Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/113/01. The contractual start date was in April 2017. The draft report began editorial review in July 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Cameron et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Unintended pregnancy is a major public health problem. Despite having one of the highest rates of modern contraceptive use worldwide, the UK has among the highest abortion rates in Europe. 1 In 2018, almost 200,000 pregnancies ended in induced abortion. 2,3 Unintended pregnancy also ends in childbirth. Around 10% of UK births are unintended and 25% are mistimed. 4 Unintended pregnancy is costly to the NHS5 and distressing for women. Unintended pregnancies are over-represented in young women from deprived backgrounds. Unintended childbirth can have both socioeconomic consequences for women and their families, and mental health consequences. 6
Emergency contraception (EC) prevents pregnancy in individual women following unprotected sex or contraceptive accidents (e.g. burst condom). Approval of EC from pharmacies and making it free of charge to all women in Scotland and Wales, and free to many women in England, has increased use and EC is now largely obtained from pharmacies. 7 However, although trials have shown that facilitating access to EC increases use of EC, these trials have failed to show an effect on unintended pregnancy rates. 8
Emergency contraception [containing levonorgestrel (LNG)] is only effective if taken within 72 hours of unprotected sex. It does not prevent conceptions from subsequent sex. The risk of pregnancy is increased up to threefold among women who have further unprotected sex in the same menstrual cycle after using EC. 9 An effective method of contraception should therefore be started as soon as possible – known as ‘quick starting’. 10 However, the only contraceptives available from pharmacies without prescription are condoms, which have high failure rates. 11 To start an effective contraceptive (i.e. hormonal or intrauterine contraception) women must visit a general practitioner (GP) or attend a sexual and reproductive health (SRH) clinic. It may take time to get an appointment and many women may lose the motivation to seek contraception, leading to an unintended pregnancy. In addition, in some UK studies, fewer than half of pharmacists gave advice about ongoing contraception after EC. 12,13
There is a lack of evidence on interventions to increase uptake of effective contraception after EC. We conducted a literature search of PubMed English-language articles from 2000 to March 2020 using search terms (((bridging) OR bridge)) AND (emergency contracept*) and identified only two relevant articles. One was a cluster randomised trial14 from Jamaica, in which women seeking EC were offered a voucher that gave them a discount on the cost of contraceptive pills. This study reported no effect of the intervention on subsequent contraceptive use. The other article15 was a pilot study of 12 pharmacies in Edinburgh, with 168 women presenting for EC randomised to receive 1 month of a progestogen-only pill (POP), rapid access to a local SRH clinic or standard care. Participants were contacted by telephone 6–8 weeks later to determine which method of contraception they were using. In the POP arm, 35 out of 39 (90%) women used the pills provided and 9 out of 28 (32%) women in the rapid-access arm attended the SRH clinic. Compared with standard care, the proportion of women using effective contraception after EC was statistically significantly greater in both the POP arm (56% vs. 16%; p = 0.001) and the rapid-access arm (52% vs. 16%; p = 0.027). The study concluded that a 1-month supply of POP after EC or rapid access to a SRH service might increase short-term uptake of effective contraception following EC, but that a high-quality, adequately powered randomised trial was needed.
Rationale for study
Unintended pregnancy remains a public health problem in the UK. Guidance from the Faculty of Sexual and Reproductive Healthcare of the Royal College of the Obstetricians and Gynaecologists (London, UK) stresses the need to ‘quick-start’ ongoing contraception after EC. 10 In 2014, National Institute for Health and Care Excellence guidance on contraceptive services for young people endorsed this recommendation. 16 In some parts of the UK, pharmacies are offering women supplies of contraceptive pills after EC use,17 despite the lack of evidence that this is an effective intervention or is cost-effective. We must show that the intervention is effective before we can recommend adoption of this approach. A recent cost-effectiveness analysis of EC estimated that, in 2011, unintended pregnancies cost the NHS over £1B. 5 It is possible that even these costs are an underestimate of the ‘real costs’, as they did not include the cost of managing medical complications of pregnancy or account for the additional costs associated with teenage pregnancy. If a community pharmacy-based intervention could be designed that was effective in reducing unintended pregnancies and was shown to be cost-effective, then this would result in savings for the health systems, which could be invested elsewhere. Women who present for EC should be given the best chance to prevent an unintended pregnancy.
Long-acting reversible contraception (LARC), which includes the implant, intrauterine contraception and injectable contraception, has been shown to be the most effective reversible method of contraception for preventing pregnancy. 18 In recognition of the effectiveness of LARC, national initiatives in the UK to increase uptake have been encouraged. 16,18 Rapid referral of women using EC to SRH services capable of initiating LARC immediately (as in this study) could do much to improve LARC uptake among women at risk of unintended pregnancy.
We therefore conducted the ‘Bridge-It’ study: a robust trial to determine whether or not a pharmacy-based intervention designed to facilitate the uptake of effective contraception after EC increases use of effective contraceptive methods, compared with standard care alone. For the Bridge-it study, we used a composite intervention of a small ‘bridging’ supply of the POP and the offer of rapid access to a participating SRH clinic. This combined both temporary contraception (giving women time to arrange an appointment with their usual contraceptive provider) with facilitated access to a specialist contraceptive service (i.e. the SRH clinic) where all methods of contraception, including the most effective LARC methods, are provided.
Chapter 2 Methods
Study design
The Bridge-it study was a pragmatic cluster randomised cohort crossover trial of community pharmacies in three regions of the UK [London (south and central), Lothian (Edinburgh and region) and Tayside (Dundee and region)]. 19 With this design, the order in which each community pharmacy provided intervention or control was randomised. There was an intervening period of at least 2 weeks (during which recruitment halted) before the pharmacy switched to the other recruitment phase. 19 The cluster design was chosen as our pilot study15 findings indicated that an individual randomised trial would not recruit sufficient numbers of women. The crossover was chosen for efficiency, with each cluster acting as its own control and fewer pharmacies required. The washout period between recruitment periods minimised any contamination effect of pharmacists between recruitment periods.
Pharmacies were chosen to be a mixture of large commercial chain stores and small independent stores. Each pharmacy had to have a sufficient volume of EC dispensing (at least 30 ECs per month) to participate.
Women receiving LNG EC from a study pharmacy were considered for study participation. The LNG EC was given in the appropriate dose (1.5 mg or 3 mg) for the woman’s weight. 10
The intervention was a composite intervention. Women received 3 months worth of the POP (75 µg of desogestrel/day) at no cost (as is the norm in the NHS) and the offer to attend a local participating SRH service to discuss and provide ongoing effective contraception, including the most effective LARC methods (i.e. intrauterine contraceptive methods and contraceptive implants). 18 The three packets of POP provide women with 3 months within which they could attend a contraceptive provider for ongoing contraception. The POP has very few absolute contraindications,20 making it safer for pharmacy provision than the combined oral contraceptive pill. The desogestrel POP was chosen as it is the market leader, the most effective POP (as it has high rates of ovulation inhibition) and is inexpensive (£9 for 3 months of the generic version). 21 Community pharmacists provided the POP using locally approved patient group directions (PGDs) (i.e. strict criteria to permit provision of specified medicines by non-prescribers). 21,22 Participating pharmacists were trained on the study protocol and use of the PGD for the POP, including information about medical contraindications to POP, potential drug interactions and ‘missed pill’ guidance. Pharmacists advised women to start the POP the day after the EC. Women in the intervention group also received a rapid-access card that on presentation to the local SRH clinic would help them to be seen as a walk-in patient to discuss contraception and obtain their preferred ongoing method. The card provided information about the location of the SRH clinic and the opening hours. The clinics (three in London, one in Lothian and two in Tayside) were located within 5 miles of the community pharmacies.
In the control group, women received standard care with the EC (i.e. usual advice about ongoing contraception). Mystery shopper visits with simulated patients12,13 were conducted in the pharmacies just before recruitment started in the control group to document the content of the standard care EC consultations (see Chapter 4).
Participants
Pharmacists assessed women’s eligibility for the study, invited them to participate, obtained written informed consent for the study (including access to SRH clinic records and data linkage with the national abortion registries) and provided a patient information sheet. Participants completed a baseline questionnaire that included demographic details, reproductive history and previous methods of contraception used, including ECs (see Appendix 3). To maximise retention in the study, participants received an online shopping voucher of £10 following recruitment. 23
Procedures
Participants in both groups of the study had a single follow-up at 4 months: either a telephone interview with a research nurse or a self-administered web-based questionnaire, according to participant preference (see Appendices 4 and 5). In addition to contraceptive use, women were asked about their interaction with the pharmacist and use of the rapid-access card (intervention group only). Participants reporting pregnancy also completed the London Measure of Unplanned Pregnancy, which is a validated questionnaire to measure the intendedness of the index pregnancy. 24,25 Serious adverse events since recruitment were also recorded.
The participating SRH clinics also searched their clinic record systems for data on if and for what reason participants in both arms of the study had used their service during the 4 months following recruitment.
Outcomes
The primary outcome was self-reported use of effective contraception (hormonal or intrauterine) at 4 months. Secondary outcomes were incidence of abortion in the 12 months following recruitment and an economic evaluation of the intervention. Both secondary outcomes will be reported later [as per agreement with the National Institute for Health Research (NIHR)].
A multimethod process evaluation was also conducted to assess implementation, mechanisms of change and context,19 and included qualitative interviews with participants, pharmacists and staff at SRH clinics. The process evaluation is reported separately in this report (see Chapter 5).
Sample size
An original power calculation for the study was based on two co-primary outcomes: (1) abortion rates at 12 months and (2) use of effective contraception at 4 months. For the abortion outcome, we required > 2000 women to be randomised in at least 26 pharmacies to have 90% power at 2.5% level of significance to detect a relative reduction of around 50. During the study, it became evident that recruiting this number of women was not feasible within the available time frame and resources. The independent oversight committees [the Trial Steering Committee (TSC) and Data Monitoring Committee (DMC)] and the funder (NIHR Health Technology Assessment programme) agreed to repower the study on a single primary outcome (i.e. use of effective contraception at 4 months). To have 90% power at a 5% level of significance and to demonstrate an increase from around 30% to 45% (absolute change 15%, relative increase 50%), the study required between 626 and 737 women, depending on the intraclass correlations (within period and between periods), which were not observable within the study at the time this change was made. 26 The sample size calculation assumed that we would not have the 4-month primary outcome data for 25% of participants and that there would be 25 participating pharmacies. For the intracluster correlations, the within-cluster, within-period correlation was 0.032 and the between-period, within-cluster correlation was also 0.032. The observed correlation between the two outcomes in the same cluster period compared with the two outcomes from different periods in the same cluster was effectively zero (< 0.00001).
Randomisation
The order of delivery of intervention or control for each pharmacy was generated using a computer software algorithm. This randomly allocated permuted blocks of size two, four and six, and blocking was used to ensure a balanced order. The randomisation file was prepared by the study statistician at the Centre for Healthcare Randomised Trials, University of Aberdeen (Aberdeen, UK), using SAS® 6.4 for Windows (SAS Institute Inc., Cary, NC, USA). Pharmacists were informed of the randomisation allocation by the study trial manager. The study was not blinded.
Statistical analysis
Baseline characteristics of participants and 4-month follow-up data were summarised using mean [standard deviation (SD)] or median (interquartile range) for continuous variables. Discrete variables were summarised with numbers and percentages. The published study protocol19 specified a hierarchical mixed-effects logistic regression on individual data for the analysis of reported use of effective contraception at 4 months, and this represented our analysis intentions at the time of submission of the study protocol. However, we revised this primary analysis in the final statistical analysis plan (accepted 5 November 2019, before any unblinded data had been seen) to use a linear model on the unweighted proportion (expressed as a percentage) at site level, following methodological guidance from Morgan et al. ,27 and we used the hierarchical mixed-effects logistic regression as a sensitivity-type analysis. Although the linear model on percentages at site level makes less use of the available information, it can be more robust, with fewer assumptions, and expresses the treatment effect as a percentage difference in proportion rather than as an odds ratio.
Ideally, the design would generate equal contributions in each time period and each site would contribute equal numbers. However, we knew from the aggregated accruing data on the database, returned to date, that sites were quite different in size and also returning different numbers in each period. Therefore, and guided by the Morgan et al. 27 paper, which indicated that the simple approach of modelling the percentages by site had the best properties in terms of control of type 1 error, we took the unusual decision to prefer the simpler approach to the more complex hierarchical approach. This resulted in forgoing the potential increase in power traded against the possibility of model misspecification, as the model assumptions (regarding the several decompositions of within and between cluster correlation, the influence of missing data, and the influence of very different sizes by period and site) might have been violated. We felt that the hierarchical approach could still usefully be reported as a sensitivity-type analysis and, as it happened, it does seem to support the simpler analysis of proportions, giving similar estimates, but with the expected increase in precision.
For the primary outcome, the percentage of women reporting use of effective contraception in each cluster period was analysed using linear regression, adjusting for period, treatment arm and centre, and with centre as a fixed effect. 27 Prespecified baseline covariates (i.e. mean age, percentage of participants currently in a sexual relationship and percentage of participants who had previously used effective contraceptive methods) were included as an additional analysis. A two-sided p-value < 0.05 was taken as statistically significant.
For the primary outcome, prespecified subgroup analysis for LARC use18 was performed using a stricter level of statistical significance (two-sided 1% significance level) and 99% confidence intervals (CIs).
The sensitivities of treatment effect estimate to missing outcome data were undertaken using multiple imputation. The missing primary outcome (i.e. uptake of effective contraception) was imputed using all baseline characteristics as predictors, except for ‘previous ectopic pregnancy’ (because of small numbers). Stata® version 16 (StataCorp LP, College Station, TX, USA) was used to impute 40 data sets.
Summary of changes to the protocol
Owing to resource and time constraints, the study was repowered on a single primary outcome (i.e. effective contraception use at 7 months). For the same reasons, the planned follow-up of participants at 12 months was removed. Abortion rates at 12 months will still be collected and these and the cost-effectiveness model will be reported separately (because of a later study end date than anticipated). A summary of changes to the protocol can be found in Box 1. The final and previous versions of the protocol can be found on the NIHR Journals Library [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1511301/#/ (accessed 15 February 2021)].
Changes were requested to the exclusion criteria and how identifiable data would be handled. The protocol was updated to version 2, 16 June 2017.
Approved.
Non-substantial amendment to protocol Protocol version 3, 16 April 2018There was a minor clarification to the wording on page 14 of the protocol to explain the time frame for the two phases of the study:
Each pharmacy will recruit on average 80 women to provide around 60 evaluable women at 12 months. 30 women will be recruited to the intervention arm and 30 women will receive standard care. In order for each pharmacy to recruit on average 80 women, pharmacies will recruit for approximately two months in each intervention or standard care phase; some pharmacies will recruit for a shorter or longer duration depending on the recruitment rate at the pharmacy and the size of the pharmacy. However, there will be a minimum break (wash-out period) of two weeks in between the two recruitment periods.
Approved.
Substantial amendment to the protocolIn December 2018, a substantial amendment was submitted to the REC.
Protocol version 4, 12 November 2018The primary and secondary end points were updated and the sample size was reduced to 626–737 participants. The trial team also requested a 6-month no-cost extension. The changes to the sample size and extension were reviewed and approved by both the TSC and the trial funder (i.e. NIHR Health Technology Assessment programme). The end date of the study was changed from 30 September 2019 to 31 March 2020.
Protocol version 5, 8 January 2019The REC reviewed this in January 2019 and requested further changes to protocol to clarify at what time GPs would be contacted.
Approved.
REC, Research Ethics Committee.
Ethics
Ethics approval was obtained from South East Scotland Research Ethics Committee in June 2017. Approvals were also obtained from NHS Research Scotland (Clydebank, UK) and Health Research Authority (London, UK).
