Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/59/06. The contractual start date was in April 2016. The draft report began editorial review in October 2019 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Duhig et al. This work was produced by Duhig et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Duhig et al.
Chapter 1 Introduction
Pre-eclampsia affects around 2–3% of all pregnancies1 and is associated with potentially serious complications for the woman and the baby, including multiple maternal organ dysfunction (severe hypertension, renal and liver impairment, abnormal clotting and stroke/seizures) and fetal morbidity and mortality. Once diagnosed, progression of the syndrome can be unpredictable, and decisions around timing of delivery need to take into account evolving maternal complications and perinatal morbidity. We have recently completed the multicentre PHOENIX (Pre-eclampsia in HOspital: Early iNductIon or eXpectant management) trial, in which we demonstrated that, in women with late preterm pre-eclampsia, a planned delivery reduces maternal morbidity, while increasing neonatal unit admissions, but with no difference in neonatal morbidity (including respiratory distress) when compared with expectant management. 2 Of the women in this gestational age window (34 to 37 weeks’ gestation) who were managed expectantly, over half required delivery for clinical indications before they reached 37 weeks’ gestation, and pregnancy was prolonged over the planned delivery date by 5 days only.
Current parameters advised by national guidelines for indicating need for delivery in pre-eclampsia are relatively blunt (e.g. uncontrolled severe maternal hypertension, abnormal maternal haematological/biochemical indices or fetal compromise on ultrasound or cardiotocography). 3 Novel prognostic models and blood biomarkers for determination of need for delivery in pregnancies with pre-eclampsia are now emerging,4,5 but their applicability to contemporaneous populations of women with late preterm pre-eclampsia needs further evaluation and validation. Existing clinical models can accurately predict the risk of complications in women with early-onset pre-eclampsia before 34 weeks’ gestation [PREP-S (Prediction models for Risk of Early-onset Pre-eclampsia – Survival)].
These models and blood markers for women with late preterm pre-eclampsia may enhance the ability of clinicians to determine who is at greatest risk of need for delivery, enabling timely surveillance and decisions around use of antenatal corticosteroids or place of care.
The aim of the study was to establish a prognostic model to inform optimal timing of delivery in women with late preterm pre-eclampsia (34 + 0 to 36 + 6 weeks’ gestation), comparing novel candidate biomarkers [e.g. placental growth factor (PlGF) concentration] with clinical and routinely collected blood/urinary parameters to determine clinically indicated need for delivery for pre-eclampsia (or related complications) within 7 days of assessment.
Chapter 2 Methods
We undertook a prospective observational cohort study that ran between February 2016 and December 2018, nested within the PHOENIX trial,2 in women with late preterm pre-eclampsia. The PHOENIX trial was a multicentre randomised controlled trial in which women from 46 units across England and Wales with preterm pre-eclampsia from 34+ 0 to 36+ 6 weeks’ gestation were randomly allocated to planned delivery or expectant management. Results of the PHOENIX trial are reported separately. 2
Development of the original PREP-S model
Prediction models for Risk of Early-onset Pre-eclampsia – Survival (PREP-S) is a prediction model that was developed and validated in early-onset pre-eclampsia arising before 34 weeks’ gestation, from 53 maternity units across the UK. The primary outcome for the PREP-S study was maternal complications of pre-eclampsia, which included maternal death; neurological, hepatic, cardiorespiratory, renal or haematological complications; or delivery before 34 weeks’ gestation. All candidate predictors identified in the development of the PREP-S as predictor variables were collected to determine the performance of PREP-S in our cohort of women with late preterm pre-eclampsia.
Women were eligible for the PEACOCK (Prognostic indicators of severe disEAse in women with late preterm pre-eClampsia tO guide deCision maKing on timing of delivery) study if they were between 34+ 0 and 36+ 6 weeks’ gestation, with a diagnosis of pre-eclampsia [as defined by the International Society for the Study of Hypertension in Pregnancy (ISSHP)],6 with a singleton or dichorionic diamniotic twin pregnancy and at least one viable fetus. Women were aged ≥ 18 years and gave written informed consent for participation. Exclusion criteria included a decision to deliver within the next 48 hours. All women eligible for the PHOENIX trial were eligible for participation in the PEACOCK study, whether they agreed or declined randomisation to the main PHOENIX trial. The study was approved by the South Central – Hampshire B Research Ethics Committee (number 13/SC/0645).
Women were approached individually and asked to provide plasma [ethylenediaminetetraacetic acid (EDTA)] and serum blood samples at the time of recruitment, which was processed within 4 hours of sampling. Samples were centrifuged at 1400 g for 10 minutes, and the separated supernatant aliquoted and stored at –80 °C. Samples were shipped back to the co-ordinating centre, thawed and processed on an electronic Triage™ instrument (Quidel Cardiovascular Inc., San Diego, CA, USA) in accordance with the manufacturer’s instructions to give a serum PlGF concentration result. The readings were not revealed to the clinical team involved in the woman’s care. Definitions and outcomes were prespecified in the study protocol (version 4.0). Outcomes were collected until the primary hospital discharge of the woman and infant.
Clinical predictor variables
Serum PlGF concentration at enrolment was evaluated as a predictor variable. PREP-S predictor variables were measured at study entry. PREP-S consisted of the following predictor variables, which were collected at diagnosis: maternal age (years), gestational age (weeks), exaggerated tendon reflexes, medical history (two or more of the following conditions: chronic hypertension, renal disease, previous history of pre-eclampsia, autoimmune disease and diabetes mellitus), systolic blood pressure (mmHg, highest over 6 hours), abnormal oxygen saturation (< 95% on air), platelet count (× 109/l), serum alanine aminotransferase level (IU/l), serum urea concentration (mmol/l), serum creatinine concentration (µmol/l), urine protein–creatinine ratio (mg/mmol), any previous treatment with oral/parenteral antihypertensives, and any previous treatment with magnesium sulphate. These were combined using the published model equation:7
§S0 (t) – baseline survival adjusted for optimism at time t.
S0 (48 hours) = 0.99142, S0 (72 hours) = 0.98542, S0 (1 week) = 0.96492, and S0 (1 month) = 0.87377.
Outcomes
The primary outcome was clinically indicated need for delivery [or delivery for related conditions, such as eclampsia or HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome] within 7 days of assessment. Secondary outcomes included clinically indicated need for delivery for pre-eclampsia within 48 hours of assessment and within 14 days of assessment, perinatal deaths and neonatal unit admission. On analysis of the main trial,2 it became clear that neonatal unit admissions did not directly reflect neonatal morbidity (as intended), but rather they reflected clinician behaviour. In the PHOENIX trial,2 the proportions of infants with confirmed morbidity diagnoses were similar, but admission for the indication of prematurity was higher in the planned delivery group. It was therefore decided that neonatal unit admission could not be used in this cohort as a surrogate marker of neonatal morbidity and further analysis of this secondary outcome was not undertaken.
Sample size estimation
It has been recommended that external validation of a prognostic model should ideally involve a minimum of 100 informative events. 8 We estimated that the primary outcome (delivery within 7 days owing to clinical indication) would occur in around 40% of women receiving expectant management, based on our previous work and other literature. 9 The sample size for estimation of the sensitivity (within 7%) and specificity (within 7%), assuming a sensitivity of 0.90, a specificity of 0.70 and 95% confidence intervals (CIs) (two-tailed), required 120 women with the primary outcome (and 180 without) in the expectant management arm, giving a minimum of 10 events per candidate variable. We estimated that two-thirds of the 500 women recruited to the PEACOCK study would receive expectant management (the group on which the model will be validated). We therefore expected 134 primary outcome events (500 × 67% × 40%).
Statistical analysis
The validation sample for the primary analysis (and secondary analysis evaluating clinically indicated need for delivery for pre-eclampsia within 14 days of assessment) was restricted to women in the PEACOCK study who underwent expectant management, that is women recruited to the PHOENIX trial (and also enrolled in the PEACOCK study) who were randomised to the expectant management arm and women who declined the PHOENIX trial and who were recruited to the PEACOCK study only who underwent the usual care strategy of expectant management. An additional analysis was conducted for evaluating clinically indicated need for delivery for pre-eclampsia within 48 hours of assessment, which included the PEACOCK women randomised to the planned delivery arm in the PHOENIX trial.
The stages of analysis were as follows: external validation of the PREP-S model, limited updating of the PREP-S model by recalibration, assessment of the model performance of the updated PREP-S model, assessment of the predictive performance of PlGF, comparison of PlGF and PREP-S, and assessment of the addition of PlGF to the PREP-S model. The performance of the models was assessed by calibration and discrimination. Model discrimination was assessed primarily using receiver operating characteristic (ROC) areas (areas under the curve), and calibration was assessed and reported graphically using calibration plots and estimated calibration slopes. The recalibrations were additionally reported graphically, with actual event rates compared with predicted rates for specified risk groups. Model performance in relation to the primary and secondary outcomes was determined using ROC areas. Test performance of PlGF was evaluated with sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios. We used a PlGF concentration cut-off point of < 100 pg/ml. This was based on the evidence that, in those presenting at < 35 weeks’ gestation, a PlGF concentration < 100 pg/ml has a high diagnostic accuracy (0.96, 95% CI 0.89 to 0.99) and negative predictive value (0.98, 95% CI 0.93– to 0.995) for determining pre-eclampsia necessitating delivery in 14 days. We have previously reported that a PlGF concentration < 100 pg/ml predicted pre-eclampsia requiring delivery within 14 days or before 37 weeks’ gestation (whichever was sooner) with sensitivity and negative predictive values similar to diagnostic accuracy estimates obtained by using a < 5th centile cut-off point. 9 Kaplan–Meier survival curves of the time from test to delivery were determined, stratified by four categories of risk determined by the PREP-S model. Assessment of PREP-S, PlGF and the combined model was conducted on the primary outcome and all secondary outcomes.
Missing data
In line with the approach used in the original Prediction models for Risk of Early-onset Pre-eclampsia (PREP) study, missing variables were handled as follows:
-
Where measurement of serum alanine aminotransferase levels were not available, aspartate aminotransferase was used instead (like for like).
-
Oxygen saturation was assumed to be normal if not recorded in clinical care.
-
No women had exaggerated tendon reflexes, which was imputed as no, as such women were ineligible for the PHOENIX trial.
-
Urinary protein-to-creatinine ratio was derived from 24-hour urinary protein excretion when there were sufficient data to derive a conversion factor.
-
Missing values for serum urea concentrations were be replaced by a value derived from serum creatinine concentrations by linear regression (serum urea = 0.053883 × serum creatinine + 0.7874831; numbers derived from linear regression, as described, in those with sufficient data, the correlation between the measurements was 0.5434 for 264 observations).
