Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/34/14. The contractual start date was in October 2014. The draft report began editorial review in May 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Simpson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Globally, it is estimated that seasonal influenza is responsible for 5 million cases of severe illness and 500,000 deaths per year, with, for example, an estimated cost to the USA of US$87B per annum. 1–3 There are also 90 million new cases of influenza and 1 million cases of influenza-associated severe acute lower respiratory infection among children. 4 National influenza vaccination programmes, delivered by primary care in the community, are important to reduce influenza-related illness, and hence the considerable investment in this approach. Previously, these programmes targeted older people (i.e. those aged ≥ 65 years) and people with chronic disease (e.g. asthma) who are susceptible to serious illness from influenza. Children are also thought to be important in the transmission of influenza to the populations at risk of serious complications from influenza, and diminished circulation of the virus has been predicted to improve herd immunity. 5 Using evidence generated from epidemiological modelling,6 and following advice from the Joint Committee on Vaccination and Immunisation, from September 2013 the seasonal influenza vaccination programme has been extended. 7 In addition to the seasonal trivalent influenza vaccine (TIV), the live attenuated influenza vaccine (LAIV) is offered to all children aged 2–11 years (except children clinically severely immunocompromised owing to conditions or immunosupressive therapy or oral steroids, and children with severe asthma) by primary care clinicians in general practice (GP) and in schools in Scotland.
Existing evidence
The evidence from clinical trials of the benefits of LAIV are largely confined to healthy children aged < 7 years (mostly for children aged < 3 years). 8,9 Efforts to estimate seasonal TIV effectiveness have been largely confined to younger healthier adults (e.g. with no randomised controlled trials showing efficacy of TIV in adults aged ≥ 65 years). 9,10 Recent observational studies have attempted to estimate the vaccine effectiveness in preventing influenza-related illness in GP patients. 3,11 Further studies have examined vaccine effectiveness with hospitalisation or death; however, these studies have suffered from bias when using non-specific outcomes,8 or have been underpowered when using more specific end points (e.g. laboratory-confirmed influenza), in particular for subgroups being targeted for vaccination (e.g. older people aged ≥ 65 years, people with at-risk disease such as asthma and pregnant women). 12 Cohort studies (with nested case–control studies) or data linkage-derived estimates of vaccine effectiveness have been undertaken, with measures taken to overcome many of the confounding issues that otherwise have limited estimations of effectiveness. 13–15 There is also a need to add to the growing body of evidence with regard to the safety of these vaccines. 16 Given the ongoing controversy regarding vaccine effectiveness and, in particular, in relation to at-risk groups (e.g. those with asthma),7 there is further need for information to help evaluate new seasonal vaccine strategies.
Chapter 2 Research questions
This research aimed to examine the vaccine effectiveness and safety of the seasonal influenza vaccines, including LAIV and TIV. The research team had access to a unique set of linked databases within a trusted research environment (TRE), which contained individual patient-level data relating to primary health care, acute hospital care data, school immunisation data, virological real-time reverse-transcription polymerase chain reaction (RT-PCR) laboratory tests and mortality. 3
In contrast to previous observational studies, these rich data sources provide information on a large number of potential confounders and highly specific laboratory outcome measures in a study cohort sampled from the general population. This assessment of the vaccine effectiveness and the public health impact of a new seasonal influenza vaccination programme seeks to clarify whether or not such a programme leads to societal benefits, therefore advancing the international evidence base.
The research questions were:
-
What was the uptake and vaccine effectiveness of LAIV administered to children (introduced to the national vaccination programme in 2013)?
-
What was the uptake and vaccine effectiveness of TIV administered to at-risk groups (e.g. those people aged ≥ 65 years and people aged < 65 years with asthma)?
-
Are laboratory-confirmed influenza tests from non-Sentinel primary care and secondary care valid (vs. Sentinel primary care practices)?
-
What was the validity of using laboratory-confirmed respiratory syncytial virus as a negative-control outcome?
-
What adverse events are associated with vaccination?
Chapter 3 Methods
Study design and population
Vaccine uptake is reported from a cross-sectional survey of all six influenza seasons. The test-negative design (TND) was used to measure vaccine effectiveness for the RT-PCR outcomes and a cohort study design was used for non-specific clinical outcomes (e.g. hospitalisation or death from influenza or pneumonia). 17
All practices in Scotland (n = 998) were invited to participate and 230 (self-selected) practices were recruited These represented 29 (65.9%) of 44 of the Community Health Partnership (CHP) areas in Scotland. Data were extracted on 1.25 million patients in Scotland into our study. Each patient contributed person-time to each influenza season while alive and fully registered with a participating GP (i.e. a person was included in the study if they were on a participating practice’s list of patients, including those who may have died or deregistered during the study period).
Three basic data sets for analysis were created:
-
all patients with a RT-PCR test
-
patients by age group (e.g. 2–4, 5–11, 12–17, 18–64 and ≥ 65 years)
-
patients at risk of serious influenza-like illnesses (ILIs) (e.g. asthma).
Databases
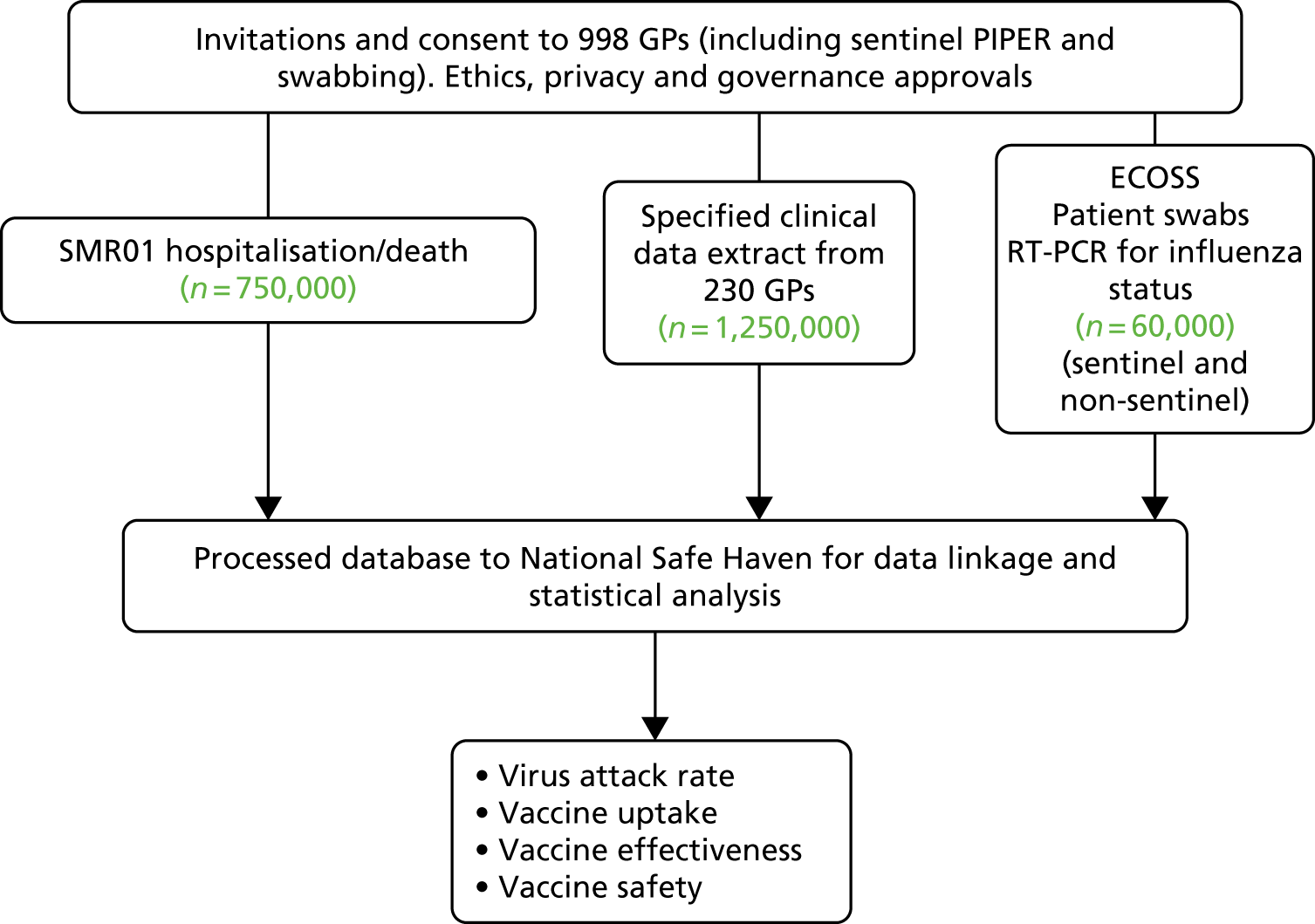
Data fields extracted from the following databases (Figure 1) were linked deterministically using the Community Health Index number; a unique identifier used by the NHS for the Scottish population. 3 The database linkage and analysis was carried out within the NHS National Services Scotland (NSS) TRE by the electronic Data Research and Innovation Service (eDRIS).
FIGURE 1.
Flow diagram for the Seasonal Influenza Vaccination Effectiveness II project. ECOSS, Electronic Communication of Surveillance in Scotland; PIPER, Pandemic Influenza Primary Care Reporting; SMR01, Scottish Morbidity Record 01.
General practice
Almost all individuals resident in Scotland are registered with a GP, which provides health-care services free of charge. Virtually all specialist hospital care services are also free of charge, usually obtained through referral from primary care or, in emergency situations, through patients attending an accident and emergency department. Primary care-based physicians co-ordinate the influenza vaccination programme for their patients and provide much of the care of patients discharged back into the community by secondary and tertiary care services. Completeness of capture of contacts and accuracy of clinical event coding (using Read codes) has been found to be > 91% among practices in Scotland. 18,19 The electronic recording of long-term prescribing information by primary care has also been found to be both accurate and complete. 20
Child Health Services Programme/Scottish Immunisation & Recall System
The Child Health Services Programme/Scottish Immunisation & Recall System database has a record of all children (used nationally from 2002) with scheduled vaccinations. Data on vaccination administration for all children in Scotland are also recorded here. 21 These data were used to determine influenza vaccinations that have been administered in schools rather than in primary care.
Electronic Communication of Surveillance in Scotland
Data on > 60,000 RT-PCR tests (including an additional 1500 tests per season funded to target 2- and 3-year-olds), collated into the Electronic Communication of Surveillance in Scotland (ECOSS) database, were used for the identification of severe disease, outbreaks and long-term trends in the incidence of laboratory-reported infections. 22
Scottish Morbidity Record
The Information Services Division (ISD) NSS maintains a database of all acute hospital discharges and deaths in Scotland, known as the Scottish Morbidity Record 01 (SMR01). All inpatient and day-case episodes of care for acute hospitals since 1981 have been recorded in the database. The database is subject to regular validation checks and the most recent quality assurance report indicated good levels of accuracy (i.e. > 90%) for the fields used in this study. 23 Diagnostic information is recorded using the International Classification of Diseases, Tenth Edition (ICD-10). There are up to six fields that can be used to record diagnoses, with one field allocated as the main reason for admission. SMR01 is linked routinely by the ISD to the Scottish death register using patient characteristics in a probabilistic matching algorithm, with a high degree of accuracy. 24 Details from death certificates issued for all deaths in Scotland are recorded in the death register, maintained by the National Records of Scotland. 25 Cause of death has been routinely coded, using ICD-10, since 2000. 3
Study period
Data from 1 September 2010 to 31 August 2016 were used. These allowed an analysis of six influenza seasons (from 2010/11 to 2015/16). Each patient contributed person-time to each influenza season while alive and registered with a participating GP. For the non-pandemic seasons, each year (i.e. 1 September to 31 August) was divided in to four periods (Figure 2). The influenza season was defined for each year using national influenza surveillance data. 26 The other periods include a pre-influenza season (starting on 1 September), a post-influenza period (which ends on 31 May each year) and a ‘non-influenza’ period (from 1 June to 31 August) (see Figure 2). Because there was a phased roll-out arrangement for influenza vaccination among children, children aged 2–3 years and primary school-aged children (for which pilots were taking place) in season 2013/14, and all preschool children aged 2–4 years and all primary school-aged children (i.e. all children aged 2–11 years) in seasons 2014/15 and 2015/16 were analysed.
FIGURE 2.
Relationship of the first influenza season (2010–11) to pre-, post- and non-influenza season periods. Reproduced from Simpson et al. 3 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.

Baseline characteristics for each patient was determined on 1 September each year. The earliest date of influenza vaccination varied for each influenza season, but always took place after 1 September.
Exposure definition
For people in at-risk groups, influenza vaccinations (TIV and LAIV for preschool children aged ≥ 2 years) are free and administered by general practitioners (Table 1). 28 Data on influenza vaccination carried out in GP (including Community Health Index number and date of administration) are recorded to enable reimbursement. Information on individuals receiving LAIV in schools is collated in the Child Health Services Programme/Scottish Immunisation & Recall Service database and was extracted for this analysis. 21 Vaccination was used to define exposure status when it was given at a time point between 1 September and the end of the influenza season (see Figure 2). An individual was defined as vaccinated 14 days after the seasonal influenza vaccine had been administered. 29 The time period from the first day of the influenza season to day 14 post vaccination was defined as ‘unexposed’ and the period from day 14 post vaccination until the end of the influenza season was defined as ‘exposed’. Therefore, those people vaccinated between the start of the pre-influenza period up until 14 days before the influenza season were defined as ‘exposed’ for the duration of the influenza season. 30
| Variable | Data | |
|---|---|---|
| Item | Source | |
| Age, sex | SMR0127 and GP27 | |
|
Hospital admission type: emergency/routine admission Date of first admission Length of stay |
SMR0127 | |
| Clinical condition codinga | Influenza vaccination | GP27 |
| Recorded given | Influenza vaccination | ISD Scotland21 |
| Clinical condition codinga | Pneumococcal vaccination | GP27 |
| SIMD: rural/urbanb | GP27 | |
| Prescription | Antiviral prescriptions | GP27 |
| Prescription | Asthma- and COPD-related prescriptions | GP27 |
| Clinical condition codinga | Clinical at-risk groups (chronic respiratory disease, chronic heart disease, chronic kidney disease, chronic liver disease, chronic neurological disease, immunosuppression, diabetes mellitus, pregnancy) | GP27 |
| Diagnosis fields | Charlson Comorbidity Index comorbidities | SMR0127 |
| Prescription, clinical attendance | Number of previous GP consultations, prescribed drugs | GP27 |
| Clinical condition codinga | Smoking,b exercise statusb | GP27 |
| Number of previous hospital admissions | SMR0127 | |
| Clinical condition codinga | Pregnancy | GP27 |
| Quality and Outcomes Framework exception reported (patient unsuitable, etc.) | GP27 | |
| Clinical condition codinga | Home oxygen | GP27 |
| Clinical condition coding,a diagnosis | Trauma | SMR0127 and GP27 |
| Clinical condition coding,a diagnosis | ILI | SMR01,27 GP27 and death records27 |
| Clinical condition coding,a diagnosis | Asthma and COPD symptoms and exacerbations | SMR0127 and GP27 |
| RT-PCR swab results | ECOSS virology database | |
Study outcomes
Real-time reverse-transcription polymerase chain reaction
Data are collated by Health Protection Scotland (HPS) on patients having had swab samples RT-PCR tested in primary and secondary care for routine diagnostic purposes outside the Sentinel scheme. All RT-PCR data on both positive and negative tests are held by HPS in the national laboratory database (the ECOSS database). From 1999, the RT-PCR testing used to confirm respiratory virus type has been found to be highly sensitive for influenza A (H3, H1) and B diagnosis. 3,31 Improvements to RT-PCR, since 2003, include the development of multiplex testing, which increases the number of pathogens tested per assay. However, the high sensitivity of these tests remains unchanged. 32
Primary care practices involved in the HPS Sentinel swabbing scheme are encouraged to obtain nasal/throat swabs from patients of all ages who have symptoms suggestive of influenza. Each GP was requested to submit five swab samples per week (seven in season 2015/16) to the West of Scotland Specialist Virology Centre (WoSSVC), Glasgow Royal Infirmary, for RT-PCR testing for a range of respiratory pathogens on any patient presenting for consultation in the practice with influenza symptoms across all ages, independent of whether the patient has or has not been vaccinated. The WoSSVC is a World Health Organization-accredited national influenza centre, which participates in the quality assurance programme to maintain this status.
Non-specific clinical outcomes
To determine the effect of vaccination status on influenza-related primary care consultations, hospital admissions and deaths, secondary analyses were undertaken using non-specific clinical outcomes derived from primary and secondary care. Data on ILI consultation were derived from the GP database. Data on hospitalisation and cause of death from influenza or pneumonia were derived from SMR01.
Confounding factors
Key characteristics of each identified patient characteristics present in each season of the cohort were included as confounders in the analyses. These were defined in each year on the first day of the pre-influenza season (i.e. on 1 September).
Demographics
Sex, age band and socioeconomic status were included in all analyses; socioeconomic status was measured using quintiles of the Scottish Index of Multiple Deprivation (SIMD). SIMD is an area-based measure of deprivation derived from seven domains, including income, employment and education. 3,33 SIMD identifies small-area concentrations of multiple deprivation across all of Scotland in a consistent way. SIMD ranks small areas (called data zones) from the most deprived (ranked 1) to the least deprived (ranked 6976), and this was mapped onto postcode and then split into quintiles of socioeconomic status. For this project, SIMD was derived from an individual patient’s full postcode. Rurality in terms of urban/rural location (one large urban and eight remote rural areas) was also included in the analysis, and this was classified in this project using an individual patient’s postcode. 34
At-risk groups
At-risk patients are those with certain comorbidities for whom seasonal influenza vaccination is indicated. Patients were defined as high risk according to national guidance if they had one or more of the following conditions:28
-
asthma
-
chronic heart disease
-
chronic kidney disease (including renal transplantation), stages 1 and 2 and 3–5
-
chronic liver disease
-
chronic neurological diseases
-
chronic respiratory diseases
-
conditions or drugs causing impaired immune function
-
diabetes mellitus.
Chronic diseases
This was included for our non-specific clinical outcomes and adverse events. Comorbidity was defined by the 17 disease categories that constituted the Charlson Comorbidity Index. 35 This index has been validated in a number of different databases using codes from health-care databases. 36 A study has mapped Read codes from a UK GP database to the relevant Charlson Comorbidity Index comorbid disease groups, resulting in a model that performed well in the prediction of 5-year mortality. 37 These codes were used to identify comorbidities that are present in a patent’s record prior to the start of each pre-influenza season (i.e. on 1 September).
Smoking status
Smoking status was derived from primary care data (current smoker, ex-smoker, non-smoker) and determined on 1 September each year.
Previous vaccinations
A variable was included for patients who have received seasonal influenza vaccination in previous seasons to account for the possibility of persisting vaccine effectiveness in the subsequent year. 38,39 Adjustment for previous pneumococcal vaccination at any time in the primary care record prior to 1 September each year was also undertaken.
Previous health-care utilisation
Measures of previous health-care resource use was used to capture other aspects of chronic health status and included previous years’ GP consultations, prescriptions (repeat) and number of admissions to hospital.
Functional status
There is no direct measure of functional status made in any of these national databases. However, individuals who were resident in some form of institutional care setting were identified from the primary care database. This was used as an indicator of more severe functional limitation.
Sensitivity analyses
Simonsen et al. ’s40 framework was used to consider the role of confounding.
Seasonality
Vaccine effectiveness should be highest during the influenza season and lower pre and post season (see Figure 2).
Vaccine match
Vaccine effectiveness should be lower in years during which the influenza vaccine was a poor match for the circulating virus.
Severity of influenza season
Vaccine effectiveness should be greater in years during which the circulating virus caused a large excess mortality during the influenza season.
Age
It is thought that influenza vaccine is less effective in the oldest age groups because of immune senescence.