Chapter 3 Results
Parts of this chapter have been reproduced from Cameron et al. 28 in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Recruitment and participant flow
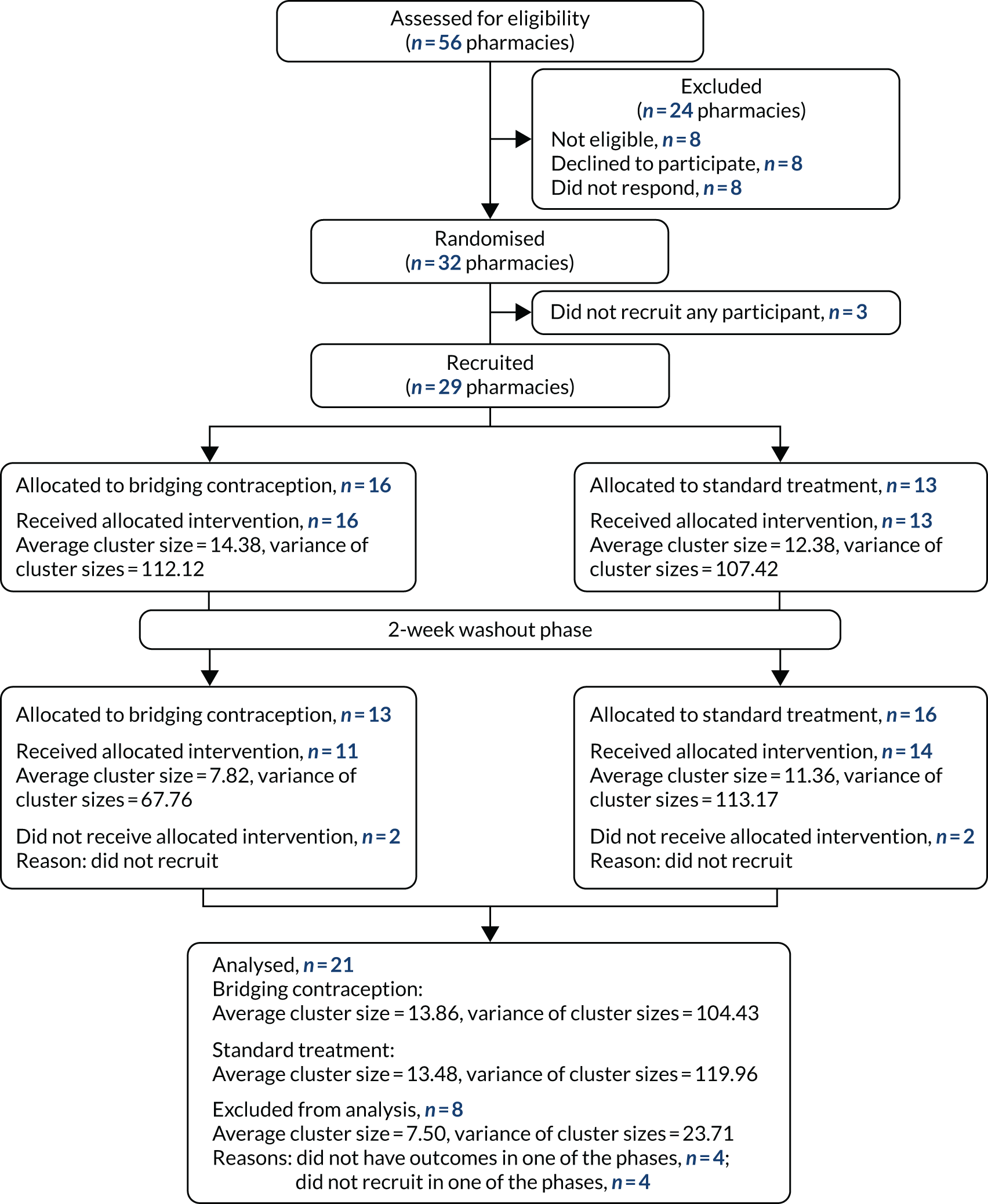
Fifty-six pharmacies were approached to participate and 32 agreed to take part in the trial (Figure 1). Twenty-nine out of the 32 pharmacies recruited participants (14 in London, 12 in Lothian and three in Tayside). Participants were recruited between 19 December 2017 and 26 June 2019. A total of 1252 participants were screened, of whom 762 were eligible and 636 consented and were randomised. Box 2 shows the inclusion and exclusion criteria.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) flow chart.
Eligible for LNG EC confirmed.
Able to give informed consent to participate in and adhere to trial requirements.
Aged ≥ 16 years.
Willing to give contact details and be contacted at 4 months by telephone, SMS, e-mail or post.
Willing to give identifying data sufficient to allow data linkage with NHS registries.
Exclusion criteriaContraindications to POPs.
Taking medication that interacts with POPs.
Already using hormonal contraception.
Requires interpreting services.
Pharmacist has concerns about non-consensual sex.
SMS, short message service.
Table 1 shows reasons for ineligibility and Figure 2 shows participant flow. The number of participants recruited per pharmacy ranged from 2–35 in the pharmacies’ first recruitment period and from 0–38 in the second period.
| Reason | n (%) |
|---|---|
| Does not require EC | 93 (19.0) |
| Lacks capacity to give informed consent | 64 (13.1) |
| Aged < 16 years | 10 (2.0) |
| Unwilling to give contact details and be contacted for follow-up | 264 (53.9) |
| Unwilling to give identifying data sufficient to allow data linkage with NHS registries | 262 (53.5) |
| Contraindication to POPs | 15 (3.1) |
| Medication that interacts with POPs | 5 (1.0) |
| Already using hormonal contraception | 156 (31.8) |
| Needs an interpreter | 10 (2.0) |
| Pharmacist concerns about non-consensual sex | 2 (0.4) |
FIGURE 2.
Participant flow chart. n, number of women who returned a 4-month questionnaire; N, number of women recruited; P, number of pharmacies.

Table 2 summarises the baseline characteristics of participants by randomised group and recruitment period. The mean age of participants was 22.7 (SD 5.7) years and 22.6 (SD 5.1) years in the intervention and control groups, respectively. There were no statistically significant differences between groups.
| Baseline characteristic | Intervention | Control | ||
|---|---|---|---|---|
| Period 1 (N = 229) | Period 2 (N = 86) | Period 1 (N = 161) | Period 2 (N = 155) | |
| Age (years), mean (SD) | 23.2 (6.0) | 21.4 (4.8) | 22.2 (4.4) | 22.9 (5.8) |
| Methods of contraception ever used, n (%) | ||||
| Combined hormonal contraception | 114 (49.8) | 41 (47.7) | 99 (61.5) | 94 (59.9) |
| POP | 39 (17.0) | 19 (22.1) | 35 (21.7) | 32 (20.4) |
| Male condom | 189 (82.5) | 66 (76.7) | 117 (72.7) | 135 (86.0) |
| Progestogen-only injectable | 14 (6.1) | 6 (7.0) | 10 (6.2) | 18 (11.5) |
| Progestogen-only implant | 29 (12.7) | 10 (11.6) | 23 (14.3) | 19 (12.1) |
| Cu-IUD | 6 (2.6) | 0 | 4 (2.5) | 4 (2.5) |
| LNG-IUS | 0 | 1 (1.2) | 4 (2.5) | 2 (1.3) |
| Withdrawal method | 65 (28.4) | 28 (32.6) | 52 (32.3) | 67 (42.7) |
| Other methodsa | 10 (4.4) | 6 (7.0) | 7 (4.3) | 9 (5.7) |
| Never used any method | 8 (3.5) | 4 (4.7) | 10 (6.2) | 2 (1.3) |
| Past birth: yes, n (%) | 30 (13.1) | 5 (5.8) | 7 (4.3) | 13 (8.3) |
| Past termination: yes, n (%) | 38 (16.6) | 6 (7.0) | 22 (13.7) | 27 (17.2) |
| Past miscarriage: yes, n (%) | 17 (7.4) | 5 (5.8) | 10 (6.2) | 6 (3.8) |
| Current sexual relationship: yes, n (%) | 176 (76.9) | 55 (64.0) | 104 (64.6) | 111 (70.7) |
| First time ever use of EC: yes, n (%) | 52 (22.7) | 22 (25.6) | 28 (17.4) | 32 (20.4) |
| Number of times used EC in the last 12 months | ||||
| Mean (SD) | 1.4 (1.4) | 1.5 (1.5) | 1.5 (1.2) | 1.7 (2.0) |
| Median (25th, 75th centile) | 1.0 (0.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) |
| Minimum, maximum | 0.0, 8.0 | 0.0, 9.0 | 0.0, 6.0 | 0.0, 20.0 |
| Ethnic background, n (%) | ||||
| White | 157 (68.6) | 60 (69.8) | 98 (60.9) | 114 (72.6) |
| Asian or Asian British | 21 (9.2) | 6 (7.0) | 8 (5.0) | 21 (13.4) |
| Black or black British | 29 (12.7) | 12 (14.0) | 36 (22.4) | 15 (9.6) |
| Mixed or other | 19 (8.3) | 6 (7.0) | 17 (10.6) | 6 (3.8) |
| Missing | 3 (1.3) | 2 (2.3) | 2 (1.2) | 1 (0.6) |
Four-month follow-up
Follow-up data at 4 months were available for 198 out of 315 (62.9%) women and 208 out of 318 (65.4%) women in the intervention and control groups, respectively. Follow-up rates did not differ statistically between groups nor between those recruited in the first and second recruitment periods of pharmacies. There were no substantial differences in any baseline characteristics between responders and non-responders (Table 3). No significant adverse events were reported.
| Characteristic (at baseline) | Non-responders (N = 227) | Responders (N = 406) |
|---|---|---|
| Age (years), mean (SD) | 22.3 (5.0) | 22.8 (5.7) |
| Methods of contraception ever used, n (%) | ||
| Combined hormonal contraception | 118 (52.0) | 230 (56.7) |
| POP | 46 (20.3) | 79 (19.5) |
| Male condom | 157 (69.2) | 350 (86.2) |
| Progestogen-only injectable | 19 (8.4) | 29 (7.1) |
| Progestogen-only implant | 29 (12.8) | 52 (12.8) |
| Cu-IUD | 3 (1.3) | 11 (2.7) |
| LNG-IUS | 2 (0.9) | 5 (1.2) |
| Female condom | 0 | 3 (0.7) |
| Cap/diaphragm | 0 | 6 (1.5) |
| Vasectomy | 1 (0.4) | 3 (0.7) |
| Withdrawal method | 62 (27.3) | 150 (36.9) |
| Natural family planning | 7 (3.1) | 13 (3.2) |
| Other method | 1 (0.4) | 2 (0.5) |
| Never used any method | 11 (4.8) | 13 (3.2) |
| Past birth: yes, n (%) | 16 (7.0) | 39 (9.6) |
| Past abortion: yes, n (%) | 36 (15.9) | 57 (14.0) |
| Past miscarriage: yes, n (%) | 16 (7.0) | 22 (5.4) |
| Current sexual relationship: yes, n (%) | 154 (67.8) | 292 (71.9) |
| First time ever use of EC: yes, n (%) | 44 (19.4) | 90 (22.2) |
| Number of times used EC in the last 12 months | ||
| Mean (SD) | 1.5 (1.2) | 1.5 (1.7) |
| Median (25th, 75th centile) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) |
| Minimum, maximum | 0.0, 6.0 | 0.0, 20.0 |
| Ethnic background, n (%) | ||
| White | 134 (59.0) | 295 (72.7) |
| Asian or Asian British | 25 (11.0) | 31 (7.6) |
| Black or black British | 42 (18.5) | 50 (12.3) |
| Mixed or other | 23 (10.1) | 25 (6.2) |
| Missing | 3 (1.3) | 5 (1.2) |
Primary outcome: effective contraception use at 4 months
The proportion of women using effective contraception (hormonal and intrauterine contraception) was 20.1% greater (95% CI 5.2% to 35.0%) in the intervention group (58.4%, SD 21.6) than in the control group (40.5%, SD 23.8) (adjusted for recruitment period, treatment arm and centre; p = 0.011) (Table 4).
| Analysis | Intervention, n; mean (SD) | Control, n; mean (SD) | Estimate | 95% CI | p-value |
|---|---|---|---|---|---|
| Primary outcomea | 21; 58.4 (21.6) | 21; 40.5 (23.8) | 20.1 | 5.2 to 35.0 | 0.011 |
| Primary outcome adjustedb | 21; 58.4 (21.6) | 21; 40.5 (23.8) | 14.5 | 0.9 to 28.2 | 0.038 |
Use of effective contraception remained statistically significantly higher in the intervention group when adjusted for recruitment period, treatment group, study centre, age, whether or not currently in a sexual relationship and history of past use of effective contraception, and was robust to the missing data (Tables 4–6) using a multiple imputation approach under an assumption of missing at random.
| Sensitivity analysisa | Intervention, n; mean (SD) | Control, n; mean (SD) | Estimate | 95% CI | p-value |
|---|---|---|---|---|---|
| Omit cluster with fewer than three responses | 14; 60.2 (19.7) | 14; 42.9 (13.8) | 15.2 | 2.3 to 28.1 | 0.026 |
| Omit cluster with > 30% individuals with missing data (excluded centre from the model) | 5; 58.5 (14.4) | 5; 44.1 (6.6) | 14.7 | 1.1 to 28.2 | 0.040 |
| Including percentage missing and number of responders in the model | 21; 58.4 (21.6) | 21; 40.5 (23.8) | 15.3 | –0.1 to 30.6 | 0.051 |
| Including percentage missing in the model | 21; 58.4 (21.6) | 21; 40.5 (23.8) | 15.0 | 0.3 to 29.7 | 0.046 |
| Including number of responders in the model | 21; 58.4 (21.6) | 21; 40.5 (23.8) | 15.0 | 0.6 to 29.4 | 0.042 |
| Multiple imputation | 15.0 | –2.0 to 32.0 | 0.076 |
| Analysisa | Intervention, n/N (%) | Control, n/N (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Fixed effects for cluster | 112/198 (56.6) | 85/208 (40.9) | 1.93 | 1.21 to 3.09 | 0.006 |
Contraceptive use and long-acting reversible contraception use at 4 months
The methods of contraception used at 4 months after EC are shown in Table 7. The most common methods used were oral contraceptive pills (POPs in the intervention group and the combined hormonal contraceptive pills in the control group). There were no sterilisations or women relying on vasectomy. Use of LARC methods (e.g. implant, intrauterine and injectable) did not differ between the intervention group (13/198, 6.6%) and control group (23/208, 11.1%) (95% CI –10.04% to 1.05%; p = 0.112).
| Variable | Intervention (N = 198), n/N (%) | Control (N = 208), n/N (%) |
|---|---|---|
| Methods of contraception used nowa | ||
| Combined hormonal contraception | 28/198 (14.1) | 47/208 (22.6) |
| POP | 71/198 (35.9) | 15/208 (7.2) |
| Male condom | 31/198 (15.7) | 63/208 (30.3) |
| Progestogen-only injectable | 4/198 (2.0) | 4/208 (1.9) |
| Progestogen-only implant | 3/198 (1.5) | 11/208 (5.3) |
| Cu-IUD | 3/198 (1.5) | 4/208 (1.9) |
| LNG-IUS | 3/198 (1.5) | 4/208 (1.9) |
| Other method | 0 | 1/208 (0.5) |
| Not using any method | 57/198 (28.8) | 62/208 (29.8) |
| LARCb | 13/198 (6.5) | 23/208 (11.1) |
| When did they start using this method? | ||
| Same day as EC | 18/141 (12.8) | 14/146 (9.6) |
| Day after EC | 38/141 (27.0) | 7/146 (4.8) |
| With start of period after EC | 13/141 (9.2) | 23/146 (15.8) |
| Other | 48/141 (34.0) | 62/146 (42.5) |
| Missing | 24/141 (17.0) | 40/146 (27.4) |
| Where did they get contraception from? | ||
| GP clinic | 74/141 (52.5) | 53/146 (36.3) |
| SRH clinic | 34/141 (24.1) | 35/146 (24.0) |
| Other | 21/141 (14.9) | 40/146 (27.4) |
The most common reason given by respondents for not using effective contraception at 4 months was that they were not currently sexually active (Table 8). Significantly fewer women in the intervention group than in the control group had used EC again since recruitment [20/198 (10.1%) vs. 37/208 (17.8%)] (95% CI –15.38% to –1.48%; p = 0.018) (see Table 8).
| Reasons for not using effective contraception | Intervention (N = 57), n/N (%) | Control (N = 62), n/N (%) |
|---|---|---|
| Not currently sexually active | 27/57 (47.4) | 28/62 (45.2) |
| Worried about side effects with contraception | 12/57 (21.1) | 21/62 (33.9) |
| Medical reasons | 1/57 (1.8) | 2/62 (3.2) |
| Not decided on method to be used | 9/57 (15.8) | 11/62 (17.7) |
| Difficult to get appointment | 8/57 (14.0) | 4/62 (6.5) |
| Difficult to find time to get to GP or SRH clinic | 6/57 (10.5) | 4/62 (6.5) |
| Trying for baby | 1/57 (1.8) | 0 |
| Other | 8/57 (14.0) | 5/62 (8.1) |
| Further use of EC: yes | 20/198 (10.1) | 37/208 (17.8) |
A significantly larger proportion of women in the intervention group reported that the pharmacist had advised them about starting ongoing contraception (98% vs. 75.5%; p < 0.001) (Table 9).
| Contraceptive advice | Intervention (N = 198), n (%) | Control (N = 208), n (%) | Effect size |
|---|---|---|---|
| Did pharmacist provide information about starting contraception? | |||
| No | 2 (1.0) | 46 (22.1) | 0.216 (0.156 to 0.277); p < 0.001 |
| Yes | 194 (98.0) | 157 (75.5) | |
| Missing | 2 (1.0) | 5 (2.4) | |
| Did pharmacist provide information about where to get contraception? | |||
| No | 19 (9.6) | 67 (32.2) | 0.237 (0.159 to 0.315); p < 0.001 |
| Yes | 178 (89.9) | 134 (64.4) | |
| Missing | 1 (0.5) | 7 (3.4) | |
A total of 158 out of 198 (79.8%) respondents in the intervention group reported that they used some of the study POP that the pharmacist provided. Table 10 provides data on when women started the POP, the number of packets of POPs used and reasons for non-use or discontinuation of the POP.