Chapter 3 Results
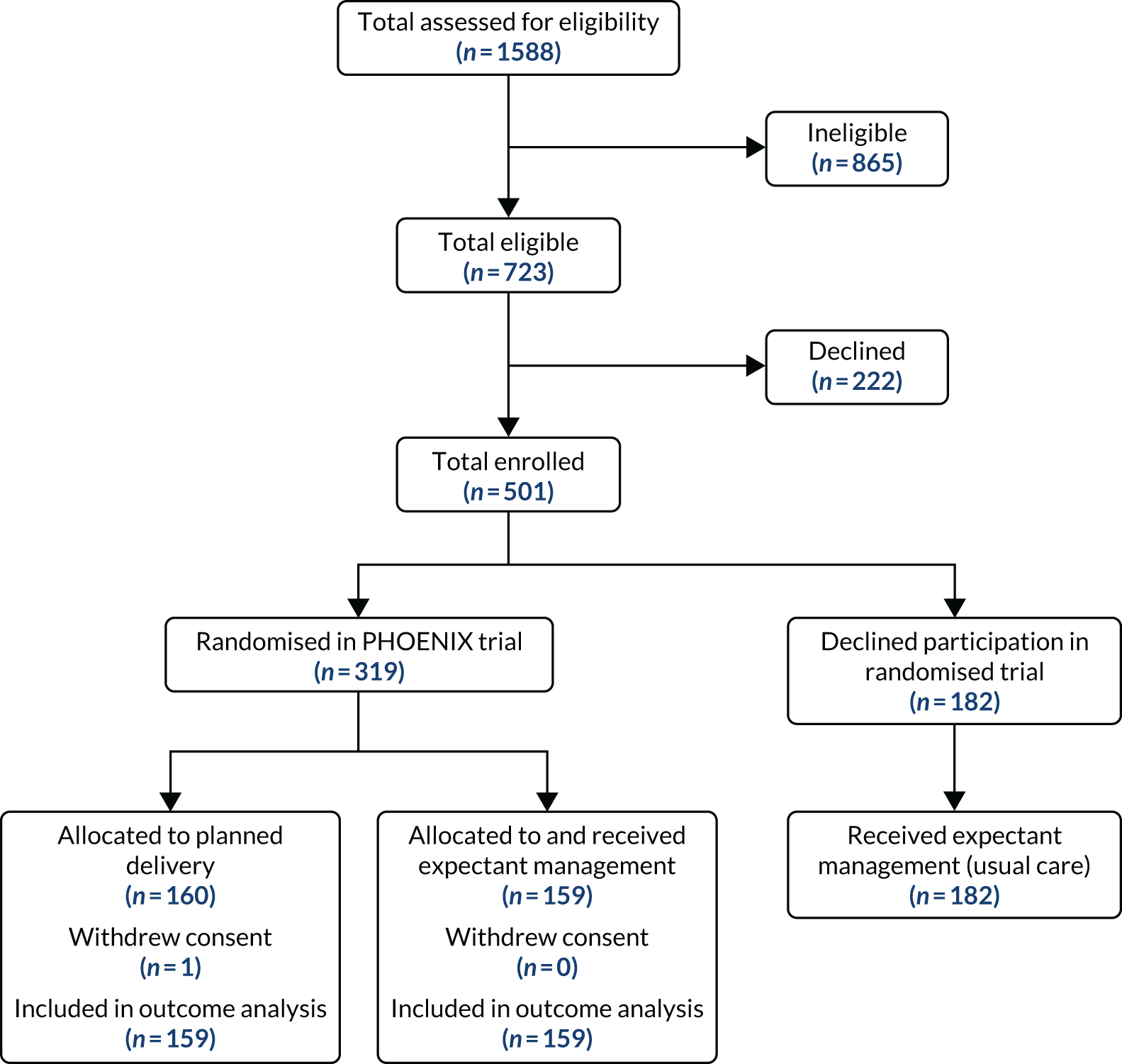
Between 27 April 2016 and 24 December 2018, we recruited 501 women to the PEACOCK study, across 36 maternity units in England and Wales (Figure 1). Across the participants who received expectant management as usual care outside the trial (n = 182) and within the PHOENIX trial allocation arm (n = 159), there were no statistically or clinically relevant differences (Table 1). Women in the two PHOENIX trial allocation arms (presented here as women in the planned delivery group included for secondary analysis) were, as expected, balanced for baseline characteristics (Table 2).
FIGURE 1.
The flow diagram of participants. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
| Non-randomised (expectant management) (N = 182) | Randomised (expectant management) (N = 159) | Comparison (95% CI) | All (expectant management) (N = 341) | |
|---|---|---|---|---|
| Maternal age (years), mean (SD) | 32.7 (5.3) | 31.1 (6.1) | MD 0.8 (0.2 to 1.5) | 31.9 (5.7) |
| Ethnicity, n (%) | ||||
| White | 122 (67.0) | 114 (71.7) | – | 236 (69.4) |
| Black | 24 (13.2) | 18 (11.4) | – | 42 (12.4) |
| Asian | 28 (15.4) | 13 (8.2) | – | 41 (12.1) |
| Mixed | 4 (2.2) | 8 (5.1) | – | 12 (3.5) |
| Other | 4 (2.2) | 5 (3.2) | – | 9 (2.6) |
| Non-white ethnicity | 60 (33.0) | 44 (27.8) | RR 1.18 (0.85 to 1.64) | 104 (30.6) |
| Multiparous, n (%) | 90 (49.5) | 83 (52.2) | RR 0.95 (0.77 to 1.17) | 173 (50.7) |
| Body mass index (kg/m2), mean (SD) | 30.2 (6.5) | 30.1 (7.9) | MD 0.1 (–0.7 to 0.8) | 30.2 (7.2) |
| Smoking, n (%) | ||||
| Never | 146 (80.2) | 114 (71.7) | – | 260 (76.2) |
| Quit before pregnancy | 21 (11.5) | 32 (20.1) | – | 53 (15.5) |
| Smoking at booking | 12 (6.6) | 11 (6.9) | – | 23 (6.7) |
| Unknown | 3 (1.6) | 2 (1.3) | – | 5 (1.5) |
| Smoking ever | 33 (18.1) | 43 (27.0) | RR 0.67 (0.45 to 1.00) | 76 (22.6) |
| Maternal history of pre-eclampsia, n (%) | 28 (31.1) | 29 (34.9) | RR 0.84 (0.53 to 1.35) | 57 (32.9) |
| Chronic hypertension, n (%) | 20 (11.0) | 25 (15.7) | RR 0.70 (0.40 to 1.21) | 45 (13.2) |
| Chronic kidney disease, n (%) | 3 (1.6) | 2 (1.3) | RR 1.31 (0.22 to 7.74) | 5 (1.5) |
| Maternal history of autoimmune disease, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Maternal medical comorbidities, n (%) | ||||
| 0 | 132 (72.5) | 113 (71.1) | – | 245 (71.8) |
| 1 | 35 (19.2) | 29 (18.2) | – | 64 (18.8) |
| 2 | 14 (7.7) | 16 (10.1) | – | 30 (8.8) |
| 3 | 1 (0.5) | 1 (0.6) | – | 2 (0.6) |
| Aspirin use, n (%) | 75 (41.4) | 68 (42.8) | RR 0.97 (0.75 to 1.24) | 43 (42.1) |
| Gestation at enrolment (weeks), mean (SD) | 35.4 (0.86) | 35.5 (0.89) | MD –0.1 (–0.2 to 0.04) | 35.4 (0.88) |
| Gestation at enrolment (weeks), n (%) | ||||
| 34+ 0–34+ 6 | 69 (37.9) | 51 (32.1) | – | 120 (35.2) |
| 35+ 0–35+ 6 | 55 (30.2) | 48 (30.2) | – | 103 (30.2) |
| 36+ 0–36+ 6 | 58 (31.9) | 60 (37.7) | – | 118 (34.6) |
| Singleton, n (%) | 174 (95.6) | 147 (92.5) | – | 321 (94.1) |
| Twin, n (%) | 8 (4.4) | 12 (7.5) | RR 0.76 (0.49 to 1.18) | 20 (5.9) |
| Maternal blood pressure 48 hours prior to enrolment (mmHg), mean (SD) | ||||
| Systolic | 153 (15) | 155 (16) | MD –0.8 (–2.4 to 0.8) | 154 (15) |
| Diastolic | 94 (10) | 95 (11) | MD –0.3 (–1.4 to 0.8) | 94 (10) |
| Highest urinary protein-to-creatinine ratio | ||||
| Number with measurement, mean (SD) | 177, 145 (238) | 156, 189 (337) | MD –22 (–54 to 10) | 333, 166 (289) |
| Suspected fetal growth restriction on ultrasound, n (%) | 22 (12.1) | 25 (15.7) | RR 0.77 (0.45 to 1.31) | 47 (13.8) |
| Maternal hyperreflexia, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Maternal blood oxygen level < 95%, n (%) | 8 (4.4) | 2 (1.3) | RR 3.49 (0.75 to 16.2) | 10 (2.9) |
| Maternal platelet count (109/l) | ||||
| Number with measurement, mean (SD) | 182, 218 (64) | 159, 217 (61) | MD 0.6 (–6.2 to 7.2) | 341, 218 (62) |
| Maternal alanine transaminase (IU/l) | ||||
| Number with measurement, mean (SD) | 174, 30 (44) | 154, 23 (41) | MD 3.7 (–0.8 to 8.3) | 341, 27 (43) |
| Maternal urea (mmol/l) | ||||
| Number with measurement, mean (SD) | 87, 4.2 (1.4) | 88, 3.9 (1.5) | MD 0.1 (–0.03 to 0.2) | 175, 4.1 (1.4) |
| Maternal creatinine (µmol/l) | ||||
| Number with measurement, mean (SD) | 182, 64 (19) | 159, 61 (14) | MD 1.4 (–0.4 to 3.2) | 341, 62 (17) |
| Maternal PlGF (pg/ml) | ||||
| Number with measurement | 178 | 157 | MD –3.3 (–18.5 to 11.9) | 335 |
| Mean (SD) | 37.1 (134.1) | 43.6 (146.7) | 40.16 (140.00) | |
| Median (IQR) | 12.0 (12.0–18.6) | 12.6 (12.0–24.1) | 12.0 (12.0–20.6) | |
| PlGF concentration ≥ 100 pg/ml, n (%) | 9 (5.1) | 7 (4.5) | RR 1.60 (0.64 to 4.02) | 18 (5.8) |
| PlGF concentration 12–100 pg/ml, n (%) | 67 (37.6) | 75 (47.8) | RR 0.91 (0.71 to 1.16) | 142 (45.7) |
| PlGF concentration < 12 pg/ml, n (%) | 76 (42.7) | 75 (47.8) | RR 1.03 (0.82 to 1.30) | 151 (48.6) |
| Randomised (planned delivery) (N = 160) | Randomised (expectant management) (N = 159) | Comparison (95% CI) | All (N = 319) | |
|---|---|---|---|---|
| Maternal age (years), mean (SD) | 30.3 (6.0) | 31.1 (6.1) | MD –0.7 (–2.0 to 0.6) | 30.7 (6.0) |
| Ethnicity, n (%) | ||||
| White | 123 (76.9) | 114 (71.7) | – | 237 (74.5) |
| Black | 17 (10.6) | 18 (11.4) | – | 35 (11.0) |
| Asian | 16 (10.0) | 13 (8.2) | – | 29 (9.1) |
| Mixed | 3 (1.9) | 8 (5.1) | – | 11 (3.5) |
| Other | 1 (0.6) | 5 (3.2) | – | 6 (1.9) |
| Non-white ethnicity | 37 (23.1) | 44 (27.8) | RR 0.83 (0.57 to 1.21) | 81 (25.5) |
| Multiparous, n (%) | 99 (61.9) | 83 (52.2) | RR 1.19 (0.98 to 1.44) | 182 (57.1) |
| Body mass index (kg/m2), mean (SD) | 30.6 (8.1) | 30.1 (7.9) | MD 0.4 (–1.3 to 2.2) | 30.36 (8.0) |
| Smoking, n (%) | ||||
| Never | 120 (75.0) | 114 (71.7) | – | 234 (73.4) |
| Quit before pregnancy | 26 (16.3) | 32 (20.1) | – | 58 (18.2) |
| Smoking at booking | 14 (8.8) | 11 (6.9) | – | 25 (7.8) |
| Unknown | 0 (0.0) | 2 (1.3) | – | 2 (0.6) |
| Smoking ever | 40 (25.0) | 43 (27.0) | RR 0.91 (0.63 to 1.32) | 83 (26.2) |
| Maternal history of pre-eclampsia, n (%) | 30 (30.3) | 29 (34.9) | RR 1.03 (0.65 to 1.63) | 59 (32.4) |
| Chronic hypertension, n (%) | 18 (11.3) | 25 (15.7) | RR 0.72 (0.41 to 1.26) | 43 (13.5) |
| Chronic kidney disease, n (%) | 2 (1.3) | 2 (1.3) | RR 0.99 (0.14 to 6.97) | 4 (1.3) |
| Maternal history of autoimmune disease, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Maternal medical comorbidities, n (%) | ||||
| 0 | 114 (71.3) | 113 (71.1) | – | 227 (71.2) |
| 1 | 33 (20.6) | 29 (18.2) | – | 62 (19.4) |
| 2 | 12 (7.5) | 16 (10.1) | – | 28 (8.8) |
| 3 | 1 (0.6) | 1 (0.6) | – | 2 (0.6) |
| Aspirin use, n (%) | 67 (41.9) | 68 (42.8) | RR 0.98 (0.76 to 1.27) | 135 (42.3) |
| Gestation at enrolment (weeks), mean (SD) | 35.5 (0.85) | 35.5 (0.89) | MD 0.02 (–0.2 to 0.2) | 35.49 (0.87) |
| Gestation at enrolment (weeks), n (%) | ||||
| 34+ 0–34+ 6 | 48 (30.0) | 51 (32.1) | – | 99 (31.0) |
| 35+ 0–35+ 6 | 52 (32.5) | 48 (30.2) | – | 100 (31.3) |
| 36+ 0–36+ 6 | 60 (37.5) | 60 (37.7) | – | 120 (37.6) |
| Singleton, n (%) | 147 (91.9) | 147 (92.5) | – | 294 (92.2) |
| Twin, n (%) | 13 (8.1) | 12 (7.5) | RR 1.08 (0.51 to 2.29) | 25 (7.8) |
| Maternal blood pressure 48 hours prior to enrolment (mmHg), mean (SD) | ||||
| Systolic | 154 (15) | 155 (16) | MD –1.1 (–4.4 to 2.3) | 154 (15) |
| Diastolic | 96 (10) | 95 (11) | MD 1.0 (–1.3 to 3.3) | 95 (10) |
| Highest urinary protein-to-creatinine ratio | ||||
| Number with measurement, mean (SD) | 158, 149 (168) | 156, 189 (337) | MD –39 (–97 to 19) | 314, 169 (266) |
