Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/04/22. The contractual start date was in November 2014. The draft report began editorial review in September 2020 and was accepted for publication in February 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Norman et al. This work was produced by Norman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Norman et al.
Chapter 1 Introduction
Some material in this chapter has been reproduced with permission from Norman et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Some material in this chapter has been reproduced with permission from Norman et al. 2 © 2021 Norman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Scientific background
Around 16 out of every 1000 women giving birth in England and Wales have a multiple pregnancy. 3 Rates of multiple pregnancy increased progressively from around 9.5 per 1000 women in the mid-1970s until 2009, when a rate of 16.4 per 1000 women was recorded. Since then, multiple pregnancy rates have been fairly stable. 3 The vast majority of multiple pregnancies are twin pregnancies.
Multiple pregnancies are associated with higher rates of stillbirth, neonatal and infant mortality, and child disability. 4 Much of this excess mortality and morbidity is a direct consequence of higher rates of preterm birth in twin pregnancies than in singleton pregnancies. 4 Unfortunately, there are no therapies that are effective in preventing preterm birth in twin pregnancy. 4
The purpose of this research was to test the clinical effectiveness, acceptability and cost-effectiveness of the Arabin (cervical) pessary for preterm birth prevention in women with twin pregnancy.
Cervical pessary in singleton pregnancy
The most recent systematic review5 of the effectiveness of the cervical pessary (against no treatment) in the prevention of preterm birth in unselected or singleton participants suggests that a cervical pessary reduces preterm birth before 34 weeks’ gestation [relative risk (RR) 0.65, 95% confidence interval (CI) 0.44 to 0.96] and increases gestational age at delivery (weighted mean difference 1.03 weeks, 95% CI 0.37 to 1.70 weeks) but has no impact on perinatal outcomes. Earlier reviews,6 including a Cochrane review7 and a review restricted to women with a short cervix,8 showed no benefit.
Cervical pessary in multiple pregnancy
A literature search on cervical pessary AND preterm birth AND twin pregnancy OR multiple pregnancy identified two systematic reviews9,10 that evaluated the effectiveness of the cervical pessary in women with twins or higher-order births. Both reviews9,10 were restricted to women with a short cervix. In neither systematic review was the pessary associated with any benefit. 9,10 A search on cervical pessary AND preterm birth AND randomised trial identified four unique trials: the PoPPT (Prevention of Preterm birth with Pessary in Twins) trial,11 the ProTWIN trial,12 PECEP-Twins13 and the trial by Nicolaides et al. 14 Two other ongoing trials were also identified: NTR4414 and NCT01334489. Table 1 summarises the primary analysis results of the existing trials.
| Trial (publication date) | Population | Number of participants | Primary outcome | RR (95% CI) |
|---|---|---|---|---|
| PoPPT trial (2017)11 | Women with twin pregnancy and a CL of ≤ 30 mm at 18+ 0–27+ 6 weeks’ gestation | 46 | PTB < 34 weeks’ gestation | 1.13 (0.53 to 2.40) |
| ProTWIN trial (2013)12 | Women with multiple pregnancy at 12–20 weeks’ gestation | 813 | Poor perinatal outcome (composite) | 0.98 (0.69 to 1.39) |
| Nicolaides et al. (2016)14 | Women with twin pregnancy at 20+ 0–24+ 6 weeks’ gestation | 1180 | Spontaneous PTB < 34 weeks’ gestation | 1.05 (0.79 to 1.41) |
| PECEP-Twins (2016)13 | Women with twin pregnancy with a CL of ≤ 25 mm at 18–22 weeks’ gestation | 137 | Spontaneous PTB < 34 weeks’ gestation | 0.41 (0.22 to 0.76) |
Several subgroup analyses of the ProTWIN trial12 have been performed. In a ‘prespecified’ subgroup analysis of women with a short cervix, use of the pessary was associated with a reduction in preterm birth. However, the importance of this finding is uncertain. Although the ‘short cervix subgroup’ analysis was prespecified, the threshold length used to define ‘short cervix’ was altered during data analysis. A per-protocol analysis of the ProTWIN trial12 has subsequently been published and shows a reduction in poor perinatal outcome (RR 0.32, 95% CI 0.13 to 0.78) and birth at < 32 weeks’ gestation (RR 0.41, 95% CI 0.20 to 0.87) in the ‘per-protocol’ treatment group.
Cervical length threshold
In 2014, when STOPPIT 2 was designed, available evidence from the ProTWIN trial12 suggested that a cervical pessary might reduce preterm birth in women with twin pregnancy and a short cervix, but not in unselected women. 12 STOPPIT 2 was designed to test the hypothesis that the Arabin pessary was effective in women with a short cervix. In deciding the cervical length threshold that would define the cervix as ‘short’, we reviewed data from the existing trial and information on the distribution of cervical lengths in the population we planned to recruit.
In the ProTWIN trial,12 the cervical length defined as ‘short’ was prespecified as the 25th centile. When the ProTWIN trial12 was designed, it was thought that the 25th centile would correspond to a cervical length of 25 mm, but on completion of the trial it was found to be 38 mm. On this basis, a subgroup analysis of women with a short cervix was performed for women with a cervical length of < 38 mm. When STOPPIT (STudy Of Progesterone for the Prevention of Preterm Birth In Twins) was being designed, UK data (derived from a prospective study of over 1000 women with twin pregnancy) suggested that 25 mm, 28 mm and 30 mm corresponded to the 14th, 25th and 30th centile of cervical length, respectively. 15 To be inclusive, and to ensure that STOPPIT 2 had wide applicability, we opted to recruit women with a cervical length threshold of 30 mm, which we anticipated was the 30th centile.
Further information16 published after the initial design of the STOPPIT 2 protocol, and a review of the cervical length in the first 20 women recruited into in STOPPIT 2, suggested that the 30th centile of cervical length was nearer to 35 mm. Accordingly, we expanded eligibility for randomisation to include all women with a cervical length of ≤ 35 mm. 16
Rationale for research
Although the ProTWIN trial12 suggested that a cervical pessary might prevent preterm birth in women with a twin pregnancy, we were firmly of the belief that further effectiveness studies were needed before adoption into UK practice. First, the subgroup of women in whom the effectiveness of the pessary was tested were (arguably) not clearly defined prior to data analysis. Second, the relevance of this subgroup to a UK population was uncertain, given the differences in population cervical length centiles described above. Third, given that the pessary was ‘taken out’ (for a variety of reasons, including pain) or ‘fell out’ in > 20% of participants, we believed that formal analysis of acceptability was required. Last, although the pessary itself is relatively cheap (approximately €40), we were keen to evaluate cost-effectiveness to assist decision-making by bodies that develop guidelines, such as the National Institute for Health and Care Excellence (NICE).
Aim and objectives
The aim of STOPPIT 2 was therefore to test the hypothesis that the Arabin cervical pessary is acceptable to pregnant women and reduces preterm birth in women with a twin pregnancy and a short cervix (i.e. ≤ 30 mm), reducing adverse neonatal outcomes and health-care costs. We preplanned subgroup analyses to determine effectiveness in women with cervical lengths of ≤ 25 mm and ≤ 28 mm, and in women with a dichorionic pregnancy.
Chapter 2 Trial design and methods
Some material in this chapter has been reproduced with permission from Norman et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Some material in this chapter has been reproduced with permission from Norman et al. 2 © 2021 Norman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Study design
STOPPIT 2 aimed to determine whether or not the Arabin cervical pessary is clinically useful in preventing preterm birth in women with a twin pregnancy and a short cervix. STOPPIT 2 was an open-label, multicentre, randomised controlled trial. The intervention group was Arabin pessary plus standard care and the control group was standard care alone. Participants were initially recruited into the screening phase of the study, when cervical length was measured. Women with a measured cervical length of ≤ 35 mm were then recruited into the treatment phase of the study.
We also performed an economic evaluation within the trial to consider the cost-effectiveness to the NHS of providing the intervention compared with usual standard care alone (see Chapter 5). A qualitative study to explore the views and experiences of participants and clinicians involved in the study is described in Chapter 4. A description of the trial protocol has already been published. 1
Ethics approval and research governance
Ethics approval for the study was given by the South East Scotland Ethics Committee 02 on 29 August 2014 (reference 14/SS/1031) and in Belgium on 21 September 2016 (reference S58820). The trial was registered with the International Standard Randomised Controlled Trial Register (reference ISRCTN98835694) and also with the Clinical Trials.gov (reference NCT02235181). A summary of the changes made to the original protocol is given in Table 2.
| Protocol version | Date | Reason for update | Substantial amendment number | Summary of changes |
|---|---|---|---|---|
| 2 | 2 June 2015 |
Text reformatted and clarified, information updated Questionnaires created and updated |
1 | General updates/reformatting
|
| 3 | 5 August 2015 | Change of measurement that defines ‘short cervix’ and confers eligibility for randomisation | 2 | Change of measurement that defines a short cervix from ≤ 30 mm to ≤ 35 mm. The anticipated population centile (≤ 30th centile) is unchanged |
| 4 | 10 January 2018 | Clarification of text, modification of the data collection and safety | 5 |
|
| 5 | 23 August 2018 | Clarification of outcomes and analysis methods | 6 |
|
Participants
The study sought to recruit women with a twin pregnancy and a short cervix from antenatal clinics in the UK and Europe.
Inclusion criteria
The inclusion criteria for the screening and the treatment phase comprised all of the following (i.e. all criteria had to be fulfilled):
-
twin pregnancy (monochorionic or dichorionic)
-
known chorionicity (as defined by first trimester ultrasound screening)
-
gestation established by scan at ≤ 16 weeks’ gestation (in accordance with NICE guidelines4)
-
aged ≥ 16 years
-
willingness to participate in both the screening and randomisation phase of the study.
Exclusion criteria
Patients were ineligible for the screening phase if they fulfilled any of the following criteria:
-
unable to give written informed consent
-
known significant congenital structural or chromosomal fetal anomaly at the time of inclusion
-
existing or planned cervical cerclage in the current pregnancy
-
existing or planned (prior to 20+ 6 weeks’ gestation) treatment for twin-to-twin transfusion syndrome in the current pregnancy
-
suspected or proven rupture of the fetal membranes at the time of recruitment
-
bulging fetal membranes at the time of recruitment
-
singleton pregnancy or higher-order multiple pregnancies
-
women who have had any fetal death (i.e. fetal heartbeat previously detected) in the index pregnancy (prior to randomisation)
-
known sensitivity, contraindication or intolerance to silicone
-
involved in a clinical trial of an investigational medicinal product, a phase 1 study or an investigation of a treatment for the prevention of preterm birth
-
monochorionic, monoamniotic pregnancy
-
heavy bleeding due to a low-lying placenta prior to randomisation.
Inclusion criteria: screening phase
-
Cervical length of ≤ 35 mm at 18+ 0–20+ 6 weeks’ gestation confirmed by an accredited clinician.
In addition, patients were ineligible for the treatment phase of the study if they fulfilled any of the following:
-
cervical length of > 35 mm at 18+ 0–20+ 6 weeks’ gestation
-
cervical length not measured at 18+ 0–20+ 6 weeks’ gestation
-
bulging fetal membranes at the time of pessary insertion
-
suspected or proven rupture of the fetal membranes at the time of pessary insertion.
Recruitment procedure
Women with a twin pregnancy were usually identified because they were attending an antenatal clinic or a specialist twin clinic, or after an ultrasound scan confirmed a twin pregnancy. Women with a twin pregnancy were given verbal and written information about the study by the investigator team at each study site. Women were then given time to consider participation. Unless they chose to waive this opportunity, women were given at least 24 hours to read the information sheet prior to giving written consent (or declining) to participate in the study. The case notes of women who indicated interest in the study were reviewed to identify inclusion and exclusion criteria.
Informed consent
Informed written consent was obtained for both the screening and the treatment stages of the study prior to recruitment. At the end of the screening phase, women who wished to continue to the treatment phase of the study were asked to (verbally) confirm their consent to continue participation. Permission was also required for future long-term follow-up of the women and babies via record linkage into national (health, social and educational) databases for any future studies.
The original signed and dated consent forms were filed in the patient case record, with a copy held securely as part of the trial site file and another copy sent to the trial office to be checked for completeness. A copy was given to the participant for their records.
Randomisation, concealment and blinding
Eligible and consenting women were recruited to the screening phase of the study. Women who were found on screening to be eligible for the treatment phase of the study and who agreed to continue were randomised to one of two treatment groups: (1) Arabin pessary plus standard care or (2) standard care alone. Randomisation was carried out by contacting the central randomisation facility at the study data centre through a web portal. Participants were assigned a unique study identifier by the web portal and staff were required to enter minimal patient details prior to randomisation. The allocation sequence was generated by computer at the Centre for Healthcare Randomised Trials (CHaRT; Aberdeen, UK). Randomisation was minimised with a random element rather than stratified, given the properties of minimisation in ensuring comparability between the groups. Minimisation variables were study centre and chorionicity (mono- or dichorionic).
Women who consented to the screening phase of the study but who were ineligible for randomisation were asked to consent to the collection of labour and delivery outcome data to facilitate calculation of positive and negative likelihood ratios for spontaneous preterm birth (SPB) before 34 weeks’ gestation for a variety of cervical length measurements.
Participants and caregivers were immediately informed of treatment allocation. It was not considered possible to conceal treatment allocation from participants, caregivers or those performing outcome assessments, and there was therefore no attempt to do so.
Screening and treatment procedures
Screening phase
To assess eligibility for the treatment phase, all women who wished to participate and who fulfilled the inclusion criteria for the screening phase were invited to undergo a transvaginal ultrasound measurement of cervical length between 18+ 0 and 20+ 6 weeks’ gestation by an accredited operator (see bullet list). If convenient, cervical length measurement was performed at the time of the routine fetal anomaly scan. In centres where cervical length measurements were part of routine clinical care, women could be consented after the cervical length and were not asked to have a further transvaginal scan, provided:
-
the transvaginal measurement was performed between 18+ 0 and 20+ 6 weeks’ gestation by an accredited operator [i.e. someone who had completed the Cervical Length Education and Review programme (Oklahoma City, OK, USA) or Fetal Medicine Foundation (London, UK) cervical length training] who had been delegated by the principal investigator
-
the woman was eligible for participation (as per the above inclusion/exclusion criteria) and the pregnancy was not ≥ 21 weeks’ gestation.
Images of the cervical length measurements were anonymised and uploaded to the electronic case report form (eCRF). Women with a cervical length of ≤ 35 mm were invited to continue to the treatment phase and randomised to either the intervention or the comparator group, as described below.
Intervention group (treatment phase)
The intervention was the Arabin pessary, which was inserted through the vagina and around the cervix between 18+ 0 and 20+ 6 weeks’ gestation by a trained member of the team. The Arabin pessary was given in addition to all standard-care measures. Unless indicated earlier, the pessary was removed at 35+ 0 to 36+ 6 weeks’ gestation.
Video instructions for pessary insertion were circulated and formed part of the study protocol training given to clinicians. Meetings about the trial gave clinicians the opportunity to practise pessary insertion on anatomical models. There were no prohibited cotreatments.
Comparator group (treatment phase)
The comparator was standard care only. There were no prohibited cotreatments. The Arabin pessary was not in general use in women with twin pregnancy in participating hospitals and so use of the pessary outside the trial (and outside allocation to the intervention group) was not anticipated.
Data collection and management
Data were collected from the participant’s notes or from observation by authorised study personnel. These data were entered directly by study site staff into the eCRF (developed in accordance with CHaRT software development standards at the University of Aberdeen).
Validation checks were created and run in real time on the eCRF. These checks included the identification of missing data, out-of-range values, illogical entries and invalid responses.
Users were informed of data issues for essential data items when the eCRF page was saved, providing users with the opportunity to correct the data immediately, where possible. The eCRF system generated a missing data query for all items not completed at the time. Cross-checks comparing data items across different forms were also performed and queries were issued to study site staff in batches for response.
The collection and management of data for health economics and for the qualitative study are described in Chapter 5.
Baseline assessment
In addition to eligibility criteria, the following baseline information was collected on all women: age, ethnicity, employment status, years of full-time education, date of last menstrual period, estimated date of delivery, chorionicity, cervical length, current smoking (tobacco and e-cigarettes), current recreational drug use, current alcohol use, obstetric history (parity, miscarriage) and ongoing medical conditions (e.g. hypertension, insulin-dependent diabetes, respiratory disease, cardiac disease, neurological disease, skin condition, thrombophilia). We also collected information on the current pregnancy, including the results of any fetal anomaly scans for each twin and the results of amniocentesis on each twin. All baseline assessments were conducted between April 2015 and February 2019.
Follow-up
Follow-up visits to review pregnancy and fetal well-being were conducted at approximately 4-weekly intervals (plus or minus 1 week) following randomisation until pessary removal at around 36 weeks’ gestation. Women randomised to the pessary and those undergoing standard care were all invited to participate in study visits. These study visits could be face to face, by telephone or by other means of communication (e.g. by letter or e-mail). At each study visit, we collected information on any pregnancy complications (i.e. serious adverse events, including bleeding), experience of the pessary during the previous 4 weeks (if applicable) and reason for and duration of any hospital admissions.
Measures
Primary outcome
There were two primary outcomes for women in the treatment phase of the study: (1) an obstetric primary outcome and (2) a neonatal primary outcome.
The obstetric primary outcome was all births before 34+ 0 weeks’ gestation following the spontaneous onset of labour. Preterm prelabour rupture of membranes at < 34 weeks’ gestation with or without contractions was included in this definition of spontaneous onset of labour. Iatrogenic delivery due to maternal or fetal conditions was not considered to fulfil the criteria for the primary outcome.
The neonatal primary outcome was a composite of adverse outcomes, including stillbirth or neonatal death, periventricular leukomalacia, early respiratory morbidity (defined as any need for supplemental oxygen > 30%, continuous positive airway pressure or intratracheal ventilation or surfactant replacement therapy within the first week of life), intraventricular haemorrhage, necrotising enterocolitis or proven sepsis, all measured up to 28 days after the expected date of delivery.
Secondary outcomes
Key obstetric secondary outcomes were mean gestation at delivery, incidence of all births before 37+ 0 weeks’ gestation, adverse events (including infection and cervical trauma) and acceptability of the pessary, as determined by participant questionnaire and experience of the device throughout the trial and pessary removal.
Key neonatal secondary outcomes were incidence of each of the individual components of the primary neonatal outcome, median weight (in grams) of the newborn at birth, any deaths of liveborn babies within the first 28 days after birth and discrete episodes of bloodstream or central nervous system infection (i.e. positive blood or cerebrospinal fluid culture), categorised by timing (either within the first 72 hours or between 72 hours and discharge).
Other outcomes
In addition to the primary and key secondary outcomes, we collected information on the obstetric outcomes of incidence of all births before each of 28+ 0, 32+ 0 and 34+ 0 weeks’ gestation, incidence of all births before 28, 32, 34 and 37 weeks’ gestation preceded by the spontaneous onset of labour, incidence of preterm birth before 34 weeks’ gestation preceded by preterm premature membrane rupture, method of delivery (in three categories: spontaneous vaginal delivery or vaginal breech, forceps or ventouse and caesarean section), duration of labour overall and of each of the first and second stages of labour, duration of stay in hospital, other adverse events (including haemorrhage, tachycardia, vaginal injury and other trauma) and serious maternal adverse events up to 28 days after discharge from the hospital.
We also collected information on key neonatal outcomes, including birthweight centile (for gestation) within 4 weeks after expected date of delivery, death of liveborn babies within the first 28 days after estimated date of delivery, cord pH and Apgar score at 1 minute and 5 minutes.
Outcomes for screening phase only
For women recruited to the screening phase but who were not randomised, the primary outcome measure was delivery before 34 weeks’ gestation.
