Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/01/04. The contractual start date was in January 2014. The draft report began editorial review in September 2018 and was accepted for publication in June 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Wilson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Some parts of this chapter have been reproduced from the Trial Of Proton Pump Inhibitors in Throat Symptoms (TOPPITS) study protocol (Watson et al. 1). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
TOPPITS addresses the problem of adults with persistent throat symptoms, such as globus pharyngeus (hereafter referred to as ‘globus’), catarrh, throat discomfort, clearing, recurring dysphonia or excess mucus. In one UK survey,2 6% of the middle-aged female population reported a persistent feeling of something in the throat (globus) in the previous 3 months. Globus is also reported to account for up to 4% of ear, nose and throat (ENT) referrals to secondary care. 3 Throat clearing is the commonest single symptom in any voice clinic. Equally familiar are intermittent hoarse voice and postnasal drip. 4 It is claimed that 55% of patients referred to a voice clinic have symptoms of extraesophageal reflux (EOR), and an English study of primary care attenders indicated that 25% had recent experience of persistent upper respiratory symptoms. 5 In the general population, the lifetime incidence of milder variants of globus is > 40%. 6 There were 1,142,404 first ENT consultations in England in 2010–11. 7 A conservative estimate is that 5% of these patients were referred for very common throat symptoms, such as throat clearing, fluctuating voice change, catarrh and chronic throat discomfort, which equates to over 57,000 NHS patients referred to secondary care that year in England alone. 7 Some patients experience anxiety as they fear that they may have throat cancer. Even if they have no features and no risk factors for cancer, they may be referred in for urgent ENT clinic assessment, a process that prolongs the anxiety and, at times, the symptoms. In the absence of good-quality treatment algorithms, patients also undergo invasive and costly assessments, such as rigid endoscopic examination of the upper aerodigestive tract under general anaesthesia, which typically reveals no significant abnormality, and empiric trials of acid suppression, typically with proton pump inhibitors (PPIs).
Rationale
Upper airway symptoms are known to have a strong placebo response. 8 Early evidence from animal experiments gave rise to the term ‘acid laryngitis’ 40 years ago. 9 Intracellular reactivation of acidified pepsin may explain pepsin activity at weakly acid pH levels. 10,11 Despite a growing trend to treat throat symptoms empirically with PPIs, controlled studies fail to demonstrate a significant benefit of PPI over placebo. 12–14 An evidence-based medicine EOR conference concluded that work assessing PPIs in throat symptoms had variable study design and quality, small numbers and heavy selection bias, with inconsistent treatment regimes, and that the small proportion of controlled studies demonstrating overall benefit of PPI over placebo15 may reflect the prompt response of heartburn to antacid treatment. 16,17 There was little evidence on other pharmaceuticals, such as H2 antagonists. 18,19 In the patient and public involvement background work for this proposal, individual interviews were conducted with several patients, encompassing both young professionals and the retired. All fully supported the research proposal. It was also clear, even from a small sample, that patient views on PPIs vary widely, but all had been treated at some point with PPIs, sometimes on more than one occasion.
Over half of UK otolaryngologists prescribe PPIs for persistent throat symptoms in the absence of structural pathology. 20 Our early systematic review12 of studies that used PPIs as an empiric treatment modality for suspected laryngopharyngeal reflux (LPR) identified 14 uncontrolled studies, one unblinded, non-randomised study with a control group of healthy volunteers and six double-blind, placebo-controlled randomised trials from 1994 to 2004. A lack of common outcome measures, selection bias and inadequate blinding of the results were among the typical limitations. Although uncontrolled series reported positive results, randomised controlled trials (RCTs) demonstrated no statistically significant differences for changes in severity or frequency of throat symptoms between PPIs and placebo. It appeared that empiric treatment of suspected LPR with PPIs, by far the most common ENT practice in the UK, is based on poor levels of evidence from uncontrolled studies. A later meta-analysis21 included further studies, notably that by Vaezi et al. ,22 and concluded that PPI therapy ‘may offer a modest but non-significant clinical benefit’ over placebo. The authors also concluded that validated diagnostic guidelines may facilitate the recognition of likely responders. The 2007 meta-analysis13 included five RCTs, only two of which had more than 22 participants, and only one randomised over 100 participants. The conclusion was that there was no overall benefit of therapy and that further work was needed to identify likely responders. 13 Finally, the most recent meta-analysis included seven placebo-controlled trials totalling 396 participants with varying doses over 4 to 16 weeks, and again showed that PPI therapy lacked evidence of efficacy in those suspected to have LPR. Rather, high placebo response levels suggested a more complex and multifactorial pathophysiology. 23 Like previous authors, the reviewers concluded that further studies are needed to characterise subgroups of patients with reflux-associated laryngeal symptoms who might benefit from treatment with PPI.
The perception in primary care is that PPIs are a reasonable ‘empirical’ treatment strategy for this group of patients. Almost since their introduction in the late 1990s, PPIs have constituted the largest part of the NHS community drugs bill: £238M in 1999 (5.6%). 24 PPIs are highly efficient in reducing gastric acid secretion. The annual NHS expenditure on PPIs is > £300M (generic omeprazole, lansoprazole and pantoprazole are the NHS Quality, Innovation, Productivity and Prevention-endorsed low-cost PPIs). The practice of giving ‘all comers’ with upper respiratory symptoms anti-reflux therapy misses the opportunity to explore other potentially beneficial approaches, such as speech therapy or management of fatigue. 25
Measuring treatment responses in throat symptoms
The most frequently used primary outcome measure in the assessment of persistent, hard-to-explain throat symptoms is the Reflux Symptom Index (RSI). This nine-item, self-administered questionnaire is scored on a Likert scale with a total score of 0–45. 26 A higher score indicates more severe symptoms. The nine-item RSI total score allows comparison with previous studies as it offers 10 years of comparative data in the literature. The RSI remains the ‘area standard’ and, despite well-rehearsed limitations, remained our chosen primary outcome measure. Some reported studies have a baseline RSI only just above the normal level and others have a considerably higher baseline RSI. An observational study included 455 participants in South Korea, in whom the mean RSI score fell from 15 at baseline to 5.6 after 12 weeks of the PPI rabeprazole. 27 Baseline RSI scores in a much smaller but comparative study of 62 participants treated with esomeprazole were considerably higher (> 20). 28 On the other hand, a rabeprazole RCT,15 like the Korean descriptive study, had baseline RSI scores around 14, closer to those of Lee et al. 27 Despite these differences in baseline severity, both of these more recent trials showed a benefit from a 3-month trial of acid suppression, but Lam et al. 15 continued follow-up for a further 6 weeks, when the effect disappeared, whereas Reichel et al. ’s28 final measurement point was the end of therapy.
The RSI has a number of limitations, which we have addressed in derivation of our own participant-reported outcome measure: the Comprehensive Reflux Symptom Score (CReSS). 29 The CReSS is a 34-item questionnaire of oesophageal and extraesophageal symptoms, which has been tested on groups of ‘throat’ patients, healthy controls and those attending for an upper gastrointestinal endoscopy. It has three statistically robust symptom factors: (1) gastrointestinal; (2) an upper airway factor relating to cough, breathing, mucus and hoarseness; and (3) an obstruction/choking globus factor. The continuing use of the RSI alongside other variables by ourselves and others has at least allowed the summation of studies in some of the prior attempted evidence synthesis exercises. One factor to be borne in mind in the application of any throat symptom questionnaire, however, is the baseline incidence of throat symptoms in the community. The upper limit of normal in the RSI is said to be 12 in the general population. The first UK study to assess RSI scores in general practice attenders identified 252 participants with a score of > 10. 5 However, only 29% had a zero rating on the integral heartburn/dyspepsia item (which accounts for up to 5 of the 45 points), which is as one would expect given that about 30% of the population have some symptoms of lower gastroesophageal reflux.
When this gastroesophageal item was excluded from the RSI analysis, 8% of general practice attenders had a RSI of > 10 owing to the remaining extraesophageal items. A more recent UK report of the population distribution of RSI values sampled 2000 adults who were also questioned on their health and lifestyle. 30 The mean RSI score was 8.3; 30% of participants had a RSI score of > 10, of whom 25% had a zero score on the gastroesophageal reflux disease (GORD) item, thus giving a 7.5% overall prevalence of suspected LPR, similar to that observed in general practice attenders. 5 Over the past 5 years, we have continued to refine our improved participant report tool, the CReSS. 29 We have demonstrated wide separation of 103 volunteers, with a mean score of < 7, from 177 throat participants, with a mean score of 31 [95% confidence interval (CI) 28 to 35]. Factor analysis in a total of 422 participants shows the CReSS to have three subscales. The greater level of detail of the CReSS and the likely better discrimination of normal from abnormal scores at baseline make it an invaluable secondary outcome variable. Such an approach addresses the research need identified in prior review work, namely that of better characterising the subgroup of suspected LPR patients who may benefit from acid suppression therapy.
Varying average baseline Reflux Symptom Index scores in different reported series
-
A small trial (of fewer than 50 participants) showed some benefit from acid suppression in postnasal drip, but only in individual symptom items, and the method of recruitment was not a pragmatic reflection of patients in normal day-to-day practice. 4
-
A large observational study of 455 participants in South Korea, most with globus sensation, throat clearing and dysphonia, was undertaken. 27 In this cohort, the mean RSI score fell from 15 at baseline to 5.6 after 12 weeks of the PPI rabeprazole. 27 In 75% of this cohort, there was a reduction of > 50% in RSI, but the proportions in the abnormal range pre and post therapy are not clear. In comparison, Reichel et al. 28 recruited 62 participants to an esomeprazole study randomised against placebo; the baseline mean RSI levels in the two groups (23 and 21, respectively) were considerably higher than those in the Korean descriptive series,27 as were those for the cohort described in an early report by the authors of the RSI, whose mean participant baseline RSI score was also 20. 26
-
In a RCT of 82 participants randomised to placebo versus rabeprazole,15 mean baseline RSI scores were closer to those of Lee et al. 27 (around 14). Understandably, therefore, as there is a baseline incidence of throat symptoms in the general population, this study appears to show a floor effect with a much smaller decrement in RSI total scores than was observed in the Reichel et al. 28 cohort, who had ‘further to fall’.
In other words, some reported studies have a baseline RSI score only just above the normal level and others have a baseline RSI score that is considerably higher. Despite these differences in baseline severity, both of these most recent trials15,28 showed a benefit from a 3-month trial of acid suppression. Lam et al. 15 continued follow-up for a further 6 weeks, when the effect disappeared, whereas the Reichel et al. 28 final measurement point was the end of therapy. The RSI remains the ‘area standard’ and, although others have attempted to introduce other questionnaires, their uptake has been patchy and many studies have reverted to single-item visual analogue scales. As discussed previously, the RSI has been applied in numerous prior studies and, despite well-rehearsed limitations,29 remains our chosen primary outcome.
The nine-item RSI total score allows comparison with previous studies as it offers 10 years of comparative data in the literature.
Description of the Comprehensive Reflux Symptom Score questionnaire
The CReSS29,31 is a 34-item questionnaire of oesophageal and extraesophageal symptoms that has been tested on groups of ‘throat’ patients, healthy controls and those attending for an upper gastrointestinal endoscopy. It has three subscales [oesophageal (17 items), upper airway (nine items) and pharyngeal (five items)] on a large-scale factor analysis:
-
The total score has 34 items, each scored from 0 to 5, so the range is 0–170. Higher values indicate worse symptoms.
-
The oesophageal subscale has 17 items – heartburn, flatulence, regurgitation, acid/sour taste in mouth, gurgling, nausea, vomiting, bloating, belching, pressure in the chest, low appetite, feeling full too early in a meal, indigestion, stomach acid, back pain, headache and bad breath (each item is scored from 0 to 5, so the total range for the subscale is 0–85).
-
The upper airway subscale has nine items – throat clearing, excess mucus, mucous drip, coughing when upright, coughing after eating, coughing when lying down, wheezing, difficulty breathing and hoarseness (each item is scored from 0 to 5, so the total range for the subscale is 0–45).
-
The pharyngeal subscale has five items – lump in the throat, swallowing food, swallowing liquid, throat pain and feeling of things stuck in throat (total score out of 0–25).
Quality-of-life impact of throat symptoms: the Laryngopharyngeal Reflux Health Related Quality of Life questionnaire
Patient-reported generic health-related quality-of-life scores are abnormal in patients with throat symptoms,32 who show abnormalities of health-related quality of life in social functioning, pain and general health perception,33 but there is a perceived need for a disease-specific instrument to assess the impact of reflux on health-related quality of life. 34 This need led to the development of the Laryngopharyngeal Reflux – Health Related Quality of Life (LPR-HRQL),35 which has been validated in a Swedish population. 36 Its 43 items are grouped into four domains and an overall impact category (which includes general questions on relationships, sleep and lifestyle). The LPR-HRQL has been used in at least one prior RCT22 and shown to respond to change, and is a secondary outcome measure of TOPPITS.
The questionnaire contains questions about LPR (acid reflux into the upper throat and how it affects the patient).
Most questions are scored from 0 to 6, describing how often the patient experiences that symptom. The code for the scores is as follows:
-
0 = none of the time (never in the past month)
-
1 = rarely (once in the past month)
-
2 = a little of the time (2–3 days in the past month)
-
3 = some of the time (about once a week)
-
4 = a lot of the time (about 2–3 days a week)
-
5 = most of the time (4–5 days a week)
-
6 = nearly all of the time or always (6–7 days a week).
After a set of questions that relate to a particular symptom, there is a question rated on a scale from 1 to 10 known as a ‘thermometer’, which asks the patient to summarise the overall impact of those symptoms on their life, in which 1 represents ‘no effect’ and 10 represents ‘an enormous effect’ on their quality of life.
Finally, at the end of the questionnaire, there are a number of questions that use the 1–10 scale. These seek to quantify how much the symptoms effect energy levels, productivity at work, social relationships, marital relationships, sexual relationships, sleeping, ability to lie comfortably in bed, the way they feel about themselves, lifestyle (such as exercising, eating and drinking) and ability to do the things they enjoy.
A summary of how these component questions are combined to assess quality of life is given in the following section.
Laryngopharyngeal Reflux – Health Related Quality of Life questionnaire components
The total for each domain is scored by taking the total score, subtracting the mean and dividing by the standard deviation – to give the z-score for each domain. All ‘thermometer’ scales can be reported alone or alongside the relevant scale.
Voice
The voice scale consists of the first 12 questions in the LPR-HRQL questionnaire. They are all scored from 0 to 6 on a Likert scale. Note that the second question (I feel satisfied with the way my voice sounds) must be reversed before adding up the total voice score.
If any patient is missing fewer than six items, impute the mean item scores based on the sample being analysed, then compute the voice scale for that patient. If any patient is missing six or more items (out of the 12), they should be treated as missing. The voice scale is calculated by adding the 12 items together, resulting in a score between 0 and 72. The 13th item in the ‘Voice’ section is the voice thermometer.
Coughing
The cough scale consist of questions 14–19 in the LPR-HRQL questionnaire. They are all scored from 0 to 6 on a Likert scale. If any patient is missing fewer than three items, impute the mean item scores based on the sample being analysed, then compute the cough scale for that patient. If any patient is missing three or more items (out of the six), they should be treated as missing. The cough scale is calculated by adding the six items together, resulting in a score between 0 and 36. The 20th item is the cough thermometer.
Clear throat
The clear throat scale consist of questions 21–26 in the LPR-HRQL questionnaire. They are all scored from 0 to 6 on a Likert scale. If any patient is missing fewer than three items, impute the mean item scores based on the sample being analysed, then compute the clear throat scale for that patient. If any patient is missing three or more items (out of the six), they should be treated as missing. The clear throat scale is calculated by adding the six items together, resulting in a score between 0 and 36. The 27th item is the clear throat thermometer.
General
The general scale consist of questions 28–32 in the LPR-HRQL questionnaire. They are all scored from 0 to 6 on a Likert scale. If any patient is missing fewer than three items, impute the mean item scores based on the sample being analysed, then compute the general scale for that patient. If any patient is missing three or more items (out of the six), they should be treated as missing. The general scale is calculated by adding the six items together, resulting in a score between 0 and 30. The 33rd item is the general thermometer.
Overall score
The overall score is calculated by adding the four thermometer scores (questions 13, 20, 27 and 33) AND the domain scores (questions 34–43). They are all scored from 1 to 10 on a Likert scale. The overall score is calculated by adding the 14 items together, resulting in a score between 14 and 140. To ease interpretation, this is rescaled to a score out of 100 by subtracting 14 and dividing by 126.
Trial objectives
TOPPITS aimed to quantify, and to characterise, the effect of PPI therapy compared with placebo. Our comprehensive package of patient-centred outcomes allows us to assess which specific throat symptoms respond, to assess whether or not any patient characteristics can predict any measured treatment response, to derive improved estimates of impact on quality of life and to define the proportion of likely non-responders for whom alternative therapeutic approaches may be more appropriate. Definitions in the RSI literature to characterise responders include 50% reduction and final score within the normal range. Here we use a normal-range end point for response.
Primary objective
To compare the symptomatic response as measured by the RSI in patients with persistent throat symptoms at the end of 16 weeks’ treatment with lansoprazole versus placebo.
Secondary objectives
-
To explore recruitment feasibility using an internal pilot.
-
To evaluate the symptom response at 12 months in comparison with that at 16 weeks.
-
To determine the utility of the RSI questionnaire,26 the CReSS questionnaire31 items and subscales and endolaryngeal examination findings as scored by the Reflux Finding Score (RFS)37 as well as the value of patient demographics including age, sex, smoking status and body mass index (BMI)38,39 as potential baseline determinants of treatment response.
-
To assess side effects, treatment compliance and use of self-pay medications.
-
To compare changes in LPR-HRQL (i.e. disease-specific quality of life).
Treatment choice in TOPPITS
Proton pump inhibitors suppress gastric acid secretion by specific inhibition of the H+/K+-ATPase enzyme pump at the secretory surface of the gastric parietal cell. There is now a wide range of available PPIs. The best-value PPIs, and the most prescribed in the UK, are omeprazole and lansoprazole. The class of drugs is generally well tolerated. The frequency of adverse effects associated with PPIs (5%) is similar to that of placebo. The commonest complaints are headache, diarrhoea, abdominal pain and nausea. Except for diarrhoea, whose incidence is < 5%, the adverse effects of PPIs seem to be independent of age, dosage or duration of treatment. The diarrhoea seems to be due to altered gut microbiome secondary to the loss of acid secretion.
The clinical side effects of PPI use include rebound hypersecretion after cessation of PPI therapy, making it hard to wean some patients off of PPIs, and, of course, reinforcing the notion that they were necessary in the first place. 40,41 Rarer side effects include pneumonia,42 Clostridium difficile, infections, acute renal inflammation43 and fractures of hip, wrist and spine. 44
If a PPI is considered appropriate, there is no evidence that any one PPI is more effective than another, when used at therapeutically equivalent doses, but newer agents are considerably more costly:
-
We used lansoprazole in TOPPITS as it is among those frequently recommended by commissioning groups45 and formularies; the choice is justified through its inclusion in National Institute for Health and Care Excellence guidance. 46
-
In TOPPITS, as is typical of LPR studies, we used twice-daily treatment to minimise the risk of ‘breakthrough’ gastroesophageal refluxes occurring at night. 47
-
Lansoprazole has a lower unit cost than omeprazole – NHS prescribing data (January to March 2010) indicate that omeprazole is the most commonly prescribed PPI (4.9 million items, unit cost £4.90, total cost £24.2M); lansoprazole totalled 3.8 million items, at a somewhat lower unit cost of £3.00 (total cost £11.4M). 48
Chapter 2 Methods
Parts of this chapter are reproduced from Watson et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Setting and conduct
This multicentre RCT was conducted at eight UK NHS sites, recruiting patients from 28 April 2014 to 28 February 2017. The final study visit was on 23 March 2018. The trial was conducted in accordance with the principles of International Conference on Harmonisation good clinical practice, received a favourable ethics opinion from the National Research Ethics Service Committee North East – Tyne and Wear South (reference 13/NE/0336) on 2 December 2013 and clinical trial authorisation from the Medicines and Healthcare products Regulatory Agency on 12 February 2014. The trial was managed by the Newcastle Clinical Trials Unit (NCTU) with a Trial Management Group, together with an independent Trial Steering Committee (TSC) and Data Monitoring Committee (DMC). Site monitoring was undertaken by staff at NCTU and the trial was audited by the sponsor, The Newcastle upon Tyne Hospitals NHS Foundation Trust. The clinical trial protocol was published in 2016. 1 A statistical analysis plan was in place prior to any comparative analyses. 49
Patient and public involvement
Several patients contributed to the design of the study and the development of the second-stage application. To canvass the views of a range of patients with different experiences of chronic throat symptoms and treatments, the patient contributors were young professionals and retired patients. These patients were identified from clinics in Sunderland by James O’Hara. Individual consultations with four patients with LPR were conducted by James O’Hara. Two of these four patients agreed to join the TSC.
There was full patient support for the research proposal; the length of time taken to treat this problem and the lack of knowledge of throat symptoms on the part of clinicians is a source of frustration. When asked about the collection of follow-up data, there was a preference for this to be conducted face to face. When discussing outcome measures, patients felt that the CReSS had greater symptom coverage than the RSI, which the team believed justified its inclusion as a secondary outcome measure. Other changes as a result of patient and public involvement are listed below:
-
Duration of therapy. This was originally planned to be 2 months; however, our patient panel felt that this was too short and that a longer period (16 weeks) would provide a more definitive result.
-
Number of follow-up visits. The patient and public involvement group had a preference for more-frequent follow-up visits but these were reduced to two in line with the Health Technology Assessment panel feedback.
-
Data collection – remote versus face to face. The patient and public involvement group believed that, rather than complete questionnaires in their homes, people would prefer – and would be willing – to attend a clinic to complete the questionnaires.
-
Outcome questionnaires. Some members of the patient and public involvement group felt that there was some ambiguity in the wording of the questionnaires. This was improved as far as possible, bearing in mind that the RSI in particular needed to be comparable to previously published studies.
Planned patient and public involvement for the duration of the trial
The plan at the application stage for patient and public involvement throughout the trial was to build on the individual consultations conducted and convene a trial user forum to obtain a group perspective. Once funding was confirmed, those involved in the consultations would be invited to join the TOPPITS User Forum (TUF). It was considered that the forum would yield better outputs if it were user led. We hoped for a group of six individuals, aimed to encourage a cross-section of views and experience and hoped to recruit representation from the voluntary sector (e.g. the British Voice Association and the British Laryngological Association).
This forum would be facilitated by the local network patient and public involvement lead. Although the frequency of meetings would ultimately be decided by the forum members, it was initially thought that they should meet prior to the TSC meetings. This would enable a member of the forum to attend the TSC, to represent the group and raise any issues or queries. The intention was that the TUF would assist the trial team to:
-
develop the recruitment strategy
-
inform the adequacy and accessibility of patient information
-
encourage the TSC to stay focused on the needs of patients
-
disseminate the findings – users may be co-authors and participate in presentations
-
link to the voluntary sector (as described previously).
In return, the TUF would be offered:
-
a group and personal role specification, so that we could go through the implications of joining with patients
-
a meeting venue with project secretary support, ideally in a community setting, approximately three times per annum
-
meeting support from the project secretary
-
examples of user group formats from similar groups
-
encouragement to develop its own pages on the trial website (www.TOPPITS.co.uk) and a link from this website to the INVOLVE website (www.invo.org.uk/)
-
a discussion of learning and development requirements with the trial team, and regular feedback.
Attempt to implement the TOPPITS User Forum
At the project launch in April 2015, the TUF was discussed in order to seek the views of attendees on recruiting patients. The two patients who had taken part in the initial consultations during the development of the trial and funding application – who had also joined the TSC – were no longer able to be involved. James O’Hara, one of three head and neck otolaryngologists in the Sunderland site, was leading on patient and public liaison and it was agreed that another member of the team (JL) would provide support with patient and public involvement. The following steps were suggested to identify and recruit TUF members:
-
Sunderland and Newcastle site staff to approach pilot trial patients at their final follow-up appointment to ask if they would be willing to join the TUF.
-
Provide patient and public involvement information (overview and specification) and ask if Jan Lecouturier can contact them by telephone to discuss further.
-
If they are happy to be contacted further, site staff to pass name and telephone number to Jan Lecouturier.
-
Jan Lecouturier to contact them after 1 week to discuss.
-
Discuss terms of reference, availability and preferences for meeting.
-
Set up first meeting.
The first steps were for Jan Lecouturier to develop materials for the TUF and amend the patient and public involvement specification and send it on to site staff. Local site staff (Sunderland and Newcastle) agreed to identify and approach patients to ask if they would be willing to join the TUF. The relevant materials were sent to the Sunderland research nurses in the first instance. By early August 2015, it transpired that only two patients had completed follow-up and neither had expressed an interest in the TUF. It was decided to ask Newcastle research nurses to approach patients, again those attending for their final follow-up appointment. The Newcastle site team asked for packs to send out to patients (at that stage, there were six patients who had completed the trial). Only one patient had expressed an interest in joining the TUF by October 2015. This patient was contacted to thank them and to indicate that the team would be in touch further when more members had been recruited to the TUF.
The problem in recruiting patients to the TUF was escalated to the Newcastle research team lead for otolaryngology. In January 2016, the research team lead – who thought that the problem with recruiting to TUF was due to the small number of patients who had completed the trial – agreed to send the materials to any patients remaining who had completed the trial. Unfortunately, this strategy attracted no further interest in joining the TUF.
Our initial outline design included a qualitative study comprising in-depth face-to-face interviews with 15 patients to explore compliance, use of other medication and symptoms experienced during the trial. These discussions might have additionally fostered higher levels of patient engagement with interviewees who might then have joined our TUF. However, that component of the study was not funded and this inference is only speculative.
Trial design
This was a multicentre, Phase III, randomised, double-blind, placebo-controlled trial, with an internal feasibility pilot, carried out in secondary care. Patients with persistent throat symptoms were identified and recruited from NHS ENT clinics. This was a pragmatic trial designed to mirror current NHS clinical practice, and so patients were not subjected to the gamut of aerodigestive tract investigations that characterise many studies but do not routinely inform the patient pathway outside a research context. Patients were randomised in a double-blind fashion between two treatment groups in a 1 : 1 ratio, stratified by centre and baseline severity [on the basis of the RSI score omitting item 9: ‘Heart burn, chest pain, indigestion, or stomach acid coming up’ – hereinafter referred to as RSI-HB (Reflux Symptom Index minus the heartburn/dyspepsia item) (range 0–40)]. The ‘mild’ cohort had RSI-HB scores of 10–20 (inclusive) and the ‘severe’ cohort had scores of > 20. The heartburn/dyspepsia item was omitted to focus on throat symptoms rather than gastrointestinal symptom burden, although the total RSI was used as the primary outcome. Following successful demonstration of recruitment in three sites in the internal pilot, the main component of the trial recruitment was conducted over a total of 30 months (inclusive of the pilot phase).
Patients
Patients were adults who had been newly referred to secondary care otolaryngology clinics with persistent (> 6 weeks) unexplained throat symptoms, principally dysphonia, throat pain, globus sensation (feeling of something stuck in the throat), throat clearing, postnasal drip or mucus excess, and also night-time unexplained cough or choking. The clinics were in the following trial site hospitals:
-
Newcastle upon Tyne – Freeman Hospital (The Newcastle upon Tyne Hospitals NHS Foundation Trust)
-
Sunderland – Sunderland Royal Hospital (City Hospitals Sunderland NHS Foundation Trust)
-
Nottingham – Queen’s Medical Centre (Nottingham University Hospitals NHS Trust)
-
Brighton – Royal Sussex County Hospital (Brighton and Sussex University Hospitals NHS Trust)
-
Glasgow – Glasgow Royal Infirmary (NHS Greater Glasgow and Clyde)
-
Manchester – Manchester Royal Infirmary (Manchester University NHS Foundation Trust)
-
Birmingham – Queen Elizabeth Hospital (University Hospitals Birmingham NHS Foundation Trust)
-
Stockport – Stepping Hill Hospital (Stockport NHS Foundation Trust).
Inclusion criteria
-
Referred with a persistent primary throat symptom: globus, hoarseness, throat clearing, throat discomfort, choking spasms, excess mucus/postnasal drip, otherwise unexplained night-time cough or choking of ≥ 6 weeks’ duration.
-
Those with a score of ≥ 10 on the non-heartburn items of the RSI.
-
Capacity to provide fully informed consent to participate in the study.
Exclusion criteria
-
Patients whose RSI-HB score was < 10.
-
Those unable to complete the TOPPITS questionnaires.
-
Patients aged < 18 years.
-
Patients who were unwilling to undergo flexible endoscopy to establish the findings below.
-
Observed endoscopic laryngopharyngeal pathology that would typically require specific surgical intervention or investigations (e.g. suspected neoplasia/dysplasia, prominent Reinke’s oedema or unilateral vocal fold polyp, vocal cord palsy or chronic inflammatory diseases).
-
Confirmed or suspected current or prior malignancy of the head and neck or oesophagus.
-
Performing voice users: singers, actors, media workers.
-
Pregnant or lactating woman. Woman of childbearing potential must be using adequate contraception.
-
Currently on acid suppressants, acid neutralisers and alginates and unwilling to discontinue these for (1) a 4-week pre-study washout period in the case of PPI usage or (2) a 24-hour period for alginate or acid neutraliser. For those discontinuing PPI, ad hoc alginate use was allowed until the final 24–48 hours of the washout period prior to reassessment to confirm ongoing eligibility.
-
Prior adverse reaction to PPIs.
-
Severe hepatic dysfunction.
-
Patients taking warfarin, phenytoin, digoxin, ciclosporin, methotrexate, erlotinib, lapatinib, tacrolimus, sucralfate, citalopram, escitalopram, fluvoxamine, St John’s wort, clozapine, ulipristal acetate, cilostazol or systemic antifungals (itraconazole, ketoconazole, posaconazole or voriconazole).
-
Human immunodeficiency virus-positive patients/patients taking antiviral medications (atazanavir, nelfinavir, raltegravir, saquinavir or tipranavir).
-
Use of other investigational study drugs within the preceding 30 days.
Patients meeting the eligibility criteria were provided with a patient information sheet (PIS) and were given time to consider participation and the opportunity to ask questions before agreeing to take part. Consent was obtained by a member of the site trial team, delegated with that task on the delegation log and reasons for non-participation were recorded on a screening log at each trial site.
Some patients were identified from routine ENT, voice or 2-week wait cancer clinics. Any patient scoring ≥ 10 on the RSI-HB, where this was in routine clinical use, and who was potentially interested in participating in TOPPITS was given a PIS. The potential participant was then invited to attend a dedicated TOPPITS clinic after a cooling-off interval of ≥ 48 hours. Some potential participants were also identified through primary care referral letters. The principal investigators at each site were responsible for posting an invitation letter and PIS detailing the study along with a clinic appointment card.
A screening log was completed for all potential participants who were screened, including the reason for ineligibility and/or refusal to participate.
Recruitment
Screening
At the dedicated TOPPITS trial clinic, any outstanding queries were answered and there was an opportunity to review the randomisation video, if this had not already been accessed online from the TOPPITS website (www.TOPPITS.co.uk), with time to discuss the study further in this clinic. At the first trial clinic appointment, a more detailed confirmatory eligibility screen was completed by the investigator to document patients’ fulfilment of the eligibility criteria for all patients considered for the study and subsequently included or excluded. Owing to the small patient population, the PIS, consent form and questionnaires for the study were available in the English language only.
Patients taking acid suppressants, acid neutralizers or alginates prior to involvement in/being approached to take part in TOPPITS were required to undergo a 4-week washout period of PPIs, or 24 hours for alginates or acid neutralisers, before randomisation and commencement of TOPPITS trial medication (see Appendix 2, Tables 36 and 37). Written consent was obtained prior to this washout period. The eligibility of any patient undergoing a 4-week washout period was reconfirmed before prescribing trial medication.
Consent
Informed consent discussions were undertaken by appropriate site staff (as per the delegation log) involved in the study, including medical staff and research nurses, with the opportunity for patients to ask any questions. Those wishing to take part gave informed consent by signing and dating the study consent form, which was witnessed and dated by a member of the research team with documented, delegated responsibility to do so. Occasionally, a patient wishing to have further time to consider the trial also attended a subsequent clinic. The original signed consent form was retained in the investigator site file (ISF) with a copy in the clinical notes, and a copy was provided to the patient. Each patient specifically consented to their general practitioner (GP) being informed of their participation in the study. The right to refuse to participate without giving reasons was respected.
Randomisation
A blocked allocation (permuted random blocks of variable length) system was used to allocate patients in a 1 : 1 ratio stratified by centre and baseline severity (two groups, on the basis of the RSI-HB score: group 1, ≤ 20; group 2, > 20). The overall RSI-HB range was 10–38.
Allocation concealment mechanism
The treatment allocation was kept blind from the patients and investigators. Randomisation was conducted via the NCTU secure web-based randomisation service by a computer-generated allocation list utilising random permuted blocks to ensure concealment of allocation. The blinded randomisation system generated a unique treatment number for each patient that linked to a corresponding allocated trial drug (blinded) in accordance with block size and strata. The treatment number was clearly documented by the investigator on the trial prescription to ensure that the pharmacist dispensed the correct medication.
Implementation
The random computer-generated allocation sequence was produced by a statistician not furthermore involved with the trial in order to ensure concealment of allocation. Randomisation was administered centrally via the NCTU using a secure web-based system. The principal investigator or delegated personnel named on the delegation log obtained a unique trial number via this system, which was available 24 hours a day. Details of a nominated NCTU contact for randomisation was provided to sites.
Patients were enrolled at trial sites by staff members who were delegated the task by inclusion on the delegation log. These were generally the principal investigators (clinicians) and research nurses.
Medication
The trial medication was manufactured by Piramal Pharma Solutions (Morpeth, UK); its provision was administered by MODEPHARMA Limited (Beckenham, UK). Three campaigns of trial medication, blinded and randomised at the point of manufacture, were used in the trial. Management and handling comprised:
-
ensuring that sites had enough kits to ensure continuous provision to patients over three campaigns and 33 months
-
co-ordination of requirements at sites with drug expiry dates
-
liaison with site pharmacy staff to ensure accurate drug accountability record keeping
-
organisation of the management of returns and their subsequent destruction at sites
-
organisation of the destruction of the remaining kits at the end of the trial
-
in-person monitoring of site pharmacies
-
documentation of all stages of provision, dispensing, return and destruction of trial medication, at sites and centrally.
Blinding
Both the patients and the investigators/assessors were blinded to assignment to either the lansoprazole group or the placebo group (double-blind). A set of sealed code-break envelopes was kept either in the pharmacy or in the ISF at participating hospitals; these envelopes were opened only in an emergency, with authorisation from the chief investigator. When the code was broken, details including the patient number, who broke the code, why and when were recorded and maintained in the ISF. Code breaks were not routinely carried out for patients who completed study treatment. There were no code breaks throughout the duration of the trial.
At the second, end-of-therapy, visit, the integrity of the blind was assessed by a questionnaire item asking the patient if they thought that they had been taking lansoprazole or placebo or if they did not know.
The blind was maintained until all trial data were collected and the database was locked. Patients were offered the opportunity at their final visit to be informed of their allocated group, once data analysis was complete.
The active intervention was a 16-week course of a 30-mg twice-daily dose of the PPI lansoprazole. The control group received a 16-week course of twice-daily matched placebo. Patients received the intervention in capsule form and swallowed the capsules in the morning and in the evening. The allocation was blind to patients and research team staff and this was maintained throughout the trial.
Outcomes
The primary outcome measure was the raw total RSI score at 16 weeks after randomisation, which was collected at the first follow-up ENT outpatient visit. The RSI score is calculated from a nine-item, self-administered questionnaire scored on a Likert scale, with each item score ranging from 0 to 5, giving a total score range of 0–45. A higher score indicates more severe symptoms. Any missing component deems the total score missing. The nine-item RSI total score allows comparison with previous studies as it offers 10 years’ worth of comparative data in the literature. The RSI remains the ‘area standard’ and, despite well-rehearsed limitations, remained our chosen primary outcome. The treatment period of 16 weeks was selected on the basis of evidence that after 8 weeks there was very little further symptomatic improvement (as measured by RSI) in a validation sample of 40 patients. 50 A period of 8 weeks might, therefore, have seemed justified. However, owing to the fairly small and highly selected sample (all pH-metry positive) of this early report, we opted for a 16-week period in our definitive, larger study, with a view to maximising the impact and uptake of the TOPPITS findings. In other words, we chose this longer period so that we would not be open to criticism for having discontinued therapy too early.