| Suspected fetal growth restriction on ultrasound, n (%) | 25 (15.6) | 25 (15.7) | RR 0.99 (0.60 to 1.65) | 50 (15.7) |
| Maternal hyperreflexia, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Maternal blood oxygen level < 95%, n (%) | 1 (0.6) | 2 (1.13) | – | 3 (0.9) |
| Maternal platelet count (109/l) | ||||
| Number with measurement, mean (SD) | 160, 225 (87) | 159, 217 (61) | MD 8.0 (–8.6 to 24.6) | 319, 221 (75) |
| Maternal alanine transaminase (IU/l) | ||||
| Number with measurement, mean (SD) | 155, 23 (24) | 154, 23 (41) | MD 0.4 (–7.8 to 7.0) | 309, 23 (34) |
| Maternal urea (mmol/l) | ||||
| Number with measurement, mean (SD) | 92, 4.0 (1.5) | 88, 4.0 (1.5) | MD –0.05 (–0.3 to 0.2) | 180, 4.0 (1.5) |
| Maternal creatinine (µmol/l) | ||||
| Number with measurement, mean (SD) | 160, 60 (15) | 159, 61 (14) | MD –1.5 (–4.7 to 1.7) | 319, 60 (14) |
| Maternal PlGF (pg/ml) | ||||
| Number with measurement | 154 | 157 | MD 4.3 (–29.5 to 38.0) | 311 |
| Mean (SD) | 47.9 (155.5) | 43.6 (146.7) | 45.7 (150.9) | |
| Median (IQR) | 12.3 (12.0–25.3) | 12.6 (12.0–24.1) | 12.0 (12.0–20.6) | |
| PlGF concentration ≥ 100 pg/ml, n (%) | 11 (7.1) | 7 (4.5) | RR 1.60 (0.64 to 4.02) | 18 (6) |
| PlGF concentration 12–100 pg/ml, n (%) | 67 (43.5) | 75 (47.8) | RR 0.91 (0.71 to 1.16) | 142 (46) |
| PlGF concentration < 12 pg/ml, n (%) | 76 (49.4) | 75 (47.8) | RR 1.03 (0.82 to 1.30) | 151 (49) |
There were similar outcomes in women randomised to the expectant management group and those participating in the non-randomised expectant management group (Table 3), whereas outcomes in the planned delivery and randomised expectant management groups (Table 4) reflect those in the larger PHOENIX trial, with earlier gestation at delivery, as expected. 2 In women receiving expectant management, 81 (50.9%) of those randomised and 95 (52.2%) of those non-randomised (i.e. outside the trial) had indicated delivery due to clinical concerns for maternal or fetal well-being. Among women managed expectantly, 211 out of 341 (61.9%) delivered within 7 days. There were no perinatal deaths in the study.
| Non-randomised (expectant management) (N = 182) | Randomised (expectant management) (N = 159) | Comparison (95% CI) | All (expectant management) (N = 341) | |
|---|---|---|---|---|
| Mean number of weeks’ gestation at delivery (SD) | 36.4 (1.05) | 36.5 (1.00) | MD –0.02 (–0.1 to –0.1) | 36.48 (1.03) |
| Preterm delivery before 37 weeks’ gestation, n (%) | 101 (55.5) | 84 (52.8) | RR 1.05 (0.86 to 1.28) | 185 (54.3) |
| Delivery within 7 days, n (%) | 108 (59.3) | 103 (64.8) | – | 211 (61.9) |
| Delivery within 2 days, n (%) | 28 (15.4) | 29 (18.2) | – | 57 (16.7) |
| Delivery within 14 days, n (%) | 158 (86.8) | 141 (88.7) | – | 299 (87.7) |
| Antenatal systolic blood pressure > 160 mmHg, n (%) | 106 (58.9) | 98 (61.6) | – | 204 (60.2) |
| Postpartum systolic blood pressure ≥ 160 mmHg, n (%) | 67 (37.9) | 61 (38.4) | – | 128 (38.1) |
| Antihypertensive medication prior to delivery, n (%) | 170 (93.4) | 145 (91.2) | RR 1.02 (0.96 to 1.09) | 315 (92.4) |
| Onset of labour, n (%) | ||||
| Spontaneous | 11 (6.0) | 7 (4.4) | – | 18 (5.3) |
| Induced | 110 (60.4) | 101 (63.5) | – | 211 (61.9) |
| Prelabour caesarean section | 61 (33.5) | 50 (31.4) | – | 111 (32.6) |
| PROM and augmentation | 0 (0.0) | 1 (0.6) | – | 1 (0.3) |
| Required indicated delivery, n (%) | 95 (52.2) | 81 (50.9) | 176 (51.6) | |
| Indication for delivery, n (%) | ||||
| Severe maternal hypertension | 40 (22.0) | 45 (28.3) | – | 85 (24.9) |
| Maternal haematological abnormality | 9 (4.9) | 6 (3.8) | – | 15 (4.4) |
| Maternal biochemical abnormality | 21 (11.5) | 21 (13.2) | – | 42 (12.3) |
| Fetal concerns on US | 30 (16.5) | 17 (10.7) | – | 47 (13.8) |
| Fetal concerns on CTG | 24 (13.2) | 18 (11.3) | – | 42 (12.3) |
| Severe maternal symptoms | 13 (7.1) | 17 (10.7) | – | 30 (8.8) |
| Reaching 37 weeks’ gestation | 76 (41.8) | 71 (44.7) | – | 147 (43.1) |
| Mean infant birthweight (grams) (SD) | 2489 (558) | 2513 (520) | MD –12 (–70 to 45) | 2500 (556) |
| Mean intergrowth centile (SD) | 32.2 (31) | 31.6 (23) | MD 0.3 (–2.8 to 3.4) | 31.89 (29.61) |
| Intergrowth SGA < 10th centile, n (%) | 69 (36.5) | 50 (29.2) | RR 1.18 (0.95 to 1.47) | 119 (33.1) |
| Intergrowth SGA < 3rd centile, n (%) | 30 (15.9) | 12 (7.0) | RR 1.58 (1.12 to 2.24) | 42 (11.7) |
| Randomised (planned delivery) (N = 159) | Randomised (expectant management) (N = 159) | Comparison (95% CI) | All (randomised) (N = 318) | |
|---|---|---|---|---|
| Mean number of weeks’ gestation at delivery (SD) | 35.9 (0.88) | 36.5 (1.00) | MD –0.6 (–0.8 to –0.4) | 36.2 (0.99) |
| Preterm delivery before 37 weeks’ gestation, n (%) | 139 (87.4) | 84 (52.8) | RR 1.65 (1.41 to 1.94) | 223 (70.1) |
| Delivery within 7 days, n (%) | 154 (99.4) | 103 (64.8) | – | 257 (80.8) |
| Delivery within 2 days, n (%) | 77 (48.4) | 29 (18.2) | – | 106 (33.3) |
| Delivery within 14 days, n (%) | 158 (99.4) | 141 (88.7) | – | 299 (94.0) |
| Antenatal systolic blood pressure > 160 mmHg, n (%) | 67 (42.1) | 98 (61.6) | – | 165 (51.9) |
| Postpartum systolic blood pressure ≥ 160 mmHg, n (%) | 50 (31.4) | 61 (38.4) | RR 0.71 (0.60 to 0.85) | 111 (34.9) |
| Antihypertensive medication prior to delivery, n (%) | 138 (86.8) | 145/159 (91.2) | RR 0.95 (0.88 to 1.03) | 283 (89.0) |
| Onset of labour, n (%) | ||||
| Spontaneous | 0 (0.0) | 7 (4.4) | – | 7 (2.2) |
| Induced | 108 (67.9) | 101 (63.5) | – | 209 (65.7) |
| Prelabour caesarean section | 50 (31.4) | 50 (31.4) | – | 100 (31.4) |
| PROM and augmentation | 1 (0.6) | 1 (0.6) | – | 2 (0.6) |
| Required indicated delivery, n (%) | – | 81 (50.9) | – | – |
| Indication for delivery, n (%) | ||||
| Severe maternal hypertension | 4 (2.5) | 45 (28.3) | – | 49 (15.4) |
| Maternal haematological abnormality | 0 (0.0) | 6 (3.8) | – | 6 (1.9) |
| Maternal biochemical abnormality | 5 (3.1) | 21 (13.2) | – | 26 (8.2) |
| Fetal concerns on US | 3 (1.9) | 17 (10.7) | – | 20 (6.3) |
| Fetal concerns on CTG | 12 (7.5) | 18 (11.3) | – | 30 (9.4) |
| Severe maternal symptoms | 3 (1.9) | 17 (10.7) | – | 20 (6.3) |
| Reaching 37 weeks’ gestation | 1 (0.6) | 71 (44.7) | – | 72 (22.6) |
| Trial allocation | 159 (100) | 0 (0.0) | – | 159 (50.0) |
| Mean infant birthweight (grams) (SD) | 2450 (465) | 2513 (520) | MD –63 (–168 to 42) | 2482 (494) |
| Mean intergrowth centile (SD) | 36.8 (29) | 31.6 (23) | MD 5.3 (–0.9 to 11.3) | 34.2 (28.8) |
| Intergrowth SGA < 10th centile, n (%) | 41 (24.0) | 50 (29.2) | RR 0.82 (0.58 to 1.17) | 91 (26.6) |
| Intergrowth SGA < 3rd centile, n (%) | 12 (7.0) | 12 (7.0) | RR 1.00 (0.46 to 2.16) | 24 (7.0) |
The test performance for PlGF in determining the need for delivery within 7 days at low (< 100 pg/ml) and very low PlGF concentrations (< 12 pg/ml) is shown in Table 5. The sensitivity of placental growth factor concentration < 100 pg/ml in determining need for delivery within 7 days was 97.9% (95% CI 94.8% to 99.4%), the negative predictive value was 71.4% (95% CI 41.9% to 91.6%) and the specificity of 8.4% (95% CI 4.1% to 14.9%). Similar test performance statistics for determining need for delivery within 14 days are shown in Table 6 and need for delivery within 2 days are shown in Table 7 (n = 501 women). Although the test had high sensitivity for delivery within 7 days, the negative predictive value was only 71% and the specificity was low (8%).