Specificity of outcome measure
Vaccine effectiveness should be greatest for the most specific outcome (i.e. laboratory-confirmed influenza infection) and lowest for the less specific outcomes [e.g. general practice acute respiratory infection (ARI) consultations].
Unmeasured confounding
The robustness of the results were assessed by modelling the effect of an unmeasured confounder, such as frailty, on the vaccine effectiveness estimates in a sensitivity analysis; an approach adopted to help explain the role of unknown confounding in observational analyses. 40 Three factors were varied: (1) the prevalence of the confounder in the vaccinated population, (2) its prevalence in the unvaccinated population and (3) the increased risk of the outcome attributable to the confounder. 41
Instrumental variable analyses
The use of influenza vaccine coverage by geographical area has been found to be a strong and valid instrumental variable, which can be used to account for confounding. 3,42 Rather than comparing patients with respect to whether or not they received influenza vaccination, this instrumental variable behaves like natural randomisation of patients to regional vaccination groups that differ in their likelihood of receiving influenza vaccination. The NSS TRE is an important development in this respect, and permissions were received to extract granular postcode/geocoding data required to test the validity of this instrumental variable analysis in the Seasonal Influenza Vaccination Effectiveness II (SIVE II) database. Therefore, the use of vaccination uptake in geographically distinct CHP areas or other suitable health board areas as a suitable instrumental variable was explored. Because there are only 14 health boards and > 30 CHPs, the latter was used as the geographical area. To be valid, this instrumental variable needed to be related to exposure status (i.e. vaccination status) and not have an independent effect on outcome other than by ways mediated through the exposure. 43 Furthermore, the instrumental variable should not be related to any variables that confound the relationship between exposure and outcome. If an association with confounders is demonstrated, it is assumed that the instrumental variable is associated with unmeasured confounders and is therefore not valid. If the instrumental variable fulfils these criteria, it can be used in analyses to produce unbiased estimates of vaccine effectiveness by accounting for unmeasured confounding.
Adverse events associated with vaccination
A self-controlled study design was used to estimate the risk of adverse events associated with influenza vaccination. 44 The assumption underlying this design is that in the situation in which the adverse event is related to vaccination, the occurrence of an adverse event in the period after vaccination is greater than periods in the same patient that are temporally unrelated to vaccination. 45 This method has the advantage of controlling for all fixed individual-level confounders as comparisons are within the same individual, rather than between vaccinated and unvaccinated populations. The time period at risk for an adverse event (risk interval) and time period not at risk (control interval) were determined separately for each outcome. 46 For virtually all adverse events, the at-risk period was 14 days following receipt of the vaccination and the pre-risk period was the 90-day period prior to the 14 days before the vaccine (i.e. days 104 to 15 before receipt of the vaccine). The post-risk period was also 90 days and began on day 15 following vaccination. The main comparisons are with the rate of adverse events in the risk period compared with (1) the pre-risk period and (2) the post-risk period.
The self-controlled case series design uses data from only those with the adverse event and who are vaccinated. For some of the adverse events, there is the possibility of a temporal change in the risk over the ≥ 200 days of observation periods. To take this into account, data were also included from unvaccinated individuals who experienced the adverse event. These individuals were assigned a pseudo date of vaccination based on the median date of vaccination for the age and season. Interaction tests were then used to compare the rates of adverse events in (1) the risk period compared with the pre-risk period, and (2) the risk period compared with the post-risk period, among vaccinated and unvaccinated individuals. If there is evidence of a significant interaction with a higher risk ratio among vaccinated individuals, then this suggests that there is a potential adverse event associated with vaccination. Because there were a large number of adverse events being tested, the Benjamini–Hochberg false discovery rate was used to adjust for multiple testing.
The analysis of the self-controlled case series was undertaken using a stratified analysis in which the comparisons of the different risk periods were made within individuals. This was achieved by using matched logistic regression, with an offset for the length of the risk period. To avoid biases, the risk periods were not censored at death or when an individual left a practice.
Statistical analysis
A 5% significance level was used for hypothesis tests for the primary outcome. All p-values were two sided. All analyses were undertaken in R, version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria). Ninety-five per cent confidence intervals (CIs) were also calculated. Logistic regression was used to investigate vaccine uptake. A generalised additive model was used to estimate the vaccine effect within the TND. Splines were used to model the effect of age and time within the season. A time-dependent Cox model was used to estimate the effect of vaccination on the consultation, hospitalisation and mortality end points. The receipt of vaccination was a time-dependent covariate. Summary statistics for this analysis are based on the person-time at risk.
Annual and pooled analyses
The study initially analysed each of the six influenza seasons separately for the primary outcome. However, a pooled analysis was carried out, in which increased precision was required (particularly for analysing subgroups of patients). In the TND, the pooled analysis used a separate spline term for days within each season.
Vaccine uptake and vaccine effectiveness
Vaccine uptake was calculated for all age groups per season as a percentage uptake.
Real-time reverse-transcription polymerase chain reaction outcomes
For vaccine effectiveness, using information from linked virological RT-PCR swab data (a binary event), a nested case test-negative control study was carried out. 47 Influenza positivity was compared with no influenza among patients who had influenza-like symptoms. The primary analysis utilised a logistic generalised additive model, in which the effects of sex, age, socioeconomic status (via the SIMD) and being in an at-risk morbidity group were adjusted for (TND study). 3,33 A spline function for time during each season was included to model the background rate of influenza and correct for any potential bias associated with the proportions of test-negative and test-positive patients in different periods. Vaccine effectiveness was measured by comparing the results from swabs taken after vaccination among those patients vaccinated, with swabs taken from those unvaccinated patients at the time the swab was collected. Vaccination was used to define exposure status if it was given at a time point between 1 September and the end of the influenza season (see Figure 2). The adjusted estimate of vaccine effectiveness was calculated using (1 – OR) × 100, in which the odds ratio (OR) was derived from the coefficient of vaccine status in the model. In the main analysis, the first dose was assessed only when two doses were given. An analysis, stratified by influenza A (H1, including pandemic influenza and H3 subtype where recorded) and influenza B, was carried out.
In addition, a number of sensitivity analyses for the primary end point were carried out.
Non-Sentinel versus Sentinel
We explored the validity of using laboratory-confirmed influenza tests from non-Sentinel primary care and secondary care sources compared with Sentinel primary care practices. Patient characteristics of individuals swabbed in non-Sentinel primary care practices and secondary care were described and any interaction between the source of the swab and the outcome was tested.
Negative controls
The use of laboratory-confirmed infections (currently 10 respiratory viruses and an infection, including rhinovirus and adenovirus) was explored using multiplex RT-PCR at the same time as the influenza RT-PCR.
Non-specific clinical outcomes
Vaccine effectiveness was estimated for non-specific clinical outcomes: primary care consultations for ILI and ARI; and emergency hospitalisation and death due to influenza/pneumonia. Hospital admissions and consultations can have multiple events and each event was counted.
Methods that were found in previous studies to be optimal for measuring vaccine effectiveness and accounting for bias and confounding were adopted. 30,47,48 Adjusted risk ratios (RRs) of vaccine effectiveness for prevention of hospitalisation/death/GP consultation were derived from time-dependent Cox models, taking into account the time at risk and the possibility of multiple events (not for death). Models did not include a cluster term to account for intrapractice correlation, as a practice code was not available in the analysis. Practice code not being available ensures that the identity of the practices in the study is hidden from the researchers. The models adjusted for sex, age, deprivation and clinical risk group, and exposure to vaccination in each season was included as a time-dependent covariate. For each season, individuals began in the unvaccinated group (and accumulated time at risk) until 14 days after the receipt of the vaccine, and then they switched to the vaccinated group.
In all models used to estimate the vaccine effectiveness, variables associated with the receipt of a vaccination and effect modifiers, such as vaccinations, consultations and hospitalisation in the previous influenza season, SIMD, urban/rural status, smoking status and Charlson Comorbidity Index score, were adjusted for. The main analysis for the non-specific clinical outcomes was a covariate adjustment.
Sample size
A final total sample size of up to 1.25 million people, from 230 practices, was expected. Using data from the Pandemic Influenza Primary Care Reporting (PIPER) 2014/15 study cohort, which had 263,000 individuals (of whom 16% were aged 2–17 years and 18% were aged ≥ 65 years),49 vaccine uptake among children aged 3–12 years was 60% and vaccine uptake among people aged ≥ 65 years was 70%. Linked to this PIPER cohort from all virology tests in Scotland were, overall, 1745 RT-PCR tests, comprising 331 RT-PCR tests among 2- to 17-year-olds and 366 RT-PCR tests among people aged ≥ 65 years. This gave a multiplier ratio of around 5 : 1 from the PIPER cohort to the SIVE II cohort, and this was used to estimate the number of RT-PCR tests expected each year. This study expected 1800 RT-PCR tests per year among people aged ≥ 65 years and 1650 laboratory tests per year among children aged 2–17 years. The study expected 630 (i.e. 1745/12 × 5) asthma patients swabbed per year, because approximately 12% of the population was treated for asthma.
Using data generated from the Seasonal Influenza Vaccine Effectiveness in the community (SIVE) project, the study estimated a vaccination rate of 60% among children targeted for receipt of LAIV and a swab positivity rate of 20% among unvaccinated children. 13 This gave 90% power to detect a vaccine effectiveness of 31% based on 1650 swabs in one season. Pooling data over two seasons gave an estimated 3300 swabs in children eligible for vaccination and a 90% power to detect a vaccine effectiveness of 22%.
For those people aged ≥ 65 years targeted for receipt of TIV, for whom there was a vaccination rate of 70% and a swab positivity rate of 10%, among the unvaccinated individuals the study anticipated an 80% power to detect a vaccine effectiveness of 39%. It was estimated that there would be a need for 1800 swabs each year in the later years. During the peak influenza activity, when swab positivity might have increased to 20%, there is a 90% power to detect a vaccine effectiveness of 31%. Approximately 1 in 12 of the population is treated for asthma and it is anticipated that 1260 swabs are needed among patients with asthma in the final two seasons. 50 Assuming that 40% are vaccinated and that the swab positivity is around 15% gives 80% power for a vaccine effectiveness of 35%. These powers do not take into account design effects for the clustering of patients within GPs. Analyses of the historic PIPER cohorts has revealed a design effect of < 7% and this serves to increase the detectable vaccine effectiveness by about 2 percentage points.
Ethics and governance processes
Permissions were obtained from the Privacy Advisory Committee (NSS) (68/14), the National Research Ethics Committee West Midlands – Edgbaston (15/WM/0035), the National Caldicott Guardian and General Practice Data Custodians. Ms Elisabeth Ehrlich was the study’s public and patient involvement lead and helped with the grant application. From a lay perspective, Ms Ehrlich helped to guide the team, ensuring that the work was relevant to the interests and needs of the public and patients. The study was supported by members of the Asthma UK Centre for Applied Research Patient Advisory Group, which comprises > 60 people (including parents of children). This group helped advise on, and contribute to, study materials and it was invited to comment on the study from the patient and family perspective. An Independent Steering Group was convened, with public and patient involvement, to oversee this work, which comprised Neil Kelly (Chairperson, General Practitioner), Jonathan Van Tam (Professor of Health Protection), Punam Mangtani (Associate Professor Clinical) and Elisabeth Ehrlich (Public and Patient Involvement Representative).
Chapter 4 Results
Vaccine uptake
The uptake of LAIV among the children registered with the 230 practices taking part in this project increased over the study period (Table 2), with nearly two-thirds of primary school-aged children vaccinated by 2015/16. TIV uptake among at-risk patients aged 12–65 years was highest in those aged 55–64 years, with nearly two-thirds being vaccinated. Among those aged ≥ 65 years, vaccine uptake was highest in the 75–84 years age group.
| Season | Age group (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2–4a | 5–11a | 12–17b | 18–54b | 55–64b | 65–74 | 75–84 | ≥ 85 | |
| 2010/11 | – | – | 21.74 | 29.97 | 62.78 | 60.40 | 66.20 | 62.62 |
| 2011/12 | – | – | 21.64 | 30.29 | 63.19 | 60.65 | 66.93 | 63.65 |
| 2012/13 | – | – | 22.32 | 30.69 | 63.48 | 61.40 | 67.74 | 64.90 |
| 2013/14 | 32.20c | – | 23.55 | 30.62 | 63.53 | 61.87 | 68.54 | 65.98 |
| 2014/15 | 39.33 | 58.76 | 24.94 | 29.52 | 61.70 | 61.16 | 67.95 | 65.25 |
| 2015/16 | 40.12 | 59.61 | 23.84 | 28.15 | 59.90 | 59.26 | 67.06 | 65.33 |
In all but two age groups (i.e. 2–4 and ≥ 85 years), more females than males received the influenza vaccine. High levels of vaccine uptake were found among the least deprived children, people living in remote and rural areas of Scotland, people with chronic diseases, people with an emergency hospital admission in the past year (specifically for preschool-aged children and 11- to 17-year-olds), and people with a prior ARI GP consultation in the past year (Table 3). The least deprived at-risk 18- to 55-year-olds were less likely to receive the vaccine. For all those people aged ≥ 65 years and eligible to receive TIV, levels of uptake were similar to levels among children, with higher levels of uptake among the least deprived groups, those people living in rural and remote areas, those people with comorbidities and a prior ARI GP consultation. Those people with an emergency hospitalisation in the last year were less likely to receive the vaccine.
| Variable | Age group (years), OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2–4 | 5–11 | 11–17a | 18–54a | 55–64a | 65–74 | 75–84 | ≥ 85 | |
| Males (vs. females) | 1.01 (0.98 to 1.03) | 0.96 (0.95 to 0.97) | 0.90 (0.86 to 0.95) | 0.59 (0.58 to 0.59) | 0.85 (0.83 to 0.87) | 0.87 (0.86 to 0.88) | 0.95 (0.94 to 0.97) | 1.03 (1.00 to 1.06) |
| SIMD quintile | ||||||||
| 2 (vs. 1) | 1.10 (1.06 to 1.14) | 1.07 (1.05 to 1.09) | 1.10 (1.02 to 1.19) | 0.96 (0.94 to 0.98) | 1.01 (0.98 to 1.05) | 0.95 (0.93 to 0.97) | 0.96 (0.93 to 0.98) | 0.97 (0.93 to 1.02) |
| 3 (vs. 1) | 1.25 (1.21 to 1.30) | 1.17 (1.14 to 1.19) | 1.02 (0.94 to 1.10) | 0.92 (0.90 to 0.94) | 1.01 (0.98 to 1.05) | 1.00 (0.98 to 1.02) | 0.96 (0.93 to 0.99) | 0.92 (0.88 to 0.97) |
| 4 (vs. 1) | 1.42 (1.37 to 1.47) | 1.23 (1.21 to 1.26) | 1.22 (1.13 to 1.32) | 0.91 (0.89 to 0.93) | 1.00 (0.97 to 1.04) | 1.03 (1.01 to 1.06) | 1.08 (1.04 to 1.11) | 1.02 (0.98 to 1.07) |
| 5 (vs. 1)b | 1.76 (1.70 to 1.82) | 1.46 (1.43 to 1.49) | 1.36 (1.26 to 1.47) | 0.86 (0.84 to 0.88) | 1.00 (0.96 to 1.03) | 1.13 (1.11 to 1.16) | 1.19 (1.16 to 1.23) | 1.22 (1.17 to 1.27) |
| UR8FOLD | ||||||||
| UR8FOLD 2 (vs. 1)c | 1.01 (0.99 to 1.04) | 1.24 (1.22 to 1.26) | 0.79 (0.75 to 0.84) | 0.84 (0.82 to 0.85) | 0.84 (0.81 to 0.87) | 1.10 (1.09 to 1.12) | 1.00 (0.98 to 1.02) | 1.02 (0.99 to 1.05) |
| factor(UR8FOLD)3 (vs. 1) | 1.03 (0.98 to 1.07) | 1.32 (1.29 to 1.36) | 0.82 (0.75 to 0.89) | 0.90 (0.88 to 0.93) | 0.88 (0.85 to 0.92) | 1.07 (1.05 to 1.10) | 0.94 (0.91 to 0.97) | 0.79 (0.76 to 0.83) |
| factor(UR8FOLD)4 (vs. 1) | 0.89 (0.83 to 0.95) | 1.22 (1.17 to 1.27) | 0.92 (0.79 to 1.06) | 0.74 (0.70 to 0.78) | 0.59 (0.55 to 0.63) | 0.86 (0.83 to 0.89) | 0.92 (0.88 to 0.96) | 0.89 (0.83 to 0.95) |
| factor(UR8FOLD)5 (vs. 1) | 1.09 (0.99 to 1.20) | 1.64 (1.55 to 1.75) | 1.12 (0.92 to 1.35) | 0.98 (0.92 to 1.05) | 1.00 (0.91 to 1.10) | 0.67 (0.64 to 0.70) | 0.68 (0.64 to 0.72) | 0.72 (0.66 to 0.79) |
| factor(UR8FOLD)6 (vs. 1) | 1.15 (1.10 to 1.20) | 1.27 (1.24 to 1.30) | 0.93 (0.85 to 1.01) | 0.90 (0.87 to 0.92) | 0.84 (0.81 to 0.87) | 1.19 (1.17 to 1.22) | 1.30 (1.26 to 1.34) | 1.50 (1.42 to 1.58) |
| factor(UR8FOLD)7 (vs. 1) | 1.18 (1.10 to 1.28) | 1.26 (1.20 to 1.32) | 1.24 (1.08 to 1.42) | 0.99 (0.94 to 1.04) | 0.78 (0.73 to 0.83) | 1.10 (1.07 to 1.14) | 1.17 (1.11 to 1.23) | 1.48 (1.36 to 1.61) |
| factor(UR8FOLD)8 (vs. 1) | 1.54 (1.43 to 1.65) | 1.74 (1.66 to 1.81) | 1.17 (1.03 to 1.33) | 1.17 (1.12 to 1.23) | 1.05 (0.99 to 1.12) | 1.34 (1.30 to 1.38) | 1.27 (1.21 to 1.33) | 1.26 (1.18 to 1.36) |
| Asthma vs. no asthma | 1.94 (1.76 to 2.15) | 1.69 (1.64 to 1.74) | 1.92 (1.65 to 2.23) | 1.35 (1.31 to 1.39) | 1.33 (1.29 to 1.37) | 2.70 (2.63 to 2.77) | 2.73 (2.63 to 2.84) | 2.33 (2.19 to 2.47) |
| Chronic heart disease vs. no chronic heart disease | 1.68 (1.49 to 1.90) | 1.70 (1.58 to 1.84) | 2.48 (2.13 to 2.89) | 2.64 (2.55 to 2.73) | 2.31 (2.24 to 2.39) | 2.99 (2.92 to 3.06) | 3.14 (3.06 to 3.22) | 2.77 (2.68 to 2.87) |
| Chronic liver disease vs. no chronic liver disease | 2.45 (0.53 to 11.24) | 1.74 (0.97 to 3.12) | 9.35 (4.98 to 17.54) | 1.78 (1.69 to 1.88) | 1.48 (1.38 to 1.58) | 1.90 (1.75 to 2.06) | 2.08 (1.84 to 2.35) | 1.30 (1.03 to 1.64) |
| Chronic neurological disease vs. no chronic neurological disease | 2.03 (1.29 to 3.20) | 1.84 (1.43 to 2.36) | 2.19 (1.66 to 2.89) | 2.08 (1.99 to 2.18) | 1.57 (1.51 to 1.64) | 2.09 (2.03 to 2.15) | 2.24 (2.17 to 2.31) | 2.14 (2.05 to 2.22) |
| COPD vs. no COPD | 1.02 (0.06 to 16.41) | 0.70 (0.33 to 1.46) | 0.93 (0.80 to 1.10) | 0.78 (0.76 to 0.81) | 0.79 (0.76 to 0.82) | 1.18 (1.13 to 1.24) | 1.10 (1.04 to 1.15) | 0.95 (0.87 to 1.03) |
| Diabetes mellitus vs. no diabetes mellitus | 2.73 (1.64 to 4.55) | 2.53 (2.13 to 2.99) | 0.58 (0.23 to 1.43) | 2.36 (2.23 to 2.49) | 2.02 (1.93 to 2.12) | 3.14 (3.06 to 3.21) | 2.85 (2.77 to 2.94) | 2.24 (2.13 to 2.35) |
| Immunosuppression vs. no immunosuppression | 1.54 (0.45 to 5.25) | 1.40 (0.99 to 1.98) | 8.71 (7.33 to 10.35) | 5.06 (4.90 to 5.22) | 3.00 (2.91 to 3.10) | 2.96 (2.71 to 3.25) | 2.67 (2.39 to 2.97) | 2.02 (1.73 to 2.37) |
| Total number of emergency hospitalisations per yeard | 1.10 (1.06 to 1.14) | 1.02 (0.98 to 1.06) | 3.75 (2.67 to 5.27) | 2.57 (2.42 to 2.74) | 2.01 (1.84 to 2.19) | 0.81 (0.79 to 0.82) | 0.73 (0.72 to 0.74) | 0.75 (0.74 to 0.77) |
| Total number of ARI GP consultations per yeard | 1.16 (1.13 to 1.19) | 1.10 (1.07 to 1.12) | 1.24 (1.15 to 1.35) | 1.11 (1.09 to 1.13) | 0.98 (0.95 to 1.00) | 1.37 (1.33 to 1.40) | 1.36 (1.31 to 1.41) | 1.32 (1.25 to 1.39) |
Laboratory-confirmed influenza
Live attenuated influenza vaccine effectiveness
A statistically significant adjusted vaccine effectiveness among children (aged 2–11 years) for preventing RT-PCR laboratory-confirmed influenza was found in 2015/16 [for all influenza, influenza A, influenza B and the significant dominant influenza A(H1N1) subtypes]. This was not, however, evident in 2014/15 (Table 4). Vaccine effectiveness was higher for influenza B than for influenza A subtypes. There were insufficient numbers of children receiving a second dose of LAIV to include in the statistical model.
| Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | ||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | ||||
| Season: 2014/15 | |||||||
| Influenza Ac | 44/131 | 33.59 | 342/1272 | 26.89 | 9.34 | 11.09 (–33.87 to 40.95) | 11.36 (–35.31 to 41.93) |
| A(H1N1) | 3/4 | 75.00 | 383/1399 | 27.38 | 0.29 | –474.59 (–5469.42 to 40.72) | –468.38 (–5949.37 to 46.60) |
| A(H3) | 33/109 | 30.28 | 353/1294 | 27.28 | 7.77 | 27.04 (–14.77 to 53.62) | 30.67 (–10.95 to 56.68) |
| Influenza B | 3/18 | 16.67 | 383/1385 | 27.65 | 1.28 | 62.50 (–31.42 to 89.30) | 69.56 (–9.04 to 91.50) |
| Influenza positived | 47/149 | 31.54 | 339/1254 | 27.03 | 10.62 | 18.72 (–19.91 to 44.91) | 20.54 (–18.53 to 46.73) |
| Season: 2015/16 | |||||||
| Influenza Ac | 42/176 | 23.86 | 410/1513 | 27.10 | 10.42 | 51.62 (28.95 to 67.06) | 46.38 (19.77 to 64.16) |
| A(H1N1) | 36/143 | 25.17 | 416/1546 | 26.91 | 8.47 | 46.27 (18.90 to 64.40) | 40.39 (8.21 to 61.29) |
| A(H3) | 1/5 | 20.00 | 451/1684 | 26.78 | 0.30 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) |
| Influenza B | 5/43 | 11.63 | 447/1646 | 27.16 | 2.55 | 82.86 (50.51 to 94.07) | 88.27 (63.86 to 96.19) |
| Influenza positived | 46/217 | 21.20 | 406/1472 | 27.58 | 12.85 | 57.71 (39.45 to 70.46) | 58.09 (39.08 to 71.17) |
Trivalent influenza vaccine effectiveness
Among at-risk 18- to 64-year-olds, a significant TIV effectiveness was found for four of the six seasons (Table 5). For the two seasons with no significant vaccine effectiveness, low levels of circulating influenza virus were present (2011/12, 5%; 2013/14, 9%). Unlike the pattern in children, there was an inconsistent observation of vaccine effectiveness being greater against B subtypes of influenza than against A subtypes of influenza. Among those people aged ≥ 65 years, significant vaccine effectiveness of 57% was found for the 2013/14 season (Table 6); however, no other season had significant vaccine effectiveness. No obvious pattern of vaccine effectiveness by influenza subtype was evident between the seasons.
| Dominant circulating strain(s) | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Season: 2010–11 | ||||||||
|
A/California/07/2009 (H1N1)pdm2009 B/Brisbane/60/2008 |
Influenza Ac | 39/118 | 33.05 | 156/380 | 41.05 | 23.69 | 46.03 (7.66 to 68.45) | 45.34 (1.37 to 69.71) |
| A(H1N1) | 33/104 | 31.73 | 162/394 | 41.12 | 20.88 | 51.25 (14.27 to 72.28) | 49.01 (5.67 to 72.44) | |
| A(H3) | 0/0 | NA | 195/498 | 39.16 | 0.00 | –1.62E–12 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 6/26 | 23.08 | 189/472 | 40.04 | 5.22 | 73.38 (29.90 to 89.89) | 71.17 (22.61 to 89.26) | |
| Influenza positived | 45/144 | 31.25 | 150/354 | 42.37 | 28.92 | 54.74 (26.91 to 71.98) | 53.04 (21.41 to 71.94) | |
| Season: 2011–12 | ||||||||
| A/Victoria/208/2009 (H3N2)a | Influenza Ac | 12/22 | 54.55 | 131/379 | 34.56 | 5.49 | –34.00 (–236.15 to 46.58) | –65.86 (–333.81 to 36.59) |
| A(H1N1) | 0/0 | NA | 143/401 | 35.66 | 0.00 | –4.66E–13 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| A(H3) | 7/12 | 58.33 | 136/389 | 34.96 | 2.99 | –47.99 (–401.76 to 56.35) | –103.89 (–645.92 to 44.27) | |
| Influenza B | 0/0 | NA | 143/401 | 35.66 | 0.00 | –2.52E–11 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza positived | 12/22 | 54.55 | 131/379 | 34.56 | 5.49 | –34.00 (–236.15 to 46.58) | –65.86 (–333.81 to 36.59) | |
| Season: 2012–13 | ||||||||
| B/Wisconsin/1/2010 | Influenza Ac | 32/91 | 35.16 | 238/562 | 42.35 | 13.94 | 46.14 (12.98 to 66.66) | 45.82 (10.59 to 67.16) |
| A(H1N1) | 1/15 | 6.67 | 269/638 | 42.16 | 2.30 | 92.18 (39.64 to 98.99) | 89.53 (17.41 to 98.67) | |
| A(H3) | 21/49 | 42.86 | 249/604 | 41.23 | 7.50 | 15.71 (–54.02 to 53.87) | 22.60 (–46.92 to 59.23) | |
| Influenza B | 16/43 | 37.21 | 254/610 | 41.64 | 6.58 | 42.47 (–10.78 to 70.12) | 33.19 (–31.48 to 66.05) | |
| Influenza positived | 48/134 | 35.82 | 222/519 | 42.77 | 20.52 | 46.43 (19.06 to 64.55) | 43.50 (13.16 to 63.24) | |
| Season: 2013–14 | ||||||||
| A/California/07/2009 (H1N1)pdm09 | Influenza Ac | 25/55 | 45.45 | 262/608 | 43.09 | 8.30 | 31.29 (–24.55 to 62.10) | 25.41 (–42.75 to 61.02) |
| A(H1N1) | 18/39 | 46.15 | 269/624 | 43.11 | 5.88 | 27.25 (–43.77 to 63.19) | 37.50 (–27.22 to 69.29) | |
| A(H3) | 2/7 | 28.57 | 285/656 | 43.45 | 1.06 | 68.90 (–64.61 to 94.12) | 46.91 (–205.10 to 90.76) | |
| Influenza B | 1/2 | 50.00 | 286/661 | 43.27 | 0.30 | 5.11 (–1482.68 to 94.31) | 66.91 (–641.63 to 98.52) | |
| Influenza positived | 26/57 | 45.61 | 261/606 | 43.07 | 8.60 | 29.88 (–25.65 to 60.87) | 31.07 (–26.85 to 62.54) | |
| Season: 2014–15 | ||||||||
|
A/Texas/50/2012 (H3N2)a B/Yamagata/16/88 |
Influenza Ac | 82/180 | 45.56 | 407/887 | 45.89 | 16.87 | 27.06 (–3.77 to 48.73) | 31.47 (1.31 to 52.42) |
| A(H1N1) | 5/9 | 55.56 | 484/1058 | 45.75 | 0.84 | 18.53 (–232.68 to 80.05) | 23.21 (–219.16 to 81.53) | |
| A(H3) | 62/140 | 44.29 | 427/927 | 46.06 | 13.12 | 28.57 (–5.27 to 51.54) | 29.84 (–4.80 to 53.03) | |
| Influenza B | 14/39 | 35.90 | 475/1028 | 46.21 | 3.66 | 62.08 (22.19 to 81.52) | 64.95 (26.82 to 83.21) | |
| Influenza positived | 95/217 | 43.78 | 394/850 | 46.35 | 20.34 | 33.75 (8.91 to 51.82) | 38.75 (14.88 to 55.92) | |
| Season: 2015–16 | ||||||||
|
A/California/07/2009 (H1N1)pdm09 B/Victoria/2/87 |
Influenza Ac | 55/149 | 36.91 | 434/1101 | 39.42 | 11.92 | 39.11 (11.26 to 58.21) | 42.27 (14.87 to 60.84) |
| A(H1N1) | 44/111 | 39.64 | 445/1139 | 39.07 | 8.88 | 30.00 (–6.47 to 53.97) | 32.85 (–3.26 to 56.33) | |
| A(H3) | 2/4 | 50.00 | 487/1246 | 39.09 | 0.32 | –227.57 (–12,600.90 to 91.55) | –195,334.91 (–3.78E+26 to 100.00) | |
| Influenza B | 16/47 | 34.04 | 473/1203 | 39.32 | 3.76 | 54.53 (11.05 to 76.76) | 41.12 (–19.54 to 70.99) | |
| Influenza positived | 69/193 | 35.75 | 420/1057 | 39.74 | 15.44 | 41.51 (18.16 to 58.20) | 40.88 (16.43 to 58.17) | |
| Dominant circulating strain(s) | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Season: 2010–11 | ||||||||
|
A/California/07/2009 (H1N1)pdm2009 B/Brisbane/60/2008 |
Influenza Ac | 25/53 | 47.17 | 234/458 | 51.09 | 10.37 | 31.84 (–24.33 to 62.63) | 42.76 (–13.13 to 71.03) |
| A(H1N1) | 19/39 | 48.72 | 240/472 | 50.85 | 7.63 | 28.99 (–43.07 to 64.75) | 28.18 (–53.73 to 66.45) | |
| A(H3) | 0/0 | NA | 259/511 | 50.68 | 0.00 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 14/28 | 50.00 | 245/483 | 50.72 | 5.48 | 41.79 (–33.29 to 74.58) | 35.01 (–64.89 to 74.46) | |
| Influenza positived | 37/78 | 47.44 | 222/433 | 51.27 | 15.26 | 36.20 (–5.77 to 61.52) | 43.38 (–0.15 to 67.99) | |
| Season: 2011–12 | ||||||||
| A/Victoria/208/2009 (H3N2)a | Influenza Ac | 15/23 | 65.22 | 280/475 | 58.95 | 4.62 | –3.28 (–157.62 to 58.60) | –6.80 (–187.75 to 60.89) |
| A(H1N1) | 0/0 | NA | 295/498 | 59.24 | 0.00 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| A(H3) | 8/12 | 80.00 | 287/488 | 58.81 | 2.01 | –104.95 (–892.87 to 57.70) | –87.17 (–867.16 to 63.78) | |
| Influenza B | 0/2 | 0.00 | 295/496 | 59.48 | 0.40 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 15/24 | 62.50 | 280/474 | 59.07 | 4.82 | 9.08 (–120.05 to 62.44) | 6.50 (–136.90 to 63.10) | |
| Season: 2012–13 | ||||||||
| B/Wisconsin/1/2010 | Influenza Ac | 78/134 | 58.21 | 450/818 | 55.01 | 0.48 | –2.29 (–50.49 to 30.47) | 13.95 (–31.48 to 43.68) |
| A(H1N1) | 8/8 | 37.50 | 525/944 | 55.61 | 5.25 | 60.32 (–69.43 to 90.71) | 64.10 (–69.39 to 92.39) | |
| A(H3) | 50/78 | 64.10 | 478/874 | 54.69 | 0.00 | –38.27 (–126.17 to 15.47) | –23.86 (–111.01 to 27.30) | |
| Influenza B | 29/53 | 54.72 | 499/899 | 55.51 | 2.02 | 14.98 (–51.13 to 52.17) | 15.03 (–59.74 to 54.80) | |
| Influenza positived | 107/186 | 57.53 | 421/766 | 54.96 | 8.57 | 4.41 (–34.24 to 31.93) | 15.67 (–21.76 to 41.60) | |
| Season: 2013–14 | ||||||||
| A/California/07/2009 (H1N1)pdm09 | Influenza Ac | 31/53 | 58.49 | 618/1058 | 58.41 | 4.77 | 31.18 (–22.14 to 61.22) | 55.47 (17.53 to 75.96) |
| A(H1N1) | 24/39 | 61.54 | 625/1072 | 58.30 | 3.51 | 16.92 (–61.47 to 57.25) | 39.51 (–22.62 to 70.16) | |
| A(H3) | 2/5 | 40.00 | 647/1106 | 58.50 | 0.45 | 69.14 (–89.40 to 94.97) | 100.00 (–1187.54 to 100.00) | |
| Influenza B | 0/1 | 0.00 | 649/1110 | 58.47 | 0.09 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 31/54 | 57.41 | 618/1057 | 58.47 | 4.86 | 33.06 (–18.01 to 62.03) | 56.63 (20.26 to 76.42) | |
| Season: 2014–15 | ||||||||
|
A/Texas/50/2012 (H3N2)a B/Yamagata/16/88 |
Influenza Ac | 282/488 | 57.79 | 1127/2037 | 55.33 | 19.33 | 12.11 (–9.10 to 29.21) | 13.58 (–8.92 to 31.43) |
| A(H1N1) | 9/12 | 75.00 | 1400/2513 | 55.71 | 0.48 | –124.89 (–754.55 to 40.82) | –145.32 (–1171.67 to 52.68) | |
| A(H3) | 224/393 | 57.00 | 1185/2132 | 55.58 | 15.56 | 15.96 (–6.05 to 33.39) | 15.84 (–8.03 to 34.43) | |
| Influenza B | 54/84 | 64.29 | 1355/2441 | 55.51 | 3.33 | –14.76 (–86.81 to 29.50) | –1.82 (–76.04 to 41.11) | |
| Influenza positived | 335/568 | 58.98 | 1074/1957 | 54.88 | 22.50 | 8.39 (–11.93 to 25.02) | 11.90 (–9.24 to 28.96) | |
| Season: 2015–16 | ||||||||
|
A/California/07/2009 (H1N1)pdm09 B/Victoria/2/87 |
Influenza Ac | 113/183 | 61.75 | 1264/2350 | 53.79 | 7.22 | –5.26 (–44.95 to 23.57) | 7.61 (–30.39 to 34.53) |
| A(H1N1) | 81/133 | 60.90 | 1296/2400 | 54.00 | 5.25 | 1.47 (–42.04 to 31.66) | 10.21 (–33.15 to 39.45) | |
| A(H3) | 2/3 | 66.67 | 1375/2530 | 54.35 | 0.12 | –179.37 (–4614.03 to 83.44) | –34.41 (–2046.34 to 91.58) | |
| Influenza B | 47/82 | 57.32 | 1330/2451 | 54.26 | 3.24 | 11.48 (–40.96 to 44.42) | 4.02 (–57.78 to 41.61) | |
| Influenza positived | 159/263 | 60.46 | 1218/2270 | 53.66 | 10.38 | 0.76 (–30.05 to 24.28) | 7.31 (–23.73 to 30.57) | |
At-risk comorbidity vaccine effectiveness
A pooled analysis over six seasons found a significant positive vaccine effectiveness for preventing RT-PCR laboratory-confirmed influenza for those aged people ≥ 65 years with asthma (Table 7). Significant positive vaccine effectiveness was found for preventing laboratory-confirmed influenza A among those people aged ≥ 65 years with chronic heart disease. Generally, vaccine effectiveness was non-statistically higher against influenza B than against influenza A.
An analysis of age groups found significant vaccine effectiveness for (1) 18- to 54-year-olds with asthma, impaired immune function and a body mass index (BMI) of > 25 kg/m2 (Table 8); (2) 55- to 64-year-olds with asthma (Table 9); (3) 65- to 74-year-olds with asthma and chronic kidney disease (overall and for influenza A) (Table 10); and (4) 75- to 84-year-olds with chronic respiratory disease [against influenza A(H3N2)] and asthma (against influenza B) (Table 11). Among the oldest age group (i.e. those people aged ≥ 85 years), significant vaccine effectiveness was found for those with chronic respiratory disease (overall and for influenza A and B), chronic heart disease (overall), asthma [overall, for influenza A and B, and for influenza A(H3N2)], diabetes mellitus (overall and for influenza A) and impaired immune function (overall) (Table 12).
