Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/210/07. The contractual start date was in April 2016. The draft report began editorial review in October 2019 and was accepted for publication in October 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Halligan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
The research detailed in this monograph is a response to the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme call of 2014, HTA 14/210, ‘Prognostic markers in early Crohn’s disease’. Some of the text presented in this section has been published previously as part of the study protocol. 1
The commissioning brief stated that the NHS decision problem turned on the fact that:
Some patients with Crohn’s disease follow a relatively limited course with rapid response to relatively simple treatment and few subsequent acute flare-ups. Others progress rapidly to severe disease needing escalation of medical treatment or surgery: as many as one-third of patients require surgery within a year of beginning oral steroids. Rapid disease control may allow sufferers to return to work and other normal activities more rapidly and may reduce the need for surgery. NICE [National Institute for Health and Care Excellence] does not currently recommend early use of anti-TNF [tumour necrosis factor] drugs although there is some evidence of better early outcomes with their use. Rapid escalation of medical treatment is allowed for within current NICE guidelines. Identification of patients who might benefit from early intensive treatment is needed before the cost-effectiveness of treatment strategies can be determined.
Reproduced with permission, 2021, NIHR HTA programme2
The HTA programme stipulated that the commissioned research should comprise a systematic review that evaluated ‘clinical criteria for treatment and/or use of biomarkers or biopsy informatics to develop a clinical tool to identify patients most likely to benefit from early intensive treatment’. Outcomes of the research would therefore be ‘[a]n overview of current evidence’ combined with ‘a signal as to which biomarkers may be most useful’. It was also noted that, contingent on the findings, any prediction tool that was developed during the research may be subsequently tested in a prospective study, in research commissioned by the HTA programme.
Crohn’s disease and modern treatment strategy
This section is reproduced with permission from Halligan et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Crohn’s disease is an inflammatory ulcerative enteropathy that generally affects young adults and can be extremely debilitating. There is no cure and treatment is traditionally applied in a ‘bottom-up’ fashion, aimed at treating symptoms as and when they arise, with treatment escalated when symptoms worsen. However, newer biological therapies appear to ameliorate the ultimate disease trajectory in addition to treating symptoms. This attribute raises the possibility that adopting these agents early in a ‘top-down’ paradigm could ‘stop the disease in its tracks’. The first disease-modifying biological agent was infliximab, which is a monoclonal antibody against the cytokine tumour necrosis factor (TNF) alpha that binds with it and prevents receptor binding. A randomised trial of infliximab versus placebo found that, of those patients who responded to an initial dose, half achieved complete mucosal healing after 1 year and stayed in remission longer and discontinued steroids earlier than control patients. 3 Biologicals also appear incrementally more effective when used in combination with other immunomodulators, such as azathioprine,4 especially when administered in a ‘top-down’ fashion. 5,6 Newer agents, such as adalimumab, are also effective. 7
The REACT (Early combined immunosuppression for the management of Crohn’s disease) study randomised patients to either conventional ‘bottom-up’ therapy or ‘early combined immunosuppression’ and found that major complications, hospitalisation and surgery were significantly reduced at 24 months for the intervention clusters. 8 Accordingly, current thinking is that early aggressive biological treatment combined with immunomodulation will prevent future disease and is preferable to merely responding to symptoms. However, administering biologicals early to all patients is unwise because these agents may precipitate serious infection, are hepatotoxic and can cause demyelination, lupus syndrome and lymphoma. 9 Biologicals are also very expensive. A strategy that could identify new diagnoses of Crohn’s disease who are destined to develop into severe disease in the future would have considerable clinical utility through ‘personalised and targeted therapy’, that is directing these patients to early biological treatment while avoiding unnecessary over-treatment in others.
Diagnostic and prognostic biomarkers of disease activity
According to the US National Institutes of Health, a biomarker is a measurable characteristic that indicates a biological process that may reflect pathology and pharmacological response to treatment. 10 Biomarkers in Crohn’s disease may indicate the presence or absence of disease, as well as its severity. It is also important to understand the distinction between a ‘diagnostic’ biomarker and a ‘prognostic’ biomarker. While a reliable diagnostic biomarker may reflect the presence/absence/severity of current disease, a prognostic biomarker may reflect the presence/absence/severity of future subsequent disease (or of response to therapy, etc.). A clinically useful biomarker may be diagnostic, prognostic or a combination of both.
In our research, we have not been too restrictive when labelling an intervention or characteristic as a ‘biomarker’. Although the term is associated with novel diagnostic technologies, simple and effective biomarkers have been used for decades. For example, stool frequency directly reflects colonic inflammation and should not be excluded from systematic review simply because it is not perceived as ‘novel’. Smoking is believed to have a profound effect on disease outcome and should also be included, although smoking in and of itself is not a marker of disease activity. Several studies have investigated simple clinical factors that are predictive of an ‘aggressive’ disease course and Markov modelling of these factors has shown that disease activity over the year following diagnosis is predictive of the clinical course of the disease over the following decade. 11
For the commissioned research we, therefore, aimed to identify the whole range of potential biomarkers used in Crohn’s disease, including clinical (both clinician and self-reported outcomes), endoscopic, radiological, faecal, urinary, serological (including the range from basic tests to antibodies), genetic and histological biomarkers. For example, C-reactive protein (CRP) is an acute-phase protein expressed by the liver that is widely used in clinical practice. Calprotectin, a protein that is released in inflamed gut epithelium, is a more recent biomarker that has also reached daily practice. Calprotectin levels change with treatment. Lactoferrin is a similar protein biomarker. The diagnostic accuracy of such biomarkers has already been subject to systematic review and meta-analysis. For example, one such review12 aimed to determine if calprotectin levels could differentiate between inflammatory and irritable bowel disease in children.
Chapter 2 Research questions
Our overarching research question was to identify biomarkers that are potentially able to predict which patients with Crohn’s disease are destined to subsequently develop severe disease. This question (and related others) was answered through the research objectives listed below.
Primary research objective
-
To carry out a systematic review of the literature, including meta-analysis where possible, that covers all potential biomarker areas to assess their predictive capability for severe Crohn’s disease. This review will determine the breadth, depth and quality of currently available evidence, and meta-analysis will identify which biomarkers may be sufficiently predictive for use in a subsequent prognostic model.
Secondary objectives
-
To compare potential predictors by direct and indirect comparison of study results. Direct comparisons between predictors from the same study constitute stronger evidence and will be preferred over indirect comparisons across different studies.
-
To explore heterogeneity among studies.
-
To identify those predictors that appear useful for the development and validation of a prognostic model to identify patients who are destined to develop severe Crohn’s disease.
-
To examine and validate any existing models identified by the systematic review.
The ability to examine primary and secondary objectives will be highly dependent on the availability and quality of data from published studies.
Chapter 3 Review methods
At the design stage, we anticipated that there would be many potential biomarkers, which could make for an unwieldy review. We, therefore, set a priori quality/quantity thresholds for review inclusion to prevent extraction of data for biomarkers that have not been sufficiently studied for the findings to be deemed generalisable/reproducible and/or that have weak methodology. For example, at the time of writing the review protocol, more than 70 separate genes had been implicated in Crohn’s disease. 13 Given that genetic sequencing is currently very expensive and there are multiple potential individual genetic predictors, we anticipated that few genetic predictors will have been studied in sufficient depth. However, sequencing will probably become increasingly cost-effective in the near future. Our systematic review, therefore, considered only those genes for which sufficient primary studies exist to provide a useful signal of their prognostic potential.
Genetic makeup is also linked to response to biological therapy. Given that genetic makeup is fixed, these factors need to be measured only once (in contrast to other biomarkers that fluctuate with disease activity). There are also multiple antibody candidates, and prognostic strategies have focused on both titres of individual antibodies and the number of different antibodies. For example, patients with three or more positive antibodies are eight times more likely to need surgery than patients who are antibody negative. 14
Ethics review
Ethics permission is not required by our institution for systematic review of available medical literature.
Search strategy
The protocol for this research was published. 1 Our review question(s) and data extraction were guided by the CHARMS (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies) checklist. 15 Darren Boone, a clinical researcher experienced in systematic review and multidisciplinary Crohn’s disease management, designed and carried out the search and was supervised by co-researchers, including statisticians with extensive prior experience of systematic review of prognostic markers (LA and SM), methodology experts (SH and SM) and disease experts (SH, TA, SB, MRJ and SAT). Uncertainties were resolved by face-to-face discussion.
Our scoping search found that individual studies frequently reported combinations of predictive biomarker groups. 1 For example, articles primarily reporting genetic predictors often included serological and/or clinical predictors. Therefore, we adopted an inclusive search strategy to identify all potential biomarker groups for severe Crohn’s disease. We combined five clusters of search terms: (1) identification of Crohn’s disease research; (2) identification of severe, disabling or complicated disease; (3) a panel of candidate biomarkers; (4) a panel of keywords and medical subject headings (MeSH) to identify prediction/prognosis; and (5) a panel of headings to exclude animal research, narrative reviews and editorials. Searches were combined using Boolean operators. We then tested the search string through its ability to identify key papers that were nominated by the authors. If unidentified, article keywords and MeSH were interrogated and the search string was refined until papers were captured. The search string used is shown in Table 1.
| Search number | Search string | Number of hits on PubMed | Number of hits on EMBASE |
|---|---|---|---|
| 1 | (crohn*) | 41,703 | 60,679 |
| 2 | Aggressiv* OR Sever* OR Disabling OR Montreal OR Beaugerie OR Liege OR Flare OR Penetrat* OR Strictur* OR Resection OR Surgical OR Surgery OR Stoma OR Failure OR Active OR Adverse OR Harvey-Bradshaw OR HBI OR CDAI OR index OR Perianal OR Complex | 6,329,437 | 4,441,705 |
| 3 | Biomark* OR Marker OR Assay OR Imaging OR Radiolog* OR Genetic OR Examination OR Serum OR Blood OR Serolog* OR Stool OR Faecal OR fecal OR feces OR faeces OR Frequency OR Urin* OR Endoscop* OR histolog* OR histopathol* OR antibod* OR age OR Smoking OR Test | 9,823,770 | 5,491,617 |
| 4 | course OR prognos* OR outcome OR cohort OR progres* OR Predict* OR Risk* OR Outcome OR onset OR Biomarker* OR Natural history OR Predict*[tiab] OR Predictive value of tests[mh] OR Scor*[tiab] OR Observ*[tiab] OR Observer variation[mh] OR risk prediction model[tiab] OR predictive model[tiab] OR predictive equation[tiab] OR prediction model[tiab] OR risk calculator[tiab] OR prediction rule[tiab] OR risk model[tiab] OR statistical model[tiab] OR cox model[tiab] OR multivariable[tiab]OR validate OR nomogram OR predictive model OR validation OR prognostic model OR prognostic scor* OR prognostic index OR predictor OR diagnos* | 7,516,769 | 5,683,139 |
| 5 | Review[Publication Type] OR Bibliography[Publication Type] OR Editorial[Publication Type] OR Letter[Publication Type] OR News[Publication Type] | 2610 | 3843 |
| 6 | 1&2&3&4 | 13,905 | 22,498 |
| 7 | 6 NOT 5 | 11,295 | 18,655 |
Search terms were identified by hand-searching Crohn’s literature and guidelines from established clinical associations [e.g. the European Crohn’s and Colitis Organisation (Vienna, Austria), the European Society of Gastrointestinal and Abdominal Radiology (Vienna, Austria)], and via a multidisciplinary panel.
Search process
Using the search string, Darren Boone queried the PubMed and EMBASE databases for literature published from inception to 1 January 2016 and screened the titles and abstracts of identified primary studies to assess their eligibility. Grey literature was identified by hand-searching conference proceedings from 2012–15, inclusive (European Crohn’s and Colitis Organisation, United European Gastroenterology Week and Digestive Disease Week). An update was performed up to 1 January 2018 for serological biomarkers. We initially intended to search eight individual databases;1 however, because our search of PubMed and EMBASE alone yielded nearly 30,000 citations, we decided that searching other databases would have been unduly burdensome in return for identifying little useful additional research.
Eligibility criteria
-
Target condition: primary studies had to report patients with proven new or established diagnoses of Crohn’s disease for which at least one biomarker was used to predict subsequent development of severe disease.
-
Definition of Crohn’s disease: diagnosis used standard clinical, endoscopic and pathological criteria. Studies reporting various severities were eligible if severe subgroups could be extracted.
-
Participants: no age restriction was applied and paediatric studies (defined as aged < 16 years) were noted for subgroup analysis. All ethnicities, races and religious groups were eligible.
-
Language: no language restriction was applied and translation was used where necessary.
-
Inclusion criteria for biomarkers: to identify biomarkers with sufficient evidence, we stipulated that potential individual predictors must have been reported in at least five individual studies. We made an exception for ‘new but exceptionally promising predictors’, which were chosen by an expert panel from a list of all candidates identified by the review. The panel were also asked to indicate any predictor that was already in widespread use and which they deemed too important to be omitted from the review.
-
Definitions of severe disease and outcomes: no universally accepted definition of ‘severe’ Crohn’s disease exists. Surgery is often used as a surrogate for severe disease but, at the same time, early ileocolonic resection may be curative in some patients (and so these patients no longer have ‘severe’ disease). Immunomodulatory requirement as a surrogate for severe disease ignores the fact that some patients achieve complete mucosal healing and avoid complications, and that these drugs are used increasingly early in the disease trajectory. Although fistulae or penetrating disease form the backbone of the severe ‘disease behaviour’ domains of the Montreal,16 Vienna16 and Paris17 classifications, perianal fistula is inconsistently considered, resulting in heterogeneous severity within those exhibiting ‘complicated’ features.
To avoid discarding potentially important research, we included studies that used a broad range of definitions. We also included studies that reported intestinal and perianal surgery [excluding appendicectomy, non-inflammatory bowel disease (IBD) surgery and simple perianal drainage]. Studies for which the end point was relapse or flare were excluded unless these aligned with our definitions of severe disease. Likewise, treatment change alone did not meet our criteria given that this is not necessarily associated with severe disease. We did not investigate lack of response to, for example, anti-tumour necrosis factor (anti-TNF) therapy or antibody development because these are related to treatment and are not in and of themselves surrogates for severe disease. Articles using treatment costs as surrogate for disease severity were excluded. Table 2 shows varying definitions of severe disease from Beaugerie et al. ,18 Montreal behaviour classification,19 Liege criteria20 and the National Institute for Health and Care Excellence (NICE) Technology Appraisal (TA) 187. 21
| Beaugerie et al.18 ‘disabling disease’ | Montreal behaviour classification19 | Liege criteria20 | NICE TA18721 |
|---|---|---|---|
| Three or more steroid courses or steroid dependence; hospitalisation for flare or Crohn’s disease complications; ≥ 12 months of disabling symptoms (nocturnal diarrhoea, urgency, abdominal pain, fever, fatigue, joint pain, uveitis or pyoderma); need for immunosuppression; intestinal resection; surgery for perianal disease | B1: inflammatory; B2: stricturing; B3: penetrating (p = perianal modifier). B2/B3 = severe Crohn’s disease | Complex perianal disease; any colonic resection; two or more SB resections (or a single SB resection of ≥ 50 cm); construction of a definitive stoma | CDAI score of ≥ 300 or Harvey–Bradshaw Index score of ≥ 8–9 |
We stipulated a minimum of 3 months between biomarker measurement and outcome measurement to ensure that the biomarker was prognostic rather than diagnostic. Therefore, although studies exploring development of severe disease following intestinal surgery were eligible (noting that surgery would define severe disease in some classifications), prediction of early postoperative complications was excluded. Likewise, because the stability of serological markers changes over disease course,22 we required contemporaneous serum draw and outcome measurement.
Data extraction
Full-text articles were examined by Darren Boone and queries were initially raised with Lucinda Archer, followed by other collaborators where necessary. Ultimately, Sue Mallett examined all full-text articles selected for the review and verified data extraction, so that all results data were double screened and extracted. Data were extracted into a data sheet that incorporated measures developed from the CHARMS checklist15 and Prediction model Risk Of Bias Assessment Tool (PROBAST),23 with additional fields specific to our review.
We extracted author, journal, design (e.g. cohort, randomised controlled trial, retrospective database), methods, setting/context (organisation/service type, country), participants (including age, range, gender), time since diagnosis (symptom duration and/or time since diagnosis for established disease), markers of severe disease, symptom severity, disease location and burden, disease complications, Crohn’s activity indices, surgical details, perianal disease and continence outcomes. In addition, we extracted data specific to the prognostic marker investigated, including measurement (methods, frequency), adverse events, reliability and reproducibility, and costs where available. We recorded study interventions and outcomes (including definitions, thresholds for severity/remission and whether or not they were prespecified) and median follow-up time with interquartile range (IQR).
For models, we documented study type (development, internal/external validation), included predictors (including measurement methods, categorisation of continuous outcomes, blinding to outcome assessment and predictor variables), sample size (number of participants with events and included in the modelling), statistical modelling methods (including fitting, missing data treatment, methods to adjust for overfitting), model performance (discrimination, calibration, sensitivity, specificity, net benefit, reclassification), model estimates and 95% confidence intervals (CIs) (e.g. univariable unadjusted or adjusted estimates for predictors, adjusted coefficients for predictors in multivariable models). Where available, we extracted estimates with 95% CIs, including odds ratios (ORs), risk ratios and hazard ratios.
Risk-of-bias assessment
The risk of bias (ROB) for each included study was assessed via PROBAST. 23 PROBAST has five broad domains: patient selection, predictors, outcome, sample size and participant flow, and analysis. Domains were combined to give an overall ROB.
Patient and public involvement
We included an ‘expert’ patient representative to help form our research proposal. The impact of patient and public involvement (PPI) was minimal in the systematic review and meta-analysis phases of this research given that data identification, extraction and analysis are largely independent of ‘opinion’. However, as described in Chapter 7, we anticipate that PPI input will be vital for future research because a non-medical perspective will be required to implement any model in daily clinical practice. Weighting the presumed benefits of early biological treatment against the risk of side effects must be considered against the predictive capabilities of the model, all from a patient perspective.
Statistical analysis
Where possible, participants with and without events (i.e. severe disease) were extracted into 2 × 2 contingency tables for each study. The number of studies and effect estimates for each predictor are reported in Table 7. Studies were grouped for meta-analysis by their effect estimates (e.g. ORs and hazard ratios). Meta-analysis was considered when there were three or more individual studies of a biomarker from which data could be extracted using the same effect estimate.
We looked for predictor association with subsequent severe disease rather than precise estimates of strength or interpredictor comparison. We anticipated that varied designs would cause varied results; therefore, meta-analysis reflects evidence across all studies which provided informative data regarding specific situations, measurements and thresholds. Studies were grouped for meta-analysis by prediction estimate type and, where sufficient, by definition of severe disease. We grouped studies that were ‘adequately adjusted’, which was defined by clearly reported adjustment factors including at least one of the following confounders: age at diagnosis, perianal disease, steroids for first flare, disease location and/or behaviour, smoking, surgery and family history. Forest plots present study results (see Figures 6–19). Meta-analysis was considered for biomarkers with three or more studies using the same effect estimate; excessive heterogeneity precluded combination. Each study was included once per meta-analysis; where studies reported estimates at different definitions of severe disease, we selected the highest ranking (order: surgery, B2/B3, B3, B2, other definition). To avoid confounding, B2 and B3 were identified as predictors for which surgery was the outcome. We did not use B3 as a predictor for B3 or B2/B3 as an outcome.
Random-effect inverse variance meta-analysis24 was used to pool ORs. Meta-analysis was performed using ‘metan’ (Stata 14, StataCorp LP, TX, USA) because 2 × 2 tables were not always available.
Owing to the paucity of results for children alone, results were analysed across all ages, with age groups indicated on forest plots.
Chapter 4 Studies included in the reviews
This review is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines; the PRISMA flow chart is illustrated in Figure 1. In total, 29,947 abstracts were identified (PubMed, n = 11,292; EMBASE, n = 18,655). A total of 15,923 duplicates were removed and 14,024 abstracts were screened. After applying the eligibility criteria, 247 full-text articles were assessed, which included four of the biomarkers that were considered essential by the expert panel. Ultimately, 71 articles were included in the review, which corresponded to 56 non-overlapping patient cohorts. Reasons for exclusions are given in Figure 1.
FIGURE 1.
The PRISMA flow chart. Some articles were included in more than one review. a, Hand-searching of conference proceedings failed to yield any additional research.

