Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/07. The contractual start date was in January 2014. The draft report began editorial review in June 2019 and was accepted for publication in October 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Jayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Treatment options for faecal incontinence and rationale for SaFaRI clinical trial
Much of the text included in this chapter has been taken from the SaFaRI protocol. 1 The research team has previously published the protocol in the International Journal of Colorectal Disease. Reproduced with permission from Williams et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Introduction
Faecal incontinence (FI) is a distressing condition that affects between 5% and 10% of the adult population. It is more common in female patients and with advancing age, and is the second most common cause of admission to a nursing home. It has an impact on social, physical and mental well-being and is a substantial burden on NHS resources.
Current treatment options
Current treatment strategies for adult FI are summarised in the National Institute for Health and Care Excellence (NICE) 2014 guidance. 2 All patients should undergo a thorough history and physical examination to determine the nature and severity of the problem, and to identify a probable aetiological cause. Initial management consists of a combination of patient education, dietary modification and antidiarrhoeal medication. If this is unsuccessful, investigation in the form of endoscopic visualisation of the colorectum, anorectal manometry (pudendal nerve testing optional), and endoanal ultrasound is performed to further characterise the underlying disorder and inform treatment options.
Conservative therapies
Conservative therapies include pelvic floor retraining, with or without biofeedback therapy, and irrigation techniques (rectal or antegrade irrigation). Biofeedback therapy aims to increase the patient’s awareness of the muscles of continence and rectal sensation. Incontinent symptoms are improved in around 50% of patients, although there appears to be a significant placebo effect, with a marked decrease in efficacy on long-term follow-up. 3 Rectal irrigation, for example using the Peristeen® system (Coloplast, Humlebæk, Denmark), aims to clear the rectum and lower colon of faecal residue. In the short term it can have beneficial effects, but as a long-term solution patients frequently find it unacceptably time-consuming and inconvenient. Recently, there has been interest in the use of bulking agents to augment the anal sphincter. Data on the efficacy of these agents is limited, but they may have a role in controlling minor incontinence or ‘seepage’, or where an isolated sphincter defect is causing incomplete closure of the anal canal. 4
Surgical interventions
Surgical interventions are indicated for those patients with moderate to severe FI that is resistant to the conservative therapies listed in Conservative therapies.
Anterior sphincteroplasty, artificial bowel sphincter and dynamic graciloplasty
Anterior sphincteroplasty may be considered for patients with discrete sphincter defects, which occur typically as a result of obstetric injury. Through a perineal incision, the disrupted sphincter muscle is isolated and an overlapping sutured repair performed. Short-term results are reasonable, with 70% of patients reporting an improvement in continence; however, there is a drop-off in the longer term, with fewer than 50% of patients experiencing a benefit at 5 years. 5 Patients who do worse following anterior sphincteroplasty include those with coexistent pudendal neuropathy, multiple sphincter defects or sphincter atrophy, and irritable bowel syndrome. Because of the poor long-term results, there has been a move away from sphincter repair, except in well-defined cases, and an increased enthusiasm for sacral nerve stimulation (SNS).
Another surgical intervention which may be considered to treat FI is the artificial bowel sphincter (ABS). The ABS consists of (1) a fluid-filled silicone cuff placed around the anus, (2) a fluid-filled, pressure-regulating balloon positioned in the abdominal wall and (3) a manual pump connecting these components, placed in either the labia majora or the scrotum. When the cuff is inflated, the anal canal is sealed. The fluid is transferred to the balloon by the manual pump, deflating the cuff and opening the anal canal to allow defaecation. A successfully functioning device improves continence and quality of life (QoL); however, it is expensive, with the device alone costing around £4000. The main problem with the ABS is the high complication rate. Revisional surgery is needed in between 12.5% and 50% of cases, with explantation rates between 16.7% and 41.2%. 6 The majority of revisions are for cuff leaks that are thought to arise from microperforations caused by repeated cycles of inflation and deflation over a number of years. Most explantations are for infective complications. As a consequence, the ABS is not in common usage.
Dynamic graciloplasty involves mobilisation of the gracilis muscle from the inner thigh and wrapping around the anus to augment sphincter function. A neurostimulation device with an impulse generator is implanted to adapt the type II, fast-twitch muscle fibres to type I, slow twitch, fatigue-resistant fibres. The patient uses an external programming device to deactivate the electrical stimulation, relaxing the muscular contraction and enabling defaecation at a voluntary time. The success rate of the operation is between 40% and 60%. 7 Like the ABS, the main problem is the high complication (infections, 28%; device malfunction, 15%; and leg pain, 13%) and reintervention rates. The use of dynamic graciloplasty in the UK has largely been superseded by SNS.
Sacral nerve stimulation
Sacral nerve stimulation for FI was first described in 19958 and has grown in popularity, gaining NICE recognition as a minimally invasive treatment for moderate to severe FI. SNS works by a combination of anal sphincter augmentation and modulation of spinal/supraspinal pathways. It benefits from a two-stage procedure, which enables the patient to assess acceptability and the clinician to evaluate efficacy prior to commitment to a permanent and expensive implant. An initial percutaneous nerve evaluation, or temporary stimulation, is performed under local, regional or general anaesthetic as a day-case procedure. A fine needle is inserted percutaneously into the sacral foramina (S3 or S4) on both sides to determine the best response in terms of anal sphincter contraction and dorsiflexion of the great toe (S3 stimulation). Once a satisfactory response is obtained, the temporary electrode is inserted, secured to the skin and connected to an external test stimulator, allowing the patient to alter the stimulation voltage. The patient is asked to keep a bowel diary for the 2–3 weeks of stimulation, which allows the clinician to quantify the degree of response. A positive response is defined as a reduction in incontinence episodes or incontinence score of ≥ 50% during the stimulation period.
Around 70% of patients have a good response and proceed to a permanent implant. Of these, 10% never gain any significant improvement and 26% experience loss of efficacy, usually within the first year. 5,9–11 A further 2–5% suffer irresolvable complications and undergo explantation. Thus, from a decision-to-treat perspective, the long-term efficacy is around 45–50%. Overall, only 50% of patients thought to be eligible for SNS have a functioning device in the long term.
The reasons for loss of efficacy are not clear, but may relate to device malfunction or fibrosis of the stimulating electrode leading to loss of conduction. Pain or discomfort at the stimulator site, down the leg or into the vagina is another commonly reported complication, experienced by 38.1% of patients. Overall, only 58.5% of patients who have a permanent implant have a good or acceptable result in the medium term. 5
Although SNS is an effective treatment for FI, it is also very costly. The component costs alone (excluding other direct and indirect medical costs) are £200 for the test stimulation and £9393 for the permanent stimulator. 12 A European study has calculated the 5-year cumulative costs for SNS at €22,150 per patient, which compares with €33,996 for a colostomy and €3234 for conservative treatment. 13 Despite this, SNS has been shown to be cost-effective. The incremental cost-effectiveness ratio (ICER) for SNS is £25,070 per quality-adjusted life-year (QALY) gained, which is within the £30,000 per QALY threshold recommended by NICE as an effective use of NHS resources.
NICE first issued its guidance on SNS for FI in 200414 and concluded that current evidence on safety and efficacy appeared to support its use, but that the procedure should only be performed in specialist units by clinicians with a particular interest in the condition. A systematic review at that time included six case series and 266 patients. In patients who had permanent implants, complete continence was achieved in 41 to 75%, while 75 to 100% of patients experienced a decrease of ≥ 50% in the number of incontinent episodes. Improvements were noted in both disease-specific and general QoL scores. The most recent review, including 13 studies and 929 patients, has confirmed the short-term efficacy of SNS. 15 Although the extent of the therapeutic effect varied between studies, a significantly beneficial effect was noted. Functional improvement was observed in 77% with idiopathic FI, 76% in sphincter rupture/episiotomy, 78% after anal repair, and 73% after neurological injury. The benefit was not restricted to improved continence, with several studies showing a significant improvement in QoL. 9,10,13
FENIX™ continence restoration system (FENIX™ magnetic sphincter augmentation)
The FENIX™ continence restoration system, or FENIX™ magnetic sphincter augmentation (MSA) (Torax Medical, Minneapolis, MN, USA), is a device that has been designed to reinforce the native sphincter for the treatment of FI that is resistant to conservative therapies. It consists of a ring of 14–20 titanium beads with magnetic cores that are linked together to form a structure to be surgically placed around the anal sphincter complex. To defecate, the patient strains in a normal way and the force generated separates the beads to open the anal canal. Continence is restored by means of passive attraction of the beads. Once implanted, the device does not require patient input to function.
The FENIX MSA costs £4000. Data on efficacy are limited, but they suggest a ≥ 50% improvement in continence in 70% of patients. Complications can occur in around 20% of patients, leading to explantation in around 10%.
Preliminary results are promising, with 70% of patients reporting a benefit; however, studies have been small and a more rigorous evaluation is required prior to its widespread adoption.
The device is manufactured in different lengths to accommodate variations in anal canal circumference, and has been CE (Conformité Européenne) marked since November 2011. FENIX MSA has been used in selected European and US centres to support a feasibility trial and was first used in the NHS in 2013.
The available evidence on safety and efficacy is limited but encouraging. Barussaud et al. 16 published data on a series of 24 patients who were implanted with FENIX between 2008 and 2012. All patients were female, with a mean age of 64 years (range 35–78 years) and the mean duration of FI being 8.8 years (range 1–40 years). The mean follow-up was 17.6 months. There was one immediate postoperative complication: cardiac arrest due to drug intolerance. The patient recovered without further sequelae. Two patients (8.7%) had the device explanted, one for device separation and one for perineal abscess at 6 months post implant. The procedure was considered a failure for five patients (21%) due to a lack of improvement in FI symptoms. Bowel diary results showed a significant improvement in the number of weekly FI episodes, decreasing from 32 to 8 in a 3-week diary. The mean Wexner score [Cleveland Clinic Incontinence Score (CCIS)] was reduced significantly from 16 points at baseline to 7 points, 8 points and 5 points at 12, 24 and 36 months, respectively. All four domains of the faecal incontinence quality-of-life (FIQoL) questionnaire scores significantly improved and remained stable postoperatively compared with the score at baseline.
A retrospective, case-matched comparison of the FENIX MSA with the ABS (Acticon® Neosphincter; American Medical Systems, Minneapolis, MN, USA) in 20 patients with severe FI17 showed that the FENIX MSA and ABS produced similar significant improvements in FI and QoL. Compared with the ABS, the FENIX MSA was associated with a significantly shorter operating time (FENIX MSA: 62 minutes vs. ABS: 97.5 minutes; p = 0.0273) and length of hospitalisation (FENIX MSA: 4.5 days vs. ABS: 10 days; p = 0.001). No difference was observed in postoperative complications. The ABS was associated with more explants/revisions (FENIX MSA: 1 vs. ABS: 4; p = 0.830), a greater incidence in postoperative constipation, and was more expensive.
Permanent stoma
For patients for whom the above surgical attempts fail to restore normal continence, the options are limited. A permanent stoma (usually colostomy) is often the last resort for patients with intractable FI. It is an effective strategy, but one that carries psychological and physical morbidity. Although most patients adapt to a permanent stoma, there is a continual fear of appliance leakage that can have an impact on social functioning. Around 50% of permanent stomas are complicated by parastomal herniation that may require surgical intervention. Moreover, a stoma is not a cheap intervention, with the 5-year cumulative costs estimated at £28,000. 13
Rationale for the SaFaRI trial
New technologies have often been introduced into clinical practice without rigorous evaluation of safety, efficacy and cost-effectiveness. Objective assessment has been overlooked because of the intrinsic appeal of new innovation, the need to be a part of a ‘pioneering group’ or, worse, because of the financial incentives from industry. Once introduced, low-grade observational evidence is often used to keep practices going. As a result, it has often been easier to ‘stop them starting’ than to ‘start them stopping.’18 Ideally, any new technology introduced into clinical practice should be simultaneously evaluated, and in most cases the best way of doing this is by randomised comparison with an already established technique. The National Institute for Health Research Horizon Scanning Centre (NIHR HSC) was established to ‘supply timely information to key health policy and decision-makers within the NHS about emerging health technologies that may have a significant impact on patients or the provision of health services in the near future.’19 In May 2012, the NIHR HSC reported on the FENIX Continence Restoration System (FENIX MSA) and concluded that ‘in order to determine its potential place in the pathway of care for FI larger long term studies of the safety, effectiveness and cost-effectiveness of FENIX in comparison to existing treatments are needed.’20 Therefore, although FENIX MSA may have a role to play in the treatment of FI, the evidence was not robust enough to support its widespread adoption.
The SaFaRI trial was thus designed to undertake a rigorous, prospective assessment of the new FENIX MSA as it was adopted into the NHS. The aim was for reliable data, collected independently from commercial interests, to be made available on the safety and efficacy of the device. This would include information on safety, efficacy, QoL and cost-effectiveness. Important information would be gained on the costs associated with the device, enabling the ICER per QALY to be determined. This would allow health-care providers to make informed decisions about value for money and future provision of the technology.
Sacral nerve stimulation was chosen as the comparator to FENIX MSA as SNS is currently the preferred, and NICE-recommended,2 surgical intervention for FI that is resistant to conservative therapies; the NIHR HSC report19 from May 2012 also identified SNS as the preferred comparator for any randomised comparison with FENIX MSA.
Furthermore, the SaFaRI trial was designed to collect additional, important data about SNS. SNS is a costly yet effective treatment for FI; however, concerns have been expressed about the lack of efficacy when analysed on an intention-to-treat basis and the loss of efficacy on longer-term follow-up. The SaFaRI study provided an additional opportunity to clarify the indications for SNS and the indicators of success.
The opportunity also presented itself to comprehensively document, for the first time, the treatment and associated costs for patients for whom either SNS or FENIX MSA is not successful. In effect, these patients would provide comparative, longitudinal data of the patient pathway where FENIX MSA or SNS is either unsuitable or unavailable.
In addition to the costs detailed above, the health economics would provide data on the short- and long-term cost-effectiveness of FENIX MSA compared with SNS. Within the analyses, use of two measures of health-related QoL to produce QALYs, the Short Form questionnaire-12 items (SF-12) together with the EuroQol-5 Dimensions (EQ-5D), would allow assessment of the sensitivity of the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) to detect changes in FI, which is to date unproven. The disease-specific questionnaire chosen to assess QoL, the FIQoL questionnaire, collects important information on many social and psychological aspects of FI (shame, depression, enjoyment, etc.). These aspects of FI have received little previous recognition in the literature and remain poorly defined.
Methods
Aim and objectives
The overall objectives of the study were to:
-
determine the short-term safety and efficacy of FENIX MSA and SNS in adult FI
-
assess FENIX MSA and SNS in terms of impact on QoL and cost-effectiveness.
Aim
The aim was to conduct a thorough evaluation of the FENIX MSA device, compared with SNS, for the treatment of adult FI.
Primary outcome measure
The primary outcome measure was success, defined as device in use and ≥ 50% improvement in the participant-reported CCIS at 18 months post randomisation.
Secondary outcome measures
-
Length of hospital stay.
-
Complications.
-
Reinterventions.
-
Constipation.
-
QoL.
-
Cost-effectiveness.
Trial design
SaFaRI was a prospective, UK multisite, parallel-group, randomised clinical study investigating the safety and efficacy of the FENIX MSA for adult FI. The comparator was SNS, a preferred treatment recommended by NICE for the treatment of FI that is resistant to conservative therapies. 2 Participants were randomised on a 1 : 1 basis to receive either FENIX MSA or SNS.
Prior to randomising participants, all participating surgeons had to have performed a minimum of 10 permanent SNS implantations, observed a minimum of one FENIX MSA procedure and performed two FENIX MSA procedures under proctorship.
A registration phase was incorporated into the study design to enable surgeons without the required FENIX MSA experience prior to study participation to gain the relevant experience within the scope of the study. Within the registration phase, the first two eligible patients providing consent were registered to the study under the training surgeon’s name and received FENIX MSA implants (there was no randomisation in the registration phase); these two operations, which were performed under proctorship, were considered study training cases and were not included in the main study/main trial analysis. Once the required FENIX MSA experience had been obtained, the surgeon could progress to the randomisation phase.
The trial received national ethics approval in the UK. The trial conduct was overseen by an independent Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC). The trial had public and patient involvement during the trial design phase and throughout the course of the study. The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) register (16077538).
Participants
The inclusion criteria were as follows:
-
aged ≥ 18 years
-
able to provide written informed consent
-
FI for > 6 months
-
incontinent episodes of ≥ 2 per week
-
suitable candidate for surgery, as judged by the operating surgeon
-
suitable for either FENIX MSA or SNS (unless the patient was being registered as a training case, in which event they only needed to be suitable for the FENIX MSA)
-
anal sphincter defect < 180° as documented on endoanal ultrasound scan
-
able and willing to comply with the terms of the protocol including QoL questionnaires.
The exclusion criteria were as follows:
-
previous interventions for FI (i.e. SNS, FENIX MSA or ABS) (unless the patient was registered as a training case, in which event they could have had previous interventions for FI)
-
chronic gastrointestinal motility disorders causing incontinence due to diarrhoea
-
obstructed defaecation, defined as an inability to satisfactorily evacuate the rectum [it was recommended that the Obstructed Defecation Score (ODS) was calculated and was ≤ 8 for trial inclusion]
-
anal sphincter defect ≥ 180°, as documented on endoanal ultrasound scan
-
an electric or metallic implant within 10 cm of anal canal
-
co-existent systemic disease (e.g. scleroderma) affecting continence
-
active anorectal sepsis
-
diagnosis of colorectal or anal cancer within previous 2 years
-
external rectal prolapse
-
significant scarring of the anorectum that, as judged by the treating surgeon, would prohibit FENIX MSA implantation or put the patient at high risk of implant erosion
-
pregnancy (it was the local surgeon’s responsibility to assess pregnancy in women of childbearing potential)
-
immunocompromised, including haematological abnormalities and treatment with steroids or other immunomodulatory medicines
-
congenital spinal abnormalities, preventing SNS implantation
-
known requirement for future magnetic resonance imaging surveillance, which would be contraindicated in the presence of metallic implant
-
suspected or known allergies to titanium.
Interventions
Preoperative investigation and preparation were as per institutional protocol, which included, as standard practice, visualisation of the colorectum (flexible sigmoidoscopy as a minimum), anorectal manometry (pudendal nerve testing optional) and endoanal ultrasound.
Sacral nerve stimulation
Sacral nerve stimulation implantation was performed in accordance with each research site’s usual practice. SNS implantation is a two-stage procedure. As per standard care, a temporary device was implanted during a day-case procedure and the degree of response to the device recorded by the participant over the course of 2 weeks. Response was assessed in accordance with each research site’s usual practice. For the purposes of the trial, the CCIS was recorded at this time point regardless of how the response was assessed locally.
As per routine care, if the response was positive (defined as a ≥ 50% improvement in incontinence episodes or ≥ 50% improvement in CCIS), then a second day-case procedure was scheduled and a permanent SNS device was implanted. If the response was negative, the temporary device was removed and the participant did not receive any further study intervention but continued follow-up for the required 18-month period. Further treatment was as per standard practice but participants were not permitted to undergo FENIX MSA implantation during the 18-month post-randomisation follow-up period.
Postoperative care was as per routine care, but participants had to be reviewed at clinic for trial purposes 2 weeks postoperatively for both temporary and permanent device implants, and at 6, 12 and 18 months post randomisation as a minimum. Any further visits were according to local standard clinical practice, but were captured on the follow-up case report forms (CRFs).
FENIX magnetic sphincter augmentation
FENIX MSA implantation was usually performed during an in-patient stay (usually of 1–3 days). Participants for whom FENIX MSA failed were not permitted to undergo SNS during the 18-month follow-up period. No postoperative care was required above routine wound care, but participants had to be reviewed for trial purposes at 2 weeks postoperatively and at 6, 12 and 18 months post randomisation as a minimum. Any further visits were performed according to local standard clinical practice and were recorded on the follow-up CRFs.
Participant-completed questionnaires
Participants completed a number of questionnaires designed to capture FI symptoms prior to randomisation (baseline), at 2 weeks post operation and at 6, 12 and 18 months post randomisation:
-
The CCIS. 21 The CCIS assesses five parameters associated with incontinence – incontinence to solid, incontinence to liquid, incontinence to gas, use of pads, and lifestyle restriction. Each parameter is scored 0–4, with ‘0’ for never and ‘4’ for every day. The five parameters are added to give a total score out of 20.
-
The ODS. 22 The ODS consists of five items: excessive straining, incomplete rectal evacuation, use of enemas and/or laxatives, vaginal-anal-perineal digitations, and abdominal discomfort and/or pain. Each item is graded from 0 to 4 with a score ranging from 0 (no symptoms) to 20 (very severe symptoms).
-
The FIQoL questionnaire. 23 This questionnaire is composed of 29 items that make up four scales: lifestyle (10 items), coping/behaviour (9 items), depression/self-perception (7 items) and embarrassment (3 items). Scoring is derived from a participant-completed questionnaire that assesses the impact of FI on four domains of QoL. Scales range from 1 to 5, with 1 indicating a lower functional QoL. Scale scores are derived by averaging the response to all items in the scale.
-
Health and Social Care Resource Use. The questionnaire is composed of questions related to contact with primary, community and social care services. The questionnaire consists primarily of ‘tick-box’ completion questions.
-
SF-12. 24 The SF-12 is a 12-item subset of the Short Form questionnaire-36 items version 2 that measures the same eight domains of health. It is a brief, reliable measure of overall health status. It is useful in large population health surveys and has been used extensively as a screening tool.
-
EQ-5D-5L. 25 This is a well-validated questionnaire used to assess generic QoL; it provides a simple descriptive profile and a single index value for health status.
Participants completed all of the above listed questionnaires at baseline and at 6, 12 and 18 months post randomisation. In addition to these time points, participants completed the CCIS and the Health and Social Care Resource Use questionnaire at 2 weeks postoperatively (for temporary SNS and FENIX MSA only). For the permanent SNS, participants completed the Health and Social Care Resource Use questionnaire at 2 weeks postoperatively.
Summary of protocol changes
A summary of all substantial amendments to the SaFaRI protocol can be found in Table 1.
| Version and date | Summary of changes |
|---|---|
| V1.0, 9 April 2014 | N/A: original protocol submitted for ethics review |
| V2.0, 10 October 2014 |
|
| V3.0, 30 April 2015 |
|
| V4.0, 7 March 2016 |
|
| V4.0, 7 March 2016 | No change to protocol. Substantial amendment submitted to formally notify the REC of the early trial closure owing to the withdrawal from the market of the FENIX MSA device |
Early trial closure
On 23 March 2017, the SaFaRI trial team received formal notice from Torax Medical (the manufacturer of the FENIX MSA device) that the decision had been made to suspend the commercial sale of the FENIX MSA device in the UK and other European countries for ‘strategic and business reasons’. As a result of this, recruitment into the SaFaRI trial, regrettably, had to cease with immediate effect.
Following the withdrawal of the FENIX MSA device and with approval from the Research Ethics Committee, all patients who had consented for the trial up to 23 March 2017, and had been randomised to the FENIX MSA arm but had not yet had surgery, were given the chance to have the FENIX MSA device implanted if they still wished to proceed with the operation as randomised. Any patients not wishing to undergo a FENIX MSA implantation, in the light of the fact that the device had been withdrawn, were offered alternative FI treatment as per local standard practice (this included the option of undergoing the alternative study intervention, SNS).
In total, 99 participants were randomised into the SaFaRI trial and 23 participants were registered as FENIX MSA training cases. A consequence of recruiting only 99 patients out of the target sample size of 350 is that the study is substantially underpowered to detect differences between the treatment arms, in particular with respect to the primary end point. Although the recruitment total was significantly less than the originally planned sample size of 350 participants, it was felt that continuing to follow up all randomised participants until the end of the planned follow-up period (i.e. 18 months post randomisation) would still provide valuable data that, at the very least, could provide some initial evidence; at the time of trial closure, SaFaRI was also the largest randomised trial of SNS to date.
The National Institute for Health Research was amenable to the trial team’s proposal to continue with the planned follow-up period, and, as a result, all patients who had been randomised into the SaFaRI trial continued to be followed up until 18 months post randomisation. The last participant’s final follow-up took place in September 2018.
End points
Primary end point
The primary end point was success, defined as device in use and ≥ 50% improvement (between the baseline and 18-month scores) in the participant-reported CCIS, at 18 months post randomisation.
Secondary end points
Secondary end points included:
-
the safety of FENIX MSA or SNS, as judged by explant rates, operative (this included those occurring during theatre time and post-surgery hospital stay) and postoperative (up to and including 12 months from the date of the last study surgery) complications
-
change from baseline in generic and disease-specific QoL as measured by CCIS, ODS, FIQoL, EQ-5D-5L and SF-12 at 6, 12 and 18 months post randomisation
-
cost-effectiveness
-
success at 6 and 12 months as defined in the primary end point.
Sample size
A total of 350 participants were required to detect at least a 20% difference in the percentage of successes at 18 months post randomisation (where success was defined as device in use and ≥ 50% CCIS improvement from baseline) between FENIX MSA and SNS at a 5% level of significance, with 90% power, assuming approximately 40% success in the SNS arm and allowing for 20% loss to follow-up. However, the number of patients recruited was 99.
Randomisation
Following confirmation of written informed consent and eligibility, patients were randomised into the trial by authorised members of staff at the trial sites. Randomisation was performed centrally using the Clinical Trials Research Unit (CTRU) automated 24-hour telephone randomisation system. Authorisation codes and personal identification numbers (PINs), provided by the CTRU, were required to access the randomisation system.
Participants were randomised on a 1 : 1 basis to receive either FENIX MSA or SNS, and were allocated a unique study number. A computer-generated minimisation programme that incorporated a random element was used with the following minimisation factors:
-
treating surgeon
-
participant sex (male or female)
-
severity of incontinence (CCIS)
-
mild to moderate: CCIS ≤ 10 points
-
moderate to severe: CCIS > 10 points.
-
-
degree of anal sphincter defect on endoanal ultrasound
-
no anal sphincter defect
-
anal sphincter defect ≤ 90°
-
> 90° anal sphincter defect < 180°.
-
Blinding
The study was not blinded to participants, medical staff or clinical trial staff because of the difference between the two devices being compared (SNS treatment requires a temporary implant followed by a permanent implant if successful and involves patient input to function).
Statistical methods
Unless otherwise stated, all analyses were prespecified and conducted on the intention-to-treat population (i.e. all randomised participants were categorised into treatment groups based on their randomisation regardless of what treatment they subsequently received). All hypothesis tests were two-sided and conducted at the 5% level of significance. Estimates and their corresponding 95% confidence intervals (CIs) and p-values are presented for fixed effects. For all end points, missing outcome data were assumed to be missing at random, and the treatment effect was therefore estimated via maximum likelihood estimation using all participants with non-missing outcome data for non-longitudinal end points (this is referred to as a complete case analysis for the remainder of the report). All models were fitted using SAS® v9.4 (SAS Institute Inc., Cary, NC, USA). (SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.)
Primary end point: device in use and ≥ 50% improvement in CCIS at 18 months post randomisation
The primary analysis was a complete case analysis. Multilevel logistic regression was used to estimate the odds ratios between treatment groups for a ‘success’ in terms of the primary end point, adjusting for all minimisation factors. All minimisation factors were included as fixed effects, except randomising surgeon, which was included as a random effect. A random intercept model was fitted using maximum likelihood via adaptive quadrature, and all modelling was performed using the SAS v9.4 glimmix procedure.
A sensitivity analysis was performed to consider additional covariates in the primary analysis regression model that were thought to be related to patient outcome. These covariates were:
-
age (years)
-
body mass index (BMI)
-
American Society of Anesthesiologists (ASA) grade
-
aetiology of incontinence (obstetric trauma, idiopathic, iatrogenic, neurological conditions)
-
type of incontinence (urge predominant, passive predominant, mixed urge and passive)
-
ODS
-
FI medication.
Secondary end point: device in use and ≥ 50% improvement in CCIS at 6 months or 12 months post randomisation
Success at 6 or 12 months was analysed using a multilevel logistic regression model, adjusting for all of the minimisation factors, to estimate the odds ratios. All minimisation factors were included as fixed effects, except randomising surgeon, which was included as a random effect.
Secondary end point: intraoperative complications
Intraoperative complications were modelled using a multilevel logistic model to estimate the odds ratio between the treatment groups for whether or not participants had an intraoperative complication, adjusting for all minimisation factors. All minimisation factors were included as fixed effects, except randomising surgeon, which was included as a random effect with a random intercept.
Secondary end point: postoperative complications and reinterventions
Postoperative complications were modelled using a multilevel logistic model to estimate the odds ratio between the treatment groups for whether participants had a postoperative complication or not, adjusting for all minimisation factors. All minimisation factors were included as fixed effects, except randomising surgeon, which was included as a random effect with a random intercept and slope.
Secondary end point: device explants
The number of explants was analysed using a multilevel logistic regression model, adjusting for all of the minimisation factors, to estimate the odds ratios. All minimisation factors were included as fixed effects, except randomising surgeon, which was included as a random effect.
Quality-of-life end points
All QoL end points were modelled using a three-level multilevel model to account for the hierarchical nature of the repeated measures data and also for the clustering effect of the operating surgeon. All models were adjusted for the minimisation factors, with the minimisation factors included as fixed effects, except for the randomising surgeon, which was included as a random intercept.