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 178/287 | 62.02 | 1332/2209 | 60.30 | 11.50 | 13.60 (–13.84 to 13.21) | 13.21 (–14.46 to 34.19) |
| A(H1N1) | 62/92 | 67.39 | 1448/2404 | 60.23 | 3.69 | –0.92 (–61.95 to 37.11) | –2.93 (–66.33 to 32.29) | |
| A(H3) | 79/132 | 59.85 | 1431/2364 | 60.53 | 5.29 | 12.09 (–31.45 to 41.21) | 12.48 (–30.89 to 41.48) | |
| Influenza B | 39/62 | 62.90 | 1471/2434 | 60.44 | 2.48 | 15.38 (–48.88 to 51.91) | 19.04 (–43.25 to 54.24) | |
| Influenza positived | 217/347 | 62.54 | 1293/2149 | 60.17 | 13.90 | 15.06 (–9.15 to 33.90) | 15.72 (–8.43 to 34.50) | |
| Chronic heart disease | Influenza Ac | 193/256 | 75.39 | 1249/1688 | 73.99 | 13.17 | 31.95 (4.22 to 51.65) | 33.12 (5.72 to 52.56) |
| A(H1N1) | 51/60 | 85.00 | 1391/1884 | 73.83 | 3.09 | –33.65 (–182.87 to 6.86) | –31.91 (–181.45 to 38.18) | |
| A(H3) | 94/128 | 73.44 | 1348/1816 | 74.23 | 6.58 | 36.47 (–1.44 to 60.21) | 35.74 (–2.79 to 59.82) | |
| Influenza B | 42/50 | 84.00 | 1400/1894 | 73.92 | 2.57 | 1.58 (–122.38 to 56.44) | 8.34 (–110.18 to 60.03) | |
| Influenza positived | 232/303 | 76.57 | 1210/1641 | 73.74 | 15.59 | 28.71 (1.78 to 48.25) | 30.88 (–4.45 to 49.94) | |
| Asthma | Influenza Ac | 114/148 | 77.03 | 748/998 | 74.95 | 12.91 | 43.06 (6.27 to 65.41) | 44.93 (8.73 to 66.77) |
| A(H1N1) | 40/49 | 81.63 | 822/1097 | 74.93 | 4.28 | 12.72 (–95.99 to 61.13) | 12.70 (–100.41 to 61.97) | |
| A(H3) | 54/65 | 83.08 | 808/1081 | 74.75 | 5.67 | –14.47 (148.84 to 47.34) | –3.95 (–128.44 to 52.69) | |
| Influenza B | 24/34 | 70.59 | 838/1112 | 75.36 | 3.06 | 65.33 (19.31 to 85.10) | 66.34 (19.99 to 85.84) | |
| Influenza positived | 137/181 | 75.69 | 725/965 | 75.13 | 7.07 | 48.77 (20.91 to 66.81) | 50.69 (23.26 to 68.31) | |
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 27/74 | 36.49 | 182/496 | 36.69 | 12.98 | 20.12 (–45.75 to 56.22) | 24.43 (–41.03 to 59.51) |
| A(H1N1) | 11/32 | 34.38 | 198/538 | 36.80 | 5.61 | 31.01 (–84.47 to 74.20) | 26.50 (–87.95 to 71.25) | |
| A(H3) | 9/26 | 34.62 | 200/544 | 36.76 | 4.56 | 30.75 (–86.64 to 74.30) | 38.10 (–83.17 to 79.08) | |
| Influenza B | 6/20 | 30.00 | 203/550 | 36.91 | 3.51 | 61.28 (–24.82 to 87.99) | 62.37 (–23.32 to 88.52) | |
| Influenza positived | 33/94 | 35.11 | 176/476 | 36.97 | 16.49 | 27.59 (–22.60 to 57.24) | 29.70 (–20.53 to 59.00) | |
| Chronic heart disease | Influenza Ac | 12/29 | 41.38 | 86/204 | 42.16 | 12.45 | 24.83 (–104.11 to 72.31) | 32.86 (–85.58 to 75.71) |
| A(H1N1) | 5/14 | 35.71 | 93/219 | 42.47 | 6.01 | –158.96 (–4645.69 to 85.87) | 90.67 (–13,107.43 to 99.99) | |
| A(H3) | 5/10 | 50.00 | 93/223 | 41.70 | 4.29 | 18.20 (–1897.24 to 96.65) | 100.00 (–7.08E+11 to 100.00) | |
| Influenza B | 6/10 | 60.00 | 92/223 | 41.26 | 4.29 | 31.84 (–430.94 to 91.25) | 100.00 (–inf to 100.00) | |
| Influenza positived | 16/37 | 43.24 | 82/196 | 41.84 | 15.88 | 4.15 (–116.09 to 57.49) | 7.83 (–108.90 to 59.33) | |
| Asthma | Influenza Ac | 72/280 | 25.71 | 546/1600 | 34.13 | 14.89 | 50.88 (32.30 to 64.36) | 52.42 (33.50 to 65.95) |
| A(H1N1) | 33/141 | 23.40 | 585/1739 | 33.64 | 7.50 | 49.47 (19.92 to 68.12) | 55.98 (28.33 to 72.97) | |
| A(H3) | 22/83 | 26.51 | 596/1797 | 33.17 | 4.41 | 59.78 (29.76 to 76.97) | 53.36 (17.59 to 73.61) | |
| Influenza B | 21/82 | 25.61 | 597/1798 | 33.20 | 4.36 | 58.40 (28.08 to 75.93) | 62.41 (33.79 to 78.66) | |
| Influenza positived | 92/361 | 25.48 | 526/1519 | 34.63 | 19.20 | 52.21 (36.38 to 64.10) | 54.74 (38.97 to 66.43) | |
| Chronic liver disease | Influenza Ac | 5/15 | 33.33 | 53/145 | 36.55 | 9.38 | 32.90 (–140.51 to 81.28) | 37.88 (–130.17 to 83.24) |
| A(H1N1) | 2/6 | 33.33 | 56/154 | 36.36 | 3.75 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| A(H3) | 1/5 | 20.00 | 57/155 | 36.77 | 3.13 | 19.06 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 3/5 | 60.00 | 55/155 | 35.48 | 3.13 | 0.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 8/20 | 40.00 | 50/140 | 35.71 | 12.50 | 22.92 (–130.59 to 74.23) | 32.61 (–109.70 to 78.34) | |
| Chronic neurological disease | Influenza Ac | 12/14 | 64.29 | 52/109 | 47.71 | 11.38 | –267.18 (–12,633.70 to 89.41) | –4.24E+5 (–inf to 100.00) |
| A(H1N1) | 4/9 | 44.44 | 57/114 | 50.00 | 7.32 | 79.30 (–52.98 to 97.20) | 100.00 (–inf to 100.00) | |
| A(H3) | 1/1 | 100.00 | 60/122 | 49.18 | 0.81 | –2.8E+11 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 0/2 | 0.00 | 61/121 | 50.41 | 1.63 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 9/16 | 56.25 | 52/107 | 48.60 | 13.01 | 60.34 (–74.89 to 91.00) | 59.10 (–182.08 to 94.07) | |
| Diabetes mellitus | Influenza Ac | 29/56 | 51.79 | 206/379 | 54.35 | 12.87 | 27.04 (–34.28 to 60.36) | 30.00 (–30.15 to 62.35) |
| A(H1N1) | 13/23 | 56.52 | 222/412 | 53.88 | 5.29 | –3.30 (–171.77 to 60.73) | 11.16 (–182.82 to 72.09) | |
| A(H3) | 11/19 | 57.89 | 224/416 | 53.85 | 4.37 | –45.50 (–420.43 to 59.32) | –48.82 (–447.01 to 59.51) | |
| Influenza B | 9/17 | 52.94 | 226/418 | 54.07 | 3.91 | 79.64 (11.24 to 95.33) | 80.48 (–9.48 to 96.52) | |
| Influenza positived | 38/73 | 52.05 | 197/362 | 54.42 | 16.78 | 33.74 (–13.99 to 61.49) | 33.87 (–14.78 to 61.90) | |
| Impaired immune function | Influenza Ac | 6/20 | 30.00 | 88/234 | 37.61 | 7.87 | 80.71 (–25.42 to 97.03) | 100.00 (–2.4E+10 to 100.00) |
| A(H1N1) | 1/5 | 20.00 | 93/249 | 37.35 | 1.97 | 99.09 (–6.4E+8 to 100.00) | 100.00 (–1.85E+13 to 100.00) | |
| A(H3) | 4/10 | 40.00 | 90/244 | 36.89 | 3.94 | 70.38 (–81.40 to 95.16) | 64.44 (–121.43 to 94.29) | |
| Influenza B | 5/12 | 41.67 | 89/242 | 36.78 | 4.72 | 64.12 (92.08 to 4.72) | 67.42 (–57.79 to 93.27) | |
| Influenza positived | 11/32 | 34.38 | 83/222 | 37.39 | 12.60 | 66.46 (13.45 to 87.00) | 67.51 (15.08 to 87.57) | |
| Chronic kidney disease | Influenza Ac | 10/26 | 38.46 | 80/157 | 50.96 | 14.21 | 98.42 (74.77 to 99.90) | 100.00 (–262E+23 to 100.00) |
| A(H1N1) | 3/10 | 30.00 | 87/173 | 50.29 | 5.46 | 100.00 (–2.25E+25 to 100.00) | 100.00 (–1.29E+30 to 100.00) | |
| A(H3) | 6/12 | 50.00 | 84/171 | 49.12 | 6.56 | 49.55 (–239.03 to 92.49) | 92.70 (–182.86 to 99.81) | |
| Influenza B | 4/4 | 100.00 | 86/179 | 48.04 | 2.19 | –1.26E+22 (–inf to 100.00) | –1.21E+30 (–inf to 100.00) | |
| Influenza positived | 14/30 | 46.67 | 76/153 | 49.67 | 16.39 | 52.28 (–30.13 to 82.50) | 69.12 (–45.74 to 93.46) | |
| eBMI > 25 kg/m2 | Influenza Ac | 6/45 | 15.56 | 75/267 | 28.09 | 14.42 | 70.94 (25.87 to 88.61) | 78.52 (39.16 to 92.42) |
| A(H1N1) | 6/31 | 19.35 | 76/281 | 27.05 | 9.94 | 60.15 (–10.00 to 85.56) | 64.10 (–12.07 to 88.50) | |
| A(H3) | 1/1 | 100.00 | 81/311 | 26.05 | 0.32 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 3/14 | 21.43 | 79/298 | 26.51 | 4.49 | 65.65 (–63.58 to 92.79) | 49.38 (–200.13 to 91.46) | |
| Influenza positived | 9/58 | 15.52 | 73/254 | 28.74 | 18.59 | 65.04 (23.95 to 83.93) | 68.35 (24.52 to 86.72) | |
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 60/102 | 58.82 | 283/583 | 48.54 | 14.89 | –7.33 (–73.60 to 33.65) | –12.43 (–83.74 to 31.20) |
| A(H1N1) | 26/44 | 59.09 | 317/641 | 49.45 | 6.42 | –11.59 (–127.76 to 45.21) | –22.93 (–157.48 to 41.31) | |
| A(H3) | 21/37 | 56.76 | 322/648 | 49.69 | 5.40 | 23.85 (–71.39 to 66.16) | –5.09 (–142.55 to 54.47) | |
| Influenza B | 7/14 | 50.00 | 336/671 | 50.07 | 2.04 | 55.98 (–46.59 to 86.78) | 60.06 (–35.65 to 88.24) | |
| Influenza positived | 67/116 | 57.76 | 276/569 | 48.51 | 16.93 | 1.56 (–54.40 to 37.24) | –1.72 (–60.85 to 35.67) | |
| Chronic heart disease | Influenza Ac | 27/44 | 61.36 | 184/297 | 61.95 | 12.90 | 49.15 (–9.49 to 76.39) | 49.09 (–9.66 to 76.37) |
| A(H1N1) | 12/21 | 57.14 | 199/320 | 62.19 | 6.16 | 58.83 (–19.25 to 85.79) | 61.84 (–16.59 to 87.51) | |
| A(H3) | 8/14 | 57.14 | 203/327 | 62.08 | 4.11 | 47.05 (–76.42 to 84.11) | 47.66 (–77.55 to 84.57) | |
| Influenza B | 5/7 | 71.43 | 206/334 | 61.68 | 2.05 | 7.37 (–573.80 to 87.27) | –2157.56 (–209,967.00 to 75.74) | |
| Influenza positived | 32/51 | 62.75 | 179/290 | 61.72 | 14.96 | 41.64 (–18.00 to 71.14) | 39.89 (–21.35 to 70.23) | |
| Asthma | Influenza Ac | 42/75 | 56.00 | 265/494 | 53.64 | 13.18 | 30.80 (–19.82 to 60.03) | 35.62 (–13.78 to 63.57) |
| A(H1N1) | 17/32 | 53.13 | 290/537 | 54.00 | 5.62 | 35.26 (–63.07 to 74.29) | 35.68 (–68.54 to 75.45) | |
| A(H3) | 18/29 | 62.07 | 289/540 | 53.52 | 5.10 | 8.52 (–138.11 to 64.85) | 16.28 (–130.89 to 69.64) | |
| Influenza B | 12/15 | 13.33 | 305/554 | 55.05 | 2.64 | 92.95 (66.23 to 98.53) | 94.77 (71.65 to 99.04) | |
| Influenza positived | 44/90 | 48.89 | 263/479 | 54.91 | 15.82 | 51.21 (19.24 to 70.52) | 55.48 (24.93 to 73.60) | |
| Chronic liver disease | Influenza Ac | 5/12 | 41.67 | 32/60 | 53.33 | 16.67 | 82.66 (–140.51 to 81.28) | 100.00 (–inf to 100.00) |
| A(H1N1) | 1/4 | 25.00 | 36/68 | 52.94 | 5.56 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| A(H3) | 4/7 | 57.14 | 33/65 | 50.77 | 9.72 | 61.95 (–737.95 to 98.27) | 100.00 (–inf to 100.00) | |
| Influenza B | 0/2 | 0.00 | 37/70 | 52.86 | 2.78 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 5/13 | 38.46 | 32/59 | 54.24 | 18.06 | 88.74 (–81.98 to 99.30) | 100.00 (–inf to 100.00) | |
| Chronic neurological disease | Influenza Ac | 19/28 | 67.86 | 83/159 | 52.20 | 14.97 | –2.2E+17 (–2.16E+17 to 99.78) | –6.7E+6 (–3.67E+17 to 100.00) |
| A(H1N1) | 8/10 | 80.00 | 94/177 | 53.11 | 5.35 | –8.3E+14 (–1.10E+10 to 100.00) | –2.53E+12 (–inf to 100.00) | |
| A(H3) | 9/13 | 69.23 | 93/174 | 53.45 | 6.95 | –1227.12 (–23,294.75 to 24.72) | –1.43E+34 (–inf to 100.00) | |
| Influenza B | 2/3 | 66.67 | 100/184 | 54.35 | 1.60 | –285.94 (–15,731.02 to 90.59) | –2.65E+5 (–1.34E+29 to 100.00) | |
| Influenza positived | 21/31 | 67.74 | 81/156 | 51.92 | 16.58 | –524.31 (–2945.94 to 27.96) | –1007.43 (–6944.49 to –74.09) | |
| Diabetes mellitus | Influenza Ac | 39/59 | 66.10 | 187/306 | 61.11 | 16.16 | 32.65 (–45.22 to 68.77) | 34.65 (–46.44 to 70.84) |
| A(H1N1) | 14/24 | 58.33 | 212/341 | 62.17 | 6.58 | –24.77 (478.667 to 73.10) | 26.51 (–179.83 to 80.70) | |
| A(H3) | 16/23 | 69.57 | 210/342 | 61.40 | 6.30 | 0.04 (–233.25 to 70.02) | 23.17 (–188.28 to 79.52) | |
| Influenza B | 3/6 | 50.00 | 223/359 | 62.12 | 1.64 | 100.00 (–inf to 100.00) | 55.39 (–397.51 to 96.00) | |
| Influenza positived | 42/64 | 65.63 | 184/301 | 61.13 | 17.53 | 32.92 (–31.24 to 65.72) | 36.40 (–29.72 to 68.82) | |
| Impaired immune function | Influenza Ac | 12/16 | 75.00 | 65/138 | 47.10 | 10.39 | –106.35 (–807.45 to 53.08) | –97.56 (–860.25 to 59.36) |
| A(H1N1) | 3/4 | 75.00 | 74/150 | 49.33 | 2.60 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| A(H3) | 7/8 | 87.50 | 70/146 | 47.95 | 5.19 | –3.67E+82 (–inf to 100.00) | –2.25E+68 (–inf to 100.00) | |
| Influenza B | 0/3 | 0.00 | 77/151 | 50.99 | 1.95 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 12/19 | 63.16 | 65/135 | 48.15 | 12.34 | 28.43 (–165.04 to 80.68) | 27.79 (–193.54 to 82.24) | |
| Chronic kidney disease | Influenza Ac | 20/29 | 68.97 | 110/158 | 69.62 | 15.51 | 32.90 (–79.79 to 74.96) | 22.56 (–114.57 to 72.05) |
| A(H1N1) | 6/10 | 60.00 | 124/177 | 70.06 | 5.35 | 67.92 (–553.88 to 98.43) | –4.5E+19 (–inf to 100.00) | |
| A(H3) | 10/14 | 71.43 | 120/173 | 69.36 | 7.49 | 26.65 (–546.44 to 91.68) | –8.52 (–901.07 to 88.24) | |
| Influenza B | 3/5 | 60.00 | 127/182 | 69.78 | 2.67 | 100.00 (–inf to 100.00) | –1.6E+14 (–inf to 100.00) | |
| Influenza positived | 23/33 | 69.70 | 107/154 | 69.48 | 17.65 | 74.94 (–21.45 to 94.83) | 67.38 (–77.57 to 94.01) | |
| eBMI > 25 kg/m2 | Influenza Ac | 9/20 | 45.00 | 72/134 | 53.73 | 12.99 | 62.18 (–7.14 to 86.65) | 61.62 (–27.75 to 88.47) |
| A(H1N1) | 6/13 | 46.15 | 75/141 | 53.19 | 8.44 | 56.94 (–55.13 to 88.05) | 69.12 (–43.86 to 93.37) | |
| A(H3) | 1/1 | 100.00 | 80/153 | 52.29 | 0.65 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza B | 2/2 | 100.00 | 79/152 | 51.97 | 1.30 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Influenza positived | 11/22 | 50.00 | 70/132 | 53.03 | 14.29 | 57.20 (–19.30 to 84.65) | 57.81 (–34.78 to 86.79) | |
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 90/142 | 63.38 | 591/1024 | 57.71 | 12.18 | 5.15 (–41.73 to 36.53) | 6.96 (–39.56 to 37.98) |
| A(H1N1) | 37/58 | 63.79 | 644/1108 | 58.12 | 4.97 | 6.54 (–67.71 to 47.92) | 10.54 (–61.81 to 50.54) | |
| A(H3) | 35/51 | 68.63 | 646/1115 | 57.94 | 4.37 | –59.82 (–215.67 to 19.09) | –57.72 (–212.17 to 20.31) | |
| Influenza B | 20/30 | 66.67 | 661/1136 | 58.19 | 2.57 | 5.29 (–123.24 to 59.82) | 12.59 (–110.36 to 63.68) | |
| Influenza positived | 110/172 | 63.95 | 571/994 | 57.44 | 14.75 | 6.52 (–36.77 to 36.10) | 9.21 (–33.29 to 38.16) | |
| Chronic heart disease | Influenza Ac | 61/78 | 78.21 | 436/591 | 73.77 | 11.66 | 18.54 (–53.83 to 56.86) | 22.75 (–46.68 to 59.32) |
| A(H1N1) | 22/29 | 75.86 | 475/640 | 74.22 | 4.33 | 18.15 (–119.09 to 69.42) | 41.19 (–72.66 to 79.97) | |
| A(H3) | 21/27 | 77.78 | 476/642 | 74.14 | 4.04 | 20.35 (–122.69 to 71.51) | 21.11 (–121.12 to 71.86) | |
| Influenza B | 15/19 | 78.95 | 482/650 | 74.15 | 2.84 | 22.97 (–159.73 to 77.16) | 29.22 (–145.98 to 79.64) | |
| Influenza positived | 76/97 | 78.35 | 421/572 | 73.60 | 14.50 | 19.97 (–40.43 to 54.39) | 23.50 (–34.88 to 56.62) | |
| Asthma | Influenza Ac | 49/71 | 69.01 | 343/465 | 73.76 | 13.25 | 51.09 (4.24 to 75.02) | 52.41 (6.50 to 75.78) |
| A(H1N1) | 22/30 | 73.33 | 370/506 | 73.12 | 5.60 | 49.37 (–33.86 to 80.85) | 51.88 (–32.58 to 82.53) | |
| A(H3) | 18/24 | 75.00 | 374/512 | 73.05 | 4.48 | –3.42 (–225.38 to 67.13) | 0.43 (–212.35 to 68.26) | |
| Influenza B | 12/13 | 61.54 | 384/523 | 73.42 | 2.43 | 62.96 (–25.05 to 89.03) | 58.66 (–41.25 to 87.90) | |
| Influenza positived | 57/84 | 67.86 | 335/452 | 74.12 | 15.67 | 52.24 (12.13 to 74.04) | 52.82 (12.90 to 74.44) | |
| Chronic liver disease | Influenza Ac | 6/8 | 75.00 | 41/62 | 66.13 | 11.43 | 23.75 (–1005.13 to 94.74) | –130.96 (–6164.46 to 91.48) |
| A(H1N1) | 3/4 | 75.00 | 44/66 | 66.67 | 5.71 | –34.53 (–10,492.47 to 98.29) | 100.00 (–3.43E+53 to 100.00) | |
| A(H3) | 2/2 | 100.00 | 45/68 | 66.18 | 2.86 | –8.41E+27 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) | |