| Use of POP | Intervention (N = 198), n/N (%) |
|---|---|
| Used any of the POPs that pharmacist provided? | |
| Yes | 158/198 (79.8) |
| No | 35/198 (17.7) |
| Missing | 5/198 (2.5) |
| If not, why?a | |
| Not a regular partner | 8/35 (22.9) |
| Not requiring regular contraception | 7/35 (20.0) |
| Worried regarding side effects | 10/35 (28.6) |
| Did not understand how to use it | 1/35 (2.9) |
| Preferred another contraceptive | 6/35 (17.1) |
| Used POP in the past and it did not agree | 1/35 (2.9) |
| Preferred to see GP for contraception | 2/35 (5.7) |
| Other | 10/35 (28.6) |
| When did they start POPs? | |
| Same day as EC | 27/158 (17.1) |
| Day after EC | 93/158 (58.9) |
| With start of period after EC | 19/158 (12.0) |
| Other | 16/158 (10.1) |
| Missing | 3/158 (1.9) |
| Number of packets of POPs used | |
| Less than one | 15/158 (9.5) |
| One | 17/158 (10.8) |
| Less than two | 13/158 (8.2) |
| Two | 8/158 (5.1) |
| Less than three | 10/158 (6.3) |
| Three | 70/158 (44.3) |
| Still taking POP | 22/158 (13.9) |
| Missing | 3/158 (1.9) |
| Main reason for stopping POPs before the supply ran out | |
| Side effects | 40/158 (25.3) |
| Started another method | 6/158 (3.8) |
| Other | 22/158 (13.9) |
| Missing | 90/158 (57.0) |
Rapid-access card and sexual and reproductive health clinic utilisation
At the 4-month follow-up interview, 137 out of 198 (69.2%) respondents in the intervention group could recall receiving the rapid-access card, but only 31 respondents (15.6%) reported that they attended the SRH clinic. Only two respondents used the rapid-access card within 1 month of recruitment. Four out of 31 respondents (12.9%) received a LARC method at the SRH clinic (Table 11).
| Variable | Period 1 (N = 147), n/N (%) | Period 2 (N = 51), n/N (%) |
|---|---|---|
| Did pharmacist provide a rapid-access card? | ||
| Yes | 105/147 (71.4) | 32/51 (62.7) |
| No | 25/147 (17.0) | 5/51 (9.8) |
| I cannot remember | 12/147 (8.2) | 12/51 (23.5) |
| Missing | 5/147 (3.4) | 2/51 (3.9) |
| Did they attend the SRH clinic? | ||
| Yes | 25/117 (21.4) | 6/44 (13.6) |
| No | 90/117 (76.9) | 35/44 (79.5) |
| Missing | 2/117 (1.7) | 3/44 (6.8) |
| If no, why?a | ||
| Not requiring contraception | 19/90 (21.1) | 9/35 (25.7) |
| Preferred to see a GP for contraception | 45/90 (50.0) | 17/35 (48.6) |
| Preferred to attend another SRH clinic for contraception | 5/90 (5.6) | 0 |
| Other | 22/90 (24.4) | 8/35 (22.9) |
| When did they attend the SRH clinic? | ||
| Same day as EC | 1/25 (4.0) | 0 |
| < 1 month after EC | 1/25 (4.0) | 0 |
| 1–2 months after EC | 2/25 (8.0) | 1/6 (16.7) |
| 2–3 months after EC | 12/25 (48.0) | 3/6 (50.0) |
| 3–4 months after EC | 4/25 (16.0) | 1/6 (16.7) |
| Other | 4/25 (16.0) | 0 |
| Missing | 1/25 (4.0) | 1/6 (16.7) |
| Did they remember to take rapid-access card to the SRH clinic? | ||
| Yes | 14/25 (56.0) | 3/6 (50.0) |
| No | 11/25 (44.0) | 2/6 (33.3) |
| Missing | 0 | 1/6 (16.7) |
| If no rapid-access card, were they refused an appointment? | ||
| Yes | 1/11 (9.1) | 0 |
| No | 10/11 (90.9) | 2/2 (100.0) |
| How long did they wait at the SRH clinic? | ||
| < 30 minutes | 8/25 (32.0) | 3/6 (50.0) |
| < 1 hour | 7/25 (28.0) | 1/6 (16.7) |
| 1–2 hours | 8/25 (32.0) | 1/6 (16.7) |
| Other | 2/25 (8.0) | 0 |
| Missing | 0 | 1/6 (16.7) |
| Did the SRH clinic provide a method of contraception? | ||
| Yes | 19/25 (76.0) | 5/6 (83.3) |
| No | 6/25 (24.0) | 0 |
| Missing | 0 | 1/6 (16.7) |
| Did the SRH clinic provide the participant’s preferred method? | ||
| Yes | 16/25 (64.0) | 5/6 (83.3) |
| No | 8/25 (32.0) | 0 |
| Missing | 1/25 (4.0) | 1/6 (16.7) |
| If no, why?a | ||
| Not enough staff or time | 2/8 (25.0) | 0 |
| Risk of pregnancy | 1/8 (12.5) | 0 |
| Other | 6/8 (75.0) | 0 |
| Method of contraception received | ||
| Progestogen-only implant | 1/25 (4.0) | 0 |
| Progestogen-only injectable | 0 | 0 |
| Cu-IUD | 1/25 (4.0) | 0 |
| LNG-IUS | 2/25 (8.0) | 1/6 (16.7) |
| Combined hormonal contraception | 3/25 (12.0) | 0 |
| POP | 12/25 (48.0) | 4/6 (66.7) |
| Male condom | 2/25 (8.0) | 0 |
| Experience of the rapid access to study SRH clinic | ||
| Smooth | 16/25 (64.0) | 3/6 (50.0) |
| Neither/nor | 6/25 (24.0) | 2/6 (33.3) |
| Problematic | 1/25 (4.0) | 0 |
| Missing | 2/25 (8.0) | 1/6 (16.7) |
Data obtained from the SRH clinic databases showed that similar proportions of participants in both the intervention and control groups actually attended the SRH clinics within 4 months of recruitment [52/305 (17%) intervention group vs. 43/309 (13.9%) control group, 95% CI –2.60% to 8.87%; p = 0.284]. A total of 41 out of 95 (43.1%) attendees received contraception and the methods supplied are shown in Table 12. There was no statistically significant difference in LARC provision to women in each group.
| Variable | Intervention (N = 305), n/N (%) | Control (N = 309), n/N (%) |
|---|---|---|
| Attended the SRH clinic? | ||
| No | 253/305 (83.0) | 266/309 (86.1) |
| Yes | 52/305 (17.0) | 43/309 (13.9) |
| Method of contraception provided at this visit? | ||
| Yes | 26/52 (50.0) | 15/43 (34.9) |
| Method provided | ||
| Progestogen-only implant | 2/26 (7.7) | 3/15 (20.0) |
| Cu-IUD | 1/26 (3.8) | 4/15 (26.7) |
| LNG-IUS | 1/26 (3.8) | 3/15 (20.0) |
| Progestogen-only injectable | 0 | 0 |
| Combined hormonal contraception | 3/26 (11.5) | 1/15 (6.7) |
| POP | 16/26 (61.5) | 2/15 (13.3) |
| Male condom | 3/26 (11.5) | 3/15 (20.0) |
| Other method | 1/26 (3.8) | 0 |
A total of 75 women both attended the SRH clinic and provided data at the 4-month interview. For 34 of these women, the SRH clinic provided a contraceptive method. Thirty out of 34 (88%) women reported using the same method of contraception at 4 months as the method provided by the SRH clinic.
Pregnancies in the 4 months since emergency contraception
Nineteen respondents (4.7%) (intervention, n = 9; control, n = 10) reported that they had been pregnant (n = 17) or were currently pregnant (n = 2). The outcome of the pregnancies that had ended were abortion in 10 women (58.8%) and miscarriage in six women (35.3%); this information was unknown for one woman (5.9%). Based on the London Measure of Unintended Pregnancy24,25 scores from the questionnaire, 15 out of 19 pregnancies (78.9%) had been unintended (seven in the intervention group and eight in the control group).
Chapter 4 Mystery shopper exercise
Parts of this chapter have been reproduced from Glasier et al. 13 in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Mystery shopper exercise
Pharmacists were advised that one or two mystery shopper visits would take place before the control group of the study started, but they were not told when these would occur. Visits took place between April 2018 and January 2019. Research nurses recruited female volunteers (aged ≥ 16 years) to be mystery shoppers and instructed them on the aims of the exercise, the scenario to be followed and the data to be collected. The members of the patient and public involvement (PPI) group for the study assisted in identifying potential mystery shoppers. The shoppers received £20 for each completed visit. They followed a scenario (a single episode of unprotected sex within 72 hours) relating to a request for EC. The scenario had been approved by the PPI group. Immediately after leaving the pharmacy, shoppers completed a data collection pro forma, recording the time of arriving and leaving the pharmacy, duration of the consultation and where the consultation about EC took place. They also noted any information provided by the pharmacist, including advice on ongoing contraception. Data from the proforma were entered into an Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) database and descriptive statistics were conducted for each question.
Results
A total of 55 mystery shopper visits were conducted at the 30 study pharmacies participating in this part of the trial. The mean total time reported spent in the pharmacy was 12 (range 1–47) minutes. The median reported duration of the consultation with the pharmacist was 6 (range 1–18) minutes. Consultations took place in a private room with 34 women (62%) and the remaining consultations took place at the counter.
Eleven mystery shoppers (20%) were unable to get EC from the pharmacy. Seven mystery shoppers reported that they were told that no trained pharmacist was available to provide EC. Four mystery shoppers were told that EC was not in stock. Two of the latter women were aged 16 years and visited the same pharmacy on the same day and both were advised to return 90 minutes later. A further two mystery shoppers were advised that a copper intrauterine device would be more effective and were directed to a SRH clinic for insertion, but neither was offered oral EC as an interim measure. Eight mystery shoppers were told that they had to swallow the EC tablets in the pharmacy and seven mystery shoppers (in London) were told that the pharmacy could not offer free EC. Three mystery shoppers were asked to provide proof of identity and age to get free EC. Table 13 shows the results for information provided by the pharmacist around EC or ongoing contraception.
| Information provided or discussed | Yes (n) | No (n) | Not completed (n) |
|---|---|---|---|
| Reason for needing EC | 43 | 12 | |
| Information about accessing ongoing contraception | 30 | 25 | |
| Oral information on importance of ongoing contraception | 27 | 27 | 1 |
| Oral advice to conduct a pregnancy test in 3 weeks | 22 | 32 | 1 |
| Written information on importance of ongoing contraception | 5 | 50 | |
| Written advice to conduct a pregnancy test in 3 weeks | 2 | 53 |
A total of 27 out of 55 (49%) mystery shoppers received advice (oral or written) about ongoing contraception. Forty-eight of the mystery shoppers made comments about their experience. Analysis of the free-text comments showed four common themes: (1) the manner of the pharmacist, (2) being requested to take the EC on the premises, (3) pharmacy not able to provide EC and (4) concerns about privacy. Examples are shown in Box 3.
He did however request for me to take the pill in front of him to make sure it is for me and not someone else. He said this is required legally. I then stated that I am uncomfortable with it, apologised for any inconvenience and left the pharmacy.
Mystery shopper B
They made me wait for a while and then fill out a form, which they kept. I was then told that they only had one EC pill and they needed to give me two because the law had changed so I should go elsewhere. I don’t think I spoke to a pharmacist at all, just a man who worked there?
Mystery shopper C
Initially asked at counter and the lady said to wait for the pharmacist. Had to repeat to pharmacist that I needed EC. Quite a few people around.
Mystery shopper D
Discussion
The mystery shopper exercise was undertaken to describe ‘standard care’ for the control group regarding the request for EC at a community pharmacy. The exercise showed that, although pharmacists were generally helpful, getting EC (for which all of the women were eligible) was not always easy (1/5 did not get EC). In addition, waiting times were sometimes long, consultations short and privacy was not always guaranteed. In a few cases, women were asked for proof of identity/age, which was not a requirement of the local PGD in place for supplying EC. In addition, fewer than half of pharmacists in the mystery shopper exercise gave any advice about ongoing contraception. These findings are similar to a previous mystery shopper study from Edinburgh, published 10 years ago. 12 Although the current mystery shopper exercise was conducted in only 30 UK pharmacies and may therefore not be fully representative of care across the UK, all participating pharmacists had recently undergone training for the Bridge-it study and so one might have expected that the performance of these pharmacies regarding advice around ongoing contraception would have been higher.
These findings suggest that opportunities to provide EC to women and to prevent unintended pregnancy are currently being missed in community pharmacies across the UK. Furthermore, opportunities for pharmacists to discuss ongoing contraception with women provided with EC are also being missed.
This adds to the debate of whether or not EC should be available on the general sales list (i.e. available without the need for a consultation and purchasable from a range of outlets). According to the Medicines and Healthcare products Regulatory Agency (London, UK), the general sales list is appropriate for medicines that can, with reasonable safety, be sold or supplied in ways other than by or under the supervision of a pharmacist. 29 The term ‘with reasonable safety’ has been defined as ‘where the hazard to health, the risk of misuse, or the need to take special precautions in handling is small and where wider sale would be a convenience to the purchaser’ (contains public sector information licensed under the Open Government Licence v3.0. URL: https://nationalarchives.gov.uk/doc/open-government-licence/version/3/). 29
It is now more than 20 years since EC was first approved as a pharmacy medicine in the UK. 7 There is abundant evidence to demonstrate that it does not present a hazard to health, nor is it widely misused, and does not need special precautions. If ECs were a general sales list medicine, then there would be more opportunities to obtain it, which would benefit women and, possibly, public health.
Chapter 5 Process evaluation
The Bridge-it study included a multimethod process evaluation to assess implementation, mechanisms of change and context to better understand the effectiveness of the trial (see Table 14). This chapter will report the methods employed and key findings relating to the following:
-
Implementation – was the intervention implemented as planned?
-
Mechanisms of change – how did the delivered intervention produce change in and impact on contraceptive practices?
-
Context – how did the local and broader context affect implementation and outcomes?
Findings are common across trial sites, unless indicated otherwise. Throughout the chapter, we highlight those findings that are pertinent to future implementation of POP provision with EC.
Methods: data sources and analysis
We employed a range of quantitative and qualitative methods, including qualitative interviews with providers (pharmacists and SRH staff) and participants, monitoring of pharmacy selection and training observations, analysis of relevant data from the 4-month follow-up questionnaire and monitoring of contemporaneous events (e.g. relevant media coverage). Further details on each data collection method and analysis approach are provided in Appendix 1, Table 16. Collection of such data allowed us to assess the role of our critical assumptions, mediators of change and intermediate outcomes that could impact on the effectiveness of the intervention.
All process data were analysed independently from the outcome data and documented before the outcomes were known. The process evaluation team (SP and LMcD) discussed the progress of data collection and analysis on a regular basis, allowing any issues that were encountered to be resolved (Table 14).
| Measure | Questions to be answered | Method/source |
|---|---|---|
| Implementation | Fidelity:
|
Qualitative interviews with pharmacists SRH providers and participants Observation of pharmacist training Monitoring and screening logs Notes from TMG and TSC meetings |
| Mechanisms of impact | Experiences of the intervention:
|
4-month intervention questionnaire Qualitative interviews with pharmacists, SRH providers and participants |
| Context | How did the local and broader context affect implementation and outcomes?
|
Qualitative interviews with pharmacists, SRH providers and participants Monitoring of contemporaneous events Notes from TMG and TSC meeting |
Qualitative interviews with pharmacists, sexual and reproductive health providers and participants
Qualitative interviews were conducted with those responsible for delivering the study (i.e. pharmacists and SRH providers) and those receiving the intervention (i.e. Bridge-it Study participants). The breakdown of recruitment by site is detailed in Figure 3. Specific details about sampling, recruitment, topics covered and analysis for each group is detailed below.
FIGURE 3.
Process evaluation flow chart.

Semistructured qualitative telephone interviews were conducted with pharmacists and SRH providers to explore the acceptability of the intervention and training received (pharmacists), the perceptions of barriers to and facilitators of implementation, and existing contextual challenges within their services (see Appendix 2). In total, 22 pharmacists were interviewed (12 from Lothian, three from Tayside and seven from London-based pharmacies). The aim had been to interview one pharmacist from each participating pharmacy in the study. The main pharmacies not represented (n = 7) are based in London (south), as it had not been possible to conduct interviews before the study was discontinued. Interviews were conducted between July 2018 and July 2019, with most interviews taking place once recruitment had ended in their pharmacy.
As well as interviewing pharmacists involved in the study, we interviewed five SRH providers in three out of four participating NHS sites (two in Lothian, two in Tayside and one in London) between May and October 2019. Our original aim had been to interview three or four staff members from each service; however, recruitment was challenging, particularly because of low Bridge-it study participant attendance at SRH clinics. Interviews were conducted 4–6 months after study recruitment had ended to allow time for SRH staff to have experience of participants attending their service.
