Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR129932. The contractual start date was in October 2019. The draft report began editorial review in July 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Duarte et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Stable angina is a type of chest pain caused by insufficient blood supply to the heart, brought on by physical activity or emotional stress, which goes away with rest. It is the key symptom of coronary artery disease (CAD), which remains one of the main causes of morbidity and mortality in high-income countries. Complications include unstable angina, heart failure, myocardial infarction (MI) and sudden death.
To alleviate symptoms, patients may receive ‘revascularisation’ to open damaged, obstructed or blocked arteries. This most commonly consists of inserting a small tube or ‘stent’ into the artery to keep it open and allow blood flow. Patients who might need revascularisation undergo a number of tests to identify blocked arteries, including coronary computed tomography angiography (CCTA) and other non-invasive tests. If these tests are inconclusive, more invasive tests are needed, for example invasive coronary angiography (ICA), where a contrast medium is injected through a catheter into the coronary arteries and radiographic images (angiograms) are taken.
Angiograms have limited ability to differentiate between arteries with inadequate blood supply (which need revascularisation) and those with adequate supply that do not need treatment. To address this, the procedure may be combined with an invasive measurement of blood flow, such as invasive fractional flow reserve (FFR) assessment. During this procedure, the blood flow is measured by inserting a wire into the coronary arteries after the patient has taken drugs to dilate the artery. The procedure is invasive and, therefore, carries some risks and may have side effects.
The Health Survey for England 2017: Adult Social Care1 reported that the prevalence among adults of ever having ischaemic heart disease (including MI and angina) was 4%. The prevalence was higher among men (6%) than women (3%) and increased with age (3% in people aged 45–54 years, 16% in people aged > 75 years). Prevalence of angina and history of angina among all adults was 3%.
Description of the technologies under assessment
Non-invasive imaging tests have been proposed to precede or replace invasive FFR, by using the existing angiograms to determine blood flow, without inserting a wire.
QAngio XA 3D/QFR
QAngio® XA 3D/QFR® (three-dimensional/quantitative flow ratio) (Medis Medical Imaging Systems BV, Leiden, the Netherlands) imaging software is used to perform quantitative flow ratio (QFR) assessment of coronary artery obstructions. It is designed to be used with all ICA systems: biplane or monoplane. It uses two standard two-dimensional (2D) angiographic projections, taken at least 25° apart – ideally between 35° and 50° apart – to create a three-dimensional (3D) reconstruction of a coronary artery; this shows the QFR values across the artery. QFR is an assessment (by frame count) of the pressure (blood flow velocity) drop over the artery, with a value of 1 representing a normally functioning artery with no pressure drop. A drop of ≥ 20 mmHg in blood pressure (QFR value of ≤ 0.8) is considered a significant obstruction where revascularisation should be considered. QAngio XA 3D/QFR software is installed on a laptop or workstation that is connected to the ICA system. The Digital Imaging and Communication in Medicine (DICOM) data from ICA projections are immediately uploaded and viewable on the connected workstation. The total time for data acquisition and analysis is about 4 to 5 minutes (as reported by the company).
AngioPLUS [Pulse Medical Imaging Technology (Shanghai) Co. Ltd, Shanghai, China] is an equivalent Conformité Européenne-marked version marketed in Asia.
The QAngio XA 3D/QFR software offers two different flow models to calculate QFR:
-
fixed-flow quantitative flow ratio (fQFR), using fixed-flow velocity
-
contrast-flow quantitative flow ratio (cQFR), using contrast frame count in an angiogram without hyperaemia.
Fixed-flow quantitative flow ratio is faster to compute, but may be less accurate than cQFR.
Furthermore, the QAngio XA 3D/QFR software provides four different QFR indices along the analysed coronary segment:
-
vessel quantitative flow ratio (vQFR): the QFR value at the distal location of the analysed vessel segment
-
index quantitative flow ratio (iQFR): a point that can be moved along the QFR pullback curve
-
lesion quantitative flow ratio (lQFR): the contribution to the QFR drop by the selected lesion alone
-
residual vQFR: an indication of the vQFR, if the selected lesion is resolved.
CAAS vFFR
The CAAS® vFFR® (vessel fractional flow reserve) (Pie Medical Imaging BV, Maastricht, the Netherlands) workflow builds a 3D reconstruction of a coronary artery based on two standard angiograms and assesses the pressure drop across the stenosis, and quantitative coronary arteriography (QCA) determines a vFFR value. It gives both anatomical and functional assessment of the stenosis and can be integrated into catheter laboratories. According to the company, the total time for analysis is approximately 2 minutes per artery.
All available versions of CAAS (i.e. 8.0, 8.1 and 8.2) use the same algorithm for calculating vFFR. The CAAS workstation provides various modules (e.g. QCA and left ventricular analysis), and the vFFR module can be added to the CAAS workstation. In addition to the vFFR, CAAS vFFR provides measurements at the end of the lesion and at a chosen position in the coronary artery.
Comparators
Invasive coronary angiography may differentiate between arteries with inadequate blood supply (which need revascularisation) and those with adequate supply that do not need treatment.
During an ICA procedure, a coronary diagnostic catheter is inserted into an artery and moved up the aorta and into the coronary arteries. A special type of dye called contrast medium is injected through the catheter into the coronary artery and angiograms are taken. Although providing valuable information on coronary artery anatomy, visual assessment of angiograms taken during ICA may have limited ability to differentiate between functionally significant (causing inadequate blood supply) and non-significant (not significantly affecting blood supply) coronary stenoses.
When ICA is inconclusive, it may be combined with the invasive measurement of FFR. In these procedures FFR is assessed invasively by advancing a pressure wire towards the stenosis and measuring the ratio in pressure between the two sides of the stenosis during maximum blood flow (induced by adenosine infusion). This is associated with risks related to the passage of a guide wire, side effects of adenosine and additional radiation exposure. The invasive FFR measurement is also associated with increased procedural time and costs compared with ICA alone. As an alternative to invasive FFR, the instantaneous wave-free ratio (iFR) may be used. This also uses inserted pressure wires to assess flow but does not require vasodilator drugs, such as adenosine.
Current service provision and care pathways
Patients who experience chest pain and may need revascularisation will be assessed for angina and other cardiovascular conditions. When clinical assessment alone is insufficient for a diagnosis, patients are referred for a 64-slice (or above) CCTA as the first-line diagnostic test.
Patients may go on to further diagnostic testing. National Institute for Health and Care Excellence (NICE) guidance2 recommends offering non-invasive functional imaging for myocardial ischaemia if a 64-slice (or above) CCTA has shown CAD of uncertain functional significance, or is non-diagnostic. This could include:
-
myocardial perfusion scintigraphy (MPS) with single-photon emission computed tomography (SPECT)
-
stress echocardiography
-
first-pass contrast-enhanced magnetic resonance (MR) perfusion
-
MR imaging for stress-induced wall motion abnormalities.
In addition, NICE’s medical technologies guidance3 recommends that HeartFlow FFRCT (HeartFlow, Inc., Redwood City, CA, USA) should be considered as an option for patients with stable, recent-onset chest pain who are offered 64-slice (or above) CCTA. It provides both functional and anatomical assessment of coronary arteries and has better diagnostic performance than CCTA alone or other non-invasive or invasive tests. If these tests are also inconclusive, ICA is offered as a third-line diagnostic tool.
A diagnosis of stable angina is made when clinical symptoms are present and:
-
Significant CAD is found during ICA or 64-slice (or above) CCTA. This is usually defined as ≥ 70% diameter stenosis (DS) of at least one major epicardial artery segment, or ≥ 50% DS in the left main coronary artery.
-
Reversible myocardial ischaemia is found during non-invasive functional imaging.
Sometimes ICA is also used to guide treatment strategies for people with a confirmed diagnosis of stable angina whose symptoms are not satisfactorily controlled with optimal medical treatment (OMT), and so may require revascularisation. ICA may differentiate between arteries with inadequate blood supply (which need revascularisation) and those with adequate supply that do not need treatment. When ICA is used to determine the presence and severity of coronary stenosis and it is inconclusive, it may be combined with the invasive measurement of FFR using a pressure wire, as recommended by the European Society of Cardiology4 and American College of Cardiology. 5 Lesions with a FFR of ≤ 0.80 are functionally significant and revascularisation may be considered. Should iFR be used, a measure of ≤ 0.89 is considered functionally significant.
Invasive coronary angiography is performed either in diagnostic-only ICA laboratories or in interventional catheter laboratories as part of the initial stenosis assessment prior to percutaneous coronary intervention (PCI). In diagnostic-only laboratories, patients in whom ICA alone is inconclusive might be referred to an interventional laboratory for a FFR or iFR assessment. In interventional laboratories a FFR or iFR assessment can be performed immediately after ICA, if needed.
The British Cardiovascular Intervention Society (BCIS)’s audit reports that 244,332 ICA procedures took place in the UK in 2017/18 in NHS and private facilities, with 35,017 procedures performed in diagnostic-only catheter laboratories.
There is substantial regional variation in the diagnostic pathway for stable angina, due in part to the availability of imaging modalities at each centre, and experience (or preferences) of the cardiologists referring for the test. Clinical advisors noted that the pathway recommended by NICE is widely recognised as current best practice.
Position of the technology in the diagnostic pathway
Either QFR or vFFR could potentially replace pressure wire FFR, or iFR, by providing a non-invasive means to assess FFR as part of an ICA assessment in people with stable chest pain of recent onset. Visual assessment of angiograms taken during ICA may be limited in its ability to differentiate between functionally significant (causing inadequate blood supply) and non-significant (not significantly affecting blood supply) coronary stenoses. Alternatively, they may be used as a precursor to invasive FFR, with the invasive procedure used when QFR or vFFR is inconclusive.
In addition, QFR may be used in other aspects of decision-making, including whether to stent more than one vessel or to select a stent type or other interventional device for revascularisation.
QAngio XA 3D/QFR and CAAS vFFR could also be used in diagnostic-only laboratories, possibly reducing the need for referrals to interventional laboratories.
The QAngio XA 3D/QFR instructions recommend the following approach:
-
QFR < 0.78 – treat the patient in the catheter laboratory
-
QFR > 0.84 – follow the patient medically
-
QFR 0.78–0.84 (grey zone) – verify by invasive FFR measurement.
Following request for clarification, Pie Medical Imaging stated that it recommends the same hybrid approach for CAAS vFFR.
The likely pathway leading to invasive FFR, and including the probable placement of QFR and vFFR, is summarised in Figure 1.
FIGURE 1.
Diagnostic pathway for stable angina, including QFR or vFFR (from the NICE Diagnostics Assessment Programme 48 final scope). 6 a, Non-invasive functional imaging includes MPS with SPECT, stress echocardiography, first-pass contrast-enhanced MR perfusion and MR imaging for stress-induced wall motion abnormalities. © NICE [2019] QAngio XA 3D/ QFR and CAAS vFFR Imaging Software for Assessing the Functional Significance of Coronary Obstructions During Invasive Coronary Angiography. Final Scope. Available from www.nice.org.uk/guidance/dg43/documents/final-scope All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.

Chapter 2 Aims and objectives
The aim of the project is to determine the clinical effectiveness and cost-effectiveness of non-invasive assessment of the functional significance of coronary stenoses, using QAngio XA 3D/QFR and CAAS vFFR imaging software.
To achieve this, the following objectives were set:
-
Clinical effectiveness –
-
To perform a systematic review and meta-analysis of the diagnostic accuracy and, where feasible, clinical efficacy of the QAngio XA 3D/QFR imaging software and CAAS vFFR software used during ICA for assessing the functional significance of coronary obstructions in people with stable chest pain whose angiograms show intermediate coronary stenosis.
-
To perform a narrative systematic review of the clinical efficacy and practical implementation of QAngio XA 3D/QFR and CAAS vFFR imaging software. This includes assessment of the associated revascularisation rates, mortality and morbidity, patient-centred outcomes, adverse events and acceptability to clinicians and patients.
-
-
Cost-effectiveness –
-
To perform a systematic review of published cost-effectiveness studies of the use of the QAngio XA 3D/QFR and CAAS vFFR imaging software for assessing the functional significance of coronary stenosis in people with stable chest pain whose angiograms show intermediate stenosis.
-
To develop a decision model to estimate the cost-effectiveness of the QAngio XA 3D/QFR and CAAS vFFR imaging software used during ICA to indicate whether or not coronary obstructions are functionally significant. Consideration will be given to differences in the cost-effectiveness of the technologies in diagnostic-only or in interventional catheter laboratories.
-
The decision model will link the diagnostic accuracy of QFR derived from the QAngio XA 3D/QFR imaging software, and vFFR derived from the CAAS vFFR software, to short-term costs and consequences (e.g. the impact on the number of revascularisations needed, the proportion of people who need invasive functional assessment of stenosis, time to test results, and associated risks of the diagnostic intervention). It will link the short-term consequences to potential longer-term costs and consequences (e.g. major cardiovascular events such as MI and sudden cardiac death, adverse events related to revascularisation and diagnosis, and mortality) using the best-available evidence.
-
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
The systematic review was conducted following the general principles recommended in the Centre for Reviews and Dissemination (CRD) guidance and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 7
Searches
Comprehensive searches of the literature were conducted to systematically identify all studies relating to QAngio XA 3D/QFR and CAAS vFFR imaging software.
The searches were carried out during October 2019, with a further updated search undertaken on 2 January 2020. The following databases were searched: MEDLINE, PubMed, EMBASE™ (Elsevier, Amsterdam, the Netherlands), the Science Citation Index (Clarivate Analytics, Philadelphia, PA, USA), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Health Technology Assessment (HTA) database and EconLit (American Economic Association, Nashville, TN, USA).
Ongoing and unpublished studies were identified by searches of ClinicalTrials.gov; Conference Proceedings Citation Index: Science (Clarivate Analytics); EU Clinical Trials Register; Open Access Theses and Dissertations; ProQuest® (ProQuest LLC, Ann Arbor, MI, USA) Dissertations & Theses A&I; PROSPERO; the World Health Organization’s International Clinical Trials Registry Platform portal; and manufacturer websites. Abstracts from any recent conferences that are thought to be relevant to the review were also consulted.
A search strategy for Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands) MEDLINE is reported in Appendix 1. The MEDLINE strategy was translated to run appropriately on the other databases and resources. No language or date restrictions were applied to the searches. No study design search filters were used.
Reference lists of relevant recent reviews8 were checked to identify additional potentially relevant reports.
Database searches were carried out to identify cost-effectiveness studies where ICA (alone and/or with FFR) was one of the interventions under comparison. The following databases were searched: EconLit, EMBASE, HTA database, MEDLINE and NHS Economic Evaluation Database (NHS EED). The search strategies for EconLit, EMBASE and MEDLINE are reported in Appendix 1.
Pragmatic supplementary PubMed and Google Scholar (Google Inc., Mountain View, CA, USA) searches were carried out to identify studies of diagnostic data on ICA compared with FFR.
Contact with study authors and manufacturers and request for individual participant data
An individual participant data (IPD) meta-analysis of four studies that has previously been performed was eligible for this review. 8 The review authors contacted the study authors prior to commencing this assessment, and the study authors agreed, in principle, to share the collected IPD with the review authors for the purposes of this work. However, because of the slow response from the study authors, the IPD could not be supplied in time for this report, and the decision was made not to pursue an IPD analysis. Instead, published data and data presented in figures were used. Where possible, IPD-equivalent data were extracted from plots using a digitising software. See Data extraction for further detail.
Selection criteria
Two reviewers independently screened all titles and abstracts. Full papers of any titles and abstracts that were thought to be relevant were obtained where possible, and the relevance of each study assessed independently by two reviewers according to the criteria below. Any disagreements were resolved by consensus or by consulting a third reviewer. Conference abstracts were included where sufficient data were reported to confirm eligibility. Authors were contacted where insufficient data were reported to confirm inclusion (for instance, to clarify what index test was used in the study, or to provide complete 2 × 2 data) and where it was unclear whether or not the same diagnostic accuracy results were presented in more than one report (e.g. conference abstracts linked to a publication).
Diagnostic accuracy
Included were diagnostic accuracy and correlation studies in which QFR using any version of the QAngio® system (Medis Medical Imaging Systems BV) or CAAS vFFR were performed in addition to invasive FFR (or iFR) as a reference standard in the same patients. Only prospective and retrospective cohorts were included. Case–control studies, letters, editorials and reviews were excluded.
Clinical effectiveness/implementation
Included were observational studies where QFR or vFFR (with or without invasive FFR) have been used and that report relevant clinical outcomes as detailed. Relevant publications reporting issues related to implementation of, or practical advice for, QFR or vFFR and their use in clinical practice were also eligible. Case reports and studies focusing only on technical aspects of QFR or vFFR (such as technical descriptions of the testing process or specifications of machinery and software) were excluded.
Participants
Patients with intermediate stenosis (however defined) who are referred for ICA to assess coronary stenosis and the need for revascularisation were included. Although the main focus of this assessment was on patients with stable chest pain (either suspected stable angina or confirmed angina that is not adequately controlled by treatment), patients with all types of angina (including unstable, non-specific and atypical) were eligible for inclusion. Patients with acute MI [ST segment elevation myocardial infarction (STEMI) and non-ST segment elevation myocardial infarction (NSTEMI) < 72 hours] were also included provided QFR was performed in non-culprit vessels.
Interventions
All versions of QAngio XA 3D/QFR (including AngioPlus) and CAAS vFFR imaging software used in conjunction with ICA to allow simulation of FFR were included.
All submeasurements of QFR were eligible, including cQFR and fQFR. Eligible health-care settings were diagnostic-only and interventional catheter laboratories.
Reference standard
The reference standard was FFR assessed using an invasive pressure wire with or without adenosine. iFR, which was found to be non-inferior to FFR for predicting cardiovascular events and all-cause mortality,9 was also accepted as a reference standard.
Outcomes
The eligible outcome measures relating to diagnostic accuracy were:
-
sensitivity and specificity of QAngio XA 3D/QFR and CAAS vFFR
-
positive predictive values (PPVs) and negative predictive values (NPVs)
-
estimates of difference in measurements between QFR or vFFR and invasive FFR/iFR (including Bland–Altman assessments)
-
correlation between QFR or vFFR and invasive FFR/iFR measurements.
Some studies reported differences or concordance between QFR or vFFR and invasive FFR/iFR in numerous ways, including inter- and intra-rater differences in measurements, mean differences (MDs), correlation coefficients, sensitivity and specificity, or receiver operating characteristic (ROC) curves. All relevant outcome definitions and cut-off points were extracted and their applicability to the decision problem accounted for when presenting the results. Diagnostic accuracy results of ICA alone were considered if reported alongside QFR or CAAS vFFR.
In addition, the following clinical outcomes were eligible:
-
morbidity, mortality and major adverse cardiac events (MACEs) (e.g. MI, heart failure)
-
adverse events related to the diagnostic procedure (e.g. pressure wire damage, adenosine side effects, stroke)
-
adverse events related to revascularisation
-
distress, anxiety and similar harms caused by QAngio XA 3D/QFR, CAAS vFFR, invasive FFR or iFR
-
subsequent use of invasive pressure wire FFR or iFR
-
subsequent revascularisation procedures performed (including unscheduled revascularisations)
-
number of vessels with stent placements
-
health-related quality of life (HRQoL)
-
radiation exposure.
Eligible outcomes related to the implementation of the interventions of interest and related practical issues included:
-
acceptability of QFR, vFFR and invasive FFR (to clinicians and patients)
-
test failure rates
-
inconclusive test rates
-
inter-observer variability
-
timing of results from data acquisition
-
referral times
-
patient satisfaction
-
training requirements
-
uptake and compliance.
Data extraction
A standardised data extraction form was developed, piloted and finalised to data-extract both study and patient characteristics and eligible outcomes. For studies reporting diagnostic accuracy data, the number of true-positive (TP), true-negative (TN), false-positive (FP) and false-negative (FN) results were extracted for each index test evaluated in each study, along with sensitivity and specificity data, the area under the curve (AUC) and 95% confidence intervals (CIs) and PPVs and NPVs. Whether diagnostic accuracy was determined per patient, vessel or lesion was recorded.
Where not reported, sensitivity and specificity were calculated if data allowed. Further data were requested from study authors when required. Correlation and MD between QFR/vFFR and FFR were recorded along with reasons for any excluded, failed or inconclusive results and any other relevant clinical outcomes from the studies.
As IPD could not be supplied, digitised data were extracted using WebPlotDigitizer (Ankit Rohatg, Pacifica, CA, USA) software to approximately reconstruct the individual-level data from included studies. Data were extracted for all studies that presented a Bland–Altman or correlation plot. Bland–Altman plots were preferred for extraction, as these were found to be generally clearer and easier to extract. The extracted averages and differences between QFR and FFR were converted into QFR and corresponding FFR values for each study. For some studies, the quality of published figures was not sufficient to extract data.
Data were extracted by one reviewer using a standardised data extraction form and independently checked by a second reviewer. Discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. Data from relevant studies with multiple publications were extracted and reported as a single study. The most recent or most complete publication was used in situations where we could not exclude the possibility of overlapping populations across separate study reports.
Critical appraisal
The quality of the diagnostic accuracy studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. The QUADAS-2 tool evaluates both risk of bias (associated with the population selection, index test, reference standard and patient flow) and study applicability (population selection, index test and reference standard) to the review question. The tool was piloted on a sample of studies. Signalling questions and criteria for decisions were finalised following piloting.
The quality assessments were performed by one reviewer and independently checked by a second reviewer. Disagreements were resolved through consensus and, when necessary, by consulting a third reviewer.
Methods of data synthesis
The results of data extraction were presented in structured tables and as a narrative summary, grouped by population and test characteristics. The diagnostic accuracy was calculated for each study based on extracted data, using the usual index test of QFR ≤ 0.8 and reference standard of FFR ≤ 0.8 as defining patients in need of stenting. Where sufficient clinically and statistically homogenous data were available, data were pooled using appropriate meta-analytic techniques. Studies that did not report sufficient information to derive 2 × 2 data (from tables, text or plots) were not included in the meta-analysis and were synthesised narratively.
Statistical analysis of diagnostic accuracy
Meta-analysis using 2 × 2 diagnostic data
The primary meta-analyses in this report were based on studies that reported 2 × 2 diagnostic data, or where data could be reconstructed from tables. Both univariate meta-analysis and bivariate meta-analysis of sensitivity and specificity10 were performed and compared, categorised according to ‘mode’ of QFR used: either fQFR, cQFR or non-specified QFR (referred to as QFR). These analyses included all patients, vessels and lesions. Results are reported in forest plots and summarised in tables and ROC plots.
Separate (univariate) meta-analyses were performed for each diagnostic outcome [sensitivity, specificity, PPV and NPV, diagnostic odds ratio (DOR), area under ROC curve, correlation between QFR and FFR, and MD between QFR and FFR] and presented in forest plots.
A hierarchical bivariate generalised linear mixed model, as described by Simmonds et al. ,11 was fitted to the data to calculate summary estimates of sensitivity and specificity and the associated 95% CIs. The same model was used to produce summary ROC curves, using the Rutter and Gatsonis formulation for the hierarchical summary receiver operating characteristic (HSROC) curve. 11,12 Results are presented in ROC plots. Unless otherwise specified, all analyses used a cut-off point for the index test of QFR ≤ 0.8 and reference standard of FFR ≤ 0.8 as defining patients in need of revascularisation.
As some studies reported data on two or more tests (e.g. QFR and ICA or fQFR and cQFR), the bivariate model was extended to include diagnostic accuracy parameters for multiple tests, which allowed for formal comparison between models in terms of sensitivity and specificity. 11
Investigation of heterogeneity and subgroup analyses
For diagnostic accuracy data, we visually inspected the forest plots and ROC space to check for heterogeneity between study results. To assess the impact of patient factors, we performed meta-regressions of sensitivity, specificity and DOR against key patient parameters reported in papers. All meta-regressions were univariate analyses (i.e. one patient parameter per metaregression).
Where available, we considered the following factors as potential sources of heterogeneity:
-
type and severity of stenosis (e.g. high percentage DS)
-
multivessel CAD
-
diffuse CAD
-
multiple stenoses in one vessel
-
microvascular dysfunction (e.g. caused by diabetes)
-
chronic total occlusion
-
diabetes
-
sex
-
age
-
ethnicity (or study location as a proxy for ethnicity)
-
results of previous non-invasive tests
-
use of fQFR compared with cQFR (QAngio XA 3D/QFR)
-
previous MI.
For these analyses fQFR was not separated from cQFR; instead, one test per study (cQFR for preference) was analysed to maximise data. This was judged to be reasonable given that diagnostic accuracy did not appear to vary substantially according to the type of QFR used.
Where studies reported the factors of interest separately by subgroup, these subgroup results were compared; however, these were too sparsely reported to permit any meta-analysis. For patient factors where data did not allow for metaregression, a narrative synthesis of the impact of covariates has been provided.
Sensitivity analyses
We conducted sensitivity analyses to explore the robustness of the results according to study quality based on QUADAS-2 domain results (e.g. risk of incorporation bias) and study design (e.g. in-procedure compared with retrospective evaluation of index test results) for diagnostic accuracy studies. ROC plots of sensitivity and specificity according to risk of bias were produced to visually assess possible bias. Where feasible, bivariate meta-analyses were repeated, subgrouped according to the assessed risk of bias.
Meta-analysis of data extracted from figures
Using data extracted from figures, estimates of sensitivity and specificity were calculated and presented on forest plots and in the ROC space to examine the variability in diagnostic test accuracy within and between studies. These were compared with the diagnostic accuracy results from 2 × 2 table to investigate whether or not the extracted data could be used for analysis. The bivariate meta-analyses performed using 2 × 2 data were repeated using the extracted figure data.
Grey-zone analysis
Extracted figure data were used to conduct an analysis where testing includes a grey zone of intermediate QFR values for which a FFR would be performed as a confirmatory test. The grey-zone diagnostic procedure considered, following the QAngio XA 3D/QFR instructions, was:
-
perform the QFR
-
if the QFR is > 0.84, continue without stenting/bypass (test negative)
-
if the QFR is ≤ 0.78, proceed to stenting/bypass (test positive)
-
if the QFR is between 0.78 and 0.84, perform a FFR test and proceed to stenting/bypass if the FFR ≤ 0.80 (the grey zone).
For the grey-zone analysis, it was assumed that anyone in the grey zone has perfect diagnostic accuracy (because all received a ‘gold standard’ FFR test); therefore, FPs and FNs are present only in patients outside the grey zone. The impact of using the grey zone on the diagnostic accuracy of QAngio XA 3D/QFR was assessed. The effect of using different FFR thresholds on the diagnostic accuracy of QAngio XA 3D/QFR was also assessed. Owing to the limited data on CAAS vFFR, no such analyses were performed for this technology.
Narrative synthesis
Evidence related to clinical effectiveness and implementation of QFR, vFFR and invasive FFR were too limited to allow meta-analysis. Results were tabulated and presented narratively. Conclusions of these studies suggested consequences for QFR and ICA and recommendations for practice, and suggested needs for further research were summarised.
Narrative summaries were used for any diagnostic accuracy outcomes where meta-analyses or other statistical analyses were not feasible. This included tabulating or plotting results as reported in studies, and narratively describing and comparing these results.
Statistical analysis of clinical effectiveness
The systematic review identified very few published data on the clinical impact of using QFR and QAngio screening. In particular, very few data were found on the impact that QFR (with or without a grey zone) might have on future incidence and prevention of coronary events. Therefore, to investigate what the clinical impact of using QFR testing might be, a simulation study was performed to identify the impact that QFR and invasive FFR assessment might have on the number of revascularisations performed, and on morbidity and mortality and other longer-term outcomes. This simulation used two key sources of data:
-
The data on FFR and QFR measurements extracted from published Bland–Altman figures were used as a representative population of patients with intermediate stenosis, with FFR and QFR measurements for each patient.
-
The IRIS-FFR study13 reported the association between FFR and coronary events in patients who are revascularised and in patients where revascularisation is deferred. These data were used to calculate the risk of coronary events, and then to simulate events for each patient in our sample population (from point 1), given their observed FFR measurement.
Combining these two data sources produced a simulated data set where each patient had the following data:
-
a FFR measurement
-
the associated QFR measurement
-
the risk of a coronary event if revascularisation were performed
-
the risk of a coronary event if revascularisation were deferred
-
whether or not the patient had a coronary event (if revascularised)
-
whether or not the patient had a coronary event (if deferred).
Three strategies for deciding on whether or not to revascularise were considered:
-
FFR only – perform FFR on all and revascularise if the FFR is ≤ 0.8
-
QFR only – perform QFR on all and revascularise if the QFR is ≤ 0.8, without FFR measurement
-
grey zone – perform a QFR and:
-
revascularise if the QFR is ≤ 0.78
-
defer if the QFR is > 0.84
-
if the QFR is between 0.78 and 0.84, perform FFR and revascularise if the FFR is ≤ 0.8.
-
Applying these strategies to the simulated data set, the following data were calculated for each strategy:
-
the proportion of patients who would be revascularised
-
the total number of coronary events
-
the proportion of patients who would undergo unnecessary revascularisation (i.e. revascularised, but would not have had an event if revascularisation were deferred)
-
the proportion of patients in whom revascularisation prevented an event (i.e. are revascularised, and would have had an event if revascularisation were deferred)
-
the proportion of patients in whom revascularisation caused an event (i.e. who would have an event after being revascularised, but would not have had an event if revascularisation were deferred).
These results were then compared across strategies to investigate how the differing strategies might alter the incidence of coronary events.
Detailed simulation methods
The sample population for the simulation was taken to be the data extracted from published Bland–Altman figures. For this analysis, fQFR data were excluded and only cQFR or non-specified QFR data were used, making a total of 3193 patients, each with a FFR measurement and its associated QFR measurement. As these data were extracted from figures, they may not be a perfect representation of the actual study patients (see Data extraction). The simulation did not differentiate between studies, so the patients were treated as if they came from a single ‘mega-study’.
To predict coronary outcome in this sample population, the results of the recent IRIS-FFR registry report were used, representing 5846 patients who were either ‘revascularised’ (stent or bypass surgery) or ‘deferred’ (continued with current management without surgery) based on their measured FFR result. The IRIS-FFR study13 used major cardiovascular events (MACE, a composite of cardiac death, MI and repeated/emergency revascularisation) as its primary outcome. The mean incidence rate from MACE in deferred patients was 1.44 events per 100 lesion-years. For simplicity, it was assumed that each person has one lesion, equating to a 1.44% risk in 1 year. Based on data reported in the publication, this equated to a risk of 0.64% at a FFR of exactly 1. According to IRIS-FFR, most of these events are later revascularisations. The hazard ratio (HR) for MACE was estimated as 1.06 per 0.01 decrease in FFR. It was assumed that the 1-year relative risk (RR) is the same as this HR. In patients with revascularisations, the mean risk of MACE was 2.4% in 1 year, with a HR of 1 (so risk is the same regardless of FFR value).
Based on those risks, the predicted risk of MACE for every person in the sample population was calculated using their reported FFR measurement (this means that risk is not dependent on QFR). A risk of event if revascularised and a risk if deferred was calculated. A Monte Carlo simulation was then used to simulate whether or not each person had a MACE if they were ‘deferred’ or if they were revascularised, based on the calculated risks. Therefore, the incidence of simulated events is solely a function of FFR values and knowing that the QFR has no impact on risk of MACEs. The simulation process was repeated 10,000 times to produce a reasonable sample of plausible simulations.
For each simulated sample, who would and would not be revascularised was determined for each of the three strategies listed above. Given that, and the known MACE status for each patient, the five statistics in the list above were calculated. The results were pooled across simulations to find median values across simulations and to plot distributions across all simulations.
All statistical analyses were conducted in R software, version 3.6 or later (The R Foundation for Statistical Computing, Vienna, Austria).
Quantity and quality of evidence available
A total of 1248 unique references were screened for eligibility, and 41 unique studies were included in the systematic review. 14–54 A total of 39 studies evaluated QAngio XA 3D/QFR,14–17,20–55 and three studies evaluated CAAS vFFR. 18,19,26 One study directly compared CAAS vFFR with QAngio XA 3D/QFR. 26 Full lists of all included references, ongoing studies and studies excluded at full-text screening stage are presented in Appendix 2, Tables 33–35.
Two studies did not report diagnostic accuracy data, but included other eligible outcomes. 25,30 All other studies were included in the diagnostic accuracy review, of which 33 were included in a meta-analysis. 15–21,23,24,26–32,34,35,37,39–46,48–54 Seventeen were conference abstracts. 14,16,18,22,25–31,35,36,38,39,44,54 Figure 2 presents an overview of the study selection process.
FIGURE 2.
Study selection process: PRISMA flow diagram.

Characteristics of included studies
Table 1 presents the characteristics of the studies included in the systematic review. Only seven studies used QFR prospectively as part of the ICA examination preceding FFR. 14,44,48–52 Fifteen studies were conducted in multiple centres. 15,22,24,26,34,37,38,41,45–47,50–52
| Main studies | Single/multicentre | Country | Population | Number of patients (vessels or lesions) | Age (years), mean (SD) | Male, % | Diabetes, % | Acute MI, % | FFR, mean (SD) or median (IQR) | Mean DS, % | Stable angina, % | Stable CAD, % | Previous MI, % | Previous PCI, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QAngio XA 3D/QFR studies | ||||||||||||||
| Cliff (2019),16 conference abstract | Single | Singapore | Acute MI and non-acute | 33 (41) | 59 (20) | 69 | 30 | 5 | NR | NR | NR | NR | NR | NR |
| Cortés et al. (2019)17 | Single | Spain | STEMI, > 50% DS in non-culprit arteries | 10 (12) | 70 (9) | 75 | NR | 100 | 0.87 (0.06) | NR | NR | 0 | NR | NR |
| Emori et al. (2018)20 | Single | Japan | Intermediate stenosis, prior/non-prior MI related | 75 (75) | 70 (9) | 77 | 47 | 0 | 0.79 (0.11)a/0.76 (0.13)a | 53 (14)a/54 (14)a | NR | NR | 50 | 51 |
| Emori et al. (2018)21 | Single | Japan | Intermediate stenosis | 100 (100) | 70 (10) | 71 | 48 | NR | 0.75 (0.10) | 55 (10) | NR | NR | 22 | NR |
| FAVOR II China: Xu et al. (2017)52 | Multi | China | CAD (suspected or known) | 308 (332) | 61 (10) | 74 | 86 | 14 | 0.82 (0.12) | 46.5 (11.3) | 23 | 34 | 48 | 65 |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | Multi | Italy, the Netherlands, Germany, Poland, Spain, Japan, Denmark | Stable angina or secondary evaluation post MI | 272 (317) | 67 (10) | 72 | 29 | 2 | 0.83 (0.09) | 45 (10) | NR | NR | NR | 40 |
| FAVOR pilot: Tu et al. (2016)46 | Multi | Belgium, Italy, the Netherlands, Germany, China, Japan, USA | Stable angina, referred for ICA and FFR | 73 (84) | 66 (9) | 84 | 27 | 0 | 0.84 (0.08) | 64.5 (4.5) | 100 | 100 | 32 | 38 |
| Goto et al. (2019),22 conference abstract | Multi | Spain, Japan, the Republic of Korea | Intermediate left main stenosis | 62 (NR) | NR | NR | NR | NR | 0.76 (0.11) | 44.1 (13,331.1) | NR | NR | NR | NR |
| Hamaya et al. (2019)23 | Single | Japan | Stable CAD, three-vessel disease | NR (154) | 68 (10) | 76 | 38 | 0 | NR | 36.8 (14.4) | NR | 100 | 23 | NR |
| Hwang et al. (2019)24 | Multi | The Republic of Korea | Intermediate stenosis, stable angina or acute MI (NCLs) | 264 (358) | 61 (13) | 77 | 33 | 31 | 0.80 (0.13) | 0.531 | NR | 69 | 6 | NR |
| Kajita et al. (2019),27 conference abstract | Single | Brazil | Stable CAD, intermediate lesions | 24 (34) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kameyama et al. (2016),28 conference abstract | Single | Japan | ACS, emergency ICA, NCLs | 25 (26) | NR | NR | NR | 100 | NR | NR | NR | NR | NR | NR |
| Kanno et al. (2019),29 conference abstract | Single | Japan | Intermediate stenosis | 95 (NR) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kanno et al. (2019),30 conference abstract | Single | Japan | Intermediate stenosis, de novo, deferred revascularisation | 212 (NR) | NR | NR | NR | NR | 0.87 (0.84–0.90) | NR | NR | NR | NR | NR |
| Kirigaya et al. (2019),31 conference abstract | Single | Japan | Stable CAD | 95 (NR) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kołtowski et al. (2018)32 | Single | Poland | Stable CAD | 268 (306) | 66 (10) | 72 | 28 | 0 | 0.80 (0.10) | 51.3 (10.2) | NR | 100 | 48 | 59 |
| Kleczyński et al. (2019)33 | Single | Poland | Stable angina, intermediate stenosis | 50 (123) | 66 (9) | 72 | NR | 0 | 0.82 (0.10) | 44.2 (11.7) | 100 | 100 | NR | NR |
| Liontou et al. (2019)34 | Multi | Spain, Japan, the Republic of Korea | Intermediate in-stent restenosis | 73 (78) | 68 (11) | 81 | 30 | 6 | 0.79 (0.09) | 51 (9) | 69 | 69 | 58 | 100 |
| Liu et al. 2017,35 conference abstract | Single | The Netherlands | Stable angina | NR (45) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Mehta et al. (2019),36 conference abstract | Single | Australia | NR | NR (85) | NR | NR | NR | NR | 0.86 (0.09) | NR | NR | NR | NR | NR |
| Mejia-Renteria et al. (2019)37 | Multi | Spain, the Republic of Korea, the Netherlands | Intermediate stenosis, stable angina and acute coronary syndrone (including MI patients, non-culprit arteries in staged procedure) | 248 (300) | 64 (10) | 76 | 38 | 17 | 0.80 (0.11) | 52 (12) | 70 | 70 | 14 | NR |
| Neylon et al. (2016), 38 conference abstract | Multi | France | NR | 36 (38) | 64 (18) | 66 | NR | NR | 0.88 (0.11) | NR | NR | NR | NR | NR |
| Sato et al. (2018),39 conference abstract | Single | Japan | Intermediate stenosis | 68 (70) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Smit et al. (2019)40 | Single | The Netherlands | Referred for FFR following ICA in diagnostic-only setting | 290 (334) | 67 (9) | 69 | 24 | 0 | 0.85 (0.01) | 43.1 (8.5) | NR | NR | 16 | 33 |
| Spitaleri et al. (2018),41 reproducibility cohort | Multi | Italy | ACS, multivessel disease, staged procedure | 31 (34) | 64 (12) | 81 | 10 | 100 | NR | 59 (13) | NR | NR | 10 | 19 |
| Spitaleri et al. (2018),41 diagnostic accuracy cohort | Multi | Italy | STEMI, multivessel disease | 45 (49) | 62 (11) | 80 | 9 | 100 | 0.84 (0.11) | 66 (10) | NR | 0 | 4 | 4 |
| Spitaleri et al. (2018),41 clinical outcomes cohort | Multi | Italy, Spain, the Netherlands | STEMI, multivessel disease | 110 (NR) | 64 (12) | 81 | 22 | 100 | NR | 62 (11) | NR | NR | 8 | 6 |
| Stähli et al. (2019)42 | Single | Germany | Intermediate and less severe stenosis (DS 40–70%), stable and unstable angina | 436 (516) | 72 | 68 | 23 | 4.1 (NSTEMI) | 0.88 (0.82–0.92) | 41 (median) | NR | 72 | 33 | 55 |
| SYNTAX II: Asano et al. (2019)15 | Multi | Belgium, the Netherlands, Spain, UK | Three-vessel disease | 386 (836) | 67 (10) | 93 | 32 | NR | 0.78 (0.73–0.84) | NR | NR | NR | 13 | NR |
| Ties et al. (2018)43 | Single | The Netherlands | Stable and unstable CAD | 96 (101) | 64 (10) | 60 | 25 | 16.7 (NSTEMI) | 0.87 (0.08) | 43.4 (8.4) | NR | 51 | NR | 24 |
| Toi et al. (2018),44 conference abstract | Single | Japan | Stable angina, intermediate stenosis | 50 (NR) | 69 (11) | 78 | 43 | NR | 0.81 (0.09) | NR | NR | NR | NR | NR |
| Tu et al. (2014)45 | Multi | Belgium, Hungary, China | Stable and unstable CAD, intermediate stenosis, de novo lesions | 68 (77) | 62 (9) | 69 | 29 | 0 | 0.82 (0.11) | 46.6 (7.3) | 77 | 87 | NR | 32 |
| Van Diemen et al. (2019),47 conference abstract | Multi | The Netherlands, Canada, UK | NR | NR (286) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| van Rosendael et al. (2017)48 | Single | The Netherlands | Non-acute, eligible for FFR | NR (15) | 64 (11) | 71 | 6 | 0 | NR | 38.7 (8.6) | NR | 100 | 6 | 24 |
| Watari et al. (2019)49 | Single | Japan | Stable CAD, intermediate stenosis | 121 (150) | 71 (11) | 68 | 36 | 3 (NSTEMI/unstable angina) | 0.81 (0.12) | 49 (9) | 35 | 97 | 21 | 36 |
| WIFI II: Westra et al. (2018)51 | Multi | Denmark | CAD, Referred from CCTA | 172 (240) | 61 (8) | 67 | 10 | NR | 0.82 (0.11) | 50 (12) | 31 | NR | NR | NR |
| WIFI prototype study: Andersen et al. (2017),14 conference abstract | NR | Denmark (plus China and the Netherlands) | Stable angina and secondary evaluation after acute MI | 93 (NR) | NR | NR | NR | NR | 0.81 (0.09) | 47 (9) | NR | NR | NR | NR |
| Yazaki et al. (2017)53 | Single | Japan | Stable angina and asymptomatic CAD | 142 (151) | 73 (10) | 70 | 29 | 0.7 (NSTEMI/unstable angina) | 0.84 (0.08) | 48.8 (8.2) | 51 | 99 | 21 | 41 |
| Ziubryte et al. (2019),54 conference abstract | Single | Lithuania | Intermediate stenosis | 62 (69) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CAAS vFFR studies | ||||||||||||||
| FAST-EXTEND: Daemen et al. (2019)18 | Single | The Netherlands | Stable or unstable angina or NSTEMI | 303 (NR) | 65 (11) | 67 | NR | NR | 0.84 (0.07) | NR | NR | NR | NR | NR |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | Single | USA | Stable CAD, unstable angina and non-STEMI undergoing PCI. FFR measured pre and post PCI | 115 (115) | 65 (10) | 76 | 37 | 11 | 0.76 (0.12) | 53.3 (18.2) | 63 | 67 | 24 | NR |
| Jin et al. (2019),26 conference abstract | Multiple | China, UK | Intermediate stenosis | 82 (101) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Most studies were conducted in Asia, including Japan (33 studies),20–24,26–53 China (five studies),14,26,45,46,52 the Republic of Korea22,24,34,37 and Singapore. 16
Twenty-one studies were conducted in Europe, including Belgium,15,45,46 Denmark,14,50,51 France,38 Germany,42,46,50 Hungary,45 Italy,41,46,50 Lithuania,54 the Netherlands,15,18,35,37,40,41,43,46–48,50 Poland,32,33,50 Spain15,22,34,37,41,50 and the UK. 15,26,47 Two studies were conducted in the USA,19,46 one in Brazil,27 one in Australia36 and one in Canada. 47 Eleven studies included an international cohort. 14,15,22,26,34,37,41,45–47,50
The QAngio XA 3D/QFR studies analysed a total of 5440 patients (over 6524 vessels or lesions), and CAAS vFFR studies analysed a total of 500 patients (over 519 vessels or lesions). Most studies included a mixed population of stable and unstable CAD, although 11 studies focused only on patients with stable CAD. 23,27,31–33,35,44,46,48,49,53 Three studies evaluated non-culprit vessels in patients with MI,17,28,41 two focused exclusively on patients with three-vessel disease,15,23 one study included only patients with intermediate left main stenosis (mostly left main bifurcation)22 and one focused specifically on in-stent restenosis. 34 Where reported, mean age ranged from 59.0 to 72.5 years, and most participants were male (60–93%). Patient history and stenosis severity varied widely across studies. The prevalence of diabetes ranged from 6% to 48%, rates of previous MI from 4% to 58%, and previous PCI 4% to 65% (not accounting for one study with 100% in-stent restenosis). 34 The mean/median FFR ranged from 0.75 to 0.88, and mean DS from 37% to 66%.
Quality of diagnostic accuracy studies
Table 2 summarises the results of the risk-of-bias and applicability assessment for QAngio XA 3D/QFR for the 24 diagnostic accuracy studies reported in a full-text manuscript, with further details reported in Appendix 3, Tables 36 and 37. The risk of bias from the 15 studies included in the diagnostic accuracy review that were reported only as conference abstracts was not formally assessed because of insufficient reporting. 14,16,18,22,25–31,35,36,38,39,44,54 Just as with FAST-EXTEND,18 the extension of the FAST study was reported as conference abstract only; only the quality of the earlier FAST study was assessed. 56
| Study | Risk of bias | Applicability | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow | Patient selection | Index test | Reference standard | |
| Cortés et al. (2019)17 | – | + | + | ? | – | – | + |
| Emori et al. (2018)20 | + | + | + | + | ? | – | + |
| Emori et al. (2018)21 | + | + | + | + | ? | – | + |
| FAVOR II China: Xu et al. (2017)52 | + | + | + | + | – | + | + |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | + | + | + | + | + | + | + |
| FAVOR pilot: Tu et al. (2016)46 | + | + | + | + | + | – | + |
| Hamaya et al. (2019)23 | – | + | + | + | + | – | + |
| Hwang et al. (2019)24 | – | + | + | + | – | – | + |
| Kleczyński et al. (2019)33 | + | – | + | + | + | – | + |
| Kołtowski et al. (2018)32 | – | + | + | + | + | – | + |
| Liontou et al. (2019)34 | ? | + | + | + | – | – | + |
| Mejia-Renteria et al. (2019)37 | + | + | + | + | + | – | + |
| Smit et al. (2019)40 | + | – | – | + | + | – | + |
| Spitaleri et al. (2018)41 (cohort B, diagnostic accuracy) | + | + | + | + | – | – | + |
| Stähli et al. (2019)42 | + | + | + | + | + | – | + |
| SYNTAX II: Asano et al. (2019)15 | + | + | + | – | – | – | + |
| Ties et al. (2018)43 | ? | + | + | + | – | – | + |
| Tu et al. (2014)45 | + | + | + | + | + | – | + |
| van Rosendael et al. (2017)48 | ? | – | – | ? | + | + | + |
| Watari et al. (2019)49 | + | + | + | + | + | + | + |
| WIFI II: Westra et al. (2018)51 | + | + | + | + | – | + | + |
| Yazaki et al. (2017)53 | + | + | – | + | + | – | + |
| CAAS vFFR | |||||||
| ILUMEN I: Ely Pizzato et al. (2019)19 | – | + | + | + | + | – | + |
| FAST: Masdjedi et al. (2020)56 | – | + | + | + | + | – | + |
A total of 11 out of the 22 QAngio XA 3D/QFR studies were rated as being at low risk of bias across all domains. 20,21,37,41,42,45,46,49–52 The main source of bias was related to study participant selection; four studies were considered at high risk of patient selection bias because of high rates of patient exclusions or significant exclusion of potentially harder to diagnose patients,17,23,24,32 and three studies did not provide sufficient information on patient selection to assess risk of selection bias (unclear risk). 34,43,48 Exclusion rates and reasons are reported in Appendix 3, Table 37. Risk of bias was rated as being generally low for other domains, although three studies were rated as being at high risk of bias because of the conduct of the index test or reference standard (e.g. no reporting of blinding between QFR and FFR results),33,48,53 and one study was rated as being at high risk of bias because of patient flow concerns, as FFR was performed only in iFR grey-zone patients. 15
The ILUMIEN I19 trial was the only CAAS vFFR complete study with a full-text manuscript. The study was rated as being at high risk of bias because of the large percentage of lesions excluded from the study (65%). In an earlier published report of the FAST-EXTEND study, Masdjedi et al. 56 also reported a high rate of exclusions (54%). Although most of these failed tests appear to have been due to angiographic image processing issues rather than limitations inherent in CAAS vFFR (see Test failures: rates and reasons), the large exclusion rates reported mean that the risk of selection bias cannot be excluded.
Only three studies raised no concerns about their applicability to the review question. 48–50 The main concern about applicability related to QFR being used retrospectively (offline) rather than as part of the ICA examination and before FFR; only five studies (all of QAngio XA 3D/QFR) were conducted prospectively and raised no significant concerns regarding the applicability of the index test. 48–52 There were no significant concerns regarding the applicability of the reference standard in any of the studies. A total of 12 out of the 22 QAngio XA 3D/QFR studies did not raise significant concerns about the applicability of their population to the review question;23,32,33,37,40,42,45,46,48–50,53 concerns about study population applicability were primarily related to the under-representation of patients with stable CAD. We note that, because only patients with a FFR measurement could be included in the diagnostic accuracy review, a subset of patients with intermediate stenosis (including those examined in a diagnostic-only setting, or with a counter-indication to adenosine) are not represented in the included evidence.
Seven studies of QAngio XA 3D/QFR14,15,41,45,48,51,52 and one of CAAS vFFR18 reported a conflict of interest with their respective manufacturers.
Overview of the meta-analyses (QAngio XA 3D/QFR)
Meta-analysis of the included studies is focused on the diagnostic accuracy of QFR (measured using QAngio XA 3D/QFR) to detect lesions or vessels requiring intervention (defined as having a FFR ≤ 0.8). There were insufficient data to perform meta-analyses of any clinical outcomes; these are discussed in Clinical outcomes.
Diagnostic accuracy of QFR was analysed in two ways. The first, and primary, analysis consists of a meta-analysis of reported diagnostic accuracy data (TPs, TNs, FPs and FNs) in studies where these data were reported or could be derived from reported estimates of sensitivity and specificity. The second approach was to extract data on FFR and QFR values in each study from published Bland–Altman plots, or plots of FFR compared with QFR, and to use these to calculate diagnostic accuracy. This approach may be less accurate, because extracting data from figures is imperfect, but it allowed for a wider range of analyses, such as considering different QFR and FFR cut-off points, and the impact of using a grey zone where patients with intermediate QFR values go on to receive confirmatory FFR. This second approach is considered in Meta-analyses of data extracted from figures (QAngio XA 3D/QFR).
Of all the included studies of QAngio XA 3D/QFR, 26 reported sufficient diagnostic accuracy data to be included in the primary meta-analysis of diagnostic accuracy (four studies23,34,44,48 were included only in analyses of data extracted from plots). These are divided into three ‘modes’ of QFR: fQFR, cQFR and studies where the type of QFR was not specified (listed as QFR or non-specified QFR). Most studies included in the primary analysis used FFR as the reference standard for determining whether or not intervention was required, all of these used a cut-off FFR point of 0.8. One study49 used iFR as the reference standard.
Figure 3 shows the general sensitivity and specificity estimates for each study, assuming an index test cut-off point of QFR ≤ 0.8 and a reference standard cut-off point of FFR ≤ 0.8. The results are plotted separately for each mode of QFR testing. This suggests that specificity is uniformly high and generally > 75% (except for two fQFR studies). Sensitivity is more heterogeneous, but is also > 75% in most studies (except for fQFR). There are no immediately apparent differences in accuracy between the three modes.
FIGURE 3.
Sensitivity and specificity estimates for each study, by mode of QFR: (a) cQFR; (b) fQFR; and (c) QFR.



Univariate meta-analyses (QAngio XA 3D/QFR)
Figure 4 shows the forest plot for the univariate meta-analysis of sensitivity, and Figure 5 the same for specificity. For the random-effect analyses, these show high sensitivity (82–85%) and high specificity (89–91%) for all three models of QFR. cQFR had a sensitivity of 85% (95% CI 78% to 90%) and specificity of 91% (95% CI 85% to 95%); fQFR had a sensitivity of 82% (95% CI 68% to 91%) and specificity of 89% (95% CI 77% to 95%). Studies that did not specify the mode of QFR had a sensitivity of 84% (95% CI 78% to 89%) and specificity of 89% (95% CI 87% to 91%). Across-study heterogeneity was moderate to high (e.g. for cQFR sensitivity, I2 = 81%), but there does not appear to be any clear evidence that the mode of QFR (fQFR vs. cQFR) makes a difference to diagnostic accuracy.
FIGURE 4.
Univariate meta-analysis of sensitivity. FAVOR, Functional Assessment by Virtual Online Reconstruction; WIFI, wire-free invasive functional imaging.
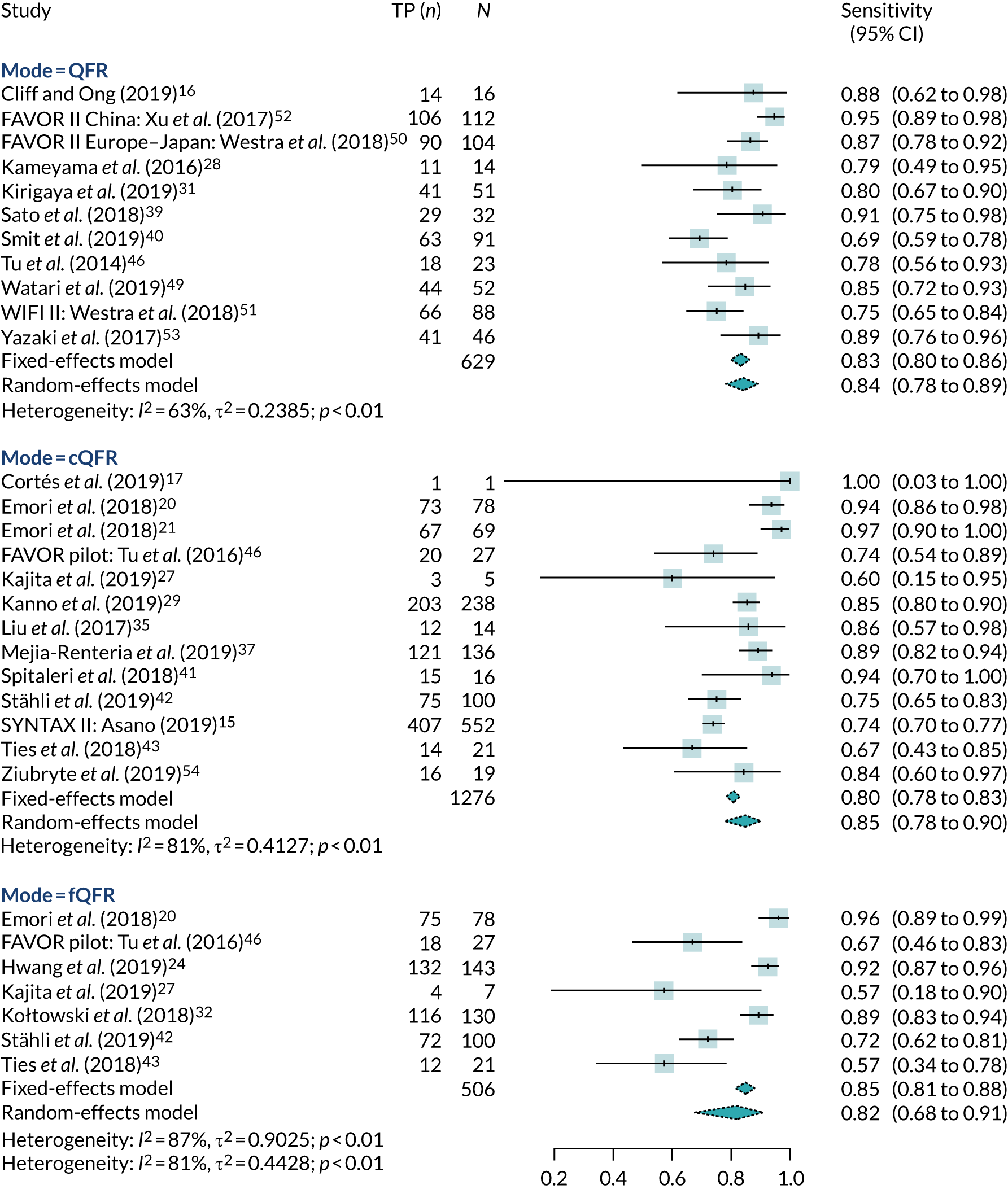
FIGURE 5.
Univariate meta-analysis of specificity. FAVOR, Functional Assessment by Virtual Online Reconstruction; WIFI, wire-free invasive functional imaging.

