Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/26/01. The contractual start date was in June 2014. The draft report began editorial review in April 2020 and was accepted for publication in August 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Goldstein et al. This work was produced by Goldstein et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Goldstein et al.
Chapter 1 Introduction
Background to this project
In 2012, a call was issued by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme for a multicentre, two-arm randomised controlled trial (RCT) to answer the question ‘What is the clinical and cost-effectiveness of cognitive–behavioural therapy (CBT) for psychogenic non-epileptic seizures (PNESs)?’ (see Appendix 1). The call specified that the CBT should be tailored for adult PNES patients whose condition persisted beyond diagnosis by a neurologist or neuropsychiatrist. The trial would be conducted in outpatient or community healthcare settings.
The decision to include patients with comorbid epilepsy would be considered by the applicants; consideration would also be given to psychiatric comorbidities. CBT would be compared with standard medical care, which was to be defined by the research team; however, it was recognised that this might include a patient information leaflet. Potentially important outcomes were identified as seizure frequency and severity, as well as duration of seizure freedom. Other outcomes identified were psychiatric symptomatology, psychosocial functioning, quality of life, healthcare utilisation, cost-effectiveness and socioeconomic factors (i.e. disability and return to work). The minimum follow-up duration was set as 1 year post randomisation.
The call acknowledged that although psychotherapy is deemed the first-line intervention for PNESs, there is limited evidence of its effectiveness, despite reports that CBT has been shown to be successful in treating other somatoform conditions. A pilot RCT demonstrated that a version of CBT designed specifically for patients with PNESs was able to reduce seizure frequency over standard care. 1 The call highlighted the requirement for a fully powered RCT to evaluate the clinical effectiveness and cost-effectiveness of CBT tailored for PNESs. The current study is a response to the call made by NIHR, proposing the expansion of the earlier pilot1 into a multicentre RCT recruiting patients from neurology services. The call recognised that PNESs are often referred to by other names. We adopted the term dissociative seizures (DSs) for reasons discussed in the next section (see Dissociative seizures). The trial was subsequently named ‘COgnitive behavioural therapy vs. standardised medical care for adults with Dissociative non-Epileptic Seizures: a multicentre randomised controlled trial (CODES)’.
Dissociative seizures
Dissociative seizures are paroxysmal behavioural events that resemble epileptic seizures or syncope; they are, however, not a result of epilepsy or any other medical condition. They are widely understood as involuntary episodes that arise via dissociative mechanisms and as a disorder sitting at the interface between neurology and psychiatry. 1–3 DSs are currently classified as conversion (functional neurological symptom) disorder4 and as a dissociative neurological symptom disorder. 5 For the purposes of the current study, DSs were defined as events leading to a transient loss of consciousness or apparent altered responsiveness. Such episodes would be clinically incompatible with recognised neurological or general medical conditions, and not better explained by another physical or psychiatric disorder. Symptoms or deficits would also cause significant distress, psychosocial impairment or warrant medical evaluation.
Dissociative seizures are also referred to as ‘pseudoseizures’, ‘functional seizures’, ‘psychogenic non-epileptic seizures’, ‘non-epileptic events/spells’ or ‘non-epileptic attack disorder’ among other names. Currently, the most prominent term used is PNESs, although it may have negative connotations for some patients,6,7 with the term ‘psychogenic’ potentially implying that the attacks are ‘deliberate’ or ‘imagined’ despite the intentions of healthcare professionals (HCPs). We chose the term ‘dissociative seizures’ in line with the International Classification of Diseases and Related Problems, Tenth Revision, classification that was current at the time that the project started,8 as it encourages a positive diagnosis and discussion of an aetiological mechanism.
The prevalence of DSs is estimated as 2–33 per 100,000 people,9 although more recent assessments place this estimate closer to 50 per 100,000 people. 10 A recent UK study2 documented the incidence rate as 4.9 per 100,000 people per year. Women form around three-quarters of all patients with DSs,11 with onset typically occurring in the late teens/twenties,12 although DSs can occur in both the old and the young. 13–15 It is thought that around 12–20% of patients presenting at epilepsy clinics may have DSs. 16
Video electroencephalography (EEG) is the gold standard diagnostic technique for DSs, with the key signs17 being the absence of typical epileptic EEG abnormalities and the presence of an intact alpha rhythm. However, a careful history alone should usually provide sufficient grounds to suspect DSs. 18 It is estimated that about 22% of DS patients have comorbid epileptic seizures,19 which may create problems in diagnosis and management. 18
Up to 80% of patients with DSs have a history of other functional somatic symptoms and disorders, including other functional neurological symptoms. 20 There are high levels of psychiatric comorbidity in patients with DSs,21–23 including maladaptive personality traits, post-traumatic stress disorder (PTSD), anxiety and depression, with patients demonstrating less adaptive coping styles. 24,25 It has also been reported that patients with DSs might have a slightly higher risk of mortality; however, this is not directly related to the seizures. 26
A recent meta-analysis has shown a clear association between the presence of childhood stressors and the presence of adulthood stressors in patients with DSs, with an odds ratio of 3.1 [95% confidence interval (CI) 1.7 to 5.6]. 27 In addition, the perceived impact of traumatic events appears greater. 28 Individual studies have shown an association between history of trauma and DSs, with 44–100% of patients reporting a history of trauma and 23–77% of patients reporting being physically and/or sexually abused. 29 This suggests that these experiences may be potential risk factors for DSs for a significant proportion of patients. The frequency of childhood sexual abuse is higher among females with DSs,30 and it has been reported that, for example, in one DS population studied, 41% of females had suffered from sexual abuse. 31 This is a much higher rate than the estimated rate of sexual abuse (15–25%) in the general female population. 32 Reports of sexual abuse increase the likelihood of a diagnosis of DSs by approximately threefold. 28,33 Importantly, however, a diagnosis of DSs is not dependent on a history of abuse. 22 In addition, patients with DSs report more life events than those with epilepsy and motor conversion disorder in the 12 months prior to DS onset,34,35 and it has been considered that repeated adverse experiences over the longer term may be as relevant as acute life events in the development of DSs. 22
In addition to trauma, two-thirds of patients with DSs report significant problems in family and social environments. 31 When compared with patients with epilepsy, marital and family problems have been reported to be more prevalent in patients with DSs. 36 Individuals with DSs have reported viewing their family as having a dysfunctional communication style,37 which may contribute to DS symptomology through distress, criticism and a tendency to somatise. 38 As a result, individuals with DSs sometimes perceive their families as lacking in commitment and support. 36
Quality of life in individuals with DSs is lower than in those who are diagnosed with epilepsy,39 which may in part be because of the association between quality of life and depression, dissociation, somatic symptoms, escape–avoidance coping strategies and family dysfunction. 40 Patients with DSs can experience very restricted lives and demonstrate high levels of avoidance behaviour41–43 owing to fears of having a seizure. Individuals with DSs may also experience high levels of stigma related to their seizures,44 which can lead to individuals isolating themselves to avoid any adverse social reactions. 45,46 In addition, family members can influence quality of life in patients with DSs. Those who have a family environment characterised by high levels of criticism and lack of interest and support have lower health-related quality of life (HRQoL). 47
In a study of longer-term outcome of 50 patients that was undertaken by retrospective analysis after an average of 2 years, over half of the patients were found to be in either a poor or a very poor state because of a combination of physical, psychological and social issues. 48 In addition to a potentially low quality of life, around 70% of individuals with DSs have poor long-term outcomes, including chronic disability and welfare dependence. 49 Studies suggest that around half of patients are receiving or dependent on disability/state benefits during follow-up. 49,50 In conjunction with this, it has been surmised51 that the societal costs of having DSs can be considerable and can, as a result of rates of unemployment and disability, approximate the costs associated with intractable epilepsy. There is also some evidence that disability and welfare dependence can continue even if patients become seizure free. 52,53
The most consistent predictive factor of poor outcome, defined in different ways in different studies, for people with DSs appears to be the duration of the symptoms: the longer the duration, the more likely the negative outcome. 48,54,55 Further factors that negatively predict outcomes include receiving social security benefits;56 the presence of previous psychiatric diagnoses,56 with evidence specifically relating to the presence of depression56,57 and anxiety;56 poor psychopathology inventory scores;49 and a difficulty in forming relationships. 58 Personality factors may also be associated with different patterns of outcome. 49,57,59 Factors predictive of good outcomes have variously been found to include employment;2,60 higher educational achievement49,61 and intelligence quotient;62 low somatisation scores;49 and being accompanied to the first clinic visit. 61 There have also been indications, of varying strength, that hyperkinetic DS semiology is associated with a less good outcome than hypokinetic DS semiology. 48,49,54,61
Health economic aspects of dissociative seizures
There is a lack of research investigating the healthcare costs incurred by people with DSs. However, the literature often refers to the prevalence of unnecessary tests and treatments that patients can undergo that are expensive and potentially harmful. 63 A study in the USA64 reported the pre-diagnosis cost of this patient group (excluding diagnostic tests) as potentially exceeding US$25,000 per patient, based on an average of 6 years to arrive at the correct diagnosis. A similar investigation in Ireland65 reported an annual pre-diagnosis cost of €5429.30, assuming that it takes an average of 5 years to reach a DS diagnosis. A correct diagnosis of DSs appears to lead to a decrease in health service use and a subsequent reduction in costs. 66,67 Although it is possible that patients with DSs may later develop other medically unexplained symptoms,20 which may also have financial implications, there is no evidence to predict whether or not this likelihood is reduced by psychological interventions.
Treatment for dissociative seizures
Although some research in the last 10 years has investigated pharmacological treatment for DSs using antidepressants,68,69 the treatment of choice in both clinical and research settings remains psychotherapy. 70,71 There has been some research on the beneficial use of psychodynamic therapy,72,73 group psychotherapy74 and psychoeducational approaches. 75,76 However, CBT has the most substantial body of data suggesting effectiveness in treating a range of somatoform disorders,77,78 although effect sizes have been noted to be small to medium. 78 More specifically, with respect to treating patients with DSs, the evidence for DS reduction comes from small, open-label studies and pilot RCTs. 79,80 Carlson and Nicholson Perry79 reviewed 13 studies of mixed quality and somewhat differing interventions that demonstrated that 47% of patients were reported to be seizure free at the end of the psychological intervention; they also reported that, considering data available from 10 studies, 82% of patients had shown at least a 50% reduction in DS occurrence at the end of the intervention.
Martlew et al. 80 reviewed data from eight open-label studies and four RCTs, and concluded that the strongest evidence for DS reduction at that point came from a pilot RCT. 1 This RCT, based on a previous single case study81 and an open-label study,82 was undertaken at just one clinical site and compared a manualised CBT treatment for DSs plus standard medical care with standard medical care alone. Standard medical care was provided within a specialist tertiary neuropsychiatry service. A total of 66 patients were allocated at random to two equally sized treatment arms. Patients who were allocated to receive CBT plus standard medical care were offered 12 sessions of DS-specific CBT (plus up to two booster sessions), including handouts supporting the session content. The structure of the treatment package was described. 1,83,84 Intention-to-treat analyses showed a significant reduction in DSs within the CBT arm at the end of treatment; at follow-up, the CBT arm tended to have fewer seizures than the arm receiving standard medical care only. In addition, at follow-up the CBT arm tended to be more likely to be seizure free for the last 3 months of the study than the standard medical care arm. Both trial arms showed a degree of improvement in some measures of health service use, as well as on the Work and Social Adjustment Scale; however, anxiety, depression and employment status showed no change. Although this study provided preliminary evidence of the efficacy of CBT for this patient group, it lacked independent outcome assessors and demonstration of effectiveness across multiple sites and practitioners.
Additional evidence for the efficacy of a largely CBT-informed approach in the treatment of DSs has been found within a small, four-arm, pilot RCT. 68 Thirty-eight patients (of whom 34 provided outcome data) from three sites were randomised to four treatment arms: 12 sessions of CBT-informed psychotherapy (CBT-ip) (n = 9), 12 sessions of CBT-ip plus flexible low-dose sertraline (n = 9), sertraline alone (n = 9) and treatment as usual (n = 7). CBT-ip was heavily informed by principles of CBT but also included other therapeutic techniques derived from mindfulness, dialectical behaviour therapy and psychodynamic approaches. The study was not powered to allow between-condition comparisons for the primary outcome of DS frequency; therefore, comparisons were made within treatment allocation. The two treatment arms incorporating CBT-ip demonstrated significant seizure reduction and improvement in functioning and scores on symptom scales, whereas the sertraline-alone arm showed a trend towards DS reduction but no change in secondary outcomes. No significant changes were found in the treatment-as-usual arm. There were also significant reductions in clusters of DSs in the two trial arms delivering CBT-ip, although the extent of the reduction depended on how clusters were defined. 85
Nonetheless, despite showing potential efficacy of a CBT approach to the reduction of DS occurrence, neither pilot RCT was an adequately powered effectiveness study that provided a sufficiently robust evidence base on which to make treatment recommendations.
Although the literature seems to suggest that psychotherapy (and, potentially, specifically CBT) should be the treatment of choice for patients with DSs, there is no uniform, recommended care pathway for these patients in the UK. 71 Furthermore, the availability of psychiatric and psychological services for the assessment, diagnosis and management of DSs is highly variable, despite the potential impact of DSs on patients, their families and society. 70 Mayor et al. 71 found that 15% of HCPs who responded to their survey indicated that they had nowhere to refer their patients; only one-third indicated that they would be able to refer patients with DSs for psychotherapy. Such psychotherapy might be further limited, given that these professionals reported that under half of their patients would be offered at least one psychotherapy session. Thus, although evidence exists for the benefit of psychotherapeutic input, the lack of an evidence-based care pathway and of evidence from larger robust treatment trials means that many patients may not be funded by their Clinical Commissioning Groups to receive psychotherapy. In addition, the geographical distribution of the patients means that in outside specialist centres there may be limited knowledge or willingness to enable these patients to be seen, resulting in inequalities in healthcare provision. A stronger evidence base would provide the basis on which policy changes to the service provision for DS patients could be made. This need is highlighted by the National Institute for Health and Care Excellence86 and the Scottish Intercollegiate Guidelines Network,87 which indicate the need for psychiatric and psychological input for DS patients. In addition, the International League Against Epilepsy,88 US National Institutes of Health89 and the National Institute for Neurological Disorders and Stroke90 have all identified the need to develop effective methods for treating DSs.
Summary and methodological rationale
The CODES trial was designed to evaluate the clinical effectiveness and cost-effectiveness of CBT for DSs within a care pathway involving neurology, neuropsychiatry and psychotherapy. It offers a potential template for future services and the commissioning of DS treatment. In addition, it can form the basis for the more extensive training of therapists to work with patients with DSs and support the importance of psychiatrists in treating this patient group, who often present with complex mental health needs. 70 Preliminary evidence of efficacy had been obtained through a proof of principle RCT1 and, therefore, this study was the next step in examining the clinical effectiveness and cost-effectiveness and generalisability of CBT as an intervention for DSs through a pragmatic, adequately powered, multicentre RCT.
Research objectives
The main aim of this research, as determined by the NIHR HTA programme commissioning brief, was to evaluate the effectiveness of a psychological intervention (DS-specific CBT) plus standardised medical care (SMC) (i.e. CBT + SMC) compared with SMC alone in improving DS control and a range of psychosocial outcomes and in reducing health service use and costs.
The primary objective was to assess the effectiveness of CBT + SMC compared with SMC alone in reducing monthly DS frequency at 12 months post randomisation.
Secondary objectives were to assess the effectiveness of CBT + SMC compared with SMC alone at 12 months post randomisation in relation to:
-
reductions in DS severity
-
improvements in seizure freedom, psychosocial and psychological well-being and HRQoL
-
participants’ global clinical improvement [Clinical Global Impression of Change (CGI)]
-
participants’ satisfaction with treatment
-
reductions in health service use
-
cost-effectiveness of CBT + SMC compared with SMC alone.
In addition, we sought to characterise:
-
patients’ subjective experiences of either the CBT or SMC treatment
-
subjective experiences of HCPs (neurologists, psychiatrists and CBT therapists) when delivering SMC or CBT (as relevant)
-
treatment fidelity of the manualised CBT treatment and the implications for roll-out in the NHS.
The completion of the CODES trial involved a number of stages in accordance with Medical Research Council guidelines,91 and these included our earlier work. 1,81,82 We refined our CBT manual and patient handouts based on our experience from our previous RCT. 1 We then trained CBT therapists) to deliver the intervention. We also developed our protocol and materials for neurologists and psychiatrists involved in diagnosing and treating patients in the study, with service user (SU) input into patient materials, and trained the research workers who would assess participants. At an early stage in the project we developed and published both our protocol for this complex intervention study84 and our statistical analysis plan (SAP)92 prior to undertaking any data analysis. We described the participants initially recruited to the study in neurology/specialist epilepsy services and then those participating in the RCT. We undertook an analysis of the fidelity with which the CBT was delivered, and conducted process analyses in the form of in-depth qualitative interviews with patients in both treatment arms. We also explored the experiences of neurologists, psychiatrists and CBT therapists delivering care during the study. We collected and analysed clinical effectiveness and cost-effectiveness follow-up data for both trial arms, with a view to disseminating the results and implications of the analyses once the study had been completed.
Chapter 2 Trial design and methods
Patient and public involvement
We incorporated patient and public involvement (PPI) throughout the course of the study, with the aim of including PPI at the stages of study design, management and dissemination.
When we were developing the project in response to the HTA commissioned call, we presented our research questions to and consulted with the local NIHR Biomedical Research Centre (BRC) Service User Research Enterprise Advisory Group (SUAG) and SUs with DSs from our clinical service at the South London and Maudsley NHS Foundation Trust. Information was also sought via a postal/online survey, for which we contacted SUs from the SUAG and our clinical service. SUs confirmed the importance of this research.
Suggestions made by SUs with DSs were used to guide how we explained the study to potential recruits and the methods used to facilitate retention in the study. These included, for example, regular reminders to record seizure frequency; updates on study progress; a project team member to contact to discuss attendance difficulties; reimbursing transport and parking costs; flexible use of paper/electronic methods to collect seizure data; limiting the length of outcome measure completion time; offering a voucher for follow-up data completion; and providing feedback for participants at the study end as part of dissemination. SUs commented on and informed our choice of outcome measures. We consulted further with SUs with DSs prior to the submission of a full application and to guide our responses to feedback/queries from the HTA Board. SUs provided feedback on the information leaflets to be provided in the neurology and psychiatry clinics.
Subsequently, we identified four people (one from the SUAG and three SU representatives), three of whom had a diagnosis of DSs. Two became members of our Trial Management Group (TMG) and two joined our Trial Steering Committee (TSC). We provided a training session in June 2014 for all four people as the study commenced. This was facilitated by a former staff member from Epilepsy Action (Leeds, UK), which is a user-led organisation that had an active training programme and had committed to offering such training.
The chief investigator and the trial manager also held an interim face-to-face meeting with one SU representative from each committee in September 2017 to review their experiences in the study committees and to consider the challenges that they felt in participating in the study and how we might address these. The PPI representatives on each committee received all relevant trial paperwork and were given their own standing agenda item where they could comment on trial matters if they had not already done so as part of the meeting. They provided feedback on relevant paperwork and, early in the project, on our trial website. The trial manager acted as the main point of contact and would discuss with them the agenda and arising issues before or after the meetings, as appropriate.
Our SU representatives have taken an active role in advising on study progress and on means to improve follow-up rates, and supported our earlier need to extend recruitment and follow-up periods. They have made contributions to dissemination and commented on our communications with participants about study progress. They have advised on the wording of study outputs (e.g. papers and feedback to study participants) and two are co-authors on this report. We have been very fortunate to maintain the active input of all four individuals who initially joined our TMG and TSC (allowing for any times when their own circumstances made this difficult), and we feel that their willingness to give clear opinions on what we have been doing has benefited the study considerably.
We have also shared our experience of PPI within our institution. One of the SU members of the TSC worked with the CODES trial manager and a research associate in the NIHR Maudsley BRC to develop new guidelines for SU involvement on steering and advisory committees for clinical trials and other research projects. These are now available for researchers on the NIHR Maudsley BRC website (www.maudsleybrc.nihr.ac.uk/patients-public/support-for-researchers/involvement-guidelines/; accessed 10 January 2021). This SU member also gave a presentation to the NIHR BRC’s SUAG about her experience on the CODES TSC to encourage other SU representatives to become involved in committees for future clinical trials.
Study design
The CODES study consisted of two phases: an observational screening phase that lasted approximately 3 months after patients had initially been recruited in neurology/specialist epilepsy clinics and then, for eligible and willing patients, an intervention phase, namely a multicentre, pragmatic, two-arm RCT with assessor (research worker and statistician) blinding. The observational phase was to allow for patients who experienced rapid spontaneous remission following diagnosis, as well as to allow time for psychiatric assessments to be scheduled. Patients with DSs can show early remission after communication of the diagnosis alone,93 and we did not want to confound our evaluations by including people whose DSs remitted quickly. Participants were randomised at the beginning of the intervention phase using a 1 : 1 ratio, stratified by site, into two treatment arms. One treatment arm consisted of CBT plus SMC (CBT + SMC) and the other treatment arm consisted of SMC alone. SMC was standardised across the trial and was not simply the treatment that would usually be delivered at a particular centre. Although some demographic data were collected at the initial recruitment into the screening phase, further measures were collected at baseline (prior to randomisation) and at two follow-up assessments (at 6 and 12 months post randomisation).
Trial approval and monitoring
The trial was approved by London – Camberwell St Giles Research Ethics Committee (REC) (REC reference 13/LO/1595). It was also approved by local research and development departments at each NHS trust. The trial was monitored by the TSC and the Data Monitoring and Ethics Committee (DMEC). Both committees met at least once per year and at most twice per year during trial set-up, recruitment and follow-up. The DMEC monitored all serious adverse events (SAEs) within the RCT as they were reported. The TMG (comprising the chief investigator, co-investigators, PPI representatives and the junior statistician and junior health economist) met at regular intervals during the study to review ongoing progress and other relevant issues such as funding and dissemination.
Study settings and care pathway adopted in the study
The trial was run from the Institute of Psychiatry, Psychology and Neuroscience, King’s College London, with the chief investigator, trial manager and some research workers based at this location. Research workers were also located at the University of Edinburgh and the University of Sheffield.
Our study design was based on a care pathway incorporating neurology and liaison/neuropsychiatry settings. After an initial assessment with a neurologist who established the diagnosis of DSs and provided the patient with an explanation for their DSs, the patient was referred to a psychiatrist, who carried out a detailed clinical assessment. The purpose of this was to review the diagnosis, establish a formulation of the patient’s problems, determine the existence of any psychiatric comorbidities and establish eligibility for the RCT. In some cases, pharmacological treatment of anxiety and/or depression was considered. We decided that the psychiatrists would be best placed to provide SMC follow-up sessions given that, in many settings, neurologists discharge patients following DS diagnosis, although SMC provision by neurologists was not proscribed. Most sites that were involved provided only neurology or only psychiatry input, but a small number of sites had both services within the same NHS trust (Table 1). Where sites comprised neurology services only, they referred patients on to designated psychiatry services, either following normal commissioning routes or following agreement for the study.
| Site location | Study phase | |
|---|---|---|
| Screening: neurology service | Intervention: psychiatry service | |
| Barts Health NHS Trust | ✗ | |
| Brighton and Sussex University Hospitals NHS Trust | ✗ | |
| Cambridge University Hospitals NHS Foundation Trust | ✗ | |
| Chesterfield Royal Hospital NHS Foundation Trust | ✗ | |
| Croydon Health Services NHS Trust | ✗ | |
| Dartford and Gravesham NHS Trust | ✗ | |
| East Kent Hospitals University NHS Foundation Trust | ✗ | |
| East Sussex Healthcare NHS Trust | ✗ | |
| Guy’s and St Thomas’ NHS Foundation Trust | ✗ | |
| Imperial College Healthcare NHS Trust | ✗ | |
| King’s College Hospital NHS Foundation Trust | ✗ | |
| Leeds Teaching Hospitals NHS Trust | ✗ | |
| Lewisham and Greenwich NHS Trust | ✗ | |
| Maidstone and Tunbridge Wells NHS Trust | ✗ | |
| Medway NHS Foundation Trust | ✗ | |
| Newcastle upon Tyne Hospitals NHS Foundation Trust | ✗ | |
| Royal Berkshire NHS Foundation Trust | ✗ | |
| Sheffield Teaching Hospitals NHS Foundation Trust | ✗ | |
| St George’s University Hospitals NHS Foundation Trust | ✗ | |
| University Hospital Birmingham NHS Foundation Trust | ✗ | |
| Western Sussex Hospitals NHS Foundation Trust | ✗ | |
| Berkshire Healthcare NHS Foundation Trust | ✗ | |
| Cambridgeshire and Peterborough NHS Foundation Trust | ✗ | |
| Derbyshire Community Health Services NHS Foundation Trust | a | |
| Derbyshire Healthcare NHS Foundation Trust | ✗ | |
| East London NHS Foundation Trust | ✗ | |
| Kent and Medway NHS and Social Care Partnership Trust | ✗ | |
| Leeds and York Partnership NHS Foundation Trust | ✗ | |
| Northumberland, Tyne and Wear NHS Foundation Trust | ✗ | |
| Sheffield Health and Social Care NHS Foundation Trust | ✗ | |
| South London and Maudsley NHS Foundation Trust | ✗ | |
| South West London and St George’s Mental Health NHS Trust | ✗ | |
| Sussex Partnership NHS Foundation Trust | ✗ | |
| West London Mental Health NHS Trust | ✗ | |
| Birmingham and Solihull Mental Health NHS Foundation Trust | ✗ | ✗ |
| Cardiff and Vale University Health Board | ✗ | ✗ |
| NHS Lothian | ✗ | ✗ |
| Royal Free London NHS Foundation Trust | ✗ | b |
| University College London Hospitals NHS Foundation Trust | ✗ | ✗ |
| University Hospital Southampton NHS Foundation Trust | ✗ | ✗ |
Screening and recruitment
Eligibility for the trial was ascertained at two stages. Participants were initially consented into the screening phase on the basis that the RCT was to be conducted on patients whose seizures persisted beyond diagnosis, and we judged this in relation to receipt of diagnosis by the neurologists. At the time of trial set-up, research indicated that only ≈ 14% of DS patients were likely to achieve seizure freedom 3 months after diagnosis. 93 Sufficient time before entering the RCT was, therefore, built into the protocol to ensure that patients continued to experience DSs before being randomised to a treatment arm within the RCT, as well as to allow sufficient time for appointments to be arranged with psychiatrists.
Screening phase
Participants were initially identified in neurology/specialist epilepsy outpatient clinics. Neurologists would make and explain the DS diagnosis (see Neurologists’ delivery of standardised medical care) and give the patient a booklet with further information about their condition (downloadable from www.codestrial.org/information-booklets/4579871164; accessed 10 January 2021). A protocol was developed for this process, as follows. If patients met the eligibility criteria and were interested in participating, the patient consented to the neurologist forwarding their contact details to the CODES team. A research worker would then contact the participant, explain the trial in more depth and cover the material in the participant information sheet (see Report Supplementary Material 1). If the patient was interested in proceeding, the research worker confirmed eligibility and obtained informed consent. This was carried out mostly in person but occasionally by post. Basic demographic information and a very brief medical history pertaining to the patient’s condition were obtained, and the patient was instructed how to keep seizure diaries. Patients were referred to the designated liaison/neuropsychiatry service by the neurologist. During this intervening period, a research worker contacted the participant fortnightly by telephone, text message or e-mail (based on participants’ preferences) to obtain seizure diary data, comprising the number of seizures experienced per week and how many were severe.
Intervention phase
A liaison or neuropsychiatrist assessed the patient approximately 3 months after their initial neurology diagnosis. This appointment was used in part to undertake further screening for eligibility for the RCT. The appointments included a reiteration of diagnostic points, provision of a more in-depth booklet about DSs (downloadable from www.codestrial.org/information-booklets/4579871164; accessed 10 January 2021) and a detailed clinical psychiatric assessment. A guide was written for the psychiatrist to facilitate consistency and good communication. If the patient met the eligibility criteria and was interested in participating in the RCT, with the patient’s consent, the psychiatrist informed the research worker, who then contacted the participant to explain the RCT further. If the participant still wished to participate, the research worker met with them and covered the material in the participant information sheet for the RCT (see Report Supplementary Material 2). The research worker confirmed eligibility, obtained informed consent and completed the baseline assessments with the patient. Participants were then randomised to one of the two treatment conditions. Enrolled patients were asked to consent to the research team contacting a carer/informant who could provide their own perspective on the participant’s DS frequency at the follow-up stages. If they agreed to this, carers/informants received a participant information sheet (see Report Supplementary Material 3) and, if they agreed to participate, they completed a consent form either at a face-to-face meeting or by post.
Eligibility criteria
Inclusion criteria (screening phase)
-
Adults aged at least 18 years old who had experienced DSs within the previous 8-week period and whose diagnosis was corroborated by either video EEG or if not available, clinical consensus (see Neurologists’ delivery of standardised medical care).
-
No recorded history of intellectual disability.
-
Had the ability to keep seizure diaries and fill out questionnaires.
-
Showed readiness to keep seizure diaries on a regular basis and attend a psychiatric assessment 3 months following receipt of their DS diagnosis in the study.
-
Were able to provide informed consent.
Exclusion criteria (screening phase)
-
A diagnosis of currently occurring epileptic seizures in addition to DSs (‘current’ is characterised as an epileptic seizure occurring in the prior year).
-
Lacking the ability to independently maintain seizure records or fill out questionnaires.
-
Met criteria for current alcohol or drug dependency in line with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)94 criteria since this might, among other things, make attendance and symptom reporting less reliable.
-
Insufficient fluency in English to complete questionnaires or later undergo CBT without an interpreter.
-
Currently undergoing CBT for another diagnosis, if this intervention would still be ongoing by the time the psychiatry assessment takes place.
-
Having previously had a CBT-based treatment for DSs at one of the trial participating centres.
Inclusion criteria for randomised controlled trial (intervention phase)
-
Adults aged at least 18 years old who had been recruited into the study in the screening phase following their diagnosis.
-
Indicated willingness to continue filling out seizure diaries and complete questionnaires.
-
Had given the research team data about their seizure occurrence on a regular basis since receiving their diagnosis of DSs in the screening phase.
-
Indicated that if they were allocated to CBT, they would be willing to attend weekly or biweekly therapy sessions.
-
Both the participant and their clinician believed that randomisation was acceptable.
-
Ability to provide written informed consent.
Exclusion criteria for randomised controlled trial (intervention phase)
-
Was currently experiencing epileptic seizures in addition to DSs.
-
DS had not been experienced during the 8-week period leading up to the psychiatry assessment.
-
Had previously had a CBT-based intervention for DSs at one of the centres taking part in the RCT.
-
Was currently undergoing a CBT intervention for another condition.
-
Was experiencing active psychosis.
-
Met criteria for current alcohol or drug dependence according to DSM-IV criteria since, in addition to making symptom recording and session attendance less reliable, it might be used to reduce anxiety and would reduce the impact of exposure during CBT, and have possible impact on patients’ memory for sessions.
-
Evidence of current use of benzodiazepines that exceeded the equivalent dose of 10 mg of diazepam per day, for reasons similar to those for alcohol or drug dependence.
-
Was at high risk of imminent self-harm, following the psychiatry assessment or according to the results of the structured psychiatric assessment [the Mini-International Neuropsychiatric Interview (M.I.N.I.)] administered by the research worker, subsequently followed up by a discussion with the relevant psychiatrist.
-
Had received a diagnosis of factitious disorder.
Randomisation
Randomisation took place following participants’ consent and completion of baseline assessments. Participants were randomised in a 1 : 1 ratio between the SMC-alone treatment arm and the CBT + SMC treatment arm, using randomly varying block sizes and stratified by site. This was intended to ensure a 1 : 1 allocation in each location in which patients were recruited. Participants were enrolled by research workers and randomised using the online randomisation system at the King’s Clinical Trials Unit (CTU) at the Institute of Psychiatry, Psychology and Neuroscience. For each participant, the relevant research worker entered participant information into the randomisation system that then generated confirmation of randomisation e-mails. Research workers received blinded confirmation and the trial manager and chief investigator received unblinded confirmation with the participants’ treatment allocation. The chief investigator was unblinded for practical and administrative reasons.
Blinding and protection from bias
To protect from bias, research workers collecting outcome data and the trial statisticians were kept blind to participant treatment allocation throughout the trial. In addition to the chief investigator, the trial manager was unblinded so that they could contact participants to inform them of their treatment allocation and inform therapists about which participants to contact to arrange CBT appointments. Participants were asked not to inform research workers of their treatment allocation when completing follow-up assessments. If participants had a treatment-related question, they would contact the trial manager. To evaluate whether or not the research workers remained blind, they completed a ‘treatment guess’ form at the 12-month follow-up or point of withdrawal. If for any reason a research worker became unblinded to a participant’s treatment allocation, they reported this to the trial manager so that the outcome assessments could be completed by a blinded research worker. Participating clinicians and patients were not blinded to treatment allocations.
Interventions
The intervention was DS-specific CBT plus SMC, or SMC alone. SMC is described below from initial diagnosis with the neurologist to the end of the trial and, as both trial arms received this, it is described first.
Control intervention
Across the UK, medical and psychological care for DSs is variable, with different specialties contributing in specific ways. 71 To produce a broadly consistent treatment environment across the trial, key approaches were employed to create what we termed ‘standardised medical care’ for patients with DSs. This included providing briefing sessions to the clinicians (e.g. at site initiation visits), a detailed leaflet about how they might explain the diagnosis to patients, crib sheets containing the essential information that they should provide to patients during sessions (see, for example, Appendix 2 for the crib sheet for psychiatrists) and sets of frequently asked questions for clinicians providing SMC (see Report Supplementary Material 4–8 for the remainder of these materials). These are techniques that have been found to be acceptable in other studies. 93 It was intended that SMC would be provided by both neurologists and psychiatrists, and would start from the initial meeting with the neurologist at diagnosis. There was no mandatory number of SMC sessions; however, after the initial neurology and psychiatry assessment, we estimated that there would be up to two SMC sessions with the neurologist and three to four sessions with the psychiatrist. However, owing to local service procedures and clinical need, we could not be prescriptive about this.
One important component of SMC was the provision of information. We created two information booklets about DSs to be given at different stages of SMC to supplement the information given to patients by their medical clinicians. These booklets were devised by the clinical members of the project team, with input from SUs with DSs and a hospital information officer. The two booklets were:
-
Dissociative seizures factsheet (neurology). This was to be given to patients by their neurologists when the diagnosis of DSs was first communicated. It was also downloadable from www.codestrial.org/information-booklets/4579871164 (accessed 10 January 2021).
-
Dissociative seizures factsheet (psychiatry). This included content from the neurology leaflet but also provided more extensive information that could help provide further details relevant to psychiatric assessment and treatment. This was to be given to patients when they attended their psychiatric assessment. It has subsequently been made available at www.codestrial.org/information-booklets/4579871164 (accessed 10 January 2021).
When research workers contacted patients about potential participation in the study phases, they asked whether or not these factsheets had been provided; if not, they ensured that participants received the relevant factsheet.
Neurologists’ delivery of standardised medical care
The key elements of SMC provided by neurologists included making a firm diagnosis and explaining it, giving the patient the factsheet on DSs and referring the patient to the study psychiatrist. When making the diagnosis of DSs, neurologists were asked to undertake their usual assessments to clarify the nature of their patient’s seizure disorder to establish the diagnosis. We acknowledged that neurologists may sometimes make the patient’s diagnosis based on clinical history, physical assessment and information provided by informants. In some cases, this had been supplemented by mobile phone recordings of seizures but also by EEG or video encephalographic data. Although video EEG is the ‘gold standard’ for diagnosing DSs, yielding least diagnostic uncertainty,17 we acknowledged that this was not always readily available to a clinical service or deemed cost-effective when other clinical information led to a high level of diagnostic certainty. Thus, video EEG was not essential in the study. If video EEG was not undertaken, a diagnostic consensus was required either between two neurologists in the patient’s clinical service pathway or between the patient’s neurologist and a neurologist in the study team, who reviewed the patient’s clinical records and all relevant investigations. We anticipated that neuroimaging would be conducted only when clinically necessary.
Neurologists were asked to explain the disorder to the patient using the guidelines provided in a crib sheet (see Report Supplementary Material 4 and 6) and explained in detail elsewhere. 84 A key aspect of the neurologist’s role at this stage was to explain the referral to psychiatry and why this was appropriate, including the potential benefits of being seen by a psychiatrist. 84 Additional information given to patients by the neurologists that was likely to be tailored to the individual included explaining (1) that anti-epileptic drugs (AEDs) are not effective in treating DSs; (2) that talking therapies might be helpful, although there is currently insufficient evidence and this is why the trial was being undertaken; (3) the disorder to significant others, and how to best respond to the DSs; and (4) driving regulations. They might also possibly discuss distraction techniques in general. Although neurologists were not expected to undertake the equivalent of a psychiatric assessment, if risks relating to self-harm, harm of others or psychosis were identified they were expected to refer patients to relevant services or instruct the patient’s general practitioner (GP) to do so if necessary.
After the initial diagnosis session, it was recommended that neurologists offer a minimum of one follow-up appointment at which any of the following might be included:
-
assessment of patient progress
-
reviewing the patient’s understanding of their diagnosis and, if necessary, going through this again
-
if appropriate, supervising the withdrawal of AEDs
-
managing comorbid physical disorders that required interventions
-
re-evaluating major psychiatric risks that might require interventions
-
consideration of the value of prescribing antidepressant or anti-anxiety medication where this was indicated on clinical grounds
-
completing any forms required by government departments if required.
However, given service and clinical limitations, this follow-up did not always occur.
Psychiatrists’ delivery of standardised medical care
The psychiatrists’ delivery of SMC was scheduled to begin with a clinical psychiatric assessment approximately 3 months following the neurological assessment and diagnosis. Where patients did not attend the first scheduled appointment, attempts were made to reschedule appoints as often as possible, allowing for service regulations regarding non-attendances and discharge.
This pre-randomisation assessment was intended to perform a partly educational function and cover several important aspects. Psychiatrists were asked to follow specific communication guidelines (see Report Supplementary Material 5 and Appendix 2); restate the points covered by the neurologist to reinforce and further explain the diagnosis; provide patients with a more detailed booklet on DSs (as indicated above); and acknowledge any fears that patients might have about being given a psychiatric diagnosis. The assessment would include a clinical assessment of relevant Axis I and Axis II psychiatric diagnoses and an assessment for risks related to self-harm and suicide; active suicidality would require exclusion from the trial and urgent treatment. It was anticipated that psychiatrists would explain and treat any other psychiatric or other functional somatic symptoms (using psychopharmacological approaches or referral to physiotherapy where relevant), and discuss any factors of possible aetiological significance that were elicited from the clinical history. Other components could include:
-
providing information concerning DS warning symptoms and the possibility of distraction without providing specific interventional techniques
-
liaising with other mental health clinicians involved in the provision of the patient’s care without referring for psychotherapy, instead focusing on psychoeducation and management of comorbid psychiatric conditions, as would normally be undertaken
-
encouraging the person to engage in or resume social activities and/or return to college/work where relevant, and liaising with the appropriate settings to facilitate this
-
involving family or friends in these areas as appropriate
-
completing forms for government departments as necessary.
Further follow-up appointments with the psychiatrist were intended to include general review and support, considering any psychiatric comorbidities and pharmacological treatment according to clinical need. Psychiatrists were instructed that no additional CBT techniques should be employed during the delivery of SMC.
Cognitive–behavioural therapy for dissociative seizures
Cognitive–behavioural interventions have generally now been shown to have positive benefits for a range of medically unexplained symptoms, as reported in a number of systematic reviews;78,95–99 however, these are not specifically studies of DSs and have not adopted specific models to underpin treatment of DSs. Although mechanisms of change have been studied in adults with medically unexplained symptoms,100 this work has not included adults with DSs. Recent conceptualisations of factors giving rise to DSs have, nonetheless, been developed and include an integrative cognitive model that seeks to explain DSs as automatic activations of a central representation of seizures (referred to as a seizure scaffold) in the context of dysfunction of inhibitory processing. 101 The seizure representation may be shaped by different factors that are relevant to seizures in the person’s life and yet further factors, such as chronic stress and arousal, may compromise adequate inhibitory processing. This and other conceptualisations of DSs (e.g. ‘panic without panic’43) offer the possibility of employing cognitive–behavioural interventions to address DS occurrence.
However, although acknowledging the richness of relatively recent models,101 the development of our CBT approach, which predates such models, stems from a single case study81 and was further refined in an open-label study82 and then in our pilot RCT. 1 A further description of the approach is given elsewhere. 83 A finding of increased symptoms of autonomic arousal as relevant to the concept of ‘panic without panic’43 is not in conflict with the approach taken in these studies.
Our cognitive–behavioural model incorporated the fear escape–avoidance model. 102,103 We considered DSs to represent a dissociative response to heightened arousal accompanying cognitive/emotional/physiological or environmental cues that may or may not be associated with previous/current distressing or life-threatening experiences. Alternatively, DSs may have occurred after events, such as panic attacks or syncope. All of these events may previously have led to unbearable feelings of distress and/or fear.
The treatment broadly consisted of engagement and rationale giving; helping the patient develop and use seizure control techniques; helping the person reduce avoidance behaviours via exposure; helping the person tackle maladaptive cognitions associated with seizure occurrence and facilitate emotional processing; dealing with trauma; and planning for relapse prevention. The key elements of the conceptualisation of the disorder and the treatment elements incorporated in the approach used here are illustrated in Figure 1.
FIGURE 1.
Model showing responses associated with DSs and the CBT techniques used to target these in our DS-specific CBT. ABC, antecedents, behaviours, consequences.

Cognitive–behavioural therapy was intended to be delivered in 12 sessions (plus one booster session) informed by a treatment manual. This number of sessions was based on our previous work1,82 that we were seeking to extend here; in our 2010 pilot RCT1 that provided preliminary evidence for efficacy, not all patients attended both booster sessions so, for easier implementation for the therapists, we limited this to one booster session. The DS-specific CBT was designed to help facilitate the patient to:
-
develop an understanding of their seizure disorder
-
develop an understanding of how cognitive, emotional, physiological and behavioural aspects of their DSs are related
-
understand factors that led to the persistence of their DSs
-
learn how to prevent DSs by interrupting behaviours, cognitions or physiological responses occurring before or at the start of their DSs
-
improve their lifestyle by undertaking previously avoided activities
-
address thought patterns and attributions about their disorder that act to maintain their DSs
-
address and deal with previous traumas, poor mood, anxiety or reduced self-esteem, where relevant
-
increase their levels of independence and help them to comprehend the contribution of significant others to their disorder.
The CBT sessions included usual CBT components, such as session agendas and planning and reviewing homework activities, but also required patients to complete seizure diaries and undertake activities designed to help with seizure control. Although treatment was manualised, there was room for flexibility, allowing individual formulations. Participants receiving CBT were given a booklet (the ‘Manual for Patients Attending CBT’) that contained written material to supplement the therapy sessions and included pages for making notes. The titles of the individual topics covered in this manual were Introduction to cognitive behaviour therapy and dissociative seizures; A guide for other people; Distraction and re-focusing techniques; Progressive muscle relaxation exercises; Breathing exercises; Graded exposure; Trauma in the context of dissociative seizures; Identifying negative automatic thoughts; Alternatives to negative thoughts; Preparing for the future; and Discharge plan.
Intervention training
Before treating any patients, CBT therapists who had been identified as potentially delivering therapy to trial patients attended a 3-day workshop. The therapists were provided with information relating to the administration and running of the trial, including reporting any SAEs. We asked therapists to report suicidal ideation, suicide attempts and new deliberate self-harm. The workshop also included teaching on DSs and dissociation; a cognitive–behavioural model of DSs; eliciting information about DS occurrence/triggers/perpetuating factors; coping behaviours and avoidance; how to convey the rationale for treatment and engagement of patients; how to deal with DSs occurring in sessions; developing seizure control techniques; using graded exposure to deal with avoided activities; challenging unhelpful thoughts; dealing with trauma and facilitating emotional processing; and ending therapy.
Teaching was supplemented by Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) slides and academic papers relating to the content of the treatment and our group’s prior work in this area. Skills specific to DSs were role-played. Three sets of workshops were held: 5–7 November 2014, 14–16 January 2015 and 14–16 October 2015. A total of 59 therapists attended these workshops. The first workshop was video-recorded in its entirety; the videos and accompanying Microsoft PowerPoint slides were edited to be viewable in manageable sections and were made available to all therapists via secure internet links as a means of reviewing the course content. This material was also made available to four additional therapists who joined later at different times during the study and for whom it was not possible to arrange full workshops. They watched the videos and had discussion sessions with the chief investigator, the lead for the intervention (Trudie Chalder) and the trial manager.
During the trial, therapists received group (and occasionally individual) supervision every 4–6 weeks. The supervisors were three senior therapists who were experienced in delivering the treatment model to patients with DSs (median experience 10 years; range 10–30 years). We expected that therapists would receive general service-related supervision in their workplace and supervision specifically related to trial patients from trial supervisors. Part of this supervision involved using recordings of therapy sessions to provide feedback to therapists and to ensure that they were adhering to the treatment model. A therapist rating scale based on the University College London CBT Competency Framework104 was used to rate one session from the initial patient for each therapist (focusing on the treatment rationale). The supervisors scored the therapists and fed back whether or not they were within the predetermined competency levels on the scale. Regular supervision allowed therapist competence and adherence to the manualised therapy to be monitored throughout the trial.
Intervention delivery
The CBT was planned to be delivered as an outpatient service at clinical centres, although occasional telephone sessions were utilised where necessary; we did not set out to evaluate the impact of telephone sessions on outcome, but telephone delivery has been shown to be feasible and effective in other disorders. 105,106 Session delivery was recorded in therapy logs. It was intended that the 12 sessions of CBT would be scheduled to occur over 4–5 months, with a further booster session being offered at approximately 9 months after randomisation. Therapists recorded and monitored attendance; reasons for rescheduling and non-attendances; disruption of therapy or injuries owing to DSs; and participants’ completion of homework and adherence to the therapeutic model on a session-by-session basis in a therapy log. We collected demographic details of those therapists who delivered the therapy. Each therapist was allocated a therapist identification number via the MACRO randomisation system (MACRO electronic data capture system, version 4, Elsevier) that was used on the therapy logs to ensure anonymity.