Qualitative interviews were also conducted with a purposive sample of 36 intervention participants (24 participants in Lothian, nine participants in London and three participants in Tayside) to explore intervention acceptability, experiences of bridging from EC to effective contraception, facilitators of and barriers to continued uptake and the wider context of their contraceptive experiences. Unintended consequences were documented through the process evaluation, including asking participants whether or not they perceived any unintended negative outcomes resulting from participation in the trial. Research nurses asked participants for consent to be contacted by the process evaluation research assistant for a qualitative interview at the end of the 4-month follow-up questionnaire, and interviews were conducted between November 2018 and October 2019. Purposive sampling was employed to recruit a representative and diverse sample, with participants sampled by area, age, use of the study POP and attendance at a SRH clinic. The characteristics of the participants who were interviewed are shown in Table 15.
| Characteristic | Participants (n) |
|---|---|
| Age group (years) | |
| 18–19 | 9 |
| 20–24 | 14 |
| 25–29 | 5 |
| 30–37 | 8 |
| Location | |
| Lothian | 24 |
| Tayside | 3 |
| London | 9 |
| Contraceptive use at time of interview | |
| POP | 10 |
| Combined pill | 4 |
| Intrauterine contraception | 1 |
| Implant | 1 |
| None | 20 |
| Contraceptive service attendeda | |
| GP | 14 |
| SRH clinic | 5 |
| No service attended | 13 |
| Unknown | 6 |
| Previous effective contraceptive use | |
| Yes | 22 |
| No | 13 |
| Missing | 1 |
All provider and participant interview data were audio-recorded, transcribed verbatim and anonymised. Data management was assisted by ethnographic software (NVivo 10, QSR International, Warrington, UK). Transcription and analysis (proceeding case by case) began with the first interview and was ongoing during data collection, allowing emergent themes to be identified and explored in future interviews. Data analysis was undertaken using ‘framework analysis’, which is a method of proven validity and reliability in which data are coded, indexed and charted systematically, then organised using a matrix or framework. 30 Such a method helps to ensure systematic thematic analysis and facilitates the synthesis of key themes across the data set. Constant comparison was carried out to ensure that the analysis represents all perspectives and negative (‘deviant’) cases.
Monitoring of pharmacy selection and observations of training
To evaluate reach and recruitment, pharmacy selection was recorded using a standardised template format. Study team members responsible for recruiting pharmacies routinely recorded decision-making that contributed to pharmacy selection, including number of contacts made, responses from potential pharmacies, rationales for inclusion/exclusion and reasons for acceptance/refusal.
To shed a light on intervention fidelity and delivery, and the potential impact of contextual factors, all intervention and training materials were reviewed and observations of training sessions were conducted by the process evaluation research assistant, where feasible. A standardised training observation form was used to guide observations, with a particular focus on the way key processes of the intervention were presented to and understood by pharmacists. In total, 13 training sessions were observed by the process evaluation research assistant in Scotland. In London, the study trainers completed the observation forms after each training session. Written observational data were transcribed into Microsoft Word, thematic analysis was conducted and descriptive summaries were written.
Monitoring of contemporaneous events
To monitor local, national and international contemporaneous events that could have an impact on contraceptive use and behaviour over the course of the study, high-coverage media stories relating to contraception were monitored and recorded over the study period (i.e. July 2017–December 2019). Weekly Google Alerts (Google Inc., Mountain View, CA, USA) were set up using relevant search terms (e.g. ‘contraception’ and ‘morning-after pill’) and key information was recorded in a Microsoft Excel workbook (e.g. date of publication, news source, headline and topic focus). Relevance was assessed primarily through the scale of coverage and source of publication. Articles were included if they were published by a mainstream media source, such as widely read print/online titles (e.g. the Daily Mail, the Guardian and the Metro), popular magazines (e.g. Time and Cosmopolitan), popular social media news sources (e.g. HuffPost, LADbible and BuzzFeed) and other relevant and widely used internet sources (e.g. BBC news website, Google News and Ofcom). Over the period of the study, 736 articles were identified from mainstream media sources in the UK and were organised into key topic areas (e.g. negative focus, personal stories, emerging contraceptive methods, accessibility of contraception, contraceptive behaviour trends and general informative pieces). Descriptive summaries of key topics identified and scale of coverage were created in Microsoft Word.
Quantitative data collected as part of the main study
Data from the pharmacist eligibility screening logs (n = 599) and relevant data from the 4-month follow-up surveys (n = 406) were drawn on as part of the process evaluation to shed a further light on acceptability, fidelity of implementation, barriers to participation and the uptake of routine contraception. As part of the study, pharmacists were asked to screen all of those attending for EC and to fill out a standardised screening form detailing reasons for ineligibility. Relevant data collected from the 4-month follow-up survey included participation in the intervention (e.g. if the woman used EC/POP, accessed a SRH service, started effective contraception, and if not then why not), delivery of key mechanisms by pharmacists and SRH providers (e.g. information provided on where to access further contraception, rapid-access card received and length of time at SRH service) and changes in circumstances that could influence contraceptive use (e.g. change of partner or pregnancy). Data were entered into statistical software package IBM SPSS Statistics and descriptive analysis conducted.
Researcher field notes and meeting minutes
Throughout the study, researcher field notes and reflections were documented, and meeting minutes were analysed to record factors that may have influenced the consistency or quality of data, and contextual factors that may have had an impact on implementation.
Synthesis of multiple data sources
After independent analysis of each data strand was completed, the findings from the multisource process evaluation were integrated to shed a light on implementation, mechanisms of change and the role of contextual factors on implementation and outcomes. To achieve this, key findings from each stage of data collection were synthesised in an analytical integration table. The table was organised to allow consideration of findings from each stage, with each strand being positioned in a single matrix for each PE component (e.g. implementation, mechanisms and context) (see Appendix 1). This method facilitated the systematic synthesis of the process data, drawing out synergistic interpretations to reveal a broader holistic perspective on how the intervention worked in practice.
Implementation
Pharmacist and sexual and reproductive health provider acceptability of the intervention
The majority of pharmacists and SRH providers were very positive about the premise of the intervention and the role of pharmacy in developing and improving contraceptive services. Bridging in community pharmacies was seen as an important way to improve access to contraception and to reduce repeat EC use and unwanted pregnancies:
. . . it shows that people are taking the issue of unwanted pregnancy seriously and they’re trying to improve, you know, the accessibility of services to women . . . and the choice that’s available to them.
Pharmacist 18, Lothian
As well as the pharmacy setting being viewed favourably, the EC consultation was seen as a particularly suitable and opportune time to offer access to routine contraception:
I also think it’s quite an opportune time when somebody comes in for emergency contraception, to pick up on, you know, sort of routine contraception. You know, the fact that they need emergency contraception, obviously something has gone wrong, so it’s a good time to be able to kind of go, well actually, have you had a think about this, or how can you deal with this kind of thing?
SRH worker 1, Lothian
It was suggested that approaching women in this way could break down some barriers (e.g. lack of time, difficulties accessing contraceptive appointments and avoidance) to accessing routine contraception at critical times.
Acceptability of taking part in a research study
Figure 4 shows the pharmacist support and training that was provided for delivering the Bridge-it study. During training sessions, the majority of pharmacists seemed enthusiastic and motivated to take part in the study and often reflected on this in interviews, expressing a desire to take part in more research:
I’m kind of like, I’d love to be involved in more things so I’m always welcome . . . you know, I’m welcome to do more kind of projects like this.
Pharmacist 20, London
FIGURE 4.
A flow chart of pharmacist training and support for delivering the Bridge-it study.

In particular, pharmacists who had been involved in the earlier pilot study15 or who had previous experience of pharmacy-based interventions were particularly enthused and seemed to be more accepting of the pharmacist role in the study, potentially because of familiarity with the kinds of processes and documentation required for trials. It is important to note that pharmacists who were particularly enthusiastic about the benefits of bridging through community pharmacies for patients, and about taking part in the study, were those who tended to recruit the most participants, perhaps highlighting the importance of ‘buy-in’ to the premise of the intervention.
Although most pharmacists reflected positively on the premise of the intervention and on taking part in research to expand contraceptive services, some concerns were raised during training. Pharmacists’ concerns typically focused on worries relating to managing the additional time and workload pressures of taking part in a research study (rather than of delivering POP per se), scepticism that additional time would be set aside to accommodate this, and concerns about how the study would fit with existing practices and changing guidelines relating to EC. These concerns are detailed in The impact of context on the Bridge-it study implementation and outcomes and focus on facilitators of and barriers to delivery and implementation in practice.
Pharmacist and sexual and reproductive health provider awareness of the study and preparedness to deliver
Pharmacists’ perceptions of and satisfaction with training
Observation of training demonstrated fidelity across sites, with sessions typically delivered as per the training guidance, covering all components and being consistent in content. Adaptations to training typically related to the time spent on particular components, fuelled by contextual factors such as lack of time. The majority of pharmacists were positive about the training received for the Bridge-it study and described the study as ‘informative’, ‘concise’, ‘well-designed’ and ‘convenient’. Pharmacists typically described being satisfied with the training staff:
. . . it was good, they were very clear, lovely manner the ladies had, so you didn’t feel daft asking a number of questions . . .
Pharmacist 8, Tayside
The venue, composition and timing of training also seemed to be acceptable to the majority of pharmacists, with different options available, small-group learning-facilitating dialogue and well-timed training encouraging confidence:
I think it was good. It wasn’t a particularly large group of us doing it. I think there were about sort of 8–10 so we were able to ask questions and chat and things which was quite good .
Pharmacist 11, London
I think it worked really well, I could start it straight away and it would be fresh in my mind because the training was a week before they would launch it, so it was good.
Pharmacist 2, Lothian
Pharmacists were also generally satisfied with the content of training and the study resources provided:
. . . the training was pretty good, pretty informative. And I think the material provided . . . stuff that they provided for reading was pretty informative as well.
Pharmacist 18, Lothian
The majority of pharmacists that were interviewed indicated that the training provided prepared them to deliver the intervention as planned, but acknowledged that time and practice were necessary to be fully prepared:
Obviously just with any of the new services it does take you just a . . . to get into a rhythm of like filling in all the paperwork and things like that.
Pharmacist 6, Lothian
However, some ways in which the training could have been improved to bolster preparedness were highlighted, including through drawing on pharmacists’ expertise in designing the training, increasing practice-based and role-play learning, and providing formalised refresher training between phases.
Awareness in sexual and reproductive health centres
Sexual and reproductive health providers were made aware of the study through management in their services. SRH providers interviewed described a lack of familiarity with their role in the study and a general lack of awareness of the study in their service:
I’m aware of a lot of studies but I wasn’t really aware of this one too much. I never saw any of the signs. You know? I never saw anything.
SRH provider 5, London
Most SRH providers indicated awareness of the study waning over time and stressed the need for reminders and easy and accessible study information to be available for all staff (including front-line reception staff).
Fidelity of intervention delivery
Experiences of delivery in participating pharmacies
Pharmacists’ descriptions of delivery and women’s accounts of their experiences in participating pharmacies suggest that fidelity of delivery was, on the whole, achieved. Although pharmacists’ descriptions of the recruitment process suggested adherence to the training protocol, there was some evidence of fatigue with research procedures, with failure to complete screening paperwork or enter data into the database relatively common across sites. Therefore, the added burden of the research context was apparent, typically extending EC consultations by approximately 15–20 minutes:
It’s a time-consuming process. So sometimes you need to manage your time really, really well and be very tight with time to fit it all in.
Pharmacist 1, Lothian
Research nurses from all sites reported having to spend a substantial amount of effort on the ground to encourage pharmacists to recruit and to assist with paperwork and database entry. Such experiences suggest the need for a more streamlined process with less paperwork and repetition, and the need for a high level of in-person support during implementation. However, the nurses noted that most of this related to taking part in a research study rather than the provision of POP as a bridging method itself. The impact of the pharmacy context on implementation is described in more detail in The impact of context on the Bridge-it study implementation and outcomes.
Women typically discussed positive and informative experiences in participating pharmacies:
. . . the lady who gave me all the advice on it, she was really, really thorough at explaining everything. She helped me out with the forms as well.
Participant 15, London
Data from the 4-month follow-up survey demonstrated that 90% (n = 178) of intervention participants recalled being given information about accessing further contraception during the EC consultation, in contrast to 64% (n = 134) of control participants. Most of the participants interviewed recalled being advised to access further contraception through either the participating SRH clinic (with rapid-access card) or their GP, typified here by participant 1, Lothian:
. . . so he said that I could either go into the clinic, and he gave me one of the ticket things [rapid-access card] to hand them [ . . . ] and he also said that if I told my GP about the study and what pill it was, they’d be able to give it to me as well.
Therefore, participants’ accounts highlighted that information around further access to contraception was predominantly accurate, consistent and clear, and may have contributed to many going on to successfully access further contraception. It is important to note that most women accessed further contraception through their GP (74/141) rather than a SRH clinic (21/141), and there was no difference in the proportion of women in either the intervention group or the control group who accessed the SRH service.
Although the majority of participants described being satisfied with the information provided in the pharmacy context, some inconsistencies in information provision emerged. Some indicated that they would have benefited from more in-depth information about the study and process involved:
I wouldn’t say from the initial chat with the pharmacist that I got a totally clear picture.
Participant 12, Lothian
It was not uncommon for participants to think that the aim of the study was to test out a new contraceptive pill, rather than about increasing access to further routine contraception:
It would be because you’re testing out a new drug to give out at pharmacies and GPs [ . . . ] and there was loads of girls getting asked to be part of the project to do statistics and see how it was affecting certain people.
Participant 10, Lothian
This framing of the intervention and lack of understanding about the aim may have had an impact on decision-making around accessing further contraception and motivations to do so. Around one-quarter of intervention participants (n = 54) could not recall being given a rapid-access card, typified by participant 35:
Do you remember if you were given a study card?
No, I don’t have a study card.
Were you told where to go if you had any issues with the contraception or if you wanted to get more?
Yes, he said that I could go there [pharmacy] and get my card and then obviously keep getting the pill but he wasn’t there when I last went so . . .
Such accounts highlight that there were some inconsistencies in information provided to participants in participating pharmacies, which resulted in confusion and, in some cases, trouble accessing further contraception.
Experiences within participating sexual and reproductive health clinics
As already highlighted, the SRH providers interviewed described a general lack of awareness of the study in their services, and few SRH providers interviewed described encountering Bridge-it study participants. In contrast to participants’ reflections on experiences in participating pharmacies, those who attended participating SRH clinics as part of the rapid-access component of the intervention reported less positive experiences, with a general lack of awareness among SRH staff encountered:
I think I spoke to someone who didn’t know what I was talking about, because when I went over and was like, can I have these . . . I was like, I’m with the study, can I please get some more contraceptive? And I don’t think she really knew, she was like, oh make an appointment with your GP.
Participant 5, Lothian
Such an experience was not uncommon, with four out of the five interviewed participants who attended their local SRH centre reporting difficulties accessing an appointment for further routine contraception in this way. Two participants were unable to access rapid appointments because the services were too busy. Although one participant overcame the time-related barriers and returned on another day, another participant was asked to wait for 2–3 hours and was unable to, and thus failed to access further contraception:
So waiting for 2 hours and being a working individual where clinics aren’t open 24 hours either, I just think, you know, some things you just have to bite your tongue with. So to cut a long story short, I’m pregnant.
Participant 23, London
Barriers to recruitment
During the Bridge-it study period, recruitment was slow and initial recruitment targets were not met, both for recruiting pharmacies and participants to take part in the study. This section describes the challenges of recruiting pharmacies and explore barriers to participation in the study that may have implications for wider implementation of bridging as a service in pharmacies.
Branches of large chain pharmacies and smaller independent pharmacies were approached to take part in the study because of their proximity to the participating SRH services, large footfall and high EC dispensing rates. Initially, it was decided to approach only those pharmacies with a dispensing rate of > 30 ECs per month, which ruled out many of the smaller independent pharmacies that appeared to be easier to set up. However, because of delays with setting up some of the larger chain pharmacies, the pharmacy recruitment strategy was adapted (September 2017) to allow consideration of pharmacies with lower EC dispensing rates. In total, 21 pharmacies that were initially approached were excluded or refused to take part. Barriers to pharmacy selection and recruitment included low EC distribution, charging for EC, already being commissioned to give out oral contraception after EC, lack of interest/enthusiasm and pharmacy environment/workload (e.g. too busy, understaffed and other disruptions).
Barriers to participation
Analysis of screening log data and pharmacist accounts in interviews indicated a variety of barriers to participation. In screening logs, pharmacists most commonly recorded unwillingness to give contact details for follow-up (n = 264) or data linkage (n = 262); in qualitative interviews, pharmacists expanded on these research-related barriers to participation, highlighting the time-consuming nature of paperwork and worries relating to confidentiality of data:
. . . lots of them were concerned about confidentiality, they were scared that I could just share the data with the GP.
Pharmacist 3, Lothian
Uncertainty around bridging as a new practice was also highlighted as a barrier to participation:
I mean, a lot of people were a little bit . . . they weren’t too sure about it I suppose, but they’re usually going to their doctor, so there was maybe issues there that they were just a little bit uncertain about the process of getting this through the pharmacy.