Summary PPVs (see Appendix 4, Figure 18) were 77% (95% CI 69% to 83%) for fQFR, 85% (95% CI 80% to 89%) for cQFR and 80% (95% CI 76% to 84%) for non-specified QFR. Summary NPVs (see Appendix 4, Figure 19) were 92% (95% CI 89% to 94%) for fQFR, 91% (95% CI 85% to 94%) for cQFR and 91% (95% CI 87% to 93%) for non-specified QFR. It should be noted that PPV and NPV depend on the distribution of FFR in each study, so summary results may not represent PPV or NPV in an ‘average’ study.
Meta-analyses of AUCs and DORs were also performed (see Appendix 4, Figures 20 and 21). Summary AUCs were 87% (95% CI 83% to 92%) for cQFR, 89% (95% CI 86% to 92%) for fQFR and 92% (95% CI 90% to 94%) for non-specified QFR. Summary DORs were 3.51 (95% CI 2.71 to 4.30) for fQFR, 3.76 (95% CI 3.01 to 4.52) for cQFR and 3.71 (95% CI 3.27 to 4.15) for non-specified QFR.
As both FFR and QFR are continuous measurements, it is also important to consider the agreement between FFR and QFR, in terms of the MD and variation between them, and their correlation. We meta-analysed reported MDs between FFR and QFR measurements and reported correlations. Where studies did not report the standard deviation (SD) of the MD, it was imputed by taking the average value from studies that did report SDs.
The MD between QFR and FFR was almost exactly zero for all three modes of QFR testing (see Appendix 4, Figure 22) [MD 0 (95% CI –0.05 to 0.06) for fQFR, –0.01 (95% CI –0.06 to 0.04) for cQFR; and MD 0.01 (95% CI –0.03 to 0.05) for non-specified QFR]. FFR and QFR were highly correlated in all studies (see Appendix 4, Figure 23): correlation coefficient 0.78 (95% CI 0.72 to 0.82) for fQFR, 0.78 (95% CI 0.70 to 0.85) for cQFR and 0.79 (95% CI 0.73 to 0.83) for non-specified QFR. We note that correlation coefficients are not a good measure of agreement between diagnostic tests; this meta-analysis is included here for information only.
Bivariate meta-analysis (QAngio XA 3D/QFR)
The results of the full bivariate meta-analysis are summarised in Table 3 and Appendix 4, Figure 24. The results are almost identical to the univariate analyses, with no evidence of differences between fQFR and cQFR.
| Mode | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| cQFR | 84.32 (77.29 to 89.48) | 91.40 (84.96 to 95.24) |
| fQFR | 81.61 (66.97 to 90.66) | 89.43 (77.58 to 95.38) |
| Non-specified QFR | 84.25 (78.51 to 88.68) | 88.95 (87.02 to 90.61) |
| cQFR or non-specified QFR | 84.34 (80.04 to 87.85) | 89.80 (86.36 to 92.45) |
To include all studies in a single meta-analysis, and given the similarity of results across modes of QFR, we performed a further bivariate meta-analysis that combined all studies using only a single ‘mode’ of QFR from each. In practice, this meant combining studies with cQFR results with studies not specifying how QFR was performed (and, as a result, excluding fQFR assessments). This might be expected to give the most ‘optimistic’ estimate of diagnostic accuracy because fQFR is excluded. The results for this combined analysis are also shown in Table 3. The results are, inevitably, very similar to those for cQFR or non-specified QFR, but with narrower CIs. We note that this arguably represents the best summary of the diagnostic accuracy of QFR, as it based on the maximum number of studies, but it is a post hoc analysis not specified in the protocol.
The summary results and HSROC curves in Appendix 4, Figure 24, demonstrate the high diagnostic accuracy of QFR and the similarity between the three analysed modes. The HSROC curve for fQFR lies consistently below that for cQFR, suggesting a possibility that fQFR may have slightly inferior diagnostic accuracy, but this difference is well within the bounds of uncertainty. This is in line with the expected use of QFR, where cQFR is calculated when the fQFR is in the range of 0.70–0.85.
Meta-analysis of invasive coronary angiography studies
Five studies included in the meta-analysis also reported 2 × 2 table data on the diagnostic accuracy of using ICA alone, using 50% DS as the cut-off point with FFR < 0.8 as the reference standard. These five studies are summarised in Table 4. We note that reporting of diagnostic data on ICA may be subject to selection bias, as only a small subset of studies reported it, and they are likely to do so to demonstrate the superiority of using QFR over relying on ICA alone.
| Study | 2D or 3D | n | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|---|
| FAVOR II China: Xu et al. (2017)52 | 2D | 332 | 62.5 (53.5 to 71.5) | 58.2 (51.7 to 64.7) |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | 2D | 317 | 44.2 (34.7 to 53.8) | 76.5 (70.8 to 82.2) |
| FAVOR pilot: Tu et al. (2016)46 | 3D | 84 | 44.4 (25.7 to 63.2) | 78.9 (68.4 to 89.5) |
| Mejia-Renteria et al. (2019)37 | 3D | 300 | 69.9 (62.1 to 77.6) | 70.7 (63.8 to 77.7) |
| Stähli et al. (2019)42 | 3D | 516 | 34.0 (24.7 to 43.3) | 91.6 (88.9 to 94.3) |
Given the limited number of studies, and because 2D and 3D ICAs may have very different performance levels, no bivariate meta-analysis of these data are presented here. Based on the results of individual studies, the diagnostic accuracy of ICA appears to be poorer than that of QFR.
Twelve included studies reported AUC estimates for diagnostic accuracy of using ICA alone. A meta-analysis of these studies gave a summary AUC for 3D ICA of 0.71 (95% CI 0.66 to 0.76). For 2D ICA, the summary AUC was 0.63 (95% CI 0.59 to 0.67). Both 2D and 3D ICA have lower AUC values than QFR, and it appears that 2D ICA may be inferior to 3D ICA.
Bivariate meta-analysis to compare tests
Eight studies in the meta-analysis compared two or more testing approaches: five of these compared using 2D or 3D ICA to QFR, and five compared fQFR to cQFR. A ROC plot of results from studies reporting two or more tests is shown in Appendix 4, Figure 25. In all five studies, ICA performed more poorly than QFR, with lower sensitivity and specificity. Differences between fQFR and cQFR were more mixed, with three studies suggesting that cQFR has slightly higher sensitivity than fQFR, but the other two were not consistent with this.
An indirect comparative bivariate meta-analysis accounting for these comparisons between studies is presented in Table 5 and Figure 6. These analyses show the clear inferiority of using ICA alone when compared with FFR as a reference standard. It is clearly inferior to using QFR in both sensitivity and specificity, with a sensitivity of only 51.2% and a specificity of 71.0%.
| Mode | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| cQFR | 83.97 (78.32 to 88.37) | 89.59 (85.15 to 92.82) |
| fQFR | 83.32 (76.42 to 88.50) | 83.91 (76.91 to 89.08) |
| QFR | 85.20 (79.76 to 89.38) | 90.09 (85.80 to 93.19) |
| ICA | 51.16 (41.86 to 60.38) | 70.99 (62.39 to 78.30) |
FIGURE 6.
A ROC plot of bivariate with comparisons of tests.

Unlike the earlier bivariate meta-analysis (see Appendix 4, Figure 24), the comparative analysis suggests that fQFR is slightly inferior to cQFR, mainly due to an inferior specificity (83.9% instead of 89.6%). This suggests that fQFR produces slightly too many FP results (where QFR ≤ 0.8 but FFR > 0.8). This might suggest that if an initial fQFR produces a result less than 0.8 it should be followed up by a confirmatory cQFR.
Impact of patient and study characteristics (QAngio XA 3D/QFR)
Impact of study characteristics
Receiver operating characteristic plots differentiating between studies reporting at patient, vessel or lesion level found no evidence that this affects diagnostic accuracy (see Appendix 4, Figure 26). There was also no evidence of any impact on diagnostic accuracy in studies where more than one approach was reported. We note that, where there was more than one lesion assessed, ‘by-patient’ and ‘by-vessel’ analyses selected a single lesion (either at random or based on clinical importance), so a lack of difference is unsurprising, as it would only arise if the choice of lesion was biased. It should also be noted that by-lesion analysis could be biased because of correlation between lesions within patients. Without full patient-level data, the impact this might have cannot be assessed.
There was no evidence of difference in diagnostic accuracy between prospective and retrospective analyses of QFR (see Appendix 4, Figure 27).
Impact of patient factors
Few studies reported diagnostic accuracy data in any form according to different patient characteristics (such as distinguishing between people with and without diabetes, or with and without multivessel disease). The limited evidence reported is discussed in Clinical outcomes.
Given this lack of evidence, to investigate the impact on diagnostic accuracy of key patient factors we have performed meta-regressions of sensitivity, specificity and DOR against the mean value of these factors, where reported in papers. These analyses are obviously limited by being meta-regressions of study-level proportions, rather than true analyses of patient-level data, and because of limited reporting of these factors across studies. For these analyses we did not separate fQFR from cQFR but used one test per study (cQFR for preference) to maximise data. This was considered reasonable given that diagnostic accuracy does not strongly depend on the mode of QFR used.
Appendix 4, Table 38, shows the regression parameter estimates (change in log-DOR, sensitivity or specificity per unit of the covariate), their 95% CIs and p-values from these metaregression analyses. For most parameters there is no evidence of any association with diagnostic accuracy. However, this may be due to a lack of data rather than no association.
Four patient factors (i.e. diabetes, stable CAD, multivessel disease and mean FFR) suggest a possibility of association, as all have at least one p-value below 0.05. Plots of the proportions of patients with these factors, against estimated sensitivity, specificity and log-DOR are shown in Appendix 4, Figures 28–31.
The association between diabetes and diagnostic accuracy is partly driven by one study where nearly all patients had diabetes, but the trend for studies with more diabetic patients to have higher sensitivity and DOR remains even if that study is removed. There is a trend for specificity and DOR to decline as higher proportions of patients have stable CAD. Conversely, specificity and DOR increase as more patients have multivessel disease (although this is based on only five studies32,37,41,42,51).
There is evidence that the lower the average FFR in a study, the higher the sensitivity and the lower the specificity (but with no impact on the overall accuracy in terms of the DOR). We might therefore also expect some variation in diagnostic accuracy with any factor that lowers FFR (DS, medical history, etc.) but the data are too limited to confirm this.
Subgroup analyses
Eleven studies reported diagnostic accuracy results stratified by patient or vessel characteristics20,21,24,29,32,38,42,51,52,57,58 and four studies reported results of multivariate regression analyses of predictors of QFR/FFR discrepancies. 15,37,50,51 All studies were of QAngio XA 3D/QFR.
The number of subgroup analyses was too small to allow meta-analysis and results are summarised narratively, and in figures. None of the analyses reported in the included studies was prespecified in a prospectively registered protocol. All patient characteristics for which subgroup data were reported were specified in the review protocol [high/low index of microcirculatory resistance (IMR) small/non-small vessel diameter, multiple/single lesion, diabetes/no diabetes, MI history], except three [left anterior descending (LAD)/no LAD vessel, chronic kidney disease (CKD) and acute MI], which are presented for the sake of completion.
Appendix 4, Figure 32, shows a ROC plot for five studies reporting sensitivity and specificity by subgroups, and Appendix 4, Figure 33, shows the DORs for the same studies. The results of subgroup analyses reported in included diagnostic accuracy studies are summarised in Appendix 5, Tables 44 and 45.
Microcirculatory resistance
Two studies explored the effect of microcirculatory resistance on the accuracy of QAngio XA 3D/QFR and showed inconsistent results. 29,57 In both studies, patient populations were stratified according to microcirculatory status, defined by the IMR, the product of hyperemic Tmn and hyperemic distal arterial pressure and measured by pressure wire. Microcirculatory dysfunction was defined as ≥ 23 U (predefined as 75th centile of IMR values) in one study57 and as ≥ 25 U in the other. 29 Results differed significantly between the two studies. Although both found a statistically significant difference in diagnostic accuracy between high- and low-IMR groups, one study found that the accuracy of QAngio XA 3D/QFR was reduced in patients with high IMR compared with low IMR [sensitivity 86% vs. 90%, specificity 69% vs. 94%, AUC 0.88 vs. 0.96, odds ratio (OR) of misclassification 1.05 (95% CI 1.02 to 1.08)],57 whereas the other29 found that QAngio XA 3D/QFR had higher sensitivity but lower specificity in the high-IMR group (sensitivity 96.7% vs. 81.5%, specificity 64.2% vs. 77.2%).
Vessel characteristics and location
There was limited evidence that vessel characteristics and location were associated with different rates of QFR/FFR discrepancies, although two studies reported that vessels with bifurcation/trifurcation lesions were associated with poorer diagnostic accuracy than other vessels. The SYNTAX59 trial found that bifurcation/trifurcation were independent predictors for the increased incidence of FP QFR (OR 1.81, 95% CI 1.10 to 2.98), and one small study of 38 vessels reported that bifurcations lesions accounted for five out of six (83.3%) false measurements. 38 One study22 that included only patients with left main stenosis (85% left main bifurcation) had high sensitivity (84.8%) and moderate specificity (68.2%) (AUC 0.82, 95% CI 0.71 to 0.93). No other studies reported on the potential impact of left main stenosis on diagnostic accuracy.
Results from studies evaluating the effect of small vessel disease on diagnostic accuracy were mixed. One study found higher sensitivity and AUC for cQFR in patients with small-vessel disease (≤ 2.8 mm reference diameter), than in other patients [sensitivity 80.0% vs. 65.7%, specificity 98.5% vs. 97.2%, AUC 0.89 (95% CI 0.85 to 0.93) vs. 0.81 (95% CI 0.76 to 0.86)],60 whereas another study found that small-vessel disease (≤ 2.5 mm reference diameter) was associated with an increased incidence of FN QFR (OR 1.67, 95% CI 1.14 to 2.44) in a multivariate analysis. 15
One study found that found no significant differences in QAngio XA 3D/QFR accuracy between subgroups with LAD and non-LAD coronary arteries,21 although a multivariate analysis from SYNTAX II15 found a non-statistically significant trend suggesting LAD may be associated with a higher rate of FPs (OR 0.53, 95% CI 0.27 to 1.04), and that lesions located in side branches were associated with a higher rate of FP QFR (OR 2.07, 95% CI 1.14 to 3.76) and FNs (OR 0.47, 95% CI 0.28 to 0.81).
One study found no significant difference in MDs between QFR and FFR per lesion in patients with single and multiple lesions,51 and multivessel disease was not a significant predictor of QFR/FFR discrepancy in a multivariate analysis conducted by another study. 37 Functional Assessment by Virtual Online Reconstruction (FAVOR) II-China52 found that the accuracy of QAngio XA 3D/QFR in patients with DS 40–80% did not differ from the whole study population results.
Comorbidities and other patient characteristics
There was also limited evidence on the impact of patient comorbidities on the accuracy of QAngio XA 3D/QFR. Two studies found no difference in diagnostic accuracy in subgroup analyses comparing patients with and without diabetes. 42,58 Smit et al. 58 found similar accuracy in diagnostic accuracy between diabetics and non-diabetics (sensitivity: 71.0% vs. 69.0%; specificity: 95.0% vs. 91.0%; AUC: 0.91 vs. 0.93; per-vessel analysis). The results of per-patient analyses were also not statistically significant. Stähli et al. 42 also found no statistically significant difference in AUC between patients with and without diabetes [AUC 0.84 (95% CI 0.76 to 0.90) vs. 0.87 (95% CI 0.83 to 0.90)]. On the other hand, FAVOR II Europe–Japan50 found in a multivariate regression that diabetes was associated with an increased chance of discrepancy between QFR and FFR (OR 2.88, 95% CI 1.30 to 6.46). 50 One study found a larger mean discrepancy between QFR and FFR in a small subgroup of 21 patients with diabetes (MD –0.059 ± 0.07) compared with 173 non-diabetic patients (MD –0.027 ± 0.074);32 the difference between the subgroups was statistically significant (p = 0.039), although no further diagnostic accuracy results were reported.
One study that compared results for vessels of stable CAD patients with non-culprit vessels in MI patients found no significant difference in diagnostic accuracy between the two groups (sensitivity: 90.1% vs. 96.2%; specificity: 89.5% vs. 90.6%; AUC: 0.946 vs. 0.967). 24 However, in a multivariate analysis another study found that acute coronary syndrome was associated with a significantly higher rate of misclassification between QFR and FFR (OR 3.97, 95% CI 1.78 to 8.86). 37 One study retrospectively compared single vessels in groups of 75 patients with and without prior MI and found no significant difference for cQFR and fQFR between the two groups. 20 One study found a statistically significant difference in AUC between patients with and without CKD [AUC: 0.67 (95% CI 0.46 to 0.88) vs. 0.89 (95% CI 0.84 to 0.94); p = 0.05].
No subgroup data were reported for the following review protocol variables: diffuse CAD, multiple stenosis in one vessel, chronic total occlusion, sex, age, ethnicity and results of previous non-invasive tests, although sex, age and chronic total inclusion were reported as non-significant variables in reported regression analyses (see Appendix 5, Table 46).
Overall, results from subgroup and regression analyses were limited by the number of studies and design issues such as small sample size and risk of confounding and should therefore be interpreted with caution. There was some evidence suggesting that diagnostic accuracy of QFR is reduced in bifurcation/trifurcation lesions. However, because of limited and sometimes inconsistent data there is insufficient evidence to conclude that patient or lesion characteristics significantly affect the diagnostic accuracy of QAngio XA 3D/QFR.
Sensitivity analyses
We performed a number of sensitivity analyses to examine the impact of QUADAS-2 risk-of-bias assessment, QUADAS-2 applicability assessment and other potential causes of bias on the diagnostic accuracy meta-analyses.
As noted in Impact of study characteristics there was no evidence that diagnostic accuracy varied by whether studies collected data prospectively or retrospectively, or if the analysis was performed at patient, vessel or lesion level.
Repeating the main bivariate meta-analyses [as in Bivariate meta-analysis (QAngio XA 3D/QFR)] by whether the QUADAS-2 assessment was high risk of bias, low risk of bias or unclear (note that all conference abstracts were classified as unclear in this analysis) for each QUADAS-2 category found no evidence of bias in diagnostic accuracy (see Appendix 4, Figure 34). There is no obvious pattern in the data for each study (small dots) and bivariate meta-analyses by risk-of-bias assessment (larger dots with CIs) are consistent between categories, and with the overall analysis.
Similar repetitions of the bivariate meta-analysis by applicability concerns from the QUADAS-2 assessment (see Appendix 4, Figure 35), and by other factors that might bias results (see Appendix 4, Figure 36), likewise showed no evidence of actual bias in any analysis. One possible exception is that studies in which patients did not have stable CAD had higher estimated sensitivity, but this was based on three small studies. Removal of these studies would not meaningfully alter the main results.
Meta-analyses of data extracted from figures (QAngio XA 3D/QFR)
To further investigate the diagnostic properties of QFR, we digitally extracted data from all papers that presented either a plot of FFR against QFR, or a Bland–Altman plot of QFR and FFR. We preferred Bland–Altman plots for extraction, as these were found to be generally clearer and easier to extract. All extraction was performed by a single reviewer using the WebPlotDigitizer software.
We used digitised data extraction to reconstruct, approximately, the individual-level data for all included studies that presented a suitable figure. The extracted averages and differences between QFR and FFR from Bland–Altman plots were converted into their equivalent QFR and FFR values. This extraction generated, approximately, the FFR and corresponding QFR for each participant in each study.
The extraction could not be perfect; the digitally extracted points were placed with some minor errors owing to overlap and low image quality. This meant that the number of extracted points was smaller than the total number of participants. We note also that the set of studies is not the same as in previous sections because some studies presented diagnostic accuracy results, but no figure, or vice versa. In all analyses, we focus on cQFR or non-specified QFR; fQFR is excluded.
Figure 7 shows the complete extracted data for QFR compared with FFR, and Figure 8 a Bland–Altman plot of all data. The different colours for the dots represent the separate studies. The pattern of data is similar to that observed in most individual studies, with FFR and QFR being highly correlated (the correlation coefficient across all data being 0.803). The distribution of data appears homogeneous across studies; the data are centred around the line where FFR = QFR (black line in figures). The data seem to broadly fit a highly correlated bivariate normal distribution, truncated at QFR and FFR values of 1.
FIGURE 7.
Fractional flow reserve against QFR for data extracted from figures. Each colour represents a separate study. The black line indicates where FFR = QFR. The upper-left shaded region shows the FNs where QFR > 0.8 but FFR ≤ 0.8 (6.5% of patients); the lower-right shaded region shows the FPs where QFR ≤ 0.8 but FFR > 0.8 (8.2% of patients).
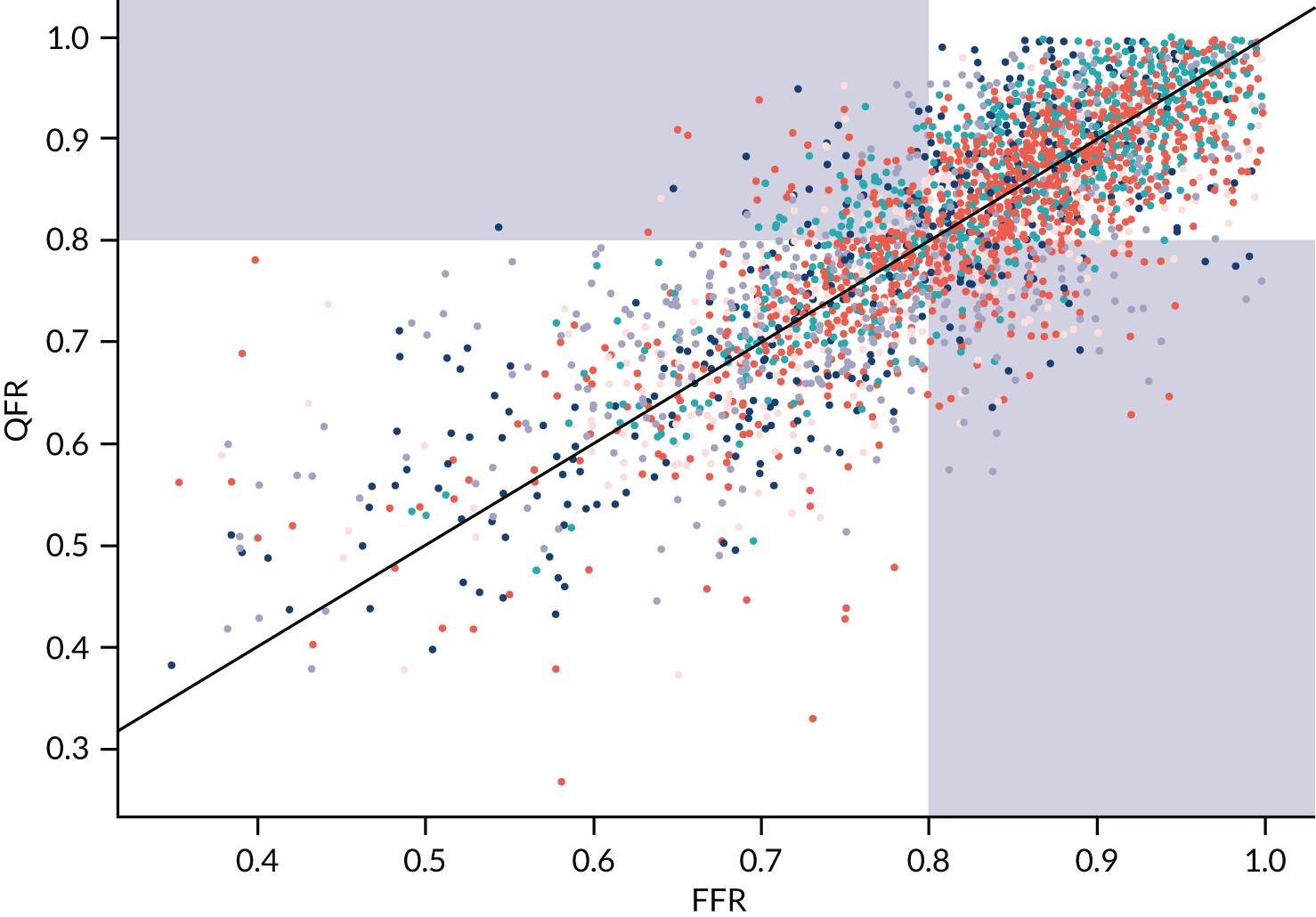
FIGURE 8.
Bland–Altman plot for data extracted from figures. Each colour represents a separate study. Black solid line indicates the mean difference between FFR and QFR; black dotted lines indicate the 95% range of differences.
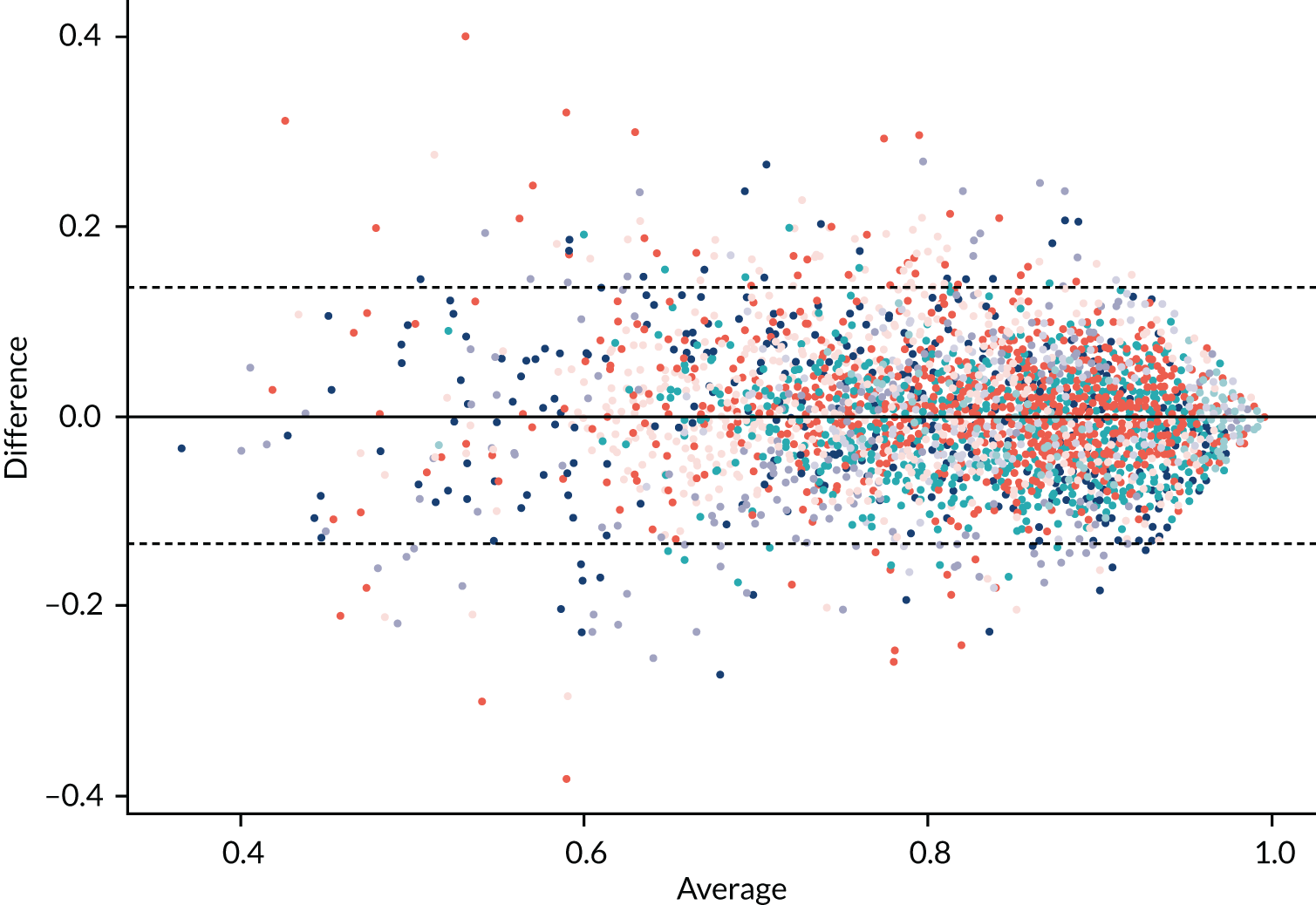
In Figure 7, the upper-left shaded region shows the FNs where QFR > 0.8 but FFR ≤ 0.8 (6.5% of patients); the lower-right shaded region shows the FPs where QFR ≤ 0.8 but FFR > 0.8 (8.2% of patients). Therefore, a minority of patients fall in these regions.
The Bland–Altman plot shows that QFR and FFR values are generally similar: the average difference between QFR and FFR is 0.001; 95% of QFR values are within 0.14 of the FFR, 90% are within 0.11 and 50% are within 0.04.
Meta-analysis of diagnostic accuracy
We calculated the diagnostic accuracy for each study based on extracted data, using the usual index test of QFR ≤ 0.8 and reference standard of FFR ≤ 0.8 as defining patients in need of coronary intervention. To investigate whether or not the extracted data could be used for analysis, we compared these diagnostic accuracy results to the results from 2 × 2 tables [used in previous meta-analyses in Bivariate meta-analysis (QAngio XA 3D/QFR)]. The extracted sensitivity and specificity estimates are summarised in Appendix 4, Table 39. Overall, 30 studies reported either 2 × 2 table data or data that could be extracted from a figure. Nine studies did not present an extractable figure, and three studies presented a figure, but no summary data.
In general, the number of data points from the extracted figure data was smaller than that reported in the studies. This is to be expected, as overlapping points will be missed when extracting from figures. There is mostly good agreement in diagnostic accuracy between the data sources, except for a few cases where the figure data have lower sensitivity and mostly lower specificity. 24,40,52,61 Only one study28 had better diagnostic accuracy when using data extracted from figures. This consistency suggests that using the extracted figure data for diagnostic analysis is reasonable, even though it represents a smaller sample size. The results of performing a bivariate meta-analysis for diagnostic accuracy using the extracted figure data are shown in Appendix 4, Figure 37. The black points are the results in each study, and the blue dot is the result of the meta-analysis (with its HSROC curve). The summary sensitivity is 84.6% (95% CI 80.7% to 87.8%) and specificity is 87.2% (95% CI 83.4% to 90.3%). This is similar to the results from the main analysis when cQFR and non-specified QFR were combined (sensitivity 84.3%, specificity 89.8%), albeit with slightly lower specificity, further confirming that analysing the extracted figure data are reasonable.
We note that this bivariate meta-analysis is presented to confirm that the extracted data reasonably represent the properties of the included studies; the bivariate meta-analyses in Bivariate meta-analysis (QAngio XA 3D/QFR) should be taken as the primary analyses.
Grey-zone analysis
The main purpose of extracting data from figures is to permit an analysis where testing includes a grey zone of intermediate QFR values for which a FFR would be performed as a confirmatory test. The grey-zone diagnostic procedure is:
-
perform QFR
-
if the QFR is > 0.84, continue without stenting/bypass and defer FFR (test negative)
-
if the QFR is ≤ 0.78, proceed directly to stenting/bypass without FFR (test positive)
-
otherwise, perform a FFR and proceed based on that result (i.e. at a 0.8 cut-off point) (the grey zone).
This means that for anyone within the grey zone there is perfect diagnostic accuracy, so FPs and FNs occur only in patients outside the grey zone.
Appendix 4, Figure 38, shows the FFR and QFR data again, with the proposed grey zone highlighted. In total, across studies, 20.1% of all patients lie within the grey zone (accordingly, up to 79.9% of patients would theoretically have a wire-free and adenosine-free procedure in this scenario). Of these grey-zone patients, 19.1% are TPs with both QFR and FFR below 0.8, and 50.2% are TNs with both tests above 0.8. Only 18.3% are FNs and 12.4% FPs. Hence, only 30.4% of patients in the grey zone have discordant FFR and QFR results (relative to the 0.8 threshold).
Within the grey zone, differences between FFR and QFR are small. This is shown in Appendix 4, Figure 39, categorised by TPs, FPs, etc. Very few patients in the grey zone differ in test values by > 0.1, and most differ by ≤ 0.05.
The diagnostic accuracy when using the grey zone improves, as would be expected, to a sensitivity of 93.1% (95% CI 90.1% to 94.9%) and a specificity of 92.1% (95% CI 88.3% to 94.5%). Appendix 4, Figure 40, shows the result of this meta-analysis (with its HSROC curve) compared with the meta-analysis without the grey zone presented in Bivariate meta-analysis (QAngio XA 3D/QFR). Clearly using the grey zone improves diagnostic accuracy compared with QFR alone because of the 3.7% of patients reclassified from test negative to positive and 2.5% who are reclassified in the opposite direction. However, this improvement depends on assuming that the 0.8 threshold of FFR genuinely separates those who need intervention from those who do not.
As an alternative to using the manufacturer-specified grey zone, we also examined what grey-zone thresholds would be required to achieve a sensitivity and specificity of 90% and 95% respectively. This is summarised in Appendix 4, Table 40. This suggests that the manufacturer-recommended grey zone favours high sensitivity over high specificity.
Alternative fractional flow reserve thresholds
The IRIS-FFR study13 found that only for a FFR ≤ 0.75 did the risk of MACEs become significantly lower in patients with revascularised lesions than in those in whom revascularisation was deferred. This suggests that the current threshold of 0.8 for planning revascularisation may not be clinically appropriate. Using the extracted figure data, we can investigate the diagnostic accuracy of QFR compared with FFR at other thresholds. For example, if the threshold for both QFR and FFR is 0.75 then the diagnostic accuracy becomes a sensitivity of 75.4% (95% CI 69.0% to 80.8%) and a specificity of 90.6% (95% CI 87.9% to 92.7%). This is compared with the previous meta-analysis at the threshold of 0.8 in Appendix 4, Figure 41. Using a 0.75 threshold leads to slightly lower sensitivity, but higher specificity. The two ROC curves, however, are almost identical, suggesting no overall change in diagnostic accuracy.
Meta-analysis of extracted figure data for two-dimensional invasive coronary angiography
To inform the economic analyses an additional pragmatic search for studies that compared 2D ICA with FFR assessment was performed to identify studies that presented sufficient granular data (such as scatterplots or Bland–Altman plots) from which ICA and FFR data could be extracted. This search identified four such studies (see Appendix 4, Table 41). 62–65
Figure 9 shows the plot of all extracted data from these four studies. The colours of the dots indicate the studies. It can be seen that, when compared with the equivalent figure for QFR (see Figure 7), 2D ICA is much more weakly correlated with FFR (correlation coefficient –0.432). There are many FNs (bottom-left shaded region, 13.0% of patients) and FPs (top-right shaded region, 25.5% of patients) when using 50% DS and the index test and FFR ≤ 0.8 as the reference standard.
FIGURE 9.
Extracted data on 2D ICA compared with FFR. Each colour represents a separate study. Black line indicates where FFR = ICA. The lower-left shaded region shows the FNs where ICA < 50% but FFR ≤ 0.8; the upper-right shaded region shows the FPs where ICA > 50% but FFR > 0.8.
We performed a bivariate meta-analysis of these extracted data, using the same approach as for QAngio XA 3D/QFR. The summary sensitivity was 62.6% (95% CI 51.5% to 72.5%) and specificity was 61.6% (95% CI 53.1% to 69.4%). This is a substantially lower diagnostic accuracy than for QAngio XA 3D/QFR.
QAngio XA 3D/QFR: studies not included in meta-analysis
Appendix 5, Table 42, presents results from the six studies of QAngio XA 3D/QFR that reported diagnostic accuracy results but were not included in the meta-analysis because of insufficient data. 14,22,33,36,38,47 All studies were reported as conference abstracts only (although one was subsequently published after the cut-off date for conducting meta-analyses). 33 One QAngio XA 3D/QFR prototype study recorded QAngio XA 3D/QFR analyses prospectively (on-site analysis), and re-ran analyses retrospectively after ‘essential modifications’ (no further details reported). All other studies were retrospective and did not report which version of QAngio XA 3D/QFR was used. 22,33,36,38,47
The results broadly reflected the findings of the meta-analysis. All studies reported moderate to high diagnostic accuracy for QAngio XA 3D/QFR compared with FFR. There was significant heterogeneity in reported diagnostic accuracy estimates. Sensitivity ranged from 64.0% to 91.8%, and specificity from 68.2% to 97.3%. Where reported, PPV estimates ranged from 74.0% to 84.8% and NPV from 68.2% to 93.0%. AUC ranged from 0.77 (95% CI 0.67 to 0.87) to 0.99 (95% CI 0.97 to 1.00). Correlation coefficients (r) also varied significantly, ranging from 0.578 to 0.801.
The wire-free invasive functional imaging (WIFI) prototype study14 reported moderate sensitivity (0.64, 95% CI 0.48 to 0.77) and specificity (0.8, 95% CI 0.66 to 0.89) in its initial analyses. Following ‘essential modifications’ (no further details reported) a blinded in-centre core laboratory reanalysis was performed, and this improved both sensitivity (0.66, 95% CI 0.51 to 0.79) and specificity (0.86, 95% CI 0.73 to 0.93).
QAngio XA 3D/QFR: other modes
Three studies reported results for QAngio XA 3D/QFR modes other than cQFR and fQFR. 32,46,48 Their results are presented in Appendix 5, Table 43. Two small studies (n = 15 and 84 vessels) reported results for adenosine–flow quantitative flow ratio (aQFR),46,48 and one larger study (n = 306 lesions) tested iQFR, lQFR and vQFR. 32 Sensitivity of aQFR per vessel ranged from 78% to 100%, and specificity from 91% to 93%; one study reported a high AUC (0.90, 95% CI 0.81 to 0.96) for aQFR, and similar results in per-patient analyses. One study found that iQFR had higher diagnostic accuracy overall (sensitivity: 83.3%; specificity: 86.6%; AUC: 0.936) than vQFR (sensitivity: 90.5%; specificity: 69.7%; AUC: 0.900) and lQFR (sensitivity: 91.1%; specificity: 71.7%; AUC: 0.822).
CAAS vFFR
The review identified four publications reporting the diagnostic accuracy of CAAS vFFR. 18,19,26,56 One is the original FAST study of vFFR,56 and one is a conference abstract reporting an update to FAST (FAST-EXTEND). 18 There were two other independent studies, one of which has only been published as a conference abstract. 26 All studies performed CAAS vFFR analyses retrospectively (offline), and two were conducted in a single centre. 18,19 One study was funded by the CAAS vFFR manufacturer. 18 All studies compared CAAS vFFR with FFR as reference standard. 18,19 One study was funded by the CAAS vFFR manufacturer. 18 Two studies included a mixed population of stable angina, unstable angina or NSTEMI. 18,19
We included only studies that explicitly reported that the CAAS system was used, or where this was confirmed by the authors. Other studies of vFFR were identified but were not included if other technologies were used or the precise technology used could not be determined. Further details on excluded studies are reported in Appendix 2, Table 35. Only one of the studies19 reported a 2 × 2 table of diagnostic accuracy, and only one56 presented a Bland–Altman plot, which we digitally extracted to calculate diagnostic accuracy. The two conference abstracts reported only sensitivity and specificity without CIs. To construct approximate CIs, we assumed that the proportion of patients with FFR ≤ 0.8 was 29% (the rate observed in the FAST study56), and constructed 2 × 2 diagnostic data under that assumption.
Table 6 summarises the properties of the CAAS vFFR studies. The sensitivity and specificity from all publications is summarised in Figure 10. There is notable heterogeneity across even this small number of studies. In particular, the ILUMIEN I study19 found considerably lower sensitivity and specificity that the FAST studies,18,56 and the Jin et al. study26 had lower sensitivity, but slightly higher specificity.
| Study | n | Test | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | Correlation, r |
|---|---|---|---|---|---|---|---|---|
| Jin et al. (2019),26 conference abstract | 101 vessels (82 patients) | CAAS vFFR | 68.2 | 87.3 | NR | NR | 0.719 (0.621 to 0.804) | NR |
| QAngio XA 3D/QFR (cQFR)a | 83.5 | 31.9 | NR | NR | 0.886 (0.807 to 0.940) | NR | ||
| QAngio XA 3D/QFR (fQFR)a | 72.7 | 89.9 | NR | NR | 0.882 (0.803 to 0.938) | NR | ||
| ILUMIEN I: Ely Pizzato et al. (2019)19 | 115 lesions (115 patients) | CAAS vFFR 8.1 | 75.0* | 46.5 | 70.1a | 52.6a | NR | 0.449 (95% CI 0.290 to 0.584; p < 0.0001) |
| FAST: Masdjedi et al. (2020)56 | 100 patients | CAAS vFFR | NR | NR | NR | NR | 0.93 (0.88 to 0.97) | 0.89 |
| 3D ICA | NR | NR | NR | NR | 0.66 (0.55 to 0.77) | |||
| FAST-EXTEND: Daemen et al. (2019),18 conference abstract | 303 patients | CAAS vFFR 8.0 | 97 | 74 | 85 | 89 | 0.95 (0.93 to 0.98) | 0.89 |
| 3D ICA | NR | NR | NR | NR | 0.63 (0.55 to 0.67) | NR |
FIGURE 10.
Sensitivity and specificity of CAAS vFFR studies.

