the CRC decision-analytic model: Hamilton et al. 13 and Emery et al. 35
In the study by Hamilton et al. ,13 seven English cancer networks were selected for using the RAT (mouse mat or desktop flip chart versions) for colorectal and lung cancer, to provide the underdiagnosed cancer risk estimates in individuals aged > 40 years presenting to primary care with symptoms. The authors13 evaluated the changes of 2WW referrals and request of colonoscopies by GPs for the two 6-month periods before and after the distribution of the RAT. A total of 614 GPs from 165 practices were recruited. The RAT for CRC was used and completed 1521 times, and was associated with increases of 26% (from 1173 to 1477) and 15% (from 1762 to 2032) in 2WW referrals and colonoscopies performed, respectively; these increases resulted in 10 new additional cases identified (from 134 to 144). Unfortunately, the authors13 were unable to distinguish which of the additional 270 colonoscopies were ordered for patients for whom the RAT was used. However, the number of 2WW, routine and urgent referrals reportedly accounted, respectively, for 49% (702/1433), 12.7% (182/1433) and 10% (144/1433) of patients evaluated with the CRC RAT.
The study by Emery et al. 35 used a 2 × 2 factorial cluster RCT design in rural Western Australia. The objective of the study was to measure the effect on the TDI of a community-based symptom awareness and general practice-based educational interventions for individuals presenting with symptoms of breast, prostate, colorectal or lung cancers. For the decision model, the intervention relevant is the one at GP level, which consisted of a GP resource card with symptom risk assessment charts (RAT) and local cancer referral pathways in which the primary outcome was the duration of the TDI. As mentioned in SR1, for colorectal cancer, the authors did not find statistically significant differences in the median TDI (GP-based intervention, n = 124; GP control, n = 122) or in the log-transformed (ln) mean difference (0.3, 95% CI –0.51 to 0.45; p = 0.42) for the GP-based intervention versus control, nor did they find it when the factorial design was analysed by tumour group or subintervals of the TDI.
Critique of identified data
It is important to note that the data on the diagnostic accuracy for the two tools for which relevant evidence was identified, QCancer and RAT, are derived from low-risk patient populations, which NICE has previously defined as constituted by those patients presenting to primary care without symptoms requiring 2WW referral and whose symptoms place them in the positive predictive value (PPV) range of 0.1–3.0% risk band. 140 The diagnostic accuracy values for QCancer were estimated from a sample whose prevalence, as determined by a recorded diagnosis of CRC over the 2-year follow-up period after the first appearance of symptoms, was 0.16% after excluding the red-flag symptoms, in a sample of 1,223,192 patients.
The prevalence over which the diagnostic accuracy of the RAT was estimated could not be calculated from the available report. 141 However, the recommended use of the tool by its developers is for patients presenting with signs and symptoms that have a PPV of > 2% to follow the 2WW referral pathway, those with symptoms having a PPV of 1–2% to be referred for primary care investigations and those with signs and symptoms having a PPV of < 1% to be managed in primary care using the GP’s clinical judgement. When patients with signs and symptoms with a PPV of ≥ 3% are excluded because they are high risk, the resulting prevalence in patients with all the symptoms considered by the RAT should fall between 0% and 3%. This would be consistent with NICE guideline6 definitions of a low-risk population of symptomatic patients presenting to primary care, and reflect the range of risk underlying the reported sensitivity and specificity values for the RAT (Hamilton141).
Systematic review 2
Table 12 summarises the relevant evidence on predictive accuracy of externally validated models in UK patients, as described in Chapter 4. Of the four models, one reports no diagnostic accuracy data (the Netherlands model55), and another reports no specific diagnostic threshold to define positive findings (the BB equation22). A third model has no reported diagnostic accuracy estimates in UK patients at a specific threshold (the RAT74). However, these data are available from a doctoral thesis dissertation, identified after SR2 was completed (William Hamilton, University of Exeter, 2019, personal communication), in which sensitivity of 69% and specificity of 77% are reported for a score of 35, corresponding to a threshold PPV of 2%. 141 The remaining model QCancer, provides sensitivity and specificity parameter estimates by sex, which may be used to populate a decision model of decision-making for suspected cancers. However, these values include patients with red-flag symptoms, which may be deemed appropriate for 2WW referrals, so a more appropriate value for the low-risk patient primary care population of interest to our study would exclude these cases. Therefore, we calculate the sensitivity and specificity of QCancer for the top 10% risk threshold, after excluding the top 1% risk observations (i.e. subtracting the published number of patients and CRC cases in the top 1% risk from the top 10% risk and cases total, respectively) from the published data,77 to be 61% and 91%, respectively. These values are derived from analysis of a large database of electronic medical records of patients seen in routine practice.
| Study | Number and category of descriptors | Study design | Source | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|
| BB equation | 8; symptoms, test results (no threshold given) | Retrospective case–control | Marshall 201122 (validated in Dutch sample, Elias 201750) | 33.5a | 97.7a |
| Netherlands model | 3; symptoms, patient demographics | Prospective cohort | Hodder 200577 | N/A | N/A |
| QCancer | 6 (females) and 7 (males); symptoms, medical history, test results | Prospective cohort | Hippisley-Cox 201280 and Collins 201215 |
|
|
| RAT | 10; symptoms, test results | Case–control prospective cohort | Hamilton 200581 (version in Marshall 201126 validated in Dutch sample, Elias 201750) |
|
|
Updated review
As discussed in Chapter 5, the evidence identified in the updated review of the relationship between diagnostic delay and cancer patient outcomes does not allow the derivation of valid estimates on the effect of expeditious CRC diagnosis on cancer stage or patient survival. In particular, the documented non-monotonic (e.g. U-shaped) relationship between diagnostic interval and mortality does not infer an effect mediated through the impact of diagnostic interval on stage of diagnosis. This is because shorter diagnostic intervals are associated with unobserved differences in cancer stage and tumour aggressiveness, and are affected by lead time and immortal time biases whereby deaths from cancer may occur outside the observed follow-up at varying degrees across the duration of the diagnostic interval. Similarly, the observed relationship between diagnostic interval and cancer stage may be affected by unobserved variation in underlying disease severity and cancer aggressiveness across the range of the diagnostic interval.
Given the lack of valid surrogate or clinical outcome data for building an economic model of diagnostic tools or decision-aid models in primary care, our cost-effectiveness analysis was limited to an exploration of the expected effects of prolonged intervals to diagnosis associated with the relative diagnostic accuracy of diagnostic tools. This limited aim will naturally account for more sources of uncertainty than an analysis based on clinical surrogate outcomes, but reflects a realistic approach given the quality of the data available.
Systematic review of existing economic decision-analytic models for colorectal cancer
Methods
The systematic review of decision models and evidence used to populate their parameters used a rigorous but pragmatic approach because of the time constraints of developing a de novo model consistent with good modelling practice. 142,143
Search strategy
The search strategy was designed to retrieve economic decision models in CRC. The following sources were searched: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and Health Management Information Consortium (HMIC) [all via Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands)], NHS Economic Evaluation Database (NHS EED) and the Health Technology Assessment (HTA) Database (both via the Cochrane Library) and EconLit [via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA)]. The searches were developed and run by an information specialist (SR) in September 2017 and updated in September 2018. A search filter was used to limit the searches to economic studies. No date or language limits were used. The database search results were exported to, and de-duplicated using, EndNote X7. De-duplication was also performed using manual checking. The search strategies for each database are detailed in Appendix 5. Studies were selected using predefined inclusion and exclusion criteria, which are presented in the following sections.
Inclusion criteria
The studies were included if the:
-
population considered was adults (aged ≥ 18 years) with symptomatic or asymptomatic CRC, or at risk of developing CRC
-
interventions included diagnosis CRC models, screening programmes to identify CRC in the population (as long as they also included a disease progression model), or treatment of CRC
-
comparator was current clinical practice or other
-
outcomes included resource use, costs and health outcomes [which included life-years gained, quality-adjusted life-years (QALYs) gained, CRC cases prevented, etc.]
-
studies were trial- or model-based that included a CRC disease model using the Dukes’ staging or the Classification of Malignant Tumours of the American Joint Committee on Cancer (AJCC), that is, a tumour node metastasis (TNM)-based classification staging system (cost-effectiveness, cost–utility) [both systems are commonly used in the UK and by NICE, although the AJCC’s system is mostly known in the UK as TNM (classification of malignant tumours). Further explanation of these classification systems can be found in Appendix 5.].
Exclusion criteria
Studies were excluded if one or more of the eligibility criteria were not met, if the studies were cost of illness or burden of diseases, if they reported only methodological issues, or if they were abstracts, reviews, commentaries, letters or editorials.
Selection of studies
Titles and abstracts obtained from the search were screened independently by two reviewers (PL and BG) using the inclusion/exclusion criteria. All potential included studies were then screened by a third reviewer (AML). Full articles were screened for eligibility by two reviewers (PL and AML). Any disagreement was resolved by consensus.
Data extraction
Standardised data forms were used to extract the relevant information for each of the included studies. One reviewer (PL) extracted the data and the information extracted was checked by a second reviewer (AML). The data extracted included author, year, type of model (diagnostic, screening, disease progression) and study question (Table 13), perspective, population, outcome measure, model duration, type of uncertainty analysis, discount rate, base year and prices (Table 14). Additional data extraction for sensitivity analyses was carried out to assess which parameters had the changes in value that most affected the incremental cost-effectiveness ratios (ICERs); this information is presented in Appendix 5.
| Study | Country/region | Pathway of care (screening/diagnostic/treatment) | Objective |
|---|---|---|---|
| Allen 2005144 | USA | Diagnosis and natural history | To compare the cost-effectiveness of four diagnostic strategies for evaluating rectal bleeding |
| Tappenden 2007145 | England | Disease progression | To develop a state-transition model to simulate the life experience of a cohort of individuals without polyps or cancer through the development of adenomatous polyps and malignant carcinoma and subsequent death in the general population |
| Tsoi 2008146 | Asian countries | Screening and disease progression | To evaluate the cost-effectiveness of FOBTs, FS and colonoscopy on the basis of disease prevalence, compliance rate and cost of screening procedures in Asian countries |
| Zauber 2008147 | USA | Screening and disease progression | To assess life-years gained and colonoscopy requirements for CRC screening strategies and identify a set of recommendable screening strategies |
| Heitman 2010148 | Canada | Screening and disease progression | To perform an economic evaluation of CRC screening in average-risk North American individuals considering all relevant screening modalities and current CRC treatment costs |
| Lee 2010149 | UK | Screening and disease progression | To assess the cost-effectiveness of three-dimensional CTC vs. OC for colonic imaging of symptomatic gastroenterology patients |
| Knudsen 2012150 | USA | Screening and disease progression | To assess the clinical effectiveness and costs of colonoscopy vs. other rescreening strategies |
| Sharp 2012151 | Ireland | Screening and disease progression | To evaluate the cost-effectiveness of a population-based screening programme |
| Whyte 2012152 | England | Screening and disease progression | To use newly available data to estimate the cost-effectiveness and endoscopy requirements of screening options for CRC to inform screening policy in England |
| Goede 2013153 | The Netherlands | Screening and disease progression | To evaluate if two-sample FIT screening is cost-effective compared with one-sample FIT |
| Gomes 2013154 | The UK | Screening and disease progression | To assess the cost-effectiveness of CTC for CRC screening |
| Tappenden 2013155 | England | Diagnosis and disease progression | To assess the feasibility and value of simulating whole disease and treatment pathways within a single model to provide a common economic basis for informing resource allocation decisions |
| Whyte 2014156 | England | Awareness campaign, screening and disease progression | To estimate the clinical effectiveness of a CRC awareness campaign |
| Cantor 2015157 | USA | Screening using patient decision aids | To provide a framework for analysing the cost-effectiveness of decision aid for CRC screening |
| Pil 2016158 | Belgium | Screening and disease progression | To assess the cost-effectiveness and budget impact analyses of the CRC screening |
| Wong 2016159 | Hong Kong | Screening and disease progression | To evaluate the cost-effectiveness, by age and sex, of CRC screening |
| Coldman 2017160 | Canada | Disease progression | To develop a simulation model to predict the effect of threshold selection on outcomes including life-years gained, CRC incidence and mortality, and costs |
| Westwood 2017161 | England and Wales | Diagnosis and disease progression | To assess the clinical effectiveness of FITs for primary care triage of people with low-risk symptoms |
| Study | Perspective | Population | Diagnostic strategies | Outcome measure | Model structure | Model duration | Uncertainty analysis | Discount rate (%) | Base year and currency |
|---|---|---|---|---|---|---|---|---|---|
| Allen 2005144 | Modified societal | Patients aged 55 years presenting one or more episodes of rectal bleeding |
|
QALYs | Markov state-transition model, annual cycles | Lifetime | Two-way sensitivity and scenario analyses | 3.5 | 2001; USD |
| Tappenden 2007145 | Assumed to be UK NHS | General population of England aged 50–60 years | N/A | QALYs | Markov state-transition model, annual cycles | Lifetime | One-way and probabilistic sensitivity analyses | 3.5 | Not explicit (2004); GBP |
| Tsoi 2008146 | Unclear | Aged 50 years | N/A | Life-years | Decision tree model | 80 years of age | One-way and two-way sensitivity analyses | 3 | Not clear or explicit (1996, 2003); USD |
| Zauber 2008147 | Societal | US average-risk population aged 40 years | N/A | Life-years | Microsimulation model | Lifetime | One-way sensitivity analysis | Not reported | Not reported |
| Heitman 2010148 | Third-party payer | Average-risk individuals aged 50–75 years | N/A | QALYs | Markov model | Lifetime | Univariate sensitivity and probabilistic sensitivity analyses | 5 | 2008; CAD |
| Lee 2010149 | UK NHS | Aged 60–69 years | N/A | Life-years, QALYs | Markov state-transition model | Lifetime | Univariate sensitivity and probabilistic sensitivity analyses | 3.5 | 2007; GBP |
| Knudsen 2012150 | Societal | Aged ≥ 50 years, with no adenomas or cancer detected | N/A | Lifetime measures per CRC, life expectancy | Simulation model | Lifetime | One-way sensitivity analysis | 3 | 2007; USD |
| Sharp 2012151 | Third-party payer |
|
N/A | QALYs | Markov state-transition model, annual cycles | Lifetime | Probabilistic sensitivity analyses | 4 | 2008; EUR |
| Whyte 2012152 | UK NHS | Individuals aged 30 years in the general population of England with normal colon epithelium | N/A | QALYs | State-transition model, annual cycles | Lifetime | One-way sensitivity analyses and probabilistic sensitivity analyses | 3.5 | Not clear or explicit (2008 or 2010); GBP |
| Goede 2013153 | Health-care system | From birth to death | N/A | Life-years | Microsimulation | Lifetime | Univariate sensitivity analysis | 3 | 2010; EUR |
| Gomes 2013154 | UK NHS | Aged ≥ 50 years | N/A | Life-years, QALYs | Markov state-transition model | Lifetime | Univariate sensitivity and probabilistic sensitivity analyses | 3.5 | 2010; GBP |
| Tappenden 2013155 | UK NHS | General population, includes diagnosis in primary care | RAT | QALYs | Discrete event simulation model | Lifetime | Constrained maximisation analysis | 3.5 | 2011; GBP |
| Whyte 2014156 | UK NHS | General population | N/A | Cancer mortality, QALYs | State-transition model with annual cycles | Lifetime | Two-way sensitivity and probabilistic sensitivity analyses | 3.5 | 2012; GBP |
| Cantor 2015157 | Not explicit | General population (adults aged ≥ 50 years). Eligible for screening | N/A | Life-years | Decision tree model | Lifetime | One-way sensitivity analysis | Not reported | 2013; USD |
| Pil 2016158 | Societal | Adults aged between 56 and 64 years eligible for CRC screening | N/A | Predicted mortality, QALYs | Decision tree and Markov models | 20 years | One-way sensitivity and probabilistic sensitivity analyses |
|
2014; EUR |
| Wong 2016159 | Not explicit | Asymptomatic subjects aged 50 years | N/A | Life-years | Markov model | 20 years | One-way sensitivity and probabilistic sensitivity analyses |
|
Not clear or explicit (1996, 2003 or 2008); USD |
| Coldman 2017160 | Third-party payer (NHS) | General population aged 45 years | N/A | Life-years gained, CRC incidence, CRC mortality | Simulation model | Lifetime | Sensitivity analysis (not clear) | 3 | 2014; CAD |
| Westwood 2017161 | UK NHS | Symptomatic patients aged ≥ 40 years presenting to primary care who are at low risk of CRC |
|
QALYs, life-years | Markov state-transition model with annual cycles | Lifetime | Scenario analysis and probabilistic sensitivity analysis | 3.5 | 2015; GBP |
Critical appraisal
The methodological quality of the studies included was assessed in detail by two reviewers (PL and AML) following the checklist for model studies by Philips et al. 162
Results
Studies identified
A first electronic search retrieved a total of 2730 hits; after de-duplication, 2129 were screened, of which 2109 hits were excluded. In addition, we were aware of two key cost-effectiveness studies that were missed in the search and decided to run a new search with a more refined set of search terms (for the revised search, see Appendix 5) to identify more cost-effectiveness models. We assessed the full text of 20 studies from the first search, seven additional studies from the revised search and one new study from the updated search. After a more in-depth screening of these 28 studies, 13 papers were excluded: for nine papers, the disease model description was not based on the Dukes’ or AJCC staging; three screening models did not include a full disease model; and the last excluded study was a costing study of setting up a screening programme. Reference-searching led us to three additional studies; 18 studies, in total, met the inclusion criteria for the review. Further details are presented in Figure 4.
FIGURE 4.
Economic decision-analytic models: the PRISMA flow diagram. a, Revised strategy; b, updated search using the revised strategy; c, a total of 546 hits were screened with the updated search; and d, further exclusion details are presented in Appendix 5.
Study characteristics
Table 13 provides the overview of the included models. Over 50% (10/18) of the studies were conducted in Europe (seven of these were in the UK), six in North America (four in the USA and two in Canada) and two in Asia.
Over 80% (15/18) of the studies were two-part models, mainly screening and full disease progression models (12/18); only three studies were diagnosis and natural history disease models. The other three models included in this review were disease progression models only. Two exceptions were made of studies that did not meet the inclusion criteria. The study by Cantor et al. 157 evaluated a patient decision aid for CRC screening; it was reviewed as it was deemed to be important for developing the diagnosis part of the model. Similarly, the model by Zauber et al. ,147 which excluded resource use and costs, was included despite not meeting the inclusion criteria.
In Table 14, the study characteristics are presented. Westwood et al. 161 is the only study of a diagnostic strategy in the population of interest to our study, namely a comparison of faecal immunochemical tests (FITs) at various thresholds against faecal occult blood tests (FOBTs) in a low-risk symptomatic population in a primary care setting. Of the two remaining studies of diagnostic strategies, only one144 compared two or more diagnostic strategies, and, although it is conducted in a primary care setting, it is concerned with a high-risk symptom only (rectal bleeding in a person aged 55 years) in US primary care. The remaining study, by Tappenden et al. ,155 is a whole-disease model, which captures the complete pathway of CRC patients from a healthy state to clinical disease down to the detail of treatment sequences used by age and disease stage. This study’s aim is not sufficiently detailed to account for the diagnostic pathway of low-risk symptoms, although it accounts for the role of a RAT in the transition from asymptomatic to clinical disease,39 and its main interest to our purposes lies in the methods used to build the disease history model.
Further, the disease history model in the study by Tappenden et al. 145 is used by most of the included screening studies145,149,154,156 summarised in Table 14 with few modifications. In common with most models, this model used a lifetime time horizon and considered cases aged 50–60 years.
None of the identified studies evaluated a diagnostic tool. Therefore, attention will be devoted in the rest of this chapter to reviewing the suitability of identified models to accommodate any evaluation of such tools, and the quality of the available evidence synthesised by existing models to inform the assessment of tools using any suitable existing or new model.
Critical appraisal: key model features
The critical appraisal data extracted for the 18 included model-based economic evaluation studies included are presented in Appendix 5, and the findings are summarised in this section.
Over 60% (11/18) of the studies considered the NHS perspective and only four studies144,147,150,158 included a societal perspective. The perspective in three studies146,157,159 was not explicitly stated, but, given the costs included, it appears to be their respective health systems’ perspectives.
There were marked differences between the models’ assumptions for the long-term rate of CRC disease progression. In 40% (8/18) of the studies,145,147,148,151,152,155,156,161 the most common assumption was to apply an annual disease progression rate, the choice of which was clearly defined by the history of the disease. The selection of key parameters was justified in all the studies, although the quality of the chosen parameters was not fully discussed in one study. 150
Fifty per cent of the studies (9/18) used the QALY as an outcome measure; life-years were used in six studies,146,147,150,153,157,159 and three studies149,154,161 included both QALYs and life-years. The parameterisation into the models was described with sufficient detail and with all the relevant sources of data in most of the studies (14/18). However, the relevance of the assumptions was difficult to understand in two studies,144,160 and how the data were incorporated into the model was unclear in two studies. 159,160
Over 80% (15/18) of the studies addressed properly the different types of uncertainty; in particular, point estimates and values for sensitivity analyses were stated and justified in most of the studies, except for three studies150,159,160 in which assumptions were less clearly described. All studies except for Pil et al. 158 justified the values used for the sensitivity analysis.
Probabilistic sensitivity analysis was undertaken by 55% (10/18) of studies, and one study153 justified the absence of probabilistic sensitivity analysis based on the lack of evidence on sampling variation of parameter values. In contrast, this information was vaguely stated in, or missing from, seven studies. 144,146,147,150,157,159,160 The choice of parameter distributions used was clearly reported in 50% (9/18) of the studies; however, five studies144,154,157,159,160 omitted the information with respect to second-order uncertainty.
The internal consistency was evaluated in 10 studies only. 144,145,147–149,152,153,155,156,161 In the absence of data on key parameters, the model was calibrated against independent data in eight studies,145,147,148,151–153,155,156 such as the rate of disease progression in asymptomatic CRC states.
Modelling approaches
Five different types of decision models were identified in the review: (1) 11 Markov state-transition models,144–146,148,149,151,152,154,156,159,161 (2) four microsimulation models,147,150,153,160 (3) one Markov model combined with a decision tree,158 (4) one decision tree157 and (5) one discrete event simulation model. 155 The colorectal health cancer states were modelled using the AJCC staging (9/18) or the Dukes’ staging systems (8/18). The model diagnosis by Cantor et al. 157 did not consider any staging.
Markov models were preferred because of their flexibility and simplicity in populating and calibration processes. Specifically, the transition within health states can be easily represented by a cohort in a Markov model. Given the limited number of data on key disease history model parameters, a Markov model may be more suitable to synthesise the clinical and economic evidence for evaluating diagnostic, screening and surveillance strategies in CRC than other types of models such as microsimulation and discrete event simulation models. The only study that used a discrete event simulation model used such an approach to accommodate the distribution of time to event survival and disease progression outcomes of CRC treatment, which were found to have limited importance in the results of the only diagnostic study of a low risk of suspected cancer in primary care (Westwood et al. ;161 see Appendix 5). Likewise, the three studies150,153,160 that used microsimulation models used such an approach for its convenience to account for the interactions between individual characteristics and risk factors of the screening tests and combining cohorts with different characteristics to produce results averaged across subgroups of the patient population.
Detailed review of individual models
UK models
Tappenden et al. 145 constructed a model for England incorporating three elements: (1) a screening intervention model that included subsequent colonoscopy surveillance; (2) a state-transition model that simulated the natural history of colorectal neoplasia from normal epithelium, to adenoma and carcinoma sequence; and (3) a mortality model that considered age-specific other causes mortality, CRC mortality and mortality from perforation due to endoscopic procedures. Transitions between model health states were calculated using an annual cycle length until the entire model cohort was absorbed into the dead state. The model assumed that the incidence of adenomas was 1.60% and that the incidence of cancer from adenomas was 3.26%. Five screening options versus no screening were evaluated: biennial FOBTs, for people aged 50–69 and 60–69 years; two once-only flexible sigmoidoscopies, for people aged 55 and 60 years; and the fifth option was once-only flexible sigmoidoscopy for individuals aged 60 years, followed by biennial FOBTs for individuals aged 61–70 years.
The UK model developed by Lee et al. 149 was a two-part model: (1) the evaluation of screening strategies for CRC and (2) a state-transition Markov model representing the natural history of colorectal neoplasia (from normal colorectal epithelium to adenoma–carcinoma sequence, to death). The four screening strategies evaluated were (1) FOBT every 2 years, (2) flexible sigmoidoscopy every 10 years, (3) optical colonoscopy every 10 years and (4) computerised tomography colonography (CTC) every 10 years. The authors used data from the study by Tappenden et al. 163 and recalibrated the data to adjust for estimates from recent observational data on CRC incidence, mortality and staging of cancer at time of diagnosis. The progression from normal epithelium to low-risk adenoma, low-risk adenoma to high-risk adenoma, and high-risk adenoma to Dukes’ stage A CRC was 1.2%, 2.4% and 3.4%, respectively. The mortality for other causes was modelled as an age-dependent probability based on UK life tables,164 and the probability of dying as a result of endoscopic perforation from either the screening procedure or the polyp removal (polypectomy) was included in the model as a potential complication of the interventions.
The two interlinked models developed by Whyte et al. 152 had (1) a model that described the screening interventions and surveillance of CRC (from invitation to screening, screening test, follow-up, diagnostic tests and surveillance) and (2) a natural history of colorectal neoplasia model (from normal colorectal epithelium to adenoma–carcinoma sequence to death). The screening intervention used the following diagnostic strategies: (1) no screening; 2) biennial guacal faecal occult blood test (gFOBT) at 60–69 years; (3) biennial gFOBT at 60–74 years; (4) immunochemical faecal occult blood test (iFOBT) at 60, 65 and 70 years; (5) biennial iFOBT at 60–69 years; (6) biennial iFOBT at 60–74 years; (7) flexible sigmoidoscopy at age 55 years; (8) flexible sigmoidoscopy at age 55 and 65 years; (9) flexible sigmoidoscopy at age 55 years and biennial gFOBT at ages 66–74 years; (10) flexible sigmoidoscopy at age 55 years and biennial iFOBT at ages 66–74 years; (11) flexible sigmoidoscopy at age 55 and iFOBT at ages 60, 65 and 70 years; (12) flexible sigmoidoscopy at age 55 years and biennial iFOBT at ages 60–74 years; and (13) flexible sigmoidoscopy at age 55 years and biennial iFOBT at ages 56–74 years. The authors assumed that probabilities associated with the transition to low-risk adenomas, the transition from low- to high-risk adenomas and the transition from high-risk adenomas to Dukes’ stage A CRC were age dependent.
An interconnected model developed by Gomes et al. 154 comprised (1) diagnostic test and the subsequent adenoma surveillance model for symptomatic patients in secondary care, and (2) a Markov model, based on CRC natural history in the UK simulating the development of CRC in the population (from normal colorectal epithelium to adenoma–carcinoma sequence to death). The model assumes a variable annual rate of polyp development of 1.9% to 3.3% and an annual incidence of CRC from adenomas of 3.4%. The model evaluated the impact of three-dimensional CTC versus optical colonoscopy to improve the detection of adenomas and CRC.
Tappenden et al. 155 developed a patient-level model that simulates the disease progression and treatment pathways from pre-clinical disease through detection, diagnosis, adjuvant/neoadjuvant treatments, follow-up, curative/palliative treatments for metastases, supportive care and eventual death. The authors assumed a detailed age-specific incidence of CRC and adenoma prevalence based on Bayesian Markov chain Monte Carlo methods. The whole-disease model considered all the processes that are linked to CRC from screening, surveillance and treatment.
The model developed by Whyte et al. 156 comprised (1) the evaluation of the effect of a campaign to increase the awareness of the signs and symptoms of CRC and encourage self-presentation to a GP, and (2) a model to represent the development of CRC in the population (from normal colorectal epithelium to adenoma–carcinoma sequence to death). The authors assumed an increased presentation rate of 10% in each CRC Dukes’ stage and an age-dependent incidence of adenomas (low and high risk) and CRC.
Westwood et al. 161 constructed a model for England incorporating three elements: (1) a decision model reflecting the diagnosis of CRC, (2) a Markov state-transition model to estimate the long-term costs and effects related to treatment and progression of CRC and (3) a Markov state-transition model to estimate effects for patients without CRC. The transitions between the model health states of the two Markov models were calculated using an annual cycle length until the entire model cohort was absorbed into the dead state. The model assumed the incidence of adenomas and the incidence of cancer (from adenomas reported in Tappenden et al. 145) to be 1.60% and 3.26%, respectively. The model evaluated three options of symptomatic individuals that presents to primary care: (1) FITs, (2) gFOBTs or (3) no triage tests at all (referral straight to colonoscopy).
European models
The Irish study by Sharp et al. 151 was a Markov state-transition model with three interlinked components: (1) the impact of screening and subsequent adenoma surveillance, (2) a natural history of colorectal neoplasia model (from normal colorectal epithelium to adenoma–carcinoma sequence to death) and (3) the impact on mortality. The authors assumed that 14% of individuals progressed from normal epithelium to stage I cancer. The second model component used gFOBT, FIT and flexible sigmoidoscopy for screening, and the diagnosis used the sensitivity and specificity of colonography and CTC. Finally, mortality in the model was assumed to be from CRC, endoscopic bowel perforation or other causes; other causes were obtained from Irish life tables and were modelled to be age dependent within each Markov cycle. The risk of dying from endoscopic perforation was considered only when using flexible sigmoidoscopy, diagnostic investigations and adenoma surveillance. The model assumed that individuals had a higher probability of dying if they had a more advanced cancer stage.
The work by Goede et al. 153 was based on the MIcrosimulation SCreening ANalysis (MISCAN) microsimulation model, which had three integrated components: (1) a model evaluating the screening strategies of one-sample and two-sample FIT screening with variable intervals, age ranges and cut-off levels (age at which to start screening: 45, 50, 55 and 60 years; age at which to stop screening: 70, 75 and 80 years; and screening interval at 1, 1.5, 2 and 3 years); (2) the natural history component part that simulates the development of CRC in the population (from normal colorectal epithelium to adenoma–carcinoma sequence to death); and (3) a demography component that simulates individual life histories without CRC to form a population. Authors assumed an age-dependent adenoma incidence and adenoma progression, whereas the CRC incidence rate was based on the observed incidence rate in the Netherlands.
The model developed by Pil et al. 158 comprised two interconnected submodels: (1) a decision tree to simulate the screening process based on FITs and (2) a state-transitional Markov model simulating the natural progression of the disease (from normal colorectal epithelium to adenoma–carcinoma sequence, diagnosis and treatment, follow-up to death). The authors assumed a sex- and age-dependent adenoma prevalence varying from 1.6% to 6.01%, and a CRC prevalence ranging from 0.11% to 0.84%.
North American models
Allen et al. 144 used a Markov decision model composed of three elements: (1) a model to evaluate four diagnostic strategies of a patient with rectal bleeding, (2) a model to simulate the natural history of colorectal neoplasia for patients with rectal bleeding (from no serious pathology to adenoma–carcinoma sequence and surveillance to death) and (3) a mortality model to estimate mortality among patients with CRC. The authors assumed that the progression of smaller adenomatous polyps to large polyps would take an average of 10 years and that the progression of large adenomatous polyps to invasive cancer would also take an average of 10 years. Once invasive cancer was detected, the average transition of 2 years, 1 year, and 1 year for Dukes’ stage A to B, B to C, and C to D staging, respectively, was assumed. It was also assumed that 7% of individuals progressed from adenomas to Dukes’ stage A and that the prevalence of adenomas was 16%. The diagnostic model evaluated (1) watchful waiting, (2) flexible sigmoidoscopy, (3) flexible sigmoidoscopy with consequent air-contrast barium enema and (4) colonoscopy. Finally, the mortality estimates used within each stage of cancer were obtained from the Surveillance, Epidemiology, and End Results (SEER) cancer registry.
Zauber et al. 147 used two previous developed microsimulation models [Simulation Model of Colorectal Cancer (SimCRC) and MISCAN] to reproduce the natural history of colorectal neoplasia (from no lesion to adenoma–carcinoma sequence to death). The semi-Markov microsimulation model simulated the effect of screening and other interventions on the incidence and mortality of CRC. It is a hybrid model, made of a Markov model and a discrete event simulation that described the progression of the underlying colorectal disease (e.g. from adenoma to carcinoma sequence) in an unscreened population. Authors assumed an age-dependent variability of adenoma prevalence varying from 10.2% to 36.7% and a CRC incidence varying from 5.3% to 7.3%. The model evaluated the following screening strategies: (1) no screening, (2) colonoscopy, (3) FOBT, (4) flexible sigmoidoscopy with biopsy and (5) flexible sigmoidoscopy combined with FOBT. For each strategy, individuals were evaluated at the following ages: 40, 50, 60, 75 and 85 years. FOBT strategies considered screening intervals of 1, 2 and 3 years; for the sigmoidoscopy and colonoscopy strategies, the intervals considered were 5, 10 and 20 years. The prevalence of pre-clinical cancer and adenomas was assumed to be zero.
The two-part model in Heitman et al. 148 included (1) the impact evaluation of screening strategies on average risk in a North American population and (2) a Markov model reproducing the natural history of the colorectal neoplasia (from normal colon to non-advanced/advanced adenomas, to CRC, to death). The authors assumed a prevalence of 17% of non-advanced adenomas, 3.8% for advanced adenomas and 0.1% for CRC. The screening strategies evaluated were (1) gFOBT or FIT annually, (2) faecal deoxyribonucleic acid (DNA) test every 3 years, (3) flexible sigmoidoscopy or CTC every 5 years and (4) colonoscopy every 10 years. The mortality rate was derived from an age-dependent population from Canada and the CRC mortality rates observed for patients with CRC according to their stage at diagnosis.
Knudsen et al. 150 developed a microsimulation model based on a previous study147 (SimCRC) to represent the natural history of colorectal neoplasia (from normal colon to non-advanced/advanced adenomas, to CRC, to death) and the relative effects of screening strategies. The prevalence, size, location and multiplicity of adenomas and the incidence of CRC was assumed from the SEER programme. The model evaluated five rescreening strategies for individuals with a negative colonoscopy result at 50 years of age: (1) no further screening, (2) continuing colonoscopy every 10 years, (3) rescreening with annual highly sensitive guaiac-based FOBT, 4) annual FIT or (5) CTC every 5 years. Rescreening was assumed to begin at 60 years (10 years after the negative colonoscopy result) for all strategies.
Cantor et al. 157 developed a decision-analytic model comprising a decision tree to evaluate the impact of a decision aid for CRC screening. The study does not present a disease model and there is no indication of patient characteristics. The model evaluated two strategies: use of decision aid and no use of the decision aid. The decision aid supported the selection of the patients who would undergo screening. Each patient undergoing screening can have one of three diagnostic tests: (1) flexible sigmoidoscopy, (2) FOBT or (3) colonoscopy, or not undertake screening. The study assumed a change in the preferences of the screening, with a consequent increase in the number of tests, number of patients undergoing the screening and costs.
The model by Coldman et al. 160 (OncoSim-CRC) comprised (1) a model to predict the outcomes of biennial screening in a cohort for eight FIT threshold values of between 50 and 225 ng/ml and (2) a model simulating the natural history for the development of CRC (from normal colorectal epithelium to adenoma–carcinoma sequence, to death). The authors assumed that the prevalence of adenomas was age, sex and size dependent. The age- and sex-specific CRC incidence and mortality rates were derived from the Canadian population.
Asian models
Tsoi et al. 146 developed a Markov model comprising three submodels that evaluated which primary screening procedure should be adopted to reduce CRC incidence. The diagnostic tests considered were (1) FOBT, (2) flexible sigmoidoscopy and (3) colonoscopy. The annual mortality rates of CRC diagnosed at different stages were based on the Hong Kong Cancer Registry,165 and the mortality due to perforation of endoscopic tests was considered to be 10%. The authors assumed a prevalence of 10% for the first stage of CRC and, that 14% of individuals progressed from normal epithelium to stage I cancer.
Wong et al. 159 used an approach similar to that of Tsoi et al. ,146 but focused on developing a Markov process model to evaluate five screening strategies: (1) flexible sigmoidoscopy as a primary screening test every 5 years at the ages of 50, 55, 60, 65 and 70 years; (2) colonoscopy as a primary screening test every 10 years at the ages of 50, 60 and 70 years; (3) flexible sigmoidoscopy for each female subject every 5 years at the ages of 50 and 55 years; (4) flexible sigmoidoscopy for each female subject every 5 years at the ages of 50, 55, 60 and 65 years; and (5) flexible sigmoidoscopy for each female subject every 5 years at the ages of 50, 55, 60, 65 and 70 years. The age-stratified incidence of CRC of the Hong Kong population was based on reports from the Hong Kong Cancer Registry.
Model population and time horizon
UK models
In the models developed by Tappenden et al. ,145 Lee et al. 149 and Whyte et al. ,156 individuals were aged 30 years. In the model developed by Westwood et al. ,161 individuals entered the model at age 40 years, and in the models developed by Whyte et al. 152 and Gomes et al. ,154 individuals were aged 50 years. The only study that included individuals from birth is Tappenden et al. 155 All of the UK models followed individuals until death.
European models
In line with the UK models, in two of the European models, by Sharp et al. 151 and Pil et al. ,158 individuals entered the model at the ages of 30 and 50 years, respectively. However, only the model developed by Sharp et al. 151 followed up individuals until death, whereas, in the model developed by Pil et al. ,158 individuals were followed up until the age of 70 years. In comparison, the study by Goede et al. 153 modelled the age distribution of the Dutch population in 2005 and all the cohort individuals were followed up until death.
North American models
In two of the US models,144,147 the cohorts entered at age 40 years and were followed up to the age of 100 years or death. However, Heitman et al. 148 and Knudsen et al. 150 considered older cohorts (aged 50 years); both studies followed up individuals to death. Although the cohort of the Canadian model148 entered at the age of 45 years and was followed until death, Cantor et al. 157 did not specify any age for their cohort.
Critique
For the purposes of the current research questions, the review has helped to identify the Westwood et al. 161 model as a useful precursor for the diagnostic phase. This model is important from two perspectives: first, it is the only evaluation of primary diagnostic tests for CRC identified by our review, and, second, the population included are the low-risk symptomatic population of interest. The focus of the critique will therefore be its methodology, as well as the source of the evidence used to populate its key parameters to inform the approach taken in the novo model. In particular, we will review the mechanisms used to link the diagnostic pathway to the Markov model of disease treatment used by Westwood et al. 161 in terms of their suitability for incorporating a diagnostic phase that has enough granularity to allow an evaluation of costs and health benefits of tool-based diagnostic strategies.
The analysis by Westwood et al. 161 informed the NICE Diagnostic Guideline Number 30140 and assessed the relevant low-risk population for using the diagnostic tools of interest to our study. A strength of the model is that it considers an intervention, FIT, that has now become the recommended standard practice in the UK. The model has policy appeal as it considers the option of referring all symptomatic patients directly to secondary care, a natural option for a country where policy-makers are concerned about expediting access to diagnosis and treatment as a way to improve cancer survival outcomes.
An attractive feature of the Westwood et al. 161 model is that it shows that detecting more CRC cases with diagnostic tests comes at the cost of imposing more unnecessary use of health-care resources and health risks from invasive investigations to the great majority of patients who do not have CRC. When the costs and life-years are calculated over the denominator of all patients presenting with symptoms of suspected CRC, the importance of the trade-off between sensitivity and specificity becomes apparent. The costs of colonoscopies and their associated risk are modelled in the Westwood et al. 161 model to explore the trade-offs between gains in sensitivity of diagnostics in primary care at the expense of exposing more false positives to invasive tests. Our de novo model analysis will follow a similar approach, which will serve to highlight that gains in sensitivity of diagnostic strategies are achieved at the expense of their specificity, especially among younger patients who are at lower risk of CRC. When modelling the impact on colonoscopies, Westwood et al. 161 make the simplifying assumption that the costs of CRC treatment are not linked to the clinical outcomes, but this simplification does not affect the results in a significant way because of the low proportion of cancer patients.
A limitation of the Westwood et al. 161 model is the quality of the evidence on the diagnostic accuracy of FITs, which was obtained from high-risk patient populations included in studies that, for the most part, were not conducted in a primary care setting. This weakness is problematic because the values of FIT of 10 µg haemoglobin (Hb)/g faeces, the recommended new standard, has sensitivity values close to 100%, which effectively limits the ability of any diagnostic tool to add to the primary care diagnostic yield (see Chapter 7). Furthermore, although referring all patients to secondary care may be the least risky option, the low prevalence of CRC means such a strategy is unaffordable and is not used in practice. 140
In terms of methods, the analysis by Westwood et al. 161 adopts a linked-data approach, whereby survival outcomes of FOBTs are driven by the ability of the test to correctly identify patients with CRC before the disease has progressed to advanced disease stages and become less responsive to treatment. The model uses disease progression data from the disease history model developed by Tappenden et al. 145 and Whyte et al. 156 to determine the extent to which delays in referral from lack of diagnostic accuracy would affect long-term life expectancy. The limitation of this approach is the high degree of uncertainty in the rates of disease progression underlying any such analysis, which were derived from calibrations of the disease history model to observed data on CRC incidence and distribution of stage at diagnosis. We adopt a similar approach in our model, given the lack of more reliable data on the relationship of delayed diagnosis and stage at diagnosis, but raise this weakness as an area that needs further research.
A further limitation of the diagnostic phase of the Westwood et al. 161 model is the way the time to referral is analysed. In particular, the delays incurred by false-negative cases are not explicitly modelled in terms of diagnostic accuracy of the tests being compared, but simply assumed to last for an arbitrary amount of time, which, in their base-case analysis, was 6 months. This adds a high degree of uncertainty in the absence of any data used by the authors to justify the extent of the delay. We sought to address this limitation by building a de novo model of the diagnostic pathway that was fully consistent between the diagnostic accuracy and the number of visits and the referral interval.
In terms of the granularity of the model used to link the diagnostic pathway to the Markov model of disease treatment, the Westwood et al. 161 model uses an annual cycle length to model health transitions. A limitation of an annual cycle is that it may not have sufficient granularity to measure the predicted outcomes, especially given small differences involved in accuracy and referral intervals between strategies. We addressed this limitation in the de novo model by using a 28-day cycle to link the diagnostic pathway to the Markov disease model.
The Westwood et al. 161 model did not account for the short-term survival benefits of reducing the time to referral and treatment, as symptomatic patients with CRC who are undiagnosed, and thus untreated, are subject to increased death risks, relative to those who have been diagnosed and started treatment, especially in disease stages II/III (Dukes’ stage B/C). We addressed this limitation by building a de novo model of the diagnostic pathway that accounts for the short-term survival benefits of reducing the time to referral and treatment.
The effect of delays in diagnosis on the mental health of a patient with CRC is also omitted from the Westwood et al. 161 model. No evidence on the magnitude of such an impact was found in the literature reviewed for this study, and the studies reviewed failed to explore the importance of that effect for the cost-effectiveness of diagnostic strategies in primary care. Our de novo model, therefore, is unable to incorporate these effects. Measures of health-related quality of life among undiagnosed and untreated CRC patients are lacking, and are difficult to measure because of the very nature of studying undiagnosed patients.
Conclusions
The review highlights differences between existing economic models of cancer diagnosis and screening. A common approach to the problem of lack of evidence on clinical outcomes of diagnostic and screening strategies has been to model long-term outcomes of CRC using a model of CRC disease progression in asymptomatic disease obtained by calibrating a disease history model to data on CRC incidence and disease stage at diagnosis. 145,147,148,151,152,155,156,161 We applied the same approach in our de novo model, given that the updated review provided no direct evidence on clinical outcomes of diagnosis, but we recommend that further research on this issue is undertaken.
Our review found no evidence on the cost-effectiveness of diagnostic tools for managing patients in primary care with suspected CRC, but identified one study of FITs161 in the low-risk population of interest that modelled the diagnostic phase. The model explored the trade-offs between gains in sensitivity of diagnostics in primary care at the expense of exposing more false positives to invasive tests, and is a useful precursor for the diagnostic phase of our de novo model. In addition, our critique of the Westwood et al. 161 model identified three areas in which we could make improvements to the diagnostic phase:
-
Time to referral – time to referral will be explicitly modelled to be consistent with diagnostic accuracy of the respective test and the distribution of the number of visits before referrals in electronic health records or retrospective reports from surveys of patients’ experiences. This will include modelling delays incurred by false negatives. By coherently modelling the sensitivity of primary care diagnostic strategies and the delay in referrals and treatment, the uncertainty in relative clinical effectiveness between diagnostic strategies is reduced relative to arbitrary estimates of diagnostic delay used by the existing model in this area.
-
Cycle length to model health transitions – our model used a 28-day, rather than annual, cycle to get accurate measure of predicted outcomes, especially because of the small differences involved in accuracy and referral intervals between strategies.
-
Cancer-related mortality – the short-term survival benefits of reducing the time to referral and treatment will be explicitly modelled.
The review of existing models also highlighted that the health-related quality-of-life outcomes in the diagnostic phase have received very limited attention. Our de novo model is similarly unable to explore the health-related quality-of-life outcomes in the diagnostic phase, as much of the policy focus has been devoted to improving cancer survival.
Chapter 7 Economic decision-analytic model for colorectal cancer
Aim and objectives
The earlier chapters highlight the uncertainty in the evidence base for decision tools, which make it difficult to assess the cost-effectiveness of the use of risk tools. In this chapter, we use a decision-analytic model to demonstrate the uncertainty inherent in the current evidence base, and show the probable impact that use of the tools in clinical practice may have on patient outcomes and NHS resources. The primary role of the decision modelling is not the estimation of the single most likely point estimates of costs per QALY associated with each diagnostic tool. Instead, the decision-analytic model and the evidence that is available are used in this chapter to explore the probable range of costs per QALY, and to ask questions about the likely impact of the diagnostic tools, given the current evidence base.
The objectives of the decision-analytic model were to examine the following questions:
-
What are the possible impacts on patient quality of life or survival if the diagnostic tools reduce time to diagnosis?
-
Will the benefit to cancer patients who are identified earlier by diagnostic tools outweigh any disutility in extra patients who do not have cancer being referred for further investigation?
-
How big an improvement in quality of life would be needed to warrant the use of these tools if there are no survival impacts associated with the diagnostic tools? Would this quality-of-life improvement be justifiable given the evidence we have?
-
Could a cancer diagnostic tool be considered cost-effective if it reduces the period of extreme anxiety for patients by expediting investigation and management?
-
Where are the gaps in the evidence base and where is more research needed?
Furthermore, we developed a model that anticipates evidence development and can be used alongside any studies measuring impact on patient outcome directly in the future to explore implications for cost-effectiveness. We will also be able to use the model to identify the parameters that contribute most to the overall decision uncertainty about the cost-effectiveness of decision tools and where additional research might be targeted using expected value of partial perfect information. 166–168 If considered effective and cost-effective, now or in the future, the model could also be developed to assess the budget impact of introducing cancer diagnostic tools in different populations.
It was not feasible to model the impact of diagnostic tools for all common cancer types for which diagnostic tools exist. Instead, we chose to model one common cancer: CRC.
Colorectal cancer was chosen, based on the following criteria, to provide a best-case example of how the diagnostic tools could affect patient outcomes:
-
Tools exist – to compare diagnostic tools with each other and with no tool, we need diagnostic tools to be available for the specific cancer type. There are existing tools for CRC, as identified in SR2. In particular, there are RATs75,81 and QCancer80 for CRC, which are now within GP systems and, therefore, potentially available for GPs to use.
-
Common cancer – CRC is the third most common cancer in the UK, accounting for 11% of cancers in women and 13% in men. 169
-
Whole-disease models exist – it is important that whole-disease models exist, and, although not made available by the original authors, could be replicated by published methods and adapted to incorporate the diagnostic pathways of interest.
-
Wide agreement on patient management –it will be important that there is wide agreement on the treatment and management of individuals to minimise uncertainties elsewhere in the clinical pathway. CRC is a cancer for which there is wide agreement on the treatment and management of individuals.
-
Evidence that use of diagnostic tools changes practice – to explore the impact of the tools, it will be important to identify a cancer type for which there is existing evidence to show where the diagnostic tools will impact, and what that impact will be. Only a few studies assess the impact of RATs in SR1 that were related to CRC. These studies reported that increased cancer referrals and investigations for CRC were associated with use of tools. 13,35
-
Evidence that change in practice affects patient outcomes – it is not enough that increased referrals and investigations are associated with use of the tools; there also needs to be existing evidence of the impact of earlier diagnosis on patient outcomes. This might include earlier stage at diagnosis, higher resection rates or improved survival, as well as improved quality of life of patients. There is some evidence to show that long (and very short) diagnostic intervals are associated with increased mortality in CRC. 99,135,137 However, as indicated in Chapter 5 and summarised in Chapter 6, Updated review, there is great uncertainty associated with estimates of the effect of expeditious CRC diagnosis on cancer stage or patient survival.
Although the implications of prolonged time to tests, diagnosis and treatment vary by cancer type, among the low-risk symptoms there are many similarities in the diagnostic options within primary care (i.e. refer all, watch and wait, refer for further tests). Therefore, the model developed can be viewed as a template for modelling the effect of diagnostic tools in cancers other than CRC, and can be revised to include prevalence and test sensitivity/specificity and costs specific to other cancers.
Methods
Conceptualisation of the model
Patient and public involvement
Involving members of the public in health research projects is becoming standard practice, but there are fewer reported examples of patient and public involvement (PPI) in health economic or modelling studies. 170,171
At the outset of putting together the diagnostic pathway of the model, we organised a workshop to meet with patients who have experienced bowel cancer diagnosis to seek their advice on our understanding of the current diagnosis pathway and to listen to their experiences of being diagnosed. This was facilitated by Dr Emma Cockcroft from the PPI team from the Peninsula Collaboration for Leadership in Applied Health Research and Care, which is based at the University of Exeter Medical School. This part of the study did not require separate ethics approval because it was underwritten by the current ethics approval granted to the PPI group at the University of Exeter.
Dr Cockcroft wrote an invitation to the Bowel Cancer West cancer treatment centre in Plymouth to participate in the PPI, which was published on their Facebook page (Facebook, Inc., Menlo Park, CA, USA). After several weeks, two patients contacted us and we opted to have a session in which we listened to their experiences and journeys through the diagnosis pathway and asked them to tell us their opinion about our diagrammatic representation and understanding of the diagnostic pathway. We then asked their opinions about the diagnostic pathway within primary care and our diagrammatic representation of this.
Two individuals diagnosed with bowel cancer, who were brother and sister, attended the meeting. Their bowel cancers were diagnosed through a test to assess whether or not they had inherited the gene after their father passed away from bowel cancer. Both diagnoses occurred at secondary care, so neither of them had any experience of diagnosis practice in primary care. Although at this point we knew that we would not obtain information on the diagnostic pathway in primary care, we decided to take this opportunity to discuss our understanding and modelled representation of the diagnostic pathway. After listening to their descriptions of the pathway and their very difficult experiences with multiple operations, we asked them if they could identify any potential misrepresentation of reality in an animated presentation covering the progression through the diagnostic pathway in stages, from the patient attending general practice owing to experienced symptoms to the moment when the GP decides to refer the patient to secondary care for a colonoscopy. We received very encouraging, although limited, feedback on our diagnosis pathway.
Other letters of invitation to Bowel Cancer West were published via Facebook, but, after weeks of not receiving any messages, we decided to focus on the other key stakeholder, the GP.
We opted to conduct this process in two steps. First, we opted to consult with a recently retired GP from Somerset to assess whether or not our explanations of key economic concepts and our diagnostic pathway were clear and suitable for presentation to a wider number of health professionals. As a warming-up task, a short interview was carried out in which we focused on the number of years of practice by the respondent and her experience with cancer diagnosis, that is number of patients diagnosed per year, some issues with cases diagnosed at late stage and similar topics, which allowed us to build a clear picture on the process and interactions taking place at the general practice involving symptomatic patients with suspected cancer.
What we learnt from this interview corroborated the evidence available:
-
too many guidelines, so it is difficult to keep all of them in mind
-
very few patients per year picked up at early staging in primary care
-
symptoms easily wrongly attributed to other diseases, mainly in those aged > 65 years with comorbidities.
We then moved to have a more in-depth conversation of the diagnostic pathway with the same retired GP. However, we changed our prior strategy and let her tell us the different steps that a GP would take if they suspect that a patient may have bowel cancer. When the reasoning behind the choices was unclear, we asked for further clarification. Notes were taken that were compared with our preconceived diagnostic pathway, thereby permitting us to have a better understanding of the current pathway for bowel cancer at general practices.
A third workshop was organised at a local general practice in Exeter. We prepared a modified diagnosis pathway, constructed using the current NICE guidelines172 and complemented with the strategies learnt from the previous interactions with the patients and the retired GP. To this diagnosis pathway we added the strategy of the ‘diagnostic tool’. The diagnostic pathway was printed on colour A7 pages that we brought with us for discussion with the GPs.
All the practice’s GPs (n = 7) attended the workshop, as well as two nurses, and the group discussion was transcribed. The GPs had very different levels of experience in terms of number of years of practice. Some of their opinions were as follows:
Tools would be useful if you could use as a justification for referral – but currently still have to meet NICE criteria to get onto 2WW. The criteria are ‘rigid’ and some referrals might get bounced back if the results from a blood test are borderline.
We use a ‘tool’ which is a calculated risk in our head.
Consider litigation – normally more likely to get sued for not doing something.
Borderline’ cases often bounced back by secondary care – they would need to accept the ‘clout’ of the diagnostic tool/aid.
We also asked them if there was any reason why they would not consider using the tool. The answers were as follows:
Stuck in ways.
Doing similar anyway – just in heads.
Don’t have the tools ‘to hand’.
We asked in what circumstances they would consider using the diagnostic tool:
To verify the ‘gut feeling’ was true, not only for referral, but for reassurance, when deciding against urgent referral or more tests, etc.
A follow-up question was if the diagnostic tool would help them to decide whether or not a patient needed referral:
Seemed this would only be the case if a tool had NICE backing.
After these questions, all the different strategies in our diagnosis pathway were discussed, which resulted in Figure 5. An additional one-to-one meeting was held with one of the GPs to ask for further clarifications.
FIGURE 5.
Diagnosis pathway model.

The diagnostic pathway
A decision-analytic model was used to investigate the key areas of uncertainty in the economic evaluation of primary care tools for informing decisions on referrals of suspected cancer patients from primary to secondary care in England.
The analysis compares costs and clinical outcomes between a diagnostic strategy whereby primary care doctors use the diagnostic algorithm in conjunction with information from investigations in primary care to decide whether or not a patient presenting with low-risk symptoms needs to be referred to secondary care for further investigations, and a strategy of primary care investigations alone. We compare the intervention strategy of RATs (CAPER)75 or QCancer80 as a triage to primary care investigations (which, in the model, is represented by FITs) or direct referral to secondary care (intervention) with the following comparator strategies:
-
ordinary referral to secondary care without prior primary care investigations for all patients (‘refer all’ strategy)
-
primary care investigations alone
-
send home/wait (‘send home and wait’ strategy).
In Figure 5, we illustrate in the diagnosis pathway for patients with low-risk symptoms who do not fulfil the 2WW criteria.
In the absence of clinical studies providing evidence on the clinical outcomes of these strategies, the effect of the diagnostic algorithm on patient management is modelled using a linked-data approach, which translates evidence of diagnostic accuracy into clinical outcomes in terms of life expectancy, health-related quality of life and health-care costs. In the model, the gain in sensitivity with the algorithm may cause an increase in the number of patients being referred to secondary care, which, in turn, results in the earlier identification of CRC cases; this is the main driver of outcomes in the model. An increase in sensitivity may also change the composition of diagnosis by disease stage, but this is harder to model without further data. The clinical benefits of the diagnostic tools depend on detecting cases at earlier disease stages than those that would occur under the status quo, without use of the diagnostic tools.
Population
To implement our model-based analysis, we define the relevant ‘low-risk’ population as those symptomatic patients presenting for the first time to a GP to seek care for their symptoms. We use prevalence estimates from Hippsley-Cox and Coupland,80 who obtained data on the number of identified CRC cases from the electronic records of 224 primary care practices in the UK over a 2-year period (from January 2000 to April 2002) following the first visit of a patient with eligible symptoms to a GP. When patients in the top 1% risk stratum are excluded, the estimated CRC prevalence rate in this population is 0.16%. 80 As the mean age in the sample was 50.1 years, and prevalence reportedly increased ‘steeply’ with age, for the case of our modelled base-case analysis, aged 70 years, a prevalence rate of 1.5% is used, which is consistent with the age effects in the risk calculator published by the same authors80 and is consistent with previous analyses informing policy. 140 No other sources of reliable prevalence data were identified.
This patient population is akin to the ‘low risk but not no-risk symptoms’75 population of the CAPER study. 75 The patient population includes the majority of symptomatic patients who do not present with high-risk symptoms, such as rectal bleeding or severe anaemia, and, therefore, are not diagnosed through the 2WW referral pathway. These are the populations the tools were developed for.
Interventions
The intervention involves use of the diagnostic tool and referral of those individuals above the threshold score of 35, which corresponds, approximately, to 2% in RAT and 0.5% in QCancer, to secondary care for investigation, which, in the model, is assumed to be colonoscopy. Although some patients undergo alternative tests such as flexible sigmoidoscopy, CTC and barium enema because of the presence of certain symptoms, older age or patient choice, only colonoscopy was modelled, as it is the most common test used. 155 This assumption was intended to avoid unnecessary complexity, given the exploratory aims of the model. For those individuals with a score below the threshold, the diagnostic tool is followed by primary care investigations, which, in the model, are assumed to consist of FITs at the 20-µg threshold (hereafter referred to as FIT). A subgroup of patients may also be sent home without a FIT if the presenting symptoms result in a score below a certain minimum level. We do not have adequate information on diagnostic accuracy for this group of patients deemed to be at too low a risk to warrant investigation; therefore, it is assumed that all patients below the threshold undergo a FIT. As a result, our analysis assumes that using the tools will always result in some further action. Figure 6 shows the decision model for patients with CRC and Figure 7 shows the decision model for patients without CRC.
FIGURE 6.
Diagnostic decision model for patients with CRC. p1, number of cases with a positive test result using the diagnostic tool/total number of CRC cases; p2, number of cases with a positive test result using the FIT test/total number of CRC cases.

FIGURE 7.
Diagnostic decision model for patients without CRC. p3, number of patients with a negative test result using the diagnostic tool/total number of patients without CRC; p4, number of patients with a negative test result using the FIT test/total number of patients without CRC.
A key difference between the diagnostic model for CRC patients and the model for non-CRC patients in Figures 6 and 7 is that the proportion of patients referred to secondary care is based on the sensitivity (p1 and p2) of the tests for CRC patients and specificity (p3 and p4) of the tests for patients without CRC. In addition, patients without CRC are assumed not to return to general practice after a negative test result.
Comparators
Faecal immunochemical test given to all
Based on clinical judgement, the standard diagnostic practice involves referral to secondary care after conducting FITs for all patients. Pragmatically, we assume that the referral decision rule consists of referring those patients who would have a positive FIT result, and sending home patients with negative results for watchful waiting. We chose to use this as the relevant comparator based on advice from local practitioners and current NICE Diagnostic Guideline recommendations,140 and guided by the forthcoming roll-out of this test as a screening test nationally. Thus, we acknowledge that at the time this report is published FIT is not yet universally used.
Send home/wait
General practitioners may send a patient home without undertaking any primary care investigations, and wait until the symptoms fail to subside and the patient makes a repeat visit before ordering such investigations. We have evaluated this option as a scenario involving an initial visit whereby the patient is sent home without having undergone primary care investigation and a return visit the following month where all (undiagnosed) CRC cases and none of the non-CRC patients return for a FIT. We vary the proportion of non-CRC patients who return for a second visit in sensitivity analyses. All outcomes and patient management pathway from the second visit are as in the ‘FIT given to all’ pathway.
After the referral decision, those with correct negative results, the true negatives, are assumed not to return to the general practice. The same applies for those with incorrect positive results, the false positives. As for CRC patients with incorrect negative test results, the ‘false-negative’ cases, it is assumed that they will return 1 month later with persistent symptoms. The pathway for these patients and those correctly identified, the ‘true-positive’ cases, is further determined by the disease model described further on.
Diagnostic pathway model
To predict the diagnostic interval associated with each of the diagnostic strategies, we made the following assumptions:
-
In the strategies involving testing, namely the two intervention strategies involving a tool plus FIT and the FIT-alone investigation strategy, patient management is determined by the result of the diagnostic test. In our base-case analysis, we allow for partial adherence to the diagnostic protocol, whereby 20% of positive results do not lead to referral or the referral is not realised.
-
In the intervention strategies whereby the tool produces a score below the threshold so that there is a sequence of two tests, the tool first and FIT second, we assume that the sensitivity and specificity of the second test in the sequence is independent of the outcome of the first test. This is likely to be an optimistic assumption, as discussed further on.
-
A CRC patient who remains undiagnosed after a first symptomatic presentation to a GP will have repeat monthly GP visits until diagnosis or death. In scenario analyses, we limit the number of repeat monthly visits to a maximum of 12, after which the GP refers the patient to secondary care without any further testing.