Table 3 shows the predictors that were deemed important for meta-analysis by the expert panel. Ultimately, this involved the addition of only one predictor: CRP. Nine predictors that were deemed important were already included. Seven predictors that were deemed important yielded too few individual studies to be viable for meta-analysis. For example, there were no prognostic studies of faecal calprotectin. ‘Severe endoscopic lesions’ were deemed important but prognostic data were provided by only two studies.
| Factor chosen by the experts | Inclusion in overview |
|---|---|
| CRP | Added |
| Azathioprine/biologicals | Confounding treatment |
| Use of TPN | Confounding treatment |
| Low adherence to medication | Confounding treatment |
| Upper disease | ‘Location’ included already |
| Bowel stenosis | Included already |
| Internal fistula | Included already |
| Stricture | Included already |
| Development of strictures during follow-up | Included already |
| Development of fistulae during follow-up | Included already |
| Jejunal involvement | Included already |
| ASCA-IgG | Included already |
| ASCA-IgA | Included already |
| Flares per year | Insufficient to include (n = 0) |
| Faecal calprotectin | Insufficient to include (n = 0) |
| Weight loss | Insufficient to include (n = 1) |
| FOXO3a | Insufficient to include (n = 1) |
| Systematic manifestations | Insufficient to include (n = 2) |
| Severe endoscopic lesions | Insufficient to include (n = 2) |
| Albumin | Insufficient to include (n = 2) |
| Ethnic origin | Insufficient to include and diverse (n = 2) |
Ultimately, the number of papers included in the three reviews were as follows:
-
general overview – 58 papers (including 11 contained in the genetic review and six in the serological review)
-
genetic review – 17 papers (including 11 contained in the general overview and two in the serological review)
-
serological review – 13 papers (including six contained in the general overview and two in the genetic review).
Chapter 5 Results of the reviews
Most of the studies were European (37/71, 52%), with 14 (20%) from the USA/Canada. A total of 40 (56%) studies were multicentre (Table 4). In total, 36 (51%) studies were prospective, 33 (46%) were retrospective and two (3%) were unclear. Of 71 studies, 11 (16%) were paediatric only, 23 (32%) were adults only and 37 (52%) were mixed. Individual study characteristics are shown in Table 4.
| Study characteristic | Total studies (N = 71) |
|---|---|
| Prospective study design (n = 36) | |
| Prospective clinic | 17 |
| Prospective cohort | 8 |
| Prospective inception cohort | 1 |
| Prospective registry | 10 |
| Retrospective study design (n = 33) | |
| Retrospective cohort | 5 |
| Retrospective clinic notes | 17 |
| Retrospective registry, databank | 8 |
| Retrospective case–control | 3 |
| Unclear study design | 2 |
| Multicentre | 40 |
| Region | |
| Europe | 38 |
| USA/Canada | 14 |
| Other | 19 |
| Participants | |
| Children only | 11 |
| Children and adults | 37 |
| Adults only | 23 |
| Recruitment dates reported | 49 |
| Number of patients, median (IQR) [range] | 248 (159–555) [70–3118] |
| Number of events (severe disease), median (IQR) [range]a | 76 (51–70) [14–981] |
| Follow-up (years), median (IQR) [range] | 8 (5–10) [0.8–18] |
The recruitment dates varied (see Table 4), with 22 (31%) studies not reporting recruitment dates. Follow-up also varied (median 8 years, IQR 5–10 years, range 0.8–18 years; Table 4). Nine studies allowed extraction of prediction of severe disease events at multiple time points during follow-up. 25–33 Studies frequently presented several definitions of severe disease (Table 5).
| Study (first author and year) | Country | Multicentre | Study design | Dates of recruitment | Follow-up and range (years) | Follow-up statistics | Number of participants | Number of events | Definition of serious disease | Adults and/or children | Marker | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Serology | Genetic | Clinical | ||||||||||
| Aldhous 200734 | Scotland | No | Prospective clinic | NR. Pre-2007 | 10 (0.3–55) | Median (range) | 274 | 105 | 113 | Surgery, B2/B3 | Adults | No | No | Yes |
| Alvarez-Lobos 200535 | Spain | No | Prospective clinic | 2002–4 | 7.4 (SD 6.1) | Mean (SD) | 159 | 59 | 70 | Surgery | Adults | No | Yes | No |
| Annese 200536 | Italy | Yes | Prospective cohort | NR. Pre-2005 | Sporadic 7 (SD 4); familial 9 (SD 6) | Mean (SD) | 316 | 87 | 151 | Surgery | Adults | Yes | Yes | Yes |
| Beaugerie 200618 | France | No | Retrospective clinic records | 1985–98 | 5 | Fixed time period | 1123 | 957 | 957 | Other | Both | No | No | Yes |
| Brant 200337 | USA | Yes | Retrospective genetic cohort | NR. Pre-2003 | B1 > 8 | Minimum time for B1 | 257 | 49 | 183 | Surgery | Adults | No | Yes | Yes |
| Büning 200438 | Germany | No | Prospective clinic | NR. Pre-2004 | 6 (SD 7.0) | Mean (SD) | 180 | 51 | 51 | Surgery | Adults | No | Yes | No |
| Charpentier 201439 | France | Yes | Retrospective from registry | 1988–2006 | 6 (2–11) | Median (range) | 367 | 103 | 103 | Surgery | Adults | No | No | Yes |
| Chatzicostas 200640 | Greece | Yes | Prospective registry, with additional clinic cohort | NR. Pre-2006 | 10.5 (SD 6.4) | Mean (SD) | 80 | 38 | 38 | B2/B3 | Both | No | No | Yes |
| Chen 201541 | The People’s Republic of China | No | Retrospective clinic database | 1992–2012 | 7 (0–19) | Median (range) | 197 | 37 | 64 | Surgery | Both | No | No | Yes |
| Choung 201642 | USA | Yes | Prospective registry | 1990–NR (pre-2015) | 6.0 (IQR 5.6–8.1) | Median (IQR) | 100 | 21 | 24 | Other | Adults | Yes | No | Yes |
| Cleynen 201343 | EUa | Yes | Retrospective hospital clinics | NR. Pre-2015 | 18 (10–62) | Median (range) | 1528 | 600 | 844 | Surgery | Both | No | Yes | Yes |
| Degenhardt 201644 | Germany | No | Retrospective serum bank | 2000–6 | 12 | Median | 70 | 20 | 20 | Other | Adults | Yes | No | No |
| Dubinsky 200845 | USA | Yes | Unclear. 21 hospital sites | NR. Pre-2008 | 2.5 (0.1 to 19.6) | Median (range) | 592 | 37 | 61 | Surgery, B2/B3 | Children | Yes | No | No |
| Goel 201346 | India | No | Retrospective clinic records | 1995–2008 | 1.5 (0.1–16.5) | Median (range) | 223 | 73 | 93 | Surgery, B2/B3 | Adults | No | No | Yes |
| Gupta 200647 | USA | Yes | Prospective registry | 2000–3 | 3.6 (SD 3.1) | Mean (SD) | 989 | 128 | 128 | Surgery | Children | No | No | Yes |
| Henckaerts 200948 | Belgium | Yes | Retrospective registry | 1996–2009 | 14 (IQR 7–22) | Median (IQR) | 666 | 349 | 432 | Surgery | Adults | No | Yes | Yes |
| Israeli 201425 | Canada | No | Retrospective clinic records | 1993–2012 | 11.1 (1–58) | Median (range) | 379 | 19 | 167 | Surgery | Both | No | No | Yes |
| Jauregui-Amezaga 201549 | Spain | No | Prospective clinic | 2007–11 | 4.1 (1.8–5.4) | Median (IQR) | 112 | 29 | 29 | Surgery | Both | Yes | No | Yes |
| Kugathasan 201750 | USA and Canada | Yes | Prospective inception cohort | 2008–12 | 3.6 (3–4.3) | Unclear | 913 | 78 | 78 | B2/B3 | Children | Yes | No | No |
| Kugathasan 200451 | USA | No | Prospective clinic | 2003 | 3.3 (0.5–7.3) | Mean (range) | 138 | 49 | 49 | B2/B3 | Children | No | Yes | No |
| Kwon 201652 | The Republic of Korea | Yes | Retrospective clinic records | 1982–2008 | 7.1 (3.1–10.8) | Median (unclear) | 705 | 156 | 156 | Surgery | Both | Yes | No | No |
| Lacher 201053 | Germany | No | Prospective clinic | 2000–9 | 4.8 (0.3–13.1) | Mean (range) | 171 | 32 | 32 | Surgery | Children | No | Yes | No |
| Laghi 200554 | Italy | No | Retrospective clinic records | NR. Pre-2005 | 10.1 (SD 8.1) | Mean (SD) | 193 | 145 | 187 | Surgery | Adults | No | Yes | Yes |
| Lakatos 200555 | Hungary | Yes | Prospective cohort | 2003–5 | 8.2 (3.2–13.2) | Unclear | 527 | 220 | 311 | Surgery | Adults | No | Yes | No |
| Lakatos 200956 | Hungary | No | Retrospective clinic records | NR. Pre-2009 | 9.4 (1.9–16.9) | Unclear | 198 | 61 | 61 | B2/B3 | Adults | No | No | Yes |
| Law 201357 | The People’s Republic of China | No | Retrospective clinic records | 2000–12 | 8 (5) | Median (IQR) | 79 | 22 | 34 | Surgery, other | Both | No | No | Yes |
| Loly 200820 | Belgium | Yes | Retrospective clinic records | NR. Pre-2006 | 5 | Minimum follow-up | 361 | 135 | 209 | Other | Both | Yes | No | Yes |
| Louis 200326 | France | No | Prospective clinic | NR. Pre-2001 | No information | 90 | 18 | 53 | B2/B3 | Both | Yes | Yes | Yes | |
| Lovasz 201327 | Hungary | Yes | Prospective cohort | 1977–2008 | 13.5 (6–19.5) | Median (IQR) | 287 | 33 | 110 | B2/B3 | Both | No | No | Yes |
| Lunney 201558 | Australia | Yes | Prospective registry | 1955–2012 | 9 (3–16) | Median (IQR) | 622 | 212 | 212 | Surgery | Both | No | No | Yes |
| Malmborg 201559 | Sweden | Yes | Retrospective clinic records | 1990–2007 | 8.8 (1.0–20.8) | Median (range) | 161 | 25 | 25 | B2/B3/surgery | Children | No | No | Yes |
| Mazor 201160 | Israel | No | Prospective clinic | 2000–10 | 12 | Mean | 146 | 65 | 65 | B2/B3/surgery | Both | No | No | Yes |
| Moon 201461 | The Republic of Korea | Yes | Retrospective hospital clinics | 1987–2012 | 4.4 (2.8) | Mean (SD) | 728 | 126 | 126 | Surgery | Adults | No | No | Yes |
| Nasir 201362 | New Zealand | Yes | Retrospective genetic case–control | 2003–13 | 9 | Mean | 503 | 240 | 240 | Surgery | Both | No | Yes | Yes |
| Nasir 201363 | New Zealand | Yes | Retrospective genetic case control | 2003–13 | 9 | Mean | 503 | 240 | 240 | Surgery | Both | No | Yes | Yes |
| Nunes 201364 | Spain | Yes | Retrospective registry | NR. 2006– | 7.6 (3–13) | Median (IQR) | 3118 | 649 | 1313 | Surgery, B2 | Adults | No | No | Yes |
| Odes 200165 | Israel | Yes | Prospective cohort | NR. Pre-2000 | 7.1 (8.0) | Mean (SD) | 208 | 64 | 64 | Surgery | Adults | No | No | Yes |
| Oh 201766 | The Republic of Korea | No | Prospective registry | 2008–10 | 7.9 (6.8–8.0) | Median (IQR) | 339 | 59 | 59 | Surgery | Adults | Yes | No | No |
| Oriuchi 200367 | Japan | No | Retrospective clinic records | 1965–98 | 9.9 (7.5) | Mean (SD) | 146 | 44 | 44 | Surgery | Both | No | No | Yes |
| Pandey 201568 | Singapore | Yes | Retrospective clinic records | 1970–2013 | 7.3 (2.9–13.0) | Median (range) | 430 | 75 | 112 | Surgery | Both | No | No | Yes |
| Papi 200569 | Italy | No | Retrospective clinic records | 1993–2001 | 4.84 (1–23.2) | Mean (range) | 139 | 47 | 47 | B3 | Both | No | No | Yes |
| Park 201370 | The Republic of Korea | No | Retrospective registry | 1989–2010 | 5.4 | Median | 1403 | 471 | 471 | Surgery | Both | No | No | Yes |
| Pigneur 201071 | France | Yes | Retrospective registry | 2000–7 | 14.7 (10.8–45.2) | Median (range) | 618 | 374 | 374 | Surgery | Both | No | No | Yes |
| Pittet 201328 | Switzerland | Yes | Retrospective clinic cohort | 2006–13 | > 10 | Range | 1026 | 83 | 463 | Surgery | Both | No | No | Yes |
| Polito 199672 | USA | No | Retrospective clinic records | 1985–91 | 14 | Mean | 555 | 85 | 334 | Surgery | Both | No | No | Yes |
| Posovszky 201373 | Germany | No | Prospective clinic | NR. Pre-2013 | 15 (11) | Mean (SD) | 202 | 30 | 109 | Surgery | Both | No | Yes | Yes |
| Protic 200874 | Serbia | No | Prospective clinic | 2005–6 | 7 (0–30) | Median (range) | 131 | 33 | 81 | Surgery | Adults | No | Yes | No |
| Renda 200875 | Italy | No | Prospective clinic | NR. Pre-2008 | 6.2 | Median | 182 | 110 | 110 | Surgery | Both | No | Yes | Yes |
| Rieder 201076 | Germany | No | Prospective clinic | 2000–6 | 0.8 (0.1–4.4) | Median (IQR) | 76 | 14 | 14 | Surgery | Adults | Yes | No | No |
| Romberg-Camps 200977 | The Netherlands | Yes | Prospective registry | 1991–2003 | 7.5 (0.2–15.4) | Median (range) | 476 | 133 | 207 | Surgery | Both | No | No | Yes |
| Ryan 201378 | Canada | Yes | Prospective registry | NR. Pre-2013 | 9.3 | Median | 86 | 21 | 100 | Surgery | Adults | Yes | No | Yes |
| Sabate 200879 | France | No | Prospective clinic | 2001–2 | 9.3 (7.7) | Mean (SD) | 225 | 83 | 83 | B2 | Adults | No | No | Yes |
| Sands 200380 | USA | Yes | Retrospective clinic cohort | 1991–7 | 3 | Minimum follow-up | 251 | 40 | 69 | Surgery | Both | No | No | Yes |
| Savoye 201281 | France | Yes | Retrospective registry | 1988–2004 | 8 (7–12) | Median (range) | 309 | 115 | 237 | Other | Children | No | No | Yes |
| Schaefer 201082 | USA | Yes | Prospective registry | 2002–8 | 2.0 (1.8–2.3) | Median (IQR) | 845 | 57 | 72 | Surgery | Children | No | No | Yes |
| Shaoul 200983 | Israel | Yes | Prospective clinic | NR. Pre-2009 | 4.9 (3.6) | Mean (SD) | 121 | 28 | 38 | Surgery, B2/B3 | Children | No | Yes | Yes |
| Siegel 201184 | USA | Yes | Unclear. 21 hospital sites | NR. Pre-2008 | 2.8 (0.1–19.6) | Median (range) | 579 | 67 | 67 | B2/B3 | Children | No | No | Yes |
| Siegel 201685 | USA | No | Prospective clinic | NR. Pre-2015 | 6.1 (0.3–15) | Median (range) | 243 | 142 | 142 | B2/B3/Surgery | Adults | Yes | No | Yes |
| Smith 200429 | Scotland | No | Prospective clinic | NR. Pre-2004 | 11.5 (6.7–20) | Median (IQR) | 90 | 28 | 62 | B2/B3 | Adults | No | Yes | Yes |
| Solberg 200786 | Norway | Yes | Prospective cohort | 1990–4 | 10.3 (9–12) | Median (range) | 237 | 34 | 85 | Surgery | Both | No | No | Yes |
| Solberg 201430 | Norway | Yes | Prospective cohort | 1990–4 | 10 | Minimum follow-up | 111 | 48 | 77 | B2/B3/surgery | Both | No | No | Yes |
| Song 201187 | The People’s Republic of China | No | Retrospective clinic cohort | 2000–9 | 4 (1–21) | Median (range) | 167 | 42 | 79 | Surgery | Both | No | No | Yes |
| Tarrant 200888 | New Zealand | Yes | Retrospective genetic case–control | 2003–5 | 6.5 (0.1–65) | Median (range) | 715 | 50 | 85 | B2/B3 | Both | No | No | Yes |
| Thia 201089 | USA | Yes | Retrospective cohort | 1970–2004 | 8.5 (0.01–36) | Median (range) | 248 | 50 | 139 | B2/B3 | Both | No | No | Yes |
| van der Heide 200990 | The Netherlands | No | Prospective clinic | 1995–2005 | 9.4 (4.9–19.0) | Median (IQR) | 258 | 130 | 220 | B2/B3 | Both | No | No | Yes |
| Van Limbergen 200831 | Scotland | Yes | Prospective cohort | 2000–8 | Adult 10.3 (3.8–20.6); children 3.7 (1.7–6.0) | Median (IQR) | 774 | 100 | 305 | Surgery | Both | No | No | Yes |
| Vernier-Massouille 200891 | France | Yes | Prospective registry | 1988–2002 | 7.0 (4.3–10.3) | Median (range) | 394 | 176 | 176 | Surgery | Children | No | No | Yes |
| Vester-Andersen 201492 | Denmark | Yes | Prospective cohort | 2003–4 | 7.7 (7.1–8.4) | Median (IQR) | 213 | 49 | 49 | Surgery | Both | No | No | Yes |
| Yaari 201693 | Israel | No | Retrospective clinic records | 2006–14 | 1 | Minimum follow-up | 126 | 59 | 59 | Surgery | Both | Yes | No | No |
| Yang 201132 | The People’s Republic of China | Yes | Retrospective clinic records | NR. Pre-2011 | NR | 207 | 147 | 166 | Other | Both | No | No | Yes | |
| Zabana 201333 | Spain | Yes | Prospective registry | 1994–2003 | 7.6 (5.8–11.6) | Median (IQR) | 246 | 43 | 109 | Surgery | Both | No | No | Yes |
The forest plots for predictors indicate data characteristics, including event, paediatric versus adult versus mixed population, and whether or not patients with severe disease were included at baseline. For each predictor meta-analysis, we ensured that patients were included in analyses only once. Figure 2 presents the ROB summarised across all studies.
FIGURE 2.
Risk of bias summarised across all studies.

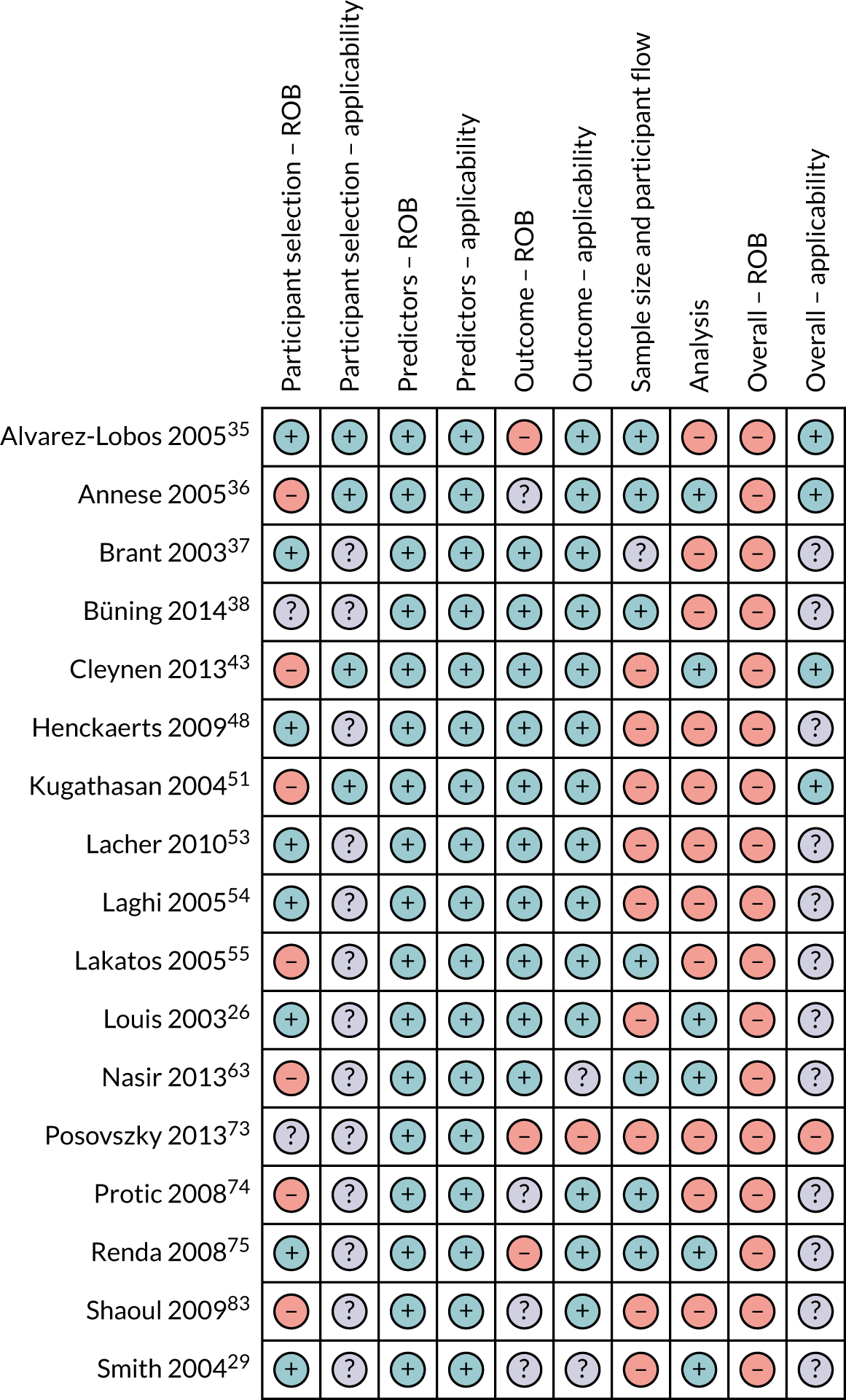
Most of the studies were rated as having high ROB in at least one domain. Accordingly, ‘Overall ROB’ was rated as ‘high’ in 65 (92%) studies. Only three studies were rated as having ‘low’59,89,91 ROB and three were rated as ‘unclear’. 28,58,79 Concern regarding ‘Overall applicability’ was rated as ‘low’ or ‘unclear’, except for five studies. 30,46,60,67,73 ROB and applicability for individual studies are presented in Box 1 and Figures 3–5.