The treatment manual given to therapists was used as a guide, as treatment needed to be individually tailored to recognise that not all elements may be applicable to all participants and different participants may progress through the stages at different speeds or, if necessary, in a different order. The manual outlined what could potentially be covered within each session.
Completion of follow-ups
Follow-up collections of measures were conducted at 6 and 12 months post randomisation. The 6-month follow-up, undertaken to maintain participant involvement in the study and to improve data modelling, was usually conducted by post, and the 12-month follow-up was generally completed in person; a flexible approach was employed to ensure retention and ease for the participant. Efforts were made to minimise dropout rates. A large part of this was the regular contact attempted with participants throughout the trial; we also used procedures that have improved response rates when posting questionnaires, such as enclosing a personalised letter, providing a Freepost envelope for its return and using colour printing for the 6-month questionnaire packs. 107 Participants were contacted before the 6-month follow-up to inform them that packs would be arriving by post. The 12-month follow-ups were scheduled well in advance of the required time point wherever possible. Researchers also phoned participants to confirm receipt of posted questionnaire packs and to offer assistance completing the questionnaires. Participants received a £10 ‘Thank you’ shopping voucher for completing the 6-month follow-up questionnaires and a £15 shopping voucher for completing the 12-month follow-up questionnaires.
Remuneration
In addition to the ‘thank you’ shopping vouchers for completing the follow-up measures, we offered participants a maximum of £25 towards the cost of travel to the initial psychiatric assessment, towards attendance at each CBT session and towards any travel incurred in the follow-up (i.e. data collection) assessments.
Adverse event reporting and serious deterioration in health
Information regarding adverse events (AEs) that may have arisen during the intervention was collected over the entire 12 months following randomisation. If a participant indicated a change to health status, the research worker, research nurse or clinical studies officer would ask for further information to determine if the event met the criteria for an AE. Although we may have been made aware of AEs throughout participants’ time in the study, given the frequent contact with participants owing to seizure diary collection, research workers specifically asked at the 6- and 12-month follow-ups about changes in health and these were all self-reported by the participant.
An AE was defined as any health event reported by a participant that was a change from baseline but did not fulfil the criteria for a SAE. This included events where the participant consulted their GP or another medical advisor or took medication. Any AEs that were fatal, life-threatening or disabling, required hospitalisation or prolongation of hospitalisation, jeopardised the participant in a way that may result in one of the above outcomes without medical or surgical intervention or were new episodes of deliberate self-harm or suicidal ideation or a suicide attempt were classed as SAEs.
Seizures were specifically excluded from AE reporting, except where other criteria, such as hospitalisation, were met. Clinicians also reported AEs. During the treatment phase of the trial, any event reported in CBT sessions or during a SMC session was ultimately reported by the SMC doctor. This was to ensure accurate reporting, but also to maintain the blinding of the research workers and the statisticians by removing mentions of CBT. All AEs and SAEs were reported to the trial manager. They were reviewed by the chief investigator and the DMEC. The latter reviewed all SAEs in the treatment phase individually, but remotely, and they were unblinded to treatment arm after reviewing the incident.
To provide an independent assessment of AEs/SAEs in the study, we recruited three clinicians who were experienced in working with patients with DSs to review the AEs; they were initially blinded to treatment allocation. The three clinicians (two consultant neurologists and one consultant psychiatrist who had not been involved in recruiting or treating any study patients) were sent spreadsheets of accounts of entirely anonymised AEs, including information about events, the phase of the study in which these events had occurred, the body system involved and whether or not these events were DS related. They were asked to judge whether or not these met the criteria for SAEs and to rate the severity (mild, moderate or severe) of each event. For both AEs and SAEs, the raters were sent information about the treatment allocation and the initial assessment of relatedness to the intervention. They were then asked to rate the AEs/SAEs for relatedness to the treatment intervention. In undertaking the ratings, the independent clinicians were asked to consider whether or not the event met the protocol definition of ‘serious’ because, if not, the event would be classified as an AE. They were also asked to consider whether or not any SAEs needed to be upgraded to serious adverse reactions (SARs)/suspected unexpected serious adverse reactions (SUSARs) and whether or not any SARs/SUSARs needed to be downgraded to SAEs. Majority decisions were adopted with discussion if there was disagreement over the ratings of severity and relatedness.
We defined a serious deterioration in health as the occurrence of any of the following outcomes: (1) a decrease of 20 points [i.e. a drop by two standard deviations (SDs)] in the Short Form questionnaire-12 items, version 2 (SF-12v2), Physical Component Summary score between baseline and both the 6-month and the 12-month follow-up assessments; (2) participant-rated scores of ‘much worse’ or ‘very much worse’ on the CGI scale; or (3) a SAR.
Evaluation of treatment fidelity
With participants’ consent, CBT sessions were recorded using high-quality digital voice recorders and uploaded remotely onto a secure password-controlled audio-upload system housed by King’s CTU. Recordings were then deleted from the voice recorders following successful upload.
We had initially anticipated that around 15 therapists would treat the study patients. 84 Eventually, given local service differences in how therapists participated in the RCT and the overall increase in the number of participants (see Summary of changes to the project protocol), 39 CBT therapists were allocated patients in the RCT. Thus, the independent raters were asked to evaluate one session from each therapist from whom we had usable recordings (not all patients gave their consent to record sessions and some recordings were not uploaded owing to recording errors). We finally had usable recordings from 36 therapists. For each therapist, we chose one patient at random (where the therapist had treated more than one patient) and then chose, on a pseudorandom basis, one of two randomly selected sessions (the third or seventh session) to be rated by each independent rater, so that an equal number of the two sessions were assessed. Sessions were stratified according to whether they had occurred earlier or later in the trial’s progression. If the specific session was missing and at least one other patient’s recordings existed for that therapist, we looked to rate the same session from either the other patient or another randomly selected patient seen by that therapist.
Two independent and experienced CBT practitioners undertook ratings of the integrity of treatment delivery from a sample of these recordings. They were asked to rate the extent to which specific CBT skills were used in the context of working with patients with DSs; whether or not therapists adhered to the therapy, as described in the treatment manual, and were seen to be delivering CBT; and the quality of therapeutic alliance. The individual items rated for each selected therapy session are shown in Appendix 3.
An initial training session was held with the two raters, the chief investigator, the trial manager and Trudie Chalder. The raters listened to and rated a randomly selected session (not included in the main rating exercise), discussed the items and clarified their meanings with the other team members. The ratings were then piloted on four randomly selected sessions and a discussion of the ratings was held to further identify difficulties in ratings and further clarify the meaning of individual items to improve the clarity of coding rules, and achieve rating scores from each rater that fell within one scale point of each other. Further discussions were held after sets of 10 recordings to enable recalibration and to prevent rater drift; discussions were held when item scores differed by more than one scale point.
Raters were blind to the identity of the patient, the treating HCP and the trial outcome. Ratings were made independently; raters were asked to avoid using mid scores as far as possible. Scores were then converted to standardised scores out of 100. For the single-item subscales (i.e. overall therapist adherence, therapeutic alliance and overall CBT delivery), scores were calculated by dividing the score by 7 and multiplying by 100. For the specific DS skills scale (i.e. DS skill items 3–6 in Appendix 3), scores were totalled and then divided by [7 × number of relevant (‘yes’ rated) items scored] × 100 to generate standardised scores.
Summary of changes to the project protocol
At an early stage, we sought ethics approval to complete initial consents into the screening phase of the study by post or telephone if it was difficult to arrange face-to-face visits. At the 6-month follow-up assessments occurring early in the study, it became apparent that not all of the participants were willing to complete the questionnaire pack and return it by post or complete it by telephone; thus, we obtained approval to offer face-to-face data completion. Similarly, we gained approval for completion of 12-month follow-ups by post or telephone where it proved difficult to arrange a face-to-face appointment with the participant.
As the study progressed, we found that our clinical colleagues requested clarification of certain inclusion and exclusion criteria.
In particular, clinicians in the different services asked for further clarity over the length of DS freedom before patients became ineligible for the first phase of the study. We therefore changed our first inclusion criterion to indicate that the person should have been having DSs in the previous 8 weeks. In addition, it was considered more appropriate to exclude patients from the first phase of the study if it was already known that they had previously experienced a CBT-based treatment for DSs at a trial participating centre, rather than applying this criterion immediately prior to consenting to the second phase of the study. Similarly, it was considered more appropriate to exclude patients from the first phase of the study if they were known to be undergoing CBT for another condition (unless this would have finished by the time of the psychiatric assessment, when eligibility for the RCT would be considered).
Finally, we amended a previous exclusion criterion concerning the patient being thought to be at ‘imminent risk of self-harm, after (neuro)psychiatric assessment or structured psychiatric assessment by the research worker with the M.I.N.I.’; in practice, if the psychiatrist felt that the patient was at imminent risk of self-harm the patient would not be considered to be eligible and so would not be assessed on the M.I.N.I. by the research worker, so both conditions would not occur. Instead, we changed this criterion to read ‘the patient is thought to be at imminent risk of self-harm, after (neuro)psychiatric assessment or structured psychiatric assessment by the research worker with the M.I.N.I., followed by consultation with the psychiatrist’. In this way, if the patient reported a high risk of self-harm on the M.I.N.I. to the research worker, the research worker would then consult the psychiatrist about the patient’s suitability for the study.
In the early stages of the 6-month follow-up data collection, it appeared that follow-up rates might be lower than estimated. We, therefore, sought HTA and ethics approval to extend our sample sizes in both stages of the study. This was also necessary because, although we had anticipated that approximately 60% of patients in the screening phase would subsequently enter the RCT, the figure consistently hovered around 51%. We gained permission to recruit 698 (rather than 501) participants into the screening phase to take into account a lower than expected rate of participants progressing from phase 1 to phase 2 (≈51% instead of ≈60%) and to randomise 356 (rather than 298) into the RCT to allow for the initially larger loss to follow-up at 6 months than expected (a conservative estimate of ≈30% rather than the expected ≈17%).
We initially intended that our nested qualitative study would focus on participants in the RCT only. With ethics approval, we extended this qualitative work to include a sample of CBT therapists delivering therapy in the RCT as well as a sample of liaison/neuropsychiatrists delivering SMC. We also obtained approval to undertake an online survey of the neurologists participating in the study.
Although we had considered obtaining Hospital Episode Statistics (HES) data for not only baseline but the full post-randomisation period84 (and as suggested in our initial trial registration on ClinicalTrials.gov), we subsequently obtained ethics approval to obtain these data for baseline and for only the last 6 months of the follow-up period for practical reasons (these data are reported in Chapter 4).
Measures
Clinical and demographic information
We collected the following clinical and demographic information: date of birth, gender, ethnicity, living arrangements, marital status, dependants, attained qualifications, employment status, receipt of disability benefit, previous epilepsy diagnosis, prescription of AEDs, age at first seizure and whether or not the person had previously sought medical help for a mental health concern. Postcodes were collected from all participants to derive an Index of Multiple Deprivation (IMD), which provides a score that is indicative of the level of deprivation in a specific area. The separate databases for England (IMD), Scotland (Scottish IMD) and Wales (Welsh IMD) were published in different years and were based on slightly differing numbers of domains used to derive the scores. We used the versions in use at the time of the first recruitment into the study108–110 and, for consistency, we used the same versions throughout the study. We allocated IMD scores to quintiles ordered across the three databases so that the lowest quintile reflected the least deprivation.
At recruitment to the screening phase, participants rated how strongly they believed that they had been given the correct diagnosis (0 = not at all, 10 = extremely strongly). At recruitment into the intervention phase, self-report information was collected on other medical conditions with which participants were currently diagnosed, along with their treatment preference for CBT + SMC or SMC alone or whether they had no preference. In addition, expectation of the outcome of treatment was assessed by questions on how logical treatment seemed and how confident they were that the treatment would help them when considering CBT, being seen by a neurologist and being seen by a psychiatrist (see Appendix 4).
As well as demographic data, at recruitment into the intervention stage psychiatric comorbidities were assessed using a structured screening instrument (the Mini-International Neuropsychiatric Interview; M.I.N.I. v6.0)111 and a screening measure of personality [the Standardised Assessment of Personality Abbreviated Scale, Self-Report (SAPAS-SR)]. 112 We included a measure of personality in the light of accounts of personality disorder or personality clusters in people with DSs49,57 and to allow potential future examination of the effect of personality in moderating outcome.
The M.I.N.I. is a commonly used, structured, psychiatric diagnostic interview and is divided into modules that correspond to diagnostic categories including major depressive episode; suicidality; manic and hypomanic episodes; panic disorder; agoraphobia; social phobia; obsessive–compulsive disorder; PTSD; alcohol dependence/abuse; substance dependence/abuse; psychotic disorders and mood disorder with psychotic features; anorexia nervosa; bulimia nervosa; generalised anxiety disorder; and antisocial personality disorder. Research staff who administered the M.I.N.I. attended a 1-day training event that was led by one of the psychiatrists in the project team (Nick Medford). In addition to didactic teaching, role plays were held. Follow-up consultations with Nick Medford were arranged to address administration/scoring difficulties.
The eight-item SAPAS-SR poses questions about how the person sees themselves. ‘Yes’ responses are scored as 1 and ‘no’ responses are scored as 0, giving a final score between 0 and 8 with a cut-off score of 4 for the presence of personality disorder. It has high test–retest reliability; the cut-off score of 4 demonstrates a sensitivity of 0.83 and specificity of 0.8112 when identifying the presence of personality disorder. As we did not then undertake formal assessment of personality disorder for those with scores of ≥ 4, we interpret these scores as indicative of maladaptive personality traits rather than frank personality disorder.
Primary outcome measure
The primary outcome measure used in the trial was self-reported monthly DS frequency at 12 months post randomisation. This enabled us to include all participants’ outcomes irrespective of their improvement or otherwise during the trial. DSs were recorded by patients in a seizure diary that was collected by research workers every 2 weeks, using the format most acceptable to the participant (e.g. via paper diary, text or e-mail). In addition, participants were asked about seizure occurrence at baseline, 6 months and 12 months by a single-item measure for which they were asked to write down how many seizures they had experienced in the previous 4 weeks. The gold standard measure for the primary outcome was to calculate it as the sum of four seizure diary entries for weeks 49–52 (post randomisation). Where these data were not available, full details about constructing the primary outcome can be found in Appendix 5.
Secondary outcome measures
A number of measures were used to assess secondary outcomes. These are grouped together with measures of the same general psychological or other process in the following sections.
Seizure experiences
Secondary outcomes that were directly related to DSs measured seizure severity, seizure reduction and seizure freedom. Two items on the Seizure Severity Scale113 measured subjective severity of DSs and how bothersome DSs were judged to be. Seizure reduction was measured from seizure diaries and self-report questions at baseline, 6 months and 12 months, as appropriate. Seizure freedom was measured in two ways. First, we determined the presence of seizure freedom in the penultimate 3 months of the study. Second, we examined the longest duration of seizure freedom between the 6-month and 12-month follow-up points. We also determined whether or not there was a > 50% reduction in DS frequency at 12 months. To obtain a further measure of seizure reduction, participants were asked to consent to our asking an informant to rate the difference in the participant’s seizures since the beginning of the trial.
Health-related quality of life
Health-related quality of life was assessed by the SF-12v2 Health Survey114 that provides two overall physical and mental health summary scores: the Physical Component Summary and the Mental Component Summary. Both have high internal consistency reliability. 115 Higher scores indicate better HRQoL. The scores are norm-based T-scores and are constructed to have a mean value of 50 points and a SD of 10 points. Scoring was undertaken using QualityMetric Health Outcomes™ Scoring Software 4.5 (QualityMetric Incorporated, Lincoln, RI, USA). We also measured HRQoL using the visual analogue scale (VAS), measuring current health from the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),116 on a scale of 0–100 (where higher scores represent better health). While the five domains measured by the EQ-5D-5L were used for the health economics analysis (see Chapter 4), we adopted the VAS as a clinical secondary outcome as it was readily quantifiable and meaningful.
Psychosocial functioning
Psychosocial function was measured by the Work and Social Adjustment Scale (WSAS),117 a five-item self-report scale that was used here to measure patients’ perceptions of the functional impact of DSs on their lives in terms of work; home management; social leisure and private leisure activities; family; and other relationships. 117 Scores of > 20 are considered to represent moderately severe impairment or greater; scores between 10 and 20 represent significant functional impairment, but less severe clinical symptomatology; and scores of < 10 are thought to represent subclinical populations. 117
Psychiatric symptoms, psychological distress and somatic symptom burden
Four self-report questionnaires were used to investigate psychiatric symptoms, psychological distress and somatic symptom burden: Generalised Anxiety Disorder-7 (GAD-7),118 Patient Health Questionnaire-9 (PHQ-9),119 the Clinical Outcomes in Routine Evaluation-10 (CORE-10)120 and the modified Patient Health Questionnaire-15 (PHQ-15). 121,122
The GAD-7 is a seven-item scale that is used for screening generalised anxiety and assessing its severity. It has good reliability,118 as well as criterion, construct, factorial and procedural validity. Higher scores indicate higher levels of anxiety. Scores of ≥ 10 indicate cases of generalised anxiety disorder. 118
The PHQ-9 is a nine-item scale that is used to measure depression based on DSM-IV criteria. It can be used to make criteria-based diagnosis of depressive disorders and measure depression severity. It has high internal reliability and test–retest reliability. 119 Scores of ≥ 10 indicate a diagnosis of depressive disorders.
The CORE-10 is a 10-item general measure of psychological distress. 120 Scores of ≥ 11 are in the clinical range for distress. The CORE-10 has good internal consistency and correlates well with other measures of depression, anxiety and overall mental health. 120
A modified version of the PHQ-15 was used to measure other somatic symptoms experienced by participants. 121 This version measures whether or not patients had ‘been bothered a lot’ by a list of symptoms over the previous month. Symptoms included the 15 most common physical symptoms of patients presenting to primary care (excluding upper respiratory tract infections),123 the 10 most common ‘neurological’ symptoms124 and the five psychological symptoms taken from the PRIME-MD Questionnaire,123 including worrying about a lot of different things; feeling down, depressed or hopeless; and ‘nerves’ or feeling anxious or on edge. Total scores were measured.
Clinical impression of improvement
A scale that was derived from the CGI125 was used as a self-rated global measure of change for participants; it asked them to rate how much they felt that their health had changed since the start of the study, using a seven-point scale from 0 = very much worse to 6 = very much better. This was also completed by clinicians (mainly psychiatrists) at the 12-month follow-up and by the CBT therapists at the end of the 12th treatment session, at which they were asked to rate how much the participant had changed since the start of the study on the same seven-point scale. Because the therapists administered this measure only to the arm receiving CBT + SMC, the therapist rating cannot be evaluated formally as a secondary outcome.
Satisfaction with treatment
Patients rated their satisfaction with treatment via a single-item measure on a seven-point scale from 0 = very dissatisfied to 6 = very satisfied.
Mediators and moderators
A number of other measures [Beliefs about Emotions Scale,126 a locally devised measure of avoidance behaviour (Avoidance of People, Places and Situations) and a measure of belief in the diagnosis of DSs and belief in having been given the correct treatment] were identified as potential mediators or moderators of outcome rather than main secondary outcomes. However, our current analysis was designed around our identified secondary outcomes, so these other variables will instead be dealt with in secondary analyses to be documented elsewhere.
The timing of the administration of the measures above is summarised in Table 2.
Health economics
These measures are described in Chapter 4.
| Variable | Standardised measure or how data were collected | Time point | ||
|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||
| Seizure frequency | Seizure diary and self-report | ✗ | ✗ | ✗ |
| Seizure experience | Seizure severity and bothersomeness | ✗ | ✗ | ✗ |
| Longest period of seizure freedom in the last 6 months | ✗ | ✗ | ||
| Seizure freedom for last 3 months of study | ✗ | |||
| > 50% reduction in seizure frequency | ✗ | ✗ | ||
| Informants’ rating of patients’ seizures | ✗ | ✗ | ||
| HRQoL | SF-12v2 | ✗ | ✗ | ✗ |
| EQ-5D-5L VAS | ✗ | ✗ | ✗ | |
| Psychosocial functioning | Work and Social Adjustment scale | ✗ | ✗ | ✗ |
| Psychiatric symptoms, psychological distress and somatic symptom burden | GAD-7 | ✗ | ✗ | ✗ |
| PHQ-9 | ✗ | ✗ | ✗ | |
| CORE-10 | ✗ | ✗ | ✗ | |
| Modified PHQ-15 | ✗ | ✗ | ✗ | |
| Clinical impression of improvement | CGI (self-reported by patients) | ✗ | ✗ | |
| CGI rated by the patient’s SMC clinician (neurologist or psychiatrist) | ✗ | |||
| Satisfaction with treatment | Single item measuring patient’s satisfaction with their treatment | ✗ | ✗ | |
Nested qualitative studies and survey of neurologists
As part of attempting to gain further insights into reasons for treatment outcomes and to identify issues that may be relevant to the roll-out of interventions, we undertook a mixed-methods approach and conducted three nested qualitative studies to understand patient participants’ experiences of CBT and SMC, the experiences of psychiatrists delivering SMC and the experiences of CBT therapists delivering the psychological intervention in the study, with a view to triangulating the findings. The study methods and results from these nested studies are presented in Chapter 5. We also undertook an online survey of neurologists participating in the study to understand further the impact of CODES on their practice and their evaluation of the care pathway and treatment elements employed in the study. Methods and results are similarly presented in Chapter 5.
Data management
Data were collected on paper source data worksheets and were transferred to an online data collection system for clinical trials (MACRO), which was hosted on a dedicated server at King’s College London and managed by the King’s CTU. Source data worksheets were kept in a research office with restricted access in locked filing cabinets. Research workers entered data and the trial manager performed data entry checks on a minimum of 30% of all data and on up to 50% of specific measures.
The trial statistician received blinded data extracts from MACRO and the randomisation system throughout the trial (e.g. to compile DMEC reports) and ran systematic data queries (such as flagging up discrepancies and missing values) that were sent to the trial manager, who resolved them by either referring to source data or contacting sites. This data checking process was repeated prior to the database lock in an iterative process until the data were deemed ‘clean’ and ready for analysis. Once the data were locked, they were sent to the trial statistician and were imported, labelled, scored and reshaped in Stata® (StataCorp LP, College Station, TX, USA). While the trial statistician undertook this process for the clinical outcome data, checking of the health economics data was undertaken by the junior health economist in the team who then analysed those data.
Participants’ contact information was kept on a secure central network server with access granted to study staff only. All computers used were password protected and held in an office with restricted access. Audio-recordings of CBT sessions were uploaded from digital voice-recorders at individual sites to a bespoke audio-upload system that was devised and housed by King’s CTU; although recordings could be listened to by specific people via password-controlled access, downloading recordings was not possible.
Power calculations and sample size
The sample size calculation was based on the results from our pilot RCT,1 which was the largest study at the time comparing CBT and standard medical care in a comparable patient population. We reported a large, standardised effect size of Cohen’s d = 0.75 for reduction in seizure frequency in the arm that received CBT [plus standard medical (i.e. neuropsychiatric) care] compared with standard medical care alone at the time point corresponding to the end of CBT treatment, after controlling for pre-randomisation seizure frequency. We also reported a moderate effect size after a further 6 months (Cohen’s d = 0.42).
In other studies of CBT-based psychotherapy for functional symptoms, a moderate effect size is common. 127,128 For this reason, it was decided to base the power calculation on detecting a more conservative moderate effect size, comparable to those found in other CBT-based interventions with patients with functional symptoms.
Initial calculations suggested that to detect an effect size of d = 0.42 with 90% power at the 5% significance level, using a two-sided t-test for logarithmic frequencies, 121 participants per trial arm would be required. However, adjustments had to be made owing to therapist effects, using pre-randomisation seizures as a covariate and rates of attrition. First, calculations of potential therapist effects were based on the assumption of around 15 therapists delivering the CBT, so an intraclass correlation coefficient (ICC) of 0.002 was applied, based on a typical therapist ICC. 129 This increased the sample size to 149 participants per treatment arm to achieve 92.6% power (using the cluspower command in Stata, allowing for clustering in only one trial arm). Second, because the use of pre-randomisation seizures as a covariate increases the precision of intervention effect estimates, a deflation factor of 0.83 was applied. This was based on a correlation between pre-randomisation DS frequency and follow-up DS frequency of r = 0.42. 1 Finally, we needed to account for attrition. We reported an 11% loss to follow-up in the pilot RCT;1 however, a more conservative attrition rate of 17% at the 12-month follow-up time point was decided on for the trial. The final sample size was, therefore, 149 participants per arm: 298 participants in total.
To obtain the target sample of 298 randomised participants, a larger number of patients was required from which to recruit into the RCT. To calculate the number of participants to be recruited into the screening phase, several factors had to be considered. One factor was that eligible participants might not wish to be randomised; from data collected in the pilot RCT,1 this was predicted to be approximately 30%. This meant that 426 participants who were still having DSs would need to be seen by psychiatrists. This number represents 85% of those initially diagnosed with DSs, as some participants may be seizure free at 3 months post diagnosis (approximately 15%93). An estimation of approximately 25% of newly diagnosed eligible patients with DSs was made to account for those who might decline to participate in the study at the point of diagnosis. This led to a final target sample size of 698 eligible patients diagnosed with DSs from whom to recruit and identifying a total pool of 1108 newly diagnosed DS patients. As noted earlier, these values were subsequently revised when the trial was under way.
Trial outcome analysis
The analyses followed the SAP that was agreed by the TSC and was published before database lock. 92
We stated in our protocol paper84 (page 8) and in the update in which we published our SAP92 (page 2) that both the primary outcome measure (seizure frequency) and the secondary outcome measures would be evaluated at 12 months post randomisation. In addition, our ethics-approved protocol made it clear that our outcome variables were to be evaluated at 12 months post randomisation. Although we appreciate that there may have been some lack of clarity in the initial completion of the trial registration, which we have subsequently tried to rectify, and in our protocol paper,84 in terms of distinguishing outcome variable measurements from trial end points, this was not meant to indicate evaluation of 6-month variables as trial outcomes. Our current analysis is clearly based on the pre-trial intention to evaluate outcome variables only at 12 months and not also at 6 months post randomisation.
Significance testing or construction of CIs for the difference between the trial arms at baseline were not carried out, as per the SAP. This is because randomisation of participants to intervention arms should have ensured that any imbalance over all measured and unmeasured baseline characteristics was because of chance. 130 The formal statistical analyses estimated the differences in relevant summaries (incidence rates, means and proportions) between patients randomised to CBT + SMC and patients randomised to SMC alone with an intention-to-treat (ITT) approach, that is all those with data were analysed in groups as randomised irrespective of treatment received.
Generalisability of the trial sample
To assess whether or not our trial sample of participants (n = 368) was similar to those who were eligible for the trial but did not go on to be randomised (n = 58), we compared key characteristics measured after consent to screening. Variables were chosen for this comparison if they might predict outcome in patients with DSs, that is to check that our randomised sample of 368 participants did not differ from the 58 participants in factors that might later be relevant to the outcome in the RCT. Factors that may predict outcome in patients with DSs more generally include symptom duration, receipt of social security benefits, having previous psychiatric diagnoses, employment status, educational achievement and gender (see Chapter 1). Although we tested a wider range of variables to encompass age, gender, social relationships, diagnostic method, predominant DS semiology, previous diagnosis of epilepsy, current prescription of AEDs, belief in diagnosis of DS and previous medical help-seeking for a mental health problem, to compare these subgroups more widely we did not compare the two subgroups on all of our demographic variables to avoid excessive testing. We employed Wilcoxon’s rank-sum test and Fisher’s exact tests for continuous and categorical variables, respectively. The variables incorporated in such comparisons are indicated in Chapter 3, Tables 6–8.
Descriptive statistics
In Chapter 3, descriptive statistics for all baseline, primary and secondary outcomes are reported by trial arm and overall for all of the time points at which data were collected (baseline, 6 months and 12 months); a formal analysis of a treatment difference was conducted for outcomes at 12 months only. Medians [interquartile range (IQR)] are used to describe count outcomes (seizure frequency or seizure freedom) owing to potential skewness, means (SD) are used to describe all other continuous outcomes, and frequencies (%) are used to report categorical and binary outcomes. All totals (n) are reported. One of the secondary outcomes (informants’ rating of patient’s seizures at 12 months post randomisation) could not be formally analysed because it was completed by only a small proportion (7.3%) of participants.
Predictors of loss to follow-up and the multivariate imputation by chained equations procedure
Prior to unblinding the trial statisticians, a binary variable was created to indicate whether or not participants provided complete 12-month follow-up data for the primary outcome (1 = provided primary outcome data, 0 = did not provide primary outcome data). This binary variable was sent to an independent King’s CTU-affiliated statistician along with the CODES therapy database; the independent statistician then created a second binary variable to indicate treatment compliance. As per the SAP, this was defined as attending at least nine sessions of CBT if allocated to the CBT + SMC arm (1 = nine or more CBT sessions attended, 0 = fewer than nine CBT sessions attended). This number of sessions was chosen to be consistent with our pilot RCT. 1 The independent statistician then ran a chi-squared test to assess whether or not treatment compliance within the intervention arm was predictive of missing 12-month primary outcome data. The chi-squared test confirmed this association to be statistically significant (p < 0.001): 94% (131/140) of participants in the intervention arm who were compliant with CBT provided primary outcome data at 12 months, compared with 54% (25/46) of those who were non-compliant. Therefore, as described in the TSC-agreed and published SAP,92 multivariate imputation via chained equations (MICE) was deemed appropriate and necessary for the main analysis to produce inferences valid under the detected missing-at-random (MAR) data-generating process. This meant that missing outcome variables at all time points were imputed instead of using the mixed-modelling approach originally suggested in the brief (pre SAP) analysis section of the protocol. 84 Multiple imputation (MI) consists of an imputation step and an analysis step. First, missing values in specified variables are multiply imputed. Second, an analysis model is fitted to each imputed data set and the analysis results are combined using Rubin’s rules. 131 This two-stage procedure provides inferences that are valid under a MAR missing data mechanism. Importantly, as defined by the imputation model, the observed variables are allowed to drive missingness at 12 months.
To inform the imputation model, logistic regression methods were used to detect which baseline variables were associated with missing follow-up at 12 months in each randomisation stratum (sites). Almost all baseline variables were included in this process, with just a few exceptions for the following reasons: (1) if measured only in small subgroups (IMD quintile Wales, type of dependant, type of carer and status of previous epilepsy diagnosis); (2) if small subgroups were merged to reduce the chance of perfect prediction (ethnicity was grouped into white, black or other; relationship status was dichotomised into married or living with partner vs. single or other; binary variables were used for ‘any current M.I.N.I. diagnosis’ and ‘any previous M.I.N.I. diagnosis’); or (3) if measured more than once (continuous score of SAPAS-SR was used, so the binary version was not included).
First, unadjusted logistic regression was implemented, with the binary follow-up variable as the outcome and the baseline measures were each tested separately. Seven variables were found to be significantly associated at the α < 0.05 level: modified PHQ-15 score (p = 0.002), at least one current M.I.N.I. diagnosis (p = 0.003), number of days seizure free in the last 6 months (p = 0.005), having a carer (p = 0.007), relationship status (p = 0.023), previously sought help for a mental health problem (p = 0.035) and SF-12v2 Mental Component Summary score (p = 0.047).
Second, manual forward stepwise regression was used, with a liberal inclusion threshold of α < 0.1. The process was as follows: a logistic regression on binary follow-up was run fixing psychiatrist site as a covariate because randomisation stratified on this; each of the seven variables above, found to be univariately associated with missing primary outcome data, was then included one by one in a step-wise fashion; all of the p-values were compared and the variable with the lowest p-value (if p < 0.1) was added. This process was repeated until no further variables could be added (i.e. p > 0.1).
The variables that were found to be associated with loss to follow-up (within sites) were number of somatic symptoms on the modified PHQ-15 – those with fewer symptoms were more likely to provide primary outcome data (17 vs. 19.7 median symptoms; p = 0.0030); carer – those with a carer were more likely than those without a carer to provide primary outcome data (91.3% vs. 80.8%; p = 0.0021); relationship status – those who were single were more likely than those married/living with partner to provide primary outcome data (89.6% vs. 81.0%; p = 0.0078); number of days seizure free in the last 6 months in the study – those with longer periods of time without a seizure were more likely to provide primary outcome data (9.5 vs. 7 median days; p = 0.0078); and previously sought help for a mental health problem – those who had never sought help were more likely than those who had to provide primary outcome data (90.6% vs. 82.2%; p = 0.037).
Once the baseline predictors of missingness had been established, the MICE procedure132 to impute missing values for each outcome could continue. We generated 100 imputed data sets for each outcome and combined the analysis results according to Rubin’s rules,131 where each imputed data set was analysed according to the corresponding analysis model.
The primary outcome imputation model included dummy variables for treatment compliance in the CBT + SMC arm and included the five baseline predictors of dropout, as above. All dummy variables were coded 0 and 1. The imputation model also included all variables of the analysis model because MI theory requires all variables of the analysis model to be included in the imputation model. Any previous (or ‘auxiliary’) measures of the primary outcome (e.g. baseline and 6 months) were included to ensure that the observed values of the same outcome measured at other time points could contribute to the prediction of missing values in the MICE procedure. Unless complete, all auxiliary variables were imputed. For the primary outcome, incomplete auxiliary variables were 6-month seizure frequency, longest period of time seizure free in the last 6 months and baseline modified PHQ-15 score.
The three count variables (baseline and 6-month seizure frequency, and days of seizure freedom) were log-transformed prior to the MI step (plus 1 to avoid logging zero). The reason for modelling the longest period of seizure freedom (days) in the last 6 months on the log-scale was because of its inverse relation to the primary outcome and, although it refers to a 6-month period, there is no maximum value owing to its self-reported nature. Baseline and 6-month seizure frequency were also log-transformed because the relationships between them and 12-month seizure frequency are multiplicative, translating into an additive effect of log-counts on the linear prediction scale. Predictive mean matching was used to impute both incomplete count variables because of zero-inflated distributions.
Continuous outcomes were imputed using linear regression. Psychiatry sites were included as random intercepts in the analysis model; therefore, we included dummy variables for fixed effects of psychiatry site in the imputation model. This was acceptable because a fixed-effects model is more general than a random-effects model. To avoid perfect prediction and overparameterisation, some small categories were merged within dummy variables (e.g. one site had only one participant and another had three participants).
Both the imputation and the analysis model assumed a negative binomial distribution because monthly seizure frequency (count) was very overdispersed (i.e. the variance was much greater than the mean). Otherwise, a Poisson model may have been appropriate. Count outcomes are constructed using the number of events (count) over an exposure period (time): for us, this corresponded to the number of seizures over number of days (7, 14, 21 or 28 days from weekly diaries).
For the primary outcome, the analysis model hence included trial arm and baseline monthly seizure frequency as the independent variables for the following reasons, respectively: to formally assess treatment arm difference and because baseline values of the outcome variable are known to be predictive of the post-randomisation outcome. Psychiatry site needed to be conditioned on because it was the randomisation stratification factor, and we chose to model the effects by site-varying random intercepts rather than by fixed effects. This approach allowed us to estimate the treatment effect in the population from which the trial sites are a sample, which provides better generalisability than if we had used a fixed-effects approach in this multicentre trial.
We also considered the random effects for SMC doctors and CBT therapists (in the CBT + SMC arm). SMC doctors (generally psychiatrists) were nested within psychiatry sites, which meant that there was commonly only one or two doctors per site. Therefore, SMC doctor effects were not distinguishable from site effects and were not included. Therapist effects were assessed empirically and, again, no evidence for their existence was found. This was evaluated using complete cases (CCs): a likelihood ratio (LR) test was run for each outcome that compared the log-likelihoods of the models including therapist effects (in the intervention arm) with the models that did not include therapist effects. None of the LR tests was significant at the α < 0.1 level and half of the tests reported a p-value equal to 1.0000 [LR χ2(1) = 0]. For some outcomes, the LR test could not even be run [i.e. p-value = not applicable (N/A)] because the model with the therapist effects could not converge; our interpretation here was that therapist effects were not helping to explain the variability in the outcome. Most therapists were also nested within psychiatry sites (in the CBT + SMC arm only), so this may have influenced the LR tests.
Given the random effects for site, the primary outcome analysis model was a mixed-effects negative binomial model that was fitted using the Stata command ‘menbreg’. The dependent variable (monthly seizure frequency at 12 months) was constructed as a count and exposure period, and the two independent variables were constructed as one dummy variable (CBT + SMC vs. SMC) and one log-transformed continuous variable (baseline monthly seizure frequency). As explained above, random intercepts for psychiatry site were included to account for common site experiences. The inbuilt Stata option ‘cmdok’ was used to allow estimation of the mixed-effects negative binomial model with multiply imputed data. Incidence rate ratios (IRRs) were reported.
All secondary outcomes were analysed following the same approach: using MI to allow for the detected MAR process. For all outcomes, the imputation model included the five predictors of missingness at 12 months; a treatment compliance dummy variable in the CBT + SMC arm; a trial arm dummy variable; psychiatry site dummy variables, and any previous measures of the same variable. A few secondary outcomes were strongly correlated and had different missing patterns, so were imputed simultaneously (i.e. imputations for both outcome variables were generated from a single, larger, imputation model). This was the case for seizure severity and bothersomeness, and self-report and doctor-rated CGI scale score change.
For the one other count outcome, the longest period of time (consecutive days) seizure free in the last 6 months, a mixed-effects negative binomial model (with site-varying random intercepts) was also used. For the secondary outcomes that were treated as continuous, mixed-effects linear regression (with site-varying random intercepts) was used: seizure severity, seizure bothersomeness, Physical Component Summary score (SF-12v2), Mental Component Summary score (SF-12v2), health today (EQ-5D-5L VAS), impact on functioning (WSAS), anxiety (GAD-7), depression (PHQ-9), distress (CORE-10), other somatic symptoms (modified PHQ-15), CGI scale change self-report, CGI scale change doctor rated and satisfaction with treatment. Finally, mixed-effects logistic regression (with site-varying random intercepts) was used for the secondary binary outcomes: seizure freedom in the last 3 months of the study and > 50% reduction in seizure frequency.
Owing to the different scales of the secondary outcomes, all estimated treatment effects were standardised to aid comparisons. For outcomes with a baseline measure, this was calculated by dividing the estimated difference between arms on the original scale by the baseline SD or by the pooled SD of the outcome. None of the p-values was adjusted for multiple testing; therefore, interpretation of statistically significant (p < 0.05) secondary outcomes should be undertaken with caution.
Checking of regression assumptions and multiply imputed data
As written in the SAP,92 regression assumptions were checked for all 17 outcomes to ensure that imputed values looked sensible. First, to check the multiply imputed data, the 100 MI data sets were saved for each outcome and summary statistics were used to compare the imputed values with the observed data; namely, the minimum, mean and maximum values of m = 1 to m = 100 were compared with these summary statistics from the observed sample to check that they were reasonably similar.
Second, a subsample of MI data sets were used to perform MI diagnostics of all count or continuous outcomes: this consisted of every 10th MI data set (m = 10, 20, . . ., 90, 100). Kernel density estimates were plotted for these 10 MI data sets against the observed and completed data sets. This meant that the distributions of the observed, imputed and completed values could be compared graphically. Kernel density plots are similar to histograms in that the y-axis depicts the density function but, compared with a histogram that uses bins (or bars), they plot the distribution using smooth lines [similar to a Locally Weighted Scatterplot Smoothing (LOWESS) curve].
For the primary outcome and other count variables, the reason for using negative binomial models instead of Poisson models was the violation of the assumption that the variance is equal to the mean; for example, for our primary outcome data the mean was 37.6 and the variance was 8611.7. It was not possible to test whether or not each seizure was independent of each other, but it was reasonable to make this assumption.
For all continuous variables, the following regression assumptions were checked:
-
Homoscedasticity of residuals – the constant variance of residuals across all data points on regression line. This is checked by inspecting scatterplots of fitted values versus standardised residuals.
-
Linearity of independent variables – the linear relationship between dependent and independent variables. For those outcomes with a corresponding baseline variable that was adjusted for in the model, this was checked by inspecting scatterplots using Stata’s inbuilt ‘avplot’ command.
-
Normality of residuals – error terms display a normal distribution. This was checked by inspecting box plots of the residuals.
Agreement between auxiliary measures and sensitivity analysis
Given that there were two different methods of recording the primary outcome measure, the diary (which allowed a 1- to 4-week exposure period) and the single measure (which specifically asked about a 4-week period), we tested whether or not there was sufficient agreement between the two variable types at the primary outcome time point (12 months post randomisation). Both versions were log-transformed (after adding 1 to avoid logging 0) so that the agreement could be measured using log-frequencies (i.e. on the log-scale). An ICC was calculated by treating the log-transformed seizure diary (pro rata 4-weekly seizure frequency) as one ‘rater’ and the log-transformed single measure (seizure freedom question) as a second ‘rater’. The Stata command ‘kappaetc’ was used to measure inter-rater reliability.
In addition to calculating the ICC, a sensitivity analysis was run to check whether the size, direction or level of significance of treatment effect was ‘sensitive’ to how the primary outcome was constructed. To do this, the gold standard measure was treated as the only accepted measure for monthly seizure frequency: all participants who had not provided seizure diary data at 12 months but had provided the single measure were treated as missing follow-up. The same MICE procedure was then followed.
Complete-case and complier-average causal effect analysis
As well as the main ITT analysis and the sensitivity analysis, each outcome was analysed with just the CCs, that is without any MI and without adjusting for baseline predictors of missingness. The same analyses models as for the MI were used. This was carried out as a ‘reality check’ to see whether or not the results differed in comparison with those derived from the imputed data; if a difference was found in terms of the statistical significance, direction of effect or size of effect, then it may highlight the bias that has been corrected for by the MICE procedure.
Similarly, to assess the efficacy of the CODES intervention (CBT) in the presence of non-compliance, we ran a complier-average causal effect (CACE) analysis for the primary outcome, as stipulated by the SAP. This analysis models the effect of receiving the intervention (at least nine sessions of CBT) and aims to estimate the efficacy of the trial, rather than the effectiveness, which is estimated by ITT analysis. Treatment receipt regardless of trial arm allocation was used as the explanatory variable of interest. The same imputation step was used as for the main ITT analysis, but this time the log-transformed primary outcome was modelled and an instrumental-variables regression was run using the two-stage least squares estimator.
Chapter 3 Recruitment, intervention delivery and clinical outcomes
In this chapter we will describe recruitment to CODES, the baseline characteristics of our sample, treatment delivery and fidelity, and the clinical outcomes.
Recruitment and sample characteristics
Introduction
Participants were initially identified and then recruited from neurology/specialist epilepsy services in 27 NHS trusts. Of the 698 initially recruited patients, 568 patients attended psychiatry assessments at a neuropsychiatry/liaison psychiatry service in 1 of 17 NHS trusts around 3 months later, and 368 patients were subsequently randomised into the trial. CBT was delivered in 1 of 18 NHS trusts linked to the psychiatry services for the purpose of the study (see Chapter 2 for the list of sites contributing to the different aspects of the study).
Participant flow through the study
Recruitment
Recruitment into the screening phase of the study (i.e. from neurology/specialist epilepsy clinics) ran from October 2014 to February 2017. Randomisation from this pool of patients into the RCT ran from January 2015 to May 2017. As a result of initial concerns regarding loss to follow-up rates, these timelines included an extension of recruitment to both phases of the study by 3 months.
Neurologists recruiting patients for the study were asked to provide information on all patients with DSs attending their clinics. Although this may be an underestimate of the number of DS patients attending neurology/epilepsy clinics, 901 patients were identified whose eligibility details were provided [the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for CODES, part 1; Figure 2]. Recruitment in the liaison/neuropsychiatry settings involved psychiatrists further assessing patients’ eligibility for the RCT. This was undertaken for the 568 patients attending their psychiatric assessment session.
FIGURE 2.
The CONSORT flow diagram: part 1.
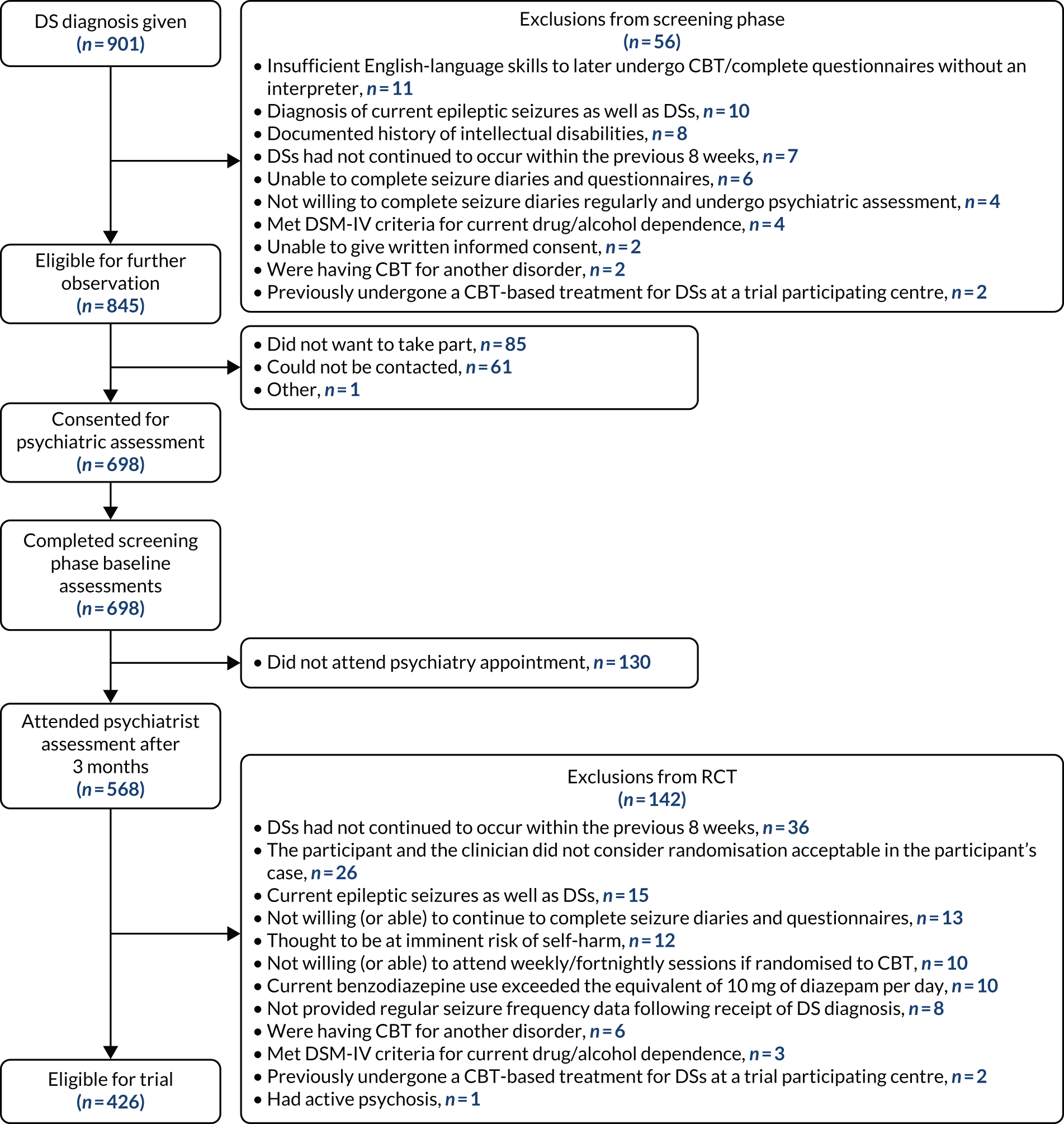
Enrolment
Of the 901 patients identified in neurology settings, 698 were consented to the screening phase of the study (i.e. consented for psychiatric assessment) (see Figure 2). This was the upper limit of the extended recruitment target for this phase of the study and was possible because of a surge in recruitment in the last month of recruitment. In addition to not meeting eligibility criteria (n = 56), as seen in Figure 2, the majority (n = 85) of the 147 patients not enrolled did not want to take part in the study, and 61 patients did not respond to attempts to make contact.