-
The sensitivity of the overall strategies determines the number of visits and monthly cycles before referral in the model. This assumption is what ultimately drives the clinical effectiveness in the model, which may be justified by the evidence available from Lyratzopoulos et al. 173 showing that the number of GP visits before diagnosis of CRC patients is positively associated with the median time to referral.
-
In the absence of data, we assume that the accuracy of diagnostic tests is independent of the disease stage at presentation. Therefore, the same intervention effect on the diagnostic interval relative to the comparators arising from assumptions 1, 2 and 3 leads to different clinical outcomes across initial disease stages, given their different rates of disease progression and responsiveness to treatment (see Disease history model linking test results and clinical outcomes). We obtain an aggregate predicted outcome for an initial distribution of disease stages in a simulated incidental cohort from our replication of the model described by Tappenden et al. 145
Disease history model linking test results and clinical outcomes
To model the implications of avoidable delays in referral and diagnosis, we employed the updated version of the disease model developed by Tappenden et al. ,145 as described in Whyte et al. 156 As we could not obtain the model from Whyte et al. ,156 we replicated it from information published on its methods. 145,156,174,175 As this disease history model was not developed for evaluating diagnostic strategies for identifying cancer patients in primary care, we adapted it to focus entirely on the symptomatic stage population of present interest. An illustration of the adapted Markov model of health-state transitions experienced by CRC patients visiting their GP with symptomatic disease is presented in Figure 8. Briefly, an undiagnosed patient presents to primary care with symptoms at one of the four possible disease stages and is managed according to one of the diagnostic strategies investigated in this study. The patient’s disease is detected and he/she is referred to secondary care for investigation with a certain probability (p), which we assume is equal to the sensitivity of the strategy, or is missed with a probability of 1 – p. Once in secondary care, the patient undergoes colonoscopy and, because this investigation has sensitivity approaching 100%, the transition probability to a cancer diagnosis from being undiagnosed equals the overall sensitivity of the strategy, p, times the probability of surviving until the end of the cycle, 1 – d.
FIGURE 8.
Markov model of health-state transitions for CRC cases.
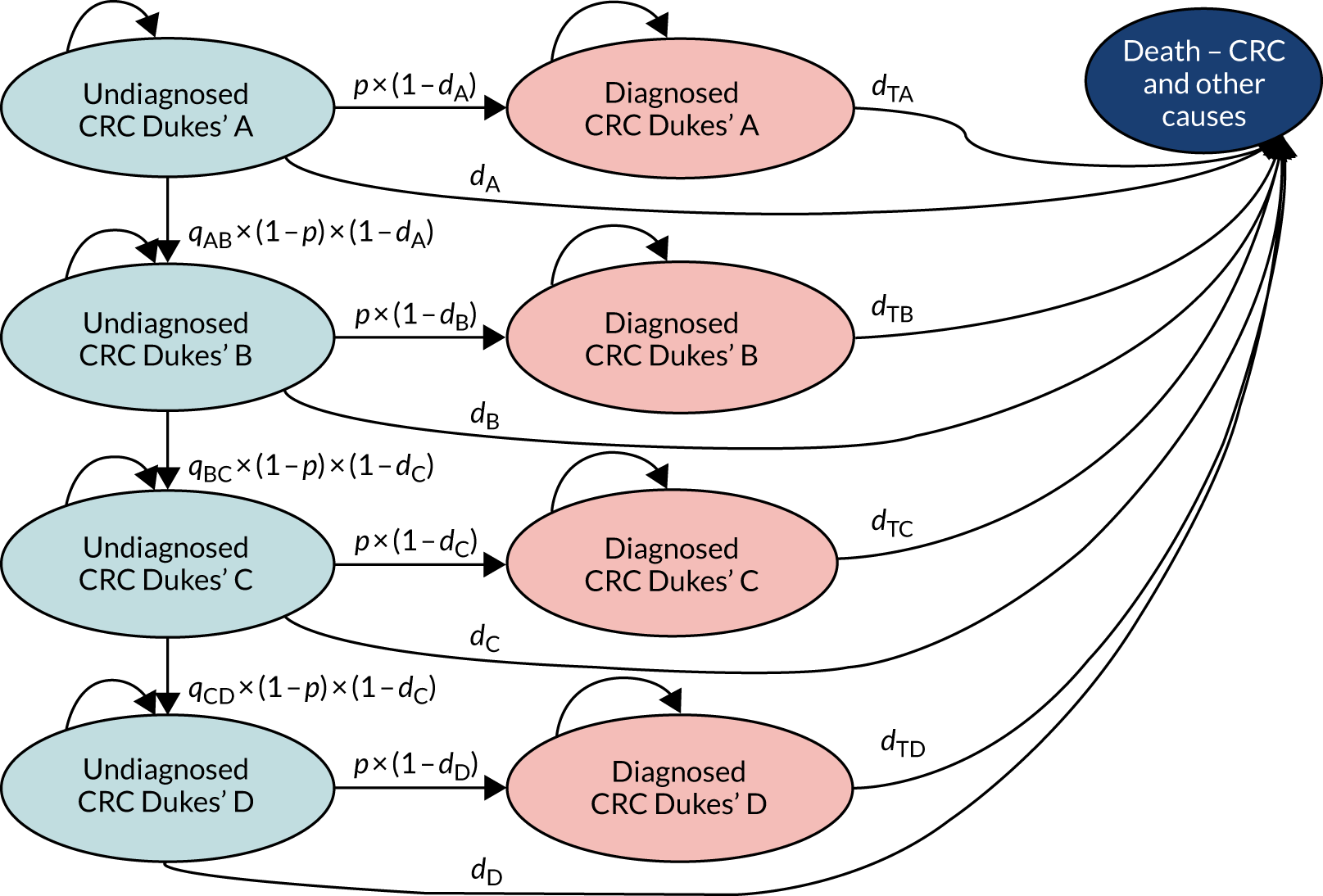
Colorectal cancer patients missed by the diagnostic strategy remain untreated and are at risk of progressing to the next stage of disease severity. The probability of disease progression from one cycle to the next is equal to the probability of a false-negative result with the diagnostic strategy times the probability of remaining alive by the end of the cycle times the probability of disease progression (q), q × (1 – d) × (1 – p). If, after repeated cycles of visits to the GP, the patient remains undiagnosed, the disease stage D would be reached, for which the only possible transition, other than to starting treatment, is to death from CRC or other causes.
In this model, a patient with CRC who, under the intervention, is identified earlier than under the control, say Dukes’ stage A or B instead of C or D, derives both short-term and long-term health benefits. The short-term benefits occur as soon as treatment starts in the form of death risk reduction, whereas the longer-term benefits arise from avoiding disease progression to Dukes’ stages C or D, for which treatment options are less effective. In terms of Figure 8, the excess death risk of not receiving treatment is captured by the relative magnitude of death probabilities on and off treatment for each of the Dukes’ disease stages A, B, C and D: dTA < dA, dTB < dB, dTC < dC and dTD < dD, respectively. The long-term survival loss from delays in diagnosis is measured by the positive relationship between the probability of CRC-related death and disease severity, that is dTA < dTB < dTC < dTD. The reduction in death risk is assumed to continue for the rest of the patient’s life, apart from the maximum survival ceiling imposed by the background mortality risks included in the model, as measured in life tables176 for the general English population of the same age and sex. In the scenario analysis, we explore the effects of adopting the assumption in Whyte et al. ,152 whereby patients who remain alive at the end of the 5-year period after diagnosis have, from then on, the same cycle probability of death as that of the general population of the same age and sex, that is negligible CRC death risk.
For patients with no CRC, a two-state Markov model was used that followed the initial decision tree of Figure 5. After the initial decision of whether or not to refer those patients, those referred undergo colonoscopy and are exposed to its associated small risk of adverse events, whereas those not referred are spared such exposure. Patients who are alive after colonoscopy or not referred to colonoscopy are in the alive health state and may transition to death from non-CRC causes, as determined by the death risks in English life tables, or remain alive at the start of the next cycle.
Mechanism of effect
In the absence of direct clinical effectiveness data, we assume a relationship between the sensitivity of both the algorithms and routine referral practice and the time to diagnosis. We break the time from symptom presentation to diagnosis into (1) the time from presentation to primary care to referral for investigation (the ‘referral interval’ in Chapter 5) and (2) the time from referral to specialist care to diagnosis and treatment, and assume that the diagnostic algorithms affect only the referral interval, while the time from referral to diagnosis and treatment remains fixed. Thus, in our analysis, a reduction in the diagnostic interval associated with any improvement in primary care diagnostic accuracy is equal to the reduction in the time from presentation to referral that is mediated through the number of primary care visits before referral.
Given our chosen population, we model the clinical effectiveness as a function of the diagnostic outcome of the first visit, assuming that, under current clinical practice, true-positive cases are referred after two visits, to allow for primary care investigations to be conducted and discussed between the patient and the doctor. False-negative cases would see their symptoms persist and would return for a third, or possibly more, visits. Because we have no obvious way to infer how a change in primary care diagnostic accuracy would affect the distribution of second and subsequent visits, we assume that the sensitivity and specificity are independent across multiple visits to primary care. This may be incorrect because individuals who are missed on an initial visit are more likely to be missed on a second visit, and so on. Indeed, we infer the probable extent of this in primary care referral practice in Table 15 and investigate the implications in sensitivity analyses (see Scenario analyses). Thus, a probability of referral after two, three, four and five or more visits is calculated, respectively, as:
| Referral after | Actual frequencya | Median days to referrala | Implicit sensitivity by visitb | Predicted frequencyc | Median days to referrald | Source |
|---|---|---|---|---|---|---|
| 2 visits | 0.76 | 18 | 0.76 | 0.76d | 18 | Analysis of data from the English National Audit of Cancer Diagnosis in Primary Care 2009–10, on 1170 general practices (≈ 14% of all English practices)173 |
| 3 visits | 0.12 | 39 | 0.50 | 0.18 | 39 | |
| 4 visits | 0.05 | 56 | 0.42 | 0.045 | 56 | |
| ≥ 5 visits | 0.06 | 122 | 1.0 | 0.01d | 122 | |
| Weighted average | 1 | 29 | N/A | 1.0 | 25 |
Under the diagnostic sensitivity of 0.76 implicit in the results reported by Lyratzopoulos et al. ,173 the predicted frequency for three, four and five or more visits is 18%, 4% and 1%, respectively, which is in contrast with the actual frequencies of 12%, 5% and 6%, respectively. Consequently, the predicted median referral interval of 25 days is lower than the actual figure of 29 days (see Table 15).
The length of time to referral (i.e. diagnosis in our model) is derived from the number of primary care visits from previous research. 173 Time to referral and the number of primary care visits before referral are strongly positively associated, with a threshold of 16 or 17 days discriminating between cancer patients referred after three or more and two or fewer visits. 173
In our Markov model of 28-day cycles, the length of the referral interval is equal to the number of initial repeat GP visits times 28 days (Table 16). The resulting times to referral implicit in the sensitivity values of the tests in each strategy adopted for the model are presented in Table 16. These base-case values correspond to sensitivity and specificity values of 69% and 77%,141 61% and 91%80 and 53% and 99%175 for the RAT, QCancer and FIT of 20 µg Hb/g faeces, respectively, after adjusting for the 80% compliance with diagnostic results, so that 20% of those testing positive would not be referred but sent home. These values are an approximation to the model results, which also account for the attrition caused by death from CRC or other causes before referral, so that the values in Table 16 are greater than the model outputs by 2–2.5 days. When the sensitivity values implicit in the number of pre-referral visits reported in a retrospective patient experience survey (Lyratzopoulos et al. 173) are used for FITs, the reduction in the time to referral relative to the comparator is only 7 days for QCancer and 8 days for the RAT (see Appendix 5).
| Referral after | QCancera | Comparatorb | Referral after | RATa | Comparatorb | ||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted frequencyc | Mean time to referral (days) | Predicted frequencyd | Mean time to referral (days) | Predicted frequencyc | Mean time to referral (days) | Predicted frequencyd | Mean time to referral (days) | ||
| 1 visit | 0.49 | 28 | N/A | 28 | 1 visit | 0.55 | 28 | N/A | 28 |
| 2 visits | 0.27 | 28 | 0.42 | 28 | 2 visits | 0.21 | 28 | 0.42 | 28 |
| 3 visits | 0.12 | 56 | N/A | N/A | 3 visits | 0.13 | 56 | N/A | N/A |
| 4 visits | 0.07 | 56 | 0.24 | 56 | 4 visits | 0.05 | 56 | 0.24 | 56 |
| ≥ 5 visits | 0.06 | 93 | 0.34 | 123 | ≥ 5 visits | 0.06 | 93 | 0.34 | 123 |
| Weighted average | 1 | 37 | 1 | 67 | Weighted average | 1 | 37 | 1 | 67 |
A note of warning is due as to the lack of comparability between the accuracies of the RAT and QCancer. Their specificity and sensitivity values were derived from studies using different designs (a case–control study for the RAT and cohort data from electronic records for QCancer) and populations. This would render any comparison of the tools biased. We note that similar arguments could also be raised in relation to the comparison of each tool with the comparator, as this is also based on an indirect comparison of heterogeneous single-arm accuracy studies; this fact reflects the low quality of data available for analysis.
To complete the link from diagnostic accuracy outcomes and number of cycles before referral (which determines the time to treatment because the time from referral to diagnosis and treatment is assumed to be unaffected) with survival outcomes, we use the relationship between time to diagnosis and disease stage at diagnosis. The only available source for this unobservable parameter is provided by a calibration exercise of a decision model based on Tappenden et al. 155 It reports rates of progressive transition between (non-clinical) Dukes’ stages A to B, B to C and C to D, and from each of these to death, which, for stages earlier than D, was exclusively unrelated to cancer. We assume that the disease progression rates from this source, which were intended to depict the experience of patients in asymptomatic states, apply to our symptomatic population, but combine these with CRC mortality rates reported by disease stage in untreated patients (Liu et al. 177) Table 17 presents the mean time to progression from Dukes’ stages A to B, B to C and C to D implied by the disease progression rates adopted.
| Dukes’ stage transition | Annual transition probability (%) | Mean time to progression (months) | Source |
|---|---|---|---|
| A to B | 58.29 | 12.3 | Authors’ calculations from reported model-calibrated dataa |
| B to C | 65.55 | 9.0 | |
| C to D | 86.48 | 4.3 |
The reductions in time to diagnosis and treatment made possible by the smaller number of initial repeat visits to the GP have the ultimate impact of increasing the likelihood of starting treatment at a stage of disease for which treatment is more effective. We employ data on relative survival (with respect to the general population) by disease stage at diagnosis, depicted in Figure 9, to model this longer-term health benefit of earlier access to treatment with more accurate GP diagnosis and referral. 178 We present the expected survival over time of an individual who is diagnosed while at Dukes’ stage B and the counterfactual, improved survival, scenario that would have been observed had this simulated case been diagnosed earlier while still in Dukes’ stage A (Figure 10).
FIGURE 9.
Relative survival of CRC patients diagnosed in 1996–2002 in England. Survival curves by disease stage. Source: National Cancer Registration and Analysis Service. 178

FIGURE 10.
Actual and counterfactual survival curves for a patient who is diagnosed at Dukes’ stage B.
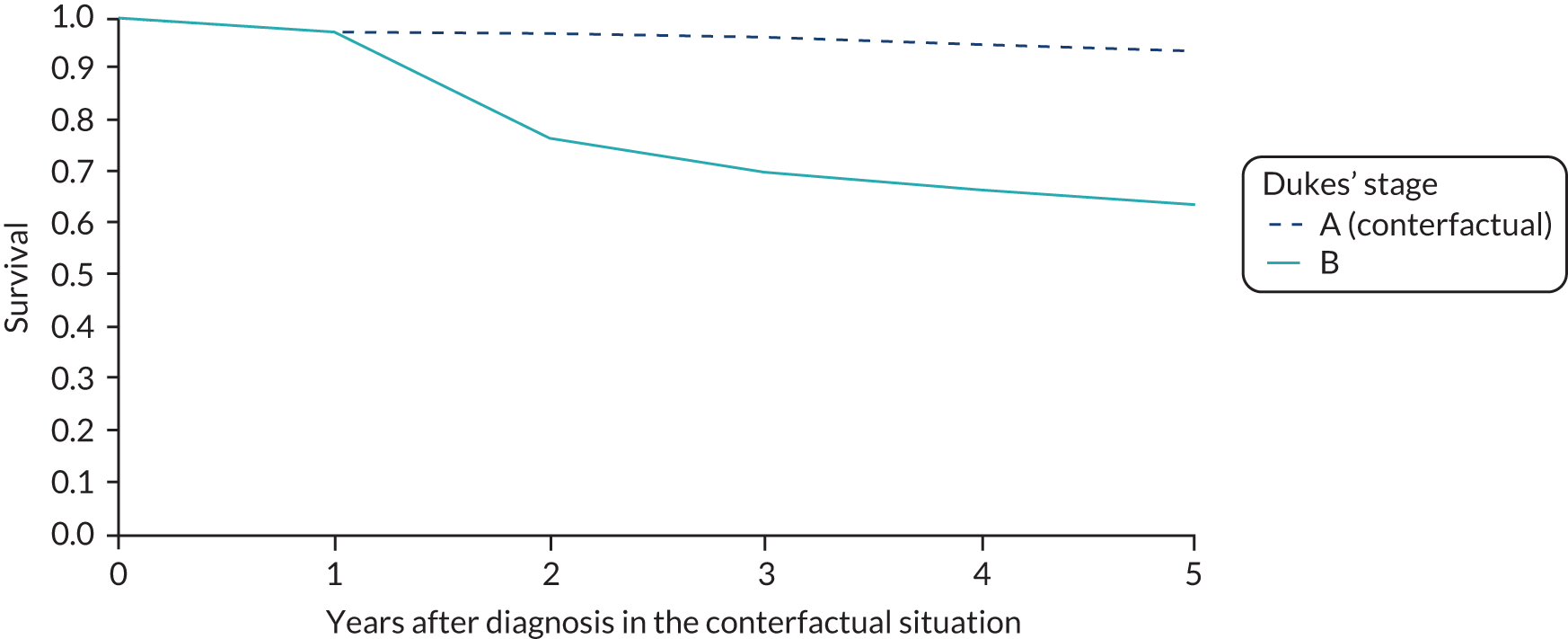
In Table 18, the link between diagnostic accuracy and disease stage at diagnosis, which is mediated by the number of pre-referral visits (cycles) and underpins the clinical effectiveness in the model, is made explicit for each strategy. The more accurate intervention strategies shift the distribution of disease stage at diagnosis towards the earlier disease stages of Dukes’ stages A and B, and C (not shown in the table). The overall percentage of stages A/B presented in the table do not account for background mortality effects; therefore, they differ by about 1 percentage point from the final model estimates, which do account for such mortality. When the sensitivity values implicit in the number of pre-referral visits reported in a retrospective patient experience survey (i.e. Lyratzopoulos et al. 173) are used for FITs, there is a 1-percentage-point gain in the proportion of patients with Dukes’ stages A/B at diagnosis with both QCancer and the RAT, relative to the comparator (see Appendix 5). Therefore, an improvement in the sensitivity of primary care diagnostic testing would be expected to reduce the time to referral and, provided this is translated into a one-to-one reduction in the time to diagnosis and treatment, increase the relative frequency of earlier disease stages at diagnosis. Stage at diagnosis would then determine the long-term clinical outcomes and costs in the model.
| Referral after | QCancer | Comparator | Referral after | RAT | Comparator | ||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted frequency | Dukes’ stage A/B (%) | Predicted frequency | Dukes’ stage A/B (%) | Predicted frequency | Dukes’ stage A/B (%) | Predicted frequency | Dukes’ stage A/B (%) | ||
| 1/2 visits | 0.65 | 54 | 0.42 | 54 | 1/2 visits | 0.68 | 54 | 0.42 | 54 |
| 3/4 visits | 0.23 | 51 | 0.24 | 51 | 3/4 visits | 0.22 | 51 | 0.24 | 51 |
| 5/6 visits | 0.08 | 48 | 0.14 | 48 | 5/6 visits | 0.07 | 48 | 0.14 | 48 |
| 7/8 visits | 0.03 | 45 | 0.08 | 45 | 7/8 visits | 0.02 | 45 | 0.08 | 45 |
| 9/10 visits | 0.01 | 42 | 0.05 | 42 | 9/10 visits | 0.01 | 42 | 0.05 | 42 |
| ≥ 10 visits | 0.01 | ≤ 40 | 0.07 | ≤ 40 | ≥ 10 visits | 0.00 | ≤ 40 | 0.07 | ≤ 40 |
| Weighted average | 1 | 52 | 1 | 50 | Weighted average | 1 | 53 | 1 | 50 |
The values of key model parameters are presented in Table 19. These values were identified from reviews of previous decision models in CRC, described in Chapter 6. This choice of data sources was partly determined by the fact that, in contrast to our research plans, the decision model of Whyte et al. 156 was not made available to our study. As a result, we had to reallocate the research resources that had been originally assigned for conducting a systematic review of model parameter values to building the model from scratch by replicating its published methods. Further details of the methods and data used to populate the disease model parameters are given in Appendix 5.
| Parameter | Value | Comment | Source |
|---|---|---|---|
| Clinical | |||
| Starting age, 70 years | |||
| Prevalence of CRC | 0.015 | Symptomatic low-risk population, as in analysis informing NICE Diagnostic Guideline Number 30140 | Hippsley-Cox 201280 |
| Disease stage at presentation | Age-specific distribution by Dukes’ stage | Simulated by authors from replicated disease history model (Tappenden 2007145) | |
| Sensitivity of QCancer | 0.610 | Based on the top 10% risk score in their tool, which gives a risk threshold of 0.5% (PPV 1.5%). We recalculated their sensitivity and specificity of this threshold to exclude observations in the top 1% risk score range (5.2% risk threshold) and thus get closer to the low-risk population (0.1–3.0% prevalence); unfortunately, the source does not give the required breakdown of results to exclude people above the 3% threshold | Hippsley-Cox 201280 |
| Specificity of QCancer | 0.910 | ||
| Sensitivity of the RAT | 0.69 | Based on the 2% risk threshold | Hamilton 2005141 |
| Specificity of the RAT | 0.77 | ||
| Sensitivity of the FIT | 0.526 | FIT 20 µg Hb/g faeces | Murphy 2017175 |
| Specificity of the FIT: 50–69 years old | 0.988 | ||
| Specificity FIT: ≥ 70 years old | 0.963 | ||
| Compliance with colonoscopy referral | 0.80 | Tappenden 2007145 | |
| Colonoscopy sensitivity for CRC | 1 | Murphy 2017175 reports a value of 0.966 from a systematic review (i.e. van Rijn 2006179) | Assumption |
| Colonoscopy specificity for CRC | 1 | Assumption | Murphy 2017175 |
| Probability of perforation with colonoscopy | 0.0017 | Atkin 2002180 | |
| Probability of death with perforation | 0.0582 | Gatto 2003181 | |
| Probability of bleeding | 0.0044 | Atkin 2002180 | |
| Transition probabilities | 4 weekly | Whyte 2014156 | |
| Dukes’ stage A to B | 0.0651 | Reported model calibration to CRC incidence and stage at diagnosis | Tappenden 2007145 |
| Dukes’ stage B to C | 0.0787 | ||
| Dukes’ stage C to D | 0.1427 | ||
| CRC death risk Dukes’ stage A | 0.00 | Post Dukes’ stage A at diagnosis | Exponential hazard function fitted to digitised Kaplan–Meier curves up to 5-year relative survival for CRC patients (diagnosed 1996–2002) by stage at diagnosis in England178 |
| CRC death risk Dukes’ stage B | 0.00 | Post Dukes’ stage B at diagnosis | |
| CRC death risk Dukes’ stage C | 0.01 | Post Dukes’ stage C diagnosis | |
| CRC death risk Dukes’ stage D | 0.07 | Post Dukes’ stage D at diagnosis | |
| HR untreated CRC in Dukes’ stage A | 1 | Survival of patients refusing treatment with newly diagnosed CRC in Taiwan (Province of China) during 2004–8. Treatment refusal was defined as ‘not undergoing any cancer treatment within 4 months of confirmed cancer diagnosis177’ | Liu 2014177 |
| HR untreated CRC in Dukes’ stage A | 1.22 | ||
| HR untreated CRC in Dukes’ stage A | 1.22 | ||
| HR untreated CRC in Dukes’ stage A | 1.22 | ||
| Costs | |||
| Cost of FIT | £3 | Threshold 20 µg Hb/g faeces. Published data, excludes costs of screening campaign in the original source | Murphy 2017175 |
| Cost of GP visit | £31 | PSSRU’s unit cost per surgery consultation lasting 9.22 minutes, without qualification costs and including direct care staff costs | Curtis 2018182 |
| Cost of colonoscopy | £615 | Costs of colonoscopy with or without polypectomy | Whyte 2014,156 NHS reference costs, screening centre estimates152 |
| Cost of treating bowel perforation | £5559 | Major surgery | Whyte 2014,156 NHS reference costs |
| Cost of bleeding | £304 | Cost of admittance for bleeding (overnight stay on medical ward), NHS reference costs | Whyte 2014156 |
| Health-care cost, Dukes’ stage A | £3178 |
|
Whyte 2012174 |
| Health-care cost, Dukes’ stage B | £3455 | ||
| Health-care cost, Dukes’ stage C | £4485 | ||
| Health-care cost, Dukes’ stage D | £4365 | ||
| Utilities | |||
| Cancer free | 0.91 | Whyte 2014.156 General population utility decline with age by sex was accounted for using the linear model proposed by Ara 2010183 | Ness 1999184 |
| Dukes’ stage A | 0.74 | ||
| Dukes’ stage B | 0.70 | ||
| Dukes’ stage C | 0.50 | ||
| Dukes’ stage D | 0.25 | ||
Calculations of costs and benefits in the model
Our Markov model consists of a repeated iteration of 28-day cycles, whereby patients accrue costs and benefits depending on the health state occupied in each iteration. We chose this cycle length as the most appropriate for capturing the frequency with which patients with unresolved symptoms return to their GPs for care. By aggregating the proportion of patients occupying the various health states in each 28-day cycle across the modelled time horizon of 30 years, we calculated the total time alive and total time spent in each state. We applied costs and health-state utility values corresponding to the different states and aggregated across health states and time periods to calculate total health-care costs and total QALYs. The exception to this method is the estimation of health-care costs of cancer treatment, which are implemented as a single lifetime cost payoff incurred at diagnosis, as in the original model by Whyte et al. 156 The modelled accumulated total life-years, QALYs and lifetime health-care costs for the intervention and the status quo strategies, over a period of 30 years after an initial presentation with symptoms to the GP, are presented for a hypothetical cohort aged 70 years at first presentation. We initially focus on the predicted clinical outcomes for the subgroup of CRC cases and then present the results for the overall cohort presenting to their GP with symptoms suggestive of CRC.
Table 20 compares the main features of our model with existing models relevant to our study question. Common to ours and the previous models is the use of a disease history model developed by Tappenden et al. 145 Whyte et al. ’s156 model is a model of screening and therefore does not address the question of interest here. The main differences between our and Westwood et al. ’s161 work is that we explicitly model the time to referral consistent with the diagnostic accuracy of the respective test strategies and the distribution of the number of visits before referrals; we chose a shorter cycle length (28 days vs. annual) to allow greater granularity for predicting outcomes; and we model the short-term survival benefits of reducing the time to referral and treatment. Like Westwood et al. ’s161 and Whyte et al. ’s156 models, we have implemented a probabilistic sensitivity analysis, but with the caveat that this type of sensitivity analysis was of limited relevance because most of the uncertainty derives from structural assumptions, such as how the length of the diagnostic and treatment interval is determined, defining the status quo diagnostic practice and measuring its diagnostic accuracy. Consequently, we did not conduct any value-of-information analysis.
| Study | Perspective | Population | Diagnostic strategies | Outcome measure(s) | Model structure | Model duration | Uncertainty analysis | Discount rate (%) | Base year and currency |
|---|---|---|---|---|---|---|---|---|---|
| Our current model | UK NHS | Symptomatic patients aged ≥ 40 years presenting to primary care who are at low risk of CRC |
|
Cancer mortality, disease stage at diagnosis, life-years, QALYs | Markov model with monthly cycles | Lifetime |
|
3.5 | 2018; GBP |
| Whyte 2014156 | UK NHS | General population | N/A | Cancer mortality, QALYs | State-transition model with annual cycles | Lifetime | Two-way sensitivity and probabilistic sensitivity analyses | 3.5 | 2012; GBP |
| Westwood 2017161 | UK NHS | Symptomatic patients aged ≥ 40 years presenting to primary care who are at low risk of CRC |
|
QALYs, life-years | Markov state-transition model with annual cycles | Lifetime | Scenario analysis and probabilistic sensitivity analysis | 3.5 | 2015; GBP |
Deterministic sensitivity analysis
To investigate areas of uncertainty in our model, we arbitrarily varied clinical parameters by 20% above and 20% below their base-case analysis values. In addition, we undertook scenario analyses for the following parameters:
-
alternative sensitivity and specificity values for the comparator (inferred from the number of GP visits before referral173 and from receiver operating characteristic data for version 3 of NICE guidelines22)
-
assuming 100% compliance with the test result (i.e. all positive cases are referred and all negative cases are not referred to secondary care)
-
no mortality benefit of treatment in stages A/B, by setting the hazard ratio to 1
-
restricting excess CRC-related death risk to first 5 years156
-
limit time horizon of analysis to 5 years
-
alternative utility values adopting a common value for all CRC disease stages145
-
alternative ‘send home/wait’ comparator strategy, whereby, at the initial visit, the GP sends the patient home without conducting a FIT; from the second visit onwards, they manage the patient as in the ‘FIT given to all’ strategy
-
modified intervention strategy whereby patients who are above the risk threshold of the tool are referred directly to secondary care, as in the original intervention strategy (see Figure 2), whereas those below the threshold are sent home, rather than being subjected to primary care investigations, as assumed in our base-case analysis
-
assuming no survival gains and maximum quality-of-life (utility) gains (i.e. zero utility values for false-negative cases until diagnosis) from more expeditious referral and diagnosis in patients with CRC.
We present our results in 2017/18 prices. We provide tentative estimates of incremental costs per life-year gained. We also present results in terms of incremental cost per QALY gained, discounting costs and QALYs at an annual rate of 3.5%.
Results
Colorectal cancer patients
According to our model predictions, using the diagnostic tool triage interventions allow for sensitivity of 82–85%, compared with 53% for the current standard strategy of FIT for all. 175 The additional sensitivity of diagnosis would reduce the number of mean visits from 5.6 to 2.6. This, in turn, would lead to fewer patients dying undiagnosed (from 12% in the status quo to 8% with the diagnostic tools) and one additional case being diagnosed and treated in disease stages A/B for every 42 CRC patients [1/(0.009 + 0.015)] presenting to primary care with symptoms and suspected CRC (Table 21). The largest gains in life for a person aged 70 years occurs among individuals presenting with undiagnosed CRC of Dukes’ stage B (0.26 years) and stage C (0.31 years), whereas the smallest gain occurs among those presenting with stage D CRC, who derive a gain of 14 days with either diagnostic tool.
| Primary care diagnostic strategy | Overall sensitivity (%) | Mean number of GP visits | Dukes’ stage at diagnosis | Percentage dying without being diagnosed | Life expectancy by Dukes’ stage at first presentation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | Weighted mean | ||||
| FIT | 53 | 5.6 | 16.9 | 32.3 | 24.0 | 14.7 | 12.1 | 13.52 | 9.29 | 4.47 | 0.95 | 7.22 |
| QCancer + FIT | 82 | 2.6 | 17.9 | 33.8 | 25.4 | 14.9 | 8.0 | 13.68 | 9.55 | 4.77 | 0.99 | 7.43 |
| Difference: QCancer – FIT | N/A | 6.4 | 0.9 | 1.5 | 1.4 | 0.0 | –4.1 | 0.16 | 0.26 | 0.31 | 0.04 | 0.21 |
| RAT + FIT | 85 | 2.4 | 18.0 | 34.0 | 25.6 | 14.9 | 7.6 | 13.69 | 9.56 | 4.79 | 0.99 | 7.44 |
| Difference: RAT – FIT | N/A | 5.0 | 1.0 | 1.7 | 1.6 | 0.1 | –4.5 | 0.17 | 0.28 | 0.33 | 0.04 | 0.23 |
Use of a diagnostic tool is predicted to reduce the amount of time spent in untreated health states by about 0.6 months (18 days), and to increase the amount of time alive while on treatment by 3 months, mostly after diagnosis at Dukes’ stage A or B (Table 22). The net effect is to increase life expectancy by 2.4 months, at an extra cost of between £29 and £36. These figures do not account for costs and health outcomes in patients without CRC, which are included in the whole-population analysis next.
| Primary care diagnostic strategy | Mean number of GP visits | Mean time spent in each health state (months) | Life-years | QALYs | Cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | TA | TB | TC | TD | |||||
| FIT alone | 5.6 | 0.2 | 0.4 | 0.3 | 0.2 | 29.1 | 39.0 | 15.2 | 2.2 | 7.22 | 3.65 | 4428 |
| QCancer + FIT | 2.6 | 0.1 | 0.2 | 0.1 | 0.1 | 30.3 | 40.4 | 15.8 | 2.2 | 7.43 | 3.87 | 4458 |
| QCancer + FIT minus FIT alone | –3.0 | –0.1 | –0.2 | –0.2 | –0.1 | 1.2 | 1.4 | 0.6 | 0.0 | 0.21 | 0.22 | 29 |
| RAT + FIT | 2.4 | 0.1 | 0.1 | 0.1 | 0.1 | 30.3 | 40.5 | 15.9 | 2.2 | 7.44 | 3.89 | 4464 |
| RAT + FIT minus FIT alone | –3.2 | –0.1 | –0.3 | –0.2 | –0.1 | 1.2 | 1.5 | 0.7 | 0.0 | 0.23 | 0.24 | 36 |
Whole-population analysis
When the costs and life-years are calculated over the denominator of all patients presenting with symptoms of suspected CRC, the importance of the trade-off between sensitivity and specificity becomes apparent. Detecting more CRC cases with diagnostic tool triage comes at the cost of imposing more unnecessary use of health-care resources and health risks from invasive investigations on the great majority of patients who do not have CRC. The costs associated with false-positive cases drives the cost difference between the diagnostic tool plus FIT and FIT alone, resulting in an extra cost of £42 or £105, depending on the diagnostic tool, per patient presenting with symptoms of suspected CRC. At the 1.5% prevalence rate of this low-risk population, the (undiscounted) additional cost per life-year saved is (42/0.0031) £13,548 with QCancer and (105/0.0032) £32,813 with the RAT (Table 23).
| Outcome | QCancer + FIT | FIT alone | Difference: QCancer + FIT minus FIT alone | RAT + FIT | FIT alone | Difference: RAT + FIT minus FIT alone |
|---|---|---|---|---|---|---|
| Costs by initial test result (£) | ||||||
| TN | 58 | 62 | –4 | 51 | 62 | –11 |
| FP | 65 | 20 | 45 | 135 | 20 | 114 |
| TP | 44 | 29 | 16 | 46 | 29 | 18 |
| FN | 23 | 37 | –14 | 21 | 37 | –16 |
| Total | 190 | 148 | 42 | 252 | 148 | 105 |
| Life-years | ||||||
| TN | 13.8023 | 14.8650 | –1.0627 | 12.1507 | 14.8650 | –2.7143 |
| FP | 1.5159 | 0.4534 | 1.0626 | 3.1674 | 0.4534 | 2.7140 |
| TP | 0.0732 | 0.0472 | 0.0260 | 0.0766 | 0.0472 | 0.0294 |
| FN | 0.0382 | 0.0610 | –0.0228 | 0.0350 | 0.0610 | –0.0260 |
| Total | 15.4297 | 15.4265 | 0.0031 | 15.4297 | 15.4265 | 0.0032 |
| QALYs | ||||||
| TN | 10.1048 | 10.8827 | –0.7780 | 8.8956 | 10.8827 | –1.9872 |
| FP | 1.1098 | 0.3319 | 0.7779 | 2.3189 | 0.3319 | 1.9870 |
| TP | 0.0399 | 0.0257 | 0.0142 | 0.0417 | 0.0257 | 0.0160 |
| FN | 0.0182 | 0.0290 | –0.0108 | 0.0166 | 0.0290 | –0.0123 |
| Total | 11.2727 | 11.2694 | 0.0033 | 11.2729 | 11.2694 | 0.0035 |
Colonoscopies contribute the largest source of difference in discounted costs, reflecting differences in specificity between strategies. The difference in colonoscopy costs relative to FIT alone is £42.34 for the QCancer plus FIT strategy, and £107.67 for the RAT plus FIT strategy (Table 24). Both intervention strategies have additional costs due to perforation and bleeding with colonoscopy, relative to FIT alone. Furthermore, there is an accompanying loss of QALYs of 0.0002 (equivalent to a loss of 1.75 full health-hours of life) associated with this unnecessary exposure to risks from colonoscopy in patients who do not have CRC, as a result of using the tools.
| Costs and QALYs | FIT alone | QCancer + FIT | Difference | RAT + FIT | Difference |
|---|---|---|---|---|---|
| Costs pre diagnosis (£) | |||||
| GP visits | 62.69 | 59.65 | –3.04 | 56.17 | –6.52 |
| FITs | 3.14 | 2.86 | –0.28 | 2.51 | –0.63 |
| Colonoscopy | 26.68 | 69.02 | 42.34 | 134.36 | 107.67 |
| Adverse events with colonoscopy | 0.47 | 1.21 | 0.74 | 2.36 | 1.89 |
| Costs post diagnosis (£) | |||||
| Lifetime cost of treatment | 54.84 | 56.83 | 1.99 | 56.97 | 2.13 |
| Total | 147.82 | 189.58 | 41.75 | 252.37 | 104.54 |
| QALYs | |||||
| No CRC | 8.8281 | 8.8281 | –0.0001 | 8.8280 | –0.0002 |
| CRC pre diagnosis | 0.0007 | 0.0003 | –0.0004 | 0.0003 | –0.0005 |
| CRC post diagnosis | 0.0482 | 0.0501 | 0.0019 | 0.0502 | 0.0021 |
| Total | 8.8770 | 8.8785 | 0.0015 | 8.8785 | 0.0015 |
| ICER (£) | 28,704 | 71,863 | |||
Tornado analysis
A tornado analysis is used to provide a graphical representation of the degree to which the incremental cost per QALY gained from tools is sensitive to independent variation by 20% of clinical parameters (Figure 11). The tornado analysis shows that the source of greatest uncertainty in the incremental cost per QALY originates from the sensitivity of the standard test strategy. A reduction of 20% in the false-negative rate, that is an increase in the sensitivity of the FIT from its base-case value of 53% to 62%, increases the discounted cost per QALY gained with the QCancer tool from £28,704 to somewhere above £48,633 (see Figure 11). A reduction of 20% in the same parameter reduces that figure to £17,842 per QALY gained. The algorithm’s specificity is the second most important economic parameter, as it determines how much of the increased detection of CRC by the primary care diagnostic workup is obtained at the expense of patients without CRC who are referred, but have no need, for colonoscopy. Naturally, the cost of colonoscopy is the single most influential cost parameter in the results; reducing its cost from £615 to £492 reduces the cost per QALY gained with QCancer to £22,883.
FIGURE 11.
Sensitivity of results to arbitrary increases and decreases of 20% in model parameter values.

Probabilistic analysis
A probabilistic sensitivity analysis was conducted for the base-case analysis by accounting for sampling uncertainty in the key model parameters (see the tornado plot in Figure 11 and see Appendix 5, Table 66, for information on the distributions used for those parameters). The first and third quartiles of the incremental discounted costs’ distribution were £40 and £44 per patient, respectively, whereas those for incremental discounted QALYs were 0.0012 and 0.0017, respectively (Figure 12). The probabilistic estimate of the incremental cost per QALY gained with the tool using the case of QCancer is £28,522, which is very similar to the deterministic estimate of £28,703.
FIGURE 12.
Cost-effectiveness plane: diagnostic tool (QCancer) vs. no tool.

Figure 13 presents the probability of the tool being cost-effective as a function of the willingness to pay for one QALY gained. This figure implies a 95% CI for the incremental cost per QALY gained with the tool of £17,000–50,000. A note of caution is warranted as this figure does not capture the uncertainty inherent in the assumption that the health benefits with the tool are due to its increased sensitivity, as it translates to fewer visits and, therefore, shorter times to referral, and that these reduced times in turn translate into diagnoses at earlier disease stages. Owing to this underestimation of the uncertainty in this probabilistic sensitivity analysis, we did not attempt to analyse the expected value of additional research information.
FIGURE 13.
Cost-effectiveness acceptability curve: tool (QCancer) vs. no tool.
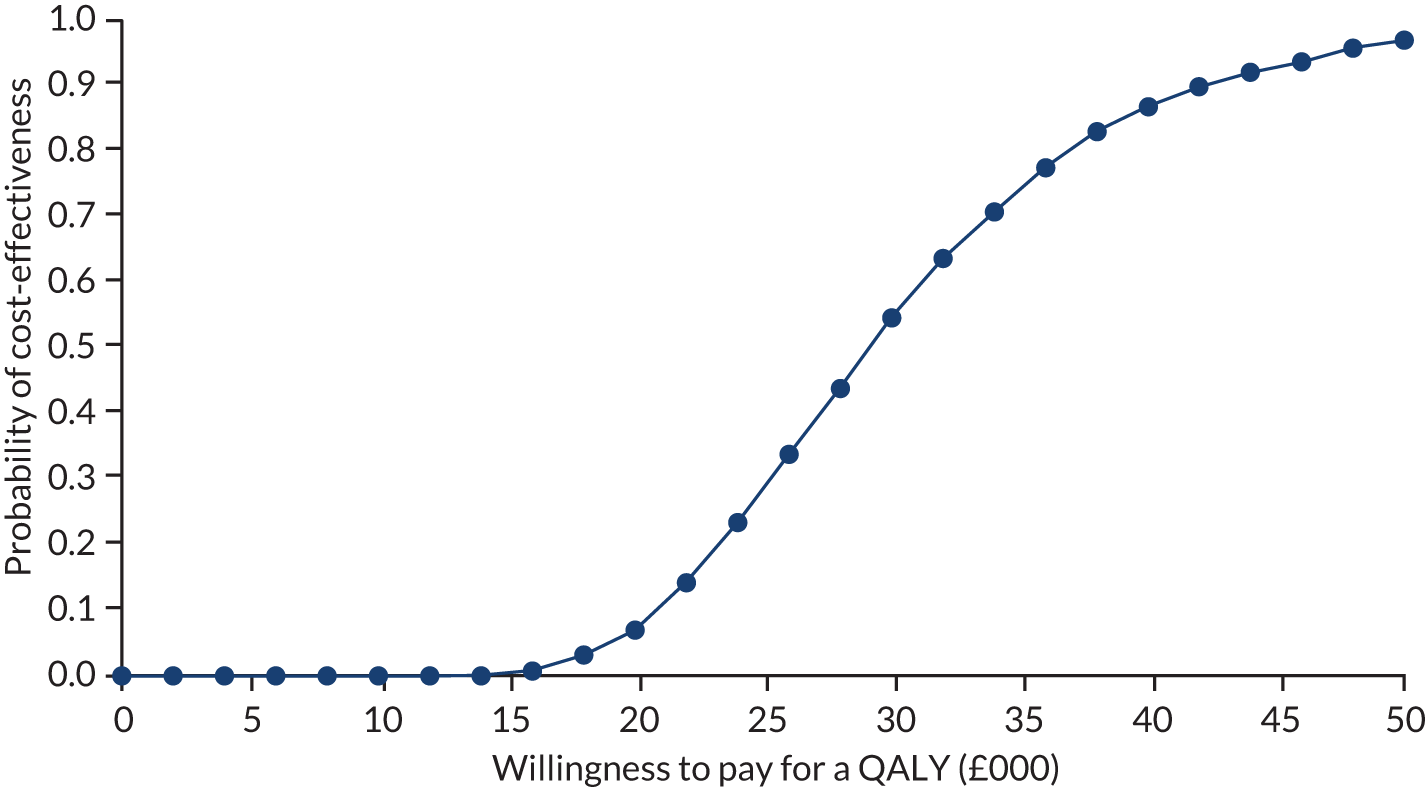
Threshold analysis
The tornado analysis has highlighted the sensitivity of the cost per QALY gained to the sensitivity of the FITs and the specificity of the diagnostic tools. Given the key role of these parameters in the results, Figure 14 depicts the levels of specificity of the triage tool required for the intervention to meet the £30,000 threshold of cost-effectiveness, at varying levels of sensitivity of the FIT. At the levels of sensitivity of ≥ 50% that have been reported for a FIT of 20 µg Hb/g faeces,175 the minimum level of specificity needed by the tool to serve as a cost-effective triage intervention in primary care is 0.89.
FIGURE 14.
Threshold analysis.
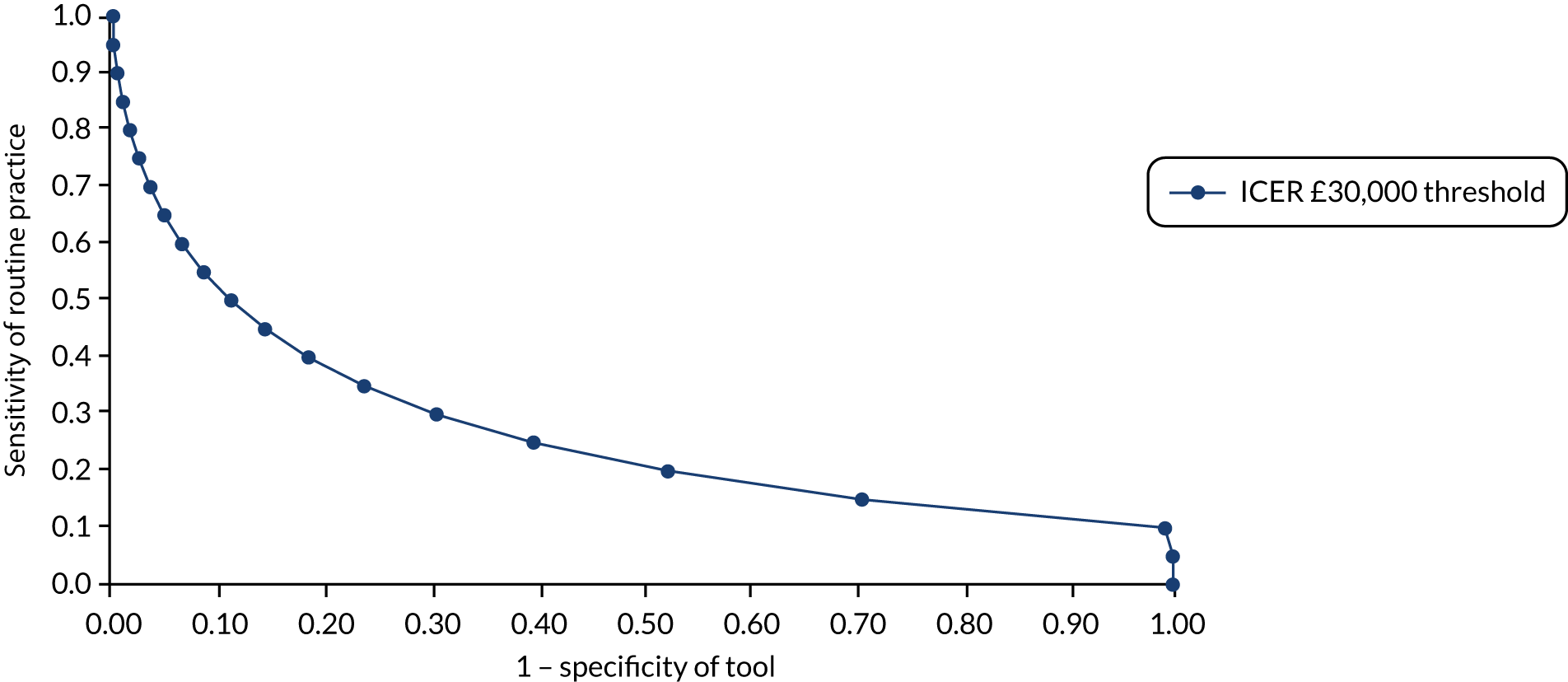
Scenario analyses
A key area of uncertainty in the economic analysis of the diagnostic tools is the diagnostic sensitivity of current standard practice. The only evidence available on sensitivity is reported from a retrospective survey,173 in which a sensitivity of primary care referral in routine practice of 76% is implicit. In the absence of data from this source,173 we assume the specificity of current practice equals the specificity that corresponds to its sensitivity value in the receiver operating characteristic curve reported for the NICE version 3 referral guidelines. 22 Using these sensitivity and specificity parameters, the intervention produces negligible and possibly negative (discounted) QALY effects (Table 25). A similar outcome would arise in younger suspected cancer patients, presenting at 55 years of age. Limiting of the analytical time horizon to 5 years, instead of the 30-year time horizon in our base-case analysis, increases the cost per QALY gained by 73–89%, depending on the tool. Imposing a maximum of 12 primary care visits before referral would have no significant effect on the cost per QALY gained by the tools.
| Scenario | RAT | QCancer | ||||
|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Cost per QALY gained (£) | Incremental cost (£) | Incremental QALYs | Cost per QALY gained (£) | |
| Base case | 104.54 | 0.0015 | 71,863 | 41.75 | 0.0015 | 28,704 |
| Higher prevalence rate (2%) | 104.52 | 0.0020 | 52,470 | 42.02 | 0.0020 | 21,439 |
| Compliance of 100% with test result | 129.87 | 0.0008 | 162,183 | 51.45 | 0.0009 | 58,313 |
| Sensitivity of 76% and specificity of 52% for standard practicea | 52.99 | 0.0003 | 167,972 | 20.85 | 0.0003 | 62,368 |
| FIT of 10 µg Hb/g faeces; sensitivity of 92% and specificity of 86% | 91.34 | –6 × 10–5 | Dominated | 35.66 | 1.6 × 10–5 | 2.26M |
| Age 55 years at presentation | 110.08 | 0.0019 | 57,767 | 45.38 | 0.0019 | 23,516 |
| No mortality benefit of treatment in stages A/B (HR = 0) | 104.45 | 0.0014 | 76,930 | 41.66 | 0.0014 | 30,552 |
| Limit CRC-related death risk to 5 years | 104.54 | 0.0018 | 57,485 | 41.75 | 0.0018 | 23,234 |
| Limit time horizon of analysis to 5 years | 104.54 | 0.0007 | 148,294 | 41.75 | 0.0007 | 59,902 |
| Restrict maximum number of GP visits to 12 | 104.54 | 0.0015 | 71,979 | 41.75 | 0.0015 | 28,749 |
| Restrict maximum number of GP visits to 12 | 104.36 | 0.0013 | 81,505 | 41.57 | 0.0013 | 32,408 |
| Same CRC stage utilities across disease stages | 104.54 | 0.0017 | 63,046 | 41.75 | 0.0016 | 25,333 |
| Disutility of colonoscopy (0.0075) | 104.54 | 0.0014 | 77,211 | 41.75 | 0.0014 | 29,507 |
In addition, we explored the uncertainty in the cost-effectiveness of the tools from allowing for a ‘send home/wait’ comparator strategy, whereby, at the initial visit, the GP sends the patient home without conducting a FIT; from the second visit onwards, they manage the patient as in the ‘FIT given to all’ strategy. This is a highly uncertain comparator to model as a result of the absence of any information on the rate of return of both CRC and non-CRC patients after an initial visit resulting in neither investigation in primary care nor referral to secondary care. To simplify the analysis, we have assumed that all CRC patients return the following month to seek care for their unresolved symptoms, whereas only a proportion of non-CRC patients do so. Thus, we show how the cost-effectiveness of the tool triage strategies vary with the proportion of CRC-negative patients who return to the GP after the first month. When 0%, 50% and 100% of these patients return, the incremental discounted cost per QALY gained with the RAT relative to this ‘send home and wait for second visit before FIT’ comparator is £50,605, £37,245 and £23,993, respectively. The corresponding figures for QCancer are £30,472, £17,194 and £4023.
We also explored a modified intervention strategy whereby patients who are above the risk threshold of the tool are referred directly to secondary care, as in the original intervention strategy (see Figure 2),whereas those below the threshold are sent home, rather than being subjected to primary care investigations, as assumed in our base-case analysis. In this scenario, the incremental cost per QALY gained with the tools over the FIT-alone strategy was predicted to be £40,140 for QCancer and £96,285 for the RAT.
We also investigated whether or not there would be any possible value of quality-of-life gains from more expeditious referral and diagnosis that would be sufficient to make the tools cost-effective, in a scenario in which more expeditious referrals do not result in survival gains. We found that, even at zero utility values of time spent in the false-negative health state, that is with undiagnosed CRC, the tools would not be cost-effective. At such extremely low values, the ICER for QCancer was £68,102 and that for the RAT was £190,294.
Discussion
In this chapter, we present exploratory analyses based on a simple model of the diagnostic pathway that has as its starting point symptomatic patients presenting to primary care and undergoing an initial clinical assessment, which is the same starting point considered by NICE Guideline Number 126 and the Diagnostic Guideline Number 30. 140 We then combine the model with an adapted disease history model for CRC from published studies (see Chapter 6), using a shorter cycle length and accounting for the excess risk of mortality due to undiagnosed CRC. By developing these features in our model, we were able to produce predictions in terms of meaningful clinical outcomes. The model was then used to identify the parameters that contribute most to the overall decision uncertainty about the cost-effectiveness of decision tools, which are good candidates for assessment as research priorities using expected value of partial perfect information. 166–168 Our model is expected to undergo further refinement as new evidence emerges, thus becoming an effective live research resource for evaluation and decision-making analysis.
Our analysis of key areas of uncertainty in the economic evaluation of the diagnostic tools led us to the following findings. First, a key determinant for the relative clinical effectiveness and cost-effectiveness of a diagnostic tool-based pathway is the diagnostic accuracy of current standard practice, which is currently unknown. We can only infer what this accuracy might be from the limited data available in a single report of findings from a retrospective survey. 173 Alternatively, as we have also done in this chapter, one may assume that patients are being managed by the results of the FIT, using the diagnostic accuracy estimates for this test available in the literature. 175 This may be a more consistent approach, but limits the scope allowed for accounting for clinician’s own judgement in the referral decision-making process.
Second, there is a lack of evidence for evaluating the impact of diagnostic tools in clinical practice. It is not known how these tools would be used in actual practice and how any impact they might have on referral intervals would affect clinical outcomes. We have opted for modelling the expected accuracy of using the diagnostic tools to triage patients for primary care investigation with a FIT, under the strong assumption that the diagnostic accuracy of FITs does not depend on the outcome of the diagnostic tool used for triage.
Third, there is little evidence on the prevalence of cancer in the low-risk population in which the diagnostic tools are intended for use. The value of this parameter is crucial for the cost-effectiveness of using the tool to detect more cancer patients early, which depends on limiting the cost imposed on the overwhelming majority of patients who present with symptoms of suspected cancer but turn out later not to have the disease. Previous analyses, including those informing the NICE Guideline Number 126 and Diagnostic Guideline Number 30,140 have assumed that the CRC prevalence in the low-risk population of interest here is 1.5% based on expert opinion, which, naturally, is highly uncertain. We have thus adopted this value in our base-case analyses. In any case, our exploratory analysis suggested that the specificity of the tools will also determine whether or not these may be used efficiently, without incurring excessive costs of colonoscopies performed in people without cancer, and are sustainable for a universal public health system such as the English NHS.
Fourth, the absence of data on the rate of CRC disease progression prior to diagnosis is also of importance. In our analysis, the slower the rate of progression, the lower the relative clinical effectiveness and cost-effectiveness of diagnostic tool triage to primary and secondary care investigations, as the tool triage would detect fewer extra cases before they had reached advanced disease stages, for which treatment is less effective, relative to those currently being detected in routine practice. However, the tornado analysis revealed that the sensitivity of current primary care practice, the specificity of the tool and the way clinicians would use the tools are more important sources of uncertainty than the disease progression in the evaluation of these tools.
Fifth, uncertainty regarding the decision-making process currently used in primary care for referring patients to secondary care owing to suspected CRC prevents drawing conclusions about the optimal intervention strategy. When we compared the intervention with a strategy of sending symptomatic patients home on the first visit and using the FIT-alone strategy for those patients who return with unresolved symptoms for a second visit, the proportion of non-CRC patients who return for the second visit determines whether or not a diagnostic tool approaches levels of cost-effectiveness. Thus, the economic value of the tools will depend on the clinical effectiveness of delaying access to colonoscopy as the alternative strategy for identifying patients who need referral, which may avoid unnecessary use of health-care resources and health risks of invasive investigations, at the cost of putting some patients with CRC at risk by delaying their identification and treatment.
Another limitation in our analysis is the lack of evidence on diagnostic accuracy from any direct comparative study of the modelled strategies. Thus, our sensitivity and specificity values are derived from single studies that are likely to be different in terms of population, as reflected, for example, in the underlying prevalence rate, or study design; for example, the evidence from one of the tools is derived from a case–control study, whereas the evidence for the other tool was obtained from a cohort study. Therefore, we cannot directly compare QCancer and RATs, as this is likely to lead to biased results.
In addition, we have not modelled the heterogeneity in current standard practice; therefore, we have not accounted for the variation in the costs and benefits of using the tools depending on local situations. It is conceivable that practices with robust referral systems may have little to benefit from using the tools, whereas areas without a sound referral system may find them beneficial. The model assumes 100% compliance of GPs with the strategies (both FITs and decision tools). However, it is clear that, currently, not all GPs use decision tools. More research is therefore needed to understand how compliance with strategies varies across localities.
The model does not account for the resource constraint effects imposed by limited capacity to do colonoscopies. Thus, although our model accounts for the benefits of faster access to colonoscopy, it does not include the opportunity costs of those patients whose access to colonoscopy will be delayed as a consequence of further demands placed on such service with limited capacity. This is an area of further research.
Conclusion
The decision-analytic model was developed to analyse the uncertainty inherent in the current evidence base, and to ask questions about the probable impact of the diagnostic tools, given the current lack of evidence. The model showed the importance of accounting for the excess mortality effects of CRC in symptomatic patients who are undiagnosed, and extends previous models in CRC by explicitly modelling a simple diagnostic referral pathway in primary care. Despite the high degree of uncertainty on evaluating the effect of the diagnostic tool on primary care referral of suspected CRC and health outcomes, our model has helped us to identify the parameters for which uncertainties contribute most to the overall decision uncertainty about the cost-effectiveness of decision tools, namely the sensitivity of current standard practice, the specificity of the diagnostic tool, the prevalence of CRC in the relevant primary care patient population and the cost of colonoscopy.
In the concluding section, we return to the original questions about the probable impact of the diagnostic tools on the costs and effects for patients presenting with symptoms of suspected CRC. For each question we summarise the initial findings from the model and highlight where the uncertainties still remain.
-
What are the possible impacts on patient quality of life or survival if use of diagnostic tools reduces time to diagnosis?
The model-based analysis suggests that the diagnostic tools would reduce the number of CRC patients who die without a diagnosis from 12% to 8%. The increased accuracy of the tool relative to the comparator also reduces the referral interval, which equals a reduction in the time to diagnosis and treatment by assumption. Owing to the challenges of measuring the effect on health-related quality of life post diagnosis, the amount of this type of benefit is highly uncertain. Overall, our modelled analysis suggests that, depending on the sensitivity of current practice, the diagnostic tools may extend life expectancy by somewhere between 4 days and 2 months among symptomatic patients aged 70 years presenting to their GP with undiagnosed CRC, relative to a hypothetical current practice of management according to results of a FIT alone. This range of variation in benefit highlights the importance of establishing current practice and its primary care diagnostic accuracy.
-
Will the benefit to cancer patients identified earlier by diagnostic tools outweigh any disutility in extra patients referred for further investigation who do not have cancer?
In the absence of evidence, our analysis suggests that the tools may help to improve diagnostic accuracy in primary care, and result in earlier referrals to secondary care. If the reduction in the referral interval results in a shortening of the diagnostic interval of the same magnitude, our model predicts that the amount of benefit to patients with CRC identified earlier outweighs the loss in quantity and quality of life in those patients referred for further investigation who do not have CRC. However, although the benefit to cancer patients would more than compensate the risks to life from exposing the overwhelming majority of patients without cancer to the risks of colonoscopy, it is unlikely to justify the additional health-care costs associated with such exposure.
A sensitivity analysis was used to explore the uncertainty around the estimated cost-effectiveness results and to highlight the parameter values that were driving the results. The sensitivity analysis revealed that the cost-effectiveness results were particularly sensitive to uncertainty around the sensitivity of current standard practice and the specificity of the tool. In a further threshold analysis, we explored the levels of sensitivity of current standard practice and the specificity of the tool in which the decision tool would meet the £30,000 cost-effectiveness threshold. At the levels of sensitivity of ≥ 50% that have been reported for a FIT of 20 µg Hb/g faeces,175 the minimum level of specificity needed by the tool to serve as a cost-effective triage intervention in primary care is 0.89.