| Influenza B | 2/2 | 100.00 | 45/68 | 66.18 | 2.86 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza positived | 8/10 | 80.00 | 39/60 | 65.00 | 14.29 | 37.22 (–2021.30 to 98.14) | 22.24 (–4917.79 to 98.80) | |
| Chronic neurological disease | Influenza Ac | 34/44 | 77.27 | 201/298 | 67.45 | 12.87 | –51.60 (–271.90 to 38.20) | –46.06 (–264.66 to 41.50) |
| A(H1N1) | 12/15 | 80.00 | 223/327 | 68.20 | 4.39 | –66.92 (–619.06 to 61.25) | –51.30 (–579.46 to 66.31) | |
| A(H3) | 16/19 | 84.21 | 219/323 | 67.80 | 5.56 | –253.35 (–1593.54 to 26.27) | –669.38 (–7677.05 to 23.89) | |
| Influenza B | 10/14 | 71.43 | 225/328 | 68.60 | 4.09 | 34.55 (–180.26 to 84.71) | 45.60 (–152.01 to 88.26) | |
| Influenza positived | 44/58 | 75.86 | 191/284 | 67.25 | 16.96 | –25.43 (–175.38 to 42.87) | –17.04 (–161.96 to 47.70) | |
| Diabetes mellitus | Influenza Ac | 38/52 | 73.08 | 329/429 | 76.69 | 10.81 | 50.02 (–9.24 to 77.13) | 49.22 (–11.31 to 76.84) |
| A(H1N1) | 13/19 | 68.42 | 354/462 | 76.62 | 3.95 | 58.44 (–30.95 to 86.81) | 59.52 (–30.04 to 87.40) | |
| A(H3) | 16/18 | 88.89 | 351/463 | 75.81 | 3.74 | –37.95 (–624.27 to 73.72) | –43.05 (–651.03 to 72.75) | |
| Influenza B | 14/17 | 82.35 | 353/464 | 76.08 | 3.53 | –66.82 (–808.29 to 69.36) | –61.40 (–886.95 to 73.61) | |
| Influenza positived | 52/68 | 76.47 | 315/413 | 76.27 | 14.14 | 36.75 (–23.40 to 67.58) | 34.06 (–29.41 to 6.40) | |
| Impaired immune function | Influenza Ac | 6/8 | 75.00 | 135/183 | 73.77 | 4.19 | 67.77 (–221.12 to 96.77) | 100.00 (–inf to 100.00) |
| A(H1N1) | 3/3 | 100.00 | 138/188 | 73.40 | 1.57 | 92.84 (–inf to 100.00) | –1.14E+138 (–inf to 100.00) | |
| A(H3) | 1/1 | 100.00 | 140/190 | 73.68 | 0.52 | –8E+11 (–inf to 100.00) | –2.14E+19 (–inf to 100.00) | |
| Influenza B | 8/10 | 80.00 | 133/181 | 73.48 | 5.24 | 43.45 (–856.17 to 96.66) | 34.58 (–1089.97 to 96.40) | |
| Influenza positived | 14/18 | 77.78 | 127/173 | 73.41 | 9.42 | 59.57 (–136.58 to 93.09) | 61.62 (–148.18 to 94.07) | |
| Chronic kidney disease | Influenza Ac | 31/45 | 68.89 | 241/320 | 75.31 | 12.33 | 56.98 (5.23 to 80.47) | 60.15 (11.32 to 82.09) |
| A(H1N1) | 11/18 | 61.11 | 261/347 | 75.22 | 4.93 | 67.19 (–14.61 to 90.61) | 71.44 (–3.93 to 92.15) | |
| A(H3) | 10/14 | 71.43 | 262/351 | 74.64 | 3.84 | 62.85 (–62.04 to 91.48) | 71.24 (–37.04 to 93.96) | |
| Influenza B | 10/14 | 71.43 | 262/351 | 74.64 | 3.84 | 40.63 (–163.49 to 86.62) | 77.23 (–135.16 to 97.79) | |
| Influenza positived | 41/58 | 70.69 | 231/307 | 75.24 | 15.89 | 55.71 (10.27 to 78.14) | 59.90 (17.23 to 80.57) | |
| eBMI > 25 kg/m2 | Influenza Ac | 25/28 | 89.29 | 190/239 | 79.50 | 10.49 | 2.39 (–272.14 to 74.39) | –16.90 (–352.53 to 69.80) |
| A(H1N1) | 19/21 | 90.48 | 196/246 | 79.67 | 7.87 | –17.06 (–465.56 to 75.77) | –25.32 (–526.88 to 74.95) | |
| A(H3) | 1/1 | 100.00 | 214/266 | 80.45 | 0.37 | –3.2E+12 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza B | 9/10 | 90.00 | 206/257 | 80.16 | 3.75 | 19.89 (–616.34 to 91.04) | 32.04 (–238.13 to 68.98) | |
| Influenza positived | 34/38 | 89.47 | 181/229 | 79.04 | 14.23 | 6.45 (–204.17 to 71.23) | –2.42 (–238.13 to 68.98) | |
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 65/111 | 58.56 | 522/855 | 61.05 | 11.49 | 23.20 (–19.47 to 50.63) | 22.63 (–20.86 to 50.48) |
| A(H1N1) | 22/29 | 75.86 | 565/937 | 60.30 | 3.00 | –88.16 (–391.61 to 27.98) | –91.64 (–413.21 to 28.44) | |
| A(H3) | 29/59 | 49.15 | 558/907 | 61.52 | 6.11 | 53.55 (14.37 to 74.81) | 52.02 (11.10 to 74.10) | |
| Influenza B | 9/18 | 50.00 | 578/948 | 60.97 | 1.86 | 50.96 (–44.37 to 83.34) | 47.97 (–56.81 to 82.74) | |
| Influenza positived | 74/127 | 58.27 | 513/839 | 61.14 | 13.15 | 30.83 (–4.20 to 54.09) | 29.84 (–6.20 to 53.65) | |
| Chronic heart disease | Influenza Ac | 94/116 | 81.03 | 502/693 | 72.44 | 14.34 | –4.52 (–8186 to 39.93) | –6.93 (–87.95 to 39.17) |
| A(H1N1) | 21/22 | 95.45 | 575/787 | 73.06 | 2.72 | –520.47 (–4722.90 to 20.18) | –855.25 (–10,783.76 to 16.16) | |
| A(H3) | 50/63 | 79.37 | 546/746 | 73.19 | 7.79 | 17.73 (–17.85 to 62.37) | 17.25 (–79.69 to 61.89) | |
| Influenza B | 13/15 | 86.67 | 583/794 | 73.43 | 1.85 | –13.32 (–453.61 to 76.80) | –17.24 (–547.17 to 78.76) | |
| Influenza positived | 105/129 | 81.40 | 491/680 | 72.21 | 15.95 | –7.43 (–81.86 to 36.54) | –6.91 (–82.98 to 37.54) | |
| Asthma | Influenza Ac | 47/54 | 87.04 | 289/392 | 73.72 | 12.11 | –2.36 (–160.98 to 59.85) | 5.13 (–144.10 to 63.13) |
| A(H1N1) | 16/17 | 94.12 | 320/429 | 74.59 | 3.81 | –589.17 (–6684.50 to 29.99) | –462.54 (–5311.69 to 41.52) | |
| A(H3) | 23/27 | 85.19 | 313/419 | 74.70 | 6.05 | 59.46 (–102.83 to 91.90) | 57.84 (–123.12 to 92.03) | |
| Influenza B | 12/17 | 70.59 | 324/429 | 75.52 | 3.81 | 82.90 (31.45 to 95.73) | 85.67 (32.32 to 96.97) | |
| Influenza positived | 58/70 | 82.86 | 278/376 | 73.94 | 15.70 | 34.76 (–42.37 to 70.10) | 41.21 (–29.81 to 73.37) | |
| Chronic liver disease | Influenza Ac | 1/3 | 33.33 | 21/33 | 63.64 | 8.33 | –7.33E+172 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) |
| A(H1N1) | 0/0 | NA | 22/36 | 61.11 | 0.00 | –7.33E+172 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) | |
| A(H3) | 1/2 | 50.00 | 21/34 | 61.76 | 5.56 | –7.33E+172 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) | |
| Influenza B | 0/0 | NA | 22/36 | 61.11 | 0.00 | –7.33E+172 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) | |
| Influenza positived | 1/3 | 33.33 | 21/33 | 63.64 | 8.33 | –7.33E+172 (–inf to 100.00) | –7.33E+172 (–inf to 100.00) | |
| Chronic neurological disease | Influenza Ac | 52/69 | 75.36 | 305/411 | 74.21 | 14.38 | 40.38 (–20.90 to 70.60) | 41.42 (–20.88 to 1.61) |
| A(H1N1) | 6/9 | 66.67 | 351/471 | 74.52 | 1.88 | 13.08 (–396.56 to 84.78) | –16.11 (–640.71 to 81.80) | |
| A(H3) | 36/44 | 81.82 | 321/436 | 73.62 | 9.17 | 24.42 (–96.62 to 70.95) | 28.39 (–90.33 to 73.06) | |
| Influenza B | 4/6 | 66.67 | 353/474 | 74.47 | 1.25 | 69.59 (–161.12 to 96.46) | 80.46 (–108.59 to 98.17) | |
| Influenza positived | 55/74 | 74.32 | 302/406 | 74.38 | 15.42 | 43.71 (–9.06 to 70.95) | 45.80 (–6.61 to 72.44) | |
| Diabetes mellitus | Influenza Ac | 47/59 | 79.66 | 361/470 | 76.81 | 11.15 | 38.36 (–34.39 to 71.73) | 41.72 (–33.00 to 74.46) |
| A(H1N1) | 7/10 | 70.00 | 401/519 | 77.26 | 1.89 | 64.10 (–73.55 to 92.57) | 71.98 (–147.90 to 96.83) | |
| A(H3) | 27/33 | 81.82 | 381/496 | 76.81 | 6.24 | 28.57 (–114.68 to 76.24) | 20.23 (–155.69 to 75.11) | |
| Influenza B | 14/15 | 93.33 | 394/514 | 76.65 | 2.84 | –153.11 (2005.78 to 69.58) | –157.16 (–2141.99 to 70.50) | |
| Influenza positived | 61/74 | 82.43 | 347/455 | 76.26 | 13.99 | 24.64 (–55.97 to 63.59) | 26.73 (–58.00 to 66.02) | |
| Impaired immune function | Influenza Ac | 5/6 | 83.33 | 64/94 | 68.09 | 6.00 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) |
| A(H1N1) | 2/2 | 100.00 | 67/98 | 68.37 | 2.00 | –7.7E+14 (–inf to 100.00) | –2.20E+57 (–inf to 100.00) | |
| A(H3) | 2/2 | 100.00 | 67/98 | 68.37 | 2.00 | –2.3E+18 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Influenza B | 1/1 | 100.00 | 68/99 | 68.69 | 1.00 | –3.84E+60 (–inf to 100.00) | –4.8E+15 (–inf to 100.00) | |
| Influenza positived | 6/7 | 85.71 | 63/93 | 67.74 | 7.00 | 56.01 (–686.74 to 97.54) | 100.00 (–inf to 100.00) | |
| Chronic kidney disease | Influenza Ac | 65/79 | 82.28 | 418/559 | 74.78 | 12.38 | –3.69 (–107.95 to 48.30) | –2.72 (–110.53 to 49.89) |
| A(H1N1) | 14/17 | 82.35 | 469/621 | 75.52 | 2.66 | 26.69 (–259.64 to 85.060) | 32.97 (–272.50 to 97.94) | |
| A(H3) | 33/39 | 84.62 | 450/599 | 75.13 | 6.11 | –30.70 (–257.36 to 52.20) | –28.59 (–264.90 to 54.68) | |
| Influenza B | 14/15 | 93.33 | 469/623 | 75.28 | 2.35 | –133.50 (–1849.28 to 72.03) | –138.54 (–2232.41 to 75.60) | |
| Influenza positived | 78/93 | 83.87 | 405/545 | 74.31 | 14.58 | –12.78 (–117.03 to 41.40) | –8.23 (–115.20 to 45.57) | |
| eBMI > 25 kg/m2 | Influenza Ac | 15/18 | 83.33 | 153/192 | 79.69 | 8.57 | 49.07 (–106.34 to 87.43) | 68.17 (–60.99 to 93.71) |
| A(H1N1) | 12/13 | 84.62 | 157/197 | 79.70 | 6.19 | 40.82 (–204.51 to 88.50) | 67.98 (–110.77 to 95.14) | |
| A(H3) | 0/0 | NA | 168/210 | 80.00 | 0.00 | 1.36E–11 (–inf to 100.00) | 2.21E–12 (–inf to 100.00) | |
| Influenza B | 4/5 | 80.00 | 164/205 | 80.00 | 2.38 | 5.86 (–921.74 to 91.33) | –1024.20 (–46,864.21 to 73.09) | |
| Influenza positived | 19/23 | 82.61 | 149/187 | 79.68 | 10.95 | 39.90 (–103.98 to 82.29) | 57.17 (–58.87 to 88.45) | |
| Disease group | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectivenessa (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| Chronic respiratory disease | Influenza Ac | 249/450 | 55.33 | 1708/3274 | 52.17 | 12.08 | 15.43 (–5.18 to 32.00) | 15.42 (–5.55 to 32.23) |
| A(H1N1) | 96/170 | 56.47 | 1861/3554 | 52.36 | 4.56 | 10.27 (–26.39 to 36.30) | 8.44 (–30.05 to 35.54) | |
| A(H3) | 101/184 | 54.89 | 1856/3540 | 52.43 | 4.94 | 15.05 (–18.61 to 39.16) | 15.25 (–18.75 to 39.52) | |
| Influenza B | 47/92 | 51.09 | 1910/3632 | 52.59 | 2.47 | 37.90 (3.53 to 60.03) | 36.34 (0.68 to 59.19) | |
| Influenza positived | 296/540 | 54.81 | 1661/3184 | 52.17 | 14.50 | 20.45 (2.88 to 34.84) | 19.78 (1.77 to 34.49) | |
| Chronic heart disease | Influenza Ac | 198/289 | 68.51 | 1318/2063 | 63.89 | 12.29 | 22.44 (–4.18 to 42.26) | 30.75 (5.81 to 49.08) |
| A(H1N1) | 61/97 | 62.89 | 1455/2255 | 64.52 | 4.12 | 34.99 (–4.40 to 59.52) | 35.94 (–5.35 to 61.05) | |
| A(H3) | 85/119 | 71.43 | 1431/2233 | 64.08 | 5.06 | 12.11 (–38.70 to 44.30) | 27.83 (–15.90 to 55.06) | |
| Influenza B | 43/59 | 72.88 | 1473/2293 | 64.24 | 2.51 | 20.07 (–48.62 to 57.01) | 13.63 (–65.01 to 54.79) | |
| Influenza positived | 237/344 | 68.90 | 1279/2008 | 63.70 | 14.63 | 20.26 (–4.54 to 39.17) | 26.79 (2.76 to 44.88) | |
| Asthma | Influenza Ac | 223/534 | 41.76 | 1669/3622 | 46.08 | 12.85 | 41.37 (28.06 to 52.21) | 48.62 (35.56 to 59.03) |
| A(H1N1) | 89/232 | 38.36 | 1803/3924 | 45.95 | 5.58 | 41.73 (20.86 to 57.10) | 48.42 (27.19 to 63.45) | |
| A(H3) | 88/188 | 46.81 | 1804/3968 | 45.46 | 4.52 | 34.23 (8.81 to 52.57) | 36.69 (9.68 to 55.63) | |
| Influenza B | 53/167 | 31.74 | 1839/3989 | 46.10 | 4.02 | 66.51 (52.07 to 76.60) | 65.46 (49.30 to 76.47) | |
| Influenza positived | 274/696 | 39.37 | 1618/3460 | 46.76 | 16.75 | 48.47 (38.18 to 57.04) | 53.56 (43.30 to 61.96) | |
| Chronic liver disease | Influenza Ac | 17/39 | 43.59 | 154/312 | 49.36 | 11.11 | 50.22 (–9.54 to 77.38) | 54.37 (–6.38 to 80.43) |
| A(H1N1) | 6/14 | 42.86 | 165/337 | 48.96 | 3.99 | 62.62 (–49.99 to 90.68) | 59.06 (–72.08 to 90.26) | |
| A(H3) | 5/17 | 47.06 | 163/334 | 48.80 | 4.84 | 60.93 (–131.78 to 93.41) | 54.38 (–158.14 to 91.94) | |
| Influenza B | 5/9 | 55.56 | 166/342 | 48.54 | 2.56 | –10.94 (–504.25 to 79.63) | –3.23 (–681.43 to 86.36) | |
| Influenza positived | 22/47 | 46.81 | 149/304 | 49.01 | 13.39 | 43.28 (–15.18 to 72.07) | 48.89 (–7.70 to 75.74) | |
| Chronic neurological disease | Influenza Ac | 120/165 | 72.73 | 688/1065 | 64.60 | 13.41 | 4.49 (–42.98 to 36.21) | 7.23 (–40.00 to 38.52) |
| A(H1N1) | 31/45 | 68.89 | 777/1185 | 65.57 | 3.66 | 14.78 (–74.67 to 58.42) | 9.53 (–88.14 to 56.50) | |
| A(H3) | 64/80 | 80.00 | 744/1150 | 64.70 | 6.50 | –46.06 (–172.98 to 21.84) | –31.40 (–152.54 to 31.63) | |
| Influenza B | 18/28 | 64.29 | 790/1202 | 65.72 | 2.28 | 56.39 (–4.76 to 81.85) | 54.69 (–12.49 to 81.75) | |
| Influenza positived | 137/192 | 71.35 | 671/1038 | 64.64 | 15.61 | 13.67 (–25.79 to 40.74) | 15.42 (–24.37 to 42.47) | |
| Diabetes mellitus | Influenza Ac | 156/232 | 67.24 | 1119/1643 | 68.11 | 12.37 | 35.42 (9.87 to 53.73) | 34.82 (8.00 to 53.82) |
| A(H1N1) | 49/78 | 62.82 | 1226/1797 | 68.22 | 4.16 | 37.49 (–7.04 to 63.49) | 34.04 (–15.49 to 62.33) | |
| A(H3) | 71/96 | 73.96 | 1204/1779 | 67.68 | 5.12 | 2.54 (–67.55 to 43.31) | 1.85 (–72.18 to 4.05) | |
| Influenza B | 41/57 | 71.93 | 1234/1818 | 67.88 | 3.04 | 29.19 (–37.35 to 63.50) | 23.64 (–52.19 to 61.69) | |
| Influenza positived | 197/287 | 68.64 | 1078/1588 | 67.88 | 15.31 | 35.56 (12.88 to 52.33) | 33.61 (9.28 to 51.42) | |
| Impaired immune function | Influenza Ac | 35/57 | 61.40 | 392/734 | 53.41 | 7.21 | 52.67 (7.98 to 75.66) | 49.52 (–4.19 to 75.54) |
| A(H1N1) | 10/15 | 66.67 | 417/776 | 53.74 | 1.90 | 54.69 (–106.62 to 90.06) | 43.43 (–21.36 to 89.72) | |
| A(H3) | 17/24 | 70.83 | 410/767 | 53.46 | 3.03 | 7.56 (–147.54 to 65.48) | –26.33 (–257.22 to 55.33) | |
| Influenza B | 18/31 | 58.06 | 409/760 | 53.82 | 3.92 | 70.04 (25.45 to 87.96) | 46.72 (–20.33 to 76.40) | |
| Influenza positived | 53/88 | 60.23 | 374/703 | 53.20 | 11.13 | 48.60 (14.52 to 69.09) | 41.87 (2.56 to 65.33) | |
| Chronic kidney disease (stage 3–5) | Influenza Ac | 134/188 | 71.28 | 903/1278 | 70.66 | 12.82 | 32.97 (1.01 to 54.62) | 33.14 (–0.54 to 55.54) |
| A(H1N1) | 35/56 | 62.50 | 1002/1410 | 71.06 | 3.82 | 60.06 (23.62 to 79.11) | 56.31 (14.17 to 77.76) | |
| A(H3) | 64/85 | 75.29 | 973/1381 | 70.46 | 5.80 | 12.83 (–58.12 to 51.94) | 3.43 (–82.66 to 48.94) | |
| Influenza B | 33/40 | 82.50 | 1004/1426 | 70.41 | 2.73 | –40.33 (–283.72 to 48.68) | –44.59 (–299.58 to 47.69) | |
| Influenza positived | 166/225 | 73.78 | 871/1241 | 70.19 | 15.35 | 27.94 (49.89 to 15.35) | 27.50 (–6.01 to 50.41) | |
| eBMI > 25 kg/m2 | Influenza Ac | 4/5 | 80.00 | 80/101 | 79.21 | 4.72 | 81.77 (–257.22 to 99.07) | 100.00 (–inf to 100.00) |
| A(H1N1) | 3/4 | 75.00 | 81/102 | 79.41 | 3.77 | 84.24 (–141.32 to 98.97) | 100.00 (–inf to 100.00) | |
| A(H3) | 0/0 | NA | 84/106 | 79.25 | 0.00 | 1.07E–11 (–inf to 100.00) | –3.60E–12 (–inf to 100.00) | |
| Influenza B | 2/2 | 100.00 | 82/104 | 78.85 | 1.89 | –4.8E+16 (–inf to 100.00) | 98.52 (–inf to 100.00) | |
| Influenza positived | 5/6 | 83.33 | 79/100 | 79.00 | 5.66 | 68.78 (–513.65 to 98.41) | 100.00 (–2.31E+77 to 100.00) | |
Swabbing setting
No significant difference was found in vaccine effectiveness between laboratory-confirmed influenza tests from Sentinel GPs, non-Sentinel GPs and hospital care sources (Table 13). No interaction was found between the source of the swab and the outcome.