Bivariate meta-analysis (CAAS vFFR)
The results of bivariate meta-analysis of these studies are presented in Table 7. It should be noted that, because there are only three independent studies, and as data had to be imputed, meta-analyses of these studies may not be robust, and are included only to permit some comparison with QAngio XA 3D/QFR analyses.
| Analysis | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| Using FAST: Masdjedi et al. (2020)56 | 75.98 (66.86 to 83.22) | 74.38 (51.32 to 88.89) |
| Using FAST-EXTEND: Daemen et al. (2019)18 | 84.86 (61.76 to 95.11) | 72.20 (50.30 to 86.95) |
As the FAST56 and FAST-EXTEND18 studies overlap, we report meta-analysis using each of these (and excluding the other). Although diagnostic accuracy is reasonable in both analyses, CIs are wide, reflecting the limited data and high heterogeneity. In both cases, specificity is lower than estimated for QFR (around 89%). When using FAST-EXTEND, sensitivity is similar to QFR (around 84%), but when using the earlier FAST study, sensitivity for CAAS vFFR is lower than for QFR. These meta-analyses should be interpreted with caution because they required imputation of data for two studies on the prevalence of FFR results below and above the cut-off point of ≤ 0.80, and because of the high heterogeneity across studies.
Only one study26 has directly compared CAAS vFFR with QFR, and this is currently reported only as a conference abstract. That study concluded that diagnostic performance of vFFR was poorer than for QFR, with AUCs of 0.719 (95% CI 0.621 to 0.804) for vFFR and 0.886 (95% CI 0.807 to 0.940) for cQFR.
Subgroup and sensitivity analyses (CAAS vFFR)
There were insufficient data to conduct any subgroup analyses or meta-regressions to investigate whether the diagnostic accuracy of CAAS vFFR varied by patient or study characteristics. Sensitivity analyses according to study quality were not feasible.
As only one study presented a figure with extractable data, analyses of these data were not performed. No further data suitable for narrative review or synthesis were identified.
Clinical outcomes
Morbidity, mortality and major adverse cardiac events
Three cohort studies reported mortality or major clinical outcomes in eligible patients with QFR (QAngio XA 3D/QFR) measurements. 30,41 All found that a clinically significant QFR was associated with a higher incidence of long-term MACEs. No data were reported for CAAS vFFR. Results are summarised in Appendix 5, Table 48, and below.
Spitaleri et al. 41 included patients with multivessel disease who underwent revascularisation as part of a large randomised trial of PCI in 1498 STEMI patients in whom at least one non-culprit lesion (NCL) was left untreated. 66 QFR was calculated in NCLs in a subgroup of 110 patients following revascularisation. Patients with QFR values > 0.80 in all NCLs were classified as having functional complete revascularisation (n = 54), and those with at least one NCL with QFR value ≤ 0.80 were classified as having functional incomplete revascularisation (n = 56). Patient-oriented cardiac events (POCEs, defined as cumulative occurrence of all-cause death, any MI and any coronary revascularisation) were measured at the 5-year follow-up. A total of 39 (35%) patients experienced an adverse event. The cumulative incidence of POCEs was higher in the group with QFR ≤ 0.80 (46%) than in the group with QFR > 0.80 (24%) (HR 2.3, 95% CI 1.2 to 4.5; p = 0.01). Further individual POCE outcomes are reported in Appendix 5, Table 48.
Kanno et al. 30 (conference abstract only) evaluated 212 de novo intermediate coronary lesions in 212 patients with deferred revascularisation based on FFR values above 0.80. Baseline and physiological indices including cQFR were compared between patients with and without MACEs (cardiovascular death, non-fatal MI, target vascular revascularisation and non-target vascular revascularisation) during the 4-year follow-up. MACE incidence at the 4-year follow-up was 5.7%. In patients with MACEs, cQFR was lower than that in patients without MACEs (mean or median 0.80 vs. 0.88; p = 0.030). On logistic regression analysis, cQFR≤ 0.8 was a significant predictor of MACEs (OR 5.60, 95% CI 1.69 to 18.6; p = 0.005).
Hamaya et al. 23 included a population of 549 patients with stable three-vessel disease who underwent cQFR. At the median 2.2 years’ follow-up, patients with MACEs had lower cQFR in all three vessels than those without MACEs [2.76 (95% CI 2.64 to 2.88) vs. 2.64 (95% CI 2.49 to 2.73); p < 0.001], and three-vessel cQFR was a statistically significant predictor of MACEs in multivariate analyses (HR 0.97, 95% CI 0.96 to 0.99). cQFR was also a better predictor of remote revascularisation (≥ 3 months) than DS [AUC 0.73 (95% CI 0.65 to 0.79) vs. AUC 0.66 (95% CI 0.56 to 0.74); p = 0.043].
Subsequent use of invasive pressure wire fractional flow reserve
No studies of QFR prospectively evaluated the impact of QFR use and subsequent reductions in the use of adenosine and pressure wire procedures. However, five studies included in the diagnostic accuracy review retrospectively derived a grey-zone strategy based on their diagnostic accuracy results to model a potential reduction in adenosine and FFR use. 29,37,40,50,51
Results are summarised in Appendix 5, Table 49. None of these studies used the grey-zone boundaries recommended by the manufacturer (0.78–0.84), and only two studies used the same grey zone. 40,50 All studies derived their grey-zone boundaries from their own cohort, except one40 that used boundaries defined by another study. 50 Diagnostic accuracy criteria of QFR against FFR used to derive grey-zone boundaries varied across the studies (e.g. minimum sensitivity and specificity of the grey zone was > 95% in one study50 and > 90% in another51). Each study retrospectively modelled a QFR–FFR hybrid strategy using QFR as the main diagnostic method and only performing FFR measurements in their defined grey zone. Despite the variety of choice of grey zones and how they were defined, all are broadly similar to each other and to the manufacturer-specified definition used in the meta-analysis in Bivariate meta-analysis (QAngio XA 3D/QFR).
All simulated grey-zone strategies were associated with a large percentage of adenosine/FFR procedures (hypothetically) avoided, ranging from 42% to 68%. The widest grey-zone area (0.71–0.90)51 was associated with the lowest proportion of adenosine/FFR-free procedures (42%), and the narrowest boundaries (0.77–0.86, 0.78–0.87) associated with the highest proportion of procedures avoided (61–68%). 40,50,51 None of the simulations modelled the clinical impact of delayed FFR in patients with a FFR below 0.8.
Interobserver variability
Eight studies reported outcomes data on the reproducibility of QFR readings between two different analysts (see Appendix 5, Table 50). One study directly compared QAngio XA 3D/QFR and CAAS vFFR,26 six studies evaluated QAngio XA 3D/QFR only17,24,25,27,33,45 and one CAAS vFFR only. 56 Three studies were reported only as conference abstracts. 25–27 The number of single measurements analysed ranged from 10 to 101 vessels. All QFR measurements were performed and compared retrospectively (offline) where reported. Only two studies explicitly reported blinding analysts to each other’s readings. 45,56
It was found that QFR had a moderate to high level of inter-rater reliability. Two studies of QAngio XA 3D/QFR reported MDs ≤ 0.01 in repeated QFR measurements between analysts. 24,45 One study reported a moderate correlation between three separate raters [mean intraclass correlation (ICC) 0.614, 95% CI 0.464 to 0.728]. Two studies reported high ICC results, one in stable angina patients (r = 0.990)33 and the other in NCLs of STEMI patients (r = 0.991). 17 Another study found that inter-rater reliability was higher for cQFR (R2 = 0.82) than for fQFR (R2 = 0.70) and ICA DS (R2 = 0.67). 27 One study found high inter-rater repeatability for QAngio XA 3D/QFR [fQFR 0.001 (SD 0.036) and cQFR 0.001 (SD 0.049)] as well as CAAS vFFR [0.005 (SD 0.037)] and no statistically significant differences between raters’ measurements. Inter-rater reliability was also high in the FAST study across 100 repeated CAAS vFFR measurements (r = 0.95). 56
Intraobserver variability
Eight studies reported outcomes data on intraobserver reproducibility of QFR readings (see Appendix 5, Table 51). Seven studies evaluated QAngio XA 3D/QFR only,16,17,25,27,33,45,54 and one study directly compared QAngio XA 3D/QFR and CAAS vFFR. 26 Five studies were reported only as conference abstracts. 16,25–27,54 Where reported, all measurements were performed retrospectively (offline). The time gap between initial and repeated measurements was reported in four studies and ranged from 3 days to 2 weeks. 16,27,45,54
All studies except one25 reported a high level of intrarater reliability for QFR. One study that assessed QFR readings independently by three analysts twice among 100 vessels reported a moderate ICC coefficient (r = 0.428). Where reported, r coefficients for QAngio XA 3D/QFR in other studies ranged from 0.958 to 0.997, and MDs between repeat measurements from 0.00 (SD 0.03) to 0.016 (SD 0.06). One study found that R2 was higher for fQFR (0.91) and cQFR (0.94) than DS measured by ICA (0.76). 27 One study that evaluated both QAngio XA 3D/QFR and CAAS vFFR found high levels of repeatability and no statistically significant changes between repeated tests (cQFR: MD 0.009 ± 0.053, p = 0.230; fQFR: MD 0.016 ± 0.060, p = 0.066; vFFR: MD 0.008 ± 0.040, p = 0.175).
Test failures: rates and reasons
Appendix 5, Table 52, reports rates of exclusions from diagnostic accuracy studies and reasons for exclusion. Sixteen studies did not report rates of patient exclusions or reasons for exclusion. 14,16,18,22,23,25,29,31,33,35,36,38,39,44,48,54 Exclusion rates varied widely, from 6% to 92%, although this is partly due to differences in patient selection criteria, reporting and methods of calculating exclusions rates (e.g. out of total population considered for eligibility vs. out of total number of patients with FFR). This limits the comparability of exclusion rates across the studies.
Issues with the acquisition and quality of angiographic images (e.g. lack of at least two projections with a 25% degree angle in between, or poor image quality) were the most reported cause of exclusion, with 15 studies reporting it as their main reason for excluding patients from QFR analyses. 15,17,19–21,24,28,32,37,42,43,46,49,50,56 Anatomical features of arteries (e.g. excessive overlapping or foreshortening, ostial lesions, severe tortuosity) were the second most commonly listed reason for exclusion. Rates of exclusions were higher overall in retrospective studies (median 28%, range 6–92%) compared with prospective studies (median 17%, range 7–52%). This may be partly explained by the fact that ICA images in retrospective studies were less likely to have been collected following manufacturer instructions to acquire images suitable for QFR.
Both CAAS vFFR that reported reasons for exclusion reported high exclusion rates (63% and 65%), although both studies were retrospective. 19,56 In both studies, the majority of exclusions were explained by angiographic image processing issues (rather than CAAS vFFR directly). For instance, 83% of exclusions in ILUMIEN I19 were due to the lack of at least two angiographic projections, table movement during ICA or pixel resolution incompatibility. ILUMIEN I19 concluded that careful adaptions in acquisitions of ICA images could reduce test failure.
Other outcomes
No evidence was reported in QAngio XA 3D/QFR and CAAS vFFR studies for any of the following protocol-specified outcomes: impact of QFR on the rate revascularisation procedures, adverse events related to the diagnostic procedure, adverse events related to revascularisation, distress, anxiety and similar harms caused by QFR, vFFR, invasive FFR or iFR, number of vessels with stent placements, HRQoL and radiation exposure.
Simulation study of clinical effectiveness
Given the very limited data on clinical effectiveness of QAngio XA 3D/QFR reported in publications, we performed a simulation study to investigate the possible impact of using QAngio XA 3D/QFR, compared with FFR, on actual coronary outcomes. This simulation study treats the complete data extracted from figures (3192 observations) as a representative sample from the true population of FFR and QFR measurements. To predict coronary outcomes we used the results of the recent IRIS-FFR registry report, representing 5846 patients who were either revascularised (stent or bypass surgery) or deferred (continued with current management without surgery) based on their measured FFR result. The full methods are set out in Statistical analysis of clinical effectiveness.
The IRIS-FFR study used major cardiovascular events (MACE, a composite of cardiac death, MI and repeated/emergency revascularisation) as its primary outcome. The reported hazard of MACEs by FFR value was used to estimate the risk for each person in the extracted data. Based on those risks we simulated whether or not each person had a MACE if they were ‘deferred’ or if they were revascularised. Note that this assumes that risk is solely a function of FFR values, and that knowing the QFR has no impact on risk of MACEs.
We investigated three strategies for deciding on whether or not to revascularise:
-
FFR only – perform FFR on all and revascularise if the FFR is ≤ 0.8
-
QFR only – perform QFR on all and revascularise if the QFR is ≤ 0.8, without FFR measurement
-
grey zone – perform a QFR and:
-
revascularise if the QFR is ≤ 0.78
-
defer if the QFR is > 0.84
-
if the QFR is between 0.78 and 0.84, perform FFR and revascularise if the FFR is ≤ 0.8.
-
Results of the simulation study
Appendix 6, Figure 42, presents an example simulation, showing the distribution of simulated MACEs according to FFR and QFR. For ease of interpretation, the majority of patients with no MACE are excluded and only patients with MACE are shown. Preventable MACEs (i.e. patients who would have MACE if not revascularised) are evenly distributed across both FFR and QFR ranges. MACEs caused by revascularisation (i.e. where MACE occurs if revascularised, but would be avoided if deferred) are concentrated above values of 0.75 for both FFR and QFR, in line with the suggestion in IRIS-FFR that deferral is preferable for a FFR > 0.75.
In Appendix 6, Figure 42, most events occur in the white regions, where the same revascularisation decision would be made using either FFR or QFR. There are few patients, and hence few MACEs, in the FP region (upper-left shaded area), where patients would be revascularised based on FFR but not if using QFR. Hence, using QFR would miss out on preventing some events in this region (green dots) but equally would avoid causing MACEs due to revascularisation (blue dots).
In Appendix 6, Figure 42, in the FP region (lower-right shaded area), where patients would be revascularised based on QFR but not if using FFR, there are also few events. QFR prevents some events in this region (green dots) that would be missed by FFR, but equally would cause MACEs due to revascularisation (blue dots). The ‘preventable’ and ‘caused’ events in these two regions approximately balance each other out.
Based on the data extracted from figures, if using the ‘FFR only’ strategy 40.2% of patients would be revascularised; using the ‘QFR-only’ strategy 42.0% would be revascularised; and using the grey-zone strategy 43.2% would be revascularised. So, using QFR moderately increases the revascularisation rate, and using it in combination with a grey zone increases it further.
Table 8 summarises the key results of the simulation. FFR is slightly more effective at preventing MACEs, but QFR leads to only about one extra unprevented MACE per 1000 patients. If the grey zone is used the total number of MACEs is closer to that of using FFR for everyone. Using QFR results in around 1.3 to 1.6 more revascularisations to prevent one MACE.
| Strategy | Percentage with MACE | Percentage with prevented MACE | Percentage with MACE caused by revascularisation | Percentage with unprevented MACE | Number of revascularisations per MACE prevented |
|---|---|---|---|---|---|
| FFR | 1.75 | 1.60 | 0.91 | 0.78 | 25.18 |
| QFR | 1.85 | 1.57 | 0.97 | 0.81 | 26.80 |
| Grey zone | 1.82 | 1.63 | 1.00 | 0.75 | 26.50 |
Using QFR with or without with a grey zone leads to more revascularisations, and so more MACEs caused by revascularisation (6 or 9 per 10,000 more, respectively), which leads to a larger number of revascularisations per MACE prevented.
Table 8 presents only the median values across all simulations. Figure 11 shows the distribution of revascularisations per MACE prevented. Appendix 6, Figures 43–45, show the prevented and unprevented events, and events caused by revascularisation across all simulations. These show the substantial overlap between the distributions, so, although the results in Table 8 suggest some difference between strategies, it is not clear if these are genuine differences that would be observed in actual clinical practice.
FIGURE 11.
Estimated revascularisations per MACE prevented across all simulations.
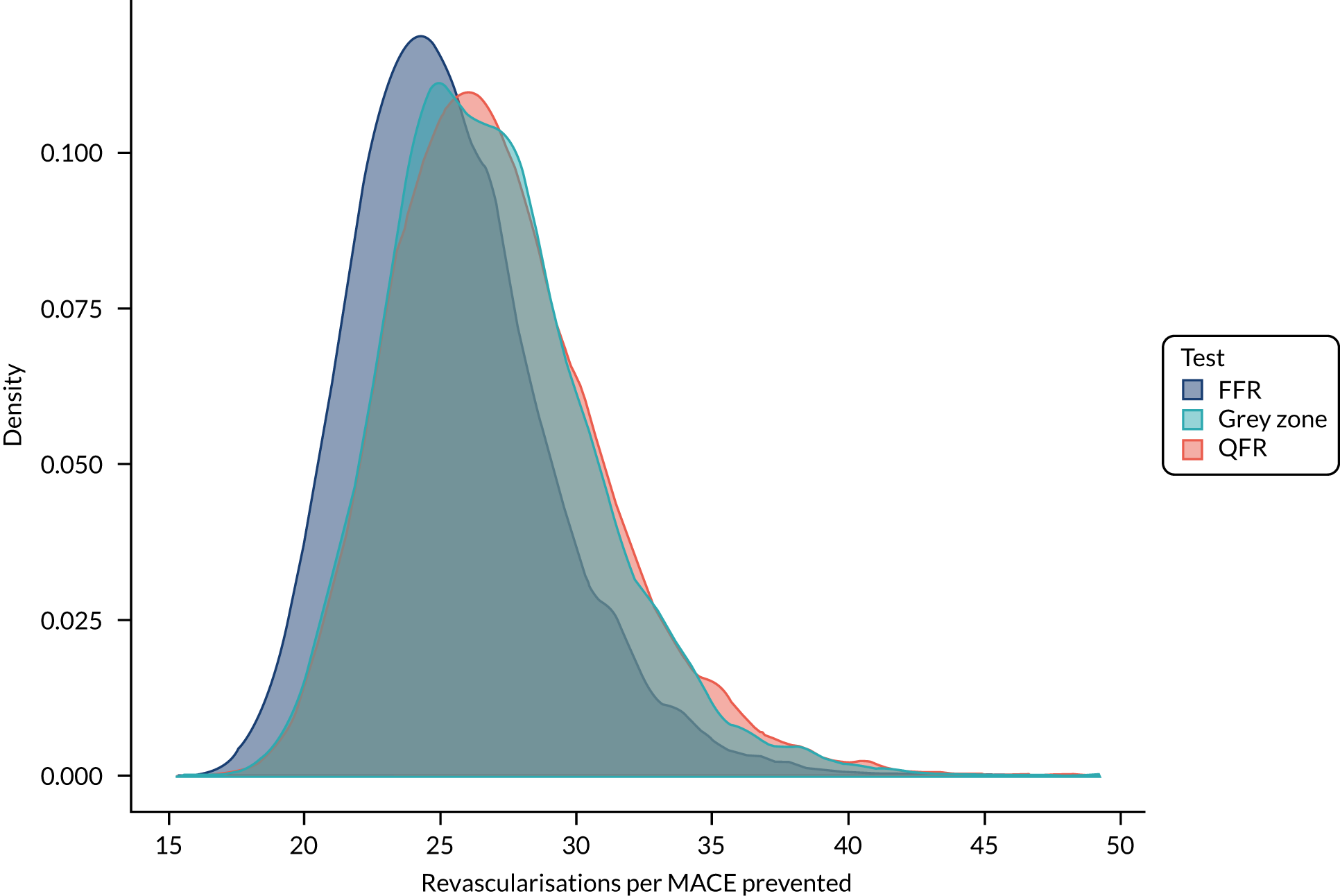
Overall, these simulations suggest that there is little conclusive clinical difference between using QFR and FFR to make revascularisation decisions. Using FFR may prevent slightly more MACEs, at around 1 event per 1000 patients, but the overlap in simulated distributions means it is highly uncertain whether or not the difference is genuine. By contrast, the simulation suggests that QAngio XA 3D/QFR increases the number of revascularisations performed, without substantially improving the number of MACEs prevented.
The simulation has numerous limitations as a result of its assumptions. Most important is that the risk of MACE depends only on a patient’s FFR. The simulation could not account for any other key patient factors, and there is the possibility that knowing the QFR as well as the FFR might alter the predicted risk. The IRIS-FFR risks may not match the risks in the UK population eligible for FFR or QFR assessment. The simulation is also based only on the data extracted from figures, which is a small sample and may not represent the patients seen in practice. The simulation considers only a single lesion per patient, when QFR may be used to assess multiple stenoses in a patient.
Implementation evidence
Timing of results from data acquisition
Six studies of QAngio XA 3D/QFR reported measuring the time required to complete QFR analysis. 14,32,45,50,52,53 The results are summarised in Appendix 5, Table 53. Two studies were prospective,14,52 and one was reported only as a conference abstract. 14 Sample size ranged from 68 to 268 patients. The reporting of methods for calculating time to QFR acquisition differed among the studies. For instance, only two studies specified that calculations included time required to select appropriate angiographic images for generating 3D images. 50,53
Time to QFR data acquisition ranged from an average of 2 minutes 7 seconds to 10 minutes (SD 3 minutes). One study of 268 patients reported that time to image acquisition significantly decreased with the number of ICAs analysed, from 5 minutes 59 seconds to 2 minutes 7 seconds, between the first and last 50 cases. One conference abstract of an earlier prototype version of QAngio XA 3D/QFR reported a mean total time to QFR of 10 minutes (SD 3 minutes). The study reported that the application required essential modifications during the study and retrospective reanalysis of ICA and QFR was performed with the final version of QFR, although it was not clear which analysis was used to derive mean time to data acquisition.
Other outcomes
No evidence was found for any of the following review protocol-specified implementation outcomes: acceptability of QFR, vFFR and invasive FFR (to clinicians and patients), referral times, patient satisfaction, training requirements, test uptake and compliance.
Conclusions and recommendations for research from included studies
Most studies concluded that QAngio XA 3D/QFR had good diagnostic accuracy for detecting significant coronary stenosis and good correlation and agreement with both wire-based FFR14,15,20,21,23,24,26–30,32,34–46,48,50–54,56,67 and iFR,16,21,24,36,49 and is able to improve angiographic assessment for evaluation of intermediary coronary artery stenosis. 14,50,52
Studies of CAAS also concluded that QFR had good correlation and agreement with wire-based FFR,18,19,24,56 although one concluded that only one-third of routinely acquired coronary angiographic images were appropriate for retrospective vFFR analysis. 19
Studies conducted in patients with acute coronary syndrome concluded that QFR was safe and accurate in assessment of non-culprit vessels. 17,28,31,41 Some studies suggested that diagnostic accuracy of QFR may be affected by specific clinical characteristics, namely small vessels,15,32 presence of bifurcated lesions and trifurcated lesions,15,38 left main stenosis,22 prior MI-related coronary arteries20 and microvascular function. 29,41
Several studies concluded that QFR may be a good alternative tool for identifying significant coronary stenosis in various clinical settings or may complement invasive wire-based options;23,31,37,49 it is applicable to patients allergic to adenosine and adenosine triphosphate (ATP) vasodilators and may avoid procedural risks or patient discomfort associated with invasive wire-based options. 23,52 Some studies noted that QFR may reduce procedure time, be associated with reduced cost and allow for wider adoption of functional assessment of coronary stenosis. 23,33,46
Some studies recommended using a hybrid approach to reduce the need for invasive FFR, although there was no consensus on an optimal grey zone. 16,33,43 In some cases, patients may be unsuitable for evaluation of stenosis severity using angiography, including diffuse tandem disease, tandem lesions, lesions with angiographic haziness caused by calcification or thrombus and lesions with ulceration cause by plaque rupture. 20 Diagnostic accuracy could be affected in patients with bifurcation lesions,32,50 patients with prior MI-related coronary arteries20 and patients with left main location of stenoses. 22 Some studies suggested that confirmation with FFR may be required close to values of 0.8. 33,53 One CAAS study noted that careful adaptations in image acquisition will be required to reduce the risk of test failures if used in daily clinical practice.
Further prospective online investigation into the clinical benefit of QFR-based revascularisation was recommended by multiple studies,17,37,42,43,45,46,48,50 including using appropriately powered randomised controlled trials (RCTs) with relevant clinical end points before implementing the device as a definite alternative to invasive FFR,24,32,37,40,50 such as the ongoing FAVOR III China trial (NCT03656848). Some recommended further testing of modelled hybrid QFR/FFR approaches. 51
A number of studies recommended that that testing of the diagnostic accuracy and feasibility of QFR in clinical practice in different settings is needed. 17,32,36–38,40,43,45,48,49
Further investigation of diagnostic precision and the application of the current QFR methodology in patients with different lesion subtypes,38 including bifurcation lesion,32,50 patients with prior MI-related coronary arteries20 and patients with left main location of stenoses22 was recommended.
Clinical effectiveness summary and conclusions
The diagnostic accuracy of QAngio XA 3D/QFR has been widely studied in 39 studies to date with a total of 5949 patients (7034 vessels or lesions).
At a cut-off point of 0.8, QFR has good diagnostic accuracy to predict FFR (also at a cut-off point of 0.8) with sensitivity around 84% and specificity around 89%. Although this means there is some discordance between QFR and FFR, most FPs or FNs arise near the boundary (e.g. where one is 0.81 and the other 0.79), and the discordance may not be clinically meaningful. Data on how this accuracy may vary by key patient characteristics was very limited, and no conclusive variation could be found. QFR, as measured using QAngio XA 3D/QFR, is generally similar to FFR measured with an invasive pressure wire. The average difference between the two values was 0.001, and values rarely differed by more than 0.14, and, in 50% of patients, by less than 0.04.
The use of a grey zone, where patients with intermediate QFR values go on to have confirmatory FFR, was found to increase diagnostic accuracy. Around 20% of patients fall in the grey zone and would receive confirmatory FFR. Of these, only around 30% have discordant FFR and QFR results, so the confirmatory FFR is unnecessary for the majority of patients in the grey zone.
Diagnostic accuracy data for CAAS vFFR were limited to only three studies. The results from the studies were heterogeneous, limiting meta-analysis and a full evaluation of CAAS vFFR. Hence its diagnostic value is currently uncertain, but it may be a potential alternative to QAngio XA 3D/QFR.
This report did not perform a full systematic review of 2D or 3D ICA, but in those studies that we did identify, the diagnostic accuracy of ICA was substantially inferior to QAngio XA 3D/QFR, with DS from ICA being poorly correlated with FFR.
There were very few reported data on clinical effectiveness and implementation outcomes when using QAngio XA 3D/QFR, as nearly all studies published to date have focused on diagnostic accuracy. What data there is suggests that the QFR results of 0.80 or below for QAngio XA 3D/QFR may be significant predictors of subsequent MACE, and that a grey-zone strategy is likely to lead to substantial reductions in adenosine and FFR procedures. Timing of results, inter-rater and intrarater reliability were generally acceptable for QAngio XA 3D/QFR, indicating that the technology is feasible in a clinical context. However, data were limited and quality of blinding uncertain, so levels of inter-rater reliability in general use remain unclear. The feasibility of CAAS vFFR is uncertain notably because of lack of evidence on repeatability within and between raters and the high rate of patient exclusions from retrospective evidence.
The simulation study to investigate the clinical impact of using QAngio XA 3D/QFR found that QAngio XA 3D/QFR may lead to a slight increase in revascularisations compared with using FFR, but both methods prevent broadly the same number of MACEs. Up to 1 person in 1000 may have a MACE if using QAngio XA 3D/QFR that could have been prevented with FFR, but this is highly uncertain. Using a grey zone seems to lead to an increase in the number of revascularisations, but with no improvement in MACE prevention compared with using FFR alone or QFR alone.
Overall, this review suggests that making decisions on revascularisation in patients with intermediate stenosis using QFR as measured by QAngio XA 3D/QFR is a reasonable diagnostic strategy, and so QFR assessment may be a reasonable alternative to invasive FFR. The trade-off appears to be a balance between avoiding the side effects of FFR (particularly adenosine use) at a cost of possibly slightly more revascularisation procedures. The use of QFR appears to be conclusively preferable to using DS measured by standard ICA alone.
The review did not find a strong case for consistently using FFR in patients in whom QFR is borderline (i.e. around 0.8, the grey-zone approach). This seems to place too strong an emphasis on patients close to the 0.8 threshold. Most patients in this region have similar FFR and QFR results (within 0.05), and so any discordance between QFR and FFR may not be clinically meaningful. A large proportion of people who go on to receive FFR have the same conclusion as their original QFR, exposing them to a potentially harmful, unnecessary test. This conclusion, however, does not prevent the use of FFR when clinicians might think it necessary for reasons other than the QFR being close to 0.8.
Data on CAAS vFFR are currently too limited and heterogeneous to draw any useful conclusions on its clinical value.
Chapter 4 Assessment of existing cost-effectiveness evidence
This chapter provides an overview of existing cost-effectiveness evidence on the use of the QAngio XA 3D/QFR and CAAS vFFR imaging software for assessing the functional significance of coronary obstructions in patients with suspected stable chest pain whose angiograms show intermediate stenosis and who may require revascularisation. The literature was systematically searched to identify and describe relevant evidence on the cost-effectiveness of the two new technologies within the indication for which these are being evaluated. This systematic review also aimed to identify the central issues associated with adapting existing decision models to address the current decision problem and to assist in the development of a new decision model drawing on the issues identified in the clinical effectiveness and cost-effectiveness review. Given that the two technologies under assessment have only recently been commercialised, it was anticipated that there would be a dearth of relevant economic evidence. Therefore, to assist the development of a new decision-analytic model, a pragmatic review of published cost-effectiveness studies evaluating ICA (alone and/or with FFR) in the management of CAD was also conducted.
Methodology of the cost-effectiveness review of QAngio XA 3D/QFR and CAAS vFFR
Searches
The bibliographic search detailed in Chapter 3, Searches, was used to identify studies reporting on the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR.
Selection process
The review considered a broad range of economic studies including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. The inclusion criteria considered were full economic evaluations comparing two or more alternatives and considering both costs and consequences (i.e. cost-minimisation, cost-effectiveness, cost–utility and cost–benefit analyses).
The protocol for the selection of relevant studies defined two selection stages: (1) assessment and screening for possible inclusion of titles and abstracts identified by the search strategy, and (2) acquisition and screening for inclusion of the full texts of potentially relevant studies. Two researchers independently screened the titles and abstracts of all reports identified by the bibliographic searches. Full-text papers were to be subsequently obtained for assessment and screened by at least two researchers, with any disagreement resolved by consensus.
Results of the cost-effectiveness review of QAngio XA 3D/QFR and CAAS vFFR
The initial search identified a total of 1243 records (after deduplication). No studies were identified as potentially relevant from their titles and/or abstracts, as none evaluated the cost-effectiveness of either QAngio XA 3D/QFR or CAAS vFFR.
Methodology of the review of decision models evaluating invasive coronary angiography
Given the lack of cost-effectiveness studies evaluating QAngio XA 3D/QFR and CAAS vFFR used during ICA, a pragmatic review of published cost-effectiveness studies evaluating ICA (alone and/or with FFR) in the management of CAD was conducted. The search targeted cost-effectiveness studies where ICA was one of the interventions under comparison. The aim of the review was to help inform the conceptualisation of the decision problem and identify any relevant sources of evidence. Importantly, the review aimed to assess how the link between short-term diagnostic outcomes and longer-term impact and subsequent prognosis associated with the diagnostic pathways in the management of CAD and associated costs and outcomes had been established in the literature. Given that the purpose of the review was broader than to inform specific inputs of the cost-effectiveness model, it was not considered appropriate to conduct a full systematic review.
Searches
Targeted searches were conducted in October 2019 in the following databases: MEDLINE databases (i.e. MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily, and Ovid MEDLINE), EconLit, EMBASE, NHS EED and the HTA database. Search strategies are detailed in Appendix 1.
Study selection
Cost-effectiveness studies published after the year 2000 where ICA (alone and/or with FFR) was one of the interventions under comparison were considered for inclusion. Only cost-effectiveness, cost–utility and cost–benefit analyses were considered eligible. Studies that presented results as a cost per diagnosis were not considered for inclusion, as the key aim of the review was to assess how the link between short-term diagnostic outcomes and longer-term impact and subsequent prognosis associated with the diagnostic pathways in the management of CAD and associated costs and outcomes had been established in the literature. The patient population of this review was defined as patients with stable chest pain and suspected or known CAD. Studies in patients with acute coronary syndromes and NSTEMI as the primary diagnosis were excluded. The inclusion criteria further specified that only titles in English would be considered eligible. Titles that were books, editorials, letters to the editor and reviews that did not include a de novo model were excluded from the review.
One researcher (AD) conducted the two-step selection process consisting of screening for inclusion (1) the titles and abstracts of studies identified by the bibliographic searches, and (2) the full-text articles identified at the previous step as potentially relevant.
Results of the review of decision models evaluating invasive coronary angiography
A total of 1740 records were identified during the initial search of economic databases, of which 1264 remained after deduplication. The first step of screening identified 25 titles as potentially relevant based on their titles and/or abstracts. After the full-text articles of these records were obtained and assessed for eligibility, 21 studies68–88 were considered to meet the selection criteria and included in the review. The studies are summarised in detail in Appendix 7, Table 56. Results of the searches and the list of excluded studies are presented in Appendix 7, Tables 54 and 55.
Given the aim of the review, a formal assessment using checklists to assess the quality of the included cost-effectiveness studies was not conducted. Instead, a narrative review of key model features, including testing and management strategies, and assumptions to support the conceptualisation and development of a de novo analytical model is presented below.
The majority of studies68–72,75,77,79,81,83,85,86,88 used a decision tree to model the diagnostic pathway and short-term outcomes, and a long-term Markov model (or multiple Markov models) to characterise disease progression. Two studies used microsimulation models74,82 that also combined a decision tree structure to model diagnostic outcomes followed by a lifetime disease progression state-transition model. Of the five studies that modelled the full-time horizon with a decision tree model, three models71,73,80 captured only short-term outcomes (1-year time horizon), whereas two others comprised longer time horizons (10 years84 and lifetime87). One study78 used a Bayesian mathematical model based on two equations to estimate costs and quality-adjusted life-years (QALYs) for each strategy under comparison over a 10-year time horizon. The equations appear to be equivalent to the calculations in a decision tree’s rollback algorithm.
Among the 21 studies, two models77 were considered to be good examples of alternative ways to evaluate diagnostic strategies in patients with suspected stable angina. These studies were selected on the basis that they encompassed many of the features identified in the other studies. The two models differed in terms of how they modelled the diagnostic pathway and subsequent long-term risks of major cardiovascular-related events and associated costs and outcomes. The first study77 was a cohort model that estimated outcomes for an average patient in clinical practice, and the second study74 was a microsimulation model that estimated outcomes for hypothetical patients at different levels of disease severity (defined in terms of number of coronary vessels affected and whether or not patients have ischaemia). A key difference of the two models was the approach taken to assess the long-term impact of the diagnostic strategies on the risk of major cardiovascular events. In one study,77 the model transition probabilities were based on risk prediction equations and patient covariates from a previously published model on angina, which allowed estimation of the occurrence of a primary cardiovascular event (with risk conditioned on factors such as age and sex) and of subsequent events conditional on having and surviving a first cardiovascular event. By contrast, the second study74 estimated the risk of primary and subsequent cardiovascular events dependent on disease severity, based on the rates of MACEs from the literature. A summary of both models is presented below.
Walker et al.77
Walker et al. 77 developed a decision tree and Markov model structure to evaluate the cost-effectiveness of eight alternative testing sequences, including different combinations of exercise treadmill testing, SPECT, cardiovascular MR and coronary angiography, to identify patients with angina who require revascularisation (i.e. those with significant stenosis) derived from the CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease) trial. 89 The study population included patients with angina (with and without significant stenosis) and those without angina, based on characteristics of patients in the CE-MARC trial. 89 The base-case analysis considered the case of a 60-year-old man, classified as grade 2 on the Canadian Cardiovascular Society (CCS) scale, with a prior likelihood of significant stenosis requiring revascularisation of 39.5%. Patients with angina were assumed to have had no previous MI. The Markov model had a 50-year time horizon with a 3-month cycle length. The perspective of the study was NHS and Personal Social Services (PSS), and health outcomes were measured in terms of QALYs. Costs were expressed in term Great British pounds (GBP) (2010/11 price year), and costs and health outcomes were discounted at a rate of 3.5% per annum.
The aim of the diagnostic testing was to identify patients with significant coronary artery stenosis who require revascularisation [either PCI or coronary artery bypass graft (CABG)]. It was assumed that all patients suspected of having significant coronary stenosis would undergo coronary angiography as a definitive test before revascularisation. ICA was considered the reference standard test with perfect sensitivity and specificity. As ICA was performed on all patients indicated for revascularisation, the model did not consider any FP test results. The diagnostic component of the model divided the patient cohort according to their underlying disease status based on characteristics of patients in the CE-MARC trial,89 survival to interventional and diagnostic procedures, test results and subsequent clinical management conditional on test results. All patients with positive and inconclusive test results progressed to a further test in the sequence, although the type of the next test depended on whether the result was positive or inconclusive for some strategies (e.g. in strategy 8 a positive exercise treadmill test result would be followed by ICA, whereas inconclusive test results would be followed by a SPECT test). Patients whose overall testing sequence resulted in a positive result were managed with either PCI or CABG. The relative proportion of patients who underwent each type of revascularisation was sourced from UK clinical registries. Patients who tested negative at any point in the test sequence were managed with optimal medication if they had angina, or with no further medical therapy for those without angina. The decision tree captures mortality associated with both invasive tests and revascularisation, and separately applies procedure-specific mortality rates for ICA, PCI and CABG. At the end of the decision tree, patients with significant stenosis could be classified as TP, FN or dead. Patients without significant stenosis could be classified as TN with angina, TN without angina or dead. All testing strategies are assumed to take the same time and do not account for delays to revascularisation resulting from strategies that involve more tests.
The diagnostic accuracy estimates for the different tests considered in the alternative strategies were conditional on positive/inconclusive results in previous tests in the strategy, thus accounting for correlations between tests within diagnostic strategies. This is possible only with access to IPD from studies that include all the tests used across the full set of diagnostic strategies, as was the case for the CE-MARC study,89 which informed diagnostic accuracy in this model. However, the people interpreting each test were blinded to the results of previous tests in each diagnostic sequence, so the data would not have captured the influence of knowledge on previous tests on the diagnostic accuracy estimates of subsequent tests.
The long-term model is composed of three submodels. Patients with significant stenosis enter one submodel at either the TP or FN state. The key difference between TP and FN patients is that TP patients have undergone revascularisation. In the base-case analysis, the treatment effect of revascularisation is limited to a reduction from angina symptoms, with improved HRQoL for TP patients compared with FN patients, whereas the same baseline risk of cardiovascular events is applied for TP and FN patients. A proportion of FN patients are assumed be correctly diagnosed over time (conditional on their CCS grade), and transition to the TP health state. Patients can remain event free, have a primary non-fatal cardiovascular event, or die from a cardiovascular event or other causes. Patients who survive a primary non-fatal cardiovascular event transition to the non-fatal cardiovascular event state and have an increased risk of further cardiovascular events for 12 months, after which they transition to the non-fatal event post 12 months state. The risk of cardiovascular events in this state is lower than in the non-fatal event post 12 months state, but higher than the baseline risk (TP and FN states). Patients in all health states are subject to a mortality risk from non-cardiovascular death, which is sourced from UK life tables (with cardiovascular deaths removed to avoid double counting). A similar submodel to the one described above, this is used to estimate the cost and health outcomes of TN patients with angina. TN patients without angina go into a two health states (alive and dead) submodel that derived transition probabilities from sex- and age-adjusted UK life tables for all-cause mortality.
The probabilities of fatal and non-fatal cardiovascular events in the submodels for patients with angina were estimated based on risk equations from the EUROPA (EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery) trial. 90 This study estimated risk equations to predict (1) the risk of a first primary event, cardiovascular death, MI or cardiac arrest (see equation 1), (2) the odds of that event being fatal (see equation 2) and (3) the risk of a further primary event in the first year after a first non-fatal event (see equation 3). The equations allow for the adjustment of the rate of events dependent on the patient characteristics (age, sex, medication, comorbidities, etc.) and, importantly, accounting for the occurrence of previous MI. Walker et al. 77 applied a fourth equation to model the risk of secondary cardiovascular events, which captures the excess cardiovascular risk for patients who had had a previous MI.
The model also considers cancer-related mortality due to radiation exposure during some testing procedures (ICA and SPECT) and PCI (assumed to be performed at the same time as ICA). The model quantified the average radiation exposure in each test sequence; these radiation dosages were then combined with cancer incidence and mortality estimates from the literature to calculate lifetime incidence and mortality conditional on the patient’s age when they were tested. The costs and morbidity associated with cancer were not modelled.
The HRQoL in the model was dependent on age, sex, CCS grade and whether or not the patient had undergone revascularisation. EuroQol-5 Dimensions (EQ-5D) utility weights by CCS grade from a study on angina were combined with UK-population norm EQ-5D estimates by age and sex to obtain age- and CCS-specific HRQoL estimates. The underlying assumption was that the relative impact of CCS grade on HRQoL compared with the population is the same across all age groups.
One important base-case assumption of the Walker et al. 77 model is that revascularisation has no impact on the risk of cardiovascular events, and provides relief only from angina symptoms (captured by change in CCS score). HRQoL scores for patients with angina (with and without significant stenosis) are based on age- and sex-adjusted UK population scores with a relative adjustment made based on CCS grade. Data from a RCT comparing coronary angioplasty with medical management was used to link CCS scores at baseline and 6 months after intervention with the two treatments. Patients with angina and significant stenosis who receive revascularisation (TP) are attributed the HRQoL based on the average CCS grade of those following treatment with angioplasty conditional on initial CCS grade. Patients with angina and significant stenosis who are misclassified (FN) are attributed the HRQoL based on the average CCS grade of those following treatment with medical management conditional on initial CCS grade. It was assumed that angina patients without significant stenosis received the same HRQoL as FN patients, whereas the HRQoL of the other TN patients without angina was based on age- and sex-adjusted UK population scores.
Costs included in the model were those of tests and interventional procedures, treatment costs in the long-term model and health-state costs (namely fatal and non-fatal cardiovascular events, and other-cause mortality). Treatment and health-state costs were also sourced from the EUROPA trial90 (with a price year inflation adjustment). Background treatment costs were the same for all patients with angina and an additional background cost was applied for patients after a cardiovascular event. Patients without angina were assumed to have no costs in the long-term model.
The authors considered uncertainty by performing probability sensitivity analysis and scenario analysis where they varied assumptions on baseline characteristics (CCS grade, sex and age), prior likelihood of coronary heart disease requiring revascularisation, rediagnosis rate of FN patients, clinical management of TP patients, the impact of radiation exposure on cancer (risk assumed to be zero), risk of cardiovascular events following revascularisation (treatment effect from the EUROPA trial90), HRQoL decrements and the cost of diagnostic tests. The model was sensitive to prior likelihood of disease, reducing the starting age and increasing baseline CCS grade in the model, use of absolute HRQoL decrements by CCS grade, allowing for a proportion of TP patients to not receive revascularisation, reidentification rate of FN patients, and costs of tests. The prior likelihood of coronary heart disease requiring revascularisation was considered a key driver of cost-effectiveness.
Genders et al.74
The model developed by Genders et al. 74 was a microsimulation model comprising a decision tree and a lifetime state-transition model to assess the cost-effectiveness of invasive and non-invasive testing strategies for patients with stable chest pain. The base-case population consisted of 60-year-old patients with a 30% pretest probability of obstructive CAD (defined as ≥ 50% stenosis on at least one vessel) who had never undergone revascularisation procedures and had no prior history of CAD. The study presents cost-effectiveness results for the separate jurisdictions. We refer here to inputs and results specific to the analyses under the UK NHS perspective, as they are more relevant to our study. Costs were calculated in GBP (2011 price year), and health outcomes were calculated as QALYs. Both costs and QALYs were discounted at an annual rate of 3.5%.
The diagnostic strategies in the model are evaluated under two different diagnostic workups. In the invasive workup, patients with obstructive CAD on CCTA and patients with inducible ischaemia on cardiac stress imaging were referred for ICA prior to a decision regarding medical management. In the conservative workup, only patients identified as having higher CAD severity by CCTA or cardiac stress imaging would be referred to ICA and patients with milder forms of the disease managed with OMT. Patients with normal arteries or mild CAD (< 50% stenosis) received no further testing under either diagnostic workup.
The decision tree starts by classifying patients according to eight categories of disease severity based on percentage stenosis, number of vessels affected, location of lesion (left main trunk or not) and severity of inducible stenosis (where present). Patient distribution across disease severity categories was sourced from hospital records for patients who had undergone CCTA and ICA. Diagnostic accuracy estimates derived from published meta-analyses were then applied to split patients according to the test results for each diagnostic strategy. For the purpose of applying these estimates, patients who were considered correctly classified with a negative result had normal coronary arteries or mild CAD (< 50% stenosis). Patients correctly identified with a positive result had moderate CAD, severe CAD or three-vessel disease/left main coronary stenosis. The model did not consider inconclusive test results. The authors assumed independence of diagnostic accuracy estimates for CCTA and cardiac stress imaging, and further assumed that FP results were possible only for mild CAD and mild inducible ischaemia (under the conservative diagnostic workup). Patients with FP results are assumed to receive unnecessary optimal medication for the full time horizon, incurring a treatment cost and utility decrement in the long-term model. As in Walker et al. ,77 adverse events from testing and revascularisation procedures were considered. However, in this model adverse events are not limited to procedural mortality, but also include non-fatal MI with ICA. This adverse event had a cost attributed to it, but did not translate into an increased risk of further events in the long-term model.
The decision tree splits the patient population according to disease severity, test results and survival to testing (ICA and FFR) and revascularisation procedures. It also allows quantifying the average exposure to radiation with the different tests and PCI.
In the ICA strategy, all patients were tested with ICA. Those who tested negative received risk factor management and those who tested positive would be tested with FFR to decide treatment. ICA is assumed to be a perfect test, and FFR appears to allow prefect distinction between disease severity categories, although this is not explicitly stated in the paper. OMT was then given to patients with mild ischaemia and moderate to severe CAD, PCI was given to patients with severe CAD and severe ischaemia, and CABG was given to patients with three-vessel disease or left main coronary stenosis. Revascularised patients would also receive OMT, and all individuals in the model received risk factor management.
Subsequent to the decision tree, patients entered a state-transition model comprising three health states: alive, post MI and dead. Patients enter the model through the alive state, where they could remain until death or suffering a non-fatal MI. Patients who suffered a non-fatal MI would transition to the post-MI state, where they could remain or transition to the dead state. The transition probabilities were derived from published trial data that reported risk of MACE (cardiovascular death, non-fatal MI and repeated revascularisation) in patients treated with CABG, PCI and OMT. The rates of MACE were dependent on disease severity and whether patients were treated with optimal medication or revascularisation. All FN patients were assumed to be correctly identified and treated by the end of the first year, with the exception of those with moderate CAD without ischaemia, of whom only 25% were rediagnosed. Patients who experienced a primary cardiovascular event would have a higher risk of subsequent cardiovascular events, which was modelled by applying a HR of 1.44 to their baseline risk. The model also considered mortality from non-cardiovascular causes. This was estimated based on age- and sex-specific general mortality data from which deaths attributed to cardiovascular causes had been removed to avoid double counting. The mortality, morbidity and costs due to cancer incidence were not modelled, although the model calculated cumulative radiation exposure over the time horizon.
The risk of MACE was estimated from the trial data separately for the first year and all subsequent years to allow for a higher event rate in the first year after starting treatment. The rates were estimated based on the CABG arm of the SYNTAX trial59 for patients with three-vessel disease or left main coronary stenosis, and the optimal medication and PCI arms of the COURAGE trial91 for the patients with suspected or mild inducible ischaemia and moderate to severe CAD (treated with optimal medication) and patients with severe CAD and severe inducible ischaemia (treated with PCI). The reciprocal of the treatment HR was applied to this risk to estimate the baseline probability of cardiovascular events for untreated patients (FN), who have a higher rate of events until they are correctly diagnosed. A single treatment effect hazard for optimal medication, PCI and CABG (HR 0.70) was sourced from three meta-analyses of OMT comparing treatment with no treatment, but it is unclear how this estimate was calculated. The rates of MACE applied in the model are summarised in Appendix 7, Table 57.
We note that the MACE rates without treatment seem counterintuitive (e.g. higher MACE rates for untreated moderate CAD with mild ischaemia compared with untreated severe CAD with severe ischaemia). The authors did not comment on the MACE rates.
If the treatment effect applied in the model is indeed the same for optimal medication and revascularisation, this is similar to the absence of a treatment effect of revascularisation in addition to optimal medication in Walker et al. 77 This is an important interpretation of the clinical evidence on the treatment effect of revascularisation, and one that is discussed further in Chapter 5, Treatment effect of revascularisation. In previous studies where a treatment effect on the rate of cardiovascular events for revascularisation compared with optimal medication was considered explicitly for comparable patients (e.g. same disease severity), seven models included the existence of a treatment effect68,69,76,83,85,86,88 and six studies did not,70–72,79,84,87 in line with Walker et al. 77 and Genders et al. 74
The HRQoL in the model was assigned to individuals according to disease severity and treatment received. Patients without CAD or inducible ischaemia were assumed to have the HRQoL of the general population based on age- and sex-specific EQ-5D estimates for the US population. For patients with CAD and inducible ischaemia who underwent active treatment (optimal medication or revascularisation), mapped EQ-5D utility decrements were applied to the general population HRQoL estimates. In the first year of treatment, the utility decrements of treatment relative to the general population were derived from the average utility decrement as observed in the same trial data that informed the rates of MACE for treated patients, whereas for the subsequent year the last observed value in the trials was carried forward. The authors state that a disutility was considered for patients with FP results. It was not clear how the utility decrements for FN patients were estimated. Appendix 7, Table 58, summarises the utility values for the start age in the model conditional on treatment and disease severity. The HRQoL estimates for the first year and subsequent years of treatment are presented for the same age solely for ease of comparison.
The model considers costs of tests, test adverse events, medication, MI in the long-term model, and incidental findings from CCTA. Unit costs were mostly sourced from UK published data. Based on the description of the unit cost selected for PCI, this procedure was assumed to take place in an outpatient setting. It is not, however, clear what assumptions were made regarding the setting for ICA, CABG and treatment of non-fatal MIs. The unit cost for FFR was sourced from a previous cost-effectiveness study in a US setting. 87 An annual cost of medication was included in the model according to disease severity and treatment received (OMT, PCI or CABG). The resource use assumed for patients who received optimal medication alone and in addition to PCI was sourced from the COURAGE trial,91 whereas for those who received CABG and optimal medication it was taken from the SYNTAX trial. 59 The distribution of medication use applied in the model is shown in Appendix 7, Table 59.
Model parameters were entered as distributions, and probabilistic sensitivity analysis was performed to incorporate joint parameter uncertainty. Scenario analysis was performed to test assumptions on diagnostic accuracy of stress echocardiography, cost of tests, alternative diagnostic pathways, probability of CAD, time to rediagnose FN patients, and treatment effect of optimal medication for FP patients. A subgroup analysis by sex was also performed. The authors do not identify any drivers of cost-effectiveness, but note that the assumption that FP patients will remain misclassified over the time horizon and that FN patients will be rediagnosed after 1 year is likely to have biased results against strategies with low specificity.
Conclusions of the assessment of existing cost-effectiveness evidence
The review did not identify any studies that evaluated the cost-effectiveness of QAngio XA 3D/QFR or CAAS vFFR. A supplementary review of published cost-effectiveness studies evaluating ICA (alone and/or with FFR) in the management of CAD identified 21 relevant studies. Two studies were considered to be particularly good examples of alternative modelling approaches to establish the link between short-term diagnostic outcomes and the longer-term impact and subsequent prognosis associated with the diagnostic pathways in the management of CAD and associated costs and outcomes. The modelling approaches identified in Results of the review of decision models evaluating invasive coronary angiography were used to inform the conceptualisation of the de novo model described in Chapter 5, Model structure, and allowed identifying relevant evidence sources to inform model inputs and assumptions.
Chapter 5 Independent economic assessment: York model
Overview
The review of cost-effectiveness studies in Chapter 4 identified no studies evaluating the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR for assessing the functional significance of coronary stenosis. Therefore, a de novo decision-analytic model was developed to formally estimate the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR for assessing the functional significance of coronary obstructions during ICA in patients with stable angina and intermediate stenosis, relative to the comparators of invasive FFR or iFR measurement or clinical decision-making based on visual interpretation of ICA alone, alongside clinical judgement, in the UK NHS.
In developing and populating the decision model, three issues are considered central to the approaches and methods employed:
-
the need to link the diagnostic accuracy of QFR and vFFR to short-term costs and consequences [e.g. the impact on the proportion of patients who need revascularisation (percutaneous or surgical), the proportion of patients who need invasive functional assessment of stenosis using FFR or iFR, and adverse event rates and HRQoL associated with the diagnostic interventions]
-
the need to link the short-term consequences to potential longer-term costs and consequences (e.g. the risk of major adverse cardiovascular events such as MI, sudden cardiac death and need for urgent/unplanned revascularisations) using the best-available evidence to ensure that differences in costs, life-years gains, and QALYs are appropriately quantified over a lifetime horizon
-
the need to ensure that the data inputs and assumptions are relevant to inform current NHS practice, with particular consideration given to any differences in the cost-effectiveness of the technologies in diagnostic-only laboratories or interventional catheter laboratories.
The decision-analytic model provides a framework for combining the diagnostic outcomes and the subsequent prognosis associated with the diagnostic outcomes over the long term, and other inputs reflecting current NHS practice. The model evaluates costs from the perspective of the NHS and PSS, expressed in GBP (2018/19 price year). Outcomes in the model are expressed in terms of QALYs. Both costs and outcomes are discounted using a 3.5% annual discount rate, in line with current NICE guidelines. 92 The model was developed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
The model is probabilistic in that uncertainty in input parameters are reflected through the use of appropriate probability distributions, rather than using fixed mean estimates for input parameters. 93 A Monte Carlo simulation is used to propagate uncertainty in input parameters through the model to capture the uncertainty in overall results. Scenario analyses are undertaken to explore the robustness of the cost-effectiveness results to changes in the parameter inputs and assumptions of the model.
The following sections outline the decision problem and the structure of the model, and provide an overview of the key assumptions and data used to populate the model.
Decision problem and population
The decision problem addressed by the model relates to the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR imaging software used during ICA for assessing the functional significance of coronary stenosis in patients with stable angina whose angiograms show intermediate stenosis.
The model considers this in the context of the NICE Clinical Guideline Pathway,94 where ICA is used to guide the treatment strategy for patients with a confirmed diagnosis of stable CAD of uncertain functional significance and whose symptoms are not satisfactorily controlled with OMT and so may require revascularisation.
QAngio XA 3D/QFR and CAAS vFFR can be used in the same clinical settings where ICA is performed. These settings include diagnostic-only laboratories and interventional catheter laboratories. One key difference between the two settings is that assessments with FFR/iFR are performed only in interventional catheter laboratories. When patients assessed in a diagnosis-only laboratory require a FFR/iFR measurement because of inconclusive ICA results, they have to be referred to an interventional catheter laboratory. Therefore, inconclusive results obtained with QAngio XA 3D/QFR and CAAS vFFR that require confirmation with FFR/iFR would also need to be referred to an interventional catheter laboratory. In contrast, a FFR/iFR assessment can be performed immediately after ICA, QAngio XA 3D/QFR or CAAS vFFR in an interventional catheter laboratory if needed.
The target population of the model consists of patients with stable CAD whose angiograms taken during ICA show intermediate stenosis. Although various definitions of intermediate stenosis exist, the modelled population considers intermediate stenosis to be any stenosis where there is clinical uncertainty about its functional significance and the potential appropriateness of revascularisation.
No subgroup data are available to permit a separate consideration of subpopulations.
Diagnostic strategies
The aim of diagnostic testing is to identify patients with functionally significant coronary stenosis who would benefit from revascularisation (PCI or CABG), in addition to OMT. As ICA is required to show intermediate stenosis, the starting diagnostic test is visual assessment with ICA. In the absence of other tests, clinical decision-making would be based on visual interpretation of the images taken during ICA, alongside clinical judgement. However, ICA alone is not sufficient to indicate whether anatomical obstructions are functionally significant or functionally non-significant; therefore, confirmatory FFR/iFR is considered the reference standard test for functional assessment of coronary obstructions. Because FFR/iFR is regarded as the ‘gold standard’ diagnostic test for assessing functional significance of stenosis, it is assumed to have perfect sensitivity and specificity of 100%. This means that all patients would receive an appropriate treatment based on the results of FFR/iFR (either revascularisation for TP test results or OMT for TN test results). The comparator diagnostic tests for QAngio XA 3D/QFR and CAAS vFFR are ICA alone (without the functional assessment of coronary obstructions) or ICA, followed by invasive FFR/iFR measurement using pressure wire.
The interventions are clinical decision-making based on QAngio XA 3D/QFR and CAAS vFFR (used during ICA), alongside clinical judgement. These technologies are alternatives to pressure wire FFR/iFR and provide a non-invasive means to simulate FFR measurement during ICA assessment. The technologies may also be used as a precursor to invasive FFR/iFR, with the invasive pressure wire FFR/iFR only used as a confirmatory procedure when QFR or vFFR test results are inconclusive.
The decision model evaluates the following five diagnostic strategies:
-
ICA alone (i.e. visual interpretation of angiographic images taken during ICA without additional testing to assess the functional significance of intermediate stenosis)
-
ICA, followed by confirmatory FFR/iFR (reference standard)
-
ICA with QFR using QAngio XA 3D/QFR
-
ICA with QFR using QAngio XA 3D/QFR, followed by confirmatory FFR/iFR if the QFR is inconclusive
-
ICA with vFFR using CAAS vFFR.
The strategy of ICA alone, referred herein as strategy 1, is based on the use of a diagnostic threshold of 50% DS to define the need for revascularisation (i.e. ≥ 50% DS in the left main coronary artery, alongside clinical judgement, is sufficient to indicate the need for revascularisation). Strategy 1 means that treatment decisions based on DS and clinical judgement alone are used to stratify treatment decisions. However, it is widely accepted that DS has only modest correlation with physiological indexes of myocardial ischaemia such as FFR/iFR. Therefore, the more appropriate comparator strategy for the new technologies is ICA followed by confirmatory FFR/iFR, referred herein as strategy 2. The diagnostic threshold for strategy 2 is a FFR value of 0.8, where revascularisation can be safely deferred for stenoses with a FFR > 0.8, whereas stenoses with a FFR ≤ 0.8 are functionally significant and should be considered for revascularisation.
The strategy of ICA with QFR, referred herein as strategy 3, is based on the use of a single diagnostic threshold of 0.8 to define functionally significant and non-significant stenoses. A QFR value of ≤ 0.8 is considered a significant obstruction and revascularisation should be considered, whereas stenoses with a QFR value > 0.8 are considered to be functionally non-significant and revascularisation can be deferred (i.e. patients receive OMT alone).
The strategy of ICA with QFR, followed by confirmatory FFR/iFR when QFR is inconclusive, referred herein as strategy 4, is considered an alternative strategy to strategy 3. In strategy 4 a dual threshold is used to represent a ‘hybrid’ approach of QFR, followed by FFR when the test results of QFR are inconclusive (grey zone). A QFR value below 0.78 is considered to have sufficiently high accuracy to indicate functionally significant stenosis and that revascularisation should be considered, whereas a QFR value above 0.84 is considered to have sufficiently high accuracy to indicate functionally non-significant stenosis and that revascularisation may be deferred (i.e. patients receive OMT alone). QFR values that are inconclusive and lie in the grey-zone region (0.78–0.84) should be verified by invasive FFR/iFR measurement before a decision is taken on the need for revascularisation. This strategy uses the same grey-zone region as described in the QAngio XA 3D/QFR instructions and corresponds to Medis Medis Medical Imaging Systems BV’s recommended use of the technology.
The strategy of ICA with vFFR, referred herein as strategy 5, is the same as strategy 3 but with the alternative technology CAAS vFFR rather than QAngio XA 3D/QFR. A vFFR value of ≤ 0.8 is considered a significant obstruction and indicates revascularisation should be considered, whereas stenoses with a vFFR value of > 0.8 are considered to be functionally non-significant and indicate revascularisation can be deferred.
Note that it is not possible to consider a sixth strategy using CAAS vFFR, followed by confirmatory FFR/iFR, when vFFR is inconclusive because there is no diagnostic accuracy data available to inform this strategy (and it is not possible to infer diagnostic information from the very limited diagnostic data available for vFFR) (see Chapter 3, CAAS vFFR).
For all strategies, patients who receive revascularisation (TP and FP patients) are assumed to be treated with PCI or CABG. The proportion of patients receiving either procedure is independent of the diagnostic strategy with fixed proportions assumed of PCI (87%) and CABG (13%) for all strategies (see Complications due to revascularisation).
Model structure
The model is made up of two components: a diagnostic element that characterises the diagnostic outcomes and costs and consequences associated with diagnostic testing and revascularisation, and a longer-term prognostic element that considers the subsequent prognosis associated with the diagnostic outcomes and associated costs and consequences of treatment over the remaining lifetime of a patient.
The period represented by the diagnostic element (referred herein as diagnostic model) takes account of the diagnostic accuracy of the non-invasive functional tests (QAngio XA 3D/QFR and CAAS vFFR used during ICA) relative to the reference standard measurement using the invasive test of FFR/iFR (assumed to have a sensitivity and specificity of 100%). Patients correctly identified as having functionally significant stenosis (TP result) will progress to revascularisation, in addition to OMT, whereas patients correctly identified as having functionally non-significant stenosis (TN result) will receive OMT without the need for revascularisation. Patients incorrectly identified as having functionally significant stenosis (FP result) will undergo unnecessary revascularisation, whereas patients incorrectly identified as not having functionally significant stenosis (FN result) will not receive an appropriate revascularisation procedure. The non-invasive tests may also lead to inconclusive results about the functional significance of stenosis, which leads to further invasive testing with pressure wire FFR/iFR to confirm whether or not there is a need for revascularisation.
The longer-term prognostic element of the model (referred herein as prognostic model) takes account of the impact and subsequent prognosis associated with the diagnostic outcomes and models the risk of MACEs such as MI, sudden cardiac death and need for urgent/unplanned revascularisation, as well as adverse events related to revascularisation and MI. The costs and HRQoL implications of treatment are modelled over a lifetime horizon.
Diagnostic model
Diagnostic outcomes are modelled with a decision tree, which takes account of the diagnostic accuracy of the tests and subsequent treatment pathway. The decision tree is constructed to compare the TP, FP, TN and FN rates of the alternative diagnostic strategies.
The decision tree starts with the alternative diagnostic strategies that are used to diagnose the functional significance of stenosis. The outcomes of each strategy are governed by the sensitivity and specificity of the particular test strategy. The accuracy of the tests is defined independently of disease prevalence (i.e. underlying prevalence of functionally significant stenosis in the population); however, the expected proportion of tests with positive and negative results in the population is dependent on the underlying prevalence. Therefore, for all strategies, patients are separated into their ‘true’ status of either functionally significant stenosis or functionally non-significant stenosis based on the distribution of the population with a FFR value ≤ 0.8. Patients with functionally significant stenosis requiring revascularisation are allocated to one of three outcome states as a result of the diagnostic strategy: (1) TP, who are correctly identified and treated with revascularisation, (2) FN, who are misidentified and do not receive revascularisation, and (3) death as a result of the mortality risks associated with the diagnostic and revascularisation procedures. Patients without functionally significant stenosis who do not require revascularisation are also allocated to one of three outcome states as a result of the diagnostic strategy: (1) TN, who are correctly identified and treated with OMT, (2) FP, who are misidentified and receive an inappropriate revascularisation, and (3) death as a result of the mortality risks associated with the diagnostic and revascularisation procedures. For revascularisation, a proportion of patients are assumed to be treated with either PCI or CABG, in addition to OMT.
A schematic of the diagnostic model for each of the five strategies is shown in Figures 12–16 (with outcome of death not shown in the figures for simplicity). Strategies 1 (ICA alone), 3 (ICA plus QFR) and 5 (ICA plus vFFR) have four possible diagnostic test results of TP, FN, FP and TN based on the diagnostic accuracy of the tests relative to the reference standard test of FFR ≤ 0.8. Strategy 2 is the reference standard test (ICA plus FFR), and TP and TN are the only diagnostic outcomes under this strategy because FFR/iFR is assumed to be a perfect test (100% sensitivity and specificity). Strategy 4 is the hybrid approach of QFR where the possible diagnostic outcomes are TP, FN, FP and TN for those considered to have conclusive QFR (values outside the grey zone), whereas TP and TN are the only possible outcomes for those with inconclusive QFR (grey zone) because these patients undergo confirmatory FFR/iFR. Death can occur as a result of the diagnostic and revascularisation procedures.
FIGURE 12.
Strategy 1 of ICA alone, without additional testing to assess the functional significance of stenosis.
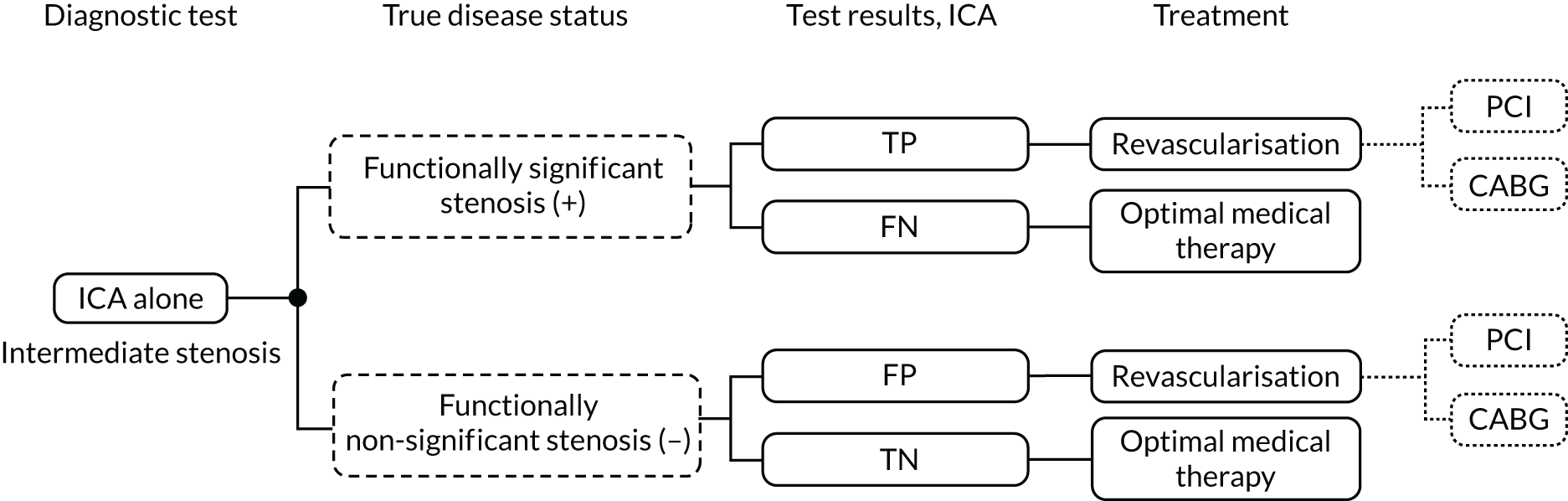
FIGURE 13.
Strategy 2 of ICA, followed by confirmatory FFR/iFR.
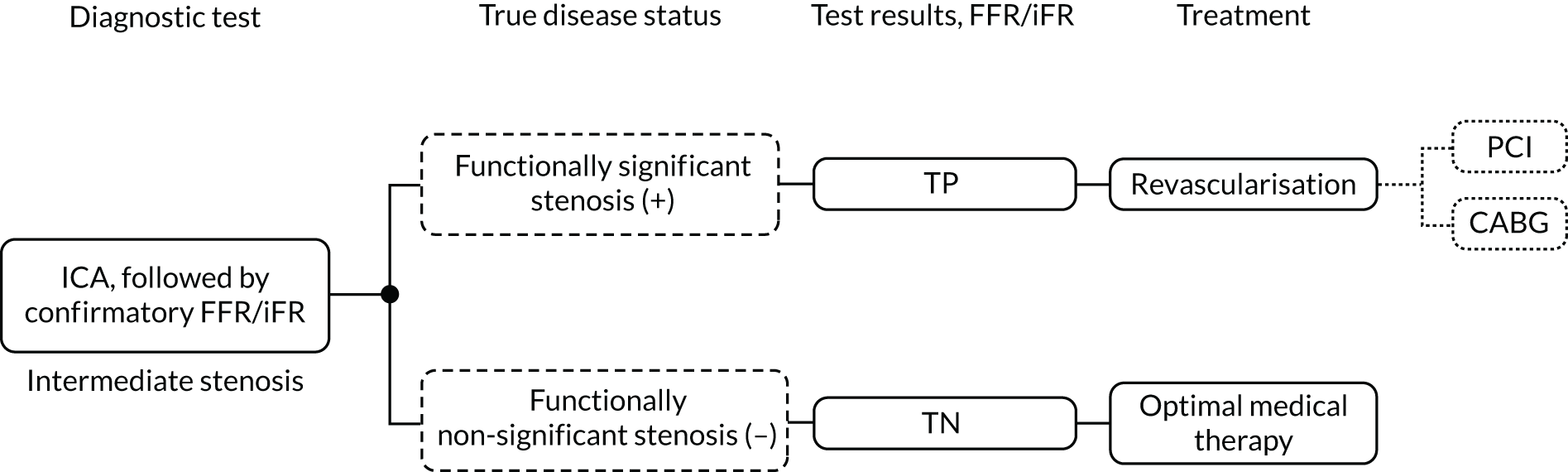
FIGURE 14.
Strategy 3 of ICA with QFR.
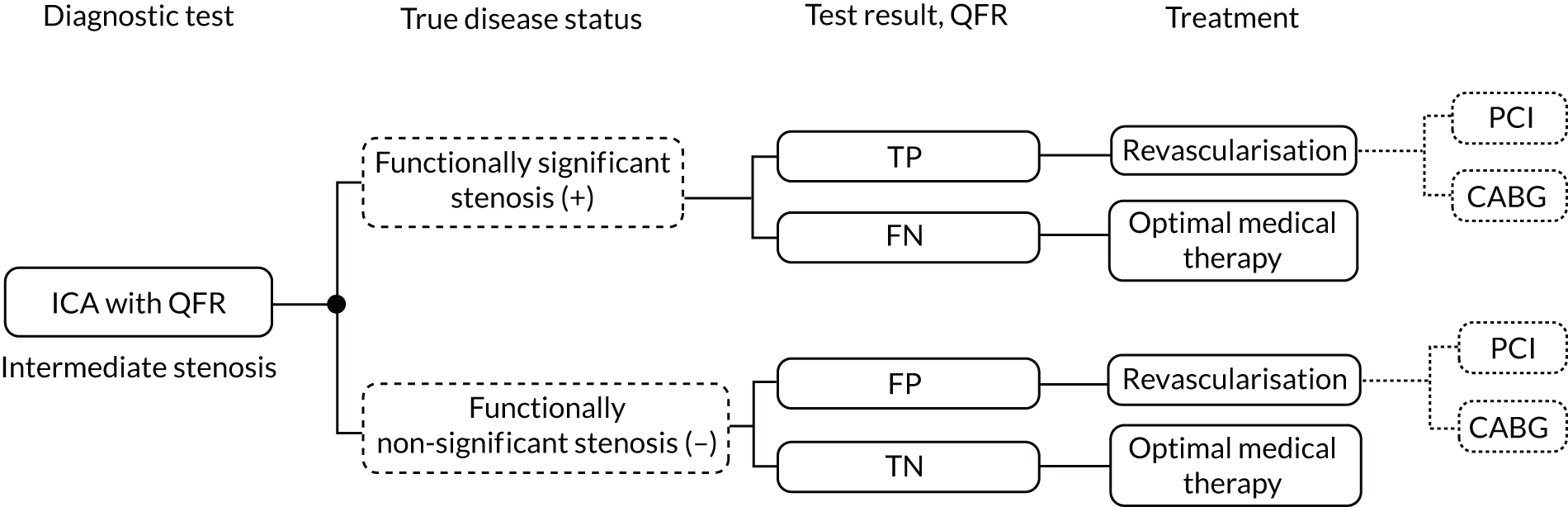
FIGURE 15.
Strategy 4 of ICA with QFR, followed by confirmatory FFR/iFR when QFR is inconclusive.
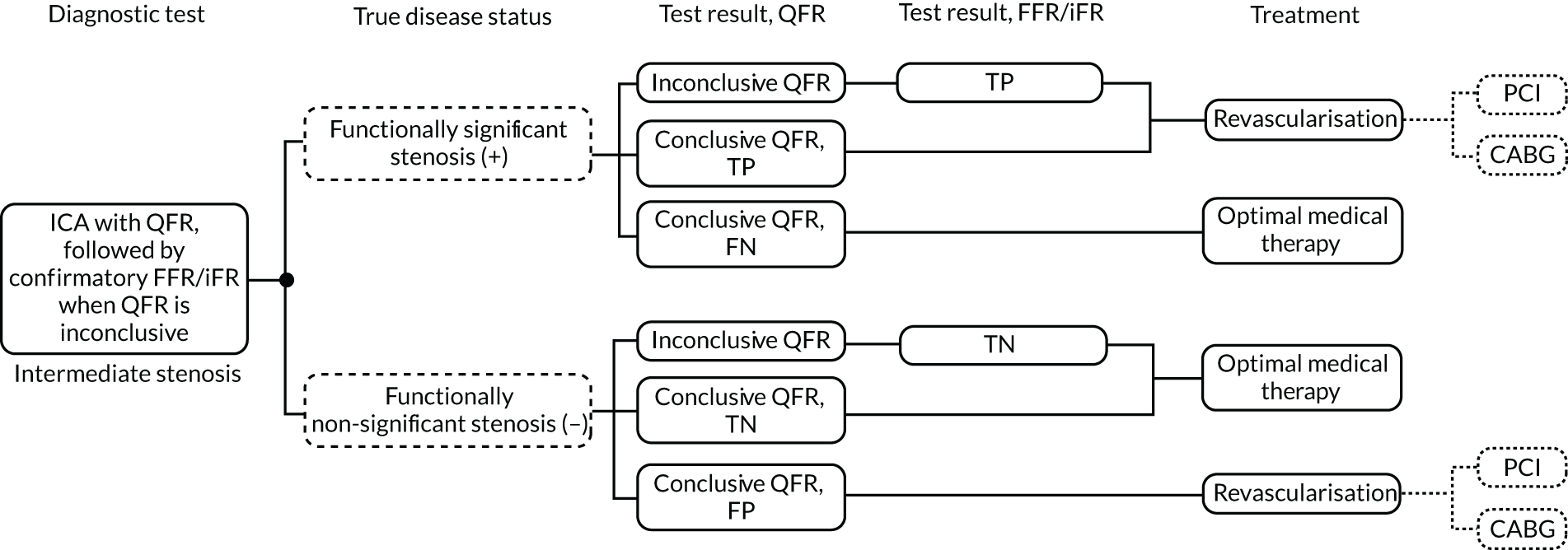
FIGURE 16.
Strategy 5 of ICA with vFFR.
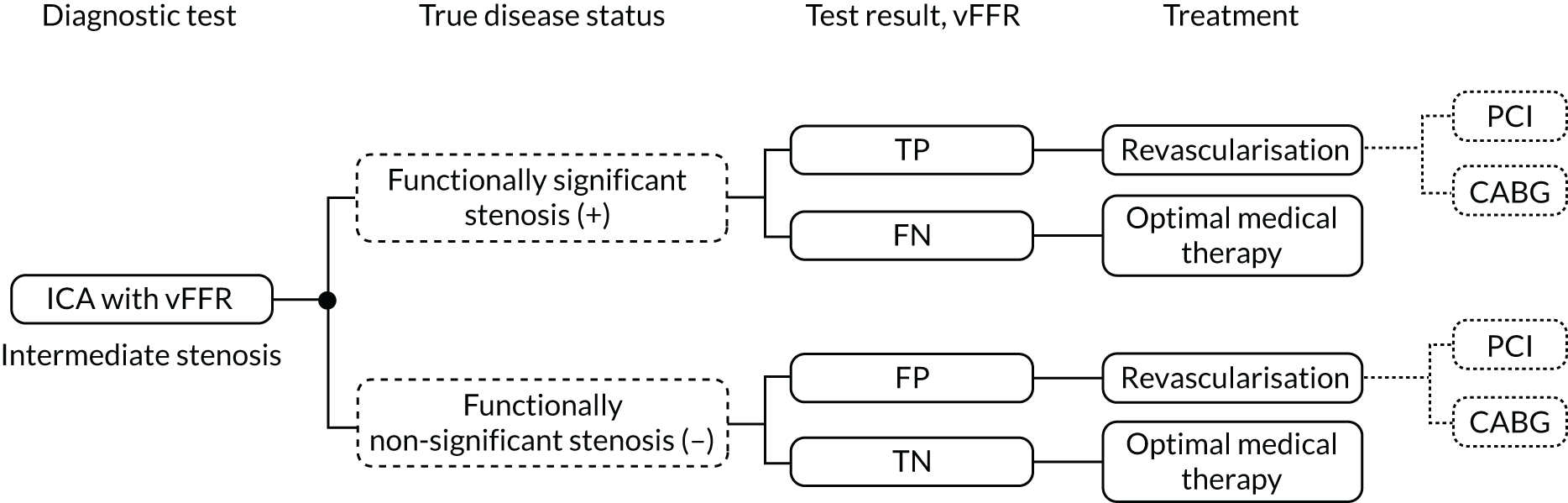
The model assumes that all diagnostic tests in each strategy are performed in the same medical appointment, and that revascularisation procedures are performed either immediately after testing or without a delay that might lead to a deterioration of the patient’s condition. Therefore, the base-case analysis is more representative of an interventional setting. The assumption that all diagnostic tests in each strategy are performed in the same medical appointment is relaxed in a scenario analysis so as to explore the cost-effectiveness of the strategies in a diagnostic-only setting.
For the diagnostic model, costs are incurred according to the type of diagnostic test, adverse events associated with FFR, and treatment received. Procedural HRQoL loss is included for FFR and revascularisation. Costs, HRQoL and mortality effects associated with ICA are excluded from the model because these are incurred equally across all strategies.
The diagnostic model represents the start of the long-term prognostic model. The proportion of patients starting in the health states in the prognostic model is based on the expected proportion of tests with positive and negative results in the population (TP, TN, FP, FN).
Prognostic model
The prognostic implications of receiving treatment (either revascularisation in addition to OMT or OMT alone) based on being in one of the four diagnostic outcome states (TP, FN, FP or TN) is quantified using a Markov model that captures the progression of disease through the risk of MACEs and associated costs and consequences, and the risk of death from non-cardiac causes, over a lifetime horizon. A cycle length of 1 year is used in the model. A schematic of the model structure is shown in Figure 17.
FIGURE 17.
Schematic of prognostic model. CV, cardiovascular.
Patients with stable CAD and intermediate stenosis enter the model in one of four diagnostic outcome health states: TN (functionally non-significant stenosis with FFR > 0.8 and received OMT), FN (functionally significant stenosis with FFR ≤ 0.8 and received OMT), TP (functionally significant stenosis with FFR ≤ 0.8 and have undergone revascularisation), FP (functionally non-significant stenosis with FFR > 0.8 and have undergone revascularisation) or death.
All patients alive may remain in their initial health state over time with no MACEs, or have a primary MACE. The primary MACE is defined as cardiovascular death, non-fatal MI and unplanned (urgent) revascularisation, where the risk differs between the first year and subsequent years from model entry. Each MACE is assumed to be mutually exclusive. If the primary MACE is fatal, the patient enters an absorbing state of cardiovascular death. If the event is a non-fatal MI, they enter the post-MI health state where the risk of a subsequent cardiovascular event (cardiovascular death or MI) is increased as a result of having had a previous MI. Patients in the post-MI state are assumed to not be at further risk of unplanned revascularisation. If the primary MACE is an unplanned revascularisation, it is assumed that patients will receive an appropriate revascularisation procedure, with the same associated costs and risks as those patients who entered the model in the TP state (i.e. it is assumed that patients who require a subsequent targeted revascularisation have the same risk of MACE as patients who enter the model in the TP state, under the assumption that the need for urgent revascularisation is indicative of functionally significant stenosis with FFR ≤ 0.8). Patients enter the unplanned revascularisation event state for one cycle only and, for the following 12 months, are at an increased risk of cardiovascular death or MI compared with subsequent years. Those who have a cardiovascular death or non-fatal MI during this period enter the cardiovascular death state or post-MI state, respectively. If no event occurs in 12 months following an unplanned revascularisation, the patient moves into the post-revascularisation health state, where the risk of cardiovascular death or MI relates to the risk post 12 months from the TP state. From any of the states in the model where patients are alive, there is a competing risk of a non-cardiovascular death.
Model input parameters
Patient population
The population consists of patients with stable CAD whose angiograms taken during ICA show intermediate stenosis. The age and sex distribution varies across the studies informing the diagnostic accuracy of the technologies (mean age ranging from 61 to 72 years and proportion of males from 67% to 81%). The IRIS-FFR registry is the largest registry to investigate the prognosis of coronary stenosis assessed by FFR. The mean age and proportion of males in the IRIS-FFR registry was 64 years and 72%, respectively. These values are within the range reported in the diagnostic accuracy studies and the patient populations of the largest RCTs undertaken to evaluate clinical outcomes of revascularisation for patients with stable CAD [e.g. the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA),95 mean age 64 years and 77% male; the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial,96 mean age 62 years and 70% male; and the COURAGE trial,91 mean age 62 years and 85% male]. These studies, however, were mainly or wholly undertaken outside the UK. The smaller ORBITA (Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina) trial,97 which enrolled patients with stable angina and angiographically severe single-vessel CAD at five UK sites, had a mean age of 66 years and was 73% male. The IRIS-FFR registry includes mostly patients with stable angina (76%), although 18% had unstable angina and nearly 6% had NSTEMI/STEMI.
Given that the IRIS-FFR registry is the largest registry to investigate the prognosis of coronary stenosis assessed by FFR and that the mean age and proportion of males is very similar to the ORBITA trial,97 this is used to inform the base-case population in the model (mean age 64 years and 72% male).
Prevalence of functionally significant stenosis
The prior likelihood of functionally significant stenosis in the population is based on the distribution of FFR values ≤ 0.8 in the recreated individual-level patient data used to inform the diagnostic accuracy of QFR compared with FFR [see Chapter 3, Meta-analyses of data extracted from figures (QAngio XA 3D/QFR)].
In the absence of alternative individual-level data to provide the underlying distribution of FFR/iFR values in the population, the analysis assumes that the population in the QAngio XA 3D/QFR studies is reflective of the UK population in terms of underlying prevalence of functionally significant stenosis for which the technologies (QAngio XA 3D/QFR and CAAS vFFR) would be used.
In the base-case analysis, the FFR distribution is based on the subset of studies that jointly reported values of FFR and cQFR or non-specified QFR to be consistent with the set of studies informing the diagnostic accuracy for strategy 4, which considers QFR followed by FFR when the test results of QFR are inconclusive (see QAngio XA 3D/QFR and CAAS vFFR). This distribution of FFR values suggests a prior likelihood of functionally significant stenosis of 40.2% based on the proportion of participants who had a FFR ≤ 0.8.
Patient throughput