Secondary outcome measures
-
RSI changes at 12 months after randomisation.
-
RSI score omitting item 9 (heartburn, chest pain, indigestion or stomach acid coming up), as the inclusion of dyspepsia symptoms has the potential to skew the results in favour of PPIs in past small trials.
-
CReSS total subscales: oesophageal (14 items), upper airway (eight items) and pharyngeal (seven items).
-
Quality of life: change in LPR-HRQL total score and subscales at 16 weeks and 12 months.
-
Laryngeal mucosal changes recorded by RFS (total range 0–29), scored by an independent observer, outside any of the recruiting sites, who was blind to treatment group.
-
The ability of RFS and patient characteristics (age, sex, smoking status and BMI) to predict any observed responses.
-
Side effects, adverse events (AEs) and serious adverse events (SAEs).
-
Use of over-the-counter medication.
-
Patient-reported satisfaction with the trial using a five-point overall satisfaction scale. This asked if the patient was very dissatisfied, dissatisfied, neither dissatisfied nor satisfied, satisfied or very satisfied. Additional comments were invited.
-
Patient accuracy in determining which treatment they had received.
Laryngoscopy
Laryngoscopy assessment of the larynx and pharynx was undertaken at baseline by the recruiting clinician using a laryngoscope. The appearances contributed to the eligibility criteria. An image was captured to allow the independent clinician to score the appearances using the RFS reported for the analysis at the end of the trial. The scoring scheme for the RFS assessment (as originally published by Belafsky et al. 37) is given in Appendix 3, Table 40. The images were anonymised, and stored and transferred securely. When possible, all imaging was captured using a video endoscope (digital) instrument rather than the poorer-definition fibrescope. The RFS is not credited with much validity by scientific scrutiny in the diagnosis of LPR. 17,51–53 However, the findings are important because non-specific laryngeal redness is often used by less experienced practitioners and, at times, speech therapists to presume a diagnosis of reflux. Hence, commentary on the significance of the images and their scores is of interest and impact. Scores can range from 0 to 26, with higher scores indicating more intense and/or widespread laryngeal inflammation.
Sample size
The primary outcome measure was the change in the RSI score between baseline and the 16-week assessment. A mean difference of 3 points in RSI score at 16 weeks was felt to be a clinically relevant target based on clinical and co-applicant experience and prior LPR therapy studies. A mean difference of 3.1 points, with an assumed SD of 7.754 equates to a standardised mean effect size of 0.4. Furthermore, a 0.4 effect size is of smaller magnitude than the effect of phonomicrosurgery or speech therapy. 55 A total of 332 patients (166 in each group of the study) were required, to provide 266 patients (133 in each group) completing the trial intervention, to detect a standardised mean effect size of 0.4 with 90% power and 5% significance level allowing for 20% loss to follow-up. A 20% loss to follow-up rate was believed to be a realistic estimate. NHS experience of the investigators in TOPPITS suggested that this was overly optimistic for a trial of this kind.
There were no planned formal interim analyses or stopping rules.
Statistical methods
Descriptive statistics are used to summarise patient characteristics, treatment compliance, RSI and other secondary measures at randomisation and 16 weeks. The descriptive analysis is based on presenting:
-
Underlying distribution of RSI reported graphically (histograms with normal curve overlaid).
-
RSI scores at baseline and 16 weeks summarised by randomised treatment group, and overall, using descriptive statistics. Means, SDs, medians, interquartile ranges (IQRs) and ranges are reported, as the score was treated as a continuous measure but is integer in nature.
-
RSI scores inside versus outside the published normal range. The percentage of patients in and out of normal range. The upper limit of normal in the RSI is said to be 12 in the general population. 26
The primary outcome measure was RSI score after 16 weeks. An unadjusted univariate analysis of 16-week RSI is presented. The primary analysis is a multivariable analysis using analysis of covariance (ANCOVA) and multilevel mixed-effect linear regression to compare the RSI at 16 weeks while adjusting for potential confounders. Although ANCOVA is generally robust to departures from normality, transformation is investigated and, if applied, is retained in the analyses throughout. Primary analysis adjusts for effects of stratification factors at randomisation [centre, as a random effect in the regression modelling, and baseline severity (mild RSI-HB of ≤ 20, severe RSI-HB of > 20), as a fixed effect in the regression modelling].
Data were analysed using a statistical software package [Stata® version 14 (StataCorp LP, College Station, TX, USA)]. The primary hypothesis tested was H0: the mean RSI at 16 weeks in the lansoprazole group is equal to the mean RSI at 16 weeks in the placebo group after adjustment for baseline stratification factors. Secondary analyses of the primary outcome measure considered adjustment for important clinical and demographic baseline factors, specifically sex, age, BMI, smoking status, alcohol consumption, baseline laryngeal appearance scores using the RFS, CReSS total and subscales and categories of symptoms. Three models were derived:
-
Model 1 – adjusted for stratification factors used at randomisation [recruiting centre (as a random effect) and baseline severity, as defined by the binary RSI-HB cut-off value of 20 (as a fixed effect)].
-
Model 2 – adjusted for baseline severity with RSI-HB as a continuous measure.
-
Model 3 – adjusted for baseline severity (RSI-HB as a continuous measure) and other important clinical and demographic baseline factors, specifically age, sex, smoking status and BMI.
Continuous covariates were investigated for non-linear relationships with outcome using first-order fractional polynomial transformations, which were used if they substantially improved model fit based on the Akaike information criterion (AIC). The optimal model was derived using a forward selection method with comparison of –2log-likelihood for variable inclusion. Analyses were conducted at a two-sided 5% level of significance throughout. The impact of removing any covariates from the final model was assessed in order to derive the most parsimonious model.
The analysis of secondary outcomes followed a broadly similar strategy for the questionnaire scores. Safety data were not subject to statistical comparison. Analyses were carried out on a complete-case basis. Missing data were described. The use of multiple imputation techniques was considered for the primary outcome and covariate data, should data be missing for patients completing the study to a sufficient extent (approximately > 10%) and deemed missing at random.
No formal interim analyses were planned. A statistical analysis plan was in place prior to any comparative analyses.
Definition of ‘compliant intention-to-treat group’
Primary statistical analyses were based on a compliant intention-to-treat (ITT) group. The DMC recommended that a distribution approach should be employed to determine a clinically relevant compliance window to maximise inclusion of patients and exclude only significant outliers. Thus, the compliant ITT group was defined, prior to comparative analyses and documented in the statistical analysis plan, as those with all ineligible and protocol violator patients included in the analysis in their randomised treatment group and attending their 16-week follow-up visit between 14 and 20 weeks. The distribution of time from randomisation to completion of the follow-up visits is reported graphically in Figure 1 (see also Appendix 1, Table 29).
FIGURE 1.
Histogram of time between randomisation and the 16-week follow-up visit in 283 patients.
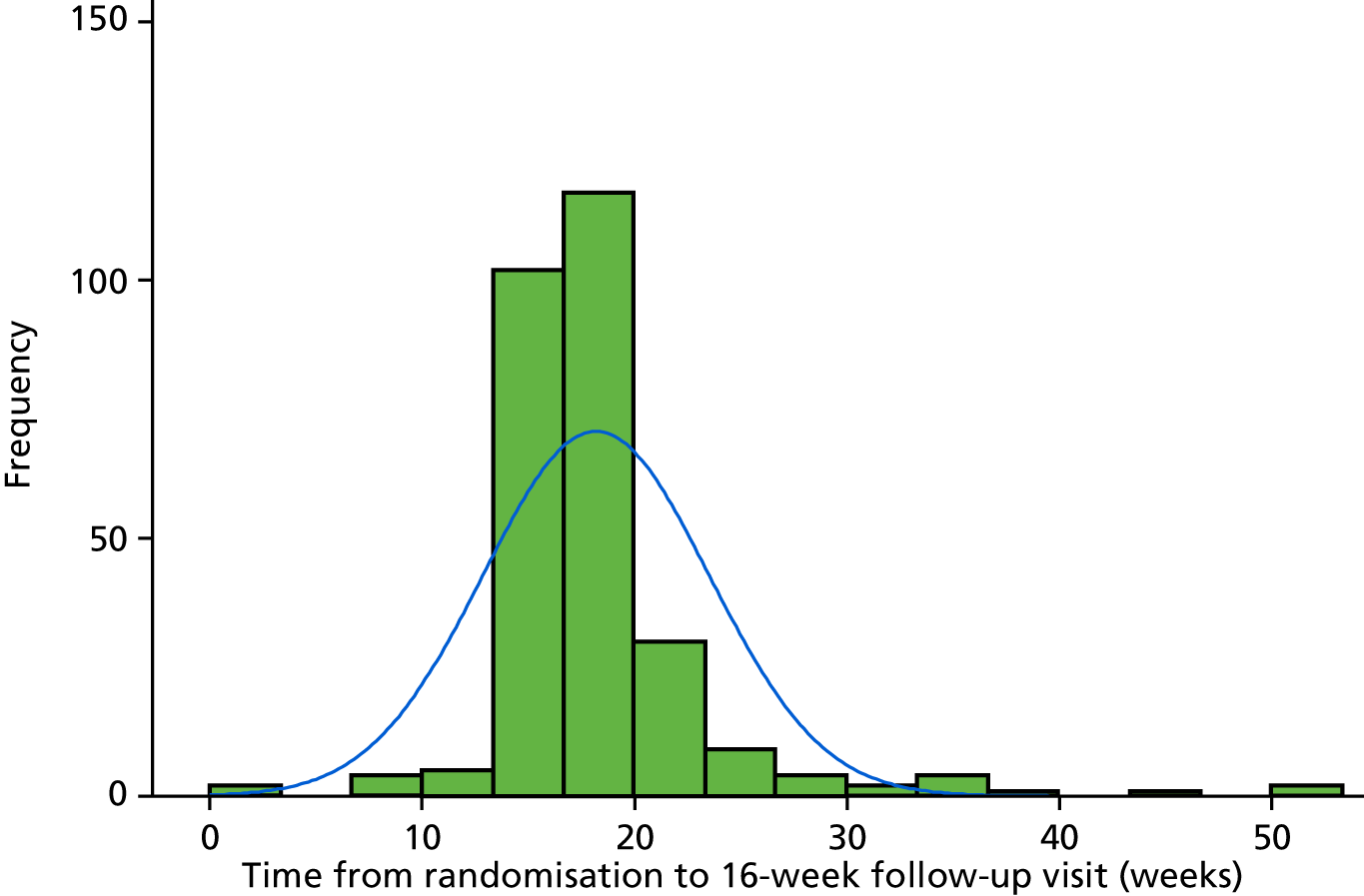
Sensitivity analyses are based on a pragmatic ITT group, with all ineligible and protocol violator patients included in the analysis on an ITT basis with patients kept in their randomised treatment group. This includes outcome measures completed at any time point.
On this basis, the power achieved was as follows:
-
compliant ITT group – 82% power based on a minimum of 102 (lansoprazole) and 118 (placebo) participants
-
pragmatic ITT group – 89% power based on a minimum of 127 (lansoprazole) and 140 (placebo) participants.
The per-treatment analysis of safety data reports the AEs that are related to the treatment. All randomised patients who start treatment were included in the analysis according to the treatment they received.
Data management
A study-specific database was designed, built and tested using Elsevier’s MACRO Electronic Data Capture (InferMed, London, UK) electronic data collection system. MACRO uses a secure web-based interface for data entry; no data are stored on computers at the site. Data for individual patients were entered by each principal investigator, or his/her delegated nominee, into the electronic case report forms. Each MACRO user is assigned role-based permissions specific to their site and role. MACRO has an inbuilt back-up facility, through Elsevier’s hosting partner Rackspace’s (Rackspace Ltd, Hayes, UK) secure premises in London, which is managed and supported by the Rackspace team.
Data entry and quality were monitored regularly by the Trial Management Group and sites were encouraged to enter all data as soon as possible. Frequent checks were made for missing and contradictory data, as well as ensuring that the data entered into the randomisation system matched those in MACRO. Data queries were raised with the sites on an ongoing basis, as necessary.
Chapter 3 Results
Recruitment to the trial is reported in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 2).
FIGURE 2.
The CONSORT flow diagram. IMP, investigational medicinal product.

Screening and recruitment
Screening and recruitment activity are summarised in Appendix 1, Tables 27 and 28.
The trial opened to recruitment on 28 April 2014, but owing to slower-than-expected recruitment a no-cost extension was obtained; hence, the trial closed to recruitment on 28 February 2017. The original target recruitment figure was 332; this was revised following DMC recommendation on 29 January 2016 to allow recruitment to continue to 28 February 2017, at which point the number recruited was 346. This ensured that the numbers of participants reaching the 4- and 12-month trial visits were sufficient to satisfy the trial protocol (version 5.0, January 2017).
Recruitment overview
-
Trial status: closed to recruitment (28 February 2017).
-
Grant awarded: 27 June 2013.
-
Ethics approval awarded: 2 December 2013.
-
Number of sites: eight.
-
Date first site opened: 28 April 2014 (site initiation date).
-
Date first patient randomised: 27 May 2014.
-
Date of data locks: 3 May 2018.
-
Total number of patients randomised before recruitment closed: 346.
-
Date last patient randomised: 24 February 2017.
-
Last follow-up: 23 March 2018.
Randomisation
A blocked allocation (permuted random blocks of variable length) system was used to allocate patients in a 1 : 1 ratio, stratified by centre and baseline severity (RSI-HB mild, ≤ 20; RSI-HB severe, > 20).
Balance was confirmed by stratification factors. See Appendix 1, Table 30, for stratification by baseline severity in terms of RSI-HB and stratification by site in Report Supplementary Material 1, Table 1. Appendix 1, Table 31, gives details of patients who were mis-stratified.
Four ineligible patients were reported (see Appendix 1, Table 32).
Withdrawals
There were 125 patients who were reported as withdrawals (including losses to follow-up) with specified dates. Two patients did not complete the trial but did not have a withdrawal date and two patients did not have trial completion status confirmed (i.e. no date for completion of or withdrawal from the trial). A summary of the timing of withdrawals from the trial, with respect to the primary end-point visit, is given in Appendix 1, Table 33. Descriptive statistics for time to withdrawal from randomisation are given in Appendix 1, Table 34.
Baseline data
Demographic baseline characteristics (Table 1) show the two treatment groups to be balanced. The age and sex statistics are consistent with the population of participants with chronic throat symptoms.
| Variable | All trial patients (N = 346) | Pragmatic ITT group (N = 267) | ||||
|---|---|---|---|---|---|---|
| Treatment group | Total | Treatment group | Total | |||
| Lansoprazole | Placebo | Lansoprazole | Placebo | |||
| Sex, n (%) | ||||||
| Male | 71 (41) | 79 (45) | 150 (43) | 49 (39) | 65 (46) | 114 (43) |
| Female | 101 (59) | 95 (55) | 196 (57) | 78 (61) | 75 (54) | 153 (57) |
| Age (years) | ||||||
| Mean (SD) | 53.5 (13.3) | 50.8 (13.9) | 52.2 (13.7) | 54.8 (12.8) | 52.3 (13.7) | 53.5 (13.3) |
| Range | 21–84 | 20–80 | 20–84 | 23–84 | 21–80 | 21–84 |
| Weight (kg) | ||||||
| n | 169 | 170 | 339 | 125 | 140 | 265 |
| Mean (SD) | 79.4 (18.2) | 79.3 (16.8) | 79.4 (17.5) | 78.0 (18.5) | 79.3 (16.1) | 78.7 (17.3) |
| Range | 43.8–142.0 | 42.5–140.3 | 42.5–142.0 | 43.8–142.0 | 48.0–140.3 | 43.8–142.0 |
| Height (m) | ||||||
| n | 170 | 171 | 341 | 126 | 140 | 266 |
| Mean (SD) | 1.68 (0.12) | 1.68 (0.10) | 1.68 (0.11) | 1.67 (0.1) | 1.68 (0.1) | 1.67 (0.1) |
| Range | 1.43–2.50 | 1.45–1.92 | 1.43–2.50 | 1.43–2.50 | 1.45–1.91 | 1.43–2.50 |
| BMI (kg/m2) | ||||||
| n | 169 | 170 | 339 | 125 | 140 | 265 |
| Mean (SD) | 28.2 (5.9) | 28.1 (5.3) | 28.1 (5.6) | 28.1 (6.3) | 28.1 (5.3) | 28.1 (5.8) |
| Range | 11.3–56.9 | 18.3–49.1 | 11.3–56.9 | 11.3–56.9 | 18.3–49.1 | 11.3–56.9 |
| Smoking (pack-years) | ||||||
| n | 168 | 171 | 339 | 124 | 140 | 264 |
| Median (IQR) | 0 (0–5) | 0 (0–0.5) | 0 (0–3) | 0 (0–3) | 0 (0–0.75) | 0 (0–2.5) |
| Mean (SD) | 4.7 (9.7) | 3.9 (10.4) | 4.3 (10.0) | 4.1 (8.7) | 4.2 (11.1) | 4.2 (10.0) |
| Range | 0–51 | 0–76 | 0–76 | 0–51 | 0–76 | 0–76 |
| Alcohol consumption (units per week) | ||||||
| n | 169 | 167 | 336 | 125 | 138 | 263 |
| Median (IQR) | 4 (0–10) | 3 (0–10) | 4 (0–10) | 4 (0–10) | 3 (0–10) | 3 (0–10) |
| Mean (SD) | 8.7 (12.2) | 6.6 (9.0) | 7.7 (10.8) | 8.3 (11.2) | 6.8 (9.4) | 7.5 (10.3) |
| Range | 0–80 | 0–60 | 0–80 | 0–50 | 0–60 | 0–60 |
| Baseline RSI-HB severity | ||||||
| n | 171 | 171 | 342 | 127 | 140 | 267 |
| Mean (SD) | 20.0 (6.8) | 20.1 (6.5) | 20.1 (6.6) | 20.0 (6.9) | 20.0 (6.5) | 20.0 (6.7) |
| Range | 10–38 | 10–38 | 10–38 | 10–38 | 10–38 | 10–38 |
| Site, n (%) | ||||||
| Birmingham | 5 (3) | 5 (3) | 10 (3) | 5 (4) | 4 (3) | 9 (3) |
| Brighton | 5 (3) | 4 (2) | 9 (3) | 5 (4) | 2 (1) | 7 (3) |
| Glasgow | 18 (10) | 21 (12) | 39 (11) | 7 (6) | 18 (13) | 25 (9) |
| Manchester | 15 (9) | 12 (7) | 27 (8) | 14 (11) | 9 (6) | 23 (9) |
| Newcastle | 66 (38) | 67 (39) | 133 (38) | 46 (36) | 51 (36) | 97 (36) |
| Nottingham | 34 (20) | 36 (21) | 70 (20) | 31 (24) | 33 (24) | 64 (24) |
| Stockport | 5 (3) | 6 (3) | 11 (3) | 5 (4) | 6 (4) | 11 (4) |
| Sunderland | 24 (14) | 23 (13) | 47 (14) | 14 (11) | 17 (12) | 31 (12) |
Scrutiny of Table 2 confirms that the compliant ITT group is a representative subsample of the total trial population.
| Variable | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (N = 102) | Placebo (N = 118) | ||
| Sex, n (%) | |||
| Male | 38 (37) | 56 (47) | 94 (43) |
| Female | 64 (63) | 62 (53) | 126 (57) |
| Age (years) | |||
| Mean (SD) | 55.3 (12.8) | 53.8 (13.4) | 54.5 (13.1) |
| Range | 23–84 | 21–80 | 21–84 |
| Weight (kg) | |||
| Mean (SD) | 78.9 (19.6) | 80.1 (16.2) | 79.5 (17.8) |
| Range | 43.8–142.0 | 50.6–140.3 | 43.8–142.0 |
| Height (m) | |||
| Mean (SD) | 1.67 (0.13) | 1.68 (0.10) | 1.67 (0.12) |
| Range | 1.43–2.50 | 1.45–1.91 | 1.43–2.50 |
| BMI (kg/m2) | |||
| Mean (SD) | 28.5 (6.7) | 28.4 (5.4) | 28.5 (6.1) |
| Range | 11.3–56.9 | 18.3–49.1 | 11.3–56.9 |
| Smoking (pack-years) | |||
| n | 101 | 118 | 219 |
| Median (IQR) | 0 (0–3) | 0 (0–0) | 0 (0–1) |
| Mean (SD) | 4.3 (9.0) | 4.3 (11.8) | 4.3 (10.6) |
| Range | 0–51 | 0–76 | 0–76 |
| Alcohol consumption (units per week) | |||
| Median (IQR) | 4 (0–10) | 3 (0–10) | 4 (0–10) |
| Mean (SD) | 8.0 (10.5) | 7.1 (9.7) | 7.5 (10.0) |
| Range | 0–45 | 0–60 | 0–60 |
| Baseline RSI-HB severity | |||
| Mean (SD) | 20.3 (7.4) | 19.8 (6.6) | 20.0 (7.0) |
| Range | 10–38 | 10–38 | 10–38 |
| Site, n (%) | |||
| Birmingham | 5 (5) | 4 (3) | 9 (4) |
| Brighton | 3 (3) | 2 (2) | 5 (2) |
| Glasgow | 5 (5) | 14 (12) | 19 (9) |
| Manchester | 9 (9) | 6 (5) | 15 (7) |
| Newcastle | 30 (29) | 39 (33) | 69 (31) |
| Nottingham | 31 (30) | 32 (27) | 63 (29) |
| Stockport | 5 (5) | 4 (3) | 9 (4) |
| Sunderland | 14 (14) | 17 (14) | 31 (14) |
Data quality
Data were received for analysis from:
-
MACRO – the trial data
-
the NCTU randomisation service – randomisation data including details of stratification (Microsoft Excel®, Microsoft Corporation, Redmond, WA, USA)
-
RFS assessment – from an independent reviewer, validated by NCTU (using Microsoft Excel) (see Appendix 3, Table 41, for the RFS scoring scheme).
Data sets were merged according to the unique identifier allocated at randomisation.
Questionnaires and forms returned, by visit
Data were collected using case report forms. Case report forms were completed and collated in the following order:
-
registration and randomisation form – completed prior to treatment allocation (baseline and demographic data for potential covariates collected)
-
questionnaires completed at baseline visit – RSI (primary), CReSS (secondary), LPR-HRQL (secondary)
-
AEs and concomitant medication use recorded at baseline visit
-
trial questionnaires again completed at visit 2 (16-week follow-up) as at the baseline visit
-
returned medication – capsule count carried out at visit 2
-
electronic case report form completed at visit 2 showing which treatment (trial drug or placebo) the patient believed they were randomised to
-
AEs and concomitant medication use recorded at visit 2
-
questionnaires completed at visit 3 as at the baseline and 16-week follow-up visits
-
AEs and concomitant medication use recorded at visit 3.
The three trial questionnaires (RSI, CReSS and LPR-HRQL) were completed at all three visits:
-
visit 1 – baseline visit (data locked: 3 May 2018)
-
visit 2 – primary end-point, 16-week follow-up visit (data locked: 3 May 2018)
-
visit 3 – 12-month follow-up visit (data locked: 3 May 2018).
Laryngoscopy assessment
The RFS data were included in the analysis to explore whether or not laryngeal appearance predicts outcome (i.e. response to lansoprazole). The image assessments were carried out (by PC) in four blocks: in December 2015, December 2016, June 2017 and December 2017.
Treatment received
The analysis set is the per-treatment analysis group defined in the statistical methods. Inclusion in the per-treatment set was based on capsule return at the 16-week primary end point. In total, 265 out of 346 participants (77%) had information on returned medication (including returned empty packaging for those who took the full course). Three patients did not take any trial medication and were removed from the per-treatment analysis group.
In total, 262 out of 346 trial participants (76%) are assumed to have taken at least one capsule of their trial medication and are included in the per-treatment analysis group [126 out of 172 (73%) in the lansoprazole group; 136 out of 174 (78%) in the placebo group]. Further details of dose taken are given in Appendix 2, Table 38.
Protocol treatment schedule
Treatment was with either a 30-mg (twice-daily) dose of the PPI lansoprazole or placebo for 16 weeks. The kits contained 17 weeks’ supply so each patient received 238 capsules of either lansoprazole or placebo. The treatment was double blind; both patients and clinicians were blind to treatment.
Trial medication was prescribed by a clinician, and dispensed to the patient or clinical staff in accordance with local pharmacy policy.
Patients who were still in possession of any medication returned all leftover trial supplies in their original packaging (even if empty) to the clinician or pharmacist at the 16-week follow-up visit (primary end point).
Medication received
At 16 weeks (± 2 weeks in protocol) following recruitment, the primary end-point clinic (visit 2) took place at the TOPPITS clinic. A patient who completed the course of medication in exactly 16 weeks would have taken 16 × 7 × 2 = 224 doses of lansoprazole or placebo. The dose supplied at randomisation comprised 238 capsules, enough for 17 weeks, with two capsules per day as per the protocol. Forty-two per cent of participants reported taking the full dose, balanced across randomised groups, and 70% patients reported taking ≥ 90% of the dose, balanced across groups. The median percentage protocol dose is 99% (IQR 86–100%).
Appendix 2 contains further details of doses taken.
Proton pump use and concomitant medication
Recent PPI usage at randomisation was collected at baseline and is presented in Appendix 2.
A total of 377 concomitant medication uses were reported during the trial among the per-treatment analysis group, in 143 unique patients (Table 3).
| Participant concomitant medication use | Visit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (baseline) | 2 (primary end point: 16 weeks) | 3 (12-month follow-up) | |||||||
| Treatment group | Total | Treatment group | Total | Treatment group | Total | ||||
| Lansoprazole | Placebo | Lansoprazole | Placebo | Lansoprazole | Placebo | ||||
| Total number of participants reporting medications | 108 | 73 | 181 | 38 | 40 | 78 | 57 | 56 | 113 |
| Number of unique participants reporting medications | 41 | 33 | 74 | 26 | 25 | 51 | 32 | 34 | 66 |
Awareness of treatment group by patients
Each patient was asked which drug they believed that they had been taking at the 16-week follow-up visit. Fourteen per cent were not able to identify (or attempt to identify) which treatment they had been randomised to and answered ‘Don’t know’ (Table 4).
| Participant opinion of drug taken | Actual randomised treatment, n (%) | Total (N = 262), n (%) | |
|---|---|---|---|
| Lansoprazole (N = 126) | Placebo (N = 136) | ||
| Lansoprazole | 53 (42) | 43 (32) | 96 (37) |
| Placebo | 51 (40) | 76 (56) | 127 (48) |
| Don’t know | 21 (17) | 15 (11) | 36 (14) |
| Total number assessed | 125 (99) | 134 (99) | 259 (99) |
Three assessments were missing: one for a participant randomised to lansoprazole and two for participants randomised to placebo.
Outcomes and estimation of treatment effects
Primary outcome measure
Compliance was assessed in relation to the time frame of 14–20 weeks post randomisation. The distribution of time from randomisation to the primary end-point 16-week follow-up visits (visit 2) is reported graphically in Figure 3. The graph shows how much time has elapsed since randomisation to the 16-week follow-up visit for all patients who attended the visit (n = 283). The dashed lines show patients included in the compliant ITT group (n = 220). Further information on compliance is given in Appendix 1, Tables 29 and 35. See Appendix 2, Tables 39 and 40, for details of individual RSI items.
FIGURE 3.
Histogram of time between randomisation and the primary end-point 16-week follow-up visit (overall). The dashed lines show patients included in the compliant ITT group (n = 220).

Baseline itemised severity scores: Reflux Symptom Index in all patients and the compliant group
The item descriptive statistics for RSI are listed in Appendix 3, Table 39. The top five RSI items, for both the trial population and the compliant group, in ranked mean endorsement severity were:
-
lump in the throat
-
throat clearing
-
excess mucus
-
troublesome cough
-
hoarseness.
Reflux Symptom Index score at baseline for the compliant analysis group (n = 220)
The means (SDs) are reported with medians, IQRs and ranges as the (total) RSI score is treated as a continuous measure but is integer in nature (Table 5).
| Baseline RSI | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | 20.5 (15–28) | 21.5 (16–27) | 21 (15.5–27) |
| Mean (SD) | 22.0 (8.0) | 21.7 (7.1) | 21.9 (7.5) |
| 95% CI of mean | 20.4 to 23.6 | 20.5 to 23.0 | 20.9 to 22.9 |
| Range | 10–41 | 10–43 | 10–43 |
The summary statistics show that the randomised groups are similar at baseline.
The underlying distribution of the RSI at baseline visits is reported graphically in Figure 4 in the form of a histogram with both groups combined (n = 220) and normal curve overlaid.
FIGURE 4.
Underlying distribution of RSI at baseline (compliant ITT group).

Reflux Symptom Index score at 16 weeks (primary end point) for the compliant analysis group
Figure 5 and Table 6 show the underlying distribution and summary statistics of the RSI score at the primary end point of 16 weeks for the 220 patients in the compliant ITT group.
FIGURE 5.
Underlying distribution of RSI at the primary end-point visit (compliant ITT group).

| RSI at 16 weeks | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | 16 (9–26) | 14 (7–23) | 15 (9–24.5) |
| Mean (SD) | 17.4 (9.9) | 15.6 (9.8) | 16.4 (9.9) |
| 95% CI of mean | 15.5 to 19.4 | 13.8 to 17.3 | 15.1 to 17.7 |
| Range | 0–41 | 0–44 | 0–44 |
A higher RSI score indicates more severe symptoms. RSI reduced overall from a median score of 21 at baseline to a median score of 15 at 16 weeks. RSI reduced overall from a mean score of 21.9 (SD 7.5) at baseline to a mean score of 16.4 (SD 9.9) at 16 weeks. The reduction in RSI is observed in both randomised treatment groups. The lansoprazole group has a mean 16-week score 1.8 points higher than that of the placebo group [17.4 (95% CI 15.5 to 19.4) vs. 15.6 (95% CI 13.8 to 17.3)], with overlapping CIs, indicating no statistical difference between the groups.
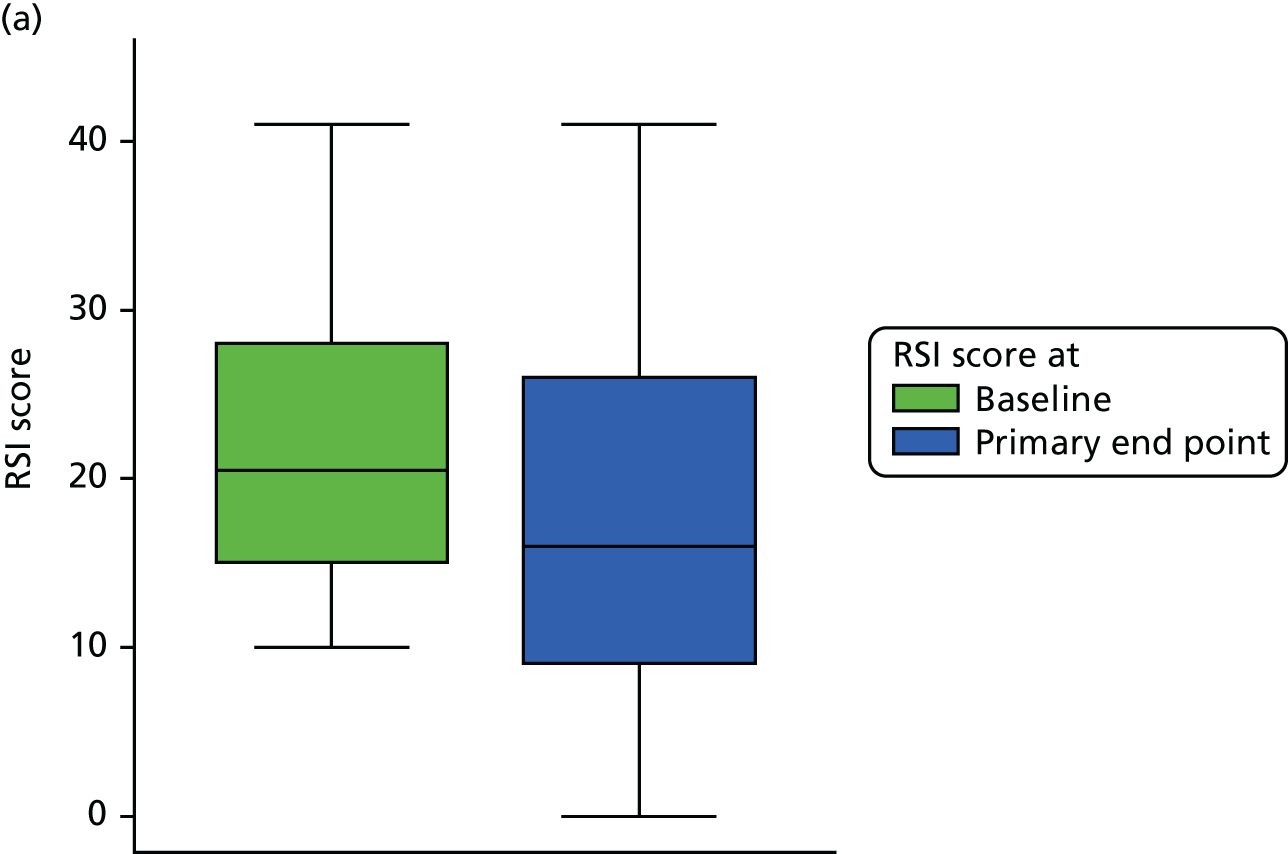
The primary outcome data for the compliant ITT group are presented graphically in Figure 6.
FIGURE 6.
Box plots showing medians, IQRs and overall ranges of RSI score at baseline and primary end-point visit (compliant ITT group). (a) Lansoprazole group (n = 102); and (b) placebo group (n = 118).

Univariate analysis of unadjusted primary outcome measure for the compliant analysis group
The underlying distribution of the primary outcome measure (see Figure 5) appears sufficiently normally distributed (overall mean = 16.4, median = 15) for parametric analysis of the primary outcome. The primary hypothesis to be tested is H0: the mean RSI scores at the primary end point (16-week visit) are equal for both groups (lansoprazole vs. placebo). A two-sided significance level of p < 0.05 is used throughout. The null hypothesis is no difference between means for PPI (17.4, 95% CI 15.5 to 19.4) versus placebo (15.6, 95% CI 13.8 to 17.3). The test statistic was t = 1.402 and the two-sided p-value was 0.162, leading to the conclusion that there was no statistically significant difference in the RSI score at 16 weeks between lansoprazole and placebo.
Table 7 and Figure 7 show the change in RSI score (change RSI = 16-week RSI – baseline RSI) to the primary end-of-treatment end point.
| Change in RSI scores (baseline to 16 weeks) | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | –4 (–10 to 1) | –5 (–13 to 1) | –5 (–11 to 1) |
| Mean (SD) | –4.6 (8.0) | –6.2 (9.0) | –5.4 (8.6) |
| 95% CI of mean | –6.2 to –3.0 | –7.8 to –4.5 | –6.6 to –4.3 |
| Range | –31 to 19 | –29 to 11 | –31 to 19 |
FIGURE 7.
Box plots showing medians, IQRs and overall ranges for change in RSI score from baseline to the 16-week follow-up (compliant ITT group). (a) Lansoprazole group (n = 102); and (b) placebo group (n = 118).