Recruitment into the screening stage occurred across all sites in parallel (Table 3). As anticipated, sites varied considerably in the number of patients recruited; this was, in part, influenced by the number of clinics held at each site.
| Site | Number of participants identified, n (N = 901) | Number of participants consented, n (%) (N = 698) | Date first participant consented | Date last participant consented |
|---|---|---|---|---|
| 1 | 46 | 26 (3.7) | 13 October 2014 | 24 February 2017 |
| 2 | 72 | 51 (7.3) | 17 October 2014 | 24 February 2017 |
| 3 | 26 | 17 (2.4) | 21 October 2014 | 10 January 2017 |
| 4 | 118 | 113 (16.2) | 21 October 2014 | 28 February 2017 |
| 5 | 51 | 32 (4.6) | 27 October 2014 | 24 February 2017 |
| 6 | 21 | 21 (3.0) | 28 October 2014 | 23 November 2016 |
| 7 | 63 | 45 (6.4) | 28 October 2014 | 15 February 2017 |
| 8 | 49 | 42 (6.0) | 1 November 2014 | 16 December 2016 |
| 9 | 22 | 15 (2.1) | 3 November 2014 | 22 February 2016 |
| 10 | 42 | 29 (4.2) | 4 November 2014 | 27 February 2017 |
| 11 | 71 | 55 (7.9) | 4 November 2014 | 20 January 2017 |
| 12 | 68 | 52 (7.4) | 12 November 2014 | 28 February 2017 |
| 13 | 20 | 16 (2.3) | 13 November 2014 | 4 August 2015 |
| 14 | 13 | 13 (1.9) | 1 December 2014 | 23 February 2017 |
| 15 | 18 | 17 (2.4) | 5 December 2014 | 9 December 2016 |
| 16 | 41 | 16 (2.3) | 10 December 2014 | 25 August 2016 |
| 17 | 30 | 25 (3.6) | 12 December 2014 | 28 February 2017 |
| 18 | 18 | 17 (2.4) | 12 December 2014 | 16 December 2016 |
| 19 | 37 | 27 (3.9) | 15 December 2014 | 20 February 2017 |
| 20 | 17 | 17 (2.4) | 24 December 2014 | 26 September 2016 |
| 21 | 7 | 7 (1.0) | 16 January 2015 | 23 November 2016 |
| 22 | 14 | 12 (1.7) | 26 January 2015 | 25 November 2016 |
| 23 | 6 | 6 (0.9) | 3 February 2015 | 13 September 2016 |
| 24 | 8 | 6 (0.9) | 12 February 2015 | 19 January 2017 |
| 25 | 9 | 7 (1.0) | 22 June 2015 | 20 December 2016 |
| 26 | 13 | 13 (1.9) | 29 February 2016 | 21 February 2017 |
| 27 | 1 | 1 (0.1) | 29 March 2016 | 29 March 2016 |
The 27 neurology/specialist epilepsy sites (NHS trusts) have been listed in order of the date that the first participant consented rather than by name to help anonymise data from participants from sites with small numbers of participants (fewer than participants). In alphabetical order, the neurology/specialist epilepsy services were at the following NHS trusts: Barts Health NHS Trust; Birmingham and Solihull Mental Health NHS Foundation Trust; Brighton and Sussex University Hospitals NHS Trust; Cambridge University Hospitals NHS Foundation Trust; Cardiff and Vale University Health Board; Chesterfield Royal Hospital NHS Foundation Trust; Croydon Health Services NHS Trust; Dartford and Gravesham NHS Trust; East Kent Hospitals University NHS Foundation Trust; East Sussex Healthcare NHS Trust; Guy’s and St Thomas’ NHS Foundation Trust; Imperial College Healthcare NHS Trust; King’s College Hospital NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Lewisham and Greenwich NHS Trust; Maidstone and Tunbridge Wells NHS Trust; Medway NHS Foundation Trust; Newcastle upon Tyne Hospitals NHS Foundation Trust; NHS Lothian; Royal Berkshire NHS Foundation Trust; Royal Free London NHS Foundation Trust; Sheffield Teaching Hospitals NHS Foundation Trust; St George’s University Hospitals NHS Foundation Trust; University College London Hospitals NHS Foundation Trust; University Hospital Southampton NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; and Western Sussex Hospitals NHS Foundation Trust.
Once patients consented to the screening phase of the study in the neurology/specialist epilepsy clinics, research workers maintained fortnightly contact with the patients wherever possible to obtain seizure diary data and remind patients about their psychiatry assessment. A total of 130 people did not attend their psychiatric assessment appointment, so could not be considered for the RCT. Of the 568 people who were assessed by a psychiatrist, 426 were considered eligible for the RCT and 368 were consented to the RCT (Figure 3). The number consented to the RCT slightly exceeded our extended target (n = 356); however, we could not ethically refrain from recruiting some rather than other eligible participants. Our recruitment graphs for both phases of the study are shown in Figure 4.
FIGURE 3.
The CONSORT flow diagram: part 2. a, One participant allocated to SMC received CODES CBT by mistake. b, Treatment receipt of CBT (compliance) was defined as receiving at least nine sessions.

FIGURE 4.
Cumulative target vs. achieved recruitment and randomisation. Note that recruitment targets were subsequently revised from November 2016 to a total of 698 in phase 1 with an extended recruitment period to February 2017 and a revised RCT total of 356 (phase 2), similarly extending recruitment by 3 months from February to May 2017.
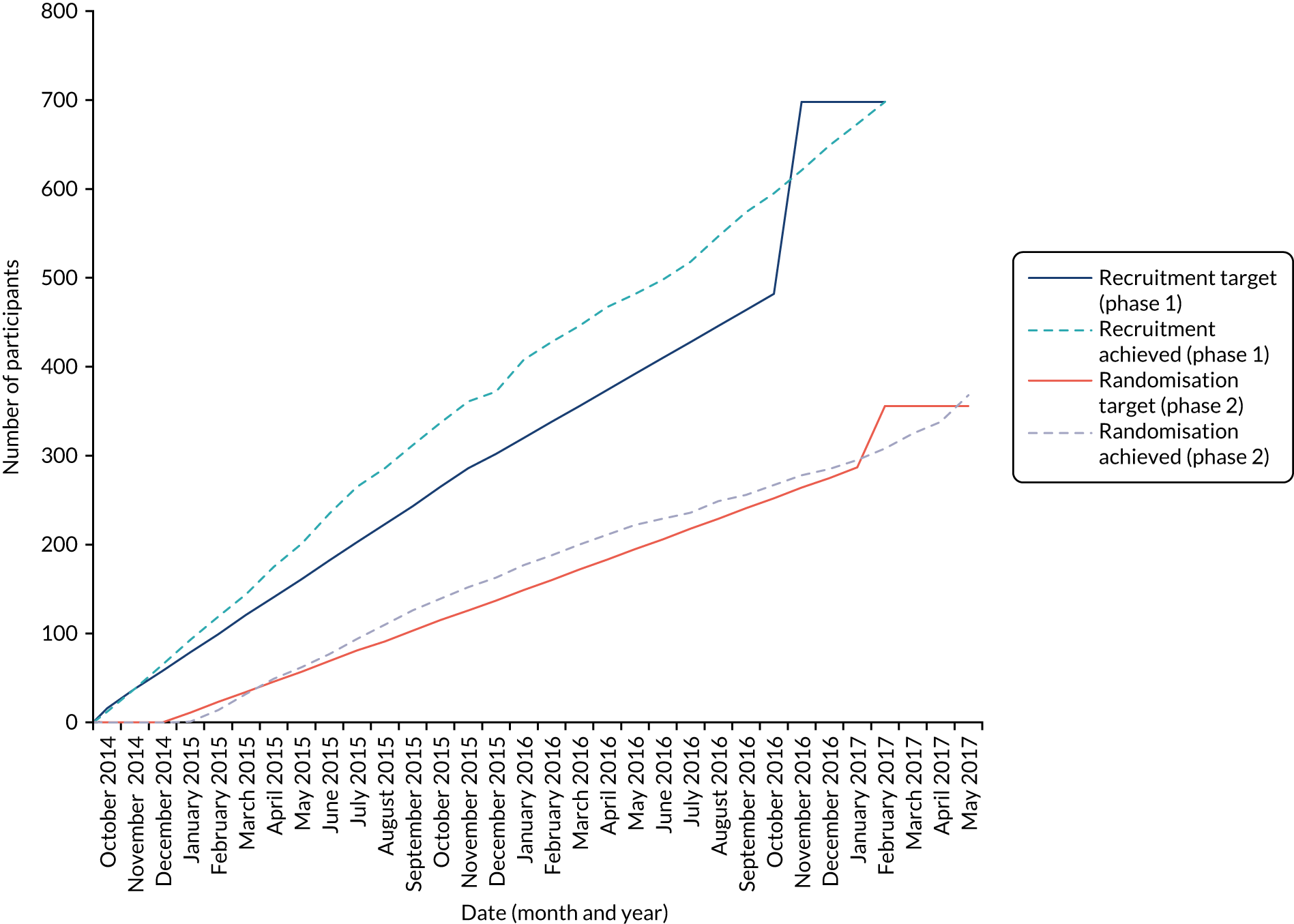
Enrolment into the RCT took place across all sites in parallel and was completed at the end of May 2017 (Table 4).
| Site | Number of participants randomised, n (%) (N = 368) | Date first participant randomised | Date last participant randomised | Date last participant followed up |
|---|---|---|---|---|
| 1 | 58 (15.8) | 16 January 2015 | 30 May 2017 | 25 May 2018 |
| 2 | 12 (3.3) | 10 February 2015 | 31 January 2017 | 9 January 2018 |
| 3 | 30 (8.2) | 10 February 2015 | 30 May 2017 | 12 June 2018 |
| 4 | 15 (4.1) | 23 February 2015 | 1 February 2016 | 20 February 2017 |
| 5 | 77 (20.9) | 23 February 2015 | 31 May 2017 | 26 June 2018 |
| 6 | 48 (13.0) | 4 March 2015 | 17 May 2017 | 2 May 2018 |
| 7 | 24 (6.5) | 6 March 2015 | 18 April 2017 | 12 April 2018 |
| 8 | 16 (4.3) | 9 March 2015 | 30 May 2017 | 8 June 2018 |
| 9 | 18 (4.9) | 17 March 2015 | 10 May 2017 | 24 April 2018 |
| 10 | 15 (4.1) | 23 March 2015 | 17 May 2017 | 24 May 2018 |
| 11 | 19 (5.2) | 25 March 2015 | 30 May 2017 | 4 June 2018 |
| 12 | 9 (2.4) | 16 April 2015 | 2 May 2017 | 18 April 2018 |
| 13 | 7 (1.9) | 30 April 2015 | 2 December 2016 | 23 November 2017 |
| 14 | 6 (1.6) | 6 May 2015 | 9 May 2017 | 9 May 2018 |
| 15 | 3 (0.8) | 18 November 2015 | 28 March 2017 | 11 April 2018 |
| 16 | 1 (0.3) | 22 June 2016 | 22 June 2016 | 16 June 2017 |
| 17 | 10 (2.7) | 12 July 2016 | 31 May 2017 | 7 June 2018 |
The 17 psychiatry sites (NHS trusts) have been listed in order of the date that the first participant was randomised rather than by name to help anonymise data of participants from sites with small numbers of participants (fewer than five participants). In alphabetical order, the psychiatry sites are as follows: Berkshire Healthcare NHS Foundation Trust; Birmingham and Solihull Mental Health Foundation Trust; Cambridgeshire and Peterborough NHS Foundation Trust; Cardiff and Vale University Health Board; Derbyshire Healthcare NHS Foundation Trust; East London NHS Foundation Trust; Kent and Medway NHS and Social Care Partnership Trust; Leeds and York Partnership NHS Foundation Trust; NHS Lothian; Northumberland Tyne and Wear NHS Foundation Trust; Sheffield Health and Social Care NHS Foundation Trust; South London and Maudsley NHS Foundation Trust; Southwest London and St George’s Mental Health NHS Trust; Sussex Partnership NHS Foundation Trust; University College London Hospitals NHS Foundation Trust; University Hospital Southampton NHS Foundation Trust; and West London NHS Trust.
Unblinding
Table 5 reports the data from the study research worker (RW) treatment guess forms to assess whether or not blinding of treatment allocation was successful. Forms were completed at 12 months post randomisation or at participant withdrawal. Forms were available for 342 out of 368 participants. The data in Table 5 imply that blinding was generally successful, with only eight randomised participants being reported to have unblinded RW(s) in some way. Most guesses were completely at random.
| Question | Total, n (%) (N = 342) |
|---|---|
| When research worker completed treatment guess | |
| 12 months post randomisation | 330 (96.5) |
| At participant withdrawal | 12 (3.5) |
| Treatment guess | |
| Strongly think he/she was allocated CBT | 20 (5.8) |
| Think he/she was allocated CBT | 98 (28.6) |
| Think he/she was allocated SMC only | 201 (58.8) |
| Strongly think he/she was allocated SMC only | 23 (6.7) |
| Treatment guess was | |
| Completely random (I may as well have tossed a coin) | 209 (61.1) |
| An educated guess (patient’s clinical condition has influenced my response) | 125 (36.5) |
| Already know (cannot guess as already unblinded) | 8 (2.3) |
To formally test whether or not trial arm predicted type of guess, ‘Strongly think he/she was allocated CBT’ was combined with ‘Think he/she was allocated CBT’, and ‘Strongly think he/she was allocated SMC only’ was combined with ‘Think he/she was allocated SMC only’. A Fisher’s exact test was then run to compare guesses between trial arms and indicated that there was an association between trial arm and RW guess (p < 0.001). For participants allocated to the SMC-alone arm, RWs guessed that the majority were allocated to SMC alone (81.3%), whereas this percentage fell to 49.7% for the CBT + SMC arm. This implies that the RWs may have been able to guess when participants were having CBT to some extent.
Baseline characteristics
We gained clinical and other demographic data at initial recruitment of the 698 participants into the study from neurology clinics, and again prior to randomisation into the RCT for 368 participants. Some data were obtained only once, whereas some data were gained at both time points. Tables 6–8 present clinical and demographic data for those consented to the screening phase (n = 698), those subsequently eligible for the RCT (having attended the psychiatric assessment, n = 426) and those randomly allocated to the two treatment arms (n = 368). Although there were some fluctuations in terms of values across study stages, Tables 6–8 indicate that those randomised into the RCT were very similar to the initially recruited sample (n = 698), and characteristics were similar for those randomised to the two treatment arms.
As described in Chapter 2, key characteristics that were measured after consent to the screening phase were compared using statistical tests between participants in our trial sample (n = 368) and participants who were eligible for the trial but did not go on to be randomised (n = 58). None of the comparisons was significant at the p-value < 0.05 level.
| Characteristic | Consented to screening phase (N = 698) | Subsequently eligible for RCT | Trial arm | ||
|---|---|---|---|---|---|
| Not randomised (N = 58) | Randomised (N = 368) | SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| Age (years), mean (SD); median (IQR) [range]a | 37.1 (14.5); 34.5 (24–48) [18–84]b | 35.8 (14.7); 30 (24–47) [18–73]b | 37.2 (14.3); 35 (24–47.5) [18–78]b | 37.7 (14.5); 35 (25–49) [18–77] | 37.3 (14.2); 35 (25–47) [18–78] |
| Gender, n (%)a,b | |||||
| Female | 515 (73.8) | 48 (82.8) | 266 (72.3) | 126 (69.2) | 140 (75.3) |
| Male | 183 (26.2) | 10 (17.2) | 102 (27.7) | 56 (30.8) | 46 (24.7) |
| Ethnicity, n (%)a,b | |||||
| Total | n = 697 | n = 57 | n = 368 | n = 182 | n = 186 |
| White | 616 (88.4) | 44 (77.2) | 330 (89.7) | 163 (89.6) | 167 (89.8) |
| Asian | 15 (2.2) | 2 (3.5) | 6 (1.6) | 4 (2.2) | 2 (1.1) |
| Black | 14 (2.0) | 2 (3.5) | 6 (1.6) | 1 (0.5) | 5 (2.7) |
| Mixed | 37 (5.3) | 6 (10.5) | 17 (4.6) | 9 (4.9) | 8 (4.3) |
| Other | 15 (2.2) | 3 (5.3) | 9 (2.4) | 5 (2.7) | 4 (2.2) |
| IMD quintile – England (N = 569), n (%) | |||||
| 1 (least deprived) | 57 (10.0)b | 6 (12.0)b | 32 (11.3)b | 15 (10.8) | 16 (11.0) |
| 2 | 66 (11.6)b | 4 (8.0)b | 39 (13.7)b | 18 (12.9) | 19 (13.1) |
| 3 | 104 (18.3)b | 7 (14.0)b | 52 (18.3)b | 22 (15.8) | 32 (22.1) |
| 4 | 178 (31.3)b | 17 (34.0)b | 83 (29.2)b | 40 (28.8) | 40 (27.6) |
| 5 (most deprived) | 163 (28.7)b | 16 (32.0)b | 78 (27.5)b | 44 (31.7) | 38 (26.2) |
| IMD quintile – Scotland (N = 113), n (%) | |||||
| 1 (least deprived) | 15 (13.3)b | 2 (28.6)b | 10 (13.0)b | 6 (15.4) | 4 (10.5) |
| 2 | 19 (16.8)b | 0 (0.0)b | 15 (19.5)b | 6 (15.4) | 9 (23.7) |
| 3 | 19 (16.8)b | 1 (14.3)b | 14 (18.2)b | 6 (15.4) | 7 (18.4) |
| 4 | 24 (21.2)b | 3 (42.9)b | 15 (19.5)b | 5 (12.8) | 11 (28.9) |
| 5 (most deprived) | 36 (31.9)b | 1 (14.3)b | 23 (29.9)b | 16 (41.0) | 7 (18.4) |
| IMD quintile – Wales (N = 16), n (%) | |||||
| 1 (least deprived) | 2 (12.5)b | 0 (0.0)b | 1 (14.3)b | 1 (25.0) | 0 (0.0) |
| 2 | 1 (6.3)b | 0 (0.0)b | 0 (0.0)b | 0 (0.0) | 0 (0.0) |
| 3 | 3 (18.8)b | 0 (0.0)b | 0 (0.0)b | 0 (0.0) | 0 (0.0) |
| 4 | 3 (18.8)b | 0 (0.0)b | 1 (14.3)b | 0 (0.0) | 1 (33.3) |
| 5 (most deprived) | 7 (43.8)b | 1 (100.0)b | 5 (71.4)b | 3 (75.0) | 2 (66.7) |
| Relationship status, n (%)a | |||||
| Single | 302 (43.3)b | 25 (43.1)b | 153 (41.6)b | 75 (41.2) | 74 (39.8) |
| Married/cohabiting | 336 (48.1)b | 22 (37.9)b | 191 (51.9)b | 97 (53.3) | 98 (52.7) |
| Separated | 19 (2.7)b | 2 (3.4)b | 9 (2.4)b | 3 (1.6) | 4 (2.2) |
| Divorced | 29 (4.2)b | 7 (12.1)b | 10 (2.7)b | 4 (2.2) | 8 (4.3) |
| Widowed | 12 (1.7)b | 2 (3.4)b | 5 (1.4)b | 3 (1.6) | 2 (1.1) |
| Living arrangements, n (%)a | |||||
| Living alone | 105 (15.0)b | 5 (8.6)b | 53 (14.4)b | 24 (13.2) | 28 (15.1) |
| Living with others | 593 (85.0)b | 53 (91.4)b | 315 (85.6)b | 158 (86.8) | 158 (84.9) |
| Has dependants, n (%)a | |||||
| No | 476 (68.2)b | 36 (62.1)b | 260 (70.7)b | 125 (68.7) | 122 (65.6) |
| Yes | 222 (31.8)b | 22 (37.9)b | 108 (29.3)b | 57 (31.3) | 64 (34.4) |
| If participant has dependents they are . . .,c n (%) | |||||
| Total dependents | n = 222 | n = 22 | n = 108 | n = 57 | n = 64 |
| Partner | 6 (2.7)b | 2 (9.1)b | 2 (1.9)b | 2 (3.5) | 4 (6.3) |
| Child | 211 (95.0)b | 20 (90.9)b | 103 (95.4)b | 55 (96.5) | 59 (92.2) |
| Parent | 1 (0.5)b | 0 (0.0)b | 1 (0.9)b | 0 (0.0) | 3 (4.7) |
| Other | 7 (3.2)b | 2 (9.1)b | 3 (2.8)b | 3 (5.3) | 3 (4.7) |
| Does participant have a carer? n (%)a | |||||
| Total | n = 693 | n = 56 | n = 367 | n = 182 | n = 186 |
| No | 446 (64.4)b | 35 (62.5)b | 227 (61.9)b | 113 (62.1) | 106 (57.0) |
| Yes | 247 (35.6)b | 21 (37.5)b | 140 (38.1)b | 69 (37.9) | 80 (43.0) |
| If participant has a carer they are . . .,c n (%) | |||||
| Total | n = 247 | n = 21 | n = 140 | n = 69 | n = 80 |
| Partner | 124 (50.2)b | 11 (52.4)b | 72 (51.4)b | 33 (47.8) | 45 (56.3) |
| Child | 27 (10.9)b | 2 (9.5)b | 17 (12.1)b | 10 (14.5) | 7 (8.8) |
| Parent | 72 (29.1)b | 7 (33.3)b | 38 (27.1)b | 17 (24.6) | 18 (22.5) |
| Friend | 21 (8.5)b | 1 (4.8)b | 12 (8.6)b | 5 (7.2) | 8 (10.0) |
| Paid | 21 (8.5)b | 2 (9.5)b | 14 (10.0)b | 10 (14.5) | 8 (10.0) |
| Other | 29 (11.7)b | 2 (9.5)b | 20 (14.3)b | 11 (15.9) | 10 (12.5) |
| Highest qualifications (based on UK educational system), n (%)a | |||||
| Total | n = 687 | n = 55 | n = 366 | n = 181 | n = 186 |
| None | 107 (15.6)b | 8 (14.5)b | 49 (13.4)b | 21 (11.6) | 22 (11.8) |
| Secondary | 180 (26.2)b | 15 (27.3)b | 94 (25.7)b | 41 (22.7) | 48 (25.8) |
| Vocational | 192 (27.9)b | 14 (25.5)b | 111 (30.3)b | 66 (36.5) | 54 (29.0) |
| Furtherd | 111 (16.2)b | 7 (12.7)b | 57 (15.6)b | 28 (15.5) | 28 (15.1) |
| Highere | 97 (14.1)b | 11 (20.0)b | 55 (15.0)b | 25 (13.8) | 34 (18.3) |
| Current employment status, n (%)a,b | |||||
| Total | n = 94 | n = 58 | n = 365 | n = 180 | n = 185 |
| Not employed or in education | 467 (67.3) | 41 (70.7) | 242 (66.3) | 122 (67.8) | 120 (64.9) |
| Employed or in education | 227 (32.7) | 17 (29.3) | 123 (33.7) | 58 (32.2) | 65 (35.1) |
| Receiving disability benefits if of working age (aged < 65 years) and not working, n (%)a,b | |||||
| Total | n = 446 | n = 40 | n = 233 | n = 115 | n = 118 |
| No | 121 (27.1) | 10 (25.0) | 68 (29.2) | 29 (25.2) | 39 (33.1) |
| Yes | 325 (72.9) | 30 (75.0) | 165 (70.8) | 86 (74.8) | 79 (66.9) |
| Receiving disability benefits if of working age (aged < 65 years) and working, n (%)a,b | |||||
| Total | n = 205 | n = 15 | n = 110 | n = 58 | n = 52 |
| No | 165 (80.5) | 13 (86.7) | 92 (83.6) | 45 (77.6) | 47 (90.4) |
| Yes | 40 (19.5) | 2 (13.3) | 18 (16.4) | 13 (22.4) | 5 (9.6) |
| Category | Consented to screening phase (N = 698) | Subsequently eligible for RCT | Trial arm | ||
|---|---|---|---|---|---|
| Not randomised (N = 58) | Randomised (N = 368) | SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| Diagnosis of DS made by video EEG, n (%)a | |||||
| No | 324 (46.4) | 20 (34.5) | 173 (47.0) | 88 (48.4) | 85 (45.7) |
| Yes | 374 (53.6) | 38 (65.5) | 195 (53.0) | 94 (51.6) | 101 (54.3) |
| Age (years) at first DSa | |||||
| Total | n = 669 | n = 55 | n = 365 | n = 181 | n = 184 |
| Mean (SD); median (IQR) [range] | 30.8 (14.3); 28 (19–41) [1–80] | 30.4 (13.7); 26 (20–41) [9–64] | 30.9 (14.1); 29 (19–42) [1–76] | 30.9 (14.6); 29 (19–42) [5–76] | 31.0 (13.5); 29 (19–41.5) [1–67] |
| Number of years between onset of DSs and current diagnosisa | |||||
| Total | n = 669 | n = 55 | n = 365 | n = 181 | n = 184 |
| Mean (SD); median (IQR) [range] | 6.3 (9.1); 3 (1–7) [0–65] | 5.6 (9.7); 2 (1–6) [0–64] | 6.2 (8.8); 3 (1–8) [0–65] | 6.5 (9.7); 3 (1–8) [0–65] | 5.9 (7.8); 3 (1–7.5) [0–44] |
| Predominant seizure type (clinician reported), n (%)a | |||||
| Total | n = 692 | n = 58 | n = 366 | n = 181 | n = 185 |
| Hypokinetic | 221 (31.9) | 18 (31.0) | 130 (35.5) | 60 (33.1) | 70 (37.8) |
| Hyperkinetic | 471 (68.1) | 40 (69.0) | 236 (64.5) | 121 (66.9) | 115 (62.2) |
| Previous diagnosis of epilepsy (clinician reported), n (%)a | |||||
| No | 510 (73.1) | 48 (82.8) | 279 (75.8) | 129 (70.9) | 150 (80.6) |
| Yes | 188 (26.9) | 10 (17.2) | 89 (24.2) | 53 (29.1) | 36 (19.4) |
| If participant has a previous diagnosis of epilepsy, what is the status of this diagnosis? (Clinician reported), n (%) | |||||
| Total | n = 179 | n = 10 | n = 85 | n = 51 | n = 34 |
| Patient still has epilepsy (but no epileptic seizures have occurred in past year) | 15 (8.4) | 2 (20.0) | 7 (8.2) | 4 (7.8) | 3 (8.8) |
| Patient had epilepsy, but now has only DSs | 20 (11.2) | 0 (0.0) | 9 (10.6) | 4 (7.8) | 5 (14.7) |
| Patient was previously misdiagnosed with epilepsy | 80 (44.7) | 3 (30.0) | 43 (50.6) | 25 (49.0) | 18 (52.9) |
| Not possible to determine validity of earlier diagnosis on basis of records | 64 (35.8) | 5 (50.0) | 26 (30.6) | 18 (35.3) | 8 (23.5) |
| Previous diagnosis of epilepsy (participant reported), n (%)a | |||||
| Total | n = 697 | n = 58 | n = 368 | n = 182 | n = 186 |
| No | 486 (69.7) | 43 (74.1) | 266 (72.3) | 130 (71.4)b | 137 (73.7)b |
| Yes | 211 (30.3) | 15 (25.9) | 102 (27.7) | 52 (28.6)b | 49 (26.3)b |
| Currently prescribed epilepsy drugs (participant reported), n (%)a | |||||
| Total | n = 696 | n = 58 | n = 368 | n = 182 | n = 186 |
| No | 481 (69.1) | 41 (70.7) | 272 (73.9) | 146 (80.2)b | 146 (78.5)b |
| Yes | 215 (30.9) | 17 (29.3) | 96 (26.1) | 36 (19.8)b | 40 (21.5)b |
| Category | Consented to screening phase (N = 698) | Subsequently eligible for RCT | Trial arm | ||
|---|---|---|---|---|---|
| Not randomised (N = 58) | Randomised (N = 368) | SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| Belief in diagnosis score (continuous: out of 10) | |||||
| Total | n = 692 | n = 57 | n = 366 | n = 181 | n = 185 |
| Median (IQR) [range] | 8 (6–10) [0–10] | 8 (5–10) [0–10] | 8 (7–10) [0–10] | 8 (7–10) [0–10] | 8 (7–10) [0–10] |
| Previous CBT for DSs (participant reported), n (%) | |||||
| Total | n= 696 | n = 58 | n = 368 | n = 181 | n = 185 |
| No | 680 (97.7) | 58 (100.0) | 362 (98.4) | 179 (98.4) | 183 (98.4) |
| Yes | 16 (2.3) | 0 (0.0) | 6 (1.6) | 3 (1.6) | 3 (1.6) |
| Previously sought help for mental health problem (participant reported), n (%)a | |||||
| Total | n = 697 | n = 58 | n = 368 | n = 182 | n = 186 |
| No | 244 (35.0) | 19 (32.8) | 134 (36.4) | 66 (36.3)b | 61 (32.8)b |
| Yes | 453 (65.0) | 39 (67.2) | 234 (63.6) | 116 (63.7)b | 125 (67.2)b |
| Currently suffering from any other medical problem (participant reported), n (%) | |||||
| Total | – | – | – | n = 181 | n = 184 |
| No | – | – | – | 50 (27.6)c | 54 (29.3)c |
| Yes | – | – | – | 131 (72.4)c | 130 (70.7)c |
Our participants had a median age in the mid-30s, and the proportion of women in the randomised subsample, at around three-quarters, was in line with expectations and was very similar to the proportion of women in the overall pool of 698 participants from which the subsample was drawn (see Table 6). We have described the total sample of 698 participants in detail elsewhere133 and reported that, although overall there were more women than men, women were more likely than men to develop DSs at a younger age, whereas men were equally likely to develop DSs across the age span.
The IMD scores, reflecting levels of deprivation based on participants’ postcodes, indicated that, when examining quintiles, > 50% of participants from England, Scotland and Wales fell in the two quintiles indicating areas of the highest levels of deprivation.
The majority of the participants were white, and > 50% had attained secondary-level education or vocational qualifications. Across all subgroups, approximately two-thirds of participants were not employed or in education. We defined ‘unemployed and not in education’ as including those who were unemployed, employed full-time/part-time but off sick, students whose studies were interrupted because of illness, retired owing to age or ill-health, or househusbands/housewives. Those categorised as being employed (approximately one-third of cases) were employed full- or part-time (and working), students or self-employed. The clear majority of those aged < 65 years and not working were receiving disability benefits, in contrast to those of working age and working. Most participants lived with other people, although roughly equal proportions were either married/cohabiting or single. Most (> 60%) reported having no dependants; around one-third (> 35%) reported having a carer, with this most often being a partner.
For the most part, at each stage of the study just over 50% of the patients had received their DS diagnosis based on video EEG (see Table 7). The median age at onset of DSs in our initial sample and those subsequently randomised was late 20s (not all participants were able to say when their DSs had started). The median duration of experiencing DSs until the delivery of the diagnosis in this study was mostly 3 years. Around two-thirds of patients were reported to have predominantly hyperkinetic DSs. The percentage of patients self-reporting a previous diagnosis of epilepsy was generally slightly higher than the percentage of patients thought by clinicians to have had a previous epilepsy diagnosis. Clinicians indicated that an appreciable number of patients were thought to have been previously misdiagnosed with epilepsy, and indicated that for between one-quarter and half of those patients it was not possible to verify the previous diagnosis. Although around one-third (30.3%) of the initially recruited (n = 698) sample self-reported that they were taking AEDs (30.9%), this percentage was nearer 20% in those entering the RCT.
The majority of patients indicated a strong belief in their diagnosis (scores between 8 and 10), with a median score of 8 (out of a maximum of 10) across all subgroups. A negligible percentage of participants had previously received CBT for their DSs. Around two-thirds had previously sought help for a mental health problem and > 70% of those entering the RCT reported suffering from another medical problem.
Psychological comorbidities and other characteristics measured pre randomisation
The Mini-International Neuropsychiatric Interview and Standardised Assessment of Personality Abbreviated Scale, Self-Report
The M.I.N.I. diagnoses are shown in Table 9 for those randomised overall and by trial arm. Of note, although this is not a diagnosis as such, 232 people (63.0%) overall met criteria for suicidality and 136 (37.0%) did not. The suicidality risk level was low for 151 (65.1%), moderate for 29 (12.5%) and high for 52 (22.4%) participants indicating suicidality, although none of the high-level risk participants was judged to be at imminent risk of harm by their psychiatrists. The most common diagnosis was a previous diagnosis of major depressive disorder and the most common current diagnosis was agoraphobia, followed by a current diagnosis of major depressive disorder, generalised anxiety disorder, PTSD and social anxiety disorder, all of which were reported by > 20% of participants.
| Category | Trial arm, n (%) | Overall, n (%) (N = 368) | |
|---|---|---|---|
| SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| M.I.N.I. | |||
| Major depressive disorder (current) | |||
| Yes | 53 (29.1) | 61 (32.8) | 114 (31.0) |
| Major depressive disorder (past) | |||
| Yes | 87 (47.8) | 106 (57.0) | 193 (52.4) |
| Suicidality | |||
| Yes | 105 (57.7) | 127 (68.3) | 232 (63.0) |
| Suicidality risk level | |||
| Total | n = 105 | n = 127 | n = 232 |
| Low (1–8) | 71 (67.6) | 80 (63.0) | 151 (65.1) |
| Moderate (9–16) | 14 (13.3) | 15 (11.8) | 29 (12.5) |
| High (≥ 17) | 20 (19.0) | 32 (25.2) | 52 (22.4) |
| Manic episode (current) | |||
| Yes | 7 (3.8) | 4 (2.2) | 11 (3.0) |
| Manic episode (past) | |||
| Yes | 10 (5.5) | 19 (10.2) | 29 (7.9) |
| Hypomanic episode (current) | |||
| Yes | 2 (1.1) | 3 (1.6) | 5 (1.4) |
| Hypomanic episode (past) | |||
| Yes | 8 (4.4) | 9 (4.8) | 17 (4.6) |
| Bipolar I disorder (current) | |||
| Yes | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Bipolar I disorder (past) | |||
| Total | n = 182 | n = 185 | n = 367 |
| Yes | 8 (4.4) | 14 (7.6) | 22 (6.0) |
| Bipolar II disorder (current) | |||
| Yes | 1 (0.5) | 2 (1.1) | 3 (0.8) |
| Bipolar II disorder (past) | |||
| Yes | 6 (3.3) | 4 (2.2) | 10 (2.7) |
| Bipolar disorder NOS (current) | |||
| Total | n = 182 | n = 185 | n = 367 |
| Yes | 2 (1.1) | 1 (0.5) | 3 (0.8) |
| Bipolar disorder NOS (past) | |||
| Total | n = 182 | n = 184 | n = 366 |
| Yes | 4 (2.2) | 2 (1.1) | 6 (1.6) |
| Panic disorder (lifetime) | |||
| Yes | 55 (30.2) | 51 (27.4) | 106 (28.8) |
| Panic disorder (current) | |||
| Yes | 27 (14.8) | 30 (16.1) | 57 (15.5) |
| Agoraphobia (current) | |||
| Yes | 83 (45.6) | 82 (44.1) | 165 (44.8) |
| Social phobia (social anxiety disorder) (current) | |||
| Yes | 34 (18.7) | 41 (22.0) | 75 (20.4) |
| Obsessive–compulsive disorder (current) | |||
| Total | n = 181 | n = 186 | n = 367 |
| Yes | 16 (8.8) | 18 (9.7) | 34 (9.3) |
| PTSD (current) | |||
| Yes | 41 (22.5) | 45 (24.2) | 86 (23.4) |
| Alcohol dependence (current) | |||
| Yes | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Alcohol abuse (current) | |||
| Yes | 3 (1.6) | 2 (1.1) | 5 (1.4) |
| Substance dependence (current)a | |||
| Yes | 0 (0.0) | 2 (1.1) | 2 (0.5) |
| Substance abuse (current)b | |||
| Yes | 1 (0.5) | 0 (0.00) | 1 (0.3) |
| Psychotic disorder (current) | |||
| Yes | 4 (2.2) | 6 (3.2) | 10 (2.7) |
| Psychotic disorder (lifetime) | |||
| Yes | 6 (3.3) | 15 (8.1) | 21 (5.7) |
| Mood disorder with psychotic features (lifetime) | |||
| Yes | 4 (2.2) | 15 (8.1) | 19 (5.2) |
| Mood disorder with psychotic features (current) | |||
| Yes | 4 (2.2) | 7 (3.8) | 11 (3.0) |
| Anorexia nervosa (current) | |||
| Yes | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Bulimia nervosa (current) | |||
| Yes | 7 (3.8) | 6 (3.2) | 13 (3.5) |
| Anorexia nervosa (binge eating/purging) (current) | |||
| Yes | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Generalised anxiety disorder (current) | |||
| Yes | 49 (26.9) | 59 (31.7) | 108 (29.3) |
| Antisocial personality disorder (lifetime) | |||
| Yes | 7 (3.8) | 9 (4.8) | 16 (4.3) |
| Self-report SAPAS-SR | |||
| Total | n = 181 | n = 182 | n = 363 |
| Total score, mean (SD) [range] | 4.0 (2.0) [0–8] | 3.9 (1.9) [0–8] | 3.9 (2.0) [0–8] |
Although some patients met the diagnosis criteria for current substance (n = 2; 0.5%) or alcohol (n = 4; 1.1%) dependence, these diagnoses had not been identified by the diagnosing neurologists or psychiatrists during their assessments of the patients.
On the SAPAS-SR, 211 (58.1%) participants had scores that potentially indicated the presence of maladaptive personality traits.
Treatment preference and expectations of outcome
Patients were asked to indicate their treatment preferences prior to randomisation. Of 367 patients responding, 228 (62.1%) indicated a preference for CBT, 15 (4.1%) indicated a preference for SMC and 124 (33.8%) did not know which treatment they would prefer. There were no clear differences in preference proportions among actual allocations, although for both the CBT + SMC and the SMC-alone treatment arms, around 60% indicated a preference for CBT + SMC. Around 3% of the SMC-alone arm and 5% of the CBT + SMC arm indicated a pre-randomisation preference to be allocated to the SMC-alone arm (see Table 10).
Expectations of treatment outcome prior to randomisation are shown in Table 10. Median ratings of how logical the different treatments seemed and how confident participants were that they would be helped by them were more positive than negative, and were similar for all three treatment arms.
| Question | Trial arm | Overall (N = 368) | |
|---|---|---|---|
| SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| Preferred treatment arm | |||
| Total | n = 182 | n = 185 | n = 367 |
| CBT | 108 (59.3) | 120 (64.9) | 228 (62.1) |
| SMC | 6 (3.3) | 9 (4.9) | 15 (4.1) |
| Do not know | 68 (37.4) | 56 (30.3) | 124 (33.8) |
| Expectation of treatment outcome (CBT)a | |||
| Total | n = 179 | n = 180 | n = 359 |
| Median (IQR) [range] | 6 (5–7) [2–8] | 6 (5–7) [1–8] | 6 (5–7) [1–8] |
| Expectation of treatment outcome (neurologist)a | |||
| Total | n = 179 | n = 180 | n = 359 |
| Median (IQR) [range] | 6 (4–7) [0–8] | 6 (4–7) [0–8] | 6 (4–7) [0–8] |
| Expectation of treatment outcome (psychiatrist)a | |||
| Total | n = 179 | n = 181 | n = 360 |
| Median (IQR) [range] | 6 (5–7) [1–8] | 6 (5–7) [1–8] | 6 (5–7) [1–8] |
For each treatment (CBT, treatment by neurologist and treatment by psychiatrist), scores are combined for how logical the treatment seems and how confident the person is that this will help them.
Treatment delivery and fidelity
Healthcare practitioners involved in delivering CODES
A wide range of neurologists, psychiatrists and therapists participated in the CODES trial; SMC was delivered via local permutations of neurology and psychiatry appointments. Of the 63 CBT therapists who were trained to deliver CODES CBT, 39 were subsequently allocated patients. Reasons for non-allocation of patients to trained therapists included therapists changing job between training and patient randomisation, therapists’ change of job role at the site and parental leave.
The demographics and clinical experience of the three types of HCPs are summarised in Appendix 6.
Numbers of sessions attended for each intervention
Table 11 presents the number of SMC appointments that participants were offered and received across both trial arms. SMC consisted of neurology and/or psychiatry appointments. All participants allocated to CBT + SMC were offered up to 13 CBT sessions, whereas the number of SMC appointments that were offered varied.
| Appointment type | Trial arm | Overall (N = 368) | |
|---|---|---|---|
| SMC alone (N = 182) | CBT + SMC (N = 186) | ||
| Neurology SMC appointments, median (IQR) [range], n | |||
| Offered | 1 (0–2) [0–6], 129 | 1 (0–1) [0–16], 129 | 1 (0–2) [0–16], 258 |
| Attended | 1 (0–2) [0–6], 129 | 1 (0–1) [0–14], 129 | 1 (0–1) [0–14], 258 |
| Psychiatry SMC appointments, median (IQR) [range], n | |||
| Offered | 4 (3–5) [0–13], 182 | 3 (2–5) [0–10], 186 | 4 (2–5) [0–13], 368 |
| Attended | 3 (2–4) [0–12], 182 | 3 (1–4) [0–8], 186 | 3 (1–4) [0–12], 368 |
| Total SMC appointments, median (IQR) [range], n | |||
| Offered | 5 (3–6) [0–13], 182 | 4 (2–6) [0–20], 186 | 4.5 (3–6) [0–20], 368 |
| Attended | 4 (2–5) [0–12], 182 | 3 (2–5) [0–19], 186 | 3 (2–5) [0–19], 368 |
Table 11 indicates that, as anticipated, participants in both trial arms were offered more psychiatry SMC appointments than neurology SMC appointments. The median number of neurology and psychiatry SMC appointments attended was one and three, respectively, in line with expectations. There were some participants who were offered and attended > 10 SMC appointments because of clinical need; however, overall, the number of SMC appointments offered and attended was similar across trial arms.
The median time between randomisation and the first CBT session was 38.5 days (IQR 26–59 days). Over half (55.9%) of participants who were randomised to receive CBT attended all 13 sessions; only eight (4.3%) participants did not attend any, and the median number of attended CBT sessions was the full course of 13. Three-quarters (75.3%) of participants attended at least nine CBT sessions, which meant that the majority were defined as treatment compliant. One participant received three extra sessions beyond the 13 offered in the trial design because they were considered to be at high risk by their therapist. For this participant, there was also no interval between session 12 and the booster session. Figure 5 illustrates the distribution of the number of CBT sessions attended in the CBT + SMC arm (n = 186). One participant who was randomised to the SMC arm was mistakenly offered the CODES CBT and subsequently attended all 13 sessions.
FIGURE 5.
Number of CBT sessions attended in the CBT + SMC arm.

Over 700 CBT sessions were scheduled and missed; around 30–40 participants missed different sessions. Although most missed appointments were cancelled in advance, over one-quarter (28.4%) were non-attendances without notification. Of 410 appointments cancelled by patients, feeling unwell (43%) and other (work and family) commitments (22%) were the most common reasons for participant non-attendance; however, nearly 12% of cancelled sessions were because of patients reporting being unable to travel alone and 7% were cancelled because of seizures. Nearly all sessions (95.8%) were attended face to face, and most participants (78.6%) attended alone. Only 10 (5.4%) participants of the total allocated to the CBT + SMC arm informed their therapist that they wished to withdraw from therapy, which included three participants who had not attended any sessions and seven participants who had attended more than one CBT session (range 2–12 sessions).
For the second CBT session onwards, therapists were asked to complete a series of questions about participant adherence to treatment. Of these responses (n = 1725), 84.4% were reported to have implemented therapy techniques well from the previous session (moderately well, very well or completely) and 80.9% were perceived by the therapists to have completed at least half of their homework. The mean duration of the therapy sessions (therapist reported) was 60.5 minutes; we did not collect data concerning the time of day that the sessions were held.
Information collected on participants who experienced a seizure during therapy indicated that only 42 (2.2%) of the attended CBT sessions were significantly disrupted by a person having a seizure; this corresponded to 18 participants in total. Most of these seizures were between 3 minutes and 10 minutes in duration, and only four resulted in the participant suffering physical injury.
Therapy protocol deviations
We observed only two deviations from the therapy protocol, one in each treatment arm. In the SMC arm, one participant received CODES CBT in error. In the CBT + SMC arm, one participant received more than 13 sessions of CBT; the participant had an additional three sessions after the standard 12th session and booster session because of clinical need.