The cost of the diagnostic tests (QAngio XA 3D/QFR and CAAS vFFR) per patient depends on the average annual throughput per centre. To estimate this throughput, assumptions about patient eligibility for testing with FFR/iFR are combined with data from the BCIS audit return (data from the 2017/18 BCIS audit return98 are used, as the information in the 2018/19 audit99 is only partially reported).
Patients with stable CAD are expected to constitute approximately one-third of patients who undergo ICA in the UK (Dr Gerald Clesham, Essex Cardiothoracic Centre, Mid and South Essex NHS Foundation Trust, 2020, personal communication). In 2017/18, and in an interventional setting, the average annual number of patients undergoing ICA in the NHS was 205,085, and the average number of ICA procedures per centre was 2093 (see Appendix 8, Table 60). 98
Under a very broad definition of intermediate stenosis, where all stable CAD patients would undergo confirmatory testing with FFR/iFR, the average annual throughput for an NHS interventional centre could be as much as one-third of the average number of ICA procedures per centre, which would yield an expected upper bound of 698 patients per centre for annual throughput in an NHS interventional setting (which is the setting considered in the base-case analysis; see Diagnostic model and Setting).
A more realistic assumption would be to consider that only patients with stable CAD who currently undergo FFR/iFR have intermediate stenosis. According to the BCIS audit returns,98 21,098 pressure wire procedures were performed annually in the UK in 2017/18 (11,726 for diagnostic-only purposes and the rest followed by PCI). This gives an average of 215 pressure wire procedures per intervention centre per year. In the base-case analysis, it is assumed that the majority of these procedures are performed in stable CAD in patients with intermediate stenosis. An average annual throughput of 200 patients per centre is assumed in the base case. Alternative throughput assumptions are considered in a scenario analysis.
Setting
In UK clinical practice, ICA can be used in two settings: diagnostic-only and interventional catheter laboratories. QAngio XA 3D/QFR and CAAS vFFR can be used in the same settings as ICA, whereas FFR/iFR is currently performed only in an interventional setting.
The base-case analysis assumes that the tests in each of the five diagnostic strategies are performed at the same medical appointment and, thus, implicitly assumes an interventional setting. Although the large majority of ICA procedures in the NHS are conducted in an interventional setting (see Appendix 8, Table 60),98 it is important to consider the differences between settings that may affect the cost-effectiveness of the strategies under comparison.
The key difference between diagnostic-only and interventional catheter laboratories is that FFR/iFR can be performed only in the latter. In a diagnostic-only setting, strategies that include FFR/iFR in the testing sequence (strategies 2 and 4) require that at least some patients undergo two separate diagnostic catheterisation procedures. The initial catheterisation corresponds to the ICA that is common to all strategies in the model and is performed in the diagnostic-only catheter laboratory. Patients who have an inconclusive QFR measurement with QAngio XA 3D/QFR (patients in the grey zone for strategy 4) and all patients in strategy 2 are referred to an interventional catheter laboratory where they undergo a second catheterisation to obtain a FFR/iFR measurement. This is in contrast with how patients would be tested in an interventional setting, as all tests could be performed with a single catheterisation, and at the same point in time. One of the implications of conducting two separate catheterisations is that strategies that involve FFR/iFR will be more costly in a diagnostic-only setting than in an interventional setting. Another potential consequence of this is that the condition of patients initially tested in a diagnostic-only setting deteriorates while waiting for the referral to the interventional catheter laboratory and subsequent clinical management with revascularisation where appropriate. However, the delays to patient management are unlikely to result in significant condition deterioration with impact on patients’ health outcomes (Dr Gerald Clesham, personal communication). This is supported by evidence of the ORBITA trial97 (see Treatment effect of revascularisation), which showed that PCI compared with a placebo (mock PCI) did not demonstrate a statistically significant increase in the exercise time or change in HRQoL of patients with medically treated angina and severe coronary stenosis at 6 weeks post procedure. 97
Another difference between settings is the expected annual patient throughput in diagnostic-only compared with interventional catheter laboratories. Appendix 8, Table 60, shows that, on average, 584 patients undergo ICA in a diagnostic-only setting compared with 2093 in an interventional setting. However, the proportion of patients with stable CAD who undergo ICA in a diagnostic-only setting is likely to be higher than in an interventional catheter laboratory. The expected patient throughput in a diagnostic-only setting is unknown but needs to be considered when evaluating the cost-effectiveness of the alternative diagnostic strategies, as it determines the cost of the QAngio XA 3D/QFR and CAAS vFFR tests. This is discussed further in Test costs.
It is possible that the diagnostic accuracy of QAngio XA 3D/QFR and CAAS vFFR is also linked to the diagnostic setting. The diagnostic accuracy studies identified in Chapter 3 do not provide evidence to ascertain whether or not there are any differences in the diagnostic accuracy of these technologies across settings, as the patient population in the review may not represent patients examined in a diagnostic-only setting. This is because only patients with a FFR measurement could be included in the diagnostic accuracy review (see Chapter 3, Selection criteria).
Finally, it is possible that there are additional training requirements for QAngio XA 3D/QFR and CAAS vFFR in diagnostic-only catheter laboratories, as staff in these centres may need more training and support to correctly calculate and interpret the QFR and vFFR measurements. It is, however, uncertain what resource use is associated with these additional training requirements, so this could not be reflected in the cost-effective analysis.
The base-case scenario assumes that all diagnostic procedures take place in an interventional setting. The diagnostic-only setting is considered in scenario analyses where the impact on cost-effectiveness estimates of the following is explored: (1) additional costs due to the need to refer patients who require FFR/iFR measurements to an interventional catheter laboratory and (2) alternative throughput assumptions (see Results of the alternative scenario analyses).
Diagnostic accuracy
The model considers the diagnostic accuracy of ICA, QFR and vFFR, and FFR/iFR is the reference standard test with 100% sensitivity and specificity. The diagnostic accuracy of iFR is assumed to be equivalent to that of FFR. The definition of functionally significant stenosis is based on a FFR value ≤ 0.8. The following sections present the diagnostic accuracy estimates used in the model.
QAngio XA 3D/QFR and CAAS vFFR
For strategies 3 (QFR) and 5 (vFFR) that consider a single diagnostic threshold of 0.8, the test results are dichotomous (either positive or negative for functionally significant stenosis) based on the estimates of sensitivity and specificity of the tests relative to the reference standard of FFR ≤ 0.8. For strategy 4, where a hybrid QFR and FFR approach is considered, the QFR test results are no longer classified as dichotomous. Under this strategy, test results are classified as positive (QFR < 0.78), negative (QFR > 0.84) or inconclusive (QFR 0.78–0.84) based on the dual thresholds of 0.78 and 0.84. Diagnostic accuracy of QFR in strategy 4 is informed by the joint probabilities of having a FFR measurement below or above the 0.8 threshold and a QFR measurement within the intervals defined by the dual thresholds of 0.78 and 0.84.
Single diagnostic threshold: sensitivity and specificity estimates
The diagnostic accuracy of QFR for strategy 3 is informed by the results of the bivariate meta-analysis reported in Chapter 3, Bivariate meta-analysis (QAngio XA 3D/QFR), which combined results of studies that reported cQFR or non-specified QFR. Alternative estimates for sensitivity and specificity based on studies reporting fQFR only and cQFR only are considered in separate scenario analyses. Table 9 presents the diagnostic accuracy estimates for QFR in strategy 3 used in the base-case analysis and alternative scenarios.
| Test | Analysis | Sensitivity (%) | Specificity (%) | Source |
|---|---|---|---|---|
| QAngio XA 3D/QFR | Base case | 84.34 | 89.80 | Bivariate meta-analysis (see Table 3) for combined cQFR and non-specified QFR |
| Scenario 1 | 81.61 | 84.93 | Bivariate meta-analysis (see Table 3) for fQFR | |
| Scenario 2 | 84.32 | 91.40 | Bivariate meta-analysis (see Table 3) for cQFR | |
| CAAS vFFR | Base case | 97.00 | 74.00 | FAST-EXTEND (2019)18 |
| Scenario 4 | 75.00 | 46.50 | ILUMIEN I (2019)19 | |
| Scenario 5 | 68.20 | 87.30 | Jin et al. (2019)26 |
The diagnostic accuracy of vFFR for strategy 5 is informed by the sensitivity and specificity estimates of the FAST-EXTEND study,18 an update on the FAST study. 56 This study is chosen to represent the base-case analysis as it is the largest (n = 303) of the four included CAAS vFFR diagnostic accuracy studies (see Chapter 3, CAAS vFFR). 18,19,26,56 A pooled meta-analysis is not considered appropriate because of the limited reported data, wide CIs, and high heterogeneity across the limited number of vFFR studies. The limited number of vFFR studies means that the diagnostic accuracy estimates for strategy 5 are highly uncertain and the outcomes of this strategy must be interpreted with caution. The robustness of the cost-effectiveness results to alternative estimates about the diagnostic accuracy of vFFR is considered in scenario analyses, whereby the estimates are informed by the remaining vFFR studies. Table 9 presents the diagnostic accuracy estimates for vFFR in strategy 5 used in the base-case analysis and alternative scenarios.
The sensitivity and specificity estimates in the model are randomly drawn from probability distributions to reflect uncertainty in these parameters. Where diagnostic accuracy was sourced from meta-analyses, the log-odds sensitivity and specificity (with CIs) and the correlation between these two test accuracy dimensions were used to inform multivariate log-normal distributions from which the probabilistic estimates are drawn. For individual studies, beta-distributions were fitted to the sensitivity and specificity estimates. To preserve the correlation between sensitivity and specificity, the diagnostic accuracy 2 × 2 tables for each study were recreated (assuming a common prevalence for functionally significant stenosis of 0.402 (see Prevalence of functionally significant stenosis) and used to inform the alpha and beta parameters of the beta distributions.
Probability of quantitative flow ratio in the hybrid approach with confirmatory fractional flow reserve
The diagnostic accuracy of QFR in strategy 4 was based on the joint distribution of QFR and FFR measurements in the extracted individual-level patient data (n = 3194) (see Chapter 3, Grey-zone analysis) for the combined cQFR and non-specified QFR data. The probabilities of QFR test results being positive (QFR < 0.78), negative (QFR > 0.84) or inconclusive (QFR 0.78–0.84) were conditional on FFR values above and below 0.8. Table 10 presents the diagnostic accuracy estimates for strategy 4.
| QAngio XA 3D/QFR test result | QFR | Positive | Negative |
|---|---|---|---|
| FFR ≤ 0.80 | FFR > 0.8 | ||
| Positive | < 0.78 | 0.744 | 0.095 |
| Inconclusive (grey area) | 0.78–0.84 | 0.188 | 0.212 |
| Negative | > 0.84 | 0.069 | 0.693 |
In the probabilistic analysis, the joint QFR and FFR probabilities in Table 10 were sampled from a set of 5000 simulated values. These values were derived from 5000 simulations of the joint distribution of FFR and QFR, generated by bootstrapping the extracted individual-level data from which the probabilities in Table 10 were derived.
The diagnostic accuracy of an equivalent hybrid diagnostic approach for vFFR was not possible because of data limitations. The diagnostic accuracy data for vFFR are very scarce (see Chapter 3, CAAS vFFR), and only 81 data points for the joint FFR and vFFR distribution were available from one single study. 56 Furthermore, the underlying distribution of FFR values in this single study was considerably different from that of the data extracted for QFR (probability of FFR ≤ 0.80 was 0.296 in the single vFFR study, compared with 0.402 across 3194 data points in the QFR studies).
Invasive coronary angiography
The base-case diagnostic accuracy of ICA was informed by the bivariate meta-analysis of extracted data presented in Meta-analysis of extracted figure data for two-dimensional invasive coronary angiography. The model considered ICA to have a sensitivity and a specificity of 62.61% and 62.59%, respectively, when using a diagnostic threshold of 50% DS. Alternative sensitivity and specificity estimates based on a meta-analysis by Danad et al. 100 (per-vessel analysis) for diagnostic performance of ICA compared with FFR are used in a scenario analysis. In this study, ICA was found to have a sensitivity of 71% and a specificity of 66% based on a diagnostic threshold of 50% DS.
Procedural adverse events
Procedures involving catheterisation for diagnostic testing (ICA and FFR/iFR) or revascularisation (PCI and CABG) have associated complications that may result in health-care resource and HRQoL loss. The diagnostic model considers the impact of serious procedural complications from FFR/iFR and revascularisation. The procedural complications of ICA are excluded from the model because all patients undergo this procedure in all strategies and, therefore, procedural complications associated with ICA do not result in differences in costs and HRQoL across strategies.
The diagnostic pathway explicitly distinguishes between complications associated with invasive testing with FFR/iFR and revascularisation so that the potential benefits of less invasive testing can be captured (i.e. non-invasive testing with QFR and vFFR in strategies 3 and 5, respectively). However, revascularisation is often performed immediately after FFR/iFR and, therefore, the rates are often not reported separately in the literature by type of procedure.
Complications due to fractional flow reserve/instantaneous wave-free ratio
Three studies were identified that reported procedural complication rates in a format suitable to inform those associated with FFR/iFR alone (i.e. unrelated to the revascularisation procedure). The RIPCORD trial101 compared the clinical management (OMT alone or in addition to PCI or CABG) of patients with stable chest pain with ICA compared with pressure wire FFR assessment. 101 The placebo arm of the ORBITA trial97 is also potentially relevant to inform the rates of FFR-/iFR-related complications. In this study, patients were randomised to either PCI or a placebo procedure for angina relief, with all patients undergoing FFR/iFR prior to randomisation. Thus, patients who had serious periprocedural complications in the placebo arm had undergone FFR/iFR but not PCI. The IRIS-FFR registry data also reports serious complications associated with FFR measurement. 13 The rates of serious events reported in the three studies are summarised in Appendix 8, Table 61.
Data from the IRIS-FFR registry are used to inform the base-case analysis because this registry is considerably larger than the other studies and is used as a source of baseline clinical effectiveness in the prognostic model (see Baseline risk of major adverse cardiac events). A scenario analysis uses the alternative source of data from the RIPCORD trial101 because this is a UK study and the patient population appears comparable to that of the base-case population (mean age 64 years and 75% male).
The majority of complications reported in the ORBITA trial97 appear to be related to ICA (major bleeding and pulmonary oedema) and not to FFR/iFR based on the description of the complications reported in the manuscript’s supplementary materials. The conversion to PCI as a result of procedural complications in ORBITA97 appears to be because of coronary dissection caused by the pressure wire, and suggests a much higher rate for this complication than that reported in the IRIS-FFR registry. The patient population in ORBITA97 may represent a more severe population (mean baseline FFR 0.69 ± 0.16) than the population in the IRIS-FFR registry (mean baseline FFR 0.83 ± 0.11). Therefore, the rate of procedural adverse events in ORBITA97 is expected to be an overestimate of the complication rates in the base-case population.
None of the studies above reported procedural mortality as a result of FFR/iFR. In the IRIS-FFR registry, deaths due to FFR may have been captured within the rates of MACEs, but this is unclear. A procedural death rate associated with FFR/iFR of 0.015% is included in the diagnostic model based on an estimate sourced from Fearon et al. ,87 which was the only study identified in the review of decision models evaluating ICA (see Results of the review of decision models evaluating invasive coronary angiography) to include FFR-specific procedural death. The rates of FFR/iFR procedural complications applied in the base-case analysis are summarised in Appendix 8, Table 73.
Note that, although patients underwent iFR and FFR in ORBITA,97 all patients underwent FFR only in the IRIS-FFR registry and RIPCORD. 101 The base-case analysis assumes that there are no differences in the rates of procedural complications because of FFR and iFR, that is the complication rates associated with pressure wire FFR in IRIS-FFR are also reflective of the average rates of iFR as an alternative to FFR in UK clinical practice.
Probabilistic estimates of the FFR procedural complication rates were obtained by randomly sampling from independent beta distributions for each event rate.
Complications due to revascularisation
Death was the most common revascularisation complication reported in the cost-effectiveness models reviewed in Results of the review of decision models evaluating invasive coronary angiography. Two studies also considered non-fatal MI, but one reports complication rates jointly for ICA and revascularisation74 and the other88 sources complication rates from a very early 1996 study.
The IRIS-FFR registry does not report procedural complications associated with revascularisation separate from the risk of MACEs. The rate of procedural deaths associated with revascularisation is sourced from UK audit data. A 0.99% death risk for non-emergency CABG102 and 0.17% for in-hospital mortality for PCI103 are applied in the diagnostic model. The mortality rate associated with revascularisation is estimated as a weighted average of the mortality rates for PCI and CABG, where the weights correspond to the relative proportion of PCI and CABG procedures. In the base-case analysis, 87% of revascularisation procedures are assumed to be PCI, whereas the remaining 13% are CABG based on BCIS audit returns. 98 The External Assessment Group (EAG) did not find data suggesting these proportions differ depending on the diagnostic pathway (e.g. patients for whom the revascularisation decision is made based on ICA results only vs. on ICA plus FFR/iFR results). It is also unknown whether or not these proportions may differ for patients tested with QAngio XA 3D/QFR or CAAS vFFR. This area of uncertainty was not explored in the analysis, as it would require strong assumptions on clinician behaviour without data to inform a plausible range of alternative assumptions. The EAG notes, however, that the impact of varying the relative proportion of PCI and CABG is likely to be limited given the high rate of PCI in clinical practice.
Other procedural adverse events: radiation exposure
Patients who undergo cardiac catheterisation are exposed to ionising radiation, which may increase the lifetime risk of malignancy and associated mortality. Some of the previous cost-effectiveness models of ICA68,71,72,74,77,79 reviewed in Results of the review of decision models evaluating invasive coronary angiography considered radiation exposure due to ICA testing and revascularisation.
QAngio XA 3D/QFR or CAAS vFFR may reduce the magnitude of radiation exposure by reducing the procedural time to less than that of FFR/iFR. However, radiation exposure even with FFR/iFR is expected to be very low, and the reduction in exposure through the use of QFR or vFFR is expected to be very marginal (Dr Gerald Clesham, personal communication). Therefore, the impact of radiation exposure on cost-effectiveness is expected to be very minimal and is not quantified in the model. This is supported by the previous cost-effectiveness models; for example, Walker et al. 77 explicitly modelled an increased risk of cancer death conditional on the amount of radiation exposure for several different diagnostic strategies that included ICA, and found that the cost-effectiveness results were robust to the exclusion of radiation effects.
Risk of major adverse cardiac events and treatment effects of revascularisation
Baseline risk of major adverse cardiac events
The benefits of treatment by correctly identifying patients suitable for revascularisation, or to have their ischaemia treated by OMT, are modelled through the impact on risk of MACEs and HRQoL. The baseline risk of MACEs in the absence of revascularisation depends on disease severity as measured by FFR/iFR, where lower FFR values are indicative of a higher cardiovascular event rate, and higher FFR values of a lower cardiovascular event rate. 104 Thus, there is an inverse relationship between FFR value and subsequent outcomes.
The IRIS-FFR registry is the largest registry to prospectively evaluate the natural history of lesions after measurement of FFR in routine clinical practice. 13 Revascularisation was deferred in 6468 lesions (75%) and performed in 2165 lesions (25%) after FFR assessment. Treatment with revascularisation was generally recommended in participating centres when the FFR was ≤ 0.75, and deferred when the FFR was > 0.8. For FFR values between 0.75 and 0.8 the decision regarding revascularisation was left to the operator’s discretion. Of the deferred lesions, 85.1% had a FFR value > 0.8, 9.2% had a FFR value of between 0.76 and 0.8, and 5.7% had a value of ≤ 0.75. The reasons for deferred lesions despite low FFR, that is, ≤ 0.75, included minimal coronary artery stenosis on ICA, diffuse disease without focal stenosis, no symptoms, small myocardial territory or unsuitability for PCI. 13
The primary end point in the IRIS-FFR registry was MACE arising from FFR-measured lesions, which was a composite of cardiac death, MI and repeat revascularisation. Cardiac death was defined as any death caused by a proximate cardiac cause, including cardiac arrest, MI and fatal arrhythmia. MI was defined as a non-fatal MI event within the first 48 hours of the procedure or ≥ 48 hours after the procedure accompanied by ischaemic symptoms. Repeat revascularisation was defined as any PCI or CABG of a lesion with an index FFR measurement. 13 The registry data provide a source of baseline risk of MACE according to FFR value in deferred lesions in the absence of revascularisation.
The overall incidence rate of MACE in the IRIS-FFR registry across the range of FFR values was 1.44% (95% CI 1.15% to 1.73%) during the median follow-up of 1.9 years in deferred lesions. The corresponding incidence rates of clinical events were 0.09% for cardiac death, 0.14% for MI, and 1.34% for repeat revascularisation. When the 5.7% of deferred lesions with a FFR value of ≤ 0.75 were excluded, the overall incidence rate of MACE was 1.24% (95% CI 0.96% to 1.52%).
The risk of MACE in deferred lesions increased significantly, whereas FFR decreased. The adjusted HR for the risk of MACE when the FFR was included as an independent predictor in deferred lesions was 1.06 (95% CI 1.05 to 1.08) per 0.01 decrease in the FFR (using FFR values ≥ 0.91 as a reference). The corresponding adjusted HRs for the risk of clinical events were 1.06 (95% CI 0.99 to 1.13) for cardiac death, 1.09 (95% CI 1.05 to 1.14) for MI and 1.07 (95% CI 1.06 to 1.09) for revascularisation.
The reported 1-year and long-term (up to 3 years) cumulative incidence of MACE in the IRIS-FFR registry for deferred lesions is used in the model to provide an estimate of the baseline risk of MACE (i.e. MACE risk in the absence of revascularisation procedure) for the first year and subsequent years. The risk of MACE and the associated consequences in terms of health-care resource use and HRQoL is modelled by stratifying patients into subgroups of FFR values, to take account of the relationship linking FFR value to subsequent prognosis. To adjust for competing risks across cardiac events, the rate of each MACE component was divided by the sum of the rates of the components. The resulting proportions (6% for cardiac death, 9% for MI and 85% for repeat revascularisation) were multiplied by the baseline rate of the composite MACE outcome for the reference group with FFR values ≥ 0.91, which resulted in rates adjusted for competing hazards from the MACE components.
The baseline risk of MACE used in the model for individuals in the group with highest FFR values (FFR values ≥ 0.91) is 0.64% per annum in the first year and 0.32% per annum in subsequent years. This risk was used as a reference to compute the baseline risk of MACE components in categories with lower FFR values (i.e. < 0.91), using the adjusted HRs of 1.06, 1.09 and 1.07 per 0.01 decrease in FFR for cardiac death, MI and revascularisation, respectively. The corresponding annual baseline risk of MACE in deferred lesions by FFR value and by component of the composite outcome are shown in Appendix 8, Figure 46, for the first year. The values for subsequent years are approximately half of the values for the first year.
This approach implies that the baseline risk of MACE used in the model is conditional on FFR value and that the distribution of FFR values differs by diagnostic strategy. Therefore, the baseline risk of MACE from the TN and FN entry states in the model (in the absence of revascularisation) for each diagnostic strategy is based on the joint conditional distribution of FFR values for the diagnostic strategy across the FFR categories shown in Appendix 8, Figure 46; that is, the expected proportions of TN outcomes with a FFR 0.81–0.85, FFR 0.86–0.90 and FFR ≥ 0.91; and FN outcomes with a FFR 0.76–0.80, FFR 0.71–0.75 and FFR ≤ 0.70 for each diagnostic strategy are dependent on the underlying distribution of FFR values across these categories in the population. The same approach is used to establish the risk of MACE following revascularisation for the TP and FP entry states in the model (see Treatment effect of revascularisation). An alternative assumption is considered in a scenario analysis in which the risk of MACE is assumed to be completely independent of FFR and diagnostic test results.
The main limitation of using the IRIS-FFR registry data as a source of baseline risk of MACE for the model is that unobserved selection factors may have influenced the decision to perform or not to perform revascularisation, which, in turn, may have exerted a modifying effect on outcomes. In addition, the registry is based outside the UK in participating centres in the Republic of Korea. In the absence of an alternative source of data that evaluates the natural history of lesions after measurement of FFR in routine UK clinical practice, the model assumes that any potential selection factors in the IRIS-FFR registry have no causal relation to outcomes and that any underlying reasons would not be expected to differ from UK practice.
Treatment effect of revascularisation
The treatment effect of revascularisation on MACE in patients with stable CAD is highly uncertain and has been an area of considerable debate over the past decades. Early RCTs examining the benefit of PCI and CABG surgery compared with OMT suggested a survival benefit for revascularisation. However, more recently, the benefits of revascularisation have been questioned as a result of similar rates of death and MI observed in patients who have been optimally pharmacologically managed without PCI.
Appendix 8, Table 62, summarises the main findings of recent RCTs (post year 2000) in stable CAD that have compared revascularisation (in addition to OMT) with OMT alone. The focus on studies after the year 2000 is because of the changes in interventions over the past decades. For example, PCI using bare metal stents or early-generation drug-eluting stents have now been shown to be less safe and effective than currently available second-generation stents,105 whereas pharmacological interventions have changed over time.
The definition of MACE differs across the studies, but it is generally defined as a composite of cardiovascular death (or all-cause mortality), MI and hospital admission, with or without the need for revascularisation. The RCTs that showed a statistically significant difference in MACE between revascularisation and medical therapy were the Trial of Invasive versus Medical therapy in the Elderly (TIME),106 Medicine, Angioplasty, or Surgery Study II (MASS II)107 and BARI 2D96 for CABG surgery; COURAGE91 and the Fractional flow reserve versus Angiography for Multivessel Evaluation (FAME) II trial108 for repeat revascularisation; and the DEFER (DEFERral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis) trial109 in low FFR (i.e. < 0.75).
In TIME,106 305 patients aged 75 years and older with stable CAD of at least Canadian Cardiac Society Class II, despite at least two antianginal drugs, were randomly assigned to PCI, CABG or OMT. A significant difference in MACE rate between the revascularisation (19%) and medical therapy (49%) groups was observed over a mean follow-up of 184.4 days. This difference was mainly due to higher rates of hospital admission for acute coronary syndrome that required revascularisation.
In MASS II,107 611 patients with proximal multivessel stenosis and documented ischaemia were randomly assigned to PCI, CABG or OMT. At the 5-year follow-up, a significant difference in MACE was observed for CABG (21.2%) compared with PCI (32.7%) and OMT (36%). This difference suggests a protective effect of CABG, but no significant difference in MACE was observed between PCI and OMT. The difference in MACE for CABG was because of a significant difference in the need for repeat revascularisation and MI; however, there was no significant difference in overall mortality. In the BARI 2D trial,96 2368 patients with stable CAD and type 2 diabetes were randomly assigned to PCI, CABG or OMT. 110 At the 5-year follow-up, patients in the CABG stratum, who had more advanced CAD than those in the PCI stratum, had a significantly lower rate of MACE (22.4%) than the medical therapy group (30.5%), which was largely driven by fewer MIs.
In the COURAGE trial,91 2287 patients with stable CAD were randomly assigned to PCI or OMT. A statistically significant difference in the cumulative rate of additional revascularisation at 4.6 years was observed between PCI (21.1%) and OMT (32.6%). The corresponding HR was 0.60 (95% CI 0.51 to 0.71). In the FAME II trial,108 888 patients with stable CAD were randomly assigned to FFR-guided PCI for patients in whom at least one stenosis was functionally significant (i.e. FFR ≤ 0.80) or OMT. At the 2-year follow-up, a significantly lower rate of MACE was observed in the PCI group (8.1%) than in the OMT group (19.5%), which was largely driven by a lower rate of urgent revascularisation in the PCI group. The corresponding HR was 0.39 (95% CI 0.26 to 0.57). In the DEFER trial,109 325 patients with stable CAD and intermediate stenosis were randomly assigned to PCI for a FFR ≥ 0.75, PCI for a FFR < 0.75 or OMT. 109 At the 2-year follow-up, a significantly lower rate of MACE was observed in the PCI group with FFR < 0.75 (78%) than in the OMT group (89%) and the PCI group with a FFR ≥ 0.75 (83%), suggesting that FFR identifies those who will benefit the most from revascularisation in terms of MACE outcomes.
The RCTs that showed a non-statistically significant difference in MACE between revascularisation and medical therapy were MASS II107 and BARI 2D96 for PCI, COURAGE91 for the outcomes of mortality and MI, and Japanese Stable Angina Pectoris (JSAP)111 and ISCHEMIA. 95
In the MASS II trial107 at the 5-year follow-up, there was no statistically significant difference in MACE between PCI (32.7%) and OMT (36%), whereas in the BARI 2D trial96 there was no statistically significant difference in MACE between the revascularisation group (77.2%) and the OMT group (75.9%). In the COURAGE trial,91 there was no statistically significant difference in 4.6-year cumulative event rates for the composite end point of all-cause mortality and MI (HR 1.05 for PCI, 95% CI 0.87 to 1.27).
In the JSAP trial111, 384 patients with stable CAD and ≥ 75% coronary stenosis were randomly assigned to PCI or OMT. 111 At the 3.3-year follow-up, there was no statistically significant difference in MACE between the PCI group (2.9%) and OMT group (3.9%). In the largest and most recent ISCHEMIA,95 5179 patients with stable CAD and moderate or severe ischaemia were randomly assigned to a revascularisation group (74% PCI and 26% CABG) and OMT group. 95 Over a median follow-up of 3.2 years, 318 primary outcome events (composite of death from cardiovascular causes and MI, or hospitalisation for unstable angina, heart failure or resuscitated cardiac arrest) occurred in the revascularisation group and 352 occurred in the OMT group. At 6 months, the cumulative event rate was 5.3% in the revascularisation group and 3.4% in the OMT group, whereas at 5 years the cumulative event rate was 16.4% in the revascularisation group and 18.2% in the OMT group. The trial findings were sensitive to the definition of MI and the timing of results; procedural MI was increased with revascularisation, and spontaneous MI was reduced with revascularisation; thus, the net effect of MI was dependent on the time point at which it was measured. The evidence from the trial suggests that there is no statistically significant difference in MACE between revascularisation and OMT.
There have been several meta-analyses that have synthesised the results of trials examining the treatment effect of revascularisation on MACE in patients with stable CAD. 112–119 Of the most recent meta-analyses that include new-generation stents, the following findings were identified:
-
Pursnani et al. 119 included RCTs comparing revascularisation with PCI with OMT in patients with stable CAD, dating from 1980 until 2012. This study found that PCI was associated with a non-statistically significant improvement in mortality (RR 0.85, 95% CI 0.71 to 1.01), cardiac death (RR 0.71, 95% CI 0.47 to 1.06), non-fatal MI (RR 0.93, 95% CI 0.70 to 1.24) or repeat revascularisation (RR 0.93, 95% CI 0.76 to 1.14), when compared with OMT. These results were consistent across different follow-up time points.
-
Thomas et al. 115 included RCTs comparing revascularisation with PCI with OMT in patients with stable CAD, dating from 1980 until 2011. When compared with OMT, PCI was associated with no statistically significant improvement in all-cause mortality (RR 0.97, 95% CI 0.84 to 1.12), cardiac death (RR 0.91, 95% CI 0.70 to 1.17) and non-fatal MI (RR 1.09, 95% CI 0.92 to 1.29).
-
Stergiopoulos et al. 118 included RCTs comparing revascularisation with PCI with OMT in patients with stable CAD, dating from 1970 until 2012. When compared with OMT, PCI was associated with no statistically significant improvement in all-cause mortality (OR 0.90, 95% CI 0.71 to 1.16), non-fatal MI (OR 1.24, 95% CI 0.99 to 1.56) and unplanned revascularisation (OR 0.64, 95% CI 0.35 to 1.17).
-
Windecker et al. 117 undertook a Bayesian network meta-analysis comparing revascularisation (PCI or CABG) with OMT among patients with stable CAD, dating from 1980 until 2013. CABG was associated with a survival benefit (RR 0.80, 95% CI 0.70 to 0.91) compared with OMT. New generation drug-eluting stents [everolimus-eluting Xience/Promus™ stent (Boston Scientific, Marlborough, MA, USA): RR 0.75, 95% CI 0.59 to 0.96; zotarolimus-eluting Resolute™ stent (Medtronic plc, Dublin, Ireland): RR 0.65, 95% CI 0.42 to 1.00], but not balloon angioplasty (RR 0.85, 95% CI 0.68 to 1.04), bare-metal stents (RR 0.92, 95% CI 0.79 to 1.05) or early-generation drug-eluting stents [paclitaxel-eluting Taxus® stent (Boston Scientific): RR 0.92, 95% CI 0.75 to 1.12; sirolimus-eluting Cypher™ stent (Cordis Corporation, Hialeah, FL, USA): RR 0.91, 95% CI 0.75 to 1.10; zotarolimus-eluting Endeavor® stent (Medtronic plc): RR 0.88, 95% CI 0.69 to 1.10], were associated with improved survival compared with OMT. CABG reduced the risk of MI compared with OMT (RR 0.79, 95% CI 0.63 to 0.99), and everolimus-eluting stents showed a trend towards a reduced risk of MI (RR 0.75, 95% CI 0.55 to 1.01). The risk of subsequent revascularisation was noticeably reduced by CABG (RR 0.16, 95% CI 0.13 to 0.20) followed by new-generation drug-eluting stents (zotarolimus-eluting Resolute stent: RR 0.26, 95% CI 0.17 to 0.40; everolimus-eluting Xience/Promus stent: RR 0.27, 95% CI 0.21 to 0.35), early-generation drug-eluting stents (zotarolimus-eluting Endeavor stent: RR 0.37, 95% CI 0.28 to 0.50; sirolimus-eluting Cypher stent: RR 0.29, 95% CI 0.24 to 0.36; paclitaxel-eluting Taxus stent: RR 0.44, 95% CI 0.35 to 0.54) and bare-metal stents (RR 0.69, 95% CI 0.59 to 0.81) compared with OMT.
-
Chacko et al. 116 included RCTs comparing PCI with OMT in patients with stable and unstable CAD, dating from 1992 until 2019, which included ISCHEMIA,95 to examine the effects on death and MI. For stable CAD, PCI did not show a statistically significant reduction in mortality (RR 0.98, 95% CI 0.87 to 1.11), cardiac death (RR 0.89, 95% CI 0.71 to 1.12) or MI (RR 0.96, 95% CI 0.86 to 1.08).
The IRIS-FFR registry13 prospectively evaluated the natural history of lesions after measurement of FFR in routine clinical practice in those revascularised [95.7% PCI, with the majority new-generation drug-eluting stents (85.1%) and 4.3% CABG] compared with deferred lesions. The overall incidence rate of MACE in revascularised lesions was 2.4%, compared with 1.44% in deferred lesions during the median follow-up of 1.9 years. The corresponding incidence rates of clinical events were 0.71% for cardiac death or MI and 1.83% for repeat revascularisation compared with 0.21% and 1.34% in deferred lesions, respectively. However, unlike the deferred lesions, the risk of MACE was not associated with FFR measurement. After adjustment for independent predictors of MACE, the risk of MACE was not statistically significantly different between revascularised and deferred lesions for FFR values ≥ 0.76 (HR 0.83, 95% CI 0.46 to 1.50 for a FFR 0.76–0.80 and HR 1.21, 95% CI 0.44 to 3.36 for a FFR 0.81–0.85). Revascularisation was associated with improved MACE rates, compared with deferred lesions, for lesions with FFR ≤ 0.75 (HR 0.47, 95% CI 0.24 to 0.89 for a FFR 0.71–0.75 and HR 0.47, 95% CI 0.26 to 0.84 for a FFR ≤ 0.70). The findings from the IRIS-FFR registry are consistent with the study by Johnson et al. ,104 which examined the prognostic value of FFR on clinical outcomes. Johnson et al. 104 undertook a meta-analysis of study-level (9173 lesions) and patient-level (6961 lesions) data investigating prognoses of MACE outcomes after FFR measurement by revascularisation compared with OMT. The study-level metaregression indicated that revascularisation was associated with a lower normalised 1-year MACE rate (composite of death, MI and repeat revascularisation), than OMT, when the FFR was ≤ 0.75. The corresponding patient-level metaregression after adjustment for DS indicated that revascularisation was associated with a lower 1-year MACE rate, than OMT, when the FFR was ≤ 0.76.
In summary, the treatment effect of revascularisation on MACE in patients with stable CAD is highly uncertain. The primary aim of the largest and most recent ISCHEMIA trial,95 which included UK centres, was to address the limitations of previous trials by determining whether or not revascularisation plus OMT, compared with a conservative strategy of OMT alone, would reduce the primary composite outcome of death from cardiovascular causes or MI, and hospitalisation for unstable angina, heart failure or resuscitated cardiac arrest in patients with stable ischaemic heart disease with moderate or severe ischaemia. As indicated above, the trial did not find evidence that revascularisation reduced the risk of MACE. Therefore, it seems appropriate for the base-case analysis to consider that the benefits of the diagnostic tests in identifying the appropriateness for revascularisation confers no benefit on MACE outcomes. This means that in the base-case analysis the risk of MACE following revascularisation for the TP and FP entry states in the model is the same as the baseline risk of MACE conditioned on FFR value. That is, the expected proportion of TP outcomes with a FFR of 0.76–0.80, 0.71–0.75 and ≤ 0.70, and FP outcomes with a FFR of 0.81–0.85, 0.86–0.90 and ≥ 0.91 for each diagnostic strategy, is dependent on the underlying distribution of the FFR values across these categories in the population.
Alternative scenarios are considered in the model where revascularisation does confer a benefit on MACE outcomes compared with OMT. Three alternative scenarios are considered:
-
A significant reduction in MACE only for a FFR ≤ 0.76, in line with the findings of the IRIS-FFR registry. In this scenario, the HR for revascularisation is set equal to 0.47 (95% CI 0.24 to 0.89 for a FFR 0.71–0.75 and 95% CI 0.26 to 0.84 for a FFR ≤ 0.70) for all components of MACE for a FFR ≤ 0.75, whereas the HR is set equal to 1 for a FFR > 0.75.
-
A significant reduction in the component of MACE of unplanned revascularisation only and no reduction for cardiac death or MI. This is in line with the findings of the trials that found there was a positive effect of revascularisation on MACE outcomes but that this positive effect was largely determined by a reduction in the number of repeat/emergency or unplanned revascularisations rather than cardiac death or MI. In this scenario, the HR is set equal to 0.26 (95% credible interval 0.17 to 0.40) for repeat revascularisation from the meta-analysis by Windecker et al. 117 for new-generation drug-eluting stents, whereas the HR is set equal to 1 for cardiac death or MI.
-
A non-statistically significant reduction in MACE for all components. This is in line with the findings of the trials that found a modest improvement in MACE outcomes for revascularisation compared with OMT, but this improvement was not statistically significant. In this scenario, the HR is set equal to 0.71 (95% CI 0.47 to 1.06) for cardiac death, 0.93 (95% CI 0.70 to 1.24) for non-fatal MI and 0.93 (95% CI 0.76 to 1.14) for repeat revascularisation based on the meta-analysis by Pursnani et al. 119
Additional sensitivity analyses using the results from the individual RCTs and meta-analyses reported above are considered to assess the impact of alternative assumptions about the treatment effect of revascularisation for MACE outcomes on the cost-effectiveness results.
Other-cause mortality
Mortality due to non-cardiovascular causes was estimated based on age- and sex-specific UK life tables120 and by deducting the mortality due to ischaemic heart disease (International Classification of Diseases, codes 20–25). 121 The age-specific probability of death was estimated as a weighted average of male and female mortality.
Health-related quality of life
To estimate QALYs, it is necessary to quality-adjust the period of time for which the average patient is alive within the model using an appropriate utility or preference score. QALYs are calculated by summing the time spent in a health state weighted by the utility value associated with the health state. Additional adjustments are made to QALYs to reflect a decrement in utility associated with an acute or adverse event. The NICE methods guide advocates a preference for EQ-5D data with utility values using UK population weights when available. 92
In the diagnostic model a one-off utility decrement is applied to patients undergoing invasive FFR/iFR and those who undergo revascularisation (known as procedural disutility). At the end of the diagnostic model, patients who survive enter the long-term prognostic model in one of the four health states of TP, FN, FP or TN. The implications on HRQoL of receiving treatment (either revascularisation in addition to OMT or OMT alone) based on being in one of the four diagnostic health states is quantified by attaching a utility value to each of the health states. A one-off utility decrement is also applied in the prognostic model to those who experience a non-fatal MI or require an unplanned revascularisation. To reflect a decrease in HRQoL for those with a history of MI, a separate utility decrement is applied to the post-MI health state. For those patients who experience an unplanned revascularisation, the utility value associated with the TP health state is applied based on the assumption that patients who undergo a targeted revascularisation achieve the same benefits of revascularisation, in terms of symptom relief, as patients who had a successful initial revascularisation procedure.
The utility values are used to calculate the expected number of QALYs for each diagnostic strategy over the duration of the model.
Procedural disutility
The model considers the procedural disutility associated with FFR/iFR, PCI and CABG. The disutility associated with ICA is not included because all patients undergo the procedure. Targeted literature searches were used to identify sources to inform procedural disutility parameters.
No studies reporting HRQoL loss associated with FFR/iFR were identified. However, FFR/iFR, particularly FFR, which requires the administration of a hyperaemic agent, can cause discomfort to patients. The administration of the most commonly used hyperaemic agent, adenosine, can result in chest pain, dyspnoea, bronchospasm, conduction disturbances, facial flushes, headaches and hypotension. 122–125 Patient discomfort can therefore arise from adverse events associated with the hyperaemic agent, but also relate to vasodilation that is a consequence of inducing a hyperaemic state to perform FFR. In the absence of suitable estimates to inform the disutility associated with FFR/iFR, a disutility equivalent to that of a PCI procedure is assumed. Furthermore, this is assumed to apply to all patients undergoing FFR/iFR and not just to those who incurred procedural complications (see Complications due to FFR/iFR). An additional disutility resulting from procedural complications was not included in the model to avoid double counting. It is expected that iFR is more tolerable to patients as it does not require the administration of a hyperaemic agent; however, a specific disutility estimate for iFR was not identified. Furthermore, the proportion of patients with intermediate stenosis who undergo iFR in UK clinical practice is also unknown. Therefore, the base-case analysis makes a simplifying assumption that the QALY loss estimate applied for FFR/iFR is representative of both types of pressure wire procedures.
One UK study126 was identified as relevant to inform the procedural disutility of revascularisation (PCI and CABG). Bagust et al. 126 conducted a cost-effectiveness study comparing drug-eluting stents with conventional stents for the treatment of symptomatic CAD. The model considered the QALY loss per percutaneous transluminal coronary angioplasty (PTCA) and per CABG by combining EQ-5D data from two clinical trials in multivessel CAD that compared alternative revascularisation procedures (PTCA with bare and drug-eluting stents, and CABG) with assumptions on the duration of the disutility. The disutility for each procedure was estimated as the difference in utility scores before and after the revascularisation procedure. The disutility was assumed to be incurred for 1 month for PTCA and 6 months for CABG, resulting in a QALY loss of 0.0056 and 0.033 QALYs per PTCA and CABG procedure, respectively. The authors noted, however, that the QALY losses may be an overestimate as all patients had multivessel disease. These QALY loss estimates are used to inform the base-case scenario. Appendix 8, Table 63, summarises the QALY loss estimates associated with each procedure in the diagnostic model. The QALY loss associated with PCI and CABG is applied in the model as a weighted average, assuming that 87% of revascularisation procedures are PCI and 13% CABG (see Complications due to revascularisation). The QALY loss associated with revascularisation is also applied in the prognostic model to capture the HRQoL impact of unplanned revascularisation. Gamma distributions were fitted to the procedural HRQoL loss to generate probabilistic estimates.
Health state utilities
The benefits of the diagnostic tests in identifying the appropriateness for revascularisation can confer health benefits through greater symptom relief and, therefore, higher HRQoL. Given that the base-case analysis assumes that there is no treatment effect of revascularisation on MACE, the improvement in symptom relief is the only benefit of revascularisation. Of the recent RCTs (post year 2000) in stable CAD that have compared revascularisation with OMT (see Treatment effect of revascularisation), there was a general trend towards significantly greater improvement in HRQoL after revascularisation. In TIME,106 after 6 months, angina severity decreased and measures of HRQoL using the Short Form questionnaire-36 items (SF-36), Duke Activity Score Index (DASI) and Rose Angina Questionnaire showed a significantly greater improvement after revascularisation compared with OMT. 106 In the MASS II trial,107 HRQoL using the SF-36 instrument was better in both the CABG and PCI groups compared with OMT at the 1-year follow-up, with the CABG group presenting the greater and progressive improvement in HRQoL. 127 In the COURAGE trial,91 HRQoL using the Seattle Angina Questionnaire (SAQ) showed a very modest improvement in SAQ score for the PCI group, compared with OMT, at the 1-year follow-up. 128 In the BARI 2D trial,96 HRQoL was reported using the DASI, RAND 36-Item Health Survey and patients’ self-rated health, and demonstrated that, compared with OMT, revascularisation was associated with significantly greater improvements in DASI, energy and self-rated health components but not health distress. 129 The HRQoL effects of revascularisation in BARI 2D96 were largely maintained over a 4-year follow-up. In the FAME II trial,108 HRQoL was measured using EQ-5D at the 1-month and 1-year follow-ups and reported based on patients’ index FFR measurement. 130 The results of the trial showed that HRQoL improved significantly from baseline after PCI, with the largest marked improvement in those with a lower FFR value, whereas it did not change significantly in the OMT group. In ISCHEMIA,95 HRQoL using the SAQ at 3, 12 and 36 months showed a significant, durable improvement in angina control and HRQoL with revascularisation compared with OMT. 131 The smaller ORBITA trial,97 which reported HRQoL using SAQ and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) visual analogue scale, was the only study that demonstrated a non-statistically significant difference in HRQoL for PCI compared with OMT. 97
In summary, the evidence from the most recent RCTs suggests that revascularisation has a significantly greater improvement in HRQoL than OMT alone. This is supported by a recent systematic review and meta-analysis summarising the evidence to determine the impact of coronary revascularisation on HRQoL, which showed that both PCI and CABG had significantly greater effects on HRQoL than medication, but the effects of the revascularisation procedures did not differ significantly from each other. 132
Of the most recent RCTs, only one trial, FAME II,108 measured improvement in HRQoL using EQ-5D. This trial was a subsequent RCT to the FAME I trial133 that compared two different revascularisation strategies in patients with stable CAD and multivessel disease using standard ICA-guided revascularisation of lesions with a > 50-mm DS and a FFR-guided revascularisation approach for lesions with a FFR ≤ 0.80. The FAME II trial108 was designed to clarify whether PCI of functionally significant stenoses (i.e. lesions with FFR ≤ 0.80) combined with OMT would be superior to OMT alone in patients with stable CAD. In a study by Nishi et al. ,130 patient-level pooled analysis from the FAME I and II trials was used to assess whether or not the benefit to HRQoL after PCI depends on the severity of the stenosis as determined by FFR measurement. The study is based on patients with stable CAD who underwent PCI with a FFR ≤ 0.80 from the FFR-guided arm of the FAME I trial and the PCI arm of the FAME II trial. 108 The study reports the change from baseline in EQ-5D utility values by FFR 0.70–0.80, FFR 0.51–0.69 and FFR ≤ 0.50. The study also reports the change from baseline in EQ-5D utility values for lesions with a FFR ≤ 0.80 that were treated medically from the OMT arm of the FAME II trial. 108 The results of this study are directly relevant for informing the HRQoL improvement from revascularisation compared with OMT in the model.
Table 11 presents the change in EQ-5D utility values from baseline to 1 month and 1 year in the PCI (in addition to OMT) group and the OMT-alone group by FFR value based on Nishi et al. 130 The EQ-5D improved significantly from baseline after PCI in all FFR subgroups at both the 1-month and the 1-year follow-ups, with a progressive improvement with lower FFR values. The EQ-5D improved slightly from baseline in the OMT group to both 1 month and 1 year, but this improvement was not statistically significant (note that the results in Table 11 exclude crossovers to PCI in the OMT group).
| Time point | EQ-5D value (95% CI) | |||
|---|---|---|---|---|
| Revascularisation | OMT | |||
| FFR 0.70–0.80 | FFR 0.51–0.69 | FFR ≤ 0.50 | FFR ≤ 0.80 | |
| At 1 month | 0.039 (0.019 to 0.059) | 0.056 (0.036 to 0.075) | 0.080 (0.058 to 0.101) | 0.003 (–0.012 to 0.017) |
| At 1 year | 0.038 (0.013 to 0.063) | 0.057 (0.037 to 0.078) | 0.065 (0.040 to 0.089) | 0.015 (–0.004 to 0.033) |
The utility values in Table 11 are used to represent the change in baseline utility for the TP and FN health states. For the TP health state, the utility value for revascularisation is used to provide a weighted change in utility value based on the expected proportion of TP outcomes with a FFR 0.70–0.80, 0.51–0.69 and ≤ 0.50, which differs by diagnostic strategy and is dependent on the underlying distribution of FFR values across these categories in the population. For the FN health state, the utility value for OMT with a FFR ≤ 0.80 is used to provide the change in baseline utility. For the FP and TN health states with a FFR > 0.80 (functionally non-significant stenosis), it is assumed that there is no change in baseline utility for patients with intermediate stenosis.
The underlying baseline utility for a 64-year-old UK patient with stable CAD is also taken from Nishi et al. ,130 where the average age of patients in the FAME trials108 was the same as the modelled population. To reflect the decreasing utility of patients as they age through the model, age- and sex-adjusted EQ-5D norms for the UK based on Ara et al. 134 were adjusted to reflect the existence of stable CAD. The adjustment factor was estimated by comparing the baseline utility of Nishi et al. 130 to the average utility of a 64-year-old UK patient, derived from a nationally representative UK sample using EQ-5D.
Patients who experience a non-fatal MI receive a one-off utility decrement, whereas those in the post-MI health state are subject to a decrease in HRQoL for the duration of time spent in this state. Both these utility decrements were sourced from Sullivan et al. 135, a study that estimated a catalogue of marginal disutilities for a wide range of health conditions based on UK-specific health preferences. In this study, marginal disutilities were estimated as EQ-5D index score decrements adjusted for patient characteristics (age, comorbidity, sex, ethnicity, income and education). The marginal disutility for ‘acute MI’ (International Classification of Diseases, Ninth Edition, code 410) informed the utility decrement for non-fatal MI events [–0.0626, standard error (SE) 0.0132], and the estimate for previous MI informed the post-MI health state (–0.0368, SE 0.0252). Gamma distributions were fitted to the utility decrements for the uncertainty analysis.
Resource use and costs
This section details the resource use and costs applied in the model. The diagnostic model considers the costs of diagnostic testing, revascularisation and treatment of procedural complications. The prognostic model considers the costs of OMT, health state and clinical events. Costs in the model are fixed estimates. Details by category of resource use and costs are presented in the sections below.
Test costs
QAngio XA 3D/QFR costs
The costs of QAngio XA 3D/QFR include the cost of the software licence, and training and certification fees. These costs are summarised in Appendix 8, Table 64 (adapted from the company’s response to NICE’s information request and additional EAG questions). Costs were originally reported in euros, and have been converted to GBP at an exchange rate of 0.86295, based on the average exchange rate between 25 August 2019 and 19 February 2020. 136
The costs of the QAngio XA 3D/QFR software licence are dependent on the number of patients tested (‘per-patient-study basis’). The cost per patient of the software licence is the same for the 10-patient and 50-patient vouchers, whereas the 100-patient voucher offers a discount of £8.63 per patient compared with the other two options (assuming the same 100 patients are tested). To simplify cost calculations, it is assumed that annual throughput is a multiple of 10. It is further assumed that an NHS trust would purchase the least costly combination of vouchers that would cover the expected annual throughput. For example, if annual expected throughput was 180 patients, the trust would purchase one 100-patient voucher and eight 10-patient vouchers. For the base-case annual throughput of 200 patients, the trust would purchase two 100-patient vouchers, at a total cost of £84,569.10 (approximately £423 per patient).
The cost of the 100-patient voucher also covers the training and certification of up to four QAngio XA 3D/QFR users. The cost of the 10-patient and 50-patient vouchers does not include any training and certification. When training and certification are required and this cost is not covered by the voucher, a fee of £3020 for up to four software users applies for both on-site and online training. It was assumed that for every 100 patients, four members of staff would require training, and so the training fee would, therefore, be covered by the voucher for an average annual throughput ≥ 100. It is only when annual throughput is lower than 100 patients and, therefore, the trust does not purchase at least one 100-patient voucher, that this cost will be incurred. In addition to the training and certification fees charged by the company, the staff time costs required for training activities should also be considered. According to Medis Medical Imaging Systems BV, on-site training is currently available only for groups of a minimum of 10 participants, but an e-learning platform is being developed to deliver training. E-learning is expected to be available before the fourth quarter of 2020. Another alternative for large research groups is to have training delivered over 1.5 days at Leiden but without travel and accommodation costs included. Medis Medical Imaging Systems BV states that using the e-learning platform training should require 5 hours of staff time, whereas certification would be done on a cloud-based solution, taking 1–2 hours. This training would typically be required by an observer or technician in the catheterisation laboratory, who uses the QFR software solution and carries out the analyses. The company also has a shortened e-learning module for the interventional cardiologist that should take 30 minutes to complete. As more details have been provided for online training, and on-site training appears to require a large number of participants, we have estimated costs of training assuming that this is delivered online.
It was assumed that for each four-member group of staff requiring training, one would be an interventional cardiologist and the other three cardiac physiologists. The cardiac physiologists are assumed to be the software operators, and thus undergo both the training and the certification module. The first module takes 5 hours to complete, whereas the second requires 1.5 hours (mid-point of the range of time provided by the company). Unit costs for staff time were taken from the Personal Social Services Research Unit (PSSRU) costs. 137 The cost per working hour of a surgical consultant was assumed to cost the same as a cardiologist’s time, and that of an allied professional (band 5) for a cardiac physiologist’s time. Staff time and costs associated with NHS staff training and certification per 100 patients are shown in Appendix 8, Table 65.
It is assumed that the additional staff time and cost of that time will increase at the same rate as throughput, which corresponds to a staff cost per patient of £7.76. We assume the same staff cost per patient for an annual throughput lower than 100 patients. This simplifying assumption effectively implies that staff time is independent of throughput.
The costs of QAngio XA 3D/QFR disaggregated by cost element are presented in Appendix 8, Table 66, for the base-case throughput assumption of 200 patients per year. The cost per patient tested with QAngio XA 3D/QFR is £430.61.
CAAS vFFR costs
The costs of CAAS vFFR include the cost of (1) the software licence, (2) training and (3) annual maintenance. Pie Medical Imaging BV has two different pricing models for CAAS vFFR, which are summarised in Appendix 8, Table 67 (adapted from the company’s response to NICE’s information request). Costs were originally reported in euros, and have been converted to GBP at the same exchange rate used to estimate the costs of QAngio XA 3D/QFR. 136
The cost of the software licence for CAAS vFFR per patient tested varies according to the pricing model selected. Under pricing model 1, the software licence costs £31,929 and is described as perpetual. The total number of tests covered by the licence depends on the lifespan of the technology, which is unknown. For the purpose of determining a cost per patient tested, it is conservatively considered that the perpetual licence covers the annual patient throughput independently of its size (i.e. the lifespan of the technology is 1 year). Under pricing model 2, the cost per patient tested is fixed at £172.59. Assuming that annual throughput is a multiple of 10 (as assumed for QAngio XA 3D/QFR cost calculations), the software licence cost per patient is lower for pricing model 2 than for pricing model 1 only when the annual throughput is ≤ 10 patients. For the base-case assumption of 200 patients per year, the software licence cost per patient tested with pricing model 1 is £159.65.
CAAS vFFR training can be delivered through three alternative platforms: e-learning, WebexTM (Cisco Systems, Milpatas, CA, USA) or on-site training. The training fee depends on the delivery platform, with e-learning offered at no cost and Webex and on-site training offered at a cost of £215.74 and £2157.38, respectively. Pie Medical Imaging BV does not specify if there is a maximum number of NHS staff covered by the training fee. It is assumed that only one training session by either Webex or on-site delivery would be required independent of annual throughput, and that any additional training would be delivered online at no additional cost. We calculated the cost of the training fee as the average between on-site and Webex training costs (£1186.56).
Pie Medical Imaging BV states that the same training should be delivered to staff using the CAAS vFFR software and the interventional cardiologist who interprets the result. Training delivered through e-learning and Webex requires 2 hours per member of staff, whereas on-site training requires 4 hours. We made the same assumptions regarding numbers of staff members who would require training as for QAngio XA 3D/FR, that is one cardiologist and three cardiac physiologists would require training for every 100 patients tested. We also assumed that the additional staff training time and cost of that time would increase at the same rate as the throughput. Thus, the staff training cost per patient is independent of throughput, but depends on the training platform. The same unit costs were applied for the calculation of staff training costs as for QAngio XA 3D/QFR. Appendix 8, Table 68, shows the staff training cost per 100 patients tested with CAAS vFRR.
The staff training cost per patient is £4.40 when delivered online (Webex or e-learning) and £8.80 when delivered on site. In the base case we assumed that the staff training cost per patient tested would correspond to £6.60, the average cost of online and on-site staff training.
Pie Medical Imaging BV charges a fee for annual maintenance of CAAS vFFR. The cost of maintenance varies by pricing model. For pricing model 1 there is no maintenance cost in the first year, and an annual cost of £4746.23 applies for subsequent years. For pricing model 2 there is an annual cost of £3023.44 incurred every year. As we have made a simplifying assumption that the lifespan of the technology is 1 year when considering the licence fee costs, we did not include an annual maintenance cost for pricing model 1.
We assumed that the NHS trust would select the pricing model that would result in fewer costs according to the expected annual throughput. Pricing model 1 results in a lower cost per patient tested when annual throughput is greater than 10. The costs of CAAS vFFR broken down by cost element are presented in Appendix 8, Table 69, for the base-case assumption of 200 patients per year. The cost per patient tested with CAAS vFFR is £172.18.
Comparison of QAngio XA 3D/QFR and CAAS vFFR testing costs
The cost per patient tested with QAngio XA 3D/QFR and with CAAS vFFR varies with annual throughput, as shown in Appendix 8, Figure 47. The cost per patient tested with CAAS vFFR under pricing model 1 is more sensitive to alternative throughput estimates than QAngio XA 3D/QFR, especially for throughputs lower than 100 patients. The cost of QAngio XA 3D/QFR is robust to alternative throughput estimates for throughputs greater than 100 patients. This cost is also consistently higher than that of CAAS vFFR for throughputs greater than 70 patients. Appendix 8, Figure 47, shows two curves for the cost per patient tested with CAAS vFFR to illustrate the difference between assuming that training is delivered over Webex and assuming it is delivered on site. The cost per patient tested with CAAS vFFR seems consistently similar for the two modes of training delivered; therefore, an average between the two modes was used to estimate the cost per patient tested with CAAS vFFR.
Invasive coronary angiography and fractional flow reserve
The unit costs used to estimate the costs of catheterisation tests currently used in NHS clinical practice were sourced from NHS reference costs 2017/18138 and uprated to 2018/19 prices. 137 The cost of ICA was calculated as the activity-weighted average of the Healthcare Resource Group (HRG) codes for simple catheterisation. However, the model did not consider a cost for ICA because all patients who enter the diagnostic model undergo this test. In line with the NICE scope, the unit cost for FFR/iFR, that is, £436.80, was estimated as the difference between the activity-weighted average of the HRG codes for complex (£2202.26; currency codes: EY42A–D) and standard cardiac catheterisation (£1765.46; currency codes: EY43A–F). This difference represents the incremental cost of FFR/iFR compared with ICA alone. The cost of iFR was not estimated separately, as it was assumed that this cost was already captured in the complex cardiac catheterisation HRG codes.
Revascularisation costs
Patients who test positive at the last step of each testing strategy undergo revascularisation with either PCI or CABG. The unit cost for these procedures was sourced from NHS reference costs 2017/18138 and uprated to 2018/19 prices. 137 We estimated the activity-weighted average cost of (1) complex and standard percutaneous transluminal coronary angioplasty and (2) complex major and standard CABG across all HRGs to inform the unit cost of PCI and CABG, respectively. Costs applied in the model and the NHS currency codes used to inform these are presented in Appendix 8, Table 70.
As the costs of PCI and CABG were estimated across all HRGs, their unit cost reflects the cost of the procedures across all settings under which the procedures are performed in the NHS (adjusted by activity). We considered this to be the most appropriate assumption in the absence of clear evidence on the proportion of procedures per setting for patients in our study population. However, patients who are treated electively for stable angina do not usually require an overnight stay. Despite the NHS target to have at least 75% of elective PCIs performed as a day case, recent audit data show that there is extremely wide variation in day-case rates139 and that only 30.4% of PCI is performed as day cases. 98 Some centres will perform PCI almost exclusively as a day case, whereas others will require patients to stay overnight in hospital following the procedure. Given the considerable variation in the NHS in terms of the need for overnight stays following PCI, we considered only the cost of PCI to correspond to that of a day-case admission in a scenario analysis.
In the base-case scenario, patients revascularised after their index testing were assumed to incur a cost of £3005 and £10,899 if they underwent PCI and CABG, respectively. This resulted in a cost per revascularisation of £4031.22, assuming that 87% of revascularisation procedures are PCI and 13% are CABG (see Complications due to revascularisation).
Procedural complications costs
The cost of procedural complications for FFR/iFR was calculated based on the rates of complications in Appendix 8, Table 71. Unit costs were sourced from NHS reference costs 2017/18138 and uprated to 2018/19 prices. 137 These costs are summarised in Appendix 8, Table 71, and range between £834.57 for bronchospasm and £3005.07 for coronary dissection. Patients who die are assumed to incur no additional costs.
Optimal medical treatment costs
It was assumed that all patients in the prognostic model are treated with OMT for stable angina. The NICE existing guidance was reviewed to define OMT. The NICE Clinical Guideline on the management of stable angina (i.e. CG126)140 recommends that patients with stable angina receive a beta-blocker or a calcium channel blocker in either monotherapy or combination therapy as the first line of treatment. The alternative to the first-line treatment for patients who cannot tolerate it is monotherapy with a long-acting nitrate or one of the novel anti-ischaemic drugs (ivabradine, nicorandil or ranolazine). The use of three anti-angina drugs is not recommended unless patients are not satisfactorily controlled with two anti-angina drugs or are waiting for revascularisation or cannot undergo revascularisation. Drugs for secondary prevention of cardiovascular disease should also be considered for patients with stable angina. These drugs include aspirin, angiotensin-converting enzyme (ACE) inhibitors, other antihypertension drugs and statins. The guideline does not make specific recommendations regarding medication for patients who have undergone PCI or CABG, but revascularised patients will still need to receive anti-angina and secondary prevention drugs after the procedures. We have, thus, considered that OMT comprises both anti-angina secondary prevention drugs. Given that revascularisation will resolve or reduce some of the angina symptomology, it is likely that the composition of OMT varies depending on whether or not patients have received revascularisation and on the type of procedure received.
One of the models74 described in Results of the review of decision models evaluating invasive coronary angiography assumed that the relative proportion of each drug class comprised by OMT would vary depending on the patient’s index treatment. We assumed the same, but sourced data on anti-angina medication use where available from Nishi et al. ,130 the same study from which we sourced estimates of HRQoL (see Results of the review of decision models evaluating invasive coronary angiography). Nishi et al. 130 reported anti-angina medication use at 1 year post diagnosis for patients treated with OMT in isolation (TN) or in addition to PCI (TP). This study also reported the use of these drugs for patients treated with OMT in isolation despite requiring revascularisation (FN). As the study did not include patients who had undergone CABG, data on the medication use for these groups (also TPs) were taken from the same source used in Genders et al. ,74 namely the SYNTAX trial. 59 As Nishi et al. 130 did not report the use of secondary prevention drugs, data on medication use for these drugs were taken from the SYNTAX trial59 and the COURAGE trial91 (also used in Genders et al. 74).
The cost of medication was estimated by combining the proportion of medication use for each type of treatment [OMT alone for patients who did not required revascularisation (TNs) and those who did (FNs), OMT for patients who underwent PCI (TPs) and CABG (TPs)], with unit costs from the British National Formulary (BNF). 141 Patients who underwent revascularisation without needing it (FPs) were assumed to have the same medication use as the corresponding TP patients. The active substances and dosages selected, as well as proportion of medication use, were validated by the clinical adviser to the EAG. Medication use and estimated costs per annum of OMT conditional on patient clinical management after diagnosis are summarised in Table 12. These costs are applied in the prognostic model at each annual cycle, and it was assumed that if the patient underwent revascularisation in the model, the cost of OMT would then correspond to OMT in addition to revascularisation regardless of the patient classification at the start of the prognostic model (TN, FN, TP or FP).
| Treatment type | Drug and dosage | Unit cost (£)a | Source | Medication use | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OMT (%) | Source | PCI and OMT (%) | Source | OMT (FN) (%) | Source | CABG and OMT (%) | Source | ||||
| Anti-angina | |||||||||||
| Beta-blocker | Bisoprolol, 5 mg/day | 0.17 | aBNF141 | 75.0 | Nishi et al. (2018)130 | 77.0 | Nishi et al. (2018)130 | 81.0 | Nishi et al. (2018)130 | 77.2 | SYNTAX (2011)59 |
| Calcium channel blocker | Amlodipine, 5 mg/day | 0.17 | 40.0 | 27.0 | 32.0 | 22.7 | |||||
| Long-acting nitrate | Isosorbide mononitrate, 60 mg/day | 0.18 | 36.0 | 23.0 | 47.0 | 8.0 | |||||
| Secondary prevention | |||||||||||
| Aspirin | Aspirin, 75 mg/day | 0.02 | aBNF141 | 95.0 | COURAGE (2007)91 | 95.0 | COURAGE (2007)91 | 95.0 | Assumed the same as for OMT (TN) | 83.3 | SYNTAX (2011)59 |
| Statin | Atorvastatin, 80 mg/day | 0.02 | 95.0 | 93.0 | 95.0 | 85.5 | |||||
| ACE inhibitor | Ramipril, 10 mg/day | 0.24 | 62.0 | 64.0 | 62.0 | 52.5 | |||||
| Cost (£) per year | 163.63 | 150.10 | 169.68 | 126.27 | |||||||
Health state and clinical event costs
The costs associated with health state membership and clinical events in the prognostic model are summarised in Appendix 8, Table 72.
It was assumed in the base-case analysis that only MI and unplanned revascularisation events would incur costs. This equates to assuming that all health-care resource use other than anti-angina and secondary prevention medication use (see Optimal medical treatment costs) is the same across health states. The unit costs of MI and unplanned revascularisation events were sourced from the NHS reference costs 2017/18138 and uprated to the 2018/19 price year. 137 The cost of a MI was estimated as the activity-weighted average across all HRG codes for actual or suspected MI (EB10A–E). The cost of an unplanned revascularisation (£4812.23) comprises the cost of PCI (activity-weighted average of EY40A–D and EY41A–D codes: £3923) and CABG (activity-weighted average of ED26A–C, ED27A–C and ED28A–C codes: £10,762) for non-elective long stays, and assumes that 87% of revascularisation performed is PCI (see Complications due to revascularisation).
Analytic methods
Overview
The cost-effectiveness of the QAngio XA 3D/QFR and CAAS vFFR imaging software used during ICA for assessing the functional significance of coronary obstructions in patients with intermediate stenosis is evaluated by comparing the total expected costs and QALYs with those obtained using pressure wire FFR/iFR measurement or visual interpretation of angiographic images alone.
Summarising cost-effectiveness using conventional incremental cost-effectiveness ratios (ICERs) requires consideration of which pairwise comparisons are appropriate when calculating incremental costs and effects. With multiple strategies there are several different comparisons that could be made, each resulting in different incremental QALYs, costs and ICERs. In this case, a fully incremental ICER comparison is required to rule out any strategies based on the principles of dominance or extended dominance. However, even with a fully incremental ICER comparison, strategies that are dominated and would not be considered cost-effective may be ‘better’ than other non-dominated (but not cost-effective) strategies. The ICER comparison is also particularly sensitive to small differences between the strategies (e.g. small changes to the denominator in terms of QALY differences may result in highly variable ICERs). For these reasons, the cost-effectiveness of the interventions is summarised in terms of net benefit, which applies a single unambiguous decision rule when there are multiple strategies; the cost-effective strategy is the one with the highest net benefit for a given cost-effectiveness threshold.
In this analysis, the net benefit is expressed in terms of net health benefit (NHB), estimated by rearranging the ICER equation into the following equation:
Strategies are ranked in terms of NHB from highest to lowest, which is used to identify the cost-effective strategy (highest NHB), but NHB is also used to interpret the next best choice (second highest NHB) and so on. A cost-effectiveness threshold of £20,000 per additional QALY is used in the analysis.
Uncertainty in the estimates of cost-effectiveness of the alternative strategies is reflected in probabilistic analysis, and the probability that each strategy is more cost-effective than the others at the cost-effectiveness threshold of £20,000 per QALY is presented for the base-case scenario.
Base-case analysis
The base-case parameters and associated assumptions are listed alongside their sources in Appendix 8, Table 73.
Scenario analyses
Alternative assumptions to those of the base-case analysis are made in a number of scenarios analyses. The aim of these analyses is to assess the robustness of the base-case results to alternative assumptions and sources of data used to parametrise the model.
Table 13 summarises the alternative scenarios analyses undertaken. The position in the base-case analysis and the alternative assumption applied is described for each element.
| Scenario | Element | Position in base-case analysis | Variation in scenario analysis |
|---|---|---|---|
| Diagnostic accuracy | |||
| 1 | Diagnostic accuracy of QAngio XA 3D/QFR | Sensitivity and specificity estimates based on the bivariate meta-analysis for all studies using cQFR or non-specified QFR | Sensitivity and specificity estimates based on studies reporting fQFR only |
| 2 | Sensitivity and specificity estimates based on studies reporting cQFR only | ||
| 3 | Perfect sensitivity and specificity for QFR (i.e. the same as FFR) | ||
| 4 | Diagnostic accuracy of CAAS vFFR | Sensitivity and specificity estimates based on the largest study of vFFR, FAST-EXTEND18 | Sensitivity and specificity estimates based on ILUMIEN I19 |
| 5 | Sensitivity and specificity estimates based on Jin et al.26 | ||
| 6 | Same sensitivity and specificity for vFFR and QFR | ||
| 7 | Diagnostic accuracy of ICA | Sensitivity and specificity estimates based on the bivariate meta-analysis of studies comparing 2D ICA with FFR | Sensitivity and specificity estimates based on meta-analysis by Danad et al.100 for diagnostic performance of ICA compared with FFR |
| 8 | Diagnostic threshold of QFR and FFR | Diagnostic threshold of 0.8 used to define functionally significant stenosis for QFR and FFR | An alternative diagnostic threshold of 0.75 used for FFR and QFR based on the findings of the IRIS-FFR registry data (note it is not possible to explore an alternative diagnostic threshold for vFFR because of an absence of data) |
| 9 | Grey-zone boundary for hybrid QFR and confirmatory FFR strategy | Grey-zone boundary of 0.78–0.84 for QFR as recommended by the manufacturer of QAngio XA 3D/QFR | A wider grey-zone boundary of 0.70–0.90 for strategy 4 of QFR plus confirmatory FFR when QFR is inconclusive |
| Risk of MACEs | |||
| 10 | Baseline risk of MACE | The baseline risk of MACE in the absence of revascularisation depends on disease severity as measured by FFR, whereas the distribution of FFR values differs by diagnostic strategy | The baseline risk of MACE is independent of FFR and diagnostic test results |
| 11 | Treatment effect of revascularisation on MACE | No treatment effect of revascularisation on risk of MACE based on the findings from ISCHEMIA95 | A significant reduction in the risk of MACE for revascularisation in FFR values < 0.76 based on the findings of the IRIS-FFR registry data |
| 12 | A significant reduction in the risk of unplanned revascularisation and no reduction for cardiac death or MI based on the findings of trials that showed a positive effect of revascularisation on MACE for repeat/emergency or unplanned revascularisation rather than cardiac death or MI | ||
| 13 | A reduction in the risk of MACE for all components based on the findings of trials that reported a modest (but non-statistically significant) improvement in MACE for revascularisation | ||