Sample size
Original sample size justification
We aimed to randomise women with a cervical length of ≤ 35 mm, which we believed to be around the 30th centile. We originally calculated that we needed a sample size of 1850 women recruited to the screening phase, which would allow us to randomise 500 women in the treatment phase (250 in each group), assuming a 15–20% drop-out between screening and randomisation. We assumed a RR reduction of 0.6 in the primary obstetric outcome (i.e. spontaneous preterm labour leading to preterm birth before 34 weeks’ gestation) in the intervention group. We considered our estimate of RR reduction to be conservative, given a RR of 0.49 for delivery before 32 weeks’ gestation and 0.47 for delivery before 34 weeks’ gestation (21% vs. 42%) in the ProTWIN trial12 (S Liem, Academic Medical Center, University of Amsterdam, 2013, personal communication). We anticipated that 35% of women in the control group would deliver before 34 weeks’ gestation. Again, we believed that this was a conservative estimate, given that a systematic review indicated that 34.9% of women with a cervical length of ≤ 35 mm (when scanned at 20 weeks’ gestation) will deliver preterm before 32 weeks’ gestation. 16 Assuming a baseline rate of 35% and a RR of 0.6, we calculated that a sample size of 500 women would provide 94% power to detect a difference at the 5% significance level. If the preterm birth rate before 34 weeks’ gestation was only 30%, the power would drop to 88%. Both estimates allowed for losses to follow-up and imperfect compliance. For the primary neonatal outcome, Liem et al. 12 showed an effect size of 0.42 at the baby level and an incidence of 24% in the control group. We powered our study for a RR of 0.6 for the primary neonatal outcome. Assuming prevalence rates as in the ProTWIN trial,12 our study would have 97% power. In practice, our post-screening groups of women with cervical length of ≤ 35 mm are probably at lower risk than women in the ProTWIN trial12 group (cervical length of < 38 mm), given comparisons of the rate of preterm delivery in each of the control groups. Therefore, if we assume a lower rate of the neonatal primary outcome of (say) 18%, we still have 88.4% power to detect a RR of 0.6 in the intervention group. Such a calculation assumed that analysis at the baby level was appropriate for the neonatal outcome. For the subgroup of women with cervical length of ≤ 25 mm, the anticipated rate of the primary obstetric outcome in the control group was 51% (82/159). 15 The study had 85% power to detect a RR of 0.6 in this group, with a likely sample size of 234, given that 25 mm was likely to be around the 14th centile. 15
Revised sample size justification
Following a reanalysis of the data (masked to treatment allocation) after 29 months of screening, with data on 1214 women screened (September 2017), we estimated that to randomise 500 women the sample size for screening needed to be increased to 2500.
Statistical analysis
The analysis and reporting of this trial were undertaken in accordance with CONSORT (Consolidated Standards of Reporting Trials) guidelines. 17,18 All statistical analyses were undertaken in Stata® 15 (StataCorp LP, College Station, TX, USA), following a predefined analysis plan agreed with the Trial Steering Committee (TSC). [For more information please see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/130422/#/ (accessed 22 April 2021).] Data were primarily analysed according to the intention-to-treat principle without imputation, with all participants remaining in their allocated group for analysis. Statistical significance was at the 5% level with corresponding 95% CIs derived. Baseline and follow-up measures were described using mean [standard deviation (SD)], median (interquartile range) and counts (with percentages), where appropriate.
The frequencies of the two primary outcomes in the study groups were compared in an intention-to-treat analysis without imputation, using logistic regression with a fixed effect for the minimisation covariate, chorionicity, and a random effect for centre to derive adjusted odds ratios (ORs) and 95% CIs of treatment effect. We intended to use multinomial logistic regression for secondary outcomes with more than one category and linear regression for continuous secondary outcomes, adjusting for chorionicity and clustering within twins. However, our planned three-level linear regression model for the primary neonatal outcome (i.e. babies nested within mother nested within centre, adjusting for chorionicity) failed to converge. We therefore used standard logistic regression for the neonatal primary outcome, adjusting for chorionicity, with a robust variance estimator clustered by woman (mother). For primary outcomes, predefined subgroup analyses were performed in women with monochorionic pregnancies, a cervical length of ≤ 25 mm and a cervical length of ≤ 28 mm. For these statistical analyses, significance was set at the 1% level and presented as 95% CIs. All analyses were performed in Stata 15. We also calculated likelihood ratios for delivery before 34 weeks’ gestation for women with cervical length of ≤ 35 mm.
A post hoc comparison was performed between participants who had membrane rupture and those who did not. A per-protocol analysis (including those in the intervention group who were adherent and excluding those in the control group who had a pessary inserted) was also performed. No formal interim analyses were undertaken.
Missing data
We believed that, if data were missing, then this was likely to be because of a miscarriage, stillbirth, or neonatal or maternal death. We therefore agreed a priori that missing data would not be imputed.
Subgroup analyses
We undertook predefined subgroup analyses of the primary outcome by monochorionicity and by cervical length (≤ 25 mm and ≤ 28 mm). In the subgroup analyses, statistical significance was set at the 1% level with corresponding 99% CIs, presented as 95% CIs.
Protocol amendments
Changes to the protocol are listed in Table 2. Most of these changes are self-explanatory. The rationale for the change in measurement that defines a short cervix (from 30 mm to 35 mm) is described in Chapter 1. Essentially, we aimed to recruit women with a cervical length threshold at or below the 30th centile. New information that became available after the start of the trial, together with information on the first 20 subjects recruited, indicated that the ‘true’ 30th centile was 35 mm and not 30 mm. 16
Chapter 3 Trial results
Centre recruitment
A total of 87 centres expressed interest in or were approached to participate in the study. The following selection criteria were applied to participating centres: number of annual deliveries, number of twin deliveries in the preceding year, current or planned participation in studies involving twins, facilities to perform cervical length scanning and estimated recruitment per month. A total of 57 centres gained local approval to recruit and their main characteristics are shown in Table 3.
| Characteristic | Number of centres |
|---|---|
| ≤ 2500 deliveries per annum | 3 |
| 2501–5000 deliveries per annum | 17 |
| 5001–7500 deliveries per annum | 29 |
| 7501–10,000 deliveries per annum | 7 |
| ≥ 10,001 deliveries per annum | 1 |
| Dedicated twin clinic | 34 |
| No twin clinic | 23 |
Flow of participants through the trial
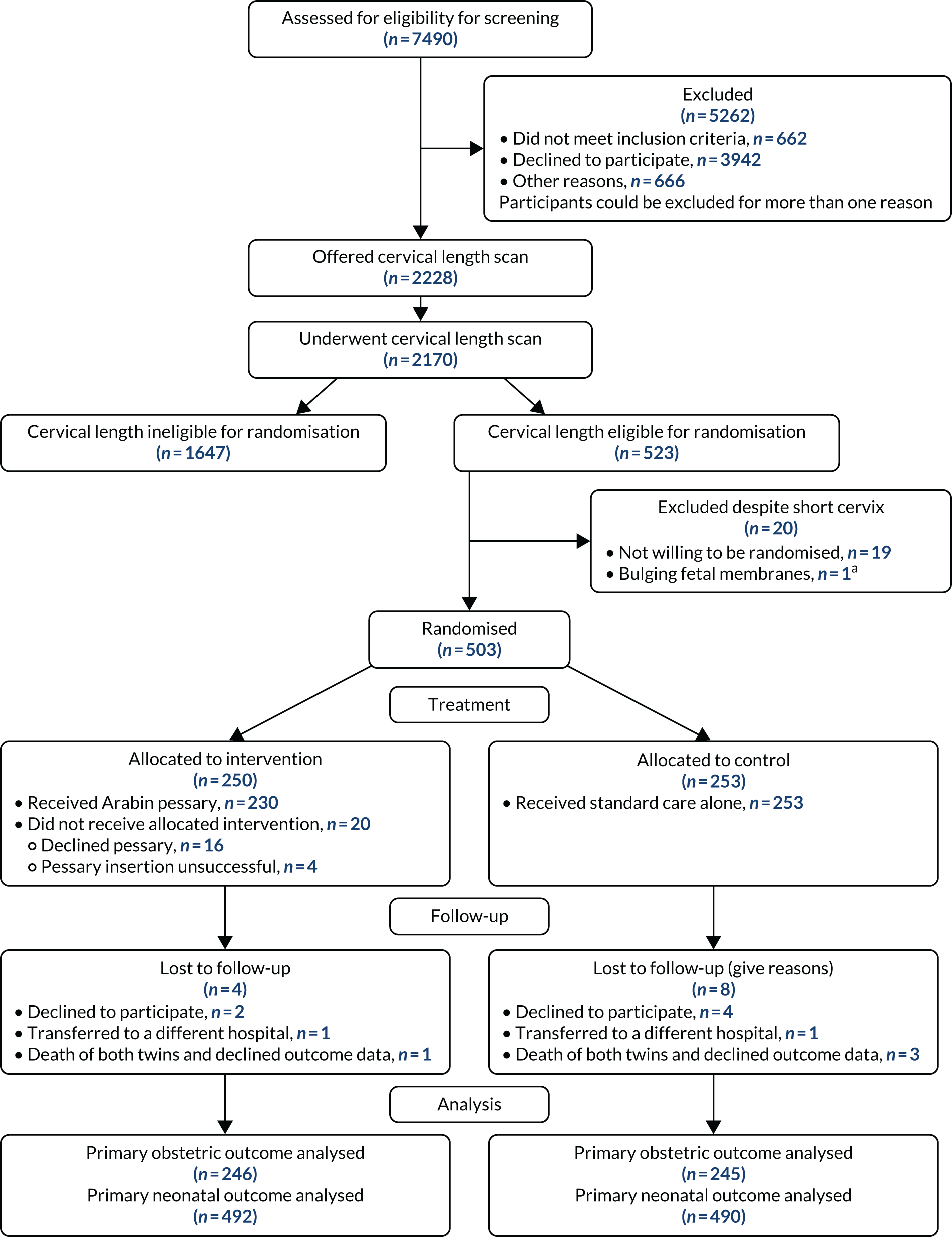
A total of 2228 participants were recruited to the screening phase of the trial between 1 April 2015 and 14 February 2019. Of these participants, 2170 had a cervical length scan. A total of 523 participants had a cervical length that conferred eligibility for the randomisation phase. Nineteen participants declined randomisation and one was found to be ineligible for randomisation, leaving 503 participants, of whom 250 were randomised to the intervention (Arabin pessary) group and 253 were randomised to the control (standard care) group. The last participant visit was on 2 August 2019.
The CONSORT flow diagram, showing participant throughput from referral to completion of follow-ups (including withdrawals and losses to follow-up), is shown in Figure 1.
FIGURE 1.
A CONSORT flow diagram. a, Bulging fetal membranes noted at time of planned pessary insertion.
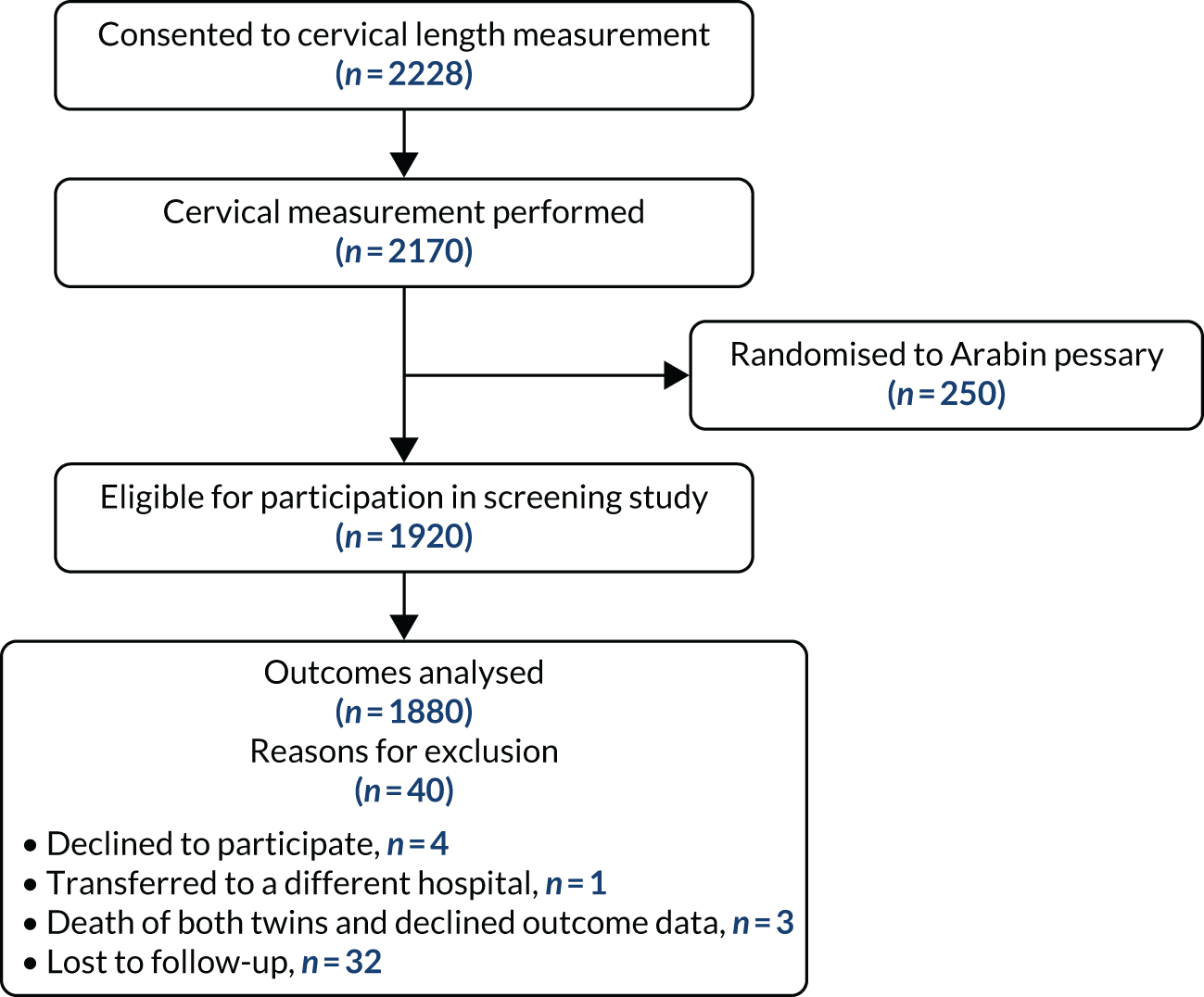
An additional flow chart showing follow-up and analysis of data in the screening study is provided in Figure 2.
FIGURE 2.
Flow of participants through screening study.
Baseline comparability
Table 4 shows the baseline characteristics of the intervention and control groups. There were no obvious differences between the groups. Formal statistical comparisons were not performed.
| Characteristic | Intervention (N = 250) | Control (N = 253) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 32.4 (5.8) | 32.7 (5.4) |
| Minimum, maximum | 17.0, 51.0 | 17.0, 50.0 |
| Cervical length (mm) | ||
| Mean (SD) | 28.8 (5.8) | 29.5 (5.1) |
| Minimum, maximum | 3.0, 35.0 | 7.0, 35.0 |
| Current smoking | ||
| Yes, n (%) | 21 (8.4) | 20 (7.9) |
| Current alcohol | ||
| Yes, n (%) | 1 (0.4) | 3 (1.2) |
| Obstetric history | ||
| Parity, n (%) | ||
| None | 150 (60.0) | 135 (53.4) |
| One | 60 (24.0) | 77 (30.4) |
| Two | 17 (6.8) | 27 (10.7) |
| Three | 12 (4.8) | 8 (3.2) |
| Four | 7 (2.8) | 3 (1.2) |
| Five | 3 (1.2) | 3 (1.2) |
| Six | 1 (0.4) | 0 (0.0) |
| Miscarriage, n (%) | ||
| None | 60 (24.0) | 65 (25.7) |
| One | 50 (20.0) | 49 (19.4) |
| Two | 17 (6.8) | 29 (11.5) |
| Three | 7 (2.8) | 6 (2.4) |
| Four | 6 (2.4) | 1 (0.4) |
| Five | 1 (0.4) | 3 (1.2) |
| Six | 2 (0.8) | 1 (0.4) |
| No previous pregnancies | 107 (42.8) | 99 (39.1) |
| Obstetric conditions, n (%) | ||
| Hypertension | ||
| Yes | 4 (1.6) | 8 (3.2) |
| Insulin-dependent diabetes | ||
| Yes | 2 (0.8) | 3 (1.2) |
| Respiratory disease | ||
| Yes | 11 (4.4) | 13 (5.1) |
| Cardiac disease | ||
| Yes | 5 (2.0) | 3 (1.2) |
| Neurological disease | ||
| Yes | 3 (1.2) | 3 (1.2) |
| Skin condition | ||
| Yes | 3 (1.2) | 3 (1.2) |
| Thrombophilia | ||
| Yes | 1 (0.4) | 3 (1.2) |
| Current pregnancy, n (%) | ||
| Fetal anomaly scan: twin 1 | ||
| Normal | 198 (79.2) | 209 (82.6) |
| Defined abnormality | 4 (1.6) | 2 (0.8) |
| Uncertain abnormality | 2 (0.8) | 1 (0.4) |
| Not carried out | 43 (17.2) | 40 (15.8) |
| Fetal anomaly scan: twin 2 | ||
| Normal | 199 (79.6) | 211 (83.4) |
| Defined abnormality | 0 | 0 |
| Uncertain abnormality | 3 (1.2) | 1 (0.4) |
| Not carried out | 43 (17.2) | 40 (15.8) |
Losses to follow-up
Both the number of women and the number of babies providing primary outcome data for the obstetric and neonatal outcomes was 491 (97.6%), with 12 women lost to follow-up (see Figure 1). The proportions of women with secondary outcomes are shown in the relevant data tables (see Tables 8 and 9).