Low ROB (n = 41) was determined when patients with severe disease could be excluded at baseline in data extraction (n = 42) and the study design was not a case–control design (n = 68). Results are at ROB when included patients have severe disease at baseline (n = 12 included; n = 16 unclear), as the results refer to a mixture of prediction of severe disease and risk of diagnosis.
Fifty studies were rated as being of low concern for applicability. Twenty studies were rated as unclear because of poor reporting and one study was rated as being of high concern owing to restricting enrolment to Indian patients who had received previous antituberculous therapy.
Predictor domainLow ROB, for which measurement of predictors would not cause ROB, was identified in 69 studies. In two studies,66,78 there is a potential ROB related to serological biomarker measurement because of the time of serum collection.
Seventy studies were rated as being of low concern for predictor assessment applicability. One study78 was rated as unclear for applicability because over 50% of patients had severe disease at enrolment when the serum sample was taken.
Outcome domainForty-seven studies were rated as being at a low ROB. Studies were rated as at a high ROB when the definition of severe disease did not correspond to our standard definitions, sometimes included steroid dosing (n = 7), or where perianal surgery was included as surgery (n = 12).
Sixty-one studies were rated as being of low concern for outcome applicability, six were rated as being unclear for applicability of outcome definitions because of poor reporting and four were rated as being of high concern as a result of less relevant outcome definitions.
Flow and timing domainThirty-seven studies had a low ROB rating, based on an average time to event of 5–10 years and more than 20 events in the analysis. A total of 31 studies were rated as being at high ROB (studies could be rated as at high ROB for more than one reason): 30 studies because the average time to event was not between 5 and 10 years, two studies, in addition, had fewer than 20 events; and one study’s time to event was unclear as well as having fewer than 20 events. In three studies, the ROB was unclear as time to event was unclear and there were more than 20 events in the analysis.
Applicability is not assessed for this domain.
Analysis domainThirty-one studies were rated as being at low ROB in the analysis because non-significant results were reported for at least three standard predictors. Forty studies were rated at high ROB because fewer than three standard predictors were reported with significant results. Standard predictors were defined as those included in this overview.
Applicability is not assessed for this domain.
OverallThree studies were rated as being at low ROB in all domains59,89,91 and in three the ROB was unclear. 28,58,79 A total of 65 studies were rated as being at high ROB in at least one domain, mostly owing to ROB in either flow and timing, or the analysis domain.
Forty-five studies were rated as having an overall low concern for applicability, with 21 having an unclear rating and five having a high concern for applicability in at least one domain. The studies that were rated as having a high concern were four studies with composite outcomes defined in a slightly non-standard way and one study that enrolled Indian patients who had received antituberculous therapy only.
FIGURE 3.
Risk-of-bias and applicability concerns summary: review of author judgements regarding each domain for each individual study in the clinical review. Orange circles indicate high ROB; blue circles indicate low ROB; purple circles indicate uncertain ROB.
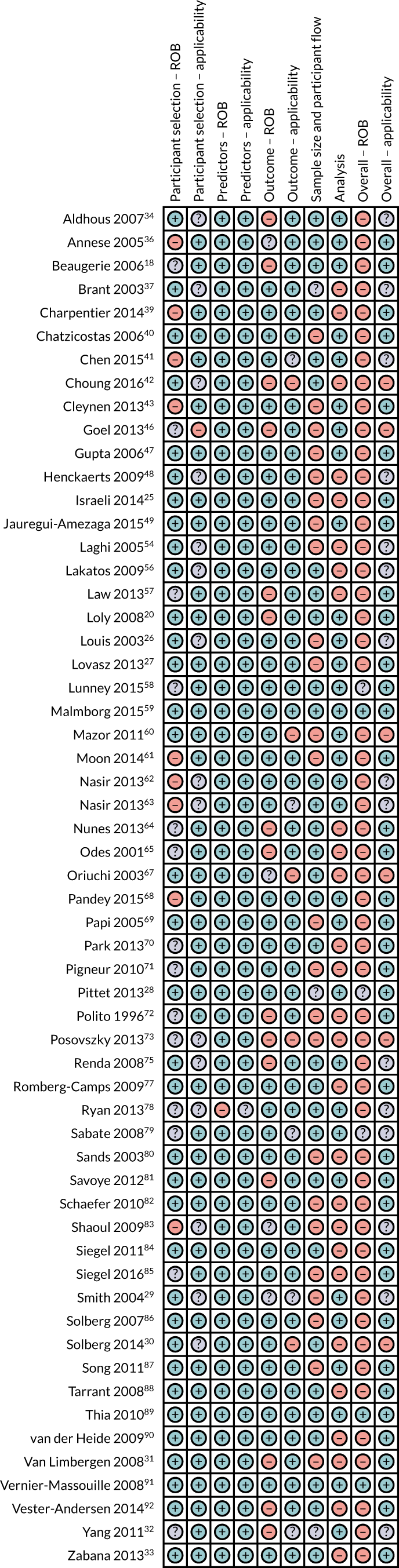
FIGURE 4.
Risk-of-bias and applicability concerns summary: review of author judgements regarding each domain for each individual study in the serological review. Orange circles indicate high ROB; blue circles indicate low ROB; purple circles indicate uncertain ROB.
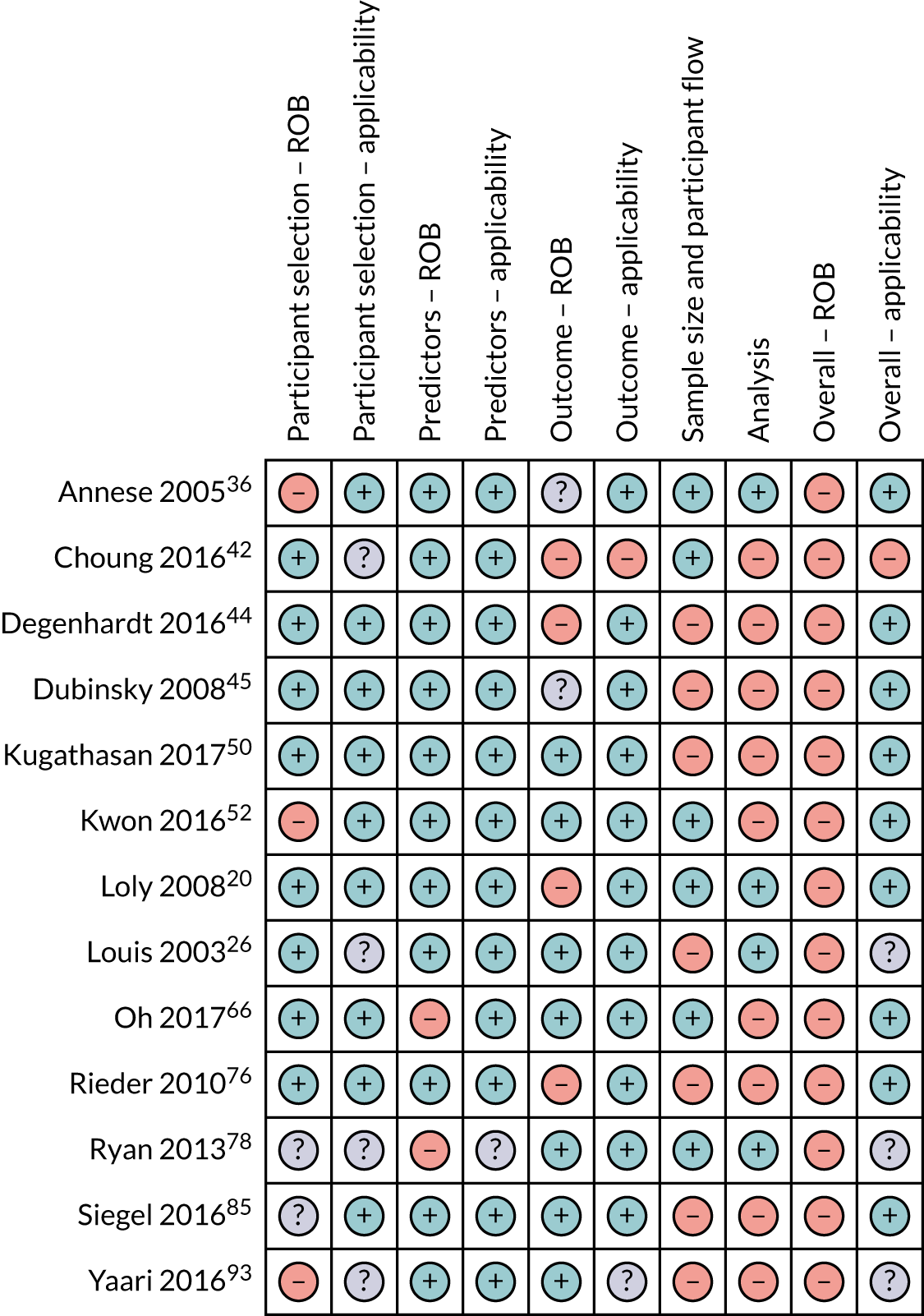
FIGURE 5.
Risk-of-bias and applicability concerns summary: review of author judgements regarding each domain for each individual study in the genetic review. Orange circles indicate high ROB; blue circles indicate low ROB; purple circles indicate uncertain ROB.
Overall, we identified 12 individual predictors eligible for inclusion: three serological, one genetic and eight clinical. There were no radiological, endoscopic or histological predictors that met our criteria for inclusion in the review.
Paediatric data are presented in the forest plots, but there were insufficient for subgroup analysis.
Table 6 shows the summary of findings across the meta-analyses performed.
| Biomarker | Overall number of participants; events expressed as minimum (studies) | Estimates reporteda | Meta-analysis estimate (95% CI); n studies | Notes |
|---|---|---|---|---|
| ASCA | 2559; 369 (10) |
|
OR 2.29 (1.31 to 3.99), 6 | Five studies report increased severe disease with presence of ASCA |
| Anti-CBir1 | 1878; 302 (5) |
|
OR 1.91 (0.85 to 4.31), 3 | Two studies report increased risk with presence of anti-CBir1 |
| CRP | 1170; 274 (3) |
|
OR 1.17 (0.85 to 1.61), 3 | Two studies report increased severe disease with presence of CRP |
| NOD2 any variant | 5526; 2683 (17) |
|
Surgery OR 1.69 (1.43 to 2.00), 16 | 14 studies report increased severe disease with presence of the NOD2 variant |
| Disease behaviour | 8678; 3142 (16) |
|
|
All studies report increased severe disease with presence of B2, B3 or B2/B3 compared with B1 |
| Age at diagnosis | 19,623; 7010 (43) | All age data:
|
|
Age comparisons limited by data cut-off points reported in studies. Aged < 17 years at diagnosis has lower OR for severe disease than that for older age |
| Duration of disease | 8690; 1714 (14) | All time points:
|
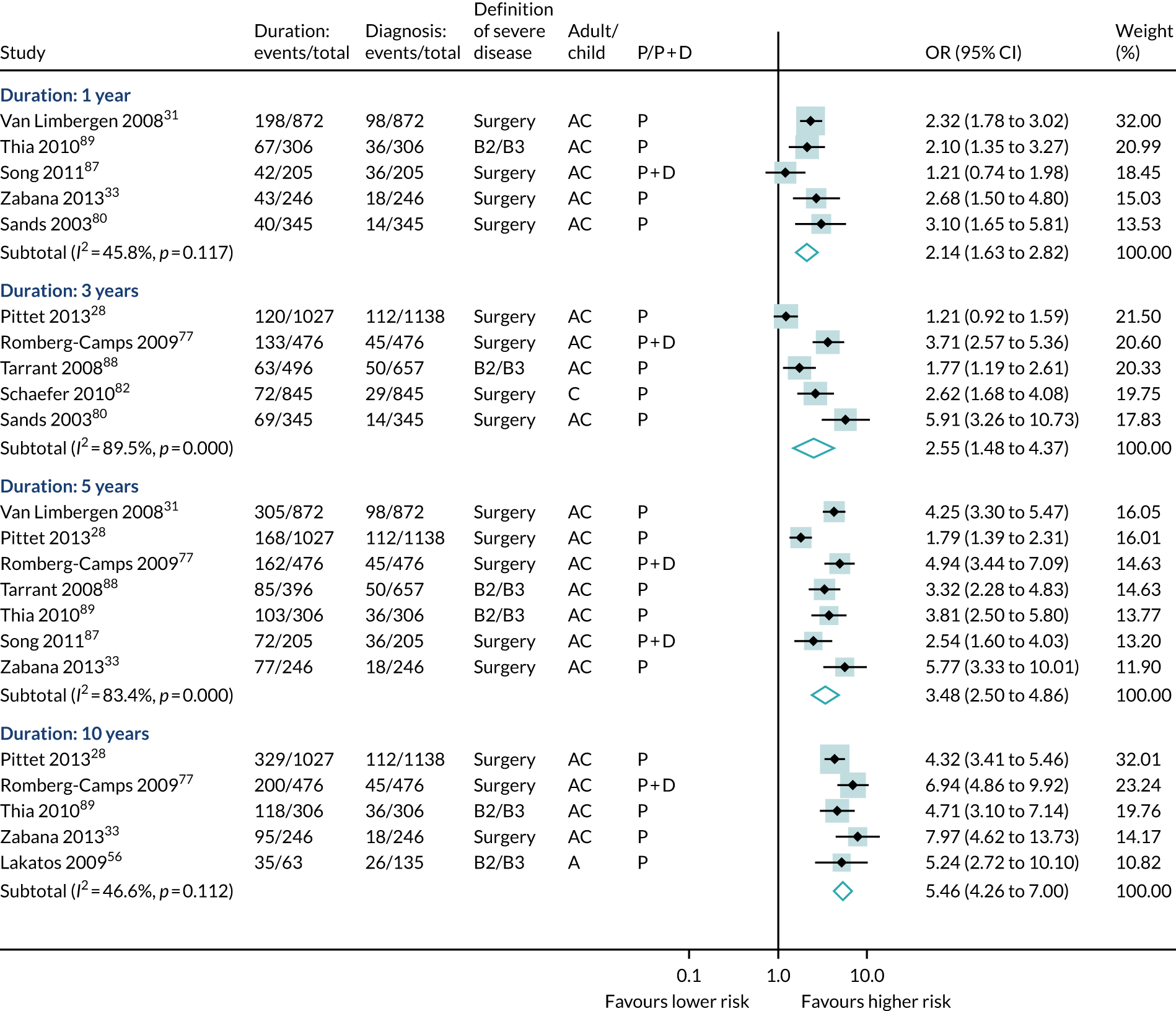
5 years: OR 3.48 (2.50 to 4.86), 7 | Based on seven studies, risk of severe disease increases with time from diagnosis |
| Location of disease |
|
|
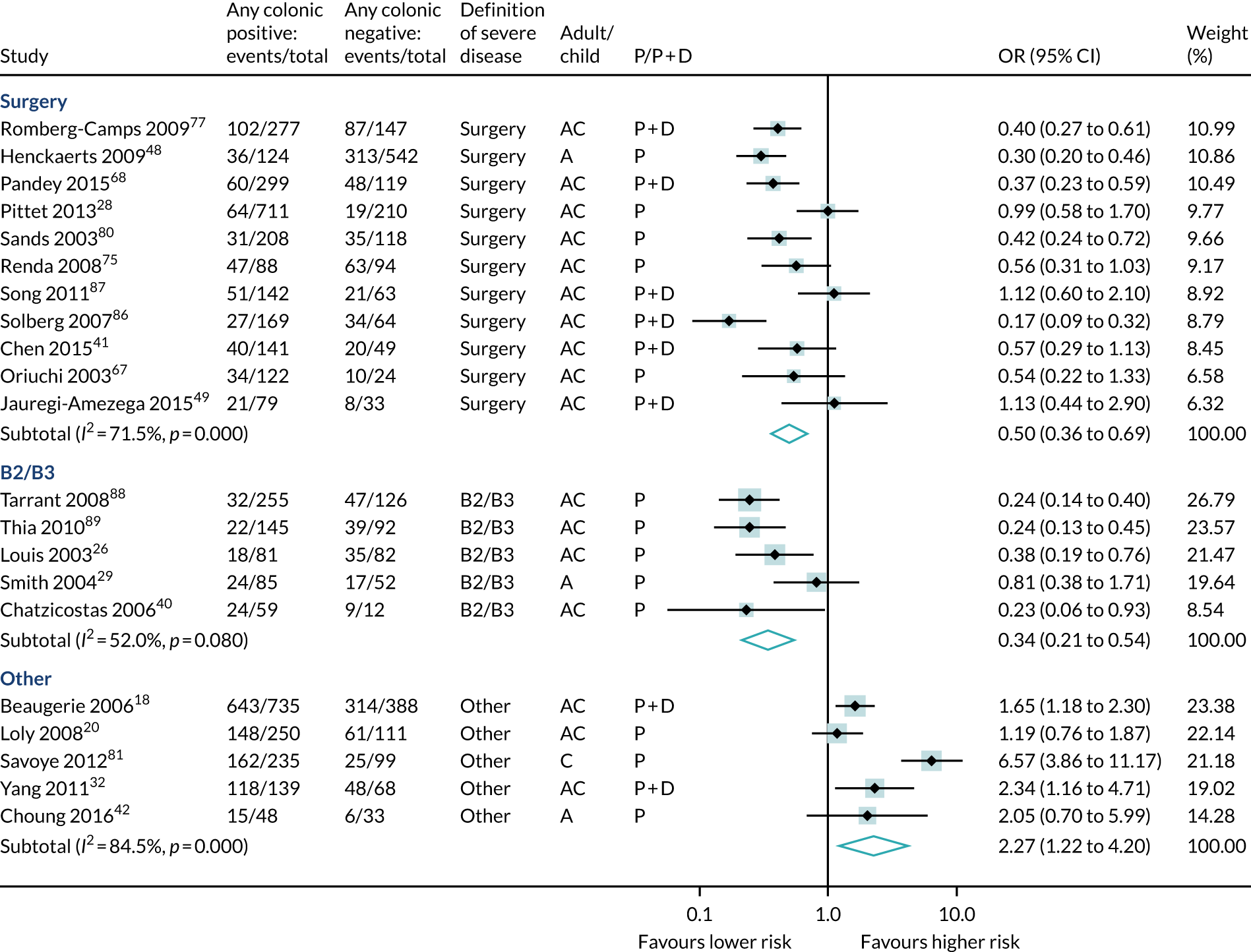
Disease confined to the colon is a predictor for less severe disease based on surgical outcome than having disease at any other location. Data synthesis only feasible for colonic only or colonic any | |
| Perianal disease | 13,483; 5510 (24) |
|
OR 1.84 (1.29 to 2.62); 13 | 10 studies report increased severe disease with presence of perianal disease |
| Smoking | 11,475; 5097 (34) |
|
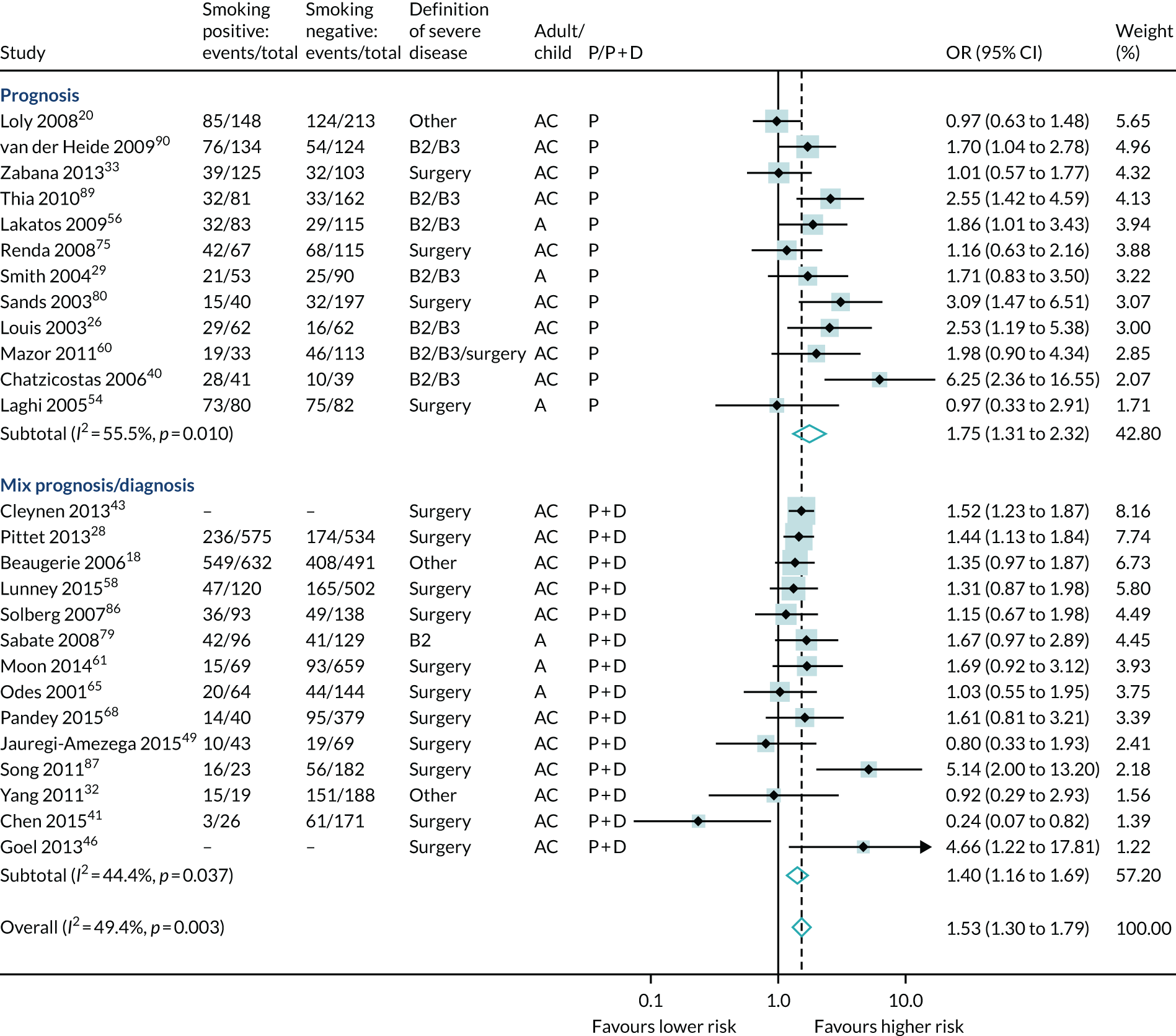
OR 1.53 (1.30 to 1.79); 26 | 21 studies report increased severe disease with current smoking |
| Sex | 14,489; 5350 (35) |
|
OR 1.14 (0.98 to 1.31); 23 | Sex has a non-significant association with severe disease. Approximately half of the studies favour men and half favour women for lower risk |
| Family history (Crohn’s disease or IBD) | 5687; 1413 (18) |
|
OR 1.05 (0.81 to 1.36); 12 | Family history has a non-significant association with severe disease. Five studies associated family history with increased risk while seven associated no family history with increased risk |
Meta-analysis of clinical predictors
The following clinical predictors had prognostic data available from fewer than five studies and, therefore, did not meet the inclusion criteria for the review: submucosal plexitis, fever, weight loss, poor growth, medication, upper disease, Jewish ethnicity, joint problems, abdominal pain, oral contraception (women), ethnic origin, systematic manifestations, systematic steroid use, azathioprine/biologicals, granuloma, depression, cancer, diarrhoea, time to diagnosis from symptom onset, severe endoscopic lesions, visceral fat area, rectal bleeding, fatigue, use of total parenteral nutrition, bowel stenosis, internal fistula, alternative initial diagnosis, abscess, stricture, development of strictures during follow-up, development of fistulae during follow-up, initial diagnosis at surgery, steroids per year, flares per year, married/common law, low conscientiousness, high neuroticism, low adherence to medication, adverse childhood experience, childhood sexual abuse, childhood physical abuse, high childhood adversity score, asthma, eczema, glandular fever, kidney stones, liver disease, mental illness, bronchiectasis, tonsillectomy, chole, grommet, immunised against measles, immunised against mumps, immunised against tuberculosis, antibiotic consumption, medication consumption, breastfed, alcohol, vegetarian, takeaways, public swimming, sand pit, farm, shared bedroom, continuous course, frequent relapse, symptoms at diagnosis, symptoms at 1 year, persistent mucosal erosions, jejunal involvement, Crohn’s Disease Activity Index (CDAI), educational level, type of centre, nausea/vomiting, diabetes mellitus, coronary artery disease and hypertension.