The limited available data on current practice in the UK suggests that the sensitivity of primary care referrals is already too high for the diagnostic tools to make a significant impact on CRC patient survival. Although our base-case analysis produced an estimate of 2 months of life extension with the tools when compared with a FIT of 20 µg Hb/g faeces, this gain is reduced to 2 weeks when the sensitivity value derived from the number of visits before CRC diagnosis in routine practice is used, and to 4 days against a FIT of 10 µg Hb/g faeces. Given the very low prevalence of CRC in the relevant primary care population, this amount of benefit may be insufficient to outweigh the risks to life from exposing a vast number of patients without CRC to colonoscopy. Furthermore, whether or not the tools are cost-effective will depend on the prevalence and the sensitivity of current referral practice and the specificity of the tool.
-
How big an improvement in quality of life would be needed to warrant the use of these tools if there are no survival impacts associated with the diagnostic tools?
We investigated this question by assuming that the increased accuracy of the tool relative to the comparator does not result in any survival benefit but would avoid any loss in quality of life arising from false-negative results, whereas the quality of life of a false-positive result remains unaffected. The benefits gained from the tools are then largest when the losses from false-negative results are the biggest, as would occur when quality of life of these false-negative patients falls to zero. Our analysis, however, shows that even preventing these extreme losses in quality of life for false-negative patients is insufficient to make the diagnostic tools cost-effective (the ICER for QCancer is then £68,102 and for the RAT is £190,294). Thus, without survival benefits, the quality-of-life benefits of the tools alone would be unlikely to be sufficient to justify their costs.
-
Could a cancer diagnostic tool be considered cost-effective if it reduces the period of extreme anxiety by expediting investigation and management in patients, even if it made no impact on patient outcome?
The model results do not account for any negative impact of referrals on a patient’s quality of life due to anxiety, or the impact of colonoscopy on patient quality of life, for which we found no evidence in the literature. Therefore, we cannot provide an answer to our study question of whether or not a cancer diagnostic tool reduces the period of extreme anxiety associated with an uncertain CRC diagnosis by expediting investigations and management. Answering this question would require prospective studies measuring the outcomes of patients in primary care, but it is questionable whether or not such a study is feasible given the difficulty to define the patient population and the low number of cases expected in a single practice each year. Nevertheless, our exploratory analyses suggest that, even if plausible reductions in the diagnostic interval brought about by the use of the tools were to equate to reductions in the amount of time patients experience extreme anxiety, the tool would not be cost-effective without extending patient survival.
-
Where are the gaps in the evidence base and where is more research needed?
The updated review showed that the statistical relationship between diagnostic delay and cancer patient outcomes is not suitable for deriving valid estimates of the effect of expeditious CRC diagnosis on cancer stage or patient survival. In particular, the documented non-monotonic (e.g. U-shaped) relationship between diagnostic interval and mortality does not imply an effect mediated through the impact of diagnostic interval on stage of diagnosis. Given the lack of valid surrogate or clinical outcome data for building an economic model of diagnostic tools or decision-aid models in primary care, our cost-effectiveness analysis was limited to an exploration of the expected effects of time delays to diagnosis associated with the relative accuracy of the diagnostic tools. This limited aim will naturally be subject to more sources of uncertainty than an analysis based on clinical surrogate outcomes, but reflects a pragmatic approach given the quality of the data available.
In the absence of direct clinical effectiveness data, we assumed a relationship between the sensitivity of both the diagnostic tool and current routine referral practice and the time to diagnosis. In the model, the time from symptom presentation to diagnosis consists of (1) the time from presentation in primary care to referral for investigation (the referral interval; see Chapter 5) and (2) the time from referral to specialist care to diagnosis. We then assumed that the diagnostic algorithm affects only the referral interval, while the time from referral to diagnosis and treatment remains fixed. Thus, in our analysis, we assume that the improvement of primary care diagnostic accuracy with the tool results in fewer visits to the GP before referral, and that the reduction in the accompanying time to referral is equal to the reduction in the time to diagnosis and treatment. We have explicitly modelled the average time to referral and diagnosis and treatment, based on the number of repeat appointments to make the mechanisms of effect transparent and open to scrutiny. The relationship between the number of repeat appointments and the time to referral is supported by evidence from a retrospective patient experience survey. 173 More research is needed on the impact of the different strategies on the number of GP appointments before referral and diagnosis.
The review highlighted that there was a lack of direct comparative accuracy evidence between the strategies/tests involved in the model. Our model is therefore based on indirect comparisons of single-arm accuracy studies and assumptions about how the tools would be used to triage patients for investigations in secondary care. There is limited evidence on the diagnostic accuracy of decision aid tools in the UK low-risk population of interest, and the methods used to evaluate the tools that have any relevant evidence, QCancer and the RAT, differ in terms of how the relevant low-risk population is defined, that is ‘top 1 to 9% risk’ versus ‘low risk but not no-risk’,75 and the study design used to develop or evaluate them, that is observational cohort versus case–control, respectively. As QCancer was developed from a large cohort from electronic medical records, data were not prospectively collected and may thus lack relevant predictors to primary care practitioners. The RAT, in contrast, warrants validation in a large representative UK primary care population, because evidence on its diagnostic accuracy is based on a retrospective patient selection design. In addition, the added value of these tools is highly uncertain as the status quo has not been defined or rigoroursly evaluated, as reflected by the poor quality of the evidence informing the latest NICE guidance (Diagnostic Guideline Number 303), including the FIT, which has been recommended by such guidance but is not yet universal at the time of writing.
More research is also needed to verify the relationship between diagnostic delay and cancer patient outcomes. This is a difficult area to study because of unobserved confounding factors, but one for which access to electronic medical health records with information to construct detailed symptoms libraries has opened new opportunities for fruitful research.
The sensitivity analysis revealed that the cost-effectiveness results were particularly sensitive to uncertainty around the diagnostic accuracy of current standard practice and the specificity of the tool. We assume that all patients in the population of interest, who we defined as those with ‘low risk but not no-risk symptoms’,75 are akin to the CAPER study population. 75 This patient population includes the majority of symptomatic patients who do not present with high-risk symptoms such as rectal bleeding or severe anaemia, and therefore are not diagnosed through the 2WW referral pathway. The best available evidence on accuracy of the tool is for the QCancer tool, as it is based on a large cohort analysis. However, even these sensitivity and specificity parameters were not for the same population of interest here, as QCancer values include patients with red-flag symptoms, who may be deemed appropriate for 2WW referrals. Therefore, in our model, we calculated the sensitivity and specificity of QCancer for the top 10% risk threshold after excluding the top 1% risk observations from the published data77 to be 61% and 91%, respectively. In a further threshold analysis, we explored the levels of accuracy of current standard practice and the specificity of the tool in which the decision tool would meet the £30,000 cost-effectiveness threshold. At the levels of sensitivity of ≥ 50% that have been reported for a FIT of 20 µg Hb/g faeces,175 the minimum level of specificity needed by the tool to serve as a cost-effective triage intervention in primary care is 0.89. More research is clearly needed on the specificity of these tools among the population of interest.
In addition, we have not modelled the heterogeneity in current standard practice; therefore, we have not accounted for the variation in the costs and benefits of using the tools depending on local situations. It is conceivable that practices with robust referral systems may have little to gain from using the tools, whereas areas without a sound referral system may find them beneficial. The model assumes 100% compliance of GPs with the strategies (both FITs and decision tools). However, it is clear that, currently, not all GPs use decision tools. More research is therefore needed to understand how compliance with strategies varies across localities.
The model does not account for the resource constraint effects imposed by limited capacity for colonoscopies. Thus, although our model accounts for the benefits of faster access to colonoscopy, it does not include the opportunity costs of those patients whose access to colonoscopy will be delayed as a consequence of further demands placed on such service with limited capacity. This is an area of further research.
Finally, there were insufficient data to evaluate one of the a priori aims of the model, which was to explore whether or not the tools will become cost-effective if they reduce the period of extreme anxiety, even if they made no further impact on patient outcome. Answering this question would require prospective studies measuring the outcomes of patients in primary care, but is questionable whether or not such a study is feasible given the difficulty to define the patient population and the low number of cases expected in a single practice each year.
Chapter 8 General practitioner survey
Introduction
Diagnosing cancer quickly once patients become symptomatic remains an important priority in the UK. 185–187 A key part of the UK’s strategy for early cancer diagnosis was to lower the threshold for fast-track referrals of symptomatic patients for investigation from an estimated 5% to an explicit 3% risk of undiagnosed cancer. This was implemented via the 2015 revision of NICE guidelines on recognition and referral for suspected cancer. 6 A potential source of diagnostic delay remains the UK’s ‘gatekeeper’ system, whereby a GP decides whether or not a symptomatic patient meets the criteria for investigation for suspected cancer. 188 Indeed, fast-track referrals for cancer are less likely when patients present with ‘low-risk but not no-risk’75 symptoms of cancer than when they present with ‘alarm’ symptoms. 189
A number of models and algorithms have been developed to quantify the risk of an undiagnosed malignancy in symptomatic patients (see Chapters 3 and 4). 42,44 Some of these models have been translated into cancer decision support tools for clinical use, helping GPs to identify patients who warrant investigation for suspected cancer.
Systematic review 1 (see Chapter 3) identified two tools that are available to support doctors assess the risk of undiagnosed skin cancer. 36–38 These tools are adapted to Australian guidelines and have limited applicability in the UK. For the internal cancers, there are two main types of cancer decision support tools available in the UK: RATs and QCancer. RATs are available for a number of specific cancer sites, and use symptoms and test results to estimate the risk that a patient has an underlying cancer. 51,66,69–71,73,81,83,90 They were identified in SR1 (see Chapter 3) as having been evaluated in their mouse mat and desktop forms. QCancer tools estimate the risk of undiagnosed cancer based on symptoms, test results and patient risk factors. They are available for specific cancer sites,32,52,80,82,84,85 and also in sex-specific, person-centred forms, which estimate an individual’s risk that they have a number of different cancers. 53,54 QCancer tools were identified as validated cancer prediction models in SR2 (see Chapter 4).
In 2013, Macmillan Cancer Support, BMJ Informatica, the Department of Health and Social Care, and Cancer Research UK collaborated to incorporate RATs and QCancer into GP IT systems, renaming them collectively as ‘electronic cancer decision support tools’. RATs are fully integrated with the GP IT software INPS Vision (In Practice Systems Ltd, London, UK) and will soon be available via SystmOne (The Phoenix Partnership, Leeds, UK). QCancer is available via EMIS Web (EMIS Health, Leeds, UK), and QCancer tools are also freely available on the internet (www.qcancer.org). NHS Digital data indicate that, together, EMIS Web and Vision had 62% of the market share of GP IT systems in 2015. 190 In addition, RATs are available in mouse mat and flip chart forms; in January 2012, they were distributed to all 10,000 general practices in England as part of the National Cancer Action Team’s Supporting Primary Care Project. 185 Information about QCancer and RATs is available via the Cancer Research UK and Macmillan Cancer Support websites. 191,192
The electronic cancer decision support tools have three main functions:
-
alert/prompt – cancer risk scores appear automatically on opening a patient’s record
-
cancer risk assessment – GPs can request a patient’s cancer risk score, using the symptom checker
-
searches/report – GPs can routinely search records and produce summaries indicating patients who may need follow-up (‘safety-netting’).
Existing research
There is little existing research on the clinical effectiveness and cost-effectiveness of cancer decision support tools (see Chapters 3 and 4).
Independent and external validations of the QCancer algorithms for colorectal,15 pancreatic,14 renal tract,91 gastro-oesophageal88 and ovarian89 cancers concluded that these tools are useful for identifying undetected cases of these cancers in primary care (see Chapter 4). The performance of the CRC RAT was compared with that of the 2005 NICE guidelines for referral for suspected cancer. 172 The RAT (area under the receiver operating characteristic curve 0.91, 95% CI 0.89 to 0.93) performed better than the 2005 NICE guidelines (area under the receiver operating characteristic curve 0.75, 95% CI 0.72 to 0.79) in discriminating patients with undiagnosed CRC. 22,172 No comparison with the current NICE guidelines6 has been carried out; however, these guidelines draw heavily on an evidence base dominated by RATs and QCancer publications.
A cohort study compared the numbers of cancer investigations and diagnoses before and after the introduction of CRC and lung cancer RATs in primary care in the UK. The introduction of RATs was associated with increased diagnostic activity and additional diagnoses of lung and colorectal cancer. 13 By contrast, a 2 × 2 design trial of a GP intervention, which included the colorectal and lung cancer RATs, did not report any evidence that the GP intervention was associated with faster time to diagnosis of cancer in rural Australia. 35 The clinical utility of cancer decision support tools in primary care remains uncertain. 42 No studies have investigated the association between the use of cancer decision support tools and the use of the urgent referral pathway for suspected cancer.
Qualitative studies of cancer decision support tools in UK primary care suggest that they are more likely to be embedded in clinical practice if they are perceived to support, but not supersede, a doctor’s clinical judgement, and with better training and improvements in design. In particular, their use of screen prompts added to the barrage of alerts generated by GP software systems, thereby increasing the risk of ‘prompt fatigue’ and disengagement. Positive aspects of the tools included raising awareness of cancer and of its symptoms, and prompting GPs to reflect on their decision-making around referrals. 13,16,17 A recent qualitative study aimed to improve the understanding of how cancer decision support tools are used by GPs. It analysed responses from a convenience sample of 126 GPs, most of whom worked in the South West Peninsula. The study reported that 18.3% of GPs used either a RAT or a QCancer, and that awareness of these tools was low (Chisnell et al. , submitted).
No studies have quantified the proportion of general practices and GPs in the UK that have access to and use cancer decision support tools. This is essential for increasing our understanding of the tools’ clinical effectiveness and cost-effectiveness.
Research aims
The primary aims of this study were to quantify the proportion of:
-
general practices and GPs in the UK that have access to the two main types of cancer decision support tools, either through a paper-based desktop tool or via their IT software (i.e. RATs and QCancer)
-
general practices where at least one GP reports using these tools.
Secondary aims of the study were to investigate the association between access to cancer decision support tools and cancer diagnostic activity. Diagnostic activity was assessed using two indicators. The first is a diagnostic process indicator, namely the age- and sex-adjusted number of 2WW referrals per 100,000 head of population for the general practice. The second is a diagnostic outcome indicator, namely the proportion of patients referred via the urgent 2WW pathway who are subsequently diagnosed with cancer. 193
Methods
A cross-sectional postal survey was designed and conducted in line with conduct and reporting guidelines for surveys. 194
Questionnaire design
The questionnaire was drawn up by the lead researcher (SP) and was refined following feedback from Professors Hamilton, Hippisley-Cox and Coupland (the originators of RATs and QCancer), and members of the research team. Images of the paper-based tools and screenshots of the electronic cancer decision support tools were included in the questionnaire (courtesy of In Practice Systems Ltd and EMIS Health) to ease their identification by the participants. Copies of the questionnaire, covering letter and information sheet are included in Appendix 6. Each questionnaire included the general practice identifier, but not the name of the responding GP.
The questionnaire was finalised following feedback from five local GPs in terms of its utility, clarity and design. The final questionnaire comprised 10 questions. Questions 1–3 asked GPs about desktop RATs, including whether or not they were available and how likely they were to be used. Questions 4–8 asked about electronic cancer decision support tools (i.e. RATs and QCancer), again focusing on availability and likely use, and training. Questions 9 and 10 asked how many years the GP had been practising and how many sessions per week they worked at the practice. Participants were also asked to select, from a list, aspects of the tools that they found helpful. The contents of the list were informed by the qualitative study findings on the positive aspects of the lung cancer and CRC tools discussed above. 13,16,17
Sample
The survey was conducted at the practice level, reflecting how decisions are made about the IT software and whether or not to download or activate electronic cancer decision support tools.
The target population was general practices in the UK and the GP partners/principals, sessional GPs (including salaried and locum GPs) and GP registrars working there. GPs who had retired or who were not currently practising were excluded.
We estimated that a sample size of 392 general practices would be large enough for a 95% CI to have a margin of error of no more than 5%. We assumed a response rate of 40%, which is considerably less than the mean value of 61% (95% CI 59% to 63%) reported in a pooled analysis of 361 postal surveys published between 2000 and 2009. 195
Survey administration
The postal survey was administered by Binley’s (Basildon, UK; www.binleys.com). This commercial company was selected because it maintains a database of all 46,000 GPs in the UK, and all general practices, which is verified every 6 months. This enabled us to obtain a random probability sample of 975 general practices. Questionnaires were sent to all the GPs (n = 4350) and GP registrars (n = 250) working in these practices. Copies for GP registrars were colour coded, to facilitate separate analyses of their responses, as these GPs are not on the General Medical Council’s GP register. The questionnaires were sent on 5 July 2017, with a follow-up questionnaire pack sent to non-responding practices on 2 August 2017. The data collection stopped on 15 November 2017. To incentivise participation, a charitable donation of £7.50 was made per completed questionnaire, capped at the first 400. The £3000 donation was shared equally between Cancer Research UK and Macmillan Cancer Support.
Data entry and coding
The data were entered into a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet by Binley’s, and double-checked for accuracy by a second data entry analyst at Binley’s. The questionnaire did not include a free-text comments section; however, any comments that were written on the returned questionnaires, or sent by e-mail or telephone, were recorded and are reported in Appendix 6. The paper copies of the questionnaire were sent to the University of Exeter for reference and archiving. The numbers of general practices and individual GPs in a practice who failed to respond were recorded. If one GP reported that they had access to cancer decision support tools, it was assumed that this was true for all other GPs at that practice.
Analyses
Descriptive statistics are reported for the responses to each question, along with the numbers of missing data. GP-level data were used to estimate the proportion (95% CI) of GPs in the UK with access to the tool. Responding practices were categorised in two ways: first, by whether or not at least one GP had access to a tool, and, second, by whether or not at least one GP used a tool. From this, we estimated the proportion (with 95% CI) of UK general practices with access to a cancer decision support tool, and the proportion of UK general practices that use the tool.
Two subanalyses examined data from general practices in England only.
Ordinary least squares regression analyses tested the association between access to cancer decision support tools and a practice-level diagnostic process indicator, namely the number of urgent 2WW referrals for suspected cancer. The dependent variable was the number of sex- and age-adjusted 2WW referrals for suspected cancer per 100,000 head of population. The independent variable was a dummy variable indicating whether or not at least one GP at the practice had access to a tool. The analysis adjusted for the general practice’s Index of Multiple Deprivation (IMD). This is a population-weighted composite measure of 37 different measures of deprivation, taking a value between 0 and 100. The higher the score, the higher the level of deprivation. 196,197
Ordinary least squares regression was also used to test the association between access to cancer decision support tools and a practice-level diagnostic outcome indicator, namely the proportion of 2WW referrals that result in a cancer diagnosis (also known as the conversion rate). This also adjusted for the practice-level IMD. 196,197
The 2WW and conversion rate data and practice-level IMD data for the period 2016–17 are published by Public Health England and are publicly available at http://fingertips.phe.org.uk/profile/cancerservices (accessed December 2019). Survey responses were mapped to Public Health England data using the general practice identifier.
Results
Sample characteristics
Responses were received from 473 GPs and from three GP registrars based in 227 practices. Responses from the GP registrars were not analysed separately, because of the small number of GP registrars providing responses. The response rate at the practice level was 23.3%; at the practitioner level, it was 10.3%. The responding practices had a median of 6 [interquartile range (IQR) 4–8] GPs, of whom a median of 2 (IQR 1–3) responded to the survey. The mean within-practice response rate was 43.7% (95% CI 39.3% to 48.1%). Unprompted comments written on incomplete surveys returned by practice managers, or messages received by e-mail or telephone (see Data entry and coding) indicated that lack of time (n = 12) and lack of awareness of the tools (n = 6) were the most common reasons for non-response.
Of the 476 respondents, 294 (61.8%) had been practising as a GP for ≥ 11 years, and 299 (62.8%) worked between five and eight sessions per week (Table 26).
| Demographic information | GPs | |
|---|---|---|
| n | % | |
| Years in practice | ||
| < 1 | 20 | 4.2 |
| 1–5 | 57 | 12.0 |
| 6–10 | 57 | 12.0 |
| 11–20 | 145 | 30.5 |
| 21–30 | 111 | 23.3 |
| > 30 | 38 | 8.0 |
| Missing | 48 | 10.1 |
| Total | 476 | 100.00 |
| Number of consultations sessions worked per week | ||
| 1–2 | 10 | 2.1 |
| 3–4 | 70 | 14.7 |
| 5–6 | 140 | 29.4 |
| 7–8 | 159 | 33.4 |
| 9–10 | 40 | 8.4 |
| > 10 | 5 | 1.1 |
| Missing | 52 | 11.0 |
| Total | 476 | 100.0 |
EMIS Web was the most frequently used IT software (96/227, 42.3%), followed by TPP SystmOne (74/227, 32.6%) and then INPS Vision (32/227, 14.1%). These relative frequencies largely reflect the national market share (Table 27).
| General practice IT software (eCDS tool) | Responding general practice | National market share (%) | |
|---|---|---|---|
| n | % | ||
| EMIS Web (QCancer) | 96 | 42.3 | 52.4 |
| TPP SystmOne (none) | 74 | 32.6 | 33.0 |
| INPS Vision (RAT) | 32 | 14.1 | 9.9 |
| Egton Medical Information Systems PCS (none) (EMIS Health) | 11 | 4.9 | 0.01 |
| Egton Medical Information Systems LV (none) (EMIS Health) | 5 | 2.2 | 0.02 |
| Other (none) | 6 | 2.2 | 3.3 |
| Microtest (none)a | 3 | 1.3 | 1.4 |
| Total | 227 | 100 | 100 |
The IMD profile of the sample is generally representative of that of the national population (Figure 15).
FIGURE 15.
Index of Multiple Deprivation profiles.
Paper-based risk assessment tools
Access to a paper-based RAT in mouse mat or flip chart form was reported by 63 of the 476 (13.2%) GPs. At the practice level, at least one GP reported access to a tool in 51 of the 227 (22.5%, 95% CI 17.2% to 28.5%) responding general practices (Table 28). The ‘other’ tools are listed in Appendix 6; all were national guidelines, or summaries thereof, rather than cancer decision support tools.
| Question category | GPs | |
|---|---|---|
| n | % | |
| Format of RAT | ||
| Mouse mat or flip chart | 63 | 13.2 |
| Other | 30 | 6.3 |
| None of these | 326 | 68.5 |
| Missing, not answered | 57 | 12.0 |
| Total | 476 | 100.0 |
| Likelihood of using paper-based RAT | ||
| Very likely | 5 | 7.9 |
| Likely | 14 | 22.2 |
| Unlikely | 29 | 46.0 |
| Very unlikely | 10 | 15.9 |
| Missing, not answered | 5 | 7.9 |
| Total | 63 | 100.0 |
Of the 63 GPs with access to a mouse mat or flip chart, 39 (61.9%) reported that they were unlikely or very unlikely to use it, and 19 (30.2%) reported that they were likely or very likely to use it (see Table 28).
The most popular probable uses of the paper-based RATs were for:
-
assessing cancer risk in patients with non-specific (n = 28/63, 44.0%) or multiple (n = 25/63, 40.0%) symptoms
-
increasing the GP’s certainty of decision-making (n = 25/63, 40.0%).
Other likely uses were less popular, namely:
-
discussing cancer risk (n = 19/63, 30.2%)
-
reassuring anxious patients (n = 16/63, 25.4%)
-
increasing awareness of cancer as a possible diagnosis (n = 17/63, 27.0%) and awareness of cancer symptoms (n = 10/63, 15.9%)
-
prompting referrals the GP would not otherwise have made (n = 13/63, 20.6%), or investigation (n = 9/63, 14.3%)
The option ‘none of these reasons’ was selected by 8 of the 63 (12.7%) GPs.
Electronic clinical decision support tools
The electronic clinical decision support tool was downloaded or activated on the IT system of 58 of the 476 responding GPs (12.2%) (Table 29), which equates to a practice level of 42 out of 227 (19.0%, 95% CI 14.0% to 24.6%). Practices using EMIS Web and INPS Vision were equally likely to have downloaded/activated the software (EMIS Web: n = 32/96, 33.3%; INPS Vision: n = 10/32, 31.3%) (Table 30). Of the 476 GPs, 174 (36.6%) were unaware of the electronic tools, and 39 (8.2%) reported that they would like to have them but they are not available for their system.
| Option | Frequency | |
|---|---|---|
| n | % | |
| Unaware of eCDS | 174 | 36.6 |
| eCDS is downloaded/activated for my IT system | 58 | 12.2 |
| eCDS is available for my IT system, but my practice has not downloaded/activated it | 23 | 4.8 |
| eCDS is available for my IT system, and my practice has plans to download/activate it in future | 6 | 1.3 |
| To my knowledge, eCDS is not available for my IT system | 108 | 22.7 |
| eCDS is not available for my IT system but I would like to have it | 39 | 8.2 |
| Missing, not answered | 68 | 14.3 |
| Total | 476 | 100.0 |
| General practice IT software | Access to electronic clinical decision support, n (%) | Total (n) | |
|---|---|---|---|
| No | Yes | ||
| EMIS Web (QCancer) | 64 (66.7) | 32 (33.3) | 96 |
| EMIS LV (none) | 5 (100.0) | 0 (0.0) | 5 |
| EMIS PCS (none) | 11 (100.0) | 0 (0.0) | 11 |
| INPS Vision (RAT) | 22 (68.8) | 10 (31.3) | 32 |
| TPP SystmOne (none) | 73 (98.7) | 1 (1.3)a | 74 |
| Microtest (none) | 3 (100.0) | 0 (0.0) | 3 |
| Other (none) | 5 (80.0) | 1 (20.0)a | 6 |
| Total | 183 (80.6) | 44 (19.4) | 227 |
Of the 58 GPs with access to the electronic clinical decision support tools, 17 (29.3%) reported having integrated it into their practice, and nine (15.5%) reported receiving training. The proportion who had both received training and had integrated the tool into their practice was low (5/58, 8.6%). At the practice level, training had been received by at least one GP in six of the 42 (14.3%) practices with access to the tool. The tool was integrated into the practice of at least one GP in 15 of the 42 (35.7%) practices.
The ‘alert prompt’ and ‘symptom checker’ functions were ranked as being the most useful by 16 (27.6%) and 14 (24.1%), respectively, of the 58 GPs with access to the tool. The ‘searches report’ was ranked least useful by 12 of the 58 GPs (20.7%)
Functions of the electronic clinical decision support tools that were considered helpful included:
-
assessing cancer risk in patients with non-specific symptoms (20/58, 34.5%), or with multiple symptoms (21/58, 36.2%)
-
discussing cancer risk with a patient (17/58, 29.3%).
Other functions were less popular:
-
increasing the awareness of cancer as a possible diagnosis (13/58, 22.4%)
-
reassuring anxious patients (10/58, 17.2%)
-
prompting referrals that would otherwise have not have made (8/58, 13.8%)
-
increasing the certainty of clinical decision-making (7/58, 12.1%)
-
increasing the awareness of cancer symptoms (7/58, 12.1%).
Discussing investigation with symptomatic patients was not a popular function (3/58, 5.2%). Approximately one-third of GPs opted to select none of the functions listed (17/58, 29.3%). When asked how likely they were to use the electronic clinical decision support tool to assess a patient whose symptoms may be caused by cancer, 39 (67.2%) reported that they would be unlikely or very unlikely to do so.
Combined tools
Of the 476 GPs, 112 (23.5%, 95% CI 19.7% to 27.6%) had access to a cancer decision support tool in either paper or electronic format, or both. At the practice level, this equates to at least one GP with access in 83 of the 227 practices (36.6%, 95% CI 30.3% to 43.1%). Of the 227 general practices, 38 (16.7%, 95% CI 12.1% to 22.2%) contained at least one GP who had access to the tools and was likely or very likely to use them.
Association between the use of tools and 2-week-wait referral activity
Regression analyses were carried out for the 173 practices in England, for which Public Health England publish age- and sex-adjusted 2WW referral and conversion rates. Of these 173 practices, 68 had access to either a paper RAT or an electronic clinical decision support tool. There was no difference in the mean 2WW referral rate between practices that do and practices that do not have access to either type of tool, after adjusting for IMD (mean difference 1.8 referrals per 100,000 head of population, 95% CI –6.7 to 10.3 per 100,000 head of population) (Table 31).
| Referral rate (per 100,000) | p-value | ||
|---|---|---|---|
| Mean difference | 95% CI | ||
| Availability of tool (yes/no) | 1.81 | –6.72 to 10.34 | 0.676 |
| IMD | 0.63 | 0.25 to 1.01 | 0.001 |
The normal probability plot of the residuals was approximately linear (data are not shown), supporting the assumption of linear regression that the error terms are normally distributed.
Access to either type of tool was not associated with a change in the proportion of 2WW referrals that resulted in a diagnosis of cancer (the conversion rate), after adjusting for IMD (mean difference –0.2, 95% CI –1.0 to 0.6) (Table 32).
| Proportion of 2WW referrals resulting in a cancer diagnosis | p-value | ||
|---|---|---|---|
| Mean difference | 95% CI | ||
| Availability of tool (yes/no) | –0.18 | –0.97 to 0.62 | 0.663 |
| IMD | –0.05 | –0.09 to –0.02 | 0.005 |
Discussion
To the best of our knowledge, this is the first UK-wide survey of the availability of cancer decision support tools. Based on the responses received, cancer decision support tools, in either paper or electronic format, are available to GPs in approximately one-third (36.6%, 95% CI 30.3% to 43.1%) of UK practices. The proportion of general practices where at least one GP had access to the tools and was likely or very likely to use them was 16.7% (95% CI 12.1% to 22.2%). There are no current plans to re-release the paper-based tools, so the expectation is that electronic clinical decision support tools will become the norm. For this reason, it is useful to report separately the availability of electronic clinical decision support tools in UK general practices, which was 19.0% (95% CI 14.0% to 24.6%). Currently, the tools are available only via EMIS Web and INPS Vision, and approximately one-third of the practices using these software systems had opted to download or activate them. The software will shortly be integrated into SystmOne, which is estimated to have 33% of the market share in the UK. Between them, EMIS Web, SystmOne and INPS Vision represent > 95% of the general practice software systems available;190 therefore, in the near future, it is reasonable to assume that nearly 100% of GPs will have access to the electronic clinical decision support tools, should they choose to download/activate them.
There was no evidence that access to the tools was associated with a change in either the rate of 2WW referrals or the proportion of those who were referred transpiring to have cancer.
Strengths and limitations
The main strength of this study is that we used random probability sampling methods, allowing for every general practice in the UK to have an equal chance of receiving the survey. This increases the generalisability of our results to the UK. Further strengths include the thorough preparation and testing of the survey before distribution to ensure that it was short, clear and user friendly, including images for easy identification of the cancer decision support tools in question. Postal surveys, with personalised letters and prepaid return envelopes, plus a financial incentive by way of a charity donation, were also used, as recognised methods of increasing the response rate. 198
Despite these steps, our sample size fell short of the intended size, resulting in wider CIs than planned. The main reason for the low response rate was probably large GP workloads, as volunteered by practice managers and reported elsewhere. 199 The potential for responder bias is an important issue to consider here, particularly because the response rate was low. If responders were more likely to have access to, and be engaged with, the tools than non-responders, then our results will be overestimates. One way to explore this is to look at the number of practices with access to computer systems that support the electronic tools to see if they were over-represented in our sample. As seen in the results, the electronic tools were available in only two (Vision and EMIS Web) of the three (Vision, EMIS Web and SystmONE) main general practice IT systems in the UK. In our sample, ≈ 57% of practices had Vision and EMIS systems; this is very similar to the national picture of 62%, and would suggest that the response rate was not related to access to the tools.
Our subanalyses did not provide any evidence of an association between access to the tools and practice-level diagnostic activity. This is perhaps not surprising, given that the electronic tools, in particular, have been available for a only short time and our survey suggests that they are not yet embedded in clinical practice. However, for the conversion rate analyses, there is a separate issue of the reliability of this diagnostic outcome indicator. This metric has drawn criticism for not being able to distinguish reliably between general practices because of the small numbers of cancer diagnosed per practice per year. 193 Future analyses should therefore focus on diagnostic process indicators, such as the age- and sex-adjusted 2WW referral rate, which distinguish between practices reliably.
What this research adds
To our knowledge, there is no comparable literature on the uptake of cancer decision support tools in the UK or elsewhere. Existing qualitative studies shed light on why the uptake of cancer diagnostic tools is relatively low: they suggest that improvements in design and training may improve the uptake of tools, which is in line with our finding of relatively low levels of training in the use of electronic clinical decision support tools. 13,16,19 Indeed, there was some variability within a general practice in terms of its response to the question asking about tool availability. Some GPs in a practice reported that they have access to the tools and others reported that they are unaware of them. This suggests that there is the potential to increase uptake through increased training in use and awareness. Any training should encourage GPs to maximise the amount of information coded into a patient’s records. This is because the algorithms rely solely on coded data, and omission of data recorded in text fields is associated with bias. 200
Conclusion
Our survey indicates that cancer clinical decision support tools are currently not widely used in the UK. This may contribute to our findings in SR1 (see Chapter 3) and SR2 (see Chapter 4) that there is limited evidence that these tools have an impact on patient outcomes. The upcoming integration of the tools into SystmONE will further increase the potential use of these tools.
As the levels of uptake are relatively low, it remains possible to carry out a RCT to assess whether or not these tools are genuinely helpful in improving the selection of patients for investigation. The attendant benefits of improved selection would include better targeting of investigation resources, potentially earlier diagnosis and reduced treatment costs. 13–15,19,88,89,91,186 Such a trial should include a study of barriers to tool use, and ways to overcome them, as well as a health economics arm.
Chapter 9 Discussion
To our knowledge, this is the first piece of work to bring together evidence on the validation clinical effectiveness, cost-effectiveness, and availability and use of cancer diagnostic tools to aid decision-making in primary care in the UK. To better understand the clinical effectiveness and cost-effectiveness of cancer diagnostic tools to aid decision-making in primary care, SR1, SR2 and the updated review followed prespecified systematic review protocols. Alongside these reviews, a decision model was developed to explore the likely trade-offs in using cancer diagnostic tools in practice, and will be available for updating when new evidence becomes available. Finally, to explore the extent to which GPs currently have access to and use cancer diagnostic tools, a survey of GPs was undertaken. The main findings are discussed in this chapter, as well as gaps in the literature and the strengths and limitations of the research.
What evidence exists on the clinical effectiveness and cost-effectiveness of cancer diagnostic tools in primary care? (Systematic review 1)
Systematic review 1 identified a limited number of heterogeneous studies that provided weak evidence of the impact of these diagnostic tools on patient-related outcomes or referral patterns. This finding is in line with previous reviews across different types of cancers,40,44 confirming the uncertainty surrounding the clinical utility of diagnostic tools in primary care. Only two RCTs were identified, and the only existing trial of RATs found no statistically significant difference in time to diagnosis between the intervention and control groups. No studies evaluating the cost-effectiveness of these tools was identified, even though the definition of diagnostic tool used in SR1 was extended to include the implementation of any diagnostic model or algorithm.
The results of SR1 are limited by heterogeneity between the studies in terms of the tools evaluated, the populations and the outcomes used. Furthermore, the findings are constrained by the design of the included studies, for example non-randomised designs and infrequent use of patient-reported outcomes.
What diagnostic prediction models exist in the literature that have the potential to be used as tools in primary care? (Systematic review 2)
Eleven site-specific cancer diagnostic prediction models were identified, and covered a range of common and less common cancer sites. These included different versions of the QCancer and RAT models, both developed in the UK. All of the models identified had been developed in Europe.
The risk of bias across studies reporting the development or validation of models was mixed. Notably, the QCancer models were developed using cohort data, and most have been externally validated, but lack impact assessment. In comparison, the RAT models have gathered more evidence of impact on practice, but have been developed from case–control studies, and with limited external validation. With the exception of the CRC RAT model, there were no reports to suggest that models were being updated based on new available data.
What evidence exists on the association between different diagnostic interval durations and patient outcomes? (Updated review)
The findings of a previous systematic review25 were updated (see Chapter 5) to further examine the association between different durations of time from first symptom to diagnosis or treatment and clinical outcomes across all major cancers. A more in-depth evaluation was conducted of CRC to inform the model.
The literature suggests a U-shaped relationship between diagnostic interval and unfavourable outcomes, which suggests the clinical need to expedite the diagnosis of symptomatic patients to avoid the preventable delays that some patients continue to experience. The review also identified important biases and other factors that may affect the findings of studies in this field.
The majority of the CRC studies found ‘no association’ between various intervals and patient outcomes. A small number of studies (n = 4, although three used the same or overlapping population) reported a positive association between shorter intervals and patient outcomes, but, paradoxically, a small number of studies (n = 3) also found a negative association.
What is the likely impact that use of the tools in clinical practice may have on patient outcomes and NHS resources? (Economic model)
There are clear gaps and uncertainties in the evidence base that need to be addressed to fully assess the clinical effectiveness and cost-effectiveness of the tools. There is no available evidence that using the tools has an impact on clinical practice.
To explore trade-offs in the benefits, harms and associated NHS resources related to the use of diagnostic tools in primary care, we developed a simple model of the primary care diagnostic pathway for patients with symptoms suspected to be caused by CRC. Exploration of the model indicates uncertainty as to whether or not the amount of benefit to patients who are identified earlier by diagnostic tools and confirmed as having cancer outweighs the loss in quantity and quality of life in those patients referred for further investigation who do not have cancer. In the absence of good-quality evidence, our model predicts that use of a diagnostic algorithm would extend life expectancy by between 4 days and approximately 2 months among symptomatic patients aged 70 years presenting to their GP with undiagnosed CRC, relative to a hypothetical current practice of management according to results of a FIT alone. Although this amount of benefit may be larger than the loss of life from exposing the overwhelming majority of patients without cancer to the risks of colonoscopy, it is unlikely to justify the additional £25 incurred in health-care costs with the tool per low-risk patient presenting to primary care suspected to have CRC.
The ability to fully evaluate the cost-effectiveness of using diagnostic tools in primary care is restricted because of a lack of evidence on their impact on clinical practice and diagnostic intervals, as well as on current standard practice. In the absence of such evidence, our analysis had to rely on two key assumptions: first, about the relationship between the sensitivity of the test and number of GP visits before referral, which has previously been shown to correlate with time to referral, and, second, about the relationship between the time to referral and time to diagnosis and treatment, so that a reduction in the referral interval was assumed to correspond to an equal reduction in the time to diagnosis and treatment.
Because of the uncertainty behind these assumptions, cost-effectiveness analysis was not the objective of our study. However, we were able to identify that, in addition to research to verify the validity of these assumptions, other key sources of uncertainty in the evidence should be the focus of research, including an evaluation of the tools in comparative studies in low-risk populations and relative to current primary care diagnostic practice. It is noted that the diagnostic accuracy studies should be conducted across the low-risk subpopulation, defined by age and prevalence, and that the sensitivity of standard practice and the specificity of triage tools are two of the most influential parameters for cost-effectiveness, which is also driven by the cost of colonoscopy.
To what extent do general practitioners have access to and use existing cancer decision support tools? (General practitioner survey)
We conducted a UK-wide survey of GPs on the availability of cancer decision support tools. Cancer decision support tools, in either paper or electronic format, were available to GPs in 36.6% (95% CI 30.3% to 43.1%) of UK practices. The proportion of general practices where at least one GP has access to the tools and was likely or very likely to use them was 16.7% (95% CI 12.1% to 22.2%). The proportion of general practices in the UK with access to an electronic clinical decision support tool was 19.0% (95% CI 14.0% to 24.6%).
The survey indicates that cancer clinical decision support tools are currently not widely used in the UK. There was no evidence that access to the tools was associated with a change in the rate of 2WW referrals, or the proportion of those referrals transpiring to have cancer. The subanalyses also did not provide any evidence of an association between access to the tools and practice-level diagnostic activity. This is, perhaps, not surprising, given that the electronic tools, in particular, have been available for only a short time and our survey suggests that they are not yet embedded in clinical practice.
Strengths
Systematic reviews 1 and 2, and the updated review, followed prespecified systematic review protocols, and the team conducting the reviews are independent and experienced in systematic review methodology. The decision model was specifically developed to reflect the primary care pathway for patients with symptoms suspected to be caused by CRC. We were able to represent key relationships required to produce predictions in terms of meaningful clinical outcomes and identify the main parameters contributing to uncertainty in the model. The model is expected to undergo further refinement as new evidence emerges, and to be used as a template for modelling the effect of diagnostic tools in other cancers.
The GP survey is the first UK-wide survey of the availability and use of cancer decision support tools. It is also the first national study to attempt to link use of the tools with changes in the rate of 2WW referrals or cancer diagnoses.
Limitations
The reviews and decision model were limited by a lack of evidence, and poor study quality. The best-quality study in SR135 was conducted in Australia, and used a composite intervention including RATs (with older versions of several instruments that were developed using populations from a different country). Not only does this limit the generalisability of results, but it also hinders examination of why no impact of patient outcomes was found. For instance, could it be due to low uptake, poor implementation or limited marginal contribution of the tools in assessing the risk of cancer?
Systematic review 2 identified a number of potential models for use in primary care, yet considerable gaps in the literature exist. There is a disparity in the available evidence on the impact of the tools and the extent to which models have been validated (e.g. whether or not they have been validated at all, and if so, if it has been in the appropriate populations). Comparison of the identified models is limited by heterogeneity between different prediction models for different cancers.
Because there was heterogeneity between studies in the updated review of diagnostic intervals, a ‘vote-counting’ approach was taken, which does not account for the magnitude of any effect. The potential for selective outcome reporting also limits the interpretation of the evidence on an association between duration of pre-diagnostic intervals and patient outcomes. It is therefore very difficult to say whether or not there is an association between different interval durations and patient outcomes, although the more recent evidence of a U-shaped relationship requires further examination.
The decision model has several limitations as it relies on a linked-data approach in a context of little available evidence. In particular, in the absence of direct clinical effectiveness data, we assume a relationship between the sensitivity of both the algorithm and routine referral practice and the number of primary care visits before referral, which has been shown to be associated with the referral interval, and that any difference between diagnostic strategies in the implied referral interval equals their difference in time to diagnosis and treatment. The lack of relevant data on the diagnostic accuracy of the current referral practice in primary care, and on the prevalence of cancer in populations at low risk are identified, not surprisingly, to be crucial in calculating the magnitude of the trade-offs in the decision model. Crucially, we do not know how the tools are being used and our main analysis of a diagnostic tool that is used to select patients for direct referral to secondary care and manage the rest by standard investigation may not necessarily be relevant to many localities using the tool. Furthermore, there were insufficient data to evaluate the effect of the tools on quality of life that is mediated by a reduction in the diagnostic interval and associated experience of severe anxiety by patients and their relatives, although our optimistic exploratory analysis revealed that this effect is unlikely to render the tools cost-effective, without further impact on patient survival.
The survey was limited by a lower than expected response rate, with a small likelihood of response bias (those aware and using the tools may have been more likely to respond). This may limit the ability to conclude that the tools do not lead to increased diagnostic activity or, indeed, the ability to characterise referral systems that may be able to benefit more from the tools.
Uncertainties
There are clear gaps and uncertainties in the evidence base that need to be addressed to fully assess the clinical effectiveness and cost-effectiveness of the tools. There is uncertainty about the relative accuracy of the tools, as there is no single study that has compared them, or evaluated any one of them relative to standard practice or against any comparator. The tools that we focused on had evidence from single-arm studies of different designs and population prevalence. Our model-based evaluation was undertaken in the context of the recent publication of a policy recommendation for FITs to replace FOBTs as routine tests. In primary care, the evidence on FITs is limited as the tests have not been evaluated in the population relevant to our study. This means that even the evidence from indirect comparisons of the tools with FITs is limited to diagnostic accuracy data that may not be valid for our study purposes.
Moreover, there is uncertainty about the interaction between GP, tool and the patient. The intervention is not just the tool, but how the GP interacts with the tool. A better understanding of this may help to disentangle uncertainties on whether or not a lack of evidence of the clinical effectiveness of the tools is due to poor implementation, insufficient uptake or the assumptions on which the tools are based.
Given the uncertainities in the evidence base, it is also possible that rapid referral can do more harm than good, or at least more harm than people might expect. As well as the false positives, there is the likelihood that expediting investigation in one group will lead to fewer resources being available for the group that are not expedited. The group not expedited will contain cancer cases, as sensitivity is around 50%, and slower diagnosis could lead to worse outcomes for these cases. These uncertainties and the associated potential for an inappropriate allocation of resources suggests the need for future research to address these issues, as outlined in Chapter 10, Recommendations for research.
Given the difficulty of conducting robust clinical studies of diagnostic tools for suspected cancer referrals in primary care, evaluations may pragmatically focus on intermediate outcomes, such as referral intervals, time to diagnosis and treatment initiation. In addressing the structural uncertainty arising from translating an effect on referral intervals into an effect on the time interval until diagnosis, observational studies may fruitfully exploit routine electronic health-care records in which, for example, changes in referral policy guidance could opportunistically be used as a natural experiment to assess the impact of earlier referrals on the diagnostic interval.
Chapter 10 Conclusions
Current evaluations of feature-based cancer diagnostic tools in primary care provided limited and mixed evidence of the impact on patient outcomes. QCancer and RATs are the dominant risk prediction tools identified for use in primary care for any cancer. These tools have the potential to be used by GPs on a wider scale once the knowledge gaps highlighted in the review are addressed. The research has been conducted in the UK, the Netherlands and Australia, suggesting limited geographical application.
The lack of robust clinical effectiveness data is an important limiting factor in assessing the cost-effectiveness of diagnostic tools. Better-quality and more research is needed to provide these data, for example more robust study designs and a wider selection of outcomes. However, identifying the ideal approach may not be straightforward. For example, there is an ongoing debate as to the most appropriate outcome for evaluating interventions to improve cancer diagnosis and referral. 201–203 Moreover, by comparing average times to diagnosis, patients not prioritised for quick referrals are at increased risk of being missed.
The cancer clinical decision support tools are not widely used, and this could explain the limited evidence evaluating their clinical effectiveness. This paucity of evidence, however, makes assessment of the impact of their use on patient outcomes difficult. The levels of uptake of the tools are relatively low, leaving sufficient numbers of general practices to act as controls in future RCTs to assess whether or not these tools are genuinely helpful in improving the selection of patients for investigation.
Recommendations for practice
The uncertainties about the clinical effectiveness of cancer diagnostic tools in primary care, compounded by continued lack of clarity about the relationship between delay to diagnosis or treatment and outcome, suggest that further recommendations for increased use of these tools may be premature. It may be that the limited uptake of the tools observed in our survey is appropriate, given the limited and mixed evidence base we have found.
Recommendations for research
It is undeniable that the rationale for cancer diagnostic tools in primary care is strong. Therefore, the priority should be for further research to confirm or refute the potential of cancer diagnostic tools to improve cancer outcomes. QCancer and RATs are well-established tools. We therefore tentatively suggest that further research should concentrate on QCancer and RATs.
The research on these tools should include:
-
Continued model validation – RATs should try to emulate the validation methods employed by QCancer. Adhering to good conduct and reporting guidance on studies of prediction tools is essential to maximise the value of the studies and to improve the ability to review them.
-
QCancer versus RATs – the performance of the two tools should be compared. It would be particularly helpful to see how similar the risks predicted by the two tools are for identical patients.
-
Assessment of clinical effectiveness – although some assessment of the clinical effectiveness of RATs has been performed, this has been inconclusive, so there is a need for this to be performed for both tools. Studies should collect information on time to diagnosis and treatment and stage at diagnosis, as well as health outcomes (if feasible). In terms of design, cluster randomised trials have been shown to be feasible, and are preferable to quasi-experimental designs already attempted. Given the likely small effect on health outcomes in particular, it is essential that the sample size of any study is appropriately large. A Phase II cluster RCT evaluating the impact of RATs for gastro-oesophageal cancer is ongoing. 20
-
Assessment of the tool in GP patient consultations – as the tool is not used in isolation, research to understand the complex nature of the intervention is warranted to investigate the interaction between GP, tool and patient. This may be part of an interventional study.
-
Assessment of quality-of-life implications of delayed and incorrect diagnosis. We do not know what the effect of delayed diagnosis is on the health-related quality of life of cancer patients and their families during the diagnostic delay and after delayed diagnosis. The anxiety caused by persistent unresolved symptoms would require retrospective observational studies recruiting subjects in large secondary care centres.
-
Assessment of cost-effectiveness – although the main current limitation is the absence of clinical effectiveness evidence, researchers should anticipate how cost-effectiveness will be addressed when such evidence becomes available. Incorporating assessment of cost-effectiveness into trials may be one approach, but experience suggests that, to generalise and extrapolate from the trial results, continued enhancement of the economic model developed in this project should be pursued and used to synthesise the latest evidence from points 1 to 3 above. Thus, our model may provide an iterative framework for informing the design of studies described in point 3, and be populated by the results of those studies.
-
Assessment of impact on other referral pathways – clinical effectiveness studies should consider not just the referral/diagnosis time in expedited pathways, but also the impact on the other pathways, as cancer cases might be slowed by a shift in resources to the expedited pathway.
-
Finally, the challenges of interpreting observational data exploring the relationship between delay and outcome continues, as demonstrated in the updated review. For further studies answering this question to be useful, they need to address issues of unmeasured confounding such as grade and stage, as well as problems of survivor time or selection bias implicit in the period of follow-up over which the studies are conducted. These are problems limiting the quality of existing evidence, some of which may be improved by developing agreed standards for providing complete and accurate reporting of all methods used and outcomes measured.
Acknowledgements
We would like to acknowledge the instrumental support of Ms Louise Crathorne and Professor Ruben E Mujica-Mota. We gratefully thank Ms Jenny Lowe for all the support received during this project, and Ms Barbara France for her help with the updated review. We also thank the external reviewers, who provided valuable advice on a complex project. We thank the patients who agreed to participate in a research workshop, as well as the GPs who provided invaluable information for understanding the CRC diagnosis pathway. Our special thanks go to Dr Emma Cockcroft, who undertook the task of organising the meetings with the patients and the GPs.
Contributions of authors
Professor Antonieta Medina-Lara (https://orcid.org/0000-0001-7325-8246), Associate Professor in Health Economics, acted as the project lead, worked on the systematic review of the economic models evidence and on the development of the de novo economic model, and supported the GP survey.
Dr Bogdan Grigore (https://orcid.org/0000-0003-4241-7595), Postdoctoral Research Associate, led SR1 and SR2.
Ms Ruth Lewis (https://orcid.org/0000-0003-0745-995X), Senior Lecturer, led the updated review and worked on SR1 and SR2.
Dr Jaime Peters (https://orcid.org/0000-0003-1778-3518), Senior Research Fellow, supported SR1 and SR2 and the writing-up of the economic model.
Dr Sarah Price (https://orcid.org/0000-0002-2228-2374), Research Fellow, undertook the design, data collection and analysis, and the writing-up of the GP survey.
Dr Paolo Landa (https://orcid.org/0000-0001-6532-6747), Research Fellow in Health Economics, worked on the systematic review of the economic models and on the de novo model.
Ms Sophie Robinson (https://orcid.org/0000-0003-0463-875X), Information Specialist, carried out the electronic searches for SR1, SR2, the review of economic models and the review of parameter values for the de novo economic model.
Professor Richard Neal (https://orcid.org/0000-0002-3544-2744), Professor of Primary Care Oncology, and Professor William Hamilton (https://orcid.org/0000-0003-1611-1373), Professor of Primary Care Diagnostics, provided support to the project on the clinical aspects of the project.
Professor Anne E Spencer (https://orcid.org/0000-0002-8163-3103), Associate Professor in Health Economics, provided senior advice for the review of the economic models, the de novo model and the GP survey.
Data-sharing statement
Requests for survey data or a copy of the model should be submitted to the corresponding author for consideration. Access to anonymised data or a dummy-data populated model may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE – 5 – a population-based study. Lancet Oncol 2014;15:23-34. https://doi.org/10.1016/S1470-2045(13)70546-1.
- Ades AE, Biswas M, Welton NJ, Hamilton W. Symptom lead time distribution in lung cancer: natural history and prospects for early diagnosis. Int J Epidemiol 2014;43:1865-73. https://doi.org/10.1093/ije/dyu174.
- Cole SR, Tucker GR, Osborne JM, Byrne SE, Bampton PA, Fraser RJ, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust 2013;198:327-30. https://doi.org/10.5694/mja12.11357.
- Richards MA. The National Awareness and Early Diagnosis Initiative in England: assembling the evidence. Br J Cancer 2009;101:1-4. https://doi.org/10.1038/sj.bjc.6605382.
- Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, et al. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. Br J Cancer 2012;107:1220-6. https://doi.org/10.1038/bjc.2012.408.
- National Institute for Health and Care Excellence . Suspected Cancer: Recognition and Referral n.d. www.nice.org.uk/guidance/ng12 (accessed 16 March 2019).
- Langton S, Siau D, Bankhead C. Two-week rule in head and neck cancer 2000–14: a systematic review. Br J Oral Maxillofac Surg 2016;54:120-31. https://doi.org/10.1016/j.bjoms.2015.09.041.
- Hiom SC. Diagnosing cancer earlier: reviewing the evidence for improving cancer survival. Br J Cancer 2015;112:1-5. https://doi.org/10.1038/bjc.2015.23.
- Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353-65. https://doi.org/10.1016/S1470-2045(12)70041-4.
- Hendriksen JM, Geersing GJ, Moons KG, de Groot JA. Diagnostic and prognostic prediction models. J Thromb Haemost 2013;11:129-41. https://doi.org/10.1111/jth.12262.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. https://doi.org/10.7326/M14-0698.
- Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001381.
- Hamilton W, Green T, Martins T, Elliott K, Rubin G, Macleod U. Evaluation of risk assessment tools for suspected cancer in general practice: a cohort study. Br J Gen Pract 2013;63:e30-6. https://doi.org/10.3399/bjgp13X660751.
- Collins GS, Altman DG. Identifying patients with undetected pancreatic cancer in primary care: an independent and external validation of QCancer® (pancreas). Br J Gen Pract 2013;63:e636-42. https://doi.org/10.3399/bjgp13X671623.
- Collins GS, Altman DG. Identifying patients with undetected colorectal cancer: an independent validation of QCancer (colorectal). Br J Cancer 2012;107:260-5. https://doi.org/10.1038/bjc.2012.266.
- Green T, Martins T, Hamilton W, Rubin G, Elliott K, Macleod U. Exploring GPs’ experiences of using diagnostic tools for cancer: a qualitative study in primary care. Fam Pract 2015;32:101-5. https://doi.org/10.1093/fampra/cmu081.
- Dikomitis L, Green T, Macleod U. Embedding electronic decision-support tools for suspected cancer in primary care: a qualitative study of GPs’ experiences. Prim Health Care Res Dev 2015;16:548-55. https://doi.org/10.1017/S1463423615000109.
- Chiang PP, Glance D, Walker J, Walter FM, Emery JD. Implementing a QCancer risk tool into general practice consultations: an exploratory study using simulated consultations with Australian general practitioners. Br J Cancer 2015;112:77-83. https://doi.org/10.1038/bjc.2015.46.
- Moffat J, Ironmonger L, Green T. Clinical Decision Support Tool for Cancer (CDS) Project: Evaluation Report to the Department of Health 2014. www.cancerresearchuk.org/sites/default/files/cds_final_310714.pdf (accessed January 2020).
- Moore HJ, Nixon C, Tariq A, Emery J, Hamilton W, Hoare Z, et al. Evaluating a computer aid for assessing stomach symptoms (ECASS): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1307-3.
- Iyen-Omofoman B, Tata LJ, Baldwin DR, Smith CJ, Hubbard RB. Using socio-demographic and early clinical features in general practice to identify people with lung cancer earlier. Thorax 2013;68:451-9. https://doi.org/10.1136/thoraxjnl-2012-202348.
- Marshall T, Lancashire R, Sharp D, Peters TJ, Cheng KK, Hamilton W. The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance. Gut 2011;60:1242-8. https://doi.org/10.1136/gut.2010.225987.
- Bourne P, Rosendahl C, Keir J, Cameron A. BLINCK-A diagnostic algorithm for skin cancer diagnosis combining clinical features with dermatoscopy findings. Dermatol Pract Concept 2012;2. https://doi.org/10.5826/dpc.0202a12.
- Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 2004;291:2705-12. https://doi.org/10.1001/jama.291.22.2705.
- Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112:92-107. https://doi.org/10.1038/bjc.2015.48.
- Bouwmeester W, Zuithoff NP, Mallett S, Geerlings MI, Vergouwe Y, Steyerberg EW, et al. Reporting and methods in clinical prediction research: a systematic review. PLOS Med 2012;9:1-12. https://doi.org/10.1371/journal.pmed.1001221.
- Reilly BM, Evans AT. Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Ann Intern Med 2006;144:201-9. https://doi.org/10.7326/0003-4819-144-3-200602070-00009.
- Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009;338. https://doi.org/10.1136/bmj.b606.
- Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A, et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-40.
- Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001744.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed January 2020).
- Hippisley-Cox J, Coupland C. Identifying patients with suspected pancreatic cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2012;62:e38-45. https://doi.org/10.3399/bjgp12X616355.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Chichester: John Wiley & Sons; 2011.
- Emery JD, Gray V, Walter FM, Cheetham S, Croager EJ, Slevin T, et al. The Improving Rural Cancer Outcomes Trial: a cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural cancer patients in Western Australia. Br J Cancer 2017;117:1459-69. https://doi.org/10.1038/bjc.2017.310.
- English DR, Burton RC, del Mar CB, Donovan RJ, Ireland PD, Emery G. Evaluation of aid to diagnosis of pigmented skin lesions in general practice: controlled trial randomised by practice. BMJ 2003;327. https://doi.org/10.1136/bmj.327.7411.375.
- Del Mar CB, Green AC. Aid to diagnosis of melanoma in primary medical care. BMJ 1995;310:492-5. https://doi.org/10.1136/bmj.310.6978.492.
- Gulati A, Harwood CA, Rolph J, Pottinger E, McGregor JM, Goad N, et al. Is an online skin cancer toolkit an effective way to educate primary care physicians about skin cancer diagnosis and referral?. J Eur Acad Dermatol Venereol 2015;29:2152-9. https://doi.org/10.1111/jdv.13167.
- Hamilton W. The CAPER studies: five case–control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 2009;101:80-6. https://doi.org/10.1038/sj.bjc.6605396.
- Williams TG, Cubiella J, Griffin SJ, Walter FM, Usher-Smith JA. Risk prediction models for colorectal cancer in people with symptoms: a systematic review. BMC Gastroenterol 2016;16. https://doi.org/10.1186/s12876-016-0475-7.
- Schmidt-Hansen M, Berendse S, Hamilton W, Baldwin DR. Lung cancer in symptomatic patients presenting in primary care: a systematic review of risk prediction tools. Br J Gen Pract 2017;67:e396-e404. https://doi.org/10.3399/bjgp17X690917.
- Usher-Smith J, Emery J, Hamilton W, Griffin SJ, Walter FM. Risk prediction tools for cancer in primary care. Br J Cancer 2015;113:1645-50. https://doi.org/10.1038/bjc.2015.409.
- Walker JG, Licqurish S, Chiang PP, Pirotta M, Emery JD. Cancer risk assessment tools in primary care: a systematic review of randomized controlled trials. Ann Fam Med 2015;13:480-9. https://doi.org/10.1370/afm.1837.
- Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk prediction models for colorectal cancer: a systematic review. Cancer Prev Res 2016;9:13-26. https://doi.org/10.1158/1940-6207.CAPR-15-0274.
- Adams ST, Leveson SH. Clinical prediction rules. BMJ 2012;344. https://doi.org/10.1136/bmj.d8312.
- Wolff R, Whiting P, Mallett S, Riley R, Westwood M, Kleijnen J, et al. PROBAST: A Risk of Bias Tool for Prediction Modelling Studies. Filtering the Information Overload for Better Decisions n.d.
- Debray TP, Damen JA, Snell KI, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017;356. https://doi.org/10.1136/bmj.i6460.
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51-8. https://doi.org/10.7326/M18-1376.
- Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1-W33. https://doi.org/10.7326/M18-1377.
- Elias SG, Kok L, Witteman BJM, Goedhard JG, Romberg-Camps MJL, Muris JWM, et al. Published diagnostic models safely excluded colorectal cancer in an independent primary care validation study. J Clin Epidemiol 2017;82:149-57. https://doi.org/10.1016/j.jclinepi.2016.09.014.
- Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case–control study. Thorax 2005;60:1059-65. https://doi.org/10.1136/thx.2005.045880.
- Hippisley-Cox J, Coupland C. Identifying patients with suspected lung cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2011;61:e715-23. https://doi.org/10.3399/bjgp11X606627.
- Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2013;63:e11-21. https://doi.org/10.3399/bjgp13X660733.
- Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2013;63:e1-10. https://doi.org/10.3399/bjgp13X660724.