Adherence to intervention
The number of women who did not adhere to the allocated treatment was 20 out of 250 in the intervention group and none in the control group. In the intervention group, 16 women declined to have the pessary inserted and 26 women asked to have it removed before the scheduled date of removal. The pessary fell out at least once in a further 13 women, and in 5 out of these 13 women it was reinserted (Table 5).
| Compliance | Intervention (N = 250), n (%) | Control (N= 253), n (%) |
|---|---|---|
| Received standard care only | 253 (100.0) | |
| Pessary insertion declined | 16 (6.4) | |
| Pessary insertion successful | 230 (92.0) | |
| Pessary insertion unsuccessful | 4 (1.6) |
Primary outcomes
Obstetric primary outcome
In total, 46 out of 250 women in the intervention group and 52 out of 253 women in the control group had preterm birth before 34+ 0 weeks following the spontaneous onset of labour. The adjusted OR of the treatment effect was 0.866 (95% CI 0.546 to 1.375; p = 0.542) (Table 6).
| Outcome | Intervention (N = 250) | Control (N = 253) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| All pregnancies | ||||
| Primary obstetric outcome: proportion of women delivering before 34 weeks’ gestation, n (%) | 46 (18.4) | 52 (20.6) | 0.866 (0.546 to 1.375) | 0.542a |
| Subgroup analyses, n/N (%) | ||||
| Monochorionic pregnancy | 10/49 (20.4) | 6/49 (12.2) | 1.572 (0.344 to 7.180) | 0.443 |
| Dichorionic pregnancy | 36/197 (18.3) | 46/196 (23.5) | 0.766 (0.391 to 1.498) | 0.305 |
| Cervical length of ≤ 28 mm | 26/90 (28.9) | 25/73 (34.3) | 0.737 (0.292 to 1.863) | 0.396 |
| Cervical length of > 28 mm | 19/160 (11.9) | 29/177 (16.4) | 0.717 (0.308 to 1.670) | 0.310 |
| Cervical length of ≤ 25 mm | 17/55 (30.9) | 18/39 (46.2) | 0.497 (0.151 to 1.633) | 0.130 |
| Cervical length of > 25 mm | 29/191 (15.2) | 34/206 (16.5) | 0.930 (0.446 to 1.938) | 0.798 |
In total, 67 out of 500 babies in the intervention group and 76 out of 506 babies in the control group had the composite neonatal primary outcome of stillbirth, neonatal death, periventricular leukomalacia, early respiratory morbidity, interventricular haemorrhage, necrotising enterocolitis or proven sepsis. The unadjusted OR for the primary neonatal outcome was 0.88 (95% CI 0.61 to 1.25; p = 0.46). Our planned three-level linear regression model for the primary neonatal outcome (babies nested within mother nested within centre, adjusting for chorionicity) failed to converge. We therefore used standard logistic regression for the neonatal primary outcome, adjusting for chorionicity and clustering at the mother level, giving an adjusted OR of 0.86 (95% CI of 0.54 to 1.36; p = 0.52). The rates of components of the neonatal primary outcome and the adjusted ORs of the treatment effect are shown in Table 7. As described in the statistical analysis plan, the level of statistical significance was set at 1% for subgroup analyses, but the adjusted OR is presented as 95% significance levels.
| Outcome | Intervention (N = 500) | Control (N = 506) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| All pregnancies | ||||
| Primary neonatal outcome: composite outcome, n (%) | 67 (13.4) | 76 (15.0) | 0.86 (0.54 to 1.36) | 0.515 |
| All pregnancies: individual components of the primary neonatal outcome, n (%) | ||||
| Stillbirth or neonatal death | 22 (4.4) | 28 (5.5) | ||
| Periventricular leukomalacia | 5 (1.0) | 1 (0.2) | ||
| Early respiratory morbidity | 36 (7.2) | 46 (9.1) | ||
| Intraventricular haemorrhage | 9 (1.8) | 6 (1.2) | ||
| Necrotising enterocolitis | 2 (0.4) | 10 (2.2) | ||
| Proven sepsis | 9 (1.8) | 4 (0.8) | ||
| Subgroup analyses, n/N (%) | ||||
| Monochorionic pregnancy | 22/100 (22.0) | 13/102 (12.7) | 1.89 (0.51 to 7.00) | 0.209 |
| Dichorionic pregnancy | 45/400 (11.3) | 63/404 (15.6) | 0.67 (0.34 to 1.34) | 0.140 |
| Cervical length of ≤ 28 mm | 41/178 (23.0) | 28/142 (19.7) | 1.19 (0.47 to 3.00) | 0.633 |
| Cervical length of > 28 mm | 26/322 (8.1) | 48/364 (13.2) | 0.57 (0.24 to 1.33) | 0.088 |
| Cervical length of ≤ 25 mm | 29/116 (25.0) | 20/78 (25.6) | 1.04 (0.32 to 3.33) | 0.931 |
| Cervical length of > 25 mm | 38/384 (9.9) | 56/428 (13.1) | 0.70 (0.34 to 1.46) | 0.214 |
Subgroup analyses
There was no evidence that the presence or absence of any of the subgroup criteria (i.e. monochorionicity, dichorionicity, cervical length of ≤ 25 mm or cervical length of ≤ 28 mm) had any impact on treatment effectiveness for either the primary obstetric or the primary neonatal outcome.
Secondary outcomes
Obstetric secondary outcomes
Obstetric secondary outcomes are shown in Tables 8 and 9. There are no differences between the groups for any of the obstetric secondary outcomes.
| Outcome | Intervention (N = 250), mean (SD) [n] | Control (N = 253), mean (SD) [n] | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Gestational age at delivery (weeks) | 34.8 (3.7) [246] | 34.5 (4.0) [245] | 0.2 (–0.6 to 1.1) | 0.495 |
| Duration | ||||
| Labour stage 1 (minutes) | 403.9 (510.8) [81] | 326.0 (255.5) [81] | 77.1 (–85.2 to 239.4) | 0.221 |
| Labour stage 2 (minutes) | 80.0 (90.7) [77] | 101.1 (202.3) [80] | –21.3 (–85.7 to 43.1) | 0.394 |
| Labour overall (minutes) | 333.4 (485.1) [123] | 325.7 (439.9) [117] | 5.4 (–147.5 to 158.3) | 0.927 |
| Hospital stay (days) | 5.5 (7.2) [243] | 5.6 (5.4) [242] | –0.1 (–1.6 to 1.4) | 0.865 |
| Outcome | Intervention, n (%) | Control, n (%) | χ2 | p-value |
|---|---|---|---|---|
| Method of delivery twin 1 | ||||
| Spontaneous vaginal delivery | 62 (24.8) | 63 (24.9) | χ2(3) = 0.835 | 0.841 |
| Vaginal breech | 3 (1.2) | 4 (1.6) | ||
| Forceps or ventouse | 20 (8.0) | 15 (5.9) | ||
| Caesarean | 160 (64.0) | 159 (62.8) | ||
| Method of delivery twin 2 | ||||
| Spontaneous vaginal delivery | 48 (19.2) | 45 (17.8) | χ2(3) = 3.338 | 0.342 |
| Vaginal breech | 13 (5.2) | 23 (9.1) | ||
| Forceps or ventouse | 15 (6.0) | 12 (4.7) | ||
| Caesarean | 169 (67.6) | 162 (64.0) | ||
| Outcome | Intervention, n (%) | Control, n (%) | Adjusted OR (95% CI) | p-value |
| Incidence of all births | ||||
| Before 28+ 0 weeks | 17 (6.8) | 24 (9.5) | 0.67 (0.27 to 1.64) | 0.248 |
| Before 32+ 0 weeks | 35 (14.0) | 41 (16.2) | 0.83 (0.42 to 1.63) | 0.470 |
| Before 34+ 0 weeks | 62 (24.8) | 66 (26.1) | 0.90 (0.52 to 1.57) | 0.640 |
| Before 37+ 0 weeks | 158 (63.2) | 161 (63.6) | 0.95 (0.57 to 1.58) | 0.786 |
| Incidence of births preceded by spontaneous onset of labour | ||||
| All births | 61 (24.4) | 71 (28.1) | 0.82 (0.48 to 1.41) | 0.342 |
| Before 28+ 0 weeks | 13 (5.2) | 19 (7.5) | 0.64 (0.23 to 1.77) | 0.261 |
| Before 32+ 0 weeks | 26 (10.4) | 32 (12.6) | 0.79 (0.37 to 1.68) | 0.427 |
| Before 34+ 0 weeks | 37 (14.8) | 46 (18.2) | 0.77 (0.40 to 1.47) | 0.295 |
| Before 37+ 0 weeks | 56 (22.4) | 66 (26.1) | 0.81 (0.47 to 1.41) | 0.324 |
| pPROM | 13 (5.2) | 6 (2.4) | 1.95 (0.52 to 7.34) | 0.196 |
| Incidence of birth before 34+ 0 weeks preceded by pPROM | 8 (3.2) | 5 (2.0) | 1.61 (0.36 to 7.14) | 0.407 |
| Adverse events | ||||
| Infection | 12 (4.8) | 10 (4.0) | 1.25 (0.39 to 3.95) | 0.623 |
| Haemorrhage | 115 (46.0) | 105 (41.5) | 1.19 (0.73 to 1.94) | 0.347 |
| Tachycardia | 6 (2.4) | 7 (2.8) | 0.70 (0.12 to 4.17) | 0.611 |
Neonatal secondary outcomes
Neonatal secondary outcomes are shown in Tables 10 and 11. Again, there are no significant differences in outcomes between the two treatment groups.
| Outcome | Intervention (n = 500) | Control (n = 506) | Difference in means (95% CI) | p-value |
|---|---|---|---|---|
| Birthweight < 10th centile, mean (SD) | 104 (20.8) | 97 (19.2) | 1.09 (0.69 to 1.72) | 0.642 |
| Birthweight (g), mean (SD) | 2170 (659) [n = 488] | 2142 (686) [n = 485] | 27 (–120 to 174) | 0.636 |
| Cord pH (venous), median (IQR) | 7.3 (3.4–7.8) [n = 212] | 7.3 (3.3–7.4) [n = 192] | 0.0 (–0.1 to 0.0) | 0.516 |
| Cord pH (arterial), median (IQR) | 7.3 (7.0–7.4) [n = 199] | 7.3 (3.4–8.3) [n = 177] | 0.0 (–0.0 to 0.1) | 0.094 |
| Apgar score at 1 minute, median (minimum, maximum) | 9.0 (0, 10) [n = 472] | 9.0 (0, 10) [n = 470] | 0.1 (–0.3 to 0.6) | 0.459 |
| Apgar score at 5 minutes, median (minimum, maximum) | 9.0 (0, 10) [n = 468] | 9.0 (0, 10) [n = 467] | 0.1 (–0.3 to 0.5) | 0.544 |
| Days of oxygen therapy, mean (SD) | 21.5 (32.9) [n = 36] | 9.3 (15.0) [n = 45] | 12.9 (–4.0 to 29.8) | 0.049 |
| Level of care days, mean (SD) | 22.0 (27.5) [n = 245] | 25.0 (31.8) [n = 225] | –4. 3 (–13.0 to 4.5) | 0.208 |
| Outcome | Intervention (N = 500), n (%) | Control (N = 506), n (%) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Received resuscitation | 119 (23.8) | 125 (25.0) | 0.93 (0.57 to 1.52) | 0.707 |
| Fetal or neonatal death within the first 28 days after birth | 4 (0.8) | 8 (1.6) | 0.49 (0.07 to 3.25) | 0.334 |
| Received surfactant | 39 (7.8) | 40 (87.9) | 0.97 (0.45 to 2.08) | 0.915 |
| Bronchopulmonary dysplasia | 6 (1.2) | 3 (0.6) | 2.00 (0.24 to 16.58) | 0.397 |
| Necrotising enterocolitis | 2 (0.4) | 10 (2.0) | 0.20 (0.03 to 1.50) | 0.039 |
| Daily level of care | ||||
| Normal care | 67 (13.4) | 59 (11.7) | 1.15 (0.61 to 2.16) | 0.564 |
| Special care | 208 (41.6) | 197 (38.9) | 1.09 (0.70 to 1.69) | 0.613 |
| High dependency care | 87 (17.4) | 108 (21.3) | 0.76 (0.45 to 1.28) | 0.175 |
| Intensive care | 72 (14.4) | 72 (14.2) | 1.00 (0.54 to 1.82) | 0.984 |
| Rate of major adverse neonatal outcomes before discharge from hospital | 121 (24.2) | 128 (25.3) | 0.92 (0.57 to 1.50) | 0.669 |
Safety issues
No significant issues were identified from the list of predefined safety outcomes (Table 12).
| Type | Intervention (N = 250), n/N (%) | Control (N = 253), n/N (%) | p-value from exact test |
|---|---|---|---|
| Mother dieda | 0/250 (0.0) | 0/253 (0.0) | |
| Any baby death | 22/500 (4.4) | 28/506 (5.5) | 0.388 |
| Intrauterine death | |||
| Stillbirth | 6/500 (1.2) | 4/506 (0.8) | 0.753 |
| Miscarriage | 12/500 (2.4) | 16/506 (3.2) | 0.451 |
| Neonatal death | 4/500 (0.8) | 8/506 (1.6) | 0.248 |
| Involved or prolonged inpatient maternal hospitalisationa | 11/250 (4.4) | 8/253 (3.2) | 0.641 |
| Involved persistent/significant maternal disabilitya/incapacity | 0/250 (0.0) | 0/253 (0.0) | |
| Life-threateninga | 1/250 (0.4) | 1/253 (0.4) | 1.000 |
| Chorioamnionitis or intrauterine infectiona | 12/250 (4.8) | 13/253 (5.1) | 0.841 |
| Congenital anomaly/birth defecta | 1/250 (0.4) | 0/253 (0.0) | 1.000 |
Device experience
Device fitting
Questionnaire responses on women’s experience of having the device fitted are shown in Table 13. The majority of women (158/234, 67.5%) found pessary fitting either painless or only slightly uncomfortable, and clinicians found device fitting easy or moderately easy (202/234, 86.3%).
| Experience of pessary at fitting | n/N (%) |
|---|---|
| Maternal experience of having device fitted | |
| Painless | 36/234 (15.4) |
| Slightly uncomfortable | 122/234 (52.1) |
| Uncomfortable | 45/234 (19.2) |
| Very uncomfortable | 23/234 (9.8) |
| Worst pain imaginable | 2/234 (0.9) |
| No response | 6/234 (2.6) |
| Clinical team experience of fitting device | |
| Easy | 158/234 (67.5) |
| Moderately easy | 44/234 (18.8) |
| Neither easy nor difficult | 8/234 (3.4) |
| Difficult | 15/234 (6.4) |
| Very difficult | 3/234 (1.3) |
| Impossible | 1/234 (0.4) |
| No response | 5/234 (2.1) |
The majority of women who provided a response about the experience of the pessary said that they ‘never’ or ‘only a few times’ felt it and that it was ‘never’ or ‘only a few times’ uncomfortable (Table 14). Experiencing vaginal discharge was common, but bleeding was uncommon.
| Maternal experience of device during pregnancy (as reported at the 36-week questionnaire) | n/N (%) |
|---|---|
| I could feel the pessary . . . | |
| Never | 83/230 (36.0) |
| A few times | 42/230 (18.3) |
| At least once every week | 3/230 (1.3) |
| Every day | 7/230 (3.0) |
| All the time | 5/230 (2.2) |
| No response | 90/230 (39.1) |
| I found the pessary uncomfortable . . . | |
| Never | 101/230 (43.9) |
| A few times | 30/230 (13.0) |
| At least once every week | 4/230 (1.7) |
| Every day | 2/230 (0.9) |
| All the time | 4/230 (1.7) |
| No response | 89/230 (38.7) |
| I found the pessary painful . . . | |
| Never | 145/230 (63.0) |
| A few times | 11/230 (4.8) |
| At least once every week | 1/230 (0.4) |
| Every day | 2/230 (0.9) |
| All the time | 1/230 (0.4) |
| No response | 70/230 (30.4) |
| I had vaginal discharge . . . | |
| Never | 7/230 (3.0) |
| A few times | 20/230 (8.7) |
| At least once every week | 20/230 (8.7) |
| Every day | 55/230 (23.9) |
| All the time | 38/230 (16.5) |
| No response | 90/230 (39.1) |
| I had vaginal bleeding . . . | |
| Never | 117/230 (50.9) |
| A few times | 20/230 (8.7) |
| At least once every week | 1/230 (0.4) |
| All the time | 2/230 (0.9) |
| No response | 90/230 (39.1) |
| I had to use panty liners/sanitary towels because of the vaginal discharge/bleeding . . . | |
| Never | 26/230 (11.3.) |
| A few times | 21/230 (9.1) |
| At least once every week | 12/230 (5.2) |
| Every day | 38/230 (16.5) |
| All the time | 44/230 (19.1) |
| No response | 113/230 (49.1) |
| The vaginal discharge was . . . | |
| The same as before the pessary | 16/230 (7.0) |
| A little more than before the pessary | 41/230 (17.8) |
| A lot more than before the pessary | 76/230 (33.0) |
| No response | 97/230 (42.1) |
| The vaginal discharge was . . . | |
| The same as in my last pregnancy | 5/230 (2.2) |
| A little more than in my last pregnancy | 16/230 (7.0) |
| A lot more than in my last pregnancy | 33/230 (14.3) |
| This is my first pregnancy | 63/230 (27.4) |
| No response | 113/230 (49.1) |
| The vaginal bleeding was . . . | |
| The same as before the pessary | 30/230 (13.0) |
| A little more than before the pessary | 7/230 (3.0) |
| A lot more than before the pessary | 7/230 (3.0) |
| No response | 186/230 (80.9) |
| The vaginal bleeding was . . . | |
| The same as in my last pregnancy | 12/230 (5.2) |
| A little more than in my last pregnancy | 2/230 (0.9) |
| A lot more than in my last pregnancy | 3/230 (1.3) |
| This is my first pregnancy | 33/230 (14.3) |
| No response | 180/230 (78.3) |
Device experience throughout the trial
Women’s experiences of the device during the trial are shown in Table 14.
Experience of device removal
Questionnaire responses on women’s experience of having the device removed are shown in Table 15. Of those who responded, the majority of women found pessary removal painless or only slightly uncomfortable and the majority of clinicians found it easy or fairly easy.
| Experience of device removal | n/N (%) |
|---|---|
| Maternal experience of having device removed | |
| Painless | 46/230 (20.0) |
| Slightly uncomfortable | 49/230 (21.3) |
| Uncomfortable | 36/230 (15.7) |
| Very uncomfortable | 44/230 (19.1) |
| Worst pain imaginable | 11/230 (4.8) |
| No response | 44/230 (19.1) |
| Clinical team experience of device removal | |
| Easy | 104/230 (45.2) |
| Moderately easy | 30/230 (13.0) |
| Neither easy nor difficult | 16/230 (7.0) |
| Difficult | 21/230 (9.1) |
| Very difficult | 3/230 (1.3) |
| No response | 56/230 (24.3) |
Outcomes of screening study
We calculated the sensitivity, specificity, positive and negative likelihood ratios of SPB before 34 weeks’ gestation for a variety of cervical length thresholds (35 mm, 30 mm, 28 mm, 25 mm and 15 mm) (Table 16). Positive likelihood ratios for spontaneous birth before 34 weeks’ gestation were < 5 for cervical lengths of ≤ 30 mm and 28 mm and between 5 and 10 for cervical lengths of ≤ 25 mm and 20 mm. Negative likelihood ratios were > 0.5 for all cervical lengths.
| Outcome for specified cervical length | Spontaneous birth before 34 weeks’ gestation (95% CI) | |
|---|---|---|
| Cervical length of 35 mm | ||
| Cervical length of 35 mm | Yes | No |
| Less than equal to threshold | TPs 58 | FPs 223 |
| Greater than threshold | FNs 146 | TNs 1453 |
| Total | 204 | 1676 |
| Sensitivity | 0.284 (0.222 to 0.346) | |
| Specificity | 0.867 (0.851 to 0.883) | |
| Positive likelihood ratio | 2.137 (1.665 to 2.743) | |
| Negative likelihood ratio | 0.826 (0.756 to 0.902) | |
| Cervical length of 30 mm | ||
| Cervical length of 30 mm | Yes | No |
| Less than or equal to threshold | TPs 35 | FPs 88 |
| Greater than threshold | FNs 169 | TNs 1588 |
| Total | 204 | 1676 |
| Sensitivity | 0.172 (0.120 to 0.223) | |
| Specificity | 0.947 (0.937 to 0.958) | |
| Positive likelihood ratio | 3.268 (2.271 to 4.701) | |
| Negative likelihood ratio | 0.874 (0.821 to 0.932) | |
| Cervical length of 28 mm | ||
| Cervical length of 28 mm | Yes | No |
| Less than or equal to threshold | TPs 25 | FPs 47 |
| Greater than threshold | FNs 179 | TNs 1629 |
| Total | 204 | 1676 |
| Sensitivity | 0.123 (0.078 to 0.168) | |
| Specificity | 0.972 (0.964 to 0.980) | |
| Positive likelihood ratio | 4.370 (2.751 to 6.943) | |
| Negative likelihood ratio | 0.903 (0.857 to 0.951) | |
| Cervical length of 25 mm | ||
| Less than or equal to threshold | TPs 20 | FPs 21 |
| Greater than threshold | FNs 184 | TNs 1655 |
| Total | 204 | 1676 |
| Sensitivity | 0.098 (0.057 to 0.139) | |
| Specificity | 0.987 (0.982 to 0.993) | |
| Positive likelihood ratio | 7.824 (4.316 to 14.184) | |
| Negative likelihood ratio | 0.913 (0.873 to 0.956) | |
| Cervical length of 20 mm | ||
| Less than or equal to threshold | TPs 10 | FPs 9 |
| Greater than threshold | FNs 194 | TNs 1667 |
| Total | 204 | 1676 |
| Sensitivity | 0.049 (0.019 to 0.079) | |
| Specificity | 0.995 (0.991 to 0.998) | |
| Positive likelihood ratio | 9.129 (3.753 to 22.201) | |
| Negative likelihood ratio | 0.956 (0.927 to 0.987) | |
Chapter 4 Health economic analysis
Introduction
The findings/conclusions from the trial clinical efficacy analysis indicated that pessary had no significant effect on the primary obstetric outcome (i.e. birth before 34 completed weeks following the spontaneous onset of labour) or on the primary neonatal outcome (i.e. composite of adverse outcomes). Given the trial evidence (i.e. that Arabin pessary is unlikely to prevent preterm birth or improve neonatal outcome in twin pregnancies with a short cervix), we considered that conducting a full within-trial cost-effectiveness analysis and a longer-term decision model-based economic evaluation was not supported/appropriate in this situation. Therefore, these analyses were not undertaken. Instead, we performed a simple cost analysis that examined the differences in costs of Arabin cervical pessary compared with conventional treatment pathways in women with twin pregnancy and a short cervix. Incremental costs were measured from the perspective of the UK NHS (hospital costs only). Mother and infant resource use from the date of randomisation to hospital discharge was used to define cumulative hospital costs over this period.