We were able to meta-analyse seven clinical markers from 58 studies. These were Montreal disease behaviour, age, disease duration, disease location, smoking, sex and family history.
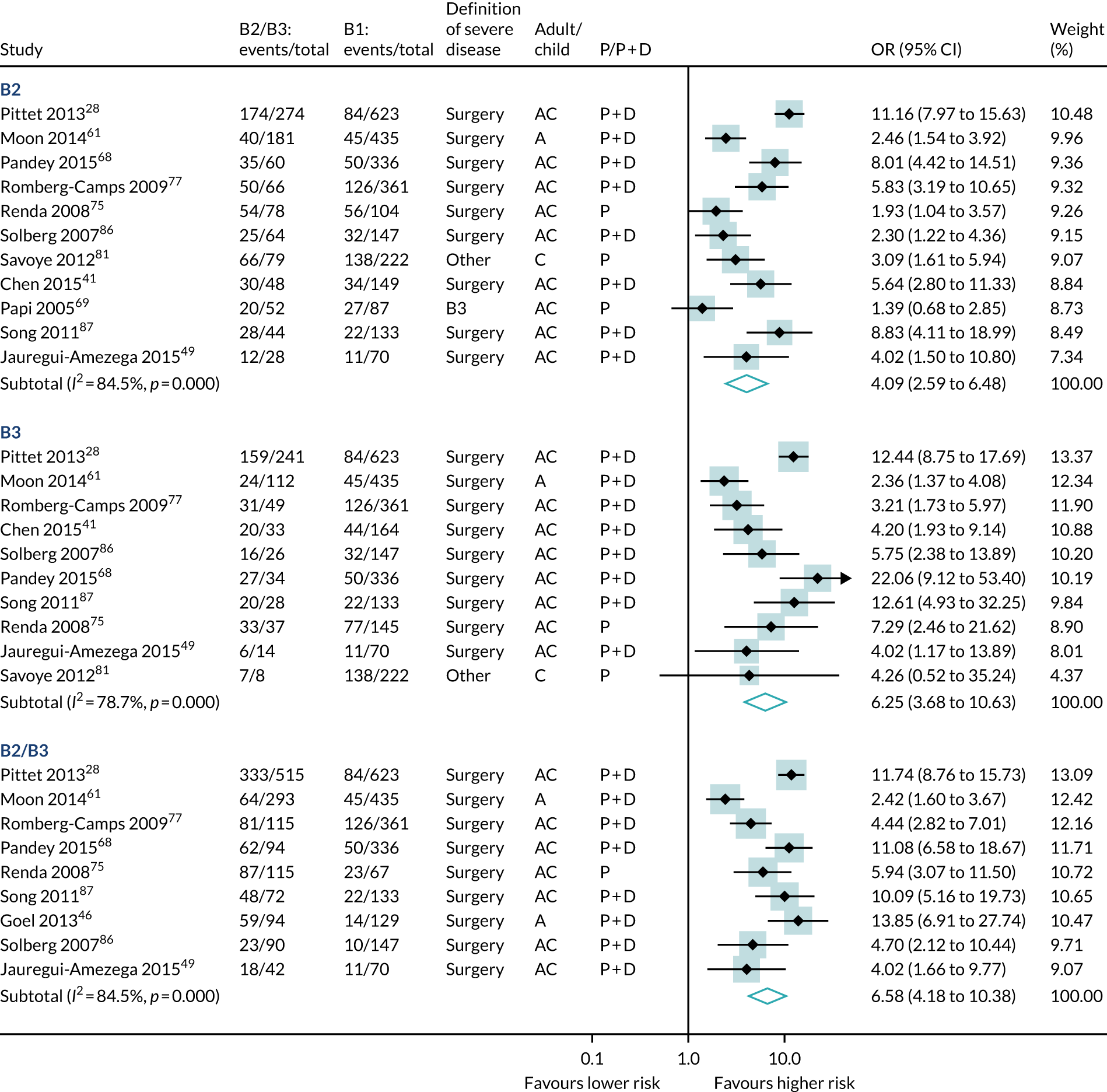
A meta-analysis of 12 studies (4376 participants, 1551 events) found that B2 stricturing, B3 fistulating and either severe or disabling disease predicted severe disease more powerfully than B1 inflammation alone (see Table 6 and Figure 6). B2 and B3 Montreal disease behaviours were the strongest predictors of subsequent severe disease in our review (B2: OR 4.09, 95% CI 2.59 to 6.48; B3: OR 6.25, 95% CI 3.68 to 10.63).
FIGURE 6.
Forest plot of unadjusted ORs investigating Montreal disease behaviour as predictive of severe Crohn’s disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
The participants’ age at diagnosis was examined in 43 studies (19,623 participants, 7010 events). Most of the studies categorised age using the Montreal/Vienna thresholds, so data were greatest for three groups: aged < 17 years, aged 17–40 years and aged > 40 years. Overall, diagnosis at < 17 years of age was associated with a lower risk of severe Crohn’s disease than other ages (see Table 6 and Figures 7–9). Disease duration was examined in 14 studies (8690 participants, 1714 events). A meta-analysis found that increased duration of disease was associated with significant risk of severe disease at all durations examined (see Table 6 and Figures 7–9).
FIGURE 7.
Forest plot of unadjusted ORs investigating age and disease duration as predictive markers for severe Crohn’s disease: age at diagnosis < 17 years compared with > 17 years, 17–40 years and > 40 years. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
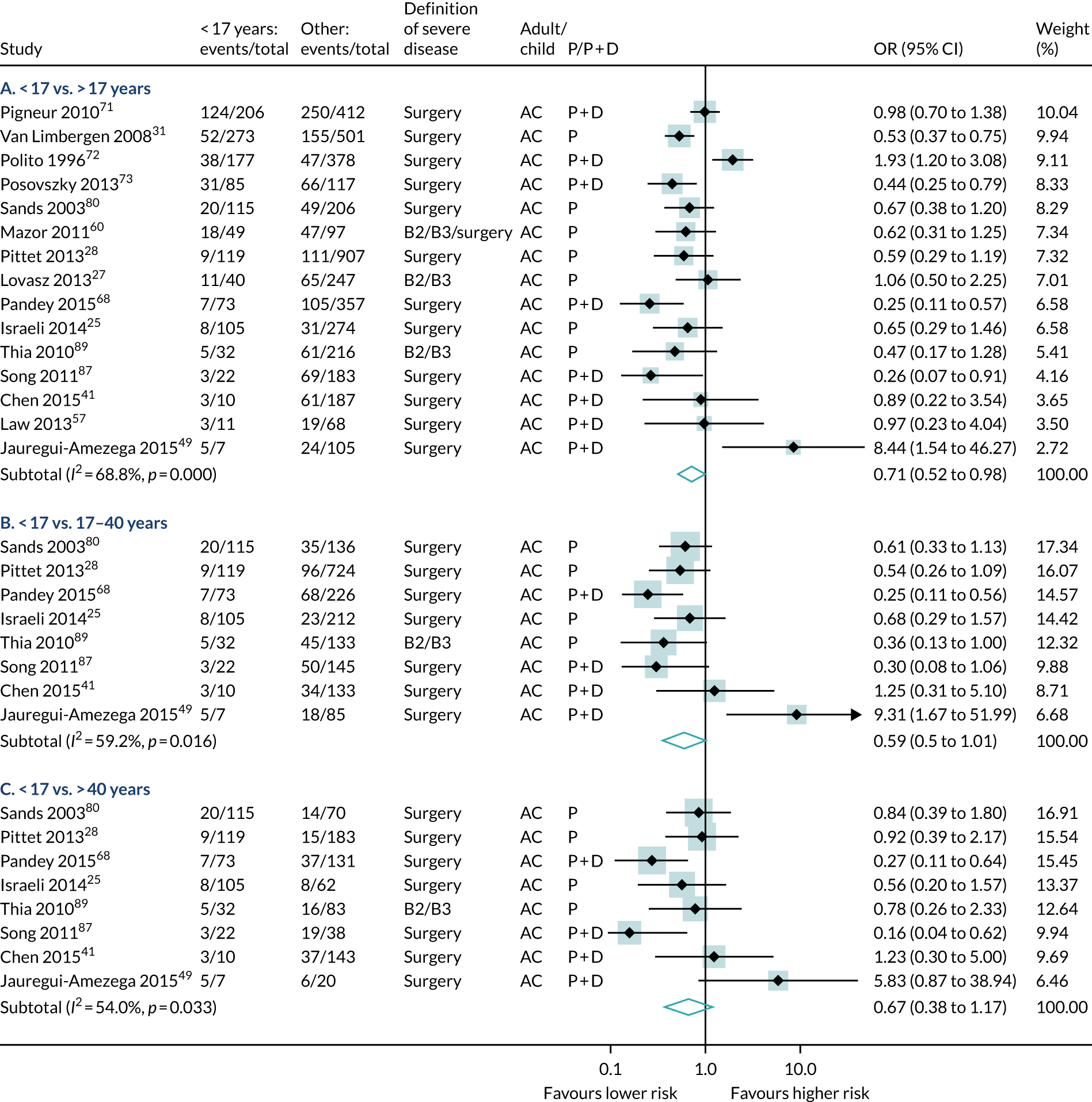
FIGURE 8.
Forest plot of unadjusted ORs investigating age and disease duration as predictive markers for severe Crohn’s disease: age at diagnosis 17–40 years compared with > 40 years. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

FIGURE 9.
Forest plot of unadjusted ORs investigating age and disease duration as predictive markers for severe Crohn’s disease: duration of disease, prognostic studies only. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Disease location was examined in 32 studies (10,877 participants, 4193 events). Studies were diverse regarding the segments and/or segment combinations described. We could analyse ‘colonic disease alone’ and ‘any colonic disease’ versus other locations. Overall, colonic disease alone conferred significantly lower surgery risk than other locations (OR 0.42, 95% CI 0.31 to 0.58) (see Table 6 and Figure 10).
FIGURE 10.
Forest plot of unadjusted ORs investigating disease location as predictive of severe Crohn’s disease: colonic disease only. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

Similarly, any colonic disease (23 studies, 7373 participants, 3086 events) predicted lower surgical or B2/B3 risk than no colonic disease (see Table 6 and Figure 11).
FIGURE 11.
Forest plot of unadjusted ORs investigating disease location as predictive of severe Crohn’s disease: any colonic involvement. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Perianal disease (24 studies, 13,483 participants, 5510 events) was associated with a significantly increased risk of subsequent severe disease overall (1.84 OR, 95% CI 1.29 to 2.62) (see Table 6 and Figure 12).
FIGURE 12.
Forest plot of unadjusted ORs investigating disease location as predictive of severe Crohn’s disease: perianal disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

A total of 34 studies reported an association of smoking with severe disease (11,475 participants, 5097 events); a meta-analysis of 26 studies found that current smoking increased the risk of severe Crohn’s disease significantly (OR 1.53, 95% CI 1.30 to 1.79) (see Table 6 and Figure 13).
FIGURE 13.
Forest plot of unadjusted ORs investigating smoking as a predictive marker for severe Crohn’s disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Sex was examined in 35 studies (14,489 participants, 5350 events) and, overall, was not a significant predictor of severe Crohn’s disease (see Table 6 and Figure 14).
FIGURE 14.
Forest plot of unadjusted ORs investigating sex as a predictive marker for severe Crohn’s disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

Family history was examined in 18 studies (5687 participants, 1413 events). A meta-analysis found no consistent direction and no significant association of family history and severe Crohn’s disease (OR 1.05, 95% CI 0.81 to 1.36) (see Table 6 and Figure 15).
FIGURE 15.
Forest plot of unadjusted ORs investigating family history as a predictive marker for severe Crohn’s disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

Meta-analysis of serological predictors
The following serological predictors had prognostic data available from fewer than five studies and, therefore, were not subject to meta-analysis: thrombocytosis, faecal calprotectin, anti-Saccharomyces cerevisiae antibodies (ASCA)-immunoglobulin (Ig)G, anti-CBir1, white blood cell (WBC), albumin, erythrocyte sedimentation rate (ESR), platelets, anaemia, CRP, haematocrit, ASCA-IgA, ferritin, anti-Fla2, anti-FlaX, anti-L IgA, anti-C IgA, anti-Saccharomyces chitobioside antibody (ACCA)-IgA, anti-Saccharomyces laminaribioside antibody (ALCA)-IgG, anti-Saccharomyces mannobioside antibody (AMCA)-IgG and anti-I2.
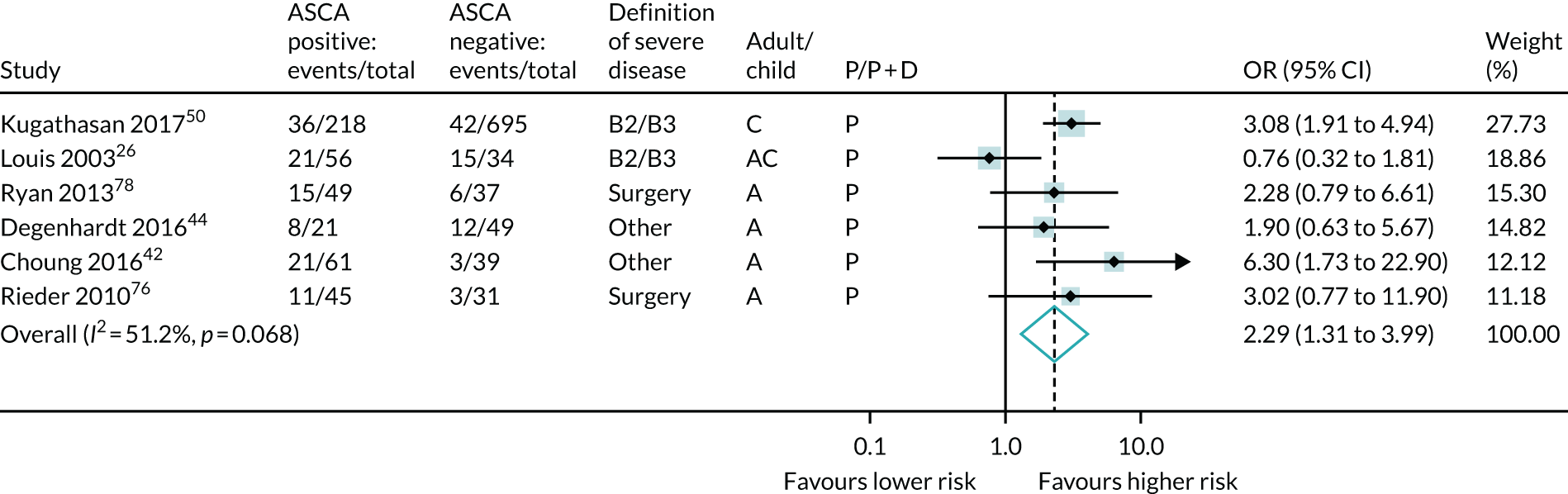
Three serological markers (ASCA, anti-CBir1 and CRP) from 13 studies were meta-analysed. ASCA and anti-CBir1 showed potential prognostic association; ASCA-positive patients had significantly increased odds of developing severe disease (OR 2.29, 95% CI 1.31 to 3.99; six studies) (see Table 6 and Figure 16).
FIGURE 16.
Forest plot of unadjusted ORs investigating serological markers as predictors of severe Crohn’s disease: ASCA. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Anti-CBir1 data were identified from five studies; meta-analysis from ORs did not reach significance (see Table 6 and Figure 17), but additional evidence from hazard ratios indicated a significant association between anti-CBir1 and severe Crohn’s disease (Table 7).
FIGURE 17.
Forest plot of unadjusted ORs investigating serological markers as predictors of severe Crohn’s disease: anti-CBir1. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.