Treatment fidelity
As described in Chapter 2, two independent CBT therapists undertook blind ratings of one session from each therapist, with half of the ratings being undertaken for session 3 and half for session 7. In total, 18 tapes for each session were rated using the rating scale described in Chapter 2, Evaluation of treatment fidelity (see Appendix 3). As indicated, scores were converted to standardised scores out of 100. For the single-item subscales (i.e. Overall Therapist Adherence, Therapeutic Alliance and Overall CBT Delivery), scores were calculated by dividing the score by 7 and then multiplying by 100. For the specific DS skills scale (i.e. DS skills items 3–6 in Appendix 3), scores were totalled and divided by [7 × number of relevant (yes-rated) items scored] × 100 to generate standardised scores. The median values are shown in Table 12.
| Measure | Median | IQR | Range |
|---|---|---|---|
| DS-specific skills | |||
| Did the therapist help the client develop some strategies for controlling the seizures, which should be implemented at the first sign of a seizure? | 50.00 | 28.57–85.71 | 14.20–100.00 |
| Did the therapist help the client challenge unhelpful beliefs related to DSs and other problems? | 57.14 | 42.86–75.00 | 14.29–100.00 |
| Did the therapist help the client ‘reclaim’ areas of their life previously avoided? | 64.29 | 37.50–83.93 | 14.29–100.00 |
| Did the therapist help the client make links between specific traumas or stressors and DSs? | 78.57 | 57.14–92.86 | 28.57–100.00 |
| Average across DS-specific skills | 58.93 | 43.30–75.89 | 25.00–100.00 |
| General skills | |||
| Overall delivery of CBT (was the therapist delivering CBT?) | 78.57 | 50.00–85.71 | 21.43–100.00 |
| Overall therapist adherence (was the therapy delivered as described in the CODES therapy manual?) | 85.71 | 51.79–91.07 | 21.43–100.00 |
| Therapeutic alliance [overall, how would you rate the therapeutic alliance (supportive encouragement, understanding, warmth, empathy)?] | 92.86 | 78.57–100.00 | 42.86–100.00 |
Overall, the median ratings for the therapy being delivered in accordance with the CODES therapy manual, the therapeutic alliance and whether or not the therapy being delivered was CBT all fell in the upper end of the scales (between considerably and extensively for adherence to the manual; just below excellent for therapeutic alliance; and between considerably and extensively for delivery of CBT). More mid-range median scores were obtained for DS-specific techniques, but it was possible that, although DS control techniques were expected to take place in session 3, by session 7 therapists may have adopted some flexibility in session content (see Wilkinson et al. 135) and teaching seizure control techniques may have become less relevant by then.
Retention of participants
As seen in the CONSORT flow diagram (see Figure 3), we obtained primary outcome data for 313 (85%) of the randomised participants. The follow-up rate did not differ in the two arms [84% (156/186) in the CBT + SMC arm; 86% (157/182) in the SMC-alone arm]. Fifty-five participants withdrew or were lost to follow-up (CBT + SMC, n = 30; SMC alone, n = 25) but in no case was withdrawal accounted for by death or other AEs (Table 13). In only one instance (in the SMC arm) was the withdrawal decision initiated by the clinician rather than the participant, but no further information was provided for this withdrawal.
| Reason | Trial arm (n) | |
|---|---|---|
| SMC alone (N = 25) | CBT + SMC (N = 30) | |
| Death of a participant | 0 | 0 |
| Adverse event | 0 | 0 |
| Unable to locate/contact participant | 0 | 0 |
| Participant not willing to complete minimum assessments (primary outcome data) | 3 | 4 |
| Other | (n = 21) | (n = 26) |
| Lost to follow-up | 15 | 23 |
| Did not want to continue | 2 | 0 |
| Participant has moved | 1 | 0 |
| Not feeling up to it psychologically or physically | 1 | 0 |
| No reason given | 2 | 3 |
| Unknown | 1 | 0 |
Clinical outcomes
Descriptive statistics for all primary and secondary outcomes are shown in Tables 14 (seizure outcomes) and 15 (other outcomes). Formal statistical comparisons of the outcome measures between the treatment arms at 12 months post randomisation are shown in Table 16. The first two sets of results (estimated trial arm difference and standardised treatment effects) were derived using the MICE procedure (100 imputations), and the third set of results was derived using the CC analysis. As explained in Chapter 2, the MI results are treated as the primary formal comparisons and CC as a sensitivity analysis. Statistical significance was assessed at the 5% test level unless stated otherwise.
| Baseline | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial arm | Overall (N = 368) | Trial arm | Overall (N = 368) | Trial arm | Overall (N = 368) | ||||
| SMC alone (N = 182) | CBT + SMC (N = 186) | SMC alone (N = 182) | CBT + SMC (N = 186) | SMC alone (N = 182) | CBT + SMC (N = 186) | ||||
| Primary outcome (evaluated at 12 monthsa), median (IQR) [range], n | |||||||||
| Monthly seizure frequency in the previous 4 weeks | 19 (5–49) [0–649], 182 | 12.5 (4–41) [0–535], 186 | 15 (4–47) [0–649], 368 | 18 (3–48) [0–640], 162 | 6 (0–24) [0–849], 161 | 9 (1–38) [0–849], 323 | 7 (1–35) [0–994], 157 | 4 (0–20) [0–571], 156 | 5 (0–27) [0–994], 313 |
| Seizure experience secondary outcomes (evaluated at 12 monthsa) | |||||||||
| Seizure severity: 1 = very mild, 7 = very severe, mean (SD) [range], n | 4.8 (1.6) [1–7], 179 | 4.7 (1.6) [1–7], 182 | 4.7 (1.6) [1–7], 361 | 4.4 (1.6) [1–7], 135 | 3.9 (1.9) [1–7], 125 | 4.1 (1.8) [1–7], 260 | 4.1 (1.8) [1–7], 130 | 3.8 (1.8) [1–7], 129 | 4.0 (1.8) [1–7], 259 |
| Seizure bothersomeness: 1 = no bother at all, 7 = very bothersome, mean (SD) [range], n | 5.4 (1.7) [1–7], 180 | 5.2 (1.7) [1–7], 182 | 5.3 (1.7) [1–7], 362 | 4.7 (2.0) [1–7], 143 | 3.9 (2.1) [1–7], 134 | 4.3 (2.1) [1–7], 277 | 4.6 (2.1) [1–7], 132 | 3.9 (2.0) [1–7], 131 | 4.2 (2.1) [1–7], 263 |
| Longest period of seizure freedom in the last 6 months (days),b median (IQR) [range], n | 7 (2–21) [0–84], 181 | 7 (2–21) [0–119], 186 | 7 (2–21) [0–119], 367 | – | – | – | 12 (3–42) [0–343], 143 | 21 (5–97.5) [0–357], 140 | 14 (3–70) [0–357], 283 |
| Seizure freedom in the last 3 months of trial, n (%) | n = 145 | n = 148 | n = 293 | ||||||
| Yes | – | – | – | – | – | – | 18 (12.4) | 29 (19.6) | 47 (16.0) |
| No | – | – | – | – | – | – | 127 (87.6) | 119 (80.4) | 246 (84.0) |
| > 50% reduction in monthly DS frequency compared with baseline, n (%) | n = 157 | n = 153 | n = 310 | n = 152 | n = 149 | n = 301 | |||
| Yes | – | – | – | 43 (27.4) | 65 (42.5) | 108 (34.8) | 60 (39.5) | 68 (45.6) | 128 (42.5) |
| No | – | – | – | 114 (72.6) | 88 (57.5) | 202 (65.2) | 92 (60.5) | 81 (54.4) | 173 (57.5) |
| Baseline | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial arm | Overall (N = 368) | Trial arm | Overall (N = 368) | Trial arm | Overall (N = 368) | ||||
| SMC alone (N = 182) | CBT + SMC (N = 186) | SMC alone (N = 182) | CBT + SMC (N = 186) | SMC alone (N = 182) | CBT + SMC (N = 186) | ||||
| HRQoL (evaluated at 12 monthsa), mean (SD) [range], n | |||||||||
| Physical Component Summary score (SF-12v2): 0 = worst health, 100 = best health | 38.8 (11.9) [13.9–65.6], 181 | 40.5 (12.4) [13.4–65.9], 185 | 39.7 (12.2) [13.4–65.9], 366 | 38.8 (11.4) [13.1–59.5], 142 | 41.5 (13.4) [15.9–66.7], 134 | 40.1 (12.4) [13.1–66.7], 276 | 38.0 (12.6) [10.4–63.7], 145 | 41.5 (13.4) [12.2–67.3], 148 | 39.8 (13.1) [10.4–67.3], 293 |
| Mental Component Summary score (SF-12v2): 0 = worst health, 100 = best health | 37.9 (11.4) [16.9–68.1], 181 | 37.7 (12.2) [13.4–67.6], 185 | 37.8 (11.8) [13.4–68.1], 366 | 37.5 (12.1) [10.5–63.0], 142 | 40.3 (11.7) [17.4–67.5], 134 | 38.8 (12.0) [10.5–67.5], 276 | 39.5 (11.8) [11.3–62.9], 145 | 41.5 (12.8) [13.9–65.7], 148 | 40.5 (12.4) [11.3–65.7], 293 |
| Health today (EQ-5D-5L VAS): 0 = worst health, 100 = best health | 54.9 (21.9) [10–100], 181 | 56.2 (24.1) [1–100], 182 | 55.5 (23.0) [1–100], 363 | 50.9 (23.1) [0–100], 143 | 58.8 (24.4) [0–100], 135 | 54.7 (24.0) [0–100], 278 | 53.4 (22.6) [5–100], 145 | 61.1 (24.0) [5–100], 148 | 57.3 (23.6) [5-100], 293 |
| Psychosocial functioning (evaluated at 12 monthsa), mean (SD) [range], n | |||||||||
| Impact of DS on functioning (WSAS) (minimum – maximum: 0–40) (higher scores = more, i.e. worse, impact) | 22.9 (10.5) [0–40], 181 | 22.5 (10.5) [0–40], 185 | 22.7 (10.5) [0–40], 366 | 22.7 (11.9) [0–40], 143 | 17.8 (13.1) [0–40], 135 | 20.3 (12.7) [0–40], 278 | 21.1 (12.7) [0–40], 145 | 16.4 (13.1) [0–40], 148 | 18.7 (13.1) [0–40], 293 |
| Psychiatric symptoms and psychological distress (evaluated at 12 monthsa), mean (SD) [range], n | |||||||||
| Anxiety (GAD-7) (minimum – maximum: 0–21) (higher scores = greater anxiety) | 10 (6.2) [0–21], 182 | 9.6 (6.2) [0–21], 186 | 9.8 (6.2) [0–21], 368 | 10.5 (6.3) [0–21], 143 | 8.1 (6.5) [0–21], 135 | 9.4 (6.5) [0–21], 278 | 9.3 (6.1) [0–21], 145 | 8.2 (6) [0–21], 148 | 8.8 (6.1) [0–21], 293 |
| Depression (PHQ-9) (minimum – maximum: 0–27) (higher scores = greater depression) | 12.6 (6.5) [0–26], 181 | 12.3 (6.7) [0–27], 186 | 12.4 (6.6) [0–27], 367 | 12.9 (7) [0–27], 142 | 11.2 (7.4) [0–27], 135 | 12.1 (7.2) [0–27], 277 | 11.7 (6.7) [0–26], 145 | 10.5 (7.5) [0–26], 148 | 11.1 (7.1) [0–26], 293 |
| Distress (CORE-10) (minimum – maximum: 0–40) (higher scores = more distress) | 18.2 (6.3) [4–34], 182 | 18.2 (6.7) [4–32], 186 | 18.2 (6.5) [4–34], 368 | 18.6 (6.6) [2.2–34], 142 | 17.2 (7.1) [0–39], 135 | 17.9 (6.9) [0–39], 277 | 18.1 (6.6) [3–33], 145 | 16.6 (6.8) [1–38], 148 | 17.3 (6.7) [1–38], 293 |
| Other somatic symptoms (modified PHQ-15) (minimum – maximum: 0–30) (higher scores = more symptoms) | 16.7 (6.2) [2–30], 181 | 16.7 (6.8) [2–30], 183 | 16.7 (6.5) [2–30], 364 | 16.8 (6.7) [0–29], 140 | 14.9 (7.4) [0–28], 135 | 15.9 (7.1) [0–29], 275 | 15.9 (6.9) [0–29], 145 | 14.1 (7.7) [0–28], 147 | 15.0 (7.4) [0–29], 292 |
| Clinical impression of improvement (evaluated at 12 monthsa), mean (SD) [range], n | |||||||||
| Self-reported change (CGI scale): 0 = very much worse, 6 = very much better | – | – | – | 3.4 (1.6) [0–6], 140 | 4.2 (1.3) [0–6], 135 | 3.8 (1.5) [0–6], 275 | 3.6 (1.8) [0–6], 145 | 4.3 (1.5) [0–6], 148 | 4.0 (1.7) [0–6], 293 |
| Clinician-rated change (CGI scale): 0 = very much worse, 6 = very much better | – | – | – | – | – | – | 3.8 (1.3) [0–6], 162 | 4.4 (1.2) [0–6], 161 | 4.1 (1.3) [0–6], 323 |
| Satisfaction with treatment (evaluated at 12 monthsa), mean (SD) [range], n | |||||||||
| Satisfaction with treatment (patient reported): 0 = very dissatisfied, 6 = very satisfied | – | – | – | 3.8 (2.0) [0–6], 140 | 5.1 (1.3) [0–6], 135 | 4.4 (1.8) [0–6], 275 | 4.2 (2.0) [0–6], 145 | 5.2 (1.4) [0–6], 148 | 4.7 (1.8) [0–6], 293 |
| Outcome | Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| MI | CC | |||||||
| Estimated trial arm differencea | 95% CI | Standardised treatment effect | 95% CI | p-value | Estimated trial arm differencea | 95% CI | p-value | |
| Monthly seizure frequency in the last 4 weeks | 0.78b | 0.56 to 1.09 | 0.144 | 0.80b | 0.58 to 1.09 | 0.156 | ||
| Seizure severityc | –0.11 | –0.50 to 0.29 | –0.07 | –0.31 to 0.18 | 0.593 | –0.20 | –0.61 to 0.22 | 0.356 |
| Seizure bothersomenessc | –0.53 | –0.97 to –0.08 | –0.30 | –0.56 to –0.05 | 0.020d | –0.65 | –1.10 to –0.19 | 0.005d |
| Longest period of seizure freedom in the last 6 months (days) | 1.64b | 1.22 to 2.20 | 0.001d | 1.63b | 1.20 to 2.22 | 0.002d | ||
| Seizure freedom in the last 3 months of trial | 1.77e | 0.93 to 3.37 | 0.083 | 1.72e | 0.90 to 3.29 | 0.098 | ||
| > 50% reduction in monthly seizure frequency relative to baseline | 1.27e | 0.80 to 2.02 | 0.313 | 1.29e | 0.81 to 2.06 | 0.279 | ||
| Physical Component Summary score (SF-12v2) | 1.78 | –0.37 to 3.92 | 0.15 | –0.03 to 0.32 | 0.105 | 2.55 | 0.52 to 4.59 | 0.014d |
| Mental Component Summary score (SF-12v2) | 2.22 | –0.30 to 4.75 | 0.15 | –0.03 to 0.33 | 0.084 | 2.22 | –0.21 to 4.66 | 0.074 |
| Health today (EQ-5D-5L VAS) | 6.16 | 1.48 to 10.84 | 0.27 | 0.06 to 0.47 | 0.010d | 6.81 | 2.15 to 11.47 | 0.004d |
| Impact of DS on functioning (WSAS)c | –4.12 | –6.35 to –1.89 | –0.39 | –0.61 to –0.18 | < 0.001d | –4.16 | –6.42 to –1.90 | < 0.001d |
| Anxiety (GAD-7)c | –1.09 | –2.27 to 0.09 | –0.18 | –0.37 to 0.01 | 0.069 | –1.06 | –2.18 to 0.07 | 0.066 |
| Depression (PHQ-9)c | –1.10 | –2.41 to 0.21 | –0.17 | –0.37 to 0.03 | 0.099 | –1.22 | –2.45 to 0.16 | 0.053 |
| Distress (CORE-10)c | –1.65 | –2.96 to –0.35 | –0.25 | –0.45 to –0.05 | 0.013d | –1.61 | –2.89 to –0.33 | 0.014d |
| Other somatic symptoms (modified PHQ-15)c | –1.67 | –2.90 to –0.44 | –0.26 | –0.45 to –0.07 | 0.008d | –1.80 | –2.96 to –0.63 | 0.003d |
| Self-reported change (CGI scale) | 0.66 | 0.26 to 1.04 | 0.39 | 0.16 to 0.62 | 0.001d | 0.78 | 0.41 to 1.15 | < 0.001d |
| Clinician-rated change (CGI scale) | 0.47 | 0.21 to 0.73 | 0.37 | 0.17 to 0.57 | < 0.001d | 0.54 | 0.28 to 0.80 | < 0.001d |
| Satisfaction with treatment (patient reported) | 0.90 | 0.48 to 1.31 | 0.50 | 0.27 to 0.73 | < 0.001d | 1.04 | 0.65 to 1.44 | < 0.001d |
Primary outcome: seizure frequency
Our primary outcome measure for the RCT was participants’ monthly DS frequency at 12 months post randomisation. As described in Chapter 2, these data were also collected at baseline and 6 months post randomisation, and these were used as auxiliary variables in the MICE procedure. The outcome was defined as DS occurrence over the previous 4 weeks and data were collected using seizure diaries. Participants were also asked how many seizures they had experienced in the past 4 weeks as a single-item measure to impute diary seizure frequency for those who had not provided it, and to help estimate the reliability of the diary data.
Both trial arms appeared to show a decrease in monthly DSs from baseline to 12 months post randomisation. An illustration of the observed change over time by trial arm is shown in Figure 6a. Geometric means have been used because they are arithmetically similar to the median and allow 95% CIs to be constructed. The between-group comparison of monthly DS frequency did not reach statistical significance at 12 months (IRR = 0.78, 95% CI 0.56 to 1.09; p = 0.144), although the inferential statistics estimated a 22% advantage for the CBT + SMC trial arm (see Table 16). When comparing the MI and CC results (see Table 16), the size, direction and significance of the treatment effect are very similar (IRR = 0.80, 95% CI 0.58 to 1.09; p = 0.156); indeed, all CC models in Table 16 lead to the same substantive conclusions as the ITT analysis models.
FIGURE 6.
Plot over time of the primary outcome and a secondary seizure experience outcome. (a) Monthly seizure frequency; and (b) longest period of seizure freedom in the last 6 months. T0, baseline; T1, 6-month follow-up; T2, 12-month follow-up. Adapted with permission from Goldstein et al. 134 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.


Furthermore, the CACE effect estimate of the primary outcome, as described in Chapter 2, leads to the same effect size as the ITT analysis (IRR = 0.78), with a similar p-value (p = 0.217). This shows that the ‘efficacy’ estimate is the same as the ‘effectiveness’ estimate. Finally, the ICC between the two methods of collecting the primary outcome (seizure diary and single measure) was 0.95, which implies very high agreement; the results of a second sensitivity analysis for the primary outcome (for which the seizure diary was treated as the only acceptable measure, resulting in 21 participants being treated as having missing outcome data) yielded a similar result again (IRR = 0.74, 95% CI 0.53 to 1.04; p = 0.086). We can, therefore, conclude that the primary outcome analysis was not sensitive to how we constructed monthly seizure frequency.
Secondary outcomes
Other aspects of seizure experience
For seizure-related secondary outcomes, all ITT analyses estimated a difference in the direction indicating an additional benefit of CBT, despite not always reaching statistical significance (see Table 16).
Seizure severity and bothersomeness
On the Seizure Severity Scale, the change in ratings of seizure severity did not reach significance (see Table 16), but the CBT + SMC arm reported their DSs as being less bothersome than the SMC-alone arm at the 12-month follow-up (standardised treatment effect –0.30, 95% CI –0.56 to –0.05; p = 0.020).
Longest seizure-free period in the last 6 months of the study
The CBT + SMC arm recorded a significantly longer seizure-free period (consecutive number of days) than the SMC-alone arm during the last 6 months of the study (IRR 1.64, 95% CI 1.22 to 2.20; p = 0.001). An illustration of the observed change over time by trial arm is shown in Figure 6b.
Seizure freedom in last 3 months of the study
The CBT + SMC arm was not significantly more likely than the SMC-alone arm to be seizure free in the last 3 months of the study.
Reduction of > 50% in dissociative seizure frequency
Although the CBT + SMC arm had higher odds than the SMC-alone arm of having a > 50% reduction in DS frequency, the odds ratio contrasting DS frequency reductions of this magnitude between trial arms was not significant.
Informant rating of dissociative seizures
As only 27 informants provided these data at 12 months post randomisation, we had insufficient data for meaningful inferential statistics to be applied. Available data are shown in Appendix 7.
Health-related quality of life
For neither the SF-12v2 Physical Component Summary score nor the Mental Component Summary score was the between-group difference at 12 months statistically significant. On the EQ-5D-5L VAS at the 12-month follow-up, the CBT + SMC arm reported higher overall health scores than the SMC-alone arm (standardised treatment effect 0.27, 95% CI 0.06 to 0.47; p = 0.010). An illustration of the observed difference between arms over time is shown in Figure 7a.
FIGURE 7.
Plot over time of HRQoL and psychosocial functioning secondary outcomes by trial arm. (a) EQ-5D-5L VAS; and (b) WSAS. T0, baseline; T1, 6-month follow-up; T2, 12-month follow-up; EQ-5D-5L VAS: 0 = worst health, 100 = best health; WSAS: 0 = minimum impact, 40 = maximum impact with higher scores reflecting worse functioning. Adapted with permission from Goldstein et al. 134 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.


Psychosocial functioning
The CBT + SMC arm had WSAS scores indicating significantly lower levels of impairment than the SMC-alone arm (standardised treatment effect –0.39, 95% CI –0.61 to –0.18; p < 0.001) at the 12-month follow-up. Although the SMC-alone arm’s score was still indicative of at least moderate-severe impairment, this was no longer the case for the CBT + SMC arm. An illustration of the observed difference between arms over time is shown in Figure 7b.
Psychiatric symptoms, psychological distress and somatic symptoms
There were no significant between-group differences on either the GAD-7 or the PHQ-9 at the 12-month follow-up. On the CORE-10, the CBT + SMC arm reported significantly lower levels of psychological distress than the SMC-alone arm at the 12-month follow-up (standardised treatment effect –0.25, 95% CI –0.45 to –0.05; p = 0.013).
In the context of similar median total numbers of symptoms at baseline, as measured by the modified PHQ-15, at the 12-month follow-up the CBT + SMC arm reported significantly fewer symptoms than the SMC-alone arm on this scale (standardised treatment effect 0.26, 95% CI –0.45 to –0.07; p = 0.008).
Clinical impression of improvement
Patient-rated improvement
The CBT + SMC arm reported significantly greater improvement in their health than the SMC-alone arm (standardised treatment effect 0.39, 95% CI 0.16 to 0.62; p = 0.001), with 52.7% of the CBT + SMC arm and 35.2% of the SMC-alone arm reporting feeling ‘much better’ or ‘very much better’.
Standardised medical care clinician-rated improvement
At the 12-month follow-up, clinician ratings indicated that the CBT + SMC arm, on average, experienced significantly greater improvement in their health than the SMC-alone arm (standardised treatment effect 0.37, 95% CI 0.17 to 0.57; p < 0.001), with 54.7% of the CBT + SMC group and 30.9% of the SMC-alone arm being rated as ‘much better’ or ‘very much better’.
CBT therapist-rated improvement
Although measured for the CBT + SMC arm only and, therefore, not formally analysed, CBT therapists rated 53.9% of their patients as ‘much better’ or ‘very much better’ at the end of treatment.
Satisfaction with treatment
At 12 months, the CBT + SMC arm participants rated themselves as significantly more satisfied with treatment than the SMC-alone arm (standardised treatment effect 0.50, 95% CI 0.27 to 0.73; p < 0.001), with 93 (62.8%) of the CBT + SMC arm and 56 (38.6%) participants in the SMC-alone arm rating themselves as ‘very satisfied’ with their treatment.
Effect sizes
To be able to compare sizes of estimated treatment effects between outcomes on different scales, we standardised all effect sizes for continuous outcomes (standardised treatment effects are shown in Table 16). Where the outcome was measured at baseline, we divided the estimated difference between arms by the SD of the baseline score; for other measures, the difference was divided by the pooled SD of the outcome.
Figure 8 displays the standardised treatment effects with 95% CIs for all continuous secondary outcomes. For outcomes in Table 16 that are interpreted as showing better outcomes from the CBT + SMC arm if the standardised treatment effect is negative (< 0), the estimated treatment effect has been reversed (multiplied by –1) in Figure 8 so that a positive standardised effect size (> 0) is in favour of CBT + SMC compared with SMC. Figure 8 shows that the estimated treatment effects for all 13 outcomes are positive (above the dashed line at d = 0), which illustrates a trend that favours the CBT + SMC arm.
FIGURE 8.
Forest plot of standardised treatment effects and 95% CIs of CBT + SMC compared with SMC alone for all outcomes analysed on a continuous scale (standardised treatment effect of > 0 favours CBT + SMC). Adapted with permission from Goldstein et al. 134 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
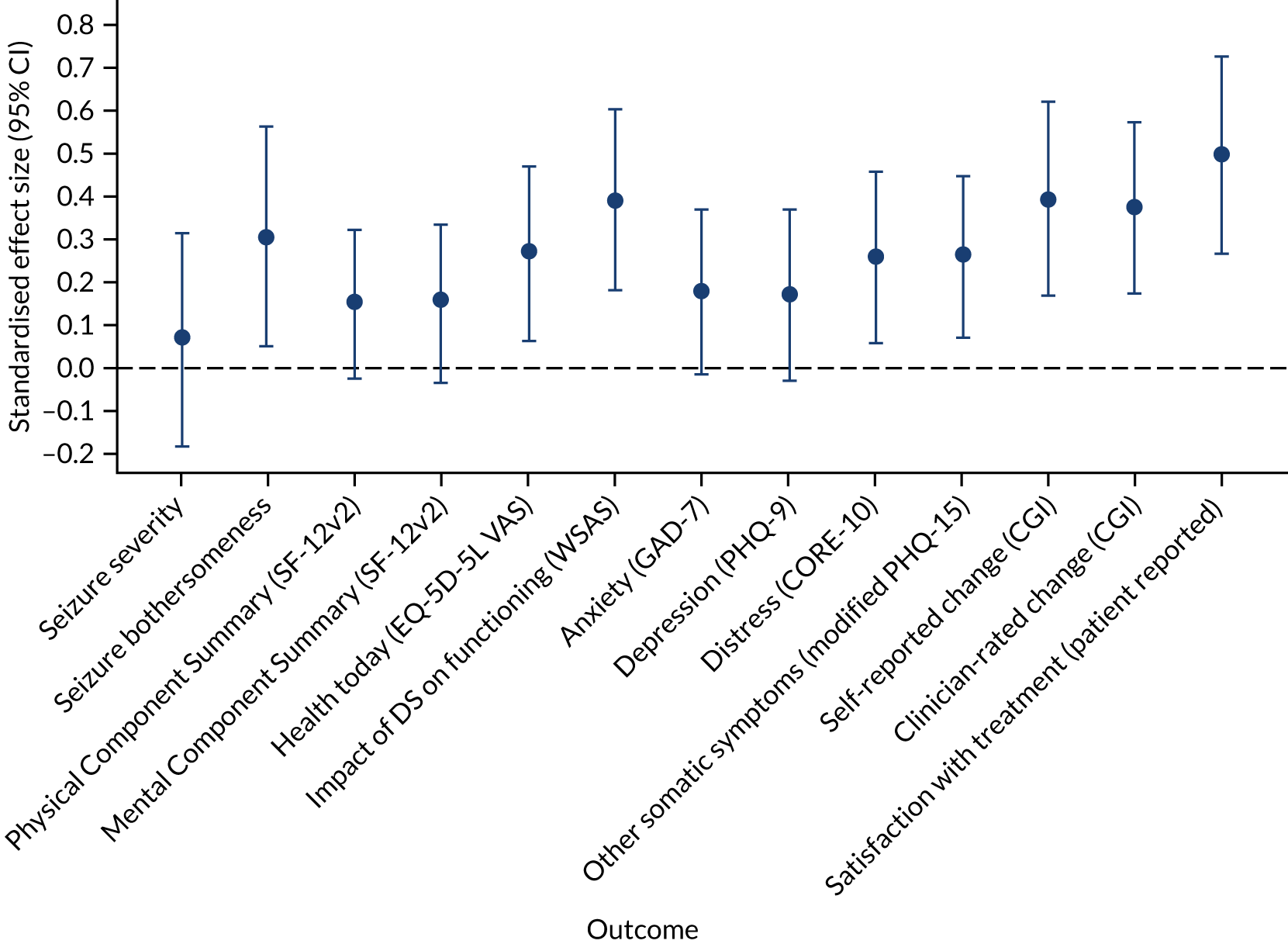
All p-values in Table 16 are prior to any adjustment for multiple testing, which is consistent with the formally agreed and published SAP. However, if conservative adjustments were to be made to the level of significance (α = 0.05), for example a Bonferroni adjustment (α/17), five of the secondary outcomes still strongly suggest that offering CBT plus SMC provided significant benefit compared with offering SMC only (i.e. at p < 0.003). Specifically, participants in the CBT + SMC arm experienced larger numbers of consecutive seizure-free days, better psychosocial functioning, better self-reported and doctor-rated CGI and greater treatment satisfaction than those in the SMC-alone arm.
Adverse events and other indices of harm
In total, 176 AEs were reported during the RCT by 110 participants (Table 17). In the CBT + SMC arm, 97 AEs were reported by 57 participants, and in the SMC-alone arm 79 AEs were reported by 53 participants; the incidence of AEs was, therefore, similar across trial arms. In the RCT, 49 out of 368 (13.3%) randomised participants reported SAEs: 31 SAEs were reported by 25 participants in the CBT + SMC arm and 27 SAEs were reported by 24 participants in the SMC-alone arm. None of the AEs or SAEs recorded in the RCT in the CBT + SMC arm was deemed likely to be related to the study intervention [likelihood of relatedness: remote, n = 33 (34%); none, n = 64 (66%)]. An example of a SAE for which the relatedness was judged as ‘remote’ was sickness and inability to eat or drink anything for several days after a seizure and resulting in the patient's admission to hospital. No AEs were considered to be SARs.
| Body system | Number of AEs (number of participants) | Number of AEs related to DSs | Number of new or worsened health problems | AE intensity | AE duration (days), minimum–maximum | Number who required hospital admission (< 24 hours; > 24 hours) | Number of AEs in CBT + SMC arm (%) | AE relatedness to CODES CBT (CBT + SMC arm only) | Number of serious AEs |
|---|---|---|---|---|---|---|---|---|---|
| Respiratory | 13 (13) | 0 | 13 | Mild, n = 9 Moderate, n = 4 | 0–84 | 1 (0; 1) | 9 (69.2) |
|
2 |
| Gastrointestinal | 14 (14) | 1 | 13 | Mild, n = 7 Moderate, n = 7 | 0–12 | 3 (0; 3) | 10 (71.4) |
|
2 |
| Genitourinary | 13 (13) | 0 | 13 | Mild, n = 8 Moderate, n = 4 Severe, n = 1 | 0–21 | 6 (3; 2) | 10 (76.9) |
|
4 |
| Haematological | 4 (3) | 0 | 4 | Mild, n = 2 Moderate, n = 2 | 3–4 | 3 (0; 3) | 0 (0.0) | N/A | 3 |
| Musculoskeletal | 29 (27) | 10 | 27 | Mild, n = 16 Moderate, n = 12 Severe, n = 1 | 0–61 | 6 (3; 3) | 12 (41.4) |
|
6 |
| Neoplasia | 2 (2) | 0 | 2 | Moderate, n = 1 Severe, n = 1 | 0 | 1 (0; 1) | 0 (0.0) | N/A | 2 |
| Neurological | 11 (7) | 8 | 11 | Mild, n = 4 Moderate, n = 7 | 0–19 | 2 (0; 1) | 3 (27.3) |
|
2 |
| Psychological | 40 (30) | 7 | 37 | Mild, n = 14 Moderate, n = 22 Severe, n = 4 | 0–327 | 8 (2; 5) | 24 (60.0) |
|
21 |
| Immunological | 5 (5) | 0 | 5 | Mild, n = 3 Moderate, n = 2 | 21 | 1 (0; 1) | 3 (60.0) |
|
2 |
| Dermatological | 4 (4) | 2 | 4 | Mild, n = 2 Moderate, n = 2 | 0 | 0 | 3 (75.0) |
|
0 |
| Allergies | 2 (2) | 0 | 2 | Mild, n = 1 Moderate, n = 1 | 1 | 1 (0; 1) | 2 (100.0) |
|
1 |
| Eyes, ear, nose and throat | 12 (12) | 1 | 11 | Mild, n = 10 Moderate, n = 1 Severe, n = 1 | 0–15 | 2 (1; 1) | 9 (75.0) |
|
2 |
| Other | 27 (25) | 11 | 24 | Mild, n = 18 Moderate, n = 8 Severe, n = 1 | 0–68 | 9 (1; 8) | 12 (44.4) |
|
11 |
Only one patient reported a decrease of 20 points on the SF-12v2 Physical Component Summary score between baseline and both the 6-month and the 12-month follow-up assessments (i.e. a change in scores of 2 SDs).
Regarding self-reported scores of ‘much worse’ or ‘very much worse’ on the participant-rated CGI scale change score at the end of the study, 25 (13.7%) participants in the SMC-alone arm and 13 (6.9%) in the CBT + SMC arm self-reported being ‘much worse’ or ‘very much worse’ on the CGI scale at the 12-month follow-up.
Discussion
Summary of results
In the CODES trial, participants with DSs who met other eligibility criteria were first recruited to a screening phase; eligible and consenting patients were subsequently randomised to receive SMC alone or CBT + SMC, with a 12-month post-randomisation follow-up. We randomised 368 people in total: 182 to the SMC-alone arm and 186 to the CBT + SMC arm. At baseline, the median 4-weekly DS frequency overall was 15 DSs (IQR 4–47 DSs), indicating often frequent DSs. There was also evidence of a high level of comorbidity and functional impairment. The study demonstrates the feasibility of delivering a CBT intervention for this group of patients across multiple centres and by therapists of different levels of seniority and experience. Patients demonstrated a high rate of belief in the diagnosis and the treatment offered. Our SMC, given the guidance we provided to clinicians in terms of its content and delivery and the information booklets provided to patients, might be suitably considered to be standardised and specialist medical care rather than standardised medical care.
The characteristics of the trial arms were generally well balanced at baseline. Our overall primary outcome follow-up rate at 12 months was 85% (313/368), balanced by treatment arm. Our analysis adopted the ITT approach with MI and followed our published SAP,92 whereby we evaluated outcomes at 12 months post randomisation only.
Regarding our primary outcome, DS frequency at 12 months post randomisation, the inferential statistics (using the MICE procedure and statistical modelling) estimated a 22% advantage for the CBT + SMC arm; however, this was not statistically significant (p = 0.144). Raw data suggested that both trial arms appeared to show an overall reduction in DS occurrence at 12 months.
All secondary outcomes for which analysis was possible were in the same direction and suggested an overall benefit of CBT + SMC over SMC alone. In 9 of the 16 comparisons this was accompanied by statistical significance (p < 0.05) and, of these, five outcomes were significantly better in the CBT + SMC arm at a p-value of ≤ 0.001. Thus, compared with the SMC-alone arm, the participants in the CBT + SMC arm were found to be less functionally impaired by their DSs, as measured by the WSAS (p < 0.001); they reported longer periods of DS-free days in the last 6 months of the study (p = 0.001), with inferential statistics estimating a 64% advantage in the CBT + SMC arm; their CGI scale scores were better when self-rated (p = 0.001) and clinician rated (p < 0.001); and their satisfaction with treatment was greater (p < 0.001). Effect sizes were moderate and, given that there is no other information on our outcomes from studies that have asked patients with DSs what might represent a clinically meaningful change for them, we cannot say whether or not these effect sizes were clinically meaningful. 136 It is the case that the CBT + SMC arm’s scores on the WSAS, for example, were in the range associated with significant functional impairment but less severe clinical symptomatology than at baseline. 117
Further considerations of the data
Raw data suggested that in the CBT + SMC arm an improvement in DS frequency was observable at the 6-month follow-up point, although our SAP did not allow formal testing at that time point. Although we cannot evaluate which component(s) of the CBT intervention offered particular benefit, our DS-specific CBT approach did include seizure control techniques, which were generally viewed positively by patients and therapists. 135,137
In a related manner, raw data suggest that the SMC-alone arm experienced a reduction in DS frequency at a later follow-up point than the CBT + SMC arm. It is not clear what led to this apparently later improvement in the SMC-alone arm and whether or not this could be attributed to discussion (but not practice) of distraction techniques during SMC sessions or whether direction to self-help websites containing descriptions of potential seizure control approaches may have resulted in the use of techniques to attempt to reduce DSs, but at a slower rate than in the CBT + SMC arm.
Considering the apparent reduction in both trial arms’ DS frequency, it is important to note that we do not know whether or not this overall pattern simply represents the natural progression of the disorder over a comparable period either in general or for people with DSs specifically involved in a RCT. Although other studies have included long-term follow-up after diagnosis and information provision,55 such data are not comparable to those for people in a RCT with frequent research team contact and in a different cultural context. We are aware that SMC might itself be considered to be an active intervention, although we had not conceived of it as such when designing the study. Thus, the material in the information booklets given to participants at both study stages may have led to a better understanding of their disorder that they could share with families (see Chapter 5). Furthermore, the frequent contact by research workers and the interest shown in the participants as a result, and the requirement to self-monitor DSs throughout the study, may have served as an unintended intervention. We know from our qualitative work that many participants also found the contact with the SMC psychiatrists to be supportive, which may have helped reduce arousal levels and DS occurrence.
We did not seek to quantitatively investigate participants’ own perceptions of any advantages deriving from more rapid seizure reduction; however, for recent-onset cases in particular, this might be hypothesised to facilitate their return to work or study and may prevent progression to chronicity, although this requires detailed empirical study. Although CBT + SMC arm participants also seemed to show an earlier decrease in how bothersome they considered their DSs to be (see Table 14), and in their WSAS scores (see Table 15 and Figure 7b), further studies could usefully consider the real-life implications of benefit of CBT + SMC on DS frequency and WSAS scores and whether these are accompanied by improved actual work status or whether any ongoing seizures continue to pose a barrier. Again, although we cannot know which components of the CBT approach were selectively effective, our DS-specific CBT did address avoidance behaviour and this may potentially relate to the improvements seen on the WSAS.
Relation to other studies
Our findings support and extend those in our pilot RCT,1 yielding a similar trajectory for DS frequency over the course of the study. Thus, this pattern of improvement in DS occurrence is not limited to a single centre or set of therapists. The current findings, from the first adequately powered trial in this area, however, indicate a wider range of benefits from CBT + SMC than from SMC alone than we were able to demonstrate previously, even if they were not reflected in our primary outcome. The only other relevant pilot RCT68 that suggested the value of what was termed CBT-informed psychotherapy for seizure reduction was conducted on a much smaller scale, was not powered to permit a comparison between groups and did not evaluate findings at 12 months post randomisation; in addition, that study excluded patients with an epilepsy diagnosis.
Our study suggests that it is possible to engage patients with DSs in psychotherapy at an acceptable level and for the most part in SMC. Our guidance to neurologists in terms of what they should tell patients about the treatment pathway included asking them to communicate to patients some of the advantages of seeing a psychiatrist. It is possible that the guidelines given to neurologists about diagnosis delivery and the information they should convey to patients communicated a level of interest in their condition by professionals that encouraged them to participate in therapy.
Strengths and limitations
We will consider the strengths and limitations of the study overall in Chapter 6, along with recommendations for future research.
Chapter 4 Health economic evaluation
This chapter presents details of the economic evaluation. It includes information on the use and cost of services and the cost-effectiveness of CBT + SMC compared with SMC alone.
Introduction
People with DSs tend to have high use of emergency medical services and other hospital-based services,138,139 particularly associated with delayed diagnosis and access to appropriate treatment. 66 There has been an increase in the diagnosis of DSs,140 which suggests a substantial burden of illness to patients, health systems, carers and society at large. 65 DS-specific CBT has been evaluated in this study as a potentially effective intervention to determine whether or not it could reduce seizure frequency and improve quality of life, as well as psychosocial functioning. However, any change to the way that care is provided is likely to have resource consequences, and this is certainly the case with psychological interventions given the limited supply of trained therapists. Therefore, it is essential to assess the cost-effectiveness of the CBT + SMC intervention compared with SMC alone. As healthcare decision-makers have to make comparisons across diverse clinical areas, it is also necessary to use a generic measure of health outcome, specifically quality-adjusted life-years (QALYs).
The aims of the health economic component are to (1) compare the service use and costs of the CBT + SMC arm with those of the SMC-alone arm at baseline and over the follow-up period, and (2) assess the cost-effectiveness of CBT + SMC relative to SMC alone at the 12-month follow-up in terms of cost per QALY gained.
Economic evaluation methods
Perspective and resource use
The primary economic evaluation adopted a health and social care perspective. This approach is in line with recommendations from the National Institute for Health and Care Excellence (NICE) and is a convention followed in most health technology assessments conducted in the UK. However, it is often the case that interventions have effects outside the health and social care system, so we approach a societal perspective in the secondary analyses. To do this, we include costs associated with lost employment and costs of care provided by family and friends alongside the health and social care costs. We do not include patient time costs, costs associated with lost leisure/recreational activities or costs of any health impacts on carers; therefore, this is not a complete societal perspective.
The number of therapist contacts was recorded as part of the study. Other health and social care service use, receipt of care from family members and time off work were measured at baseline and at 6 and 12 months’ follow-up using a bespoke version of the Client Service Receipt Inventory (CSRI). 141 The CSRI is a widely used questionnaire that is usually adapted for each specific study and allows for the collection of retrospective data on health and social care use. The CSRI listed key services and to measure contacts with individual professionals the participant was asked whether or not this had occurred, how many times in the previous 6 months and what the typical duration of the contact was. For inpatient care, the CSRI asked for information on the specialty and number of days in hospital. For outpatient and accident and emergency (A&E) care it collected information on the number of visits and those visits made by an ambulance. Medication received (for all reasons) was also recorded. If an individual had a missing number of service contacts, then the median of others with these data was used.
The questions on care from family or friends specified specific areas: help in the home, help outside the home, personal care and help with medical problems. The participant was asked to state how many hours of care per week were provided in these areas because of their health problems. Participants were asked to state how many days they had lost from work during the previous 6 months because of health problems. Some services were measured with the CSRI but not costed owing to lack of appropriate values. This included self-help groups and online resources.
In most studies, including CODES, the CSRI relies on participant self-reporting and so may be prone to recall accuracy problems. To address this, we also used data from the HES as an alternative for assessing use and estimating the hospital inpatient, outpatient and emergency care costs. Data were requested from NHS Digital, Electronic Data Research and Innovation Service (NHS Scotland) and NHS Wales Informatics Service, and permission was granted for the use of these data. Ethics approval was granted for us to apply for HES data for participants who had not formally withdrawn from the study; we supplied these organisations with the participants’ personal identifier numbers and NHS (or in Scotland Community Health Index) numbers, and we were sent the data by personal identifier number but without other identifiers. Information was recorded differently for England, Scotland and Wales, although it was still possible to combine the data.
Cost calculations
Intervention costs were derived from information on salaries, overheads, training and supervision. The salaries were based on self-reported bands of the therapists taking part in the study. Costs of other health and social care contacts were calculated by combining the service use data with recognised national unit costs. 142,143 Average wage rates144 were used to value lost work and care provided by family/friends, assuming that time spent providing this could be spent on work or leisure. The use of wage rates in principle reflects the opportunity cost of this time. Medication costs were calculated using information in the British National Formulary. 145 Nationally applicable unit costs were also applied to the HES data that consisted of inpatient days, A&E visits and outpatient contacts. Costs are reported in 2017/18 Great British pounds. The list of unit costs used here is given in Appendix 8.
Quality-adjusted life-years
The main outcome measure in the economic evaluation was QALYs derived from EQ-5D-5L. 116 QALYs are a composite measure combining HRQoL and time, and allow comparisons to be made across different conditions. The EQ-5D-5L is a short scale that is used to measure HRQoL considering five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For each domain, participants rate their current health using a five-point scale to describe the extent to which they can perform activities (ranging from ‘No problems/symptoms’ to ‘Unable to/extreme problems’). This gives rise to 3125 unique health states ranging from 11111 to 55555. The scale also includes a VAS that has been described in Chapter 2. Participants completed the EQ-5D-5L at all study points. Weights that are assumed to be proxies for ‘utilities’ were applied to the health states measured by the EQ-5D-5L. These weights are anchored by 1 (reflecting full health) and 0 (reflecting death), and were obtained from work conducted by Devlin et al. 146 QALYs were generated from the utility scores using area under the curve methods with an assumption of linear change between adjacent time points.
Analysis
Analyses were conducted using Stata software version 15. The use and cost of individual services were described but statistical tests were not conducted to analyse the group differences. Total health and social care costs and societal costs were compared between the two arms over the follow-up period. This was carried out using an ordinary least-squares linear-regression model with follow-up cost as the dependent variable and the baseline costs and group identifier included as independent variables. Cost data usually follow a skewed distribution and, therefore, bootstrapping methods were used to produce 95% CIs around the cost differences. Comparisons of QALYs over the follow-up period were also made using a regression model, controlling for baseline utility scores. 147
To assess cost-effectiveness, costs were combined with QALYs and the primary clinical outcome measure. Missing costs and QALYs were imputed using a best-subset regression procedure in Stata. Incremental costs and outcomes were estimated using regression models and 1000 differences obtained from bootstrapped resamples were plotted on a cost-effectiveness plane to investigate uncertainty around the incremental cost-effectiveness ratio obtained from point estimates. The intervention is considered ‘dominant’ if it is less expensive and more effective than SMC. If it is more expensive and more effective, incremental cost-effectiveness ratios were constructed to indicate the extra cost incurred to achieve a one-unit reduction in DS frequency or one extra QALY. To explore uncertainty around cost-effectiveness estimates (based on QALYs), we plotted cost-effectiveness planes (based on incremental cost–outcome pairs from 1000 bootstrapped resamples) and plotted cost-effectiveness acceptability curves (CEACs) (that were derived using the net benefit approach). We did not produce cost-effectiveness planes or CEACs for the analyses based on the difference in seizure frequency. CEACs reveal to decision-makers the probability of the intervention being cost-effective compared with the alternative, given different (implicit monetary) values placed on incremental improvements in the outcome measurement.