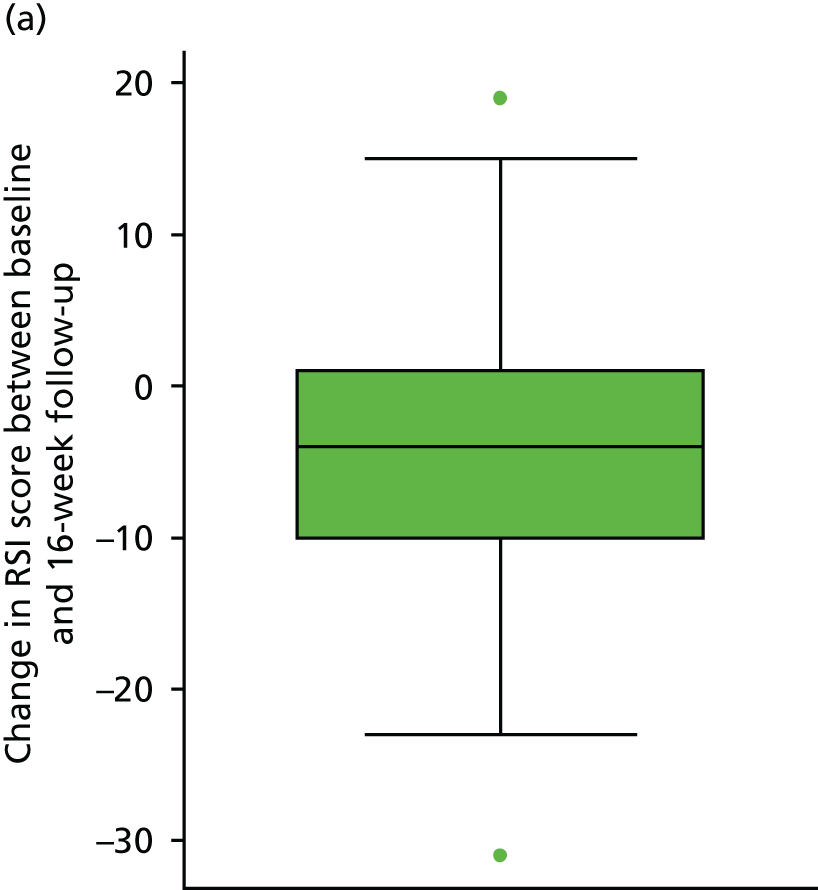
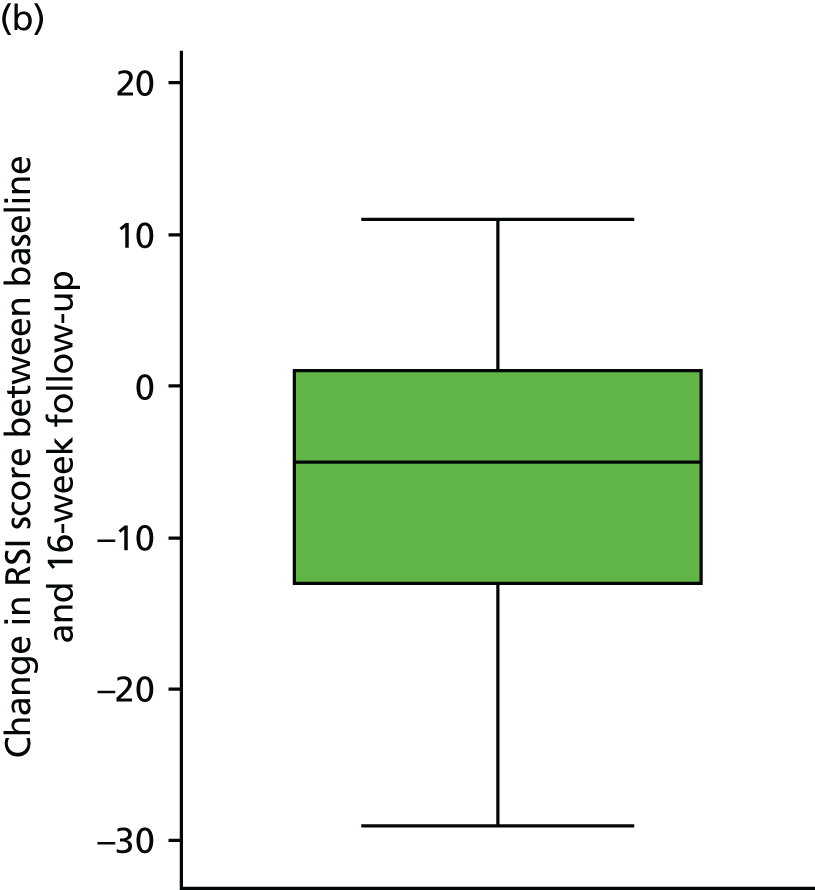
The overall mean reduction in RSI score from baseline to 16 weeks is 5.4, which is observed across both randomised groups: lansoprazole group, 4.6-point reduction; placebo group, 6.2-point reduction (with overlapping CIs indicating no statistical difference between the groups). The lansoprazole group had a mean reduction of 1.6 points less than the mean reduction observed in the placebo group.
Comparison of Reflux Symptom Index scores at baseline and 16 weeks with the published upper limit of normal range, for the compliant intention-to-treat analysis group
The upper limit of normal in the total nine-item RSI is said to be < 12 in the asymptomatic population. 26 Overall, 10% of patients are within normal range at baseline, balanced across randomised groups (this figure reflects the inclusion severity criterion, i.e. the eight-item version RSI excluding the dyspepsia item 9, ‘RSI-HB’). At 16 weeks, 43% (95% CI 37% to 50%) of participants were within the normal range, balanced across randomised groups: 41% (95% CI 31% to 51%) for the lansoprazole group versus 45% (95% CI 36% to 54%) for the placebo group. The overlapping CIs indicate no statistical difference between the groups.
Multivariable analysis of primary outcome for the compliant intention-to-treat analysis group
The primary outcome measure was RSI score after 16 weeks and was initially analysed using ANCOVA methods in order to compare the 16-week RSI scores between the treatment groups while adjusting for potential confounders, as specified in the protocol (Table 8). Although ANCOVA was generally robust to departures from normality, normality was assumed (see Figure 5).
| Source | Partial SS | df | Mean square | F-ratio | p-value |
|---|---|---|---|---|---|
| Model | 5502.119 | 9 | 611.347 | 8.14 | < 0.001 |
| Arm | 198.180 | 1 | 198.180 | 2.64 | 0.106 |
| Site | 1822.126 | 7 | 260.304 | 3.47 | 0.002 |
| Baseline severity | 3429.688 | 1 | 3429.688 | 45.68 | < 0.001 |
| Residual | 15,765.859 | 210 | 75.076 | ||
| Total | 21,267.977 | 219 | 97.114 |
There did not appear to be a statistically significant difference in RSI at 16 weeks between randomised treatment groups (p = 0.106). In ANCOVA, site is not a reliable estimate as the algorithm deals with the location numerals as if they were quantitative data. Site is more properly modelled as a random effect in a multilevel model.
Statistical modelling of the primary outcome
A multilevel mixed-effect linear regression was used to model the primary outcome measure – 16-week RSI – on a complete-case basis. Three models were developed as specified in Statistical methods (see Table 12 for a summary of the model results for both the compliant and the pragmatic ITT populations).
Model 1
Model 1 adjusted for stratification factors used at randomisation as covariates in the analysis: (1) recruiting centre (as a random effect) and (2) binary baseline severity as defined by the binary RSI-HB cut-off value of 20 (as a fixed effect) (Table 9).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 1.929 | 1.160 | 1.66 | 0.096 | –0.345 to 4.203 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –6.010 | 4.727 | –1.27 | 0.204 | –15.275 to 3.254 |
| Glasgow | –0.973 | 3.478 | –0.28 | 0.780 | –7.789 to 5.843 | |
| Manchester | 5.123 | 3.576 | 1.43 | 0.152 | –1.886 to 12.131 | |
| Newcastle | –1.871 | 3.047 | –0.61 | 0.539 | –7.843 to 4.101 | |
| Nottingham | –5.937 | 3.028 | –1.96 | 0.050 | –11.871 to –0.003 | |
| Stockport | –3.701 | 4.025 | –0.92 | 0.358 | –11.590 to 4.188 | |
| Sunderland | –3.385 | 3.216 | –1.05 | 0.293 | –9.689 to 2.919 | |
| RSI-HB baseline severity: severe (reference = mild) | Fixed | 8.173 | 1.181 | 6.92 | < 0.001 | 5.858 to 10.489 |
| Constant | 14.349 | 3.044 | 4.71 | < 0.001 | 8.383 to 20.315 | |
Modelling site as a random effect demonstrates no impact of site on RSI score at 16 weeks, as anticipated, and demonstrates the advantage of modelling these data more appropriately.
Binary RSI-HB at baseline is confirmed as being statistically significantly, related to 16-week RSI and justified as a stratification factor in the trial design. Patients in the severe severity stratum at baseline are estimated to have a RSI score that is 8 points higher (worse) at 16 weeks.
There is no statistically significant difference between randomised groups [lansoprazole compared with placebo (p = 0.096)] when adjusted for site and baseline binary RSI-HB. The difference between randomised groups, when accounting for site and baseline severity, indicates that lansoprazole patients are estimated to have RSI scores at 16 weeks that are 1.9 points higher (worse) than the scores of placebo patients (95% CI –0.3 to 4.2; p = 0.096).
Random errors are assumed to produce residuals that are normally distributed, as is the case for model 1, in which the residuals fall in a symmetrical pattern and have a constant spread throughout the range (Figures 8 and 9).
FIGURE 8.
Scatterplot of residuals for model 1 (both groups combined, n = 220) (compliant ITT group).

FIGURE 9.
Histogram of residuals for model 1 (both groups combined, n = 220) (compliant ITT group).
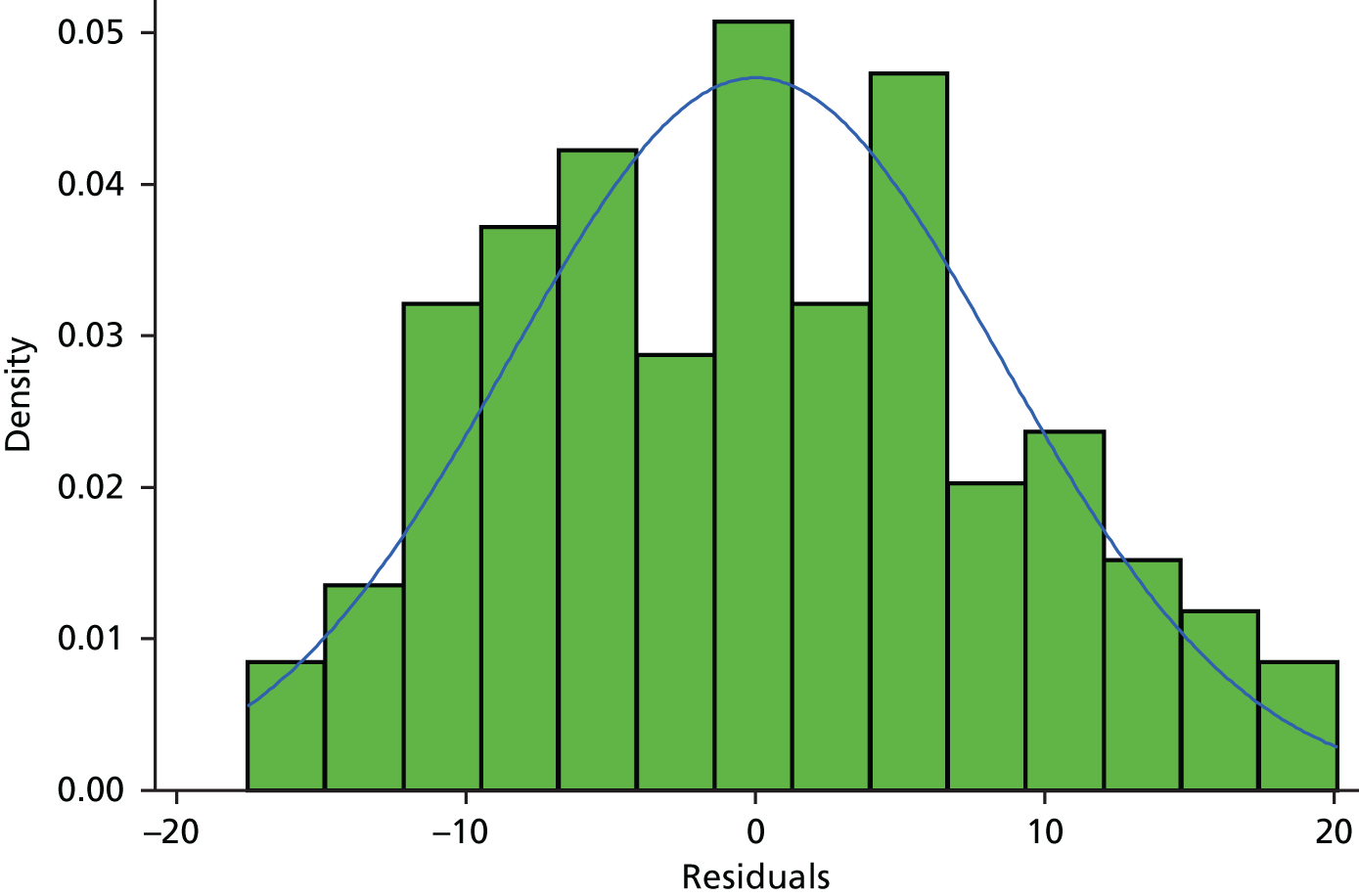
Further information on model 1 at 12 months, for the compliant ITT group, is given in Appendix 7, Table 53, and similarly for model 2 in Appendix 7, Table 54.
Model 2
Model 2 adjusted for the stratification factor recruiting centre (as a random effect) at randomisation and baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect).
The continuous covariate (RSI-HB) was explored to assess whether or not transformation of RSI-HB was a better fit to the relationship with outcome than an untransformed continuous measure based on a reduction in AIC. There was no reduction in AIC, so to build the most parsimonious, clinically interpretable model, RSI-HB was retained as an untransformed continuous covariate, under the assumption of linearity with outcome (Table 10).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 1.410 | 1.091 | 1.29 | 0.196 | –0.728 to 3.549 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –1.300 | 4.493 | –0.29 | 0.772 | –10.106 to 7.506 |
| Glasgow | –1.694 | 3.252 | –0.52 | 0.602 | –8.067 to 4.679 | |
| Manchester | 3.003 | 3.359 | 0.89 | 0.371 | –3.579 to 9.586 | |
| Newcastle | –2.785 | 2.840 | –0.98 | 0.327 | –8.351 to 2.781 | |
| Nottingham | –5.664 | 2.847 | –1.99 | 0.047 | –11.243 to –0.084 | |
| Stockport | –3.085 | 3.783 | –0.82 | 0.415 | –10.499 to 4.329 | |
| Sunderland | –2.759 | 3.027 | –0.91 | 0.362 | –8.693 to 3.174 | |
| RSI-HB continuous baseline severity | Fixed | 0.721 | 0.079 | 9.10 | < 0.001 | 0.566 to 0.877 |
| Constant | 4.320 | 3.274 | 1.32 | 0.187 | –2.098 to 10.738 | |
The log-likelihood statistics show that continuous baseline severity substantially improves the model fit (reduction in –2log-likelihood) compared with model 1, which included severity as a binary stratification factor.
A 1-unit increase in baseline severity is associated with a 0.7-unit increase (95% CI 0.6 to 0.9) in 16-week RSI.
There is no statistically significant difference between randomised groups [lansoprazole compared with placebo (p = 0.196)] when adjusted for site and baseline continuous RSI-HB. The estimated difference between randomised groups, when accounting for site and baseline severity, indicates that patients receiving lansoprazole are estimated to have RSI scores at 16 weeks that are 1.4 points higher (worse) than the scores of placebo patients (95% CI –0.7 to 3.5; p = 0.196).
Residual plots demonstrate the underlying assumptions of the model to not be substantially violated (Figures 10 and 11).
FIGURE 10.
Scatterplot of residuals for model 2 (both groups combined, n = 220) (compliant ITT group).
FIGURE 11.
Histogram of residuals for model 2 (both groups combined, n = 220) (compliant ITT group).

Model 3
Model 3 adjusted for baseline severity (RSI-HB as a continuous measure) and the baseline factors age, sex, smoking status (binary), alcohol consumption (binary) and BMI. Non-linear continuous covariates were explored but continuous covariates were retained as untransformed (Table 11).
| Covariate | n | AIC | Beta | SE | Test statistic | p-value |
|---|---|---|---|---|---|---|
| BMI | ||||||
| Continuous | 220 | 1634.002 | 0.017 | 0.110 | 0.15 | 0.878 |
| Log-transformed | 1634.017 | |||||
| Complex transformation (BMI–2) | 1633.240 | |||||
| Age | ||||||
| Continuous | 220 | 1633.977 | –0.011 | 0.051 | –0.22 | 0.826 |
| Log-transformed | 1633.791 | |||||
| Complex transformation (age–2) | 1629.129 | |||||
| Smoking (binary) | 219 | 1626.368 | 1.547 | 1.520 | 1.02 | 0.310 |
| Sex (binary – male/female) | 220 | 1632.896 | 1.423 | 1.343 | 1.06 | 0.291 |
| Alcohol consumption (binary) | 217 | 1611.289 | –1.761 | 1.483 | –1.19 | 0.236 |
As none of the potential covariates appears to have a significant univariate relationship with the RSI score at the primary end point, model 2 remains optimum.
Sensitivity analysis of the primary outcome
A sensitivity analysis based on the pragmatic ITT group including all patients with the 16-week visit at any time was also carried out. This did not change the conclusions of the trial (Table 12).
| Model | ITT population | |||
|---|---|---|---|---|
| Complaint (n = 220) | Pragmatic (n = 267) | |||
| Beta (95% CI) | p-value | Beta (95% CI) | p-value | |
| 1 | 1.9 (–0.35 to 4.20) | 0.096 | 1.5 (–0.60 to 3.53) | 0.165 |
| 2 | 1.4 (–0.73 to 3.55) | 0.196 | 1.1 (–0.83 to 3.05) | 0.264 |
| 3 | Model 2 optimum | N/A | Model 2 optimum | N/A |
Appendix 5 provides further details of the secondary analyses of the primary outcome measure (the pragmatic ITT group). Appendix 5, Table 47, gives summary statistics for the primary outcome measure: RSI for the pragmatic ITT population at baseline and primary end point. Appendix 5, Table 48, shows the ANCOVA results. Appendix 5, Table 49, shows results for model 1, primary outcome measure as response with adjustment for stratification and other baseline factors. In addition, during the DMC closed session held on 9 March 2018, the DMC discussed the different analysis groups. The chairperson felt that a sensitivity analysis looking at the per-protocol group would be useful to support the primary analysis. Details of this analysis are provided in Appendix 5. Appendix 5, Table 50, shows summary statistics at the primary end point.
Secondary analysis of the primary outcome measure using derived Reflux Symptom Index minus the heartburn/dyspepsia item
A secondary analysis of the primary outcome based on the reduced RSI score (derived from eight items excluding item 9, the dyspepsia and heartburn component – RSI-HB) was planned and undertaken. RSI-HB, scored out of 40, was calculated and analysed as was the primary outcome measure (complete nine-item RSI).
Means, SDs, medians, IQRs and ranges for RSI-HB are reported as the score is treated as a continuous measure but is integer in nature. The summary statistics show that the randomised groups are similar at baseline (Table 13).
| RSI-HB | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | 19 (14–25) | 19.5 (14–24) | 19 (14–24.5) |
| Mean (SD) | 20.3 (7.4) | 19.8 (6.6) | 20.0 (7.0) |
| 95% CI of mean | 18.8 to 21.7 | 18.6 to 21.0 | 19.1 to 20.9 |
| Range | 10–38 | 10–38 | 10–38 |
A higher RSI score indicates more severe symptoms. RSI-HB reduced overall from a median of 19 at baseline to a median of 13 at 16 weeks. RSI-HB reduced overall from a mean score of 20.0 (SD 7.0) at baseline to a mean score of 15.0 (SD 9.2) at 16 weeks (Table 14 and Figure 12). The reduction in RSI-HB is observed in both randomised treatment groups. The lansoprazole group has a mean 16-week score that is 2.4 points higher than the score for the placebo group [16.3 (95% CI 14.5 to 18.1) vs. 13.9 (95% CI 12.2 to 15.5), respectively], with overlapping CIs indicating no statistical difference between the groups. The null hypothesis was that there was no difference between the means. The test statistic was t = 1.945 and the two-sided p-value was 0.0530.
| RSI-HB | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | 14 (9–25) | 12 (7–19) | 13 (8–22) |
| Mean (SD) | 16.3 (9.4) | 13.9 (9.0) | 15.0 (9.2) |
| 95% CI of mean | 14.5 to 18.1 | 12.2 to 15.5 | 13.8 to 16.2 |
| Range | 0–39 | 0–39 | 0–39 |
FIGURE 12.
Box plots showing medians, IQRs and overall ranges of the RSI-HB score at baseline and 16-week follow-up (compliant ITT group). (a) Lansoprazole group (n = 102); and (b) placebo group (n = 118).
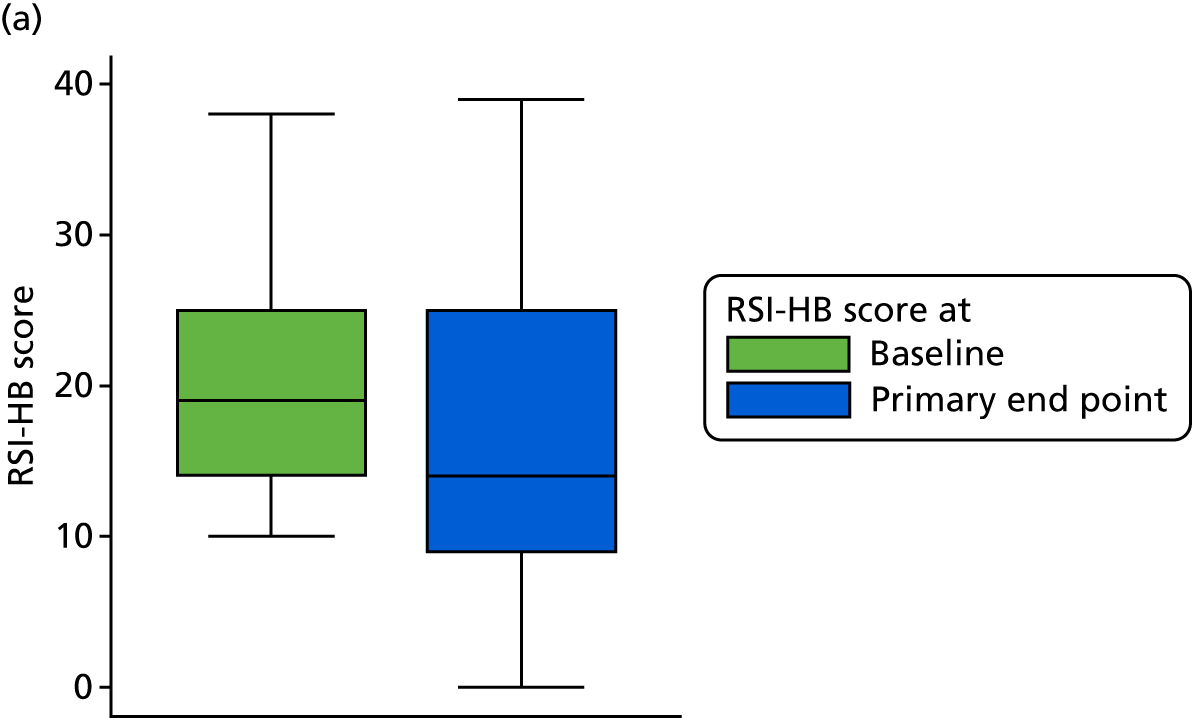
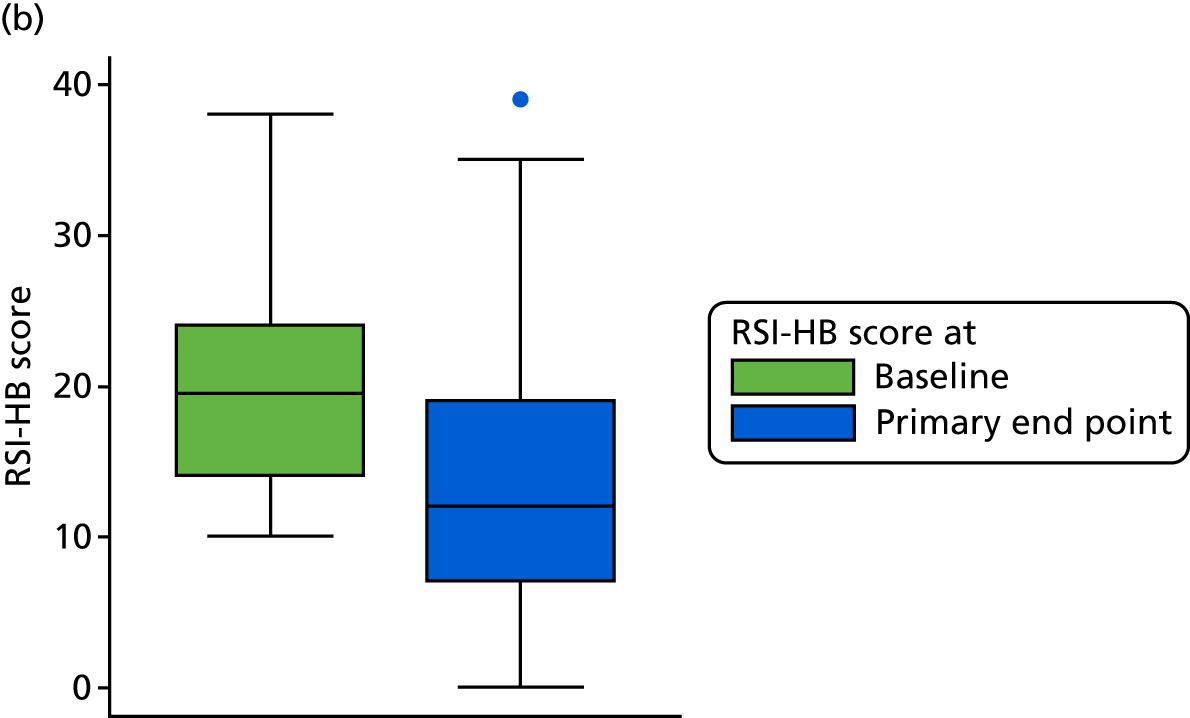
There is no statistically significant difference in the RSI-HB score at 16 weeks between the lansoprazole and placebo groups.
Change in Reflux Symptom Index minus the heartburn/dyspepsia item score from baseline to 16 weeks for the compliant intention-to-treat group
Table 15 and Figure 13 show the change in RSI-HB score (change RSI-HB = 16 weeks’ RSI-HB – baseline RSI-HB). The reduction in RSI-HB score from baseline to 16 weeks is similar between randomised groups.
| Change in RSI-HB scores (baseline to 16 weeks) | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Median (IQR) | –3 (–9 to 1) | –5.5 (–12 to 0) | –4.5 (–10 to 0.5) |
| Mean (SD) | –4.0 (7.6) | –5.9 (8.4) | –5.0 (8.1) |
| 95% CI of mean | –5.5 to –2.5 | –7.4 to –4.4 | –6.1 to –3.9 |
| Range | –31 to 17 | –27 to 12 | –31 to 17 |
FIGURE 13.
Box plots showing medians, IQRs and overall ranges of change in RSI-HB score from baseline to the 16-week follow-up (compliant ITT group). (a) Lansoprazole group (n = 102); and (b) placebo group (n = 118).
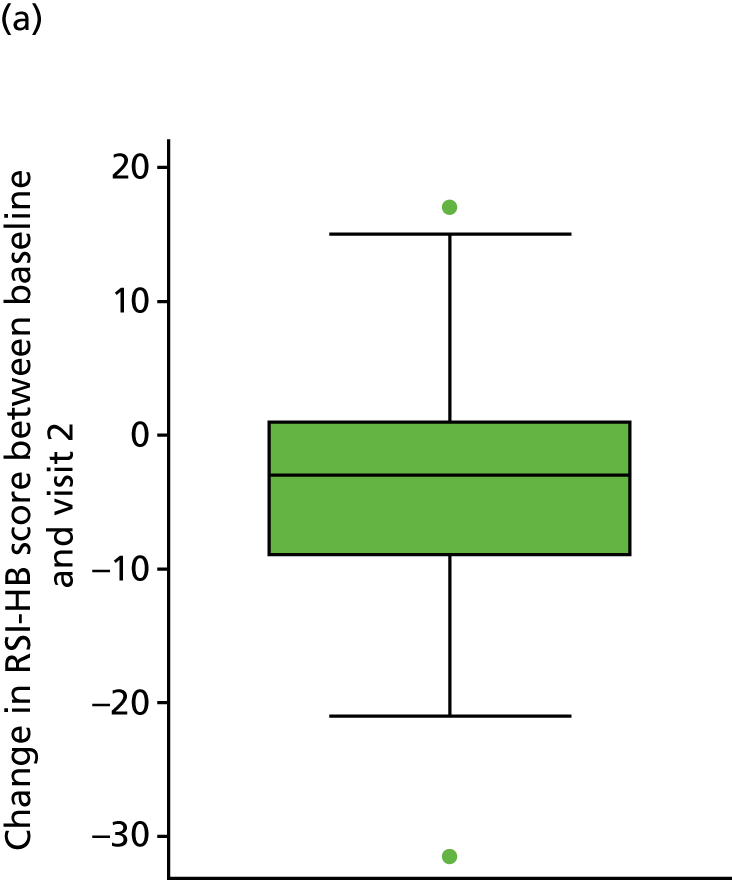
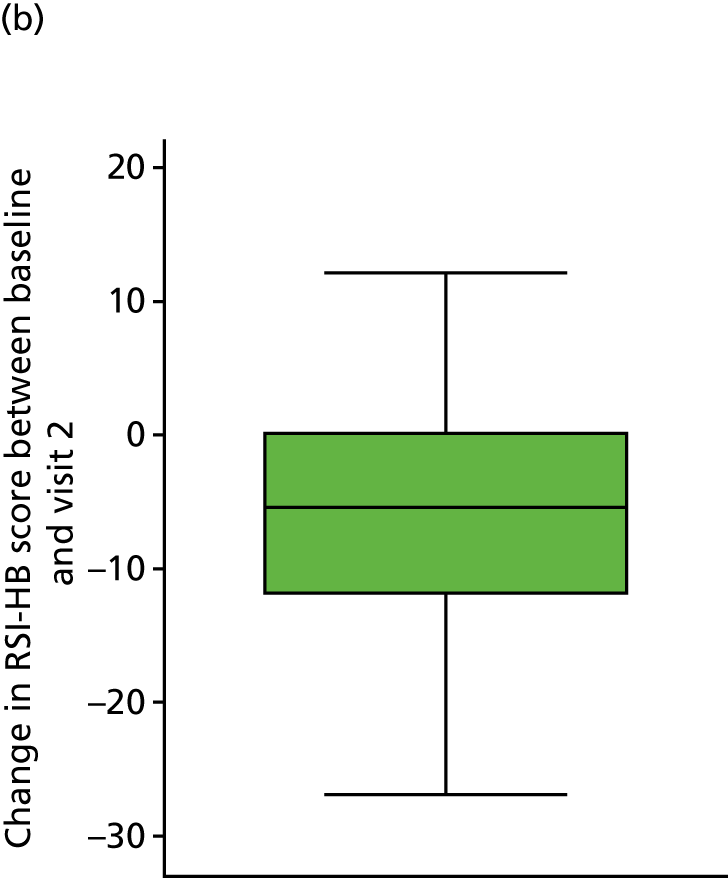
The overall mean reduction in RSI-HB score from baseline to 16 weeks is 5.0 (95% CI –6.1 to –3.9), a reduction that is observed across both randomised groups [lansoprazole, 4.0-point reduction (95% CI –5.5 to –2.5); placebo, 5.9-point reduction (95% CI –7.4 to –4.4)], with overlapping CIs indicating no statistical difference between the groups. The lansoprazole group had a mean reduction that is 1.9 points less than the mean reduction observed in the placebo group (see Figure 13).
Multivariate analysis and statistical modelling of the RSI-HB for the compliant group are given in Appendix 4. Appendix 4, Table 42, presents ANCOVA results, Table 43 presents the results of model 1 (adjusted for stratification factors) and Table 44 presents the results of model 2 (adjusted for stratification factor recruiting centre and for baseline severity in terms of RSI-HB as a continuous measure).
A sensitivity analysis based on the pragmatic ITT group including all patients with their 16-week primary end-point visit taking place at any time did not change the conclusions (see Appendix 6). Appendix 6, Table 51, shows summary statistics for RSI-HB for the pragmatic ITT group at baseline and the primary end point. In Appendix 7, Table 53, RSI-HB at baseline is confirmed as being statistically significantly related to 12-month RSI. Patients with greater severity at baseline are estimated to have RSI scores that are 8.2 points higher (worse) at 12 months.
Secondary outcome measures
Reflux Symptom Index at the 12-month follow-up for the compliant intention-to-treat group
The 12-month RSI scores were secondary outcome measures, both the complete nine-item RSI and the reduced eight-item RSI-HB. These 12-month scores were assessed descriptively in unadjusted models and in multivariable models adjusted by stratification factors (Table 16).
| RSI at 12 months | RSI 12-month follow-up (visit 3) | ||
|---|---|---|---|
| Treatment group | Total | ||
| Lansoprazole | Placebo | ||
| n | 82 | 99 | 181 |
| Median (IQR) | 15 (6–24) | 12 (6–19) | 13 (6–21) |
| Mean (SD) | 16.0 (10.8) | 13.6 (9.6) | 14.7 (10.2) |
| 95% CI of mean | 13.6 to 18.4 | 11.7 to 15.5 | 13.2 to 16.2 |
| Range | 0–43 | 0–41 | 0–43 |
The longitudinal changes in total RSI are summarised in Table 17 and Figure 14. Values show some further reduction in the post-treatment 8-month follow-up phase.
| RSI score | Time point | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | 12 months | Baseline to 16 weeks | Baseline to 12 months | ||||||
| Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | |
| n | 102 | 118 | 102 | 118 | 82 | 99 | 102 | 118 | 82 | 99 |
| Median (IQR) | 20.5 (15 to 28) | 21.5 (16 to 27) | 16 (9 to 26) | 14 (7 to 23) | 15 (6 to 24) | 12 (6 to 19) | –4 (–10 to 1) | –5 (–13 to 1) | –6 (–13 to 0) | –8 (–14 to –3) |
| Range | 10 to 41 | 10 to 43 | 0 to 41 | 0 to 44 | 0 to 43 | 0 to 41 | –31 to 19 | –29 to 11 | –34 to 15 | –27 to 22 |
FIGURE 14.
Box plots showing medians, IQRs and overall ranges of RSI score at baseline, 16 weeks and 12 months (compliant ITT group). (a) Lansoprazole group (n = 82); and (b) placebo group (n = 99).
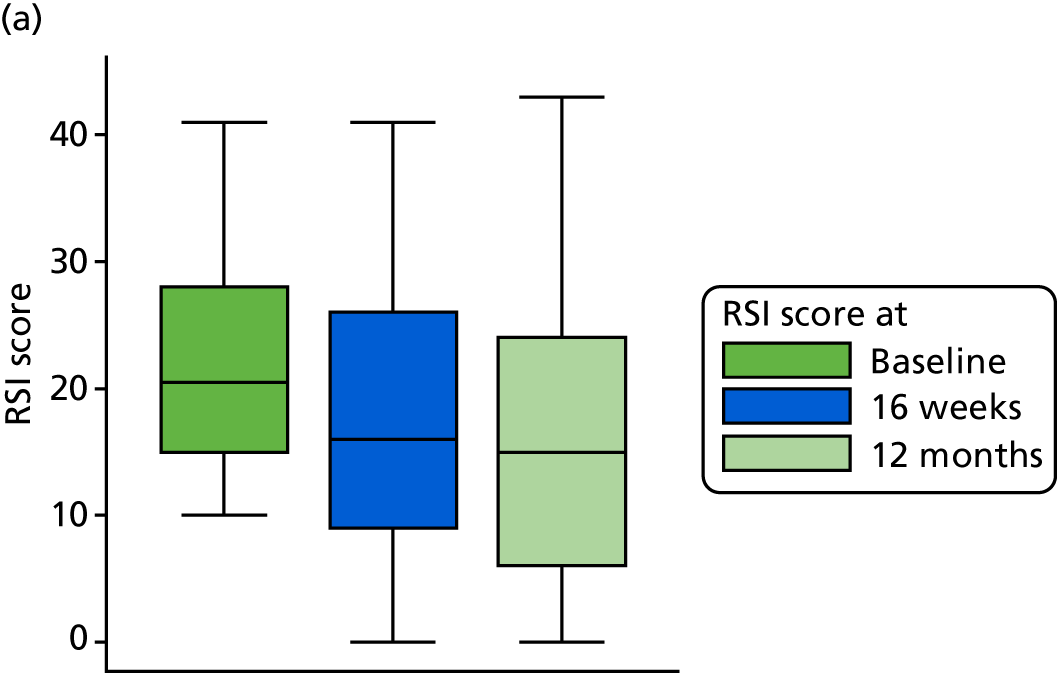
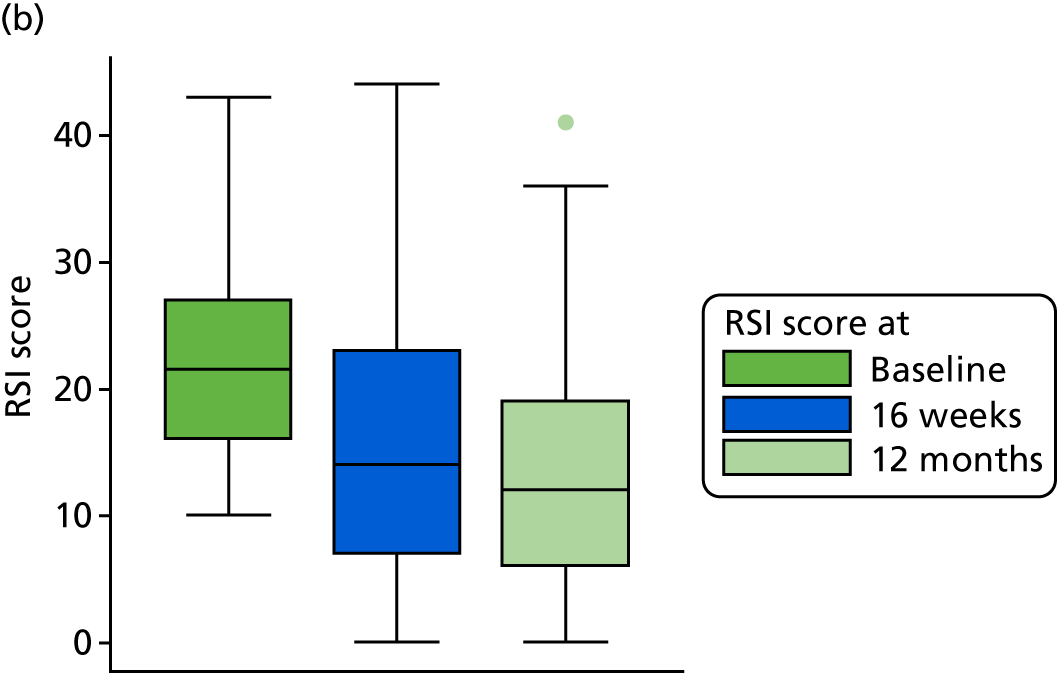
Mean RSI reduces in both groups at 16 weeks and further reduces at 12 months.