Chapter 2 Results
Recruitment
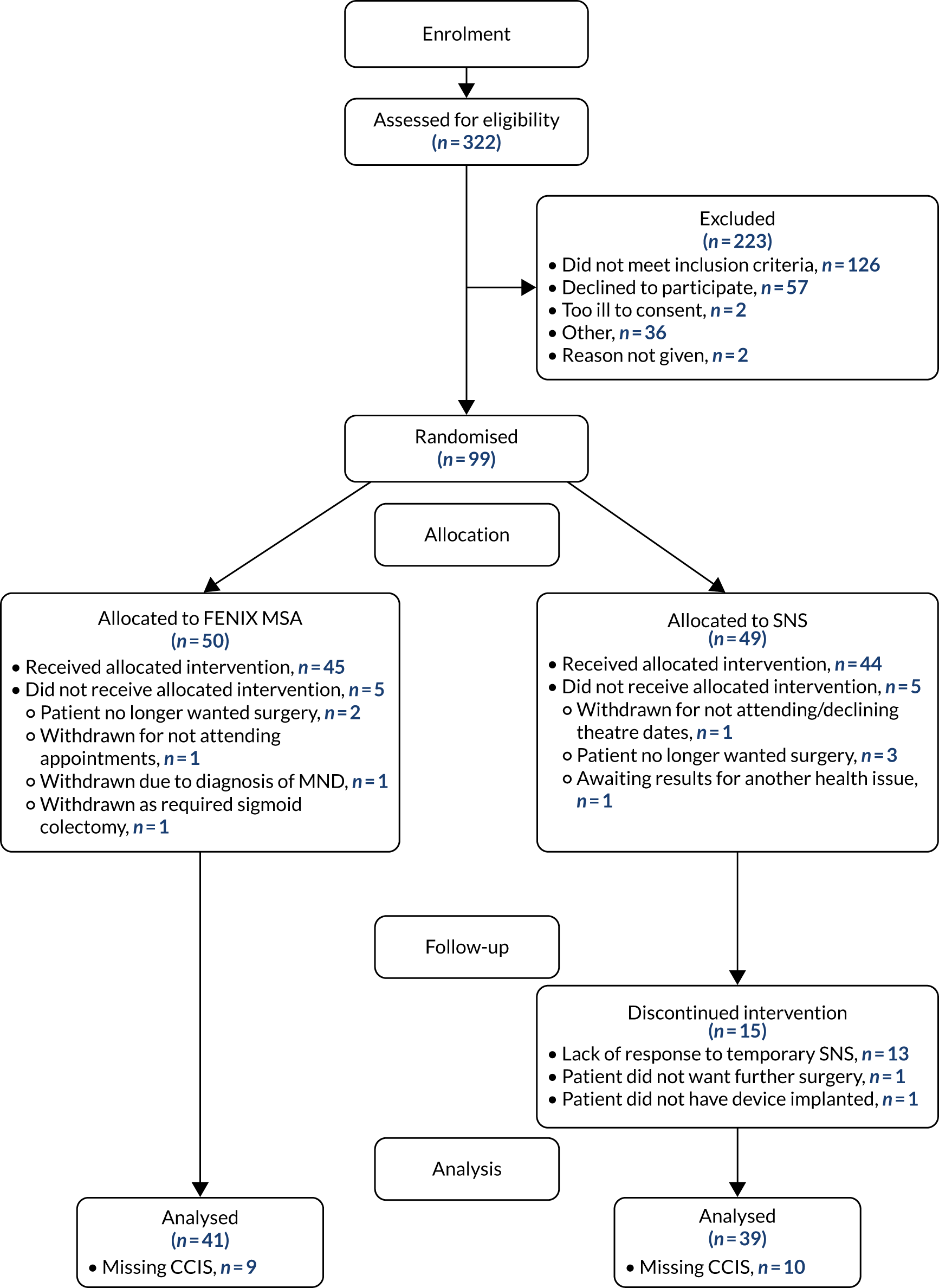
Between 30 October 2014 and 23 March 2017, 322 patients were assessed for eligibility across 18 sites. Ninety-nine of these patients were randomised into the SaFaRI study and 23 participants were registered as FENIX MSA training cases. Recruitment by site can be seen in Table 2. A Consolidated Standards of Reporting Trials (CONSORT) flow diagram showing all patients screened for eligibility can be seen in Figure 1.
| Site number | Site name | Randomised patients, n | Registered patients, n |
|---|---|---|---|
| 00050 | St James’s University Hospital | 45 | 1 |
| 00170 | University Hospital of North Durham | 4 | 1 |
| 00114 | Southampton General Hospital | 2 | 0 |
| 00232 | The Northern General Hospital | 14 | 0 |
| 00052 | St Peter’s Hospital | 5 | 0 |
| 00108 | Poole Hospital | 7 | 2 |
| 00002 | Royal Devon and Exeter Hospital | 3 | 0 |
| 00153 | The Churchill Hospital | 0 | 3 |
| 00172 | Wythenshawe Hospital | 4 | 0 |
| 00099 | Good Hope Hospital | 2 | 3 |
| 10908 | University College London Hospital | 0 | 2 |
| 00080 | Manchester Royal Infirmary | 4 | 0 |
| 00117 | Bristol Royal Infirmary | 4 | 2 |
| 00072 | Royal Victoria Infirmary | 2 | 2 |
| 00023 | Dewsbury District Hospital | 1 | 2 |
| 00317 | St Mark’s Hospital | 1 | 2 |
| 00031 | Leicester Royal Infirmary | 1 | 2 |
| 00118 | Derriford Hospital | 0 | 1 |
FIGURE 1.
The CONSORT flow diagram. MND, motor neuron disease.
Baseline data
The minimisation factors are summarised by treatment arm across all randomised patients in Table 3. Summaries of additional baseline characteristics are given in Table 4. All the minimisation factors and baseline characteristics are well balanced between the two treatment arms.
| Stratification factor | FENIX MSA, n (%) | SNS, n (%) | Total, n (%) |
|---|---|---|---|
| Surgeon ID | |||
| 2 | 10 (20.0) | 12 (24.5) | 22 (22.2) |
| 1 | 11 (22.0) | 8 (16.3) | 19 (19.2) |
| 3 | 6 (12.0) | 8 (16.3) | 14 (14.1) |
| 31 | 3 (6.0) | 4 (8.2) | 7 (7.1) |
| 26 | 2 (4.0) | 3 (6.1) | 5 (5.1) |
| 4 | 3 (6.0) | 1 (2.0) | 4 (4.0) |
| 12 | 3 (6.0) | 1 (2.0) | 4 (4.0) |
| 21 | 2 (4.0) | 2 (4.1) | 4 (4.0) |
| 30 | 2 (4.0) | 2 (4.1) | 4 (4.0) |
| 36 | 1 (2.0) | 3 (6.1) | 4 (4.0) |
| 32 | 2 (4.0) | 1 (2.0) | 3 (3.0) |
| 8 | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| 15 | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| 25 | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| 5 | 0 (0.0) | 1 (2.0) | 1 (1.0) |
| 23 | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| 999 | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| Sex | |||
| Female | 49 (98.0) | 47 (95.9) | 96 (97.0) |
| Male | 1 (2.0) | 2 (4.1) | 3 (3.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| CCIS | |||
| ≤ 10 points (mild to moderate) | 6 (12.0) | 5 (10.2) | 11 (11.1) |
| > 10 points (moderate to severe) | 44 (88.0) | 44 (89.8) | 88 (88.9) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| Anal sphincter defect | |||
| No anal sphincter defect | 27 (54.0) | 27 (55.1) | 54 (54.5) |
| ≤ 90° | 19 (38.0) | 18 (36.7) | 37 (37.4) |
| > 90° to < 180° | 4 (8.0) | 4 (8.2) | 8 (8.1) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| Characteristic | FENIX MSA | SNS | Total |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 60.6 (13.1) | 60.8 (14.3) | 60.7 (13.7) |
| Median (range) | 61.5 (30.0–82.0) | 59.0 (35.0–90.0) | 59.0 (30.0–90.0) |
| IQR | 50.0–71.0 | 52.0–72.0 | 52.0–71.0 |
| Missing | 0 | 0 | 0 |
| n | 50 | 49 | 99 |
| Ethnicity, n (%) | |||
| White | 47 (94.0) | 49 (100.0) | 96 (97.0) |
| Mixed | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Asian (Indian) | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Black | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| Incontinence type, n (%) | |||
| Mixed urge and passive | 16 (32.0) | 20 (40.8) | 36 (36.4) |
| Urge predominant | 13 (26.0) | 12 (24.5) | 25 (25.3) |
| Passive predominant | 8 (16.0) | 4 (8.2) | 12 (12.1) |
| Missing | 13 (26.0) | 13 (26.5) | 26 (26.3) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| FI aetiology, n (%) | |||
| Obstetric trauma | 29 (58.0) | 26 (53.1) | 55 (55.6) |
| Idiopathic | 11 (22.0) | 15 (30.6) | 26 (26.3) |
| Iatrogenic | 5 (10.0) | 1 (2.0) | 6 (6.1) |
| Neurological conditions | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Other | 4 (8.0) | 6 (12.2) | 10 (10.1) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| ASA grade, n (%) | |||
| 1 | 25 (50.0) | 23 (46.9) | 48 (48.5) |
| 2 | 24 (48.0) | 21 (42.9) | 45 (45.5) |
| 3 | 1 (2.0) | 5 (10.2) | 6 (6.1) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| Length of time suffered FI (months) | |||
| Mean (SD) | 71.6 (64.6) | 89.9 (90.8) | 80.6 (78.6) |
| Median (range) | 60.0 (9.0–384) | 60.0 (12.0–480) | 60.0 (9.0–480) |
| IQR | 36.0–84.0 | 27.0–114 | 36.0–96.0 |
| Missing | 0 | 1 | 1 |
| n | 50 | 48 | 98 |
| Average number of episodes per week | |||
| Mean (SD) | 7.4 (6.95) | 7.0 (7.01) | 7.2 (6.94) |
| Median (range) | 5.0 (2.0–28.0) | 4.0 (1.5–30.0) | 4.3 (1.5–30.0) |
| IQR | 3.0–7.0 | 2.0–10.0 | 2.5–8.0 |
| Missing | 1 | 0 | 1 |
| n | 49 | 49 | 98 |
| Resting pressure (cmH20) | |||
| Mean (SD) | 52.4 (28.2) | 58.4 (29.2) | 55.4 (28.7) |
| Median (range) | 48.0 (10.0–120) | 53.0 (19.0–133) | 51.5 (10.0–133) |
| IQR | 33.0–73.0 | 35.0–77.5 | 34.0–75.0 |
| Missing | 0 | 1 | 1 |
| n | 50 | 48 | 98 |
| Squeeze pressure (cmH20) | |||
| Mean (SD) | 77.0 (50.0) | 84.8 (42.0) | 80.8 (46.2) |
| Median (range) | 69.5 (7.0–269) | 85.5 (22.0–199) | 71.0 (7.0–269) |
| IQR | 44.0–105 | 49.0–116 | 46.0–109 |
| Missing | 0 | 1 | 1 |
| n | 50 | 48 | 98 |
| Threshold volume (ml) | |||
| Mean (SD) | 57.6 (35.0) | 53.1 (39.3) | 55.5 (36.9) |
| Median (range) | 52.0 (14.0–180) | 46.0 (10.0–200) | 47.0 (10.0–200) |
| IQR | 32.5–79.0 | 30.0–60.0 | 30.0–74.0 |
| Missing | 10 | 15 | 25 |
| n | 40 | 34 | 74 |
| Maximum tolerated volume (ml) | |||
| Mean (SD) | 127 (60.6) | 137 (69.1) | 131 (64.4) |
| Median (range) | 112 (35.0–270) | 117 (37.0–292) | 114 (35.0–292) |
| IQR | 85.0–160 | 77.0–195 | 80.0–180 |
| Missing | 10 | 15 | 25 |
| n | 40 | 34 | 74 |
| Internal anal sphincter defect, n (%) | |||
| Yes | 8 (16.0) | 10 (20.4) | 18 (18.2) |
| No | 41 (82.0) | 39 (79.6) | 80 (80.8) |
| Missing | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| External anal sphincter defect, n (%) | |||
| Yes | 20 (40.0) | 19 (38.8) | 39 (39.4) |
| No | 29 (58.0) | 30 (61.2) | 59 (59.6) |
| Missing | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
| ODS | |||
| Mean (SD) | 7.4 (3.78) | 7.1 (3.62) | 7.3 (3.68) |
| Median (range) | 7.0 (2.0–18.0) | 8.0 (2.0–17.0) | 7.0 (2.0–18.0) |
| Missing | 5 | 6 | 11 |
| n | 45 | 43 | 88 |
| BMI (kg/m2) | |||
| Mean (SD) | 28.8 (5.59) | 28.9 (5.78) | 28.9 (5.65) |
| Median (range) | 28.8 (19.1–53.0) | 28.7 (19.2–45.0) | 28.7 (19.1–53.0) |
| Missing | 1 | 0 | 1 |
| n | 49 | 49 | 98 |
| CCIS (points) | |||
| Mean (SD) | 14.4 (3.15) | 14.7 (2.95) | 14.6 (3.04) |
| Median (range) | 14.5 (6.0–20.0) | 15.0 (9.0–20.0) | 15.0 (6.0–20.0) |
| Missing | 4 | 3 | 7 |
| n | 46 | 46 | 92 |
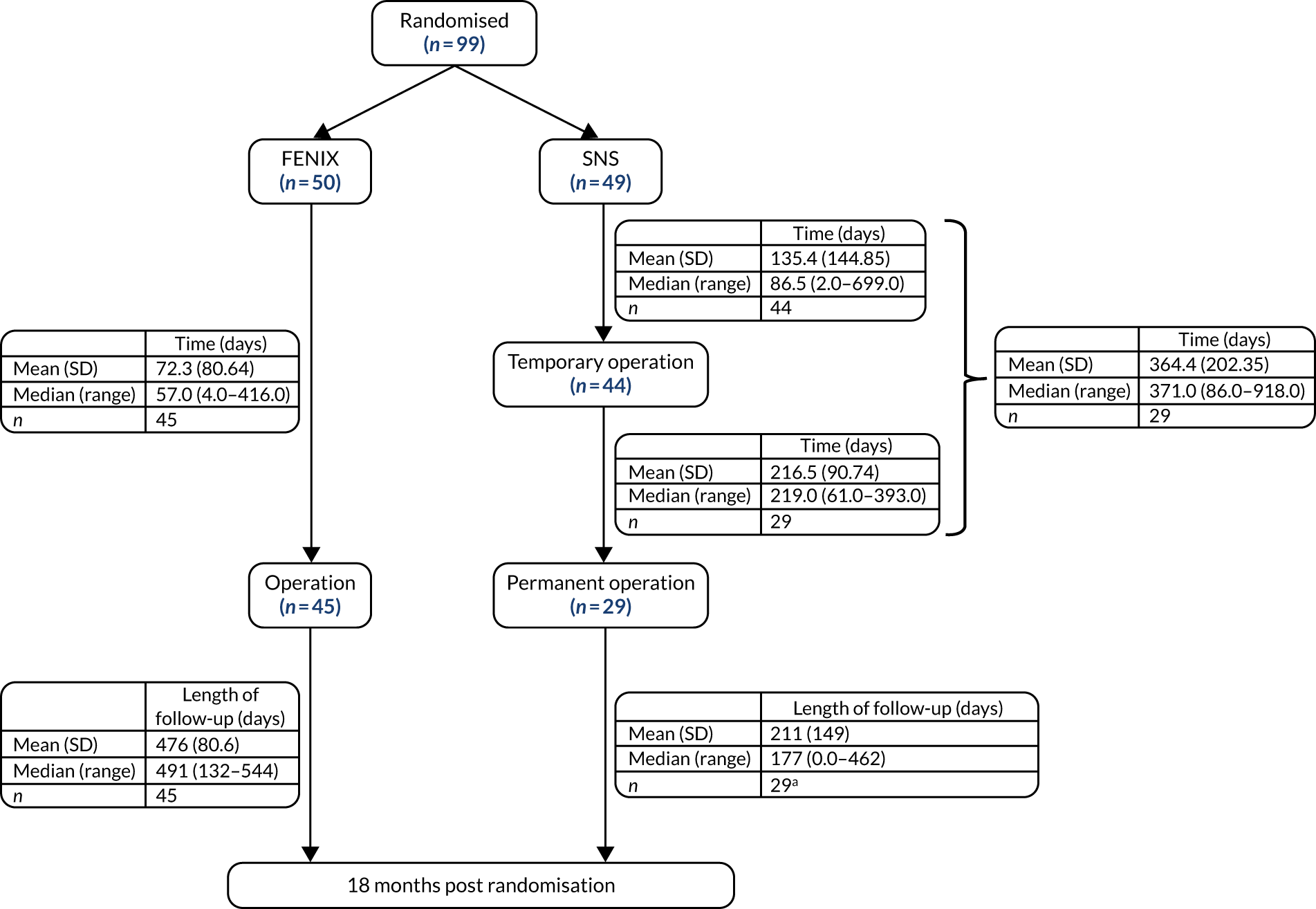
The time from randomisation to surgery can be seen in Figure 2. The time to implant of the permanent device was very different between the two treatment arms, with some SNS participants not receiving a permanent device until more than 18 months post randomisation. The reason for the delays in the SNS arm were mostly due to surgical capacity.
FIGURE 2.
Time from randomisation to surgery. a, Patients that received operation after 18 months’ follow-up have length of follow-up set to 0. SD, standard deviation.
Primary end point: device in use and ≥ 50% improvement in Cleveland Clinic Incontinence Score at 18 months post randomisation
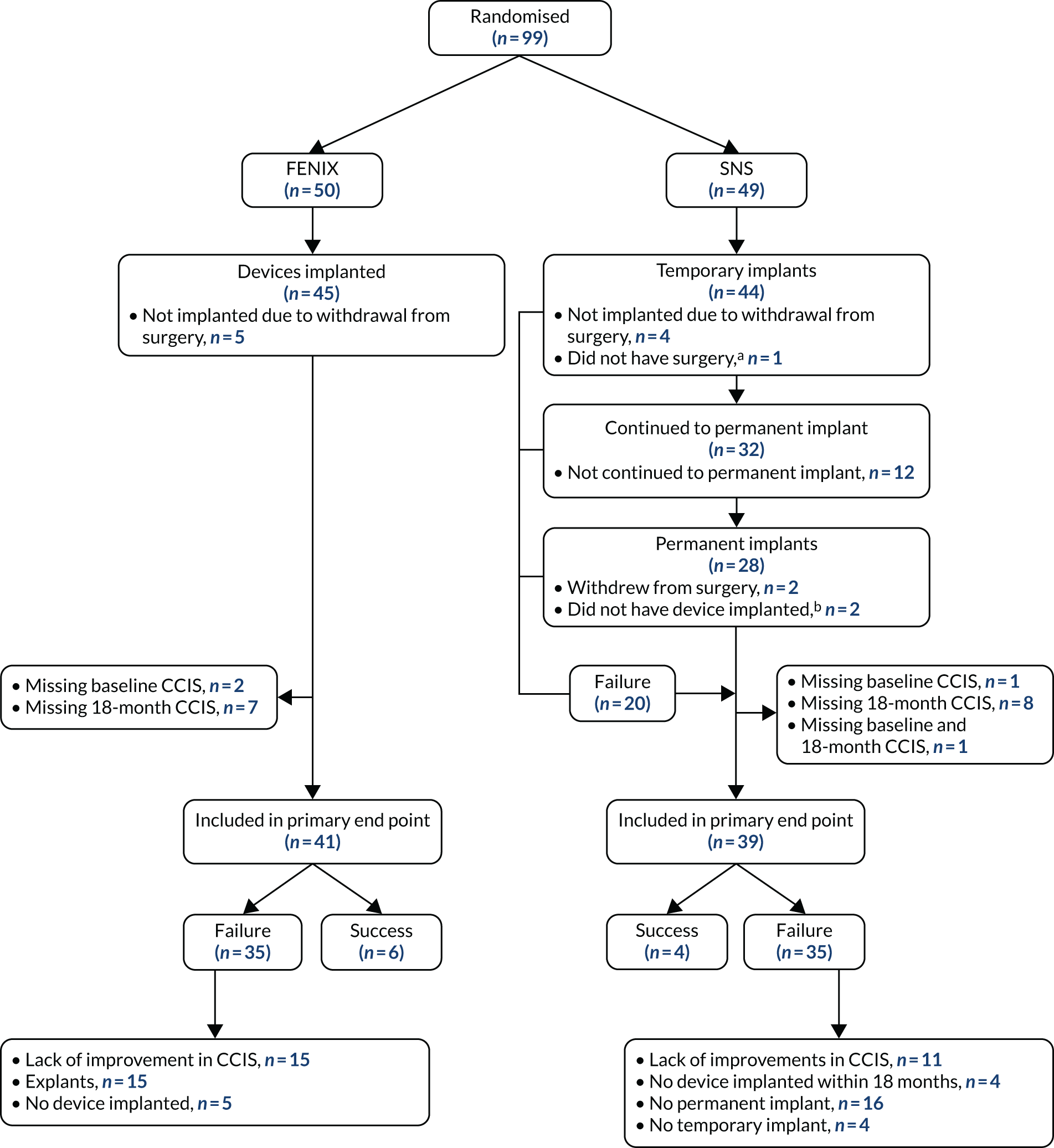
A total of 80 out of 99 (80.8%) participants were included in the primary analysis as 19 participants had missing primary outcome data. The pathway for participants in the primary analysis can be seen in Figure 3.
The characteristics for participants included in the primary analysis are presented in Table 5.
FIGURE 3.
Diagram of participants in primary analysis. a, Due to other health issues; b, due to complication during procedure.
| Variable | FENIX MSA | SNS | Total |
|---|---|---|---|
| CCIS at baseline (points) | |||
| Mean (SD) | 14.2 (3.23) | 14.6 (3.05) | 14.4 (3.13) |
| Median (range) | 14.0 (6.0–20.0) | 14.5 (9.0–20.0) | 14.0 (6.0–20.0) |
| Missing, n | 2 | 1 | 3 |
| n | 39 | 38 | 77 |
| CCIS at 18 months (points) | |||
| Mean (SD) | 11.3 (4.42) | 12.0 (4.57) | 11.7 (4.48) |
| Median (range) | 11.5 (3.0–17.0) | 12.0 (1.0–19.0) | 12.0 (1.0–19.0) |
| Missing, n | 13 | 6 | 19 |
| n | 28 | 33 | 61 |
| Sex, n (%) | |||
| Male | 1 (2.4) | 2 (5.1) | 3 (3.8) |
| Female | 40 (97.6) | 37 (94.9) | 77 (96.3) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| CCIS stratification factor, n (%) | |||
| ≤ 10 points (mild to moderate) | 6 (14.6) | 5 (12.8) | 11 (13.8) |
| > 10 points (moderate to severe) | 35 (85.4) | 34 (87.2) | 69 (86.3) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| Anal sphincter defect, n (%) | |||
| No anal sphincter defect | 22 (53.7) | 22 (56.4) | 44 (55.0) |
| ≤ 90° | 16 (39.0) | 13 (33.3) | 29 (36.3) |
| > 90° to < 180° | 3 (7.3) | 4 (10.3) | 7 (8.8) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| Randomising surgeon, n (%) | |||
| 1 | 10 (24.4) | 6 (15.4) | 16 (20.0) |
| 2 | 10 (24.4) | 10 (25.6) | 20 (25.0) |
| 3 | 5 (12.2) | 6 (15.4) | 11 (13.8) |
| 4 | 3 (7.3) | 1 (2.6) | 4 (5.0) |
| 5 | 0 (0.0) | 1 (2.6) | 1 (1.3) |
| 8 | 0 (0.0) | 1 (2.6) | 1 (1.3) |
| 12 | 2 (4.9) | 1 (2.6) | 3 (3.8) |
| 15 | 1 (2.4) | 1 (2.6) | 2 (2.5) |
| 21 | 2 (4.9) | 2 (5.1) | 4 (5.0) |
| 23 | 1 (2.4) | 0 (0.0) | 1 (1.3) |
| 25 | 0 (0.0) | 1 (2.6) | 1 (1.3) |
| 26 | 1 (2.4) | 2 (5.1) | 3 (3.8) |
| 30 | 0 (0.0) | 1 (2.6) | 1 (1.3) |
| 31 | 3 (7.3) | 2 (5.1) | 5 (6.3) |
| 32 | 2 (4.9) | 1 (2.6) | 3 (3.8) |
| Other | 1 (2.4) | 3 (7.7) | 4 (5.0) |
| Total | 41 (100) | 39 (100) | 80 (100) |
The primary end point was not evaluable for 19 out of 99 randomised patients: 9 out of 50 patients randomised to FENIX MSA and 10 out of 49 patients randomised to SNS (see Figure 3). For the remaining 80 out of 99 patients with an evaluable primary end point, the rate of ‘success’ was 10 out of 80 (12.5%) patients overall: 6 out of 41 (14.6%) patients in the FENIX MSA arm and 4 out of 39 (10.3%) patients in the SNS arm (Table 6). The unadjusted odds ratio was 1.50 (95% CI 0.39 to 5.78; p = 0.56).
| Success | FENIX MSA | SNS | Total |
|---|---|---|---|
| 18 months post randomisation, n (%) | |||
| Unsuccessful | 35 (85.4) | 35 (89.7) | 70 (87.5) |
| Successful | 6 (14.6) | 4 (10.3) | 10 (12.5) |
| Total | 41 (100) | 39 (100) | 80 (100) |
Summaries of the two individual components of the primary end point have been provided in Appendix 1, Tables 30–33.
The odds ratio adjusting for the minimisation factors was 1.45 (95% CI 0.36 to 5.83; p = 0.59).
The adjusted estimates of odds ratios and 95% CIs are presented in Table 7. The model shows no statistically significant differences between any of the minimisation factors, although this would be expected owing to the small number of patients recruited, which has led to large standard errors (SEs) of the estimates (i.e. wide CIs and underpowered hypothesis tests).
| Effect | Odds ratio | Odds ratio 95% CI | p-value |
|---|---|---|---|
| FENIX MSA (vs. SNS) | 1.453 | 0.362 to 5.827 | 0.5926 |
| Male (vs. female) | < 0.001 | < 0.001 to infinity | 0.9957 |
| Baseline CCIS > 10 points (vs. baseline CCIS ≤ 10 points) | 0.631 | 0.110 to 3.614 | 0.5993 |
| Anal sphincter defect ≤ 90° (vs. no defect) | 1.108 | 0.260 to 4.723 | 0.8883 |
| Anal sphincter defect > 90° to < 180° (vs. no defect) | 1.238 | 0.115 to 13.298 | 0.8579 |
The estimated random effects with respect to surgeons were equal to 0. The SE was not estimable owing to the low numbers of patients operated on by each surgeon (see Table 5).
In Appendix 1, Figure 30 shows the empirical probability plot for the primary analysis model, which can be used to compare actual Pearson residuals with expected Pearson residuals. The y-axis is the actual Pearson residual value, the x-axis is the empirical median Pearson residual expected under our fitted model assumptions. Each dot represents the actual Pearson residual for an individual patient. If the model fitted perfectly, we would expect all of the dots to lie on the reference line. The band in Appendix 1, Figure 30, represents the interval between the empirical 2.5th percentile and 97.5th percentile empirical Pearson residual. There are some areas where the reference line is outside the band, meaning that the model may not fit the data too well, but this is due to the small sample size and the rarity of successful outcomes.
In Appendix 1, Figure 31 presents the plot of exponentiated delta-betas (y-axis) versus patient identifier. Exponentiated delta-betas further from 1 indicate greater influence of the observation on the estimated treatment effect. Patients with a success for the primary end point are more influential than patients without success for the primary end point, which may be expected given that success was an uncommon occurrence. There does not appear to be any other observations with a large influence on the model.
Sensitivity analysis: additional covariates
Owing to the small number of ‘successes’, performing the sensitivity analysis with additional covariates that was described in the analysis plan was inappropriate.
Longitudinal analysis
As the primary end point was measured at 6, 12 and 18 months post randomisation, a longitudinal analysis using the data at each time point was performed. The model did not adjust for the stratification factors because of the added complexity and the number of missing data. In addition, the stratification factors caused the model to fail to converge.
The fixed effects of the model can be seen in Table 8. The estimated random effect caused by the operating surgeon was 1.52 (SE 0.99) and the estimated random effect caused by within patient measurements was 1.19. The model does not show a significant difference between the treatment arms (p = 0.20) or in change over time (p = 0.17), although, again, it is worth noting that the estimates have large SEs due to the small sample size.
| Effect | Estimate | Estimate 95% CI | p-value |
|---|---|---|---|
| FENIX MSA (vs. SNS) | 1.429 | 0.281 to 7.261 | 0.20 |
| Time (months) | 1.039 | 0.914 to 1.180 | 0.17 |
| Time and treatment interaction | 1.278 | 0.902 to 1.812 | 0.27 |
The model results can be seen in Figure 4.
FIGURE 4.
Longitudinal model results.

Secondary end point: device in use and ≥ 50% improvement in Cleveland Clinic Incontinence Score at 12 months post randomisation
Success at 12 months post randomisation was evaluable for 67 out of 99 (67.7%) participants. Thirteen patients (1 FENIX and 12 SNS) were not included in this analysis as they had not had a permanent device fitted within 12 months of randomisation because of surgical capacity in the trial sites. The other 19 patients were not included because of missing CCIS at baseline, at 12 months post randomisation, or at both time points.
A total of 4 out of 27 (14.8%) patients did not have a temporary SNS device fitted (and therefore did not have a permanent device implanted) because they withdrew from surgery before the temporary SNS operation. A total of 4 out of 40 (10%) patients in the FENIX arm did not have a FENIX device implanted as a result of withdrawing from surgery. Seventeen out of 27 (63%) patients did not have a permanent SNS device implanted because of either withdrawal (n = 5) or the lack of success of the temporary device (n = 12). Ten out of 40 (25%) patients who were randomised to FENIX had the device explanted; there were no explants in the SNS arm. The number of successes is summarised in Table 9. Owing to the small number of successes at 12 months, the models fitted were not meaningful and so are not presented.
| Success | FENIX MSA | SNS | Total |
|---|---|---|---|
| 12 months post randomisation, n (%) | |||
| Unsuccessful | 35 (87.5) | 26 (96.3) | 61 (91.0) |
| Successful | 5 (12.5) | 1 (3.7) | 6 (9.0) |
| Total | 40 (100) | 27 (100) | 67 (100) |
Secondary end point: device in use and ≥ 50% improvement in Cleveland Clinic Incontinence Score at 6 months post randomisation
Success at 6 months post randomisation was evaluable for 56 out of 99 (56.6%) patients. Twenty-three patients (3 FENIX and 20 SNS) were not included in this analysis because they had not had a permanent device fitted within 6 months of randomisation owing to surgical capacity at trial sites. The other 20 patients were not included because of missing CCIS at baseline, 6 months post randomisation, or at both time points.
A total of 3 out of 18 (16.7%) patients did not have a temporary SNS device implanted (and therefore no permanent SNS device was implanted) because of withdrawal from surgery. Two out of 38 (5.3%) of patients randomised to FENIX did not have a permanent device implanted because of withdrawal from surgery. Thirteen out of 18 (72.2%) patients did not have a permanent device fitted because of withdrawal (n = 4) or the lack of efficacy of the temporary SNS device (n = 9). Nine patients in the FENIX arm (76.3%) had the device explanted within 6 months of randomisation and there were no explants in the SNS arm. The number of successes in each arm is summarised in Table 10. Owing to the small number of successes at 6 months, the models fitted did not converge and so are not presented.
| Success | FENIX MSA | SNS | Total |
|---|---|---|---|
| 6 months post randomisation, n (%) | |||
| Unsuccessful | 33 (86.8) | 18 (100.0) | 51 (91.1) |
| Successful | 5 (13.2) | 0 (0.0) | 5 (8.9) |
| Total | 38 (100) | 18 (100) | 56 (100) |
Secondary end point: intraoperative complications
In total, 89 out of 99 patients (89.9%) had intraoperative complication data; 45 patients received an operation in the FENIX arm and 44 patients received an operation in the SNS arm.
There were four intraoperative complications in four patients; 3 out of 45 patients (6.7%) who were randomised to FENIX and received the operation had an intraoperative complication, and 1 out of 44 patients (2.3%) who were randomised to SNS and received at least one operation had an intraoperative complication, giving an overall complication rate of 4.5% (4/89) in randomised patients.
The complication in the SNS arm was an unexpected serious complication (USC). The details are as follows:
-
USC – anaphylaxis
-
Clavien–Dindo grade – IVb
-
USC description – patient became tachycardic, hypotensive and flushed following administration of Teicoplanin (Targocid, Sanofi, Paris, France) prior to their anaesthetic for insertion of SNS
-
Outcome – recovered.
There were three intraoperative complications in the FENIX arm, none of which was serious. The complications were bleeding, cyst found in recto vaginal septum, and rectal perforation.
