Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/130/73. The contractual start date was in July 2017. The draft report began editorial review in July 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Booth et al. This work was produced by Booth et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Booth et al.
Chapter 1 Introduction
Some material throughout this chapter has been reproduced with permission from Booth et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Scientific background and current evidence base
Urinary incontinence in care homes
Urinary incontinence (UI) is defined by the International Continence Society as ‘any involuntary loss of urine’. 2 Prevalence of UI increases with age,3 with the highest prevalence found in older adults living in residential or nursing care homes. 3 Adults living with dementia are three times more likely to have UI than individuals of equivalent age and characteristics who do not have dementia,4 with the development of UI often being a catalyst for individuals requiring full-time care. 5,6 The most common type of UI in the care home population is mixed UI,3 in which symptoms of urgency UI and stress UI occur together, and are often accompanied by additional functional leakage due to physical and cognitive frailty. Mixed UI is considered the most challenging form of incontinence to manage as it includes symptoms of both of the common types of UI (urgency and stress) and is often resistant to intervention. 7
Burden of urinary incontinence
The burden of UI is significant, and is increasing for older adults and care services. 8 It is a distressing condition for older adults, which has a negative impact on their dignity and quality of life. 9 UI is associated with cognitive and physical impairment,8,10 increased risk of falls and fractures,11 sleep disturbance,9 hygiene problems and tissue viability problems. 12 It also has a significant impact on social participation, which can lead to isolation and clinical depression. 13,14 Although the personal costs associated with reduced quality of life and social withdrawal are not quantifiable, the cost of treatment and management of UI remains high. The NHS currently spends upwards of £80M per year on absorbent pads alone for the purpose of containing UI,15 which excludes the costs of associated care needed to manage use of absorbent pads in older, frailer individuals or those living with dementia. With the number of such individuals predicted to increase rapidly as the population ages, the cost of managing UI is also set to increase, with significant implications for future care provision. 16
Management of continence in care homes
Urinary incontinence is viewed as a generic condition in care homes: assessment and identification of specific types of UI or bladder problems is not routine, and interventions targeted towards treatment of specific UI types, such as mixed or urgency UI, have not been extensively demonstrated in this setting. The most common management option for bladder and bowel incontinence in care homes is the use of absorbent products to contain the leakage, combined with scheduled toileting. 17,18 Other conservative, non-pharmacological options to aid management of UI, such as individualised voiding programmes, bladder training or pelvic floor muscle exercise programmes, are often considered unsuitable for care home environments because they are labour intensive and require a degree of co-operation and engagement, which may not be suitable for those with cognitive impairment or dementia. Antimuscarinic drugs may be prescribed for the management of urgency UI or overactive bladder problems; however, their use is associated with significant adverse effects in frail older people and may counteract the functional benefits of anticholinesterase inhibitors used in the treatment of dementia. 19,20 Active treatment of UI or the promotion of continence in care homes is rare and a containment approach predominates. 18
Use of transcutaneous posterior tibial nerve stimulation to treat urinary incontinence and possible mechanism of effect
Transcutaneous posterior tibial nerve stimulation (TPTNS) is a form of non-invasive neuromodulation used to treat the symptoms of an overactive bladder (OAB), including urgency UI and mixed UI. TPTNS uses surface electrodes applied behind the medial malleolus to electrically stimulate the tibial nerve. A full treatment programme is delivered in 30-minute bouts, twice per week over a 6-week period. Although the exact mechanism of action is yet to be fully understood, TPTNS is believed to restore the balance between excitatory and inhibitory bladder functioning by modulating the signal traffic to and from the bladder through the sacral plexus. 21 Current understanding suggests that stimulating afferent sacral nerves in the lower leg increases inhibition of the efferent pelvic nerve activity, which reduces detrusor contractility and increases bladder capacity. 21–23 By these means, TPTNS reduces the sensation of urgency and the frequency of voiding demanded, thus enabling improved bladder control. These mechanisms may also reduce the volume of urine retained in the bladder after voiding. 23 For the care home population, TPTNS may reduce the sudden urge to urinate, allowing residents more time to reach a toilet, and because the bladder capacity may increase the frequency of daily voiding may also reduce. In turn these changes may enable more appropriate use of the toilet to void, rather than relying on containment of leakage by absorbent products.
A treatment approach, as opposed to a containment approach, engenders respect for the person’s right to use a toilet. Treatment using TPTNS is distinctive because, unlike commonly used behavioural interventions, there is no requirement for the recipient to engage with the treatment. Therefore, it is highly suited to individuals who are frail and/or living with dementia, and the stimulation site at the tibial nerve in the ankle ensures that the person’s dignity and comfort are maintained.
Evidence for effectiveness of transcutaneous posterior tibial nerve stimulation
There is evidence that TPTNS can be effective at reducing symptoms of urgency or mixed UI in women, and in adults with neurogenic bladder dysfunction. 24–27 A systematic review of TPTNS for the treatment of UI using Cochrane methodology identified 10 randomised controlled trials (RCTs), with a total of 472 participants. 24 Improvements were reported in all studies in terms of bladder symptoms and/or UI-related quality of life. Although the studies had small sample sizes and contained methodological weaknesses [Grading of Recommendations Assessment, Development and Evaluation (GRADE) score gave ‘low quality’ ratings], the results indicated that TPTNS showed promise as a safe, low-cost intervention for UI. 28 One small-scale feasibility study has been undertaken in care homes29 and indicated that TPTNS had the potential to be a safe, acceptable and effective treatment for UI in this population. However, definitive evidence on the effectiveness of TPTNS is required before it can be recommended for routine practice in a care home context.
Rationale for research
Current evidence24 to date has been generated from studies based on women only, or on both men and women adult populations. The participants in these studies had both physical and cognitive capacity, and the studies were conducted in outpatient health-care contexts. One small pilot study has shown the safety and acceptability of using TPTNS in care homes,29 but no other research in this care setting has been undertaken. Given the complexity of need, and the physical and mental dependence of care home residents, and the particular challenges of UI and its management in care homes there is a need for definitive evidence on the effectiveness of TPTNS before it can be recommended for routine practice in the care home context.
Aims and objectives
Aim
The aim of the ELECTRIC (ELECtric Tibial nerve stimulation to Reduce Incontinence in Care homes) trial was to determine the clinical effectiveness of a programme of TPTNS to treat UI in care home residents, and the associated costs and consequences.
Objectives
-
To establish whether or not TPTNS is more effective than sham stimulation for reducing the volume of UI at 6, 12 and 18 weeks in care home residents.
-
To investigate mediating factors that have an impact on the effectiveness of TPTNS in a mixed-methods process evaluation involving fidelity, implementation support and qualitative components.
-
To undertake an economic evaluation of TPTNS in care homes assessing the costs of providing the programme and presenting these costs alongside the key primary and secondary outcomes in a cost–consequences analysis.
-
To explore in an interview study the experiences of TPTNS from the perspectives of care home residents, family carers, care home staff (nurses, carers and senior carers), and care home managers.
Chapter 2 Trial design and methods
Study design
The ELECTRIC trial was designed to evaluate TPTNS, an intervention to treat UI, in care home residents. It was a multicentre, pragmatic, participant and outcome assessor-blind, randomised placebo-controlled trial comparing the effectiveness of TPTNS with sham stimulation to reduce the volume of UI in care home residents. The main trial was supplemented by an economic evaluation comparing TPTNS with the usual continence care pathway using a cost–consequences analysis (see Chapter 4). A mixed-methods process evaluation explored the adherence to intervention delivery and the acceptability of the intervention and support. A qualitative study was also undertaken, which explored views and experiences with TPTNS among care home residents, their families and care home staff, and is presented in Chapter 5. An internal pilot study was conducted with 97 participants. All stop/go criteria were met, allowing progression to the full trial.
The trial was designed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist30 and the ELECTRIC trial protocol was published in an open access journal. 1
Ethics approval and research governance
Version 1.0 of the ELECTRIC trial protocol was approved by the Yorkshire and Humber Bradford Leeds Research Ethics Committee (REC) [reference 17/YH/0328, integrated research application system (IRAS) ID 233879 – see Report Supplementary Material 1) for English care home sites and by the Scotland A REC (reference 17/SS/0117, IRAS ID 224515 – see Report Supplementary Material 2) for Scottish care home sites. Ethics approval was also granted by Glasgow City Health and Social Care Partnership for involvement of care homes run by Glasgow City Council on 14 September 2018. The trial sponsor was Glasgow Caledonian University (GCU) and the ELECTRIC trial office was established at GCU. The trial was prospectively registered with Clinical Trials.gov under the registration number NCT03248362 (August 2017), and with the International Standard Randomised Controlled Trial Register (ISRCTN) under the reference number 98415244 (April 2018). A summary of the changes made to the protocol, which were approved by both RECs, is provided in Appendix 1.
Participants
The trial recruited older adults, including those with cognitive impairment, residing in nursing or residential care homes in England and Scotland who experienced UI at least weekly.
Eligibility criteria
The care home residents were eligible for inclusion if they:
-
had self- or staff-reported UI more than once per week
-
used the toilet or a toilet aid for bladder emptying, with or without assistance
-
wore absorbent pads to contain urine.
Exclusion criteria included care home residents:
-
with an indwelling urinary catheter
-
with a symptomatic urinary tract infection (UTI)
-
with a post void residual urine volume (PVRU) > 300 ml
-
with a cardiac pacemaker
-
with epilepsy that was being treated
-
with bilateral leg ulcers
-
with pelvic cancer (current)
-
with palliative care status
-
who were non-English speakers.
Recruitment procedure
Identification and informed consent
One person in each care home (usually the care home manager or a senior nurse) was assigned as the local principal investigator (PI) for that site. The PI was responsible for identifying potentially eligible participants in the care home based on the aforementioned criteria (see Report Supplementary Material 9). However, the process for recruitment differed based on the location of the care home, in accordance with statutory requirements on capacity to provide informed consent to participate in research in Scotland and England (see below). All consent procedures were approved by RECs with responsibility for reviewing applications for research involving adults who lack capacity.
Recruitment in Scotland
The PI compiled a log of potentially eligible residents in the care homes based on the trial eligibility criteria, then provided trial information to the resident if appropriate (depending on capacity), and sought permission for the trial team to contact them with the purpose of providing more detailed information about the trial and answering any questions. An aphasia-friendly version of the participant information sheet was made available when appropriate. This approach was made by the regional research assistant (RRA), who was a registered nurse working as part of the trial team. Under the Adults with Incapacity (Scotland) Act 2000,31 when a resident had a certificate of incapacity the local PI would identify and provide the study information to the resident’s welfare attorney (if one was appointed) or their nearest relative. If there was no welfare attorney identified or the resident did not have a relative who could be consulted then they were considered ineligible to participate in the study. The local PI would seek agreement from the welfare attorney/nearest relative for the RRA to speak to the resident. Following confirmation from the local PI of permission to approach the resident or welfare attorney/nearest relative, the RRA would arrange to meet them (or telephone them if a meeting was not possible) to provide a full explanation of the trial, ensure eligibility (see Report Supplementary Material 10) and seek written consent for the resident to participate. In accordance with the principles of the Adults with Incapacity (Scotland) Act 2000,31 verbal consent or assent was sought at every research contact (to ensure ongoing consent to participate) and welfare attorneys/nearest relatives could withdraw consent for the resident at any time.
Recruitment in England
In England, the PI compiled a log of potentially eligible residents and approached residents with capacity similar to the procedure described for Scotland. In accordance with the Mental Capacity Act (MCA) 2005,32 if the local PI believed a resident’s capacity was in question, they would identify and provide the information to the resident’s personal consultee (usually a family member or friend) or, if one was not available, a nominated consultee identified by the care home study team, and would seek their agreement for an approach from the RRA. The RRA then provided a full explanation of the study, ensured eligibility and sought the consultee’s advice on what they felt that the resident’s wishes would have been about taking part in the trial, if they had had capacity. The consultee would sign a declaration form if they believed that the resident would have chosen to agree to participate. Similar to Scotland, verbal consent or assent was sought at every research contact and consultees could withdraw consent for the resident at any time if they felt that the resident’s wishes had changed.
Recruitment of family carers and care home staff
In both Scotland and England, written consent to answer questions about residents was sought from family carers and care home staff by the RRAs. In addition, the qualitative researcher identified and approached a sample of residents, family members and staff to take part in the process evaluation interviews and focus groups. When not previously given, written consent was obtained prior to the interview taking part.
Baseline visit
After informed consent was obtained from the resident or relevant person, the RRA performed a bladder scan for the final assessment of eligibility (PVRU < 300 ml) at the baseline visit. Data were collected for baseline measures (see Data collection) and a 24-hour pad collection was scheduled with care home staff (see Report Supplementary Material 11).
Randomisation, allocation and blinding
Following successful completion of baseline measures, including 24-hour pad weight test (PWT) (see Data collection), remote randomisation was initiated by the RRA. Residents were randomised to one of two groups: TPTNS or sham stimulation. Randomisation was computer allocated on a one-to-one basis in random permuted blocks of two, four or six individuals, with minimisation by sex – male/female; UI severity – mild (0–200 ml per 24 hours), moderate (201–400 ml per 24 hours), severe (> 400 ml per 24 hours); centre (care home).
Information required to perform the randomisation was submitted by the RRA to a web-based randomisation system created and managed by the Centre for Healthcare Randomised Trials (CHaRT) Unit at the University of Aberdeen, which ensured allocation was concealed from both participants and RRAs. The randomisation results, along with a unique study identification number, were automatically generated and emailed to the trial office, which in turn emailed these to the relevant local PI. The local PI recorded the allocation group in a separate file and informed the care home staff of which intervention to deliver.
The RRAs (who collected the data) were blinded to the allocation group. To ensure that the participant and their relatives/carers were also blinded to the allocation group, those in the control group received sham stimulation, rather than a no treatment comparator.
Intervention
Transcutaneous posterior tibial nerve stimulation utilises peripheral neuromodulation to improve bladder function. A portable electrical stimulation machine (Neurotrac Continence,™ Verity Medical Ltd, Tagoat, Ireland) and two surface electrodes were used to electrically stimulate the tibial nerve, which runs close to the skin surface behind the medial malleolus of the ankle.
In the treatment group, one electrode (the cathode) was placed behind the medial malleolus and the other (the anode) was placed 10 cm cephalad to it (two or three fingers above and slightly towards the back of the calf) – Figure 1 shows the correct positioning. Standardised stimulation parameters were applied of 10-Hz frequency, 200-µs–1 pulse width, in continuous stimulation mode. The machines were ‘locked’ prior to use so that the only adjustable parameter was the intensity of stimulation. The intensity (mA) was adjusted according to the tolerance level of the participant, keeping the intensity as high and close to the person’s motor threshold as possible, while still remaining comfortable throughout the session.
FIGURE 1.
Positioning of electrodes for TPTNS.
Comparison
In the sham group, the electrodes were placed on the lateral aspect of the ankle so as to avoid the tibial nerve. The cathode electrode was placed behind the lateral malleolus and the anode electrode 10 cm cephalad to it – Figure 2 shows the correct positioning. The same stimulation parameters as the treatment group were applied: 10-Hz frequency, 200-µs–1 pulse width, in continuous stimulation mode and the machines were locked prior to use. The intensity of stimulation was increased until the participant was aware of the sensation of electrical stimulation and then reduced to a low-intensity, subclinical stimulation of 4 mA for the duration of the session. The participant was informed that it was quite normal not to feel any sensation from the stimulation.
FIGURE 2.
Positioning of electrodes for sham stimulation.

Treatment delivery
Participants in both the treatment and sham groups received an electrical stimulation programme comprising 12 sessions of 30 minutes’ duration each, delivered twice per week over 6 weeks. The programme was delivered by care home staff who received specific training and support. A proposed schedule for each participant was suggested at the start of the intervention, but there was flexibility around where, how and when the sessions were delivered. Stimulations delivered were logged by care home staff on a stimulation diary for each participant (see Report Supplementary Material 12).
Each stimulation session was recorded by care home staff in a stimulation diary, showing the date, time of day, intensity of stimulation and any relevant comments, such as where the session was given or what the participant was doing at the time. The stimulation (TPTNS or sham) was offered to the participant a maximum of two times in any 24-hour period and if the stimulation was refused when first offered (verbally or by non-verbal behaviour), it was postponed by at least 1 hour and then offered one further time. Records of acceptance and refusals were documented in the stimulation diary.
Training
Regional research assistant training
The RRAs were trained by the chief investigator and trial managers in the following tasks: assessing eligibility, taking consent, collecting data, completing the case report form (CRF), recording adverse events (AEs)/change of status, randomisation procedures and using the trial database. This training was accompanied by a detailed standard operating procedure (SOP) for the RRAs. Each RRA underwent good clinical practice (GCP) training and 2-yearly updates as required.
Implementation support facilitator training
The implementation support facilitators (ISFs) received training from the chief investigator and trial managers on administering TPTNS in both the treatment and sham groups (to check correct use by care home staff), checking adherence and reporting AEs/change of status. A detailed SOP was developed for ISFs. The ISFs also underwent GCP training and 2-yearly updates as required.
Care home staff training
A bespoke training programme for care home staff on how to administer the intervention was developed and delivered to small groups in each home by the chief investigator and trial managers. The half-day theory and practice-based training course included:
-
a background presentation, including the purpose of the study, participants’ individual roles, education about UI in care home residents, usual management strategies, common challenges to continence promotion, theory of TPTNS and how to implement it in care
-
an informal discussion about what the staff found difficult and challenging around UI, what they believe ‘works’ when supporting people with dementia and how this would help implement TPTNS in usual-care pathways
-
a practical demonstration of application, initiation and removal of TPTNS/sham intervention
-
experiential learning, during which all staff were provided with an electrical stimulator to become familiar with the equipment and practise administering TPTNS/sham intervention to each other under supervision of the chief investigator and trial managers.
A SOP was developed for care home staff and a signed Certificate of Competence was awarded to staff who completed the ELECTRIC trial training and achieved competency in delivering the intervention.
Following the training session(s), the ISF worked with the care home staff in a period of facilitated support to build their confidence and help them implement their learning in practice. This was followed by an individual staff competency assessment during the first 1–2 weeks of the intervention, during which the ISF corrected any errors in delivery of the intervention (TPTNS or sham), provided any remedial training and issued a further Certificate of Competence.
Care home principal investigator training
The PI in each care home underwent similar training to care home staff (described above), with additional training on identifying potential participants and completing the site screening log, reporting AEs/change of status and storing trial paperwork. A SOP was developed to provide support to PIs in this role.
Training handbook and digital versatile disc
As part of the training programme, a training handbook (see Report Supplementary Material 3) and digital versatile disc (DVD) were developed to ensure that care home staff were knowledgeable and competent to administer the intervention. These resources were available electronically and were presented to staff during training.
Adherence monitoring
Records of stimulations delivered (sham and treatment) were kept by care home staff using individual stimulation diaries for each participant. Date, time, intensity and length of stimulation were recorded, and this log was compared with data recorded automatically by the locked electrical stimulator machines at three time points (approximately once every 2 weeks) during the 6-week intervention period (see Report Supplementary Material 13). Any discrepancies between the two records were flagged and, when appropriate, additional training provided to care home staff to correct for future stimulations (e.g. if the intensity of sham stimulation was found to be higher than 4 mA, staff were advised to lower the intensity to 4 mA in future stimulations). These adherence checks were initially performed by local PIs, who were also asked to ensure correct electrode positioning (i.e. how they would be positioned during the treatment) photographically. However, during the pilot study it emerged that this was too great a burden for local PIs and adherence checks were being missed. Taking photographs of electrode positioning was also found to be problematic because the person conducting the adherence monitoring was not always present at the time of the stimulation, thus they were subsequently unable to identify participants from the photograph. Following discussions with the project management group (PMG) and approval from both RECs, the role of adherence monitoring was passed to the ISFs, who were already visiting care homes to independently witness that stimulations were being delivered according to the protocol and address additional training needs. They were thus able to directly observe electrode positioning. In addition, a field was added to the stimulation diary for care home staff to record electrode positioning as the internal or external ankle site. In the case of the final adherence check being made at the end of the stimulation period with no further stimulations due, the last adherence check was made by the trial managers in the trial office (Scottish care homes only). The ISF based in England performed all three adherence checks.
Using data from the three checks, adherence was assessed as follows:
-
Time was judged to be correct if the time on the stimulation diary was within 1 hour of that recorded on the electrical stimulator (which was only able to record in intervals of whole hours).
-
Intensity was judged to be correct for participants in the treatment group if the average measurement recorded by the electrical stimulator was higher than 10 mA and within 10 mA of that recorded in the diary.
-
Intensity was judged to be correct for participants in the sham group if the average recorded by the electrical stimulator and stimulation diary was lower than 10 mA.
-
Position was judged to be correct if the local PI/ISF witnessed electrodes positioned correctly for the group to which the participant was allocated (i.e. medial side of ankle for those in the treatment group, lateral side of ankle for those in the sham group). Position was also judged to be correct if the position field on the stimulation diary was correctly completed.
An adherence check (of which there were three) was judged to be correct if the criteria for time, intensity and position were all assessed as correct. Overall adherence was deemed to be correct for a participant if two or more adherence checks were correct.
Data collection
Baseline and outcome data were collected by the RRAs during face-to-face visits to the care homes and from accessing care home medical records. To ensure consistency, the RRAs followed a SOP for data collection. The data were entered by the data co-ordinator into a database created and managed by CHaRT, University of Aberdeen. Randomisation occurred following the baseline visit and outcomes were measured at 6, 12 and 18 weeks post randomisation.
Primary outcome
The primary outcome was the volume of urine leaked in 24 hours at 6 weeks post randomisation, measured using a 24-hour PWT. This test is based on the premise that 1 g of fluid weight is equal to 1 ml of urine and is thus an objective measure of urine leakage. To carry out a PWT, the participant (with the help of staff if required) emptied their bladder, applied a clean, dry pad at an agreed set time and then retained all used pads for the following 24 hours. Individual pads were sealed in a small plastic bag and then collected in a larger resealable bag to prevent evaporation. The RRA collected and weighed the large bag as close to the end of the 24-hour pad collection period as possible, and the dry weight of an equivalent number of pads to those collected was deducted from the total weight of the bag, to provide the 24-hour volume of urine passed as a result of UI.
Secondary outcomes
Urinary outcomes
-
The 24-hour PWT was repeated at 12 and 18 weeks to assess the sustainability of any effect of TPTNS.
-
The number of pads used in 24 hours was measured at 6, 12 and 18 weeks post randomisation and recorded by care home staff in a bladder diary.
-
The PVRU was measured at 6, 12 and 18 weeks post randomisation using a portable ultrasound bladder scanner to assess if there was any impact of TPTNS on urinary retention.
-
The Perception of Bladder Condition (PBC)33 was assessed at 6, 12 and 18 weeks post randomisation. This is a single-question global patient-reported outcome measure of perceived bladder condition, and was adapted for use with family carers and staff as well as participants.
-
The Minnesota Toileting Skills Questionnaire (MTSQ)34 was administered at 6, 12 and 18 weeks post randomisation. This is a five-question patient-reported outcome measure of degree of difficulty relating to skills necessary to use the toilet and was completed by the participant and a staff member separately.
Quality-of-life outcomes
-
Dementia Quality of Life (DEMQOL)35 was assessed at 6 and 18 weeks post randomisation. This was completed by the participant and/or an identified proxy (using DEMQOL-PROXY) for that participant. DEMQOL measures health-related quality of life in people with dementia.
Economic outcomes
-
A resource use questionnaire (RUQ) incorporated into the CRFs was administered by the RRAs at baseline, and then at 6 and 18 weeks post randomisation (see Report Supplementary Material 4–7). This recorded the participant’s usual continence care pathway, including details on aids and devices for managing incontinence, medication relating to continence and, if appropriate, how many staff were required to help with toileting.
-
Staff grades and the time required for delivering the intervention were recorded by each care home and costed using the appropriate pay scales for each site, alongside cost estimates for training materials, the trainer and training time, based on the market rates for these items. Care provided by health professionals external to the care home as a result of UI was also recorded in the CRF, along with unit costs attached to these resources using standard sources including NHS Reference Costs,36 Unit Costs of Health and Social Care 201937 and the British National Formulary. 38
Qualitative data collection
See Chapter 5 for further details.
Resident/family members
Face-to-face interviews were conducted with a sample of care home residents and/or family members at 6 weeks (i.e. immediately following the intervention) and 12 weeks to explore perspectives relating to the intervention and any noted impact on continence status and quality of life.
Care home staff
Focus group and individual interviews were conducted with care home staff who had received the ELECTRIC trial training and were involved in the direct delivery of the TPTNS/sham intervention to elicit views about the treatment, care home practices and the research processes.
Care home managers
Individual interviews with care home managers, with a range of working backgrounds and years of experience, were completed at 18 weeks, when the study concluded.
Internal pilot study
An internal pilot study was undertaken to assess the feasibility of recruitment and retention, adherence to the allocated stimulation programme and completion of the primary outcome measure. Data were collected and assessed against four preset criteria during the first 6 months of the recruitment phase (months 6–12) and reviewed by the Trial Steering Committee (TSC) and funders in month 13 to determine whether or not the ELECTRIC trial should progress to full trial. Data for each of the four criteria were categorised using a traffic light system of green (to indicate ‘continue’), amber (to indicate the need to ‘implement contingency measures’) and red (to indicate the need to ‘pause’ the trial and investigate the possibility of discontinuing the trial). Table 1 outlines the four criteria and preset thresholds for traffic light categories.
| Criterion | Target | Green: continue | Amber: implement contingency measures | Red: pause trial |
|---|---|---|---|---|
| 1. Recruitment | 100 residents during pilot | > 90 residents recruited | 76–90 residents recruited | ≤ 75 residents recruited |
| 2. Adherence to stimulation | At least 8 out of 12 stimulation sessions received by each participant | > 70% of participants receive ≥ 8 stimulation sessions | 50–70% of participants receive ≥ 8 stimulation sessions | < 50% of participants receive ≥ 8 stimulation sessions |
| 3. Completeness of 24-hour PWT at 6 weeks | A complete PWT for all randomised participants (no missing data) | > 70% of participants with complete 6-week PWT | 50–70% of participants with complete 6-week PWT | < 50% of participants with complete 6-week PWT |
| 4. Fidelity to allocated intervention | All residents remaining in trial to receive the intervention protocol associated with the group to which they were allocated, in terms of duration, intensity and correct ankle position | > 70% of participants correctly receive ≥ 8 stimulation sessions | 50–70% of participants correctly receive ≥ 8 stimulation sessions | < 50% of participants correctly receive ≥ 8 stimulation sessions |
Participant withdrawal
Participants who withdrew from the treatment/sham intervention could still participate in all other follow-up outcome data if they wished, for example questionnaires or interviews. Those who were unable to participate in some 6- or 12-week data collection measures (e.g. if they were hospitalised or too unwell) could still be followed up at subsequent visits.
Sample size
Original sample size justification
The original recruitment target was calculated to be 500 care home residents, based on a sample size of 344 needed to detect important clinical differences of 200 ml per 24 hours of urine leakage, with 90% power at the two-sided 5% alpha level, including an inflated attrition estimate of 30% to account for loss owing to death and other types of loss to follow-up. The standard deviation (SD) used in the original calculation came from a small, single-centre trial with a selected population in which the reported SD was 450 ml. 39 A 95% confidence interval (CI) was put around the SD estimate and the upper CI bound (570 ml) was used as a conservative measure for the sample size calculation to account for recruiting to a pragmatic multicentre trial.
Revised recruitment target
Following 1 year of recruitment to the ELECTRIC trial, a data cut was performed by CHaRT, University of Aberdeen, and the recruitment target was reviewed. Kieser and Friede40 recommend re-estimating the sample variance from observed data using the whole trial cohort and calculating the one-sample variance, and also an adjusted estimate to account for potential bias in the one-sample variance, under the alternative hypothesis. The required sample size was then recalculated without penalty to the type I error rate. The SDs using these two methods were 427 and 415, which indicated sample sizes of 194 and 184, respectively. Choosing the more conservative of these and applying the increase for 30% attrition gave a total of 278 participants randomised for 90% power to detect a 200 ml difference in the primary outcome. The observed attrition after 1 year of recruitment to the ELECTRIC trial was 15%, suggesting 278 participants to be a conservative upper bound estimate of the required sample size. However, to account for potential differences in variability and missingness of data at the beginning and end of the trial it was concluded that recruitment should continue for the duration of the planned recruitment period (18 months), with the aim of exceeding the minimum requirement of 278 randomised participants. Three blinded independent statisticians, the Data Monitoring and Ethics Committee (DMEC), the TSC, the PMG and the funders all agreed with the revision to the sample size and recruitment target.
