Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR130462. The protocol was agreed in September 2019. The assessment report began editorial review in March 2020 and was accepted for publication in August 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Objective
The overall objective of this project was to provide an update to National Institute for Health and Care Excellence (NICE) diagnostics guidance, published in October 2014, on early rule out of acute myocardial infarction (AMI) using high-sensitivity troponin tests [diagnostics guidance (DG) 15]. 1 Some sections of this report have been reproduced from our previous publication. 2 This update summarises the current evidence on the clinical effectiveness and cost-effectiveness of high-sensitivity troponin assays (including new assays that have become available to the NHS since the publication of DG151) for the management of adults presenting with acute chest pain, focusing on the early (i.e. within 4 hours of presentation) rule out of non-ST elevation myocardial infarction (NSTEMI). The following research questions were defined to address the objective of the review.
-
What is the clinical effectiveness of high-sensitivity cardiac troponin (hs-cTn) assays (used singly or in series) compared with conventional diagnostic assessment for achieving early discharge (within 4 hours of presentation) when NSTEMI is excluded and without increase in adverse outcomes?
-
What is the diagnostic performance of hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the early rule out of NSTEMI in adults with acute chest pain?
-
What is the accuracy of hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the prediction of major adverse cardiac events (MACEs) [e.g. cardiac death, non-fatal myocardial infarction (MI), revascularisation or hospitalisation for myocardial ischaemia] during 30-day follow-up in adults with acute chest pain?
-
What is the cost-effectiveness of using hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) compared with the current standard of serial troponin T and/or I testing on admission and at 10–12 hours post admission?
These research questions were addressed as components of a single systematic review and associated cost-effectiveness modelling.
Chapter 2 Background and definition of the decision problem(s)
Population
The primary indication for this assessment is the early rule out of AMI and consequent early discharge in people presenting with acute chest pain and suspected, but not confirmed, NSTEMI.
Acute coronary syndrome (ACS) is the term used to describe a spectrum of conditions caused by coronary artery disease (CAD). ACS arises when atheromatous plaque ruptures or erodes, leading to vasospasm, thrombus formation and distal embolisation, obstructing blood flow through the coronary arteries. It incorporates three distinct conditions: (1) unstable angina (UA), (2) ST elevation myocardial infarction (STEMI) and (3) NSTEMI. CAD and MI are a significant health burden in the UK, with Office for National Statistics mortality data for 2018 showing 19,654 deaths from AMI and 59,995 deaths from ischaemic heart disease (AMI accounted for 3.6% of all deaths recorded in 2018 and ischaemic heart disease accounted for approximately 10.3% of all deaths recorded in 2018). 3
Acute coronary syndrome usually presents as chest pain and chest pain has been reported as the most common cause of hospital admissions in the UK. 4 Hospital Episode Statistics (HES) for 2017–18 show 226,393 emergency admissions for chest pain, accounting for approximately 5% of all emergency admissions. 5 However, many people presenting with acute chest pain will have non-cardiac underlying causes, such as gastro-oesophageal disorders, muscle pain, anxiety or stable ischaemic heart disease. A 2003 study6 on the impact of cardiology guidelines on the diagnostic classification of people with ACS in the UK reported that the majority of people admitted to hospital with chest pain have either no ischaemic heart disease or stable ischaemic heart disease. 6 HES for 2017–18 remain consistent with this observation, showing diagnoses of AMI in 45,163 emergency admissions and UA in 13,056 admissions (these represent approximately 20% and 6% of emergency admissions with chest pain, respectively). 5 Accurate and prompt differentiation of ACS (in particular AMI), stable CAD and other causes of chest pain is therefore vital to ensure appropriate and timely intervention when required and to avoid unnecessary hospital admissions.
ST elevation myocardial infarction can usually be diagnosed on presentation by electrocardiogram (ECG), hence the main diagnostic challenge in the investigation of suspected ACS is the detection or rule out of NSTEMI. Investigation of ACS can also involve identification of people with UA (i.e. CAD with worsening symptoms, but no evidence of myocardial necrosis).
Since the development of protein biomarkers of myocardial damage in the 1980s, the number of biomarker assays available has proliferated, cardiac specificity has increased and the role of biomarkers in the diagnostic work-up of acute chest pain has expanded. The most recent HES show that the number of emergency department (ED) attendances where the first recorded investigation was a cardiac biomarker has risen substantially from 13,743 in 2010–11 to 28,379 in 2011–127 (recorded in our previous report for DG152) and then to 36,907 in 2017–18. 8 Cardiac troponin I (cTnI) and cardiac troponin T (cTnT), together with cardiac troponin (cTn) C, form the troponin–tropomyosin complex, which is responsible for regulating cardiac muscle contraction. cTnI and cTnT are used clinically as markers of cardiomyocyte necrosis, indicative of AMI. Troponin assays are intended for use in conjunction with clinical history taking and electrocardiography monitoring as, although specificity is high, troponins may also be elevated in many other conditions, including myocarditis, congestive heart failure, severe infections, renal disease and chronic inflammatory conditions of the muscle or skin. Standard biochemical diagnosis of NSTEMI is based on elevation of the cardiac biomarker troponin above the 99th centile of the reference range for the normal population. 9 However, the optimal sensitivity of standard troponin assays for MI occurs several hours after the onset of symptoms10 and, historically, this has been reflected in clinical guidelines (CGs), which recommended standard cTnI or cTnT testing at initial hospital assessment and again 10–12 hours after the onset of symptoms. 11,12 As the majority of people presenting with chest pain do not have NSTEMI, where presentation is within a few hours of symptom onset, delayed biomarker measurement may result in unnecessary periods of extended observation or hospitalisation, and associated costs. DG15 recommended the use of some hs-cTn assays [i.e. the Elecsys® Troponin-T high sensitive (Roche, Basel, Switzerland) and the ARCHITECT STAT High Sensitive Troponin-I (Abbott Laboratories, Abbott Park, IL, USA)] as options for the early rule out of NSTEMI in people presenting to an ED with chest pain and suspected ACS. 13 This recommendation was incorporated into the 2016 update to the NICE CG95. 14 High-sensitivity troponin assays are also now included in Scottish Intercollegiate Guidelines Network guidance on the management of ACS. 15 This updated assessment is being undertaken to ensure that guidance is based on current evidence (including new hs-cTn assays developed and marketed since the publication of DG15) and, where possible, to facilitate the provision of more detailed, evidence-based recommendations on how to use hs-cTn assays (e.g. timing of testing and use of sequential testing strategies).
Intervention technologies
High-sensitivity cTn assays that are able to detect lower levels of troponin in the blood are now available. Current generations of commercially available assays have analytical sensitivities up to 100 times greater than was the case for early troponin assays (1 ng/l vs. 100 ng/l). 16 Use of these high-sensitivity assays enables the detection of small changes in troponin levels, and may enable NSTEMI to be ruled out at an earlier time after the onset of acute chest pain. Use of the hs-cTn assays has the potential to facilitate earlier discharge for people with normal troponin levels. The recommended definition of a hs-cTn assay uses two criteria. 16,17
-
The total imprecision, coefficient of variation (CoV), of the assay should be ≤ 10% at the 99th centile value for the healthy reference population.
-
The limit of detection (LoD) of the assay should be such as to allow measurable concentrations to be attainable for at least 50% (ideally >95%) of healthy individuals.
A number of high-sensitivity cardiac troponin I (hs-cTnI) and high-sensitivity cardiac troponin T (hs-cTnT) assays are currently available for use in the NHS in England and Wales, and all are designed for use in clinical laboratory settings.
ARCHITECT STAT High Sensitive Troponin-I assay (Abbott Diagnostics)
The ARCHITECT STAT High Sensitive Troponin-I assay can be used with the ARCHITECT i2000SR and i1000SR analysers (Abbott Laboratories, Abbott Park, IL, USA). The assay is a quantitative, chemiluminescent microparticle immunoassay for serum or plasma samples. Results are available within 16 minutes. The ARCHITECT STAT High Sensitive Troponin-I assay can detect cTnI in 96% of the reference population, and has a recommended 99th centile cut-off point of 26.2 ng/l with a CoV of 4%. 18 The assay is Conformitè Europëenne (CE) marked and available to the NHS.
Alinity i STAT High Sensitivity Troponin-I assay (Abbott Diagnostics)
The Alinity i STAT High Sensitive Troponin-I assay (Abbott Laboratories, Abbott Park, IL, USA) can be used with the Alinity i analyser (Abbott Laboratories, Abbott Park, IL, USA). It is a chemiluminescent microparticle immunoassay used for the quantitative determination of troponin I in plasma and serum samples. Results are available within 18 minutes. The Alinity i STAT High Sensitive Troponin-I assay has a recommended 99th centile cut-off point of 26.2 ng/l with a CoV of 4.6%. Sex-specific 99th centile cut off points of 15.6 ng/l for females (CoV of 5.0%) and 34.2 ng/l for males (CoV of 4.5%) are also provided. 19 The assay is CE marked and available to the NHS.
Access High Sensitivity Troponin I (Beckman Coulter)
The Access High Sensitivity Troponin I (Beckman Coulter, Brea, CA, USA) assay can be used with both the Access 2 and DxI/DxC analysers (Beckman Coulter, Brea, CA, USA). The assay is a quantitative, paramagnetic particle chemiluminescent immunoassay for serum or plasma samples. The turnaround time of the assay is 17 minutes. The Access High Sensitivity Troponin I assay has a recommended 99th centile cut-off point of 17.5 ng/l for the whole population, 11.6 ng/l for females and 19.8 ng/l for males, with a CoV of < 10%. 20 The assay can detect troponin I in > 50% of the reference population. The assay is CE marked and available to the NHS.
VIDAS High sensitive Troponin I assay (bioMérieux)
The VIDAS® High sensitive Troponin I (bioMérieux SA, Marcy l'Etoile, France) assay is designed for use in a laboratory setting on the following analysers: VIDAS, MINI VIDAS and VIDAS 3 (bioMérieux SA, Marcy l'Etoile, France). It is intended for the in vitro quantitative determination of troponin I in serum and plasma (lithium heparin) samples. Test results are available in 20 minutes. It has a recommended 99th centile cut-off point of 19 ng/l. Sex-specific 99th centile cut-off points of 11 ng/l for females and 25 ng/l for males are provided. 21 The assay is CE marked and available to the NHS.
VITROS High Sensitivity Troponin I Assay (Ortho Clinical Diagnostics)
The VITROS® High Sensitivity Troponin I Assay (Ortho Clinical Diagnostics, Marlow, UK) is designed for use in a laboratory setting on the following analysers: VITROS® ECi/ECiQ/3600 Immunodiagnostic Systems (Ortho Clinical Diagnostics, Marlow, UK) and the VITROS® 5600/XT 7600 Integrated System (Ortho Clinical Diagnostics, Marlow, UK). It is an immunometric immunoassay and is intended for the in vitro quantitative determination of troponin I in serum and plasma samples. Test results are available in 15 minutes. It has a recommended 99th centile cut-off point of 11 ng/l for both lithium heparin and serum samples. Sex-specific 99th centile cut-off points of 9 ng/l (in lithium heparin and serum) for females and 13 ng/l (in lithium heparin) and 12 ng/l (in serum) for males are provided. 22 The assay can detect troponin I in > 50% of the reference population. The assay is CE marked and available to the NHS.
TriageTrue High Sensitivity Troponin I Test (Quidel)
The TriageTrue High Sensitivity Troponin I Test (Quidel, San Diego, CA, USA) can be used in a near-patient setting (i.e. the point of care) or in a laboratory with the Triage MeterPro analyser Quidel, San Diego, CA, USA). It is a fluorescence immunoassay and is intended for the in vitro quantitative determination of troponin I in ethylenediaminetetraacetic acid, anticoagulated whole blood and plasma samples. Test results are available in < 20 minutes. It has a recommended 99th centile cut-off point of 20.5 ng/l with a CoV of < 10%. Sex-specific 99th centile cut-off points of 14.4 ng/l for females and 25.7 ng/l for males are provided. 23 The test can detect troponin I in > 50% of the reference population. The test is CE marked and available to the NHS.
Elecsys Troponin-T high sensitive assay (Roche)
The Elecsys Troponin-T high sensitive assay and Elecsys cTnT-hs STAT assay can be used on the cobas e 411, e 601, e 602 and e 801 analysers (Roche, Basel, Switzerland). The assay is a quantitative, sandwich electrochemiluminescence immunoassay for serum and plasma samples. Results are available within 18 minutes with the standard assay and within 9 minutes if the STAT assay is used. Both versions of the assay can detect cTnT in 57% of the reference population and have a recommended 99th centile cut-off point of 14 ng/l with a CoV of < 10%. 24–26 Both versions of the assay are CE marked and available to the NHS.
ADVIA Centaur High-Sensitivity Troponin I assay (Siemens Healthcare)
The ADVIA Centaur® High-Sensitivity Troponin I assay (Siemens Healthcare, Erlangen, Germany) can be used with the ADVIA Centaur XP and ADVIA Centaur XPT analysers (Siemens Healthcare, Erlangen, Germany). It is a magnetic latex particle chemiluminescent immunoassay and is intended for the in vitro quantitative determination of cTnI in serum and plasma samples. Test results are available within 18 minutes. The assay has a recommended 99th centile cut-off point of 47.34 ng/l for the whole population in lithium heparin samples and of 46.47 ng/l in serum samples. 27 Sex-specific cut-off points of 36.99 ng/l for females and 57.27 ng/l for males are also recommended. 27 Each 99th centile has a CoV of < 10%. The assay can detect cTnI in > 50% of the reference population. The assay is CE marked and available to the NHS.
Atellica IM High-Sensitivity Troponin I (Siemens Healthcare)
The Atellica® IM High-Sensitivity Troponin I assay (Siemens Healthcare, Erlangen, Germany) can only be used with the Atellica® IM analyser (Siemens Healthcare, Erlangen, Germany). It is a magnetic latex particle chemiluminescent immunoassay and is intended for the in vitro quantitative determination of cTnI in serum and plasma samples. Test results are available within 10 minutes. The assay has a recommended 99th centile cut-off point of 45.2 ng/l for lithium heparin samples and 45.43 ng/l for serum samples. Each 99th centile has a CoV of < 10%. 28 The assay can detect cTnI in > 50% of the reference population. The assay is CE marked and available to the NHS.
Dimension® EXL™ hs-cTnI (Siemens Healthcare)
The Dimension® EXL™ hs-cTnI (Siemens Healthcare, Erlangen, Germany) assay is designed for use in a laboratory setting with the Dimension EXL analyser (Siemens Healthcare, Erlangen, Germany). It is a magnetic latex particle chemiluminescent immunoassay and is intended for the in vitro quantitative determination of troponin I in serum and plasma samples. Test results are available in 18 minutes. It has a recommended 99th centile cut-off point of 60.4 ng/l for lithium heparin and 58.2 ng/l for serum. 29 Sex-specific 99th centile cut-off points of 51.4 ng/l for females and 76.2 ng/l for males in lithium heparin and 47.8 ng/l for females and 71.8 ng/l for males in serum are provided. 29 Each 99th centile has a CoV of < 10%. The assay can detect troponin I in > 50% of the reference population. The assay is CE marked and available to the NHS.
Dimension Vista High-Sensitivity Troponin I assay (Siemens Healthcare)
The Dimension Vista® High-Sensitivity Troponin I assay (Siemens Healthcare, Erlangen, Germany) is designed for use in a laboratory setting with the Dimension Vista analysers (Siemens Healthcare, Erlangen, Germany). It is a magnetic latex particle chemiluminescent immunoassay and is intended for the in vitro quantitative determination of cTnI in serum and plasma samples. Test results are available within 10 minutes. The assay has a recommended 99th centile cut-off point of 58.9 ng/l for lithium heparin samples and 57.9 ng/l for serum samples. 30 Sex-specific 99th centile cut-off points of 53.77 ng/l for females and 78.5 ng/l for males are also recommended. 30 Each 99th centile has a CoV of < 10%. The assay can detect cTnI in > 50% of the reference population. The assay is CE marked and available to the NHS.
A summary of the product properties of hs-cTnI and hs-cTnT assays available in the NHS in England and Wales is provided in Table 1.
| Manufacturer | System and compatible analysers | Assay | 99th centile (ng/l) | CoV at 99th centile (%) | Proportion of reference population in which cTn is detected (%) | Turnaround time (minutes) | LoD (ng/l) | LoQ (ng/l) |
|---|---|---|---|---|---|---|---|---|
| Abbott Diagnostics | ARCHITECT i1000sr and i2000sr | ARCHITECT hs-cTnI18 |
Overall: 26.2 Female: 15.6 Male: 34.2 |
Overall: 4.0 Female: 5.3 Male: 3.5 |
9631 | 18a | 1.9 | 4.7 (10% CoV); 1.3 (20% CoV) |
| Abbott Diagnostics | Alinity i | Alinity hs-cTnI19 |
Overall: 26.2 Female: 15.6 Male: 34.2 |
Overall: 4.6 Female: 5.0 Male: 4.5 |
9631 | 18a | 1.6 | 3.7 (10% CoV); 2.1 (20% CoV) |
| Beckman Coulter | Access 2, DxI 600/800, DxC 600i/880i/860i/680i/660i | Access hs-cTnI20 |
Lithium heparin – Overall: 17.5 Female: 11.6 Male: 19.8 |
Lithium heparin – Overall: 3.7 Female: 4.2 Male: 3.6 |
> 50 | 17a | 2.3 | 2.3 |
|
Serum – Overall: 18.2 Female: 11.8 Male: 19.7 |
Serum – Overall: 6.0 Female: 6.9 Male: 5.8 |
|||||||
| bioMérieux | VIDAS, MINI VIDAS, VIDAS 3 | VIDAS hs-cTnIa |
Overall: 19 Female: 11 Male: 25 |
20 | ||||
| Ortho Clinical Diagnostics | VITROS ECi/ECiQ/3600 Immunodiagnostic Systems and the VITROS 5600/XT 7600 Integrated System | VITROS hs-cTnI22 |
Lithium heparin – Overall: 11 Female: 9 Male: 13 |
≤ 10a | > 50 | 15a | 0.39–0.86 | 1.23 |
|
Serum – Overall: 11 Female: 9 Male: 12 |
||||||||
| Quidel | Triage MeterPro | TriageTrue hs-cTnI23 |
Overall: 20.5 Female: 14.4 Male: 25.7 |
Overall: < 10 | > 50 | < 20a | Plasma: 1.6 | Plasma: 8.4 (10% CoV); 3.6 (20% CoV) |
| Whole blood: 1.9 | Whole blood: 6.2 (10% CoV); 2.8 (20% CoV) | |||||||
| Roche |
200 test pack: cobas e 411, e 601, e 602 300 test pack: cobas e 801 |
Elecsys hs-cTnT24,25 |
Overall: 14 Female: 9 Male: 16.8 |
< 10 | 57 | 18 |
3 (cobas e 801) 5 (all others) |
2.97–6.60 |
| Roche |
100 test pack: cobas e 411, e 601, e 602, 300 test pack: cobas e 801 |
Elecsys hs TnT STAT26 |
Overall: 14 Female: 9 Male: 16.8 |
< 10 | 57 | 9 |
3 (cobas e 801) 5 (all others) |
13 |
| Siemens Healthcare | Atellica | Atellica IM hs-cTnI28 |
Lithium heparin – Overall: 45.2 Female: 34.11 Male: 53.48 |
< 4 | 75 | 10 | 1.6 | 2.5 |
|
Serum – Overall: 45.43 Female: 38.64 Male: 53.53 |
||||||||
| Siemens Healthcare | Dimension EXL | Dimension EXL hs-cTnI29 |
Lithium heparin – Overall: 60.4 Female: 51.4 Male: 76.2 |
< 5 | > 50 | 10 | 2.7 | 4.0 |
|
Serum – Overall: 58.2 Female: 47.8 Male: 71.8 |
||||||||
| Siemens Healthcare | Dimension Vista | Dimension Vista hs-cTnI30 |
Lithium heparin – Overall: 58.9 Female: 53.7 Male: 78.5 |
< 5 | > 50 | 10 | 2.0 | 3.0 |
|
Serum – Overall: 57.9 Female: 51.1 Male: 74.9 |
||||||||
| Siemens Healthcare | ADVIA Centaur XP and ADVIA Centaur XPT | ADVIA Centaur hs-cTnI27 |
Lithium heparin – Overall: 47.34 Female: 36.99 Male: 57.27 |
< 4.9 | 63 | 18 | 1.6 | 2.5 (20% CoV) |
|
Serum – Overall: 46.47 Female: 39.59 Male: 58.05 |
This assessment considers hs-cTn assays used singly or in series, up to 3 hours after the onset of chest pain or up to 3 hours after presentation (as reported) for serial troponin measurements. Data for both relative and absolute change in troponin levels and peak troponin are presented.
Comparator
The comparator for this technology appraisal is serial troponin T and/or I testing (using any method not defined as a hs-cTn test) on admission and at 10–12 hours after the onset of symptoms, as used in our previous diagnostic assessment report (DAR),2 conducted to support the development of DG15. 13
Care pathway
Diagnostic assessment
The assessment of patients with suspected ACS is described in NICE CG95. 11 This has been updated since the publication of DG1513 to include recommendations on the use of high-sensitivity troponin assays. 14 The guideline specifies that initial assessment should include a resting 12-lead ECG, along with a clinical history, a physical examination and biochemical marker analysis. For people in whom a regional ST segment elevation or presumed new left branch bundle block is seen on the ECG, management should follow NICE CG167. 32 People without persistent ST elevation changes on the ECG [i.e. those with non-ST segment elevation acute coronary syndrome (NSTE-ACS)] should receive further investigation using cardiac biomarkers, with the aim of distinguishing NSTEMI from UA. NICE CG95 makes the following recommendations on the use of cardiac biomarkers. 14
-
Do not use high-sensitivity troponin tests for people in whom ACS is not suspected.
-
For people at high or moderate risk of MI (as indicated by a validated tool), perform high-sensitivity troponin tests, as recommended in the NICE diagnostics guidance on MI (DG15).
-
For people at low risk of MI (as indicated by a validated tool):
-
perform a second high-sensitivity troponin test, as recommended in the NICE diagnostics guidance on MI (DG15), if the first troponin test at presentation is positive
-
consider performing a single high-sensitivity troponin test at presentation to rule out NSTEMI if the first troponin test is below the lower LoD (i.e. negative).
-
-
Ensure that patients understand that a detectable troponin on the first high-sensitivity test does not necessarily indicate that they have had an MI. Do not use biochemical markers, such as natriuretic peptides and high-sensitivity C-reactive protein, to diagnose an ACS.
-
Do not use biochemical markers of myocardial ischaemia (such as ischaemia-modified albumin) as opposed to markers of necrosis when assessing people with acute chest pain.
-
When interpreting high-sensitivity troponin measurements, take into account:
-
the clinical presentation
-
the time from onset of symptoms
-
the resting 12-lead ECG findings
-
the pre-test probability of NSTEMI
-
the length of time since the suspected ACS
-
the probability of chronically elevated troponin levels in some people
-
that 99th centile thresholds for troponin I and T may differ between sexes.
-
Clinical guideline 95 recommends that a diagnosis of NSTEMI should be made using the universal definition of MI, which states that AMI is defined as a change in cardiac biomarker concentration and at least one cardiac biomarker concentration value above the 99th centile for the reference population, accompanied by symptoms of ischemia, an abnormal ECG, evidence of myocardial damage on imaging, or an intracoronary thrombus identified by angiography or at autopsy. 11
The Scottish Intercollegiate Guidelines Network guideline 148 provides the following recommendations in relation to cTns. 15
-
In patients with suspected ACS, serum troponin concentration should be measured at presentation to guide appropriate management and treatment.
-
Serum troponin concentration should be measured 12 hours from the onset of symptoms to establish a diagnosis of MI.
-
In patients with suspected ACS, measurement of cTn at presentation and at 3 hours after presentation with a high-sensitivity assay should be considered as an alternative to serial measurement over 10–12 hours with a standard troponin assay to rule out MI.
-
Sex-specific thresholds of cTn should be used for the diagnosis of MI in men and women.
Guidelines from the European Society of Cardiology (ESC) on the management of ACS in patients presenting without persistent ST segment elevation recommend that ‘measurement of cardiac troponins with sensitive or high-sensitivity assays to obtain results within 60 minutes’. 33 The guideline also describes 0/1- and 0/3-hour rule-out algorithms, which incorporate both high-sensitivity troponin assays and clinical risk scores. 33 For the 0/1-hour algorithm, additional troponin testing after 3–6 hours is recommended if the first two measurements are inconclusive and the clinical condition is still suggestive of ACS. 33
The guideline from the American College of Cardiology (ACC)/American Heart Association (AHA) on the management of patients with NSTE-ACS does not include any specific recommendations about the use of high-sensitivity troponin assays. 34 However, the guideline does note that ‘For patients with a TIMI [thrombolysis in myocardial infarction] risk score of 0 and normal high-sensitivity cardiac troponin 2 hours after presentation, accelerated diagnostic protocols have been developed that predict a very low rate of 30-day MACE’. 34
The 2017 publication Asia-Pacific Consensus Statement on the Optimal Use of High-Sensitivity Troponin Assays in Acute Coronary Syndromes Diagnosis: Focus on hs-cTnI makes nine recommendations. 35
-
Troponin is the preferred cardiac biomarker for diagnostic assessment of ACS and is indicated for patients with symptoms of possible ACS.
-
hs-cTn assays are recommended.
-
Serial testing is required for all patients.
-
Testing should be performed at presentation and 3 hours later.
-
Sex-specific cut-off point values should be used for hs-cTnI assays.
-
A hs-cTnI level > 10 times the upper limit of normal should be considered to ‘rule in’ a diagnosis of ACS.
-
Dynamic change > 50% in hs-cTnI level from presentation to 3-hour retest identifies patients at high risk for ACS.
-
When only point-of-care testing is available, patients with elevated readings should be considered at high risk, whereas patients with low/undetectable readings should be retested after 6 hours or sent for laboratory testing.
-
Regular education on the appropriate use of troponin tests is essential.
The rapidly expanding evidence base on hs-cTns, together with their increasing uptake and inclusion in CGs, means that an update to the NICE diagnostics guidance on early rule out of AMI using high-sensitivity troponin tests (DG15), published in October 2014,13 is now considered necessary.
Management/treatment
The NICE CG9436 provides recommendations on the management of people with suspected NSTE-ACS. The guideline states that initial treatment should include a combination of antiplatelet (e.g. aspirin, clopidogrel and glycoprotein IIb/IIIa inhibitors) and antithrombin therapy, and should take into account contraindications, risk factors and the likelihood of percutaneous coronary intervention. The following NICE guidelines are being combined and updated: Unstable Angina and NSTEMI: The Early Management of Unstable Angine and Non-ST-Segment-Elevation Myocardial Infarction (CG94),36 Myocardial Infarction: Cardiac Rehabilitation and Prevention of Further Cardiovascular Disease (CG172)37 and Myocardial Infarction with ST-segment Elevation: The Acute Management of Myocardial Infarction with ST-Segment Elevation (CG167). 32 The new guideline will be titled Acute Coronary Syndromes when published, and publication is expected in November 2020.
Longer-term follow-up of people who have had an AMI is described in full in NICE CG48 Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction. 38 This includes recommendations on lifestyle changes, cardiac rehabilitation programmes, drug therapy (including a combination of angiotensin-converting enzyme inhibitors, aspirin, beta-blockers and statins) and further cardiological assessment to determine whether or not coronary revascularisation is required. 38
A list of NICE guideline documents relevant to the management of suspected ACS is provided in Appendix 9.
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE Diagnostic Assessment process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
In addition, text in this chapter has been reproduced from Westwood et al. ,2 which contains information licensed under the Non-Commercial Government Licence v2.0.
Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care,39 the NICE Diagnostics Assessment Programme Manual40 and the Cochrane Handbook for DTA Reviews. 41 A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for this review is provided in Appendix 10. All data for studies included in our previous DAR,2 conducted to support the development of DG15,13 were taken directly from that report.
Systematic review methods
Search strategy
Search strategies utilised in the original report2 were updated with any new interventions identified in the NICE scope. Search strategies were based on the intervention (i.e. high-sensitivity troponin assays) and target condition, as recommended in the CRD’s guidance for undertaking reviews in health care39 and the Cochrane Handbook for DTA Reviews. 41
Search strategies were developed specifically for each database and the keywords associated with hs-cTnT or hs-cTnI were adapted according to the configuration of each database. No language restrictions were applied.
The following databases were searched between 20 September 2019 and 26 September 2019 for relevant studies from 2013 to the present:
-
MEDLINE ALL (Ovid) – 1946 to 24 September 2019
-
EMBASE (Ovid) – 1974 to 25 September 2019
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley) – Issue 9/September 2019
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley) – Issue 9/September 2019
-
Database of Abstracts of Reviews of Effects (DARE) (CRD) – up to March 2015
-
Health Technology Assessment (HTA) database (CRD) – up to March 2018
-
Science Citation Index (SCI) (Web of Science) – 1988 to 24 September 2019
-
Conference Proceedings Citation Index – Science (CPCI-S) (Web of Science) – 1990 to 24 September 2019
-
Latin American and Caribbean Health Sciences Literature (LILACS) (internet) – 2013 to 20 September 2019
-
National Institute for Health Research HTA programme (internet) – up to 26 September 2019
-
PROSPERO (International Prospective Register of Systematic Reviews) (internet) – up to 20 September 2019.
Completed and ongoing trials were identified by searches of the following resources (2013–present):
-
National Institutes of Health ClinicalTrials.gov (URL: www.clinicaltrials.gov/) – first posted from 1 January 2013 to 31 December 2019.
-
World Health Organization International Clinical Trials Registry Platform (URL: www.who.int/ictrp/en/) – date of registration 1 January 2013 to 25 September 2019.
The following key conference proceedings are indexed in EMBASE and so will be covered in the EMBASE search detailed above:
-
AHA Scientific Sessions.
-
American Association for Clinical Chemistry.
-
ESC.
The following conference abstracts were manually searched to compliment those conference abstracts indexed in EMBASE:
-
American Association for Clinical Chemistry (2018, 2019).
-
AHA Scientific Sessions 2017–19.
-
ESC 2019.
References in retrieved articles and relevant systematic reviews were checked.
Searches took into account generic and other product names for the intervention. All search strategies are provided in Appendix 1. The main EMBASE strategy was independently peer reviewed by a second information specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) peer review checklist. 42
Inclusion and exclusion criteria
Inclusion criteria for each of the clinical effectiveness questions are summarised in Table 2. Studies that fulfilled these criteria were eligible for inclusion in the review. Studies that were included in our previous DAR,2 conducted to support the development of DG15,13 were also included in this review.
| Question | What is the diagnostic performance of hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the early rule out of NSTEMI in adults with acute chest pain? | What is the accuracy of hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the prediction of a MACE (e.g. cardiac death, non-fatal MI, revascularisation or hospitalisation for myocardial ischaemia) during 30-day follow-up in adults with acute chest pain? | What is the effectiveness of hs-cTn assays (used singly or in series) compared with conventional diagnostic assessment for achieving successful early discharge of adults with acute chest pain within 4 hours of presentation? |
| Participants | Adults (aged ≥ 18 years) presenting with acute ‘pain, discomfort or pressure in the chest, epigastrium, neck, jaw, or upper limb without an apparent non-cardiac source’34 due to a suspected, but not proven, AMI | ||
| Setting | Secondary or tertiary care | ||
| Interventions (index test) | Any hs-cTnT or hs-cTnI test,a listed in Table 1, or hs-cTn assays (used singly or in series,b such that results were available within 3 hours of presentation) | ||
| Comparators | Any other hs-cTn test or test sequence, as specified above, or no comparator | Troponin T or I measurement on presentation and 10–12 hours after the onset of symptoms | |
| Reference standard | The third or fourth universal definition of AMI,43 including measurement of troponin T or I (using any method) on presentation and 3–6 hours later, or occurrence of a MACE (any definition used in identified studies) during 30-day follow-up | Not applicable | |
| Outcomesc | Test accuracy (i.e. the numbers of TP, FN, FP and TN test results) | Early discharge (i.e. ≤ 4 hours after initial presentation) without a MACE during follow-up; incidence of a MACE during follow-up; reattendance at or readmission to hospital during follow-up; time to discharge; patient satisfaction or HRQoL measures | |
| Study design | Diagnostic cohort studies | RCTs (CCTs will be considered if no RCTs are identified) | |
Inclusion screening and data extraction
Two out of three reviewers (MW, DF and GW) independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant were obtained and the same two reviewers independently assessed these for inclusion. Any disagreements were resolved by consensus. Details of studies excluded at the full-paper screening stage are presented in Appendix 5.
Studies cited in materials provided by the manufacturers of hs-cTn assays were first checked against the project reference database (in EndNote X8, Clarivate Analytics, Philadelphia, PA, USA) and any studies not already identified by our searches were screened for inclusion following the process described above.
The following data were extracted: study details, inclusion and exclusion criteria, participant characteristics (demographic characteristics and cardiac risk factors), target condition (NSTEMI or AMI), details of the hs-cTnT or hs-cTnI test strategy (manufacturer, number and timing of tests, and definition of positive diagnostic threshold), details of reference standard [manufacturer, timing, diagnostic threshold for conventional troponin T or I testing, clinical and imaging components of the reference standard, method of adjudication (e.g. two independent clinicians)], incidence of a MACE during 30-day follow-up and test performance outcome measures [numbers of true-positive (TP), false-positive (FP), false-negative (FN) and true-negative (TN) test results]. When studies reported data for the development and validation of hs-cTn test strategy, data were extracted for the validation cohort only. Data were extracted by one reviewer, using the data extraction forms from the original systematic review. 2 A second reviewer checked data extraction and any disagreements were resolved by consensus or discussion with a third reviewer. Full data extraction tables are provided in Appendix 2.
Quality assessment
The methodological quality of included randomised controlled trials (RCTs) was assessed using the revised Cochrane Risk-of-Bias Tool for Randomised Trials. 44 The methodological quality of included diagnostic test accuracy (DTA) studies that evaluated a single hs-cTn assay for the target conditions NSTEMI, AMI or MACEs was assessed using QUADAS-2. 45 Studies that provided data for two or more hs-cTn assays were assessed using QUADAS-2C,46 a version of the QUADAS tool that has been developed specifically for the assessment of comparative DTA studies (this tool is currently undergoing piloting and is not yet published). Quality assessments were undertaken by one reviewer and checked by a second (MW, DF and GW). Any disagreements were resolved by consensus.
The results of the quality assessments are summarised and presented in Tables 4–6 and are presented in full, by study, in Appendices 3 and 4.
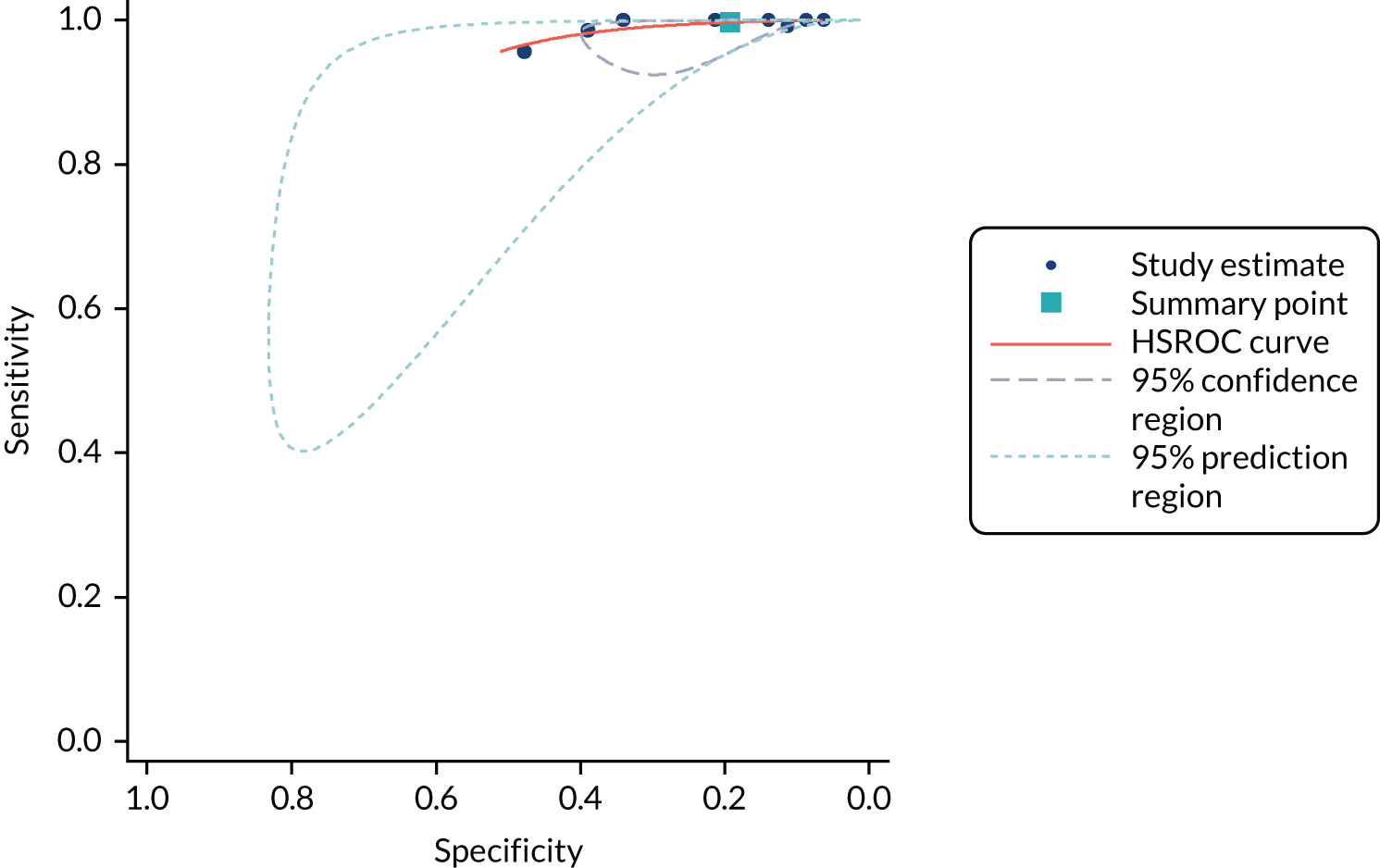
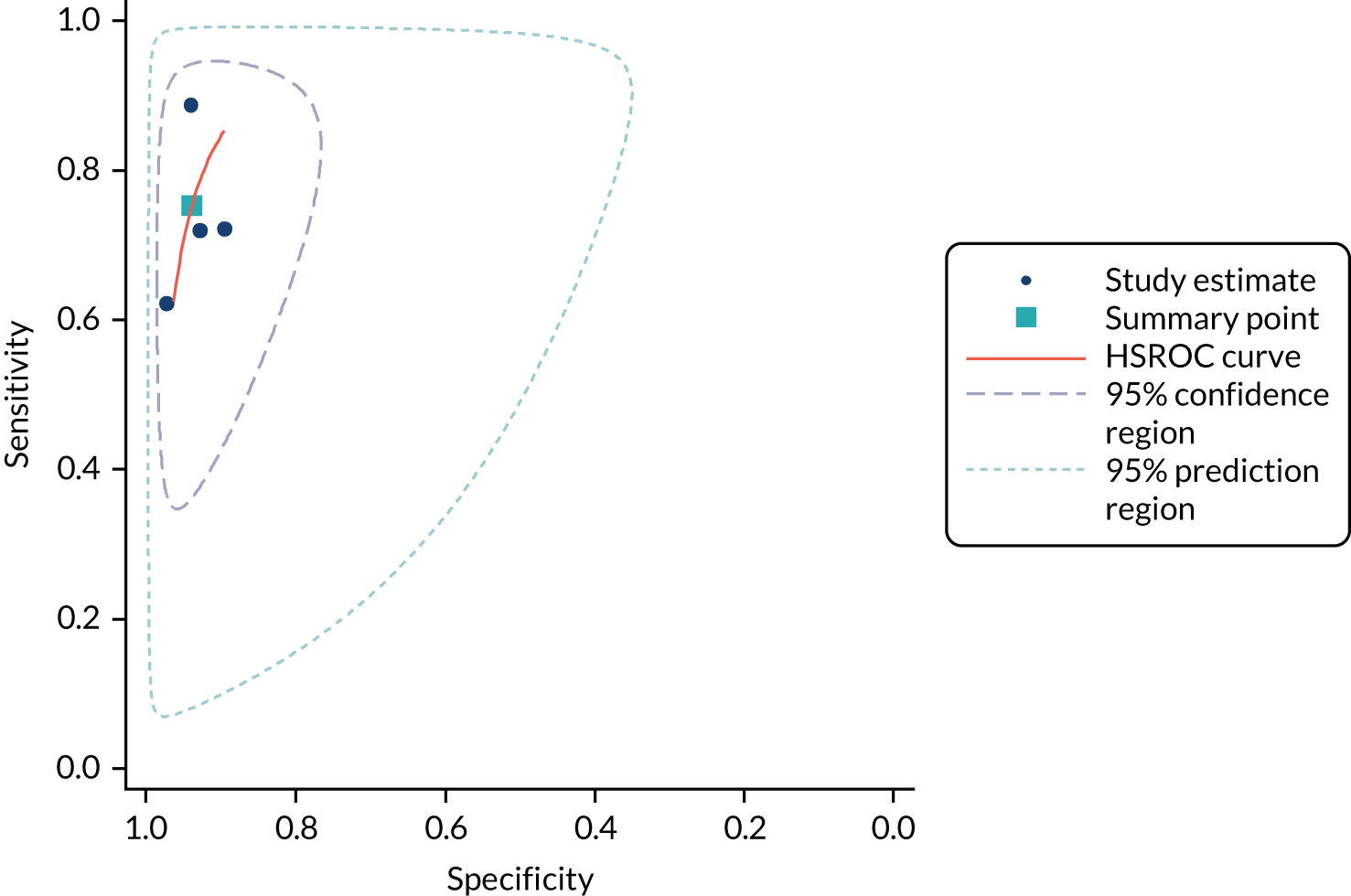
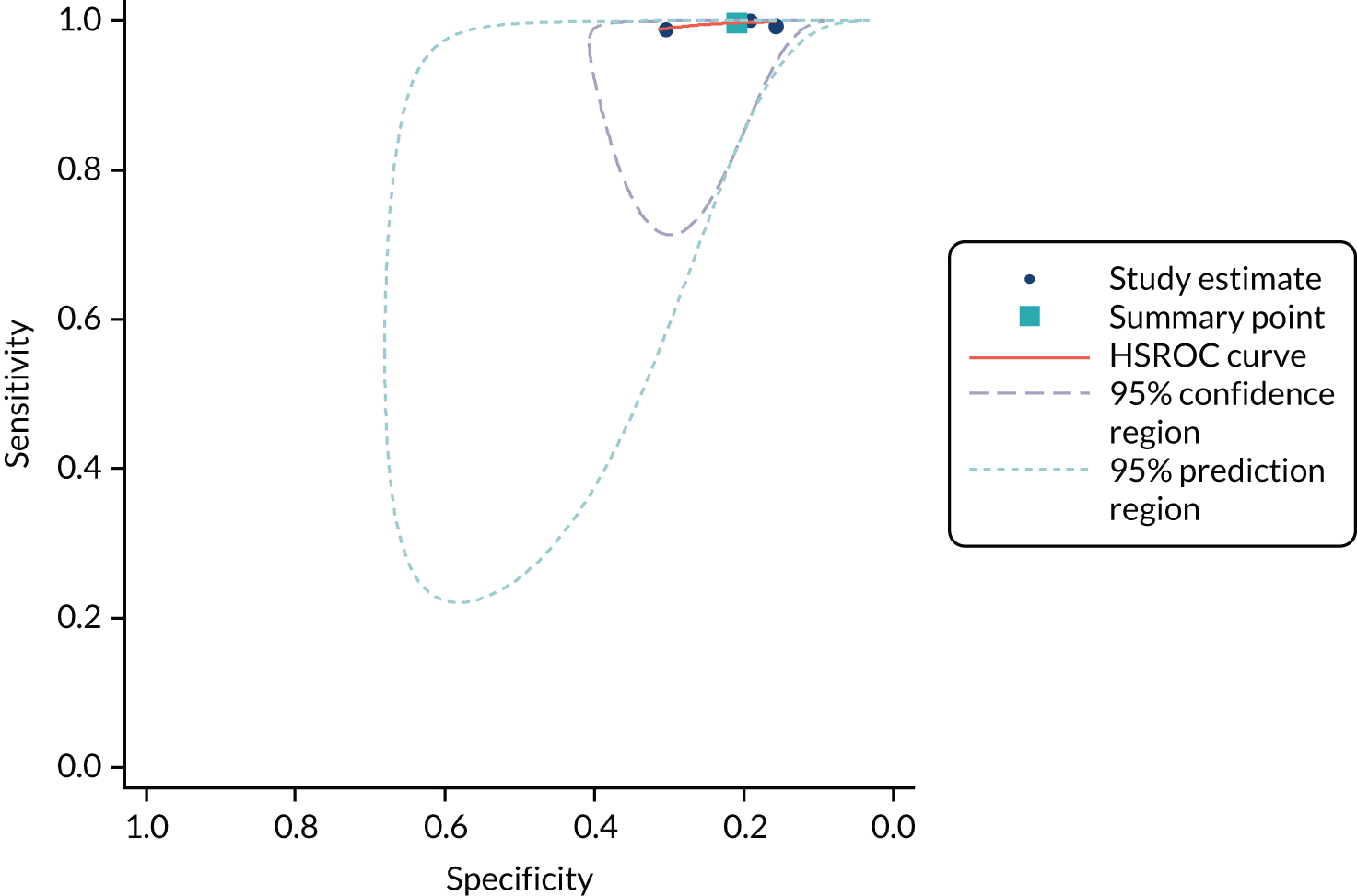
Methods of analysis/synthesis
Sensitivity and specificity were calculated for each set of 2 × 2 data and plotted in receiver operating characteristic (ROC) space. The hierarchical summary receiver operating characteristic (SROC) model was used to estimate summary sensitivity and specificity with 95% confidence intervals (CIs) and prediction regions around the summary points, and to plot hierarchical SROC curves. Pooled results were obtained only from meta-analyses involving four or more studies. 47–49 This approach allows for between-study heterogeneity in sensitivity and specificity, and for the trade-off (negative correlation) between sensitivity and specificity commonly seen in diagnostic meta-analyses. For meta-analyses with fewer than four studies, we estimated separate pooled estimates of sensitivity and specificity using random-effects logistic regression. 50 Heterogeneity was assessed visually using SROC plots and assessed statistically using the variance of logit (sensitivity) and logit (specificity), where ‘logit’ indicates the logistic function (the smaller these values were, the less heterogeneity there was between studies). Analyses were performed in Stata 13 (StataCorp LP, College Station, TX, USA), mainly using the metandi command. For analyses with fewer than four studies, we used MetaDisc. 51
Analyses were conducted separately for each hs-cTn assay. Analyses were stratified according to target condition (e.g. NSTEMI, any AMI or 30-day MACE), timing of collection of blood sample for testing and the threshold used to define a positive hs-cTn result. Stratified analyses were conducted for all time points and thresholds for which sufficient data were available.
When possible, we compared the accuracy of the included hs-cTn assays by tabulating the summary estimates from analyses for common time points and thresholds assessed for multiple assays.
Results of the assessment of clinical effectiveness assessment
The literature searches of bibliographic databases conducted for this update identified 9379 new references. After the initial screening of titles and abstracts, 212 papers were considered potentially relevant and were ordered for full-paper screening. Of these, one study52 could not be obtained from The British Library and 80 were included in the review. 53–132 In addition, 37 publications, taken from the assessment report conducted for DG15,2 were carried forward and included in this review. 133–169 All potentially relevant studies cited in documents supplied by the test manufacturers had already been identified by bibliographic database searches. Four additional publications, not identified because their publication post-dated our searches,170–173 and two further studies, which were unpublished at that time,174,175 were provided (academic in confidence) by specialist committee members. Figure 1 shows the flow of studies through the review process and Appendix 5 provides details, with reasons for exclusions, of all publications excluded at the full-paper screening stage.
FIGURE 1.
Flow of studies through the review process.
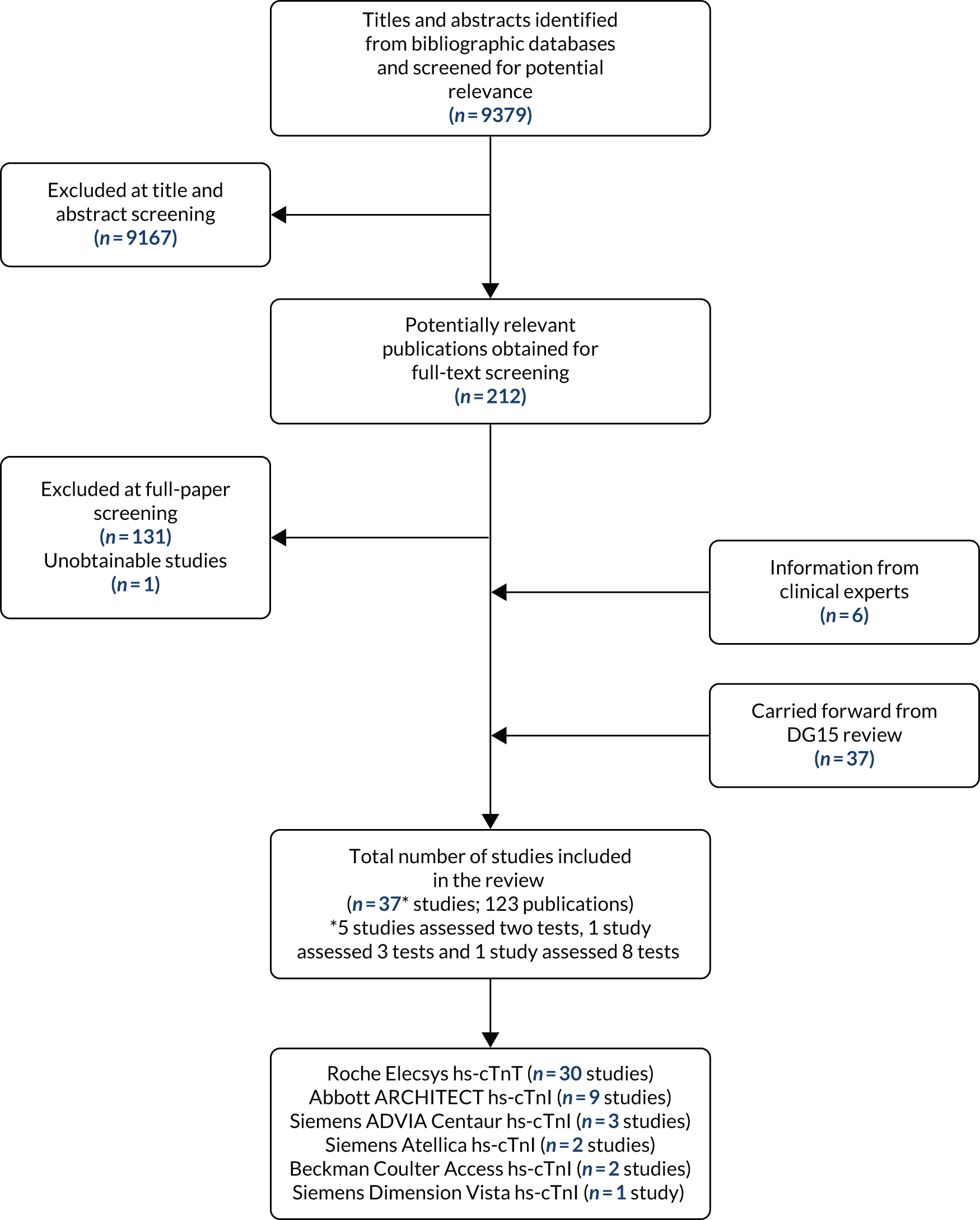
Overview of included studies
Based on the update searches and inclusion screening described above and information taken from the assessment report conducted for DG15,2 a total of 123 publications53–175 of 37 studies56,58,61,62,64,68,72,80,84,87–89,96,100–102,110,115,117,121,133,135,137,139,141,142,144,147,148,150,157,159,161,165,171,175,176 were included in the review. The results section of this report cites studies using the primary publication and, where this is different, the publication in which the referenced data were reported. Thirty studies reported accuracy data for the Roche Elecsys hs-cTnT assay,56,58,61,62,64,68,72,80,87–89,100–102,115,117,121,133,135,137,139,142,144,147,148,150,157,159,161,165 nine studies reported accuracy data for the Abbott ARCHITECT hs-cTnI assay,58,61,64,68,84,96,101,110,141 two studies reported accuracy data for Siemens Healthcare Atellica hs-cTnI,61,176 three studies reported accuracy data for Siemens Healthcare ADVIA Centaur hs-cTnI,58,115,176 two studies reported accuracy data for Beckman Coulter ACCESS hs-cTnI58,171 and one study reported accuracy data for each of Siemens Healthcare Dimension Vista hs-cTnI,58 Ortho VITROS hs-cTnI,58 bioMérieux VIDAS hs-cTnI58 and Quidel Cardiovascular TriageTrue hs-cTnI. 58 Seven studies reported accuracy data for more than one assay. 58,61,64,68,101,115,176
We did not identify any studies of the Abbott Alinity hs-cTnI and the Siemens Healthcare Dimension EXL hs-cTnI, which also met the inclusion criteria for this review. The High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome (High-STEACS) trial,61 which contributed multiple diagnostic accuracy data sets, was a stepped-wedge cluster RCT that evaluated implementation of an early rule-out pathway in hospitals in Scotland. This trial assessed rates of reclassification of patients, and subsequent incidence of MI and cardiovascular death when hs-cTnI results were made available for patients previously classified based on cTnI results (these results have been included). 99 A second stepped-wedge cluster RCT, the High-Sensitivity Cardiac Troponin On Presentation to Rule Out Myocardial Infarction (HiSTORIC) trial (unpublished report provided AiC),175 evaluated the implementation of an early rule-out pathway in hospitals in Scotland. The primary outcomes were length of stay, and MI or cardiac death after discharge (at 30 days). Publications reporting new data were identified for three of the studies included in the assessment report conducted for DG15:2 ADAPT (2-Hour Accelerated Diagnostic Protocol to Assess Patients With Chest Pain Symptoms Using Contemporary Troponins as the Only Biomarker),68 APACE (Advantageous Predictors of Acute Coronary Syndromes Evaluation)58 and QUART (QUeensland Accelerated Risk Trial). 88 Table 3 provides a summary of the included studies and related publications.
| Study | Country(s) | n | Target condition(s) reported | Subgroup(s) reported |
|---|---|---|---|---|
| Abbott ARCHITECT hs-cTnI | ||||
| BACC | Germany | 1040 | NSTEMI | None |
| aNeumann et al. 201684 | ||||
| Neumann et al. 201785 | ||||
| Neumann et al. 201786 | ||||
| a,bKeller et al. 2011141 | Germany | 1818 | AMI | None |
| bKeller et al. 2011163 | ||||
| UTROPIA | USA | 1631 | NSTEMI | |
| Dodd et al. 2019125 | ||||
| Sandoval et al. 201795 | ||||
| aSandoval et al. 201796 | ||||
| Venge et al. 2017 110 | Germany, France Austria and the Netherlands | 450 | AMI | None |
| Abbott Alinity hs-cTnI | ||||
| No studies identified | ||||
| Beckman Coulter ACCESS hs-cTnI | ||||
| ADAPT/IMPACT | Australia | 1280 | NSTEMI | None |
| Nestelberger et al. 2019171 | ||||
| Siemens Healthcare Dimension EXL hs-cTnI | ||||
| No studies identified | ||||
| Roche Elecsys hs-cTnT | ||||
| a,bAldous et al. 2012139 | New Zealand | 939 | NSTEMI; AMI | None |
| b Aldous et al. 2012 134 | ||||
| b Aldous et al. 2011 143 | ||||
| b Aldous et al. 2011 147 | New Zealand | 382 | AMI | None |
| bAldous et al. 2011162 | ||||
| bAldous et al. 2010155 | ||||
| a,bBody et al. 2011161 | UK | 703 | AMI | None |
| bBody et al. 2011153 | ||||
| bBody et al. 2010169 | ||||
| Body et al. 2015 56 | UK | 463 | AMI; 30-day MACE | None |
| Cappellini et al. 2019 62 | Italy | 3318 | NSTEMI | Sex |
| b Christ et al. 2010 150 | Germany | 137 | AMI | None |
| CORE | Sweden | 1138 | 30-day MACE | |
| Borna et al. 2018116 | ||||
| Mokhtari et al 2016119 | ||||
| aMokhtari et al. 2016121 | ||||
| Mokhtari et al 2017120 | ||||
| FASTER I and FAST II | Sweden | 360 | NSTEMI | None |
| bEggers et al. 2012137 | ||||
| a,b Freund et al. 2011142 | France | 317 | AMI | Low/moderate vs. high pre-test probability |
| bFreund et al. 2010166 | ||||
| a Huang et al. 2015 72 | China | 3458 | AMI | Renal function |
| Guangquan et al. 201673 | ||||
| b Kurz et al. 2011 148 | Germany | 94 | NSTEMI | None |
| Lin et al. 2019 117 | Singapore | 2444 | 30-day MACE | None |
| a,bMelki et al. 2011144 | Sweden | 233 | NSTEMI | None |
| bMelki et al. 2010154 | ||||
| a Peacock et al. 2018 89 | USA | 1600 | AMI | None |
| Chang et al. 2018 124 | ||||
| PITAGORAS | Spain | 446 | NSTEMI; 30-day MACE | None |
| b Sanchis et al. 2012 135 | ||||
| QUART | Australia | 764 | AMI | None |
| bParsonage et al. 2013151 | ||||
| Parsonage et al. 2013131 | ||||
| aParsonage et al. 201488 | ||||
| RATPAC (point-of-care arm) | UK | 850 | NSTEMI; 30-day MACE | None |
| a,bCollinson et al. 2013159 | ||||
| bCollinson et al. 2012164 | ||||
| bCollinson et al. 2012152 | ||||
| REACTION-US | USA | 569 | NSTEMI | None |
| aNowak 201887 | ||||
| Nowak 2018127 | ||||
| b Saenger et al. 2010 165 | USA | 288 | AMI | None |
| b Sebbane et al. 2013 157 | France | 248 | NSTEMI | None |
| Shiozaki et al. 2017 100 | Japan | 413 | NSTEMI | None |
| Slagman et al. 2017 102 | Germany | 3423 | NSTEMI | None |
| TRAPID-AMI | 1282 | NSTEMI; AMI; 30-day MACE | Sex and age (< 65 years vs. ≥ 65 years) | |
| Body et al. 2015122 | ||||
| Body et al. 2016114 | ||||
| McCord et al. 2017126 | ||||
| aMueller et al. 201680 | ||||
| Mueller-Hennessen et al. 201681 | ||||
| Mueller-Hennessen et al. 201782 | ||||
| Mueller-Hennessen et al. 201983 | ||||
| TUSCA | Spain | 358 | NSTEMI | None |
| bSantaló 2013133 | ||||
| Abbott ARCHITECT hs-cTnI and Roche Elecsys hs-cTnT | ||||
| ADAPT | Australia and New Zealand | NSTEMI; AMI; 30-day MACE | None | |
| Aldous et al. 201453 | ||||
| Boeddinghaus et al. 201657 | ||||
| bCullen et al. 2013156 | ||||
| aCullen et al. 201468 | ||||
| Eggers et al. 201669 | ||||
| Greenslade et al. 201571 | ||||
| Meller et al. 2015118 | ||||
| Parsonage et al. 2013130 | ||||
| van der Linden et al. 2018109 | ||||
| Wildi et al. 2017112 | ||||
| ROMI-3 | USA | 1137 | NSTEMI | Renal function |
| Kavasak et al. 201776 | ||||
| aShortt et al. 2017101 | ||||
| TRUST | UK | 963 (867 Abbott hs-cTnI, 959 Roche hs-cTnT) | NSTEMI | None |
| aCarlton et al. 201564 | ||||
| Carlton et al. 201563 | ||||
| Abbott ARCHITECT hs-cTnI, Siemens Healthcare Atellica hs-cTnI and Roche Elecsys hs-cTnT | ||||
| High-STEACS | UK (Scotland) | 32,837 | NSTEMI; 30-day MACE | Sex, age (< 65 years vs. ≥ 65 years), history of ischaemic heart disease |
| aBularga et al. 201961 | ||||
| Chapman et al. 201765 | ||||
| Chapman et al. 201866 | ||||
| Chapman et al. 201967 | ||||
| Miller-Hodges et al. 201879 | ||||
| Shah et al. 201598 | ||||
| Chapman et al. 2020174 | ||||
| Roche Elecsys TnT and Siemens ADVIA Centaur hs-cTnI | ||||
| BEST | UK | 665 | NSTEMI | None |
| aBody et al. 2019115 | ||||
| Body et al. 2020172 | ||||
| Siemens Healthcare Atellica hs-cTnI and ADVIA Centaur hs-cTnI | ||||
| High-US | USA | 2212 | NSTEMI; 30-day MACE | None |
| Nowak et al. 2019128 | ||||
| Nowak et al. 2019129 | ||||
| aSandoval et al. 2019176 | ||||
| Abbott ARCHITECT hs-cTnI, Roche Elecsys hs-cTnT, Siemens Healthcare ADVIA Centaur hs-cTnI, Siemens Healthcare Dimension Vista hs-cTnI, Beckman Coulter ACCESS hs-cTnI, Ortho VITROS hs-cTnI, bioMérieux VIDAS hs-cTnI and Quidel Cardiovascular TriageTrue hs-cTn | ||||
| APACE | NSTEMI; AMI; 30-day MACE | Sex, age (≤ 70 years vs. > 70 years), previous CAD, renal function | ||
| Badertscher et al. 201854 | ||||
| Badertscher et al. 20186 | ||||
| aBoeddinghaus et al. 201758 | ||||
| Boeddinghaus et al. 201859 | ||||
| Boeddinghaus et al. 201960 | ||||
| Boeddinghaus et al. 2019123 | ||||
| Boeddinghaus et al. 2019170 | ||||
| Boeddinghaus et al. 2020173 | ||||
| bCullen et al. 2013156 | ||||
| bHoeller et al. 2013168 | ||||
| bHaaf et al. 2012136 | ||||
| bHochholzer et al. 2011149 | ||||
| bIrfan et al. 2013158 | ||||
| Jaeger et al. 20162 | ||||
| Kaier et al. 201775 | ||||
| Lindahl et al. 2017132 | ||||
| bPotocki et al. 2012140 | ||||
| Reichlin et al. 201590 | ||||
| Reichlin et al. 201591 | ||||
| bReiter et al. 2011146 | ||||
| bReiter et al. 2012138 | ||||
| bReichlin et al. 2009167 | ||||
| bReichlin et al. 2011145 | ||||
| Rubini Gimenez et al. 201470 | ||||
| Rubini Gimenez et al. 201592 | ||||
| Rubini Gimenez et al. 201593 | ||||
| Rubini Giménez et al. 201694 | ||||
| Twerenbold et al. 2017105 | ||||
| Twerenbold et al. 2017103 | ||||
| Twerenbold et al. 2017104 | ||||
| Twerenbold et al. 2018106 | ||||
| Twerenbold et al. 2018107 | ||||
| Twerenbold et al. 2019108 | ||||
| Wildi et al. 2016111 | ||||
| Wildi et al. 2019113 | ||||
Twenty two56,58,61,62,64,84,102,110,115,121,133,135,137,141,142,144,148,150,157,159,161,175 of the 37 included studies were conducted in Europe (seven in the UK56,61,64,115,159,161,175), five were conducted in Australia and New Zealand,68,88,139,147,171 six were conducted in the USA,87,89,101,165,176,177 three were conducted in East Asia72,100,117 and one was a worldwide study. 80 Twenty seven of the 37 included studies reported receiving some support from test manufacturers, including supply of assay kits56,58,61,64,68,72,80,84,87-89,96,101,115,133,135,139,141,142,144,147,148,150,157,165,171,176 and three studies did not report any information on funding. 62,102,110
For DTA studies, full details of the characteristics of study participants, study inclusion and exclusion criteria, hs-cTn assay used and reference standard, and detailed results are reported in the data extraction tables presented in Appendix 2 (see Tables 35–37).