| Swab location | Influenza type and subtype | Laboratory-confirmed influenza | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Influenza-positive cases | Influenza-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| GP Sentinel | Influenza Ac | 71/399 | 17.79 | 647/3302 | 19.59 | 10.78 | 29.33 (5.47 to 47.16) | 17.62 (–17.24 to 42.12) |
| A(H1N1) | 17/181 | 9.39 | 701/3520 | 19.91 | 4.89 | 71.96 (51.03 to 83.94) | 61.13 (27.45 to 79.17) | |
| A(H3) | 52/203 | 25.62 | 666/3498 | 19.04 | 5.49 | –26.25 (–77.00 to 9.94) | –28.19 (–93.94 to 15.27) | |
| Influenza B | 30/220 | 13.64 | 688/3481 | 19.76 | 5.94 | 54.39 (29.04 to 70.68) | 31.13 (–16.05 to 59.13) | |
| Influenza positived | 98/601 | 16.31 | 620/3100 | 20.00 | 16.24 | 36.29 (18.57 to 50.16) | 19.14 (–8.80 to 39.91) | |
| GP non-Sentinel | Influenza Ac | 29/104 | 27.88 | 73/270 | 27.04 | 27.81 | 36.66 (–31.02 to 69.38) | 70.59 (19.97 to 89.19) |
| A(H1N1) | 3/23 | 13.04 | 99/351 | 28.21 | 6.15 | 69.22 (–7.88 to 91.22) | 74.75 (–14.91 to 94.45) | |
| A(H3) | 3/5 | 60.00 | 99/369 | 26.83 | 1.34 | –418.03 (–3560.82 to 26.70) | 6.05 (–1302.25 to 93.70) | |
| Influenza B | 11/41 | 26.83 | 91/333 | 27.33 | 10.96 | 74.67 (0.77 to 93.53) | 77.86 (–12.78 to 95.65) | |
| Influenza positived | 37/140 | 26.43 | 65/234 | 27.78 | 37.43 | 43.28 (–7.17 to 69.99) | 64.01 (16.87 to 84.42) | |
| Hospital | Influenza Ac | 776/2247 | 34.53 | 5819/15,439 | 37.69 | 12.70 | 26.21 (18.38 to 33.28) | 27.02 (18.13 to 34.94) |
| A(H1N1) | 259/995 | 26.03 | 6336/16,691 | 37.96 | 5.63 | 49.62 (41.54 to 56.58) | 34.73 (22.83 to 44.80) | |
| A(H3) | 349/773 | 45.15 | 6246/16,913 | 36.93 | 4.37 | –14.31 (–32.59 to 1.46) | 18.42 (3.31 to 31.18) | |
| Influenza B | 174/551 | 31.58 | 6421/17,135 | 37.47 | 3.12 | 41.47 (29.17 to 51.63) | 35.04 (19.05 to 47.88) | |
| Influenza positived | 948/2789 | 33.99 | 5647/14,897 | 37.91 | 15.77 | 29.90 (23.24 to 35.99) | 29.51 (21.78 to 36.47) | |
Negative controls
The study explored the use of laboratory-confirmed infections, tested using multiplex RT-PCR at the same time as the influenza RT-PCR, and found no significant vaccine effectiveness for non-influenza viruses (Table 14).
| Age group (years) | Virus/infection type | Laboratory-confirmed infections | Total positive (%) | Vaccine effectiveness (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Virus-positive cases | Virus-negative controls | Unadjusteda | Adjustedb | |||||
| Vaccinated/total (n) | Vaccinated (%) | Vaccinated/total (n) | Vaccinated (%) | |||||
| 18–54 | Coronavirus | 17/124 | 13.71 | 294/2624 | 11.20 | 4.51 | –30.42 (–123.90 to 24.03) | –26.55 (–118.06 to 26.56) |
| Mycoplasma pneumoniae | 9/70 | 12.86 | 305/2699 | 11.30 | 2.53 | –47.74 (–207.99 to 29.13) | –58.04 (–231.04 to 24.55) | |
| Parainfluenza type 1 | 3/25 | 12.00 | 310/2743 | 11.30 | 0.90 | –57.56 (–450.79 to 54.93) | –54.60 (–441.17 to 55.84) | |
| Parainfluenza type 2 | 4/22 | 18.18 | 310/2747 | 11.29 | 0.79 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Rhinovirus | 40/337 | 11.87 | 274/2431 | 11.27 | 12.17 | –25.91 (–82.33 to 13.05) | –33.88 (–94.46 to 7.84) | |
| Respiratory syncytial virus | 9/101 | 8.91 | 305/2667 | 11.44 | 3.65 | 18.90 (–64.31 to 59.97) | 23.60 (–55.35 to 62.43) | |
| 55–64 | Coronavirus | 13/37 | 35.14 | 142/641 | 22.15 | 5.46 | –74.31 (–264.18 to 16.57) | –64.15 (–246.37 to 22.20) |
| Mycoplasma pneumoniae | 3/14 | 21.43 | 153/668 | 22.90 | 2.05 | –44.85 (–467.39 to 63.02) | –22.00 (–409.71 to 70.80) | |
| Parainfluenza type 1 | 0/9 | 0.00 | 156/673 | 23.18 | 1.32 | 100.00 (–inf to 100.00) | 100.00 (–inf to 100.00) | |
| Parainfluenza type 2 | 1/6 | 16.67 | 155/676 | 22.93 | 0.88 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Rhinovirus | 14/67 | 20.90 | 142/614 | 23.13 | 9.84 | –35.57 (–165.50 to 30.78) | –39.63 (–175.51 to 29.23) | |
| Respiratory syncytial virus | 6/38 | 15.79 | 150/644 | 23.29 | 5.57 | 34.32 (–66.52 to 74.09) | 30.06 (–78.52 to 72.60) | |
| 65–74 | Coronavirus | 25/38 | 65.79 | 367/661 | 55.52 | 5.44 | 8.57 (–97.00 to 57.57) | 11.27 (–92.39 to 59.08) |
| Mycoplasma pneumoniae | 5/11 | 45.45 | 390/693 | 56.28 | 1.56 | 10.54 (–254.85 to 77.45) | 13.31 (–250.11 to 78.53) | |
| Parainfluenza type 1 | 4/12 | 33.33 | 391/692 | 56.50 | 1.70 | 46.80 (–111.93 to 86.64) | 49.03 (–106.45 to 87.42) | |
| Parainfluenza type 2 | 4/09 | 44.44 | 391/695 | 56.26 | 1.28 | 0.00 (–inf to 100.00) | 0.00 (–inf to 100.00) | |
| Rhinovirus | 43/83 | 51.81 | 353/622 | 56.75 | 11.77 | –9.12 (–85.25 to 35.72) | –9.67 (–86.66 to 35.57) | |
| Respiratory syncytial virus | 30/42 | 71.43 | 366/662 | 55.29 | 5.97 | –82.47 (–283.43 to 13.17) | –81.98 (–282.79 to 13.48) | |
Clinical outcomes
After adjustment for confounding, including age in the model, significant negative vaccine effectiveness was found for GP outcomes, ARI and ILI for people aged ≥ 65 years (Table 15). Significant positive vaccine effectiveness was found for emergency hospitalisation or death from influenza and pneumonia (see Table 15). Rates and relative risks can be found in Table 16.
| Outcome | Season | Vaccine effectiveness (95% CI) |
|---|---|---|
| GP consultations | ||
| ARI | 2015/16 | –47.64 (–49.61 to –45.69) |
| 2014/15 | –59.28 (–61.47 to –57.12) | |
| 2013/14 | –55.40 (–57.64 to –53.19) | |
| 2012/13 | –53.34 (–55.51 to –51.21) | |
| 2011/12 | –59.69 (–61.99 to –57.42) | |
| 2010/11 | –46.72 (–49.05 to –44.43) | |
| Influenza or pneumonia | 2015/16 | –33.66 (–39.61 to –27.97) |
| 2014/15 | –34.90 (–40.49 to –29.54) | |
| 2013/14 | –64.02 (–72.41 to –56.05) | |
| 2012/13 | –28.70 (–34.41 to –23.22) | |
| 2011/12 | –58.01 (–65.39 to –50.95) | |
| 2010/11 | –46.43 (–52.98 to –40.16) | |
| Emergency hospitalisations | ||
| Influenza or pneumococcal disease | 2015/16 | 17.28 (15.55 to 18.98) |
| 2014/15 | 23.40 (21.81 to 24.95) | |
| 2013/14 | 22.41 (20.51 to 24.27) | |
| 2012/13 | 27.56 (25.89 to 29.19) | |
| 2011/12 | 20.58 (18.69 to 22.44) | |
| 2010/11 | 21.19 (19.19 to 23.15) | |
| Deaths | 2015/16 | 31.82 (30.78 to 32.84) |
| 2014/15 | 38.57 (37.64 to 39.49) | |
| 2013/14 | 34.57 (33.50 to 35.62) | |
| 2012/13 | 33.93 (32.90 to 34.93) | |
| 2011/12 | 34.19 (33.14 to 35.22) | |
| 2010/11 | 39.78 (38.83 to 40.71) | |
| Outcome | Season | Vaccination status | Rate per 1000 patient-seasons | Relative risk | 95% CI |
|---|---|---|---|---|---|
| GP | |||||
| Influenza or pneumonia consultation | 2015/16 | Unvaccinated | 5.20 | 1.00 | NA |
| Vaccinated | 5.85 | 1.13 | 1.11 to 1.15 | ||
| 2014/15 | Unvaccinated | 5.61 | 1.00 | NA | |
| Vaccinated | 5.99 | 1.07 | 1.05 to 1.09 | ||
| 2013/14 | Unvaccinated | 4.12 | 1.00 | NA | |
| Vaccinated | 4.50 | 1.09 | 1.07 to 1.12 | ||
| 2012/13 | Unvaccinated | 4.79 | 1.00 | NA | |
| Vaccinated | 4.98 | 1.04 | 1.02 to 1.06 | ||
| 2011/12 | Unvaccinated | 4.48 | 1.00 | NA | |
| Vaccinated | 4.86 | 1.08 | 1.06 to 1.11 | ||
| 2010/11 | Unvaccinated | 3.95 | 1.00 | NA | |
| Vaccinated | 4.38 | 1.11 | 1.08 to 1.13 | ||
| ARI consultation | 2015/16 | Unvaccinated | 10.92 | 1.00 | NA |
| Vaccinated | 22.80 | 2.09 | 2.06 to 2.11 | ||
| 2014/15 | Unvaccinated | 10.85 | 1.00 | NA | |
| Vaccinated | 23.85 | 2.20 | 2.17 to 2.22 | ||
| 2013/14 | Unvaccinated | 10.57 | 1.00 | NA | |
| Vaccinated | 22.58 | 2.14 | 2.11 to 2.16 | ||
| 2012/13 | Unvaccinated | 11.57 | 1.00 | NA | |
| Vaccinated | 22.98 | 1.99 | 1.96 to 2.01 | ||
| 2011/12 | Unvaccinated | 10.73 | 1.00 | NA | |
| Vaccinated | 22.23 | 2.07 | 2.05 to 2.10 | ||
| 2010/11 | Unvaccinated | 9.67 | 1.00 | NA | |
| Vaccinated | 16.91 | 1.75 | 1.73 to 1.77 | ||
| Emergency hospitalisations | |||||
| Influenza or pneumococcal disease | 2015/16 | Unvaccinated | 1.05 | 1.00 | NA |
| Vaccinated | 1.82 | 1.73 | 1.66 to 1.79 | ||
| 2014/15 | Unvaccinated | 1.27 | 1.00 | NA | |
| Vaccinated | 2.22 | 1.76 | 1.70 to 1.82 | ||
| 2013/14 | Unvaccinated | 0.86 | 1.00 | NA | |
| Vaccinated | 1.70 | 1.98 | 1.90 to 2.07 | ||
| 2012/13 | Unvaccinated | 1.12 | 1.00 | NA | |
| Vaccinated | 1.97 | 1.75 | 1.68 to 1.82 | ||
| 2011/12 | Unvaccinated | 1.00 | 1.00 | NA | |
| Vaccinated | 2.15 | 2.15 | 2.06 to 2.23 | ||
| 2010/11 | Unvaccinated | 1.19 | 1.00 | NA | |
| Vaccinated | 2.13 | 1.79 | 1.73 to 1.85 | ||
| Deaths | 2015/16 | Unvaccinated | 11.02 | 1.00 | NA |
| Vaccinated | 9.60 | 0.87 | 0.86 to 0.88 | ||
| 2014/15 | Unvaccinated | 11.80 | 1.00 | NA | |
| Vaccinated | 9.78 | 0.83 | 0.82 to 0.84 | ||
| 2013/14 | Unvaccinated | 10.60 | 1.00 | NA | |
| Vaccinated | 8.98 | 0.85 | 0.84 to 0.86 | ||
| 2012/13 | Unvaccinated | 11.95 | 1.00 | NA | |
| Vaccinated | 10.11 | 0.85 | 0.83 to 0.86 | ||
| 2011/12 | Unvaccinated | 11.59 | 1.00 | NA | |
| Vaccinated | 9.55 | 0.82 | 0.81 to 0.84 | ||
| 2010/11 | Unvaccinated | 10.39 | 1.00 | NA | |
| Vaccinated | 9.71 | 0.93 | 0.92 to 0.95 | ||
In the sensitivity analysis, and according to Simonsen et al. ’s framework,40 vaccine effectiveness during the pre- and post-influenza season was similar to that during peak influenza season (Table 17). Little variation was found between the seasons with different poorly or well-matched vaccines, severity, or by specificity of outcome (all-cause mortality had higher vaccine effectiveness than hospitalisation for influenza and pneumonia). 37
| Outcome | Season | Season stage | Start | End | Vaccine effectiveness (95% CI) |
|---|---|---|---|---|---|
| Emergency hospitalisations: influenza or pneumococcal disease | 2010/11 | Pre | 1 September 2010 | 11 October 2010 | 27.77 (10.80 to 41.51) |
| 2010/11 | Mid | 12 December 2010 | 13 March 2011 | 22.40 (10.19 to 32.95) | |
| 2010/11 | Post | 14 March 2011 | 30 June 2012 | 15.73 (2.83 to 26.92) | |
| 2011/12 | Pre | 1 September 2011 | 19 February 2012 | 25.97 (16.43 to 34.43) | |
| 2011/12 | Mid | 20 February 2012 | 6 May 2012 | 12.84 (–2.01 to 25.54) | |
| 2011/12 | Post | 7 May 2012 | 30 June 2012 | 21.28 (4.39 to 35.19) | |
| 2012/13 | Pre | 1 September 2012 | 9 December 2012 | 26.40 (9.87 to 39.89) | |
| 2012/13 | Mid | 10 December 2012 | 21 April 2013 | 29.17 (20.97 to 36.53) | |
| 2012/13 | Post | 22 April 2013 | 30 June 2013 | 4.43 (–14.04 to 19.91) | |
| 2013/14 | Pre | 1 September 2013 | 2 February 2014 | 22.71 (11.71 to 32.34) | |
| 2013/14 | Mid | 3 February 2014 | 13 April 2014 | 9.47 (–7.19 to 23.53) | |
| 2013/14 | Post | 14 April 2014 | 30 June 2014 | 24.07 (11.02 to 35.20) | |
| 2014/15 | Pre | 1 September 2014 | 14 December 2014 | 22.71 (7.92 to 35.12) | |
| 2014/15 | Mid | 15 December 2014 | 19 April 2015 | 23.52 (15.48 to 30.80) | |
| 2014/15 | Post | 20 April 2015 | 30 June 2015 | 18.01 (4.61 to 29.53) | |
| 2015/16 | Pre | 1 September 2015 | 27 December 2015 | 11.24 (–3.12 to 23.60) | |
| 2015/16 | Mid | 28 December 2015 | 15 May 2016 | 21.14 (12.88 to 28.61) | |
| 2015/16 | Post | 16 May 2016 | 30 June 2016 | 18.05 (0.77 to 32.33) |
Instrumental variable analysis
Vaccine uptake did vary by the proposed instrumental variable community CHP areas (Table 18). However, the correlation with individual vaccine uptake and our outcome was low and, therefore, this is considered a weak instrument. Therefore, it was not possible to use this method to estimate vaccine effectiveness accounting for unmeasured confounding.
| CHP | Vaccine uptake by season (%) | |||||
|---|---|---|---|---|---|---|
| 2010/11 | 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | |
| S03000001 | 78.80 | 77.30 | 78.30 | 79.30 | 80.54 | 78.11 |
| S03000002 | 78.35 | 79.58 | 80.89 | 75.28 | 75.78 | 76.16 |
| S03000003 | 78.40 | 76.98 | 78.52 | 73.53 | 77.14 | 78.20 |
| S03000004 | 64.46 | 67.46 | 63.64 | 63.41 | 62.95 | 61.53 |
| S03000005 | 100.00 | 100.00 | NA | NA | NA | NA |
| S03000006 | 70.40 | 61.30 | 64.94 | 78.54 | 77.77 | 74.89 |
| S03000007 | 62.49 | 63.39 | 63.24 | 63.61 | 62.85 | 61.15 |
| S03000008 | 55.23 | 54.74 | 55.71 | 56.24 | 54.75 | 53.22 |
| S03000009 | 80.15 | 80.96 | 81.00 | 81.02 | 80.14 | 77.10 |
| S03000010 | 73.25 | 74.65 | 74.94 | 75.78 | 74.53 | 73.68 |
| S03000011 | 77.15 | 78.12 | 79.29 | 79.51 | 78.88 | 78.14 |
| S03000013 | 0.00 | 0.00 | 0.00 | 0.00 | 50.00 | 66.67 |
| S03000014 | 40.00 | 37.50 | 33.33 | 37.50 | 33.33 | 20.00 |
| S03000015 | 76.85 | 78.67 | 80.80 | 79.19 | 79.54 | 78.05 |
| S03000017 | 61.97 | 63.22 | 63.77 | 63.19 | 61.81 | 62.60 |
| S03000018 | 75.04 | 76.07 | 81.05 | 77.22 | 75.97 | 74.48 |
| S03000020 | 77.82 | 77.25 | 82.39 | 79.31 | 81.06 | 80.27 |
| S03000023 | 74.68 | 76.09 | 76.91 | 80.08 | 79.39 | 75.83 |
| S03000025 | 19.47 | 20.50 | 19.72 | 20.24 | 19.78 | 19.95 |
| S03000029 | 65.85 | 66.77 | 66.50 | 73.10 | 72.63 | 67.24 |
| S03000030 | 78.80 | 79.05 | 81.38 | 81.54 | 79.16 | 79.40 |
| S03000031 | 46.85 | 47.47 | 47.66 | 47.25 | 46.30 | 44.74 |
| S03000032 | 56.06 | 56.65 | 58.13 | 57.90 | 57.29 | 56.01 |
| S03000035 | 49.20 | 50.03 | 51.11 | 51.34 | 50.82 | 49.99 |
| S03000036 | 0.00 | 0.00 | 0.00 | NA | NA | NA |
| S03000037 | 76.79 | 76.67 | 81.07 | 78.16 | 79.32 | 78.62 |
| S03000038 | 76.76 | 76.04 | 78.10 | 78.51 | 77.98 | 75.19 |
| S03000039 | 72.21 | 76.20 | 76.81 | 77.54 | 76.19 | 76.48 |
| S03000040 | 73.69 | 72.60 | 72.84 | 75.05 | 73.79 | 73.85 |
| S03000041 | 0.00 | 0.00 | 0.00 | 0.00 | NA | 100.00 |
| S03000042 | 57.80 | 58.75 | 58.78 | 58.76 | 57.94 | 57.07 |
| S03000043 | 61.27 | 61.94 | 63.53 | 64.43 | 63.36 | 61.82 |
| S03000044 | 62.66 | 63.21 | 63.62 | 63.41 | 62.94 | 60.74 |
Modelling an unmeasured confounder
Estimates from published data were used to model the effect of inadequately measured confounders on vaccine effectiveness in the cohorts for emergency hospitalisations due to pneumonia or influenza. The baseline vaccine effectiveness is that derived from pooling the estimates for the six seasons in Table 12. The pooled estimate, using a random-effects meta-analysis, is 22.9% (95% CI 19.8% to 25.8%).