Pharmacist 5, Lothian
It seemed that the unfamiliar nature of this practice acted as a deterrent to some. Some thought that these particular barriers to participation would be overcome if bridging became an established pharmacy service, with less paperwork and greater advertising and awareness of the service:
I mean, if it was more widely known and became a more accepted pharmacy service, you would see more people.
Pharmacist 5, Lothian
Pharmacists talked about greater awareness resulting in more people participating, having time to decide whether or not to participate and visiting pharmacies specifically for longer-term contraception.
As well as research-related barriers, pharmacists drew attention to some barriers to participation that may impact on uptake if bridging were widely implemented as a service in this format, which therefore require consideration. Narratives of resistance to take POP specifically or hormonal contraception generally were commonly raised by pharmacists as a barrier to participation:
Do you think there’s anything that prevented people from participating?
Erm, well I’d say, the choice of it only being one pill, would definitely have been one. A lot of people didn’t like the fact that it was the progestogen-only one.
As well as a lack of choice of contraceptive bridging options available, pharmacists commonly described lack of time and a sense of rush as being common to EC consultations and a barrier to further discussion:
. . . the main problem is people are in a rush every time they come in to get the morning after pill.
Pharmacist 15, London
Pharmacists highlighted that this sense of rush often appeared to be due to busy schedules and limited time, as well as, at times, embarrassment or a desire to move on from the event:
I expect, embarrassment, that they just wanted to come in and out, you know, we are talking about something that people feel embarrassed about, they just want to come in, swallow the tablet, get out, forget the whole thing ever happened.
Pharmacist 8, Tayside
Some pharmacists also indicated that potential participants may have been deterred by lack of time to consider participation, particularly as decision-making around contraception often requires thoughtful consideration. Therefore, the immediate need to make a decision, combined with embarassment, lack of time and lack of choice, may have acted as barriers to participation in the study and could have an impact on uptake if bridging were widely implemented as a service. Suggestions to alleviate such barriers in wider implementation include provision of non-judgemental and supportive consultations, flexibility regarding accessing routine contraceptive services (e.g. option to book appointment) and increasing the number of contraception options available. In particular, the option of being able to book appointments to discuss further routine contraception with their pharmacist without the prerequisite of taking EC was favourable for many of those interviewed, making the service more feasible in the pharmacy setting and more beneficial for patients. Some of these potential solutions to increase uptake if bridging were widely implemented would be relatively straightforward to implement (e.g. a continuing professional development course on supportive consultations), whereas others would require practice or regulation changes. Box 4 provides a summary of key learning for future implementation.
-
The intervention was acceptable to pharmacists and SRH providers and was seen as an important way to develop and improve access to contraceptive services.
-
A lack of awareness in SRH services emphasised the need for integration and regular communication with all services involved in implementation and delivery.
-
Fidelity of delivery was mostly achieved and highlighted the importance of clear and consistent messaging around access to further contraception.
-
Barriers to participation included research-related factors, as well as barriers relevant to wider implementation as a service (e.g. embarrassment, lack of time and choice).
-
Suggestions to overcome barriers to participation include greater choice of contraceptive bridging methods, an option to book appointments in pharmacies and greater advertising of the service.
Mechanisms of impact: participants’ experiences of bridging and impacts on contraceptive practices
This section will focus on participants’ experiences of bridging and the impacts on contraceptive practices in the short and, potentially, long term. The aim is to shed a light on potential mechanisms of change and both facilitators of and barriers to uptake of bridging in future.
Mechanisms of change: access, knowledge, confidence and self-efficacy
Overcoming barriers to accessing regular contraception
Participants’ descriptions of their experiences suggest that bridging in the pharmacy context may have a positive impact on contraceptive knowledge and practices, in both the short and, potentially, the long term, overcoming both personal barriers (e.g. avoidance, lack of time) and structural barriers (e.g. difficulties accessing appointments) to the uptake of routine contraception. It seemed that, for many of the participants, being approached in the pharmacy setting when attending for EC provided them with the ‘push’ they needed to prompt a change in their contraceptive practices:
I think it was good because I probably would’ve taken a lot longer to figure it out and how to get it myself. And it was really convenient. It made me kind of realise that it was time to go on one and that it was something I did need to do.
Participant 10, Lothian
This also highlights the EC consultation as an opportune moment to intervene, as indicated by pharmacists and SRH providers, and how offering bridging could interrupt potential repeat EC use, which is viewed as a persistent issue in some community-based pharmacies. As one participant highlighted:
It was just a huge bonus to me having something that meant I didn’t have to wait even longer, because I probably inevitably would have . . . in that gap again, probably had to go for more emergency contraception.
Participant 18, Lothian
Participant accounts emphasised the importance of the pharmacy setting in overcoming existing access barriers to contraception in traditional contraceptive health-care settings (e.g. general practices, SRH clinics). Difficulties accessing routine contraception were commonly discussed in terms of finding the time to access contraceptive services and GP appointments, as well as in relation to the availability of services:
So what do you think makes it difficult to access effective contraception, so to have a regular contraception?
I think, well, if you go to the GP or if you go to a [SRH] clinic, it’s just the waiting time and you have to take quite a bit of your day up, to be honest. If you go via the GP, I have to book my appointment two, three weeks in advance.
Participants in London seemed to struggle particularly, as one participant said:
To be honest, getting an appointment in London is actually the worst thing ever.
Participant 35, London
Participants highlighted the convenience and availability of pharmacies as pivotal in helping to overcome such existing barriers to accessing contraception:
I know pharmacies are often open early or they’re open late and they’re open at lunch times so if I can go then and I don’t have to book time out of work to go to a GP or a doctor that’s like 09.00 to 17.00, then that would be great.
Participant 12, Lothian
As well as helping to overcome barriers of time and difficulties in accessing appointments, some described how the pharmacy context could help to alleviate the potential embarrassment of accessing contraception in more traditional ways, with many valuing the fact that pharmacy consultations tended to take place in small private rooms and were typically discreet and non-judgemental:
I think every pharmacy has a little room for you to go in and it’s quite discreet.
Participant 19, Lothian
Therefore, key mechanisms of change that are specific to the pharmacy setting were those of access and the nature of the setting, resulting in easier, convenient and potentially less embarrassing access to contraception.
In practice, pharmacists highlighted the immediate positive impact of being able to offer bridging contraception and the real need and demand for this service:
. . . there are a lot of people come in and ask about getting contraception and what you can and can’t supply and things, so some sort of extended service would probably fit in well.
Pharmacist 5, Lothian
Specific populations, including young people and students, were mentioned as being particularly prone to experiencing such difficulties and being appreciative of the service. Owing to the existence of ingrained difficulties to accessing appointments in more traditional settings and the existing demand for a more accessible service through pharmacies, pharmacists typically were optimistic about the feasibility of changing behaviour through the study, highlighting a synergy between the intervention design and patient need.
Increased knowledge, confidence and self-efficacy
As well as access being highlighted as a key mechanism of change, participants drew attention to a variety of other potential benefits related to taking part in the Bridge-it study. These included heightened awareness and knowledge of contraception and contraceptive services:
I found out more about it [contraception]. I’ve got more knowledge of that type of stuff now so that’s one of the positive things, I guess.
Participant 9, London
Some described how this increased awareness and knowledge of contraception and available services improved their confidence in both accessing contraception and in relation to protecting themselves from risk:
It’s meant that I’m on the pill, I’ve got that sorted, I know that I can go to the pharmacy to get advice, I hopefully won’t be needing the emergency contraception again, but I know that I can get it there if, for whatever reason, I need it. Yeah, I think, it’s probably given me a bit more confidence with it as well.
Participant 36, Tayside
It has definitely forced me to make me more aware, so I’ve not had unprotected sex since then. So yes and obviously now I’m a lot more open to actually go ask about it and try and get on one.
Participant 7, Tayside
Such accounts highlight key potential mechanisms of change in the uptake of effective contraception, with increased knowledge, confidence and self-efficacy possibly working to foster healthier practices and attitudes towards sexual health and risk.
Pharmacists also discussed that, although the Bridge-it study might not always be successful in driving uptake of hormonal contraceptives, the intervention likely prompted people to think more about their sexual health and consider longer-term contraception, as typified here:
And what, if any, positive effects do you think the Bridge-it intervention had?
I think it had quite a lot, to be honest. A lot of people probably thinking more about their sexual health, thinking more about longer-term contraception. A lot of them hadn’t maybe thought about starting on contraception after taking EC, so when I was explaining the importance of that, they were like, oh, you know, that’s quite a good thing to do . . . Even the people that maybe turned me down I think it still had a positive impact on them because they’ll go away and maybe think about it now, so I don’t think it was negative in any way.
This pharmacist also highlighted how further positive outcomes may be linking patients with local sexual health clinics, raising awareness of availability of testing and other related services.
Facilitators of and barriers to continued uptake of routine contraception
It is important to explore why the intervention worked for some participants and not for others. As indicated in the main outcome findings, over half (122/198) of the intervention participants remained on effective contraception at the 4-month follow-up, with 36% (71/198) remaining on POP, 14% (28/198) on the combined hormonal contraceptive pill/patch/ring and 7% (13/198) on LARC methods. However, although many participants overcame barriers to continued uptake of effective contraception, 44% (88/198) of intervention participants were not on contraception at the 4-month follow-up.
Why did participants remain on effective contraception?
Participants who remained on effective contraception typically found the process of accessing further supplies through their GP or SRH clinic straightforward, and tended to have positive or no side effects in response to taking POP:
I don’t feel that there has been any side effects, like, of like up and down moods or mood swings that some other women get on different pills, which is very positive.
Participant 1, Lothian
I really like it, so I’ve actually went and got some more of that now [ . . . ] afterwards I just went to the doctor and got some more because I don’t know, I like it.
Participant 17, Lothian
Familiarity was also highlighted as a reason for continued use of oral contraception specifically:
At the moment I do feel happy on it and it’s convenient, I’m used to taking the pill, and my friends are like, oh coil is so easy because you don’t have to think about it, but I’m used to it.
Participant 18, Lothian
A number of participants described considering changing to other options in the future. For example, participant 16 indicated that she would like to try something more long term, but had concerns relating to the implant insertion procedures:
The implant I’m a bit scared of because sort of like the injection stuff, I’m not a fan of that, but I would want to because I think I’ve heard a lot of good stuff about it, so I’m not sure, yeah. Still debating it . . .
Participant 16, Lothian
Participant 16 later elaborated that she would also struggle to find the time to access an appointment to discuss other options, stating ‘I’m just quite busy and I don’t have the time to go and get another one really’.
Some of the participants who remained on effective contraception post study were those who had no prior experience of routine contraception. For example, participant 8 (London) described herself as a frequent user of EC and highlighted barriers to accessing appointments as the primary reason for not being on routine contraception prior to taking part in the Bridge-it study:
It was just the convenience of booking an appointment. Sometimes I have to wait 3 weeks or so and then it all conflicts with my day-to-day life.
Participant 8, London
Such experiences highlight the beneficial nature of offering bridging in accessible settings such as community pharmacies in overcoming existing barriers to accessing effective contraception. As well as the Bridge-it study having an impact on previous non-users, bridging seemed to be beneficial for some participants who had previous negative experiences on other forms of hormonal contraception. A minority of participants who had previous particularly negative experiences with other hormonal contraception also found the POP to be suitable:
And to be fair these . . . the pill that I’m taking just now has been great for me. And if that hadn’t happened last year and nobody had talked to me about it, I don’t know if I would have ever tried it, I would probably still be thinking that the pill is evil.
Participant 27, Lothian
This participant had almost lost hope in the pill as a suitable method of contraception because of previous negative side effects from other oral contraception, but tried it after being approached to take part in the study and, at the time of interview, had been satisfactorily using POP since then. Such accounts highlight the barriers to accessing contraception, the sometimes time-consuming nature of finding the right contraceptive, the importance of individuals being supported in their contraceptive journeys and the benefits of offering bridging contraception through multiple routes, including community pharmacies.
Barriers to continued uptake of effective contraception
The 4-month follow-up survey and interviews with participants shed a light on a range of barriers to continued uptake of POP or to seeking alternatives, which are important to consider for future implementation. For many, personal circumstances, such as not being sexually active, their current relationship status or being pregnant, resulted in them deciding to not be on contraception. Data from the 4-month follow-up survey indicated that almost half (28/57) of intervention respondents who were not on effective contraception cited such circumstances.
As well as personal circumstances, a range of other reasons were highlighted as barriers to continued uptake of effective contraception. At follow-up, one-quarter (40/158) of respondents from the intervention group discontinued POP before their supply had run out because of worries about side effects. Participants who were interviewed described a range of adverse side effects they attributed to taking POP, including spotting, prolonged bleeding, skin problems, poor mental health and mood changes, headaches, weight gain, lowered libido and nausea. The most common side effects mentioned were spotting and prolonged bleeding, typified by participant 12 (Lothian):
There was blood every day and not much but enough to be annoying, if you know what I mean. So that’s why I only took one packet and then I stopped because I was just like I can’t . . .
Prior to taking part in the Bridge-it study, 22 of the participants interviewed had previous experiences of being on regular or long-term contraception, and the majority of them attributed no longer being on contraception at the time of entry into the study to negative side effects. Therefore, for such participants, a barrier to regular uptake of contraception was not necessarily access related, but symptom and well-being related, and such accounts highlight the persistent difficulties faced by some women in their contraceptive journeys.
Some women described the commitment required to take the POP every day as a barrier to uptake, particularly in the context of busy lives:
I think as well it can be annoying having to remember every day to take it unless you’re in a really good routine.
Participant 12, Lothian
As well as difficulties in remembering to take the POP during the Bridge-it study, some described previous experiences of struggling to remember to take oral contraception in the past:
I don’t think it’s hard to access it. I think, like the only thing I have found was just having it at the same time, like, every day.
Participant 9, London
Missed pills were a common reason for previous EC use, highlighting the recurring issue of user compliance and how this may act as a barrier to uptake of effective contraception.
Difficulties accessing GP/SRH clinic appointments or finding the time to attend such services to renew their supply or find an alternative were reported by one-quarter (14/57) of intervention participants. Some discussed not being able to continue accessing POP through the pharmacy as a barrier to getting further supplies:
It’s a bit of an annoyance having to do it especially when it’s something that you’ve been on for ages and you have to almost go back just to get it prescribed.
Participant 12, Lothian
And if I could just . . . because I don’t want to have to book an appointment at the GP, you know . . . if I could just go to the pharmacy and get something I probably would have done it.
Participant 22, Lothian
Participants also discussed how the embarrassment and shame related to attending SRH clinics may have acted as a barrier to the rapid-access component of the study:
I think I would rather go to the GP, but only because I feel like it is a little bit of a taboo to say I’m going to the [SRH] clinic.
Participant 27, Lothian
Therefore, consistent with these concerns, few intervention participants visited participating SRH clinics for further contraception (17%, n = 52), with most of those accessing additional POP or alternatives doing so through their GPs. Such experiences highlight that, although bridging in the pharmacy setting was pivotal in helping many participants to overcome initial access barriers to routine contraception, the need to access further supplies/advice in more traditional contraceptive settings created barriers for some to remaining on effective contraception. Considerations for wider implementation to overcome such challenges could include greater linkage with general practices in the bridging process, as well as the feasibility of longer-term contraceptive care in the pharmacy setting.
Although bridging using POP was effective for many in the study, including non-users and previous users of hormonal contraception, it is important to recognise that it is not a comprehensive solution, as the remaining barriers highlight. Persistent barriers to uptake relating to well-being and access of routine effective contraception remain, emphasising the need for a package of solutions to ensure that all of these diverse different needs are met. Such challenges must be acknowledged to optimise future implementation, with attention paid to making the process of accessing ongoing contraception post bridging easier, the provision of a variety of contraceptive options and the need for a central focus on the importance of well-being during contraceptive consultations. Box 5 provides a summary of key learning for future implementation.
-
Provision of a bridging supply of POP with EC from the pharmacy had a positive impact on contraceptive practices in the short term and, potentially, in the longer term.
-
Key mechanisms of change highlighted include ease of access, increased knowledge, awareness, confidence in accessing contraception and managing risk.
-
Persistent barriers to accessing and regularly using routine contraception remain, including worries about side effects, the ingrained stigma of SRH services and difficulties accessing contraceptive appointments.
-
This intervention may work well as part of a package of solutions to increase the uptake of effective contraception, ensuring that a diversity of different needs are met.
-
Suggestions to increase uptake of effective contraception include greater linkage with general practices, consideration of longer-term contraceptive care in the pharmacy setting, a key focus on side effects and the importance of well-being in contraceptive consultations.
The impact of context on the Bridge-it study implementation and outcomes
It is important to consider context in implementation and the potential impact that existing contextual challenges may have on wider implementation.
The pharmacy context: competing priorities and staffing issues
Pharmacists provided insights into their specific pharmacy contexts during training and in interviews. The pharmacists highlighted potential facilitators of recruitment (e.g. long opening hours, accessibility, high rates of EC dispensing), as well as barriers to delivery of the Bridge-it study intervention (e.g. time pressures, lack of resources, competing demands). In particular, issues of time management and competing priorities seemed to be ingrained in community pharmacy working cultures, with common narratives emerging around high workloads and expanding roles:
And what do you think are the main challenges of working in your pharmacy?