Study quality
We conducted a quality assessment of the two RCTs included in this assessment, using the revised Cochrane Risk-of-Bias Tool for Cluster Randomised Trials. 44 The results are shown in Table 4.
| Quality assessment | High-STEACS trial99 | HiSTORIC trial175 |
|---|---|---|
| Bias arising from the randomisation process | Low | NI |
| Bias arising from the timing of intervention and recruitment of individual participants in relation to randomisation | Low | Low |
| Bias due to deviations from intended interventions | Low | Low |
| Bias due to missing outcome data | Low | Low |
| Bias in measurement of the outcome | Low | Low |
| Bias in selection of the reported result | Low | Low |
| Overall bias | Low | Low |
Overall, the trials were well conducted with procedures to ensure randomisation and blinding. Patients were unaware of the intervention in both the High-STEACS99 and HiSTORIC trials. 175
The methodological quality of the included DTA studies that evaluated a single hs-cTn assay was assessed using QUADAS-2. 45 Studies that provided data for two or more hs-cTn assays were assessed using QUADAS-2C. 46 The main potential sources of bias in the included DTA studies relate to patient spectrum and patient flow. There were also concerns regarding the applicability of the patient population. There were concerns regarding the applicability of the reference standard for some studies in the previous systematic review,2 but this was not the case for any of the new studies identified for this update. The results of the QUADAS-2 and QUADAS-2C assessments are summarised in Tables 5 and 6 (full QUADAS-2 and QUADAS-2C assessments for each study are provided in Appendices 3 and 4, respectively). A summary of the risks of bias and applicability concerns within each QUADAS-2 and QUADAS-2C domain is provided below.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| ADAPT/IMPACT, Nestelberger et al. 2019171 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| aAldous et al. 2011147 | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ |
| aAldous et al. 2012139 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| BACC, Neumann et al. 201684 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| aBody et al. 2011161 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Body et al. 201556 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Cappellini et al. 201962 | ✓ | ✗ | ? | ? | ✓ | ✓ | ✓ |
| aChrist et al. 2010150 | ✓ | ✓ | ? | ✓ | ✗ | ✓ | ✗ |
| CORE, Mokhtari et al. 2016119,121 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| aFASTER I and FAST II, Eggers et al. 2012137 | ? | ✓ | ? | ✗ | ✗ | ✓ | ✗ |
| aFreund et al. 2011142 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Huang et al. 201572 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| aKeller et al. 2011141 | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ |
| aKurz et al. 2011148 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Lin et al. 2019117 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| aMelki et al. 2011144 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Peacock et al. 201889 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| aPITGORAS, Sanchis et al. 2012135 | ✗ | ✓ | ? | ✓ | ✗ | ✓ | ✓ |
| QUART, Parsonage et al. 201488 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| aRATPAC (point-of-care arm), Collinson et al. 2013159 | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ |
| REACTION-US, Nowak et al. 201887 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| aSaenger et al. 2010165 | ? | ✓ | ? | ? | ✗ | ✓ | ✗ |
| aSebbane et al. 2013157 | ? | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| Shiozaki et al. 2017100 | ✗ | ✓ | ? | ✓ | ✗ | ✓ | ✓ |
| Slagman et al. 2017102 | ? | ✓ | ✗ | ? | ? | ✓ | ? |
| TRAPID-AMI, Mueller et al. 201680 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| aTUSCA, Santaló et al. 2013133 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ? |
| UTROPIA, Sandoval et al. 201796 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Venge et al. 2017110 | ? | ✓ | ? | ✗ | ✗ | ✓ | ✓ |
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| ADAPT, Cullen et al. 201468 | |||||||
| Abbott ARCHIRECT hs-cTnI | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Roche Elecsys hs-cTnT | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abbott ARCHIRECT hs-cTnI vs. Roche Elecsys hs-cTnT | ✓ | ? | ✓ | ✓ | |||
| APACE, Boeddinghaus et al. 2018,59 Boeddinghaus et al. 2019,170 Boeddinghaus et al. 2019178 (comparison of assays using ESC 0/1-hour pathway or equivalent) | |||||||
| Abbott ARCHIRECT hs-cTnI | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Beckman Coulter ACCESS hs-cTnI | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Ortho VITROS hs-cTnI | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Roche Elecsys hs-cTnT | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Siemens ADVIA Centaur hs-cTnI | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Quidel TriageTrue hs-cTnI | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Comparison of Abbott ARCHITECT hs-cTnI, Roche Elecsys hs-cTnT and Siemens ADVIA Centaur hs-cTnI | ? | ? | ✗ | ✓ | |||
| Comparison of all tests | ? | ? | ✗ | ✗ | |||
| BEST, Body et al. 2019,115 Body et al. 2020172 | |||||||
| Roche Elecsys hs-cTnT | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Siemens ADVIA Centaur hs-cTnI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Roche Elecsys hs-cTnT vs. Siemens ADVIA Centaur hs-cTnI | ✗ | ? | ✗ | ✗ | |||
| High-STEACS, Chapman et al. 2018,66 Chapman et al. 201967 (comparison of assays using ESC 0/1-hour pathway, ESC 0/3-hour pathway and High-STEACS 0/3-hour pathway) | |||||||
| ARCHITECT hs-cTnI | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Siemens Atellica hs-cTnI | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| ARCHITECT hs-cTnI vs. Siemens Atellica hs-cTnI | ? | ? | ? | ✗ | |||
| High-US, Sandoval et al. 2019176 | |||||||
| Siemens Atellica hs-cTnI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Siemens ADVIA Centaur hs-cTnI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Siemens Atellica hs-cTnI vs. Siemens ADVIA Centaur hs-cTnI | ✓ | ? | ✓ | ✓ | |||
| ROMI-3, Shortt et al. 2017101 | |||||||
| Abbott ARCHIRECT hs-cTnI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Roche Elecsys hs-cTnT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abbott ARCHIRECT hs-cTnI vs. Roche Elecsys hs-cTnT | ✓ | ? | ✓ | ✓ | |||
| TRUST, Carlton et al. 201564 | |||||||
| Abbott ARCHIRECT hs-cTnI | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Roche Elecsys hs-cTnT | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Abbott ARCHIRECT hs-cTnI vs. Roche Elecsys hs-cTnT | ✓ | ✓ | ✗ | ✗ | |||
Patient spectrum
Eight studies87,88,100,117,121,135,139,144 assessed using QUADAS-2 were rated as having a high risk of bias for patient selection. A further nine studies80,89,102,110,137,148,157,161,165 were rated as having an unclear risk of bias because they did not provide sufficient details to make a judgement on whether or not appropriate steps were taken to minimise bias when enrolling patients. Five studies88,117,121,139,144 enrolled patients at certain times only (e.g. during office hours). This was considered to have the potential to lead to the inclusion of a different spectrum of patients than if consecutive patients had been enrolled. Two studies87,100 were rated as having a high risk of bias for patient selection because they excluded patients for reasons that were not specified in their reported methods. The last study135 that was judged as having a high risk of bias for patient enrolment excluded certain patient groups, including those with a troponin elevation in any two serial determinations and those with a prior diagnosis of ischemic heart disease, structural heart disease, concomitant heart failure or significant bradyarrhythmia.
All studies assessed using QUADAS-2C were rated as having a low risk of bias for patient selection for all individual index tests. However, one study, for which data for two hs-cTn assays were reported in separate publications,115,172 was rated as having a high risk of bias for patient selection for the comparison of the two assays. This was because the study did not set out to conduct both tests in all patients or to randomly allocate patients to one of the two tests. A further two studies, APACE59,170,178 and the High-STEACS trial,66,67 were rated as having an unclear risk of bias with respect to the comparison between hs-cTn assays.
As with our previous systematic review,2 this assessment included studies that enrolled both mixed populations (i.e. when the target condition was any AMI) and studies restricted to our primary focus of populations where patients with STEMI were excluded (i.e. target condition NSTEMI). Studies not restricted to this specific patient group were therefore considered to have high concerns regarding applicability. Only seven studies133,137,139,144,148,157,159 from our previous systematic review were restricted to patients in whom STEMI had been excluded. Three of these studies137,144,148 were restricted to patients admitted to coronary care/chest patients units, and so were considered to represent patients with more severe disease, and a further study159 had strict inclusion criteria that resulted in the inclusion of a very low-risk population. These four studies137,144,148,159 were not considered to be representative of the spectrum of patients with chest pain presenting to the ED, and so were also rated as having high concerns regarding applicability. This assessment includes a further 13 studies58,61,62,64,68,72,80,84,96,101,115,171,176 that were restricted to patients in whom STEMI had been excluded.
Index test
All but three of the studies62,68,117 were rated as having a low risk of bias for the index, as they reported data for at least one threshold that was prespecified. Two studies62,117 were rated as having a high risk of bias in this domain because they reported data for optimised thresholds that were derived in the same population. As the reference standard (i.e. the diagnosis of AMI or a MACE) was generally interpreted after the high sensitivity troponin test, blinding was not considered important for these studies. However, all but one of the studies64 that compared two or more hs-cTn assays were rated as having an unclear risk of bias with respect to the comparison, using QUADAS-2C, as no information was provided about whether or not index tests were interpreted blind to the results of other index tests. Inclusion criteria were very tightly defined in terms of the high-sensitivity troponin assays that we were interested in, and so all studies were considered to have low concerns regarding the applicability of the index test.
Reference standard
Nine studies61,62,100,110,133,135,137,150,165 were rated as having an unclear risk of bias for the reference standard because it was unclear whether or not the diagnosis of NSTEMI/AMI/MACEs was made without knowledge of the high-sensitivity troponin results. One study,115 assessed using QUADAS-2C, was rated as having a high risk of bias for one of the two hs-cTn assays assessed and for the comparison between assays (this was because the results of one of the hs-cTn assays were available to clinicians adjudicating the final diagnosis). Ten of the studies137,139,141,142,147,148,150,157,161,165 taken from our previous systematic review had high concerns regarding the applicability of the reference standard. All new studies identified for this assessment had low concerns regarding the applicability of the reference standard.
Patient flow
Six of the studies110,137,141,147,157,159 that reported data for a single hs-cTn assay, assessed using QUADAS-2, were considered as having a high risk of bias for patient flow and a further three studies62,102,165 were considered as having an unclear risk of bias. In all cases, this was related to withdrawals from the study. Verification bias was not considered to be a problem in any of the studies. All of the studies assessed using QUADAS-2C were rated as having a low risk of bias for patient flow, with respect to the individual hs-cTn assays that they assessed. However, four of these studies [APACE,59,170,178 BEST (Bedside Evaluation of Sensitive Troponin),115,172 High-STEACS66,67 and TRUST (Triage Rule-out Using Sensitive Troponin)64] were rated as having a high risk of bias with respect to at least one between-assay comparison and in all cases this was because the number of patients for whom hs-cTn results were available differed between assays.
Randomised controlled trials comparing high-sensitivity troponin assays with conventional troponin assays
Study details
Two RCTs were identified. 99,175 The High-STEACS trial, which contributed multiple diagnostic accuracy data sets, was a stepped-wedge cluster RCT that evaluated implementation of an early rule-out pathway in hospitals in Scotland. This trial assessed rates of reclassification of patients and subsequent incidence of MI and cardiovascular death when hs-cTnI results were made available for patients previously classified based on cTnI results. 99 A second stepped-wedge cluster RCT, the HiSTORIC trial (unpublished report provided AiC)175 also evaluated the implementation of an early rule-out pathway in hospitals in Scotland. The primary outcomes were length of stay and MI or cardiac death after discharge (at 30 days). A summary of study details for the High-STEACS and HiSTORIC trials is provided in Table 7.
| Study detail | High-STEACS trial99 | HiSTORIC trial175 |
|---|---|---|
| Number of patients | 48,282 (47% female) | 31,492 (45% female) |
| Location and setting | Ten secondary and tertiary care hospitals in Scotland | Seven acute hospitals in Scotland |
| Trial design | Stepped-wedge cluster RCT | |
| Study dates | June 2013 to March 2016 | December 2014 to December 2016 |
| Participant inclusion criteria | Patients presenting with suspected ACS and with paired cTn measurements from standard care and trial assay | Consecutive patients with suspected ACS and a normal troponin concentration at presentation |
| Participant exclusion criteria | Patients previously admitted during the trial period or not resident in Scotland | Patients presenting with an out-of-hospital cardiac arrest or STEMI, previously admitted during the trial or not resident in Scotland |
| High-sensitivity assay | hs-cTnI (Abbott ARCHITECT) CoV < 10% at 4.7 ng/l, and 99th centile URL of 34 ng/l in men and 16 ng/l in women | |
| Contemporary assay | cTnI (Abbott) CoV < 10% at 40 ng/l (seven sites) and 50 ng/l (three sites) at 6 and 12 hours | Serial testing at presentation and repeated 6–12 hours after onset of symptoms if indicated |
| Primary outcome | Subsequent MI (type 1 or type 4b) or cardiovascular death within 1 year following initial presentation to hospital |
Length of stay (i.e. length of time from presentation to the ED until discharge from hospital) MI (type 1, type 4b or type 4c) or cardiac death at 30 days (primary) and 1 year (secondary) |
| Other outcomes | Duration of hospital stay, MI (type 1 or 4b), unplanned coronary revascularisation, all-cause death, death from cardiovascular causes, hospital admission for heart failure and ischaemic stroke, major haemorrhage, unplanned hospital admission (excluding ACS and non-cardiovascular death) | Proportion of patients discharged from the ED, MI, cardiac death, cardiovascular death, all-cause death, unplanned coronary revascularisation and revisits for any reason after discharge at 1 year |
Both studies99,175 had large sample sizes and reported power calculations for the primary outcome. Both women and men were represented in the trials. The mean age of patients in the High-STEACS trial99 was 61 years and the mean age of patients in the HiSTORIC trial175 was 59 years. The HiSTORIC trial175 excluded patients with STEMI but the High-STEACS trial99 did not. As both trials99,175 were conducted in Scotland, they are likely to be highly relevant to UK practice.
Both trials99,175 used the Abbott ARCHITECT high-sensitivity assay. In the High-STEACS trial,99 during the validation phase of the trial (6–12 months), results of the hs-cTnI assay were concealed from the attending clinician and a contemporary cTn assay was used to guide care. A high-sensitivity test was introduced after 6 months (early implementation) or 12 months (late implementation). 99 The HiSTORIC trial175 also had a validation phase where troponin testing was performed at presentation and repeated 6–12 hours after the onset of symptoms, if indicated. In the validation phase of the HiSTORIC trial,175 the High-STEACS trial99 early rule-out pathway was used. A range of outcomes were investigated in both trials. Both trials99,175 considered MI and cardiac death at 1 year and length of stay in hospital. The HiSTORIC trial175 also investigated MI or cardiac death at 30 days.
Efficacy results
In the High-STEACS trial,99 patients reclassified by the high-sensitivity test were older [mean age 75 years, standard deviation (SD) 14 years] than those identified by a cTnI assay (mean age 70 years, SD 15 years) and more likely to be women (83% vs. 41%). They were less likely to show myocardial ischaemia on the electrocardiograph (14% vs. 36%). Other baseline characteristics were similar. In the High-STEACS trial,99 2586 (5%) patients had MI or death from cardiovascular causes at 1 year. Of the 1771 patients reclassified by the hs-cTnI assay, 105 of 720 (15%) were in the validation phase and 131 of 1051 (12%) were in the implementation phase. The adjusted odds ratio (OR) for implementation compared with validation was 1.10 (95% CI 0.75 to 1.61). 99 In the HiSTORIC trial175 (confidential information has been removed).
In the High-STEACS trial,99 patients reclassified using the high-sensitivity test, there were no differences in any of the secondary efficacy and safety outcome measures between phases, including MI (type 1 or 4b), unplanned coronary revascularisation, all-cause death, death from cardiovascular causes (cardiac and non-cardiac), hospital admission for heart failure and ischaemic stroke. 99
In the High-STEACS trial,99 the median length of stay was 7 [interquartile range (IQR) 3–24] hours in the implementation phase and 4 (IQR 3–20) hours in the validation phase. In the HiSTORIC trial175 (confidential information has been removed). 175
The authors of the High-STEACS trial99 concluded that although implementation of a hs-cTn assay resulted in reclassification of 17% of 10,360 patients with myocardial injury or infarction, only one-third had a diagnosis of type 1 MI and the incidence of subsequent MI or death from cardiovascular causes within 1 year was not affected by use of this assay. 99 (Confidential information has been removed.)175
Diagnostic accuracy of the Roche Elecsys hs-cTnT assay
Study details
Thirteen diagnostic cohort studies,133,135,137,139,142,144,147,148,150,157,159,161,165 taken from our previous systematic review,2 and a further 17 studies,56,58,61,62,64,68,72,80,87–89,100–102,115,117,121 newly identified or updated (i.e. new publications since our previous systematic review), provided data on the diagnostic performance of the Roche Elecsys hs-cTnT assay. One of these studies89 assessed the STAT version of the assay. Twenty seven56,58,61,62,64,68,72,80,87–89,100–102,115,133,137,139,142,144,147,148,150,157,159,161,165 of the 30 studies in this section assessed the diagnostic performance of the Roche Elecsys hs-cTnT assay for the detection of AMI, three studies117,121,135 assessed performance for the prediction of a MACE within 30 days of the index presentation and four studies56,58,64,89 provided data for both AMI and 30-day MACE. Eighteen studies58,62,64,68,72,80,87,100-102,115,133,137,139,144,148,157,159 provided data specific to the population of interest for this assessment (i.e. participants with STEMI were excluded and the target condition was NSTEMI rather than any AMI).
All but one62 of the 26 studies that assessed diagnostic performance for the detection of AMI reported data on the diagnostic performance of a single sample taken on presentation for at least one threshold. Twenty-two studies56,64,68,70,72,88,100–102,114,133,137,139,142,144,147,148,150,157,159,161,165 reported data for the 99th centile for the general population and 14 of these studies64,68,70,72,100–102,133,137,139,144,148,157,159 provided data for the target condition NSTEMI. Nine studies56,63,75,87,101,114,115,139,147 assessed the diagnostic performance of a LoD threshold (5 ng/l) in a single sample taken on presentation and six of these studies63,75,87,101,115,139 provided data for the target condition NSTEMI. Similarly, eight studies56,63,101,114,139,150,161,167 assessed the diagnostic performance of a limit of blank (LoB) threshold (3 ng/l) in a single sample taken on presentation and three of these studies63,101,139 provided data for the target condition NSTEMI. Studies assessing the diagnostic performance of the Roche Elecsys hs-cTnT assay for the detection of AMI (any AMI or NSTEMI) reported data for a total of 33 different testing strategies (with different combinations of sample timing and threshold). Table 8 provides summary estimates of the diagnostic performance of all combinations of population, diagnostic threshold and hs-cTnT test timing that were assessed by more than one study. Diagnostic performance estimates are also provided where combinations assessed by a single study have been selected for inclusion in the cost-effectiveness modelling conducted for this assessment. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold. Table 6 also includes diagnostic performance estimates for prespecified clinical subgroups taken from single studies. Full results (including numbers of TP, FP, FN and TN test results) for all studies and all data sets are provided in Appendix 2, Table 37.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Single sample strategies | |||||
| 99th centile threshold (14 ng/l) at 0 hours | All | Any AMI | 22 | 90 (85 to 94) | 78 (72 to 83) |
| All | NSTEMI | 14 | 90 (85 to 94) | 77 (68 to 84) | |
| All | MACE | 2 | 81 (75 to 86) | 78 (76 to 81) | |
| Age ≤ 70 years | Any AMI | 1146 | 88 (78 to 94) | 86 (83 to 89) | |
| Age > 70 years | Any AMI | 1146 | 97 (92 to 99) | 49 (44 to 55) | |
| Patients with pre-existing CAD | Any AMI | 1140 | 93 (85 to 97) | 60 (55 to 65) | |
| Patients without pre-existing CAD | Any AMI | 1140 | 94 (88 to 97) | 82 (79 to 85) | |
| Mixed; low to moderate pre-test probability | Any AMI | 1142 | 89 (70 to 97) | 85 (79 to 89) | |
| Mixed; high pre-test probability | Any AMI | 1142 | 94 (77 to 99) | 66 (50 to 79) | |
| Female | NSTEMI | 194 | 91 (85 to 96) | 79 (76 to 82) | |
| Male | NSTEMI | 194 | 91 (87 to 94) | 79 (76 to 81) | |
| Patients with an eGFR < 30 ml/minute/1.73 m2 | NSTEMI | 172 | 100 (83 to 100) | 13 (4 to 29) | |
| Patients with an eGFR 30–59 ml/minute/1.73 m2 | NSTEMI | 172 | 100 (96 to 100) | 47 (39 to 55) | |
| Patients with an eGFR 60–89 ml/minute/1.73 m2 | NSTEMI | 172 | 96 (91 to 98) | 72 (68 to 76) | |
| Patients with an eGFR > 90 ml/minute/1.73 m2 | NSTEMI | 172 | 92 (83 to 97) | 84 (80 to 87) | |
| LoD (< 5 ng/l) at 0 hours | All | Any AMI | 9 | 99 (97 to 99) | 36 (28 to 45) |
| All | NSTEMI | 6 | 99 (97 to 100) | 35 (25 to 46) | |
| All | MACE | 3 | 98 (95 to 99) | 32 (30 to 34) | |
| LoB (< 3 ng/l) at 0 hours | All | Any AMI | 8 | 100 (98 to 100) | 19 (11 to 31) |
| All | NSTEMI | 3 | 98 (96 to 99) | 21 (19 to 22) | |
| All | MACE | 3 | 96 (93 to 98) | 17 (15 to 19) | |
| 99th centile threshold (14 ng/l) at 2 hours | All | NSTEMI | 2 | 95 (92 to 96) | 81 (79 to 82) |
| Multiple sample strategies | |||||
| ESC 0/1 hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | All | NSTEMI | 1 104 | 99 (98 to 100) | 68 (67 to 70) |
| All | MACE | 2 | 99 (97 to 100) | 62 (61 to 64) | |
| Patients with normal renal function | NSTEMI | 1106 | 99 (97 to 100) | 78 (76 to 80) | |
| Patients with impaired renal function (eGFR < 60 ml/minute/1.73 m2) | NSTEMI | 1106 | 100 (98 to 100) | 26 (22 to 31) | |
| (< 14 ng/l at 0 hours AND 2 hours) AND Δ < 4 ng/l | All | NSTEMI | 2 | 98 (96 to 99) | 74 (72 to 76) |
| < 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours | All | NSTEMI | 3 | 98 (97 to 99) | 73 (71 to 74) |
| < 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours | All | NSTEMI | 1 87 | 100 (93 to 100) | 45 (40 to 49) |
| 99th centile threshold (< 14 ng/l at 0 hours AND 3 hours) | All | NSTEMI | 1 148 | 100 (89 to 100) | 77 (58 to 90) |
Single sample strategies
The summary estimates of sensitivity and specificity, where the diagnostic threshold was defined as the 99th centile for the general population, were 90% (95% CI 85% to 94%) and 78% (95% CI 72% to 83%), respectively, based on data from 22 studies56,64,68,70,72,88,100–102,114,133,137,139,142,144,147,148,150,157,159,161,165 The SROC curve for this analysis is shown in Figure 2. These estimates were similar when the analysis was restricted to studies that excluded participants with STEMI64,68,70,72,100-102,133,137,139,144,148,157,159 [summary estimates of sensitivity and specificity were 90% (95% CI 85% to 94%) and 77% (95% CI 68% to 84%), respectively]. The SROC curve is shown in Figure 3. Based on these data, it is unlikely that hs-cTnT testing on a single admission sample, using the 99th centile diagnostic threshold, would be considered adequate for rule out of any AMI or NSTEMI. The summary estimates of sensitivity and specificity, where the diagnostic threshold was defined as the 99th centile for the general population but the sample was taken 2 hours after presentation, were 95% (95% CI 92% to 96%) and 81% (95% CI 79% to 82%), respectively, based on data from three studies68,139,144 in which the target condition was NSTEMI. Later sampling appears to be associated with improved rule-out performance at this threshold.
In our previous systematic review, limited data were identified on additional clinical subgroups [i.e. age > 70 years vs. ≤ 70 years,146 without pre-existing CAD vs. with pre-existing CAD140 and high vs. low to moderate pre-test probability (determined by clinical judgement and based on cardiovascular risk factors, type of chest pain, physical findings and ECG abnormalities)142]. None of these studies excluded participants with STEMI. The study146 that stratified participants by age reported a higher estimate of sensitivity (97%, 95% CI 92% to 99%) in participants aged > 70 years than for patients aged ≤ 70 years (88%, 95% CI 78% to 94%). The estimate of sensitivity for people aged > 70 years was also higher than the corresponding summary estimates derived from all 22 studies56,64,68,70,72,88,100–102,114,133,137,139,142,144,147,148,150,157,159,161,165 that used the 99th centile diagnostic threshold. A similar pattern was apparent for people with a high pre-test probability compared with those with a low to moderate pre-test probability142 and for participants without pre-existing CAD compared with those with pre-existing CAD140 (see Table 8). As with the age stratification, the estimates of sensitivity were higher than the corresponding summary estimates derived from the 22 studies56,64,68,70,72,88,100–102,114,133,137,139,142,144,147,148,150,157,159,161,165 that used the 99th centile diagnostic threshold, for people with a high pre-test probability and for people without pre-existing CAD. Figure 4 illustrates the variation in performance characteristics of a single admission sample, using the 99th centile diagnostic threshold, when used in different clinical subgroups. These data provide some indication that hs-cTnT testing on a single admission sample, using the 99th centile diagnostic threshold, may be adequate for rule out of AMI in certain selected populations [i.e. older people (aged ≥ 70 years), those without pre-existing CAD and people classified by clinical judgement as having a high pre-test probability].
FIGURE 4.
A ROC space plot for the Roche Elecsys hs-cTnT assay using the 99th centile threshold and a presentation sample in different clinical subgroups. Reproduced from Westwood et al. 2 Contains information licensed under the Non-Commercial Government Licence v2.0.

In addition to these studies, the current assessment identified one further study72 that reported data on how the diagnostic performance of a single sample taken on presentation, and using the 99th centile for the general population as the cut-off point, varies with renal function (see Table 8). These data72 show a marked decrease in specificity as renal function decreases.
Nine studies56,63,75,87,101,114,115,139,147 assessed the diagnostic performance of a LoD threshold (5 ng/l) in a single sample taken on presentation. The summary estimates of sensitivity and specificity using this threshold were 99% (95% CI 97% to 99%) and 36% (95% CI 28% to 45%), respectively (the SROC curve for this analysis is shown in Figure 5). The summary estimates of sensitivity and specificity were similar [99% (95% CI 97% to 100%) and 35% (95% CI 25% to 46%), respectively] when the analysis was restricted to the six studies63,75,87,101,115,139 providing data for the target condition NSTEMI (the SROC curve for this analysis is shown in Figure 6). The eight studies56,63,101,114,139,150,161,167 that assessed the diagnostic performance of a LoB threshold (3 ng/l) in a single sample taken on presentation gave a similarly high summary estimate of sensitivity (100%, 95% CI 98% to 100%), which was associated with reduced specificity (19%, 95% CI 11% to 31%) (the SROC curve for this analysis is shown in Figure 7). Again, restricting the analysis to those studies that provided data for the target condition NSTEMI63,101,139 did not substantially change the summary estimates of sensitivity (98%, 95% CI 96% to 99%) and specificity (21%, 95% CI 19% to 22%). These data add to the data for these thresholds included in our previous systematic review,2 and provide some indication that hs-cTnT testing on a single admission sample may be adequate to rule out any AMI or NSTEMI when a lower diagnostic threshold (5 ng/l or 3 ng/l) is used.
Multiple sample strategies
The number of multiple sample strategies/rule-out algorithms that have been evaluated has substantially increased since our previous systematic review. 2 Our previous systematic review2 included eight studies133,139,143,145,151,158,165,168 that provided data on the performance of a variety of strategies involving multiple sampling, most commonly involving a combination of a peak hs-cTn value above the 99th centile diagnostic threshold and a 20% change in hs-cTnT over 2 or 3 hours following presentation. The current assessment includes data for a total of 23 distinct multiple sample strategies that used the Roche Elecsys hs-cTnT assay (including six for the STAT version of the assay), of which 14 were evaluated in populations that excluded patients with STEMI (target condition NSTEMI). Most strategies were evaluated by a single study (the summary sensitivity and specificity estimates for strategies that were evaluated by more than one study are provided in Table 8). Diagnostic performance estimates are also provided when combinations assessed by a single study have been selected for inclusion in the cost-effectiveness modelling conducted for this assessment. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold (see Table 8). Full results for all multiple sample strategies evaluated are provided in Appendix 2, Table 37. In general, the use of multiple sample strategies appears to offer increased specificity compared with a single sample on presentation and a very low (LoD or LoB) threshold, without substantial loss of sensitivity (see Table 6).
The ESC 0/1-hour rule-out pathway combines an initial sample and a very low (LoD of 5 ng/l) threshold in patients reporting a minimum symptom duration of 3 hours, with repeat testing at 1 hour for patients in whom the initial hs-cTnT is < 12 ng/l and in whom symptom duration is < 3 hours (i.e. it uses an ‘or’ combination). The sensitivity and specificity estimates for this strategy were 99% (95% CI 98% to 100%) and 68% (95% CI 67% to 70%), respectively, for the target condition NSTEMI (taken from the APACE study104). The overall rule-out rate for this strategy was 56.9%. It was not clear in what proportion of participants NSTEMI was ruled out using the presentation sample alone. 104 Based on data from the same study,104 the ESC 0/1-hour rule-out pathway would miss 5 of 746 (0.67%) people with NSTEMI. A further publication of the APACE study108 reported data for the performance of the ESC 0/1-hour rule-out pathway for both the target condition NSTEMI and the target condition MACE at 30-day follow-up (including MI at index admission). Data from this publication indicated that, although the ESC 0/1-hour rule-out pathway did not miss any participants with NSTEMI at the index admission, 3 of 1420 (0.21%) participants who met the rule-out criteria experienced a MACE during 30 days’ follow-up. 108
Similar estimates of diagnostic performance were obtained for strategies involving an ‘AND’ combination of initial hs-cTnT level and absolute change. The summary estimates of sensitivity and specificity for a hs-cTnT level below the 99th centile (< 14 ng/l) on presentation and at 2 hours combined with an absolute change of < 4 ng/l were 98% (95% CI 96% to 99%) and 74% (95% CI 72% to 76%), respectively (based on data from two studies57,90). Similarly, the summary estimates of sensitivity and specificity for a hs-cTnT level of < 12 ng/l on presentation combined with an absolute change of < 3 ng/l at 1 hour were 98% (95% CI 97% to 99%) and 73% (95% CI 71% to 74%), respectively (based on data from three studies80,91,100). It should be noted that this strategy is equivalent to the rule-out threshold used in the repeat testing component of the ESC 0/1-hour pathway. Comparing the sensitivity and specificity estimates for these two strategies, we can see that, although the additional very early rule-out step (i.e. hs-cTnT < 5 ng/l on presentation) in the ESC 0/1-hour pathway may facilitate earlier discharge for some patients, it does not appear to improve overall diagnostic performance.
Prognostic accuracy
A total of nine studies56,63,81,89,108,117,121,135,174 assessed the performance of one or more testing strategies using the Roche Elecsys hs-cTnT assay for the prediction of a MACE within 30 days of the index presentation. As for the target conditions any AMI and NSTEMI, Table 8 provides summary estimates of the diagnostic performance of all combinations of population, diagnostic threshold and hs-cTnT test timing that were assessed by more than one study. The sensitivity estimates for single sample strategies and the target condition (i.e. a MACE) were generally slightly lower than those for the target conditions any AMI or MACE, and specificity estimates were similar or lower. The sensitivity estimates for the ESC 0/1-hour rule-out strategy were similar for the target conditions MACE and NSTEMI, and the specificity estimate was lower for MACE than for NSTEMI (see Table 8).
Diagnostic accuracy of the Abbott ARCHITECT hs-cTnI assay
Study details
Nine diagnostic cohort studies58,61,64,68,84,96,101,110,141 provided data on the diagnostic performance of the Abbott ARCHITECT hs-cTnI assay, one141 of which was taken directly from our previous systematic review. 2 The remaining studies were newly identified or updated (i.e. new publications since our previous systematic review2). All studies in this section assessed the accuracy of the Abbott ARCHITECT hs-cTnI assay for the detection of AMI and seven studies58,61,64,68,84,96,101 provided data specific to the population of interest for this assessment (i.e. participants with STEMI excluded). Three studies58,61,68 also assessed the performance of the Abbott ARCHITECT hs-cTnI assay for the prediction of a MACE within 30 days of the index presentation.
All nine studies58,61,64,68,84,96,101,110,141 in this section reported data on the diagnostic performance of a single sample taken on presentation, for at least one threshold. Five studies58,64,68,101,110 reported data for the 99th centile for the general population and four58,64,68,101 of these studies provided data for the target condition NSTEMI. Four studies58,68,96,101 assessed the diagnostic performance of a LoD threshold (2 ng/l) in a single sample taken on presentation, all of which were for the target condition NSTEMI. Studies assessing the diagnostic performance of the Abbott ARCHITECT hs-cTnI assay for the detection of AMI (i.e. any AMI or NSTEMI) reported data for a total of 33 different testing strategies (i.e. different combinations of sample timing and threshold). Table 9 provides summary estimates of the diagnostic performance of all combinations of population, diagnostic threshold and hs-cTnI test timing that were assessed by more than one study. Diagnostic performance estimates are also provided when combinations assessed by a single study have been selected for inclusion in the cost-effectiveness modelling conducted for this assessment. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold. Table 9 also includes diagnostic performance estimates for prespecified clinical subgroups taken from single studies. Full results (including numbers of TP, FP, FN and TN test results) for all studies and all data sets are provided in Appendix 2, Table 37.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Single sample strategies | |||||
| 99th centile threshold (26.2 ng/l) at 0 hours | All | Any AMI | 5 | 75 (65 to 82) | 94 (91 to 96) |
| NSTEMI | 4 | 75 (64 to 84) | 94 (90 to 96) | ||
| Sex-specific 99th centile threshold (females 16 ng/l and males 34 ng/l at 0 hours) | Patients with an eGFR < 60 ml/minute/1.73 m2 | NSTEMI | 179 | 99 (96 to 100) | 71 (67 to 74) |
| Patients with an eGFR ≥ 60 ml/minute/1.73 m2 | 179 | 99 (97 to 100) | 92 (91 to 93) | ||
| Patients aged ≥ 65 years with an eGFR ≥ 60 ml/minute/1.73 m2 | 179 | 98 (96 to 100) | 86 (84 to 88) | ||
| Patients aged ≥ 65 years with an eGFR < 60 ml/minute/1.73 m2 | 179 | 98 (95 to 100) | 69 (65 to 73) | ||
| Patients aged < 65 years with an eGFR ≥ 60 ml/minute/1.73 m2 | 179 | 99 (97 to 100) | 96 (95 to 97) | ||
| Patients aged < 65 years with an eGFR < 60 ml/minute/1.73 m2 | 179 | 100 (88 to 100) | 82 (72 to 89) | ||
| LoD (< 2ng/l) at 0 hours | All | NSTEMI | 4 | 100 (99 to 100) | 21 (16 to 26) |
| All | MACE | 161 | 97 (95 to 98) | 39 (39 to 40) | |
| < 4 ng/l at 0 hours | All | NSTEMI | 2 | 99 (97 to 100) | 50 (48 to 52) |
| < 5 ng/l at 0 hours | All | NSTEMI | 3 | 97 (95 to 98) | 58 (57 to 59) |
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | All | NSTEMI | 2 | 99 (98 to 100) | 57 (56 to 59) |
| Normal renal function | NSTEMI | 1106 | 99 (97 to 100) | 66 (64 to 68) | |
| Impaired renal function (eGFR < 60 ml/minute/1.73 m2) | NSTEMI | 1106 | 99 (95 to 100) | 25 (20 to 30) | |
| High-STEACS trial79 pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR (≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours) | All | NSTEMI | 1 66 | 99 (97 to 100) | 76 (73 to 78) |
| Male | NSTEMI | 165 | 98 (93 to 100) | 88 (85 to 91) | |
| Female | 98 (92 to 100) | 87 (83 to 90) | |||
| Aged < 65 years | 99 (93 to 100) | 94 (92 to 96) | |||
| Aged ≥ 65 years | 97 (92 to 99) | 78 (74 to 82) | |||
| Known ischaemic heart disease | 96 (89 to 99) | 82 (78 to 86) | |||
| No known ischaemic heart disease | 100 (97 to 100) | 92 (89 to 94) | |||
| All | MACE | 166 | 98 (97 to 99) | 81 (79 to 83) | |
Single sample strategies
The summary estimates of sensitivity and specificity where the diagnostic threshold was defined as the 99th centile for the general population were 75% (95% CI 65% to 82%) and 94% (95% CI 94% to 96%), respectively, based on data from five studies58,64,68,101,110 (the SROC curve for this analysis is shown in Figure 8). These estimates were similar when the analysis was restricted to studies that excluded participants with STEMI. For these studies,58,64,68,101 the summary estimates of sensitivity and specificity were 75% (95% CI 64% to 84%) and 94% (95% CI 90% to 96%), respectively (the SROC curve for this analysis is shown in Figure 9). Based on these data, it is unlikely that hs-cTnI testing on a single admission sample using the 99th centile diagnostic threshold would be considered adequate for either rule out or rule in of any AMI or NSTEMI.
The results of subgroup analyses, using data from the High-STEACS study,79 appear to indicate that the sensitivity of a single sample taken on presentation can be markedly increased by using sex-specific 99th centile cut-off points (see Table 9). Data from this study also indicated that specificity is lower in patients with impaired renal function [estimated glomerular filtration rate (eGFR) < 60 ml/minute/1.73 m2].
Four studies58,68,96,101 assessed the diagnostic performance of a LoD threshold (2 ng/l) in a single sample taken on presentation, all of which were for the target condition NSTEMI. The summary estimates of sensitivity and specificity using this threshold were 100% (95% CI 99% to 100%) and 21% (95% CI 16% to 26%), respectively (the SROC curve for this analysis is shown in Figure 10). These data provide some indication that hs-cTnI testing on a single admission sample may be adequate to rule out NSTEMI when a lower diagnostic threshold (2 ng/l) is used.
Multiple sample strategies
The number of multiple sample strategies/rule-out algorithms that have been evaluated has substantially increased since our previous systematic review. 2 Our previous systematic review2 included only two studies141,151 that provided data on the performance of strategies involving multiple sampling. The current assessment includes data for a total of 17 distinct multiple sample strategies using the Abbott ARCHITECT hs-cTnI assay, of which 12 were evaluated in populations that excluded patients with STEMI (target condition NSTEMI). Most strategies were evaluated by a single study and the summary sensitivity and specificity estimates for strategies that were evaluated by more than one study are provided in Table 9. Diagnostic performance estimates are also provided when combinations assessed by a single study have been selected for inclusion in the cost-effectiveness modelling conducted for this assessment. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold (see Table 9). Full results for all multiple sample strategies evaluated are provided in Appendix 2, Table 37. In general, the use of multiple sample strategies appears to offer increased specificity compared with a single sample on presentation and a very low (LoD or LoB) threshold, without substantial loss of sensitivity (see Table 7).
The ESC 0/1-hour rule-out pathway combines an initial sample and a very low (LoD of 2 ng/l) threshold in patients reporting a minimum symptom duration of 3 hours, with repeat testing at 1 hour for patients in whom the initial hs-cTnI level is <5 ng/l and in whom symptom duration is < 3 hours (i.e. it uses an ‘or’ combination). The summary sensitivity and specificity estimates for this strategy were 99% (95% CI 98% to 100%) and 57% (95% CI 56% to 59%), respectively, for the target condition NSTEMI (two studies66,104). Based on data from one of these studies,66 the overall rule-out rate for this strategy was 71.4% and NSTEMI was ruled out using the single presentation sample alone in 37.7% of participants. In one study,66 no participants with NSTEMI were missed using the ESC 0/1-hour rule-out criteria, and in the second study,104 8 of 740 (1.08%) people with NSTEMI were missed, based on the ESC 0/1-hour rule-out criteria. Subgroup analysis indicated a marked reduction in specificity (25%, 95% CI 20% to 30%) when this strategy was used in people with impaired renal function (eGFR 60 ml/minute/1.73 m2). 106 The High-STEACS trial pathway utilises an initial rule-out step, based on a low (5 ng/l) threshold in a sample taken at presentation, in patients reporting a minimum symptom duration of 2 hours. Repeat testing at a later time point (3 hours) is then undertaken for patients in whom the initial hs-cTnI level is less than the sex-specific 99th centile (16 ng/l for females and 34 ng/l for males) and in whom symptom duration was < 2 hours at presentation. The High-STEACS trial pathway appears to offer a further increase in specificity for the target condition NSTEMI [the sensitivity and specificity estimates for this strategy were 99% (95% CI 97% to 100%) and 76% (95% CI 73% to 78%), respectively]. 66 The overall rule-out rate for this pathway was 64.9% and it was not clear in what proportion of participants NSTEMI was ruled-out using the presentation sample alone. 66 Based on data from the same study,66 the High-STEACS pathway would miss 2 of 275 (0.73%) patients with NSTEMI. The same publication also provided data for the target condition of a MACE at 30-day follow-up (including MI at index admission), showing that a further four participants of those who met the rule-out criteria (i.e. 4/1244, 0.32%) experienced a MACE during the follow-up period. 66
Subgroup analyses reported in a further publication of the High-STEACS trial65 indicted that the sensitivity of this pathway was consistently high (≥ 97%) across all clinical subgroups assessed (see Table 9).
Prognostic accuracy
Three studies58,61,68 assessed the performance of one or more testing strategies using the Abbott ARCHITECT hs-cTnI assay for the prediction of a MACE within 30 days of the index presentation. No single or multiple sample strategy was assessed by more than one study. Where available, sensitivity and specificity estimates from single studies for strategies corresponding to those selected for inclusion in cost-effectiveness modelling with the target condition NSTEMI estimates from single studies, have been included in Table 7. Sensitivity estimates for 30-day MACE were similar to those for NSTEMI, whereas specificity estimates were higher (see Table 7).
Diagnostic accuracy of the Beckman Coulter Access hs-cTnI assay
Study details
Two studies, the APACE study58 and ADAPT/IMPACT (Improved Assessment of Chest Pain Trial),171 provided data on the diagnostic performance of the Beckman Coulter Access hs-cTnI assay. 60,171 In both studies, patients with STEMI were excluded (i.e. the target condition was NSTEMI).
Single sample strategies
No single sample test strategies were assessed.
Multiple sample strategies
The two60,171 studies evaluating the Beckman Coulter Access hs-cTnI assay each assessed a different multiple sample strategy. One study60 reported data for a strategy that followed the structure of the ESC 0/1-hour rule-out pathway [i.e. an initial sample with a low threshold (4 ng/l) followed by repeat testing at 1 hour in patients whose initial troponin level was < 5 ng/l and who did not report a minimum symptom duration of 3 hours]. The sensitivity and specificity estimates for this strategy were 99% (95% CI 94% to 100%) and 70% (95% CI 66% to 74%), respectively. 60 The overall rule-out rate for this strategy was 60%, with NSTEMI being ruled out in 32% of participants based on the presentation sample alone. 60 In this study, 1 of 96 (1.04%) participants with NSTEMI were missed using the ESC 0/1-hour rule-out criteria. 60 The second study64 assessed a similar strategy, but with repeat testing at 2 hours. The sensitivity estimates were similar for the two strategies, but the specificity of the 2-hour repeat testing strategy was higher than that of the 1-hour strategy (Table 10). Full results (including the numbers of TP, FP, FN and TN test results) are provided in Appendix 2, Table 37. Both strategies were selected for inclusion in our cost-effectiveness modelling.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours) | All | NSTEMI | 1 60 | 99 (94 to 100) | 70 (66 to 74) |
| (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) | 1 171 | 98 (92 to 100) | 83 (81 to 86) | ||
Diagnostic accuracy of the bioMérieux VIDAS hs-cTnI assay
Study details
One diagnostic cohort study,132 which formed part of the APACE study,58 provided data on the diagnostic performance of the bioMérieux VIDAS hs-cTnI assay. This study excluded patients with STEMI (i.e. the target condition was NSTEMI).
Single sample strategies
No single sample test strategies were assessed.
Multiple sample strategies
The study132 evaluating the bioMérieux VIDAS hs-cTnI assay assessed the performance of a repeat testing strategy, with samples taken on presentation and at 2 hours (Table 11). This strategy was selected for inclusion in our cost-effectiveness modelling, as it was the only strategy evaluated for the bioMérieux VIDAS hs-cTnI assay. The reported sensitivity and specificity estimates were 98% (95% CI 92% to 100%) and 64% (95% CI 59% to 68%), respectively (see Table 11). The overall rule-out rate for this strategy was 54.6%, with NSTEMI being ruled out in 32.6% of participants, based on the presentation sample alone. 132 Using this strategy, 2 of 87 (2.29%) participants with NSTEMI were missed. 132 Full results (including the numbers of TP, FP, FN and TN test results) are provided in Appendix 2, Table 37.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Multiple sample strategies | |||||
| < 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours) | All | NSTEMI | 1 132 | 98 (92 to 100) | 64 (59 to 68) |
Diagnostic accuracy of the Ortho VITROS hs-cTnI assay
Study details
One diagnostic cohort study,170 which formed part of the APACE study,58 provided data on the diagnostic performance of the Ortho VITROS hs-cTnI assay. 170 This study assessed the accuracy of the Ortho VITROS hs-cTnI assay for the detection of AMI. Participants with STEMI were excluded (i.e. the target condition was NSTEMI rather than any AMI).
Single sample strategies
No single sample test strategies were assessed.
Multiple sample strategies
The study of Ortho VITROS hs-cTnI assay170 assessed the performance of a strategy incorporating measurements performed at baseline and at 1 hour. The strategy followed the structure of the ESC 0/1-hour rule-out pathway. The threshold used to rule out AMI was < 1 ng/l at presentation with a minimum symptom duration of 3 hours, OR < 2 ng/l at presentation together with an absolute change within 1 hour of < 1 ng/l for patients with symptom duration < 3 hours. The reported sensitivity of this strategy was 100% (95% CI 95% to 100%) and the specificity was 60% (95% CI 55% to 64%) (Table 12). The overall rule-out rate for this strategy was 52.9%, with NSTEMI being ruled out in 18% of participants based on the presentation sample alone. 170 No participants with NSTEMI were missed. 170 Full results (including the numbers of TP, FP, FN and TN test results) are provided in Appendix 2, Table 37. This strategy was selected for inclusion in our cost-effectiveness modelling, as it was the only strategy evaluated for the Ortho VITROS hs-cTnI assay.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours) | All | NSTEMI | 1 170 | 100 (95 to 100) | 60 (55 to 64) |
Diagnostic accuracy of the Quidel TriageTrue hs-cTnI assay
Study details
One diagnostic cohort study,173 which formed part of the APACE study,58 provided data on the diagnostic performance of the Quidel TriageTrue hs-cTnI assay. This study assessed the accuracy of the Quidel TriageTrue hs-cTnI assay for the detection of AMI. Participants with STEMI were excluded (i.e. the target condition was NSTEMI rather than any AMI).
Single sample strategies
No single sample test strategies were assessed.
Multiple sample strategies
One study170 assessed the performance of a Quidel TriageTrue hs-cTnI assay strategy, incorporating measurements performed at baseline and at 1 hour. The strategy followed the structure of the ESC 0/1-hour rule-out pathway. The threshold used to rule out AMI was < 4 ng/l at presentation with a minimum symptom duration of 3 hours, OR < 5 ng/l at presentation together with an absolute change within 1 hour of < 3 ng/l for patients with symptom duration < 3 hours. The reported sensitivity of this strategy was 100% (95% CI 97% to 100%) and the specificity was 66% (95% CI 62% to 70%) (Table 13). The overall rule-out rate for this strategy was 55.4%, with NSTEMI being ruled out in 45% of participants based on the presentation sample alone. 170 No participants with NSTEMI were missed. 170 Full results (including the numbers of TP, FP, FN and TN test results) are provided in Appendix 2, Table 37. This strategy was selected for inclusion in our cost-effectiveness modelling, as it was the only strategy evaluated for the Quidel TriageTrue hs-cTnI assay.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%)(95% CI) | Specificity (%)(95% CI) |
|---|---|---|---|---|---|
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | All | NSTEMI | 1 173 | 100 (97 to 100) | 66 (62 to 70) |
Diagnostic accuracy of the Siemens ADVIA Centaur hs-cTnI assay
Study details
Three studies, APACE,58 BEST115 and High-US (High-Sensitivity Cardiac Troponin I Assays in the United States),176 provided data on the diagnostic performance of the Siemens Healthcare ADVIA Centaur hs-cTnI assay. All three studies reported data for the target condition NSTEMI59,172,176 and one study176 also assessed the performance of the Siemens ADVIA Centaur hs-cTnI assay for the prediction of a MACE within 30 days of index presentation.
Single sample strategies
The BEST study172 assessed the diagnostic performance of a single sample taken at presentation and a low rule-out threshold (3 ng/l) for the target condition NSTEMI. The High-US study176 assessed the performance of three different thresholds (2 ng/l, 3 ng/l and 5 ng/l) in a single sample taken at presentation for both NSTEMI and MACEs. The 2-ng/l and the 5-ng/l thresholds were selected for inclusion in our cost-effectiveness modelling. Sensitivity and specificity estimates for these thresholds and summary estimates for the 3 ng/l threshold are provided in Table 14.
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Single sample strategies | |||||
| < 2 ng/l at 0 hours | All | NSTEMI | 1 176 | 100 (99 to 100) | 23 (21 to 25) |
| < 2 ng/l at 0 hours | All | MACE | 1176 | 100 (98 to 100) | 23 (22 to 25) |
| < 3 ng/l at 0 hours | All | NSTEMI | 2 | 99 (98 to 100) | 35 (33 to 36) |
| < 3 ng/l at 0 hours | All | MACE | 1176 | 99 (97 to 100) | 36 (33 to 38) |
| < 5 ng/l at 0 hours | All | NSTEMI | 1 176 | 99 (97 to 100) | 52 (50 to 54) |
| < 5 ng/l at 0 hours | All | MACE | 1176 | 99 (96 to 100) | 52 (50 to 54) |
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | All | NSTEMI | 1 59 | 99 (95 to 100) | 56 (52 to 60) |
| < 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours) | All | NSTEMI | 1 59 | 100 (95 to 100) | 67 (61 to 72) |
Multiple sample strategies
The APACE study59 evaluated two different multiple sample strategies using the Siemens ADVIA Centaur hs-cTnI assay. One strategy followed the structure of the ESC 0/1-hour rule-out pathway [i.e. an initial sample with a low threshold (3 ng/l) followed by repeat testing at 1 hour in patients whose initial troponin level was < 6 ng/l and who did not report a minimum symptom duration of 3 hours]. 59 The sensitivity and specificity estimates for this strategy were 99% (95% CI 95% to 100%) and 56% (95% CI 52% to 60%), respectively. The overall rule-out rate for this strategy was 46.4%, with NSTEMI being ruled out in 16% of participants based on the presentation sample alone. 59 Based on data from this study, use of the ESC 0/1-hour pathway would miss 1 of 114 (0.88%) people with NSTEMI. 59 The second study59 assessed a similar strategy, but with higher thresholds and repeat testing at 2 hours. The sensitivity estimates were similar for the two strategies, but the specificity of the 2-hour repeat testing strategy was higher than that of the 1-hour strategy (see Table 14). Full results are provided in Appendix 2, Table 37. Both strategies were selected for inclusion in our cost-effectiveness modelling.
Diagnostic accuracy of the Siemens Atellica hs-cTnI assay
Study details
Two studies, the High-STEACS trial61 and High-US,176 provided data on the diagnostic performance of the Siemens Healthcare Atellica hs-cTnI assay. Both studies67,176 reported data for the target condition NSTEMI and one study176 also assessed the performance of the Siemens Atellica hs-cTnI assay for the prediction of a MACE within 30 days of the index presentation.
Single sample strategies
The High-US study176 assessed the performance of three different thresholds (2 ng/l, 3 ng/l and 5 ng/l) in a single sample taken at presentation, for both NSTEMI and MACEs. The 2 ng/l threshold was selected for inclusion in our cost-effectiveness modelling. The sensitivity and specificity estimates for this threshold were 100% (95% CI 98% to 100%) and 26% (95% CI 24% to 28%), respectively (Table 15).
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Single sample strategies | |||||
| < 2 ng/l at 0 hours | All | NSTEMI | 1 176 | 100 (98 to 100) | 26 (24 to 28) |
| < 2 ng/l at 0 hours | All | MACE | 1176 | 99 (97 to 100) | 26 (24 to 28) |
| Multiple sample strategies | |||||
| ESC 0/1-hour pathway: (symptoms ≥ 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | All | NSTEMI | 167 | 94 (79 to 99) | 69 (64 to 74) |
| ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 34 ng/l (females), ≤ 53 ng/l (males) at 0 hours] OR [≤ 34 ng/l (females), ≤ 53 ng/l (males) at 0 and 3 hours] OR Δ < 50% of 99th centile at 0–3 hours | All | NSTEMI | 167 | 91 (87 to 94) | 74 (72 to 77) |
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours] | All | NSTEMI | 1 67 | 98 (95 to 99) | 74 (72 to 76) |
Multiple sample strategies
The High-STEACS study67 assessed the diagnostic performance of three different multiple testing strategies for the target condition NSTEMI. One strategy, defined as the ESC 0/1-hour pathway, used a combination of a minimum symptom duration of 3 hours and a low rule-out threshold (3 ng/l) on presentation, OR repeat testing in patients with a presentation troponin level < 6 ng/l AND symptom duration < 3 hours. A second strategy, defined as the ESC 0/3-hour pathway, used a combination of a minimum symptom duration of 6 hours and sex-specific thresholds, OR relative difference at 3 hours. Neither of the two ESC pathways for this assay met the minimum clinically acceptable sensitivity criterion for inclusion in cost-effectiveness modelling. The sensitivity and specificity estimates for these two strategies are provided in Table 15. The High-STEACS pathway combined an initial sample and a low (5 ng/l) threshold in patients reporting a minimum symptom duration of 2 hours with repeat testing at a later time point (3 hours) for patients in whom the initial hs-cTnI is less than the sex-specific 99th centile (i.e. 34 ng/l for females and 53 ng/l for males) and in whom symptom duration was < 2 hours. The High-STEACS pathway was selected for inclusion in our cost-effectiveness modelling. The sensitivity and specificity estimates for this strategy were 98% (95% CI 95% to 99%) and 74% (95% CI 72% to 76%), respectively. 67 The overall rule-out rate for this strategy was 64.5%, with NSTEMI being ruled out in 29.7% of participants based on the presentation sample alone. 67 In this study, application of the High-STEACS pathway missed 6 of 278 (2.16%) participants with NSTEMI. 67
Diagnostic accuracy of the Siemens Healthcare Dimension Vista hs-cTnI assay
Study details
One diagnostic cohort study,74 which formed part of the APACE study,58 provided data on the diagnostic performance of the Siemens Healthcare Dimension Vista hs-cTnI assay. This study74 assessed the accuracy of the Siemens Healthcare Dimension Vista hs-cTnI assay for the detection of AMI. Participants with STEMI were excluded (i.e. the target condition was NSTEMI rather than any AMI).
Single sample strategies
No single sample test strategies were assessed.
Multiple sample strategies
The study of Siemens Healthcare Dimension Vista hs-cTnI74 assessed the performance of a strategy incorporating measurements performed at baseline and absolute change within 1 hour. The threshold used to rule out AMI was < 5 ng/l at presentation and a change within the hour of < 2 ng/l, which was derived from a cohort of 750 patients. The strategy was validated with a further 750 patients. This strategy was selected for inclusion in our cost-effectiveness modelling, as it was the only strategy evaluated for the Siemens Dimension Vista hs-cTnI assay (Table 16).
| Test strategy | Population | Target condition | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|---|
| Multiple sample strategies | |||||
| < 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours | All | NSTEMI | 1 74 | 100 (97 to 100) | 66 (62 to 69) |
| Male | NSTEMI | 174 | 95 (87 to 99) | 62 (57 to 66) | |
| Female | 100 (89 to 100) | 73 (66 to 79) | |||
The sensitivity of the strategy was 100% (95% CI 97% to 100%) and specificity was 66% (95% CI 62% to 69%). Results were provided separately for male and female participants. Sensitivity for males was 95% (95% CI 87% to 99%) and for females it was 100% (95% CI 89% to 100%). Specificity for males was 62% (95% CI 57% to 66%) and for females it was 73% (95% CI 66% to 79%). Full results (including the numbers of TP, FP, FN and TN test results) are provided in Appendix 2, Table 37.
Comparative diagnostic accuracy for test strategies assessed for more than one assay in the same study
Seven studies58,61,64,68,101,115,176 reported accuracy data for more than one assay.
Four studies, ADAPT,68 APACE,58 ROMI-3 (Optimum Troponin Cutoffs for ACS in the ED),101 and TRUST,64 provided data to support a direct comparison between the Roche Elecsys hs-cTnT assay and the Abbott ARCHITECT hs-cTnI assay, using either the 99th centile for the general population, or LoD threshold and a single sample at presentation or both, for the target condition NSTEMI. As data for these combinations of assay threshold and timing are reported individually by a number of additional studies (see Diagnostic accuracy of the Roche Elecsys hs-cTnT assay and Diagnostic accuracy of the Abbott ARCHITECT hs-cTnI assay), it is possible to compare the estimates of relative sensitivity and specificity derived from indirect comparisons of summary estimates with those derived from direct, within-study comparisons (Table 17). Although the sensitivity estimates for the Roche Elecsys hs-cTnT assay, using the 99th centile for the general population threshold and a single sample at presentation, were higher than those for the Abbott ARCHITECT hs-cTnI assay (direct or indirect comparisons), neither assay achieved the minimum clinically acceptable sensitivity (97%). Based on these data, it is unlikely that using the 99th centile diagnostic threshold and a single sample at presentation would be considered adequate for rule out of NSTEMI. When the LoD threshold was used with a single sample at presentation, sensitivity estimates were comparable for the Roche Elecsys hs-cTnT assay and the Abbott ARCHITECT hs-cTnI assay (direct or indirect comparisons) and the sensitivity estimates were always ≥ 99%. The indirect comparison (based on summary estimates and one75 of the two direct comparisons) indicated that specificity was higher for the Roche Elecsys hs-cTnT assay (30%, 95% CI 27% to 33%) than for the Abbott ARCHITECT hs-cTnI assay (18%, 95% CI 16% to 21%). 75 The second direct comparison gave similar specificities for the Roche Elecsys hs-cTnT assay (18%, 95% CI 16% to 20%) and the Abbott ARCHITECT hs-cTnI assay (16%, 95% CI 14% to 18%). 101 These data indicate that the LoD threshold and a single sample at presentation is likely to be adequate for ruling out NSTEMI, using either the Roche Elecsys hs-cTnT assay or the Abbott ARCHITECT hs-cTnI assay. There is no clear evidence to support the choice of one assay over the other.
| Assay (threshold) | Indirect comparison | Direct comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADAPT68 | APACE70,75 | ROMI-3101 | TRUST64 | ||||||||
| n | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
| Roche Elecsys hs-cTnT (99th centile, 14 ng/l) | 14 | 90 (85 to 94) | 77 (68 to 84) | 91 (86 to 94) | 81 (79 to 83) | 92 (89 to 94) | 79 (77 to 81) | 92 (87 to 96) | 58 (55 to 62) | 84 (74 to 94) | 86 (83 to 88) |
| Abbott ARCHITECT hs-cTnI (99th centile, 26.2 ng/l) | 4 | 75 (64 to 84) | 94 (90 to 96) | 89 (84 to 93) | 94 (93 to 95) | 72 (67 to 76) | 93 (91 to 94) | 72 (64 to 80) | 90 (87 to 91) | 62 (49 to 74) | 97 (96 to 98) |
| Roche Elecsys hs-cTnT (LoD, 5 ng/l) | 6 | 99 (97 to 100) | 35 (25 to 46) | NR | 100 (97 to 100) | 30 (27 to 33) | 99 (96 to 100) | 18 (16 to 20) | NR | ||
| Abbott ARCHITECT hs-cTnI (LoD, 2 ng/l) | 4 | 100 (99 to 100) | 21 (16 to 26) | 100 (99 to 100) | 18 (16 to 21) | 99 (96 to 100) | 16 (14 to 18) | ||||
The APACE study58 provided data on the performance of the ESC 0/1-hour pathway using the rule-out thresholds specified for the Roches Elecsys hs-cTnT assay59,104 and the Abbott ARCHITECT hs-cTnI assay59,104 in the ESC 2015 guidelines for the management of ACSs in patients presenting without persistent ST segment elevation. 33 The APACE study also provided data on the performance of the ESC 0/1-hour pathway using rule-out thresholds derived for the Beckman Coulter ACCESS hs-cTnI,60 Siemens ADVIA Centaur hs-cTnI,59 Ortho VITROS hs-cTnI170 and Quidel TriageTrue hs-cTnI 173 assays. Although all six assay ESC 0/1-hour pathways were evaluated in participants from the APACE trial, only the Roche Elecsys hs-cTnT, Abbott ARCHITECT hs-cTnI and Siemens ADVIA Centaur hs-cTnI assays were evaluated in the same patient subgroup (reported in a single publication59). For this reason, the comparison of Roche Elecsys hs-cTnT, Abbott ARCHITECT hs-cTnI and Siemens ADVIA Centaur hs-cTnI assays has been rated as having a low risk of bias with respect to the flow and timing domain of QUADAS-2C, whereas the all tests comparison was rated as having a high risk of bias (see Table 6). The comparative sensitivity and specificity estimates for the rule-out threshold of the ESC 0/1-hour pathway are provided in Table 18, with those estimates that were derived from the same participant subgroup of the APACE study highlighted in bold. Data from the APACE study58 indicate that the ESC 0/1-hour rule-out pathway performs consistently across all six hs-cTn assays evaluated (sensitivity estimates were always ≥ 98%).
| Assay | Threshold | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| Roche Elecsys hs-cTnT | (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 99 (95 to 100) | 69 (65 to 73) |
| Abbott ARCHITECT hs-cTnI | (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 98 (94 to 100) | 65 (60 to 69) |
| Beckman Coulter ACCESS hs-cTnI | (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours) | 99 (94 to 100) | 70 (66 to 74) |
| Ortho VITROS hs-cTnI | (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours) | 100 (95 to 100) | 60 (55 to 64) |
| Quidel TriageTrue hs-cTnI | (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 100 (97 to 100) | 66 (62 to 70) |
| Siemens ADVIA Centaur hs-cTnI | (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 99 (95 to 100) | 56 (52 to 60) |
The High-STEACS study61 provided data on the rule-out performance of the ESC 0/1-hour pathway, the ESC 0/3-hour pathway and the High-STEACS 0/3-hour pathway, using the Abbott ARCHITECT hs-cTnI assay66 and the Siemens Atellica hs-cTnI assay. 67 As results for the two assays were published separately66,67 and neither assay was evaluated in all participants in the High-STEACS study,61 it is not clear that the same group of study participants received both assays. For this reason, the comparison has been rated as having a high risk of bias with respect to the flow and timing domain of QUADAS-2C, whereas the all tests comparison was rated high risk of bias (see Table 6). The comparative sensitivity and specificity estimates for the rule-out thresholds of each pathway and assay combination are provided in Table 19. Data from this study indicated that the sensitivity of the ESC 0/1-hour pathway was lower using the rule-out thresholds developed for the Siemens Atellica hs-cTnI assay (94%, 95% CI 79% to 99%)67 than using the recommended ESC recommended rule-out thresholds33 for the Abbott ARCHITECT hs-cTnI assay 100% (95% CI 91% to 100%). 66 The sensitivity ESC 0/1-hour rule-out pathway developed for the Siemens Atellica hs-cTnI assay did not reach the specified minimum clinically acceptable value of 97% and therefore this strategy was not included in our cost-effectiveness modelling. The sensitivity and specificity estimates for the ESC 0/3-hour rule-out pathway were similar using either the Abbott ARCHITECT hs-cTnI assay66 or the Siemens Atellica hs-cTnI assay;67 however, neither reached the specified minimum clinically acceptable value of 97%. The sensitivity and specificity estimates for the High-STEACS 0/3-hour rule-out pathway were also similar using either the Abbott ARCHITECT hs-cTnI assay66 or the Siemens Atellica hs-cTnI assay67 and both were ≥ 98%, indicating that the High-STEACS pathway is likely to be adequate for ruling out NSTEMI.
| Assay | Pathway: threshold | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| Abbott ARCHITECT hs-cTnI | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 h AND Δ < 2 ng/l at 0 to 1 hours) | 100 (91 to 100) | 78 (73 to 82) |
| Siemens Atellica hs-cTnI | ESC 0/1-hour pathway: (symptoms ≥ 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 94 (79 to 99) | 69 (64 to 74) |
| Abbott ARCHITECT hs-cTnI | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 ng/l (F) ≤ 34 ng/l (M) at 0 hours] OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 91 (87 to 94) | 74 (72 to 77) |
| Siemens Atellica hs-cTnI | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 34 ng/l (F) ≤ 53 ng/l (M) at 0 hours] OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 90 (86 to 93) | 81 (79 to 82) |
| Abbott ARCHITECT hs-cTnI | High-STEACS 0/3-hour pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l] | 99 (97 to 100) | 76 (73 to 78) |
| Siemens Atellica hs-cTnI | High-STEACS 0/3-hour pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours] | 98 (95 to 99) | 74 (72 to 76) |
The High-US study compared the performance of two Siemens hs-cTnI assays (the Atellica and ADVIA Centaur) using three low thresholds and a single sample at presentation, for the target condition NSTEMI. 176 All three of the thresholds assessed were above the LoD (1.6 ng/l) for the assays. Table 20 provides comparative sensitivity and specificity estimates for the two assays. The results of this study indicate consistent performance between the two Siemens assays evaluated for all three thresholds. The sensitivity estimates were ≥ 99% for both assays at all three thresholds, indicating that a single sample at presentation and a low threshold (above the LoD) is likely to be adequate for ruling out NSTEMI.
| Assay | Threshold | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| Siemens Atellica hs-cTnI | 2 ng/l | 100 (98 to 100) | 26 (24 to 28) |
| Siemens ADVIA Centaur hs-cTnI | 2 ng/l | 100 (99 to 100) | 23 (21 to 25) |
| Siemens Atellica hs-cTnI | 3 ng/l | 99 (97 to 100) | 37 (35 to 40) |
| Siemens ADVIA Centaur hs-cTnI | 3 ng/l | 99 (97 to 100) | 35 (33 to 37) |
| Siemens Atellica hs-cTnI | 5 ng/l | 99 (97 to 100) | 53 (51 to 55) |
| Siemens ADVIA Centaur hs-cTnI | 5 ng/l | 99 (97 to 100) | 52 (50 to 54) |
The BEST study172 provided data to compare the rule-out performance two single sample at presentation strategies based on different assays, the Siemens ADVIA Centaur assay using a threshold of 3 ng/l172 and the Roche Elecsys hs-cTnT assay using the LoD (5 ng/l) threshold. 115 Data for the two assays were reported in separate publications with different numbers of participants (subgroups of the BEST study population) and for this reason the comparison has been rated as having a high risk of bias with respect to the flow and timing domain of QUADAS-2C (see Table 6). The sensitivity estimates were similar for the Roche Elecsys hs-cTnT assay (99%, 95% CI 93% to 100%)115 and the Siemens ADVIA hs-cTnI assay (99%, 95% CI 96% to 100%),172 whereas the Roche Elecsys hs-cTnT assay had higher specificity (47%, 95% CI 43% to 51%)115 than the Siemens ADVIA Centaur hs-cTnI assay (33%, 95% CI 30% to 36%). 172
Selection of test strategies for inclusion in cost-effectiveness modelling
Test strategies for each hs-cTn assay were selected for inclusion in cost-effectiveness modelling based on optimal diagnostic performance, as indicated by data from the systematic review. Data from studies that excluded patients with STEMI (i.e. where the target condition was NSTEMI) were preferentially selected.
Each test strategy is defined by a combination of four factors: (1) assay, of which there are nine, (2) number of tests (up to two), (3) timing of tests (between 0 and 3 hours) and (4) threshold concentration, of which there are many. This implies that there are many tens of possible strategies to compare in the cost-effectiveness analysis (CEA), which would be of questionable feasibility to construct, analyse and present as a full incremental analysis. It is also unnecessary to compare strategies that could be determined to be dominated before conducting the CEA. Therefore, all dominated strategies were eliminated by considering the factors that might affect either the total cost or quality-adjusted life-years (QALYs) [i.e. sensitivity, specificity, assay (assume a different cost for each one) and number and timing of tests (the greater number and later administration implies a higher cost)]. According to these criteria, the final number of non-dominated strategies was > 40, and so deemed to be still too high. Therefore, given that the main basis of considering these strategies was the idea that they might facilitate the safe rule out of those without a NSTEMI, the clinical experts on the specialist committee for this assessment were consulted to determine whether or not there was a minimum acceptable sensitivity (i.e. a maximum FN rate). They were asked the following.
We have now reached the stage, with this assessment, where decisions need to be made regarding which test strategies will be included in our cost effectiveness modelling.
This is problematic because, as I’m sure you will be aware, the volume of data has increased markedly since our previous assessment and there remains a lack of consistency with respect to test strategies evaluated; our final data set comprises over 60 distinct combinations of assay, threshold and timing.
Given the very large number of possible strategies, we considered limiting the strategies to be included in the CEA model to those for which it can be determined, before CEA, that they are not dominated. This approach would be based on criteria that might affect either the total cost or QALYs [quality-adjusted life years]:
However, using this approach still results in around 40 non-dominated strategies.