It was found that a scenario of 15% prevalence for unaccounted frail individuals in the unvaccinated population, with frailty causing a fourfold increase in the risk of emergency hospitalisation and with a 5% presence of frailty among the vaccinated population, resulted in the vaccine no longer being effective (vaccine effectiveness 2.75, 95% CI –1.11 to 6.49). 9 A twofold increase in risk of hospitalisation would have relatively little impact, although it does reduce the magnitude of the vaccine effect estimate. By varying the prevalence of frailty and its risk on outcome, the change in vaccine effectiveness estimates for a number of scenarios (Table 19) were modelled. 27 The study also found that this model can be displayed effectively in graphical form (Figures 3 and 4). In Figures 4 and 5, the vaccine effectiveness is estimated given levels of the unmeasured confounder in the vaccinated population (x-axis) and unvaccinated population (each of the lines representing a different prevalence of the unmeasured confounder in the vaccinated population). At points where the prevalence of the unmeasured confounder is the same in the unvaccinated individuals and vaccinated individuals, then the baseline result is achieved, as there is no difference in the level of the unmeasured variable in the two groups.
| Increase in the risk of outcome on account of the confounder | Prevalence of confounder (%) | Emergency hospitalisation for pneumonia or influenza, adjusted vaccine effectiveness (95% CI) | |
|---|---|---|---|
| In unvaccinated population | In vaccinated population | ||
| – | 0 | 0 | 22.88 (19.81 to 25.84) |
| Doubled | 5 | 0 | 19.03 (15.80 to 22.13) |
| Doubled | 10 | 5 | 19.21 (15.99 to 22.31) |
| Doubled | 215 | 5 | 15.54 (12.17 to 18.77) |
| Quadrupled | 5 | 0 | 11.31 (7.78 to 14.71) |
| Quadrupled | 10 | 5 | 12.82 (–9.35 to 16.16) |
| Quadrupled | 15 | 5 | 2.75 (–1.11 to 6.49) |
FIGURE 3.
Unmeasured residual confounding and vaccine effectiveness (doubling of risk in emergency hospitalisations for influenza and pneumonia).
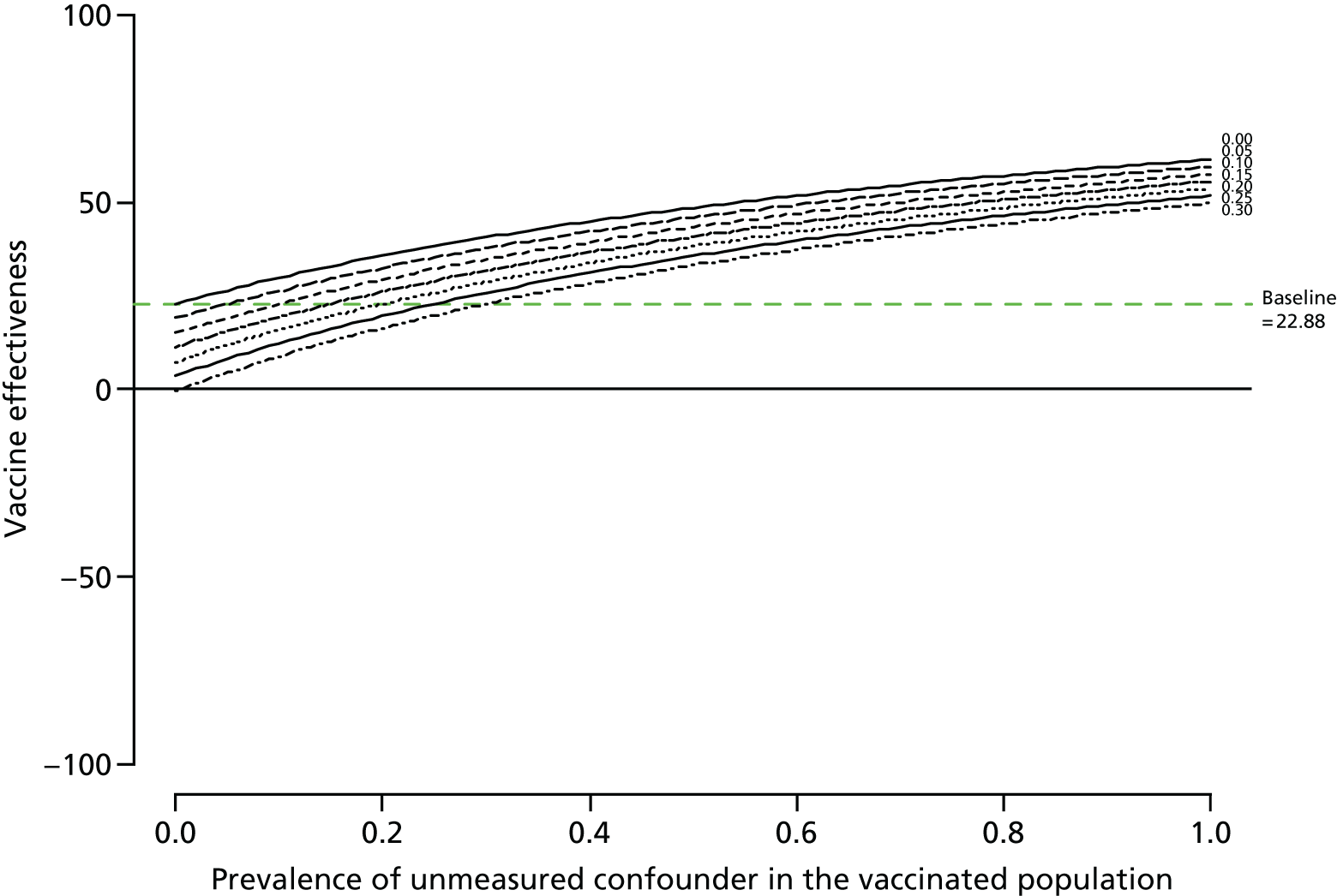
FIGURE 4.
Unmeasured residual confounding and vaccine effectiveness (quadrupling of risk in emergency hospitalisations for influenza and pneumonia).
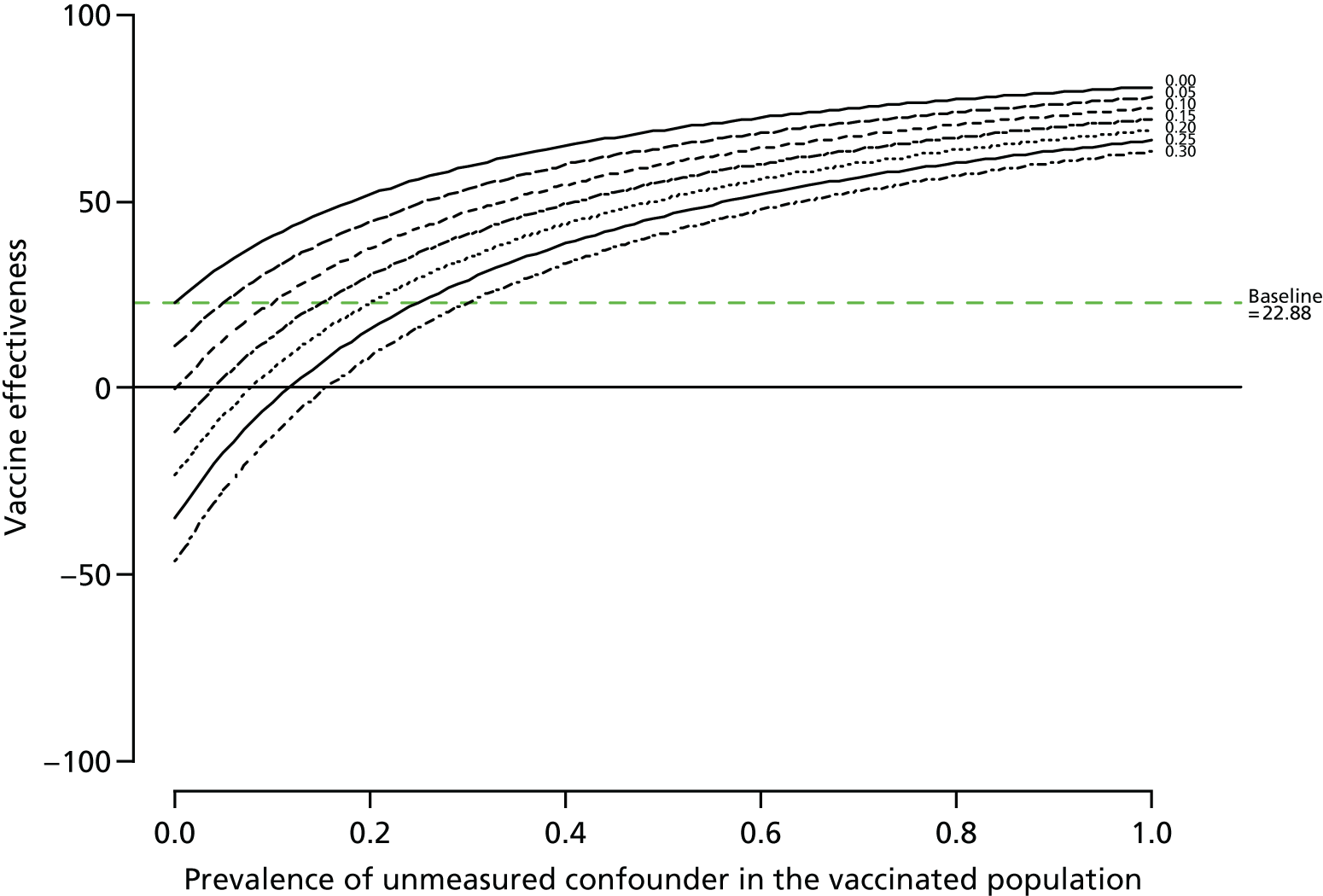
FIGURE 5.
General practice consultations: adverse events in children with LAIV interactions. Note that dots to the left of the vertical dashed line at 1 correspond to adverse events where the RR comparing the risk period with the pre- or post-risk period is lower among those children vaccinated than in those children unvaccinated. Dots to the right of the vertical dashed line at 1, and where all of the horizontal line corresponding to the 95% CI is above 1, indicate adverse events where there is a potential association with receipt of the vaccine. Some of the estimates are based on very small numbers and the right-hand side of the plot is truncated at 10.
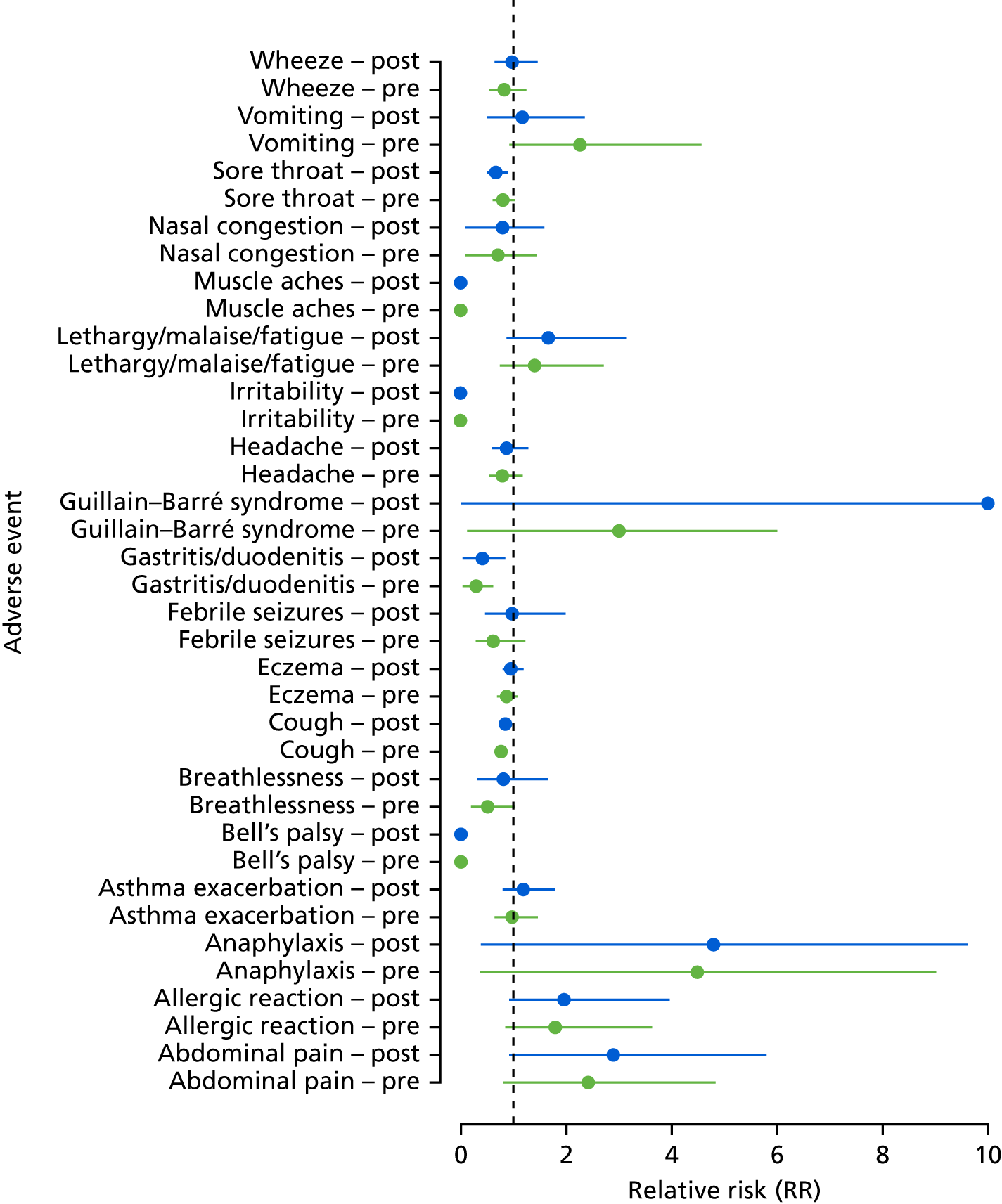
Vaccine safety
The LAIV was found to be safe in children, with no adverse reactions found (Figures 5 and 6). The number of adverse events recorded for children was very small and the CIs are wide. In most cases, fewer adverse events were reported among children who were vaccinated than in those who were unvaccinated, suggesting that relatively healthy children are the ones who received the LAIV.
FIGURE 6.
Hospitalisation: adverse events in children with LAIV interactions.

The TIV was found to be safe, with no association with adverse events (Figures 7 and 8). The exception to this finding was a statistically significant increase in allergic reaction (non-anaphylaxis) coded in the GP database; here, the TIV incidence RR (without the interaction term) was 1.33 (96% CI 1.13 to 1.56; p < 0.001). The Benjamini–Hochberg-adjusted p-value for this interaction test was 0.009 for GP consultations. This is the only adverse event for which there was a suggestion that the TIV was associated with a temporary increase in consultations following vaccination. In a majority of potential adverse events, those people who were vaccinated were at lower risk of GP consultation post vaccination than those people who were unvaccinated.
FIGURE 7.
General practice consultations: adverse events in adults with TIV interactions.

FIGURE 8.
Hospitalisation: adverse events in adults with TIV interactions.

Chapter 5 Discussion
In the first year of full rollout of LAIV to all preschool-aged and primary school-aged children in 2014/15 [an influenza season with circulating influenza A(H3N2)], vaccine effectiveness was positive (but non-significant) against RT-PCR laboratory-confirmed influenza. Highly significant vaccine effectiveness was found for season 2015/16, in which influenza A(H1N1) predominated. The LAIV was found to be safe among children, with no increased adverse reactions.
For those people considered at risk and who were aged < 65 years, TIV conferred significant protection against influenza for four of the six seasons. The two seasons in which no statistically significant vaccine effectiveness could be demonstrated coincided with seasons in which there was low levels of circulating influenza virus.
Positive significant vaccine effectiveness was found among those people aged ≥ 65 years for one season (2013/14), a less severe season in which one predominate strain [influenza A(H1N1)] circulated. TIV was found to be safe overall. The finding of increased GP consultation for (non-anaphylaxis) allergic reaction may be related to common TIV issues at the injection site (e.g. arm soreness and redness). 10
The acceptability of the LAIV programme among children registered with our practices was evidenced by high initial and then increasing uptake in both preschool- and primary school-aged children. There were also high levels of uptake of the TIV among those people aged ≥ 65 years, all of whom were able to receive the vaccine for free as part of the programme. High levels of TIV uptake was found for adults with at-risk diseases and who were aged 55–64 years. Uptake of the vaccine varied among different groups. Vaccine effectiveness, measured using hospital and GP non-specific clinical outcomes, was assessed using the Simonsen et al. 40 framework and was found to suffer from bias. The chosen instrumental variable, vaccine uptake by geographically determined health area, was determined not to be valid.