Statistical analysis
All analyses were prespecified in a statistical analysis plan (SAP) (see Report Supplementary Material 8) approved by the PMG, TSC and DMEC in advance of the analysis. A 5% two-sided significance level was used to denote statistical significance throughout, with any estimates displayed with 95% CIs and p-values. Statistical analysis was undertaken according to the intention-to-treat principle based on all participants who were randomised. All analyses were undertaken in Stata® version 15 (StataCorp LP, College Station, TX, USA).
Primary outcome
The primary outcome, total volume of urine leaked in 24 hours at 6 weeks post randomisation, was analysed using linear regression correcting for baseline 24-hour PWTs and the design covariates [severity of UI (mild/moderate/severe) and sex (male/female)]. Potential care home clustering was controlled for using a random-effects robust variance. In addition, the effects of adherence to treatment (duration, intensity and position) were explored using randomisation as the instrumental variable in a complier-average causal effect (CACE) model40 (using two-stage least squares). Compliance was determined for both the TPTNS and sham groups individually (see Figure 3). However, for the CACE analysis, compliance is defined as being compliant to the intervention (i.e. TPTNS). Consequently, in the modelling, compliance for the sham group is set to zero to reflect that the sham group is not compliant to TPTNS.
Secondary outcomes
Secondary outcomes were analysed using a similar strategy, but with models suitable for each outcome. In addition, these utilised all available follow-up data from all randomised participants using a standard time interaction model to incorporate repeated measures. These were estimated using generalised linear model linear regression for continuous data; although outcomes may be skewed, the use of baseline information as a covariate will satisfy the normal assumptions. For binary outcomes, Poisson regression models were used with a log-link function summarising the treatment effects as adjusted risk differences and adjusted relative risk ratios. All models were adjusted as described above.
All model assumptions were assessed by means of the summary statistics and/or graphical plots.
Patient-reported outcome measures
Patient-reported outcome measures (PROMs) collected using validated questionnaires were combined into an overall score and missing items were imputed using the strategies described in appendix B of the SAP (see Report Supplementary Material 8), and taking into account the level of missingness overall and within a person.
Subgroup analyses
Predefined subgroups were reported as the magnitude of the subgroup effect estimates along with their 95% CIs, and interpreted in an exploratory manner and broadly to provide recommendations for further investigations. Predefined subgroups were tested using interactions for the following:
-
sex (male/female)
-
UI severity [mild (0–200 ml per 24 hours); moderate (201–400 ml per 24 hours); severe (> 400 ml per 24 hours)]
-
functional dependency –
-
total Barthel Index score
-
Barthel Index Mobility score (immobile, wheelchair independent, walks with help of one person, independent)
-
Barthel Toilet Use score (dependent; occasional accident; continent)
-
-
clinical frailty scale – (≤ 5, 6, 7 or more)
-
on anticholinergics for incontinence (or not)
-
falls status in last 6 months
-
number residents who, at baseline, have fallen in the last 6 months [n (%)]
-
number of falls: ≤ 6 falls, > 6.
-
For identified post hoc subgroups, forest plots were used to illustrate possible effects of the following:
-
size of the centre
-
functional mobility
-
restricted communication
-
need for help to use the toilet
-
number of helpers needed to use the toilet
-
urinary urgency symptoms.
Economic evaluation
A cost–consequences analysis (CCA) approach was used to incorporate the range of costs and benefits of the TPTNS intervention (see Chapter 4). The conclusions of economic analysis were presented in a disaggregated ‘balance sheet’ form, presenting costs of providing the intervention alongside the key primary and secondary outcomes. Health-related quality of life was assessed using Dementia Quality of Life - utility index (DEMQOL-U) and Dementia Quality of Life Proxy - utility index (DEMQOL-PROXY-U) with a UK tariff. 41 Full details on how unit costs were attached to resource use are presented in Chapter 4.
Breaches
Only one protocol breach was documented during the trial (see Appendix 3). This involved the unblinding of a participant’s relative by a member of care home staff at the 12-week follow-up, who subsequently unintentionally unblinded the RRA. The incident was reported to the chief investigator, who took the decision that the RRA should continue to collect 12- and 18-week follow-up data as their ability to influence outcome measures was minimal.
Public and patient involvement
To involve users and carers in the set-up and delivery of the ELECTRIC trial, a stakeholder and public involvement group (SPIG) was established to meet every 6–9 months during the trial, undertaking the following roles:
-
advising on information and documentation, for example consent and information forms
-
assessing the acceptability of intervention and data collection processes
-
assisting with the interpretation of finding and results
-
assisting with the development and distribution of lay summaries.
Members of the SPIG included an older adult with experience of receiving TPTNS, a family carer with extensive experience of developing practice in care homes, a Scottish Care Inspectorate Health Improvement Advisor with a special interest in promoting continence, representatives from Age Scotland (Edinburgh, UK) and Alzheimer Scotland (Edinburgh, UK), an NHS Continence Service Manager, and a care home manager and senior clinical nurse with extensive educational experience. One of the members of the SPIG was invited to be a study co-applicant and joined the PMG, and another member (with experience of TPTNS) agreed to join the TSC to enable patient and public involvement (PPI) representation in these groups.
Our original research proposal included a Care Home Reference Group (CHRG), comprising residents and staff of a single BUPA UK (London, UK) care home, that was consulted during proposal development about elements of our proposed plans, particularly the outcome measures. However, because of the BUPA UK decision to close their care homes in Scotland, our planned study partnership was unable to progress.
Trial oversight
The ELECTRIC trial was managed by a PMG comprising all co-investigators and representatives from the trial office and the CHaRT trial team. The PMG met regularly face to face or by teleconference to review and direct study progress. A TSC with independent members, including PPI, oversaw the conduct and progress of the trial. The DMEC oversaw the safety of participants within the trial.
Chapter 3 Trial outcomes and results
This chapter describes the recruitment, baseline characteristics and other data on participants and the care homes that they were recruited from, and reports the results of the trial analysis as described in the SAP (see Report Supplementary Material 8).
Care home and participant recruitment
A total of 44 care homes agreed to participate in the ELECTRIC trial. Of these, 37 care homes recruited at least one resident (see Appendix 2). Between January 2018 and July 2019, 714 residents were screened for inclusion, of which 410 were eligible to be randomised.
Figure 3 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial from care home identification, through resident eligibility screening and randomisation to outcomes data collection at the three follow-up time points. Reasons for declining to participate, ineligibility and withdrawal/attrition at each outcome assessment are presented. Two residents were excluded following consent, but before randomisation, because a baseline 24-hour PWT was not available.
FIGURE 3.
A CONSORT flow diagram of care homes and participants through the ELECTRIC trial. PO, patient outcome; REL, resident eligibility log. a, Resident/welfare guardian/attorney; b, for CACE adherence to sham protocol is zero – see Primary outcome and Intervention received.

We randomised 408 participants, but two participants were classified as post-randomisation exclusions and none of their data (baseline or otherwise) is included in any tables. Both participants were in the TPTNS group, had pacemakers and were randomised in error. Both received some TPNS treatment (six and two sessions, respectively) but this was stopped immediately on discovery of the pacemaker. Neither participant experienced any AE related to receiving TPTNS. In total, 406 residents were included in the trial: 197 allocated to the TPTNS group and 209 to the sham stimulation group. Resident recruitment was completed by the end of July 2019 and all follow-up data was collected by January 2020.
Baseline characteristics
Table 2 summarises the participants’ general baseline characteristics. The two groups were comparable at baseline. Resident age ranged from 58 to 107 years and the mean age was 85 years (SD 8 years). The majority of residents in both groups were female (76.6% in the TPTNS group; 78.5% in the sham group) with moderate cognitive impairment [mean Mini Mental State Examination (MMSE) score 12.8 (SD 9.3) in the TPTNS group and 13.4 (SD 8.9) in the sham group]. Participants had similar levels of high physical dependency [mean Barthel score 7.2 (SD 3.9) in the TPTNS group and 7.9 (SD 4.0) in the sham group] and, in both groups, the majority were severely or very severely frail (54.3% in the TPTNS group; 51.2% in the sham group).
| Variable | TPTNS (N = 197) | Sham (N = 209) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Female, n (%) | 151 (76.6) | 164 (78.5) | ||||
| Age (years) | 85 | 8 | 85 | 8 | ||
| MMSE total score | 119 | 12.8 | 9.3 | 133 | 13.4 | 8.9 |
| Length of stay prior to randomisation (weeks) | 194 | 109 | 93 | 206 | 115 | 125 |
| DEMQOL | 70 | 84.6 | 14.1 | 80 | 88.7 | 14.9 |
| DEMQOL-PROXY | 193 | 98.2 | 15.2 | 208 | 99.1 | 13.7 |
| Barthel score | 188 | 7.2 | 3.9 | 197 | 7.9 | 4 |
| Clinical frailty categories, n (%) | ||||||
| Managing well | 5 (2.5) | 14 (6.7) | ||||
| Vulnerable | 12 (6.1) | 9 (4.3) | ||||
| Mildly frail | 15 (7.6) | 26 (12.4) | ||||
| Moderately frail | 58 (29.4) | 53 (25.4) | ||||
| Severely frail | 107 (54.3) | 104 (49.8) | ||||
| Very severely frail | – | 3 (1.4) | ||||
| Falls in the last 6 months | ||||||
| Number of residents who have fallen, n (%) | 91 (46.2) | 102 (48.8) | ||||
| Number of falls per resident | 197 | 1.8 | 5.5 | 208 | 1.1 | 1.8 |
Table 3 summarises the residents’ continence status at baseline and shows that UI was a chronic condition, being present for at least 18 months in the TPTNS group and 20 months in the sham group. The majority of residents (59.4% in the TPTNS group; 55.5% in the sham group) experienced severe UI of > 400 ml leakage in 24 hours and all apart from one resident in the TPTNS group wore absorbent pads continuously to contain the leakage. The number of pads used in a 24-hour period was similar for both groups. The PVRUs were relatively small (approximately 80 ml, which is within normal limits), as was the number of residents taking anticholinergic medication to treat UI. The mean total volume of urine leaked in 24 hours (the primary outcome measure) at baseline was similar between the two groups (536 ml in the TPTNS group; 560 ml in the sham group) but the SDs indicate greater variability of leakage in the sham group (370 ml in the TPTNS group; 469 ml in the sham group).
| Variable | TPTNS (N = 197) | Sham (N = 209) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Base UI severity, n (%) | ||||||
| Mild (0–200 ml per 24 hours) | 44 (22.3) | 54 (25.8) | ||||
| Moderate (201–400 ml per 24 hours) | 36 (18.3) | 39 (18.7) | ||||
| Severe (> 400 ml per 24 hours) | 117 (59.4) | 116 (55.5) | ||||
| Total volume of urine (ml) in 24 hours | 197 | 536.0 | 369.6 | 209 | 560.4 | 468.7 |
| Duration of UI (months) | 63 | 17.7 | 18.1 | 75 | 20 | 31.5 |
| Pads used (24 hours) | 197 | 3.9 | 1.5 | 209 | 3.8 | 1.7 |
| PVRU (ml) | 177 | 75.5 | 65.1 | 183 | 80.8 | 70.3 |
| UTIs treated (antibiotics) | 197 | 0.4 | 0.9 | 207 | 0.3 | 0.7 |
| Wear pad continuously, N; n (%) | 194; 193 (99.5) | 207; 207 (100.0) | ||||
| On anticholinergics to treat UI, N; n (%) | 197; 8 (4.1) | 209; 7 (3.3) | ||||
| Toilet access restrictions, n (%) | ||||||
| Mobility | 168 (85.3) | 162 (77.5) | ||||
| Problems communicating need | 86 (43.7) | 85 (40.7) | ||||
| Problems locating toilet | 54 (27.4) | 67 (32.1) | ||||
| Does not try to get to toilet | 20 (10.2) | 34 (16.3) | ||||
| Other | 16 (8.1) | 24 (11.5) | ||||
| MTSQ (resident) | 82 | 6.7 | 7.8 | 94 | 5.1 | 7.1 |
| MTSQ (staff) | 194 | 13.7 | 6.8 | 203 | 12.7 | 7 |
| PBC (resident) | 87 | 2.6 | 1.6 | 99 | 2.2 | 1.6 |
| PBC (carer) | 53 | 3.5 | 1.1 | 57 | 3.6 | 1.2 |
| PBC (staff) | 193 | 3.3 | 1.5 | 206 | 3.3 | 1.4 |
Restricted access to independent toilet use as a result of mobility problems was found for more than three-quarters of residents (85% in the TPTNS group; 78% in the sham group) and communicating need to use the toilet was an issue for > 40% of residents in both groups (44% in the TPTNS group; 41% in the sham group). Functional skills for independent toilet use were similar in both groups for the resident-reported and the staff-reported MTSQ; however, staff reported more severe functional deficits than residents (scores range from 0 to 20, with higher scores indicating more difficulty). Ratings of PBC by each of the three respondent groups (residents, family carers and staff) were similar for the TPTNS and the sham groups; however, residents rated their PBC as less severe [mean score 2.4 (minor problems to some moderate problems)] than staff (mean score 3.3) and family carers [mean score 3.6 (some moderate problems to severe problems)].
Flow of participants through the trial
At the primary outcome time point of 6 weeks, there were 7 (3.6%) and 4 (1.9%) deaths in the TPTNS and sham groups, respectively (see Figure 3). There were also 23 (11.7%) and 27 (12.9%) participants, respectively, with missing pad weight data at 6 weeks. We do not have detailed reasons for missing pad weights. There were 167 (84.8%) participants in the TPTNS group and 178 (85.2%) participants in the sham group who provided pad weight data and were included in the primary outcome analysis. Detailed information on deaths and missing pad weight data at other time points is included in Figure 3.
Intervention received
A description of the stimulation that residents received in both arms of the trial is presented in Table 4. Full details of the stimulation protocol are in Chapter 2 but are repeated here briefly. The intervention comprised a programme of 12 sessions of stimulation of 30 minutes’ duration, delivered twice per week for 6 weeks. We defined adherence in terms of intensity of stimulation, position of electrodes, duration of stimulation and number of sessions, confirmed by a minimum of two correct adherence checks made by the ISF during the 6-week intervention programme. This meant that for each group the participants received their allocated stimulation at the correct intensity [resident-directed that for comfort above 10 mA (TPTNS) or 4 mA (sham)], delivered in the correct position [medial malleolus (TPTNS) or lateral malleolus (sham)] for the correct duration (≥ 15 minutes) for the correct number of times (fidelity ≥ 8 sessions; full stimulation programme = 12 sessions). The ISF reported adherence to the allocated protocol for 154 (78.2%) participants in the TPTNS group and 149 (71.3%) participants in the sham group.
| Number of stimulation sessions | TPTNS (N = 197), n (%) | Sham (N = 209), n (%) |
|---|---|---|
| 0 | 8 (4) | 17 (8) |
| 1–4 | 11 (6) | 17 (8) |
| 5–7 | 17 (9) | 17 (8) |
| ≥ 8 | 161 (82) | 158 (76) |
| Adherent to allocated protocol | 154 (78) | 149 (71) |
In the primary outcome CACE analysis (see Chapter 2), the proportion in the TPNS arm classed as adherent to allocated intervention and for whom a 24-hour pad weight was available was 142 out of 167 (85.0%). Our fidelity checks confirmed that no participants in the sham arm received TPTNS.
Primary outcome results
Data on the primary outcome, volume of urine loss (ml) per 24 hours at the 6-week follow-up, is described in Table 5. The results of the intention-to-treat complete-case analysis show that the adjusted mean (SD) reduction in urine leakage was –5 ml (362 ml) in the TPTNS group and –66 ml (394 ml) in the sham group. The difference between the groups (adjusted for baseline leakage and covariates) was 68 ml (95% CI 0 to 136 ml; p = 0.05 ml) in favour of the sham intervention.
| Descriptive data | TPTNS (N = 197) | Sham (N = 209) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |
| Baseline volume (ml) | 536 | 370 | 197 | 560 | 469 | 209 |
| 6-week volume (ml) | 566 | 382 | 167 | 498 | 400 | 178 |
| Change from baseline (ml) | –5 | 362 | 167 | –66 | 394 | 178 |
| Model | Estimate of treatment effect (ml) | 95% CI (ml) | p-value (ml) | |||
| Complete-case ITT | 68 | 0 to 136 | 0.050 | |||
| Multiple imputation | 65 | –3 to 133 | 0.060 | |||
| Adjusted for non-compliance | 81 | 2 to 160 | 0.044 | |||
| Multiple imputation adjusted for non-compliance | 79 | –2 to 159 | 0.057 | |||
Sensitivity analysis
Sensitivity analysis to assess the robustness of the treatment effect to missing data and non-compliance to the TPTNS gave similar results to the complete-case intention-to-treat (ITT) analysis.
Subgroup analysis
Figure 4 summarises the prespecified subgroup analyses and Figure 5 presents the post hoc subgroup analyses. There were no modifying effects on the treatment effect in a subgroup analysis.
FIGURE 4.
Forest plot of subgroup analysis for the primary outcome. Clinical frailty: ≤ 5, very fit to mildly frail; 6, moderately frail; ≥ 7, severely or very severely frail. ADL, activities of daily living.
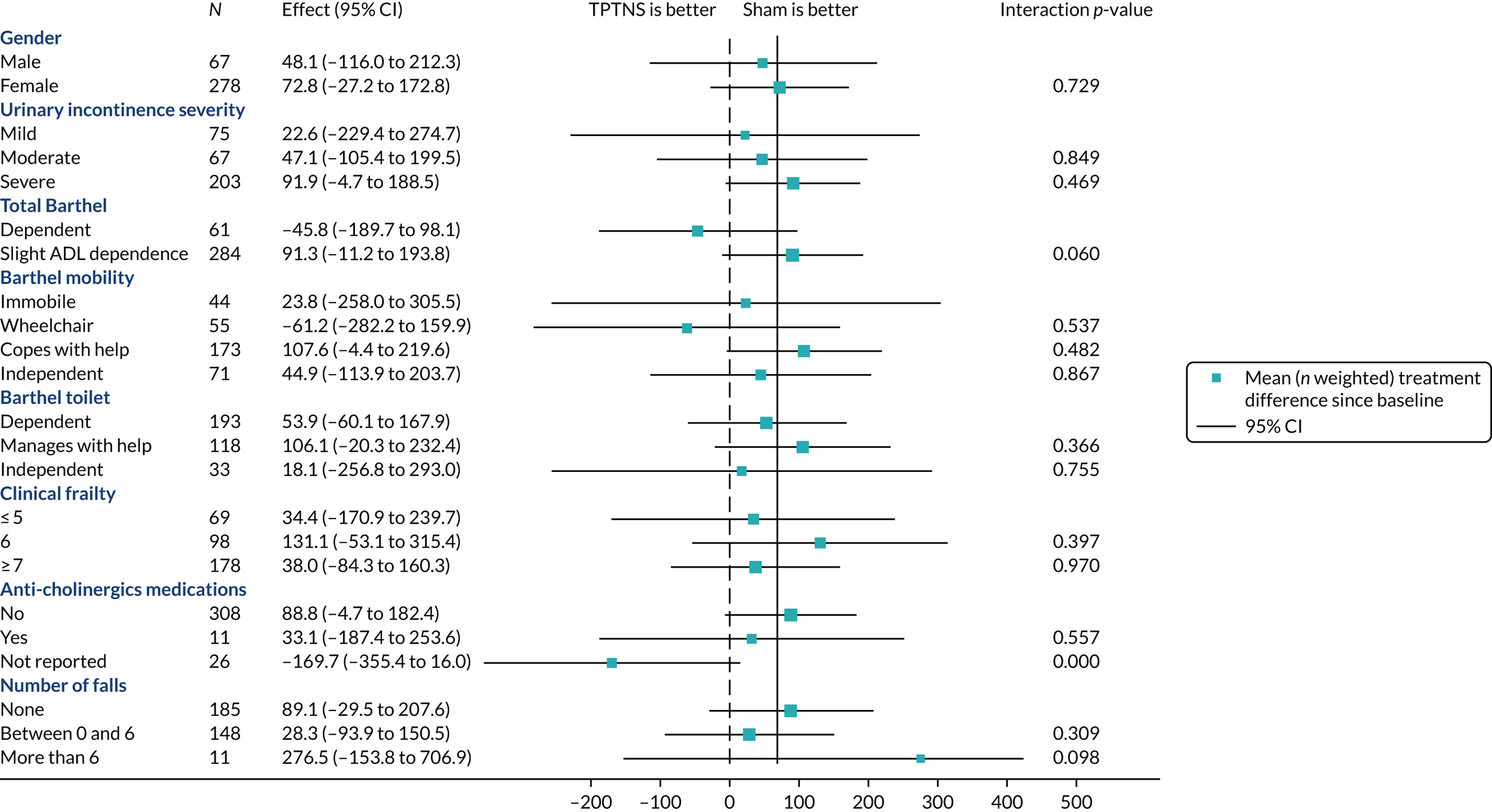
FIGURE 5.
Forest plot of post hoc subgroup analysis for the primary outcome. Effect size of total urine leaked (after 6 weeks).
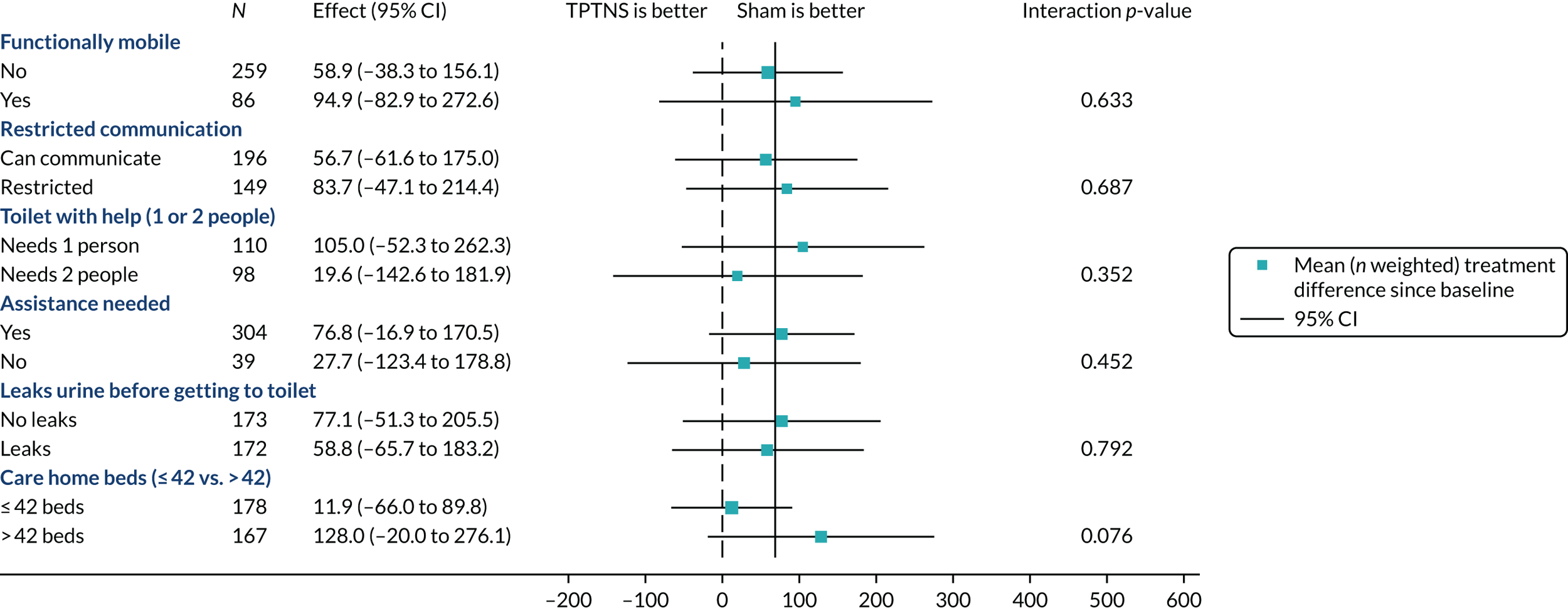
Table 6 describes total urine leakage over time. Treatment effect estimates were fairly consistent over time: incompatible with a worthwhile reduction in urine volume when receiving TPTNS. It should be noted that the treatment effect estimate at 6 weeks differs from the figure in Table 5, reflecting the longitudinal model. Secondary clinical outcomes are reported in Table 7; pad use and PVRU were similar between groups at all time points, confirming that TPTNS does not increase urinary retention in this population.
| Time | TPTNS, mean (SD); n | Sham, mean (SD); n | Estimate of treatment effect (ml) | 95% CI (ml) | p-value (ml) |
|---|---|---|---|---|---|
| Baseline | 536 (370); 197 | 560 (469); 209 | |||
| 6 weeks | 566 (382); 167 | 498 (400); 178 | 53 | –22 to 128 | 0.164 |
| 12 weeks | 585 (438); 152 | 520 (411); 157 | 70 | –9 to 148 | 0.081 |
| 18 weeks | 555 (400); 133 | 547 (454); 156 | 21 | –60 to 102 | 0.605 |
| Outcome | TPTNS, mean (SD); n | Sham, mean (SD); n | Estimate of treatment effect | 95% CI | p-value |
|---|---|---|---|---|---|
| Pads used (24 hours) | |||||
| Baseline | 3.5 (1.6); 197 | 3.4 (1.8); 209 | |||
| 6 weeks | 3.5 (1.6); 166 | 3.4 (1.7); 178 | 0.1 | –0.2 to 0.4 | 0.47 |
| 12 weeks | 3.2 (1.7); 152 | 3.1 (1.5); 156 | 0.1 | –0.2 to 0.5 | 0.54 |
| 18 weeks | 3.1 (1.5); 133 | 3.0 (1.6); 156 | 0.1 | –0.3 to 0.4 | 0.71 |
| PVRU (ml) | |||||
| Baseline | 76 (65); 177 | 81 (70) 183 | |||
| 6 weeks | 92 (81); 112 | 86 (83); 124 | 1.4 | –18 to 21 | 0.89 |
| 12 weeks | 81 (89); 83 | 86 (84); 99 | –3.5 | –25 to 18 | 0.75 |
| 18 weeks | 84 (88); 80 | 79 (76); 99 | 0.5 | –22 to 23 | 0.96 |
Resident- and staff-reported outcomes and treatment effect estimates are summarised in Table 8. Perception of bladder control was measured using the single item PBC (by residents, their family and care home staff). The rating was fairly constant over time (between minor and moderate problems) for residents and staff; CIs around treatment effects generally rule out any difference of more than one point on the item in either direction. The family ratings are few and should be interpreted with care.
| Assessment | TPTNS, mean (SD); n | Sham, mean (SD); n | Estimate of treatment effect | 95% CI | p-value |
|---|---|---|---|---|---|
| Patient PBC | |||||
| Baseline | 2.6 (1.6); 87 | 2.2 (1.6); 99 | |||
| 6 weeks | 2.3 (1.5); 69 | 2.0 (1.4); 81 | 0.2 | –0.7 to 1.1 | 0.63 |
| 12 weeks | 2.2 (1.6); 53 | 2.0 (1.4); 72 | 0.0 | –1.0 to 1.0 | 0.94 |
| 18 weeks | 2.2 (1.5); 43 | 1.9 (1.2); 58 | 0.1 | –1.0 to 1.2 | 0.84 |
| Family PBC | |||||
| Baseline | 3.5 (1.1); 53 | 3.6 (1.2); 57 | |||
| 6 weeks | 2.9 (1.7); 12 | 2.6 (1.7); 11 | 0.1 | –2.2 to 2.4 | 0.92 |
| 12 weeks | 2.0 (1.0); 3 | 2.4 (1.3); 7 | 0.1 | –3.0 to 3.2 | 0.95 |
| 18 weeks | 3.3 (0.6); 3 | 3.7 (0.6); 3 | –3.2 | –8.7 to 2.4 | 0.26 |
| Staff PBC | |||||
| Baseline | 3.3 (1.5); 193 | 3.3 (1.4); 206 | |||
| 6 weeks | 2.9 (1.4); 166 | 3.2 (1.4); 177 | –0.5 | –1.0 to -0.1 | 0.024 |
| 12 weeks | 3.2 (1.3); 147 | 3.2 (1.4); 161 | 0.0 | –0.5 to 0.4 | 0.85 |
| 18 weeks | 3.1 (1.6); 137 | 3.0 (1.5); 155 | 0.1 | –0.4 to 0.6 | 0.59 |
| MTSQ (resident) | |||||
| Baseline | 6.7 (7.8); 82 | 5.1 (7.1); 94 | |||
| 6 weeks | 6.4 (7.0); 66 | 5.4 (7.4); 78 | 0.3 | –1.5 to 2.0 | 0.78 |
| 12 weeks | 6.8 (7.4); 46 | 5.3 (7.2); 66 | 0.6 | –1.5 to 2.6 | 0.59 |
| 18 weeks | 8.7 (7.9); 41 | 5.0 (6.6); 56 | 1.0 | –1.1 to 3.1 | 0.37 |
| MTSQ (staff) | |||||
| Baseline | 13.7 (6.8); 194 | 12.7 (7.0); 203 | |||
| 6 weeks | 14.2 (6.8); 166 | 12.5 (7.4); 179 | 1.2 | 0.2 to 2.2 | 0.017 |
| 12 weeks | 14.8 (6.4); 152 | 13.6 (6.8); 166 | 0.6 | –0.5 to 1.6 | 0.30 |
| 18 weeks | 15.6 (6.0); 138 | 13.7 (6.8); 151 | 1.2 | 0.2 to 2.3 | 0.024 |
| DEMQOL | |||||
| Baseline | 84.6 (14.0); 70 | 88.7 (14.9); 80 | |||
| 6 weeks | 87.0 (15.3); 51 | 89.7 (10.9); 61 | –0.6 | –4.6 to 3.5 | 0.78 |
| 18 weeks | 85.8 (13.1); 28 | 90.3 (11.1); 42 | 0.4 | –4.7 to 5.4 | 0.89 |
| DEMQOL-PROXY | |||||
| Baseline | 98.2 (15.2); 193 | 99.1 (13.7); 208 | |||
| 6 weeks | 100.8 (11.7); 162 | 98.2 (12.9); 172 | 2.3 | –0.1 to 4.7 | 0.055 |
| 18 weeks | 103.5 (11.6); 132 | 102.2 (12.6); 152 | 1.0 | –1.6 to 3.5 | 0.46 |
The MTSQ scores range from 0 (no difficulties) to 20 (cannot do), rated by both residents and staff at the care home. For both outcomes at each time point there were small differences favouring the sham intervention.