- Fijten GH, Starmans R, Muris JW, Schouten HJ, Blijham GH, Knottnerus JA. Predictive value of signs and symptoms for colorectal cancer in patients with rectal bleeding in general practice. Fam Pract 1995;12:279-86. https://doi.org/10.1093/fampra/12.3.279.
- Muris JW, Starmans R, Fijten GH, Crebolder HF, Schouten HJ, Knottnerus JA. Non-acute abdominal complaints in general practice: diagnostic value of signs and symptoms. Br J Gen Pract 1995;45:313-16.
- Nørrelund N, Nørrelund H. Colorectal cancer and polyps in patients aged 40 years and over who consult a GP with rectal bleeding. Fam Pract 1996;13:160-5. https://doi.org/10.1093/fampra/13.2.160.
- Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ 2007;334. https://doi.org/10.1136/bmj.39171.637106.AE.
- Jordan KP, Hayward RA, Blagojevic-Bucknall M, Croft P. Incidence of prostate, breast, lung and colorectal cancer following new consultation for musculoskeletal pain: a cohort study among UK primary care patients. Int J Cancer 2013;133:713-20. https://doi.org/10.1002/ijc.28055.
- McCowan C, Donnan PT, Dewar J, Thompson A, Fahey T. Identifying suspected breast cancer: development and validation of a clinical prediction rule. Br J Gen Pract 2011;61:e205-14. https://doi.org/10.3399/bjgp11X572391.
- Kop R, Hoogendoorn M, Moons LMG, Numans ME, ten Teije A. On the advantage of using dedicated data mining techniques to predict colorectal cancer. Lect Notes Comp Sci 2015;9105:133-42. https://doi.org/10.1007/978-3-319-19551-3_16.
- Keane MG, Horsfall L, Rait G, Pereira SP. A case–control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005720.
- Holtedahl K, Hjertholm P, Borgquist L, Donker GA, Buntinx F, Weller D, et al. Abdominal symptoms and cancer in the abdomen: prospective cohort study in European primary care. Br J Gen Pract 2018;68:e301-e310. https://doi.org/10.3399/bjgp18X695777.
- Dommett RM, Redaniel T, Stevens MC, Martin RM, Hamilton W. Risk of childhood cancer with symptoms in primary care: a population-based case–control study. Br J Gen Pract 2013;63:e22-9. https://doi.org/10.3399/bjgp13X660742.
- Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract 2010;60:121-8. https://doi.org/10.3399/bjgp10X483175.
- Shephard E, Neal R, Rose P, Walter F, Hamilton WT. Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract 2013;63:e250-5. https://doi.org/10.3399/bjgp13X665215.
- Shephard EA, Neal RD, Rose PW, Walter FM, Hamilton W. Symptoms of adult chronic and acute leukaemia before diagnosis: large primary care case-control studies using electronic records. Br J Gen Pract 2016;66:e182-8. https://doi.org/10.3399/bjgp16X683989.
- Shephard EA, Neal RD, Rose P, Walter FM, Litt EJ, Hamilton WT. Quantifying the risk of multiple myeloma from symptoms reported in primary care patients: a large case–control study using electronic records. Br J Gen Pract 2015;65:e106-13. https://doi.org/10.3399/bjgp15X683545.
- Shephard EA, Stapley S, Neal RD, Rose P, Walter FM, Hamilton WT. Clinical features of bladder cancer in primary care. Br J Gen Pract 2012;62:e598-604. https://doi.org/10.3399/bjgp12X654560.
- Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of pancreatic cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br J Cancer 2012;106:1940-4. https://doi.org/10.1038/bjc.2012.190.
- Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br J Cancer 2013;108:25-31. https://doi.org/10.1038/bjc.2012.551.
- Stapley SA, Rubin GP, Alsina D, Shepard EA, Rutter MD, Hamilton WT. Clinical features of bowel disease in patients aged < 50 years in primary care: a large case–control study. Br J Gen Pract 2017;67:e336-44. https://doi.org/10.3399/bjgp17X690425.
- Walker S, Hyde C, Hamilton W. Risk of uterine cancer in symptomatic women in primary care: case–control study using electronic records. Br J Gen Pract 2013;63:e643-8. https://doi.org/10.3399/bjgp13X671632.
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng K, Marshall T. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case–control study. BMC Med 2009;7. https://doi.org/10.1186/1741-7015-7-17.
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng KK, Marshall T. The importance of anaemia in diagnosing colorectal cancer: a case–control study using electronic primary care records. Br J Cancer 2008;98:323-7. https://doi.org/10.1038/sj.bjc.6604165.
- Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of The Health Improvement Network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393-401. https://doi.org/10.1002/pds.1335.
- Hodder RJ, Ballal M, Selvachandran SN, Cade D. Pitfalls in the construction of cancer guidelines demonstrated by the analyses of colorectal referrals. Ann R Coll Surg Engl 2005;87:419-26. https://doi.org/10.1308/003588405X71018.
- Hoogendoorn M, Szolovits P, Moons LMG, Numans ME. Utilizing uncoded consultation notes from electronic medical records for predictive modeling of colorectal cancer. Artif Intell Med 2016;69:53-61. https://doi.org/10.1016/j.artmed.2016.03.003.
- Kop R, Hoogendoorn M, Teije AT, Büchner FL, Slottje P, Moons LM, et al. Predictive modeling of colorectal cancer using a dedicated pre-processing pipeline on routine electronic medical records. Comput Biol Med 2016;76:30-8. https://doi.org/10.1016/j.compbiomed.2016.06.019.
- Hippisley-Cox J, Coupland C. Identifying patients with suspected colorectal cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2012;62:e29-37. https://doi.org/10.3399/bjgp12X616346.
- Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 2005;93:399-405. https://doi.org/10.1038/sj.bjc.6602714.
- Hippisley-Cox J, Coupland C. Identifying patients with suspected gastro-oesophageal cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2011;61:e707-14. https://doi.org/10.3399/bjgp11X606609.
- Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case–control study. BMJ 2009;339. https://doi.org/10.1136/bmj.b2998.
- Hippisley-Cox J, Coupland C. Identifying women with suspected ovarian cancer in primary care: derivation and validation of algorithm. BMJ 2011;344. https://doi.org/10.1136/bmj.d8009.
- Hippisley-Cox J, Coupland C. Identifying patients with suspected renal tract cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2012;62:e251-60. https://doi.org/10.3399/bjgp12X636074.
- Hamilton W, Barrett J, Stapley S, Sharp D, Rose P. Clinical features of metastatic cancer in primary care: a case–control study using medical records. Br J Gen Pract 2015;65:e516-22. https://doi.org/10.3399/bjgp15X686077.
- Hamilton W, Kernick D. Clinical features of primary brain tumours: a case–control study using electronic primary care records. Br J Gen Pract 2007;57:695-9.
- Collins GS, Altman DG. Identifying patients with undetected gastro-oesophageal cancer in primary care: external validation of QCancer® (gastro-oesophageal). Eur J Cancer 2013;49:1040-8. https://doi.org/10.1016/j.ejca.2012.10.023.
- Collins GS, Altman DG. Identifying women with undetected ovarian cancer: independent and external validation of QCancer® (ovarian) prediction model. Eur J Cancer Care 2013;22:423-9. https://doi.org/10.1111/ecc.12015.
- Hamilton W, Sharp DJ, Peters TJ, Round AP. Clinical features of prostate cancer before diagnosis: a population-based, case-control study. Br J Gen Pract 2006;56:756-62.
- Collins GS, Altman DG. Identifying patients with undetected renal tract cancer in primary care: an independent and external validation of QCancer® (renal) prediction model. Cancer Epidemiol 2013;37:115-20. https://doi.org/10.1016/j.canep.2012.11.005.
- Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262-7. https://doi.org/10.1038/bjc.2012.68.
- Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353:1119-26. https://doi.org/10.1016/S0140-6736(99)02143-1.
- Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguiló A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer 2007;43:2467-78. https://doi.org/10.1016/j.ejca.2007.08.023.
- Ramos M, Esteva M, Cabeza E, Llobera J, Ruiz A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer 2008;44:510-21. https://doi.org/10.1016/j.ejca.2008.01.011.
- Thompson MR, Heath I, Swarbrick ET, Faulds Wood L, Ellis BG. Earlier diagnosis and treatment of symptomatic bowel cancer: can it be achieved and how much will it improve survival?. Colorectal Dis 2011;13:6-16. https://doi.org/10.1111/j.1463-1318.2009.01986.x.
- Moher D, Tsertsvadze A, Tricco AC, Eccles M, Grimshaw J, Sampson M, et al. When and how to update systematic reviews. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.MR000023.pub3.
- Cooper C, Booth A, Britten N, Garside R. A comparison of results of empirical studies of supplementary search techniques and recommendations in review methodology handbooks: a methodological review. Syst Rev 2017;6. https://doi.org/10.1186/s13643-017-0625-1.
- Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer 2013;49:2187-98. https://doi.org/10.1016/j.ejca.2013.01.025.
- Ángeles-Llerenas A, Torres-Mejía G, Lazcano-Ponce E, Uscanga-Sánchez S, Mainero-Ratchelous F, Hernández-Ávila JE, et al. Effect of care-delivery delay on the survival of Mexican women with breast cancer. Salud Publica Mex 2016;58:237-50. https://doi.org/10.21149/spm.v58i2.7793.
- Redaniel MT, Martin RM, Ridd MJ, Wade J, Jeffreys M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: historical cohort study using the Clinical Practice Research Datalink. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0126608.
- Murchie P, Raja EA, Lee AJ, Brewster DH, Campbell NC, Gray NM, et al. Effect of longer health service provider delays on stage at diagnosis and mortality in symptomatic breast cancer. Breast 2015;24:248-55. https://doi.org/10.1016/j.breast.2015.02.027.
- Pace LE, Mpunga T, Hategekimana V, Dusengimana JM, Habineza H, Bigirimana JB, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist 2015;20:780-8. https://doi.org/10.1634/theoncologist.2014-0493.
- Unger-Saldaña K, Miranda A, Zarco-Espinosa G, Mainero-Ratchelous F, Bargalló-Rocha E, Miguel Lázaro-León J. Health system delay and its effect on clinical stage of breast cancer: multicenter study. Cancer 2015;121:2198-206. https://doi.org/10.1002/cncr.29331.
- Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc B 2015;370. https://doi.org/10.1098/rstb.2014.0042.
- Helewa RM, Turner D, Park J, Wirtzfeld D, Czaykowski P, Hochman D, et al. Longer waiting times for patients undergoing colorectal cancer surgery are not associated with decreased survival. J Surg Oncol 2013;108:378-84. https://doi.org/10.1002/jso.23412.
- Murchie P, Raja EA, Brewster DH, Campbell NC, Ritchie LD, Robertson R, et al. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: effect on disease stage and survival. Br J Cancer 2014;111:461-9. https://doi.org/10.1038/bjc.2014.352.
- Dregan A, Møller H, Charlton J, Gulliford MC. Are alarm symptoms predictive of cancer survival?: population-based cohort study. Br J Gen Pract 2013;63:e807-12. https://doi.org/10.3399/bjgp13X675197.
- Pita-Fernández S, González-Sáez L, López-Calviño B, Seoane-Pillado T, Rodríguez-Camacho E, Pazos-Sierra A, et al. Effect of diagnostic delay on survival in patients with colorectal cancer: a retrospective cohort study. BMC Cancer 2016;16. https://doi.org/10.1186/s12885-016-2717-z.
- Aslam MI, Chaudhri S, Singh B, Jameson JS. The ‘two-week wait’ referral pathway is not associated with improved survival for patients with colorectal cancer. Int J Surg 2017;43:181-5. https://doi.org/10.1016/j.ijsu.2017.05.046.
- Patel R, Anderson JE, McKenzie C, Simpson M, Singh N, Ruzvidzo F, et al. Compliance with the 62-day target does not improve long-term survival. Int J Colorectal Dis 2018;33:65-9. https://doi.org/10.1007/s00384-017-2930-5.
- Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol 2017;15:728-37. https://doi.org/10.1016/j.cgh.2016.10.038.
- Leiva A, Esteva M, Llobera J, Macià F, Pita-Fernández S, González-Luján L, et al. Time to diagnosis and stage of symptomatic colorectal cancer determined by three different sources of information: a population based retrospective study. Cancer Epidemiol 2017;47:48-55. https://doi.org/10.1016/j.canep.2016.10.021.
- Janssen RM, Takach O, Nap-Hill E, Enns RA. Time to endoscopy in patients with colorectal cancer: analysis of wait-times. Can J Gastroenterol Hepatol 2016;2016. https://doi.org/10.1155/2016/8714587.
- Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. Adv Exp Med Biol 2013;788:355-62. https://doi.org/10.1007/978-94-007-6627-3_48.
- Živković D. Effect of delays on survival in patients with lung carcinoma in Montenegro. Acta Clin Croat 2014;53:390-8.
- Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, Valdes L, Carreira JM, Pose A, et al. Symptoms and reason for a medical visit in lung cancer patients. Acta Med Port 2014;27:318-24. https://doi.org/10.20344/amp.4229.
- Gildea TR, DaCosta Byfield S, Hogarth DK, Wilson DS, Quinn CC. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. Clinicoecon Outcomes Res 2017;9:261-9. https://doi.org/10.2147/CEOR.S132259.
- Kim JO, Davis F, Butts C, Winget M. Waiting time intervals for non-small cell lung cancer diagnosis and treatment in alberta: quantification of intervals and identification of risk factors associated with delays. Clin Oncol 2016;28:750-9. https://doi.org/10.1016/j.clon.2016.06.010.
- Nikonova A, Guirguis HR, Buckstein R, Cheung MC. Predictors of delay in diagnosis and treatment in diffuse large B-cell lymphoma and impact on survival. Br J Haematol 2015;168:492-500. https://doi.org/10.1111/bjh.13150.
- Goldschmidt N, Zamir L, Poperno A, Kahan NR, Paltiel O. Presenting signs of multiple myeloma and the effect of diagnostic delay on the prognosis. J Am Board Fam Med 2016;29:702-9. https://doi.org/10.3122/jabfm.2016.06.150393.
- Keizer AC, Korse TCM, Meijer WG, Tesselaar ET. The effect of delay in diagnosis in patients with neuroendocrine tumors. Int J Endocr Oncol 2016;3:33-9. https://doi.org/10.2217/ije.15.32.
- Alahapperuma LS, Fernando EA. Patient-linked factors associated with delayed reporting of oral and pharyngeal carcinoma among patients attending national cancer institute, Maharagama, Sri Lanka. Asian Pac J Cancer Prev 2017;18:321-5.
- Esmaelbeigi F, Hadji M, Harirchi I, Omranipour R, vand Rajabpour M, Zendehdel K. Factors affecting professional delay in diagnosis and treatment of oral cancer in Iran. Arch Iran Med 2014;17:253-7. https://doi.org/014174/AIM.007.
- Lim A, Mesher D, Gentry-Maharaj A, Balogun N, Widschwendter M, Jacobs I, et al. Time to diagnosis of type I or II invasive epithelial ovarian cancers: a multicentre observational study using patient questionnaire and primary care records. BJOG 2016;123:1012-20. https://doi.org/10.1111/1471-0528.13447.
- Altman AD, Lambert P, Love AJ, Turner D, Lotocki R, Dean E, et al. Examining the effects of time to diagnosis, income, symptoms, and incidental detection on overall survival in epithelial ovarian cancer: Manitoba Ovarian Cancer Outcomes (MOCO) study group. Int J Gynecol Cancer 2017;27:1637-44. https://doi.org/10.1097/IGC.0000000000001074.
- Gobbi PG, Bergonzi M, Comelli M, Villano L, Pozzoli D, Vanoli A, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. https://doi.org/10.1016/j.canep.2012.12.002.
- Jooste V, Dejardin O, Bouvier V, Arveux P, Maynadie M, Launoy G, et al. Pancreatic cancer: wait times from presentation to treatment and survival in a population-based study. Int J Cancer 2016;139:1073-80. https://doi.org/10.1002/ijc.30166.
- Gao W, Song LB, Yang J, Song NH, Wu XF, Song NJ, et al. Risk factors and negative consequences of patient’s delay for penile carcinoma. World J Surg Oncol 2016;14. https://doi.org/10.1186/s12957-016-0863-z.
- Bonfill X, Martinez-Zapata MJ, Vernooij RW, Sánchez MJ, Suárez-Varela MM, de la Cruz J, et al. Clinical intervals and diagnostic characteristics in a cohort of prostate cancer patients in Spain: a multicentre observational study. BMC Urol 2015;15. https://doi.org/10.1186/s12894-015-0058-x.
- Urakawa H, Tsukushi S, Arai E, Kozawa E, Futamura N, Ishiguro N, et al. Association of short duration from initial symptoms to specialist consultation with poor survival in soft-tissue sarcomas. Am J Clin Oncol 2015;38:266-71. https://doi.org/10.1097/COC.0b013e318295aea2.
- Goedhart LM, Gerbers JG, Ploegmakers JJ, Jutte PC. Delay in diagnosis and its effect on clinical outcome in high-grade sarcoma of bone: a referral oncological centre study. Orthop Surg 2016;8:122-8. https://doi.org/10.1111/os.12239.
- De Boer C, Niyonzima N, Orem J, Bartlett J, Zafar SY. Prognosis and delay of diagnosis among Kaposi’s sarcoma patients in Uganda: a cross-sectional study. Infect Agent Cancer 2014;9. https://doi.org/10.1186/1750-9378-9-17.
- Kobayashi K, Saito T, Kitamura Y, Nobushita T, Kawasaki T, Hara N, et al. Effect of the time from the presentation of symptoms to medical consultation on primary tumor size and survival in patients with testicular cancer: shift in the last 2 decades. Urol Oncol 2014;32:43-22. https://doi.org/10.1016/j.urolonc.2013.05.007.
- Tørring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol 2012;65:669-78. https://doi.org/10.1016/j.jclinepi.2011.12.006.
- Tørring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer 2011;104:934-40. https://doi.org/10.1038/bjc.2011.60.
- Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death?. Cancer Causes Control 2013;24:961-77. https://doi.org/10.1007/s10552-013-0172-6.
- Korsgaard M, Pedersen L, Sørensen HT, Laurberg S. Reported symptoms, diagnostic delay and stage of colorectal cancer: a population-based study in Denmark. Colorectal Dis 2006;8:688-95. https://doi.org/10.1111/j.1463-1318.2006.01014.x.
- Biswas M, Ades AE, Hamilton W. Symptom lead times in lung and colorectal cancers: what are the benefits of symptom-based approaches to early diagnosis?. Br J Cancer 2015;112:271-7. https://doi.org/10.1038/bjc.2014.597.
- National Institute for Health and Care Excellence . Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care. Diagnostics Guidance [DG30] n.d. www.nice.org.uk/guidance/dg30 (accessed 16 March 2019).
- Hamilton W. Towards Earlier Diagnosis of Cancer in Primary Care: A Population-Based Case–Control Study of Colorectal, Lung and Prostate Cancer 2005.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force . Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 1. Value Health 2012;15:796-803. https://doi.org/10.1016/j.jval.2012.06.012.
- Kaltenthaler E, Tappenden P, Paisley S, Squires H. NICE DSU Technical Support Document 13: Identifying and Reviewing Evidence to Inform the Conceptualisation and Population of Cost-effectiveness Models. Sheffield: Decision Support Unit, School of Health and Related Research, University of Sheffield; 2011.
- Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults. A cost-effectiveness analysis comparing four diagnostic strategies. J Gen Intern Med 2005;20:81-90. https://doi.org/10.1111/j.1525-1497.2005.40077.x.
- Tappenden P, Chilcott J, Eggington S, Patnick J, Sakai H, Karnon J. Option appraisal of population-based colorectal cancer screening programmes in England. Gut 2007;56:677-84. https://doi.org/10.1136/gut.2006.095109.
- Tsoi KK, Ng SS, Leung MC, Sung JJ. Cost-effectiveness analysis on screening for colorectal neoplasm and management of colorectal cancer in Asia. Aliment Pharmacol Ther 2008;28:353-63. https://doi.org/10.1111/j.1365-2036.2008.03726.x.
- Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659-69. https://doi.org/10.7326/0003-4819-149-9-200811040-00244.
- Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLOS Med 2010;7. https://doi.org/10.1371/journal.pmed.1000370.
- Lee D, Muston D, Sweet A, Cunningham C, Slater A, Lock K. Cost effectiveness of CT colonography for UK NHS colorectal cancer screening of asymptomatic adults aged 60–69 years. Appl Health Econ Health Policy 2010;8:141-54. https://doi.org/10.2165/11535650-000000000-00000.
- Knudsen AB, Hur C, Gazelle GS, Schrag D, McFarland EG, Kuntz KM. Rescreening of persons with a negative colonoscopy result: results from a microsimulation model. Ann Intern Med 2012;157:611-20. https://doi.org/10.7326/0003-4819-157-9-201211060-00005.
- Sharp L, Tilson L, Whyte S, O’Ceilleachair A, Walsh C, Usher C, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer 2012;106:805-16. https://doi.org/10.1038/bjc.2011.580.
- Whyte S, Chilcott J, Halloran S. Reappraisal of the options for colorectal cancer screening in England. Colorectal Dis 2012;14:e547-61. https://doi.org/10.1111/j.1463-1318.2012.03014.x.
- Goede SL, van Roon AH, Reijerink JC, van Vuuren AJ, Lansdorp-Vogelaar I, Habbema JD, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 2013;62:727-34. https://doi.org/10.1136/gutjnl-2011-301917.
- Gomes M, Aldridge RW, Wylie P, Bell J, Epstein O. Cost-effectiveness analysis of 3-D computerized tomography colonography versus optical colonoscopy for imaging symptomatic gastroenterology patients. Appl Health Econ Health Policy 2013;11:107-17. https://doi.org/10.1007/s40258-013-0019-z.
- Tappenden P, Chilcott J, Brennan A, Squires H, Glynne-Jones R, Tappenden J. Using whole disease modeling to inform resource allocation decisions: economic evaluation of a clinical guideline for colorectal cancer using a single model. Value Health 2013;16:542-53. https://doi.org/10.1016/j.jval.2013.02.012.
- Whyte S, Harnan S. Effectiveness and cost-effectiveness of an awareness campaign for colorectal cancer: a mathematical modeling study. Cancer Causes Control 2014;25:647-58. https://doi.org/10.1007/s10552-014-0366-6.
- Cantor SB, Rajan T, Linder SK, Volk RJ. A framework for evaluating the cost-effectiveness of patient decision aids: a case study using colorectal cancer screening. Prev Med 2015;77:168-73. https://doi.org/10.1016/j.ypmed.2015.05.003.
- Pil L, Fobelets M, Putman K, Trybou J, Annemans L. Cost-effectiveness and budget impact analysis of a population-based screening program for colorectal cancer. Eur J Intern Med 2016;32:72-8. https://doi.org/10.1016/j.ejim.2016.03.031.
- Wong MC, Ching JY, Chan VC, Lam TY, Luk AK, Wong SH, et al. Colorectal cancer screening based on age and gender: a cost-effectiveness analysis. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000002739.
- Coldman A, Flanagan W, Nadeau C, Wolfson M, Fitzgerald N, Mermon S, et al. Projected effect of fecal immunochemical test threshold for colorectal cancer screening on outcomes and costs for Canada using the OncoSim microsimulation model. J Cancer Policy 2017;13:38-46. https://doi.org/10.1016/j.jcpo.2017.07.004.
- Westwood M, Lang S, Armstrong N, van Turenhout S, Cubiella J, Stirk L, et al. Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: a systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0944-z.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Tappenden P, Eggington S, Nixon J, . Colorectal Cancer Screening Options Appraisal. Report to the English Bowel Cancer Screening Working Group 2004.
- Office of National Statistics . Mortality Statistics: Cause [series DH2, No. 32] 2006. www.statistics.gov.uk/downloads/theme_health/Dh2_32/DH2_No32_2005.pdf (accessed 22 March 2010).
- Hospital Authority . Hong Kong Cancer Registry n.d. www.ha.org.hk/cancereg (accessed May 2007).
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. https://doi.org/10.1016/S0140-6736(02)09832-X.
- Hoomans T, Seidenfeld J, Basu A, Meltzer D. Systematizing the Use of Value of Information Analysis in Prioritizing Systematic Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. https://doi.org/10.1177/0272989X04263162.
- Cancer Research UK . Cancer Incidence for Common Cancers n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared (accessed 5 May 2016).
- Goodwin E, Boddy K, Tatnell L, Hawton A. Involving members of the public in health economics research: insights from selecting health states for valuation to estimate quality-adjusted life-year (QALY) weights. Appl Health Econ Health Policy 2018;16:187-94. https://doi.org/10.1007/s40258-017-0355-5.
- Pizzo E, Doyle C, Matthews R, Barlow J. Patient and public involvement: how much do we spend and what are the benefits?. Health Expect 2015;18:1918-26. https://doi.org/10.1111/hex.12204.
- National Institute for Health and Care Excellence (NICE) . Referral Guidelines for Suspected Cancer. Clinical Guideline [CG27] 2005.
- Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer 2013;108:686-90. https://doi.org/10.1038/bjc.2013.1.
- Whyte S, Harnan S, Scope A, Simpson E, Tappenden P, Duffy S, et al. Early Awareness Interventions for Cancer: Colorectal Cancer n.d. www.eepru.org.uk/article/early-awareness-interventions-for-cancer-colorectal-cancer-policy-research-unit-in-economic-evaluation-of-health-and-care-interventions/ (accessed 16 March 2019).
- Murphy J, Halloran S, Gray A. Cost-effectiveness of the faecal immunochemical test at a range of positivity thresholds compared with the guaiac faecal occult blood test in the NHS Bowel Cancer Screening Programme in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017186.
- Office for National Statistics . National Life Tables, UK: 2015 to 2017 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015to2017 (accessed 16 March 2019).
- Liu CY, Chen WT, Kung PT, Chiu CF, Wang YH, Shieh SH, et al. Characteristics, survival, and related factors of newly diagnosed colorectal cancer patients refusing cancer treatments under a universal health insurance program. BMC Cancer 2014;14. https://doi.org/10.1186/1471-2407-14-446.
- National Cancer Registration and Analysis Service . Colorectal Cancer Survival by Stage – NCIN Data Briefing n.d. www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage (accessed 27 February 2019).
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343-50. https://doi.org/10.1111/j.1572-0241.2006.00390.x.
- Atkin WS, Cook CF, Cuzick J, Edwards R, Northover JM, Wardle J. UK Flexible Sigmoidoscopy Screening Trial Investigators . Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet 2002;359:1291-300. https://doi.org/10.1016/S0140-6736(02)08268-5.
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003;95:230-6. https://doi.org/10.1093/jnci/95.3.230.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650-7. https://doi.org/10.1111/j.1572-0241.1999.01157.x.
- Department of Health and Social Care (DHSC) . Improving Outcomes: A Strategy for Cancer 2011.
- Department of Health and Social Care (DHSC) . The Likely Impact of Earlier Diagnosis of Cancer on Costs and Benefits to the NHS 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213788/dh_123576.pdf (accessed 16 March 2019).
- Independent Cancer Taskforce . Achieving World-Class Cancer Outcomes: Taking the Strategy Forward n.d. www.england.nhs.uk/wp-content/uploads/2016/05/cancer-strategy.pdf (accessed 16 March 2019).
- Vedsted P, Olesen F. Are the serious problems in cancer survival partly rooted in gatekeeper principles? An ecologic study. Br J Gen Pract 2011;61:e508-12. https://doi.org/10.3399/bjgp11X588484.
- Zhou Y, Mendonca SC, Abel GA, Hamilton W, Walter FM, Johnson S, et al. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br J Cancer 2018;118:24-31. https://doi.org/10.1038/bjc.2017.381.
- NHS Digital . GP Systems of Choice. Practice and System Data: Market Share 2015 n.d. http://content.digital.nhs.uk/media/20723/NIC-06225-H1B9Q---GPSoC-Market-Share-Practice-and-System-data---October-2015/xls/ (accessed 16 March 2019).
- ClinRisk . Welcome to QCancer n.d. www.qcancer.org (accessed January 2020).
- Cancer Research UK . Cancer Risk Assessment Tools n.d. www.cancerresearchuk.org/sites/default/files/rats.pdf (accessed January 2020).
- Abel G, Saunders CL, Mendonca SC, Gildea C, McPhail S, Lyratzopoulos G. Variation and statistical reliability of publicly reported primary care diagnostic activity indicators for cancer: a cross-sectional ecological study of routine data. BMJ Qual Saf 2018;27:21-30. https://doi.org/10.1136/bmjqs-2017-006607.
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care 2003;15:261-6. https://doi.org/10.1093/intqhc/mzg031.
- Creavin ST, Creavin AL, Mallen CD. Do GPs respond to postal questionnaire surveys? A comprehensive review of primary care literature. Fam Pract 2011;28:461-7. https://doi.org/10.1093/fampra/cmr001.
- Abel GA, Barclay ME, Payne RA. Adjusted indices of multiple deprivation to enable comparisons within and between constituent countries of the UK including an illustration using mortality rates. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012750.
- Ministry of Housing, Communities & Local Government . English Indices of Deprivation 2015: Technical Report n.d. www.gov.uk/government/publications/english-indices-of-deprivation-2015-technical-report (accessed 16 March 2019).
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. https://doi.org/10.1002/14651858.MR000008.pub4.
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30. https://doi.org/10.1016/S0140-6736(16)00620-6.
- Price SJ, Stapley SA, Shephard E, Barraclough K, Hamilton WT. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case–control study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011664.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128-38. https://doi.org/10.1097/EDE.0b013e3181c30fb2.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. https://doi.org/10.1177/0272989X06295361.
- Debray TP, Vergouwe Y, Koffijberg H, Nieboer D, Steyerberg EW, Moons KG. A new framework to enhance the interpretation of external validation studies of clinical prediction models. J Clin Epidemiol 2015;68:279-89. https://doi.org/10.1016/j.jclinepi.2014.06.018.
- Adelstein BA, Irwig L, Macaskill P, Turner RM, Chan SF, Katelaris PH. Who needs colonoscopy to identify colorectal cancer? Bowel symptoms do not add substantially to age and other medical history. Aliment Pharmacol Ther 2010;32:270-81. https://doi.org/10.1111/j.1365-2036.2010.04344.x.
- Baicus C, Ionescu R, Tanasescu C. Does this patient have cancer? The assessment of age, anemia, and erythrocyte sedimentation rate in cancer as a cause of weight loss. A retrospective study based on a secondary care university hospital in Romania. Eur J Intern Med 2006;17:28-31. https://doi.org/10.1016/j.ejim.2005.07.009.
- Ewing M, Naredi P, Zhang C, Månsson J. Identification of patients with non-metastatic colorectal cancer in primary care: a case–control study. Br J Gen Pract 2016;66:e880-6. https://doi.org/10.3399/bjgp16X687985.
- Galvin R, Joyce D, Downey E, Boland F, Fahey T, Hill AK. Development and validation of a clinical prediction rule to identify suspected breast cancer: a prospective cohort study. BMC Cancer 2014;14. https://doi.org/10.1186/1471-2407-14-743.
- Khademi H, Radmard AR, Malekzadeh F, Kamangar F, Nasseri-Moghaddam S, Johansson M, et al. Diagnostic accuracy of age and alarm symptoms for upper GI malignancy in patients with dyspepsia in a GI clinic: a 7-year cross-sectional study. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0039173.
- Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol 2011;118:280-8. https://doi.org/10.1097/AOG.0b013e318224fce2.
- Saraiva R, Perkusich M, Silva L, Almeida H, Siebra C, Perkusich A. Early diagnosis of gastrointestinal cancer by using case-based and rule-based reasoning. Expert Systems With Applications 2016;61:192-20. https://doi.org/10.1016/j.eswa.2016.05.026.
- Reeves MJ, Osuch JR, Pathak DR. Development of a clinical decision rule for triage of women with palpable breast masses. J Clin Epidemiol 2003;56:636-45. https://doi.org/10.1016/S0895-4356(03)00123-9.
- Robertson R, Campbell C, Weller DP, Elton R, Mant D, Primrose J, et al. Predicting colorectal cancer risk in patients with rectal bleeding. Br J Gen Pract 2006;56:763-7.
- Simpkins SJ, Pinto-Sanchez MI, Moayyedi P, Bercik P, Morgan DG, Bolino C, et al. Poor predictive value of lower gastrointestinal alarm features in the diagnosis of colorectal cancer in 1981 patients in secondary care. Aliment Pharmacol Ther 2017;45:91-9. https://doi.org/10.1111/apt.13846.
- Koning NR, Moons LM, Büchner FL, Helsper CW, Ten Teije A, Numans ME. Identification of patients at risk for colorectal cancer in primary care: an explorative study with routine healthcare data. Eur J Gastroenterol Hepatol 2015;27:1443-8. https://doi.org/10.1097/MEG.0000000000000472.
- Kim MK, Kim K, Kim SM, Kim JW, Park NH, Song YS, et al. A hospital-based case–control study of identifying ovarian cancer using symptom index. J Gynecol Oncol 2009;20:238-42. https://doi.org/10.3802/jgo.2009.20.4.238.
- Høgdall C, Fung ET, Christensen IJ, Nedergaard L, Engelholm SA, Petri AL, et al. A novel proteomic biomarker panel as a diagnostic tool for patients with ovarian cancer. Gynecol Oncol 2011;123:308-13. https://doi.org/10.1016/j.ygyno.2011.07.018.
- Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol 2005;141:1008-14. https://doi.org/10.1001/archderm.141.8.1008.
- Argenziano G, Catricalà C, Ardigo M, Buccini P, De Simone P, Eibenschutz L, et al. Seven-point checklist of dermoscopy revisited. Br J Dermatol 2011;164:785-90. https://doi.org/10.1111/j.1365-2133.2010.10194.x.
- Emery JD, Hunter J, Hall PN, Watson AJ, Moncrieff M, Walter FM. Accuracy of SIAscopy for pigmented skin lesions encountered in primary care: development and validation of a new diagnostic algorithm. BMC Dermatol 2010;10. https://doi.org/10.1186/1471-5945-10-9.
- Gerbert B, Bronstone A, Maurer T, Hofmann R, Berger T. Decision support software to help primary care physicians triage skin cancer: a pilot study. Arch Dermatol 2000;136:187-92. https://doi.org/10.1001/archderm.136.2.187.
- Rogers T, Marino ML, Dusza SW, Bajaj S, Usatine RP, Marchetti MA, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med 2016;29:694-701. https://doi.org/10.3122/jabfm.2016.06.160079.
- Walter FM, Morris HC, Humphrys E, Hall PN, Prevost AT, Burrows N, et al. Effect of adding a diagnostic aid to best practice to manage suspicious pigmented lesions in primary care: randomised controlled trial. BMJ 2012;345. https://doi.org/10.1136/bmj.e4110.
- Walter FM, Prevost AT, Vasconcelos J, Hall PN, Burrows NP, Morris HC, et al. Using the 7-point checklist as a diagnostic aid for pigmented skin lesions in general practice: a diagnostic validation study. Br J Gen Pract 2013;63:e345-53. https://doi.org/10.3399/bjgp13X667213.
- Ballal MS, Selvachandran SN, Maw A. Use of a patient consultation questionnaire and weighted numerical scoring system for the prediction of colorectal cancer and other colorectal pathology in symptomatic patients: a prospective cohort validation study of a Welsh population. Colorectal Dis 2010;12:407-14. https://doi.org/10.1111/j.1463-1318.2009.01984.x.
- Selvachandran SN, Hodder RJ, Ballal MS, Jones P, Cade D. Prediction of colorectal cancer by a patient consultation questionnaire and scoring system: a prospective study. Lancet 2002;360:278-83. https://doi.org/10.1016/S0140-6736(02)09549-1.
- Smith D, Ballal M, Hodder R, Soin G, Selvachandran SN, Cade D. Symptomatic presentation of early colorectal cancer. Ann R Coll Surg Engl 2006;88:185-90. https://doi.org/10.1308/003588406X94904.
- Zortea M, Schopf TR, Thon K, Geilhufe M, Hindberg K, Kirchesch H, et al. Performance of a dermoscopy-based computer vision system for the diagnosis of pigmented skin lesions compared with visual evaluation by experienced dermatologists. Artif Intell Med 2014;60:13-26. https://doi.org/10.1016/j.artmed.2013.11.006.
- Grewal K, Hamilton W, Sharp D. Ovarian cancer prediction: development of a scoring system for primary care. BJOG 2013;120:1016-19. https://doi.org/10.1111/1471-0528.12200.
- Shahzad N, Rashid N, Zahra S, Malik A. Role of malignancy index in prediction of malignancy in ovarian masses preoperative. Med Forum Mon 2015;26:44-7.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010;102:222-9. https://doi.org/10.1093/jnci/djp500.
- Lim AW, Mesher D, Gentry-Maharaj A, Balogun N, Jacobs I, Menon U, et al. Predictive value of symptoms for ovarian cancer: comparison of symptoms reported by questionnaire, interview, and general practitioner notes. J Natl Cancer Inst 2012;104:114-24. https://doi.org/10.1093/jnci/djr486.
- Law CW, Rampal S, Roslani AC, Mahadeva S. Development of a risk score to stratify symptomatic adults referred for colonoscopy. J Gastroenterol Hepatol 2014;29:1890-6. https://doi.org/10.1111/jgh.12638.
- Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1997;15:3085-92. https://doi.org/10.1200/JCO.1997.15.9.3085.
- Esteva M, Leiva A, Ramos M, Pita-Fernández S, González-Luján L, Casamitjana M, et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer 2013;13. https://doi.org/10.1186/1471-2407-13-87.
- Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg 2013;148:516-23. https://doi.org/10.1001/jamasurg.2013.1680.
- Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol 2012;23:2731-7. https://doi.org/10.1093/annonc/mds101.
- Brazda A, Estroff J, Euhus D, Leitch AM, Huth J, Andrews V, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol 2010;17:291-6. https://doi.org/10.1245/s10434-010-1250-6.
- Eastman A, Tammaro Y, Moldrem A, Andrews V, Huth J, Euhus D, et al. Outcomes of delays in time to treatment in triple negative breast cancer. Ann Surg Oncol 2013;20:1880-5. https://doi.org/10.1245/s10434-012-2835-z.
- McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012;30:4493-500. https://doi.org/10.1200/JCO.2012.39.7695.
- Mujar M, Dahlui M, Yip CH, Tabib NA. Delays in time to primary treatment after a diagnosis of breast cancer: does it impact survival?. Prev Med 2013;56:222-4. https://doi.org/10.1016/j.ypmed.2012.12.001.
- Redaniel MT, Martin RM, Cawthorn S, Wade J, Jeffreys M. The association of waiting times from diagnosis to surgery with survival in women with localised breast cancer in England. Br J Cancer 2013;109:42-9. https://doi.org/10.1038/bjc.2013.317.
- Sue GR, Lannin DR, Killelea B, Tsangaris T, Chagpar AB. Does time to definitive treatment matter in patients with ductal carcinoma in situ?. Am Surg 2013;79:561-5.
- Ermiah E, Abdalla F, Buhmeida A, Larbesh E, Pyrhönen S, Collan Y. Diagnosis delay in Libyan female breast cancer. BMC Res Notes 2012;5. https://doi.org/10.1186/1756-0500-5-452.
- Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat 2012;136:813-21. https://doi.org/10.1007/s10549-012-2304-1.
- Wright GP, Wong JH, Morgan JW, Roy-Chowdhury S, Kazanjian K, Lum SS. Time from diagnosis to surgical treatment of breast cancer: factors influencing delays in initiating treatment. Am Surg 2010;76:1119-22.
- Wagner JL, Warneke CL, Mittendorf EA, Bedrosian I, Babiera GV, Kuerer HM, et al. Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg 2011;254:119-24. https://doi.org/10.1097/SLA.0b013e318217e97f.
- Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. West Midlands Urological Research Group . Delay and survival in bladder cancer. BJU Int 2002;89:868-78. https://doi.org/10.1046/j.1464-410X.2002.02776.x.
- Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Skolarus TA, Kim SP, et al. Delays in diagnosis and bladder cancer mortality. Cancer 2010;116:5235-42. https://doi.org/10.1002/cncr.25310.
- Mommsen S, Aagaard J, Sell A. Presenting symptoms, treatment delay and survival in bladder cancer. Scand J Urol Nephrol 1983;17:163-7. https://doi.org/10.3109/00365598309180162.
- Gulliford MC, Petruckevitch A, Burney PG. Survival with bladder cancer, evaluation of delay in treatment, type of surgeon, and modality of treatment. BMJ 1991;303:437-40. https://doi.org/10.1136/bmj.303.6800.437.
- Tokuda Y, Chinen K, Obara H, Joishy SK. Intervals between symptom onset and clinical presentation in cancer patients. Intern Med 2009;48:899-905. https://doi.org/10.2169/internalmedicine.48.1720.
- Maguire A, Porta M, Malats N, Gallén M, Piñol JL, Fernandez E. Cancer survival and the duration of symptoms. An analysis of possible forms of the risk function. ISDS II Project Investigators. Eur J Cancer 1994;30A:785-92. https://doi.org/10.1016/0959-8049(94)90293-3.
- Liedberg F, Anderson H, Månsson A, Månsson W. Diagnostic delay and prognosis in invasive bladder cancer. Scand J Urol Nephrol 2003;37:396-400. https://doi.org/10.1080/00365590310006246.
- Thompson MR, Asiimwe A, Flashman K, Tsavellas G. Is earlier referral and investigation of bowel cancer patients presenting with rectal bleeding associated with better survival?. Colorectal Dis 2011;13:1242-8. https://doi.org/10.1111/j.1463-1318.2010.02438.x.
- Terhaar sive Droste JS, Oort FA, van der Hulst RW, Coupé VM, Craanen ME, Meijer GA, et al. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC Cancer 2010;10. https://doi.org/10.1186/1471-2407-10-332.
- Neal RD, Allgar VL, Ali N, Leese B, Heywood P, Proctor G, et al. Stage, survival and delays in lung, colorectal, prostate and ovarian cancer: comparison between diagnostic routes. Br J Gen Pract 2007;57:212-19.
- Currie AC, Evans J, Smith NJ, Brown G, Abulafi AM, Swift RI. The impact of the two-week wait referral pathway on rectal cancer survival. Colorectal Dis 2012;14:848-53. https://doi.org/10.1111/j.1463-1318.2011.02829.x.
- Zafar A, Mak T, Whinnie S, Chapman MAS. The 2-week wait referral system does not improve 5-year colorectal cancer survival. Colorectal Dis 2012;14:e177-80. https://doi.org/10.1111/j.1463-1318.2011.02826.x.
- Gort M, Otter R, Plukker JT, Broekhuis M, Klazinga NS. Actionable indicators for short and long term outcomes in rectal cancer. Eur J Cancer 2010;46:1808-14. https://doi.org/10.1016/j.ejca.2010.02.044.
- Roland CL, Schwarz RE, Tong L, Ahn C, Balch GC, Yopp AC, et al. Is timing to delivery of treatment a reliable measure of quality of care for patients with colorectal adenocarcinoma?. Surgery 2013;154:421-8. https://doi.org/10.1016/j.surg.2013.04.049.
- Van Hout AM, de Wit NJ, Rutten FH, Peeters PH. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur J Gastroenterol Hepatol 2011;23:1056-63. https://doi.org/10.1097/MEG.0b013e32834c4839.
- Deng SX, An W, Gao J, Yin J, Cai QC, Yang M, et al. Factors influencing diagnosis of colorectal cancer: a hospital-based survey in China. J Dig Dis 2012;13:517-24. https://doi.org/10.1111/j.1751-2980.2012.00626.x.
- Cerdán-Santacruz C, Cano-Valderrama O, Cárdenas-Crespo S, Torres-García AJ, Cerdán-Miguel J. Colorectal cancer and its delayed diagnosis: have we improved in the past 25 years?. Rev Esp Enferm Dig 2011;103:458-63. https://doi.org/10.4321/S1130-01082011000900004.
- Valentín-López B, Ferrándiz-Santos J, Blasco-Amaro JA, Morillas-Sáinz JD, Ruiz-López P. Assessment of a rapid referral pathway for suspected colorectal cancer in Madrid. Fam Pract 2012;29:182-8. https://doi.org/10.1093/fampra/cmr080.
- Ramsay G, MacKay C, Nanthakumaran S, Craig WL, McAdam TK, Loudon MA. Urgency of referral and its impact on outcome in patients with colorectal cancer. Colorectal Dis 2012;14:e375-7. https://doi.org/10.1111/j.1463-1318.2012.02961.x.
- Guzmán Laura KP, Bolíbar Ribas I, Alepuz MT, González D, Martín M. Impact on patient care time and tumor stage of a program for fast diagnostic and treatment of colorectal cancer. Rev Esp Enferm Dig 2011;103:13-9. https://doi.org/10.4321/s1130-01082011000100003.
- Tomlinson C, Wong C, Au HJ, Schiller D. Factors associated with delays to medical assessment and diagnosis for patients with colorectal cancer. Can Fam Physician 2012;58:e495-501.
- Brocken P, Kiers BA, Looijen-Salamon MG, Dekhuijzen PN, Smits-van der Graaf C, Peters-Bax L, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336-41. https://doi.org/10.1016/j.lungcan.2011.08.017.
- Radzikowska E, Roszkowski-Śliż K, Głaz P. The impact of timeliness of care on survival in non-small cell lung cancer patients. Pneumonol Alergol Pol 2012;80:422-9.
- Loh LC, Chan LY, Tan RY, Govindaraju S, Ratnavelu K, Kumar S, et al. Time delay and its effect on survival in malaysian patients with non-small cell lung carcinoma. Malays J Med Sci 2006;13:37-42.
- Annakkaya AN, Arbak P, Balbay O, Bilgin C, Erbas M, Bulut I. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori 2007;93:61-7. https://doi.org/10.1177/030089160709300111.
- Skaug K, Eide GE, Gulsvik A. Predictors of long-term survival of lung cancer patients in a Norwegian community. Clin Respir J 2011;5:50-8. https://doi.org/10.1111/j.1752-699X.2010.00200.x.
- Pita-Fernández S, Montero-Martinez C, Pértega-Diaz S, Verea-Hernando H. Relationship between delayed diagnosis and the degree of invasion and survival in lung cancer. J Clin Epidemiol 2003;56:820-5. https://doi.org/10.1016/S0895-4356(03)00166-5.
- Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest 2008;133:1167-73. https://doi.org/10.1378/chest.07-2654.
- Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung cancer and their prognostic implications. J Thorac Oncol 2011;6:1254-9. https://doi.org/10.1097/JTO.0b013e318217b623.
- González-Barcala FJ, García-Prim JM, Alvarez-Dobaño JM, Moldes-Rodríguez M, García-Sanz MT, Pose-Reino A, et al. Effect of delays on survival in patients with lung cancer. Clin Transl Oncol 2010;12:836-42. https://doi.org/10.1007/s12094-010-0606-5.
- Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage?. Ups J Med Sci 2008;113:287-96. https://doi.org/10.3109/2000-1967-236.
- Christensen ED, Harvald T, Jendresen M, Aggestrup S, Petterson G. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg 1997;12:880-4. https://doi.org/10.1016/S1010-7940(97)00275-3.
- Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax 2004;59:45-9.
- Murai T, Shibamoto Y, Baba F, Hashizume C, Mori Y, Ayakawa S, et al. Progression of non-small-cell lung cancer during the interval before stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:463-7. https://doi.org/10.1016/j.ijrobp.2010.10.001.
- Salomaa ER, Sällinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest 2005;128:2282-8. https://doi.org/10.1378/chest.128.4.2282.
- Mohan A, Mohan C, Bhutani M, Pathak AK, Pal H, DAS C, et al. Quality of life in newly diagnosed patients with lung cancer in a developing country: is it important?. Eur J Cancer Care (Engl 2006;15:293-8. https://doi.org/10.1111/j.1365-2354.2006.00654.x.
- Prabhu M, Kochupillai V, Sharma S, Ramachandran P, Sundaram KR, Bijlani L, et al. Prognostic assessment of various parameters in chronic myeloid leukemia. Cancer 1986;58:1357-60. https://doi.org/10.1002/1097-0142(19860915)58:6<1357::aid-cncr2820580629>3.0.co;2-m.
- Friese CR, Earle CC, Magazu LS, Brown JR, Neville BA, Hevelone ND, et al. Timeliness and quality of diagnostic care for medicare recipients with chronic lymphocytic leukemia. Cancer 2011;117:1470-7. https://doi.org/10.1002/cncr.25655.
- Bertoli S, Bérard E, Huguet F, Huynh A, Tavitian S, Vergez F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood 2013;121:2618-26. https://doi.org/10.1182/blood-2012-09-454553.
- Jacobi N, Rogers TB, Peterson BA. Prognostic factors in follicular lymphoma: a single institution study. Oncol Rep 2008;20:185-93. https://doi.org/10.3892/or.20.1.185.
- Norum J. The effect of diagnostic delay in patients with Hodgkin’s lymphoma. Anticancer Res 1995;15:2707-10.
- Foulc P, N’Guyen JM, Dréno B. Prognostic factors in Sézary syndrome: a study of 28 patients. Br J Dermatol 2003;149:1152-8. https://doi.org/10.1111/j.1365-2133.2003.05677.x.
- Kim YH, Bishop K, Varghese A, Hoppe RT. Prognostic factors in erythrodermic mycosis fungoides and the Sézary syndrome. Arch Dermatol 1995;131:1003-8. https://doi.org/10.1001/archderm.1995.01690210033005.
- Kariyawasan CC, Hughes DA, Jayatillake MM, Mehta AB. Multiple myeloma: causes and consequences of delay in diagnosis. QJM 2007;100:635-40. https://doi.org/10.1093/qjmed/hcm077.
- Friese CR, Abel GA, Magazu LS, Neville BA, Richardson LC, Earle CC. Diagnostic delay and complications for older adults with multiple myeloma. Leuk Lymphoma 2009;50:392-400. https://doi.org/10.1080/10428190902741471.
- Koivunen P, Rantala N, Hyrynkangas K, Jokinen K, Alho OP. The impact of patient and professional diagnostic delays on survival in pharyngeal cancer. Cancer 2001;92:2885-91. https://doi.org/10.1002/1097-0142(20011201)92:11<2885::AID-CNCR10119>3.0.CO;2-G.
- Teppo H, Alho OP. Relative importance of diagnostic delays in different head and neck cancers. Clin Otolaryngol 2008;33:325-30. https://doi.org/10.1111/j.1749-4486.2008.01704.x.
- Teppo H, Koivunen P, Hyrynkangas K, Alho OP. Diagnostic delays in laryngeal carcinoma: professional diagnostic delay is a strong independent predictor of survival. Head Neck 2003;25:389-94. https://doi.org/10.1002/hed.10208.
- Wildt J, Bundgaard T, Bentzen SM. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci 1995;20:21-5. https://doi.org/10.1111/j.1365-2273.1995.tb00006.x.
- Seoane J, Takkouche B, Varela-Centelles P, Tomás I, Seoane-Romero JM. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol 2012;37:99-106. https://doi.org/10.1111/j.1749-4486.2012.02464.x.
- Hansen O, Larsen S, Bastholt L, Godballe C, Jørgensen KE. Duration of symptoms: impact on outcome of radiotherapy in glottic cancer patients. Int J Radiat Oncol Biol Phys 2005;61:789-94. https://doi.org/10.1016/j.ijrobp.2004.07.720.
- McGurk M, Chan C, Jones J, O’regan E, Sherriff M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br J Oral Maxillofac Surg 2005;43:281-4. https://doi.org/10.1016/j.bjoms.2004.01.016.
- Alho OP, Teppo H, Mäntyselkä P, Kantola S. Head and neck cancer in primary care: presenting symptoms and the effect of delayed diagnosis of cancer cases. CMAJ 2006;174:779-84. https://doi.org/10.1503/cmaj.050623.
- Sidler D, Thum P, Winterhalder R, Huber G, Haerle SK. Undifferentiated carcinoma of nasopharyngeal type (UCNT): a Swiss single-institutional experience during 1990-2005. Swiss Med Wkly 2010;140:273-9. https://doi.org/smw-12844.
- Caudell JJ, Locher JL, Bonner JA. Diagnosis-to-treatment interval and control of locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2011;137:282-5. https://doi.org/10.1001/archoto.2011.20.
- Brouha XDR, Op De Coul B, Terhaard CH, Hordijk GJ. Does waiting time for radiotherapy affect local control of T1N0M0 glottic laryngeal carcinoma?. Clin Otolaryngol Allied Sci 2000;25:215-18. https://doi.org/10.1046/j.1365-2273.2000.00347.x.
- Kumar S, Heller RF, Pandey U, Tewari V, Bala N, Oanh KT. Delay in presentation of oral cancer: a multifactor analytical study. Natl Med J India 2001;14:13-7.
- Brouha XD, Tromp DM, Hordijk GJ, Winnubst JA, de Leeuw JR. Oral and pharyngeal cancer: analysis of patient delay at different tumor stages. Head Neck 2005;27:939-45. https://doi.org/10.1002/hed.20270.
- Sheng L, Shui Y, Shen L, Wei Q. Effect of patient-related delay in diagnosis on the extent of disease and prognosis in nasopharyngeal carcinoma. Am J Rhinol 2008;22:317-20. https://doi.org/10.2500/ajr.2008.22.3174.
- Tromp DM, Brouha XD, Hordijk GJ, Winnubst JA, de Leeuw RJ. Patient and tumour factors associated with advanced carcinomas of the head and neck. Oral Oncol 2005;41:313-19. https://doi.org/10.1016/j.oraloncology.2004.09.008.
- Allison P, Franco E, Black M, Feine J. The role of professional diagnostic delays in the prognosis of upper aerodigestive tract carcinoma. Oral Oncol 1998;34:147-53. https://doi.org/10.1016/S1368-8375(97)00088-2.
- Al-Rajhi N, El-Sebaie M, Khafaga Y, AlZahrani A, Mohamed G, Al-Amro A. Nasopharyngeal carcinoma in Saudi Arabia: clinical presentation and diagnostic delay. East Mediterr Health J 2009;15:1301-7.
- Teppo H, Heikkinen J, Laitakari K, Alho OP. Diagnostic delays in vestibular schwannoma. J Laryngol Otol 2009;123:289-93. https://doi.org/10.1017/S0022215108003113.
- Pitchers M, Martin C. Delay in referral of oropharyngeal squamous cell carcinoma to secondary care correlates with a more advanced stage at presentation, and is associated with poorer survival. Br J Cancer 2006;94:955-8. https://doi.org/10.1038/sj.bjc.6603044.
- Vernham GA, Crowther JA. Head and neck carcinoma – stage at presentation. Clin Otolaryngol Allied Sci 1994;19:120-4. https://doi.org/10.1111/j.1365-2273.1994.tb01194.x.
- Lee AW, Foo W, Law SC, Poon YF, Sze WM, O SK, et al. Nasopharyngeal carcinoma: presenting symptoms and duration before diagnosis. Hong Kong Med J 1997;3:355-61.
- Miziara ID, Cahali MB, Murakami MS, Figueiredo LA, Guimaraes JR. Cancer of the larynx: correlation of clinical characteristics, site of origin, stage, histology and diagnostic delay. Rev Laryngol Otol Rhinol 1998;119:101-4.
- Scott SE, Grunfeld EA, McGurk M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol 2005;41:396-403. https://doi.org/10.1016/j.oraloncology.2004.10.010.
- Ho T, Zahurak M, Koch WM. Prognostic significance of presentation-to-diagnosis interval in patients with oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2004;130:45-51. https://doi.org/10.1001/archotol.130.1.45.
- Teppo H, Hyrynkangas K, Koivunen P, Jokinen K, Alho OP. Impact of patient and professional diagnostic delays on the risk of recurrence in laryngeal carcinoma. Clin Otolaryngol 2005;30:157-63. https://doi.org/10.1111/j.1365-2273.2004.00954.x.
- Umezu T, Shibata K, Kajiyama H, Yamamoto E, Mizuno M, Kikkawa F. Prognostic factors in stage IA-IIA cervical cancer patients treated surgically: does the waiting time to the operation affect survival?. Arch Gynecol Obstet 2012;285:493-7. https://doi.org/10.1007/s00404-011-1966-y.
- Fruchter RG, Boyce J. Delays in diagnosis and stage of disease in gynecologic cancer. Cancer Detect Prev 1981;4:481-6.
- Menczer J, Chetrit A, Sadetzki S. National Israel Ovarian Cancer Group . The effect of symptom duration in epithelial ovarian cancer on prognostic factors. Arch Gynecol Obstet 2009;279:797-801. https://doi.org/10.1007/s00404-008-0814-1.
- Crawford SC, Davis JA, Siddiqui NA, de Caestecker L, Gillis CR, Hole D, et al. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7357.196.
- Elit LM, O’Leary EM, Pond GR, Seow HY. Impact of wait times on survival for women with uterine cancer. J Clin Oncol 2014;32:27-33. https://doi.org/10.1200/JCO.2013.51.3671.
- Franceschi S, La Vecchia C, Gallus G, Decarli A, Colombo E, Mangioni C, et al. Delayed diagnosis of endometrial cancer in Italy. Cancer 1983;51:1176-8. https://doi.org/10.1002/1097-0142(19830315)51:6<1176::aid-cncr2820510634>3.0.co;2-o.
- Obermair A, Hanzal E, Schreiner-Frech I, Buxbaum P, Bancher-Todesca D, Thoma M, et al. Influence of delayed diagnosis on established prognostic factors in endometrial cancer. Anticancer Res 1996;16:947-9.
- Pirog EC, Heller DS, Westhoff C. Endometrial adenocarcinoma – lack of correlation between treatment delay and tumor stage. Gynecol Oncol 1997;67:303-8. https://doi.org/10.1006/gyno.1997.4874.
- Robinson KM, Christensen KB, Ottesen B, Krasnik A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: a nationwide Danish study. Qual Life Res 2012;21:1519-25. https://doi.org/10.1007/s11136-011-0077-3.
- Nagle CM, Francis JE, Nelson AE, Zorbas H, Luxford K, de Fazio A, et al. Reducing time to diagnosis does not improve outcomes for women with symptomatic ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2011;29:2253-8. https://doi.org/10.1200/JCO.2010.32.2164.
- Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer 1985;56:2727-32. https://doi.org/10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8.
- Lurie G, Wilkens LR, Thompson PJ, Matsuno RK, Carney ME, Goodman MT. Symptom presentation in invasive ovarian carcinoma by tumor histological type and grade in a multiethnic population: a case analysis. Gynecol Oncol 2010;119:278-84. https://doi.org/10.1016/j.ygyno.2010.05.028.
- Raptis DA, Fessas C, Belasyse-Smith P, Kurzawinski TR. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon 2010;8:239-46. https://doi.org/10.1016/j.surge.2010.03.001.
- McLean SR, Karsanji D, Wilson J, Dixon E, Sutherland FR, Pasieka J, et al. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J Surg Oncol 2013;107:853-8. https://doi.org/10.1002/jso.23338.
- Sundi D, Svatek RS, Margulis V, Wood CG, Matin SF, Dinney CP, et al. Upper tract urothelial carcinoma: impact of time to surgery. Urol Oncol 2012;30:266-72. https://doi.org/10.1016/j.urolonc.2010.04.002.
- Waldert M, Karakiewicz PI, Raman JD, Remzi M, Isbarn H, Lotan Y, et al. A delay in radical nephroureterectomy can lead to upstaging. BJU Int 2010;105:812-17. https://doi.org/10.1111/j.1464-410X.2009.08821.x.
- O’Brien D, Loeb S, Carvalhal GF, McGuire BB, Kan D, Hofer MD, et al. Delay of surgery in men with low risk prostate cancer. J Urol 2011;185:2143-7. https://doi.org/10.1016/j.juro.2011.02.009.
- Korets R, Seager CM, Pitman MS, Hruby GW, Benson MC, McKiernan JM. Effect of delaying surgery on radical prostatectomy outcomes: a contemporary analysis. BJU Int 2012;110:211-16. https://doi.org/10.1111/j.1464-410X.2011.10666.x.
- Sun M, Abdollah F, Hansen J, Trinh QD, Bianchi M, Tian Z, et al. Is a treatment delay in radical prostatectomy safe in individuals with low-risk prostate cancer?. J Sex Med 2012;9:2961-9. https://doi.org/10.1111/j.1743-6109.2012.02806.x.
- Hanson PR, Belitsky P, Millard OH, Lannon SG. Prognostic factors in metastatic nonseminomatous germ cell tumours. Can J Surg 1993;36:537-40.
- Fossa SD, Klepp O, Elgjo RF, Eliassen G, Melsom H, Urnes T, et al. The effect of patient’s delay and doctor’s delay in patients with malignant germ cell tumours. Int J Androl 1981;4:134-44. https://doi.org/10.1111/j.1365-2605.1981.tb00664.x.
- Huyghe E, Muller A, Mieusset R, Bujan L, Bachaud JM, Chevreau C, et al. Impact of diagnostic delay in testis cancer: results of a large population-based study. Eur Urol 2007;52:1710-16. https://doi.org/10.1016/j.eururo.2007.06.003.
- Moul JW, Paulson DF, Dodge RK, Walther PJ. Delay in diagnosis and survival in testicular cancer: impact of effective therapy and changes during 18 years. J Urol 1990;143:520-3. https://doi.org/10.1016/S0022-5347(17)40007-3.