Even if it were feasible to model this number of strategies, interpretation of CE [cost-effectiveness] results with this many comparators is very challenging, particularly where, as in this case, the differences are likely to be small.
Therefore, we would like to request your input to determine a minimum clinically acceptable sensitivity which we will then use as an initial criterion to select strategies for CE modelling. In this context, please could you provide your opinion on what should constitute the minimum sensitivity.
On the basis of the responses of the clinical experts (see Appendix 6), an additional criterion, minimum sensitivity of 97%, was applied. As a result of this, the number of strategies was reduced to a manageable number of 21 (Table 21).
| Test strategy | Number of studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| Roche Elecsys hs-cTnT | |||
| LoD (< 5 ng/l) at 0 hours | 663,75,87,101,115,139 | 99 (97 to 100) | 35 (25 to 46) |
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 1104 | 99 (98 to 100) | 68 (67 to 70) |
| < 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours | 380,91,100 | 98 (97 to 99) | 73 (71 to 74) |
| < 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours | 187 | 100 (93 to 100) | 45 (40 to 49) |
| 99th centile threshold (< 14 ng/l at 0 hours AND 3 hours) | 1148 | 100 (89 to 100) | 77 (58 to 90) |
| Abbott ARCHITECT hs-cTnI | |||
| LoD (< 2 ng/l) at 0 hours | 458,71,96,101 | 100 (99 to 100) | 21 (16 to 26) |
| < 4 ng/l at 0 hours | 271,101 | 99 (97 to 100) | 50 (48 to 52) |
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 266,104 | 99 (98 to 100) | 57 (56 to 59) |
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours] | 166 | 99 (97 to 100) | 76 (73 to 78) |
| Beckman Coulter Access hs-cTnI | |||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours) | 160 | 99 (94 to 100) | 70 (66 to 74) |
| (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) | 1171 | 98 (92 to 100) | 83 (81 to 86) |
| bioMérieux VIDAS hs-cTnI | |||
| < 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours) | 1132 | 98 (92 to 100) | 64 (59 to 68) |
| Ortho VITROS hs-cTnI | |||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours) | 1170 | 100 (95 to 100) | 60 (55 to 64) |
| Quidel TriageTrue hs-cTnI | |||
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 1173 | 100 (97 to 100) | 66 (62 to 70) |
| Siemens ADVIA Centaur hs-cTnI | |||
| < 2 ng/l at 0 hours | 1176 | 100 (99 to 100) | 23 (21 to 25) |
| < 5 ng/l at 0 hours | 1176 | 99 (97 to 100) | 52 (50 to 54) |
| ESC 0/1-hour pathway: (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 159 | 99 (95 to 100) | 56 (52 to 60) |
| < 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours) | 159 | 100 (95 to 100) | 67 (61 to 72) |
| Siemens Atellica hs-cTnI | |||
| < 2 ng/l at 0 hours | 1176 | 100 (98 to 100) | 26 (24 to 28) |
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours] | 167 | 98 (95 to 99) | 74 (72 to 76) |
| Siemens Dimension Vista hs-cTnI | |||
| < 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours | 174 | 100 (97 to 100) | 66 (62 to 69) |
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of hs-cTn assays (used up to 4 hours from the onset of chest pain/presentation) compared with the current standard of serial troponin T and/or I testing on admission and at 10–12 hours after the onset of symptoms for the early rule out of AMI in people with acute chest pain.
Review of economic analyses of hs-cTn assays
Search strategy
The search strategies used to identify clinical effectiveness studies, detailed in Chapter 3, Search strategy, were also employed to identify any cost studies since 2013. Details of the databases searched for this update are provided in Chapter 3, Search strategy, and full strategies are provided in Appendix 1. Search strategies utilised in the original report2 were updated with any new interventions identified in the NICE scope. Search strategies were based on intervention (high-sensitivity troponin assays) and target condition, as recommended in the CRD guidance for undertaking reviews in health care39 and the Cochrane Handbook for DTA Reviews. 41
Additional top-up searches were run to identify any specific cost studies from the UK that utilise a cost filter together with the NICE UK geographic filter;179,180 these strategies and the filters used are also detailed in Appendix 1.
The following databases were searched on 10 January 2020 for relevant UK cost studies from 2013 to the present:
-
MEDLINE ALL (Ovid): 1946 to 9 January 2020
-
EMBASE (Ovid): 1974 to 9 January 2020
-
American Economic Association’s electronic bibliography (EconLit) (EBSCOhost): 2013 to 9 January 2020
-
NHS Economic Evaluation Database (NHS EED) (URL: www.crd.york.ac.uk/CRDWeb/): 2013 to March 2015.
Inclusion criteria
Studies reporting a full economic analysis that explicitly related to the cost-effectiveness of hs-cTn or standard cTn (with cTn implying either cTnI or cTnT) testing, with survival and/or QALYs as an outcome measure, were eligible for inclusion. Specifically, one of the strategies had to include cTn testing. Studies that reported a cost analysis of cTn testing only were not included in the review.
Results
Five studies, identified in our previous assessment report,2 are described below and summarised in Table 22.
| Study detail | Included study | ||||
|---|---|---|---|---|---|
| Goodacre et al.;181 Fitzgerald et al.182 | Vaidya et al.183 | Thokala et al.;184 Goodacre et al.185 | CADTH report186 | Collinson et al.159 | |
| Population | People presenting to hospital with chest pain due to suspected, but not proven, AMI and no other potentially serious alternative pathology or comorbidity | Patients presenting to the hospital with chest pain | Patients attending hospital with symptoms suggesting MI, but a normal or non-diagnostic ECG and no major comorbidities requiring hospital treatment | 65-year-old patients presenting to an ED with ischemic chest pain, without an ST segment elevation ECG who require cTn testing for diagnosis of NSTEMI | Patients presenting to hospital with symptoms suggestive of MI but with no diagnostic ECG changes (ST deviation > 1 mm or T-wave inversion > 3mm), no known history of CHD and no major comorbidities requiring inpatient treatment |
| Time horizon | Lifetime | Lifetime | Lifetime | Lifetime | Lifetime |
| Objective | Estimate the cost-effectiveness of the PoC panel in terms of mean costs and QALYs accrued compared with standard care | Assess the cost-effectiveness of a hs-cTnT, alone or combined with the H-FABP assay, in comparison with the cTnT assay for the diagnosis of AMI | Estimate the incremental cost per QALY of delayed troponin testing compared with presentation testing and no testing to determine which diagnostic strategy should be recommended | To investigate the cost-effectiveness of hs-cTnT and hs-cTnI assays compared with each other, as well as with cTnI assays, in patients with suspected ACS symptoms in the ED | Assess the cost-effectiveness of measuring a combination of biomarkers compared with measurement of cTn alone |
| Source of effectiveness information | Data from within the trial up to 3 months and, beyond this, lifetime costs and QALY estimates were used from a previous economic evaluation | No information | Sensitivity and specificity were taken from the meta-analysis, as reported in the Goodacre et al. report;185 the RATPAC trial159 was used for sampling patient characteristics; the Mills et al. report187 was used to assess risk of reinfarction and death, and Polanczyk et al.188 was used for life expectancy of patients with MI and myocardial re-infarction | Sensitivity and specificity from review performed in same report. Proportion of UA and mortality estimated based on published studies, and one unpublished study. Utility decrements based on published study | Sensitivity and specificity data derived from data from the HTA (RATPAC) itself, short-term survival and probability of reinfarction based on Mills et al.187 Source for long-term survival and QALYs not specified |
| Comparators |
Diagnostic assessment using the PoC biochemical marker panel Conventional diagnostic assessment without the panel |
Conventional cTnT hs-cTnT hs-cTnT combined with the H-FABP assay |
No biochemical testing: discharge all patients without treatment (hypothetical) Standard troponin assay measured at presentation using the 10% CoV as the threshold for positivity Standard troponin assay measured at presentation using the 99th centile threshold High-sensitivity troponin assay measured at presentation using the 99th centile threshold Standard troponin assay measured at presentation and 10 hours after symptom onset using the 99th centile threshold |
hs-cTnT hs-cTnI cTnI |
No testing: discharge all patients without treatment hs-cTn at presentation: discharge home if test is negative or admit to hospital for troponin testing at 10–12 hours if positive hs-cTn and a combination of cytoplasmic or neurohormone biomarkers at presentation: discharge home if both tests are negative or admit to hospital for troponin testing at 10–12 hours if either test is positive hs-cTn at presentation and at 90 minutes, as in the RATPAC protocol: discharge home if both tests are negative or admit to hospital for troponin testing at 10–12 hours if either test is positive Standard troponin testing at 10–12 hours |
| Unit costs | Microcosting study within RATPAC and PSSRU unit costs | No information | Admission and treatment were based on the national tariff. Lifetime costs for MI patients were taken from Ward et al.189 The price of a troponin test was taken from Goodacre et al.181 | Costs of hospital admission were based on the Ontario Case Costing Initiative database and the Ontario Schedule of Benefits for Physician Services. Costs of ED visits were based on a hospital in Soutwestern Ontario, Canada, and the Ontario Schedule of Benefits. Unit prices of cTn tests were based on information provided by the manufacturers | Hospital stay and treatment for MI based on NHS reference cost and biochemical testing based on Goodacre et al.181 |
| Measure of benefit | QALYs | AMI survivor | QALYs | QALYs | QALYs |
| Study type | Trial-based economic evaluation up to 3 months, decision tree lifetime, cost–utility analysis | Model-based cost-effectiveness and cost–utility study | Model-based cost–utility analysis | Model-based cost–utility analysis | Model-based cost–utility study |
| Model assumptions |
2-hour delay between sampling and results available 4 hours after presentation at ED patients moves to inpatient department 1-hour delay between presentation and start of biomarker sampling After a short term (test–treatment–outcome), progress depends on whether or not patient had MI and whether or not this was treated only |
No information |
10-hour troponin testing has perfect sensitivity and specificity (as it is the reference standard) 2-hour delay from the time at which sampling could be performed to results available For presentation testing strategies, decision made within 1 hour of results available For 10-hour testing strategies, decision made according to scenario applied Diagnostic strategy influences outcomes among patients with MI only |
Non-NSTEMI patients are further classified into UA or non-ACS, with consequences for costs and outcome There is a small survival benefit (relative risk of 1-year mortality 1.01) of treating early compared with treating late (presentation testing vs. standard testing) |
10-hour troponin testing has perfect sensitivity and specificity (as it is the reference standard) Presentation blood tests taken in ED, and results available and decision made within 2 hours of sampling For testing at 10–12 hours, delays according to scenario used |
| Perspective | NHS | Health care | NHS | Publicly funded health-care system | NHS in England and Wales |
| Discount rate | No information | No information | No information | 5% discount rate applied to costs and QALYs | No information |
| Uncertainty around cost-effectiveness ratio expressed | Incremental cost-effectiveness plane and probability of strategy being dominated/cost-effective | CEACs (not shown in abstract) | CEACs for PSA results, per scenario | As reported in outcomes of one-way sensitivity analyses,186 and also (for the PSA) in CEACs | CEACs |
| Sensitivity analysis | PSA | One way and probabilistic | One-way sensitivity analyses, scenario analyses (doctor on demand, twice-daily ward round and once-daily ward round) and PSA | One way and probabilistic | Secondary analysis using cTnI instead of cTnT, scenario analysis (doctor on demand, once-daily ward round, twice-daily ward round) and PSA |
| Outcome (cost and life-years/QALYs) per comparator |
Empirical 3 months - PoC: £1217; QALY 0.158 Standard care: £1006; QALY 0.161 For the model, no outcomes per comparator were reported |
No information |
For doctor-on-demand scenario, per 1000 patients without known CAD No testing: £965,994; QALY 26,227 Presentation standard troponin: 10% CoV; £1,560,361; QALY 26,345 Presentation standard troponin, 99th percentile: £1,609,760; QALY 26,352 Presentation high-sensitivity troponin, 99th percentile: £1,806,910; QALY 26,279 10-hour troponin: £2,016,540; QALY 26,286 |
cTnI: CA$2018; QALY 8.1385 hs-cTnI: CA$2082; QALY 3.1389 hs-cTnT: CA$2186; QALY 8.1399 |
For doctor-on-demand scenario, per 1000 patients: No testing: £965,994; QALY 26,227 hs-cTnT at presentation: £1,581,263; QALY 26,349 hs-cTnT at presentation and 90 minutes: £1,715,526; QALY 26,354 hs-cTnT and H-FABP at presentation: £1,682,362; QALY 26,359 10-hour troponin: £2,016,540; QALY 26,386 |
| Summary of incremental analysis |
Empirical 3 months: Increment PoC vs. standard care: £211; QALY –0.00282 Probability PoC cost-effective at £20,000/QALY = 0.4% Decision model 3 months: Increment PoC vs. standard care: £169; QALY –0.002 Probability PoC cost-effective at £20,000/QALY = 22.3% Decision model lifetime: Increment PoC vs. standard care: £329; QALY –0.087 Probability PoC cost-effective at £20,000/QALY = 33.6% |
hs-cTnT vs. cTnT: incremental cost €111; 16/17 lives per 1000 AMI; ICER €3748/QALY hs-cTnT + H-FABP vs. cTnT: incremental cost €178; ICER €5717/QALY |
For doctor-on-demand scenario: Presentation standard troponin: 10% CoV vs. no testing; £5030/QALY Presentation standard troponin 99th percentile vs. presentation standard troponin: 10% CoV; £6518/QALY Presentation high-sensitivity troponin 99th percentile vs. presentation standard troponin 99th percentile: £7487/QALY 10-hour troponin vs. presentation high-sensitivity troponin 99th percentile: £27,546/QALY |
cTnI reference hs-cTnI: incremental costs CA$64; incremental QALYs 0.000352 dominated (by extension) hs-cTnT: incremental costs CA$168; incremental QALYs 0.001408; ICER CA$119,377/QALY |
No testing: reference strategy hs-cTnT compared vs. testing: ICER £5012/QALY hs-cTnT at presentation and at 90 minutes: dominated hs-cTnT and H-FABP vs. hs-cTnT at presentation: ICER £11,026/QALY (as reported, but correct value should be £10,871) 10-hour troponin vs. hs-cTnT and H-FABP: ICER £12,090/QALY Conclusion: if a rapid rule-out strategy with a sensitivity of 95% (and a specificity of around 90%) is available, then a 10-hour troponin strategy does not seem cost-effective |
Goodacre et al.181 and Fitzgerald et al.182
The study by Fitzgerald et al. 182 was based on the RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac Markers) multicentre pragmatic controlled trial. 181 An economic evaluation was undertaken to assess the cost-effectiveness of management based on testing with a panel of point-of-care cardiac markers compared with management without point-of-care panel assessment. The included population consisted of patients presenting to hospital with chest pain due to suspected, but not proven, AMI and no other potentially serious alternative pathology or comorbidity. The analysis was performed from an NHS perspective, using trial data to estimate the mean costs per patient of chest pain-related care and the mean number of QALYs accrued by patients in each arm of the trial, with a time horizon of 3 months. In addition, a decision-analytic model was constructed to duplicate (validate) trial results and extrapolate results to a longer time horizon.
Resource use data were collected for all patients. Cost and outcome data were collected using patient notes and self-completed questionnaires. Unit prices were based partly on a micro-costing study on a sample of patients, partly on a study previously undertaken by the investigators, and partly on purchase price and national unit costs. QALYs were calculated based on EuroQol-5 Dimensions measurements. In a sensitivity analysis, productivity costs were included, as reported by the patients.
As it was anticipated that the trial would have limited power to detect a difference in major adverse events, the decision-analytic model was intended to explore whether or not uncertainty around the effect of the intervention on the major adverse event rate could influence the potential cost-effectiveness of the intervention. The model used trial data to estimate costs and QALYs for up to 3 months. Beyond this, lifetime cost and QALYs were estimated from a previous study. 190 It was assumed that patients who had died at 3 months would accrue no further costs or QALYs. Those who had survived non-fatal MI would accrue costs and QALYs associated with coronary heart disease (CHD) (estimated at £10,079 and 6.829, respectively). Those without CHD were assigned zero costs and 20 QALYs.
Empirical results showed that the point-of-care test strategy was dominated by standard care, which delivered slightly more QALYs at a lower cost. The probability that point-of-care testing would be more cost-effective than standard care at a willingness-to-pay threshold of £20,000 per QALY was < 1%. The decision-analytic model, again, resulted in higher costs and less effect for the point-of-care panel assay compared with standard care, and also when extrapolated to lifetime survival. The probability of the point-of-care panel assay being cost-effective for the 3-month and lifetime model was 22.3% and 33.6%, respectively.
The main conclusion was that point-of-care panel assay testing is unlikely to be considered cost-effective in the NHS, with an 89% probability that standard care is dominant. Cost-effectiveness was mainly driven by differences in mean cost, with point estimates suggesting that, per patient, point-of-care panel assessment was £211 more expensive than standard care.
Vaidya et al.183
Vaidya et al. 183 aimed to assess the cost-effectiveness of an hs-cTnT assay, alone or in combination with the heart-type fatty acid-binding protein (H-FABP), in comparison with the conventional cTnT assay for the diagnosis of AMI in patients presenting to hospital with chest pain. A decision-analytic model was developed to perform both a cost–utility analysis (cost per QALY gained) and a CEA (cost per life-year gained and cost per AMI averted), using a health-care perspective and a lifetime time horizon. One-way and probabilistic sensitivity analyses (PSAs) were conducted.
The incremental cost-effectiveness ratio (ICER) for hs-cTnT compared with conventional cTnT was €3748 per QALY gained. For hs-cTnT in combination with H-FABP compared with conventional cTnT, the ICER was €5717 per QALY gained. For life-years and AMI averted, no ICERs were reported in the abstract. The PSA showed the hs-cTnT assay to be the preferable strategy, with a probability of > 90% at a ceiling ratio of €4800 per QALY. This led to the conclusion that the hs-cTnT assay is very cost-effective relative to the conventional cTnT assay. Combining hs-cTnT with H-FABP did not seem to offer any additional economic or health benefit over the hs-cTnT test alone.
Goodacre et al.185 and Thokala et al.184
Goodacre et al. 185 and Thokala et al. 184 aimed to estimate the cost-effectiveness of using alternative biomarker strategies to diagnose MI, and using biomarkers, computed tomography coronary angiography and exercise electrocardiography to risk-stratify troponin-negative patients. As the second aim was outside the scope of this review, we have summarised the analysis that compares the biomarker strategies for diagnosing MI only, referred to in the HTA report as ‘the diagnostic phase model’. The different diagnostic strategies were applied to a hypothetical cohort of patients attending the ED with suspected, but not proven, ACS. Patient characteristics were defined using data from the RATPAC trial,191 as well as patients’ arrival times during the day at the ED. The model assigned each patient a probability of reinfarction or death, depending on their characteristics and whether or not they had treatment. The model took a lifetime time horizon. The economic perspective was that of the NHS in England and Wales.
The following strategies were applied to each patient.
-
No testing [discharge all patients without treatment (hypothetical)].
-
Standard troponin assay measured at presentation using the 10% CoV as the threshold for positivity.
-
Standard troponin assay measured at presentation using the 99th centile threshold.
-
High-sensitivity troponin assay measured at presentation using the 99th centile threshold.
-
Standard troponin assay measured at presentation and 10 hours after symptom onset using the 99th centile threshold.
Blood tests at presentation were assumed to be taken in the ED and so a decision could be made within 1 hour of the test results becoming available. For the 10–12 hours troponin measurement, three different scenarios were tested:
-
the ‘doctor-on-demand’ scenario, with medical staff available 24 hours a day to make a disposition decision within 1 hour of the results being available
-
the twice-daily ward round scenario, with medical staff only available at twice-daily ward rounds to make disposition decisions
-
the once-daily ward round scenario, with medical staff only available at a once-daily ward round to make disposition decisions.
Sensitivity and specificity estimates for the presentation troponin tests were obtained by performing a meta-analysis of estimates from individual primary studies included in the accompanying review. The 10-hour troponin test was assumed to have perfect sensitivity and specificity, as it was the reference standard for the review. This implies that patients with a FP test on the hs-cTn testing at presentation will still be discharged home after the 10- to 12-hour troponin test, but patients with a FN test will be discharged home without treatment. The ‘discharge without testing or treatment’ by definition has perfect specificity, but a sensitivity of 0%.
The risk of reinfarction and death for patients with MI was based on a study by Mills et al. 187 Life expectancy of patients with MI and MI with reinfarction was estimated from Polanczyk et al. ,188 and the utility of patients with MI was based on Ward et al. 189 The utility of patients with reinfarction was estimated by using a multiplicative factor of 0.8 for patients with MI (expert opinion). Patients without MI were assigned the life expectancy and utility scores of the general population. Lifetime costs for patients with MI were based on Ward et al. 189 One-way sensitivity analyses were performed, as well as a PSA. In a secondary analysis, a strategy was added that involved alternative biomarkers in combination with the presentation troponin testing.
The results showed that measuring a 10-hour troponin level in all patients was the most effective strategy (ICER £27,546–103,560). However, at a threshold of £30,000 per QALY, the optimal strategy in all but one scenario was measurement of high-sensitivity troponin at presentation, with a 10-hour troponin test if positive and discharge home if negative (ICER £7487–17,191/QALY). The exception was a scenario involving patients without known CAD and a doctor available on demand to discharge the patient, when, using the £30,000 per QALY threshold, the strategy of measuring a 10-hour troponin level in all patients was optimal (ICER of £27,546/QALY). Sensitivity analyses showed the optimal strategy to vary with different levels of sensitivity and timing of the tests.
The report concluded that the additional costs that are likely to be incurred by measuring a 10-hour troponin level, compared with a presentation high-sensitivity troponin level, are unlikely to represent a cost-effective use of NHS resources in most of the scenarios tested.
CADTH optimal use report186
The CADTH report186 aimed to determine the cost-effectiveness of hs-cTnT and hs-cTnI assays compared with each other, as well as with cTnI assays in patients with suspected ACS symptoms in the ED. For this purpose, three comparators were considered: (1) hs-cTnT, (2) hs-cTnI and (3) cTnI. As cTnT is no longer available in Canada, it was not taken into account in the analysis. The target population consisted of patients aged 65 years presenting to the ED, without ST segment elevation, who required cTn testing for diagnosis of NSTEMI. For the economic evaluation, a decision tree was constructed that calculated lifetime cost per QALY from the perspective of a publicly funded health-care system.
The model consisted of a short-term part (which had a time horizon of 1 year) and a long-term part. The short-term part incorporated the testing and treatment procedures and short-term outcomes. Patients were tested at presentation at the ED and if they were not admitted to hospital after the first test they were tested again after 6 hours. When the patient was admitted after the first test, treatment was said to be initiated early, and when a patient was admitted after the second test, treatment was late. One year mortality depended on whether or not a patient had NSTEMI and whether they were treated early, treated late or untreated (in the case of FN test results). Those not suffering from NSTEMI were further stratified into UA or not having ACS. The annual probability of death in the long-term part of the model was dependent on patient age, gender and whether they had suffered an NSTEMI, UA or did not have any type of ACS in the short-term part of the model.
The sensitivity and specificity for each cTn test at presentation to the ED was derived from the systematic review that was also part of this study. In the model, patients with a negative cTn test at presentation were assumed to be observed and have a second cTn test 6 hours later. After the second cTn test, 90% of these FNs were assumed to become TPs.
Short-term mortality rates and relative risks (RRs) for treated/non-treated patients were taken from published clinical studies and one non-referenced study. The relative risk for late treatment compared with early treatment was derived from expert opinion. Long-term mortality rates were taken from published clinical studies and one non-referenced study. QALYs were calculated by incorporating an age-specific utility decrement for patients with NSTEMI. A number of one-way sensitivity analyses were performed, as well as a PSA.
The base-case results indicated that hs-cTnI was dominated by hs-cTnT, when compared to cTnI, at an ICER of CA$119,377 per QALY. The PSA showed that for willingness-to-pay thresholds up to CA$124,000, cTnI had the highest probability of being cost-effective. For willingness-to-pay thresholds > CA$124,000, hs-cTnT had the highest probability of being cost-effective. The hs-cTnI test was not likely to be cost-effective for any value of the threshold.
The authors concluded that hs-cTnT would be considered the most cost-effective testing strategy if willingness to pay for a QALY is ≥ CA$119,377, otherwise cTnI would be the most cost-effective test. However, there was a lot of uncertainty in results when model assumptions were changed.
Collinson et al.159
Collinson et al. 159 used a decision tree developed in the related HTA by Goodacre et al. 185 to compare the cost-effectiveness of five diagnostic strategies for a hypothetical cohort of patients presenting to hospital with symptoms suggestive of MI but with no diagnostic ECG changes, no known history of CHD and no major comorbidities requiring inpatient treatment. Essentially, this was a substudy of the point-of-care arm of the RATPAC trial. All methods and model inputs were identical to the study by Thokala et al. 184 and the HTA report by Goodacre et al. ,185 but with slightly different strategies applied to the cohort of patients.
-
No testing: discharge all patients without treatment (theoretical ‘zero’ option).
-
hs-cTnT at presentation: discharge home if test is negative or admit to hospital for troponin testing at 10–12 hours if positive.
-
hs-cTnT and H-FABP at presentation: discharge home if both tests are negative or admit to hospital for troponin testing at 10–12 hours if either test is positive.
-
hs-cTnT at presentation and at 90 minutes, as in the RATPAC protocol: discharge home if both tests are negative or admit to hospital testing at 10–12 hours if either test is positive.
-
Standard troponin testing at 10–12 hours.
The difference with the other studies is in the addition of H-FABP in the third strategy, and in the second high-sensitive troponin test at 90 minutes in the fourth strategy. In a secondary analysis, cTnT was replaced by cTnI. Sensitivity and specificity of presentation biochemical testing were estimated using data from within the study (RATPAC). Standard troponin testing at 10–12 hours was assumed to have perfect sensitivity and specificity, as this was the reference standard.
At the £20,000 per QALY threshold, 10-hour troponin testing was cost-effective (£12,090/QALY) in the doctor-on-demand scenario, but not in the other scenarios (once-daily ward round and twice-daily ward rounds), where hs-cTnT and H-FABP measurement at presentation was cost-effective. At the £30,000 per QALY threshold, 10-hour troponin testing was cost-effective in the doctor-on-demand scenario and twice-daily ward rounds scenario (£24,600/QALY), whereas the hs-cTnT and H-FABP measurement at presentation strategy was cost-effective (£14,806/QALY) in the once-daily ward round scenario. Secondary analysis using cTnI instead of cTnT showed that cTnI testing at presentation and at 90 minutes was cost-effective in all three scenarios at the £20,000 per QALY threshold and in two of the scenarios at the £30,000 per QALY threshold, with 10-hour troponin being cost-effective in the doctor-on-demand scenario only (£24,327/QALY). The overall conclusion was that 10-hour troponin testing is only likely to be cost-effective compared with rapid rule-out strategies if patients can be discharged as soon as a negative result is available and a £30,000 per QALY threshold is used.
The targeted literature search conducted for this assessment retrieved 98 records. After removing 63 duplicates, this resulted in 35 remaining records. After initial screening of titles and abstracts, one paper192 was considered to be potentially relevant. Hand-searching identified an additional seven potentially relevant papers, but after title and abstract screening these were excluded as they were not full cost-effectiveness studies (n = 4)193–196 or were cost-effectiveness studies not focused on the UK (n = 3). 197–199
Ambavane et al.192
The Ambavane et al. 192 study used patients [enrolled in the TRAPID-AMI (High Sensitivity Cardiac Troponin T Assay for Rapid Rule-out of Acute Myocardial Infarction) study] who presented to the ED with acute chest pain to assess the cost-effectiveness of a 1-hour rule-out and rule-in algorithm, using hs-cTnT testing, in comparison with standard care. The study reported that the 1-hour algorithm had higher sensitivity (87% vs. 69%) but lower specificity (96% vs. 97%) than standard care. Total costs were reduced for the 1-hour algorithm compared with standard care (£2480 vs. £4561) and this was mainly driven by a shorter length of stay in the ED.
Summary of studies included in the cost-effectiveness review
Most of the studies identified in this review found that the question of whether or not hs-cTn testing is cost-effective cannot be answered unequivocally. In favour of hs-cTn testing, the abstract by Vaidya et al. 183 concluded that hs-cTnT testing is ‘very cost effective’ and the study by Goodacre et al. 185 concluded that ‘the optimal strategy in all but one scenario was high-sensitivity troponin at presentation, with a 10 hour troponin test if positive and discharge home if negative’. 185 The other papers reported ICERs that were considerably higher and with substantial uncertainty. The accuracy of high-sensitive tests and the efficiency of decision-making based on test results were important drivers of cost-effectiveness.
Model structure and methodology
Troponin testing strategies considered in the model
The health economic analysis will estimate the cost-effectiveness of different troponin testing strategies for diagnosing or ruling out NSTEMI in patients presenting at the ED with suspected NSTE-ACS who have no major comorbidities requiring hospitalisation (e.g. as heart failure or arrhythmia) and in whom STEMI has been ruled out. Those diagnosed with NSTEMI will then be admitted to the hospital for AMI treatment and those diagnosed as without NSTEMI can be discharged without AMI treatment and further hospital stay. AMI treatment might include aspirin, statins and angiotensin-converting enzyme inhibitors, and consideration of coronary revascularisation for high-risk cases. 185 Initiating AMI treatment for NSTEMI will reduce the probability of MACEs, particularly cardiac death and reinfarction.
Standard serial troponin testing for patients with acute chest pain due to possible ACS does not achieve optimal sensitivity in detecting AMI until 10–12 hours after onset of symptoms. Waiting for 10–12 hours after symptoms onset is burdensome for patients and induces additional health-care costs. Therefore, various alternatives have been proposed that use more sensitive troponin tests for the early rule out of NSTEMI (within the 4-hour NHS ED target). 200
Chapter 3 of this report summarises evidence about the clinical effectiveness of the various hs-cTn test strategies reported in the literature and describes the process used to select strategies for inclusion in the economic model. For the economic model, only those high-sensitivity troponin tests that had a sensitivity of ≥ 97% were selected (based on expert opinion indicating that sensitivity should minimally be 97% to be acceptable for clinicians). This resulted in the following high-sensitivity troponin strategies being evaluated in the economic model.
-
Roche Elecsys hs-cTnT:
-
99th centile threshold (< 14 ng/l at 0 hours AND 3 hours)
-
LoD (< 5 ng/l) at 0 hours
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)
-
(< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours)
-
(< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours).
-
-
Siemens Dimension Vista hs-cTnI:
-
(< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours).
-
-
Abbott ARCHITECT hs-cTnI:
-
LoD (< 2 ng/l) at 0 hours
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours)
-
High-STEACS pathway – (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l]
-
< 4 ng/l at 0 hours.
-
-
Siemens ADVIA Centaur hs-cTnI:
-
< 2 ng/l at 0 hours
-
< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0–2 hours)
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)
-
< 5 ng/l at 0 hours.
-
-
Siemens Atellica hs-cTnI:
-
< 2 ng/l at 0 hours
-
High-STEACS pathway – (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours AND Δ < 3 ng/l].
-
-
Beckman Coulter Access hs-cTnI:
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)
-
[(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)].
-
-
Ortho VITROS hs-cTnI:
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours).
-
-
bioMérieux VIDAS hs-cTnI:
-
< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours).
-
-
Quidel TriageTrue hs-cTnI:
-
ESC 0/1-hour pathway – (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours).
-
In the base case, it was assumed that standard troponin had perfect sensitivity and specificity (reference case) for diagnosing AMI. Using this assumption, all patients testing positive on a hs-cTn test but negative on the standard troponin would be classified as FPs. This implies that their risk for adverse events would be the same as for those patients testing negative on both the hs-cTn test and the standard troponin, and that they ought to be discharged home without further immediate treatment. However, there is evidence to suggest that patients with a negative standard troponin and a positive hs-cTn may be at higher long-term risk for adverse events than patients who test negative on both the standard and the high-sensitive troponin. 201 A secondary analysis was therefore performed, which attributed a higher risk of adverse events (e.g. MI and mortality) to a proportion of patients testing FP on the hs-cTn test.
Based on the available evidence, two analyses were performed.
-
The base-case analysis.
-
A secondary analysis. This analysis assumes that patients testing FP in the hs-cTn testing strategies do not have the same risk for adverse events as patients testing TN. Instead, these patients were assigned a higher risk for (re)infarction and death to reflect the idea that, when the hs-cTn test gives a positive result, in some cases this must be caused by a disease process, whether or not the strict definition of AMI is met. The risk of adverse events in patients testing positive on a hs-cTn test but negative on the standard troponin is higher than the risk of adverse events for patients testing negative on both the hs-cTn test and the standard troponin, and lower than the risk of adverse events in patients diagnosed with NSTEMI (i.e. testing positive on both a hs-cTn and standard troponin).
Model structure
An identical model structure to the one reported in the initial DAR2 is used. This model structure was developed using the HTA report by Goodacre et al. 185 as a starting point and adapted to better fit the scope of the current assessment. In the health economic model, the mean expected costs (NHS and Personal Social Services perspective) and QALYs were calculated for each alternative strategy. These long-term consequences were estimated based on the accuracy of the different testing strategies followed by AMI treatment or discharge from the hospital without AMI treatment for patients presenting at the ED with suspected NSTE-ACS, including patients with NSTEMI and patients without NSTEMI, who are further subdivided into ‘no ACS, no UA’ and ‘UA’. For this purpose, a decision tree and a state–transition model were developed. The decision tree was used to model the 30-day outcomes after presentation, based on test results and the accompanying treatment decision. These outcomes consisted of ‘no ACS, no UA’, ‘UA’, ‘non-fatal AMI (untreated)’, ‘non-fatal AMI (treated)’ and ‘death’. The decision tree is shown in Figure 11 and the state–transition model is shown in Figure 12.
FIGURE 11.
Decision tree structure.

FIGURE 12.
State–transition model structure. During the first year post AMI a distinction is made between treated and untreated AMI.

The long-term consequences in terms of costs and QALYs were estimated using a state–transition cohort model (see Figure 15) with a lifetime time horizon (60 years). The cycle time was 1 year, except for the first cycle, which was adjusted to 335.25 days (i.e. 365.25 days – 30 days) to ensure that the decision tree period (30 days) and the first cycle summed to 1 year. The following health states were included:
-
no ACS and no UA
-
UA
-
post AMI (treated and untreated)
-
post AMI with reinfarction
-
death.
In short, patients presenting at the ED with suspected NSTEMI were classified as TP, FP, FN or TN. TP patients were considered to be correctly treated for AMI, whereas TNs were considered not to be treated for AMI (TN patients can be with or without UA). FP patients were considered to be those who have no AMI, but who did not meet early rule-out criteria. It was assumed that FP patients would remain in the hospital longer (i.e. as long as it would take for the standard troponin test results to become available), but would not be treated for AMI. Consequently, the life expectancy and quality of life for FP patients was, in the base-case analysis, equal to the life expectancy and quality of TN patients. Finally, FN patients were assumed to have untreated AMI with consequently increased reinfarction and mortality probabilities for 1 year.
Model parameters
Estimates for the model input parameters were retrieved from the literature and by consulting experts. Accuracy estimates were derived from the systematic review component of this assessment (see Chapter 3).
Transition probabilities
An overview of transition probabilities is provided in Table 23.
| Transition probability | Estimate | SE/95% CI | Distribution | Source |
|---|---|---|---|---|
| Decision tree (short term) | ||||
| Proportion of AMI of all chest pain emergency admissions | 0.199 | 0.001 | Beta | HES5 |
| Proportion of NSTEMIs of all confirmed cases of heart attack | 0.613 | 0.002 | Beta | Healthcare Quality Improvement Programme202 |
| NSTEMI prevalencea | 0.122 | Calculated | ||
| Proportion of UA (of all non-NSTEMI patients) | 0.160 | 0.038 | Beta | CADTH report186 |
| Decision tree (30-day) probabilities | ||||
| Mortality: treated AMI | 0.097 | 0.012 | Beta | Pope et al.203 |
| Mortality: untreated AMI | 0.105 | 0.069 | Beta | Pope et al.203 |
| Mortality: treated UA | 0.021 | 0.005 | Beta | Pope et al.203 |
| Mortality: no ACS | b | Fixed | ONS204 | |
| State–transition model (long term) | ||||
| AMI incidence | c | Fixed | British Heart Foundation205 | |
| Annual reinfarction (treated)d | 0.023 | 0.001 | Beta | Smolina et al.206 |
| RR reinfarction (untreated vs. treated)e | 2.568 | 1.366 to 5.604 | Log-normal | Mills et al.187 |
| Annual mortality no ACS | b | Fixed | ONS204 | |
| Annual mortality post MId | 0.066 | 0.000 | Beta | Smolina et al.206 |
| Annual mortality post reinfarctiond | 0.142 | 0.002 | Beta | Smolina et al.206 |
| HR mortality (UA vs. NSTEMI) | 0.781 | 0.581 to 1.053 | Log-normal | Allen et al.207 |
| RR mortality (untreated vs. treated)d | 1.877 | 0.951 to 4.239 | Log-normal | Mills et al.187 |
| Secondary analysis (adjusted RR for patients tested FP) | ||||
| OR: AMIf | 1.210 | 0.830 to 1.760 | Log-normal | Liplinski et al.201 |
| OR: deathf | 1.600 | 1.140 to 2.240 | Log-normal | Liplinski et al.201 |
| Proportion of AMIg | 0.109 | 0.011 | Beta | Liplinski et al.201 |
| Proportion of deathg | 0.110 | 0.011 | Beta | Liplinski et al.201 |
| RR: AMIf,h | 0.842 | Calculated | Liplinski et al.201 | |
| RR: deathf,h | 0.652 | Calculated | Liplinski et al.201 | |
Decision tree
The proportions of patients testing positive or negative (and therefore commencing AMI treatment or being discharged from the hospital) were based on the estimated accuracy of the testing strategies considered (Table 24) and the estimated prevalence of NSTEMI in the UK (12.2%) (see Table 23). The proportion of TPs, FPs, FNs and TNs were calculated (Table 25) as follows:
-
TP = NSTEMI prevalence × sensitivity.
-
FP = (1 – NSTEMI prevalence) × (1 – specificity).
-
FN = NSTEMI prevalence × (1 – sensitivity).
-
TN = (1 – NSTEMI prevalence) × specificity.
| Test strategy | Sensitivity (SE)a | Specificity (SE)a | Distribution | Source |
|---|---|---|---|---|
| Standard troponin (at presentation and after 10–12 hours) | 1.00 (–) | 1.00 (–) | Fixed | Assumption |
| 1. Roche Elecsys hs-cTnT: 99th centile | 1.00 (0.03) | 0.77 (0.08) | Multivariate normal | See Chapter 3 |
| 2. Roche Elecsys hs-cTnT: LoD | 0.99 (0.01) | 0.35 (0.05) | Multivariate normal | See Chapter 3 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 0.99 (0.01) | 0.68 (0.01) | Multivariate normal | See Chapter 3 |
| 4. Roche Elecsys hs-cTnT (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 1.00 (0.02) | 0.45 (0.02) | Multivariate normal | See Chapter 3 |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 0.98 (0.01) | 0.73 (0.01) | Multivariate normal | See Chapter 3 |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 1.00 (0.02) | 0.66 (0.02) | Multivariate normal | See Chapter 3 |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 1.00 (0.00) | 0.21 (0.03) | Multivariate normal | See Chapter 3 |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 0.99 (0.00) | 0.57 (0.01) | Multivariate normal | See Chapter 3 |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 0.99 (0.01) | 0.76 (0.01) | Multivariate normal | See Chapter 3 |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 0.99 (0.01) | 0.50 (0.01) | Multivariate normal | See Chapter 3 |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 1.00 (0.00) | 0.23 (0.01) | Multivariate normal | See Chapter 3 |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 1.00 (0.01) | 0.67 (0.03) | Multivariate normal | See Chapter 3 |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 0.99 (0.01) | 0.56 (0.02) | Multivariate normal | See Chapter 3 |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 0.99 (0.01) | 0.52 (0.01) | Multivariate normal | See Chapter 3 |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 1.00 (0.01) | 0.26 (0.01) | Multivariate normal | See Chapter 3 |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 0.98 (0.01) | 0.74 (0.01) | Multivariate normal | See Chapter 3 |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 0.99 (0.02) | 0.70 (0.02) | Multivariate normal | See Chapter 3 |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 0.98 (0.02) | 0.83 (0.01) | Multivariate normal | See Chapter 3 |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 1.00 (0.01) | 0.60 (0.02) | Multivariate normal | See Chapter 3 |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 0.98 (0.02) | 0.64 (0.02) | Multivariate normal | See Chapter 3 |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 1.00 (0.01) | 0.66 (0.02) | Multivariate normal | See Chapter 3 |
| Test strategy | TP | FP | FN | TN | PPV | NPV |
|---|---|---|---|---|---|---|
| Standard troponin (at presentation and after 10–12 hours) | 0.12 | 0.00 | 0.00 | 0.88 | 1.00 | 1.00 |
| 1. Roche Elecsys hs-cTnT: 99th centile | 0.12 | 0.20 | 0.00 | 0.68 | 0.38 | 1.00 |
| 2. Roche Elecsys hs-cTnT: LoD | 0.12 | 0.57 | 0.00 | 0.31 | 0.18 | 1.00 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 0.12 | 0.28 | 0.00 | 0.60 | 0.30 | 1.00 |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 0.12 | 0.48 | 0.00 | 0.40 | 0.20 | 1.00 |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 0.12 | 0.24 | 0.00 | 0.64 | 0.33 | 1.00 |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 0.12 | 0.30 | 0.00 | 0.58 | 0.29 | 1.00 |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 0.12 | 0.69 | 0.00 | 0.18 | 0.15 | 1.00 |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 0.12 | 0.38 | 0.00 | 0.50 | 0.24 | 1.00 |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 0.12 | 0.21 | 0.00 | 0.67 | 0.36 | 1.00 |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 0.12 | 0.44 | 0.00 | 0.44 | 0.22 | 1.00 |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 0.12 | 0.68 | 0.00 | 0.20 | 0.15 | 1.00 |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 0.12 | 0.29 | 0.00 | 0.59 | 0.30 | 1.00 |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 0.12 | 0.39 | 0.00 | 0.49 | 0.24 | 1.00 |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 0.12 | 0.42 | 0.00 | 0.46 | 0.22 | 1.00 |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 0.12 | 0.65 | 0.00 | 0.23 | 0.16 | 1.00 |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 0.12 | 0.23 | 0.00 | 0.65 | 0.34 | 1.00 |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 0.12 | 0.26 | 0.00 | 0.61 | 0.31 | 1.00 |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 0.12 | 0.15 | 0.00 | 0.73 | 0.45 | 1.00 |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 0.12 | 0.35 | 0.00 | 0.53 | 0.26 | 1.00 |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 0.12 | 0.32 | 0.00 | 0.56 | 0.27 | 1.00 |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 0.12 | 0.30 | 0.00 | 0.58 | 0.29 | 1.00 |
After treatment, TP patients in the decision tree were allocated to ‘non-fatal AMI (treated)’ and FP patients were further subdivided between ‘no ACS, no UA’ and ‘UA’ (based on the proportion of UA among non-NSTEMI patients) (see Table 23). After being discharged, TN patients were also subdivided between ‘no ACS, no UA’ and ‘UA’, whereas FN patients were allocated to ‘non-fatal AMI (untreated)’. The proportions of FNs (reported in Table 25) can be considered as the proportion of AMIs that would have been missed when assuming that standard troponin testing had perfect accuracy. Finally, to calculate the total number of deaths in the decision tree, the probability of 30-day mortality was assigned based on above mentioned subdivision (see Table 23). It was assumed that UA was always correctly diagnosed and therefore the mortality probability for treated UA was used.
State–transition model
The age-dependent AMI incidence in the UK205 was used to model the occurrence of AMI for patients in the health states ‘no ACS,’ and ‘UA’. It was assumed that all AMIs in the state–transition model were diagnosed correctly and therefore received treatment. For patients in the ‘post-MI’ health state, the probability of reinfarction after treated AMI was retrieved from a UK record linkage study (n = 387,452) that assessed long-term survival and recurrence after AMI. 206 For this purpose, the probabilities for females and males were weighted according to the estimated proportion of females and males in the population (males = 58.1%). 185 The reinfarction probability for the ‘post-MI with reinfarction’ health state is equal to the reinfarction probability for the ‘post-MI’ health state. The reinfarction relative risk (RR) for people with untreated compared with treated AMI was calculated from a study by Mills et al. 187 and based on patients with a troponin concentration of 5–19 ng/l. This RR was assumed for the first year after presentation at ED only, after which no increased risk was assumed (i.e. RR equals 1.0 for untreated vs. treated AMI after year 1).
Age-dependent mortality from the general population was used for patients in the ‘no ACS, no UA’ health state. 204 For the ‘post-MI’ and ‘post-MI with reinfarction’ health states, mortality was extracted from the record linkage study. 206 Again, the study by Mills et al. 187 was used to calculate the mortality RR for untreated compared with treated AMI for the first year, after which an RR of 1.0 was used. Finally, a multivariate adjusted mortality hazard ratio for UA compared with NSTEMI was retrieved from a study by Allen et al. 207 to calculate mortality after UA.
All input parameters for the state–transition model are reported in Table 23.
Health state utilities
Age-dependent utility scores from the UK general population were calculated for patients in the ‘no ACS, no UA’ health state based on a linear regression model. 189 These age-dependent utility scores were combined with age-dependent disutilities for AMI186 to calculate utilities for the ‘post-MI’ health states (with or without reinfarction). Utility scores for the ‘UA’ health state were calculated based on post-MI utility scores and a utility increment of 0.010189 (Table 26).
| Utility score | Estimate | SE | Distribution | Source |
|---|---|---|---|---|
| No ACS, no UA | ||||
| Intercept | 1.060 | 0.029 | Normal | Ward et al.189 |
| Disutility for age | 0.004 | 0.001 | Normal | Ward et al.189 |
| Post MI (disutility vs. no ACS by age in years) | ||||
| 45 | 0.060 | 0.001 | Normal | Ward et al.189 |
| 55 | 0.051 | 0.001 | Normal | Ward et al.189 |
| 65 | 0.025 | 0.001 | Normal | Ward et al.189 |
| 75 | 0.007 | 0.001 | Normal | Ward et al.189 |
| UA | ||||
| Utility increment vs. AMI | 0.010 | 0.042 | Normal | Ward et al.189 |
Resource use and costs
Test-specific resource use consisted of the number of tests performed and the duration of hospital stay (hours) before discharge/AMI treatment (Table 27). For test strategies that involved a subsequent test conditional on the outcomes of the first test, the rule-out rate for the presentation sample was used to calculate number of subsequent tests.
| Test | Estimate | Range | Distribution | Source |
|---|---|---|---|---|
| Number of tests | ||||
| Standard troponin (at presentation and after 10–12 hours) | 2.00 | Fixed | Assumption | |
| 1. Roche Elecsys hs-cTnT: 99th centile | 2.00 | Fixed | Assumption | |
| 2. Roche Elecsys hs-cTnT: LoD | 1.00 | Fixed | Assumption | |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 1.75 | Fixed | Assumption | |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 2.00 | Fixed | Assumption | |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 2.00 | Fixed | Assumption | |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 2.00 | Fixed | Assumption | |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 1.00 | Fixed | Assumption | |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 1.62 | Fixed | Assumption | |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 1.41 | Fixed | Assumption | |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 1.00 | Fixed | Assumption | |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 1.84 | Fixed | Assumption | |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 1.84 | Fixed | Assumption | |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 2.00 | Fixed | Assumption | |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 1.00 | Fixed | Assumption | |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 1.00 | Fixed | Assumption | |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 1.70 | Fixed | Assumption | |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 1.68 | Fixed | Assumption | |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l and Δ < 5 ng/l at 0 to 2 hours)] | 1.68 | Fixed | Assumption | |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 1.82 | Fixed | Assumption | |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 1.67 | Fixed | Assumption | |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 1.55 | Fixed | Assumption | |
| Hospital stay (hours) before discharge/AMI treatmenta | ||||
| Standard troponin (at presentation and after 10–12 hours) | 14 | 13–15 | Beta PERT | Assumption |
| 1. Roche Elecsys hs-cTnT: 99th centile | 6 | Fixed | Assumption | |
| 2. Roche Elecsys hs-cTnT: LoD | 3 | Fixed | Assumption | |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 4 | Fixed | Assumption | |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 3.5 | Fixed | Assumption | |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 4 | Fixed | Assumption | |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 4 | Fixed | Assumption | |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 3 | Fixed | Assumption | |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 4 | Fixed | Assumption | |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 6 | Fixed | Assumption | |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 3 | Fixed | Assumption | |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 3 | Fixed | Assumption | |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 5 | Fixed | Assumption | |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 4 | Fixed | Assumption | |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 3 | Fixed | Assumption | |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 3 | Fixed | Assumption | |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 6 | Fixed | Assumption | |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 4 | Fixed | Assumption | |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 5 | Fixed | Assumption | |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 4 | Fixed | Assumption | |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 5 | Fixed | Assumption | |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 4 | Fixed | Assumption | |
Health state costs were retrieved from a study published by Danese et al. ,209 which was a retrospective cohort study using Clinical Practice Research Datalink records to identify UK individuals who had their first cardiovascular event between 2006 and 2012. Direct medical costs were estimated for 24,093 patients.
Additionally, costs of fatal events were accumulated for all fatal AMIs. For this purpose, it was assumed that all 30-day deaths after ‘true’ NSTEMIs were due to a fatal AMI event. To calculate the hospital stay costs for patients, based on the number of hours before the test results become available, non-elective inpatient stays (i.e. short stays) were retrieved from the Personal Social Services Research Unit and divided by 24 (to calculate hourly costs). For the calculation of hospital stay duration, it was assumed that doctors were available on demand and the time to discharge was delayed because of the time between arrival at the ED and the start of first sampling (1 hour), and the time between sampling and the results being available (2 hours). In the case of multiple testing, the 1-hour delay between arrival at the ED and start of sampling was applied to the first test only; however, this also affected the timing of the second test, if applicable. The 2-hour delay before test results become available applies to all tests performed.
Although information was provided by test manufacturers to calculate test-dependent costs, based on clinical expert input, it was assumed that the costs per test would be identical for all tests (i.e. £2.50, which is consistent with the test cost information submitted by the manufacturers), except for the point-of-care test (i.e. Quidel). For this test, we assumed a cost of £25.00 (based on cost information submitted by the manufacturers). However, scenario analyses were performed using test-specific costs. For these scenario analyses it should be noted that the information received from the manufacturers did not allow us to incorporate costs related to the analyser (e.g. capital, service, maintenance and training costs) nor the personnel costs (implicitly assuming that these costs would be identical for all test strategies).
All costs were inflated to the 2018–19 price level (Table 28).
| Cost | Estimate (£) | SE/range (£) | Distribution | Source |
|---|---|---|---|---|
| Health state costs | ||||
| No ACS, no UA first year | 2403.70 | 175.36 | Gamma | Danese et al.209 |
| No ACS, no UA subsequent year | 2403.70 | 175.36 | Gamma | Danese et al.209 |
| UA first year | 4427.02 | 74.54 | Gamma | Danese et al.209 |
| UA subsequent year | 2208.02 | 69.16 | Gamma | Danese et al.209 |
| Post MI first year | 6865.23 | 151.42 | Gamma | Danese et al.209 |
| Post MI subsequent years | 2493.13 | 176.95 | Gamma | Danese et al.209 |
| Post re-MI first year | 8197.80 | 611.91 | Gamma | Danese et al.209 |
| Post re-MI subsequent years | 4123.37 | 968.43 | Gamma | Danese et al.209 |
| Event costs | ||||
| AMI treatment costs | 2496.48 | Fixed | NHS Reference Costs 2017–18 210 | |
| Costs of fatal AMI | 1539.75 | 10.56 | Gamma | Walker et al.211 |
| Unit prices | ||||
| Hospital stay costs (per hour)c | 26.08 | Fixed | PSSRU212 | |
| Test costsa | 2.50 | 1.85–6.00 | Beta PERT | Expert opinion, information submitted by manufacturer and assumptions |
| Test costs (point of care) | 25.00 | 1.85–26.00 | Beta PERT | |
Overview of main model assumptions
The main assumptions in the health economic analyses are as follows.
-
Serial troponin testing (comparator) has perfect accuracy (sensitivity = 1.0 and specificity = 1.0).
-
The life expectancy, quality of life and cost for FP patients is, in the base-case analysis, equal to the life expectancy, quality of life and cost of TN patients. This assumption was amended in the secondary and sensitivity analyses.
-
In contrast with AMIs occurring during the decision tree period, all AMIs (either first or reinfarction) occurring in the state–transition model are diagnosed correctly and therefore treated.
-
UA is always correctly diagnosed and therefore treated.
-
The reinfarction probability for the ‘post MI with reinfarction’ health state is equal to the reinfarction probability for the ‘post-MI’ health state.
-
The increased post-MI reinfarction and mortality probabilities for untreated AMI were assumed to last 1 year and afterwards a RR of 1.0 was applied (for untreated vs. treated AMI).
-
There is no additional benefit of starting treatment early and so treatment effect for high-sensitive strategies is equal to treatment effect for standard troponin strategy.
-
All 30-day deaths (after presentation at the ED) are due to fatal AMI events and will receive the associated costs.
Model analyses
Expected costs, life-years and QALYs were estimated for all strategies. Discount rates of 3.5% and a half-cycle correction were applied for both costs and effects. Incremental cost and QALYs for each strategy compared with standard troponin and with the next best alternative were calculated. The ICER was then calculated by dividing the incremental costs by the incremental QALYs. PSAs (10,000 simulations) were performed and cost-effectiveness acceptability curves (CEACs) were constructed.
Secondary analysis
For the base case, it was assumed that patients who tested negative on standard troponin and positive on hs-cTn tests would experience life expectancy and quality of life equal to TN patients. This assumption is, however, debatable. A meta-analysis by Liplinski et al. 201 showed that patients with a negative standard troponin test and positive hs-cTn test have an increased risk of (re)infarction and mortality compared with those who test negative on both the standard troponin and hs-cTn tests. Although this risk was not as high as in patients with both positive standard troponin and positive hs-cTn tests, it could still be considered prognostically important. Therefore, in this secondary analysis, the risk of MI and mortality was adjusted for patients who tested FP (see Table 23). It was assumed for this proportion of patients that the relative treatment benefit would be equal to that for TP patients. As the prevalence of this ‘higher-risk subgroup’ is likely to be the same for all comparators, it was assumed that this proportion was equal to the lowest proportion of FP patients for all hs-cTn tests (0.15) (see Table 25). This ‘higher-risk subgroup’ was assumed to be treated for all hs-cTn tests (as they tested positive with these tests) and untreated for the standard troponin test (as they tested negative with this test), therefore affecting the probability of adverse outcomes (according to the RR of reinfarction and mortality) (see Table 23) and treatment costs (see Table 28). In addition, the post-MI utility and health state costs were used for this ‘higher-risk subgroup’.
Sensitivity and scenario analysis
For both the base-case and the secondary analyses, one-way sensitivity analyses were performed and included all probabilistic parameters (NHS reference costs were included by ± 20%), creating tornado diagrams for the relevant comparisons on the cost-effectiveness frontier. Additionally, the following scenario analyses were performed.
-
AMI treatment costs (£2496 based on NHS reference costs) are applied for patients who tested FP rather than using no treatment costs, as assumed in the base-case analysis.
-
The assumption that the increased post AMI reinfarction and mortality probabilities for untreated AMI lasts for only 1 year was replaced by the assumption that these probabilities would remain elevated for a lifetime.
-
The assumption of equal test costs was relaxed and test-dependent costs were incorporated (based on the information provided by manufacturers). The assay-specific test costs were (unit price per test):
-
Roche Elecsys hs-TnT – £6.05
-
Abbott ARCHITECT hs-TnI – £4.17
-
Siemens ADVIA Centaur hs-TnI – £2.00
-
Siemens Atellica hs-TnI – £2.00
-
Siemens Dimension Vista hs-TnI – £2.00
-
Beckman Coulter ACCESS hs-TnI – £2.75
-
Ortho VITROS hs-TnI – £1.85
-
BioMérieux VIDAS hs-TnI – £6.05
-
Quidel TriageTrue hs-TnI (point of care) – £25.00.
-
In addition to the abovementioned scenario analyses, the base-case and secondary analyses results were also considered in comparing different strategies per assay (in case of multiple strategies).
Results of cost-effectiveness analyses
This section describes the results using deterministic and probabilistic analyses for the base-case analysis and the secondary analysis. Scenario analyses (deterministic) and sensitivity analyses are described here and results of these are presented in tabulated form in Appendices 6 and 7.
Base-case analysis
The base-case analysis includes 22 test strategies. Tables 29 and 30 show the deterministic and probabilistic cost-effectiveness results of these comparisons, respectively. Standard troponin (at presentation and after 10–12 hours) testing was the most effective (probabilistic 15.5331 life-years; 12.0825 QALYs) and the most expensive strategy (£38,871). However, other testing strategies with a sensitivity of 100% (subject to uncertainty) were almost equally as effective, resulting in the same life-year and QALY gain at up to four decimal places. These were (starting with the cheapest) Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours); Ortho VITROS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours)]; Siemens ADVIA Centaur hs-cTnI [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)]; Roche Elecsys hs-cTnT [99th centile threshold (< 14 ng/l at 0 hours AND 3 hours)]; Quidel TriageTrue hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)]; Roche Elecsys hs-cTnT (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours); Siemens Atellica hs-cTnI (< 2 ng/l at 0 hours); and Siemens ADVIA Centaur hs-cTnI (< 2 ng/l at 0 hours). Owing to the few differences in outcomes between these strategies, some of these appear to be on the cost-effectiveness frontier, even when they are not (Figure 13). The CEAC for the base-case analysis is shown in Figure 14.
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,666 | 12.0763 | –210 | –0.0011 | 188,819 | Cheapest |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,669 | 12.0765 | –206 | –0.0009 | 218,065 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,678 | 12.0768 | –198 | –0.0006 | 355,439 | £22,200 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,683 | 12.0768 | –193 | –0.0006 | 346,892 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,693 | 12.0774 | –183 | 0.0000 | 328,961,202 | £26,504 |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,702 | 12.0768 | –173 | –0.0006 | 311,539 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,704 | 12.0763 | –171 | –0.0011 | 154,010 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,705 | 12.0768 | –171 | –0.0006 | 307,326 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,705 | 12.0763 | –171 | –0.0011 | 153,650 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,706 | 12.0774 | –170 | 0.0000 | 305,073,895 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,706 | 12.0767 | –170 | –0.0007 | 234,660 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,708 | 12.0768 | –168 | –0.0006 | 302,200 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,709 | 12.0774 | –167 | 0.0000 | 300,489,458 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,709 | 12.0774 | –167 | 0.0000 | 299,391,873 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,711 | 12.0768 | –165 | –0.0006 | 296,376 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,726 | 12.0774 | –149 | 0.0000 | 268,289,079 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,734 | 12.0774 | –142 | 0.0000 | 254,650,046 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,746 | 12.0769 | –130 | –0.0005 | 259,678 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,773 | 12.0774 | –103 | 0.0000 | 185,244,726 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,782 | 12.0774 | –93 | 0.0000 | 167,886,624 | Dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,784 | 12.0772 | –92 | –0.0002 | 550,577 | Dominated |
| Standard troponin (at presentation and after 10–12 hours) | 38,876 | 12.0774 | 0 | 0.0000 | NA | £328,961,202 |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,625 | 12.0768 | –246 | –0.0058 | 42,753 | Cheapest |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,650 | 12.0790 | –221 | –0.0036 | 62,121 | Extendedly dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,662 | 12.0798 | –209 | –0.0027 | 77,589 | Extendedly dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,662 | 12.0764 | –209 | –0.0061 | 34,307 | Dominated |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,663 | 12.0813 | –208 | –0.0012 | 169,682 | £8455 |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,678 | 12.0794 | –193 | –0.0032 | 60,899 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,681 | 12.0795 | –190 | –0.0030 | 63,659 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,684 | 12.0791 | –187 | –0.0034 | 54,645 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,688 | 12.0825 | –183 | 0.0000 | 36,842,603 | £20,190 |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,698 | 12.0811 | –173 | –0.0014 | 119,994 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,699 | 12.0815 | –171 | –0.0010 | 169,198 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,701 | 12.0825 | –170 | 0.0000 | 28,179,082 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,702 | 12.0818 | –169 | –0.0007 | 233,736 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,704 | 12.0825 | –167 | 0.0000 | 25,072,373 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,706 | 12.0825 | –165 | 0.0000 | 15,661,356 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,721 | 12.0825 | –149 | 0.0000 | 28,167,521 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,729 | 12.0825 | –142 | 0.0000 | 17,442,604 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,738 | 12.0817 | –132 | –0.0008 | 169,952 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,768 | 12.0825 | –103 | 0.0000 | 21,210,686 | Extendedly dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,777 | 12.0825 | –94 | 0.0000 | 31,584,800 | Extendedly dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,778 | 12.0823 | –93 | –0.0002 | 381,602 | Dominated |
| Standard troponin (at presentation and after 10–12 hours) | 38,871 | 12.0825 | 0 | 0.0000 | NA | £36,842,603 |
FIGURE 13.
The cost-effectiveness frontier for the base-case analysis (based on PSA). CEF, cost-effectiveness frontier.
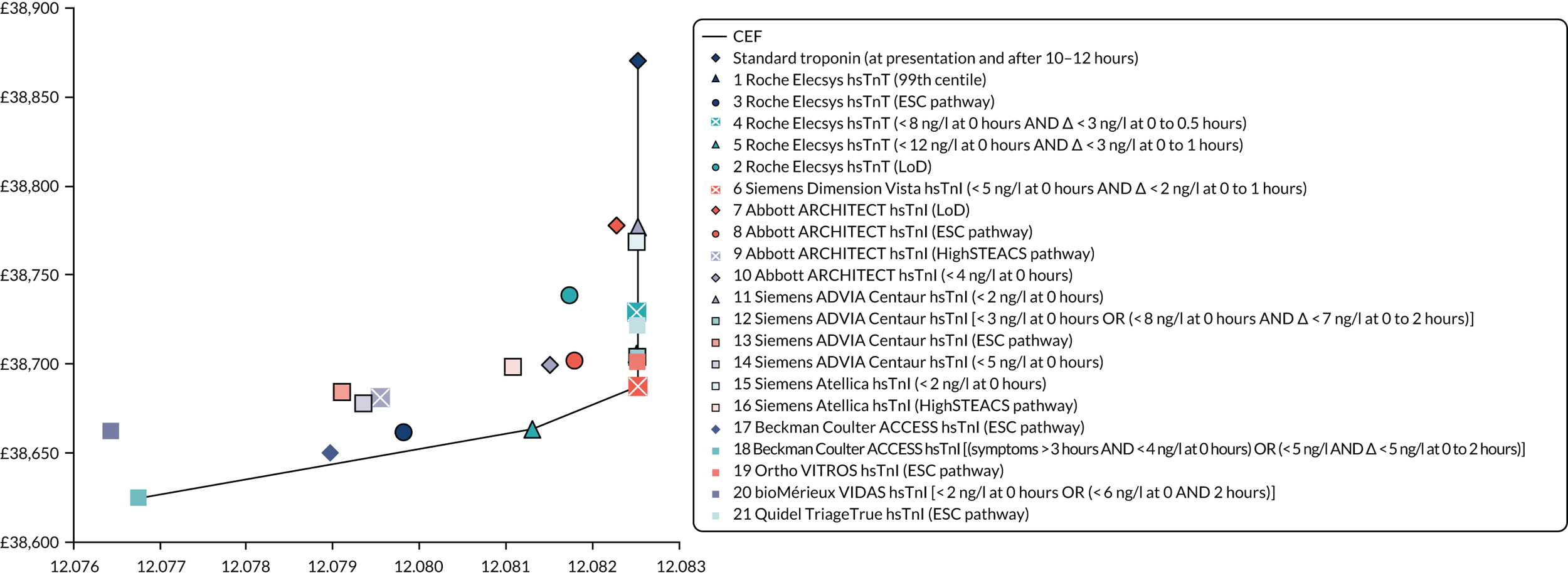
FIGURE 14.
A CEAC for the base-case analysis.
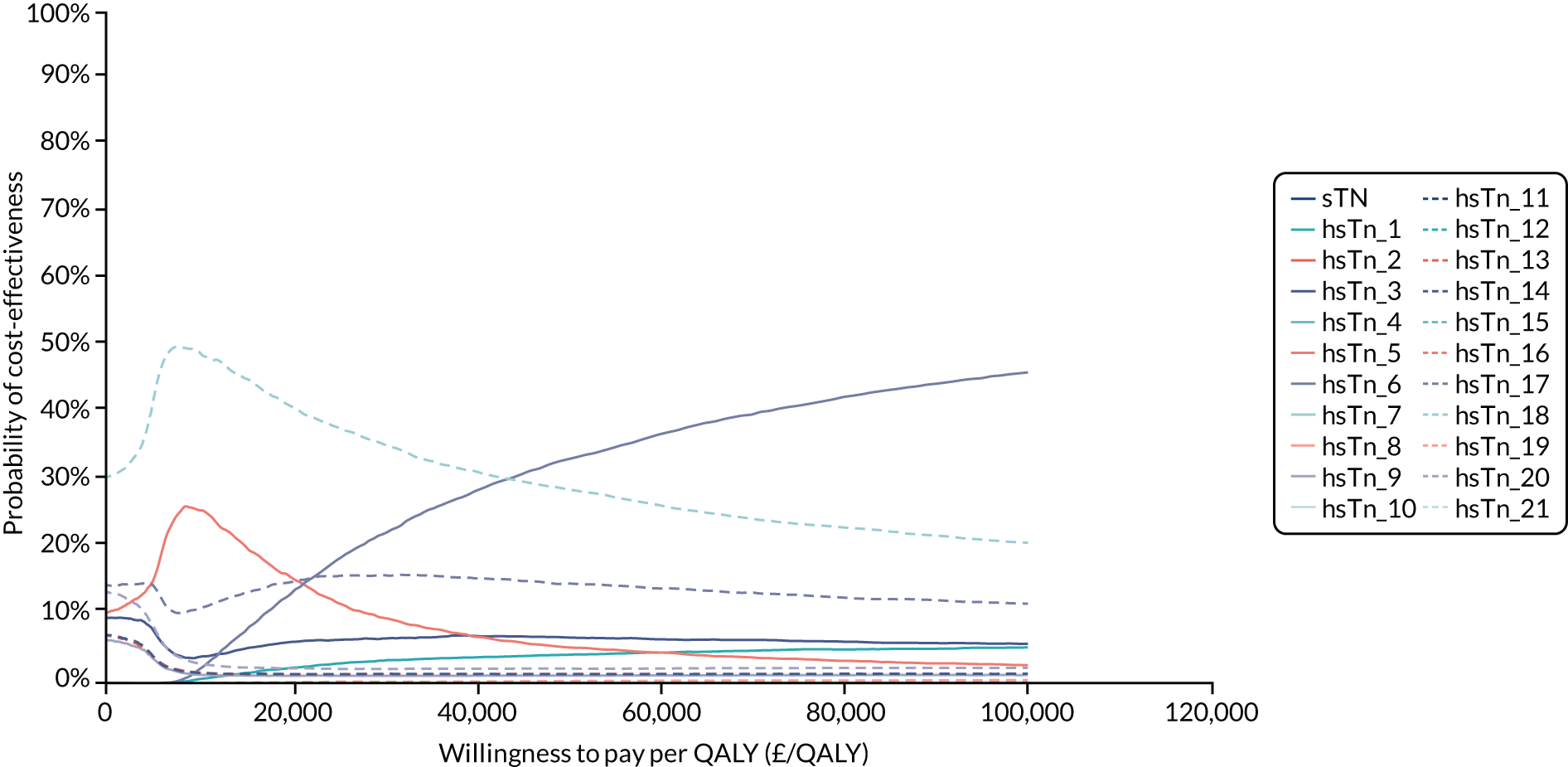
Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours), the test strategy with the highest specificity [83 (95% CI 81 to 86)], was the cheapest (probabilistic analysis £38,625), but it was also amongst the least effective (15.5254 life-years and 12.0768 QALYs), owing to a sensitivity of 98 (95% CI 92 to 100). Compared with standard troponin testing, hs-cTn testing resulted in probabilistic ICERs ranging between £34,307 and £36,842,603 savings per QALY lost.