Univariate analysis RSI scores were reported at 12 months for the compliant ITT group, in which the null hypothesis is of no difference between means. The test statistic was t = 1.583 and the two-sided p-value was 0.115, concluding that there is no statistically significant difference in the RSI score at 12 months between the lansoprazole and placebo groups.
Reflux Symptom Index scores at the 12-month follow-up within the published normal range (< 12) for the compliant intention-to-treat group
Overall, 10% of patients were within the normal range at baseline, balanced across randomised groups (the inclusion criterion being the eight-item version of the RSI excluding the dyspepsia item 9). At 16 weeks, 43% (95% CI 37% to 50%) of participants are within the normal range. There is a slight increase at 12 months to 48% (95% CI 41% to 55%) of participants being within the normal range, balanced across randomised groups: lansoprazole 40% (95% CI 29% to 51%) versus placebo 55% (95% CI 45% to 65%). The overlapping CIs indicate no statistical difference between the groups.
Multivariable analysis of Reflux Symptom Index at 12 months for the compliant intention-to-treat group
Note that ANCOVA for RSI at 12 months (see Appendix 7) showed no statistically significant difference in RSI at 12 months between randomised treatment groups (p = 0.256), but site and baseline severity remain significantly related to 12-month RSI. Age and sex also appear to be significantly related to 12-month RSI when all are included in the ANCOVA analysis. The statistical modelling of the 12-month RSI for the compliant ITT group is also included in Appendix 7 and was conducted similarly to the prior modelling exercises.
The difference between randomised groups, when accounting for site and binary baseline severity, indicates that lansoprazole participants are estimated to have RSI scores at 12 months that are 2.5 points higher (worse) than those of placebo participants (95% CI –0.1 to 5.0; p = 0.06).
The underlying assumptions of the model were not substantially violated. As none of the potential covariates appears to have a significant univariate relationship with the 12-month RSI score, model 2 with continuous baseline severity (RSI-HB) and site remains the optimum. There was no statistically significant difference between the lansoprazole group and the placebo group when adjusted for site and baseline continuous RSI-HB. There is an estimated difference that lansoprazole participants have RSI scores at 12 months that are 1.7 points higher (worse) than those of placebo participants (95% CI –0.7 to 4.1 points; p = 0.157). Further information is given in Appendix 7, Table 54.
Reflux Finding Score for the compliant intention-to-treat group
The RFS was independently scored by a single expert rater (PC) who was blind to patient treatment group. Images were scored (Table 18) from 0 to 26 and assessed as a potential predictor of RSI at 16 weeks. There were a total of 256 RFSs for unique participants (see Appendix 3), of which 167 were for participants in the compliant ITT group.
| RFSs of images | Treatment group | Total (N = 220) | |
|---|---|---|---|
| Lansoprazole (N = 102) | Placebo (N = 118) | ||
| n (%) with RFSs | 82 (80) | 85 (72) | 167 (76) |
| Median (IQR) | 9 (7–12) | 9 (7–11) | 9 (7–12) |
| Mean (SD) | 9.7 (4.1) | 9.2 (3.8) | 9.4 (3.9) |
| 95% CI of mean | 8.8 to 10.6 | 8.4 to 10.0 | 8.8 to 10.0 |
| Range | 0–20 | 0–18 | 0–20 |
Reflux Finding Score at baseline for the compliant intention-to-treat group
The means, SDs, medians, IQRs and ranges are reported as the score is treated as a continuous measure but is integer in nature (see Table 18).
A higher RFS indicates more severe laryngeal inflammation. Overall, RFS at baseline is, on average, 9.4 points (95% CI 8.8 to 10.0 points). The summary statistics show the number and value of RFSs to be similar in both treatment groups at baseline. Usable RFS assessments were available for 76% of participants in the compliant ITT analysis group.
The utility of the Reflux Finding Score as a response predictor for the compliant intention-to-treat group
The use of both first-order fractional polynomial transformations and a fourth model of primary outcome baseline RFS failed to find any significant relationship of baseline RFS with the RSI score at the primary end point (see Appendix 8).
Comprehensive Reflux Symptom Score
Comprehensive Reflux Symptom Score total and subscale scores baseline descriptive analysis: compliant intention-to-treat analysis group
The CReSS at baseline was assessed as a potential predictor of RSI at 16 weeks (Table 19). A higher CReSS indicates worse symptoms. There was a 98% completion rate for CReSS at baseline in the compliant ITT analysis set. The score is treated as a continuous measure but is integer in nature. The summary statistics and box plots for CReSS total (see Appendix 9, Figure 17) and subscale (see Appendix 9, Figures 18–20) scores, and information on missing data, are included in Appendix 9. Missing observations were few and so multiple imputation was not used.
| Descriptive statistic | CReSS total (range: 0–170) | CReSS oesophageal subscale (range: 0–85) | CReSS upper airway subscale (range: 0–45) | CReSS pharyngeal subscale (range: 0–25) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Total (N = 220) | Treatment group | Total (N = 220) | Treatment group | Total (N = 220) | Treatment group | Total (N = 220) | |||||
| Lansoprazole (N = 102) | Placebo (N = 118) | Lansoprazole (N = 102) | Placebo (N = 118) | Lansoprazole (N = 102) | Placebo (N = 118) | Lansoprazole (N = 102) | Placebo (N = 118) | |||||
| n (% of compliant group) | 100 (98) | 115 (97) | 215 (98) | 101 (99) | 115 (97) | 216 (98) | 101 (99) | 116 (98) | 217 (99) | 102 (100) | 116 (98) | 218 (99) |
| Median (IQR) | 47.5 (27.5–69) | 50 (33–69) | 49 (30–69) | 20 (9–29) | 19 (11–27) | 19 (10–29) | 16 (10–24) | 17 (10–25.5) | 17 (10–25) | 9 (5–13) | 9.5 (4.5–14) | 9 (5–14) |
| Mean (SD) | 50.3 (27.4) | 51.1 (25.7) | 50.7 (26.4) | 20.7 (14.9) | 21.2 (14.1) | 21.0 (14.5) | 17.3 (9.9) | 17.9 (9.9) | 17.6 (9.9) | 9.4 (5.8) | 9.4 (5.6) | 9.4 (5.7) |
| 95% CI of mean | 44.9 to 55.7 | 46.4 to 55.8 | 47.2 to 54.3 | 17.8 to 23.7 | 18.6 to 23.8 | 19.0 to 22.9 | 15.4 to 19.3 | 16.1 to 19.7 | 16.3 to 19.0 | 8.2 to 10.5 | 8.4 to 10.4 | 8.6 to 10.2 |
| Range | 2–142 | 8–141 | 2–142 | 0–76 | 0–63 | 0–76 | 0–45 | 0–44 | 0–45 | 0–22 | 0–22 | 0–22 |
Appendix 9 shows the similarity of 16-week and 12-month outcomes in total and subscale CReSSs between those treated with lansoprazole and those treated with placebo (see Appendix 9, Tables 58–62).
Baseline itemised severity score: Comprehensive Reflux Symptom Score
The item descriptive statistics for CReSS are listed in Report Supplementary Material 1, Table 12, for the whole trial population and in Report Supplementary Material 1, Table 13, for the compliant group.
The top five CReSS items, for both the trial population and the compliant group, in ranked mean endorsement severity were:
-
throat clearing
-
feeling things stuck in throat
-
lump in throat
-
excess mucus
-
hoarseness.
The relationship between the RSI at baseline and total CReSS at baseline for the compliant ITT group is shown in Figure 15, which demonstrates a linear relationship (i.e. an increased CReSS is associated with increased RSI score). (It should be remembered that the RSI symptom items were all purposively included in the CReSS and so the two are not independent of each other.)
FIGURE 15.
Scatterplot of the relationship between the total CReSS and RSI at baseline (compliant ITT group, n = 215).

Comprehensive Reflux Symptom Score subscale scores as covariates for Reflux Symptom Index at 16 weeks
The reduction in AIC through simple log or complex (fractional polynomial) transformations was not substantial (see Appendix 9, Table 59). In order to build the most parsimonious, clinically interpretable model, the CReSS total and subscale scores were retained as untransformed continuous covariates, under the assumption of linearity with outcome. The optimum predictive model based on CReSS incorporated the upper airway subscale, but this performed less well than inclusion of RSI-HB in the predictive model.
Laryngopharyngeal Reflux – Health Related Quality of Life questionnaire
Patient quality of life was assessed using the LPR-HRQL. Higher scores indicate worse LPR quality of life. The total LPR-HRQL score and subscales at baseline and 16-week and 12-month follow-ups are presented for the compliant ITT population. Missing item rules dictate that three questionnaires with missing items can be imputed [two at baseline and one at the primary end point (16 weeks)].
Baseline Laryngopharyngeal Reflux – Health Related Quality of Life scores
Table 20 shows baseline scores (raw and standardised) for overall score and the voice, cough, clear throat and general subscales. Each remaining item not included in the subscales is reported separately.
| Scale and descriptive statistics | Scores | |||||
|---|---|---|---|---|---|---|
| Raw | Standardised | |||||
| Treatment group | Total | Treatment group | Total | |||
| Lansoprazole | Placebo | Lansoprazole | Placebo | |||
| Voice (raw range: 0–72) | ||||||
| Median (IQR) | 8 (6 to 22) | 7.5 (6 to 15.5) | 8 (6 to 19) | –0.46 (–0.59 to 0.45) | –0.40 (–0.52 to 0.19) | –0.44 (–0.59 to 0.38) |
| Mean (SD) | 15.1 (15.4) | 12.9 (13.4) | 13.9 (14.4) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 12.1 to 18.1 | 10.5 to 15.4 | 12.0 to 15.9 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 67 | 0 to 62 | 0 to 67 | –0.98 to 3.37 | –0.96 to 3.65 | –0.98 to 3.65 |
| Cough (raw range: 0–36) | ||||||
| Median (IQR) | 6 (1 to 15) | 5 (0 to 13) | 5 (1 to 13) | –0.35 (–0.87 to 0.59) | –0.32 (–0.88 to 0.56) | –0.34 (–0.87 to 0.56) |
| Mean (SD) | 9.3 (9.5) | 7.9 (9.0) | 8.6 (9.3) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 7.5 to 11.2 | 6.2 to 9.6 | 7.3 to 9.8 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 34 | 0 to 35 | 0 to 35 | –0.98 to 2.58 | –0.88 to 3.00 | –0.98 to 3.00 |
| Clear (raw range: 0–36) | ||||||
| Median (IQR) | 8 (3 to 14) | 7 (2.5 to 13.5) | 8 (3 to 14) | –0.16 (–0.81 to 0.63) | –0.23 (–0.83 to 0.65) | –0.16 (–0.81 to 0.63) |
| Mean (SD) | 9.2 (7.6) | 8.7 (7.4) | 8.9 (7.5) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 7.7 to 10.7 | 7.3 to 10.0 | 7.9 to 9.9 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 35 | 0 to 33 | 0 to 35 | –1.21 to 3.39 | –1.17 to 3.27 | –1.21 to 3.39 |
| General (raw range: 0–30) | ||||||
| Median (IQR) | 5.5 (2 to 10) | 5 (1 to 11) | 5 (1 to 11) | –0.22 (–0.77 to 0.48) | –0.26 (–0.91 to 0.71) | –0.26 (–0.91 to 0.64) |
| Mean (SD) | 6.9 (6.4) | 6.6 (6.2) | 6.8 (6.3) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 5.7 to 8.2 | 5.5 to 7.8 | 5.9 to 7.6 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 27 | 0 to 26 | 0 to 27 | –1.08 to 3.14 | –1.08 to 3.14 | –1.08 to 3.14 |
| Overall (raw rescaled range: 0–100) | ||||||
| Median (IQR) | 24 (10 to 45) | 22 (8 to 41) | 23 (9 to 42) | –0.23 (–0.84 to 0.74) | –0.20 (–0.86 to 0.68) | –0.20 (–0.84 to 0.70) |
| Mean (SD) | 28.9 (22.2) | 26.5 (21.7) | 27.6 (21.9) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 24.5 to 33.3 | 22.5 to 30.5 | 24.7 to 30.6 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 95 | 0 to 87 | 0 to 95 | –1.30 to 2.99 | –1.22 to 2.80 | –1.30 to 2.99 |
Higher scores indicate worse LPR quality of life.
Raw scores appear skewed. Each subscale is reported as a standardised ‘z-score’ calculated by subtracting the means and dividing by SD, which appear less skewed. Each subscale section also has an extra question labelled ‘the thermometer’, reported individually and with the appropriate subscale (see Appendix 10, Tables 62 and 63).
Laryngopharyngeal Reflux – Health Related Quality of Life at primary end point (16-week follow-up)
The results are shown in Table 21.
| Scale and descriptive statistics | Scores | |||||
|---|---|---|---|---|---|---|
| Raw | Standardised | |||||
| Treatment group | Total | Treatment group | Total | |||
| Lansoprazole | Placebo | Lansoprazole | Placebo | |||
| Voice (raw range: 0–72) | ||||||
| Median (IQR) | 7 (6 to 18) | 6 (6 to 14) | 6.3 (6 to 15) | –0.47 (–0.54 to 0.35) | –0.39 (–0.39 to 0.32) | –0.39 (–0.54 to 0.32) |
| Mean (SD) | 13.3 (13.4) | 10.4 (11.2) | 11.7 (12.4) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 10.6 to 16.0 | 8.3 to 12.4 | 10.1 to 13.4 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 59 | 0 to 67 | 0 to 67 | –0.99 to 3.40 | –0.92 to 5.04 | –0.99 to 5.04 |
| Cough (raw range: 0–36) | ||||||
| Median (IQR) | 4 (0 to 12) | 2 (0 to 7) | 2 (0 to 10) | –0.39 (–0.85 to 0.54) | –0.41 (–0.69 to 0.28) | –0.41 (–0.69 to 0.42) |
| Mean (SD) | 7.4 (8.6) | 5.0 (7.2) | 6.1 (8.0) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 5.7 to 9.1 | 3.6 to 6.3 | 5.0 to 7.1 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 31 | 0 to 34 | 0 to 34 | –0.85 to 2.74) | –0.69 to 4.04 | –0.85 to 4.04 |
| Clear (raw range: 0–36) | ||||||
| Median (IQR) | 5 (1 to 11) | 3 (0 to 10) | 4 (0 to 11) | –0.31 (–0.84 to 0.49) | –0.36 (–0.80 to 0.66) | –0.31 (–0.80 to 0.52) |
| Mean (SD) | 7.3 (7.6) | 5.5 (6.8) | 6.3 (7.2) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 5.8 to 8.8 | 4.2 to 6.7 | 5.4 to 7.3 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 30 | 0 to 34 | 0 to 34 | –0.97 to 3.00 | –0.80 to 4.18 | –0.97 to 4.18 |
| General (raw range: 0–30) | ||||||
| Median (IQR) | 2.5 (0 to 6) | 2 (0 to 7) | 2 (0 to 7) | –0.35 (–0.75 to 0.21) | –0.45 (–0.80 to 0.41) | –0.45 (–0.75 to 0.37) |
| Mean (SD) | 4.7 (6.2) | 4.6 (5.8) | 4.6 (6.0) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 3.5 to 5.9 | 3.6 to 5.7 | 3.8 to 5.4 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 29 | 0 to 29 | 0 to 29 | –0.75 to 3.92 | –0.80 to 4.23 | –0.80 to 4.23 |
| Overall (raw rescaled range: 0–100) | ||||||
| Median (IQR) | 11 (3 to 33) | 10 (2 to 25) | 10 (2 to 27) | –0.42 (–0.79 to 0.57) | –0.36 (–0.71 to 0.36) | –0.42 (–0.74 to 0.47) |
| Mean (SD) | 20.5 (22.4) | 17.1 (20.9) | 18.7 (21.6) | 0 (1) | 0 (1) | 0 (1) |
| 95% CI | 16.1 to 25.0 | 13.3 to 21.0 | 15.8 to 21.6 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 |
| Range | 0 to 83 | 0 to 96 | 0 to 96 | –0.92 to 2.80 | –0.82 to 3.77 | –0.92 to 3.77 |
The raw scores again appear skewed, and the standardised scores less so. The 16-week scores (see Table 21) show a marked reduction from baseline across all domains and across both treatment groups. The equivalent thermometers are given in Appendix 10, Table 65.
The median overall LPR-HRQL scale at the 16-week primary end point reduced (improved) by around half, with almost identical reductions in the two treatment groups.
Laryngopharyngeal Reflux – Health Related Quality of Life at 12-month follow-up (visit 3)
Corresponding data provided at 12-month follow-up are shown in Appendix 10, Table 64, with thermometer scores in Appendix 10, Table 65.
Plots of laryngopharyngeal reflux health-related quality of life
Plots of LPR-HRQL (subscales and corresponding thermometer scores) are shown in Report Supplementary Material 1, Figures 1–8. Report Supplementary Material 1, Figures 9–18, shows the LPR-HRQL domain score graphs at baseline, 16 weeks and 12 months.
Overall subscale values are shown in Figure 16.
FIGURE 16.
The LPR-HRQL overall subscale scores aggregated at visits, by treatment group. (a) Lansoprazole group; and (b) placebo group.
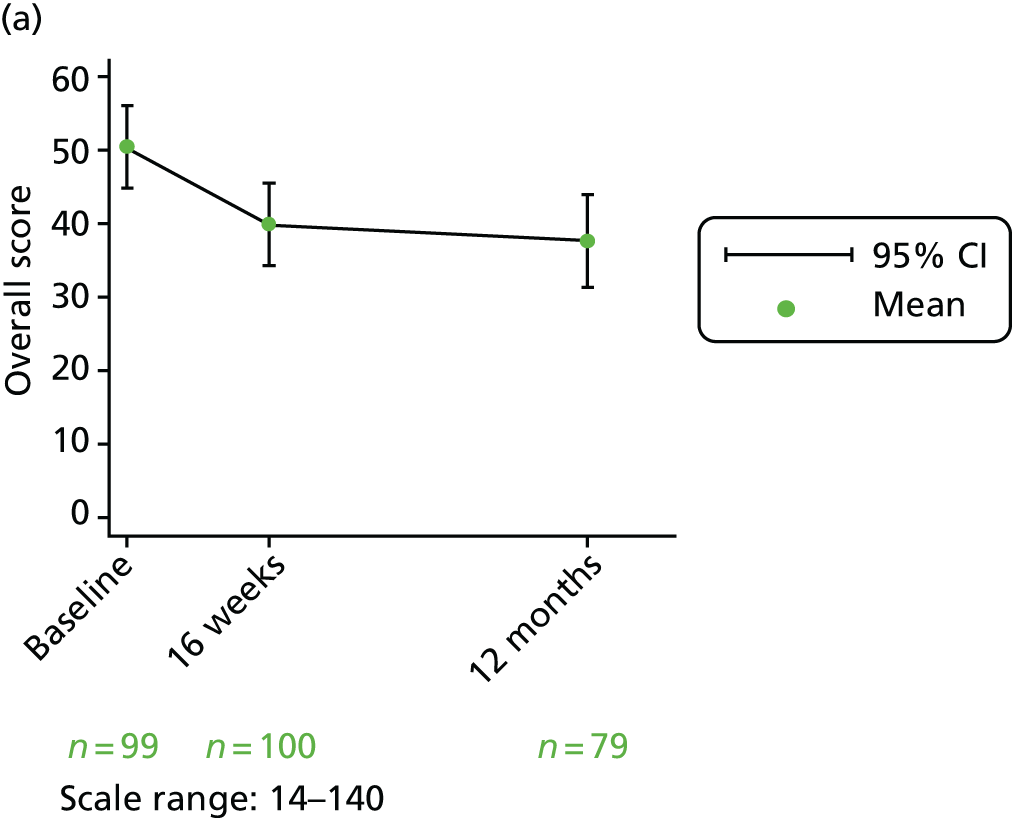
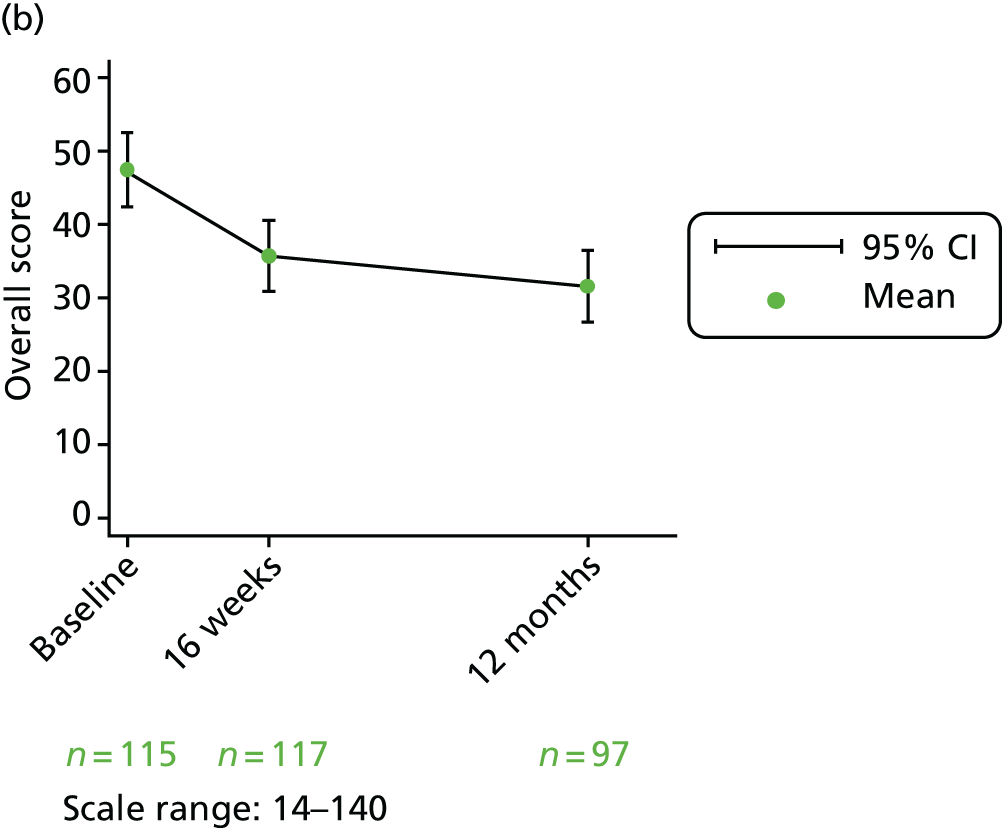
The overall score is calculated by adding the thermometer scores [voice (question 13), coughing (question 20), clear throat (question 27) and general (question 33)] and the remaining domain scores (questions 34–43).
The mean voice, coughing, clear throat, general subscale scores and overall scores were relatively low at baseline and had reduced by 16 weeks in both groups, with further reduction at the 12-month visits. The same patterns are also reflected in the median thermometers, when a score of 3 or 4 indicates a small effect and scores of 2 and 1 indicate minimal to no effect on quality of life.
Each of the remaining domain scores used to calculate the overall score is also presented individually at baseline and 16-week and 12-month follow-up visits in Report Supplementary Material 1, Figures 9–18.
There is only a small effect on overall quality of life relating to individual domain scores.
The IQRs of the domain scores generally decrease from baseline levels by 16 weeks in both treatment groups.
None of the baseline median LPR-HRQL domain scores reflects a large impact of symptoms on the individual domains and the small reductions (improvements) by 16 weeks are similar across treatment groups.
Standardised area under the curve for overall Laryngopharyngeal Reflux – Health Related Quality of Life
A standardised area under the curve (SAUC) is calculated for each patient, defined as area under the curve as a proportion of time observed, and reported descriptively. A total of 213 out of the 220 participants in the compliant ITT group are included in SAUC analysis (for lansoprazole, 75 participants have LPR-HRQL scores at all three time points, 22 participants have LPR-HRQL scores at visits 1 and 2 and one participant has LPR-HRQL scores at visits 1 and 3; for placebo, 94 participants have LPR-HRQL scores at all three time points, 20 participants have LPR-HRQL scores at visits 1 and 2 and one participant has LPR-HRQL scores at visits 1 and 3). The distribution is skewed.
Median SAUC over 12 months is 32.6 (IQR 22.5–53.1) for lansoprazole and 29.9 (IQR 19.9–50.4) for placebo. In other words, patients reported approximately 30% of a maximum 100 LPR-HRQL score, similar across groups over 12 months’ follow-up.
Repeated-measures mixed model
A repeated-measures mixed-effects multilevel model accounts for longitudinal data within patients over time (Table 22).
| Model | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | 2.903 | 3.656 | 0.79 | 0.427 | –4.262 to 10.069 |
| Time | |||||
| 16 weeks | –11.719 | 2.069 | –5.66 | < 0.001 | –15.775 to –7.664 |
| 12 months | –14.826 | 2.209 | –6.71 | < 0.001 | –19.155 to –10.497 |
| Interaction: group*time | |||||
| Lansoprazole*16 weeks | 1.302 | 3.048 | 0.43 | 0.669 | –4.673 to 7.276 |
| Lansoprazole*12 months | 2.111 | 3.290 | 0.64 | 0.521 | –4.338 to 8.560 |
| Constant | 47.209 | 2.489 | 18.97 | < 0.001 | 42.331 to 52.087 |
There is a significant decrease (improvement in quality of life) from baseline to the 16-week and 12-month follow-up visits. The lansoprazole group had an overall LPR-HRQL score that was, on average, 2.9 points higher (worse) than that for the placebo group (95% CI –4.3 to 10.1; p = 0.427), increasing from 16 weeks to 12 months.
Summary of Reflux Symptom Index, total Comprehensive Reflux Symptom Score and Laryngopharyngeal Reflux – Health Related Quality of Life scores for the compliant group at all three time points
Table 23 provides a summary of total RSI, CReSS and LPR-HRQL scores at three time points.
| Questionnaire | Scores, mean (95% CI) | ||
|---|---|---|---|
| Treatment group | Total (N = 220) | ||
| Lansoprazole (n = 102) | Placebo (n = 118) | ||
| Baseline | |||
| RSI | 22.0 (20.4 to 23.6) | 21.7 (20.5 to 23.0) | 21.9 (20.9 to 22.9) |
| CReSS total | 50.3 (44.9 to 55.7) | 51.1 (46.4 to 55.8) | 50.7 (47.2 to 54.3) |
| LPR-HRQL | 28.9 (24.5 to 33.3) | 26.5 (22.5 to 30.5) | 27.6 (24.7 to 30.6) |
| 16 weeks | |||
| RSI | 17.4 (15.5 to 19.4) | 15.6 (13.8 to 17.3) | 16.4 (15.1 to 17.7) |
| CReSS total | 38.9 (33.4 to 44.3) | 34.7 (29.6 to 39.9) | 36.6 (32.9 to 40.4) |
| LPR-HRQL | 20.5 (16.1 to 25.0) | 17.1 (13.3 to 21.0) | 18.7 (15.8 to 21.6) |
| 12 months | |||
| RSI | 16.0 (13.6 to 18.4) | 13.6 (11.7 to 15.5) | 14.7 (13.2 to 16.2) |
| CReSS total | 36.6 (29.8 to 43.5) | 31.8 (26.6 to 36.9) | 33.9 (29.8 to 38.1) |
| LPR-HRQL | 18.8 (13.7 to 23.8) | 13.9 (10.0 to 17.8) | 16.0 (13.0 to 19.2) |
Patient satisfaction with TOPPITS
At the 12-month follow-up (visit 3), 213 out of 346 (62%) participants answered the satisfaction question, of whom 115 (54%) were very satisfied, 59 (28%) were satisfied, 29 (14%) were neither satisfied nor dissatisfied, five (2%) were dissatisfied and five (2%) were very dissatisfied.
Safety
Information was gathered on AEs at all three TOPPITS clinic visits. We recorded any untoward medical occurrence in a patient to whom a medicinal product had been administered, including occurrences that were not necessarily caused by or related to that product. In the protocol,1 it was stated that causality would be graded on a five-point scale [unrelated, unlikely, possible, probable and definitely related to the investigational medicinal product (IMP)]. MACRO was set up for data collection on a three-point scale (not related, possibly related and probably related). A file note was added to the trial master file detailing this change from the protocol.
Adverse event causality was reported on the three-point scale available from MACRO. The numbers of patients reporting any AE are reported, clarifying those deemed to be treatment related. SAEs and occurrence of suspected unexpected serious adverse reactions are also recorded by group. The clinical side effects of PPI use include gastrointestinal disturbance and rebound hypersecretion after cessation of PPI therapy. Rarer side effects include pneumonia, C. difficile infections, acute renal inflammation and fractures of the hip, wrist and spine.
Adverse events for the per-treatment analysis group
There were 112 reported AEs in the per-treatment group (Tables 24 and 25) in 74 unique patients.
| Status | AEs, n (%) | ||
|---|---|---|---|
| Treatment group | Total (N = 112) | ||
| Lansoprazole (N = 60) | Placebo (N = 52) | ||
| Patient taking treatment? Yes | 42 (70) | 38 (73) | 80 (71) |
| Patient taking treatment when AE occurred? No | 15 (25) | 14 (27) | 29 (26) |
| AE dates missing | 3 (5) | 0 (0) | 3 (3) |
| Related to treatment? | AEs, n (%) | |||||
|---|---|---|---|---|---|---|
| Treatment group | Total | |||||
| Lansoprazole | Placebo | |||||
| During treatment | After treatment | During treatment | After treatment | During treatment | After treatment | |
| Probably | ||||||
| Severe | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Moderate | 5 (12) | 0 (0) | 0 (0) | 0 (0) | 5 (6) | 0 (0) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Possibly | ||||||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderate | 5 (12) | 0 (0) | 3 (8) | 0 (0) | 8 (10) | 0 (0) |
| Mild | 7 (17) | 0 (0) | 3 (8) | 0 (0) | 10 (13) | 0 (0) |
| Not related | ||||||
| Severe | 1 (2) | 0 (0) | 2 (5) | 0 (0) | 3 (4) | 0 (0) |
| Moderate | 11 (26) | 4 (27) | 10 (26) | 2 (14) | 21 (26) | 6 (21) |
| 1 (AE dates missing) | 1 (AE dates missing) | |||||
| Mild | 12 (29) | 10 (67) | 20 (53) | 12 (86) | 32 (40) | 22 (76) |
| 2 (AE dates missing) | 2 (AE dates missing) | |||||
| Not reported | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Total | 42a (100) | 15a (100) | 38 (100) | 14 (100) | 80a (100) | 29a (100) |
Further information from the database manager indicates that two of the three AEs with missing dates pre-date the start of the trial. One was marked ‘not available’ by the site in MACRO. More details are provided in Report Supplementary Material 1.
A check was carried out to see if any patients who attended the primary end-point visit but did not provide information on returned medication reported any AEs. These could still be attributed to the trial medication, but would not be included in the per-treatment group as no assumption on medication taken was made. There were two such events: one unrelated (dental abscess) and one possibly related to the medication (details: Patient 6009, randomised to lansoprazole, reported a mild AE – ‘confusion, weakness on left leg, chest pain after swallowing the trial drug’).
Report Supplementary Material 1 provides a line listing of the breakdown of AEs by treatment group, with categorisation in terms of relationship to medication, severity and whether or not the participants were taking the medication at the time when the AE occurred. Most of the AEs and adverse reactions were expected treatment-related toxicities.
Serious adverse events for the per-treatment analysis group
There were two SAEs, both unrelated to the trial medication. One was unintentional bleach ingestion on an overseas holiday due to an error made by a hotel staff member (PPI group) and the other was an exacerbation of pre-existing asthma (placebo group).
Chapter 4 Discussion
Terminology
Persistent throat symptoms include chronic throat clearing, hoarse voice (dysphonia), sensation of a lump in the throat (globus) and increased mucus sensation (catarrh). The symptoms most commonly experienced by the TOPPITS participants were, in order of frequency, as recorded by the RSI severity endorsement [see Report Supplementary Material 1, Tables 16 (whole population) and 17 (compliant group)]:
-
lump in the throat
-
throat clearing
-
excess mucus
-
troublesome cough
-
hoarseness.
Similar frequencies were, not surprisingly, seen with CReSS:
-
throat clearing
-
feeling things stuck in throat
-
lump in throat
-
excess mucus
-
hoarseness.
Laryngopharyngeal reflux was promoted as an umbrella term in the mid-1990s and has since grown in popularity despite ongoing debate regarding pathophysiology, diagnosis and treatment. We aimed to quantify shifts in medical terminology for throat symptoms over time. Search terms included ‘throat clearing’, ‘reflux laryngitis’, ‘acid laryngitis’, ‘globus pharyngeus’, ‘globus sensation’, ‘globus hystericus’, ‘catarrh’ or ‘laryngopharyngeal reflux’ in the title or abstract. The total number of publications per decade was derived along with subtotals for clinical research and reviews. Catarrh (n = 473) and the various terms for globus (n = 468) featured prominently for the decades 1960s to 1980s, with fewer instances of acid/reflux laryngitis (n = 96). The 209 references to throat clearing date from 1980 (numerically the commonest symptom in most detailed surveys). There has been an exponential rise in LPR from the 1990s (n = 810), although fewer than one in five reports were classed as a ‘clinical study’. The predilection for LPR is no doubt supported by the fact that > 50% of the US general population has heartburn. 56 The use of anticholinergic medications, especially in combination, increases the odds of experiencing globus by a factor of 3.52. 57 An investigation of the frequency of throat clearing and cough and how often each is associated with a positive symptom index for reflux found a low probability of objective association. This is even less likely if both symptoms are present or if patients report an ‘excessive’ cough. 58
The premise for TOPPITS was that without a robust UK trial we saw nothing to question the increasing popularity of acid suppression medications as a prime therapy for upper respiratory symptoms. If all putative LPR patients referred to otolaryngologists were treated with PPIs, the costs are estimated at £4M per annum. Although the link between LPR and upper respiratory symptoms is little more than speculative or intuitive when close enquiry of the evidence base is made, the level of acceptance in primary care is also increasing. Many patients seen by non-TOPPITS otolaryngologists at participating centres might have considered TOPPITS had they not already had substantial trials of PPIs in primary care and thus never even reached the screening clinic.