Fewer than 40% of residents in each arm were able to complete the DEMQOL health-related quality-of-life questionnaire at baseline. The proxy version of the score was completed by a family member or a member of care home staff at both 6 and 18 weeks. There was a small difference (about 0.2 SD) in favour of the TPTNS treatment at 6 weeks.
Safety
Adverse events are summarised in Table 9.
| Adverse events | TPTNS (N = 197), n (%) | Sham (N = 209), n (%) |
|---|---|---|
| UTI | 8 (4.1) | 4 (1.9) |
| UTI and chest infection | 0 (0.0) | 3 (1.4) |
| Leg pain/heavy sensationa | 0 (0.0) | 2 (1.0) |
| Reduced kidney function | 1 (0.5) | 0 (0.0) |
| Blood on absorbent pad | 0 (0.0) | 1 (0.5) |
| Participant became agitateda | 0 (0.0) | 1 (0.5) |
| Episodes – suspected seizure (not confirmed) | 0 (0.0) | 1 (0.5) |
| Total | 9 (4.6) | 12 (5.7) |
Serious adverse events
There were four serious adverse events (SAEs) that required residents to be admitted to hospital: three in the sham group (one for a kidney infection, one for a chest infection and a UTI, and one for vomiting and irregular heart rhythm); and one in the TPTNS group (for a UTI and pulmonary oedema). All four events occurred during the 6-week intervention programme; none of the events was classified as being related to the trial interventions (see Appendix 4).
Non-serious adverse events
There were a total of 21 participants who had a non-SAE, mainly a UTI (15/21), three of whom also had a potential chest infection. All non-SAEs were judged to be unrelated to the intervention, other than two in the sham group: one participant withdrew because their leg felt ‘heavy’ and they felt dizzy; one participant became agitated and distressed, with no impact on intervention delivery as the contingency plan to offer the intervention at alternative times was implemented successfully (see Appendix 4).
Routine toileting practice
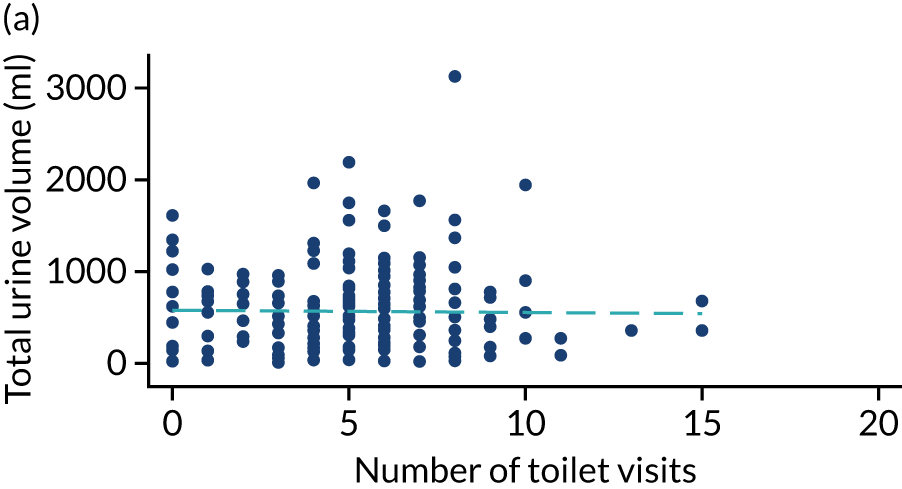
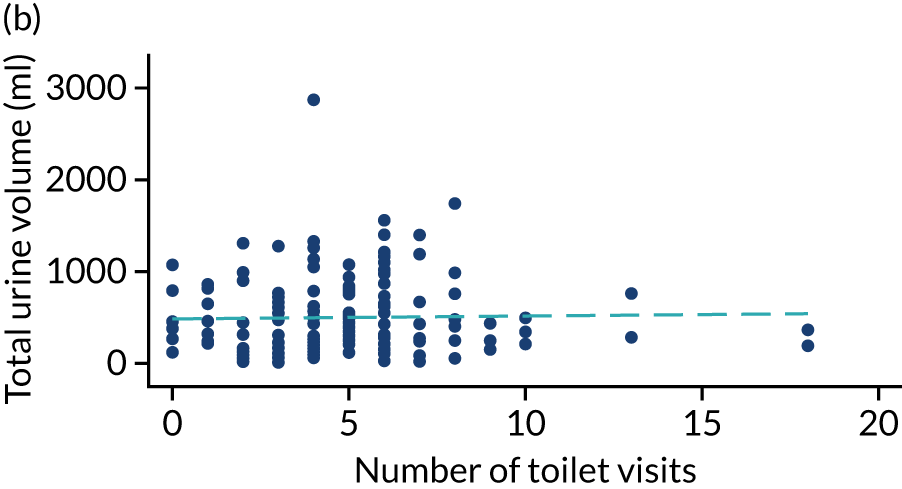
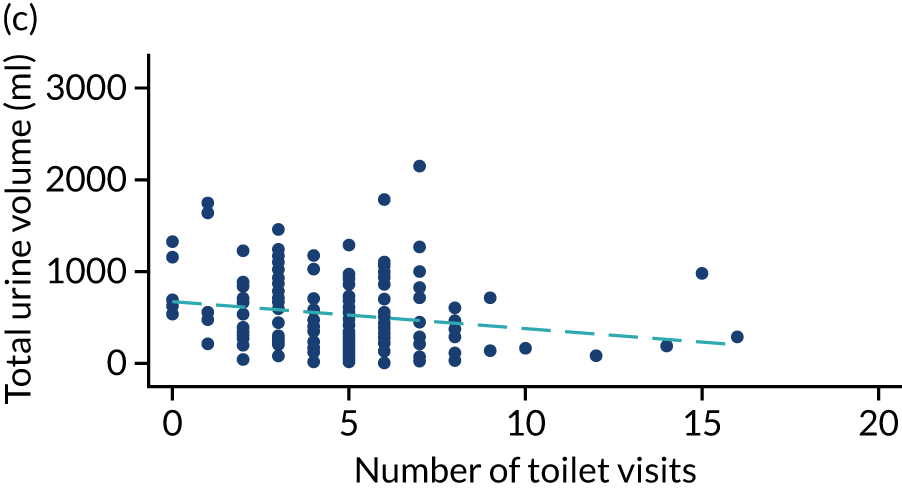
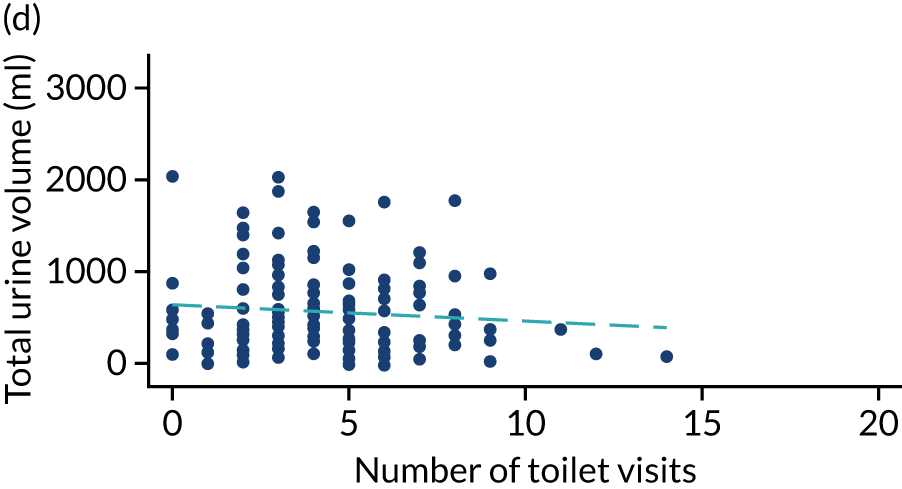
Post hoc analysis of any changes in routine toileting practices in care homes across the four time points are shown in Figures 6 and 7. The expected increase in the number of visits to the toilet at 6 weeks, associated with a reduced volume of urine leaked in the pads and increased use of the toilet to void, is not demonstrated. Routine toileting practices remained similar across the time points for both the TPTNS and sham groups.
FIGURE 6.
Urine leaked in pads (ml) against number of toilet visits in the TPTNS group over time. (a) Baseline: rho = –0.164; p = 0.022; (b) 6 weeks: rho = 0.050; p = 0.524; (c) 12 weeks: rho = 0.114; p = 0.180; and (d) 18 weeks: rho = –0.092; p = 0.303. Dashed blue line represents the line of fit.

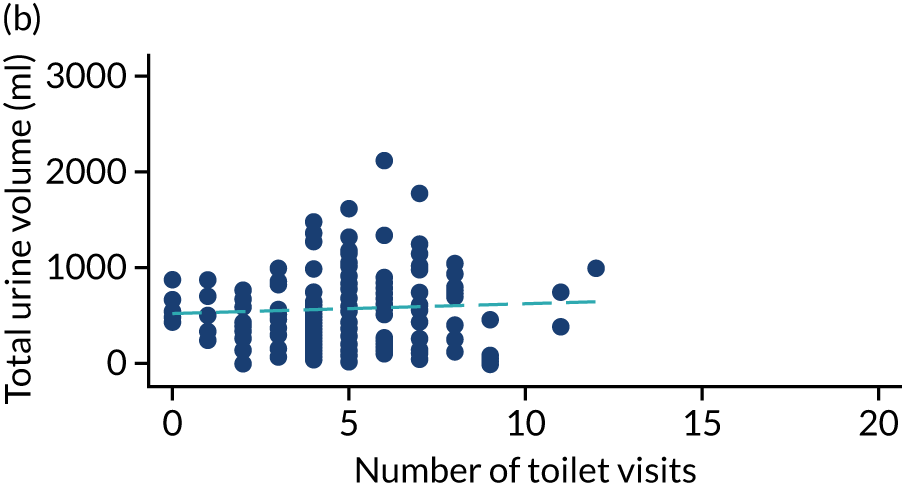


FIGURE 7.
Urine leaked in pads (ml) against number of toilet visits in the sham group over time. (a) Baseline: rho = –0.006; p = 0.930; (b) 6 weeks: rho = 0.069; p = 0.367; (c) 12 weeks: rho = –0.199; p = 0.015; and (d) 18 weeks: rho = –0.047; p = 0.572. Dashed blue line represents the line of fit.
Summary of effectiveness results
We randomised 406 care home residents recruited from 37 care homes to receive TPTNS or sham stimulation. The majority had severe UI (> 400 ml per 24 hours), all wore absorbent pads, and the two groups were comparable at baseline with regard to age, sex, and cognitive and physical impairment levels. We found no evidence of any clinically relevant reductions in 24-hour urine leakage at any time point after treatment with TPTNS. Given the challenges of undertaking research in this population, our compliance with allocated treatment was high and the number of missing data was small; furthermore, results from sensitivity analyses were consistent with the primary complete case ITT analysis, reinforcing our findings. There were no safety concerns related to TPTNS.
Chapter 4 Economic evaluation
Introduction
This chapter describes the economic evaluation that was undertaken alongside the trial. The aim of the analysis was to assess the costs of providing a programme of TPTNS, compared with the costs of providing sham treatment. The TPTNS intervention, an electrical stimulation programme comprising 12 sessions of 30 minutes’ duration each, provided twice per week over 6 weeks, was delivered by care home staff who received a specific package of training and support. To make the best use of the evidence generated during the trial and to incorporate the range of costs and benefits of the TPTNS intervention, a CCA approach was used. This presented costs of providing the programme alongside the key primary and secondary outcomes. This was conducted instead of a cost-effectiveness analysis because there was more than one multidimensional outcome of importance that was considered useful to capture. An original intention had been to complement the CCA with a further synthesis of costs and benefits between the groups in the trial using a cost-effectiveness analysis. However, as no difference was found between the two groups in DEMQOL or DEMQOL-PROXY, and no difference was found between groups in costs, none of the constituent parts indicated that this would be useful.
Methods
Economic evaluation
A CCA approach was used. 42 This involves a descriptive presentation of the range of costs and benefits of an intervention. Outcomes are reported in natural units, such as the use of incontinence products or the impact on continence care pathways, which should resonate with those involved in the planning and delivery of continence care in care homes. It also enables costs and benefits to be reflected where there is a known direction of change, but insufficient data is available to quantify that change. The use of a ‘balance sheet’ reporting approach allows the reader to form their own opinion on the relevance and relative importance of the findings to their decision-making context. 42 Disaggregated costs and benefits were descriptively presented and outcomes reported in natural units.
Perspective
A public sector payer perspective (in the UK, this is the central government Treasury), specifically in terms of NHS costs and local authority costs for care homes, was used to provide a practical assessment of resource implications and consequences of interest for those involved in care home service provision and funding decisions.
Discounting
Reporting of economic analysis was in 2018–19 Great British pounds (GBP). The time horizon for the trial was 18 weeks. As costs did not extend beyond 1 year, discounting was not required.
Quality-of-life health outcomes
Data about quality of life were collected using the DEMQOL and DEMQOL-PROXY (titled DEMQOL-Carer) questionnaires. These are condition-specific measures of health-related quality of life designed specifically for use with individuals experiencing cognitive decline and dementia. 35,43 The raw scores from each of these can be used to generate health state utility values for use in economic evaluation through conversion to DEMQOL-U and DEMQOL-PROXY-U, respectively. 41,44 DEMQOL-U consists of five domains: positive emotion, memory, relationship, negative emotion and loneliness. Four possible levels of response relating to severity are available for each domain. DEMQOL-PROXY-U consists of four domains: positive emotion, memory, appearance and negative emotion. As with DEMQOL-U, each has four possible levels of response relating to severity. DEMQOL was administered at baseline and at the 6- and 18-week follow-ups, and completed by the participant. DEMQOL-PROXY was administered at baseline and at the 6- and 18-week follow-ups, and completed with a proxy resident perspective by a single, named proxy. This could be a different proxy at each time point. Individual raw scores were converted to health state utility values using the scoring algorithm for the UK. 41
Resource use
Resources to deliver the TPTNS intervention, details about the use of absorbent pads (number and type) and other equipment, and (if appropriate) the number of staff required to assist residents to use the toilet were measured. Data on resource use were collated from the RUQ designed for this study and from data collected as part of the main trial (see Report Supplementary Material 4–7). The RUQ consisted of three questions related to medication prescribed for incontinence, resources required for toilet assistance, and aids and devices for managing incontinence. In addition, at the 6- and at 18-week follow-ups, questions about the use of primary care in the preceding 6-week period were included. Information on all consultations with health-care professionals external to the care home as a result of their UI was categorised according to who the consultation was with [general practitioner (GP), nurse, physiotherapist, etc.] and where the consultation took place (in the surgery or in the care home). Stimulation diary records were used to determine the average time taken by care home staff to complete stimulations [over 12 sessions in a 6-week period (minutes)] and the staff grade of the stimulation administrator. This was recorded on the RUQ. The RUQ was administered at baseline to establish the usual continence care pathway and completed by the RRA. The RRA was a registered nurse working in trial regions in Scotland and England. The RUQ was used again at the 6- and 18-week follow-ups.
Unit costs
The unit costs and sources used to estimate total cost per participant are given in Appendix 5. Unit costs were attached to the individual resources identified in the RUQ. Unit costs were identified using Unit Costs of Health and Social Care 201937 for staff for primary care, and British National Formulary38 for prescribed medication. Incontinence products’ market prices were identified from supplier direct websites or large chain shops with an online presence. Direct costs of interest included expenditure on absorbent pads and expenditure on other protection products to manage UI.
Intervention costs
Intervention costs included staff training (training time using staff roles reported for delivery of TPTNS/sham intervention), the trainer and the materials (TPTNS electrical stimulator machines, skin electrodes, handbook and training DVD). Costs were based on the market rates for these items. The costs of developing the handbook and DVD were not included. Cost of training facilities was not included. (It was assumed that training would not be expected to be delivered off site in normal working practice.) Equipment to deliver the intervention [cost of TPTNS Neurotrac machine, consumables (skin electrodes, wipes, batteries)] were also based on market rates for these items. Time to deliver the intervention was costed using staff time.
Data analysis
Descriptive statistics were used to assess the number of each item of resource used by participants in each group. The total cost per participant was estimated by combining the number of each item of resource used with the unit cost of that item. This provided an estimate of mean cost per participant by treatment group. Differences in mean costs associated with UI products, staff time for toilet assistance and other health-care resource use (e.g. GP visits) during routine follow-up were assessed. Independent samples t-tests were used to compare the groups at each time point for resource use. Utility change scores from baseline to 6 weeks, and from baseline to 18 weeks, were calculated and tested using the parametric paired t-test for statistically significant difference in health-related quality-of-life scores before and after TPTNS. Treatment groups were compared using independent t-tests. Although an original intention had been to estimate quality-adjusted life-year gain, this was not conducted as no differences were observed in benefits or costs. To maximise transparency and aid interpretation of results all available data were used, when possible.
Missing data
Missing data can preclude calculation of utilities for DEMQOL-U and DEMQOL-PROXY-U. No imputation was conducted. When resource use was missing for health-care NHS contacts, the participant was assumed not to have used the resource category.
Results
Intervention costs
Seventy-two bespoke training courses were delivered to 425 care home staff at care home venues. Each staff member received an online handbook and DVD to support their skills development. A total of 148 participants also received a follow-up individual staff competency assessment in their workplace. The average cost of the training and support package per staff member was estimated to be £121.03, based on the assumption that normal practice would be to make use of local trainers [10-mile radius (travel time of 30 minutes and 20-mile round-trip mileage reimbursement per person)] and excluding economic cost of venue. Although this reflects trial costs for training conducted in care homes in Scotland, it should be noted that additional costs were incurred by travel and subsistence reimbursements for training conducted in care homes in England because distances to care homes were greater.
Delivery of the intervention required one staff member for each of the 12 times that the stimulation was delivered during the trial. An average salary of £38.06 per hour was used to value staff time. This was estimated from staff roles reported in trial stimulation diary records (66% care assistants, 13% care leaders, 20% nurses). Staff did not need to be present during stimulation. Data for time to set up and take off the machine for each stimulation event was available for 92% of stimulation events. Data were missing for 8% (345/4127) stimulation events. Time to set up and take off the machine took ≤ 5 minutes for 94% (3536/3782) of stimulations, with 80% of stimulations (3042/3782) taking ≤ 3 minutes. The cost of delivery of TPTNS (excluding training) in the trial was estimated to be £81.20 per participant (Table 10).
| Item | Unit cost (£) | Numbera | Total cost (£) |
|---|---|---|---|
| TPTNS training and support package | |||
| Handbook and DVD | 11.20 | 425 | 4760.00 |
| Training events | 143.00 | 72 | 10,296.00 |
| Carer hours for intervention training | 28.00 | 170 × 2 hours | 9520.00 |
| Care leader hours for intervention training | 40.00 | 210 × 2 hours | 16,800.00 |
| Nurse hours for intervention training | 60.00 | 45 × 2 hours | 5400.00 |
| Individual staff competency assessment | 31.50 | 148 | 4662.00 |
| Average training cost per staff (n = 425) | 121.03 | ||
| TPTNS intervention | |||
| Neurotrac machine (branded) | 74.49 | 172 | 12,812.28 |
| Skin electrode pads (single use) | 2.98 | 4130 | 12,307.40 |
| Time to set up and take off the machine (per resident per stimulation event) | 38.06 | 4127 × 3 minutes (206 hours) | 7840.98 |
| Average intervention cost per participant (n = 406) | 81.20 | ||
Dementia Quality of Life and Dementia Quality of Life Proxy
A total of 406 residents from 37 care homes in the UK (23 in Scotland and 14 in England) completed DEMQOL-U or proxy-completed DEMQOL-PROXY-U for at least one measurement point. Baseline utility scores could be calculated for DEMQOL-U for 141 (35%) participants, and for DEMQOL-PROXY-U for 397 (98%) participants (Table 11). At baseline, for 257 participants (126 in the TPTNS group and 131 in the sham group) only DEMQOL-PROXY-U data were available (see Appendix 6). There were 41 participants (15 in the TPTNS group and 26 in the sham group) with both DEMQOL-U and DEMQOL-PROXY-U data available at all time points. A larger range of mean utility values was observed for DEMQOL-PROXY (0.722–0.742) than for DEMQOL (0.790–0.803), as reported by the care home resident. Minimum and maximum utility values were reported for both tools at baseline (DEMQOL-U, 0.243–0.986 and DEMQOL-PROXY-U, 0.363–0.937). No significant difference was found between participants’ scores over time, or between treatment and control groups at any time point, for either DEMQOL-U or DEMQOL-PROXY-U (Table 11).
| Assessment | TPTNS, mean (SD); n | Sham, mean (SD); n | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| DEMQOL-U | ||||
| Baseline | 0.771 (0.187); 66 | 0.826 (0.162); 75 | –0.055 (–0.113 to 0.002) | 0.062 |
| 6 weeks | 0.774 (0.203); 48 | 0.803 (0.156); 59 | –0.029 (–0.098 to 0.040) | 0.405 |
| 18 weeks | 0.778 (0.171); 28 | 0.821 (0.143); 40 | –0.042 (–0.119 to 0.034) | 0.271 |
| DEMQOL-PROXY-U | ||||
| Baseline | 0.716 (0.123); 190 | 0.727 (0.122); 207 | –0.011 (–0.036 to 0.012) | 0.340 |
| 6 weeks | 0.730 (0.122); 159 | 0.736 (0.122); 165 | –0.006 (–0.033 to 0.021) | 0.653 |
| 18 weeks | 0.741 (0.117); 129 | 0.744 (0.128); 150 | –0.002 (–0.031 to 0.027) | 0.877 |
Resource use data
Baseline resource use assessment was completed for 406 participants, at the 6-week follow-up for 370 participants and at the 18-week follow-up for 321 participants (see Appendix 7). Assessment of trial data collected about products related to incontinence management indicated no significant differences between groups at any time point (Table 12). Three-quarters of participants used between 1 and 3 items in 24 hours at an average cost of £1.19 (SD £1.51) per participant.
| Assessment time point | TPTNS | Sham | Mean difference | 95% CI | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Baseline | ||||||
| Total number of pads per day | 2.79 | 1.52 | 2.73 | 1.69 | 0.06 | –0.202 to 0.326 |
| Value of pads (£) | 1.25 | 1.42 | 1.30 | 1.53 | –0.05 | –0.297 to 0.190 |
| 6-week follow-up | ||||||
| Total number of pads per day | 2.68 | 1.59 | 2.51 | 1.54 | 0.17 | –0.104 to 0.441 |
| Value of pads (£) | 1.19 | 1.21 | 1.12 | 1.01 | 0.07 | –0.118 to 0.269 |
| 12-week follow-up | ||||||
| Total number of pads per day | 2.44 | 1.49 | 2.54 | 1.42 | –0.10 | –0.378 to 0.165 |
| Value of pads (£) | 1.05 | 0.90 | 1.13 | 0.92 | –0.08 | –0.251 to 0.087 |
| 18-week follow-up | ||||||
| Total number of pads per day | 2.53 | 1.51 | 2.40 | 1.45 | 0.13 | –0.160 to 0.416 |
| Value of pads (£) | 1.27 | 2.15 | 1.22 | 2.43 | –1.16 | –0.400 to 0.492 |
At each time point, around 85% of participants required assistance from care home staff to use a toilet. Most residents needed the assistance of one or two members of staff and visited the toilet four or five times a day (Table 13). There was no evidence of a difference between the TPTNS group and the sham group that was practicably meaningful. Assuming 5 minutes per toilet visit, and a derived average hourly pay of £36.08 per staff member (based on unit costs for proportions of staff delivering intervention), the value of staff time to assist a resident to attend the toilet was estimated as £19.17 (SD £13.22) for the TPTNS group and £17.30 (SD £13.33) for the sham group (per 24-hour period) using all available data. No significant differences were observed between groups.
| Assessment time point | TPTNS | Sham | Mean difference | 95% CI | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Baseline | ||||||
| Number of staff required per visit | 1.48 | 0.73 | 1.30 | 0.68 | 0.18 | 0.040 to 0.320 |
| Number of visits required per day | 4.07 | 2.51 | 3.88 | 2.72 | 0.19 | –0.351 to 0.740 |
| Value of staff time per 24 hours (£) | 18.86 | 12.99 | 16.38 | 12.47 | 2.48 | –0.177 to 5.130 |
| 18-week follow-up | ||||||
| Number of staff required per visit | 1.39 | 0.67 | 1.26 | 0.75 | 0.13 | –0.033 to 0.284 |
| Number of visits required per day | 4.20 | 2.70 | 3.73 | 2.71 | 0.47 | –0.152 to 1.089 |
| Value of staff time per 24 hours (£) | 18.13 | 13.06 | 16.33 | 13.21 | 1.80 | –1.208 to 4.815 |
A total of 898 items (433 in the TPTNS group, 465 in the sham group) of special equipment provided as a result of incontinence were reported for daily use (890 items) and for infrequent use (eight items: four transfer aids, two toilet aids, one mobility aid and one incontinence protection for furniture). Daily use items were predominantly related to mobility aids (n = 402), transfer aids (n = 244) and toilet aids (n = 179). Across the treatment groups, mobility aids (≈ 40%), transfer aids (≈ 25%) and toilet aids (≈ 20%) accounted for similar proportions of special equipment required. Pressure mats (n = 20); sensor alarms (n = 20); falls protection items, such as crash mat or bed rails (n = 20); and incontinence protection for furniture (n = 5) were also reported. Costs were not estimated for infrequent use items as there were so few of these items.
Cost of health-care services used
Appointments with health services staff for continence problems were available from the resource use questionnaire at 6 weeks and 18 weeks (each covering the preceding 6 weeks). The number of NHS contacts is shown (see Appendix 8). Use of NHS services were reported for 54 participants (21 in the TPTNS group; 33 in the sham group). A total of six participants (all in the sham group) used services during both follow-up periods. No significant differences were observed between groups at the 6-week or 18-week follow-up. Average costs at the 6-week follow-up were £11.88 (95% CI £5.72 to £18.77) for TPTNS, compared with £19.40 (95% CI £11.62 to £27.93) for sham. At the 18-week follow-up, the average cost was £7.66 (95% CI £2.52 to £14.25) for the TPTNS group and £12.60 (95% CI £6.81 to £19.24) for the sham group. Statistical power to detect between-arm differences is very low, and the evidence available from this analysis may have been due to the small numbers of individuals and the low reported use of these services.
Out of 406 participants, 370 had at least one medication reported at baseline (181 in the TPTNS group; 189 in the sham group). Across the three RUQ time points, a total of 336 different medications were reported. This included seven medications used directly for treatment of UI reported for 32 participants (20 in the TPTNS group; 12 in the sham group): duloxetine (Cymbalta®, Eli Lilly and Company) (n = 3), fesoterodine (Toviaz®, Pfizer Inc., New York, NY USA) (n = 1), mirabegron (Betmiga®, Astellas Pharma) (n = 7), oxybutynin (Ditropan®, Janssen Pharmaceutica) (n = 7), solifenacin (Vesicare®, Astellas Pharma) (n = 8), tolterodine (Detrusitol®, Pfizer Inc) (n = 2) and trospium chloride (Regurin®, Madaus Pharma) (n = 4) (Appendix 7). All were classed as ‘ongoing’ medication and there was very little change over the course of the 18 weeks of the study. No further analysis of cost of medications was undertaken.