| Predictor | Study (first author and year) | Definition of serious disease | Number of participants | Number of events | Outcome | Significant | Point estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| ASCA | Annese 200536 | B2 | 316 | NR | p-value | Yes | 0.046 |
| Dubinsky 200845 | Surgery | 592 | 61 | HR | Yes | 3.2 (1.1 to 9.5) | |
| Loly 200820 | Other | 73 | NR | p-value | No | NS | |
| Siegel 201685 | B2/B3/surgery | 243 | 142 | HR | Yes | 1.42 (1.24 to 1.63) | |
| CBir-1 | Dubinsky 200845 | B2/B3 | 536 | 37 | HR | Yes | 2.5 (1.2 to 5.2) |
| Siegel 201685 | B2/B3/surgery | 243 | 142 | HR | Yes | 1.47 (1.24 to 1.75) | |
| CRP | All studies in MA | ||||||
| NOD2 | Shaoul 200983 | Surgery | 119 | 38 | p-value | No | NS |
| Disease behaviour (B3) | Vester-Andersen 201492 | Surgery | 213 | 49 | p-value | No | NR |
| Park 201370 | Surgery | 1403 | 471 | Adj HR | Yes | 5.67 (4.51 to 7.11) | |
| Disease behaviour (NR) | Brant 200337 | Surgery | 257 | 183 | p-value | NR | NR |
| Disease behaviour (B2/B3) | Nunes 201364 | Surgery | 3118 | 1269 | Adj HR | Yes | 4.42 (3.87 to 5) |
| Age at diagnosis | Annese 200536 | Surgery | 316 | 87 | Adj OR | Yes | 1.37 (1.05 to 1.79) |
| Duration of disease | Renda 200875 | Surgery | 182 | 110 | HR | No | 0.9 (0.9 to 1.02) at 1.5 years |
| Goel 201346 | B2/B3 | 223 | 93 | p-value | No | > 0.3 at 6 years | |
| Location: colonic only | Malmborg 201559 | B2/B3/surgery | 161 | 25 | HR | No | 0.72 (0.33 to 1.59) |
| Aldhous 200734 | Surgery | 251 | 113 | Adj HR | Yes | 0.27 (0.16 to 0.45) | |
| Park 201370 | Surgery | 1403 | 471 | Adj HR | Yes | 0.36 (0.2 to 0.64) | |
| Location: any colonic | Papi 200569 | B3 | 139 | 47 | Adj HR | No | 0.7 (0.3 to 1.6) |
| Annese 200536 | Surgery | 316 | 87 | p-value | No | NS | |
| Perianal | Annese 200536 | Surgery | 316 | 87 | p-value | No | NS |
| Brant 200337 | Surgery | 257 | 183 | p-value | No | NS | |
| Chen 201541 | Surgery | 197 | 64 | HR | No | 0.68 (0.32 to 1.42) | |
| Law 201357 | Other | 79 | 34 | p-value | No | NS | |
| Nasir 201362 | Surgery | 503 | 240 | Adj OR | Yes | 2.84 (1.83 to 4.38) | |
| Nunes 201364 | Surgery | 3201 | 1313 | Adj HR | Yes | 1.32 (1.17 to 1.5) | |
| Park 201370 | Surgery | 1403 | 471 | Adj HR | No | 1.09 (0.91 to 1.32) | |
| Ryan 201378 | Surgery | 182 | 77 | p-value | No | 0.06 | |
| Siegel 201685 | B2/B3/surgery | 243 | 142 | HR | No | 0.86 (0.54 to 1.37) | |
| Tarrant 200888 | B2/B3 | 715 | 189 | HR | Yes | 1.62 (1.28 to 2.05) | |
| Vernier-Massouille 200891 | Surgery | 394 | 176 | HR | No | 0.94 (0.53 to 1.65) | |
| Smoking | Lovasz 201327 | B2/B3 | 287 | 110 | HR | No | 1.48 (0.96 to 2.37) |
| Law 201357 | Other | 79 | 34 | Adj HR | Yes | 4.68 (1.03 to 4.09)a | |
| Brant 200337 | Surgery | 257 | 183 | p-value | No | NS | |
| Goel 201346 | Surgery | 223 | 73 | p-value | No | > 0.3 | |
| Ryan 201378 | Surgery | 182 | 77 | p-value | No | 0.05 | |
| Vester-Andersen 201492 | Surgery | 213 | 49 | p-value | No | NS | |
| Annese 200536 | Surgery | 316 | 87 | Adj OR | Yes | 1.42 (1.06 to 1.88) | |
| Papi 200569 | B3 | 139 | 47 | Adj OR | No | 1.2 (0.5 to 2.6) | |
| Sex | Nasir 201363 | Surgery | 503 | 240 | Adj OR | No | 1.16 (0.77 to 1.74) |
| Papi 200569 | B3 | 139 | 47 | Adj OR | No | 1.67 (0.33 to 2.17) | |
| Gupta 200647 | Surgery | 989 | 128 | HR | No | 1.42 (1.00 to 2.01) | |
| Malmborg 201559 | B2/B3/surgery | 161 | 25 | HR | No | 0.59 (0.31 to 1.15) | |
| Siegel 201184 | B2/B3 | 579 | 67 | HR | No | 0.55 (0.3 to 1.01) | |
| Charpentier 201439 | Surgery | 367 | 103 | HR | No | 1.3 (0.8 to 1.9) | |
| Vernier-Massouille 200891 | Surgery | 394 | 176 | HR | No | 0.96 (0.71 to 1.3) | |
| Aldhous 200734 | B2/B3 | 274 | 105 | Adj HR | No | 0.89 (0.6 to 1.3) | |
| Annese 200536 | Surgery | 316 | 87 | p-value | No | NS | |
| Brant 200337 | Surgery | 257 | 183 | p-value | NR | NR | |
| Law 201357 | Other | 79 | 34 | p-value | No | NS | |
| Ryan 201378 | Surgery | 182 | 77 | p-value | No | NS | |
| Family history | Papi 200569 | B3 | 139 | 47 | Adj OR | No | 2 (0.5 to 7.6) |
| Malmborg 201559 | B2/B3/surgery | 161 | 17 | HR | No | 0.21 (0.03 to 1.56) | |
| Aldhous 200734 | Surgery | 251 | 113 | Adj HR | No | 1.06 (0.65 to 1.73) | |
| Brant 200337 | Surgery | 257 | 183 | p-value | No | NS | |
| Ryan 201378 | Surgery | 182 | 77 | p-value | No | NS | |
| Tarrant 200888 | B2/B3 | 715 | 85 | p-value | No | NS |
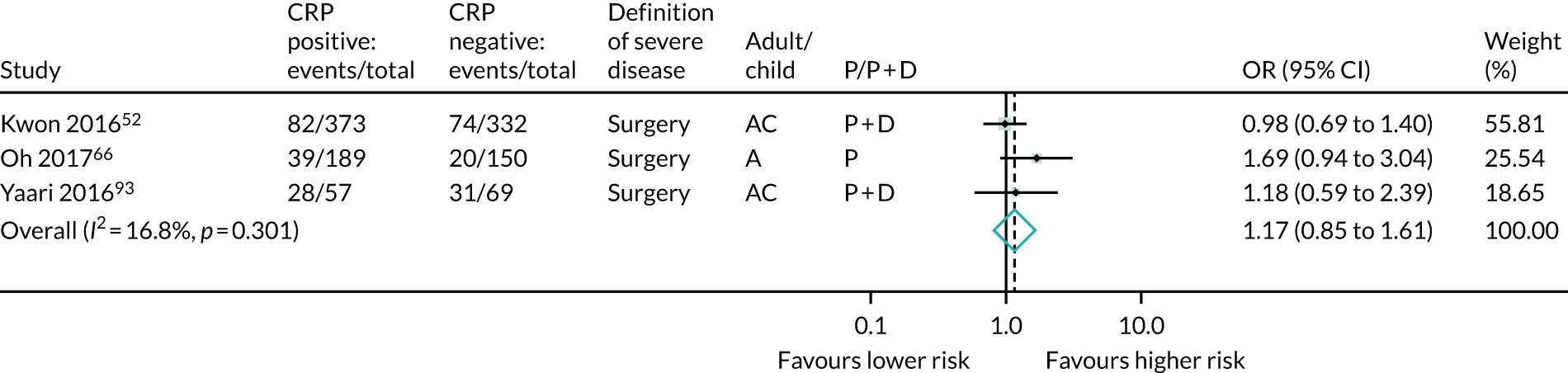
For CRP, data were identified from only three studies (the expert panel requested that CRP was included) and the results found no significant predictive association of CRP and severe Crohn’s disease (see Table 6 and Figure 18).
FIGURE 18.
Forest plot of unadjusted ORs investigating serological markers as predictors of severe Crohn’s disease: CRP. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Meta-analysis of genetic predictors
The following genetic predictors had prognostic data available from fewer than five studies and, therefore, were not subject to meta-analysis: LOC441108, TNFSF15, 5p13.1, NCF4, CX3CR1, JAK2, SBNO2, ZPBP, PTGER4, PUS10, PRDM1, C13ORF31, SLC22A23, TAB2/MAP3K7IP2, PTPN22, ICOSLG, STAT3, PTPN2, NKX2-3, POU2E1, U10, U7, AK097548, CDKAL1, HERC2, ATG4A, NALP3, IL21, CARD8, nucleotide-binding oligomerisation domain-containing protein 2 (NOD2) – all 3 versus 0, TLR4 D299G, FOXO3a, IBD5, DLG5, PAI-1, SMAD3, MMP, TIMP, ATG2A, FNBP1L and ATG4D.
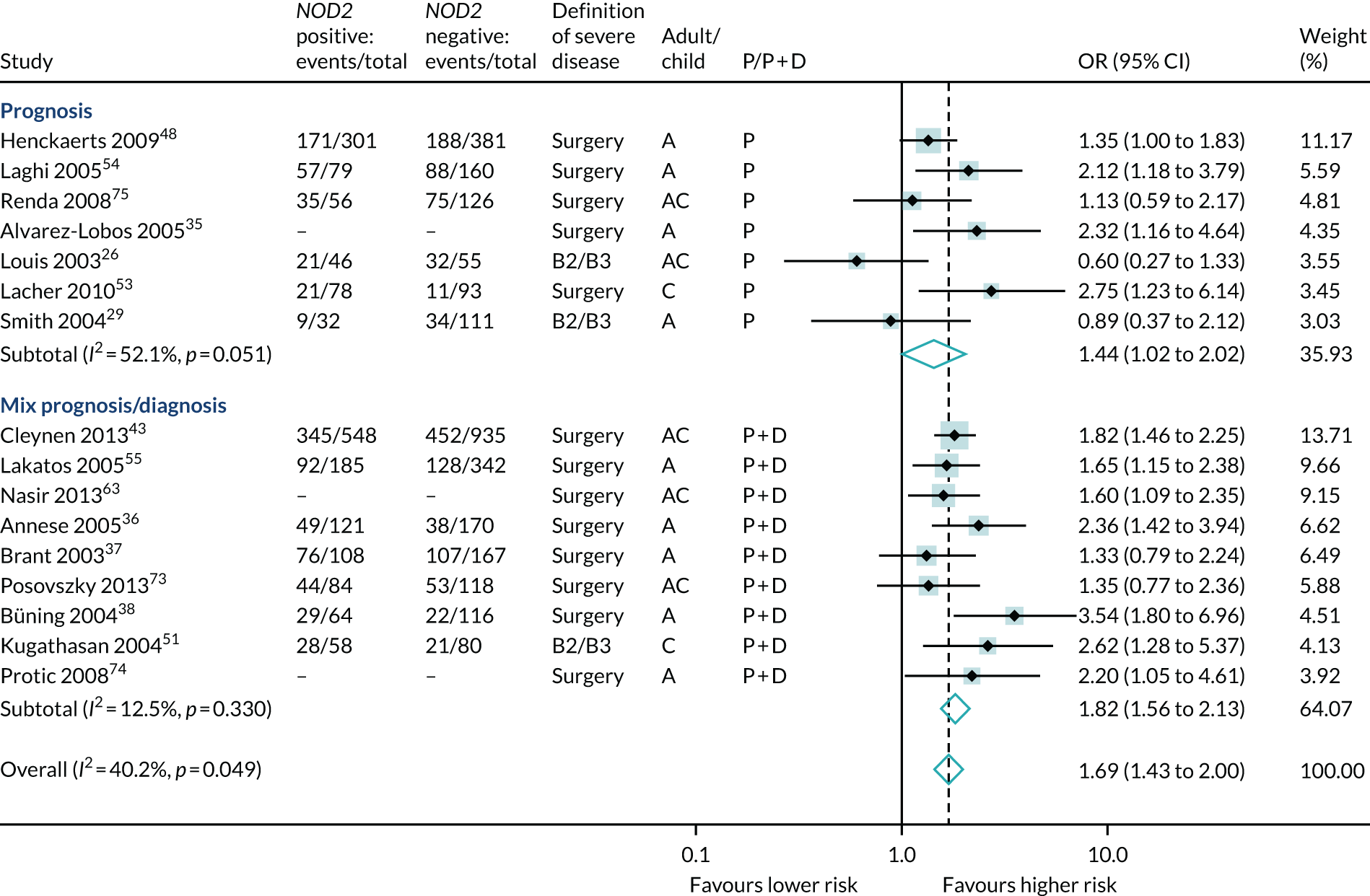
Of the genetic markers, results for NOD2 in at least one variant were reported in 17 studies, although one study reported only p-values and was excluded from meta-analysis. 83 Meta-analysis of 16 studies (5407 participants, 2645 events) suggested higher risk of severe Crohn’s disease with a NOD2 variant gene overall (OR 1.69, 95% CI 1.43 to 2.00); studies were a mix of prognosis and prognosis/diagnosis (see Table 6 and Figure 19).
FIGURE 19.
Forest plot of unadjusted ORs investigating NOD2 (any variant) as a predictor of severe Crohn’s disease. A, adult; AC, both adults and children; C, child; P, predictive effect only; P + D, predictive and diagnostic effect combined.
Identification of existing systematic reviews
During this review, we identified seven previous systematic reviews that investigated the association of mostly serological and some genetic biomarkers with severe Crohn’s disease. 94–100 Three of these reviews95,99,100 did not separate primary studies of diagnosis and prognosis. One review95 identified two studies of predictive biomarkers, one of which76 was also identified as the only prognostic study by a second review. 96 The remaining systematic review101 included three biomarker prediction studies, only one of which met our inclusion criteria.
Search update: August 2020
To obtain an estimate of new data subsequent to our original search, we repeated the search at the time of revising this monograph (August 2020). The search period was extended from 2016 to 17 August 2020, inclusive. The search string identified 4005 indexed studies from PubMed and 6878 studies from EMBASE. The researcher who performed the original search (Darren Boone) examined the abstracts of all of these studies to identify studies of biomarkers with potentially prognostic data. Ultimately, we identified 87 papers, seven of which were identified by our updated search for serological markers that extended to 1 January 2018. That is, at the time of writing there were potentially 80 additional papers that contained prognostic data relevant to our review question. All of these papers were identified by the PubMed search; once duplicates had been removed, the EMBASE search contributed no potentially relevant material. We identified no new biomarker that would satisfy our a priori threshold for inclusion of reporting in five or more individual papers. Our original search retained 29% of those papers subject to full-text review. A similar proportion applied here would suggest that around 23 of these 80 papers would be suitable for inclusion, the large majority of which examine phenotype and/or age and/or smoking. We are, therefore, as confident as we can be that at the time of writing there are insufficient indexed data to alter our conclusions relating to genetic and serological biomarkers, and that our meta-analytical data relating to clinical factors are likely to stand unchanged. Table 8 details the additional potentially relevant research that was identified, which has been split by biomarker(s) investigated.