- Harding M, Paul J, Kaye SB. Does delayed diagnosis or scrotal incision affect outcome for men with non-seminomatous germ cell tumours?. Br J Urol 1995;76:491-4. https://doi.org/10.1111/j.1464-410x.1995.tb07754.x.
- Goh J, Bayati M, Zenios SA, Singh S, Moore D. Data uncertainty in Markov chains: application to cost-effectiveness analyses of medical innovations. Oper Res 2018;66:697-715. https://doi.org/10.1287/opre.2017.1685.
- Prout GR, Griffin PP. Testicular tumors: delay in diagnosis and influence on survival. Am Fam Physician 1984;29:205-9.
- Dieckmann KP, Becker T, Bauer HW. Testicular tumors: presentation and role of diagnostic delay. Urol Int 1987;42:241-7. https://doi.org/10.1159/000281948.
- Meffan PJ, Delahunt B, Nacey JN. The value of early diagnosis in the treatment of patients with testicular cancer. N Z Med J 1991;104:393-4.
- Bosl GJ, Vogelzang NJ, Goldman A, Fraley EE, Lange PH, Levitt SH, et al. Impact of delay in diagnosis on clinical stage of testicular cancer. Lancet 1981;2:970-3. https://doi.org/10.1016/S0140-6736(81)91165-X.
- Ware SM, Al-Askari S, Morales P. Testicular germ cell tumors. Prognostic factors. Urology 1980;15:348-52. https://doi.org/10.1016/0090-4295(80)90467-7.
- Wishnow KI, Johnson DE, Preston WL, Tenney DM, Brown BW. Prompt orchiectomy reduces morbidity and mortality from testicular carcinoma. Br J Urol 1990;65:629-33. https://doi.org/10.1111/j.1464-410x.1990.tb14834.x.
- Chilvers CE, Saunders M, Bliss JM, Nicholls J, Horwich A. Influence of delay in diagnosis on prognosis in testicular teratoma. Br J Cancer 1989;59:126-8. https://doi.org/10.1038/bjc.1989.25.
- Akdaş A, Kirkali Z, Remzi D. The role of delay in stage III testicular tumours. Int Urol Nephrol 1986;18:181-4. https://doi.org/10.1007/bf02082606.
- Scher H, Bosl G, Geller N, Cirrincione C, Whitmore W, Golbey R. Impact of symptomatic interval on prognosis of patients with stage III testicular cancer. Urology 1983;21:559-61. https://doi.org/10.1016/0090-4295(83)90190-5.
- Napier MP, Rustin GJ. Diagnostic delay and risk of relapse in patients with stage I nonseminomatous germ cell tumour followed on active surveillance. BJU Int 2000;86:486-9. https://doi.org/10.1046/j.1464-410X.2000.00779.x.
- Metzger S, Ellwanger U, Stroebel W, Schiebel U, Rassner G, Fierlbeck G. Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res 1998;8:181-6. https://doi.org/10.1097/00008390-199804000-00014.
- Temoshok L, DiClemente RJ, Sweet DM, Blois MS, Sagebiel RW. Factors related to patient delay in seeking medical attention for cutaneous malignant melanoma. Cancer 1984;54:3048-53. https://doi.org/10.1002/1097-0142(19841215)54:12<3048::aid-cncr2820541239>3.0.co;2-m.
- Montella M, Crispo A, Grimaldi M, De Marco MR, Ascierto PA, Parasole R, et al. An assessment of factors related to tumor thickness and delay in diagnosis of melanoma in southern Italy. Prev Med 2002;35:271-7. https://doi.org/10.1006/pmed.2002.1067.
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res 2002;12:389-94. https://doi.org/10.1097/00008390-200208000-00012.
- Carli P, De Giorgi V, Palli D, Maurichi A, Mulas P, Orlandi C, et al. Dermatologist detection and skin self-examination are associated with thinner melanomas: results from a survey of the Italian Multidisciplinary Group on Melanoma. Arch Dermatol 2003;139:607-12. https://doi.org/10.1001/archderm.139.5.607.
- Baade PD, English DR, Youl PH, McPherson M, Elwood JM, Aitken JF. The relationship between melanoma thickness and time to diagnosis in a large population-based study. Arch Dermatol 2006;142:1422-7. https://doi.org/10.1001/archderm.142.11.1422.
- Helsing MD. Trofosfamide as a salvage treatment with low toxicity in malignant lymphoma. A phase II study. Eur J Cancer 1997;33:500-2. https://doi.org/10.1016/S0959-8049(97)89029-6.
- Richard MA, Grob JJ, Avril MF, Delaunay M, Thirion X, Wolkenstein P, et al. Melanoma and tumor thickness: challenges of early diagnosis. Arch Dermatol 1999;135:269-74. https://doi.org/10.1001/archderm.135.3.269.
- Krige JE, Isaacs S, Hudson DA, King HS, Strover RM, Johnson CA. Delay in the diagnosis of cutaneous malignant melanoma. A prospective study in 250 patients. Cancer 1991;68:2064-8. https://doi.org/10.1002/1097-0142(19911101)68:9<2064::aid-cncr2820680937>3.0.co;2-3.
- Cassileth BR, Clark WH, Heiberger RM, March V, Tenaglia A. Relationship between patients’ early recognition of melanoma and depth of invasion. Cancer 1982;49:198-200. https://doi.org/10.1002/1097-0142(19820101)49:1<198::aid-cncr2820490138>3.0.co;2-9.
- Alam M, Goldberg LH, Silapunt S, Gardner ES, Strom SS, Rademaker AW, et al. Delayed treatment and continued growth of nonmelanoma skin cancer. J Am Acad Dermatol 2011;64:839-48. https://doi.org/10.1016/j.jaad.2010.06.028.
- Renzi C, Mastroeni S, Passarelli F, Mannooranparampil TJ, Caggiati A, Potenza C, et al. Factors associated with large cutaneous squamous cell carcinomas. J Am Acad Dermatol 2010;63:404-11. https://doi.org/10.1016/j.jaad.2009.09.044.
- Lim BS, Dennis CR, Gardner B, Newman J. Analysis of survival versus patient and doctor delay of treatment in gastrointestinal cancer. Am J Surg 1974;127:210-14. https://doi.org/10.1016/0002-9610(74)90159-7.
- Ziliotto A, Kunzle JE, De Souza A, Colicchio-Filho O. Evolutive and prognostic aspects in gastric cancer. Analysis of 189 cases. Cancer 1987;59:811-17. https://doi.org/10.1002/1097-0142(19870215)59:4<811::aid-cncr2820590426>3.0.co;2-s.
- Arvanitakis C, Nikopoulos A, Giannoulis E, Theoharidis A, Georgilas V, Fotiou H, et al. The impact of early or late diagnosis on patient survival in gastric cancer in Greece. Hepatogastroenterology 1992;39:355-7.
- Martin IG, Young S, Sue-Ling H, Johnston D. Delays in the diagnosis of oesophagogastric cancer: a consecutive case series. BMJ 1997;314:467-70. https://doi.org/10.1136/bmj.314.7079.467.
- Windham TC, Termuhlen PM, Ajani JA, Mansfield PF. Adenocarcinoma of the stomach in patients age 35 years and younger: no impact of early diagnosis on survival outcome. J Surg Oncol 2002;81:118-24. https://doi.org/10.1002/jso.10157.
- Fernandez E, Porta M, Malats N, Belloc J, Gallén M. Symptom-to-diagnosis interval and survival in cancers of the digestive tract. Dig Dis Sci 2002;47:2434-40. https://doi.org/10.1023/a:1020535304670.
- Maconi G, Kurihara H, Panizzo V, Russo A, Cristaldi M, Marrelli D, et al. Gastric cancer in young patients with no alarm symptoms: focus on delay in diagnosis, stage of neoplasm and survival. Scand J Gastroenterol 2003;38:1249-55. https://doi.org/10.1080/00365520310006360.
- Haugstvedt TK, Viste A, Eide GE, Søreide O. Patient and physician treatment delay in patients with stomach cancer in Norway: is it important? The Norwegian Stomach Cancer Trial. Scand J Gastroenterol 1991;26:611-19. https://doi.org/10.3109/00365529109043635.
- Wang J, Liu F, Gao H, Wei W, Zhang X, Liang Y, et al. The symptom-to-treatment delay and stage at the time of treatment in cancer of esophagus. Jpn J Clin Oncol 2008;38:87-91. https://doi.org/10.1093/jjco/hym169.
- Sharpe D, Williams RN, Ubhi SS, Sutton CD, Bowrey DJ. The ‘two-week wait’ referral pathway allows prompt treatment but does not improve outcome for patients with oesophago-gastric cancer. Eur J Surg Oncol 2010;36:977-81. https://doi.org/10.1016/j.ejso.2010.07.002.
- Grotenhuis BA, van Hagen P, Wijnhoven BP, Spaander MC, Tilanus HW, van Lanschot JJ. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg 2010;14:476-83. https://doi.org/10.1007/s11605-009-1109-y.
- Saithna A, Pynsent PB, Grimer RJ. Retrospective analysis of the impact of symptom duration on prognosis in soft tissue sarcoma. Int Orthop 2008;32:381-4. https://doi.org/10.1007/s00264-007-0319-8.
- Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. The symptom-to-diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J Surg Oncol 2011;104:771-5. https://doi.org/10.1002/jso.22006.
- Rougraff BT, Davis K, Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival?. Clin Orthop Relat Res 2007;462:181-9. https://doi.org/10.1097/BLO.0b013e3180f62608.
- Wurtz LD, Peabody TD, Simon MA. Delay in the diagnosis and treatment of primary bone sarcoma of the pelvis. J Bone Joint Surg Am 1999;81:317-25. https://doi.org/10.2106/00004623-199903000-00003.
- Ruka W, Emrich LJ, Driscoll DL, Karakousis CP. Tumor size/symptom duration ratio as a prognostic factor in patients with high-grade soft tissue sarcomas. Eur J Cancer Clin Oncol 1988;24:1583-8. https://doi.org/10.1016/0277-5379(88)90049-1.
- Bacci G, Ferrari S, Longhi A, Forni C, Zavatta M, Versari M, et al. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. J Pediatr Hematol Oncol 2002;24:27-30. https://doi.org/10.1097/00043426-200201000-00008.
- Singal AG, Waljee AK, Patel N, Chen EY, Tiro JA, Marrero JA, et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw 2013;11:1101-8. https://doi.org/10.6004/jnccn.2013.0131.
- Holmäng S, Johansson SL. Impact of diagnostic and treatment delay on survival in patients with renal pelvic and ureteral cancer. Scand J Urol Nephrol 2006;40:479-84. https://doi.org/10.1080/00365590600864093.
- Toth-Fejel S, Pommier RF. Relationships among delay of diagnosis, extent of disease, and survival in patients with abdominal carcinoid tumors. Am J Surg 2004;187:575-9. https://doi.org/10.1016/j.amjsurg.2004.01.019.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. https://doi.org/10.1245/s10434-010-0985-4.
- Zinkin LD. A critical review of the classifications and staging of colorectal cancer. Dis Colon Rectum 1983;26:37-43. https://doi.org/10.1007/bf02554677.
- Bolin TD, Korman MG, Stanton R, Talley NJ, Newstead GL, Donnelly N, et al. Positive cost effectiveness of early diagnosis of colorectal cancer. Colorectal Dis 1999;1:113-22. https://doi.org/10.1046/j.1463-1318.1999.00028.x.
- Byers T, Gorsky R. Estimates of costs and effects of screening for colorectal cancer in the United States. Cancer 1992;70:1288-95. https://doi.org/10.1002/1097-0142(19920901)70:3+<1288::aid-cncr2820701515>3.0.co;2-1.
- Neilson AR, Whynes DK. Cost-effectiveness of screening for colorectal cancer: a simulation model. IMA J Math Appl Med Biol 1995;12:355-67. https://doi.org/10.1093/imammb/12.3-4.355.
- Gow J. Costs of screening for colorectal cancer: an Australian programme. Health Econ 1999;8:531-40. https://doi.org/10.1002/(SICI)1099-1050(199909)8:6<531::AID-HEC441>3.0.CO;2-K.
- Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care 2000;16:799-810. https://doi.org/10.1017/s0266462300102077.
- Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000;133:573-84. https://doi.org/10.7326/0003-4819-133-8-200010170-00007.
- Reyes CM, Allen BA, Terdiman JP, Wilson LS. Comparison of selection strategies for genetic testing of patients with hereditary nonpolyposis colorectal carcinoma: effectiveness and cost-effectiveness. Cancer 2002;95:1848-56. https://doi.org/10.1002/cncr.10910.
- Kievit W, de Bruin JH, Adang EM, Severens JL, Kleibeuker JH, Sijmons RH, et al. Cost effectiveness of a new strategy to identify HNPCC patients. Gut 2005;54:97-102. https://doi.org/10.1136/gut.2004.039123.
- Telford JJ, Levy AR, Sambrook JC, Zou D, Enns RA. The cost-effectiveness of screening for colorectal cancer. CMAJ 2010;182:1307-13. https://doi.org/10.1503/cmaj.090845.
- Allameh Z, Davari M, Emami MH. Cost-effectiveness analysis of colorectal cancer screening methods in Iran. Arch Iran Med 2011;14:110-14. https://doi.org/011142/AIM.008.
- Dan YY, Chuah BY, Koh DC, Yeoh KG. Screening based on risk for colorectal cancer is the most cost-effective approach. Clin Gastroenterol Hepatol 2012;10:266-71. https://doi.org/10.1016/j.cgh.2011.11.011.
- Atkin W, Brenner A, Martin J, Wooldrage K, Shah U, Lucas F, et al. The clinical effectiveness of different surveillance strategies to prevent colorectal cancer in people with intermediate-grade colorectal adenomas: a retrospective cohort analysis, and psychological and economic evaluations. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21250.
- Office for National Statistics . Cancer Diagnoses and Age-Standardised Incidence Rates for All Types of Cancer by Age, Sex and Region Including Breast, Prostate, Lung and Colorectal Cancer n.d. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2017 (accessed 1 March 2019).
- NHS Improvement . Archives Reference Costs 2020. https://improvement.nhs.uk/resources/reference-costs/ (accessed December 2020).
- GOV.UK . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed December 2020).
- Gavin DR, Valori RM, Anderson JT, Donnelly MT, Williams JG, Swarbrick ET. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013;62:242-9. https://doi.org/10.1136/gutjnl-2011-301848.
- Ara R, Brazier J. Estimating health state utility values for comorbid health conditions using SF-6D data. Value Health 2011;14:740-5. https://doi.org/10.1016/j.jval.2010.12.011.
- National Institute for Health and Care Excellence (NICE) . Colorectal Cancer 2020.
- Whyte S, Chilcott J, Essat M, Stevens J, Wong R, Kalita N. Re-Appraisal of the Options for Colorectal Cancer Screening. Report for the NHS Bowel Cancer Screening Programme 2011.
Appendix 1 Literature search strategies
Database
MEDLINE
Host: Ovid.
Date range searched: 1946 to May week 1 2017.
Date searched: 11 May 2017.
Searcher: SR.
Hits: 2008.
Search strategy
-
exp Neoplasms/
-
(cancer$ or neopla$).tw.
-
(tumour$ or tumor$).tw.
-
or/1-3
-
Primary Health Care/
-
exp General Practice/
-
General Practitioners/
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
or/5-9
-
Decision Support Systems, Clinical/
-
Decision Support Techniques/
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or ation$).tw.
-
or/11-13
-
“Early Detection of Cancer”/
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
or/15-17
-
4 and 10 and 14 and 18.
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid
Date range searched: 10 May 2017.
Date searched: 11 May 2017.
Searcher: SR.
Hits: 270.
Search strategy
-
(cancer$ or neopla$).tw.
-
(tumour$ or tumor$).tw.
-
or/1-2
-
primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
or/4-5
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw.
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
or/8-9
-
3 and 6 and 7 and 10.
EMBASE
Host: Ovid.
Date range searched: 1974 to 10 May 2017.
Date searched: 11 May 2017.
Searcher: SR.
Hits: 3867.
Search strategy
-
exp Neoplasms/
-
(cancer$ or neopla$).tw.
-
(tumour$ or tumor$).tw.
-
or/1-3
-
Primary Health Care/
-
exp General Practice/
-
General Practitioners/
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
or/5-9
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw.
-
exp decision support system/
-
11 or 12
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagnos$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
Early Cancer Diagnosis/
-
or/14-16
-
4 and 10 and 13 and 17.
Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials
Host: Cochrane Library.
Date ranges searched: Cochrane Database of Systematic Reviews (CDSR) – issue 5 of 12, May 2017; Cochrane Central Register of Controlled Trials – issue 4 of 12, April 2017.
Date searched: 11 May 2017.
Searcher: SR.
Hits: 391.
Search strategy
-
MeSH descriptor: [Neoplasms] explode all trees
-
(cancer* or neopla*):ti,ab,kw
-
(tumour* or tumor*):ti,ab,kw
-
#1 or #2 or #3
-
MeSH descriptor: [Primary Health Care] explode all trees
-
MeSH descriptor: [General Practice] explode all trees
-
MeSH descriptor: [General Practitioners] explode all trees
-
(“primary care” or “general practi*” or “family practi*”):ti,ab,kw
-
(primary near/3 (healthcare or “health care”)):ti,ab,kw
-
#5 or #6 or #7 or #8 or #9
-
MeSH descriptor: [Decision Support Techniques] this term only
-
MeSH descriptor: [Decision Support Systems, Clinical] this term only
-
(tool or tools or aid* or model or models or checklist* or “check list” * or rule or rules or algorithm* or equation*):ti,ab,kw
-
#11 or #12 or #13
-
MeSH descriptor: [Early Detection of Cancer] this term only
-
(predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagnos* or prognos*):ti,ab,kw
-
(2ww or “2 week wait” or “two week wait” or “2 week rule” or “two week rule”):ti,ab,kw
-
#15 or #16 or #17
-
#4 and #10 and #14 and #18.
Science Citation Index and Conference Proceedings Citation Index – Science
Host: Web of Science.
Date range searched: not applicable.
Date searched: 11 May 2017.
Searcher: SR.
Hits: 2816.
Search strategy
-
TS=((cancer* or neopla*))
-
TS=((tumour* or tumor*))
-
#1 or #2
-
TS=((“primary care” or “general practi*” or “family practi*”))
-
TS=((primary near/2 healthcare))
-
TS=((primary near/2 “health care”))
-
#3 or #4 or #5
-
TS= ((tool or tools or aid* or model or models or checklist* or “check list*” or rule or rules or algorithm* or equation*))
-
TS=((predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagnos* or prognos*))
-
TS=((2ww or “2 week wait” or “two week wait” or “2 week rule” or “two week rule”))
-
#9 or #10
-
#3 and #7 and #8 and #11
-
Indexes=SCI-EXPANDED, CPCI-S Timespan=2004-2011.
DocType=All document types; Language=All languages.
Search results
The following table shows the number of hits per database and in total.
| Database | Hits (n) |
|---|---|
| MEDLINE | 2008 |
| MEDLINE In-Process & Other Non-Indexed Citations | 270 |
| EMBASE | 3867 |
| Cochrane Library (CDSR and CENTRAL) | 391 |
| Web of Science (SCI and CPCI-S) | 2816 |
| Total records | 9352 |
| Duplicates | 3743 |
| Total unique records | 5609 |
The updated searches were carried out on 30 January 2018. The next table shows the number of hits per database and in total for the updated searches.
| Database | Hits (n) |
|---|---|
| MEDLINE | 168 |
| MEDLINE In-Process & Other Non-Indexed Citations | 1 |
| EMBASE | 336 |
| Cochrane Library (CDSR and CENTRAL) | 29 |
| Web of Science (SCI and CPCI-S) | 181 |
| Total records | 715 |
| Duplicates | 84 |
| Total unique records | 631 |
Appendix 2 Systematic review 1: supplementary tables
| Study | Prediction tool | Cancer type(s) | Country | Study design | Intended purpose |
|---|---|---|---|---|---|
| Del Mar 199537 | Melanoma ‘algorithm’ (plus camera) | Melanoma | Australia | Field trial | To assess the algorithm’s ability to reduce the number of benign lesions being excised without reducing the excision of invasive lesions by comparing the numbers of excised lesions with algorithm use with the number excised without algorithm use |
| English 200336 | Melanoma ‘algorithm’ (plus camera) | Melanoma | Australia | RCT | To determine whether or not an aid to the diagnosis of pigmented skin lesions reduces the ratio of benign lesions to melanomas excised after introduction in general practice |
| Gulati 201538 | GP Skin Cancer Toolkit | Skin cancer | UK | Case–control | To assess the impact of the toolkit by comparing before and after national skin cancer referral data, cross-sectional questionnaires and urgent skin cancer referral data to two NHS trusts |
| Hamilton 201313 | RAT for lung cancer and CRC in two formats: mouse mat and desktop flip chart | Lung cancer, CRC | UK | Cohort study | To compare referrals and investigations for colorectal and lung cancer before and after the implementation of a RAT |
| Emery 201735 | Composite intervention including the RAT for colorectal, lung and prostate cancers, as well as summaries of relevant guidelines for colorectal, lung, prostate and breast cancers | CRC, lung cancer, prostate cancer, breast cancer | Australia | Factorial cluster RCT | To measure the effect of community-based symptom awareness and general practice-based educational interventions on the time to diagnosis in rural patients presenting with breast, prostate, colorectal or lung cancer in Western Australia |
| Study | Population | Recruitment (plus inclusion and exclusion criteria) | Sample size | Tool description | Predictors |
|---|---|---|---|---|---|
| Del Mar 199537 | Medical practitioners, mostly in general practice, in each of two cities in tropical Queensland, Australia Examination of the histopathological reports of 5823 melanocytic skin lesions excised over the intervention period (2 years) and in the preceding 6 months | Two provincial cities in central Queensland were selected for the trial on the basis of their similarity; one city was randomly selected to implement the use of algorithm, the other city was used as a control | 53 medical practitioners (45 GPs, seven surgeons and one dermatologist) in the control community; 52 medical practitioners (48 GPs and four surgeons) in the intervention community | An algorithm for managing clinically suspicious naevi, aided by the use of a camera | Change in lesion characteristics: size, outline, colour, elevation, surface change, daughter lesions, tingling or itching; aged > 40 years |
| English 200336 | Data on examined and/or excised skin lesions suspected of being malignant, reported by general practices in Perth, WA, Australia | General practices from Perth, WA, Australia, were randomised to either control (national guidelines on managing melanoma) or intervention (diagnosis tool) |
|
An algorithm for managing clinically suspicious naevi, aided by the use of a camera | Change in lesion characteristics: size, outline, colour, elevation, surface change, daughter lesions, tingling or itching; and aged > 40 years |
| Gulati 201538 | 2WW referral data from 2456 practices were obtained for 4 months during the launch of the toolkit (July–October 2012) and compared with referral habits in the same months in the previous year (July–October 2011). Referral habits during these two time periods were also measured in 3693 practices where the GP Skin Cancer Toolkit was not used. Appropriateness of the 2WW referrals based on data from Homerton University Hospital and Royal London Hospital between July and October 2011 and between July and October 2012 | Data on participating practices were based on website usage data (www.doctors.net.uk). Cancer Waiting Times data were obtained through PHE’s National Cancer Registration Service (East Midlands) and taken from the National Cancer Waiting Times Monitoring Dataset, provided by NHS England. A total of 276 GPs referring to Barts Health NHS Trust and Homerton University Hospital NHS Foundation Trust were analysed for appropriateness according to NICE referral guidelines | Impact on referral statistics: 2456 practices that used the toolkit as cases; 3693 practices as controls. Appropriateness of urgent referral: 2011 – 365 referrals from 214 GPs; 2012 – 387 referrals from 183 GPs (114 GPs had access to toolkit) | The toolkit consisted of a referral decision aid (referral guidelines based on red flags), lesion recognition resource (a series of images), clinical cases and a quiz | NICE referral guidelines (weighted seven-point checklist) |
| Hamilton 201313 | Number of investigations (X-rays or colonoscopies) and 2WW referrals from practices and local NHS trusts for the two 6-month periods before and after the distribution of the tools | A selected GP cancer lead from seven of the 28 English cancer networks recruited local general practices to which the RATs were supplied. A total of 614 GPs from 165 practices were recruited; 2593 assessments were included | 614 GPs from 165 practices | RAT gives risk estimates for patients aged > 40 years presenting to primary care with symptoms of possible cancer, for single symptoms, pairs of symptoms and repeat attendances with the same symptom. The values are colour coded to aid interpretation | For CRC: symptoms (constipation, diarrhoea, rectal bleeding, abdominal pain, abdominal tenderness, abnormal rectal examination), loss of weight, haemoglobin (levels 10–13 g/dl; < 10 g/dl); NR for other cancers |
| Emery 201735 | Two trial areas in Western Australia | Inclusion criteria: adults aged > 18 years; diagnosed with breast, lung, colorectal or prostate cancer between 1 January 2012 and the recruitment end date of 31 March 2014; and resident of trial areas at the time of cancer diagnosis | 1358 patients (497 in trial area A, 861 in trial area B) | Resource card containing the RAT tables for colorectal, lung and prostate cancers, as well as the National Breast and Ovarian Cancer Centre guidelines for investigating new breast symptoms | NR |
| Study | Outcomes (definitions) | Outcomes | Main results |
|---|---|---|---|
| Del Mar 199537 | Percentages of benign (neither malignant nor potentially malignant) melanocytic lesions excised during the 2-year intervention period | Number of excisions performed in each city was measured at baseline (6 months before implementation of the intervention) and 2 years after; percentage of excised lesions that are neither invasive nor potentially malignant; and median number of excisions per doctor | Total number of excised lesions:
|
Percentage of excised lesions that are neither invasive nor potentially malignant:
|
|||
The median number of excisions per doctor (2.5% and 97.5% percentiles)
|
|||
| English 200336 | Malignant lesion – an in situ or invasive melanoma benign lesion – naevus (including dysplastic naevus) or a seborrhoeic keratosis | Ratio of benign pigmented lesions to melanomas excised | Number of excised skin lesions (including seborrheic keratoses):
|
| Gulati 201538 | Number of urgent (2WW) cancer referrals; proportion of appropriate urgent referrals (based on the NICE guidelines); melanoma and non-melanoma skin cancer rates | Number of urgent referrals, diagnoses of melanoma skin cancer; appropriateness of urgent referrals | 21,000 GPs were invited to use the tool; 8163 GPs accessed the tool during the 2012 period. There were no significant changes in the number of urgent GP referrals for suspected skin cancer (Spearman’s rank 0.20; p < 0.001), diagnoses of melanoma (Spearman’s rank 0.064; p < 0.001) or diagnoses of non-melanoma skin cancer (Spearman’s rank 0.068; p < 0.001) between the toolkit user and non-user groups. The proportion of appropriate referrals increased from 21.37% in 2011 to 32.3% in 2012, giving an incidence rate ratio of 3.13 (95% CI 2.21 to 4.42, z-statistic 6.46; p < 0.0001); the differences in numbers of appropriate referrals between toolkit users and non-users did not reach statistical significance by Spearman’s rank test and ANOVA |
| Hamilton 201313 | Investigation for cancer: 2-week referral to the appropriate specialty, or a chest X-ray in possible lung cancer | Number of investigations and two-week referrals | Lung cancer: 31% increase in 2-week referrals (332 before, 436 after); 4% increase in related investigations (chest X-ray: 7431 before, 7723 after) |
| Colorectal cancer: 26% increase in 2-week referrals (1173 before, 1477 after); 15% increase in colonoscopies (1762 before, 2032 after) | |||
| Emery 201735 | TDI, as the time from first symptom to cancer diagnosis | TDI, as the time from first symptom to cancer diagnosis | No significant differences in the median or ln mean TDI at either intervention level:
|
| Study | Risk of bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Baseline outcome measurements similar | Baseline characteristics similar | Incomplete outcome data | Knowledge of the allocated interventions adequately prevented during the study | Protection against contamination | Selective outcome reporting | Other risks of bias | |
| Del Mar 199537 | High risk (selection of intervention site was arbitrary) | Low risk (sites chosen sufficiently far apart that an intervention in one site was unlikely to affect clinical behaviour in the other) | Low risk | Low risk | High risk (a large proportion of GPs did not participate post randomisation) | High risk (use of the intervention was not blinded) | Low risk (‘data analysis was performed before the code identifying the city was broken’) | Low risk | Unclear risk |
| English 200336 | Low risk (intervention practices were randomly selected) | Low risk (randomisation codes in sealed envelopes) | Low risk | Low risk | Unclear risk | High risk (‘after randomisation, participants and research assistants who visited practices were not blinded to assignment’) | Low risk (‘all coding of outcome data was done blind to assignment’) | Low risk | Unclear risk |
| Gulati 201538 | High risk (voluntary selection of participants) | High risk (use of the intervention was not blinded) | Low risk | Low risk | Unclear risk | Unclear risk (based on reported use of the toolkit) | Unclear risk | Unclear risk | High risk (only 39% of GPs who had access to the toolkit used it) |
| Hamilton 201313 | High risk (not a RCT) | High risk (allocation not concealed) | N/A (cohort study) | N/A (cohort study) | Unclear risk (details not reported) | High risk (the GPs were not blinded, irrespective of whether or not they were counted) | Unclear risk (the intervention was not blinded) | Unclear risk | High risk (other important influencing factors could have occurred during the before and after. Unclear how many GPs involved) |
| Emery 201735 | Low risk (allocation of interventions was random) | High risk (allocation not concealed) | Low risk | Low risk | Low risk | Low risk (research staff who collected outcome data and the trial statistician were blinded to group allocation) | Low risk (measures were taken to avoid contamination: re-clustering of practices attended by same practitioners, use of media avoided in the control areas) | Low risk | Low risk |
Appendix 3 Systematic review 2: supplementary tables
| Study | Prediction model | Cancer type(s) | Country | Setting | Study design | Stage of development | Data source |
|---|---|---|---|---|---|---|---|
| Bladder | |||||||
| Shephard 201269 | RAT | Bladder | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Blood | |||||||
| Shephard 201667 | RAT | Leukaemia | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Shephard 201568 | RAT | Multiple myeloma | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Brain | |||||||
| Hamilton 200787 | RAT | Brain | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Breast | |||||||
| McCowan 201160 | Clinical prediction rule for breast cancer | Breast | UK | Developed in secondary care, for use in primary care | Prospective cohort | External validation | Derivation cohort: consecutive patients attending the symptomatic breast clinic at Ninewells Hospital, Dundee (Scotland), who were referred by a GP between February and June 2007 |
| Validation cohort: all women who attended for an initial consultation regarding symptomatic breast problems in 11 participating general practices in the region between January 2006 and June 2007 | |||||||
| Colorectal | |||||||
| Marshall 201122 | BB equation | Colorectal | UK | Primary care | Case–control | External validation |
Derivation cohort: THIN Validation cohort: CAPER |
| Elias 201750 | BB equation | Colorectal | The Netherlands | Primary care | Prospective cross-sectional | External validation | CEDAR study: patients referred to endoscopy centres by participating Dutch primary care practices. 2009–12 |
| Fijten 199555 | Referred to as the ‘Netherlands model’ in Hodder 200577 and Elias 201750 | Colorectal | The Netherlands | Primary care | Prospective cohort | Apparent performance | 290 consecutive patients with rectal bleeding presenting to 83 GPs in Limburg (the Netherlands) from September 1988 to April 1990. Predictors: questionnaires completed by GPs and patients, and laboratory test results |
| Hodder 200577 | Netherlands model (Fijten 199555) | Colorectal | UK | Evaluated in secondary care, model developed in primary care (Fijten 199555) | Prospective cohort | External validation | Patients referred from primary care with colorectal symptoms over a 3-year period to the Leighton Hospital, Crewe, UK |
| Elias 201750 | ‘Netherlands model’ (Fijten 199555) | Colorectal | The Netherlands | Primary care | Prospective cross-sectional | External validation | CEDAR study: patients referred to endoscopy centres by participating Dutch primary care practices. 2009–12 |
| Kop 201561 | No name (machine learning) | Colorectal | The Netherlands | Primary care | Retrospective case–control | Apparent performance | Anonymised electronic records from two general practice database systems in the Utrecht region, the Netherlands, between 1 July 2006 and 31 December 2011 |
| Nørrelund 199657 | Danish (Nørrelund) model | Colorectal | Denmark | Primary care | Prospective cohort | Apparent performance II | Patients presenting to GPs with first episode of rectal bleeding
|
| Elias 201750 | Danish (Nørrelund) model | Colorectal | The Netherlands | Primary care | Prospective cross-sectional | External validation | CEDAR study: patients referred to endoscopy centres by participating Dutch primary care practices. 2009–12 |
| Hippisley-Cox 201280 | QCancer | Colorectal | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Collins 201215 | QCancer | Colorectal | UK | Primary care | Retrospective cohort | External validation | THIN database |
| Hamilton 200581 | RAT | Colorectal | UK | Primary care | Case–control | Apparent performance | Patients attending all 21 general practices in Exeter, UK. Cases identified from the cancer registry at the Royal Devon and Exeter Hospital |
| Elias 201750 | RAT | Colorectal | The Netherlands | Primary care | Prospective cross-sectional | External validation | CEDAR study: patients referred to endoscopy centres by participating Dutch primary care practices. 2009–12 |
| Hamilton 200974 | RAT | Colorectal | UK | Primary care | Case–control | Model updating | THIN database |
| Stapley 201772 | RAT | Bowel | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Gastro-oesophageal | |||||||
| Hippisley-Cox 201182 | QCancer | Gastro-oesophageal | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Collins 201388 | QCancer | Gastro-oesophageal | UK | Primary care | Retrospective cohort | External validation | THIN database |
| Stapley 201371 | RAT | Gastro-oesophageal | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Lung | |||||||
| Iyen-Omofoman 201321 | No name | Lung | UK | Primary care | Case–control | Internal validation II | THIN database |
| Hippisley-Cox 201152 | QCancer | Lung | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Hamilton 200551 | RAT | Lung | UK | Primary care | Case–control | Apparent performance | Patients attending all 21 general practices in Exeter, UK. Cases identified from the cancer registry at the Royal Devon and Exeter Hospital |
| Ovarian | |||||||
| Hippisley-Cox 201184 | QCancer | Ovarian | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Collins 201389 | QCancer | Ovarian | UK | Primary care | Retrospective cohort | External validation | THIN database |
| Hamilton 200983 | RAT | Ovarian | UK | Primary care | Case–control | Apparent performance | Patients attending 39 general practices in Devon, UK |
| Pancreas | |||||||
| Hippisley-Cox 201232 | QCancer | Pancreas | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Collins 201314 | QCancer | Pancreas | UK | Primary care | Retrospective cohort | External validation | THIN database |
| Stapley 201270 | RAT | Pancreatic | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Keane 201462 | No name | PDAC and BTCs | UK | Primary care | Case–control | Apparent performance | THIN database |
| Prostate | |||||||
| Hamilton 200690 | RAT | Prostate | UK | Primary care | Case–control | Apparent performance | Patients attending all 21 general practices in Exeter, UK. Cases identified from the cancer registry at the Royal Devon and Exeter Hospital |
| Renal | |||||||
| Hippisley-Cox 201285 | QCancer | Renal | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Collins 201391 | QCancer | Renal | UK | Primary care | Retrospective cohort | External validation | THIN database |
| Shephard 201366 | RAT | Renal | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Uterine | |||||||
| Walker 201373 | RAT | Uterine | UK | Primary care | Case–control | Apparent performance | GPRD (now called the CPRD) |
| Metastatic | |||||||
| Hamilton 201586 | RAT | Metastatic (breast, colorectal, prostate) | UK | Primary care | Case–control | Apparent performance | Patients attending 11 general practices in Devon, UK |
| Multiple | |||||||
| Hippisley-Cox 201353 | QCancer | Females – multiple cancers (including breast, cervical, ovarian, uterine, blood, colorectal, gastro-oesophageal, lung, pancreatic, renal) | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Hippisley-Cox 201354 | QCancer | Males – multiple cancers (including lung, colorectal, gastro-oesophageal, pancreatic, renal tract, blood, prostate, testicular) | UK | Primary care | Open prospective cohort | Internal validation II | QResearch database |
| Dommett 201364 | No name | Multiple paediatric cancers | UK | Primary care | Prospective case–control | Apparent performance | CPRD |
| Muris 199556 | Netherlands (Muris 199556) model | Multiple | The Netherlands | Primary care | Prospective cohort | Apparent performance | Patients presenting to GPs for new abdominal complaints. 1989 |
| Elias 201750 | Netherlands (Muris 1995) model | Colorectal, yet Muris 1995 contains multiple cancers | The Netherlands | Primary care | Prospective cross-sectional | External validation | CEDAR study: patients referred to endoscopy centres by participating Dutch primary care practices. 2009–12 |
| Study | Participants | Candidate predictors | Outcome to be predicted | Sample size | Number/handling of missing data |
|---|---|---|---|---|---|
| Bladder | |||||
| Shephard 201269 | Inclusion criteria:
|
43 symptoms and 104 abnormal test results were considered initially. However, only six symptoms and seven abnormal test results occurred in at least 5% of cases | Bladder cancer within 1 year of presentation | 26,633 | NR |
| Exclusion criteria: metastatic cancer of the bladder from a non-bladder primary, diagnosis before 2000, or no consultations in the year before diagnosis | |||||
| Blood | |||||
| Shephard 201667 | Inclusion criteria:
|
50 symptoms were considered initially | Leukaemia diagnosis within 1 year of presentation | 4655 (2877 chronic, 937 acute) leukaemia cases and 22,852 controls (12,811 for chronic and 4214 for acute) | NR |
| Exclusion criteria: cases with reticuloendothelial cancer or thrombocytic leukaemia and their matched controls; < 1 year of records before the index date; cases without controls; controls with leukaemia; and controls who had not sought medical care after registration | |||||
| Shephard 201568 | Inclusion criteria:
|
62 symptoms and 22 abnormal test results were considered initially where they occurred in at least 2% of cases or controls | Myeloma within 1 year of presentation | 14,860 patients (2703 cases; 12,157 controls) | NR |
| Exclusion criteria: < 1 year of records before the index date; cases without controls; controls with myeloma; controls who had not sought medical care after registration | |||||
| Brain | |||||
| Hamilton 200787 | Inclusion criteria:
|
Libraries of codes for clinical variables previously described with brain tumours were assembled and occurrences of these variables in the 6 months before the index date in cases and controls were identified. Variables were retained only if they occurred in at least 1% of cases or controls | Diagnosis of brain cancer within 1 year of presentation |
|
NR |
Exclusion criteria:
|
|||||
| Breast | |||||
| McCowan 201160 | Derivation cohort:
|
Age, socioeconomic status (as assessed by the Carstairs Index), obesity classification, smoking history, menopausal status, regular periods, current pregnancy, contraception, hormone replacement therapy, hysterectomy, number of pregnancies, past breast problems history, signs after examination, lump characteristics (size, shape, mobility, texture), nipple discharge | Breast cancer diagnosis within 1 year of presentation |
|
NR |
Validation cohort:
|
|||||
| Colorectal | |||||
| Marshall 201122 | Inclusion criteria:
|
Constipation episode, laxative prescription, diarrhoea episode, antimotility prescription, change in bowel habit (diarrhoea), change in bowel habit (constipation), change in bowel habit (no diarrhoea/constipation), IBS diagnosis, antispasmodic prescription, rectal bleeding or melaena, faecal occult blood present, weight loss of > 10% in 2 years, weight loss of 5–10% in 2 years, unknown/no weight loss, abdominal pain/tenderness, abnormal rectal examination, anaemia with low levels of Hb, iron prescription, flatulence, diabetes, BMI of > 30 kg/m2, DVT/PE, abdominal mass, mean cell volume of < 80 fl | Diagnosis of CRC | Derivation: cases, 5477; controls, 38314 | NR |
| Exclusion criteria: cases with < 2 years of data before diagnosis | Discrimination based on two data sets: derivation (THIN) data set and CAPER data set (349 cases; 1744 controls) | ||||
| Elias 201750 | Inclusion criteria: lower abdominal complaints for at least 2 weeks with rectal bleeding, change in bowel habit, abdominal pain, fever, diarrhoea, weight loss, sudden onset in elderly, and/or physical examination suggestive of colorectal disease | N/A. Used BB equation (Marshall 201122) | CRC | 810, including 37 with CRC | Multiple imputation |
| Fijten 199555 | Inclusion criteria: overt rectal bleeding reason for GP visit, or history of recent rectal blood loss (within the previous 3 months) | Relevant items were identified from literature. Data collected by:
|
Diagnosis of CRC within at least 1 year of study entry, from medical records and GP | 269 patients, nine cancer diagnoses |
|
| Exclusion criteria: patients aged < 18 or > 75 years, pregnant, urgent admission to a hospital (e.g. for a massive bleeding or acute abdominal pain), follow-up data not available | |||||
| Hodder 200577 | All patients referred from primary care with colorectal symptoms from October 1999 to October 2002 | N/A. Used Fijten 199555 | Diagnosis of CRC | 3302 patients, 156 diagnosed with cancer | NR |
| Elias 201750 | Inclusion criteria: lower-abdominal complaints for at least 2 weeks with rectal bleeding, change in bowel habit, abdominal pain, fever, diarrhoea, weight loss, sudden onset in elderly, and/or physical examination suggestive of colorectal disease | N/A. Used Fijten 199555 | CRC | 810, including 37 with CRC | Multiple imputation |
| Kop 201561 | Patients aged ≥ 30 years
|
Age; sex; number of times each of 720 ICPC codes were recorded; number of times each of 86 medication (ATC) code was prescribed; number of times patient was referred to a specialist for each of the 94 referral codes; 33 attributes, each representing whether or not a patient is currently in a certain (medical) condition; number of times Hb was inside/outside threshold; number of times a patient’s cell volume value was inside/outside the desired range; number of times blood was found/was not found in a patient’s stool; whether or not patient’s medical history contains a certain temporal pattern | Diagnostic of CRC | 808 CRC diagnoses occurring in 219,447 patients | NR |
| Nørrelund 199657 | Inclusion criteria: aged ≥ 40 years, presenting with first episode of rectal bleeding within previous 6 months |
|
CRC |
|
NR |
| Study 1: 1989–91; study 2: 1991–2 | |||||
| Exclusion criteria: known inflammatory bowel disease, colonic polyps, polyposis coli, CRC, predisposition to haemorrhage (e.g. coagulation defect), melaena stool | |||||
| Conducted follow-up in 1994: 0 (study 1) and 2 (study 2) additional diagnoses of CRC observed | |||||
| Elias 201750 | Inclusion criteria: lower abdominal complaints for at least 2 weeks with rectal bleeding, change in bowel habit, abdominal pain, fever, diarrhoea, weight loss, sudden onset in elderly, and/or physical examination suggestive of colorectal disease | N/A. Used Danish (i.e. Nørrelund 199657) model | CRC | 810, including 37 with CRC | Multiple imputation |
| Hippisley-Cox 201280 | Inclusion criteria: patients aged 30–84 years registered between 1 January 2000 and 30 September 2010 |
|
Diagnosis of CRC, defined as incident diagnosis of CRC during the 2 years after study |
|
Multiple imputation to replace missing values for BMI, alcohol intake and smoking status |
| Exclusion criteria: missing a postcode-related Townsend deprivation score; history of pancreatic cancer at baseline; red-flag symptoms recorded in the 12 months before the study entry date | |||||
| Inclusion criteria: patients from general practices using EMIS computer system for a minimum of 1 year | |||||
| Collins 201215 |
|
N/A. Used QCancer (colorectal); see Hippisley-Cox 201280 | Diagnosis of CRC, defined as incident diagnosis of CRC during the 2 years after study entry | 2,135,540 patients, including 3712 cases of CRC (1676 women and 2036 men) | Multiple imputation using all predictors plus the outcome variable was used to replace missing values for alcohol consumption |
| Hamilton 200581 | Inclusion criteria:
|
All related features (symptoms and variables) were investigated; only features occurring in at least 2.5% of either cases or controls were analysed | Diagnosis of CRC within 2 years of presentation | 349 cases; 1744 controls | NR |
| Exclusion criteria (cases and controls): unobtainable records; no consultations in the 2 years before diagnosis; previous CRC; or residence outside Exeter at the time of diagnosis | |||||
| Elias 201750 | Inclusion criteria: lower-abdominal complaints for at least 2 weeks with rectal bleeding, change in bowel habit, abdominal pain, fever, diarrhoea, weight loss, sudden onset in elderly, and/or physical examination suggestive of colorectal disease | N/A. Used RAT (colorectal) | CRC | 810, including 37 with CRC | Multiple imputation |
| Hamilton 200974 | Inclusion criteria:
|
23 candidate variables (features) were identified from a review of the literature | Diagnosis of CRC within 2 years of presentation | 5477 cases; 38,314 controls | NR |
| Exclusion criteria: < 2 years of data recorded prior to the diagnosis | |||||
| Stapley 201772 | Inclusion criteria:
|
All symptoms, physical signs or abnormal investigations related to CRC/IBD that occurred in ≥ 5% of cases or controls were retained | Diagnosis of either IBD or CRC within 1 year of presentation with symptoms |
|
NR |
| Exclusion criteria: cases and controls with no consultations in the year before the index date; controls that had a previous diagnostic code of CRC/IBD, diagnosis before 2000 | |||||
| Gastro-oesophageal | |||||
| Hippisley-Cox 201182 | Inclusion criteria: patients aged 30–84 years, registered with practices between 1 January 2000 and 30 September 2010 | Current first onset of dysphagia, haematemesis, loss of appetite, weight-loss symptom or abdominal pain; recent GP consultation for tiredness; age; BMI; smoking status; alcohol status; Townsend deprivation score; family history of gastrointestinal cancer; previous diagnosis of cancer apart from gastro-oesophageal cancer; anaemia | Diagnosis of either gastric cancer or oesophageal cancer in the 2 years following presentation |
|
Multiple imputation to replace missing values for BMI, alcohol intake and smoking status |
| Exclusion criteria: patients without a postcode-related Townsend deprivation score, history of gastro-oesophageal cancer at baseline, red-flag symptoms recorded (dysphagia, haematemesis, loss of appetite, weight loss and abdominal pain) in the 12 months prior to the study entry date | |||||
| Inclusion criteria: patients from general practices using EMIS computer system for a minimum of 1 year | |||||
| Collins 201388 |
Inclusion criteria: patients registered between 1 January 2000 and 30 June 2008, and recorded on THIN database Exclusion criteria: prior diagnosis of CRC, patients registered < 12 months with the general practice, had invalid dates, aged < 30 or > 85 years |
N/A. Used QCancer (gastro-oesophageal), see Hippisley-Cox 201152 | Diagnosis of gastro-oesophageal cancer in the 2 years after study entry | 2,135,540 patients, including 1766 cases of gastro-oesophageal cancer | Multiple imputation using all predictors plus the outcome variable was used to replace missing values for alcohol consumption |
| Stapley 201371 | Inclusion criteria:
|
All symptoms, physical signs or abnormal investigations that occurred in ≥ 5% of cases or controls were retained | Gastro-oesophageal cancer diagnosis within 1 year of presentation |
Oesophageal cancer: 4854 cases, 21,506 controls Gastric cancer: 2617 cases, 11,371 controls |
NR |
| Exclusion criteria: any individuals without a consultation in the year before the diagnosis/index date; cancers from other sites that had spread to the oesophagus or stomach; controls if they had ever had oesophagogastric cancer | |||||
| Lung | |||||
| Iyen-Omofoman 201321 | Inclusion criteria:
|
|
Diagnosis of lung cancer within 12 months of presentation | 12,074 cases; 120,731 controls | Unclear |
| Hippisley-Cox 201152 |
|
Current first onset of haemoptysis, loss of appetite or weight-loss symptom; recent (within 12 months) GP consultation for cough, dyspnoea, tiredness or hoarseness; BMI; smoking status; chronic obstructive airways disease diagnosed ever; Townsend deprivation score; family history of lung cancer; previous diagnosis of cancer apart from lung cancer; asthma diagnosed ever; pneumonia diagnosed ever; asbestos exposure ever; anaemia | Diagnosis of lung cancer during the 2 years after study entry |
|
Multiple imputation to replace missing values for smoking and BMI |
| Hamilton 200551 | Inclusion criteria:
|
All related features (symptoms and variables) were investigated. Only features occurring in at least 2.5% of either cases or controls were analysed | Diagnosis of lung cancer within 2 years of presentation | 247 cases, 1235 controls | NR |
| Exclusion criteria (both): unobtainable records; no consultations in the 2 years before diagnosis; previous lung cancer; or residence outside Exeter at the time of diagnosis | |||||
| Ovarian | |||||
| Hippisley-Cox 201184 |
|
|
Diagnosis of ovarian cancer during the 2 years after study entry |
|
Multiple imputation to replace missing values for BMI, alcohol intake and smoking status |
| Collins 201389 |
|
N/A. Used QCancer (ovarian), see Hippisley-Cox 201284 | Diagnosis of ovarian cancer during the 2 years after study entry | 1,054,818 patients (1,827,997 person-years) | No missing data reported |
| Hamilton 200983 | Inclusion criteria:
|
Only symptoms occurring in at least 5% of either cases or controls were studied | Diagnosis of ovarian cancer within 1 year of presentation | 212 cases; 1060 controls |
|
| Exclusion criteria: medical record unobtainable, no entry in the records in the year before diagnosis, previous ovarian cancer or bilateral oophorectomy, or residence outside the study area at the time of diagnosis | |||||
| Pancreas | |||||
| Hippisley-Cox 201232 | Inclusion criteria: patients aged 30–84 years registered between 1 January 2000 and 30 September 2010; missing a postcode-related Townsend deprivation score; history of pancreatic cancer at baseline; red-flag symptoms recorded in the 12 months before the study entry date, aged < 30 or > 84 years |
|
Presence of pancreatic cancer |
|
Multiple imputation to replace missing values for BMI, alcohol intake and smoking status |
| Collins 201314 |
|
N/A. Used QCancer (pancreas), see Hippisley-Cox 201232 | Diagnosis of pancreatic cancer within 2 years of study entry | 2,150,322 patients (3,744,567 person-years) | Multiple imputation using all predictors plus the outcome variable was used to replace missing values for smoking status |
| Stapley 201270 | Inclusion criteria:
|
All symptoms, physical signs or abnormal investigations that occurred in ≥ 5% of cases or controls were retained | Pancreatic cancer diagnosis within 1 year of presentation | 3635 cases, 16,459 controls | NR |
| Exclusion criteria: those with no consultations in the year before cancer diagnosis/index date; controls excluded if ever had pancreatic cancer | |||||
| Keane 201462 |
|
‘Alarm’ symptoms and commonly performed blood test results were identified based on clinical knowledge and the existing literature. Only symptoms with a frequency of > 5% were identified as potential alarm symptoms. Age, sex, time period and Townsend deprivation score, smoking status and BMI were selected as potential confounders | Diagnosis of PDAC or BTC within 2 years of presentation | 2773 cases with PDAC, 848 cases with BTC and 15,395 controls | NR |
| Prostate | |||||
| Hamilton 200690 | Inclusion criteria:
|
All related features (symptoms and variables) were investigated; only features occurring in at least 2.5% of either cases or controls were analysed | Diagnosis of prostate cancer within 2 years of presentation | 217 cases, 1080 controls | NR |
| Exclusion criteria (both): unobtainable records; no consultations in the 2 years before diagnosis; previous prostate cancer; or residence outside Exeter at the time of diagnosis | |||||
| Renal | |||||
| Hippisley-Cox 201285 |
|
|
Diagnostic of renal tract cancer within 2 years of presentation |
|
Multiple imputation to replace missing values for BMI, alcohol intake and smoking status |
| Collins 201391 |
|
N/A. Used QCancer (renal), see Hippisley-Cox 201232 | Presence of renal cancer | 2,145,133 patients (3,731,312 person-years) | Multiple imputation using all predictors plus the outcome variable was used to replace missing values for smoking status |
| Shephard 201366 | Inclusion criteria:
|
|
Diagnosis of kidney cancer within 1 year of presentation | 17,240 patients (3149 cases; 14,091 controls) | NR |
| Exclusion criteria: metastatic cancer of the kidney from a non-kidney primary, diagnosis before 2000, or no consultations in the year before diagnosis | |||||
| Uterine | |||||
| Walker 201373 | Inclusion criteria:
|
All symptoms, physical signs or abnormal investigations related to uterine cancer, that occurred in ≥ 2% of cases or controls were retained | Diagnosis of uterine tumour within 1 year of presentation with symptoms | 2732 cases, 9537 controls | NR |
| Exclusion criteria: leiomyosarcoma; diagnosis before 1 January 2000; controls diagnosed with uterine cancer before the index date; metastatic cancer from a non-uterine primary cancer; women with a recorded hysterectomy before the index date; and women with no consultations in the year before the index date | |||||
| Metastatic | |||||
| Hamilton 201586 | Inclusion criteria:
|
NR. 207 separate ‘features’ were identified in > 2% of cases | Diagnosis of metastatic cancer within 6 months of diagnosis of primary cancer |
|
NR |
| Exclusion criteria: primary cancer considered incurable at the time of initial diagnosis, or metastatic spread had occurred within 6 months of diagnosis of the primary cancer; cases for whom the primary cancer was diagnosed before 40 years of age; patients whose metastases occurred before registration at the current practice; cases, the full record for which had been archived off site after death | |||||
| Multiple | |||||
| Hippisley-Cox 201353 |
|
Red-flag symptoms and more general symptoms, plus risk factors: age, BMI, smoking status, alcohol use, Townsend deprivation score (postcodes), previous diagnosis of cancer, anaemia, family history of breast cancer, family history of gastrointestinal cancer, family history of ovarian cancer, benign breast disease, chronic pancreatitis, type 1 diabetes, type 2 diabetes, endometriosis, endometrial hyperplasia or polyp, fibroid, polycystic ovarian disease, rheumatoid arthritis, systemic lupus erythematosus, a HIV infection or AIDS, oral contraceptive use, hormone replacement therapy | Diagnosis of cancer within 2 years after study entry |
|
Multiple imputation was used in the validation cohort to replace missing values for BMI, alcohol, and smoking |
| Hippisley-Cox 201354 |
|
Red-flag symptoms and more general symptoms, plus risk factors: age, BMI, smoking status, alcohol use, Townsend deprivation score (postcodes), previous diagnosis of cancer, anaemia, family history of gastrointestinal cancer, family history of prostate cancer, chronic pancreatitis, type 1 diabetes, type 2 diabetes | Diagnosis of cancer within 2 years after study entry |
|
Multiple imputation was used in the validation cohort to replace missing values for BMI, alcohol intake and smoking status |
| Dommett 201364 | Inclusion criteria:
|
Three or more consultations, upper respiratory tract infection, musculoskeletal symptoms, vomiting, cough, headache, lymphadenopathy, rash, abdominal pain, childhood infection, fever, abnormal movement, abdominal mass, pain, fatigue, lump mass swelling (below neck excluding abdomen), eye swelling, shortness of breath, bruising, pallor, bleeding, lump mass swelling of head and neck, visual symptoms, constipation | GPRD medical code for cancer (leukaemia/lymphoma, central nervous system tumours, bone tumour/soft tissue sarcoma, abdominal tumour) within 1 year of recorded symptom | 1267 cases; 15,318 controls | NR |
| Muris 199556 | Inclusion criteria: aged 18–75 years, consulting GP for new abdominal complaints lasting at least 2 weeks, consenting to participate | Age and sex. WBC count, ESR, low Hb level, positive FOBT. Low somatisation score, no depression, high self-esteem, social inadequacy, plus 23 symptoms | Multiple cancers | 933, including ≈ 18 having cancer | NR |
| Elias 201750 | Inclusion criteria: lower-abdominal complaints for at least 2 weeks with rectal bleeding, change in bowel habit, abdominal pain, fever, diarrhoea, weight loss, sudden onset in elderly, and/or physical examination suggestive of colorectal disease | N/A. Used Muris 199556 | CRC, yet Muris 199556 is multiple cancers | 810, including 37 with CRC | NR |
| Study | Model development | Model performance |
|---|---|---|
| Bladder | ||
| Shephard 201269 | Conditional logistic regression. Variables with an independent association with cancer (p < 0.001) were included in the final multivariable model. Variables were then grouped for multivariable analysis, collecting together variables that were similar (such as visible and non-visible haematuria), using a p-value threshold of ≤ 0.05. The final stage of multivariable analysis used all variables surviving the previous stages, and used a p-value threshold of 0.01. All excluded variables were checked against the final model | PPVs were estimated for predictors shown to be independently associated with cancer in the multivariable analysis. This was repeated for pairs of symptoms and for second attendances with the same symptom (see figure 2 of publication69) |
| Blood | ||
| Shephard 201667 | Conditional logistic regression. Variables with p < 0.1 from univariable analyses were grouped by similarity, those with p < 0.05 entered final model, at which point those with p < 0.01 were retained in model |
|
| Shephard 201568 | Non-parametric methods in univariable analysis, a p-value threshold of ≤ 0.1 was used to identify candidate variables for multivariable analysis These were then grouped into small clinically coherent groups containing similar variables in the first stage of multivariable analysis, with retention requiring a p-value of ≤ 0.05. A final multivariable model used the surviving variables from the previous stages, using a p-value threshold of 0.01 | PPVs were calculated (see figure 2 of publication68) |
| Brain | ||
| Hamilton 200787 | Variables associated with tumours in univariable analyses with p ≤ 0.1 were entered into the staged multivariable analyses; discarded variables were checked against the final model. Seven clinically plausible internal interactions were tested in the final model | PPVs:
|
| Breast | ||
| McCowan 201160 | Logistic regression. Univariable associations for the explanatory variables were investigated and those with a threshold of < 0.01 were used in the multivariate regression model | The regression coefficients from the derivation cohort were applied to individuals in the validation cohort and used to generate expected and observed probabilities of breast cancer. The numbers of expected vs. observed cancers were also plotted by decile of predicted risk, based on the derivation model. Hosmer–Lemeshow goodness-of-fit test for the calibration of the model showed no significant difference between expected and observed breast cancers (7.02, p = 0.73), but the plot suggested that the number of cancers was overestimated for those at highest risk |
| Colorectal | ||
| Marshall 201122 | Multivariable conditional logistic regression analysis. Initial univariable conditional logistic regression analysis was carried out with the initial predictor variables and some variables were combined. Variables associated with CRC with p < 0.1 were entered into multivariable conditional logistic regression |
ROC curves, sensitivity, LRs and PPVs also reported In the CAPER data set, AUCs: BB 0.92 (95% CI 0.91 to 0.94); CAPER 0.91 (95% CI 0.89 to 0.93); NICE guidelines 0.75 (95% CI 0.72 to 0.79) |
| Elias 201750 | N/A. Used BB equation22 |
|
| Fijten 199555 | Forward stepwise logistic regression analysis. Variables showing an association (p < 0.1) with cancer were included in the multivariate model |
|
| Hodder 200577 | Used Fijten 199555 risk prediction model. Also assessed performance of guidelines and scores that were not based on risk prediction models (results not reported here) | AUC of 0.775 (SE 0.02) |
| Elias 201750 | N/A. Used Fijten 199555 |
|
| Kop 201561 | Models were generates using logistic regression, RF, SVM and the CART algorithm | A priori algorithm: the records are scanned first to create frequent patterns of size 1. These patterns are used to generate successively larger patterns, that is k patterns are used to obtain frequent k + 1 patterns. Generating a k + 1 pattern is, however, more elaborate than for standard a priori, because the generated candidate patterns need to accommodate for multiple possible (successions) and (occuring) relations. This results in more k + 1 patterns being tested for frequency than with standard a priori, increasing the complexity |
| Hippisley-Cox 201280 |
|
Females:
|
Males:
|
||
| Collins 201215 | N/A. Used QCancer (colorectal), see Hippisley-Cox 201280 |
Men: Multiple imputation (m = 10) (n = 1,059,765)Complete case (n = 417,560) |
|
Women: Complete case (n = 1,075,775) |
||
| Nørrelund 199657 | Mann–Whitney and chi-squared tests, and logistic regression. No further details reported | Study 1 (apparent performance I):
|
Study 2 (apparent performance II), new bleeders:
|
||
Study 2 (apparent performance II), new or changed bleeders:
|
||
| Elias 201750 | N/A. Used Danish (Nørrelund 199657) model |
|
| Hamilton 200581 | Variables associated with cancer in univariable analyses, using a p-value of ≤ 0.1, entered the multivariable analysis. In the first stage, similar variables were grouped together; in the second stage, analyses were repeated with the new groups | Nine features [constipation, diarrhoea, rectal bleeding, loss of weight, abdominal pain, abdominal tenderness, abnormal rectal examination, anaemia (Hb level 10−13 g/dl; Hb < 10 g/dl) and a blood sugar concentration of > 10 mmol/l] were associated with CRC before diagnosis. The PPVs (95% CI%) of these were rectal bleeding 2.4% (1.9% to 3.2%), weight loss 1.2% (0.91% to 1.6%), abdominal pain 1.1% (0.86% to 1.3%), diarrhoea 0.94% (0.73% to 1.1%), constipation 0.42% (0.34% to 0.52%), abnormal rectal examination 4.0% (2.4% to 7.4%), abdominal tenderness 1.1% (0.77% to 1.5%), a Hb level of < 10.0 g/dl 2.3% (1.6% to 3.1%), positive FOBT 7.1% (5.1% to 10%) and a blood glucose concentration of 410 mmol/l 0.78% (0.51% to 1.1%); all p-values < 0.001. See figure 2 of the publication81 for additional PPVs |
| Elias 201750 | N/A. Used RAT (colorectal) model81 |
|
| Hamilton 200974 | Variables with a univariable association with cancer significant with a p-value of < 0.1 were entered into a staged multivariable analysis |
PPVs were estimated Six symptoms and two abnormal investigations (anaemia and microcytosis) were independently associated with CRC. The PPVs (95% CIs) of symptoms were as follows: rectal bleeding, to PPV for a male aged ≥ 80 years 4.5% (3.5% to 5.9%), change in bowel habit 3.9% (2.8% to 5.5%), weight loss 0.8% (0.5% to 1.3%), abdominal pain 1.2% (1.0% to 1.4%), diarrhoea 1.2% (1.0% to 1.5%) and constipation 0.7% (0.6% to 0.8%). PPVs were lower in females and younger patients. Only 27% of patients had reported either of the two higher-risk symptoms |
| Stapley 201772 | Conditional logistic regression. Variables independently associated with pancreatic cancer with a p-value of < 0.1 were entered into the multivariable analysis multivariable analysis performed in three stages; final model used a threshold p-value of < 0.02 | PPVs were derived, using national incidence data to estimate prior odds (see figure 2 in publication72) |
| Gastro-oesophageal | ||
| Hippisley-Cox 201182 | Cox’s proportional hazards. Rubin’s rules were used to combine the results across the imputed data sets. Fractional polynomials were used to model non-linear risk relationships with continuous variables. Fitted full model, variables retained if HR < 0.80, HR > 1.20 and p < 0.01 | Females:
|
Males:
|
||
| Collins 201388 | N/A. Used QCancer (gastro-oesophageal), see Hippisley-Cox 201182 |
Men: Multiple imputation (m = 10) (n = 1,077,977)Complete case (n = 852,532) |
|
Women: Multiple imputation (m = 10) (n = 1,062,217)Complete case (n = 1,075,775) |
||
| Stapley 201371 | Conditional logistic regression. Variables independently associated with oesophago-gastric cancer with a p-value of < 0.1 were entered into the multivariable analysis. Significant variables (at p < 0.05) were grouped together and those variables significant at a p-value of < 0.01 remained in the final model | PPVs for the risk of oesophago-gastric cancer in patients consulting in primary care. PPVs (%): dysphagia 1.5 (95% CI 1.3 to 1.8), dyspepsia 0.2 (95% CI 0.19 to 0.22), nausea/vomiting 0.18 (0.17 to 0.20), abdominal pain 0.08 (95% CI 0.08 to 0.09), reflux 0.19 (95% CI 0.18 to 0.22), chest pain 0.05 (95% CI 0.05 to 0.06), epigastric pain 0.28 (95% CI 0.24 to 0.32), weight loss 0.26 (95% CI 0.23 to 0.31), constipation 0.07 (95% CI 0.06 to 0.07), low Hb 0.07 (95% CI 0.07 to 0.08), abnormal hepatic enzymes 0.04 (95% CI 0.04 to 0.05), raised inflammatory markers 0.08 (95% CI 0.08 to 0.09) and raised cholesterol 0.02 (95% CI 0.02 to 0.02) |
| Lung | ||
| Iyen-Omofoman 201321 |
Analyses conducted separately for features 4–12 and 13–24 months before diagnosis/index date Logistic regression. Included features statistically significant (p < 0.05) in univariate analyses. From multivariate model removed variables that were not significant, and features not significant in univariate analyses were rechecked for significance in multivariate model |
Model based on features 4–12 months before diagnosis/index date. Area under the ROC curve of 0.88 Sensitivities (%) and specificities (%), respectively, reported at different risk score cut-off values: |
| Hippisley-Cox 201152 |
Cox proportional hazards models with age as the underlying time variable were used to develop separate risk equations in males and females Variables included if HR < 0.80, HR > 1.20 and a p-value of 0.01 |
Females:
|
Males:
|
||
| Hamilton 200551 |
Variables associated with cancer in univariable analyses, using a p-value of ≤ 0.1, entered the multivariable analysis 18 clinically plausible interactions were tested in the final model; analyses were repeated excluding data from the last 180 days of the 730-day period studied. PPVs for individual variables and for pairs of variables were calculated from the LR and the observed incidence of cancer during the study |
Colour-coded PPVs are reported in figure 2 of the publication51 |
| Ovarian | ||
| Hippisley-Cox 201184 | Cox’s proportional hazards model was used to estimate the coefficients for each risk factor using robust variance estimates to allow for the clustering of patients within general practices. Rubin’s rules were used to combine the results across the imputed data sets. Fractional polynomials for modelling non-linear risk relations with continuous variables. Fitted full model, variables retained if HR < 0.80, HR > 1.20 and a p-value of 0.01 |
|
| Collins 201389 | N/A. Used QCancer (ovarian), see Hippisley-Cox 201184 |
|
| Hamilton 200983 | Symptoms independently associated with the outcome, with a p-value of < 0.1, were retained for multivariable analyses. The resulting 99 variables were placed in eight groups and each group was analysed by multivariable conditional logistic regression. Symptoms still associated with cancer were rearranged into two larger groups (abdominal symptoms and other symptoms) for the final model. Discarded symptoms were checked against the final model. Five clinically plausible interactions were tested in the final model | PPVs were estimated and presented in figure 2 of the publication83 |
| Pancreas | ||
| Hippisley-Cox 201232 |
Cox proportional hazards models with age as the underlying time variable were used to develop separate risk equations in males and females Variables included if HR < 0.80, HR > 1.20 and p < 0.01 |
Females:
|
Males:
|
||
| Collins 201314 | N/A. Used QCancer (pancreas), see Hippisley-Cox 201232 | Multiple imputation (m = 10)
|
Complete case
|
||
| Stapley 201270 | Conditional logistic regression. Variables independently associated with pancreatic cancer with a p-value of < 0.1 were entered into the multivariable analysis. In the first stage, similar variables were grouped together; in the second stage analyses were repeated with the new groups (with p < 0.05); in the third stage, the final model was built with the retained variables (with p < 0.01) |
PPVs for the risk of pancreatic cancer in patients consulting in primary care were calculated using Bayes’ theorem; see figure 2 in publication70 PPVs for patients aged > 60 years were < 1%, apart from jaundice at 22% (95% CI 14% to 52%), although several pairs of symptoms had PPVs of > 1% |
| Keane 201462 |
|
NR |
| Prostate | ||
| Hamilton 200690 | Conditional logistic regression. Variables associated with cancer in univariable analyses, using a p-value of ≤ 0.1, entered the multivariable analysis. In the first stage, similar variables were grouped together; in the second stage, analyses were repeated with the new groups (with p < 0.05); in the third stage, the final model was built with the retained variables (with p < 0.01) |
PPVs were calculated from the LRs and the observed annual incidence of cancer during the study, see figure 2 in publication90 Eight features were associated with prostate cancer before diagnosis. Their PPVs against a background risk of 0.35% were as follows: urinary retention 3.1% (95% CI 1.5% to 6.0%); impotence 3.0% (95% CI 1.7% to 4.9%); frequency 2.2% (95% CI 1.3% to 3.5%); hesitancy 3.0% (95% CI 1.5% to 5.5%); nocturia 2.2% (95% CI 1.2% to 3.6%); haematuria 1.0% (95% CI 0.57% to 1.8%); weight loss 0.75% (95% CI 0.38% to 1.4%); abnormal rectal examination, deemed benign 2.8% (95% CI 1.6% to 4.6%); abnormal rectal examination, deemed malignant 12% (95% CI 5.0% to 37%). All p < 0.001, except for hesitancy (p = 0.032), nocturia (p = 0.004) and haematuria (p = 0.009) |
| Renal | ||
| Hippisley-Cox 201285 |
Cox proportional hazards models with age as the underlying time variable were used to develop separate risk equations in males and females Variables included if HR < 0.80, HR > 1.20 and a p-value of 0.01 |
Females:
|
Males:
|
||
| Collins 201391 | N/A. Used QCancer (renal), see Hippisley-Cox 201285 | Multiple imputation (m = 10)
|
Complete case
|
||
| Shephard 201366 | Conditional logistic regression: features associated with cancer with p-values of ≤ 0.1 were grouped for the first multivariable analysis, with retention requiring a p-value of ≤ 0.05. A final multivariable model was compiled from the features of the previous stage for which p < 0.01 |
|
| Uterine | ||
| Walker 201373 | Conditional logistic regression. Variables independently associated with pancreatic cancer with a p-value of < 0.1 were entered into the multivariable analysis. In the first stage, similar variables were grouped together; in the second stage, analyses were repeated with the new groups (with p < 0.05); in the third stage, the final model was built with the retained variables (with p < 0.05) | PPVs were estimated for features shown to be independently associated with uterine cancer in the multivariable analysis, using Bayes’ theorem. See figure 1 in publication73 |
| Metastatic | ||
| Hamilton 201586 | Conditional logistic regression. Univariable analyses were performed initially, retaining variables with a p-value of < 0.1 to enter into multivariable analyses; only variables that were present in > 2% of the cases were studied. Cancer controls and healthy controls were used in separate analyses, and the cancer sites were analysed separately, and also merged and a unified analysis performed. Clinically plausible interaction terms were added to each model, and LR testing was applied to test whether or not they improved the models | NR |
| Multiple | ||
| Hippisley-Cox 201353 | Multinomial logistic regression was used to estimate the coefficients for each predictor variable for each type of cancer. Fitted full model and variables retained if p ≤ 0.01. Fractional polynomials were used to model non-linear risk relationships with continuous variables |
|
| Hippisley-Cox 201354 | Multinomial logistic regression was used to estimate the coefficients for each predictor variable for each type of cancer. Fitted full model and variables retained if p ≤ 0.01. Fractional polynomials were used to model non-linear risk relationships with continuous variables |
|
| Dommett 201364 | Conditional logistic regression. Variables occurring in at least 2% of either cases or controls with a univariable p-value of ≤ 0.1 entered the multivariable conditional logistic regression. A p-value of < 0.01 was used for retention in the final model. PPVs were calculated using Bayes’ theorem | 12 predictors had a PPV of ≥ 0.04% |
| Muris 199556 | Variables for which p < 0.25 in univariate analyses were entered into multiple stepwise forward logistic regressions | NR |
| Elias 201750 | N/A. Used Muris 199656 model |
|
| Study | Model evaluation | Results | Interpretation and discussion |
|---|---|---|---|
| Bladder | |||
| Shephard 201269 | Development data set only | Symptoms:
|
Findings support investigation of all patients aged > 40 years with visible haematuria |
Disease:
|
|||
Investigations:
|
|||
| Blood | |||
| Shephard 201667 | Development data set only | Ten symptoms were independently associated with chronic leukaemia, OR (95% CIs): infection 1.5 (1.3 to 1.6), cough 1.2 (1.1 to 1.4), hypertension 1.2 (1.1 to 1.4), shortness of breath 1.3 (1.1 to 1.5), fatigue 2.1 (1.8 to 2.6), diarrhoea 1.4 (1.1 to 1.7), lymphadenopathy 22 (13 to 36), malaise 1.7 (1.3 to 2.3), weight loss 3.0 (2.1 to 4.2) and bruising 2.3 (1.6 to 3.2) | PPVs too small, with benign alternative explanations much more likely for the symptoms |
| Thirteen symptoms were independently associated with acute leukaemia, OR (95% CIs): infection 1.5 (1.3 to 1.8), shortness of breath 2.5 (1.9 to 3.2), fatigue 4.4 (3.3 to 6.0), chest pain 1.5 (1.1 to 2.1), abdominal pain 1.7 (1.2 to 2.2), diarrhoea 2.2 (1.5 to 3.1), malaise 3.4 (2.2 to 5.2), vomiting/nausea 1.8 (1.2 to 2.6), bruising 3.7 (2.3 to 5.8), fever 5.3 (2.7 to 10), nosebleeds and/or bleeding gums 5.7 (3.1 to 10), flu 3.9 (2 to 7.5) and weight loss 3 (1.5 to 5.8) | |||
| No individual symptom or combination of symptoms had a PPV of > 1% | |||
| Shephard 201568 | Development data set only | Symptoms:
|
Although no single symptom is a strong indicator of myeloma, repeated occurrences of back pain or back pain combined with nosebleeds or rib pain suggest initial testing of inflammatory markers, at the discretion of the GP |
Investigations:
|
|||
| Brain | |||
| Hamilton 200787 | Development data set only | New-onset seizure 87.0 (42.0 to 180.0), weakness 23.0 (7.1 to 77.0), headache 6.7 (5.6 to 8.0), confusion 11.0 (7.6 to 16.0), memory loss 2.7 (1.7 to 4.2), visual disorder 2.0 (1.2 to 3.3), motor loss 1.8 (1.5 to 2.2) and motor loss with weakness 0.2 (0.06 to 0.8) | Findings suggest that isolated headache presented to primary care has too small a risk of an underlying brain tumour to warrant investigation; however, new-onset seizures should be investigated |
| Breast | |||
| McCowan 201160 | External validation | Independent clinical predictors (adjusted OR and 95% CIs): increasing age by year, 1.10 (1.07 to 1.13); presence of a discrete lump, 15.20 (4.88 to 47.34); breast thickening, 7.64 (2.23 to 26.11); lymphadenopathy, 3.63 (1.33 to 9.92); and lump of 2 cm, 5.41 (2.36 to 12.38). All eight patients with skin tethering had breast cancer | The clinical prediction rule discriminates between patients at high risk and patients at low risk of breast cancer |
| Colorectal | |||
| Marshall 201122 |
|
Model predictors and ORs (95% CIs): constipation, 2.06 (1.88 to 2.26); diarrhoea, 2.38 (2.14 to 2.66); change in bowel habit, 13.83 (11.70 to 16.34); abdominal pain, 3.82 (3.49 to 4.18); rectal bleeding, 20.11 (17.35 to 23.32); Hb level of 13–13.99 g/dl, 1.33 (1.18 to 1.50); Hb level of 12–12.99 g/dl, 1.63 (1.42 to 1.87); Hb level of 11–11.99 g/dl, 2.54 (2.16 to 2.99); Hb level of 10–10.99 g/dl, 5.18 (4.19 to 6.39); Hb level of 9–9.99 g/dl, 8.08 (6.13 to 10.65); Hb level of < 9 g/dl, 15.94 (11.78 to 21.57); mean cell volume of 80–84.99 fl, 2.71 (2.30 to 3.19); mean cell volume of < 80 fl, 7.67 (6.23 to 9.44); weight loss of ≥ 10%, 2.92 (2.39 to 3.57); and weight loss of 5–10%, 1.37 (1.09 to 1.73) | Both multivariable BB and CAPER equations performed significantly better than NICE referral guidelines172 |
| Elias 201750 | External validation | Elias ranked the BB equation as sixth out of 19 models evaluated | The top-ranked model was the NICE guidelines. Note that Elias used a very broad definition of prediction model, which included guidelines and weighted scores |
| Fijten 199555 | Development data set only |
|
The combination of age, change in bowel habit and blood seen mixed with or on stool can serve as a useful diagnostic tool for the prediction of CRC (and overtly bleeding polyps) |
| Hodder 200577 | External validation | The Netherlands model had better discrimination than the Harvard model, but inferior discrimination compared with the weighted numerical score | |
| Elias 201750 | External validation | CEDAR data set included participants older than those in original Fijten 199555 derivation data set. Based on the results, Elias et al. ranked the Fijten 199555 model as 13th out of 19 models | The top-ranked model was NICE guidelines. Note that Elias 2017 used a very broad definition of prediction model, which included guidelines and weighted scores |
| Kop 201561 | Five subsets were selected and four machine-learning algorithms were applied to them in a fivefold cross-validation fashion:
|
AUCs and 95% CIs for subsets:
|
Study suggests metabolic syndrome as potential predictor |
| Nørrelund 199657 | Validated in study 2 | Only age was found to be a statistically significant predictor of cancer from study 1:
|
Reported symptoms of weight loss, abdominal pain, change in bowel habits or discomfort were not found to be predictors of CRC in either study 1 or 2 |
| No statistically significant variables were found in study 2 to predict cancer | |||
| Elias 201750 | External validation | Elias et al.50 ranked the Danish (i.e. Nørrelund et al.57) model as 18th out of 19 models evaluated | The top-ranked model was NICE guidelines. Note that Elias 2017 used a very broad definition of prediction model, which included guidelines and weighted scores |
| Hippisley-Cox 201280 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HR (95% CI):
|
State that the algorithm performed well, with good discrimination and calibration |
| Collins 201215 | External validation with a different cohort | Model calibration is very good, with close agreement between predicted and observed CRC risks across all tenths of risk | |
| Hamilton 200581 | Development data set only | ORs (95% CIs): rectal bleeding 15 (9.0 to 2.4), weight loss 2.7 (1.7 to 4.6), number of episodes of abdominal pain 2.2 (1.7 to 2.8), constipation 2.0 (1.2 to 3.3), number of episodes of diarrhoea 1.6 (1.3 to 2.0), rectal disease on examination 13 (4.7 to 37), tenderness on palpitation of abdomen 3.6 (1.7 to 7.8), positive FOBT 81 (20 to 330), Hb level of 12.0–12.9 g/dl 2.5 (0.95 to 6.8), Hb level of 10.0–11.9 g/dl 4.3 (2.1 to 9.0), Hb level of < 10 g/dl 13 (6.2 to 28), blood sugar concentration of > 10 mmol/l 2.0 (1.3 to 3.1) | 10 symptoms, signs or investigation results were independently associated with CRC. Five of these remained associated with cancer 180 days before diagnosis |
| Interaction terms, ORs (95% CIs): abdominal pain with tenderness 0.56 (0.38 to 0.82), positive FOBT with a Hb level of < 10 g/dl 0.020 (0.0015 to 0.27) | |||
| Elias 201750 | External validation | Elias ranked the RAT (CRC) model as 10th out of 19 models evaluated. The top-ranked model was the NICE guidelines | Note that Elias used a very broad definition of prediction model, which included guidelines and weighted scores |
| Hamilton 200974 | Development data set only | ORs (95% CIs): rectal bleeding 20 (17 to 23), change in bowel habit 14 (12 to 17), abdominal pain 3.9 (3.6 to 4.3), diarrhoea 2.4 (2.1 to 2.7), constipation 2.1 (1.9 to 2.3), weight loss of 5.0–9.9% 1.2 (0.99 to 1.5), weight loss of ≥ 10% 2.5 (2.1 to 3.0), Hb level of 12.0–12.9 g/dl 1.7 (1.5 to 1.9), Hb level of 11.0–11.9 g/dl 2.8 (2.4 to 3.2), Hb level of 10.0–10.9 g/dl 5.9 (4.8 to 7.2), Hb level of 9.0–9.9 g/dl 9.3 (7.1 to 12), Hb level of < 9 g/dl 18 (14 to 25), mean red cell volume of < 80 fl 6.5 (5.3 to 7.9) | There is a need to improve identification of CRC among the large number of patients presenting only with low-risk symptoms |
| Stapley 201772 | Development data set only | OR (95% CIs) for CRC: diarrhoea 7.7 (4.3 to 14), abdominal pain 6.0 (4.2 to 8.7), rectal bleeding 54 (26 to 110), change in bowel habit 58 (21 to 160), constipation 7.9 (4.3 to 14), nausea/vomiting 2.7 (1.4 to 5.1), rectal mass 190 (51 to 720), raised levels of inflammatory markers 3.1 (2.0 to 4.7), low Hb level 5.2 (3.2 to 8.5), low mean red cell volume 4.3 (2.3 to 8.0) | Rectal bleeding and change in bowel habit are strongly predictive of CRC/IBD when combined with abnormal haematology |
| Gastro-oesophageal | |||
| Hippisley-Cox 201182 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HR (95% CI)
|
The algorithm developed and validated in the study performed well in quantifying the absolute risk of having existing, but as yet undiagnosed, gastro-oesophageal cancer |
| Collins 201388 | External validation | Model calibration was good, with reasonable agreement between predicted and observed gastro-oesophageal cancer risks across all but the last tenth of risk | |
| Stapley 201371 | Development data set only | Sixteen features were independently associated with oesophagogastric cancer, OR (95% CI) (all p < 0.001): dysphagia, 139 (112 to 173); reflux, 5.7 (4.8 to 6.8); abdominal pain, 2.6 (2.3 to 3.0); epigastric pain, 8.8 (7.0 to 11.0); dyspepsia, 6 (5.1 to 7.1); nausea and/or vomiting, 4.9 (4.0 to 6.0); constipation, 1.5 (1.2 to 1.7); chest pain, 1.6 (1.4 to 1.9); weight loss, 8.9 (7.1 to 11.2); thrombocytosis, 2.4 (2.0 to 2.9); low level of Hb, 2.4 (2.1 to 2.7); low MCV, 5.2 (4.2 to 6.4); high levels of inflammatory markers, 1.7 (1.4 to 2.0); raised levels of hepatic enzymes, 1.3 (1.2 to 1.5); high WBC count, 1.4 (1.2 to 1.7); and high cholesterol, 0.8 (0.7 to 0.8) | The tool can be used to guide GPs on referral for oesophagogastric cancer. Evidence suggests that reliance on ‘red-flag’ symptoms solely has limited use |
| Lung | |||
| Iyen-Omofoman 201321 | Split sampling validation |
Clinical and sociodemographic features that were independently associated with lung cancer were patients’ age, sex, socioeconomic status and smoking history. From 4 to 12 months before diagnosis, the symptoms/clinical features that were independently predictive of lung cancer were as follows, OR (95% CI): 11–20 GP consultations 1.23 (1.16 to 1.29), > 20 GP consultations 1.36 (1.28 to 144), cough 1.63 (1.53 to 1.75), haemoptysis 8.70 (6.75 to 11.20), dyspnoea 1.41 (1.29 to 1.55), weight loss 2.66 (2.13 to 3.29), lower respiratory tract infections 1.56 (1.38 to 1.76), non-specific chest infections 1.55 (1.44 to 1.68), chest/shoulder pain 1.39 (1.28 to 1.51), hoarseness 1.79 (1.28 to 2.49), upper respiratory tract infections 1.15 (1.02 to 1.30) and chronic obstructive pulmonary disease 1.61 (1.46 to 1.78) |
Risk prediction model performed better than existing NICE guidelines |
| Hippisley-Cox 201152 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HR (95% CI):
|
The algorithm developed and validated in the study performed well in quantifying the absolute risk of having existing, but as yet undiagnosed, lung cancer |
| Hamilton 200551 | Development data set only | ORs (95% CIs): appetite loss 86 (3.6 to 2100), haemoptysis 32 (13 to 81), dyspnoea 4.7 (2.7 to 8.0), weight loss 4.3 (2.2 to 8.2), fatigue 3.2 (1.7 to 6.0), chest pain 2.9 (1.8 to 4.7), second attendance with cough 2.7 (1.7 to 4.4), finger clubbing 18 (1.7 to 190), thrombocytosis 9.3 (3.4 to 26), abnormal spirometry 7.5 (2.8 to 21), current smoker 9.7 (5.3 to 18), ex-smoker 5.9 (3.0 to 12), smoking status unknown 5.4 (2.8 to 10), dyspnoea with fatigue 0.28 (0.11 to 0.73), appetite loss in patients aged > 70 years 0.13 (0.024 to 0.76) | The study provides an evidence base for selection of patients for investigation of possible lung cancer, both for clinicians and for developers of guidelines |
| After excluding variables reported in the final 180 days before diagnosis, haemoptysis, dyspnoea and abnormal spirometry remained independently associated with cancer | |||
| Ovarian | |||
| Hippisley-Cox 201184 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HRs (95% CIs): family history of ovarian cancer 9.8 (5.4 to 17.9), Hb level of < 110 g/l in the previous year 2.3 (1.7 to 2.9), current abdominal pain 7.0 (6.1 to 8.0), current abdominal distension 23.1 (18.2 to 29.4), current appetite loss 5.2 (3.4 to 7.9), current rectal bleeding 2.0 (1.4 to 2.8), current postmenopausal bleeding 6.6 (5.1 to 8.5), current weight loss 2.0 (1.3 to 3.1) | The algorithm developed and validated in the study performed well in quantifying the absolute risk of having existing, but as yet undiagnosed, ovarian cancer |
| Collins 201389 | External validation | Model calibration was good with reasonable agreement between predicted and observed ovarian cancer risks across all tenths of risk, with a slight overprediction | |
| Good predictive ability for identifying patients with undetected ovarian cancer | |||
| Hamilton 200983 | Development data set only | Seven symptoms and one interaction term were associated with ovarian cancer in multivariable analysis. The univariable PPVs (95% CIs) and multivariable ORs (95% CIs), respectively, for these were 2.5% (1.2% to 5.9%) and 240 (46 to 1200) for abdominal distension; 0.5% (0.2% to 0.9%) and 24 (9.3 to 64) for postmenopausal bleeding; 0.6% (0.3% to 1.0%) and 17 (6.1 to 50) for loss of appetite; 0.2% (0.1% to 0.3%) and 16 (5.6 to 48) for increased urinary frequency; 0.3% (0.2% to 0.3%) and 12 (6.1 to 22) for abdominal pain; 0.2% (0.1% to 0.4%) and 7.6 (2.5 to 23) for rectal bleeding; and 0.3% (0.2% to 0.6%) and 5.3 (1.8 to 16) for abdominal bloating | Study findings suggest that early symptoms may be useful in the identification of ovarian cancer |
| Interaction term OR (95% CI): abdominal distension with urinary frequency 0.015 (0 to 0.29) | |||
| In 181 (85%) cases and 164 (15%) controls, at least one of these seven symptoms was reported to primary care before diagnosis. After exclusion of symptoms reported in the 180 days before diagnosis, abdominal distension, urinary frequency and abdominal pain remained independently associated with a diagnosis of ovarian cancer | |||
| Pancreas | |||
| Hippisley-Cox 201232 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HR (95% CI):
|
The algorithm developed and validated in the study performed well in quantifying the absolute risk of having existing, but as yet undiagnosed, pancreatic cancer |
| Collins 201314 | External validation | QCancer (pancreas) increasingly overpredicts risk, with increases across the tenths of risk | |
| Stapley 201270 | Development data set only | Nine features were associated with pancreatic cancer, OR (95% CI) [all p < 0.001 except for back pain, (p = 0.004)]; jaundice, OR 1000 (95% CI 430 to 2500); abdominal pain, 5 (4.4 to 5.6); nausea/vomiting, 4.5 (3.5 to 5.7); back pain, 1.4 (1.1 to 1.7); constipation, 2.2 (1.7 to 2.8); diarrhoea, 1.9 (1.5 to 2.5); weight loss, 15 (11 to 22); malaise, 2.4 (1.6 to 3.5); and new-onset diabetes 2.1 (1.7 to 2.5) | Most previously reported symptoms of pancreatic cancer were also relevant in primary care, providing a basis for selection of patients for investigation, especially with multiple symptoms, although predictive values were mostly small |
| Keane 201462 | Development data set only |
|
Findings could improve the symptom-based cancer decision support tools used for identification of BTC and PDAC |
|
|||
| Prostate | |||
| Hamilton 200690 | Development data set only | ORs (95% CIs): urinary retention 11 (5 to 25.5), second presentation with weight loss 9.2 (2.7 to 31), impotence 5.3 (2.8 to 9.8), frequency 3.2 (1.9 to 5.4), hesitancy 2.9 (1.1 to 7.5), nocturia 2.6 (1.3 to 5), haematuria 2.4 (1.3 to 4.7), benign abnormal rectal examination 3.7 (1.9 to 7.3), malignant abnormal rectal examination 70 (13 to 380) | The predictive values for symptoms present in most men with prostate cancer will help guide GPs and patients about the value of further investigation |
| Loss of weight, impotence, frequency and abnormal rectal examination remained associated with cancer after excluding the final 180 days from analysis | |||
| Renal | |||
| Hippisley-Cox 201285 | Split sample validation (66%/33%) | Predictors and (fully adjusted) HR (95% CI):
|
The algorithm developed and validated in the study performed well in quantifying the absolute risk of having existing, but as yet undiagnosed, renal cancer |
| Collins 201391 | External validation | Model calibration showed good agreement across those aged 30–69 years, whereas it overpredicted the risk of renal tract cancer in those aged between 70 and 84 years | |
| The model showed good predictive ability for identifying patients with suspected undiagnosed renal tract cancer who would benefit from further clinical investigation | |||
| Shephard 201366 | Development data set only | Fifteen features were independently associated with kidney cancer, OR (95% CI): visible haematuria 37 (28 to 49), abdominal pain 2.8 (2.4 to 3.4), microcytosis 2.6 (1.9 to 3.4), raised inflammatory markers 2.4 (2.1 to 2.8), thrombocytosis 2.2 (1.7 to 2.7), low level of Hb 1.9 (1.6 to 2.2), urinary tract infection 1.8 (1.5 to 2.1), nausea 1.8 (1.4 to 2.3), raised creatinine levels 1.7 (1.5 to 2.0), leukocytosis 1.5 (1.2 to 1.9), fatigue 1.5 (1.2 to 1.9), constipation 1.4 (1.1 to 1.7), back pain 1.4 (1.2 to 1.7), abnormal liver function 1.3 (1.2 to 1.5) and raised blood sugar 1.2 (1.1 to 1.4) | Visible haematuria is the commonest and most powerful single predictor of kidney cancer, and the risk rises when additional symptoms are present |
| Uterine | |||
| Walker 201373 | Development data set only | Nine features were significantly associated with uterine cancer, OR (95% CI): postmenopausal bleeding 154.5 (100.4 to 237.8), excessive vaginal bleeding 22.3 (11.9 to 41.8), irregular menstruation (one GP visit) 41.5 (27.3 to 63.0), irregular menstruation (two GP visits) 69.0 (31.6 to 150.7), vaginal discharge 13.7 (10 to 21), haematuria 8.7 (5.0 to 15.1), abdominal pain (one GP visit) 2.0 (1.4 to 2.8), abdominal pain (two GP visits) 3.4 (2.0 to 5.8), low Hb 2.1 (1.5 to 2.9), raised platelets 1.5 (1.0 to 2.3) and raised glucose 1.4 (1.1 to 1.8); all p < 0.01, other than raised platelet count (p = 0.05) and raised glucose concentrations (p = 0.02) | Findings of the study, in particular the importance of postmenopausal bleeding and haematuria, for uterine cancer may inform GPs in the selection of women for investigation |
| In the year before diagnosis, 1725 (63%) cases had a record of abnormal vaginal bleeding, compared with 135 (1%) controls. The PPV of uterine cancer with postmenopausal bleeding was 4%, and was higher in women with multiple or repeated symptoms | |||
| Metastatic | |||
| Hamilton 201586 | Development data set only | Adjusted OR (95% CI):
|
The scarcity of specific symptoms and the fairly common occurrence of non-specific symptoms (vomiting and loss of appetite) may explain delays in the diagnosis of metastases |
| Multiple | |||
| Hippisley-Cox 201353 | Split sample validation (66%/33%) | Each cancer model contained the following number of predictors (see additional tables on www.qcancer.org for details) for these cancers: breast 10, cervical 12, ovarian 11, uterine 7, blood 10, colorectal 13, gastro-oesophageal 13, lung 15, pancreatic 14, renal 11, other cancers 22 | A new algorithm designed to estimate the absolute risk of having existing but as yet undiagnosed cancer was developed and validated |
| Hippisley-Cox 201354 | Split sample validation (66%/33%) | Each cancer model contained the following number of predictors (see additional tables on www.qcancer.org for details) for these cancers: prostate 14, testicular 3, blood 14, colorectal 12, gastro-oesophageal 13, lung 17, pancreatic 15, renal tract 8, other cancers 20 | A new algorithm designed to estimate the absolute risk of having existing but as yet undiagnosed cancer was developed and validated |
| Dommett 201364 | Development data set only | Models were developed for the following cancer groups, plus all cancers: leukaemia/lymphoma (12 predictors), central nervous system tumours (seven predictors), bone tumours and soft tissue sarcomas (four predictors), abdominal tumours (seven predictors) | Twelve features of childhood cancers were identified, each of which increased the risk of cancer at least 10-fold |
Predictors, adjusted ORs (95% CIs) and PPVs (95% CIs), respectively, for all cancers’ model:
|
|||
| When each of these 12 symptoms was combined singly with at least three consultations in a 3-month period, the probability of cancer was between 11 and 76 in 10,000 | |||
| Muris 199556 | Apparent performance | Statistically significant predictors [adjusted ORs (95% CI)] were:
|
The aim of the study was to identify predictors for organic disease, with a secondary analysis looking at predictors for neoplasms |
| Elias 201750 | External validation | Elias et al.50 ranked the Muris et al.56 model as 13th out of 19 models evaluated | The top-ranked model was the NICE guidelines. Note that Elias 2017 used a very broad definition of prediction model, which included guidelines and weighted scores |
| Study | 1a. Were appropriate data sources used, e.g. cohort, RCT or nested case-control study data? | 1b. Were all inclusions and exclusions of participants appropriate? | 1c. Were participants enrolled at a similar state of health, or were predictors considered to account for differences? | I. Participant selection | 2a. Were predictors defined and assessed in a similar way for all participants in the study? | 2b. Were predictor assessments made without knowledge of outcome data? | 2c. Are all predictors available at the time the model is intended to be used? | 2d. Were all relevant predictors analysed? | II. Predictors | 3a. Was a prespecified outcome definition used? | 3b. Were predictors excluded from the outcome definition? | 3c. Was the outcome defined and determined in a similar way for all participants? | 3d. Was the outcome determined without knowledge of predictor information? | III. Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marshall 201122 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Yes | Low risk |
| McCowan 201160 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Dommett 201364 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Keane 201462 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Fijten 199555 | No | Yes | Yes | High risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hodder 200577 | No | Unclear | Yes | High risk | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Yes | No | High risk |
| Kop 201561 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201353 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201354 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Collins 201215 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201280 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Collins 201388 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201182 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 2011c52 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Collins 201389 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201184 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Collins 201314 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201232 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Collins 201391 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201285 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 201586 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Shephard 201568 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Shephard 201269 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Stapley 201772 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 200787 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 200581 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 200974 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Stapley 201371 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 2005b51 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 2009b83 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Stapley 201270 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 200690 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Walker 201373 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Shephard 201667 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Shephard 201366 | Yes | Yes | Yes | Low risk | Yes | Unclear | Yes | Yes | Unclear risk | Yes | Yes | Yes | Yes | Low risk |
| Iyen-Omofoman 201321 | Yes | Yes | Yes | Low risk | Yes | Unclear | Yes | Yes | Unclear risk | Yes | Yes | Yes | Yes | Low risk |
| Elias 201750 | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Nørrelund 199657 | Yes | Yes | Yes | Low risk | Yes | Unclear | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Unclear | Low risk |
| Muris 199556 | Yes | Unclear | Yes | Unclear risk | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Low risk |
| Study | 4a. Were there a reasonable number of outcome events? | 4b. Was the time interval between predictor assessment and outcome determination appropriate? | 4c. Were all enrolled participants included in the analysis? | 4d. Were participants who were missing data handled appropriately? | IV. Sample size and participant flow | 5a. Were non-binary predictors handled appropriately? | 5b. Was selection of predictors based on univariable analysis avoided? | 5c. Was model overfitting (optimism in model performance) accounted for, for example using bootstrapping or shrinkage techniques? | 5d. Were any complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | 5e. Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | 5f. For the model or any simplified score, were relevant performance measures evaluated, for example calibration, discrimination, (re)classification and net benefit? | 5g. Was the model recalibrated or was it likely (based on the evidence presented, e.g. calibration plot) that recalibration was not needed? | V. Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marshall 201122 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| McCowan 201160 | No | Yes | No | Yes | High risk | Yes | No | No | Yes | Yes | Yes | No | High risk |
| Dommett 201364 | Yes | Yes | Yes | Yes | Low risk | Yes | No | Unclear | Yes | Yes | Yes | Unclear | Unclear risk |
| Keane 201462 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Fijten 199555 | Unclear | Yes | Yes | Yes | Unclear risk | Yes | No | No | Yes | Yes | Yes | No | High risk |
| Hodder 200577 | Yes | Yes | Yes | Yes | Low risk | Unclear | No | No | Yes | Yes | Yes | No | Unclear risk |
| Kop 201561 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear risk |
| Hippisley-Cox 201353 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201354 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Collins 201215 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201280 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Collins 201388 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201182 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201152 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Collins 201389 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201184 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Collins 201314 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201232 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Collins 201391 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hippisley-Cox 201285 | Yes | Yes | Yes | Yes | Low risk | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Hamilton 201586 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Shephard 201568 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Shephard 201269 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Stapley 201772 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Hamilton 200787 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Hamilton 200581 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Hamilton 200974 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Stapley 201371 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Hamilton 200551 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Hamilton 200983 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Stapley 201270 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Hamilton 200690 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | Unclear | Yes | Yes | No | Unclear | High risk |
| Walker 201373 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Shephard 201667 | Yes | Yes | Yes | Unclear | Unclear risk | Yes | No | No | Yes | Yes | No | No | High risk |
| Shephard 201366 | Yes | Yes | Yes | Unclear | Unclear risk | Unclear | No | No | Unclear | Yes | No | No | High risk |
| Iyen-Omofoman 201321 | Yes | Yes | Yes | Unclear | Unclear risk | Unclear | No | No | Unclear | Yes | No | No | High risk |
| Elias 201750 | Yes | Unclear | No | Unclear | Unclear risk | Unclear | N/A | N/A | N/A | N/A | Yes | Unclear | Unclear risk |
| Nørrelund 199657 | Yes | Yes | Unclear | Unclear | Unclear risk | Unclear | Yes | No | No | No | Yes | No | High risk |
| Muris 199556 | No | Yes | Unclear | Unclear | Unclear risk | Yes | No | No | Unclear | Yes | No | No | High risk |
| Study | Prediction tool | Cancer type(s) | Country | Population |
|---|---|---|---|---|
| Adelstein 2010204 | No name (multiple variable model for CRC) | Colorectal | Australia | Patients referred for colonoscopy, NSW Australia |
| Baicus 2006205 | No name (generic cancer) | Multiple | Romania | Patients admitted to a hospital in Romania (January–September 2003) with involuntary weight loss |
| Ewing 2016206 | No name (Swedish – non-metastatic CRC) | Colorectal | Sweden | Patients with cancer |
| Galvin 2014207 | Prediction rule for breast cancer | Breast | Ireland | Prospective cohort of consecutive patients with breast cancer symptoms reviewed at the symptomatic breast units in Beaumont hospital (Ireland) |
| Khademi 2012208 | No name (risk-prediction model for upper GI cancer) | GI | Islamic Republic of Iran | Patients diagnosed with dyspepsia and referred to a tertiary referral gastroenterology clinic in Tehran (Islamic Republic of Iran) from 2002 to 2009 |
| Moore 2011209 | Risk of Ovarian Malignancy Algorithm | Ovarian | USA | Premenopausal and postmenopausal women aged ≥ 18 years presenting to a generalist (defined as a general gynaecologist, internist, family practitioner gastroenterologist or general surgeon) with an ovarian cyst or an adnexal mass and subsequently scheduled to undergo surgery |
| Saraiva 2016210 | No name (uses of AI to aid diagnosis of GI cancers) | GI | Brazil | Patients with GI cancer |
| Reeves 2003211 | BREASTAID | Breast | USA | Referred and follow-up patients |
| Robertson 2006212 | No name | Colorectal | UK | Patients referred with rectal bleeding |
| Simpkins 2017213 | No name | Colorectal | USA | Patients newly referred with GI symptoms from primary care to two secondary care centres |
| Koning 2015214 | No name | Colorectal | The Netherlands | Patients referred for colonoscopy |
| Kim 2009215 | No name | Ovarian | The Republic of Korea | All referred/screened patients |
| Høgdall 2011216 | No name | Ovarian | Denmark | Complex laboratory equipment required – Biomek® 2000 (Beckman Coulter Inc., Brea CA, USA); primary care availability unlikely |
| Study | Prediction tool | Cancer type(s) | Country | Comment |
|---|---|---|---|---|
| Bourne 201223 | BLINCK | Melanoma | Australia | Algorithm (clinical and dermatoscopic criteria) |
| Dolianitis 2005217 | Dermoscopic algorithms: seven-point checklist, the ABCD rule and the Menzies method218 | Melanoma | Australia | Algorithms |
| Emery 2010219 | PCSA | Melanoma | UK/Australia | Does not calculate risk of cancer (score) |
| Gerbert 2000220 | Decision support software | Melanoma | CA, USA | Algorithm (clinical and dermatoscopic criteria) |
| Rogers 2016221 | TADA | Melanoma | NY, USA | Algorithm (dermatoscopic criteria) |
| Walter 2012222 | MoleMate™ (MedX Health Corp., Mississauga, ON, Canada), seven-point checklist | Melanoma | UK | MoleMate is a ‘computerised diagnostic tool’ involving a novel imaging technique (SIA); seven-point checklist is an algorithm |
| Walter 2013223 | Original and weighted seven-point checklist | Melanoma | UK | Does not calculate risk of cancer (score) diagnosis of pigmented skin lesions (including melanoma) in primary care; based on RCT reported in Walter 2012,222 record 6175, currently included in SR1 |
| Hodder 200577 | Netherlands model; Harvard model; weighted numerical score | CRC | UK | Develop a system to compare and validate referral guidelines (is this SR2 external validation rather than SR1-impact assessment?) |
| Ballal 2010224 | Weighted numerical score | CRC | Wales (UK) | Does not calculate risk of cancer (score) external validation of score developed by Selvachandran 2002225 |
| Selvachandran 2002225 | Weighted numerical score | CRC | UK | Does not calculate risk of cancer (score) |
| Smith 2006226 | Weighted numerical score | CRC | UK | Does not calculate risk of cancer (score) |
| Zortea 2014227 | No name (computer image processing algorithm) | Melanoma | Germany, Norway | Image processing system for skin cancer detection |
| Grewal 2013228 | No name (scoring system derived from Hamilton 2009 et al.83 data) | Ovarian | UK | Scoring system derived from Hamilton 200983 data |
| Shahzad 2015229 | No name | Ovarian | Pakistan | Index score |
| Rossing 2010230 | No name | Ovarian | USA | Index score |
| Lim 2012231 | No name | Ovarian | UK | Scoring system |
| Law 2014232 | No name | Colorectal | Malaysia | Index score derived from Selvachandran 2002225 data |
| Study | Prediction model | Cancer type(s) | Country | Setting | Study design | Stage of development | Data source |
|---|---|---|---|---|---|---|---|
| Holtedahl 201863 | Prediction model for abdominal cancers | Abdominal [including all cancers of digestive organs, female genital organs, urinary organs (including testis)]. Other cancers were included in additional analyses if they were associated with abdominal signs or symptoms | Norway, Denmark, Sweden, Scotland, Belgium and the Netherlands | Primary care | Prospective cohort | Apparent performance | GP records |
| Study | Participants | Candidate predictors | Outcome to be predicted | Sample size | Number/handling of missing data |
|---|---|---|---|---|---|
| Holtedahl 201863 | 493 GPs recruited via Cancer and Primary Care Research International Network, February–July 2011 | Abdominal pain (lower and upper), constipation, diarrhoea, distended abdomen/bloating, increased belching/flatulence, acid regurgitation, rectal bleeding, unexpected genital bleeding, macroscopic haematuria, increased urinary frequency, other abdominal problems | Cancer diagnosis within 180 of GP survey. Diagnosis taken from GP records 8 months after GP survey. Study authors distinguished between abdominal and non-abdominal cancers | 61,802 patients, including 175 cases (0.28%) | Not reported |
| GPs recorded consultations over a period of 10 days for patients aged ≥ 16 years. If abdominal symptoms were mentioned in the consultation, specific symptom-related questions were asked using a pro forma | |||||
| GPs also identified all those with a diagnosis of abdominal cancer within 6 months after the GP survey, regardless of whether or not the patient attended during the survey period |
| Study | Model development | Model performance |
|---|---|---|
| Holtedahl 201863 | Cox proportional hazards models. Report univariate and multivariate analyses (for the most frequent symptoms and combinations of symptoms adjusted for sex) | Sensitivity, specificity, likelihood ratios and PPVs reported for individual symptoms and combinations of symptoms (by age group or sex) |
| Authors report no evidence to reject proportional hazards assumption | ||
| Main analyses included all patients with new abdominal cancer diagnosis within 180 days. Other analyses looked at all cancers, or abdominal cancers also beyond 180 days | ||
| 0.05 level of statistical significance used |
| Study | Model evaluation | Results | Interpretation and discussion |
|---|---|---|---|
| Holtedahl 201863 | Developmental data set only | HR (95% CI):
|
The strength of the association between abdominal symptoms and cancers highlights the importance of responding to these symptoms. But, as some new cancers did not involve these symptoms, clinical suspicion is needed |
| HRs (95% CIs) were also reported for combinations of symptoms – see table 4 of Holtedahl 201863 |
| Study | 1a. Were appropriate data sources used, e.g. cohort, RCT or nested case-control study data? | 1b. Were all inclusions and exclusions of participants appropriate? | 1c. Were participants enrolled at a similar state of health, or were predictors considered to account for differences? | I. Participant selection | 2a. Were predictors defined and assessed in a similar way for all participants in the study? | 2b. Were predictor assessments made without knowledge of outcome data? | 2c. Are all predictors available at the time the model is intended to be used? | 2d. Were all relevant predictors analysed? | II. Predictors | 3a. Was a prespecified outcome definition used? | 3b. Were predictors excluded from the outcome definition? | 3c. Was the outcome defined and determined in a similar way for all participants? | 3d. Was the outcome determined without knowledge of predictor information? | III. Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Holtedahl 201863 | Yes | Yes | Unclear | Unclear risk | Yes | Yes | Yes | Yes | Low risk | Unclear | Yes | Unclear | Yes | Unclear risk |
| Study | 4a. Were there a reasonable number of outcome events? | 4b. Was the time interval between predictor assessment and outcome determination appropriate? | 4c. Were all enrolled participants included in the analysis? | 4d. Were participants who were missing data handled appropriately? | IV. Sample size and participant flow | 5a. Were non-binary predictors handled appropriately? | 5b. Was selection of predictors based on univariable analysis avoided? | 5c. Was model overfitting (optimism in model performance) accounted for, for example using bootstrapping or shrinkage techniques? | 5d. Were any complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | 5e. Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | 5f. For the model or any simplified score, were relevant performance measures evaluated, for example calibration, discrimination, (re)classification and net benefit? | 5g. Was the model recalibrated or was it likely (based on the evidence presented, e.g. calibration plot) that recalibration was not needed? | V. Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Holtedahl 201863 | Yes | Unclear | No | Unclear | High risk | Unclear | Yes | Unclear | Yes | Yes | Yes | No | Unclear risk |
Appendix 4 Updated review: supplementary tables
| Author and year; country | Study aim | Patient population (n analysed) | Study design; [data sources] | Interval(s) definition (T interpretation), and how interval was analysed | Outcome measures | Comments and method of analysis | Addressed waiting time paradox? |
|---|---|---|---|---|---|---|---|
| Breast | |||||||
| Ángeles-Llerenas 2016;100 Mexico | To estimate the effect of care delivery delays on survival among women with breast cancer | Patients with histopathologically diagnosed breast cancer (2007–9) attending hospital for treatment (n = 854) |
|
|
Survival (from initiating treatment; 5 years’ follow-up) | Cox proportional risk model adjusting for, among others, stage and sign and symptoms. Analysis also stratified by stage | No evidence of this |
|
|||||||
| Murchie 2015;102 Scotland, UK | To explore whether or not longer provider delays (between first presentation and treatment) were associated with later stage and poorer survival in women with symptomatic breast cancer | Symptomatic patients diagnosed with breast cancer (1997–8) in Northern Scotland identified via linked data sets (n = 850) |
|
First presentation to primary care to first treatment or to definitive diagnosis (T9) |
|
|
No evidence of this |
| Weeks; continuous variable, and modelled as four periods: 4 (reference), 13, 26, and 39 corresponding to knots placed on distribution of interval | |||||||
| Pace 2015;103 Rwanda | To investigate the magnitude, impact of and risk factors for, patient and system delays in breast cancer diagnosis in Rwanda | Women who presented with a breast complaint to oncology clinic (2012–14) and abnormal breast examination findings (n = 144) |
|
|
Stage (advanced) | Multivariate ordinal logistic regression adjusting for clinical and demographic characteristics (did not incorporate symptoms or tumour grade) used to obtain OR (95% CI) (presented as a forest plot only) | No evidence of this |
|
|||||||
| Unger-Saldaña, 2015;104 Mexico | To determine the correlation between health system delay and clinical disease stage in patients with breast cancer |
|
|
‘Patient interval’: identification of the problem (via symptoms or screening) to first medical consultation (T3) ‘Health system interval’: first medical consultation to first treatment (T14) |
Stage (advanced) |
|
No evidence of this. Patients with delay of ≥ 50 months excluded |
| Months; continuous variable | |||||||
| Bladder | |||||||
| Bryan 2015;105 England, UK | To investigate the influence of tumour factors, patient factors, carcinogen exposure and pathway delays on the long-term outcome of urothelial bladder cancer | Patients newly diagnosed with urothelial bladder cancer (1991–2) in the West Midlands region (n = 1478) |
|
|
|
Cox regression used to identify factors associated with longer intervals, including, among others, stage and tumour size. Cox regression also used to assess predictors of poor survival, including delays, tumour stage, grade, and size | No evidence of this |
|
|||||||
| Colorectal | |||||||
| Aslam 2017;110 England, UK | To determine whether or not referral and diagnosis through the 2WW pathway confers a survival advantage on these patients | Patients diagnosed with CRC at local university hospitals (2005–12) and undergoing surgery (study included patients presenting via screening, but not in the analysis of OS; analysis also conducted excluding emergency presentations) (n = 2604) |
|
Referral to initial treatment (T12) | Survival (median OS) | Kaplan–Meier methods (log-rank test)
|
Excluded emergency presentations |
| Days; Kaplan–Meier curves developed for two groups: < 62 vs. > 62 days | |||||||
| Chen 2017;112 USA | To examine patient presentation, provider evaluation and time to diagnosis, which can affect stage and prognosis. Also compared the symptoms, clinical features, time to diagnosis and stage of young-onset (aged < 50 years) CRC with those of patients diagnosed at age ≥ 50 years | Patients seen at a cancer institute (2008–14), with pathologically confirmed CRC diagnosis; included two separate cohorts: those aged < 50 years and those aged ≥ 50 years (included emergency presentations) (n = 458) |
|
Symptom onset to diagnosis (defined as the sum of ‘symptom duration’ and ‘workup duration’) (T4) Symptom duration: symptom onset to initial visit (T1) Workup duration: first medical visit to date of pathological diagnosis (T8) |
Stage (III and IV) |
|
Did look at delay to diagnosis by CRC symptoms vs. non-specific symptoms, but do not account for this in the main analysis of delay vs. stage at diagnosis |
| Days; median intervals compared for advanced and non-advanced stage at diagnosis (but not assessed statistically) | |||||||
| Helewa 2013;106 Canada | To determine if increased wait times for CRC treatments resulted in worse survival outcomes. Secondary objectives: to determine predictors of longer waiting times and urgent presentations | Patients diagnosed with stage I–IV CRC, (2004–6) who underwent major surgical resections. Included emergency presentations, which were also analysed separately (n = 1628) |
|
|
Survival (5-year OS) |
|
Adjusted for emergency presentations |
|
|||||||
| Janssen 2016;114 Canada | To evaluate local wait times for colonoscopy in patients who were subsequently found to have CRC | Patients referred to and subsequently seen by the gastroenterologist and had a pathological diagnosis of CRC (2010–13). (Excluded emergency presentations and those seen as inpatients.) (n = 246) |
|
Primary care referral to definitive endoscopy (T11) | Node positivity, and presence of distant metastases | Assessed whether or not local wait times (from referral) were in accordance with guidelines, but also evaluated the effect of wait time on the presence of node positivity, and distant metastases at diagnosis. Compared the outcomes of patients who were seen within the guidelines with the outcomes of patients seen outside the guidelines using Fisher’s exact or chi-squared tests | Excluded emergency presentations and inpatients. Majority of patients had alarm symptoms at referral |
| Days; divided into 2 groups: < 60 vs. > 60 days | |||||||
| Leiva 2017;113 Spain | To determine the diagnostic intervals for the diagnosis of CRC by using three different sources of information and to analyse the relationship of CRC stage with the diagnostic interval according to the source of information | Patients diagnosed with CRC at one of nine public hospitals (2006–8) and registered with GP. (Included emergency presentation.) (n = 715) |
|
Diagnostic interval calculated using three different information sources: patient recall, hospital records and GP records For hospital and GP records, diagnostic interval defined as first registry of symptoms to diagnosis (T8) For patient-recall, diagnostic interval defined as onset of symptoms to diagnosis (T4) |
Stage (more advanced) |
|
No evidence of this. Main analyses of interest were unadjusted, and emergency presentations were included |
| Days; median interval estimated for each TNM stage (classified as 0, 1, 2, 3, 4, unknown), and cumulative distribution of the three intervals also plotted for four stage groupings: 0–I, II–III, IV, unknown | |||||||
| Murchie 2014;107 Scotland, UK | To explore if longer provider delays (time from presentation to treatment) were associated with more advanced stage disease at diagnosis and poorer survival | Symptomatic patients diagnosed with CRC (1997–8) in northern Scotland identified via linked data sets (data collected in 2000–1). (Included emergency presentation.) (n = 958) |
|
‘Provider delay: first presentation in primary care to treatment or definitive diagnosis (T9) |
|
|
Models adjusted for emergency admission, signs and symptoms |
| Weeks; continuous variable, and modelled as four periods: 4 (reference), 20, 40 and 60 corresponding to knots placed on distribution of interval | |||||||
| Patel, 2018;111 Scotland, UK | To determine the impact of compliance with the 62-day pathway on outcomes in patients with CRC in the long term | Patients treated for CRC at one of three district general hospitals (1999–2005). Included patients presenting electively or in an emergency setting (with patients who did not go on to have surgery within 72 hours of admission reclassified as elective). (n = 1012; 794 elective, 218 emergency) |
|
Initial GP referral to first treatment (T12) | Survival (mean OS from diagnosis based on ≥ 6 years’ follow-up); and stage (C or D vs. A or B) |
|
Excluded emergency presentations for survival outcomes |
| Days: dichotomised based on being seen within 62-day standard or not; divided into four groups: standard met (elective), standard met (emergency), standard failed (elective) and standard failed (emergency); also plotted separate Kaplan–Meier curves for ≤ 62 vs. > 62 days | |||||||
| Pita-Fernández 2016;109 Spain | To analyse the relationship between the interval from first symptom to diagnosis and survival in CRC | Patients diagnosed with CRC at a university hospital (1994–2000) (n = 942) |
|
‘Diagnostic delay’: onset of symptoms to diagnosis (biopsy or direct surgery) (T4) | Survival (5 years, from diagnosis) | Kaplan–Meier curves (log-rank test). Cox proportional hazards model with restricted cubic spline curves. Sequential adjustment for stage (initial model adjusted for age and sex). Stratified analysis colon and rectal | No evidence of this |
| Months; analysed as a continuous variable (using penalised splines), and quartiles: < 1.5, 1.5 to 3.4, 3.4 to 6.4, > 6.4 months, with Kaplan–Meier curves developed and compared for each quartile | |||||||
| Lung | |||||||
| Gildea 2017;118 USA | To assess the wait time to diagnose NSCLC and the cost of diagnosis and treatment based on the stage at diagnosis | Patients diagnosed with NSCLC (2007–11) with both medical and pharmacy benefits (health insurance) (n = 1210) |
|
First diagnostic test for lung cancer to a definitive diagnosis (T13) | Stage (IIIb–IV) |
Stratified by stage and analysed descriptively Study measured wait time to diagnosis and evaluated the cost of diagnosis and treatment based on stage of diagnosis. Assessed records for diagnostic testing in the 12 months prior to date of diagnosis Time to diagnosis from the initial diagnostic test was 5 months for stage IV patients vs. 6.2 months for stage I (mean ranged from 5 to 6.2 months for all stages). Multivariate regression analysis found that stage was the only significant independent predictor of total health-care cost (p < 0.001 for stage I vs. stage II, IIIa, IIIb, or IV) |
No evidence of this |
| Months; comparison of mean (SD) interval for each stage (I, II, IIIa, IIIb, IV); ‘long mean delay’: 5 or 6 months | |||||||
| Gonzalez-Barcala 2014;117 Spain | To analyse the delays in the diagnosis and treatment of lung cancer in our health area, the factors associated with the timeliness of care and their possible relationship with the survival of these patients | Patients with a cytohistologically confirmed diagnosis of lung cancer (2005–8) (n = 307) |
|
|
Survival (3 years’ follow-up) | Cox regression adjusted for sex, age, smoking status, stage, histology, comorbidity and general health status | No evidence of this |
|
|||||||
| Kim 2016;119 Canada | Aim to quantify diagnostic and treatment intervals and identify factors associated with delays | Patients with histologically confirmed stage I–III NSCLC diagnosed and treated in Alberta (2004–11) (n = 3009) |
|
|
Stage | Multivariable logistic regression was carried out to identify factors associated with delays (including, among others, stage and histological type). Stage III was one of the factors associated with prompt care | Adjusted analyses, but unclear if adjusted for relevant features to wait time paradox |
| Days; ‘delay’ defined as: T14 ≥ 78, T13 ≥ 38, T15 ≥ 51 days; estimated the adjusted odds of being in the delay category for each stage [stage I, II, III; reference = stage I] | |||||||
| Radzikowska 2013;115 Poland | To evaluate the influence on survival of delays in the diagnosis and treatment in an unselected population of SCLC patients | Patients admitted to pulmonary outpatients departments and registered on the National Tuberculosis and Lung Diseases Research Institute in Warsaw (1995–8). Survival data collected up until 2003 (n = 3479) |
|
‘Patient’s delay’: first symptoms to first visit to the GP (T1) ‘Doctor’s delay’; first visit to GP to diagnosis (T8) |
Survival (5 years from diagnosis) |
|
No evidence of this |
| Days; median used to divide into two groups: T1: ≤ 30 vs. > 30 days (shorter delays used as reference); T4: ≤ 42 vs. > 42 days (longer delay used as reference). [Note: ‘Doctor’s delay’ was inconsistently defined as time from first doctor/GP visit to diagnosis (T8) and treatment (T9); marked here as T8] | |||||||
| Živković 2014;116 Montenegro | To investigate whether or not waiting times and delays in diagnosis and treatment of patients with lung cancer have any bearing on prognosis and survival | Patients diagnosed and treated for lung cancer at a single hospital (2009) (n = 206) |
|
|
Survival (1 year, from symptom onset) |
Survival analysed using Kaplan–Meier methods Assessed both ‘patient delay’ (T1) and ‘total delay’ (T4), which included patient and health-care delay (primary care ‘doctor’s delay I’ and specialist medical services delay ‘doctor’s delay II’) by comparing the cumulative survival for those seen within or after 8 weeks. There were no significant difference between the Kaplan–Meier curves for the assessment of either interval (p > 0.05) |
No evidence of this |
|
|||||||
| Lymphoma | |||||||
| Nikonova 2015;120 Canada | To describe the determinants of delays in diagnosis and treatment of patients with DLBCL and the impact of delays on clinical outcomes | Patients treated for DLBCL at a single cancer centre (2002–10) (n = 278) |
|
|
Survival (5-year OS/PFS rates) |
|
No evidence of this |
Weeks/months; categorised into three or four groups. T7: 0–1 week, > 1 week to 1 month, > 1 month; T14: 0–6 weeks, > 6 weeks to 3 months, 3–6 months, > 6 months. ‘Delay’ defined as: T7 > 6 months, T14 > 4 months
|
|||||||
| Multiple sites | |||||||
| Dregan 2013;108 England, UK | To evaluate diagnostic time intervals and consultation patterns after presentation with alarm symptoms, and their association with cancer diagnosis and survival |
|
|
First consultation with alarm symptom to diagnosis (T8) | Survival (from diagnosis; up to 4 years’ follow-up) |
|
No evidence of this |
| Days; divided into five groups: < 15, 15–90 (reference), 91–180, 181–365, and > 365 days | |||||||
| Redaniel 2015;101 England, UK | To assess the association between diagnostic interval (time from presentation in primary care to diagnosis) and 5-year survival, stratified by NICE-qualifying alert and non-alert symptom presentations | Patients diagnosed with CRC, breast cancer, lung cancer or prostate cancer who presented to a GP with a cancer symptom 1 year prior to diagnosis identified (1998–2009) in England. (Excluded patients diagnosed through emergency routes)(n = 22,051: CRC, 5912; breast cancer, 8639; lung cancer, 5737; prostate cancer, 1763) |
|
Presentation in primary care to diagnosis (T8) | Survival (5 years) | EHR (95% CIs) computed using a generalised linear model with a Poisson error structure. Separate multivariable models built for each cancer site adjusting for, among others, the effects of period of cancer plan implementation, Dukes’ stage, tumour subsite (for CRC) and tumour differentiation. Also included stratified analysis by alert vs. non-alert symptoms | No evidence of adjusting for symptoms |
|
|||||||
| Myeloma | |||||||
| Goldschmidt 2016;121 Israel | To assess whether or not clinical and laboratory data could provide early clues to multiple myeloma diagnosis and whether or not time to detection affects survival | Patients diagnosed with multiple myeloma (2002–11), and matched cancer-free controls presenting with back pain, and who were insured in one of the Israeli health organisations (HMOs) (n = 107) |
|
First signs and symptoms of myeloma (‘combination’ of symptoms and laboratory results) to time of diagnosis (T8) |
|
|
No evidence of this |
| Months; mean and categorised into three groups: < 2, 2–12 and > 12 months. Also compared Kaplan–Meier curves for the three groups | |||||||
| Neuroendocrine | |||||||
| Keizer 2016;122 the Netherlands |
Note that the data extraction for this study is based on an abstract as the inter-library loan for the full text is still outstanding To investigate the association of delay in diagnosis with stage, grade, symptoms, primary site of the tumour and survival in patients with well-differentiated (grade 1, grade 2) neuroendocrine tumour |
Patients referred to the NKI-AvL and Westfriesgasthuis (2003–9) (n = 227) | Unable to assess | Onset of first symptoms to diagnosis (T4) |
|
|
Unable to assess |
| Months | |||||||