Comparisons based on the next best alternative showed that for willingness-to-pay values < £8455 per QALY, the Beckman Coulter ACCESS hs-TnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] would be cost-effective. For willingness-to-pay thresholds between £8455 and £20,190 per QALY, the Roche Elecsys hs-TnT (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) was cost-effective. For a willingness-to-pay threshold of > £20,190 per QALY, the Siemens Dimension Vista hs-TnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) would be cost-effective (see Table 30).
At a willingness-to-pay threshold of £20,000 and £30,000 per QALY, the Beckman Coulter ACCESS hs-cTnI [ESC 0/1 hour-pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] had a probability of being cost-effective of 41% and 36%, respectively. At these thresholds, the Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) had a probability of being cost-effective of 13% and 22%, respectively.
Secondary analysis
The secondary analysis includes the same test strategies. This analysis assumed that in a proportion of patients with a FP hs-cTn test (i.e. positive hs-cTn test and a negative standard troponin test), there is prognostic significance [i.e. it is associated with an increased risk of adverse events (e.g. mortality and MI)], which can be reduced by testing positive using the hs-cTn test (Tables 31 and 32).
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/ ΔQALYs | ||
|---|---|---|---|---|---|---|
| Δ Cost (£) | Δ QALY | Δ Costs/Δ QALYs (£) | ||||
| Standard troponin (at presentation and after 10–12 hours) | 37,503 | 11.3230 | 0 | 0.0000 | NA | Cheapest |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,017 | 11.4014 | 514 | 0.0784 | 6559 | Extendedly dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,022 | 11.4064 | 519 | 0.0835 | 6216 | Extendedly dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,022 | 11.4035 | 519 | 0.0805 | 6445 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,023 | 11.4147 | 520 | 0.0918 | 5668 | Extendedly dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,030 | 11.4291 | 527 | 0.1062 | 4967 | Extendedly dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,033 | 11.4313 | 530 | 0.1083 | 4894 | Extendedly dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,042 | 11.4250 | 540 | 0.1020 | 5290 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,054 | 11.4361 | 551 | 0.1132 | 4867 | Extendedly dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,054 | 11.4352 | 551 | 0.1122 | 4910 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,061 | 11.4396 | 559 | 0.1167 | 4789 | Extendedly dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,063 | 11.4469 | 561 | 0.1239 | 4523 | Extendedly dominated |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,064 | 11.4510 | 562 | 0.1280 | 4387 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,065 | 11.4488 | 562 | 0.1259 | 4465 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,067 | 11.4455 | 564 | 0.1225 | 4605 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,073 | 11.4424 | 570 | 0.1195 | 4771 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,086 | 11.4465 | 583 | 0.1235 | 4722 | Dominated |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,093 | 11.4610 | 590 | 0.1380 | 4278 | £4278 |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,101 | 11.4455 | 598 | 0.1225 | 4880 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,104 | 11.4522 | 601 | 0.1292 | 4650 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,110 | 11.4547 | 608 | 0.1317 | 4612 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,118 | 11.4562 | 615 | 0.1333 | 4615 | Dominated |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs | ||||
| Standard troponin (at presentation and after 10–12 hours) | 37,517 | 11.3340 | 0 | 0.0000 | NA | Cheapest |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,039 | 11.4463 | 522 | 0.1123 | 4648 | Extendedly dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,046 | 11.4201 | 529 | 0.0861 | 6148 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,050 | 11.4328 | 532 | 0.0988 | 5389 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,051 | 11.4249 | 534 | 0.0909 | 5868 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,051 | 11.4221 | 534 | 0.0881 | 6064 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,055 | 11.4466 | 538 | 0.1126 | 4778 | Extendedly dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,057 | 11.4497 | 540 | 0.1157 | 4662 | Extendedly dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,060 | 11.4547 | 543 | 0.1207 | 4500 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,066 | 11.4628 | 548 | 0.1288 | 4258 | Extendedly dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,070 | 11.4430 | 553 | 0.1089 | 5072 | Dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,072 | 11.4619 | 555 | 0.1279 | 4337 | Dominated |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,077 | 11.4725 | 560 | 0.1385 | 4043 | £4043 |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,079 | 11.4535 | 562 | 0.1195 | 4699 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,087 | 11.4571 | 570 | 0.1231 | 4630 | Dominated |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,088 | 11.4678 | 570 | 0.1338 | 4263 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,092 | 11.4627 | 575 | 0.1287 | 4467 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,111 | 11.4636 | 594 | 0.1296 | 4580 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,115 | 11.4691 | 598 | 0.1351 | 4425 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,126 | 11.4627 | 609 | 0.1287 | 4729 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,126 | 11.4689 | 609 | 0.1349 | 4517 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,139 | 11.4718 | 622 | 0.1378 | 4514 | Dominated |
In the secondary analysis, standard troponin (at presentation and after 10–12 hours) was the cheapest (£37,517) and the least effective (11.334 QALYs) testing strategy (probabilistic analysis). Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] was the most effective testing strategy (11.4725 QALYs) at higher costs (£38,077). All other strategies were (extendedly) dominated. The ICER of Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] compared with standard troponin (at presentation and after 10–12 hours) was £4043 per QALY gained.
At a willingness-to-pay threshold of £20,000 and £30,000 per QALY, the Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0–1 hour)] had a probability of being cost-effective of 67% and 64%, respectively (Figures 15 and 16).
FIGURE 15.
The cost-effectiveness frontier for the secondary analysis (based on PSA). CEF, cost-effectiveness frontier.

FIGURE 16.
A CEAC for the secondary analysis.

Scenario analyses
Three scenario analyses were performed deterministically and conditional on both the base-case and the secondary analyses. Results are shown in Appendix 7. Scenario 1 (see Table 39 and Figure 17) assumed that patients who tested FP would receive treatment and a treatment cost would be incurred for these patients. In this scenario and conditional on the base case, the Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours), the test strategy with the highest specificity [83 (95% CI 81 to 86)], was the cheapest. The Roche Elecsys hs-cTnT [99th centile threshold (< 14 ng/l at 0 hours AND 3 hours)] was cost-effective for thresholds > £57,659 per QALY gained and standard troponin (at presentation and after 10–12 hours) would be cost-effective at thresholds > £157,505,897 per QALY gained.
Scenario 1 was conditional on the secondary analysis (see Table 40 and Figure 18) and resulted in standard troponin (at presentation and after 10–12 hours) being the cheapest strategy. The Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) was cost-effective at and above a threshold of £4698 per QALY gained and all other test strategies were more costly and less effective.
Scenario 2 (see Table 41 and Figure 19) assumed a lifetime RR of higher mortality and reinfarction rate for those who tested FN (instead of an increased 1-year risk). Conditional on the base case, the Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) remained the cheapest, and the Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] and Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) were cost-effective at thresholds > £6962 and £7874 per QALY gained, respectively. Standard troponin (at presentation and after 10–12 hours) would be cost-effective thereafter, over thresholds of almost £70 M only.
Scenario 2 (see Table 42 and Figure 20), conditional on the secondary analysis, resulted in standard troponin (at presentation and after 10–12 hours) being the cheapest strategy. The Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) was cost-effective above a threshold of £3362 per QALY gained, and all other test strategies were less effective and therefore dominated or extendedly dominated.
Scenario 3 (see Table 43 and Figure 21) assumed differential test costs for all tests, based on information provided by the manufacturers. Conditional on the base case, the Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) remained the cheapest, and the Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] and Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) were cost-effective over thresholds of £22,200 and £23,949 per QALY gained, respectively. Standard troponin (at presentation and after 10–12 hours) would be cost-effective thereafter, above thresholds of approximately £330 M.
In scenario 3 (see Table 44 and Figure 22), conditional on the secondary analysis, standard troponin (at presentation and after 10–12 hours) remained the cheapest strategy. The Beckman Coulter ACCESS hs-cTnI (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours) was cost-effective up to a threshold of £4281 per QALY gained, and all other test strategies were less effective and therefore dominated or extendedly dominated.
Sensitivity analyses
The following input parameters had a noticeable impact on the estimated cost-effectiveness in the base-case analysis: the 30-day mortality for untreated and treated AMI (decision tree) and the mortality 1 year after treated and untreated AMI (Markov trace). Varying the remaining parameters did not have a substantial impact on the results in the comparisons between the Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours), the Roche Elecsys hs-cTnT [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)] and the Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] (see Figures 23 and 24). In the comparison between the Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) and standard troponin (at presentation and after 10–12 hours) (see Figure 25), and in addition to parameters in the other comparisons, the parameters with the most impact on results were the proportions of AMI in emergency admissions and the proportions of NSTEMI in patients with heart attack (see Appendix 8).
In the secondary analysis, the parameters with notable impact on the estimated cost-effectiveness were the 30-day mortality for untreated AMI, the mortality 1 year after treated and untreated AMI, the discount rate used for outcomes and the relative mortality for patients who tested TP compared with those who tested FP {comparison of the Beckman Coulter ACCESS hs-cTnI [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)] vs. standard troponin (at presentation and after 10–12 hours) testing} (see Appendix 8, Figure 26).
Incremental analyses per assay
Base-case analysis
The per assay analyses (Table 33) indicate that at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, the following test strategies would be the most cost-effective use of the particular assays: Roche Elecsys hs-cTnT (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours), Abbott ARCHITECT hs-cTnI (ESC pathway), Siemens ADVIA Centaur hs-cTnI [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)], Siemens Atellica hs-cTnI (High-STEACS pathway) and Beckman Coulter ACCESS hs-cTnI (ESC pathway).
| Strategy | Cost (£) | QALY | ICER |
|---|---|---|---|
| Roche Elecsys hs-cTnT assay | |||
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,662 | 12.0798 | Cheapest |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,663 | 12.0813 | £1040 |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,706 | 12.0825 | £35,140 |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,729 | 12.0825 | £9,658,481 |
| 2. Roche Elecsys hs-cTnT: LoD | 38,738 | 12.0817 | Dominated |
| Abbott ARCHITECT hs-cTnI assay | |||
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,681 | 12.0795 | Cheapest |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,699 | 12.0815 | Extendedly dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,702 | 12.0818 | £9,183 |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,778 | 12.0823 | £158,972 |
| Siemens ADVIA Centaur hs-cTnI assay | |||
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,678 | 12.0794 | Cheapest |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,684 | 12.0791 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,704 | 12.0825 | £8213 |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,777 | 12.0825 | £19,868,699 |
| Siemens Atellica hs-cTnI assay | |||
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,698 | 12.0811 | Cheapest |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,768 | 12.0825 | £48,675 |
| Beckman Coulter ACCESS hs-cTnI assay | |||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,625 | 12.0768 | Cheapest |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,650 | 12.0790 | £11,522 |
Secondary analysis
The per assay analyses (Table 34) indicate that at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, the following test strategies would be the most cost-effective use of the particular assays: Roche Elecsys hs-cTnT (99th centile), Abbott ARCHITECT hs-cTnI (High-STEACS pathway), Siemens ADVIA Centaur hs-cTnI [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)], Siemens Atellica hs-cTnI (High-STEACS pathway) and Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)].
| Strategy | Cost (£) | QALY | ICER |
|---|---|---|---|
| Roche Elecsys hs-cTnT assay | |||
| 2. Roche Elecsys hs-cTnT: LoD | 38,050 | 11.4328 | Cheapest |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,070 | 11.4430 | Extendedly dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,072 | 11.4619 | £769 |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,088 | 11.4678 | £2658 |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,139 | 11.4718 | £12,797 |
| Abbott ARCHITECT hs-cTnI assay | |||
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,046 | 11.4201 | Cheapest |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,055 | 11.4466 | £326 |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,079 | 11.4535 | Extendedly dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,115 | 11.4691 | £2666 |
| Siemens ADVIA Centaur hs-cTnI assay | |||
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,039 | 11.4463 | Cheapest |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,051 | 11.4221 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,057 | 11.4497 | Extendedly dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,111 | 11.4636 | £4140 |
| Siemens Atellica hs-cTnI assay | |||
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,051 | 11.4249 | Cheapest |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,126 | 11.4689 | £1719 |
| Beckman Coulter ACCESS hs-cTnI assay | |||
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,066 | 11.4628 | Cheapest |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,077 | 11.4725 | £1197 |
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
The evidence base relating to the use of hs-cTn assays for the early rule out of AMI in people presenting with chest pain has expanded rapidly since the publication of our previous systematic review,2 which was conducted to support the development of DG15. 13 Update searches of bibliographic databases (from 2013 to October 2019) conducted for this assessment identified a total of 9379 unique references, compared with a total of 6766 unique references identified for the 9-year period (2005 to October 2013) covered by the searches conducted for our previous systematic review. 2 This current assessment includes a total of 123 publications relating to 37 studies, compared with the 37 publications relating to 18 studies included in our previous systematic review. 2
The main areas of change are an expansion of the number of hs-cTn assays available for use in the UK NHS, an increase in the number of studies comparing the performance of different hs-cTn assays and a proliferation of studies considering how to operationalise hs-cTn assays in clinical practice (previously, the majority of studies assessed the diagnostic accuracy of a single test).
This assessment includes nine assays that were not included in the scope for DG15:13 (1) Abbott Alinity hs-cTnI, (2) Beckman Coulter Access hs-cTnI, (3) bioMérieux VIDAS hs-cTnI, (4) Ortho Clinical Diagnostics VITROS hs-cTnI, (5) Quidel Cardiovascular TriageTrue hs-cTnI, (6) Siemens Healthcare Atellica hs-cTnI, (7) Siemens Healthcare Dimension EXL hs-cTnI, (8) Siemens Healthcare Dimension Vista hs-cTnI and (9) Siemens Healthcare ADVIA Centaur hs-cTnI. One assay that was included in DG15 (i.e. the Beckman Coulter AccuTnI+3 hs-cTnI assay) is no longer available and therefore it is not included in this current assessment. As was the case in our previous systematic review,2 most results relate to two assays, the Roche Elecsys hs-cTnT assay and the Abbott Architect hs-cTnI assay. Of the studies included in this assessment, 30 provided data on the Roche Elecsys hs-cTnT assay, nine provided data on the Abbott ARCHITECT hs-cTnI assay, three provided data on the Siemens ADVIA Centaur hs-cTnI assay, two provided data on each of the Siemens Atellica hs-cTnI assay and the Beckman Coulter Access hs-cTnI assay, and one study provided data on each of the Siemens Dimension Vista hs-cTnI assay, the Ortho VITROS hs-cTnI assay, the bioMérieux VIDAS hs-cTnI assay and the Quidel TriageTrue hs-cTnI assay. We did not identify any studies that evaluated testing strategies using either the Abbott Alinity hs-cTnI assay or the Siemens Dimension EXL hs-cTnI assay.
The APACE study was the only study included in our previous systematic review2 to evaluate more than one hs-cTn assay168 (i.e. to provide data to support direct comparisons of performance between assays). This assessment includes 25 new publications,54,55,58–60,70,74,75,90–94,103–108,111,113,123,132,170,173 relating to the APACE study, which have been published since our previous systematic review. Of particular significance is the fact that eight different hs-cTn assays (i.e. the Roche Elecsys hs-cTnT, Abbott ARCHITECT hs-cTnI, the Beckman Coulter Access hs-cTnI, the bioMérieux VIDAS hs-cTnI, the Ortho VITROS hs-cTnI, the Quidel TriageTrue hs-cTnI, the Siemens ADVIA Centaur hs-cTnI and the Siemens Dimension Vista hs-cTnI) have now been evaluated in subgroups of the APACE study population. Five further studies that are included in this assessment (ADAPT,68 BEST,115 High-US,176 ROMI-3101 and TRUST64) evaluated two hs-cTn assays and one study (High-STEACS61) evaluated three assays.
Our previous systematic review included theoretical optimal testing strategies for the Roche Elecsys hs-cTnT assay and for the Abbott ARCHITECT hs-cTnI assay. These strategies used a two-step repeat-testing process, providing two potential opportunities to rule out NSTEMI and hence to discharge patients within the 4-hour window specified in the scope. Our estimates of the clinical effectiveness and cost-effectiveness of these strategies were limited by the assumption that the diagnostic performance of the second step is the same when used in people in whom NSTEMI is not ruled out by the first step, as it is when used in the whole population. This assumption was necessary because no combined test performance data were available for the proposed strategies. Indeed, there were few studies of any multiple test strategies. By contrast, this current assessment includes data for a very large number of different test strategies (e.g. unique combinations of assay, threshold and timing), which are dominated by multiple testing strategies (59 distinct multiple testing strategies). Therefore, the construction of theoretical optimised testing strategies has been rendered obsolete, and the problem has become one of determining which of the large number of strategies that have been proposed and evaluated are likely to be considered clinically acceptable and cost-effective. The process of selecting test strategies for inclusion in cost-effectiveness modelling is described in detail in Chapter 3, Selection of test strategies for inclusion in cost-effectiveness modelling.
With respect to single test strategies, the results of our previous systematic review2 indicated that very low hs-cTn levels (below a threshold that is at or near the LoD) in a single sample, taken on presentation, may be considered adequate to rule out NSTEMI. At the time of our previous review, data for an LoD threshold rule-out strategy and the target condition NSTEMI were available for the Roche Elecsys hs-cTnT assay only (threshold 5 ng/l). One study141 evaluated a LoD threshold for the Abbott ARCHITECT hs-cTnI assay (2 ng/l) for the target condition any AMI. The number of included studies reporting data for the performance of a single presentation sample rule-out strategy, using a threshold at or near to the LoD for the assay, has increased in this assessment. The summary estimates of sensitivity and specificity for the target condition NSTEMI, using the Roche Elecsys hs-cTnT assay and a threshold of 5 ng/l in a single presentation sample, were 99% (95% CI 97% to 100%) and 35% (95% CI 25% to 46%), respectively, based on data from six studies63,75,87,101,115,139 (see Table 8). The corresponding summary sensitivity and specificity estimates for the Abbott ARCHITECT hs-cTnI assay, using a 2 ng/l threshold, were 100% (95% CI 99% to 100%) and 21% (95% CI 16% to 26%), respectively, based on data from four studies58,68,96,101 (see Table 9). Of the remaining hs-cTn assays included in this assessment, only the Siemens Atellica hs-cTnI assay and the Siemens ADVIA Centaur hs-cTnI assay were evaluated using a single presentation sample rule-out strategy, with a threshold at or near to the LoD for the assay. The LoD for both of these assays is 1.6 ng/l and both assays were evaluated by the High-US study,176 using a rule-out threshold of 2 ng/l. The sensitivity and specificity estimates were 100% (95% CI 99% to 100%) and 23% (95% CI 21% to 25%), respectively, for the Siemens ADVIA Centaur hs-cTnI assay (see Table 14), and 100% (95% CI 98% to 100%) and 26% (95% CI 24% to 28%), respectively, for the Siemens Atellica hs-cTnI assay (see Table 15). 176
The majority of the multiple test strategies selected for inclusion in our cost-effectiveness modelling (see Table 21) comprised an initial rule-out step, based on hs-cTn levels in a sample taken on presentation and a minimum symptom duration, and a second stage (for patients not meeting the initial rule-out criteria), based on presentation levels of hs-cTn and absolute change in hs-cTn between presentation and a second sample taken after 1, 2 or 3 hours. The 2015 ESC guidelines33 for the management of ACSs in patients presenting without persistent ST segment elevation included 0/3- and 0/1-hour algorithms for rule-in and rule-out of AMI using hs-cTn assays. The ESC 0/1-hour algorithm incorporates separate rule-out and rule-in pathways and an intermediate ‘observe’ zone. 33 The rule-out pathway comprises an initial rule-out step, based on hs-cTn levels in a sample taken on presentation for patients who have a minimum symptom duration of 3 hours, and a second stage (for patients not meeting the initial rule-out criteria), based on presentation levels of hs-cTn and absolute change in hs-cTn between presentation and a second sample taken after 1 hour. The published ESC 0/1-hour algorithm specifies rule-out thresholds to be used with the Roche Elecsys hs-cTnT assay, the Abbott ARCHITECT hs-cTnI assay and the Siemens Dimension Vista hs-cTnI assay. 33 Subsequently, ESC 0/1-hour algorithm rule-out thresholds have been published for the Beckman Coulter Access hs-cTnI assay,60 the Ortho VITROS hs-cTnI assay,170 the Quidel TriageTrue hs-cTnI assay173 and the Siemens ADVIA Centaur hs-cTnI assay. 59 Data on the rule-out performance of the ESC 0/1-hour algorithm for the target condition NSTEMI that are included in this assessment were calculated by dichotomising at the rule-out threshold (i.e. study participants in the observe or the rule-in categories were classified as test positive). Unsurprisingly, the addition of a second rule-out step appears to offer consistently higher specificity than rule-out strategies based on very low hs-cTn levels in a single sample taken on presentation alone, and sensitivity estimates remained high. Sensitivity and specificity estimates for the ESC 0/1-hour rule-out pathways included in this assessment were:
-
99% (95% CI 98% to 100%) and 68% (95% CI 67% to 70%), respectively, for the Roche Elecsys hs-cTnT assay [rule-out threshold: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)]104
-
99% (95% CI 98% to 100%) and 57% (95% CI 56% to 59%), respectively, for the Abbott ARCHITECT hs-cTnI assay (summary estimate based on two studies66,104) [rule-out threshold: (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours)]104,213
-
99% (95% CI 94% to 100%) and 70% (95% CI 66% to 74%), respectively, for the Beckman Coulter Access hs-cTnI assay [rule-out threshold: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 4 ng/l at 0 to 1 hours)]60
-
100% (95% CI 95% to 100%) and 60% (95% CI 55% to 64%) for the Ortho VITROS hs-cTnI assay [rule out threshold: (symptoms > 3 hours AND < 1 ng/l at 0 hours) OR (< 2 ng/l at 0 hours AND Δ < 1 ng/l at 0 to 1 hours)]170
-
100% (95% CI 97% to 100%) and 66% (95% CI 62% to 70%) for the Quidel TriageTrue hs-cTnI assay [rule-out threshold: (symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)]173
-
99% (95% CI 95% to 100%) and 67% (95% CI 61% to 72%) for the Siemens ADVIA Centaur hs-cTnI assay [rule-out threshold: (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)]. 59
All of these test strategies were selected for inclusion in our cost-effectiveness modelling. Using a hypothetical cohort of 1000 patients and an NSTEMI prevalence of 12.2%, calculated by combining the HES 2017–18 prevalence of AMI in people presenting to the ED with chest pain117 and the ratio of NSTEMI to STEMI from the Myocardial Ischemia National Audit Project,202 application of the ESC 0/1-hour rule-out pathway would result in the discharge of between 500 and 615 people (depending on the hs-cTn assay used) within 2 hours of presentation (allowing for a 1-hour assay turnaround time), with a maximum of one instance of NSTEMI missed per 1000 people. Thresholds for the ESC 0/1-hour pathway using the Siemens Atellica hs-cTnI assay have also been published (rule-out threshold: (symptoms > 3 hours AND < 3 ng/l at 0 hours) OR (< 6 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours). 67 However, this strategy did not reach the specified minimum clinically acceptable sensitivity of 97%. The sensitivity and specificity estimates were 94% (95% CI 79% to 99%) and 69% (95% CI 64% to 74%), respectively, and therefore it was not included in our cost-effectiveness modelling. Two-step rule-out strategies, such as High-STEACS,61 which use a later (3-hour) second sample, offer the potential to further increase overall specificity. The sensitivity and specificity estimates for the High-STEACS pathway that have been included in this assessment were:
-
99% (95% CI 97% to 100%) and 76% (95% CI 73% to 78%), respectively, for the Abbott ARCHITECT hs-cTnI assay {rule-out threshold: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (females) ≤ 34 ng/l (males) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours]}66
-
98% (95% CI 95% to 100%) and 74% (95% CI 72% to 76%), respectively, for the Siemens Atellica hs-cTnI assay {rule-out threshold: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (females) ≤ 53 ng/l (males) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours]}. 67
Based on the hypothetical cohort of 1000 patients, described above, application of the High-STEACS rule-out pathway would result in the discharge of between 650 and 667 patients within 4 hours (allowing for a 1-hour assay turnaround time), with up to two patients with NSTEMI being erroneously discharged for every 1000 people presenting with chest pain. These findings are consistent with the conclusions from a recently published large individual patient-level analysis,214 which took data from 15 international patient cohorts (n = 22,651 patients) and used a derivation-validation design to assess multiple hs-cTn test strategies and inform the development of a risk assessment tool. This study found that patients at low risk for MI were likely to have very low concentrations of hs-cTn at presentation and small absolute changes on serial sampling, and that these patients were also at very low risk for MI or death from any cause at 30 days. 214
In addition to the changes in the evidence about diagnostic accuracy described above, two major RCTs (i.e. the High-STEACS trial99 and the unpublished HiSTORIC trial175) are included in this assessment. Both trials were stepped-wedge cluster RCTs that evaluated implementation of an early rule-out pathway in hospitals in Scotland. The primary outcomes were length of stay, and MI or cardiac death after discharge (at 30 days). 99,175 Both trials used the Abbott ARCHITECT hs-cTnI assay. In the High-STEACS trial,99 during the validation phase of the trial (6–12 months), results of the hs-cTnI assay were concealed from the attending clinician and a contemporary cTn assay was used to guide care. A high-sensitivity test was introduced after 6 months (early implementation) or 12 months (late implementation). 99 The HiSTORIC trial175 also had a validation phase where troponin testing was performed at presentation and repeated 6–12 hours after the onset of symptoms, if indicated. 175 In the validation phase of HiSTORIC trial, the High-STEACS early rule-out pathway was used. 99,175 In the High-STEACS trial, of 1771 patients reclassified by the hs-cTnI assay, 105 of 720 (15%) patients were in the validation phase and 131 of 1051 (12%) patients were in the implementation phase. The adjusted OR for implementation compared with validation was 1.10 (95% CI 0.75 to 1.61). 99 In the HiSTORIC trial175 (confidential information has been removed). In the High-STEACS trial,99 the median length of stay was 7 (IQR 3–24) hours in the implementation phase and 4 (IQR 3–20) hours in the validation phase. In the HiSTORIC trial175 (confidential information has been removed). The authors of the High-STEACS trial99 concluded that although implementation of a hs-cTn assay resulted in reclassification of 17% of 10,360 patients with myocardial injury or infarction, only one-third of the patients had a diagnosis of type 1 MI and the incidence of subsequent MI or death from cardiovascular causes within 1 year was not affected by use of this assay. 99 (Confidential information has been removed.)175 These studies represent direct, real-world evidence about the effects of implementing an early rule-out strategy based on a hs-cTn assay obtained in a UK setting.
We identified a further RCT, the RAPID-TnT (Rapid Assessment of Possible ACS In the emergency Department with high sensitivity Troponin T),215 conducted in Australia, which did not meet the inclusion criteria for this assessment as it did not compare testing with a hs-cTn assay with testing with a conventional cTn assay. Participants in the RAPID-TnT trial (n = 3378) were randomised to either the 0/1-hour Roche Elecsys hs-cTnT [reported to the LoD (< 5ng/l)] or masked Roche Elecsys hs-cTnT [reported to ≤ 29 ng/l evaluated at 0/3 hours (standard arm)]. The 30-day primary end point was all-cause death and MI. 215 Participants in the 0/1-hour arm were more likely to be discharged from the ED (45.1% vs. 32.3%) and the median length of ED stay was also shorter in the 0/1-hour arm [4.6 (IQR 3.4–6.4) hours vs. 5.6 (IQR 4.0–7.1) hours]. 215 The 0/1-hour Roche Elecsys hs-cTnT protocol was not inferior to standard care, with respect to 30-day all-cause mortality and MI [17/1646 (1.0%) in the 0/1-hour arm vs. 16/1642 (1.0%) in the standard arm, incidence rate ratio 1.06 (95% CI 0.53 to 2.11); non-inferiority was an absolute margin of 0.5% determined by poisson regression]. 215
Cost-effectiveness
In our health economic analysis, the cost-effectiveness of different testing strategies involving hs-cTn for the early rule out of AMI in people with acute chest pain presenting to the ED with suspected ACS and STEMI ruled out was assessed. In the base case, standard troponin testing at 10–12 hours was considered the reference standard, assuming perfect sensitivity and specificity. In addition to the base-case analysis, given some evidence that FPs compared with this reference standard also have an increased mortality and MI probability, a secondary analysis was conducted that assumed an increased risk of adverse events (i.e. MI and mortality) for patients with a FP hs-cTn test result.
In the base-case analysis, standard troponin testing was both most effective and most costly. The strategies considered cost-effective, depending on ICER thresholds, were the Beckman Coulter ACCESS hs-TnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] for willingness-to-pay thresholds of < £8455 per QALY gained, the Roche Elecsys hs-cTnT [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)] for thresholds between £8455 and £20,190 per QALY gained and the Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) for thresholds > £20,190 per QALY gained.
The above mentioned results should, however, be interpreted while noting that the differences between the strategies in both costs and QALYs were very small. Given these minimal differences in cost-effectiveness, it might be worthwhile to consider other aspects not captured in the economic assessment. This might include differences in the proportion of patients who are correctly ruled out (i.e. TNs). Although the cost consequences of the early rule out have been considered in the cost-effectiveness assessment, early rule out might have benefits not captured by the model (e.g. preventing unnecessary anxiety in patients without MI and making hospital resources available for other patients). It is noticeable that, in the base-case analysis, the high-sensitivity test strategies with the highest TN rates (i.e. ≥ 65%) involve high-sensitivity test strategies with a second test 2–3 hours after the initial test {i.e. the Siemens Atellica hs-cTnI (High-STEACS pathway), the Abbott ARCHITECT hs-cTnI (High-STEACS pathway), the Roche Elecsys hs-cTnT (99th centile) and the Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)]}.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts, to identify unpublished studies. Owing to the known difficulties in identifying test accuracy studies using study design-related search terms,216 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Therefore, large numbers of citations were identified and screened, relatively few of which met the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment (e.g. a significant difference between the treatment and control groups that favours treatment). This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (i.e. high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice or by retrospective review of records. Test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 217 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 41 We did not undertake a statistical assessment of publication bias in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Clear inclusion criteria were specified in the protocol for this review. The review has been registered on PROSPERO (CRD42019154716) and the protocol is available from URL: www.nice.org.uk/guidance/indevelopment/gid-dg10035/documents (accessed 20 October 2020). The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for exclusion for all of the studies that were considered potentially relevant at initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 5). The review process followed recommended methods to minimise the potential for error and/or bias. 39 Studies were independently screened for inclusion by two reviewers and data extraction and quality assessment were carried out by one reviewer and checked by a second (MW, DF and GW). Any disagreements were resolved by consensus.
Diagnostic cohort studies included in this review were assessed for risk of bias and applicability using the QUADAS-2 tool developed by the authors45 and recommended by the Cochrane Collaboration. 41 QUADAS-2 is structured into four key domains: (1) participant selection, (2) index test, (3) reference standard and (4) the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear), and the participant selection, index test and reference standard domains are also rated separately for concerns regarding the applicability of the study to the review question (low, high or unclear). Studies that provided data for two or more hs-cTn assays were assessed using QUADAS-2C,46 in place of QUADAS-2. QUADAS-2C is a version of the QUADAS tool that has been developed specifically for the assessment of comparative DTA studies. This tool is currently undergoing piloting and is not yet published. The results of the QUADAS-2 and QUADAS-2C assessments are reported, in full, for all included studies in Appendices 3 and 4, and are summarised in Chapter 3, Study quality. The methodological quality of included RCTs was assessed using the revised Cochrane Risk-of-Bias Tool for Randomised Trials. 44 The main potential sources of bias in the studies included in this assessment were related to participant spectrum and participant flow (domains 1 and 4 of QUADAS-2 and QUADAS-2C). The most common feature of studies rated as having a ‘high risk of bias’ for patient selection was the inclusion of participants based on staffing or work flow considerations (e.g. participants were excluded if they presented at night or during busy periods). 88,117,121,139,144 This was considered to have the potential to lead to the inclusion of a different spectrum of patients than if consecutive patients had been enrolled. All studies assessed using QUADAS-2C were rated as having a low risk of bias for patient selection for all individual index tests. However, one study, for which data for two hs-cTn assays were reported in separate publications,115,172 was rated as having a high risk of bias for participant selection for the comparison of the two assays. This was because the study did not set out to conduct both tests in all patients or to randomly allocate patients to one of the two tests. Six of the studies110,137,141,147,157,159 that reported data for a single hs-cTn assay, assessed using QUADAS-2, were considered as having a high risk of bias for patient flow and a further three studies62,102,165 were considered as having an unclear risk of bias. In all cases, this was related to withdrawals from the study. Verification bias was not considered to be a problem in any of the studies. All of the studies assessed using QUADAS-2C were rated as having a low risk of bias for participant flow, with respect to the individual hs-cTn assays that they assessed. However, four of these studies (APACE,59,170,178 BEST,115,172 High-STEACS66,67 and TRUST64) were rated as having a high risk of bias for participant flow, with respect to at least one between-assay comparison. In all cases, this was because the number of participants for whom hs-cTn results were available differed between assays.
As with our previous systematic review,2 this assessment included studies that enrolled both mixed populations (i.e. when the target condition was any AMI) and studies restricted to populations where patients with STEMI were excluded (i.e. the target condition was NSTEMI). Our primary focus remained the population of patients with STEMI excluded. Studies not restricted to this specific patient group were therefore considered to have high concerns regarding applicability. Seven studies133,137,139,144,148,157,159 from our previous systematic review were restricted to patients in whom STEMI had been excluded. This assessment includes a further 13 studies58,61,62,64,68,72,80,84,96,101,115,171,176 that were restricted to patients in whom STEMI had been excluded.
The most recent systematic review218 identified during this assessment aimed to compare the diagnostic performance of various accelerated algorithms using hs-cTn assays, for patients with symptoms suggestive of AMI. This review, by Lee et al. ,218 reported summary estimates of sensitivity and specificity for a ‘0-hour algorithm’, 1-hour algorithm, 2-hour algorithm and 0- to 1-hour delta algorithm. Separate estimates were reported for hs-cTnT and hs-cTnI; however, no distinction was made between different hs-cTnI assays. None of the summary estimates of sensitivity reported in the systematic review by Lee et al. 218 reached the minimum clinically acceptable sensitivity (97%) defined for this assessment.
We believe that our assessment provides information of direct relevance to UK clinical practice, as we focus on the performance of hs-cTn within the 4-hour time window corresponding to the target for NHS EDs, which specifies a maximum ED waiting time of 4 hours before admission, transfer or discharge. 200
This assessment represents an advance on our previous systematic review,2 conducted to support the development of DG15,13 in that we are now able to include data on the diagnostic performance of two-stage rule-out algorithms, which have been taken directly from large diagnostic cohort studies. In our previous systematic review, we proposed strategies for how hs-cTn assays might be applied and interpreted to maximise diagnostic performance. These strategies were devised with consideration to test timing, diagnostic threshold and interpretation of combinations of multiple test results. However, because there was no direct evidence of the performance of such strategies, our estimates of their effectiveness and cost-effectiveness relied on the assumption that the diagnostic performance of the second step would be the same when used in people in whom NSTEMI was not ruled out by the first step, as when used in the whole population. 2
A limitation of this assessment, with respect to the evaluation of the ESC 0/1-hour pathway, is our use of the rule-out threshold to dichotomise data. This approach classifies all patients in both the observe and the rule-in arms of the ESC 0/1-hour pathway as test positive and therefore does not account for potential differences in the care pathway for these two patient groups.
This assessment was further limited in that the scope21 did not include studies evaluating the use of hs-cTn assays as part of or in combination with a clinical risk score.
Our searches identified two recent systematic reviews that evaluated the History ECG Age Risk factors Troponins (HEART) score219 for risk stratification of patients presenting to the ED with chest pain220,221 and that included an assessment of the effect of using hs-cTn (vs. conventional troponins) in the heart score. Both studies used the low-risk HEART score (0–3) to define the rule-out threshold and reported accuracy data using 30-day to 6-week (short-term) MACEs as the reference standard. Van Den Verg and Body220 reported summary estimates of sensitivity and specificity of the HEART score, based on nine studies using either conventional or high-sensitivity troponin assays. The summary sensitivity estimate was 97% (95% CI 94% to 98%) and the summary specificity estimate was 47% (95% CI 41% to 54%). 220 None of the studies in this review compared the performance of the HEART score using a hs-cTn assay compared with conventional troponins. However, the review authors noted that the two studies that used a high-sensitivity assay (Roche Elecsys hs-cTnT), with the original HEART score definition and a target condition of short-term MACEs, reported differing estimates of sensitivity [93% (95% CI 84% to 98%) and 100% (95% CI 98% to 100%)]. Laureano-Phillips et al. 221 reported summary sensitivity and specificity estimates for the original HEART score and the target condition short-term MACEs using either conventional or high-sensitivity troponin assays. The summary sensitivity estimate was 97% (95% CI 94% to 98%) and the summary specificity estimate was 38% (95% CI 33% to 43%); however, the number of studies included in this analysis was unclear. 221 The only estimates of the sensitivity and specificity of the HEART score using high-sensitivity troponins, provided in this review, were for a different target condition (i.e. all-time frame MACEs). 221 The findings of these two reviews220,221 suggest that further work may be needed to validate the use of high-sensitivity troponin assays in the context of the HEART score and, potentially, other clinical risk scores that include a cTn component.
The potential use of clinical risk scores in combination with hs-cTn test strategies is distinct from the integration of hs-cTn assays into existing clinical risk scores, in place of conventional troponin assays. One of the publications of the High-STEACS study66 included in this assessment reported data on the performance of the High-STEACS pathway, using the Abbott ARCHITECT hs-cTnI assay and the rule-out threshold (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours], alone and in combination validated clinical risk scores [a HEART score of ≤ 3,219 a Global Registry of Acute Coronary Events (GRACE) score of ≤ 108,222 a Thrombolysis Myocardial Infarction score of 0 or 1,223 or and Emergency Department Assessment of Chest Pain Score (EDACS) of < 16224]. The High-STEACS pathway alone classified 1244 of 1917 (64.9%) participants as low risk (rule out) and missed instances of NSTEMI at index presentation and one further instance during the 30-day follow-up. 66 Combining the High-STEACS pathway with clinical risk scores reduced the proportion of people classified as low risk (rule out) in all instances (HEART 24.3%, GRACE 47%, Thrombolysis In Myocardial Infarction 44% and EDACS 41%) and the addition of a clinical risk score did not improve the negative predictive value of the High-STEACS pathway. 66 The same pattern was observed when the ESC 0/1-hour pathway, using the Abbott ARCHITECT hs-cTnI assay and the rule-out threshold (symptoms > 3 hours AND < 2 ng/l at 0 hours) OR (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) was assessed alone and in combination with the same set of clinical risk scores. 66 These data provide an indication that the addition of clinical risk scores to the key hs-cTn multiple test strategies considered in this assessment would be likely to reduce the proportion of patients discharged within 4 hours (ruled out), without improving safety.
Our assessment was less comprehensive for the Beckman Coulter Access hs-cTnI, bioMérieux VIDAS hs-cTnI, Ortho Clinical Diagnostics VITROS hs-cTnI, Quidel Cardiovascular TriageTrue hs-cTnI, Siemens Healthcare Atellica hs-cTnI, Siemens Healthcare Dimension Vista hs-cTnI and Siemens Healthcare ADVIA Centaur hs-cTnI assays than for the Roche Elecsys hs-cTnT and the Abbott ARCHITECT hs-cTnI assays, because available data were limited for these six assays. Furthermore, we were unable to identify any studies of either the Abbott Alinity hs-cTnI assay or the Siemens Healthcare Dimension EXL hs-cTnI assay.
Cost-effectiveness
Our CEA is, to the best of our knowledge, the most comprehensive to date in terms of the number of relevant hs-cTn test strategies for the early rule out of AMI in people presenting to the ED with acute chest pain and suspected ACS. The model was informed by a comprehensive, high-quality systematic review of DTA.
As in any economic model, a number of major and minor assumptions had to be made. It is important to understand the impact of these assumptions to correctly interpret the results of the model. The impact of most assumptions has been explored in sensitivity and scenario analyses. However, one major assumption that was maintained throughout all analyses was the conservative assumption of no health benefit of early treatment in the hs-cTn strategies, compared with ‘late’ treatment in the standard cTn strategy. Although many experts believe that there must be a benefit, at least to some extent, of treating patients early, there is no evidence to support or quantify a timing effect as of yet. In addition, there may well be adverse effects associated with early treatment (e.g. the risk of bleeding, unnecessary percutaneous coronary interventions, etc.). The Canadian HTA report186 identified in the economic review did include an advantage for early versus late treatment, based on one study, which investigated the effect of a 36-hour treatment delay. 225 The RR found in this study was then recalculated, assuming a constant effect of timing on treatment benefit, to a RR of 1.035 of mortality for a treatment delay of 6 hours versus early treatment, which was again adjusted to 1.01 based on expert opinion. Any possible adverse effect of early treatment was not considered in this analysis. A similar approach would have been possible in the present model but, in our view, this would not be informative, given the level of uncertainty underlying this final estimate. Therefore, it was decided to leave out a possible effect of timing of treatment. This could be considered a conservative approach, but even this is uncertain.
The assumption that standard troponin, as the reference standard, has perfect sensitivity and specificity was also maintained throughout all analyses. However, there is evidence that the prognostic performance of standard troponin testing may be imperfect. For example, a negative troponin test might correctly assess that a patient is not experiencing a NSTEMI, but some patients with negative test results may still benefit from treatment. A secondary analysis was performed to take this possibility into account, which resulted in the standard troponin strategy being less effective than the hs-cTn testing strategies.
In addition to the abovementioned strategies, it should be noted that not all test strategies presented in Chapter 3 are considered in the CEAs (see Chapter 3 for an overview of all high-sensitivity troponin strategies that were identified in the literature). For the economic model, only those high-sensitivity troponin tests that had a sensitivity of ≥ 97% were selected. Although some of the test strategies with lower sensitivity might potentially be cost-effective, it would be questionable whether or not these strategies would be considered acceptable for clinicians.
Uncertainties
Clinical effectiveness
A recent systematic review of sex-specific and overall 99th centiles of hs-cTnI and hs-cTnT derived from healthy reference populations226 found that 14 of 16 (87.5%) hs-cTnI studies and 11 of 18 (61.1%) hs-cTnT studies reported lower female-specific thresholds than the overall threshold for the population. Conversely, male-specific thresholds were reported as being ‘generally in line with currently used overall thresholds’. 226 In addition, the product information leaflets for all of the hs-cTn assays included in this assessment report separate female and male, as well as overall, 99th centile for the general population (see Table 1). Despite this, the clinical effectiveness and cost-effectiveness of using sex-specific thresholds for hs-cTn assay remains unclear. Although there are some subgroup data comparing the performance of a common threshold in males and females,62,65,74,79,81,94 few studies have evaluated the diagnostic performance of sex-specific thresholds. Considering those test strategies included in this assessment, which were selected for inclusion in our cost-effectiveness modelling, only the High-STEACS pathway utilises sex-specific thresholds. 66,67 It remains unclear whether or not the use of sex-specific thresholds in the High-STEACS pathway offers any advantage over the use of a single general population threshold, as no equivalent pathway (using a single general population threshold) has been evaluated.
Our previous systematic review2 identified some data on the diagnostic performance of hs-cTn testing in clinically important subgroups (e.g. older people146,168 and people with and without pre-existing CAD). 140,168 However, these data were very limited and were available for the Roche Elecsys hs-cTnT assay only. The current assessment includes some additional data about the performance of hs-cTn test strategies in people with normal renal function and those with impaired renal function,72,79,106 people with known ischemic heart disease and those with no known ischemic heart disease,65 and people aged ≥ 65 years compared with those aged < 65 years. 65 Of particular note are the renal function subgroup data for the ESC 0/1-hour pathway, using the Abbott ARCHITECT hs-cTnI assay,106 which indicate that the sensitivity of the rule-out pathway is high for both people with normal renal function (99%, 95% CI 97% to 100%) and those with impaired renal function (i.e. an eGFR < 60 ml/minute/1.73 m2) (99%, 95% CI 94% to 100%). However, the specificity of this test strategy was markedly lower in patients with impaired renal function (25%, 95% CI 20% to 30%) than in those with normal renal function (66%, 95% CI 64% to 68%). 106 Based on the hypothetical cohort of 1000 patients, described above, these data indicate that the use of the ESC 0/1-hour rule-out strategy in people with impaired renal function would not lead to any additional instances of NSTEMI being missed, but would reduce the number of people discharged within 4 hours to approximately 220. Subgroup data for the High-STEACS pathway, also using the Abbott ARCHITECT hs-cTnI assay,65 indicate that this test strategy may fall bellow the clinically acceptable threshold for sensitivity (97%) defined from this assessment, when used in people with known ischemic heart disease [96% (95% CI 89% to 99%) vs. those with no known ischemic heart disease, 100% (95% CI 97% to 100%)]. There remains some uncertainty about how the diagnostic performance of individual hs-cTn assays may vary in clinically relevant subgroups, as well as what may constitute the optimal testing strategy in these groups.
It should be noted that the performance of any test strategy that incorporates the 99th centile for the general population in the diagnostic threshold will be dependent on the characteristics of the reference population from which this value was derived. The High-STEACS pathway using the Abbott ARCHITECT hs-cTnI assay {rule-out threshold: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 16 ng/l (F) ≤ 34 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours]}66 and the High-STEACS pathway using the Siemens Atellica hs-cTnI assay {rule-out threshold: (symptoms ≥ 2 hours AND < 5 ng/l at 0 hours) OR [≤ 34 ng/l (F) ≤ 53 ng/l (M) at 3 hours AND Δ < 3 ng/l at 0 to 3 hours]}67 were the only two strategies, selected for inclusion in our cost-effectiveness modelling, to incorporate 99th centile thresholds. The product information leaflet for the Abbott ARCHITECT hs-cTnI assay describes the 99th centile as derived from a healthy US population of 1531 individuals, who had normal BNP (B-type natriuretic peptide), glycated haemoglobin and eGFR values. The leaflet also recommends that laboratories should establish their own 99th centile that is applicable to their population. 18 Similarly, the product information leaflet for the Siemens Atellica hs-cTnI assay describes the 99th centile as derived from a healthy US population of 2007 individuals, aged between 22 and 91 years. The leaflet also recommends that laboratories should establish their own 99th centile that is applicable to their population and that reflects their institutional criteria for the diagnosis of AMI. 28
Cost-effectiveness
The main uncertainties for the CEA lie in the model assumptions, particularly regarding the effect of actual clinical practice in terms of both other diagnostic information and treatment, given this information. Although many of these assumptions have been varied in one-way sensitivity analyses, the precise implication of FN test results, where patients are discharged without essential treatment, or of FP test results, where patients stay in hospital and may receive unnecessary interventions, is unknown. Given this, as well as the minimal differences between the test strategies, the results of the CEA should be interpreted in the context of potential cost and benefits (i.e. FNs/FPs) that are not captured in the economic model.
Chapter 6 Conclusions
Implications for service provision
There is evidence to indicate that high-sensitivity troponin assays can be used to rule out NSTEMI in adults presenting with acute chest pain within the 4-hour NHS ED target. Test strategies that comprise an initial rule-out step, based on low hs-cTn levels in a sample taken on presentation and a minimum symptom duration, and a second stage (for patients not meeting the initial rule-out criteria), based on low presentation levels of hs-cTn and small absolute change in hs-cTn between presentation and a second sample taken after 1, 2 or 3 hours, are likely to produce the highest rule-out rates while maintaining clinically acceptable sensitivity (very low rates of missed NSTEMI). There is a lack of evidence about the clinical effectiveness of two of the intervention technologies included in the scope of this assessment (i.e. the Abbott Alinity hs-cTnI assay and the Siemens Dimension EXL hs-cTnI assay).
From a cost-effectiveness perspective, the Roche Elecsys hs-cTnT (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) and Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) might be cost-effective for thresholds of £20,000 and £30,000 per QALY gained, respectively (base case). For the secondary analysis, the Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] was considered to be cost-effective for these thresholds. The cost-effectiveness results should, however, be interpreted while noting that the differences between the strategies in both costs and QALYs were very small. Given these minimal differences in cost-effectiveness, it might be worthwhile to consider other aspects not captured in the economic assessment. Therefore, it is worth noting that the high-sensitivity tests strategies with the highest TNs (i.e. ≥ 65%) involve high-sensitivity tests strategies with a second test 2–3 hours after the initial test {i.e. the Siemens Atellica hs-cTnI (High-STEACS pathway), the Abbott ARCHITECT hs-cTnI (High-STEACS pathway), the Roche Elecsys hs-cTnT (99th centile) and the Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)]}.
Suggested research priorities
If adoption of either the Abbott Alinity hs-cTnI assay or the Siemens Dimension EXL hs-cTnI assay is to be considered, then studies are needed to evaluate the diagnostic performance of these assays and to determine optimum test strategies and thresholds.
Further diagnostic cohort studies or subgroup analyses of existing data sets are needed to fully explore possible variation in the accuracy of hs-cTn assays and the optimal testing strategies for these assays in relevant demographic and clinical subgroups (e.g. sex, age, ethnicity, renal function, previous CAD, previous AMI).
Multivariable prediction modelling studies may be useful to assess the independent prognostic value of a positive hs-cTn test result, in the context of other clinical risk factors and tests, in patients who do not have a confirmed AMI at the index presentation.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Professor Rick Body, Clinical Professor of Emergency Medicine, Manchester Royal Infirmary/University of Manchester; Professor Nick Mills, Professor of Cardiology and Consultant Cardiologist, University of Edinburgh; Professor Adam Timmis, Professor of Clinical Cardiology, Queen Mary University of London and St Bartholomew’s Hospital, London; Professor Paul Collinson, Professor of Cardiovascular Biomarkers, St George’s University Hospitals NHS Foundation Trust; and Mr Alan Reid, scheme organiser UK NEQAS Cardiac Markers (Glasgow, UK) and principal clinical scientist, Queen Elizabeth University Hospital, Glasgow. Finally, the authors would like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Subgroup for providing input on the patients’ perspective at key stages of the assessment process.
Contributions of authors
Marie Westwood (https://orcid.org/0000-0002-6257-0653) planned and performed the systematic review and provided interpretation of evidence.
Bram Ramaekers (https://orcid.org/0000-0001-5785-9228) planned and performed the CEAs and interpreted results.
Sabine Grimm (https://orcid.org/0000-0002-2175-7999) planned and performed the CEAs and interpreted results.
Gill Worthy (https://orcid.org/0000-0003-1463-5413) planned and performed the systematic review and provided interpretation of evidence.
Debra Fayter (https://orcid.org/0000-0003-4487-9216) planned and performed the systematic review and provided interpretation of evidence.
Nigel Armstrong (https://orcid.org/0000-0002-7443-4798) contributed to planning and interpretation of CEAs, acquisition of input data and conducted model peer review.
Titas Buksnys (https://orcid.org/0000-0002-8383-7732) contributed to planning and interpretation of CEAs, acquisition of input data and conducted model peer review.
Janine Ross (https://orcid.org/0000-0002-4763-2078) devised and performed the literature searches and provided information support to the project.
Manuela Joore (https://orcid.org/0000-0002-5649-6768) provided senior advice and support to the systematic review and CEAs, respectively.
Jos Kleijnen (https://orcid.org/0000-0003-2787-7091) provided senior advice and support to the systematic review and CEAs, respectively.
All parties were involved in drafting and/or commenting on the report.
Publication
Westwood ME, Armstrong N, Worthy G, Fayter D, Ramaekers BLT, Grimm S, et al. Optimizing the use of high-sensitivity troponin assays for the early rule-out of myocardial infarction in patients presenting with chest pain: a systematic review. Clin Chem 2021;67:237–44.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Chalkidou A, Erskine J, Radhakrishnan Kartha M, Langford T, Macmillan T, Keevil S. Review Report of DG15: Myocardial Infarction (Acute): Early Rule Out Using High-Sensitivity Troponin Tests (Elecsys Troponin T High-Sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 Assays) 2017.
- Westwood M, van Asselt T, Ramaekers B, Whiting P, Thokala P, Joore M, et al. High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19440.
- Office for National Statistics . Deaths Registered in England and Wales: 2018 n.d. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-276695 (accessed 22 August 2019).
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30. https://doi.org/10.1136/hrt.2003.027599.
- NHS Digital . Hospital Admitted Patient Care Activity, 2017–18 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2017-18 (accessed 14 August 2019).
- Collinson PO, Rao AC, Canepa-Anson R, Joseph S. Impact of European Society of Cardiology/American College of Cardiology guidelines on diagnostic classification of patients with suspected acute coronary syndromes. Ann Clin Biochem 2003;40:156-60. https://doi.org/10.1258/000456303763046085.
- Health and Social Care Information Centre . Hospital Episode Statistics, Admitted Patient Care – England 2011–12 n.d. www.hscic.gov.uk/catalogue/PUB08288 (accessed 20 February 2020).
- NHS Digital . Hospital Accident and Emergency Activity, 2017–18 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-accident--emergency-activity/2017-18 (accessed 14 August 2019).
- Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95. https://doi.org/10.1016/j.jacc.2007.09.011.
- Ebell MH, Flewelling D, Flynn CA. A systematic review of troponin T and I for diagnosing acute myocardial infarction. J Fam Pract 2000;49:550-6.
- National Institute for Health and Care Excellence . Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin. NICE CG95 n.d. www.nice.org.uk/guidance/cg95 (accessed 29 January 2020).
- Scottish Intercollegiate Guidelines Network . SIGN 93. Acute Coronary Syndromes. A National Clinical Guideline 2013.
- National Institute for Health and Care Excellence . Myocardial Infarction (Acute): Early Rule Out Using High-Sensitivity Troponin Tests (Elecsys Troponin T High-Sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 Assays). Diagnostics Guidance [DG15] n.d. www.nice.org.uk/guidance/dg15 (accessed 29 January 2020).
- National Institute for Health and Care Excellence . Chest Pain of Recent Onset: Assessment and Diagnosis Of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin n.d. www.nice.org.uk/nicemedia/live/12947/47938/47938.pdf (accessed 20 February 2020).
- Scottish Intercollegiate Guidelines Network . SIGN 148. Acute Coronary Syndromes. A National Clinical Guideline 2016.
- Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem 2009;55:1303-6. https://doi.org/10.1373/clinchem.2009.128363.
- Apple FS, Collinson PO. IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. https://doi.org/10.1373/clinchem.2011.165795.
- Abbott Laboratories . ARCHITECT: STAT High Sensitive Troponin-I. Package Insert 2018.
- Abbott Laboratories . Alinity I: STAT High Sensitive Troponin-I Reagent Kit. Package Insert 2018.
- Beckman Coulter Inc . ACCESS: HsTnI High Sensitivity Tropnin I. Instructions for Use 2018.
- National Institute for Health and Care Excellence . High-Sensitivity Troponin for the Early Rule Out of Acute Myocardial Infarction. Final Scope – Guidance Update n.d. www.nice.org.uk/guidance/gid-dg10035/documents/final-scope-2 (accessed 21 January 20).
- Ortho Clinical Diagnostics . VITROS: Immunodiagnostic Products Hs Troponin I Reagent Pack. Instructions for Use 2019.
- Quidel . TriageTrue: High Sensitivity Troponin I Test. Package Insert 2019.
- Roche . Elecsys Troponin T Hs: 18 Mins. Package Insert 2019.
- Roche . Elecsys Troponin T Hs: E801. Package Insert 2019.
- Roche . Elecsys Troponin T Hs STAT. Package Insert 2019.
- Siemens . ADVIA Centaur: High-Sensitivity Tropinin I (TNIH). Package Insert 2018.
- Siemens Healthcare . Atellica IM: High-Sensitivity Tropinin I (TnIH). Package Insert 2018.
- Siemens . Dimension EXL: High Sensitivity Tropinin I. Package Insert 2018.
- Siemens . Dimension Vista: High Sensitivity Tropinin I. Package Insert 2018.
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. https://doi.org/10.1373/clinchem.2012.192716.
- National Institute for Health and Care Excellence . Myocardial Infarction With ST-Segment Elevation: The Acute Management of Myocardial Infarction With ST-Segment Elevation. NICE CG167 n.d. www.nice.org.uk/guidance/cg167 (accessed 20 February 2020).
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. https://doi.org/10.1093/eurheartj/ehv320.
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e139-e228. https://doi.org/10.1161/CIR.0000000000000134.
- Tan JWC, Lam CSP, Kasim SS, Aw TC, Abanilla JM, Chang WT, et al. Asia-Pacific consensus statement on the optimal use of high-sensitivity troponin assays in acute coronary syndromes diagnosis: focus on hs-TnI 2017;9:81-7. https://doi.org/10.1136/heartasia-2016-010818.
- National Institute for Health and Care Excellence . Unstable Angina and NSTEMI: Early Management. NICE CG94 n.d. http://guidance.nice.org.uk/CG94/NICEGuidance/pdf/English (accessed 29 January 2020).
- National Institute for Health and Care Excellence . Myocardial Infarction: Cardiac Rehabilitation and Prevention of Further Cardiovascular Disease. NICE CG172 n.d. www.nice.org.uk/guidance/cg172 (accessed 29 January 2020).
- National Institute for Health and Care Excellence . MI – Secondary Prevention: Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction. NICE CG48 n.d. www.nice.org.uk/guidance/CG48/NICEGuidance (accessed 20 February 2020).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care n.d. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 29 January 2020).
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual n.d. www.nice.org.uk/media/A0B/97/DAPManualFINAL.pdf (accessed 28 August 2013).
- Cochrane Methods Screening and Diagnostic Tests . Handbook for DTA Reviews n.d. https://methods.cochrane.org/sdt/handbook-dta-reviews (accessed 14 August 2019).
- Canadian Agency for Drugs and Technologies in Health . CADTH Peer Review Checklist for Search Strategies n.d. www.cadth.ca/en/resources/finding-evidence-is (accessed 17 July 2013).
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. https://doi.org/10.1093/eurheartj/ehs184.
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0): Additional Considerations for Cluster-Randomized Trials n.d. www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomized-trials-2016 (accessed 29 January 2020).
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- QUADAS-2C Group . Development of QUADAS-2C, A Quality Assessment Tool for Comparative Diagnostic Accuracy Studies: A Delphi Study Protocol n.d. https://osf.io/tmze9 (accessed 9 January 2020).
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103. https://doi.org/10.1016/j.jclinepi.2007.09.013.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-3.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-31.
- Velilla Moliner J, Gros Bañeres B, Povar Marco J, Santaló Bel M, Ordoñez Llanos J, Martín Martín A, et al. Diagnostic performance of high sensitive troponin in non-ST elevation acute coronary syndrome. Med Intensiva 2020;44:88-95. https://doi.org/10.1016/j.medin.2018.07.014.
- Aldous S, Mark Richards A, George PM, Cullen L, Parsonage WA, Flaws D, et al. Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction. Int J Cardiol 2014;177:182-6. https://doi.org/10.1016/j.ijcard.2014.09.026.
- Badertscher P, Boeddinghaus J, Twerenbold R, Nestelberger T, Wildi K, Wussler D, et al. Direct comparison of the 0/1h and 0/3h algorithms for early rule-out of acute myocardial infarction. Circulation 2018;137:2536-8. https://doi.org/10.1161/CIRCULATIONAHA.118.034260.
- Badertscher P, Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Sabti Z, et al. Effect of acute coronary syndrome probability on diagnostic and prognostic performance of high-sensitivity cardiac troponin. Clin Chem 2018;64:515-25. https://doi.org/10.1373/clinchem.2017.279513.
- Body R, Burrows G, Carley S, Cullen L, Than M, Jaffe AS, et al. High-sensitivity cardiac troponin t concentrations below the limit of detection to exclude acute myocardial infarction: a prospective evaluation. Clin Chem 2015;61:983-9. https://doi.org/10.1373/clinchem.2014.231530.
- Boeddinghaus J, Reichlin T, Cullen L, Greenslade JH, Parsonage WA, Hammett C, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction by use of high-sensitivity cardiac troponin I. Clin Chem 2016;62:494-50. https://doi.org/10.1373/clinchem.2015.249508.
- Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Badertscher P, Cupa J, et al. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation 2017;135:1597-611. https://doi.org/10.1161/CIRCULATIONAHA.116.025661.
- Boeddinghaus J, Twerenbold R, Nestelberger T, Badertscher P, Wildi K, Puelacher C, et al. Clinical validation of a novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem 2018;64:1347-60. https://doi.org/10.1373/clinchem.2018.286906.
- Boeddinghaus J, Nestelberger T, Twerenbold R, Koechlin L, Meier M, Troester V, et al. High-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem 2019;65:893-904. https://doi.org/10.1373/clinchem.2018.300061.
- Bularga A, Lee KK, Stewart S, Ferry AV, Chapman AR, Marshall L, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation 2019;140:1557-68. https://doi.org/10.1161/CIRCULATIONAHA.119.042866.
- Cappellini F, Falbo R, Saltafossi D, Avanzini F, Signorini S, Fania C, et al. Development of an algorithm for ruling-out non-ST elevation myocardial infarction in the emergency department using high sensitivity troponin T assay. Clin Chim Acta 2019;495:1-7. https://doi.org/10.1016/j.cca.2019.03.1625.
- Carlton EW, Cullen L, Than M, Gamble J, Khattab A, Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart 2015;101:1041-6. https://doi.org/10.1136/heartjnl-2014-307288.
- Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med 2015;66:635-45.e1. https://doi.org/10.1016/j.annemergmed.2015.07.006.
- Chapman AR, Anand A, Boeddinghaus J, Ferry AV, Sandeman D, Adamson PD, et al. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation 2017;135:1586-96. https://doi.org/10.1161/CIRCULATIONAHA.116.025021.
- Chapman AR, Hesse K, Andrews J, Ken Lee K, Anand A, Shah ASV, et al. High-sensitivity cardiac troponin I and clinical risk scores in patients with suspected acute coronary syndrome. Circulation 2018;138:1654-65. https://doi.org/10.1161/CIRCULATIONAHA.118.036426.
- Chapman AR, Fujisawa T, Lee KK, Andrews JP, Anand A, Sandeman D, et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart 2019;105:616-22. https://doi.org/10.1136/heartjnl-2018-314093.
- Cullen L, Aldous S, Than M, Greenslade JH, Tate JR, George PM, et al. Comparison of high sensitivity troponin T and I assays in the diagnosis of non-ST elevation acute myocardial infarction in emergency patients with chest pain. Clin Biochem 2014;47:321-6. https://doi.org/10.1016/j.clinbiochem.2013.11.019.
- Eggers KM, Aldous S, Greenslade JH, Johnston N, Lindahl B, Parsonage WA, et al. Two-hour diagnostic algorithms for early assessment of patients with acute chest pain – implications of lowering the cardiac troponin I cut-off to the 97.5th percentile. Clin Chim Acta 2015;445:19-24. https://doi.org/10.1016/j.cca.2015.03.002.
- Rubini Gimenez M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Schaefer M, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J 2014;35:2303-11. https://doi.org/10.1093/eurheartj/ehu188.
- Greenslade JH, Kavsak P, Parsonage W, Shortt C, Than M, Pickering JW, et al. Combining presentation high-sensitivity cardiac troponin I and glucose measurements to rule-out an acute myocardial infarction in patients presenting to emergency department with chest pain. Clin Biochem 2015;48:288-91. https://doi.org/10.1016/j.clinbiochem.2014.11.019.
- Huang H, Zhu S, Wang W, Yi H, Du X, Nie X, et al. Diagnosis of acute myocardial infarction in patients with renal insufficiency using high-sensitivity troponin T. Clin Chem Lab Med 2015;53:723-30. https://doi.org/10.1515/cclm-2014-0715.
- Guangquan L, Hualan H, Xin N, Yong H, Haolan S, Tongxing L, et al. Time from symptom onset influences high-sensitivity troponin T diagnostic accuracy for the diagnosis of acute myocardial infarction. Clin Chem Lab Med 2016;54:133-42. https://doi.org/10.1515/cclm-2014-0776.
- Jaeger C, Wildi K, Twerenbold R, Reichlin T, Rubini Gimenez M, Neuhaus JD, et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am Heart J 2016;171:92-102. https://doi.org/10.1016/j.ahj.2015.07.022.
- Kaier TE, Twerenbold R, Puelacher C, Marjot J, Imambaccus N, Boeddinghaus J, et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation 2017;136:1495-508. https://doi.org/10.1161/CIRCULATIONAHA.117.028084.
- Kavsak PA, Shortt C, Ma J, Clayton N, Sherbino J, Hill SA, et al. A laboratory score at presentation to rule-out serious cardiac outcomes or death in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chim Acta 2017;469:69-74. https://doi.org/10.1016/j.cca.2017.03.021.
- Kitamura M, Hata N, Takayama T, Hirayama A, Ogawa M, Yamashina A, et al. High-sensitivity cardiac troponin T for earlier diagnosis of acute myocardial infarction in patients with initially negative troponin T test – comparison between cardiac markers. J Cardiol 2013;62:336-42. https://doi.org/10.1016/j.jjcc.2013.06.005.
- Mahler SA, Stopyra JP, Apple FS, Riley RF, Russell GB, Hiestand BC, et al. Use of the HEART Pathway with high sensitivity cardiac troponins: a secondary analysis. Clin Biochem 2017;50:401-7. https://doi.org/10.1016/j.clinbiochem.2017.01.003.
- Miller-Hodges E, Anand A, Shah ASV, Chapman AR, Gallacher P, Lee KK, et al. High-sensitivity cardiac troponin and the risk stratification of patients with renal impairment presenting with suspected acute coronary syndrome. Circulation 2018;137:425-35. https://doi.org/10.1161/CIRCULATIONAHA.117.030320.
- Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, Defilippi C, McCord J, et al. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T presented at the European Society of Cardiology annual meeting, September 2014, Barcelona, Spain. Ann Emerg Med 2016;68:76-87. https://doi.org/10.1016/j.annemergmed.2015.11.013.
- Mueller-Hennessen M, Lindahl B, Giannitsis E, Biener M, Vafaie M, deFilippi CR, et al. Diagnostic and prognostic implications using age- and gender-specific cut-offs for high-sensitivity cardiac troponin T – Sub-analysis from the TRAPID-AMI study. Int J Cardiol 2016;209:26-33. https://doi.org/10.1016/j.ijcard.2016.01.213.
- Mueller-Hennessen M, Mueller C, Giannitsis E, Biener M, Vafaie M, DeFilippi CR, et al. Serial sampling of high-sensitivity cardiac troponin T may not be required for prediction of acute myocardial infarction diagnosis in chest pain patients with highly abnormal concentrations at presentation. Clin Chem 2017;63:542-51. https://doi.org/10.1373/clinchem.2016.258392.
- Mueller-Hennessen M, Lindahl B, Giannitsis E, Vafaie M, Biener M, Haushofer AC, et al. Combined testing of copeptin and high-sensitivity cardiac troponin T at presentation in comparison to other algorithms for rapid rule-out of acute myocardial infarction. Int J Cardiol 2019;276:261-7. https://doi.org/10.1016/j.ijcard.2018.10.084.
- Neumann JT, Sörensen NA, Schwemer T, Ojeda F, Bourry R, Sciacca V, et al. Diagnosis of myocardial infarction using a high-sensitivity troponin I 1-hour algorithm. JAMA Cardiol 2016;1:397-404. https://doi.org/10.1001/jamacardio.2016.0695.
- Neumann JT, Sörensen NA, Ojeda F, Renné T, Schnabel RB, Zeller T, et al. Early diagnosis of acute myocardial infarction using high-sensitivity troponin I. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0174288.
- Neumann JT, Sörensen NA, Ojeda F, Schwemer T, Lehmacher J, Gönner S, et al. Immediate rule-out of acute myocardial infarction using electrocardiogram and baseline high-sensitivity troponin I. Clin Chem 2017;63:394-402. https://doi.org/10.1373/clinchem.2016.262659.
- Nowak RM, Gandolfo CM, Jacobsen G, Christenson RH, Moyer M, Hudson M, et al. Ultrarapid rule-out for acute myocardial infarction using the generation 5 cardiac troponin T assay: results from the REACTION-US Study. Ann Emerg Med 2018;72:654-64. https://doi.org/10.1016/j.annemergmed.2018.06.021.
- Parsonage WA, Greenslade JH, Hammett CJ, Lamanna A, Tate JR, Ungerer JP, et al. Validation of an accelerated high-sensitivity troponin T assay protocol in an Australian cohort with chest pain. Med J Aust 2014;200:161-5. https://doi.org/10.5694/mja13.10466.
- Peacock WF, Baumann BM, Bruton D, Davis TE, Handy B, Jones CW, et al. Efficacy of high-sensitivity troponin T in identifying very-low-risk patients with possible acute coronary syndrome. JAMA Cardiol 2018;3:104-11. https://doi.org/10.1001/jamacardio.2017.4625.
- Reichlin T, Cullen L, Parsonage WA, Greenslade J, Twerenbold R, Moehring B, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med 2015;128:369-79.e4. https://doi.org/10.1016/j.amjmed.2014.10.032.
- Reichlin T, Twerenbold R, Wildi K, Gimenez MR, Bergsma N, Haaf P, et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ 2015;187:E243-E252. https://doi.org/10.1503/cmaj.141349.
- Rubini Gimenez M, Twerenbold R, Jaeger C, Schindler C, Puelacher C, Wildi K, et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med 2015;128:861-70.e4. https://doi.org/10.1016/j.amjmed.2015.01.046.
- Rubini Gimenez M, Twerenbold R, Wildi K, Wagener M, Puelacher C, Hillinger P, et al. Direct comparison of safety and efficacy of 2 rule-out strategies for AMI: undetectable levels at presentation vs. combination of 1h-algorithm and undetectable levels at presentation. Eur Heart J 2015;36.
- Rubini Giménez M, Twerenbold R, Boeddinghaus J, Nestelberger T, Puelacher C, Hillinger P, et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin T in suspected myocardial infarction. JAMA Cardiol 2016;1:912-20. https://doi.org/10.1001/jamacardio.2016.2882.
- Sandoval Y, Smith SW, Thordsen SE, Bruen CA, Carlson MD, Dodd KW, et al. Diagnostic performance of high sensitivity compared with contemporary cardiac troponin I for the diagnosis of acute myocardial infarction. Clin Chem 2017;63:1594-604. https://doi.org/10.1373/clinchem.2017.272930.
- Sandoval Y, Smith SW, Love SA, Sexter A, Schulz K, Apple FS. Single high-sensitivity cardiac troponin I to rule out acute myocardial infarction. Am J Med 2017;130:1076-83.e1. https://doi.org/10.1016/j.amjmed.2017.02.032.
- Kavsak PA, Wang X, Ko DT, MacRae AR, Jaffe AS. Short- and long-term risk stratification using a next-generation, high-sensitivity research cardiac troponin I (hs-cTnI) assay in an emergency department chest pain population. Clin Chem 2009;55:1809-15. https://doi.org/10.1373/clinchem.2009.127241.
- Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. https://doi.org/10.1016/S0140-6736(15)00391-8.
- Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. https://doi.org/10.1016/S0140-6736(18)31923-8.
- Shiozaki M, Inoue K, Suwa S, Lee CC, Chikata Y, Ishiura J, et al. Utility of the 0-hour/1-hour high-sensitivity cardiac troponin T algorithm in Asian patients with suspected non-ST elevation myocardial infarction. Int J Cardiol 2017;249:32-5. https://doi.org/10.1016/j.ijcard.2017.09.009.
- Shortt C, Ma J, Clayton N, Sherbino J, Whitlock R, Pare G, et al. Rule-in and rule-out of myocardial infarction using cardiac troponin and glycemic biomarkers in patients with symptoms suggestive of acute coronary syndrome. Clin Chem 2017;63:403-14. https://doi.org/10.1373/clinchem.2016.261545.
- Slagman A, von Recum J, Möckel M, Holert F, Meyer Zum Büschenfelde D, Müller C, et al. Diagnostic performance of a high-sensitive troponin T assay and a troponin T point of care assay in the clinical routine of an emergency department: a clinical cohort study. Int J Cardiol 2017;230:454-60. https://doi.org/10.1016/j.ijcard.2016.12.085.
- Twerenbold R, Rubini Gimenez M, Reichlin T, Boeddinghaus J, Nestelberger T, Badertscher T, et al. Performance of the ESC 0/1-hour algorithm for rapid rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin I in patients with impaired and normal renal function. Eur Heart J 2017;38:465-6. https://doi.org/10.1093/eurheartj/ehx502.P2328.
- Twerenbold R, Neumann JT, Soerensen NA, Karakas M, Rubini Gimenez M, Boeddinghaus J, et al. Validation of the European society of cardiology 0/1-hour algorithm for rule-out and rule-in of acute myocardial infarction. Eur Heart J 2017;38. https://doi.org/10.1093/eurheartj/ehx502.2271.
- Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, et al. Effect of the FDA regulatory approach on the 0/1-h algorithm for rapid diagnosis of MI. J Am Coll Cardiol 2017;70:1532-4. https://doi.org/10.1016/j.jacc.2017.07.746.
- Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Puelacher C, et al. 0/1-hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation 2018;137:436-51. https://doi.org/10.1161/CIRCULATIONAHA.117.028901.
- Twerenbold R, Boeddinghaus J, Nestelberger T, Rubini Gimenez M, Badertscher P, Puelacher C, et al. Direct comparison of three 0/1h-algorithms for rapid rule-out and rule-in of acute myocardial infarction using one ultra-sensitive and two high-sensitivity cardiac troponin assays. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy564.P828.
- Twerenbold R, Costabel JP, Nestelberger T, Campos R, Wussler D, Arbucci R, et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol 2019;74:483-94. https://doi.org/10.1016/j.jacc.2019.05.046.
- van der Linden N, Wildi K, Twerenbold R, Pickering JW, Than M, Cullen L, et al. Combining high-sensitivity cardiac troponin I and cardiac troponin T in the early diagnosis of acute myocardial infarction. Circulation 2018;138:989-99. https://doi.org/10.1161/CIRCULATIONAHA.117.032003.
- Venge P, van Lippen L, Blaschke S, Christ M, Geier F, Giannitsis E, et al. Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay. Clin Chim Acta 2017;469:119-25. https://doi.org/10.1016/j.cca.2017.03.023.
- Wildi K, Nelles B, Twerenbold R, Rubini Giménez M, Reichlin T, Singeisen H, et al. Safety and efficacy of the 0 h/3 h protocol for rapid rule out of myocardial infarction. Am Heart J 2016;181:16-25. https://doi.org/10.1016/j.ahj.2016.07.013.
- Wildi K, Cullen L, Twerenbold R, Greenslade JH, Parsonage W, Boeddinghaus J, et al. Direct comparison of 2 rule-out strategies for acute myocardial infarction: 2-h accelerated diagnostic protocol vs. 2-h algorithm. Clin Chem 2017;63:1227-36. https://doi.org/10.1373/clinchem.2016.268359.
- Wildi K, Boeddinghaus J, Nestelberger T, Twerenbold R, Badertscher P, Wussler D, et al. Comparison of fourteen rule-out strategies for acute myocardial infarction. Int J Cardiol 2019;283:41-7. https://doi.org/10.1016/j.ijcard.2018.11.140.
- Body R, Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, de Filippi CR, et al. The use of very low concentrations of high-sensitivity troponin T to rule out acute myocardial infarction using a single blood test. Acad Emerg Med 2016;23:1004-13. https://doi.org/10.1111/acem.13012.
- Body R, Twerenbold R, Austin C, Boeddinghaus J, Almashali M, Nestelberger T, et al. Diagnostic accuracy of a high-sensitivity cardiac troponin assay with a single serum test in the emergency department. Clin Chem 2019;65:1006-14. https://doi.org/10.1373/clinchem.2018.294272.
- Borna C, Kollberg K, Larsson D, Mokhtari A, Ekelund U. The objective CORE score allows early rule out in acute chest pain patients. Scand Cardiovasc J 2018;52:308-14. https://doi.org/10.1080/14017431.2018.1546891.
- Lin Z, Lim SH, Chua SJT, Tai ES, Chan YH, Richards AM. High-sensitivity troponin T and long-term adverse cardiac events among patients presenting with suspected acute coronary syndrome in Singapore. Singapore Med J 2019;60:418-26. https://doi.org/10.11622/smedj.2019013.
- Meller B, Cullen L, Parsonage WA, Greenslade JH, Aldous S, Reichlin T, et al. Accelerated diagnostic protocol using high-sensitivity cardiac troponin T in acute chest pain patients. Int J Cardiol 2015;184:208-15. https://doi.org/10.1016/j.ijcard.2015.02.006.
- Mokhtari A, Borna C, Gilje P, Tydén P, Lindahl B, Nilsson HJ, et al. A 1-h combination algorithm allows fast rule-out and rule-in of major adverse cardiac events. J Am Coll Cardiol 2016;67:1531-40. https://doi.org/10.1016/j.jacc.2016.01.059.
- Mokhtari A, Lindahl B, Schiopu A, Yndigegn T, Khoshnood A, Gilje P, et al. A 0-hour/1-hour protocol for safe, early discharge of chest pain patients. Acad Emerg Med 2017;24:983-92. https://doi.org/10.1111/acem.13224.
- Mokhtari A, Lindahl B, Smith JG, Holzmann MJ, Khoshnood A, Ekelund U. Diagnostic accuracy of high-sensitivity cardiac troponin T at presentation combined with history and ECG for ruling out major adverse cardiac events. Ann Emerg Med 2016;68:649-58.e3. https://doi.org/10.1016/j.annemergmed.2016.06.008.
- Body R, Nowak R, Lindahl B, Giannitsis E, Mueller C. The use of very low levels of high sensitivity troponin T to rule out acute myocardial infarction using a single blood test. Acad Emerg Med 2015;22:S55-S56.
- Boeddinghaus J, Nestelberger T, Twerenbold R, Rubini Gimenez M, Koechlin L, Troester V, et al. A novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2019;8. https://doi.org/10.1093/eurheartj/ehz745.0065.
- Chang AM, Hollander JE, Ostlund RE, Diercks D, Rafique Z, Ziegler A, et al. Impact of delta rules on performance of a high-sensitivity cardiac troponin T assay for diagnosis of acute myocardial infarction. Eur Heart J 2018;39:1366-7. https://doi.org/10.1093/eurheartj/ehy566.P6455.
- Dodd KW, Sandoval Y, Smith SW, Sexter A, Schulz K, Apple FS, et al. Diagnostic performance of high-sensitivity cardiac troponin I for ruling in and ruling out acute myocardial infarction. Acad Emerg Med 2019;26.
- McCord J, Moyer M, Jacobsen G, Christenson R, Hudson M, Noll S, et al. Is the European society of cardiology 0- and 1-hour algorithm guidelines for rapid evaluation of acute myocardial infarction effective at 0 hour and 30 minutes. Ann Emerg Med 2017;70. https://doi.org/10.1016/j.annemergmed.2017.07.066.
- Nowak RM, Gandolfo C, Jacobsen G, Christenson R, Moyer M, Hudson M, et al. Rapid evaluation of acute myocardial infarction in a united states population using high sensitivity cardiac troponin T and a European society of cardiology 0/1-hour algorithm guideline. Acad Emerg Med 2018;25.
- Nowak RM, Jacobsen G, McCord J, Apple FS, Christenson R, DeFilippi C, et al. High-sensitivity troponin I: two-hour evaluation for acute myocardial infarction in the united states. Acad Emerg Med 2019;26.
- Nowak RM, McCord J, Christenson R, Jacobsen G, Apple FS, DeFilippi C, et al. High-sensitivity troponin I: one-hour evaluation for acute myocardial infarction in the united states. Acad Emerg Med 2019;26.
- Parsonage W, Cullen L, Greenslade J, Aldous S, George P, Lamanna A, et al. A study comparing diagnostic accuracy of high sensitivity assays of troponin I and troponin T for myocardial infarction within two hours of presentation to the emergency room. Heart Lung Circ 2013;22:S207-S208. https://doi.org/10.1016/j.hlc.2013.05.494.
- Parsonage W, Cullen L, Greenslade J, Tate J, Ungerer J, Hammett C, et al. Comparison of highly sensitive troponin I and T results in the diagnosis of acute myocardial infarction. J Am Coll Cardiol 2013;61. https://doi.org/10.1016/S0735-1097(13)60229-6.
- Lindahl B, Jernberg T, Badertscher P, Boeddinghaus J, Eggers KM, Frick M, et al. An algorithm for rule-in and rule-out of acute myocardial infarction using a novel troponin I assay. Heart 2017;103:125-31. https://doi.org/10.1136/heartjnl-2016-309951.
- Santaló M, Martin A, Velilla J, Povar J, Temboury F, Balaguer J, et al. Using high-sensitivity troponin T: the importance of the proper gold standard. Am J Med 2013;126:709-17. https://doi.org/10.1016/j.amjmed.2013.03.003.
- Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J 2012;29:805-10. https://doi.org/10.1136/emermed-2011-200222.
- Sanchis J, Bardají A, Bosch X, Loma-Osorio P, Marín F, Sánchez PL, et al. Usefulness of high-sensitivity troponin T for the evaluation of patients with acute chest pain and no or minimal myocardial damage. Am Heart J 2012;164:194-200.e1. https://doi.org/10.1016/j.ahj.2012.05.015.
- Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation 2012;126:31-40. https://doi.org/10.1161/CIRCULATIONAHA.112.100867.
- Eggers KM, Venge P, Lindahl B. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta 2012;413:1135-40. https://doi.org/10.1016/j.cca.2012.03.011.
- Reiter M, Twerenbold R, Reichlin T, Benz B, Haaf P, Meissner J, et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J 2012;33:988-97. https://doi.org/10.1093/eurheartj/ehr376.
- Aldous SJ, Richards M, Cullen L, Troughton R, Than M. Diagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest pain. CMAJ 2012;184:E260-8. https://doi.org/10.1503/cmaj.110773.
- Potocki M, Reichlin T, Thalmann S, Zellweger C, Twerenbold R, Reiter M, et al. Diagnostic and prognostic impact of copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart 2012;98:558-65. https://doi.org/10.1136/heartjnl-2011-301269.
- Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. https://doi.org/10.1001/jama.2011.1896.
- Freund Y, Chenevier-Gobeaux C, Bonnet P, Claessens YE, Allo JC, Doumenc B, et al. High-sensitivity versus conventional troponin in the emergency department for the diagnosis of acute myocardial infarction. Crit Care 2011;15. https://doi.org/10.1186/cc10270.
- Aldous SJ, Richards AM, Cullen L, Than MP. Early dynamic change in high-sensitivity cardiac troponin T in the investigation of acute myocardial infarction. Clin Chem 2011;57:1154-60. https://doi.org/10.1373/clinchem.2010.161166.
- Melki D, Lind S, Agewall S, Jernberg T. Diagnostic value of high sensitive troponin T in chest pain patients with no persistent ST-elevations. Scand Cardiovasc J 2011;45:198-204. https://doi.org/10.3109/14017431.2011.565792.
- Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation 2011;124:136-45. https://doi.org/10.1161/CIRCULATIONAHA.111.023937.
- Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J 2011;32:1379-89. https://doi.org/10.1093/eurheartj/ehr033.
- Aldous SJ, Florkowski CM, Crozier IG, Elliott J, George P, Lainchbury JG, et al. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Ann Clin Biochem 2011;48:241-8. https://doi.org/10.1258/acb.2010.010219.
- Kurz K, Giannitsis E, Becker M, Hess G, Zdunek D, Katus HA. Comparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndrome. Clin Res Cardiol 2011;100:209-15. https://doi.org/10.1007/s00392-010-0230-y.
- Hochholzer W, Reichlin T, Stelzig C, Hochholzer K, Meissner J, Breidthardt T, et al. Impact of soluble FMS-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur Heart J 2011;32:326-35. https://doi.org/10.1093/eurheartj/ehq429.
- Christ M, Popp S, Pohlmann H, Poravas M, Umarov D, Bach R, et al. Implementation of high sensitivity cardiac troponin T measurement in the emergency department. Am J Med 2010;123:1134-42. https://doi.org/10.1016/j.amjmed.2010.07.015.
- Parsonage W, Cullen L, Greenslade J, Tate J, Ungerer J, Hammett C, et al. Comparison of highly sensitive troponin I and T results in the diagnosis of acute myocardial infarction. Presented at 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention; 9–11 Mar 2013; San Francisco: CA. J Am Coll Cardiol 2013;61.
- Collinson P, Gaze D, Thokala P, Goodacre S. To examine the diagnostic accuracy of highly sensitive troponin assays using diagnosis based on the universal definition of myocardial infarction in the unselected emergency room population. Presented at ESC Congress 2012; 25–29 Aug 2012; Munich: Germany. Eur Heart J 2012;33.
- Body R, Burrows G, Cook G, Carley SD, France M, Jarvis J, et al. High sensitivity troponin: validation and subsequent audit of a novel ‘rule out’ cut-off. Presented at College of Emergency Medicine Autumn Conference 2011; 21–23 Sept 2011; Gateshead: UK. Emerg Med J 2011;28. https://doi.org/10.1136/emermed-2011-200617.1.
- Melki D, Lind S, Agewall S, Jernberg T. High sensitive troponin T rules out myocardial infarction 2 hours from admission in chest pain patients. Presented at American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention; 14–16 Mar 2010; Atlanta: GA. J Am Coll Cardiol 2010;55. https://doi.org/10.1016/S0735-1097(10)61108-4.
- Aldous S, Florkowski C, George P, Than M, Crozier I. High sensitivity troponin assays predict major adverse events at 2 years and at levels below the 99th percentile. Presented at American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention; 14–16 Mar 2010; Atlanta: GA. J Am Coll Cardiol 2010;55. https://doi.org/10.1016/S0735-1097(10)60917-5.
- Cullen L, Mueller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013;62:1242-9. https://doi.org/10.1016/j.jacc.2013.02.078.
- Sebbane M, Lefebvre S, Kuster N, Jreige R, Jacques E, Badiou S, et al. Early rule out of acute myocardial infarction in ED patients: value of combined high-sensitivity cardiac troponin T and ultrasensitive copeptin assays at admission. Am J Emerg Med 2013;31:1302-8. https://doi.org/10.1016/j.ajem.2013.04.033.
- Irfan A, Reichlin T, Twerenbold R, Meister M, Moehring B, Wildi K, et al. Early diagnosis of myocardial infarction using absolute and relative changes in cardiac troponin concentrations. Am J Med 2013;126:781-8.e2. https://doi.org/10.1016/j.amjmed.2013.02.031.
- Collinson PO, Gaze DC, Thokala P, Goodacre S. Randomised Assessment of Treatment using Panel Assay of Cardiac markers – Contemporary Biomarker Evaluation (RATPAC CBE). Health Technol Assess 2013;17. https://doi.org/10.3310/hta17150.
- Reiter M, Twerenbold R, Reichlin T, Mueller M, Hoeller R, Moehring B, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction. Heart 2013;99:708-14. https://doi.org/10.1136/heartjnl-2012-303325.
- Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332-9. https://doi.org/10.1016/j.jacc.2011.06.026.
- Aldous SJ, Florkowski CM, Crozier IG, George P, Mackay R, Than M. High sensitivity troponin outperforms contemporary assays in predicting major adverse cardiac events up to two years in patients with chest pain. Ann Clin Biochem 2011;48:249-55. https://doi.org/10.1258/acb.2010.010220.
- Keller T, Zeller T, Echevarria FO, Tzikas S, Baldus S, Bickel C, et al. High sensitive troponin I dynamic improves early diagnosis of acute myocardial infarction. Eur Heart J 2011;32.
- Collinson P, Gaze D, Thokala P, Goodacre S. To examine the diagnostic accuracy of highly sensitive troponin assays using diagnosis based on the universal definition of myocardial infarction in the unselected emergency room population. Eur Heart J 2012;33.
- Saenger AK, Korpi-Steiner NL, Bryant SC, Karon BS, Jaffe AS. Utilization of a high sensitive troponin T assay optimizes serial sampling in the diagnosis of acute myocardial infarction compared to multiple contemporary troponin assays. Circulation 2010;122.
- Freund Y, Chenevier-Gobeaux C, Goulet H, Claessens Y, Bonnet P, Allo J, et al. Comparison of high-sensitivity cardiac troponin concentrations versus conventional troponin for the diagnosis of myocardial infarction in the emergency department. Ann Emerg Med 2010;56. https://doi.org/10.1016/j.annemergmed.2010.06.524.
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858-67. https://doi.org/10.1056/NEJMoa0900428.
- Hoeller R, Rubini Giménez M, Reichlin T, Twerenbold R, Zellweger C, Moehring B, et al. Normal presenting levels of high-sensitivity troponin and myocardial infarction. Heart 2013;99:1567-72. https://doi.org/10.1136/heartjnl-2013-303643.
- Body R, Carley SD, McDowell G, Nuttall M, Wibberley C, France M, et al. Use of low level high sensitivity troponin to rule out acute myocardial infarction in the emergency department. Eur Heart J Suppl 2010;12:F111-F112.
- Boeddinghaus J, Twerenbold R, Nestelberger T, Koechlin L, Wussler D, Meier M, et al. Clinical use of a new high-sensitivity cardiac troponin I assay in patients with suspected myocardial infarction. Clin Chem 2019;65:1426-36. https://doi.org/10.1373/clinchem.2019.304725.
- Nestelberger T, Boeddinghaus J, Greenslade J, Parsonage WA, Than M, Wussler D, et al. Two-hour algorithm for rapid triage of suspected acute myocardial infarction using a high-sensitivity cardiac troponin I assay. Clin Chem 2019;65:1437-47. https://doi.org/10.1373/clinchem.2019.305193.
- Body R, Morris N, Reynard C, Collinson PO. Comparison of four decision aids for the early diagnosis of acute coronary syndromes in the emergency department. Emerg Med J 2020;37:8-13. https://doi.org/10.1136/emermed-2019-208898.
- Boeddinghaus J, Nestelberger T, Koechlin L, Wussler D, Lopez-Ayala P, Walter JE, et al. Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol 2020;75:1111-24. https://doi.org/10.1016/j.jacc.2019.12.065.
- Chapman AR, Sandeman D, Ferry AV, Stewart S, Strachan FE, Wereski R, et al. Risk stratification using high-sensitivity cardiac troponin T in patients with suspected acute coronary syndrome. J Am Coll Cardiol 2020;75:985-7. https://doi.org/10.1016/j.jacc.2019.12.036.
- Anand A, Lee K, Chapman AR, Ferry AV, Adamson PD, Strachan FE, et al. High-sensitivity cardiac troponin on presentation to rule out myocardial infarction. Unpublished n.d.
- Sandoval Y, Nowak R, deFilippi CR, Christenson RH, Peacock WF, McCord J, et al. Myocardial infarction risk stratification with a single measurement of high-sensitivity troponin I. J Am Coll Cardiol 2019;74:271-82. https://doi.org/10.1016/j.jacc.2019.05.058.
- Pourrajab F, Torkian Velashani F, Khanaghaei M, Hekmatimoghaddam S, Rahaie M, Zare-Khormizi MR. Comparison of miRNA signature versus conventional biomarkers before and after off-pump coronary artery bypass graft. J Pharm Biomed Anal 2017;134:11-7. https://doi.org/10.1016/j.jpba.2016.11.014.
- Chopard R, Plastaras P, Jehl J, Descotes-Genon V, Seronde M-F, Janin S, et al. Abstract 12491: impact of positive thrombus retrieval during primary percutaneous coronary intervention with thrombectomy on infarct size and microvascular obstruction. Circulation 2011;124.
- Ayiku L, Levay P, Hudson T, Craven J, Barrett E, Finnegan A, et al. The medline UK filter: development and validation of a geographic search filter to retrieve research about the UK from OVID MEDLINE. Health Info Libr J 2017;34:200-16. https://doi.org/10.1111/hir.12187.
- Ayiku L, Levay P, Hudson T, Craven J, Finnegan A, Adams R, et al. The Embase UK filter: validation of a geographic search filter to retrieve research about the UK from OVID Embase. Health Info Libr J 2019;36:121-33. https://doi.org/10.1111/hir.12252.
- Goodacre S, Bradburn M, Fitzgerald P, Cross E, Collinson P, Gray A, et al. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15230.
- Fitzgerald P, Goodacre SW, Cross E, Dixon S. Cost-effectiveness of point-of-care biomarker assessment for suspected myocardial infarction: the Randomized Assessment of Treatment using Panel Assay of Cardiac markers (RATPAC) trial. Acad Emerg Med 2011;18:488-95. https://doi.org/10.1111/j.1553-2712.2011.01068.x.
- Vaidya A, Severens H, Bongaerts BWC, Cleutjens K, Hofstra L, van Dieijen-Visser M, et al. Use of high-sensitive troponin T assay for the early diagnosis of acute myocardial infarction in chest pain patients: an economic evaluation. Med Decis Making 2012;32.
- Thokala P, Goodacre SW, Collinson PO, Stevens JW, Mills NL, Newby DE, et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart 2012;98:1498-503. https://doi.org/10.1136/heartjnl-2012-302188.
- Goodacre S, Thokala P, Carroll C, Stevens JW, Leaviss J, Al Khalaf M, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17010.
- Canadian Agency for Drugs and Technologies in Health . High-Sensitivity Cardiac Troponin for the Rapid Diagnosis of Acute Coronary Syndrome in the Emergency Department: A Clinical and Cost-Effectiveness Evaluation n.d. www.cadth.ca/media/pdf/OP0511_Troponin_ScienceReport_e.pdf (accessed 20 February 2020).
- Mills NL, Churchhouse AM, Lee KK, Anand A, Gamble D, Shah AS, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16. https://doi.org/10.1001/jama.2011.338.
- Polanczyk CA, Kuntz KM, Sacks DB, Johnson PA, Lee TH. Emergency department triage strategies for acute chest pain using creatine kinase-MB and troponin I assays: a cost-effectiveness analysis. Ann Intern Med 1999;131:909-18. https://doi.org/10.7326/0003-4819-131-12-199912210-00002.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11140.
- Oluboyede Y, Goodacre S, Wailoo A. ESCAPE Research Team . Cost effectiveness of chest pain unit care in the NHS. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-174.
- Goodacre SW, Bradburn M, Cross E, Collinson P, Gray A, Hall AS. RATPAC Research Team . The Randomised Assessment of Treatment using Panel Assay of Cardiac markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart 2011;97:190-6. https://doi.org/10.1136/hrt.2010.203166.
- Ambavane A, Lindahl B, Giannitsis E, Roiz J, Mendivil J, Frankenstein L, et al. Economic evaluation of the one-hour rule-out and rule-in algorithm for acute myocardial infarction using the high-sensitivity cardiac troponin T assay in the emergency department. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0187662.
- Gamble JHP, Hutchinson T, Eayrs KE, Orr WP. A rapid chest pain assessment pathway including high-sensitivity troponin T testing reduces length of stay. Heart 2013;99. https://doi.org/10.1136/heartjnl-2013-304019.22.
- Tamimi W, Alajlan A, Alsolamy S, Julicher P. A queuing model analysis to evaluate the impact of high-sensitive troponin I on emergency department management metrics. Clin Chem 2016;62.
- Davies T, De Silva K, Haslam D, Fluck D, Williams M, Jacques A, et al. Current utilisation of high-sensitivity troponin; does it improve our accuracy in diagnosing acute myocardial infarction?. Heart 2015;101:A6-A7. https://doi.org/10.1136/heartjnl-2015-308066.10.
- Twerenbold R, Jaeger C, Rubini Gimenez M, Wildi K, Reichlin T, Nestelberger T, et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J 2016;37:3324-32. https://doi.org/10.1093/eurheartj/ehw232.
- Vaidya A, Severens JL, Bongaerts BW, Cleutjens KB, Nelemans PJ, Hofstra L, et al. High-sensitive troponin T assay for the diagnosis of acute myocardial infarction: an economic evaluation. BMC Cardiovasc Disord 2014;14. https://doi.org/10.1186/1471-2261-14-77.
- Kaambwa B, Ratcliffe J, Horsfall M, Astley C, Karnon J, Coates P, et al. Cost effectiveness of high-sensitivity troponin compared to conventional troponin among patients presenting with undifferentiated chest pain: a trial based analysis. Int J Cardiol 2017;238:144-50. https://doi.org/10.1016/j.ijcard.2017.02.141.
- Shortt C, Xie F, Whitlock R, Ma J, Clayton N, Sherbino J, et al. Economic considerations of early rule-in/rule-out algorithms for the diagnosis of myocardial infarction in the emergency department using cardiac troponin and glycemic biomarkers. Clin Chem 2017;63:593-602. https://doi.org/10.1373/clinchem.2016.261776.
- Department of Health and Social Care (DHSC) . The NHS Plan: A Plan for Investment, A Plan for Reform 2000.
- Lipinski MJ, Baker NC, Escárcega RO, Torguson R, Chen F, Aldous SJ, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J 2015;169:6-16.e6. https://doi.org/10.1016/j.ahj.2014.10.007.
- Healthcare Quality Improvement Programme . Myocardial Ischaemia National Audit Project: 2019 Summary Report (2017 18 Data n.d. www.nicor.org.uk/wp-content/uploads/2019/09/MINAP-2019-Summary-Report-final.pdf (accessed 6 February 2020).
- Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163-70. https://doi.org/10.1056/NEJM200004203421603.
- Office for National Statistics . Interim Life Tables, England &Amp; Wales, 1980–82 to 2010–12 n.d. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2010-2012/rft-ew.xls (accessed 20 February 2020).
- British Heart Foundation . Heart Statistics: Morbidity, Incidence n.d. www.bhf.org.uk/research/heart-statistics/morbidity/incidence.aspx (accessed 20 February 2020).
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes 2012;5:532-40. https://doi.org/10.1161/CIRCOUTCOMES.111.964700.
- Allen LA, O’Donnell CJ, Camargo CA, Giugliano RP, Lloyd-Jones DM. Comparison of long-term mortality across the spectrum of acute coronary syndromes. Am Heart J 2006;151:1065-71. https://doi.org/10.1016/j.ahj.2005.05.019.
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. https://doi.org/10.1001/jama.280.19.1690.
- Danese MD, Gleeson M, Kutikova L, Griffiths RI, Azough A, Khunti K, et al. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011805.
- NHS Improvement . NHS Reference Costs 2017–2018 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 19 February 2020).
- Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, et al. Long-term healthcare use and costs in patients with stable coronary artery disease: a population-based cohort using linked health records (CALIBER). Eur Heart J Qual Care Clin Outcomes 2016;2:125-40. https://doi.org/10.1093/ehjqcco/qcw003.
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2018 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 19 February 2020).
- Cottens D, Maeremans J, McCutcheon K, Lamers S, Roux L, Duponselle J, et al. Prognostic value of the high-sensitivity troponin T assay after percutaneous intervention of chronic total occlusions. J Cardiovasc Med 2018;19:366-72. https://doi.org/10.2459/JCM.0000000000000660.
- Neumann JT, Twerenbold R, Ojeda F, Sörensen NA, Chapman AR, Shah ASV, et al. Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med 2019;380:2529-40. https://doi.org/10.1056/NEJMoa1803377.
- Chew DP, Lambrakis K, Blyth A, Seshadri A, Edmonds MJR, Briffa T, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the Rapid Assessment of Possible ACS In the emergency Department with high sensitivity Troponin T (RAPID-TnT) study. Circulation 2019;140:1543-56. https://doi.org/10.1161/CIRCULATIONAHA.119.042891.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. https://doi.org/10.1016/j.jclinepi.2010.07.006.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
- Lee C-C, Huang S-S, Yeo YH, Hou Y-T, Park JY, Inoue K, et al. High-sensitivity-cardiac troponin for accelerated diagnosis of acute myocardial infarction: a systematic review and meta-analysis. Am J Emerg Med 2020;38:1402-7. https://doi.org/10.1016/j.ajem.2019.11.035.
- Backus BE, Six AJ, Kelder JC, Bosschaert MA, Mast EG, Mosterd A, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol 2013;168:2153-8. https://doi.org/10.1016/j.ijcard.2013.01.255.
- Van Den Berg P, Body R. The HEART score for early rule out of acute coronary syndromes in the emergency department: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2018;7:111-19. https://doi.org/10.1177/2048872617710788.
- Laureano-Phillips J, Robinson RD, Aryal S, Blair S, Wilson D, Boyd K, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med 2019;74:187-203. https://doi.org/10.1016/j.annemergmed.2018.12.010.
- Anderson F, FitaGerald G. Methods and Formulas Used to Calculate the GRACE Risk Scores for Patients Presenting to Hospital With an Acute Coronary Syndrome n.d. www.outcomes-umassmed.org/grace/files/GRACE_RiskModel_Coefficients.pdf (accessed 12 February 2020).
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835-42. https://doi.org/10.1001/jama.284.7.835.
- Than M, Flaws D, Sanders S, Doust J, Glasziou P, Kline J, et al. Development and validation of the emergency department assessment of chest pain score and 2 h accelerated diagnostic protocol. Emerg Med Australas 2014;26:34-4. https://doi.org/10.1111/1742-6723.12164.
- Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165-75. https://doi.org/10.1056/NEJMoa0807986.
- Kimenai DM, Janssen EBNJ, Eggers KM, Lindahl B, den Ruijter HM, Bekers O, et al. Sex-specific versus overall clinical decision limits for cardiac troponin I and T for the diagnosis of acute myocardial infarction: a systematic review. Clin Chem 2018;64:1034-43. https://doi.org/10.1373/clinchem.2018.286781.
- Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 2001;38:2114-30. https://doi.org/10.1016/S0735-1097(01)01702-8.
- Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH, Cannon CP, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem 2007;53:547-51. https://doi.org/10.1373/clinchem.2006.084715.
- Worster A, Kavsak P. High-STEACS Algorithm missed fewer patients with acute MI than the ESC Pathway in the ED. Ann Intern Med 2017;167. https://doi.org/10.7326/ACPJC-2017-167-6-034.
- Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem 2010;56:642-50. https://doi.org/10.1373/clinchem.2009.134460.
- Aguirre P, Reyes G, Blanchet J, Nacke L, Coronel ML, Macín SM, et al. The value in daily practice of high sensitive troponin T for myocardial infarction diagnosis. Insuf Card 2014;9:2-7.
- Badertscher P, Boeddinghaus J, Twerenbold R, Nestelberger T, Wussler D, Puelacher C, et al. Direct comparison of the 0/1h- and 0/3h-algorithm for early rule-out of acute myocardial infarction. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy565.P1735.
- Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014;63:2569-78. https://doi.org/10.1016/j.jacc.2014.03.017.
- Biener M, Mueller M, Vafaie M, Jaffe AS, Widera C, Katus HA, et al. Diagnostic performance of rising, falling, or rising and falling kinetic changes of high-sensitivity cardiac troponin T in an unselected emergency department population. Eur Heart J Acute Cardiovasc Care 2013;2:314-22. https://doi.org/10.1177/2048872613498517.
- Borna C, Thelin J, Ohlin B, Erlinge D, Ekelund U. High-sensitivity troponin T as a diagnostic tool for acute coronary syndrome in the real world: an observational study. Eur J Emerg Med 2014;21:181-8. https://doi.org/10.1097/MEJ.0b013e328362a71b.
- Burgio MA, Marino G. cTnT-hs in the early diagnosis of acute myocardial infarction: evaluation of rapid rule-out (0-1 h) in an emergency department population. Riv Ital Della Medicina Di Lab 2018;14:208-15. https://doi.org/10.1007/s13631-018-00214-3.
- Burgio MA, Marino G, Di Maria D. Troponin cTnT-hs: a matter of gender and age? Evaluation of differentiated cut-offs by gender and age in an emergency department population. Riv Ital Della Medicina Di Lab 2018;14:41-9. https://doi.org/10.1007/s13631-018-0184-z.
- Canadian Institutes of Health Research McMaster University . Optimum Troponin Cutoffs for ACS in the ED (ROMI-3) n.d. https://ClinicalTrials.gov/show/NCT01994577 (accessed 20 February 2020).
- Cortes MM, Lambardi F, Ariznavarreta P, Resi S, Arbucci R, Borda M, et al. Usefulness of the HEART Score with High-Sensitivity Troponin T for the evaluation of patients with chest pain. Rev Argent Cardiol 2018;86:15-24. https://doi.org/10.7775/rac.v86.i5.13326.
- Costabel JP, Conde D, Lambardi F, Barboza AC, Cobo AL, Aragon M, et al. Evaluation of a new diagnostic algorithm for acute coronary syndrome using high-sensitivity troponin T assay. Rev Argent Cardiol 2014;82:298-303.
- Costabel JP, Ariznavarreta P, Lambardi F, Arbucci R, Vergara JM, Katib C, et al. Results of the first patients with suspected acute coronary syndrome evaluated with the 1-hour algorithm proposed by the European Society of Cardiology. Rev Argent Cardiol 2019;87:193-8. https://doi.org/10.7775/rac.v87.i4.11881.
- Croce A, Brunati P, Colzani C, Terramocci R, Favero S, Bordoni G, et al. A Rational adoption of the high sensitive assay for cardiac troponin I in diagnostic routine. Dis Markers 2017;2017. https://doi.org/10.1155/2017/4523096.
- Cullen L, Parsonage WA, Greenslade J, Lamanna A, Hammett CJ, Than M, et al. Delta troponin for the early diagnosis of AMI in emergency patients with chest pain. Int J Cardiol 2013;168:2602-8. https://doi.org/10.1016/j.ijcard.2013.03.044.
- Cullen L, Parsonage W, Greenslade J, Aldous S, George P, Lamanna A, et al. Use of sex-specific cut-offs with highly sensitive troponin I assay values for the diagnosis of acute myocardial infarction in emergency patients with chest pain. Eur Heart J 2013;34:735-6. https://doi.org/10.1093/eurheartj/eht309.P4056.
- Cullen L, Greenslade J, Than M, Tate J, Ungerer JP, Pretorius C, et al. Performance of risk stratification for acute coronary syndrome with two-hour sensitive troponin assay results. Heart Lung Circ 2014;23:428-34. https://doi.org/10.1016/j.hlc.2013.11.003.
- Cullen L, Greenslade JH, Than M, Brown AF, Hammett CJ, Lamanna A, et al. The new Vancouver Chest Pain Rule using troponin as the only biomarker: an external validation study. Am J Emerg Med 2014;32:129-34. https://doi.org/10.1016/j.ajem.2013.10.021.
- Dadkhah S, Almuwaqqat Z, Sulaiman S, Husein H, Nguyen Q, Ali S, et al. Sensitive troponin I and stress testing in the emergency department for the early management of chest pain using 2-hour protocol. Crit Pathw Cardiol 2017;16:89-92. https://doi.org/10.1097/HPC.0000000000000115.
- Druey S, Wildi K, Twerenbold R, Jaeger C, Reichlin T, Haaf P, et al. Early rule-out and rule-in of myocardial infarction using sensitive cardiac Troponin I. Int J Cardiol 2015;195:163-70. https://doi.org/10.1016/j.ijcard.2015.05.079.
- Ferencik M, Mayrhofer T, Lu MT, Woodard PK, Truong QA, Peacock WF, et al. High-sensitivity cardiac troponin I as a Gatekeeper for coronary computed tomography angiography and stress testing in patients with acute chest pain. Clin Chem 2017;63:1724-33. https://doi.org/10.1373/clinchem.2017.275552.
- Gandolfo CM, Nowak R, Hudson MP, Moyer M, Christenson R, Cook B, et al. Baseline high sensitivity troponin t value below the level of detection to rule-out acute myocardial infarction in the United States. Circulation 2017;136.
- Gandolfo CM, McCord J, Hudson MP, Moyer M, Christenson R, Cook B, et al. Rapid evaluation of acute myocardial infarction using a change in high-sensitivity cardiac troponin T over 1 hour. Circulation 2017;136.
- Goorden SM, van Engelen RA, Wong LS, van der Ploeg T, Verdel GJ, Buijs MM. A novel troponin I rule-out value below the upper reference limit for acute myocardial infarction. Heart 2016;102:1721-7. https://doi.org/10.1136/heartjnl-2015-308667.
- Greenslade JH, Nayer R, Parsonage W, Doig S, Young J, Pickering JW, et al. Validating the Manchester Acute Coronary Syndromes (MACS) and Troponin-only Manchester Acute Coronary Syndromes (T-MACS) rules for the prediction of acute myocardial infarction in patients presenting to the emergency department with chest pain. Emerg Med J 2017;34:517-23. https://doi.org/10.1136/emermed-2016-206366.
- Greenslade JH, Carlton EW, Van Hise C, Cho E, Hawkins T, Parsonage WA, et al. Diagnostic accuracy of a new high-sensitivity troponin I assay and five accelerated diagnostic pathways for ruling out acute myocardial infarction and acute coronary syndrome. Ann Emerg Med 2018;71:439-51.e3. https://doi.org/10.1016/j.annemergmed.2017.10.030.
- Gunsolus I, Sandoval Y, Smith SW, Sexter A, Schulz K, Herzog CA, et al. Renal dysfunction influences the diagnostic and prognostic performance of high-sensitivity cardiac troponin I. J Am Soc Nephrol 2018;29:636-43. https://doi.org/10.1681/ASN.2017030341.
- Ichise T, Tada H, Sakata K, Kawashiri MA, Yamagishi M, Hayashi K. Impact of aging on high-sensitivity cardiac troponin T in patients suspected of acute myocardial infarction. Intern Med 2017;56:2097-102. https://doi.org/10.2169/internalmedicine.8510-16.
- Invernizzi L, Doka M, Cappellini F, Signorelli S, Falbo R, Ronzoni G, et al. Effectiveness of highly sensitive troponin T assay for early diagnosis of acute myocardial infarction (AMI). Biochim Clin 2013;37:36-9.
- Isiksacan N, Biyik I, Erturk M, Koser M, Karakurt H, Ozalp B, et al. Comparison of high sensitive and conventional troponin assays in diagnosis of acute myocardial infarction. Turk Biyokim Derg 2017;42:77-85. https://doi.org/10.1515/tjb-2016-0270.
- Isiksacan N, Biyik I, Opan S, Caglar FNT, Erturk M, Yazan S, et al. Effect of age and gender differences on high-sensitive troponin T measurement in the diagnosis of acute myocardial infarction. J Lab Med 2019;43:35-40. https://doi.org/10.1515/labmed-2018-0326.
- Poole Hospital NHS Foundation Trust . Triage Rule-Out Using Sensitive Troponin (TRUST): Study of Early Risk-Stratification of Suspected Cardiac Chest Pain and Initiation of 1-Hour High-Sensitivity Troponin Testing in Very Low and Low-Risk Emergency Department Patients n.d. http://isrctn.com/ISRCTN21109279 (accessed 20 February 2020).
- Kavsak PA, Worster A, Hill SA, Jaffe AS. Evaluation of the Siemens ADVIA Centaur high-sensitivity cardiac troponin I assay in serum. Clin Chim Acta 2018;487:216-21. https://doi.org/10.1016/j.cca.2018.10.012.
- Kavsak PA, Worster A, Shortt C, Ma J, Clayton N, Sherbino J, et al. High-sensitivity cardiac troponin concentrations at emergency department presentation in females and males with an acute cardiac outcome. Ann Clin Biochem 2018;55:604-7. https://doi.org/10.1177/0004563217743997.
- Kavsak PA, Worster A, Shortt C, Ma J, Clayton N, Sherbino J, et al. Performance of high-sensitivity cardiac troponin in the emergency department for myocardial infarction and a composite cardiac outcome across different estimated glomerular filtration rates. Clin Chim Acta 2018;479:166-70. https://doi.org/10.1016/j.cca.2018.01.034.
- Kaysak PA, Clark L, Jaffe AS. Effect of repeat measurements of high sensitivity cardiac troponin on the same sample using the European Society of Cardiology 0-hour/1-hour or 2-hour algorithms for early rule-out and rule-in for myocardial infarction. Clin Chem 2017;63:1163-5. https://doi.org/10.1373/clinchem.2017.272914.
- Kellens S, Verbrugge FH, Vanmechelen M, Grieten L, Van Lierde J, Dens J, et al. Point-of-care heart-type fatty acid binding protein versus high-sensitivity troponin T testing in emergency patients at high risk for acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:177-84. https://doi.org/10.1177/2048872615570221.
- Korley FK, Schulman SP, Sokoll LJ, DeFilippis AP, Stolbach AI, Bayram JD, et al. Troponin elevations only detected with a high-sensitivity assay: clinical correlations and prognostic significance. Acad Emerg Med 2014;21:727-35. https://doi.org/10.1111/acem.12417.
- Kovács F, Kocsis I, Varga M, Sárváry E, Bicsák G. Automated measurement of biomarkers for the diagnosis of acute myocardial infarction. Orv Hetil 2015;156:964-71. https://doi.org/10.1556/650.2015.30145.
- Lin Y, Zhang G, Feng G, Li Y, Zhu J, Zhou Z, et al. 1/3 hours rule in and rule out algorithm for NSTEMI using a high-sensitivity cardiac troponin I at emergency department in Chinese population. Clin Chem 2018;64.
- Ljung L, Lindahl B, Eggers KM, Frick M, Linder R, Löfmark HB, et al. A rule-out strategy based on high-sensitivity troponin and HEART score reduces hospital admissions. Ann Emerg Med 2019;73:491-9. https://doi.org/10.1016/j.annemergmed.2018.11.039.
- McCord J, Cabrera R, Lindahl B, Giannitsis E, Evans K, Nowak R, et al. Prognostic utility of a modified HEART score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes 2017;10. https://doi.org/10.1161/CIRCOUTCOMES.116.003101.
- McRae AD, Innes G, Graham M, Lang E, Andruchow JE, Ji Y, et al. Undetectable concentrations of a food and drug administration-approved high-sensitivity cardiac troponin T assay to rule out acute myocardial infarction at emergency department arrival. Acad Emerg Med 2017;24:1267-77. https://doi.org/10.1111/acem.13229.
- McRae AD, Innes G, Graham M, Lang E, Andruchow JE, Yang H, et al. Comparative evaluation of 2-hour rapid diagnostic algorithms for acute myocardial infarction using high-sensitivity cardiac troponin T. Can J Cardiol 2017;33:1006-12. https://doi.org/10.1016/j.cjca.2017.04.010.
- McRae A, Graham M, Abedin T, Ji Y, Yang H, Wang D, et al. Sex-specific, high-sensitivity cardiac troponin T cut-off concentrations for ruling out acute myocardial infarction with a single measurement. CJEM 2019;21:26-33. https://doi.org/10.1017/cem.2018.435.
- Mohsen M, Shawky A. The diagnostic utility of high-sensitivity cardiac troponin T in acute coronary syndrome. Egypt Heart J 2016;68:1-9. https://doi.org/10.1016/j.ehj.2014.12.003.
- Mueller T, Egger M, Peer E, Jani E, Dieplinger B. Evaluation of sex-specific cut-off values of high-sensitivity cardiac troponin I and T assays in an emergency department setting – results from the Linz Troponin (LITROP) study. Clin Chim Acta 2018;487:66-74. https://doi.org/10.1016/j.cca.2018.09.026.
- Nacke L, Blanchet J, Reyes G, Aguirre P, Zoni R, Perna ER, et al. Effectiveness of different cutoff points of high-sensitivity troponin T to diagnose myocardial infarction. Rev De La Fed Argentina De Cardiol 2014;43:141-5.
- Nasuruddin DN, Muzaini NH, Zaini IZ, Nawi AM, Hassan HHC, Choor CK, et al. Clinical comparison of two high sensitive troponin-I assays in patients suspected of acute myocardial infarction in the emergency department. Int J Cardiol 2017;249:S17-S18. https://doi.org/10.1016/j.ijcard.2017.09.073.
- Nejatian A, Omstedt Å, Höijer J, Hansson LO, Djärv T, Eggers KM, Svensson P. Outcomes in Patients with chest pain discharged after evaluation using a high-sensitivity troponin T assay. J Am Coll Cardiol 2017;69:2622-30. https://doi.org/10.1016/j.jacc.2017.03.586.
- Nestelberger T, Wildi K, Boeddinghaus J, Twerenbold R, Reichlin T, Gimenez MR, et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0 h/1 h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol 2016;207:238-45. https://doi.org/10.1016/j.ijcard.2016.01.112.
- Nestelberger T, Boeddinghaus J, Wussler D, Twerenbold R, Badertscher P, Wildi K, et al. Predicting major adverse events in patients with acute myocardial infarction. J Am Coll Cardiol 2019;74:842-54. https://doi.org/10.1016/j.jacc.2019.06.025.
- Neumann JT, Sörensen NA, Rübsamen N, Ojeda F, Schock A, Seddighizadeh P, et al. Evaluation of a new ultra-sensitivity troponin I assay in patients with suspected myocardial infarction. Int J Cardiol 2019;283:35-40. https://doi.org/10.1016/j.ijcard.2018.12.001.
- Nowak R, Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, DeFilippi C, et al. High sensitivity cardiac troponin T in patients not having an acute coronary syndrome: results from the TRAPID-AMI study. Biomarkers 2017;22:709-14. https://doi.org/10.1080/1354750X.2017.1334154.
- Papendick C, Blyth A, Seshadri A, Edmonds MJR, Briffa T, Cullen L, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: design of the Rapid Assessment of Possible ACS In the emergency Department with high sensitivity Troponin T (RAPID-TnT) study. Am Heart J 2017;190:25-33. https://doi.org/10.1016/j.ahj.2017.05.004.
- Peitsmeyer P, Schwemer T, Schluter M, Ojeda F, Wildi K, Zeller T, et al. Validated staged algorithm using high-sensitivity assayed cardiac troponin I to diagnose non-ST-segment elevation myocardial infarction in patients with acute chest pain. Circulation 2013;128.
- Peitsmeyer P, Schwemer T, Schlueter M, Ojeda F, Zeller T, Sinning C, et al. Gender-specific diagnosis of acute myocardial infarction using high-sensitivity assayed cardiac troponin I. Eur Heart J 2013;34. https://doi.org/10.1093/eurheartj/eht309.3512.
- Pettersson A, Ljung L, Johansson C, Heilborn U, Jernberg T, Frick M, et al. Experiences of a one-hour algorithm in chest pain patients with a nonelevated troponin T at presentation. Crit Pathw Cardiol 2018;17:6-12. https://doi.org/10.1097/HPC.0000000000000138.
- Pickering JW, Young JM, George P, Aldous S, Cullen L, Greenslade JH, et al. The utility of presentation and 4-hour high sensitivity troponin I to rule-out acute myocardial infarction in the emergency department. Clin Biochem 2015;48:1219-24. https://doi.org/10.1016/j.clinbiochem.2015.07.033.
- Pickering JW, Greenslade JH, Cullen L, Flaws D, Parsonage W, Aldous S, et al. Assessment of the European Society of Cardiology 0-hour/1-hour algorithm to rule-out and rule-in acute myocardial infarction. Circulation 2016;134:1532-41. https://doi.org/10.1161/CIRCULATIONAHA.116.022677.
- Pickering JW, Greenslade JH, Cullen L, Flaws D, Parsonage W, George P, et al. Validation of presentation and 3-h high-sensitivity troponin to rule-in and rule-out acute myocardial infarction. Heart 2016;102:1270-8. https://doi.org/10.1136/heartjnl-2015-308505.
- Pickering JW, Young JM, George PM, Watson AS, Aldous SJ, Troughton RW, et al. Validity of a novel point-of-care troponin assay for single-test rule-out of acute myocardial infarction. JAMA Cardiol 2018;3:1108-12. https://doi.org/10.1001/jamacardio.2018.3368.
- Reddy LL, Shah SA, Dherai AJ, Ponde CK, Ashavaid TF. Troponin T and heart type fatty acid binding protein (H-FABP) as biomarkers in patients presenting with chest pain 2016;31:87-92. https://doi.org/10.1007/s12291-015-0492-2.
- Reichlin T, Twerenbold R, Maushart C, Reiter M, Moehring B, Schaub N, et al. Risk stratification in patients with unstable angina using absolute serial changes of 3 high-sensitive troponin assays. Am Heart J 2013;165:371-8.e3. https://doi.org/10.1016/j.ahj.2012.11.010.
- Renstroum R, Tjora HL, Steiro OT, Omland T, Bjoerneklett RO, Nygaard OK, et al. Combining the European Society of Cardiology troponin algorithms and HEART Score for ruling out acute coronary syndrome in unselected patients presenting with acute chest pain: the WESTCOR study. Eur Heart J 2018;39:355-6. https://doi.org/10.1093/eurheartj/ehy565.P1739.
- Riedlinger D, Möckel M, Müller C, Holert F, Searle J, von Recum J, et al. High-sensitivity cardiac troponin T for diagnosis of NSTEMI in the elderly emergency department patient: a clinical cohort study. Biomarkers 2018;23:551-7. https://doi.org/10.1080/1354750X.2018.1460763.
- Sandoval Y, Smith SW, Shah AS, Anand A, Chapman AR, Love SA, et al. Rapid rule-out of acute myocardial injury using a single high-sensitivity cardiac troponin I measurement. Clin Chem 2017;63:369-76. https://doi.org/10.1373/clinchem.2016.264523.
- Santi L, Farina G, Gramenzi A, Trevisani F, Baccini M, Bernardi M, et al. The HEART score with high-sensitive troponin T at presentation: ruling out patients with chest pain in the emergency room. Intern Emerg Med 2017;12:357-64. https://doi.org/10.1007/s11739-016-1461-3.
- Schoenenberger AW, Stallone F, Walz B, Bergner M, Twerenbold R, Reichlin T, et al. Incremental value of heart-type fatty acid-binding protein in suspected acute myocardial infarction early after symptom onset. Eur Heart J Acute Cardiovasc Care 2016;5:185-92. https://doi.org/10.1177/2048872615571256.
- Schofer N, Brunner FJ, Schlüter M, Ojeda F, Zeller T, Baldus S, et al. Gender-specific diagnostic performance of a new high-sensitivity cardiac troponin I assay for detection of acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2017;6:60-8. https://doi.org/10.1177/2048872615626660.
- Schønemann-Lund M, Schoos MM, Iversen K, Hansen SI, Thode J, Clemmensen P, et al. Retrospective evaluation of two fast-track strategies to rule out acute coronary syndrome in a real-life chest pain population. J Emerg Med 2015;49:833-42. https://doi.org/10.1016/j.jemermed.2015.06.026.
- Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350. https://doi.org/10.1136/bmj.g7873.
- Shortt C, Phan K, Hill SA, Worster A, Kavsak PA. An approach to rule-out an acute cardiovascular event or death in emergency department patients using outcome-based cutoffs for high-sensitivity cardiac troponin assays and glucose. Clin Biochem 2015;48:282-7. https://doi.org/10.1016/j.clinbiochem.2014.11.010.
- Stallone F, Schoenenberger AW, Puelacher C, Rubini Gimenez M, Walz B, Naduvilekoot Devasia A, et al. Incremental value of copeptin in suspected acute myocardial infarction very early after symptom onset. Eur Heart J Acute Cardiovasc Care 2016;5:407-15. https://doi.org/10.1177/2048872616641289.
- Stoyanov KM, Hund H, Biener M, Gandowitz J, Riedle C, Löhr J, et al. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur Heart J Acute Cardiovasc Care 2020;9:39-51. https://doi.org/10.1177/2048872619861911.
- Su Q, Guo Y, Liu H, Qin Y, Zhang J, Yuan X, et al. Diagnostic role of high-sensitivity cardiac troponin T in acute myocardial infarction and cardiac noncoronary artery disease. Arch Med Res 2015;46:193-8. https://doi.org/10.1016/j.arcmed.2015.03.005.
- Suh D, Keller DI, Hof D, von Eckardstein A, Gawinecka J. Rule-out of non-ST elevation myocardial infarction by five point of care cardiac troponin assays according to the 0 h/3 h algorithm of the European Society of Cardiology. Clin Chem Lab Med 2018;56:649-57. https://doi.org/10.1515/cclm-2017-0486.
- Teggert A, Twerenbold R. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Ann Clin Biochem 2015;52. https://doi.org/10.1177/0004563215605692.
- Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med 2014;174:51-8. https://doi.org/10.1001/jamainternmed.2013.11362.
- Than MP, Pickering JW, Aldous SJ, Cullen L, Frampton CM, Peacock WF, et al. Effectiveness of EDACS versus ADAPT accelerated diagnostic pathways for chest pain: a pragmatic randomized controlled trial embedded within practice. Ann Emerg Med 2016;68:93-102.e1. https://doi.org/10.1016/j.annemergmed.2016.01.001.
- Thelin J, Borna C, Erlinge D. Öhlin B. The combination of high sensitivity troponin T and copeptin facilitates early rule-out of ACS: a prospective observational study. BMC Cardiovasc Disord 2013;13. https://doi.org/10.1186/1471-2261-13-42.
- Thet EM, Murphy J, Crilley J. Outcome of integration of new centaur (Siemen’s) high-sensitivity troponin i assay with heart score chest pain pathway to maximise early discharge from emergency department (ED). Heart 2019;105:A136-A137. https://doi.org/10.1136/heartjnl-2019-BCS.160.
- Twerenbold R, Meller B, Rubini M, Wildi K, Mueller M, Reichlin T, et al. One-hour rule-out and rule-in of acute myocardial infarction using siemens sensitive cardiac troponin I ultra. Eur Heart J 2013;34. https://doi.org/10.1093/eurheartj/eht307.P434.
- Twerenbold R, Reichlin T, Rubini-Gimenez M, Mueller M, Wildi K, Haaf P, et al. One-hour rule-out and rule-in of acute myocardial infarction using Siemens high-sensitivity cardiac troponin T. Eur Heart J 2013;34. https://doi.org/10.1093/eurheartj/eht307.P424.
- Twerenbold R, Neumann JT, Sörensen NA, Ojeda F, Karakas M, Boeddinghaus J, et al. Prospective validation of the 0/1-h algorithm for early diagnosis of myocardial infarction. J Am Coll Cardiol 2018;72:620-32. https://doi.org/10.1016/j.jacc.2018.05.040.
- Vigen R, Kutscher P, Fernandez F, Yu A, Bertulfo B, Hashim IA, et al. Evaluation of a novel rule-out myocardial infarction protocol incorporating high-sensitivity troponin T in a US hospital. Circulation 2018;138:2061-3. https://doi.org/10.1161/CIRCULATIONAHA.118.033861.
- Wang G, Wang J, Wu S, Zheng W, Zhang H, Ma J, et al. Clinical impact of using a more sensitive troponin assay in patients with acute chest pain. Clin Cardiol 2019;42:561-7. https://doi.org/10.1002/clc.23177.
- Wildi K, Singeisen H, Twerenbold R, Badertscher P, Wussler D, Klinkenberg LJJ, et al. Circadian rhythm of cardiac troponin I and its clinical impact on the diagnostic accuracy for acute myocardial infarction. Int J Cardiol 2018;270:14-20. https://doi.org/10.1016/j.ijcard.2018.05.136.
- Yip TP, Pascoe HM, Lane SE. Impact of high-sensitivity cardiac troponin I assays on patients presenting to an emergency department with suspected acute coronary syndrome. Med J Aust 2014;201:158-61. https://doi.org/10.5694/mja13.00117.
- Yokoyama H, Higuma T, Endo T, Nishizaki F, Hanada K, Yokota T, et al. ‘30-minute-delta’ of high-sensitivity troponin I improves diagnostic performance in acute myocardial infarction. J Cardiol 2018;71:144-8. https://doi.org/10.1016/j.jjcc.2017.08.003.
Appendix 1 Literature search strategies
EMBASE (Ovid)
Dates searched: 1974 to 25 September 2019.
Date of search: 26 September 2019.
Search strategy
-
“high sensitivity cardiac troponin T”/or high sensitivity troponin t assay/( 90)
-
“high sensitivity cardiac troponin I”/or high sensitivity troponin i assay/ (44)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (2939)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra).ti,ab,ot. (1194)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (4206)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (2415)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (6601)
-
(troponin$ adj5 (architect or elecsys or access or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)).ti,ab,hw,ot. (396)
-
(“dimension exl” or “atellica IM” or atellica-im or “alinity i” or alinity-i or “advia centaur” or “dimension vista”).ti,ab,hw,ot. (1300)
-
troponin$.mv,my. (65)
-
(elecsys$ or architect$ or centaur or vidas or vitros or atellica or alinity).dv. (2819)
-
(advia or advia120 or advia1800 or advia2120i or advia2400 or adviacentaur).dv. (972)
-
or/1-12 (12,098)
-
troponin t/or troponin I/or (60304-72-5 or 77108-40-8).rn. (38,060)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot,hw. (9,837,120)
-
14 and 15 (21,639)
-
13 or 16 (27,778)
-
thorax pain/ (84,161)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (110,352)
-
acute coronary syndrome/ (54,220)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot,hw. (67,681)
-
exp heart muscle ischemia/ (91,534)
-
exp heart infarction/ (365,052)
-
exp Unstable-Angina-Pectoris/ (23,610)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot,hw. (410)
-
Unstable angina$.ti,ab,ot. (19,196)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot,hw. (554,354)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot,hw. (163,966)
-
or/18-28 (719,484)
-
17 and 29 (14,259)
-
animal/ (1,431,471)
-
animal experiment/ (2,438,936)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,574,496)
-
or/31-33 (6,574,496)
-
exp human/ (20,203,276)
-
human experiment/ (469,138)
-
or/35-36 (20,204,702)
-
34 not (34 and 37) (5,073,475)
-
30 not 38 (13,490)
-
limit 39 to yr = “2013 -Current” (8169)
MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily (Ovid)
Dates searched: 1946 to 24 September 2019.
Date of search: 26 September 2019.
Search strategy
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (1169)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (561)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (1967)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (1117)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (3072)
-
(troponin$ adj5 (architect or elecsys or access or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)).ti,ab,hw,ot. (138)
-
(“dimension exl” or “atellica IM” or atellica-im or “alinity i” or alinity-i or “advia centaur” or “dimension vista”).ti,ab,hw,ot. (398)
-
or/1-7 (4229)
-
troponin t/or troponin I/or (60304-72-5 or 77108-40-8).rn. (12,105)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (7,169,880)
-
9 and 10 (6385)
-
8 or 11 (8437)
-
chest pain/ (12,556)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (40,643)
-
exp myocardial ischemia/ (419,151)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot. (29,162)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot. (321)
-
Unstable angina$.ti,ab,ot. (12,789)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot. (260,414)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot. (89,398)
-
or/13-20 (570,108)
-
12 and 21 (4465)
-
animals/not (animals/and humans/) (4,585,749)
-
22 not 23 (4245)
-
limit 24 to yr = “2013 -Current” (2104)
Cochrane Database of Systematic Reviews (Wiley)
Dates searched: Issue 9/September 2019.
The CDSR search retrieved four references.
Cochrane Central Register of Controlled Trials (Wiley)
Dates searched: Issue 9/September 2019
Date of search: 26 September 2019.
The CENTRAL search retrieved 567 references (436 when trials and pre 2013 records removed).
Search strategy
-
#1 (Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs):ti,ab,kw (259)
-
#2 (Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra):ti,ab,kw (108)
-
#3 ((troponin t or tnt or ctnt or tropt or trop t) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (1608)
-
#4 ((troponin I or tni or ctni or tropI or trop I) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (2893)
-
#5 (troponin* near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (623)
-
#6 (troponin* near/5 (architect or elecsys or access or unicel or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)):ti,ab,kw (17)
-
#7 #1 or #2 or #3 or #4 or #5 or #6 (3902)
-
#8 MeSH descriptor: [Troponin T] this term only (432)
-
#9 MeSH descriptor: [Troponin I] this term only (506)
-
#10 #8 or #9 (897)
-
#11 (sensitiv* or hs or early or initial or rapid or present* or ultra or “high performance” or ultrasensitive):ti,ab,kw (401,488)
-
#12 #10 and #11 (436)
-
#13 #7 or #12 (4184)
-
#14 MeSH descriptor: [Chest Pain] this term only (428)
-
#15 ((chest or thorax or thoracic) near/2 (pain* or discomfort or tight* or pressure)):ti,ab,kw (5686)
-
#16 (acute near/2 coronary near/2 syndrome*):ti,ab,kw (6420)
-
#17 MeSH descriptor: [Myocardial Ischemia] explode all trees (26,176)
-
#18 (preinfarc* Angina* or pre infarc* Angina*):ti,ab,kw (349)
-
#19 (Unstable angina*):ti,ab,kw (3941)
-
#20 ((heart* or myocardi* or cardiac or coronary) near/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*)):ti,ab,kw (41,934)
-
#21 (MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI):ti,ab,kw (17,551)
-
#22 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 (63,623)
-
#23 #13 and #22 with Cochrane Library publication date Between Sep 2013 and Dec 2019 (571)
Latin American and Caribbean Health Sciences Literature
Dates searched: 2013 to 20 September 2019.