Secondary end point: postoperative complications
A total of 85 out of 99 patients (85.9%) were included in the analysis of postoperative complications; 10 patients were not included due to not receiving an operation and four patients were not included due to missing follow-up forms. There were 42 out of 85 (49.4%) patients who experienced at least one postoperative complication: 33 out of 45 patients (73.3%) in the FENIX MSA arm and 9 out of 40 patients (22.5%) in the SNS arm (Table 11). The unadjusted odds ratio of having a complication in the FENIX MSA arm was 7.77 (95% CI 3.0 to 20.0; p < 0.001).
| Did patient experience a postoperative complication?, n (%) | FENIX MSA | SNS | Total |
|---|---|---|---|
| Yes | 33 (73.3) | 9 (22.5) | 42 (49.4) |
| No | 12 (26.7) | 31 (77.5) | 43 (50.6) |
| Total | 45 (100) | 40 (100) | 99 (100) |
Figure 2 shows the time from randomisation to operation and Table 12 shows the time from randomisation to first complication. Complications data were collected at 6, 12 and 18 months’ post-randomisation follow-up visits; therefore, patients who had their SNS devices fitted more than 18 months post randomisation will not have any complications recorded. There were five complications from the temporary SNS operation: (1) neurological, (2) haemorrhoid discomfort, (3) intermittent flare of eczema at SNS dressing site, (4) lack or loss of efficiency, and (5) device failure/separation. There were eight complications from the permanent SNS operation: (1) lead migration/fragmentation, (2) pain at battery site, (3) lack or loss of efficiency, (4) transient anal/rectal pain, (5) rectal/anal pain on defaecation, (6) neurological, (7) cardiorespiratory and (8) device reoperation: replacement of SNS wire. There were 88 complications across 33 patients in the FENIX MSA arm (Table 13).
| Parameter | FENIX MSA | SNS | Total |
|---|---|---|---|
| Mean (SD) (days) | 80.0 (148.72) | 79.3 (123.87) | 79.8 (142.33) |
| Median (range) (days) | 12.0 (0.0–540.0) | 15.0 (0.0–355.0) | 13.0 (0.0–540.0) |
| Participants, n | 33 | 9 | 42 |
| Complication | Total, n (%) |
|---|---|
| Worsening constipation/obstructed defecation | 18 (20.5) |
| Othera | 17 (19.3) |
| Transient anal/rectal pain | 14 (15.9) |
| Device erosion | 8 (9.1) |
| Wound infection | 7 (8.0) |
| Device explant/reoperation | 7 (8.0) |
| Implant infection | 5 (5.7) |
| Bleeding/wound haematoma | 4 (4.5) |
| Neurological | 3 (3.4) |
| Lack or loss of efficiency | 3 (3.4) |
| Urinary retention | 1 (1.1) |
| Device failure/separation | 1 (1.1) |
| Total | 88 (100) |
The adjusted model odds ratios can be seen in Table 14. The model results show a statistically significant difference in the odds of a complication between the two treatment arms, with an adjusted odds ratio of 12.91 (95% CI 2.75 to 60.68; p = 0.004). The random effect with respect to surgeon (i.e. the ‘random intercept’) was 0.79 (SE 1.38), and the random effect with respect to the interaction between surgeon and the difference between treatments (i.e. the ‘random slope’) was < 0.0001 (SE 2.17).
| Effect | Odds ratio | Odds ratio 95% CI | p-value |
|---|---|---|---|
| FENIX MSA (vs. SNS) | 12.91 | 2.75 to 60.68 | 0.0042 |
| Male (vs. female) | 1.04 | 0.03 to 36.87 | 0.98 |
| Baseline CCIS > 10 points (vs. baseline CCIS ≤ 10 points) | 0.79 | 0.11 to 5.59 | 0.81 |
| Anal sphincter defect ≤ 90° (vs. no defect) | 1.43 | 0.44 to 4.68 | 0.55 |
| Anal sphincter defect > 90° to ≤ 180° (vs. no defect) | 0.41 | 0.03 to 6.78 | 0.53 |
The time from randomisation to operation is presented in Figure 2. There is a large difference between the arms, with four patients in the SNS arm not having a permanent operation within 18 months of randomisation; therefore, the model results may be biased against the FENIX MSA arm as patients in this arm will have had longer postoperative follow-up and, therefore, longer exposure to the risk of postoperative complication within the trial follow-up period. However, Table 12 shows the number of days from operation to first complication and this shows that most complications occurred within 3 months, with some happening a long time after the operation. As seen in Figure 2, most SNS patients were followed up for > 3 months post randomisation.
Appendix 1, Figure 32, shows the empirical probability plot for the postoperative complications model, which can be used to compare actual Pearson residuals with expected Pearson residuals. The y-axis is the actual Pearson residual value, the x-axis is the empirical median Pearson residual expected under our fitted model assumptions. Each dot represents the actual Pearson residual for an individual patient. If the model fitted perfectly we would expect all of the dots to lie on the reference line. The band in Figure 32 represents the interval between the empirical 2.5th percentile and 97.5th percentile empirical Pearson residual. No values lie outside this region, indicating that we do not have any substantial outliers.
Appendix 1, Figure 33, presents the plot of exponentiated delta-betas (y-axis) versus patient identifier. Exponentiated delta-betas further from 1 indicate greater influence of the observation on the estimated treatment effect. There does not appear to be any unusually influential observations in the delta-beta plot.
Secondary end point: device explants
The number of devices in situ and explanted within 18 months of randomisation can be seen in Table 15. The mean number of days from device implant to explant was 164 (SD 168) and the median number of days was 112 (range 0–449).
| Device status | FENIX MSA, n (%) | SNS, n (%) | Total, n (%) |
|---|---|---|---|
| Device in situ | 30 (60.0) | 24 (49.0) | 54 (54.5) |
| Device explanted | 15 (30.0) | 0 (0.0) | 15 (15.2) |
| No device fitted | 5 (10.0) | 21 (42.9) | 26 (26.3) |
| Device implanted more than 18 months post randomisation | 0 (0.0) | 4 (8.2) | 4 (4.0) |
| Total | 50 (100) | 49 (100) | 99 (100) |
Figure 5 shows the periods of time (measured from randomisation) for which each patient had a permanent device in situ. The mean number of days from implant to explant was 164 (SD = 168) and the median number of days was 112 (range 0–449). As there were no explants in the SNS arm, the device explants end point has not been modelled because any regression models would not converge.
FIGURE 5.
Time to explant: (a) FENIX MSA; and (b) SNS. Dashed line indicates that patient had device explanted.


The reasons for explant are given in Table 16 and include participant intolerance (n = 4), device erosion/migration and infection, implant infected and visible through the skin, device eroded, infection/erosion (n = 3), rectal perforation the device was removed/no implant, chronic sinus infection, participant did not have adequate response, and device malfunction.
| Reason | Days from randomisation to explant |
|---|---|
| Participant intolerance | 257 |
| Device erosion/migration + infection | 16 |
| Implant infected and is visible through the skin | 154 |
| Participant did not have adequate response | 399 |
| Device eroded | 350 |
| Infection/erosion of FENIX beads SAE | 14 |
| Rectal perforation the device was removed/no implant | 0 |
| Infection and erosion, device explanted | 7 |
| Infection erosion | 69 |
| Chronic sinus infection | 169 |
| Participant did not have adequate response | 409 |
| Device malfunction | 449 |
Secondary end point: Cleveland Clinic Incontinence Score
The CCIS assesses five parameters associated with incontinence: incontinence to solid, incontinence to liquid, incontinence to gas, use of pads and lifestyle restrictions. Each parameter is scored 0–4 with ‘0’ for never and ‘4’ for every day. The five parameters are added to give a total score out of 20, with a lower score indicating a better QoL.
Summary measures of the CCIS split by time point and treatment arm are given in Table 17.
| CCIS (points) | FENIX MSA | SNS | Total |
|---|---|---|---|
| Baseline | |||
| Mean (SD) | 14.4 (3.15) | 14.7 (2.95) | 14.6 (3.04) |
| Median (range) | 14.5 (6.0–20.0) | 15.0 (9.0–20.0) | 15.0 (6.0–20.0) |
| Missing | 4 | 3 | 7 |
| n | 46 | 46 | 92 |
| 6 months post randomisation | |||
| Mean (SD) | 11.7 (4.61) | 13.8 (3.45) | 12.8 (4.16) |
| Median (range) | 12.0 (2.0–20.0) | 14.0 (7.0–20.0) | 13.0 (2.0–20.0) |
| Missing | 15 | 10 | 25 |
| n | 35 | 39 | 74 |
| 12 months post randomisation | |||
| Mean (SD) | 11.8 (4.86) | 12.9 (3.78) | 12.3 (4.37) |
| Median (range) | 12.0 (3.0–20.0) | 13.0 (5.0–20.0) | 12.0 (3.0–20.0) |
| Missing | 15 | 15 | 30 |
| n | 35 | 34 | 69 |
| 18 months post randomisation | |||
| Mean (SD) | 11.1 (4.35) | 12.1 (4.55) | 11.6 (4.45) |
| Median (range) | 11.0 (3.0–17.0) | 12.5 (1.0–19.0) | 12.0 (1.0–19.0) |
| Missing | 20 | 15 | 35 |
| n | 30 | 34 | 64 |
The model diagnostics can be seen in Appendix 1, Figure 34.
Secondary end point: EuroQol-5 Dimensions, five-level version
The EQ-5D-5L is made up of 5 dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each of which has 5 levels (no problems, slight problems, moderate problems, severe problems and extreme problems). These levels can be combined to calculate an index value, which ranges from 1 (best possible health), through 0 (death) to –0.594 (worse than death).
Summary measures of the EQ-5D-5L index values, and the EQ-5D-5L Visual Analogue Scale (VAS) scores, split by time point and treatment arm, are given in Table 18.
| Variable | FENIX MSA | SNS | Total |
|---|---|---|---|
| EQ-5D-5L score | |||
| Baseline | |||
| Mean (SD) | 0.666 (0.256) | 0.647 (0.261) | 0.657 (0.257) |
| Median (range) | 0.740 (–0.02–1.00) | 0.736 (–0.05–1.00) | 0.736 (–0.05–1.00) |
| Missing | 4 | 3 | 7 |
| n | 46 | 46 | 92 |
| 6 months post randomisation | |||
| Mean (SD) | 0.687 (0.225) | 0.639 (0.295) | 0.662 (0.263) |
| Median (range) | 0.736 (–0.02–1.00) | 0.732 (–0.22–1.00) | 0.736 (–0.22–1.00) |
| Missing | 12 | 7 | 19 |
| n | 38 | 42 | 80 |
| 12 months post randomisation | |||
| Mean (SD) | 0.684 (0.258) | 0.673 (0.263) | 0.679 (0.259) |
| Median (range) | 0.750 (–0.06–1.00) | 0.752 (00.023–1.00) | 0.750 (–0.06–1.00) |
| Missing | 13 | 13 | 26 |
| n | 37 | 36 | 73 |
| 18 months post randomisation | |||
| Mean (SD) | 0.660 (0.258) | 0.683 (0.255) | 0.672 (0.255) |
| Median (range) | 0.732 (0.054–1.00) | 0.743 (–0.05–1.00) | 0.735 (–0.05–1.00) |
| Missing | 20 | 17 | 37 |
| n | 30 | 32 | 62 |
| EQ-5D-5L VAS score | |||
| Baseline | |||
| Mean (SD) | 65.7 (20.3) | 66.8 (24.8) | 66.2 (22.5) |
| Median (range) | 70.0 (0.00–95.0) | 70.0 (5.00–100) | 70.0 (0.00–100) |
| Missing | 2 | 5 | 7 |
| n | 48 | 45 | 93 |
| 6 months post randomisation | |||
| Mean (SD) | 66.9 (19.6) | 72.7 (19.3) | 69.9 (19.5) |
| Median (range) | 70.0 (30.0–95.0) | 75.0 (15.0–95.0) | 75.0 (15.0–95.0) |
| Missing | 11 | 7 | 18 |
| n | 39 | 42 | 81 |
| 12 months post randomisation | |||
| Mean (SD) | 65.5 (21.1) | 73.2 (19.7) | 69.4 (20.6) |
| Median (range) | 70.0 (10.0–96.0) | 75.0 (20.0–100) | 72.5 (10.0–100) |
| Missing | 14 | 13 | 27 |
| n | 36 | 36 | 72 |
| 18 months post randomisation | |||
| Mean (SD) | 66.0 (19.6) | 72.1 (14.0) | 69.2 (17.0) |
| Median (range) | 67.5 (20.0–95.0) | 70.0 (33.0–100) | 70.0 (20.0–100) |
| Missing | 20 | 16 | 36 |
| n | 30 | 33 | 63 |
Secondary end point: faecal incontinence quality of life
The FIQoL questionnaire is composed of 29 items that make up four scales: lifestyle (10 items), coping/behaviour (9 items), depression/self-perception (7 items) and embarrassment (3 items). Scales range from 1 to 5 and scale scores are derived by averaging the response to all items in the scale. A lower score indicates a lower functional QoL.
Summary measures of the four FIQoL domains split by time point and treatment arm are provided in Table 19.
| FIQoL domain | FENIX MSA | SNS | Total |
|---|---|---|---|
| Lifestyle (baseline) | |||
| Mean (SD) | 2.17 (0.864) | 2.12 (0.887) | 2.15 (0.871) |
| Median (range) | 2.00 (1.00–3.80) | 2.00 (1.00–3.90) | 2.00 (1.00–3.90) |
| Missing | 5 | 5 | 10 |
| n | 45 | 44 | 89 |
| Lifestyle (6 months post randomisation) | |||
| Mean (SD) | 2.64 (1.09) | 2.39 (0.918) | 2.51 (1.01) |
| Median (range) | 2.70 (1.00–4.00) | 2.30 (1.00–4.00) | 2.50 (1.00–4.00) |
| Missing | 13 | 11 | 24 |
| n | 37 | 38 | 75 |
| Lifestyle (12 months post randomisation) | |||
| Mean (SD) | 2.74 (1.08) | 2.35 (1.03) | 2.56 (1.06) |
| Median (range) | 3.05 (1.00–4.00) | 2.30 (1.00–3.90) | 2.75 (1.00–4.00) |
| Missing | 14 | 17 | 31 |
| n | 36 | 32 | 68 |
| Lifestyle (18 months post randomisation) | |||
| Mean (SD) | 2.73 (1.03) | 2.62 (1.02) | 2.68 (1.02) |
| Median (range) | 2.90 (1.00–4.00) | 2.90 (1.00–4.00) | 2.90 (1.00–4.00) |
| Missing | 21 | 22 | 43 |
| n | 29 | 27 | 56 |
| Coping (baseline) | |||
| Mean (SD) | 1.44 (0.534) | 1.50 (0.616) | 1.47 (0.573) |
| Median (range) | 1.33 (1.00–3.67) | 1.33 (1.00–3.44) | 1.33 (1.00–3.67) |
| Missing | 9 | 9 | 18 |
| n | 41 | 40 | 81 |
| Coping (6 months post randomisation) | |||
| Mean (SD) | 2.04 (0.984) | 1.63 (0.610) | 1.81 (0.818) |
| Median (range) | 1.67 (1.00–4.00) | 1.56 (1.00–3.22) | 1.56 (1.00–4.00) |
| Missing | 23 | 16 | 39 |
| n | 27 | 33 | 60 |
| Coping (12 months post randomisation) | |||
| Mean (SD) | 2.24 (1.02) | 1.57 (0.671) | 1.91 (0.923) |
| Median (range) | 2.11 (1.00–4.00) | 1.44 (1.00–3.22) | 1.56 (1.00–4.00) |
| Missing | 23 | 22 | 45 |
| n | 27 | 27 | 54 |
| Coping (18 months post randomisation) | |||
| Mean (SD) | 1.94 (0.895) | 1.97 (0.820) | 1.96 (0.847) |
| Median (range) | 1.67 (1.00–3.89) | 2.11 (1.00–3.44) | 1.89 (1.00–3.89) |
| Missing | 30 | 28 | 58 |
| n | 20 | 21 | 41 |
| Depression (baseline) | |||
| Mean (SD) | 2.52 (0.956) | 2.33 (0.864) | 2.43 (0.911) |
| Median (range) | 2.14 (1.14–4.29) | 2.29 (1.00–4.29) | 2.14 (1.00–4.29) |
| Missing | 11 | 11 | 22 |
| n | 39 | 38 | 77 |
| Depression (6 months post randomisation) | |||
| Mean (SD) | 2.74 (1.07) | 2.48 (0.891) | 2.60 (0.978) |
| Median (range) | 2.36 (1.43–4.43) | 2.43 (1.14–4.14) | 2.43 (1.14–4.43) |
| Missing | 24 | 19 | 43 |
| n | 26 | 30 | 56 |
| Depression (12 months post randomisation) | |||
| Mean (SD) | 2.70 (1.11) | 2.57 (0.898) | 2.64 (1.01) |
| Median (range) | 2.29 (1.14–4.29) | 2.64 (1.29–4.00) | 2.43 (1.14–4.29) |
| Missing | 20 | 21 | 41 |
| n | 30 | 28 | 58 |
| Depression (18 months post randomisation) | |||
| Mean (SD) | 2.53 (1.01) | 2.89 (0.948) | 2.72 (0.981) |
| Median (range) | 2.43 (1.29–4.43) | 3.00 (1.43–4.43) | 2.57 (1.29–4.43) |
| Missing | 29 | 25 | 54 |
| n | 21 | 24 | 45 |
| Embarrassment (baseline) | |||
| Mean (SD) | 1.74 (0.699) | 1.64 (0.640) | 1.69 (0.668) |
| Median (range) | 1.67 (1.00–3.33) | 1.33 (1.00–3.33) | 1.67 (1.00–3.33) |
| Missing | 4 | 2 | 6 |
| n | 46 | 47 | 93 |
| Embarrassment (6 months post randomisation) | |||
| Mean (SD) | 2.12 (0.996) | 1.87 (0.789) | 1.99 (0.897) |
| Median (range) | 1.67 (1.00–4.00) | 1.67 (1.00–4.00) | 1.67 (1.00–4.00) |
| Missing | 11 | 7 | 18 |
| n | 39 | 42 | 81 |
| Embarrassment (12 months post randomisation) | |||
| Mean (SD) | 2.20 (0.970) | 1.87 (0.791) | 2.04 (0.894) |
| Median (range) | 2.33 (1.00–4.00) | 1.67 (1.00–3.67) | 1.83 (1.00–4.00) |
| Missing | 13 | 12 | 25 |
| n | 37 | 37 | 74 |
| Embarrassment (18 months post randomisation) | |||
| Mean (SD) | 2.24 (1.02) | 1.99 (0.827) | 2.11 (0.926) |
| Median (range) | 2.33 (1.00–4.00) | 1.83 (1.00–4.00) | 2.00 (1.00–4.00) |
| Missing | 21 | 17 | 38 |
| n | 29 | 32 | 61 |
Secondary end point: Obstructed Defecation Score
The ODS consists of five items: excessive straining, incomplete rectal evacuation, use of enemas and/or laxatives, vaginal-anal-perineal digitations, and abdominal discomfort and/or pain. Each item is graded from 0 to 4, with scores ranging from 0 (no symptoms) to 20 (very severe symptoms), meaning that a lower score indicates better QoL.
Summary measures of the total ODS split by time point and treatment arm are presented in Table 20.
| ODS | FENIX MSA | SNS | Total |
|---|---|---|---|
| Baseline | |||
| Mean (SD) | 7.44 (3.78) | 7.09 (3.62) | 7.27 (3.68) |
| Median (range) | 7.00 (2.00–18.0) | 8.00 (2.00–17.0) | 7.00 (2.00–18.0) |
| Missing | 5 | 6 | 11 |
| n | 45 | 43 | 88 |
| 6 months post randomisation | |||
| Mean (SD) | 9.10 (4.83) | 7.53 (3.48) | 8.40 (4.33) |
| Median (range) | 8.00 (0.000–20.0) | 7.00 (0.000–16.0) | 8.00 (0.000–20.0) |
| Missing | 10 | 17 | 27 |
| n | 40 | 32 | 72 |
| 12 months post randomisation | |||
| Mean (SD) | 8.34 (4.86) | 7.52 (3.75) | 7.94 (4.34) |
| Median (range) | 8.00 (1.00–20.0) | 8.00 (2.00–15.0) | 8.00 (1.00–20.0) |
| Missing | 15 | 16 | 31 |
| n | 35 | 33 | 68 |
| 18 months post randomisation | |||
| Mean (SD) | 8.58 (4.77) | 6.90 (4.34) | 7.67 (4.58) |
| Median (range) | 8.00 (0.000–18.0) | 6.00 (0.000–16.0) | 7.00 (0.000–18.0) |
| Missing | 24 | 18 | 42 |
| n | 26 | 31 | 57 |
Secondary end point: Short Form questionnaire-12 items
The SF-12 version 2 is a generic health survey that measures eight health domains: physical functioning, role – physical, bodily pain, general health, vitality, social functioning, role – emotional and mental health. The Physical Component Summary (PCS) and Mental Component Summary (MCS) measures are calculated using a combination of the eight domains. Scores are calibrated so that the mean score is 50 with a standard deviation (SD) of 10 in the 2009 general US population. All scores range from 0 to 100, with a higher value indicating better functioning and well-being.
Summary measures of the SF-12 PCS and MCS components split by time point and treatment arm are presented in Table 21.
| SF-12 component | FENIX MSA | SNS | Total |
|---|---|---|---|
| PCS (baseline) | |||
| Mean (SD) | 44.9 (9.36) | 46.9 (10.2) | 45.9 (9.78) |
| Median (range) | 46.5 (18.6–61.2) | 47.3 (23.6–64.0) | 47.3 (18.6–64.0) |
| Missing | 3 | 1 | 4 |
| n | 47 | 48 | 95 |
| PCS (6 months post randomisation) | |||
| Mean (SD) | 46.2 (9.96) | 47.7 (9.71) | 47.0 (9.80) |
| Median (range) | 45.6 (27.6–68.5) | 48.7 (23.9–70.0) | 47.1 (23.9–70.0) |
| Missing | 11 | 7 | 18 |
| n | 39 | 42 | 81 |
| PCS (12 months post randomisation) | |||
| Mean (SD) | 47.1 (9.64) | 47.4 (9.49) | 47.2 (9.50) |
| Median (range) | 49.0 (26.7–65.3) | 49.8 (20.3–64.1) | 49.2 (20.3–65.3) |
| Missing | 12 | 12 | 24 |
| n | 38 | 37 | 75 |
| PCS (18 months post randomisation) | |||
| Mean (SD) | 43.9 (10.9) | 47.0 (10.4) | 45.5 (10.7) |
| Median (range) | 43.9 (26.9–59.7) | 46.8 (23.7–63.3) | 46.6 (23.7–63.3) |
| Missing | 19 | 16 | 35 |
| n | 31 | 33 | 64 |
| MCS (baseline) | |||
| Mean (SD) | 41.2 (11.4) | 41.2 (11.2) | 41.2 (11.2) |
| Median (range) | 42.7 (22.8–61.8) | 40.9 (17.0–63.5) | 41.3 (17.0–63.5) |
| Missing | 3 | 1 | 4 |
| n | 47 | 48 | 95 |
| MCS (6 months post randomisation) | |||
| Mean (SD) | 41.6 (13.2) | 42.5 (12.5) | 42.0 (12.8) |
| Median (range) | 40.8 (15.8–62.0) | 45.2 (15.4–61.2) | 43.1 (15.4–62.0) |
| Missing | 10 | 7 | 17 |
| n | 40 | 42 | 82 |
| MCS (12 months post randomisation) | |||
| Mean (SD) | 41.6 (13.3) | 43.8 (11.7) | 42.7 (12.5) |
| Median (range) | 41.9 (20.5–60.5) | 43.2 (17.4–62.4) | 43.2 (17.4–62.4) |
| Missing | 12 | 12 | 24 |
| n | 38 | 37 | 75 |
| MCS (18 months post randomisation) | |||
| Mean (SD) | 43.5 (12.5) | 44.3 (12.2) | 43.9 (12.2) |
| Median (range) | 41.2 (15.2–63.1) | 46.7 (19.2–69.6) | 44.6 (15.2–69.6) |
| Missing | 19 | 16 | 35 |
| n | 31 | 33 | 64 |
Registered training cases
Prior to randomising patients in the study, all surgeons were required to have experience of a minimum of one observed FENIX MSA procedure and two FENIX MSA procedures under proctorship. Surgeons who did not have this experience before study participation joined the registration phase of the study in which the first two eligible patients providing consent were registered to receive FENIX MSA implants. These two operations, performed under proctorship, were considered training cases and were not included in the main study.
A total of 23 patients were registered as training cases for the FENIX MSA operation. Of these 23 patients, 22 received the FENIX operation and one withdrew, giving the reason as travelling scheduled for after the surgery date; the patient withdrew after discussing with a research nurse advice about travelling post surgery, and decided not to risk discomfort and complications.
There was one operative complication, with the complication described as ‘Perf skin posterior. Primary repair’.
Only complications that occurred within 30 days of the operation were reported for the registered training cases. There were 22 postoperative complications across 13 patients:
-
wound infection (n = 7)
-
transient anal/rectal pain (n = 3)
-
wound dehiscence (n = 2)
-
worsening constipation/obstructed defecation (n = 2)
-
urinary retention
-
bleeding/urinary retention
-
device explant/reoperation
-
urinary tract infection
-
incomplete emptying of bladder on micturition
-
prickly sensation
-
incomplete emptying
-
superficial wound dehiscence.
Chapter 3 Health economics
This section outlines the approach to the manipulation and analysis of the health economic data from the SaFaRI trial for the purpose of conducting an economic evaluation of the FENIX device.
The economic evaluation followed the NICE reference case26 and, hence, was a cost–utility analysis presenting cost per incremental QALY from the perspective of the health-care and personal social service’s provider. The evaluation used both trial-based and model-based analyses, and adopted a lifetime horizon. A model was required given that the likely costs and benefits of the interventions continue to accrue after the trial time horizon (18 months). Our primary analysis is based on the combined trial and model results, but we also report cost-effectiveness at the trial end.
The evaluation assumes a willingness-to-pay threshold (λ) of £20,000 per QALY gain, and estimates the incremental costs and benefits of the FENIX magnetic anal sphincter (MAS) device versus usual SNS for adult FI.
Methods: trial
Health utility and quality-adjusted life-year calculation
Health-related quality-of-life (HRQoL) data to enable QALY calculations were collected during the trial at baseline and at 6, 12 and 18 months post randomisation. Data were available on the EQ-5D-5L27,28 and the SF-12 [Short Form questionnaire-6 Dimensions (SF-6D)]. 29
The primary analysis is based on the EQ-5D-5L direct valuation. 30 The secondary analysis estimated QALYs based on the SF-12 (SF-6D) instrument. A supplementary analysis reported results based on the EQ–5D-5L crosswalk (to the EQ-5D-3L). 31
The QALY calculation was estimated on an area under the curve approach, adopting the following assumptions:
-
All follow-ups were carried out at the exact, prespecified time period (0, 6, 12 and 18 months).
-
A patient who died with their last EQ-5D-5L observation as positive had a linear fall in HRQoL from this point until death.
-
A patient who died with their last EQ-5D-5L observation as negative had this constant level of HRQoL from this point until death.
Notation: E0 = baseline utility, E6 = utility at 6 months, E12 = utility at 12 months, E18 = utility at 18 months, t = duration in each health state in days. If EQ-5D-5L was present at baseline, 6, 12 and 18 months’ follow-up, QALYs were calculated by:
Resource use and costs
The perspective for the costs was the health and social care provider (i.e. wider costs, such as productivity loss or out-of-pocket expenses, were not included).
We used a combination of patient-reported data, CRF data capture and published estimates to derive costs during the evaluation. The general approach, individual unit costs and costing assumptions were verified with and refined following input from the lead clinician on the trial. The cost categories and overall approach to establishing the cost of each intervention over the trial period and the associated source of resource use/costs is as follows:
-
device costs – taken from publications and online resources
-
procedure costs – taken from national tariffs and online resources
-
medications – based on patient report
-
primary/social care – based on patient report
-
secondary care after the initial procedure – generated by attributing costs to explants, complications and further treatments/surgery.
The strategy for secondary care resource use capture deviates from the strategy that was planned. We had originally aimed to request NHS digital data to capture this aspect of resource; however, as a result of the early stopping of the trial, the associated reduction in research funding and the resultant limited sample size, this was considered to be impractical and was not pursued. This decision was made following consultation with the trial team. Since secondary care costs (hospital visits and stay) were not captured directly elsewhere in the trial, we elected to capture them via the length of stay of the original procedure and by costing subsequent explants, complications and surgeries.
The combined device and procedure costs are included in Table 22 and include the device-specific cost and a day-case rate for insertion of a generic neurostimulator for treatment of FI costs. These costs are fixed, except for the cost of the hospital stay. Any nights spent in hospital (including the first night) were costed using the unit cost shown for an excess bed-day. Data collected in the trial (e.g. relating to theatre time during procedure) may have allowed for a more granular approach to costing the procedure; however, given the small sample sizes, it may be prone to undue bias from outliers; thus, we opted to use NHS reference cost tariffs.
| Item | Cost (2018/19 prices) | Source and assumptions | Inflated from year |
|---|---|---|---|
| SNS: temporary | |||
| Temporary device cost | £680 | Hounsome and Roukas32 | N/A |
| Temporary device procedure costs | £1819 | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Excess bed-day cost for stays > 1 day | £362 | Insertion of neurostimulator for treatment of faecal incontinence, elective short stay excess bed-day (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| SNS: permanent | |||
| Permanent device cost | £7750 | Hounsome and Roukas32 | N/A |
| Permanent procedure costs | £1819 | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Excess bed-day cost for stays > 1 day | £362 | Insertion of neurostimulator for treatment of faecal incontinence, elective short stay excess bed-day (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| FENIX: MSA | |||
| Sizing tool | £300 | URL: www.io.nihr.ac.uk/wp-content/uploads/migrated/2220.838f0fdf.FENIXforfaecalincontinenceFINAL.pdf (accessed 12 February 2021) | N/A |
| Device cost | £4000 | URL: www.io.nihr.ac.uk/wp-content/uploads/migrated/2220.838f0fdf.FENIXforfaecalincontinenceFINAL.pdf (accessed 12 February 2021) | N/A |
| Procedure cost | £1819 | Assumed same as SNS: insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Excess bed-day cost for stays > 1 day | £362 | Insertion of neurostimulator for treatment of faecal incontinence, elective short stay excess bed-day (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
An operative form was completed for each patient following the procedure. The form captures operation details, including which procedure the patient received (temporary SNS and/or permanent SNS, or FENIX MSA) and any intraoperative complications that may occur. The form also reports whether patients receive a local, general or spinal anaesthetic, and whether or not the procedure has been completed. Furthermore, a 2-week postoperative review was issued for each patient to gather information on whether or not patients progress to permanent SNS, if they were randomised to receive temporary SNS, whether or not the device they received is still in situ and a reason provided if the device has been explanted. This discharge form was also used to record whether or not patients who have been discharged receive further surgery or suffer from any postoperative complications. Follow-up forms were completed by the local research teams at 6, 12 and 18 months following randomisation and asked for similar patient information. Individual complications and further surgeries were costed using the unit costs given in Appendix 1, Tables 39 and 40, respectively. With the exception of explantation, we assumed that the costs of any intraoperative complications were captured in the length of stay of the initial procedure.
At baseline and at 6-month, 12-month and 18-month follow-up, patients were provided with a specially designed form to report on their (primary and social) health-care resource use in the past 3 months: general practitioner (GP), practice nurse, district nurse, physiotherapist, occupational therapist, counsellor in the GP surgery visits, clinic (non-hospital) by telephone/e-mail or at home. They were also asked if they received support from social services, including meals-on-wheels deliveries, laundry services, care workers/help at home or social worker visits, and the frequency of this support. Furthermore, they were asked to provide information on the use of community/residential services, including any visits and overnight stays at convalescent homes, nursing homes and day centres. Patients were also asked to note what medication relating to their FI they were taking on a regular basis. Unit costs for medications are listed in Appendix 1, Table 41, and for primary care/social care in Appendix 1, Tables 42 and 43. We assumed that any follow-up care costs associated with the interventions are captured in the self-reported primary care use data. The 3-month recall data were multiplied by two to obtain 6-month values.
In a majority of cases, unit costs were based on national resources such as the Personal Social Services Research Unit (PSSRU) report, NHS Reference Costs and the Electronic Market Information Tool (eMIT). Elsewhere, we used other web resources and previous publications as the basis for unit costs for health-care resources. All costs are presented in Great British pounds and the price year is 2018 (costs are inflated to 2018/19 prices where necessary using the Consumer Price Index for health).
Analysis
The main analysis was based on an intention-to-treat principle. The trial analysis produced ICERs over the trial period (18 months). We used seemingly unrelated regression34,35 to account for the expected correlation between costs and QALYs while controlling for any baseline imbalances. Although we had planned to use the same covariates as the statistical analysis, analyses were run with no adjustment, and subsequently adjusting for baseline EQ-5D-5L and (primary/social care) costs given the data limitations. The regression models estimated costs and QALYs that will allow the calculation of ICERs.
Our primary analysis incorporated multiple imputation (MI) to impute missing data; however, we also conducted a complete-case analysis. Individuals with missing EQ-5D-5L items were not allocated a utility index score. Where missing values could not be dealt with in the manner described above (see Health utility and quality-adjusted life-year calculation), we conducted MI as part of the model estimation procedure. Costs were imputed for each individual at each time point for the following categories: (1) total primary care and social care costs, (2) total secondary care costs and (3) total medication costs.
We present a range of sensitivity analyses to explore the impact of the assumptions made in the base case analysis on the cost-effectiveness estimates. These include the methods used to assess utility in the trial and model covariates. We also conducted non-parametric bootstrapping to calculate the probability that FENIX was cost-effective at the trial end.