| Study (first author and year) | Title | Clinical biomarker(s) | Serological biomarker(s) | Genetic biomarker(s) |
|---|---|---|---|---|
| Aaltonen 2019102 | Risk factors for proctectomy in consecutive Crohn’s colitis surgical patients in a reference colorectal centre | Duration, gender and perianal disease | ||
| Aggarwal 2017103 | Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn’s disease to assess remission and predict relapse | CDAI | Calprotectin | |
| Alexakis 2018104 | Smoking status at diagnosis and subsequent smoking cessation: associations with corticosteroid use and intestinal resection in Crohn’s disease | Smoking | ||
| Arieira 2018105 | Clinical course in Crohn’s disease: factors associated with behaviour change and surgery | Age, phenotype and smoking | ||
| Arora 2018106 | Effect of oral tobacco use and smoking on outcomes of Crohn’s disease in India | Sex and smoking | ||
| Arora 2018107 | Colonic Crohn’s disease is associated with less aggressive disease course than ileal or ileocolonic disease | Phenotype | ||
| Assa 2017108 | Perianal pediatric Crohn disease is associated with a distinct phenotype and greater inflammatory burden | Phenotype and perianal disease | ||
| Assa 2018109 | The long-term predictive properties of the Paris classification in paediatric inflammatory bowel disease patients | Phenotype | ASCA | |
| Birimberg-Schwartz 2016110 | pANCA and ASCA in children with IBD-unclassified, Crohn’s colitis, and ulcerative colitis-A longitudinal report from the IBD Porto Group of ESPGHAN | pANCA and ASCA | ||
| Bossuyt 2018111 | Risk stratification for surgery in stricturing ileal Crohn’s disease: the BACARDI risk model | Phenotype | CRP | NOD2 |
| Brückner 2018112 | Incidence and risk factors for perianal disease in pediatric Crohn disease patients followed in CEDATA-GPGE registry | Sex, family history, EIMs and phenotype | ||
| Buisson 2019113 | Faecal calprotectin is a very reliable tool to predict and monitor the risk of relapse after therapeutic de-escalation in patients with inflammatory bowel diseases | Calprotectin | ||
| Burisch 2019114 | Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study | Phenotype | ||
| Chaparro 2019115 | Differences between childhood- and adulthood-onset inflammatory bowel disease: the CAROUSEL study from GETECCU | Age at onset, sex, EIMs, FHx and smoking | ||
| Chaudhry 2017116 | A fixed stricture on routine cross-sectional imaging predicts disease-related complications and adverse outcomes in patients with Crohn’s disease | Phenotype and smoking | Haemoglobin and CRP | |
| Chen 2017117 | Performance of risk prediction for inflammatory bowel disease based on genotyping platform and genomic risk score method | Age, age at diagnosis and phenotype | GWAS | |
| Chhaya 2016118 | Emerging trends and risk factors for perianal surgery in Crohn’s disease: a 20-year national population-based cohort study | Age, sex and location | ||
| Chun 2018119 | Association of perianal fistulas with clinical features and prognosis of Crohn’s disease in Korea: results from the CONNECT study | Phenotype, age and sex | ||
| de Barros 2017120 | Evolution of clinical behavior in Crohn’s disease: factors associated with complicated disease and surgery | Age, smoking, phenotype and age at diagnosis | ||
| Dias 2017121 | The risk of disabling, surgery and reoperation in Crohn’s disease – a decision tree-based approach to prognosis | Phenotype | ||
| Dias 2017122 | Development and validation of risk matrices for Crohn’s disease outcomes in patients who underwent early therapeutic interventions | Age at diagnosis, perianal disease and phenotype | ||
| Diederen 2017123 | Raised faecal calprotectin is associated with subsequent symptomatic relapse, in children and adolescents with inflammatory bowel disease in clinical remission | PCDAI | CRP and calprotectin | |
| Dong 2019124 | A novel surgical predictive model for Chinese Crohn’s disease patients | Phenotype | CRP, WBCs and PLts | |
| Foster 2019125 | Consecutive faecal calprotectin measurements for predicting relapse in paediatric Crohn’s disease patients | PCDAI | CRP and ESR | |
| Fumery 2016126 | Natural history of Crohn’s disease in elderly patients diagnosed over the age of 70 years: a population-based study | Phenotype and EIMs | ||
| Fumery 2019127 | Long-term outcome of paediatric-onset Crohn’s disease: a population-based cohort study | Phenotype | ||
| Gasparetto 2016128 | Clinical course and outcomes of diagnosing Inflammatory Bowel Disease in children 10 years and under: retrospective cohort study from two tertiary centres in the United Kingdom and in Italy | Age at onset, EIMs and phenotype | ||
| Guizzetti 2018129 | Development of clinical prediction models for surgery and complications in Crohn’s disease | Age, gender, disease location and HBI | ||
| Herman 2017130 | The characteristics and long-term outcomes of pediatric Crohn’s disease patients with perianal disease | Age, sex and HBI | Laboratory data | |
| Herzog 2018131 | Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications | Phenotype, sex and smoking | ||
| Hou 2016132 | Characteristics and behavior of elderly-onset inflammatory bowel disease: a multi-center US study | Age at onset | ||
| Huguet 2018133 | Inflammatory bowel disease in patients over the age of 70 years. Does the disease duration influence its behavior? | Age | ||
| Hwang 2017134 | Influence of age at diagnosis on the clinical characteristics of Crohn’s disease in Korea: results from the CONNECT study | Age at onset | ||
| Jeuring 2017135 | Improvements in the long-term outcome of Crohn’s disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort | Phenotype | ||
| Jeuring 2016136 | Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity? | Age and phenotype | ||
| Jones 2019137 | Faecal calprotectin and magnetic resonance enterography in ileal Crohn’s disease: correlations between disease activity and long-term follow-up | Age and MRI | Calprotectin | |
| Kaur 2016138 | Perianal Crohn’s disease is associated with distal colonic disease, stricturing disease behaviour, IBD-associated serologies and genetic variation in the JAK-STAT pathway | Phenotype and FHx | ASCA and OmpC | Multiple Loci |
| Kayar 2019139 | Risk factors associated with progression to intestinal complications of Crohn disease | Smoking, EIMs and phenotype | ||
| Kim 2017140 | Clinical characteristics and long-term outcomes of paediatric Crohn’s Disease: a single-centre experience | Age, sex, FHx and phenotype | ||
| Kim 2017141 | Incidence of and risk factors for free bowel perforation in patients with Crohn’s disease | Sex, age at diagnosis and phenotype | ||
| Kim 2018142 | The clinical characteristics and prognosis of Crohn’s disease in Korean patients showing proximal small bowel involvement: results from the CONNECT study | Phenotype | ||
| Kostas 2017143 | Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease | CRP and calprotectin | ||
| Kühn 2018144 | [Risk factors for early surgery and surgical complications in Crohn’s disease] | Age at onset and phenotype | CRP and albumin | |
| Kunovsky 2019145 | The role of the NOD2/CARD15 gene in surgical treatment prediction in patients with Crohn’s disease | NOD2 | ||
| Kupka 2018146 | Crohn’s disease – genetic factors and progress of the disease | Phenotype | NOD2 | |
| Kwapisz 2017147 | The utility of fecal calprotectin in predicting the need for escalation of therapy in inflammatory bowel disease | Calprotectin | ||
| Levine 2020148 | Complicated disease and response to initial therapy predicts early surgery in paediatric Crohn’s disease: results from the Porto Group GROWTH study | Phenotype and PCDAI | Anti-OmpC | |
| Liu 2018149 | Lémann index at diagnosis predicts the risk of early surgery in Crohn’s disease | Lémann index at diagnosis and phenotype | ||
| Mańkowska-Wierzbicka 2016150 | C-reactive protein as a diagnostic and prognostic factor in inflammatory bowel diseases | Age | CRP | |
| Mañosa 2018151 | Phenotype and natural history of elderly onset inflammatory bowel disease: a multicentre, case–control study | Age at onset and phenotype | ||
| Mosli 2018152 | Risk stratification of patients with Crohn’s disease: a retrospective analysis of clinical decision-making and its impact on long-term outcome | Age, smoking, Montreal and age at diagnosis | ||
| Müller 2016153 | Baseline characteristics and disease phenotype in inflammatory bowel disease | PCDAI, age and phenotype | CRP | |
| Naganuma 2016154 | Endoscopic severity predicts long-term prognosis in Crohn’s disease patients with clinical remission | Phenotype | CRP | |
| Nahon 2016155 | Diagnostic delay is associated with a greater risk of early surgery in a French cohort of Crohn’s disease patients | Age at onset and phenotype | ||
| Ng 2016156 | Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia | Phenotype | ||
| Nguyen 2017157 | Risk of surgery and mortality in elderly-onset inflammatory bowel disease: a population-based cohort study | Age at onset and phenotype | ||
| Ouaz 2016158 | Changes of Crohn’s disease phenotype over time | Phenotype, smoking, age and sex | ||
| Pallotta 2018159 | A risk score system to timely manage treatment in Crohn’s disease: a cohort study | Age, sex, phenotype, CDAI and age at diagnosis | CRP | |
| Park 2019160 | Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort | |||
| Park 2017161 | Development of a novel predictive model for the clinical course of Crohn’s disease: results from the CONNECT study | Age and phenotype | ||
| Parker 2016162 | Radiologic predictors of surgery in newly diagnosed pediatric Crohn disease patients | Phenotype | ||
| Pernat Drobež 2018163 | DNA polymorphisms predict time to progression from uncomplicated to complicated Crohn’s disease | Multiple loci | ||
| Rinawi 2016164 | Evolution of disease phenotype in pediatric-onset Crohn’s disease after more than 10 years follow up – cohort study | Phenotype | ||
| Rispo 2018165 | Combined endoscopic/sonographic-based risk matrix model for predicting one-year risk of surgery: a prospective observational study of a tertiary centre severe/refractory Crohn’s disease cohort | Phenotype | ||
| Rönnblom 2017166 | Clinical course of Crohn’s disease during the first 5 years. Results from a population-based cohort in Sweden (ICURE) diagnosed 2005–2009 | Phenotype | ||
| Saad 2016167 | Age of diagnosis is associated with disease presentation and therapeutic complications in patients with Crohn’s disease | Age at onset and phenotype | ||
| Sharma 2019168 | Natural history of children with mild Crohn’s disease | Phenotype | ||
| Smids 2017169 | Candidate serum markers in early Crohn’s disease: predictors of disease course | Multiple (36 markers) | ||
| Sollelis 2019170 | Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease | CDAI | CRP and calprotectin | |
| Song 2018171 | Clinical characteristics and long-term prognosis of elderly-onset Crohn’s disease | Age at onset and phenotype | ||
| Stallmach 2019172 | Predictive parameters for the clinical course of Crohn’s disease: development of a simple and reliable risk model | Age at onset and phenotype | Haemoglobin and CRP | |
| Sun 2019173 | Clinical features and prognosis of Crohn’s disease with upper gastrointestinal tract phenotype in Chinese patients | Age at onset and phenotype | ||
| Szántó 2018174 | Biological therapy and surgery rates in inflammatory bowel diseases – data analysis of almost 1000 patients from a Hungarian tertiary IBD centre | Age at onset and phenotype | ||
| Torres 2016175 | Predicting outcomes to optimize disease management in inflammatory bowel diseases | Age at onset and phenotype | Multiple | Multiple loci |
| Wang 2018176 | Study of disease phenotype and its association with prognosis of paediatric inflammatory bowel disease in China | Porto criteria and Paris classification | IL-10 receptor A | |
| Wu 2019177 | Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric Crohn’s disease | Phenotype | ASCA-IgA and CBir | |
| Ye 2017178 | Fecal calprotectin is a strong predictive marker of relapse in Chinese patients with Crohn’s disease: a two-year prospective study | Calprotectin | ||
| Zhao 2019179 | A 10-year follow-up study of the natural history of perianal Crohn’s disease in a Danish population-based inception cohort | Phenotype | ||
| Zhulina 2016180 | The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease | Calprotectin | ||
| Ziv-Baran 2018181 | Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-onset paediatric Crohn’s disease | PCDAI, age and phenotype | CRP and calprotectin |
Chapter 6 Discussion
The research detailed in this monograph is a response to the NIHR HTA programme call of 2014, HTA 14/210, ‘Prognostic markers in early Crohn’s disease’. The outcomes stipulated by the HTA programme in their commissioning brief were ‘[a]n overview of current evidence’ and ‘[a] signal as to which biomarkers may be most useful’. Although a large amount of existing research has investigated the potential of a multitude of biomarkers to predict severe Crohn’s disease, individual studies are usually small, single centre and restricted to one or a handful of biomarkers. Furthermore, results from individual studies often diverge, providing no clarity. The consequence is that individual clinicians cannot synthesise these data in a way that is meaningful for individual patients. In response to the brief, we have carried out a comprehensive overview across all biomarker types, the findings of which are detailed in this report. Although we identified seven previous systematic reviews that investigated associations between severe Crohn’s disease and mostly serological and some genetic biomarkers, many confounded biomarker associations with both current disease (i.e. a diagnostic role) and future disease (i.e. a prognostic or predictive role), with no distinction between the two roles.
To generate meaningful results, we stipulated an a priori criterion that excluded biomarkers with insufficient evidence (defined by us as fewer than five component primary studies), and considered meta-analysis only where data for an individual biomarker could be extracted from three or more studies. Given the potential literature – we identified nearly 30,000 abstracts – we encountered relatively few genuinely prognostic studies, that is studies that investigated the development of future severe disease. We were surprised that common biomarkers, such as CRP, white blood cell count and haemoglobin, were rarely investigated despite being in widespread clinical use. We found no endoscopic, radiological or histopathological biomarkers that were sufficiently examined for meaningful meta-analysis. Although we anticipated an inevitable delay between validation of new biomarkers and subsequent prognostic studies, we were surprised that well-established biomarkers were poorly researched. This dearth of research may reflect researcher preoccupation with novel biomarkers, publication bias towards novel biomarkers or bias against studies finding no benefit.
Ultimately, across all biomarker types we identified only 71 individual studies with prognostic data, representing just 56 non-overlapping patient cohorts. From these, this report presents a meta-analysis of 11 potential predictors, of which eight displayed some evidence of predictive utility following meta-analysis. We present results for NOD2 (any variant), the genetic predictor quoted in clinical guidelines as being associated with an increased risk of severe disease. We present a meta-analysis of three serological biomarkers, finding both ASCA-positive and anti-CBir1-positive patients to be at a higher risk of severe disease. It may be that biomarker levels vary too widely to be useful; acute phase reactant levels probably represent the patient status at diagnosis, for example during a flare. The levels of antimicrobial antigens at subsequent times remain uncertain. Although studies with paired sera at 5-year intervals imply stability, others suggest that levels of antimicrobial antigens change with disease progression and treatment. We excluded research without baseline serum draw. To have clinical utility, biomarkers must be prognostic in early disease.
We meta-analysed seven clinical predictors: Montreal behaviour, age, disease duration, disease location, smoking, sex and family history. Five of these showed prognostic potential (sex and family history did not). The strongest association with subsequent severe disease across the entire review was found for Montreal B2 and B3 categories, with ORs of 4.09 and 6.25, respectively. Meta-analysis also suggested that increased risk of developing severe disease was associated with perianal disease, longer disease duration and smoking. We found that the risk of subsequent severe disease was potentially decreased in patients who were diagnosed at a younger age and in those with any colonic disease or colonic disease alone.
Our review does have limitations, the majority of which are contingent on the quality of the primary component studies and difficulties with definitions of severe disease. As noted already, we found that true prognostic research was relatively scarce and was often reported confounded with diagnosis. Moreover, methods were heterogeneous and we were obliged to meta-analyse across different methods of biomarker measurement, different positivity thresholds and different follow-up durations. Such variability probably underlies the disparity between study effect estimates displayed, as seen, for example, in the ASCA forest plot.
Because data were heterogeneous, we stress that interpretation of our findings should focus on which biomarkers were found to have predictive potential rather than the precise strength of that prediction. Readers should not draw conclusions regarding the effectiveness of different predictors given that our estimates are based on results from different studies. Particularly for serological markers, the number and quality of studies are presently such that findings from future studies will be important; the majority of genetic and serological biomarkers that were identified were examined by insufficient studies for meaningful meta-analysis.
Furthermore, results from individual studies probably differ because of varying patient populations, study designs or outcome definitions. For example, we were obliged to use a range of definitions for ‘severe’ disease because there is no single universally accepted/reported definition. Where the measure is relatively inclusive, for example Beaugerie et al. ,18 around 60% of patients will have ‘disabling disease’ within 5 years. Conversely, although the Liege criteria define severity more precisely,20 no article in our review stated the extent of intestinal surgery with detail sufficient to allow post hoc classification; single, early ileocaecal resection may be curative. Subsequently, only Loly et al. 20 (who proposed the Liege criteria) published data using this end point.
It is also possible that patients undergoing complex, expensive serological testing at diagnosis may be spectrum-biased towards severe disease. It is self-evident that biomarkers predictive of future severe disease have no clinical utility when such disease is established at diagnosis. For example, we were careful not to use Montreal B3 at baseline to predict development of B3 disease. B1, B2 and B3 are used mostly as predictors of surgery. We found that young age was not predictive of severe disease. However, children often display a panenteric phenotype that precludes surgical therapy, even when disease is severe.
We found considerable ROB, mostly because of suspected reporting bias as studies reported fewer than three standard predictors with non-significant results and results were not reported for standard follow-up times. Accordingly, no results were available for follow-up extending 5 to 10 years. Our analyses included some retrospective studies recruiting before 2004. Current treatment paradigms are evolving rapidly, and it is unclear whether or not biomarkers will behave similarly pre- and post-biologic introduction; we present individual study results with information sufficient for readers to explore this. Likewise, it was beyond our remit to consider anti-TNF response/failure to respond/loss of response. Recent studies have found that this in itself may identify patients destined to develop severe disease. 182,183 Predictive studies of multigene assays are currently under way, but we identified insufficient existing research for their inclusion in this review.
Owing to the large number of citations identified, we were unable to perform initial independent abstract screening by two reviewers; Darren Boone screened all abstracts alone. However, any uncertainty regarding eligibility, no matter how minor, was raised with Lucinda Archer (a statistician) initially and the with other members of the team if any uncertainty persisted. However, it is possible that some relevant data were discarded on the initial sift by using a single researcher. All full-text articles retrieved were screened independently by Sue Mallett (the senior statistician) to verify that data extraction for meta-analysis was correct, in particular to make the distinction between diagnostic and prognostic material.
This work has taken a considerable amount of time and resource, which was largely because of the nature of the prognostic reviews in diagnosis. Diagnostic studies remain poorly and imprecisely indexed in comparison with studies of therapeutic interventions. This problem is confounded further when the aim is to separate diagnostic from prognostic information, which is often confounded within the same article (as explained elsewhere). The result is that search strings must be inclusive to avoid missing important data, but this inflates the amount of primary research that must be examined. We carried out an updated search in August 2020 to attempt to quantify the number of potentially relevant data subsequent to our original search. We identified an additional 4005 indexed articles in PubMed and 6878 in EMBASE, yielding 80 potentially relevant full-text papers. We estimate that around 23 of these would ultimately yield useful prognostic data, the large majority dealing with clinical biomarkers. Accordingly, we do not believe that our research has missed important serological or genetic biomarker research and we believe that our findings are likely to stand overall.
In summary, we carried out a systematic review of biomarkers potentially predictive of subsequent severe Crohn’ disease, across all biomarker types, and focused on those contained in clinical guidelines. We found that prognostic research was heterogeneous and at a high ROB, and relatively few biomarkers relevant to clinical guidelines were investigated sufficiently for meta-analysis. Of those that were, we found evidence of prognostic potential for two serological (ASCA and anti-CBir1), one genetic (NOD2) and five clinical biomarkers (Montreal behaviour, age, disease duration, disease location and smoking). Considerably more prognostic research in this area is required, with methodological rigour, informed by consensus agreement around definitions of severe disease and following the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines,184 so that study results can be used by other researchers.
Chapter 7 Conclusions and recommendations for future research
We can make two broad conclusions and recommendations that arise from the research presented in this monograph. The first is that the quality and extent of prognostic research to predict future disease severity is somewhat lacking. We recommend that funders consider commissioning studies that are clearly prognostic and methodologically sound, and that adhere to established reporting guidelines for prognostic research. Because of the evidence gaps we identified, any such research should investigate all potentially important predictors, not just those that are perceived as ‘cutting-edge’ and/or ‘novel’.
The second recommendation stems from the predictors identified by our review and meta-analysis; in the face of the limitations that we describe, we have identified a limited number of relatively easily accessible predictors that could be combined and tested in a multivariable prognostic model. This model would aim to accurately predict those patients with a new diagnosis of Crohn’s disease who are destined to develop severe disease in the future, thereby facilitating individualised, early, targeted biological therapy. At the time of writing, we are currently developing and validating such a model using historic data obtained from the Royal Devon and Exeter NHS Trust; this work has been interrupted by the 2020 covid-19 pandemic that required clinical academics to return to full-time clinical work. It is our intention to complete this work when possible. If that validation proves sufficiently promising, we will ask that funders consider commissioning an external validation and/or clinical trial. Although the cleanest methodology would be to randomise application of the predictive model, this may prove ethically difficult given the widespread use of biological therapies currently. Due consideration should, therefore, be given to use of historic databases.
As noted elsewhere, because the existing literature is deficient it is likely that we have ‘missed’ some potentially important predictors simply because they have not been assessed sufficiently at the time of writing. As a result, development of a multivariable model should be flexible and not be restricted to the predictors identified by us. Development and validation could also incorporate multigene arrays, which are garnering considerable current interest: their incremental benefit (or otherwise) can then be quantified by their effect on prediction when added as one of the variables within an existing prognostic model based on more easily accessible data.
The work described in this monograph has not had substantial PPI input because it is a review of existing research performed by others. By contrast, we will seek PPI input from our expert patient collaborator when we have completed our planned work on the predictive model. Specifically, to inform clinical implementation of any model, we need to understand, from a patient perspective, how the presumed benefits of early biological therapy might be weighed against potential harmful side effects. These considerations must be made within the context of predictive accuracy, namely the degree to which the model may deny biological therapy to some deserving patients while simultaneously over-treating others.
Acknowledgements
The authors are grateful to Professor Brian Saunders, Professor Simon Travis, Dr John Hamlin and Dr Andrew Millar for their help with the expert panel.
The authors are grateful to the UK NIHR HTA programme for funding this research (project number: HTA 14/210/07).
Contributions of authors
Steve Halligan (https://orcid.org/0000-0003-0632-5108) drafted the initial manuscript.
Darren Boone (https://orcid.org/0000-0002-3178-9791) performed the literature search, performed the majority of the data extraction and revised the initial manuscript.
Lucinda Archer (https://orcid.org/0000-0003-2504-2613) performed the majority of the data extraction and the statistical analysis, and revised the initial manuscript.
Tariq Ahmad (https://orcid.org/0000-0002-6058-5528) revised the initial manuscript.
Stuart Bloom (https://orcid.org/0000-0002-6361-4662) revised the initial manuscript.
Manuel Rodriguez-Justo (https://orcid.org/0000-0001-5007-1761) revised the initial manuscript.
Stuart A Taylor (https://orcid.org/0000-0002-6765-8806) revised the initial manuscript.
Sue Mallett (https://orcid.org/0000-0002-0596-8200) performed the majority of the data extraction and the statistical analysis, and drafted the initial manuscript.
All of the authors made substantial contributions to the interpretation and intellectual content, agree to be accountable for all aspects of the work, and gave final approval of the version of the report to be published. Professor Steve Halligan is guarantor.
Data-sharing statement
The research described in this monograph is a systematic review of available indexed literature; for this reason, all of the primary studies are available, in line with the stipulations of the individual journals concerned. The complete data extraction table used for this review can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Halligan S, Boone D, Bhatnagar G, Ahmad T, Bloom S, Rodriguez-Justo M, et al. Prognostic biomarkers to identify patients destined to develop severe Crohn’s disease who may benefit from early biological therapy: protocol for a systematic review, meta-analysis and external validation. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0383-5.
- National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme . HTA No. 14 210: Prognostic Markers in Early Crohn’s Disease 2014. https://njl-admin.nihr.ac.uk/document/download/2023257 (accessed March 2021).
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541-9. https://doi.org/10.1016/S0140-6736(02)08512-4.
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383-95. https://doi.org/10.1056/NEJMoa0904492.
- D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660-7. https://doi.org/10.1016/S0140-6736(08)60304-9.
- Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010;138:463-8. https://doi.org/10.1053/j.gastro.2009.09.056.
- Feagan BG, Panaccione R, Sandborn WJ, D’Haens GR, Schreiber S, Rutgeerts PJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology 2008;135:1493-9. https://doi.org/10.1053/j.gastro.2008.07.069.
- Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015;386:1825-34. https://doi.org/10.1016/S0140-6736(15)00068-9.
- Peyrin-Biroulet L, Fiorino G, Buisson A, Danese S. First-line therapy in adult Crohn’s disease: who should receive anti-TNF agents?. Nat Rev Gastroenterol Hepatol 2013;10:345-51. https://doi.org/10.1038/nrgastro.2013.31.
- Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual frameworks. Clin Pharmacol Ther 2001;69:89-95. https://doi.org/10.1067/mcp.2001.113989.
- Blonski W, Buchner AM, Lichtenstein GR. Clinical predictors of aggressive/disabling disease: ulcerative colitis and crohn disease. Gastroenterol Clin North Am 2012;41:443-62. https://doi.org/10.1016/j.gtc.2012.01.008.
- Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17550.
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010;42:1118-25. https://doi.org/10.1038/ng.717.
- Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology 2004;126:414-24. https://doi.org/10.1053/j.gastro.2003.11.015.
- Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001744.
- Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A-36A. https://doi.org/10.1155/2005/269076.
- Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314-21. https://doi.org/10.1002/ibd.21493.
- Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology 2006;130:650-6. https://doi.org/10.1053/j.gastro.2005.12.019.
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749-53. https://doi.org/10.1136/gut.2005.082909.
- Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol 2008;43:948-54. https://doi.org/10.1080/00365520801957149.
- National Institute for Health and Care Excellence (NICE) . Infliximab and Adalimumab for the Treatment of Crohn’s Disease (TA187) 2010.
- Teml A, Kratzer V, Schneider B, Lochs H, Norman GL, Gangl A, et al. Anti-Saccharomyces cerevisiae antibodies: a stable marker for Crohn’s disease during steroid and 5-aminosalicylic acid treatment. Am J Gastroenterol 2003;98:2226-31. https://doi.org/10.1111/j.1572-0241.2003.07673.x.
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51-8. https://doi.org/10.7326/M18-1376.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Israeli E, Ryan JD, Shafer LA, Bernstein CN. Younger age at diagnosis is associated with panenteric, but not more aggressive, Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:72-9.e1. https://doi.org/10.1016/j.cgh.2013.06.027.
- Louis E, Michel V, Hugot JP, Reenaers C, Fontaine F, Delforge M, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut 2003;52:552-7. https://doi.org/10.1136/gut.52.4.552.