Date of search: 20 September 2019.
URL: http://regional.bvsalud.org/php/index.php?lang=en
Search strategy
| Terms | Records |
|---|---|
| tw:((troponin* OR mh:d05.750.078.730.825.925 OR mh:d12.776.210.500.910.925 OR mh:d12.776.220.525.825.925 OR mh:d05.750.078.730.825.962 OR mh:d12.776.210.500.910.962 OR mh:d12.776.220.525.825.962 OR mh:d05.750.078.730.825 OR mh:d12.776.210.500.910 OR mh:d12.776.220.525.825 OR hstnt OR hs-tnt OR hsctnt OR hs-ctnt OR tnt-hs OR tnths OR ctnths OR ctnt-hs OR hstni OR hs-tni OR hsctni OR hs-ctni OR tni-hs OR tnihs OR ctnihs OR ctni-hs OR ctni-ultra)) AND (db:(“LILACS”)) AND (year_cluster:[2013 TO 2019]) | 159 |
| Total | 159 |
Science Citation Index – Expanded (Web of Science)
Date range searched: 1988 to 24 September 2019.
Conference Proceedings Citation Index – Science
Date range searched: 1990 to 24 September 2019.
Date of search: 25 September 2019.
Search strategy
-
# 1 TS = (Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs) (1113)
-
# 2 TS = (Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra) (439)
-
# 3 TS = ((troponin* or tnt or ctnt or tropt or tni or ctni or tropI or “trop t” or “trop I”) NEAR/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or “high performance” or ultrasensitive)) (5176)
-
# 4 ((troponin*) NEAR/5 (architect or elecsys or access or unicel or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)) (201)
-
# 5 #4 OR #3 OR #2 OR #1 (5334)
-
# 6 TS = ((chest or thorax or thoracic) NEAR (pain* or discomfort or tight* or pressure)) (37,887)
-
# 7 TS = (acute NEAR/2 coronary NEAR/2 syndrome*) (42,293)
-
# 8 TS = (preinfarc* angina* or pre infarc* angina) (1114)
-
# 9 TS = unstable angina* (16,970)
-
# 10 TS = ((heart* or myocard* or cardiac or coronary) NEAR/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*)) (308,052)
-
# 11 TS = (MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI) (118,099)
-
# 12 #6 OR # & or #8 OR #9 OR #10 OR #11 (426,084)
-
# 13 #12 AND #5 (1897)
ClinicalTrials.gov (internet)
URL: http://clinicaltrials.gov/ct2/search/advanced
Date of search: 20 September 2019.
Expert search option.
First posted from 1 January 2013 to 31 December 2019.
Search strategy
| Search term | Records |
|---|---|
| troponin AND INFLECT (“01/01/2013” : “12/31/2019”) [STUDY-FIRST-POSTED] AND (architect OR elecsys OR access OR unicel OR centaur OR vidas OR vitros OR dimension OR vista OR triagetrue OR triage-true OR atellica OR alinity OR advia) | 55 |
| troponin AND INFLECT (“01/01/2013” : “12/31/2019”) [STUDY-FIRST-POSTED] AND (sensitive OR hs OR early OR initial OR rapid OR presentation OR ultra OR high performance OR ultrasensitive) | 618 |
| Total | 673 |
| Total after duplicates removed | 629 (44 duplicates removed) |
World Health Organization International Clinical Trials Registry Platform (internet)
Date of search: 25 September 2019.
Advanced search option: Title and Intervention combined with OR.
Date of registration limited to 1 January 2013 to 25 September 2019.
Search strategy
| Title | Condition | Intervention | Records |
|---|---|---|---|
| Troponin OR Troponins | |||
| Troponin OR Troponins | |||
| Total | 139 trials |
Health Technology Assessment database
URL: www.crd.york.ac.uk/CRDWeb/
Dates searched: up to March 2018.
Database of Abstracts of Reviews of Effects
URL: www.crd.york.ac.uk/CRDWeb/
Dates searched: up to March 2015.
Date of search: 26 September 2019.
Forty-five records after date restriction.
Search strategy
-
MeSH DESCRIPTOR Troponin EXPLODE 1 IN DARE, HTA (32)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs) IN DARE, HTA FROM 2013 TO 2019 (0)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra) IN DARE, HTA FROM 2013 TO 2019 (0)
-
(troponin t or tnt or ctnt or tropt or trop t) IN DARE, HTA FROM 2013 TO 2019 (8)
-
(troponin I or tni or ctni or tropI or trop I) IN DARE, HTA FROM 2013 TO 2019 (10)
-
(troponin or troponins) IN DARE, HTA FROM 2013 TO 2019 (29)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 (45)
PROSPERO (International Prospective Register of Systematic Reviews) (internet)
URL: www.crd.york.ac.uk/prospero/#searchadvanced
Dates searched: up to 20 September 2019.
Date of search: 20 September 2019.
Searched in ‘All fields’.
Search strategy
| Terms | Records |
|---|---|
| Troponin* | 112 |
| Limited to 2013–2019 |
National Institute for Health Research Health Technology Assessment
URL: www.nihr.ac.uk/explore-nihr/funding-programmes/health-technology-assessment.htm
Date of search: 26 September 2019.
One record (URL: www.nihr.ac.uk/documents/case-studies/trapid-ami-impact-case-study/21537www.nihr.ac.uk/documents/case-studies/trapid-ami-impact-case-study/21537).
Conference abstracts
The following conference abstracts were manually searched to compliment those conference abstracts indexed in EMBASE:
-
American Association for Clinical Chemistry 2017–19
-
AHA Scientific Sessions 2017–19
-
ESC 2019.
Additional UK-specific cost searches
EMBASE (Ovid)
Dates searched: 1974 to 9 January 2020.
Date of search: 10 January 2020.
Search strategy
-
“high sensitivity cardiac troponin T”/or high sensitivity troponin t assay/ (88)
-
“high sensitivity cardiac troponin I”/or high sensitivity troponin i assay/ (43)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (3051)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra).ti,ab,ot. (1246)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (4333)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (2510)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (6836)
-
(troponin$ adj5 (architect or elecsys or access or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)).ti,ab,hw,ot. (414)
-
(“dimension exl” or “atellica IM” or atellica-im or “alinity i” or alinity-i or “advia centaur” or “dimension vista”).ti,ab,hw,ot. (1318)
-
troponin$.mv,my. (66)
-
(elecsys$ or architect$ or centaur or vidas or vitros or atellica or alinity).dv. (2923)
-
(advia or advia120 or advia1800 or advia2120i or advia2400 or adviacentaur).dv. (1000)
-
or/1-12 (12,496)
-
troponin t/or troponin I/or (60304-72-5 or 77108-40-8).rn. (38,546)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot,hw. (10,000,737)
-
14 and 15 (22,046)
-
13 or 16 (28,399)
-
health-economics/ (32,473)
-
exp economic-evaluation/ (299,466)
-
exp health-care-cost/ (285,436)
-
exp pharmacoeconomics/ (199,679)
-
or/18-21 (634,055)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (1,023,679)
-
(expenditure$ not energy).ti,ab. (38,862)
-
(value adj2 money).ti,ab. (2361)
-
budget$.ti,ab. (37,347)
-
or/23-26 (1,058,833)
-
22 or 27 (1,380,813)
-
letter.pt. (1,099,578)
-
editorial.pt. (638,530)
-
note.pt. (785,740)
-
or/29-31 (2,523,848)
-
28 not 32 (1,262,897)
-
(metabolic adj cost).ti,ab. (1461)
-
((energy or oxygen) adj cost).ti,ab. (4231)
-
((energy or oxygen) adj expenditure).ti,ab. (30,901)
-
or/34-36 (35,509)
-
33 not 37 (1,255,639)
-
exp animal/ (24,976,369)
-
exp animal-experiment/ (2,482,604)
-
nonhuman/ (6,026,401)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,603,915)
-
or/39-42 (26,921,217)
-
exp human/ (20,412,882)
-
exp human-experiment/ (480,344)
-
44 or 45 (20,414,345)
-
43 not (43 and 46) (6,507,765)
-
38 not 47 (1,144,073)
-
17 and 48 (837)
-
limit 49 to yr = “2013 -Current” (475)
-
United Kingdom/ (385,970)
-
(national health service* or nhs*).ti,ab,in,ad. (334,600)
-
(english not ((published or publication* or translat* or written or language* or speak* or literature or citation*) adj5 english)).ti,ab. (41,191)
-
(gb or “g.b.” or britain* or (british* not “british columbia”) or uk or “u.k.” or united kingdom* or (england* not “new england”) or northern ireland* or northern irish* or scotland* or scottish* or ((wales or “south wales”) not “new south wales”) or welsh*).ti,ab,jx,in,ad. (3,091,729)
-
(bath or “bath’s” or ((birmingham not alabama*) or (“birmingham’s” not alabama*) or bradford or “bradford’s” or brighton or “brighton’s” or bristol or “bristol’s” or carlisle* or “carlisle’s” or (cambridge not (massachusetts* or boston* or harvard*)) or (“cambridge’s” not (massachusetts* or boston* or harvard*)) or (canterbury not zealand*) or (“canterbury’s” not zealand*) or chelmsford or “chelmsford’s” or chester or “chester’s” or chichester or “chichester’s” or coventry or “coventry’s” or derby or “derby’s” or (durham not (carolina* or nc)) or (“durham’s” not (carolina* or nc)) or ely or “ely’s” or exeter or “exeter’s” or gloucester or “gloucester’s” or hereford or “hereford’s” or hull or “hull’s” or lancaster or “lancaster’s” or leeds* or leicester or “leicester’s” or (lincoln not nebraska*) or (“lincoln’s” not nebraska*) or (liverpool not (new south wales* or nsw)) or (“liverpool’s” not (new south wales* or nsw)) or ((london not (ontario* or ont or toronto*)) or (“london’s” not (ontario* or ont or toronto*)) or manchester or “manchester’s” or (newcastle not (new south wales* or nsw)) or (“newcastle’s” not (new south wales* or nsw)) or norwich or “norwich’s” or nottingham or “nottingham’s” or oxford or “oxford’s” or peterborough or “peterborough’s” or plymouth or “plymouth’s” or portsmouth or “portsmouth’s” or preston or “preston’s” or ripon or “ripon’s” or salford or “salford’s” or salisbury or “salisbury’s” or sheffield or “sheffield’s” or southampton or “southampton’s” or st albans or stoke or “stoke’s” or sunderland or “sunderland’s” or truro or “truro’s” or wakefield or “wakefield’s” or wells or westminster or “westminster’s” or winchester or “winchester’s” or wolverhampton or “wolverhampton’s” or (worcester not (massachusetts* or boston* or harvard*)) or (“worcester’s” not (massachusetts* or boston* or harvard*)) or (york not (“new york*” or ny or ontario* or ont or toronto*)) or (“york’s” not (“new york*” or ny or ontario* or ont or toronto*))))).ti,ab,in,ad. (2,372,103)
-
(bangor or “bangor’s” or cardiff or “cardiff’s” or newport or “newport’s” or st asaph or “st asaph’s” or st davids or swansea or “swansea’s”).ti,ab,in,ad. (96,722)
-
(aberdeen or “aberdeen’s” or dundee or “dundee’s” or edinburgh or “edinburgh’s” or glasgow or “glasgow’s” or inverness or (perth not australia*) or (“perth’s” not australia*) or stirling or “stirling’s”).ti,ab,in,ad. (327,742)
-
(armagh or “armagh’s” or belfast or “belfast’s” or lisburn or “lisburn’s” or londonderry or “londonderry’s” or derry or “derry’s” or newry or “newry’s”).ti,ab,in,ad. (43,867)
-
or/51-58 (3,767,357)
-
(exp “arctic and antarctic”/or exp oceanic regions/or exp western hemisphere/or exp africa/or exp asia/or exp “australia and new zealand”/) not (exp united kingdom/or europe/) (2,999,470)
-
59 not 60 (3,559,996)
-
50 and 61 (67)
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily
Dates searched: 1946 to 9 January 2020.
Date of search: 10 January 2020.
Search strategy
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (1220)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (588)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (2041)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (1170)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (3211)
-
(troponin$ adj5 (architect or elecsys or access or centaur or vidas or vitros or dimension or vista or triagetrue or triage-true or atellica or alinity or advia)).ti,ab,hw,ot. (145)
-
(“dimension exl” or “atellica IM” or atellica-im or “alinity i” or alinity-i or “advia centaur” or “dimension vista”).ti,ab,hw,ot. (415)
-
or/1-7 (4401)
-
troponin t/or troponin I/or (60304-72-5 or 77108-40-8).rn. (12,356)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (7,299,032)
-
9 and 10 (6557)
-
8 or 11 (8656)
-
economics/(27,118)
-
exp “costs and cost analysis”/ (231,602)
-
economics, dental/ (1909)
-
exp “economics, hospital”/ (24,141)
-
economics, medical/ (9050)
-
economics, nursing/ (3996)
-
economics, pharmaceutical/ (2905)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (760,923)
-
(expenditure$ not energy).ti,ab. (28,754)
-
(value adj1 money).ti,ab. (33)
-
budget$.ti,ab. (28,351)
-
or/13-23 (910,365)
-
((energy or oxygen) adj cost).ti,ab. (4005)
-
(metabolic adj cost).ti,ab. (1367)
-
((energy or oxygen) adj expenditure).ti,ab. (24,380)
-
or/25-27 (28,784)
-
24 not 28 (903,751)
-
letter.pt. (1,058,044)
-
editorial.pt. (514,173)
-
historical article.pt. (356,143)
-
or/30-32 (1,909,174)
-
29 not 33 (868,281)
-
12 and 34 (241)
-
limit 35 to yr = “2013 -Current” (133)
-
exp United Kingdom/ (359,811)
-
(national health service* or nhs*).ti,ab,in. (184,469)
-
(english not ((published or publication* or translat* or written or language* or speak* or literature or citation*) adj5 english)).ti,ab. (93,416)
-
(gb or “g.b.” or britain* or (british* not “british columbia”) or uk or “u.k.” or united kingdom* or (england* not “new england”) or northern ireland* or northern irish* or scotland* or scottish* or ((wales or “south wales”) not “new south wales”) or welsh*).ti,ab,jw,in. (1,999,631)
-
(bath or “bath’s” or ((birmingham not alabama*) or (“birmingham’s” not alabama*) or bradford or “bradford’s” or brighton or “brighton’s” or bristol or “bristol’s” or carlisle* or “carlisle’s” or (cambridge not (massachusetts* or boston* or harvard*)) or (“cambridge’s” not (massachusetts* or boston* or harvard*)) or (canterbury not zealand*) or (“canterbury’s” not zealand*) or chelmsford or “chelmsford’s” or chester or “chester’s” or chichester or “chichester’s” or coventry or “coventry’s” or derby or “derby’s” or (durham not (carolina* or nc)) or (“durham’s” not (carolina* or nc)) or ely or “ely’s” or exeter or “exeter’s” or gloucester or “gloucester’s” or hereford or “hereford’s” or hull or “hull’s” or lancaster or “lancaster’s” or leeds* or leicester or “leicester’s” or (lincoln not nebraska*) or (“lincoln’s” not nebraska*) or (liverpool not (new south wales* or nsw)) or (“liverpool’s” not (new south wales* or nsw)) or ((london not (ontario* or ont or toronto*)) or (“london’s” not (ontario* or ont or toronto*)) or manchester or “manchester’s” or (newcastle not (new south wales* or nsw)) or (“newcastle’s” not (new south wales* or nsw)) or norwich or “norwich’s” or nottingham or “nottingham’s” or oxford or “oxford’s” or peterborough or “peterborough’s” or plymouth or “plymouth’s” or portsmouth or “portsmouth’s” or preston or “preston’s” or ripon or “ripon’s” or salford or “salford’s” or salisbury or “salisbury’s” or sheffield or “sheffield’s” or southampton or “southampton’s” or st albans or stoke or “stoke’s” or sunderland or “sunderland’s” or truro or “truro’s” or wakefield or “wakefield’s” or wells or westminster or “westminster’s” or winchester or “winchester’s” or wolverhampton or “wolverhampton’s” or (worcester not (massachusetts* or boston* or harvard*)) or (“worcester’s” not (massachusetts* or boston* or harvard*)) or (york not (“new york*” or ny or ontario* or ont or toronto*)) or (“york’s” not (“new york*” or ny or ontario* or ont or toronto*))))).ti,ab,in. (1,349,609)
-
(bangor or “bangor’s” or cardiff or “cardiff’s” or newport or “newport’s” or st asaph or “st asaph’s” or st davids or swansea or “swansea’s”).ti,ab,in. (52,779)
-
(aberdeen or “aberdeen’s” or dundee or “dundee’s” or edinburgh or “edinburgh’s” or glasgow or “glasgow’s” or inverness or (perth not australia*) or (“perth’s” not australia*) or stirling or “stirling’s”).ti,ab,in. (201,032)
-
(armagh or “armagh’s” or belfast or “belfast’s” or lisburn or “lisburn’s” or londonderry or “londonderry’s” or derry or “derry’s” or newry or “newry’s”).ti,ab,in. (24,860)
-
or/37-44 (2,573,849)
-
(exp africa/or exp americas/or exp antarctic regions/or exp arctic regions/or exp asia/or exp oceania/) not (exp great britain/or europe/) (2,796,611)
-
45 not 46 (2,431,577)
-
36 and 47 (27)
Economics filters
Centre for Reviews and Dissemination
NHS EED MEDLINE using OvidSP. York: CRD; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
NHS EED EMBASE using OvidSP. York: CRD; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase (accessed 2 June 2014).
UK filter
Ayiku L, Levay P, Hudson T, Craven J, Barrett E, Finnegan A, et al. The MEDLINE UK filter: development and validation of a geographic search filter to retrieve research about the UK from OVID MEDLINE. Health Info Libr J 2017;34:200–16.
Ayiku L, Levay P, Hudson T, Craven J, Finnegan A, Adams R, et al. The EMBASE UK filter: validation of a geographic search filter to retrieve research about the UK from OVID EMBASE. Health Info Libr J 2019;36:121–33.
American Economic Association’s electronic bibliography (EBSCOhost)
Dates searched: 2013 to 9 January 2020.
Date of search: 16 January 2020.
Search modes: Boolean/Phrase.
Search strategy
S1 TX Troponin* (1)
S2 TX Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs (0)
S3 TX Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni (0)
NHS Economic Evaluation Database
URL: www.crd.york.ac.uk/CRDWeb/)
Dates searched: up to March 2015.
Date of search: 16 January 2020.
Search strategy
-
MeSH DESCRIPTOR troponin EXPLODE ALL TREES IN NHSEED (15)
-
* FROM 2013 TO 2020 (25,075)
-
#1 AND #2 (3)
-
(troponin) OR (troponins) IN NHSEED FROM 2013 TO 2020 (3)
-
#3 OR #4 (3)
Appendix 2 Data extraction tables
| Study | Selection criteria | Participant details | Assay | |
|---|---|---|---|---|
|
ADAPT (ACTRN1261100106994) Aldous et al. 201453 Eggers et al. 2016 69 Greenslade et al. 2015 71 Meller et al. 2015118 Parsonage et al. 2013130 van der Linden et al. 2018 109 Wildi et al. 2017112 Country: Australia and New Zealand Funding: The manufacturers (Abbott, Roche and Siemens) provided partial funding Recruitment: November 2007 to February 2011 Number of participants: 1194 |
Inclusion criteria: Prospectively recruited adults (aged ≥ 18 years) with possible cardiac symptoms in accordance with the AHA case definitions (i.e. acute chest, epigastric, neck, jaw or arm pain; or discomfort or pressure without a clear non-cardiac source) Exclusion criteria: Clear cause, other than ACS, for symptoms; staff considered recruitment to be inappropriate (e.g. receiving palliative treatment); transfer from another hospital; pregnancy; STEMI; patients who stated that their first episode of pain commenced > 12 hours before presentation; and patients with missing zero- or 2-hour samples Patient category: NSTEMI and 30-day MACE |
Median age (years) (IQR): 61 (50–73) Male (%): 59 Previous CAD (%): 21 Previous AMI (%): 26 Previous revascularisation (%): 24 Diabetes (%): 14 Smoking (%): 18 Hypertension (%): 56 Dyslipidaemia (%): 53 |
Abbott ARCHITECT hs-cTnI; Roche Elecsys hs-cTnT | |
|
ADAPT/IMPACT (ACTRN12611001069943/ACTRN12611000206921) Nestelberger et al. 2019 171 Country: Australia Funding: ADAPT was supported by research grants from the Emergency Medicine Foundation (Milton, QLD, Australia) and the Royal Brisbane and Women’s Hospital Foundation (Brisbane, QLD, Australia), and Beckman Coulter and investigational reagents were provided by the manufacturers. No information was reported about the funding of IMPACT Recruitment: ADAPT November 2007 to February 2011; IMPACT February 2011 to March 2014 Number of participants: 1280 |
Inclusion criteria: Adults (aged ≥ 18 years) with at least 5 minutes of symptoms where the attending physician planned to perform serial cTnI tests. The AHA case definitions for possible cardiac symptoms were used (i.e. acute chest, epigastric, neck, jaw or arm pain, or discomfort or pressure without an apparent non cardiac source) Exclusion criteria: STEMI; clear cause other than ACS for the symptoms at presentation (e.g. examination findings of pneumonia); inability to provide informed consent; staff considered recruitment to be inappropriate (e.g. receiving palliative treatment); transfer from another hospital; pregnancy; previous enrolment; and inability to be contacted after discharge Patient category: NSTEMI |
Median age (years) (IQR): 51 (43–62) Male (%): 60.1 Previous AMI: 14.3 Previous CAD (%): 17.3 Previous revascularisation (%): 12.4 Diabetes (%): 12.8 Smoking (%): 27.7 Hypertension (%): 43.6 Dyslipidaemia (hypercholesterolemia) (%): 42.3 Median BMI (kg/m2) (IQR): 28.3 (25.0–32.8) |
Beckman Coulter ACCESS hs-cTnI | |
|
Country: New Zealand Funding: Funded by the National Heart Foundation (Auckland, New Zealand) and assay reagents were provided by the manufacturer (Roche). One author declared personal funding from Abbott Recruitment: November 2007 to December 2010 |
Inclusion criteria: Adults (aged ≥ 18 years) with symptoms suggestive of cardiac ischemia (i.e. acute chest, epigastric, neck, jaw or arm pain; or discomfort or pressure without an apparent non-cardiac source) Exclusion criteria: ST segment elevation on an ECG;139 unable to provide informed consent; and would not be available to follow-up |
Median age (years) (IQR): 65 (56–76) Male (%): 60 White (%): 89 Previous CAD (%): 52 Previous revascularisation (%): 30 Family history (%): 60 Diabetes (%): 17 Smoking (%): 61 Hypertension (%): 61 Dyslipidaemia (%): 58 Median BMI (kg/m2) (IQR): 28 (25, 31) Median (IQR) time (hours) to presentation: 6.3 (3.3–13.3) |
Roche Elecsys hs-cTnT | |
|
Country: New Zealand Funding: Manufacturers (Roche and Abbott) supplied the assays. The study was funded by a New Zealand National Heart Foundation grant Recruitment: November 2006 to April 2007 Number of participants: 332 |
Inclusion criteria: Consecutive patients presenting to the ED with chest pain. Participants were eligible for inclusion if the attending clinician had sufficient suspicion of ACS and serial troponins and electrocardiography were considered necessary Exclusion criteria: aged < 18 years; samples not stored for both time points (on admission and at 6–24 hours) Patient category: Mixed |
Median age (years) (IQR): 64 (53–74) Male (%): 60 White (%): 85 Previous CAD (%): 54 Family history (%): 40 Diabetes (%): 16 Smoking (%): 45 Hypertension (%): 46 Dyslipidaemia (%): 38 Median (IQR) time (hours) to presentation: 4.0 (2.0–8.6) |
Roche Elecsys hs-cTnT | |
|
APACE (NCT00470587) Badertscher et al. 201854 Badertscher et al. 201855 Boeddinghaus et al. 201859 Boeddinghaus et al. 201960 Boeddinghaus et al. 2019123 Boeddinghaus et al. 2019170 Jaeger et al. 201674 Kaier et al. 201775 Lindahl et al. 2017132 Reichlin et al. 201590 Reichlin et al. 201591 Rubini Gimenez et al. 201470 Rubini Gimenez et al. 201592 Rubini Gimenez et al. 201593 Rubini Giménez et al. 201694 Twerenbold et al. 2017105 Twerenbold et al. 2017103 Twerenbold et al. 2017104 Twerenbold et al. 2018106 Twerenbold et al. 2018107 Twerenbold et al. 2019108 Wildi et al. 2016111 Wildi et al. 2019113 Country: Czechia, Italy, Poland, Spain and Switzerland Funding: Swiss National Science Foundation (Bern Switzerland), Swiss Heart Foundation (Bern Switzerland), Department of Internal Medicine of the University Hospital Basel (Basel, Switzerland), Roche, Siemens, Abbott, Brahms, Nanosphere (Northbrook, IL, USA) and 8sense (Rosenheim, Germany) Recruitment: April 2006 to August 2011 Number of participants: 2245 |
Inclusion criteria: Consecutive adults (aged > 18 years) presenting to the ED with symptoms suggestive of AMI (e.g. acute chest pain, angina pectoris at rest, other thoracic sensations) within an onset or peak within the last 12 hours Exclusion criteria: Terminal kidney failure requiring dialysis Patient category: Mixed |
Median age (years) (IQR): 62 (49–74) Male (%): 68 Previous AMI (%): 24 Previous CAD (%): 33 Previous revascularisation (%): 27 Diabetes (%): 18 Smoking (%): 25 Hypertension (%): 61 Dyslipidaemia (hypercholesterolemia) (%): 49 Median BMI (kg/m2) (IQR): 27 (24–30) |
Roche Elecsys hs-cTnT; Abbott ARCHITECT hs-cTnI; Siemens Healthcare ADVIA Centaur hs-cTnI; Siemens Healthcare Dimension Vista hs-cTnI | |
|
BACC (NCT02355457) Neumann et al. 201785 Neumann et al. 201786 Country: Germany Funding: This study was supported by the German Center of Cardiovascular Research (Berlin, Germany) and an unrestricted grant from Abbott Recruitment: July 2013 to December 2014 Number of participants: 1040 |
Inclusion criteria: Adults (aged > 18 years) presenting to the ED with symptoms suggestive of AMI Exclusion criteria: STEMI Patient category: NSTEMI |
Median age (years) (IQR): 65 (52–75) Male (%): 64.7 Previous CAD or revascularisation (%): 33.6 Previous AMI (%): 15.6 Diabetes (%): 14.4 Smoking (%): 23.2 Hypertension (%): 69.1 Dyslipidaemia (hyperlipoproteinemia) (%): 43.8 Median BMI (kg/m2) (IQR): 26.0 (23.5–29.4) |
Abbott ARCHITECT hs-cTnI | |
|
BEST Body et al. 2020 172 Country: UK Funding: Manchester University NHS Foundation Trust (Manchester, UK). Singulex, Inc. (Alameda, CA, USA) loaned the Singulex Clarity® System and Roche provided reagents without charge for this study Recruitment: NR Number of participants: 665 |
Inclusion criteria: Adults (aged > 18 years) who presented to the ED with pain, discomfort or pressure in the chest, epigastrium, neck, jaw or upper limb without an apparent non-cardiac source, which warranted investigation for possible ACS Exclusion criteria: Patients with peak symptoms occurring > 12 hours before enrolment; those with unequivocal ST elevation MI; those with another medical condition requiring hospital admission; and patients lacking the mental capacity to provide written informed consent Patient category: NSTEMI |
Mean age (years) (SD): 56 (15) Male (%): 60.8 Previous AMI (%): 25.4 Previous revascularisation (%): 24.2 Diabetes (%): 20.5 Hypertension (%): 46.5 Dyslipidaemia (%): 37.9 |
Roche Elecsys hs-cTnT | |
|
Country: UK Funding: Central Manchester NHS Trust Recruitment: January 2006 to February 2007 Number of participants: 703 |
Inclusion criteria: Patients presenting to the ED with chest pain; aged > 25 years and chest pain within previous 24 hours that initial treating physician suspected may be cardiac in nature Exclusion criteria: Renal failure requiring dialysis; trauma with suspected myocardial contusion; another medical condition mandating hospital admission; and if the patient did not consent to and provide a blood sample for use by the research team Patient category: Mixed |
Mean age (years) (SD): 59 (14) Male (%): 61 Kidney disease (%): 1 Previous AMI (%): 24 Previous revascularisation (%): 20 Previous family history (%): 48 Diabetes (%): 18 Smoking (%): 31 Dyslipidaemia (%): 48 Median time to presentation (hours): 3.5 |
Roche Elecsys hs-cTnT | |
|
Body et al. 2015 56 Country: UK Funding: UK College of Emergency Medicine. hs-cTn kits were donated to the research team by Roche Diagnostics Recruitment: NR Number of participants: 463 |
Inclusion criteria: Adult patients presenting to the ED with chest pain suspected to be of cardiac origin Exclusion criteria: Patients requiring hospital admission for a concomitant medical condition were excluded, as well as those with renal failure needing dialysis, significant chest trauma with suspected myocardial contusion, or who were pregnant; non-English speakers; prisoners (for ethics reasons); and those in whom all means of follow-up would be impossible Patient category: Mixed and 30-day MACE |
Mean (SD): 64 (16) Male (%): 58.3 Previous AMI (%): 30 Family History (%): 36.9 Diabetes (%): 17.3 Smoking (%): 20.7 Hypertension (%): 42.5 Dyslipidaemia (%): 40.2 |
Roche Elecsys hs-cTnT | |
|
Cappellini et al. 2019 62 Country: Italy Funding: Not stated Recruitment: November 2011 to October 2015 (derivation cohort) Number of participants: 6403 (derivation cohort) |
Inclusion criteria: Adults (aged ≥ 18 years) with suspect NSTEMI arriving at the ED within a median time of 3.4 hours and with three serial time-point measures of hs-cTnT Exclusion criteria: Patients with STEMI or with unclassified AMI (because of rapid transfer to other hospitals or death occurring before AMI classification) Patient category: NSTEMI |
Median age (years) (IQR): 73 (59–82) Male (%): 55.4 White (%): NR No further participant characteristics were reported |
Roche Elecsys hs-cTnT | |
|
Country: Germany Funding: hs-cTnT test kits were provided by Roche Recruitment: 7 September 2009 to 21 September 2009 Number of participants: 137 |
Inclusion criteria: Consecutive patients with acute chest pain of possible coronary origin presenting to the ED Exclusion criteria: NR Patient category: Mixed |
Mean age (years) (SD): 66 (16) Male (%): 64 Previous AMI (%): 32 Previous CAD (%): 34 Previous revascularisation (%): 24 Family history (%): 12 Diabetes (%): 22 Smoking (%): 22 Hypertension (%): 66 Dyslipidaemia (%): 35 Mean BMI (kg/m2) (SD): 28 (5) Time to presentation: 0–2 hours 36%; 2–6 hours 22%; 6–24 hours 33%; > 24 hours 20% |
Roche Elecsys hs-cTnT | |
|
CORE Borna et al. 2018 116 Mokhtari et al. 2016119 Mokhtari et al. 2017 120 Country: Sweden Funding: The study was funded by an ALF research grant at Skåne University Hospital (Scania, Sweden) and by a grant from Region Skåne (Kristianstad, Sweden), which are national grants from the Swedish government Recruitment: February 2013 to April 2014 Number of participants: 1138 |
Inclusion criteria: Adults (aged ≥ 18 years) with a primary symptom of non-traumatic chest pain and for whom hs-cTnT was ordered at presentation (0 hours) were enrolled during weekdays between 09.00 and 21.00 Exclusion criteria: Patients with severe communication barriers, (e.g. not speaking Swedish or English, or with dementia); STEMI Patient category: 30-day MACE |
Median age (years) (IQR): 63.2 (49.1–73.7) Male (%): 54.6 Previous AMI (%): 19.9 Previous revascularisation (%): 20.3 Family history (%): 22.6 Diabetes (%): 13.9 Smoking (current or previous) (%): 56.3 Hypertension (%): 43.5 Dyslipidaemia (hypercholesterolemia) (%): 22.8 |
Roche Elecsys hs-cTnT | |
|
FASTER I and FAST II Country: Sweden Funding: Swedish Society of Medicine (Stockholm, Sweden) and the Selander Foundation (Greenwich, CT, USA) Recruitment: May 2000 to March 2001 (FAST II); October 2002 to August 2003 (FASTER I) Number of participants eligible (enrolled): 495 (360) |
Inclusion criteria: Chest pain with ≥ 15-minute duration within the last 24 hours (FAST II) or the last 8 hours (FASTER I). Analysis restricted to patients with symptom onset < 8 hours Exclusion criteria: ST segment elevation on the admission 12-lead ECG leading to immediate reperfusion therapy or its consideration was used as exclusion criterion Patient category: NSTEMI |
Median age (years) (IQR): 67 (58–76) Male (%): 66 Previous AMI (%): 38 Previous revascularisation (%): 18 Diabetes (%): 18 Smoking (%): 18 Hypertension (%): 43 Dyslipidaemia (%): 38 Delay < 4 hours (%): 40 |
Roche Elecsys hs-cTnT | |
|
Country: France Funding: Assay kits for the study were provided by the manufacturers (Roche) Recruitment: August 2005 to January 2007 Number of participants: 317 |
Inclusion criteria: Consecutive adults (aged > 18 years) presenting to the ED with chest pain suggestive of ACS (onset or peak within the previous 6 hours) Exclusion criteria: Patients with acute kidney failure requiring dialysis were excluded Patient category: Mixed (13 were STEMI and 32 NSTEMI) |
Mean age (years) (SD): 57 (17) Male (%): 65 Previous CAD (%): 26 Family history (%): 32 Diabetes (%): 14 Smoking (%): 40 Dyslipidaemia (%): 36 |
Roche Elecsys hs-cTnT | |
|
High-STEACS (NCT01852123) Chapman et al. 2017 65 Chapman et al. 2018 66 Chapman et al. 2019 67 Miller-Hodges et al. 2018 79 Shah et al. 201598 Country: UK (Scotland) Funding: This trial was funded by the British Heart Foundation (Birmingham, UK) (SP/12/10/29922), with support from a Research Excellence Award (RE/18/5/34216). CJW was supported by NHS Lothian through the Edinburgh Clinical Trials Unit. Abbott Laboratories provided cTn assay reagents, calibrators and controls without charge Recruitment: June 2013 to March 2016 Number of participants: 32,837 |
Inclusion criteria: All patients presenting to the ED were screened by the attending clinician and prospectively included in the trial if cTn was requested for suspected ACS Exclusion criteria: Patients were excluded if they had been admitted previously during the study period, were pregnant or did not live in Scotland. Patients with myocardial injury at presentation, with ≤ 2 hours of symptoms or with STEMI elevation MI were excluded Patient category: NSTEMI and 30-day MACE |
Mean age (years) (SD): 58.4 (17.1) Male (%): 53.0 Previous CAD (%): 23.0 Previous AMI (%): 8.0 Previous revascularisation (%): 8.8 Diabetes (%): 6.0 |
Abbott ARCHITECT hs-cTnI; Siemens Healthcare Atellica hs-cTnI | |
|
High-US Nowak et al. 2019128 Nowak et al. 2019129 Country: USA Funding: Siemens Healthcare Recruitment: April 2015 to April 2016 Number of participants: 2212 |
Inclusion criteria: ED patients aged ≥ 22 years with suspected AMI. Patients had to have at least one hs-cTnI concentration available at presentation, using both the Atellica and ADVIA Centaur assays Exclusion criteria: Patients in whom results were not available for either one or both assays, did not have a valid baseline hs-cTnI result, did not have a 12-lead ECG, in whom post-discharge follow-up was missing or presented with STEMI were excluded from analyses Patient category: NSTEMI |
Mean age (years) (SD): 57 (13) Male (%): 56.0 White (%): 56.0 Previous CAD (%): 38.0 Diabetes (%): 30.0 Smoking (%): 27.0 Hypertension (%): 70.0 |
Siemens Healthcare Atellica hs-cTnI; Siemens Healthcare ADVIA Centaur hs-cTnI | |
|
Huang et al. 2015 72 Guangquan et al. 201673 Country: China Funding: Roche Diagnostics GmbH in Shanghai Recruitment: July 2009 to December 2013 Number of participants: 2249 |
Inclusion criteria: Patients with a suspected diagnosis of AMI (chest pain onset < 12 hours) presenting at the ED Exclusion criteria: Patients requiring renal replacement therapy, who had metal coronary stents implanted or who had transferred from other hospitals were excluded (patients with STEMI were excluded from the NSTEMI analysis) Patient category: NSTEMI and mixed |
Mean age (years) (range): 61 (48–71) Male (%): 65 Previous CAD (%): 15 Previous revascularisation (%): 2 Diabetes (%): 12.9 Smoking (%): 31 Hypertension (%): 26 Dyslipidaemia (%): 5.4 |
Roche Elecsys hs-cTnT | |
|
Country: Germany Funding: Abbott Diagnostics provided study funding Recruitment: January 2007 to December 2008 Number of participants: 1818 |
Inclusion criteria: Consecutive adults (aged 18–85 years) presenting to three chest pain units with chest pain suggestive of ACS Exclusion criteria: Major surgery or trauma within the previous 4 weeks; pregnancy; intravenous drug abuse; and anaemia (haemoglobin < 10 g/dl) Patient category: Mixed |
Mean age (years) (SD): 61 (14) Male (%): 66 Previous CAD (%): 36 Family history (%): 32 Diabetes (%): 16 Smoking (%): 24 Hypertension (%): 74 Dyslipidaemia (%): 73 Mean BMI (kg/m2) (SD): 28 (5) |
Abbott ARCHITECT hs-cTnI | |
|
Country: Germany Funding: Investigators were supported by Roche Diagnostics and assay kits were also provided by the manufacturer Recruitment: May 2008 to December 2008 Number of participants: 94 |
Inclusion criteria: Consecutive patients admitted to a chest pain unit with symptoms suggestive of ACS Exclusion criteria: ST segment elevation; severe kidney dysfunction (eGFR < 60 ml/minute/1.73 m2); and patients undergoing PCI during follow-up sampling Patient category: NSTEMI |
Mean age (years) (SD): 66 (11) Male (%): 71 Previous AMI (%): 37 Previous CAD (%): 50 Previous revascularisation (%): 17 Family history (%): 32 Diabetes (%): 31 Smoking (%): 22 Hypertension (%): 78 Dyslipidaemia (%): 65 Median symptom onset (minutes) (IQR): 358 (152–929) Mean (SD) BMI (kg/m2): 28 (4) |
Roche Elecsys hs-cTnT | |
|
Lin et al. 2019 117 Country: Singapore Funding: This study was funded by the SingHealth Foundation Research grant (SHF/FG403P/2008) and National University of Singapore Recruitment: March 2010 to April 2014 Number of participants: 2444 |
Inclusion criteria: Adults (aged ≥ 25 years) presenting to the ED, from Monday to Friday, from 08.00 to 21.00 hours, with symptoms suggestive of ACS (e.g. chest pain or angina equivalent) Exclusion criteria: STEMI; end-stage renal failure; no cTn obtained as part of standard care; lost to follow-up Patient category: 30-day MACE |
Median age (years) (IQR): 55 (47–64) Male (%): 66.9 Previous CAD (%): 25.3 Previous AMI (%): 10.1 Previous revascularisation (%): 21.3 Family history (%): 14.7 Diabetes (%): 13.3 Smoking (current and previous) (%): 26.8 Hypertension (%): 70.9 Dyslipidaemia (%): 52.7 |
Roche Elecsys hs-cTnT | |
|
Country: Sweden Funding: Partially supported by a grant from Roche Diagnostics, who also provided reagents. Supported by the Swedish Heart and Lung Foundation (Stockholm, Sweden) and the National Board of Health and Welfare (Stockholm, Sweden) Recruitment: August 2006 to January 2008 Number of participants: 233 |
Inclusion criteria: Patients admitted to a coronary care unit with chest pain or other symptoms suggestive of ACS within 12 hours of admission Exclusion criteria: Patients with persistent ST segment elevation Patient category: NSTEMI |
Median age (years) (IQR): 65 (55–76) Male (%): 67 Previous AMI (%): 30 Previous revascularisation (%): 21 Diabetes (%): 23 Smoking (%): 17 Hypertension (%): 50 Median time (hours) from symptom onset (25th to 75th centile): 5 (3–8) |
Roche Elecsys hs-cTnT | |
|
Chang et al. 2018 124 Country: USA Funding: Roche Diagnostics Recruitment: 2011–15 Number of participants: 1679 |
Inclusion criteria: Adults (aged ≥ 21 years) presenting to one of 15 US EDs with suspected ACS, within 24 hours of symptom onset Exclusion criteria: AMI in previous 3 months; transfer from another medical facility; surgery (including percutaneous coronary intervention) or hospitalisation within the last 3 months; recent cardioversion or defibrillation, acute non-cardiac primary illness prior to enrolment (e.g. severe sepsis); cardiogenic shock; and pregnancy Patient category: Mixed and MACE |
Median age (years) (IQR): 55 (47–64) Male (%): 51.6 Previous CAD (%): 26.5 Previous AMI (%): 18.6 Previous revascularisation (%): 22.5 Diabetes (%): 26.1 Smoking (%): 30.5 Hypertension (%): 66.2 Dyslipidaemia (%): 50.1 Median BMI (kg/m2) (IQR): 29.9 (25.9–35.4) |
Roche Elecsys hs-cTnT STAT | |
|
PITAGORAS Country: Spain Funding: Supported by a grant from Roche Diagnostics Recruitment: NR Number of participants: 446 |
Inclusion criteria: Patients presenting to the ED with chest pain of possible coronary origin and onset of pain within the previous 24 hours Exclusion criteria: Persistent ST segment elevation on an ECG; troponin elevation in any of two serial determinations (at arrival and 6–8 hours later); prior diagnosis of ischemic heart disease by either the finding of significant stenosis in a prior coronary angiogram or previously documented AMI; left bundle branch block or other non-interpretable ECG or inability to perform exercise test; structural heart disease different to ischemic heart disease; concomitant heart failure or significant bradyarrhythmia (< 55 beats/minute) or tachyarrhythmia (> 110 beats/minute) at admission Patient category: NSTEMI |
Mean age (years) (SD): 60 (12) Male (%): 59 Family history (%): 14 Diabetes (%): 20 Smoking (%): 25 Hypertension (%): 54 Dyslipidaemia (%): 46 |
Roche Elecsys hs-cTnT | |
|
QUART (ACTRN12610000053022) Parsonage et al. 2013131 Country: Australia Funding: Emergency Medicine Foundation (Milton, QLD, Australia) and Roche Diagnostics Recruitment: November 2008 to February 2011 Number of participants: 764 |
Inclusion criteria: Consecutive adult patients (aged ≥ 18 years) presenting during office hours to a single, large, metropolitan tertiary hospital ED with symptoms suggestive of cardiac chest pain Exclusion criteria: A clear cause of symptoms other than ACS; inability or unwillingness to provide consent or be contacted after discharge; recruitment considered inappropriate by staff (e.g. palliative treatment); interhospital transfer; pregnancy; and previous enrolment Patient category: Mixed |
Mean age (years) (SD): 55.3 (15.1) Male (%): 61.3 Previous AMI (%): 17.9 Previous revascularisation (%): 17.1 Family history (%): 50.5 Diabetes (%): 14.7 Smoking (recent or current) (%): 31.0 Hypertension (%): 49.2 Dyslipidaemia (%): 50.9 Median (IQR) time to presentation (hours): 4.97 (1.63–20.60) |
Roche Elecsys hs-cTnT | |
|
RATPAC (point-of-care arm) Country: UK Funding: UK HTA programme Recruitment: February 2007 to June 2008 Number of participants: 850 |
Inclusion criteria: Patients presenting to the ED with chest pain due to suspected, but not proven, AMI Exclusion criteria: ECG changes diagnostic for AMI or high-risk ACS (> 1 mm ST deviation or > 3 mm inverted T waves); known CAD with prolonged (> 1 hour) or recurrent typical cardiac-type pain; proven or suspected serious non-cardiac pathology (e.g. PE); comorbidity or social problems requiring hospital admission even if AMI ruled out; obvious non-cardiac cause of chest pain (e.g. pneumothorax or muscular pain); and presentation > 12 hours after most significant episode of pain Patient category: NSTEMI |
Median age (years) (IQR): 54 (44–64) Male (%): 60 Previous AMI (%): 40 Previous revascularisation (%): 1 Diabetes (%): 8 Smoking (%): 28 Hypertension (%): 35 Dyslipidaemia (%): 24 Median (IQR) time to presentation (hours): 8.25 (5.17–12.30) |
Roche Elecsys hs-cTnT | |
|
REACTION-US Nowak et al. 2018 87 Nowak et al. 2018127 Country: USA Funding: The Henry Ford Health System (Detroit, MI, USA) and Roche Diagnostics Recruitment: NR Number of participants: 569 |
Inclusion criteria: Convenience sample (patients screened when research co-ordinators were available) of adults (aged > 21 years) presenting to the ED with symptoms suggestive of ACS and for whom a triage ECG was available Exclusion criteria: Patients with acute distress requiring immediate life-saving interventions; cardioversion or defibrillation or thrombolytic therapy within the previous 24 hours; STEMI leading to immediate reperfusion therapy; traumatic injuries; transfers from other facilities; and patients who were pregnant or breast feeding Patient category: NSTEMI |
Median age (years) (IQR): 55 (49–63) Male (%): 52 Previous CAD (%): 35.9 Previous AMI (%): 29.5 Previous revascularisation (%): 24.6 Family history (%): 38.8 Diabetes (%): 28.8 Smoking (%): 37.3 Hypertension (%): 81.5 Dyslipidaemia (hypercholesterolemia) (%): 50.3 Median (IQR) time to presentation (hours): 8.7 (2.3–41.5) |
Roche Elecsys hs-cTnT | |
|
ROMI-3 (NCT01994577) Kavasak et al. 201776 Country: Canada Funding: Canadian Institutes of Health Research (Ottawa, ON, Canada), Abbott Laboratories, Roche Diagnostics, Healthcare Diagnostics, Ortho Clinical Diagnostics, Randox Laboratories, Beckman Coulter and CADTH Recruitment: May 2013 to August 2013 Number of participants: 1137 |
Inclusion criteria: Adults (aged ≥ 18 years) presenting to the ED with symptoms of and investigated for ACS (i.e. troponin ordered by an ED physician) Exclusion criteria: Patients were excluded if they met any of the following exclusion criteria before troponin I testing: death (all cause); STEMI; and serious ventricular cardiac dysrhythmia. Patients who had any of the following health conditions within the previous 30 days were also excluded: traumatic chest pain, including surgery or cardiac manipulation; STEMI or NSTEMI; diagnosis of pulmonary embolus; known active malignancy; sepsis; patients who were previously enrolled or transferred from another primary care facility Patient category: NSTEMI |
Mean age (years) (SD): with MI 73.3 (14.1), without MI 65.8 (16.6) Male (%): 47.1 Family history (%): 54.2 Diabetes (%): 29.3 Smoking (%): 25.7 Hypertension (%): 70.7 Dyslipidaemia (hypercholesterolemia) (%): 59.5 |
Roche Elecsys hs-cTnT; Abbott ARCHITECT hs-cTnI | |
|
Country: USA Funding: Two authors declared individual funding from manufacturers (one from Roche Diagnostics and one from Beckman Coulter and Abbott) Recruitment: NR. Conference abstract only Number of participants: 288 |
Inclusion criteria: Patients presenting to the ED with symptoms suggestive of AMI Exclusion criteria: None reported Patient category: Mixed Details: NSTEMI 19% and STEMI 15% |
No further participant details reported | Roche Elecsys hs-cTnT | |
|
Country: France Funding: Study funded by the hospital, with assay reagents supplied by the manufacturers Recruitment: December 2009 to November 2011 Number of participants: 248 |
Inclusion criteria: Adults presenting to the ED with chest pain–recent (within 12 hours of presentation) Exclusion criteria: Traumatic causes of chest pain. STEMI was defined by the persistent elevation of the ST segment of at least 1 mm in two contiguous ECG leads or by the presence of a new left bundle branch block with positive cardiac enzyme results. Patients with STEMI were excluded from the analysis for our review Patient category: NSTEMI (data also reported for mixed AMI but not extracted) |
Median age (years) (IQR): 61 (48–75) Male (%): 63 |
Roche Elecsys hs-cTnT | |
|
Shiozaki et al. 2017 100 Country: Japan and Taiwan Funding: This work was supported by Japan Society for the Promotion of Science (Tokyo, Japan) Grants-in-Aid for Scientific Research (grant number JP24591070) Recruitment: November 2014 to April 2015 Number of participants: 413 |
Inclusion criteria: Patients presenting with chest pain suggestive of ACS in whom the attending physician planned to perform serial biomarker tests Exclusion criteria: STEMI; patients who staff considered recruitment inappropriate for (e.g. terminal illness); and patients with trauma that may have increased troponin levels Patient category: NSTEMI |
Median age (years) (IQR): 72 (59–81) Male (%): 60.8 Previous revascularisation (%): 24.9 Diabetes (%): 26.9 Smoking (%): 18.9 Hypertension (%): 63.9 Dyslipidaemia (%): 60.5 Median BMI (kg/m2) (IQR): 23.3 (20.6–25.8) |
Roche Elecsys hs-cTnT | |
|
Slagman et al. 2017 102 Country: Germany Funding: NR Recruitment: October 2012 to March 2013, and August 2013 to November 2013 Number of participants: 3423 |
Inclusion criteria: All patients with routine point-of-care troponin T measurement at admission, who presented to the ED of a tertiary care hospital Exclusion criteria: Patients with a final diagnosis of STEMI and patients with surgical conditions were excluded, as were patients with missing troponin values Patient category: NSTEMI |
Median age (years) (IQR): 61 (45–73) Male (%): 57.2 Family history (%): 32.0 Diabetes (%): 22.8 Smoking (%): 34.2 Hypertension (%): 18.4 Dyslipidaemia (hypercholesterolemia) (%): 9.6 Median BMI (kg/m2) (IQR): 27 (24–30) |
Roche Elecsys hs-cTnT | |
|
TRAPID-AMI Body et al. 2015122 Body et al. 2016 114 McCord et al. 2017126 Mueller-Hennessen et al. 201681 Mueller-Hennessen et al. 2017 82 Mueller-Hennessen et al. 201983 Country: Belgium, Germany, Italy, Switzerland, Spain, Sweden, UK, USA and Australia Funding: Roche Diagnostics Recruitment: April 2011 to June 2013 Number of participants: 1282 |
Inclusion criteria: Adults (aged ≥ 18 years) presenting to the ED with symptoms suggestive of AMI (such as acute chest pain and angina pectoris) and with an onset or maximum of discomfort or pain within the previous 6 hours Exclusion criteria: Patients with renal failure requiring long-term haemodialysis; those with trauma, cardioversion, defibrillation, or thrombolytic therapy before inclusion; individuals receiving coronary artery bypass grafting within the last month or hospitalised for AMI within the last 3 weeks; and pregnant and breastfeeding women Patient category: NSTEMI, mixed and 30-day MACE |
Median age (years) (IQR): 62 (50–74) Male (%): 62.8 Previous AMI (%): 24.9 Previous revascularisation (%): 30.3 Diabetes (%): 21.1 Smoking (%): 22.8 Hypertension (%): 62.8 Dyslipidaemia (hypercholesterolemia) (%): 10.8 |
Roche Elecsys hs-cTnT | |
|
TRUST (ISRCTN 21109279) Carlton et al. 2015 63 Country: UK Funding: This study was funded by the Royal College of Emergency Medicine (London, UK) and Bournemouth University (Dorset, UK). The lead author received funding from Abbott for related research Recruitment: July 2012 to August 2013 Number of participants: 963 (959 Roche hs-cTnT; 867 Abbott hs-cTnI) |
Inclusion criteria: Consecutive patients were screened and recruited 24 hours a day, 7 days a week, during the study period. Patients were included if they were aged ≥ 18 years and had at least 5 minutes of chest pain suggestive of ACS, and for whom the attending physician determined that evaluation with serial troponin testing was required. Possible cardiac symptoms included acute chest, epigastric, neck, jaw, or arm pain, or discomfort or pressure without an apparent non-cardiac source, in accordance with the AHA case definitions Exclusion criteria: Patients were excluded if any of the following were present: STEMI or left bundle branch block not known to be old; ECG changes diagnostic of ischemia (ST segment depression ≥ 1 mm or T-wave inversion); arrhythmias (new-onset atrial fibrillation, atrial flutter, sustained supraventricular tachycardia, second-degree or complete heart block, or sustained or recurrent ventricular arrhythmias); aged ≥ 80 years; atypical symptoms in the absence of chest discomfort; a clear non-ACS cause for chest pain at presentation (e.g. pulmonary embolism, pneumonia, aortic dissection); another medical condition requiring hospital admission; refusal or inability to give informed consent; non-English speaking; pregnancy; renal failure requiring dialysis; or inability to be contacted after discharge Patient category: NSTEMI |
Roche hs-cTnT cohort Mean age (years) (SD): 58.0 (13.3) Male (%): 58.8 White (%): 95.2 Previous AMI (%): 21.3 Previous revascularisation (%): 24.3 Family history (%): 36.8 Diabetes (%): 17.1 Smoking (%): 24.1 Hypertension (%): 55.1 Dyslipidaemia (%): 66.1 Median time to presentation (hours): 2.4 |
Abbott hs-cTnI cohort Mean age (years) (SD): 57.9 (13.0) Male (%): 59.4 White (%): 95.4 Previous AMI (%): 21.9 Previous revascularisation (%): 24.1 Family history (%): 37.7 Diabetes (%): 16.7 Smoking (%): 24.2 Hypertension (%): 55.0 Dyslipidaemia (%): 67.2 Median time to presentation (hours): 2.3 |
Roche Elecsys hs-cTnT; Abbott ARCHITECT hs-cTnI |
|
TUSCA Country: Spain Funding: Reagents and logistical support were provided by Roche Diagnostics Recruitment: NR Number of participants: 358 |
Inclusion criteria: Adult (aged > 18 years) described as presenting with ACSs and symptom duration ≥ 5 minutes. Population included 174 people with a final diagnosis of non-ACSs Exclusion criteria: ST segment elevation; new left bundle branch block; pre-admission thrombolytic therapy; defibrillation or cardioversion before sampling; pregnancy; renal failure requiring dialysis; UA within 2 months; and coronary artery bypass graft within 3 months Patient category: NSTEMI |
Mean age (years) (range): 69 (27–93) Male (%): 68 Previous CAD (%): 35 Diabetes (%): 26 Hypertension (%): 62 Presentation within 3 hours: 46.2% |
Roche Elecsys hs-cTnT | |
|
UTROPIA (NCT02060760) Dodd et al. 2019125 Sandoval et al. 2017 95 Country: USA Funding: Abbott Diagnostics and the Minneapolis Medical Research Foundation (Minneapolis, MN, USA) Recruitment: February 2014 to May 2014 Number of participants: 1631 |
Inclusion criteria: Consecutive unselected patients in whom initial pre-set serial troponin I measurements at 0, 3, 6 and 9 hours were ordered on clinical indication to rule in and rule out AMI. For inclusion, patients needed a baseline troponin I measurement at presentation and at least one additional troponin I measured within 24 hours of presentation before discharge and at least one 12-lead ECG Exclusion criteria: Aged < 18 years; STEMI; pregnancy; trauma; declined to participate on research, as documented on information disclosure; did not present through the ED; or were transferred from an outside hospital Patient category: NSTEMI |
Mean age (years) (SD): 57 (15) Male (%): 56 Previous CAD (%): 23 Previous AMI (%): 12 Previous revascularisation (%): 14 Diabetes (%): 43 Smoking (history of tobacco use) (%): 59 Hypertension (%): 66 Dyslipidaemia (%): 43 |
Abbott ARCHITECT hs-cTnI | |
|
Venge et al. 2017 110 Country: Germany, France, Austria and the Netherlands Funding: NR Recruitment: NR Number of participants: 450 |
Inclusion criteria: Adults (aged ≥ 18 years) presenting with symptoms suggestive of MI, presenting for the first time and < 12 hours after symptom onset Exclusion criteria: NR Patient category: Mixed |
Median age (years) (range): 62 (18–94) Male (%): 58.9 Previous CAD (%): 36.2 Previous AMI (%): 17.9 Previous revascularisation (%): 28.2 Family history (%): 28.0 Diabetes (%): 22.1 Smoking (%): 25.9 Hypertension (%): 61.1 Dyslipidaemia (%): 42.4 Median BMI (kg/m2) (range): 26.4 (15.9–50.6) |
Abbott ARCHITECT hs-cTnI | |
| Study | High-sensitivity troponin (ng/l) | Reference standard | ||||||
|---|---|---|---|---|---|---|---|---|
| Assay(s) | LoD | 99th centile | CoV | Target condition(s) | Reference standard | Standard troponin | Observer | |
|
ADAPT (ACTRN12611001069943) Aldous et al. 201453 Boeddinghaus et al. 2016 57 Eggers et al. 201669 Greenslade et al. 2015 71 Meller et al. 2015118 Parsonage et al. 2013130 van der Linden et al. 2018 109 Wildi et al. 2017112 |
Abbott ARCHITECT hs-cTnI | 1.9 | 26.2 | < 5% at 26.2 | NSTEMI; MACE |
Third Universal Definition of Myocardial Infarction 43 The criteria for a MACE included any of the following: death (excluding clearly non-cardiac); cardiac arrest; AMI; emergency revascularisation procedure; cardiogenic shock; ventricular arrhythmia requiring intervention; and a high-degree atrioventricular block requiring intervention, within 30 days after initial presentation |
Conventional troponins were measured using Abbott Diagnostics TnI (Abbott Laboratories, Abbott Park, IL, USA) (LoD 10 ng/l, 99th centile 28 ng/l, CoV < 10% at 32 ng/l, decision threshold 30 ng/l) or Beckman Coulter second generation Accutane (Beckman Coulter, Brea, CA, USA) (LoD 10 ng/l, 99th centile 40 ng/l, CoV < 10% at 60 ng/l, decision threshold 40 ng/l) Serial sampling up to at least 6 hours |
Adjudication of all cardiac end points was made by two cardiologists, with consultation of a third cardiologist in case of disagreement. Cardiologists had knowledge of the clinical record, ECG, troponin results and objective testing from standard care |
| Roche Elecsys hs-cTnT | 5 | 14 | 10% at 13 | |||||
|
ADAPT/IMPACT (ACTRN12611001069943/ACTRN12611000206921) Nestelberger et al. 2019 171 |
Beckman Coulter ACCESS hs-cTnI | 2.3 | 18; females 12; males 20 | < 10% at 18 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | NR | Two independent cardiologists not directly involved in patient care reviewed all available medical records (i.e. history, physical examination, results of laboratory testing, including hs-cTnT concentrations, radiologic testing, electrocardiography, echocardiography, cardiac exercise test, lesion severity and morphology in coronary angiography, discharge summary) pertaining to the patient from the time of ED presentation to 30-day follow-up |
| Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | NSTEMI | ACC227 |
Conventional troponins were measured using Abbott Diagnostics TnI (LoD 10 ng/l, 99th centile 28 ng/l, CoV < 10% at 32 ng/l, decision threshold 30 ng/l) Timing: On presentation and at 2 hours and 6–12 hours |
Diagnoses on admission and at follow-up were independently adjudicated by one cardiologist, who was blinded to hs-cTnT results | |
| Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | AMI | Joint ESC, ACC, AHA and WHF9 |
Conventional troponins were measured using Abbott Diagnostics TnI 2 (LoD 10 ng/l, 99th centile 28 ng/l, CoV < 10% at 32 ng/l) Change (rise or fall) in troponin I 2, or no change but no clear alternative cause of troponin elevation, were considered indicative of AMI Timing: On presentation and at follow-up (6–24 hours) |
Final diagnoses were adjudicated independently by cardiologists, blinded to patient history and hs-cTnT | |
|
APACE (NCT00470587) Badertscher et al. 201854 Badertscher et al. 201855 Boeddinghaus et al. 2017 58 Boeddinghaus et al. 2019123 b Boeddinghaus et al. 2019 170 Jaeger et al. 2016 74 Kaier et al. 2017 75 Reichlin et al. 2015 90 Reichlin et al. 2015 91 Rubini Gimenez et al. 2014 70 Rubini Gimenez et al. 2015 92 Rubini Gimenez et al. 201593 Rubini Giménez et al. 2016 94 Twerenbold et al. 2017105 Twerenbold et al. 2017103 Twerenbold et al. 2017 104 Twerenbold et al. 2018 106 Twerenbold et al. 2018107 Twerenbold et al. 2019 108 Wildi et al. 2016111 Wildi et al. 2019113 |
Roche Elecsys hs-cTnT | 5 | 14 | 10% at 13 | NSTEMI; AMI; MACE | Third Universal Definition of Myocardial Infarction 43 | Myocardial necrosis was diagnosed by at least one conventional troponin value above the 99th centile together with a significant rising or falling | Adjudication of the final diagnosis was performed by two independent cardiologists at the core laboratory (University Hospital Basel, Basel, Switzerland), applying the universal definition of AMI by using two sets of data. First, all available medical records obtained during clinical care, including history, physical examination, results of laboratory testing [including serial clinical (hs)-Tn levels], radiological testing, electrocardiography, echocardiography, cardiac exercise test, lesion severity and morphology in coronary angiography, pertaining to the patient from the time of ED presentation to 90-day follow-up. Second, study-specific assessments, including detailed chest pain characteristics using 34 predefined criteria, serial hs-cTnT concentrations and clinical follow-up by telephone. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist |
| Abbott ARCHITECT hs-cTnI | 1.9 | 26.2 | < 5% at 1.9 | |||||
| Beckman Coulter Access hs-cTnI | 2.3 | 18 | < 5% at 18 | |||||
| Siemens Healthcare ADVIA Centaur hs-cTnI | 2.2 | 47 | < 5% at 47 | |||||
| Siemens Healthcare Dimension Vista hs-cTnI | 0.5 | 9 | 10% at 3 | |||||
| Ortho VITROS hs-cTnI | 0.4 | 11 | < 7% at 11 | |||||
| bioMérieux VIDAS hs-cTnI | 1.3–3.2 | 19 | 7% at 19 | |||||
|
BACC Neumann et al. 2016 84 Neumann et al. 201785 Neumann et al. 201786 |
Abbott ARCHITECT hs-cTnI | 1.9 | 27 | 10% at 5.2 | NSTEMI | ESC33 | Roche Elecsys hs-cTnT on admission and at 3 hours | The final diagnosis was adjudicated based on all available clinical and imaging results, electrocardiography and standard laboratory testing, including hs-cTnT. The final diagnosis of all patients was made by two cardiologists independently and disagreements were resolved by consultation with a third cardiologist |
|
BEST |
Roche Elecsys hs-cTnT | 5 | 14 (16 males, 9 females) | < 10% at 5 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | Roche Elecsys hs-cTnT on admission and at 3 hours | Outcomes were adjudicated by two independent investigators based on all available clinical data up to 30 days after presentation |
| Siemens ADVIA Centaur hs-cTnI | 1.6 | 47 | < 10% at 6 | |||||
| Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 9 | AMI | Joint ESC, ACC, AHA and WHF9 |
Rise or fall of cTnT, or both, above the 99th centile (10 ng/l) in the appropriate clinical context. For patients with modest elevations of cTnT (< 0.1 ng/ml) at baseline, an absolute difference of at least 20 ng/l on serial sampling was considered to represent a significant rise, fall, or both based on the analytical performance of the cTnT assay Timing: At least 12 hours after the onset of the most significant symptoms |
Two independent investigators who had all clinical, laboratory and imaging data available for review, but who were blinded to hs-cTnT levels | |
| Body et al. 2015 56 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 12 | AMI |
AMI was diagnosed on the basis of a rise and/or fall of cTnT above the 99th centile, with a minimum change between samples of 0.02 µg/l, in conjunction with the appropriate clinical context, imaging evidence of MI or ischemic ECG changes MACE within 30 days was defined as death, incident AMI or the need for coronary revascularisation, or if the treating cardiologist reported the presence of a coronary stenosis of > 50% |
Standard troponin T (cTnT, fourth generation Elecsys, Roche Diagnostics; 99th centile 0.01 µg/l, CoV < 10% at 0.035 µg/l, LoD 0.01 µg/l) at the time of arrival in the ED and 12 hours after symptom onset | The primary outcome of AMI was adjudicated by two independent investigators, with all clinical, laboratory and imaging data (including reference standard 12-hour cTnT concentrations) available for review, but blinded to investigational assay (hs-cTnT) results. Disagreements were resolved by discussion |
| Cappellini et al. 2019 62 | Roche Elecsys hs-cTnT | 5 | 14 | NR | NSTEMI | AMI in accordance with the Third Universal Definition of Myocardial Infarction43 | NR | Final diagnoses were made by the attending ED physician if participants were not hospitalised and by a physician of the specific medical unit in the case of hospitalisation, with cardiologist consultations when required |
| a Christ et al. 2010 150 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | AMI | Joint ESC, ACC, AHA and WHF9 |
Myocardial necrosis was diagnosed on the basis of a rising and/or falling cTnT pattern (> 20% or < 20% compared with the cTnT levels admission) with at least one value above the 99th centile and an imprecision of < 10%. Myocardial necrosis not related to AMI was defined as a typical rise and fall of cTnT levels without clinical evidence of CAD, and cardiac pain without necrosis was defined as a typical patient history and clinical signs of cardiac pain without increased levels of cTnT. UA was diagnosed when a patient had normal troponin levels and typical angina at rest or exercise, or a cardiac catheterisation result compatible with the diagnosis. cTnT cut-off level of 0.04 µg/l Timing: At presentation and about 6 hours at discretion of physician |
Two independent consultants |
|
CORE Borna et al. 2018 116 Mokhtari et al. 2016119 Mokhtari et al. 2016 121 Mokhtari et al. 2017 120 |
Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 14 | MACE |
MACEs were defined as an adjudicated diagnosis of AMI, UA, cardiac arrest, cardiogenic shock, ventricular arrhythmia requiring intervention, high-degree atrioventricular block requiring intervention, or death from a cardiac or unknown cause AMI was defined in accordance with the universal definition, requiring a significant increase or decrease of hs-cTnT levels, with at least one value above the 99th centile, combined with symptoms or signs of cardiac ischaemia |
Roche Elecsys hs-cTnT | MACEs were independently adjudicated by two clinicians (internal medicine and cardiology, and emergency medicine), blinded to each other’s assessments and hs-cTnT results. Disagreements were resolved by consultation with two or three cardiologists |
|
FASTER I and FAST II |
Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | NSTEMI | Joint ESC, ACC, AHA and WHF9 |
cTnI (Stratus CS, Siemens Healthcare Diagnostics, Deerfield, IL, USA). Non-STEMI defined as cTnI above the 99th centile of 0.07 µg/l at least at one measurement together with a ≥ 20% rise and/or fall and an absolute change ≥ 0.0 µg/l within 24 hours. To allow for the calculation of relative changes, cTnI was set to 0.02 µg/l (i.e. a concentration below the lowest level of detection) when reported as 0.00 or 0.01 µg/l Timing: Eight time points during the first 24 hours following enrolment |
NR |
| Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 14 | AMI | Joint ESC, ACC, AHA and WHF9 |
cTnI (Siemens Healthcare Diagnostica Inc., Newark, NJ, USA or Access analyser Beckman Coulter Inc., Brea, CA, USA) Threshold for Siemens assay 140 ng/l and CoV ≤ 10% Threshold for Beckman assay 60 ng/l, CV 10% Timing: On presentation and at 3–9 hours if needed |
Two independent ED physicians who were blinded to hs-cTnT results. Disagreements were adjudicated by a third ED physician | |
|
High-STEACS Bularga et al. 2019 61 Chapman et al. 2017 65 Miller-Hodges et al. 2018 79 Shah et al. 201598 |
Abbott ARCHITECT hs-cTnI | 2 | 16 (females), 34 (males) | 10% at 4.7 | NSTEMI; MACE | Third Universal Definition of Myocardial Infarction 43 | NR | MI was independently adjudicated by two clinicians, based on all clinical information and serial cTn measurements, in accordance with the third universal definition of MI. MI and death after discharge were also independently adjudicated by two clinicians. Any disagreements were resolved by consultation with a third clinician |
| Siemens Healthcare Atellica hs-cTnI | 1.6 | 34 (females), 53 (males) | NR | |||||
|
High-US Nowak et al. 2019128 Nowak et al. 2019129 Sandoval et al. 2019 176 |
Siemens Healthcare Atellica hs-cTnI | NR | 45 | 20% at 1.6 | NSTEMI |
Third Universal Definition of Myocardial Infarction 43 30-day MACE: AMI or death, including index MI, within 30 days |
Local hospital standard cTn results, including both the manufacturers’ package and locally established cTn cut-off points where applicable. Assays varied across the participating sites [Abbott ARCHITECT STAT Troponin-I, seven sites; Abbott iSTAT POC Cardiac Troponin I (Abbott Laboratories, Abbott Park, IL, USA), five sites; Siemens ADVIA Centaur TnI-Ultra (Siemens Healthcare, Erlangen, Germany), six sites; Beckman Coulter Accutane (Beckman Coulter, Brea, CA, USA), two sites; Beckman Coulter AccuTnI+3 (Beckman Coulter, Brea, CA, USA), one site; Siemens Dimension Vista® LOCI® CTNI (Siemens Healthcare, Erlangen, Germany), four sites; Siemens Dimension® EXLTM LOCI® TNI (Siemens Healthcare, Erlangen, Germany), two sites; Ortho Clinical Diagnostics VITROS Troponin I ES (Ortho Clinical Diagnostics, Marlow, UK), three sites; Roche Cardiac Troponin T, Gen 4, 8 series (Roche, Basel, Switzerland); Siemens Stratus® CS High-sensitivity Troponin I (Siemens Healthcare Diagnostics, Deerfield, IL, USA), one site] | Each case was adjudicated by a unique combination of five adjudicators, with a majority rule applied to determine the final MI classification. The adjudicators were blinded to the investigational Atellica IM and ADVIA Centaur hs-cTnI results and patient diagnosis established by the treating hospital. Each adjudicator independently used their expert opinion to assess whether the requirements of an MI diagnosis were met |
| Siemens Healthcare ADVIA Centaur hs-cTnI | NR | 47 | 20% at 2.5 | |||||
|
Huang et al. 2015 72 Guangquan et al. 201673 |
Roche Elecsys hs-cTnT | 3 | 14 | 10% at 13 | AMI; NSTEMI | AMI in accordance with guidelines by Thygesen et al.43 |
Conventional cTnT (fourth generation). Diagnosis of AMI, either NSTEMI or STEMI, required a conventional cTnT above 99th centile together with at least two of the following: symptoms of ischaemia, new ST-T changes or a new Q wave on the ECG and imaging showing new loss of viable myocardium Timing: At presentation and repeated after 6 to 9 hours at the discretion of the physician in charge |
Final diagnosis was adjudicated by both emergency physician and cardiologist from the time of enrolment to discharge. A third cardiologist refereed in situations of disagreement |
| Abbott ARCHITECT hs-cTnI STAT | 3.4 | 24–30 for this study population | 10% at 5.2 | AMI | Joint ESC, ACC, AHA and WHF9 |
Conventional serial troponin T or I (no further details) Timing: On presentation and at 3 and 6 hours |
Final diagnosis adjudicated by two independent cardiologists, with disagreements referred to a third cardiologist. All three were blinded to hs-cTnI results | |
| a Kurz et al. 2011 148 | Roche Elecsys hs-cTnT | 3 | 13.5 | 8% at 10 | NSTEMI | Joint ESC, ACC, AHA and WHF9 |
Fourth generation cTnT (Roche Elecsys, Mannheim, Germany): LoD 10 ng/l, diagnostic threshold 30 ng/l Diagnosis of NSTEMI required elevated cTnT concentration in at least one of the consecutive samples collected within 24 hours of the index event Timing: On presentation, at 6 hours and at least one sample between presentation and 6 hours |
NR |
| Lin et al. 2019 117 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | MACE | MACE was defined as any of the following: cardiac death; ventricular fibrillation; MI; critical stenosis found on coronary angiography (≥ 50% for the left main coronary artery stenosis or ≥ 70% for epicardial vessel stenosis); and emergency cardiac revascularisation procedures (e.g. coronary artery bypass graft, percutaneous coronary intervention) | Roche Elecsys hs-cTnT | MACEs were independently adjudicated by an emergency medicine attending physician and an attending cardiologist based on the case records, which included investigation results and data on troponin collected during the index visit and up to 1 year of follow-up. Disagreements were resolved by consensus |
| Roche Elecsys hs-cTnT | 2 | 14 | < 10% at 13 | NSTEMI | Joint ESC, ACC, AHA and WHF9 |
Conventional troponin Roche fourth generation cTnT (LoD 10 ng/l, 10% CoV at 35 ng/l) or Beckman Coulter Access Accutane (LoD 10 ng/l, 99th centile 40 ng/l, CoV < 10% at 60 ng/l) Timing: On presentation and 9–12 hours later |
Final diagnosis determined by the individual cardiologist and then adjudicated by two independent evaluators. All three were blinded to hs-cTnT results | |
|
Peacock et al. 2018 89 Chang et al. 2018 124 |
Roche Elecsys hs-cTnT, STAT | 6 | 19 | NR | AMI; MACE |
Third Universal Definition of Myocardial Infarction 43 MACE included all post-discharge death, AMI or urgent myocardial revascularisation |
NR, presentation and at 3 hours, 6–9 hours and 12–24 hours | An independent clinical events committee, made up of two cardiologists and one emergency physician, adjudicated the rule-in AMI diagnosis. The clinical events committee had access to all clinical data (including the local troponin assay results), but was blinded to hs-cTnT results |
|
PITAGORAS |
Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 14 | MACE | MACE | NR | NR |
|
QUART Parsonage et al. 2013131 Parsonage et al. 2014 88 |
Roche Elecsys hs-cTnT | 5 | 14 | 10% at 13 | AMI | Third Universal Definition of Myocardial Infarction 43 | Local cTnI measurement at presentation and then 6 hours afterwards. The cTnI values, measured with the Access Accu-cTnI assay on a UniCel DxI 800 platform (Beckman Coulter, Brea, CA, USA) were used for adjudication. This assay had an LoD of 10 ng/l and imprecision giving a 10% CoV at 60 ng/l. The 99th centile of a healthy reference population was 40 ng/l | Final diagnoses were adjudicated independently by one of two cardiologists, with all ACS end points and 10% of non-ACS end points re-adjudicated by both cardiologists. Consensus was achieved for all end points |
|
RATPAC (point-of-care arm) |
Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | NSTEMI | Joint ESC, ACC, AHA and WHF9 |
Conventional troponins were measured using one of the following methods: Siemens cTnI Ultra (LoD 6 ng/l, 99th centile 40 ng/l, CoV 10% at 30 ng/l); Abbott cTnI (LoD 10 ng/l, 99th centile 12 ng/l, CoV 10% at 32 ng/l); Beckman Accutane (LoD 10 ng/l, 99th centile 40 ng/l, CoV 10% at 60 ng/l) and Roche cTnT (LoD 10 ng/l, 99th centile 10 ng/l, CV 10% at 30 ng/l) Timing: On presentation and at 10–12 hours |
An initial working diagnosis was recorded by the senior ED clinician and reviewed by two independent clinicians; all were blind to hs-cTnT results |
|
REACTION-US Nowak et al. 2018 87 Nowak et al. 2018127 |
Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | Siemens Centaur system TnI Ultra assay on a Centaur XP analyzer (99th centile 40 ng/l) | Adjudication of the final diagnosis of AMI was performed by a board-certified cardiologist and emergency physician working as a team, with additional review by another board-certified cardiologist in the event of disagreement. The adjudicating physicians were blinded to the hs-cTnT results |
|
ROMI-3 Kavasak et al. 201776 Shortt et al. 2017 101 |
Roche Elecsys hs-cTnT | 5 | 14 | 2.3% at 30 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | Abbott cTnI (LoD 10 ng/l, 99th centile 30 ng/l) | Outcome adjudication was led by an emergency physician and independently adjudicated by at least two other study authors. All adjudicators were blinded to the hs-cTn results |
| Abbott ARCHITECT hs-cTnI | 2 | 26 | 4.4–7.1% at 20 | |||||
| a Saenger et al. 2010 165 | Roche Elecsys hs-cTnT | NR | 14 | NR | AMI | AMI (unclear method) | NR | NR |
| a Sebbane et al. 2013 157 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | NSTEMI | Joint ESC, ACC, AHA and WHF9 |
cTnI measured using the Access2 analyser (Access Immunosystem, Beckman Instruments, Paris, France). The LoD was <10 ng/l and the decision threshold was 40 ng/l Timing: Convention cTn (cTnI) on presentation, 6 hours later and beyond as needed |
Two independent ED physicians, blinded to hs-cTnT results |
| Shiozaki et al. 2017 100 | Roche Elecsys hs-cTnT | 5 | 14 | NR | NSTEMI | Joint ESC and ACC guidelines | NR | Two senior cardiologists |
| Slagman et al. 2017 102 | Roche Elecsys hs-cTnT | 5 | 14 | 3.5% at 16 | NSTEMI | The end point (reference standard) of this study was a main hospital diagnosis of NSTEMI. Diagnoses were retrieved from the hospital information system as ICD-10 codes and were coded by treating physicians who had access to all available clinical information | Roche Elecsys hs-cTnT at 3 hours or troponin T at 6 hours | NR |
|
TRAPID-AMI Body et al. 2015122 Body et al. 2016 114 McCord et al. 2017126 Mueller et al. 2016 80 Mueller-Hennessen et al. 201681 Mueller-Hennessen et al. 2017 82 Mueller-Hennessen et al. 201983 |