| Oral | |||||||
| Alahapperuma 2017;123 Sri Lanka | To identify patient-linked delays between the time of first noticing symptoms and definitive diagnosis, and its association with stage at diagnosis of oral and pharyngeal carcinoma patients attending the National Cancer Institute | Patients with histologically confirmed carcinoma of the oral cavity or pharynx (n = 351) |
|
Symptoms’ onset to first seen by health-care practitioner: ‘patient-delay 1’ (T1) Time taken to reach a specialty centre after being referred: ‘patient-delay 2’ (T10) |
Stage [III–IV (vs. I–II)] |
|
No evidence of this |
| Months/weeks; divided into two groups based on presence or absence of ‘delay’ defined as: > 3 months for T1, > 2 weeks for T10 | |||||||
| Esmaelbeigi 2014;124 Islamic Republic of Iran | To study the situation of professional delays and stage on diagnosis of oral cancer among patients who were hospitalised in the Cancer Institute hospital, the largest referral centre which treats head and neck cancer in the Islamic Republic of Iran | Oral cancer patients admitted to the Cancer Institute (2009–10) (n = 206) |
|
Onset of symptoms to initiation of treatment (T5) | Stage (advanced) |
Multivariate logistic regression used to obtain OR (95% CI) adjusted for a number of patient characteristics (including symptoms) and grade Also assessed predictors of patient (onset of symptoms to first visit to physician) and professional (initial investigation symptoms to treatment) delay in diagnosis and treatment of oral cancer. Assessed association between ‘total delay’ (T5) and stage at diagnosis only |
Unclear if adjustment for symptoms addresses this to any extent |
| Days; stratified into two groups based on median, with higher than median (140 days) classified as ‘delay’ | |||||||
| Ovarian | |||||||
| Altman, 2017;126 Canada | To analyse data on time to diagnosis and correlate this with OS | All invasive epithelial ovarian cancer cases (diagnosed between 2004 and 2010) identified via the Manitoba Cancer Registry. Included emergency presentations and some patients whose cancer was identified incidentally (both presentation types adjusted for in analysis) (n = 601) |
|
First presentation (contact with any health-care provider as a result of any symptoms related to epithelial ovarian cancer) to diagnosis (T8) | Survival (from diagnosis; at ≥ 4 years’ follow-up) |
|
Analysis adjusted for various stage, morphology, symptoms at first presentation, and emergency presentation |
| Days; continuous variable | |||||||
| Lim 2016;125 UK | To compare time to diagnosis of the typically slow-growing type I (low-grade serous, low-grade endometrioid, mucinous, clear cell) and the more aggressive type II (high-grade serous, high-grade endometrioid, undifferentiated, carcinosarcoma) invasive epithelial ovarian cancer | Patients diagnosed with epithelial ovarian cancer (2006–8) recruited before definitive diagnosis or treatment. (Included emergency presentations.) (n = 227) |
|
First symptom to presentation (T1) First presentation to primary care to diagnosis (T8) |
Stage (advanced) |
|
No evidence of this |
| Months; categorised into four: 0 to < 3, ≥ 3 to < 6, ≥ 6 to < 9, ≥ 9 months | |||||||
| Pancreatic | |||||||
| Gobbi 2013;127 Italy | To investigate the prognostic role of diagnostic delay and clinical presentation (regarding pain, jaundice, and weight loss) in pancreatic carcinoma | Newly diagnosed and treated patients with pancreatic cancer (2001–10) (n = 170) |
|
Onset of the first symptom(s) to pathological diagnosis (T4) | Survival (cumulative; from diagnosis) |
Kaplan–Meier methods (log-rank test). Multiple regression applied to survival according to the Cox proportional hazards model. Covariates included stage (stage I–II vs. stage III–IV), among others Diagnostic delay was one of the factors with a significant and independent prognostic value; stage was not. There was a significant different between the Kaplan–Meier curves for different thresholds (p < 0.0001), with longer delays associated with poor survival |
No evidence of this |
| Weeks; continuous variable, also plotted separate Kaplan–Meier curves for three thresholds: < 4, 4–6 and > 16 weeks | |||||||
| Jooste 2016;128 France | To estimate patient and treatment delays in patients with pancreatic cancer and to measure their association with survival in a non-selected population | Patients diagnosed with pancreatic cancer for the first time (2009–11) and registered in two French digestive cancer registries. (Included emergency presentations.) (n = 298) |
|
|
Survival (3-year survival based on net survival) |
|
|
Months/days; patient and treatment delays were categorised into two: T1 < 1 or ≥ 1 month; T9 < 29 or ≥ 29 days. Overall delay (T5) analysed as four categories based on the ‘patient’ and ‘treatment’ delay categories:
|
|||||||
| Penile | |||||||
| Gao 2016;129 China | To ascertain risk factors resulting in delayed treatment-seeking and to evaluate its influence on prognosis | Patients with a histopathologic diagnosis and treated for penile cancer (2004–10) (n = 228) |
|
Symptom onset to first medical consultation (T1) |
|
|
No evidence of this |
| Months; divided into four categories: ≤ 1 [‘undelayed’ reference], 1–3, 3–6, > 6 months | |||||||
| Prostate | |||||||
| Bonfill 2015;130 Spain | To determine the clinical characteristics of prostate cancer patients, the diagnostic process and the factors that might influence intervals from consultation to diagnosis and from diagnosis to treatment | Symptomatic and asymptomatic patients diagnosed and treated for prostate cancer at one of seven tertiary care hospitals (2010–11) (n = 470) |
|
|
Stage (T2a–T4; reference: T1a–T1c) | Multivariate analysis also used to investigate association between intervals and diagnostic characteristics (potential predictors); no significant association between the interval groups and stage found in univariate analysis for T8 or T15. EMPARO-CU study | Unlikely. Measured number of symptoms, but results based on univariate analyses |
| Days; dichotomised: T8 ≤ 100 vs. > 100 days; T15 ≤ 30 vs. > 30 days | |||||||
| Sarcoma | |||||||
| De Boer 2014;133 Uganda | To measure the association between primary delay and the cancer stage of HIV Kaposi’s sarcoma patients on diagnosis of the disease by a clinician | HIV-infected adults with histologically-confirmed Kaposi’s sarcoma treated at a cancer institute (2012) (n = 161) |
|
Symptom onset to presentation to a clinician (any health professional) (T1) | Stage (poor-risk stage) | Multivariable logistic regression used to estimate OR of having poor-risk stage vs. low-risk stage at presentation, adjusted for age, sex, income, and exposure to antiviral therapy. Also evaluated factors associated with delay | No evidence. Measured pain. But it was not included in multivariate analysis |
| Months; dichotomised: ≤ 3 vs. > 3 months | |||||||
| Goedhart 2016;132 the Netherlands | To investigate delay in diagnosis by both patients and doctors, and to evaluate its effect on outcomes of high-grade sarcoma of bone | Patients with high-grade bone sarcoma diagnosed (2000–12) at a single oncological centre (n = 102) |
|
‘Total delay’: initial symptoms to histopathological diagnosis (T4) ‘Referral clinic doctor-related delay’: presentation to oncology centre and histopathological diagnosis (T11) |
|
|
No evidence of this |
| Months/days; dichotomised: T4 < 4 vs. ≥ 4 months; T11 ≤ 42 vs. > 42 days | |||||||
| Urakawa 2015;131 Japan | To determine whether or not the duration from initial symptoms to specialist consultation affects the prognosis for soft-tissue sarcoma | Patients with primary soft-tissue sarcoma seen at a single specialist hospital (2001–11). Excluded patients seen within 1 month (most were second-opinion patients) (n = 142) |
|
Onset of symptoms to specialist consultation (T3) | Survival (5-year OS rate; from first seen by specialist) |
Kaplan–Meier methods (log-rank test). Cox proportional hazard methods adjusting for multiple covariates, including, among others, tumour size, depth, and histological grade used to obtain HR (95% CIs) Data on delay available for 142 patients with soft-tissue sarcoma, of whom 142 had non-small cell sarcoma and were included in survival analysis |
Excluded patients seen within 1 month (most were second-opinion patients) |
| Months; divided into two groups: < 6 vs. ≥ 6 months (reference); also plotted separate Kaplan–Meier curves | |||||||
| Testicular | |||||||
| Kobayashi 2014;134 Japan | To clarify the effect of the time to consultation on disease extension and survival | Patients who were treated for testicular germ cell tumours at a cancer centre (1991–2010) (n = 175) |
|
Symptom onset to first medical consultation at cancer centre or associated institution (no appointment needed, so system issues were absent) (T1) |
|
|
No evidence of this |
| Months; divided into two groups: < 6 vs. ≥ 6 months (reference); also plotted separate Kaplan–Meier curves | |||||||
| Study | Outcome | Time intervala | Association | Outcome description | Statistic test/method of analysis | Results |
|---|---|---|---|---|---|---|
| Aslam 2017110 | Survival | T12 | <> | Median OS | Log-rank test comparing median survival from Kaplan–Meier curves. The study also included patients referred via screening, survival analysis limited to emergency, 2WW, urgent and routine referrals. Analysis also conducted excluding emergency | There was no significant difference in overall survival between the two interval groups (for 2WW, urgent, and routine referrals combined): < 62 days group: 7.1 years vs. > 62 days group: 6.54 years (p = 0.620) |
| Chen 2017112 | Stage | T4 | – Aged < 50 years | TNM stage: advanced (stage III–IV) vs. non-advanced (stage I–II) | Median interval (days) from symptom onset and/or workup to diagnosis were compared for advanced and non-advanced stage at diagnosis (but not statistically) within each age group | The authors noted that the longest time to diagnosis (T4) was observed in patients aged < 50 years with non-advanced CRC at diagnosis (stage I and II) followed by patients aged < 50 years with advanced CRC at diagnosis (stages III and IV) (median days 174 vs. 124)
|
| T1 | – Aged < 50 years | |||||
| T8 | <> | |||||
| Dregan 2013108 | Survival | T4 | <> | Overall mortality (from diagnosis) | Adjusted HR (95% CI) obtained from Cox regression model (adjusted for age and sex). (Also analysed risk of death according to presence or absence of alarm symptoms) |
|
| Patients with no preceding alarm symptoms had shorter survival from diagnosis than those presenting with relevant alarm symptoms | ||||||
| Helewa 2013106 | Survival | T9 | <> | 5-year OS | Adjusted HR (95% CI) and p-values obtained from multivariate Cox proportional hazards model using bootstrap resampling. Important confounders adjusted for included, among others, stage, emergency presentations and location. Emergency presentations were also analysed separately. Also conducted analysis limited to stage I–III CRC (excluding stage IV and ‘unknown’) | Increased total wait time quartile was not associated with worse survival (p = 0.4898)
|
| When total wait time was considered as a continuous variable (days), it was still not associated with worse survival (p = 0.2618) | ||||||
| Janssen 2016114 | Stage (advanced) | T11 | <> | Node positivity, and presence of distant metastases | Chi-squared tests |
|
| Leiva 2017113 | Stage (advanced) | T8 |
|
TNM tumour stage (0, 1, 2, 3, 4, unknown) | The Mann–Whitney U-test and the Kruskal–Wallis test were used to assess association between diagnostic intervals and various patient and clinical characterises (including, among others stage, emergency presentation, location, symptoms at presentation, and perceived seriousness of symptoms). The independent effect of variables was assessed using Cox regression and then the extension of the multivariate Cox proportional hazards model with time-dependent covariates |
|
| T4 | <> (patient) | TNM tumour stage (0, 1, 2, 3, 4, unknown) | As above |
|
||
| Murchie 2014107 | Stage (advanced) | T9 | <> | Dukes’ stage was collapsed into a binary variable: early (A or B) vs. advanced (C or D) | OR (99% CI) obtained from logistic regression model with a restricted cubic spline (to allow for non-linear relationship), following sequential adjustment (using 1–4 models; 1 representing unadjusted) of patient and tumour factors (including, among others, tumour grade, and signs and symptoms). Time to treatment was modelled using four knots corresponding to the 25th, 50th, 75th and 100th centile |
|
| Murchie 2014107 | Survival | T9 | <> | All-cause survival (from date of first presentation) | HR (99% CI) obtained from Cox survival models, both with a restricted cubic spline (to allow for non-linear relationship), following sequential adjustment (1–4 models) of patient and tumour factors (including, among others, tumour grade, emergency admission, and signs and symptoms). Time to treatment was modelled using four knots corresponding to the 25th, 50th, 75th and 100th centile. Stratified analysis also conducted for rectal and colon cancer | The spline curves showed a significant non-linear (p < 0.001) (and unadjusted inverse, p < 0.001) association between provider delay and mortality. In the univariate analysis, diagnostic interval of < 4 weeks was associated with poor survival, but this was no longer present after adjusting for confounders. According to the fully adjusted model, provider delays of 40 and 60 weeks were associated with later-stage disease at presentation, but the OR did not reach statistical significance
|
| Patel 2018111 | Survival | T12 | – | Long-term (mean) survival | Proportion of patients still alive at long-term follow-up (October 2011), and log-rank test comparing Kaplan–Meier curves. Also assessed 30-day postoperative mortality and cause of death |
|
| Patel 2018111 | Stage | T12 | – | Stage: Dukes’ A–B vs. C–D | Proportions compared using chi-squared test |
|
| Pita-Fernández 2016109 | Survival | T4 |
|
5-year mortality (after diagnosis) | The influence survival was analysed in two ways:
|
|
| Pita-Fernández 2016109 | Stage (advanced) | T4 | <> | TNM tumour stage (I, II–III, IV) | The association between the interval (categorised into quartiles) and tumour stage analysed using multivariate logistic regression (adjusting for age and sex) | The interval was not found to be significantly associated with stage at diagnosis [stage I: 3.6 months; stage II–III: 3.4 months; stage IV: 3.2 months (p = 0.728)], even after adjusting for age and sex |
| Pruitt 2013137 | Survival | T8 |
+ (Colon) <> (Rectal) |
All-cause mortality, and CRC-specific mortality | Cases (CRC deaths) and controls (deaths due to other causes or censored) were matched on survival time and the association of delay with death was examined using logistic regression. OR (95% CI) estimated using multivariate regression analysis adjusting for sociodemographic, tumour (histology, stage, grade, location), and treatment factors. Stratified analysis also conducted according to and stage (and presenting symptom in sensitivity analysis) |
|
| Pruitt 2013137 | Survival | T15 |
|
All-cause mortality, and CRC-specific mortality | OR (95% CI) estimated using multivariate regression analysis adjusting for sociodemographic, tumour (histology, stage, grade, location), and treatment factors. Stratified analysis also conducted according to and stage (and presenting symptom in sensitivity analysis) |
|
| Redaniel 2015101 | Survival | T8 | <> | 5-year survival | 5-year EHRs (95% CI) were computed using a generalised linear model with a Poisson error structure. Univariable and multivariable models built, for each cancer site, and stratified by the nature of the symptoms (NICE-qualifying alert vs. non-alert). Multivariable models controlled for, among others, the effects of period of cancer plan implementation, Dukes’ stage, tumour subsite (for CRC), and tumour differentiation | There was no evidence of an association between diagnostic interval and mortality. EHR based on multivariate analysis for all patients:
|
| The results showed significant lower mortality associated with longer diagnostic intervals for patients presenting with non-alert symptoms (EHR > 6 months vs. < 1 month: 0.85, 0.72 to 1.00; p = 0.049), whereas no significant associations were identified for patients presenting with NICE-qualifying alert symptoms | ||||||
| Tørring 201399 | Survival | T8 | – (Alarm symptoms, Q1);<> (Vague symptoms) | 5-year overall mortality |
|
|
| Tørring 2012135 | Survival | T8 |
–/+ (GP-based; Q1, Q4) <> (Patient-based; GP records-based) –/+ (combined; Q1, Q2) |
5-year overall mortality rate (after diagnosis) |
Data taken from three studies using different methods for identifying date of first presentation. Data analysed for each study separately and combined The data were modelled in two ways:The 5-year HR (95% CI) estimated as a function of the length of the diagnostic interval and adjusted for tumour site (colon/rectum), age and sex using proportional hazard Cox regression. In the analysis of the combined data, differences were also allowed for in study-specific baseline hazards. (Note that the GP record-based study is Tørring 2011136) |
GP-based study (n = 266) adjusted HRs for each quartile:
|
Patient-based study (n = 658) adjusted HRs for each quartile:
|
||||||
Record-based study (n = 319) adjusted HRs for each quartile:
|
||||||
Combined data (n = 1243) adjusted HRs for each quartile:
|
||||||
| The association between diagnostic interval and survival was the same for all three types of data: displaying a U-shaped association with decreasing and, subsequently, increasing mortality with longer diagnostic intervals (i.e. U-shaped association observed using three different data collection methods in different health-care systems and over different time periods) | ||||||
| Tørring 2011136 | Survival | T8 |
– (Alarm symptoms) <> (Vague symptoms) |
3-year overall mortality (after diagnosis) |
|
|
|
Results of the studies included in the updated review
The ‘abbreviated results’ presented in Table 53 represent those used to populate Table 11. The results provide a summary of the impact of ‘short intervals’ on outcome to correspond to the methods used in the original review.
| Study | Outcome | Time intervalb | Abbreviated results | Summary of results for studies reporting significant findings |
|---|---|---|---|---|
| Breast | ||||
| Ángeles-Llerenas 2016100 | Survival | T5 | <> | |
| Ángeles-Llerenas 2016100 | Survival | T1 | <> | |
| Ángeles-Llerenas 2016100 | Survival | T15 | <> | |
| Murchie 2015102 | Survival | T9 | – | Very short intervals – poor outcomes
|
| Murchie 2015102 | Stage (III–IV) | T9 | – | Very short intervals – poor outcomes
|
| Pace 2015103 | Stage (advanced) | T1 | + | Longer intervals – worse outcomes
|
| Pace 2015103 | Stage (advanced) | T8 | + | Longer intervals – worse outcomes
|
| Unger-Saldaña 2015104 | Stage (advanced) | T3 | + | Longer intervals – worse outcomes
|
| Unger-Saldaña 2015104 | Stage (advanced) | T14 | + | Longer intervals – worse outcomes
|
| Bladder | ||||
| Bryan 2015105 | Survival | T5 | <> | |
| Bryan 2015105 | Survival | T12 | <> | |
| Bryan 2015105 | Survival | T2 | <> | |
| Bryan 2015105 | Survival | T10 | <> | |
| Bryan 2015105 | Survival | T14 | <> | |
| Bryan 2015105 | Stage (advanced) | T5 | <> | |
| Bryan 2015105 | Stage (advanced) | T12 | <> | |
| Bryan 2015105 | Stage (advanced) | T2 | <> | |
| Bryan 2015105 | Stage (advanced) | T10 | <> | |
| Bryan 2015105 | Stage (advanced) | T14 | <> | |
| Bryan 2015105 | Tumour size (> 2 cm) | T14 | – | Longer intervals – better outcomes
|
| Bryan 2015105 | Tumour size (> 2 cm) | T12 | – | Longer intervals – better outcomes
|
| Colorectal | ||||
| Aslam 2017110 | Survival | T12 | <> | |
|
|
|
– (aged < 50 years) | Shorter intervals – worse outcomesAmong younger patients, those with advanced stage (stage III–IV) had shorter symptom and workup durations than early stage (stage I-II) CRC
|
|
|
|
– (aged < 50 years) | Shorter intervals – worse outcomesPatients with advanced stage had shorter symptom durations than early stage
|
| Chen 2017112 | Stage (III–IV) | T8 | <> (aged < 50/≥ 50 years) | |
| Chen 2017112 | Stage (III–IV) | T4 | <> (aged ≥ 50 years) | |
| Chen 2017112 | Stage (III–IV) | T1 | <> (aged ≥ 50 years) | |
| Helewa 2013106 | Survival | T9 | <> | |
| Janssen 2016114 | Node positivity, and presence of distant metastases | T11 | <> | |
| Leiva 2017113 | Stage (more advanced) | T8 | – (hospital) | Longer intervals – better outcomes
|
| <> (GP) | ||||
| Leiva 2017113l | Stage (more advanced) | T4 | <> | |
| Murchie 2014107 | Stage (advanced; C–D) | T9 | <> | |
| Murchie 2014107 | Survival | T9 | <> | |
| Pita-Fernández 2016109 | Survival | T4 | <> | |
| Patel 2018111 | Survival | T12 | – | Longer intervals – better outcomes
|
| Patel 2018111 | Stage | T12 | – | Longer intervals – better outcomes
|
| Multisite | ||||
| Urinary tract | ||||
| Dregan 2013108 | Survival | T8 | + | Longer intervals – worse outcomes
|
| Lung | ||||
| Dregan 2013108 | Survival | T8 | <> | |
| Gastro-oesophageal | ||||
| Dregan 2013108 | Survival | T8 | <> | |
| Colorectal | ||||
| Dregan 2013108 | Survival | T8 | <> | |
| Prostate | ||||
| Redaniel 2015101 | Survival | T8 | – | Longer intervals – better outcomesProstate cancer mortality was lower in patients with longer diagnostic intervals, regardless of type of presenting symptom
|
| Lung | ||||
| Redaniel 2015101 | Survival | T8 | – | Longer intervals – better outcomes
|
| Colorectal | ||||
| Redaniel 2015101 | Survival | T8 | <> | |
| Breast | ||||
| Redaniel 2015101 | Survival | T8 | <> | |
| Lung | ||||
| Gildea 2017118 | Stage (IIIb–IV) | T13 | <> (NSCLC) | |
| Gonzalez-Barcala 2014117 | Survival (3 years’ follow-up) | T14 | – | Longer intervals – better outcomes
|
| Gonzalez-Barcala 2014117 | Survival (3 years’ follow-up) | T15 | – | Longer intervals – better outcomes
|
| Gonzalez-Barcala 2014117 | Survival (3 years’ follow-up) | T3 | <> | |
| Gonzalez-Barcala 2014117 | Survival (3 years’ follow-up) | T11 | <> | |
| Kim 2016119 | Stage (II, III vs. I) | T13 | – (NSCLC) | Shorter intervals – worse outcomes
|
| Kim 2016119 | Stage (III vs. I) | T15 | – (NSCLC) | Shorter intervals – worse outcomes
|
| Kim 2016119 | Stage (III vs. I) | T14 | – (NSCLC) | Shorter intervals – worse outcomes
|
| Radzikowska 2013115 | Survival | T1 | <> (SCLC) | |
| Radzikowska 2013115 | Survival | T8 | – (SCLC) | Shorter intervals – worse outcomes
|
| Živković 2014116 | Survival | T1 | <> | |
| Živković 2014116 | Survival | T4 | <> | |
| Lymphoma | ||||
| Nikonova 2015120 | Survival | T7 | <> | |
| Nikonova 2015120 | Survival | T14 | <> | |
| Myeloma | ||||
| Goldschmidt 2016121 | Survival | T8 | <> | |
| Goldschmidt 2016121 | Stage | T8 | <> | |
| Neuroendocrine | ||||
| Keizer 2016122 | Survival | T4 | <> | |
| Keizer 2016122 | Stage | T4 | <> | |
| Oral | ||||
| Alahapperuma 2017123 | Stage [III–IV (vs. I–II)] | T1 | + | Longer intervals – worse outcomes
|
| Alahapperuma 2017123 | Stage [III–IV (vs. I–II)] | T10 | <> | |
| Esmaelbeigi 2014124 | Stage (advanced) | T5 | + | Longer intervals – worse outcomes
|
| Ovarian | ||||
| Altman 2017126a | Survival | T8 | + (after 30 days) |
|
| Lim 2016125 | Stage (advanced) | T1 | <> (type I and II) | |
| Lim 2016125 | Stage (advanced) | T8 | <> (type I and II) | |
| Pancreatic | ||||
| Gobbi 2013127 | Survival | T4 | + |
Longer intervals – worse outcomes Time to diagnosis (weeks) was a significant and independent prognostic factor: HR 1.02, 95% CI 1.01 to 1.04. There was a significant difference between the Kaplan–Meier curves for different thresholds (p < 0.0001): < 4, 4–6 and > 16 weeks |
| Jooste 2016128 | Survival | T5 | <> | |
| Penile | ||||
| Gao 2016129 | Survival | T1 | + (2-year OS) | Longer intervals – worse outcomes
|
| <> (5-year OS) | ||||
| Gao 2016129 | Stage (T1b–T4 vs. Tis/Ta/T1a) | T1 | + | Longer intervals – worse outcomes
|
| Gao 2016129 | Tumour size (> 2 cm) | T1 | + | Longer intervals – worse outcomes
|
| Gao 2016129 | Metastases (M1) | T1 | + | Longer intervals – worse outcomes
|
| Gao 2016129 | Lymph node involvement (N1–3) | T1 | – |
Longer intervals – worse outcomes Patients with a delay of > 6 months had significant risks for higher positive rate of regional lymph nodes (compared with patients with a delay of ≤ 1 month) |
| Prostate | ||||
| Bonfill 2015130 | Stage (T2a–T4; reference T1a–T1c) | T8 | <> | |
| Bonfill 2015130 | Stage (T2a–T4; reference T1a–T1c) | T15 | <> | |
| Sarcoma | ||||
| De Boer 2014133 | Stage [poor-risk stage (vs. good risk)]c | T1 | + (HIV Kaposi’s sacoma) | Longer intervals – worse outcomes
|
| Goedhart 2016132 | Survival | T4 | <> | |
| Goedhart 2016132 | Survival | T11 | <> | |
| Goedhart 2016132 | Metastases | T4 | <> | |
| Urakawa 2015131 | Survival | T3 | – | Shorter intervals – worse outcomes
|
| Testicular | ||||
| Kobayashi 2014134 | Survival | T1 | + | Longer intervals – worse outcomes
|
| Kobayashi 2014134 | Stage (> II) | T1 | <> | |
| Kobayashi 2014134 | Size (larger; > 2 vs. ≤ 2 cm and > 5 vs. ≤ 5 cm) | T1 | + | Longer intervals – worse outcomes
|
These abbreviated results, however, represent a very simplistic interpretation of the study findings. Some studies assessed the interval as a categorical variable, whereby not all categories were associated with a significant or the same findings. Some studies evaluated the impact of ‘long intervals’ (often referred to as ‘delay’), which were converted to represent the impact of ‘short intervals’. A brief summary of the study findings are presented in the last column of Table 53 to show how our ‘abbreviated results’ were derived from the actual study results for these studies. It was not our intention to extract detailed results of included studies for non-CRC sites; therefore, the brief summaries relate mainly to the interpretation of studies reporting statistically significant findings.
Colorectal cancer studies
| Index relating to aim of study | Definition |
|---|---|
| Interval | Evaluate the influence of delay on patient outcomes (or association between interval and outcomes) |
| Delay | Describe the intervals (or identify delays). Includes comparison of intervals with recommended guidelines also |
| Progress | Changes in delay (intervals) over time |
| Symptoms | Evaluation of which specific symptoms are associated with delay |
| Causes | Identifying which factors are associated with delay (or determinants of delay). (Predictors of delay) |
| Prognostic factors | Identifying modifiable factors associated with patient outcomes (or identifying influence of various factors). (Predictors of stage/survival) |
| Pathway | Comparing different referral ‘type’ interventions or pathways, for example fast track vs. conventional; rapid/urgent referral vs. non-urgent |
| Note that studies were excluded from the updated review for which this was the primary aim, and for which the reference details in the EndNote library did not indicate that the study also evaluated the impact of the interval on patient outcomes (survival or stage) |
| Author and year; country | Study aim category | Study design | Setting | Study population | Participants sampled (n) | Recruited or records available (n) | Analysed (n) | Data source | Interval | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|---|
| Aslam 2017;110 the UK | Pathway; interval | Cohort study (unclear if retrospective or prospective) | Secondary care (single site) | Patients diagnosed with CRC within the University Hospitals of Leicester NHS Trust, between 2005 and 2012. Interval comparison in patients undergoing surgery. Study also included patients referred via screening (n = 275), but not included in survival analysis. Included emergency admission (n = 967), but also presented survival analysis excluding these patients | 3618 (47 missing) | 3571 (967 emergency presentation; 275 screening) | 2329 | Database maintained by the NHS trust on CRC outcomes | T12 | Survival |
| Chen 2017;112 the USA | Delay; causes; interval | Retrospective comparative cohort | Secondary care (single site) | Patients diagnosed with colorectal adenocarcinoma, confirmed by pathologists, and seen at the Stanford Cancer Institute between 2008 and 2014. Compared data of patients with young-onset CRC (diagnosed at an age < 50 years) with those patients diagnosed with CRC at an age of ≥ 50 years. Excluded patients with insufficient data, and, for cohort ≥ 50 years, those who were asymptomatic. First medical visit was with a primary care provider for 70% (320/458) of patients, and emergency department for 25% (113/458) | 1759 (382 diagnosed at age < 50 years; 1377 at age ≥ 50 years) | 354 for those aged < 50 years (101 with insufficient data); 1276 for those aged ≥ 50 years, from which a random sample of 436 was taken (95 screening age, 109 with insufficient data) | 455 (253 aged < 50 years; 232 aged ≥ 50 years) | Stanford Cancer Institute Research Database (clinical records) used to identify cases, and obtain data on interval and outcome | T4, T1, T8 | Stage |
| Dregan 2013;108 the UK | Symptoms; interval | Retrospective population-based cohort study | Primary care (multicentre) | Patients presenting with a specific alarm symptom (haematuria, haemoptysis, dysphagia or rectal bleeding) or diagnosed with the corresponding cancer (urinary tract, lung, gastro-oesophageal, or colorectal, respectively) recorded between 2002 and 2006. Excluded patients with incomplete data or discrepant dates | 5791 eligible (498 CRC) | 5791 (267 excluded; 47 for CRC) | 5524 (451 CRC) | Primary care records (UK CPRD) linked with Cancer Registry data | T4 | Survival |
| Helewa 2013;106 Canada | Interval; prognostic factors; delay | Retrospective population-based cohort study | Secondary care (multicentre) | Patients diagnosed with stage I–IV CRC, between 2004 and 2006, who underwent major surgical resections in Manitoba. Included emergency presentations (n = 262), which were also analysed separately. Patients for whom dates of index contact could not be determined were excluded, as were patients seen in emergency department | 2086 diagnosed (1628 underwent surgery) | Diagnostic interval (and total wait time): diagnostic interval + treatment wait) could be determined for only 1307 out of 1628 (80.3%); treatment wait for 1628 | Unclear (1628) | Population-based Manitoba Cancer Registry (linked with other administrative databases) | T9 | Survival (adjusted for stage and emergency presentations) |
| Janssen 2016;114 Canada | Delay; interval | Retrospective chart review | Secondary care (single site) | Patients referred to, and subsequently seen by, a gastroenterologist at St. Paul’s Hospital, Vancouver, and had a pathological diagnosis of CRC, between 2010 and 2013. Patients with a mass on rectal/physical examination or a high suspicion of malignancy based on diagnostic imaging at the time of referral were excluded | 592 | 246 met inclusion criteria, but node positivity and distant metastases could not be determined in 13 patients | Unclear (246) | Patients identified via hospital pathology database and then cross-referenced against EMR to select those seen by the gastroenterologists (n = 10) at the hospital | T11 | Stage |
| Leiva 2017;234 Spain | Delay; interval; causes | Retrospective population-based multicentre cross-sectional study | Primary and secondary care (multicentre) | Consecutive series of symptomatic patients diagnosed with CRC at one of nine public hospitals from 2006 to 2008 and registered with GP | 795 | Interval data available via: interviews for 715 (61 not interviewed, 19 no onset date), hospital records for 637, and GP records for 316 | 715 for ‘patient recall’, 637 for ‘hospital records’ and 316 for ‘GP records’ | Cases of CRC identified from pathology services. Data on intervals identified using three different sources of information:
|
T4, T8 | Stage |
| Murchie 2014;107 the UK (Scotland) | Interval; prognostic factors | Retrospective population-based cohort study | Primary and secondary care (multicentre) | Symptomatic patients diagnosed with CRC between 1997 and 1998 in northern Scotland identified via CRUX data set (numbers not stated). Those with signs and symptoms > 2 years prior to treatment were excluded | NR | Unclear | 958 for survival; 868 stage | Linked data from four data sets: CRUX data sets (primary care data), Scottish Cancer Registry (for tumour stage and grade), the Scottish Death Registry and the acute hospital discharge (SMR01) data set | T9 | Survival and stage (adjusted for tumour grade, emergency admissions, and signs and symptoms) |
| Pita-Fernández 2016;109 Spain | Interval; delay; symptoms | Retrospective cohort study | Tertiary care (single site) | Patients diagnosed with CRC at the Complexo Hospitalario Universitario A Coruña (A Coruña, north-west Spain) between 1994 and 2000 | 1482 | Unclear; 942 with available data from clinical records to calculate interval | 942 | Interval data from clinical records with mortality data obtained from Galician Mortality | T4 | Survival (adjusted for stage) |
| Patel, 2018;111 the UK (Scotland) | Interval; delay | Retrospective cohort | Secondary care (multicentre) | Patients treated for CRC at one of three district general hospitals (1999–2005). Included patients presenting electively or in an emergency setting (with patients who did not go on to have surgery within 72 hours of admission reclassified as elective) (n = 1012) | 1672 (660 missing data) | 1012 (794 elective, 218 emergency) | 1012 | Patient records | T12 | Survival; stage |
| Pruitt 2013;137 the USA | Interval; delay | Retrospective population-based cohort study (matched case–control) | Secondary care (multicentre) | Older US adults (aged ≥ 66 years) with invasive colon or rectal cancer and full Medicare coverage were eligible. Patients with a relevant Mediacaid claim and CRC diagnosed between 1998 and 2005 were included. Excluded patients with pre-existing comorbidities, and patients presenting for emergency procedures | Total NR; 9663 not eligible | 10,663 eligible (excluded: 994 no symptom presentation claim; 979 no treatment) | 9669 for T8 (6702 colon, 2967 rectal) and 9684 for T15 (6698 colon, 2986 rectal) | An existing linkage of the 1998–2005 National Cancer Institute’s SEER programme data with 1997–2006 Medicare claims files from the Centers for Medicare and Medicaid Services | T8, T15 | Survival (adjusted for stage, grade, location; stratified by symptom, stage) |
| For T8, patientts without a claim for symptom presentation visit were excluded (presumed to be identified via screening), and for T15 those who did not have treatment within 12 days of diagnosis | ||||||||||
| Redaniel 2015;101 the UK (England) | Interval | Retrospective population-based cohort study | Primary care (multicentre) | Patients diagnosed with colorectal, breast, lung or prostate cancers who presented to a GP with a cancer symptom 1 year prior to diagnosis identified. Only English cancer patients diagnosed in the years covered by all four data sets (1998–2009) were included. (Excluded patients diagnosed through emergency routes) | 62,178 eligible patients (cancer diagnosis) on CPRD (45,766 linked to other databases) | 22,051 (48%) patients presented to a GP with a cancer symptom 1 year prior to diagnosis (5912 with CRC) | 22,051 (5912 CRC) | CPRD was linked to the NCDR and to the 2007 English IMD data sets. (NCDR captures data from the merged Cancer Registry, Hospital Episode Statistics and the ONS) | T8 | Survival (stratified by alert or non-alert symptom; and adjusted for stage, tumour differentiation, and subsite, among other factors) |
| Tørring 2013;99 Denmark | Interval; causes | Retrospective population-based cohort study | Primary care (multicentre) | Patients in the former Danish county of Aarhus with newly diagnosed colorectal, lung, melanoma skin, breast or prostate cancer during a 1-year period (2004–05), and whose GPs were involved in the diagnosis. (included emergency admission). (Note that the CRC cohort appears to be the same as is Tørring 2011136) | 1543 | 1376 involved GP diagnosis (GP did not participate for 248) | 1128 (268 CRC) | Consecutive cancer patients identified from the County Hospital Discharge Registry, and linked to historical database hosted at the Department of Clinical Epidemiology, Aarhus University Hospital (via civil registry number). Each patient’s GP was subsequently identified by linking the patient’s data to the Health Service Registry. Same sources used for data on covariates, interval, and death | T8 | Survival (adjusted; stratified by alarm/vague symptom, cancer) |
| Date of first presentation, symptoms, and date of diagnosis obtained via GP questionnaire (84% response rate) | ||||||||||
| Tørring 2012;135 the UK (n = 1) and Denmark (n = 2) | Interval | Three population-based cohort studies [one retrospective case–control studies (UK); and two prospective cohort studies] | Primary care (multicentre) | Analysed data from three previously described population-based studies in Denmark (n = 2) and the UK (n = 1). All newly diagnosed CRC patients aged > 39 years were included from each study. (Included emergency admission.) (Note that one of the studies, based on a GP questionnaire, is Tørring 2011136) | 1667 (361 for GP recall; 945 for ‘patient recall’; 361 GP records) |
|
1243 (266 GP-recall; 658 for patient recall; 319 GP records) |
|
T8 | Survival |
| Excludes 146 – GP not involved (37 GP recall; 79 for patient recall; 30 GP records) | ||||||||||
| Tørring 2011;136 Denmark | Interval | Prospective population-based cohort study | Primary care (multicentre) | All newly diagnosed CRC patients aged > 17 years during 1 year (1 September 2004 to 31 August 2005). Excluded patients whose GP was not involved in diagnosis. (Included emergency admission) | 363 | 326 involved GP diagnosis (GP did not participate for 58) | 268 |
|
T8 | Survival |
Is the sample representative of the relevant cancer patient population? The population may be quite specific, typified by age, stage, ethnicity or other factors.
Yes: only if this is clearly reported.
Can’t tell: if it’s reported in an ambiguous way.
Not reported: if it doesn’t say.
If none of the above – please qualify with free text (this may be a majority of studies).
Characteristics’ reportingWas the reporting of participant characteristics complete?
Yes: only if this is clearly and fully reported.
Can’t tell: is if it’s reported in an ambiguous way.
Not reported: if it doesn’t say.
If none of the above – please qualify with free text (this may be the majority of studies, e.g. those that give just age and sex).
Representativeness of participantsWere participants who participated (or whose data were used) representative of the sample from which they (or it) were sourced?
Yes: only if this is clearly and fully reported.
Can’t tell: is if it’s reported in an ambiguous way.
Not reported: if it doesn’t say.
Not applicable: if all of sample participated – for example database study.
If none of the above – please qualify with free text.
Bias minimisation in measurement of symptom durationWere steps taken (as stated by the investigators) to minimise and check for biases and inaccuracies introduced due to the method used for measurement of symptom duration?
Yes: if clear evidence of this, please list information as free text: MANDATORY.
No: if no evidence of this.
Can’t tell: if unclear (this includes where results may be reported but no mention in methods).
Independent variable assessmentWas the assessment symptom duration (explanatory variable) conducted independent of the assessment of the outcome variable?
Yes: if reported as done.
No: if clearly reported that same researcher did it.
Not reported: if it doesn’t say.
Not applicable: if method does not require this to be done, for example database study.
A priori definition of outcome variableWas the outcome variable specified/defined a priori?
Yes.
No.
Appropriate definition of outcome variableWas the outcome variable clearly defined?
Yes: for example type of stage, type of survival – not necessary to enter detail.
No: anything other than yes.
Multivariate analysisWas multivariate analysis conducted?
Yes.
No.
Prognostic adjustmentWas adjustment for important prognostic factors conducted as part of the analysis?
Yes: if clear evidence of this (e.g. performance status, age, smoking, comorbidity) please qualify with free text MANDATORY.
No: if no evidence of this.
Can’t tell: if unclear (this includes studies for which results may be reported but no mention in methods) please qualify with free text MANDATORY.
Outlier adjustment for symptom durationWas adjustment for outliers conducted as part of the analysis?
Yes: if clear evidence of this – please qualify with free text MANDATORY.
No: if no evidence of this.
Not applicable: for example if there were no symptom durations greater than 2 years for more quickly diagnosed cancers.
Can’t tell: if unclear (this includes studies for which results may be reported but no mention in methods) please qualify with free text MANDATORY.
Confounder adjustmentWas adjustment for confounders (identified in advance of the study) conducted as part of the analysis?
Yes: if clear evidence of this, please qualify with free text MANDATORY.
No: if no evidence of this.
Can’t tell: if unclear (this includes where results may be reported but no mention in methods) please qualify with free text MANDATORY.
| Study | Time intervala | Sample representativeness | Characteristics reporting | Representativeness of participants | Bias minimisation | Independent variable assessment | A priori definition | Appropriate definition | Multivariate analysis | Prognostic adjustment | Outlier adjustment | Confounder adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aslam 2017110 | T12 | Patients receiving surgery | NR | Yes | No | N/A | Yes | Yes | No | No | No | No |
| Chen 2017112 | T4, T1, T8 | Yes | Yes | Cannot tell | No | N/A | Yes | Yes | No (for outcome of interest) | No | No | No |
| Dregan 2013108 | T4 | Yes | Just age and sex | Yes | Used sensitivity analysisb | N/A | Yes | Yes | Yes | No | No | No |
| Helewa 2013106 | T9 | Patients receiving surgery | Yes | Cannot tell | No | N/A | Yes | Yes | Yes | Yes | No | Yes |
| Janssen 2016114 | T11 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | No | No | N/A | No |
| Leiva 2017113 | T8, T4 | Yes | Yes | Cannot tell | No | NR | Yes | Yes | Yes | Yes | No | Yes |
| Murchie 2014107 | T9 | Yes | Yes | Cannot tell | No | N/A | Yes | Yes | Yes | Yes | N/A | Yes |
| Patel 2018111 | T12 | Yes | Just age and ASA gradec | Cannot tell | No | N/A | Yes | Yes | No | No | No | No |
| Pita-Fernández 2016109 | T4 | Yes | Yes | Cannot tell | No | NR | Yes | Yes | Yes | No (age, sex and stage only) | No | Yes |
| Pruitt 2013137 | T8, T15 | Older Medicaid patients | Yes | Yes | Yesd | N/A | Yes | Yes | Yes | Yes (histology, grade, stage, location) | N/A | Yes |
| Redaniel 2015101 | T8 | Yes | Yes | Yes | Cannot tell | N/A | Yes | Yes | Yes | Yes | N/A | Yes |
| Tørring 2013101 | T8 | Yes | Yes | Yes (16% excluded) | No | N/A | Yes | Yes | Yes | Yes (age, sex and comorbidity only) | No | Yes |
| Tørring 2012135 | T8 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | No | Unclear | Yes |
| Tørring 2011136 | T8 | Yes | Yes | Yes | Unclear | N/A | Yes | Yes | Yes | No | Unclear | Yes |
| Study | Outcome | Time intervala | Association | Outcome description | Statistic test/method of analysis | Results |
|---|---|---|---|---|---|---|
| Aslam 2017110 | Survival | T12 | <> | Median OS | Log-rank test comparing median survival from Kaplan–Meier curves. The study also included patients referred via screening, survival analysis limited to emergency, 2WW, urgent, and routine referrals. Analysis also conducted excluding emergency | There was no significant difference in OS between the two interval groups (for 2WW, urgent, and routine referrals combined): < 62 days group: 7.1 years vs. > 62 days group: 6.54 years (p = 0.620) |
| Chen 2017112 | Stage | T4, T1, T8 | – (Aged < 50 years) | TNM stage: advanced (stage III–IV) vs. non-advanced (stage I–II) | The median interval (days) from symptom onset and/or workup to diagnosis was compared for advanced and non-advanced stage at diagnosis (but not statistically) within each age group | The authors noted that the longest time to diagnosis (T4) was observed in patients aged < 50 years with non-advanced CRC at diagnosis (stage I and II) followed by patients aged < 50 years with advanced CRC at diagnose (stages III and IV) (median days 174 vs. 124)
|
| – (Aged < 50 years) | ||||||
| <> | ||||||
| Dregan 2013108 | Survival | T4 | <> | Overall mortality (from diagnosis) | Adjusted HR (95% CI) obtained from Cox regression model (adjusted for age and sex). (Risk of death according to presence or absence of alarm symptoms was also analysed) |
|
| Helewa 2013106 | Survival | T9 | <> | 5-year OS | Adjusted HR (95% CI) and p-values obtained from multivariate Cox proportional hazards model using bootstrap resampling. Important confounders that were adjusted for included, among others, stage, emergency presentations and location. Emergency presentations were also analysed separately. An analysis limited to stage I–III CRC was also conducted (excluding stage IV and ‘unknown’) | Increased total wait time quartile was not associated with worse survival (p = 0.4898)
|
| When total wait time was considered as a continuous variable (days), it was still not associated with worse survival (p = 0.2618) | ||||||
| Janssen 2016114 | Stage (advanced) | T11 | <> | Node positivity, and presence of distant metastases | Chi-squared tests | Node positive in < 60 days group: 42/102 (41%) vs. > 60 days group: 52/144 (36%) (p = 0.42) |
| Distant metastases in < 60 days group: 12/102 (12%) vs. > 60 days group: 9/144 (6%) (p = 0.13) | ||||||
| Leiva 2017113 | Stage (advanced) | T8 | – (Hospital) | TNM tumour stage (0, 1, 2, 3, 4, unknown) | The Mann–Whitney U-test and the Kruskal–Wallis test were used to assess the association between diagnostic intervals and various patient and clinical characterises (including, among others, stage, emergency presentation, location, symptoms at presentation and perceived seriousness of symptoms). The independent effect of variables was assessed using Cox regression and then the extension of the multivariate Cox proportional hazards model with time-dependent covariates |
|
| <> (GP) | ||||||
| T4 | <> (patient) | TNM tumour stage (0, 1, 2, 3, 4, unknown) | As above |
|
||
| Murchie 2014107 | Stage (advanced) | T9 | <> | Dukes’ stage was collapsed into a binary variable: early (A or B) vs. advanced (C or D) | OR (99% CI) obtained from logistic regression model with a restricted cubic spline (to allow for non-linear relationship), following sequential adjustment (using 1–4 models; 1 representing unadjusted) of patient and tumour factors (including, among others, tumour grade, and signs and symptoms). Time to treatment was modelled using four knots corresponding to the 25th, 50th, 75th and 100th centile | The spline curves showed a significant non-linear (p = 0.04) (and unadjusted, p = 0.002) association between provider delay and stage. Delays of between 4 and 34 weeks were associated with earlier-stage disease, and intervals beyond this were associated with later-stage disease. However, the plot based on a fully adjusted model showed wide CIs |
According to the fully adjusted model, provider delays of 40 and 60 weeks were associated with later-stage disease at presentation, but the OR did not reach statistical significance
|
||||||
| Murchie 2014107 | Survival | T9 | <> | All-cause survival (from date of first presentation) | HR (99% CI) obtained from Cox survival models, both with a restricted cubic spline (to allow for non-linear relationship), following sequential adjustment (1–4 models) of patient and tumour factors (including, among others, tumour grade, emergency admission, and signs and symptoms). Time to treatment was modelled using four knots corresponding to the 25th, 50th, 75th and 100th centile. Stratified analysis also conducted for rectal and colon cancer | The spline curves showed a significant non-linear (p < 0.001) (and unadjusted inverse, p < 0.001) association between provider delay and mortality. In the univariate analysis, diagnostic interval of < 4 weeks was associated with poor survival, but this was no longer present after adjusting for confounders. According to the fully adjusted model, provider delays of 40 and 60 weeks were associated with later-stage disease at presentation, but the OR did not reach statistical significance
|
| Patel 2018111 | Survival | T12 | – | Long-term (mean) survival | Proportion of patients still alive at long-term follow-up (October 2011), and log-rank test comparing Kaplan–Meier curves; 30-day postoperative mortality and cause of death were also assessed | Long-term survival was greatest in elective patients who did not receive treatment > 62 days (52% alive), compared with elective cases treated ≤ 62 days (34% alive). This was supported by the log-rank analysis (p < 0.001) |
| Operative mortality was higher in patients treated ≤ 62 days (7% elective, 20% emergency) than in those who were treated > 62 days (4% elective, 7% emergency). The most common cause of death was CRC in all groups | ||||||
| Patel 2018111 | Stage | T12 | – | Stage (Dukes’ stage A–B vs. C–D) | Proportions compared using chi-squared test | Proportion of early-stage disease (Dukes’ stage A–B) was highest in elective patients treated > 62 days (50%) (p < 0.01) and lowest in emergencies treated ≤ 62 days (30%) (p = 0.26) |
| Later-stage disease (Dukes’ stage C–D) was most common in emergency patients treated ≤ 62 days (58%) and lowest in elective patients treated > 62 days (36%) | ||||||
| Pita-Fernández 2016109 | Survival | T4 | <> (Rectal, – not adjusted for stage) | 5-year mortality (after diagnosis) | The influence survival was analysed in two ways:
|
|
| <> (Colon) | ||||||
| <> CRC | ||||||
| Pita-Fernández 2016109 | Stage (advanced) | T4 | <> | TNM tumour stage (I, II–III, IV) | The association between the interval (categorised into quartiles) and tumour stage analysed using multivariate logistic regression (adjusting for age and sex) | The interval was not found to be significantly associated with stage at diagnosis (stage I: 3.6 months, stage II–III: 3.4 months, stage IV: 3.2 months; p = 0.728), even after adjusting for age and sex |
| Pruitt 2013137 | Survival | T8 | + (Colon) | All-cause mortality, and CRC-specific mortality | Cases (CRC deaths) and controls (deaths due to other causes or censored) were matched on survival time, and the association of delay with death was examined using logistic regression. ORs (95% CI) were estimated using multivariate regression analysis adjusting for sociodemographic, tumour (histology, stage, grade, location) and treatment factors. Stratified analysis also conducted according to and stage (and presenting symptom in sensitivity analysis) |
|
| <> (Rectal) | ||||||
| Pruitt 2013137 | Survival | T15 | – (Colon, < 1 week) | All-cause mortality, and CRC-specific mortality | OR (95% CI) estimated using multivariate regression analysis adjusting for sociodemographic, tumour (histology, stage, grade, location), and treatment factors. Stratified analysis also conducted according to stage (and presenting symptom in sensitivity analysis) |
|
| <> (Rectal) | ||||||
| Redaniel 2015101 | Survival | T8 | <> | 5-year survival | 5-year EHRs (95% CIs) were computed using a generalised linear model with a Poisson error structure. Univariable and multivariable models built, for each cancer site, and stratified by the nature of the symptoms (NICE-qualifying alert vs. non-alert). Multivariable models controlled for, among others, the effects of period of cancer plan implementation, Dukes’ stage, tumour subsite (for CRC) and tumour differentiation | There was no evidence of an association between diagnostic interval and mortality. EHR based on multivariate analysis for all patients:
|
| The results showed significant lower mortality associated with longer diagnostic intervals for patients presenting with non-alert symptoms (EHR > 6 months vs. < 1 month: 0.85, 95% CI 0.72 to 1.00; p = 0.049), whereas no significant associations were identified for patients presenting with NICE-qualifying alert symptoms | ||||||
| Tørring 201399 | Survival | T8 | – (Alarm symptoms, Q1) | 5-year overall mortality |
|
Alarm/serious symptoms (n = 201): the categorical analysis showed that both very short and long diagnostic intervals (first or fourth quartile compared with second and third) were associated with poor survival, but only the adjusted OR for the former was statistically significant –
|
| <> (Vague symptoms) | Vague symptoms (n = 67): the categorical analysis showed that the first diagnostic interval quartile was associated with poor survival, compared with second and third, whereas the fourth was not, but these findings were not statistically significant. Adjusted ORs (95% CIs) for each quartile:
|
|||||
| Tørring 2012135 | Survival | T8 | –/+ (GP based; Q1, Q4) | 5-year overall mortality rate (after diagnosis) |
Data taken from three studies using different methods for identifying date of first presentation. Data analysed for each study separately and combined The data were modelled in two ways:The 5-year HRs (95% CIs) estimated as a function of the length of the diagnostic interval and adjusted for tumour site (colon/rectum), age and sex using proportional hazard Cox regression. In the analysis of the combined data, differences were also allowed for in study-specific baseline hazards. (Note that the GP record-based study is Tørring 2011136) |
GP-based study (n = 266) adjusted HRs for each quartile:
|
| <> (Patient based; GP records-based) | Patient-based study (n = 658) adjusted HRs for each quartile:
|
|||||
| –/+ (combined; Q1, Q2) | Combined data (n = 1243) adjusted HRs for each quartile:
|
|||||
| Tørring 2011136 | Survival | T8 | – (Alarm symptoms) | 3-year overall mortality (after diagnosis) |
|
Alarm/serious symptoms (n = 201): The cubic spline regression analysis revealed that the risk of dying within 3 years decreased with diagnostic intervals of up to 5 weeks and then increased (p = 0.002). Adjusted OR for diagnostic intervals:
|
| <> (Vague symptoms) | Vague symptoms (n = 67): the cubic spline regression analysis revealed a reverse effect, with increasing risk of dying from day 1 up to ≈ 12 weeks, but the association was not statistically significant (p = 0.205). Adjusted OR for diagnostic intervals:
|
| Interval and outcome measure | Time intervala | Positive association | No association | Negative association |
|---|---|---|---|---|
| Breast | ||||
| Survival | ||||
| Diagnostic interval | T8 | Tørring 2011136 | ||
| Treatment interval | T15 | |||
| Stage | ||||
| Symptom onset to diagnosis | T4 | |||
| Treatment interval | T15 | |||
| Other | ||||
| Treatment interval and risk of recurrence | T15 | Eastman 2013238 | ||
| Bladder | ||||
| Survival | ||||
| Symptom onset to referral | T2 | Wallace 2002247 | ||
| Symptom onset to diagnosis | T4 | Hollenbeck 2010248 | ||
| Symptom onset to treatment | T5 | |||
| From referral to first seen by specialist | T10 | Gulliford 1991250 | ||
| From referral to treatment | T12 | Gulliford 1991250 | ||
| From specialist care to treatment | T14 | Gulliford 1991250 | Wallace 2002247 | |
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Symptom onset to diagnosis | T4 | Maguire 1994252 | ||
| Diagnostic interval | T8 | Liedberg 2003253 | ||
| Colorectal | ||||
| Survival | ||||
| Symptom onset to treatment | T5 | Terhaar sive Droste 2010255 – late-stage CRC | ||
| Referral interval | T6 | |||
| Diagnostic interval | T8 | Pruitt 2013137 – rectal | ||
| Treatment interval | T15 | Pruitt 2013137 – colon | ||
| Stage | ||||
| Patient interval | T1 | Van Hout 2011261 | ||
| Symptom onset to referral | T2 | Van Hout 2011261 | ||
| Symptom onset to first seen by specialist | T3 | Van Hout 2011261 | ||
| Symptom onset to diagnosis | T4 | |||
| Symptom onset to treatment | T5 | |||
| Referral interval | T6 | Neal 2007256 | ||
| Treatment interval | T15 | Guzman 2011266 – colon | Guzman 2011266 – rectal | |
| Other | ||||
| Patient interval and satisfaction | T1 | Tomlinson 2012267 | ||
| Lung | ||||
| Survival | ||||
| Patient interval | T1 | Brocken 2012268 | Radzikowska 2012269 | |
| Symptom onset to seen in specialist care | T3 | Loh 2006270 | ||
| Symptom onset to diagnosis | T4 | Maguire 1994252 | ||
| Symptom onset to treatment | T5 | Annakkaya 2007271 | ||
| Referral interval | T6 | Brocken 2012268 | Neal 2007256 | |
| Diagnostic interval | T8 | Tørring 201399 | ||
| From referral to first seen by specialist | T10 | Brocken 2012268 | ||
| Seen in specialist care to diagnosis | T13 | Brocken 2012268 | Gould 2008274 | |
| Seen in specialist care to treatment | T14 | Loh 2006270 | Gould 2008274 | |
| Treatment interval | T15 | Gonzalez-Barcala 2010276 | ||
| Stage | ||||
| Patient interval | T1 | |||
| Symptom onset to treatment | T5 | Christensen 1997278 | Yilmaz 2008277 | Myrdal 2004279 |
| Referral interval | T6 | Brocken 2012268 | Neal 2007256 | |
| First seen in primary care to specialist | T7 | Yilmaz 2008277 | ||
| Diagnostic interval | T8 | Pita-Fernández 2003273 | ||
| Seen in primary care to treatment | T9 | Yilmaz 2008277 | ||
| From referral to first seen by specialist | T10 | Brocken 2012268 | ||
| Seen in specialist care to diagnosis | T13 |
Brocken 2012268 Yilmaz 2008277 |
Gould 2008274 | |
| Seen in specialist care to treatment | T14 | Gould 2008274 | ||
| Treatment interval | T15 | Murai 2012280 |
Brocken 2012268 Yilmaz 2008277 |
Salomaa 2005281 |
| Other | ||||
| Symptom onset to diagnosis, and quality of life | T4 | Mohan 2006282 | ||
| Leukaemia | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Prabhu 1986283 – chronic myeloid | ||
| Diagnostic interval | T8 | Friese 2011284 – chronic lymphocytic | ||
| Treatment interval | T15 | Bertoli 2013285 – acute myeloid | ||
| Lymphoma | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 |
Foulc 2003288 – Sézary syndrome Kim 1995289 – Sézary syndrome |
||
| Myeloma | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Kariyawasan 2007290 | ||
| Other | ||||
| Symptom onset to diagnosis and complications at diagnosis | T4 | |||
| Neuroendocrine | ||||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Oral/head/neck | ||||
| Survival | ||||
| Patient interval | T1 | |||
| Symptom onset to diagnosis | T4 | |||
| Symptom onset to treatment | T5 | Hansen 2005297 – nasophayngeal | McGurk 2005298 – head and neck | |
| Diagnostic interval | T8 | |||
| Treatment interval | T15 | Sidler 2010300 – nasopharyngeal | ||
| Stage | ||||
| Patient interval | T1 | |||
| Symptom onset to referral | T2 | Pitchers 2006310 – oropharyngeal | Vernham 1994311 – unspecified | |
| Symptom onset to seen in specialist care | T3 | Allison 1998307 – aerodigestive tract | ||
| Symptom onset to diagnosis | T4 | |||
| Symptom onset to treatment | T5 | McGurk 2005298 – unspecified | ||
| First seen in primary care to specialist | T7 | Allison 1998307 – aerodigestive tract | ||
| Diagnostic interval | T8 | Al-Rajhi 2009308 – nasopharyngeal | ||
| Other | ||||
| Patient interval and risk of recurrence | T1 | Teppo 2005316 – laryngeal | ||
| Diagnostic interval and risk of recurrence | T8 | Teppo 2005316 – laryngeal | ||
| Cervical | ||||
| Survival | ||||
| First seen in specialist care to treatment | T14 | Umezu 2012317 | ||
| Stage | ||||
| Patent interval | T1 | Fruchter 1981318 | Tokuda 2009251 | |
| Symptom onset to diagnosis | T4 | Fruchter 1981318 | ||
| Diagnostic interval | T8 | Fruchter 1981318 | ||
| Endometrial | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Menczer 1995319 | ||
| Referral to treatment | T12 | Crawford 2002320 | ||
| Treatment interval | T15 | Elit 2003321 | ||
| Stage | ||||
| Patent interval | T1 | Tokuda 2009251 | ||
| Symptom onset to diagnosis | T4 | Pirog 1997324 | ||
| Other | ||||
| Symptom onset to treatment, quality of life and satisfaction | T5 | Robinson 2012325 | ||
| Ovarian | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Nagle 2011326 | ||
| Referral interval | T6 | Neal 2007256 | ||
| Stage | ||||
| Patient interval | T1 | |||
| Symptom onset to diagnosis | T4 | Lurie 2012328 | ||
| Other | ||||
| Symptom onset to treatment and QOL and satisfaction | T5 | Robinson 2012325 | ||
| Pancreatic | ||||
| Survival | ||||
| Symptom onset to referral | T2 | Raptis 2010329 | ||
| Symptom onset to diagnosis | T4 | Gobbi 2013127 | ||
| Treatment interval | T15 | Yun 2012236 | ||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Other | ||||
| Diagnostic interval and resectability | T8 | McLean 2013330 | ||
| Upper tract urothelial | ||||
| Survival | ||||
| Treatment interval | T15 | |||
| Stage | ||||
| Treatment interval | T15 | Waldert 2010332 | ||
| Prostate | ||||
| Survival | ||||
| Referral interval | T6 | Neal 2007256 | ||
| Diagnostic interval | T8 | Tørring 201399 | ||
| Treatment interval | T15 | O’Brien 2011333 | ||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Treatment interval | T15 | |||
| Testicular | ||||
| Survival | ||||
| Patient interval | T1 | Hanson 1993336 | Fossa 1981337 | |
| Symptom onset to diagnosis | T4 | |||
| Symptom onset to treatment | T5 | |||
| Diagnostic interval | T8 | Fossa 1981337 | ||
| Stage | ||||
| Patient interval | T1 | Fossa 1981337 | ||
| Symptom onset to diagnosis | T4 |
Harding 1995340 Moul 1990339 – seminoma only |
||
| Symptom onset to treatment | T5 | |||
| Diagnostic interval | T8 | |||
| Other | ||||
| Symptom onset to diagnosis and chance of complete remission | T4 | Akdas 1986349 | ||
| Symptom onset to diagnosis and response to treatment | T4 | Scher 1983350 | ||
| Symptom onset to treatment and relapse rate | T5 | Napier 2000351 | ||
| Melanoma | ||||
| Survival | ||||
| Diagnostic interval | T8 | |||
| Stage | ||||
| Patient interval | T1 | Richard 1999359 | ||
| Symptom onset to diagnosis | T4 | |||
| First seen in primary care to specialist | T7 | Montella 2002354 | ||
| Diagnostic interval | T8 | |||
| Referral to specialist care to treatment | T12 | Richard 1999359 | ||
| Non-melanoma skin | ||||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Other: | ||||
| Symptom onset to seen in specialist care and increase in tumour size | T3 | Alam 2011362 | ||
| Symptom onset to treatment and larger lesions | T5 | Renzi 2010363 | ||
| Gastric | ||||
| Survival | ||||
| Patient interval | T1 | Lim 1974364 | Ziliotto 1987365 | |
| Symptom onset to diagnosis | T4 | Maconi 2003370 | ||
| First (medical visit) seen in primary care to treatment | T9 | Lim 1974364 | ||
| Treatment interval | T15 | Yun 2012236 | ||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Diagnostic interval | T8 | Haugstvedt 1991371 | ||
| Oesophageal | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Fernandez 2002369 | ||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Symptom onset to diagnosis | T4 | Martin 1997367 | ||
| Symptom onset to treatment | T5 | Wang 2008372 | ||
| Gastro-oesophageal | ||||
| Survival | ||||
| Referral to treatment | T12 | Sharpe 2010373 | ||
| Other | ||||
| Treatment interval and morbidity and in-hospital mortality | T15 | Grotenhuis 2010374 | ||
| Sarcoma | ||||
| Survival: | ||||
| Symptom onset to diagnosis | T4 | |||
| Symptom onset to treatment | T5 | Ruka 1988379 – soft-tissue sarcoma | ||
| Stage | ||||
| Symptom onset to diagnosis | T4 | Bacci 2002380 – osteosarcoma | ||
| Other | ||||
| Symptom onset to treatment and risk of distant metastases | T5 | Nakamura 2011376 – soft-tissue sarcoma | ||
| Hepatocellular | ||||
| Survival | ||||
| Treatment interval | T15 | Singal 2013381 | ||
| Stage: | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Renal | ||||
| Stage | ||||
| Patient interval | T1 | Tokuda 2009251 | ||
| Symptom onset to treatment | T5 | Holmang 2006382 | ||
| Brain/central nervous system | ||||
| Stage | ||||
| Patient interval | T1 | Fruchter 1981318 | Tokuda 2009251 | |
| Symptom onset to diagnosis | T4 | Fruchter 1981318 | ||
| Diagnostic interval | T8 | Fruchter 1981318 | ||
| Carcinoid | ||||
| Survival | ||||
| Symptom onset to diagnosis | T4 | Toth-Fegel 2004383 | ||
| Stage | ||||
| Symptom onset to diagnosis | T4 | Toth-Fegel 2004383 | ||
| Multisite | ||||
| Survival | ||||
| Diagnostic interval | T8 | Tørring 201399 – breast, lung, colorectal, prostate and melanoma combined | ||
Appendix 5 Economic decision-analytic model
Search strategy for economic models in colorectal cancer
MEDLINE
Host: Ovid.