Methods
Measurement and valuation of hospital resource use
The costs of hospital stay and intervention-related costs are presented in Table 17. Resource consequences included length of stay within antenatal, postnatal and neonatal specialties (e.g. special care baby units or neonatal units), as well as any spells in intensive care for either mother or baby. The length of stays was valued using a per diem unit cost derived from NHS reference costs. 19 Costs of maternal hospital stay (per day) on different wards (i.e. labour, antenatal or other) were derived as a weighted (by activity) average from the NHS reference costs. 19 For instance, labour–delivery ward day unit costs were based on Healthcare Resource Group (HRG) codes of stays of ≥ 1 day [NZ30A to NZ51C (non-elective long stay)]. The costs of neonatal care unit admissions were also determined as weighted averages from NHS reference costs (critical care service codes CCU13–CU14, HRG codes XA01Z–XA05Z). 19 Costs related to the delivery of the Arabin pessary intervention were based on trial expenditure records for pessary device and clinician training and for outpatient attendances for pessary insertion/removal from NHS references costs [HRG code 501 (obstetrics outpatient attendance)]. 19 All costs were expressed in Great British pounds (£) at 2017/18 prices. Length of stay for the study population did not exceed 1 year and, as a result, no discounting of hospital costs was undertaken. To calculate the hospital cost of the babies, we used to two methods. Costing method 1 used the weighted average daily cost for neonatal care unit admissions across different levels of care (£725.65). Costing method 2 used the specific daily cost by level of care (i.e. normal care, special care, high-dependency care or intensive care) and assigned these to the babies’ individual number of days spent under various level of care.
| Resource use item | Unit cost (2017–18 prices) | Source/basis for estimate |
|---|---|---|
| Maternal hospitalisation | ||
| Antenatal ward per day | £838.50 (daily excess bed-day cost £497.70) |
Weighted average daily inpatient cost. Non-elective long stay. Currency codes NZ16Z–NZ24B, currency description (antenatal ward stay – various categories)19 Weighted average daily (excess) inpatient cost. Non-elective excess bed-days. Currency codes NZ16Z–NZ24B19 |
| Labour ward per day | By main method of delivery:
|
Weighted average daily inpatient cost. Non-elective inpatient stay19 Currency codes NZ30A–NZ34C (normal deliveries) Currency codes NZ40A–NZ44C (assisted deliveries) Currency codes NZ50A–NZ51C (caesarean) Weighted average daily (excess) inpatient cost. Non-elective excess bed-days. Currency codes NZ30A–NZ51C19 |
| Other ward per day | Same unit cost assumed as antenatal ward daily cost (i.e. £838.50) | Hospital ward stay recorded within STOPPIT 2 as ‘other’ occurred in all women in the prenatal phase period and so the daily unit cost for antenatal ward was assumed for stay in other ward types |
| Neonatal hospitalisation | ||
| Neonatal care unit per day |
£725.65 (weighted average across different levels of care) By specific level of care: |
Weighted average daily inpatient cost. Critical care. NHS reference costs19 codes CCU13–CU14 (neonatal intensive care unit – facility for babies on a transitional care ward). Currency codes XA01Z–XA05Z:
|
| Pessary intervention delivery-related costs | ||
| Arabin pessary device per unit | £35 | Pessary unit price based on trial finance purchase records. Assumed mid-point price (range £32–37) |
| Clinician training in Arabin pessary use | £27.42 | Actual training expenditure costs tracked in the trial (net total ≈ £6854 and 120 clinicians completed the training). Cost per patient estimated by apportioning the total cost incurred over the total number of women in the intervention group (n = 250) |
| Obstetric outpatient visit for pessary insertion/removal | £135 | Outpatient attendance, service code 501 obstetrics, consultant led19 |
Information on babies’ hospital length of stay (number of days) in the trial was recorded (1) based on the total number of days (i.e. the difference between the recorded hospital admission and discharge dates) and (2) from the number of days babies spent receiving different levels of care (i.e. intensive care, high dependency, special care or normal care). The second method of calculating the hospital costs of babies was performed as a sensitivity analysis and was undertaken to try to take into account how the distribution of total days was spread over different intensity/complexity levels of care and to examine whether or not this changed the cost estimates.
Data analysis
Complete hospital resource use data were available for 485 of 503 (96.4%) women. In the intervention and the control group, respectively, four and eight women were lost to follow-up or declined data collection and in a further three and three women, respectively, their dates of hospital admission and discharge were missing. Therefore, the maternal resource use data set comprised 243 and 242 women in the intervention and control groups, respectively. The corresponding available neonatal hospital resource data in both groups were 244 mothers and 488 babies [based on excluding the previous 12 women who declined data collection or were lost to follow-up and three further twins (two from the intervention group and one from the control group) because of incomplete dates of hospital stay and discharge, for instance because of the babies being delivered at a different site].
The cost analysis focused on calculating the hospital resource use and costs relating to:
-
hospital length of stay of the mothers in the trial and average length of stay by ward type (labour, antenatal or other)
-
total hospital costs of the mothers and the estimated total cost per randomised mother by treatment group
-
hospital length of stay of the twins and average length of stay by care level (normal care, special care, high-dependency care or intensive care)
-
total hospital costs of the twins within 28 days post estimated date of delivery and the estimated cost per baby.
Results
Table 18 shows the average hospital stay per randomised mother in the labour delivery ward and other wards by treatment group (for any reason, including for normal labour and delivery). The mean duration of labour delivery ward stay was 5.2 and 5.6 days for the intervention and control groups, respectively. The mean duration of stay on the labour ward not leading to the delivery of the babies was 0.14 and 0.18 days for the intervention and control groups, respectively. Mean duration of stay per mother in the antenatal ward was 0.9 and 0.9 days for the intervention and control groups, respectively. The mean duration of stay per mother in other wards was 0.02 and 0.04 days for the pessary and standard-care groups, respectively. The (unadjusted) mean total cost per mother associated was £8311.05 in the intervention group and £8763.59 in the control group [a difference in mean cost (saving) of £452.54].
| Resource use | Intervention (n = 243) | Control (n = 242) |
|---|---|---|
| Length of stay per randomised mother, mean (SD) [minimum, maximum] days | ||
| Labour ward (subsequent delivery of the babies) | 5.2 (4.3) [0, 5] | 5.6 (5.4) [0, 54] |
| Labour ward (no delivery) | 0.14 (0.79) | 0.18 (1.55) |
| Antenatal ward | 0.92 (4.04) [0, 44] | 0.91 (3.37) [0, 32] |
| Other ward | 0.02 (0.16) [0, 2] | 0.04 (0.46) [0, 7] |
| Hospital costs per mother, mean (SD) cost (£) | ||
| Pessary intervention delivery related costs | 316.02 (90.50) | |
| Labour ward costs (delivery) | 6794.97 (6914.52) | 7515.25 (8695.29) |
| Labour ward costs (no delivery) | 175.73 (1028.08) | 249.22 (2232.08) |
| Antenatal ward costs | 1010.52 (4973.94) | 967.93 (3968.50) |
| Other ward costs | 13.80 (131.30) | 31.18 (384.46) |
| Total hospital costs | 8311.05 (8845.15) | 8763.59 (10,008.94) |
Table 19 presents the average hospital resource use of the babies, including hospital stay (related to any neonatal unit admissions incurred) and number of days spent in various levels of care, under continuous positive airway pressure, under supplementary oxygen or on ventilations. The mean duration of hospital stay of babies was 10.88 days in the intervention group and 11.10 days in the control group. The (unadjusted) mean total cost per baby associated with each treatment group was £7892.93 and £8053.52, respectively [a difference in mean cost (saving) of £160.59]. Using costing method 2 (see Measurement and valuation of hospital resource use), the mean hospital stay cost per baby was £8678.43 and £8953.42 for the intervention and control group, respectively, with a mean cost difference of £274.99.
| Resource | Intervention (n = 244)a | Control (n = 244)a |
|---|---|---|
| Length of hospital stay per baby (days) | ||
| Mean | 10.88 | 11.10 |
| Median | 1.00 | 0 |
| Minimum, maximum | 0, 153 | 0, 161 |
| 25th, 75th percentile | 12, 13 | 13, 13 |
| Hospital costs (£) mean (SD) | 7892.23 (16,088.18) | 8053.52 (17,207.08) |
Conclusion from the economic evaluation: cost analysis
The economic evaluation did not undertake a full cost-effectiveness analysis (i.e. within-trial and model-based analyses), as the trial data found no clear indication of improved effectiveness with pessary treatment. Instead, the resource use and cost analysis aimed to give an assessment of the costs associated with Arabin pessary treatment. The simple costs analysis showed pessary treatment is no more costly than standard care. The findings point to a potential small cost advantage for both maternal and neonatal hospitalisation costs (i.e. £435.16 and £160.59, respectively) for the pessary strategy. The results are, however, surrounded by some uncertainties (e.g. in the price of the pessary and unit costing assignment methods used to value resource use). Furthermore, the current research assessed only the short-term costs and benefits of the pessary intervention. However, it could be argued that even a modest short-term difference in neurodevelopment may have profound implications clinically, as well as yielding long-term differences in costs and effectiveness, for instance measured in terms of quality-adjusted life-year gains, although this again involves some degree of uncertainty.
Chapter 5 Qualitative study and experiencing the trial
Introduction
The overall aim of the nested qualitative interview study was to understand the experiences of pregnant women and health-care professionals involved in STOPPIT 2. For pregnant women, the focus was on their decision-making, expectations about participation, perceptions of risk and engagement with the trial processes, including experience of the intervention group. For health-care professionals, the focus was on their role in recruitment, including explaining the trial, the communication of information and how staff experienced providing the intervention.
Findings are presented separately and thematically. First, findings are presented for pregnant women and include the following themes: experience of twin pregnancy, deciding to take part, experiencing the trial (including transvaginal ultrasound), risk and randomisation, and experience with the pessary. Second, findings are presented for health-care professionals and include the following themes: experience of trial processes, recruitment and supporting women.
Methods
Recruitment and sampling: pregnant women
All of the women who took part in the interview study had previously agreed to being approached by a qualitative researcher when they were recruited into the main trial.
Seventy women were invited to take part by letter, with up to two follow-up telephone calls. Two women declined to participate, two women were hospitalised and so were not followed up and 20 women could not be contacted. Forty-six women agreed to be interviewed and 41 were successfully interviewed (five women could not be contacted on the agreed day). Women who took part in the interview were sent two consent forms, one to keep and one to return.
The women were recruited from 14 of the 57 UK sites taking part in the trial. These sites were chosen to reflect the diversity of hospital settings and UK regions. The sample included women from each arm of the trial [i.e. women who were screened but not eligible to take part (n = 11), women randomised to standard care (n = 10) and women on the intervention group (n = 20)]. The sample included women across a wide range of cervical measurements, ranging from 10 to 60 mm.
Many of the women who took part in the qualitative study were highly motivated to become pregnant and had also experienced multiple disappointments in attempting to conceive and carry a fetus to term. Table 20 details the sample characteristics.
| Participant characteristic | Category | Number of participants |
|---|---|---|
| Age range (years) | 22–42 | 41 |
| Employment | Full-time employment | 30 |
| Part-time employment | 8 | |
| Unemployed | 3 | |
| Ethnicity | White British | 27 |
| White other (French, German, New Zealand, Polish) | 5 | |
| African | 1 | |
| Afro-Caribbean | 2 | |
| Indian/Pakistani/Bangladeshi | 4 | |
| South American | 1 | |
| Mixed-race white/African | 1 | |
| Marital status | Married/cohabiting | 41 |
| Sexuality | Same-sex parents | 2 |
| Reproductive history: disclosed during interview, all that apply | IVF pregnancy: first attempt | 3 |
| IVF pregnancy: second to eighth attempt | 14 | |
| Treatment for PCOS this pregnancy | 3 | |
| Pregnancy via intrauterine insemination | 1 | |
| Unplanned pregnancy | 1 | |
| Previous molar pregnancy | 1 | |
| Previous ectopic pregnancy (×2) | 2 | |
| Previous early miscarriage | 1 | |
| Previous multiple early miscarriages (2–5) | 7 | |
| Previous preterm birth at 24 weeks’ gestation | 1 | |
| Previous preterm birth at 31 weeks’ gestation | 1 | |
| No miscarriage disclosed | 19 | |
| Children at home (if any) | One | 5 |
| Two | 2 | |
| Four | 1 | |
| Cervical length | Non-eligible cervical range (37–60 mm) | 11 |
| Standard-care cervical range (21–34 mm) | 10 | |
| Arabin pessary cervical range (10–33 mm) | 20 |
Recruitment and sampling: health-care professionals/trial staff
Seventeen health-care professionals involved with the trial were interviewed, either face to face or by telephone, from 10 trial sites with differing resources and services, for example dedicated twin clinics. Sites also varied in the configurations of trial teams and with different levels of recruitment. The research midwives (RMs) (n = 8) who took part had the most contact with women and were involved in trial recruitment, consent processes, administering the survey and follow-up of women during the trial. We interviewed two consultant obstetricians (COs) and one clinical research fellow (CRF) who were involved in transvaginal scanning and pessary fitting and one midwife who had conducted transvaginal scanning. One consultant midwife (CM) was interviewed. The senior RMs (n = 5) had additional responsibilities in relation to trial administration and the work allocation of RMs (Table 21).
| Site number | Role |
|---|---|
| 1 | RM |
| CO | |
| 2 | RM |
| CM | |
| CRF | |
| Senior RM | |
| 3 | RM |
| 4 | RM |
| 5 | RM |
| 6 | Senior RM |
| Senior RM | |
| 7 | RM |
| Senior RM | |
| 8 | RM |
| CO | |
| 9 | RM |
| 10 | Senior RM |
The interviews: pregnant women
The interviews took place between 24 and 34 weeks’ gestation and, wherever possible, before 28 weeks’ gestation. A semistructured topic guide combined consistency and flexibility. The topic guides were designed to explore how women experienced trial participation in the context of their pregnancies to develop a nuanced understanding of the experience of the trial in relation to a pregnancy at risk of preterm labour. The use of prompts within topic areas aided the generation of data from the woman’s point of view and her experience. [For more information please see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/130422/#/ (accessed 22 April 2021).]
The women were offered face-to-face or telephone interviews; however, all the women preferred to take part in a telephone interview. These interviews were timed to fit around the women’s family and work commitments, and it was clear that some of the women who took part had gone to some lengths to fit in the interview around their busy lives. Five interviews took place during the pilot phase of the study late in 2015, and these interviews informed the topic guides for the main qualitative study. The remaining interviews were conducted between November 2017 and May 2018. Interviews lasted between 24 and 70 minutes and were fully transcribed. Follow-up interviews were conducted with five women in the intervention group after the removal of the pessary. Three of the women interviewed from the intervention group requested early pessary removal. Therefore, these interviews also captured the experience of having the pessary removed.
The interviews: health-care professionals
Three interviews were completed during the pilot phase of the trial in October 2015. The remaining interviews were carried out between January and September 2018. These interviews lasted between 19 and 83 minutes and were fully transcribed. The timing of the interviews allowed early interviews to inform the later interviews, and the analysis of both women’s and health-care professionals’ data sets to inform each other as the study progressed. As with the women’s interviews, the health-care professionals’ interviews took a semistructured approach, enabled by a topic guide. [For more information please see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/130422/#/ (accessed 22 April 2021).]
The topic guides were designed to explore health-care professionals’ experience of administering trial processes, according to individual roles and responsibilities. The RMs’ interviews tended to be longer than those with the COs and clinicians, who were mainly involved with pessary fitting rather than more extensive contact with the women. Many of the RMs were involved at each stage of women’s journey through the trial and they were also involved in the ongoing support and follow-up of women. The topic guide included exploration of professionals’ experience of working within clinical research, moving to a more specific focus on the trial itself, and also incorporated each aspect of the trial process as relevant to individual professional experience.
The majority of these interviews were conducted by telephone; however, some were conducted face to face at two of the study sites. Again, the health-care professionals who took part did so in the context of busy work schedules, but they were keen to share their thoughts and experiences of the trial.
Process of analysis
All interviews were digitally recorded and transcribed verbatim. Analysis began early into the data collection period to help to shape the interviews that were conducted in the later stages of the data collection period. This approach helped to explore the emergent themes in more depth before deciding on a final coding structure. An inductive, cross-sectional thematic approach was utilised in analysing the entire data set, whereby index coding was applied to reveal patterns and themes within the data (e.g. for each trial arm in relation to the women). 20 This method was selected as it was particularly suitable in identifying individual women’s experience of each arm of the trial in the context of their current and previous experience of pregnancy. A similar approach was taken in relation to the health-care professional data set, which facilitated an exploration of professionals’ accounts in conjunction with their individual roles and responsibilities.
NVivo Pro (version 11; QSR International, Warrington, UK) computer software was used to manage and support the coding of data. Initial codes were created around the main interview questions and comparison made across the women’s experience on each arm of the trial and health-care professionals’ accounts. The research team members were involved in ongoing discussions as data collection and analysis progressed. The use of computer software helped to facilitate a critical and rigorous approach to the assignment and with the interpretation of the codes and emergent themes. 21 A two-cycle approach to coding was undertaken. 22 First-cycle coding involved applying structural coding, which facilitated an approach whereby the data were coded according to broad topics [i.e. experience of transvaginal ultrasonography (TVU)]. The segments of data were then subject to further analysis and comparison. Analysis and interpretation were supported by analytic memo writing on emergent patterns and themes. Second-cycle coding further refined the original codes and memos into a smaller number of categories that were subject to further analysis and interpretation. This stage of the analysis involved a team approach, helping to improve the rigour of the findings. 23
Findings: women’s experiences
The following abbreviations are used in quotation sources to identify characteristics of participants: cervix (Cx) length in mm, non-eligible trial arm (NE), intervention group (P) and control group (SC).
Twin pregnancy
The backdrop to all the women’s experiences, as elicited in the interviews, was that of a twin pregnancy and the concerns this generates. Many had complex obstetric histories and, for some of the women, the current pregnancy represented what they saw as their last chance to become a parent. With such high stakes, many of the women encountered significant levels of anxiety about fetal development and the ability of their bodies to support a twin pregnancy.
Participants described a variety of emotional responses and different means of dealing with a pregnancy vulnerable to complications. Excitement was tempered by worry and apprehension and information was experienced as both reassuring and anxiety provoking:
I was very excited, I think I got less excited when I got home and had a little bit of the look at the information [. . .]. So, we were initially very excited, but then I got myself really quite worried.
NE6-Cx38
Many of the women described strategies with which they managed the anxiety they faced in the context of obstetric consultations that focused on potential complications of twin pregnancies. On the one hand, the women described feeling grateful for the additional monitoring their pregnancies incurred, whereas, on the other hand, some described having to manage high levels of anxiety due to continual monitoring, which provided a constant reminder of what might go wrong. Although notions of risk shaped the women’s pregnancies, managing their time served to control their anxiety and keep an emotional distance from the fetuses:
I just kind of live in those 2 weeks . . . and get to the scan, and have the usual anxiety, worry, before I go in there [. . .] and then I kind of move onto the next 2 weeks. So I actually, I never look too far ahead [. . .] [I’ve had] two unsuccessful pregnancies . . . in between . . . [. . .] I think it definitely has added to the anxiety and the worry [. . .] I’m just, I’m not waiting for something to go wrong, but I just feel nervous, to think too far ahead.