- Lovasz BD, Lakatos L, Horvath A, Szita I, Pandur T, Mandel M, et al. Evolution of disease phenotype in adult and pediatric onset Crohn’s disease in a population-based cohort. World J Gastroenterol 2013;19:2217-26. https://doi.org/10.3748/wjg.v19.i14.2217.
- Pittet V, Rogler G, Michetti P, Fournier N, Vader JP, Schoepfer A, et al. Penetrating or stricturing diseases are the major determinants of time to first and repeat resection surgery in Crohn’s disease. Digestion 2013;87:212-21. https://doi.org/10.1159/000350954.
- Smith BR, Arnott ID, Drummond HE, Nimmo ER, Satsangi J. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn’s disease. Inflamm Bowel Dis 2004;10:521-8. https://doi.org/10.1097/00054725-200409000-00005.
- Solberg IC, Cvancarova M, Vatn MH, Moum B. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s Disease (the IBSEN study). Inflamm Bowel Dis 2014;20:60-8. https://doi.org/10.1097/01.mib.0000436956.78220.67.
- Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114-22. https://doi.org/10.1053/j.gastro.2008.06.081.
- Yang CH, Ding J, Gao Y, Chen X, Yang ZB, Xiao SD. Risk factors that predict the requirement of aggressive therapy among Chinese patients with Crohn’s disease. J Dig Dis 2011;12:99-104. https://doi.org/10.1111/j.1751-2980.2011.00484.x.
- Zabana Y, Garcia-Planella E, Van Domselaar M, Mañosa M, Gordillo J, López San Román A, et al. Does active smoking really influence the course of Crohn’s disease? A retrospective observational study. J Crohns Colitis 2013;7:280-5. https://doi.org/10.1016/j.crohns.2012.03.020.
- Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott ID, Satsangi J. Does cigarette smoking influence the phenotype of Crohn’s disease? Analysis using the Montreal classification. Am J Gastroenterol 2007;102:577-88. https://doi.org/10.1111/j.1572-0241.2007.01064.x.
- Alvarez-Lobos M, Arostegui JI, Sans M, Tassies D, Plaza S, Delgado S, et al. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg 2005;242:693-700. https://doi.org/10.1097/01.sla.0000186173.14696.ea.
- Annese V, Lombardi G, Perri F, D’Inca R, Ardizzone S, Riegler G, et al. Variants of CARD15 are associated with an aggressive clinical course of Crohn’s disease – an IG–IBD study. Am J Gastroenterol 2005;100:84-92. https://doi.org/10.1111/j.1572-0241.2005.40705.x.
- Brant SR, Picco MF, Achkar JP, Bayless TM, Kane SV, Brzezinski A, et al. Defining complex contributions of NOD2/CARD15 gene mutations, age at onset, and tobacco use on Crohn’s disease phenotypes. Inflamm Bowel Dis 2003;9:281-9. https://doi.org/10.1097/00054725-200309000-00001.
- Büning C, Genschel J, Bühner S, Krüger S, Kling K, Dignass A, et al. Mutations in the NOD2/CARD15 gene in Crohn’s disease are associated with ileocecal resection and are a risk factor for reoperation. Aliment Pharmacol Ther 2004;19:1073-8. https://doi.org/10.1111/j.1365-2036.2004.01967.x.
- Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423-32. https://doi.org/10.1136/gutjnl-2012-303864.
- Chatzicostas C, Roussomoustakaki M, Potamianos S, Paspatis G, Mouzas I, Romanos J, et al. Factors associated with disease evolution in Greek patients with inflammatory bowel disease. BMC Gastroenterol 2006;6. https://doi.org/10.1186/1471-230X-6-21.
- Chen M, Yi F, Zhou F, Huang M, Li J, Yan W, et al. Risk factors for initial surgery in patients with Crohn’s disease in Central China. Surg Today 2015;45:1002-8. https://doi.org/10.1007/s00595-014-1093-z.
- Choung RS, Princen F, Stockfisch TP, Torres J, Maue AC, Porter CK, et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol Ther 2016;43:1300-10. https://doi.org/10.1111/apt.13641.
- Cleynen I, González JR, Figueroa C, Franke A, McGovern D, Bortlík M, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut 2013;62:1556-65. https://doi.org/10.1136/gutjnl-2011-300777.
- Degenhardt F, Dirmeier A, Lopez R, Lang S, Kunst C, Roggenbuck D, et al. Serologic anti-GP2 antibodies are associated with genetic polymorphisms, fibrostenosis, and need for surgical resection in Crohn’s disease. Inflamm Bowel Dis 2016;22:2648-57. https://doi.org/10.1097/mib.0000000000000936.
- Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, et al. Increased immune reactivity predicts aggressive complicating Crohn’s disease in children. Clin Gastroenterol Hepatol 2008;6:1105-11. https://doi.org/10.1016/j.cgh.2008.04.032.
- Goel A, Dutta AK, Pulimood AB, Eapen A, Chacko A. Clinical profile and predictors of disease behavior and surgery in Indian patients with Crohn’s disease. Indian J Gastroenterol 2013;32:184-9. https://doi.org/10.1007/s12664-012-0293-y.
- Gupta N, Cohen SA, Bostrom AG, Kirschner BS, Baldassano RN, Winter HS, et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology 2006;130:1069-77. https://doi.org/10.1053/j.gastro.2006.02.003.
- Henckaerts L, Van Steen K, Verstreken I, Cleynen I, Franke A, Schreiber S, et al. Genetic risk profiling and prediction of disease course in Crohn’s disease patients. Clin Gastroenterol Hepatol 2009;7:972-80. https://doi.org/10.1016/j.cgh.2009.05.001.
- Jauregui-Amezaga A, Rimola J, Ordás I, Rodríguez S, Ramírez-Morros A, Gallego M, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut 2015;64:1397-402. https://doi.org/10.1136/gutjnl-2014-308101.
- Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710-18. https://doi.org/10.1016/S0140-6736(17)30317-3.
- Kugathasan S, Collins N, Maresso K, Hoffmann RG, Stephens M, Werlin SL, et al. CARD15 gene mutations and risk for early surgery in pediatric-onset Crohn’s disease. Clin Gastroenterol Hepatol 2004;2:1003-9. https://doi.org/10.1016/S1542-3565(04)00452-5.
- Kwon JH, Im JP, Ye BD, Cheon JH, Jang HJ, Lee KM, et al. Disease phenotype, activity and clinical course prediction based on C-reactive protein levels at diagnosis in patients with Crohn’s disease: results from the CONNECT study. Gut Liver 2016;10:595-603. https://doi.org/10.5009/gnl15411.
- Lacher M, Helmbrecht J, Schroepf S, Koletzko S, Ballauff A, Classen M, et al. NOD2 mutations predict the risk for surgery in pediatric-onset Crohn’s disease. J Pediatr Surg 2010;45:1591-7. https://doi.org/10.1016/j.jpedsurg.2009.10.046.
- Laghi L, Costa S, Saibeni S, Bianchi P, Omodei P, Carrara A, et al. Carriage of CARD15 variants and smoking as risk factors for resective surgery in patients with Crohn’s ileal disease. Aliment Pharmacol Ther 2005;22:557-64. https://doi.org/10.1111/j.1365-2036.2005.02629.x.
- Lakatos PL, Lakatos L, Szalay F, Willheim-Polli C, Osterreicher C, Tulassay Z, et al. Toll-like receptor 4 and NOD2/CARD15 mutations in Hungarian patients with Crohn’s disease: phenotype-genotype correlations. World J Gastroenterol 2005;11:1489-95. https://doi.org/10.3748/wjg.v11.i10.1489.
- Lakatos PL, Czegledi Z, Szamosi T, Banai J, David G, Zsigmond F, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol 2009;15:3504-10. https://doi.org/10.3748/wjg.15.3504.
- Law ST, Li KK. Age-related differences in the clinical course of Crohns disease in an Asian population: a retrospective cohort review. Indian Pediatr 2013;50:1148-52. https://doi.org/10.1007/s13312-013-0301-z.
- Lunney PC, Kariyawasam VC, Wang RR, Middleton KL, Huang T, Selinger CP, et al. Smoking prevalence and its influence on disease course and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2015;42:61-70. https://doi.org/10.1111/apt.13239.
- Malmborg P, Grahnquist L, Ideström M, Lindholm J, Befrits R, Björk J, et al. Presentation and progression of childhood-onset inflammatory bowel disease in Northern Stockholm County. Inflamm Bowel Dis 2015;21:1098-108. https://doi.org/10.1097/MIB.0000000000000356.
- Mazor Y, Maza I, Kaufman E, Ben-Horin S, Karban A, Chowers Y, et al. Prediction of disease complication occurrence in Crohn’s disease using phenotype and genotype parameters at diagnosis. J Crohns Colitis 2011;5:592-7. https://doi.org/10.1016/j.crohns.2011.06.002.
- Moon CM, Park DI, Kim ER, Kim YH, Lee CK, Lee SH, et al. Clinical features and predictors of clinical outcomes in Korean patients with Crohn’s disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol 2014;29:74-82. https://doi.org/10.1111/jgh.12369.
- Nasir BF, Griffiths L, Nasir A, Roberts R, Barclay M, Gearry R, et al. Perianal disease combined with NOD2 genotype predicts need for IBD-related surgery in Crohn’s disease patients from a population-based cohort. J Clin Gastroenterol 2013;47:242-5. https://doi.org/10.1097/MCG.0b013e318258314d.
- Nasir BF, Griffiths LR, Nasir A, Roberts R, Barclay M, Gearry RB, et al. An envirogenomic signature is associated with risk of IBD-related surgery in a population-based Crohn’s disease cohort. J Gastrointest Surg 2013;17:1643-50. https://doi.org/10.1007/s11605-013-2250-1.
- Nunes T, Etchevers MJ, Domènech E, García-Sánchez V, Ber Y, Peñalva M, et al. Smoking does influence disease behaviour and impacts the need for therapy in Crohn’s disease in the biologic era. Aliment Pharmacol Ther 2013;38:752-60. https://doi.org/10.1111/apt.12440.
- Odes HS, Fich A, Reif S, Halak A, Lavy A, Keter D, et al. Effects of current cigarette smoking on clinical course of Crohn’s disease and ulcerative colitis. Dig Dis Sci 2001;46:1717-21. https://doi.org/10.1023/a:1010609722315.
- Oh K, Oh EH, Baek S, Song EM, Kim GU, Seo M, et al. Elevated C-reactive protein level during clinical remission can predict poor outcomes in patients with Crohn’s disease. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0179266.
- Oriuchi T, Hiwatashi N, Kinouchi Y, Takahashi S, Takagi S, Negoro K, et al. Clinical course and longterm prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol 2003;38:942-53. https://doi.org/10.1007/s00535-003-1177-9.
- Pandey A, Salazar E, Kong CSC, Lim WC, Ong J, Ong DEH, et al. Risk of major abdominal surgery in an Asian population-based Crohn’s disease cohort. Inflamm Bowel Dis 2015;21:2625-33. https://doi.org/10.1097/MIB.0000000000000525.
- Papi C, Festa V, Fagnani C, Stazi A, Antonelli G, Moretti A, et al. Evolution of clinical behaviour in Crohn’s disease: predictive factors of penetrating complications. Dig Liver Dis 2005;37:247-53. https://doi.org/10.1016/j.dld.2004.10.012.
- Park SK, Yang SK, Park SH, Park SH, Kim JW, Yang DH, et al. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol 2013;47:400-8. https://doi.org/10.1097/MCG.0b013e3182705f9e.
- Pigneur B, Seksik P, Viola S, Viala J, Beaugerie L, Girardet JP, et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953-61. https://doi.org/10.1002/ibd.21152.
- Polito JM, Childs B, Mellits ED, Tokayer AZ, Harris ML, Bayless TM. Crohn’s disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology 1996;111:580-6. https://doi.org/10.1053/gast.1996.v111.pm8780560.
- Posovszky C, Pfalzer V, Lahr G, Niess JH, Klaus J, Mayer B, et al. Age-of-onset-dependent influence of NOD2 gene variants on disease behaviour and treatment in Crohn’s disease. BMC Gastroenterol 2013;13. https://doi.org/10.1186/1471-230x-13-77.
- Protic MB, Pavlovic ST, Bojic DZ, Krstic MN, Radojicic ZA, Tarabar DK, et al. CARD15 gene polymorphisms in Serbian patients with Crohn’s disease: genotype-phenotype analysis. Eur J Gastroenterol Hepatol 2008;20:978-84. https://doi.org/10.1097/MEG.0b013e328302f45e.
- Renda MC, Orlando A, Civitavecchia G, Criscuoli V, Maggio A, Mocciaro F, et al. The role of CARD15 mutations and smoking in the course of Crohn’s disease in a Mediterranean area. Am J Gastroenterol 2008;103:649-55. https://doi.org/10.1111/j.1572-0241.2007.01589.x.
- Rieder F, Schleder S, Wolf A, Dirmeier A, Strauch U, Obermeier F, et al. Serum anti-glycan antibodies predict complicated Crohn’s disease behavior: a cohort study. Inflamm Bowel Dis 2010;16:1367-75. https://doi.org/10.1002/ibd.21179.
- Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MA, Cilissen M, Engels LG, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol 2009;104:371-83. https://doi.org/10.1038/ajg.2008.38.
- Ryan JD, Silverberg MS, Xu W, Graff LA, Targownik LE, Walker JR, et al. Predicting complicated Crohn’s disease and surgery: phenotypes, genetics, serology and psychological characteristics of a population-based cohort. Aliment Pharmacol Ther 2013;38:274-83. https://doi.org/10.1111/apt.12368.
- Sabate JM, Ameziane N, Lamoril J, Jouet P, Farmachidi JP, Soule JC, et al. The V249I polymorphism of the CX3CR1 gene is associated with fibrostenotic disease behavior in patients with Crohn’s disease. Eur J Gastroenterol Hepatol 2008;20:748-55. https://doi.org/10.1097/MEG.0b013e3282f824c9.
- Sands BE, Arsenault JE, Rosen MJ, Alsahli M, Bailen L, Banks P, et al. Risk of early surgery for Crohn’s disease: implications for early treatment strategies. Am J Gastroenterol 2003;98:2712-18. https://doi.org/10.1111/j.1572-0241.2003.08674.x.
- Savoye G, Salleron J, Gower-Rousseau C, Dupas JL, Vernier-Massouille G, Fumery M, et al. Clinical predictors at diagnosis of disabling pediatric Crohn’s disease. Inflamm Bowel Dis 2012;18:2072-8. https://doi.org/10.1002/ibd.22898.
- Schaefer ME, Machan JT, Kawatu D, Langton CR, Markowitz J, Crandall W, et al. Factors that determine risk for surgery in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2010;8:789-94. https://doi.org/10.1016/j.cgh.2010.05.021.
- Shaoul R, Karban A, Reif S, Weiss B, Shamir R, Tamir A, et al. Disease behavior in children with Crohn’s disease: the effect of disease duration, ethnicity, genotype, and phenotype. Dig Dis Sci 2009;54:142-50. https://doi.org/10.1007/s10620-008-0326-7.
- Siegel CA, Siegel LS, Hyams JS, Kugathasan S, Markowitz J, Rosh JR, et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn’s disease. Inflamm Bowel Dis 2011;17:30-8. https://doi.org/10.1002/ibd.21386.
- Siegel CA, Horton H, Siegel LS, Thompson KD, Mackenzie T, Stewart SK, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther 2016;43:262-71. https://doi.org/10.1111/apt.13460.
- Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430-8. https://doi.org/10.1016/j.cgh.2007.09.002.
- Song XM, Gao X, Li MZ, Chen ZH, Chen SC, Hu PJ, et al. Clinical features and risk factors for primary surgery in 205 patients with Crohn’s disease: analysis of a South China cohort. Dis Colon Rectum 2011;54:1147-54. https://doi.org/10.1097/DCR.0b013e318222ddc3.
- Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082-93. https://doi.org/10.1111/j.1572-0241.2008.02212.x.
- Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147-55. https://doi.org/10.1053/j.gastro.2010.06.070.
- van der Heide F, Dijkstra A, Weersma RK, Albersnagel FA, van der Logt EM, Faber KN, et al. Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2009;15:1199-207. https://doi.org/10.1002/ibd.20884.
- Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 2008;135:1106-13. https://doi.org/10.1053/j.gastro.2008.06.079.
- Vester-Andersen MK, Vind I, Prosberg MV, Bengtsson BG, Blixt T, Munkholm P, et al. Hospitalisation, surgical and medical recurrence rates in inflammatory bowel disease 2003–2011 – a Danish population-based cohort study. J Crohns Colitis 2014;8:1675-83. https://doi.org/10.1016/j.crohns.2014.07.010.
- Yaari S, Benson A, Aviran E, Lev Cohain N, Oren R, Sosna J, et al. Factors associated with surgery in patients with intra-abdominal fistulizing Crohn’s disease. World J Gastroenterol 2016;22:10380-7. https://doi.org/10.3748/wjg.v22.i47.10380.
- Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn’s disease: a meta-analysis. Am J Gastroenterol 2011;106:699-712. https://doi.org/10.1038/ajg.2011.19.
- Bonneau J, Dumestre-Perard C, Rinaudo-Gaujous M, Genin C, Sparrow M, Roblin X, et al. Systematic review: new serological markers (anti-glycan, anti-GP2, anti-GM-CSF Ab) in the prediction of IBD patient outcomes. Autoimmun Rev 2015;14:231-45. https://doi.org/10.1016/j.autrev.2014.11.004.
- Kaul A, Hutfless S, Liu L, Bayless TM, Marohn MR, Li X. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:1872-84. https://doi.org/10.1002/ibd.22862.
- Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18:1894-9. https://doi.org/10.1002/ibd.22861.
- Prideaux L, De Cruz P, Ng SC, Kamm MA. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis 2012;18:1340-55. https://doi.org/10.1002/ibd.21903.
- Xiong Y, Wang GZ, Zhou JQ, Xia BQ, Wang XY, Jiang B. Serum antibodies to microbial antigens for Crohn’s disease progression: a meta-analysis. Eur J Gastroenterol Hepatol 2014;26:733-42. https://doi.org/10.1097/MEG.0000000000000102.
- Zhang Z, Li C, Zhao X, Lv C, He Q, Lei S, et al. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn’s disease: a meta-analysis. Dig Dis Sci 2012;57:2944-54. https://doi.org/10.1007/s10620-012-2244-y.
- Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, et al. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol 2006;101:360-7. https://doi.org/10.1111/j.1572-0241.2006.00456.x.
- Aaltonen G, Carpelan-Holmström M, Keränen I, . Risk factors for proctectomy in consecutive Crohn’s colitis surgical patients in a reference colorectal centre. Int J Colorectal Dis 2019;34:1401-6. https://doi.org/10.1007/s00384-019-03337-8.
- Aggarwal V, Day AS, Connor S, Leach ST, Brown G, Singh R, et al. Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn’s disease to assess remission and predict relapse. Gastrointest Endosc 2017;86:1070-8. https://doi.org/10.1016/j.gie.2017.09.011.
- Alexakis C, Saxena S, Chhaya V, Cecil E, Majeed A, Pollok R. Smoking status at diagnosis and subsequent smoking cessation: associations with corticosteroid use and intestinal resection in Crohn’s disease. Am J Gastroenterol 2018;113:1689-700. https://doi.org/10.1038/s41395-018-0273-7.
- Arieira C, Cúrdia Gonçaves T, Dias de Castro F, João Moreira M, Cotter J. Clinical course in Crohn’s disease: factors associated with behaviour change and surgery. Scand J Gastroenterol 2018;53:1222-7. https://doi.org/10.1080/00365521.2018.1503709.
- Arora U, Ananthakrishnan AN, Kedia S, Bopanna S, Mouli PM, Yadav DP, et al. Effect of oral tobacco use and smoking on outcomes of Crohn’s disease in India. J Gastro Hepatol 2018;33:134-40. https://doi.org/10.1111/jgh.13815.
- Arora U, Kedia S, Garg P, Bopanna S, Jain S, Yadav DP, et al. Colonic Crohn’s disease is associated with less aggressive disease course than ileal or ileocolonic disease. Dig Dis Sci 2018;63:1592-9. https://doi.org/10.1007/s10620-018-5041-4.
- Assa A, Amitai M, Greer ML, Castro DA, Kuint RC, Martínez-León M, et al. Perianal pediatric Crohn disease is associated with a distinct phenotype and greater inflammatory burden. J Pediatr Gastroenterol Nutr 2017;65:293-8. https://doi.org/10.1097/MPG.0000000000001484.
- Assa A, Rinawi F, Shamir R. The long-term predictive properties of the paris classification in paediatric inflammatory bowel disease patients. J Crohns Colitis 2018;12:39-47. https://doi.org/10.1093/ecco-jcc/jjx125.
- Birimberg-Schwartz L, Wilson DC, Kolho KL, Karolewska-Bochenek K, Afzal NA, Spray C, et al. pANCA and ASCA in children with IBD-unclassified, Crohn’s colitis, and ulcerative colitis-a longitudinal report from the IBD Porto Group of ESPGHAN. Inflamm Bowel Dis 2016;22:1908-14. https://doi.org/10.1097/MIB.0000000000000784.