The final cost and QALY estimates at 18 months were added to the beginning of the decision model time horizon, and additional costs and benefits were estimated in the model from 18 months over a lifetime.
Methods: decision model
We generated a de novo decision-analytic model to estimate cost per QALY estimates over a lifetime horizon. In addition to extending the evaluation period to a relevant horizon, the model also allows the exploration of uncertainty in the parameter estimates, individually and jointly, as part of a probabilistic sensitivity analysis (PSA). The latter will allow the production of cost-effectiveness acceptability curves (CEACs)36 and estimates of the value of information (further research). The modelling was conducted in Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Model type, cycle length, structure and health states
The model is a Markov model describing a simplified patient pathway from the end of the trial period (18 months) over a lifetime in terms of discrete health states. The average age of the patients at the model start reflected that of the trial participants (62 years). Patients move between these health states at the end of every model cycle (in this case, 1 year) according to prespecified transition probabilities.
The structure of the decision model is shown in Figure 6. The structure was informed by other models used in this population32,37 and was finalised following input from the lead clinician.
FIGURE 6.
Decision model structure.

In the model, we defined the health states as being related to effectiveness according to the percentage improvement on baseline CCIS (100–76% improvement, 75–51% improvement, ≤ 50% improvement) and whether or not the device is still in situ (device explant). ‘Better’ health states (i.e. higher percentage of improvement) are associated with lower costs and higher QoL. Although we did not a priori expect the interventions to have an impact on survival, because the model is over a lifetime horizon, we also modelled background, all-cause mortality (dead health state).
Each health state is associated with a specific average cost and HRQoL (utility value). A hypothetical cohort of FI patients for each treatment arm was distributed between these health states at model start with the distribution reflecting that of the trial sample and intervention effectiveness/functionality at 18 months. These patients moved between health states at the end of each model cycle, with the likelihood of this movement determined by transition probabilities that reflected treatment effectiveness – more specifically, loss of effectiveness over time – and mortality rates.
We assumed that 18 months after initial implant, effectiveness could only deteriorate, and thus patients would be transited downwards in terms of percentage improvement from baseline. We also assumed that once explantation had occurred, another device (either SNS or FENIX) would not be implanted. Patients receiving FENIX initially are unlikely to receive another FENIX device following explantation owing to clinical viability. While it is possible that initial FENIX implants could be replaced by SNS devices and that initial SNS devices could be replaced by either another SNS device or a FENIX device, the patient numbers on these pathways would be relatively small. Incorporating these possibilities would require additional modelling complexity, and the additional burden of data requirements (e.g. effectiveness of second implants) was unlikely to be met, and thus additional assumptions would be required. We also included in the explant health state those in the SNS group who did not have a permanent implant. The explant health state was therefore capturing ‘conservative management’ for those without a device in situ.
The durability of the FENIX device is unknown and the device will in theory continue to function in the long term until a fault or infection occurs. However, the SNS device has a finite battery life (approximately between 5 and 7 years), at which point explantation, battery replacement and implantation is required. We reflected this in a submodel for SNS. After battery replacement, patients were subject to the same distribution among effectiveness categories as observed in the trial arm at 18 months (rather than immediately post procedure) for those that had the permanent device implanted. We did not include another battery replacement at 14 years in the base-case model because of the added modelling complexity and the small proportion likely to have a device in situ but we tested this in simplified sensitivity analyses. Other modelling assumptions are set out in the parameter tables.
Parameter values: transition probabilities
The decision model required a set of parameter values that defined the movement of the cohort around the model (transition probabilities are shown in Table 23). Where possible, the transition rates for the model were estimated from the randomised controlled trial (RCT) data (observed data rather than imputed). We used the end distribution of trial patients per arm across percentage improvement groups as the starting proportions in these groups at model start. We used trial data on transition rates between these percentage improvement groups during the trial to specify a rate beyond 18 months. As a result of the limited sample size, we were unable to do this in a robust way per intervention arm, so we assumed these rates to be the same across arms.
| Parameter | Value | SE | Distributiona | Source/reference |
|---|---|---|---|---|
| Common parameters | ||||
| Starting age of model cohort (years) | 62 | N/A | Fixed | Mean of the trial sample |
| Survival | Age dependent | N/A | Fixed | Taken from Office for National Statistics life tables38 |
| FI population annual incidence in England (for EVPI) | 13,126 | N/A | Fixed |
The NIHR Horizon Scanning Centre (2012) News Brief: FENIX™ Continence Restoration System for severe chronic faecal incontinence20 80 new adults per 100,000 population 30 of these referred to surgical specialist Adult population in England = 43,752,473 |
| Number of years for which the decision problem is relevant (for value of information) | 10 | N/A | Fixed | Assumed |
| FENIX | ||||
| Starting proportions | Trial data at 18 months | |||
| 100–76% improvement | 0.00 | N/A | Dirichlet | |
| 75–51% improvement | 0.16 | N/A | Dirichlet | |
| ≤ 50% improvement | 0.39 | N/A | Dirichlet | |
| Device explant | 0.42 | N/A | Dirichlet | |
| Dead | 0.03 | N/A | Dirichlet | |
| Transition between % improvement group | Trial data | |||
| 100–76% to 75–51% | 0.167 | N/A | Dirichlet | |
| 100–76% to ≤ 50% | 0.667 | N/A | Dirichlet | |
| 75–51% to ≤ 50% | 0.800 | N/A | Dirichlet | |
| Probability of explant | 0.15 | N/A | Dirichlet | |
| SNS | ||||
| Starting proportions | Trial data at 18 months | |||
| 100–76% improvement | 0.026 | N/A | Dirichlet | |
| 75–51% improvement | 0.105 | N/A | Dirichlet | |
| ≤ 50% improvement | 0.342 | N/A | Dirichlet | |
| Device explant | 0.526 | N/A | Dirichlet | |
| Dead | 0.000 | N/A | Dirichlet | |
| Transition between % improvement group | Trial data | |||
| 100–76% to 75–51% | 0.167 | N/A | Dirichlet | |
| 100–76% to ≤ 50% | 0.667 | N/A | Dirichlet | |
| 75–51% to ≤ 50% | 0.800 | N/A | Dirichlet | Survival curve based on trial data |
| Probability of explant | 0.15 | N/A | Dirichlet | Assumed same rate as FENIX |
| Probability of battery replacement | 1 | N/A | Fixed | Battery replacement every 7 years based on triangulation of several sources and clinical opinion32,39,40 |
We applied parametric survival curves to the time-to-explant data from the trial and extrapolated this beyond 18 months. The survival analysis indicated no increase in hazard over time, and thus we estimated a constant hazard rate using the exponential distribution in Stata® version 15 (StataCorp LP, College Station, TX, USA). There were no explants in the SNS group, but a clinical expert felt that this was an artefact of the sample size; hence, we used the same explant rate for both intervention groups. Following input from the same clinical expert, we assumed that survival in the FI population was not significantly different from survival in the general population and, thus, used Office for National Statistics mortality data38 to model this. We also assumed that neither the interventions nor the percentage improvement had an impact on survival.
Parameter values: health state utilities
Economic evaluations are designed to inform resource allocation decisions, thus the model used the QALY outcome measure as prescribed by NICE. The estimation of QALYs requires the production of utility weights for health states; these data were derived from the RCT (Table 24). We used linear regression predicting EQ-5D-5L utility weights and health state groups as an explanatory categorical variable. The regression used multiple observations per person and robust SEs to reflect this approach.
| Parameter | Value | SE | Distribution | Source/reference |
|---|---|---|---|---|
| Health state utility | ||||
| 100–76% improvement | 0.96 | 0.0304 | Beta | Trial data |
| 75–51% improvement | 0.90 | 0.0429 | Beta | Trial data |
| ≤ 50% improvement | 0.75 | 0.0275 | Beta | Trial data |
| Device explant (subsequently, conservative management) | 0.64 | 0.0801 | Beta | Trial data |
| Health state costs (per year) | ||||
| 100–76% improvement | £90.67 | 79.39 | Gamma | Trial data |
| 75–51% improvement | £248.99 | 154.45 | Gamma | Trial data |
| ≤ 50% improvement | £384.07 | 131.16 | Gamma | Trial data |
| Device explant | £631.91 | 451.92 | Gamma | Trial data |
| Procedure and device costs | ||||
| Device explant: procedure cost | £1819 | N/A | Fixed | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) (assumed same for FENIX and SNS) |
| Battery replacement: procedure cost | £1819 | N/A | Fixed | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) (assumed same for FENIX and SNS) |
| Battery replacement: SNS device cost | £7750 | N/A | Fixed | Hounsome and Roukas32 |
As the interventions were assumed not to influence survival, it was not considered necessary to adjust utility values for age over the time horizon. There was no utility decrement for battery replacement as this was assumed to be a ‘tunnel state’ straight to percentage CCIS improvement groups (i.e. patients did not stay in this state for a sufficient period to incur substantive HRQoL loss).
Parameter values: costs
We generated health state costs from the trial data (see Table 24). As we expected, costs were at their highest immediately after the initial procedure and then fell over time (with the exception of the battery replacement for SNS). We used the 12-month and 18-month resource use data to estimate ongoing health-care costs in the model. As it was unclear whether or not the trial forms captured ongoing (conservative management) costs for the no device group, we generated a monthly cost following discussion with the lead clinician (detailed in Table 24) and tested this in a sensitivity analysis. We used the full set of cost data to estimate costs immediately post battery replacement, and this was anticipated to capture any complication and additional surgery costs. The explantation of a device or replacement of a battery led to the procedure costs outlined in Table 24.
Relevant costs for the evaluation include the FENIX device/SNS acquisition and procedure (including length of hospital stay), costs of treating adverse events/complications, costs of device explants and other surgery, and (for SNS) the cost of battery replacement. These are in addition to the health state costs that were obtained from the trial data. All costs relating to other surgeries, explants and complications within 18 months were observed/captured in the trial data, and thus were not modelled here. After 18 months, complication costs were assumed to be captured within the other health states.
Unit costs were obtained from national sources including the PSSRU,41 the British National Formulary42 (BNF) and the NHS Reference Cost33 database. Internet searches were conducted for other cost elements.
Validation
We validated the model by checking the face validity of the model structure and parameter values with other health economists and with the lead clinician. We tested the internal validity of the model by checking that model outputs followed expectations when changes were made to certain parameter values (e.g. that the cost-effectiveness of FENIX improved following an increase in the SNS device cost or reduced following an increase in the probability of explant in the FENIX arm).
Analysis
The average costs and QALYs accrued during the trial period were inputted at the start of the model, with further costs and benefits added to these over the model horizon. We generated a deterministic ICER per QALY gained and a net monetary benefit (NMB) estimate at the model end. We tested these estimates using prespecified sensitivity analyses to identify drivers of cost-effectiveness. We specified distributions for certain parameter values (denoted in the parameter tables) and ran a PSA where random parameters were selected from these distributions and inputted into the model over 10,000 Monte Carlo simulations. The 10,000 costs and QALY estimates generated from the PSA were plotted on a cost-effectiveness plane and the average of these was used to calculate the probabilistic ICER. We used estimates of NMB to determine the probability that FENIX was cost-effective compared with SNS over a range of willingness-to-pay values on the CEAC. Since the CEAC indicates the probability of cost-effectiveness, we can calculate the probability that the optimal treatment will not be cost-effective. Using this information and the NMB provided by the alternative treatment (the net benefit loss), we used the value of information framework43 to estimate the total population expected value of perfect information (EVPI). The population EVPI is estimated using the expected net benefit loss per person from the decision, the number of people with FI that would be eligible for the treatments per year (annual incidence is estimated to be 13,126) and the period for which the FENIX versus SNS decision is still relevant (here, we assumed this to be 10 years). The higher the EVPI value, the greater the potential cost of uncertainty and the greater the incentive to conduct additional research (e.g. further trials) to reduce that uncertainty prior to making a decision.
We used a NICE willingness-to-pay threshold of £20,000 per QALY to determine cost-effectiveness (or NMB value of > 0). We used a half-cycle correction to reflect the expectation that health state transitions can happen at any time during a model cycle. In accordance with current NICE recommendations, costs and outcomes post year 1 were discounted at 3.5% per annum. The perspective remained that of the health and personal social services provider.
Results
Trial analysis
Descriptive results for the costs (and cost category) are shown by arm in Table 25. There is a relatively modest difference in total cost between arms. However, there are notable differences in the individual cost categories, with the FENIX procedure being much cheaper than the SNS procedure, but being associated with significantly higher secondary care costs (for other complications and surgeries). Descriptive statistics on costs (and by cost category) and utility by arm, time-point and sample are included in Appendix 1, Table 44.
| Cost categorya | FENIX (£) | SNS (£) | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Procedure | 6190.55 | 274.96 | 8176.49 | 926.80 |
| Medication | 65.48 | 20.02 | 31.47 | 7.91 |
| Explants | 541.83 | 122.65 | 503.13 | 119.97 |
| Primary care | 701.30 | 115.38 | 549.32 | 102.40 |
| Secondary care | 2663.85 | 1119.02 | 597.11 | 283.86 |
| Total | 10,163.01 | 1266.37 | 9857.51 | 888.44 |
The length of stay varied between intervention arms (SNS: range 0 to 59 days, median 0 days, mean 1.43 days; FENIX: range 0 to 15 days, median 1 day, mean 1.39 days).
The economic evaluation results for the 18-month trial period are included in Table 26. Results are presented for complete case and following MI using EQ-5D-5L valuation, EQ-5D-5L to 3L cross-walk and SF-6D utility scores for alternative adjusted models.
| Analysis | Costs (£) | QALYs | ICER | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | FENIX (95% CIs) | n | SNS (95% CIs) | Incremental (95% CIs) | n | FENIX (95% CIs) | n | SNS (95% CIs) | Incremental (95% CIs) | Unadjusted | Adjusted for baseline utility + age | Adjusted for baseline utility + age + surgeon | |
| Complete-case analysis | |||||||||||||
| EQ-5D-5L | 24 | 11,360.25 (6506.76 to 16,213.74) | 23 | 10,913.08 (7870.44 to 13,955.73) | 447.17 (–4912.45 to 5806.79) | 24 | 1.157 (1.042 to 1.273) | 23 | 1.148 (1.018 to 1.277) | 0.010 (–0.150 to 0.170) | 44,051.88 (n = 47) | 117,153.71 (n = 47) | 440.57 (n = 46) |
| EQ–5D-3L cross-walk | 24 | 11,360.25 (6506.76 to 16,213.74) | 23 | 10,913.08 (7870.44 to 13,955.73) | 447.17 (–4912.45 to 5806.79) | 24 | 1.030 (0.895 to 1.166) | 23 | 1.023 (0.888 to 1.159) | 0.007 (–0.170 to 0.185) | 62,121.75 (n = 47) | Dominated (n = 47) | 303.05 (n = 46) |
| SF-6D | 24 | 11,546.51 (6685.34 to 16,407.68) | 23 | 9597.22 (6342.64 to 12,851.81) | 1949.28 (–3517.45 to 7416.02) | 24 | 0.975 (0.890 to 1.051) | 23 | 1.038 (0.950 to 1.125) | –0.062 (–0.169 0.045) | Dominated (n = 47) | Dominated (n = 47) | Dominated (n = 45) |
| Imputed data set | |||||||||||||
| EQ-5D-5L | 47 | 10,163.01 (7610.86 to 12,715.17) | 47 | 9857.51 (8067.04 to 11,647.99) | 305.50 (–2694.59 to 3305.58) | 47 | 1.129 (1.050 to 1.207) | 47 | 1.124 (1.039 to 1.209) | 0.005 (–0.106 to 0.115) | 67,697.50 (n = 94) | 44,785.62a (n = 94) | 124,387.65 (n = 87) |
| EQ–5D-3L cross-walk | 47 | 10,164.88 (7613.59 to 12,716.16) | 47 | 9869.06 (8077.85 to 11,660.27) | 295.82 (–2702.91 to 3294.54) | 47 | 1.019 (0.934 to 1.103) | 47 | 0.986 (0.890 to 1.082) | 0.032 (–0.091 to 0.156) | 9193.58 (n = 94) | 12,804.46 (n = 94) | Dominated (n = 87) |
| SF-6D | 47 | 10,152.95 (7607.32 to 12,698.58) | 47 | 9853.67 (8062.22 to 11,645.12) | 299.28 (–2696.15 to 3294.72) | 47 | 0.994 (0.940 to 1.049) | 47 | 0.997 (0.944 to 1.050) | –0.003 (–0.076 to 0.070) | Dominated (n = 94) | 344,418.94 (n = 94) | 100,461.45 (n = 87) |
For the EQ-5D-5L and SF-6D analyses, there were a total of 47 cases across both arms that met the complete-case criteria (of which 42 cases were common across both the EQ-5D-5L and the SF-6D complete cases), whereas the MI analysis sample total was 94 cases. Five of the original 99 cases were dropped because they had no baseline data or only baseline data. In all analyses, FENIX was more expensive in the trial period than SNS, although this was reduced in the imputed analyses. In all of the EQ-5D-5L analyses, except for the complete-case, 3L cross-walk with adjustment (for age and baseline utility), FENIX led to a QALY benefit compared with SNS. We present the results for adjustment for operating surgeon, but because of the small sample sizes these figures are highly variable and cannot be considered robust.
The ICER for the primary analysis (MI, direct EQ-5D-5L valuation with adjustment for age and baseline utility) is £44,785.62 per QALY gained and is well above the £20,000 threshold. In this analysis, FENIX was associated with modest additional costs and negligible incremental QALYs compared with SNS. Uncertainty in the results was represented in the CEAC (Figure 7). At a willingness-to-pay threshold of £20,000 per QALY gain, FENIX has a 41.3% chance of being cost-effective.
FIGURE 7.
Cost-effectiveness acceptability curve.

The supplementary analysis using MI and a cross-walk to derive EQ-5D-3L values yields a much higher QALY gain and lower ICER for FENIX. The analyses based on the SF-6D indicated a QALY loss associated with FENIX, which was therefore dominated.
The proportion of patients distributed between the CCIS percentage improvement groups is shown in Table 23 (parameter table). For both arms, a majority of patients were either in the < 50% improvement group or did not have a device in situ. Where the device was in situ, the results appear to indicate a slight benefit for the SNS group, with more patients in the 50–75% and > 75% improvement groups. One patient in the FENIX arm had died by 18 months and, although this is unlikely to be attributable to randomisation arm, we retained this patient in the analysis.
The costs and benefits estimated during the trial period were added to the start of the model and assumed to be fixed in the base case deterministic analysis. In each simulation of the probabilistic analysis, we used a bootstrapped cost and QALY generated from the trial data.
Decision modelling
The model Markov trace is included in Figure 8. This gives an indication of the predicted intervention effectiveness and survival over time. There are very few noticeable differences in the curves over the lifetime horizon. The kinks in the SNS figure show where those patients with a device in situ receive a battery replacement at 7 years and the redistribution among CCIS percentage improvement groups.
FIGURE 8.
Markov model traces: (a) SNS; and (b) FENIX.


The deterministic model results, including lifetime costs, QALYs and ICER, are presented in Table 27. Estimated lifetime costs are higher in the SNS group, as are the QALYs. The ICER and NMB values indicate that FENIX is not cost-effective over this time horizon (SNS is the optimal strategy). These results are mirrored by the probabilistic results, where the ICER for SNS versus FENIX drops.
| Intervention | Costs (discounted) | QALYs (discounted) | Incremental cost (discounted) | Incremental QALYs (discounted) | ICER | NMB |
|---|---|---|---|---|---|---|
| Deterministic | ||||||
| FENIX | £18,657 | 10.12 | £183,806 | |||
| SNS | £19,972 | 10.33 | £1315 | 0.20 | £6508 | £186,531 |
| Probabilistic | ||||||
| FENIX | £18,668 | 10.09 | £183,130 | |||
| SNS | £19,975 | 10.32 | £1306 | 0.23 | £5694 | £186,413 |
Figure 9 plots the 10,000 costs and QALYs from the Monte Carlo simulations. The results highlight significant uncertainty in the model outputs. The ICER of the mean value falls just below the willingness-to-pay threshold (for SNS minus FENIX). The uncertainty in the results is further represented in the CEAC (Figure 10). At a willingness-to-pay threshold of £20,000 per QALY gain, SNS has a 55% chance of being the optimal strategy (and, therefore, FENIX has a 45% chance of being the optimal strategy).
FIGURE 9.
Cost-effectiveness plane: SNS vs. FENIX. The orange dot represents the ICER using the mean from the cost and QALY simulations.
FIGURE 10.
Cost-effectiveness acceptability curve.

Table 28 includes a range of deterministic sensitivity analyses. The analyses include using complete-case costs and QALYs from the trial, changing the year at which battery replacement is conducted in the SNS group, changes to health state costs and utilities, and assuming equivalent effectiveness across interventions at 18 months. In most analyses, the decision is unchanged (i.e. SNS remains the optimal strategy). A significant drop in the utility estimates for those without a device in situ substantially reduces the QALY gain from SNS over time and changes the decision such that FENIX would be the optimal strategy. FENIX also becomes the optimal strategy (cost saving but marginally less effective) when the proportions of patients across CCIS percentage improvement and explant groups are assumed to be equivalent at the trial end.
| Intervention | Costs | QALYs | Incremental cost | Incremental QALYs | ICER | NMB |
|---|---|---|---|---|---|---|
| Base case scenario | ||||||
| FENIX | £18,657 | 10.12 | £183,806 | |||
| SNS | £19,972 | 10.33 | £1315 | 0.20 | £6508 | £186,531 |
| Complete-case scenario | ||||||
| FENIX | £19,855 | 10.15 | £183,189 | |||
| SNS | £21,027 | 10.35 | £1173 | 0.20 | £5973 | £185,943 |
| Battery replacement at year 5 | ||||||
| FENIX | £18,657 | 10.12 | £183,806 | |||
| SNS | £20,681 | 10.33 | £2023 | 0.21 | £9830 | £185,899 |
| Additional battery replacement at year 14 (only cost implications) | ||||||
| FENIX | £18,657 | 10.12 | £183,806 | |||
| SNS | £20,300 | 10.33 | £1643 | 0.20 | £8134 | £186,203 |
| 50% increase in FI cost after explant (£1264) | ||||||
| FENIX | £25,486 | 10.12 | £176,977 | |||
| SNS | £27,555 | 10.33 | £2069 | 0.20 | £10,245 | £178,948 |
| Cost of FI assuming 100% use pads, 7.5% use irrigation, 2.5% use anal plug and 7.5% have a stoma (£1895 on average per year; base case £632) | ||||||
| FENIX | £32,303 | 10.12 | £170,160 | |||
| SNS | £34,798 | 10.33 | £2495 | 0.20 | £12,353 | £171,705 |
| 25% reduction in the utility for patients after device explanted | ||||||
| FENIX | £18,657 | 8.32 | £147,792 | |||
| SNS | £20,300 | 8.43 | £1643 | 0.10 | £15,784 | £148,230 |
| 50% reduction in the utility for patients after device explanted | ||||||
| FENIX | £18,657 | 6.52 | £111,777 | |||
| SNS | £20,300 | 6.53 | £1643 | 0.01 | £265,313 | £110,258 |
| FENIX proportion of patients at end of trial equal to SNS | ||||||
| FENIX | £18,904 | 10.32 | £187,542 | |||
| SNS | £20,300 | 10.33 | £1397 | 0.00 | £489,114 | £186,203 |
Table 29 includes the per patient and population EVPI. These values reflect a monetary cost (attaching a value of £20,000 to a QALY) of the uncertainty in the current decision problem. At the NICE willingness-to-pay per QALY threshold of £20,000, there was a 55% and a 45% chance that SNS and FENIX, respectively, were the optimal intervention and, hence, there was significant uncertainty remaining in the decision. Given the large number of potential intervention recipients, the cost of this uncertainty over 1 year and 10 years is considerable. The EVPI estimates indicate that further research in this area is warranted to reduce the level of decision uncertainty.
| Parameter | Value |
|---|---|
| Per person EVPI | £9630 |
| FI annual incidence | 13,126 |
| Total population EVPI at 1 year | £126,403,677 |
| Total population EVPI at 10 years | £1,264,036,774 |
Chapter 4 Discussion
Although the SaFaRI trial failed to recruit the planned 350 participants, it is nevertheless the largest reported evaluation of the MAS (FENIX) device to our knowledge, providing important information about circumanal implants to augment anal sphincter function and treat FI. Despite initial encouraging results in small, single-centre studies, which provided the rationale for this larger randomised clinical trial, the FENIX device was taken off the market in 2017, when the manufacturing company (Torax Medical, Minneapolis, MN, USA) was purchased by Johnson and Johnson (New Brunswick, NJ, USA). The decision to discontinue marketing the FENIX device was said to be strategic, with Johnson and Johnson continuing to market a related device (LINX®) for the treatment of gastro-oesophageal reflux.
The SaFaRI trial involved 18 pelvic floor centres, with colorectal surgeons from across the UK who were members of the Association of Coloproctology of Great Britain and Ireland. The majority of cases were recruited from a single site (St James’s University Hospital, Leeds), which may have an impact on the generalisability of the results. In mitigation, strenuous attempts were made to standardise the technique for MAS implantation with preceptorship of training cases performed to manufacturer guidelines. The technique for SNS implantation was left to individual surgeons to reflect normal practice.
The study population was typical of that suffering from moderate to severe FI, with the majority of participants being female and a median age of 59.0 years. In accordance with the known aetiology of FI, previous obstetric injury accounted for the majority of cases, with mixed passive and urge incontinence being the predominant symptomatology.
Previous studies of SNS have reported outcomes at time points relative to the patient’s operation (either temporary or permanent SNS), which makes comparison with the results of the SaFaRI trial difficult. In the SaFaRI trial, the patient outcomes were measured at time points relative to randomisation (i.e. from the key decision-making event, rather than from operation). This approach evaluates the effect of the decision of which intervention to give the patient. It provides data that is more informative to clinicians and patients when weighing up the risks and benefits of surgical treatment for FI, and is preferred over the previously reported per-protocol analyses.
The 18-month follow-up post randomisation was chosen with the expectation that definitive device operations (FENIX and permanent SNS) would occur within around 6 months post randomisation, giving patients around 12 months’ follow-up post operation. The median time of 57.0 days from randomisation to implantation of a FENIX device probably conforms to normal NHS practice. The median time of 371 days from randomisation to implantation of a permanent SNS device, although longer than our a priori expectation, might not be unusual in the NHS, where there are surgical capacity issues and benign conditions tend to be given low priority. This delay in undertaking permanent SNS implantation had a bearing on the number of patients who had a device in situ and had completed 18 months’ follow-up at the time of stopping the study.
In total, five (10%) participants withdrew from surgery in the FENIX group and did not undergo device implantation. This compared with five (10.2%) participants not receiving a temporary SNS device, four (8.2%) participants not undergoing implantation, and a further 12 (24.5%) participants not proceeding from a temporary device to a permanent device owing to lack of efficacy. This gives an intention-to-treat figure of 57.1% and a per-protocol figure of 62.2% of patients progressing to a permanent implant, which is generally in keeping with the literature. However, the success rate at 18 months, as judged by the intention-to-treat analysis, was very low at 8.2%. Even on a per-protocol basis, of the 28 participants who received a permanent SNS implant, only four (14.3%) reported a successful outcome with the device in situ and sustained benefit at 18 months’ follow-up. This contrasts with the literature, which generally reports success rates between 50 and 70%. In a long-term analysis of 407 patients undergoing SNS, Altomare et al. 44 reported progression from temporary to permanent implant in 66.8% of patients. At a median of 84 months’ follow-up, success was maintained in 71.3% of patients in per-protocol analysis and 47.7% on intention-to-treat analysis. 44 Similar results were reported in the systematic review published by Thin et al.,45 with the median success rate of SNS, on intention to treat, being 63%, 58% and 54% on short-, medium-, and long-term follow-up, respectively.
The reason for the large discrepancy between the success rates observed in SaFaRI and those reported in the literature is not clear. One possible explanation is the rigour of data collection within the context of a RCT, giving a more accurate reflection of true success. It is possible that the large number of sites participating in SaFaRI, compared with the single-centre studies reported in the literature, may have captured data from widely different practices, although all participating surgeons were experienced with the SNS technique prior to participation in the study. The proportion of patients who progressed from the temporary SNS device to the permanent SNS device is similar to the proportions seen in the literature. The discrepancy is seen in the number of successes in the patients who received the permanent SNS implant. Four patients did not receive the permanent SNS device before 18 months post randomisation, and so were classed as ‘failures’ according to the definition of the primary end point, which may explain some of the discrepancy. Most other studies have used a 50% reduction in the number of FI episodes per week as their end point rather than 50% improvement in CCIS, which was used in SaFaRI. This could also explain some of the discrepancy between the results of SaFaRI and those reported in the literature.
A similar low success rate was observed with the FENIX device, with only six (13.3%) successes out of 45 participants who underwent device implantation. Again, this is much lower than in the reported literature. Pakravan et al. 46 reported a success rate of 76% in 18 patients undergoing FENIX implantation, defined as a ≥ 50% reduction in FI rates per week, with no explants. In a longer-term study, Sugrue et al. 47 reported on 37 FENIX patients with a median follow-up of 5 years. Therapeutic success rates at 1, 3 and 5 years were 63%, 66% and 53%, respectively. The lower success rate of the FENIX device in SaFaRI appears to have been because of a higher proportion of patients not experiencing an improvement in CCIS (36.6% of the 41 patients with complete data) and a higher explant rate (33% of the 45 implants).
Both the FENIX and the SNS interventions proved to be relatively safe, with few intraoperative complications. However, the postoperative complications were high, particularly in the FENIX group (73.3%), with participants receiving the FENIX intervention being 11 times more likely to suffer a postoperative complication, a difference that did not appear to be related to the effect of the operating surgeon. Notably, the most commonly reported complication in the FENIX group was worsening symptoms of obstructed defaecation (20.5%). Recommendation was made that patients should have an ODS of < 8 for entry to SaFaRI. The baseline characteristics for the whole group showed a median ODS of 7.0 (range 2.0–18.0), and perhaps with more stringent inclusion criteria this complication would have been reduced. However, there was no difference in ODS between the two treatment arms and having a device in situ did not have a significant effect on ODS. It is possible that patients in the FENIX group were more likely to attribute any obstructed defaecation symptoms to the implanted device. The high complication rate with the FENIX device is mirrored in the literature. Sugrue et al. 47 reported 30 adverse events in 35 patients undergoing FENIX implantation, with the most common being defaecatory dysfunction (20%), followed by pain (14%), erosion (11%) and infection (11%). 47 In Pakravan et al. ’s46 smaller study of 18 patients, the most common complication was postoperative pain (29%). The FENIX explant rate observed in SaFaRI was much higher than previously reported, with 15 out of the 45 implants (33.3%) being explanted by the 18-month time point, with the majority explanted within 6 months. Similarly, the complication rate in the SNS group (22.5%) was higher than might be expected from the literature. This might be a result of rigorous data collection within a randomised trial and the fact that all possible complications were recorded, which is not always the case in single-centre cohort studies.
For those patients who retained a device, there was a statistically significant benefit in terms of reduction in CCIS, but there was no difference between the treatment groups, with the effect sustained throughout follow-up. This benefit was independent of baseline CCIS. Likewise, for patients who retained a device there was a sustained improvement in QoL as calculated by the EQ-5D-5L score, but not the VAS, with no difference between the treatment groups. The FIQoL showed a similar pattern, with improvement in all QoL domains if the device remained in situ. Both Pakravan et al. 46 and Sugrue et al. 47 reported improvement in FIQoL scores across all domains following FENIX implantation, although several studies attest to the benefit of SNS in improving FIQoL. 48–50 The benefit from a functioning FENIX or SNS device appears to be more on physical than mental abilities, as judged by the SF-12 v2 analysis.
An economic evaluation of FENIX compared with SNS was undertaken from the perspective of the social and health-care provider. Estimates of cost-effectiveness were produced over the trial period and over a lifetime horizon using a de novo decision model.