NE11-Cx38
The women were keen to learn the signs that might indicate pregnancy complications and, for some, this was also a means of gaining some control over uncertainty:
If we did have symptoms of things and I would know that it wasn’t necessarily normal and it was worth getting checked out. Quite a lot of the symptoms are normal pregnancy symptoms for twin-to-twin transfusion syndrome. So, the things like your tummy getting bigger, but a lot bigger, and your back hurting and needing to drink more. So, it’s quite, I found it . . . or at times I think is this a symptom? Is it not? [. . .] They will check you out and if it’s actually fine, that’s great, but it’s better to be precautious. So, it does give me an element of control because it means I know what I’m looking out for, rather than just blindly going between scans not knowing if everything is OK or not, erm . . . possibly.
NE6-Cx38
Women also drew attention to the role of midwives in relation to the emotional support they provided:
The midwives are lovely, ‘cause they’re very friendly and have got good people skills. All the ones I’ve seen, they’ve made me feel really comfortable, and it’s all normal and absolutely fine. They’ve been very good at making me feel comfortable with things.
P13-Cx33
Deciding to take part
The majority of women heard about the trial during their first obstetric antenatal consultation at 12–16 weeks’ gestation. One woman described this consultation as the ‘everything that can go wrong meeting’ (NE6-Cx38). Many of the women talked about feeling overwhelmed by the sheer volume of information provided. The timing of recruitment was important to many of the women, who felt acutely concerned about the nature of the risks involved in their pregnancies. The women observed that recruiters were careful about how the trial was explained to them:
We were given a lot of information and that was part of it, but it wasn’t kind of billed as everything can go wrong and this may help. It was just ‘you might want to consider this, it’s a research project that the hospital are taking part in, so it would be something to consider and have a look at’.
NE6-Cx38
Although this participant seemed to be clear about the nature and purpose of the trial at the point of recruitment, the picture becomes less clear-cut as women progress in trial participation and the associated decision-making.
Overall, most of the women framed participation in medical research in positive terms, for example ‘[we were] very, very happy to be involved, because anything to . . . medical enhancement and helping the cause, that kind of thing’ (NE8-Cx46). By contrast, others described feeling more cautious about the implications of becoming involved in research, as suggested by the following participant:
My husband was a little bit more concerned about [. . .] if it was like a research study that was done by a private company, big pharma, or if it was something that it was going to be done through the NHS.
P6-Cx26
Women expressed increased confidence in taking part in research that was conducted in the NHS and by doctors whom they felt they could trust. Women who had experienced in vitro fertilisation were aware of the achievements of reproductive research; as one woman put it:
If you’re willing to take it, you’ve got to be willing to give something back yourself give something back.
P12-Cx23
Many of the women also expressed trust in the clinical trial research processes:
. . . they wouldn’t give it to us if they thought it was going to harm the baby.
NE6-Cx38
They also expressed confidence in the trial itself:
I found out that it was . . . that you already tested it for singleton and it worked, so it felt OK.
P15-Cx31
How women viewed medical research and its relation to clinical practice appeared to influence how they made the decision to take part. Although most women described the decision as straightforward, even logical, there was nonetheless hidden complexity. Their decisions were tinged with hopes and concerns and affected by perceptions of risk and understandings of trial methodology.
Overall, the most common reasons women gave for taking part was that doing so might prevent SPB in their own pregnancies (a risk factor that they understood as being otherwise beyond their control) and that the research would potentially help other couples in the future. Many of the women taking part in the trial described the decision to do so as an easy or logical decision. This was based on the reasoning that prolonging the pregnancy, even by a week, was beneficial to the fetuses. For some women, the decision was reached quickly, and they reported making this decision without consulting others. By contrast, some women sought further information, including from the trial team. Concerns related to additional appointments, any risks of TVU and, for a few, the pessary fitting itself.
For most women, the decision to take part was supported by the belief that this would do no harm to the fetuses. In this respect, trial participation was believed to be less risky than other types of research participation, as it involved a removable medical device rather than a drug or surgical procedure:
There’s no harm in doing it [. . .] if I was in the group who could be helped by it then it would be worthwhile [. . .] but there would be no harm in kind of taking part [. . .] [my partner and I] chatted about whether we would want to take part [. . .] what would the right reasons for taking part [. . .] we couldn’t see any negatives to me being scanned [. . .] no harm would be caused [. . .] so we felt it was worth trying.
NE6-Cx38
Although the reasons women gave for taking part suggest that decision-making was mostly a straightforward process, with women reporting few anxieties about participation at the point of recruitment, some women also talked about feeling unable not to take part. This was an emotional imperative, where the trial was seen as a means to gain reassurance or emotional support in relation to their own body’s capacities to support the pregnancy or to give their fetuses the best chance:
The approach bit of the trial just explained obviously it was to try and stop early labour in twins, would we like to take part? And at first both of us . . . said it was my choice because it was my body if I had to get the pessary inserted. So I thought about it and at first I said no. And then I went to . . . my friend’s got twins and I went to their first birthday party [. . .] they still had tubes, they can eat, but the fluids they have to have through tubes. So that made me think more into it. I thought for the best for my babies I would rather just go ahead and do it just for peace of mind.
NE2-Cx39
Fear of future regret freighted what was otherwise portrayed as quite a straightforward decision:
. . . having to live with the guilt of something happening to the babies.
NE3-Cx46
For some, the trial offered the opportunity to learn something new about their bodies; as one woman commented:
I was quite keen to have the scan [. . .] because that’s not something you’d normally be able to find out about in a pregnancy.
NE11-Cx38
Wanting to know, and hoping to be reassured about, cervical length was talked about by many women. Many talked about cervical length screening in positive terms, hoping to discover ‘a nice long cervix’ (NE11-Cx38). Some women also admitted that, at the time of consenting to take part in the study, they assumed that they would receive reassuring results:
I guess I didn’t really assume that I’d have a short cervix, I just assumed [. . .] I’d be told it was normal and I’d be off on my way. So yeah, I didn’t really think too much of it at that time.
P14-Cx33-P removed
Alternatively, they would feel reassured that enhanced monitoring or treatment would ensue if they did have a short cervix; for example, ‘even if I didn’t get the pessary thing that they were putting in, then they’d still monitor me if I had a short cervix, [. . .] and then I’d be more prepared if I was to go in to labour early’ (NE7-Cx60).
Overall, the women were knowledgeable about the aims of the trial, the eligibility criteria and the purpose of randomisation. Their accounts show that their preparedness to participate was shaped by their overall experience of twin pregnancy, their positive regard for research and their sense of doing something to support their pregnancies and fetuses, and to help others in the future.
Experience of transvaginal ultrasonography
Thirty-six of the 41 women who took part in the interview study had encountered TVU during assistive conception treatments or during early miscarriage, and those women expressed no concerns about the procedure itself. Many of the women framed vaginal medical procedures as ‘something you get used to’ or ‘to be expected in pregnancy’. Overall, women described the physical aspect of the procedure as ‘not as bad as a smear’ or ‘not uncomfortable’. One woman admitted that, although she had received information about the scan prior to the procedure, she ‘didn’t sort of think about in my own mind about an internal scan, let alone how they would do it, [. . .] probably naivety on my own part’ (P20-Cx25). From this participant’s description of the procedure, it was evident that trial staff offered further information and, aside from feeling a little embarrassed, she was happy to go ahead.
Although women were accepting of TVU as an aspect of the trial, partners were occasionally uncertain or embarrassed about the procedure, as the following quotation demonstrates:
He was the one in the room that was a bit like, oh, he didn’t know what do with himself. He was like, ‘do you want me to go and hide and stand behind the curtain?’ He said to the lady, she was like, ‘no just go up to the head.’ And I was thinking like, ‘Christ it’s not you that’s getting it.’ You know what I mean? I was like, ‘it’s fine,’ you know, I suppose you just have to get used to these things, don’t you?
P18-Cx25
Most women felt reassured that the procedure posed no risk to fetal development. However, a small number of women still felt worried on the day of their scan:
I was just thinking, ‘oh gosh, [. . .] I hope you don’t touch [my cervix] [. . .]’ because when you have your smear test, they always warn you if you’re pregnant, you know, this can lead to miscarriage, so you need to make sure you’re not pregnant; so those kind of warnings from before made me a bit more cautious.
NE5-Cx38
Overall, the women felt happy with the assurances they were given by trial staff and felt more confident that the procedure was safe:
. . . they talk to you about that [TVU] first, and you know, reassure you that it’s all safe.
P17-Cx28
I asked the question as to whether it was safe to have an internal scan at that point, and I think if the answer had been, ‘well we don’t really know,’ or ‘no’, then it would have influenced my decision.
NE9-Cx37
Some of the women who lacked experience of TVU felt concern about whether or not TVU would be ‘as rough’ as abdominal scanning. Some also made reference to ‘trust in the doctor’ and others remarked at how gentle the procedure was:
. . . [it] wasn’t an unpleasant experience either. It wasn’t like a smear test. It was much more comfortable.
SC9-Cx26
Other women described the TVU operator as ‘gentle and really kind’ (SC4-Cx29).
It was also evident that, although most of the women were comfortable with the prospects of undergoing TVU, at the actual point of having the scan some women described feeling a sense of anticipation in relation to what it would reveal:
I just wanted to, almost get on with it, and have the scan, and know one way or the other.
NE11-Cx38
As noted earlier, anticipating knowledge of cervical length formed part of the process of decision-making regarding the trial. The possibility of entering the trial and receiving the pessary affected reaction to receiving the measurement, as outlined below.
Reaction to cervical length
Women reported that they were given the results of the scan either during the procedure or soon afterwards. Women in all arms of the trial drew on the language trial staff used to explain the results, but varied in their responses to their cervical length measurement.
Non-eligible arm
Women in this arm of the trial interpreted longer cervical length in positive terms, as the following quotation illustrates:
She was quite quick to reassure me that she thought I had a very long cervix [. . . it was] like, a huge sigh of relief, that kind of, oh, OK, that’s a good sign. And although I didn’t have the numbers at that point, it was that kind of relief that . . . it, it was just reassuring in general. Because like I say, I’ve been so worried about so many things in the pregnancy. And actually, to be told that you were lower risk for something, was quite nice, after I’d been told that, you know, everything was high risk about the pregnancy, and then to be told you were low risk for this particular, erm, study. It was, it was really reassuring. So I found that I could relax that little bit more.
NE11-Cx38
Longer cervical length was seen as some good news in the context of an otherwise high-risk pregnancy, and related to their bodily competence (i.e. their ability to ‘hold on to the babies’):
It’s made me relax a lot more. Because, obviously, I know that my cervix is strong [. . .] it’s not too short, or anything. So that’s took a lot of worry, from me. Because, then, I know within myself that I’ve got a good strong, basically, like body, that I can hold onto my babies as long as I can, you know. I would say that made me feel more at ease, knowing that my cervix was fine, and that, you know, everything was OK, knowing that my cervix was nice and strong, that was me feeling a lot more settled within myself, and within my pregnancy, you know.
NE4-Cx46
Others seemed less reassured, noting that cervical length is just one risk factor:
It meant there was one less thing to worry about and one less thing to think about, I knew that my body was going to do what it could. Because the babies are inside . . . obviously there’s other things that could go wrong and I’m not naïve to think that just because I’ve got a long cervix that my babies are going to stay in there until full term. But it meant that it was one less thing for me to think about. It gave me a bit of reassurance, knowing that my body obviously will be able to keep hold of the babies in that sense.
NE7-Cx60
Intervention group
The women’s descriptions of how the results were explained suggested that one of the ways trial staff did this was to use eligibility as a means of framing the results:
. . . they confirmed that it was shorter and that I would be eligible for the trial.
P20-Cx25
They said if it’s under 35 mm you can enter and mine was 33.5 or something, so it was just in.
P8-CV33-P removed
The meaning of cervical length for these participants seemed to be guided by eligibility criteria and shortness was therefore given a trial-specific meaning:
It was shorter than average, but it wasn’t short enough for them to intervene in a normal circumstance. [. . .] it was short for this study rather than short, if that makes sense.
P4-Cx31
How women interpreted the results of screening represented a critical juncture in relation to continuing participation, as the following quotation demonstrates:
It was something that I hadn’t anticipated [. . .]. I had no way of knowing what the best thing was to do. And I felt very much whichever decision I made, could it have been the right one, could it have been the wrong one. It was quite a difficult decision to make and one that . . . it was harder than I thought it would be when I initially went for that cervical length scan.
P4-Cx31
Other participants whose cervical measurement was just on the borderline for eligibility seemed to be somewhat less committed to the study or concerned about the possibility of SPB. One of these participants described not persevering with an uncomfortable pessary:
I had the pessary in for like a week and then had it taken out because it was too painful, so obviously I’m not that worried about preterm birth, otherwise I would have persisted harder and harder.
P8-Cx33-P removed
Some of the women in the intervention group reported being given a cervical measurement that they found difficult to recall at the time of the interview, suggesting that the measurement was not important for everybody:
I think it was like 31 mm or something like that, so they said it’s under 35.
P15-Cx31
One woman with a shorter cervix of 25 mm understood that ‘it wasn’t actually short, but I went into randomisation or something like that. It was just pretty regular I think’ (P18-Cx25). Another participant took a pragmatic approach to her results, saying:
I found it helpful to kind of know what the result meant, but there was also, sort of, it doesn’t mean a lot to me. You know, it’s just a number and in the absence of knowing what to compare it to, so it was helpful when the hospital staff kind of, told me what the results meant. Yes, so they said there was basically nothing to worry about.
P11-Cx31
For other women, the actual cervical length held considerable significance, sometimes because of their previous obstetric history. For example, the following woman was familiar with cervical length measurement, having experienced monitoring of her cervix during her second pregnancy. Her previous experience of SPB with two singleton pregnancies, including a neonatal death, meant that she was highly concerned about SPB in her twin pregnancy. Cervical length measurement was therefore highly emotive and relevant for her:
I knew at 20 weeks, 2.6 was quite short because . . . well obviously like I said at 24 weeks my daughter, it were 3.3. And, then I also knew a few weeks before, well 18 weeks, with the twins my, my cervix was 3.5, so I knew that in 2 weeks my cervix had reduced by almost a centimetre. So, I’m thinking in another 2 weeks it’s going to reduce by another centimetre. And, then I’m going to have nothing left and go into labour.
P16-Cx26
Although this participant was highly concerned about SPB, her experience was complicated by her fear of having the pessary fitted. She wanted to be randomised to the device in the hope that it might help prevent SPB, but she was also concerned that having the pessary fitted would trigger labour.
Cervical length measurement was linked by some to expectations regarding trial participation, holding surprise results for some, as the following quotation suggests:
When she finished measuring it, she said my cervix is 10 mm. I didn’t have any sense of what that meant, if it was a good thing, if it was a bad thing. Then, when she’d finished, she kind of looked at me and said, ‘yes I’m sorry, your cervix is only 10 mm’. I said, ‘OK, so what does that mean?’ She said like, ‘well you . . . the chances of you having a miscarriage and going into early labour are quite high’. So, I said, ‘OK, what does that mean, what’s going to happen now?’ She said, ‘oh well, there’s nothing we can do, we’ll . . . are you still happy to take part in the trial?’ I said, ‘well yes, of course. You’ve just told me my cervix is 10 mm,’ and she said ‘yes, but we’ll only fit you with the pessary if you’re randomised by the computer, we don’t choose’.
P1-Cx10
In exploring her feelings about receiving this result, this participant went on to say:
Well, to be quite honest, I didn’t think I would even be randomised. I didn’t think that our cervix would be so short. So, it didn’t really hit me until we actually had the scan of my cervix.
P1-Cx10
Although this participant was randomised to the intervention group, the impact of knowledge of cervical length measurement had a significant influence on her experience of the remainder of her pregnancy. One of the ways that this participant dealt with the knowledge that she had a short cervix was to follow her doctor’s recommendation to rest in bed. Another participant self-imposed bed rest:
. . . they didn’t say to me you’ve got to be on confined bed rest, but I was like, there’s no other place I’m going, that was it, I’m lying down until I get to like 30 weeks.
P12-Cx23
Other participants framed cervical length measurement as important information that would help them prepare for a future in which SPB was a possibility:
I’m one of those people if . . . I like to know all the different things that could go wrong and for me to know that actually I have got a shorter cervix than what is normal, you know, I just to know and I just . . . I’m able to . . . I was . . . I knew that I could prepare myself should anything else go wrong, you know?
P2-Cx28
Responses to receiving a cervical length measurement short enough for entry into the trial varied, depending partly on obstetric history, on how the information was presented, and on expectations of both the trial/treatment and the risks of stillbirth.
Control group
As noted above, trial staff’s interpretations of the results helped women make sense of their cervical length measurement. The following participant understood that her cervical length was ‘longer’ than the actual numerical value:
If I’d had a shorter cervix it might have been more of a worry but because I was only slightly on the shorter side . . . I felt OK with that [randomisation to the control group] decision. I suppose since then I have thought to myself, ‘oh, what is the likelihood of . . . of them coming prematurely now, you know?’ [. . .] not much has happened since in terms of us, we’ve just carried on. We’ve gone, ‘OK, we’re part of a . . . the group that doesn’t have the pessary so we’ll get on with our lives and not think too much or worry.’ [. . .] I suppose my one concern would be is if I had a short cervix and I was in a control group I might be a little bit more worried and then you . . . that adds to all the other worries of pregnancy. [. . .] some women might prefer to be in ignorance because their babies might come prematurely anyway. Rather than have to then worry about it along with all the other worries of pregnancy.
SC9-Cx26
As with some of the participants on the intervention group, when women discussed how they felt about cervical length measurement, many of them couched their results in terms of trial eligibility. When results were framed in this manner, neither cervix length nor being in the control group seemed to cause particular concern:
Although it was within the boundaries to be applicable, it was still on the longer scale, so there wasn’t hopefully too much to worry about there. [. . .] on the whole it’s a decent-ish size [laughs], so I’m not going to worry too much. I’m not going to worry about it too much, about having the pre-term labour.
SC10-Cx31
The sonographer first said it looks pretty normal and she told us the measurements and said you’re just, just into the trial. [. . .] So it wasn’t overly short and it wasn’t long. It was just, a regular [laugh] size, maybe just slightly on the shorter side. So I felt reassured when I was randomised to not have the pessary that at least my cervix wasn’t so short that it was so dangerous.
SC9-Cx26
Not all participants were completely reassured by the measurement and sometimes they were unable to make sense of what they were told, as the following quotation suggests:
I remember them telling me a number. The length. I just can’t remember what it is. She said, ‘oh you’re just on borderline’. I was just thinking, ‘well what does she mean borderline?’ Borderline small or borderline long? [laughs].
SC1-Cx34
A few were wavering in their acceptance of reassurance from their health-care professionals:
I knew I couldn’t do anything about it and I was fine but I asked the doctor, the doctor said, ‘No. It’s fine.’ So I said, ‘OK, so . . . yes.’ I thought, ‘OK. I can cope with it.’ [. . .] He was not concerned at all I would say, really at all [. . .] but if I think about it then I get a little bit scared, because I say, I don’t know, yeah, I was really all right? Was it true? It was true for sure but I don’t have any means of comparison, so I don’t know if that is really the right measurements or is not the right measurements for that gestation . . . like . . . but I’m trying not to think about it [small laugh].
SC4-Cx29
Not all of the women were happy to be in the control group, although efforts were made to reassure them, as the following quotation illustrates:
[The midwife] did tell me that the cervix, it changes all the time. Erm, and really, at that point I was a bit disappointed, ‘cause I was thinking, ‘right, OK, now I’ve had that scan, and I know, really now, that my cervix is short, you know, and the babies can come at, kind of like, any time.’ So, it was a bit worrying, but she did sit me down, and we did have a good chat. Erm, and she said, you know, if I wanted to speak to the consultant who could explain it more and stuff. And I said, no, ‘it’s fine, you know, I knew from the start what I was getting myself into, and I knew what the process was’ [. . .] I did, I really wanted that pessary [laughing] I did talk to her, and I told her that I was really disappointed.