It is time I would say, it is the ever-increasing amount of roles that we have taken on and having the time to do them properly.
Many described a move towards transferring services from general practices, resulting in increasing responsibilities and pressures on pharmacist time. Such pressures were often exacerbated by a lack of resources and staffing issues:
. . . it never feels like you have enough people.
Pharmacist 12, Lothian
This seemed to be common across the participating pharmacies, fuelled by staff holidays, understaffing, high staff turnover and absence. Although such existing challenges at times contributed to low recruitment rates and a lack of engagement with the Bridge-it study, pharmacists were typically enthusiastic about embedding bridging into everyday pharmacy practice. However, it is evident that if bridging were widely implemented as a service, accounts of widespread contextual challenges highlight the need for additional resources because of the potentially time-intensive nature of such a service.
The sexual and reproductive health clinic context: funding cuts and changing service provision
Sexual and reproductive health providers described SRH services being overwhelmed by different contextual factors, including patient needs and demand, staff availability, funding cuts and changing service provision. Similarly to pharmacy staff, SRH workers described continually trying to manage priorities to cope with staff shortages:
I think that across the whole of the NHS, resources is a real issue. You know, if staff are off sick, we kind of struggle to cover things. You know, we’re constantly trying to juggle, and constantly trying to desperately figure out if we take somebody off this clinic then maybe we could cover that clinic . . .
SRH worker 1, Lothian
Accounts highlighted the impact this could have on meeting patient expectations and needs, in relation to both the availability of appointments and the time provided for those appointments:
We don’t have enough time for appointments but also, we don’t have enough appointments for the demand. You know? And because . . . well, you know, services have been cut and staff [have] been cut and there’s only so much, really, that you can give.
SRH worker 5, London
As well as services being cut, SRH staff also spoke of services being reshaped to accommodate limited funding and resources. Two sites highlighted a move to triaging and from walk-in clinics to priority access clinics. An increased focus on young people’s services was evident, as was a move away from provision of routine contraception to a focus on more specialised services, as indicated by SRH worker 3 (Tayside):
Because obviously we were providing the more specialist stuff, whereas people that would be looking just for routine contraception would be encouraged to attend their GPs, rather than come to the specialist service, just because [of] the lack of capacity.
These existing challenges and practice priorities had potential implications relating to the implementation of the Bridge-it study intervention and potential wider implementation of bridging as a service. Concerns were raised relating to SRH services having the resources to cope with rapid-access appointments, as well as the lack of fit with existing service provision and practice priorities. One SRH worker highlighted how exceptions to normal practice would be made for research studies:
I think if they showed the card, though, and I’ve had that situation before, it’s slightly different, if they have evidence that they participate in that study, so that would be slightly different.
SRH worker 2, Lothian
Despite this, concerns were raised about the potential for Bridge-it study participants to be turned away or missed because of a lack of fit with current practice:
And although the nurses were trying to get the information from patients if they had been involved in the Bridge-it study, if the patient didn’t specifically explain that, they probably wouldn’t have been able to get into the clinic that easily.
SRH worker, Tayside
As already highlighted, this did happen in practice, demonstrating how existing contextual circumstances in the SRH context influenced implementation in practice. It is also important to consider how a lack of fit with existing service provision and lack of resources raises issues around how the study would fit in practice if it were widely implemented as a service in this format. If provision of routine contraception to all age groups is not a practice priority, it may make it difficult for bridging to become embedded in routine practice. Some suggested redesigning the study to refer women to general practices instead:
I mean perhaps them going to a general practice setting would be more appropriate than directing them to sexual health, given the situation that sexual health is in nowadays, if you know what I mean. Because it is a bit more of a specialist service.
SRH worker 3, Tayside
However, it was also highlighted that this would not be a simple solution because of an already overburdened general practice service.
Changing contraceptive guidelines and potential impact on policy
Pharmacists raised concerns during training of how the study would fit with existing practices and changing guidelines relating to EC. Throughout the study period, a number of contraceptive guidelines were updated, influencing contraceptive practice and, consequently, the implementation of the study. In March 2018, new EC guidelines came into effect,10 recommending another type of EC, ulipristal acetate (ellaOne®, HRA Pharma, Paris, France), as the first option rather than LNG. This had an impact on recruitment, as, if given ulipristal acetate, patients would not be eligible for the Bridge-it study. Some pharmacists described this new guidance as a barrier to recruitment and stopped recruiting participants:
So because we’d had some additional training with [local SRH clinic] I would always offer people [ulipristal acetate] rather than [LNG] because some people would just say, you just have to have [LNG], but for me as a pharmacist and I guess as a woman as well, I would want to be giving the patient the most appropriate medicine . . .
Pharmacist 19, Lothian
As well as acting as a barrier to recruitment in the Bridge-it study, pharmacists highlighted how this guidance could have implications for bridging as a practice in pharmacies if widely implemented:
I think with the push towards [ulipristal acetate] that’ll, kind of, throw a spanner in the works for this idea. Yes. Because it obviously, you’re not going to be able to take it straight away.
Pharmacist 21, Tayside
Box 6 provides a summary of key learning for future implementation.
-
Existing challenges in the participating pharmacy and SRH contexts (e.g. lack of resources and changing practice priorities) influenced implementation of the study in practice, with the deprioritisation of screening potential participants, fatigue with research procedures and participants being missed or turned away from SRH centres.
-
Updated contraceptive guidance had an impact on recruitment into the study and has potential implications for the wider implementation of bridging in the current format.
-
Suggested learning for wider implementation includes the need for sufficient resources and time to administer this service, and a need to consider changing service provision in SRH contexts.
Process evaluation summary and key implications
Summary of findings
The intervention was acceptable to pharmacists and SRH providers, was seen as an important way to develop and improve access to contraceptive services, and a way to reduce repeat EC use. Pharmacists were generally satisfied with the training received and reported that it prepared them for delivering the intervention; however, the apparent lack of awareness in SRH centres emphasised the need for regular communication. Reflections of implementation indicate that the fidelity of delivery was, on the whole, achieved in the pharmacy context, highlighting adherence to the protocol and the typically clear and consistent messaging around accessing further contraception. Analysis of screening log data and pharmacist accounts during interviews shed a light on a variety of barriers to participation in the study. Some specifically relate to the research context, whereas others are important to consider with regard to the wider implementation as a service.
Participants’ accounts highlight that providing a bridging supply of POP with EC from the pharmacy as a practice may have a positive impact on knowledge of contraception and contraceptive practices in the short term and, potentially, in the longer term through overcoming existing barriers to access, increasing confidence in accessing contraception and managing risk. The process evaluation highlighted potential mechanisms of change in helping to overcome some well-established barriers to accessing longer-term contraception, including ease of access and increased knowledge, awareness of services, motivation, confidence and perceptions of self-efficacy. However, persistent barriers to accessing and using routine effective contraception remain, including worries about side effects, concerns about the commitment required, ingrained stigma related to accessing SRH clinics and difficulties accessing repeat prescriptions and appointments for continued contraceptive care. Such barriers are important to consider for future contraceptive trials and in relation to wider implementation of bridging as a service.
A broad range of factors influenced the implementation of the intervention in practice, including the context of participating pharmacies and SRH clinics, and broader changes to contraceptive guidelines. A range of cross-cutting challenges to implementation emerged that were specific to the community pharmacy and SRH context (e.g. high workloads, understaffing and changing priorities), which had an impact on delivery, with evidence of deprioritisation of screening and recruitment, fatigue with research procedures and participants being missed or turned away at SRH centres. Implementation was also influenced by key changes to contraceptive guidelines that had implications for pharmacy practice. It is important to understand such key contextual challenges to implementation and the potential impact on outcomes to shed a light on how these issues may be alleviated if bridging were widely implemented as a service.
Key learning and implications
Why did the intervention work?
The findings from the process evaluation support the conceptual framework underpinning the intervention that the offer of a bridging contraception during EC consultations along with signposting to SRH services would encourage and prompt uptake of ongoing effective contraception. As well as having a positive impact on the uptake of effective contraception, the intervention potentially contributed to an impact for some participants in the longer term through increasing their knowledge of contraception, awareness of services, and confidence in accessing contraception and managing risk. The process evaluation shed a light on potential key mechanisms of change, including ease of access, increased knowledge and awareness of services, motivation and perceptions of self-efficacy. In particular, the convenience and accessibility of pharmacies seemed pivotal in overcoming some well-established barriers to contraception.
Accounts highlighted an existing demand for greater access to effective contraception through pharmacies, and the provision of POPs as a bridging method of contraception seemed to be generally accepted and welcomed by pharmacists, SRH providers and participants, highlighting a synergy between intervention design and patient need. As well as being acceptable to pharmacists, SRH providers and participants, bridging as a practice also seemed to be feasible in the pharmacy setting, despite existing contextual challenges, suggesting that the practice could be widely implemented and routinely embedded in community pharmacies (albeit with some adaptations to alleviate challenges and encourage greater uptake).
Although prompting positive change in contraceptive practices for many participants, it is important to acknowledge that barriers to accessing and remaining on effective contraception persist, including worries about side effects, ingrained stigma relating to accessing contraception (particularly in SRH services) and difficulties accessing appointments for continued contraceptive care. Such challenges require consideration in the design of future contraceptive trials and in the wider implementation of bridging contraception as a service, and emphasise the need for a package of solutions to ensure that this diverse range of different needs are met.
Implications for wider implementation
The process evaluation shed a light on important implications for wider implementation of bridging as a service and these are detailed below. It is important to consider ways to address key implementation challenges and overcome persistent barriers to accessing and using effective contraception.
Key learning for wider implementation as a service
-
Adaptations to the intervention should be made to recognise existing contextual challenges in the pharmacy and SRH context, changing contraceptive guidelines and recurrent barriers to long-term use of effective contraception.
-
Suggestions to increase uptake of bridging contraception in the pharmacy setting include greater advertising and promotion of the service, provision of non-judgemental and supportive contraceptive consultations, an option to book routine contraceptive consultations in pharmacies outside of EC consultations and increasing the bridging contraceptive options available.
-
Suggestions to increase continued uptake of effective contraception include clear and consistent information provision about further contraceptive access, greater linkage with general practices, easier processes for obtaining repeat prescriptions and consideration of longer-term contraceptive care in the pharmacy setting. In addition, there should be a focus on both the side effects and the importance of well-being during contraceptive consultations.
-
Existing contextual challenges in the pharmacy and SRH context, including lack of resources and changing practice priorities, highlight the need for sufficient resources and time to administer this service for it to be embedded in routine practice.
Chapter 6 Discussion of the Bridge-it study
Interpretation
The Bridge-it study demonstrated that this community pharmacist-delivered intervention of provision of a small bridging supply of oral contraception along with EC and the invitation to a SRH clinic resulted in a significantly larger proportion of women using effective contraception 4 months later than standard care alone. The difference in uptake of effective contraception between the groups was large. Furthermore, significantly fewer women in the intervention group than in the control group sought EC again during the study. In addition, consistent with the known safety of the POP,20 no serious adverse events were reported. These findings are clinically important. Given that use of effective contraception prevents unintended pregnancies, the public health benefit of this simple and safe intervention is potentially considerable.
Why did the intervention work?
The increase in use of effective contraception at 4 months (1 month after bridging supplies of POP in intervention group had run out) seemed to be largely related to continued use of the POP, with further supplies mostly obtained from the GP. There was evidence from the process evaluation that women who received and used the POP liked it and decided to continue with it. The POP has not traditionally been a common choice for contraception in the UK. It is estimated to be used by only 5.6%31 of contraception users and is typically reserved for those with contraindications to combined hormonal contraception. 20
Did advice from the pharmacist play a role?
A larger proportion of women in the intervention group recalled that the pharmacist discussed contraception with them. The proportion receiving contraceptive advice in both groups of the study was much larger than in our mystery shopper exercise (49% of mystery shoppers received advice),13 suggesting a possible effect of the study on pharmacist behaviour. It is possible, therefore, that the relative impact of the intervention compared with standard care in ‘real life’ might be greater than observed in our study.
It was a disappointment that the rapid access invitation to SRH services did not increase the proportion of women in the intervention group accessing the SRH services compared with the control group. The rapid access to a SRH clinic was used by fewer than one out of five women in the study and only two women (< 1%) used the rapid access within 1 month of EC. In our earlier pilot study, 32% of women used the rapid access to a SRH clinic. 12
The process evaluation highlighted some of the difficulties facing SRH services during the study, including funding cuts to SRH services (London) that may have restricted access for these women. It was also suggested that many women prefer to access contraception from their GP and that the perceived stigma of attending a SRH service is still a barrier for some. We had included the rapid-access invitation in our study design to facilitate access to a specialist contraceptive service where all methods could be provided. Our earlier pilot study had suggested that this might increase access to and uptake of LARC;15 however, we did not observe this in the current study, in which rates of LARC uptake were similar between groups. Although the rapid access may help some women to access effective contraception, the findings do suggest that this may be a less important component of the intervention than the supply of POP. Given the difficulties facing SRH services and some of the complexities highlighted by the process evaluation around SRH services being able to make rapid access available, community pharmacies signposting to contraceptive services may suffice.
Generalisability and limitations
The Bridge-it study was a well-designed trial, with a sufficiently large sample size to show the expected effect with sufficient power. The primary outcome findings also remained consistent, even when adjusted for a range of factors and the missingness of data. The study was conducted at three different sites across the UK and the trends were consistent at each site. The Bridge-it study findings are also reassuringly consistent with the findings of our earlier pilot study. 15
The pharmacies in the Bridge-it study included a mix of small independent pharmacies and large chain pharmacies, and so should reflect EC service provision across the UK. In addition, the women who participated in the Bridge-it study were of similar age and shared similar characteristics to EC users in previous studies in the UK. 32 The findings should therefore be applicable across the UK.
The intervention tested was a simple one (i.e. a 3-month supply of POP provided by PGDs). In addition, the intervention should not be costly, as POP is an inexpensive drug. However, this will be determined by our cost-effectiveness analysis.
This was a difficult study to undertake. Pharmacists are not usually familiar with undertaking research. The pharmacy is a commercial setting. Requiring pharmacists to recruit participants, provide study information and complete additional paperwork as part of a research study was an added burden to EC provision. Although cluster randomised trials are generally susceptible to bias as allocation is known to participants at consent, our pilot study informed us that a study design of individual randomisation in the community pharmacy setting would not work. 15 The study design meant that different groups of women participated in the two periods. This mix of factors contributed to varying recruitment rates across pharmacies and between periods, resulting in different lengths of required recruitment and not all pharmacies successfully recruiting in both periods. Although this added to the complexity of the study and its management, it produces more robust and generalisable findings.
The main limitation of this study (as for many contraceptive studies) is that the outcome was the uptake of effective contraception rather than the number of unintended pregnancies. We had originally intended to examine abortion rates in each group as a co-primary outcome, but during the course of the study it became evident that we could not recruit sufficient numbers of participants within a realistic time frame, and so we chose to focus solely on uptake of effective contraception, which required smaller sample size. Use of effective contraception should prevent unintended pregnancy; however, the association is more complex, particularly as the use of a method of contraception does not always equate to correct and consistent use. 33 Thousands of participants would have been required for this study to have the power to demonstrate an effect of the intervention on unintended pregnancies. Although participants gave consent to allow subsequent data linkage with abortion registries, the sample size may be too small to show any difference between the groups.
Another consideration is that contraceptive use was self-reported. However, there is some evidence that women’s self-reporting of contraceptive method is reliable. 34 The findings from the limited validation exercise among those women who both obtained contraception from the SRH clinic and responded to follow-up showed that 88% of women were provided with the same method as they had reported. This provides some support for the reliability of self-reporting of contraception. Loss to follow-up for the study was 35%, which was larger than the 25% we expected. However, there was no differential loss to follow-up between groups and the characteristics of women with and without follow-up data were similar.
This study was undertaken with EC containing LNG, which was the most commonly used EC at that time and suitable for immediate start of oral contraception. 35 However, any future rollout of the intervention does not need to be limited to this EC, as it would also be possible for community pharmacists to provide bridging contraception of the POP containing ulipristal acetate (a more effective EC). 36 The only issue would be that women would be advised to wait 5 days after taking ulipristal acetate before starting the POP (rather than immediately or the next day after EC containing LNG) because it has been shown that starting hormonal contraception sooner than 5 days after ulipristal acetate might potentially impair the ability of ulipristal acetate to delay ovulation and therefore render EC less effective. 10,37,38
Overall evidence
With the exception of our earlier pilot study,15 to the best of our knowledge, the only published study that examined the uptake of effective contraception after EC offered women (in Jamaica) a discount on the subsequent cost of contraceptive pills and had no effect on subsequent contraceptive use. 14 The only other intervention that has demonstrated a large impact on the use of effective contraception was the Contraceptive CHOICE Project in the USA,39 in which women requiring contraception in a range of settings were counselled about LARC and provided with their chosen method free of charge in a setting where contraception is usually not free. LARC uptake was 67% in the Contraceptive CHOICE Project cohort, compared with 3% nationally. The Bridge-it study, by comparison, assessed a simple intervention delivered by community pharmacists at EC request.