The HES data were analysed by examining the numbers of participants receiving inpatient care, receiving outpatient care or visiting A&E departments at baseline and in the 6 months prior to the 12-month follow-up, and making comparisons between the two arms. Numbers of contacts/days were also compared, as were mean costs. Owing to restrictions agreed when applying for the data, we did not link HES data to any variables other than participant ID and group idenifier. For this reason, these could not be directly used in the cost-effectiveness analysis.
Sensitivity analyses
In the sensitivity analyses, the Short Form questionnaire-6 Dimensions (i.e. utility score) (SF-6D), generated from the SF-12v2, was used to derive QALYs. 148 The SF-12v2 contains a wider range of items than the EQ-5D-5L and, thus, could reflect the impact of DSs more appropriately. A comparison of HRQoL has demonstrated that QALYs derived from the SF-6D performed well for people with epilepsy in discriminating between those with and those without seizures over a 2-year follow-up period,149 although there was no relevant follow-up data for DS patients. Further sensitivity analyses were conducted by increasing and decreasing the intervention costs by 25% and 50%. We examined the impact on societal costs of using alternative unit costs for informal care and lost employment. Specifically, we used the healthcare worker unit cost (£26 per hour) to derive informal care costs and a minimum wage of £7.50 for informal care and lost employment.
Results
Service use
Service use during the 6-month period prior to baseline and at the 6- and 12-month follow-up period is described in Table 18. At baseline, > 80% of the participants in each arm had contact with GPs. Other community services were used by far fewer participants, with the next most commonly used being practice nurses. The mean number of contacts with community services was largest for GPs, followed by home helps. There were no obvious differences in the use of community services between the arms. During the 12-month follow-up period, around three-quarters of each trial arm had contacts with GPs. Other community services were again used by relatively few participants, although there were some differences between arms with the CBT + SMC arm participants more likely to have contact with practice nurses and the SMC-alone arm more likely to have contact with physiotherapists. It is also evident that a small number of participants were reporting receipt of CBT at baseline in each of the arms. At follow-up, this occurred for the CBT + SMC arm only. It may be that the seven participants who reported such contacts were referring to the actual trial intervention, or it may be that they were in fact receiving non-intervention CBT.
| Service category | Unit of measure | Baseline (N = 367) | 6-month follow-up (N = 277) | 12-month follow-up (N = 293) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMC alone (N = 182) | CBT + SMC (N = 185) | SMC alone (N = 143) | CBT + SMC (N = 134) | SMC alone (N = 145) | CBT + SMC (N = 148) | ||||||||
| Users, n (%) | Contacts for all, mean (SD) | Users, n (%) | Contacts for all, mean (SD) | Users, n (%) | Contacts for all, mean (SD) | Users, n (%) | Contacts for all, mean (SD) | Users, n (%) | Contacts for all, mean (SD) | Users, n (%) | Contacts for all, mean (SD) | ||
| Community services | |||||||||||||
| GP | Contact | 147 (81) | 5.0 (9.3) | 164 (88) | 5.4 (10.1) | 106 (63) | 3.1 (4.6) | 100 (59) | 3.2 (5.2) | 117 (71) | 3.3 (3.7) | 123 (75) | 3.5 (4.0) |
| Practice nurse | Contact | 65 (36) | 0.7 (1.4) | 74 (40) | 1.1 (2.2) | 48 (29) | 0.6 (1.1) | 45 (27) | 1.0 (1.7) | 46 (28) | 0.7 (1.9) | 65 (40) | 1.0 (1.8) |
| Epilepsy nurse | Contact | 7 (4) | 0.1 (0.5) | 5 (3) | 0.2 (1.9) | 6 (4) | 0.1 (0.4) | 3 (2) | 0.04 (0.3) | 3 (2) | 0.02 (0.1) | 3 (2) | 0.04 (0.3) |
| Physiotherapist | Contact | 20 (11) | 0.6 (2.9) | 33 (18) | 0.7 (2.3) | 16 (10) | 0.4 (2.2) | 19 (11) | 0.7 (2.8) | 21 (22) | 0.5 (1.4) | 18 (11) | 0.5 (2.2) |
| Social worker | Contact | 8 (4) | 0.1 (0.5) | 13 (7) | 0.3 (2.4) | 10 (6) | 0.2 (1.0) | 11 (7) | 0.9 (6.4) | 13 (8) | 0.2 (0.8) | 15 (9) | 1.2 (11.5) |
| Counsellor/psychologist | Contact | 27 (15) | 1.0 (7.0) | 27 (15) | 0.8 (3.3) | 23 (14) | 0.6 (2.3) | 17 (10) | 1.0 (3.5) | 22 (13) | 0.8 (2.8) | 17 (10) | 0.8 (3.4) |
| Other CBT | Contact | 10 (6) | 0.4 (1.9) | 9 (5) | 0.4 (1.9) | 7 (4) | 0.2 (1.0) | 9 (5) | 0.4 (1.9) | 0 (0.0) | 0 (0.0) | 7 (4) | 0.3 (1.4) |
| Other talk therapy | Contact | 18 (10) | 1.2 (7.4) | 21 (11) | 1.1 (3.8) | 2 (1) | 0.1 (1.2) | 7 (4) | 0.7 (3.5) | 7 (4) | 0.2 (1.3) | 5 (3) | 0.4 (2.3) |
| Home help: household tasks | Contact | 11 (6) | 4.0 (21.9) | 12 (6) | 2.6 (15.6) | 33 (20) | 5.8 (26.9) | 50 (30) | 4.7 (22.0) | 14 (8) | 9.3 (35.6) | 13 (8) | 6.5 (26.1) |
| Home help: personal care | Contact | 5 (3) | 2.8 (18.5) | 8 (4) | 3.0 (17.3) | 7 (4) | 3.4 (22.4) | 10 (17) | 7.6 (30.5) | 7 (4) | 8.1 (46.4) | 10 (6) | 8.0 (46.7) |
| Other community services | Contact | 21 (12) | 0.7 (3.0) | 20 (11) | 0.7 (3.2) | 16 (10) | 0.6 (1.9) | 18 (11) | 0.5 (1.7) | 18 (11) | 0.7 (2.6) | 21 (13) | 1.4 (7.2) |
| Medication | Number | 139 (77) | 4.3 (4.0) | 157 (85) | 4.5 (4.1) | 121 (72) | 4.8 (4.6) | 107 (63) | 4.5 (3.8) | 132 (81) | 4.5 (4.5) | 117 (71) | 3.9 (4.0) |
| Hospital-based services | |||||||||||||
| Inpatient | Length of stay (days) | 64 (36) | 2.3 (5.7) | 67 (36) | 2.3 (5.4) | 28 (17) | 0.5 (1.8) | 22 (13) | 0.7 (3.3) | 40 (24) | 1.1 (2.8) | 28 (17) | 1.5 (7.8) |
| Outpatient | Contact | 66 (37) | 1.1 (2.6) | 64 (35) | 1.0 (2.4) | 49 (29) | 0.7 (1.2) | 52 (31) | 1.2 (3.0) | 53 (32) | 1.1 (2.8) | 52 (32) | 1.1 (2.7) |
| Ambulance use | Contact | 78 (43) | 1.0 (1.5) | 65 (35) | 1.5 (4.4) | 40 (24) | 0.9 (3.0) | 27 (16) | 0.6 (1.5) | 38 (23) | 0.5 (1.4) | 33 (20) | 0.4 (1.1) |
| A&E visits | Contact | 104 (57) | 1.5 (2.4) | 87 (47) | 2.1 (5.2) | 57 (34) | 1.3 (3.9) | 48 (28) | 0.9 (1.8) | 67 (41) | 0.9 (1.5) | 56 (34) | 0.9 (2.2) |
| Clinical Decision Unit | Contact | 25 (14) | 0.5 (4.1) | 22 (12) | 0.2 (0.8) | 15 (9) | 0.4 (1.0) | 13 (8) | 0.3 (0.7) | 14 (8) | 0.4 (0.7) | 12 (7) | 0.3 (0.4) |
| Psychiatric appointment | Contact | 140 (78) | 1.0 (1.5) | 140 (76) | 1.0 (1.1) | 76 (46) | 1.1 (1.6) | 75 (44) | 1.3 (1.5) | 86 (52) | 1.3 (1.5) | 81 (50) | 1.4 (2.3) |
| Neurology appointment | Contact | 152 (84) | 1.4 (1.3) | 153 (83) | 1.5 (1.6) | 71 (43) | 0.8 (1.2) | 64 (38) | 0.9 (1.8) | 67 (41) | 0.6 (0.9) | 52 (32) | 0.5 (0.7) |
| Other hospital care | Contact | 23 (13) | 0.4 (1.7) | 24 (13) | 0.6 (2.5) | 23 (14) | 1.0 (6.3) | 17 (10) | 0.7 (3.2) | 17 (10) | 0.7 (3.2) | 17 (10) | 0.4 (2.3) |
| Informal care | |||||||||||||
| Personal care | Hours per week | 84 (47) | 7.0 (25.1) | 85 (46) | 6.9 (22.9) | 66 (40) | 4.0 (8.2) | 51 (30) | 8.8 (2.9) | 60 (36) | 4.5 (17.8) | 54 (33) | 5.5 (23.9) |
| Medical procedures | Hours per week | 42 (23) | 2.2 (12.9) | 34 (18) | 2.8 (15.8) | 38 (23) | 1.9 (14.1) | 28 (17) | 5.3 (26.4) | 38 (23) | 4.1 (22.2) | 33 (20) | 3.9 (23.7) |
| Help in home | Hours per week | 115 (64) | 10.9 (28.3) | 103 (56) | 9.6 (24.1) | 81 (49) | 12.3 (32.0) | 68 (40) | 11.4 (32.0) | 87 (53) | 9.9 (26.2) | 82 (50) | 9.4 (28.0) |
| Help outside home | Hours per week | 109 (61) | 4.8 (17.8) | 87 (52) | 6.7 (23.3) | 83 (50) | 6.4 (22.7) | 71 (42) | 7.4 (25.5) | 88 (53) | 7.1 (27.2) | 75 (46) | 6.1 (22.2) |
| Time spent ‘on-call’ | Hours per week | 72 (40) | 43.5 (70.2) | 66 (36) | 37.3 (65.2) | 37 (22) | 28.9 (60.5) | 34 (20) | 29.5 (59.7) | 57 (35) | 49.5 (72.8) | 48 (29) | 46.6 (73.2) |
| Productivity loss | |||||||||||||
| Days off work owing to ill health | Days | 61 (34) | 31 (60) | 64 (35) | 27 (58) | 43 (26) | 11.7 (39.2) | 40 (24) | 10.5 (37.1) | 24 (15) | 10.4 (39.0) | 26 (15) | 6.0 (25.8) |
| Hours off work owing to ill health | Hours per week | 10 (6) | 0.6 (2.9) | 7 (2) | 0.4 (2.1) | 9 (5) | 1.1 (5.4) | 7 (4) | 0.8 (4.9) | 6 (4) | 0.7 (4.1) | 4 (2) | 0.6 (4.5) |
Turning to hospital use, Table 18 shows that around one-third of each arm had inpatient stays during the baseline period, with a similar average number of inpatient days. Outpatient appointments also occurred for about one-third of each arm. Use of an ambulance was slightly more likely in the SMC-alone arm, as were visits to A&E departments. Contacts with psychiatrists occurred for about three-quarters of each arm and contacts with neurologists for > 80%. During the entire 12-month follow-up period, there was less use of inpatient care, especially in the CBT + SMC arm. Outpatient use remained similar to baseline and there was reduction for both trial arms in visits to A&E departments. There were also reductions for both trial arms in contacts with psychiatrists and neurologists.
Receipt of informal care was very high in both trial arms, with nearly half of the participants at baseline reporting having received help with personal care and over half reporting having received help inside and outside the home. Over one-third reported that informal carers spent some time ‘on-call’ (i.e. had to remain with the participant in the home even if no care was being directly provided). When this occurred, it accounted for the most hours of care received per week. During the follow-up period, levels of informal care remained high and with no clear differences between the arms.
Service costs
The mean number of CBT sessions attended by participants in the CBT + SMC arm was 10. Combined with the unit cost of a clinical psychologist (used because this is the largest professional grouping of the current therapists) produced associated mean costs of £1064. One participant in the SMC-alone arm had access to the intervention and the mean cost for the arm was estimated at £7.
Mean costs of community services during the baseline period were highest for GP contacts and home help providing both personal care and support for household tasks (Table 19). The latter was more expensive for the SMC-alone arm. At follow-up, intervention costs were the highest healthcare cost for the CBT + SMC arm. At both the 6-month and the 12-month follow-up, GPs and home help service items again accounted for relatively high costs. Medication costs at baseline were similar between the arms, whereas at both follow-up points they were somewhat higher for the SMC-alone arm. Inpatient costs were similar at baseline but higher at follow-up for the CBT + SMC arm. However, the difference at 12 months of £332 is equivalent to < 1 extra inpatient day. Other hospital costs were relatively similar between trial arms at both baseline and follow-up, as were the total hospital costs.
| Cost component | Time point, mean cost (SD) (£) | |||||
|---|---|---|---|---|---|---|
| Baseline (N = 367) | 6-month follow-up (N = 277) | 12-month follow-up (N = 293) | ||||
| SMC alone (n = 182) | CBT + SMC (n = 185) | SMC alone (n = 143) | CBT + SMC (n = 134) | SMC alone (n = 145) | CBT + SMC (n = 148) | |
| Intervention | – | – | 7 (99) | 1064 (411) | ||
| Community services | ||||||
| GP | 250 (385) | 272 (559) | 159 (232) | 148 (255) | 188 (368) | 168 (247) |
| Practice nurse | 5 (11) | 8 (20) | 5 (12) | 5 (11) | 6 (17) | 8 (19) |
| Epilepsy nurse | 4 (25) | 10 (108) | 2 (11) | 6 (60) | 0.5 (3) | 1 (9) |
| Physiotherapist | 17 (81) | 23 (84) | 11 (53) | 20 (69) | 16 (61) | 13 (47) |
| Social worker | 3 (16) | 13 (85) | 9 (44) | 21 (139) | 7 (33) | 30 (250) |
| Counsellor/psychologist | 98 (619) | 97 (409) | 83 (314) | 123 (503) | 104 (408) | 103 (510) |
| Other CBT | 35 (180) | 27 (175) | 19 (101) | 46 (207) | 0 (–) | 28 (148) |
| Other talk therapy | 111 (604) | 150 (698) | 12 (106) | 70 (456) | 30 (155) | 39 (236) |
| Home help: household tasks | 297 (1886) | 146 (915) | 358 (2339) | 586 (2417) | 379 (2123) | 368 (2186) |
| Home help: personal care | 412 (2978) | 142 (1011) | 111 (821) | 216 (1017) | 172 (999) | 188 (1021) |
| Other community services | 100 (405) | 90 (434) | 76 (259) | 69 (238) | 93 (357) | 203 (999) |
| Total community costs | 1332 (4222) | 978 (1901) | 845 (2527) | 1310 (3386) | 995 (2664) | 1149 (3102) |
| Medication | 277 (786) | 253 (621) | 528 (1866) | 384 (943) | 552 (1125) | 343 (858) |
| Hospital-based services | ||||||
| Inpatient care | 1506 (3664) | 1485 (3483) | 344 (1161) | 435 (2120) | 460 (1114) | 792 (5047) |
| Outpatient care | 145 (350) | 140 (324) | 95 (168) | 171 (408) | 156 (390) | 147 (374) |
| Ambulance use | 118 (182) | 176 (520) | 107 (356) | 67 (182) | 65 (161) | 47 (129) |
| A&E visits | 227 (351) | 306 (761) | 200 (573) | 137 (260) | 138 (222) | 131 (319) |
| Clinical Decision Unit | 79 (602) | 32 (113) | 63 (141) | 43 (102) | 53 (101) | 38 (65) |
| Psychiatrist appointment | 113 (160) | 99 (120) | 122 (169) | 154 (239) | 139 (162) | 149 (250) |
| Neurology appointment | 239 (216) | 247 (264) | 114 (170) | 120 (252) | 89 (119) | 63 (99) |
| Other hospital care | 57 (234) | 82 (348) | 142 (857) | 101 (432) | 36 (181) | 56 (309) |
| Total hospital costs | 2484 (4046) | 2567 (4006) | 1187 (1826) | 1228 (2548) | 1136 (1409) | 1423 (5153) |
| Health and social care costs | 4092 (5840) | 3798 (4797) | 2560 (3712) | 2922 (4795) | 2683 (3676) | 2915 (6346) |
| Informal care (hours per week) | ||||||
| Personal care | 2470 (8896) | 2457 (8140) | 1423 (2932) | 3121 (10,293) | 1607 (6355) | 1959 (8502) |
| Medical procedures | 774 (4552) | 1011 (5617) | 689 (5017) | 1874 (9388) | 1452 (7902) | 1370 (8443) |
| Help in home | 3907 (10,017) | 3401 (8581) | 4388 (11,387) | 4045 (11,369) | 3505 (9302) | 3350 (9975) |
| Help outside home | 1702 (6303) | 2357 (8279) | 2263 (8080) | 2622 (9082) | 2176 (7895) | 2511 (9691) |
| Time spent ‘on-call’ | 17,223 (25,844) | 13,254 (23,176) | 10,282 (21,514) | 10,506 (21,241) | 17,602 (25,910) | 16,587 (26,043) |
| Total informal care costs | 26,045 (40,986) | 22,480 (38,769) | 19,045 (29,996) | 22,168 (47,513) | 26,342 (42,484) | 25,777 (49,441) |
| Productivity costs | ||||||
| Days off work owing to illness | 3117 (6178) | 2783 (5955) | 1206 (4043) | 1081 (3821) | 1070 (4017) | 619 (2661) |
| Hours off work owing to illness | 7 (39) | 5 (31) | 17 (79) | 12 (72) | 10 (60) | 9 (66) |
| Total productivity costs | 3124 (6179) | 2788 (5954) | 1223 (4039) | 1093 (3843) | 1080 (4015) | 628 (2659) |
| Total societal costs | 33,261 (43,242) | 29,066 (41,208) | 22,828 (30,641) | 26,183 (49,145) | 30,105 (43,245) | 29,320 (50,176) |
Informal care costs were substantial at all time periods and for both trial arms. No major differences between the arms were apparent. Lost employment costs were higher for the SMC-alone arm at both baseline and follow-up.
The total mean health and social care costs at baseline were £4092 for the SMC-alone arm and £3798 for the CBT + SMC arm. From baseline to the 6-month follow-up, the costs were £2560 and £2922, respectively, whereas at the 12-month follow-up they were £2683 and £2915, respectively. The difference in health and social care costs over the entire follow-up period, adjusted for baseline, was £1834 (bootstrapped 95% CI £478 to £3475), which is around £840 more than the extra cost accounted for by the intervention itself.
The mean societal costs at baseline were £33,261 for the SMC-alone arm and £29,066 for the CBT + SMC arm. At 6 months, the societal costs were £22,828 for the SMC-alone arm and £26,183 for the CBT + SMC arm. During the combined 12-month follow-up period, the societal costs were £55,503 and £52,933, respectively. The imputed difference, adjusted for baseline costs, was £6566 (bootstrapped 95% CI –£5909 to £18,919).
Quality-adjusted life-years
Table 20 reports the utility scores and QALYs derived from both the EQ-5D-5L and the SF-12v2 measures. At baseline and at the 6- and 12-month follow-ups, the CBT + SMC arm had a slightly higher utility score. The CC analysis shows a QALY difference of 0.0416 in favour of the CBT + SMC arm. After imputation, the difference is similar at 0.0457. However, the QALY difference after controlling for baseline is less: 0.0152 for imputed cases (bootstrapped 95% CI –0.0106 to 0.0392).
| Measure | Trial arm, n; mean (SD) | |
|---|---|---|
| SMC alone | CBT + SMC | |
| EQ-5D-5L utility at baseline | 181; 0.5847 (0.3315) | 182; 0.6172 (0.3090) |
| EQ-5D-5L utility at 6 months | 143; 0.5755 (0.3200) | 134; 0.6005 (0.3399) |
| EQ-5D-5L utility at 12 months | 144; 0.5644 (0.3090) | 148; 0.6362 (0.3176) |
| EQ-5D-5L-based QALYs (CC) | 127; 0.5711 (0.2910) | 123; 0.6127 (0.3086) |
| EQ-5D-5L-based QALYs (imputed) | 182; 0.5710 (0.3086) | 185; 0.6167 (0.2874) |
| SF-6D utility at baseline | 181; 0.5680 (0.1291) | 182; 0.5778 (0.1263) |
| SF-6D utility at 6 months | 142; 0.5744 (0.1336) | 132; 0.5957 (0.1446) |
| SF-6D utility at 12 months | 144; 0.5715 (0.1412) | 148; 0.6179 (0.1561) |
| SF-6D-based QALYs (CC) | 125; 0.5708 (0.1173) | 122; 0.6001 (0.1303) |
| SF-6D-based QALYs (imputed) | 182; 0.5683 (0.1100) | 185; 0.5916 (0.1167) |
| EQ–5D crosswalk utility at baseline | 181; 0.4842 (0.3358) | 182; 0.5172 (0.3412) |
| EQ–5D crosswalk utility at 6 months | 143; 0.4825 (0.3392) | 134; 0.4921 (0.3878) |
| EQ–5D crosswalk utility at 12 months | 144; 0.4515 (0.3458) | 148; 0.4515 (0.3633) |
| EQ–5D crosswalk QALYs (CC) | 127; 0.4722 (0.3087) | 123; 0.5076 (0.3483) |
| EQ–5D crosswalk QALYs (imputed) | 182; 0.4735 (0.2926) | 185; 0.5092 (0.3203) |
When the SF-12v2 was used to generate the SF-6D, it was shown from the CC analysis that CBT + SMC accrued 0.0293 more QALYs than SMC alone. After imputation, the difference was 0.0233 QALYs. When baseline utility was controlled for, the differences became 0.0231 and 0.0157 QALYs, respectively. We also used the crosswalk method150 to convert the EQ-5D-5L scores to tariffs derived for the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). This resulted in lower utility scores for both trial arms and an incremental QALY gain for CBT + SMC of 0.0089 QALYs.
Cost-effectiveness results
After imputation and controlling for baseline costs and utility, the CBT + SMC arm was shown to have incremental costs of £1834 and incremental QALYs based on the EQ-5D-5L of 0.0152 (Table 21). This gives an incremental cost per QALY of £120,658 compared with SMC alone. The incremental QALYs based on the SF-6D were 0.0157 QALYs, resulting in an incremental cost per QALY of £116,815.
| Measure | NHS perspective | |
|---|---|---|
| Imputed (adjusted) | CC (adjusted) | |
| Incremental cost (intervention – control) | £1834 (95% CI £478 to £3475) | £1251 (95% CI –£299 to £2960) |
| Incremental QALYs: EQ-5D-5L (intervention – control) | 0.0152 (95% CI –0.0106 to 0.0392) | 0.0152 (95% CI –0.0230 to 0.0518) |
| Incremental cost-effectiveness ratio: EQ-5D-5L | £120,658 per QALY | £82,303 per QALY |
| Incremental QALYs: SF-6D (intervention – control) | 0.0157 (95% CI 0.0053 to 0.0271) | 0.0231 (95% CI 0.0067 to 0.0395) |
| Incremental cost-effectiveness ratio SF-6D | £116,815 per QALY | £54,156 per QALY |
| Incremental QALYs: EQ-5D-3L crosswalk (intervention – control) | 0.0089 (95% CI –0.0202 to 0.0393) | 0.0089 (95% CI –0.0349 to 0.0509) |
| Incremental cost-effectiveness ratio: EQ-5D-3L crosswalk | £206,067 per QALY | £140,562 per QALY |
| Incremental seizures (intervention – control) | 3 | 3 |
| Incremental cost-effectiveness ratio: seizures reduced | £611 per reduced seizure | £417 per reduced seizure |
Uncertainty around the cost-effectiveness results based on the EQ-5D-5L is depicted in Figure 9. Most of the bootstrapped replications fall in the top-right quadrant in which costs and QALYs are higher for the CBT + SMC arm. The percentage of replications falling below the red line indicating that the intervention is cost-effective based on a threshold of £20,000 per QALY is 27%. The corresponding CEAC (Figure 10) indicates that the probability of cost-effectiveness increases as the threshold value on a QALY rises, but is relatively low. Similar results apply to the cost-effectiveness results derived from the SF-6D (Figures 11 and 12).
FIGURE 9.
Cost-effectiveness plane using the EQ-5D-5L and costs measured from the health and social care perspective.
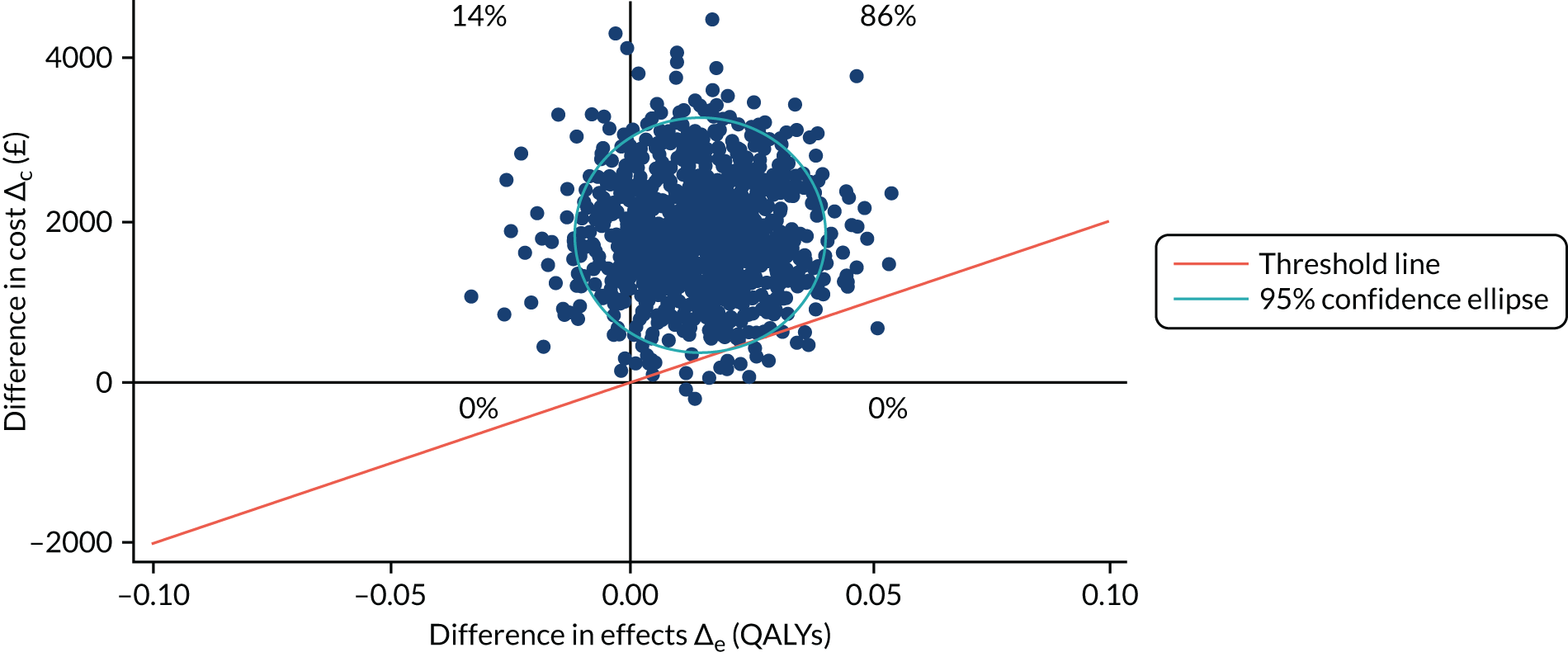
FIGURE 10.
Cost-effectiveness acceptability curve using the EQ-5D-5L and costs measured from the health and social care perspective.

FIGURE 11.
Cost-effectiveness plane using the SF-6D measure derived from the SF-12v2 and costs measured from the health and social care perspective.
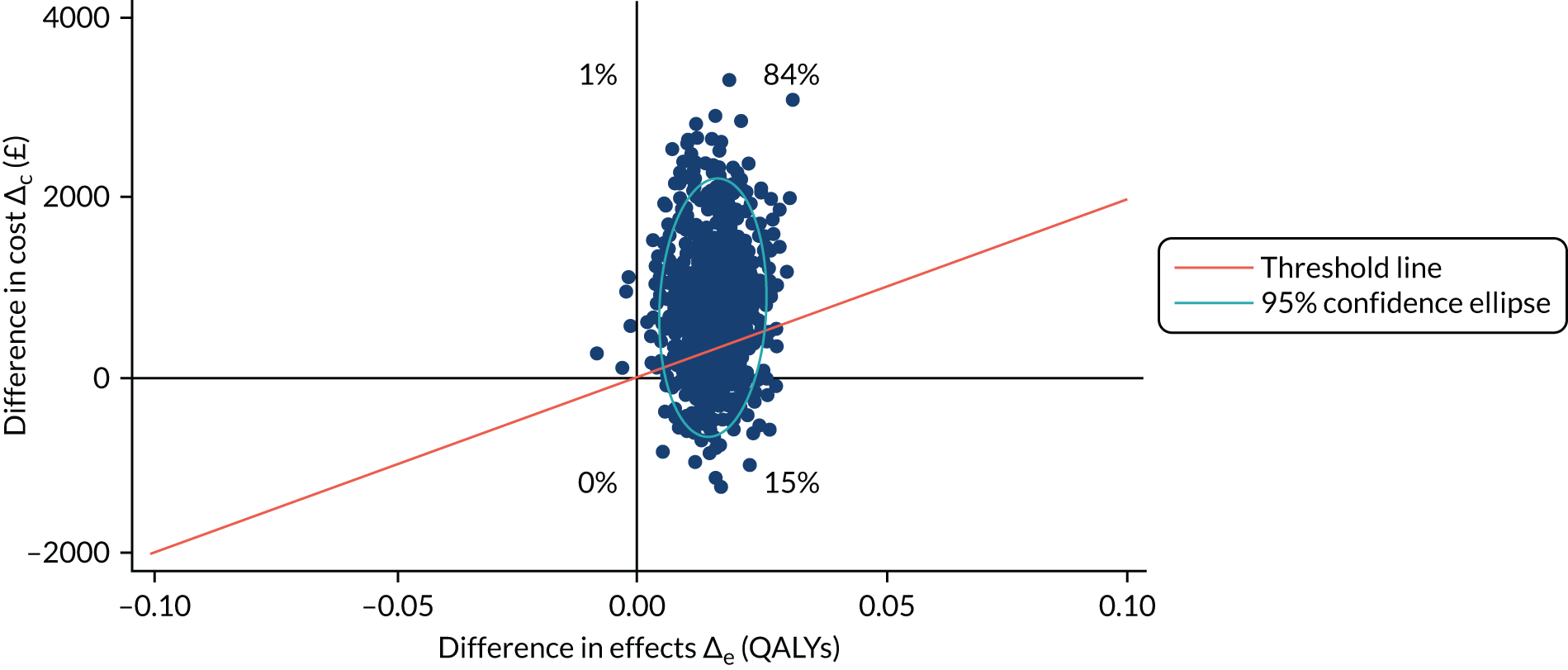
FIGURE 12.
Cost-effectiveness acceptability curve using the SF-6D derived from the SF-12v2 and costs measured from the health and social care perspective.

With the crosswalk method used for the utility scores, the incremental cost-effectiveness ratio (ICER) for CBT + SMC increased to £206,067 per QALY. When the intervention costs were reduced by 25%, the ICER fell to £103,224 per QALY (Table 22) and further to £85,724 when the costs were reduced by 50%. By definition, the ICERs rise with higher intervention costs to £138,158 and £155,592 for 25% and 50% increases in intervention costs, respectively.
| Measure | NHS perspective | |
|---|---|---|
| Imputed (unadjusted) | Imputed (adjusted) | |
| Incremental cost (intervention – control): minus 25% of the intervention costs | £1526 (95% CI £125 to £3050) | £1569 (95%CI £197 to £3179) |
| Incremental QALYs: EQ-5D-5L (intervention – control) | 0.0441 (95% CI –0.0123 to 0.1007) | 0.0152 (95% CI –0.0106 to 0.0392) |
| Incremental cost-effectiveness ratio: EQ-5D-5L | £34,603 per QALY | £103,224 per QALY |
| Incremental cost (intervention – control): plus 25% of the intervention costs | £2057 (95% CI £690 to £3510) | £2100 (95% CI £743 to £3551) |
| Incremental QALYs: EQ-5D-5L (intervention – control) | 0.0441 (95% CI –0.0123 to 0.1007) | 0.0152 (95% CI –0.0106 to 0.0392) |
| Incremental cost-effectiveness ratio: EQ-5D-5L | £46,644 per QALY | £138,158 per QALY |
| Incremental cost (intervention – control): minus 50% of the intervention costs | £1261 (95% CI –£140 to £2813) | £1303 (95% CI £119 to £2902) |
| Incremental QALYs: EQ-5D-5L (intervention – control) | 0.0441 (95% CI –0.0123 to 0.1007) | 0.0152 (95% CI –0.0106 to 0.0392) |
| Incremental cost-effectiveness ratio: EQ-5D-5L | £28,594 per QALY | £85,724 per QALY |
| Incremental cost (intervention – control): plus 50% of the intervention costs | £2323 (95% CI £941 to £4024) | £2365 (95% CI £1029 to £3925) |
| Incremental QALYs: EQ-5D-5L (intervention – control) | 0.0441 CI (95% CI –0.0123 to 0.1007) | 0.0152 (95% CI –0.0106 to 0.0392) |
| Incremental cost-effectiveness ratio: EQ-5D-5L | £52,676 per QALY | £155,592 per QALY |
We have shown that societal costs are higher by £6566 for the CBT + SMC arm. With our main analysis showing a QALY difference of 0.0152, this results in an ICER of £431,974 per QALY.
Applying the unit cost of a healthcare worker resulted in increased informal care costs for both trial arms (£66,239 for the SMC-alone arm and £65,486 for the CBT + SMC arm). The difference between the arms (£2359) was not statistically significant (95% CI –£19,583 to £24,280). Applying the minimum wage of £7.50 to informal care would reduce the informal care cost to £19,209 and £18,990, respectively. Lost employment costs would be reduced from £2303 to £1174 for the SMC-alone arm and from £1721 to £903 for the CBT + SMC arm using the minimum wage.
Cost-effectiveness based on the number of seizures
The difference in median seizure rate between the two arms at 12 months is three seizures (see Table 14). The difference in costs is £1251 for the CC analysis and £1834 after imputation, producing ICERs of £41,740 and £611, respectively. The results imply that the cost for an avoided seizure over the 4 weeks prior to the 12-month follow-up is £611. There is, however, no accepted (cost-effectiveness) value of a seizure avoided and, therefore, it is simply a value judgement as to whether or not this represents value for money.
Hospital Episode Statistics analysis
The findings from the analysis of the HES are shown in Table 23. Between baseline and 6 months, > 80% of each arm had outpatient contacts, which fell substantially for the follow-up period. The number of outpatient contacts was very similar between arms and over time. Direct comparison with the CSRI data is difficult when we look at all outpatient contacts because we separated out psychiatrist and neurologist contacts with the CSRI data. At baseline, the CSRI data report 76% of patients having psychiatrist contacts in the CBT + SMC arm and 78% in the SMC-alone arm. The figures for neurologist contacts are 83% and 84%, respectively. The figure for other hospital care is 13% for both trial arms. Although all participants should have had neurologist and psychiatrist contacts, both the CSRI and the HES data indicate similar levels of use, and this is < 100%. Between one-fifth and one-quarter of patients in both trial arms at both time points have missed appointments. This information is not provided in the CSRI.
| Service | Baseline | Months 7–12 of follow-up | ||
|---|---|---|---|---|
| SMC alone | CBT + SMC | SMC alone | CBT + SMC | |
| Outpatient: attended | ||||
| n (%) | 153 (84) | 165 (89) | 104 (57) | 118 (63) |
| Mean (SD) contacts by users | 4.3 (4.2) | 4.0 (3.5) | 4.5 (3.9) | 5.0 (4.5) |
| Mean (SD) cost (2017/18, £) | 498 (574) | 487 (478) | 350 (507) | 436 (594) |
| Outpatient: DNA | ||||
| n (%) | 35 (19) | 47 (25) | 41 (23) | 50 (27) |
| Mean (SD) times by users | 1.5 (0.9) | 1.4 (0.7) | 1.5 (0.7) | 1.7 (0.9) |
| A&E | ||||
| n (%) | 81 (45) | 85 (46) | 65 (36) | 70 (38) |
| Mean (SD) visits by users | 2.1 (1.8) | 2.6 (2.3) | 2.3 (2.1) | 2.1 (1.7) |
| Mean (SD) cost (2017/18, £) | 141 (235) | 174 (294) | 119 (248) | 116 (214) |
| Inpatient stay | ||||
| n (%) | 57 (31) | 62 (33) | 39 (21) | 42 (23) |
| Mean (SD) days by users | 4.8 (6.5) | 4.7 (6.0) | 3.3 (4.8) | 3.2 (5.3) |
| Mean (SD) cost (2017/18, £) | 935 (2672) | 977 (2562) | 445 (1623) | 451 (1768) |
If we combine the cost of outpatient care from Table 23 with the psychiatrist and neurologist costs (these categories should be mutually exclusive), we find similarities with the costs in Table 19. (Other hospital costs are not included here.) At baseline, this cost is £497 for SMC alone and £491 for CBT + SMC (whereas the HES data show costs of £498 and £487, respectively). In the 6 months prior to the 12-month follow-up, the costs are £384 for SMC alone and £359 for CBT + SMC. The HES-based costs are £350 and £436, respectively. Therefore, there is reasonably good agreement between the CSRI and the HES data for outpatient care, except for CBT + SMC for which HES gives higher costs. At follow-up, the CBT + SMC arm does make more use of this than the SMC-alone arm, but the difference is not large.
The HES data also show very similar rates of A&E department contacts between trial arms, with participants of both arms less likely to attend in the follow-up period. The rate of use at baseline for the CBT + SMC arm is very similar when comparing HES (46%) with CSRI (47%) data; there is more of a difference for the SMC-alone arm (45% and 57%, respectively). The HES-based costs of A&E care are rather different at baseline from the CSRI-based costs (with higher costs for the CSRI), particularly for the CBT + SMC arm. However, at the 12-month follow-up there are very similar costs (£119 and £138 for the SMC-alone arm and £116 and £131 for the CBT + SMC arm, respectively).
At baseline, HES report that 31% of the SMC-alone arm and 33% of the CBT + SMC arm use inpatient care. The CSRI data are 36% for each arm. However, the costs of care are noticeably different when using the CSRI or the HES data. Costs based on the former are higher at baseline for both trial arms and for the CBT + SMC arm at follow-up. The implication is that although participants report a similar rate of inpatient use to HES, they also report more days in hospital. The difference at baseline between methods is comparable for the two arms, whereas at follow-up it seems to result in costs that are potentially overestimated by £341 for the CBT + SMC arm. If we were to reduce the overall incremental costs of £1834 for the CBT + SMC arm compared with the SMC-alone arm by this amount, the ICER is reduced to £95,096 per QALY (based on the EQ-5D-5L).
Discussion
Main findings
To our knowledge, this is the first economic evaluation of CBT for people with DSs participating in an RCT evaluating a psychotherapeutic intervention. The main clinical results of the study have shown that although CBT + SMC was superior to SMC alone on most of the secondary outcomes, the primary outcome was not significantly different between the trial arms (see Chapter 3). In this chapter, we have found that CBT + SMC produces slightly more QALYs than SMC alone, but that the difference is not substantial. This is the case whether the EQ-5D-5L or the SF-6D was used to generate QALYs. The costs for the CBT + SMC arm were higher than those for the SMC-alone arm, with most of the extra cost accounted for by the intervention itself. It was apparent that the intervention did not seem to offset costs in other sectors. It is not unusual for interventions such as this to result in higher health costs than usual care, and this is not in itself evidence against cost-effectiveness. It is recognised that to achieve improved outcomes it is often necessary to have higher costs of care and this is where the incremental cost per QALY is relevant. Interventions that are assessed by NICE are usually considered to be cost-effective if they achieve a cost per QALY below £20,000–30,000. In this study, the cost per QALY for CBT + SMC was £120,658 compared with SMC alone. The figure was similar when QALYs were generated from the SF-6D. For this reason, the addition of CBT to SMC would be unlikely to be considered cost-effective. Costs were not markedly different between the trial arms when informal care and lost employment costs were included.
It was of interest that costs in both trial arms fell for some services. This is not unusual in trials, given that recruitment often requires various investigations, and health improvements (often experienced by receiving care in the control groups) should lead to reductions in use. The study was not, however, designed to explore changes in costs. There were not obvious cost differences between the two arms (other than for the therapy itself). However, medication costs were higher for SMC alone. This may be because of compensation for the lack of CBT in that arm.
Limitations
The analyses were conducted in a way that is consistent with similar NIHR-funded economic evaluations. However, a number of limitations need to be considered when interpreting these results. First, we relied on self-report data for the evaluation. The CSRI asked participants to recall what services they had received in the previous 6 months, which may have led to inaccuracies. There is, however, no reason to suppose that potential inaccuracy would differ between arms, and for most services that we wanted to collect data on it was the only option. The possibility of errors did, however, lead us to examine HES data in addition to those collected with the CSRI. For outpatient use, the findings from the CSRI and from HES were remarkably similar. For A&E visits, the costs were somewhat different at baseline but at follow-up were very similar using the different methods. The methods produced the largest discrepancies for inpatient care, with higher costs indicated by the CSRI. When patients are admitted they are likely to be particularly unwell and recollection of exact numbers of days may be exaggerated. When we produced an ICER taking this into account, it fell to £95,096.
What is clear from the analyses is that both the CSRI and the HES suggest that fewer than 100% of participants had psychiatrist and neurologist contacts during the baseline period. The trial procedures were that such contacts should take place for recruitment to occur. The CSRI data could quite easily be underestimates of real use, but HES data seem to validate the CSRI. Although HES should capture all contacts, it is still reliant on contacts being recorded and reported, which may not always have occurred. Some of the missed appointments reported in HES will also have been for neurologists and psychiatrists. One clinician in one site provided both neurological and psychiatric input (see Appendix 6), but it is unlikely that would be the reason for the figures reported here.
Second, although CSRI data covered baseline and the whole follow-up period, the HES data covered the baseline period and the 6 months prior to the 12-month follow-up. This means that we are not able to make full comparisons between the two methods. However, the comparisons we have made are still informative and largely validate the CSRI method.
Third, the outcome measure used in the economic evaluation was QALYs generated with the EQ-5D-5L. This is the approach recommended by NICE and used by most UK health economists. However, it is unclear whether or not the EQ-5D-5L is a sensitive measure in this patient group, and given that most secondary outcomes favoured CBT + SMC we might have expected larger differences. Use of the SF-6D-based QALYs revealed smaller differences between groups.
Fourth, the tariffs that were attached to the health states were taken from work conducted by Devlin et al. 146 Since we embarked on this study, concerns have been raised about this value set,151 with some arguing for conversion to tariffs derived for the EQ-5D-3L. 151
Fifth, the time horizon may be too limited to fully assess cost-effectiveness; it may be that the effects of CBT continue long term. Although a 1-year follow-up is reasonable for a trial, we might wish to conduct modelling work to determine the effects beyond this period. For CBT + SMC to be cost-effective, there would need to be ongoing differences in HRQoL compared with SMC alone. If ‘top-ups’ were required, then this would have cost implications. Continued health improvements would hopefully lead to future reductions in health service use that would also improve cost-effectiveness. We addressed this in sensitivity analyses and the ICERs rose substantially when using the crosswalk method.
Finally, there was one person in the SMC-alone arm who received the intervention. There were also participants in both trial arms who reported other CBT and talk therapy. However, the mean costs were low (see Table 19) and this would not substantially affect the results. In addition, we were not able to verify the nature of this additional therapy owing to the risk of unblinding the research workers. We cannot therefore be sure as to its nature or extent and had no analogous HES data against which to compare it.
Conclusions
The study found that CBT when added to SMC resulted in increased healthcare costs. QALYs were also increased but not sufficiently for the intervention to be considered cost-effective at the 12-month follow-up.
Chapter 5 Process evaluation of the CODES interventions
Introduction
Three nested qualitative studies were completed during the study to provide further valuable information regarding the subjective experiences of the patients and HCPs involved in the trial. The overarching aim of these three nested studies was to explore the views of (1) the patients involved in the CODES interventions, (2) the psychiatrists delivering SMC and (3) the therapists delivering the CBT intervention. We recognised that the experience of each of these three groups in the trial would differ and that we could not have an identical questioning approach (and, therefore, overarching theoretical framework) that could apply to each group; however, we aimed to reveal aspects of the different treatment arms that patients and clinicians found useful and to identify potential barriers to change. Given that each of these studies could yield complex data that could be described in detail separately, we further synthesised the results from these studies for the purpose of the current report. Thus, triangulation of the findings of each of these studies was undertaken to help clarify any practical implications of this research. We then undertook a predominantly quantitative survey of neurologists involved in the study and report this (and its methods) after the qualitative study (see Neurologists’ experiences of participating in the CODES trial). We have documented aspects of this work elsewhere. 135,137,152,153
There is very little research exploring the experiences of HCPs working with patients with DSs. Existing quantitative research indicates that there is a gap in the knowledge among medical professionals who regularly encounter the condition. 154 Many HCPs report having a poorer understanding of DSs than of epilepsy, with some considering the condition as untreatable or within the patient’s control. 155–157 Negative experiences with patients are frequently reported157 and clinicians often describe a feeling of hopelessness. 158 HCPs’ perceptions are likely to have an impact on how patients experience their condition, influencing how far they accept their diagnosis and comply with psychological interventions.
Owing to the complexity of the interventions tested in the CODES trial and, for some, the novelty of the care pathway that was introduced, a qualitative approach was used to capture patients’ and clinicians’ views more comprehensively. Using interview methods allowed patients and clinicians to elaborate on their responses, unconstrained by a questionnaire, and adopting a mixed-methods approach in a RCT has been shown to provide essential insight into how and why an intervention is effective. 159,160 Given the large number of neurologists involved in the study, it was not feasible to undertake in-depth interviews with a sufficiently sized representative sample; therefore, we gathered, via an online survey, predominantly quantitative information on neurologists’ experiences of their involvement in the CODES care pathway.