SC8-Cx24
Although staff were very conscious of women’s feelings, women found it difficult to imagine how they might feel as participation in the trial progressed, as the following quotation demonstrates:
. . . the research midwife did say to me, like, you know, how does that make you feel, to which the answer was it didn’t really make me feel anything because I don’t . . . I just wasn’t expecting to . . . I wasn’t really expecting, I hadn’t thought about what length my cervix might possibly be.
SC3-Cx27
Other participants seemed to take the news of assignment to the control group in their stride, especially when they felt that their pregnancies were going well.
Some women’s accounts of being randomised to standard care were bound up with their motivations for participating, invoking an altruistic discourse of helping, although they were not receiving the treatment themselves:
I came out in the controlled, don’t do anything group, which was fine because, you know, I then still feel like I’m sort of helping in some way [laughs], you’re at least part of the study, which will hopefully help people later on if this technique works.
SC3-Cx27
Others appeared to regret that they had not been randomised to the intervention group and, despite understanding the nature and purpose of the trial, they would have preferred to have been ‘doing something’ to support their pregnancy and the research:
I just feel it was a little bit unfair, because I was willing, and I wanted to do it, I felt like, you know, maybe there should have been . . . I, I can totally understand why it was randomised, and you can understand that feature of it, because it makes it totally anonymous. It makes it, even it makes it credible, you know, for whatever hospital, or whatever place it’s taking place in. But I just felt like, I actually wanted to do it, and I felt like if there were a chance, an opportunity, for somebody who actually wants to do it, then they should be able to take part in the study.
SC8-Cx24
The effects of randomisation had to be managed. Sometimes women in the control group were offered further monitoring, involving repeat TVU and what one participant described as the ‘swab test’ (SC3-Cx27). One participant described a situation whereby a TVU 4 weeks after the original one suggested that her cervix had shortened. As this was worrying, a further swab test was carried out, which was normal, and she was given instructions to return in 2 weeks. She interpreted this situation as positive, in that although she had been randomised to the control group she was still receiving care. Another participant received the news that her cervix was 21 mm, which was not what she had been expecting to find out. When she was randomised to the control group the obstetrician discussed the three options of treatment with her:
We could have a stitch put into the cervix or we could try progesterone pessaries that we put in each day, and we could come back in a week and have it rescanned and just see what had happened. So, we decided on the progesterone pessaries and to come back in a week and have it rescanned. She said, so you will be in the trial. I was quite surprised then, because I just expected it to come back that it would be longer and that I wouldn’t be in the trial. We carried it on until 30 weeks.
SC2-Cx21
The participant went on to explain that, when the consultant saw her the following week, her cervix had increased in length to 29 mm. This news surprised her and made her doubt the first measurement. She continued with the progesterone pessaries until she ‘ran out of them at 30’ (SC2-Cx21).
At the stage of receiving the results from randomisation, very few participants described feeling concerned:
I suppose . . . I began to worry a little bit about having a pessary, just thinking, after, ‘well, if I am randomised to have it then I’ve got to come back in to have it fitted.’
SC9-Cx26
Although women’s reactions to cervical length measurement and randomisation varied and results and participation were interpreted contextually, many women in both arms of the trial noted and appreciated the additional support they received from RMs. Most often this support took the form of access to a RM via a telephone call if they were worried about the well-being of the pregnancy. This participant’s comment was typical of many of the women’s feelings:
. . . the care received from the people at the trial has been great, because when I was having problems, I was able to call someone. And the research midwife was very good, she’d always answer the phone or call me back even in the evening, and arrange pretty quickly for me to be able to go back in and be seen when I needed to be.
P9-Cx25-P removed
Experience in the intervention group
Reaction to the pessary
Nine of the 20 women in the intervention group of the study talked about what it was like seeing the pessary for the first time. One participant remarked ‘we just had a look at the leaflet and we’re like, what! It’s such a huge pessary, it’s really weird’ (P14-Cx33-P removed). It seemed to be hard for women to imagine the device inside their bodies. One aspect of their concerns was that, despite reassurances from the health-care staff about the pliability of the device, the women remained sceptical about whether or not the pessary could be passed through the vagina to reach the cervix without causing significant discomfort.
Seeing the pessary for the first time also seemed to make some women waver a little about their decision to take part, as the quotation below implies:
After they spoke to me, I was already considering could I do this? And then I agreed to it, because I thought, ‘yes I could have a pessary.’ And, because that was the only thing that was worrying me, was having a strange object in my body. And I wasn’t sure [what] it would be doing. But that’s what we were trying to find out, so I’d already decided in my head that I wanted to do it, and have the pessary. So, I suppose, yeah, I was already prepared in my head that it was going to happen.
P13-Cx33
Some women also found it difficult to imagine that the pessary would not interfere with bladder and bowel functions, and others could not imagine how it could be fitted:
. . . straight away, as soon as they gave me the information, the leaflets we were reading. They got a sample one out and showed me it. You were able to look at it, feel it. I was like, ‘oh my god, how do they get that in?’
P12-Cx23
Of the nine women who talked about their reaction to the pessary, only one participant took a more matter-of-fact approach to the pessary. This participant drew on her experience of using the menstrual cup and therefore seemed to have little difficulty in imagining the pessary being fitted inside her body:
She just showed me the thing and then she just inserted it.
P15-Cx31
Most women, therefore, had to overcome some concerns evoked by seeing the pessary or imagining it in their bodies.
Pessary fitting
Overall, the women’s descriptions of the procedure varied considerably on a continuum from ‘a wee bit uncomfortable’ (P20-Cx25) through to ‘insanely painful’ (P8-Cx33-P removed). Fourteen of the 20 women described the experience using words such as ‘uncomfortable’ or ‘slightly painful’, whereas six women described it as ‘very painful’. Some described how they needed emotional support from the RMs to help them relax:
It was probably, I’d have to say, a little bit uncomfortable when they were just putting that in but, you know, I had the lady that was there for me . . . that was there with me from the beginning. She was holding my hand. You know, she was just saying to me, ‘don’t tense up, it’s going to hurt more, you know, relax.’ She was keeping me relaxed.
P2-Cx28
For some women, the concerns that they had about the size of the pessary may have made the challenge of ‘relaxing’ during pessary fitting difficult and in these instances participants tended to blame themselves for the discomfort they experienced during pessary fitting.
In trying to make sense of the pessary fitting, women often used experiences of TVU or cervical smear testing as a baseline for comparisons:
A couple of doctors came in, they showed me what the pessary was, they told me what they were about to do, and then they . . . they basically fitted the . . . the pessary. Again, a procedure similar to that that you’d have as a . . . as a smear test is the only thing I’ve thought, you know, that I can really compare it to. A wee bit uncomfortable, not the most pleasant experience in the world but nothing, you know, not . . . not outrageous.
P20-Cx25
Although this participant found the experience no more than ‘a wee bit uncomfortable’, women’s accounts of the fitting procedure demonstrated wide variation in relation to how this was experienced. One woman described it as ‘one of the most uncomfortable experiences I’ve gone through’ (P9-Cx25-P removed). Another participant described this as ‘being put through the mill’ (P18-Cx25). By contrast, others evaluated the procedure as ‘a lot quicker and easier than I thought’ (P13-Cx33).
Some of the women also expressed gratitude that no instruments were involved in fitting the pessary:
I was happy with it, because he didn’t use any devices, as far as I could see, he put it in with his hand. So in my head, I thought, well, it can’t be going that near the babies if it’s only going as far as his hand can reach. So that did actually reassure me that it wasn’t . . . you know, it was that way and, therefore, it couldn’t have been near them. So I think it was actually . . . it actually reassured me to know how he was putting it in.
P4-Cx31
For others, the involvement of the doctor’s hand was disturbing:
Cause it’s someone’s hand, that’s the thing, as opposed to, you know, when they are measuring your cervix, you know. I was surprised when someone putting their hand, you know [laughs]. So, that was probably, probably a bit sore. [Participant’s husband’s name] had already left at this stage.
P18-Cx25
Dealing with pessary insertion required ‘emotion work’ on the part of the women to relax and, as discussed in Supporting women through trial processes, from the health-care professionals involved.
Living with the pessary
Vaginal discharge
Some women were concerned about the quantity and nature of vaginal discharge they experienced. One participant remarked:
. . . it’s just not what you think a discharge would be [. . .] it seemed to not create discharge throughout the day [. . .] and then the discharge all comes at once.
P7-Cx34
She asked herself ‘is it definitely my pessary or could it possibly be my waters broken?’ Other participants questioned whether or not they were leaking urine.
Women who experienced increased discharge frequently sought medical advice to reassure themselves that what they were experiencing was a consequence of the pessary rather than a leak of amniotic fluid. It was not so much the quantity but the fluidity of the discharge that was concerning to women, for example:
. . . it’s so watery sometimes, you get that feeling that it’s fluid that’s leaking or something.
P19-Cx31
After seeking advice and undergoing a test to establish the nature of her discharge, this participant later explored the possibility of purchasing her own home testing kit. When she discovered that this would be difficult, she sought reassurance at her fortnightly ultrasound examinations, saying ‘the only reason why I’m really calm at the moment is that I had my water levels checked on the scan’.
Other participants who experienced vaginal discharge noted that had they known that the discharge they might experience would be ‘watery’ in consistency they would have felt less anxious about it. For example, this participant said that she knew ‘the discharge would be increased, but I’ve not been really told that it might look so much different in the consistency’ (P19-Cx31).
One participant described a situation in which she had been worried about low abdominal pain and vaginal bleeding and on arriving at her local hospital encountered staff who had no experience of the Arabin pessary:
I was 27 plus six and just all of a sudden I felt some pain, which felt similar to getting your period, and so now not pregnancy-like pain, so I got a bit suspicious about that. I went to bed and just waited if it would disappear, well, it didn’t, and then I had some bleeding additionally, so we took a taxi, went to the hospital, the new hospital then, which is nearby, which . . . where they hadn’t heard of the pessary before, and it turned out that I lost some water and blood, so they had to remove the pessary just because it turned into a risk of infection. And then they just monitored me and the babies for 3 or 4 days, but since there is no additional water and no additional blood, they said fine to go home now, and just take it easy.
P14-Cx33-P removed
Another participant experienced a similar situation in which she encountered medical staff who were unfamiliar with the pessary:
I was at home and I’d felt like a gush of water come out, [. . .] it was quite a lot, sort of had a panic and then it happened again, more came out. So I rang the midwife, I was quite upset because it was quite a lot and I explained what had happened and she said to come in. [. . .] they put a speculum in and when they put the speculum in it all filled up with water and loads of water came out and they said, ‘yeah, your waters have gone,’ [. . .] so it was like a big panic. But then they’d spoken to somebody else [. . .] was familiar with the pessary – this is after, sorry, they came back and said, ‘we’re going to take your pessary out,’ they removed it, but then another consultant had started a shift and he’d researched it a bit more and said that it was common with the pessary, could be the build-up of fluid and things. [. . .] I’ve not had any leaking since so it must have been the pessary.
P10-Cx33-P removed
The thought of having the pessary removed was on the mind of some women, especially those who had found insertion painful. Some women talked about seeking information about removal from internet forums where the topic of pessary removal had created a good deal of interest among women with a pessary:
I read that people find it a bit uncomfortable, but most of them were actually more afraid of having the pessary removed.
P14-Cx33-P removed
I Googled [Google Inc., Mountain View, CA, USA] it and then I found on BabyCentre they had forums other women had posted on, but I could also find the study online and I could read about it online, so I was like, when do they take it out, what happens if it needs to be taken out early, you know, could that compromise your cervix or anything.
P10-Cx33-P removed
In addition to these two women, two others had the pessary removed, both because they found that it was uncomfortable and interfered with their daily activities.
Sexual relations after pessary fitting
Women varied in their experience of and approach to sexual relations after the pessary had been fitted. Only a few described no problems in maintaining their usual sexual relations after having the pessary fitted. One described a situation in which she had worried slightly about whether or not her partner would notice the pessary during sexual intercourse:
My husband said he thinks he’s noticed it, but he’s not . . . but again almost not sure. If he can feel it it’s not every time, and it’s not a barrier.
P3-Cx33
Other participants had tried to continue with sexual intercourse, but discontinued because of a fear that doing so posed a risk to the pregnancy:
I think we both were aware of it, and also I’m so fragile, the whole experience, that we wouldn’t risk it, I think, so we were again very careful.
P14-Cx33 removed
Others made their decision not to have sexual intercourse after they had the pessary fitted: some worried that it would induce labour, some decided that it was just something to avoid (a kind of sacrifice) and others had made a decision to abstain from sexual intercourse at the beginning of pregnancy for fear of inducing a miscarriage, as the following woman describes:
We haven’t . . . I’ve not had sex for. . . well since like 8 weeks pregnant or something.
P12-Cx23
On being fitted with the pessary later in her pregnancy, this participant goes on to say that, although the risk of miscarriage had passed, contemplating sexual intercourse was ‘just too scary. I just can’t . . . I couldn’t do it. I couldn’t . . . yes, it would just be weird I think. Oh no, there’s a big blue pessary in there . . . I’m like, oh, I couldn’t do it, it’s so weird’ (P12-Cx23).
Only one participant said that she had been advised to abstain from sexual intercourse:
I was actually told, because of my risks of preterm labour, and stuff, that I should try and abstain from it. So, from the beginning I have stopped because I didn’t want to risk, you know, going into preterm labour, or anything. So, it wasn’t only . . . it wasn’t the pessary that stopped me from doing that. It was from beforehand even where, you know, midwives, and even research that I’d done myself which said, you know, with a twin pregnancy they do, you know, recommend that you get advice about, you know, sexual activity, and stuff.
P2-Cx28
Some talked about the pessary affecting their or their partner’s experience of sex and a few said that more information would have been helpful, especially if they were trying to maintain their sex lives:
. . . the information does say that you can still have sex. But because it felt so low down I just felt like that wasn’t an option. So for quite a few months it just . . . I think it’s probably mind over matter. But . . . just didn’t want any intercourse at all to be honest. And then as I’ve settled down we have sort of managed to have . . . to do sort of a little bit. But it’s still different. It was still very conscious that there’s a pessary . . . if he knocks it or . . .
P7-Cx34
I think it’s incredibly important, throughout pregnancy we’ve been intimate, the whole way through, there’s not a moment where we haven’t been at all, or not felt like we wanted to, because yeah, I think it’s something that’s important to us, to stay close [. . .] [it] helps you through the harder times as well, when things are difficult. So you just remember that we’re doing this because we really want to do it, and we want it for our babies, but we were really happy before this as well, and going through it all, it helps if you feel close and can support each other.
P13-Cx33
Findings: health-care professionals
Overall, the health-care professionals interviewed were supportive of the trial and hoped that it would prevent preterm births:
I would quite like it if the pessary did work. And we could prevent preterm births.
RM1
The need for good evidence was considered important, and this trial was viewed as well designed:
. . . a well-designed study [. . .] [answering] a very good clear question, [without] the bias issues in other [similar] trials that have been done.
CRF1
As one RM noted:
I just think it is something that’s so worthwhile investigating. So, erm, to, to me, if it was me that was in that situation, I, I would . . . and was, you know, randomised the pessary, I would take that without a shadow of a doubt. So I think if you feel enthusiastic about something – not that you push anything onto anybody.
RM1
Likewise, enthusiasm from clinicians was considered important – the ‘doctor effect’ on recruitment:
I wonder if they get the feeling of the consultant supports the study.
RM7
However, a research culture and integrating trial protocols into a busy clinical environment require careful management and resources.
Trial processes
Motivation and commitment are required to enable a trial to work and this commitment has to extend beyond the trial staff. As the following interviewee noted:
. . . it requires a sequence of events that probably . . . than you have to be really, really motivated to make it . . . to deliver the trial [. . .] we manage to do it because we have certain things that pull together, but we also have a number of staff who give their time, that are not paid [. . .] you know, they’re using that time . . . time that they could be doing other things.
CRF1
Much thought had gone into integrating trial protocols so that neither women nor centres had additional work/appointments. In the bigger centres, staff talked of carefully negotiated logistics, involving both the research teams and the clinical areas working together. Many of the RMs identified the successes they had in recruitment to be partly influenced by having the support of clinical staff who were keen to promote research.
There was also evidence that trial centres adapted care pathways to accommodate recruitment. For example, in the centre where RM8 worked, women with dichorionic diamniotic pregnancies were offered an obstetric appointment at 16 weeks rather than at 20 weeks. Behind-the-scenes planning involved high levels of communication and a commitment to making time for the trial in the context of other equally pressing commitments. Commitment to research and evidence-based practice seemed to shape the trial’s success, but access to resources was also crucial:
. . . there’s only two of us who do accredited cervical length scans, so actually that burden falls on people who aren’t actually directly having STOPPIT in their job description or in their job planning. So, there’s that motivation from them and that’s because the unit, as a whole, thinks that research is important and wants to take part [. . .] Then, the people involved in putting the pessaries in, there’s me and . . . all the other doctors who run the twins clinic [. . .] but when they’re in a busy clinic, they still . . . even if they’re over-running in their clinic and there’s a woman waiting for a pessary insertion, they don’t . . . they still come and do it, if that makes sense? They’re still committed to creating time or making space, for those women to have the process.
CRF1
Building a research culture requires understanding of the research process and roles of RMs in that process. Some RMs felt frustration that midwife colleagues misunderstood the role of the RM as ‘sitting up there and doing all research and statistics all day’ (RM6), whereas RMs saw their role as more clinical, dealing with the complexities of talking to people about research and adapting communications for each patient. As is evident from the accounts of women participating, the information and reassurance provided by health-care professionals was influential in decisions to participate and in women’s experiences of the trial process.
Recruitment of women to the trial
In most centres, the recruitment was led by the consultant and the initial introduction to the trial was during the first obstetric antenatal appointment. When recruitment was described as a smooth process, it was frequently reported by midwives to be because of ‘flexible consultants’ (RM7) and good research team communication and organisation [relating to identifying potential participants, accessing resources when required (i.e. scanning rooms) and the means to access clinical staff who could perform the scans and fit the pessary while women were attending antenatal appointments]. With the woman’s permission, a RM contacted a woman by telephone after she had considered participation:
[CO] is the one that approaches. She sees them at the, erm, our twin clinic. When they’re scanned, and they’re diagnosed, [CO] sees them. She speaks to them about the study, . . . gives them the patient information leaflet, then . . . tells them that I’ll follow it up with a phone call. She gives me a date and time, and I then phone, answer any more questions that need to be answered. And then, they, if they want more time to think about it, that’s fine. If they’re happy to go ahead, then I give them the date and time. If they decline, then that’s fine as well.
RM1
In centres where women attended specialised twin clinics, RMs’ accounts suggested that recruiting from a specialist twin pregnancy clinic was more straightforward than trying to identify eligible women across several clinics. In centres with no such facility, analysis suggests that midwives experienced additional workloads, in that more clinicians were involved in recruitment and the RM spent time reminding clinical staff should an eligible woman be attending a clinic. RMs said that recruiting from a specific clinic per week also helped with the logistics of pre-screening potential participants prior to the clinic beginning, making them feel fully prepared with all the information they required to ensure that the process was as smooth as possible. One midwife contrasted STOPPIT 2 with another trial, saying:
. . . you feel like you’re ready . . . rather than getting a phone call saying, ‘can you come down now because this women’s here, we’re going to consent this woman’ . . . and you’re grabbing all your bits and pieces and running down and you’re think, ‘oh, I don’t know anything about her, I’ve not had a chance to look on the computer.’