Cost–consequences analysis
Table 14 presents a descriptive comparison of the costs and outcomes of TPTNS compared with usual continence care pathways. The balance sheet reports both the costs and benefits mix of qualitative and quantitative findings, including financial and non-financial outcomes, to provide a more representative reflection of the impact of TPTNS on care home residents. Different stakeholders will find different parts to be of more direct relevance. The economic analysis indicated that there was no significant change in resource use over time (pads, toileting assistance, health-care use) and there was no evidence of impact on health-related quality of life (as measured using DEMQOL-U/DEMQOL-PROXY-U). This was in line with the main findings of the study, which reported that TPTNS was not found to be clinically effective during the trial, and that toilet use routines and absorbent pad use did not change.
| In favour of TPTNS | In favour of current practice |
|---|---|
| Non-invasive, acceptable to care home resident and tolerated well | ITT complete-case analysis favoured the sham intervention (see Chapter 3, Trial outcomes and results) |
| Staff time to set up and take off the machine was ≤ 5 minutes for 94% of stimulations. Staff are not required to be present for the duration of treatment | |
| TPTNS is a treatment seeking to attempt to address the cause of UI. Usual continence care seeks to contain UI | |
| Neither in favour of nor against TPTNS | |
| No impact on absorbent product use. Average cost of £1.19 (SD £1.51) per participant in 24 hours, during trial. [Mean difference (95% CI) between TPTNS and sham at 18 weeks: number of pads per day, 0.13 (95% CI –0.160 to 0.416); value of pads (£), –1.16 (95% CI –0.400 to 0.492)] | |
| No evidence of impact on health-related quality of life (as measured using DEMQOL-U/DEMQOL-PROXY-U). [Mean difference (95% CI) between TPTNS and sham at 18 weeks: DEMQOL-U –0.042 (95% CI –0.119 to 0.034); DEMQOL-PROXY-U –0.002 (95% CI –0.031 to 0.027)] | |
| No impact on resources (staff time, equipment) required for residents’ toilet assistance. Dependency on staff for toilet assistance was indicative of labour intensity of continence care. [Estimated mean value of staff time per 24-hour period, assuming 5 minutes per visit: £19.17 (SD £13.22) in the TPTNS group; £17.30 (SD £13.33) in the sham group] | |
| Care home involvement in research motivated staff and improved confidence in knowledge about UI (see Chapter 5, Qualitative study). Cost of training and support estimated as £121.03 per staff member | |
| Additional cost per resident to receive TPTNS (cost of machine and pads) was estimated as £81.20. Active reuse of skin electrodes would lower this cost | |
Discussion
The economic evaluation examined the costs of delivering TPTNS and presented a balance sheet account of several impacts of the TPTNS intervention. The results of the trial did not indicate that TPTNS effectively reduced UI for this group of residents, and the evaluation of resource use also did not demonstrate differences between the randomised groups. No statistically significant difference in improvement in health-related quality of life between baseline and the 18-week follow-up was found between participants’ scores over time, or between the TPTNS and sham groups, using DEMQOL-U or DEMQOL-PROXY-U. The resource use data collected highlighted participants’ dependency on staff to take them to the toilet, and that the use of transfer and mobility aids was a daily and ongoing requirement. Incontinence in care homes is usually managed using absorbent pads. These can be costly to the care home and health service providers. It was noted that practice in terms of usage of continence products to manage UI did not change between time points. Although costs were slightly lower for residents receiving TPTNS than the sham treatment, differences were small and neither clinically nor statistically significant. Only a small number of individuals reported consulting primary care health professionals for incontinence-related issues. Similarly, a small number of participants were reported as receiving medications prescribed for UI. The absence of anticholinergic medications and the very low reported use of the NHS continence service during the trial potentially indicates that the usual continence care approach is to seek to contain UI rather than instigate active treatment for UI. Use of pads to manage UI was commonplace, with almost all trial participants reported as wearing pads continuously (see Chapter 3, Trial results). For the economic analysis, retail prices were used to cost continence products. In practice, lower unit costs per product could be attainable by care homes if they have supplier agreements in place.
Average costs were estimated for staff training to deliver TPTNS and delivery of the intervention to each participant in the trial. Training and support costs were estimated at £121.03 per staff member. Delivery of TPTNS was estimated at £81.20 per participant. In practice, although the time taken by staff members to deliver TPTNS would not be expected to fluctuate much, the proportional cost per resident for the Neurotrac machine would change depending on both the lifetime of the machine and the number of residents that might be expected to use the machine. Changes to the usual-care pathway for continence care during the trial were delivery of TPTNS/sham intervention and 24-hour pad collection, the latter being a trial-specific activity that would not continue in normal working practice. In practice, continence training could become part of continuing professional development training and development packages. The evidence from interviews (see Chapter 5, Qualitative study) about staff appreciation of the training indicated that continence training was beneficial not just for the technical delivery of TPTNS, but also to improve staff knowledge and understanding about causes and types of incontinence, effects of incontinence on residents and different management strategies. This could encourage more UI assessment in care homes so that residents’ incontinence can be fully assessed, type of incontinence identified and appropriate treatment implemented. A practical constraint on training, which has resource implications, should be highlighted. During the trial there were no local staff in England to deliver the training. Although the additional travel and subsistence costs incurred during the trial have not been included, managers or commissioners may wish to note this potential additional financial demand if local trainers were not available.
Limitations
Generally, CCA approaches have limited generalisability of findings because they are unable to provide specific or definitive guidance about the cost-effectiveness of an intervention. As no significant difference in primary outcomes or outcomes used for cost-effectiveness were observed, it was decided that it was inappropriate to continue to produce incremental cost-effectiveness ratios between the two groups. Although data about quality of life were successfully collected using both DEMQOL-U and DEMQOL-PROXY-U, completion of DEMQOL-U by participants was lower than completion of DEMQOL-PROXY-U. A limitation highlighted by the developers of the DEMQOL utility index is that the health state classification system may be inadequate for individuals with severe dementia. 41 A further challenge is that DEMQOL-U and DEMQOL-PROXY-U have been suggested to be more responsive to changes in dementia symptoms than to physical changes. 45 It should also be noted that as the proxy form could be completed by a different person each time, consistency of responses cannot be assumed.
In the current study, the use of CCA has been useful to indicate which costs and outcomes will be most relevant to future continence trials in care homes. Future studies using a resource use questionnaire may wish to note that using open responses for medications resulted in > 7000 items being reported. Although UI-specific medications could be identified from this, it was not possible to determine whether or not the use of the additional generic medications that were reported (e.g. painkillers) may be related to the condition being studied. For an economic evaluation, it may be more efficient to use a combination of preidentified items of interest with space for free-text additions to determine whether or not there is any change in specific medication patterns following treatment.
Another potential resource saving was in terms of facilities. Qualitative evidence indicated that a positive impact on laundry (a reduction in items requiring laundering because of UI) had been noted by one care home manager. Data had not been collected about this during the trial and future studies may wish to consider including the collection of such information.
Summary implications for policy-makers
Although TPTNS was not found to be clinically effective for this particular group of care home residents, it was notable that continence care was labour intensive and required both staff time and the use of supportive aids for the mobility and transfer of residents. Training provided to care home staff as part of the trial was positively received. Feedback indicated that the value lay in gaining skills to discuss incontinence and management thereof with care home residents and their families. Availability of local trainers to deliver this is key to managing costs, but there is potential for this to be cost neutral if routine education of care home staff about UI, its causes, and different treatment and management approaches is incorporated into continuous professional development packages.
Overall, the evidence from the CCA of the ELECTRIC trial does not suggest that there is an economic case for TPTNS. However, the positive reception of UI knowledge for care home staff suggests that there may a case for considering routine education as part of continuous professional development (CPD) packages.
Chapter 5 Qualitative study
Introduction
Qualitative methods integrated in a RCT allow an understanding of the process and progress of a trial. 46 Qualitative findings may also enhance understanding of the effectiveness of treatments and interventions, and the organisational and social contexts in which they are tested. 47 The qualitative study nested within the ELECTRIC trial enabled exploration of residents’, family members’ and care home staff’s perspectives and experiences of the research processes and TPTNS intervention.
The objectives of the qualitative investigation were to explore:
-
experiences of the TPTNS intervention from the perspectives of residents, family members and care home staff
-
factors affecting intervention implementation in the care home context and optimisation for sustainability
-
care home staff perspectives of participation in the research study and operationalisation of the research processes.
Methods
Participants
Sampling
Purposive sampling was used to gain a ‘whole-team’ perspective on experiences of the TPTNS intervention, its impact and any associated changes in practice in specific care home settings (i.e. large, small, residential, nursing and social care homes). To achieve this, three distinct groups of participants (residents/family members, care home staff and care home managers) were recruited from care homes purposively selected to reflect the full range of settings.
Residents/family members
A target sample of 40 residents, or family member surrogates, was planned. The residents (n = 30 in the TPTNS group; n = 10 in the sham group) participated in a focus group or interview at a single time point – at 6 weeks (immediately following the intervention) or at 12 weeks. Maximum variation was sought in relation to care home location (Scotland/England), sex, age, bladder symptoms, cognitive and functional status, and resident or carer status. The original protocol was amended to enable residents without capacity to participate in interviews. Previous research indicates that care home residents with varying degrees of dementia are capable of participating in research during delivery of an intervention and may provide relevant and pertinent data. 48
Care home staff
A target sample of 60–100 care home staff who had received the ELECTRIC trial training and were directly involved in delivery of the TPTNS/sham intervention was planned. Representation was sought across a range of grades, years of experience, time working in care homes and roles [registered nurses (RNs), senior care assistants (SCAs) and care assistants (CAs) working in nursing/residential care homes, and social care workers and social care assistants (SocCAs) working in local authority care homes]. In addition, a further 20 individual interviews (maximum) were planned to explore views that staff may be reluctant to share in a focus group.
Care home managers
A target sample of 20 care home managers who represented a range of care home environments and years of experience in care home management was planned.
Recruitment
Residents
At baseline, all participants gave consent (or had welfare guardian consent) to be approached for interview (see Chapter 2, Recruitment procedure). The qualitative researcher liaised with care home staff to arrange a suitable date and time to conduct interviews and focus groups. On the agreed date, care home staff assessed potential participants to ascertain whether or not they were well and able to take part in the planned interview, before asking them if they were agreeable. The qualitative researcher who had Protecting Vulnerable Groups clearance (Scotland and England) then introduced themselves and reminded the resident that they had previously consented to be interviewed, provided assurances of confidentiality and anonymity, answered any questions and checked verbal consent to proceed. No residents declined to participate at this point.
Family members
If no residents with capacity could be identified from a selected care home, the qualitative researcher contacted family members by telephone and invited them to be interviewed at home, at the care home or over the telephone, according to their preference, at a mutually convenient date and time.
Residents without capacity
Care home staff identified residents without capacity but with welfare guardian consent, who they considered well and able to take part in a small group discussion.
Care home staff
All care home staff completing the ELECTRIC trial training had given informed consent to be invited for interview. The qualitative researcher telephoned selected care homes (those that were at appropriate time points in the trial, i.e. 6 weeks or 12 weeks) and liaised with the care home manager or designated ELECTRIC trial ‘champion’ to arrange a mutually convenient date and time to visit. The use of focus groups or interviews was a pragmatic, mutually agreed decision made on the day, reflecting care home staff workload and availability.
Care home managers
Managers working in care homes where the ‘whole-team’ approach was possible were invited to participate in a telephone interview, as close as possible to the end of the care home’s participation in the trial.
Data collection: interviews and focus groups
Qualitative data were collected using semistructured focus groups and individual interviews, facilitated by the qualitative researcher. Participants were reassured that anonymity and confidentiality would be maintained. All interviews and focus groups were digitally recorded and transcribed verbatim.
Residents
To allow concurrent collection of qualitative and quantitative data, residents were interviewed at one of two time points – at 6 weeks or at 12 weeks following completion of the intervention.
Residents without capacity
TPTNS was administered during mixed-capacity focus group interviews to prompt responses to and reflections on the treatment and any treatment impact or effects. A second, and sometimes third, researcher (JB, ML) helped facilitate these focus groups, supporting participation and making field observations. Care home staff were also present for the reassurance of ‘a familiar face’ and to attend to residents, if required.
Care home staff
Focus group and individual interviews were undertaken with care home staff who had received the ELECTRIC trial training and were involved in the direct delivery of the TPTNS/sham intervention in the workplace, at a mutually convenient time.
Care home managers
Interview and focus groups, conducted in the care home or by telephone, were held at a mutually convenient time.
Topic guides
Semistructured topic guides were developed for the various participant groups (see Appendix 9).
Residents/family members
Perspectives were sought on the ELECTRIC trial, reactions to TPTNS and any impact on continence status or quality of life. Data relating to residents’ perspectives of urinary problems and the impact on their lives of living in a care home setting were also collected. Family members provided their perspectives of UI and its effect on their relative, as well as their views on the ELECTRIC trial and any interaction they had had with the resident and/or care home staff in relation to the ELECTRIC trial and intervention.
Care home staff
Key elements of the capability, opportunity, motivation and behaviour (COM-B) model of behaviour,49 which enables analysis of behaviour (practice) and identification of the mechanisms that need to be changed to bring about behaviour change, formed the theoretical underpinning of the staff topic guide. The COM-B model49 purports that human behaviour (B) results from the interaction between personal, physical and psychological capabilities (C), social and environmental opportunities (O) used, and reflective or automatic motivators (M). In relation to the elements of this model, to illuminate potential influences on behaviour change in relation to TPTNS use, perspectives were sought on the organisation of care, how management works with care staff, staff turnover, how continence care is organised within care home routines, ELECTRIC trial training, trial activity and any resultant changes in practice. Staff were also asked for their insights into residents’ responses to UI and its effects on their daily life, and their view on the ELECTRIC trial and the TPTNS intervention.
Care home managers
Perspectives were sought on culture and management values, perceived effects of TPTNS at an organisational level, any impact on culture, quality of care and economic impact, along with strategic considerations for intervention rollout and sustainability in the event of TPTNS being effective.
Pilot testing
To test the sampling framework and the feasibility and appropriateness of planned qualitative data collection and analysis methods, a pilot was conducted in a single care home. Three members of care home staff and three residents (n = 2 in the TPTNS group; n = 1 in the sham group) were interviewed at 6 and 12 weeks after completing the intervention; the care home manager was interviewed at week 12. The pilot demonstrated that at 6 weeks, residents had better recall of the research processes and had received more information from the care home staff relating to the trial than at 12 weeks. To maximise quality of responses and qualitative data, the decision was made to conduct interviews at the 6-week time point, whenever possible. These pilot data are included in the qualitative results.
Analysis
The complex nature of the data generated by the ‘whole-team’ approach required use of an analysis framework that would support understanding and facilitate presentation of data in a format that would enable subsequent optimisation and operationalisation of the ELECTRIC trial.
As described above, the COM-B model of behaviour49 was used to inform development of the topic guides for care home staff to elicit understanding of current practice (behaviour) and identify mechanisms and strategies to promote and support behaviour change. The theoretical domains framework (TDF) (v2)50 comprises 14 domains (knowledge, skills, social/professional role and identity, beliefs about capabilities, optimism, beliefs about consequences, reinforcement, intentions, goals, memory, attention and decision processes, environmental context and resources, social influences, emotions and behavioural regulation), providing a comprehensive, theory-informed approach to identifying determinants of behaviour. The TDF aligns with the elements of the COM-B49,50 and is used in this study to structure the analytic framework. 50
Framework analysis allowed comparison of data at two levels, individual (interviews and focus groups) and global (across the entire data set), and enables generation of themes in and between cases. 51 The TDF was used explicitly to inform understanding of factors affecting implementation of TPTNS in a care home context. Inductive codes were also developed and used to explore the residents’ data.
Analysis was conducted following Richie and Spencer’s52 five phases of analysis:
-
familiarisation
-
identifying a thematic framework
-
indexing
-
charting
-
mapping and interpretation.
The QSR NVivo (version 11) (QSR International, Warrington, UK) data management and analysis software was used to support the analysis process. Individual frameworks were developed for each distinct set of data (residents/family members, staff, care home managers). Specific analytic intentions were associated with each data set derived from the qualitative study objectives and areas of questioning for each group of participants. All transcripts were summarised, coded and informed by key concepts. For the care home residents and family members, the framework was based on the questions about their experiences of UI, associated care practices and views about the ELECTRIC trial. For the staff the key concepts of the TDF formed the analytic framework, charted by the qualitative researcher (see Appendix 10). The range and diversity of the themes was mapped, followed by a process of interpretation in which patterns of association were investigated and possible reasons for these explored. Two reviewers (CoD and LM) independently analysed 52% of transcripts. Iterative rounds of discussion between ELECTRIC trial researchers (qualitative researcher, JB, ML and CoD) led to agreement on the coding matrix and analysis of the inductive themes. This iterative group approach enhanced reliability and reduced the risk of lone researcher bias. 53
Reporting the results
Seven TDF domains significantly captured the involvement of care home staff within the ELECTRIC trial: knowledge, skills, social/professional role and identity, beliefs about capabilities, beliefs about consequences, goals, and environmental context and resources. Themes were inductively derived from the populated TDF domains. The challenges experienced and facilitators of implementing TPTNS into routine practice and evidence of behaviour change required to achieve this are explored. Direct anonymised quotations are used to illustrate themes. To indicate the source of each quotation, a different respondent code is used, comprising the code for level of staff, care home identification number and order of recruitment to the study. For example, ‘RN, 26016’ denotes a RN from care home 260, recruitment number 16. Residents are identified by sex, the care home identification number and order of recruitment for example, ‘Female 18001’ denotes a female resident in care home 180, participant number 01. ‘FM’ before an identification number denotes a family member.
The results of the analysis are presented in two sections: the first addresses objective 1, and describes the views and experiences of care home residents, family members and care home staff on the TPTNS intervention. The second addresses objectives 2 and 3 conjointly. These data, although not directly aligned with the TDF, have been analysed thematically and integrated into this report of the findings to enhance understanding of factors affecting the intervention in the care home context, optimisation strategies, and perceptions and experiences of care home staff regarding participation in the ELECTRIC trial.
Findings
Recruitment
Participants (residents, family members and care home staff) were recruited from care homes (n = 23) participating in the ELECTRIC trial in Scotland (n = 15) and England (n = 8). Care homes were categorised as nursing, residential or local authority, that is owned and run by the local city council (Scotland only), and small (< 42 residents) or large (≥ 42 residents).
The participant sample results follow, and the framework analysis of interview and focus group data are then reported. Table 15 provides an overview of the total participation in interviews and focus groups. This includes participation by some people in more than one interview or focus group (see Participants).
| Residents (n) | Family members (n) | Resident/family member focus groups (number of participants by category) | Care home staff (n) | |
|---|---|---|---|---|
| Interviews | 18a | 4 | N/A | 50 |
| Focus groups | 18b | N/A |
Focus group 1: residents, n = 1; family members, n = 2 Focus group 2: residents, n = 1; family members, n = 1 |
21 |
| Total number of participants | 36 | 4 | 5 (residents, n = 2; family members, n = 3) | 71 |
| Total number of focus groups and interviews = 114 + 5 second interviews = 119 | ||||
Residents/family members
Forty residents participating in the ELECTRIC trial were purposively sampled and recruited for qualitative interviews/focus groups. Eighteen of these residents participated in four focus groups of between three and five participants with other residents residing in the same care home; one resident participated in a focus group with two of their family members, and one with one family member. Eighteen residents took part in individual interviews. Two of these interviews were follow-up interviews with individual residents who had participated in a focus group in which the other residents were people with dementia. The two residents were followed up and interviewed at a later date as their views and opinions could not be completely captured during the focus group because of the challenging nature of the other participants. Three residents were interviewed twice (at 6 weeks and 12 weeks) in the initial qualitative pilot study (see Methods). Four family members were interviewed separately from their participating relative (at their own request): two in their own homes and two at the care home where their relative was in residence. Participant characteristics are detailed in Table 16.
| Resident baseline demographic details | Participants |
|---|---|
| Total number of residents | 40 |
| Female, n (%) | 36 (90) |
| Mean age (years); range | 84; 73–94 |
| Certificate of incapacity, n (%) | 20 (50) |
| MMSE score, n (%) | |
| 24–30, no cognitive impairment | 12 (30) |
| 18–23, mild cognitive impairment | 8 (20) |
| 0–17, severe cognitive impairment | 11 (27.5) |
| No score available | 9 (22.5) |
| Total | 40 (100) |
| Level of UI severity, n (%) | |
| Mild, 0–200 ml per 24 hours | 8 (20) |
| Moderate, 201–400 ml per 24 hours | 10 (25) |
| Severe > 400 ml per 24 hours | 22 (55) |
| Total | 40 (100) |
| Randomised to the TPTNS group, n (%) | 23 (57.5) |
| Family member relationship with resident, n | |
| Spouse/partner | 2 |
| Son/daughter | 4 |
| Other | 1 |
| Total | 7 |
| Care home geographical location/type, n | |
| Scotland/nursing | 6 |
| Scotland/residential | 4 |
| Scotland/local authority | 16 |
| England/nursing | 7 |
| England/residential | 7 |
| Total | 40 |
Care home staff
Seventy-one participating care home staff were purposively sampled and recruited. Care home staff who had completed the TPTNS training were interviewed if they were on duty on the agreed day/time of the qualitative researcher’s visit. Professional roles included care home managers, RNs, SCAs, CAs, social workers (from local authority care homes in Scotland) and SocCAs (also from local authority care homes in Scotland). A breakdown of care home staff is provided in Table 17. Participants had completed the ELECTRIC trial TPTNS training and all participating staff, with the exception of care home managers, were involved in delivering the intervention. Eight focus groups of between two and four care home staff were conducted (n = 21 participants); 50 staff participated in individual interviews (including one telephone interview).
| Care home staff interviewed and workplace setting | Number of staff |
|---|---|
| Care home managers: years of experience (mean/range) | 10.5 years/1 month–30 years |
| Nursing | 11 |
| Residential | 7 |
| Total | 18 |
| RNs: years of experience (mean/range) | 4.4 years/2 months–11 years |
| Nursing | 10 |
| Residential | 1 |
| Total | 11 |
| SCAs: years of experience (mean/range) | Not available at time of writing owing to COVID restrictions |
| Nursing | 11 |
| Residential | 5 |
| Total | 16 |
| CAs: years of experience | Not available at time of writing |
| Nursing | 7 |
| Residential | 4 |
| Total | 11 |
| Total number of nursing/residential staff | 56 |
| Local authority homes | |
| Care home managers | 0 |
| Social care workers | 6 |
| Social care assistants | 9 |
| Total number of local authority staff | 15 |
| Total number of care home staff interviewed | 71 |
A total of 114 participants took part in focus groups and interviews (residents/family members and care home staff); participations totalled 119 (see Table 14). The ‘whole-team’ approach to data collection, whereby the care home manager, at least two members of staff and at least one resident were interviewed, was achieved in 10 of the 23 participating care homes. Seven of these were ‘small’ care homes (four nursing care, three residential care), and three were ‘large’ (all nursing care).
Data collection
Residents and family members
Residents and family members participated in one-to-one interviews; however, residents without capacity participated in ‘mixed capacity’ focus groups.
Care home staff
When small staff numbers and busy workloads compromised the release of more than one staff member at a time, care homes opted for individual interviews (n = 35) rather than the planned focus groups.
Care home managers
Care home managers usually opted to participate in face-to-face interviews (n = 14) at their care home rather than the planned telephone interviews. Some (n = 3) chose to take part with a member of staff who had first-hand knowledge of the running of the trial. One care home manager opted for a telephone interview. The findings of care home managers and all other care home staff focus groups and interviews were analysed as a complete data set, rather than separating the care home managers as had been originally planned. This accommodated the varying roles and responsibilities of care home staff.
Findings
Experiences and perceptions of residents and family members
Many of the residents could provide limited responses only. This was mainly because of decreased cognitive ability; even those deemed to have capacity sometimes had difficulty recalling the trial and/or receiving TPTNS. Others, however, were able to express their views and perceptions, and family members of residents with dementia shared opinions relating to their relatives.
The effect of urinary incontinence on residents’ health and well-being
Residents spoke openly about their UI problems. They described areas in which their quality of life was adversely affected through loss of dignity:
I had to ring for help, I didn’t much like that. It’s not what ladies do!
Female, 18001
Participants also described the adverse effect on their ability to socialise:
I can’t really say ‘Nip to the loo’, I can’t ‘nip to the loo’, I have to be there about 10 minutes [laughter] because I go and stop, and I think it’s finished. I might get up and then go ‘Oh no!’ and I have to go back again. I am conscious of me bladder all the time, it’s sore.
Female, 51025
It’s going to other people’s houses that bothers you, you are always frightened in case you get up and go to the toilet . . . in case you wet the settee or something.
Female, 44018
Family members of residents with dementia commented on the progression of their UI:
I think now she is at the stage where before she would have got up and tried to go to the toilet, she will just sit and let it come away from her.
Male FM, 22504
Now it [UI] doesn’t bother him because he is not really aware. I think he is aware when he does it because we have walked in many a time and he has pulled the pad out and done various things with it.
Female FM, 21501
Wearing pads
Residents spoke about wearing incontinence pads; it was an important aspect of UI for many. This participant had accepted wearing pads since moving into the care home:
As soon as you come into the home you wear pads, it’s the ‘done thing’.
Male, 43014
Another participant viewed wearing pads as a consequence of growing older:
Supposing I was somewhere I couldnae get to the toilet, I wouldn’t completely wet myself but I might dribble a bit, so it is a safety thing. I put it doon [down] to getting older.
Female, 35004
However, another participant did not completely understand why they had to wear pads:
I don’t know why they [care home staff] . . . why they do it [give her a pad]. I think sometimes I get a bit loose . . . me bladder. It just comes on all of a sudden and perhaps I am a bit too slow, so they put the pads on now. [Shrugs and sighs] I don’t mind. It’s alright.
Female, 38021
Residents’ impressions of the trial
Although residents could often reflect on their UI, recalling their initial reaction to the trial varied owing to cognitive ability. However, sometimes there was wholehearted acceptance, often accompanied by enthusiasm:
As soon as somebody mentioned [the trial] I said, ‘You can put my name down for that, I definitely want to be on it, I am adamant about it’.
Female, 51025
It feels great [to be part of a trial]. It feels you are helping other folk as well as yourself.
Female, 44017
There was some understanding of how TPTNS works:
They put electric lines on your heel and it travels up your bones and to your bladder and tightens up the muscles.
Female, 51023
However, other participants perceived they were primarily helping in a more general sense:
Well it is more to help you . . . and the staff to understand what it [UI] is all about.
Female, 44019
Not very much really other than that it chartered the urinary incontinence with placement of the electrodes, so you knew you were getting them in the right place.
Female, 14005
Family members’ experiences of the trial
Family members whose relatives had dementia appeared to be most concerned about potential distress:
Anything that doesn’t freak her out I am all for it. She can get nervous of the least wee [little] thing now. I suppose it is ‘the condition’ [dementia] that causes this.
Male FM, 22504
Reassurance and information giving was also important:
She [trial manager] talked about an ankle monitor, I couldn’t understand how that [TPTNS] could have anything to do with incontinence. But she assured me that it was part and parcel of the trial, that Mum wouldn’t be forced to wear it, it would depend how well she tolerated it. So that was good, they wanted to gather data to see whether this had any benefits.
Female FM, 21503
Ultimately, family members were interested in the outcome of the trial and whether or not there could be any benefit of TPTNS to UI:
Once the trial is over, I would like to know whether there’s been ‘Yes it has been successful’ or ‘No’ or ‘There’s other work taken from it’. Just a couple of lines just to keep in touch would be nice.
Female FM, 35506
Residents’ perceptions of transcutaneous posterior tibial nerve stimulation
None of the participating residents who recalled TPTNS viewed it as an inconvenience or annoyance. They recounted how they incorporated the sessions into their everyday life:
I was sitting watching my telly so it [TPTNS] wisnae [wasn’t] any inconvenience. You know I sat and watched my telly.
Female, 35004
Oh, I can’t remember how long [I had TPTNS for]. It never troubled me or anything, I just rested for that time. I had no problems at all.