There is no agreed, standard, core patient self-report outcome even for lower oesophageal reflux; the only COMET website reference to reflux is to a workshop that concluded that members strongly supported the development of validated patient-reported outcome instruments. This lack of a pre-eminent outcome tool led us to develop the CReSS. 59 The CReSS covers all oesophageal and extraesophageal reflux symptoms by incorporating all items from the original Gastrointestinal Symptom Assessment Score,60 together with all components of the RSI. CReSS totals are independent of age, and all items were endorsed by ≥ 17% of throat symptom patients. 61
The inexorable rise in popularity of LPR as the default term for persistent throat symptoms appears to have fuelled huge popularity for PPI therapy despite a lack of evidence. Attempts to reverse this trend, in view of recent adverse PPI publicity, will be supported by the overall negative finding of the TOPPITS data. Globus pharyngeus was described as a ‘tainted term’ in a recent opinion article due to its connotation of ‘globus hystericus’. 62 The authors propose that a feeling of having a lump in the throat on dry swallow is a normal sensation, accentuated by circumstances. Their proposed contemporary term is ‘troublesome Throat Awareness’ (tTA). However, this idiosyncratic acronym, although functionally useful, will not readily supplant the classical term.
Recent reports on epidemiology of persistent throat symptoms
The 1982 ENT outpatient globus prevalence figure of 4.2%3 has fallen little in the ensuing decades, being 3.8% in a recent report. 63 Despite its name, there is little evidence linking the symptom items of the RSI with any demonstrable oesophagopharyngeal reflux. Indeed, the very first attempt – almost 30 years ago – to treat a similar list of symptoms with H2 antagonists yielded a negative result. 19 The RSI has nonetheless been used to assess ‘LPR’ in a number of recent studies. A population prevalence study approached 2000 UK-dwelling adults, who were sent the RSI and questions on their health and lifestyle. The mean RSI was 8.3; 30% had a RSI score of > 10, of whom 75% had symptoms of GORD. Patients with depression and irritable bowel syndrome were more likely to have higher RSI scores. In other words, in this population survey, correlates of the RSI questionnaire score appear to be gastroesophageal reflux, depression and irritable bowel syndrome. 30 In the general Greek population, the prevalence of ‘LPR’ was 18.8%, similar in both sexes (p > 0.05), with a peak age of 50–64 years and with a positive association with tobacco smoking and alcohol consumption. 64 A total of 3006 individuals surveyed in Guangdong Province, China,65 completed questionnaires about their physical and psychological characteristics and globus symptomatology, and our own, physician-generated Glasgow Edinburgh Throat Scale66 with questionnaires on quality of sleep and life. In this survey, those with no history of GORD, dysphagia, odynophagia or alerting symptoms, such as weight loss and hoarseness, were diagnosed as having globus. The overall lifetime prevalence of globus so defined was 21.5%, with a peak age at disease onset of 35–54 years, commoner in urban dwellers and with no sex bias. Anxiety (40%), depression (31%) and sleep disorders (24%) in respondents with globus were significantly commoner than in those without globus. 65 A more informal enquiry made by our group many years ago in a high street survey of UK shoppers indicated a (within the last 3 months) female population prevalence of globus in 6% of those questioned. 2
Perception of reflux causation of persistent throat symptoms in general practice
The risk of overattribution of throat symptoms to so-called LPR has been recognised in the specialist ENT literature for some time. A retrospective chart review analysis identified 105 patients in a private laryngology clinic setting with a previous diagnosis of LPR as the cause of hoarseness. After anti-reflux therapy, 82% had no improvement or felt worse, leading the authors to conclude that LPR was overdiagnosed, especially when no other explanation was readily apparent. 67 A similar reliance on a putative LPR diagnosis in the absence of clear evidence has filtered into the wider, non-ENT, medical community. A US web-based survey of 314 (13%) doctors (46% family practitioners) showed that 64% preferred to treat rather than immediately refer a patient with chronic (> 6 weeks’) unexplained hoarseness, most often with anti-reflux medication (86%), even in the absence of GORD symptoms and antihistamines (54%). 68 Pre-treatment appears to significantly delay diagnosis. A retrospective case review of 755 patients referred to a voice service in Kansas City69 reported that 244 patients (32%) received a diagnosis before attending, this being LPR in over half, and, of those, > 65% had no GORD symptoms. Over 80% of diagnoses changed following attendance at the voice clinic and so those with prior treatment had a median duration of symptoms that was 6 weeks longer than those without (p = 0.04). 69
Similar trends seem to prevail in the UK. Although only 27% of TOPPITS recruits had taken PPIs in the preceding 12 months (see Appendix 2, Table 36), many others who had previously had this treatment were understandably reluctant either to discontinue it simply to enter a RCT of the same medication or to return to it when it had previously failed, and so the figure of 27% under-represents the general laryngology referral population usage of PPIs.
Misuse of PPIs is also documented internationally in a wider context. Only 34% of Swiss GPs are reported to attempt dose reduction in long-term users. 70 An Italian primary care study found that almost 15% of a sample of over 6300 patients were taking PPIs – over 30% potentially inappropriately. 71 A Canadian study of over 870 patients presenting to the emergency department of a teaching hospital also found PPI prescription to be inappropriate in > 30% of participants. 72 An observational study of the patients admitted to an acute hospital in Ireland in February 201873 identified that almost half of the sample of 1764 patients were regular PPI users. Almost 55% had no documented indication. Polypharmacy in the elderly gave particular cause for concern. 73 Pre-admission and discharge prescriptions were reviewed in a tertiary teaching hospital in Singapore to determine continuation of pre-admission and new PPI prescriptions at discharge. 74 Of 150 patients, 53% received prescriptions for PPIs, of whom > 80% had no valid reason. 74
What does TOPPITS tell us about the impact of proton pump inhibitor treatment on throat symptoms?
The key messages from the trial are:
-
There was no statistically significant difference between randomised groups, lansoprazole compared with placebo, when adjusted for site and baseline binary RSI-HB. This difference indicates that lansoprazole patients are estimated to have RSI scores at 16 weeks that are 1.9 points higher (worse) than placebo patients (95% CI –0.3 to 4.2; p = 0.096) (see Table 9).
-
There is no statistically significant difference in the mean RSI score at 16 weeks between lansoprazole (17.4, 95% CI 15.5 to 19.4) and placebo (15.6, 95% CI 13.8 to 17.3) (p = 0.162). (See the t-test results in Chapter 3, Univariate analysis of unadjusted primary outcome measure for the compliant analysis group.)
-
At 16 weeks, 43% of participants had RSI scores within the normal range, with no significant difference between the lansoprazole (41%) and placebo (45%) groups (see Report Supplementary Material 1, Table 15).
-
Patient demographics39,75 were explored as potential baseline determinants of treatment response but age, sex, smoking status, alcohol consumption and BMI did not improve the RSI 16-week prediction (model 3; see Table 11).
-
At 12 months, there is a further minor drop in RSI scores (see Table 17 and box plots in Figure 14).
-
Baseline RFS does not predict PPI response (see Appendix 8, Table 56).
TOPPITS is a considerably larger trial than the majority of series included in recent meta-analyses. Pre TOPPITS, a trial in 146 selected patients, all with signs of posterior laryngitis,22 showed no symptom benefit from PPI therapy over placebo, but did not report RSI scores. Lee et al. 76 noted less marked PPI responses in older patients (to a lower-dose PPI regimen than was used in TOPPITS). However, this may have reflected the fact that the oldest cohort had the highest baseline RSI levels and/or that the authors used a dichotomous definition of response (i.e. a 50% drop in RSI) rather than a continuous variable assessment. Baseline severity was a major determinant of end-point severity in TOPPITS.
Awareness of treatment group by patients
We were interested to explore patient perception of their treatment group as there was a concern that those with more severe heartburn might recognise a prompt heartburn response as implying that they were in the PPI group. However, the awareness (true-positive opinion) of group was broadly equal in the cohorts (see Table 4). Of the PPI cohort, 42% correctly called their allocation, compared with 56% of placebo participants.
The recent TOPPITS context
The premise for TOPPITS was that, without a robust UK trial, we saw nothing to stop the increasing ‘belief’ in LPR as a prime cause of upper respiratory symptoms, as the theory was supported by prominent laryngological enthusiasts, some respiratory medicine experts and, of course, the pharmaceutical industry. If all putative LPR patients referred to otolaryngologists were treated with PPI, we would calculate the costs to be approximately £4M per annum. Although the link between LPR and upper respiratory symptoms is little more than speculative or intuitive when close enquiry of the evidence base is made, the level of acceptance in primary care is also increasing. Many patients seen by non-TOPPITS otolaryngologists at participating centres might have considered TOPPITS had they not already had substantial trials of PPI in primary care and thus never even reached the screening clinic.
A 2016 review52 of the evidence linking reflux and voice changes concluded that the association lacked supporting data from clinical trials. The authors describe using the Bradford Hill criteria to support their conclusion that ‘the evidence toward causality between reflux and voice is insufficient’. 52 Tantalisingly, however, the authors conclude by saying that a relationship ‘does exist which deserves careful consideration’ – despite having concluded that:
To date, neither clinical trials nor comparative observational studies have been able to demonstrate a strong dose response between reflux and voice disorders, temporality (reflux precedes dysphonia), consistent treatment effects or strength of association between anti-reflux treatment and improved voice among patients with presumed LPR.
Schneider et al. 52
Three meta-analyses have been reported since TOPPITS was designed. A 2014 meta-analysis of eight papers comprising 370 patients found no statistical difference in effect between PPIs and placebo. 77 Separate assessment of the impact on cough was also not statistically significant. 77 This result was in disagreement with two meta-analyses published in 2016. 78,79 Both of these reports include a subset of studies with RSI as primary outcome. Wei78 examined pooled relative risks for the response rate and standardised mean differences (SMDs) for RSI and RFS. Of 13 RCTs, five reported RSI (total n = 277, ranging from only 37 to 8215 participants per study). Pooled results are reported to demonstrate that the total RSI improved significantly more for PPI therapy than for placebo (SMD 3.65, 95% CI 1.56 to 5.75), although no significant difference was found in response rate (0.04, 95% CI –0.06 to 0.14) or RFS (SMD 0.91, 95% CI –0.53 to 2.35). Scrutiny of the tabulated and forest plot findings, however, indicates that, of the five trials in the RSI analysis, three favour PPI, but with highly significant heterogeneity (i.e. variability in results across studies), questioning the pooling of the data in the first place. The smallest mean difference in RSI was 0.26. The greatest SMD total RSI score (14.21 in 60 patients) is reported in Chinese with an English-language abstract. However, this was not a study comparing PPIs with placebo but of ‘golden voice capsule’ plus or minus PPI. 80 This study was omitted when Guo et al. 79 carried out a similar RSI meta-analysis on the remaining four studies (total n = 217). Despite dropping the outlying ineligible study, there remained highly significant heterogeneity (p < 0.00001), and the SMD had fallen to 1.65 (95% CI 0.15 to 3.14).
Reichel et al. 81 carried out a RCT of PPI in patients with throat symptoms, although the citation in Wei’s78 bibliography is of their publication of 49 patients on the lack of correlation between the symptom change after PPI with measurable 24-hour pH monitoring change. The actual RCT carried out by this group had RSI as the principal outcome in 58 patients. 28 (Moreover, the reference numbering in the table of all 13 included studies is awry78). However, the representation of Reichel et al. ’s81 data in the appropriate paper is accurate. Reichel et al. 81 included only patients with a RFS of > 7 and their sample showed a RSI SMD of 3.85 at 3 months. The TOPPITS median RFS was 9 at baseline (IQR 7–12) (see Table 18) (i.e. a majority of patients would meet Reichel et al. ’s81 qualifying criterion). The end of treatment mean change from baseline in the total RSI was 14.3 in the esomeprazole group, which was statistically greater than in the placebo group (7.8, SMD 3.85). 28 The authors note the marked placebo response, which was indeed greater than that in either group of the much larger TOPPITS cohort at 4 months (mean change from baseline in all patients = 5.3).
Other authors have considered whether or not oropharyngeal pH monitoring (Restech, Respiratory Technology Corporation, Houston, TX, USA) may predict PPI response. RSI, laryngoscopy and 24-hour oropharyngeal pH monitoring were undertaken in 22 patients who were given a 3-month course of 40 mg of pantoprazole twice a day. 82 A symptom response was defined as a 5-point fall in the RSI score. Laryngoscopic findings did not correlate with response and only nine patients (40.9%) had abnormal pH-metry, all being responders. Four pH-metry negative patient were also responders. Thus, oropharyngeal pH monitoring showed a sensitivity of 69% and a specificity of 100%. 82 In another report of 24-hour multichannel intraluminal oesophageal impedance/pH-metry, GORD was confirmed in < 40% of patients previously labelled as having LPR, which the authors attribute to ‘the low specificity of the laryngoscopic findings’. 83
Optimal administration of PPI usually takes place 30 minutes before food; > 30% of patients are suboptimal users. 84 (Esomeprazole seems not to lose efficacy when administered after meals. 85) Fifty-one patients with throat symptoms completed a face-to-face semistructured interview, a questionnaire and the RSI. Over 62% of participants described an incorrect routine in taking their PPI (taking it with other pills, taking it with food/drink and uncertainty about which pill is for reflux), although this did not have an impact on the RSI. RSI scores were moderately correlated with patient-reported reflux severity (r = 0.62, p < 0.0001, R2 = 0.34). Even when PPI compliance was adequate, symptoms such as globus, mucus, voice dysfunction and dysphagia persisted. In addition to the dubious effect size of PPI therapy, there are now also increasing concerns about the safety of PPIs.
Proton pump inhibitor risk
The US veterans study86 of the impact of PPI use on all-cause mortality in almost 350,000 individuals found that, over a median follow-up of 5.71 years, PPI use was associated with an increased risk of death compared with the use of H2 antagonist blockers [hazard ratio (HR) 1.25]. The risk of death was increased when considering PPI use versus no PPI and no H2 blockers (HR 1.23). Among new PPI users, there was a graded association between the duration of exposure and the risk of death. The authors concluded that limiting PPI use and duration to instances when it is medically indicated may be warranted. 86 A systematic review of PPIs and cardiovascular events analysed five studies that directly compared the effect of PPI use on mortality and/or cardiovascular morbidity (including 22,427 patients in mortality data sets and 354,446 patients in morbidity data sets). For patients taking PPIs, all-cause mortality [odds ratio (OR) 1.68] and rate of major cardiovascular events (OR 1.54) were significantly higher, indicating a significant increase in morbidity due to cardiovascular disease. 87 Long-term PPI use has also been linked to acute interstitial nephritis, fractures, and C. difficile-associated diarrhoea, suggesting a need for cautious prescribing and regular monitoring, especially in older adults. 88
Gastric cancer risk with PPIs was assessed in over 63,000 individuals in Hong Kong who were given clarithromycin-based triple therapy for Helicobacter pylori. 89 During a median follow-up of 7.6 years, 153 people (0.24%) developed gastric cancer (HR 2.44); this was not observed with those taking H2 antagonists (HR 0.72). The risk increased with duration of PPI use: the HR was 5.04 at 1 year and 8.34 after 3 years. The adjusted absolute risk difference for PPI use versus non-PPI use was a 4.29-fold excess of gastric cancer per 10,000 person-years. 89 Similar conclusions were reached in a contemporaneous meta-analysis of almost 1 million individuals in west China. 90
Using data from Danish nationwide registries and Cox models regressing for both propensity scores and drug use, a Danish group91 explored the current practice of using PPIs as an adjunctive therapy in cancer patients. Cancer-specific and non-cancer death among PPI users (two or fewer prescriptions within 6 months after diagnosis, n = 36,066) were compared with non-users (fewer than two prescriptions, n = 311,853) or users of histamine H2-receptor antagonists (n = 5152). Adjusted HRs for cancer-specific mortality among postdiagnostic PPI users as compared with non-users or H2-receptor antagonist users were 1.29 and 1.15, respectively, and the risk was greatest for ovarian cancer. Although the associations were stronger among new PPI users after cancer diagnosis, indicating potential confounding, and the impact of PPIs on mouse tumour models was variable, the authors raise concerns about PPI safety among cancer patients. 91
Alginate/alkaline water
The alginate preparation Gaviscon Advance [Reckitt Benckiser Healthcare (UK) Ltd] was assessed alone versus co-prescription with a PPI in an open-label study of ‘100 consecutive LPR patients’ with a RSI of > 10 attending a joint voice clinic. 92 All were treated with Gaviscon Advance four times daily. If patients had already been started on a PPI, this was optimised to a twice-daily dosing regimen. Follow-up RSI scores in 72 patients showed no significant RSI differences from the additional use of PPI.
At the cellular level, tissue-bound pepsin is fundamental to reflux pathophysiology. Human pepsin remains stable at a pH level of 7.4 and may be reactivated by hydrogen ions from any source. Most tap and bottled waters (typically with pH levels of 6.7–7.4) would not be expected to affect pepsin stability. Artesian well water containing natural bicarbonate (pH level of 8.8) irreversibly inactivated human pepsin (in vitro). In addition, it has good acid-buffering capacity. Thus, the consumption of alkaline water may have therapeutic benefits for reflux patients. 93 In vitro tests of the proprietary alginate Gaviscon Advance show that it can specifically remove both pepsin and bile acids, limit their diffusion and affect enzymatic activity of pepsin, hence the potential for oesophageal protection. 94 Gaviscon Advance, it should be noted, relies principally on its alginate raft and not on an acid-buffering capacity.
The Comprehensive Reflux Symptom Score
The advantages of CReSS include standardised, comprehensive capture of patient experience; discriminant validity of ENT and GORD patients from volunteers; and discrete oesophageal, pharyngeal and upper airway subscales. 31 The CReSS and RSI are correlated (see Figure 15) as they are not independent, the CReSS incorporating all RSI symptoms. However, the addition of the CReSS upper airway domain to RSI-HB improved the ability to predict RSI at 16 weeks (see Appendix 9, Table 62).
The Reflux Finding Score is unreliable
We felt that it was important to include a report on the endolaryngeal examination findings as scored by the RFS because of its common use in clinical practice. This is despite limited evidence to suggest that laryngeal mucosal changes – increased ‘Reflux Finding’ Scores – discriminate between ‘normals’ and general throat symptom patients. 95 Furthermore, the RFS has not been proven to be a valid tool to diagnose LPR. 17,51–53 In our study, the RFS was independently scored 0–26 and assessed as a potential response predictor (alongside patient characteristics of age, sex, smoking status and BMI), comparing it with the RSI at 16 weeks. The number and value of RFSs were similar in both treatment groups at baseline. Adjusted for important baseline factors and RFS baseline assessment, the RFS was not suitable for first-order fractional polynomial transformations and not considered for inclusion in the multivariable model. Thus, the total RFS was not subsequently considered as a predictive factor of treatment response. Several authors have suggested that one feature in the RFS – presence of pseudosulcus – is the best predictor of EOR,96,97 but this level of individual items analysis was not possible. Pseudosulcus has been reported generally as a rare finding. 97 Others have found that RFS raters are influenced by patient symptoms and so the reliability and objectivity of the RFS is questionable. 98 The RFS is also insensitive to postfundoplication symptomatic benefit. 99
In a selected series of 78 patients with chronic cough and/or suspected vocal cord dysfunction,100 87% had a RSI score of > 13 and 51% had a RFS of > 7. Salivary pepsin had a sensitivity of 78% and specificity of 53% for predicting a high RFS. There were significant correlations between the RSI and RFS (r = 0.51) and between the severity of laryngeal inflammation and the concentration of pepsin (r = 0.28). All cases investigated with pH-impedance study had objective evidence of proximal reflux. 100 However, these findings in selected patients do not mirror observations in the generality of those presenting to clinics with non-specific throat symptoms, who are the focus of TOPPITS.
Update on pH-metry and manometry in throat symptoms
Up to 50% of the patients suspected of reflux laryngitis syndrome fail to respond to acid suppression therapy. This has led to measurement of acid exposure times in an attempt to improve the specificity of the diagnosis. Using distal and cervical oesophageal pH-metry in 46 ‘LPR’ patients and 58 healthy control patients, the percentage of time that the pH level is < 4 and the number of LPR episodes emerged as diagnostic LPR criteria. 101 Up to 50% of the patients suspected of reflux laryngitis syndrome fail to respond to acid suppression therapy. Laryngopharyngeal bolus exposure time during multichannel intraluminal impedance and pH (MII-pH) testing was the best response predictor in one study. 102 Of 109 patients evaluated by MII-pH, 47% were classed as ‘positive’ for evidence of significant LPR, with an average of 46 episodes of proximal reflux exposure (either acid or non-acid). RSI scores were significantly different between positive and negative studies, whereas RFSs were not. 103
High-resolution manometry metrics in 24 globus patients were compared with 24 age-matched and sex-matched subjects with non-obstructive dysphagia and 21 healthy control patients. 104 In a multivariate model, receiver operating characteristic analysis identified a threshold of 0.4-mmHg upper oesophageal sphincter (UOS) residual pressure in globus patients (sensitivity 66.7%, specificity 71.5%, positive predictive value 55.2%, negative predictive value 80.0%). 104 Those globus patients who also have GORD may also have a longer UOS zone. 105 Repeated reactive dry swallowing and/or throat clearing might contribute to these observations, which may have a historical correlate in the so-called ‘inferior constrictor strain swallow’ described by Gray. 106
There is recent interest in ambulatory 24-hour oropharyngeal aerosol acid exposure assessment, which was used in 101 patients with GORD symptoms and cough, hoarseness, asthma symptoms or globus sensation alongside concomitant oesophageal pH monitoring. Oesophageal 24-hour pH-metry was positive in 66 patients (65%), of whom 39% also had evidence of aerosol oropharyngeal acid. Only eight of those with normal oesophageal pH-metry had oropharyngeal acid exposure. 107 Our own prior work shows that aspiration of bile acids may induce airway fibrosis through the production of transforming growth factor beta 1 and fibroblast proliferation. Early intervention to attenuate these processes may reduce fibrogenesis in various airway diseases associated with GORD. 108
Throat symptom patient-reported outcome tools
Three patient-reported outcome measures were utilised in TOPPITS. The RSI was considered the ‘market-leader’ in terms of popularity and its seemingly ubiquitous association with ‘LPR’. There was concern from the research team that the ninth questionnaire item groups four traditional GORD symptoms (heartburn, indigestion, chest pain and stomach acid coming up). It was hypothesised that this item could lead to overall PPI treatment response reporting given that these symptoms are commonly treated with PPIs, hence the rationale to refine the RSI into a score including and excluding this item for the purposes of baseline stratification. The CReSS questionnaire unbundles this item into distinct questions and has additional items cited as important by patients. The CReSS was included to assess whether or not the more comprehensive symptom profiling would allow greater sensitivity to change than the RSI does. In a study of the quality-of-life impact of throat symptoms in 80 patients, females experienced a greater impact on vitality and mental health than males did. 109 A South Korean study explored the added impact of throat symptoms (RSI) on quality of life in 300 GORD patients. Those with supra-oesophageal symptoms had lower quality-of-life scores proportional to the symptom severity. 110 The LPR-HRQL was included in recognition of the lack of quality-of-life measures or published outcomes in the field of chronic throat symptoms.
All three outcome measures show significant improvement in both the treatment group and the placebo group from baseline to 16 weeks and from baseline to 12 months. The ninth RSI item did not affect treatment reporting between the two groups, as was considered by the research team. The quality-of-life measure (LPR-HRQL) also demonstrated proportionate reductions at 16 weeks and 12 months. This again proves the significant throat symptom placebo effect (Reichel et al. 28). Presumably, both the experience of being in a trial and the additional clinical team contact contributed to the pharmacological component of the placebo response. Evidence suggests that a substantial proportion of patients with unexplained throat symptoms are susceptible to additional, unrelated medically unexplained symptoms. This would accord with the magnitude of the placebo effect in TOPPITS. 23 A person’s locus of control is either internal (believes to personally control his or her life) or external (believes to be controlled by others, by environmental factors that cannot be influenced or by chance). An external locus of control has been found to be related to higher placebo response. 111 Contrary to expectation, somatisation in a primary care study was found not to correlate with locus of control,112 and this whole territory in throat symptoms warrants further investment. Although antidepressants modulate oesophageal sensation and reduce functional chest pain,113 throat symptom patients regularly discontinue therapy. 114
Psychological aspects
In a study of US Vietnam War veterans,115 the prevalence of globus was 6.4%. Those men with globus had increased risks of being diagnosed with somatisation disorder, major depression, generalised anxiety disorder, post-traumatic stress disorder and drug abuse or dependence (OR 1.89), giving rise to the hypothesis that men may develop globus to ‘represent’ other, related and treatable psychopathology. 115 More widely, evidence suggests that a substantial proportion of patients with unexplained throat symptoms are susceptible to additional, unrelated medically unexplained symptoms. The propensity to multiple unexplained physical symptoms can be quantified by completion of a Review Of Symptoms or similar checklist. 116
In a recent therapy trial, 148 refractory globus patients were randomised to receive 6 weeks of paroxetine, amitriptyline or lansoprazole. Response (> 50% reduction on the Glasgow Edinburgh Throat Scale) was achieved by 72% of paroxetine users, 46% of those on amitriptyline and only 14% of the lansoprazole group. 117 In our own early study, 24 globus patients were assessed for current and past psychiatric illness and then received amitriptyline or placebo using a double-blind design. Nine patients met the criteria for psychiatric disorder in the past; six had suffered from panic disorder. Two further patients had been troubled by classic panic attacks. Nine of the 12 patients treated with amitriptyline and two of the placebo group discontinued treatment. Thus, the association of pathological anxiety with globus was associated with a high incidence of tricyclic antidepressant treatment failure. 114 Antidepressants modulate oesophageal sensation and reduce functional chest pain113 but more research is required on their potential in throat symptoms.
Psychophysiological mechanisms
Recent work from China118,119 suggested that serotonin transporter gene (SLC6A4) polymorphism may be associated with both globus pharyngeus and its antidepressant action. Globus patients were randomised into paroxetine or amitriptyline groups for 6 weeks. Treatment response was defined as a > 50% reduction in the Glasgow Edinburgh Throat Scale scores. A significant association between the genotype and the response to antidepressant treatment was observed. 118,119 A recent study of UOS and oesophageal body sensitivity and compliance between globus patients and healthy control patients found that somatisation, panic disorder and post-traumatic stress severity were significantly associated with globus. UOS compliance and somatisation were also found to be independently associated with globus, implying that globus is a complex disorder of the brain–gut axis rather than a ‘psychosomatic’ disorder or a peripheral oesophageal disorder. 120 Transnasal oesophagoscopy, high-resolution manometry and 24-hour MII-pH monitoring were conducted on 30 globus referrals in Helsinki. 121 There was no evidence of acid or non-acid GORD or elevated UOS pressure. 121
Potential methods to address the limitations encountered in TOPPITS patient and public involvement
The patient and public involvement strand of TOPPITS was drawn up with high hopes, and nurtured by the ongoing efforts of our qualitative researcher, Jan Lecouturier, yet proved relentlessly disappointing. The following proposals might have, on refection, mitigated the low patient and public involvement input:
-
Enhanced budget to fund a team member to attend clinics alongside the research nurses to explore reasons for non-engagement.
-
Much of the allocated patient and public involvement budget went towards developing the website.
-
Enhanced sampling strategy seeking participation – targeting TOPPITS pilot study patients was not productive; we might have approached those who declined TOPPITS or patients with other throat problems.
-
Enhanced buy-in from the site staff (i.e. research staff there lead on developing the materials?). Would this have raised the profile of the patient and public involvement and/or resulted in greater commitment?
Generalisability
TOPPITS deserves a high profile as the results are generalisable for the following reasons:
-
It had a large sample compared with prior single trials and even the total numbers in several meta-analyses.
-
It was multicentre, UK-wide and pragmatic.
-
The spread of baseline RSI scores was similar to the range in other publications in the field.
-
The sex balance and age profile were typical of reports of persistent throat symptoms in the West.
-
Key patient symptoms (lump in the throat, throat clearing, excess mucus, troublesome cough, hoarseness, feeling things stuck in throat) incorporate the majority of those seen in NHS ENT clinics.
-
The message for primary and secondary care resonates with other messages concerning overuse of PPIs when evidence is weak or lacking. 122
-
This is a topical subject as some of the epidemiological adverse data on long-term PPI use including impact on life expectancy are only now emerging.
Interpretation
Given the lack of any trend in treatment response in TOPPITS presented in this report, there is a limit to the conclusions that can be drawn regarding the utility and merit of the questionnaires employed. It could be argued that the inclusion of three patient-reported outcome measures was unnecessary and that the RSI alone would have sufficed, providing a rapid measure, with a published literature context, patients’ acceptability and demonstrable sensitivity to change. However, the lack of any trend towards a treatment effect in TOPPITS is the very reason why it is so important that TOPPITS presents the most comprehensive evidence base possible. The use of multiple tools also offers the opportunity for the research team to analyse patients’ symptoms in further detail in the near future. Many patients present with throat symptoms. TOPPITS has clearly demonstrated that the common practice of treating patients with PPIs is ineffective. Optimum management should focus on patients’ symptoms and concerns, and address how these should be managed. The TOPPITS patients provide a wealth of patient-reported symptom data that will facilitate future research and clinical pathways.
The study was not powered for subgroup analysis, and, as a clinical trial of an investigational medicinal product, this TOPPITS main report rightly limits itself to the analysis presented to the DMC and TSC in the agreed statistical analysis plan. However, group data sets may contain identifiable subgroups of individuals who potentially stand to gain more from a trial therapy. We propose to explore the data distribution in greater detail, looking for any potential clustering of symptoms, to see whether or not there may be merit in pursuing anti-reflux approaches (not necessarily PPIs for the safety reasons outlined previously) or indeed whether or not future efforts would be more logically applied to different aetiological theories and therapeutic pathways, including psychological treatments.
Potential bias
Did patients guess whether or not they were taking the active drug? Apparently not, as the correct identification rate was similar in both groups (see Table 4). We were fortunate that our mucosal rater (PC) was working in Australia at the time and so had no way of judging the future allocation of therapy for any individual. The outcomes were all patient self-reported. This may have had some minor impact on outcomes measurement overall but not in any systematic way so as to influence one therapy over another.
Bias is minimised through ITT analyses retaining ineligible and protocol-violator patients in the analysis according to their randomised treatment group. The trial assumed a level of drop-out owing to the primary outcome measure being patient reported, and the sample size was inflated accordingly to retain power. Observed drop-out was as anticipated and was not differential across treatment groups. It was demonstrated that the patient characteristics of the pragmatic and compliant ITT groups were representative of the trial population. Planned secondary sensitivity analyses, based on a pragmatic ITT group, confirmed the conclusions of the primary analyses, based on the compliant ITT group.
Chapter 5 Conclusions
Health-care implications
TOPPITS is a robust clinical trial that confirms the findings of other work (which found no evidence to support PPI treatment benefit in cohort studies of those with persistent throat symptoms). De-prescribing, defined as lowering dosage, switching to as-needed use or complete discontinuation, should be considered for many PPI users. 88 Current NHS guidance for the management of persistent throat symptoms is lacking. The TOPPITS outcome stands to contribute to future throat symptom guidance, particularly given the current popularity for primary PPI treatment. The TOPPITS results provide evidence that patients and clinicians need to consider in making shared decisions about PPI use for persistent throat symptoms.
This is important as the substantial gamut of adverse consequences of PPI treatment is only now becoming apparent. A reversal of the terminology trend towards LPR as the terminology of choice for persistent throat symptoms is also worthy of consideration. 122
Pursuit of alternative treatments
Mucosal protection
Sucralfate offers a safe, locally active treatment by protective adherence to denuded mucosal surfaces and also has bile salt-binding properties, with GORD efficacy comparable to that of H2-receptor blockade. 123 To date, no studies comparing sucralfate with PPIs have been reported in the medical literature. 124 It could be argued that this is of little relevance in throat symptoms as we have shown that PPIs are irrelevant in any case. However, mucosal protection agents might play a part in the control of sensory symptoms, possibly even in the absence of pathological levels of acid exposure.
Lifestyle modification
The most significant lifestyle modification for persistent throat symptoms is weight loss. Additional lifestyle interventions include upright head-of-bed elevation, avoiding meals close to bedtime and avoiding high-fat meals within 2–3 hours of reclining. These simple low-cost interventions have understandably had relatively little research investment. A systematic review of lifestyle interventions in GORD concluded that weight loss and tobacco smoking cessation should be recommended when relevant, and that avoiding late-evening meals and head-of-bed elevation were effective in nocturnal GORD. 125
In a study of supra-oesophageal reflux prevalence, timing and response to head-of-bed elevation by 6 inches,126 nasopharyngeal pH-metry detected acid in 113 (48%) participants, with over half being only in the supine position. Sequential overnight nasopharyngeal pH monitoring before and after head-of-bed elevation was obtained in only 13 of those with supine reflux. Ten subjects demonstrated significant improvement, with complete resolution in most. 126 However, some bed types, such as storage divans, do not lend themselves easily to elevation and so the use of a mattress-top wedge is becoming increasing popular in the commercial sector (e.g. https://putnams.co.uk/products/memory-foam-bed-wedge; accessed 30 October 2019). Such wedges allow the sufferer to elevate the chest on a gentle slope while not affecting the sleep position of any bed partner.
Other pathways/future work
Primary care offers unique insights into an individual’s history of multiple prior somatisation episodes; however, the rarity of laryngoscopic facilities in primary care distorts the management pathway for persistent throat symptoms. There is reassurance from adequate explanation of the vicious behavioural cycles that can perpetuate throat symptoms (dry swallow, throat clearing), together with an indication to patients of the common occurrence of their problems. All are preferable to universal PPI prescription for undiagnosed throat symptoms.
When there are atypical features, early referral for laryngopharyngoscopy may be unavoidable. Secondary care offers a more straightforward locus for research owing to the concentration of patients seen in every general ENT clinic. Psychological approaches may be offered by speech therapists or as primary therapy or pharmacological interventions in their own right. Cognitive–behavioural therapy could feasibly be part of a package for treating functional dysphonia,25,127–129 but the efficacy of cognitive–behavioural therapy in dysphonia and globus remains to be addressed in a substantive trial. The placebo phenomenon111 might also usefully be exploited by the use of traditional voice care measures: hydration, steam and honey. More work is needed on the role of antidepressants. 113,114,117,118,130 Speech therapy delivered by specialist practitioners can be a primary globus therapy,131 but needs to be explored in a wider range of practitioners.
Acknowledgements
This trial was sponsored by The Newcastle upon Tyne Hospitals NHS Foundation Trust (research and development reference number 6831).
Contributions of authors
Professor Janet A Wilson (https://orcid.org/0000-0002-6416-5870) was the chief investigator and designed and developed the trial protocol. She oversaw the running of the trial, the data analysis and the preparation of the final report and subsequent outputs.
Professor Deborah D Stocken (https://orcid.org/0000-0001-8031-1738) was the senior clinical trials statistician and contributed to the design, analysis, interpretation and reporting of the trial.
Miss Gillian C Watson (https://orcid.org/0000-0003-2466-0268) managed the trial on a day-to-day basis and contributed substantially to the preparation of the final report.
Mr Tony Fouweather (https://orcid.org/0000-0002-2292-0495) was the clinical trials statistician and contributed to the design and analysis of the trial and the presentation of data for this paper.
Mr Julian McGlashan (https://orcid.org/0000-0001-6716-6089) was the principal investigator at the Nottingham site and contributed to the trial design, interpretation and dissemination.
Mr Kenneth MacKenzie (https://orcid.org/0000-0002-2952-7717) was the principal investigator at the Glasgow site and contributed to the design, interpretation and dissemination of the trial.
Professor Paul Carding (https://orcid.org/0000-0002-4206-1827) provided the blinded assessment of baseline digital images and contributed to the design, interpretation and dissemination of the trial.
Mr Yakubu Karagama (https://orcid.org/0000-0002-6257-905X) was the principal investigator at the Manchester site and contributed to the design, interpretation and dissemination of the trial.
Mr Meredydd Harries (https://orcid.org/0000-0002-3026-5662) was the principal investigator at the Brighton site and contributed to data interpretation.
Dr Stephen Ball (https://orcid.org/0000-0002-5804-4466) was the principal investigator at the Sunderland site and contributed to data interpretation.
Ms Sadie Khwaja (https://orcid.org/0000-0001-7129-8789) was the principal investigator at the Stockport site and contributed to data interpretation.
Mr Declan Costello (https://orcid.org/0000-0001-6063-3211) was the principal investigator at the Birmingham site and contributed to data interpretation.
Ms Ruth Wood (https://orcid.org/0000-0002-8296-1774) designed and managed the database.