Date range searched: 1946 to September week 3 2017.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 685.
Search strategy
-
exp Economics/
-
Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
exp Economics, Hospital/
-
(economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti,kf.
-
exp “Fees and Charges”/
-
(fee or fees or charge$ or preference$).tw.
-
(fiscal or funding or financial or finance).tw.
-
exp “Costs and Cost Analysis”/
-
exp Health Care Costs/
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
exp Decision Support Techniques/
-
exp Models, Economic/
-
economic model*.ab,kf.
-
(decision adj2 (tree$ or analy$ or model$)).ti,ab,kf.
-
exp Decision Theory/
-
markov.ti,ab,kf.
-
markov chains/
-
monte carlo method/
-
monte carlo.ti,ab,kf.
-
(survival adj3 analy$).tw.
-
exp Health Expenditures/
-
Uncertainty/
-
exp Budgets/
-
Decision Support Systems, Clinical/
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw.
-
or/1-28
-
exp Colorectal Neoplasms/
-
((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw.
-
(MCRC or CRC).tw.
-
30 or 31 or 32
-
29 and 33
-
Early Detection of Cancer’/
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
35 or 36 or 37
-
34 and 38
-
Primary Health Care/
-
exp General Practice/
-
General Practitioners/
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
39 or 40 or 41 or 42 or 43
-
39 and 44
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Date range searched: not applicable.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 72.
Search strategy
-
(economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti,kf.
-
(fee or fees or charge$ or preference$).tw.
-
(fiscal or funding or financial or finance).tw.
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
economic model*.ab,kf.
-
(decision adj2 (tree$ or analy$ or model$)).ti,ab,kf.
-
markov.ti,ab,kf.
-
monte carlo.ti,ab,kf.
-
(survival adj3 analy$).tw.
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw.
-
or/1-11
-
((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw.
-
(MCRC or CRC).tw.
-
13 or 14
-
12 and 15
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
17 or 18
-
16 and 19
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
21 or 22
-
20 and 23
EMBASE
Host: Ovid.
Date range searched: 1974 to 28 September 2017.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 1177.
Search strategy
-
exp Economics/
-
exp Health Economics/
-
(economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti,kw.
-
(fee or fees or charge$ or preference$).tw
-
(fiscal or funding or financial or finance).tw.
-
Cost/
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
Statistical Model/
-
economic model*.ab,kw.
-
markov.ti,ab,kw
-
markov chain/
-
monte carlo method/
-
monte carlo.ti,ab,kw.
-
(decision adj2 (tree$ or analy$ or model$)).ti,ab,kw.
-
Decision Theory/
-
(survival adj3 analy$).tw.
-
Budget/
-
Decision Support System/
-
(tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).tw.
-
or/1-20
-
exp Colorectal Cancer/
-
((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw.
-
(MCRC or CRC).tw.
-
22 or 23 or 24
-
21 and 25
-
Early Cancer Diagnosis/
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
27 or 28 or 29
-
exp Primary Health Care/
-
General Practice/
-
General Practitioner/
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
31 or 32 or 33 or 34 or 35
-
30 and 36
Health Management Information Consortium
Host: Ovid.
Date range searched: 1979 to July 2017.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 154.
Search strategy
-
exp Colorectal Cancer/
-
((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or rcinoma* or adenocarcinoma* or “small cell” or squamous)).tw.
-
(MCRC or CRC).tw.
-
1 or 2 or 3
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
5 or 6
-
4 and 7
-
exp Primary Care/
-
exp General Practice/
-
exp General Practitioners/
-
(primary care or general practi$ or family practi$).tw.
-
(primary adj3 (healthcare or health care)).tw.
-
9 or 10 or 11 or 12 or 13
-
8 and 14
Science Citation Index and Conference Proceedings Citation Index – Science
Host: Web of Science.
Date range searched: not applicable.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 616.
Search strategy
-
TS=((pharmacoeconomic* or socioeconomics or economic* or pric* or cost* or cba or cea or cua or “health utilit*” or “value for money”))
-
TS=((colorectal or colon* or rect* or bowel*) near/2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TS=(MCRC or CRC)
-
TS=(predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagno* or prognos*)
-
TS=. (2ww or 2 week wait or two week wait or 2 week rule or two week rule)
-
TS=. (primary care or general practi* or family practi*)
-
TS=(primary near/2 healthcare)
-
TS=(primary near/2 health care)
-
#3 OR #2
-
#5 OR #4
-
#8 OR #7 OR #6
-
#11 AND #10 AND #9 AND #1
NHS Economic Evaluation Database and Health Technology Assessment Database
Host: Cochrane Library.
Date range searched: HTA, Issue 4 of 4, October 2016; NHS EED, 2 of 4, April 2015.
Date searched: 29 September 2017.
Searcher: SR.
Hits: HTA: 0, NHS EED; total = 0.
Search strategy
-
((colorectal or colon* or rect* or bowel*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,kw
-
MeSH descriptor: [Colorectal Neoplasms] explode all trees
-
(MCRC or CRC):ti,ab,kw
-
#1 or #2 or #3
-
MeSH descriptor: [Primary Health Care] explode all trees
-
MeSH descriptor: [General Practice] explode all trees
-
MeSH descriptor: [General Practitioners] explode all trees
-
(“primary care” or “general practi*” or “family practi*”):ti,ab,kw
-
(primary near/3 (healthcare or “health care”)):ti,ab,kw
-
#5 or #6 or #7 or #8 or #9
-
MeSH descriptor: [Decision Support Techniques] this term only
-
MeSH descriptor: [Decision Support Systems, Clinical] this term only
-
(tool or tools or aid* or model or models or checklist* or “check list” * or rule or rules or algorithm* or equation*):ti,ab,kw
-
#11 or #12 or #13
-
MeSH descriptor: [Early Detection of Cancer] this term only
-
(predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagnos* or prognos*):ti,ab,kw
-
(2ww or “2 week wait” or “two week wait” or “2 week rule” or “two week rule”):ti,ab,kw
-
#15 or #16 or #17
-
#4 and #10 and #14 and #18
EconLit
Host: EBSCOhost.
Date range searched: not applicable.
Date searched: 29 September 2017.
Searcher: SR.
Hits: 226.
Search strategy
-
TX ((colorectal or colon* or rect* or bowel*) N2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TX (MCRC or CRC)
-
TX (predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagno* or prognos*)
-
TX (2ww or 2 week wait or two week wait or 2 week rule or two week rule)
-
1 or 2
-
3 or 4
-
5 and 6
Number of hits per database and in total
| Database | Hits (n) |
|---|---|
| MEDLINE | 685 |
| MEDLINE In-Process & Other Non-Indexed Citations | 72 |
| EMBASE | 1177 |
| HMIC | 154 |
| Web of Science (SCI and CPCI-S) | 616 |
| Cochrane Library – HTA and NHS EED | 0 |
| EconLit | 226 |
| Total records | 2730 |
| Duplicates | 601 |
| Total unique records | 2129 |
Revised search strategy for economic models in colorectal cancer
MEDLINE
Host: Ovid.
Date range searched: 1946 to November week 4 2017.
Date searched: 11 December 2017.
Searcher: SR.
Hits: 574.
Search strategy
| 1 | (economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti. |
| 2 | (fee or fees or charge$ or preference$).ti. |
| 3 | (fiscal or funding or financial or finance).ti. |
| 4 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ti. |
| 5 | (value adj2 (money or monetary)).ti. |
| 6 | economic model*.ti. |
| 7 | (decision adj2 (tree$ or analy$ or model$)).ti. |
| 8 | markov.ti. |
| 9 | monte carlo.ti. |
| 10 | (survival adj3 analy$).ti. |
| 11 | (tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).ti. |
| 12 | or/1-11 |
| 13 | exp Colorectal Neoplasms/ |
| 14 | ((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw. |
| 15 | (MCRC or CRC).tw. |
| 16 | or/13-15 |
| 17 | “Early Detection of Cancer”/ |
| 18 | (predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw. |
| 19 | (2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw. |
| 20 | or/17-19 |
| 21 | 12 and 16 and 20 |
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Date range searched: not applicable.
Date searched: 11 December 2017.
Searcher: SR.
Hits: 71.
Search strategy
| 1 | (economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti. |
| 2 | (fee or fees or charge$ or preference$).ti. |
| 3 | (fiscal or funding or financial or finance).ti. |
| 4 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ti. |
| 5 | (value adj2 (money or monetary)).ti. |
| 6 | economic model*.ti. |
| 7 | (decision adj2 (tree$ or analy$ or model$)).ti. |
| 8 | markov.ti. |
| 9 | monte carlo.ti. |
| 10 | (survival adj3 analy$).ti. |
| 11 | (tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).ti. |
| 12 | or/1-11 |
| 13 | ((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw. |
| 14 | (MCRC or CRC).tw. |
| 15 | 13 or 14 |
| 16 | (predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw. |
| 17 | (2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw. |
| 18 | 16 or 17 |
| 19 | 12 and 15 and 18 |
EMBASE
Host: Ovid.
Date range searched: 1974 to 9 December 2017.
Date searched: 11 December 2017.
Searcher: SR.
Hits: 658.
Search strategy
| 1 | (economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti. |
| 2 | (fee or fees or charge$ or preference$).ti. |
| 3 | (fiscal or funding or financial or finance).ti. |
| 4 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ti. |
| 5 | (value adj2 (money or monetary)).ti. |
| 6 | economic model*.ti. |
| 7 | (decision adj2 (tree$ or analy$ or model$)).ti. |
| 8 | markov.ti. |
| 9 | monte carlo.ti. |
| 10 | (survival adj3 analy$).ti. |
| 11 | (tool or tools or aid$ or model or models or checklist$ or check list$ or rule or rules or algorithm$ or equation$).ti. |
| 12 | or/1-11 |
| 13 | exp colorectal cancer/ |
| 14 | ((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw. |
| 15 | (MCRC or CRC).tw. |
| 16 | or/13-15 |
| 17 | early cancer diagnosis/ |
| 18 | (predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw. |
| 19 | (2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw. |
| 20 | or/17-19 |
| 21 | 12 and 16 and 20 |
Health Management Information Consortium
Host: Ovid.
Date range searched: 1979 to September 2017.
Date searched: 11 December 2017.
Searcher: SR.
Hits: 32.
Search strategy
-
exp Colorectal Cancer/
-
((colorectal or colon* or rect* or bowel*) adj3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)).tw.
-
(MCRC or CRC).tw.
-
1 or 2 or 3
-
(predict$ or assess$ or scor$ or risk$ or validat$ or decision$ or identif$ or diagno$ or prognos$).tw.
-
(2ww or 2 week wait or two week wait or 2 week rule or two week rule).tw.
-
5 or 6
-
4 and 7
NHS Economic Evaluation Database and Health Technology Assessment Database
Host: Cochrane Library.
Date range searched: Health Technology Assessment, issue 4 of 4, October 2016; NHS EED, 2 of 4, April 2015.
Date searched: 11 December 2017.
Searcher: SR.
Hits: HTA: 0; NHS EED: 0; total = 0.
Search strategy
-
((colorectal or colon* or rect* or bowel*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,kw
-
MeSH descriptor: [Colorectal Neoplasms] explode all trees
-
(MCRC or CRC):ti,ab,kw
-
#1 or #2 or #3
-
MeSH descriptor: [Early Detection of Cancer] this term only
-
(predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagnos* or prognos*):ti,ab,kw
-
(2ww or “2 week wait” or “two week wait” or “2 week rule” or “two week rule”):ti,ab,kw
-
#5 or #6 or #7
-
#4 and #8
EconLit
Host: EBSCOhost.
Date range searched: not applicable.
Date searched: 11 December 2017.
Searcher: SR.
Hits: 1.
Search strategy
-
TX ((colorectal or colon* or rect* or bowel*) N2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TX (MCRC or CRC)
-
TX (predict* or assess* or scor* or risk* or validat* or decision* or identif* or diagno* or prognos*)
-
TX (2ww or 2 week wait or two week wait or 2 week rule or two week rule)
-
1 or 2
-
3 or 4
-
5 and 6
Number of hits per database and in total
| Database | Hits (n) |
|---|---|
| MEDLINE | 574 |
| MEDLINE In-Process & Other Non-Indexed Citations | 71 |
| EMBASE | 658 |
| HMIC | 32 |
| Cochrane Library – HTA and NHS EED | 0 |
| EconLit | 1 |
| Total records | 1336 |
| Duplicates | 564 |
| Total unique records | 772 |
Updated searches, 27 September 2018
| Database | Hits (n) |
|---|---|
| MEDLINE | 249 |
| MEDLINE In-Process & Other Non-Indexed Citations | 28 |
| EMBASE | 351 |
| HMIC | 0 |
| Cochrane Library – HTA and NHS EED | 1 |
| EconLit | 0 |
| Total records | 629 |
| Duplicates | 83 |
| Total unique records | 546 |
American Joint Committee on Cancer and Dukes’ classification systems
The decision models in the literature are based on two different classification systems of CRC stages. These are the AJCC and Dukes.
The American Joint Committee on Cancer staging system
The AJCC’s staging system384 classifies cancer following the TNM system, in which the first consideration is the size and extent of the main tumour (or primary tumour), the second refers to the number of nearby lymph nodes with cancer, and the third one refers whether or not the cancer has metastasised, if the cancer has spread from the primary tumour to other parts of the body. The TNM system is the most widely used cancer staging system (see www.cancer.gov/about-cancer/diagnosis-staging/staging; accessed January 2019).
Primary tumours can be graded in six levels:
-
T0, no evidence of primary tumour
-
Tis, carcinoma in situ: intraepithelial or invasion of the lamina propria
-
T1, the tumour is confined to the submucosa
-
T2, the tumour has grown into (but not through) the muscularis propria
-
T3, the tumour has grown into (but not through) the serosa
-
T4, the tumour directly invades other organs or structures, and/or perforates visceral peritoneum.
The levels for lymph nodes are as follows:
-
N0, no regional nodes involved
-
N1, 1–3 regional nodes involved
-
N2, four or more regional nodes involved.
Finally, metastasis can be:
-
M0: no distant metastasis
-
M1: distant metastasis present.
Tumour, number, metastasis combinations were grouped into five stages:
-
Stage 0, cancer is defined by Tis N0 M0, called carcinoma in situ, is related to the presence of abnormal cells, but they have not spread to nearby tissue.
-
Stage I, a cancer defined by T1 N0 M0 or T2 N0 M0.
-
Stage II, a cancer defined by T3–4 N0 M0.
-
Stage III, represented by cancer defined by any T1–2, N1, M0, any T3–4, N1 M0, any T N2 M0.
-
Stage IV, represented by a cancer with any T, any N, M1, where the cancer has spread to distant parts of the body.
The Dukes’ staging system
The other staging system was initially defined by Dukes in 1932,385 but this has been revised several times. This system has now largely been replaced by the TNM staging system, but it is currently used in many of the English and UK economic models. This system has four stages:
-
Dukes’ A: invasion into but not through the bowel wall (90% chance of 5-year survival)
-
Dukes’ B: invasion through the bowel wall but not involving lymph nodes (70% chance of 5-year survival)
-
Dukes’ C: involvement of lymph nodes (30% chance of 5-year survival)
-
Dukes’ D: widespread metastases.
Comparison of the American Joint Committee on Cancer and Dukes’ staging systems
A conversion of the Dukes’ stages into TNM is represented in Table 59.
| Stage | Dukes’ | TNM |
|---|---|---|
| Tumour invasion confined to the mucosa | A | Tis, N0 |
| Tumour invasion limited to the submucosa, no lymph node involvement | A | T1, N0 |
| Tumour invasion limited to the submucosa, lymph node involvement | C | T1, N1–2 |
| Limited tumour invasion into the muscle layer, no lymph node involvement | A | T2, N0 |
| Limited tumour invasion into the muscle layer, lymph node involvement | C | T2, N1–2 |
| During the whole muscle layer tumour involvement, no lymph node involvement | B | T3, N0 |
| During the whole muscle layer tumour involvement, lymph node involvement | C | T3, N1–2 |
| Tumours have kept the neighbouring organs, no lymph node involvement | B | T4, N0 |
| Tumours have kept the neighbouring organs, lymph node involvement | C | T4, N1–2 |
| Other factors notwithstanding distant metastases | D | T1–4, N0–2, M1 |
Excluded studies of full-text articles assessed for eligibility
Thirteen full texts were excluded because the models were not based on the Dukes’ or AJCC classification staging (9/13), the models were screening models without a full disease progression model component (3/13) or the study was a costing analysis for a screening programme (1/13). Further details are chronologically summarised in this section.
Bolin et al. 386 compared the costs of different strategies for CRC screening, including colonoscopy, to provide a decision on early diagnosis for CRC in Australia. This model was based on a model developed by the Office of Technology Assessment (OTA). 246 Both models were excluded because the staging was grouped into A + B and C + D, and did not use the Dukes’ full staging classification. The model by Byers and Gorsky387 was also excluded because it too used the OTA model, which considers only two stages based on an incomplete Dukes’ classification system.
Neilson and Whynes388 reported the development of a simulation model for assessing the cost-effectiveness of CRC in the UK. The model was not included as it considers only two health states for CRC (early- and late-detected CRC), which does not correspond with the Dukes’ staging classification.
Gow’s389 study was excluded on the basis of being a costing study of a programme in Australia for screening CRC.
Khandker et al. 390 evaluated the guidelines of the American Gastroenterological Association for CRC screening and surveillance programmes, using a cost-effectiveness framework for people at average risk and increased risk in the USA. This study was not included because the stages of CRC were dependent on anatomical extent (local, regional and distant) that only conceptually parallels with three stages (A, B and C) of the Dukes’ staging classification.
The study by Sonnenberg et al. 391 compared the cost-effectiveness of faecal occult blood testing, flexible sigmoidoscopy and colonoscopy as strategies for screening CRC. The study was excluded because the Markov models were for the screening strategies only and did not include a full disease model component.
Reyes et al. 392 was excluded because the authors were evaluating the clinical effectiveness and incremental cost-effectiveness of four strategies to detect hereditary non-polyposis colorectal carcinoma (HNPCC) gene carriers in individuals with CRC and the authors excluded a full disease progression model.
Kievit et al. 393 determined the clinical effectiveness, efficiency and feasibility of a new strategy based on microsatellite instability analysis for the detection of HNPCC in the Netherlands. This study was excluded because the Markov model included only one health state for CRC, without considering the different stages of the disease.
Telford et al. 394 estimated the incremental cost-effectiveness of 10 strategies for CRC screening in Canada. The study was not included because the Dukes’ staging or other classification system that was part of the inclusion criteria was not used.
The study by Allameh et al. 395 was excluded because this was another screening cost-effectiveness model that did not include a whole-disease progression model.
The study by Dan et al. 396 was excluded because the cost-effectiveness analysis of single endoscopic examination screening within a Markov framework was based on the risk for the population of Singapore and based on a single health state (progression to CRC).
The methodological paper by Goh et al. 341 was excluded because the study aimed to compute the maximal and minimal values for the discounted value of the Markov chain for the transition parameters of CRC disease in the USA. The staging used in this model was localised, regional and distant cancer sites, which differs from the Dukes’ or other system classification specified in the inclusion criteria.
Finally, a 2017 study by Atkin et al. 397 was also excluded because the authors considered five mutually exclusive health states (no adenomas, adenomas, preclinical CRC, diagnosed CRC and dead), and thus omitted any type of staging classification.
Sensitivity analysis, parameter specification and incremental cost-effectiveness ratio variation
| Study | Type of analysis | Parameters affecting ICER | ICER variation |
|---|---|---|---|
| Allen 2005144 |
|
|
|
| Tappenden 2007145 |
|
|
|
| Tsoi 2008146 |
|
|
|
| Zauber 2008147 | N/A | N/A | N/A |
| Heitman 2010148 |
|
|
|
| Lee 2010149 |
|
|
|
| Knudsen 2012150 | USA |
|
|
| Sharp 2012151 |
|
|
|
| Whyte 2012152 |
|
|
|
| Goede 2013153 | Digital Subtraction Angiography |
|
|
| Gomes 2013154 |
|
|
|
| Tappenden 2013155 | No sensitivity analysis | The authors provided constrained maximisation analysis | N/A |
| Whyte 2014156 |
|
|
|
| Cantor 2015157 |
|
|
|
| Pil 2016158 |
|
|
|
| Wong 2016159 |
|
|
|
| Coldman 2017160 | DSA |
|
|
| Westwood 2017161 |
|
|
|
Critical appraisal of model-based economic evaluations
| Question | Allen et al.144 | Tappenden et al.145 | Tsoi et al.146 | Zauber et al.147 | Heitman et al.148 | Lee et al.149 | Knudsen et al.150 | Sharp et al.151 | Whyte et al.152 | Goede et al.153 | Gomes et al.154 | Tappenden et al.155 | Whyte and Harnan156 | Cantor et al.157 | Pil et al.158 | Wong et al.159 | Coldman et al.160 | Westwood et al.161 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | ||||||||||||||||||
| S1: statement of decision problem/objective | ||||||||||||||||||
| Is there a clear statement of the decision problem? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the primary decision-maker specified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S2: statement of scope/perspective | ||||||||||||||||||
| Is the perspective of the model stated clearly? | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | Yes | Yes |
| Are the model inputs consistent with the stated perspective? | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Has the scope of the model been stated and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S3: rationale for model structure | ||||||||||||||||||
| Has the evidence regarding the model structure been described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Have any competing theories regarding model structure been considered? | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Are the sources of data used to develop the structure of the model specified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the causal relationships described by the model structure justified appropriately? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S4: structural assumptions | ||||||||||||||||||
| Are the structural assumptions transparent and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S5: strategies/comparators | ||||||||||||||||||
| Is there a clear definition of the option under evaluation? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Have all feasible and practical options been evaluated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is there justification for the exclusion of feasible options? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| S6: model type | ||||||||||||||||||
| Is the chosen model type appropriate given the decision problem and specified causal relationship with the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S7: time horizon | ||||||||||||||||||
| Is the time horizon of the model sufficient to reflect all important differences between options? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the time horizon of the model, and the duration of treatment and treatment effect described and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S8: disease states/pathways | ||||||||||||||||||
| Do the disease states (state-transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of the interventions? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S9: cycle length | ||||||||||||||||||
| Is the cycle length defined and justified in terms of the natural history of the disease? | No | Yes | No | Yes | Yes | No | No | Yes | Yes | No | No | No | No | N/A | No | No | No | Yes |
| Data | ||||||||||||||||||
| D1: data identification | ||||||||||||||||||
| Are the data identification methods transparent and appropriate given the objectives of the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Where choices have been made between data sources, are these justified appropriately? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Has particular attention been paid to identifying data for the important parameters in the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Has the quality of the data been assessed appropriately? | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Where expert opinion has been used, are the methods described and justified? | No | Yes | N/A | N/A | N/A | N/A | No | Yes | Yes | N/A | N/A | Yes | No | N/A | Yes | N/A | Yes | Yes |
| D2: pre-model data analysis | ||||||||||||||||||
| Are the pre-model data analysis methodology based on justifiable statistical and epidemiological techniques? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| D2a: baseline data | ||||||||||||||||||
| Is the choice of baseline date described and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes |
| Are transition probabilities calculated appropriately? | NR | Yes | Yes | Yes | Yes | N/A | N/A | Yes | Yes | N/A | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes |
| Has a half cycle correction been applied to both cost and outcome? | NR | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| If not, has this omission been justified? | NR | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| D2b: treatment effects | ||||||||||||||||||
| If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Have alternative assumptions been explored through sensitivity analysis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No | N/A | N/A | N/A | N/A | N/A | No | N/A |
| D2c: quality-of-life weights (utilities) | ||||||||||||||||||
| Are the utilities incorporated into the model appropriate? | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Is the source for the utility weights referenced? | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Are the methods of derivation for the utility weights justified? | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Yes | No | Yes |
| D3: data incorporation | ||||||||||||||||||
| Have all the data incorporated into the model been described and referenced in sufficient detail? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate?) | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | N/A | Yes | No | Yes |
| Is the process of data incorporation transparent? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | No | No | Yes |
| If the data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | No | Yes | N/A | N/A | N/A | N/A | N/A | Yes | Yes | N/A | Yes | No | N/A | No | Yes | No | No | Yes |
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | No | Yes | N/A | N/A | N/A | N/A | N/A | Yes | Yes | N/A | No | Yes | N/A | No | Yes | No | No | Yes |
| D4: assessment of uncertainty | ||||||||||||||||||
| Have the four principal types of uncertainty been addressed? | No | Yes | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | No | No | No |
| If not, has the omission of particular forms of uncertainty been justified? | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | No | No |
| Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | No | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | No | No |
| D4c: heterogeneity | ||||||||||||||||||
| Has heterogeneity been dealt with by running the model separately for different subgroups? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| D4d: parameter | ||||||||||||||||||
| Are the methods of assessment of parameter uncertainty appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| Has probabilistic sensitivity analysis been done; if not, has this been justified? | No | N/A | No | No | N/A | N/A | No | N/A | N/A | Yes | N/A | N/A | N/A | No | N/A | N/A | No | Yes |
| If the data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Yes | N/A | Yes | N/A | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes | N/A | No | N/A | N/A | Yes |
| Consistency | ||||||||||||||||||
| C1: internal consistency | ||||||||||||||||||
| Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | Yes | N/A | N/A | Yes | Yes | Yes | N/A | N/A | Yes | Yes | N/A | Yes | N/A | N/A | N/A | N/A | N/A | N/A |
| C2: external consistency | ||||||||||||||||||
| Are the conclusions valid given the data presented? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are any counterintuitive results from the model explained and justified? | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| If the model has been calibrated against independent data, have any differences been explained and justified? | N/A | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | No | No | No | N/A | No | N/A | No | No |
| Have the results of the model been compared with those of previous models and any differences in results explained? | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
Probabilities and costs of adverse events of diagnostic tests in included studies
| Study | Type (cost/utility/probability) | Description | Value |
|---|---|---|---|
| Allen 2005144 | Probability | Procedure complication rates for haemorrhage from colonoscopy | 0.007 |
| Procedure complication rates for perforation from colonoscopy | 0.004 | ||
| Mortality rate for colonoscopy | 0.0001 | ||
| Procedure complication rates for perforation from FS | 0.001 | ||
| Mortality rate for FS | 0.00000006 | ||
| Procedure complication rates for perforation from ACBE | 0.0001 | ||
| Mortality rate for ACBE | 0.00002 | ||
| Cost | Costs of procedural complications of perforation | US$13,598 | |
| Costs of procedural complications of haemorrhage | US$4561 | ||
| Tappenden 2007145 | Probability | Probability of perforation from colonoscopy (without polypectomy) | 0.0008 |
| Probability of perforation from colonoscopy (with polypectomy) | 0.0017 | ||
| Probability of death following perforation for colonoscopy | 0.0582 | ||
| Probability of perforation (without polypectomy) from FS | 0.000025 | ||
| Probability of perforation (with polypectomy) from FS | 0.000025 | ||
| Probability of death following perforation for FS | 0.0582 | ||
| Probability of bleeding following FS | 0.000295 | ||
| Probability of bleeding following colonoscopy | 0.00439 | ||
| Cost | Cost of treating bowel perforation (major surgery) | £5407.74 | |
| Cost of admittance for bleeding | £250.21 | ||
| Heitman 2010148 | Probability | Risk of bleeding for diagnostic colonoscopy | 0.0003 |
| Risk of bleeding for therapeutic colonoscopy | 0.005 | ||
| Risk of perforation of diagnostic colonoscopy | 0.0009 | ||
| Risk of perforation of therapeutic colonoscopy | 0.0024 | ||
| Risk of perforation after FS | 0.0002 | ||
| Risk of death after endoscopic perforation | 0.049 | ||
| Cost | Cost of complication for perforation | CAN$31,223 | |
| Cost of complication for bleeding | CAN$3194 | ||
| Lee 2010149 | Probability | Risk of bleeding from screening FOBT | 0 |
| Risk of bleeding from screening FS | 0.000295 | ||
| Risk of bleeding from screening colonoscopy | 0.00439 | ||
| Risk of bleeding from screening CTC | 0 | ||
| Risk of perforation from screening FOBT | 0 | ||
| Risk of perforation from screening FS | 0.000025 | ||
| Risk of perforation from screening colonoscopy | 0.0008 | ||
| Risk of perforation from screening CTC | 0.0006 | ||
| Risk of perforation from polypectomy FOBT | 0.0017 | ||
| Risk of perforation from polypectomy FS | 0.000025 | ||
| Risk of perforation from polypectomy colonoscopy | 0.0017 | ||
| Risk of perforation from polypectomy CTC | 0.0017 | ||
| Risk of mortality following perforation | 0.00582 | ||
| Knudsen 2012150 | Probability | Risk of perforation from colonoscopy, by age group (years) | |
| 50–65 | 0.00036 | ||
| 66–69 | 0.00036 | ||
| 70–74 | 0.00042 | ||
| 75–79 | 0.00052 | ||
| 80–84 | 0.00064 | ||
| ≥ 85 | 0.00087 | ||
| Risk of bleeding with transfusion from colonoscopy, by age group (years) | |||
| 50–65 | 0.00089 | ||
| 66–69 | 0.00089 | ||
| 70–74 | 0.00103 | ||
| 75–79 | 0.00127 | ||
| 80–84 | 0.00156 | ||
| ≥ 85 | 0.00214 | ||
| Risk of bleeding without transfusion from colonoscopy, by age group (years) | |||
| 50–65 | 0.00245 | ||
| 66–69 | 0.00245 | ||
| 70–74 | 0.00284 | ||
| 75–79 | 0.00351 | ||
| 80–84 | 0.00430 | ||
| ≥ 85 | 0.00589 | ||
| Risk of other gastrointestinal events from colonoscopy, by age group (years) | |||
| 50–65 | 0.00320 | ||
| 66–69 | 0.00320 | ||
| 70–74 | 0.00400 | ||
| 75–79 | 0.00540 | ||
| 80–84 | 0.00730 | ||
| ≥ 85 | 0.00880 | ||
| Risk of perforation from CTC, by age group (years) | |||
| 50–65 | 0.00005 | ||
| 66–69 | 0.00005 | ||
| 70–74 | 0.00005 | ||
| 75–79 | 0.00005 | ||
| 80–84 | 0.00005 | ||
| ≥ 85 | 0.00005 | ||
| Cost | Cost of perforation from colonoscopy | US$15,985 | |
| Cost of bleeding with transfusion from colonoscopy | US$7784 | ||
| Cost of bleeding without transfusion from colonoscopy | US$1775 | ||
| Cost of other gastrointestinal events from colonoscopy | US$1195 | ||
| Cost of perforation from CTC | US$15,985 | ||
| Sharp 2012151 | Probability | Probability of perforation from FS with polypectomy | 0.00002 |
| Probability of perforation from FS without polypectomy | 0.00002 | ||
| Probability of death after perforation FS | 0.06452 | ||
| Probability of (major) bleeding following FS | 0.00029 | ||
| Probability of perforation from colonoscopy with polypectomy | 0.00216 | ||
| Probability of perforation from colonoscopy without polypectomy | 0.00107 | ||
| Probability of death after following perforation | 0.05195 | ||
| Probability of (major) bleeding following colonoscopy | 0.00379 | ||
| Cost | Cost of treating bowel perforation | €10,200 | |
| Cost of admittance for bleeding | €3079 | ||
| Whyte 2012152 | Probability | Perforation rate from colonoscopy with polypectomy | 0.003 |
| Perforation rate from colonoscopy without polypectomy | 0 | ||
| Probability of death after perforation colonoscopy | 0.052 | ||
| Perforation rate from FS with polypectomy | 0.001 | ||
| Perforation rate from FS without polypectomy | 0 | ||
| Probability of death after perforation FS | 0.065 | ||
| Probability of hospitalisation for bleeding after FS | 0.0003 | ||
| Probability of hospitalisation for bleeding after colonoscopy | 0.003 | ||
| Cost | Cost of treating bowel perforation (major surgery) | £2164 | |
| Cost of admittance for bleeding (overnight stay on medical ward) | £278 | ||
| Goede 2013153 | Probability | Fatal complications rate after colonoscopy | 0.0001 |
| Cost | Costs complications after colonoscopy | €1250 | |
| Gomes 2013154 | Probability | Rate of complications from colonoscopy bleeding | 0.0044 |
| Rate of complications from colonoscopy perforation | 0.002 | ||
| Rate of polypectomy bleeding rate | 0.02 | ||
| Rate of polypectomy perforation | 0.0038 | ||
| FS bleeding rate | 0.0003 | ||
| Risk of death after perforation | 0.0582 | ||
| Cost | Costs of admittance for bleeding | £295 | |
| Cost of treatment of bowel perforation | £5822 | ||
| Tappenden 2013155 | Probability | Probability of perforation from colonoscopy | 0.0013 |
| Probability surgery for perforation | 0.66 | ||
| Probability FS perforation | 0.2 × 10–4 | ||
| Probability DCBE perforation | 0.4 × 10–4 | ||
| Probability CTC perforation | 0.0005 | ||
| Probability patient dies during perforation surgery | 0.06 | ||
| Probability patient dies during polyp surgery | 0.04 | ||
| Probability patient undergoes stenting | 0.20 | ||
| Probability stenting successful | 0.72 | ||
| Probability stent-related mortality | 0.0025 | ||
| Cost | Emergency surgery postoperative mortality rate | £294.77 | |
| Cost emergency surgery | £7079.93 | ||
| Cost emergency surgery for perforation | £5088.91 | ||
| Cost conservative management of perforation | £881.99 | ||
| Cost stent insertion | £1012.73 | ||
| Whyte 2014156 | Probability | Probability of perforation rate of colonoscopy (with polypectomy) | 0.003 |
| Probability of perforation rate of colonoscopy (without polypectomy) | 0 | ||
| Probability of death following perforation from colonoscopy | 0.052 | ||
| Probability of hospitalisation for bleeding from colonoscopy | 0.003 | ||
| Cost | Cost of treating bowel perforation (major surgery) | £5089 | |
| Cost of admittance for bleeding (overnight stay on medical ward) | £278 | ||
| Cantor 2015157 | Not included | Not included | |
| Pil 2016158 | Not included | Not included | |
| Wong 2016159 | Probability | Bleeding rate | 0.002 |
| Perforation rate | 0.0002 | ||
| Morality due to perforation | 0.1 | ||
| Cost | Cost of bleeding | US$3320 | |
| Cost of perforation | US$10,790 | ||
| Coldman 2017160 | Not included | Not included | |
| Westwood 2017161 | Probability | Bleeding rate after colonoscopy | 0.0026 |
| Perforation rate after colonoscopy | 0.0005 | ||
| Death rate after colonoscopy | 0.000029 | ||
| Cost | Cost of bleeding | £603 | |
| Cost of perforation | £3228 | ||
| Cost of death | £0 | ||
Decision model data and assumptions
Clinical effectiveness parameters
Distribution of disease stages at presentation
As, by definition, no data exist on the distribution of symptomatic patients by disease stage at first presentation to the GP, we replicated the disease history model by Tappenden et al. 145 and generated the distribution of disease stages at presentation by age from that model. The model by Tappenden et al. 143 was calibrated to data on CRC prevalence and incidence (see Chapter 6), and we verified that predictions from our replication of that model matched the national mortality data on CRC incidence in England. 398
Diagnostic accuracy of the faecal immunochemical test
A systematic review of diagnostic accuracy for FIT found no diagnostic accuracy studies for FIT at thresholds of 10 µg Hb/g faeces and 20 µg Hb/g faeces evaluated in the low-risk population of interest here, which was defined by the NICE Guideline Number 126 as that with a prevalence of 0.1–3%. 161 Furthermore, only one study of those identified was conducted in a primary care setting, but, in this study, the FIT was evaluated in patients who were about to be referred to secondary care. The meta-analysed values from four studies for FITs of 10 µg Hb/g faeces with the OC-Sensor (Palex Medical S.A., Sant Cugat del Vallès, Spain ) were 92.1% (86.9% to 95.3%) for sensitivity and 85.8% (78.3% to 91.0%) for specificity;161 sensitivity and specificity of 100% and 76.6%, respectively, for HM-JACKarc (Hitachi Chemical Co., Ltd, Tokyo, Japan) from one study were also reported, but are not considered here as they were based on only 11 CRC cases. As these estimates may overestimate the sensitivity of the test, we use these values for the sensitivity analysis. For our base-case analysis, we chose to use instead the values reported by Murphy et al. 175 for a screening programme for which a FIT of 20 µg Hb/g faeces, the threshold with the highest sensitivity of those investigated, was reported to have sensitivity of 52.6% and specificity of 98.8% for those aged 50–69 years, and specificity of 96.3% for those aged ≥ 70 years.
Excess mortality of symptomatic undiagnosed patients
Patients with false-negative results are exposed to delays in diagnosis and treatment, and we accounted for the excess risks of death from lack of treatment experienced by an as yet undiagnosed CRC patient. The only source of evidence that we identified was a study of newly diagnosed CRC patients who refused treatment in Hong Kong and whose survival outcomes were compared with patients who accepted treatment for CRC. 177 The study subjects included patients with CRC at disease stages I–IV, in whom a hazard ratio death of 2.66 (95% CI 2.49 to 2.84) was reported for refusing treatment. The study did not report a treatment effect by disease stage. Therefore, we used this estimate for all disease stages except for Dukes’ stage A, for which we conservatively assume that lack of treatment has no immediate effect on survival, in line with previous models. 145
Relative mortality of symptomatic undiagnosed patients
We estimate the CRC-related death probabilities from the relative survival curves by Dukes’ stage depicted in Figure 9. We digitised the curves and fitted exponential survival models to the extracted data (R2 values of 0.98, 0.99, 0.99 and 0.97 for relative survival curves of Dukes’ stages A, B, C and D, respectively). The resulting estimates are presented in Tables 6 and 7. Admittedly, these estimates, which were obtained from data of patients diagnosed between 1996 and 2002, are outdated, as 5-year survival in CRC patients has increased since the time the data were collected. However, this is the most recent available data by disease stage, and further sensitivity analyses revealed that this parameter had limited influence on the study results.
It must be noted that using the same data Whyte et al. 152 have reported net survival curves by disease stage at diagnosis and age. Given the time constraints we faced and the limited influence that these age-specific CRC survival data would have on the results, we did not incorporate these data into the model. We note that, by using relative survival estimates instead of net survival ones, we implicitly assume that there is no heterogeneity in survival outcomes, which is a strong assumption, but one with no consequence to our results.
Costs
Faecal immunochemical test
Our unit cost estimate for a FIT was obtained from a screening programme evaluation. 161 The figure used for the model excludes the costs of research. This is lower than the £4.53 and £6.03 figures for OC-Sensor and HM-JACKarc, respectively, used by a previous study. 161 We explore the effect of these alternative cost estimates in sensitivity analyses.
Colonoscopy
We obtained the costs of colonoscopy from the data reported by Whyte and Harnan. 156 The original figure of £563 was obtained from NHS reference costs399 and data from a single screening centre. 156 The figure reflated to 2018 prices used in the model for this parameter is presented in Tables 6 and 7. A previous study adopted a much lower cost of £372 from an average of outpatient diagnostic colonoscopy with and without biopsy in NHS reference costs of 2014–15. 161,400 We retained the higher cost for our base-case analysis and used the lower cost for the sensitivity analysis.
We use the same source to account for the costs of adverse events with colonoscopy, namely perforation and bleeding. 156 The NHS reference costs reported by the source, £5089 and £278 for perforation and bleeding, respectively, are different from those used in the study by Westwood et al. 161 of £3228 and £603, respectively, which were derived from length of hospital stay estimates of those events reported in a 2012 study (1.7 and 9.1 days, respectively161,401). We use the former set of figures for the base-case analysis, and the latter for the sensitivity analysis.
Treatment of colorectal cancer
Lifetime costs of treatment by Dukes’ disease stage and age were obtained from a previous study. 152 This source derived its estimates from simulations of the disease history model by Tappenden et al. 145 The estimates used in the model are those figures reflated to 2018 prices, and presented in Tables 6 and 7. Although no better source of estimates was identified, the value of these parameters was found to have negligible influence on the study results.
Utilities
We adopted utility values by CRC disease stage used in the model by Tappenden et al. ,145 which obtained them from the study by Ness et al. 184 These values were used by the existing study in the field. 161 These values were derived from individual interviews of 81 patients who had previously undergone removal of colorectal adenoma; during the interviews, participants were presented with stage-dependent outcome states and asked to assess their relative value using the standard gamble technique. In the absence of utility data for treated patients, we assumed that these values are applicable to both untreated and treated patients. In sensitivity analyses, we found that the choice of values for these parameters did not affect the result in a significant way.
In addition, utility values for both patients with CRC and healthy individuals are applied background utilities from the model by Ara and Brazier,402 which accounts for the non-linear decline of utility with age, and differentiates between age and sex.
We found no available estimate on the negative impact on health-related quality of life of colonoscopy or its adverse events. Therefore, we limited our analysis to explore the effect of a disutility of colonoscopy of 0.0075 in the month when referral takes place, that is the equivalent of a loss of life in full health of 5 hours.
Assumptions in Westwood et al.’s161 model versus assumptions in our model
Table 63 presents a comparison of the assumptions used in the previous diagnostic model161 relevant to our analysis and our own model. Our modelled cycle length is 28 days, as opposed to Westwood et al. ’s161 yearly length, which is too long to capture the small differences in the referral interval between the diagnostic strategies that we investigated. We modelled the probability of delayed referral and diagnosis in patients with a false-negative outcome after the initial tests in primary care, as opposed to the previous model’s161 arbitrary assumption of a 6-month delay to diagnosis. In contrast to Westwood et al. ’s161 model, in our model an account is made for excess mortality during the time spent in a false-negative state.
| Assumption number | Westwood et al.161 model | Our model |
|---|---|---|
| 1 | A lifetime horizon with a 1-year cycle length captures the probability of progression for treated and untreated patients | Lifetime horizon with 4-weekly cycles is required to measure outcomes with sufficient accuracy |
| 2 | Any differences in costs between the tests in patients without CRC were assumed to occur only in the first year | Any differences in costs between the tests in patients without CRC were assumed to occur only in the first month |
| 3 | Any differences in life expectancy between intervention and comparator for patients without CRC are due only to the difference in mortality due to colonoscopy/CTC | Same assumption |
| 4 | Any differences in costs between intervention and comparator for patients without CRC are only due to difference in cost of gFOBTs and colonoscopy/CTC | Any differences in costs between intervention and comparator for patients without CRC are only due to difference in cost of FIT and colonoscopy |
| 5 | Testing has no long-term (after 1 year) effect on costs or QALYs in disease-negative people. Thus, in patients without CRC, FOBTs would not significantly delay diagnosis of the underlying cause of presenting symptoms, and hence would not incur any extra cost or effect on mortality | Same assumption but applies after 1 month |
| Diagnostic model | ||
| 6 | A time frame of 1 year was assumed for the diagnostic model | Integrated within Markov model |
| 7 | A positive FIT/gFOBT result in referral to colonoscopy | A positive (above the risk threshold) tool assessment results in referral to colonoscopy, as does a positive FIT result after a negative tool result in the intervention or, in the control arm, in all patients |
| 8 | A negative FIT or gFOBT result in a watchful waiting strategy, in which a colonoscopy/CTC will be performed when symptoms persist. A repeat FIT/gFOBT might also be performed, but referral to colonoscopy/CTC is not modelled as being contingent on the results of the repeat test | A negative FIT, in the control, or both tool and FIT, in the intervention, results in a watchful waiting strategy, in which repeat testing will be performed when symptoms persist (which is assumed to occur in all CRC cases and none of the non-CRC patients), and referral to colonoscopy is modelled as being contingent on the results of the repeat testing |
| 9 | The sensitivity and specificity of colonoscopy for detection of CRC is 100% | Same assumption |
| 10 | The symptoms of all those patients with CRC who receive a false-negative result will persist such that they will all receive a colonoscopy and thus be diagnosed (within 1 year) should they survive (NG1516/expert opinion403) | Same assumption, but < 1% of patients will receive colonoscopy beyond the 1 year – as determined by the probability of a positive result after repeated monthly trials of repeat visit and testing according to sensitivity of the test. This corresponds with data available to authors from the electronic primary care record |
| 11 | Patients who had a false-negative gFOBT or FIT result, and whose symptoms persisted, have an increased probability of progressing to a worse cancer state because of the delay in diagnosis | Same, all false-negative patients have persistent symptoms |
| 12 | Probability of delayed diagnosis of CRC was assumed to be the probability of progression within Dukes’ stages at 6 months | The probability of time to diagnosis was determined by the sensitivity of the overall strategy, with the probability of diagnosis at cycle t being given by the Bernoulli formula for ‘success’ out of t trials (cycles), that is [(1 – sensitivity)t − 1] × sensitivity, which, when combined with the rate of disease progression over Dukes’ disease stages, determined the disease stage at diagnosis |
| 14 | Only those patients with a negative test result, and whose symptoms do not persist, do not receive a colonoscopy/CTC | Patients with a negative test result who have CRC have persistent symptoms and return every month for testing and get referred to receive colonoscopy with a probability determined by the sensitivity of the strategy (see 13). All negative-testing patients without CRC are assumed to clear the symptoms after the initial test result and not to return to the GP |
| 15 | CT of the chest, abdomen and pelvis is performed for all of the patients testing positive for CRC after colonoscopy or CTC, to estimate the stage (Dukes’ A–D) of the disease (NG126) | This cost element is not considered, but has a negligible effect on the results |
| 16 | For the base-case scenario, we considered a threshold of 10 µg Hb/g faeces, or equivalent, for the detection of CRC using a single faecal sample. Other options were explored in sensitivity analyses | For the base-case scenario, we considered a threshold of 20 µg Hb/g faeces, as the evidence on 10 µg Hb/g faeces was derived from a population different from that of interest |
| 17 | The prevalence of CRC in the base-case population was 1.5%, as in NG126 | The prevalence of CRC in the base-case population was 1.5% |
| CRC Markov model | ||
| 18 | After the initial distribution of patients in the CRC model is determined, patients may stay in their current health state, progress to the health state representing the next worsening in the condition or die (from CRC or another cause) (NG126) | Same specification |
| 19 | Costs associated with the health states of the CRC Markov model were estimated as lifetime costs (i.e. one-off cost) | Same specification |
| Healthy population Markov model | ||
| 20 | Patients entering this model can either die of all of the causes or stay in the ‘alive’ health state | Same specification |
| 21 | For the base-case scenario, patients aged ≥ 40 years (NG126) | For the base-case analysis, patients aged 70 years |
| 22 | The adverse events included in the diagnostic model are bowel bleeding, perforation and death (literature) | Same specification |
| Adverse events | ||
| 23 | Reduction of quality of life due to adverse events is assumed to be negligible within a lifetime (assumption) | Same assumption |
| 24 | No costs of patients who die owing to adverse events of colonoscopy | Same assumption |
| 25 | Adverse events due to CTC were assumed to be the same as those of colonoscopy | CTC was not included in the model |
| Test costs | ||
| 26 | Costs of laboratory staff to analyse the test were assumed to be the same for FITs and gFOBTs | Irrelevant; FOBT was not considered as is not used in practice |
| 27 | Training costs and the costs of the laboratory staff for analysing the test results were not included in the total costs because it was assumed that these are the same for FITs and gFOBTs | Laboratory staff costs for analysis were included in the cost of a FIT; training costs were not included, to correspond with a measure of marginal costs |
| 28 | Costs of the material needed to analyse a sample include costs of the reagents, buffer, reaction cells and analyser cups | Same cost elements were included |
| 29 | Costs of colonoscopy/CTC, adverse event costs and CT costs were included in the diagnostic model (NG126) | Same costs except CTC were included in the model |
| 30 | We assumed that the cost of colonoscopy/CTC includes the costs of a follow-up appointment with a gastroenterologist (assumption/expert opinion) | The cost of a follow-up appointment with gastroenterologist is implicit in our chosen unit cost of colonoscopy (£563 in 2011 prices, £596 after reflation to 2014–15 prices), which was taken from Whyte and Harnan156 and compares with Westwood et al.’s161 £372 (in 2014–15 prices); the difference (£224) is £89 higher than the £135 unit cost of a gastroenterology outpatient appointment used by Westwood et al.161 Whyte and Harnan’s156 reported source for the cost of colonoscopy is the NHS reference costs (no further details provided), as is Westwood et al.’s.161 |
| 31 | For test-negative patients whose symptoms persist, an additional GP appointment cost was considered | Negative-testing patients whose symptoms persist are all false negatives and keep returning each month to the GP until they test positive or die without referral and diagnosis |
| 32 | Indirect costs parameters were not included in the model, given the perspective of the NHS | Same specification |
| 33 | Zero excess mortality during delayed diagnosis in CRC patients | Positive excess mortality during delayed diagnosis in CRC patients |
In terms of resource use, our cost of colonoscopy (£596 in 2014/15 prices), based on Whyte and Harnan,156 is higher than that used by Westwood et al. 161 (£372), despite the fact that both are derived from NHS reference costs. However, the difference is reduced to £89 because Westwood et al. 161 include an additional cost of £135 for a follow-up appointment with a gastroenterologist to discuss the colonoscopy results, which is not explicitly considered by Whyte and Harnan156 (who provide no further detail). Unlike the Westwood et al. 161 model, investigations for determining disease stage in patients diagnosed with CRC are not considered in our analysis, but sensitivity analyses indicate that these have a negligible effect on the results.
Sensitivity analyses
| Referral after | QCancer | Comparatora | RAT | |||
|---|---|---|---|---|---|---|
| Predicted frequencyb,c | Mean time to referral (days) | Predicted frequencyc,d | Mean time to referral (days) | Predicted frequencyb,c | Mean time to referral (days) | |
| 1 visit | 0.49 | 28 | N/A | 28 | 0.55 | 28 |
| 2 visits | 0.24 | 28 | 0.61 | 28 | 0.19 | 28 |
| 3 visits | 0.13 | 56 | N/Ae | N/A | 0.14 | 56 |
| 4 visits | 0.07 | 56 | 0.24 | 56 | 0.05 | 56 |
| ≥ 5 visits | 0.08 | 95 | 0.15 | 102 | 0.07 | 94 |
| Weighted average | 1 | 39 | 1 | 46 | 1 | 38 |
| Referral after | QCancer | Comparatora | RAT | |||
|---|---|---|---|---|---|---|
| Predicted frequency | Dukes’ A/B (%) | Predicted frequency | Dukes’ A/B (%) | Predicted frequency | Dukes’ A/B (%) | |
| 1/2 visits | 0.73 | 54 | 0.61 | 54 | 0.74 | 54 |
| 3/4 visits | 0.20 | 51 | 0.24 | 51 | 0.19 | 51 |
| 5/6 visits | 0.05 | 48 | 0.09 | 48 | 0.05 | 48 |
| 7/8 visits | 0.01 | 45 | 0.04 | 45 | 0.01 | 45 |
| 9/10 visits | 0.00 | 42 | 0.01 | 42 | 0.00 | 42 |
| > 10 visits | 0.00 | 40 | 0.01 | ≤ 40 | 0.00 | 40 |
| Weighted average | 1 | 53 | 1 | 52 | 1 | 53 |
Probabilistic sensitivity analyses
| Parameter | Value | Distribution | Source |
|---|---|---|---|
| Clinical | |||
| Prevalence of CRC | 0.015 | Beta(1838,121683) | Hippsley-Cox 201285 |
| Sensitivity of QCancer | 0.610 | Beta(1198,765) | Hippsley-Cox 201285 |
| Specificity of QCancer | 0.910 | Beta(1111261,109968) | |
| Sensitivity of FIT | 0.526 | Beta(73,65) | FIT of 20 µg Hb/g faeces; Murphy 2017175 |
| Specificity of FIT: 50–69 years old | 0.988 | Beta(10148,123) | |
| Specificity FIT: ≥ 70 years old | 0.963 | Beta(9891,380) | |
| Compliance with colonoscopy referral | 0.80 | Beta(80,20) | Assumption |
| Transition probabilities | 4-weekly | Whyte 2014156 | |
| Dukes’ A to Dukes’ B | 0.0651 | Beta(651,9349) | Assumption. Tappenden 2007145 reported model calibration to CRC incidence and stage at diagnosis |
| Dukes’ B to Dukes’ C | 0.0787 | Beta(787,9213) | |
| Dukes’ C to Dukes’ D | 0.1427 | Beta(1427,8573) | |
| Costs | |||
| Cost of colonoscopy | £615 | Uniform(555,676) | Assumption, ± 10% following Whyte 2011,404 appendix 4 |
Appendix 6 General practitioner survey
Cover letter
Participant information
Aids to cancer diagnosis in primary care survey
Other types of cancer decision support tools named by general practitioners in the free text as being available to them
Cancer guideline summaries
-
BMJ cancer guidelines chart
-
BMJ flow charts on computer desktop
-
BMJ Informatica
-
Booklet from a GP ‘Update’ course
-
Fast-track referral guidance/NICE guidelines
-
GP update summary of cancer guidelines NICE 2015
-
Macmillan Rapid Referral guidelines
-
NICE guidelines 2WW form
-
NICE/BMJ cancer assessment/referral guidelines poster
-
NICE: suspected cancer recognition and referral
-
Pathfinder (Northants)
-
Scottish Referral Guidelines for suspected cancer (quick reference guide)
-
Secure Grampian Cancer Network chart (NHS Grampian)
Comments received
-
GP unable to complete at present.
-
GP unable to complete at present.
-
GP unable to complete at present.
-
GP unable to complete at present.
-
Registrar – unable to complete at present.
-
GPs do not use these tools.
-
GPs do not use these tools.
-
GPs do not use these tools.
-
GPs do not use these tools.
-
GPs do not use these tools.
-
GPs do not use these tools.
-
Registrar + 3 Year of military primary care experience.
-
Unfortunately no time.
-
Incomplete as GP has left.
-
Q1 comment – Have Macmillan guidance.
-
Tool itself was excellent. Did have Macmillan Tool kit for few months but abandoned – IT (local) support inadequate.
-
The surgery have advised that they have not heard of the cancer tools so will not complete the survey.
-
Never heard of QCancer I was unaware of it but having checked the computer it is there and available.
-
Likely if I am told that it picks up more cancer or reduces referrals in trials using eCDS. Also likely if, when using, the tool, I can quote on the referral that the eCDS recommends referral and this would be actioned by secondary care and not ignored.
-
The practice have advised that they use SystmOne so do not have acess to the electronic cancer decision support tools. Because of this they will not be returning the survey.
-
I haven’t been using one regularly but would be interested in using a mousemat tool.
-
Sorry GPs do not wish to participate.
-
Declined.
-
Q7 – I use it off the internet QCancer.
-
Q2 – would if I had one – mousemat would be useful.
-
Q4 – I tried to use when I was a GP in Wirral on EMIS Web but not successful & Q6 – I have seen in action at Macmillan conference.
-
Q1 – Did have mousemat but don’t any longer.
-
Not completed – too time-consuming.
-
Not completed – too time-consuming.
-
Not completed – too time-consuming.
-
Not completed – too time-consuming.
-
I raised this as an option & past partners felt concerned that not acting on a certain risk would make them more likely to be sued. It never went any further.
-
Flipchart – had one but too much paper – not practical for daily use, too ‘fussy’.
Copy of email received
List of abbreviations
- AJCC
- American Joint Committee on Cancer
- BB
- Bristol–Birmingham
- CAPER
- Cancer Prediction in Exeter
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CRC
- colorectal cancer
- CTC
- computerised tomography colonography
- eCDS
- electronic clinical decision support
- FIT
- faecal immunochemical test
- FOBT
- faecal occult blood test
- gFOBT
- guacal faecal occult blood test
- GP
- general practitioner
- Hb
- haemoglobin
- HMIC
- Health Management Information Consortium
- HNPCC
- hereditary non-polyposis colorectal carcinoma
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- iFOBT
- immunochemical faecal occult blood test
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IT
- information technology
- MeSH
- medical subject heading
- MISCAN
- MIcrosimulation SCreening ANalysis
- NAEDI
- National Awareness and Early Diagnosis Initiative
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- OTA
- Office of Technology Assessment
- PPI
- patient and public involvement
- PPV
- positive predictive value
- PROBAST
- Prediction model Risk Of Bias ASsessment Tool
- QALY
- quality-adjusted life-year
- RAT
- risk assessment tool
- RCT
- randomised controlled trial
- SEER
- Surveillance, Epidemiology, and End Results
- SimCRC
- Simulation Model of Colorectal Cancer
- SR
- systematic review
- TDI
- total diagnostic interval
- THIN
- The Health Improvement Network
- TNM
- tumour node metastasis
- WW
- week wait