Female, 38201
This resident perceived they should receive a stronger dose (they were in the sham group), because they sensed TPTNS was not having any effect. They showed a desire to keep going with treatment:
When they put the machines on up to [stimulation strength of] 4, I need it up higher to feel the tingle. I think I need perhaps a bit stronger [dose] and for a bit longer. It doesn’t matter [which group] I was in; I am going to do it for longer to see.
Female, sham group, 51025
Others perceived a positive effect on their UI status as a result of their TPTNS:
I think it has slowed down in some ways. I think I have slowed down from what it was at the beginning. I don’t have to think about having a wee as many times and stuff like that.
Male, TPTNS group, 18002
I am lasting longer; I am not going all the time. Sometimes I forget to go. I think ‘Oh gosh I better go,’ and I go. Because I have more control.
Female, TPTNS group, 14005
Although this participant was in the sham group, they perceived that their UI had improved:
But I feel it [UI] has got better. I think I have got more control of it; I can hold it a bit better. I feel it gives me more time to get to the toilet.
Female, sham group, 44018
A family member who was asked if there were any notable change in their mother’s UI status perceived that it was the CAs (not the RNs) who would notice any changes:
Have I noticed? I think that would have to be the carers that would need to tell you; they see to her personal [UI] care. I don’t know.
Female FM, 22505
The residents and family members showed an interest in the trial, although their knowledge was limited. For family members, interest was usually focused on ensuring that, owing to the cognitive status of their relative, they would come to no harm, rather than any particular interest in the trial per se. None of the residents spoke unfavourably about the trial or TPTNS; it did not interfere with their daily life, rather they were able to continue with their routine undisturbed, and some perceived that they had noticed a positive change in their UI status.
Experiences and perceptions of care home staff and managers
For the ELECTRIC trial to be successful, changes to continence care practices in care homes were required. These changes took the form of two additions to practice, together with the need to change current routine toileting activities, to reflect individual resident continence and voiding needs. The two additions were (1) the delivery of the TPTNS/sham intervention and (2) the implementation of 24-hour pad collections. Evidence of these changes in care home staff behaviour and the factors affecting the nature and extent of the changes in the particular context of care homes is provided, based on the underpinning TDF and COM-B model and subsequent thematic analysis. Data coding and analysis in terms of the TDF domains is shown in Appendix 11 and the thematic analysis is presented here, with the relevant TDF domains indicated in brackets as numbers between 1 and 14.
Gaining knowledge (theoretical domains framework 1 – knowledge)
The desire to gain new knowledge encompasses initial impressions of the trial and the TPTNS training, and perceptions of gaining new knowledge. Scepticism, reservation, enthusiasm and curiosity in relation to their new knowledge are described. How prepared care home staff were for the ELECTRIC trial varied between care homes, with some staff being well informed prior to the ELECTRIC trial training and others having no knowledge of the trial until they were allocated to attend the ELECTRIC trial training by the care home managers. Many care home managers chose not to attend the training and cascaded responsibility for the trial to others from the outset.
Initial impressions (theoretical domains framework 12 – social influences)
I wasn’t quite sure how it was going to work.
RN, 22808
Prior to commencement of the trial, care home managers were often well informed about its purpose and the processes it would involve, having attended external regional meetings and forums and from speaking to the trial managers and chief investigator:
Our clinical director is very keen on research and making things happen, and change and innovation within care homes. She actually introduced the ELECTRIC trial to the group.
Care home manager, 43913
And the [trial manager] that we discussed how we felt and that was quite good. But it was also very interesting to get that information.
Care home manager, 29914
This SCA received information about the trial directly from the care home manager and felt well informed prior to TPTNS training:
Well, [our care home manager] came to us and told us that this trial was coming on and that it was a nationwide thing to see if they can, not get rid of incontinence, but lessen the impact that it has on [residents] through . . . stimulating a nerve in the leg, and obviously all these things need to be tested.
SCA, 41725
However, the above participant was in the minority: most care home staff described being unprepared, with some having little or no knowledge about the trial until they were sent for TPTNS training, often having received a directive from management to attend without prior warning:
When I came to do the training, I had never heard of it before.
RN, 14816
I only heard about it actually at the training. When I was told I was going to the training, that was the first time I heard about it.
SocCA, 36621
Having attended the training, care home staff views were mainly very positive, and they described how they approached the training with an attitude of curiosity in anticipation of potential benefits to residents with UI:
I was curious about it as I had never heard of TNS [TPTNS] before . . . I thought it was a good idea.
SCA 34726
My initial reaction was ‘It would be absolutely amazing if we could find something to help the residents’.
CA, 51853
Learning about research
Looking at the broad picture of the trial.
Care home manager, 37915
The ELECTRIC trial training provided learning around the purpose and processes of the trial, and specific knowledge and skills training, including learning around UI and TPTNS. Responses to the two distinct elements of the training differed, with UI education and TPTNS skills being mentioned more frequently than the research elements. Staff appeared to have knowledge relating to understanding UI and delivery of TPTNS, but not necessarily of their input and contribution to a large trial and the importance of maintaining rigour.
However, some staff enjoyed being involved in the broader aspects of the trial. For instance, this SocCA appreciated being consulted about potential participants by the trial RRA:
I think having input into who went on the trial, certainly in my unit . . . the girl that came from the University [the RRA] she said to me ‘Who would be suitable, who wouldn’t be suitable?’ and helped me pick the residents that it was suitable for . . .
SocCA, 36622
Care home staff also referred to the professionalism of the trainers and some were able to appreciate that they were participating in a wider trial because of feedback from the trial staff about their contribution:
We were one of the first homes, we were almost one of the guinea pigs in it, and it was really good to get the feedback from both [chief investigator and RRA] that a lot of the stuff that we had kind of thought about and put forward as ideas was used for other homes, so that they kind of changed things. So that was actually good to actually be part of the beginning of it and also . . . you know, help the trial go further because of things that we had identified and changed.
Care home manager, 43913
It is always good even being part of research programmes – it is really interesting.
RN, 35815
And there was a sense of understanding of the wider purpose of the trial:
. . . the [trial] staff that came down and did the training were very friendly, very professional, and it was a very enjoyable session. All the staff from here who took part said they really felt they had learned a lot around incontinence and why the trial was going to take place.
Care home manager, 14905
Finally, the training gave a more in-depth insight to UI than previous training they had received:
. . . it’s opened . . . my eyes, because the continence training we had previously was how to apply the pad and how absorbent is the pad. This is something more, it’s extending the subject, so it was interesting.
SCA, 500744
Enthusiasm (theoretical domains framework 5)
I mean if it works, it is going to be fantastic.
SCA, 44638
Many care home staff were optimistic and hopeful about participating in the trial and the possible impact it could have on UI care. On completion of training, staff recalled how they felt enthused and motivated to put their training into practice:
I think what [ELECTRIC is] doing is absolutely wonderful because a lot of our time works around incontinence, if you like. And if you can cut that wee [small] part out, it is more time with the resident to give them more activities, more outdoor things. I just think if it works it will be absolutely wonderful.
CA, 43629
Providing underpinning knowledge that was so often lacking gave staff much needed encouragement:
The carers haven’t actually got that knowledge, and I think the training gave them the underpinning knowledge [they needed] . . . it gave them a boost and I think that’s really important.
Care home manager, 43913
Scepticism (theoretical domains framework 5)
There’s no way in a million years that will work!
SCA 50746
Some care home staff expressed scepticism about the concept of TPTNS after the training. Although they had been taught the principles and simplicity of TPTNS, some could not conceive how it could work and believed that it would be time-consuming:
I didnae [did not] believe it would work. I was a non-believer . . . I thought, ‘I don’t know how can a machine stop you from peeing? [urinating]’ I just couldnae [could not] fathom that oot [out], how a machine could stop you from peeing.
SocCA, 36624
I thought it was going to be an absolute pain, if I am honest, I thought we would be putting it on, and we would have to sit there for half an hour and keep talking the resident into keeping it on. I thought it was going to be really time consuming . . .
SCA, 22709
Often, once the care home staff had experienced delivery of TPTNS, these concerns were resolved:
But it turns out it’s not [time-consuming], and [the residents] all took it quite well.
SCA, 22709
Concern about involving people with dementia in research (theoretical domains framework 6 – beliefs about consequences)
I was kind of not sure whether I agreed with it, to be honest.
Care home manager, 29914
Trial training included discussions with staff about involving people with dementia in research and the reasons for the wide eligibility criteria for the ELECTRIC trial. This included discussion of whether or not it was ethical to not offer people with dementia the opportunity to take part in research from which they may benefit. The majority of staff were in favour of including people with dementia and those who voiced concerns were very much in the minority; no one declined to participate on these grounds:
I wasn’t that comfortable – but not as uncomfortable as not to take part.
Care home manager, 29914
However, it was important to acknowledge staff concerns:
I didn’t think it was a guid [good] thing. Because most of oor [our] residents have got dementia or whatever and I just thought, ‘I don’t get this at all.’ Because I am still in the opinion that these people [residents with dementia], they don’t even understand – do you know what I mean? So sorry I jist cannae [just cannot] get my heed [head] round it.
SCA, 450751
Ethically, I was struggling . . . yeah. There was always that part of me that was, ‘She doesn’t even really know what I am doing.’ I struggled with that a little bit.
Care home manager, 29914
Staff views and opinions of the training was generally positive. However, there was some evidence of ethics concerns and, with regards to trial processes, there was limited interest in the outcome measures; for example, interest was focused on learning how to deliver TPTNS.
Developing skills (theoretical domains framework 2)
Specific skills training based on understanding the mechanism of urinary incontinence
You get an insight into how the body is working.
SCA, 41725
Stuff like your stress incontinence and like that . . . there is an urge to go . . . and they [trial staff] were saying like, ‘Has anybody heard of medication to stop incontinence?’ and I was like, ‘I didn’t know there was an actual thing for that!’
SCA, 18702
Skills training in the delivery of TPTNS began in the ELECTRIC trial training sessions and was continued into the practice area by ISFs. During the ELECTRIC trial training, different types of UI were discussed and the underpinning mechanism of action of TPTNS was explained so that care home staff could understand the reasons for conducting the trial and the theory about how TPTNS might work. Care home staff were given the opportunity to try TPTNS on themselves, an activity they enjoyed, and they appreciated experiencing how it might feel for the residents:
You could feel what it felt like, so you knew what [the residents] were going to expect, so that was quite good.
Care home manager, 34908
Completing the task correctly
Just double checked we were doing it right.
SCA, 22709
Input from the ISFs was frequently commented on as valuable and helpful. Their role was multifaceted: they continued the TPTNS skills training in the practice arena and carried out competency assessments. They encouraged staff and provided support, instilling confidence where individuals had concerns about ‘getting it right’ or ‘doing it wrong’ (see Becoming confident below). Staff described the TPTNS competency training as a check to ensure that they were completing the task correctly:
. . . [the ISF] came back in to see if we were doing it right . . . and it was fine yeah.
SCA, 500745
However, although ISFs were able to oversee intervention delivery and adherence checking, they were not involved in trial data collection. Responsibility for many research aspects of the trial (e.g. completing outcomes measures accurately, pad collections, bladder diaries) fell to care home staff and the success of these activities was often dependent on professional roles, responsibilities, and care culture within the individual homes.
The importance of leadership (theoretical domains framework 3)
The complexities of the care home setting, the various grades of staff, care home culture and the importance of leadership all had an impact on staff attitudes towards UI and UI care, efficient running of the trial and, ultimately, on whether or not change in practice could take place. Care homes varied in the way they organised trial-related work. Some appointed a member of care home staff as a ‘champion’, who was wholly responsible for organising trial activity (n = 8), or the care home manager (n = 3) assumed the ‘champion’ role. Other nursing homes (n = 4) afforded shared responsibility to SCAs and CAs, with limited input from RNs. In residential care homes and local authority care homes CAs (n = 8) were sometimes completely responsible for the trial activities. Care home managers’ roles ranged from being remote to wholly engaged, enthusiastic and ‘hands on’.
Strong leadership: working as a team
We are working together to benefit the residents.
SCA, 500744
Strong leadership that actively encouraged teamwork was identified as a key factor in running the trial successfully within the care home setting. There were care homes who described a cohesive team approach, with staff of all levels being involved in the trial. Staff described a welcome lack of ‘rank’, with all care home staff being aware of the trial:
I think it is good as well that there’s been no . . . rank difference, so it is anybody from [care home manager] down to the care assistants and nurses, everybody has been involved in it.
RN, 14816
The staff are all aware that this trial has to take place and what we are doing. It is not just us as nurse assistants, [name] as a senior is doing it . . . Everybody is aware.
SCA, 41725
Care homes adopting this team approach frequently appointed a ‘champion’ who took responsibility for organising the smooth running of the trial. As this champion was often a respected member of the staff and a good communicator, this approach resulted in good teamwork. This ‘continence champion’ within a larger care home gave an encouraging response:
I am the continence champion here. [The trial chief investigator] came and spoke to me, the care home manager and deputy care home manager, you could see how excited she was about it as well, and that made me excited . . . and later we [care home staff] all sat together . . . discussing things and how this could make a difference.
RN, 22808
Sometimes, in smaller homes, the care home managers oversaw the trial work and this was readily accepted by the staff, who were clearly used to strong leadership:
The manager oversaw it all so . . . when the team were coming and saying, ‘Oh we need so many [TPTNS] doing and we have got a little behind with this . . .,’ whoever was on duty, we just rolled it out. It was very much a team effort which is really, really good.
RN, 14816
And this care home manager, also in a smaller care home, related an important lesson learnt when they initially tried a team-focused approach that did not work and they subsequently felt compelled to take charge:
I completely controlled it [laughter] . . . initially we kind of said, ‘Right these are your residents, you work out when your treatments are’. But actually, it wasn’t working, it needed more control. So, I think that one of the learnings for me was that you really need somebody to lead it, you really need a champion.
Care home manager, 43913
Enjoying the trial through feeling valued
I just sort of kind of blossomed through it!
RN, 50847
Care home staff described a sense of feeling valued not only by management but also through being part of the ELECTRIC trial, which emerged as a key factor in staff investment in the research. Differences between care homes were described. Those that adopted a cohesive team approach and/or had appointed a ‘champion’ comprised staff who perceived they were valued, and felt themselves and their care home to be contributing to a worthwhile project:
I would say that the staff who were trained, really had a feel-good factor and it made them feel very valued and that it was an important job that they were doing.
Care home manager, 21903
Absence of support or leadership
The most they will do is they will write in the diary your name and ‘ELECTRIC trial’.
SocCA, 36624
Although strong leadership was evident in some care homes and was associated with positive experiences of the trial, there were care homes in which leadership was weak or virtually absent and this resulted in more negative experiences. Here, staff described managers that were remote and uncommunicative. And, although staff had enjoyed the training and demonstrated a positive attitude towards the trial, they struggled under demanding workloads to maintain trial-related responsibilities. Staff described feeling that they were part of a ‘tick-box’ exercise and that there was lack of concern for trial processes or for their engagement in the trial from managers who communicated by telephone or through checklists in a diary only:
When they [‘seniors’] phone for the report and you get, ‘. . . and your ELECTRIC trial, did you do them [the TPTNS in the diary for that day]?’ ‘Yes.’ It is like a tick box . . . it is a tick box kind of thing for them.
SocCA, 36622
This staff member went on to recount how their senior, who was rarely involved in the trial activities, had delivered the wrong TPTNS using a dose appropriate for someone randomised to the TPTNS group to a resident randomised to the sham group (no harm came to this resident):
She had it [TPTNS] [and had documented as such] on the wrong bit of the leg [laughter] and had it up to 34 [a therapeutic stimulation strength, not sham] [laughter]. So, the Seniors no, they’re not involved enough.
SocCA, 36622
One SocCA had little or no contact with their senior, was never asked how they was getting on or whether or not they needed support in the trial and was left largely unsupervised:
No, he has no’ [not] asked me. Our senior . . . doesn’t work my shift pattern so . . . I very rarely see him.
SocCA, 44637
Whose role is urinary incontinence care? (theoretical domains framework 3)
Delivery of UI care was usually seen as the role of CAs and SCAs. In nursing homes, RNs were viewed as being too busy with other responsibilities, one of the reasons SCAs and CAs were more involved in the ELECTRIC trial than originally anticipated:
It is usually just us like, yeah just us.
CA, 14618
As previously described, in the nursing homes senior staff (RNs and care home managers) were not as involved in the trial as anticipated, one reason being that they did not view UI care as part of their role. In many care homes, senior staff described delegating UI care, describing CAs as the main UI caregivers. This RN was asked whether or not they had noticed any change in residents’ UI status since starting TPTNS:
As I say . . . we maybe don’t notice it [UI care] day-to-day because obviously we are not as ‘hands on’ . . . as the girls are.
RN, 26832
And this care home manager:
I think probably . . . the carers benefited most [from participating in the trial] because they are the ones that are doing the . . . continence work.
Care home manager, 45909
This CA corroborated that understanding:
[junior members of staff] . . . [are] the ones that can do it [UI care] and sometimes the seniors [SCAs] as well, but then not so much the nurses . . . they have to do their own thing [laughter].
CA, 14618
This care home manager commented that in their care home the RNs were not enthusiastic about participating in the trial, and it was agreed that the SCAs and CAs should mainly carry out trial activities:
The nurses heaved a sigh of relief . . . ‘Oh, I can go and get so and so to do it?’ [laughter] . . . we didn’t see the point in it only being nurses, you know, because it wasn’t something that particularly needed to be done by the nurses.
Care home manager, 22907
They went on to say that being part of the trial was a good thing for the care staff because it gave them a sense of self-worth:
But then as they [RNs] were able to pass that duty down to the care staff I think it gave the care staff a sense of self-worth that they were able to do it.
Care home manager, 22907
Disinclined to participate in the trial
The care staff did more of the trial because I was just doing other things.
RN, 21806
The role of UI care frequently fell to junior care home staff. Often, if SCAs and CAs took on UI care, it seemed to follow that they would also be responsible for the trial activities. This RN, a ‘champion’, comments that although they appreciated that other RNs had many other tasks to carry out, they also sensed that they preferred not to be involved:
It was actually the nurses were not too sure. We had senior carers and nurses trained up, and the senior carers seem to just run with it, maybe because they are ‘at the coal face’ [carrying out UI care] more than the nurses, but they seem to just grab the idea and run with it. But the nurses . . . it was just another added thing onto their role, it was another thing to do.
RN, 50847
This SCA was quite clear about who engaged with the trial:
I haven’t seen one nurse do it. I don’t really know I have never really heard any of them [RNs] say anything about [the trial]. They are always too busy [with] meds and dressings and stuff like that but . . . pretty much yes, it’s down to the care staff.
SCA, 18702
Clearly, junior staff were frequently responsible for most of the trial-related work, including co-ordinating TPTNS, completing pad collections and maintaining bladder diaries within their routine practice. The impact of this varied; some junior staff were able to cope well whereas others found it stressful.
In summary, most care homes perceived UI care as the role of junior staff (CAs and SCAs) and senior staff were better placed continuing with their normal work, allowing the CAs/SCAs to manage the trial activity. However, although TPTNS training was well received and implementation was efficiently monitored by the ISFs, junior staff may have required more support in managing the other trial activities (see Making the trial work).
Becoming confident (theoretical domains framework 4)
An important feature of participating in the trial from the perspective of care staff was their new learning, including knowledge about UI and skills to implement TPTNS, but their confidence to implement this new learning in practice was also essential. Engaging with the ELECTRIC trial was seen as ‘more than just learning a new task’ and:
It’s not just a physical thing, it is also a mental thing.
SCA, 51752
Some staff admitted to feeling somewhat uncertain moving forward in delivering the intervention. This care home manager recognised the important role training had in teaching staff the right skills and enabling them to have confidence in delivering the intervention:
Obviously, it was a wee [little] bit fearful at first because you have always got this thing, ‘Oh, what if I do something wrong?’ And to actually have the training and be reassured that basically, you know, that the staff are comfortable and confident using this, that was probably the best thing.
Care home manager, 35917
The ISFs played a key role in instilling confidence and reinforcing new skills, being available for care home staff as they implemented TPTNS into everyday practice. Many care home staff had not learnt a new skill for some time, nor had they participated in research before. Having support from the ISFs reinforced the learning from the training sessions and ensured that staff were competent and confident to deliver the intervention. The ISFs were often present in the care home during that time, ready with advice and guidance:
Because sometimes you worry, and you are not sure, ‘Is that right? Was it three fingers I was doing or one?’ So it was good to have somebody come in and go, ‘No’ and put us straight. So that was good there was a wee [little] bit of reassurance so that we got it right. I appreciated that [laughter].
SCA, 22709
. . . [the ISF] would say, ‘You are very good, this is great, you have got it all right.’ It was good, we were well supported.
Care home manager, 450748
The training and subsequent support from trial staff visiting the care homes gave care home staff the confidence to put new learning into practice and engage in TPTNS/sham provision.
This SCA had also been able to put their learning into practice, recognising that this helped not only the resident, but also their own practice, knowing that they was providing effective treatment for UI:
I have learned. . . that a wee [little] lady gets ‘functionally incontinent’, so [that] is helping me. [Residents] get really distressed because they still know they need to go to the toilet; they just can’t locate where the toilet is. So now that you can pre-empt that happening, it’s much better for them and for us, you are stopping somebody from being incontinent in a corridor, their dignity is not compromised . . .
SocCA, 36622
Potential consequences of the ELECTRIC trial (theoretical domains framework 6)
Care home staff views about what might be the outcomes of engaging with the ELECTRIC trial were varied and seemed to depend on the level of reflection of each particular staff member following training.
Potential consequences were both negative and positive, particularly with regard to attitudes towards UI care in a care home context.
Proactive treatment for urinary incontinence
There’s some hope out there; there’s something that will help, because UI has never been a high priority. You know ‘I have got this and this is me for life.’ But you know that’s maybe not the case then?
Care home manager, 26911
Well because there isn’t anything out there for a start other than just popping a pad on them. Is there? You know.
Care home manager, 26911
This is the first I have ever heard of any kind of continence trials or anything like that and like I say I have been nursing for years and I have never heard of anything like this. So this is why again I jumped at the chance of being involved in it.
SCA, 510752
Care home staff commented on the training and the impact their learning had on their UI practice. This care home manager talked about the key message and the change in practice they had implemented:
It is not the fact that ‘I am incontinent there’s nothing I can do’ it is the fact that ‘Yes you are incontinent but we are going to try and help you, we are going to try and do something else’.
Care home manager, 14905
If the residents ask for the toilet themselves – brilliant, go to the toilet and empty their bladder. Less with pads, less with clothing, less work to change the whole resident. So it’s working for them and for us as well.
SCA, 50744
A positive impact on some residents’ UI status was perceived following TPTNS, and this RN describes the ‘light bulb moment’ when CAs realised the change in one resident:
The staff were coming to me and going, ‘There’s a problem with this lady, her pads are not wet, her pads are just not wet!’ and I said, ‘Are you taking her to the toilet, is she actually passing urine in the toilet?’ ‘Yes, but her pads are not wet,’ and I said, ‘Well, why do you think that might be?’ and they were like, ‘OH!’ [laughter], like a light bulb moment for them.
RN, 50847
Care home staff acknowledged that improving residents’ UI status would have an impact on their quality of life, both socially and psychologically. This RN identifies the potential effects on residents’ psychological well-being:
Reduced anxiety for them . . . because I think that is what I see in a lot of the residents, is that anxiety about going to the toilet or needing to go to the toilet.
RN, 22808
There were also accounts of residents having an improvement socially, such as this resident who was able to go on holiday and be less focused on their UI:
She actually went on holiday just after [TPTNS] and we did notice a change in her going to the bathroom and that as well. Whereas before she would be more toilet focused but she didn’t have to be. It meant that she could go on holiday and enjoy it a wee [little] bit better.
Care home manager, 35917
Finally, this RN summed up their views that this research could help residents return to being able to manage their own UI care:
I thought it was great, yes. I think that anything that can give them that wee [little] bit of self-respect and dignity back is always worth researching and giving them that hope that, ‘Yes, we can help you with this wee [little] thing.’ That they can self-manage their personal care again. It is a small thing, but it is a big issue in their lives.
RN, 35815
Person-centred changes to practice
Let’s have an improved quality of life instead of ‘Just let’s do toileting’.
RN, 44733
Initiating a move from task orientation to person-centred care is summarised by this care home manager, who had noticed positive changes in residents’ UI status:
Now we are trying to get away from task orientation and [TPTNS] is helping because the residents who were badly incontinent . . . we know when they need to go. So, they are not getting taken to the toilet because it’s time you know, it’s part of the task. They are going because it is time for them to go.
Care home manager, 45909
The knowledge gained from the training had brought about change in practice to a more person-centred approach according to this care home manager:
Even if it means that you might still be incontinent but more aware . . . so we can . . . take people to the toilet at specific times. Or they may have a bit more warning so that they can say, ‘I think I need to go to the toilet now’.
Care home manager, 14905
Making the trial work in care homes (theoretical domains framework 11, theoretical domains framework 12)
In terms of the trial-related activities the smooth running of the research varied across care homes, with some reporting an easy adjustment and others experiencing difficulties:
You just organise yourself at the end of the day.
SCA, 51752
Care homes that were well organised, with a ‘champion’ and/or strong team approach, managed day-to-day trial-related activities well. This was not only delivering the TPTNS, but also completing pad collections, maintaining bladder diaries and assigning trial tasks to staff:
I used to put it in the diary, ‘Tuesday, the ELECTRIC trial day’. You know, you just organise yourself and then you don’t get stressed. And if you did have a busy day on that day, you just think, ‘Well, that’s OK, I will just do it tomorrow then.’ It was never stressful for me and it wasn’t a big deal.
SCA, 51752, appointed ‘champion’
Other staff were left to organise their own work, without supervision:
My thing then was finding the time because I had five [residents] on it. So, it’s quite a lot. But I found if I did them all at the one time it was so much better.
SocCA, 36622
Communication breakdowns regularly occurred, which could cause distress and make the organisation of the trial activities challenging for staff. For example, a failure to pass on information in the diary about starting stimulation undermined this socCA’s confidence:
We have got three shift patterns . . .and it [communication] sort of broke down with the other shift, it jist didnae [just did not] seem to follow through . . . I usually follow this other team, I don’t follow [name’s] team . . . so this is where it wisnae [was not] left . . . there were no notes [in the diary] . . .
SocCA, 44637
Perceived ‘excuse-making’ was a frustration for other CAs:
They say, ‘Oh, I have never done it,’ [TPTNS] and I am like, ‘Well why? Why? There’s no excuse! Why?’ ‘I am too busy’. But . . . please excuse my French, but it is crap because all it takes is one minute to put on and then they can walk away for half an hour!
CA, 18601
Prioritising trial outcomes measures: the role of good leadership and teamwork
This is important, it is important, and it could have an impact.
Care home manager, 50918
The organisation of trial-related tasks worked well with good leadership and the engagement of the whole team. This care home manager recognised the importance of accurate recording of research data and successfully conveyed this to staff:
The staff have got involved with the pad changes, including the staff on nights. . . . the important thing was recording on the charts, you have got to record everything and not miss anything out. And . . . I am proud to say, I think there has only been one session that was missed.
Care home manager, 51904
And some care home staff organised efficient pad collections:
We were remembering [pad collections] because we were putting them in the residents’ rooms. The bags were in their rooms, so it was a case of when you took [a pad] off the certain person you went straight to their room, so we all knew how to do that.
CA, 150643
In some care homes, staff worked in partnership with residents who had capacity, so that a small number were able to self-manage their pad collections:
The staff tell me when . . . aye, they leave me a bag and I put the pads in.
Female, 35004
I do a check sheet and save my front pads.
Male, 18002
This resident corrected staff when they almost used the wrong pad collection bag:
The first time we lost the bag. This time they wanted to put the pad in a yellow bag. I said, ‘But they wanted it in an orange bag.’ . . . [laughter]!’
Female, 18001
Organisation of the trial also included recording bladder diaries, and again, in some care homes, this was conducted efficiently:
. . . [The bladder diaries] are all in their little packets, so the residents all have their own little packets that go into the [bed]room and then we used to sit and write it in there with them.
CA, 14618
We got really good feedback from [the ISF] that the bladder diaries were perfect [laughter].
Care home manager, 29914
In care homes where organisation and leadership were less clear, uncertainty with bladder diaries and pad collection processes was apparent:
I don’t know about that [bladder diaries]. No, I think . . . were they just popped in the, em . . . that’s interesting, I don’t know then?
RN, 35815
This SocCA, who delivered TPTNS and was responsible for the trial in their care home section, had not heard of the bladder diaries either:
No we have not done that. We don’t do that.
SocCA, 44638
Bladder diaries were sometimes difficult to maintain accurately because they were moved from room to room and residents would go to the toilet without the care home staffs’ knowledge:
The [bladder] diary is more of a nightmare because it can be in like a dining room because we are all having to take them back and forward. Trying to keep track because a couple [of residents] obviously go [to the toilet] themselves and then trying to remember all the ones that [have bladder diaries].