| Costs of diagnostic tests | |||
| 14 | Patient throughput | Costs of QFR and vFFR based on an average annual throughput of 200 patients | Alternative average annual throughput of 100 patients for QFR and vFFR |
| Costs of revascularisation | |||
| 15 | Cost of PCI | Cost of PCI across all HRGs | Cost of PCI based on day cases only |
| 16 | Revascularisation procedure | Proportion of revascularisations assumed to be PCI in 87% of cases and CABG in 13% based on BCIS audit data | Alternative assumption of 75% PCI and 25% CABG |
| HRQoL | |||
| 17 | Duration of HRQoL benefits | HRQoL benefits of revascularisation and OMT observed at 1 year based on the findings of Nishi et al.130 for the TP and FN health states applied for a lifetime duration | Alternative assumptions about the duration of HRQoL benefits of revascularisation and OMT |
| 18 | Magnitude of HRQoL benefits | No HRQoL benefits associated with treatment based on the findings from ORBITA97 | |
| 19 | Procedural disutility | Procedural disutility associated with FFR, equivalent to that of PCI | Higher procedural disutility associated with FFR, equivalent to that of CABG |
| Procedural complications associated with FFR | |||
| 20 | FFR procedural death rate | Procedural death risk sourced from Fearon et al.87 | No procedural death risk from FFR |
| 21 | FFR adverse event rates | Adverse event rates sourced from IRIS-FFR registry13 | Adverse event rates sourced from RIPCORD101 |
| 22 | Adverse event rates sourced from ORBITA97 | ||
| Setting | |||
| 23 | Cost of FFR/iFR | Unit cost of FFR/iFR corresponds to the incremental cost of FFR/iFR compared with ICA alone (i.e. difference between a complex and a standard catheterisation) | Unit cost of FFR/iFR corresponds to the cost of a complex catheterisation |
| 24 | Patient throughput | Costs of QFR and vFFR based on an average annual throughput of 200 patients | Costs of QFR and vFFR based on an average annual throughput of 500 patients |
Model validation
The model was developed in Microsoft Excel by two analysts (AD and LS) and the programming checked by a third analyst (CR). As part of an overall quality assurance process, the internal validity of the model was assessed by extensively exploring logical consistency in the model results. A separate version of the prognostic model was also independently programmed by the third analyst (CR) to successfully replicate the base-case results.
Results of the independent economic assessment
Results of the base-case scenario
Deterministic and probabilistic cost-effectiveness results expressed in terms of NHB at a cost-effectiveness threshold of £20,000 per additional QALY for the base-case scenario are presented in Tables 14 and 15, respectively. Strategy ranking from highest to lowest NHB is presented in both tables. The incremental net health benefit (INHB) is calculated for each strategy relative to ICA alone. The probability that each strategy is cost-effective at a threshold of £20,000 per additional QALY is presented in Table 15 for the probabilistic analysis. The results are consistent for both the deterministic and probabilistic analyses.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa,b | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank | Probability cost-effective at £20,000/QALY |
|---|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.039 | 4696 | 10.804 | – | 5 | 0.100 |
| 2 | ICA plus FFR | 11.073 | 4825 | 10.831 | 0.027 | 1 | 0.278 |
| 3 | ICA plus QFR | 11.065 | 4813 | 10.824 | 0.020 | 2 | 0.218 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.070 | 5020 | 10.819 | 0.015 | 4 | 0.199 |
| 5 | ICA plus vFFR | 11.076 | 5119 | 10.820 | 0.016 | 3 | 0.204 |
The strategy with the highest NHB is strategy 2 (ICA plus FFR) but the difference between all the strategies is small. Strategy 2 is also the strategy with the highest probability of being cost-effective (27.8%). The least costly strategy is strategy 1 (ICA alone), which also has the lowest QALY gain, whereas the most costly strategy is strategy 5 (ICA plus vFFR) but this has the highest QALY gain. The INHB per patient diagnosed for each of the strategies relative to ICA alone is 0.027 QALYs (or equivalently £544) for ICA plus FFR; 0.020 QALYs (or equivalently £400) for ICA plus QFR; 0.015 QALYs (or equivalently £298) for ICA plus QFR, followed by confirmatory FFR when QFR is inconclusive; and 0.016 QALYs (or equivalently £316) for ICA plus vFFR.
To understand the difference in NHB between the alternative strategies, the disaggregated results for total expected costs and QALYs are informative. Table 16 shows the expected costs and QALYs for each strategy from the diagnostic and prognostic components of the model separately. The diagnostic model includes the costs of the diagnostic tests, revascularisation, and costs associated with treating adverse events related to FFR, and the prognostic model includes costs related to unplanned/repeat revascularisation, MI events and long-term OMT use. The diagnostic model includes a procedural disutility associated with invasive FFR/iFR and revascularisation, and the prognostic model includes the long-term symptom-free benefits of revascularisation, a disutility associated with unplanned/repeat revascularisation, MI events and history of MI, applied to a baseline utility for patients with intermediate stenosis. Note that costs, adverse events and disutility associated with invasive ICA are excluded from the model because all strategies undergo ICA. The disaggregated costs and QALYs from the diagnostic component of the model are shown in Table 17, and the proportion of patients who enter the long-term prognostic model in each of the TN, FN, TP and FP entry states for each of the alternative strategies (based on the diagnostic accuracy results) is shown in Table 18.
| Strategy | Identification | Model | Total model results | ||||
|---|---|---|---|---|---|---|---|
| Diagnostic | Prognostic | ||||||
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | ||
| 1 | ICA alone | 1940 | –0.0044 | 2757 | 11.043 | 4696 | 11.039 |
| 2 | ICA plus FFR | 2059 | –0.0093 | 2766 | 11.082 | 4825 | 11.073 |
| 3 | ICA plus QFR | 2044 | –0.0037 | 2769 | 11.069 | 4813 | 11.065 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 2259 | –0.0051 | 2761 | 11.075 | 5020 | 11.070 |
| 5 | ICA plus vFFR | 2373 | –0.0050 | 2747 | 11.081 | 5119 | 11.076 |
| Strategy | Identification | Costs (£) | QALY loss | |||
|---|---|---|---|---|---|---|
| Testing | Revascularisation | Adverse events (FFR) | Testing (FFR) | Revascularisation | ||
| 1 | ICA alone | – | 1940 | – | – | –0.00440 |
| 2 | ICA plus FFR | 437 | 1620 | 1.49 | –0.00559 | –0.00367 |
| 3 | ICA plus QFR | 431 | 1613 | – | – | –0.00365 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 519 | 1739 | 0.21 | –0.00113 | –0.00394 |
| 5 | ICA plus vFFR | 172 | 2199 | – | – | –0.00498 |
| Strategy | Identification | Diagnostic accuracy | Percentage of revascularisation | |||||
|---|---|---|---|---|---|---|---|---|
| TN (n) | FN (n) | TP (n) | FP (n) | PPV (%) | NPV (%) | |||
| 1 | ICA alone | 0.368 | 0.150 | 0.251 | 0.229 | 52.3 | 71.0 | 48.0 |
| 2 | ICA plus FFR | 0.598 | 0.000 | 0.401 | 0.000 | 100 | 100 | 40.1 |
| 3 | ICA plus QFR | 0.537 | 0.063 | 0.338 | 0.061 | 84.8 | 89.5 | 39.9 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 0.541 | 0.028 | 0.373 | 0.057 | 86.8 | 95.2 | 43.0 |
| 5 | ICA plus vFFR | 0.443 | 0.012 | 0.389 | 0.155 | 71.5 | 97.3 | 54.4 |
Certain strategies have among the highest diagnostic model costs, as they result in a greater proportion of revascularisations. The percentage of revascularisation is dependent on the rate of TP and FP results. The PPV is highest for strategies involving QFR [strategies 3 (84.8%) and 4 (86.8%)], compared with vFFR (71.5%) or ICA alone (52.3%), whereas FFR is assumed to have perfect PPV. This means that there are more unnecessary revascularisations for vFFR and ICA alone, than for QFR or FFR, which increases the costs of revascularisation for these tests. Some of the total diagnostic model cost is offset by differences in costs of testing. The costs of the diagnostic tests are dependent on the level of patient throughput, and for the base-case scenario (throughput assumed to be 200) vFFR has lower costs (£172 per patient tested) than QFR (£431) and FFR (£437). The cost of adverse events associated with FFR increases the total cost of FFR but this is very marginal because of a low likelihood of adverse events occurring. The difference in total diagnostic model costs between strategies 2 (ICA plus FFR) and 3 (ICA plus QFR) is £15 per patient diagnosed, whereas the total diagnostic model cost for strategy 4, with the addition of FFR when QFR is inconclusive, increases the cost of a QFR strategy by £215 per patient because of a higher rate of TP results. The difference in total diagnostic model costs of strategy 5 relative to strategies 2 and 3 is £314 and £329 per patient, respectively, and this is because of a higher percentage of revascularisation as a result of more FP test results. The procedural QALY loss associated with invasive FFR and revascularisation ranges from 0.0037 for ICA plus QFR (no additional adverse events for QFR relative to ICA) to 0.0093 QALYs for ICA plus FFR.
The difference in total costs between the strategies from the prognostic model is much smaller than the diagnostic model because the base-case scenario assumes that there is no treatment effect associated with revascularisation on the rates of MACE. The only difference between the strategies in terms of costs associated with MACE is because of the inverse relationship between underlying FFR and subsequent risk of MACE, that is those with lower FFR have a higher risk of a cardiovascular event, whereas those with higher FFR have a lower risk of a cardiovascular event. The prognostic model also includes a difference in annual costs for OMT between those who receive OMT in addition to revascularisation (£150 for PCI and £126 for CABG) and those who receive OMT alone (£163 for TN and £169 for FN). Strategies 5 (ICA plus vFFR) and 1 (ICA alone) have the lowest prognostic costs, which is mainly due to the lower total costs of OMT associated with a greater number of revascularisations in these strategies. The difference in total prognostic costs between strategy 3 (ICA plus QFR), with the highest cost, and strategy 5 (ICA plus vFFR), with the lowest cost, is £22 per patient.
The difference in total QALYs between the strategies from the prognostic model is largely due to the HRQoL gain associated with TP test results. The base-case scenario assumes that there is no change in baseline HRQoL associated with TN and FP test results, whereas there is a small non-statistically significant change associated with FN test results. This means that the total expected QALYs is greater for strategies with more TP test results (better sensitivity), whereas there are no HRQoL benefits associated with more TN test results (better specificity). As a result, strategies 2 (ICA plus FFR) and 5 (ICA plus vFFR) have the highest prognostic model QALY gains, and strategy 1 (ICA alone) has the lowest. These QALY gains, however, are offset by the disutility associated with diagnostic testing that is highest for strategies 2 and 5 and lowest for strategy 1.
The benefits of revascularisation, in terms of improved HRQoL, mean that the sensitivity of test results is a more important driver of cost-effectiveness than specificity because TP test results translate into higher QALY gains than mismanagement of FN results. The base-case cost-effectiveness results are largely driven by the balance between the costs of the diagnostic tests and the costs and benefits of revascularisation.
Results of the alternative scenario analyses
Diagnostic accuracy
Scenarios 1–3: using alternative sensitivity and specificity estimates for quantitative flow ratio
The sensitivity (84.3%) and specificity (89.8%) estimates for QFR, which are used to inform strategy 3 in the base-case scenario, are based on the primary bivariate meta-analysis described in Chapter 3, Bivariate meta-analysis (QAngio XA 3D/QFR), from all studies that reported diagnostic accuracy data for cQFR mode or non-specified QFR. Two separate scenarios consider the impact on cost-effectiveness of small differences in diagnostic accuracy by mode of QFR: scenario 1 uses sensitivity (81.6%) and specificity (89.4%) estimates for QFR based on studies that report only fQFR mode, whereas scenario 2 uses sensitivity (84.3%) and specificity estimates (91.4%) for QFR based on studies that report only cQFR mode. Table 19 presents the cost-effectiveness results for scenarios 1 and 2.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 1: sensitivity and specificity estimates based on fQFR mode only for strategy 3 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.084 | 4778 | 10.845 | 0.019 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
| Scenario 2: sensitivity and specificity estimates based on cQFR mode only for strategy 3 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.088 | 4775 | 10.849 | 0.023 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
In scenario 1, there is a reduction in both the PPV (from 84.8% to 83.8%) and NPV (from 89.5% to 87.9%) for strategy 3, ICA plus QFR, compared with the base-case scenario. This results in a marginally smaller reduction in total QALYs (0.003 QALYs per patient) and a decrease in total costs (£34 per patient) for strategy 3 compared with the base case, with no change in the overall ranking of NHB across strategies. In scenario 2, there is an increase in both the PPV (from 84.8% to 86.8%) and NPV (from 89.5% to 89.7%) for strategy 3 compared with the base case, due to more TN test results as a result of better specificity. This results in a marginally small increase in total QALYs (0.001 QALYs per patient) and a decrease in total costs (£37 per patient) for strategy 3 compared with the base-case scenario. This means that the sensitivity and specificity estimates used in scenario 2 are slightly more favourable for the cost-effectiveness of QFR compared with scenario 1 or the base-case scenario; however, the more favourable estimates do not result in a change in NHB ranking across the strategies.
To understand the trade-off in diagnostic test costs and adverse events associated with FFR/iFR (strategy 2) compared with QFR (strategy 3), scenario 3 considers the impact on cost-effectiveness when QFR and FFR are assumed to be a perfect test (i.e. 100% sensitivity and specificity and the same underlying distribution of FFR values are used as in the base case for strategy 2). The total number of QALYs and costs for strategy 3 increase by 0.017 QALYs and £6 per patient from the base-case scenario, which is largely due to a small increase in the number of revascularisations (from 39.9% to 40.2%). With QFR assumed to be a perfect test, strategy 3 has a higher NHB than strategy 2 (an increase of 0.008 QALYs per patient) and is ranked the most cost-effective strategy. The increase in NHB is largely due to greater total QALYs gained for strategy 3 compared with strategy 2, whereas the difference in total costs between the strategies is small (£7 per patient). The difference in total costs is small because QFR and FFR have similar costs of diagnostic testing (£431 for QFR vs. £437 for FFR/iFR), the number of revascularisations is the same under this scenario and the costs associated with treating adverse events of FFR/iFR are small (average of £1.49 per patient tested). The difference in total QALYs is due to the disutility associated with FFR/iFR and an increased risk of procedural mortality for FFR/iFR.
Scenarios 4–6: using alternative sensitivity and specificity estimates for vFFR
The sensitivity (97.0%) and specificity (74.0%) estimates for vFFR, which are used to inform strategy 5 in the base-case scenario, are based on the largest (303 patients) study of vFFR. As noted in Chapter 3, CAAS vFFR, there are only three independent studies of CAAS vFFR, one of which was published only as a conference abstract. 26 As reported in Chapter 3, Bivariate meta-analysis (CAAS vs. vFFR), the bivariate meta-analysis of CAAS vFFR studies should be interpreted with caution owing to limited data and high heterogeneity across studies. Therefore, this meta-analysis was not used to inform the economic model. Two scenarios consider the impact on cost-effectiveness of different diagnostic accuracy estimates for vFFR based on the two studies not included in the base-case scenario: scenario 4 uses sensitivity (75%) and specificity (46.5%) estimates for vFFR based on the ILUMIEN I study,19 and scenario 5 uses sensitivity (68.2%) and specificity (87.3%) estimates for vFFR based on the Jin et al. 26 conference abstract. Table 20 presents the cost-effectiveness results for scenarios 4 and 5.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 4: sensitivity and specificity estimates based on the ILUMIEN I study19 for vFFR in strategy 5 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 4 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.065 | 4813 | 10.824 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.065 | 5412 | 10.795 | –0.031 | 5 |
| Scenario 5: sensitivity and specificity estimates based on the Jin et al.26 for vFFR in strategy 5 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.065 | 4813 | 10.824 | 0.020 | 3 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 4 |
| 5 | ICA plus vFFR | 11.068 | 4360 | 10.850 | 0.024 | 2 |
In scenario 4, there is decrease in both the PPV (from 71.5% to 48.5%) and NPV (from 97.3% to 73.5%) for strategy 5 compared with the base-case scenario. This results in a substantial decrease in total QALYs (0.033 QALYs per patient) and an increase in total costs (£294 per patient) for strategy 5 compared with the base case. Strategy 5 is now ranked the least cost-effective strategy (lowest in terms of NHB), with lower NHB than in strategy 1 of ICA alone (a reduction of 0.031 QALYs per patient, equivalent to £620 per patient diagnosed at a cost-effectiveness threshold of £20,000 per QALY). In scenario 5, there is an increase in the PPV (from 71.5% to 78.3%) but a decrease in the NPV (from 97.3% to 80.3%) for strategy 5 owing to lower sensitivity and better specificity than the base-case scenario. This results in a substantial decrease in both total QALYs (0.030 QALYs per patient) and total costs (£758 per patient) for strategy 5 compared with the base-case scenario. Strategy 5, ICA plus vFFR, now appears a more cost-effective strategy than strategies 3 and 4 based on QFR and is ranked second in terms of NHB, with FFR remaining the strategy with the highest NHB.
To understand the impact of differences in diagnostic test costs between vFFR and QFR, scenario 6 considers the impact on cost-effectiveness by assuming that both tests have the same diagnostic accuracy as in strategies 3 and 5 (i.e. the sensitivity and specificity for vFFR is set equal to the base-case scenario for QFR). Table 21 presents the cost-effectiveness results for scenario 6.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 2 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 3 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.016 | 4 |
| 5 | ICA plus vFFR | 11.087 | 4554 | 10.860 | 0.034 | 1 |
In scenario 6, the total QALYs and costs are reduced for strategy 5, largely owing to a decrease in the number of revascularisations (from 54.5% to 40.0%). The only difference between strategies 5 and 3 is the difference in the costs of diagnostic testing of £258 less per patient for vFFR compared with QFR for the base-case throughput assumption of 200 patients per year. This difference in the cost of testing between vFFR and QFR, under the scenario of equivalent diagnostic accuracy, is sufficient to change the ranking of NHB across the strategies, with strategy 5 now ranked with the highest NHB.
Scenario 7: using alternative sensitivity and specificity estimate for invasive coronary angiography
The sensitivity (62.6%) and specificity (61.6%) estimates for ICA, which are used to inform strategy 1 in the base-case scenario, are based on the bivariate meta-analysis in Chapter 3, Meta-analysis of extracted figure data for two-dimensional invasive coronary angiography, from studies that reported diagnostic accuracy data for 2D ICA compared with FFR. Scenario 7 is used to assess the impact on cost-effectiveness of an alternative (higher) estimate of diagnostic accuracy for ICA based on a meta-analysis by Danad et al. 100 for diagnostic performance of ICA compared with FFR (sensitivity 71% and specificity 66% from per-vessel analysis). Table 22 presents the cost-effectiveness results for scenario 7.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.073 | 4726 | 10.837 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.018 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.010 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.006 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.005 | 4 |
In scenario 7, there is an increase in both the PPV (from 52.3% to 58.4%) and NPV (from 71.0% to 77.2%) for strategy 1 compared with the base-case scenario. This results in an increase in total QALYs (0.012 QALYs per patient) and a marginal increase in total costs (£29 per patient) for strategy 1 but with no change in the NHB ranking, with ICA alone ranked the least cost-effective strategy.
Scenario 8: using an alternative diagnostic threshold for fractional flow reserve and quantitative flow ratio
The base-case scenario uses a diagnostic threshold of FFR ≤ 0.8 to define functionally significant stenosis. In scenario 8, an alternative diagnostic threshold of FFR ≤ 0.75 is used to assess the impact on cost-effectiveness results for strategies 1, 2 and 3 compared with the base-case scenario (note that there were insufficient diagnostic accuracy data for vFFR to inform an alternative diagnostic threshold for strategy 5, whereas strategy 4 uses a hybrid approach rather than a single diagnostic threshold). In scenario 8, the diagnostic accuracy of QFR is based on the estimates reported in Chapter 3, Alternative FFR thresholds, for this alternative threshold (sensitivity 75.4% and specificity 90.6%), whereas the diagnostic accuracy of ICA was estimated at FFR ≤ 0.75 and ≥ 50% DS using the approach described in Chapter 3, Meta-analysis of extracted figure data for two-dimensional invasive coronary angiography (sensitivity 74.0% and specificity 56.4%). The use of an alternative threshold also changes the prior probability of functionally significant stenosis from 40% in the base-case scenario to 25% in scenario 8. This means that the underlying distribution of FFR values for the different diagnostic outcomes (TN, FN, TP and FP) shifts for these strategies, as the category of FFR 0.76–0.80 is ‘moved’ from the distribution of FFR in patients with functionally significant stenosis (TP and FN) to those without (TN and FP). This shift in the FFR distribution also changes the utility increment for TP, slightly increasing it for all strategies (the size of the increment varies by strategy as it is dependent on the underlying FFR distribution). The utility increment for FN was assumed to remain the same, even though this increment was estimated in patients with FFR ≤ 0.80 who did not receive revascularisation.
Table 23 presents the cost-effectiveness results for scenario 8. The NHB decreases for all three strategies but the ranking of strategies follows the same order as the base-case scenario, with strategy 2 ranked as the most cost-effective option. The total QALYs for all three strategies are reduced owing to the reduction in prior probability of functionally significant stenosis, which means that fewer patients are able to benefit from revascularisation. The proportion of patients who undergo revascularisation is reduced for both strategies 2 and 3 compared with the base-case scenario. The changes in diagnostic accuracy of strategy 3 (lower sensitivity and increased specificity) combined with lower prior probability of significant stenosis compared with the base-case results in a lower PPV (72.8% vs. 84.8%) and higher NPV (91.7% vs. 89.5%). In this scenario, the greater increase in FP compared with FN for strategy 3 results in a greater number of revascularisations for this strategy compared with strategy 2 (25.9% vs. 25%). The diagnostic accuracy of strategy 1 changes in the opposite way to strategy 3 (i.e. the sensitivity increases and the specificity decreases). Despite the lower prior probability of functionally significant stenosis, this results in more revascularisation procedures overall (51.2% vs. 48.1%), with associated higher costs and greater QALY loss compared with the base-case scenario. In the prognostic model, all three strategies accrue more costs and fewer QALYs than the base-case scenario. The lower QALY gains and higher costs result from fewer patients with TP results entering the prognostic model in all three strategies, so fewer patients benefit from the utility increment and lower medication costs associated with a TP test result.
Scenario 9: using an alternative definition of the grey zone for strategy 4
In the base-case scenario, a hybrid approach is used for strategy 4, with QFR followed by confirmatory FFR when the results of QFR are inconclusive (grey zone). The definition of the grey zone is based on the manufacturer’s recommendation of QFR 0.78–0.84. In scenario 9, an alternative wider definition is used for the grey zone of between 0.70 and 0.90 to assess the impact on the cost-effectiveness of strategy 4.
Table 24 presents the cost-effectiveness results for scenario 9.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.061 | 4697 | 10.826 | 5 | |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.097 | 5097 | 10.842 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
The wider definition of the grey zone in scenario 9 increases the proportion of patients in the grey zone compared with the base-case scenario from 20.2% to 61.6%, which changes the diagnostic accuracy of strategy 4 with more confirmatory FFR tests. The PPV and NPV of strategy 4 increases by 10.9% and 3.7%, respectively, compared with the base-case scenario. By widening the grey-zone definition, more patients undergo FFR, which increases the costs of testing for strategy 4 by £181 compared with the base-case scenario. However, an increase in FFR also results in fewer revascularisations than in the base-case scenario (reduced from 43.1% in the base-case scenario to 40.4% in scenario 9 for strategy 4), with a corresponding reduction in revascularisation costs of £108. The reduction in revascularisations also reduces the QALY loss owing to this procedure compared with the base-case scenario, but this is offset by the increase in QALY loss owing to FFR. In the diagnostic model, strategy 4 is more costly (£73 per patient) and incurs more QALY loss (0.0021 QALYs per patients) compared with the base-case scenario. The reduced misclassification and consequent improved clinical management of patients results in higher QALY gains (0.005 QALYs per patient) for strategy 4 in the prognostic model, than in the base-case scenario.
Overall, both the total QALYs (0.04 QALYs per patient) and total costs (£78 per patient) are higher for strategy 4 compared with the base-case scenario, which results in a small reduction in NHB (0.001 QALYs), compared with the base-case scenario, which leads to the same NHB for strategies 4 and 5.
Risk of major adverse cardiovascular events
Scenario 10: baseline risk of major adverse cardiac events independent of fractional flow reserve and diagnostic test results
The baseline risk of MACE in the base-case scenario depends on disease severity as measured by FFR value, where lower FFR values are indicative of a higher cardiovascular event rate and higher FFR values of a lower rate, where the distribution of FFR values differs by diagnostic strategy. In scenario 10, the dependency on FFR is removed and the impact on cost-effectiveness is assessed by considering the baseline risk of MACE to be completely independent of FFR and diagnostic test results.
Table 25 presents the cost-effectiveness results for scenario 10.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.109 | 4573 | 10.881 | – | 5 |
| 2 | ICA plus FFR | 11.176 | 4698 | 10.942 | 0.061 | 1 |
| 3 | ICA plus QFR | 11.154 | 4688 | 10.920 | 0.039 | 4 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.168 | 4893 | 10.923 | 0.042 | 2 |
| 5 | ICA plus vFFR | 11.172 | 4979 | 10.923 | 0.042 | 3 |
In scenario 10, the overall risk of MACE is 1.44% in the first year and 0.72% in subsequent years based on the IRIS-FFR registry data across all FFR values, whereas in the base-case scenario it ranges from 4.33% for FFR values < 0.70 to 0.64% for FFR values > 0.90. In scenario 10, this results in an increase in total QALYs (0.048 for strategy 1, 0.080 for strategy 2, 0.067 for strategy 3, 0.075 for strategy 4 and 0.074 for strategy 5) and a decrease in total costs per patient for each strategy (£124 for strategy 1, £127 for strategy 2, £124 for strategy 3, £126 for strategy 4 and £139 for strategy 5) compared with the base-case scenario. The corresponding NHB ranking changes because strategies 3–5 are very similar in terms of NHB (10.920 QALYs for strategy 3 and 10.923 QALYs for strategies 4 and 5), and strategy 2 remains the most cost-effective option and strategy 1 the least. The removal of dependency on FFR values appears to have the greatest impact on strategy 5, ICA plus vFFR. This is largely because this strategy has more TP test results (better sensitivity) than strategies 3 and 4 using QFR. In scenario 10, the baseline risk of MACE is lower for FFR values ≤ 0.80 and greater for FFR values > 0.80, compared with the baseline risk of MACE used in the base-case scenario. This means that strategies with more TN test results (better specificity) are penalised with a higher risk of MACE, and strategies with more TP test results (better sensitivity) benefit from a lower risk of MACE. As a result, the NHB for strategy 5 improves more than for strategies 3 and 4. This is in line with the base-case conclusion that sensitivity of test results is a more important driver of cost-effectiveness than specificity because TP test results translate into higher QALY gains than mismanagement of FN results.
Scenarios 11–13: treatment effect of revascularisation on major adverse cardiac event
The base-case scenario assumes that there is no treatment effect associated with revascularisation on the risk of MACE based on the findings of ISCHEMIA95 (i.e. revascularisation does not confer additional benefits over and above OMT on the risk of MACE). Three separate scenarios are used to consider the impact on cost-effectiveness of alternative assumptions about the benefits of revascularisation on MACE outcomes compared with OMT: scenario 11 considers a significant reduction in the risk of MACE for revascularisation in patients with a FFR value < 0.76, based on the findings of the IRIS-FFR registry data, which show that there was a statistically significant reduction in the risk of clinical outcomes only for lesions with a FFR < 0.76; scenario 12 considers a statistically significant reduction in the risk of unplanned revascularisation, and there is no reduction for cardiac death or MI, based on the findings of trials that showed a positive effect of revascularisation on MACE for repeat/emergency or unplanned revascularisation rather than cardiac death or MI; and scenario 13 considers a reduction in the risk of MACE for all components (unplanned revascularisation, cardiac death or MI) based on the findings of trials that reported a modest (but non-statistically significant) improvement in MACE for revascularisation compared with OMT. Table 26 presents the cost-effectiveness results for scenarios 11–13.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 11: a significant reduction in the risk of MACE for FFR values < 0.76 for revascularised lesions | ||||||
| 1 | ICA alone | 11.080 | 4606 | 10.850 | – | 5 |
| 2 | ICA plus FFR | 11.118 | 4709 | 10.882 | 0.032 | 1 |
| 3 | ICA plus QFR | 11.108 | 4704 | 10.873 | 0.023 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.114 | 4908 | 10.869 | 0.019 | 4 |
| 5 | ICA plus vFFR | 11.122 | 4993 | 10.872 | 0.022 | 3 |
| Scenario 12: a significant reduction in the risk of unplanned revascularisation following an index revascularisation procedure | ||||||
| 1 | ICA alone | 11.062 | 4542 | 10.835 | – | 5 |
| 2 | ICA plus FFR | 11.097 | 4590 | 10.868 | 0.033 | 1 |
| 3 | ICA plus QFR | 11.088 | 4609 | 10.858 | 0.023 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.094 | 4799 | 10.854 | 0.019 | 4 |
| 5 | ICA plus vFFR | 11.099 | 4885 | 10.855 | 0.020 | 3 |
| Scenario 13: a modest reduction in the risk of MACE following an index revascularisation procedure | ||||||
| 1 | ICA alone | 11.073 | 4686 | 10.838 | – | 5 |
| 2 | ICA plus FFR | 11.112 | 4807 | 10.871 | 0.033 | 1 |
| 3 | ICA plus QFR | 11.101 | 4797 | 10.861 | 0.023 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.108 | 5003 | 10.858 | 0.020 | 4 |
| 5 | ICA plus vFFR | 11.114 | 5101 | 10.859 | 0.021 | 3 |
In scenario 11, the HR for revascularisation compared with OMT is set equal to 0.47 for the risk of MACE (across all components) for FFR values ≤ 0.75, whereas the HR is set equal to 1 for FFR values > 0.75. Thus, strategies with more TP test results and a higher proportion of lower FFR values are expected to have better outcomes than the base-case scenario. Scenario 11 results in an increase in total QALYs (0.019 for strategy 1, 0.022 for strategy 2, 0.021 for strategy 3, 0.021 for strategy 4 and 0.024 for strategy 5) and a decrease in total costs per patient for each strategy (£91 for strategy 1, £116 for strategy 2, £108 for strategy 3, £111 for strategy 4 and £125 for strategy 5) compared with the base-case scenario. The corresponding NHB ranking switches for strategies 4 and 5, with strategy 5 appearing marginally more cost-effective than strategy 4. This is largely because strategy 5 has marginally more TP test results, which benefit from a lower risk of MACE, compared with strategy 4.
In scenario 12, the HR for revascularisation compared with OMT for lesions with a FFR ≤ 0.8 is set equal to 0.26 for the risk of unplanned revascularisation, whereas the HR is set equal to 1 for cardiac death or MI. In this scenario, there is very little impact on total QALYs across strategies, whereas the total costs per patient decrease for each strategy (£155 for strategy 1, £235 for strategy 2, £203 for strategy 3, £220 for strategy 4 and £233 for strategy 5) compared with the base-case scenario. The corresponding NHB ranking switches for strategies 4 and 5, with strategy 5 appearing marginally more cost-effective than strategy 4. Again, this is largely because strategy 5 has marginally more TP test results, which benefit from a lower risk of revascularisation, compared with strategy 4.
In scenario 13, the HR for revascularisation compared with OMT for lesions with a FFR ≤ 0.8 is set equal to 0.71 for the risk of cardiac death, 0.93 for non-fatal MI and 0.93 for unplanned revascularisation. Scenario 11 results in an increase in total QALYs (0.012 for strategy 1, 0.016 for strategy 2, 0.014 for strategy 3, 0.015 for strategy 4 and 0.016 for strategy 5) and a decrease in total costs per patient for each strategy (£11 for strategy 1, £18 for strategy 2, £15 for strategy 3, £16 for strategy 4 and £17 for strategy 5), compared with the base-case scenario. The corresponding NHB ranking switches for strategies 4 and 5, with strategy 5 appearing marginally more cost-effective than strategy 4 (see Table 26). Again, strategies with more TP test results benefit most from improved clinical outcomes than strategies with more TN test results.
Scenario 14: costs of diagnostic tests
The cost per test of QFR and vFFR in the base-case scenario is based on an average annual throughput of 200 patients per centre, which corresponds to an average cost of £431 per patient for QFR and £172 per patient for vFFR. Based on the base-case assumptions, the average cost per test of QFR is constant for an annual throughput > 100 patients, and it is expected to range from £473 (throughput of 90 patients) to £741 (throughput of 10 patients) per patient for a throughput < 100 patients. For vFFR, the average cost per test varies for throughput < 200 patients, with an average cost per test of £338 for throughput of 100 patients and increasing to £2153 for a throughput of 10 patients per centre. Scenario 14 considers the impact on cost-effectiveness of an alternative average annual throughput of 100 patients for QFR and vFFR. Table 27 presents the cost-effectiveness results for scenario 14.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4825 | 10.855 | 0.029 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.021 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5019 | 10.843 | 0.017 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5283 | 10.834 | 0.008 | 4 |
Scenario 14 results in an increase in the total costs of strategy 5, which is £166 per patient. The corresponding NHB ranking of strategies is unchanged. As an additional exploratory analysis, the average annual throughput for vFFR was varied to establish the point of indifference in NHB between strategies 3 and 5, with everything else held equal to the base-case scenario. The NHB for strategies 3 and 5 are equal (and ranked as the second highest NHB across strategies after ICA plus FFR) at an average annual throughput of 500 patients per centre, where the average cost per test of QFR is expected to be £431, while it is only £73 for vFFR.
Scenarios 15 and 16: costs of revascularisation
In the base-case scenario, the costs of revascularisation for PCI and CABG are based on a weighted average of NHS reference costs across all HRGs, whereas the proportion of patients who undergo PCI and CABG as the index procedure is based on BCIS audit data. Two alternative scenarios are used to consider the impact on cost-effectiveness of a variation in the costs of revascularisation: scenario 15 considers a lower cost of PCI (reduced from £3005 to £2179 per patient) based on day cases only from NHS reference costs, and scenario 16 considers an alternative assumption of PCI in 75% of cases (reduced from 87% in the base case) and CABG in 25% of cases (increased from 13% in the base case), which increases the average cost of revascularisation from £4031 to £4978 per patient. Table 28 presents the cost-effectiveness results for scenarios 15 and 16.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 15: cost of PCI based on day cases only | ||||||
| 1 | ICA alone | 11.061 | 4351 | 10.844 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4536 | 10.869 | 0.025 | 1 |
| 3 | ICA plus QFR | 11.087 | 4525 | 10.861 | 0.017 | 3 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 4709 | 10.858 | 0.014 | 4 |
| 5 | ICA plus vFFR | 11.098 | 4726 | 10.862 | 0.018 | 2 |
| Scenario 16: proportion of revascularisations assumed to be PCI in 75% of cases and CABG in 25% | ||||||
| 1 | ICA alone | 11.054 | 5218 | 10.793 | – | 5 |
| 2 | ICA plus FFR | 11.090 | 5274 | 10.826 | 0.033 | 1 |
| 3 | ICA plus QFR | 11.081 | 5259 | 10.818 | 0.025 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.087 | 5495 | 10.812 | 0.019 | 3 |
| 5 | ICA plus vFFR | 11.090 | 5699 | 10.805 | 0.012 | 4 |
In scenario 15, the total QALYs are unchanged, whereas the total costs per patient for each strategy are reduced compared with the base-case scenario (£346 for strategy 1, £289 for strategy 2, £287 for strategy 3, £310 for strategy 4 and £392 for strategy 5). The corresponding NHB ranking for strategies 3, 4 and 5 are changed, with strategy 5 appearing marginally more cost-effective than strategies 3 and 4. This is largely because strategy 5 has more TP and FP test results that benefit from a lower cost of revascularisation compared with strategies 3 and 4, which have more TN test results (better specificity).
In scenario 16, the total QALYs for each strategy are marginally reduced (ranging from 0.006 to 0.008 QALYs per patient) owing to a higher procedural disutility associated with revascularisation as a result of an increase in CABG surgery, whereas the total costs for each strategy are increased as a result of higher costs associated with CABG compared with PCI (£521 for strategy 1, £449 for strategy 2, £447 for strategy 3, £476 for strategy 4 and £581 for strategy 5). The corresponding NHB ranking of strategies is unchanged.
Scenarios 17–19: health-related quality of life
In the base-case scenario, improvement in symptom relief is the only benefit of revascularisation compared with OMT, which is assumed to be maintained over a lifetime duration. The HRQoL benefits in the FAME I and II trials108,133 observed at 1 year for the TP (revascularised with a FFR ≤ 0.8) and FN (OMT with a FFR ≤ 0.8) health states are applied in the model over a lifetime duration. Two separate scenarios are used to consider the impact on cost-effectiveness of both the duration and magnitude of HRQoL benefits of revascularisation: scenario 17 considers the impact on cost-effectiveness of the duration of HRQoL benefits by assuming that benefits are maintained for a limited duration of 5 years only and then return to baseline, whereas scenario 18 considers the impact of assuming no HRQoL benefits associated with revascularisation, over and above OMT, based on the findings of the ORBITA trial. 97 Table 29 presents the cost-effectiveness results for scenarios 17 and 18.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 17: HRQoL benefits associated with revascularisation and OMT maintained for 5 years only | ||||||
| 1 | ICA alone | 10.903 | 4697 | 10.668 | – | 3 |
| 2 | ICA plus FFR | 10.913 | 4825 | 10.672 | 0.004 | 2 |
| 3 | ICA plus QFR | 10.915 | 4812 | 10.674 | 0.006 | 1 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 10.915 | 5019 | 10.664 | –0.004 | 4 |
| 5 | ICA plus vFFR | 10.913 | 5118 | 10.657 | –0.011 | 5 |
| Scenario 18: no HRQoL benefits associated with revascularisation | ||||||
| 1 | ICA alone | 10.843 | 4697 | 10.608 | – | 1 |
| 2 | ICA plus FFR | 10.840 | 4825 | 10.598 | –0.010 | 3 |
| 3 | ICA plus QFR | 10.847 | 4812 | 10.606 | –0.002 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 10.844 | 5019 | 10.593 | –0.015 | 4 |
| 5 | ICA plus vFFR | 10.839 | 5118 | 10.583 | –0.025 | 5 |
Scenario 17 results in a significant reduction in total QALYs for each strategy compared with the base-case scenario (0.158 QALYs for strategy 1, 0.183 QALYs for strategy 2, 0.172 QALYs for strategy 3, 0.178 QALYs for strategy 4 and 0.185 QALYs for strategy 5), while the total costs remain unchanged. The corresponding impact on NHB is a change in ranking, with strategy 3 now ranked marginally more cost-effective than strategy 2, and strategy 5 is ranked the least cost-effective strategy. In fact, the shorter the duration of HRQoL benefits, the more cost-effective strategy 3 appears relative to strategy 2. This is because the benefits of revascularisation need to be maintained for longer to offset the procedural disutility associated with FFR. The point of indifference in duration of HRQoL benefits between strategies 2 and 3, with everything else held the same as the base-case scenario, is 7 years (i.e. strategy 2 appears the most cost-effective strategy only if the HRQoL benefits associated with revascularisation are maintained for at least 7 years to offset the procedural disutility associated with FFR).
Scenario 18 results in a significant reduction in total QALYs across all strategies compared with the base-case scenario (0.218 QALYs for strategy 1, 0.256 QALYs for strategy 2, 0.240 QALYs for strategy 3, 0.249 QALYs for strategy 4 and 0.259 QALYs for strategy 5), and the total costs remain unchanged. The smallest reduction in QALYs is for strategies 1 and 3 because these strategies have the lowest proportion of TP test results compared with the other strategies. The corresponding impact on NHB is a change in ranking, with strategy 1 now ranked the most cost-effective strategy, followed by strategy 3, and strategy 5 is ranked the least cost-effective strategy. ICA alone appears the most cost-effective option because there are no benefits associated with revascularisation compared with OMT and, therefore, limited benefits associated with diagnostic testing to correctly identify patients suitable for revascularisation.
An additional scenario 19 is used to assess the impact on cost-effectiveness of assuming a higher procedural disutility associated with FFR compared with the base-case scenario. In the absence of identifying an estimate of EQ-5D disutility associated with FFR, the base-case scenario assumes that the procedural disutility for FFR is equivalent to the disutility associated with PCI (a decrement of 0.0056 QALYs). In scenario 19, the impact on cost-effectiveness is assessed by considering a higher procedural disutility associated with FFR, which is equivalent to that of CABG surgery (a decrement of 0.0330 QALYs). Table 30 present the cost-effectiveness results for scenario 19.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.069 | 4825 | 10.828 | 0.002 | 4 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.021 | 1 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.088 | 5019 | 10.837 | 0.011 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 2 |
Scenario 19 results in a change in the NHB of strategies 2 and 4 with FFR. The total QALYs for both strategies are reduced, with the largest reduction, as expected, in strategy 2 (0.027 QALYs per patient for strategy 2 and 0.005 QALYs per patient for strategy 4). This changes the NHB ranking such that strategy 2 is now only marginally more cost-effective than strategy 1 of ICA alone, and strategy 3 is ranked the most cost-effective option. As an additional exploratory analysis, the procedural disutility associated with FFR was varied to establish the point of indifference in NHB between strategies 2 and 3, with everything else held equal to the base-case scenario. The NHB for strategies 2 and 3 are equal (and ranked the highest across all strategies) at a procedural disutility of 0.014 QALYs for FFR, which is 2.5 times greater than the disutility associated with PCI but less than half the disutility associated with CABG.
Scenarios 20–22: using alternative sources to inform fractional flow reserve/instantaneous wave-free ratio procedural complication rates
The base-case scenario assumes that procedural death associated with FFR/iFR is 0.015% based on Fearon et al. ,87 and the rates of other adverse events associated with FFR/iFR are taken from the IRIS-FFR study. 13 Three separate scenarios are used to explore the impact on cost-effectiveness of alternative assumptions and data sources used to inform procedural complication rates of FFR/iFR: scenario 20 sets the procedural death rate equal to zero, whereas scenarios 21 and 22 use the rates informed by the RIPCORD101 and ORBITA97 trials, respectively (both these studies were identified in Complications due to FFR/iFR (subsection of procedural adverse events) as potentially relevant data sources). In scenario 21, a procedural complication rate of 0.5% is assumed for the following adverse events: ventricular arrhythmia, vessel occlusion, coronary dissection and deep-vein thrombosis. The unit cost applied for vessel occlusion is the same as for CABG (£10,896) because this was the procedure used to treat this adverse event in the RIPCORD trial. 101 For deep-vein thrombosis, a unit cost of £997.40 was estimated based on the activity weighted average of currency codes for deep-vein thrombosis (YQ51A–E) across all HRG codes from NHS reference costs 2017/18138 and uprated to 2018/19 prices. 137 All other adverse events use the same unit costs as the base-case scenario. Scenario 22 considers only a 4.21% rate of coronary dissection, as observed in the ORBITA trial. 97 Table 31 presents the cost-effectiveness results for scenarios 20–22.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 20: no procedural death with FFR/iFR | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.098 | 4825 | 10.857 | 0.030 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.094 | 5019 | 10.843 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
| Scenario 21: FFR/iFR complication rates from RIPCORD101 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4875 | 10.853 | 0.026 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5026 | 10.842 | 0.016 | 4 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
| Scenario 22: FFR/iFR complication rates from ORBITA97 | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 5 |
| 2 | ICA plus FFR | 11.096 | 4899 | 10.851 | 0.025 | 1 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5029 | 10.842 | 0.016 | 3 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 4 |
Scenario 20 results in only a very small impact on the cost-effectiveness results. The NHB of strategies 2 and 4 increase by 0.002 and 0.0004 QALYs, respectively, compared with the base-case scenario because more patients survive FFR procedure to receive revascularisation. The small increase in revascularisations leads to more QALYs and costs accrued for both strategies, compared with base-case scenario. These differences have no impact on the ranking of NHB across strategies.
Scenarios 21 and 22 lead to an increase in the costs of adverse events for strategy 2 compared with the base-case scenario (£50 and £74 per patient, respectively), and the cost increase for strategy 4 compared with the base case is £6 and £9, respectively. Overall, this translates into a small decrease in the NHB of strategies 2 and 4, with no change in the ranking of NHB across strategies.
Scenarios 23 and 24: diagnostic-only setting
Setting details key differences between diagnostic-only and interventional settings. Scenarios 23 and 24 reflect the diagnostic-only setting by considering (1) the additional costs due to the need to refer patients who require FFR/iFR measurements to an interventional catheter laboratory and (2) alternative throughput assumptions.
In scenario 23, the unit cost of FFR/iFR corresponds to the cost of a complex catheterisation (£2202) so as to account for the additional catheterisation that would be required under this scenario (see Invasive coronary angiography and fractional flow reserve). In the base-case scenario the cost of FFR/iFR only includes the incremental cost of FFR/iFR compared with ICA (£437), as a single catheterisation allows both procedures to be performed. Scenario 24 builds on the assumptions of scenario 23 on the cost of FFR/iFR and further assumes an average annual throughput of 500 patients per diagnostic-only centre. This is the average annual throughput value at which (with everything else held equal to the base-case scenario) strategies 3 and 5 became equivalent in terms of NHB, as identified by a previous exploratory analysis. This throughput estimate is close to the average annual number of ICA procedures per diagnostic-only centre (584; see Model input parameters, Patient population, Patient throughput). Cost-effectiveness results for scenarios 23 and 24 are presented in Table 32.
| Strategy | Identification | Total QALYs | Total costs (£) | NHBa | INHBa | NHB rank |
|---|---|---|---|---|---|---|
| Scenario 23: cost of FFR/iFR accounts for additional catheterisation in a diagnostic-only setting | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 3 |
| 2 | ICA plus FFR | 11.096 | 6590 | 10.767 | –0.060 | 5 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 1 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5376 | 10.825 | –0.002 | 4 |
| 5 | ICA plus vFFR | 11.098 | 5118 | 10.842 | 0.016 | 2 |
| Scenario 24: throughput of 500 patients per year for QFR and vFFR, and cost of FFR/iFR accounts for additional catheterisation in a diagnostic-only setting | ||||||
| 1 | ICA alone | 11.061 | 4697 | 10.826 | – | 3 |
| 2 | ICA plus FFR | 11.096 | 6590 | 10.767 | –0.060 | 5 |
| 3 | ICA plus QFR | 11.087 | 4812 | 10.847 | 0.020 | 2 |
| 4 | ICA plus QFR plus confirmatory FFR (grey zone) | 11.093 | 5376 | 10.825 | –0.002 | 4 |
| 5 | ICA plus vFFR | 11.098 | 5018 | 10.847 | 0.021 | 1 |
In scenario 23, the cost of testing increases considerably for strategies 2 (£1765) and 4 (£357) compared with the base-case scenario. The sharp increase in cost for these strategies reduces their NHB, with strategy 1 now having the lowest NHB followed by strategy 4. In this scenario, strategy 3 has the highest NHB across all strategies.
In scenario 24, in addition to the changes to the testing costs of strategy 2 and 4 found for scenario 23, the increase of annual patient throughput reduces the testing costs of strategy 5 (£99 less than in the base-case scenario), and the testing costs of strategy 3 remain £431. The difference in total costs between strategy 5 and strategy 3 is reduced to £206, which is offset by the QALY gains of strategy 5 compared with strategy 3 (0.011). In this scenario, strategy 5 has the highest NHB across all strategies, but this result needs to be interpreted cautiously given the uncertainties in the diagnostic accuracy of CAAS vFFR.
The deterministic cost-effectiveness results of the base-case and scenario analyses are summarised in Appendix 9, Table 74.
Discussion of the independent economic assessment
The decision problem addressed by the model relates to the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR imaging software used during ICA for assessing the functional significance of coronary stenosis in patients with stable angina whose angiograms show intermediate stenosis. Five diagnostic strategies were addressed: strategy 1 of ICA alone (visual interpretation of angiographic images without additional testing to assess the functional significance of intermediate stenosis), strategy 2 of ICA followed by confirmatory FFR/iFR (reference standard), strategy 3 of ICA with QFR, strategy 4 of ICA with QFR, followed by confirmatory FFR/iFR when QFR is inconclusive, and strategy 5 of ICA with vFFR. The decision model considered the diagnostic accuracy of the non-invasive functional tests (QAngio XA 3D/QFR and CAAS vFFR) and ICA relative to the reference standard of pressure wire FFR/iFR to determine whether or not patients were correctly identified as having functionally significant stenosis and should progress to revascularisation (in addition to OMT) or functionally non-significant stenosis and should receive OMT without the need for revascularisation. The short-term costs and consequences associated with diagnostic testing and revascularisation were considered. These short-term consequences were then linked to longer-term costs and consequences associated with the diagnostic outcomes and treatment by modelling the risk of major adverse cardiovascular events (MI, sudden cardiac death and need for urgent/unplanned revascularisation) over a lifetime horizon.
The cost-effectiveness of the diagnostic strategies was assessed by ranking the strategies in terms of NHB from highest to lowest. The strategy with the highest NHB represents the cost-effective strategy, and the ranking is used to interpret the next best choice (second highest NHB) and so on. A cost-effectiveness threshold of £20,000 per additional QALY was used to determine cost-effectiveness. The strategy with the highest NHB was strategy 2, FFR, and the strategy ranked with the lowest NHB was strategy 1, ICA alone. The strategy with the second highest NHB was strategy 3, QFR, and the next best strategies were either strategy 4, QFR and confirmatory FFR, or strategy 5, vFFR. Strategy 2 was also the strategy with the highest probability of being cost-effective (27.8%) and strategy 1 had the lowest probability (10.0%), whereas strategies 3–5 had similar probabilities of cost-effectiveness (21.8% for strategy 3, 19.9% for strategy 4 and 20.4% for strategy 5).
The difference in NHB between strategies 2 (cost-effective) and 3 (next best strategy) was 0.007 QALYs, which is equivalent to £140 per patient diagnosed at a cost-effectiveness threshold of £20,000 per QALY gained. The diagnostic test costs for QFR and FFR were similar (£437 per test for FFR vs. £431 per test for QFR). The difference between the two strategies was largely driven by the trade-off in HRQoL between the procedural disutility associated with FFR/iFR and the HRQoL benefits associated with revascularisation. The procedural QALY loss associated with FFR/iFR was not sufficient to offset the higher QALY gains associated with revascularisation for FFR owing to more TP test results for strategy 2 than for strategy 3.
The difference in NHB between strategies 3 and 4 was 0.005 QALYs, which is equivalent to £100 per patient diagnosed at a cost-effectiveness threshold of £20,000 per QALY gained. This difference was largely driven by the additional costs of testing for strategy 4 (£519 per patient tested, with QFR followed by confirmatory FFR when QFR is conclusive), compared with strategy 3 (£431 per test for QFR without additional testing). The higher QALY gains associated with more TP test results for strategy 4 (due to the addition of confirmatory FFR in the inconclusive QFR test results) was not sufficient to offset the additional costs of testing with both QFR and FFR in strategy 4.
The difference in NHB between strategies 4 and 5 was minimal, at 0.001 QALYs (or equivalently £20 per patient diagnosed at a cost-effectiveness threshold of £20,000 per QALY gained), and the difference in NHB between strategies 3 and 5 was 0.004 QALYs (or equivalently £80 per patient diagnosed). The diagnostic test costs for vFFR were smaller than QFR (£172 per test for vFFR vs. £431 per test for QFR), and the PPV was lower and the NPV higher in strategy 5 than in strategy 3. The higher QALY gains associated with more TP test results, and the lower diagnostic testing costs, for strategy 5, compared with strategy 3, was not sufficient to offset the additional costs associated with unnecessary revascularisations due to a greater number of FP test results in strategy 5. Therefore, the benefits of improved test sensitivity of vFFR in strategy 5 are not sufficient to offset the better test specificity with QFR.
The cost-effectiveness results for strategy 5 should be interpreted with caution because of the limited availability of diagnostic accuracy studies for vFFR. The estimates of sensitivity and specificity for vFFR in strategy 5 were based on one study with 303 patients, whereas the diagnostic accuracy estimates for QFR were based on 26 studies and > 3000 lesions, which was used to inform strategies 3 and 4.
A number of alternative scenarios were considered in which the assumptions used as part of the base-case results were varied. These analyses were undertaken to assess the robustness of the base-case results to variation in the sources of data used to populate the model and alternative assumptions. These alternative scenarios showed that the cost-effectiveness results for strategy 3 were robust to the mode of flow used for QFR measurement, contrast-flow QFR or fixed-flow QFR. The results were also robust to the use of an alternative diagnostic threshold of 0.75 for FFR and QFR in strategies 2 and 3, and to a wider definition of the grey-zone region for confirmatory FFR in strategy 4. The use of different diagnostic accuracy estimates for vFFR based on two alternative studies highlighted the substantial uncertainty surrounding the cost-effectiveness of vFFR in strategy 5. In particular, the diagnostic accuracy results reported for vFFR in a conference abstract were much more favourable than in the largest single study used in the base-case analysis, which resulted in strategy 5 being ranked the second best cost-effective strategy after strategy 2.
To understand the trade-off in costs and benefits associated with strategies 2 (cost-effective) and 3 (next best strategy), a scenario was undertaken that considered both tests, FFR and QFR, to have the same diagnostic accuracy (i.e. both tests perfect, with the same underlying distribution of FFR values). In this case, the total QALYs and costs for strategy 3 increased by 0.017 QALYs and £6 per patient from the base-case scenario. Strategy 3 became cost-effective with the highest NHB (an increase of 0.008 QALYs per patient for strategy 3 compared with strategy 2). The increase in NHB was largely due to greater total QALYs gained for strategy 3 compared with strategy 2, with the difference mainly due to the procedural disutility associated with FFR/iFR and, to a lesser extent, the increased risk of procedural mortality for FFR/iFR.
In an additional exploratory scenario, the procedural disutility associated with FFR/iFR was also varied to establish the point of indifference in cost-effectiveness between strategies 2 and 3, with the diagnostic accuracy for QFR the same as used in the base case (and all other parameters the same as base case). The NHB for strategies 2 and 3 were equal (and ranked the highest across all strategies) at a procedural disutility of 0.014 QALYs for FFR/iFR, which is 2.5 times greater than the procedural disutility associated with PCI but less than half the disutility associated with CABG surgery.
A scenario was also undertaken to assess the impact on cost-effectiveness of the duration of HRQoL benefits associated with revascularisation. This scenario highlighted that the benefits need to last for at least 7 years to offset the disutility associated with FFR/iFR in the base case for strategy 2 to remain cost-effective above strategy 3.
The benefits of revascularisation, in terms of improved HRQoL, suggest that the sensitivity of test results is a more important driver of cost-effectiveness than specificity because TP test results translate into higher QALY gains than mismanagement of FN test results. This was further supported by scenario analyses that included a benefit of revascularisation on the risk of MACE. Furthermore, strategy 1 appeared cost-effective relative to the alternative strategies only when it was assumed that there were no benefits of revascularisation (i.e. no impact on risk of MACE or HRQoL gain).
When considering a diagnostic-only setting, the large additional costs of repeating diagnostic catheterisation in a subsequent health-care contact in an interventional laboratory for strategies involving a FFR/iFR measurement (strategies 2 and 4) favoured the cost-effectiveness of strategies without this testing component. Strategy 3 (QFR alone) became the strategy with the highest net benefit, followed by strategy 5 (vFFR) alone.
Conclusions from cost-effectiveness results
The base-case cost-effectiveness results showed that the test strategy with the highest net benefit (most cost-effective strategy) was ICA followed by confirmatory FFR/iFR, for a cost-effectiveness threshold of £20,000 per QALY gained. However, the difference in net benefit between this strategy and the next best strategies for the assessment of functional significance of coronary obstructions was relatively small at 0.007 QALYs (£140) for ICA with QFR, 0.012 QALYs (£240) for ICA with QFR, followed by confirmatory FFR/iFR when QFR is inconclusive, and 0.011 QALYs (£220) for ICA with vFFR. The cost-effectiveness results for the strategy of ICA with vFFR must be interpreted with caution owing to very limited data available from diagnostic accuracy studies of vFFR. In addition, there was no diagnostic information available to inform a strategy of ICA with vFFR followed by confirmatory FFR/iFR when vFFR is inconclusive.
The key drivers of cost-effectiveness were (1) the sensitivity (rather than specificity) of the tests because TP test results translated into higher QALY gains than mismanagement of FN test results, (2) the procedural QALY loss associated with FFR/iFR, (3) the magnitude and duration of the QALY gains associated with revascularisation and (4) the additional costs associated with confirmatory testing with FFR/iFR.
Chapter 6 Discussion
Statement of principal findings
Diagnostic accuracy
The diagnostic accuracy of QFR has been widely studied, with 39 studies in this review, including 5940 patients (over 7043 vessels or lesions). QFR, as measured using QAngio, is highly correlated with FFR measured with an invasive pressure wire. The average difference between FFR and QFR measurements is almost zero, and they rarely differ by more than 0.1, with about 50% of measurements differing by less than 0.04.
QAngio XA 3D/QFR at a cut-off point of 0.8 has good diagnostic accuracy to predict FFR (also at a cut-off point of 0.8), cQFR mode had a sensitivity of 85% (95% CI 78% to 90%) and a specificity of 91% (95% CI 85% to 95%) and fQFR mode had a sensitivity of 82% (95% CI 68% to 91%) and a specificity of 89% (95% CI 77% to 95%). Although there is some discordance between QFR and FFR, most FPs or FNs arise near the boundary (e.g. where one is 0.81 and the other 0.79), and the discordance may not be clinically meaningful. Data on how this accuracy may vary by key patient characteristics was very limited, and no conclusive variation could be found.
The use of a ‘grey-zone’ strategy, where patients with a QFR between 0.78 and 0.84 receive confirmatory FFR, improves diagnostic accuracy, compared with using QFR alone, to a sensitivity of 93.1% and a specificity of 92.1%. However, this improvement is dependent on assuming that the exact FFR cut-off point of 0.8 is clinically meaningful. Most FFR and QFR values differ by 0.05 or less; therefore, the grey-zone approach is mainly identifying discordant FFR and QFR results very close to the 0.8 boundary; 30.4% of patients with QFR results in the grey zone have results that are discordant with their FFR.
Data on the diagnostic accuracy of CAAS vFFR were limited to only three studies. Owing to variable reporting of results and apparent substantial heterogeneity in results across studies, a full meta-analysis was not feasible.
Although assessing the diagnostic accuracy of using standard ICA alone was not the focus of this report, studies that reported data on ICA, and targeted searches for additional data, found that ICA alone had poor diagnostic accuracy when compared with FFR. All studies that compared QFR with ICA found QFR to be superior in diagnostic accuracy.
Clinical value and implementation
This review found limited evidence on the clinical impact of using QFR. The use of a grey zone could significantly reduce the proportion of adenosine and pressure-wire-free procedures compared with universal use of FFR, without significantly affecting diagnostic accuracy. Evidence on the applicability of QAngio XA 3D/QFR suggests that the technology is applicable in a clinical context.
Given the limitations in the evidence, a simulation study was performed to investigate the impact of using QFR, with or without a grey zone, on future coronary events. The simulation found that using QFR slightly increased the revascularisation rate compared with using FFR for all, from 40.2% to 42%. Using a grey-zone strategy increased it further to 43.2%. However, all three strategies had similar numbers of resulting coronary events, suggesting that all have a broadly similar benefit when making decisions as to who should receive revascularisation.
Although CAAS vFFR appears promising, its clinical value is currently uncertain because of limited evidence and a lack of on-site prospective studies.
Cost-effectiveness
The base-case cost-effectiveness results showed that the test strategy with the highest net benefit (most cost-effective strategy) was ICA followed by confirmatory FFR/iFR (strategy 2) at a cost-effectiveness threshold of £20,000 per QALY gained. However, the difference in net benefit between this strategy and the next best strategies was relatively small at 0.007 QALYs (or equivalently £140) per patient diagnosed for ICA with QFR (strategy 3), 0.012 QALYs (or equivalently £240) per patient diagnosed for ICA with QFR followed by confirmatory FFR/iFR when QFR is inconclusive (strategy 4) and 0.011 QALYs (or equivalently £220) per patient diagnosed for ICA with vFFR (strategy 5).
A number of alternative scenarios were considered in which the assumptions used as part of the base-case results were varied. These alternative scenarios showed that the cost-effectiveness results were robust to the mode of QFR measurement (contrast-flow QFR or fixed-flow QFR), the use of an alternative diagnostic threshold of 0.75 for FFR and QFR, the use of a wider definition of the grey-zone region for confirmatory FFR/iFR when QFR is inconclusive, throughput assumptions for QFR and vFFR, alternative estimates of procedural complication rates for FFR/iFR, and dependency of MACE risk on FFR. The scenarios were also used to identify the main drivers of cost-effectiveness. The key drivers identified were (1) the sensitivity (rather than specificity) of test results because TP test results translated into higher QALY gains than mismanagement of FN test results, (2) the procedural QALY loss associated with FFR/iFR, (3) the magnitude and duration of the QALY gains associated with revascularisation and (4) the additional costs associated with confirmatory testing with FFR/iFR in strategy 4. Strategy 1 of ICA alone, without additional testing, appeared cost-effective relative to the other strategies only when it was assumed that there were no benefits of revascularisation.
Overall, the differences in net benefit at a cost-effectiveness threshold of £20,000 per QALY between ICA followed by confirmatory FFR/iFR (strategy 2), and ICA with QFR (strategy 3) are small in an interventional setting. When considering a diagnostic-only setting, ICA with QFR may result in higher net benefit at a cost-effectiveness threshold of £20,000 per QALY than strategy 1, assuming QAngio XA 3D/QFR has similar diagnostic accuracy across settings.
Strengths and limitations of the assessment
Strengths
This review includes a comprehensive systematic review of all the published literature on QFR as assessed by QAngio XA 3D/QFR and CAASS vFFR technologies, and has been conducted following recognised guidelines to ensure high quality.
The review identified a substantial literature on the diagnostic accuracy of QAngio XA 3D/QFR (37 studies and > 5000 patients) and, despite evidence of heterogeneity and variable quality in the evidence, future research is unlikely to significantly change the overall diagnostic accuracy review findings. Study authors were contacted to provide additional data, and the review includes additional data from published studies and data from as yet unpublished studies.
This review has made best use of all available data, including extracting data from published figures, to maximise the range of analyses, including analysing the diagnostic impact of using a grey zone with QFR, and performing a simulation study to assess the clinical impact of QFR on future coronary events. To our knowledge, this goes beyond any previous review or meta-analysis in the field.
This is the first study, to our knowledge, to assess the cost-effectiveness of QAngio XA 3D/QFR and CAAS vFFR. The decision model comprehensively assessed both the short-term costs and consequences associated with diagnostic testing and the longer-term impact of treatment on both costs and consequences to ensure that lifetime differences (e.g. the risk of major adverse cardiovascular events and HRQoL benefits associated with revascularisations) were appropriately quantified.
Limitations
Evidence on the CAAS vFFR technology was limited to four studies, which varied in their reporting, and appeared to have heterogeneous results. This prevented any full meta-analyses of diagnostic accuracy for CAAS vFFR or any assessment of its clinical effectiveness.
There were insufficient data allowing exploration of the impact of key patient characteristics (such as multivessel disease or diabetes) on diagnostic accuracy or clinical effectiveness, so these could not be fully investigated.
As is common in reviews of diagnostic tests, data beyond basic diagnostic accuracy, such data on the clinical impact of QAngio XA 3D/QFR, or its practical implementation, were extremely limited and could not be fully reviewed. Although a simulation study was performed to address this, it was innately limited by having to make strong assumptions about the relevant population, and the risk of events, in that population.
The cost-effectiveness results for strategy 5 with vFFR must be interpreted with caution because of very limited data available from diagnostic accuracy studies of vFFR. The use of alternative diagnostic accuracy estimates for vFFR highlighted the substantial uncertainty surrounding the cost-effectiveness of vFFR. The cost-effectiveness results were very sensitive to the procedural disutility assumed in the model for FFR/iFR and the duration of HRQoL benefits associated with revascularisation.
Uncertainties
Although there is substantial evidence of the diagnostic accuracy of QFR assessment using QAngio, it remains largely unclear which patient or lesion characteristics might significantly affect the diagnostic accuracy of QAngio XA 3D/QFR.
The clinical value of QAngio XA 3D/QFR to support decision-making on revascularisation remains uncertain, particularly what impact it might have on preventing or causing future coronary events, and whether the 0.8 cut-off point, or the proposed grey zone, is clinically appropriate. However, it appears unlikely that its clinical value or use will differ substantially from widespread use of FFR.
Prospective evidence for the clinical benefit of QFR-guided PCI is lacking. Results from the large RCTs FAVOR III Europe–Japan142 (non-inferiority trial comparing QFR with standard FFR-guided PCI) and FAVOR III China143 (superiority trial comparing QFR with angiography-alone-guided PCI), with a target recruitment of 2000 and 3860 and due to be completed in March 2022 and February 2023, respectively, will be informative.
Current evidence on CAAS vFFR is very limited, so its diagnostic accuracy, clinical value and cost-effectiveness are highly uncertain.
Chapter 7 Conclusions
Implications for health care
Clinical implications
The results of this review suggest that making revascularisation decisions using QFR as measured with QAngio XA 3D/QFR is preferable to making decisions based on DS assessment using standard ICA alone.
The high level of correlation between QFR and FFR, and the high level of diagnostic accuracy of QFR, suggest that QFR assessment could potentially replace FFR entirely, and hence remove the need for invasive pressure wire and adenosine use. Simulations suggest that replacing FFR with QFR entirely might slightly increase the number of patients who are revascularised, but would have minimal or no impact on the incidence of coronary events.
The use of a grey zone, where patients with borderline QFR values proceed to a FFR assessment, would require around 20% of patients to have a FFR. However, for around 70% of these patients the FFR assessment would agree with the existing QFR assessment. The use of a grey zone might increase the number of patients being revascularised but would appear to have almost the same future incidence of coronary events as if FFR had been used in all patients.
The current evidence on CAAS vFFR appears to be too limited for it to be used in clinical practice at this time.
Economic implications
The economic evidence suggests small differences in net benefit at a cost-effectiveness threshold of £20,000 per QALY between ICA followed by confirmatory FFR/iFR (strategy 2), and ICA with QFR (strategy 3) in an interventional setting. In a diagnostic-only setting, ICA with QFR may yield a higher net benefit at a cost-effectiveness threshold of £20,000 per QALY than ICA followed by confirmatory FFR/iFR, provided that the diagnostic accuracy of QAngio XA 3D/QFR is comparable across settings. Therefore, the use of QAngio XA 3D/QFR in line with strategy 3 is potentially a good use of NHS resources, particularly in a diagnostic-only setting.
Suggested research priorities
The substantial existing evidence for diagnostic accuracy of QAngio XA 3D/QFR suggests that further studies of diagnostic accuracy are not required. However, further prospective investigation of the diagnostic accuracy of QAngio XA 3D/QFR in patients with different lesion subtypes, including bifurcation lesion and left main location stenoses, or with three-vessel disease, may be needed to confirm trends reflected in existing evidence.
Large, prospective studies are required to assess the diagnostic accuracy and clinical feasibility of CAAS vFFR. Ideally, these should compare CAAS vFFR with ICA assessment and, if possible, with QFR.
Randomised controlled trials are required to investigate whether or not the use of QFR-guided PCI (with or without a grey zone) results in improved clinical outcomes. Such studies should follow up patients for all key coronary events, including events caused by unnecessary revascularisation and report rates of and reasons for test failure in a clinical setting and in a wide range of patients with intermediate stenosis. Results from the FAVOR III Europe–Japan142 and FAVOR III China143 will be informative.
Acknowledgements
We would like to thank Dr Gerald Clesham for his helpful clinical advice during the project, and the following authors of included studies for providing clarification or additional data: Professor Andreas Baumbach, Dr Joost Daemen, Dr Tsunekazu Kakuta, Dr Paul Knaapen, Professor Jacek Legutko, Dr Catherine Liontou, Dr Ojas Mehta, Dr Kozo Okada and Dr Greta Žiubrytė. We would also like to thank Dr Marta Soares and Dr Robert Hodgson for providing valuable comments on aspects of the economic analysis.
Contributions of authors
Ana Duarte (https://orcid.org/0000-0002-0528-4773) (Research Fellow, Health Economist) developed the economic model, contributed to the writing of the cost-effectiveness sections of the report and the economic analysis.
Alexis Llewellyn (https://orcid.org/0000-0003-4569-5136) (Research Fellow, Systematic Reviewer) contributed to the protocol, performed the systematic review and wrote most of the sections on clinical effectiveness.
Ruth Walker (https://orcid.org/0000-0003-2765-7363) (Research Fellow, Systematic Reviewer) contributed to the protocol, performed the systematic review and wrote the background and methods sections.
Laetitia Schmitt (https://orcid.org/0000-0003-1052-488X) (Research Fellow, Health Economist) contributed to the development of the economic model and commented on drafts of the cost-effectiveness sections of the report.
Kath Wright (https://orcid.org/0000-0002-9020-1572) (Information Specialist) produced the database search strategies and performed the searches.
Simon Walker (https://orcid.org/0000-0002-5750-3691) (Senior Research Fellow, Health Economist) contributed to the economic model development and commented on drafts of the cost-effectiveness sections of the report.
Claire Rothery (https://orcid.org/0000-0002-7759-4084) (Senior Research Fellow, Health Economist) contributed to the protocol, the writing of the cost-effectiveness sections, the economic analysis, economic model development and validation, and had overall responsibility for the cost-effectiveness sections of the report.
Mark Simmonds (https://orcid.org/0000-0002-1999-8515) (Senior Research Fellow, Statistician) contributed to the protocol, performed the meta-analyses and oversaw the conduct and writing of the clinical effectiveness sections and the report as a whole.
Data-sharing statement
This report does not include primary data. All data are taken from publications, as cited in the report. Full data extraction forms and the economic model are available from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Brown L, Morris S, Neave A. Health Survey for England 2017: Adult Social Care. London: NHS Digital; 2018.
- National Institute for Health and Care Excellence (NICE) . Recent-Onset Chest Pain of Suspected Cardiac Origin: Assessment and Diagnosis. Clinical Guideline [CG95] n.d. www.nice.org.uk/guidance/cg95 (accessed 26 May 2021).
- National Institute for Health and Care Excellence (NICE) . HeartFlow FFRCT for Estimating Fractional Flow Reserve from Coronary CT Angiography. Medical Technologies Guidance [MTG32] n.d. www.nice.org.uk/guidance/mtg32 (accessed 26 May 2021).
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407-77. https://doi.org/10.1093/eurheartj/ehz425.
- Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44-16. https://doi.org/10.1016/j.jacc.2012.07.013.
- National Institute for Health and Care Excellence (NICE) . QAngio XA 3D QFR and CAAS VFFR Imaging Software for Assessing the Functional Significance of Coronary Obstructions During Invasive Coronary Angiography. Final Scope 2019. www.nice.org.uk/guidance/dg43/documents/final-scope (accessed 8 September 2021).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Westra J, Tu S, Campo G, Qiao S, Matsuo H, Qu X, et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: an individual patient-data meta-analysis. Catheter Cardiovasc Interv 2019;94:693-701. https://doi.org/10.1002/ccd.28283.
- Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017;376:1813-23. https://doi.org/10.1056/NEJMoa1616540.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Simmonds MC, Higgins JP. A general framework for the use of logistic regression models in meta-analysis. Stat Methods Med Res 2016;25:2858-77. https://doi.org/10.1177/0962280214534409.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, et al. Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017;135:2241-51. https://doi.org/10.1161/CIRCULATIONAHA.116.024433.
- Andersen BK, Vestergaard M-B, Andreasen LN, Tu S, Krusell L, Maeng M, et al. CRT-400.12 Feasibility and diagnostic precision of in-procedure computed fractional flow reserve: the wire-free invasive functional imaging (WIFI) Study. JACC Cardiovasc Interv 2017;10. https://doi.org/10.1016/j.jcin.2016.12.177.
- Asano T, Katagiri Y, Chang CC, Kogame N, Chichareon P, Takahashi K, et al. Angiography-derived fractional flow reserve in the SYNTAX II trial: feasibility, diagnostic performance of quantitative flow ratio, and clinical prognostic value of functional SYNTAX score derived from quantitative flow ratio in patients with 3-vessel disease. JACC Cardiovasc Interv 2019;12:259-70. https://doi.org/10.1016/j.jcin.2018.09.023.
- Cliff Li KF, Ong PJL. TCTAP A-127 correlation of quantitative flow ratio assessment against instantaneous wave-free ratio in hemodynamic evaluation of coronary lesions. J Am Coll Cardiol 2019;73. https://doi.org/10.1016/j.jacc.2019.03.173.
- Cortés C, Rodríguez-Gabella T, Gutiérrez H, Arnold R, Serrador AM, Ramos B, et al. Quantitative flow ratio in myocardial infarction for the evaluation of non-infarct-related arteries. The QIMERA pilot study. REC Interv Cardiol 2019;1:13-20. https://doi.org/10.24875/RECICE.M19000007.
- Daemen J, Masdjedi K, Balbi M, Nuis RJ, van Zandvoort L, Ligthart J, et al. Extended validation of novel 3-dimensional quantitative coronary angiography (3D-QCA) based software to calculate vessel fractional flow reserve (vFFR): the FAST-EXTEND study. JACC Cardiovasc Interv 2019;12. https://doi.org/10.1016/j.jcin.2019.01.066.