The LAIV uptake among 2- to 4-year-olds in the SIVE II practices in the 2014/15 season (39.3%) was similar to uptake in England and Wales (38.7%). 51 The modest uptake that was found among at-risk patients (particularly those aged between 18 and 55 years) has been found previously in our SIVE study and in other data sets (e.g. QResearch and questionnaire surveys). 52,53 The uptake in this study for older adults was lower than that found using GP routine reporting in Scotland [e.g. 59.2–67.1% vs. 74.5% (for those aged ≥ 65 years) in 2015–16]. 54
When comparing different groups of patients stratified by age band, there was greater power in this study than in the SIVE study to determine if uptake differed among groups of patients. 47 This study found, for example, that children in the least deprived quintile were more likely to receive influenza vaccine than those in the most deprived quintile; a similar pattern was found in uptake across deprivation groups using England and Wales GP data. 51 As in the SIVE study, it was also found that older people who had an emergency admission were less likely to be vaccinated;47 however, this may be an example of the healthy vaccine effect bias in which less sick older people are more likely to be vaccinated. 40
The findings of positive vaccine effectiveness (against RT-PCR-confirmed influenza) for LAIV in the two seasons for which there was full vaccination rollout to primary school-aged and preschool-aged children (significantly positive in 2015/16) have been found elsewhere. For example, this study’s 2015/16 results confirm those of a UK study (which included Scottish GPs) [overall vaccine effectiveness 58.1% vs. 57.6%; influenza B 88.3% vs. 81.4%; and influenza A(H1N1) 40.4% vs. 41.5%, respectively]. 55 However, the study’s results were in contrast to those from the US Centers for Disease Control and Prevention, which found that no protection was conveyed to children vaccinated with LAIV in 2014/15 (21% vs. −3%) and in 2015/16 (58% vs. 3%). 56,57
Trivalent influenza vaccine effectiveness among adults was the lowest when the predominant circulating subtype of influenza was H3N2 and highest when the predominant circulating subtype of influenza was H1N1. This was found for the pre-2009/10 seasons in the SIVE study. 47 Low vaccine effectiveness for H3N2 has been found elsewhere. 58 Reasons for poor vaccine effectiveness for H3N2 seasons have been postulated. Chicken eggs are used for culturing clinical isolates and for large-scale production of vaccines. However, influenza virus can mutate while being grown in chicken eggs, which could have an influence on antigenicity and lead to decreasing vaccine effectiveness. 59 Others have suggested that low H3N2 vaccine effectiveness in adults may not be due to egg adaptation, but rather caused by low vaccine immunogenicity in a subset of the population. 60 The finding of reduced TIV effectiveness in those people aged ≥ 65 years reinforces the evidence base on which the Joint Committee on Vaccination and Immunisation has made a recommendation of the preferential use of an adjuvanted seasonal influenza vaccine for the 2018/19 season. 61
When compared with vaccine effectiveness in other vaccines administered to children and adults, influenza vaccine effectiveness was found to be lower. For example, rotavirus vaccination among young children was 90% effective in children aged > 12 months;62 vaccination for measles, mumps, rubella and varicella was 85% effective against varicella;63 and herpes zoster vaccine was 69% effective in adults aged ≥ 60 years. 64
In contrast to Wong et al. ,42 who determined that geographical zones could be used as an instrumental variable, this study’s use of the geographical area – CHP – did not result in a strong instrumental variable. 43
The well-known safety profile of the TIV has been confirmed in this study using a novel observational study design to reduce bias. 9,10,46 However, there is less evidence regarding the safety of LAIV in children. 8 Previously reported short-term adverse effects of LAIV from clinical trials include bronchospasm, headache, nausea, vomiting and diarrhoea. Serious adverse events include pneumonia, bronchopneumonia, bronchiolitis and bronchitis. This study found no increase in adverse harms (mild and serious) from the LAIV used.
Study limitations
The estimates of vaccine uptake (particularly for those people aged ≥ 65 years) are lower relative to those reported by HPS, which are based on vaccine claim counts by all GPs in Scotland. SIVE II study practices may have been located in areas with a low level of vaccine uptake (e.g. more deprived areas). However, in this study it was not possible to check this as the study did not have practice identifiers. In similar, previous studies with smaller numbers of practices,30,47,65,66 but using similar data sets, vaccine uptake among those people aged ≥ 65 years was closer to national estimates of 75%. If SIVE II practices have actual lower levels of vaccine uptake, then this study’s estimates of vaccine effectiveness will be unbiased. If this study has missing data on vaccination, then the effect of the misclassification of some vaccinated individuals as unvaccinated will have moved the estimates of vaccine effectiveness towards zero, and so the vaccine effectiveness reported in this study may be too low. However, the estimates of vaccine effectiveness are unlikely to be substantially lower, as the pooled estimates for the TND for any influenza among those people aged ≥ 65 years over the six seasons is 17.9% (95% CI 5.2% to 28.7%), whereas from the cohort for emergency hospitalisation for pneumonia or influenza the vaccine effectiveness is 22.9% (95% CI 19.8% to 25.8%). Therefore, there was little scope for big increases in the estimated vaccine effectiveness, assuming that all of the misclassified unvaccinated individuals did not experience an event. The study was unable to calculate whether or not there was any indirect effect of the LAIV. The indirect impact of LAIV, defined as reduction in cumulative disease incidence over the same period between pilot and non-pilot areas in non-target age groups (< 4 years of age and > 11 years of age), was measured in England; however, no statistically significant indirect protection to other age groups was found. 67 The work of other groups exploring this issue will continue to be monitored. Owing to the observational nature of this study, which uses routinely collected data, residual confounding may be present or unaccounted for.
A no-cost extension to this project was granted: this was due to the complexity of extracting and linking data from a number of disparate data sets (challenging in terms of putting the necessary governance permissions in place and technical processes required for data extraction and linkage). Future projects requiring data linkage should build adequate time to address governance and technical challenges.
The study recruited 230 practices, which was fewer than the 500 practices (of a total of 998 Scottish GPs) originally stated in the grant application. Substantial resource was committed to the process of recruitment (e.g. presentation to GP national users groups, GP newsletter advertisements, e-mail and telephone recruitment, and several reminders). Despite the lower than expected final number of GPs participating in this project, to date this is one of the largest individual patient-level primary care recruitment and data extractions to take place in Scotland (with one-fifth of practices participating). Only a small number of practices responded to turn down the offer to participate (almost all with no explanation). With the introduction of national strategies to use primary care data for research purposes [e.g. the Scottish Primary Care Information Resource; URL: www.spire.scot (accessed 1 October 2018)], it is hoped that ambitious recruitment targets, such as in this study, will be seen as more feasible.
Recommendations for further research
The monitoring of the LAIV programme with enhanced Sentinel swabbing of preschool- and primary school-aged children should continue. Future studies should be planned to further monitor the seasonal influenza vaccine in at-risk patients and such studies will continue to require expertise in data linkage and advanced analyses of these complex data sets. Replication of vaccine effectiveness and safety in LAIV and TIV in other countries (that have these influenza vaccine programmes) is also needed to confirm this study’s results. A future study will be required to monitor the safety and effectiveness of the adjuvanted TIV available to older people for the 2018/19 season. 61 Any future evaluation work should contain a health economics analysis to help understand at what level of vaccine effectiveness it becomes cost-effective to recommend vaccination or continue to vaccinate.
Conclusions
Using these data, it was found that LAIV was safe and effective in decreasing RT-PCR-confirmed influenza in children. TIV was safe and significantly effective (in most seasons) for those people aged 18–64 years with positive vaccine effectiveness and in most seasons for those people aged ≥ 65 years (although this was statistically significant in only one season). Higher vaccine effectiveness was found among younger adults with asthma. Few countries’ health systems allow for the integrated and accessible data recording that made this study possible and which made it feasible to centrally collate almost all hospitalisations and deaths attributed to influenza, allowing for completeness of reporting. LAIV was found to be safe, with vaccine effectiveness comparable to that found for TIV. TIV immunisation for at-risk adults in primary health-care settings is effective.
Acknowledgements
The authors would like to thank staff at Albasoft Ltd, HPS, the WoSSVC, the Asthma UK Centre for Applied Research and the ISD electronic Data Research and Innovation Service team, and would also like to thank the GPs contributing data to the study and members of the Independent Steering Committee and Asthma UK Centre for Applied Research patient and public involvement team for overseeing this work.
Contributions of authors
Colin R Simpson (Professor of Population Health) was the principal investigator and led the writing of this report.
Nazir I Lone (Senior Clinical Fellow, Intensive Care) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Kim Kavanagh (Senior Lecturer) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Tanya Englishby (Research Fellow) carried out the analyses and helped to design the study and write the report.
Chris Robertson (Professor of Statistics) carried out the analyses and helped to design the study and write the report.
Jim McMenamin (Consultant Epidemiologist) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Beatrix von Wissman (Specialist Trainee in Public Health) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Eleftheria Vasileiou (PhD student) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Christopher C Butler (Professor of Primary Care) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Lewis D Ritchie (Professor of Primary Care) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Rory Gunson (Consultant Clinical Scientist) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Jürgen Schwarze (Edward Clark Chair of Child Life and Health) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Aziz Sheikh (Professor of Primary Care Research and Development) helped design the study, draft and write the project protocol and revise the report for important intellectual content.
Publications
Simpson CR, Lone NI, Kavanagh K, Robertson C, McMenamin J, von Wissman B, et al. Evaluating the effectiveness, impact and safety of live attenuated and seasonal inactivated influenza vaccination: protocol for the Seasonal Influenza Vaccination Effectiveness II (SIVE II) study. BMJ Open 2017;7:e014200.
Vasileiou E, Sheikh A, Butler CC, Robertson C, Kavanagh K, Englishby T, et al. Seasonal influenza vaccine effectiveness in people with asthma: a national test-negative design case-control study. Clin Infect Dis 2020;71:e94–e104.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179-86. https://doi.org/10.1001/jama.289.2.179.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007;25:5086-96. https://doi.org/10.1016/j.vaccine.2007.03.046.
- Simpson CR, Lone NI, Kavanagh K, Robertson C, McMenamin J, von Wissman B, et al. Evaluating the effectiveness, impact and safety of live attenuated and seasonal inactivated influenza vaccination: protocol for the Seasonal Influenza Vaccination Effectiveness II (SIVE II) study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014200.
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011;378:1917-30. https://doi.org/10.1016/S0140-6736(11)61051-9.
- Cauchemez S, Valleron AJ, Boëlle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature 2008;452:750-4. https://doi.org/10.1038/nature06732.
- Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001527.
- Department of Health and Social Care . CVI Statement on the Routine Annual Influenza Vaccination Programme n.d. www.gov.uk/government/publications/jcvi-statement-on-the-routine-annual-influenza-vaccination-programme (accessed 17 June 2015).
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36-44. https://doi.org/10.1016/S1473-3099(11)70295-X.
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD004879.pub4.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010;7. https://doi.org/10.1002/14651858.CD001269.pub4.
- Valenciano M, Ciancio B. I-MOVE study team . I-MOVE: a European network to measure the effectiveness of influenza vaccines. Euro Surveill 2012;17. https://doi.org/10.2807/ese.17.39.20281-en.
- Doshi P. Influenza: marketing vaccine by marketing disease. BMJ 2013;346. https://doi.org/10.1136/bmj.f3037.
- Simpson CR, Lone N, Kavanagh K, Ritchie LD, Robertson C, Sheikh A, et al. Trivalent inactivated seasonal influenza vaccine effectiveness for the prevention of laboratory confirmed influenza in a Scottish population 2000–2009. Euro Surveill 2015;20:ii-21043. https://doi.org/10.2807/1560-7917.ES2015.20.8.21043.
- Kavanagh K, Robertson C, McMenamin J. Assessment of the variability in influenza A(H1N1) vaccine effectiveness estimates dependent on outcome and methodological approach. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0028743.
- Kavanagh K, Robertson C, McMenamin J. Estimates of influenza vaccine effectiveness in primary care in Scotland vary with clinical or laboratory endpoint and method – experience across the 2010/11 season. Vaccine 2013;31:4556-63. https://doi.org/10.1016/j.vaccine.2013.07.056.
- Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M. SNIFFLE Study Investigators . Safety of live attenuated influenza vaccine in atopic children with egg allergy. J Allergy Clin Immunol 2015;136:376-81. https://doi.org/10.1016/j.jaci.2014.12.1925.
- De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013;18. https://doi.org/10.2807/1560-7917.ES2013.18.37.20585.
- Information Services Division . Practice Team Information (PTI) Statistics 2010.
- McAlister FA, Murphy NF, Simpson CR, Stewart S, MacIntyre K, Kirkpatrick M, et al. Influence of socioeconomic deprivation on the primary care burden and treatment of patients with a diagnosis of heart failure in general practice in Scotland: population-based study. BMJ 2004;328. https://doi.org/10.1136/bmj.38043.414074.EE.
- Whitelaw FG, Nevin SL, Milne RM, Taylor RJ, Taylor MW, Watt AH. Completeness and accuracy of morbidity and repeat prescribing records held on general practice computers in Scotland. Br J Gen Pract 1996;46:181-6.
- Information Services Division . Scottish Immunisation &Amp; Recall System n.d. www.isdscotland.org/Health-Topics/Child-Health/Child-Health-Programme/Scottish-Immunisation-Recall-System.asp (accessed 25 November 2015).
- Health Protection Scotland . Surveillance Data and Systems n.d. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2104/documents/1_2012-05-17.pdf (accessed 17 June 2015).
- Information Services Division . NHS Hospital Data Quality: Towards Better Data from Scottish Hospitals 2007.
- Kendrick S, Clarke J. The Scottish Record Linkage System. Health Bull 1993;51:72-9.
- Scottish Public Health Observatory . Overview of Key Data Sources: ISD Linked Database 2010.
- Health Protection Scotland . Influenza Surveillance Systems n.d. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2524/documents/1_2009-05-19.pdf (accessed 23 November 2015).
- Simpson CR, Lone N, McMenamin J, Gunson R, Robertson C, Ritchie LD, et al. Early estimation of pandemic influenza Antiviral and Vaccine Effectiveness (EAVE): use of a unique community and laboratory national data-linked cohort study. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19790.
- Chief Medical Officer . Seasonal Influenza Vaccination Programme 2015–16 2015.
- Salisbury D, Ramsay M, Noakes K. Immunisation Against Infections Disease. London: The Stationery Office; 2006.
- Lone NI, Simpson C, Ritchie LD, Robertson C, Sheikh A, McMenamin J, et al. Seasonal Influenza Vaccine Effectiveness in the community (SIVE): exploitation of a unique national linked dataset. BMJ Open 2012;15. https://doi.org/10.1136/bmjopen-2012-001019.
- Wallace LA, McAulay KA, Douglas JD, Elder AG, Stott DJ, Carman WF. Influenza diagnosis: from dark isolation into the molecular light. West of Scotland Respiratory Virus Study Group. J Infect 1999;39:221-6. https://doi.org/10.1016/S0163-4453(99)90053-1.
- Gunson RN, Bennett S, Maclean A, Carman WF. Using multiplex real time PCR in order to streamline a routine diagnostic service. J Clin Virol 2008;43:372-5. https://doi.org/10.1016/j.jcv.2008.08.020.
- National Statistics . Scottish Index of Multiple Deprivation: 2009 General Report 2009.
- Scottish Government . Scottish Government Urban Rural Classification n.d. www.gov.scot/Topics/Statistics/About/Methodology/UrbanRuralClassification (accessed 14 August 2018).
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol 2000;29:891-8. https://doi.org/10.1093/ije/29.5.891.
- Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010;11. https://doi.org/10.1186/1471-2296-11-1.
- McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014;59:1375-85. https://doi.org/10.1093/cid/ciu680.
- Skowronski DM, Chambers C, Sabaiduc S, De Serres G, Winter AL, Dickinson JA, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–15 season. Clin Infect Dis 2016;63:21-32. https://doi.org/10.1093/cid/ciw176.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007;7:658-66. https://doi.org/10.1016/S1473-3099(07)70236-0.
- Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948-63. https://doi.org/10.2307/2533848.
- Wong K, Campitelli MA, Stukel TA, Kwong JC. Estimating influenza vaccine effectiveness in community-dwelling elderly patients using the instrumental variable analysis method. Arch Intern Med 2012;172:484-91. https://doi.org/10.1001/archinternmed.2011.2038.
- Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000;29:722-9. https://doi.org/10.1093/ije/29.4.722.
- Glanz JM, McClure DL, Xu S, Hambidge SJ, Lee M, Kolczak MS, et al. Four different study designs to evaluate vaccine safety were equally validated with contrasting limitations. J Clin Epidemiol 2006;59:808-18. https://doi.org/10.1016/j.jclinepi.2005.11.012.
- Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine 2004;22:2064-70. https://doi.org/10.1016/j.vaccine.2004.01.017.
- Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine 2009;27:2114-20. https://doi.org/10.1016/j.vaccine.2009.01.125.
- Simpson CR, Lone N, Kavanagh K, Ritchie LD, Robertson C, Sheikh A, et al. Seasonal Influenza Vaccine Effectiveness (SIVE): an observational retrospective cohort study – exploitation of a unique community-based national-linked database to determine the effectiveness of the seasonal trivalent influenza vaccine. Health Serv Deliv Res 2013;1.
- Kotz D, Viechtbauer W, Simpson C, van Schayck OC, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med 2015;3:761-8. https://doi.org/10.1016/S2213-2600(15)00320-3.
- Health Protection Scotland . Influenza Surveillance Systems n.d. www.hps.scot.nhs.uk/a-to-z-of-topics/influenza (accessed 1 April 2016).
- Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J R Soc Med 2010;103:98-106. https://doi.org/10.1258/jrsm.2009.090348.
- Hardelid P, Rait G, Gilbert R, Petersen I. Factors associated with influenza vaccine uptake during a universal vaccination programme of preschool children in England and Wales: a cohort study. J Epidemiol Community Health 2016;70:1082-7. https://doi.org/10.1136/jech-2015-207014.
- Coupland C, Harcourt S, Vinogradova Y, Smith G, Joseph C, Pringle M, et al. Inequalities in uptake of influenza vaccine by deprivation and risk group: time trends analysis. Vaccine 2007;25:7363-71. https://doi.org/10.1016/j.vaccine.2007.08.032.
- Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009;58:446-58. https://doi.org/10.1016/j.jinf.2009.04.001.
- Health Protection Scotland . Monthly National Influenza Report Week Ending 8 May 2016 n.d. www.hps.scot.nhs.uk/resourcedocument.aspx?id=4356 (accessed 17 June 2018).
- Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill 2016;21:pii-30348. https://doi.org/10.2807/1560-7917.ES.2016.21.38.30348.
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016;63:1564-73. https://doi.org/10.1093/cid/ciw635.
- Centers for Disease Control and Prevention (CDC) . ACIP Votes Down Use of LAIV for 2016–2017 Flu Season. Media Statement 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html (accessed 17 June 2018).
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016;16:942-51. https://doi.org/10.1016/S1473-3099(16)00129-8.
- Wu NC, Zost SJ, Thompson AJ, Oyen D, Nycholat CM, McBride R, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLOS Pathog 2017;13. https://doi.org/10.1371/journal.ppat.1006682.
- Cobey S, Gouma S, Parkhouse K, Chambers BS, Ertl HC, Schmader KE, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–13. Clin Infect Dis 2018;67:327-33. https://doi.org/10.1093/cid/ciy097.
- Joint Centre for Vaccination and Immunisation . Draft Minute of the Meeting on 7 February 2018 n.d. https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/284102495624 (accessed 29 May 2018).
- Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarró M, Heylen E, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 2012;345. https://doi.org/10.1136/bmj.e4752.
- Seward JF, Marin M, Vázquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis 2008;197:82-9. https://doi.org/10.1086/522145.
- Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis 2016;213:1872-5. https://doi.org/10.1093/infdis/jiw047.
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza – primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14340-05.
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: a retrospective observational cohort study. Lancet Infect Dis 2012;12:696-702. https://doi.org/10.1016/S1473-3099(12)70133-0.
- Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, Bawa Z, et al. Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013/14 influenza season. Euro Surveill 2014;19. https://doi.org/10.2807/1560-7917.ES2014.19.22.20823.
List of abbreviations
- ARI
- acute respiratory infection
- BMI
- body mass index
- CHP
- Community Health Partnership
- CI
- confidence interval
- ECOSS
- Electronic Communication of Surveillance in Scotland
- GP
- general practice
- HPS
- Health Protection Scotland
- ICD-10
- International Classification of Diseases, Tenth Edition
- ILI
- influenza-like illness
- ISD
- Information Services Division
- LAIV
- live attenuated influenza vaccine
- NSS
- NHS National Services Scotland
- OR
- odds ratio
- PIPER
- Pandemic Influenza Primary Care Reporting
- RR
- risk ratio
- RT-PCR
- real-time reverse-transcription polymerase chain reaction
- SIMD
- Scottish Index of Multiple Deprivation
- SIVE
- Seasonal Influenza Vaccine Effectiveness in the community
- SIVE II
- Seasonal Influenza Vaccination Effectiveness II
- SMR01
- Scottish Morbidity Record 01
- TIV
- trivalent influenza vaccine
- TND
- test-negative design
- TRE
- trusted research environment
- WoSSVC
- West of Scotland Specialist Virology Centre