CA, 15643
Uncertainty as to who was responsible for completing the bladder diaries could occur:
I think the care staff probably did the bulk of them [bladder diaries]. Obviously, they can go back and ask certain ones but it’s the [care] staff did them.
RN, 26832
And sometimes the bladder diaries were completed all at one time:
Yeah I jist [just] sat and done it . . . we used to bring the packs out and then jist [just] sit and fill them in . . . jist [just] sit and fill it all in and that was that.
Social care worker, 44735
However, the method of completing the diaries ‘all at once’ described in the quotation above was contrary to the directions for bladder diary completion, which were designed to capture the individual’s information at the time it occurred to accurately reflect toileting activities and times for each trial participant.
It was often difficult to record the bladder diary accurately when residents used the toilet independently and may leave the care home for periods of the day:
It impacted . . . it is a wee bit time consuming the bladder diary, and plus the difficulty with that was two ladies who have capacity, they toilet independently. So you didn’t want to interfere with their daily routine so it’s very difficult to get a true reflection recorded. Also her family take her out for the best part of the day. It wasn’t a true record of how she was managing and unfortunately she is the lady who is [in the TPTNS group].
Social care worker, 44734
Recording outcome measures could be problematic
Probably the most onerous part for us was the pad weighs – we tried our best.
Care home manager, 21903
Some care homes found organising the trial difficult, with the most commonly recounted problems being forgetting and/or throwing away pads during collection times:
I have to confess I have thrown a couple of the pads out; I totally forgot.
SocCA, 36624
The stress for me was just when we were doing the pad collections in case there were any mistakes or people were forgetting . . .
Care home manager, 31910
Senior staff were sometimes aware that junior staff had thrown pads away:
Sometimes when you go to the toilet with somebody the pad goes straight into the bin [rather than being saved for pad collection]. Sometimes the carers might have forgotten to collect the pads . . .
RN, 21807
Staff also described how residents could be unpredictable and either throw pads away or hide them, making accurate pad collections problematic:
The hardest part was just the pad collection, because you never know when residents are going to take a pad off or where they are going to put it. So keeping track of all the people who were [in ELECTRIC] was . . . interesting.
SCA, 37740
There are a few times we have gone in [the resident’s room] and the pads have been removed in the most obscure places, so it’s obvious that he has removed them himself. So that’s been quite frustrating, in the fact he doesn’t want to take part, he will say, ‘Yes’ and then he will sneak the pad off. So, we have not really got fantastic readings.
SCA, 41725
Other factors also disrupted smooth running of pad collections, such as having to rely on agency staff:
Obviously if you have last minute sickness and you were doing a pad collection and you have agency staff on, and they don’t know . . . or they are not as aware, they will just whizz it [the pad] in the bin . . . it kind of put us on the back foot a little bit, I felt.
Care home manager, 37915
Implementing essential aspects of the research (e.g. completing outcomes measures), although managed efficiently in some care homes, was problematic for others. Challenging, sometimes complex, care home working conditions meant that research activities proved onerous, often because staff forgot, or were not told, when to begin pad collections/bladder diaries, or were not sufficiently aware of the importance of outcomes measures and focused their efforts on effective delivery of the TPTNS, to the detriment of the outcomes collection activities. The heavy workload, and sometimes a lack of support, confounded the efficacy of the research for some, but were dealt with more easily by other care homes.
Impact of the research on staff time and resources
The trial has become part of our routine; you quickly adapt to routine.
SCA, 41725
Care homes that adapted well to the trial had good leadership and were well organised, with staff who understood their roles and tended not to feel compromised by conflicting priorities. Staff expressed a willingness to participate and did not feel under pressure of time:
Over time the staff got more confident with the [TPTNS] and it didn’t take them as long, and the same with pad collections, they were a bit . . . time consuming but staff were making sure that they had the right pads, the right times and things. But we got more confident with it over time.
RN, 22808
No, it’s not been stressful in the slightest. It’s all just quick to do and [TPTNS] is easy obviously because you can leave the machine sitting with [residents] for half an hour and then just go back and take it off.
SCA, 50746
Residents also commented that the trial did not impact on their daily routine:
I can’t remember much about it quite honestly, it [TPTNS] was just a routine thing we had to do.
Female, 34008
However, some staff members described finding difficulty in coping with their heavy workload:
One thing that went against us was because we are so busy in here and we only have two staff. And some of the residents who were in the trial . . . maybe couldn’t be left alone for the half an hour, so finding the time has been the main issue.
SocCA, 44638
The same member of staff went on to recount how they had missed a TPTNS session:
I was under pressure because I thought, ‘I need to get this done before the time is up,’ but . . . I felt guilty about missing it, but there was really nothing I could have done.
SocCA, 44638
Being the only member of staff who had done the training became burdensome for this SCA:
I will try and get the night staff to do it, like, ‘Can you do this?’ and it’s like, ‘Well, we have not had the training.’ That [lack of other staff being trained] then does put pressure on me to try and get [TPTNS] on then and I will be leaving work late.
SCA, 18702
Although not the only member of staff who had been trained, being solely responsible for delivering the intervention during a shift was perceived as challenging to the point that intervention delivery could not be completed. The practice of non-completion because of lack of time and potential deferment to the following day was context dependent, being acceptable in some care homes and not others:
At times I have been like, ‘Oh I have got this bloody ELECTRIC trial to do!’ [laughter] steam coming out my ears. Oh, the residents are all going off their nuts and I am like, ‘Oh, my good God in Govan! 7 o’clock at night I am running about sticking pads to people’s feet!’ [laughter]. The most management will do is write in the diary . . . but I have just been scoring through it and saying, ‘Too busy; far too busy!’
SocCA, 35613
Potential benefits to care home resources
Although care home managers observed that the most likely positive impact on resources would be a reduction in the quantity of incontinence aids/pads used, they did not anticipate reducing their orders in the near future:
Yes, less [sic] pads, I would think so, yeah . . . And maybe [reduced] pad absorbencies . . . as well, in the future.
Care home manager, 50918
We daren’t reduce the amount of continence aids or pads that we get from the company because trying to get them increased again – it’s like gold dust.
Care home manager, 21903
Another care home manager identified laundry as another resource impact, as residents who were perceived to have benefited from TPTNS were not requiring such frequent changes of clothing:
It’s affected the laundry staff as well because they are not going through as many clothes for the residents . . . one of the [residents], it could have been twice or three times a day – a full set of clothes, and that’s reduced completely.
Care home manager, 45909
What happens next? (theoretical domains framework 9 – goals)
Care home staff spoke about their intention to carry on with TPTNS treatment after the trial finished. They specifically identified residents who had been in the sham group, initially refused to participate or not been able to provide consent in time for the trial:
[Name of resident] is always trotting to the loo and she refused to go on the trial. But I think she’s got most of her faculties and she will be looking to see how the others get on and then she will come forward and it’s good to have a machine because then we can try it on her.
Care home manager, 51904
For residents who were allocated to the sham group, staff posited that offering them TPTNS would lessen the disappointment of discovering they were not in the treatment group:
She might be a bit disappointed when I tell her that it was the sham that she was on, but I think that, you know, when she was so excited about trying it anyway, I think she would be quite keen to try . . .
RN, 26832
Did care homes continue with transcutaneous posterior tibial nerve stimulation?
Care homes that had received Neurotrac machines following trial completion gave a mixed response. One care home manager who had been enthusiastic about participating in the trial admitted that the machines had not been used since the trial had finished, and that they felt ‘a bit disappointed in myself . . . that I didn’t continue with it’ (care home manager, 43913):
Do you know that way, we did the trial, it’s done, it’s ticked? We have got two machines which are probably [still] in the box. We shouldn’t have seen it as a beginning and an end, we should have seen it as a continuing thing, and we didn’t. I think we need to actually think about this, and actually go and try . . . even if it’s two residents that we identify.
Care home manager, 43913
However, another care home had continued for a number of weeks after the trial finished:
We had amazing results from the trial. We are using the machines on a couple of residents that have got capacity, but we haven’t had much of a success with them yet though, but we will keep going.
Care home manager, 45909
Future strategies for training and continuing with transcutaneous posterior tibial nerve stimulation
Some care homes reported that they wanted to wait for the trial results before planning future strategies for continuing with TPTNS. This care home manager commented that they hoped to identify residents with UI problems earlier:
I think it would be easier to [use TPTNS] now because you would be identifying [residents] at an early stage [in their UI status], instead of having to identify all of them, all at once. We haven’t used the machines yet, not off our own back, kind of thing, I don’t know why; really probably because of waiting for the outcome of the trial.
Care home manager, 34908
This care home manager felt that it was important to have external support if they were going to continue using TPTNS:
I think that maybe with that there would have to be things like a nurse specialist or something like that within the community to be that ‘go to’ [person] to . . . monitor it rather than just . . . sticking electrodes on people willy nilly, somebody coming in just to say ‘Yeah.’ I think otherwise it is just going to be misused and it will . . . be phased out rather than being implemented.
Care home manager, 37915
Figure 8 shows the themes identified in the qualitative analysis of care home staff interviews mapped to domains in the TDF. Colour coding (orange, light orange, light blue) indicates how the themes also relate to the key concepts of the COM-B. Where more than one domain of the TDF maps to a single qualitative theme, the colour used for that theme indicates which COM-B concept the theme aligns with most strongly.
FIGURE 8.
Themes mapped to the TDF domains and COM-B system. Light orange, capability; orange, opportunity; light blue, motivation.
Discussion
The ELECTRIC qualitative study aimed to explore (1) the experiences of participating in the ELECTRIC trial from the perspectives of residents, families, care home staff and care home managers, and (2) the influencing factors on implementation and sustainability, using the guiding framework of the TDF and COM-B system49 to aid understanding.
The residents’ experiences were wholly positive in terms of the ELECTRIC intervention and trial processes, but their responses to the questions about their experiences of UI and its management did highlight the general need for greater attention to be paid to better understanding of UI and continence management in the care home context. Residents described UI experiences and care home practices that had an impact on their quality of life, including the indiscriminate use of absorbent pads. They described routine UI care that included wearing absorbent pads, which some perceived to be for convenience to save care home staff time rather than reflecting their needs. Care home staff may lack understanding of the impact UI has on residents’ quality of life and assume that wearing pads is acceptable, even when they may not be required. This practice can result in uncertainty, discomfort and loss of dignity, and does not reflect person-centred continence care.
The qualitative study showed that care home staff developed their capabilities through gaining knowledge about UI from the ELECTRIC trial training and developing skills to implement TPTNS, with support from the ISFs. The ISFs were crucial to the staff attaining confidence to apply their new knowledge and skills in practice. It was clear that UI and associated care was largely undertaken by and the responsibility of CAs and SCAs in all types of care home. Unexpectedly, the RNs were not universally involved in the trial, despite initial expectations for them to be leaders. The findings indicated that the RN role in continence care is unclear. Many care homes did not view continence care as a priority for RNs, and individual care home approaches to allocating responsibility for implementing the ELECTRIC trial seemed to be influenced more by care home culture than any deliberations on the most appropriate grade of staff to lead the project. Where RNs took the lead, successful organisation and implementation of research activities was evident, and teamwork was strong.
In those residential care homes (without RNs) that appointed ‘champions,’ similar success was demonstrated. Those ‘strong leadership’ care homes perceived that the trial had raised awareness of UI; many reported that they had changed their practice through providing residents with more person-centred care, far removed from routinised toileting practices. The remaining care homes and all of the local authority care homes that did not prioritise leadership in terms of a ‘champion’ (or a care home manager who prioritised this role) often struggled to organise effective implementation of trial activities, and many care staff displayed stress and feelings of resentment owing to the additional workload. Unsurprisingly, when carers or senior care staff were left unsupported and unsupervised, commitment to the trial was affected. These staff are likely to have limited knowledge, and no or inadequate prior education/training in providing high-quality person-centred continence care, with most of their knowledge being derived from the specific product training provided by commercial companies. Yet the findings provide evidence of both their enthusiasm for further education in the area of UI and their ability to respond to the ELECTRIC trial training in terms of successfully implementing an intervention that they recognise might be helpful to their residents.
The qualitative study highlighted the need for strong leadership across all aspects of the research, and the consequences of poor or absent leadership were seen in the acknowledged inadequate data collection by some care staff. Thus, the motivation to change behaviour and practice was apparent, but was more successful when accompanied by good leadership and support. In particular, when the potential consequences of the ELECTRIC trial were considered, these were overwhelmingly positive in terms of TPTNS as a new ‘treatment’. This could potentially change staff attitudes and responses to UI from being focused on the task to a more person-centred approach to care that is focused on the resident’s quality of life.
However, there were a number of barriers to implementing the ELECTRIC trial. Barriers were mainly around the outcomes measurement activities of collecting pads for the PWTs and completing bladder diaries accurately, both of which proved challenging for a number of staff and care homes. Poor understanding of the purpose and processes of these research activities, and a lack of leadership were reasons offered. However, the care home context in terms of routine practices (task-focused UI care), communication difficulties, and time and staff resource constraints all contributed to the challenges. Using the TDF and COM-B to support the analysis for this qualitative study has shown that the TPTNS/sham interventions were universally well received and implemented in the care homes; however, the specific trial-related research activities were less so.
Strengths and limitations of the qualitative study
A key strength of the qualitative study linked to trials in general is that it allowed the voices of those the intervention aimed to help to be heard and represented. Residents and family members not only articulated the often profound impact of UI on their lives, but were also encouraged that research was focusing on the issue of continence. They spoke not only in terms of appreciating the potential personal gain from the trial, but also in contributing to the wider research community and helping others in the future. The residents evidently felt valued through participating in the trial and care home staff often commented that residents had felt ‘special’ to be a part of it.
The adopted ‘whole-team’ approach comprehensively captured the views and opinions of care home staff across all grades/levels. This approach also enabled comparisons between types/size of care homes and the impact of leadership styles that were often indicative of the success of the research activities. Although this was not achieved in all care homes, the ‘whole-teams’ approach provided a full, in-depth insight into care home culture that helped to inform the qualitative study as a whole.
The use of specific time points in the research trajectory to target care homes to participate in the qualitative study was based on qualitative researcher availability and allowed for a degree of randomness in recruitment, preventing any systematic or deliberate selection of care homes based on particular criteria. The qualitative researcher worked independently of the trial management team, communicating directly with care homes to accomplish a large number of interviews across England and Scotland, with a range of care home types, to achieve maximum variability in the sampling. The qualitative researcher was a RN and had recent experience of working in the care home setting; therefore, they were well placed to respond effectively to the care home environment, the staff and the residents.
The qualitative study sought to capture the views of people with dementia, an innovative and wholly inclusive approach not often attempted in care home research. The positive reactions to TPTNS of people with dementia at the time of delivery supported other residents’ (who had capacity) responses that TPTNS did not cause discomfort or distress and, with occasional support from care home staff, it could be tolerated for the duration of delivery without any problem. Without this inclusive approach the responses of people with dementia would not have been captured. Family members also confirmed that they did not perceive any detriment from TPTNS for their relatives with dementia and were enthusiastic to learn the outcome of the research.
Some limitations were observed. Inconsistency and changeability in residents’ cognitive capacity frequently resulted in a lack of recall of the trial or the intervention effects, and, therefore, had an impact on the overall quality of data obtained. This was often because of the timing of interviews in terms of the length of time that had passed, however short, between TPTNS and interviews, but was also affected by resident illness or fatigue. It was noted that interviews conducted at different times during the day yielded different data quality. Although determined on an individual basis, the qualitative research fellow was frequently advised to interview residents in the morning to avoid the risk of fatigue, because as the day progressed their ability to concentrate may be compromised. Other reasons to avoid interviews later in the day were pragmatic, usually because the occurrence of visitors and social activities increased throughout the afternoon.
Staff availability in terms of their time and willingness to engage in focus groups/interviews was a significant negative factor in data collection – staff often cancelled their appointment last minute or failed to attend without providing a reason. This was especially so with some care home managers, who agreed to be interviewed and then offered last-minute reasons to withdraw and/or failed to reschedule. The qualitative researcher was unable to secure an interview with any local authority care home managers, none of whom attended interviews, despite agreeing to numerous appointments. After repeated attempts to rearrange with the same outcome, further attempts to interview local authority care home managers were ceased as it was considered futile to persist. This resulted in a lack of ‘whole-team’ data for the local authority care homes, although the other care home staff and residents were enthusiastic and forthcoming with their views. The absence of local authority care home manager interviews was unfortunate as care home staff who reported a lack of or breakdown in communication with senior staff, and the negative impact this had on the way the trial was organised, were often from local authority care homes. It would therefore have been advantageous to elicit the perceptions of local authority care home managers on this aspect of the trial.
It is possible that only those staff who were positive about the trial may have been willing to attend for interview, although care home staff with sceptical views did not seem to be deterred and were willing to share their views, as evidenced in the findings.
Summary
The qualitative study showed that residents and their families were highly receptive to participating in the trial. With the additional inclusion of people with dementia in focus groups, as well as those residents with no cognitive impairment, it was surmised that residents found TPTNS acceptable and tolerable. Some residents perceived that they had experienced positive changes to their UI status as a result of TPTNS and expressed that they would be happy to continue to use it in the future, if required.
All grades of care home staff demonstrated enthusiasm towards increasing their learning about UI and continence care in the ELECTRIC trial training, although implementation of trial activities varied considerably throughout the care homes. Strong leadership and teamwork were key to the effective organisation of the trial; indeed, the accurate recording and collection of outcomes measures were dependent on it. In care homes where coherent leadership and organisation were lacking, and where junior staff often carried out trial activities unsupervised, undefined roles and lack of communication were challenging and often resulted in inconsistently completed outcomes measures.
Similarly, the care homes that benefited most from the trial were those with strong leadership, often reporting that changes in practice had occurred through person-centred care that prioritised the individual resident rather than routine toileting activity. When considering future continence practice and possible use of TPTNS for their residents, care homes with strong leadership had considered strategies for training staff and initiating TPTNS for residents as early as possible. However, there would not be any reduction in the ordering of UI absorbent products and aids as it was felt that it was important to maintain the current supply and avoid potential shortfalls for residents in the future.
Chapter 6 Discussion and conclusions
Statement of principal findings/summary of findings
The results of the ELECTRIC trial are very clear. There was no evidence that TPTNS is effective in reducing UI in older care home residents at our primary outcome time point of 6 weeks post randomisation, or over the 18-week duration of the study. No evidence of an effect in favour of TPTNS was found across any of the continence-related outcomes, including absorbent pad use; PBC by residents, family members and staff; skills for using the toilet; or PVRU.
Adherence to the intervention protocols, a potential mediator of better clinical outcomes, was good overall, with 78% of residents being fully adherent to the TPTNS programme and 71% of residents being fully adherent to the sham stimulation. Given the challenges of delivering a minimum of eight stimulation sessions over a 6-week period in the context of a busy care home, this adherence rate was considered successful and further supports a conclusion of no benefit from the TPTNS intervention.
There were no serious AEs related to the TPTNS and only two non-serious related AEs, both in the sham group: one participant complained of a ‘heavy leg’ and ‘feeling dizzy’, and the other became aggressive when the attempt to set up the sham stimulation was made.
Evaluation of resource use did not demonstrate differences between the randomised groups. Levels of ongoing dependency on mobility and transfer aids, and on staff assistance to visit the toilet were high in both groups. The cost of delivering the programme of TPTNS in a care home was estimated as £201 per participant, which comprised training and support costs of £121 for each staff member and TPTNS delivery costs of £81 for each participant. No statistically significant difference in improvement in health-related quality of life using DEMQOL-U or DEMQOL-PROXY-U was found.
The qualitative study, which took a ‘whole-team’ perspective to explore experiences of TPTNS, showed that care home residents and their family members thought that TPTNS was acceptable and it was well tolerated, even in those with severe dementia. Some residents and staff perceived improvements in UI as a result of TPTNS and said that they would be happy to continue the intervention in the future if required.
All grades of staff demonstrated enthusiasm for learning about continence in the ELECTRIC trial training; however, implementation of trial activities varied considerably across the care home sites. Strong leadership and teamwork were key to the effective organisation and implementation of the trial; indeed, accurate recording and collection of outcomes measures were dependent on it. In care homes where coherent leadership and organisation were lacking, and where junior staff often carried out trial activities unsupervised, undefined roles and a lack of communication presented challenges that frequently resulted in inconsistently completed outcome measures.
The care homes that benefited most from the trial were those with strong leadership, often reporting that changes in practice had occurred through improved person-centred care that prioritised the individual resident rather than routine toileting activity. In considering their future continence care practices and possible use of TPTNS for residents, care homes with strong leadership had considered strategies for training staff and initiating TPTNS for residents as early as possible. However, any reduction in ordering of absorbent products to contain UI would be avoided to preserve the supply chain and prevent shortfalls for residents in the future.
The ELECTRIC trial results in the context of other studies
The ELECTRIC trial was designed to test the effectiveness of TPTNS in the context of nursing and residential care homes in England and Scotland. This was considered important for a number of reasons: the prevalence of UI is high in the care home setting,16,54 and care homes provide care to those with the most severe level of both physical and mental frailty and have continuously high dependency as a result, particularly as the prevalence of those with dementia increases. They also have the lowest proportion of care delivery by registered nursing staff and limited or often no access to therapy, and no on-site medical care. These features of care homes mean that they cannot be considered in the same light as other health-care settings, thus interventions that show promise of effectiveness in acute or community care settings require full testing for effectiveness in care home contexts.
The evidence for effectiveness of TPTNS as an intervention for urgency/mixed UI in adults is accruing but remains equivocal. The majority of studies involve adult women with an OAB/mixed UI25–27,55,56 and are relatively small (fewer than 100 participants). To our knowledge, there have been no published systematic reviews of the TPTNS literature since our 2018 publication, which included 10 RCTS and three prospective cohort studies. 24 However, a further six RCTS have been reported57–62 involving 340 adults, 195 of whom received TPTNS (176 were women) and 72 of whom were older women, all independently living in their own homes. Only one trial was a sham controlled study;62 two compared TPTNS and percutaneous tibial nerve stimulation (PTNS),59,60 one compared TPTNS with PTNS plus an additional drug,61 one compared lifestyle interventions and pelvic floor muscle exercises with these plus TPTNS,58 and one compared two different intensities of TPTNS stimulation with a no treatment control. 57 All of the trials were small and of variable quality, with a number of different primary outcomes and follow-up time points reported. Risk of bias was low in one study,62 it was unclear in another61 and there were concerns about the risk of performance and detection bias due to a lack of blinding of participants, care providers and outcomes assessors in the remaining four trials. 57–60
As was the case in the previous systematic review, four of the six trials reported positive results for TPTNS in terms of improvements in urinary symptoms57,59–61 and five trials reported positive results for quality of life. 57–61 One RCT62 reported no effect of TPTNS compared with sham stimulation on PBC or secondary outcomes of 24-hour pad weight, parameters of voiding diaries or patient-reported symptoms scores, including for UI, in a trial of 50 adults with idiopathic OAB and neurogenic bladder. The study concluded that TPTNS is not an effective treatment for OAB or neurogenic bladder dysfunction and, to our knowledge, it is the only published trial to date to indicate no effect of TPTNS. However, the participants were all patients with bladder dysfunction that was resistant to a number of previous interventions, including pelvic floor muscle exercise programmes and antimuscarinic medications (mean of 3 in each of the TPTNS and sham groups for the OAB group), making them a particularly challenging group to treat and dissimilar to the other trial participants in this important respect.
Two were non-inferiority trials, one60 comparing PTNS and TPTNS for the treatment of OAB in adults and the second59 comparing long-term maintenance treatment using self-managed TPTNS at home with staff-applied PTNS in a hospital setting. Both trials reported equally positive outcomes for TPTNS as for PTNS. Only one trial57 specifically recruited older women, aged ≥ 60 years, to explore the effectiveness of TPTNS delivery at two different stimulation intensity parameters compared with a no intervention control. The women were community living, with no history of previous treatment for OAB, in contrast to some of the other trials in which TPTNS was implemented as a third-line intervention. 59–62 Compared with the control group, urinary symptoms and bladder diary results in both TPTNS groups were significantly improved by more than half at both stimulation intensities. To our knowledge, there have been no other studies undertaken involving any type of electrical stimulation treatment for UI in care homes during this period.
The ELECTRIC trial results conflict with all currently published trials of TPTNS for treating UI, except the trial by Welks and McKibbon. 62
Interpretation of results
To our knowledge, the ELECTRIC trial is the largest sufficiently powered pragmatic trial currently available, comparing TPTNS stimulation with a sham stimulation, the highest level of comparator. Adherence to the intervention programme was high (78%) and we are confident that those randomised to the TPTNS group received the correct intervention. Retention of participants was high across the 18-week intervention period and missing data were relatively low and randomly distributed across the arms and study sites.
We are confident that our results are robust. However, given the discrepancy with all other reported trials bar one,62 we must consider possible explanations.
It is arguable that all other trials have inherent methodological and design flaws, such that the results are rendered universally questionable. The risk of bias in all 16 published trials identified (10 in our previous systematic review,24 a further six trials identified since the last search in 201757–62) is unclear or high in all but one. 62
Alternatively, as the ELECTRIC trial is the only powered trial undertaken across a total of 37 nursing and residential care homes, we may infer that the context of care homes may underpin the explanation and, therefore, warrants further exploration to better understand the ELECTRIC trial results.
We consider care home context in terms of two types of explanatory factors.
Resident-related factors
Not unexpectedly for a care home population, our participants were highly dependent (mean Barthel Index score 7.6) and more than half were severely or very severely frail, with moderate to severe dementia as indicated by the mean MMSE score of 13 (12–20 indicates moderate dementia; < 12 indicates severe dementia). More than 80% of participants had restricted access to the toilet because of mobility reasons and 42% were unable to communicate their need to use the toilet. Thus, these participants were almost exclusively dependent on staff for their toileting and continence care and were unlikely to initiate toileting, as indicated in the qualitative data in which regular toileting routines, rather than individualised routines, were highlighted as the norm for this care context.
The mean number of toilet visits per resident for the total sample across all time points was 4.15 (SD 2.68) in 24 hours (CCA data), and Figures 6 and 7 show that, for both groups, there was no change in routine practice in the care homes regarding toileting throughout the study. Thus, even if residents had experienced any reduced sensation of urinary urgency and increased warning time for the need to void (which should occur if TPTNS is effective), no additional toilet visits were provided to enable residents to void in a toilet, as opposed to using the absorbent pads to void. Without associated changes to toileting practices, any effects produced by TPTNS would be unlikely to be recognised. Furthermore, fewer than five toilet visits per day is insufficient for frail older adults to maintain independent continence, as a normal adult voiding frequency ranges between six and eight voids per 24 hours. 63 Participants would thus be forced to void in their pads on at least some occasions.
This situation contrasts markedly with participants in all other TPTNS studies, who are fully independent regarding their toilet use and continence management and can therefore adjust to any changes in their bladder sensation and function accordingly, without having to rely on anyone else for support.
In addition, participants in other TPTNS studies have full mental capacity and cognitive abilities to understand, respond to and complete standardised PROMs that are routinely used in continence trials. The ELECTRIC trial participants were largely unable to do this, and a number of outcome measures were completed by proxies who were family members or, in the majority of cases, members of staff who knew the resident well. For example, only 150 (37%) residents completed the DEMQOL and 176 (43%) residents completed the MTSQ. Reliance on proxies for judgements about personal functions, such as PBC or quality of life, is less than ideal and there is no way to determine degree of accuracy; however, there was no alternative for this frail, dependent care home population. This feature of research in care homes was understood from the outset and influenced the design of the study, particularly the choice of primary outcome, which, for the reasons outlined previously, could not be a PROM. It needed to be objectively measurable, hence the use of absorbent pad weights to determine 24-hour urinary leakage. However, there were challenges associated with this outcome, which are discussed in Methodological considerations.
Poor information about the type of UI is a further resident-related factor that may help to explain the ELECTRIC trial results. TPTNS specifically targets symptoms of urinary urgency, frequency and nocturia, and was used in this trial because these symptoms occur in mixed UI, the most common type of UI in older adults. 3,16 Type of incontinence was recorded in the residents’ notes for only 135 residents, 33% of the sample, and there was no way to determine whether or not these residents did indeed experience overactive bladder symptoms of urgency, frequency or nocturia. Furthermore, the levels of dependency suggest that regardless of any other urinary symptoms, functional UI was the most prevalent clinical diagnosis in this sample. Earlier research supports this conclusion,64,65 in which 60–90% of care home residents with incontinence had significant mobility issues and severe cognitive impairment, similar to our results. The difficulties associated with enabling people with functional UI to use a toilet reinforces the previous interpretation that a lack of change to toileting practice will mask any effects of TPTNS that may have occurred.