- Bossuyt P, Debeuckelaere C, Ferrante M, de Buck van Overstraeten A, Vanbeckevoort D, Billiet T, et al. Risk stratification for surgery in stricturing ileal Crohn’s disease: the BACARDI risk model. J Crohn’s Colitis 2018;12:32-8. https://doi.org/10.1093/ecco-jcc/jjx110.
- Brückner A, Werkstetter KJ, de Laffolie J, Wendt C, Prell C, Weidenhausen T, et al. Incidence and risk factors for perianal disease in pediatric crohn disease patients followed in CEDATA-GPGE registry. J Pediatr Gastroenterol Nutr 2018;66:73-8. https://doi.org/10.1097/MPG.0000000000001649.
- Buisson A, Mak WY, Andersen MJ, Lei D, Kahn SA, Pekow J, et al. Faecal calprotectin is a very reliable tool to predict and monitor the risk of relapse after therapeutic de-escalation in patients with inflammatory bowel diseases. J Crohns Colitis 2019;13:1012-24. https://doi.org/10.1093/ecco-jcc/jjz023.
- Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2019;68:423-3. https://doi.org/10.1136/gutjnl-2017-315568.
- Chaparro M, Garre A, Ricart E, Iglesias-Flores E, Taxonera C, Domènech E, et al. Differences between childhood- and adulthood-onset inflammatory bowel disease: the CAROUSEL study from GETECCU. Aliment Pharmacol Ther 2019;49:419-28. https://doi.org/10.1111/apt.15114.
- Chaudhry NA, Riverso M, Grajo JR, Moser PP, Zou F, Homsi M, et al. A fixed stricture on routine cross-sectional imaging predicts disease-related complications and adverse outcomes in patients with Crohn’s disease. Inflamm Bowel Dis 2017;23:641-9. https://doi.org/10.1097/MIB.0000000000001054.
- Chen GB, Lee SH, Montgomery GW, Wray NR, Visscher PM, Gearry RB, et al. Performance of risk prediction for inflammatory bowel disease based on genotyping platform and genomic risk score method. BMC Med Genet 2017;18. https://doi.org/10.1186/s12881-017-0451-2.
- Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, et al. Emerging trends and risk factors for perianal surgery in Crohn’s disease: a 20-year national population-based cohort study. Eur J Gastroenterol Hepatol 2016;28:890-5. https://doi.org/10.1097/MEG.0000000000000651.
- Chun J, Im JP, Kim JW, Lee KL, Choi CH, Kim H, et al. Association of perianal fistulas with clinical features and prognosis of Crohn’s disease in Korea: results from the CONNECT study. Gut Liver 2018;12:544-54. https://doi.org/10.5009/gnl18157.
- de Barros KSC, Flores C, Harlacher L, Francesconi CFM. Evolution of clinical behavior in Crohn’s disease: factors associated with complicated disease and surgery. Dig Dis Sci 2017;62:2481-8. https://doi.org/10.1007/s10620-017-4685-9.
- Dias CC, Pereira Rodrigues P, Fernandes S, Portela F, Ministro P, Martins D, et al. The risk of disabling, surgery and reoperation in Crohn’s disease - A decision tree-based approach to prognosis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0172165.
- Dias CC, Rodrigues PP, Coelho R, Santos PM, Fernandes S, Lago P, et al. Development and validation of risk matrices for Crohn’s disease outcomes in patients who underwent early therapeutic interventions. J Crohns Colitis 2017;11:445-53.
- Diederen K, Hoekman DR, Leek A, Wolters VM, Hummel TZ, de Meij TG, et al. Raised faecal calprotectin is associated with subsequent symptomatic relapse, in children and adolescents with inflammatory bowel disease in clinical remission. Aliment Pharmacol Ther 2017;45:951-60. https://doi.org/10.1111/apt.13950.
- Dong Y, Xu L, Fan Y, Xiang P, Gao X, Chen Y, et al. A novel surgical predictive model for Chinese Crohn’s disease patients. Medicine (Baltimore) 2019;98. https://doi.org/10.1097/MD.0000000000017510.
- Foster AJ, Smyth M, Lakhani A, Jung B, Brant RF, Jacobson K. Consecutive fecal calprotectin measurements for predicting relapse in pediatric Crohn’s disease patients. World J Gastroenterol 2019;25:1266-77. https://doi.org/10.3748/wjg.v25.i10.1266.
- Fumery M, Pariente B, Sarter H, Charpentier C, Armengol Debeir L, Dupas JL, et al. Natural history of Crohn’s disease in elderly patients diagnosed over the age of 70 years: a population-based study. Inflamm Bowel Dis 2016;22:1698-707. https://doi.org/10.1097/MIB.0000000000000821.
- Fumery M, Pariente B, Sarter H, Savoye G, Spyckerelle C, Djeddi D, et al. Long-term outcome of pediatric-onset Crohn’s disease: A population-based cohort study. Dig Liver Dis 2019;51:496-502. https://doi.org/10.1016/j.dld.2018.11.033.
- Gasparetto M, Guariso G, Pozza LV, Ross A, Heuschkel R, Zilbauer M. Clinical course and outcomes of diagnosing Inflammatory Bowel Disease in children 10 years and under: retrospective cohort study from two tertiary centres in the United Kingdom and in Italy. BMC Gastroenterol 2016;16. https://doi.org/10.1186/s12876-016-0455-y.
- Guizzetti L, Zou G, Khanna R, Dulai PS, Sandborn WJ, Jairath V, et al. Development of clinical prediction models for surgery and complications in Crohn’s disease. J Crohns Colitis 2018;12:167-77. https://doi.org/10.1093/ecco-jcc/jjx130.
- Herman Y, Rinawi F, Rothschild B, Nir O, Shamir R, Assa A. The characteristics and long-term outcomes of pediatric Crohn’s disease patients with perianal disease. Inflamm Bowel Dis 2017;23:1659-65. https://doi.org/10.1097/MIB.0000000000001171.
- Herzog D, Fournier N, Buehr P, Rueger V, Koller R, Heyland K, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol 2018;30:598-607. https://doi.org/10.1097/MEG.0000000000001072.
- Hou JK, Feagins LA, Waljee AK. Characteristics and behavior of elderly-onset inflammatory bowel disease: a multi-center US study. Inflammatory Bowel Diseases 2016;22:2200-5. https://doi.org/10.1097/MIB.0000000000000849.
- Huguet JM, Iborra M, Bosca-Watts MM, Maroto N, Gil R, Cortes X, et al. Inflammatory bowel disease in patients over the age of 70 y. Does the disease duration influence its behavior?. Scand J Gastroenterol 2018;53:1079-84. https://doi.org/10.1080/00365521.2018.1501603.
- Hwang SW, Kim JH, Im JP, Ye BD, Koo HS, Huh KC, et al. Influence of age at diagnosis on the clinical characteristics of Crohn’s disease in Korea: results from the CONNECT study. J Gastroenterol Hepatol 2017;32:1716-22. https://doi.org/10.1111/jgh.13775.
- Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, et al. Improvements in the long-term outcome of Crohn’s disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol 2017;112:325-36. https://doi.org/10.1038/ajg.2016.524.
- Jeuring SF, van den Heuvel TR, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity?. Inflamm Bowel Dis 2016;22:1425-34. https://doi.org/10.1097/MIB.0000000000000738.
- Jones GR, Fascì-Spurio F, Kennedy NA, Plevris N, Jenkinson P, Lyons M, et al. Faecal calprotectin and magnetic resonance enterography in ileal Crohn’s disease: correlations between disease activity and long-term follow-up. J Crohns Colitis 2019;13:442-50. https://doi.org/10.1093/ecco-jcc/jjy187.
- Kaur M, Panikkath D, Yan X, Liu Z, Berel D, Li D, et al. Perianal Crohn’s disease is associated with distal colonic disease, stricturing disease behavior, IBD-associated serologies and genetic variation in the JAK-STAT pathway. Inflamm Bowel Dis 2016;22:862-9. https://doi.org/10.1097/MIB.0000000000000705.
- Kayar Y, Baran B, Ormeci AC, Akyuz F, Demir K, Besisik F, et al. Risk factors associated with progression to intestinal complications of Crohn disease. Chin Med J (Engl 2019;132:2423-9. https://doi.org/10.1097/CM9.0000000000000489.
- Kim HJ, Oh SH, Kim DY, Lee HS, Park SH, Yang SK, et al. Clinical characteristics and long-term outcomes of paediatric Crohn’s disease: a single-centre experience. J Crohns Colitis 2017;11:157-64. https://doi.org/10.1093/ecco-jcc/jjw146.
- Kim JW, Lee HS, Ye BD, Yang SK, Hwang SW, Park SH, et al. Incidence of and risk factors for free bowel perforation in patients with Crohn’s disease. Dig Dis Sci 2017;62:1607-14. https://doi.org/10.1007/s10620-017-4539-5.
- Kim OZ, Han DS, Park CH, Eun CS, Kim YS, Kim YH, et al. The clinical characteristics and prognosis of Crohn’s disease in Korean patients showing proximal small bowel involvement: results from the CONNECT study. Gut Liver 2018;12:67-72. https://doi.org/10.5009/gnl16500.
- Kostas A, Siakavellas SI, Kosmidis C, Takou A, Nikou J, Maropoulos G, et al. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol 2017;23:7387-96. https://doi.org/10.3748/wjg.v23.i41.7387.
- Kühn F, Nixdorf M, Schwandner F, Klar E. Risikofaktoren für einen frühen OP-Zeitpunkt und chirurgische Komplikationen bei Morbus Crohn [Risk factors for early surgery and surgical complications in Crohn’s disease]. Zentralbl Chir 2018;143:596-602. https://doi.org/10.1055/a-0645-1489.
- Kunovsky L, Kala Z, Marek F, Dolina J, Poredska K, Kucerova L, et al. The role of the NOD2/CARD15 gene in surgical treatment prediction in patients with Crohn’s disease. Int J Colorectal Dis 2019;34:347-51. https://doi.org/10.1007/s00384-018-3122-7.
- Kupka T, Simova J, Dvorackova J, Martinek L, Motyka O, Uvirova M, et al. Crohn’s disease – genetic factors and progress of the disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018;162:139-43. https://doi.org/10.5507/bp.2017.058.
- Kwapisz L, Gregor J, Chande N, Yan B, Ponich T, Mosli M. The utility of fecal calprotectin in predicting the need for escalation of therapy in inflammatory bowel disease. Scand J Gastroenterol 2017;52:846-50. https://doi.org/10.1080/00365521.2017.1315740.
- Levine A, Chanchlani N, Hussey S, Ziv-Baran T, Escher JC, Amil Dias J, et al. Complicated disease and response to initial therapy predicts early surgery in paediatric Crohn’s disease: results from the Porto Group GROWTH study. J Crohns Colitis 2020;14:71-8. https://doi.org/10.1093/ecco-jcc/jjz111.
- Liu W, Zhou W, Xiang J, Cao Q, Zhu J, Qi W, et al. Lémann Index at diagnosis predicts the risk of early surgery in Crohn’s disease. Dis Colon Rectum 2018;61:207-13. https://doi.org/10.1097/DCR.0000000000000930.
- Mańkowska-Wierzbicka D, Karczewski J, Poniedziałek B, Grzymisławska M, Staszewski R, Królczyk A, et al. C-reactive protein as a diagnostic and prognostic factor in inflammatory bowel diseases. Postepy Hig Med Dosw 2016;70:1124-30. https://doi.org/10.5604/17322693.1223798.
- Mañosa M, Calafat M, de Francisco R, García C, Casanova MJ, Huelín P, et al. Phenotype and natural history of elderly onset inflammatory bowel disease: a multicentre, case-control study. Aliment Pharmacol Ther 2018;47:605-14. https://doi.org/10.1111/apt.14494.
- Mosli M, Sabbahi H, Alyousef H, Abdulhaq M, Hadadi A, Aljahdali E, et al. Risk stratification of patients with Crohn’s disease: a retrospective analysis of clinical decision making and its impact on long-term outcome. Dig Dis 2018;36:49-55. https://doi.org/10.1159/000477613.
- Müller KE, Lakatos PL, Kovacs JB, Arato A, Varkonyi A, Nemes E, et al. Baseline characteristics and disease phenotype in inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2016;62:50-5. https://doi.org/10.1097/MPG.0000000000000885.
- Naganuma M, Hisamatsu T, Matsuoka K, Kiyohara H, Arai M, Sugimoto S, et al. Endoscopic severity predicts long-term prognosis in Crohn’s disease patients with clinical remission. Digestion 2016;93:66-71. https://doi.org/10.1159/000441767.
- Nahon S, Lahmek P, Paupard T, Lesgourgues B, Chaussade S, Peyrin-Biroulet L, et al. Diagnostic delay is associated with a greater risk of early surgery in a french cohort of Crohn’s disease patients. Dig Dis Sci 2016;61:3278-84. https://doi.org/10.1007/s10620-016-4189-z.
- Ng SC, Zeng Z, Niewiadomski O, Tang W, Bell S, Kamm MA, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology 2016;150:86-95.e3. https://doi.org/10.1053/j.gastro.2015.11.019.
- Nguyen GC, Bernstein CN, Benchimol EI. Risk of surgery and mortality in elderly-onset inflammatory bowel disease: a population-based cohort study. Inflamm Bowel Dis 2017;23:218-23. https://doi.org/10.1097/MIB.0000000000000993.
- Ouaz A, Fekih M, Labidi A, Ben Mustapha N, Serghini M, Zouiten L, et al. Changes of Crohn’s disease phenotype over time. Tunis Med 2016;94:167-70.
- Pallotta N, Vincoli G, Pezzotti P, Giovannone M, Gigliozzi A, Badiali D, et al. A risk score system to timely manage treatment in Crohn’s disease: a cohort study. BMC Gastroenterol 2018;18. https://doi.org/10.1186/s12876-018-0889-5.
- Park SH, Aniwan S, Scott Harmsen W, Tremaine WJ, Lightner AL, Faubion WA, et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm Bowel Dis 2019;25:1054-60. https://doi.org/10.1093/ibd/izy329.
- Park Y, Cheon JH, Park YL, Ye BD, Kim YS, Han DS, et al. Development of a novel predictive model for the clinical course of Crohn’s disease: results from the CONNECT study. Inflamm Bowel Dis 2017;23:1071-9. https://doi.org/10.1097/MIB.0000000000001106.
- Parker D, Karmazyn B, Steiner SJ. Radiologic predictors of surgery in newly diagnosed pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 2016;63:e182-5. https://doi.org/10.1097/MPG.0000000000001217.
- Pernat Drobež C, Repnik K, Gorenjak M, Ferkolj I, Weersma RK, Potočnik U. DNA polymorphisms predict time to progression from uncomplicated to complicated Crohn’s disease. Eur J Gastroenterol Hepatol 2018;30:447-55. https://doi.org/10.1097/MEG.0000000000001055.
- Rinawi F, Assa A, Hartman C, Mozer Glassberg Y, Nachmias Friedler V, Rosenbach Y, et al. Evolution of disease phenotype in pediatric-onset Crohn’s disease after more than 10 years follow up – Cohort study. Dig Liver Dis 2016;48:1444-50. https://doi.org/10.1016/j.dld.2016.08.118.
- Rispo A, Imperatore N, Testa A, Bucci L, Luglio G, De Palma GD, et al. Combined endoscopic/sonographic-based risk matrix model for predicting one-year risk of surgery: a prospective observational study of a tertiary centre severe/refractory Crohn’s disease cohort. J Crohns Colitis 2018;12:784-93. https://doi.org/10.1093/ecco-jcc/jjy032.
- Rönnblom A, Holmström T, Karlbom U, Tanghöj H, Thörn M, Sjöberg D. Clinical course of Crohn’s disease during the first 5 years. Results from a population-based cohort in Sweden (ICURE) diagnosed 2005-2009. Scand J Gastroenterol 2017;52:81-6. https://doi.org/10.1080/00365521.2016.1230777.
- Saad AM, Czul F, Sakuraba A, Rubin DT, Cohen RD. Age of diagnosis is associated with disease presentation and therapeutic complications in patients with Crohn’s disease. Inflamm Bowel Dis 2016;22:1027-31. https://doi.org/10.1097/MIB.0000000000000732.
- Sharma Y, Bousvaros A, Liu E, Bender Stern J. Natural history of children with mild Crohn’s disease. World J Gastroenterol 2019;25:4235-45. https://doi.org/10.3748/wjg.v25.i30.4235.
- Smids C, Horjus Talabur Horje CS, Nierkens S, Drylewicz J, Groenen MJM, Wahab PJ, et al. Candidate serum markers in early Crohn’s disease: predictors of disease course. J Crohns Colitis 2017;11:1090-100. https://doi.org/10.1093/ecco-jcc/jjx049.
- Sollelis E, Quinard RM, Bouguen G, Goutte M, Goutorbe F, Bouvier D, et al. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J Gastroenterol 2019;25:2354-64. https://doi.org/10.3748/wjg.v25.i19.2354.
- Song EM, Kim N, Lee SH, Chang K, Hwang SW, Park SH, et al. Clinical characteristics and long-term prognosis of elderly-onset Crohn’s disease. Scand J Gastroenterol 2018;53:417-25. https://doi.org/10.1080/00365521.2018.1437927.
- Stallmach A, Bokemeyer B, Helwig U, Lügering A, Teich N, Fischer I, et al. Predictive parameters for the clinical course of Crohn’s disease: development of a simple and reliable risk model. Int J Colorectal Dis 2019;34:1653-60. https://doi.org/10.1007/s00384-019-03369-0.
- Sun XW, Wei J, Yang Z, Jin XX, Wan HJ, Yuan BS, et al. Clinical features and prognosis of Crohn’s disease with upper gastrointestinal tract phenotype in chinese patients. Dig Dis Sci 2019;64:3291-9. https://doi.org/10.1007/s10620-019-05651-1.
- Szántó K, Nyári T, Bálint A, Bor R, Milassin Á, Rutka M, et al. Biological therapy and surgery rates in inflammatory bowel diseases - Data analysis of almost 1000 patients from a Hungarian tertiary IBD center. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0200824.
- Torres J, Caprioli F, Katsanos KH, Lobatón T, Micic D, Zerôncio M, et al. Predicting Outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis 2016;10:1385-94. https://doi.org/10.1093/ecco-jcc/jjw116.
- Wang XQ, Xiao Y, Xu X, Yu Y, Shan CY, Guo Y, et al. Study of disease phenotype and its association with prognosis of paediatric inflammatory bowel disease in China. BMC Pediatr 2018;18. https://doi.org/10.1186/s12887-018-1212-x.
- Wu J, Lubman DM, Kugathasan S, Denson LA, Hyams JS, Dubinsky MC, et al. Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric Crohn’s disease. Am J Gastroenterol 2019;114:777-85. https://doi.org/10.14309/ajg.0000000000000237.
- Ye L, Chen BQ, Wang SD, Shi H, Yang Z, Wang FY. Fecal calprotectin is a strong predictive marker of relapse in Chinese patients with Crohn’s disease: a two-year prospective study. Scand J Gastroenterol 2017;52:1113-19. https://doi.org/10.1080/00365521.2017.1346704.
- Zhao M, Lo BZS, Vester-Andersen MK, Vind I, Bendtsen F, Burisch J. A 10-year follow-up study of the natural history of perianal Crohn’s disease in a Danish population-based inception cohort. Inflamm Bowel Dis 2019;25:1227-36. https://doi.org/10.1093/ibd/izy374.
- Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther 2016;44:495-504. https://doi.org/10.1111/apt.13731.
- Ziv-Baran T, Hussey S, Sladek M, Amil Dias J, Martin de Carpi J, Miele E, et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-onset paediatric Crohn’s disease. Aliment Pharmacol Ther 2018;48:1242-50. https://doi.org/10.1111/apt.15016.
- Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther 2014;40:620-8. https://doi.org/10.1111/apt.12869.
- Zitomersky NL, Atkinson BJ, Fournier K, Mitchell PD, Stern JB, Butler MC, et al. Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis 2015;21:307-14. https://doi.org/10.1097/MIB.0000000000000284.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55-63. https://doi.org/10.7326/M14-0697.
List of abbreviations
- anti-CBir1
- anti-flagellin antibody
- anti-TNF
- anti-tumour necrosis factor
- ASCA
- anti-Saccharomyces cerevisiae antibodies
- CDAI
- Crohn’s Disease Activity Index
- CHARMS
- CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies
- CI
- confidence interval
- CRP
- C-reactive protein
- ESR
- erythrocyte sedimentation rate
- HTA
- Health Technology Assessment
- IBD
- inflammatory bowel disease
- Ig
- immunoglobulin
- IQR
- interquartile range
- JAK
- Janus kinase
- MeSH
- medical subject heading
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NOD2
- nucleotide-binding oligomerisation domain-containing protein 2
- OR
- odds ratio
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST
- Prediction model Risk Of Bias Assessment Tool
- ROB
- risk of bias
- STAT
- signal transducer and activator of transcription
- TA
- Technology Appraisal
- TNF
- tumour necrosis factor
- TRIPOD
- Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis
- WBC
- white blood cell