| Delivery within 7 days | |
|---|---|
| < 100 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 97.9 (94.8 to 99.4); 133/135 |
| Specificity (%) (95% CI); n/N | 8.4 (4.1 to 14.9); 10/119 |
| Positive predictive value (%) (95% CI); n/N | 63.3 (57.5 to 68.8); 188/297 |
| Negative predictive value (%) (95% CI); n/N | 71.4 (41.9 to 91.6); 10/14 |
| Positive likelihood ratio (95% CI) | 1.07 (1.01 to 1.13) |
| Negative likelihood ratio (95% CI) | 0.25 (0.08 to 0.77) |
| < 12 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 62.0 (54.7 to 68.9); 119/192 |
| Specificity (%) (95% CI); n/N | 55.5 (46.1 to 64.6); 66/119 |
| Positive predictive value (%) (95% CI); n/N | 69.2 (61.7 to 76.0); 119/172 |
| Negative predictive value (%) (95% CI); n/N | 47.5 (39.0 to 51.6); 66/139 |
| Positive likelihood ratio (95% CI) | 1.39 (1.11 to 1.75) |
| Negative likelihood ratio (95% CI) | 0.69 (0.54 to 0.87) |
| Delivery within 14 days | |
|---|---|
| < 100 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 97.4 (94.8 to 99.0); 266/273 |
| Specificity (%) (95% CI); n/N | 18.4 (7.7 to 34.3); 7/38 |
| Positive predictive value (%) (95% CI); n/N | 89.6 (85.5 to 92.8); 266/297 |
| Negative predictive value (%) (95% CI); n/N | 50.0 (23.0 to 77.0); 7/14 |
| Positive likelihood ratio (95% CI) | 1.19 (1.03 to 1.39) |
| Negative likelihood ratio (95% CI) | 0.14 (0.05 to 0.38) |
| < 12 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 58.6 (52.5 to 64.5); 160/273 |
| Specificity (%) (95% CI); n/N | 68.4 (51.3 to 82.5); 26/39 |
| Positive predictive value (%) (95% CI); n/N | 93.0 (88.1 to 96.3); 160/172 |
| Negative predictive value (%) (95% CI); n/N | 18.7 (12.6 to 26.2); 26/139 |
| Positive likelihood ratio (95% CI) | 1.86 (1.15 to 2.99) |
| Negative likelihood ratio (95% CI) | 0.60 (0.47 to 0.78) |
| Delivery within 2 days | |
|---|---|
| < 100 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 95.2 (89.8 to 98.2); 119/125 |
| Specificity (%) (95% CI); n/N | 5.6 (3.4 to 8.7); 19/337 |
| Positive predictive value (%) (95% CI); n/N | 27.2 (23.1 to 31.7); 119/337 |
| Negative predictive value (%) (95% CI); n/N | 76.0 (54.9 to 90.6); 19/25 |
| Positive likelihood ratio (95% CI) | 1.01 (0.96 to 1.06) |
| Negative likelihood ratio (95% CI) | 0.85 (0.35 to 2.08) |
| < 12 pg/ml | |
| Sensitivity (%) (95% CI); n/N | 54.4 (45.3 to 63.3); 68/125 |
| Specificity (%) (95% CI); n/N | 47.2 (41.7 to 52.7); 159/338 |
| Positive predictive value (%) (95% CI); n/N | 27.6 (22.2 to 33.7); 68/246 |
| Negative predictive value (%) (95% CI); n/N | 73.6 (67.2 to 79.4); 159/216 |
| Positive likelihood ratio (95% CI) | 1.03 (0.85 to 1.24) |
| Negative likelihood ratio (95% CI) | 0.97 (0.77 to 1.21) |
For evaluation of the PREP-S prognostic model in this cohort, baseline predictor variables were assessed in the PEACOCK study cohort and the original PREP-S cohort (Table 8). There were important differences between the two cohorts, particularly relating to gestation at enrolment, definitions used for and, therefore, incidence of adverse maternal outcomes.
| Variable | PEACOCK: all expectant management (N = 341) | PREP-S cohort (N = 954) | Comparison |
|---|---|---|---|
| Maternal age (years) | |||
| Number with measurement, mean (SD) | 341, 31.9 (5.7) | 954, 30.2 (6.1) | p < 0.0001 |
| Multiparous | |||
| Number with measurement, n (%) | 341, 173 (50.7) | 954, 403 (42) | p = 0.006 |
| Maternal history of pre-eclampsia | |||
| Number with measurement, n (%) | 341, 57 (32.9) | 336, 169 (43) | p < 0.0001 |
| Chronic hypertension | |||
| Number with measurement, n (%) | 341, 45 (13.2) | 944, 139 (15) | p = 0.43 |
| Maternal medical comorbidities | |||
| Number with measurement | 341 | 953 | p < 0.0009 |
| 1 +, n (%) | 96 (27.9) | 352 (37) | p = 0.53 |
| 2 +, n (%) | 32 (9.6) | 101 (11) | |
| Maternal blood pressure 48 hours prior to enrolment (mmHg) | |||
| Number with measurement | 341 | 949 | p < 0.0001 |
| Systolic, mean (SD) | 154 (15) | 159 (19) | p < 0.0001 |
| Diastolic, mean (SD) | 94 (10) | 99 (12) | |
| Gestation at enrolment (weeks) | |||
| Number with measurement, mean (SD) | 341, 35.4 (0.88) | 954, 30.5 (2.9) | p < 0.0001 |
| Maternal hyperreflexia | |||
| Number with measurement, n (%) | – | 601, 147 (24) | |
| Maternal blood oxygen level < 94% | |||
| Number with measurement, n (%) | 177, 3 (1.7) | 433, 4 (0.9) | p = 0.23 |
| Highest urinary protein-to-creatinine ratio | |||
| Number with measurement, mean (SD) | 333, 166 (289) | 845, 273 (492) | p < 0.0001 |
| Maternal platelet count (109/l) | |||
| Number with measurement, mean (SD) | 341, 218 (62) | 913, 226 (78) | p = 0.006 |
| Maternal alanine transaminase (IU/l) | |||
| Number with measurement, mean (SD) | 341, 27 (43) | 879, 31 (71) | p < 0.0001 |
| Maternal urea (mmol/l) | |||
| Number with measurement, mean (SD) | 175, 4.1 (1.4) | 884, 4.6 (4.4) | p < 0.0001 |
| Maternal creatinine (µmol/l) | |||
| Number with measurement, mean (SD) | 341, 62 (17) | 916, 61 (18) | p = 0.54 |
| Baseline treatment with magnesium | |||
| Number with measurement, n (%) | – | 954, 144 (15) | – |
| Delivery before 34 weeks’ gestation (%) | – | 61.3 | – |
| Delivery before 37 weeks’ gestation (%) | 54.3 | – | – |
| PEACOCK adverse maternal outcome (%) | 19.5a | – | – |
| PREP-S adverse maternal outcome (%) | – | 15.1b | – |
The ROC areas for PlGF and PREP-S are shown in Table 9 and Figure 2, with consideration of the PREP-S model for a dichotomised end point (delivery within 7 days), not a time-to-survival model as originally described, and assessment of PlGF concentration and PREP-S in combination, treating PREP-S as a single predictor. 4 The area under the curve for the clinical prediction model (PREP-S) concentration and PlGF in this cohort in determining need for delivery within 7 days was 0.64 [standard error (SE) 0.03] and 0.60 (SE 0.03), respectively, and 0.65 (SE 0.03) in combination. Both PREP-S (when used to determine a binary outcome and PlGF concentration have limited clinical applicability in this cohort in determining need for delivery within 7 days.
| ROC area (SE) (95% CI) | Harrell’s C-index (95% CI) | Comparison (vs. PREP-S alone) | |
|---|---|---|---|
| PREP-S alone | 0.64 (0.03) (0.58 to 0.71) | 0.61 (0.57 to 0.64) | – |
| PlGF alone | 0.60 (0.03) (0.54 to 0.66) | – | p = 0.314 |
| PREP-S + PlGF | 0.65 (0.03) (0.58 to 0.71) | – | p = 0.776 |
FIGURE 2.
The ROC areas for PlGF and PREP-S in determining need for delivery within 7 days. AUC, area under the curve. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.