RM6
Some of the data reflected a high level of concern for women’s feelings throughout the trial and particularly in relation to the type of information given to them in the early stages of pregnancy:
. . . we tried for a little while [to recruit] [. . .] at 12 weeks [. . .] but we felt in the end that that was a bit too early [. . .] Because it is quite a big thing to take in, isn’t it? [. . .] we don’t want them to Dr Google lots of stuff necessarily.
RM8
Despite the care some centres had given to ensuring that women were not inconvenienced by additional clinic attendance and the care given to considering when it was appropriate to discuss the trial, some RMs reported that it was a ‘hard sell’ (RM8) when trying to recruit women. Some noted that women were sometimes reluctant to increase their involvement with the clinical setting and/or found the notion of cervical length measurement and the fitting of the pessary unacceptable:
They absolutely cringe at the thought of the pessary but I would say probably most of those are probably primips. I think it’s an easier sell maybe to multips who have already had babies, probably already had a smear, you know, are used to that kind of gynae stuff, maybe. Whereas like an 18-year-old, first pregnancy, twins, I mean, firstly they’re in utter shock, aren’t they?
RM8
Many of the RMs felt that, as women had a high level of contact with maternity services because of the high-risk status of their pregnancy, some women were reluctant to participate as they were concerned that doing so would increase their level of clinical contact. Often this was expressed in simple terms, for example: ‘[women] don’t necessarily want extra visits’ (RM8). Some centres had taken significant steps to mitigate this issue by reassuring women that involvement in the trial would not incur additional appointments, although appointments may be extended because of screening and pessary fitting, etc.
Analysis suggested that one of the ways that some of the RMs helped to support the recruitment of women was by viewing participation incrementally, starting with screening. Some talked about offering support by stressing the ongoing voluntary nature of participation:
. . . we go through everything, and I say, we will talk about that again, if you have a short cervix, I make the point that we will address that again.
RM7
This particular midwife viewed the flexibility offered by the trial design as an important means by which to meet her own and the women’s emotional needs, as this quotation illustrates:
. . . it feels much more comfortable for me, being involved in this, that we can say – ‘yeah, you can see how you feel, you don’t know how you might feel until it happens, about having a short cervix.’
RM7
Relatedly, when considering issues to do with recruitment and retention of women, the trial was viewed by this midwife, and others, as particularly good in that there was no pressure to ensure continuing participation:
From a target point of view, if anyone agrees to have the initial scan, and then they chose not to be randomised, we still get the accrual for that, so we haven’t got the pressure of the figures, to encourage you.
RM7
The study design, therefore, seemed to support the midwives, who felt concerned about women’s reaction to cervical measurement results and ongoing participation. Cervical measurement was conveyed as providing useful information in itself.
Barriers to recruitment
Analysis has already alluded to why, from the RMs’ perspectives, women might not want to participate, and how both clinic factors and their own communicative practices could support recruitment. Focusing further on perceived barriers, RMs felt that they encountered barriers at the level of language, despite the availability of interpreters (sometimes a family member), understanding of trial processes and of the intervention itself:
I think that quite a lot of the women . . . hear the word research and then they think it’s going to be something that, ‘oh, I couldn’t possibly understand that.’
RM6
It was difficult to explain the concept of internal scanning and to ascertain whether or not women had understood the nature of the procedure. Some midwives felt that women chose not to participate because they had concerns about TVU scanning. One midwife describes the point of explaining the scan to women as the point that women say ‘no, I don’t want it’ (RM6). Others said that they suspected women were concerned about the effect of TVU on the pregnancy:
. . . maybe they worry that having a scan is going to stir things up and maybe cause them to miscarry.
RM8
I think it’s a pretty well-run trial. And, erm, it, it’s probably not the easiest to, erm, approach women on, because of the intimacy things . . . and things like that. But I, I do think, as time goes on, everyone’s gaining experience in it.
RM1
The size of the pessary was also considered a difficult aspect to manage:
I felt that people might be quite unwilling to accept the intervention [. . .] The pessary, it’s the pessary itself, um, I felt was quite big . . . and sort of bulky-looking, and I thought . . . I don’t . . . I don’t know whether to show it to people, whether not to show it to people. Um, did a bit of experimentation with that, er, and, I mean, I was still learning, I’m still deciding. But I think with the majority of people, it’s better not to show it at that initial chat.
RM3
The RMs worked hard to build their own confidence in talking about the pessary. After visiting a centre that was more familiar with the device, RM3 noted that:
. . . it’s interesting because you notice a spike in recruitment. And em, that’s because I was more confident then, telling people about the trial and selling the trial, and selling the . . . the Arabin pessary as a real potential for preventing preterm delivery.
RM3
Supporting women through trial processes
As noted, trial participation depended on several inter-related factors, including clinic logistics, health-care professional commitment and reassuring communication at every stage. Midwives also discussed how they found their own role in STOPPIT 2 enjoyable, as they had increased contact with ‘high-risk’ women over the course of their pregnancies:
. . . you do get continuity, because you’re at the antenatal clinics. So, when you recruit somebody, or you’ve given them some information, you, you see them quite frequently.
RM1
. . . you have more time to spend with women and . . . as . . . in a midwifery role, it’s always rushed and you spend your entire time saying, ‘I’ll be with you in a moment’, and apologising, but it’s nice in research to have, um, to spend the time . . . with the women, um, and have time to talk to them, and because obviously they always . . . they know you’re a midwife, they always talk to you about other issues as well and ask you questions that are midwifery related, um, so it’s nice to have that time to spend with them.
RM2
Overall, RMs’ accounts of communicating the results to women demonstrated that they felt concerned about how women would respond to the news. One way in which they supported women, should they be found to have a shorter cervix, was to prepare them in advance through a carefully planned dialogue, whereby the information that would reassure women was ‘planted’ at an earlier stage of the trial process, linking the meaning of the measurement to the trial, as the following quotation illustrates:
Even those that have been found to have the short cervix, none of them have been particularly fazed about it [. . .] I think the way they’ve been counselled – ‘So, yes, you will have a short cervix by definition for the study but actually if it’s not less than 30, do you know what I mean, we would consider that normal by our normal standards’. However for the study it’s considered short so I think maybe they’re a bit more reassured.
RM8
Health-care professionals found that responding to women and discussing the results of cervical screening measurement involved a high level of emotional engagement and attention to an ongoing awareness of women’s emotional response to the information, as this quotation suggests:
I do it case by case really, see how they react, and how much reassurance they’re going to require.
CM1
Cervical length measurement provided reassurance to the midwives, as well as to the women:
. . . the vibe is after a scan when we have a long cervix, the consultants give a feeling of, yeah, there’s a reassurance, yeah. Although they do clearly say, as well, ‘we wouldn’t expect you to have a very early preterm delivery, but we can’t exclude it.’
RM7
Staff also felt that women sought participation as a means to gain reassurance:
. . . they want the reassurance of knowing that the cervix is alright.
CM1
Like the women, many of the midwives also expressed the notion that taking cervical measurements was a useful screening tool. On the one hand, taking a measurement meant that women could be reassured if they were found to have a long cervix, whereas, on the other hand, women with shorter cervices could be offered ‘serial scans or more regular appointments, or you know, you might be able to do something a little bit more to reassure those ladies who are found to have a short cervix’ (RM4).
There was also a perception that women were curious about their cervical length, but not as keen to be randomised to receive the pessary:
I think a lot of women, possibly, are interested in finding out their cervical length, but may not be quite so interested if they’re randomised to get the pessary.
RM1
Randomisation was identified as a point in the study when RMs felt that they had to exert caution in terms of the information and reassurance they offered women, not least because women with a short cervix would be worried if they were randomised to standard care:
. . . you have to be very careful how you word things with the ladies that don’t get randomised to the pessary, you know, because often they will be quite anxious and you have to reiterate the fact that the reason we’re doing the study is to find out if this pessary is effective.
RM4
Some of the RMs raised concerns about how they imagined women might feel about being randomised to the control group. When asked about how she would feel about a woman with a cervical length of 25 mm being randomised to standard care, one RM said that even if the woman was unconcerned she would feel compelled to check on the progress of the pregnancy:
I’d be thinking, ‘oh my God.’ I’d probably be thinking, ‘yes, I’m definitely going to be ringing you every 4 weeks,’ but you see if she hadn’t gone into the study we would never have known. Even if she did show signs of preterm labour at say 25 weeks, yes, we might discover she’s got a short cervix on speculum but potentially if it wasn’t for the study we wouldn’t know.
RM8
This account suggests that the RM draws on the logic that knowledge can potentially ‘make people extra vigilant if the woman felt something was amiss’ (RM8).
Reassurance could be offered in several ways, including giving direct emotional support:
The second lady who got randomised for control, um, she really wanted the pessary, she said, ‘um, so that wasn’t, you know, that wasn’t very nice.’ I think I gave her a hug and said . . . and made sure she was OK, and said, ‘do you feel OK,’ and she went . . . and she said that yeah, she felt really gutted that she didn’t get it, um, but she was OK and she under . . . and she went, ‘I know, I understand, you know, um . . . that that’s just the way it goes, I understand it’s, um’ . . . I think she, you know, she understood about, like, the randomisation, really, so she was . . . you know, she under . . . I think she said, ‘I understand that you know, it’s not your decision,’ um, so, um . . . yeah, that was OK as well [. . .] I was more worried how she would go home and react to it.
RM2
The RMs anticipated women’s concerns and sought to smooth their experience of the trial and their pregnancy. This required optimising their own strategies, learning how best to provide information, seeking support from the trial team, if necessary, and adjusting their practice accordingly. One RM noted that she altered what she said to women about pessary fitting based on her observations of the fitting procedure, from being ‘quick and easy’ to ‘it can be quite uncomfortable when it’s being fitted’ (RM4).
The RMs’ concerns extended to those who did not continue their participation, especially after the TVU had identified a short cervix, anticipating that the woman would feel guilty should anything happen, something some of the women interviewed noted as a reason to participate. RMs also expressed relief when women with shorter cervices were randomised to the intervention group, as this quotation suggests:
. . . some that have been around 24, 26, who have been randomised to pessary, so it’s kind of almost a relief when I’ve found that, and they have been randomised to pessary, you think, oh well that’s good.
CM1
Conclusion
The experiences of both women and health-care professionals involved in the trial suggest that research is experienced and enacted in the context of care. This is manifested in different ways, from adapting clinic routines to minimise the impact of trial participation on women and to support recruitment, through providing information and reassurance, to ensuring follow-up care for those randomised to standard care. The ever-present risks inherent in twin pregnancies are amplified for those with difficult obstetric histories. Cervical measurements and pessary fitting are deeply embodied experiences: women think about the impact on their bodies and their fetuses and are mindful of their decisions on their overall experience of their pregnancies, including ensuring that they are doing everything they can while not thinking too far into the future.
The communication of information to women, and how this is taken up and used by them, is the result of careful assessment and crafting by the RMs. They talk about how they assess each woman’s need for information and likely reaction, yet also are honest about their own initial reactions to the intervention and to their role in promoting it.
Trials require all sorts of labour to ensure that they progress. This work extends beyond the trial team and encompasses institutional culture, clinical support, midwives’ caring practices and women’s own cognitive and emotional labour. All of those interviewed were participants in the trial, and most had thought with their ‘head’ and ‘heart’ about participation. This blending of ‘objective’ information with affect and care characterises the experience of the trial from both the women’s and the health-care professionals’ perspectives.
Chapter 6 Patient and public involvement
Aim
The aim of STOPPIT 2 patient and public involvement (PPI) was to set up two Patient Advisory Groups (PAGs) (one in Edinburgh, led by SCB, and one in Exeter, led by Dr Andrew Gibson). The Edinburgh PAG was a new group and the Exeter PAG used the existing expertise of the Applied Research Collaboration South West Peninsula (PenARC, formerly PenCLAHRC), with the idea that each of the PAGs would contribute to the development of study documents and advise on the project direction and on publishing the project to potential beneficiaries.
Independent PPI representatives (not members of the PAGs) were also planned as members of the study oversight committees: the TSC and the Trial Management Group (TMG).
Methods
Patient Advisory Groups
Following several face-to-face and teleconference meetings in December 2014, it was decided that the most efficient and cost-effective way of managing the PPI would be for Dr Andrew Gibson (collaborator) to run one PAG, consisting of approximately eight members and meeting in London every 6–9 months. However, Dr Gibson left the project team (April 2015) to take up a new post and Dr Kath Maguire of the PenCLAHRC PPI team agreed to take on the role.
Dr Maguire determined that she would use the PenCLAHRC group and set up a PAG using the existing members who were located throughout the south-west of England. Dr Maguire recruited six members to the STOPPIT 2 PAG and provided each member with training about trial processes to facilitate discussion of issues identified as being key to a particular stage of the project. For instance, contribution to development of the patient information sheet and consent form, with suggested strategies to improve the acceptability of the intervention. It was also agreed that the group would help develop lay summaries of the research and a plan for dissemination to service users and families. Between the face-to-face meetings, the group received e-mail updates about the progress of the project.
Trial Oversight Group patient and public involvement representation
Jane Denton (a study collaborator) was also the Multiple Births Foundation (MBF) (London, UK) representative on the TMG.
Independent patient and public involvement
The Twins and Multiple Births Association (TAMBA), now known as Twins Trust, provided Mr Keith Reed as a member of the TSC.
Members of our TMG, TAMBA and MBF offered to assist in nominating and identifying individuals for the TSC. Initially, four parents volunteered to join the TSC and each had a lived experience of either preterm birth or twin pregnancy within the last 10 years. It was clear that the commitment and training required was too much for many of the volunteers. Two volunteers declined to participate and after a year one member withdrew. However, Mrs Andrea Hall provided continued support throughout the project as a member of the TSC.
Jane Denton, Keith Reed and Mrs Hall each offered extensive lay and patient involvement experience, acting as the voice of women with experience of twin births. They were involved in the design of the study, including the protocol, patient information sheets and the development of strategies for participant communication.
Study results
Outcomes
The PAG met regularly between September 2015 and September 2017, contributing to the development of the participant information documents. [For more information please see NIHR Journals Library, URL: www.journalslibrary.nihr.ac.uk/programmes/hta/130422/#/ (accessed 22 April 2021).] During the process of determining the need for an extension to the study, Dr Maguire informed the TMG that she was no longer able to continue to support the PPI on this project. After moving to Cornwall, Dr Maguire had found it difficult supporting the PAG, but this became even more difficult as she was no longer working with the groups who previously attended workshops and the continuity of the relationship could not be maintained.
Funding for the PAG was re-allocated to Edinburgh and the team determined that a new advisory group was required. The Edinburgh trial team implemented a number of strategies to engage the public and set up a new PAG. A request for volunteers was placed on the Edinburgh Tommy’s Midwives Facebook page [Facebook, Inc., Menlo Park, CA, USA, URL: www.facebook.com/tommysmidwivesedin/ (accessed 23 April 2021)]. TAMBA and the MBF were asked to see if any of their members would be willing to become PAG members. We initially received 13 responses; however, only three women engaged in further conversations and did not want to commit to anything on a regular basis, as they had young families and work commitments.
The STOPPIT 2 group felt that it was important that patients were supported to build confidence, facilitate effective communication and enable involvement to represent a true partnership between research and its participants. PPI is a crucial part of this support to ensure relevant and acceptable research goals. Funding was allocated for PPI from within STOPPIT 2 and from QUIDS [Health Technology Assessment programme (UK) REC 17/WS/0081 ISRCTN41598423, see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/143201/#/ (accessed 23 April 2021)], a trial that was also struggling to have effective PPI representation and so resources were jointly pooled. The STOPPIT 2 trial administrator was asked to be the co-ordinator for the PAG within the Medical Research Council (MRC) Centre for Reproductive Health (Edinburgh, UK), assisted by the QUIDS study administrator. The group was renamed Patient Involvement in Clinical Trials Research (PICTR).
Recruitment for the proposed group took place at the Baby & Toddler Show, SEC Centre, Glasgow, UK, 27 April 2018. A total of 162 people agreed to sign up to become part of the PICTR group. This included 34 men, some of whom were very keen to be involved at all levels, including being part of a grant application. One man informed us that we were the first people to have asked for his opinion about any aspect of pregnancy and childbirth, as all the focus had been on his partner. The group seemed keen to share the knowledge they had gained from their own experience of pregnancy and birth, and were pleased to be asked to participate in an advisory capacity.
Initial contact was made with the PICTR group by e-mail and further correspondence was conducted in this way. This included asking for member input concerning various research questions for the study plus other projects. Regular newsletters were also sent. To engage with members of the group in person, it was decided that all the volunteers who had become part of the PICTR group should be invited to a meeting in Edinburgh Royal Infirmary, Edinburgh, UK, on Friday 17 August 2018 to ‘meet the researchers and their teams’.
From the substantial list of names, of which around 20% lived in, or around, Edinburgh, we had one definite acceptance. It was clear that this format for discussing research is not suitable for parents with small children. We were asking parents for a commitment at one of the busiest times of their lives (i.e. bringing up small babies and children). This initial meeting was organised for a Friday afternoon within a hospital setting. Owing to the poor response to our invitation, the proposed meeting was cancelled and we discussed further what other opportunities could we possibly offer parents with small children to facilitate easy attendance at any kind of organised event.
It was decided to hold a children’s party, with refreshments and children’s entertainment laid on, so that the study researchers could talk to parents directly. We also felt that this kind of event was giving something back to parents in return for their involvement in the PICTR group. The party was organised to take place on a Saturday afternoon at a local church hall with excellent facilities.
Patient Involvement in Clinical Trials Research party (Edinburgh, January 2019)
The invitation was extended to all members of the PICTR group and initial acceptance was good (14 responses). Actual attendance totalled 10 parents bringing seven children, who varied in age from 7 months to 7 years. Members of the research team attending were two professors, with a PhD student and medical student also attending. It was possible for each researcher to speak individually to the PICTR members.
All research staff involved in reproductive health were also invited to attend, but their acceptance was limited. It appears there is a disjunction between the ability (or willingness) of both parents and staff to attend arranged events, depending on the venue and timing. It became clear that the informality of a children’s party is a much more sympathetic space in which to discuss various reproductive health issues. One mother told us that she did not wish to come to a meeting to sit in a circle while being expected to speak, as this would be too intimidating. A meeting, possibly including presentations, on a weekday within a hospital setting is perhaps not ideal and too formal (although this suited the research staff). Parking at the hospital is also limited and expensive.
The parents who did attend the party were very keen to become involved in advising on our reproductive health research. We subsequently wrote to each parent individually with information about the ongoing trials in the Centre for Reproductive Health. We consulted with the group on several issues concerned with our research.
Overall, our resource has identified PPI representatives to act:
-
on the TSC for Pravastatin For The Treatment Of Preterm Labour (PIPIN) study
-
as a co-applicant on a grant to investigate whether or not delaying removal of long-acting reversible contraception in obese women may reduce weight prior to pregnancy
-
on the Trial Oversight Committee for the CASSAVA trial.
Discussion and reflections
It is beneficial to have an ‘end-user’ group that can give honest advice about the practical impact of the minutiae involved in the trial requirements and procedures. Conversely, it is frustrating when PPI representatives are unable to be involved in various trial events, such as milestone meetings.
The PPI group were very good at giving advice on literature given out to participants during the study, as well as being involved in the set-up stages. For example, the STOPPIT 2 information leaflet for partners was developed in consultation with the initial PAG in Exeter.
The first PAG members were invaluable in looking at the patient literature and consent forms, with many useful comments about the timing of information given out, the study consent procedure, cervical measurement and the pessary itself.
The parent representative (who herself had twins) identified to sit on the TSC was excellent, attending most of the arranged TSC meetings, attending the STOPPIT 2 workshops and speaking about her ‘twin experience’ at one meeting. It was extremely useful to have input from a parent who had been through a twin pregnancy (plus associated preterm birth consequences) to put the patient view across. The staff who attended the meeting where our representative spoke commented that they found this point of view extremely illuminating and requested the opportunity to hear from other women at future meetings.
One study participant also volunteered to be part of the National Institute for Health Research experience in research: ‘Claire Bowley’s Story’. [For more information please see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/130422/#/ (accessed 22 April 2021).]