At the end of the trial, slightly lower costs were observed in the SNS group despite the fact that intervention costs were significantly higher in this group. There were notably higher secondary care costs in the FENIX arm, which appears to be driven by the complication and other surgery rates. There were also higher (self-reported) primary care costs in the FENIX arm.
There was a small QALY intervention differential at 18 months in favour of FENIX; however, at 18 months, the ICERs for FENIX compared with SNS were > £20,000, except for analyses using cross-walking to EQ-5D-3L. The patient proportions across health state groups at the end of the trial (feeding into the decision model) slightly favoured SNS, despite this group having a higher proportion of patients without a device in situ. The trial results were highly sensitive to choice of estimation model and utility derivation strategy.
The lifetime expected costs and QALYs generated by the decision model were a reversal of those observed at 18 months; that is, in the long term, FENIX was less costly but also less effective. The base case analysis still indicated that FENIX was not cost-effective compared with SNS and that the latter strategy was optimal. This finding held in most sensitivity analyses, except where there were significant reductions in the explanted group QoL and where the effectiveness at trial end was assumed to be equivalent between arms.
Our model estimated lifetime costs similar to the 5 years’ costs (€22,150) estimated elsewhere for SNS,13 and it is unclear what drives this disparity. Another evaluation of SNS also used a 5-year time horizon32 yielding similar 5-year SNS costs (£13,829–19,153). There are few other published economic evaluations in this population37,51 and none comparing SNS with FENIX. One abstract reports this direct comparison over 1 year and finds similar results to ours (FENIX being more effective and more costly) over this period, but finding an ICER of < £20,000. 52
Limitations
The SaFaRI study was limited by the small number of patients recruited as a consequence of the FENIX device being taken off the market less than one-third of the way through the study. Any conclusions therefore have to be taken with caution, given that the sample size was inadequate to show a difference between the two treatments. Despite the small numbers, important information has been gained, particularly relating to the patient pathways, delays in treatment and the complication profiles of the two treatments.
As expected, given the small sample and loss to follow-up, there is significant uncertainty in the cost-effectiveness results. The 18 months’ results were influenced by the approach to dealing with missing data and utility assessment. It is possible that the difference in post-procedure follow-up timing across arms may have affected the relative costs and QALYs captured, increasing uncertainty. The longer-term results were heavily reliant on the effectiveness data from the trial, which are relatively weak. A number of assumptions were necessary in the decision modelling (not all of which were incorporated in sensitivity analyses) and these may have a significant impact on the model outputs. A larger sample size would have permitted greater confidence in the model parameter values generated from the trial data. More nuanced approaches may also have been enabled (e.g. having differing effectiveness decay rates for the two interventions). Our analyses were limited to the health-care perspective; taking a broader view (e.g. including patient out-of-pocket costs) may have affected results, especially if those without a device in situ covered a proportion of conservative management costs themselves.
Given these factors and the level of uncertainty in the results, it is difficult to commend one intervention over another on the basis of the evidence presented. The results from the value of information analysis suggest that further research is warranted.
Future research
Future research might include longer follow-up of SaFaRI participants to ensure accurate capture of efficacy and explant rates in the longer term. There would be scope for further economic modelling to include standard (non-operative) care and additional data from other studies, and new devices for FI. Because SaFaRI was terminated early, as a result of a buyout of the manufacturer, the sample size is greatly reduced from that originally planned. There is a possible option of undertaking a meta-analysis of a similar study undertaken in France52 to gain a better understanding of the efficacy and safety of the FENIX and SNS devices.
Chapter 5 Conclusions
The SaFaRI study suggests that neither FENIX nor SNS is as effective in treating FI of moderate to severe severity as previously reported in the literature. The FENIX device was associated with a high postoperative morbidity, including the risk of explantation within the first year. The FENIX device was withdrawn from the market in 2017 and the results from SaFaRI would not support its reintroduction in its current form. Similar high rates of complications and explantation have been observed with other circumanal sphincter augmentation devices, such as the Acticon Neosphincter® (American Medical Systems, Minneapolis, MN, USA). New strategies are needed for anal sphincter augmentation that avoid the use of prosthetic implants that can become infected and erode.
Although the results for SNS, as observed in SaFaRI, were disappointing, it remains a recommended treatment by NICE for patients with moderate to severe FI that is resistant to medical management. It is unlikely, given its established place in surgical practice, that the use of SNS will decline as a consequence of SaFaRI. However, more research is required to unravel why the pathways for SNS are so inefficient, with patients suffering long waiting times for treatment of a disabling condition. The technique for SNS continues to evolve and new devices are coming to the market. The NIHR SUBSoNIC study is currently recruiting and investigating the efficacy and mechanism of subsensory (optimised) neuromodulation in adults with FI. 53 It will be interesting to see how the outcomes of this study will compare with those of SaFaRI.
Use of our health economics model to incorporate synthesised evidence from other studies and explore other scenarios (e.g. new devices with longer battery lives) may be informative in the future. Previous research indicated that SNS was only marginally cost-effective compared with conservative management12 and perhaps the latter strategy could be included in a wider evaluation.
Acknowledgements
We are indebted to the patients who participated in this trial. We would also like to thank the TSC (Charles Knowles, Doreen McClurg, Graeme MacLennan and Tara Willson), the DMEC (Paul-Antoine LeHur, Natalie Rowland and Lynn Shaw), additional members of the Trial Management Group (Gregory Taylor, Sushil Maslekar, David Meads, Armando Vargas-Palacios, Alison Smith, Debbie Beirne, Adam Douglas, Jacqueline Emkes, Catherine Moriarty, David Protheroe, Jen Lodge and Karen Nugent) and the CTRU Project Team for their important contributions. Sarah Abraham provided assistance during the economic evaluation.
Patient and public involvement
The SaFaRI study had patient and public involvement (PPI) throughout its lifetime, from the initial stages of protocol development through to trial completion. Adam Douglas and Jacqueline Emkes served on the Trial Management Group and Tara Willson was a member of the TSC.
All three PPI representatives regularly attended trial oversight committee meetings and were actively involved in trial discussions, providing valued opinions and ideas from a patient and public perspective. PPI input also fed into the protocol design and the drafting/review of participant information resources.
The following institutions and surgeons participated in the trial:
Bristol Royal Infirmary: Jonathan Randall; The Churchill Hospital: Helen Jones, Ian Lindsey and Kim Gorissen; Derriford Hospital: Chris Oppong; Dewsbury and District Hospital: Adeshina Fawole; Good Hope Hospital: Haney Youssef and Mark Chapman; Leicester Royal Infirmary: Andrew Miller; Manchester Royal Infirmary: Finlay Curran; Northern General Hospital: Steve Brown; Poole Hospital: Andrew Clarke; Royal Devon and Exeter Hospital: Patricia Boorman; Royal Victoria Infirmary: Stefan Plusa; Southampton General Hospital: Sophie Pilkington; St James’s University Hospital: David Jayne, Julian Hance and Sushil Maslekar; St Mark’s Hospital: Carolynne Vaizey; St Peter’s Hospital: Pasha Nisar; University College London Hospital: Richard Cohen and James Crosbie; University Hospital of North Durham: Susan Green; Wythenshawe Hospital: Karen Telford.
Contributions of authors
David G Jayne (https://orcid.org/0000-0002-8725-3283) (Consultant Colorectal Surgeon), Neil Corrigan (https://orcid.org/0000-0002-1424-9830) (Statistician), Julie Croft (https://orcid.org/0000-0001-7586-3394) (Clinical Triallist), Vicky Napp (https://orcid.org/0000-0001-6726-2222) (Clinical Triallist) and Julia M Brown (https://orcid.org/0000-0002-2719-7064) (Statistician) designed the study and were involved in study co-ordination.
Annabelle E Williams (https://orcid.org/0000-0001-8832-6686) (ST7 Specialist Trainee in Colorectal and General Surgery) was the Clinical Research Fellow for the study, and provided invaluable clinical input during site set-up and the initial stages of recruitment.
Neil Corrigan, Alison Pullan (https://orcid.org/0000-0001-6060-9123) (Statistician) and Julia M Brown were responsible for the statistical analysis.
Rachel Kelly (https://orcid.org/0000-0002-2912-2859) (Clinical Triallist) was responsible for data management and was involved in trial co-ordination.
David Meads (https://orcid.org/0000-0003-1369-2483) (Health Economist), Armando Vargas-Palacios (https://orcid.org/0000-0002-6503-0134) (Health Economist) and Adam Martin (https://orcid.org/0000-0002-2559-6483) (Health Economist) conducted the cost analysis and reporting.
Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Health Economist) was involved in the design of the health economics component of the study.
David G Jayne, Steven R Brown (https://orcid.org/0000-0002-0980-2793) (Consultant Colorectal Surgeon), Sushil Maslekar (https://orcid.org/0000-0002-8233-8480) (Consultant Colorectal Surgeon), Andrew Clarke (https://orcid.org/0000-0003-3585-8704) (Consultant Colorectal Surgeon) and Pasha Nisar (https://orcid.org/0000-0001-8374-6761) (Consultant Colorectal Surgeon) made significant contributions to participant recruitment.
Karen Nugent (https://orcid.org/0000-0002-0408-2950) (Consultant Colorectal Surgeon), Jen Lodge (https://orcid.org/0000-0002-5814-6630) (Senior Community Incontinence Advisor), David Protheroe (https://orcid.org/0000-0001-9139-0726) (Consultant Liaison Psychiatrist) contributed to the trial design and sat on the SaFaRI Trial Management Group.
Steven R Brown also contributed to the trial design.
All authors contributed to data interpretation and the writing and review of the manuscript.
Publication
William AE, Croft J, Napp V, Corrigan N, Brown JM, Hulme C, et al. SaFaRI: sacral nerve stimulation versus the FENIX magnetic sphincter augmentation for adult faecal incontinence: a randomised investigation. Int J Colorectal Dis 2016;31:465–72.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Williams AE, Croft J, Napp V, Corrigan N, Brown JM, Hulme C, et al. SaFaRI: sacral nerve stimulation versus the FENIX magnetic sphincter augmentation for adult faecal incontinence: a randomised investigation. Int J Colorectal Dis 2016;31:465-72. https://doi.org/10.1007/s00384-015-2492-3.
- National Institute for Health and Care Excellence (NICE) . Faecal Incontinence in Adults [QS54] 2014. www.nice.org.uk/guidance/qs54 (accessed 11 May 2019).
- Ferrara A, De Jesus S, Gallagher JT, Williamson PR, Larach SW, Pappas D, et al. Time-related decay of the benefits of biofeedback therapy. Tech Coloproctol 2001;5:131-5. https://doi.org/10.1007/s101510100014.
- Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD007959.pub2.
- Maeda Y, Lundby L, Buntzen S, Laurberg S. Suboptimal outcome following sacral nerve stimulation for faecal incontinence. Br J Surg 2011;98:140-7. https://doi.org/10.1002/bjs.7302.
- Mundy L, Merlin TL, Maddern GJ, Hiller JE. Systematic review of safety and effectiveness of an artificial bowel sphincter for faecal incontinence. Br J Surg 2004;91:665-72. https://doi.org/10.1002/bjs.4587.
- Chapman AE, Geerdes B, Hewett P, Young J, Eyers T, Kiroff G, et al. Systematic review of dynamic graciloplasty in the treatment of faecal incontinence. Br J Surg 2002;89:138-53. https://doi.org/10.1046/j.1365-2168.2002.02018.x.
- Matzel KE, Stadelmaier U, Hohenfellner M, Gall FP. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet 1995;346:1124-7. https://doi.org/10.1016/S0140-6736(95)91799-3.
- Melenhorst J, Koch SM, Uludag O, van Gemert WG, Baeten CG. Sacral neuromodulation in patients with faecal incontinence: results of the first 100 permanent implantations. Colorectal Dis 2007;9:725-30. https://doi.org/10.1111/j.1463-1318.2007.01241.x.
- Tjandra JJ, Lim JF, Matzel K. Sacral nerve stimulation: an emerging treatment for faecal incontinence. ANZ J Surg 2004;74:1098-106. https://doi.org/10.1111/j.1445-1433.2004.03259.x.
- Altomare DF, Ratto C, Ganio E, Lolli P, Masin A, Villani RD. Long-term outcome of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2009;52:11-7. https://doi.org/10.1007/DCR.0b013e3181974444.
- Dudding TC, Meng Lee E, Faiz O, Parés D, Vaizey CJ, McGuire A, et al. Economic evaluation of sacral nerve stimulation for faecal incontinence. Br J Surg 2008;95:1155-63. https://doi.org/10.1002/bjs.6237.
- Hetzer FH, Bieler A, Hahnloser D, Löhlein F, Clavien PA, Demartines N. Outcome and cost analysis of sacral nerve stimulation for faecal incontinence. Br J Surg 2006;93:1411-17. https://doi.org/10.1002/bjs.5491.
- National Institute for Health and Care Excellence (NICE) . Interventional Procedure Guidance 99: Sacral Nerve Stimulation for Faecal Incontinence 2004. www.nice.org.uk/guidance/ipg99 (accessed 7 February 2021).
- Matzel KE. Sacral nerve stimulation for faecal incontinence: its role in the treatment algorithm. Colorectal Dis 2011;13:10-4. https://doi.org/10.1111/j.1463-1318.2010.02519.x.
- Barussaud ML, Mantoo S, Wyart V, Meurette G, Lehur PA. The magnetic anal sphincter in faecal incontinence: is initial success sustained over time?. Colorectal Dis 2013;15:1499-503. https://doi.org/10.1111/codi.12423.
- Wong MT, Meurette G, Stangherlin P, Lehur PA. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum 2011;54:773-9. https://doi.org/10.1007/DCR.0b013e3182182689.
- Gray M. How to Get Better Value Health Care. Witney: Alden Press; 2007.
- National Institute for Health Research Horizon Scanning Centre . FENIX® Continence Restoration System for Severe Chronic Faecal Incontinence n.d. www.hsc.nihr.ac.uk/topics/fenix-continence-restoration-system-for-severe-chr/.
- NIHR Horizon Scanning Centre . FENIX® Contience Restoration System for Severe Chronic Faecal Incontinence 2012. http://io.nihr.ac.uk/wp-content/uploads/migrated/2220.838f0fdf.FENIXforfaecalincontinenceFINAL.pdf (accessed 3 February 2020).
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77-9. https://doi.org/10.1007/BF02050307.
- Renzi A, Brillantino A, Di Sarno G, d’Aniello F. Five-item score for obstructed defecation syndrome: study of validation. Surg Innov 2013;20:119-25. https://doi.org/10.1177/1553350612446354.
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 2000;43:9-16. https://doi.org/10.1007/BF02237236.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- The EuroQol Group . EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013: Process and Methods (PMG9) 2013. https://nice.org.uk/process/pmg9/chapter/foreword (accessed 3 April 2019).
- Brooks RG, Jendteg S, Lindgren B, Persson U, Björk S. EuroQol: health-related quality of life measurement. Results of the Swedish questionnaire exercise. Health Policy 1991;18:37-48. https://doi.org/10.1016/0168-8510(91)90142-K.
- Kind P, Dolan P. The effect of past and present illness experience on the valuations of health states. Med Care 1995;33:A255-63.
- Brazier JE, Roberts J, Deverill M. The estimation of a preference based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Hounsome N, Roukas C. Cost-effectiveness of sacral nerve stimulation and percutaneous tibial nerve stimulation for faecal incontinence. Therap Adv Gastroenterol 2018;11. https://doi.org/10.1177/1756284818802562.
- NHS Improvement . NHS Reference Costs 2017–2018 n.d. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed 31 May 2019).
- Fiebig DG, Baltagi B. A Companion to Theoretical Econometrics. Malden, MA: Blackwell Publishing Ltd; 2001.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- van Wunnik BP, Visschers RG, van Asselt AD, Baeten CG. Cost-effectiveness analysis of sacral neuromodulation for faecal incontinence in The Netherlands. Colorectal Dis 2012;14:e807-14. https://doi.org/10.1111/codi.12002.
- Office for National Statistics (ONS) . National Life Tables: UK 2020. https://ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 3 February 2020).
- NHS England . Clinical Commissioning Policy: Sacral Nerve Stimulation (SNS) for Faecal Incontinence (Adult) 2013. www.england.nhs.uk/wp-content/uploads/2013/08/a08-p-b.pdf (accessed 26 April 2019).
- National Institute for Health and Care Excellence . Axonics Sacral Neuromodulation System for Overactive Bladder and Faecal Incontinence. Medtech Innovation Briefing [MIB164] 2018. www.nice.org.uk/advice/mib164/chapter/The-technology (accessed 26 April 2019).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 31 May 2019).
- Wilson EC. A practical guide to value of information analysis. Pharmacoeconomics 2015;33:105-21. https://doi.org/10.1007/s40273-014-0219-x.
- Altomare DF, Giuratrabocchetta S, Knowles CH, Muñoz Duyos A, Robert-Yap J, Matzel KE. European SNS Outcome Study Group . Long-term outcomes of sacral nerve stimulation for faecal incontinence. Br J Surg 2015;102:407-15. https://doi.org/10.1002/bjs.9740.
- Thin NN, Horrocks EJ, Hotouras A, Palit S, Thaha MA, Chan CL, et al. Systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg 2013;100:1430-47. https://doi.org/10.1002/bjs.9226.
- Pakravan F, Helmes C. Magnetic anal sphincter augmentation in patients with severe fecal incontinence. Dis Colon Rectum 2015;58:109-14. https://doi.org/10.1097/DCR.0000000000000263.
- Sugrue J, Lehur PA, Madoff RD, McNevin S, Buntzen S, Laurberg S, et al. Long-term experience of magnetic anal sphincter augmentation in patients with fecal incontinence. Dis Colon Rectum 2017;60:87-95. https://doi.org/10.1097/DCR.0000000000000709.
- Duelund-Jakobsen J, Lehur PA, Lundby L, Wyart V, Laurberg S, Buntzen S. Sacral nerve stimulation for faecal incontinence – efficacy confirmed from a two-centre prospectively maintained database. Int J Colorectal Dis 2016;31:421-8. https://doi.org/10.1007/s00384-015-2411-7.
- Irwin GW, Dasari BV, Irwin R, Johnston D, Khosraviani K. Outcomes of sacral nerve stimulation for faecal incontinence in Northern Ireland. Ulster Med J 2017;86:20-4.
- Moya P, Arroyo A, Lacueva J, Candela F, Soriano-Irigaray L, López A, et al. Sacral nerve stimulation in the treatment of severe faecal incontinence: long-term clinical, manometric and quality of life results. Tech Coloproctol 2014;18:179-85. https://doi.org/10.1007/s10151-013-1022-y.
- Indinnimeo M, Ratto C, Moschella CM, Fiore A, Brosa M, Giardina S. Sacral neuromodulation for the treatment of fecal incontinence: analysis of cost-effectiveness. Dis Colon Rectum 2010;53:1661-9. https://doi.org/10.1007/DCR.0b013e3181f46309.
- Bulsei J, Lehur P, Durand-Zaleski I. A Comparison of Magnetic Anal Sphincter and Sacral Neuromodulation for Fecal Incontinence: the MOS-STIC Study Cost-effectiveness Results. European Society of Coloproctology; 2018.
- National Institute for Health Research . SUBSoNIC Clinical Trial n.d. www.qmul.ac.uk/pctu/about-us/clinical-strengths-and-studies/our-studies/subsonic/ (accessed 19 May 2019).
- National Institute for Health and Care Excellence (NICE) . Peristeen Transanal Irrigation System for Managing Bowel Dysfunction 2018. www.nice.org.uk/guidance/mtg36 (accessed 10 February 2021).
- National Institute for Health and Care Excellence (NICE) . Infliximab, Adalimumab and Golimumab for Treating Moderately to Severely Active Ulcerative Colitis After the Failure of Conventional Therapy 2015. www.nice.org.uk/guidance/ta329/resources/ (accessed 10 February 2021).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
Appendix 1
Summaries of components of the ‘success’ end point at 6, 12 and 18 months post randomisation
| Success | FENIX MSA, n (%) | SNS, n (%) | Total, n (%) |
|---|---|---|---|
| 6 months post randomisation | |||
| Unsuccessful | 33 (86.8) | 18 (100.0) | 51 (91.1) |
| Successful | 5 (13.2) | 0 (0.0) | 5 (8.9) |
| Total | 38 (100) | 18 (100) | 56 (100) |
| 12 months post randomisation | |||
| Unsuccessful | 35 (87.5) | 26 (96.3) | 61 (91.0) |
| Successful | 5 (12.5) | 1 (3.7) | 6 (9.0) |
| Total | 40 (100) | 27 (100) | 67 (100) |
| 18 months post randomisation | |||
| Unsuccessful | 35 (85.4) | 35 (89.7) | 70 (87.5) |
| Successful | 6 (14.6) | 4 (10.3) | 10 (12.5) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| ≥ 50% improvement in CCIS? | FENIX MSA, n (%) | SNS, n (%) | Total, n (%) |
|---|---|---|---|
| 6 months post randomisation | |||
| No | 33 (86.8) | 18 (100.0) | 51 (91.1) |
| Yes | 5 (13.2) | 0 (0.0) | 5 (8.9) |
| Total | 38 (100) | 18 (100) | 56 (100) |
| 12 months post randomisation | |||
| No | 35 (87.5) | 25 (92.6) | 60 (89.6) |
| Yes | 5 (12.5) | 2 (7.4) | 7 (10.4) |
| Total | 40 (100) | 27 (100) | 67 (100) |
| 18 months post randomisation | |||
| No | 34 (82.9) | 34 (87.2) | 68 (85.0) |
| Yes | 7 (17.1) | 5 (12.8) | 12 (15.0) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| Device in use? | FENIX MSA, n (%) | SNS, n (%) | Total, n (%) |
|---|---|---|---|
| 6 months post randomisation | |||
| Yes | 27 (71.1) | 5 (27.8) | 32 (57.1) |
| No | 11 (28.9) | 13 (72.2) | 24 (42.9) |
| Total | 38 (100) | 18 (100) | 56 (100) |
| 12 months post randomisation | |||
| Yes | 25 (62.5) | 11 (40.7) | 36 (53.7) |
| No | 15 (37.5) | 16 (59.3) | 31 (46.3) |
| Total | 40 (100) | 27 (100) | 67 (100) |
| 18 months post randomisation | |||
| Yes | 21 (51.2) | 16 (41.0) | 37 (46.3) |
| No | 20 (48.8) | 23 (59.0) | 43 (53.8) |
| Total | 41 (100) | 39 (100) | 80 (100) |
| Improvement in continence | Device in use? | ||
|---|---|---|---|
| No, n (%) | Yes, n (%) | Total, n (%) | |
| FENIX MSA (6 months post randomisation) | |||
| ≥ 50% improvement in CCIS? | |||
| No | 11 (100.0) | 22 (81.5) | 33 (86.8) |
| Yes | 0 (0.0) | 5 (18.5) | 5 (13.2) |
| Total | 11 (100) | 27 (100) | 38 (100) |
| SNS (6 months post randomisation) | |||
| ≥ 50% improvement in CCIS? | |||
| No | 13 (100.0) | 5 (100.0) | 18 (100.0) |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 13 (100) | 5 (100) | 18 (100) |
| FENIX MSA (12 months post randomisation) | |||
| ≥ 50% improvement in CCIS? | |||
| No | 15 (100.0) | 20 (80.0) | 35 (87.5) |
| Yes | 0 (0.0) | 5 (20.0) | 5 (12.5) |
| Total | 15 (100) | 25 (100) | 40 (100) |
| SNS (12 months post randomisation) | |||
| ≥ 50% improvement in CCIS? | |||
| No | 15 (93.8) | 10 (90.9) | 25 (92.6) |
| Yes | 1 (6.3) | 1 (9.1) | 2 (7.4) |
| Total | 16 (100) | 11 (100) | 27 (100) |
| FENIX MSA (18 months post randomisation) | |||
| CCIS improvement? | |||
| No | 19 (95.0) | 15 (71.4) | 34 (82.9) |
| Yes | 1 (5.0) | 6 (28.6) | 7 (17.1) |
| Total | 20 (100) | 21 (100) | 41 (100) |
| SNS (18 months post randomisation) | |||
| CCIS improvement? | |||
| No | 22 (95.7) | 12 (75.0) | 34 (87.2) |
| Yes | 1 (4.3) | 4 (25.0) | 5 (12.8) |
| Total | 23 (100) | 16 (100) | 39 (100) |
Exploratory analyses: additional covariates
Cleveland Clinic Incontinence Score
There were 96 out of 99 (97%) patients included in the analysis, meaning that they each provided their CCIS at least once at one of the baseline, 6-, 12- or 18-month time points.
The CCIS data were modelled using a three-level multilevel model, with time points clustered within patients and patients clustered within surgeons. The estimates of the fixed effects can be seen in Table 34. The random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 5.93 (SE 1.26). The residual of the random effects was 7.13 (SE 0.71).
| Covariate | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Intercept | 11.1227 | 1.1579 | < 0.0001 | 8.6546 to 13.5907 |
| FENIX MSA vs. SNS | 0.1052 | 0.6643 | 0.8744 | –1.2048 to 1.4152 |
| Male vs. female | 1.0012 | 1.6859 | 0.5533 | –2.3234 to 4.3258 |
| Baseline CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | 3.4638 | 1.1137 | 0.0021 | 1.2677 to 5.6600 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 0.5030 | 0.6384 | 0.4316 | –0.7547 to 1.7624 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | 0.7415 | 1.1433 | 0.5174 | –0.7558 to 1.7619 |
| Time (months) | –0.07833 | 0.07518 | 0.2987 | –1.5131 to 2.9961 |
| Device in use | –3.0386 | 0.7209 | < 0.0001 | –4.4601 to –1.6171 |
| Time and baseline CCIS interaction (CCIS > 10 points vs. CCIS ≤ 10 points) | 0.03473 | 0.07793 | 0.6564 | –0.1189 to 0.1884 |
| Device in use and treatment interaction | –0.1304 | 0.8309 | 0.8755 | –1.7688 to 1.5080 |
Patients who were randomised to the FENIX MSA arm have a baseline CCIS 0.11 points lower than patients randomised to SNS; however, this result is not statistically significant (p = 0.9). Patients having a device in use reduces the CCIS by 3.04 points, a result that is statistically significant (p < 0.0001); however, the interaction term is not statistically significant (p = 0.9). The estimated change in CCIS over time is small; there is a reduction of 0.078 points per month on average, although the CI around this estimate is large owing to the small number of patients. The baseline CCIS (≤ 10 points or > 10 points) has a significant effect on the CCIS (p = 0.005), as would be expected due to them using the same measure; however, the interaction between baseline CCIS and time was not significant (p = 0.8).
The results of the model can be seen in Figure 11. This figure shows the difference between two identical patients, with the only difference being the randomised treatment, and assuming they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 11.
Cleveland Clinic Incontinence Score model results.

EuroQol-5 Dimensions, five-level version
There were 96 out of 99 (97%) patients included in the analysis, meaning that 96 patients provided a score at least once at one of the baseline, 6-, 12- or 18-month time points.
The EQ-5D-5L score was modelled using a three-level multilevel model, with time points clustered within patients and patients clustered within surgeons. The fixed effects of the model are presented in Table 35. The random effect caused by surgeon was 0.00 (SE 0.003) and the random effect caused by within-patient measurements was 0.04 (SE 0.007). The residual of the random effects was 0.03 (SE 0.003). The model did not show a significant difference in the score between treatment arms (p = 0.76) or over time (p = 0.18). The device being in use has a statistically significant difference of 0.13 (p = 0.004), meaning that patients with their device in use have an EQ-5D-5L score 0.13 points higher than patients without the device in use, and this difference is the same for patients randomised to both treatment arms, as can be seen by the non-significance of the device in use and treatment interaction (p = 0.2). There is no indication that there is a clustering effect caused by the randomising surgeon.
| Covariate | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| EQ-5D-5L score model | ||||
| Intercept | 0.7408 | 0.07531 | < 0.0001 | 0.5803 to 0.9013 |
| FENIX MSA vs. SNS | 0.01453 | 0.04771 | 0.7610 | –0.07953 to 0.1086 |
| Male vs. female | 0.03300 | 0.1249 | 0.7918 | –0.2132 to 0.2792 |
| Baseline CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –0.1180 | 0.06974 | 0.0922 | –0.2555 to 0.01950 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 0.01844 | 0.04755 | 0.6986 | –0.07530 to 0.1122 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –0.02196 | 0.08345 | 0.7927 | –0.1865 to 0.1426 |
| Time (months) | –0.00250 | 0.001854 | 0.1785 | –0.00616 to 0.001152 |
| Device in use | 0.1300 | 0.04494 | 0.0042 | 0.04144 to 0.2186 |
| Device in use and treatment interaction | –0.06466 | 0.05166 | 0.2121 | –0.1665 to 0.03718 |
| EQ-5D-5L VAS model | ||||
| Intercept | 81.1491 | 6.4029 | < 0.0001 | 67.5016 to 94.7967 |
| FENIX MSA vs. SNS | –4.3211 | 3.7654 | 0.2525 | –11.7444 to 3.1022 |
| Male vs. female | –4.9907 | 9.9532 | 0.6166 | –24.6127 to 14.6313 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –14.1538 | 6.1549 | 0.0225 | –26.2878 to –2.0199 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 1.4308 | 3.7453 | 0.7028 | –5.9529 to 8.8144 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | 2.7676 | 6.7168 | 0.6807 | –10.4741 to 16.0093 |
| Time (months) | –0.02979 | 0.3702 | 0.9359 | –0.7596 to 0.7001 |
| Device in use | 5.8162 | 3.5563 | 0.1035 | –1.1947 to 12.8272 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | 0.04212 | 0.3831 | 0.9126 | –0.7131 to 0.7973 |
| Device in use and treatment interaction | –4.6053 | 4.0771 | 0.2600 | –12.6431 to 3.4324 |
The results of the model can be seen in Figure 12, which presents estimates and 95% CIs for the EQ-5D-5L score at 6, 12 and 18 months post randomisation for two patients who are identical except for their randomised treatment, and assuming that they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 12.
EQ-5D score model results.
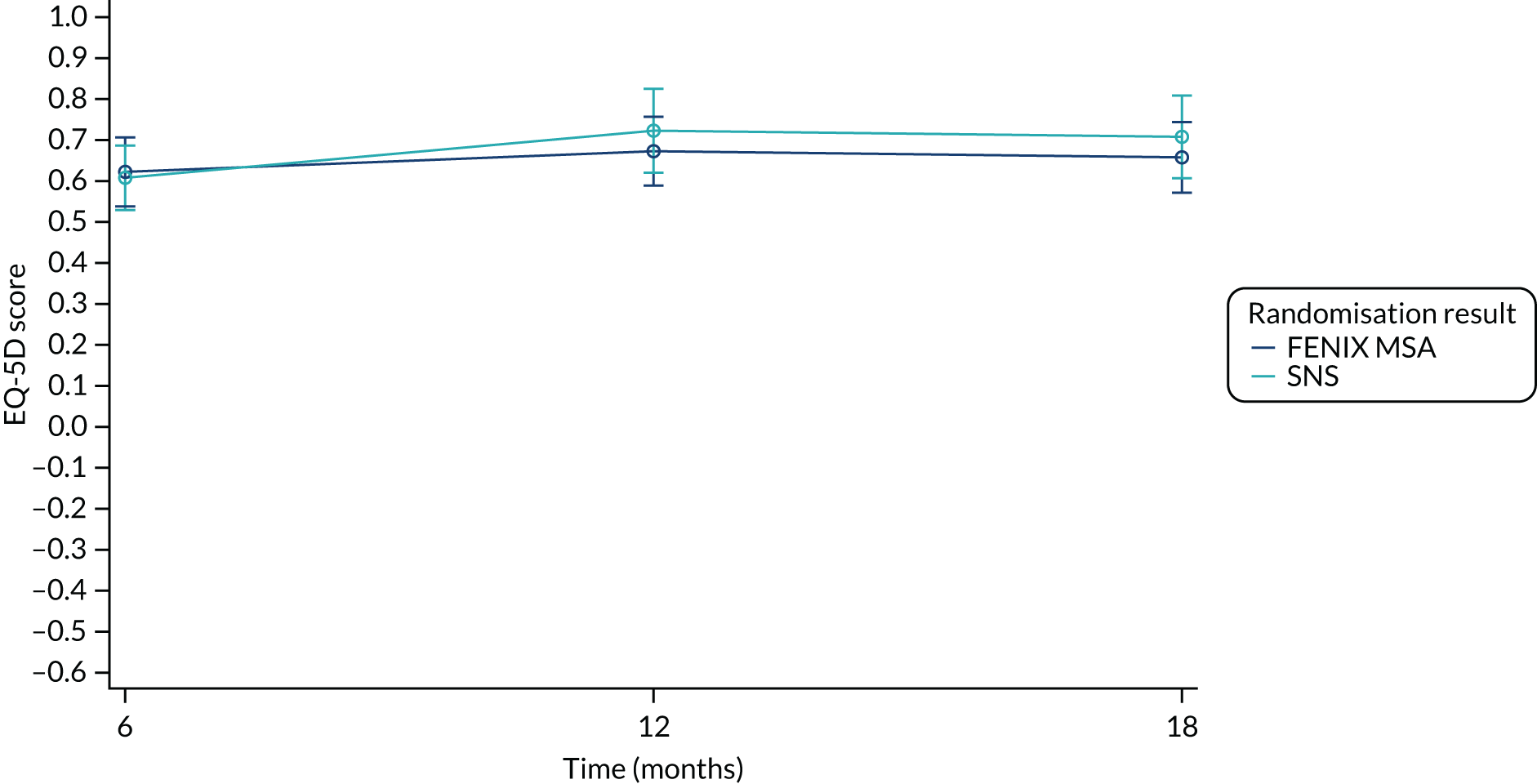
The EQ-5D-5L questionnaire also records the respondent’s self-rated health on a vertical VAS ranging from 0–100, in which higher numbers indicate better health.