- Ely Pizzato P, Samdani AJ, Vergara-Martel A, Palma Dallan LA, Tensol Rodrigues Pereira G, Zago E, et al. Feasibility of coronary angiogram-derived vessel fractional flow reserve in the setting of standard of care percutaneous coronary intervention and its correlation with invasive FFR. Int J Cardiol 2019;301:45-9. https://doi.org/10.1016/j.ijcard.2019.10.054.
- Emori H, Kubo T, Kameyama T, Ino Y, Matsuo Y, Kitabata H, et al. Diagnostic accuracy of quantitative flow ratio for assessing myocardial ischemia in prior myocardial infarction. Circ J 2018;82:807-14. https://doi.org/10.1253/circj.CJ-17-0949.
- Emori H, Kubo T, Kameyama T, Ino Y, Matsuo Y, Kitabata H, et al. Quantitative flow ratio and instantaneous wave-free ratio for the assessment of the functional severity of intermediate coronary artery stenosis. Coron Artery Dis 2018;29:611-17. https://doi.org/10.1097/MCA.0000000000000650.
- Goto S, Lauri F, Mejia-Renteria H, Liontou C, Lee H, Tanigaki T, et al. Angiography-Derived Functional Assessment of Left Main Coronary Stenoses n.d.
- Hamaya R, Hoshino M, Kanno Y, Yamaguchi M, Ohya H, Sumino Y, et al. Prognostic implication of three-vessel contrast-flow quantitative flow ratio in patients with stable coronary artery disease. EuroIntervention 2019;15:180-8. https://doi.org/10.4244/EIJ-D-18-00896.
- Hwang D, Choi KH, Lee JM, Mejia-Renteria H, Kim J, Park J, et al. Diagnostic agreement of quantitative flow ratio with fractional flow reserve and instantaneous wave-free ratio. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.118.011605.
- Ishihara T, Takahara M, Tsujimura T, Kurata N, Iida O, Asai M, et al. Retrospective assessments of intra- and inter-rater reliability of quantitative flow ratio. J Am Coll Cardiol 2019;73. https://doi.org/10.1016/S0735-1097(19)31772-3.
- Jin CY, Ramasamy A, Bourantas CV, Safi H, Kilic Y, Tufaro V, et al. Diagnostic accuracy of quantitative flow ratio (QFR) and vessel fractional flow reserve (vFFR) compared with fractional flow reserve (FFR) based on 7.5 frames/second coronary angiography. Eur Heart J 2019;40. https://doi.org/10.1093/eurheartj/ehz748.1037.
- Kajita A, Bezerra CG, Ozaki Y, Dan K, Melaku GD, Pinton FA, et al. Comparison between fractional flow reserve (FFR) vs. computational fractional flow reserve derived from three-dimensional intravascular ultrasound (IVUSFR) and quantitative flow ratio (QFR). JACC Cardiovasc Interv 2019;12. https://doi.org/10.1016/j.jcin.2019.01.146.
- Kameyama T, Kubo T, Emori H, Ino Y, Matsuo Y, Yamano T, et al. Usefulness of QFR measurement for non-culprit lesion of ACS patients. J Am Coll Cardiol 2016;68. https://doi.org/10.1016/j.jacc.2016.09.679.
- Kanno Y, Hoshino M, Hamaya R, Sugiyama T, Kanaji Y, Usui E, et al. Functional classification discordance in intermediate coronary stenoses between fractional flow reserve and angiography-based quantitative flow ratio. Open Heart 2020;7. https://doi.org/10.1136/openhrt-2019-001179.
- Kanno Y, Hoshino M, Sugano A, Kanaji Y, Yamaguchi M, Sumino Y, et al. Prognostic value of contrast-flow quantitative flow ratio in patients with revascularization deferral based on preserved fractional flow reserve. J Am Coll Cardiol 2019;73. https://doi.org/10.1016/S0735-1097(19)31778-4.
- Kirigaya H, Okada K, Hibi K, Akiyama E, Matsuzawa Y, Iwahashi N, et al. TCT-325 quantitative flow ratio for assessment of nonculprit coronary lesions in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019;74. https://doi.org/10.1016/j.jacc.2019.08.405.
- Kołtowski Ł, Zaleska M, Maksym J, Tomaniak M, Soliński M, Puchta D, et al. Quantitative flow ratio derived from diagnostic coronary angiography in assessment of patients with intermediate coronary stenosis: a wire-free fractional flow reserve study. Clin Res Cardiol 2018;107:858-67. https://doi.org/10.1007/s00392-018-1258-7.
- Kleczyński P, Dziewierz A, Rzeszutko L, Dudek D, Legutko J. Is quantitative flow ratio enough to accurately assess intermediate coronary stenosis? A comparison study with fractional flow reserve. Cardiol J 2019;26:793-5. https://doi.org/10.5603/CJ.2019.0116.
- Liontou C, Mejía-Renteria H, Lauri FM, Goto S, Lee HJ, Nakayama M, et al. Quantitative flow ratio for functional evaluation of in-stent restenosis. EuroIntervention 2021;17:e396-8.
- Liu T, Somi S, Bleeker G, Gotte M. Functional Assessment of Coronary Stenoses by Quantitative Flow Ratio: A Non-Invasive Alternative for Standard Fractional Flow Reserve n.d. https://doi.org/10.1093/eurheartj/ehx502.P2375.
- Mehta O, Hay M, Ihdayhid AR, Cameron J, Wong D. TCT-333 Comparison of diagnostic accuracy between quantitative flow ratio, instantaneous wave-free ratio and DILEMMA score against fractional flow reserve. J Am Coll Cardiol 2019;74. https://doi.org/10.1016/j.jacc.2019.08.414.
- Mejia-Renteria H, Lauri FM, Lee JM, McInerney A, van der Hoeven NW, de Waard GA, et al. Interindividual variations in the adenosine-induced hemodynamics during fractional flow reserve evaluation: implications for the use of quantitative flow ratio in assessing intermediate coronary stenoses. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.119.012906.
- Neylon A, Roy A, Hovasse T, Chevalier B, Garot P, Benamer H, et al. Novel non-invasive quantitative flow ratio for estimating fractional flow reserve. J Am Coll Cardiol 2016;68. https://doi.org/10.1016/j.jacc.2016.09.658.
- Sato Y, Tanaka T, Koseki K, Okuno T, Koike H, Sato K, et al. Comparison Between Quantitative Flow Ratio and Fractional Flow Reserve in Intermediate Coronary Stenosis n.d. https://doi.org/10.1016/S0735-1097(18)31722-4.
- Smit JM, Koning G, van Rosendael AR, El Mahdiui M, Mertens BJ, Schalij MJ, et al. Referral of patients for fractional flow reserve using quantitative flow ratio. Eur Heart J Cardiovasc Imaging 2019;20:1231-8. https://doi.org/10.1093/ehjci/jey187.
- Spitaleri G, Tebaldi M, Biscaglia S, Westra J, Brugaletta S, Erriquez A, et al. Quantitative flow ratio identifies nonculprit coronary lesions requiring revascularization in patients with ST-segment-elevation myocardial infarction and multivessel disease. Circ Cardiovasc Interv 2018;11. https://doi.org/10.1161/CIRCINTERVENTIONS.117.006023.
- Stähli BE, Erbay A, Steiner J, Klotsche J, Mochmann HC, Skurk C, et al. Comparison of resting distal to aortic coronary pressure with angiography-based quantitative flow ratio. Int J Cardiol 2019;279:12-7. https://doi.org/10.1016/j.ijcard.2018.11.093.
- Ties D, van Dijk R, Pundziute G, Lipsic E, Vonck TE, van den Heuvel AFM, et al. Computational quantitative flow ratio to assess functional severity of coronary artery stenosis. Int J Cardiol 2018;271:36-41. https://doi.org/10.1016/j.ijcard.2018.05.002.
- Toi S, Iijima R, Hara H, Nakamura M. Usefulness of contrast FFR and Quantitative Flow Ratio(QFR) in intermediate coronary artery stenosis. Eur Heart J 2018;39:759-60. https://doi.org/10.1093/eurheartj/ehy563.P3653.
- Tu S, Barbato E, Köszegi Z, Yang J, Sun Z, Holm NR, et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv 2014;7:768-77. https://doi.org/10.1016/j.jcin.2014.03.004.
- Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv 2016;9:2024-35. https://doi.org/10.1016/j.jcin.2016.07.013.
- Van Diemen PAA, Driessen RS, Kooistra RA, Stuijfzand WJ, Raijmakers PG, Schumacher SP, et al. A comparison between the diagnostic performance of quantitative flow ratio and non-invasive imaging modalities for diagnosing myocardial ischemia defined by FFR, a PACIFIC-trial interim analysis. Eur Heart J 2019;40. https://doi.org/10.1093/eurheartj/ehz748.0038.
- van Rosendael AR, Koning G, Dimitriu-Leen AC, Smit JM, Montero-Cabezas JM, van der Kley F, et al. Accuracy and reproducibility of fast fractional flow reserve computation from invasive coronary angiography. Int J Cardiovasc Imaging 2017;33:1305-12. https://doi.org/10.1007/s10554-017-1190-3.
- Watarai M, Otsuka M, Yazaki K, Inagaki Y, Kahata M, Kumagai A, et al. Applicability of quantitative flow ratio for rapid evaluation of intermediate coronary stenosis: comparison with instantaneous wave-free ratio in clinical practice. Int J Cardiovasc Imaging 2019;35:1963-9. https://doi.org/10.1007/s10554-019-01656-z.
- Westra J, Andersen BK, Campo G, Matsuo H, Koltowski L, Eftekhari A, et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared with pressure-derived fractional flow reserve: the FAVOR II Europe–Japan study. J Am Heart Assoc 2018;7. https://doi.org/10.1161/JAHA.118.009603.
- Westra J, Tu S, Winther S, Nissen L, Vestergaard MB, Andersen BK, et al. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: the WIFI II Study (Wire-Free Functional Imaging II). Circ Cardiovasc Imaging 2018;11. https://doi.org/10.1161/CIRCIMAGING.117.007107.
- Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol 2017;70:3077-87. https://doi.org/10.1016/j.jacc.2017.10.035.
- Yazaki K, Otsuka M, Kataoka S, Kahata M, Kumagai A, Inoue K, et al. Applicability of 3-dimensional quantitative coronary angiography-derived computed fractional flow reserve for intermediate coronary stenosis. Circ J 2017;81:988-92. https://doi.org/10.1253/circj.CJ-16-1261.
- Ziubryte G, Jarusevicius G, Unikas R. Agreement Between Quantitative Flow Ratio and FFR in Assessment of Functional Significance of Intermediate Coronary Artery Stenosis n.d.
- Legutko J, Kleczynski P, Dziewierz A, Rzeszutko L, Bartus S, Bagienski M, et al. Correlation between quantitative flow ratio (QFR) and fractional flow reserve (FFR). Eur Heart J 2017;38:481-2. https://doi.org/10.1093/eurheartj/ehx502.P2378.
- Masdjedi K, van Zandvoort LJC, Balbi MM, Gijsen FJH, Ligthart JMR, Rutten MCM, et al. Validation of 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve: fast assessment of stenosis severity (FAST)-study. EuroIntervention 2020;16:591-9. https://doi.org/10.4244/EIJ-D-19-00466.
- Mejia-Renteria H, Lee JM, Lauri F, van der Hoeven NW, de Waard GA, Macaya F, et al. Influence of microcirculatory dysfunction on angiography-based functional assessment of coronary stenoses. JACC Cardiovasc Interv 2018;11:741-53. https://doi.org/10.1016/j.jcin.2018.02.014.
- Smit JM, El Mahdiui M, van Rosendael AR, Jukema JW, Koning G, Reiber JHC, et al. Comparison of diagnostic performance of quantitative flow ratio in patients with versus without diabetes mellitus. Am J Cardiol 2019;123:1722-8. https://doi.org/10.1016/j.amjcard.2019.02.035.
- Kappetein AP, Feldman TE, Mack MJ, Morice MC, Holmes DR, Ståhle E, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J 2011;32:2125-34. https://doi.org/10.1093/eurheartj/ehr213.
- Erbay A, Steiner J, Lauten A, Landmesser U, Leistner DM, Stahli BE. Assessment of intermediate coronary lesions by fractional flow reserve and quantitative flow ratio in patients with small-vessel disease. Catheter Cardiovasc Interv 2020;96:743-51. https://doi.org/10.1002/ccd.28531.
- Kołtowski L, Maksym J, Zaleska M, Tomaniak M, Puchta D, Opolski G, et al. Diagnostic accuracy of quantitative flow ratio – a wire-free fractional flow reserve derived from diagnostic coronary angiography. Circulation 2017;136.
- Ding D, Yang J, Westra J, Chen Y, Chang Y, Sejr-Hansen M, et al. Accuracy of 3-dimensional and 2-dimensional quantitative coronary angiography for predicting physiological significance of coronary stenosis: a FAVOR II substudy. Cardiovasc Diagn Ther 2019;9:481-91. https://doi.org/10.21037/cdt.2019.09.07.
- Kim HY, Lim HS, Doh JH, Nam CW, Shin ES, Koo BK, et al. Physiological severity of coronary artery stenosis depends on the amount of myocardial mass subtended by the coronary artery. JACC Cardiovasc Interv 2016;9:1548-60. https://doi.org/10.1016/j.jcin.2016.04.008.
- Mejia-Renteria H, Lauri F, Lee JM, Van Der Hoeven N, De Waard G, De Hoyos A, et al. Implementation of computational fluid dynamics increases the diagnostic performance of angiography-derived indices of stenosis severity. J Am Coll Cardiol 2017;70:B74-5. https://doi.org/10.1016/j.jacc.2017.09.242.
- Sejr-Hansen M, Westra J, Winther S, Tu S, Nissen L, Gormsen L, et al. Comparison of quantitative flow ratio and fractional flow reserve with myocardial perfusion scintigraphy and cardiovascular magnetic resonance as reference standard. A Dan-NICAD substudy. Int J Cardiovasc Imaging 2020;36:395-402. https://doi.org/10.1007/s10554-019-01737-z.
- Sabaté M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Jiménez-Quevedo P, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 2016;387:357-66. https://doi.org/10.1016/S0140-6736(15)00548-6.
- van Diemen P, Driessen R, Stuijfzand W, Schumacher S, Bom M, Everaars H, et al. TCT-112 comparison between the diagnostic performance of quantitative flow ratio and myocardial perfusion imaging for detecting myocardial ischemia. J Am Coll Cardiol 2019;74. https://doi.org/10.1016/j.jacc.2019.08.158.
- Bertoldi EG, Stella SF, Rohde LE, Polanczyk CA. Long-term cost-effectiveness of diagnostic tests for assessing stable chest pain: modeled analysis of anatomical and functional strategies. Clin Cardiol 2016;39:249-56. https://doi.org/10.1002/clc.22532.
- Amemiya S, Takao H. Computed tomographic coronary angiography for diagnosing stable coronary artery disease: a cost-utility and cost-effectiveness analysis. Circ J 2009;73:1263-70. https://doi.org/10.1253/circj.CJ-08-1186.
- Min JK, Gilmore A, Jones EC, Berman DS, Stuijfzand WJ, Shaw LJ, et al. Cost-effectiveness of diagnostic evaluation strategies for individuals with stable chest pain syndrome and suspected coronary artery disease. Clin Imaging 2017;43:97-105. https://doi.org/10.1016/j.clinimag.2017.01.015.
- Burgers LT, Redekop WK, Al MJ, Lhachimi SK, Armstrong N, Walker S, et al. Cost-effectiveness analysis of new generation coronary CT scanners for difficult-to-image patients. Eur J Health Econ 2017;18:731-42. https://doi.org/10.1007/s10198-016-0824-z.
- Pletscher M, Walker S, Moschetti K, Pinget C, Wasserfallen JB, Greenwood JP, et al. Cost-effectiveness of functional cardiac imaging in the diagnostic work-up of coronary heart disease. Eur Heart J Qual Care Clin Outcomes 2016;2:201-7. https://doi.org/10.1093/ehjqcco/qcw008.
- Lee SP, Jang EJ, Kim YJ, Cha MJ, Park SY, Song HJ, et al. Cost-effectiveness of coronary CT angiography in patients with chest pain: comparison with myocardial single photon emission tomography. J Cardiovasc Comput Tomogr 2015;9:428-37. https://doi.org/10.1016/j.jcct.2015.02.008.
- Genders TS, Petersen SE, Pugliese F, Dastidar AG, Fleischmann KE, Nieman K, et al. The optimal imaging strategy for patients with stable chest pain: a cost-effectiveness analysis. Ann Intern Med 2015;162:474-84. https://doi.org/10.7326/M14-0027.
- Goeree R, Blackhouse G, Bowen JM, O’Reilly D, Sutherland S, Hopkins R, et al. Cost-effectiveness of 64-slice CT angiography compared with conventional coronary angiography based on a coverage with evidence development study in Ontario. Expert Rev Pharmacoecon Outcomes Res 2013;13:675-90. https://doi.org/10.1586/14737167.2013.838079.
- Espinosa G, Annapragada A. Cost-effectiveness of a novel blood-pool contrast agent in the setting of chest pain evaluation in an emergency department. AJR Am J Roentgenol 2013;201:710-19. https://doi.org/10.2214/AJR.12.9946.
- Walker S, Girardin F, McKenna C, Ball SG, Nixon J, Plein S, et al. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart 2013;99:873-81. https://doi.org/10.1136/heartjnl-2013-303624.
- Boldt J, Leber AW, Bonaventura K, Sohns C, Stula M, Huppertz A, et al. Cost-effectiveness of cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary artery disease in Germany. J Cardiovasc Magn Reson 2013;15. https://doi.org/10.1186/1532-429X-15-30.
- Westwood M, Al M, Burgers L, Redekop K, Lhachimi S, Armstrong N, et al. A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17090.
- Priest VL, Scuffham PA, Hachamovitch R, Marwick TH. Cost-effectiveness of coronary computed tomography and cardiac stress imaging in the emergency department: a decision analytic model comparing diagnostic strategies for chest pain in patients at low risk of acute coronary syndromes. JACC Cardiovasc Imaging 2011;4:549-56. https://doi.org/10.1016/j.jcmg.2011.03.008.
- Min JK, Gilmore A, Budoff MJ, Berman DS, O’Day K. Cost-effectiveness of coronary CT angiography versus myocardial perfusion SPECT for evaluation of patients with chest pain and no known coronary artery disease. Radiology 2010;254:801-8. https://doi.org/10.1148/radiol.09090349.
- Ladapo JA, Jaffer FA, Hoffmann U, Thomson CC, Bamberg F, Dec W, et al. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol 2009;54:2409-22. https://doi.org/10.1016/j.jacc.2009.10.012.
- Genders TS, Meijboom WB, Meijs MF, Schuijf JD, Mollet NR, Weustink AC, et al. CT coronary angiography in patients suspected of having coronary artery disease: decision making from various perspectives in the face of uncertainty. Radiology 2009;253:734-44. https://doi.org/10.1148/radiol.2533090507.
- Kreisz FP, Merlin T, Moss J, Atherton J, Hiller JE, Gericke CA. The pre-test risk stratified cost-effectiveness of 64-slice computed tomography coronary angiography in the detection of significant obstructive coronary artery disease in patients otherwise referred to invasive coronary angiography. Heart Lung Circ 2009;18:200-7. https://doi.org/10.1016/j.hlc.2008.10.013.
- Hernández R, Vale L. The value of myocardial perfusion scintigraphy in the diagnosis and management of angina and myocardial infarction: a probabilistic economic analysis. Med Decis Making 2007;27:772-88. https://doi.org/10.1177/0272989X07306111.
- Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8300.
- Fearon WF, Yeung AC, Lee DP, Yock PG, Heidenreich PA. Cost-effectiveness of measuring fractional flow reserve to guide coronary interventions. Am Heart J 2003;145:882-7. https://doi.org/10.1016/S0002-8703(03)00072-3.
- Hayashino Y, Nagata-Kobayashi S, Morimoto T, Maeda K, Shimbo T, Fukui T. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with type 2 diabetes and additional atherogenic risk factors. J Gen Intern Med 2004;19:1181-91. https://doi.org/10.1111/j.1525-1497.2004.40012.x.
- Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453-60. https://doi.org/10.1016/S0140-6736(11)61335-4.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6. https://doi.org/10.1136/hrt.2005.086728.
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16. https://doi.org/10.1056/NEJMoa070829.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Andrew B, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- National Institute for Health and Care Excellence . Chest Pain Overview 2020. http://pathways.nice.org.uk/pathways/chest-pain (accessed 6 April 2020).
- Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395-407. https://doi.org/10.1056/NEJMoa1915922.
- Mori Brooks M, Barsness G, Chaitman B, Chung S-C, Faxon D, Feit F, et al. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J 2008;156:528-36. https://doi.org/10.1016/j.ahj.2008.05.015.
- Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31-40. https://doi.org/10.1016/S0140-6736(17)32714-9.
- Ludman PF. BCIS National Audit. Adult Interventional Procedures 1st April 2018 to 31st March 2019. Lutterworth: British Cardiovascular Intervention Society; 2020.
- Ludman PF. BCIS Audit Returns Adult Interventional Procedures 2017–18. Lutterworth: British Cardiovascular Intervention Society; 2019.
- Danad I, Szymonifka J, Twisk JWR, Norgaard BL, Zarins CK, Knaapen P, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J 2017;38:991-8. https://doi.org/10.1093/eurheartj/ehw095.
- Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ Cardiovasc Interv 2014;7:248-55. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000978.
- National Institute for Cardiovascular Outcomes Research . National Adult Cardiac Surgery Audit 2019. Summary Report (2015 16–2017 18 Data) 2019.
- National Institute for Cardiovascular Outcomes Research . 2019 NCAP Annual Report. Improving Cardiovascular Outcomes: Timely, Specialist, Evidence-Based Care 2019.
- Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641-54. https://doi.org/10.1016/j.jacc.2014.07.973.
- Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ 2013;347. https://doi.org/10.1136/bmj.f6625.
- Pfisterer M. Trial of invasive versus medical therapy in the elderly (TIME). Heart Drug 2001;1:144-47. https://doi.org/10.1159/000048951.
- Hueb W, Lopes NH, Gersh BJ, Soares P, Machado LA, Jatene FB, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2007;115:1082-9. https://doi.org/10.1161/CIRCULATIONAHA.106.625475.
- Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503-15. https://doi.org/10.1056/NEJMoa0805796.
- De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208-17. https://doi.org/10.1056/NEJMoa1408758.
- Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928-34. https://doi.org/10.1161/01.cir.103.24.2928.
- Nishigaki K, Yamazaki T, Kitabatake A, Yamaguchi T, Kanmatsuse K, Kodama I, et al. Percutaneous coronary intervention plus medical therapy reduces the incidence of acute coronary syndrome more effectively than initial medical therapy only among patients with low-risk coronary artery disease a randomized, comparative, multicenter study. JACC Cardiovasc Interv 2008;1:469-79. https://doi.org/10.1016/j.jcin.2008.08.002.
- Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol 2008;52:894-90. https://doi.org/10.1016/j.jacc.2008.05.051.
- Jeremias A, Kaul S, Rosengart TK, Gruberg L, Brown DL. The impact of revascularization on mortality in patients with nonacute coronary artery disease. Am J Med 2009;122:152-61. https://doi.org/10.1016/j.amjmed.2008.07.027.
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet 2009;373:911-18. https://doi.org/10.1016/S0140-6736(09)60319-6.
- Thomas S, Gokhale R, Boden WE, Devereaux PJ. A meta-analysis of randomized controlled trials comparing percutaneous coronary intervention with medical therapy in stable angina pectoris. Can J Cardiol 2013;29:472-82. https://doi.org/10.1016/j.cjca.2012.07.010.
- Chacko L, P Howard J, Rajkumar C, Nowbar AN, Kane C, Mahdi D, et al. Effects of percutaneous coronary intervention on death and myocardial infarction stratified by stable and unstable coronary artery disease: a meta-analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes 2020;13. https://doi.org/10.1161/CIRCOUTCOMES.119.006363.
- Windecker S, Stortecky S, Stefanini GG, da Costa BR, daCosta BR, Rutjes AW, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 2014;348. https://doi.org/10.1136/bmj.g3859.
- Stergiopoulos K, Boden WE, Hartigan P, Möbius-Winkler S, Hambrecht R, Hueb W, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med 2014;174:232-40. https://doi.org/10.1001/jamainternmed.2013.12855.
- Pursnani S, Korley F, Gopaul R, Kanade P, Chandra N, Shaw RE, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv 2012;5:476-90. https://doi.org/10.1161/CIRCINTERVENTIONS.112.970954.
- Office for National Statistics . National Life Tables: England and Wales 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables (accessed February 2020).
- Office for National Statistics . Mortality Statistics – Underlying Cause, Sex and Age n.d. www.nomisweb.co.uk/query/construct/summary.asp?mode=construct%26version=0%26dataset=161 (accessed February 2020).
- McGeoch RJ, Oldroyd KG. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter Cardiovasc Interv 2008;71:198-204. https://doi.org/10.1002/ccd.21307.
- Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol 2006;533:77-88. https://doi.org/10.1016/j.ejphar.2005.12.056.
- Schlundt C, Bietau C, Klinghammer L, Wiedemann R, Rittger H, Ludwig J, et al. Comparison of intracoronary versus intravenous administration of adenosine for measurement of coronary fractional flow reserve. Circ Cardiovasc Interv 2015;8. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001781.
- Rudzinski W, Waller AH, Rusovici A, Dehnee A, Nasur A, Benz M, et al. Comparison of efficacy and safety of intracoronary sodium nitroprusside and intravenous adenosine for assessing fractional flow reserve. Catheter Cardiovasc Interv 2013;81:540-4. https://doi.org/10.1002/ccd.24652.
- Bagust A, Grayson AD, Palmer ND, Perry RA, Walley T. Cost effectiveness of drug eluting coronary artery stenting in a UK setting: cost–utility study. Heart 2006;92:68-74. https://doi.org/10.1136/hrt.2004.053850.
- Favarato ME, Hueb W, Boden WE, Lopes N, Nogueira CR, Takiuti M, et al. Quality of life in patients with symptomatic multivessel coronary artery disease: a comparative post hoc analyses of medical, angioplasty or surgical strategies – MASS II trial. Int J Cardiol 2007;116:364-70. https://doi.org/10.1016/j.ijcard.2006.06.001.
- Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, Spertus JA, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes 2008;1:12-20. https://doi.org/10.1161/CIRCOUTCOMES.108.798462.
- Brooks MM, Chung SC, Helmy T, Hillegass WB, Escobedo J, Melsop KA, et al. Health status after treatment for coronary artery disease and type 2 diabetes mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation 2010;122:1690-9. https://doi.org/10.1161/CIRCULATIONAHA.109.912642.
- Nishi T, Piroth Z, De Bruyne B, Jagic N, Möbius-Winkler S, Kobayashi Y, et al. Fractional flow reserve and quality-of-life improvement after percutaneous coronary intervention in patients with stable coronary artery disease. Circulation 2018;138:1797-804. https://doi.org/10.1161/CIRCULATIONAHA.118.035263.
- Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med 2020;382:1408-19. https://doi.org/10.1056/NEJMoa1916370.
- Takousi MG, Schmeer S, Manaras I, Olympios CD, Makos G, Troop NA. Health-related quality of life after coronary revascularization: a systematic review with meta-analysis. Hellenic J Cardiol 2016;57:223-37. https://doi.org/10.1016/j.hjc.2016.05.003.
- Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816-21. https://doi.org/10.1016/j.jacc.2009.11.096.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Exchangerates.org.uk . Euro (EUR) to British Pound (GBP) Exchange Rate History n.d. www.exchangerates.org.uk/EUR-GBP-exchange-rate-history.html (accessed February 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- Department of Health and Social Care . National Schedule of NHS Costs 2017 18 2020. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed February 2020).
- National Institute for Cardiovascular Outcomes Research . National Audit for Percutaneous Coronary Intervention 2019 Summary Report (2017 18 Data) 2019.
- National Institute for Health and Care Excellence (NICE) . Stable Angina: Management 2016.
- BMJ Group and Pharmaceutical Press . British National Formulary (online) n.d. https://bnf.nice.org.uk/ (accessed February 2020).
- Christiansen EH. Functional Assessment by Virtual Online Reconstruction. The FAVOR III Europe Japan Study (FAVOR III EJ) 2018. www.clinicaltrials.gov/ct2/show/NCT03729739 (accessed June 2021).
- Bo X. The FAVOR III China Study (FAVOR III) 2018. https://clinicaltrials.gov/ct2/show/NCT03656848 (accessed June 2021).
- Cliff Li KF, Chuang TH, Ong PJL. TCTAP A-131 First report on the use and repeatability of quantitative flow ratio in a South East Asian population. J Am Coll Cardiol 2019;73:S68-9. https://doi.org/10.1016/j.jacc.2019.03.177.
- Cortes Villar C, Vera Vera S, Goncalves LR, Ramos B, Serrador A, Gutierrez H, et al. Functional evolution of non-culprit lesions in acute myocardial infarction. A quantitative flow ratio study. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy563.P4625.
- Cortes C, Vera S, Catala P, Gutierrez H, Arnold R, Hinojosa W, et al. Quantitative flow ratio in myocardial infarction for the evaluation of non-infart related arteries: the QIMERA pilot study. REC Interv Cardiol 2019;1:13-20.
- Emori H, Kubo T, Kameyama T, Ino Y, Matsuo Y, Tanaka A, et al. The correlation between quantitative flow ratio (QFR) and fractional flow reserve (FFR). J Am Coll Cardiol 2016;68:B213-14. https://doi.org/10.1016/j.jacc.2016.09.666.
- Emori H, Kubo T, Tanigaki T, Kawase Y, Shiono Y, Shimamura K, et al. TCT-328 diagnostic performance of quantitative flow ratio from angiography versus fractional flow reserve from computed tomography. J Am Coll Cardiol 2019;74. https://doi.org/10.1016/j.jacc.2019.08.408.
- Tanigaki T, Emori H, Kawase Y, Kubo T, Omori H, Shiono Y, et al. QFR versus FFR derived from computed tomography for functional assessment of coronary artery stenosis. JACC Cardiovasc Interv 2019;12:2050-9. https://doi.org/10.1016/j.jcin.2019.06.043.
- Daemen J. Fast Assessment of STenosis Severity – FASTII Study n.d. https://clinicaltrials.gov/ct2/show/NCT03791320 (accessed June 2021).
- Chang Y, Chen L, Westra J, Sun Z, Guan C, Zhang Y, et al. Reproducibility of quantitative flow ratio: an inter-core laboratory variability study. Cardiol J 2018;20.
- Fuwai Hospital . The FAVOR II China Study 2017. https://clinicaltrials.gov/ct2/show/NCT03191708 (accessed June 2021).
- Zhang Y, Zhang S, Westra J, Ding D, Zhao Q, Yang J, et al. Automatic coronary blood flow computation: validation in quantitative flow ratio from coronary angiography. Int J Cardiovasc Imaging 2019;35:587-95. https://doi.org/10.1007/s10554-018-1506-y.
- Holm NR. Diagnostic Accuracy of On-Line Quantitative Flow Ratio (QFR). FAVOR II Europe–Japan (FAVOR II EJ) 2016. https://clinicaltrials.gov/ct2/show/NCT02959814 (accessed June 2021).
- Tu S, Westra J, Yang J, Birgelen Cv, Ferrara A, Pellicano M, . Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary X-ray angiography in the international multicenter FAVOR (Functional Assessment by Various FlOw Reconstructions) pilot study. J Am Coll Cardiol Intv 2016;9:2024-35.
- Hamaya R, Hoshino M, Kanno Y, Yamaguchi M, Fukuda T, Ohya H, et al. Prognostic implication of three-vessel three-dimensional quantitative coronary angiography-based contrast-flow quantitative flow ratio in patients with stable coronary artery disease. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy563.P4596.
- Choi KH. Clinical Relevance of Functional Angiography for Non-Culprit Stenosis in Patients With Acute Myocardial Infarction n.d.
- Palma Dallan LA, Pizzato P, Rodrigues Pereira GT, Vergara-Martel A, Zago E, Zimin V, et al. Application of virtual fractional flow reserve analysis in real world invasive procedures: insights from ILUMIEN-I Trial. JACC Cardiovasc Interv 2019;12:S1-2. https://doi.org/10.1016/j.jcin.2019.01.005.
- Hideo-Kajita A, Ozaki Y, Bezerra C, Dan K, Melaku GD, Waksman R, et al. Quantitative flow ratio (QFR) inter- and intra-observer reproducibility assessed at baseline and after 1 week. JACC Cardiovasc Interv 2019;12. https://doi.org/10.1016/j.jcin.2019.01.118.
- Sugiyama T, Kanno Y, Hamaya R, Hoshino M, Usui E, Kanaji Y, et al. Determinants of visual-functional mismatches as assessed by coronary angiography and 3-D angiography-based quantitative flow ratio. Eur Heart J 2019;40. https://doi.org/10.1093/eurheartj/ehz745.0439.
- Kanno Y, Hoshio M, Sugiyama T, Kanaji Y, Yamaguchi M, Hada M, et al. Hybrid QFR-FFR decision making strategy for revascularization. Eur Heart J 2019;40. https://doi.org/10.1093/eurheartj/ehz748.1022.
- Kanno Y, Yonetsu T, Kanaji Y, Usui E, Hoshino M, Hada M, et al. Accuracy of Quantitative Flow Ratio Obtained from 3D Computational Quantitative Coronary Angiography in Comparison With Invasive Fractional Flow Reserve As a Reference n.d. https://doi.org/10.1016/S0735-1097(18)31529-8.
- Zaleska M, Koltowski L, Maksym J, Chabior AK, Pohadajło A, Soliński M, et al. Quantitative flow ratio and fractional flow reserve mismatch – clinical and biochemical predictors of measurement discrepancy. Postepy Kardiol Interwencyjnej 2019;15:301-7. https://doi.org/10.5114/aic.2019.87883.
- Zaleska M, Koltowski L, Maksym J, Tomaniak M, Chabior A, Pohadajlo A, et al. Influence of diabetes mellitus and chronic kidney disease on diagnostic accuracy of quantitative flow ratio (QFR). J Am Coll Cardiol 2018;72. https://doi.org/10.1016/j.jacc.2018.08.1821.
- Mehta O, Lim R, Hay M, Ildayhid A, Zhang M, Cameron J, et al. Diagnostic accuracy of quantitative flow ratio (QFR) compared with instantaneous flow wave free ratio (iFR) and DILEMMA score to predict fractional flow reserve (FFR). Heart Lung Circ 2019;28. https://doi.org/10.1016/j.hlc.2019.06.607.
- Liontou C, Mejia-Renteria H, Goto S, Lee H, Lauri F, Macaya F, et al. Functional assessment of in-stent restenosis with quantitative flow ratio (QFR). A comparison with de novo coronary stenoses. J Am Coll Cardiol 2018;72. https://doi.org/10.1016/j.jacc.2018.08.1122.
- Mejia-Renteria H, Lauri F, Lee JM, Van Der Hoeven N, De Waard G, De Hoyos A, et al. Influence of microcirculatory resistance on the assessment of coronary stenosis severity with quantitative flow ratio (QFR): results of an international multicentre study. J Am Coll Cardiol 2017;70:B30-1. https://doi.org/10.1016/j.jacc.2017.09.121.
- Mejia-Renteria H, Lauri F, Macaya F, Liontou C, Lee JM, van der Hoeven N, et al. Evaluation of the diagnostic performance of the quantitative flow ratio (QFR) according to the inter-individual variations in the adenosine response during fractional flow reserve (FFR) measurement. J Am Coll Cardiol 2018;72. https://doi.org/10.1016/j.jacc.2018.08.1773.
- Mejia-Renteria H, Lauri F, Macaya F, Ryan N, Nombela-Franco L, Gonzalo N, et al. Diagnostic performance of the novel quantitative flow ratio to predict significant coronary stenoses. Eur Heart J 2017;38. https://doi.org/10.1093/eurheartj/ehx502.P2380.
- Macaya F, Lauri F, Mejia-Renteria H, Pareek N, Goto S, Liontou C, et al. Angiography-derived functional assessment of non-culprit stenoses with quantitative flow ratio at the time of ST-elevation myocardial infarction. J Am Coll Cardiol 2018;72. https://doi.org/10.1016/j.jacc.2018.08.1446.
- Lauri FM, Mejia-Renteria H, Lee JM, Van Der Hoeven N, De Waard G, MacAya F, et al. Improving the diagnostic accuracy of quantitative flow ratio (QFR): a proposal of QFR-fractional flow reserve (FFR) hybrid approach. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy566.P5511.
- Smit J, Koning G, van Rosendael A, El Mahdiui M, Mertens B, Jukema J, et al. Referral of Patients for Fractional Flow Reserve Using Coronary Contrast-Flow Quantitative Flow Ratio n.d. https://doi.org/10.1016/S0735-1097(18)32118-1.
- Smit JM, El Mahdiui M, van Rosendael AR, Jukema JW, Koning G, Reiber JH, et al. Diagnostic performance of quantitative flow ratio in diabetic and non-diabetic patients. Circulation 2018;138.
- Smit JM, Koning G, van Rosendael AR, El Mahdiui M, Jukema JW, Reiber JHC, et al. Diagnostic performance of quantitative flow ratio in diabetic and non-diabetic patients. Eur Heart J 2018;39.
- Erbay A, Steiner J, Lauten A, Landmesser U, Leistner D, Stahli BE. Assessment of intermediate coronary lesions by fractional flow reserve and quantitative flow ratio in patients with small-vessel disease. Eur Heart J 2018;39. https://doi.org/10.1093/eurheartj/ehy563.P4611.
- Erbay A, Steiner J, Fröhlich G, Lauten A, Landmesser U, Leistner D, et al. Quantitative Flow Ratio in the Evaluation of Intermediate Coronary Lesions in Diabetic Versus Non-Diabetic Patients n.d.
- Leistner DM, Erbay A, Steiner J, Lauten A, Landmesser U, Stahli B. Diagnostic performance of quantitative fow ratio in intermediate coronary artery lesions: a real-world single-centre experience. Kardiovask Medizin 2018;21.
- Kobayashi Y, Fearon WF. Simultaneous anatomic and physiologic assessment of coronary artery disease with coronary angiography alone. JACC Cardiovasc Interv 2019;12:271-3. https://doi.org/10.1016/j.jcin.2018.10.032.
- Asano T, Katagiri Y, Chang CC, Kogame N, Chichareon P, Takahashi K, et al. Angiography-derived fractional flow reserve in the SYNTAX II trial: diagnostic accuracy of QFR and clinical prognostic value of functional SYNTAX score derived from QFR. J Am Coll Cardiol 2018;72. https://doi.org/10.1016/j.jacc.2018.08.1449.
- Kogame N, Takahashi K, Tomaniak M, Chichareon P, Modolo R, Chang CC, et al. Clinical implication of quantitative flow ratio after percutaneous coronary intervention for 3-vessel disease. JACC Cardiovasc Interv 2019;12:2064-75. https://doi.org/10.1016/j.jcin.2019.08.009.
- Kogame N, Takahashi K, Tomaniak M, Chichareon P, Modolo R, Katagiri Y, et al. TCT-111 clinical implication of quantitative flow ratio after percutaneous coronary intervention for three vessel disease. J Am Coll Cardiol 2019;74. https://doi.org/10.1016/j.jacc.2019.08.157.
- Westra JS, Andersen BK, Vestergaard MB, Winther S, Nissen L, Boetker HE, et al. Resting Pd/Pa and FFR discordance: effect on the diagnostic performance of quantitative flow ratio (QFR) with FFR as reference standard. Eur Heart J 2017;38. https://doi.org/10.1093/eurheartj/ehx502.P2381.
- Westra J, Tu S, Nissen L, Winther S, Britt M, Andersen BK, et al. Physiological testing of coronary artery stenosis by computation of invasive coronary angiography. The wire-free functional imaging (WIFI-II) study. J Am Coll Cardiol 2016;68:B4-5. https://doi.org/10.1016/j.jacc.2016.09.887.
- Andreasen LN, Andersen BK, Vestergaard MB, Tu S, Westra JS, Reiber JHC, et al. Feasibility and diagnostic precision of in-procedure computed FFR: the wire-free invasive functional imaging (WIFI) study. EuroIntervention 2016.
- Holm NR. The Wire-Free Invasive Functional Imaging (WIFI) Study 2016. https://clinicaltrials.gov/ct2/show/NCT02795585 (accessed June 2021).
- Otsuka M, Goto M, Kataoka S, Kahata M, Kumagai A, Inoue K, et al. FFRQCA: FFR Computation Derived from 3-Dimensional Quantitative Coronary Angiography n.d.
- Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2010;122:949-57. https://doi.org/10.1161/CIRCULATIONAHA.109.911669.
- Dagenais GR, Lu J, Faxon DP, Kent K, Lago RM, Lezama C, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation 2011;123:1492-500. https://doi.org/10.1161/CIRCULATIONAHA.110.978247.
- Hochman JS. International Study Of Comparative Health Effectiveness With Medical And Invasive Approaches (ISCHEMIA): Primary Report of the Clinical Outcomes n.d.
- Spertus JA. International Study of Comparative Health Effectiveness With Medical and Invasive Approaches. Primary Report of Quality of Life Outcomes n.d.
Appendix 1 Clinical review literature search strategies
MEDLINE (via OVID)
Date searched: 1 October 2019.
Date range searched: 1946 to 25 September 2019.
Search strategy
-
QANGIO$.ti,ab,kw. (8)
-
quantitative flow ratio$.ti,ab,kw. (36)
-
QFR.ti,ab,kw. (82)
-
“3D/QFR”.ti,ab,kw. (1)
-
aQFR.ti,ab,kw. (2)
-
adenosine-flow QFR.ti,ab,kw. (2)
-
cQFR.ti,ab,kw. (6)
-
contrast-flow QFR.ti,ab,kw. (7)
-
fQFR.ti,ab,kw. (5)
-
fixed-flow QFR.ti,ab,kw. (5)
-
iQFR.ti,ab,kw. (1)
-
index QFR.ti,ab,kw. (1)
-
LQFR.ti,ab,kw. (4)
-
lesion QFR.ti,ab,kw. (1)
-
vQFR.ti,ab,kw. (1)
-
vessel QFR.ti,ab,kw. (1)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 (99)
-
vessel FFR.ti,ab,kw. (11)
-
vFFR.ti,ab,kw. (8)
-
CAAS vFFR.ti,ab,kw. (0)
-
18 or 19 or 20 (19)
-
17 or 21 (118)
-
animals/not (humans/and animals/) (4,586,208)
-
22 not 23 (107)
-
“quinol:fumarate reductase”.ti,ab. (29)
-
24 not 25 (88).
Appendix 2 Included, excluded and ongoing studies
| Main studies | Linked studies |
|---|---|
| Cliff and Ong (2019),16 conference abstract | Cliff et al.144 |
| Cortés et al. (2019)17 | Cortes Villar et al.145 and Cortes et al.146 |
| Emori et al. (2018)20 | Emori et al.20 |
| Emori et al. (2018)21 | Emori et al.147,148 and Tanigaki et al.149 |
| FAST-EXTEND: Daemen et al. (2019),18 conference abstract | Masdjedi et al.56 and Daemen150 |
| FAVOR II China: Xu et al. (2017)52 | Ding et al.,62 Chang et al.,151 Fuwai Hospital152 and Zhang et al.153 |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | Holm154 |
| FAVOR pilot: Tu et al. (2016)46 | Tu et al.155 |
| Goto et al. (2019),22 conference abstract | |
| Hamaya et al. (2019)23 | Hamaya et al.156 |
| Hwang et al. (2019)24 | Choi157 |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | Palma Dallan et al.158 |
| Ishihara et al. (2019),25 conference abstract | |
| Jin et al. (2019),26 conference abstract | |
| Kajita et al. (2019),27 conference abstract | Hideo-Kajita et al.159 |
| Kameyama et al. (2016),28 conference abstract | |
| Kanno et al. (2019),29 conference abstract | Sugiyama et al.160 and Kanno et al.161,162 |
| Kanno et al. (2019),30 conference abstract | |
| Kirigaya et al. (2019),31 conference abstract | |
| Kołtowski et al. (2018)32 | Kołtowski et al.61 and Zaleska et al.163,164 |
| Kleczyński et al. (2019)33 | Legutko et al.55 |
| Liontou et al. (2019)34 | |
| Liu et al. (2017),35 conference abstract | |
| Mehta et al. (2019),36 conference abstract | Mehta et al.165 |
| Mejia-Renteria et al. (2019)37 | Xu et al.,57 Mejia-Renteria et al.,64,167–169 Liontou et al.,166 Macaya et al.170 and Lauri et al.171 |
| Neylon et al. (2016),38 conference abstract | |
| Sato et al. (2018),39 conference abstract | |
| Smit et al. (2019)40 | Smit et al.40,58,172–174 |
| Spitaleri et al. (2018)41 | |
| Stähli et al. (2019)42 | Erbay et al.60,175,176 and Leistner et al.177 |
| SYNTAX II: Asano et al. (2019)15 | Kobayashi and Fearon,178 Asano et al.179 and Kogame et al.180,181 |
| Ties et al. (2018)43 | |
| Toi et al. (2018),44 conference abstract | |
| Tu et al. (2014)45 | |
| Van Diemen et al. (2019)47 | van Diemen et al.67 |
| van Rosendael et al. (2017)48 | van Rosendael et al.48 |
| Watari et al. (2019)49 | |
| WIFI II: Westra et al. (2018)51 | Westra et al.182,183 |
| WIFI Prototype study: Andersen et al. (2017),14 conference abstract | Andreasen et al.184 and Holm185 |
| Yazaki et al. (2017)53 | Otsuka et al.186 |
| Ziubryte et al. (2019),54 conference abstract |
| Setting | Recruitment years | Interventions | Main indications | Responsible party | Registration number | N |
|---|---|---|---|---|---|---|
| RCTs | ||||||
|
|
|
|
EH Christiansen, Aarhus University Hospital, Skejby | NCT03729739 | Target: 2000 |
|
|
|
|
Bo Xu, China National Centre for Cardiovascular Diseases | NCT03656848 | Actual: 3860 |
|
|
|
|
X Qu, Shanghai Chest Hospital | ChiCTR-INR-17011360 | Target: 100 |
|
|
|
|
Qiang Zhao, Ruijin Hospital | NCT03977129; 2018CR001 | Target: 792 |
| Single-arm diagnostic accuracy studies | ||||||
|
|
|
|
Z Zhang, Department of cardiovascular medicine, Hospital of Third Military Medical University | ChiCTR1800014516 | Target: 200 |
|
|
|
|
Pulse Medical Imaging Technology (Shanghai) Co. Ltd (Shanghai, China) | NCT03405506; CARDIAC201701 | Target: 69 |
|
|
|
|
C Jensen, Contilia Clinical Research Institute | NCT03420131; U1111–1199–4364; DRKS00012757 | Target: 250–80 |
|
|
|
|
C Kiyuk, Seoul St Mary’s Hospital | NCT04102917; XC18REDI0035 | Actual: 915 |
|
|
|
|
University of Aarhus | NCT03481712 | Target: 2000 |
|
|
|
|
Joo Myung Lee, Samsung Medical Centre | NCT03791788 | Target: 524 |
|
|
|
|
Carlos Baladron, Hospital Clínico Universitario de Valladolid | NCT04200469; CASVE-PI-19–1515 | Target: 100 |
| Reason for exclusion | Study |
|---|---|
| Population not eligible | Adjedj J, Hyafil F, Aminfar F, Farnoud A, Rubimbura V, Fournier S, et al. Hemodynamic and clinical impact in adult patients with anomalous aortic origin of the coronary artery evaluated with quantitative flow reserve. Eur Heart J 2019;40(Suppl. 1):1687 |
| Adjedj J, Hyafil F, Muller O, Aubry P. Hemodynamic and Clinical Impact in Adult Patients with Anomalous Aortic Origin of the Right Coronary Artery Evaluated with Quantitative Flow Ratio. Paper presented at EuroPCR, 21–24 May 2019, Paris, France | |
| Biscaglia S. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. Paper presented at EuroPCR, 21–24 May 2019, Paris, France | |
| Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, et al. Prognostic value of QFR measured immediately after successful stent implantation: the international multicenter prospective HAWKEYE study. JACC Cardiovasc Interv 2019;12:2079–88 | |
| Zhu Y. Effect of QFR-guided Revascularisation on 30-day mortality in Patients Undergoing Valve Surgery With Concomitant Coronary Artery Disease. 2018. URL: www.chictr.org.cn/com/25/showprojen.aspx?proj=30416 | |
| Ishibashi Y, Grundeken MJ, Nakatani S, Iqbal J, More MA, Genereux P, et al. In vitro validation and comparison of different software packages or algorithms for coronary bifurcation analysis using calibrated phantoms: implications for clinical practice and research of bifurcation stenting. Catheter Cardiovasc Interv 2015;85:554–63 | |
| Masdjedi K, Ligthart J, Witberg K, Tomaniak M, Zandvoort L, Diletti R, et al. The prognostic value of angiography-based vessel-FFR after successful percutaneous coronary intervention: the FAST outcome study. J Am Coll Cardiol 2019;74(Suppl.):B110 | |
| Campo G. Angio-based Fractional Flow Reserve to Predict Adverse Events After Stent Implantation. 2016. URL: https://clinicaltrials.gov/show/NCT02811796. | |
| Renji Hospital. Early Prediction of QFR in STEMI-I. 2018. URL: https://clinicaltrials.gov/show/NCT03780335 | |
| Renji Hospital. Early Assessment of QFR in STEMI-II. 2019. URL: https://clinicaltrials.gov/show/NCT03910400 | |
| Ozaki Y, Gonzalo N, Salazar CH, Kuku KO, Mejia-Renteria H, Hideo-Kajita A, et al. Comparison of quantitative flow ratio value of left anterior descending and circumflex coronary artery in patients with Takotsubo syndrome. Int J Cardiovasc Imaging 2019;36:3–8 | |
| Rubimbura V, Guillon B, Fournier S, Amabile N, Chi Pan C, Combaret N, et al. Validation of Quantitative Flow Reserve and Residual Quantitative Flow Reserve to Predict FFR Post-Stenting from the Does Optical Coherence Tomography Optimise Results of Stenting Study (DOCTORS) Population. Paper presented at EuroPCR, 21–24 May 2019, Paris, France | |
| Solanki R, Gosling R, Rammohan V, Hose R, Lawford P, Gunn J, et al. Assessing the accuracy of a novel in silico imaging tool for the 3D reconstruction of coronary vasculature in the context of virtual fractional flow reserve. Heart 2019;105:A14 | |
| Suzuki N, Nishide S, Kimura T, Aoyagi T, Kanamori K, Shiratori Y, et al. Relationship of quantitative flow ratio after second-generation drug-eluting stent implantation to clinical outcomes. Heart Vessels 2020;35:743–9 | |
| Tar B, Bakk S, Beres Z, Molnar F, Santa J, Svab M, et al. Calculation of the residual pressure gradient after stent implantation of the coronary lesions on the basis of 3D coronary angiography and fluid dynamic equations. Eur Heart J 2014;1:810–11 | |
| Tu S, Koszegi Z, Tar B, Reiber J. Calculation of hyperemic stenosis resistance and myocardial resistance using computational fluid dynamics combined with three-dimensional angiographic reconstruction and intracoronary pressure measurement. EuroIntervention 2013;9:93 | |
| Vedia OVC, Macaya FMT, Lauri LF, Mejia-Renteria MH, Gonzalo GN, Trigo TM, et al. Diagnostic performance of quantitative flow ratio in predicting fractional flow reserve in patients with takotsubo syndrome. Eur Heart J 2018;39(Suppl. 1):1151 | |
| Waksman R, Ozaki Y, Gonzalo N, Trivino CS, Kuku K, Renteria HM, et al. Assessment of microvascular dysfunction using quantitative flow ratio in patients with Takotsubo syndrome. J Am Coll Cardiol 2019;73(Suppl. 1):1630 | |
| Zhuk S, Smith O, Thondapu V, Halupka K, Moore S. Using contrast motion to generate patient specific blood flow simulations during invasive coronary angiography. J Biomech Eng 2019;142:021001 | |
| No eligible index test | Boogers MJ, Schuijf JD, Broerse A, Kitslaar PH, Van Velzen JE, Dijkstra J, et al. Automated quantification of area stenosis using a novel dedicated registration algorithm: a feasibility study with multi-detector row computed tomography and intravascular ultrasound. Eur Heart J 2012;33:1007–16 |
| Boogers MM, Schuijf JD, Van Werkhoven JM, Kitslaar PH, Frenay M, Dijkstra J, et al. Novel dedicated approach for automatic quantification of the degree of coronary artery stenosis on 64-slice multi-slice computed tomography: a comparison with quantitative coronary angiography. Eur Heart J 2009;1:484 | |
| Chung WY, Choi BJ, Lim SH, Matsuo Y, Lennon RJ, Gulati R, et al. Three dimensional quantitative coronary angiography can detect reliably ischaemic coronary lesions based on fractional flow reserve. J Korean Med Sci 2015;30:716–24 | |
| Chung WY, Lim SH, Matsuo Y, Gulati R, Sandhu G, Lerman A. Three dimensional quantitative coronary angiography can detect reliably ischaemic coronary lesions based on fractional flow reserve. J Am Coll Cardiol 2012;59:E172 | |
| Cook C, Kousera C, Ahmad Y, Nijjer S, Petraco R, Al-Lammee R, et al. Can computational fluid dynamics (CFD) predictions of FFR agree with invasive FFR in intermediate stenoses? Lessons from a study using OCT and invasive measures of coronary flow. EuroIntervention 2016;France.:230 | |
| Dębski M, Kruk M, Bujak S, Dzielinska Z, Demkow M, Kepka C. Coronary computed tomography angiography equals invasive angiography for the prediction of coronary revascularisation. Postepy Kardiol Interwencyjnej 2019;15:308–13 | |
| Ejlersen JA, Poulsen SH, Mortensen J, May O. Accuracy of adenosine 2D strain stress echocardiography in the detection of coronary artery disease in patients with chest pain. Eur Heart J 2015;1:601 | |
| Ferencik M, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Liu T, et al. Computed tomography-based high-risk coronary plaque score to predict acute coronary syndrome among patients with acute chest pain – results from the ROMICAT II trial. J Cardiovasc Comput Tomogr 2015;9:538–45 | |
| Gosling R, Morris P, Lawford P, Hose R, Gunn J. Virtual (computed) FFR and virtual coronary intervention (VCI) vs angiography for guiding PCI: a virtual study. Heart 2019;105:A45 | |
| Gosling RC, Morris PD, Silva Soto DA, Lawford PV, Hose DR, Gunn JP. Virtual coronary intervention: a treatment planning tool based upon the angiogram. JACC Cardiovasc Imaging 2019;12:865–72 | |
| Hebsgaard L, Nielsen TM, Tu S, Krusell LR, Maeng M, Veien KT, et al. Advanced Angiography Compared with OCT for Sizing of Coronary Stents. A Does Optical Coherence Tomography Optimise Revascularisation (DOCTOR) Fusion Substudy. Paper presented at EuroPCR, 20–23 May 2014, Paris, France | |
| Ishibashi Y, Onuma Y, Nakatani S, Morel MA, Girasis C, Wentzel JJ, et al. In Vitro Validation of Two Bifurcation Algorithms of Quantitative Coronary Angiography in Calibrated Phantoms: Comparison with a CAAS System and with a QAngio XA. Paper presented at EuroPCR, 20–23 May 2014, Paris, France | |
| Johnson NP, Matsumura M, Achenbach S, Engstrom T, Assali A, Jeremias A, et al. Angiography-derived fractional flow reserve versus invasive nonhyperemic pressure ratios. J Am Coll Cardiol 2019;73:3232–3 | |
| Kashiwabara K, Shinozaki T, Kozuma K, Oba K, Matsuyama Y. Two-by-two cross-over study to evaluate agreement between versions of a quantitative coronary analysis system (QAngio XA). Int J Cardiovasc Imaging 2017;33:779–87 | |
| Kornowski R, Vaknin-Assa H, Assali A, Greenberg G, Valtzer O, Lavi I. Online angiography image-based FFR assessment during coronary catheterization: a single-center study. J Invasive Cardiol 2018;30:224–9 | |
| Lal K, Gunn J, Morris P, Gosling R, Lawford P, Hose R, et al. Computational modelling of fractional flow reserve from coronary angiography: expert training required. Heart 2019;105:A15–16 | |
| Lee JH, Yoon MH, Tahk SJ, Shin JH, Hwang GS, Choi SY, et al. Comparision of 3-dimensional quantitative coronary angiography and intravascular ultrasound for detecting functionally significant coronary lesions. Eur Heart J 2018;39(Suppl. 1):525 | |
| Lee JM, Koo BK, Shin ES, Nam CW, Doh JH, Hu X, et al. Clinical outcomes of deferred lesions with angiographically insignificant stenosis but low fractional flow reserve. J Am Heart Assoc 2017;6:e006071 | |
| Lee JM, Koo BK, Shin ES, Nam CW, Doh JH, Hwang D, et al. Clinical implications of 3-vessel fractional flow reserve measurement in patients with coronary artery disease. J Am Coll Cardiol 2017;70(Suppl. 1):B137–8 | |
| Lee JM, Koo BK, Shin ES, Nam CW, Doh JH, Hwang D, et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J 2018;39:945–51 | |
| Leone AM, De Caterina AR, De Maria GL, Scalone G, Tagliaferro F, Gardi A, et al. Three-dimensional quantitative coronary angiography and quantification of jeopardised myocardium to predict functional significance of intermediate coronary artery stenosis. EuroIntervention 2015;11:308–18 | |
| Li J, Gong Y, Yi T, Hong T, Liu Z, Zheng B, et al. TCT-323 angiography-derived contrast fractional flow reserve from a specially designed computational fluid dynamic method. J Am Coll Cardiol 2019;74(Suppl.):B321 | |
| Li S, Chin C, Thondapu V, Poon EKW, Monty JP, Li Y, et al. Numerical and experimental investigations of the flow-pressure relation in multiple sequential stenoses coronary artery. Int J Cardiovasc Imaging 2017;33:1083–8 | |
| Mangiacapra F, Conte M, Tu S, Peace AJ, Di Serafino L, Ntarladinias I, et al. Performance of three-dimensional vs. two-dimensional quantitative coronary angiography in discriminating functionally significant coronary stenoses according to fractional flow reserve. EuroIntervention 2011;M:M144 | |
| Matar F, Falasiri S, Caruncho C, Leung C, Glover C, Sullebarger JT. When should FFR be used during coronary angiography? The importance of left anterior descending artery and minimal lumen diameter measurement. Catheter Cardiovasc Interv 2015;2:S67–8 | |
| Mohee K, Mynard J, Dhunnoo G, Halcox J, Obaid D. Diagnostic performance of virtual FFR derived from routine coronary angiography using 1D flow modelling: validation against fractional flow reserve. J Am Coll Cardiol 2017;70(Suppl. 1):B306–7 | |
| Morris PD, Ryan D, Morton AC, Lycett R, Lawford PV, Hose DR, et al. Virtual fractional flow reserve from coronary angiography: modelling the significance of coronary lesions: results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc Interv 2013;6:149–57 | |
| Morris PD, Silva Soto DA, Feher JFA, Rafiroiu D, Lungu A, Varma S, et al. Fast virtual fractional flow reserve based upon steady-state computational fluid dynamics analysis: results from the VIRTU-Fast study. JACC Basic Transl Sci 2017;2:434–46 | |
| Koo BK. Clinical Implication of 3-vessel Fractional Flow Reserve (FFR). URL: https://clinicaltrials.gov/ct2/show/NCT01621438 (accessed June 2021) | |
| Newcombe R, Gosling R, Gunn J, Narracott A, Hose R, Morris P, et al. An atlas of computed FFR in common patterns of coronary artery disease. Heart 2019;105:A51–2 | |
| Nishi T, Kitahara H, Fujimoto Y, Nakayama T, Sugimoto K, Takahara M, et al. Comparison of 3-dimensional and 2-dimensional quantitative coronary angiography and intravascular ultrasound for functional assessment of coronary lesions. J Cardiol 2017;69:280–6 | |
| Nishi T, Nakayama T, Fujimoto Y, Kobayashi Y. Comparison of three-dimensional and two-dimensional quantitative coronary angiography and intravascular ultrasound for the functional assessment of coronary lesions. Circulation 2014;130:A17712 | |
| Papafaklis M, Muramatsu T, Ishibashi Y, Tsirka G, Bourantas C, Fotiadis D, et al. Virtual resting Pd/Pa from 3-dimensional quantitative coronary angiography and flow modelling: comparison against fractional flow reserve in real-world patients. J Am Coll Cardiol 2016;67:370 | |
| Papafaklis MI, Muramatsu T, Ishibashi Y, Bourantas CV, Fotiadis DI, Brilakis ES, et al. Virtual resting Pd/Pa from coronary angiography and blood flow modelling: diagnostic performance against fractional flow reserve. Heart Lung Circ 2018;27:377–80 | |
| Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, et al. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire – fractional flow reserve. EuroIntervention 2014;10:574–83 | |
| Peña J, Shaw L, Lin F, Andreini D, Cademartiri F, Chinnaiyan K, et al. The association of acute coronary syndrome and coronary plaque features by sex: the iconic study. J Am Coll Cardiol 2018;71(Suppl.):A1632 | |
| Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv 2017;10:e005233 | |
| Pyxaras SA, Tu S, Barbati G, Barbato E, Di Serafino L, De Vroey F, et al. Quantitative angiography and OCT for the functional assessment of mild-to-moderate coronary stenoses: comparison with FFR. EuroIntervention 2013;9:144 | |
| Pyxaras SA, Tu S, Barbati G, Barbato E, Di Serafino L, Toth G, et al. Quantitative angiography and optical coherence tomography for the functional assessment of mild-to-moderate coronary stenoses: comparison with fractional flow reserve. Eur Heart J 2013;34(Suppl. 1):706 | |
| Pyxaras SA, Tu S, Barbato E, Barbati G, Di Serafino L, De Vroey F, et al. Quantitative angiography and optical coherence tomography for the functional assessment of nonobstructive coronary stenoses: comparison with fractional flow reserve. Am Heart J 2013;166:1010–18.e1 | |
| Saad M, Toelg R, Khattab AA, Kassner G, Abdel-Wahab M, Richardt G. Determination of haemodynamic significance of intermediate coronary lesions using three-dimensional coronary reconstruction. EuroIntervention 2009;5:573–9 | |
| Tar B, Koszegi Z. Smart calculation of the virtual functional assessment index with separate determination of the laminar and turbulent flow resistance. EuroIntervention 2016;France:297 | |
| Tu S, Echavarria-Pinto M, von Birgelen C, Holm NR, Pyxaras SA, Kumsars I, et al. Fractional flow reserve and coronary bifurcation anatomy: a novel quantitative model to assess and report the stenosis severity of bifurcation lesions. JACC Cardiovasc Interv 2015;8:564–74 | |
| Yong AS, Ng AC, Brieger D, Lowe HC, Ng MK, Kritharides L. Three-dimensional and two-dimensional quantitative coronary angiography, and their prediction of reduced fractional flow reserve. Eur Heart J 2011;32:345–53 | |
| Yu W, Huang JY, Jia D, Chen SL, Raffel C, Ding DX, et al. Diagnostic accuracy of intracoronary optical coherence tomography-based quantitative flow ratio for assessment of coronary stenosis. J Am Coll Cardiol 2018;72(Suppl.):B18 | |
| Zhang J, Wu W, Zou H, Fam J, Luo T, Lomarda A, et al. Diagnostic performance of fractional flow reserve derived from simplified modelling and computed tomography coronary angiography on discriminating ischaemic coronary lesions. J Cardiovasc Comp Tomogr 2017;11(Suppl. 1):S36–7 | |
| Zhang JM, Zhong L, Luo T, Lomarda AM, Huo Y, Yap J, et al. Simplified models of non-invasive fractional flow reserve based on CT images. PLOS ONE 2016;11:e0153070 | |
| No eligible reference standard | Adjedj J, Hyafil F, Muller O, Aubry P. Hemodynamic and Clinical Impact in Adult Patients with Anomalous Aortic Origin of the Right Coronary Artery Evaluated with Quantitative Flow Ratio. Paper presented at EuroPCR, 21–24 May 2019, Paris, France |
| Erbay A, Penzel L, Heuberger A, Steiner J, Lauten A, Skurk C, et al. The Diagnostic Reliability of Quantitative Flow Ratio (QFR) for Assessment of Non-Culprit Lesions in ACS Patients. Paper presented at the Annual Meeting of the German Society of Cardiology, 24–27 April 2019, Mannheim, Germany | |
| Hamaya R, Horie T, Yonetsu T, Sugano A, Kanaji Y, Usui E, et al. High-sensitivity cardiac troponin decrease after percutaneous coronary intervention in patients with stable coronary artery disease. Heart Vessels 2019;34:948–56 | |
| Lauri F, Macaya F, Mejia-Renteria H, Goto S, Yeoh J, Nakayama M, et al. Angiography-derived functional assessment of non-culprit coronary stenoses in primary percutaneous coronary intervention. EuroIntervention 2020;15:e1594–601 | |
| Liu L, Yang W, Nagahara Y, Li Y, Lamooki SR, Muramatsu T, et al. The impact of image resolution on computation of fractional flow reserve: coronary computed tomography angiography versus 3-dimensional quantitative coronary angiography. Int J Cardiovasc Imaging 2016;32:513–23 | |
| Di Girolamo D. Non Culprit Functional Evaluation With 3D Angio QFR in STEMI PCI Procedure. 2016. URL: https://clinicaltrials.gov/show/NCT02998853 (accessed June 2021) | |
| Okamoto H, Kume T, Yamada R, Imai K, Neishi Y, Uemura S. Comparison of optical coherence tomography measurements with 3-dimensional quantitative coronary angiography-derived quantitative flow ratio. Eur Heart J 2018;39(Suppl. 1):1379–80 | |
| Okamoto H, Kume T, Yamada R, Neishi Y, Uemura S. Comparison of Optical Coherence Tomography Measurements with 3-Dimensional Quantitative Coronary Angiography – Derived Computed Fractional Flow Reserve. Journal of the American College of Cardiology. Conference: 67th Annual Scientific Session of the American College of Cardiology, 10–12 March 2018, Orlando, FL, USA | |
| Qi Q, Liu G, Yuan Z, Liu L, Tu S, Zhao Q. Quantitative Flow Ratio-guided surgical intervention in symptomatic myocardial bridging. Cardiol J 2020;27:685–92 | |
| Sejr-Hansen M, Westra J, Thim T, Christiansen E, Eftekhari A, Kristensen SD, et al. Comparison of quantitative flow ratio and instantaneous wave-free ratio for immediate assessment of non-culprit lesions in patients with ST-segment elevation myocardial infarction an iSTEMI substudy. J Am Coll Cardiol 2018;72(Suppl.):B248–9 | |
| Sejr-Hansen M, Westra J, Winther S, Tu S, Nissen L, Gormsen L, et al. Comparison of QFR and FFR with myocardial perfusion scintigraphy and cardiovascular magnetic resonance as reference standard. A Dan-NICAD substudy. Int J Cardiovasc Imaging 2020;36:395–402 | |
| Smit JM, Koning G, van Rosendael AR, Dibbets-Schneider P, Mertens BJ, Delgado V, et al. The relationship between contrast-flow quantitative flow ratio and ischaemia assessed by SPECT MPI. Eur Heart J 2017;38(Suppl. 1):825 | |
| Smit JM, Koning G, van Rosendael AR, Dibbets-Schneider P, Mertens BJ, Jukema JW, et al. Relationship between coronary contrast-flow quantitative flow ratio and myocardial ischaemia assessed by SPECT MPI. Eur J Nucl Med Mol Imaging 2017;44:1888–96 | |
| Smit JM, van Rosendael AR, Koning G, Reiber JH, Dibbets-Schneider P, Mertens BJ, et al. The relationship between contrast-flow QFR and ischaemia assessed by SPECT MPI. Eur Heart J Cardiovasc Imaging 2017;18(Suppl. 1):i45–6 | |
| Suetomi T, Okamura T, Nakao F, Yamada J, Oda T, Mochizuki M, et al. Impact of Jailing Configuration and Bifurcation Angle on Incomplete Stent Apposition After Single Crossover Stenting with Final Kissing Balloon Dilatation, Assessed by Three-Dimensional OCT. Paper presented at EuroPCR, 19–22 May 2015, Paris, France | |
| Takahashi K, Kogame N, Tomaniak M, Chichareon P, Chang CC, Modolo R, et al. TCT-326 diagnostic performance of angiography-based quantitative flow ratio with respect to fractional flow reserve derived from computed tomography angiography: insight from the SYNTAX III trial. J Am Coll Cardiol 2019;74(Suppl.):B324 | |
| Tomaniak M, Masdjedi K, Zandvoort L, Neleman T, Tovar M, Vermaire A, et al. TCT-331 correlation between 3D-QCA-based FFR and quantitative lumen assessment by IVUS for left main coronary stenoses: the FAST Left Main study. J Am Coll Cardiol 2019;74(Suppl.):B329 | |
| Westra J, Winther S, Tu S, Nissen L, Gormsen L, Petersen S, et al. Comparison of quantitative flow ratio and fractional flow reserve to identify myocardial ischaemia: validation with myocardial perfusion scintigraphy and cardiovascular magnetic resonance. J Am Coll Cardiol 2017;70(Suppl. 1):B30 | |
| No eligible outcome | Kanno Y, Hoshino M, Sugano A, Kanaji Y, Yamaguchi M, Sumino Y, et al. Optical coherence tomography-defined plaque vulnerability in relation to functional stenosis severity stratified by fractional flow reserve and contrast quantitative flow ratio. J Am Coll Cardiol 2019;73(Suppl. 1):1172 |
| Kanno Y, Hoshino M, Sugiyama T, Kanaji Y, Yamaguchi M, Hada M, et al. Impact of subtended myocardial mass on the assessment of functional ischaemia as evaluated by FFR and QFR. Eur Heart J 2019;40(Suppl. 1):1654 | |
| Kleczyński P, Dziewierz A, Wiktorowicz A, Bartus S, Rzeszutko L, Bagienski M, et al. Contrast medium Pd/Pa ratio in comparison to fractional flow reserve, quantitative flow ratio and instantaneous wave-free ratio – a comprehensive assessment. Postepy w Kardiologii Interwencyjnej 2017;13:356 | |
| Sejr-Hansen M, Westra J, Thim T, Christiansen EH, Eftekhari A, Kristensen SD, et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction – an iSTEMI substudy. Catheter Cardiovasc Interv 2019;94:686–92 | |
| Yamaguchi M, Hoshino M, Horie T, Yuki H, Kanno Y, Hirano H, et al. Evaluation of non-culprit lesions in acute coronary syndromes using quantitative flow ratio. J Am Coll Cardiol 2019;73(Suppl. 1):1473 | |
| Study design not eligible | Accuracy of angiography-derived fractional flow reserve: a systematic review and meta-analysis. PROSPERO 2017 CRD42017084512 Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017084512 |
| Anonymous. Corrigendum to: diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J 2019;40:356 | |
| Asano T, Katagiri Y, Collet C, Tenekecioglu E, Miyazaki Y, Sotomi Y, et al. Functional comparison between BuMA Supreme biodegradable polymer sirolimus-eluting and durable polymer zotarolimus-eluting coronary stents using quantitative flow ratio: PIONEER QFR Substudy. EuroIntervention 2018;14:e570–9 | |
| Chahour K, Aboulaich R, Habbal A, Abdelkhirane C, Zemzeme N. Numerical simulation of the fractional flow reserve (FFR). Math Model Nat Phenom 2018;13 | |
| Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, et al. Diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J 2018;39:3314–21 | |
| Dan K, Shinoda A, Tsuzura D, Garcia-Garcia HM. Triple coronary vessel disease including double vessel chronic total occlusion: quantitative flow ratio minimises injury of the single vessel that provides collaterals. Cardiol J 2019;26:407–9 | |
| Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B et al. Corrigendum to: Diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J 2019;40:356–56 | |
| Erbay A, Abdelwahed YS, Stahli BE, Landmesser U, Leistner DM. The danger lurks dastardly in the coronary vessel wall: spotlight on patients’ vulnerability. Eur Heart J 2018;39:1656 | |
| Cesaro A, Gragnano F, Di Girolamo D, Moscarella E, Diana V, Pariggiano I, et al. Functional assessment of coronary stenosis: an overview of available techniques. Is quantitative flow ratio a step to the future? Expert Rev Cardiovasc Ther 2018;16:951–62 | |
| Giddens DP. Computing fractional flow reserve during coronary angiography: how good is good enough? JACC Cardiovasc Interv 2016;9:2036–8 | |
| Guillon B, Rubimbura V, Fournier S, Eeckhout E, Schiele F, Muller O, et al. Validation of Quantitative Flow Reserve and Residual Quantitative Flow Reserve To Predict Fractional Flow Reserve Post Stenting from the DOCTORS (Does Optical Coherence Tomography Optimise Results of Stenting) Study Population. Paper presented at the Joint Annual Meeting of the Swiss Society of Cardiology and the Swiss Society of Cardiac Surgery, 19–21 June 2019, Interlaken, Switzerland | |
| Hirshfeld Jr JW, Nathan AS. QFR and FFRCT: accurate enough? JACC Cardiovasc Interv 2019;12:2060–3 | |
| Howard JP, Murthy VL. A song of pressure and flow, or there and back again. JACC Cardiovasc Interv 2018;11:754–6 | |
| Lansky AJ, Pietras C. Fractional flow reserve from 3-dimensional quantitative coronary angiography: fresh light through an old window. JACC Cardiovasc Interv 2014;7:778–80 | |
| Mejia-Renteria H, Tu S, Macaya F, Escaned J. Influence of coronary microcirculatory dysfunction on FFR calculation based on computational fluid dynamics. Eur Heart J Cardiovasc Imaging 2017;18:1066 | |
| Morris PD, van de Vosse FN, Lawford PV, Hose DR, Gunn JP. ‘Virtual’ (computed) fractional flow reserve: current challenges and limitations. JACC Cardiovasc Interv 2015;8:1009–17 | |
| Nørgaard BL, Ko B. Angiography based quantitative flow ratio in coronary artery disease: mimic of FFR – ready for clinical use? Int J Cardiol 2019;279:29–30 | |
| Rasmussen LD, Winther S, Westra J, Isaksen C, Ejlersen JA, Brix L, et al. Danish study of Non-Invasive testing in Coronary Artery Disease 2 (Dan-NICAD 2): study design for a controlled study of diagnostic accuracy. Am Heart J 2019;215:114–28 | |
| Rubimbura V, Guillon B, Fournier S, Amabile N, Chi Pan C, Combaret N, et al. Validation of Quantitative Flow Reserve and Residual Quantitative Flow Reserve to Predict FFR Post-stenting from the Does Optical Coherence Tomography Optimise Results of Stenting Study (DOCTORS) Population. Paper presented at EuroPCR, 21–24 May 2019, Paris, France. | |
| Tang CX, Wang YN, Zhou F, Schoepf UJ, Assen MV, Stroud RE, et al. Diagnostic performance of fractional flow reserve derived from coronary CT angiography for detection of lesion-specific ischemia: a multi-center study and meta-analysis. Eur J Radiol 2019;116:90–7 | |
| Tu S, Bourantas C, Nørgaard B, Kassab G, Koo B-K, Reiber J. Image-based assessment of fractional flow reserve. EuroIntervention 2015;11(Suppl. V):V50–4 | |
| Tu S, Westra J, Yang J, Li Y, Holm N, Reiber J. Functional Coronary Assessment Based on Three-Dimensional Quantitative Coronary Angiography. In Escaned J, Serruys PW, editors. Coronary Stenosis Imaging, Structure and Physiology, Part IV. London: PCR Publishing; 2010 | |
| Vestergaard MB, Andersen BK, Westra JS, Christiansen EH, Holm NR. Optimal assessment of lesion severity in the left anterior descending artery by quantitative flow ratio. The wire-free invasive functional imaging WIFI LAD study. JACCCardiovasc Interv 2017;10(Suppl.):S50–1 | |
| Westra J, Tu S, Campo G, Qiao S, Matsuo H, Qu X, et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: an individual patient-data meta-analysis. Catheter Cardiovasc Interv 2019;94:693–701 | |
| Xing Z, Pei J, Huang J, Hu X, Gao S. Diagnostic performance of QFR for the evaluation of intermediate coronary artery stenosis confirmed by fractional flow reserve. Braz J Cardiovasc Surg 2019;34:165–72 | |
| Zaleska M, Koltowski L, Maksym J, Tomaniak M, Opolski M, Kochman J. Alternative methods for functional assessment of intermediate coronary lesions. Cardiol J 2019;27:825–35 | |
| Zhang J, Zhang R, Guo L. Diagnostic potency of contrast induced fractional flow reserve versus quantitative flow ratio for assessing the functional significance of coronary stenosis: a meta-analysis. J Interv Cardiol 2020;2020:7352150 | |
| Zhenhua X. Diagnostic performance of quantitative flow ratio (QFR) for the evaluation of intermediate coronary stenosis severity confirmed by fractional flow reserve. 2018 | |
| Zuo W, Yang M, Chen Y, Xie A, Chen L, Ma G. Meta-analysis of diagnostic performance of instantaneous wave-free ratio versus quantitative flow ratio for detecting the functional significance of coronary stenosis. BioMed Res Int 2019;2019:5828931 |
Appendix 3 Risk-of-bias and applicability assessment: Quality Assessment of Diagnostic Accuracy Studies-2
Risk-of-bias assessment
Signalling question (SQ)1: was a consecutive or random sample of patients enrolled?
SQ2: did the study avoid inappropriate exclusions? (Note exclusion of tandem lesion and percentage.)
Risk of bias (participant selection): could the selection of patients have introduced bias?
SQ3: was QFR performed during the same exam as angiography (i.e. online)?
SQ4: was the same cut-off point used for the index test and FFR?
SQ5: was interpretation of QFR blinded to FFR? (Note that, if done prospectively, and if FFR was done by separate staff/in separate room, then fine.)
Risk of bias (index test): could the conduct or interpretation of the index test have introduced bias?
SQ6: is the reference standard likely to measure FFR accurately enough?
SQ7: was interpretation of FFR blinded to QFR?
Risk of bias (reference standard): could the conduct or interpretation of the reference standard test have introduced bias?
SQ8: did all patients analysed receive both the index test and FFR (or some received iFR only)?
SQ9: were all or nearly all patients included in the analysis?
SQ10: were QFR and FFR measured during the same examination?
Risk of bias (flow and timing): could the patient flow have introduced bias?
| Study | Comments | SQ1 | SQ2 | PS | SQ3 | SQ4 | SQ5 | IT | SQ6 | SQ7 | RS | SQ8 | SQ9 | SQ10 | FT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortés et al. (2019)17 | Very small sample of 12 patients with FFR. Retrospective, offline QFR. Exclusions and reasons for exclusion not reported. Only 12 patients included. Exclusion of patients not reported. FFR blinded because performed before QFR (retrospective) | N | UC | – | N | Y | Y | + | Y | Y | + | Y | N | N | ? |
| Emori et al. (2018)20 | If lesions in multiple arteries, only one (most severe stenosis) was selected as the target vessel. FFR blinded because performed before QFR (retrospective). A total of 15% excluded (reasons provided) | Y | Y | + | N | Y | Y | + | Y | Y | + | Y | Y | N | + |
| Emori et al. (2018)21 | Retrospective but consecutive, with few exclusions (reasons provided). FFR blinded because performed before QFR (retrospective) | Y | Y | + | N | Y | Y | + | Y | Y | + | Y | Y | N | + |
| FAST: Masdjedi et al. (2020)56 [CAAS vFFR] | Large number excluded, notably owing to lack of two adequate orthogonal views (58), overlap or foreshortening (35) and inadequate pressure waveform (25). FFR blinded because performed before QFR (retrospective) | Y | UC | – | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| FAVOR II China: Xu et al. (2017)52 | Consecutive, prospective. No significant concerns | Y | Y | + | Y | Y | Y | + | Y | Y | + | Y | Y | Y | + |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | UC if consecutive | UC | Y | + | Y | Y | Y | + | Y | Y | + | Y | Y | Y | + |
| FAVOR pilot: Tu et al. (2016)46 | Excluded ostial left main or ostial right (unclear how many) | N | UC | + | N | Y | Y | + | Y | Y | + | Y | Y | Y | + |
| Hamaya et al. (2019)23 | Retrospective selection of subgroup of 154 vessels with FFR (out of 549 patients and 1595 vessels). Large proportion excluded owing to anatomy: 140 vessels excluded owing to small (< 2 mm) right coronary artery or left circumflex coronary artery (52), arrhythmia during ICA (32), ineligible coronary anatomy (98) or insufficient image quality (10). FFR blinded because performed before QFR (retrospective). Large proportion excluded owing to anatomy | N | UC | – | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Hwang et al. (2019)24 | Large number of vessels excluded (124; 25.7%) because of calibration failure (49), ostium lesion (35) or insufficient projection (25). FFR blinded because performed before QFR (retrospective). A total of 25.7% excluded (reasons provided) | Y | N | – | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | Large percentage (65%) of lesions excluded. FFR blinded because performed before QFR (retrospective) | UC | UC | – | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Kleczyński et al. (2019)33 | The analysis was conducted twice by two analyzers and the mean value (from four calculations) was used for further analysis. This may have reduced the risk of inter-rater and intrarater variability. Results per analyser not reported | Y | UC | + | N | Y | Y | – | Y | Y | + | Y | UC | N | + |
| Kołtowski et al. (2018)32 | Retrospective selection; large number excluded, including 299 owing to lack of proper ICA projections (34.9%), bifurcation lesions (5%), tandem lesions (2.5%) or ostial lesion (3%) | N | Y | – | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Liontou et al. (2019)34 | Large number of vessels excluded (124/202 or 61.4%); reasons provided. Unclear how many ostial lesions, overlapping and tortuous vessels were excluded. FFR blinded because performed before QFR (retrospective) | UC | N | ? | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Mejia-Renteria et al. (2019)37 | Retrospective; large number of exclusions (101), including ostial in left main or right coronary artery (10), grafted target vessels (2), inadequate projections (28), significant overlapping (17), inadequate ICA quality (19), resting haemodynamic data not available (6) and contrast filling not optimal for TIMI frame count analysis (15) | N | Y | + | Y | Y | Y | + | Y | Y | + | Y | N | N | + |
| Smit et al. (2019)40 | Retrospective but consecutive. A total of 13.5% vessels excluded but reasons reported and acceptable. Unlikely to have been blinded. Mean time between ICA and invasive FFR 22.8 ± 25.1 days. The majority of patients included in our study (97%) underwent FFR measurement within 3 months after the initial ICA. A total of 13% of patients excluded | Y | Y | + | N | Y | UC | – | Y | UC | – | Y | N | N | + |
| Spitaleri et al. (2018)41 [cohort B, diagnostic accuracy] | Vessels with diffuse disease excluded, but relatively low (n = 8/76; 11% of otherwise eligible patients) and no other significant concerns. FFR blinded because performed before QFR (retrospective) | Y | N | + | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Stähli et al. (2019)42 | Retrospective, but limited exclusions with acceptable reasons reported. All pressure tracings were reviewed for high signal quality, and FFR and resting Pd/Pa ratio analysed offline by experienced investigators blinded to QFR. A total of 8.7% excluded (reasons provided) | Y | Y | + | N | Y | Y | + | Y | Y | + | Y | Y | N | + |
| SYNTAX II: Asano et al. (2019)15 | Large percentage (29%) of lesions excluded owing to lack of appropriate projections, but no other significant concerns. FFR blinded because performed before QFR (retrospective). FFR performed only in iFR grey zone (0.86–0.93) patients | N | UC | + | N | Y | Y | + | UC | Y | + | N | N | N | – |
| Ties et al. (2018)43 | Very large proportion of excluded vessels (69.6%), mostly owing to ‘lack of basic requirements’ (insufficient details). FFR blinded because performed before QFR (retrospective). A total of 69.7% excluded (reasons provided) | UC | N | ? | N | Y | Y | + | Y | Y | + | Y | N | N | + |
| Tu et al. (2014)45 | Three were excluded; reasons included (1) the interrogated vessel had too much overlap or foreshortening (> 90%), (2) the image quality of the hyperemic projection was not sufficient to evaluate by frame count and (3) the mean pressure of the guiding catheter or blood haematocrit value was not documented | Y | UC | + | N | Y | Y | + | Y | Y | + | Y | Y | N | + |
| van Rosendael et al. (2017)48 | Prospectively recruited. No notes on how many were excluded (and reasons for exclusion). Very small sample (n = 17 patients). No reporting of blinding. No reporting on number excluded and reasons for exclusion | UC | UC | ? | Y | Y | UC | – | Y | UC | – | Y | UC | Y | ? |
| Watari et al. (2019)49 | Consecutive, prospective. iFR was the only reference standard | Y | N | + | Y | Y | Y | + | UC | Y | + | N | Y | Y | + |
| WIFI II: Westra et al. (2018)51 | Intention to exclude as few as possible based on impaired angiographic quality | Y | Y | + | Y | Y | Y | + | Y | Y | + | Y | Y | Y | + |
| Yazaki et al. (2017)53 | Consecutive, prospective | Y | Y | + | Y | Y | Y | + | Y | UC | – | Y | Y | Y | + |
Applicability assessment
Applicability concern 1: are there concerns that the included patients do not match the review question?
Applicability concern 2: are there concerns that the index test, its conduct or interpretation differ from the review question (e.g. QFR cut-off point not 0.8 or unusual QFR mode used for main analysis)?
Applicability concern 3: are there concerns that the reference standard, its conduct or interpretation differ from the review question?
| Study | Comments | Patient selection | Index test | Reference standard |
|---|---|---|---|---|
| Cortés et al. (2019)17 | Minority with intermediate stenosis and stable CAD. A total of 100% STEMI with > 50% DS in non-culprit arteries. Offline assessment | – | – | + |
| Emori et al. (2018)20 | Percentage of stable CAD unknown; only most severe arteries selected in case of multiple lesions. Offline assessment | ? | – | + |
| Emori et al. (2018)21 | Percentage of stable CAD unknown. Offline assessment | ? | – | + |
| FAST: Masdjedi et al. (2020)56 [CAAS vFFR] | Majority with intermediate stenosis and stable CAD. A total of 60% stable CAD; 26% acute intermediate stenosis. Large number of exclusions. Offline assessment | – | – | + |
| FAVOR II China: Xu et al. (2017)52 | Minority with intermediate stenosis and stable CAD. A total of 34% stable CAD; 61% unstable angina. Online assessment | – | + | + |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | Majority with intermediate stenosis and stable CAD. Online assessment | + | + | + |
| FAVOR pilot: Tu et al. (2016)46 | Majority with intermediate stenosis and stable CAD. All stable angina. Offline assessment | + | – | + |
| Hamaya et al. (2019)23 | Majority with intermediate stenosis and stable CAD. 100% stable CAD; only 10% with three-vessel disease. Offline assessment | + | – | + |
| Hwang et al. (2019)24 | Majority with intermediate stenosis and stable CAD. A total of 69% stable CAD, 31% acute; however, the large rate of exclusion is potentially concerning. Offline assessment | – | – | + |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | Majority with intermediate stenosis and stable CAD. A total of 63% stable angina, 22.1% unstable; 10.9% STEMI. Offline assessment | + | – | + |
| Kleczyński et al. (2019)33 | Majority with intermediate stenosis and stable CAD. Offline assessment | + | – | + |
| Kołtowski et al. (2018)32 | Majority with intermediate stenosis and stable CAD. A total of 100% stable CAD. Offline assessment | + | – | + |
| Liontou et al. (2019)34 | Majority with intermediate stenosis and stable CAD. Different population: all in-stent restenosis, ≥ 50% DS in stent, although 69% had stable angina (26% unstable angina, 6% acute MI). Offline assessment | – | – | + |
| Mejia-Renteria et al. (2019)37 | Majority with intermediate stenosis and stable CAD. A total of 70% stable angina; 30% ACS. Offline assessment | + | – | + |
| Smit et al. (2019)40 | Majority with intermediate stenosis and stable CAD. All referred from diagnostic-only setting, so likely to be stable. All intermediate stenoses (30–90%). Offline assessment | + | – | + |
| Spitaleri et al. (2018)41 [cohort B, diagnostic accuracy] | Minority with intermediate stenosis and stable CAD. All STEMI with multivessel disease patients (assessment of non-culprit vessels). Offline assessment | – | – | + |
| Stähli et al. (2019)42 | Majority with intermediate stenosis and stable CAD. A total of 72% stable CAD; 4% acute; most/all other unstable angina. All intermediate stenoses. Offline assessment | + | – | + |
| SYNTAX II: Asano et al. (2019)15 | All three-vessel disease (potentially harder to diagnose). Stable or unstable angina, or atypical chest pain (n/% not reported). Offline assessment | – | – | + |
| Ties et al. (2018)43 | Majority with intermediate stenosis and stable CAD. A total of 51% stable CAD; 16.7% acute (NSTEMI). Most patients excluded. Offline assessment | – | – | + |
| Tu et al. (2014)45 | Majority with intermediate stenosis and stable CAD. A total of 76.5% stable angina; 8.8% silent ischaemia; 64% bifurcation lesions. Offline (China, Hungary) and online (Belgium) assessment | + | – | + |
| van Rosendael et al. (2017)48 | Majority with intermediate stenosis and stable CAD. All stable CAD, though DS low. Online assessment | + | + | + |
| Watari et al. (2019)49 | Majority with intermediate stenosis and stable CAD. Online assessment | + | + | + |
| WIFI II: Westra et al. (2018)51 | Minority with intermediate stenosis and stable CAD. A total of 31% stable angina; 34% atypical angina; 31% non-specific angina. Online assessment | – | + | + |
| Yazaki et al. (2017)53 | Majority with intermediate stenosis and stable CAD. A total of 50.7% stable angina; 99.3% CAD. Offline assessment | + | – | + |
Appendix 4 Further meta-analysis results
FIGURE 18.
Meta-analysis of PPVs.