Dr Jan Lecouturier (https://orcid.org/0000-0002-5191-5804) advised on the patient and public involvement aspects of the trial.
Dr James O’Hara (https://orcid.org/0000-0002-4096-3296) assisted in the design and development of the protocol and was, for a time, the principal investigator at the Sunderland site.
Publications
Watson G, O’Hara J, Carding P, Lecouturier J, Stocken D, Fouweather T, Wilson J. TOPPITS: Trial of Proton Pump Inhibitors in Throat Symptoms. Study protocol for a randomised controlled trial. Trials 2016;17:175–83.
O’Hara J, Stocken DD, Watson GC, Fouweather T, McGlashan J, MacKenzie K, et al. Use of proton pump inhibitors to treat persistent throat symptoms: multicentre, double blind, randomised, placebo controlled trial. BMJ 2021;372:m4903.
Data-sharing statement
Anonymised data from this study may be available to the scientific community subject to regulatory and ethics approval. Requests for data should be directed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Watson G, O’Hara J, Carding P, Lecouturier J, Stocken D, Fouweather T, et al. TOPPITS: Trial Of Proton Pump Inhibitors in Throat Symptoms. Study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1267-7.
- Deary IJ, Wilson JA, Kelly SW. Globus pharyngis, personality, and psychological distress in the general population. Psychosomatics 1995;36:570-7. https://doi.org/10.1016/S0033-3182(95)71614-0.
- Moloy PJ, Charter R. The globus symptom. Incidence, therapeutic response, and age and sex relationships. Arch Otolaryngol 1982;108:740-4. https://doi.org/10.1001/archotol.1982.00790590062017.
- Pawar S, Lim HJ, Gill M, Smith TL, Merati A, Toohill RJ, et al. Treatment of postnasal drip with proton pump inhibitors: a prospective, randomized, placebo-controlled study. Am J Rhinol 2007;21:695-701. https://doi.org/10.2500/ajr.2007.21.3098.
- Lowden M, McGlashan JA, Steel A, Strugala V, Dettmar PW. Prevalence of symptoms suggestive of extra-oesophageal reflux in a general practice population in the UK. Logoped Phoniatr Vocol 2009;34:32-5. https://doi.org/10.1080/14015430902735847.
- Thompson WG, Heaton KW. Heartburn and globus in apparently healthy people. Can Med Assoc J 1982;126:46-8.
- NHS Digital . Hospital Outpatient Activity – 2010–11 2011. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/hospital-outpatient-activity-2010-11 (accessed 22 November 2019).
- Noordzij JP, Khidr A, Evans BA, Desper E, Mittal RK, Reibel JF, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 2001;111:2147-51. https://doi.org/10.1097/00005537-200112000-00013.
- Delahunty JE. Acid laryngitis. J Laryngol Otol 1972;86:335-42. https://doi.org/10.1017/S0022215100075356.
- Franchi A, Brogelli B, Massi D, Santucci M, De Campora E, Gallo O. Dilation of intercellular spaces is associated with laryngo-pharyngeal reflux: an ultrastructural morphometric analysis of laryngeal epithelium. Eur Arch Otorhinolaryngol 2007;264:907-11. https://doi.org/10.1007/s00405-007-0295-z.
- Johnston N. Review article: uptake of pepsin at pH 7 – in non-acid reflux – causes inflammatory, and perhaps even neoplastic, changes in the laryngopharynx. Aliment Pharmacol Ther 2011;33:13-20.
- Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope 2006;116:144-8. https://doi.org/10.1097/01.mlg.0000191463.67692.36.
- Gatta L, Vaira D, Sorrenti G, Zucchini S, Sama C, Vakil N. Meta-analysis: the efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007;25:385-92. https://doi.org/10.1111/j.1365-2036.2006.03213.x.
- Sen P, Georgalas C, Bhattacharyya AK. A systematic review of the role of proton pump inhibitors for symptoms of laryngopharyngeal reflux. Clin Otolaryngol 2006;31:20-4. https://doi.org/10.1111/j.1749-4486.2006.01134.x.
- Lam PK, Ng ML, Cheung TK, Wong BY, Tan VP, Fong DY, et al. Rabeprazole is effective in treating laryngopharyngeal reflux in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol 2010;8:770-6. https://doi.org/10.1016/j.cgh.2010.03.009.
- Ah-See K, Wilson J. Extra-oesophageal reflux: state of the knowledge base. Clin Otolaryngol 2012;37:9-16. https://doi.org/10.1111/j.1749-4486.2012.02443.x.
- Powell J, Cocks HC. Mucosal changes in laryngopharyngeal reflux-prevalence, sensitivity, specificity and assessment. Laryngoscope 2013;123:985-91. https://doi.org/10.1002/lary.23693.
- Hanson DG, Kamel PL, Kahrilas PJ. Outcomes of antireflux therapy for the treatment of chronic laryngitis. Ann Otol Rhinol Laryngol 1995;104:550-5. https://doi.org/10.1177/000348949510400709.
- Kibblewhite DJ, Morrison MD. A double-blind controlled study of the efficacy of cimetidine in the treatment of the cervical symptoms of gastroesophageal reflux. J Otolaryngol 1990;19:103-9.
- Karkos PD, Benton J, Leong SC, Karkanevatos A, Badran K, Srinivasan VR, et al. Trends in laryngopharyngeal reflux: a British ENT survey. Eur Arch Otorhinolaryngol 2007;264:513-17. https://doi.org/10.1007/s00405-006-0222-8.
- Qadeer MA, Phillips CO, Lopez AR, Steward DL, Noordzij JP, Wo JM, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006;101:2646-54. https://doi.org/10.1111/j.1572-0241.2006.00844.x.
- Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006;116:254-60. https://doi.org/10.1097/01.mlg.0000192173.00498.ba.
- Reimer C, Bytzer P. Management of laryngopharyngeal reflux with proton pump inhibitors. Ther Clin Risk Manag 2008;4:225-33. https://doi.org/10.2147/TCRM.S6862.
- Hungin AP, Rubin GP, O’Flanagan H. Long-term prescribing of proton pump inhibitors in general practice. Br J Gen Pract 1999;49:451-3.
- O’Hara J, Miller T, Carding P, Wilson J, Deary V. Relationship between fatigue, perfectionism, and functional dysphonia. Otolaryngol Head Neck Surg 2011;144:921-6. https://doi.org/10.1177/0194599811401236.
- Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice 2002;16:274-7. https://doi.org/10.1016/S0892-1997(02)00097-8.
- Lee YS, Choi SH, Son YI, Park YH, Kim SY, Nam SY. Prospective, observational study using rabeprazole in 455 patients with laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol 2011;268:863-9. https://doi.org/10.1007/s00405-010-1475-9.
- Reichel O, Dressel H, Wiederänders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg 2008;139:414-20. https://doi.org/10.1016/j.otohns.2008.06.003.
- Papakonstantinou L, Leslie P, Gray J, Chadwick T, Hudson M, Wilson JA. Laryngopharyngeal reflux: a prospective analysis of a 34 item symptom questionnaire. Clin Otolaryngol 2009;34:455-9. https://doi.org/10.1111/j.1749-4486.2009.01998.x.
- Kamani T, Penney S, Mitra I, Pothula V. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol 2012;269:2219-25. https://doi.org/10.1007/s00405-012-2028-1.
- Drinnan M, Powell J, Nikkar-Esfahani A, Heading RC, Doyle J, Griffin SM, et al. Gastroesophageal and extraesophageal reflux symptoms: similarities and differences. Laryngoscope 2015;125:424-30. https://doi.org/10.1002/lary.24950.
- Siupsinskiene N, Adamonis K, Toohill RJ. Quality of life in laryngopharyngeal reflux patients. Laryngoscope 2007;117:480-4. https://doi.org/10.1097/MLG.0b013e31802d83cf.
- Cheung TK, Lam PK, Wei WI, Wong WM, Ng ML, Gu Q, et al. Quality of life in patients with laryngopharyngeal reflux. Digestion 2009;79:52-7. https://doi.org/10.1159/000205267.
- Lenderking WR, Hillson E, Crawley JA, Moore D, Berzon R, Pashos CL. The clinical characteristics and impact of laryngopharyngeal reflux disease on health-related quality of life. Value Health 2003;6:560-5. https://doi.org/10.1046/j.1524-4733.2003.65243.x.
- Carrau RL, Khidr A, Gold KF, Crawley JA, Hillson EM, Koufman JA, et al. Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg 2005;131:315-20. https://doi.org/10.1001/archotol.131.4.315.
- Andersson O, Ryden A, Ruth M, Ylitalo Moller R, Finizia C. Validation of the Swedish translation of LPR-HRQL. Med Sci Monit 2010;16:CR480-7.
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001;111:1313-17. https://doi.org/10.1097/00005537-200108000-00001.
- Saruç M, Aksoy EA, Vardereli E, Karaslaan M, Ciçek B, Ince U, et al. Risk factors for laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 2012;269:1189-94. https://doi.org/10.1007/s00405-011-1905-3.
- Gao L, Weck MN, Rothenbacher D, Brenner H. Body mass index, chronic atrophic gastritis and heartburn: a population-based study among 8936 older adults from Germany. Aliment Pharmacol Ther 2010;32:296-302. https://doi.org/10.1111/j.1365-2036.2010.04334.x.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing?. Cleve Clin J Med 2011;78:39-4. https://doi.org/10.3949/ccjm.77a.10087.
- Reimer C, Søndergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009;137:80-7. https://doi.org/10.1053/j.gastro.2009.03.058.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004;292:1955-60. https://doi.org/10.1001/jama.292.16.1955.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol 2009;104:27-32. https://doi.org/10.1038/ajg.2009.49.
- Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010;170:765-71. https://doi.org/10.1001/archinternmed.2010.94.
- NHS . Proton Pump Inhibitor (PPI) Guidance April 2017 n.d. www.gwh.nhs.uk/media/295818/ppi-guidance-apr-17-post-gwh-comments.pdf (accessed 22 November 2019).
- National Institute for Health and Care Excellence (NICE) . Gastro-Oesophageal Reflux Disease and Dyspepsia in Adults: Investigation and Management n.d. www.nice.org.uk/guidance/cg184 (accessed 22 November 2019).
- Weigt J, Kandulski A, Busch F, Malfertheiner P. Nocturnal gastric acid breakthrough is not associated with night-time gastroesophageal reflux in GERD patients. Dig Dis 2009;27:68-73. https://doi.org/10.1159/000210107.
- NHS Business Services Authority . Information Services n.d. www.nhsbsa.nhs.uk/Documents/Apr_-_Jun_10_Gastro-intestinal.pdf.
- Gamble C, Krishan A, Stocken D, Lewis S, Juszczak E, Doré C, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA 2017;318:2337-43. https://doi.org/10.1001/jama.2017.18556.
- Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 2001;111:979-81. https://doi.org/10.1097/00005537-200106000-00009.
- Vaezi MF. Sensitivity and specificity of reflux-attributed laryngeal lesions: experimental and clinical evidence. Am J Med 2003;115:97-104. https://doi.org/10.1016/S0002-9343(03)00205-5.
- Schneider GT, Vaezi MF, Francis DO. Reflux and voice disorders: have we established causality?. Curr Otorhinolaryngol Rep 2016;4:157-67. https://doi.org/10.1007/s40136-016-0121-5.
- Kelchner LN, Horne J, Lee L, Klaben B, Stemple JC, Adam S, et al. Reliability of speech-language pathologist and otolaryngologist ratings of laryngeal signs of reflux in an asymptomatic population using the reflux finding score. J Voice 2007;21:92-100. https://doi.org/10.1016/j.jvoice.2005.09.004.
- McGlashan JA, Johnstone LM, Sykes J, Strugala V, Dettmar PW. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 2009;266:243-51. https://doi.org/10.1007/s00405-008-0708-7.
- Steen IN, MacKenzie K, Carding PN, Webb A, Deary IJ, Wilson JA. Optimising outcome assessment of voice interventions, II: sensitivity to change of self-reported and observer-rated measures. J Laryngol Otol 2008;122:46-51. https://doi.org/10.1017/S0022215107007839.
- Cohen E, Bolus R, Khanna D, Hays RD, Chang L, Melmed GY, et al. GERD symptoms in the general population: prevalence and severity versus care-seeking patients. Dig Dis Sci 2014;59:2488-96. https://doi.org/10.1007/s10620-014-3181-8.
- Haft S, Carey RM, Farquhar D, Mirza N. Anticholinergic medication use is associated with globus pharyngeus. J Laryngol Otol 2016;130:1125-9. https://doi.org/10.1017/S002221511600918X.
- Abdul-Hussein M, Freeman J, Castell DO. Cough and throat clearing: atypical GERD symptoms or not GERD at all?. J Clin Gastroenterol 2016;50:e50-4. https://doi.org/10.1097/MCG.0000000000000384.
- Nikkar A, James P, Powell J, Millar I, Carding P, Leslie P, et al. Reflux symptomatology: towards better definition of the patient experience. Clin Otolaryngol 2012;37:128-9.
- Rothman M, Farup C, Stewart W, Helbers L, Zeldis J. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci 2001;46:1540-9. https://doi.org/10.1023/A:1010660425522.
- Powell J, Nikkar A, Doyle J, Wilson J. Extraesophageal reflux: throat and gastrointestinal patients. Otolaryngol Head Neck Surg 2012;147. https://doi.org/10.1177/0194599812451438a116.
- Doody J, Fenton JE. Troublesome Throat Awareness (tTA) as a contemporary alternative to ‘globus pharyngeus’. Surgeon 2017;15:183-5. https://doi.org/10.1016/j.surge.2017.05.001.
- Rasmussen ER, Schnack DT, Ravn AT. A prospective cohort study of 122 adult patients presenting to an otolaryngologist’s office with globus pharyngeus. Clin Otolaryngol 2018;43:854-60. https://doi.org/10.1111/coa.13065.
- Spantideas N, Drosou E, Bougea A, Assimakopoulos D. Laryngopharyngeal reflux disease in the Greek general population, prevalence and risk factors. BMC Ear Nose Throat Disord 2015;15. https://doi.org/10.1186/s12901-015-0020-2.
- Tang B, Cai HD, Xie HL, Chen DY, Jiang SM, Jia L. Epidemiology of globus symptoms and associated psychological factors in China. J Dig Dis 2016;17:319-24. https://doi.org/10.1111/1751-2980.12354.
- Deary IJ, Wilson JA, Harris MB, MacDougall G. Globus pharyngis: development of a symptom assessment scale. J Psychosom Res 1995;39:203-13. https://doi.org/10.1016/0022-3999(94)00104-D.
- Thomas JP, Zubiaur FM. Over-diagnosis of laryngopharyngeal reflux as the cause of hoarseness. Eur Arch Otorhinolaryngol 2013;270:995-9. https://doi.org/10.1007/s00405-012-2244-8.
- Ruiz R, Jeswani S, Andrews K, Rafii B, Paul BC, Branski RC, et al. Hoarseness and laryngopharyngeal reflux: a survey of primary care physician practice patterns. JAMA Otolaryngol Head Neck Surg 2014;140:192-6. https://doi.org/10.1001/jamaoto.2013.6533.
- Holcomb AJ, Hamill CS, Irwin T, Sykes K, Garnett JD, Kraft S. Practice patterns of referring physicians in management of the dysphonic patient. Otolaryngol Head Neck Surg 2018;158:1072-8. https://doi.org/10.1177/0194599818758958.
- Selby K, Cornuz J, Cohidon C, Gaspoz JM, Senn N. How do Swiss general practitioners agree with and report adhering to a top-five list of unnecessary tests and treatments? Results of a cross-sectional survey. Eur J Gen Pract 2018;24:32-8. https://doi.org/10.1080/13814788.2017.1395018.
- Tosetti C, Nanni I. Use of proton pump inhibitors in general practice. World J Gastrointest Pharmacol Ther 2017;8:180-5. https://doi.org/10.4292/wjgpt.v8.i3.180.
- Nguyen PV, Tamaz R. Inappropriate prescription of proton pump inhibitors in a community setting. Can J Hosp Pharm 2018;71:267-71. https://doi.org/10.4212/cjhp.v71i4.2828.
- Ali O, Poole R, Okon M, Maunick S, Troy E. Irrational use of proton pump inhibitors in general practise. Ir J Med Sci 2018;188:541-4. https://doi.org/10.1007/s11845-018-1891-1.
- Akram F, Huang Y, Lim V, Huggan PJ, Merchant RA. Proton pump inhibitors: are we still prescribing them without valid indications?. Australas Med J 2014;7:465-70. https://doi.org/10.4066/AMJ.2014.2093.
- Halum SL, Postma GN, Johnston C, Belafsky PC, Koufman JA. Patients with isolated laryngopharyngeal reflux are not obese. Laryngoscope 2005;115:1042-5. https://doi.org/10.1097/01.MLG.0000162656.05715.57.
- Lee YC, Lee JS, Kim SW, Kwon KH, Eun YG. Influence of age on treatment with proton pump inhibitors in patients with laryngopharyngeal reflux disease: a prospective multicenter study. JAMA Otolaryngol Head Neck Surg 2013;139:1291-5. https://doi.org/10.1001/jamaoto.2013.5556.
- Liu C, Wang H, Liu K. Meta-analysis of the efficacy of proton pump inhibitors for the symptoms of laryngopharyngeal reflux. Braz J Med Biol Res 2016;49. https://doi.org/10.1590/1414-431X20165149.
- Wei C. A meta-analysis for the role of proton pump inhibitor therapy in patients with laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 2016;273:3795-801. https://doi.org/10.1007/s00405-016-4142-y.
- Guo H, Ma H, Wang J. Proton pump inhibitor therapy for the treatment of laryngopharyngeal reflux: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2016;50:295-300. https://doi.org/10.1097/MCG.0000000000000324.
- Luo H, Ma S, Gao Y, Yan J, Hou J, Xu M. The therapeutic effect of proton pump inhibitor on alleviation of hoarseness symptoms in patients with laryngopharyngeal reflux. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2015;29:997-1001.
- Reichel O, Keller J, Rasp G, Hagedorn H, Berghaus A. Efficacy of once-daily esomeprazole treatment in patients with laryngopharyngeal reflux evaluated by 24-hour pH monitoring. Otolaryngol Head Neck Surg 2007;136:205-10. https://doi.org/10.1016/j.otohns.2006.10.011.
- Vailati C, Mazzoleni G, Bondi S, Bussi M, Testoni PA, Passaretti S. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy?. J Voice 2013;27:84-9. https://doi.org/10.1016/j.jvoice.2012.08.006.
- de Bortoli N, Nacci A, Savarino E, Martinucci I, Bellini M, Fattori B, et al. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related?. World J Gastroenterol 2012;18:4363-70. https://doi.org/10.3748/wjg.v18.i32.4363.
- Sheikh I, Waghray A, Waghray N, Dong C, Wolfe MM. Consumer use of over-the-counter proton pump inhibitors in patients with gastroesophageal reflux disease. Am J Gastroenterol 2014;109:789-94. https://doi.org/10.1038/ajg.2013.421.
- Boltin D, Zvidi I, Raskin M, Kayless H, Schmilovitz-Weiss H, Gingold-Belfer R, et al. Effect of postprandial administration of esomeprazole on reflux symptoms in gastroesophageal reflux disease: a randomized, controlled trial. Dig Dis 2018;36:257-63. https://doi.org/10.1159/000489557.
- Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015735.
- Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ 2018;27:443-50. https://doi.org/10.1016/j.hlc.2017.10.020.
- Pezeshkian S, Conway SE. Proton pump inhibitor use in older adults: long-term risks and steps for deprescribing. Consult Pharm 2018;33:497-503. https://doi.org/10.4140/TCP.n.2018.497.
- Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 2018;67:28-35. https://doi.org/10.1136/gutjnl-2017-314605.
- Wan Q-Y, Wu X-T, Li N, Du L, Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926 386 participants. Gut 2018;68:762-4. https://doi.org/10.1136/gutjnl-2018-316416.
- Tvingsholm SA, Dehlendorff C, Østerlind K, Friis S, Jäättelä M. Proton pump inhibitor use and cancer mortality. Int J Cancer 2018;143:1315-26. https://doi.org/10.1002/ijc.31529.
- Wilkie MD, Fraser HM, Raja H. Gaviscon Advance alone versus co-prescription of Gaviscon Advance and proton pump inhibitors in the treatment of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 2018;275:2515-21. https://doi.org/10.1007/s00405-018-5079-0.
- Koufman JA, Johnston N. Potential benefits of pH 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. Ann Otol Rhinol Laryngol 2012;121:431-4. https://doi.org/10.1177/000348941212100702.
- Strugala V, Avis J, Jolliffe IG, Johnstone LM, Dettmar PW. The role of an alginate suspension on pepsin and bile acids – key aggressors in the gastric refluxate. Does this have implications for the treatment of gastro-oesophageal reflux disease?. J Pharm Pharmacol 2009;61:1021-8. https://doi.org/10.1211/jpp/61.08.0005.
- Milstein CF, Charbel S, Hicks DM, Abelson TI, Richter JE, Vaezi MF. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs. flexible laryngoscope). Laryngoscope 2005;115:2256-61. https://doi.org/10.1097/01.mlg.0000184325.44968.b1.
- Ylitalo R, Lindestad PA, Hertegard S. Is pseudosulcus alone a reliable sign of gastroesophago-pharyngeal reflux?. Clin Otolaryngol Allied Sci 2004;29:47-50. https://doi.org/10.1111/j.1365-2273.2004.00778.x.
- Belafsky PC, Postma GN, Koufman JA. The association between laryngeal pseudosulcus and laryngopharyngeal reflux. Otolaryngol Head Neck Surg 2002;126:649-52. https://doi.org/10.1067/mhn.2002.125603.
- Chang BA, MacNeil SD, Morrison MD, Lee PK. The reliability of the Reflux Finding Score among general otolaryngologists. J Voice 2015;29:572-7. https://doi.org/10.1016/j.jvoice.2014.10.009.
- Weber B, Portnoy JE, Castellanos A, Hawkshaw MJ, Lurie D, Katz PO, et al. Efficacy of anti-reflux surgery on refractory laryngopharyngeal reflux disease in professional voice users: a pilot study. J Voice 2014;28:492-500. https://doi.org/10.1016/j.jvoice.2013.12.009.
- Spyridoulias A, Lillie S, Vyas A, Fowler SJ. Detecting laryngopharyngeal reflux in patients with upper airways symptoms: symptoms, signs or salivary pepsin?. Respir Med 2015;109:963-9. https://doi.org/10.1016/j.rmed.2015.05.019.
- Fusconi M, De Virgilio A, Conte M, Colicchio MG, Gallo A, Greco A, et al. The importance of the number of reflux episodes in the diagnosis of laryngopharyngeal reflux disease. Otolaryngol Head Neck Surg 2013;148:261-6. https://doi.org/10.1177/0194599812466534.
- Wang AJ, Liang MJ, Jiang AY, Lin JK, Xiao YL, Peng S, et al. Predictors of acid suppression success in patients with chronic laryngitis. Neurogastroenterol Motil 2012;24:432-7. https://doi.org/10.1111/j.1365-2982.2011.01873.x.
- Cumpston EC, Blumin JH, Bock JM. Dual pH with multichannel intraluminal impedance testing in the evaluation of subjective laryngopharyngeal reflux symptoms. Otolaryngol Head Neck Surg 2016;155:1014-20. https://doi.org/10.1177/0194599816665819.
- Peng L, Patel A, Kushnir V, Gyawali CP. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. J Clin Gastroenterol 2015;49:95-100. https://doi.org/10.1097/MCG.0000000000000078.
- Tang Y, Huang J, Zhu Y, Qian A, Xu B, Yao W. Comparison of esophageal motility in gastroesophageal reflux disease with and without globus sensation. Rev Esp Enferm Dig 2017;109:850-5. https://doi.org/10.17235/reed.2017.4449/2016.
- Gray LP. The relationship of the ‘inferior constrictor swallow’ and ‘globus hystericus’ or the hypopharyngeal syndrome. J Laryngol Otol 1983;97:607-18. https://doi.org/10.1017/S0022215100094688.
- Fuchs HF, Müller DT, Berlth F, Maus MK, Fuchs C, Dübbers M, et al. Simultaneous laryngopharyngeal pH monitoring (Restech) and conventional esophageal pH monitoring-correlation using a large patient cohort of more than 100 patients with suspected gastroesophageal reflux disease. Dis Esophagus 2018;31. https://doi.org/10.1093/dote/doy018.
- Perng DW, Chang KT, Su KC, Wu YC, Wu MT, Hsu WH, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest 2007;132:1548-56. https://doi.org/10.1378/chest.07-1373.
- Lechien JR, Huet K, Khalife M, Fourneau AF, Finck C, Delvaux V, et al. Gender differences in the presentation of dysphonia related to laryngopharyngeal reflux disease: a case-control study. Eur Arch Otorhinolaryngol 2018;275:1513-24. https://doi.org/10.1007/s00405-018-4951-2.
- Gong EJ, Choi KD, Jung HK, Youn YH, Min BH, Song KH, et al. Quality of life, patient satisfaction, and disease burden in patients with gastroesophageal reflux disease with or without laryngopharyngeal reflux symptoms. J Gastroenterol Hepatol 2017;32:1336-40. https://doi.org/10.1111/jgh.13716.
- Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 2015;12:472-85. https://doi.org/10.1038/nrgastro.2015.117.
- Garcia-Campayo J, Sanz-Carrillo C. A review of the differences between somatizing and psychologizing patients in primary care. Int J Psychiatry Med 1999;29:337-45. https://doi.org/10.2190/FMJ2-UK3Y-FKB8-DCGN.
- Weijenborg PW, de Schepper HS, Smout AJPM, Bredenoord AJ. Effects of antidepressants in patients with functional esophageal disorders or gastroesophageal reflux disease: asystematic review. Clin Gastroenterol Hepatol 2015;13:251-9.e1. https://doi.org/10.1016/j.cgh.2014.06.025.
- Deary IJ, Wilson JA. Problems in treating globus pharyngis. Clin Otolaryngol Allied Sci 1994;19:55-60. https://doi.org/10.1111/j.1365-2273.1994.tb01148.x.
- Gale CR, Wilson JA, Deary IJ. Globus sensation and psychopathology in men: the Vietnam experience study. Psychosom Med 2009;71:1026-31. https://doi.org/10.1097/PSY.0b013e3181bc7739.
- Okland TS, Gonzalez JR, Ferber AT, Mann SE. Association between patient review of systems score and somatization. JAMA Otolaryngol Head Neck Surg 2017;143:870-5. https://doi.org/10.1001/jamaoto.2017.0671.
- Chen D-Y, Jia L, Gu X, Jiang S-M, Xie H-L, Xu J. Comparison of paroxetine and amitriptyline in the treatment of refractory globus pharyngeus. Dig Liver Dis 2016;48:1012-7. https://doi.org/10.1016/j.dld.2016.05.025.
- Liu Y, Jia L, Jiang SM, Chen DY, Song JS, Xu J. Serotonin Transporter Gene (SLC6A4) polymorphism may be associated with Chinese globus pharyngeus and its antidepressant effects. Digestion 2018;97:146-53. https://doi.org/10.1159/000484202.
- Zhou W-C, Jia L, Chen D-Y, Liu Y, Liu J, Jiang SM, et al. The effects of paroxetine and amitriptyline on the upper esophageal sphincter (UES) pressure and its natural history in globus pharyngeus. Dig Liver Dis 2017;49:757-63. https://doi.org/10.1016/j.dld.2017.02.008.
- Rommel N, Van Oudenhove L, Arts J, Caenepeel P, Tack J, Pauwels A. Esophageal sensorimotor function and psychological factors each contribute to symptom severity in globus patients. Am J Gastroenterol 2016;111:1382-8. https://doi.org/10.1038/ajg.2016.302.
- Nevalainen P, Walamies M, Kruuna O, Arkkila P, Aaltonen LM. Supragastric belch may be related to globus symptom – a prospective clinical study. Neurogastroenterol Motil 2016;28:680-6. https://doi.org/10.1111/nmo.12764.
- Zerbib F, Dulery C. Facts and fantasies on extraesophageal reflux: a gastroenterologist perspective. J Clin Gastroenterol 2017;51:769-76. https://doi.org/10.1097/MCG.0000000000000918.
- Tytgat GN. Clinical efficacy of sucralfate in reflux oesophagitis. Scand J Gastroenterol Suppl 1987;140:29-31.
- Savarino E, Zentilin P, Marabotto E, Pellegatta G, Coppo C, Brunacci M, et al. Drugs for improving esophageal mucosa defense: where are we now and where are we going?. Ann Gastroenterol 2017;30:585-91. https://doi.org/10.20524/aog.2017.0187.
- Ness-Jensen E, Hveem K, El-Serag H, Lagergren J. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016;14:175-82.e1. https://doi.org/10.1016/j.cgh.2015.04.176.
- Scott DR, Simon RA. Supraesophageal reflux: correlation of position and occurrence of acid reflux-effect of head-of-bed elevation on supine reflux. J Allergy Clin Immunol Pract 2015;3:356-61. https://doi.org/10.1016/j.jaip.2014.11.019.
- Daniilidou P, Carding P, Wilson J, Drinnan M, Deary V. Cognitive behavioral therapy for functional dysphonia: a pilot study. Ann Otol Rhinol Laryngol 2007;116:717-22. https://doi.org/10.1177/000348940711601002.
- Deary V, Miller T. Reconsidering the role of psychosocial factors in functional dysphonia. Curr Opin Otolaryngol Head Neck Surg 2011;19:150-4. https://doi.org/10.1097/MOO.0b013e328346494d.
- Deary V, McColl E, Carding P, Miller T, Wilson J. A psychosocial intervention for the management of functional dysphonia: complex intervention development and pilot randomised trial. Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-018-0240-5.
- Martín-Merino E, Ruigómez A, García Rodríguez LA, Wallander MA, Johansson S. Depression and treatment with antidepressants are associated with the development of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;31:1132-40. https://doi.org/10.1111/j.1365-2036.2010.04280.x.
- Khalil HS, Reddy VM, Bos-Clark M, Dowley A, Pierce M, Morris C, et al. Speech therapy in the treatment of globus pharyngeus: how we do it. Clin Otolaryngol 2011;36:388-92. https://doi.org/10.1111/j.1749-4486.2011.02326.x.
Appendix 1 Screening, recruitment and withdrawal data
The site and time course of screening and recruitment are summarised in Tables 26 and 27.
| Site | Number of patients | |
|---|---|---|
| Screened | Recruited (based on MACRO) | |
| Birmingham | 31 | 10 |
| Brighton | 50 | 9 |
| Glasgow | 462 | 39 |
| Manchester | 116 | 27 |
| Newcastle | 399 | 133 |
| Nottingham | 178 | 70 |
| Stockport | 17 | 11 |
| Sunderland | 174 | 47 |
| Total | 1427 | 346 |
| Site | Year | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | Total | |||||||||||||||||||||||||||||||
| May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | ||
| Newcastle | 1 | 3 | 2 | 3 | 4 | 5 | 4 | 5 | 4 | 3 | 1 | 7 | 7 | 6 | 6 | 3 | 4 | 6 | 1 | 7 | 4 | 6 | 2 | 3 | 7 | 0 | 4 | 5 | 6 | 2 | 6 | 1 | 5 | 0 | 133 |
| Sunderland | 3 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 | 2 | 4 | 8 | 0 | 1 | 0 | 5 | 2 | 0 | 1 | 1 | 47 | ||
| Nottingham | 1 | 3 | 7 | 4 | 3 | 4 | 2 | 2 | 0 | 6 | 0 | 1 | 3 | 4 | 1 | 4 | 2 | 4 | 3 | 1 | 2 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 70 | |||
| Brighton | 2 | 3 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | |||||||||||
| Manchester | 1 | 1 | 0 | 1 | 2 | 0 | 3 | 0 | 0 | 0 | 2 | 2 | 3 | 1 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 27 | |||||||||||
| Glasgow | 2 | 0 | 3 | 3 | 4 | 3 | 1 | 2 | 4 | 2 | 0 | 1 | 5 | 2 | 1 | 0 | 2 | 3 | 1 | 39 | |||||||||||||||
| Birmingham | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 10 | ||||||||||||||||||||
| Stockport | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 11 | ||||||||||||||||||||||
| Month total | 1 | 3 | 5 | 6 | 9 | 14 | 10 | 9 | 8 | 5 | 7 | 10 | 17 | 8 | 8 | 10 | 8 | 14 | 9 | 13 | 14 | 13 | 14 | 15 | 14 | 13 | 12 | 14 | 12 | 15 | 10 | 6 | 13 | 7 | 346 |
Numbers analysed
Those participants included in each of the analysis sets are summarised in Table 28.
| Time from randomisation to primary end-point visit (visit 2) | Overall | Treatment group | ||||
|---|---|---|---|---|---|---|
| Lansoprazole | Placebo | |||||
| Number of participants | Number with RSI at visit 2 | Number of participants | Number with RSI at visit 2 | Number of participants | Number with RSI at visit 2 | |
| < 10 weeks | 6 | 3 | 2 | 1 | 4 | 2 |
| Between 10 and 14 weeks | 5 | 5 | 2 | 2 | 3 | 3 |
| Between 14 and 18 weeks (protocol compliance) | 185 | 178 | 87 | 82 | 98 | 96 |
| Between 18 and 20 weeks | 44 | 42 | 21 | 20 | 23 | 22 |
| Compliant ITT group (14 to 20 weeks) | 229 | 220 | 108 | 102 | 121 | 118 |
| Between 20 and 25 weeks | 25 | 25 | 14 | 14 | 11 | 11 |
| Between 25 and 30 weeks | 8 | 8 | 3 | 3 | 5 | 5 |
| > 30 weeks | 10 | 6 | 6 | 5 | 4 | 1 |
| Pragmatic ITT group (all visit 2 at any time) | 283 | 267 | 135 | 127 | 148 | 140 |
| Missing (16-week visit, RSI visit 2 = RSI at 16 weeks) | 63 | 79 | 37 | 45 | 26 | 34 |
| Total number randomised | 346 | 346 | 172 | 172 | 174 | 174 |
The stratified subgroups are summarised in Table 29. Table 29 shows the balance over treatment groups in terms of the baseline severity measured by RSI-HB, with a cut-off score of 20.
| Baseline RSI-HB severity | Participants, n (%) | ||
|---|---|---|---|
| Treatment group | Total (N = 346) | ||
| Lansoprazole (N = 172) | Placebo (N = 174) | ||
| Mild (≤ 20) | 91 (53) | 93 (53) | 184 (53) |
| Severe (> 20) | 81 (47) | 81 (47) | 162 (47) |
During the course of the trial, during analysis of recruitment data for DMC meetings, it was discovered that some patients were mis-stratified in terms of baseline severity, defined by their RSI-HB scores. This affected approximately 10% of patients, as outlined in Table 30.
| Randomised as | Stratification status | |
|---|---|---|
| Correct | Mis-stratified | |
| RSI-HB ‘mild’ (≤ 20) | ||
| n | 169 | 12a |
| Range | 10–20 | 21–34 |
| RSI-HB ‘severe’ (> 20) | ||
| n | 137 | 24a |
| Range | 21–38 | 10–20 |
Ineligible patients
Despite treatment withdrawal, patients were followed up in the trial whenever possible. All primary statistical analyses were carried out on an ITT basis, retaining patients in their randomised treatment groups and including protocol-violator and ineligible patients. Ineligible patients were classed as those randomised patients who were subsequently found not to have adhered to the eligibility criteria of the trial. Four ineligible patients were reported (Table 31).
| Identifier | Reason patient was ineligible | Number ineligible (%) |
|---|---|---|
| 5010 | Ineligible – this patient was randomised in error, then found to be ineligible. No medication received; no outcome data | 4 (1) |
| 5101 | It was confirmed that the patient did not fulfil exclusion criteria as escitalopram long-term user | |
| 1062 | Patient was randomised in error at the start of their washout period and was then found to be ineligible | |
| 2106 | Patient was consented and randomised in error, as it was discovered that they were ineligible, so withdrawn immediately |
A sensitivity analysis was not carried out as the ineligibility rate was not excessive (1%).