Qualitative studies
Methods
Recruitment
Patient participants
Trial participants were asked at the end of the 12-month follow-up if they were amenable to participating in a qualitative study about their experiences in the trial and of having DSs. The sampling frame created for the study was based on ensuring that we interviewed those reflecting the larger study sample in terms of gender, location and age. We initially planned to interview 20 individuals who received CBT + SMC and 10 individuals who received SMC alone. Participants who were ambivalent about, resistant to or did not engage with treatment were also deliberately solicited to ensure that a range of responses was represented. We did not include any participants who refused follow-up. Interviews were undertaken by three researchers between February 2016 and April 2018; two participants refused to take part.
Psychiatrist participants
Of the 29 psychiatrists involved in the CODES trial, we used purposive sampling to select 10 psychiatrists, including people from a range of sites and reflecting their range of experience in treating functional neurological disorders (FNDs), particularly DSs. We excluded psychiatrists who were CODES trial grant holders from those interviewed to avoid bias from those involved in designing the trial. The selected psychiatrists worked at nine NHS trusts across England and worked in either liaison or neuropsychiatry specialties. Interviews were undertaken by one researcher between June and September 2017; there were no refusals to participate.
CBT therapist participants
Participants were recruited from the pool of 39 CBT therapists who treated patients in the CODES RCT. These therapists were situated across 18 NHS trusts throughout the UK and came from a range of professional backgrounds. The therapists provided a variety of outpatient services from the context of employment in hospital-based services and specialist community settings. Participants were selected to ensure a variety of both professional backgrounds and experiences of working with people with DSs, including within the trial. Sixteen potential participants were invited via e-mail to participate; of these, 12 clinicians from 10 NHS trusts participated. Interviews were conducted by one researcher between May and November 2017.
Interview procedure
From recognising the different experiences of the different participant groups within the trial, the interview schedules for each participant group were developed iteratively through discussion by members of the CODES research team. 135,137,152 These members of the team, who were not involved in conducting the interviews, provided both clinical expertise, in terms of the topics covered, and methodological guidance. With respect to the patient participants, the topics were designed to explore their experience of receiving the diagnosis, the impact of DSs on their life, the experience of the study treatment(s) that they received and their thoughts about participating in the study. For the psychiatrists, topics were designed to focus on their experiences of delivering SMC within the CODES trial and their participation in the RCT more generally. Elsewhere, we have reported on the psychiatrists’ general experiences of working with patients with DSs. 152 The interviews with the CBT therapists focused on their experiences of delivering the DS-specific CBT within the RCT and explored the value of certain aspects of the intervention. Furthermore, interviews explored the potential of effectively applying our manualised intervention to patients with DSs in the context of other difficulties. We also set out to understand whether or not the therapists felt that SMC sufficiently prepared their patients for therapy.
The semistructured interviews with the patients took between 45 and 90 minutes, whereas those with psychiatrists and CBT therapists lasted between 40 and 96 minutes. All of the interviews followed the relevant predetermined interview schedules (see Appendix 9). During the interviews, the participants were asked to illustrate their responses with examples where relevant. Probing techniques were employed by the interviewer to encourage the interviewees to expand on responses161 and to provide negative as well as positive reflections. Participants were interviewed face to face where possible; however, three CBT therapist interviews were held via teleconference owing to geographical distance and the impracticality of travelling to see the therapists in their place of work. In all other instances, the face-to-face interviews were undertaken at a time and a location to suit the interviewee.
Interviewers
None of the individuals conducting the interviewers was a qualified professional. The person interviewing the CBT therapists was a male, trainee clinical psychologist who was completing the interviews as part of the research component of their doctorate and who had experience in conducting in-depth interviews. Those interviewing the patients and psychiatrists were female research workers employed on the CODES project; one of the female researchers interviewed all of the psychiatrists and a subset of the patients. All were graduates and had experience of previous qualitative research. Although none was professionally qualified, all understood the condition being treated in the trial and, particularly in the context of the patient interviews, were able to be empathic with the interviewees. The person who was interviewing the therapists did not know these professionals prior to conducting the interviews. Those interviewing the patients and psychiatrists had prior working relationships with some but not all of the interviewees, which may have influenced the interviewees’ responses. All interviewers were able to reflect on the possibility that being a representative of the trial may have elicited positive responses about the trial and the intervention. However, adhering to the interview schedules and, as with the interviews of the therapists, encouraging negative as well as positive reflections were strategies employed to avoid bias and the eliciting of only positive reflections on aspects of the study. In addition, the fact that three individuals conducted the interviews with patients will have reduced any inadvertent bias from a specific interviewer.
All of the interviews were conducted, transcribed and coded before the main trial quantitative results were known; however, in the case of the patient interviews, the interviewer would have obtained information from the interview about that person’s outcome. Although all but one of the individuals conducting the interviews were involved in the coding process, other members of the research team contributed to the coding and interpretation of the results.
Analysis
Data analysis for the interviews with the patient participants and CBT therapists followed thematic framework analysis, which is deemed useful where groups of researchers work together. 162 Thematic analysis163 was employed for the psychiatrists’ interviews. In all cases, the interviews were digitally recorded and transcribed verbatim by members of the CODES research team, resulting in anonymised transcripts that were then checked against the original recordings to verify their accuracy. NVivo 11 software (QSR International, Warrington, UK) was used to code all sets of data.
Thematic framework analysis began with familiarisation of the data by pairs of researchers (a lead researcher and another member of the team), for which the transcripts were read several times to establish initial ideas for recurring themes, in line with the a priori aims of each study. Researchers then allocated labels to sections of the text to indicate their understanding of the content. Meetings between members of the research team were held for each set of data (patients and therapists) and indicated considerable agreement in how material was coded as well as the definition of themes that arose from how the codes had been grouped into categories. In each case, the grouping of ideas into common categories or themes was used to create a theoretical framework. This framework was then applied back to the transcripts to establish whether or not the framework applied suitably to the raw data, referred to as indexing. Data were then charted to visualise the main themes and consider any interactions between them.
For the thematic analysis that was used to analyse the psychiatrists’ interviews, two researchers initially coded three randomly selected transcripts and initial codes and themes were discussed by the research team. All 10 transcripts were then coded independently by the two researchers, recording the coding in NVivo 11. The researchers identified themes to represent the interview content, with new themes added as subsequent interviews were analysed. Agreements with the coding and the establishment of the parameters of major themes were arrived at by holding regular meetings. Where both researchers identified the same overarching themes, they were then combined; subthemes were then organised under the relevant overarching theme.
For both types of analyses (thematic framework analysis and thematic analysis) the researchers coding the data used a deductive approach, despite that those undertaking the thematic analysis of the psychiatrists’ interviews did not chart the data. We then undertook a triangulation approach using the different data sources (i.e. data from the different sets of interviews) to identify common themes that emerged from the three sets of interviews. This approach is one of the four types of triangulation previously identified. 164,165
Two members of the research team who were initially familiar with at least one set of transcripts and their resulting themes then undertook an iterative process of discussions and reduction and analysis of data from the three sets of interviews (patients, psychiatrists and CBT therapists). This was undertaken with the aim of looking for areas of agreement and divergence of opinion between the different groups. Further team discussions were held to further conceptualise the arising themes and supporting quotations were selected for each group of participants when documenting the themes; those undertaking the selection of quotations were mindful of selecting negative as well as positive quotations and to avoid presenting a biased summary of the findings.
Results
Participants
Patient participants
Twenty-two participants who had received CBT + SMC and eight who had received SMC alone were interviewed. Of the total sample, 21 (70%) participants were women and nine (30%) were men. Only two participants were not white British in terms of ethnicity. At the time of the interview, 10 (33.3%) participants were aged between 18 and 30 years, six (20%) were aged between 31 and 40 years, seven (23.3%) were aged between 41 and 50 years, two (6.7%) were aged between 51 and 60 years, four (13.3%) were aged between 61 and 70 years and one (3.3%) was aged between 71 and 80 years. Eleven participants were in full- or part-time employment or study. The range of time since onset of DSs (as reported by participants) was considerable, with five participants (16.7%) having experienced DSs for < 1 year, 15 (50%) having experienced DSs for between 1 and 9 years, six (20%) having experienced DSs for between 10 and 19 years, two (6.7%) having experienced DSs for between 20 and 29 years and one (3.3%) having experienced DSs for either 30–39 years or 40–44 years.
Of the people interviewed who had been allocated to receive CBT, 14 (63.6%) had attended all 12 sessions plus the booster session, one (4.5%) had attended 12 sessions, three (13.6%) had attended 11 sessions, one (4.5%) had attended 10 sessions and one (4.5%) had attended nine sessions. Thus, 20 out of 22 (91%) participants were compliant with CBT, having attended at least nine sessions. 92 Of those participants not meeting this definition of compliance, one had received five CBT sessions and the remaining person had received only one session. Interviewed patients who were allocated to the SMC-alone arm had attended a median of four SMC sessions (range 2–6 sessions). More specifically, of those allocated to the SMC-alone arm, four had attended four sessions, three had attended three sessions and one had attended just one session. The patients allocated to the CBT + SMC arm attended a median of 3.5 SMC sessions (range 0–10 sessions), that is they attended a wider range of SMC sessions. Thus, four patients attended four SMC sessions, three patients each attended one, two, three and five sessions, two patients attended six sessions and one patient each attended nine and 10 sessions. Two patients attended no SMC sessions. None of the patients interviewed here reported any previous knowledge about DSs at the time of their diagnosis by a CODES neurologist. Of the currently interviewed sample, one person had concurrent controlled epilepsy. For eight people there was a record of epilepsy having been previously misdiagnosed. A further individual reported feeling that their seizures could be a mixture of DSs and epilepsy, although clinical opinion was that the diagnosis was DSs only.
Psychiatrists
Ten psychiatrists were interviewed: five women and five men. Of the total sample, six were working in liaison psychiatry, one in neuropsychiatry and three people had dual accreditation in both liaison psychiatry and neuropsychiatry. The psychiatrists’ ages ranged between 31 and 60 years, with 8 out of the 10 aged 41–50 years. Six psychiatrists worked in London and the remainder worked elsewhere in England. The group had between 11 and 28 years of clinical experience since their General Medical Council registration, with five psychiatrists (50%) reporting between 11 and 15 years of experience, two (20%) reporting between 16 and 20 years of experience, one (10%) reporting between 21 and 25 years of experience and two (20%) reporting experience falling within the range of 26–30 years. All reported experience working with patients with DSs and medically unexplained symptoms.
CBT therapists
Twelve interviews were conducted with therapists delivering CBT. The majority of these therapists (n = 10, 83.3%) were female. Five therapists were aged between 31 and 40 years, while the remainder were aged between 41 and 50 years. The majority (n = 9, 75%) reported that they had previous experience of working with patients with DSs, whereas 10 (83.3%) said that they had previous experience of working with patients with medically unexplained symptoms. Six individuals reported having spent between 0 and 5 years practising as a CBT therapist, two people had practised for between 6 and 10 years, two people had practised for between 11 and 15 years and two people had practised for between 16 and 20 years. The therapists’ professional backgrounds were self-reported as follows: clinical psychologist (n = 4, 33.3%), counselling psychologist (n = 2, 16.7%), psychotherapist (n = 2, 16.7%), CBT therapist (n = 1, 8.3%), neurological physiotherapist (n = 1, 8.3%), nursing (n = 1, 8.3%) and occupational therapist (n = 1, 8.3%). In terms of prior CBT qualifications, seven (58.3%) had no specific CBT qualification, two (16.7%) indicated that they had a diploma in CBT, two (16.7%) reported having a Master of Science in CBT and one (8.3%) had a Bachelor of Science in CBT. Six therapists were accredited with the British Association for Behavioural and Cognitive Psychotherapies. Four (33.3%) of the therapists worked in Greater London, three (25%) worked in north-east England, two (16.7%) worked in south-east England, two (16.7%) worked in south-east Scotland and one (8.3%) worked in the Midlands. At the time of the interview, the median number of trial patients treated by each therapist was 8.5 patients (IQR 3.25–12.75 patients).
Findings from the three participant groups
The overview of the themes from the different sets of interviews led to the classification of findings according to the following main themes: experience of receiving and delivering the diagnosis; CODES dissociative seizures factsheets; CBT engagement and delivery; and delivery, content and quality of SMC. Within these themes, we identified subthemes where relevant (Table 24). We have expanded on some of these findings elsewhere135,137,152 and noted that within the psychiatrist interviews in particular there was a consistent degree of agreement between respondents on the majority of topics. In the themes in the following sections we have reflected where divergent beliefs emerged.
| Theme | Subthemes |
|---|---|
| Experience of receiving and delivering the diagnosis |
Receiving the initial diagnosis Approaches to and perceptions of diagnosis delivery |
| CODES dissociative seizures factsheets |
Improving understanding Useful professional resource |
| CBT engagement and delivery |
Helpful CBT techniques Family presence – help or hindrance? Concerns and dislikes concerning therapy Challenges to delivering and receiving therapy |
| Delivery, content and quality of SMC |
Providing SMC Benefits of the multidisciplinary care pathway Therapeutic relationship Progress during SMC |
Experience of receiving and delivering the diagnosis
For our 30 trial participants, there was a very variable length of time prior to receiving the DS diagnosis. For many participants, this period was characterised by frequent trips to A&E departments and hospitalisations during which they reported that they had sometimes received traumatic and unnecessary medical interventions, AEDs and, not uncommonly, a misdiagnosis of epilepsy.
Prior to being assessed by a CODES neurologist, the generalised lack of knowledge surrounding DSs among HCPs, combined with a potential misdiagnosis or uncertain diagnosis, often left patient participants feeling bewildered and desperate for clear information and guidance. Against this backdrop, we found that the CODES trial participants interviewed here reported that the delivery of the diagnosis of DSs by CODES neurologists was the first time that they had ever heard of this diagnosis.
Participants recalled a range of emotions on receiving the diagnosis from relief (that they had a diagnosis and/or that it was not epilepsy) to disbelief (that it was psychological), as well as anger and frustration at there being no simple cure. A minority described feeling abandoned or cut adrift, sensing that the neurologists could not help them any further. Understanding the diagnosis and its implications took time. One participant who had been unable to accept the diagnosis from a neurologist (outside the trial) found that, having been referred to a CODES neurologist, their explanation of DSs finally made sense:
I think because she’d [neurologist] seen part of one [a seizure] and she explained it in the way that we would talk. And she just really explained how it actually happens and how they work. And for the first time I thought that’s me . . . and it all started to fall into place, make sense. ‘Cos it had been so long not knowing what was going on and thinking I was having every kind of whatever . . . and then to understand. It was fantastic.
Female participant, CBT + SMC, interview 3
The complexity of both delivering and receiving the DS diagnosis continues to be an area of ongoing concern. 166 Given the absence of clear, uniform care pathways across the UK for people with DSs, CODES psychiatrists reported that, by the time they reach psychiatric services, patients are often sceptical of or struggling with the diagnosis. When explaining the DS diagnosis, most of the psychiatrists felt that the explanation should be specific to the individual patient so that it was meaningful to them. A way of doing this was to relate the explanation of the diagnosis to the patient’s personal life experience. This link to a personal life event could assist patients who struggled with emotional literacy to understand dissociation and the DS diagnosis.
The majority of the psychiatrists commented on the need for caution when explaining the diagnosis to the patient. This meant being careful with their choice of words and taking cues from the patient themselves as to what was likely to upset or cause offence:
. . . for other people anxiety can be a problem. I’m careful about using that word if they have been very clear with me they don’t think they are anxious.
Female psychiatrist, liaison psychiatry, interview 6
It was also important not to draw conclusions too quickly as to how the DSs had developed. From a clinical perspective, psychiatrists felt that it was important to move at the patient’s own pace in developing their understanding of the condition.
The CODES psychiatrists noted that the patients who were referred to them in the study were, overall, more accepting than those outside the trial of their DS diagnosis. The psychiatrists seemed to attribute this to patients having received a more detailed delivery and explanation of the diagnosis from neurologists. This may have been influenced by better resources being available to neurologists, such as the CODES Dissociative Seizure Factsheet (Neurology), as well as guidelines for delivering the diagnosis and a clear care pathway:
I did notice as well that everybody coming to see me was much more OK about their diagnosis than was previously the case . . . previously I used to get a lot more people who were unhappy with the diagnosis.
Female psychiatrist, liaison psychiatry, interview 4
Nevertheless, therapists felt that the complexity of the diagnosis remained a challenge, and some patients appeared to remain unclear about their diagnosis and treatment. Where there was poor diagnostic understanding, however, therapists did not necessarily feel that this was because of a lack of effort by neurologists and psychiatrists to explain the condition. Therapist interviewees noted the complicated nature of a DS diagnosis and the difficulty involved in relating such a difficult concept in a comprehensible way in a short time:
. . . so I don’t think it’s that they didn’t hear it. I think they heard it and they tried their best, to try and get that over, but it’s such a hard concept – ‘so what I just collapse?’, ‘how does that happen?’, you know ‘my brain just shuts down? how does that happen?’.
Female CBT therapist, interview 5
CODES dissociative seizure factsheets
According to many trial participants, aside from the explanation of DSs by the neurologist, it was the CODES factsheet given to them at that initial appointment that often marked a turning point in their improved understanding of DSs. Participants reported that the booklet not only offered hope that there were HCPs in CODES who understood but who could also potentially help them.
One participant recalled her reaction to reading the CODES Dissociative Seizure Factsheet (Neurology):
It was like a reassurance. So this is what’s happening and that’s OK. When I got those that’s when I gave them to my parents. I copied them and gave them to my parents and said this is what’s wrong with me and they were really good about it actually. It sort of made sense to them.
Female participant, CBT + SMC, interview 20
Thus another benefit was that they could refer to the factsheets subsequently when trying to explain DSs to others. Patient participants often expressed how hard DSs had been to describe to both family and friends, and said that they had given the booklets to others, including the GP, as they found the condition so hard to explain themselves. Two out of the 30 patients interviewed admitted not really reading the factsheets: one because she found it hard to read anything in book form and the other because he did not want to.
CBT therapists placed great value on the factsheets, and the majority said the fact that participants received a prescribed explanation of their diagnosis from neurologists and psychiatrists, as well as receiving educational materials, meant that those participants randomised to CBT seemed to attend therapy with more diagnostic understanding than those seen outside the RCT.
Analysis from the interviews of psychiatrists showed broad consensus that the dissociative seizures factsheets given to participants were very beneficial. Psychiatrists seemed to enjoy reading the Dissociative Seizures Factsheet (Psychiatry) themselves and found this factsheet a useful reference point for medical trainees and their own work. There was a general agreement that having something prepared and in colour to give to patients during or at the end of an appointment was valuable, as the patient and others could refer to the materials outside the clinic. They also felt that the anxiety and avoidance commonly found in this patient group may mean that they struggle to take in everything that is being said during a consultation, so the booklets could increase the amount of good-quality information to which they can refer.
Cognitive–behavioural therapy engagement and delivery
The majority of the CBT therapists reported that they found the seizure control techniques recommended in the therapy manual to be helpful in working with their trial patients. From the therapists’ point of view, it seemed that trial patients initially felt that they had no control over their seizures. Therapists introduced participants to a range of CBT techniques to help with seizure management, including (but not exclusively) progressive muscle relaxation, breathing, distraction and refocusing, visualisation and graded exposure:
It can give them a sense of control and obviously it could make a difference if, if they know that they are not going to have it in an embarrassing situation, that if they know, say if they are going out to, I don’t know a wedding.
Female CBT therapist, interview 3.
As these techniques were introduced, a number of those randomised to CBT were able to use them to potentially delay a seizure or even stop one completely. The clear majority of the 22 CBT participants said that they had found a CBT technique that was effective and which they were still practising long after therapy had ended. Eighteen of the 22 CBT participants found that controlling and slowing down their breath by breathing from the stomach helped them to relax and even to divert a seizure. One of the 18 participants found that breathing through stomach pain (a warning sign of a seizure) helped her in seizure management. However, two of the CBT participants said that the breathing technique made them feel dizzy and that they could not progress with it. Distraction techniques, such as tuning into sound or tapping or counting down from 100 to 1, were also named as effective at managing seizures. Utilising breathing techniques had really helped one SMC participant after they had found these on a website. Participants seemed to understand that the techniques were there to be tried and tested, and if they did not like a technique or it did not work it was worth trying another one.
In line with the fear–avoidance model of DSs used in the current trial, the identification of avoidance behaviours and the use of graded exposure to address them were key tools of the intervention. Therapists deemed graded exposure to be useful depending on the presence and nature of avoidance, finding behavioural avoidance more easily addressable within the therapy than emotional avoidance:
Once they started to do some behavioural stuff and, and and if they . . . went out and did something and found that their anxiety went down, that was a ‘light-bulb moment’ for some people.
Female CBT therapist, interview 7.
This view was supported by several participants who received CBT and who reported that although initially very anxious and thereby avoidant of travelling to the therapy sessions alone on public transport, they were progressively able to use travelling to therapy as a goal set as part of their homework. To consolidate what they were learning in therapy, several CBT participants reflected that homework had been an essential part of their progress and that they had understood the rationale underpinning it:
Oh it [homework] definitely helps. The key to having successful CBT is repetition cos once you’ve had that repetition – it’s a bit like practice makes perfect – cos once you’ve had that repetition it’s programmed in your muscle memory and you can recall these techniques or just go back to thinking what you’ve learnt in those sessions.
Male participant, CBT + SMC, interview 12
One of the CBT participants, however, found homework too emotionally challenging:
I didn’t like any of it. Well, some of it was OK but the writing down the thoughts and feelings and all that I hate. I’m not good at that because that just brings everything back and it just makes me feel worse. We got round that by doing other things.
Female participant, CBT + SMC, interview 26
A number of CBT therapists also recalled that homework was too challenging for some participants because of high levels of avoidance among those who did not want to take the ‘work’ outside the sessions. Equally, there were those participants at the opposite end of the spectrum who did not appear to be avoiding anything and so setting avoidance-orientated homework goals proved challenging for therapists.
CBT therapists felt that the participant handbooks (i.e. the ‘Manual for Patients Attending CBT’ containing chapters supplementing the content of the CBT sessions) were helpful. They recalled participants’ positive feedback about certain sections, particularly those on trauma and distraction/refocusing. Therapists found it helpful to be able to refer to these handbooks in sessions to help explain homework. Of the trial participants, five mentioned that they remembered receiving other handouts from the CBT therapists to help with homework techniques between sessions, but referred to them and the handbook in general terms only rather than describing specific sections. For one participant, they were a mitigating factor against an impaired memory:
There was quite a few worksheets and bits that she did which were really helpful because then I could take them away. My problem is that I have such a bad memory that if I just have a talking therapy I can’t always remember what she said to do or to work on. And a week gets so busy in my life with having children and everything else that I often forgot, so by having something visual I could refer back to it and that was really good.
Female participant, CBT + SMC, interview 23
The role of family members and partners, both at the point of diagnosis and later in therapy sessions, emerged as a key factor in CBT therapists’ interviews but to a much lesser degree in those of the trial participants. A few participants felt that it was vital to have someone else there when seeing neurologists or other doctors because they reflected that they would have felt unable to process all of the information in these consultations, citing poor memory, high emotion or anxiety.
Involving family members in the recovery process was prescribed in the therapists’ manual. Participants were asked to bring a family member or partner to session 3 to explore whether or not there were factors in their lives that may be perpetuating their problems.
Seven CODES therapists expressed their approval of incorporating family members within the therapy protocol. There was broad consensus among them that family members can play a marked role in helping relatives to recover from DSs. Interviewees commented that participants’ families had often experienced DSs to be distressing episodes and that their potentially overprotective responses may be counterproductive to recovery. Therapists believed that providing the family with a rationale of the treatment and introducing the family to a more ‘hands-off’ approach early on was helpful:
So I think that culturally, generally, you know, they are of a culture where family gets very involved and helps out and jump in and do things for each other in general, and whoever is the sick one in the family, you know they will help out. But on the positive side, I think that they were actually quite ready and maybe, you know, happy to, you know use the information to take a little bit of a step back.
Female CBT therapist, interview 11
However, 3 of the 12 therapists observed that this new approach to managing DSs, by changing habits that may be quite entrenched, could be difficult for family members to adjust to. Almost half of the therapists, however, found that the chapter for family members in the patient handbook was a helpful tool in supporting them to understand the recommended approach to DSs.
Only five of the patient interviewees who had received CBT talked about family members attending therapy and, although generally positive, there were some mixed feelings about it. Although initially welcoming the idea of bringing in his wife, one participant said that he did not feel that her presence was ultimately helpful to him because he had started to censor what he said in the sessions. However, for the remaining four participants, the involvement of parents, carers and other family members had been positive. For them, the sessions with a family member were times when difficult conversations could take place, with the therapist acting almost as family mediator or at least providing a safe, neutral space. For one participant, inviting a mother and later on a carer into the session was the opportunity to explore ways of relaxing the overprotective ‘rules’ that had been deemed to keep the participant ‘safe’ and to allow them to perform small chores as recovery progressed:
But then [CODES CBT therapist] as well invited Mum because some of the things he asked me to do freaked my Mum out like using a kettle. I mean I’d dropped a bottle of wine before and she was really worried but [CODES CBT therapist] reassured her and when she saw my progress and stuff, she was encouraging me. Obviously she was still concerned.
Female participant, CBT + SMC, interview 17
A number of psychiatrists thought that participants may be deterred by difficult issues that could emerge in CBT or the SMC sessions. They cited a range of reasons that might deter participants from the challenges of CBT, such as emotional illiteracy, avoidance, ambivalence towards the DS diagnosis or being unable to identify a reason for the onset of DSs.
Conversely, a few psychiatrists felt that simply signing up to the CODES trial meant that participants had a certain desire to engage with a psychological understanding of DSs and in that sense were a self-selecting group. One psychiatrist also felt that the clarity of the study protocol was a way of engaging participants in therapy.
As for the patient participants, when asked about their pre-randomisation treatment preference, many of the participants in the CBT arm said that when they were first told that they had been randomised to therapy they had initially reacted with fear, thinking that sessions would involve ‘sitting around in groups’ or being forced to relive painful childhood experiences. Many of the CBT participants reflected afterwards that these fears had proved unfounded. Two of the SMC participants recalled being glad that they were not assigned to CBT because they felt that they would never have been able to talk at length to someone on a weekly basis.
For patient participants who did not attend all 13 sessions (i.e. 12 sessions plus the booster session) or who disengaged from CBT, there were specific issues such as not liking the CBT techniques or finding the behavioural experiments extremely challenging. One person who disengaged after session 9 felt that CBT techniques, such as breathing, were meaningless, as he had no warnings of a seizure so there was no point in trying to control them. He was also frustrated by the session being limited to 1 hour:
It’s never long enough and it’s never in-depth enough. As I said she had a schedule to work with and it really did, not sort of upset me but it was always, there was so much more I wanted to say or do but couldn’t because she’d say right your hour’s up . . .
Male participant, CBT + SMC, interview 21
Another CBT participant who stopped attending after session 10 felt that she was not learning anything new from the CODES CBT, as she had previously received CBT for depression. A second CBT participant, who had been frequently housebound and had high levels of social anxiety, was adamant that CBT had helped in terms of seizure reduction and her approach to DSs. She had, however, attended only five sessions owing to serious family illness. Separately, however, she recalled her fear of undertaking behavioural experiments, such as walking around in public with the therapist at her side:
And we walked from the hospital to the town and that was just, yeah, mental. I actually feel like I could have strangled her [the therapist] . . . You’re not just frightened of the seizure but what other people are going to do. Are they going to hurt you, are they going to kick you? . . . And are cars going to run you over and are people going to look at you?
Female participant, CBT + SMC, interview 13.
For the CBT therapists, having managed to engage participants in therapy, there was an enormous range of complexities and comorbidities that complicated a structured therapy and even occasionally derailed it. Among the reasons cited by therapists were housing or family issues; self-harm; suicidal tendencies; physical health problems, such as comorbid controlled epilepsy; or an ongoing major depression that needed to be tackled with goal-setting (e.g. ‘getting dressed each day’) before starting any graded exposure for the seizures. Therapists generally felt that the structure of the manualised therapy allowed them to get things ‘back on track’ from the aforementioned obstacles. However, once engaged, therapists sometimes found that convincing participants to desist from using behavioural coping strategies that were keeping them ‘safe’ could be challenging for patients.
Given the complex presentations seen in the patients, three therapists indicated that it might be important that an intervention, such as that employed here, warranted the involvement of suitably qualified and experienced clinicians:
But again we come back to therapist skill. That, yeh, I do think you’re working with very complex people . . . I don’t think being a kind of newly qualified CBT therapist would manage it.
Female CBT therapist, interview 2
From the patients’ perspective, problems surrounding transport were the predominant barrier to attending appointments. Given the high levels of dependent living and the distance initially reported by patient participants, making long journeys to hospital appointments often meant relying on the availability (and goodwill) of family, friends, hospital transport or a combination of these. Twenty out of the 30 patient participants mentioned that transport was a barrier to attending appointments; only five out of the 20 participants had been able to travel on public transport on their own. These concerns were incorporated into the treatment by some therapists. Fear of travelling alone could be used to devise a CBT homework goal and three of the CBT participants found that the confidence that they gained from therapy enabled them to start travelling to sessions independently.
The CODES psychiatrists also felt that the lack of local service provision for people with DSs meant that being faced with long distances to attend SMC appointments was a definite deterrent; the trial did not reimburse travel costs to these appointments. They were aware that when the study stopped in some centres there would be no local provision for patients with DSs, which presents a significant challenge to a group of patients who can often be quite disabled. However, the psychiatrists also cited other practical issues for patients, such as work commitments, child care, hospital parking and other comorbidities.
Although noting that travel was an issue, the therapists more readily identified other concerns as being related to dropout, such as disagreement with the diagnosis.
Delivery, content and quality of standardised medical care
Owing to the pragmatic nature of the study, the study protocol anticipated (rather than prescribed) three to four psychiatry follow-up sessions. 84 From the psychiatrists’ perspective, offering SMC appointments with participants could prove challenging, mainly because of service pressures, policies and variations in structure. Pressures on resources meant that some sites would not usually offer more than a set maximum number of follow-up appointments; others would not necessarily follow-up patients with DSs at all, unless they had clear psychiatric comorbidities. Other services struggled or were unable to offer more than one follow-up appointment over the year following randomisation.
Many psychiatrists felt that the CODES approach to SMC was very similar to what they were doing in their daily practice with this group of patients anyway. However, some changes to practice were required. Psychiatrists were asked in the CODES SMC guidance document to omit the use of CBT techniques; however, they could give basic advice on breathing and distraction methods. This was felt to be slightly restrictive, especially if the psychiatrist had CBT training or CBT formed a core part of their usual practice:
We did diaries, we did quite a lot of um behavioural and cognitive advice . . . also sort of stress management techniques or distraction techniques, grounding and all that, so we used to do that in our clinic [describing usual SMC practice outside CODES].
Male psychiatrist, neuropsychiatry, interview 10
In terms of longer-term roll-out, psychiatrists indicated the challenge of creating a care package that could be widely implemented with homogeneity in terms of the content and quality of SMC provided. As well as this, some psychiatrists felt that SMC could not be sufficiently standardised owing to the variable levels of experience among clinicians working with the disorder:
There’s no standard across different hospitals or different services or different centres . . . or in fact probably there was not any standard medical care between clinicians to clinicians, different people at different levels depending on their exposure to the condition.
Male psychiatrist, neuropsychiatry, interview 10
Related to this was the perceived lack of education and training both for psychiatrists and for other healthcare providers with respect to working with patients with DSs or FND more generally, and the harm that this could do:
I’ve found people have found it very upsetting . . . find it more difficult to accept the diagnosis if they kind of been shunted around different services and people are like ‘Oh what’s this I’ve never heard of it’.
Female psychiatrist, liaison psychiatry, interview 4
All but one of the 12 CBT therapists provided positive feedback on aspects of SMC. Several indicated that their patients had reported positive interactions with psychiatrists or neurologists and that their concerns had been listened to. Six therapists acknowledged the value of the close working relationships that they had experienced with their neurology and psychiatry colleagues within CODES. They felt that study patients had benefited from their own cohesive working with neurologists and psychiatrists and that this had helped diminish any sense that patients may have had of being ‘abandoned’ by these professionals following their allocation to CBT. Six therapists said that they felt that the quality of medical care delivered to trial participants was more favourable than that which they might have otherwise received. In some cases, therapists indicated that for patients not in such a trial there might be accounts of far less positive experiences of medical care prior to therapy, and that this could hamper engagement in therapy. Therapists described aspects of the SMC used in CODES that had appeared to facilitate this process, including, as indicated above, the CODES factsheets given to patients by neurologists and psychiatrists and the standardisation of the explanation given to participants.
Patients’ views of SMC were, overall, positive and many people welcomed an additional level of help and care, understanding that these sessions would comprise a general review of their DSs or therapy or a chance to talk about medication. For two participants who had received CBT and one in the SMC-alone arm, the relationship with the psychiatrist had been vital to their recovery. For the two receiving CBT, therapy had led to the disclosure of trauma that, in turn, had almost derailed therapy. At these points, having a psychiatrist who they could trust as well as a therapist had allowed them to feel that there was an integrated team looking after them; they both reported that this had been invaluable. For the participant in SMC alone, the psychiatrist was the person who, he felt, had really confirmed the neurologist’s diagnosis of DSs and he felt able to confide in him:
It’s completely different speaking to someone that you know than to speaking to a stranger. With a stranger you can be your true self even if it was the angry, closed-off person that was me.
Male participant, SMC, interview 2
One SMC-alone female participant said that she felt unsupported by both the neurologist and the psychiatrist in her coming to terms with a DS diagnosis, after being originally misdiagnosed with epilepsy and having taken AEDs for years. She was one of two out of the eight participants in the SMC-alone arm for whom the relationship with the psychiatrist appeared to be overshadowed by a previous diagnosis of epilepsy, leaving both participants resistant to the idea that DSs was a psychological disorder. In contrast to the majority of the participants, they both resisted any suggestion that the seizures could be possibly connected to difficult life events and viewed SMC appointments as a waste of time:
I’m not a down person 24/7 so I don’t need to be speaking about, you know he couldn’t like see no problems or, there’s nothing that could be pinpointed, I said to him time and time again I’ve spoken to everybody I can think of and there’s no reasons why people can think of me having them.
Male participant, SMC, interview 4
In addition, several of the participants felt that the length of time between the SMC appointments was disappointing.
The psychiatrists reported that some patients improved with SMC appointments and without CBT or other therapy. Some of the varying examples of positive progress included persuading a participant to start an antidepressant when there was a significant anxiety disorder present, leading to an improvement in mental state and subsequent reduction in seizures, as well as reducing focus on symptoms and A&E attendance:
Because of there was obviously something; some containment going on there . . . there has actually been an improvement.
Female psychiatrist, liaison psychiatry, interview 5
Psychiatrists were at times surprised by the progress made by seemingly very complex patients; factors contributing to this could be a good working alliance and an effective holistic assessment of the patient and their strengths.
The psychiatrists differed to some extent in terms of who they felt benefited from SMC and particularly whether or not those who benefited from SMC had to be more or less psychologically minded; several clinicians said that they felt that high-risk patients with distinct psychiatric comorbidity may be better suited to SMC than CBT:
Where there is a clearly defined anxiety disorder or depressive disorder which you think you can treat effectively with medication, they’re quite good to be seen in SMC.
Female psychiatrist, liaison psychiatry, interview 6
Summary
The CODES psychiatrists and CBT therapists felt that the patients who were referred to them in the study on the whole had a better understanding of the DS diagnosis than patients who might normally be seen outside the trial, owing to the more detailed delivery and explanation of the diagnosis from neurologists; this suggested that this early stage in the care pathway was important to the subsequent engagement of patients. There was consensus among trial participants, CBT therapists and psychiatrists that the dissociative seizures factsheets helped in understanding the diagnosis and were a valuable resource, providing clear information that could be understood by family and friends and other HCPs. Many of the participants randomised to CBT reflected very positively on their experience of treatment and felt that they had developed useful CBT skills to help with seizure management; however, treatment was not without its challenges for both the participants and the therapists and not all techniques were equally well experienced or implemented in all cases, highlighting the need to have a range of strategies to offer to patients. SMC was also positively received by most patients, and the psychiatrists noted that it could by itself lead to improvement. For the psychiatrists, keeping CBT techniques out of SMC appointments could be difficult, especially because all the psychiatrists interviewed had some therapy training.
Limitations
We acknowledge limitations to our qualitative evaluations. We interviewed only 10 psychiatrists and 12 therapists; psychiatrists working in Scotland and Wales were not interviewed and we did not interview therapists from all participating centres, meaning that some service-related biases may have been present. All healthcare provider participants were involved in the CODES trial and knew either the interviewer or other members of the project team; although it is possible that this may have influenced their responses, as the interviews did not exclusively ask about the CODES trial, other responses may have been less likely to be affected by these professional relationships. Patient participants were, as intended, mostly chosen to enable reflections to be gathered on CBT; however, we may not have adequately sampled those failing to complete treatment and we cannot be certain that we would not have elicited further themes had we interviewed more patients in the SMC-alone arm.
Neurologists’ experiences of participating in the CODES trial
Introduction
Within the CODES trial, neurologists were required to assume an important role in the treatment pathway. The aim of this process study was to explore neurologists’ experiences of their often more-than-usual in-depth role within the treatment of DSs and their thoughts on working with patients recruited to the CODES trial. More specifically, we investigated neurologists’ knowledge of DSs and their clinical practice for the condition before and after their involvement in the trial, as well as their opinions about CODES-related components. As we could not undertake a representative sample of qualitative interviews because 91 neurologists/epilepsy specialists had participated in the study, clinicians were asked to complete an online survey comprising 43 questions yielding qualitative and quantitative data.
Methods
Recruitment
We invited 84 neurologists/epilepsy specialists via e-mail to participate in this process evaluation (all 91 participating CODES neurologists/epilepsy specialists were e-mailed initially, but the e-mail addresses of seven were no longer active). We employed the Bristol Online Survey Tool to distribute a specially designed 43-item questionnaire, with all items outlined elsewhere. 153 Clinicians were sent a reminder to complete the survey 2 weeks after the initial invitation. All data were provided anonymously.
Questionnaire
The survey was developed by members of the CODES team. The questions covered the following: (1) demographics (six items) – gender, age, clinical role, years of experience in clinical neurology and number of patients with DSs diagnosed per month and under their care; (2) knowledge about DSs and interventions prior to and after their participation in the CODES trial (four items); (3) clinical practice before, during and since participation in the CODES trial (22 items); and (4) their views about CODES-related components (11 items).
We included predominantly quantitative (Likert) scales with multiple options (1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree and 5 = strongly agree), as well as open-ended questions.
Data analysis
Descriptive statistics were reported for quantitative data. Data comparing self-report scores before and after CODES participation were analysed using the Wilcoxon signed-rank test. Statistical significance was assessed at a p-value < 0.05. Data were analysed using SPSS 24 (Statistical Product and Service Solutions Inc., IBM Corporation, Armonk, NY, USA). Qualitative data from open-ended questions were grouped thematically; further specific quotations are presented elsewhere. 153
Results
Respondents’ demographics
Forty-three (51%) out of the 84 contacted neurologists completed the survey. Most were consultant neurologists (n = 40); the remaining three were one specialty registrar, one staff grade and one GP with a special interest in neurology. Most of the clinicians were male (60.5%) and aged 41–60 years (81.4%). The median number of years of clinical experience in neurology was 18 years (IQR 13–22 years). Respondents indicated that the median number of patients who they would typically diagnose with DSs per month was three (IQR 2–5 patients), and the median number of patients with DSs under their care at any one time was 20 (IQR 9.5–50.0 patients).
Knowledge about dissociative seizures
Neurologists generally reported a good level of knowledge about DSs before their involvement in the CODES trial (median 4, IQR 4.0–5.0). This did not change as a result of their participation in the trial (median 5, IQR 4.0–5.0; p = 0.76). Similarly, doctors self-reported a good level of knowledge of the therapeutic potential of psychological interventions for DSs before (median 4, IQR 4.0–5.0) and after their involvement in the trial (median 4, IQR 4.0–5.0; p = 0.67).
Participants’ clinical practice when working with patients with dissociative seizures
Thirty per cent of the neurologists reported that participating in the CODES trial had changed their practice regarding referring participants to psychological interventions. This included making earlier and more direct referrals for DSs, and the development of a DS pathway within their organisation as a consequence of the study. Of those who had not changed their practice, some already had local and well-developed specialised psychotherapy services in place for DSs. Others were still unable to refer to psychotherapy for DSs following the trial. During the trial, there was an improved ability to refer patients who lived outside the practice area to psychological services.
Around half (53.5%) of the respondents reported being able to refer patients to DS-specific psychological intervention before the CODES trial. This figure did not change after the conclusion of the trial. The availability of services appeared an important determinant. Doctors who had access to DS-specific psychological intervention prior to the trial referred around 78% of patients to these services. The comparable estimate from those who could not directly refer patients was 12%.
Sixteen per cent of neurologists reported that their involvement in the CODES trial changed their referral practice to psychiatrists/neuropsychiatrists for DSs. Both before and after their involvement in the CODES trial, the majority of neurologists agreed that psychiatry services should be involved in the care of all patients with DSs (before: median 4, IQR 3–5; after: median = 4, IQR 4–5; p = 0.1). However, only 55% said that they would have referred patients with DSs to psychiatrists/neuropsychiatrists before their involvement in CODES; for several respondents, this was because of a lack of local experts and resources.
Unsurprisingly, before their participation in the CODES trial, neurologists reported many different referral practices (Figure 13). Most neurologists referred patients with DSs directly to psychological interventions or to another professional who might then make this referral.
FIGURE 13.
Referral practice prior to involvement in the CODES trial. (Note that multiple responses were allowed.)

Neurologists’ confidence in recommending psychological therapy for DSs did not change as a result of their involvement in the CODES trial (before: median 4, IQR 4–5; during: median 4, IQR 4–5; p = 0.16). Although doctors reported having a good level of knowledge before the CODES trial of how psychological intervention may benefit patients with DSs, their ability to describe the psychological therapy offered to help patients with DSs improved during the study (before: median 3, IQR 2–4; during: median 4, IQR 3–4; p = 0.001).
Clinicians were asked to indicate their usual practice with respect to following up patients with DSs before, during and after their participation in the CODES trial (Figure 14). Overall, participation in the trial did not appear to affect how patients were followed up. Nonetheless, 26% of doctors indicated that the study had an impact on their practice relating to patients with DSs in other ways. Frequently reported changes included having more detailed conversations with patients; being better able to signpost patients and provide them with information; having greater confidence in the exploration of aetiological factors with patients with DSs; employing the CODES factsheet; making direct referrals for psychological treatment; making a greater number of referrals to other professionals; and feeling more confident making and delivering the diagnosis without stigma.
FIGURE 14.
Neurologists’ usual follow-up practice for patients with DSs before, during and after their involvement in CODES. (Note that respondents could indicate more than one option.)

CODES trial-related components
Doctors were asked their opinion on the usefulness of the different elements of the CODES care pathway and materials provided (Figure 15). Most doctors found all elements ‘very’ or ‘extremely’ useful, with particular emphasis on CBT for those patients who received it (86.1% of doctors) and the dissociative seizures factsheet (81.4%).
FIGURE 15.
Ratings of the usefulness of CODES study elements.

In addition, most neurologists reported that patient satisfaction for the psychiatric care (69.7%) and CBT (79.1%) within CODES was ‘very good’ (Figure 16), with 90% reporting that they would like this pathway to continue within their service. Overall, 91% of doctors also said that they would like to continue using the factsheet, with one specifically deeming it ‘extremely useful’ and suggesting that it offered ‘therapeutic benefit’ in itself. In addition, 84% of respondents reported referring their patients to relevant websites ‘often’ or ‘very frequently’.
FIGURE 16.
Ratings of CODES study components.

Around 84% of the neurologists reported ease of recruitment of patients with DSs to the study, as well as a general satisfaction of patients with their participation (> 69%). Despite this, doctors expressed more uncertainty with regard to keeping patients engaged (around 35% neither agreed nor disagreed), alluding to a level of difficulty in maintaining some patients’ engagement in the trial.
When providing additional thoughts on the CODES trial, positive comments included reference to raising awareness and understanding of DSs and that the pathway allowed patients quicker access to assessment and treatment of the disorder, avoiding existing waiting lists. The study also facilitated multicentred collaboration that was considered as a ‘lasting legacy of the trial’.
Two doctors said that they would like additional clinical resources to meet the increased clinical demand resulting from the CODES trial; another mentioned the problems faced by patients who had to travel long distances to receive treatment during the study.
Limitations
We acknowledge that there are certain limitations within this aspect of the process evaluation. There was a moderate response rate, with only 51% of the neurologists completing the online survey; consequently, this sample is likely to be biased towards those with a keener interest in the study and DSs and, therefore, the views reported here might not be entirely representative of the wider group of participating CODES neurologists.
Owing to responses being anonymous, we were unable to link responses back to practice in the study. Thus, for example, responses from individuals recruiting only small numbers of patients would have been based on limited experience within the study. In addition, results are exclusively based on self-report and may not reflect their actual practice.
Overall conclusions
Our process studies of patients, neurologists, psychiatrists and CBT therapists participating in the CODES trial highlight several common themes across the groups that have potential implications for future clinical practice in this area. These are in the context of an overall study that incorporated a care pathway, often specially devised for the project in certain services, that incorporated the involvement of neurologists/epilepsy specialists, liaison or neuropsychiatrists and CBT therapists (of varying levels of experience and professional backgrounds). Despite a general agreement among neurologists that their role in the care of DSs does not extend beyond diagnosis,167,168 it was not difficult to recruit a large number of these clinicians to take part in a trial that involved them playing a potentially larger-than-usual role in patient management. This suggests that previous research indicating neurologists’ resistance to be involved in the DS treatment process may be because of a lack of treatment resources rather than an objection to being involved in treating these patients or to psychotherapy per se. 169 Although the pathway streamlined the service provision for patients, providing in many cases therapy that might not otherwise have been available, in some cases it led to increased service demand or demand for CBT that could no longer be met after recruitment ended.