FIGURE 19.
Meta-analysis of NPVs.

FIGURE 20.
Meta-analysis of DORs.
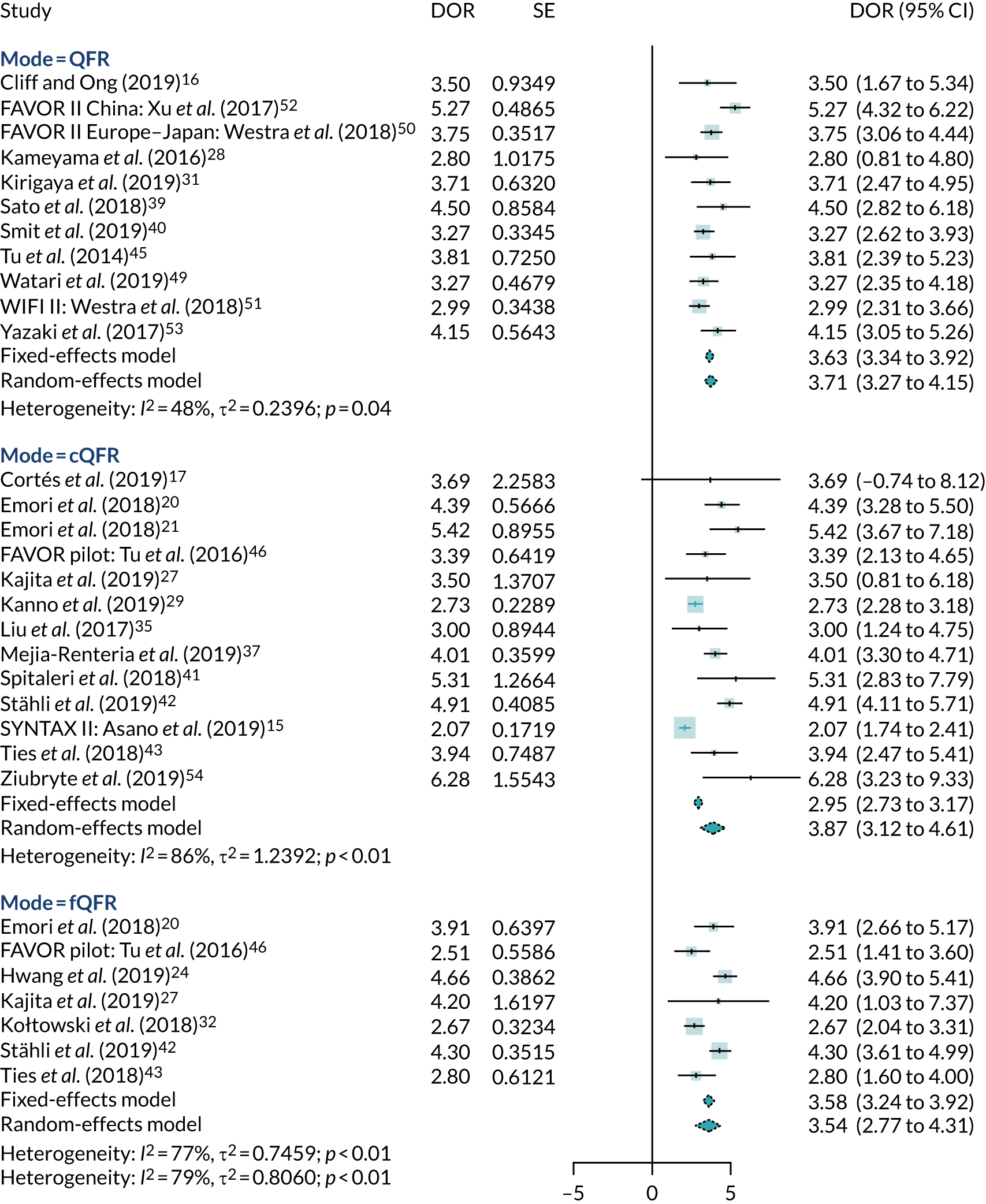
FIGURE 21.
Meta-analysis of AUC.