Performance of the PREP-S model and PlGF concentration is similar in determining delivery in 2 and 14 days in this cohort (Table 10 and Figures 3 and 4), and these predictors have limited clinical applicability in this setting.
| Delivery time | ROC area (SE) (95% CI) | Comparison (vs. PREP-S alone) |
|---|---|---|
| In 14 days | ||
| PREP-S alone | 0.72 (0.05) (0.63 to 0.82) | |
| PlGF alone | 0.67 (0.05) (0.58 to 0.77) | p = 0.352 |
| PREP-S + PlGF | 0.74 (0.05) (0.65 to 0.83) | p = 0.080 |
| In 2 days | ||
| PREP-S alone | 0.71 (0.04) (0.64 to 0.79) | |
| PlGF alone | 0.53 (0.04) (0.45 to 0.61) | p = 0.0002 |
| PREP-S + PlGF | 0.72 (0.04) (0.64 to 0.79) | p = 0.639 |
FIGURE 3.
The ROC areas for PlGF and PREP-S in determining need for delivery within 2 days. AUC, area under the curve. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
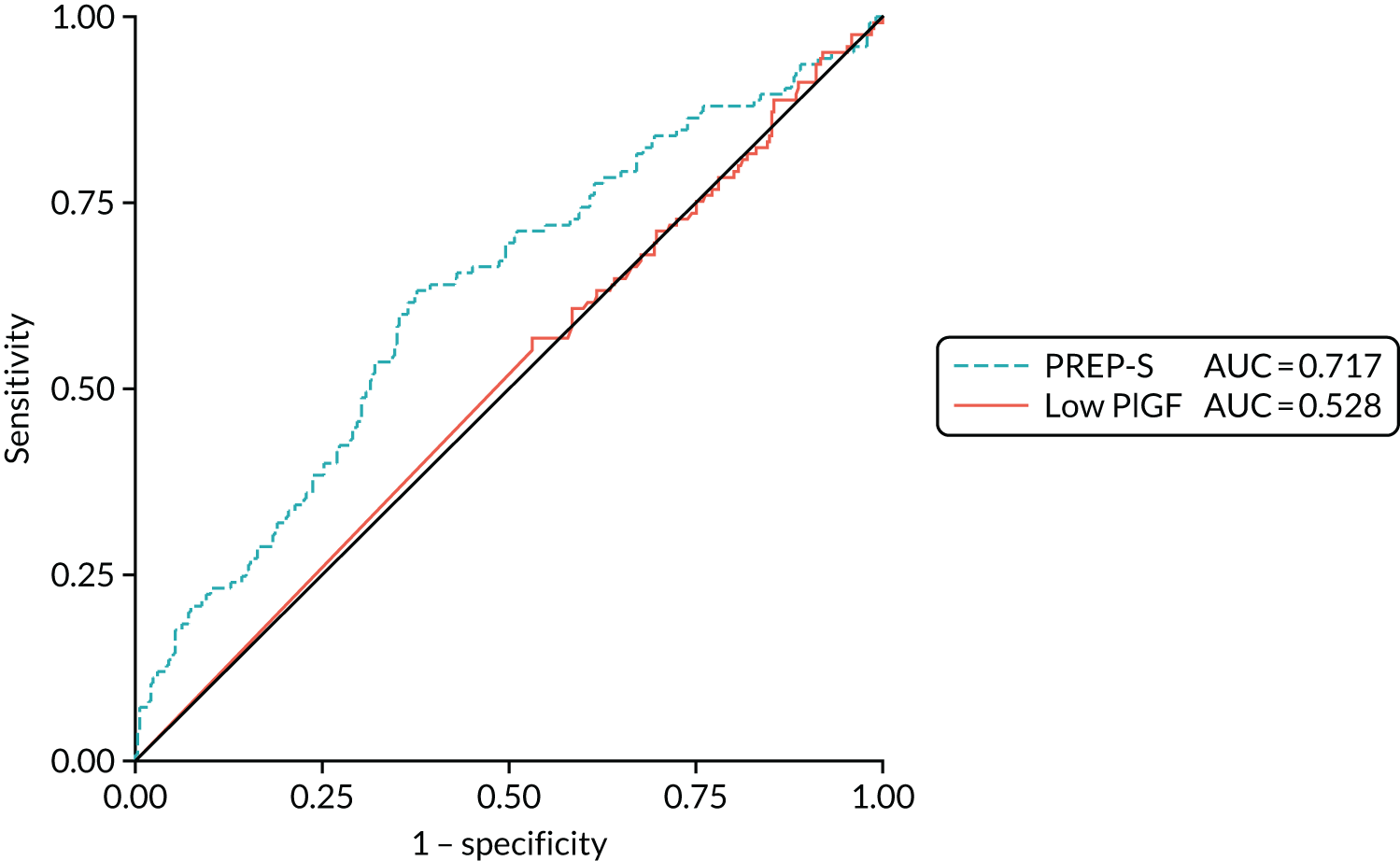
FIGURE 4.
The ROC areas for PlGF and PREP-S in determining need for delivery within 14 days. AUC, area under the curve. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.

The Kaplan–Meier time-to-delivery estimates for women in the expectant management groups, stratified by four PREP-S risk categories (as observed), are shown in Figure 5, and the recalibrated estimates are shown in Figure 6.
FIGURE 5.
Observed risks for time-to-delivery Kaplan–Meier failure estimates by four 7-day PREP-S risk categories. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
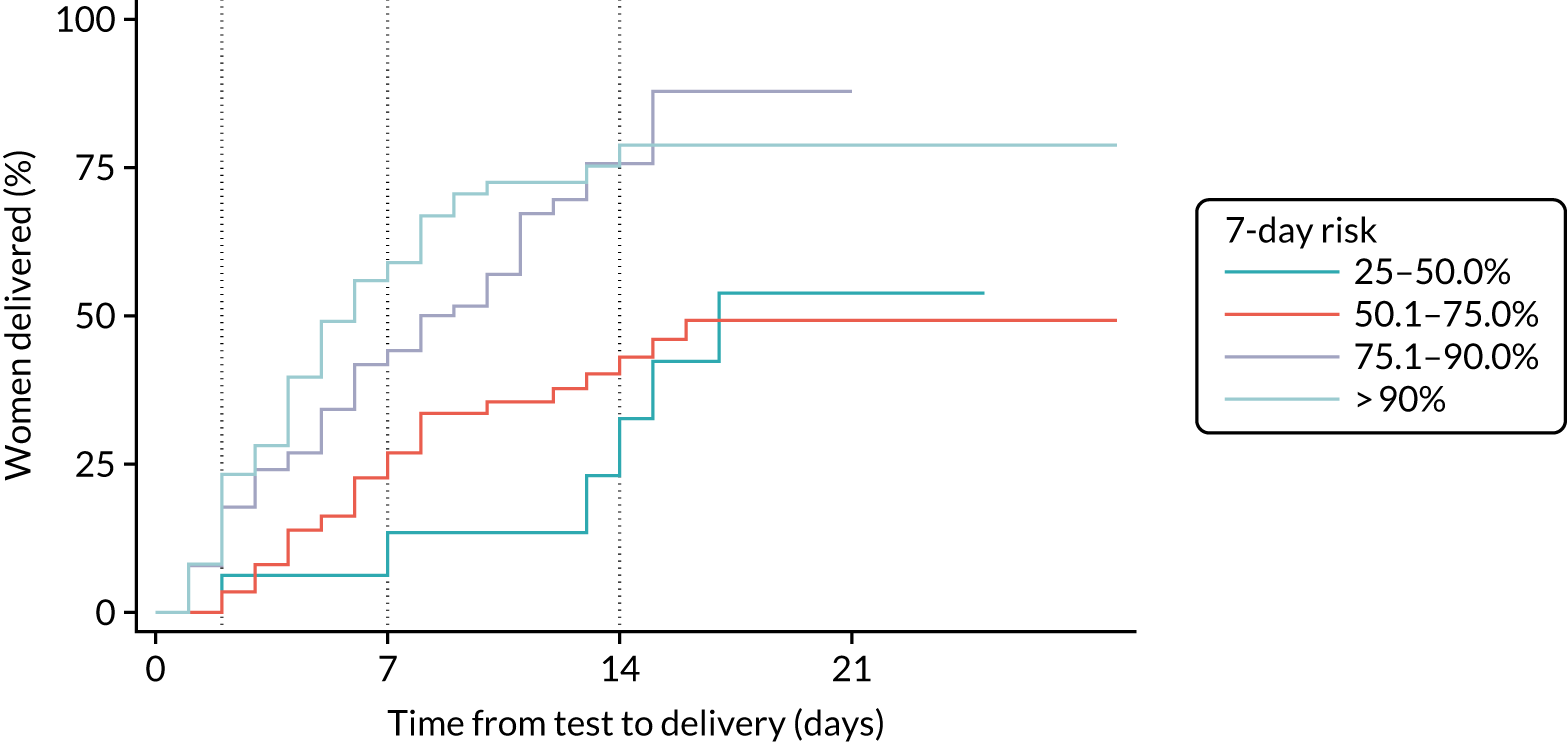
FIGURE 6.
Recalibrated risks for time-to-delivery Kaplan–Meier failure estimates by four 7-day PREP-S risk categories. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.

Calibration of the PREP-S model is shown in Table 11, with calibration in the large and of the slope assessed for predicting delivery for pre-eclampsia within 7 days. Calibration of the PREP-S model in this cohort was less good than that achieved in the original PREP-S cohorts. Overall, approximately the same number of women did have the outcome that was predicted by the model (expected value 0; calculated value –0.13; not significantly different). However, calibration of the slope was 0.375 (expected value 1.0), suggesting that the difference between adverse outcome event rates between low- and high-risk groups was not as great as the PREP-S model suggested, with PREP-S consistently overpredicting the adverse event rate in the higher-risk groups. Recalibration of the model had no impact on the ROC areas, but slightly improved the calibration of the PREP-S probabilities so that the notional probabilities were slightly closer to the actual event rate in the various subgroups.