Withdrawals
In Table 32, the frequency column shows the number fitting the visit status in relation to the primary end point. The compliant column shows those with a primary outcome measure within the compliance window, as specified in Chapter 2, Statistical methods. The pragmatic column shows those with a primary outcome measure at any time, as specified in statistical methods section.
| Status in relation to primary end-point visit | Treatment group | Combined participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lansoprazole | Placebo | ||||||||
| n | Primary outcome (RSI) (n) | n | Primary outcome (RSI) (n) | n | Primary outcome (RSI) (n) | ||||
| Compliant | Pragmatic | Compliant | Pragmatic | Compliant | Pragmatic | ||||
| Visit 2 attended; not withdrawn | 103 | 82 | 100 | 109 | 95 | 107 | 212 | 177 | 207 |
| No visit 2 but not withdrawn | 6 | 0 | 0 | 3 | 0 | 0 | 9 | 0 | 0 |
| Withdrew before visit 2 | 7 | 2 | 4 | 8 | 4 | 5 | 15 | 6 | 9 |
| Withdrew at visit 2 | 6 | 3 | 4 | 10 | 4 | 9 | 16 | 7 | 13 |
| Withdrew and no visit 2 | 31 | 0 | 0 | 23 | 0 | 0 | 54 | 0 | 0 |
| Withdrew after visit 2 | 19 | 15 | 19 | 21 | 15 | 19 | 40 | 30 | 38 |
| Total withdrawn | 63 | 62 | 125 | ||||||
| Total | 172 | 102 | 127 | 174 | 118 | 140 | 346 | 220 | 267 |
It is seen that 267 patients provided the primary outcome measure (RSI at visit 2), of whom 220 were compliant within 14–20 weeks of randomisation. In total, 125 participants withdrew/were lost to follow-up over the duration of the trial. Sixty of these provided the primary outcome measure. Of 60 withdrawals providing the primary outcome, 43 were compliant within 14–20 weeks of randomisation. The timing of loss or withdrawal is shown in Table 33.
| Descriptive statistics | Treatment group | Overall | |
|---|---|---|---|
| Lansoprazole | Placebo | ||
| n | 63 | 62 | 125 |
| Median (IQR) | 19 (8–54) | 25 (13–53) | 21 (9–53) |
| Mean (SD) | 30.9 (24.8) | 31.7 (22.9) | 31.3 (23.8) |
| Range | 0–83 | 0–84 | 0–84 |
Missing data in the primary outcome
The RSI data points that were missing are summarised in Table 34.
| Visit | RSI completeness (total = nine items) | Participants, n (%) | ||
|---|---|---|---|---|
| Treatment group | Total (N = 346) | |||
| Lansoprazole (N = 172) | Placebo (N = 174) | |||
| Baseline (visit 1) | 9 items | 171 (99) | 171 (98) | 342 (99) |
| 1–8 items | 0 (0) | 2 (1) | 2 (< 1) | |
| Missing | 1 (< 1) | 1 (< 1) | 2 (< 1) | |
| 16-week visit (visit 2) (per protocol), 14–18 weeks post randomisation | 9 items | 82 (48) | 96 (55) | 178 (51) |
| Missinga | 5 (3) | 2 (1) | 7 (2) | |
| 16-week visit (visit 2) (compliant ITT), 14–20 weeks post randomisation | 9 items | 102 (59) | 118 (68) | 220 (64) |
| Missinga | 6 (3) | 3 (2) | 9 (3) | |
| 16-week visit (visit 2) (pragmatic ITT group), any time | 9 items | 127 (74) | 140 (80) | 267 (77) |
| Missinga | 8 (5) | 8 (5) | 16 (5) | |
| No visit 2 at 16 weeks | Missingb | 37 (22) | 26 (15) | 63 (18) |
Appendix 2 Details of patients in relation to per-treatment analysis and concomitant medications
Per-treatment analysis
Issue
In most cases in the per-treatment analysis group, medication was dispensed on the same day as randomisation. Four patients did not receive their trial medication at the baseline visit and in a further six there was no note of IMP being issued. However, details of returned medication at the primary end point show that all 10 did receive IMP and they are included in the per-treatment set.
Washout period for prior anti-reflux medication
Recent PPI use is summarised in Table 35.
| PPI use | Participants, n (%) | ||
|---|---|---|---|
| Treatment group | Total (N = 262) | ||
| Lansoprazole (N = 126) | Placebo (N = 136) | ||
| PPI used in last 12 months? | |||
| Yes | 31 (25) | 39 (29) | 70a (27) |
| No | 95 (75) | 97 (71) | 192 (73) |
Full washout data were available for 64 out of 68 participants who reported discontinuing PPI use in the previous 12 months (Table 36).
| Washout period (weeks) | Treatment group | Total (N = 64) | |
|---|---|---|---|
| Lansoprazole (N = 30) | Placebo (N = 34) | ||
| Washout period, n | 30 | 34 | 64 |
| Median (IQR) | 5.9 (4.6–8.6) | 6.9 (5.0–11.1) | 6.1 (4.9–9.8) |
| Mean (SD) | 8.5 (8.5) | 9.6 (7.1) | 9.1 (7.8) |
| Range | 0.1–44.4 | 0.6–27.6 | 0.1–44.4 |
The median washout period was 6 weeks. In total, five participants started the trial treatment < 4 weeks after stopping using another PPI. Two were excluded from per-treatment analysis; the other three had between 1 day and 1 week of washout and were included.
Compliance with dosage of investigational medicinal product
The time (number of days) on the treatment (T) was calculated as the date of the 16-week follow-up clinic visit minus the date of randomisation, when the trial drug was dispended. The assumed dose is time on the treatment (T) multiplied by 2 to establish the number of capsules expected to have been taken from commencement of treatment (nE). The number of capsules received by patients (nP) after randomisation (238 in each kit) was not explicitly recorded in MACRO and was therefore imputed for each patient. The number of capsules returned (nR) at the 16-week follow-up visit was recorded in MACRO. The observed dose is the number of capsules taken during the course of treatment (nO) is calculated as (nP) – (nR). The percentage of the protocol dose taken is calculated as (nO)/(nE) × 100.
Note that it is possible to have an assumed percentage dose of ≥ 100% if time on treatment × 2 is greater than the number of capsules provided in the kit. This is truncated to be 100% in Table 37, which shows assumed doses taken based on time between dispensing and primary end-point visit, when unused capsules were returned.
| Dose | Treatment group | Total (N = 262) | |
|---|---|---|---|
| Lansoprazole (N = 126) | Placebo (N = 136) | ||
| n (%) of patients assumed to have taken full or partial dose | |||
| 100% | 50 (40) | 61 (45) | 111 (42) |
| 90–99% | 35 (28) | 38 (28) | 73 (28) |
| < 90% | 41 (33) | 37 (27) | 78 (30) |
| Assumed doses used | |||
| Median (IQR) | 222 (190–238) | 228 (206–238) | 224 (198–238) |
| % protocol dose | |||
| Median (IQR) | 97 (83–100) | 99 (89–100) | 99 (86–100) |
Forty-two per cent of patients reported taking the full dose, balanced across randomised groups, and 70% of patients reported taking ≥ 90% of the dose, balanced across groups. The median percentage taking the protocol dose is 99% (IQR 86–100%).
The maximum time on treatment is 17 weeks, as per IMP supplied. There were 81 participants, 45 in the lansoprazole group, who did not have any information for the number of capsules returned so were omitted from the per-treatment analysis group. The 81 participants comprised:
-
57 who consented to the trial but did not attend primary end-point visit 2
-
13 who attended the primary end-point visit, some with primary outcome measure, but had no information on returned capsules
-
six who did not attend the primary end-point visit, had no primary outcome measure or any information on returned capsules
-
five others, of whom three stopped owing to requirement for other medications; one had only a 2-day suspension of treatment; one was lost.
There were five participants with reasons for stopping taking trial medication but no indication as to how many capsules had been taken, or were returned. Three patients took no medication.
Reasons for discontinuing medication
Common reasons for discontinuing or interrupting IMP were as follows:
-
lansoprazole, n = 33 (some multiple)
-
rash, n = 3
-
upper gastrointestinal symptoms, n = 8
-
lower gastrointestinal symptoms, n = 6
-
holidays, n = 5 (mostly interruptions)
-
headaches; unrelated new medical event – miscarriage/diagnosis polyarthritis; erratic eating patterns; concern about medication load; lack of benefit; 1 week’s antibiotics, GP prescribed another PPI, belief that symptoms were endocrine in origin.
-
-
placebo, n = 30
-
upper gastrointestinal symptoms, n = 3
-
lower gastrointestinal symptoms, n = 2
-
holidays, n = 9 (mostly interruptions)
-
headaches (n = 3); breathing difficulty; erratic eating pattern; concern about medication load; lack of benefit; unrelated new medical events (n = 2); antibiotics or other temporary therapy needed (n = 3), prescribed another PPI.
-
Concomitant medication
Some form of antacid medication was taken by five participants out of the total cohort at baseline (four in the lansoprazole group). At the end of therapy, six of the lansoprazole group and seven of the placebo group were taking antacid remedies of one sort or another. This had changed to 30 and 25, respectively, at 12 months – a notable rise bearing in mind the smaller total sample at this later time point.
Appendix 3 Baseline data: itemised severity scores for Reflux Symptom Index; Reflux Finding Score scoring
Table 38 gives the itemised scores for the total population at baseline.
| RSI item | Mean | SD | Median | IQR |
|---|---|---|---|---|
| 1 – hoarseness | 2.4 | 1.6 | 3 | 1–4 |
| 2 – throat clearing | 3.4 | 1.3 | 4 | 3–4 |
| 3 – excess throat mucus | 2.9 | 1.6 | 3 | 2–4 |
| 4 – difficulty swallowing | 1.7 | 1.6 | 2 | 0–3 |
| 5 – coughing after eating or lying down | 2.1 | 1.6 | 2 | 0–3 |
| 6 – breathing difficulties | 1.6 | 1.6 | 1 | 0–3 |
| 7 – troublesome cough | 2.5 | 1.7 | 3 | 1–4 |
| 8 – lump in throat | 3.5 | 1.4 | 4 | 3–5 |
| 9 – heartburn | 1.8 | 1.6 | 1.5 | 0–3 |
There were a few very minor differences when considering the compliant population baseline RSI scores (Table 39).
| RSI item | Mean | SD | Median | IQR |
|---|---|---|---|---|
| 3 – excess throat mucus | 1.5 | |||
| 4 – difficulty swallowing | 1.6 | 2 | ||
| 5 – coughing after eating or lying down | 2.2 | 1.7 | 2 | 1–3.5 |
| 8 – lump in throat | 1.5 | |||
| 9 – heartburn | 1.9 | 2 |
Reflux Finding Score scoring schema and data recording
The RFS total values range between 0 and 26, including items for different subsites of the larynx, indicating level of abnormality. An outline of these areas and the range of scores for each item are shown in Table 40. 37 The ranking for each subsite is seen to be inconsistent. Some items have dichotomous scoring, others a 5-point score. The maximum score per feature is not uniform.
| Assessment item | Scoring options |
|---|---|
| Subglottic oedema | 0 absent, 2 present |
| Ventricular obliteration | 0 absent, 2 partial, 4 complete |
| Erythema/hyperaemia | 0 absent, 2 arytenoids involved, 4 diffuse |
| Vocal fold oedema | 0 absent, 1 mild, 2 moderate, 3 severe, 4 polypoid |
| Diffuse laryngeal oedema | 0 absent, 1 mild, 2 moderate, 3 severe, 4 obstructing |
| Posterior commissure hypertrophy | 0 absent, 1 mild, 2 moderate, 3 severe, 4 obstructing |
| Granuloma/granulation | 0 absent, 2 present |
| Thick mucus | 0 absent, 2 present |
Not all laryngeal images were rated using the RFS. Some images were unusable owing to poor quality, particularly from one of the sites where equipment was of a lower grade. In the early stages of the trial, there was a problem with matching the TOPPITS trial identifier to patients’ names and this created potential transcription errors before independent assessment. This issue was addressed in the early phase of the trial. Any rating with uncertainty of the identified subject was excluded from the analysis of the trial. Some images were submitted to the secure server twice and were consequently rated twice. When there were two ratings and confidence in the identified trial number, the chronologically first rating was used throughout.
In all, 363 assessments had both a validated identifier and a RFS. Of these, 107 were duplicates, leaving 256 unique identifiable RFS assessments.
In summary, the patient identifier attributable to a small number of images was not certain. There were also some unusable images owing to poor image quality, particularly from one of the sites where equipment was of a lower grade.
Appendix 4 Multivariable analysis of Reflux Symptom Index minus the heartburn/dyspepsia item for the compliant intention-to-treat group
Analysis of covariance for secondary analysis Reflux Symptom Index minus the heartburn/dyspepsia item: compliant intention-to-treat group
The outcome measure RSI-HB score after 16 weeks was initially analysed using ANCOVA methods in order to compare the 16-week RSI-HB scores between the treatment groups while adjusting for potential confounders. The ANCOVA results are shown in Table 41.
| Source | Partial SS | df | Mean square | F-ratio | p-value |
|---|---|---|---|---|---|
| Model | 4854.794 | 9 | 539.422 | 8.20 | < 0.001 |
| Arm | 329.700 | 1 | 329.700 | 5.01 | 0.026 |
| Site | 1646.931 | 7 | 235.276 | 3.58 | 0.001a |
| Baseline severity | 2880.361 | 1 | 2880.361 | 43.80 | < 0.001 |
| Residual | 13,809.206 | 210 | 65.758 | ||
| Total | 18,664.000 | 219 | 85.224 |
Adjusted for the effects of site and baseline severity, there is evidence of statistically significant difference in RSI-HB at 16 weeks between randomised treatment groups (p = 0.026) but not in favour of active intervention. There is evidence of dependence on baseline severity (p < 0.001). The randomised treatment group is not statistically significant when adjusted for other baseline factors. ANCOVA adjusted for stratification variables and other important baseline factors of RSI-HB for the compliant ITT group showed that the average RSI-HB score at 16 weeks in the lansoprazole and placebo groups remained no different once taking into account age, sex, smoking status, alcohol consumption and BMI.
Modelling was then undertaken as with the total RSI score.
Model 1
Model 1 adjusted for stratification factors used at randomisation [recruiting centre (as a random effect) and baseline severity as defined by the binary RSI-HB cut-off value of 20 (as a fixed effect) as covariates in the analysis] (Table 42).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 2.489 | 1.086 | 2.29 | 0.022 | 0.360 to 4.617 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –4.468 | 4.424 | –1.01 | 0.312 | –13.138 to 4.202 |
| Glasgow | 0.273 | 3.255 | 0.08 | 0.933 | –6.106 to 6.653 | |
| Manchester | 6.265 | 3.347 | 1.87 | 0.061 | –0.294 to 12.825 | |
| Newcastle | –0.542 | 2.852 | –0.19 | 0.849 | –6.131 to 5.047 | |
| Nottingham | –4.423 | 2.833 | –1.56 | 0.118 | –9.977 to 1.130 | |
| Stockport | –2.671 | 3.767 | –0.71 | 0.478 | –10.054 to 4.712 | |
| Sunderland | –1.992 | 3.010 | –0.66 | 0.508 | –7.892 to 3.907 | |
| RSI-HB baseline severity: severe (reference = mild) | Fixed | 7.490 | 1.106 | 6.77 | < 0.001 | 5.323 to 9.657 |
| Constant | 11.681 | 2.849 | 4.10 | < 0.001 | 6.097 to 17.264 | |
There is no statistically significant benefit of lansoprazole, although there is an apparent difference between randomised treatment groups when adjusted for site and baseline binary RSI-HB score. The estimated difference between randomised groups, when accounting for site and baseline severity, estimates that lansoprazole patients have RSI-HB scores at 16 weeks that are 2.5 points higher (worse) than those of placebo patients (95% CI 0.4 to 4.6; p = 0.02). RSI-HB at baseline is confirmed as being statistically significantly related to 16-week RSI-HB score and is justified as a stratification factor in the trial design. Patients with severe RSI-HB at baseline are estimated to have RSI-HB scores that are 7.5 points higher (worse) at 16 weeks. The underlying assumptions of the model were not substantially violated.
Model 2
Model 2 adjusted for the stratification factor recruiting centre (as a random effect) at randomisation and for baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect) (Table 43).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 2.011 | 1.020 | 1.97 | 0.049 | 0.012 to 4.010 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –0.094 | 4.201 | –0.02 | 0.982 | –8.327 to 8.139 |
| Glasgow | –0.363 | 3.040 | –0.12 | 0.905 | –6.321 to 5.596 | |
| Manchester | 4.317 | 3.140 | 1.37 | 0.169 | –1.838 to 10.472 | |
| Newcastle | –1.355 | 2.655 | –0.51 | 0.610 | –6.559 to 3.849 | |
| Nottingham | –4.155 | 2.662 | –1.56 | 0.119 | –9.371 to 1.062 | |
| Stockport | –2.067 | 3.537 | –0.58 | 0.559 | –8.999 to 4.865 | |
| Sunderland | –1.398 | 2.831 | –0.49 | 0.621 | –6.946 to 4.150 | |
| RSI-HB continuous baseline severity | Fixed | 0.668 | 0.074 | 9.01 | < 0.001 | 0.523 to 0.813 |
| Constant | 2.337 | 3.061 | 0.76 | 0.445 | –3.664 to 8.337 | |
When adjusted for site and continuous baseline severity RSI-HB, the placebo group again shows a greater reduction in symptoms. The estimated difference between randomised groups, when accounting for site and baseline severity, indicates that lansoprazole patients have RSI-HB scores at 16 weeks that are 2.0 points higher (worse) than those of placebo patients (95% CI 0.0 to 4.0; p = 0.05). A 1-unit increase in baseline severity is associated with a 0.67 increase (95% CI 0.5 to 0.8) in 16-week RSI-HB. As before, it is baseline RSI-HB score that remains the most important (positive) correlate of 16-week RSI-HB. The underlying assumptions of the model were not substantially violated.
Model 3
Model 3 adjusted for baseline severity (RSI-HB as a continuous measure) and age, sex, smoking status (binary), alcohol consumption (binary) and BMI (Table 44).
| Covariate | AIC | Beta | SE | Test statistic | p-value |
|---|---|---|---|---|---|
| Age | |||||
| Continuous | 1605.288 | –0.003 | 0.048 | –0.06 | 0.949 |
| Log-transformed | 1605.177 | ||||
| Complex transformation (age–2) | 1604.592 | ||||
| BMI | |||||
| Continuous | 1605.208 | 0.030 | 0.103 | 0.29 | 0.773 |
| Log-transformed | 1605.292 | ||||
| Complex transformation (BMI–2) | 1604.590 | ||||
| Sex (binary – male/female) | 1604.122 | 1.356 | 1.258 | 1.08 | 0.282 |
| Binary smoking (n = 219) | 1597.239 | 1.831 | 1.422 | 1.29 | 0.199 |
| Binary alcohol consumption (n = 217) | 1582.310 | –1.803 | 1.387 | –1.30 | 0.195 |
The reduction in AIC through simple log or complex (fractional polynomial) transformation was not substantial. In order to build the most parsimonious, clinically interpretable model, age and BMI were retained as untransformed continuous covariates, under the assumption of linearity with outcome. As none of the potential covariates appears to have a significant univariate relationship, model 2 with continuous baseline severity (RSI-HB) and site as covariates remains the optimal model. A sensitivity analysis based on the pragmatic ITT group including all patients with their 16-week primary end-point visit taking place at any time did not change the conclusions.
Reflux Finding Score was not included in the multivariable model as it added no further beneficial fit to the data.
The results of the multilevel mixed-effect linear regression (model 2) for RSI-HB (see Table 44) was extended to include RFS baseline assessment scores and report the change in –2log-likelihood and significance of RFS as a covariate in the model.
Model 4
Model 4 is adjusted for baseline RSI-HB (continuous measure), other important baseline factors and RFS baseline assessment. RFS was explored for suitable first-order fractional polynomial transformations (Table 45).
| Covariate | AIC | Beta | SE | Test statistic | p-valuea |
|---|---|---|---|---|---|
| RFS | |||||
| Continuous | 1237.800 | 0.195 | 0.193 | 1.01 | 0.314 |
| Log-transformed (RFS + 0.0001) | 1236.033 | ||||
| Complex transformation (RFS0) | 1236.830 | ||||
As the reduction in AIC through transformation was not substantial, the RFS was retained as an untransformed continuous covariate, under the assumption of linearity with outcome.
Building the optimal model was based on a forward selection method; –2log-likelihood was compared with a chi-squared distribution to assess variable inclusion. As the relationship is not significantly related to the RSI score at the primary end point, RFS was not included in the multivariable model because it added no further beneficial fit to the data.
Appendix 5 Secondary analysis of the primary outcome measure (Reflux Symptom Index): pragmatic and per-protocol groups
Pragmatic intention-to-treat group
The pragmatic analysis group comprises those participants completing the primary outcome visit at any time point after randomisation. All 283 participants had second visits, of whom 267 (94%) had usable RSI data. Of these, 135 (48%) were in the lansoprazole group and 148 (52%) were in the placebo group. All total RSI scores were in the range of 0–44, with 40% falling within the RSI population reference range (0–12), balanced across randomised groups. The means and SDs are reported (Table 46), with medians, IQRs and ranges, as the RSI score is treated as a continuous measure but is integer in nature.
| RSI | Time point | |||
|---|---|---|---|---|
| Baseline (visit 1) | 16 weeks (visit 2) | |||
| Lansoprazole (n = 127) | Placebo (n = 140) | Lansoprazole (n = 127) | Placebo (n = 140) | |
| Median (IQR) | 21 (16–26) | 22 (16–27) | 15 (9–25) | 15 (8–23) |
| Mean (SD) [95% CI] | 21.7 (7.4) | 21.9 (7.0) | 17.1 (9.6) [15.5 to 18.8] | 16.0 (9.5) [14.4 to 17.6] |
| Range | 10–41 | 10–43 | 0–41 | 0–44 |
The RSI appears to have reduced from a median of 21 at baseline to a median of 15 at 16 weeks in both randomised groups. The underlying RSI distribution appeared to be sufficiently normally distributed (overall mean 16.5, median 15) for parametric analysis of the primary outcome (H0 = mean RSI scores at 16 weeks are equal for both groups; two-tailed p < 0.05).
The test statistic t = 1.020 and p = 0.1544, concluding that there is no statistically significant difference in the RSI score at 16 weeks between lansoprazole and placebo. As predicted, therefore, the reduction in RSI score from baseline to the primary end point was similar across the randomised groups (mean –4.6 in lansoprazole, –5.9 in placebo).
Multivariable analysis of the pragmatic intention-to-treat group
The RSI score after 16 weeks was initially analysed using ANCOVA methods in order to compare the 16-week RSI scores between the treatment groups while adjusting for the effects of the stratification factors at randomisation: recruitment centre and baseline severity (mild: RSI-HB ≤ 20, severe: RSI-HB > 20) (Table 47). The null hypothesis of equality, p = 0.05, applied throughout.
| Source | Partial SS | df | Mean square | F-ratio | p-value |
|---|---|---|---|---|---|
| Model | 5021.445 | 9 | 557.938 | 7.46 | < 0.001 |
| Arm | 138.600 | 1 | 138.600 | 1.85 | 0.175 |
| Site | 1726.855 | 7 | 246.694 | 3.30 | 0.002a |
| Baseline severity | 3475.861 | 1 | 3475.861 | 46.50 | < 0.001 |
| Residual | 19,211.146 | 257 | 74.752 | ||
| Total | 24,232.592 | 266 | 91.100 |
A further ANCOVA analysis considered age, sex, smoking status, alcohol consumption and BMI.
The hypothesis to be tested is H0: the average RSI scores at 16 weeks are equal for both groups (lansoprazole vs. placebo) with adjustment for baseline stratification variables and also other clinical and demographic baseline factors. A two-sided significance level of p < 0.05 is used throughout. Age and BMI were sufficiently normal to include as untransformed data. Alcohol consumption and smoking status had excessive zero scores, thus skewed to the left and so binary alcohol and smoking variables were created with response ‘yes’ or ‘no’ (Table 48).
| Source | Partial SS | df | Mean square | F-ratio | p-value |
|---|---|---|---|---|---|
| Model | 5184.009 | 14 | 370.286 | 4.86 | < 0.001 |
| Arm | 82.303 | 1 | 82.303 | 1.08 | 0.300 |
| Site | 1605.675 | 7 | 229.382 | 3.01 | 0.005a |
| Baseline severity | 3298.724 | 1 | 3298.724 | 43.32 | < 0.001 |
| Age | 21.802 | 1 | 21.802 | 0.29 | 0.593 |
| Sex | 166.950 | 1 | 166.950 | 2.19 | 0.140 |
| Binary smoking | 14.841 | 1 | 14.841 | 0.19 | 0.659 |
| Binary alcohol | 36.450 | 1 | 36.450 | 0.48 | 0.490 |
| BMI | 11.665 | 1 | 11.665 | 0.15 | 0.696 |
| Residual | 18,809.583 | 247 | 76.152 | ||
| Total | 23,993.592 | 261 | 91.929 |
Statistical modelling of the pragmatic intention-to-treat group
The analysis set is the pragmatic ITT group (n = 267). Analysis is carried out on a complete-case basis (analysing only those cases with complete information on all variables). Missing observations were examined to determine both the extent of and the reason for such omissions. Any missing data are described for each model. The use of multiple imputation techniques was considered for the secondary outcome data and covariate data. The rationale was that missing data to a sufficient extent of > 10% would necessitate imputation. Data were not missing to this extent for the pragmatic ITT group so multiple imputation was not used.
Multilevel mixed-effect linear regression was used to model the 16-week RSI outcome measure for the pragmatic group. Three models were developed.
Model 1
Model 1 adjusted for stratification factors used at randomisation: recruiting centre (as a random effect) and binary baseline severity as defined by the binary RSI-HB cut-off value of 20 (as a fixed effect) as covariates in the analysis. Modelling site as a random effect demonstrates no impact of site on RSI score at 16 weeks, as anticipated, and demonstrates the advantage of modelling these data more appropriately.
The RSI-HB score at baseline is confirmed as being statistically significantly related to 16-week RSI scores, and justified as a stratification factor in the trial design. Patients with ‘severe’ RSI scores at baseline are estimated to have RSI scores that are 7 points higher (worse) than patients with mild severity scores at baseline at 16 weeks.
There is no statistically significant difference between randomised groups, lansoprazole compared with placebo (p = 0.165), when adjusted for site and baseline binary RSI-HB.
Model 2
Model 2 adjusted for the stratification factor recruiting centre (as a random effect) at randomisation and for baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect). A 1-unit increase in baseline RSI-HB severity was associated with a 0.7-unit increase (95% CI 0.6 to 0.9) in 16-week RSI. There was no statistically significant difference between randomised groups, lansoprazole compared with placebo (p = 0.264), when adjusted for site and baseline continuous RSI-HB.
Model 3
Model 3 adjusted for baseline severity (RSI-HB as a continuous measure) and other important clinical and demographic baseline factors, specifically age, sex, smoking status, alcohol consumption and BMI. (Alcohol and smoking were treated as binary variables; see above.)
Conclusion
As none of the potential covariates appears to have a significant univariate relationship with the RSI score at the primary end point (p > 0.1 for all), model 2 with continuous baseline severity (RSI-HB) and site remains the optimal model.
Per-protocol population
During the DMC closed session held on 9 March 2018, the DMC discussed the different analysis groups. The chairperson felt that a sensitivity analysis including only patients who complied with the protocol (16 weeks ± 2 weeks) could be useful to support the primary analysis. The numbers available for this analysis are 178 out of 346 (51% of the trial population), with 82 out of 172 (48%) in the lansoprazole treatment group and 96 out of 174 (55%) in the placebo group.
The summary statistics showed the randomised groups to be similar at baseline and for the RSI score to reduce similarly in both randomised groups at 16 weeks (the primary outcome) (Table 49).
| RSI | Primary outcome measure: RSI 16-week follow-up (visit 2) | ||
|---|---|---|---|
| Treatment group | Total | ||
| Lansoprazole | Placebo | ||
| n | 82 | 96 | 178 |
| Median (IQR) | 15 (9–26) | 13 (7–23) | 13.5 (8–24) |
| Mean (SD) | 16.9 (10.0) | 15.4 (10.1) | 16.1 (10.1) |
| 95% CI of mean | 14.7 to 19.1 | 13.4 to 17.5 | 14.6 to 17.6 |
| Range | 0–41 | 0–44 | 0–44 |
Overall, 47% of participants were within the normal range at the primary end point for the per-protocol population, balanced across randomised treatment groups. Proceeding to the previously employed multivariate strategies, the conclusion is not affected: site and baseline severity remain significantly related to 16-week RSI. Adjusted for the effects of stratification variables and other baseline factors, there was no statistically significant difference in 16-week RSI between randomised treatment groups (p = 0.586).
Statistical modelling of the per-protocol group
In the statistical modelling, as before, model 2 adjusted for the stratification factor recruiting site (as a random effect) at randomisation and for baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect). The log-likelihood statistics show that the continuous baseline severity substantially improves the model fit (reduction in –2log-likelihood) compared with the previous model with binary severity stratification factor above. A 1-unit increase in baseline severity is associated with a 0.7-unit increase (95% CI 0.5 to 0.9) in 16-week RSI. There is no statistically significant difference between randomised groups, lansoprazole compared with placebo (p = 0.522), when adjusted for site and baseline continuous RSI-HB. The estimated difference between randomised groups, when accounting for site and baseline severity, indicates that lansoprazole patients are estimated to have RSI scores at 16 weeks that are 0.81 points higher (worse) than placebo (95% CI –1.7 to 3.3; p = 0.52). As none of the potential covariates appeared to have a significant univariate relationship with the RSI score at the primary end point (p > 0.1 for all), model 2 with continuous baseline severity (RSI-HB) and site remains the optimal model.
Appendix 6 Secondary analysis of the primary outcome measure having excluded the heartburn component of Reflux Symptom Index (Reflux Symptom Index minus the heartburn/dyspepsia item): pragmatic group
Means, SDs, medians, IQRs and ranges are reported (Table 50) as the score is treated as a continuous measure but is integer in nature.
| RSI-HB | Time point | |||
|---|---|---|---|---|
| Baseline (visit 1) | 16 weeks (visit 2) | |||
| Lansoprazole group (n = 127) | Placebo group (n = 140) | Lansoprazole group (n = 127) | Placebo group (n = 140) | |
| Median (IQR) | 19 (15–24) | 20 (15–24) | 14 (9–23) | 13 (7.5–19.5) |
| Mean (SD) | 20.0 (6.9) | 20.0 (6.5) | 16.0 (9.0) | 14.3 (8.8) |
| 95% CI of mean | 18.8 to 21.2 | 18.9 to 21.1 | 14.4 to 17.5 | 12.8 to 15.8 |
| Range | 10–38 | 10–38 | 0–39 | 0–39 |
The randomised groups are similar in both treatment groups at baseline. A comparison of differences of unadjusted 16-week RSI-HB scores for pragmatic ITT group tested H0: the mean RSI-HB scores at 16 weeks are equal for both groups (lansoprazole vs. placebo; two-sided p < 0.05). The test statistic, t = 1.518, p = 0.130, concludes that there is no statistically significant difference in the RSI-HB scores at 16 weeks between lansoprazole and placebo.
As before, ANCOVA multivariate analysis was followed by statistical modelling. Model 2 adjusted for the stratification factor recruiting centre (as a random effect) at randomisation and for baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect). As none of the potential covariates appears to have a significant univariate relationship with the RSI-HB score at the primary end point (p > 0.1 for all), model 2 with continuous baseline severity (RSI-HB) and site remains the optimal model.
Appendix 7 Analysis of covariance of Reflux Symptom Index at 12 months
The outcome measure is RSI score after 12 months and it was initially analysed using ANCOVA methods, as detailed previously, in order to compare the 12-month RSI scores between the treatment groups while adjusting for potential confounders. Adjusted for the stratification effects of site and baseline severity, there was no statistically significant difference in RSI at 12 months between randomised treatment groups (p = 0.069). A secondary ANCOVA analysis considered age, sex, smoking status, alcohol consumption and BMI (Table 51).
| Source | Partial SS | df | Mean square | F-ratio | p-value |
|---|---|---|---|---|---|
| Model | 6034.890 | 14 | 431.064 | 5.66 | < 0.001 |
| Arm | 99.122 | 1 | 99.122 | 1.30 | 0.256 |
| Site | 2660.900 | 7 | 380.129 | 4.99 | < 0.001a |
| Baseline severity | 3019.474 | 1 | 3019.474 | 39.62 | < 0.001 |
| Age | 444.648 | 1 | 444.648 | 5.83 | 0.017 |
| Sex | 298.691 | 1 | 298.691 | 3.92 | 0.049 |
| Binary smoking | 4.754 | 1 | 4.754 | 0.06 | 0.803 |
| Binary alcohol | 58.667 | 1 | 58.667 | 0.77 | 0.382 |
| BMI | 123.473 | 1 | 123.473 | 1.62 | 0.205 |
| Residual | 12,422.082 | 163 | 76.209 | ||
| Total | 18,459.972 | 177 | 104.277 |
There was no statistically significant difference in RSI at 12 months between randomised treatment groups on ANCOVA (p = 0.256), but site and baseline severity remain significantly related to 12-month RSI. Age and sex also appear to be significantly related to 12-month RSI when all stratification and baseline characteristics are included in the model.
Model 1
Model 1 adjusted for stratification factors used at randomisation [recruiting centre (as a random effect) and binary baseline severity as defined by the binary RSI-HB cut-off value of 20 (as a fixed effect)] as covariates in the analysis (Table 52).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 2.469 | 1.311 | 1.88 | 0.060 | –0.100 to 5.038 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –19.058 | 6.094 | –3.13 | 0.002 | –31.003 to –7.113 |
| Glasgow | –8.713 | 4.942 | –1.76 | 0.078 | –18.400 to 0.973 | |
| Manchester | –0.717 | 4.923 | –0.15 | 0.884 | –10.367 to 8.933 | |
| Newcastle | –8.616 | 4.453 | –1.93 | 0.053 | –17.344 to 0.112 | |
| Nottingham | –13.138 | 4.461 | –2.95 | 0.003 | –21.881 to –4.395 | |
| Stockport | –10.709 | 5.177 | –2.07 | 0.039 | –20.856 to –0.563 | |
| Sunderland | –9.029 | 4.626 | –1.95 | 0.051 | –18.096 to 0.037 | |
| RSI-HB baseline severity: severe (reference = mild) | Fixed | 8.233 | 1.314 | 6.27 | < 0.001 | 5.658 to 10.808 |
| Constant | 19.149 | 4.405 | 4.35 | < 0.001 | 10.515 to 27.784 | |
There is no statistically significant difference between randomised groups, lansoprazole compared with placebo, when adjusted for site and baseline binary RSI-HB. The difference between randomised groups, when accounting for site and binary baseline severity, indicates that lansoprazole patients are estimated to have RSI scores at 12 months that are 2.5 points higher (worse) than those of placebo patients (95% CI –0.1 to 5.0; p = 0.06). The underlying assumptions of the model were not substantially violated.
Model 2
Model 2 adjusted for the stratification factor recruiting centre (as a random effect) at randomisation and for baseline severity in terms of RSI-HB as a continuous measure (as a fixed effect) (Table 53).
| Model | Type | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| Group: lansoprazole (reference = placebo) | Fixed | 1.730 | 1.222 | 1.42 | 0.157 | –0.666 to 4.126 |
| Site (reference = Birmingham) | ||||||
| Brighton | Random | –12.195 | 5.701 | –2.14 | 0.032 | –23.369 to –1.022 |
| Glasgow | –7.452 | 4.611 | –1.62 | 0.106 | –16.489 to 1.585 | |
| Manchester | –1.609 | 4.592 | –0.35 | 0.726 | –10.608 to 7.391 | |
| Newcastle | –7.999 | 4.153 | –1.93 | 0.054 | –16.139 to 0.141 | |
| Nottingham | –11.125 | 4.161 | –2.67 | 0.007 | –19.280 to –2.970 | |
| Stockport | –8.324 | 4.842 | –1.72 | 0.086 | –17.813 to 1.165 | |
| Sunderland | –6.729 | 4.313 | –1.56 | 0.119 | –15.182 to 1.724 | |
| RSI-HB continuous baseline severity | Fixed | 0.739 | 0.087 | 8.51 | < 0.001 | 0.569 to 0.909 |
| Constant | 7.188 | 4.483 | 1.60 | 0.109 | –1.598 to 15.974 | |
A 1-unit increase in baseline severity of RSI-HB is associated with a 0.7-unit increase in 12-month RSI score (95% CI 0.6 to 0.9). There is no statistically significant difference between randomised groups, lansoprazole compared with placebo, when adjusted for site and baseline continuous RSI-HB. The difference between randomised groups, when accounting for site and baseline severity, indicates that lansoprazole patients are estimated to have RSI scores at 12 months that are 1.7 points higher (worse) than those of placebo patients (95% CI –0.7 to 4.1; p = 0.157). The underlying assumptions of the model were not substantially violated.