A total of 97 out of 99 (98%) patients were included in the VAS analysis, meaning that they provided a VAS score at least at one time point.
The VAS was modelled using a three-level multilevel model with time points clustered within patients and patients clustered within surgeons. The fixed effects of the model can be seen in Table 35. The random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 233.05 (SE 44.06). The residual of the random effects was 169.71 (SE 16.62). The randomised treatment has no significant effect on the VAS score (p = 0.25) and there is no significant difference over time between the two treatment arms (p = 0.9). The baseline CCIS minimisation factor has a statistically significant effect (p = 0.023); however, the effect over time is not significant, meaning that the baseline VAS score is higher for patients who have a baseline CCIS ≤ 10 points, but the VAS score changes at the same rate for patients with a baseline CCIS ≤ 10 points and > 10 points. The device being in use does not have a significant effect on the VAS score (p = 0.1) and the effect is not significantly different between the treatment arms (p = 0.3) There is no indication of a clustering effect caused by the randomising surgeon.
The results of the model can be seen in Figure 13, which presents estimates and 95% CIs for the EQ-5D-5L VAS score at 6, 12 and 18 months post randomisation for two patients who are identical except for their randomised treatment, and assuming that they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 13.
EQ-5D VAS model results.
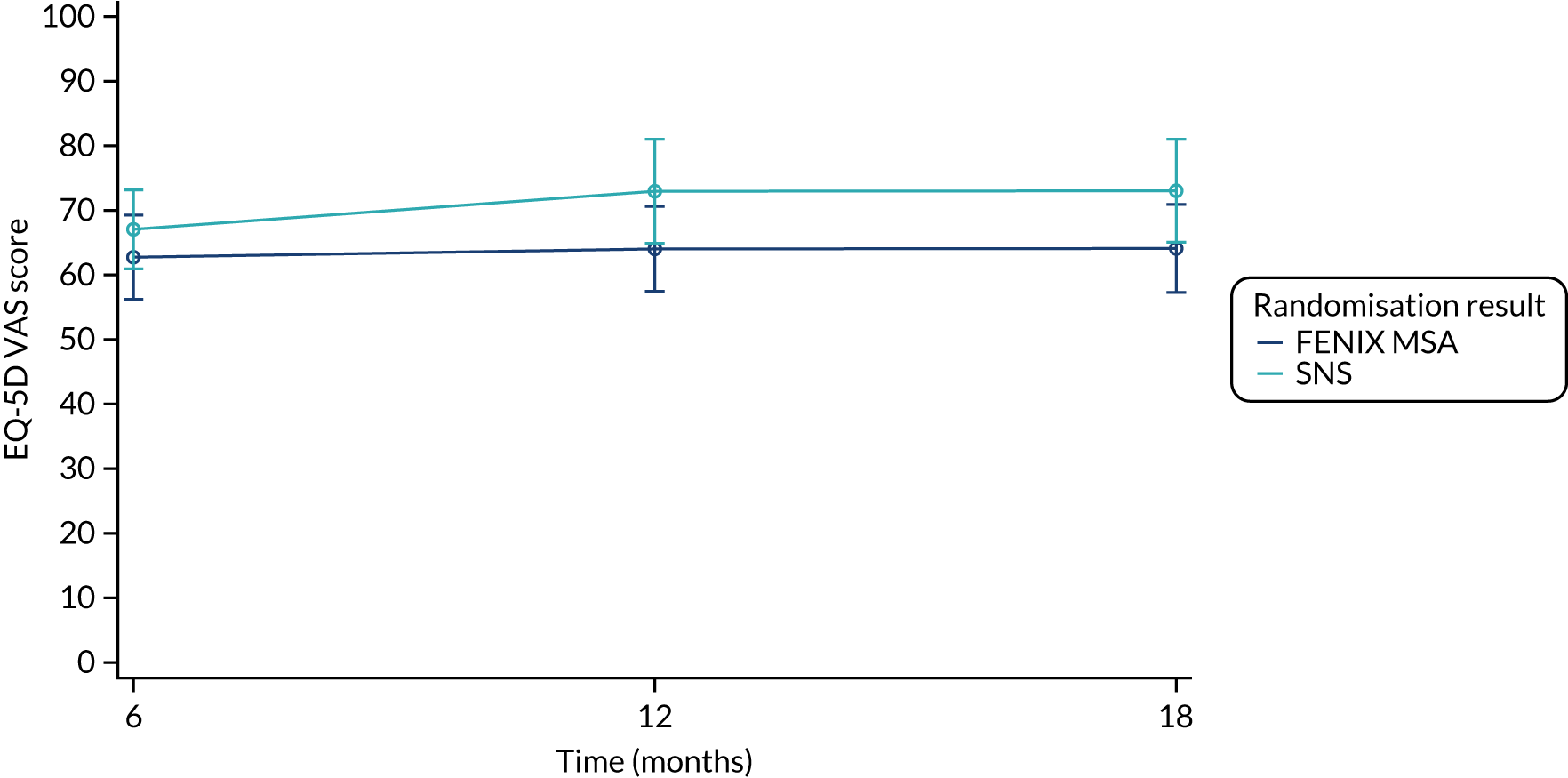
The model diagnostics can be seen in Figures 14 and 15.
FIGURE 14.
Model residuals for EQ-5D-5L score. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.
FIGURE 15.
Model residuals for EQ-5D VAS. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.

Faecal incontinence quality of life
The results of the models fitted are summarised in Table 36. None of the domains shows any significant difference between the treatment arms. All of the domains show a statistically significant improvement in FIQoL when the device is in use and there is no significant difference in the improvement between the two treatment arms.
| Covariate | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Lifestyle domain | ||||
| Intercept | 2.9449 | 0.3039 | < 0.0001 | 2.2971 to 3.5927 |
| FENIX MSA vs. SNS | 0.01600 | 0.1824 | 0.9302 | –0.3437 to 0.3757 |
| Male vs. female | –0.4174 | 0.4905 | 0.3958 | –1.3850 to 0.5501 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –0.8378 | 0.2893 | 0.0042 | –1.4085 to –0.2672 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | –0.03691 | 0.1849 | 0.8420 | –0.4017 to 0.3278 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –0.01219 | 0.3427 | 0.9717 | –0.6882 to 0.6639 |
| Time (months) | 0.000148 | 0.000462 | 0.7491 | –0.00076 to 0.001059 |
| Device in use | 0.4124 | 0.1526 | 0.0075 | 0.1113 to 0.7135 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | 0.000119 | 0.000482 | 0.8051 | –0.00083 to 0.001070 |
| Device in use and treatment interaction | 0.06514 | 0.1701 | 0.7022 | –0.2705 to 0.4007 |
| Coping domain | ||||
| Intercept | 2.0434 | 0.2566 | < 0.0001 | 1.4931 to 2.5937 |
| FENIX MSA vs. SNS | 0.004001 | 0.1468 | 0.9783 | –0.2862 to 0.2942 |
| Male vs. female | 0.1544 | 0.3750 | 0.6811 | –0.5869 to 0.8958 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –0.6468 | 0.2415 | 0.0083 | –1.1242 to –0.1694 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 0.03141 | 0.1497 | 0.8341 | –0.2645 to 0.3273 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –0.03069 | 0.2533 | 0.9037 | –0.5314 to 0.4700 |
| Time (months) | 0.000235 | 0.000544 | 0.6666 | –0.00084 to 0.001311 |
| Device in use | 0.5340 | 0.1441 | 0.0003 | 0.2493 to 0.8188 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | 0.000168 | 0.000568 | 0.7685 | –0.00096 to 0.001291 |
| Device in use and treatment interaction | 0.2217 | 0.1693 | 0.1926 | –0.1131 to 0.5564 |
| Depression domain | ||||
| Intercept | 3.2273 | 0.3340 | <0.0001 | 2.5109 to 3.9436 |
| FENIX MSA vs. SNS | 0.05262 | 0.1948 | 0.7874 | –0.3323 to 0.4376 |
| Male vs. female | 0.01222 | 0.4939 | 0.9803 | –0.9638 to 0.9882 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –0.8544 | 0.3116 | 0.0069 | –1.4701 to –0.2387 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | –0.1388 | 0.2016 | 0.4924 | –0.5372 to 0.2597 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –0.1428 | 0.3481 | 0.6823 | –0.8307 to 0.5452 |
| Time (months) | –6.32E-6 | 0.000557 | 0.9910 | –0.00111 to 0.001095 |
| Device in use | 0.4244 | 0.1617 | 0.0096 | 0.1048 to 0.7439 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | 0.000017 | 0.000581 | 0.9772 | –0.00113 to 0.001165 |
| Device in use and treatment interaction | –0.04992 | 0.1872 | 0.7901 | –0.4199 to 0.3201 |
| Embarrassment domain | ||||
| Intercept | 2.3581 | 0.2489 | < 0.0001 | 1.8276 to 2.8886 |
| FENIX MSA vs. SNS | –0.01209 | 0.1465 | 0.9343 | –0.3008 to 0.2766 |
| Male vs. female | 0.3032 | 0.3921 | 0.4402 | –0.4697 to 1.0761 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –0.7196 | 0.2382 | 0.0067 | –1.1891 to –0.2500 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 0.003584 | 0.1475 | 0.9806 | –0.2873 to 0.2945 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –0.2464 | 0.2634 | 0.3505 | –0.7656 to 0.2727 |
| Time (months) | 0.000492 | 0.000441 | 0.2662 | –0.00038 to 0.001361 |
| Device in use | 0.3192 | 0.1252 | 0.0115 | 0.07244 to 0.5659 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | –0.00038 | 0.000457 | 0.4107 | –0.00128 to 0.000525 |
| Device in use and treatment interaction | 0.2677 | 0.1433 | 0.0632 | –0.01482 to 0.5503 |
In the lifestyle domain, the random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 0.61 (SE 0.10). The residual of the random effects was 0.25 (SE 0.03).
In the coping domain, the random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 0.32 (SE 0.06). The residual of the random effects was 0.22 (SE 0.03).
In the depression domain, the random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 0.60 (SE 0.11). The residual of the random effects was 0.27 (SE 0.03).
In the embarrassment domain, the random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 0.38 (SE 0.07). The residual of the random effects was 0.21 (SE 0.02).
The results of the models can be seen in Figures 16–19, which present estimates and 95% CIs for each of the FIQoL domains at 6, 12 and 18 months post randomisation for two patients who are identical except for their randomised treatment, and assuming that they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 16.
The FIQoL lifestyle model results.
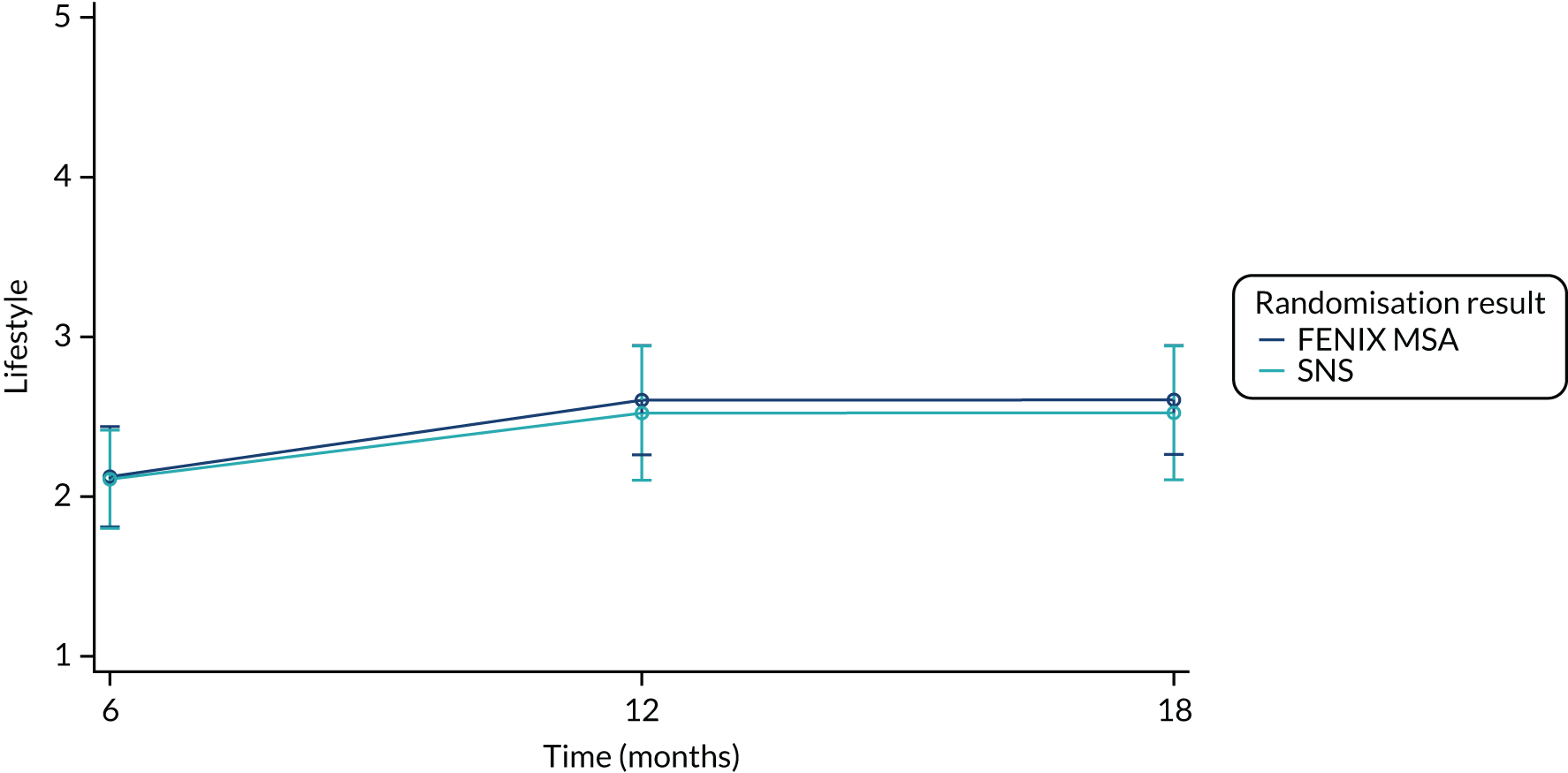
FIGURE 17.
The FIQoL coping model results.
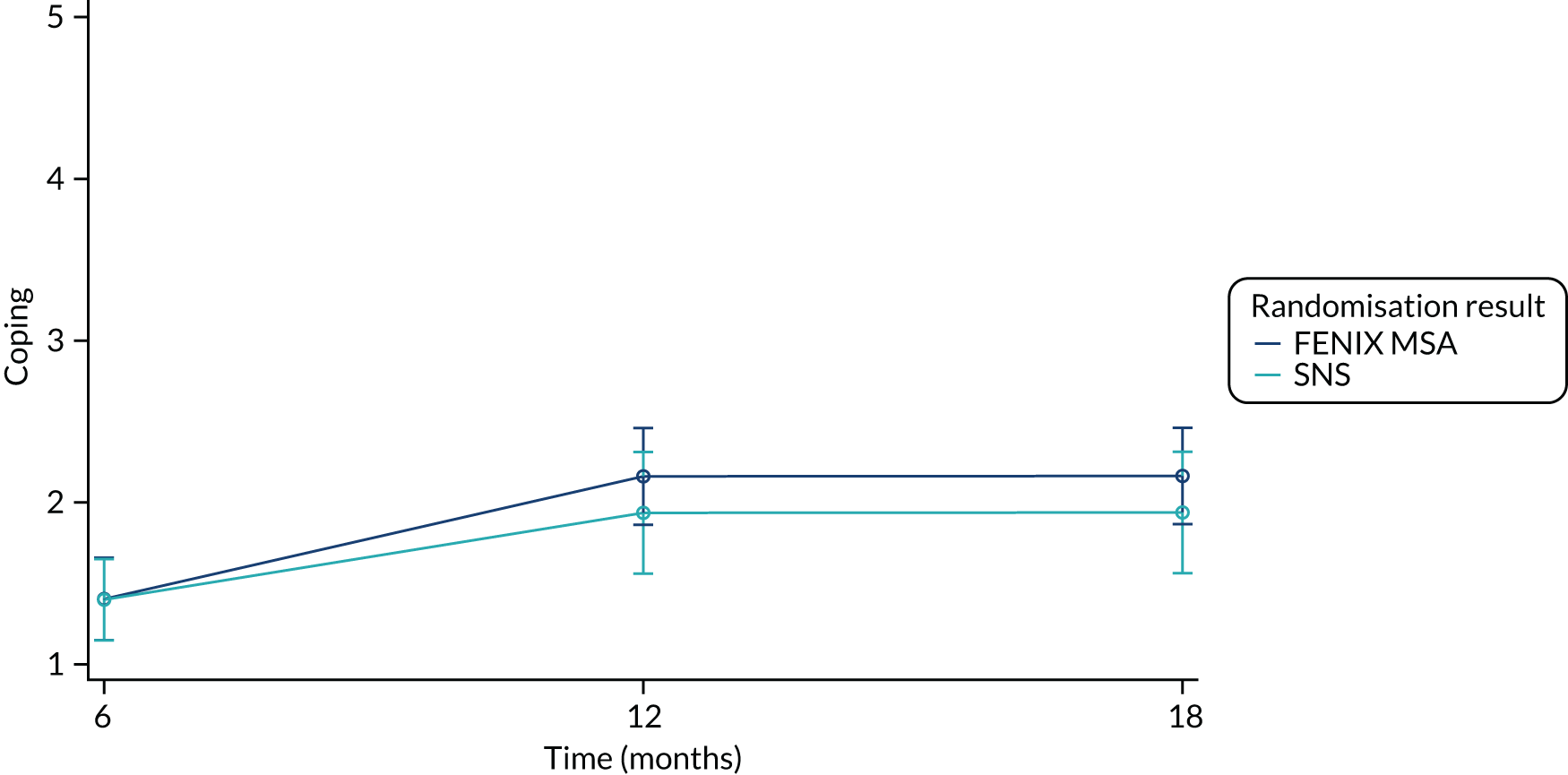
FIGURE 18.
The FIQoL depression model results.
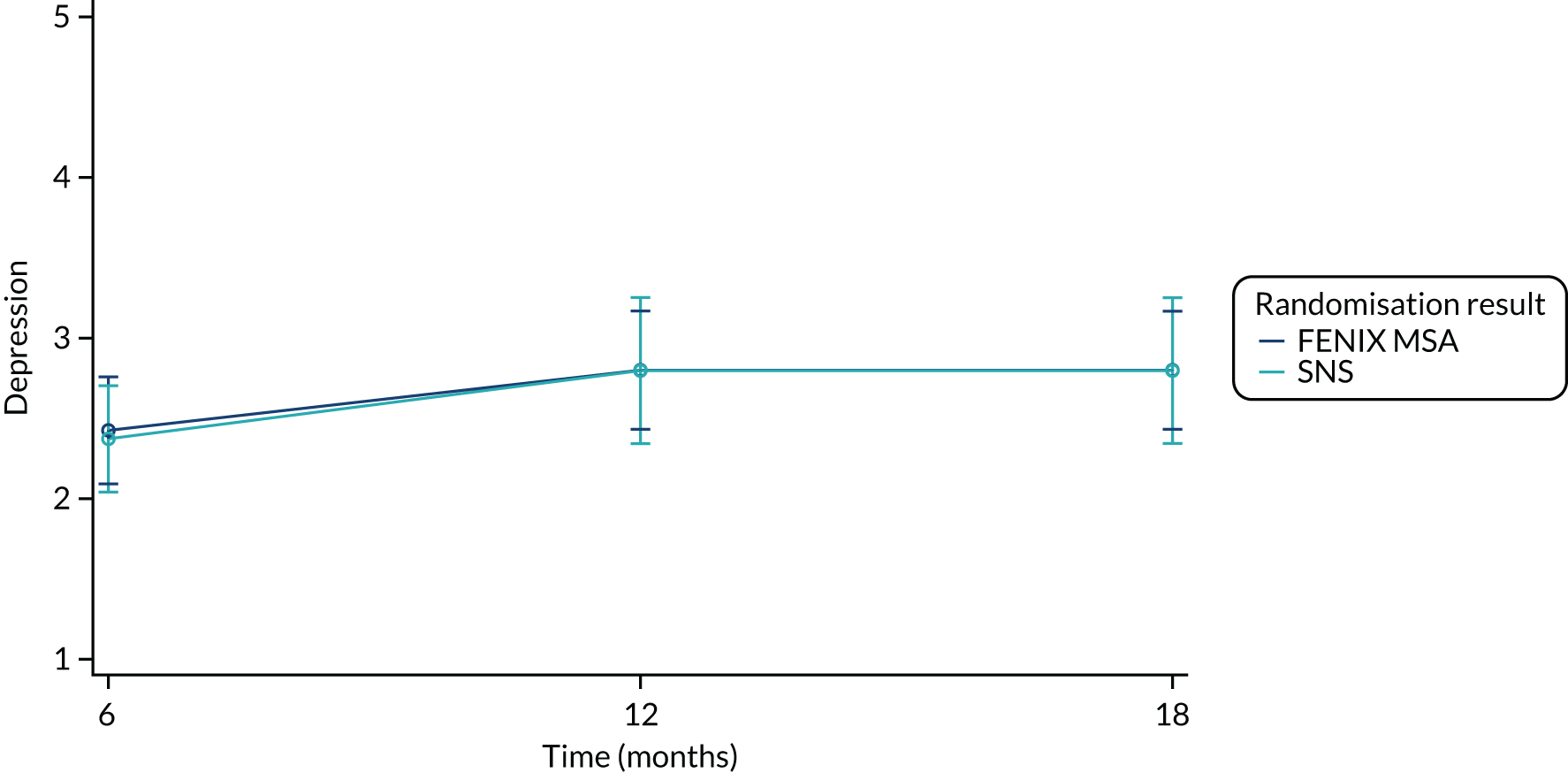
FIGURE 19.
The FIQoL embarrassment model results.
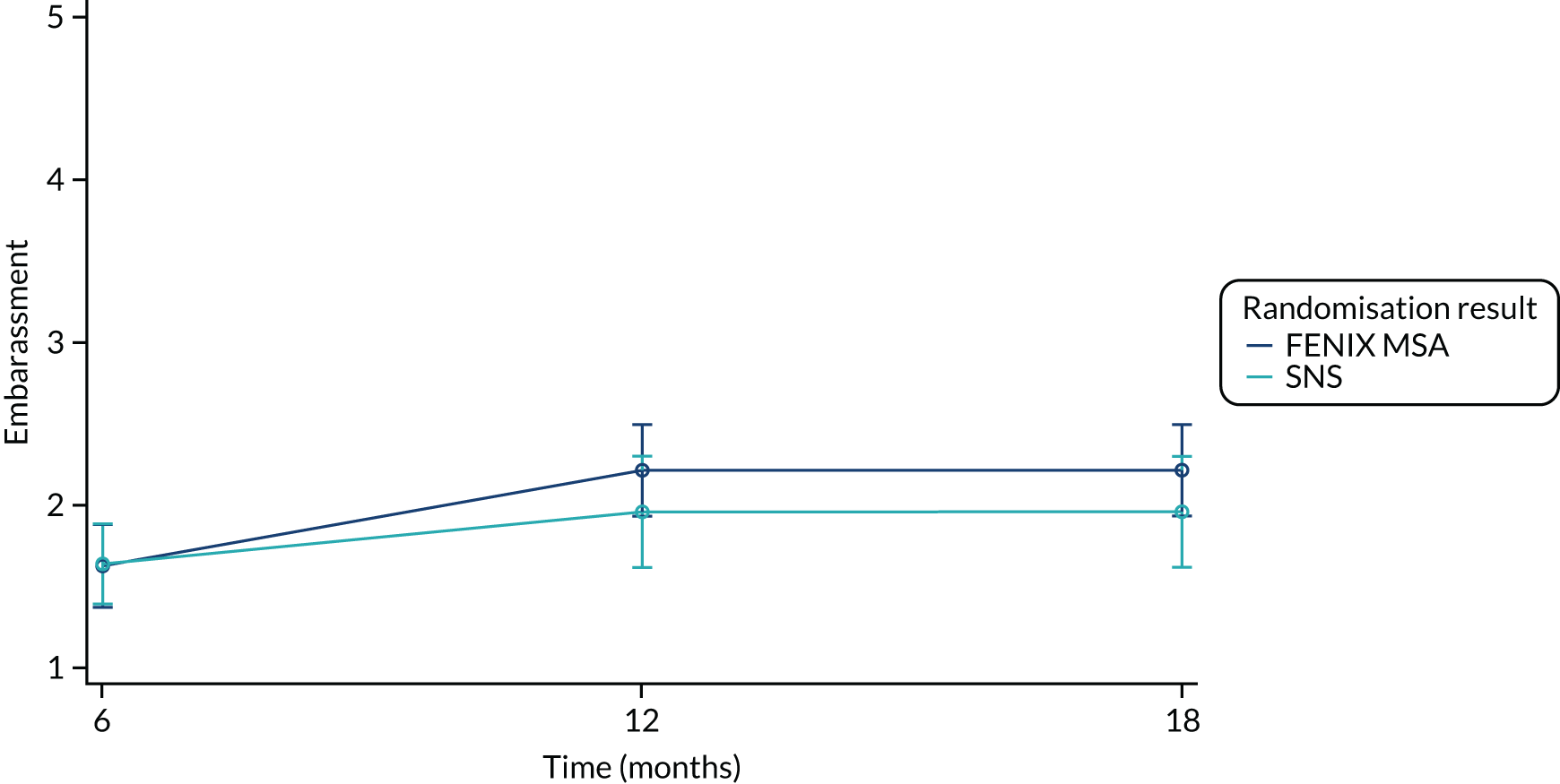
The model diagnostics corresponding to models of the lifestyle, coping, depression and embarrassment domains of the FIQoL can be seen in Figures 20–23, respectively.
FIGURE 20.
Model residuals for FIQoL: lifestyle. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.

FIGURE 21.
Model residuals for FIQoL: coping. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.
FIGURE 22.
Model residuals for FIQoL: depression. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.

FIGURE 23.
Model residuals for FIQoL: embarrassment. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.
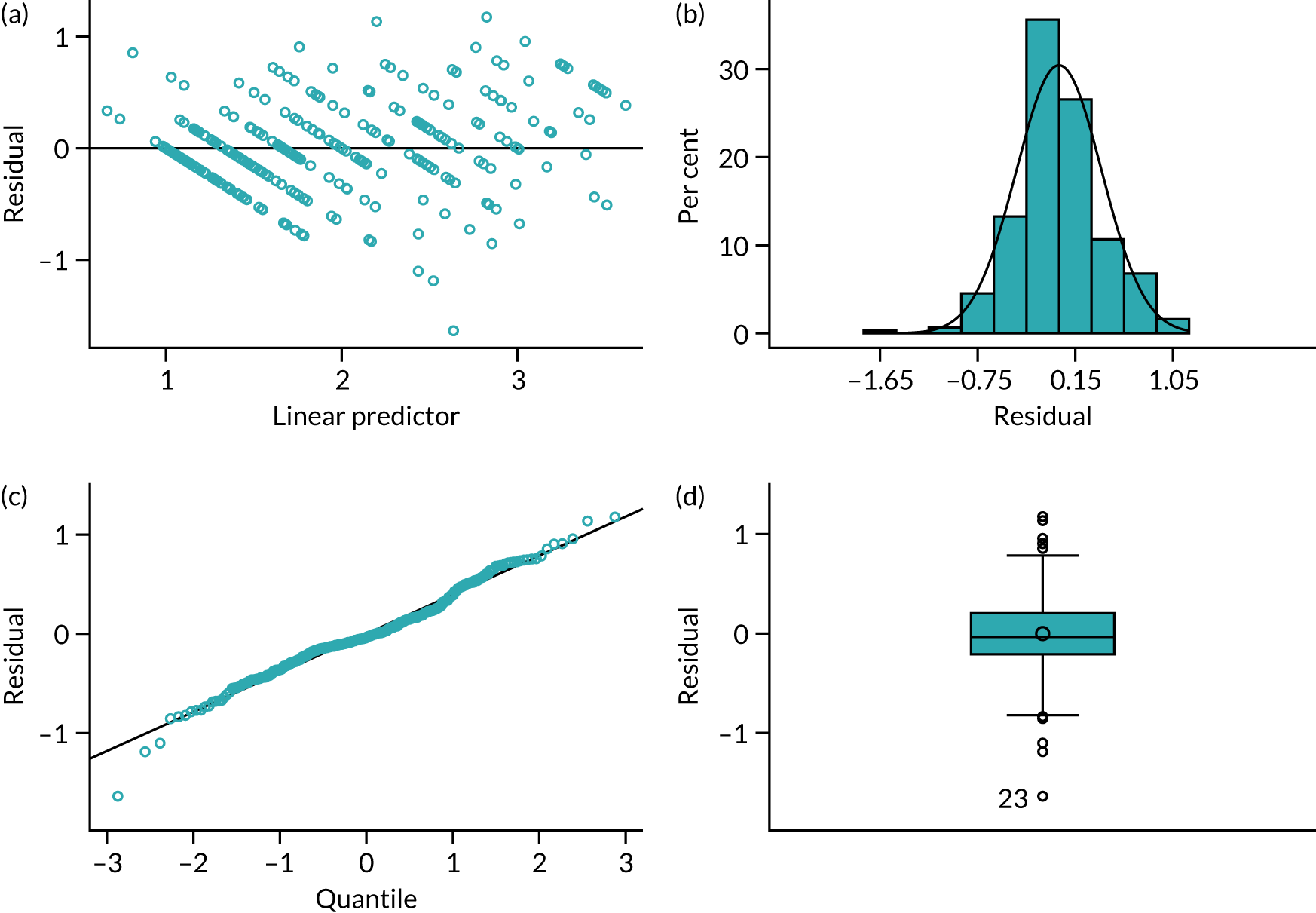
Obstructed Defecation Score
The results of the model fitted to the ODS data can be seen in Table 37. There is no significant difference in the ODS between the two treatment arms (p = 0.64). Whether or not the patient has the device in use does not have a statistically significant difference on the ODS (p = 0.58).
| Covariate | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Intercept | 5.2302 | 1.2865 | 0.0010 | 2.4882 to 7.9723 |
| FENIX MSA vs. SNS | 1.1086 | 0.7205 | 0.1256 | –0.3130 to 2.5301 |
| Male vs. female | 0.3308 | 2.0760 | 0.8736 | –3.7648 to 4.4264 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | 1.8101 | 1.2449 | 0.1476 | –0.6458 to 4.2661 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | 0.5190 | 0.7554 | 0.4929 | –0.9713 to 2.0092 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –1.0357 | 1.3198 | 0.4336 | –3.6394 to 1.5680 |
| Time (months) | –0.00207 | 0.002504 | 0.4098 | –0.00701 to 0.002871 |
| Device in use | 0.3027 | 0.5458 | 0.5798 | –0.7740 to 1.3795 |
| Time and CCIS interaction (CCIS ≤ 10 points vs. CCIS > 10 points) | 0.002587 | 0.002631 | 0.3267 | –0.00260 to 0.007777 |
The random effect caused by surgeon was 0 and the random effect caused by within patient measurements was 8.63 (SE 1.73). The residual of the random effects was 8.08 (SE 0.83).