| Delivery within 7 days | Delivery within 2 days | Delivery within 14 days | |
|---|---|---|---|
| In the large | –0.13 (p = 0.52) | –1.07 (p < 0.0001, Z –5.78) | 0.41 (p = 0.29) |
| In the large – recalibrated | 0.24 (p = 0.075) | 0.10 (p = 0.79) | 0.89 (p = 0.001, Z = 3.32) |
| Of the slope | 0.375 (p < 0.00001, Z = –5.7) | 1.18 (p = 0.483) | 0.49 (p < 0.0001, Z = –4.06) |
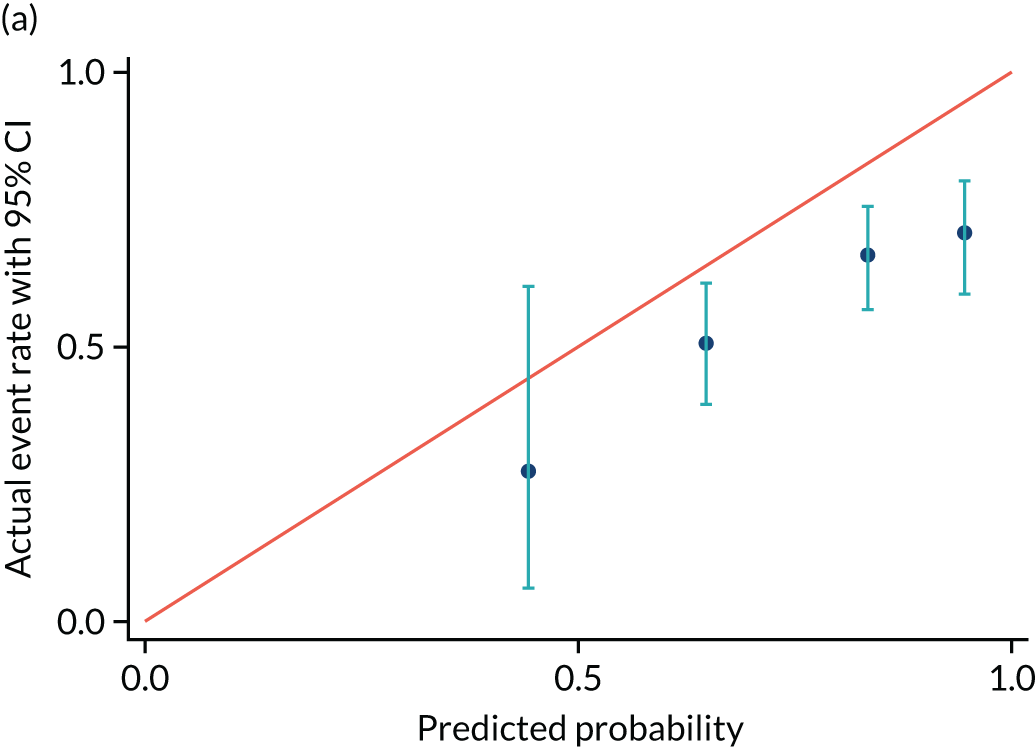
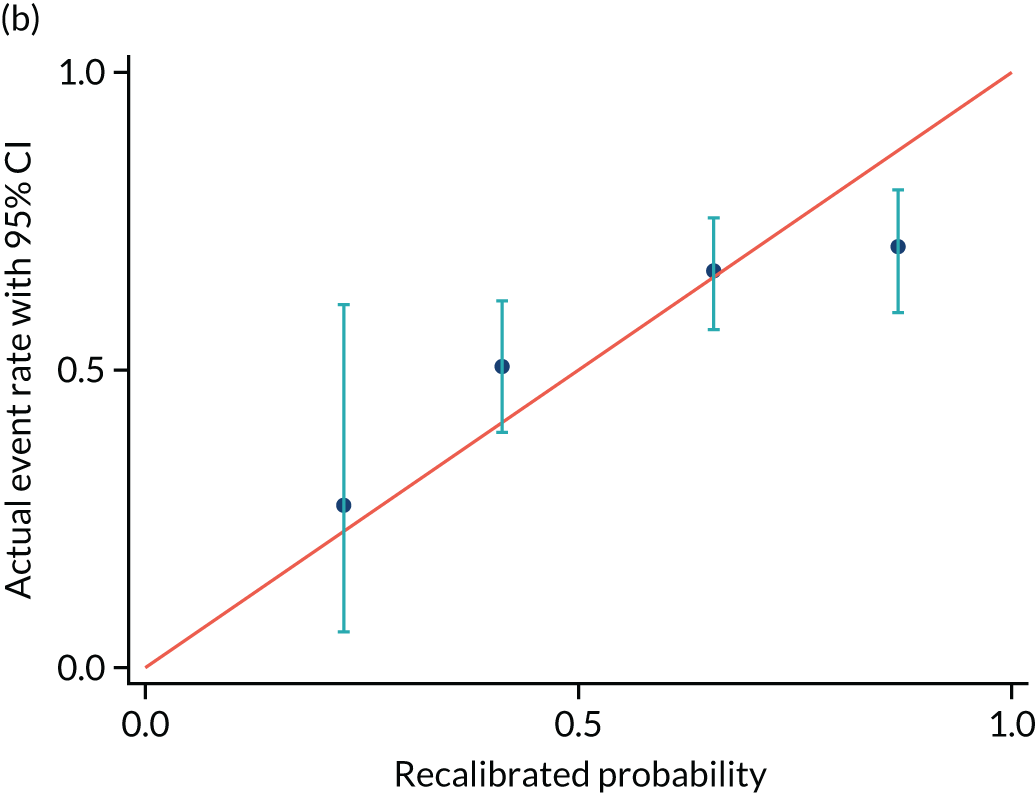
Calibrations plots are shown for PREP-S for delivery within 7 days (Figure 7 and Table 12), 2 days (Figure 8 and Table 13) and 14 days (Figure 9 and Table 14). These are used as a prognostic model to determine time to delivery within a certain number of days as a binary outcome, not as a time-to-survival model. Without recalibration, there is poor agreement between the predicted and the actual event rates in each risk group. After recalibration, there is some improvement, particularly in the overall average, but substantial differences remain. For example, in Figure 7b, only the third group is correctly aligned, and the fourth group remains substantially unaligned.
FIGURE 7.
Calibration plot for delivery within (a) 7 days and (b) recalibrated. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
| Event rate | Predicted probability group | |||
|---|---|---|---|---|
| 0.25–0.50 | 0.51–0.75 | 0.76–0.90 | > 0.90 | |
| Number of women | 11 | 85 | 105 | 82 |
| Original predicted event rate, mean (95% CI) | 0.44 (0.36 to 0.50) | 0.65 (0.50 to 0.75) | 0.83 (0.75 to 0.90) | 0.95 (0.90 to 1.00) |
| Recalibrated predicted event rate, mean (95% CI) | 0.23 (0.17 to 0.27) | 0.41 (0.27 to 0.53) | 0.66 (0.53 to 0.77) | 0.87 (0.77 to 0.99) |
| Actual event rate, proportion (n) | 0.27 (3) | 0.51 (167) | 0.67 (70) | 0.71 (58) |
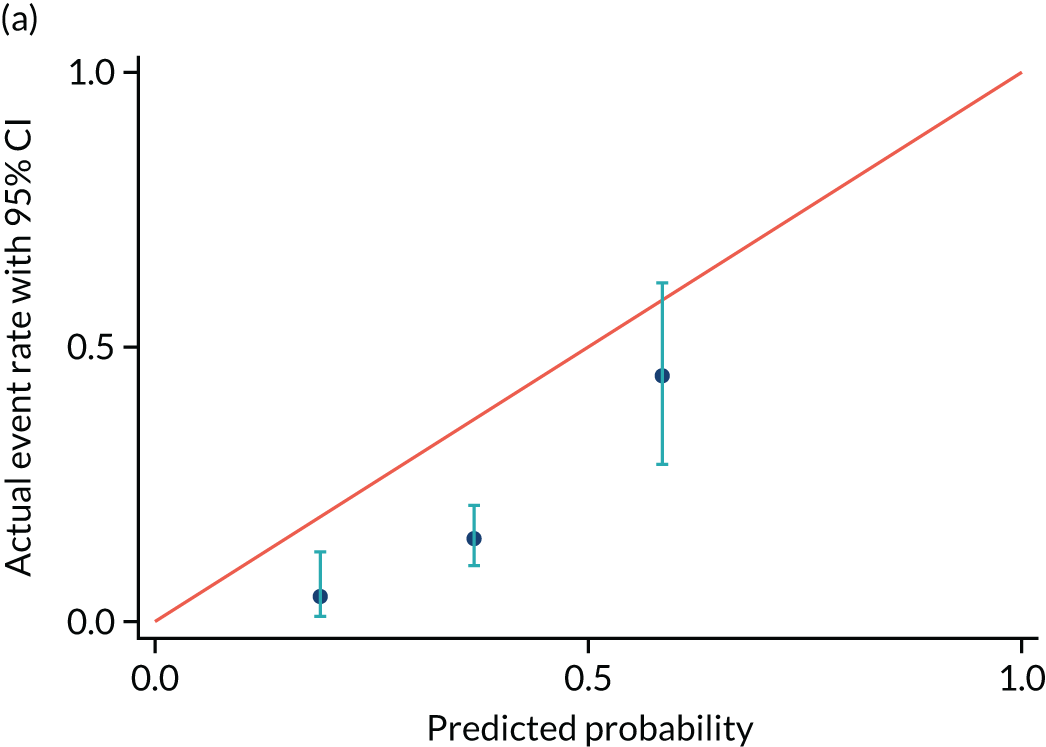
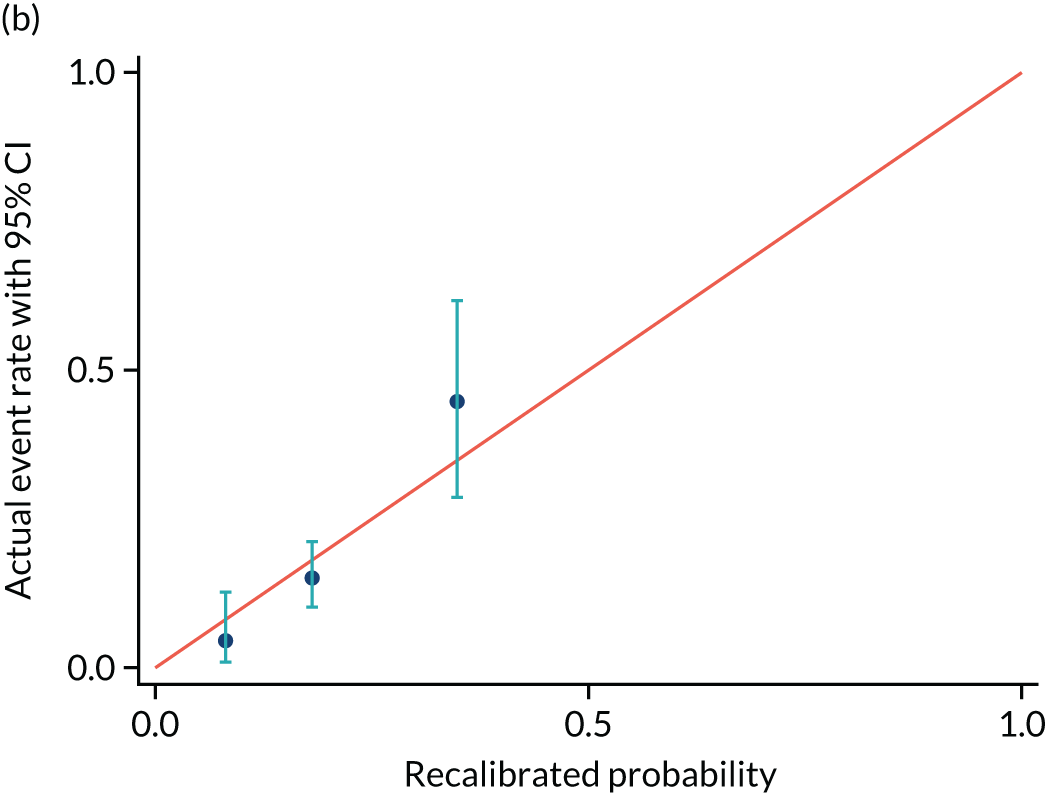
FIGURE 8.