Recommendations for research
Future research should be directed at a robust evaluation of the impact of the Bridge-it study intervention following any wide-scale implementation (to include impact on abortion rates, cost-effectiveness and on experiences of women and community pharmacists following implementation). This should be cognisant of existing contextual challenges in the pharmacy and SRH context.
The findings of the Bridge-it study are particularly relevant to the NHS during the current Covid-19 pandemic. Pharmacists are already embedded in SRH care in the UK and could provide an alternative way for women to access regular effective contraception. 40 As part of the NHS Long Term Plan, NHS England plans to work with the government to make greater use of community pharmacists’ skills and opportunities to engage patients. 41,42 As part of the deal, the NHS is introducing an expanded clinical role for local pharmacists, beginning ‘a revolution in patient care’ that could see community pharmacy becoming the first port of call for minor illness and health advice.
Therefore, we propose that future research should also focus on the feasibility, acceptability and impact of more contraceptive services being delivered from community pharmacies. Our process evaluation has highlighted the need for sufficient resources for such services to be embedded in routine practice. In doing so, and alongside appropriate advertising and promotion of the service and an emphasis on well-being during contraceptive consultations, services could overcome persistent barriers to accessing and using effective contraception.
Future research should also consider ways to better support research in the community pharmacy setting.
Implications for decision-makers
What if offering a 3-month supply of the progestogen-only pill with emergency contraception became standard practice throughout the UK?
Emergency contraception users are a group who are at high risk of unintended pregnancy. A subsequent increase of 20% in contraceptive uptake is a large and clinically important increase. Pharmacy provision of POP is inexpensive. A 3-month supply of the desogestrel POP costs the NHS approximately £3 and the estimated cost of the pharmacist’s time is around £30. 21,43 Provision of a bridging supply of POP with EC has received support in surveys of EC users and also from members of the Faculty of Sexual and Reproductive Healthcare. 32,44 In addition to increasing uptake of effective contraception, it is likely that widely implementing bridging POP might also reduce rates of repeat EC use (as observed in the Bridge-it study), which would also generate savings (through reduced consultations for EC).
Some may be concerned that the intervention could discourage use of LARC. However, there were no significant differences between the two arms of the study in uptake of LARC after EC use. Existing evidence plus the data from interviews with participants, pharmacists and SRH clinic staff, conducted as part of the process evaluation, showed that not all woman wish to use LARC45 and many value the control they have with a method such as a pill, which allows them to start and stop the contraception themselves. Furthermore, mathematical modelling from the USA suggests that relatively more unintended pregnancies at population level would be prevented by efforts focused on encouraging women who are not using an effective contraceptive method to use one (e.g. POP), compared with switching those already using an effective method to a more effective LARC method. 46
We chose to provide the POP as bridging contraception over the more commonly used combined oral contraceptive pill because the latter has more contraindications and health risks. 20 The POP used in the study (i.e. 75 µg of desogestrel/day) consistently inhibits ovulation in almost all users (in contrast to older POPs), and so is likely to be as effective as the combined oral contraceptive and is easier to use as it is taken every day without a break.
This study has enormous implications for public health policy, as it provides evidence for a simple, effective strategy that could prevent more unintended pregnancies for women.
Unintended pregnancies may end in abortion, but may also result in miscarriage or an unplanned birth. Better pregnancy planning can improve outcomes for babies and mothers.
Although this intervention has the potential to be cost-effective, this will not be known until the results of the planned health economics analysis has been completed.
In addition, we are aware of pharmacies in some settings already supplying an oral contraceptive pill along with EC, yet without any robust evidence to support this practice. 17 The Bridge-it study provides the robust evidence required and also the evidence to support any future wide-scale implementation.
Chapter 7 Conclusions
Provision of a bridging supply of POP with EC from a pharmacist clinic provides temporary contraception (POP) to prevent unintended pregnancies from unprotected sex in the same cycle and subsequent cycles. The provision of a rapid-access card to a SRH clinic was designed to facilitate access to contraceptive services (SRH clinic), but we did not find evidence that this approach was effective.
The Bridge-it study showed that this intervention resulted in a significant increase in subsequent reported use of effective contraception, which has the potential to prevent unintended pregnancies for women after EC. A reduction in unintended pregnancies would also save costs for the NHS and a cost-effectiveness analysis will report on this.
Evaluation of the impact of this intervention would be important following any future wide-scale implementation.
Impact of patient and public involvement, challenges of patient and public involvement and lessons learnt
Pre-funding study design and protocol development
We used the local PPI network at the SRH service in Lothian to help inform and improve the study design and recruitment plan. We also held small group meetings with women attending our specialist contraceptive services, teenage mothers attending toddlers groups, pregnant women in the Family Nurse Partnership programme and members of the local abortion rights group. The study name, protocol and original Health Technology Assessment programme application were reviewed and approved by the chairperson and members of the PPI group. The Plain English summary of the application was edited by a PPI member (a 16-year-old woman) and was improved as a result. We also used our PPI network to help address questions from the Health Technology Assessment board.
Mystery shopper exercise
We used our PPI members from the TSC and local PPI members at each site to help identify potential ‘mystery shoppers’ for the pharmacy visits.
Trial Steering Committee and key project decisions
Patient and public involvement members commented on the participant information sheet and consent documentation, and, in response to this, some changes were made and these documents were improved as a result. The input of the PPI members on key recruitment issues arising during the course of the study (relating to slower than expected recruitment) was highly valuable, as was their positive voice on the decision to repower the study on the primary outcome of effective contraception use at 4 months (confirmed by PPI members as an important outcome for women).
The PPI members have also approved drafts of papers and reports. The PPI network (including a 17-year-old woman) and the PPI representatives on our TSC also made suggestions to improve the Plain English summary in this report.
Dissemination
The PPI members will be involved in dissemination of the main study findings through their networks and social media.
The findings of the mystery shopper exercise have already been published13 (online early) and a PPI member is writing a patient opinion piece to be submitted to the same journal and hopefully published alongside this.
Acknowledgements
We wish to thank the women who participated in this study, community pharmacists at the study pharmacies who recruited women to the study, and health-care professionals at the study SRH clinics who assisted with the implementation of the study (see below). Thanks to research staff Deirde Sally, Nicola Stewart and Maria Nunez (University College London, London, UK) for their support with implementation of the study at local sites in London, and Kristina Saunders (University of Glasgow, Glasgow, UK) for support with process evaluation. Thanks to Sarah Cameron and Lorna Aucott (senior statistician, Centre for Healthcare Randomised Trials) for support. Thanks to Katherine Lewis, Laura Flett and Judith Parker (Edinburgh Clinical Trials Unit, Edinburgh, UK) for trial management support. Thanks also to Katherine Lewis for assistance in the compilation of this report.
List of study pharmacies
Newington Pharmacy, Edinburgh; Boots Princes Street, Edinburgh; Boots Shandwick Place, Edinburgh; Boots Earl Grey Street, Edinburgh; Boots Gyle, Edinburgh; Boots St Patrick Street, Edinburgh; Boots Multrees Walk, Edinburgh; Boots Ocean Terminal, Edinburgh; Boots Edinburgh Fort Retail Park, Edinburgh; Boots Cameron Toll, Edinburgh; Boots Craigleith, Edinburgh; Bristo Square Pharmacy, Edinburgh; Asda Pharmacy, Perth; Boots High Street, Dundee; Boots Perth Road, Dundee; Peace Pharmacy, London; Westbury Chemist, London; Baba Chemist, London; Lings Chemist, London; Streatham Day Lewis, London; Morrisons, Aylesham Centre, London; Evergreen Pharmacy, London; Greenlight Pharmacy, London; Sandylight Pharmacy, London; Greenfields (Day Lewis), London; JP Pharmacy, London; Boots Goodge Street, London; Boots Tottenham Court Road, London; and Boots Holborn, London.
List of study sexual and reproductive health clinics
Chalmers Sexual and Reproductive Health Service, Edinburgh; Tayside Sexual and Reproductive Health Service, Ninewells Hospital, Dundee; Camberwell Sexual Health Centre, London; and The Margaret Pyke/Mortimer Market Centres or the Archway Centre, London.
Collaborators
The Bridge-it study TSC provided oversight for this trial on behalf of the sponsor (University of Edinburgh and NHS Lothian) and the funder. The members are Peter Brocklehurst (chairperson), Birmingham Clinical Trials Unit; Lucy Michie, NHS Ayrshire and Arran; Kaye Wellings, London School of Hygiene and Tropical Medicine; Joanna Loudon, PPI member, Edinburgh; Kirsten Stuart, PPI member, Edinburgh; and Emily Whitaker, PPI member, Edinburgh.
The DMC is an independent multidisciplinary group, consisting of clinicians and statisticians. The members are Claire Anderson (chairperson), University of Nottingham; Elizabeth Allen, London School of Hygiene and Tropical Medicine; and Caroline Moreau, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA. A copy of the DMC charter is held in Edinburgh Clinical Trials Unit.
The study has co-sponsorship between the University Court of the University of Edinburgh and Lothian Health Board. The sponsors’ representative is the Academic and Clinical Central Office for Research and Development (ACCORD) (Edinburgh, UK).
Contributions of authors
Sharon T Cameron (https://orcid.org/0000-0002-1168-2276) was responsible for the study design, wrote the first draft of the report, with significant input from all authors at all stages, and contributed to, read and approved the final report.
Anna Glasier (https://orcid.org/0000-0002-5697-255X) was responsible for the study design, wrote the first draft of the report, with significant input from all authors at all stages, and contributed to, read and approved the final report.
Lisa McDaid (https://orcid.org/0000-0002-7711-8723) was responsible for the study design, wrote the process evaluation component, and contributed to, read and approved the final report.
Andrew Radley (https://orcid.org/0000-0003-4772-2388) was responsible for the study design, and contributed to, read and approved the final report.
Susan Patterson (https://orcid.org/0000-0003-1846-5749) contributed to later stages of study design and analysis, wrote the process evaluation component, and contributed to, read and approved the final report.
Paula Baraitser (https://orcid.org/0000-0002-3354-6494) was responsible for the study design, and contributed to, read and approved the final report.
Judith Stephenson (https://orcid.org/0000-0002-8852-0881) was responsible for the study design, and contributed to, read and approved the final report.
Richard Gilson (https://orcid.org/0000-0002-9572-193X) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Claire Battison (https://orcid.org/0000-0001-9549-4522) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Kathleen Cowle contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Thenmalar Vadiveloo (https://orcid.org/0000-0001-5531-6289) was responsible for the statistical analyses and contributed to data interpretation, and contributed to, read and approved the final report.
Anne Johnstone (https://orcid.org/0000-0003-1886-2138) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Alessandra Morelli (https://orcid.org/0000-0002-9803-2136) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Beatriz Goulao (https://orcid.org/0000-0003-1490-7183) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Mark Forrest (https://orcid.org/0000-0002-2395-8823) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) contributed to later stages of study design and analysis, and contributed to, read and approved the final report.
John Norrie (https://orcid.org/0000-0001-9823-9252) was responsible for the study design, wrote the first draft of the report, with significant input from all authors at all stages, and contributed to, read and approved the final report.
Publications
Cameron ST, Baraitser P, Glasier A, McDaid L, Norrie J, Radley A, et al. Pragmatic cluster randomised cohort cross-over trial to determine the effectiveness of bridging from emergency to regular contraception: the Bridge-It study protocol. BMJ Open 2019;9:e029978.
Cameron ST, Glasier A, McDaid L, Radley A, Baraitser P, Stephenson J, et al. A pragmatic cluster randomised crossover trial to determine use of effective contraception following provision of the progestogen-only pill to women presenting to community pharmacies for emergency contraception: the Bridge-it study. Lancet 2020;396:1585–94.
Glasier A, Baraitser P, McDaid L, Norrie J, Radley A, Stephenson JM, et al. Emergency contraception from the pharmacy 20 years on: a mystery shopper study. BMJ Sex Reprod Health 2021;47:55–60.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Sedgh G, Singh S, Shah IH, Ahman E, Henshaw SK, Bankole A. Induced abortion: incidence and trends worldwide from 1995 to 2008. Lancet 2012;379:625-32. https://doi.org/10.1016/S0140-6736(11)61786-8.
- Department of Health and Social Care (DHSC) . Abortion Statistics, England and Wales 2014.
- Information Services Division . Abortion Statistics 2018.
- Wellings K, Jones KG, Mercer CH, Tanton C, Clifton S, Datta J, et al. The prevalence of unplanned pregnancy and associated factors in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet 2013;382:1807-16. https://doi.org/10.1016/S0140-6736(13)62071-1.
- Thomas CM, Cameron S. Can we reduce costs and prevent more unintended pregnancies? A cost of illness and cost-effectiveness study comparing two methods of EHC. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003815.
- Smith Battle L, Leonard V. Inequities compounded: explaining variations in the transition to adulthood for teen mothers’ offspring. J Fam Nurs 2012;18:409-31. https://doi.org/10.1177/1074840712443871.
- Marston C, Meltzer H, Majeed A. Impact on contraceptive practice of making emergency hormonal contraception available over the counter in Great Britain: repeated cross sectional surveys. BMJ 2005;331. https://doi.org/10.1136/bmj.38519.440266.8F.
- Polis CB, Schaffer K, Blanchard K, Glasier A, Harper CC, Grimes DA. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD005497.pub2.
- Cheng L, Che Y, Gülmezoglu AM. Interventions for emergency contraception. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD001324.pub4.
- Faculty of Sexual and Reproductive Healthcare . Emergency Contraception Guidance 2012. www.fsrh.org (accessed 11 January 2016).
- Trussell J. Contraceptive Efficacy 2014. www.glowm.com/section_view/heading/contraceptive-efficacy/item/374 (accessed 18 February 2021).
- Glasier A, Manners R, Loudon JC, Muir A. Community pharmacists providing emergency contraception give little advice about future contraceptive use: a mystery shopper study. Contraception 2010;82:538-42. https://doi.org/10.1016/j.contraception.2010.05.008.
- Glasier A, Baraitser P, McDaid L, Norrie J, Radley A, Stephenson JM, et al. Emergency contraception from the pharmacy 20 years on: a mystery shopper study. BMJ Sex Reprod Health 2021;47:55-60. https://doi.org/10.1136/bmjsrh-2020-200648.
- Chin-Quee DS, Wedderburn M, Otterness C, Janowitz B, Chen-Mok M. Bridging emergency contraceptive pill users to regular contraception: results from a randomized trial in Jamaica. Contraception 2010;81:133-9. https://doi.org/10.1016/j.contraception.2009.08.015.
- Michie L, Cameron ST, Glasier A, Larke N, Muir A, Lorimer A. Pharmacy-based interventions for initiating effective contraception following the use of emergency contraception: a pilot study. Contraception 2014;90:447-53. https://doi.org/10.1016/j.contraception.2014.05.004.
- National Institute for Health and Care Excellence (NICE) . Contraceptive Services for Under 25s 2014.
- Parsons J, Adams C, Aziz N, Holmes J, Jawad R, Whittlesea C. Evaluation of a community pharmacy delivered oral contraception service. J Fam Plann Reprod Health Care 2013;39:97-101. https://doi.org/10.1136/jfprhc-2012-100304.
- National Institute for Health and Care Excellence (NICE) . Long-Acting Reversible Contraception 2005.
- Cameron ST, Baraitser P, Glasier A, McDaid L, Norrie J, Radley A, et al. Pragmatic cluster randomised cohort cross-over trial to determine the effectiveness of bridging from emergency to regular contraception: the Bridge-It study protocol. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029978.
- Faculty of Sexual and Reproductive Healthcare . UK Medical Eligibility Criteria for Contraceptive Use 2016.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 9 March 2021).
- Medicines and Healthcare products Regulatory Authority (MHRA) . Patient Group Directions – Who Can Use Them 2017.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.MR000032.pub2.
- Cameron ST, Glasier A. Identifying women in need of further discussion about the decision to have an abortion and eventual outcome. Contraception 2013;88:128-32. https://doi.org/10.1016/j.contraception.2012.10.032.
- Hall JA, Stephenson J, Barrett G. On the stability of reported pregnancy intentions from pregnancy to 1 year postnatally: impact of choice of measure, timing of assessment, women’s characteristics and outcome of pregnancy. Maternal Child Health J 2019;23:1177-86. https://doi.org/10.1007/s10995-019-02748-x.
- Arnup SJ, McKenzie JE, Hemming K, Pilcher D, Forbes AB. Understanding the cluster randomised crossover design: a graphical illustration of the components of variation and a sample size tutorial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2113-2.
- Morgan KE, Forbes AB, Keogh RH, Jairath V, Kahan BC. Choosing appropriate analysis methods for cluster randomised cross-over trials with a binary outcome. Stat Med 2017;36:318-33. https://doi.org/10.1002/sim.7137.