Considerable value was placed across the different respondent groups on the quality of the diagnosis and its delivery, and the impact that this then had on patients’ apparent readiness for therapy. The fact that the diagnosis was delivered by neurologists and then restated by psychiatrists meant that therapists generally felt that patients coming for CBT were much better informed about the disorder than might normally have been the case. Psychiatrists also felt that the initial diagnosis delivery generally helped their engagement of patients. In addition to the usefulness of the diagnosis communication guidelines for neurologists, considerable value was placed on the two dissociative seizure factsheets. Neurologists wished to continue to be able to use these, and psychiatrists and patients recognised their potential value as being a resource to which patients could refer to help explain their disorder to others outside sessions. The factsheets were materials that psychiatrists found valuable for their teaching.
Neurologists responding to the survey clearly found the psychiatric assessment component of the care pathway useful; they were not specifically asked about the specialist background of those who might undertake such assessments, but interviews with both psychiatrists and CBT therapists gave the impression that those working with people with DSs should receive sufficient education/training in working with patients with DSs and FNDs more generally, often because of the complexity of patients’ presentations. We have commented elsewhere152 on the importance of the education of HCPs to avoid, for example, unnecessary mention of epilepsy in a manner that can derail engagement with psychiatric interventions as well as psychotherapy. Similarly important is education to prevent professional isolation170 and to prevent professionals holding negative attitudes to patients with DSs and functional disorders more generally. 169
Importantly, before their involvement in CODES, the majority of participating neurologists were experienced in diagnosing and managing DSs and reported having a good understanding of how psychological intervention may be beneficial in this disorder. Nonetheless, despite their rates of patient diagnosis both before and after the trial, only around 50% were able to make direct referrals to a DS-specific psychological intervention. These results illustrate the need for more widespread resources specific to the disorder. Potentially related to this is the observation that 14 doctors (32.6%) reported that, during the trial, they were more likely to consider a DS diagnosis at an earlier stage of the assessment process. This may indicate that previously documented diagnostic delays for DS patients have been because of a lack of awareness of the disorder or access to treatment provision. 171,172 Our findings indicate that many neurologists within the trial lost their ability to refer DS patients to psychological therapy once recruitment to the study had ended. This further demonstrates the issues surrounding a lack of access to psychological treatment for DSs and the importance of working to expand resources. It is noteworthy, therefore, that the vast majority (90%) of participating doctors reported that they would like the CODES care pathway to continue in their clinical service. Practical considerations for service roll-out, whether considering SMC alone or SMC with CBT longer term, would also include the need to deliver assessments and interventions that are local to patients, as travel was identified as a key barrier to involvement in sessions, made all the more relevant by the complexity of patients’ presentations and avoidance behaviour.
Patients and CBT therapists identified materials and techniques that were of clinical value and reflected, mostly positively, on the value of involving family members in the therapy, highlighting the potential role of significant others in maintaining a restrictive lifestyle for patients. Having written materials supported the content of therapy sessions and helped overcome patients’ memory difficulties.
Some of the psychiatrists said that they found it difficult to be asked to refrain from including CBT techniques in their interactions with trial participants. It would seem unlikely that these individuals, many of whom had some CBT training as part of their professional development, would opt to exclude these elements when working with patients with DSs in the future, although how this would be received both by patients and by therapists involved in delivering CBT to these patients, and how the intervention advice might conflict, cannot be determined from our current evaluation. Nonetheless, the involvement of a psychiatrist as well as a therapist potentially offered additional support for patients when addressing emotionally very difficult material, and again supports the impression of the importance of the entire care pathway. Close interprofessional working was also recognised as an important aspect of the study by CBT therapists.
Despite the disadvantage of not having been able to undertake in-depth interviews with a purposive sample of the neurologists involved in the study, overall our process evaluation does provide support for the overarching care pathway adopted in the study and for its individual components.
Chapter 6 Overall discussion and conclusions
The CODES trial set out to explore the clinical effectiveness and cost-effectiveness of SMC compared with DS-specific CBT + SMC at 12 months post randomisation in the treatment of adults with DSs. Although this was a pragmatic trial in routine NHS care settings, patients had to be assessed, treated and followed up following guidelines that did not invariably coincide with approaches to clinical care that are usually implemented in the participating services. The process evaluation undertaken with trial participants and HCPs added insights into the experiences of participants and clinicians with implications for what might be carried forward from the study.
Principal outcomes
We evaluated all outcomes at 12 months post randomisation, in line with our published SAP. 92 In terms of clinical outcomes, all estimated trial arm differences were in favour of CBT + SMC. For our primary outcome, both trial arms appeared to show decreased DS frequency over the course of the trial, but the between-group difference was not statistically significant; inferential statistics nonetheless suggested a 22% advantage for the CBT + SMC arm. For continuous secondary outcomes, this pattern of results favouring the CBT + SMC arm is illustrated in Figure 8. Although 9 out of 16 secondary outcomes were better in the CBT + SMC arm at a p-value < 0.05, five of these reached significance at the more conservative level of a p-value ≤ 0.001. This included one seizure-related outcome (longest period of DS freedom in months 7–12 of the study) as well as clinically important aspects of psychosocial function, global outcome and satisfaction with treatment. Compliance with CBT sessions by those allocated to receive them was good and similar numbers of SMC sessions were offered to the two trial arms. There was no evidence that the CBT + SMC arm experienced greater levels of harm than the SMC-alone arm during the trial, suggesting that our approaches can be offered safely within the NHS.
Health service use decreased in both trial arms over the course of the trial, but there was no overall comparative benefit from CBT + SMC; owing to the therapy cost, the total NHS costs were higher in the CBT + SMC arm than in the SMC-alone arm, and the cost of the therapy did not offset other changes in service use. The addition of CBT to SMC did lead to increased QALYs, but the difference between arms was small. The ICER for CBT + SMC compared with SMC alone was £120,658, and was £116,815 when the SF-6D was used. We estimated that the cost of a reduction in monthly seizure frequency was £611 per seizure. Whether or not this represents ‘value for money’ is unclear. We would need to have an estimate from participants themselves concerning the value of a reduction in one seizure per month to understand its worth and the possible benefit of such a reduction on psychological and psychosocial functioning. From a societal perspective, we noted the often high level of informal care reported by participants at baseline. Within the timescale of this study, it may have been unrealistic to assume that this would reduce significantly given the potential range of other comorbidities in this patient group and family support systems that may have developed.
In addition to the self-report health and informal care use measures, we obtained objective measures of health service use (A&E, outpatient and inpatient use) and found a generally similar pattern of results to that shown by the CSRI for A&E and outpatient use. There were slightly higher costs estimated for inpatient stays based on the CSRI than on HES data. To our knowledge, this is the first time that HES data have been analysed for patients with DSs. Although the numbers of patients contributing to the results from the CSRI and HES data differed slightly, the current data suggest that DS patients are able to provide broadly accurate accounts of their hospital health service use. It is possible, however, that the length of hospital stays was slightly over-reported. It is not possible in any case to be sure that HES data are themselves entirely accurate given that their completion depends on the diligence of the many different people who complete the records. We suggest, therefore, that future studies rely mostly on self-report measures of health service use unless very fine-grained analyses are required. Certainly, HES data do not permit the estimation of medication costs, and these were of relevance in this sample.
The current study has yielded data showing areas of differential clinical effectiveness in favour of CBT + SMC when considering secondary outcomes. We cannot determine whether or not the current high cost-effectiveness ratio of £120,658 per QALY is due to the relatively short follow-up period. Both costs and QALYs were measured for the whole follow-up period, but we do not know what longer-term impact of CBT there may be. If gains were prolonged, this would reduce the cost-effectiveness ratio. However, this assumes that no ‘top-ups’ are provided. For both trial arms, patients may have received SMC sessions as a function of what was recommended as part of the trial rather than what may have occurred in everyday clinical practice. Taking into consideration scores on the modified PHQ-15 and the number of SAEs (of which relatively few were related to DSs), it is clear that there was a wide range of reported comorbid medical disorders, many requiring medical service input, and it is perhaps unlikely that our CBT intervention would have reduced these. Patients were also characterised at baseline by high levels of psychiatric comorbidity on the M.I.N.I. (see Table 9); therefore, it is possible that at follow-up the failure to show reduced costs may be a function of this comorbidity having been identified during the trial, with participants being directed for further treatment. The fact that we evaluated our DS-specific intervention in a sample of people with DSs characterised by generally high levels of deprivation and low quality of life at baseline may also have had an effect on our outcomes.
Despite the lack of demonstrable cost-effectiveness, according to NICE guidelines it is important to note what patients reported about their experiences. There was greater satisfaction with treatment in the CBT + SMC arm than in the SMC arm (see Chapter 3). Relatively few AEs (30/118) and SAEs (10/58) were deemed to have been related to DSs. In addition to the value placed on seizure control techniques by those receiving them, there was a perception that different members of the multidisciplinary care team involved in the study could potentially offer a greater range of support than would have been available in the SMC-alone arm (see Chapter 5). The value of the multidisciplinary care pathway implemented for the CODES trial was acknowledged by the neurologists, psychiatrists and therapists, and supports the view that clear interdisciplinary communication is important for the management of patients with FND. A clear difficulty was that after their involvement in the trial it was not possible for all services to maintain this multidisciplinary care pathway (see Chapter 5), which has implications for service availability after this study. Nonetheless, the value placed on aspects of the diagnostic process, the guidance given to neurologists in terms of diagnosis delivery and the written materials made available for neurologists and psychiatrists to give to patients suggest that there are aspects of our SMC that could be adopted more routinely in clinical services. However, the use of such material may benefit from enhanced education of professionals about FND more generally and DSs specifically. 152
It is important to note that our analyses reflect group outcomes and hide variability within the different outcomes. Given that we followed our published SAP,92 we have not yet examined whether there were subgroups of patients showing different patterns of outcome either overall or at different times, or whether there were factors clearly moderating outcome. We have commented (see Chapter 3) on the fact that our DS-specific CBT was not a trauma-focused intervention and that in certain cases patients may have been deemed to need further therapy at the end of their time in the study. Although we did not formally monitor this clinical need, we are also aware that we did not systematically measure participants’ abuse history. We chose not to administer a trauma/life events checklist at baseline because we did not want to cause distress to participants or make them feel that disclosing abuse was essential early in the study. We acknowledge that we cannot examine whether or not abuse histories relate to outcome. We did, however, have a proxy measure of abuse history, the PTSD subscale of the M.I.N.I. (see Chapter 3), although this will not necessarily reflect the range and impact of different histories of abuse that may have been present in this population. We know from our qualitative work137 that some patients were apprehensive about being allocated to the CBT + SMC intervention arm (see also Chapter 5). CBT therapists were not able to undertake an assessment of individuals’ suitability for a CBT intervention prior to them starting therapy. Being able to do this in routine practice may have led to patients being better prepared for therapy and all it entails, and may have allowed treatment to be deferred where this may have allowed better engagement, something not possible in a RCT. Nevertheless, patients had provided informed consent to be randomised possibly to a talking therapy, indicating a willingness to engage to some extent in change.
We are aware that some within-group variability may have come from the fact that for some patients a longer time between diagnosis delivery and start of therapy may have suited their personal and other family circumstances better in terms of attending treatment sessions, rather than having to work within the timelines of the funded study. Of note, however, is the observation (see Chapter 3) from our CACE analysis that the effectiveness of the intervention (i.e. the outcome seen for all randomised patients allocated treatment) was the same as its efficacy (i.e. the outcome seen in those receiving at least nine CBT sessions), which suggests that being compliant with attending CBT sessions was not straight-forwardly related to outcome. This indicates that a more fine-grained analysis of patients’ pattern of improvement could be informative.
Strengths and limitations
To the best of our knowledge, the CODES trial is by far the largest trial to have been undertaken worldwide in the treatment of DSs (n = 368). It is a study that has attracted international interest. It had high levels of PPI/SU input to study governance via our oversight committees throughout the study. The study recruited people from 27 neurology/epilepsy services and randomised patients in 17 liaison/neuropsychiatry services across the UK, thereby removing the bias associated with single centres that may attract patient referrals corresponding to specific clinicians’ interests, although we did involve services with interest and experience in working with patients with DSs. However, the broad spread of recruitment sites, with different organisational infrastructure, may have made it difficult to get accurate estimates of the overall number of patients initially considered for eligibility to the first phase of the study. We experienced excellent recruitment and retention by randomising above target and obtaining primary outcome data (monthly DS frequency at 12 months) for 85% of those randomised. We maintained a high level of masking for our data collectors and statisticians. We recruited patients with a wide range of comorbid psychopathology and other background characteristics that posed a stringent test of our psychotherapeutic intervention. Our baseline measures were well balanced across the trial arms. Our SMC materials were developed by experts in the field, but there was also SU input and advice from a hospital information officer to ensure the readability of clinical materials for participants.
The trial demonstrated that the CBT intervention could be applied satisfactorily by a range of therapists with diverse backgrounds who, following CODES-specific training, displayed a level of adherence to the therapy manual rated at 86% while the delivery of CBT was rated at 79%; therapists’ therapeutic alliance with participants was high. It is possible that higher levels of fidelity with the seizure-related components could have led to better effectiveness; however, our current trial design does not permit evaluation of the relative importance of the DS-specific components compared with a more generic or transdiagnostic CBT approach. Supervision of therapists was provided by experts in the application of our therapy model to patients with DSs, although their many years of experience with the treatment model may not be on offer elsewhere from future supervisors. Our therapists were from a range of professional backgrounds and levels of clinical experience; therefore, we were not testing our CBT using only therapists with high levels of prior experience in working with patients with DSs, which is important for generalisability. Given that we involved substantially more therapists than initially anticipated, this may mean that some therapists had insufficient opportunity to undertake the therapy with enough patients to develop confidence in applying the DS-specific CBT skills.
We commented earlier (Chapter 3) that our SMC might be better conceptualised as specialist and standardised medical care. Therefore, a possible weakness of the study is that, with respect to SMC, we may have inadvertently created an intervention in its own right, rather than allowing services to continue to treat patients as they would usually have done. Some services changed what they generally offered patients during the study (e.g. one site began to offer a psychoeducation group to patients with FND following diagnosis in neurology and prior to individual psychotherapy). By referring to the need to provide standardised rather than usual care, it was possible to explain the need for them to keep consistent what was offered to all of that site’s patients in the trial rather than starting mid-trial to offer potential CODES patients other interventions. Thus, it was possible to maintain consistency within the trial.
It is also the case that the frequent seizure diary data collection and contact with the research team, which would not occur normally in clinical practice over such a long period, may also have served as an intervention. It may have regularly focused patients’ attention on their disorder and its perceived severity. The quality of SMC might, however, have been such that a particularly high level of belief in diagnosis was enabled in the RCT patients, which may have rendered them less typical than other patients with DSs.
Given our inclusion and exclusion criteria, there will be some other limits to the generalisability of our findings. We recruited participants into the RCT according to a strict care pathway, including only people who had initially received their diagnosis from neurologists/epilepsy specialists and who had attended their psychiatric assessment around 3 months later. Thus, people with DSs who presented initially to other services or who were referred directly to secondary care CODES psychiatrists by their GP were not included. Including both neurology and psychiatry services in the study meant that, in some settings, we needed to create care pathways that did not exist prior to the study and that ceased to function after the study ended (see Chapter 5). The care pathways, therefore, offered benefit to patients who might not otherwise have had treatment opportunities but makes our findings less generalisable to settings in which both treatment components are not available.
The study was conducted in the context of the NHS where treatment provision is free at the point of use; therefore, this health context may not generalise to other healthcare systems. Similarly, healthcare costs may reflect service characteristics that are specific to the NHS and social care context; state financial benefits changed during the course of the study and we did not, therefore, estimate these. At times it was necessary to encourage services not to discharge patients for non-attendance at early psychiatry or CBT sessions where waiting list pressures might otherwise have led to discharge. Therefore, it is possible that our uptake rates might have been lower had we not been running a trial and encouraging services to engage reluctant or non-attenders, although we cannot estimate by how much. Similarly, we offered patients up to £25 towards travel to the initial psychiatry assessment and the CBT sessions and, although some patients able to receive hospital transport might not have claimed this money, this option might also have increased CBT attendance by others.
We excluded people with documented intellectual disabilities because we wished to implement our CBT package without further modification and make it more uniform; we similarly excluded people with insufficient fluency in English, as we could not guarantee the availability of interpreters and our outcome measures were all standardised in English. The majority of patients were white, with very few from other ethnic backgrounds, possibly as a result of this exclusion criterion. The delivery of our intervention, therefore, occurred within a relatively limited cultural context. The level of flexibility required by therapists to take account of cultural beliefs or other influences was, therefore, not tested.
Several baseline (pre-randomisation) measures suggested an appreciable level of psychopathology in our sample. We used the SAPAS-SR112 as a screen for maladaptive personality traits rather than frank personality disorder, but note that the mean value obtained for our sample was very similar to that observed in people with generalised anxiety disorder. 173 This finding may reflect the confounding problem of anxiety symptoms in our sample rather than providing a meaningful estimate of maladaptive personality characteristics.
A particular limitation to the discussion of the findings presented here is that, although we adhered to our SAP92 and focused our outcome evaluation at the 12-month post-randomisation time point (see explanations in Chapter 2), we were not able to evaluate the statistical significance of any between-group differences on our many measures at the 6-month post-randomisation time point. We chose to evaluate outcomes at 12 months post randomisation to test our intervention in a stringent manner, to minimise the number of comparisons undertaken and to be in line with the commissioning brief, which stipulated at least 12 months of follow-up. Although we presented the two arms’ 6-month data (see Tables 14 and 15, and Figures 6 and 7), we cannot comment here on between-group differences at this time point using our current statistical modelling. However, it will be important for the clinical field to know the limits, in terms of both time and scope, of any intervention offered to patients with DSs, and we would recommend that any future trials formally evaluate outcomes at a point broadly consistent with the end of treatment as well as after longer-term follow-up. It might be appropriate, therefore, to avoid having to adjust statistically for a large number of related analyses, for future work to evaluate fewer outcomes but at both time points.
The funder had identified DS frequency as an important outcome and we adopted this as we could conduct a power calculation based on our previous findings. 1 However, others have questioned whether or not DS frequency is a useful outcome measure52 and whether or not economic productivity might be more informative, despite the fact that it may be challenging to improve this in a short period of time. It is also possible that functional status (e.g. as measured by the WSAS in our study) might offer a more informative measure.
We did not insist that all participants had received their diagnosis of DSs based on video EEG to be eligible for the trial; just over half of the sample was diagnosed in this manner, which is broadly consistent with management of patients with DSs in the UK. 71 Video EEG is not always available or deemed cost-effective in clinical services. Others have considered it appropriate to initiate treatments with greater diagnostic uncertainty. 17 We overcame some of this difficulty by accepting a consensus diagnosis or by facilitating review where the patient had been diagnosed by only one clinician without video EEG, but we acknowledge the possibility of misdiagnosis in some cases. The psychiatric assessment some weeks later allowed for further scrutiny of patients’ presentations and led to some exclusions. We did not, however, classify diagnostic levels of certainty according to recommended suggestions, so our data cannot be compared more widely on this basis. 17
In this trial, patients were unblinded to the treatment arm allocation. We did not attempt to blind treating clinicians and we cannot be sure that doctors did not change their practice depending on the treatment their patients received in the study. In particular, this could have been relevant to the use of pharmacological interventions as part of SMC. LaFrance et al. 68 studied the effect of antidepressant medication on DS occurrence, and, although we indicated that psychopharmacological interventions could be implemented by the SMC doctors, we did not formally record when these were prescribed or what they were. Therefore, we cannot know whether or not there was a difference in specific types of medication use between arms.
Given the trial’s pragmatic design, we did not attempt to control for therapists’ attention and time between the two treatment arms. Although our CBT package did permit therapists to address trauma in patients’ histories, this was not a specific trauma-focused therapy and after the 12-month follow-up point additional therapy to address trauma may have been warranted for some patients.
Implications for health care
Outside the CODES study, clinical services for people with DSs remain variable (see Chapter 5, Rawlings et al. 153 and Hingray et al. 155). The CODES trial shows that within an NHS context and through the provision of materials, guidelines and training, it is possible to facilitate diagnosis delivery and subsequent medical plus psychotherapeutic care in a manner that leads to a number of benefits in patients’ psychological and psychosocial functioning. Our 12-session intervention was based on our previous work regarding numbers of sessions. 1,82 However, there may remain patients who require further treatment, for example for complex trauma, and services need to consider remaining open to patients who need ongoing clinical input. Equally, our CACE analysis (see Chapter 3) suggests that in clinical applications of the intervention, patient-by-patient assessment of the number of sessions needed to bring about benefit in terms of DS reduction may be necessary and it cannot automatically be assumed that better results are achieved with more sessions. Clinicians, as well as patient participants, valued the provision of written educational materials, suggesting that these could helpfully be used in routine clinical practice. It is possible that greater education about DSs (and FND in general) as part of HCPs’ training152 will lay the groundwork for better-quality service delivery more broadly. Wider possibilities for delivering DS-specific CBT (e.g. via Improving Access to Psychological Therapies services) could be explored and training provided, but such services could then be encouraged to foster firm links with referrers (neurologists, psychiatrists and GPs) to provide ‘joined up’ care rather than simply accepting referrals and then managing patients completely independently. We did not record whether or not all CBT sessions were offered during standard working hours and, although we consider it highly likely that they were, future service provision might consider whether or not people with DSs who are able to maintain active employment or study despite their seizures might be disadvantaged by therapy that is provided in normal working hours only.
Recommendations for future research
Further research is needed to clarify who benefits most from DS-specific CBT and what mediates change. Planned explorations of mediators and moderators of outcome in this study will address this issue. In addition, given that it is not possible to determine this from the current study of our complex intervention, future research could determine which components of the DS-specific CBT are important in improving functioning in patients and whether briefer interventions could yield similar or greater benefits and, if so, for whom. Our qualitative data (see Chapter 5, Wilkinson et al. ,135 Read et al. ,137 and Jordan et al. 152) may also offer helpful insights into aspects of future interventions that might enhance uptake and allow realistic expectations of what participants should expect from treatment. Other, third-wave, CBT approaches (e.g. acceptance and commitment therapy) for patients with DSs174 could possibly be a useful comparison with our DS-specific CBT as a means of comparing and evaluating therapeutic modalities. Future research could also try to establish estimates of clinically meaningful change for people with DSs in a variety of outcomes, such as those measured here. This could lead to improvement of future treatment studies in this area.
Finally, the cost-effectiveness analysis was based entirely on trial data and future research might also use modelling to investigate longer-term cost-effectiveness.
Acknowledgements
We are very grateful to the members and particularly the chairpersons of the TSC and DMEC for their invaluable advice and support throughout the study.
Trial Steering Committee members
Professor Christopher Williams (chairperson), Professor Garry Barton, Professor Steve Jones, Professor Edward J Watkins, Professor Adam Zeman and our service user representatives (Ms Lorna Sprague and another member who wishes to remain anonymous).
Data Monitoring and Ethics Committee members
Professor Karina Lovell (chairperson), Dr John Paul Leach, Professor Tony Marson and Dr Ruth Pickering.
Staff at collaborating hospitals
Anne-Mary Abe, Naghme Adab, Niruj Agrawal, Holger Allroggen, Debie Alvares, Thomasin Andrews, Heather Angus-Leppan, Julia Aram, Richard Armstrong, Antonio Atalaia, Manny Bagary, Masha Bennett, Tamsin Black, Daniel Blackburn, Mayur Bodani, Mark Broadhurst, Elisa Bruno, Mary Buckley, Richard Chalmers, Sam Chong, Muhammad Chowdhury, Fahmida Chowdury, Katia Cikurel, Giovanni Cocco, Hannah Cock, Sarah Cooper, Sarah Cope, Amy Copping, Robert Delamont, Gary Dennis, Christopher Derry, Rebecca Devlin, Jon M Dickson, Beate Diehl, Clare Donnelly, Susan Duncan, Mark Edwards, Shan Ellawella, Cathy Ellis, Jennifer Elvish, Robert Elwes, Sandra Eriemo, Sofia Eriksson, Kerry Evans, Rafey Faruqui, Gerald Finnerty, Lorena Flores, Nick Firth, Robert Fung, Paula Gardiner, Christopher Graham, Zayn Green-Thompson, Richard Grunewald, Robert Hadden, Khalid Hamandi, Rachel Harding, Sreedharan Harikrishnan, Simon Harrison, Helen Healy, Channa Hewamadduma, Stephen Higgins, Stephen Howell, Heloise Hunt, Abrar Hussain, Motchila Innocente, Graham Jensch, Michael Johnson, Joanna Karlsson, Andrew Kelso, Steven Kemp, Jonathan Knibb, Norman Kock, Michael Koutroumanidis, Stjepana Kovac, Guru Kumar, Andy Laker, Guy Leschziner, Rebecca Liu, Dora Lozsadi, Lea Ludwig, Bridget MacDonald, Lindsey MacGregor, Melissa Maguire, Mark Manford, Davide Martino, Dougall McCorry, Kenneth McKeown, Fiona McKevitt, Anna Meadow, Shafqat Memon, Ana Miorelli, Clinton Mitchell, Tejal N Mitchell, Virginia Moffitt, Nicholas Moran, Aimee Morgan-Boon, John Moriarty, Marco Mula, Nandini Mullatti, Lina Nashef, Daniel O’Hara, Louise Oakley, Suzanne O’Sullivan, Lisa Page, Darshna Patel, Panayiota Petrochilos, Danielle Phoenix, William Pickerell, Thirza Pieters, Gary Price, David Protheroe, Patrick Pullicino, Jennifer Quirk, Sanjeev Rajakulendran, Basil Ridha, Claire Rockliffe-Fidler, Carrie Rowbottom, Fergus Rugg-Gunn, Amrit Sachar, Romi Saha, Gerard Saldanha, Shanika Samarasekera, Violeta Sanchez Sanchez, Alastair Santhouse, Karen Scholes, Abhijeeth Shetty, Paul Shotbolt, Rachel Simkiss, Jasvinder Singh, Jananee Sivagnanasundaram, Sean Slaght, Philip Smith, Dilraj Sokhi, Biba Stanton, Liya Suvorova, Tayyeb Tahir, Ruth Taylor, Lara Teare, Lorenza Tedesco, James Teo, John Thorpe, Laura Toplis, Myrto Tsakopoulou, Ivona Tylova, Tracey Vick, Jane Vinnicombe, Matthew Walker, Cathy Walsh, Gillian Watson, Thomas Webb, Tim Wehner, Killian Welch, Kirstin Weyrich, Margaret Whittaker, Mirdhu Wickremaratchi, Louise Wicks and Mahinda Yogarajah.
Cognitive–behavioural therapy fidelity raters
Alison Griffiths and Sarah Lack.
Contributions of authors
Laura H Goldstein (https://orcid.org/0000-0001-9387-3035) (Professor of Clinical Neuropsychology) was the chief investigator, led the trial at all stages and led the drafting of the full report.
Emily J Robinson (https://orcid.org/0000-0002-6692-5415) (Research Fellow in Medical Statistics) wrote the statistical analysis plan and designed and conducted the analyses of the clinical quantitative data.
Izabela Pilecka (https://orcid.org/0000-0001-7350-4873) (Research Associate) was the trial manager who managed the later stages of the study.
Iain Perdue (https://orcid.org/0000-0002-8078-8146) (Research Associate) was the trial manager who oversaw most aspects of the conduct of the trial, supporting sites and staff on a day-to-day basis.
Iris Mosweu (https://orcid.org/0000-0001-6797-2478) (Research Associate, Health Economics) undertook the main health economics analysis and contributed to the drafting of the health economics results.
Julie Read (https://orcid.org/0000-0003-4450-4529) (Research Worker) contributed to data collection and the conduct, analysis and reporting of qualitative work.
Harriet Jordan (https://orcid.org/0000-0002-7268-7310) (Research Worker) contributed to data collection and the conduct, analysis and reporting of qualitative work.
Matthew Wilkinson (https://orcid.org/0000-0002-7309-2909) (Clinical Psychologist) contributed to data collection and the conduct, analysis and reporting of qualitative work.
Gregg Rawlings (https://orcid.org/0000-0003-4962-3551) (Trainee Clinical Psychologist) contributed to data collection, analysis and reporting of the neurologists’ survey.
Sarah J Feehan (https://orcid.org/0000-0003-3986-2698) (Research Worker) contributed to data collection and the conduct, analysis and reporting of qualitative work.
Hannah Callaghan (https://orcid.org/0000-0002-4864-6666) (Research Worker) contributed to data collection and the drafting of the report.
Elana Day (https://orcid.org/0000-0002-7840-2320) (Research Worker) contributed to analysis and reporting of qualitative work and other aspects of the report.
James Purnell (https://orcid.org/0000-0003-2448-8824) (Administrator) contributed to analysis and reporting of qualitative work and other aspects of the report.
Maria Baldellou Lopez (https://orcid.org/0000-0003-0001-2573) (Administrator) contributed to the drafting of the report.
Alice Brockington (https://orcid.org/0000-0001-8469-9231) (Consultant in Neurology) undertook blind ratings of adverse events.
Christine Burness (https://orcid.org/0000-0001-9062-0630) (Consultant in Neurology) undertook blind ratings of adverse events.
Norman A Poole (https://orcid.org/0000-0002-3187-6430) (Consultant in Neuropsychiatry) undertook blind ratings of adverse events.
Carole Eastwood (https://orcid.org/0000-0002-8173-0376) (PPI representative) was involved in the conception, design and conduct of the study, and advised on issues relevant to service users, including dissemination.
Michele Moore (https://orcid.org/0000-0002-9686-1504) (PPI representative) was involved in the conception, design and conduct of the study, and advised on issues relevant to service users, including dissemination.
John DC Mellers (https://orcid.org/0000-0002-1253-2835) (Consultant Neuropsychiatrist) was a co-applicant and was involved in the conception, design and conduct of the study.
Jon Stone (https://orcid.org/0000-0001-9829-8092) (Professor of Neurology) was a co-applicant and was involved in the conception, design and conduct of the study.
Alan Carson (https://orcid.org/0000-0002-7425-0964) (Professor of Neuropsychiatry) was a co-applicant and was involved in the conception, design and conduct of the study.
Nick Medford (https://orcid.org/0000-0001-6739-6001) (Consultant Neuropsychiatrist) was a co-applicant and was involved in the conception, design and conduct of the study.
Markus Reuber (https://orcid.org/0000-0002-4104-6705) (Professor of Neurology) was a co-applicant and was involved in the conception, design and conduct of the study.
Paul McCrone (https://orcid.org/0000-0001-7001-4502) (Professor of Health Economics) was a co-applicant and was involved in the conception, design and conduct of the study. He also had overall responsibility for the health economics component and its analysis.
Joanna Murray (https://orcid.org/0000-0002-7348-7505) (Senior Lecturer) was a co-applicant and was involved in the conception, design and conduct of the study (especially the qualitative studies).
Mark P Richardson (https://orcid.org/0000-0001-8925-3140) (Paul Getty III Professor of Epilepsy) was a co-applicant and was involved in the conception, design and conduct of the study.
Sabine Landau (https://orcid.org/0000-0002-3615-8075) (Professor of Biostatistics) was a co-applicant, was involved in the conception, design and conduct of the study and supervised the clinical quantitative analyses.
Trudie Chalder (https://orcid.org/0000-0003-0775-1045) (Professor of Cognitive Behavioural Psychotherapy) was a co-applicant, was involved in the conception, design and conduct of the study, developed the cognitive–behavioural intervention and oversaw the manual writing, training and supervision of therapists.
Publications
Goldstein LH, Mellers JDC, Landau, S, Stone J, Carson A, Medford N, et al. Cognitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurol 2015;15:98.
Robinson EJ, Goldstein LH, McCrone P, Perdue I, Chalder T, Mellers JDC, et al. Cognitive behavioural therapy versus standardised medical care for adults with dissociative non-epileptic seizures (CODES): statistical and economic analysis plan for a randomised controlled trial. Trials 2017;18:258.
Goldstein LH, Robinson EJ, Reuber M, Chalder T, Callaghan H, Eastwood C, Landau S, et al. Characteristics of 698 patients with dissociative seizures: a UK multicenter study. Epilepsia 2019;60:2182–93.
Jordan H, Feehan, S, Perdue I, Murray J, Goldstein LH. Exploring psychiatrists’ perspectives of working with patients with dissociative seizures in the UK healthcare system as part of the CODES trial – a qualitative study. BMJ Open 2019;9:e026493.
Rawlings G, Perdue I, Goldstein LH, Carson AJ, Stone J, Reuber M. Neurologists’ experiences of participating in the CODES study – a multicentre randomised controlled trial comparing cognitive behavioural therapy vs standardised medical care for dissociative seizures. Seizure: Eur J Epilepsy 2019;71:8–12.
Goldstein LH, Robinson EJ, Mellers JDC, Stone J, Carson A, Chalder T, et al. Psychological and demographic characteristics of 368 patients with dissociative seizures: data from the CODES cohort. Psychol Med 2020;1–13.
Goldstein LH, Robinson EJ, Mellers JDC, Stone J, Carson A, Reuber M, et al. Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry 2020;7:491–505.
Read J, Jordan H, Perdue I, Purnell J, Murray J, Chalder T, et al. The experience of trial participation, treatment approaches and perceptions of recovery among participants with dissociative seizures within the CODES randomised controlled trial: a qualitative study. Epilepsy Behav 2020;111:107230.
Stone J, Callaghan H, Robinson EJ, Carson A, Reuber M, Chalder T, et al. Predicting first attendance at psychiatry appointments in patients with dissociative seizures. Seizure: Eur J Epilepsy 2020;74:93–8.
Wilkinson M, Day E, Purnell J, Pilecka I, Perdue I, Murray J, et al. The experiences of therapists providing cognitive behavioural therapy (CBT) for dissociative seizures in the CODES randomized controlled trial: a qualitative study. Epilepsy Behav 2020;105:106943.
Published conference abstracts
Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Magill N, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic seizures (CODES): an RCT protocol. J Neurol Neurosurg Psychiatry 2015;86(9). (Conference: British Neuropsychiatry Association, London, February 2015.)
Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Medford N, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): progress in a randomised controlled trial. Epilepsia 2016;57:23. (Conference: 12th European Congress on Epileptology, Prague, September 2016.)
Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Medford N, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic seizures (CODES): update on a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 2017;88:A26. (Conference: British Neuropsychiatry Association, London, February 2017.)
Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Medford N, et al. COgnitive behavioural therapy vs. standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): baseline characteristics of participants in a randomised controlled trial. Epilepsia 2018;59:S239. (Conference: 13th European Congress on Epileptology, Vienna, August 2018.)
Protocol available
Goldstein LH, Mellers JDC, Landau, S, Stone J, Carson A, Medford N, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurol 2015;15:98.
See also update (statistical analysis plan)
Robinson EJ, Goldstein LH, McCrone P, Perdue I, Chalder T, Mellers JDC, et al. COgnitive behavioural therapy versus standardised medical care for adults with Dissociative non-Epileptic seizures (CODES): statistical and economic analysis plan for a randomised controlled trial. Trials 2017;18:258.
Data-sharing statement
In the first instance all data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following this.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Goldstein LH, Chalder T, Chigwedere C, Khondoker MR, Moriarty J, Toone BK, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010;74:1986-94. https://doi.org/10.1212/WNL.0b013e3181e39658.
- Duncan R, Razvi S, Mulhern S. Newly presenting psychogenic nonepileptic seizures: incidence, population characteristics, and early outcome from a prospective audit of a first seizure clinic. Epilepsy Behav 2011;20:308-11. https://doi.org/10.1016/j.yebeh.2010.10.022.
- Lesser RP. Psychogenic seizures. Neurology 1996;46:1499-507. https://doi.org/10.1212/WNL.46.6.1499.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 2013. https://doi.org/10.1176/appi.books.9780890425596.
- World Health Organization . ICD 11 for Mortality and Morbidity Statistics (ICD 11 MMS) 2018.
- Scull DA. Pseudoseizures or non-epileptic seizures (NES); 15 synonyms. J Neurol Neurosurg Psychiatry 1997;62. https://doi.org/10.1136/jnnp.62.2.200-a.
- Stone J, Campbell K, Sharma N, Carson A, Warlow CP, Sharpe M. What should we call pseudoseizures? The patient’s perspective. Seizure 2003;12:568-72. https://doi.org/10.1016/S1059-1311(03)00055-4.
- World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Description and Diagnostic Guidelines 1992.
- Benbadis SR, Hauser WA. An estimate of the prevalence of psychogenic non-epileptic seizures. Seizure 2000;9:280-1. https://doi.org/10.1053/seiz.2000.0409.
- Kanemoto K, LaFrance WC Jr, Duncan R, Gigineishvili D, Park SP, Tadokoro Y, et al. PNES around the world: where we are now and how we can close the diagnosis and treatment gaps-an ILAE PNES Task Force report. Epilepsia Open 2017;2:307-16. https://doi.org/10.1002/epi4.12060.
- Gates JR. Nonepileptic seizures: Classification, coexistence with epilepsy, diagnosis, therapeutic approaches, and consensus. Epilepsy Behav 2002;3:28-33. https://doi.org/10.1006/ebeh.2001.0310.
- Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav 2015;46:60-5. https://doi.org/10.1016/j.yebeh.2015.03.015.
- Acar G, Salinsky MC. Demographic and historical backgrounds of the elderly with nonepileptic seizures: a comparative study. Neurol India 2010;58:48-52. https://doi.org/10.4103/0028-3886.60396.
- Duncan R, Oto M, Martin E, Pelosi A. Late onset psychogenic nonepileptic attacks. Neurology 2006;66:1644-7. https://doi.org/10.1212/01.wnl.0000223320.94812.7a.
- Patel H, Scott E, Dunn D, Garg B. Nonepileptic seizures in children. Epilepsia 2007;48:2086-92. https://doi.org/10.1111/j.1528-1167.2007.01200.x.
- Angus-Leppan H. Diagnosing epilepsy in neurology clinics: a prospective study. Seizure 2008;17:431-6. https://doi.org/10.1016/j.seizure.2007.12.010.
- LaFrance WC Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013;54:2005-18. https://doi.org/10.1111/epi.12356.
- Mellers JDC. The approach to patients with ‘non-epileptic seizures’. Postgrad Med J 2005;81:498-504. https://doi.org/10.1136/pgmj.2004.029785.
- Kutlubaev MA, Xu Y, Hackett ML, Stone J. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav 2018;89:70-8. https://doi.org/10.1016/j.yebeh.2018.10.010.
- McKenzie PS, Oto M, Graham CD, Duncan R. Do patients whose psychogenic non-epileptic seizures resolve, ‘replace’ them with other medically unexplained symptoms? Medically unexplained symptoms arising after a diagnosis of psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2011;82:967-9. https://doi.org/10.1136/jnnp.2010.231886.
- Bermeo-Ovalle A, Kanner AM, LaFrance WC, Schachter SC. Gates and Rowan’s Nonepileptic Seizures. Cambridge: Cambridge University Press; 2018.
- Brown RJ, Reuber M. Psychological and psychiatric aspects of psychogenic non-epileptic seizures (PNES): a systematic review. Clin Psychol Rev 2016;45:157-82. https://doi.org/10.1016/j.cpr.2016.01.003.
- Walsh S, Levita L, Reuber M. Comorbid depression and associated factors in PNES versus epilepsy: systematic review and meta-analysis. Seizure 2018;60:44-56. https://doi.org/10.1016/j.seizure.2018.05.014.
- Frances PL, Baker GA, Appleton PL. Stress and avoidance in pseudoseizures: testing the assumptions. Epilepsy Res 1999;34:241-9. https://doi.org/10.1016/S0920-1211(98)00116-8.
- Goldstein LH, Drew C, Mellers J, Mitchell-O’Malley S, Oakley DA. Dissociation, hypnotizability, coping styles and health locus of control: characteristics of pseudoseizure patients. Seizure 2000;9:314-22. https://doi.org/10.1053/seiz.2000.0421.
- Duncan R, Oto M, Wainman-Lefley J. Mortality in a cohort of patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2012;83:761-2. https://doi.org/10.1136/jnnp-2012-302900.
- Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018;5:307-20. https://doi.org/10.1016/S2215-0366(18)30051-8.
- Pick S, Mellers JD, Goldstein LH. Dissociation in patients with dissociative seizures: relationships with trauma and seizure symptoms. Psychol Med 2017;47:1215-29. https://doi.org/10.1017/S0033291716003093.
- Fiszman A, Alves-Leon SV, Nunes RG, D’Andrea I, Figueira I. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav 2004;5:818-25. https://doi.org/10.1016/j.yebeh.2004.09.002.
- Beghi M, Cornaggia I, Magaudda A, Perin C, Peroni F, Cornaggia CM. Childhood trauma and psychogenic nonepileptic seizures: a review of findings with speculations on the underlying mechanisms. Epilepsy Behav 2015;52:169-73. https://doi.org/10.1016/j.yebeh.2015.09.007.
- Reuber M, Howlett S, Khan A, Grünewald RA. Non-epileptic seizures and other functional neurological symptoms: predisposing, precipitating, and perpetuating factors. Psychosomatics 2007;48:230-8. https://doi.org/10.1176/appi.psy.48.3.230.
- Leserman J. Sexual abuse history: prevalence, health effects, mediators, and psychological treatment. Psychosom Med 2005;67:906-15. https://doi.org/10.1097/01.psy.0000188405.54425.20.
- Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev 2006;26:1020-40. https://doi.org/10.1016/j.cpr.2005.11.011.
- Binzer M, Stone J, Sharpe M. Recent onset pseudoseizures – clues to aetiology. Seizure 2004;13:146-55. https://doi.org/10.1016/S1059-1311(03)00184-5.
- Stone J, Sharpe M, Binzer M. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics 2004;45:492-9. https://doi.org/10.1176/appi.psy.45.6.492.
- Moore PM, Baker GA, McDade G, Chadwick D, Brown S. Epilepsy, pseudoseizures and perceived family characteristics: a controlled study. Epilepsy Res 1994;18:75-83. https://doi.org/10.1016/0920-1211(94)90035-3.
- Krawetz P, Fleisher W, Pillay N, Staley D, Arnett J, Maher J. Family functioning in subjects with pseudoseizures and epilepsy. J Nerv Ment Dis 2001;189:38-43. https://doi.org/10.1097/00005053-200101000-00007.
- Wood BL, McDaniel S, Burchfiel K, Erba G. Factors distinguishing families of patients with psychogenic seizures from families of patients with epilepsy. Epilepsia 1998;39:432-7. https://doi.org/10.1111/j.1528-1157.1998.tb01396.x.
- Al Marzooqi SM, Baker GA, Reilly J, Salmon P. The perceived health status of people with psychologically derived non-epileptic attack disorder and epilepsy: a comparative study. Seizure 2004;13:71-5. https://doi.org/10.1016/S1059-1311(03)00158-4.
- Jones B, Reuber M, Norman P. Correlates of health-related quality of life in adults with psychogenic nonepileptic seizures: a systematic review. Epilepsia 2016;57:171-81. https://doi.org/10.1111/epi.13268.
- Cullingham T, Kirkby A, Sellwood W, Eccles FJR. Avoidance in nonepileptic attack disorder: a systematic review and meta-analyses. Epilepsy Behav 2019;95:100-11. https://doi.org/10.1016/j.yebeh.2019.03.004.
- Dimaro LV, Dawson DL, Roberts NA, Brown I, Moghaddam NG, Reuber M. Anxiety and avoidance in psychogenic nonepileptic seizures: the role of implicit and explicit anxiety. Epilepsy Behav 2014;33:77-86. https://doi.org/10.1016/j.yebeh.2014.02.016.
- Goldstein LH, Mellers JD. Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry 2006;77:616-21. https://doi.org/10.1136/jnnp.2005.066878.
- Robson C, Myers L, Pretorius C, Lian OS, Reuber M. Health related quality of life of people with non-epileptic seizures: the role of socio-demographic characteristics and stigma. Seizure 2018;55:93-9. https://doi.org/10.1016/j.seizure.2018.01.001.
- Fairclough G, Fox J, Mercer G, Reuber M, Brown RJ. Understanding the perceived treatment needs of patients with psychogenic nonepileptic seizures. Epilepsy Behav 2014;31:295-303. https://doi.org/10.1016/j.yebeh.2013.10.025.
- Karterud HN, Haavet OR, Risor MB. Social participation in young people with nonepileptic seizures (NES): a qualitative study of managing legitimacy in everyday life. Epilepsy Behav 2016;57:23-8. https://doi.org/10.1016/j.yebeh.2016.01.009.
- LaFrance WC Jr, Alosco ML, Davis JD, Tremont G, Ryan CE, Keitner GI, et al. Impact of family functioning on quality of life in patients with psychogenic nonepileptic seizures versus epilepsy. Epilepsia 2011;52:292-300. https://doi.org/10.1111/j.1528-1167.2010.02765.x.
- Lempert T, Schmidt D. Natural history and outcome of psychogenic seizures: a clinical study in 50 patients. J Neurol 1990;237:35-8. https://doi.org/10.1007/BF00319665.
- Reuber M, Pukrop R, Bauer J, Helmstaedter C, Tessendorf N, Elger CE. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305-11. https://doi.org/10.1002/ana.3000.
- Quigg M, Armstrong RF, Farace E, Fountain NB. Quality of life outcome is associated with cessation rather than reduction of psychogenic nonepileptic seizures. Epilepsy Behav 2002;3:455-9. https://doi.org/10.1016/S1525-5050(02)00524-3.
- Martin RC, Bell B, Hermann B, Mennemeyer S, Prigatano GP, Pliskin NH. Clinical Neuropsychology and Cost Outcome Research: A Beginning. New York, NY: Psychology Press; 2003.
- Reuber M, Mitchell AJ, Howlett S, Elger CE. Measuring outcome in psychogenic nonepileptic seizures: how relevant is seizure remission?. Epilepsia 2005;46:1788-95. https://doi.org/10.1111/j.1528-1167.2005.00280.x.
- Walczak TS, Papacostas S, Williams DT, Scheuer ML, Lebowitz N, Notarfrancesco A. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia 1995;36:1131-7. https://doi.org/10.1111/j.1528-1157.1995.tb00472.x.
- Selwa LM, Geyer J, Nikakhtar N, Brown MB, Schuh LA, Drury I. Nonepileptic seizure outcome varies by type of spell and duration of illness. Epilepsia 2000;41:1330-4. https://doi.org/10.1111/j.1528-1157.2000.tb04613.x.