Calibration plot for delivery within (a) 2 days and (b) recalibrated. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
| Event rate | Predicted probability group | ||
|---|---|---|---|
| < 0.25 | 0.25–0.50 | > 0.50 | |
| Number of women | 66 | 179 | 38 |
| Original predicted event rate, mean (95% CI) | 0.19 (0.10 to 0.25) | 0.37 (0.25 to 0.50) | 0.59 (0.50 to 0.74) |
| Recalibrated predicted event rate, mean (95% CI) | 0.08 (0.40 to 0.11) | 0.18 (0.11 to 0.27) | 0.35 (0.27 to 0.51) |
| Actual event rate, proportion (n) | 0.05 (3) | 0.15 (27) | 0.45 (17) |
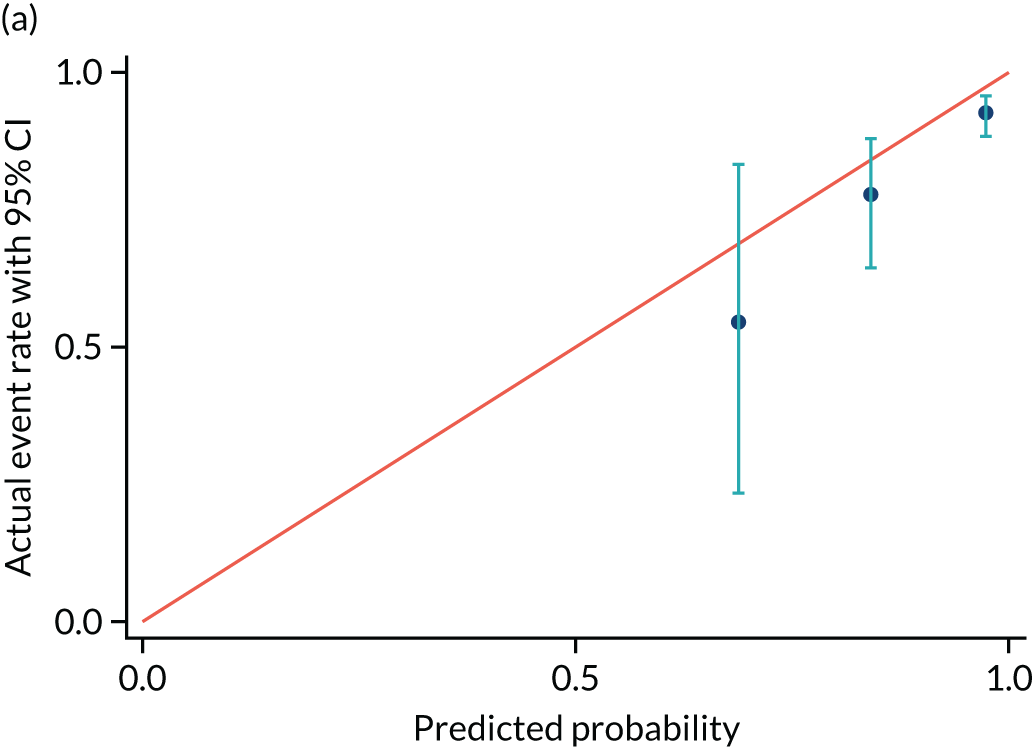
FIGURE 9.
Calibration plot for delivery within (a) 14 days and (b) recalibrated. Reproduced with permission from Duhig et al. 10 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original.
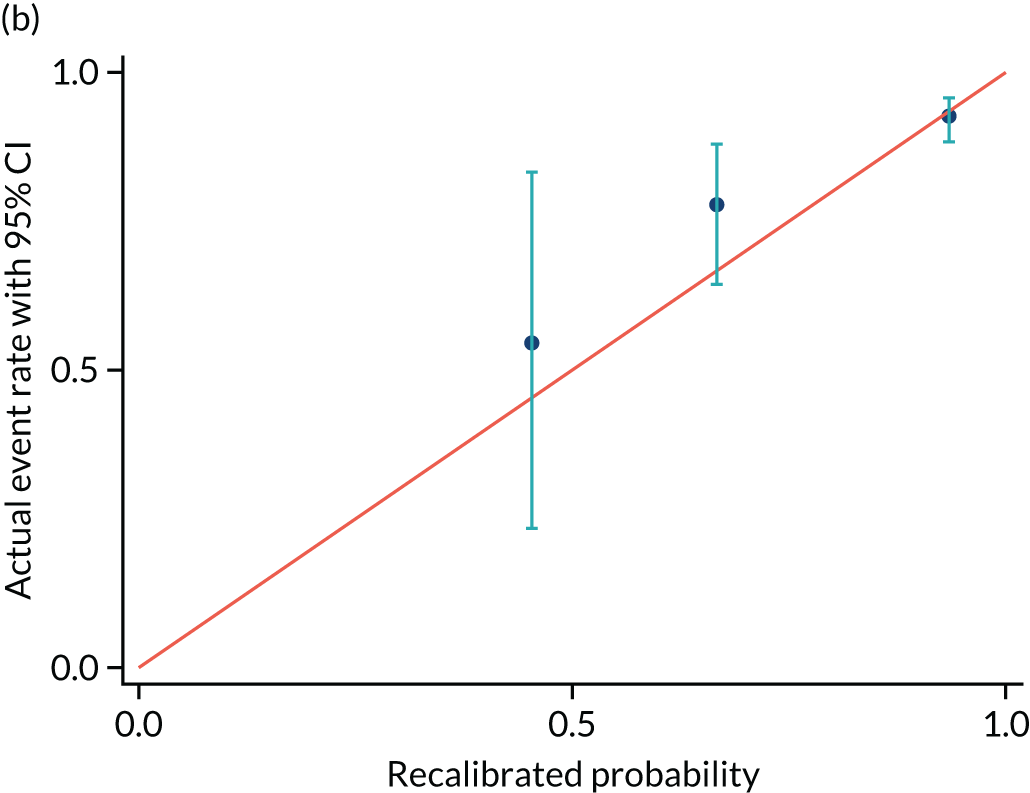
| Event rate | Predicted probability group | ||
|---|---|---|---|
| 0.50–0.75 | 0.76–0.90 | > 0.90 | |
| Number of women | 11 | 54 | 218 |
| Original predicted event rate, mean (95% CI) | 0.69 (0.59 to 0.75) | 0.84 (0.75 to 0.90) | 0.97 (0.91 to 0.99) |
| Recalibrated predicted event rate, mean (95% CI) | 0.45 (0.36 to 0.53) | 0.67 (0.53 to 0.77) | 0.93 (0.78 to 0.99) |
| Actual event rate, proportion (n) | 0.55 (6) | 0.77 (42) | 0.93 (203) |
Evaluation of other thresholds (undertaken post hoc following a reviewer request) for PlGF did not substantially improve test performance over and above the prespecified thresholds (Table 15).
| Threshold for PlGF (pg/ml) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| < 20 | 56.6 (51.2 to 62.0) | 54.5 (45.2 to 63.5) | 77.4 (71.7 to 82.5) | 31.3 (25.2 to 38.0) |
| < 30 | 74.9 (70.0 to 79.5) | 35.8 (27.3 to 44.9) | 76.3 (71.3 to 80.7) | 34.1 (26.0 to 43.0) |
| < 40 | 81.7 (77.2 to 85.7) | 25.2 (17.8 to 33.8) | 75.1 (70.3 to 79.4) | 33.3 (23.9 to 43.9) |
| < 50 | 86.4 (82.3 to 89.9) | 18.7 (12.2 to 26.7) | 74.6 (69.9 to 78.8) | 33.3 (22.4 to 45.7) |
| < 60 | 88.8 (84.9 to 91.9) | 17.1 (10.9 to 24.9) | 74.7 (70.1 to 78.9) | 35.6 (23.6 to 49.1) |
| < 70 | 92.0 (88.6 to 94.7) | 15.4 (9.6 to 23.1) | 75.0 (70.5 to 79.1) | 41.3 (27.0 to 56.8) |
| < 80 | 92.0 (88.6 to 94.7) | 13.8 (8.3 to 21.2) | 74.6 (70.2 to 78.7) | 38.6 (24.4 to 54.5) |
| < 90 | 93.8 (90.7 to 96.1) | 10.6 (5.7 to 17.4) | 74.3 (69.9 to 78.4) | 38.2 (22.2 to 56.4) |
Chapter 4 Discussion
Statement of principal findings
The findings from this research indicate that, in this group of women with late preterm pre-eclampsia, PlGF measurement and the PREP-S model are not likely to add to the current clinical assessment to help plan care for these women around timing of delivery.
Strengths and weaknesses of the study
The PEACOCK study was nested within a larger trial (PHOENIX), which evaluated timing of delivery in women with late preterm pre-eclampsia. We were necessarily constrained by the design of the PHOENIX trial such that we studied women who had reached a higher number of gestational weeks (from 34 up to 37 weeks’ gestation) than those in the original PREP study (who had reached up to 34 weeks’ gestation). In addition, we chose a different, binary, outcome (clinically indicated need for delivery by 7 days) and a different initial statistical analysis (presenting ROC areas). Further statistical analysis is under way to derive the predicted Kaplan–Meier estimates for the PREP-S categories for comparison with the observed estimates. These methods will provide further measure of the PREP-S model using a time-to-survival approach (as originally described). 4 We originally chose measurement of PlGF concentrations as a potential predictor, based on our other work describing strong test performance of PlGF concentrations in women with suspected pre-eclampsia. 9 However, the distribution of PlGF concentrations in women with confirmed pre-eclampsia is very different from the distribution of those presenting with suspected disease, with a high proportion of women (> 90%) having low or very low PlGF results. Although sensitivity of the test remains high, specificity, predictive values and likelihood ratios are all suboptimal, and the areas under the ROC curves for determining need for delivery in 7 days are too low to be clinically useful.
Placental growth factor is a biomarker that is considered reasonably ‘upstream’ in the pathophysiological process of the development of pre-eclampsia. The poor prognostic performance in this group may be because the need for delivery from pre-eclampsia within 7 days is associated with a variety of multiorgan, end-stage clinical parameters and, therefore, an ‘upstream’ biomarker such as PlGF is unable to discriminate which individuals are at a higher risk than others. In addition, clinicians act on the early signs of impending clinical deterioration (e.g. abnormal liver transaminases) to avoid severe hepatic dysfunction (as used in the original PREP-S study). If these blood test results are considered good at predicting severe adverse outcomes, then the treatment paradox (e.g. decision for delivery based on early derangement of liver transaminases) could have an impact on the performance of prognostic markers or models, as women will have the primary outcome (clinically indicated need for delivery within 7 days) without necessarily going on to develop severe maternal adverse outcomes. Although our chosen primary outcome (need for delivery for pre-eclampsia within 7 days) acts as a surrogate to represent clinician concern of substantial fetal or maternal compromise, the suboptimal performance of PlGF for predicting delivery in this group may also reflect the complex, multipathological nature of this end point, and that a single biomarker is unable to determine both fetal and maternal compromise, which have considerably different pathology (albeit the same clinical end point of early delivery). It remains to be determined if PlGF may perform better at predicting fetal indications for delivery such as fetal growth restriction or acute placental compromise, given that fetal complications reflect a placental phenotype of pre-eclampsia. Although PlGF measurements have shown considerable potential as a diagnostic adjunct in women with suspected disease,11 and the distribution of low and very low PlGF concentrations in the PEACOCK cohort confirms that we had participating women with placental dysfunction, the findings suggest that this test does not appear to have strong prognostic value (for need for delivery) in this setting.