Although we were keen to be inclusive and show that a dementia diagnosis should not exclude care home residents from participating in continence research, we should consider that many of these participants may have been too cognitively and physically frail to benefit from any effects of TPTNS on their UI because of their total dependence on staff for all continence activities. Such dependence makes sustainable changes to toileting practices unrealistic for large numbers of highly dependent residents in the majority of care homes. Nevertheless, TPTNS was shown to be well tolerated and safe, even for this frail population. Given the evidence from a number of other published trials and the divergence between these and the ELECTRIC trial results, we conclude that TPTNS is not effective in the care home context partly because of residents’ inability to independently use/express their need to use a toilet and their reliance on staff for all continence care. Such high levels of care input cannot be consistently provided or sustained in practice. In addition, the lack of effectiveness of TPTNS was shown across the entire resident population and reflected in the subgroup analyses, in which no evidence for any prespecified or post hoc grouping variable was found.
Care home-/organisation-related factors
In these care homes, continence care was seen as part of the everyday working routines and not something that necessarily needed to change. Qualitative findings suggested that incontinence was not seen as a health condition or a problem to be dealt with, but more an inherent characteristic of the resident. They were considered to be ‘incontinent of urine’ and this was their established bladder status, which was seen by some as inevitable with ageing, particularly in those residents with dementia. As such, it was accepted, indeed expected in some care homes, that residents wore absorbent pads to contain their incontinence, despite some residents, as described in Chapter 5, not understanding the reasons for this or agreeing with the practice. Containing the urine leakage, together with regular toilet visits, formed the bulk of the continence care practices in the care homes. This is in line with evidence from other care home studies. 66–68
Because managing UI was seen as ‘care’ it follows that it was considered the role of the CAs to manage it and this was not the realm of the RNs. This offers one explanation as to why the RNs did not engage with the ELECTRIC trial as originally anticipated. It also potentially explains why the continence care and toileting routines did not change during the trial. As CAs are not equipped or expected to make changes to practice, and as identified in the qualitative study, (1) the care was often entrenched and so much a fundamental part of daily work for the CAs as to be habitual and, therefore, subconsciously executed; (2) there was a lack of time and resources to increase the number of toilet visits should the need have been recognised by the residents or the care staff; and (3) there was no expectation of changes to continence status for the residents.
However, the qualitative study also found a high level of appreciation among the CAs and SCAs for the education about incontinence that was provided as part of the ELECTRIC trial training and enthusiasm to apply their new knowledge to improve their residents’ individual continence condition. For this to happen universally, changes to practice would be required that would need a person with the authority to effect these changes to engage with the project and lead it. The qualitative study raised the key role of leadership to the ELECTRIC trial, highlighting the impact of strong leadership on successful delivery of not only the TPTNS and sham interventions, but also the completion of the trial outcomes. For those care homes where the manager and/or senior nurse championed the ELECTRIC trial, the research process was generally smooth and all trial activities were absorbed into the care home working routines with little apparent difficulty. However, in situations where the care staff were ‘left to get on with it’, there was evidence of more negative experiences for staff and no sense of any comprehensive approach to implementation – ‘doing ELECTRIC’ meant demonstrably completing the stimulations, with little attention paid to toileting practices or outcomes collection activities. This was particularly the case for the local authority care homes where managers did not actively engage with the research processes and responsibility for all ELECTRIC trial activities was delegated to a senior carer, some of whom struggled to cope.
Such disparity in leadership may reveal fundamental beliefs about the status of continence care in this context. In some care homes, the ELECTRIC trial was an opportunity for the care staff to have something of their own, and this was reflected in the carers’ expressed sense of feeling valued, but whether there was real belief or confidence in any potential changes was questionable, and may not have translated into necessary changes to practice. Care home managers were not prepared to reduce their pad orders even if there was evidence of effect from the ELECTRIC trial. This was a point of interest in the qualitative findings and may reflect underlying doubt about any likely changes to residents’ continence status, as well as an understandable reluctance to decrease orders, based on pragmatic recognition that once given up, absorbent pad supplies may be difficult to reinstate. Regardless of any reductions in absorbent pad usage that may or may not have been observed during the ELECTRIC trial, care home managers stated that they would not be making any changes to their continence product orders. This is reasonable given the different systems and challenges associated with accessing continence products that many homes experience. The inflexibility and poor responsiveness of supply systems, together with limited individual resident product prescriptions, mean that care homes are unlikely to make any changes that may reduce the availability of products for their residents in the longer term.
Methodological considerations
Strengths and limitations
The ELECTRIC trial was a multicentre, pragmatic, participant- and outcome assessor-blind randomised placebo-controlled trial with a high follow-up rate for the primary outcome. The design included an extensive qualitative study involving a ‘whole-team’ approach that enabled a comprehensive understanding of the trial results from different perspectives.
The conduct and reporting of the trial followed the CONSORT recommendations (www.consort-statement.org/). We used a central web-based computer randomisation application developed by CHaRT, accessed remotely by trial staff, to assign group allocation and delivery to care home sites electronically, thus minimising the risk of selection bias. Analyses were conducted on an ITT basis and in accordance with a prespecified SAP (see Report Supplementary Material 8) agreed by the TSC.
We recruited participants from a range of different types and sizes of nursing and residential care homes in England and Scotland and achieved real diversity, adding to the generalisability of the findings. Ultimate variability in care home sites was greater than originally planned because of the early withdrawal of our main partner, BUPA UK, before recruitment began as a result of their decision to sell their care home business. Thus, alternative care home sites had to be found to replace the 14 large (> 100 beds) care homes originally proposed. The multiple sites recruited were so diverse that we are confident of representativeness.
At baseline, the two groups were highly comparable indicating the success of the allocation process. Follow up was high, with primary outcome data for 85% of participants at 6 weeks, 76% of participants at 12 weeks and 71% of participants at 18 weeks, and missing data were well balanced, suggestive of a low risk of attrition bias. Sensitivity analyses exploring potential effects of missingness on the primary outcome did not affect our conclusions.
To minimise the risk of performance bias, elements of the trial delivery were standardised as far as possible. This included the ELECTRIC trial training, which was delivered to all staff by two out of three trainers (the chief investigator and two trial managers) and used a standard training pack, which was delivered face to face and then provided in paper format to each member of staff who attended. Every care home was also given copies of DVDs demonstrating the techniques for TPTNS and sham stimulation.
The intervention was standardised as far as is possible. Identical equipment was used in a similar pattern over the 6-week intervention period for both groups. The only differences between groups were the electrode position on the ankle (medial malleolus in the TPTNS group, lateral malleolus in the sham group) and intensity of stimulation (highest comfortable intensity > 10 mA in the TPTNS group; 4 mA in the sham group).
The participants and outcomes assessors (ELECTRIC trial RRAs) were blind to the allocated group and thus the risk of detection bias was low. However, it was not possible to blind those delivering the intervention as they needed to deliver a different stimulation protocol depending on the participants’ allocated group. To ensure adherence to the relevant protocol, a minimum of two adherence checks were undertaken by the ISFs, who were part of the research trial staff, not the care home team, and whose roles were solely to support those delivering the intervention to do so correctly and to complete trial outcome measures correctly. Adherence to the intervention was 78% in the TPTNS group and 71% in the sham group, which was considered good when accounting for the number of sessions required and the complexity of delivery in the care home context.
Overall, the attrition rates in the ELECTRIC trial, including the number of deaths, were lower than anticipated and formed the basis of a recruitment target revision that took place after 12 full months of recruitment had been completed. There were fewer deaths in the trial cohort than expected. The original sample size calculation included a potential attrition of 30%, based on the experience of the EPIC trial69 [National Institute for Health Research (NIHR) Health Technology Assessment (HTA) 11/15/13] led by Professor Claire Surr, an ELECTRIC trial co-investigator. The revised sample size required for the ELECTRIC trial to be sufficiently powered reduced the sample size from 500 participants to having primary outcome data for a minimum of 278 participants. However, to allow the sample size to be adequate to conduct meaningful subgroup analyses, recruitment was continued according to the original planned timelines, which resulted in our final sample of 406 participants.
Potential limitations are recognised in the ELECTRIC trial. Despite the relatively low rates of missing data at each outcomes time point, detailed reasons were not recorded, other than the number of withdrawals, which were 27 (6.7%), 44 (10.8%) and 50 (12.3%) participants at 6, 12 and 18 weeks, respectively. Primary outcome data were missing for 23 (5.7%), 35 (8.6%) and 38 (9.4%) participants at 6, 12 and 18 weeks, respectively, and, although balanced across the two groups, a detailed breakdown of specific reasons would have been informative. Nevertheless, feedback from the research assistants, together with qualitative findings that highlighted the challenges associated with pad collections, suggests that the majority of missing data can be expected to be the result of failure to complete the pad collections by care homes. This was an issue for a number of care homes, as discussed previously, particularly where leadership from senior staff was limited or absent and communication between care team members was inadequate for instructions to cross shifts for 24 hours.
The 24-hour PWT is considered the most accurate and reliable way to objectively assess UI. 70 It requires collection of the total pad usage over the allotted time period, which can be easily attainable for a competent individual; however, for the ELECTRIC trial participants it meant depending on whole teams of staff spanning two or three care shifts to retain pads, often for a number of individuals, and put them in the correct collection bag. Co-operation and good communication between staff were essential to complete these PWTs; however, evidence from the qualitative interviews suggested that this could be challenging for some care homes. Discrepancies between bladder diary information and numbers of pads in some collection bags corroborated the difficulties encountered, which included forgetting to retain all pads for a participant, inadvertently throwing some pads in the clinical waste and failing to locate pads that were discarded by the resident. We also do not know what happened to soiled pads as this information was rarely provided on the bladder diaries. Staff were asked to discard solid material but include the pads in the collection; however, we do not know if this happened, and it may explain some of the small pad numbers in the collections. The care home environment itself could also present challenges for accurate pad collections. The collection bags were usually stored in the participant’s bathroom; however, the participant was not always taken back to their own room to use the toilet. Therefore, some pads were removed elsewhere in the care home, which could cause difficulties for staff in ensuring that the used pad was added to the correct collection bag in the correct room.
A diminishing interest in the trial may have occurred over the 18-week course, as indicated by the balanced reduction in pads collected at the 12- and 18-week time points, although it was not accompanied by a reduced 24-hour leakage or increased number of toilet visits. This suggests that participants had fewer pad changes over time.
The qualitative findings suggest that some staff did not understand the outcomes measurement aspects of the ELECTRIC trial. Although large numbers of care home staff received training, it was not possible to train all staff who were dealing with ELECTRIC trial participants; therefore, understanding and interest in the study processes was likely to be variable across staff. Equally, although the ELECTRIC trial training focused on the skills to both apply TPTNS/sham and complete the outcomes measures, the care home staff focused more on the intervention delivery than the outcomes assessment. It seemed from the qualitative interviews that many did not understand the purpose of the pad weights or the methods for accurate pad collection. This happened despite clear verbal and written instructions on each occasion and individual support from the research assistants, who confirmed what needed to be done and by whom when they delivered the equipment to complete the pad collection the day before it was due to begin.
We acknowledge that completion rates by care home residents were low for the self-reported secondary outcomes of PBC, MTSQ and DEMQOL. Although a limitation in terms of interpreting the responses, the low rates were not unexpected and were offset by proxy completion by participants’ family members or care home staff. In addition, information on use of these measures in people with moderate to severe cognitive impairment has been provided, which will be of potential benefit for future research in care home contexts.
A limitation of the qualitative study was the smaller than expected number of interviews undertaken with residents who had received TPTNS treatment. We aimed to recruit 75% of residents from the TPTNS group; however, only 57% were recruited from this group. This was because there were fewer residents with cognitive capacity in the treatment group. Therefore, the views of residents on their subjective experiences with TPTNS were more limited than planned.
Resident and public involvement
A SPIG and CHRG were set up during the initial planning stages of this research. The SPIG comprised an older adult with recent experience of the TPTNS intervention, a family member caring for a person with dementia, a care home manager, a community continence team lead, a person with dementia, a Scottish Care Inspectorate health improvement advisor, and representatives from Age Scotland and Alzheimer Scotland. The CHRG were staff and residents of a local care home. Both groups contributed to the development of the outline proposal and full NIHR proposal, were consulted particularly on the outcomes measures, and advised on the acceptability and practicability of the 24-hour PWT as the choice of primary outcome measure. Unfortunately, owing to the closure of BUPA UK care homes in Scotland, the CHRG did not continue to advise the ELECTRIC trial in the funded research period.
The SPIG met three times during the trial and provided advice to the ELECTRIC trial team on a number of trial elements. They reviewed trial documentation, including participant information leaflets (standard and aphasia-friendly) and outcome measures; commented on the acceptability of the intervention and advised on appropriate contingency measures for the stop/go criteria in the internal pilot; made suggestions to enhance effective data collection processes, including PWTs; and advised on the presentation of lay summaries. One member resigned after 2 years because their personal circumstances had changed, and the COVID-19 pandemic prevented the final SPIG meeting from taking place. The ELECTRIC trial team feel that the SPIG played an invaluable role in the conduct of the trial and that their contributions enhanced both the recruitment and retention of participants.
Conclusions
The ELECTRIC trial showed that in the care home context, which has a large proportion of older residents with poor cognitive capacity and limited independent mobility, TPTNS was not effective in reducing UI. The evidence suggests that there is no beneficial effect of TPTNS on any continence-related outcomes or resident quality of life, despite good adherence to the intervention protocol by care home staff. TPTNS was acceptable as an intervention for UI in all care home residents, including those with dementia. The cost of delivering a programme of TPTNS was estimated to be £201 per participant, comprising training and support costs of £121 per staff member and delivery of TPTNS costs of £80 per participant. Care home staff were enthusiastic to learn about continence care, particularly the care staff.
Implications for health care in care homes
The evidence indicates that care home staff can be confident that use of TPTNS in care homes, which have a large proportion of elderly, frail residents lacking cognitive capacity, will confer no reduction in UI for their residents.
The evidence from the CCA of the ELECTRIC trial does not suggest that there is an economic case for TPTNS. However, the positive reception of UI learning for care home staff suggests that there may a case for considering education as part of routine CPD packages.
Recommendations for research
Research to investigate TPTNS in care home residents with OAB/urgent UI who also have the capability to independently use the toilet could be useful to determine whether or not targeted TPTNS is effective in this population.
Investigation of other approaches to treating UI in care home residents should be considered, based on clinical assessment of type of UI, to target interventions at those most likely to benefit from them.
Research to explore the effects of in-depth training for care home staff regarding urinary continence on changes to practice in how it is managed and continence experiences and outcomes for residents could add greater understanding on improving continence care in the context of care homes.
The ELECTRIC trial showed that care homes engage and can participate well in large research trials. Care homes could be viewed and developed further as potential settings for health-care research, especially as the care home context differs from NHS health-care settings and confers unique features that enable a more comprehensive consideration of research conduct and findings of relevance to frail older people.
Acknowledgements
The authors are grateful to the ELECTRIC trial office team members, who were instrumental to the success of the trial, including Mrs Jacqueline Gray (data co-ordinator); Dr Bridget Davis (qualitative research assistant); Mrs Ann Dolan, Dr Caroline Miller and Ms Deana Whalley (RNs); and Mrs Pia Ashton and Mrs Brenda Bain (ISFs).
We would like to extend our gratitude and thanks to all of the participants and their families, proxies and legal guardians, where appropriate, who took part in this trial.
We are grateful to all of the staff, managers and owners of the care homes who participated in the trial, particularly those who took on the role of PI (listed below).
Thanks to Janice Allan (Pacific Care Ltd, Glasgow, UK), Jackie Weston (Care Concern Group) and Brian Polding-Clyde (local integration lead) who assisted with the recruitment of care homes in Scotland. Thanks also to Julie Arrowsmith (Century Healthcare, Lytham Saint Annes, UK) and NHS Fylde and Wyre Clinical Commissioning Group (Blackpool, UK) for assistance in recruiting homes in England.
Many thanks to the team at CHaRT in Aberdeen, including Mark Forrest, Brian Taylor and Alina Uyazina, for developing and maintaining the ELECTRIC trial website and database.
The authors would like to thank the members of the ELECTRIC trial SPIG for their advice and support with the trial: Jenny Ackland (Age Scotland), Rona Agnew (NHS Greater Glasgow and Clyde SPHERE Bladder and Bowel Service, Glasgow, UK), Janice Allan, Jackie Dennis (Care Inspectorate Scotland, Dundee, UK), Joyce Goel (lay member), Archie Noone (lay member), Andrew Lowndes (Playlist for Life, Glasgow, UK and family carer) and Susan Rendell (Alzheimer Scotland).
We would also like to thank the members of the TSC for their advice, guidance and support during the trial: Dr Lorna Aucott (CHaRT, Aberdeen), Professor Francine Cheater (lay member and family carer), Dr Seonaidh Cotton (CHaRT, Aberdeen), Professor Pip Logan (TSC chairperson, University of Nottingham), Dr Chris Sutton (University of Manchester), Mr Joby Taylor (Consultant Urological Surgeon, Forth Valley Royal Hospital) and Professor Lois Thomas (University of Central Lancashire). Thanks also to the DMEC comprising Dr Terence Quinn as chairperson (University of Glasgow), Professor Adam Gordon (University of Nottingham) and Professor Simon Skene (University of Surrey).
Our grateful thanks to the residents and staff of the following care homes for their contributions to the ELECTRIC trial.
Care homes: Scotland
| Care home name | Care home provider/owner | PI |
|---|---|---|
| Balquhidder House | Balquhidder Care Ltd | Victoria Reilly |
| Burnfoot Care Home | West Coast Care | Linda Cunningham/Gillian Sloane |
| Campbell Snowdon House | Abbeyfield Strathgryffe Society | Jim Melville |
| Craigielea Care Home | The Holmes Care (Group) Ltd | Sheila Inshaw |
| Davislea Care Home | Glasgow City Council | Karen Magennis |
| Doonbank House Care Home | West Coast Care | Gail McClure |
| Drumry House Care Home | Glasgow City Council | Alyson Parker |
| Glebe House Care Home | West Coast Care | Dana Gilchrist |
| Glenfairn House Nursing Home | Glenfairn Limited | Alice Thomas |
| Howard House Nursing and Residential Home | Lorimer Care Homes Limited | Mary Kerr |
| Larkfield View Care Centre | The Holmes Care (Group) Ltd | Elsie Maclennan |
| Lillyburn Care Home | Pacific Care Ltd | Claire Selbie |
| Mosswood Care Home | Pacific Care Ltd | Lesley Murtie |
| Orchard Grove Residential Care Home | Glasgow City Council | Fiona Wells/Therese Fallon |
| Ranfurly Care Home | Silverline Care | Fiona Devine |
| Redford Nursing Home | Redford Nursing Home | Jeanette Henderson |
| Riverside Care Home | Glasgow City Council | Evelyn Downie |
| Stanely Park Care Home | Pacific Care Ltd | Sue Kedley |
| Strathleven Care Home | Pelan Ltd | Ainsley Clark |
| Sun Court Nursing Home | Sun Court Healthcare Ltd | Maryjane Nicholson |
| Templeton House Care Home | Windyhall Care Home plc | Katrina Thompson |
| The Grange Care Home | Grange Care Home Ltd | Mhairi Findlay |
| Westbank Nursing | Westbank Care Home Ltd | Debbie McMaster |
Care homes: England
| Care home name | Care home provider/owner | PI |
|---|---|---|
| Bankhouse Care Home | HC-One | Jackie Salisbury |
| Brooklands House Rest Home | Mr and Mrs P Gilligan | Carol Gilligan |
| Cross and Passion Convent | Sisters of the Cross and Passion | Anna England |
| Kepplegate House Care Home | Kepplegate Ltd | Karen Shaw |
| Mariners Court Care Home | Century Healthcare Ltd | Carolanne Kyle |
| New Thursby Nursing Care Home | Century Healthcare Ltd | Deborah Thompson |
| Pilling Nursing Home | Zion Care Ltd | Kirsty Miller/Michelle Alty |
| Priory Court Nursing Care Home | Century Healthcare Ltd | Samantha Pollitt |
| St Alban’s Nursing Home | Zion Care Ltd | Laura Haymes |
| St George’s Nursing Care Home | Century Healthcare Ltd | Tammy Faulkner/Selena Mountford |
| Stella Matutina Care Home | Sisters of Charity of Jesus and Mary | Janet Chandler |
| The Conifers Nursing Home | Conifers Care Ltd | Karen Burns |
| The Hamptons Care Centre | New Care Lytham (OPCO) Ltd | Lorraine Disley/Suzanne Scholz |
| The Willows Care Home | Olivia Josephine Care Ltd | Tracey Otterman |
Contributions of authors
Joanne Booth (https://orcid.org/0000-0002-7870-6391) (Chief Investigator, Professor of Rehabilitation Nursing) conceived the study and was responsible for the study and report overall. She led the protocol development and contributed to recruitment, data collection, analysis and interpretation of results, and writing/editing of the report.
Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Senior Statistician, Centre for Health Care Randomised Trials) led the statistical protocol development and analysis, and writing/editing of the report.
Seonaidh Cotton (https://orcid.org/0000-0002-7883-0608) (Deputy Senior Trial Manager, Centre for Health Care Randomised Trials) contributed to the protocol development, management of the trial, interpretation of results and report writing.
Bridget Davis (https://orcid.org/0000-0001-6935-0891) (Qualitative Research Assistant) and Maggie Lawrence (https://orcid.org/0000-0002-1685-4639) (Professor of Nursing) were responsible for the qualitative research aspects of the trial, including protocol development, data collection, analysis and interpretation of results, and writing/editing of the report.
Linda Fenocchi (https://orcid.org/0000-0003-2536-8234) (Post-doctoral Researcher in Health Economics) and Helen Mason (https://orcid.org/0000-0002-9303-2794) (Professor of Health Economics) were responsible for the health economic aspects of protocol development, analysis and interpretation of results, and writing/editing of the report.
Claire Goodman (https://orcid.org/0000-0002-8938-4893) (Professor of Nursing) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Suzanne Hagen (https://orcid.org/0000-0002-9741-9160) (Professor and Deputy Director of the NMAHP Research Unit) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Danielle Harari (https://orcid.org/0000-0003-3596-6510) (Consultant Physician in Geriatric Medicine) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Andrew Lowndes (Deputy Chairperson, Playlist for Life) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing the report, and was a member of the SPIG.
Lisa Macaulay (https://orcid.org/0000-0003-2906-8757) and Catriona O’Dolan (https://orcid.org/0000-0002-9765-2464) (Co-Trial Managers) led the day-to-day management of the trial and contributed to recruitment, data collection, interpretation of results and writing/editing of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor of Medical Statistics, Director of the Centre for Health Care Randomised Trials) contributed to statistical aspects of protocol development, conduct of the trial, analysis and interpretation of results and writing/editing of the report.
Doreen McClurg (https://orcid.org/0000-0002-2872-1702) (Professor of Pelvic Floor Physiotherapy) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor of Medical Statistics and Trial Methodology, Director of Edinburgh Clinical Trials Unit) contributed to statistical aspects of protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Christine Norton (https://orcid.org/0000-0003-2259-0948) (Professor of Clinical Nursing Research) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Dawn Skelton (https://orcid.org/0000-0001-6223-9840) (Professor of Ageing and Health) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Claire Surr (https://orcid.org/0000-0002-4312-6661) (Professor of Dementia Studies, Director of the Centre for Dementia Research) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Shaun Treweek (https://orcid.org/0000-0002-7239-7241) (Professor of Health Services Research) contributed to protocol development, conduct of the trial, interpretation of results and writing/editing of the report.
Publication
Booth J, Aucott L, Cotton S, Goodman C, Hagen S, Harari D, et al. ELECtric Tibial nerve stimulation to Reduce Incontinence in Care homes: protocol for the ELECTRIC randomised trial. Trials 2019;20:723.
Data-sharing statement
Any data-sharing requests are to be made to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Booth J, Aucott L, Cotton S, Goodman C, Hagen S, Harari D, et al. ELECtric Tibial nerve stimulation to Reduce Incontinence in Care homes: protocol for the ELECTRIC randomised trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3723-7.
- D’Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn 2019;38:433-77. https://doi.org/10.1002/nau.23897.
- Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric 2019;22:217-22. https://doi.org/10.1080/13697137.2018.1543263.
- Grant RL, Drennan VM, Rait G, Petersen I, Iliffe S. First diagnosis and management of incontinence in older people with and without dementia in primary care: a cohort study using The Health Improvement Network primary care database. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001505.
- Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing 1997;26:367-74. https://doi.org/10.1093/ageing/26.5.367.
- Morrison A, Levy R. Fraction of nursing home admissions attributable to urinary incontinence. Value Health 2006;9:272-4. https://doi.org/10.1111/j.1524-4733.2006.00109.x.
- Sung VW, Borello-France D, Newman DK, Richter HE, Lukacz ES, Moalli P, et al. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs. surgery alone on incontinence symptoms among women with mixed urinary incontinence: the ESTEEM randomized clinical trial. JAMA 2019;322:1066-76. https://doi.org/10.1001/jama.2019.12467.
- Schumpf LF, Theill N, Scheiner DA, Fink D, Riese F, Betschart C. Urinary incontinence and its association with functional physical and cognitive health among female nursing home residents in Switzerland. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0414-7.
- Dubeau CE, Simon SE, Morris JN. The effect of urinary incontinence on quality of life in older nursing home residents. J Am Geriatr Soc 2006;54:1325-33. https://doi.org/10.1111/j.1532-5415.2006.00861.x.
- Rait G, Fletcher A, Smeeth L, Brayne C, Stirling S, Nunes M, et al. Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing 2005;34:242-8. https://doi.org/10.1093/ageing/afi039.
- Chiarelli PE, Mackenzie LA, Osmotherly PG. Urinary incontinence is associated with an increase in falls: a systematic review. Aust J Physiother 2009;55:89-95. https://doi.org/10.1016/S0004-9514(09)70038-8.
- Beeckman D. A decade of research on Incontinence-Associated Dermatitis (IAD): evidence, knowledge gaps and next steps. J Tissue Viability 2017;26:47-56. https://doi.org/10.1016/j.jtv.2016.02.004.
- Yip SO, Dick MA, McPencow AM, Martin DK, Ciarleglio MM, Erekson EA. The association between urinary and fecal incontinence and social isolation in older women. Am J Obstet Gynecol 2013;208:146-7. https://doi.org/10.1016/j.ajog.2012.11.010.
- de Vries HF, Northington GM, Bogner HR. Urinary incontinence (UI) and new psychological distress among community dwelling older adults. Arch Gerontol Geriatr 2012;55:49-54. https://doi.org/10.1016/j.archger.2011.04.012.
- NHS England . Excellence in Continence Care: Practical Guidance for Commissioners, and Leaders in Health and Social Care 2018.
- Wagg A, Chen L, Johnson T, Kirschner-Hermanns R, Kuchel G, Markland A, et al. Incontinence 6th Edition. Bristol: International Continence Society; 2017.
- Omli R, Skotnes LH, Romild U, Bakke A, Mykletun A, Kuhry E. Pad per day usage, urinary incontinence and urinary tract infections in nursing home residents. Age Ageing 2010;39:549-54. https://doi.org/10.1093/ageing/afq082.
- Roe B, Flanagan L, Jack B, Barrett J, Chung A, Shaw C, et al. Systematic review of the management of incontinence and promotion of continence in older people in care homes: descriptive studies with urinary incontinence as primary focus. J Adv Nurs 2011;67:228-50. https://doi.org/10.1111/j.1365-2648.2010.05481.x.
- Sink KM, Thomas J, Xu H, Craig B, Kritchevsky S, Sands LP. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc 2008;56:847-53. https://doi.org/10.1111/j.1532-5415.2008.01681.x.
- Zarowitz BJ, Allen C, O’Shea T, Tangalos EG, Berner T, Ouslander JG. Challenges in the pharmacological management of nursing home residents with overactive bladder or urinary incontinence. J Am Geriatr Soc 2015;63:2298-307. https://doi.org/10.1111/jgs.13713.
- Choudhary M, van Mastrigt R, van Asselt E. Inhibitory effects of tibial nerve stimulation on bladder neurophysiology in rats. SpringerPlus 2016;5. https://doi.org/10.1186/s40064-016-1687-6.
- Tai C, Shen B, Chen M, Wang J, Liu H, Roppolo JR, et al. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int 2011;107:303-9. https://doi.org/10.1111/j.1464-410X.2010.09358.x.
- Amarenco G, Ismael SS, Even-Schneider A, Raibaut P, Demaille-Wlodyka S, Parratte B, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol 2003;169:2210-15. https://doi.org/10.1097/01.ju.0000067446.17576.bd.