- Cameron ST, Glasier A, McDaid L, Radley A, Baraitser P, Stephenson J, et al. A pragmatic cluster randomised crossover trial to determine use of effective contraception following provision of the progestogen-only pill to women presenting to community pharmacies for emergency contraception: the Bridge-it study. Lancet 2020;396:1585-94. https://doi.org/10.1016/S0140-6736(20)31785-2.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Guidance. Medicines: Reclassify Your Product 2014.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Cea-Soriano L, García Rodríguez LA, Machlitt A, Wallander MA. Use of prescription contraceptive methods in the UK general population: a primary care study. BJOG 2014;121:53-60. https://doi.org/10.1111/1471-0528.12465.
- Michie L, Cameron ST, Glasier A, Greed E. Contraceptive use among women presenting to pharmacies for emergency contraception: an opportunity for intervention. J Fam Plann Reprod Health Care 2014;40:190-5. https://doi.org/10.1136/jfprhc-2013-100730.
- Wellings K, Brima N, Sadler K, Copas AJ, McDaid L, Mercer CH, et al. Stopping and switching contraceptive methods: findings from Contessa, a prospective longitudinal study of women of reproductive age in England. Contraception 2015;91:57-66. https://doi.org/10.1016/j.contraception.2014.09.008.
- Smith C, Edwards P, Free C. Assessing the validity and reliability of self-report data on contraception use in the MObile Technology for Improved Family Planning (MOTIF) randomised controlled trial. Reprod Health 2018;15. https://doi.org/10.1186/s12978-018-0494-7.
- Baird AS. Use of ulipristal acetate, levonorgestrel and the copper-intrauterine device for emergency contraception following the introduction of new FSRH guidelines. J Fam Plann Reprod Health Care 2013;39:264-9. https://doi.org/10.1136/jfprhc-2012-100467.
- Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010;375:555-62. https://doi.org/10.1016/S0140-6736(10)60101-8.
- Cameron ST, Berger C, Michie L, Klipping C, Gemzell-Danielsson K. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, double-blind parallel-arm, placebo-controlled study. Hum Reprod 2015;30:1566-72. https://doi.org/10.1093/humrep/dev115.
- Brache V, Cochon L, Duijkers IJ, Levy DP, Kapp N, Monteil C, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod 2015;30:2785-93. https://doi.org/10.1093/humrep/dev241.
- Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol 2012;120:1291-7. https://doi.org/10.1097/AOG.0b013e318273eb56.
- Heller R, Johnstone A, Cameron ST. The feasibility of contraceptive injections at the community pharmacy. Eur J Contracept Reprod Health Care 2017;22:327-33. https://doi.org/10.1080/13625187.2017.1357808.
- Iacobucci G. Pharmacists could be allowed to prescribe high dose statins under NHS plan. BMJ 2019;366. https://doi.org/10.1136/bmj.l5447.
- NHS England . A Summary of the Community Pharmacy Contractual Framework for 2019 20 to 2023 24: Supporting Delivery for the NHS Long Term Plan 2019.
- NHS National Services Scotland . Emergency Hormonal Contraception (EHC) n.d. https://nhsnss.org/services/practitioner/pharmacy/pharmacy-services/public-health-services/emergency-hormonal-contraception-ehc/ (accessed 3 June 2020).
- Boog K, Chen ZE, Cameron S. Sexual and reproductive healthcare providers’ opinions on expansion of pharmacy-led provision of contraception. BMJ Sex Reprod Health 2019;45:183-9. https://doi.org/10.1136/bmjsrh-2018-200252.
- Glasier A, Scorer J, Bigrigg A. Attitudes of women in Scotland to contraception: a qualitative study to explore the acceptability of long-acting methods. J Fam Plann Reprod Health Care 2008;34:213-17. https://doi.org/10.1783/147118908786000497.
- Bullard KA, Edelman AB, Williams SM, Rodriguez MI. Ulipristal acetate compared to levonorgestrel emergency contraception among current oral contraceptive users: a cost-effectiveness analysis. Contraception 2019;100:222-7. https://doi.org/10.1016/j.contraception.2019.05.004.
- BBC News . Boots Apologises for Morning-After Pill Response 2017. www.bbc.co.uk/news/uk-40689763 (accessed April 2021).
Appendix 1 Supplemental table
| Source | Implementation | Mechanisms of impact | Context |
|---|---|---|---|
| Qualitative interviews |
Provider acceptability: bridging seen as an important method of developing pharmacy services, overcoming access barriers and reducing EC use. EC consultation opportune time for intervention Concerns raised: additional time/workload pressures; fit with existing practices/guidelines Training: study staff approachable and clear; venue, composition and timing suitable; content and resources adequate. Most felt that training prepared them for delivery, but could have benefited from pharmacist expertise and role-play and refresher sessions. SRH staff received no formal training so lacked awareness of study Barriers to participation: research barriers (e.g. confidentiality of data, paperwork); uncertainty about bridging; common barriers included lack of time/embarrassment; and not wanting to take POP/hormonal contraception. Suggestions to alleviate barriers: option to return/book appointments; more contraception options Fidelity of delivery (pharmacy): descriptions suggest adherence to protocol, although some fatigue with process. Participants mostly reported positive experiences and clear/consistent information about accessing further contraception. Confusion regarding the study aim was common and there were some inconsistencies relating to the rapid-access component Fidelity of delivery (SRH centre): few encountered any Bridge-it study participants. Participants mostly described negative experiences (4/5 struggled to get further contraception), reporting lack of awareness, being advised to attend a general practice and clinics being too busy |
Being approached acted as ‘prompt to change contraceptive practices’. Helped to overcome existing barriers (avoidance, lack of time and difficulties accessing appointments) Pharmacy setting accessible, convenient and less embarrassing compared with traditional settings. Other benefits: increased awareness/knowledge of contraception/services and improved confidence in accessing and using contraception Participants currently on effective contraception: had positive/no side effects; found it easy to access further contraception; familiarity. Some on effective contraception post study had no prior experience on contraception (due to lack of need and access barriers), whereas a small number had previous negative experiences and found POP suitable Participants not on effective contraception because of a range of reasons: personal circumstances (e.g. not sexually active, no partner, pregnant or planning pregnancy); worries and experiences of side effects (e.g. prolonged bleeding, mood changes, skin problems); commitment because of busy schedules/forgetting; difficulties accessing GP/SRH clinics or finding time to attend. Side effects from HC commonly mentioned as barrier post study and pre study. Twenty-two women interviewed said they were not on contraception pre study because of previous negative experiences. Not being able to get further contraception through pharmacies was a barrier. Embarrassment/shame of accessing contraception from SRH clinics commonly mentioned |
Pharmacy context: existing challenges common across sites included competing priorities, high workloads, lack of resources and expanding roles. Pressures exacerbated at particular times (e.g. in winter, flu clinics take priority). Existing challenges had an impact on delivery, with deprioritisation of screening at busy times. New contraceptive guidelines regarding ulipristal acetate acted as a barrier to delivery for some and concerns were raised about future implementation. Despite challenges, pharmacists were typically positive about embedding bridging as a service SRH context: existing challenges across sites included lack of resources, funding cuts and changing service provision. Services being cut and reshaped (two sites moved to providing walk-in consultations only to those who were triaged as a priority). Focus moved away from provision of routine contraception to young people and specialised services. Some worried that participants might be turned away/missed because of a lack of fit with practice priorities and a lack of resources. Some suggested sending to general practices instead Broader cultural context: most participants did not express being consciously aware of any media coverage about contraceptives. Participants mostly described seeing coverage relating to the new male contraceptive pill, and articles focusing on negative side effects and general ‘horror stories’. Some did talk about media coverage leading to particular contraceptives potentially getting negative reputations and how this could impact on decision-making around contraception |
| Quantitative data (4-month survey; screening logs) |
Fidelity of delivery: 90% (n = 178) of intervention participants and 64% (n = 134) of control participants provided with information about accessing further contraception. Fifty-four intervention participants could not recall being given a rapid-access card. Most were seen at SRH clinic in < 1 hour (15/25). Sixty-four per cent (16/25) had smooth experiences of the rapid-access system to study SRH clinic Acceptability: most women accessed further contraception through their GP (74/141) (vs. from SRH, 21/141). Only 17% of women attended the participating SRH centre and 50% preferred accessing contraception from a GP. Thirty-two per cent of women were not provided with their preferred method of contraception Barriers to participation (screening logs): not willing to give contact details and be followed up (54%, 264/490); not willing to give identifying data sufficient to allow data linkage with NHS registries (54%, n = 262); already using a hormonal method of contraception (32%, n = 156); does not require EC (19%, n = 93); and does not have capacity to give informed consent (13%, n = 64) |
Uptake of effective contraception: 62% (122/198) of intervention participants remained on effective contraception at 4-month follow-up – POP, 36% (71/198); combined pill/patch/ring, 14% (28/198); and LARC methods, 7% (13/198). Forty-four per cent (88/198) of intervention participants were not on effective contraception at 4-month follow-up Reasons for not using effective contraception at 4 months: not currently sexually active, 47% (27/57); worries about side effects, 21% (12/57); not decided on method to use, 16% (7/57); difficult to get appointment with GP or a SRH clinic, 14% (8/57); and difficult to find time to get to GP or a SRH clinic, 11% (6/57) Eighteen per cent (35/198) of women did not use any POP because of worries about side effects (29%, 10/35); they were not with a regular partner (23%, 8/35); they did not require regular contraception (20%, 7/35); or they preferred to start another contraceptive (17%, 6/35) For those who took POP, main reason for stopping before supply ran out: side effects (25%, 40/158) and started another method (4%, 6/158) Ten per cent (20/198) of intervention participants had used EC post study vs. 18% (37/208) of control participants |
|
| Training observations and pharmacy selection |
Location/format of training: most (n = 27) training conducted in pharmacies (e.g. consultation/training/break rooms) and seven were at SRH sites. On average, one to three pharmacists were present. Training lasted approximately 75–80 minutes Fidelity: consistency across sites, sessions mostly delivered as per training guidance, covering all components. Some adaptations were made, mostly relating to contextual factors (e.g. lack of time) that had an impact on particular components Acceptability: majority of pharmacists appeared enthusiastic about the study and engaged well with content, asking for clarification if unsure. Most seemed confident and accepting of their role. Implementation concerns included lack of staff/resources, volume of paperwork and availability of rapid-access appointments Barriers to participation highlighted in training: reluctance to take POP, lack of time and worries about data confidentiality (particularly from younger participants) Pharmacy selection/recruitment: initially approached those dispensing > 30 ECs per month, but adapted to consider those dispensing < 30 ECs per month to include more independent pharmacies/increase recruitment Barriers to selection: low EC dispensing, charging for EC, commissioned for bridging, lack of interest and too busy |
Pharmacists’ perspectives: sought-after service, real demand for easier access through pharmacies, often have patients looking for this service Highlighted additional benefits: raising awareness of local sexual health clinics and awareness of testing services |
Training sessions, at times, provided insights into other contextual factors that may influence implementation, including the specific pharmacy context, typical clientele and current EC practice/changing guidelines. Pharmacists frequently mentioned high workloads, lack of resources and reliance on locums as potential barriers to delivery |
| Monitoring of contemporaneous events/changing guidelines | Contraceptive guidelines: March 2018 – new EC guidelines recommending ulipristal acetate (ellaOne) as first option. If this was provided, then the woman was no longer eligible for study. October 2018 – new weight guidance requiring double dose of LNG if weight > 75 kg | Media coverage of contraception: July 2017–December 2019 – 736 articles identified from mainstream media sources. Topics included personal accounts of negative experiences; emerging contraceptive methods (e.g. male contraceptives, contraceptive digital applications); accessibility of contraception (e.g. barriers to access and use); contraceptive behaviour trends; and general informative pieces. Sustained coverage on negative side effects and personalised ‘horror stories’ detailing fatal or life-threatening impacts. Over the 3-year study period, the number of articles almost tripled, from 35 in 2017 to 94 in 2019. A prominent and relevant story during the study was the widespread coverage relating to the cost and accessibility of the pill in a major chain pharmacy in the UK (n = 64), which was criticised for refusing to reduce EC cost for fear of ‘incentivis[ing] inappropriate use’.47 The pharmacy subsequently said it ‘sincerely’ apologised for its ‘poor choice of words’ over emergency contraception pricing47 | |
| Researcher fieldnotes/meeting minutes |
Initial set-up took longer than anticipated, which had consequences for project staffing, pharmacy set-up, training and recruitment. Finalising PGDs and SS for each site was time-consuming and problematic, complicated by differences in health boards. Other factors contributing to delays included changing data protection laws, difficulties organising training sessions, particularly in busy periods, and research staffing issues Research nurses from all sites reported receiving few e-mail responses and having to spend a substantial amount of effort on the ground to encourage pharmacists to recruit, retrain and assist with paperwork. Reported evident fatigue with research process Some major London pharmacies were already commissioned for oral bridging |
Pharmacists reported a decline in EC requests during the summer and winter holidays, particularly pronounced in areas normally densely populated by students Slow recruitment was fuelled by understaffing, reliance on locums and other operational challenges (e.g. prioritisation of flu clinics) Protocol amendment submitted to allow new weight guidance to be part of study; however, changing guidance did cause some confusion and some pharmacists continued to exclude potential participants based on this new guidance Owing to commissioning of a SRH bid, KCH site stopped recruiting and participating pharmacies were removed from the study |
|
| Interpretation and synthesis | The intervention was acceptable to providers and was seen as an important way to improve access to contraception and to reduce repeat EC use. Training was considered to be satisfactory, although suggested improvements included drawing on pharmacist expertise, more practice-based learning and formal refresher training. Pharmacists seemed accepting of their role in the study and felt prepared for delivery, although some had concerns relating to workload pressures. Fidelity of delivery was mostly achieved in the pharmacy context, with typically clear and consistent messaging around accessing further contraception. Accounts highlighted a lack of awareness in SRH centres and participants reported unsatisfactory experiences, indicating the need for greater integration of all services involved. A variety of barriers to participation were highlighted, some specific to the research context and others relevant to wider implementation (e.g. embarrassment, reluctance to take POP) | Bridging may have a positive impact on contraceptive practices and knowledge in the short term and potentially longer term. Potential key mechanisms of change highlighted include ease of access, increased knowledge, awareness and confidence in accessing contraception and managing risk. A key mechanism specific to the pharmacy setting was ease of access. Accounts highlighted the real need and demand for this service, suggesting synergy in intervention design and patient need. Persistent barriers to accessing and regularly using routine contraception remain, including worries about side effects, ingrained stigma of SRH services and difficulties accessing contraceptive appointments. Although the study was effective for some (including non-users and previous users), it was not a comprehensive solution and the remaining challenges highlight the need for a package of solutions to ensure that their diverse range of needs are met | A broad range of contextual factors influenced the implementation of the study, including the context of participating pharmacies and SRH centres, broader policy and cultural factors, and the research context. Existing challenges in provider contexts, including lack of resources and changing practice priorities, influenced the implementation of the study, with screening deprioritisation and participants being missed or turned away from SRH centres. Such existing challenges meant a high level of in-person study support was required to motivate staff to recruit. Despite challenges, pharmacists were enthusiastic about embedding bridging as routine practice; however, accounts highlight the need for additional resources because of existing time pressures. There was sustained negative media coverage of contraception during the study, which may have had an impact on decision-making around participating and contraceptive use. Updated contraceptive guidance had an impact on recruitment to the study and has potential implications for wider implementation in the current format |
| Key learning and recommendations |
Suggestions to increase uptake of bridging contraception in the pharmacy setting and overcome barriers to participation include greater advertising and promotion of the service; provision of non-judgemental and supportive contraceptive consultations; an option to book routine contraceptive consultations in pharmacies outside of EC consultations; and increasing the bridging contraceptive options available Learning for future trials: need for streamlined process with condensed paperwork, adequate staff for in-person support, and integration and regular communication with all services involved in implementation and delivery |
Suggestions to increase continued uptake of effective contraception include clear and consistent information provision regarding further contraceptive access, greater linkage with general practices, easier processes for obtaining repeat prescriptions and consideration of longer-term contraceptive care within the pharmacy setting |
Existing contextual challenges in the pharmacy and SRH context, including lack of resources and changing practice priorities, highlight the need for sufficient resources and time to administer this service for bridging to be embedded in routine practice Challenges in study set-up and implementation highlight the importance of flexibility and adaptability, and the importance of in-person support from study staff throughout |
Appendix 2 Process evaluation topic guides
Appendix 3 Baseline questionnaire
Appendix 4 Four-month questionnaire for control group
Appendix 5 Four-month questionnaire for intervention group
List of abbreviations
- CI
- confidence interval
- DMC
- Data Monitoring Committee
- EC
- emergency contraception
- GP
- general practitioner
- LARC
- long-acting reversible contraception
- LNG
- levonorgestrel
- NIHR
- National Institute for Health Research
- PGD
- patient group direction
- POP
- progestogen-only pill
- PPI
- patient and public involvement
- SD
- standard deviation
- SRH
- sexual and reproductive health
- TSC
- Trial Steering Committee