Roche Elecsys hs-cTnT | 5 | 14 | 10% at 13 | AMI; NSTEMI; MACE | Third Universal Definition of Myocardial Infarction43 and ESC guidelines | s-cTnI ultra (ADVIA Centaur, 99th centile 40 ng/l) at baseline, 1 hour, 2 hours and 4–14 hours | Each patient was adjudicated by two independent cardiologists. Adjudicators reviewed all available medical records [i.e. patient history; physical examination results; results of laboratory testing, including levels of s-cTnI ultra, local cTn obtained before the first or after the last blood draw for the study if available, creatinine, cystatin C, free haemoglobin (to quantify haemolysis), and NT-proBNP; radiologic imaging; electrocardiography; echocardiography; cardiac stress test; and lesion severity and morphology in coronary angiography] pertaining to the patient from ED presentation to 30-day follow-up, blind to hs-cTnT. Discrepancies were solved by discussion with a third cardiologist |
|
TRUST Carlton et al. 2015 63 |
Roche Elecsys hs-cTnT | NR | 14 | < 10% at 9 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | Roche Elecsys hs-cTnT at presentation and after 6 hours | Adjudication of the end point was carried out by two local cardiologists blinded to all risk scores, but who had access to the clinical record, ECG results and serial high-sensitivity troponin T results |
| Abbott ARCHITECT hs-cTnI | 1.9 | 26.2 | 5% at 1.9 | |||||
|
TUSCA |
Roche Elecsys hs-cTnT | NR | 14 | 10% at 9.3 | NSTEMI | National Academy of Clinical Biochemistry and International Federation of Clinical Chemistry Committee228 |
Roche Elecsys hs-cTnT. NSTEMI was defined as cTnT > 10 ng/l and Δ cTnT > 20% Timing: 30 minutes after arrival and at 2, 4 and 6–8 hours or until discharge |
Final diagnosis was made by an adjudication committee |
|
UTROPIA Dodd et al. 2019125 Sandoval et al. 2017 95 Sandoval et al. 2017 96 |
Abbott ARCHITECT hs-cTnI | 1.9 |
Female: 16 Male: 34 |
5.3% at 15 | NSTEMI | Third Universal Definition of Myocardial Infarction 43 | Abbott ARCHITECT contemporary cTnI | Final diagnosis was adjudicated by two clinicians after review of all available medical records, including 12-lead ECG, echocardiography, angiography, hs-cTnI values and clinical presentation |
| Venge et al. 2017 110 | Abbott ARCHITECT hs-cTnI | NR | 26.2 | NR | AMI | Third Universal Definition of Myocardial Infarction 43 |
Roche Elecsys hs-cTnT, measured at a central laboratory Diagnosis of an MI required at least one troponin T result above the 99th centile upper reference limit Timing: Presentation and at 2–4 hours and 6–24 hours |
Final diagnosis was adjudicated by two independent cardiologists, with access to electrocardiography, clinical records and hospital standard TnT results. Disagreements were resolved by consultation with a third cardiologist |
| Study | Publication | Assay | Participants | Threshold (ng/l) | Target condition | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADAPT | Boeddinghaus et al. 201657 | Abbott ARCHITECT hs-cTnI | All | (< 6 at 0 hours AND 2 hours) AND Δ < 2 at 0 to 2 hours | NSTEMI | 254 | 325 | 2 | 713 | 99 (97 to 100) | 69 (66 to 72) |
| < 26.2 at 0 hours AND 2 hours | 150 | 65 | 12 | 967 | 93 (87 to 96) | 94 (92 to 95) | |||||
| Roche Elecsys hs-cTnT | (< 14 at 0 hours AND 2 hours) AND Δ < 4 at 0 to 2 hours | 140 | 233 | 5 | 775 | 97 (92 to 99) | 77 (74 to 79) | ||||
| Greenslade et al. 201571 | Abbott ARCHITECT hs-cTnI | < 2 at 0 hours | 182 | 979 | 0 | 251 | 100 (98 to 100) | 20 (18 to 23) | |||
| < 4 at 0 hours | 180 | 530 | 2 | 700 | 99 (96 to 100) | 57 (54 to 60) | |||||
| Cullen et al. 201468 | < 26.2 at 0 hours | 181 | 83 | 23 | 1284 | 89 (84 to 93) | 94 (93 to 95) | ||||
| < 26.2 at 0 hours AND 2 hours | 195 | 103 | 9 | 1264 | 96 (92 to 98) | 92 (91 to 94) | |||||
| < 26.2 at 2 hours | 94 | 1273 | 93 (92 to 94) | ||||||||
| Roche Elecsys hs-cTnT | < 14 at 0 hours | 185 | 262 | 19 | 1105 | 91 (86 to 94) | 81 (79 to 83) | ||||
| < 14 at 2 hours | 191 | 258 | 13 | 1109 | 94 (89 to 97) | 81 (79 to 83) | |||||
| < 14 at 0 hours AND 2 hours | 192 | 287 | 12 | 1080 | 94 (90 to 97) | 79 (77 to 81) | |||||
| Eggers et al. 201669 | Abbott ARCHITECT hs-cTnI | < 15.5 at 0 hours AND 2 hours | 221 | 497 | 4 | 902 | 98 (96 to 100) | 64 (62 to 67) | |||
| van der Linden et al. 2018109 | Abbott ARCHITECT hs-cTnI and Roche Elecsys hs-cTnT | < 4 at 0 hours AND < 9 at 0 hours | 403 | 1046 | 5 | 1083 | 99 (97 to 100) | 51 (49 to 53) | |||
| Cullen et al. 2013156 | Abbott ARCHITECT hs-cTnI | < 26.2 at 0 hours AND 2 hours | MACE | 227 | 96 | 20 | 1292 | 92 (88 to 95) | 93 (92 to 94) | ||
| ADAPT/IMPACT | Nestelberger et al. 2019171 | Beckman Coulter ACCESS hs-cTnI | (< 4 at 0 hours AND symptoms > 3 hours) OR (< 5 at 0 hours AND Δ < 5 at 0 to 2 hours) | NSTEMI | 86 | 197 | 2 | 995 | 98 (92 to 100) | 83 (81 to 86) | |
| APACE | Kaier et al. 201775 | Abbott ARCHITECT hs-cTnI | < 2 at 0 hours | 224 | 881 | 0 | 199 | 100 (99 to 100) | 18 (16 to 21) | ||
| Roche Elecsys hs-cTnT | < 5 at 0 hours | 218 | 763 | 1 | 326 | 100 (97 to 100) | 30 (27 to 33) | ||||
| Boeddinghaus et al. 201960 | Beckman Coulter ACCESS hs-cTnI | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 at 0 hours) OR (< 5 at 0 hours AND Δ < 4 at 0 to 1 hours) | 95 | 176 | 408 | 99 (94 to 100) | 70 (66 to 74) | ||||
| Boeddinghaus et al. 210758 | Abbott ARCHITECT hs-cTnI | < 2 at 0 hours | 451 | 1924 | 0 | 453 | 100 (99 to 100) | 19 (17 to 21) | |||
| < 5 at 0 hours | 438 | 874 | 13 | 1503 | 97 (95 to 98) | 63 (61 to 65) | |||||
| < 5 at 0 hours AND Δ < 2 at 0–1 hour | 444 | 925 | 7 | 1452 | 98 (97 to 99) | 61 (59 to 63) | |||||
| < 2 at 0 hours OR (< 5 at 0 hours AND Δ < 2 at 0 to 1 hours) | 921 | 1456 | 61 (59 to 63) | ||||||||
| Boeddinghaus et al. 201859 | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 at 0 hours) OR (< 5 at 0 hours AND Δ < 2 at 0 to 1 hours) | 112 | 195 | 2 | 355 | 98 (94 to 100) | 65 (60 to 69) | ||||
| Roche Elecsys hs-cTnT | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 at 0 hours) OR (< 12 at 0 hours AND Δ < 3 at 0 to 1 hours) | 113 | 169 | 1 | 381 | 99 (95 to 100) | 69 (65 to 73) | ||||
| Siemens ADVIA Centaur hs-cTnI | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 3 at 0 hours) OR (< 6 at 0 hours AND Δ < 3 at 0 to 1 hours) | 243 | 307 | 56 (52 to 60) | |||||||
| < 3 at 0 hours OR (< 8 at 0 hours AND Δ < 7 at 0 to 2 hours) | 61 | 100 | 0 | 200 | 100 (95 to 100) | 67 (61 to 72) | |||||
| Boeddinghaus et al. 2020173 | Quidel TriageTrue | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 4 at 0 hours) OR (< 5 at 0 hours AND Δ < 3 at 0 to 1 hours) | 88 | 155 | 0 | 302 | 100 (97 to 100) | 66 (62 to 70) | |||
| Twerenbold et al. 2019108 | Roche Elecsys hs-cTnT | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 at 0 hours) OR (< 12 at 0 hours AND Δ < 3 at 0 to 1 hours) | MACE | 228 | 648 | 3 | 1417 | 99 (96 to 100) | 69 (67 to 71) | ||
| NSTEMI | 224 | 652 | 0 | 1420 | 100 (99 to 100) | 69 (66 to 71) | |||||
| Twerenbold et al. 2017104 | Abbott ARCHITECT hs-cTnI | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 at 0 hours) OR (< 5 at 0 hours AND Δ < 2 at 0 to 1 hours) | 732 | 1628 | 8 | 1982 | 99 (98 to 100) | 55 (53 to 57) | |||
| Roche Elecsys hs-cTnT | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 at 0 hours) OR (< 12 at 0 hours AND Δ < 3 at 0 to 1 hours) | 741 | 1136 | 5 | 2468 | 68 (67 to 70) | |||||
| Rubini Giménez et al. 201694 | Female | < 14 at 0 hours | 116 | 156 | 11 | 593 | 91 (85 to 96) | 79 (76 to 82) | |||
| < 9 at 0 hours | 127 | 284 | 2 | 463 | 98 (95 to 100) | 62 (58 to 65) | |||||
| Male | < 14 at 0 hours | 313 | 325 | 32 | 1188 | 91 (87 to 94) | 79 (76 to 81) | ||||
| < 15.5 at 0 hours | 304 | 276 | 40 | 1238 | 88 (85 to 92) | 82 (80 to 84) | |||||
| Rubini Gimenez et al. 201470 | Abbott ARCHITECT hs-cTnI | All | < 26.2 at 0 hours | 287 | 132 | 112 | 1695 | 72 (67 to 76) | 93 (91 to 94) | ||
| Roche Elecsys hs-cTnT | < 14 at 0 hours | 367 | 387 | 32 | 1440 | 92 (89 to 94) | 79 (77 to 81) | ||||
| Reichlin et al. 201590 | (< 14 at 0 hours AND 2 hours) AND Δ < 4 at 0 to 2 hours | 188 | 277 | 1 | 682 | 99 (97 to 100) | 71 (68 to 74) | ||||
| Reichlin et al. 201591 | < 12 at 0 hours AND Δ < 3 at 0 to 1 hours | 228 | 306 | 785 | 100 (98 to 100) | 72 (69 to 75) | |||||
| Rubini Gimenez et al. 201592 | Abbott ARCHITECT hs-cTnI | < 5 at 0 hours AND Δ < 2 at 0–1 hour | 163 | 285 | 2 | 455 | 99 (96 to 100) | 61 (58 to 65) | |||
| Boeddinghaus et al. 2019170 | Ortho VITROS hs-cTnI | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 1 at 0 hours) OR (< 2 at 0 hours AND Δ < 1 at 0 to 1 hours) | 61 | 184 | 0 | 275 | 100 (95 to 100) | 60 (55 to 64) | |||
| Cullen et al. 2013156 | Abbott ARCHITECT hs-cTnI | < 26.2 at 0 hours AND 2 hours | MACE | 129 | 62 | 27 | 691 | 83 (76 to 88) | 92 (90 to 94) | ||
| Lindahl et al. 2017132 | bioMérieux VIDAS hs-cTnI | < 2 at 0 hours OR (< 6 at 0 hours AND 2 hours) | NSTEMI | 85 | 184 | 2 | 321 | 98 (92 to 100) | 64 (59 to 68) | ||
| Reichlin et al. 2009167 | Abbott ARCHITECT hs-cTnI | ≤ 10 at 0 hours | AMI | 116 | 77 | 7 | 518 | 94 (89 to 98) | 87 (84 to 90) | ||
| Roche Elecsys hs-cTnT | ≤ 2 at 0 hours | 123 | 512 | 0 | 83 | 100 (98 to 100) | 14 (11 to 17) | ||||
| Reiter et al. 2011146 | > 70 years | < 14 at 0 hours | 96 | 157 | 2 | 151 | 98 (93 to 100) | 49 (43 to 55) | |||
| < 5 at 0 hours | 98 | 305 | 0 | 3 | 100 (97 to 100) | 1 (0 to 3) | |||||
| ≤ 70 years | < 14 at 0 hours | 54 | 87 | 7 | 533 | 89 (78 to 95) | 86 (83 to 89) | ||||
| Potocki et al. 2012140 | With pre-existing CAD | 73 | 142 | 5 | 213 | 94 (86 to 98) | 60 (55 to 65) | ||||
| Without pre-existing CAD | 100 | 114 | 6 | 517 | 94 (88 to 98) | 82 (79 to 85) | |||||
| Hochholzer 2011149 | All | < 11 at 0 hours | 129 | 177 | 3 | 454 | 98 (94 to 100) | 72 (68 to 75) | |||
| NSTEMI | 90 | 97 (91 to 99) | 72 (68 to 75) | ||||||||
| Reichlin et al. 2011145 | Δ 30% at 0–2 hours | 43 | 84 | 24 | 439 | 64 (52 to 76) | 84 (81 to 87) | ||||
| APACE | Twerenbold et al. 2018106 | Abbott ARCHITECT hs-cTnI | Normal renal function | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 2 at 0 hours) OR (< 5 at 0 hours AND Δ < 2 at 0 to 1 hours) | 326 | 730 | 4 | 1444 | 99 (97 to 100) | 66 (64 to 68) | |
| Renal dysfunction (eGFR < 60 ml/minute/1.73 m2) | 141 | 227 | 2 | 75 | 99 (95 to 100) | 25 (20 to 30) | |||||
| Roche Elecsys hs-cTnT | Normal renal function | ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 at 0 hours) OR (< 12 at 0 hours AND Δ < 3 at 0 to 1 hours) | 360 | 528 | 4 | 1875 | 99 (97 to 100) | 78 (76 to 80) | |||
| Renal dysfunction (eGFR < 60 ml/minute/1.73 m2) | 150 | 249 | 0 | 88 | 100 (98 to 100) | 26 (22 to 31) | |||||
| Jaeger et al. 201674 | Siemens Dimension Vista hs-cTnI | All | < 5 at 0 hours AND Δ < 2 at 0–1 hour | 98 | 224 | 428 | 100 (97 to 100) | 66 (62 to 69) | |||
| Female | 25 | 57 | 152 | 100 (89 to 100) | 73 (66 to 79) | ||||||
| Male | 72 | 168 | 4 | 272 | 95 (87 to 99) | 62 (57 to 66) | |||||
| Hoeller et al. 2011168 | Abbott ARCHITECT hs-cTnI | All | < 26.2 at 0 hours | AMI | 240 | 93 | 71 | 1163 | 77 (72 to 82) | 93 (91 to 94) | |
| Roche Elecsys hs-cTnT | < 14 at 0 hours | 398 | 363 | 46 | 1265 | 90 (86 to 92) | 78 (76 to 80) | ||||
| BACC | Neumann et al. 201684 | Abbott ARCHITECT hs-cTnI | ≤ 27 at 0 hours AND 3 hours | NSTEMI | 161 | 74 | 23 | 725 | 88 (82 to 92) | 91 (89 to 93) | |
| ≤ 6 at 0 hours | 170 | 312 | 14 | 487 | 92 (88 to 96) | 61 (57 to 64) | |||||
| ≤ 6 at 0 hours AND 1 hour | 180 | 373 | 4 | 426 | 98 (95 to 99) | 53 (50 to 57) | |||||
| ≤ 6 at 0 hours AND 3 hours | 182 | 402 | 2 | 397 | 99 (96 to 100) | 50 (46 to 53) | |||||
| ≤ 27 at 0 hours AND 1 hour | 143 | 59 | 41 | 740 | 78 (71 to 84) | 93 (91 to 94) | |||||
| BEST | Body et al. 2019115 | Roche Elecsys hs-cTnT | < 5 at 0 hours | 76 | 313 | 1 | 275 | 99 (93 to 100) | 47 (43 to 51) | ||
| Body et al. 2020172 | Siemens ADVIA Centaur hs-cTnI | < 3 at 0 hours | 131 | 580 | 287 | 99 (96 to 100) | 33 (30 to 36) | ||||
| Body 2015 | Body et al. 201556 | Roche Elecsys hs-cTnT | < 14 at 0 hours | AMI | 75 | 106 | 4 | 278 | 95 (88 to 99) | 72 (68 to 77) | |
| MACE | 88 | 92 | 10 | 272 | 90 (82 to 95) | 75 (70 to 79) | |||||
| < 3 at 0 hours | AMI | 79 | 360 | 0 | 24 | 100 (96 to 100) | 6 (4 to 9) | ||||
| MACE | 99 | 352 | 13 | 100 (97 to 100) | 4 (2 to 6) | ||||||
| < 5 at 0 hours | AMI | 78 | 289 | 1 | 95 | 99 (93 to 100) | 25 (21 to 29) | ||||
| MACE | 97 | 270 | 99 (94 to 100) | 26 (22 to 31) | |||||||
| Cappellini 2019 | Cappellini et al. 201962 | < 14 at 0 hours AND Δ ≤ 4 at 0–3 hours | NSTEMI | 473 | 3178 | 2 | 2758 | 100 (98 to 100) | 46 (45 to 48) | ||
| All | < 14 at 0 hours AND Δ ≤ 3 at 0–1 hour | 471 | 3284 | 4 | 2652 | 99 (98 to 100) | 45 (43 to 46) | ||||
| Female | 189 | 1560 | 0 | 1109 | 100 (98 to 100) | 42 (40 to 43) | |||||
| < 14 at 0 hours AND Δ ≤ 4 at 0–3 hours | 1496 | 1173 | 44 (42 to 46) | ||||||||
| Male | < 14 at 0 hours AND Δ ≤ 3 at 0–1 hour | 282 | 1702 | 4 | 1565 | 99 (96 to 100) | 48 (46 to 50) | ||||
| < 14 at 0 hours AND Δ ≤ 4 at 0–3 hours | 285 | 1714 | 1 | 1553 | 100 (98 to 100) | 48 (46 to 49) | |||||
| CORE | Borna et al. 2018116 | All | ≤ 14 at 0 hours AND 2 hours | MACE | 78 | 152 | 12 | 509 | 87 (78 to 93) | 77 (74 to 80) | |
| Mokhtari et al. 2017120 | < 5 at 0 hours OR (< 12 at 0 hours AND Δ < 3 at 0 to 1 hours) | 117 | 471 | 2 | 430 | 98 (94 to 100) | 48 (44 to 51) | ||||
| Mokhtari et al. 2016121 | < 5 at 0 hours | 121 | 674 | 4 | 339 | 97 (92 to 99) | 33 (31 to 36) | ||||
| ≤ 14 at 0 hours | 93 | 206 | 32 | 807 | 74 (66 to 82) | 80 (77 to 82) | |||||
| < 12 at 0 hours AND Δ < 3 at 0–1 hour | 117 | 163 | 2 | 146 | 98 (94 to 100) | 47 (42 to 53) | |||||
| High-STEACS | Bularga et al. 201961 | Abbott ARCHITECT hs-cTnI | < 2 at 0 hours | 4289 | 27,857 | 24 | 14,931 | 99 (99 to 100) | 35 (34 to 35) | ||
| < 5 at 0 hours | 4215 | 15,386 | 98 | 27,402 | 98 (97 to 98) | 64 (64 to 64) | |||||
| Analysis population (excluding patients with cTn > 99th centile at presentation, presenting ≤ 2 hours from symptom onset, with STEMI, with missing presentation hs-cTnI) | < 2 at 0 hours | 502 | 19,619 | 15 | 12,701 | 97 (95 to 98) | 39 (39 to 40) | ||||
| < 5 at 0 hours | 462 | 9115 | 55 | 23,205 | 89 (86 to 92) | 72 (71 to 72) | |||||
| Chapman et al. 2020174 | Roche Elecsys hs-cTnT | All | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Chapman et al. 201967 | Siemens Atellica hs-cTnI | ESC 0/1-hour pathway: (symptoms ≥ 3 hours AND < 3 at 0 hours) OR (< 6 at 0 hours AND Δ < 3 at 0 to 1 hours) | NSTEMI | 29 | 115 | 2 | 260 | 94 (79 to 99) | 69 (64 to 74) | ||
| ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 34 (F) ≥ 53 (M) at 0 hours] OR [≥ 34 (F) ≥ 53 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 252 | 420 | 25 | 1223 | 91 (87 to 94) | 74 (72 to 77) | |||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 34 (F) ≤ 53 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 272 | 430 | 6 | 1212 | 98 (95 to 99) | 74 (72 to 76) | |||||
| Chapman et al. 201866 | Abbott ARCHITECT hs-cTnI | ESC 0/1-hour pathway: (symptoms ≥ 3 hours AND < 3 at 0 hours) OR (< 6 at 0 hours AND Δ < 3 at 0 to 1 hours) | 33 | 83 | 0 | 290 | 100 (91 to 100) | 78 (73 to 82) | |||
| ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | MACE | 327 | 231 | 49 | 1279 | 87 (83 to 90) | 85 (83 to 86) | ||||
| NSTEMI | 244 | 314 | 27 | 1301 | 90 (86 to 93) | 81 (79 to 82) | |||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | MACE | 378 | 295 | 6 | 1238 | 98 (97 to 99) | 81 (79 to 83) | ||||
| NSTEMI | 273 | 400 | 2 | 1242 | 99 (97 to 100) | 76 (73 to 78) | |||||
| Chapman et al. 201765 | Age < 65 years | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 72 | 29 | 7 | 593 | 91 (83 to 96) | 95 (93 to 97) | |||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 78 | 39 | 1 | 583 | 99 (93 to 100) | 94 (92 to 96) | |||||
| Age ≥ 65 years | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 99 | 57 | 13 | 348 | 88 (81 to 94) | 86 (82 to 89) | ||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 109 | 88 | 3 | 317 | 97 (92 to 99) | 78 (74 to 82) | |||||
| Female | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 61 | 48 | 5 | 362 | 92 (83 to 97) | 88 (85 to 91) | ||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 65 | 54 | 1 | 356 | 98 (92 to 100) | 87 (83 to 90) | |||||
| Known ischaemic heart disease | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 73 | 52 | 16 | 377 | 82 (72 to 89) | 88 (84 to 91) | ||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 85 | 77 | 4 | 352 | 96 (89 to 99) | 82 (78 to 86) | |||||
| Male | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 110 | 38 | 15 | 579 | 88 (81 to 93) | 94 (92 to 96) | ||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 122 | 73 | 3 | 544 | 98 (93 to 100) | 88 (85 to 91) | |||||
| No known ischaemic heart disease | ESC 0/3-hour pathway: [symptoms ≥ 6 hours AND ≤ 16 (F) ≤ 34 (M) at 0 hours] OR [≤ 16 (F) ≤ 34 (M) at 3 hours] OR Δ < 50% of 99th centile at 0 to 3 hours | 95 | 33 | 4 | 548 | 96 (90 to 99) | 94 (92 to 96) | ||||
| High-STEACS pathway: (symptoms ≥ 2 hours AND < 5 at 0 hours) OR [≤ 16 (F) ≤ 34 (M) at 3 hours AND Δ < 3 at 0 to 3 hours] | 99 | 48 | 0 | 533 | 100 (97 to 100) | 92 (89 to 94) | |||||
| Miller-Hodges et al. 201879 | Female patients with an eGFR < 60 ml/minute/1.73 m2 | < 16 at 0 hours | 105 | 121 | 1 | 243 | 99 (95 to 100) | 67 (62 to 72) | |||
| Female patients with an eGFR ≥ 60 ml/minute/1.73 m2 | 160 | 156 | 1269 | 99 (97 to 100) | 89 (87 to 91) | ||||||
| Male patients with an eGFR < 60 ml/minute/1.73 m2 | < 34 at 0 hours | 98 | 82 | 2 | 252 | 98 (93 to 100) | 75 (70 to 80) | ||||
| Male patients with an eGFR ≥ 60 ml/minute/1.73 m2 | 280 | 109 | 4 | 1843 | 99 (96 to 100) | 94 (93 to 95) | |||||
| Patients aged < 65 years with an eGFR < 60 ml/minute/1.73 m2 | < 16 (females), < 34 (males) at 0 hours | 23 | 17 | 0 | 76 | 100 (88 to 100) | 82 (72 to 89) | ||||
| Patients aged < 65 years with an eGFR ≥ 60 ml/minute/1.73 m2 | 197 | 75 | 1 | 1926 | 99 (97 to 100) | 96 (95 to 97) | |||||
| Patients aged ≥ 65 years with an eGFR < 60 ml/minute/1.73 m2 | 180 | 186 | 3 | 419 | 98 (95 to 100) | 69 (65 to 73) | |||||
| Patients aged ≥ 65 years with an eGFR ≥ 60 ml/minute/1.73 m2 | 243 | 190 | 4 | 1186 | 98 (96 to 100) | 86 (84 to 88) | |||||
| Patients with an eGFR < 60 ml/minute/1.73 m2 | < 1.2 at 0 hours | MACE | 224 | 661 | 0 | 19 | 100 (99 to 100) | 3 (2 to 4) | |||
| < 16 (females), < 34 (males) at 0 hours | NSTEMI | 203 | 203 | 3 | 495 | 99 (96 to 100) | 71 (67 to 74) | ||||
| < 5 at 0 hours | MACE | 222 | 525 | 2 | 155 | 99 (97 to 100) | 23 (20 to 26) | ||||
| Patients with an eGFR ≥ 60 ml/minute/1.73 m2 | < 1.2 at 0 hours | 455 | 2739 | 3 | 625 | 99 (98 to 100) | 19 (17 to 20) | ||||
| < 16 (females), < 34 (males) at 0 hours | NSTEMI | 440 | 265 | 5 | 3112 | 99 (97 to 100) | 92 (91 to 93) | ||||
| < 5 at 0 hours | MACE | 451 | 1227 | 7 | 2137 | 98 (97 to 99) | 64 (62 to 65) | ||||
| High-US | Sandoval et al. 2019176 | Siemens ADVIA Centaur hs-cTnI | All | < 2 at 0 hours | 276 | 1481 | 1 | 454 | 100 (98 to 100) | 23 (22 to 25) | |
| NSTEMI | 259 | 1498 | 0 | 455 | 100 (99 to 100) | 23 (21 to 25) | |||||
| < 3 at 0 hours | MACE | 274 | 1248 | 3 | 687 | 99 (97 to 100) | 36 (33 to 38) | ||||
| NSTEMI | 257 | 1265 | 2 | 688 | 35 (33 to 37) | ||||||
| < 5 at 0 hours | MACE | 273 | 924 | 4 | 1011 | 99 (96 to 100) | 52 (50 to 54) | ||||
| NSTEMI | 257 | 940 | 2 | 1013 | 99 (97 to 100) | 52 (50 to 54) | |||||
| Siemens Atellica hs-cTnI | < 2 at 0 hours | MACE | 275 | 1432 | 503 | 26 (24 to 28) | |||||
| NSTEMI | 258 | 1449 | 1 | 504 | 100 (98 to 100) | 26 (24 to 28) | |||||
| < 3 at 0 hours | MACE | 273 | 1207 | 4 | 728 | 99 (96 to 100) | 38 (35 to 40) | ||||
| NSTEMI | 256 | 1224 | 3 | 729 | 99 (97 to 100) | 37 (35 to 40) | |||||
| < 5 at 0 hours | MACE | 274 | 899 | 4 | 1036 | 99 (96 to 100) | 54 (51 to 56) | ||||
| NSTEMI | 256 | 916 | 3 | 1037 | 99 (97 to 100) | 53 (51 to 55) | |||||
| Huang et al. 201572 | Huang et al. 201572 | Roche Elecsys hs-cTnT | ≤ 14 at 0 hours | AMI | 1064 | 331 | 44 | 810 | 96 (95 to 97) | 71 (68 to 74) | |
| NSTEMI | 308 | 13 | 96 (93 to 98) | 71 (68 to 74) | |||||||
| Patients with an eGFR ≥ 90 ml/minute/1.73 m2 | AMI | 363 | 70 | 19 | 367 | 95 (92 to 97) | 84 (80 to 87) | ||||
| NSTEMI | 59 | 5 | 370 | 92 (83 to 97) | 84 (80 to 87) | ||||||
| Patients with an eGFR of 30–59 ml/minute/1.73 m2 | AMI | 197 | 87 | 2 | 75 | 99 (96 to 100) | 46 (38 to 54) | ||||
| NSTEMI | 78 | 86 | 0 | 77 | 100 (96 to 100) | 47 (39 to 55) | |||||
| Patients with an eGFR of 60–89 ml/minute/1.73 m2 | AMI | 462 | 148 | 19 | 362 | 96 (94 to 98) | 71 (67 to 75) | ||||
| NSTEMI | 156 | 142 | 7 | 364 | 96 (91 to 98) | 72 (68 to 76) | |||||
| Patients with an eGFR < 30 ml/minute/1.73 m2 | AMI | 46 | 28 | 0 | 4 | 100 (94 to 100) | 13 (4 to 29) | ||||
| NSTEMI | 16 | 100 (83 to 100) | 13 (4 to 29) | ||||||||
| Lin et al. 2019117 | Lin et al. 2019117 | All | < 10 at 0 hours | MACE | 165 | 328 | 108 | 1843 | 60 (54 to 66) | 85 (83 to 86) | |
| < 20 at 2 hours | 163 | 161 | 110 | 2010 | 93 (91 to 94) | ||||||
| < 5 at 0 hours AND 2 hours | 185 | 367 | 88 | 1804 | 68 (62 to 73) | 83 (81 to 85) | |||||
| Δ < 10 at 0–2 hours | 115 | 63 | 158 | 2108 | 42 (36 to 48) | 97 (96 to 98) | |||||
| Peacock et al. 2018 | Chang et al. 2018124 | Roche Elecsys hs-cTnT STAT | < 19 at 0 hours | AMI | 125 | 164 | 8 | 1058 | 94 (88 to 97) | 87 (85 to 88) | |
| Δ ≤ 10% at 0–3 hours AND < 19 at 3 hours | 129 | 549 | 4 | 673 | 97 (92 to 99) | 55 (52 to 58) | |||||
| Δ ≤ 2 at 0–3 hours AND < 19 at 3 hours | 127 | 263 | 6 | 959 | 95 (90 to 98) | 78 (76 to 81) | |||||
| Δ ≤ 50% at 0–3 hours AND < 19 at 3 hours | 125 | 187 | 8 | 1035 | 94 (88 to 97) | 85 (83 to 87) | |||||
| Δ ≤ 8 at 0–3 hours AND < 19 at 3 hours | 169 | 1053 | 86 (84 to 88) | ||||||||
| Peacock et al. 201989 | < 19 at 0 hours AND 3 hours | 178 | 1044 | 85 (83 to 87) | |||||||
| MACE | 8 | 282 | 7 | 967 | 53 (27 to 79) | 77 (75 to 80) | |||||
| < 6 at 0 hours AND 3 hours | AMI | 131 | 610 | 2 | 612 | 98 (95 to 100) | 50 (47 to 53) | ||||
| MACE | 11 | 694 | 4 | 555 | 73 (45 to 92) | 44 (42 to 47) | |||||
| QUART | Parsonage et al. 201488 | Roche Elecsys hs-cTnT | ≤ 14 at 0 hours | AMI | 52 | 113 | 595 | 93 (83 to 98) | 84 (81 to 87) | ||
| ≤ 14 at 2 hours | 54 | 116 | 2 | 592 | 96 (88 to 100) | 84 (81 to 86) | |||||
| ≤ 14 at 0 hours OR 2 hours | 123 | 585 | 83 (80 to 85) | ||||||||
| REACTION-US | Nowak et al. 201887 | < 6 at 0 hours | NSTEMI | 44 | 361 | 0 | 164 | 100 (93 to 100) | 31 (27 to 35) | ||
| < 8 at 0 hours AND Δ < 3 at 0–0.5 hours | 274 | 221 | 45 (40 to 49) | ||||||||
| ROMI-3 | Shortt et al. 2017101 | Abbott ARCHITECT hs-cTnI | < 1 at 0 hours | 132 | 920 | 1 | 84 | 99 (96 to 100) | 8 (7 to 10) | ||
| < 15 at 0 hours | 110 | 216 | 23 | 788 | 83 (75 to 89) | 78 (76 to 81) | |||||
| < 2 at 0 hours | 132 | 846 | 1 | 158 | 99 (96 to 100) | 16 (14 to 18) | |||||
| < 26 at 0 hours | 96 | 105 | 37 | 899 | 72 (64 to 80) | 90 (87 to 91) | |||||
| < 3 at 0 hours | 132 | 691 | 1 | 313 | 99 (96 to 100) | 31 (28 to 34) | |||||
| < 4 at 0 hours | 131 | 586 | 2 | 418 | 98 (95 to 100) | 42 (39 to 45) | |||||
| < 5 at 0 hours | 129 | 504 | 4 | 500 | 97 (92 to 99) | 50 (47 to 53) | |||||
| Roche Elecsys hs-cTnT | < 12 at 0 hours | 126 | 476 | 7 | 528 | 95 (89 to 98) | 53 (49 to 56) | ||||
| < 14 at 0 hours | 123 | 417 | 10 | 587 | 92 (87 to 96) | 58 (55 to 62) | |||||
| < 24 at 0 hours | 108 | 229 | 25 | 775 | 81 (74 to 87) | 77 (74 to 80) | |||||
| < 3 at 0 hours | 132 | 891 | 1 | 113 | 99 (96 to 100) | 11 (9 to 13) | |||||
| < 5 at 0 hours | 824 | 180 | 18 (16 to 20) | ||||||||
| < 8 at 0 hours | 129 | 638 | 4 | 366 | 97 (92 to 99) | 36 (33 to 40) | |||||
| Shortt et al. 2017101 | Abbott ARCHITECT hs-cTnI | < 7 at 0 hours | 126 | 393 | 7 | 611 | 95 (89 to 98) | 61 (58 to 64) | |||
| Shiozaki et al. 2017100 | Shiozaki et al. 2017100 | Roche Elecsys hs-cTnT | < 13 at 0 hours | 57 | 246 | 0 | 110 | 100 (95 to 100) | 31 (26 to 36) | ||
| < 13 at 0 hours AND Δ < 3 at 0–1 hour | 120 | 236 | 66 (61 to 71) | ||||||||
| Slagman et al. 2017100 | Slagman et al. 2017102 | < 14 at 0 hours | 115 | 1086 | 9 | 2213 | 93 (87 to 97) | 67 (65 to 69) | |||
| TRAPID-AMI | Body et al. 2016114 | AMI | 189 | 198 | 24 | 871 | 89 (84 to 93) | 81 (79 to 84) | |||
| < 3 at 0 hours | 210 | 653 | 3 | 416 | 99 (96 to 100) | 39 (36 to 42) | |||||
| < 5 at 0 hours | 209 | 513 | 4 | 556 | 98 (95 to 99) | 52 (49 to 55) | |||||
| Mueller et al. 201680 | < 12 at 0 hours AND Δ < 3 at 0–1 hour | 206 | 263 | 7 | 806 | 97 (93 to 99) | 75 (73 to 78) | ||||
| NSTEMI | 185 | 96 (93 to 99) | 75 (73 to 78) | ||||||||
| Mueller-Hennessen et al. 2017229 | ≤ 14 at 0 hours AND Δ < 9.2 at 0–1 hour | AMI | 98 | 9 | 115 | 1060 | 46 (39 to 53) | 99 (98 to 100) | |||
| ≤ 14 at 0 hours AND Δ < 9.2 at 0–2 hours | 126 | 13 | 87 | 1056 | 59 (52 to 66) | 99 (98 to 99) | |||||
| ≤ 14 at 0 hours AND Δ < 20% at 0–1 hour | 83 | 28 | 130 | 1041 | 39 (32 to 46) | 97 (96 to 98) | |||||
| ≤ 14 at 0 hours AND Δ < 20% at 0–2 hours | 119 | 46 | 94 | 1023 | 56 (49 to 63) | 96 (94 to 97) | |||||
| Mueller-Hennessen et al. 201781 | Aged < 65 years | (≤ 14 at 0 hours AND 1 hour) AND Δ < 20% at 0 to 1 hours | MACE | 76 | 23 | 79 | 547 | 49 (41 to 57) | 96 (94 to 97) | ||
| Aged ≥ 65 years | 123 | 43 | 102 | 289 | 55 (48 to 61) | 87 (83 to 90) | |||||
| (≤ 28 at 0 hours AND 1 hour) AND Δ < 20% at 0 to 1 hours | 92 | 10 | 133 | 322 | 41 (34 to 48) | 97 (95 to 99) | |||||
| Female | (≤ 14 at 0 hours AND 1 hour) AND Δ < 20% at 0 to 1 hours | 62 | 17 | 37 | 361 | 63 (52 to 72) | 96 (93 to 97) | ||||
| (≤ 9 at 0 hours AND 1 hour) AND Δ < 20% at 0 to 1 hours | 71 | 37 | 28 | 341 | 72 (62 to 80) | 90 (87 to 93) | |||||
| Male | (≤ 14 at 0 hours AND 1 hour) AND Δ < 20% | 137 | 49 | 144 | 475 | 49 (43 to 55) | 91 (88 to 93) | ||||
| (≤ 15.5 at 0 hours AND 1 hour) AND Δ < 20% at 0 to 1 hours | 129 | 41 | 152 | 483 | 46 (40 to 52) | 92 (90 to 94) | |||||
| TRUST | Carlton et al. 201564 | Abbott ARCHITECT hs-cTnI | All | ≤ 26.2 at 0 hours | NSTEMI | 41 | 22 | 25 | 779 | 62 (49 to 74) | 97 (96 to 98) |
| Roche Elecsys hs-cTnT | ≤ 14 at 0 hours | 66 | 127 | 13 | 753 | 84 (74 to 91) | 86 (83 to 88) | ||||
| Carlton et al. 201563 | < 3 at 0 hours | MACE | 94 | 755 | 1 | 72 | 99 (94 to 100) | 9 (7 to 11) | |||
| NSTEMI | 78 | 771 | 0 | 73 | 100 (96 to 100) | 9 (7 to 11) | |||||
| < 5 at 0 hours | MACE | 92 | 560 | 3 | 267 | 97 (91 to 99) | 32 (29 to 36) | ||||
| NSTEMI | 78 | 574 | 0 | 270 | 100 (96 to 100) | 32 (29 to 35) | |||||
| UTROPIA | Sandoval et al. 201796 | Abbott ARCHITECT hs-cTnI | < 1.9 at 0 hours | 168 | 1018 | 2 | 443 | 99 (96 to 100) | 30 (28 to 33) | ||
| < 5 at 0 hours | 161 | 657 | 9 | 804 | 95 (90 to 98) | 55 (52 to 58) | |||||
| Sandoval et al. 201795 | Males < 34 at 0 hours AND females < 16 at 0 hours | 113 | 191 | 57 | 1270 | 66 (59 to 74) | 87 (85 to 89) | ||||
| Males < 34 AND females < 16 at 0 AND 3 hours | 104 | 137 | 5 | 822 | 95 (90 to 98) | 86 (83 to 88) | |||||
| Venge et al. 2017110 | Venge et al. 2017110 | < 26.2 at 0 hours | AMI | 46 | 28 | 18 | 325 | 72 (59 to 82) | 92 (89 to 95) | ||
| < 26.2 at 2–4 hours | 52 | 27 | 6 | 268 | 90 (79 to 96) | 91 (87 to 94) | |||||
| Aldous et al. 2011147 | Aldous et al. 2011147 | Roche Elecsys hs-cTnT | < 13 at 0 hours | 92 | 38 | 18 | 184 | 84 (75 to 90) | 83 (77 to 88) | ||
| < 14 at 0 hours | 36 | 186 | 84 (78 to 88) | ||||||||
| < 15 at 0 hours | 93 | 29 | 17 | 193 | 85 (76 to 91) | 87 (82 to 91) | |||||
| < 5 at 0 hours | 106 | 131 | 4 | 91 | 96 (91 to 99) | 41 (34 to 48) | |||||
| Aldous et al. 2012 | Aldous et al. 2011143 | Peak < 14 at 0–2 hours | NSTEMI | 189 | 149 | 11 | 590 | 95 (90 to 97) | 80 (77 to 83) | ||
| < 14 at 0–2 hours AND Δ < 20% at 0–2 hours | 99 | 43 | 101 | 696 | 50 (42 to 57) | 94 (92 to 96) | |||||
| < 14 at 0–2 hours OR Δ < 20% at 0–2 hours | 195 | 260 | 5 | 479 | 98 (94 to 99) | 65 (61 to 68) | |||||
| Aldous et al. 2012134 | < 14 at 0 hours | AMI | 74 | 54 | 8 | 249 | 90 (82 to 96) | 82 (77 to 86) | |||
| < 14 at 0 AND 2 hours | 78 | 74 | 4 | 229 | 95 (88 to 99) | 76 (70 to 80) | |||||
| < 14 at 0–1 hour | 77 | 63 | 5 | 240 | 94 (86 to 98) | 79 (74 to 84) | |||||
| < 14 at 0–2 hours | 78 | 67 | 4 | 236 | 95 (88 to 99) | 78 (73 to 82) | |||||
| < 14 at 0 hours AND Δ < 20% at 0–2 hours | 49 | 81 | 33 | 222 | 60 (48 to 70) | 73 (68 to 78) | |||||
| < 14 at 0 hours OR Δ < 20% at 0–2 hours | 81 | 131 | 1 | 172 | 99 (93 to 100) | 57 (51 to 62) | |||||
| Aldous et al. 2012139 | < 14 at 0 hours | NSTEMI | 181 | 134 | 24 | 600 | 88 (83 to 92) | 82 (79 to 84) | |||
| < 3 at 0 hours | 196 | 383 | 9 | 351 | 96 (92 to 98) | 48 (44 to 52) | |||||
| < 5 at 0 hours | 192 | 305 | 13 | 429 | 94 (89 to 97) | 58 (55 to 62) | |||||
| < 14 at 2 hours | 189 | 149 | 16 | 585 | 92 (88 to 95) | 80 (77 to 83) | |||||
| < 5 at 2 hours | 196 | 340 | 9 | 394 | 96 (92 to 98) | 54 (50 to 57) | |||||
| < 3 at 2 hours | 201 | 424 | 4 | 310 | 98 (95 to 99) | 42 (39 to 46) | |||||
| Body et al. 2011161 | Body et al. 2011161 | < 14 at 0 hours | AMI | 111 | 101 | 199 | 472 | 36 (30 to 41) | 82 (79 to 85) | ||
| < 3 at 0 hours | 130 | 378 | 0 | 195 | 100 (98 to 100) | 34 (30 to 38) | |||||
| Christ et al. 2010150 | Christ et al. 2010150 | < 14 at 0 hours | 19 | 45 | 1 | 72 | 95 (75 to 100) | 62 (52 to 70) | |||
| < 3 at 0 hours | 20 | 92 | 0 | 25 | 100 (86 to 100) | 21 (14 to 30) | |||||
| FASTER I and FAST II | Eggers et al. 2012137 | < 14 at 0 hours | NSTEMI | 101 | 59 | 27 | 173 | 79 (71 to 86) | 75 (68 to 80) | ||
| < 45.7 at 0 hours | 65 | 11 | 63 | 221 | 51 (42 to 60) | 95 (92 to 98) | |||||
| Freund et al. 2011142 | Freund et al. 2011142 | < 14 at 0 hours | AMI | 42 | 48 | 3 | 224 | 93 (82 to 99) | 82 (77 to 87) | ||
| Low/moderate pre-test probability | 20 | 36 | 2 | 200 | 91 (71 to 99) | 85 (80 to 89) | |||||
| High pre-test probability | 22 | 12 | 1 | 24 | 96 (78 to 100) | 67 (49 to 81) | |||||
| Keller et al. 2011141 | Keller et al. 2011141 | Abbott ARCHITECT hs-cTnI | All | < 3.4 | 282 | 633 | 0 | 345 | 100 (99 to 100) | 35 (32 to 38) | |
| 959 | 19 | 2 (1 to 3) | |||||||||
| < 3 | 232 | 77 | 50 | 901 | 82 (77 to 87) | 92 (90 to 94) | |||||
| < 30 | 277 | 94 | 5 | 884 | 98 (96 to 99) | 90 (88 to 92) | |||||
| Δ < 20% at 0–3 hours | 218 | 723 | 64 | 255 | 77 (72 to 82) | 26 (23 to 29) | |||||
| < 3.4 at 0 hours AND Δ < 20% at 0–3 hours | 254 | 454 | 54 | 498 | 82 (78 to 87) | 52 (49 to 56) | |||||
| < 30 at 3 hours AND Δ < 20% at 0–3 hours | 187 | 34 | 110 | 929 | 63 (57 to 68) | 96 (95 to 98) | |||||
| (< 30 at 0 AND 3 hours) AND Δ < 20% at 0 to 3 hours | 52 | 26 | 4 | 869 | 93 (83 to 98) | 97 (96 to 98) | |||||
| Kurz et al. 2011148 | Kurz et al. 2011148 | Roche Elecsys hs-cTnT | < 14 at 0 hours | NSTEMI | 16 | 7 | 10 | 24 | 62 (41 to 80) | 77 (59 to 90) | |
| < 9.5 at 0 hours | 38 | 11 | 8 | 37 | 83 (69 to 92) | 77 (63 to 88) | |||||
| < 14 at 0 AND 3 hours | 26 | 7 | 0 | 23 | 100 (89 to 100) | 77 (58 to 90) | |||||
| < 14 at 0 hours AND Δ <20% at 0–3 hours | 11 | 27 | 15 | 3 | 42 (23 to 63) | 10 (2 to 27) | |||||
| Melki et al. 2011144 | Melki et al. 2011144 | < 14 at 0 hours | 112 | 21 | 2 | 98 | 98 (94 to 100) | 82 (74 to 89) | |||
| < 14 at 2 hours | 114 | 25 | 0 | 94 | 100 (97 to 100) | 79 (71 to 86) | |||||
| PITAGORAS | Sanchis et al. 2012135 | < 3 at 0 hours | MACE | 53 | 207 | 9 | 177 | 85 (74 to 93) | 46 (41 to 51) | ||
| RATPAC (point-of-care arm) | Collinson et al. 2013159 | < 14 at 0 hours | NSTEMI | 33 | 14 | 733 | 79 (67 to 88) | 96 (94 to 97) | |||
| Peak < 14 at 0–1.5 hours | 57 | 43 | 11 | 736 | 84 (73 to 92) | 94 (93 to 96) | |||||
| Saenger et al. 2010165 | Saenger et al. 2010165 | < 14 at 0 hours | AMI | 92 | 38 | 6 | 152 | 94 (87 to 98) | 80 (74 to 85) | ||
| Δ < 8 at 0–3 hours | 94 | 9 | 4 | 181 | 96 (90 to 99) | 95 (91 to 98) | |||||
| Sebbane et al. 2013157 | Sebbane et al. 2013157 | < 14 at 0 hours | NSTEMI | 19 | 25 | 6 | 142 | 76 (55 to 91) | 85 (79 to 90) | ||
| < 18 at 0 hours | 17 | 150 | 90 (84 to 94) | ||||||||
| TUSCA | Santaló et al. 2013133 | < 14 at 0 hours | 71 | 80 | 8 | 199 | 90 (81 to 96) | 71 (66 to 77) |
Appendix 3 QUADAS-2 assessments
Study: ADAPT/IMPACT, Nestelberger et al. 2019171
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Adults presenting to the ED with possible cardiac symptoms | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Patients with STEMI excluded (target condition NSTEMI) | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Beckman Coulter ACCESS hs-cTnI, reference standard adjudication occurred after the index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| AMI (third universal definition), with access to clinical records, ECG and conventional troponin and hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Aldous et al. 2011147
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive adults presenting to the ED with chest pain were eligible for inclusion | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
|
Roche Elecsys hs-cTnT on admission and after 6 hours. Data reported for admission for four thresholds No details of interpretation reported. One threshold was derived from ROC analysis and primary analysis based on 99th centile |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
|
Reference standard diagnosis of AMI based on joint ESC and ACC criteria, and included serial conventional cTnI (10- to 12-hour time point not specified) Determination of diagnosis was made blind to hs-cTnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Participants for whom stored samples were not available at both time points were excluded | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Study: Aldous et al. 2012139
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Patients presenting to the ED between 05.30 and 20.00 with chest pain | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Patients with ST segment elevation excluded | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
|
Roche Elecsys hs-cTnT Data reported for multiple thresholds based on predetermined properties of the assay Frozen samples used, unclear whether or not interpretation of index test was blind to reference standard |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Reference standard was final diagnosis of AMI based on ACC criteria and including the results of serial conventional cTnI (10- to 12-hour time point not specified), but blinded to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Biomarkers in Acute Cardiac Care (BACC), Neumann et al. 201684
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Prospective recruitment of adult patients presenting to the ED with acute chest pain. Patients with STEMI (ECG) were excluded from the analysis | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Patients with chest pain, STEMI excluded | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Abbott ARCHITECT hs-cTnI on admission and at 1 and 3 hours, adjudication of diagnosis made at a later time | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| 2015 ESC guidelines and the third universal definition of AMI, including 0- and 3-hour troponins measured using Roche Elecsys TnT. Adjudication made by two independent cardiologists who were unaware of the hs-cTnI results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Body et al. 2011161
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Prospective enrolment of patients; unclear if consecutive | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Mixed chest pain | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT. Threshold: 99th centile cut-off point and LoD. Blinding not reported. Objective test interpreted prior to reference standard and so unlikely to have been influenced by knowledge of reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Universal definition of AMI. Time point not specified. Clinicians were blinded to Hs-cTn | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| 301 patients were excluded prior to enrolment. All patients enrolled were included in the 2 × 2 table | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Body et al. 201556
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive adult patients presenting to the ED with chest pain suspected to be of cardiac origin. Patients requiring hospitalisation for a concomitant medical condition and those with renal failure needing dialysis or chest trauma were excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Target condition was mixed AMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Reference standard determined after the index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3 reference standard
| A. Risk of bias | |
|---|---|
| AMI diagnosis made based on cTnT (0 and 12 hours), ECG and all clinical and imaging data. Clinicians adjudicating AMI were blind to the hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Cappellini et al. 201962
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| All cases of suspected AMI arriving at the ED | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| All cases of suspect AMI in patients arriving at the ED. Patients with STEMI excluded from the analysis (target condition NSTEMI) | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| 2 × 2 data were available for the derivation cohort only (i.e. the cohort in which the optimised threshold/algorithm was derived) | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: high |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Third universal definition of MI. The hs-cTnT could have been included in the reference standard. Time point not specified | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Different physicians made decisions on the AMI depending on whether or not the patient was hospitalised | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: unclear |
Study: Christ et al. 2010150
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Retrospective analysis of consecutive patients presenting to ED with chest pain | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Patients with general chest pain symptoms. Includes participants with a final diagnosis of STEMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT. Threshold was the 99th centile cut-off point. Blinding not reported. It was retrospective analysis and so disease status may have been known when interpreting results. However, it was an objective test and so unlikely to have been influenced by knowledge of disease state | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Joint ESC and ACC criteria. Time point not specified. Unclear whether or not clinicians were blinded to hs-cTn. A second consensus diagnosis incorporating hs-cTn was also made and so clinicians may have been aware of the result for the first consensus diagnosis based only on standard troponin | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| No dropouts reported, all included patients accounted for in flow diagram and numbers suggest that troponin results were available for all | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Clinical Objective Rule-out Evaluation (CORE), Mokhtari et al. 2016/17119,121
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Patients were only enrolled between 09.00 and 21.00 on weekdays. Patients with STEMI or who did not speak Swedish or English were excluded | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Patients who present at nights and at weekends may differ from those recruited | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| MACEs were adjudicated after the index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| The reference standard was adjudicated independently by multiple clinicians who were blind to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients were assessed for 30-day MACE using the same process | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Fast Assessment of Thoracic Pain by nEuRal networks I (FASTER I) and Fast Assessment of Thoracic Pain II (FAST II), Eggers et al. 2012137
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Unclear whether consecutive or random patients were enrolled | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Non-STEMI patients with chest pain presenting to coronary care/chest pain unit | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT. A threshold of 99th centile cut-off point and 95% specificity value. Blinding not reported. The objective test interpreted prior to reference standard and so unlikely to have been influenced by knowledge of reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Joint ESC and ACC criteria. Time point not specified. Unclear whether or not clinicians were blinded to Hs-cTn. A second consensus diagnosis | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Only 360 patients out of 495 who fulfilled inclusion criteria had all biochemical tests performed and were included in the analysis. Reasons for not performing tests were not reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Study: Freund et al. 2011142
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive adults presenting to the ED with chest pain (onset or peak within previous 6 hours). Patients with acute kidney failure requiring dialysis were excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Unselected ED chest pain population. Includes participants with a final diagnosis of STEMI. Data also presented for subgroups with low–moderate and high pre-test probability | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and at 3–9 hours, if available. Reference standard (final diagnosis) adjudicated by two independent physicians after acute episode. Threshold was 99th centile | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Reference standard was final diagnosis based on joint ESC and ACC criteria and included conventional cTnI on admission and at 3–9 hours, if needed (10- to 12-hour time point not specified). Clinicians adjudicating final diagnosis were blind to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Huang et al. 201572
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| A consecutive sample of patients with suspected AMI were enrolled. Patients requiring renal replacement therapy who had metal coronary stents implanted or who had transferred from other hospitals were excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| A consecutive sample of patients with suspected AMI were enrolled. Results were also reported for NSTEMI (patients with STEMI excluded from the analysis) | |
| Do the included patients match the question? Yes | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT. Threshold was the 99th centile cut-off point. Blinding not reported. The objective test was interpreted prior to reference standard and so was unlikely to have been influenced by knowledge of reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Conventional cTnT (fourth generation). Diagnosis of AMI, either NSTEMI or STEMI required a conventional cTnT above the 99th centile together with at least two of the following: symptoms of ischaemia, new ST-T changes or a new Q wave on the ECG and imaging showing new loss of viable myocardium. Attending physicians were blinded to the hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Final diagnosis was adjudicated by both an emergency physician and a cardiologist from the time of enrolment to discharge. A third cardiologist refereed in situations of disagreement. All patients appear to be included in the analysis | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Keller et al. 2011141
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive patients presenting to chest pain units | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| General chest pain populations. Some participants had a final diagnosis of STEMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Abbott Architect STAT hs-cTnI on admission and at 3 hours. Reference standard (final diagnosis) was adjudicated after hs-cTnI testing. Thresholds based on test properties appeared to be prespecified | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
|
Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT (10- to 12-hour time point not specified) Determination of diagnosis was made blind to hs-cTnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| None of the analyses included all study participants (558 or 867 participants missing) | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Study: Kurz et al. 2011148
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive patients admitted to a chest pain unit. 206 patients not included because of ‘technical reasons’ (not fully defined, e.g. venepuncture not possible) | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Appears to be an unselected chest pain population, STEMI excluded. Second publication230 is for a retrospectively selected subgroup of participants with a diagnosis of NSTEMI or UA. Patients were admitted to chest pain units | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
|
Roche Elecsys hs-cTnT. Data reported for admission, 3- and 6-hour samples (6-hour data not extracted) Reference standard troponin testing occurred after hs-cTnT. Threshold was prespecified for data extracted from Giannitsis et al. ,230 but not from Kurz et al. 148 (low risk of bias for Giannitsis et al. 230 data) |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
|
Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT (10- to 12-hour time point not specified) Unclear whether or not determination of diagnosis was made blind to hs-cTnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Lin et al. 2019117
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Convenience sample of patients presenting Monday to Friday, from 08.00 to 21.00, with suspected ACS. Patients who did not have any data on cTn obtained as part of standard care, as well as those lost to follow-up and patients with STEMI were excluded | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Patients presenting at night and weekends may differ from those recruited | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| MACEs were adjudicated after the index test. Optimised thresholds were derived from ROC analyses conducted as part of the study | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: high |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| hs-cTnT results were known to clinicians who adjudicated MACEs | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | No |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: high |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All study participants appear to have been assessed for 30-day MACEs using the same procedure | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Melki et al. 2011144
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Recruitment described as ‘consecutive except for temporary interruptions of the study due to high work load in the coronary care unit’ | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Chest pain patients admitted to chest pain unit, excluding ST segment elevation | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and at 2 hours. Reference standard (final diagnosis) determined after hs-cTnT testing. Threshold based on assay characteristics and appears predetermined | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
|
Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT or cTnI (9- to 12-hour time point specified) Determination of diagnosis was made blind to hs-cTnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Peacock et al. 201889
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Patients with suspected ACS presenting to 1 of 15 US EDs within 24 hours of symptom onset. Exclusion criteria were AMI within the last 3 months, transfer from another medical facility, surgery (including percutaneous coronary intervention) or hospitalisation within the last 3 months, recent cardioversion or defibrillation, acute non-cardiac primary illness prior to enrolment (e.g. severe sepsis), cardiogenic shock and pregnancy | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Target condition mixed AMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Reference standard adjudicated after index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Third universal definition of AMI. Reference standard adjudicated blind to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: PITGORAS, Sanchis et al. 2012135
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Patients excluded because of troponin elevation in any of two serial determinations (at arrival and 6–8 hours later) and prior diagnosis of ischemic heart disease. No details on how patients were selected for the study | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Selected low-risk population | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and at 6–8 hours (data reported for admission and peak values). Reference standard (30-day composite) occurred after testing. Thresholds were reported as prespecified | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Composite 30-day end point of AMI, death and revascularisation | |
| Not clear whether or not those adjudicating AMI were aware of hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appeared to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: QUART, Parsonage et al. 201488
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive adult patients presenting to the ED during office hours with symptoms suggestive of cardiac chest pain. Exclusion criteria were reported and were appropriate | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Target condition mixed (any AMI) | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Index test conducted before reference standard adjudication | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Third universal definition of AMI. Results of the investigational hs-cTnT assay were not available at the time of adjudication | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: RATPAC (point-of-care arm), Collinson et al. 2013159
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Participants with chest pain and suspected AMI. Study uses a subgroup of one arm of a RCT. Patients at high risk of NSTEMI were excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Chest pain patients, excluding those with diagnostic ECG changes | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and at 90 minutes. Reference standard (final diagnosis) determined after hs-cTnT. Threshold based on assay characteristics, including the 99th centile | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT or cTnI (10- to 12-hour time point specified). Determination of diagnosis was made blind to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| 1125 patients enrolled: 25 no samples collected and 250 samples taken but study samples not collected | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Study: Rapid Evaluation of Acute Myocardial Infarction in the United States (REACTION-US), Nowak et al. 201887
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Convenience sample (patients screened when research co-ordinators were available). Patients with STEMI, acute distress requiring life-saving interventions in the previous 24 hours, or who were transferred from another hospital or were pregnant were excluded. The results section indicates that some patients who did not meet the limited exclusion criteria were excluded | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case-control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Target condition was NSTEMI, but patients screened may not be representative of all patients presenting with suspected ACS | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| The reference standard was adjudicated after the index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Third universal definition of AMI, adjudicated by a panel of clinicians who were blinded to the hs-TnT result | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard. Thirty (5%) patients were not included in the 30-minute Δ analysis | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: low |
Study: Saenger et al. 2010165
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| No details on how patients were selected. No exclusion criteria reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| No exclusion criteria reported. The reference standard was AMI (diagnosis method not specified). Diagnoses included STEMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and after 3 hours. Data reported for admission and Δ 0–3 hours. No details of interpretation reported. Threshold for Δ value derived from ROC analysis. The 99th centile was also used | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Reference standard diagnosis of AMI (no details reported) | |
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| No withdrawals reported | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Unclear |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | Risk: unclear |
Study: Sebbane et al. 2013157
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| No details on how patients were selected for inclusion | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Unselected cohort of adult patients presenting with chest pain of recent onset (within 12 hours) | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission or from sample taken during pre-hospital management. Final diagnosis adjudicated 1 month after acute episode. Optimal diagnostic thresholds were determined using within-study ROC analyses. The 99th centile was also reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Diagnosis determined by two independent ED physicians, based on joint ESC and ACC criteria. Reference standard included cTnI taken on admission, at 6 hours and beyond, as needed (10- to 12-hour time point not specified). Physicians had access to serial cTnI results, but were blinded to hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Fifty-four patients were excluded from the analyses because of missing data, including lack of copeptin, hs-cTnT and cTnI measurements | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Study: Shiozaki et al. 2017100
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Patients with chest pain suggestive of ACS, STEMI or trauma that could elevate troponins were excluded. Thirty patients aged > 90 years and 16 with a poor prognosis were excluded (the reasons were not specified in the methods). An additional 21 patients were excluded for additional unspecified reasons | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | Risk: high |
| B. Applicability | |
| Target condition was NSTEMI, but exclusions may mean that the study is not representative of the population presenting with suspected ACS | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| The reference standard diagnosis was adjudicated after the index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Adjudicated by two cardiologists based on ESC/ACC guidelines. Unclear whether or not this was carried out with knowledge of the hs-TnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Slagman et al. 2017102
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| All patients with a routine point-of-care troponin T measurement at admission (presenting symptoms unclear). Patients with a final diagnosis of STEMI and patients with surgical conditions were excluded, as were patients with missing troponin values | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Target condition was NSTEMI, but presenting symptoms unclear | |
| Do the included patients match the question? | Concerns: unclear |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Reference standard diagnosis adjudicated after index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Clinicians adjudicating the reference standard diagnosis had access to all clinical information, including hs-cTnT results. Reference standard diagnosis was retrieved for International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in hospital records | |
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | No |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: high |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: unclear |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients appear to have been included in the analysis | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: unclear |
Study: TRAPID-AMI, Mueller et al. 201680
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Adults presenting to the ED with symptoms suggestive of AMI within the previous 6 hours. Exclusion criteria were listed and were appropriate | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Primary target condition was mixed (any AMI). A subgroup analysis excluding patients with STEMI (target condition NSTEMI) was reported | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| The index test was conducted before reference standard adjudication | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it pre-specified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Third universal definition of AMI and ESC guidelines. Information available to clinical adjudicators was listed and did not include hs-cTnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: UltraSensitive Troponin in Acute Coronary syndromes (TUSCA), Santalo et al. 2013133
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive adult patients presenting to the ED | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Appears to be an unselected ED chest pain population | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Roche Elecsys hs-cTnT on admission and at 2, 4 and 6–8 hours or until discharge (data reported for admission and Δ values). Unclear whether or not hs-cTnT was interpreted blind to cTnT | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Final diagnosis adjudicated by a committee, based on Roche cTnT at admission and 2, 4 and 6–8 hours or until discharge (10- to 12-hour time point not specified). NSTEMI defined as cTnT > 10 ng/l and Δ cTnT > 20%, also 99th centile. Unclear whether or not adjudicators were blinded to hs-cTnT | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: unclear |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Use of TROPonin In Acute coronary syndromes (UTROPIA), Sandoval et al. 201796
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive, unselected patients with suspected AMI | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Patients with STEMI excluded (target condition was NSTEMI) | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Final diagnosis adjudicated after the index test. Prespecified thresholds (LoD and High-STEACS) used | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Final diagnosis made with knowledge of hs-cTnI results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | No |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: high |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients were included in the analyses and the final diagnosis was reached using the same process in all cases | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: low |
Study: Venge et al. 2017110
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Prospective enrolment of adult patients with suspected MI. No exclusion criteria listed | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Setting is inconsistently described (‘Ed’ or ‘ED’ and ‘coronary care/chest pain units’). Target condition was mixed AMI | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Reference standard troponin T was assessed at a central laboratory (after index test) | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Reference standard included troponin T results at 2–4 and 6–24 hours, as well as clinical information and MI, and was adjudicated by a panel of cardiologists. Not clear if cardiologists adjudicating final diagnosis had access to hs-cTnI results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the same reference standard. The study compared Abbott ARCHITECT hs-cTnI with a conventional cTnI assay and a point-of-care assay (these assays are not included in the scope of this review). Patients who did not have data for all three assays were excluded from the analyses. | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | Risk: high |
Appendix 4 QUADAS-2C assessments
Study: ADAPT, Cullen et al. 201468
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Yes | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Low | |
| Domain: index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | Unclear |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Unclear | Unclear |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Unclear | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | Yes | Yes | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | Low | Low |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition, as defined by the reference standard, does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | Yes | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | Low | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | Yes | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | Low | |
Study: APACE, Boeddinghaus et al. 2018/19,59,170,178 (comparison of assays using ESC 0/1-hour pathway or equivalent)
| Domain: patient selection | |||||||
|---|---|---|---|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Beckman Coulter ACCESS hs-cTnI | Answers for the Ortho VITROS hs-cTnI | Answers for the Quidel TriageTrue hs-cTnI | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | Yes | Yes | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low | Low | Low | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low | Low | Low | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of all tests | ||||||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |||||
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Unclear | ||||||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||||||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||||||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Unclear | |||||
| Domain: index tests | |||||||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Beckman Coulter ACCESS hs-cTnI | Answers for the Ortho VITROS hs-cTnI | Answers for the Quidel TriageTrue hs-TnI | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | Yes | Yes | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low | Low | Low | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low | Low | Low | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of all tests | ||||||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |||||
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||||||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||||||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||||||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |||||
| Domain: reference standard | |||||||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Beckman Coulter ACCESS hs-cTnI | Answers for the Ortho VITROS hs-cTnI | Answers for the Quidel TriageTrue hs-cTnI | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | No | No | No | No | No | No | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | High | High | High | High | High | High |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low | Low | Low | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of all tests | ||||||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | No | |||||
| 3.6 Did the reference standard avoid incorporating any of the index tests? | No | ||||||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | High | |||||
| Domain: flow and timing | |||||||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Beckman Coulter ACCESS hs-cTnI | Answers for the Ortho VITROS hs-cTnI | Answers for the Quidel TriageTrue hs-cTnI | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | Yes | Yes | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low | Low | Low | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of all tests | ||||||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |||||
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||||||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||||||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | No. (Yes for comparison of Abbott ARCHITECT hs-cTnI, Roche Elecsys hs-cTnT and Siemens ADVIA Centaur hs-cTnI) | ||||||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | High. (Low for comparison of Abbott ARCHITECT hs-cTnI, Roche Elecsys hs-cTnT and Siemens ADVIA Centaur hs-cTnI) | |||||
Study: BEST, Body et al. 2019/20115,172
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Roche Elecsys hs-cTnT with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | No | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | High | |
| Domain: index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Roche Elecsys hs-cTnT with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | No | Yes | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | High | Low |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Roche Elecsys hs-cTnT with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | No | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | No | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | High | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the Roche Elecsys hs-cTnT | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Roche Elecsys hs-cTnT with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | No | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | High | |
Study: High-STEACS, Chapman et al. 2018/1966,67
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Siemens Atellica hs-cTnI | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Siemens Atellica hs-cTnI | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Unclear | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Unclear | |
| Domain: Index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the ARCHITECT hs-cTnI | Answers for the Siemens Atellica hs-cTnI | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Siemens Atellica hs-cTnI | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the ARCHITECT hs-cTnI | Answers for the Siemens Atellica hs-cTnI | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | Unclear | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | Unclear | Unclear |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Siemens Atellica hs-cTnI | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Unclear | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | Unclear | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | Unclear | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the ARCHITECT hs-cTnI | Answers for the Siemens Atellica hs-cTnI | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the ARCHITECT hs-cTnI with the Siemens Atellica hs-cTnI | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | No | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | High | |
Study: High-Sensitivity Cardiac Troponin I Assays in the United States (High-US), Sandoval et al. 2019176
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Siemens Atellica hs-cTnI | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Siemens Atellica hs-cTnI with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Yes | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Low | |
| Domain: index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the Siemens Atellica hs-cTnI | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Siemens Atellica hs-cTnI with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the Siemens Atellica hs-cTnI | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | Yes | Yes | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | Low | Low |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Siemens Atellica hs-cTnI with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | Yes | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | Low | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the Siemens Atellica hs-cTnI | Answers for the Siemens ADVIA Centaur hs-cTnI | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Siemens Atellica hs-cTnI with the Siemens ADVIA Centaur hs-cTnI | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | Yes | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | Low | |
Study: ROMI-3, Shortt et al. 2017101
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Yes | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Low | |
| Domain: index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Unclear | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Unclear | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | Yes | Yes | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | Low | Low |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | Yes | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | Low | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | Yes | Yes | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | Yes | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | Low | |
Study: TRUST, Carlton et al. 201564
| Domain: patient selection | |||
|---|---|---|---|
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 1.1 Was a consecutive or random sample of patients enrolled? | Yes | Yes |
| 1.2 Was a case–control design avoided? | Yes | Yes | |
| 1.3 Did the study avoid inappropriate exclusions? | Yes | Yes | |
| Risk of bias | 1.4 Could the selection of patients have introduced bias? | Low | Low |
| Concerns regarding applicability | 1.5 Are there concerns that the included patients do not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 1.6 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 1.7 Was the intention for patients either to receive all index tests or to be randomly allocated to index tests? | Yes | ||
| 1.8 If patients were randomised, was the allocation sequence random? | NA | ||
| 1.9 If patients were randomised, was the allocation sequence concealed until patients were enrolled and assigned to index tests? | NA | ||
| Risk of bias | 1.10 Could the selection of patients have introduced bias in the comparison? | Low | |
| Domain: index tests | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 2.1 Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes |
| 2.2 If a threshold was used, was it prespecified? | Yes | Yes | |
| Risk of bias | 2.3 Could the conduct or interpretation of the index test have introduced bias? | Low | Low |
| Concerns regarding applicability | 2.4 Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 2.5 Was risk of bias for this domain judged ‘low’ for all index tests? | Yes | |
| 2.6 If patients received multiple index tests, were test results interpreted without knowledge of the results of the other index test(s)? | Yes | ||
| 2.7 If patients received multiple index tests, is undergoing one index test unlikely to affect the performance of the other index test(s)? | Yes | ||
| 2.8 Were differences in the conduct or interpretation between the index tests unlikely to advantage one of the tests? | Yes | ||
| Risk of bias | 2.9 Could the conduct or interpretation of the index tests have introduced bias in the comparison? | Low | |
| Domain: reference standard | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 3.1 Is the reference standard likely to correctly classify the target condition? | Yes | Yes |
| 3.2 Were the reference standard results interpreted without knowledge of the results of the index test? | No | No | |
| Risk of bias | 3.3 Could the reference standard, its conduct or its interpretation have introduced bias? | High | High |
| Concerns regarding applicability | 3.4 Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 3.5 Was risk of bias for this domain judged ‘low’ for all index tests? | No | |
| 3.6 Did the reference standard avoid incorporating any of the index tests? | No | ||
| Risk of bias | 3.7 Could the reference standard, its conduct or its interpretation have introduced bias in the comparison? | High | |
| Domain: flow and timing | |||
| Single test accuracy (QUADAS-2) | Answers for the Abbott ARCHITECT hs-cTnI | Answers for the Roche Elecsys hs-cTnT | |
| Signalling questions | 4.1 Was there an appropriate interval between index tests and reference standard? | Yes | Yes |
| 4.2 Did all patients receive a reference standard? | Yes | Yes | |
| 4.3 Did all patients receive the same reference standard? | Yes | Yes | |
| 4.4 Were all patients included in the analysis? | No (10% missing) | No (< 1% missing) | |
| Risk of bias | 4.5 Could the patient flow have introduced bias? | High | Low |
| Comparative accuracy (QUADAS-2C) | Answers for the comparison of the Abbott ARCHITECT hs-cTnI with the Roche Elecsys hs-cTnT | ||
| Signalling questions | 4.6 Was risk of bias for this domain judged ‘low’ for all index tests? | No | |
| 4.7 Was there an appropriate interval between the index tests? | Yes | ||
| 4.8 Was the same reference standard used for all index tests? | Yes | ||
| 4.9 Are the proportions and reasons for missing data similar across index tests? | No | ||
| Risk of bias | 4.10 Could the patient flow have introduced bias in the comparison? | High | |
Appendix 5 Details of excluded studies with rationale
To be included in the review studies had to fulfil the criteria below.