- Booth J, Connelly L, Dickson S, Duncan F, Lawrence M. The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: a systematic review. Neurourol Urodyn 2018;37:528-41. https://doi.org/10.1002/nau.23351.
- Schreiner L, dos Santos TG, Knorst MR, da Silva Filho IG. Randomized trial of transcutaneous tibial nerve stimulation to treat urge urinary incontinence in older women. Int Urogynecol J 2010;21:1065-70. https://doi.org/10.1007/s00192-010-1165-6.
- Manríquez V, Guzmán R, Naser M, Aguilera A, Narvaez S, Castro A, et al. Transcutaneous posterior tibial nerve stimulation versus extended release oxybutynin in overactive bladder patients. A prospective randomized trial. Eur J Obstet Gynecol Reprod Biol 2016;196:6-10. https://doi.org/10.1016/j.ejogrb.2015.09.020.
- Souto SC, Reis LO, Palma T, Palma P, Denardi F. Prospective and randomized comparison of electrical stimulation of the posterior tibial nerve versus oxybutynin versus their combination for treatment of women with overactive bladder syndrome. World J Urol 2014;32:179-84. https://doi.org/10.1007/s00345-013-1112-5.
- Guyatt G, Oxman A, Aklm E, Kunz R, Vist G, Brozeka J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Booth J, Hagen S, McClurg D, Norton C, MacInnes C, Collins B, et al. A feasibility study of transcutaneous posterior tibial nerve stimulation for bladder and bowel dysfunction in elderly adults in residential care. J Am Med Dir Assoc 2013;14:270-4. https://doi.org/10.1016/j.jamda.2012.10.021.
- SPIRIT . SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) n.d. www.spirit-statement.org/ (accessed June 2020).
- Great Britain . Adults With Incapacity (Scotland) Act 2000 2000. www.legislation.gov.uk/asp/2000/4/contents (accessed 11 June 2020).
- Great Britain . Mental Capacity Act 2005 2005. www.legislation.gov.uk/ukpga/2005/9 (accessed 11 June 2020).
- Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 2006;49:1079-86. https://doi.org/10.1016/j.eururo.2006.01.007.
- Talley KM, Wyman JF, Olson-Kellogg BG, Bronas UG, McCarthy TC. Reliability and validity of two measures of toileting skills in frail older women without dementia [published online ahead of print 3 June 2016]. J Gerontol Nurs 2016. https://doi.org/10.3928/00989134-20160531-02.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9100.
- NHS Improvement . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed July 2020).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2019 2019. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ (accessed July 2020).
- Joint Formulary Committee . British National Formulary. n.d. www.medicinescomplete.com/#/ (accessed 27 May 2020).
- Vinsnes AG, Helbostad JL, Nyrønning S, Harkless GE, Granbo R, Seim A. Effect of physical training on urinary incontinence: a randomized parallel group trial in nursing homes. Clin Interv Aging 2012;7:45-50. https://doi.org/10.2147/CIA.S25326.
- Kieser M, Friede T. Simple procedures for blinded sample size adjustment that do not affect the type I error rate. Stat Med 2003;15:3571-81. https://doi.org/10.1002/sim.1585.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Rowen D, Labeit A, Stevens K, Elliott J, Mulhern B, Carlton J, et al. Estimating a preference-based single index measuring the quality-of-life impact of self-management for diabetes. Med Decis Making 2018;38:699-707. https://doi.org/10.1177/0272989X18784291.
- Ratcliffe J, Flint T, Easton T, Killington M, Cameron I, Davies O, et al. An empirical comparison of the EQ-5D-5L, DEMQOL-U and DEMQOL-Proxy-U in a post-hospitalisation population of frail older people living in residential aged care. Appl Health Econ Health Policy 2017;15:399-412. https://doi.org/10.1007/s40258-016-0293-7.
- Murtagh MJ, Thomson RG, May CR, Rapley T, Heaven BR, Graham RH, et al. Qualitative methods in a randomised controlled trial: the role of an integrated qualitative process evaluation in providing evidence to discontinue the intervention in one arm of a trial of a decision support tool. Qual Saf Health Care 2007;16:224-9. https://doi.org/10.1136/qshc.2006.018499.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Lindeloef N, Lundin-Olsson L, Skelton DA, Lundman B, Rosendahl E. Experiences of older people with dementia participating in a high-intensity functional exercise program in nursing homes: ‘while it’s tough, it’s useful’. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0188225.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0605-9.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Bowling A. Research Methods in Health: Investigating Health and Health Services. Maidenhead: Open University Press; 2014.
- Offermans MP, Du Moulin MF, Hamers JP, Dassen T, Halfens RJ. Prevalence of urinary incontinence and associated risk factors in nursing home residents: a systematic review. Neurourol Urodyn 2009;28:288-94. https://doi.org/10.1002/nau.20668.
- Bartley J, Gilleran J, Peters K. Neuromodulation for overactive bladder. Nat Rev Urol 2013;10:513-21. https://doi.org/10.1038/nrurol.2013.143.
- Ammi M, Chautard D, Brassart E, Culty T, Azzouzi AR, Bigot P. Transcutaneous posterior tibial nerve stimulation: evaluation of a therapeutic option in the management of anticholinergic refractory overactive bladder. Int Urogynecol J 2014;25:1065-9. https://doi.org/10.1007/s00192-014-2359-0.
- Teixeira Alve A, Azevedo Garcia P, Henriques Jácomo R, Batista de Sousa J, Borges Gullo Ramos Pereira L, Barbaresco Gomide Mateus L, et al. Effectiveness of transcutaneous tibial nerve stimulation at two different thresholds for overactive bladder symptoms in older women: a randomized controlled clinical trial. Maturitas 2020;135:40-6. https://doi.org/10.1016/j.maturitas.2020.02.008.
- Bykoviene L, Kubilius R, Aniuliene R, Bartuseviciene E, Bartusevicius A. Pelvic floor muscle training with or without tibial nerve stimulation and lifestyle changes have comparable effects on the overactive bladder. A randomized clinical trial. Urol J 2018;15:186-92. https://doi.org/10.22037/uj.v0i0.4169.
- Martin-Garcia M, Crampton J. A single-blind, randomized controlled trial to evaluate the effectiveness of transcutaneous tibial nerve stimulation (TTNS) in Overactive Bladder symptoms in women responders to percutaneous tibial nerve stimulation (PTNS). Physiotherapy 2019;105:469-75. https://doi.org/10.1016/j.physio.2018.12.002.
- Ramírez-García I, Blanco-Ratto L, Kauffmann S, Carralero-Martínez A, Sánchez E. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: randomized control trial. Neurourol Urodyn 2019;38:261-8. https://doi.org/10.1002/nau.23843.
- Abulseoud A, Moussa A, Abdelfattah G, Ibrahim I, Saba E, Hassouna M. Transcutaneous posterior tibial nerve electrostimulation with low dose trospium chloride: could it be used as a second line treatment of overactive bladder in females. Neurourol Urodyn 2018;37:842-8. https://doi.org/10.1002/nau.23361.
- Welk B, McKibbon M. A randomized, controlled trial of transcutaneous tibial nerve stimulation to treat overactive bladder and neurogenic bladder patients. Can Urol Assoc J 2020;14:E297-E303. https://doi.org/10.5489/cuaj.6142.
- Lukacz ES, Sampselle C, Gray M, MacDiarmid S, Rosenberg M, Ellsworth P, et al. A healthy bladder: a consensus statement. Int J Clin Pract 2011;65:1026-36. https://doi.org/10.1111/j.1742-1241.2011.02763.x.
- Schnelle JF, MacRae PG, Ouslander JG, Simmons SF, Nitta M. Functional incidental training, mobility performance, and incontinence care with nursing home residents. J Am Geriatr Soc 1995;43:1356-62. https://doi.org/10.1111/j.1532-5415.1995.tb06614.x.
- Schnelle JF, Alessi CA, Simmons SF, Al-Samarrai NR, Beck JC, Ouslander JG. Translating clinical research into practice: a randomized controlled trial of exercise and incontinence care with nursing home residents. J Am Geriatr Soc 2002;50:1476-83. https://doi.org/10.1046/j.1532-5415.2002.50401.x.
- Flanagan L, Roe B, Jack B, Barrett J, Chung A, Shaw C, et al. Systematic review of care intervention studies for the management of incontinence and promotion of continence in older people in care homes with urinary incontinence as the primary focus (1966–2010). Geriatr Gerontol Int 2012;12:600-11. https://doi.org/10.1111/j.1447-0594.2012.00875.x.
- Ostaszkiewicz J, Dunning T, Hutchinson A, Wagg A, Gwini S, Dickson-Swift V. The development and validation of instruments to measure dignity-protective continence care for care-dependent older people in residential aged care facilities: a study protocol. Neurourol Urodyn 2020;39:1363-70. https://doi.org/10.1002/nau.24343.
- Mangnall J, Taylor P, Thomas S, Watterson L. Continence problems in care homes: auditing assessment and treatment. Nurs Older People 2006;18:20-2. https://doi.org/10.7748/nop2006.03.18.2.20.c2414.
- Surr CA, Holloway I, Walwyn REA, Griffiths AW, Meads D, Kelley R, et al. Dementia Care Mapping to reduce agitation in care home residents with dementia: the EPIC cluster RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24160.
- Groutz A, Blavais J, Chaikin D, Resnick N, Engleman K, Anzalone D, et al. Non-invasive outcome measures of urinary incontinence and lower urinary tract symptoms: a multicenter study of micturition diary and pad tests. J Urol 2000;164:698-701. https://doi.org/10.1016/S0022-5347(05)67284-9.
- Curtis LA. Unit Costs of Health and Social Care 2013 2013. http://pssru.ac.uk/project-pages/unit-costs/2013 (accessed July 2020).
Appendix 1 Summary of changes made to the protocol during the ELECTRIC trial
| Protocol version number | Summary of changes | Protocol date |
|---|---|---|
| 2.0 |
Number of care homes in study changed from 20 to > 20 Proportion of 500 residents recruited in England vs. Scotland changed from 50 : 50 to 33 : 67 72-hour bladder diary replaced with 24-hour bladder diary Responsibility of fidelity checks transferred from local PI to ISF SOP for adverse event/serious adverse event reporting clarified Section describing the internal pilot study added Section detailing process evaluation data analysis added References to Data Protection Act 1998 updated to GDPR |
27 August 2018 |
| 3.0 | Sample size recalculated based on lower than anticipated attrition. New recruitment target changed to a minimum of 278 care home residents | 29 May 2019 |
Appendix 2 Recruitment by centre
| Centre (care home) | Location | Randomisations |
|---|---|---|
| Lillyburn Care Home | Scotland | 22 |
| Mosswood Care Home | Scotland | 15 |
| New Thursby Nursing Care Home | England | 17 |
| Stanely Park Care Home | Scotland | 23 |
| Priory Court Nursing Care Home | England | 7 |
| St George’s Nursing Care Home | England | 3 |
| The Hamptons Care Centre | England | 14 |
| Stella Matutina Care Home | England | 12 |
| Ranfurly Care Home | Scotland | 14 |
| Craigielea Care Home | Scotland | 23 |
| Balquhidder House | Scotland | 16 |
| Templeton House Care Home | Scotland | 4 |
| Campbell Snowdon House | Scotland | 9 |
| Doonbank House Care Home | Scotland | 9 |
| Burnfoot Care Home | Scotland | 6 |
| Glebe House Care Home | Scotland | 3 |
| Cross and Passion Convent | England | 4 |
| Mariners Court Care Home | England | 12 |
| Strathleven | Scotland | 4 |
| The Willows Care Home | England | 8 |
| Larkfield View Care Centre | Scotland | 10 |
| Orchard Grove Residential Care Home | Scotland | 28 |
| Pilling Nursing Home | England | 7 |
| Kepplegate House Care Home | England | 6 |
| The Conifers Nursing Home | England | 6 |
| Westbank Nursing | Scotland | 5 |
| St Alban’s Nursing Home | England | 8 |
| Sun Court Nursing Home | Scotland | 11 |
| Davislea Care Home | Scotland | 9 |
| Riverside Care Home | Scotland | 34 |
| Redford Nursing Home | Scotland | 8 |
| Drumry House Care Home | Scotland | 6 |
| Glenfairn House Nursing Home | Scotland | 15 |
| Bankhouse Care Home | England | 6 |
| Howard House Nursing and Residential Home | Scotland | 7 |
| Brooklands House Rest Home | England | 9 |
| The Grange Care Home | Scotland | 8 |
| Alexandra | England | 0 |
| Woodsidea | Scotland | 0 |
| Windy Halla | Scotland | 0 |
| Castleviewa | Scotland | 0 |
| Beechwood Parka | Scotland | 0 |
| Birdstona | Scotland | 0 |
| Total | 408 |
Appendix 3 Protocol breaches/post randomisation exclusions
| Centre affected | Date of breach | Description of breach | Assessment/outcome |
|---|---|---|---|
| 14 | 6 April 2018 | Participant 14002 was randomised to treatment group on 29 March 2018. On 6 April 2018 the RRA discovered the participant had a pacemaker, which was part of the exclusion criteria of the study. RRA immediately informed trial manager and chief investigator. The participant had been given two stimulation sessions by the date of the breach discovery | Post randomisation exclusion. Stimulations were stopped and participant was immediately withdrawn from the trial |
| 18 | 12 February 2019 | Participant 18025 was randomised to treatment group on 1 November 2018, but was later discovered to have a pacemaker fitted. RRA immediately informed trial manager and chief investigator. The participant had been given six stimulation sessions by the date of the breach discovery | Post randomisation exclusion. Stimulations were stopped and participant was immediately withdrawn from the trial |
| 44 | 11 November 2019 | Accidental unblinding (sham group) by care home staff to the RRA and participant’s family | As this participant was past the primary outcome data collection point of 6 weeks, data collection was continued at 12 and 18 weeks |
Appendix 4 The ELECTRIC trial serious adverse events and adverse events
| Trial ID | Date of event | SAE/AE | Details | Severity | Relation to the ELECTRIC trial | Trial intervention |
|---|---|---|---|---|---|---|
| 15018 | 22 April 2018 | SAE | Vomiting, shaking and irregular heart rhythm – admitted to hospital overnight. Two stimulations before and 10 stimulations after hospital admission | Moderate | Unlikely | No change |
| 35011 | 3 February 2019 | SAE | Kidney infection – admitted to hospital within 6 weeks. Four stimulations before and zero stimulations after hospital admission | Moderate | Unrelated | Stopped |
| 15023 | 12 September 2019 | SAE | UTI and pulmonary oedema, in hospital with end-of-life care. Eight stimulations before and zero stimulations after hospital admission | Moderate | Unlikely | Stopped |
| 26009 | 15 December 2018 | SAE | UTI and chest infection within 6 weeks – admitted to hospital. Four stimulations before and five stimulations after hospital admission (received only nine stimulations in total) | Moderate | Unlikely | Interrupted |
| 12002 | 27 February 2018 | AE | UTI and cough within 6 weeks | Mild | Unlikely | No change |
| 12002 | 22 March 2018 | AE | UTI and chest infection within 6 weeks | Mild | Unlikely | No change |
| 12017 | 22 March 2018 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 13013 | 1 April 2018 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 13020 | 5 April 2018 | AE | UTI on day stimulation was received | Mild | Unlikely | No change |
| 13018 | 8 April 2018 | AE | Left leg pain (not reported until after electrodes removed) | Moderate | Unrelated | No change |
| 15011 | 26 April 2018 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 15011 | 26 April 2018 | AE | Leg felt heavy and felt dizzy – resident withdrew from stimulations | Mild | Probably | Stopped |
| 13002 | 2 May 2018 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 13002 | 4 May 2018 | AE | Reduced kidney function within 6 weeks | Moderate | Unrelated | No change |
| 15017 | 4 May 2018 | AE | Blood observed on incontinence pad | Mild | Unlikely | No change |
| 15013 | 13 May 2018 | AE | Participant became aggressive, stimulation sessions not interrupted as received four stimulations after this incident (received all 12 stimulations) | Mild | Related | No change |
| 25010 | 13 September 2018 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 31004 | 14 December 2018 | AE | Participant suffered two ‘episodes’. RA thinks these may have been seizures. The resident had three stimulation sessions before and nine stimulation sessions after the incident | Moderate | Unlikely | No change |
| 26005 | 14 January 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 34010 | 11 February 2019 | AE | UTI and chest infection within 6 weeks | Mild | Unlikely | No change |
| 48011 | 27 April 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 48004 | 3 May 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 48010 | 8 May 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 48007 | 23 May 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
| 15023 | 1 September 2019 | AE | UTI within 6 weeks | Mild | Unlikely | No change |
Appendix 5 Unit costs and sources
| Resource | Unit cost in 2019 prices (£) | Data source for unit cost valuation | Assumption for units used in calculations | Applied unit cost (£) per recorded item |
|---|---|---|---|---|
| TPTNS training and support package | ||||
| Trainers | 25.00 | Trial records (estimation) | Based on two trainers for 2 hours per event, plus additional travel time and mileage reimbursement per person of 30 minutes for an average round trip of 20 miles (£0.45 per mile) | 143.00 |
| Assessors | 15.00 | Trial records (estimation) | Based on one assessor for 1 hour per individual staff competency assessment (plus travel cost) | 31.50 |
| Training handbook and DVD | 11.20 | Trial records (estimation) | Per pack per trainee | 11.20 |
| TPTNS intervention | ||||
| Neurotrac machine (branded) | 74.49 | Amazon.co.uk (Amazon.com, Inc., Bellevue, WA, USA) | A total of 172 units were used during the ELECTRIC trial | 74.49 |
| PP3 alkaline battery [Energizer (Energizer Holdings, Inc., St. Louis, MO, USA)] | 1.70 | Amazon.co.uk | Based on assumption of replacement (one battery) every 10 hours of use | 1.70 |
| Skin electrode pads (single use) | 1.49 | boots.com (Boots UK Limited, Nottingham, UK) | Based on two pads per stimulation episodea | 2.98 |
| Incontinence products | ||||
| Pads | 0.105–1.425 | Supplier websitesb | Based on average cost | 0.377 |
| Adult pants | 0.306–1.460 | Supplier websitesb | Based on average cost | 0.906 |
| Slips | 0.461–1.114 | Supplier websitesb | Based on average cost | 0.728 |
| Bed and chair protection | 6.860 | Supplier websitesb | Based on average cost | 6.860 |
| Community-based social care staff | ||||
| Care assistant | 28.00 | PSSRU Unit Costs 201937 | Based on face to face, per hour, weekday | 28.00 |
| Care leader | 40.00 | PSSRU Unit Costs 201937 | Per hour | 40.00 |
| AfC band 5 nurse | 60.00 | PSSRU Unit Costs 201937 | Per hour of patient-related work | 60.00 |
| Community-based health-care staff | ||||
| GP | 39.00 | PSSRU Unit Costs 201937 | Based on 9.22-minute consultation | 39.00 |
| GP care home visit | 4.30 | PSSRU Unit Costs 201937 | Per minute of patient contact. Based on assumed duration of 23.4-minute consultation (PSSRU Unit Costs 2013)71 | 100.62 |
| Practice nurse | 37.00 | PSSRU Unit Costs 201937 | Based on assumed duration of 15-minute consultation | 9.25 |
| Practice nurse care home visit | 37.00 | PSSRU Unit Costs 201937 | Based on 27-minute consultation | 16.65 |
| District nurse (AfC band 6) | 84.00 | PSSRU Unit Costs 201937 | Based on assumed duration of 27 minutes (as per practice nurse home visit) of patient-related work | 37.80 |
| Physiotherapist (community services) | 46.00 | PSSRU Unit Costs 201937 | Per hour | 46.00 |
| Occupational therapist | 44.00 | PSSRU Unit Costs 201937 | Per hour | 44.00 |
| Continence nurse | 113.00 | PSSRU Unit Costs 201937 | Based on assumed duration of 27 minutes (as per practice nurse home visit), costed as the time of one hospital-based nurse specialist (AfC band 6) | 50.85 |
| Medications used to treat UI (unit cost per tablet) | ||||
| Duloxetine | 3.43 | Indicative Drug Tariff Price38 | 20 mg, 28 capsules | 0.12 |
| Fesoterodine fumarate | 25.78 | Indicative Drug Tariff Price38 | 4 mg, 28 tablets | 0.92 |
| Mirabegron | 29.00 | Indicative Drug Tariff Price38 | 25 mg, 30 tablets | 0.97 |
| Oxybutynin hydrochloride | 1.60 | Indicative Drug Tariff Price38 | 2.5 mg, 84 tablets | 0.02 |
| Solifenacin succinate | 27.62 | Indicative Drug Tariff Price38 | 5 mg, 30 tablets | 0.92 |
| Tolterodine tartrate | 29.03 | Indicative Drug Tariff Price38 | 1 mg, 56 tablets | 0.52 |
| Trospium chloride | 26.00 | Indicative Drug Tariff Price38 | 20 mg, 60 tablets | 0.43 |
Appendix 6 Data completeness for calculation of utility values
| DEMQOL scores | Baseline (N = 406) | 6 weeks (N = 365) | 18 weeks (N = 310) | |||
|---|---|---|---|---|---|---|
| Valid | Missing | Valid | Missing | Valid | Missing | |
| All participants | ||||||
| DEMQOL-U | 141 | 265 | 107 | 258 | 68 | 242 |
| DEMQOL-PROXY-U | 397 | 9 | 324 | 41 | 279 | 31 |
| TPTNS (n = 197) | ||||||
| DEMQOL-U | 66 | 131 | 48 | 129 | 28 | 115 |
| DEMQOL-PROXY-U | 190 | 7 | 159 | 18 | 129 | 14 |
| Sham (n = 209) | ||||||
| DEMQOL-U | 75 | 134 | 59 | 129 | 40 | 127 |
| DEMQOL-PROXY-U | 207 | 2 | 165 | 23 | 150 | 17 |
Appendix 7 Resource use data
| Resources | Time pointa (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| TPTNS | Sham | |||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Trial data about incontinence management products (number and brand/type) | 197 | 166 | 152 | 133 | 209 | 178 | 156 | 156 |
| Total pads in 24 hours | 694 | 556 | 497 | 412 | 693 | 569 | 466 | 458 |
| Total adult pants in 24 hours | 36 | 43 | 46 | 44 | 68 | 55 | 52 | 42 |
| Total slip in 24 hours | 35 | 24 | 15 | 15 | 42 | 37 | 34 | 30 |
| Total bed and chair protection in 24 hours | 4 | 2 | 0 | 5 | 3 | 0 | 0 | 6 |
| RUQb | 197 | 180 | 151 | 209 | 190 | 170 | ||
| UI medication reported | 19 | 14 | 11 | 10 | 8 | 10 | ||
| Duloxetine | 2 | 2 | 2 | 1 | 1 | 1 | ||
| Fesoterodine fumarate | 0 | 0 | 0 | 1 | 1 | 1 | ||
| Mirabegron | 5 | 4 | 3 | 2 | 0 | 1 | ||
| Oxybutynin hydrochloride | 5 | 4 | 2 | 1 | 1 | 2 | ||
| Solifenacin succinate | 4 | 2 | 2 | 3 | 3 | 3 | ||
| Tolterodine tartrate | 2 | 2 | 2 | 0 | 0 | 0 | ||
| Trospium chloride | 1 | 0 | 0 | 2 | 2 | 2 | ||
| Use of special equipment reportedc | 105 | 103 | 66 | 117 | 112 | 86 | ||
| Alarm | 6 | 2 | 1 | 8 | 2 | 1 | ||
| Falls protection | 4 | 2 | 3 | 6 | 0 | 5 | ||
| Incontinence protection for furniture | 2 | 0 | 1 | 3 | 0 | 0 | ||
| Mobility aid | 79 | 62 | 48 | 86 | 65 | 63 | ||
| Toilet aid | 36 | 31 | 18 | 36 | 29 | 31 | ||
| Transfer aid | 60 | 39 | 29 | 63 | 32 | 25 | ||
| Ulcer prevention | 7 | 3 | 0 | 4 | 4 | 2 | ||
| Contact with NHS for UI reportedd | NC | 13 | 8 | NC | 24 | 15 | ||
Appendix 8 Resource use: total number of contacts in the 6 weeks preceding data collection with each NHS service by groups, using all available data
| NHS Service | TPTNS | Sham | Total contacts (n) | Unit cost (£) | Total cost (£) | ||
|---|---|---|---|---|---|---|---|
| 6-week follow-up (n = 13) | 18-week follow-up (n = 8) | 6-week follow-up (n = 24) | 18-week follow-up (n = 15) | ||||
| GP SA | 16 | 9 | 33 | 17 | 75 | 39.00 | 2925.00 |
| GP CHV | 12 | 7 | 17 | 13 | 49 | 100.62 | 4930.38 |
| Practice nurse SA | 1 | 0 | 0 | 1 | 2 | 9.25 | 18.50 |
| Practice nurse CHV | 2 | 0 | 1 | 1 | 4 | 16.65 | 66.60 |
| District nurse | 7 | 0 | 6 | 0 | 13 | 37.80 | 491.40 |
| Physiotherapist | 0 | 0 | 1 | 0 | 1 | 46.00 | 46.00 |
| Occupational therapist | 0 | 0 | 1 | 1 | 2 | 44.00 | 88.00 |
| Continence service | 0 | 2 | 7 | 2 | 11 | 50.85 | 559.35 |
| Total contacts | 38 | 18 | 66 | 35 | 157 | 9125.23 | |
Appendix 9 Topic areas explored with participant groups
| Participant group | Topic area |
|---|---|
| Residents/family members |
|
| Care home staff |
|
| Care home managers |
|
Appendix 10 The ELECTRIC trial qualitative themes using theoretical domains framework V3 (March 2020)
| Care home staff perception of trial (TDF Framework domains 1–14)50 (working document: updated April 2020) | ||||||
|---|---|---|---|---|---|---|
| 1. Knowledge | 2. Skills | 3. Social/professional role and identity | 4. Beliefs about capabilities | 5. Optimism | 6. Beliefs about consequences | |
| TDF domain |
|
|
|
|
|
|
| ELECTRIC coding (NVivo) associated with each domain |
|
|
Identity within professional role
|
|
|
|
| Care home staff perception of trial (TDF Framework domains 1–14)50 (working document: updated April 2020) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 7. Reinforcement | 8. Intentions | 9. Goals | 10. Memory, attention and decision processes | 11. Environmental context and resources | 12. Social influences | 13. Emotion | 14. Behavioural regulation | |
| TDF domain |
|
|
|
|
|
|
|
|
| ELECTRIC coding (NVivo) associated with each domain |
|
|
|
|
|
|
|
|
Appendix 11 Section of the analytic framework demonstrating the application of the theoretical domains framework knowledge domain to the care home staff data set (using NVivo 11)
List of abbreviations
- AE
- adverse event
- CA
- care assistant
- CACE
- complier-average causal effect
- CCA
- cost–consequences analysis
- CHaRT
- Centre for Healthcare Randomised Trials
- CHRG
- care home reference group
- CI
- confidence interval
- COM-B
- capability, opportunity, motivation and behaviour
- CONSORT
- Consolidated Standards of Reporting Trials
- CPD
- continuous professional development
- CRF
- case report form
- DEMQOL
- Dementia Quality of Life
- DEMQOL-PROXY
- Dementia Quality of Life Proxy
- DEMQOL-PROXY-U
- Dementia Quality of Life Proxy – utility index
- DEMQOL-U
- Dementia Quality of Life – utility index
- DMEC
- Data Monitoring and Ethics Committee
- DVD
- digital versatile disc
- ELECTRIC
- ELECtric Tibial nerve stimulation to Reduce Incontinence in Care homes
- GCP
- good clinical practice
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HTA
- Health Technology Assessment
- IRAS
- integrated research application system
- ISF
- implementation support facilitator
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- MCA
- Mental Capacity Act
- MMSE
- Mini Mental State Examination
- MTSQ
- Minnesota Toileting Skills Questionnaire
- NIHR
- National Institute for Health Research
- OAB
- overactive bladder
- PBC
- Perception of Bladder Condition
- PI
- principal investigator
- PMG
- project management group
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PTNS
- percutaneous tibial nerve stimulation
- PVRU
- post void residual urine volume
- PWT
- pad weight test
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RN
- registered nurse
- RRA
- regional research assistant
- RUQ
- resource use questionnaire
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SCA
- senior care assistant
- SD
- standard deviation
- SocCA
- social care assistant
- SOP
- standard operating procedure
- SPIG
- stakeholder and public involvement group
- TDF
- theoretical domains framework
- TPTNS
- transcutaneous posterior tibial nerve stimulation
- TSC
- Trial Steering Committee
- UI
- urinary incontinence
- UTI
- urinary tract infection
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25410).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