The results of the model can be seen in Figure 24, which presents estimates and 95% CIs for each of the ODSs at 6, 12 and 18 months post randomisation for two patients who are identical except for their randomised treatment, and assuming that they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 24.
Obstructed Defecation Score model results.
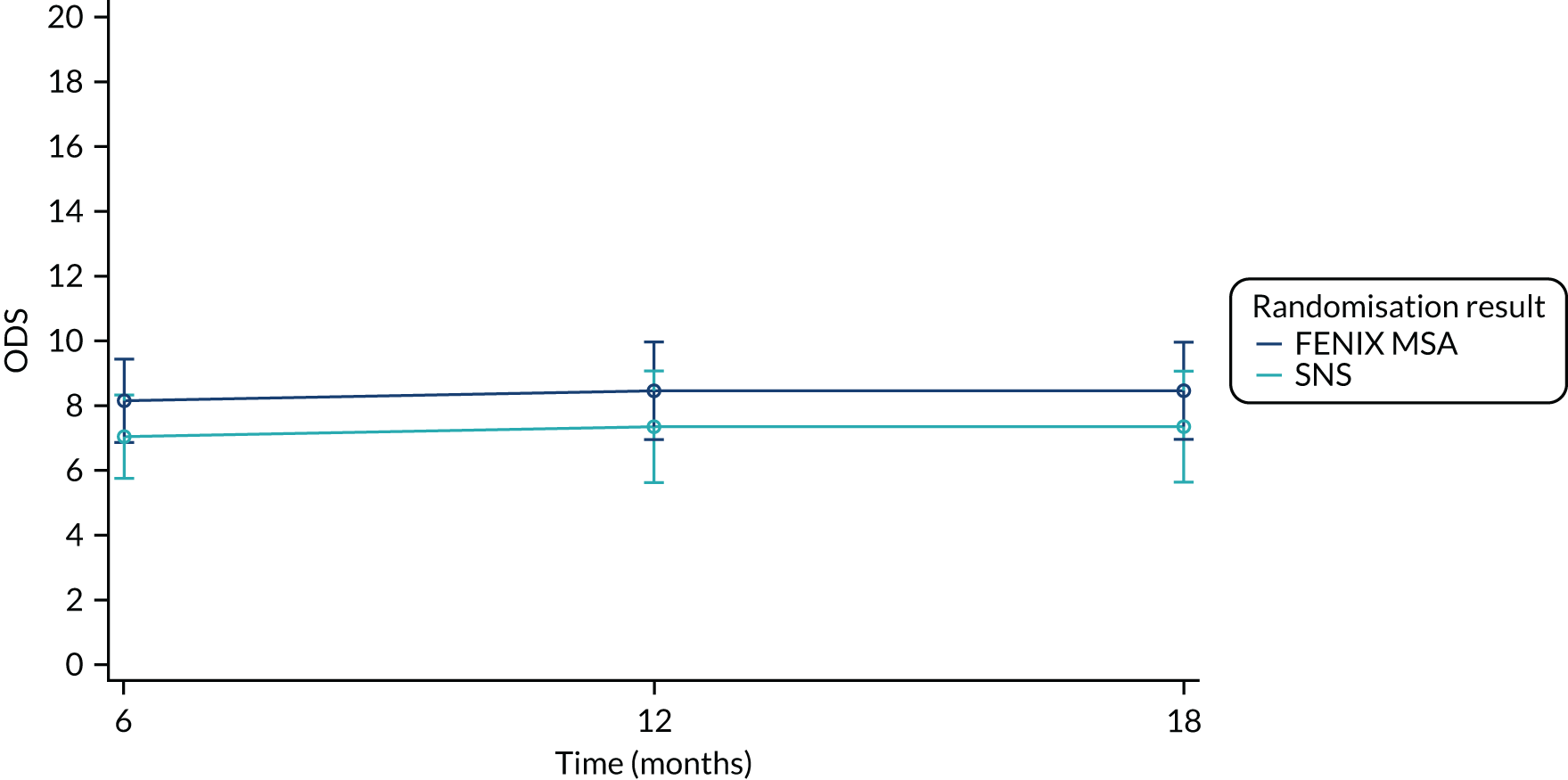
The model diagnostics can be seen in Figure 25.
FIGURE 25.
Model residuals for ODS. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.

Short Form questionnaire-12 items version 2
The fixed effects of the multilevel model fitted to the data can be seen in Table 38. Both models show that there is no difference between the PCS/MCS for patients randomised to the two treatment arms. The PCS model shows a statistically significant increase in score of 3.07 if the patient has the device in use (p = 0.04), and this difference is not significantly different for the treatment arms (p = 0.6). The MCS model does not show any statistically significant difference in the score if the patient has the device in use. The MCS model shows a significant difference between the scores for patients who have mild to moderate CCIS versus moderate to severe CCIS (p = 0.004); however, this effect is not significantly different over time (p = 0.48).
| Covariate | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| PCS model | ||||
| Intercept | 51.5532 | 3.0005 | < 0.0001 | 45.1578 to 57.9486 |
| FENIX MSA vs. SNS | –1.8281 | 1.8383 | 0.3211 | –5.4514 to 1.7953 |
| Male vs. female | 1.6534 | 5.0070 | 0.7416 | –8.2158 to 11.5225 |
| CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –4.8303 | 2.7803 | 0.0838 | –10.3104 to 0.6498 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | –0.7844 | 1.8763 | 0.6763 | –4.4827 to 2.9139 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | –1.4812 | 3.3644 | 0.6602 | –8.1126 to 5.1502 |
| Time (months) | –0.1045 | 0.05933 | 0.0797 | –0.2214 to 0.01247 |
| Device in Use | 3.0712 | 1.4691 | 0.0377 | 0.1756 to 5.9668 |
| Device in use and treatment interaction | –0.9068 | 1.7147 | 0.5975 | –4.2866 to 2.4729 |
| MCS model | ||||
| Intercept | 51.4858 | 3.7708 | < 0.0001 | 43.4485 to 59.5231 |
| FENIX MSA vs. SNS | –1.6192 | 2.1049 | 0.4426 | –5.7680 to 2.5295 |
| Male vs. female | –1.5167 | 5.8981 | 0.7973 | –13.1419 to 10.1085 |
| Baseline CCIS > 10 points (moderate to severe) vs. ≤ 10 points (mild to moderate) | –10.7417 | 3.6346 | 0.0035 | –17.9056 to –3.5778 |
| Anal sphincter defect ≤ 90° vs. no anal sphincter defect | –0.4134 | 2.2128 | 0.8520 | –4.7749 to 3.9481 |
| Anal sphincter defect > 90° to < 180° vs. no anal sphincter defect | 2.1652 | 3.9816 | 0.5871 | –5.6826 to 10.0130 |
| Time (months) | –0.1289 | 0.2084 | 0.5368 | –0.5397 to 0.2818 |
| Device in use | 2.4745 | 1.3824 | 0.0749 | –0.2503 to 5.1993 |
| Time and baseline CCIS interaction | 0.1546 | 0.2168 | 0.4764 | –0.2726 to 0.5819 |
For the PCS model, the random effect caused by surgeon was 2.45 (SE 5.84) and the random effect caused by within-patient measurements was 61.14 (SE 11.45). The residual of the random effects was 30.52 (SE 2.93).
For the PCS model, the random effect caused by surgeon was 0 and the random effect caused by within-patient measurements was 82.30 (SE 15.16). The residual of the random effects was 58.73 (SE 5.62).
The results of the models can be seen in Figures 26 and 27, which present estimates and 95% CIs for each of the PCS and MCS components of the SF-12 at 6, 12 and 18 months post randomisation for two patients who are identical except for their randomised treatment, and assuming that they received the device after 6 months and it remained in use at 12 and 18 months post randomisation.
FIGURE 26.
Physical Component Summary model results.
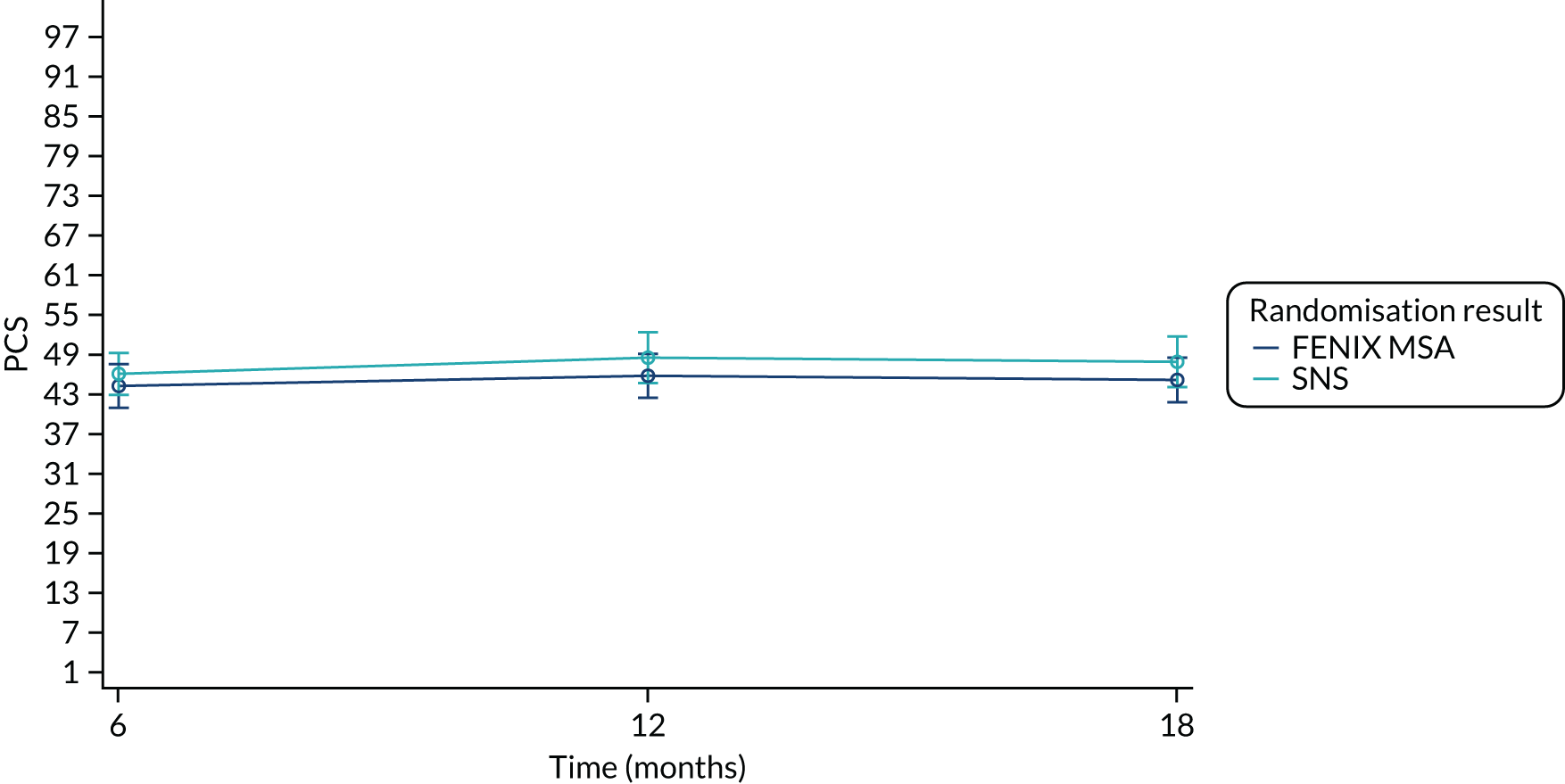
FIGURE 27.
Mental Component Summary model results.

The model diagnostics can be seen in Figures 28 and 29.
FIGURE 28.
Model residuals for SF-12: PCS. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.
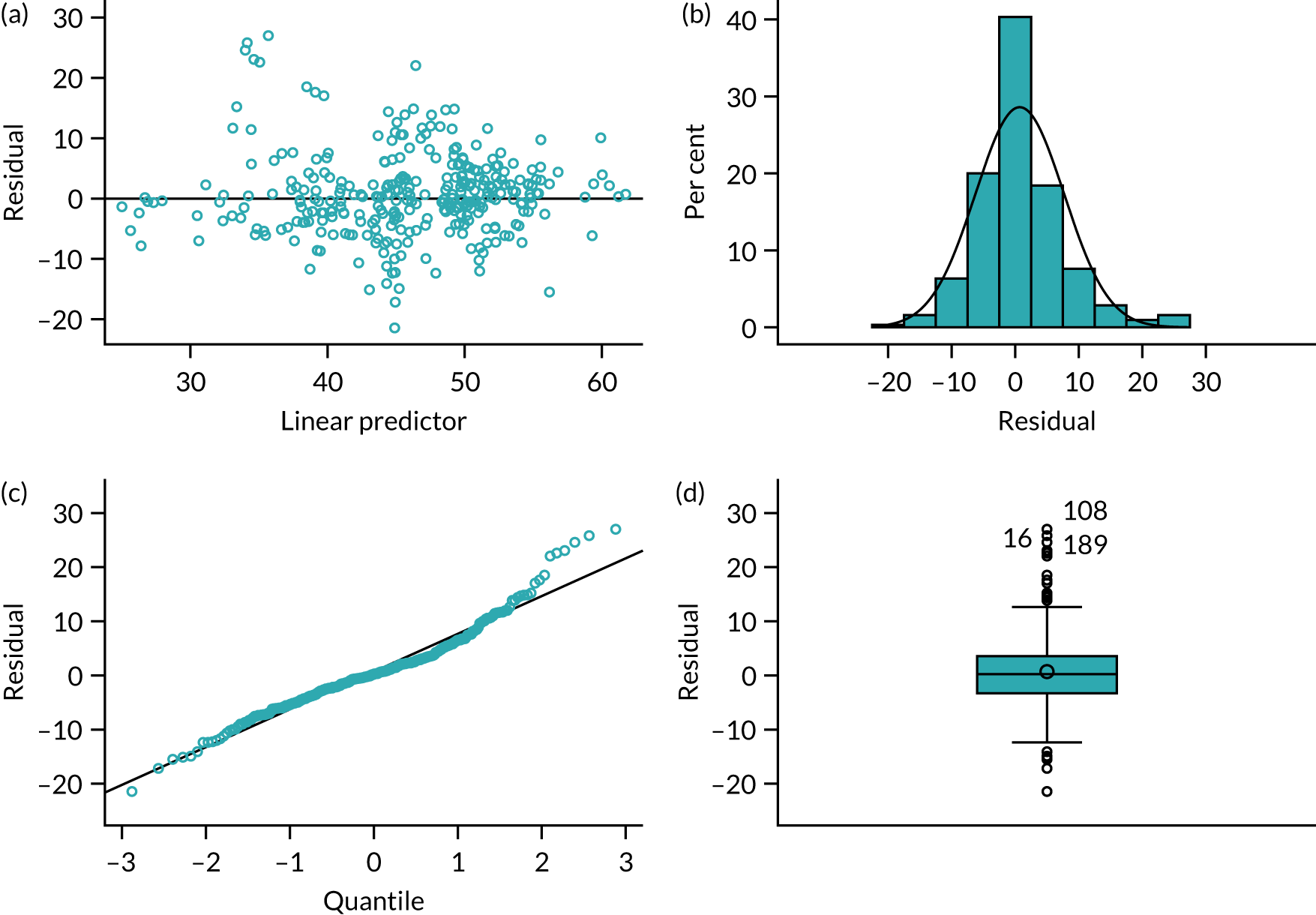
FIGURE 29.
Model residuals for SF-12: MCS. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.
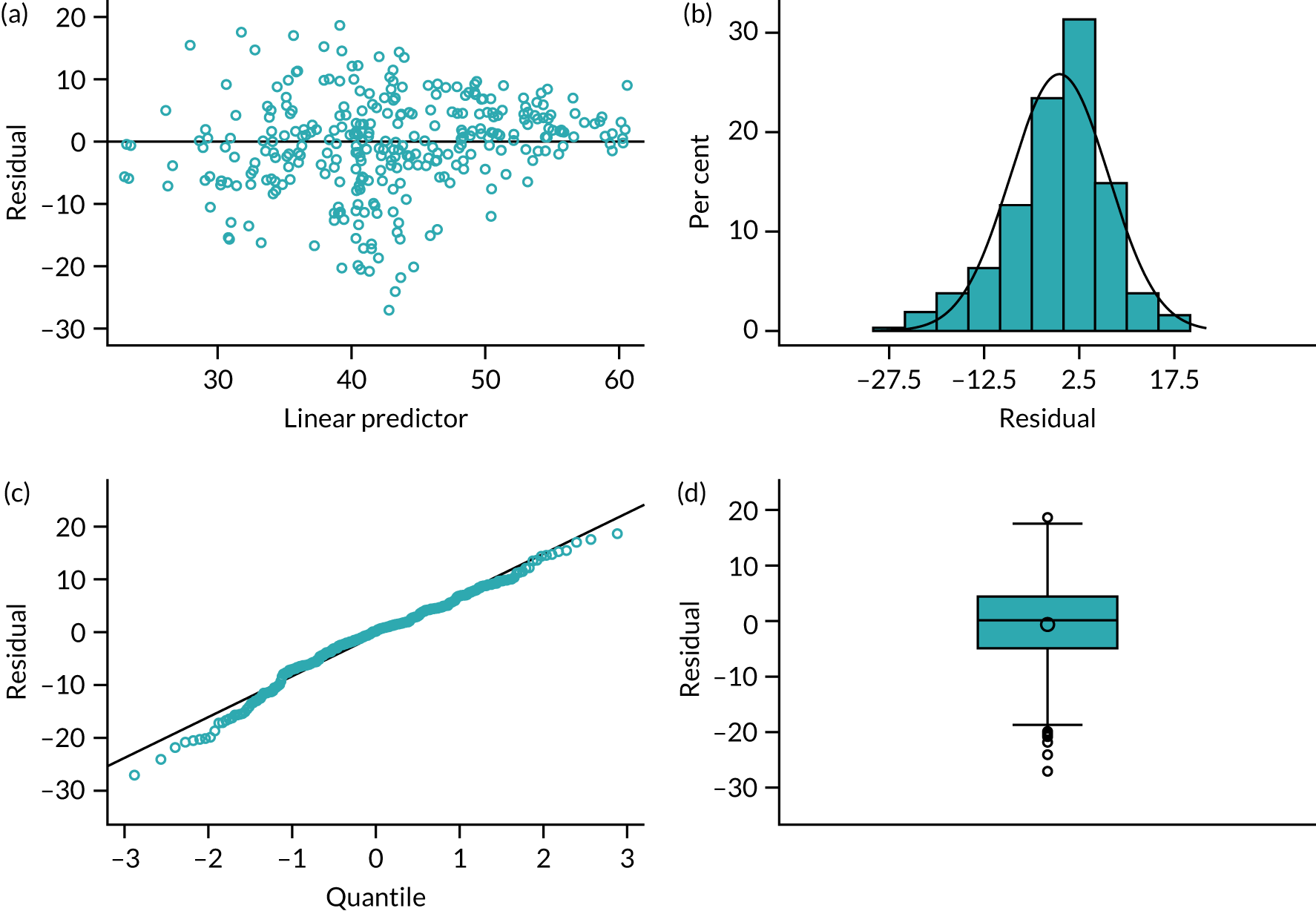
Model diagnostics
FIGURE 30.
Empirical probability plot: primary end point.
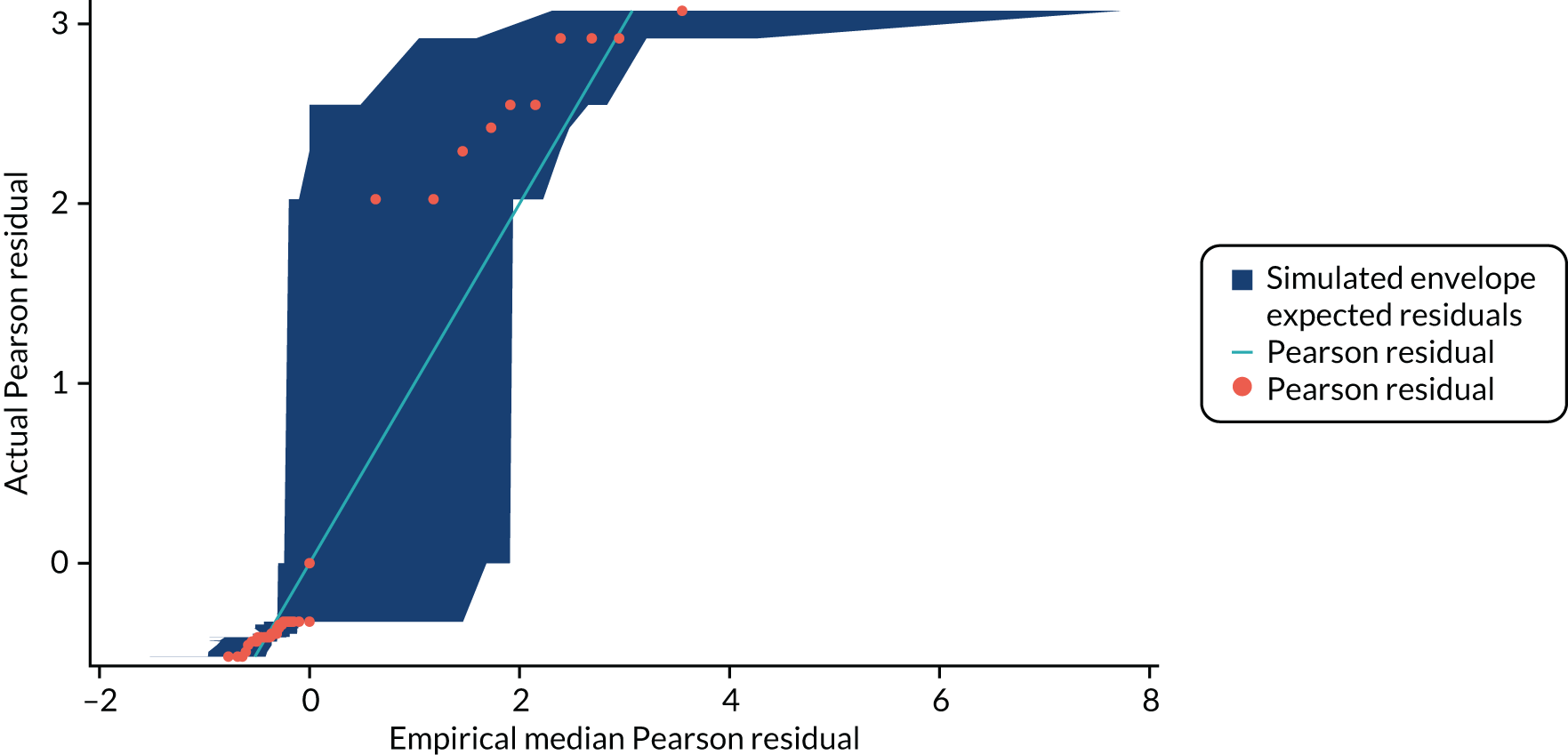
The observations that lie furthest from the line are those patients who have a higher probability of a successful outcome owing to the stratification factors entered at randomisation.
FIGURE 31.
Delta-betas: primary end point.
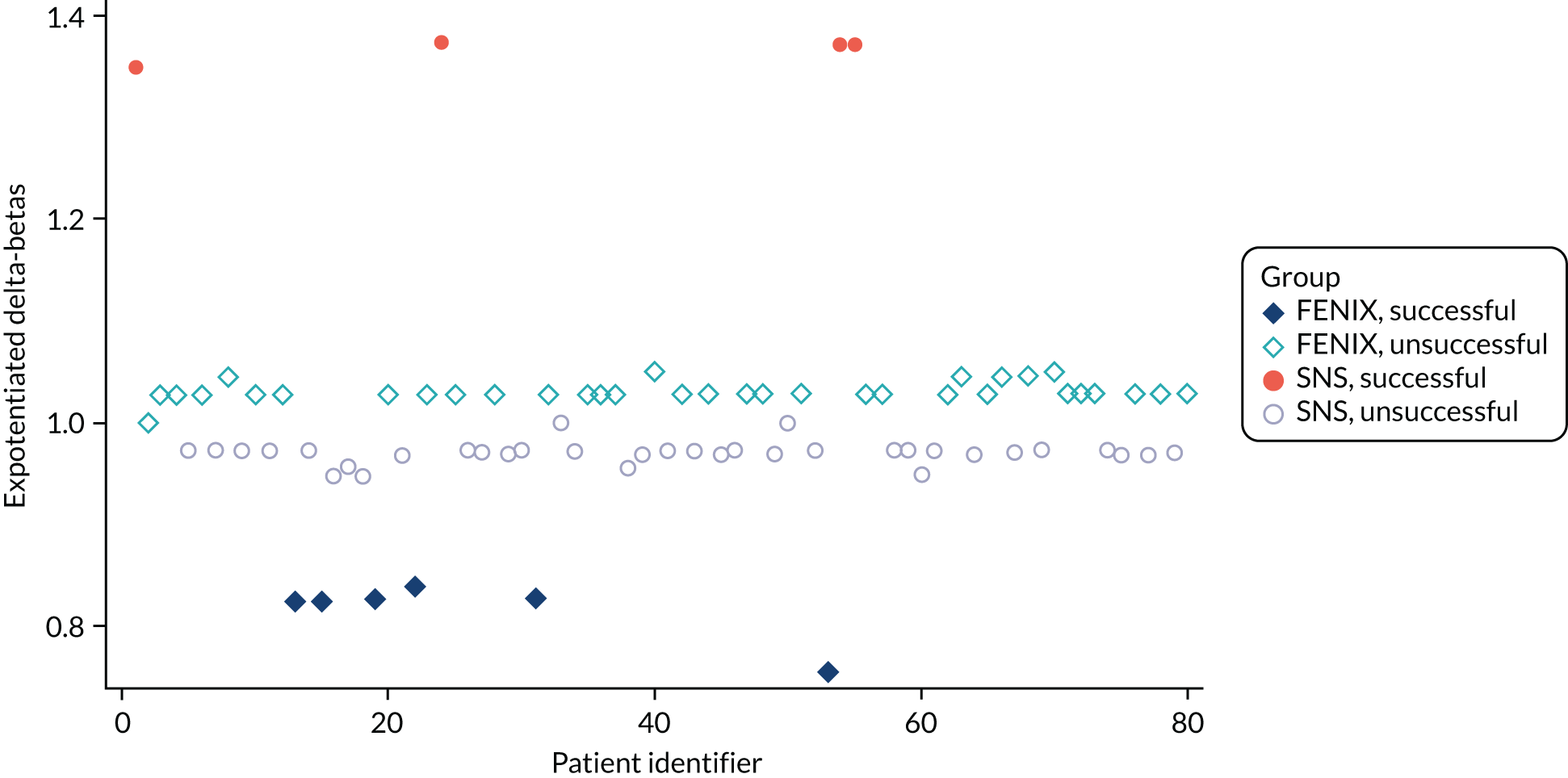
FIGURE 32.
Empirical probability plot: postoperative complications.
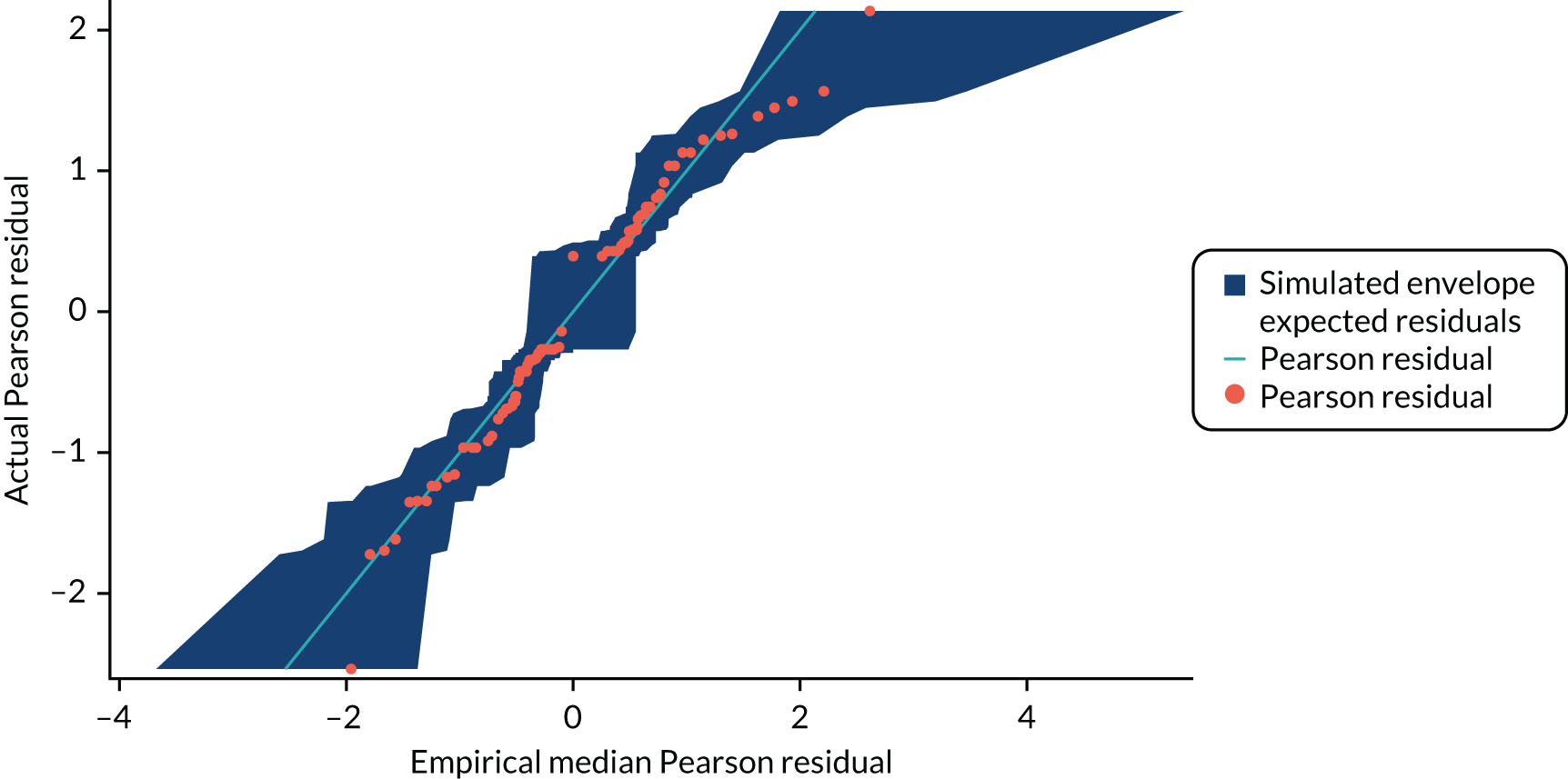
FIGURE 33.
Delta-betas: postoperative complications.

The observations with the greatest delta-betas are FENIX patients who did not have a complication, where the operation was performed by a surgeon that had a slightly higher probability of a complication occurring.