Claire’s narrative informed potential participants about her personal experience of participating in research. Staff reported that women found this very helpful as they had often requested the opportunity to speak to others who had participated in the study but because of data protection laws were not able to facilitate these requests. Instead, they were able to share information via reading about Claire’s experience.
Overall, PPI representation has meant that we have truly gained an invaluable point of view about trial participation. We have usefully considered the impact of the trial process on our participants’ lives in terms of our study literature, procedures and practical applications.
In conclusion, we feel that PPI engagement is easier to obtain than maintain. Parents are very keen to give their views on research and like the idea of being involved, but this does not always follow through when it comes to practical involvement, such as attending a meeting or giving time to a grant application. The PPI engagement process is also time-consuming for the co-ordinators and requires a lot of thought and input.
Prior to the conclusion of the project in July 2019, PICTR group management was transferred to the care of the PPI Advisor (Education Core) from the Usher Institute of Population Health Sciences and Informatics, Edinburgh, UK. It is hoped that there will be a long and lasting relationship with the PICTR members. Even after the project funding concluded, further requests for PPI involvement in reproductive health studies were made to the PICTR group, including the development of a results letter for STOPPIT 2.
Chapter 7 Conclusions
Summary of findings
The results of STOPPIT 2 are clear. The Arabin pessary does not prevent SPB in women with twin pregnancy, nor does it improve neonatal outcome. There was no evidence of benefit in neonatal outcomes in the planned subgroups of women with a monochorionic pregnancy, those with a cervical length of ≤ 25 mm and those with a cervical length of ≤ 28 mm. Therefore, a trend to a reduction in preterm birth in these subgroups is not likely to be clinically useful. It is possible that there is benefit in other subgroups, as yet unidentified, and individual patient-level data from this and other studies could usefully address this issue. The pessary was well tolerated in the majority of women, with the majority finding insertion and removal painless or only slightly uncomfortable. In addition, the majority of professionals described insertion and removal of the pessary as ‘easy’ or ‘fairly easy’. However, a significant minority disagreed, with 10% of women describing pessary insertion as very painful or the worst pain imaginable.
In parallel with the clinical trial, we conducted in-depth interviews with women and clinicians about their experiences with the pessary and participation in the trial. These interviews gave rich information on the views of both groups, the impact of a ‘short cervix’ diagnosis in women with twin pregnancy and the ‘labour’ involved in recruiting women into a trial and supporting them through participation in a randomised trial.
The findings point to a potential small cost advantage in terms of both maternal and neonatal hospitalisation costs (i.e. £435.16 and £160.59, respectively) for the pessary strategy. The results are, however, surrounded by some uncertainties (e.g. in the price of the pessary and unit costing assignment methods used to value resource use).
The screening study allowed us to define population parameters for cervical length in women with twin pregnancy and positive and negative likelihood ratios for SPB before 34 weeks’ gestation for a variety of measurements. None of the negative likelihood ratios was < 0.5. Positive likelihood ratios were < 5 for all lengths above 25 mm, and between 5 and 10 for cervical lengths of ≤ 25 and 20 mm. These data suggest that cervical length measurements are not a particularly good test for predicting preterm birth in women with twin pregnancy. A ‘long’ cervix has little value for reassurance and, in the absence of any effective therapies, there is little utility in the positive likelihood ratios associated with cervical lengths of ≤ 25 mm.
Reflections on study results
To some, it will be surprising that STOPPIT 2 did not show a benefit from the Arabin pessary. This surprise will be felt most strongly by those with a prior belief that the pessary ‘ought’ to work. They may be prompted to consider alternative pessary designs, to consider alternative intervention groups or subgroups and may wish to conduct research on this. Others may suggest that the concept that a mechanical device placed around the cervix could prevent either the pathways that trigger preterm labour or cervical opening when preterm labour is initiated is a simplistic one and may have little merit as an ‘a priori’ hypothesis.
It is not clear why our study showed contrasting results to those of Liem et al. ,12 although the target ‘short cervix’ population in our study was larger and the cervical length cut-off point was unequivocally described prior to analysis. Hence, we believe that it is more likely that the subgroup analysis in the study by Liem et al. 12 is a type I error than that the null results in our study are a type II error.
Strengths
The strengths of STOPPIT 2 are that it is a large ‘real-world’ study and used a population threshold to define a short cervix group of women randomised to pessary of placebo. All of the ‘short cervix’ pessary studies of twins (including STOPPIT 2) were open label; however, to the best of our knowledge, STOPPIT 2 had a larger number of events than any of the other studies published to date. 11–14 Only the Goya et al. 13 and Liem et al. 12 studies (the two smallest studies) suggested benefit in the short cervix subgroup. Adherence was reasonably good. Ninety-two per cent of women in the intervention group had the pessary successfully inserted, although 10.4% of all women allocated to the intervention group subsequently asked for the pessary to be removed prior to the scheduled date, and in a further 5.2% the pessary fell out.
Limitations
The limitations of STOPPIT 2 are that it was underpowered to identify smaller treatment effects than originally anticipated (i.e. 40% reduction in preterm birth before 34 weeks’ gestation) and/or small subgroup effects. An additional limitation is the open-label design of this study. Although a single- or double-masked design would have been extremely challenging and our outcome is unlikely to be influenced by ascertainment bias, it is possible that the open-label approach led to unintended and undocumented differences in treatment between the two groups.
Implications for health care
-
The Arabin pessary should not be used in women with twin pregnancy for the prevention of preterm labour.
-
A ‘long’ cervical length measurement should not be used to reassure women with twin pregnancy about their risk of SPB.
Future research implications
-
Women with twin pregnancies have high rates of preterm birth (19.4%), with many having at least one serious neonatal outcome. Further work is required to find effective therapies.
-
Individual patient data meta-analysis of STOPPIT 2 and other published trials should be conducted to determine if there are (as yet unidentified) subgroups of women with twin pregnancy who benefit from Arabin pessary insertion.
Acknowledgements
We thank the members of the TSC (Andrew Whitelaw, Gary Mires, Keith Reed and Rebecca Cannings-John), our PPI representative (Mrs Andrea Hall), Data Monitoring Committee (Ed Juszczak, Peter Fowlie and Tim Overton) and the STOPPIT 2 trial support team (Lorraine Adamson, Angela Niven, Gayle Beveridge, Tariq Derdeb, Amy Witherspoon and Glynis Omond).
We thank the principal investigators from each site who participated for the duration of the study: Miss Bini Ajay, Croydon Hospital; Mr Yinka Akinfenwa, Homerton Hospital; Mr George Attilakos, University College Hospital London; Professor Phil Bennett, Imperial College Healthcare NHS Trust (Queen Charlotte and St Mary’s Hospitals); Dr Sara Brigham, Countess of Chester Hospital; Mr George Bugg, Nottingham City Hospital; Miss Vedrana Caric, James Cook University Hospital (South Tees); Miss Santhi Chidambaram, Chesterfield Royal Hospital; Mr David Churchill, New Cross Hospital (Wolverhampton); Mr Vaideha Deshpande, Queen Elizabeth Hospital Gateshead; Dr Emma Ferriman, Sheffield Teaching Hospitals NHS Foundation Trust (Jessop Wing); Dr Michael Egbor, Epsom and St Helier University Hospital (St Helier); Mr Jonathan Ford, Leighton Hospital Crewe; Dr Katarzyna Gajewska-Knapik, Addenbrooke’s Hospital; Dr Beth Gibson, Norfolk and Norwich University Hospital; Mr Kim Hinshaw, Sunderland Royal Hospital; Mr Rob Holmes, Royal Cornwall Hospital; Mr David Howe, Princess Anne Hospital Southampton; Miss Nikki Jackson, Hillingdon Hospital; Dr Dawn Kernaghan, Princess Royal Maternity Hospital Glasgow; Professor Asma Khalil, St George’s Hospital London; Professor Mark Kilby, Birmingham Women’s Hospital; Dr Bea Knight, Royal Devon and Exeter Hospital; Miss Rukhsana Kousar, Pinderfields Hospital Mid Yorkshire; Dr Marie-Anne Ledingham, Queen Elizabeth University Hospital Glasgow; Professor Dr Liesbeth Lewi, Universitaire Ziekenhuizen Leuven Belgium; Dr Alec McEwan, Nottingham Queen’s Medical Centre; Dr Louise Melson, Poole Hospital; Mr Basem Muammar, Russells Hall Hospital Dudley; Miss Mani Malarselvi, Birmingham Heartlands Hospital; Miss Raji Myagerimath, Arrowe Park Hospital; Ms Neena Navaneetham, George Eliot Hospital NHS Trust; Dr Justine Nugent, Lancashire Women and Newborn Centre Burnley General Hospital (East Lancashire Hospitals NHS Trust); Mr Moboladale Ojutiku, Basildon and Thurrock University Hospital; Ms Vinita Raheja, Wansbeck General Hospital Ashington; Dr Sunit Rane, St John’s Hospital Livingston; Mrs Sandhya Rao, Whiston Hospital (St Helens and Knowsley NHS Teaching Hospital); Dr Andrew Sharp, Liverpool Women’s Hospital; Dr Andrew Shennan, St Thomas’s Hospital London; Dr Ashalatha Shetty, Aberdeen Royal Maternity Hospital; Mr Nigel Simpson, Leeds Teaching Hospital; Dr Fatimah Soydemir, Royal Preston Hospital; Dr Sarah Stock, Edinburgh Simpson Centre for Reproductive Health; Dr John Tomlinson, Royal Bolton Hospital; Mrs Kalpana Upadhyay, Wrexham Maelor; Miss Luxmi Velauthar, Barts Health NHS Trust London (Newham Royal London Whipps Cross Hospital); Mr Stuart Verdin, Salisbury Hospital Maternity Unit; Miss Radhika Viswanatha, Epsom and St Helier University Hospital (Epsom); Dr Lesley Walker, Royal Victoria Infirmary Newcastle; Professor Ross Welch, Derriford Hospital Plymouth; Dr Melissa Whitworth, St Mary’s Hospital Manchester; Mrs Janet Wright, Bradford Royal Infirmary and Mr Peter Young, Royal Stoke University Hospital.
In addition, we thank the principal investigators from each site who participated for and those who participated for only part of the study: Mr Clive Aldrich, Northampton General Hospital; Miss Hind Al-Husain, Northampton General Hospital; Dr Helene Brandon, Queen Elizabeth Hospital Gateshead; Dr Helen Cameron, Sunderland Royal Hospital; Dr Simona Cicero, Homerton Hospital; Mr Kausik Das, Birmingham Heartlands; Dr Hany Lashen, Sheffield Teaching Hospitals NHS Foundation Trust; Mr Jim McCormack, Countess of Chester Hospital; Miss Stella Mwenechanya, Arrowe Park Hospital; Ms Surabhi Nanda, Liverpool Women’s Hospital; and Dr Alex Patience, Royal Victoria Infirmary Newcastle.
We also thank the midwives, sonographers and research and development offices that contributed to the study. We are grateful to Dr Arabin for her support and advice during the trial set-up. We are also grateful to the many people who helped in this study but who we have been unable to name and, in particular, all the women (and their babies) who participated in STOPPIT 2.
Contributions of authors
Jane E Norman (https://orcid.org/0000-0001-6031-6953) (Dean of the Faculty of Health Sciences) had overall responsibility for the study, drafted all but Chapters 4 and 5 of the final report, and made comments on the interpretation of the data and the final draft of the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Director of CHaRT, University of Aberdeen) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, was responsible for the statistical analysis plan and analysis of the clinical outcome data, and made comments on the interpretation of the data and the final draft of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor of Medical Statistics and Director, CHaRT, University of Aberdeen) was responsible for the statistical analysis plan and analysis of the clinical outcome data, and made comments on the interpretation of the data and the final draft of the report.
David Cooper (https://orcid.org/0000-0002-9361-4399) (Research Fellow in Statistics, University of Aberdeen) was responsible for the statistical analysis plan and analysis of the clinical outcome data, and made comments on the interpretation of the data and the final draft of the report.
Sonia Whyte (https://orcid.org/0000-0003-0878-4244) (Trial Manager) was responsible for the day-to-day organisation and delivery of the study, supported by Mrs Lorraine Adamson (Trial Administrator), and made comments on the interpretation of the data and the final draft of the report.
Sushila Chowdhry (https://orcid.org/0000-0002-2766-1808) (Research Assistant, University of Edinburgh) designed the qualitative work, conducted the interviews described in Chapter 5 and generated the first draft of this chapter, and made comments on the interpretation of the data and the final draft of the report.
Sarah Cunningham-Burley (https://orcid.org/0000-0002-0009-7653) (Professor of Medical and Family Sociology, University of Edinburgh) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, designed the qualitative work, provided input to the interviews described in Chapter 5 and helped to generated the first draft of this chapter, and made comments on the interpretation of the data and the final draft of the report.
Aileen R Neilson (https://orcid.org/0000-0003-3758-0566) (Senior Health Economist, University of Edinburgh) was responsible for the health economic work, conducted the health economic analysis described in Chapter 4 and generated the first draft of this chapter, and made comments on the interpretation of the data and the final draft of the report.
Xue W Mei (https://orcid.org/0000-0002-6279-4884) (Research Fellow in Medical Statistics and Epidemiology, University of Oxford) was responsible for the health economic work, and made comments on the interpretation of the data and the final draft of the report.
Joel BE Smith (https://orcid.org/0000-0002-5096-1462) (Health Economist, University of Edinburgh) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, was responsible for the health economic work, and made comments on the interpretation of the data and the final draft of the report.
Andrew Shennan (https://orcid.org/0000-0001-5273-3132) (Professor of Obstetrics, King’s College London) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Stephen C Robson (https://orcid.org/0000-0001-7897-7987) (Professor of Obstetrics, University of Newcastle) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Steven Thornton (https://orcid.org/0000-0001-7613-0408) (Dean of Exeter Medical School) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Mark D Kilby (https://orcid.org/0000-0001-9987-4223) (Professor of Fetal Medicine, University of Birmingham) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Neil Marlow (Professor of Neonatal Medicine, University College London) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Sarah J Stock (https://orcid.org/0000-0003-4308-856X) (Clinical Lecturer in Maternal and Fetal Medicine, University of Edinburgh) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Philip R Bennett (https://orcid.org/0000-0002-6253-4919) (Professor of Obstetrics and Gynaecology, Imperial College London) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Jane Denton (https://orcid.org/0000-0002-0877-1008) (Director of the MBF) was involved in the initial conception, design and funding acquisition for the study, and for management as the study progressed, and made comments on the interpretation of the data and the final draft of the report.
Publication
Norman JE, Norrie J, MacLennan G, Cooper D, Whyte S, Chowdhry S, et al. Evaluation of the Arabin cervical pessary for prevention of preterm birth in women with a twin pregnancy and short cervix (STOPPIT-2): an open-label randomised trial and updated meta-analysis. PLOS Med 2021;18:e1003506.
Data-sharing statement
Data are available from CHaRT, Aberdeen University, and requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Norman JE, Norrie J, Maclennan G, Cooper D, Whyte S, Cunningham Burley S, et al. Open randomised trial of the (Arabin) pessary to prevent preterm birth in twin pregnancy with health economics and acceptability: STOPPIT-2-a study protocol. BMJ Open 2018;8.
- Norman JE, Norrie J, MacLennan G, Cooper D, Whyte S, Chowdhry S, et al. Evaluation of the Arabin cervical pessary for prevention of preterm birth in women with a twin pregnancy and short cervix (STOPPIT-2): an open-label randomised trial and updated meta-analysis. PLOS Med 2021;18. https://doi.org/10.1371/journal.pmed.1003506.
- Office for National Statistics (ONS) . Birth Characteristics in England and Wales: 2017 2019.
- National Collaborating Centre for Women’s and Children’s Health . Multiple Pregnancy: The Management of Twin and Triplet Pregnancies in the Antenatal Period. 2011.
- Zheng L, Dong J, Dai Y, Zhang Y, Shi L, Wei M, et al. Cervical pessaries for the prevention of preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32:1654-63. https://doi.org/10.1080/14767058.2017.1414795.
- Liem SM, van Pampus MG, Mol BW, Bekedam DJ. Cervical pessaries for the prevention of preterm birth: a systematic review. Obstet Gynecol Int 2013;2013. https://doi.org/10.1155/2013/576723.
- Abdel-Aleem H, Shaaban OM, Abdel-Aleem MA. Cervical pessary for preventing preterm birth. Cochrane Database Syst Rev 2013;5. https://doi.org/10.1002/14651858.CD007873.pub3.
- Saccone G, Ciardulli A, Xodo S, Dugoff L, Ludmir J, Pagani G, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analysis. J Ultrasound Med 2017;36:1535-43. https://doi.org/10.7863/ultra.16.08054.
- Thangatorai R, Lim FC, Nalliah S. Cervical pessary in the prevention of preterm births in multiple pregnancies with a short cervix: PRISMA compliant systematic review and meta-analysis. J Matern Fetal Neonatal Med 2018;31:1638-45. https://doi.org/10.1080/14767058.2017.1319930.
- Saccone G, Rust O, Althuisius S, Roman A, Berghella V. Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand 2015;94:352-8. https://doi.org/10.1111/aogs.12600.
- Berghella V, Dugoff L, Ludmir J. Prevention of preterm birth with pessary in twins (PoPPT): a randomized controlled trial. Ultrasound Obstet Gynecol 2017;49:567-72. https://doi.org/10.1002/uog.17430.
- Liem S, Schuit E, Hegeman M, Bais J, de Boer K, Bloemenkamp K, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): a multicentre, open-label randomised controlled trial. Lancet 2013;382:1341-9. https://doi.org/10.1016/S0140-6736(13)61408-7.
- Goya M, de la Calle M, Pratcorona L, Merced C, Rodó C, Muñoz B, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: a multicenter randomized controlled trial (PECEP-Twins). Am J Obstet Gynecol 2016;214:145-52. https://doi.org/10.1016/j.ajog.2015.11.012.
- Nicolaides KH, Syngelaki A, Poon LC, de Paco Matallana C, Plasencia W, Molina FS, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. Am J Obstet Gynecol 2016;214:3-9. https://doi.org/10.1016/j.ajog.2015.08.051.
- To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol 2006;194:1360-5. https://doi.org/10.1016/j.ajog.2005.11.001.
- Kindinger LM, Poon LC, Cacciatore S, MacIntyre DA, Fox NS, Schuit E, et al. The effect of gestational age and cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta-analysis. BJOG 2016;123:877-84. https://doi.org/10.1111/1471-0528.13575.
- Altman DG. Better reporting of randomised controlled trials: the CONSORT statement. BMJ 1996;313:570-1. https://doi.org/10.1136/bmj.313.7057.570.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Department of Health and Social Care . NHS Reference Costs 2017–18 2018. https://webarchive.nationalarchives.gov.uk/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 26 April 2021).
- Mason J. Qualitative Researching. London: SAGE Publications Ltd; 2018.
- Silverman D. Doing Qualitative Research: A Practical Handbook. London: SAGE Publications Ltd; 2000.
- Saldana J. The Coding Manual for Qualitative Researchers. London: SAGE Publications Ltd; 2009.
- MacQueen KM, McLellan-Lemal E, Bartholow K, Milstein B, Guest G, MacQueen KM. Handbook for Team-Based Qualitative Research. Lanham, MD: AltaMira Press; 2008.
List of abbreviations
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CM
- consultant midwife
- CO
- consultant obstetrician
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- clinical research fellow
- eCRF
- electronic case report form
- HRG
- Healthcare Resource Group
- MBF
- Multiple Births Foundation
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PAG
- Patient Advisory Group
- PenCLAHRC
- Applied Research Collaboration South West Peninsula
- PICTR
- Patient Involvement in Clinical Trials Research
- PPI
- patient public involvement
- RM
- research midwife
- RR
- relative risk
- SD
- standard deviation
- SPB
- spontaneous preterm birth
- TAMBA
- Twins and Multiple Births Association
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TVU
- transvaginal ultrasonography