The PREP-S model was developed in an early-onset pre-eclampsia population (prior to 34 weeks’ gestation), whereas the PEACOCK population was women with late preterm pre-eclampsia (34 to 37 weeks’ gestation). The underlying pathophysiology of the condition is likely to vary across these two groups, and hence the model cannot automatically be transferred for use in the different population. PREP-S was mainly developed to predict neurological, hepatic, renal, haematological and cardiorespiratory complications, and delivery before 34 weeks’ gestation, as clinicians would consider delivery before 34 weeks’ gestation only if the risks of complications are considered to outweigh the risks of prematurity. In the PEACOCK study, we assessed women with late preterm pre-eclampsia, including only those who either developed pre-eclampsia after 34 weeks’ gestation or remained undelivered after 34 weeks’ gestation if they did develop pre-eclampsia prior to this gestation. Both of these groups are different from those in which the model was developed for PREP-S. Importantly, clinicians are likely to have lower threshold for delivery in women with late preterm pre-eclampsia than early-onset pre-eclampsia because the risk of prematurity-related complications is lower for births after than before 34 weeks’ gestation. Although the PREP-S model has consistently shown accurate performance both in the development data set and in two separate validation data sets of early-onset pre-eclampsia,4 we found that the model cannot be transferred to a late preterm pre-eclampsia population to predict a different outcome.
There was a very small proportion of missing data. We chose a pragmatic approach to reflect the scenario of using a prediction model with an individual woman in clinical practice. For a scenario such as having an aspartate aminotransferase result (rather than a serum alanine aminotransferase result), single-value substitution was more appropriate than multiple imputation, which was not practical in clinical practice.
Strengths and weaknesses in relation to other studies, discussing important differences in results
At the time of conception of this study, there were a number of studies suggesting strong test performance for angiogenic factors measured in pregnancy, but the majority of the studies focused on women with suspected pre-eclampsia and the role of measurement in confirmed pre-eclampsia was underexplored. One early study by Verlohren et al. 12 assessed the ratio of the soluble fms-like tyrosine kinase-1 (sFlt-1) to PlGF in 95 women with pre-eclampsia after 34 weeks’ gestation and compared duration of remaining pregnancy between women in the upper and lowest quartiles of the sFlt-1/PlGF ratio (but did not report other test performance statistics for this outcome). They reported that women with pre-eclampsia with a sFlt-1/PlGF ratio in the upper quartile had a significantly reduced duration of pregnancy. However, a more recent study by Lou et al. 13 found that, in women with pre-eclampsia after 34 weeks’ gestation, there was no significant difference in sFlt-1/PlGF ratio between those who delivered within 7 days and those who delivered later. Meler et al. 14 similarly concluded that the role of a low PlGF concentration in predicting maternal complications in early-onset pre-eclampsia is limited because of both its low specificity and its low positive predictive value.
Meaning of the study
PlGF testing and the PREP-S prediction model cannot be recommended to help plan care for late preterm pre-eclampsia regarding timing of delivery. This is important and timely information given the current NHS-wide adoption of PlGF testing as a diagnostic adjunct in the assessment of women with suspected pre-eclampsia, a different population of women in this study, who had confirmed pre-eclampsia. Despite the confirmed diagnostic utility of PlGF in women with suspected pre-eclampsia, PlGF does not appear to have a role in assisting clinicians in determining timing of delivery in women with established preterm pre-eclampsia. The PREP-S model both alone and in combination with PlGF appears to have limited clinical applicability for determining which women would require delivery in 7 days (from date of assessment), in women with late preterm pre-eclampsia.
Unanswered questions and future research
Statistical modelling
Further statistical analysis of these data, considering the PREP-S model with a time-to-event analysis (as the model originally described) could be undertaken. We will consider the addition of PlGF to this model, and derive the predicted Kaplan–Meier estimates for the PREP-S categories for comparison with the observed estimates. These methods may provide further measures of the PREP-S model. We will consider rebuilding the PREP-S model within this data set, using PlGF as a candidate predictor.
Subgroup analysis
Given that PlGF is associated with placental pathology, and angiogenic factors have been observed to be imbalanced in pregnancies complicated by fetal growth restriction,9,15 we will undertake a subgroups analysis for the primary outcome in determining need for delivery in 7 days for fetal indications.
Angiogenic marker assessment
Maternal serum and urinary sFlt-1 and sFlt-1/PlGF ratios have been shown to be correlated with pre-eclampsia disease severity in some small studies. 16 Work is also under way to assess the performance of sFlt-1 and the sFlt-1/PlGF ratio to determine if this has superior performance to PlGF alone in predicting the primary outcome in this cohort.
Patient and public involvement
The research question for the PEACOCK study was identified with the involvement of women from our Hypertension in Pregnancy patient and public involvement (PPI) group, alongside the charity Action on Pre-eclampsia. With their input, we have identified a research question related to the uncertainties of the clinical course of pre-eclampsia that they deem extremely relevant to the physical and emotional well-being of women. Given that there is no reliable, robust way of determining which women and babies will become seriously unwell from pre-eclampsia, there is significant anxiety for women, with uncertainty as to who will deteriorate (and in what time frame). This also results in prolonged hospital stays for women who remain well, which has an impact on their existing family life.
Action on Pre-eclampsia were consulted on an ongoing basis for the duration of the PHOENIX trial and the PEACOCK study, advising on the execution of the study, particularly relating to approaching women for participation. We will consult with our PPI group and the charity when the results of the PEACOCK study are ready for dissemination.
Acknowledgments
Contributions of authors
Kate Duhig (https://orcid.org/0000-0001-9176-5671) (Clinical Research Fellow) contributed to the analysis and writing of the study. She also wrote the report.
Paul T Seed (https://orcid.org/0000-0001-7904-7933) (Statistician) wrote the later versions of the statistical analysis plan and undertook the statistical analysis.
Anna Placzek (https://orcid.org/0000-0002-6745-5996) (Study Manager) assisted with day-to-day running of the trial.
Jenie Sparkes (https://orcid.org/0000-0002-9973-544X) (Research Midwife) supported site research midwives and related activities.
Carolyn Gill (https://orcid.org/0000-0003-0012-5105) (Senior Research Technician) co-ordinated sample movement and analysis.
Anna Brockbank (https://orcid.org/0000-0002-2764-0556) (Research Technician) undertook sample logistics and analysis.
Andrew Shennan (https://orcid.org/0000-0001-5273-3132) (Professor, Obstetrics) conceived the study, contributed to study design and interpretation, and was involved in securing funding for the study.
Shakila Thangaratinam (https://orcid.org/0000-0002-4254-460X) (Professor, Obstetrics) conceived the study, contributed to study design, analysis and interpretation, and was involved in securing funding for the study.
Lucy C Chappell (https://orcid.org/0000-0001-6219-3379) (NIHR Research Professor, Obstetrics) conceived the study, was the chief investigator and was involved in securing funding for the study. She also wrote the report.
All authors reviewed, contributed to and approved the final version of the report.
Contributions of others
Pollyanna Hardy was listed as a co-investigator on the initial grant application, but moved to Birmingham CTU soon after the study commenced and handed over to Louise Linsell. Louise Linsell and Virginia Chiocchia wrote early drafts of a statistical analysis plan. The statistical analysis then transferred to King’s College London and new drafts of the statistical analysis plan were written by Paul Seed.
Publication
Duhig KE, Seed PT, Placzek A, Sparkes J, Hendy E, Gill C, et al. Prognostic indicators of severe disease in late preterm pre-eclampsia to guide decision making on timing of delivery: the PEACOCK study. Pregnancy Hypertens 2021;24:90–5.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol 2018;51:743-50. https://doi.org/10.1002/uog.19039.
- Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet 2019;394:1181-90. https://doi.org/10.1016/S0140-6736(19)31963-4.
- National Institute for Health and Care Excellence . Hypertension in Pregnancy: Diagnosis and Management. 2019.
- Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0827-3.
- von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, Côté AM, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet 2011;377:219-27. https://doi.org/10.1016/S0140-6736(10)61351-7.
- Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104. https://doi.org/10.1016/j.preghy.2014.02.001.
- Thangaratinam S, Allotey J, Marlin N, Mol BW, Von Dadelszen P, Ganzevoort W, et al. Development and validation of Prediction models for Risks of complications in Early-onset Pre-eclampsia (PREP): a prospective cohort study. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21180.
- Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med 2016;35:214-26. https://doi.org/10.1002/sim.6787.
- Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013;128:2121-31. https://doi.org/10.1161/CIRCULATIONAHA.113.003215.
- Duhig KE, Seed PT, Placzek J, Sparkes J, Hendy E, Gill C, et al. Prognostic indicators of severe disease in late preterm pre-eclampsia to guide decision making on timing of delivery: the PEACOCK study. Pregnancy Hypertens 2021;24:90-5. https://doi.org/10.1016/j.preghy.2021.02.012.
- Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 2019;393:1807-18. https://doi.org/10.1016/S0140-6736(18)33212-4.
- Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 2012;206:58-8. https://doi.org/10.1016/j.ajog.2011.07.037.
- Lou WZ, Jiang F, Hu J, Chen XX, Song YN, Zhou XY, et al. Maternal serum angiogenic factor sFlt-1 to PlGF ratio in preeclampsia: a useful marker for differential diagnosis and prognosis evaluation in Chinese women. Dis Markers 2019;2019. https://doi.org/10.1155/2019/6270187.
- Meler E, Scazzocchio E, Peguero A, Triunfo S, Gratacos E, Figueras F. Role of maternal plasma levels of placental growth factor for the prediction of maternal complications in preeclampsia according to the gestational age at onset. Prenat Diagn 2014;34:706-10. https://doi.org/10.1002/pd.4390.
- Herraiz I, Quezada MS, Rodriguez-Calvo J, Gómez-Montes E, Villalaín C, Galindo A. Longitudinal change of sFlt-1/PlGF ratio in singleton pregnancy with early-onset fetal growth restriction. Ultrasound Obstet Gynecol 2018;52:631-8. https://doi.org/10.1002/uog.18894.
- Tang P, Xu J, Xie BJ, Wang QM. Use of serum and urinary soluble sFlt-1 and PLGF in the diagnosis of preeclampsia. Hypertens Pregnancy 2017;36:48-52. https://doi.org/10.1080/10641955.2016.1237642.
List of abbreviations
- CI
- confidence interval
- HELLP
- haemolysis, elevated liver enzymes, low platelets
- NIHR
- National Institute for Health Research
- PEACOCK
- Prognostic indicators of severe disEAse in women with late preterm pre-eClampsia tO guide deCision maKing on timing of delivery
- PHOENIX
- Pre-eclampsia in HOspital: Early iNductIon or eXpectant management
- PlGF
- placental growth factor
- PPI
- patient and public involvement
- PREP
- Prediction models for Risk of Early-onset Pre-eclampsia
- PREP-S
- Prediction models for Risk of Early-onset Pre-eclampsia – Survival
- ROC
- receiver operating characteristic
- SE
- standard error
- sFlt-1
- soluble fms-like tyrosine kinase-1