- Asadi-Pooya AA, Bahrami Z, Homayoun M. Natural history of patients with psychogenic nonepileptic seizures. Seizure 2019;66:22-5. https://doi.org/10.1016/j.seizure.2019.02.006.
- McKenzie P, Oto M, Russell A, Pelosi A, Duncan R. Early outcomes and predictors in 260 patients with psychogenic nonepileptic attacks. Neurology 2010;74:64-9. https://doi.org/10.1212/WNL.0b013e3181c7da6a.
- Kanner AM, Parra J, Frey M, Stebbins G, Pierre-Louis S, Iriarte J. Psychiatric and neurologic predictors of psychogenic pseudoseizure outcome. Neurology 1999;53:933-8. https://doi.org/10.1212/wnl.53.5.933.
- Ettinger AB, Dhoon A, Weisbrot DM, Devinsky O. Predictive factors for outcome of nonepileptic seizures after diagnosis. J Neuropsychiatry Clin Neurosci 1999;11:458-63. https://doi.org/10.1176/jnp.11.4.458.
- Reuber M, Pukrop R, Bauer J, Derfuss R, Elger CE. Multidimensional assessment of personality in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2004;75:743-8. https://doi.org/10.1136/jnnp.2003.013821.
- Carton S, Thompson PJ, Duncan JS. Non-epileptic seizures: patients’ understanding and reaction to the diagnosis and impact on outcome. Seizure 2003;12:287-94. https://doi.org/10.1016/S1059-1311(02)00290-X.
- Arain AM, Hamadani AM, Islam S, Abou-Khalil BW. Predictors of early seizure remission after diagnosis of psychogenic nonepileptic seizures. Epilepsy Behav 2007;11:409-12. https://doi.org/10.1016/j.yebeh.2007.07.017.
- McDade G, Brown SW. Non-epileptic seizures: management and predictive factors of outcome. Seizure 1992;1:7-10. https://doi.org/10.1016/1059-1311(92)90047-5.
- Krahn LE, Reese MM, Rummans TA, Peterson GC, Suman VJ, Sharbrough FW, et al. Health care utilization of patients with psychogenic nonepileptic seizures. Psychosomatics 1997;38:535-42. https://doi.org/10.1016/S0033-3182(97)71398-7.
- Martin RC, Gilliam FG, Kilgore M, Faught E, Kuzniecky R. Improved health care resource utilization following video-EEG-confirmed diagnosis of nonepileptic psychogenic seizures. Seizure 1998;7:385-90. https://doi.org/10.1016/S1059-1311(05)80007-X.
- Magee JA, Burke T, Delanty N, Pender N, Fortune GM. The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav 2014;33:45-8. https://doi.org/10.1016/j.yebeh.2014.02.010.
- Ahmedani BK, Osborne J, Nerenz DR, Haque S, Pietrantoni L, Mahone D, et al. Diagnosis, costs, and utilization for psychogenic non-epileptic seizures in a US health care setting. Psychosomatics 2013;54:28-34. https://doi.org/10.1016/j.psym.2012.08.005.
- Razvi S, Mulhern S, Duncan R. Newly diagnosed psychogenic nonepileptic seizures: health care demand prior to and following diagnosis at a first seizure clinic. Epilepsy Behav 2012;23:7-9. https://doi.org/10.1016/j.yebeh.2011.10.009.
- LaFrance WC Jr, Baird GL, Barry JJ, Blum AS, Frank Webb A, Keitner GI, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014;71:997-1005. https://doi.org/10.1001/jamapsychiatry.2014.817.
- LaFrance WC, Keitner GI, Papandonatos GD, Blum AS, Machan JT, Ryan CE, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology 2010;75:1166-73. https://doi.org/10.1212/WNL.0b013e3181f4d5a9.
- Agrawal N, Fleminger S, Ring H, Deb S. Neuropsychiatry in the UK: national survey of existing service provision. Psychiatric Bulletin 2008;32:288-91. https://doi.org/10.1192/pb.bp.107.018424.
- Mayor R, Smith PE, Reuber M. Management of patients with nonepileptic attack disorder in the United Kingdom: a survey of health care professionals. Epilepsy Behav 2011;21:402-6. https://doi.org/10.1016/j.yebeh.2011.05.019.
- Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychother Theor Res Pract Train 2009;46:125-38. https://doi.org/10.1037/a0015138.
- Russell LA, Abbass AA, Allder SJ, Kisely S, Pohlmann-Eden B, Town JM. A pilot study of reduction in healthcare costs following the application of intensive short-term dynamic psychotherapy for psychogenic nonepileptic seizures. Epilepsy Behav 2016;63:17-9. https://doi.org/10.1016/j.yebeh.2016.07.017.
- Zaroff CM, Myers L, Barr WB, Luciano D, Devinsky O. Group psychoeducation as treatment for psychological nonepileptic seizures. Epilepsy Behav 2004;5:587-92. https://doi.org/10.1016/j.yebeh.2004.03.005.
- Chen DK, Maheshwari A, Franks R, Trolley GC, Robinson JS, Hrachovy RA. Brief group psychoeducation for psychogenic nonepileptic seizures: a neurologist-initiated program in an epilepsy center. Epilepsia 2014;55:156-66. https://doi.org/10.1111/epi.12481.
- Wiseman H, Mousa S, Howlett S, Reuber M. A multicenter evaluation of a brief manualized psychoeducation intervention for psychogenic nonepileptic seizures delivered by health professionals with limited experience in psychological treatment. Epilepsy Behav 2016;63:50-6. https://doi.org/10.1016/j.yebeh.2016.07.033.
- Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007;69:881-8. https://doi.org/10.1097/PSY.0b013e31815b00c4.
- van Dessel N, den Boeft M, van der Wouden JC, Kleinstäuber M, Leone SS, Terluin B, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD011142.pub2.
- Carlson P, Nicholson Perry K. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure 2017;45:142-50. https://doi.org/10.1016/j.seizure.2016.12.007.
- Martlew J, Pulman J, Marson AG. Psychological and behavioural treatments for adults with non-epileptic attack disorder. Cochrane Database Syst Rev 2014;2. https://doi.org/10.1002/14651858.CD006370.pub2.
- Chalder T. Non epileptic attacks: a cognitive behavioural approach in a single case with a four year follow up. Clin Psychol Psychother 1996;3:291-7. https://doi.org/10.1002/(SICI)1099-0879(199612)3:4<291::AID-CPP72>3.0.CO;2-H.
- Goldstein LH, Deale AC, Mitchell-O’Malley SJ, Toone BK, Mellers JDC. An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures: a pilot study. Cogn Behav Neurol 2004;17:41-9. https://doi.org/10.1097/00146965-200403000-00005.
- Goldstein LH, LaFrance WC, Mellers JDC, Chalder T, LaFrance WC, Schachter SC. Gates and Rowan’s Nonepileptic Seizures. Cambridge: Cambridge University Press; 2018.
- Goldstein LH, Mellers JDC, Landau S, Stone J, Carson A, Medford N, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0350-0.
- Baird GL, Harlow LL, Machan JT, LaFrance WC Jr. Cluster reduction in patients in a pilot treatment trial for psychogenic nonepileptic seizures. Epilepsy Behav 2017;73:273-9. https://doi.org/10.1016/j.yebeh.2017.04.015.
- National Institute for Health and Care Excellence (NICE) . Epilepsies: Diagnosis and Management. Clinical Guideline 2012.
- Scottish Intercollegiate Guidelines Network . Diagnosis and Management of Epilepsy in Adults. Guidelines 70 2005.
- Kerr M, Mensah S, Besag F, De Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia 2011;52:2133-8. https://doi.org/10.1111/j.1528-1167.2011.03276.x.
- LaFrance WC, Alper K, Babcock D, Barry JJ, Benbadis S, Caplan R, et al. Nonepileptic seizures treatment workshop summary. Epilepsy Behav 2006;8:451-61. https://doi.org/10.1016/j.yebeh.2006.02.004.
- Kelley MS, Jacobs MP, Lowenstein DH. NINDS Epilepsy Benchmark Stewards. The NINDS epilepsy research benchmarks. Epilepsia 2009;50:579-82. https://doi.org/10.1111/j.1528-1167.2008.01813.x.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Robinson EJ, Goldstein LH, McCrone P, Perdue I, Chalder T, Mellers JDC, et al. COgnitive behavioural therapy versus standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): statistical and economic analysis plan for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2006-4.
- Hall-Patch L, Brown R, House A, Howlett S, Kemp S, Lawton G, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia 2010;51:70-8. https://doi.org/10.1111/j.1528-1167.2009.02099.x.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 1994.
- Jones B, de C, Williams AC. CBT to reduce healthcare use for medically unexplained symptoms: systematic review and meta-analysis. Br J Gen Pract 2019;69:e262-e269. https://doi.org/10.3399/bjgp19X701273.
- Kleinstäuber M, Witthöft M, Steffanowski A, van Marwijk H, Hiller W, Lambert MJ. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD010628.pub2.
- Liu J, Gill NS, Teodorczuk A, Li Z-J, Sun J. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord 2019;245:98-112. https://doi.org/10.1016/j.jad.2018.10.114.
- Menon V, Rajan TM, Kuppili PP, Sarkar S. Cognitive behavior therapy for medically unexplained symptoms: a systematic review and meta-analysis of published controlled trials. Indian J Psychol Med 2017;39:399-406. https://doi.org/10.4103/IJPSYM.IJPSYM_17_17.
- Sumathipala A. What is the evidence for the efficacy of treatments for somatoform disorders? A critical review of previous intervention studies. Psychosom Med 2007;69:889-900. https://doi.org/10.1097/PSY.0b013e31815b5cf6.
- Pourová M, Klocek A. Řiháček T, Čevelíček M. Therapeutic change mechanisms in adults with medically unexplained physical symptoms: a systematic review. J Psychosom Res 2020;134. https://doi.org/10.1016/j.jpsychores.2020.110124.
- Brown RJ, Reuber M. Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clin Psychol Rev 2016;47:55-70. https://doi.org/10.1016/j.cpr.2016.06.003.
- Lang PJ, Shilen JM. Research in Psychotherapy (Vol III). Washington, DC: American Psychological Association; 1968.
- Mowrer OH. Learning Theory and Behaviour. New York, NY: Wiley; 1960.
- Roth AD. A new scale for the assessment of competences in cognitive and behavioural therapy. Behav Cogn Psychother 2016;44:620-4. https://doi.org/10.1017/S1352465816000011.
- Burgess M, Andiappan M, Chalder T. Cognitive behaviour therapy for chronic fatigue syndrome in adults: face to face versus telephone treatment: a randomized controlled trial. Behav Cogn Psychother 2012;40:175-91. https://doi.org/10.1017/S1352465811000543.
- Mohr DC, Vella L, Hart S, Heckman T, Simon G. The effect of telephone-administered psychotherapy on symptoms of depression and attrition: a meta-analysis. Clinical Psychol (New York) 2008;15:243-53. https://doi.org/10.1111/j.1468-2850.2008.00134.x.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7347.1183.
- Ministries of Housing, Communities and Local Government . English Indices of Deprivation 2010 2010. www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 15 March 2019).
- Scottish Government . Scottish Index of Multiple Deprivation 2012. www.gov.scot/Topics/Statistics/SIMD/DataAnalysis/Background-Data-2012/Background2SIMD2012.
- Welsh Government . Welsh Index of Multiple Deprivation 2011. https://gov.wales/statistics-and-research/welsh-index-multiple-deprivation/?tab=previous%26lang=en (accessed 15 March 2019).
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Germans S, Van Heck GL, Moran P, Hodiamont PPG. The self-report standardized assessment of personality-abbreviated scale: preliminary results of a brief screening test for personality disorders. Personal Ment Health 2008;2:70-6. https://doi.org/10.1002/pmh.34.
- Cramer JA, Baker GA, Jacoby A. Development of a new seizure severity questionnaire: initial reliability and validity testing. Epilepsy Res 2002;48:187-97. https://doi.org/10.1016/S0920-1211(02)00003-7.
- Ware J, Kosinski M, Keller S. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res 2009;18:727-35. https://doi.org/10.1007/s11136-009-9483-1.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. https://doi.org/10.1192/bjp.180.5.461.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Barkham M, Bewick B, Mullin T, Gilbody S, Connell J, Cahill J, et al. The CORE-10: A short measure of psychological distress for routine use in the psychological therapies. Couns Psychother Res 2013;13:3-13. http://doi.org/10.1080/14733145.2012.729069.
- Carson AJ, Stone J, Hansen CH, Duncan R, Cavanagh J, Matthews K, et al. Somatic symptom count scores do not identify patients with symptoms unexplained by disease: a prospective cohort study of neurology outpatients. J Neurol Neurosurg Psychiatry 2015;86:295-301. https://doi.org/10.1136/jnnp-2014-308234.
- Kroenke K, Spitzer RL, deGruy FV III, Swindle R. A symptom checklist to screen for somatoform disorders in primary care. Psychosomatics 1998;39:263-72. https://doi.org/10.1016/S0033-3182(98)71343-X.
- Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV III, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749-56. https://doi.org/10.1001/jama.1994.03520220043029.
- Lempert T, Dieterich M, Huppert D, Brandt T. Psychogenic disorders in neurology: frequency and clinical spectrum. Acta Neurol Scand 1990;82:335-40. https://doi.org/10.1111/j.1600-0404.1990.tb03312.x.
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
- Rimes KA, Chalder T. The Beliefs about Emotions Scale: validity, reliability and sensitivity to change. J Psychosom Res 2010;68:285-92. https://doi.org/10.1016/j.jpsychores.2009.09.014.
- White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet 2011;377:823-36. https://doi.org/10.1016/S0140-6736(11)60096-2.
- Sharpe M, Walker J, Williams C, Stone J, Cavanagh J, Murray G, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77:564-72. https://doi.org/10.1212/WNL.0b013e318228c0c7.
- Baldwin SA, Murray DM, Shadish WR, Pals SL, Holland JM, Abramowitz JS, et al. Intraclass correlation associated with therapists: estimates and applications in planning psychotherapy research. Cogn Behav Ther 2011;40:15-33. https://doi.org/10.1080/16506073.2010.520731.
- Altman DG, Doré CJ. Baseline comparisons in randomized clinical trials. Stat Med 1991;10:797-9. https://doi.org/10.1002/sim.4780100514.
- Little RJA, Rubin DB. Statistical Analysis with Missing. New York, NY: John Wiley & Sons Ltd; 2002.
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219-42. https://doi.org/10.1177/0962280206074463.
- Goldstein LH, Robinson EJ, Reuber M, Chalder T, Callaghan H, Eastwood C, et al. Characteristics of 698 patients with dissociative seizures: a UK multicenter study. Epilepsia 2019;60:2182-93. https://doi.org/10.1111/epi.16350.
- Goldstein LH, Robinson EJ, Mellers JDC, Stone J, Carson A, Reuber M, et al. Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry 2020;7:491-505. https://doi.org/10.1016/S2215-0366(20)30128-0.
- Wilkinson M, Day E, Purnell J, Pilecka I, Perdue I, Murray J, et al. The experiences of therapists providing cognitive behavioral therapy (CBT) for dissociative seizures in the CODES randomized controlled trial: a qualitative study. Epilepsy Behav 2020;105. https://doi.org/10.1016/j.yebeh.2020.106943.
- Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol 2005;58:1217-19. https://doi.org/10.1016/j.jclinepi.2005.07.009.
- Read J, Jordan H, Perdue I, Purnell J, Murray J, Chalder T, et al. The experience of trial participation, treatment approaches and perceptions of change among participants with dissociative seizures within the CODES randomized controlled trial: a qualitative study. Epilepsy Behav 2020;111. https://doi.org/10.1016/j.yebeh.2020.107230.
- Perez DL, LaFrance WC Jr. Nonepileptic seizures: an updated review. CNS Spectr 2016;21:239-46. https://doi.org/10.1017/S109285291600002X.
- Fritzsche K, Baumann K, Götz-Trabert K, Schulze-Bonhage A. Dissociative seizures: a challenge for neurologists and psychotherapists. Dtsch Arztebl Int 2013;110:263-8. https://doi.org/10.3238/arztebl.2013.0263.
- Ladha H, Gupta S, Pati S. Trends in hospital inpatient costs of psychogenic nonepileptic seizures (P3.230). Neurology 2017;88.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Department of Health and Social Care (DHSC) . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed 7 April 2021).
- Office for National Statistics . UK Labour Market: Jan 2017. Estimates of Employment, Unemployment, Economic Inactivity and Other Employment-Related Statistics for the UK 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/bulletins/uklabourmarket/jan2017 (accessed 15 March 2019).
- Joint Formulary Committee . British National Formulary 2019.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Langfitt JT, Vickery BG, McDermott MP, Messing S, Berg AT, Spencer SS, et al. Validity and responsiveness of generic preference-based HRQOL instruments in chronic epilepsy. Qual Life Res 2006;15:899-914. https://doi.org/10.1007/s11136-005-5231-3.
- van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 13 March 2020).
- Jordan H, Feehan S, Perdue I, Murray J, Goldstein LH. Exploring psychiatrists’ perspectives of working with patients with dissociative seizures in the UK healthcare system as part of the CODES trial: a qualitative study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026493.
- Rawlings GH, Perdue I, Goldstein LH, Carson AJ, Stone J, Reuber M. Neurologists’ experiences of participating in the CODES study – a multicentre randomised controlled trial comparing cognitive behavioural therapy vs standardised medical care for dissociative seizures. Seizure 2019;71:8-12. https://doi.org/10.1016/j.seizure.2019.05.020.
- Worsely C, Whitehead K, Kandler R, Reuber M. Illness perceptions of health care workers in relation to epileptic and psychogenic nonepileptic seizures. Epilepsy Behav 2011;20:668-73. https://doi.org/10.1016/j.yebeh.2011.01.029.
- Hingray C, El-Hage W, Duncan R, Gigineishvili D, Kanemoto K, LaFrance WC Jr, et al. Access to diagnostic and therapeutic facilities for psychogenic nonepileptic seizures: An international survey by the ILAE PNES Task Force. Epilepsia 2018;59:203-14. https://doi.org/10.1111/epi.13952.
- Kanaan RA, Armstrong D, Wessely SC. Neurologists’ understanding and management of conversion disorder. J Neurol Neurosurg Psychiatry 2011;82:961-6. https://doi.org/10.1136/jnnp.2010.233114.
- Rawlings GH, Reuber M. What patients say about living with psychogenic nonepileptic seizures: a systematic synthesis of qualitative studies. Seizure 2016;41:100-11. https://doi.org/10.1016/j.seizure.2016.07.014.
- Karterud HN, Knizek BL, Nakken KO. Changing the diagnosis from epilepsy to PNES: patients’ experiences and understanding of their new diagnosis. Seizure 2010;19:40-6. https://doi.org/10.1016/j.seizure.2009.11.001.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16. https://doi.org/10.1186/s13063-015-0709-y.
- Rubin HJ, Rubin IS. Qualitative Interviewing: The Art of Hearing Data. Thousand Oaks, CA: SAGE Publications Ltd; 1995.
- Furber C. Framework analysis: a method for analysing qualitative data. Afr J Midwifery Womens Health 2010;4:97-100. https://doi.org/10.12968/ajmw.2010.4.2.47612.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Denzin NK. Sociological Methods. A Sourcebook. New York, NY: MacGraw-Hill; 1978.
- Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34:1189-208.
- LaFrance WC Jr, Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia 2013;54:53-67. https://doi.org/10.1111/epi.12106.
- Dworetzky BA. What are we communicating when we present the diagnosis of PNES?. Epilepsy Curr 2015;15:353-7. https://doi.org/10.5698/1535-7511-15.6.353.
- Sahaya K, Dholakia SA, Lardizabal D, Sahota PK. Opinion survey of health care providers towards psychogenic non epileptic seizures. Clin Neurol Neurosurg 2012;114:1304-7. https://doi.org/10.1016/j.clineuro.2012.03.047.
- Rawlings GH, Reuber M. Health care practitioners’ perceptions of psychogenic nonepileptic seizures: a systematic review of qualitative and quantitative studies. Epilepsia 2018;59:1109-23. https://doi.org/10.1111/epi.14189.
- Rommelfanger KS, Factor SA, LaRoche S, Rosen P, Young R, Rapaport MH. Disentangling stigma from functional neurological disorders: conference report and roadmap for the future. Front Neurol 2017;8. https://doi.org/10.3389/fneur.2017.00106.
- Asadi-Pooya AA, Tinker J. Delay in diagnosis of psychogenic nonepileptic seizures in adults: a post hoc study. Epilepsy Behav 2017;75:143-5. https://doi.org/10.1016/j.yebeh.2017.08.005.
- Reuber M, Fernández G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58:493-5. https://doi.org/10.1212/wnl.58.3.493.
- Mahoney AEJ, Hobbs MJ, Newby JM, Williams AD, Andrews G. Psychometric properties of the worry behaviors inventory: replication and extension in a large clinical and community sample. Behav Cogn Psychother 2018;46:84-100. https://doi.org/10.1017/S1352465817000455.
- Cope SR, Poole N, Agrawal N. Treating functional non-epileptic attacks – should we consider acceptance and commitment therapy?. Epilepsy Behav 2017;73:197-203. https://doi.org/10.1016/j.yebeh.2017.06.003.
Appendix 1 Psychotherapy for psychogenic non-epileptic seizures (Health Technology Assessment programme)
This information can be found at www.fundingawards.nihr.ac.uk/award/12/26/01 (accessed on 11 March 2021), under Commissioning Brief.
Appendix 2 Contents of the communication protocol (crib sheet) for psychiatrists
(Note that formatting has been changed for current purposes.)

Communication protocol for psychiatrists
Explaining the diagnosis
-
Name the condition DISSOCIATIVE SEIZURES is preferable here.
-
Real attacks – not ‘imagined’ or ‘put on’ – can be frightening and disabling.
-
Tell them what they do not have and why – i.e. why tests etc. show this is not epilepsy.
-
Common and recognised condition diagnosed on positive grounds.
What are dissociative seizures?
-
Dissociation is the medical word for a ‘trance-like’ state or ‘switching off’.
-
‘Normal’ examples of dissociation (e.g. day-dreaming, divided attention, ‘shock’).
What causes dissociative seizures?
-
Causes often not obvious: may be related to ‘stress’ but this is often not apparent.
-
Discuss aetiological factors emerging from clinical history. Relevance of predisposing, precipitating and perpetuating factors.
-
Provide a model for the attacks – e.g. brain becomes overloaded and shuts down.
Treatment
-
Potentially reversible condition.
-
Information available on the CODES leaflet (e.g. self-help websites).
-
Anti-epileptic drugs are not effective and usually stopped.
-
Treatment of psychiatric comorbidity.
Information about the CODES study at point of randomisation
-
We do not know what treatment is best for dissociative seizures.
-
Understanding and acceptance of the diagnosis is often very helpful in its own right.
-
We know that many people get better when they are given information about the condition and receive support from a specialist doctor (known here as ‘Standardised Medical Care’).
-
We are doing the CODES study to find out if CBT is any better than ‘Standardised Medical Care’ (SMC) as we don’t know that CBT is actually better than SMC alone.
-
Explain randomisation.
Record if ELIGIBLE? and WILLING TO BE CONTACTED? by researcher

Definitions, inclusion and exclusion criteria for psychiatrists
How are you defining dissociative (non-epileptic) seizures?
1. Episodes of apparent altered responsiveness or ‘transient loss of consciousness’ [if in doubt refer/discuss with trial team (for example a paroxysmal movement disorder with some impairment of responsiveness would count even if the patient can remember the event)] 2. Clinical findings that demonstrate incompatibility between the episodes and recognised neurological or general medical conditions (e.g. typical features of a prolonged motionless episodes or a prolonged generalised thrashing attack with eyes tightly closed and a normal EEG) 3. Not better explained by another medical or mental disorder 4. The symptom or deficit causes significant distress, psychosocial impairment, or warrants medical evaluation.
Important FAQs
I think the patient needs to be seen by a community mental health team. What should I tell them about treating the seizures?
You should refer them in the normal way. The patient should receive normal care for problems such as self-harm or depression. The local team need to know that treatment of the seizures remains your responsibility as part of a trial which the patient has consented to, and should not form part of their work with the patient.
Can I give the patient medication for anxiety or depression?
There is no recognised drug treatment for non-epileptic seizures, so medication should not be prescribed for the seizures themselves. However, some patients may have co-morbid anxiety or depression that warrants prescription of medication and in such cases medication may be given, as per normal clinical practice.
Appendix 3 Treatment fidelity rating scale
| Therapy items to be rated | Question | Rating scale | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall therapist adherence | 1. Was the therapy delivered as described in the CODES therapy manual? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Not at all | Somewhat | Considerably | Extensively | |||||
| Therapeutic alliance | 2. Overall, how would you rate the therapeutic alliance (supportive encouragement, understanding, warmth, empathy)? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Very poor | Fair | Good | Excellent | |||||
| Competence in terms of specific change techniques for working with DSs |
3. Did the therapist help the client develop some strategies for controlling the seizures, which should be implemented at the first sign of a seizure?a Was this relevant to the session (yes/no)? |
1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Not at all | Some | Considerably | Extensively | |||||
|
4. Did the therapist help the client make links between specific traumas or stressors and dissociative seizures?a Was this relevant to the session (yes/no)? |
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Not at all | Some | Considerably | Extensively | |||||
|
5. Did the therapist help the client challenge unhelpful beliefs related to dissociative seizures and other problems?a,b Was this relevant to the session (yes/no)? |
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Not at all | Some | Considerably | Extensively | |||||
|
6. Did the therapist help the client ‘reclaim’ areas of their life previously avoided?a Was this relevant to the session (yes/no)? |
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Not at all | Some | Considerably | Extensively | |||||
| Overall delivery of CBT | 7. Was the therapist delivering CBT? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Not at all | Somewhat | Considerably | Extensively | |||||
Appendix 4 Expectation of treatment outcome
Please circle one answer for each question that best represents your feelings about the treatment outcome.
| 1. How logical does CBT as a treatment seem to you? | ||||
|---|---|---|---|---|
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
| 2. How confident are you that this treatment would help your illness? | ||||
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
| 3. How logical does treatment by a neurologist seem to you? | ||||
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
| 4. How confident are you that this treatment will help your illness? | ||||
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
| 5. How logical does treatment by a psychiatrist seem to you? | ||||
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
| 6. How confident are you that this treatment will help your illness? | ||||
| Extremely | Moderately | Somewhat | Only slightly | Not at all |
Appendix 5 Construction of the primary outcome
Adapted with permission from Goldstein et al. 134 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
At each of the three time points there were two methods of collecting the primary outcome measure of monthly (4-weekly) seizure frequency. Seizure diaries recorded weekly counts, which was the gold standard measure. Ideally the 12-month primary outcome was calculated as the sum of weeks 49–52 (the previous four diary weeks at 1 year post randomisation).
If this was not possible, that is at least one diary week was missing between 49 and 52 weeks, the previous week, if recorded (week 48), was included instead. Furthermore, if week 48 was missing but week 47 was recorded, this was included instead. It was not possible to include weeks 53 or 54 because the CODES database was set up to record diary entries up to 52 weeks (1 year). Participants were, therefore, recorded as having complete follow-up at 12 months post randomisation if they had provided 1–4 seizure diary weeks between weeks 47 and 52.
If no seizure diary weeks were provided between weeks 47 and 52 but participants had completed the self-report questions about seizures at 12 months, data were used from these to estimate diary seizure frequency. One of these questions asked ‘In the last 3 months, have you had a seizure?’. If this was answered yes, the next question was opened up: ‘How many seizures have you had in the past 4 weeks?’. These auxiliary measures were, therefore, used to approximate the gold standard measure, and the participant could be recorded as completing follow-up. Given that the question refers to the past 4 weeks, the observation period for these participants was set to 28 days (4 weeks).
An identical method was used for monthly seizure frequency at 6 months post randomisation. Here, the gold standard was the sum of weeks 23–26 (the previous four diary weeks at 6 months post randomisation).
Again, if this was not possible, that is at least one diary week was missing between weeks 23 and 26, the previous week, if recorded (i.e. week 22), was included instead. Furthermore, if week 22 was also missing but week 21 was recorded, this was included instead. Unlike at 12 months, a 2-week visit window was allowed on either side of 6 months. This meant that week 27 was included if a diary entry was missing between weeks 21 and 26 and fewer than four were complete in total; similarly, week 28 was included if recorded and fewer than 4 weeks were complete between weeks 21 and 27. Participants were, therefore, recorded as having completed follow-up at 6 months post randomisation if they had provided 1–4 weekly diary entries between diary weeks 21 and 28, inclusive.
The same self-report questions about seizures were collected at 6 months and, as with those at 12 months, were used to approximate diary seizure frequency if completed and seizure diary entries 21–28 were missing.
The baseline measure for monthly seizure frequency was collected before randomisation, in between the neurology and psychiatry appointments. The gold standard method was the sum of diary weeks –1 to –4, where –1 was the week prior to psychiatry assessment. This was when the participants’ eligibility for the trial was reassessed, which included having dissociative seizures in the 8 weeks prior to psychiatric assessment.
Diary weeks that commenced after the date of randomisation or included date of randomisation were dropped and could not be used. If a diary entry between –1 and –4 was missing and –5 was recorded, this was included instead; if an entry was missing between weeks –1 and –5, the number of entries recorded was less than four, and –6 was recorded, that was included instead. This process was continued up to week –8 (56 days prior to randomisation). Participants were, therefore, treated as having complete baseline seizure diary if they provided 1–4 diary entries between weeks –1 and –8. Since the diary was self-report, sometimes consecutive week numbers did not include all consecutive days.
Participants were also asked to give an estimate of seizure frequency at time of consent to the trial; once again this self-reported single measure was used to approximate baseline diary seizure frequency for those who did not complete any of the relevant diary weeks –8 to –1.
For the reasons described above, there were some participants with a baseline measure for monthly seizure frequency of zero; these participants may have had no seizures in the 4 weeks prior to psychiatric assessment, but they had all experienced at least one seizure in the 8 weeks prior to psychiatric assessment and were, therefore, all eligible for the trial.
For the purpose of descriptive statistics throughout the report, if 1–3 weeks of seizure diary had been provided at any of the three time points, a pro rata monthly frequency was calculated; for example, for a total of six seizures in weeks 49–51, with nothing recorded for weeks 47, 48 or 52, a participant’s pro rata monthly seizure frequency would be 8 = (6/3) × 4.
Appendix 6 Self-reported demographics of the neurologists, psychiatrists and CBT therapists taking part in the study
| Characteristic of professional group | Participants |
|---|---|
| Neurologists (N = 91)a | |
| Gender (n = 89), n (%) | |
| Female | 34 (38.2) |
| Male | 55 (61.8) |
| Age (years) (n = 89), n (%) | |
| 21–40 | 21 (23.6) |
| 41–60 | 63 (70.8) |
| 61–70 | 5 (5.6) |
| Current clinical appointment (n = 89), n (%) | |
| Consultant | 78 (87.6) |
| Specialist registrar | 4 (4.5) |
| Other | 7 (7.8) |
| Length of experience in psychiatry following registration with the GMC (years) (n = 88), mean (SD) [range] | 16.3 (7.6) [4–43] |
| Subspecialist interest in epilepsy (n = 89), n (%) | |
| Yes | 62 (69.7) |
| Psychiatrists (N = 29) | |
| Gender (n = 29), n (%) | |
| Female | 8 (27.6) |
| Male | 21 (72.4) |
| Age (years) (n = 29), n (%) | |
| 21–40 | 4 (13.8) |
| 41–60 | 25 (86.2) |
| Current clinical appointment (n = 29), n (%) | |
| Consultant | 29 (100.0) |
| Subspecialist accreditation (n = 29), n (%) | |
| Neuropsychiatry | 8 (27.6) |
| Liaison psychiatry | 12 (41.4) |
| Dual accreditation | 9 (31.0) |
| Length of experience in psychiatry following registration with the GMC (years) (N = 29), mean (SD) [range] | 17.8 (6.1) [4–31] |
| Previous experience working with patients with DS (n = 29), n (%) | |
| Yes | 28 (96.6) |
| Previous experience working with patients with medically unexplained symptoms (n = 29), n (%) | |
| Yes | 28 (96.6) |
| CBT therapists (N = 39) | |
| Gender (n = 39), n (%) | |
| Female | 31 (79.5) |
| Male | 8 (20.5) |
| Age (years) (n = 39), n (%) | |
| 21–40 | 14 (35.9) |
| 41–60 | 25 (64.1) |
| BABCP accreditation (n = 39), n (%) | |
| Yes | 10 (25.6) |
| Professional background (n = 39), n (%) | |
| Clinical psychology | 20 (51.3) |
| Nursing | 8 (20.5) |
| Occupational therapy | 3 (7.7) |
| Counselling psychology | 2 (5.1) |
| High-intensity therapy | 1 (2.6) |
| Other | 5 (12.8) |
| Post-core professional training in CBT (highest CBT qualification) (n = 37), n (%) | |
| Diploma | 10 (27.0) |
| Doctorate | 9 (24.3) |
| Master of Science | 5 (13.5) |
| Bachelor of Science | 4 (10.8) |
| Certificate | 2 (5.4) |
| Other | 7 (18.9) |
| Length of CBT training (months) (n = 23) | |
| Mean (SD) [range] | 20.2 (12.5) [0–40] |
| Monthly CBT-specific clinical supervision (hours) (n = 31) | |
| Mean (SD) [range] | 2.0 (0.9) [0–5] |
| Experience practising as a CBT therapist (years) (n = 33) | |
| Mean (SD) [range] | 10.8 (7.3) [0–24] |
| Previous experience working with patients with DSs (n = 36), n (%) | |
| Yes | 29 (80.6) |
| Previous experience working with patients with medically unexplained symptoms (n = 36), n (%) | |
| Yes | 33 (91.7) |
| NHS job banding (higher number banding = more senior post) (n = 36), n (%) | |
| Band 7 | 15 (41.7) |
| Band 8a | 13 (36.1) |
| Band 8b | 5 (13.9) |
| Band 8c | 1 (2.8) |
| Band 8d | 2 (5.6) |
Appendix 7 Informants’ ratings of patients’ dissociative seizures (where available)
| Ratinga | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Trial arm | Overall (N = 368) | Trial arm | Overall (N = 368) | |||
| SMC alone (N = 182) | CBT + SMC (N = 186) | SMC alone (N = 182) | CBT + SMC (N = 186) | |||
| n = 15 | n = 20 | n = 35 | n = 10 | n = 17 | n = 27 | |
| Worse | 3 (20.0) | 5 (25.0) | 8 (22.9) | 3 (30.0) | 3 (17.6) | 6 (22.2) |
| Same | 4 (26.7) | 6 (30.0) | 10 (28.6) | 4 (40.0) | 2 (11.8) | 6 (22.2) |
| Better | 6 (40.0) | 9 (45.0) | 15 (42.9) | 3 (30.0) | 11 (64.7) | 14 (51.9) |
| Seizure free | 2 (13.3) | 0 (0.0) | 2 (5.7) | 0 (0.0) | 1 (5.9) | 1 (3.7) |
Appendix 8 List of unit costs used in costing services
| Item | Unit cost | Source |
|---|---|---|
| Community services | ||
| GP | £33 per 9 minutes | GP: per patient per minute – excluding direct care staff costs – with qualification costs. PSSRU142 |
| Practice nurse | £36 per hour | Nurse (GP practice). PSSRU142 |
| Epilepsy nurse | £77 per hour | Other specialist nursing, adult, face to face. PSSRU142 |
| Physiotherapist | £33 per hour | Scientific and professional staff – band 5. PSSRU142 |
| Social worker | £43 per hour | Social worker (adult services). PSSRU142 |
| Counsellor/psychologist | £106 per hour | Counsellor/psychologist. PSSRU142 |
| CBT | £100 per contact | CBT. PSSRU142 |
| Other talk therapy | £100 per hour | CBT. PSSRU142 |
| Home help: household tasks (hours per week) | £26 per hour | Home care worker, face to face: weekday. PSSRU142 |
| Home help: personal care (hours per week) | £26 per hour | Home care worker, face to face: weekday. PSSRU142 |
| Other community services | £137 per contact | Weighted average of all outpatient attendances. NHS reference costs143 |
| Drugs | Varied | British National Formulary. 77 ed, March–September 2019145 |
| Hospital services | ||
| Inpatient (length of stay) | £648 per night | Non-elective inpatient stays (short stay). NHS reference costs143 |
| Outpatient | £137 per contact | Weighted average of all outpatient attendances. NHS reference costs143 |
| Ambulance use | £119 per contact | NHS reference costs143 |
| A&E visits | £148 per episode | A&E. NHS reference costs143 |
| Clinical decision unit | £148 per episode | A&E. NHS reference costs143 |
| Psychiatric appointment | £108 per contact | Doctors: consultant psychiatry. NHS reference costs143 |
| Neurology appointment | £138 per contact | Outpatient: neurology. NHS reference costs143 |
| Other hospital care | £137 per contact | Weighted average of all outpatient attendances. NHS reference costs143 |
| Informal care | ||
| Personal care | £13.68 per hour | Used the AWE of £513 per 37.5 hours. Office for National Statistics – AWE and the Annual Survey of Hours and Earnings144 |
| Medical procedures | £13.68 per hour | Used the AWE of £513 per 37.5 hours. Office for National Statistics144 |
| Help in home | £13.68 per hour | Used the AWE of £513 per 37.5 hours. Office for National Statistics144 |
| Help outside home | £13.68 per hour | Used the AWE of £513 per 37.5 hours. Office for National Statistics144 |
| Time spent ‘on-call’ | £13.68 per hour | Used the AWE of £513 per 37.5 hours. Office for National Statistics144 |
| Productivity loss | ||
| Days off work because of ill health | £103 per day | Office for National Statistics144 |
| Hours off work because of ill health | £14.71 per hour | Office for National Statistics144 |
Appendix 9 Qualitative interview schedules
Interviews with patient participants
Reproduced with permission from Read et al. 137 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Section 1: impact of seizures and diagnosis
-
How did you become involved in the study?
-
What impact did the seizures have on day-to-day life?
-
How did your family and friends respond to you in relation to the seizures?
-
Did you think of it as a seizure at the beginning?
-
How did you react to the neurologist’s diagnosis of DSs and how did you explain it to others?
-
Did the diagnosis make sense to you?
-
Other physical or mental health problems?
-
Did you have any previous knowledge of DSs?
-
What did the diagnosis mean for the future?
Section 2: CODES study materials and treatment
-
What did you make of the CODES blue and purple booklets?
-
Did you seek information from anywhere else, such as websites?
-
Did you understand the study process of information sheets, consent forms and randomisation?
-
Was there anything beneficial about the treatment (CBT or SMC) that you received?
-
How did you get on with the CBT therapist and neuropsychiatrist (if they received CBT)/CODES neuropsychiatrist (if they received SMC)?
-
Were there any light-bulb moments in either CBT or SMC?
-
Was there anything unhelpful about treatment?
-
What was a typical CBT/SMC session like?
-
How many sessions of CBT/SMC did you attend?
-
What was your view of the content of the CBT sessions – homework tasks, seizure diary/record keeping?
-
Were there CBT techniques that worked or did not work in terms of recovery?
-
Are you continuing to use any techniques (either CBT or SMC)?
-
What impact did the treatment have on your understanding of DSs?
-
How did you find the materials/handouts used in the CBT sessions?
-
Would you have preferred any other treatment?
-
How did sessions with the therapist make you feel?
-
What did you think about having CBT sessions recorded?
-
What was your view of the content of the SMC sessions?
-
Were you given any materials/handouts in SMC (other than the CODES booklets)?
-
Were there any barriers to attending treatment? Could it have been made easier?
Section 3: recovery process
-
How are things now?
-
How do your seizures impact on day-to-day life? Any changes from before?
-
(If seizures reduced, did you feel an increase in anxiety or depression?)
-
Any change in comorbidities?
-
Any changes in your relationships with family/friends from before?
-
What was your experience of the study as a whole and what value did it have?
-
What would/does getting better look like – what is the value of the outcome?
-
Is there any ongoing support you have sought or you would like to be available to you?
Interviews with CODES psychiatrists
Reproduced with permission from Jordan et al. 152 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Section 1: background and specific issues relating to the CODES randomised controlled trial
-
How does CODES SMC differ from the techniques you would usually use to treat patients with DSs?
-
If SMC is shown to reduce seizures, what do you think will be most difficult about making SMC standard across services?
-
Were there any parts of the CODES SMC approach that proved more challenging?
-
How did you manage significant deterioration in a participant’s mental health during their time in the CODES study?
-
How did you feel about not referring to other types of therapy while a participant was in CODES?
Section 2: experience of the intervention
-
Did the way patients engaged with SMC seem to change over time?
-
Were there any ‘light-bulb moments’ in the course of SMC where patients appeared to have a sudden understanding of their condition?
Section 3: individual psychological, social or health-related differences and impact on treatment
-
Do you think that there were any factors that may have affected patients’ understanding of their diagnosis?
-
Were there any patients who may have been more suitable than others to receive SMC alone? If so, what distinguished these types of clients?
-
Were there issues that you had to address to improve engagement? Or were there any barriers to patients engaging with SMC?
-
Could sessions ever become side-tracked/derailed by other issues? For example, social issues, safeguarding or health-related concerns?
Interviews with CODES CBT therapists
Reproduced with permission from Wilkinson et al. 135 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Section 1: what opinion do CBT therapists have regarding the efficacy, flexibility and design of this cognitive–behavioural therapy intervention?
-
How did you find the therapy manual and associated materials?
-
Possible subquestions if needed:
-
How was it using the therapy manual/the structure of the intervention on a sessional basis?
-
What did you think about the ordering of the sessions?/Was any re-ordering necessary?
-
Which aspects of the intervention did you feel were easier/more difficult to deliver?
-
Did you tend to direct clients to particular readings in the booklet for clients?/Were any of the chapters in the clients’ booklet more/less useful?
-
-
How was it working within the CBT protocol? What would you say about the flexibility of this approach? (or was that what you meant by the question I added in above?)
-
If this CBT intervention were to be rolled out across other services, what changes, if any, would you make to it?
-
If you were not working under the constraints of the trial, would you have applied a different therapeutic model, and if so what? Did that cause any tension for you?
Section 2: what experience do CBT therapists believe their clients had of the intervention? What experience did CBT therapists have of delivering the intervention?
-
What would you say about your clients’ ability to relate the CBT model to their difficulties? How satisfying/meaningful did clients tend to find this as an explanation for their problem?
-
Were there any ‘light-bulb moments’ in the course of treatment where clients appeared to have a sudden understanding of their treatment? (Prompt if needed: if so, at what point in treatment did this occur?/Could you describe the nature of this moment?)
-
Did the way clients engaged with therapy seem to change over time? (Prompt if needed: could you say something about the nature of this change?)
-
Did your experience of providing this intervention change over the course of the trial? (Prompt if needed: if so, in what ways did this change?)
Section 3. What psychological processes did CBT therapists think that they were targeting in the intervention? Did therapists perceive individual psychological, social or health-related differences between clients that made it easier or more difficult for them to benefit from the cognitive–behavioural therapy intervention?
-
What psychological processes did you think that you were targeting (directly or indirectly) in the intervention?
-
Possible sub-questions:
-
Did fear avoidance feature in your clients’ presentations?
-
If trauma was a significant feature of your client’s presentation, how did you approach it in the context of this intervention?
-
-
Were there characteristics of clients that made it easier or harder for them to work with the treatment? (Prompt: what were these characteristics? How did they affect the course of treatment? If we think about a particular client . . .)
-
Were there issues that you had to address to improve engagement? (Prompt: could you provide any examples of this? Were there any issues regarding timing/location/travel/child care/need for relative support?)
-
Could sessions ever become side-tracked/derailed by other issues? For example, social issues, safeguarding or health-related concerns? (Prompt: could you give any examples of this? How easy was it to come back to the focus of treatment?)
Section 4. How did CBT therapists experience the overall care pathway, and how well integrated, in their opinion, were the cognitive–behavioural therapy and standardised medical care aspects of treatment?
-
What did you think about the overall care pathway? (Prompt: how did SMC sit alongside the CBT intervention?/What would you say about the integration of these two aspects of treatment?)
-
Did clients discuss their experiences of SMC in CBT sessions, and if so what did they report? In what ways did this seem to influence their understanding of their condition?
-
Do you feel that your clients understood their diagnosis? What do you think this diagnosis meant for them?
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AED
- anti-epileptic drug
- BRC
- Biomedical Research Centre
- CACE
- complier-average causal effect
- CBT
- cognitive–behavioural therapy
- CBT-ip
- CBT-informed psychotherapy
- CC
- complete case
- CEAC
- cost-effectiveness acceptability curve
- CGI
- Clinical Global Impression of Change
- CI
- confidence interval
- CODES
- COgnitive behavioural therapy vs. standardised medical care for adults with Dissociative non-Epileptic Seizures: a multicentre randomised controlled trial
- CONSORT
- Consolidated Standards of Reporting Trials
- CORE-10
- Clinical Outcome Routine Evaluation-10
- CSRI
- Client Service Receipt Inventory
- CTU
- Clinical Trials Unit
- DMEC
- Data Monitoring and Ethics Committee
- DS
- dissociative seizure
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- EEG
- electroencephalography
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FND
- functional neurological disorder
- GAD-7
- General Anxiety Disorder-7
- GP
- general practitioner
- HCP
- healthcare professional
- HES
- Hospital Episode Statistics
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IRR
- incidence rate ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LR
- likelihood ratio
- MAR
- missing at random
- MI
- multiple imputation
- MICE
- multivariate imputation via chained equations
- M.I.N.I.
- Mini-International Neuropsychiatric Interview
- N/A
- not applicable
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PHQ-9
- Patient Health Questionnaire-9
- PHQ-15
- Patient Health Questionnaire-15
- PNES
- psychogenic non-epileptic seizure
- PPI
- patient and public involvement
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RW
- research worker
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAPAS-SR
- Standardised Assessment of Personality Abbreviated Scale, Self-Report
- SAR
- serious adverse reaction
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions (i.e. utility score)
- SF-12v2
- Short Form questionnaire-12 items, version 2
- SMC
- standardised medical care
- SU
- service user
- SUAG
- Service User Research Enterprise Advisory Group
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WSAS
- Work and Social Adjustment Scale
Notes
-
Participant Information Sheet for the initial phase of the study
-
Potential frequently asked questions for neurologists delivering SMC
-
Potential frequently asked questions for psychiatrists delivering SMC
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25430).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