Model 3
Model 3 adjusted for baseline severity (RSI-HB as a continuous measure) and other important clinical and demographic baseline factors, specifically age, sex, smoking status, alcohol consumption and BMI (Table 54).
| Covariate | AIC | Beta | SE | Test statistic | p-value |
|---|---|---|---|---|---|
| Age | |||||
| Continuous | 1330.032 | 0.013 | 0.05 | 0.22 | 0.828 |
| Log-transformed | 1330.079 | ||||
| Complex transformation (age3) | 1329.639 | ||||
| BMI | |||||
| Continuous | 1329.971 | 0.042 | 0.128 | 0.33 | 0.743 |
| Log-transformed | 1330.079 | ||||
| Complex transformation (BMI–2) | 1329.417 | ||||
| Sex (binary – male/female) | 1330.058 | 0.224 | 1.527 | 0.15 | 0.884 |
| Binary alcohol consumption (n = 176) | 1315.883 | –1.781 | 1.690 | –1.05 | 0.293 |
| Binary smoking (n = 177) | 1321.686 | 2.307 | 1.737 | 1.33 | 0.186 |
The reduction in AIC through simple log or complex (fractional polynomial) transformation was not substantial and continuous covariates were therefore retained as untransformed. As none of the potential covariates appears to have a significant univariate relationship with the 12-month RSI score, model 2 with continuous baseline severity (RSI-HB) and site remains the optimum model.
Appendix 8 Consideration of the weight of baseline Reflux Finding Score in modelling the primary outcome: Reflux Symptom Index at 16 weeks
Reflux Symptom Index was not included in the multivariable model because it added no further beneficial fit to the data.
The multilevel mixed-effect linear regression (model 2) for RSI-HB (see Table 53) was extended to include RFS baseline assessment scores and report the change in –2log-likelihood and significance of RFS as a covariate in the model.
Model 4 is adjusted for baseline RSI-HB (continuous measure), other important baseline factors and RFS baseline assessment. RFS was explored for suitable first-order fractional polynomial transformations (Table 55).
| Covariate | AIC | Beta | SE | Test statistic | p-valuea |
|---|---|---|---|---|---|
| RFS | |||||
| Continuous | 1237.800 | 0.195 | 0.193 | 1.01 | 0.314 |
| Log-transformed (RFS + 0.0001) | 1236.033 | ||||
| Complex transformation (RFS0) | 1236.830 | ||||
As the reduction in AIC through transformation was not substantial, RFS was retained as an untransformed continuous covariate, under an assumption of linearity with outcome.
Building the optimal model was based on a forward selection method; –2log-likelihood was compared against a chi-squared distribution to assess variable inclusion. As the relationship is not significantly related to the RSI score at the primary end point, RFS was not included in the multivariable model because it added no further beneficial fit to the data.
Appendix 9 Comprehensive Reflux Symptom Score total and subscale 16-week follow-up scores descriptive analysis: compliant intention-to-treat analysis group (n = 220)
The CReSS total and subscale scores at the primary end point (16 weeks) are summarised by randomised treatment group and overall using descriptive statistics in Table 56 and at the 12-month follow-up in Table 57. The means, SDs, medians, IQRs and ranges are reported as the score is treated as a continuous measure but is integer in nature. Box plots showing CReSS total and subscales at the three time points are shown in Figures 17–20.
| CReSS | Total (range: 0–170) | Oesophageal subscale (range: 0–85) | Upper airway subscale (range: 0–85) | Pharyngeal subscale (range: 0–25) | ||||
|---|---|---|---|---|---|---|---|---|
| Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | |
| n | 102 | 118 | 102 | 118 | 102 | 118 | 102 | 118 |
| Median (IQR) | 36 (15 to 55) | 27.5 (14 to 48) | 12 (4 to 26) | 11.5 (5 to 22) | 12 (5 to 21) | 8 (4 to 18) | 6 (2 to 10) | 4 (2 to 8) |
| Mean (SD) | 38.9 (27.7) | 34.7 (28.3) | 16.4 (14.7) | 16.2 (14.9) | 13.6 (9.8) | 11.3 (10.0) | 7.0 (5.6) | 5.7 (5.2) |
| 95% CI | 33.4 to 44.3 | 29.6 to 39.9 | 13.5 to 19.3 | 13.5 to 18.9 | 11.6 to 15.5 | 9.5 to 13.2 | 5.9 to 8.1 | 4.7 to 6.6 |
| Range | 2–140 | 0–158 | 0–75 | 0–76 | 0–38 | 0–45 | 0–21 | 0–23 |
| Factors | AIC | Beta | SE | Test statistic | p-valuea |
|---|---|---|---|---|---|
| Total CReSS | |||||
| Continuous | 1543.889 | 0.175 | 0.023 | 7.76 | < 0.001 |
| Log-transformed | 1550.044 | ||||
| Complex transformation (total CReSS0.5) | 1543.519 | ||||
| Oesophageal | |||||
| Continuous | 1571.588 | 0.230 | 0.044 | 5.22 | < 0.001 |
| Log-transformed | 1592.283 | ||||
| Complex transformation (oesophageal0.5) | 1569.072 | ||||
| Upper airway | |||||
| Continuous | 1534.297 | 0.502 | 0.059 | 8.53 | < 0.001 |
| Log-transformed | 1585.946 | ||||
| Complex transformation (upper airway2) | 1532.443 | ||||
| Pharyngeal | |||||
| Continuous | 1579.617 | 0.491 | 0.114 | 4.29 | < 0.001 |
| Log-transformed | 1590.116 | ||||
| Complex transformation (pharyngeal1) | 1579.617 | ||||
FIGURE 17.
The CReSS total scores at baseline and follow-up visits for the compliant ITT population (median, IQR and overall range). (a) Lansoprazole; and (b) placebo.

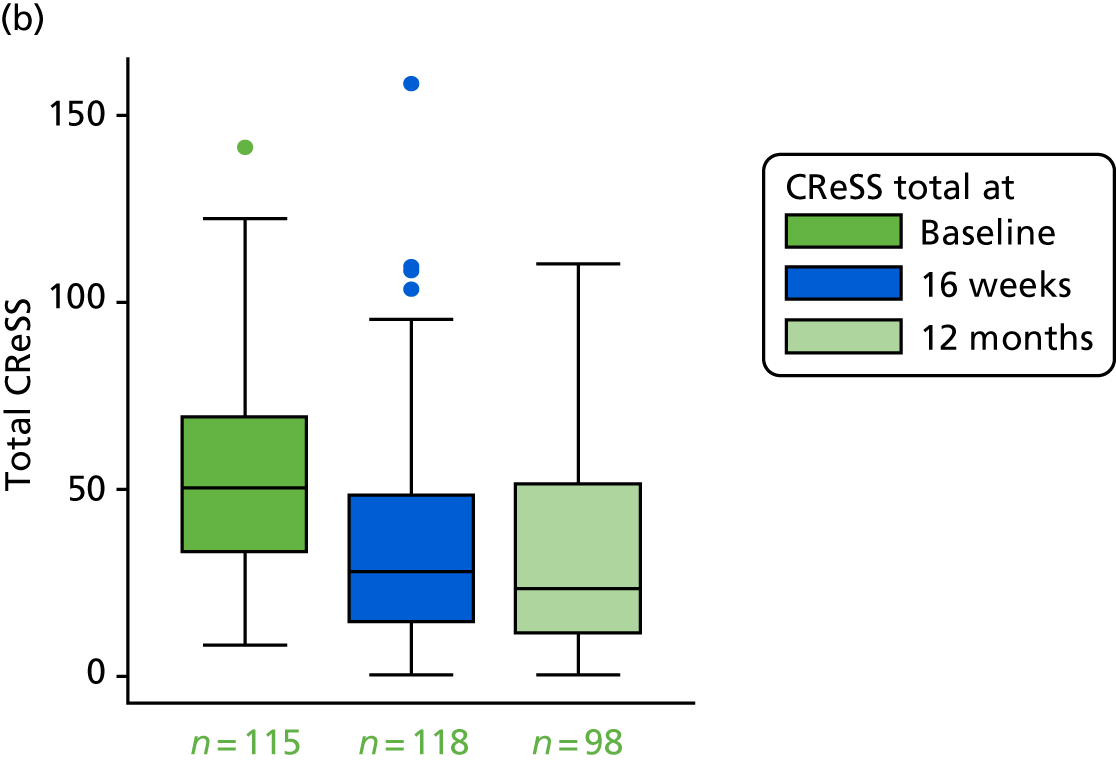
FIGURE 18.
The CReSS oesophageal subscale scores at baseline and follow-up for the compliant ITT population. (a) Lansoprazole; and (b) placebo. The 16-week and 12-month CReSS oesophageal subscale score is lower than at baseline in both treatment groups.
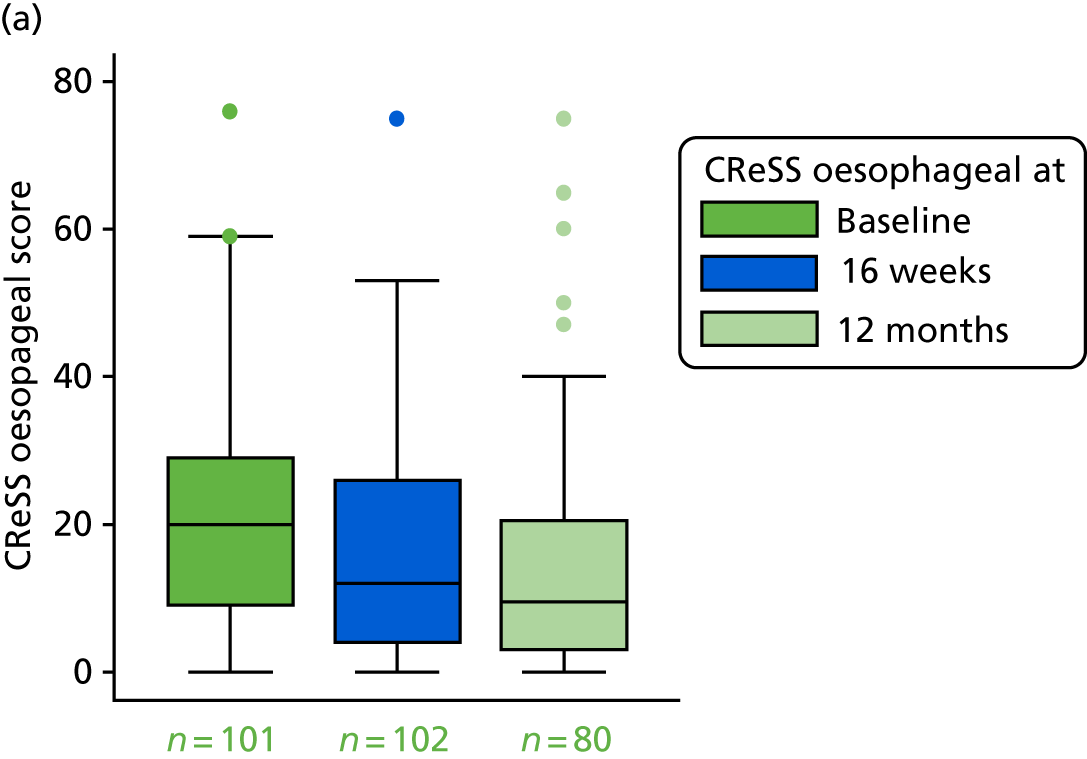
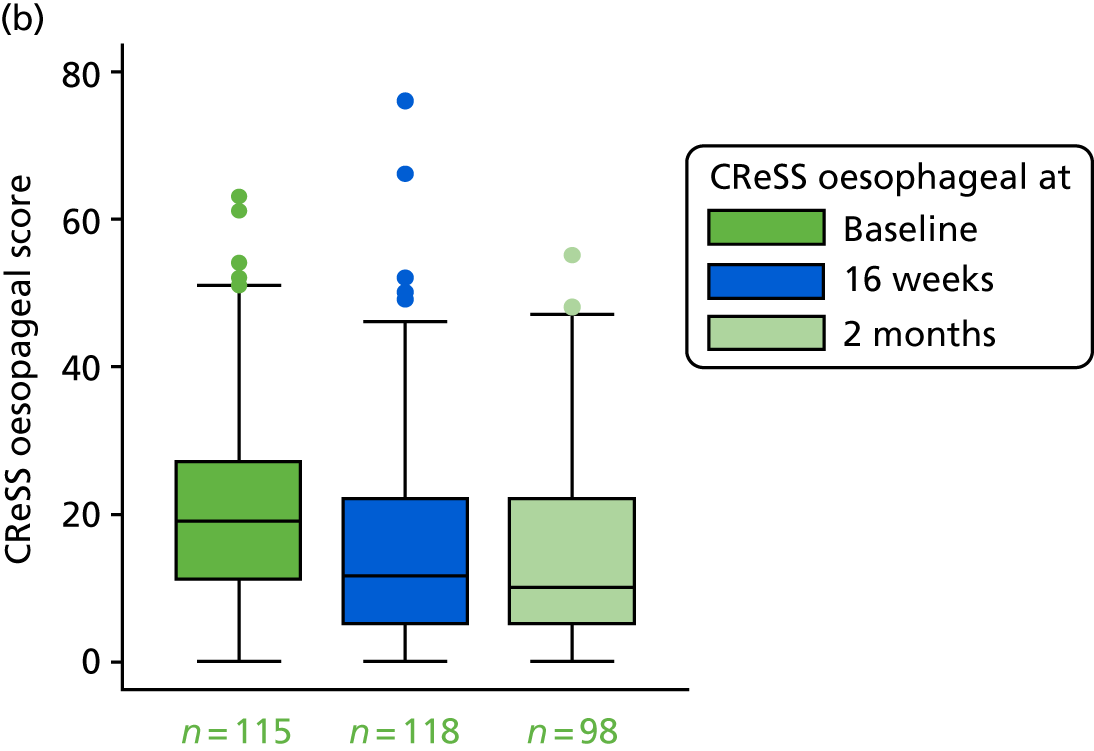
FIGURE 19.
The CReSS upper airway subscale scores at baseline and follow-up visits for the compliant ITT population. (a) Lansoprazole; and (b) placebo. The 16-week and 12-month CReSS upper airway score is lower than at baseline in both treatment groups.
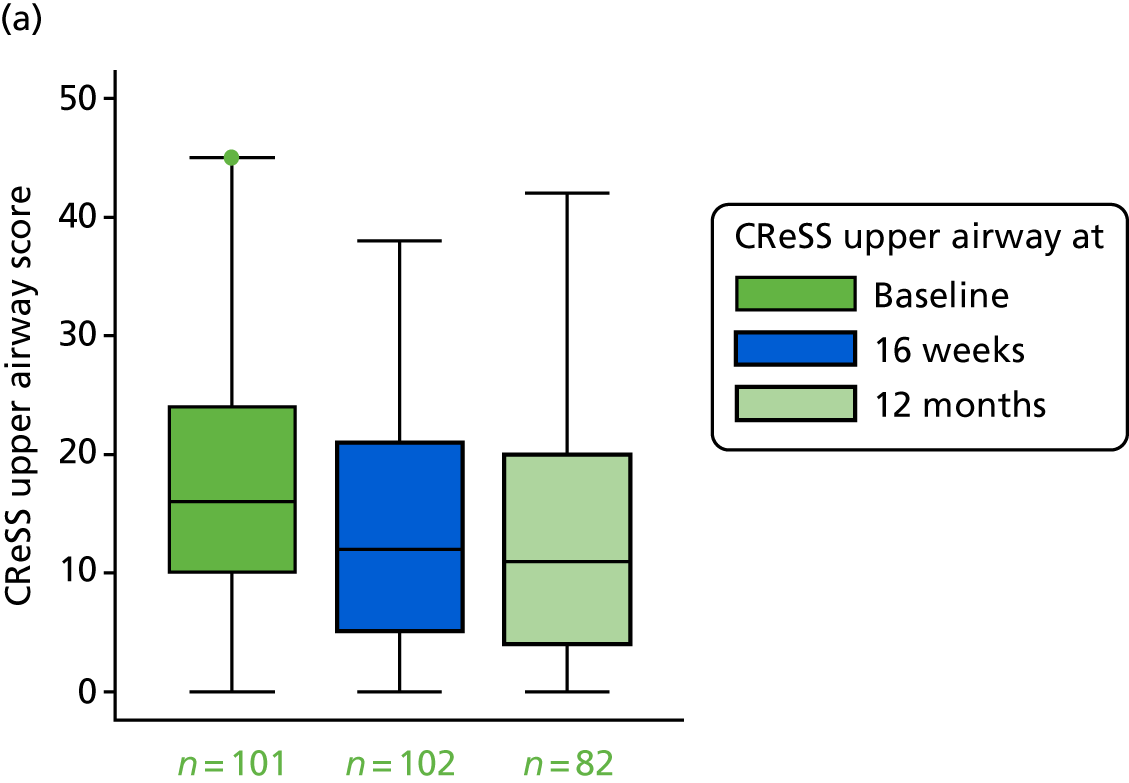
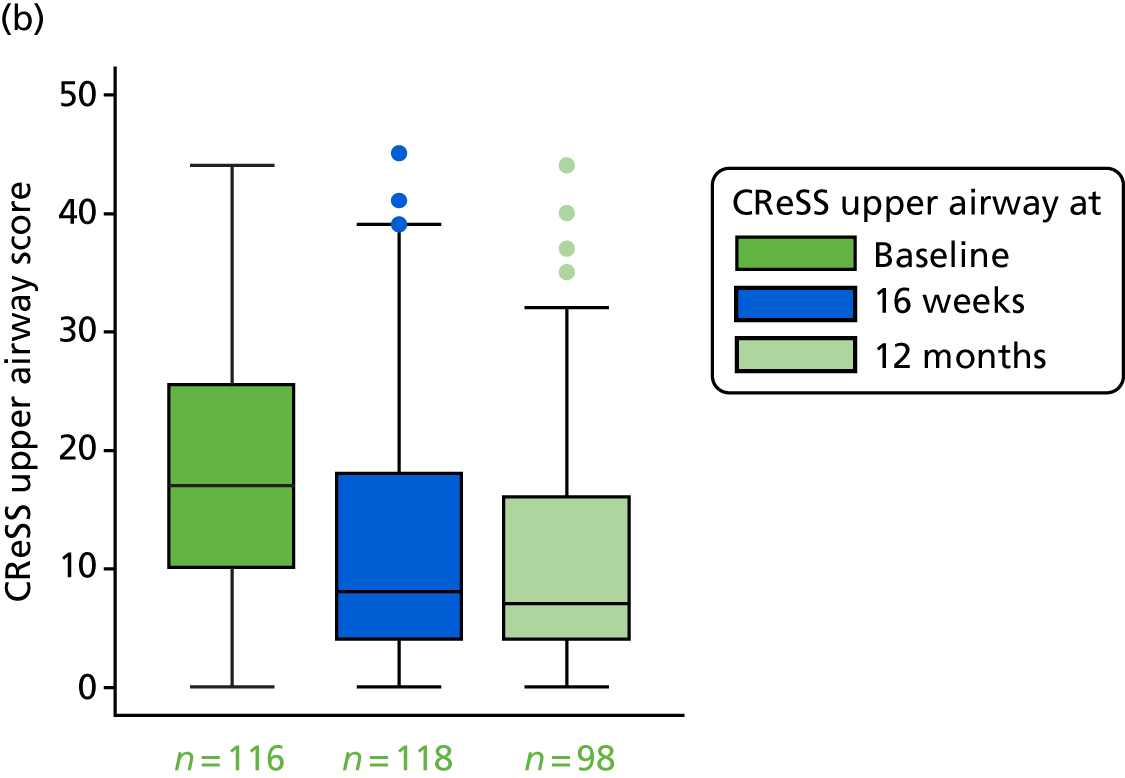
FIGURE 20.
The CReSS pharyngeal subscale scores at baseline and follow-up for the compliant ITT population. (a) Lansoprazole; and (b) placebo. The 16-week and 12-month CReSS pharyngeal subscale score is lower than at baseline in both treatment groups.


A higher CReSS indicates more severe symptoms. The CIs for lansoprazole and placebo overlap for all scales, showing that the differences are not significant. Summary statistics show CReSS total and subscale scores to reduce in both randomised groups at 16 weeks. Summary statistics also show CReSS total and subscale scores to continue to reduce in both randomised groups by 12 months (Table 58).
| CReSS | 12-month follow-up (visit 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (range: 0–170) | Oesophageal subscale (range: 0–85) | Upper airway subscale (range: 0–45) | Pharyngeal subscale (range: 0–25) | |||||
| Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | Lansoprazole group | Placebo group | |
| n (%) | 80 (78) | 98 (83) | 80 (78) | 98 (83) | 82 (80) | 98 (83) | 82 (80) | 98 (83) |
| Median (IQR) | 28 (15–53) | 23 (11–51) | 9.5 (3–20.5) | 10 (5–22) | 11 (4–20) | 7 (4–16) | 5 (2–10) | 3 (1–8) |
| Mean (SD) | 36.6 (30.7) | 31.8 (25.9) | 15.4 (16.0) | 15.3 (13.5) | 12.9 (11.0) | 10.3 (10.0) | 6.3 (5.8) | 4.6 (4.7) |
| 95% CI | 29.8 to 43.5 | 26.6 to 36.9 | 11.8 to 18.9 | 12.6 to 18.0 | 10.5 to 15.3 | 8.3 to 12.3 | 5.0 to 7.6 | 3.7 to 5.6 |
| Range | 0–153 | 0–110 | 0–75 | 0–55 | 0–42 | 0–44 | 0–23 | 0–22 |
The 16-week and 12-month total CReSS is lower than at baseline in both groups. The three subscale CReSSs are shown in Figures 18–20. All box plots show medians, IQRs and overall ranges.
There were two participants in the compliant ITT group with CReSS total scores completely missing at baseline. A further three participants had partially missing data. Two participants had CReSS oesophageal scores completely missing at baseline. Two participants had partially missing data. Two participants had CReSS upper airway scores completely missing at baseline. Another participant had partially missing data. Two participants in the compliant ITT group had CReSS pharyngeal scores completely missing at baseline.
The box plots show how the CReSS total and subscales change over the course of the trial visits, shown separately for the treatment groups. For all CReSS scales, a similar larger drop is seen between baseline and 16 weeks for both treatment groups.
Relationship of Comprehensive Reflux Symptom Score and Reflux Symptom Index (primary outcome)
Simple log and fractional polynomial transformations were considered for CReSS total and subscale scores to investigate better fit than under the assumption of linearity with RSI at 16 weeks (see Table 57), when the best-fitting transformation is selected based on a reduction in AIC. The reduction in AIC through simple log or complex (fractional polynomial) transformations was not substantial. In order to build the most parsimonious, clinically interpretable model, the CReSS total and subscale scores were retained as untransformed continuous covariates, under the assumption of linearity with outcome.
Comprehensive Reflux Symptom Score univariate analysis
The univariate analysis is based on fitting a univariate model of each baseline CReSS domain score as a potential predictor of RSI at 16 weeks (Table 59).
| Baseline severity measure | AIC | Beta | SE | Test statistic | p-value |
|---|---|---|---|---|---|
| Total CReSS | 1543.889 | 0.175 | 0.023 | 7.76 | < 0.001 |
| CReSS oesophageal | 1571.588 | 0.230 | 0.044 | 5.22 | < 0.001 |
| CReSS upper airway | 1534.297 | 0.502 | 0.059 | 8.53 | < 0.001 |
| CReSS pharyngeal | 1579.617 | 0.491 | 0.114 | 4.29 | < 0.001 |
| RSI-HB | 1524.924 | 0.754 | 0.082 | 9.24 | < 0.001 |
Baseline CReSS total and subscales appear to be significant predictors of the primary outcome (RSI) at 16 weeks. All increases in CReSS domain scores result in an increase in RSI. A 1-unit increase in (1) baseline CReSS total results in a 0.18-unit increase in RSI at 16 weeks, (2) baseline CReSS oesophageal subscale results in a 0.23-unit increase, (3) baseline CReSS upper airway subscale results in a 0.50-unit increase and (4) baseline CReSS pharyngeal results in a 0.49-unit increase. It appears that baseline RSI-HB remains the single most important predictor of the primary outcome – a 1-unit increase results in a 0.75-unit increase in 16-week RSI.
Comprehensive Reflux Symptom Score multivariable analysis
Table 60 summarises each of the baseline CReSS domains’ individual significance (presented with RSI-HB) when included individually in a multivariable model for RSI at 16 weeks, adjusted by site and baseline RSI-HB.
| CReSS domains in modela | AIC | Beta | SE | Test statistic | p-value | 95% CI (beta) |
|---|---|---|---|---|---|---|
| None | 1525.460 | |||||
| RSI-HB | 0.723 | 0.080 | 9.02 | < 0.001 | 0.565 to 0.879 | |
| Total CReSS | 1522.308 | 0.064 | 0.028 | 2.28 | 0.022 | 0.009 to 0.119 |
| RSI-HB | 0.568 | 0.104 | 5.47 | < 0.001 | 0.365 to 0.772 | |
| Oesophageal | 1523.397 | 0.085 | 0.042 | 2.03 | 0.043 | 0.003 to 0.168 |
| RSI-HB | 0.654 | 0.086 | 7.60 | < 0.001 | 0.485 to 0.822 | |
| Upper airway | 1521.063 | 0.206 | 0.081 | 2.55 | 0.011 | 0.047 to 0.364 |
| RSI-HB | 0.518 | 0.112 | 4.62 | < 0.001 | 0.299 to 0.738 | |
| Pharyngeal | 1527.460 | 0.001 | 0.113 | 0.01 | 0.995 | –0.220 to 0.221 |
| RSI-HB | 0.721 | 0.093 | 7.79 | < 0.001 | 0.540 to 0.903 | |
Baseline CReSS total and subscales, with the exception of the pharyngeal subscale, are significant predictors of RSI at 16 weeks, independent of and in addition to RSI-HB. A 1-unit increase in all baseline CReSS domains results in an increase in RSI at 16 weeks (total score, 0.06 increase; oesophageal subscale, 0.09 increase; upper airway, 0.2 increase).
Comparison of Comprehensive Reflux Symptom Score total and subscales and Reflux Symptom Index minus the heartburn/dyspepsia item as baseline severity measures
The baseline severity as provided by the baseline CReSS total and subscale scores were investigated to see if they explained variability in the data better than baseline RSI-HB. The modelling process for the compliant ITT group was repeated including CReSS baseline severity as an alternative to RSI-HB (Table 61).
| Multivariable modela baseline severity measure | AIC | Beta | SE | Test statistic | p-value |
|---|---|---|---|---|---|
| Total CReSS | 1548.332 | 0.164 | 0.023 | 7.18 | < 0.001 |
| CReSS oesophageal | 1572.542 | 0.210 | 0.044 | 4.81 | < 0.001 |
| CReSS upper airway | 1539.431 | 0.471 | 0.059 | 7.92 | < 0.001 |
| CReSS pharyngeal | 1578.926 | 0.444 | 0.110 | 4.02 | < 0.001 |
| RSI-HB | 1525.460 | 0.723 | 0.080 | 9.02 | < 0.001 |
The results in Table 61 show that all baseline CReSS total and subscales are significant predictors of the primary outcome when adjusted by site.
The model with a substantially better fit to the data lowest AIC is that including baseline RSI-HB severity measure with an AIC of 1525.4. The best-fitting model based on only baseline CReSS severity measures is the model including CReSS upper airway with an AIC of 1539.4. This covariate explains more variability in the RSI score at 16 weeks than the total CReSS but nevertheless performs less well than RSI-HB. The addition of CReSS upper airway score to RSI-HB, in a model adjusted by site, results in a better-fitting model with the lowest AIC of 1521.1 (see Table 60).
Appendix 10 Laryngopharyngeal Reflux – Health Related Quality of Life tabulated thermometer and domain scores
The groups are seen to be balanced, with similar scores across treatment groups and quality-of-life domains at baseline in the ‘thermometers’, which are part of the scoring system; a score of 3 or 4 indicates a small effect and scores of 2 and 1 indicate minimal to no effect on quality of life (Table 62).
| Scale | Treatment group | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lansoprazole | Placebo | ||||||||
| n | Median (IQR) | Range | n | Median (IQR) | Range | n | Median (IQR) | Range | |
| Voice | 101 | 2 (1–5) | 1–10 | 116 | 2 (1–5) | 1–10 | 217 | 2 (1–5) | 1–10 |
| Cough | 99 | 3 (1–6) | 1–10 | 116 | 2.5 (1–5) | 1–10 | 215 | 3 (1–5) | 1–10 |
| Clear | 101 | 4 (2–6) | 1–10 | 116 | 3 (1–6) | 1–10 | 217 | 4 (2–6) | 1–10 |
| General | 102 | 4 (2–7) | 1–10 | 116 | 3 (2–6) | 1–10 | 218 | 3.5 (2–6) | 1–10 |
At the end of treatment (primary end point) (Table 63), mean voice, coughing, clear throat, general subscale scores and overall scores were reduced in both groups.
| Scale | Treatment group | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lansoprazole | Placebo | ||||||||
| n | Median (IQR) | Range | n | Median (IQR) | Range | n | Median (IQR) | Range | |
| Voice | 100 | 2 (1–4) | 1–10 | 117 | 1 (1–3) | 1–10 | 217 | 1 (1–3) | 1–10 |
| Cough | 102 | 2 (1–4) | 1–10 | 117 | 1 (1–3) | 1–10 | 219 | 2 (1–4) | 1–10 |
| Clear | 102 | 2 (1–5) | 1–10 | 117 | 2 (1–5) | 1–10 | 219 | 2 (1–5) | 1–10 |
| General | 102 | 2 (1–5) | 1–10 | 117 | 2 (1–5) | 1–10 | 219 | 2 (1–5) | 1–10 |
There is a further reduction in the 12-month visits for scale and thermometer scores (Tables 64 and 65).
| Scale | Descriptive statistics | Scores | |||||
|---|---|---|---|---|---|---|---|
| Raw | Standardised | ||||||
| Treatment group | Total (n = 220) | Treatment group | Total | ||||
| Lansoprazole | Placebo | Lansoprazole | Placebo | ||||
| Voice (raw range: 0–72) | Number complete | 82 | 99 | 181 | 82 | 99 | 181 |
| Median (IQR) | 6 (6 to 11) | 6 (3 to 12) | 6 (6 to 11) | –0.42 (–0.42 to –0.11) | –0.32 (–0.68 to 0.41) | –0.32 (–0.42 to 0.05) | |
| Mean (SD) | 12.8 (16.0) | 8.6 (8.3) | 10.5 (12.5) | 0 (1) | 0 (1) | 0 (1) | |
| 95% CI | 9.3 to 16.3 | 7.0 to 10.3 | 8.7 to 12.3 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 | |
| Range | 0 to 68 | 0 to 39 | 0 to 68 | –0.80 to 3.46 | –1.04 to 3.67 | –1.04 to 3.67 | |
| Cough (raw range: 0–36) | Number complete | 82 | 99 | 181 | 82 | 99 | 181 |
| Median (IQR) | 3 (0 to 11) | 1 (0 to 6) | 2 (0 to 9) | –0.44 (–0.76 to 0.44) | –0.50 (–0.64 to 0.19) | –0.50 (–0.64 to 0.44) | |
| Mean (SD) | 7.0 (9.2) | 4.6 (7.2) | 5.7 (8.2) | 0 (1) | 0 (1) | 0 (1) | |
| 95% CI | 5.0 to 9.0 | 3.2 to 6.0 | 4.5 to 6.9 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 | |
| Range | 0 to 36 | 0 to 36 | 0 to 36 | –0.76 to 3.17 | –0.64 to 4.37 | –0.76 to 4.37 | |
| Clear (raw range: 0–36) | Number complete | 82 | 99 | 181 | 82 | 99 | 181 |
| Median (IQR) | 4.5 (0 to 10) | 2 (0 to 6) | 3 (0 to 9) | –0.32 (–0.87 to 0.35) | –0.39 (–0.70 to 0.25) | –0.39 (–0.70 to 0.25) | |
| Mean (SD) | 7.1 (8.2) | 4.4 (6.3) | 5.7 (7.3) | 0 (1) | 0 (1) | 0 (1) | |
| 95% CI | 5.3 to 8.9 | 3.2 to 5.7 | 4.5 to 6.7 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 | |
| Range | 0 to 30 | 0 to 26 | 0 to 30 | –0.87 to 2.80 | –0.70 to 3.41 | –0.87 to 3.41 | |
| General (raw range: 0–30) | Number complete | 81 | 99 | 180 | 81 | 99 | 180 |
| Median (IQR) | 3 (0 to 6) | 2 (0 to 6) | 2 (0 to 6) | –0.30 (–0.78 to 0.19) | –0.30 (–0.69 to 0.48) | –0.30 (–0.69 to 0.32) | |
| Mean (SD) | 4.8 (6.2) | 3.6 (5.1) | 4.1 (5.6) | 0 (1) | 0 (1) | 0 (1) | |
| 95% CI | 3.5 to 6.2 | 2.5 to 4.6 | 3.3 to 5.0 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 | |
| Range | 0 to 28 | 0 to 28 | 0 to 28 | –0.78 to 3.76 | –0.69 to 4.77 | –0.78 to 4.77 | |
| Overall (raw rescaled range: 0–100) | Number complete | 79 | 97 | 176 | 79 | 97 | 176 |
| Median (IQR) | 12 (2 to 22) | 7 (1 to 18) | 8 (2 to 20) | –0.30 (–0.73 to 0.15) | –0.35 (–0.68 to 0.23) | –0.35 (–0.69 to 0.23) | |
| Mean (SD) | 18.8 (22.5) | 13.9 (19.2) | 16.0 (20.8) | 0 (1) | 0 (1) | 0 (1) | |
| 95% CI | 13.7 to 23.8 | 10.0 to 17.8 | 13.0 to 19.2 | –0.2 to 0.2 | –0.2 to 0.2 | –0.1 to 0.1 | |
| Range | 0 to 91 | 0 to 94 | 0 to 94 | –0.83 to 3.23 | –0.72 to 4.15 | –0.83 to 4.15 | |
| Scale | Treatment group | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lansoprazole | Placebo | ||||||||
| n | Median (IQR) | Range | n | Median (IQR) | Range | n | Median (IQR) | Range | |
| Voice | 80 | 1 (1–3) | 1–10 | 98 | 1 (1–2) | 1–10 | 178 | 1 (1–3) | 1–10 |
| Cough | 81 | 2 (1–4) | 1–10 | 98 | 1 (1–2) | 1–10 | 179 | 1 (1–3) | 1–10 |
| Clear | 81 | 2 (1–5) | 1–10 | 99 | 1 (1–3) | 1–10 | 180 | 2 (1–4) | 1–10 |
| General | 81 | 2 (1–4) | 1–10 | 99 | 2 (1–3) | 1–9 | 180 | 2 (1–4) | 1–10 |
List of abbreviations
- AE
- adverse event
- AIC
- Akaike information criterion
- ANCOVA
- analysis of covariance
- BMI
- body mass index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CReSS
- Comprehensive Reflux Symptom Score
- DMC
- Data Monitoring Committee
- ENT
- ear, nose and throat
- EOR
- extraesophageal reflux
- GORD
- gastroesophageal reflux disease
- GP
- general practitioner
- HR
- hazard ratio
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ISF
- investigator site file
- ITT
- intention to treat
- LPR
- laryngopharyngeal reflux
- LPR-HRQL
- Laryngopharyngeal Reflux – Health Related Quality of Life
- MII-pH
- multichannel intraluminal impedance and pH
- NCTU
- Newcastle Clinical Trials Unit
- OR
- odds ratio
- PIS
- patient information sheet
- PPI
- proton pump inhibitor
- RCT
- randomised controlled trial
- RFS
- Reflux Finding Score
- RSI
- Reflux Symptom Index
- RSI-HB
- Reflux Symptom Index minus the heartburn/dyspepsia item
- SAE
- serious adverse event
- SAUC
- standardised area under the curve
- SD
- standard deviation
- SMD
- standardised mean difference
- TOPPITS
- Trial Of Proton Pump Inhibitors in Throat Symptoms
- TSC
- Trial Steering Committee
- TUF
- TOPPITS User Forum
- UOS
- upper oesophageal sphincter
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25030).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