FIGURE 34.
Model residuals for CCIS end point. (a) Raw residuals vs. linear predictor value; (b) histogram of the raw residuals with normal density overlay for reference; (c) normal Q-Q plot of the raw residuals; and (d) box plot of the raw residuals.

Health economics
| Item | Cost (2018/19 prices) | Source and assumptions | Inflated from year |
|---|---|---|---|
| Postoperative complications: listed | |||
| Cardiorespiratory: serious | £1056 | Non-elective short stay (DZ19 K): other respiratory disorders with single intervention, with CC score 0–4 (NHS Reference Costs 2017–201833) | N/A |
| Urinary retention: serious | £359 | Non-elective short stay (LB16 K): urinary incontinence or other urinary problems, without interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | N/A |
| Neurological pain: serious | £358 | Non-elective short stay (WH08B): unspecified pain with CC score 0 (NHS Reference Costs 2017–201833) | N/A |
| Bleeding/wound haematoma: serious | £1069 | Non-elective short stay (FD03E): gastrointestinal bleed with single intervention, with CC score 0–4 (NHS Reference Costs 2017–201833) | N/A |
| Wound infection: serious | £967 | Non-elective short stay (WH07D): infection or other complications of procedures, with single interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | N/A |
| Implant infection: serious | £967 | Non-elective short stay (WH07D): infection or other complications of procedures, with single interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | N/A |
| Lead migration/fragmentation: serious or not serious | £1100 | Minor anal procedures, ≥ 19 years, day case (FF42 A) (NHS Reference Costs 2017–201833) | N/A |
| Pain at battery site (permanent SNS) due to non-infective cause (e.g. battery rotation): serious or not serious | £1100 | Minor anal procedures, ≥ 19 years, day case (FF42 A) (NHS Reference Costs 2017–201833) | N/A |
| Lack or loss of efficiency: serious or not serious | N/A | Assumed cost to be explant, which is included separately | N/A |
| Transient anal/rectal pain: serious | £585 | Minimal anal procedure, day case (FF43Z) (NHS Reference Costs 2017–201833) | N/A |
| Device failure/separation: serious or not serious | £1100 | Minor Anal Procedures, ≥ 19 years, day case (FF42 A) (NHS Reference Costs 2017–201833) | N/A |
| Device erosion: serious or not serious | £1819 | Assume explant occurs and same cost as insertion: insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Device explant/reoperation: serious or not serious | £1819 | Assume explant occurs and same cost as insertion: insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Worsening constipation/obstructive defaecation: serious | £585 | Minimal anal procedure, day case (FF43Z) (NHS Reference Costs 2017–201833) | N/A |
| Other serious complications | |||
| Wound still not healed following explant, fistula | £967 | Non-elective short stay (WH07D): infection or other complications of procedures, with single interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | N/A |
| Wound dehiscence | £967 | Non-elective short stay (WH07D): infection or other complications of procedures, with single interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | N/A |
| Covering stoma | £1093 | Minor anal procedures, ≥ 19 years, non-elective short stay (FF42 A) (NHS Reference Costs 2017–201833) | N/A |
| Presented with constipation | £585 | Minimal anal procedure, day case (FF43Z) (NHS Reference Costs 2017–201833) | N/A |
| All non-serious complications (listed and other) | |||
| All other non-serious complications | £105 | Colorectal surgery, consultant-led, non-admitted face-to-face attendance, follow-up (WF01 A) (NHS Reference Costs 2017–201833) | N/A |
| Item | Cost (2018/19 prices) | Source and assumptions | Inflated from year |
|---|---|---|---|
| Explants | |||
| Explant of device: temporary or permanent – SNS | £1819 | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Explant of device: temporary or permanent – FENIX | £1819 | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) (Assumed same as SNS) | N/A |
| Further surgery and treatments | |||
| Biofeedback therapy | £345 | Initial consultation (£75) and three subsequent 30-minute sessions (£90 each) (BrainTrain UK; URL: www.braintrainuk.com/about-us/prices/; last accessed 20 February 2021) | |
| Colostomy formed | £13,851 |
Colectomy £9218 complex large intestine procedures, ≥ 19 years, with CC score 3–5 (FF31C) (NHS Reference Costs 2017–201833) Ileoanal pouch formation/reversal of ileostomy £4633 major small intestine procedures, ≥ 19 years, with CC score 0–1 (FF22D) (NHS Reference Costs 2017–201833) |
|
| EUA of rectum and manual evacuation | £1100 | ‘Minor anal procedure’. (NHS Reference Costs 2017–201833) | |
| EUA of rectum and evacuation of haematoma | £1100 | ‘Minor anal procedure’. (NHS Reference Costs 2017–201833) | |
| Insertion of temporary SNS | £3692 |
£680 temporary device cost (Hounsome and Roukas32) £1819 insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) £1193 insertion of neurostimulator electrodes for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) |
|
| Insertion of permanent SNS | £10,762 |
£7750 permanent device cost (Hounsome and Roukas32) £1819 insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) £1193 Insertion of neurostimulator electrodes for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) |
|
| Laparoscopic insertion of pudendal nerve stimulator | £10,762 |
£7750 permanent device cost (Hounsome and Roukas32) £1819 insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) £1193 insertion of neurostimulator electrodes for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) |
|
| Laparoscopic sigmoid colectomy | £13,851 |
Colectomy £9218 complex large intestine procedures, ≥ 19 years, with CC score 3–5 (FF31C) (NHS Reference Costs 2017–201833) Ileoanal pouch formation/reversal of ileostomy £4633 major small intestine procedures, ≥ 19 years, with CC score 0–1 (FF22D) (NHS Reference Costs 2017–201833) |
|
| Laparoscopic ventral mesh rectopexy | £1300 | Major anal procedures, ≥ 19 years, with CC score 0 (FF40B) (NHS Reference Costs 2017–201833) | |
| Physiotherapy | £57 | Physiotherapist, adult, one to one, allied health professionals, community health services (A08A1) (NHS Reference Costs 2017–201833) | |
| Posterior tibial nerve stimulation | £2579 | Hounsome and Roukas32 | |
| Rectal irrigation | £76.28 | NICE54 | |
| Removal of FENIX | £1819 | Insertion of neurostimulator for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) (Assumed same as SNS) | |
| Replacement of SNS wire | £1193 | Insertion of neurostimulator electrodes for treatment of faecal incontinence, day case (FF47Z) (NHS Reference Costs 2017–201833) | N/A |
| Sigmoidoscopy | £402 | Diagnostic flexible sigmoidoscopy, ≥ 19 years, day case (FE35Z) (NHS Reference Costs 2017–201833) | |
| Stoma | £13,851 |
Colectomy £9218 complex large intestine procedures, ≥ 19 years, with CC score 3–5 (FF31C) (NHS Reference Costs 2017–201833) Ileoanal pouch formation/reversal of ileostomy £4633 major small intestine procedures, ≥ 19 years, with CC score 0–1 (FF22D) (NHS Reference Costs 2017–201833) |
|
| Annual ongoing stoma care | £2444 | NICE55 | 2014–15 |
| Wound closed up | £967 | Non-elective short stay (WH07D): infection or other complications of procedures, with single interventions, with CC score 0–1 (NHS Reference Costs 2017–201833) | |
| Excluded as unrelated to FI and the interventions | |||
| Bilateral foot surgery | |||
| Bilateral left and right knee repair | |||
| Intramedullary nailing surgery | |||
| Left shoulder surgery | |||
| Right submandibular gland excision | |||
| Right total knee replacement | |||
| Item | Cost (2018/19 prices) | Source and assumptions |
|---|---|---|
| Abena pads | £10.40 | Pack of 30, premium Abena Abri-San-7–premium. URL: www.allaboutincontinence.co.uk/incontinence-brands/abena/abena-abri-san (accessed 12 February 2021) |
| Adcal | £2.95 | Adcal-D3 750-mg/200-unit caplets (Kyowa Kirin Ltd, Galashiels, UK), 112 tablets (BNF42) |
| Alendronic acid | £1.59 | Alendronic acid 10-mg tablets (AAH Pharmaceuticals Ltd, Coventry, UK). 28 tablets (BNF42) |
| Alverine citrate | £3.60 | Alverine 60-mg capsule (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Amitriptyline (10 mg) | £0.91 | Amitriptyline 10-mg tablets (AAH Pharmaceuticals Ltd) 28 tablets (BNF42) |
| Amitriptyline (25 mg) | £0.72 | Amitriptyline 25-mg tablets (AAH Pharmaceuticals Ltd) 28 tablets (BNF42) |
| Anal plug | £66.17 | Peristeen anal plug 1450 small: 12–37 mm. URL: www.clearchemist.co.uk/peristeen-anal-pl-1450-s-12–37mm.html?gclid=EAIaIQobChMIq6v-5ene4AIVE4XVCh0gXw1LEAQYASABEgKAePD_BwE (accessed 1 February 2019) |
| Andrews Original Salts | £5.49 | 250 mg. URL: www.boots.com/andrews-original-salts-250g-10007017 (accessed 1 February 2019) |
| Aqua flush | £33.95 | URL: www.bcapformulary.nhs.uk/2118-trans-anal-irrigation-systems (accessed 1 February 2019) |
| Aspirin (75 mg) | £1.13 | Aspirin 75-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Atorvastatin (20 mg) | £0.78 | Atorvastatin 20 mg (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Braltus® | £25.80 | Braltus 10-mg inhalation powder capsules with Zonda inhaler (Teva UK Ltd, Castleford, UK). 30 capsules (BNF42) |
| Buscopan (10 mg) | £3.00 | Buscopan 10-mg tablets (Sanofi, Reading, UK). Hyoscine butylbromide 10 mg. 56 tablets (BNF42) |
| Colpermin | £3.77 | Colpermin gastro-resistant modified-release capsules (McNeil Products Ltd, High Wycome, UK). Peppermint oil 200 µl, 20 capsules (BNF42) |
| Candesartan (2 mg) | £2.02 | Candesartan 2-mg tablets (AAH Pharmaceuticals Ltd). Candesartan cilexetil 2 mg. 7 tablets (BNF42) |
| Cefalexin | £1.57 | Cefalexin 250-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Celluvisic® dry eye drops | £4.80 | Celluvisic 0.5% eye drops 0.4-ml unit dose (Allergan Ltd, Marlow, UK). Carmellose sodium 5 mg per 1 ml. 30 unit dose (BNF42) |
| Cetirizine | £0.81 | Cetirizine 10-mg tablets. Cetirizine hydrochloride 10 mg (BNF42) |
| Cholestyramine | £10.76 | Questran 4-g oral powder sachets (Bristol-Myers Squibb Pharmaceuticals Ltd, Uxbridge, UK). Colestyramine anhydrous 4 g (BNF42) |
| Cinnarizine | £5.06 | Cinnarizine 15-mg tablets (AAH Pharmaceuticals Ltd). Cinnarizine 15 mg. 84 tablets (BNF42) |
| Ciprofloxacin | £2.11 | Ciprofloxacin 100-mg tablets [Alliance Healthcare (Distribution) Ltd, Chessington, UK]. Ciprofloxacin hydrochloride 100 g. 6 tablets (BNF42) |
| Citalopram (20 mg) | £1.00 | Citalopram 20-mg tablets (AAH Pharmaceuticals Ltd). Citalopram hydrochloride. 28 tablets (BNF42) |
| Clindamycin | £3.45 | Clindamycin 150-mg capsules (AAH Pharmaceuticals Ltd). Clindamycin hydrochloride 150 mg. 24 capsules (BNF42) |
| Clonidine (25 mg) | £4.50 | Clonidine 25-mg tablets (AAH Pharmaceuticals Ltd). Clonidine hydrochloride 25 mg. 112 tablets (BNF42) |
| Co-amoxiclav | £1.77 | Co-amoxiclav 250-mg/125-mg tablets (AAH Pharmaceuticals Ltd). Amoxicillin (as amoxicillin trihydrate) 250 mg, Clavulanic acid (as potassium clavulanate) 125 mg. 21 tablets (BNF42). Co-codamol |
| Co-codamol (30 mg) | £3.90 | Co-codamol 30-mg/500-mg caplets (AAH Pharmaceuticals Ltd). 30 tablets (BNF42) |
| Codeine | £0.74 | Codeine 15-mg tablets (AAH Pharmaceuticals Ltd). Codeine phosphate 15 mg. 28 tablets (BNF42) |
| Co-dydramol (10 mg/500 mg) | £0.71 | Co-dydramol 10-mg/500-mg tablets (AAH Pharmaceuticals Ltd). 30 tablets (BNF42) |
| Colecalciferol (1.5 mg) | £3.65 | Adcal-D3 lemon chewable tablets (Kyowa Kirin Ltd). Calcium carbonate 1.5 g, colecalciferol 400 unit. 56 tablets (BNF42) |
| Colestyramine | £10.76 | Questran 4-g oral powder sachets (Bristol-Myers Squibb Pharmaceuticals Ltd). 50 sachets (BNF42) |
| Colpermin | £3.77 | Colpermin gastro-resistant modified release capsules (McNeil Products Ltd). Peppermint oil 200 µl. 20 capsules (BNF42) |
| Coloplast anal catheters | £76.28 | NICE54 |
| Cream for piles | £2.49 | Anusol® HC ointment (Church & Dwight UK Ltd, Folkestone, UK). 30 g (BNF42) |
| Peristeen irrigation | £76.28 | NICE54 |
| Dioctyl | £2.09 | Dioctyl 100-mg capsules (UCB Pharma Ltd, Slough, UK). Docusate sodium 100 mg. 30 capsules (BNF42) |
| Doxycycline | £17.30 | Periostat® 20-mg tablets (Alliance Pharmaceuticals Ltd). 56 tablets (BNF42) |
| Dulcolax® | £2.35 | Dulcolax 10-mg suppositories (Sanofi). 12 suppository (BNF42) |
| Entrolax | £2.35 | Dulcolax 10-mg suppositories (Sanofi). 12 suppository (BNF42) |
| Estradiol pessaries (10 mg) | £16.72 | Vagifem® 10-mg vaginal tablets (Novo Nordisk Ltd, Gatwick, UK). 24 pessary (BNF42) |
| Femigel | £10.99 | URL: www.amazon.co.uk/Australian-Bodycare-Femigel-Vaginal-Moisturiser/dp/B01LXQPO79/ref=sr_1_1?keywords=femigel%26qid=1552575258%26s=drugstore%26sr=1–1-catcorr (accessed 12 February 2021) |
| Fittleworth sense catheters | £1.72 | Brand cost not found: assumed same as Lofric Sense. URL: www.supplychain.nhs.uk/savings/price-ranking/∼/media/Files/Price%20Ranking/Price%20Ranking%20Urology%20March%202018.ashx (accessed 1 February 2019) |
| Flucloxacillin | £1.00 | Flucloxacillin 250-mg capsules (AAH Pharmaceuticals Ltd) 28 capsules (BNF42) |
| Fluoxetine (40 g) | £1.80 | Fluoxetine 40-mg capsules [Alliance Healthcare (Distribution) Ltd]. 30 capsules (BNF42) |
| Folic acid | £2.93 | Folic acid 400-µg tablets (Phoenix Healthcare Distribution Ltd, Runcorn, UK). 90 tablets (BNF42) |
| Furosemide (20 mg) | £2.10 | Furosemide 20-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Fybogel | £2.73 | Fybogel 3.5-g effervescent granules sachets plain SF [Reckitt Benckiser Healthcare (UK) Ltd, Slough, UK]. 30 sachets (BNF42) |
| GAVISCON® | £4.46 | GAVISCON Advance Mint chewable tablets [Reckitt Benckiser Healthcare (UK) Ltd]. 24 tablets (BNF42) |
| Glycerin suppositories | £1.04 | Glycerol 1-g suppositories (DE Pharmaceuticals, Prudhoe, UK). 12 suppository (BNF42) |
| Ibuprofen | £3.60 | Ibuprofen 600-mg tablets (AAH Pharmaceuticals Ltd). 84 packets (BNF42) |
| ICaps® | £13.49 | 30 tablets URL: www.boots.com/icaps-extra-lutein-tablets-30s-10114811 (accessed 1 February 2019) |
| IMODIUM® | £4.21 | IMODIUM plus caplets (McNeil Products Ltd). 12 tablets (BNF42) |
| IMODIUM | £1.17 | IMODIUM 1-mg/5-ml oral solution (Janssen–Cilag Ltd, High Wycombe, UK). 100 ml (BNF42) |
| Infliximab | £377.00 | FLIXABI® 100-mg powder for concentrate for solution for infusion vials (Biogen Idec Ltd, Maidenhead, UK). Infliximab 100 mg. 1 vial (BNF42) |
| Infliximab + B12 injection | £14.50 | Cytamen 1000-µg/1-ml solution for injection ampoules (RPH Pharmaceuticals AB, Ashton-under-Lyne, UK). Five ampoules (BNF42) |
| Irrigation | £76.28 | NICE54 |
| Kira® Menopause relief (6.5 mg) | £10.29 | URL: www.hollandandbarrett.com/shop/product/kira-menopause-relief-tablets-6–5mg-60032265 (accessed 1 February 2019) |
| Lactulose | £2.28 | Lactulose 3.1–3.7-g/5-ml oral solution (Phoenix Healthcare Distribution Ltd). 500 ml. (BNF42) |
| Lansoprazole | £0.65 | Lansoprazole 15-mg gastro-resistant capsules (AAH Pharmaceuticals Ltd). 28 capsules (BNF42) |
| Lascido® | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Laxicol® | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Laxido® | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Laxiolo® | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Laxulo® | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Lercanidipine (10 mg) | £5.34 | Lercanidipine 10-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Levothyroxine (100 mg) | £0.99 | Levothryoxine sodium 100-µg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Lisinopril | £0.82 | Lisinopril 2.5-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Loperamide | £2.93 | Loperamide 2-mg tablets (AAH Pharmaceuticals Ltd). 30 tablets. 30 tablets (BNF42) |
| Lactulose | £2.28 | Lactulose 3.1–3.7-g/5-ml oral solution (Phoenix Healthcare Distribution Ltd). 500 ml. (BNF42) |
| Macrogol | £2.63 | Macrogol compound oral powder sachets sugar free [Alliance Healthcare (Distribution) Ltd]. 20 sachets (BNF42) |
| Magnesium hydroxide | £5.31 | Magnesium hydroxide 7.45–8.35% oral suspension BP (AAH Pharmaceuticals Ltd). 500 ml (BNF42) |
| MOVICOL® | £5.41 | MOVICOL oral powder 13.8-g sachets lemon and lime (Forum Health Products Ltd, Redhill, UK). 20 sachets (BNF42) |
| Mebeverine | £6.00 | Mebeverine 135-mg tablets (AAH Pharmaceuticals Ltd). 100 tablets (BNF42) |
| Methocarbamol | £12.65 | Methocarbamol 750-mg tablets (AAH Pharmaceuticals Ltd). 100 tablets (BNF42) |
| Metronidazole (400 mg) | £18.00 | Metronidazole (AAH Pharmaceuticals Ltd). 21 tablets (BNF42) |
| Mini irrigation system | £76.28 | Assumed: NICE54 |
| Mirabegron | £29.00 | Betmiga 25-mg modified-release tablets (Astellas Pharma Ltd, Woking, UK). Mirabegron 25 mg. 30 tablets (BNF42) |
| Movelat | £8.39 | Gel: 80 g URL: www.boots.com/movelat-relief-gel-80g-10023902 (accessed 1 February 2019) |
| MoviCell | £25.55 | Converted from Euros. URL: www.iafstore.com/uk/promopharma/movicell-drena-plus-codp33303 (accessed 1 February 2019) |
| MOVICOL | £8.11 | MOVICOL chocolate oral powder 13.9-g sachets (Forum Health Products Ltd). 30 sachets (BNF42) |
| Naproxen (500 mg) | £12.00 | Naproxen 500-mg tablets (AAH Pharmaceuticals Ltd)/28 tablets (BNF42) |
| Nitrofurantoin (500 mg) | £15.42 | Nitrofurantoin 50-mg capsules (AAH Pharmaceuticals Ltd). 30 capsules (BNF42) |
| Nortriptyline | £8.55 | Nortriptyline 10-mg tablets (AAH Pharmaceuticals Ltd). 30 tablets (BNF42) |
| Octasa (400 mg) | £16.58 | Octasa 400-mg MR gastro-resistant tablets (Tillotts Pharma UK Ltd, Wellingore, UK). 90 tablets (BNF42) |
| Omacor (1000 mg) | £6.00 | Omega 3-acid-ethyl esters 1000-mg capsules (Glenmark Pharmaceuticals Europe Ltd, Harrow, UK) 28 tablets (BNF42) |
| Omeprazole (10–40 mg) | £5.79 | Omeprazole 20-mg gastro-resistant (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| OTC laxatives | £1.00 | Senna 7.5-mg tablets (AAH Pharmaceuticals Ltd). 20 tablets (BNF42) |
| Oxybutynin hydrochloride | £3.05 | Oxybutynin 2.5-mg tablets (AAH Pharmaceuticals Ltd). 56 tablets (BNF42) |
| Zapain® (500 mg) | £3.85 | Zapain 30-mg/500-mg capsules (Advanz Pharma, London, UK). 100 capsules (BNF42) |
| Pantoprazole | £0.80 | Pantoprazole 20-mg gastro-resistant tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Paracetamol | £1.53 | Paracetamol 500-mg caplets (AAH Pharmaceuticals Ltd). 100 tablets (BNF42) |
| Peristeen bowel wash | £76.28 | NICE54 |
| Pregabalin | £3.43 | Pregabalin 25-mg capsules (AAH Pharmaceuticals Ltd). 56 capsules (BNF42) |
| Proctosedyl Ointment | £10.34 | Proctosedyl Ointment (Sanofi). 30 g (BNF42) |
| Procyclidine | £3.47 | Procyclidine 5-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Questran® powder | £10.76 | Questran 4-g oral powder sachets (Bristol-Myers Squibb Pharmaceuticals Ltd). 50 sachets (BNF42) |
| Questran | £10.76 | Questran 4-g oral powder sachets (Bristol-Myers Squibb Pharmaceuticals Ltd). 50 sachets (BNF42) |
| Quinine | £2.04 | Quinine sulfate 200-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Qufora® IrriSedo Mini system | £59.00 | URL: www.stomacarehandbook.com/product/2470/qufora_irrisedo_mini_system (accessed 1 February 2019) (includes 15 cones) |
| Ranitidine | £1.50 | Ranitidine 150-mg tablets (AAH Pharmaceuticals Ltd). 60 tablets (BNF42) |
| Replens MD™ Vaginal Moisturiser | £11.49 | Replens MD Vaginal Moisturiser: 35 g. URL: www.boots.com/replens-md-vaginal-moisturiser-35g-10025232 (accessed 1 February 2019) |
| Senna | £1.00 | Senna 7.5-mg tablets (AAH Pharmaceuticals Ltd). 20 tablets (BNF42) |
| Senokot | £3.23 | Senokot Max Strength 15-mg tablets [Reckitt Benckiser Healthcare (UK) Ltd] (BNF42) |
| Sertraline | £0.76 | Sertraline 50-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Simvastatin | £0.55 | Simvastatin 10-mg tablets (AAH Pharmaceuticals Ltd) 28 tablets (BNF42) |
| Suppositories (generic) | £3.49 | URL: www.boots.com/boots-constipation-relief-12-suppositories-10006837 (accessed 12 February 2021) (12 tablets) |
| Telmisartan | £6.00 | Telmisartan 20-mg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Thyroxine | £1.20 | Levothyroxine sodium 12.5-µg tablets (AAH Pharmaceuticals Ltd). 28 tablets (BNF42) |
| Trajenta | £33.26 | Trajenta 5-mg tablets (Boehringer Ingelheim Ltd, Bracknell, UK). 28 tablets (BNF42) |
| Tramadol | £0.76 | Tramadol 50-mg capsules (AAH Pharmaceuticals Ltd). 30 capsules (BNF42) |
| TRUSOPT® eye drops | £6.33 | TRUSOPT 20-mg/-ml eye drops (Santen UK Ltd, St Albans, UK). 5 ml (BNF42) |
| Vitamineral Green | £98.22 | 500 g. URL: www.amazon.co.uk/Healthforce-Vitamineral-Green-Powder-500-Grams/dp/B001H0T4TA/ref=sr_1_fkmrnull_1?keywords=vitamineral±green%26qid=1552578134%26s=gateway%26sr=8–1-fkmrnull (accessed 1 February 2019) |
| Item | Cost (2018/19 prices) | Source and assumptions | Inflated from year |
|---|---|---|---|
| GP, home visit (face to face) | £95.47 | Curtis and Burns56 estimates for costs/minute and Curtis and Burns57 estimates for duration | 2017 |
| GP, surgery visit (face to face) | £37.00 | Curtis and Burns56 estimates for a consultation duration of 9.22 minutes | 2017 |
| GP telephone/e-mail | £28.40 | Curtis and Burns56 estimates for costs/minutes and Curtis and Burns57 lasting 7.1 minutes at a per patient contact time of £4. Curtis and Burns57 estimates for duration. The same cost is assumed for any values entered for telephone contacts in the telephone contracts in the final column for all of ‘GP surgery visit’, ‘GP home visit’, ‘GP out-of-hours home visit’ | 2017 |
| District nurse (face to face) | £38.45 | ‘District nurse, adult, face to face’ (NHS Reference Costs 2017–201833) under community health services | |
| District nurse (telephone/e-mail) | £18.88 | ‘District nurse, adult, none face to face’ (NHS Reference Costs 2017–201833) under community health services | |
| GP out of hours (face to face) | £124.06 | Curtis and Burns56 estimates for travelling time costs/minute and National Audit Office (2014) estimates for out-of-hours consultations | 2017 |
| Practice nurse (face to face) | £14.11 | Curtis and Burns41 estimate per hour £54.60 (£42 × 1.30 ratio for direct contact time). An average consultation of 15.5 minutes (PSSRU, 2014/2015) | |
| Practice nurse (telephone/e-mail) | £4.26 | Curtis and Burns41 page 182 under ‘Time use of community care professionals’ states that GP practice nurses dedicate 5.3% of their time to telephone consultations | |
| Occupational physiotherapist (face to face) | £57 | ‘Physiotherapist, adult, one to one’ (NHS Reference Costs 2017–201833) | |
| Occupational physiotherapist, telephone/e-mail | £33.06 | ‘Physiotherapy, non-admitted non-face-to-face attendance, follow-up’, using volume-weighted average of consultant-led and non-consultant-led costs (NHS Reference Costs 2017–201833) | 2016 |
| Occupational therapist, face to face | £81.66 | ‘Occupational therapist, adult, one to one’ (NHS Reference Costs 2017–201833) | 2016 |
| Occupational therapist, telephone/e-mail | £43.64 | ‘Occupational therapy, non-admitted none face-to-face attendance, follow-up’, (consultant-led and non-consultant-led reference cost identical) (NHS Reference Costs 2017–201833) | 2016 |
| Counsellor (face to face) | £44 | Curtis and Burns41 counsellor (band 6) under ‘scientific and professional staff’ | 2018 |
| Counsellor (telephone/e-mail) | £23.32 | Assumed same ratio face to face: telephone as occupational therapist (53%) |
| Item | Cost (£, 2018/19 prices) | Source and assumptions | Inflated from year |
|---|---|---|---|
| Other support from personal social services | |||
| Meals on wheels (frozen) | £4.65 | URL: www.leeds.gov.uk/adult-social-care/help-at-home (accessed 3 April 2019). Assumed cost of £3.65 for a main course and £1.00 for dessert (uppermost values) | 2018 |
| Meals on wheels (hot) | £8.80 | URL: www.leeds.gov.uk/adult-social-care/help-at-home (accessed 3 April 2019). £6.00 for the hot main meal and dessert and a cost of £2.80 for the tea | 2018 |
| Laundry services | £14.00 | URL: www.laundryheap.co.uk/ (accessed 3 April 2019). £14 per 6 kg wash | 2018 |
| Home help, face to face (assumed same cost as that of cleaner, carer, home care and health and social care) | £58.54 | ‘Health visitor, other clinical interventions’ (NHS Reference Costs 2017–201833) | 2017 |
| Community and residential based services | |||
| Nursing home/hospice stay, per day | £468.26 | ‘Inpatient day in hospice care’ (Public Health England, 2017) | 2012 |
| Convalescent care | £158.00 | Per day permanent resident week ‘Local authority own-provision residential care for older people (age 65+)’ from Curtis and Burns41 | 2017–18 |
| Group | Number and mean | All data collected as part of the trial | Complete-case analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baselinea | 6 months | 12 months | 18 months | Baselinea | 6 months | 12 months | 18 months | ||
| Utilities: EQ-5D-5L | |||||||||
| Total | n | 89 | 80 | 73 | 63 | 47 | 47 | 47 | 47 |
| Mean | 0.762 | 0.754 | 0.769 | 0.750 | 0.798 | 0.756 | 0.793 | 0.770 | |
| FENIX | n | 44 | 38 | 37 | 31 | 24 | 24 | 24 | 24 |
| Mean | 0.761 | 0.771 | 0.777 | 0.731 | 0.802 | 0.772 | 0.792 | 0.757 | |
| SNS | n | 45 | 42 | 36 | 32 | 23 | 23 | 23 | 23 |
| Mean | 0.763 | 0.738 | 0.760 | 0.768 | 0.794 | 0.740 | 0.794 | 0.783 | |
| Costs: health and social care | |||||||||
| Total | n | 81 | 76 | 67 | 47 | 47 | 47 | 47 | |
| Mean | £116.72 | £126.34 | £121.02 | £103.95 | £121.71 | £142.50 | |||
| FENIX | n | 39 | 38 | 32 | 24 | 24 | 24 | ||
| Mean | £107.70 | £125.26 | £176.15 | £110.45 | £128.64 | £203.39 | |||
| SNS | n | 42 | 38 | 35 | 23 | 23 | 23 | ||
| Mean | £125.10 | £127.41 | £70.62 | £97.17 | £114.48 | £78.97 | |||
| Costs: secondary care | |||||||||
| Total | n | 94 | 92 | 91 | 47 | 47 | 47 | ||
| Mean | £416.83 | £501.89 | £131.52 | £716.18 | £464.17 | £69.36 | |||
| FENIX | n | 47 | 46 | 45 | 24 | 24 | 24 | ||
| Mean | £603.07 | £847.81 | £180.45 | £954.11 | £820.70 | £86.13 | |||
| SNS | n | 47 | 46 | 46 | 23 | 23 | 23 | ||
| Mean | £230.60 | £155.97 | £83.66 | £467.91 | £92.14 | £51.87 | |||
| Costs: medications | |||||||||
| Total | n | 81 | 76 | 67 | 47 | 47 | 47 | ||
| Mean | £9.75 | £7.52 | £10.84 | £8.60 | £9.46 | £12.87 | |||
| FENIX | n | 39 | 38 | 32 | 24 | 24 | 24 | ||
| Mean | £10.75 | £9.15 | £20.22 | £11.87 | £12.77 | £23.14 | |||
| SNS | n | 42 | 38 | 35 | 23 | 23 | 23 | ||
| Mean | £8.81 | £5.89 | £2.27 | £5.19 | £6.00 | £2.15 | |||
List of abbreviations
- ABS
- artificial bowel sphincter
- ASA
- American Society of Anesthesiologists
- BMI
- body mass index
- BNF
- British National Formulary
- CCIS
- Cleveland Clinic Incontinence Score
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVPI
- expected value of perfect information
- FI
- faecal incontinence
- FIQoL
- faecal incontinence quality of life
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- MAS
- magnetic anal sphincter
- MCS
- Mental Component Summary
- MI
- multiple imputation
- MSA
- magnetic sphincter augmentation
- NICE
- National Institute for Health and Care Excellence
- NIHR HSC
- National Institute for Health Research Horizon Scanning Centre
- NMB
- net monetary benefit
- ODS
- Obstructed Defecation Score
- PCS
- Physical Component Summary
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SNS
- sacral nerve stimulation
- TSC
- Trial Steering Committee
- USC
- unexpected serious complication
- VAS
- Visual Analogue Scale