-
Population: adults (aged ≥ 18 years) presenting with acute pain, discomfort or pressure in the chest, epigastrium, neck, jaw or upper limb without an apparent non-cardiac source due to a suspected, but not proven, AMI.
-
Setting: secondary or tertiary care.
-
Index test: the Abbott ARCHITECT hs-cTnI; the Abbott Alinity hs-cTnI; the Beckman Coulter Access hs-cTnI; the bioMérieux VIDAS hs-cTnI; the Ortho VITROS hs-cTnI; the Quidel Triage True hs-cTnI Roche Elecsys (cTnT-hs or cTnT-hs STAT); the Siemens Atellica hs-cTnI; the Siemens Dimension EXL hs-cTnI; the Siemens Dimension Vista hs-cTnI; and the Siemens ADVIA Centaur hs-cTnI. Results were to be available within 3 hours.
-
Reference standard: the third or fourth universal definition of AMI,43 including measurement of troponin T or I (using any method) on presentation and 3–6 hours later, or occurrence of a MACE (any definition used in identified studies) during the 30-day follow-up.
-
Outcome: sufficient data to construct a 2 × 2 table of test performance.
Table 38 summarises studies that were screened for inclusion based on full-text publication, but did not fulfil one or more of the above criteria. Studies were assessed sequentially against criteria (as soon as a study had failed based on one of the criteria it was not assessed against subsequent criteria). Table 38 shows which of the criteria each study fulfilled (‘yes’) and on which criterion it failed (‘no’) or was unclear.
| Study | Study design | Setting | Population | Index test | Reference standard | Outcome |
|---|---|---|---|---|---|---|
| Aguirre et al. 2014231 | Yes | Yes | Yes | Yes | Yes | No |
| Ambavane et al. 2017192 | Yes | Yes | Yes | Yes | Unclear | No |
| Badertscher et al. 2018232 | Yes | Yes | Unclear | Unclear | Unclear | Yes |
| Bandstein et al. 2014233 | Yes | Yes | Yes | Unclear | Yes | No |
| Biener et al. 2013234 | Yes | Yes | Yes | No | ||
| Borna et al. 2014235 | Yes | No | ||||
| Burgio and Marino 2018236 | No | |||||
| Burgio et al. 2018237 | Yes | Yes | Yes | No | ||
| Canadian Institutes of Health Research McMaster University, 2017238 | No | |||||
| Chew et al. 2019215 | Yes | Yes | Yes | Yes | Yes | No |
| Cortes et al. 2018239 | Yes | Yes | Yes | Yes | Yes | No |
| Costabel et al. 2014240 | Yes | Yes | Yes | Yes | No | |
| Costabel 2019241 | Yes | Yes | Yes | Unclear | ||
| Croce et al. 2017242 | Yes | Yes | No | |||
| Cullen et al. 2013243 | Yes | Yes | Yes | No | ||
| Cullen et al. 2013244 | Yes | Yes | Yes | Unclear | Unclear | No |
| Cullen et al. 2014245 | Yes | Yes | Yes | No | ||
| Cullen et al. 2014246 | Yes | Yes | Yes | Yes | Yes | No |
| Dadkhah et al. 2017247 | Yes | Yes | Yes | No | ||
| Druey et al. 2015248 | Yes | Yes | Yes | No | ||
| Ferencik et al. 2017249 | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Gandolfo et al. 2017250 | Yes | Yes | Yes | Unclear | Yes | |
| Gandolfo et al. 2017251 | Unclear | Yes | Unclear | Yes | Unclear | No |
| Goorden et al. 2016252 | Yes | Yes | Yes | Yes | Unclear | Yes |
| Greenslade et al. 2017253 | No | |||||
| Greenslade et al. 2018254 | No | |||||
| Gunsolus et al. 2018255 | Yes | Yes | Unclear | Unclear | Unclear | No |
| Hoeller et al. 2013168 | Yesa | Yes | Yes | Yes | Yes | Yes |
| Ichise et al. 2017256 | Yes | Yes | Yes | Yes | Unclear | No |
| Invernizzi et al. 2013257 | Yes | Yes | Yes | Unclear | No | |
| Isiksacan et al. 2017258 | Yes | Yes | Yes | Yes | No | |
| Isiksacan et al. 2019259 | Yes | Yes | Yes | Yes | No | |
| Poole Hospital NHS Foundation Trust, 2013260 | Yesb | Yes | Yes | Yes | Yes | Yes |
| Kavsak et al. 2018261 | No | |||||
| Kavsak et al. 2018262 | Yes | Unclear | Yes | Yes | Yes | No |
| Kavsak et al. 2018263 | Yes | Yes | Yes | Yes | Yes | No |
| Kaysak et al. 2017264 | Unclear | No | ||||
| Kellens et al. 2016265 | Yes | Yes | Unclear | Yes | Yes | Unclear |
| Kitamura et al. 201377 | Yes | Yes | No | |||
| Korley et al. 2014266 | Yes | Yes | Yes | Yes | No | |
| Kovács et al. 2015267 | Yes | Yes | Yes | Yes | Yes | No |
| Lin et al. 2018268 | Yes | Yes | Yes | Yes | Yes | No |
| Ljung et al. 2019269 | No | |||||
| Mahler et al. 201778 | Yes | Yes | Yes | Yes | Yes | No |
| McCord et al. 2017270 | Yes | Yes | Yes | Yes | Yes | No |
| McRae et al. 2017271 | No | |||||
| McRae et al. 2017272 | No | |||||
| McRae et al. 2019273 | No | |||||
| Mohsen and Shawky 2016274 | Yes | Yes | Yes | No | ||
| Mueller et al. 2018275 | No | |||||
| Nacke et al. 2014276 | Yes | Yes | Yes | No | ||
| Nasuruddin et al. 2017277 | Yes | Yes | Yes | Yes | Unclear | No |
| Nejatian et al. 2017278 | Yes | Yes | No | Unclear | ||
| Nestelberger et al. 2016279 | Yes | Yes | Yes | Yes | Yes | No |
| Nestelberger et al. 2019280 | Yes | Yes | Yes | No | ||
| Neumann et al. 2019214 | No | |||||
| Neumann et al. 2019281 | Yes | Yes | Yes | No | ||
| Nowak et al. 2017282 | Yes | Yes | Yes | Yes | Yes | No |
| Papendick et al. 2017283 | Yes | Yes | Yes | No | ||
| Peitsmeyer et al. 2013284 | Yes | Yes | Yes | Yes | No | |
| Peitsmeyer et al. 2013285 | Yes | Yes | Yes | No | ||
| Pettersson et al. 2018286 | Yes | Yes | Yes | Yes | No | |
| Pickering et al. 2015287 | Yes | Yes | Yes | Yes | Yes | No |
| Pickering et al. 2016288 | No | |||||
| Pickering et al. 2016289 | No | |||||
| Pickering et al. 2018290 | Yes | Yes | Yes | No | ||
| Reddy et al. 2016291 | Yes | Yes | Yes | Yes | No | |
| Reichlin et al. 2013292 | Yes | Yes | Yes | Yes | No | |
| Renstroum et al. 2018293 | Yes | Yes | Unclear | Unclear | Unclear | No |
| Riedlinger et al. 2018294 | No | |||||
| Sandoval et al. 2017295 | Yes | Yes | Yes | Yes | No | Unclear |
| Santi et al. 2017296 | Yes | Yes | Yes | Yes | Yes | No |
| Schoenenberger et al. 2016297 | No | |||||
| Schofer et al. 2017298 | Yes | Yes | Yes | No | ||
| Schønemann-Lund et al. 2015299 | Yes | No | ||||
| Shah et al. 2015300 | Yes | Yes | Yes | Yes | No | |
| Shortt et al. 2015301 | Yes | Yes | Yes | No | ||
| Stallone et al. 2016302 | No | |||||
| Stoyanov et al. 2019303 | No | |||||
| Su et al. 2015304 | Yes | Yes | Yes | Yes | Unclear | No |
| Suh et al. 2018305 | Yes | No | ||||
| Teggert and Twerenbold 2015306 | Yes | Yes | Yes | No | ||
| Than et al. 2014307 | Yes | Yes | Yes | No | ||
| Than et al. 2016308 | Yes | Yes | Yes | No | ||
| Thelin et al. 2013309 | Yes | Yes | Yes | Yes | No | |
| Thet et al. 2019310 | Yes | Yes | No | |||
| Twerenbold et al. 2013311 | Yes | Yes | Yes | No | ||
| Twerenbold et al. 2013312 | Yes | Yes | Yes | Yes | Unclear | Yes |
| Twerenbold et al. 2018313 | No | |||||
| Vigen et al. 2018314 | Yes | Yes | Yes | Unclear | Yes | Yes |
| Wang et al. 2019315 | No | |||||
| Wildi et al. 2018316 | Yes | Yes | No | |||
| Yip et al. 2014317 | No | |||||
| Yokoyama et al. 2018318 | Yes | Yes | Yes | Yes | No |
Appendix 6 Selection of test strategies for cost-effectiveness modelling: responses of specialist committee members
The following responses were received from specialist committee members, regarding setting a minimum clinically acceptable sensitivity for hs-cTn-based rule-out strategies.
Response 1
Priority is minimising false negatives and the suggestion of including only strategies that provide NPV [negative predictive value] > 99% or sensitivity > 97% is sensible if only to limit the number of strategies to model.
Gold standard should be a high-sensitivity assay using the 99th centile this time around but could be flexible about when this is measured.
For cost-effectiveness it might make sense to compare one test and two test strategies.
Need to consider whether strategies that use a single test with a risk tool [HEART, TMACs (Troponin-only Manchester Acute Coronary Syndromes), EDACS (Emergency Department Assessment of Chest Pain Score)] should be considered separately – no additional cost, but differences in terms of effectiveness and safety.
We should also consider whether to update our recommendations on the use of the 99th centile, and in particular whether we recommend sex-specific thresholds or not.
Response 2
Most of the rule out strategies are modelled at 99% NPV [negative predictive value]. Modelling including a 99% sensitivity is probably desirable but may be not feasible. I am not sure troponin testing alone will achieve > 99% sensitivity. But would be delighted to be proved wrong.
Choice of assay in the lab is not determined by analytical performance of the cTn assay but by a range of factors as it is one of approximately 200 assays considered as part of a lab automation package.
The choice of pathway is between the ESC approach and High-STEACS. All use admission measurement then a follow up measurement, a decision limit and a delta. Pragmatically, although retest at 1 h [hour] is suggested this is unlikely to be achieved in practice so a 1- to 2-hour second sample is more realistic.
If faced between waiting 1 hour for an answer or 4 hours I know what ED patients will choose. I know I would.
So while I understand the desire to be inclusive it is also desirable to be pragmatic. Current evidence favours admission sampling for rule out then repeat sampling for rule in/rule out/further testing. Troponin testing is NOT a standalone and there are 1–3 time points for decisions all with the same choice. Do I send the patient home (God takes care of him) admit him to the cardiologists or medics (smart doc takes over) or hang on to do more tests. This occurs at presentation and at the retest time(s).
Response 3
I’d say the very minimum should be 95%. However, we could even push that further and go to 97%. Even though clinicians will generally say that they wouldn’t accept sensitivities less than 99%, I’d probably err against going much further than 97%, to be honest. Otherwise, we will essentially just be picking the strategy with highest specificity, without balancing the two based on the economic modelling. Risk aversion may ultimately not be the best strategy overall because it, too, has an important cost and risk associated with it.
I’d also stratify the analysis by assay and timing. Running a lifetime model is likely to find that more conservative serial sampling strategies will dominate the faster strategies. For example, if you test troponin on arrival and at 4 hours, it is likely to have slightly higher sensitivity than testing on arrival and at 3 hours (assuming you optimise the cut-offs at each time point with equal rigor).
Running a lifetime economic model would always therefore tend to lead us to issuing conservative recommendations – e.g. 4-hour testing over 3-hour testing. The traditional lifetime model doesn’t capture the granular costs of ED visits and certainly doesn’t capture the risks of waiting on a trolley in an ED corridor because so many patients are waiting for inpatient tests.
We need to account for that, and we also need to account for the fact that serial testing strategies could be run together, e.g.:
I would suggest collating the evidence we have for each assay. Then, we could perhaps consider using network met-analysis (or similar) to construct the optimal serial testing strategy for each assay.
Alternatively, putting it more simply, we could directly compare the cost-effectiveness of single-test strategies; then (separately) 0/1-hour strategies; then 0/2-hour strategies [and each would be compared against the reference standard to ensure that it isn’t dominated]. That would help avoid the potential bias towards making conservative recommendations.
Response 4
A few thoughts on sensitivity. False-negative results, are clearly more dangerous for patients with suspected ACS than false-positive results, particularly if they result in patients being discharged from A&E [accident and emergency] departments with reassurance. On that basis we should probably only consider test strategies that deliver a high level of sensitivity – say > 85% or 90%.
Appendix 7 Scenario analyses
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,724 | 12.0763 | –152 | –0.0011 | 136,383 | Cheapest |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,764 | 12.0765 | –112 | –0.0009 | 118,636 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,781 | 12.0768 | –95 | –0.0006 | 170,370 | Extendedly dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,787 | 12.0768 | –89 | –0.0006 | 159,271 | Extendedly dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,788 | 12.0774 | –88 | 0.0000 | 157,505,897 | £57,659 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,793 | 12.0768 | –83 | –0.0006 | 149,485 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,794 | 12.0763 | –82 | –0.0011 | 73,814 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,809 | 12.0774 | –66 | 0.0000 | 119,216,717 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,822 | 12.0774 | –54 | 0.0000 | 96,913,928 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,828 | 12.0763 | –47 | –0.0011 | 42,608 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,843 | 12.0774 | –33 | 0.0000 | 58,544,594 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,843 | 12.0774 | –32 | 0.0000 | 58,315,678 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,855 | 12.0768 | –21 | –0.0006 | 36,935 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,862 | 12.0768 | –14 | –0.0006 | 24,942 | Dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,867 | 12.0768 | –9 | –0.0006 | 15,429 | Dominated |
| Standard troponin (at presentation and after 10–12 hours) | 38,876 | 12.0774 | 0 | 0.0000 | NA | £157,505,897 |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,878 | 12.0767 | 2 | –0.0007 | –2607 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,923 | 12.0774 | 47 | 0.0000 | –84,642,503 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,969 | 12.0769 | 93 | –0.0005 | –185,857 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 39,027 | 12.0774 | 151 | 0.0000 | –271,257,977 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 39,047 | 12.0774 | 171 | 0.0000 | –307,122,945 | Dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 39,055 | 12.0772 | 179 | –0.0002 | –1,073,915 | Dominated |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| Standard troponin (at presentation and after 10–12 hours) | 37,503 | 11.3230 | 0 | 0.0000 | NA | Cheapest |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,151 | 11.4610 | 648 | 0.1380 | 4698 | £4698 |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,158 | 11.4510 | 655 | 0.1280 | 5117 | Dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,167 | 11.4488 | 664 | 0.1259 | 5277 | Dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,172 | 11.4469 | 670 | 0.1239 | 5403 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,183 | 11.4455 | 680 | 0.1225 | 5551 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,192 | 11.4547 | 689 | 0.1317 | 5233 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,192 | 11.4522 | 690 | 0.1292 | 5336 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,196 | 11.4424 | 693 | 0.1195 | 5799 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,196 | 11.4562 | 694 | 0.1333 | 5204 | Dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,196 | 11.4313 | 694 | 0.1083 | 6405 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,198 | 11.4396 | 695 | 0.1167 | 5958 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,198 | 11.4465 | 696 | 0.1235 | 5633 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,200 | 11.4361 | 698 | 0.1132 | 6162 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,201 | 11.4291 | 698 | 0.1062 | 6572 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,204 | 11.4352 | 701 | 0.1122 | 6247 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,217 | 11.4455 | 714 | 0.1225 | 5826 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,230 | 11.4250 | 727 | 0.1020 | 7129 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,244 | 11.4147 | 742 | 0.0918 | 8083 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,274 | 11.4064 | 771 | 0.0835 | 9239 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,284 | 11.4035 | 782 | 0.0805 | 9705 | Dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,286 | 11.4014 | 784 | 0.0784 | 9994 | Dominated |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,654 | 12.0721 | –222 | –0.0053 | 42,267 | Cheapest |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,659 | 12.0729 | –216 | –0.0045 | 48,464 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,672 | 12.0748 | –204 | –0.0026 | 77,572 | £6962 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,677 | 12.0748 | –199 | –0.0026 | 75,761 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,692 | 12.0721 | –183 | –0.0053 | 34,891 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,693 | 12.0774 | –183 | 0.0000 | 69,706,183 | £7874 |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,693 | 12.0721 | –183 | –0.0053 | 34,815 | Dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,696 | 12.0748 | –179 | –0.0026 | 68,270 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,698 | 12.0740 | –177 | –0.0034 | 51,980 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,699 | 12.0748 | –177 | –0.0026 | 67,378 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,702 | 12.0748 | –174 | –0.0026 | 66,291 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,705 | 12.0748 | –171 | –0.0026 | 65,057 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,706 | 12.0774 | –170 | 0.0000 | 64,644,677 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,709 | 12.0774 | –167 | 0.0000 | 63,673,276 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,709 | 12.0774 | –167 | 0.0000 | 63,440,707 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,726 | 12.0774 | –149 | 0.0000 | 56,850,305 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,734 | 12.0774 | –142 | 0.0000 | 53,960,316 | Extendedly dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,740 | 12.0750 | –135 | –0.0024 | 57,282 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,773 | 12.0774 | –103 | 0.0000 | 39,253,952 | Dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,782 | 12.0766 | –94 | –0.0008 | 118,920 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,782 | 12.0774 | –93 | 0.0000 | 35,575,926 | Dominated |
| Standard troponin (at presentation and after 10–12 hours) | 38,876 | 12.0774 | 0 | 0.0000 | NA | £69,706,183 |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| Standard troponin (at presentation and after 10–12 hours) | 36,496 | 10.9853 | 0 | 0.0000 | NA | Cheapest |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,015 | 11.4007 | 1519 | 0.4155 | 3656 | Extendedly dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,017 | 11.4128 | 1521 | 0.4276 | 3558 | Extendedly dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,022 | 11.4064 | 1525 | 0.4211 | 3622 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,022 | 11.4035 | 1526 | 0.4182 | 3648 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,022 | 11.4265 | 1526 | 0.4412 | 3460 | Extendedly dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,027 | 11.4292 | 1531 | 0.4439 | 3448 | Extendedly dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,042 | 11.4250 | 1546 | 0.4397 | 3517 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,048 | 11.4341 | 1552 | 0.4488 | 3457 | Extendedly dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,048 | 11.4331 | 1552 | 0.4478 | 3465 | Dominated |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,054 | 11.4475 | 1558 | 0.4622 | 3371 | Extendedly dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,057 | 11.4448 | 1561 | 0.4595 | 3397 | Dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,059 | 11.4468 | 1563 | 0.4615 | 3386 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,061 | 11.4383 | 1565 | 0.4530 | 3454 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,061 | 11.4396 | 1565 | 0.4544 | 3445 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,067 | 11.4455 | 1571 | 0.4602 | 3413 | Dominated |
| 18. Beckman Coulter ACCESS hs-cTnI: hsTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,081 | 11.4568 | 1585 | 0.4716 | 3362 | £3362 |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,086 | 11.4465 | 1590 | 0.4612 | 3447 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,092 | 11.4481 | 1596 | 0.4628 | 3448 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,101 | 11.4455 | 1605 | 0.4602 | 3487 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,104 | 11.4526 | 1608 | 0.4674 | 3441 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,118 | 11.4562 | 1622 | 0.4710 | 3444 | Dominated |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,666 | 12.0763 | –210 | –0.0011 | 188,442 | Cheapest |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,677 | 12.0765 | –199 | –0.0009 | 210,557 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,678 | 12.0768 | –197 | –0.0006 | 354,684 | £22,200 |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,689 | 12.0768 | –187 | –0.0006 | 335,724 | Dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,692 | 12.0774 | –184 | 0.0000 | 330,758,895 | £23,949 |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,702 | 12.0768 | –174 | –0.0006 | 312,438 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,704 | 12.0763 | –172 | –0.0011 | 154,774 | Dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,705 | 12.0774 | –171 | 0.0000 | 307,200,566 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,707 | 12.0768 | –169 | –0.0006 | 303,093 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,708 | 12.0774 | –168 | 0.0000 | 302,143,335 | Dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,708 | 12.0767 | –168 | –0.0007 | 232,351 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,710 | 12.0768 | –166 | –0.0006 | 298,174 | Dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,710 | 12.0768 | –165 | –0.0006 | 297,336 | Dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,711 | 12.0763 | –165 | –0.0011 | 148,321 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,716 | 12.0774 | –159 | 0.0000 | 286,628,255 | Dominated |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,726 | 12.0774 | –149 | 0.0000 | 268,289,079 | Dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,741 | 12.0774 | –135 | 0.0000 | 241,886,429 | Dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,749 | 12.0769 | –126 | –0.0005 | 252,587 | Dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,772 | 12.0774 | –104 | 0.0000 | 186,143,573 | Dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,781 | 12.0774 | –94 | 0.0000 | 169,540,501 | Dominated |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,785 | 12.0772 | –90 | –0.0002 | 540,570 | Dominated |
| Standard troponin (at presentation and after 10–12 hours) | 38,876 | 12.0774 | 0 | 0.0000 | NA | £330,758,895 |
| Strategy | Cost (£) | QALY | Compared with standard troponin | Full incremental ICER: Δ costs/Δ QALYs | ||
|---|---|---|---|---|---|---|
| Δ Costs (£) | Δ QALYs | Δ Costs/Δ QALYs (£) | ||||
| Standard troponin (at presentation and after 10–12 hours) | 37,503 | 11.3230 | 0 | 0.0000 | NA | Cheapest |
| 7. Abbott ARCHITECT hs-cTnI: LoD | 38,019 | 11.4014 | 516 | 0.0784 | 6580 | Extendedly dominated |
| 11. Siemens ADVIA Centaur hs-cTnI: < 2 ng/l at 0 hours | 38,021 | 11.4035 | 518 | 0.0805 | 6434 | Extendedly dominated |
| 15. Siemens Atellica hs-cTnI: < 2 ng/l at 0 hours | 38,021 | 11.4064 | 518 | 0.0835 | 6210 | Extendedly dominated |
| 2. Roche Elecsys hs-cTnT: LoD | 38,026 | 11.4147 | 524 | 0.0918 | 5706 | Extendedly dominated |
| 10. Abbott ARCHITECT hs-cTnI: < 4 ng/l at 0 hours | 38,032 | 11.4291 | 529 | 0.1062 | 4982 | Extendedly dominated |
| 14. Siemens ADVIA Centaur hs-cTnI: < 5 ng/l at 0 hours | 38,032 | 11.4313 | 530 | 0.1083 | 4889 | Extendedly dominated |
| 4. Roche Elecsys hs-cTnT: (< 8 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 0.5 hours) | 38,050 | 11.4250 | 547 | 0.1020 | 5360 | Dominated |
| 13. Siemens ADVIA Centaur hs-cTnI: ESC pathway | 38,053 | 11.4352 | 550 | 0.1122 | 4901 | Extendedly dominated |
| 8. Abbott ARCHITECT hs-cTnI: ESC pathway | 38,056 | 11.4361 | 554 | 0.1132 | 4891 | Extendedly dominated |
| 19. Ortho VITROS hs-cTnI: ESC pathway | 38,060 | 11.4396 | 558 | 0.1167 | 4778 | Extendedly dominated |
| 17. Beckman Coulter ACCESS hs-cTnI: ESC pathway | 38,065 | 11.4488 | 562 | 0.1259 | 4468 | Extendedly dominated |
| 6. Siemens Dimension Vista hs-cTnI: (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) | 38,066 | 11.4455 | 563 | 0.1225 | 4596 | Dominated |
| 3. Roche Elecsys hs-cTnT: ESC pathway | 38,069 | 11.4469 | 567 | 0.1239 | 4573 | Dominated |
| 5. Roche Elecsys hs-cTnT: (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours) | 38,072 | 11.4510 | 569 | 0.1280 | 4442 | Extendedly dominated |
| 20. bioMérieux VIDAS hs-cTnI: [< 2 ng/l at 0 hours OR (< 6 ng/l at 0 AND 2 hours)] | 38,079 | 11.4424 | 576 | 0.1195 | 4821 | Dominated |
| 12. Siemens ADVIA Centaur hs-cTnI: [< 3 ng/l at 0 hours OR (< 8 ng/l at 0 hours AND Δ < 7 ng/l at 0 to 2 hours)] | 38,085 | 11.4465 | 582 | 0.1235 | 4714 | Dominated |
| 18. Beckman Coulter ACCESS hs-cTnI: [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] | 38,094 | 11.4610 | 591 | 0.1380 | 4281 | £4281 |
| 21. Quidel TriageTrue hs-cTnI: ESC pathway | 38,101 | 11.4455 | 598 | 0.1225 | 4880 | Dominated |
| 16. Siemens Atellica hs-cTnI: High-STEACS pathway | 38,103 | 11.4522 | 600 | 0.1292 | 4643 | Dominated |
| 9. Abbott ARCHITECT hs-cTnI: High-STEACS pathway | 38,113 | 11.4547 | 610 | 0.1317 | 4630 | Dominated |
| 1. Roche Elecsys hs-cTnT: 99th centile | 38,125 | 11.4562 | 622 | 0.1333 | 4669 | Dominated |
FIGURE 17.
Scenario 1 conditional on the base-case cost-effectiveness frontier. CEF, cost-effectiveness frontier.
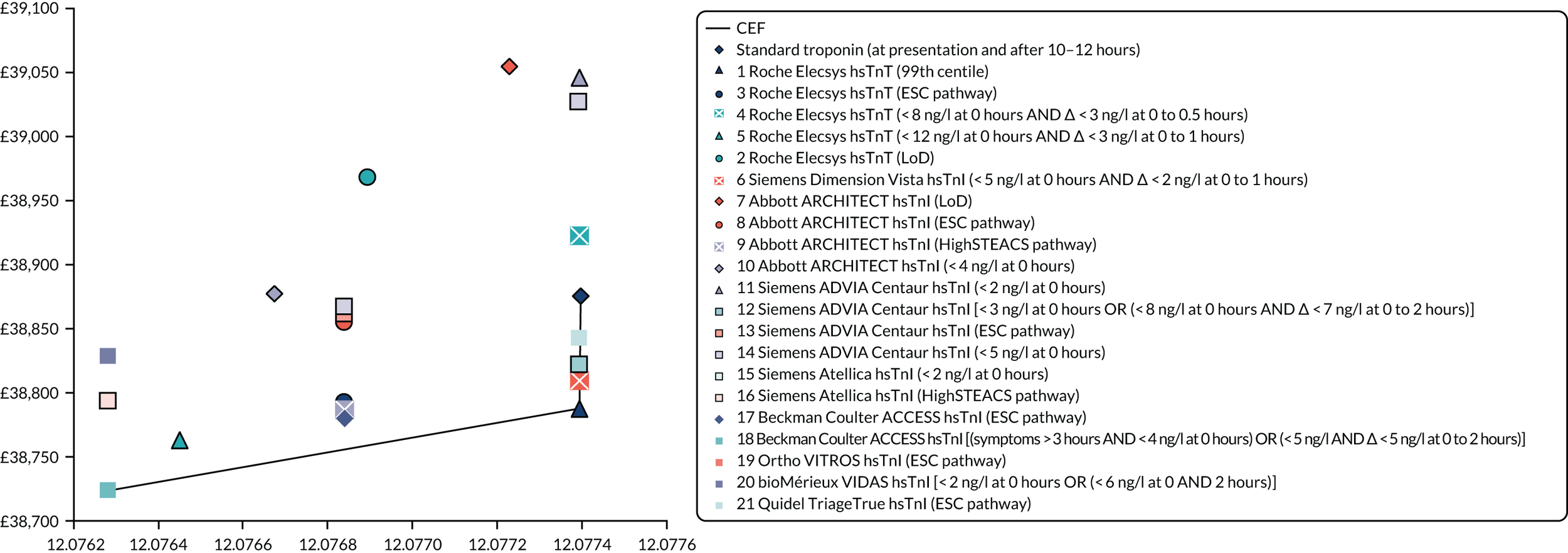
FIGURE 18.
Scenario 1 conditional on the secondary analysis cost-effectiveness frontier. CEF, cost-effectiveness frontier.
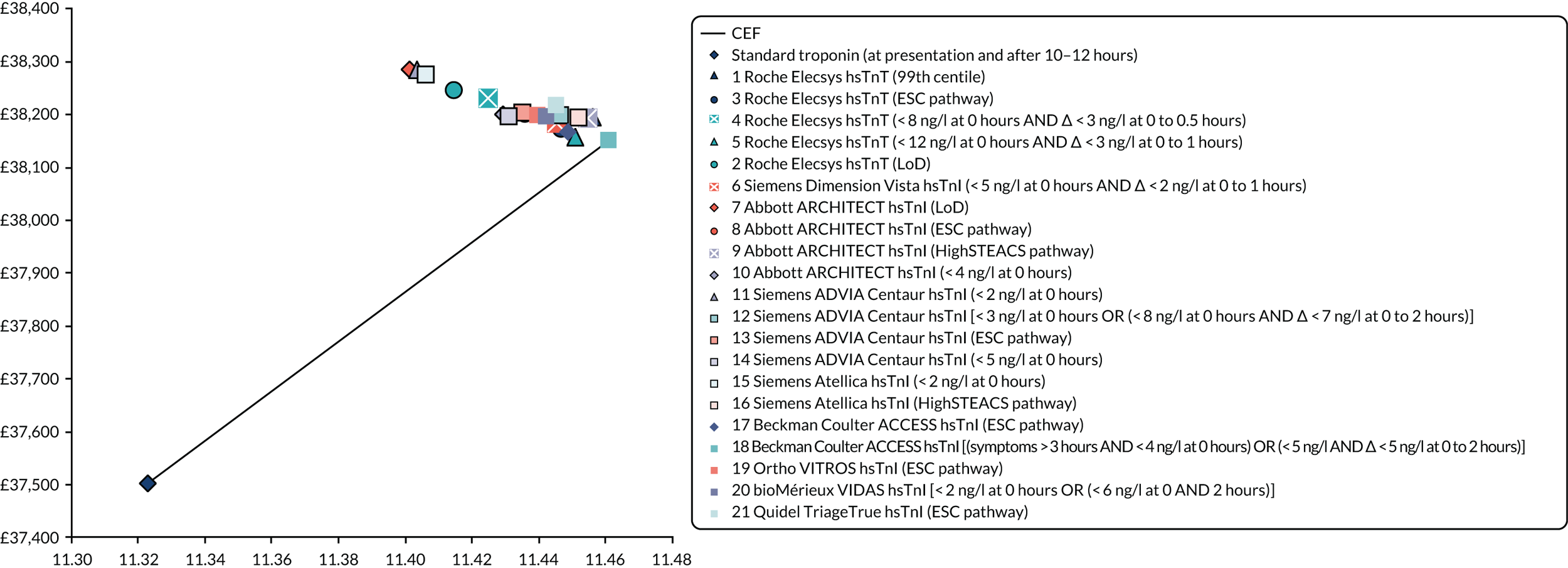
FIGURE 19.
Scenario 2 conditional on the base-case cost-effectiveness frontier. Cost-effectiveness frontier.
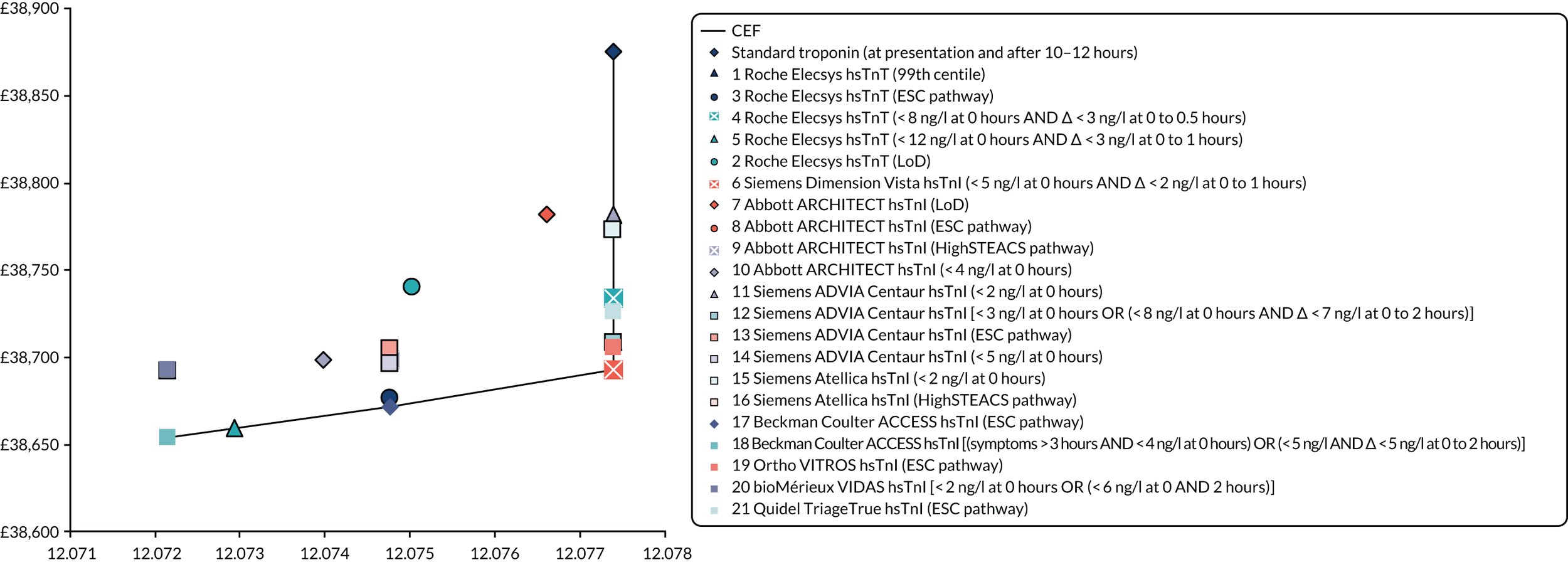
FIGURE 20.
Scenario 2 conditional on the secondary analysis cost-effectiveness frontier. CEF, cost-effectiveness frontier.

FIGURE 21.
Scenario 3 conditional on the base-case cost-effectiveness frontier. CEF, cost-effectiveness frontier.

FIGURE 22.
Scenario 3 conditional on the secondary analysis cost-effectiveness frontier. CEF, cost-effectiveness frontier.
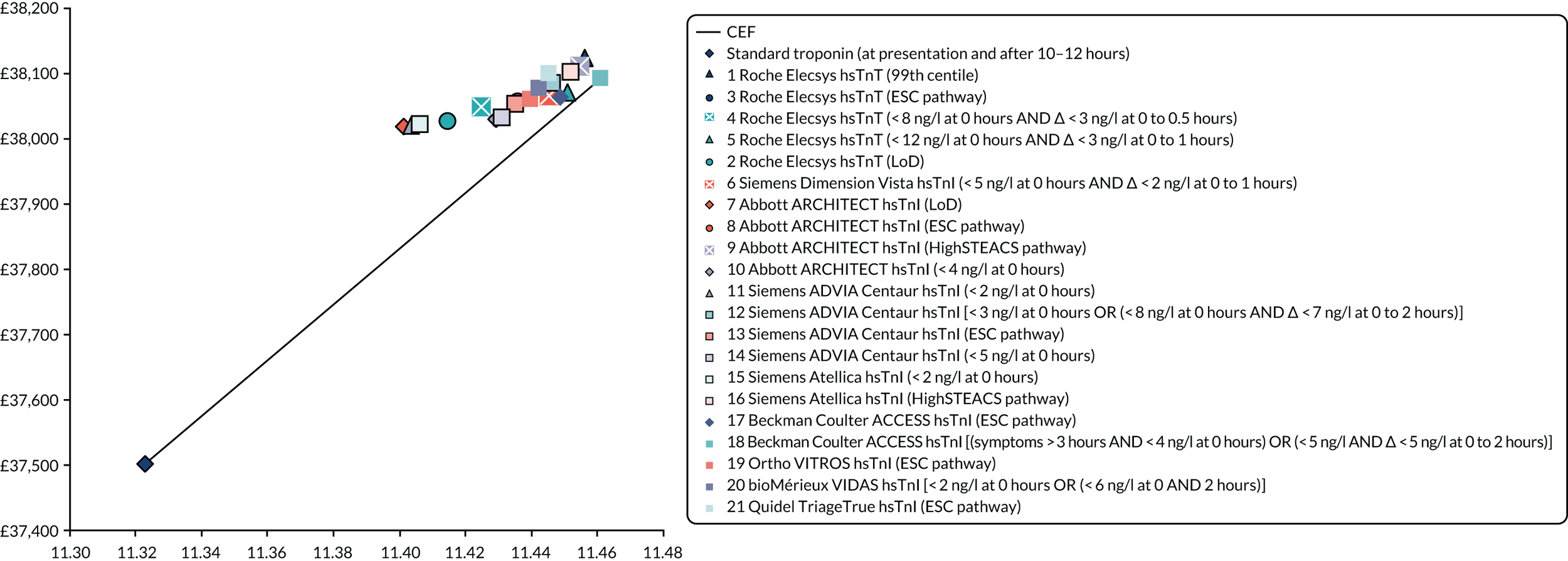
Appendix 8 Deterministic one-way sensitivity analyses
Deterministic one-way sensitivity analyses for the base case (based on incremental net benefit)
FIGURE 23.
Tornado diagram for the comparison of the Roche Elecsys hs-cTnT [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)] with the Beckman Coulter ACCESS hs-cTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)].

FIGURE 24.
Tornado diagram for the comparison of the Siemens Dimension Vista hs-cTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours) with the Roche Elecsys hs-cTnT [ESC 0/1-hour pathway: (symptoms > 3 hours AND < 5 ng/l at 0 hours) OR (< 12 ng/l at 0 hours AND Δ < 3 ng/l at 0 to 1 hours)]. DOWSA, deterministic one-way sensitivity analysis.
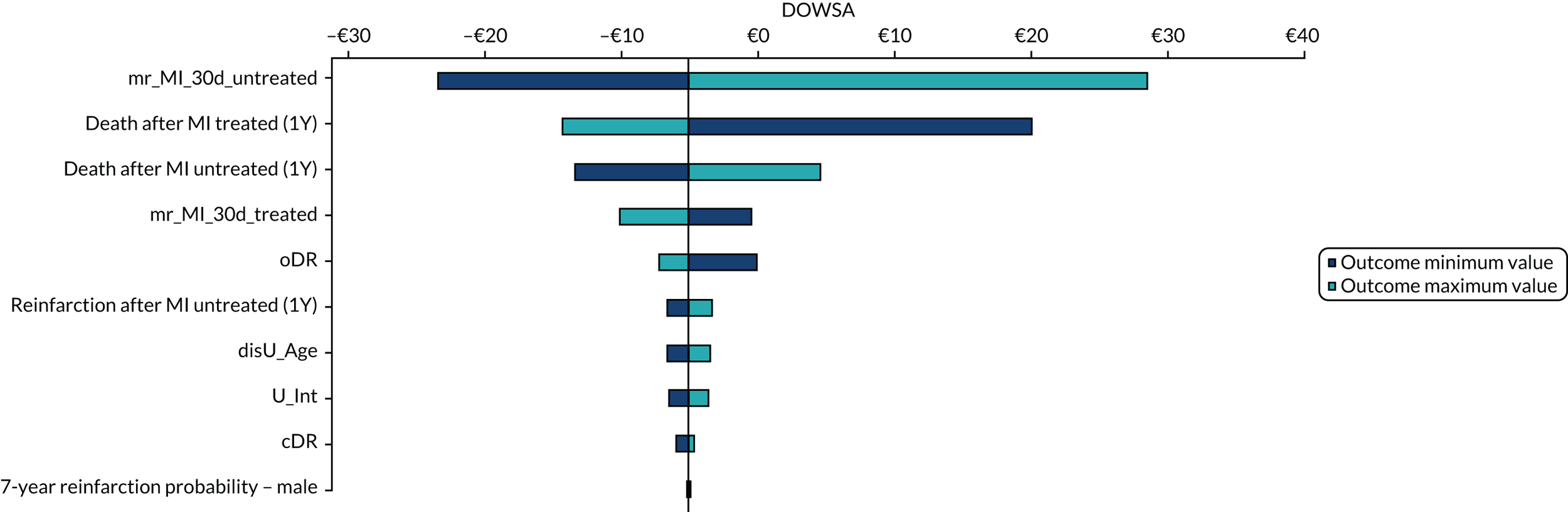
FIGURE 25.
Tornado diagram for comparison of standard troponin (at presentation and after 10–12 hours) with the Siemens Dimension Vista hsTnI (< 5 ng/l at 0 hours AND Δ < 2 ng/l at 0 to 1 hours). DOWSA, deterministic one-way sensitivity analysis.
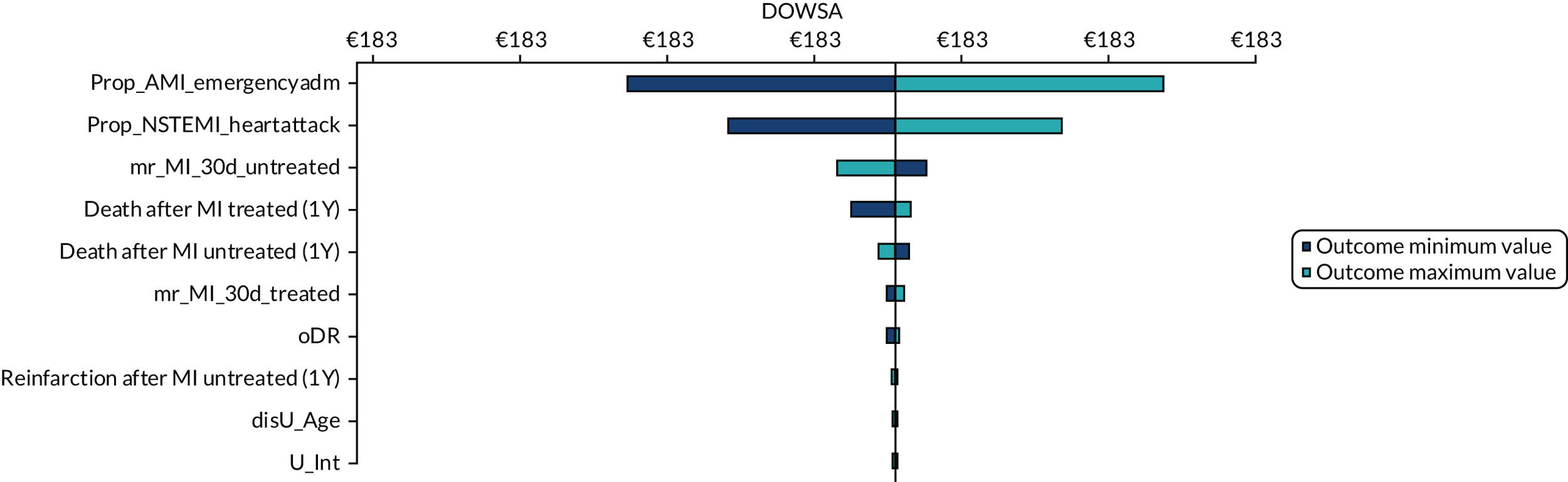
Deterministic one-way sensitivity analysis for the secondary analysis (based on incremental net benefit)
FIGURE 26.
Tornado diagram for comparison of the Beckman Coulter ACCESS hsTnI [(symptoms > 3 hours AND < 4 ng/l at 0 hours) OR (< 5 ng/l AND Δ < 5 ng/l at 0 to 2 hours)] with standard troponin (at presentation and after 10–12 hours). DOWSA, deterministic one-way sensitivity analysis.

Appendix 9 NICE guidance relevant to the management of suspected acute coronary syndrome
Myocardial Infarction: Cardiac Rehabilitation and Prevention of Further Cardiovascular Disease. NICE CG172. URL: www.nice.org.uk/guidance/cg172 (accessed 29 January 2020).
Chest Pain of Recent Onset: Assessment and Diagnosis Of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin. NICE CG95. URL: www.nice.org.uk/guidance/cg95 (accessed 29 January 2020).
Unstable Angina and NSTEMI: Early Management. NICE CG94. URL: http://guidance.nice.org.uk/CG94/NICEGuidance/pdf/English (accessed 29 January 2020).
Myocardial Infarction with ST-Segment Elevation: The Acute Management of Myocardial Infarction with ST-Segment Elevation. NICE CG167. URL: www.nice.org.uk/guidance/cg167 (accessed 20 February 2020).
Myocardial Infarction (Acute): Early Rule Out Using High-Sensitivity Troponin Tests (Elecsys Troponin Thigh-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 Assays). Diagnostics Guidance [DG15]. URL: www.nice.org.uk/guidance/dg15 (accessed 29 January 2020).
CG94, CG172 and CG167 are currently under revision to become a single guideline. Expected publication date is November 2020 (GID-NG10085).
Appendix 10 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist
| Section/topic | # | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis or both | Page 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary, including (as applicable) background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; and systematic review registration number | Pages 14–16 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Objective, page 27 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS | Objective and definition of decision problem, pages 27–41 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address) and, if available, provide registration information including registration number | PROSPERO registration, page 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | Systematic review methods, inclusion and exclusion criteria, page 43 and Table 2 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Systematic review methods, search strategy, pages 42 and 43 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review and, if applicable, included in the meta-analysis) | Systematic review methods, inclusion screening and data extraction, page 46 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Systematic review methods, inclusion screening and data extraction, page 46 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | Systematic review methods, inclusion screening and data extraction, page 46 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether or not this was done at the study or outcome level) and how this information is to be used in any data synthesis | Systematic review methods, quality assessment, pages 46 and 47 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | Systematic review methods, methods of analysis/synthesis, page 47 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | Systematic review methods, methods of analysis/synthesis, page 47 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | NA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | NA |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Results of the assessment of clinical effectiveness, overview of included studies, pages 47–9 and Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | Results of the assessment of clinical effectiveness, pages 62–93, Table 3 and Appendix 2 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Results of the assessment of clinical effectiveness, study quality, pages 57–62 and Appendix 3 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study (a) a simple summary data for each intervention group (b) effect estimates and CIs, ideally with a forest plot | Results of the assessment of clinical effectiveness, pages 62–93 and Appendix 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | Results of the assessment of clinical effectiveness, pages 65–81 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | NA |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see item 16)] | NA |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings, including the strength of evidence for each main outcome, and consider their relevance to key groups (e.g. health-care providers, users and policy-makers) | Discussion, pages 146–51 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias) and at review level (e.g. incomplete retrieval of identified research, reporting bias) | Discussion, pages 152–9 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research | Conclusions, pages 161 and 162 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data) and the role of funders for the systematic review | Page 2 |
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False negative
- An incorrect negative test result (i.e. the number of diseased persons with a negative test result).
- False positive
- An incorrect positive test result (i.e. the number of non-diseased persons with a positive test result).
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test that is being evaluated.
- Likelihood ratio
- The likelihood ratio describes how many times more likely it is that a person with the target condition will receive a particular test result than a person without the target condition.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality-adjusted life-year
- A measure of health gain used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best, currently available, method for diagnosing the target condition. The index test is compared against this to allow calculation of estimates of accuracy.
- Sensitivity
- The proportion of people with the target disorder who have a positive test result.
- Specificity
- The proportion of people without the target disorder who have a negative test result.
- State–transition model
- A model in which individuals move (transition) between disease states as their condition changes over time. Time spent in each disease state for a single model cycle (and transitions between states) is associated with a cost and a health outcome.
- True negative
- A correct negative test result (i.e. the number of non-diseased persons with a negative test result).
- True positive
- A correct positive test result (i.e. the number of diseased persons with a positive test result).
List of abbreviations
- ACC
- American College of Cardiology
- ACS
- acute coronary syndrome
- ADAPT
- 2-Hour Accelerated Diagnostic Protocol to Assess Patients With Chest Pain Symptoms Using Contemporary Troponins as the Only Biomarker
- AHA
- American Heart Association
- AiC
- academic in confidence
- AMI
- acute myocardial infarction
- APACE
- Advantageous Predictors of Acute Coronary Syndromes Evaluation
- BEST
- Bedside Evaluation of Sensitive Troponin
- CAD
- coronary artery disease
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CDSR
- Cochrane Database of Systematic Reviews
- CE
- Conformitè Europëenne
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CG
- clinical guideline
- CHD
- coronary heart disease
- CI
- confidence interval
- CoV
- coefficient of variation
- CRD
- Centre for Reviews and Dissemination
- cTn
- cardiac troponin
- cTnI
- cardiac troponin I
- cTnT
- cardiac troponin T
- DAR
- diagnostic assessment report
- DARE
- Database of Abstracts of Reviews of Effects
- DG
- diagnostics guidance
- DTA
- diagnostic test accuracy
- ECG
- electrocardiogram
- ED
- emergency department
- EDACS
- Emergency Department Assessment of Chest Pain Score
- eGFR
- estimated glomerular filtration rate
- ESC
- European Society of Cardiology
- FN
- false negative
- FP
- false positive
- GRACE
- Global Registry of Acute Coronary Events
- HEART
- History ECG Age Risk factors Troponins
- HES
- Hospital Episode Statistics
- High-STEACS
- High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome
- High-US
- High-Sensitivity Cardiac Troponin I Assays in the United States
- HiSTORIC
- High-Sensitivity Cardiac Troponin On Presentation to Rule Out Myocardial Infarction
- hs-cTn
- high-sensitivity cardiac troponin
- hs-cTnI
- high-sensitivity cardiac troponin I
- hs-cTnT
- high-sensitivity cardiac troponin T
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMPACT
- Improved Assessment of Chest Pain Trial
- IQR
- interquartile range
- LoB
- limit of blank
- LoD
- limit of detection
- MACE
- major adverse cardiac event
- MI
- myocardial infarction
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NSTE-ACS
- non-ST segment elevation acute coronary syndrome
- NSTEMI
- non-ST elevation myocardial infarction
- OR
- odds ratio
- PROSPERO
- International Prospective Register of Systematic Reviews
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUART
- QUeensland Accelerated Risk Trial
- RATPAC
- Randomised Assessment of Treatment using Panel Assay of Cardiac Markers
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- ROMI-3
- Optimum Troponin Cutoffs for ACS in the ED
- RR
- relative risk
- SD
- standard deviation
- SROC
- summary receiver operating characteristic
- STEMI
- ST elevation myocardial infarction
- TN
- true negative
- TP
- true positive
- TRAPID-AMI
- High Sensitivity Cardiac Troponin T Assay for Rapid Rule-out of Acute Myocardial Infarction
- TRUST
- Triage Rule-out Using Sensitive Troponin
- UA
- unstable angina
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Diagnostic Advisory Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.