FIGURE 22.
Meta-analysis of MD between FFR and QFR.

FIGURE 23.
Meta-analysis of correlation between QFR and FFR.

FIGURE 24.
Receiver operating characteristic plot of bivariate meta-analysis.

FIGURE 25.
Receiver operating characteristic plot of studies comparing ICA, fQFR and cQFR.
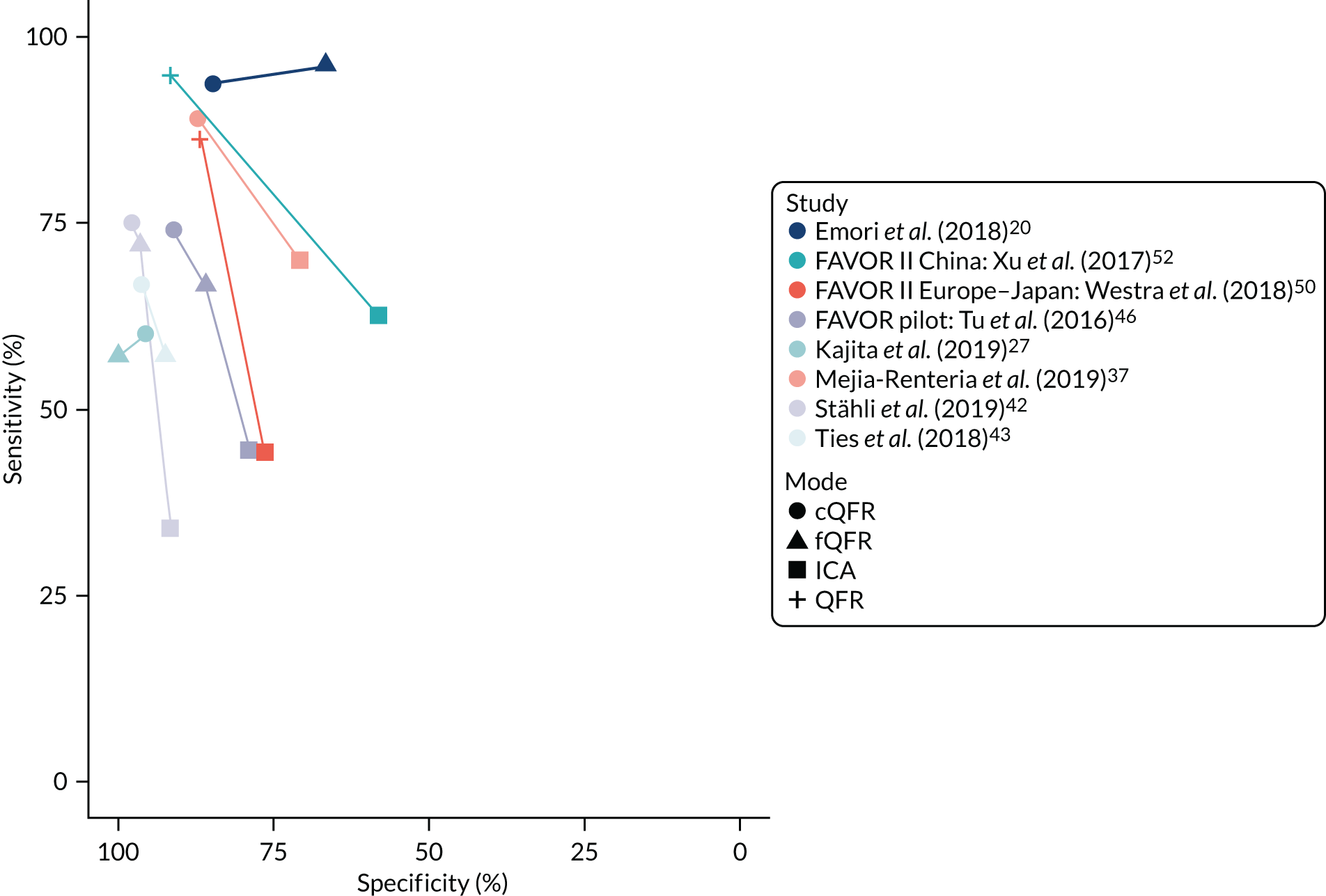
FIGURE 26.
Bivariate meta-analysis by unit of analysis.
FIGURE 27.
Bivariate meta-analysis by study type.
FIGURE 28.
Metaregression of sensitivity, specificity and DOR by proportion with diabetes: (a) sensitivity; (b) specificity; and (c) log-DOR.
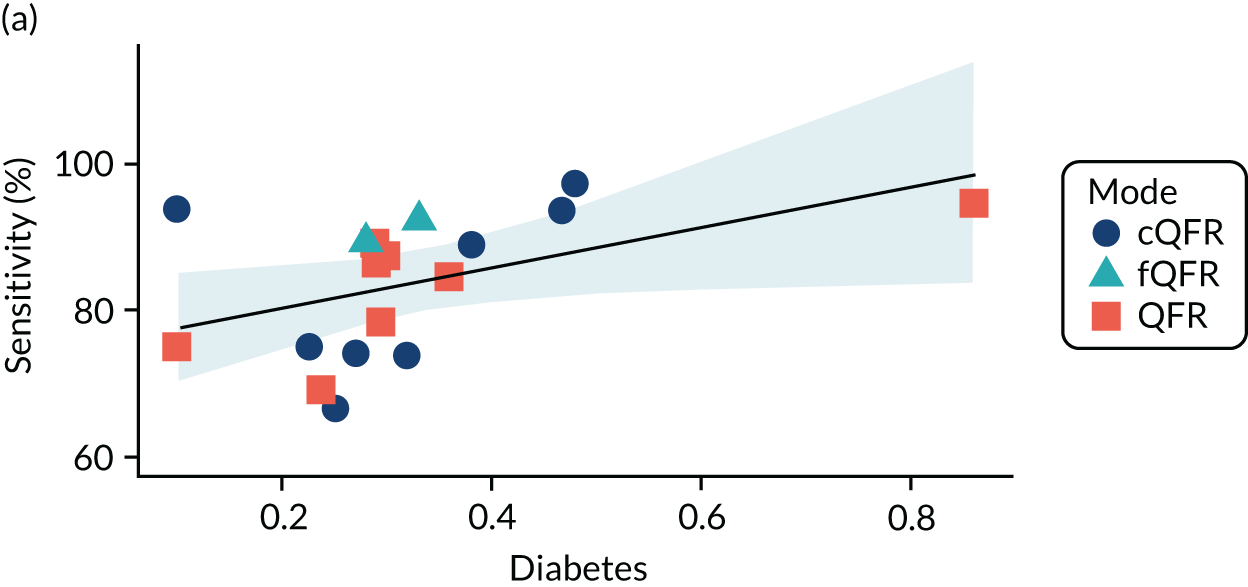

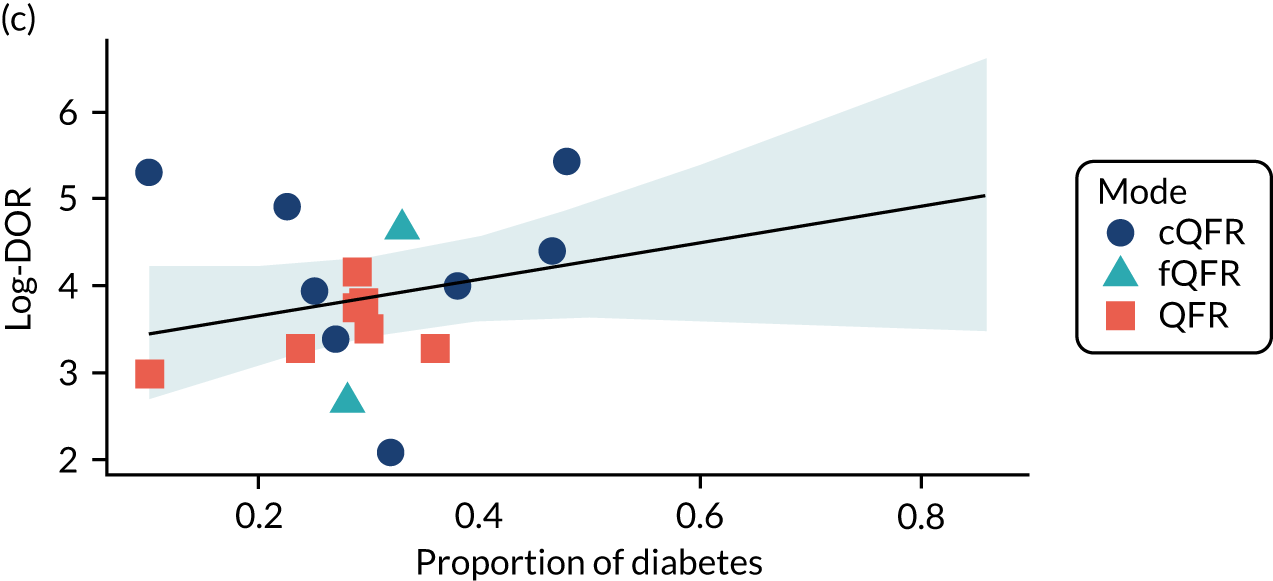
FIGURE 29.
Metaregression of sensitivity, specificity and DOR by proportion with stable CAD: (a) sensitivity; (b) specificity; and (c) log-DOR.
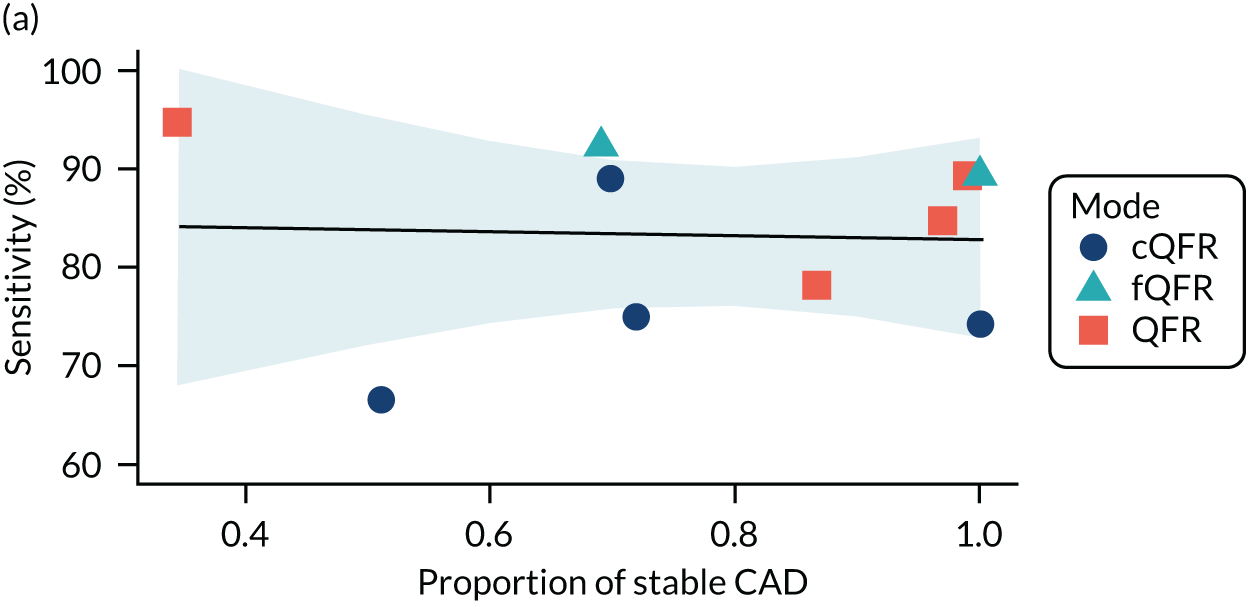
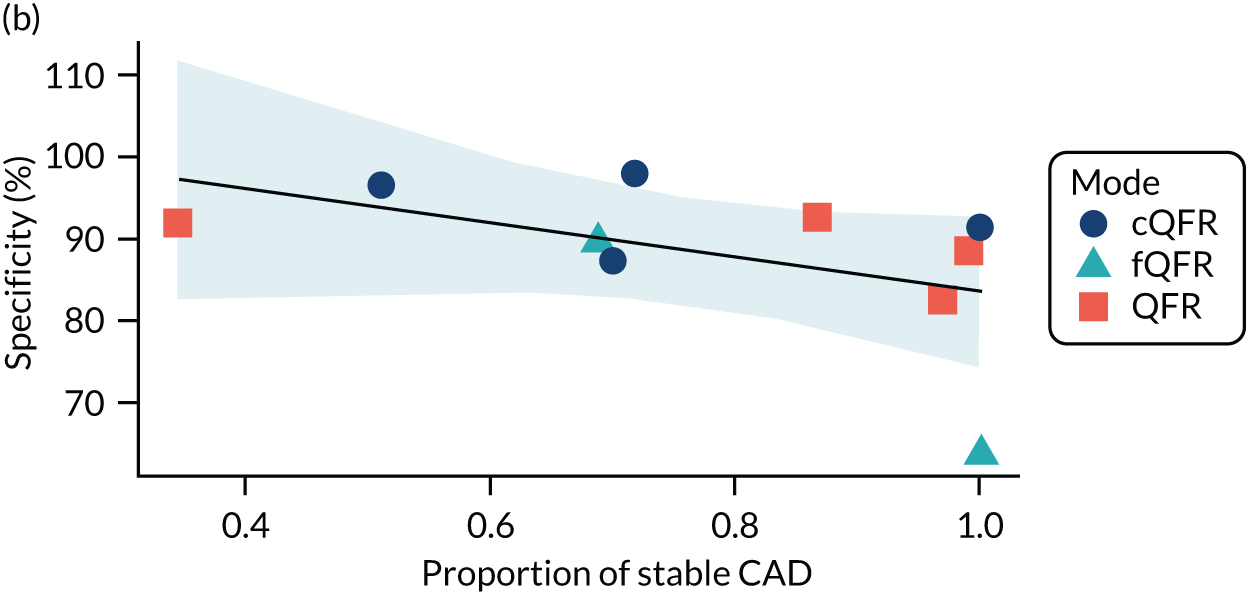

FIGURE 30.
Metaregression of sensitivity, specificity and DOR by proportion with multivessel disease: (a) sensitivity; (b) specificity; and (c) log-DOR.
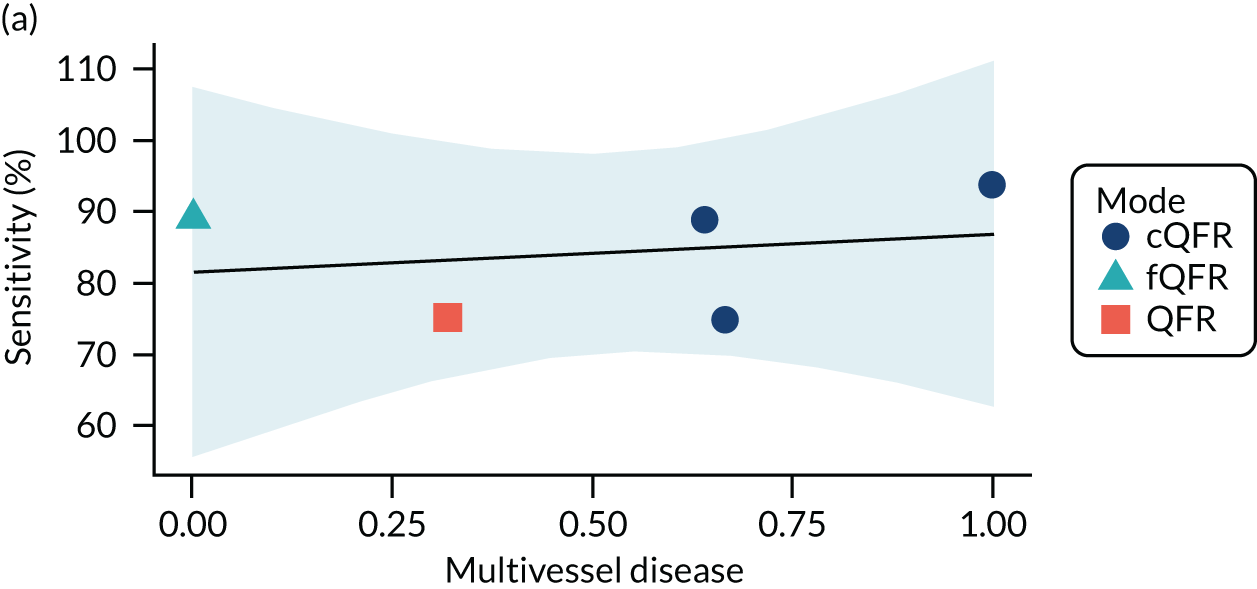
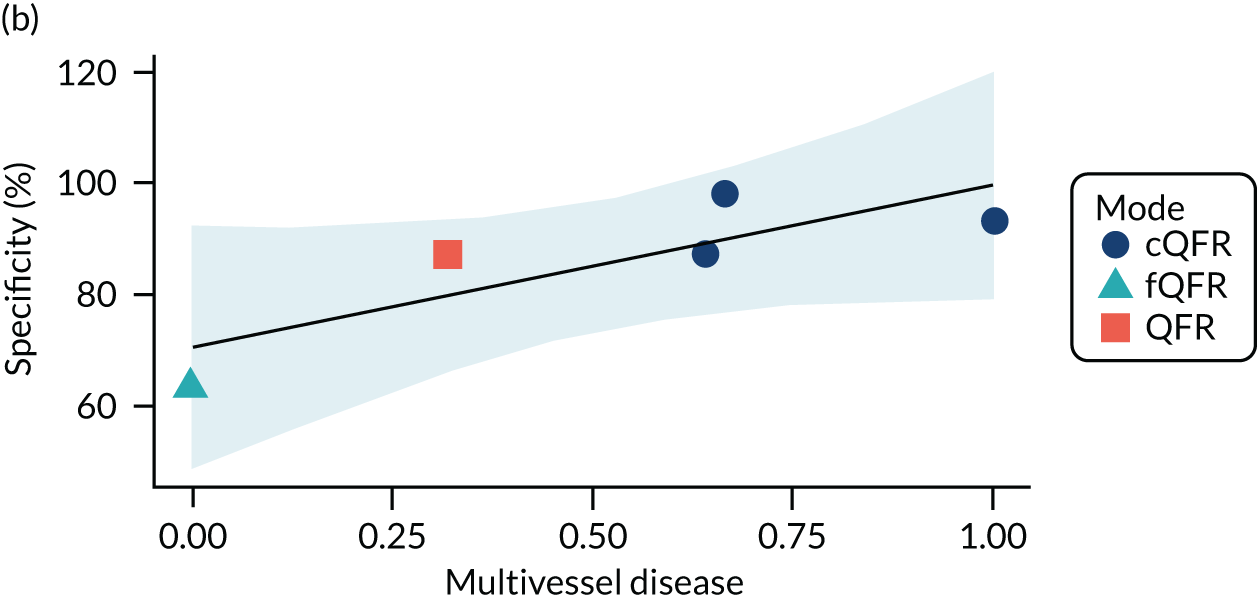
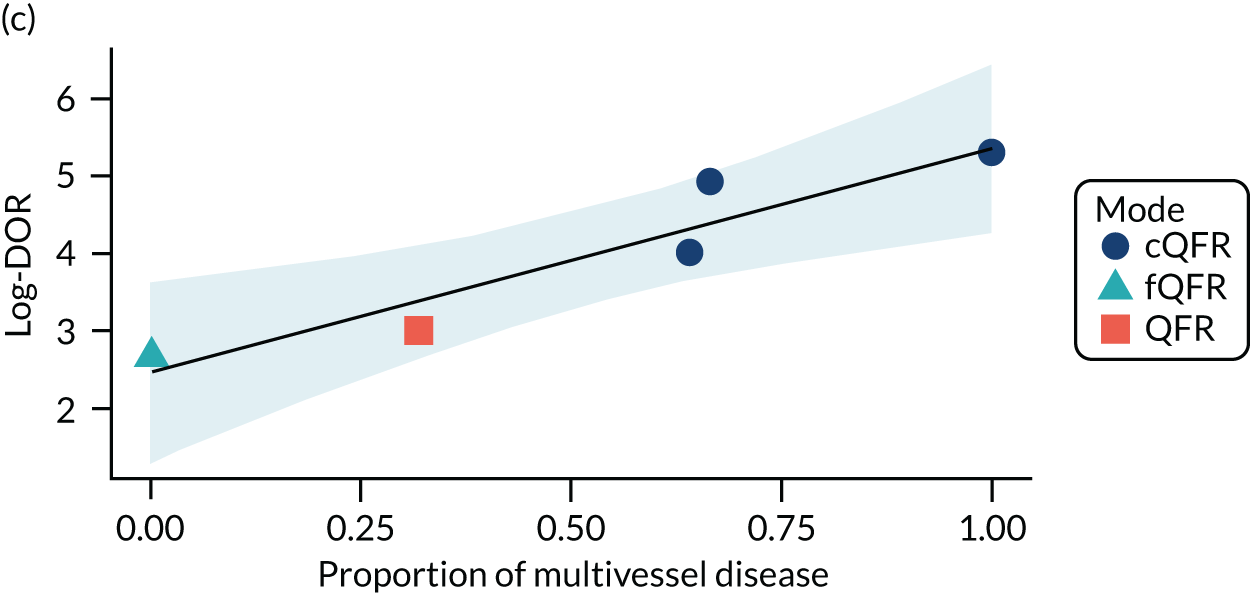
FIGURE 31.
Metaregression of sensitivity, specificity and DOR by mean FFR: (a) sensitivity; (b) specificity; and (c) log-DOR.
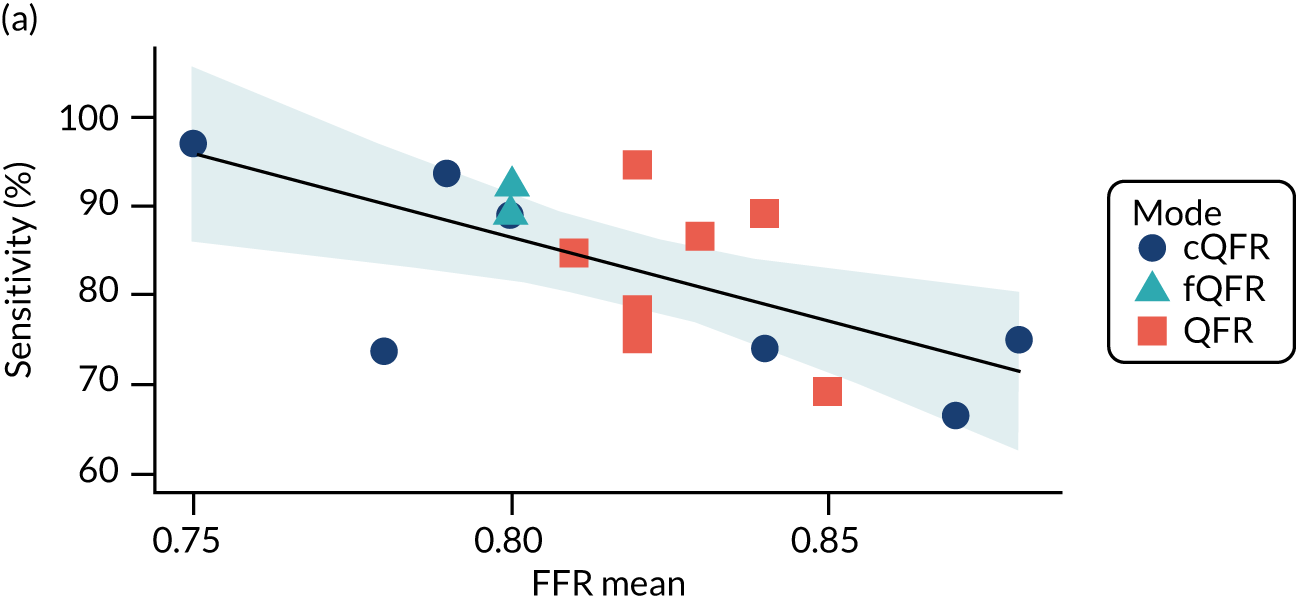
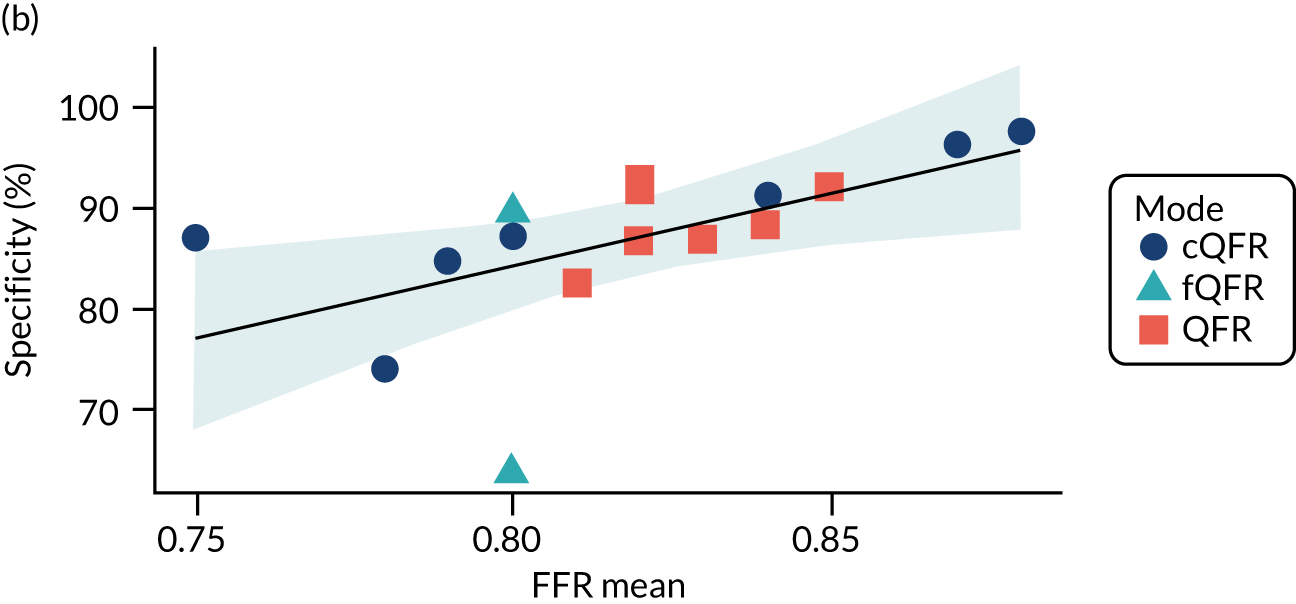

| Variable | Log-DOR (95% CI; p-value) | Sensitivity (95% CI; p-value) | Specificity (95% CI; p-value) |
|---|---|---|---|
| Mean age, years | 0.01 (–0.1 to 0.1; 0.822) | 0.05 (–1.11 to 1.11; 0.936) | –0.12 (–1.1 to 1.1; 0.813) |
| Sex | –2.14 (–8.23 to 8.23; 0.501) | 19.99 (–42.18 to 42.18; 0.538) | –33.84 (–84.29 to 84.29; 0.207) |
| Diabetes | 2.07 (–0.51 to 0.51; 0.136) | 27.6 (2.8 to –2.8; 0.044) | –1.53 (–25.35 to 25.35; 0.901) |
| Mean DS | 0.01 (–0.06 to 0.06; 0.753) | 0.6 (–0.17 to 0.17; 0.149) | –0.25 (–0.89 to 0.89; 0.466) |
| Stable angina | –0.7 (–2.99 to 2.99; 0.574) | –13.8 (–35.41 to 35.41; 0.266) | 5.25 (–4.62 to 4.62; 0.345) |
| Stable CAD | –2.57 (–4.18 to 4.18; 0.014) | –1.89 (–29.97 to 29.97; 0.898) | –20.69 (–46.25 to 46.25; 0.151) |
| Previous MI | 0.28 (–3.89 to 3.89; 0.899) | 10.67 (–25.87 to 25.87; 0.579) | –14.04 (–48.13 to 48.13; 0.437) |
| Acute MI | 0.23 (–0.99 to 0.99; 0.719) | 4.85 (–8.74 to 8.74; 0.495) | –0.3 (–12.51 to 12.51; 0.963) |
| Multivessel disease | 2.87 (1.74 to –1.74; 0.016) | 5.28 (–20.21 to 20.21; 0.712) | 29.05 (7.64 to –7.64; 0.076) |
| Diffuse CAD | –7.76 (–15.72 to 15.72; 0.307) | –28.98 (–299.8 to 299.8; 0.868) | –59.17 (–165.3 to 165.3; 0.472) |
| Previous PCI | 0.37 (–3.34 to 3.34; 0.848) | 27.43 (–13.32 to 13.32; 0.216 | –25.99 (–62.63 to 62.63; 0.195) |
| Mean FFR (per 0.1 FFR) | 0.01 (–1.45 to 1.45; 0.988) | –18.77 (–30.62 to 30.62; 0.008) | 14.59 (3.84 to –3.84; 0.019) |
| Percentage with a FFR < 0.8 | –0.01 (–0.04 to 0.04; 0.595) | 0.32 (0.06 to –0.06; 0.026) | –0.3 (–0.52 to 0.52; 0.012) |
FIGURE 32.
Sensitivity and specificity by patient subgroups.
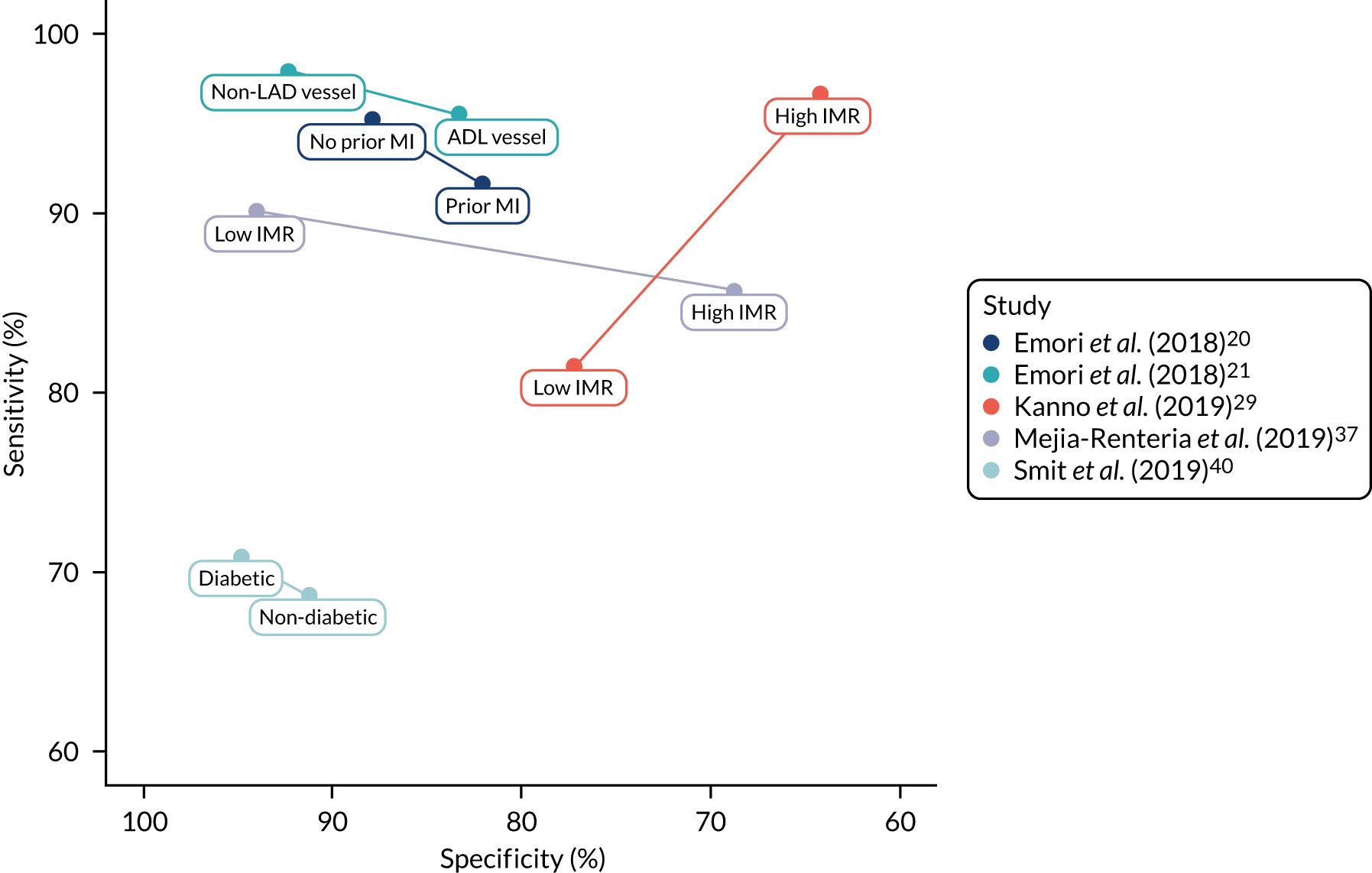
FIGURE 33.
Diagnostic odds ratios by patient subgroups.
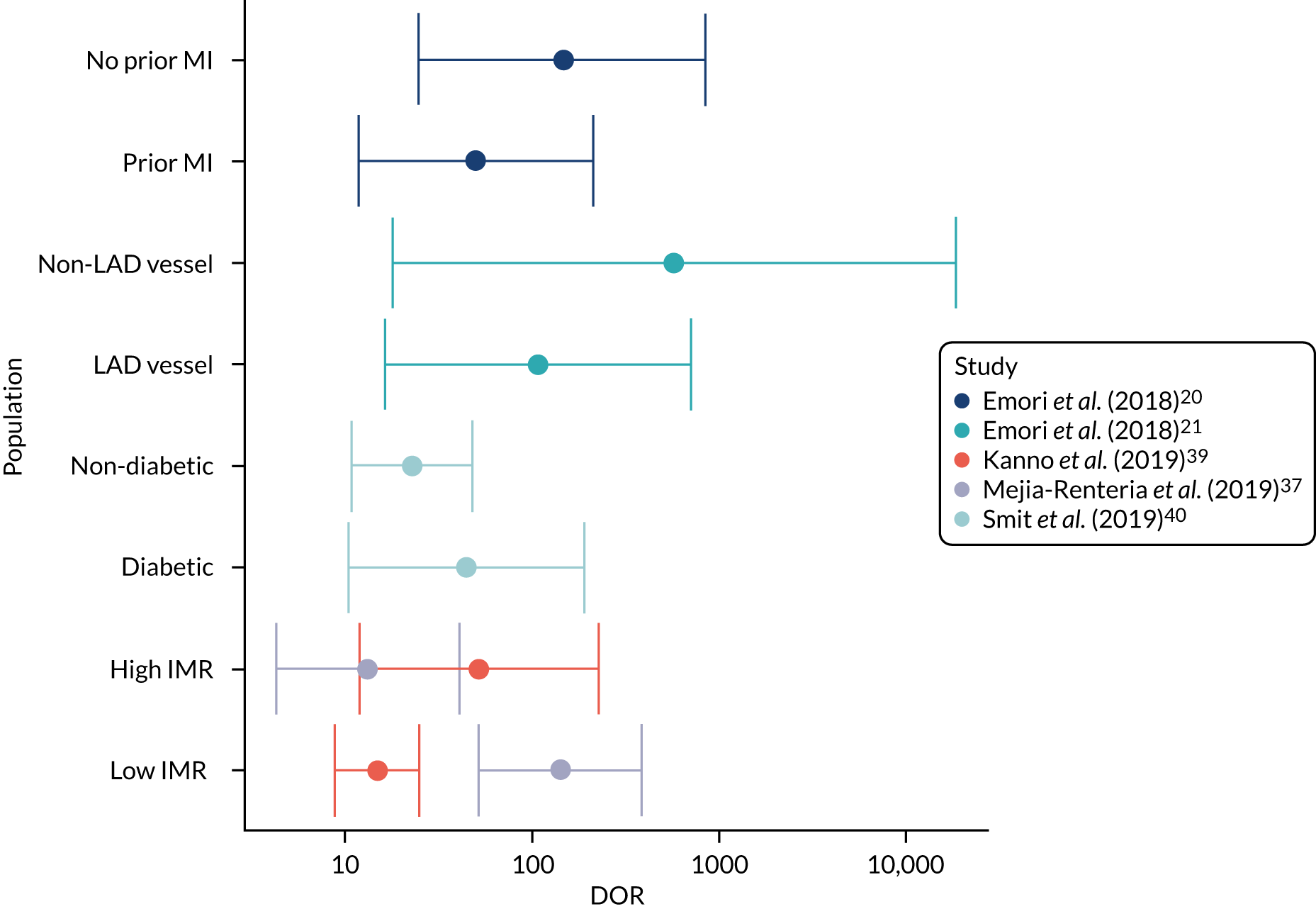
FIGURE 34.
Bivariate meta-analyses according to QUADAS-2 risk-of-bias classification: (a) flow; (b) index text; (c) patient selection; and (d) reference standard.
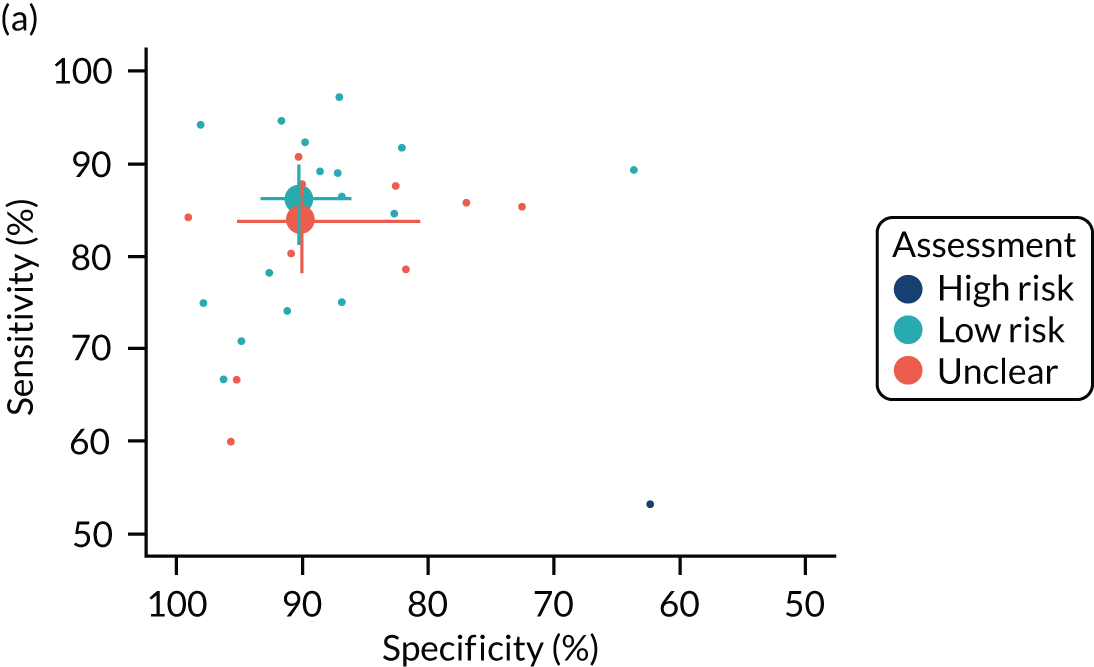
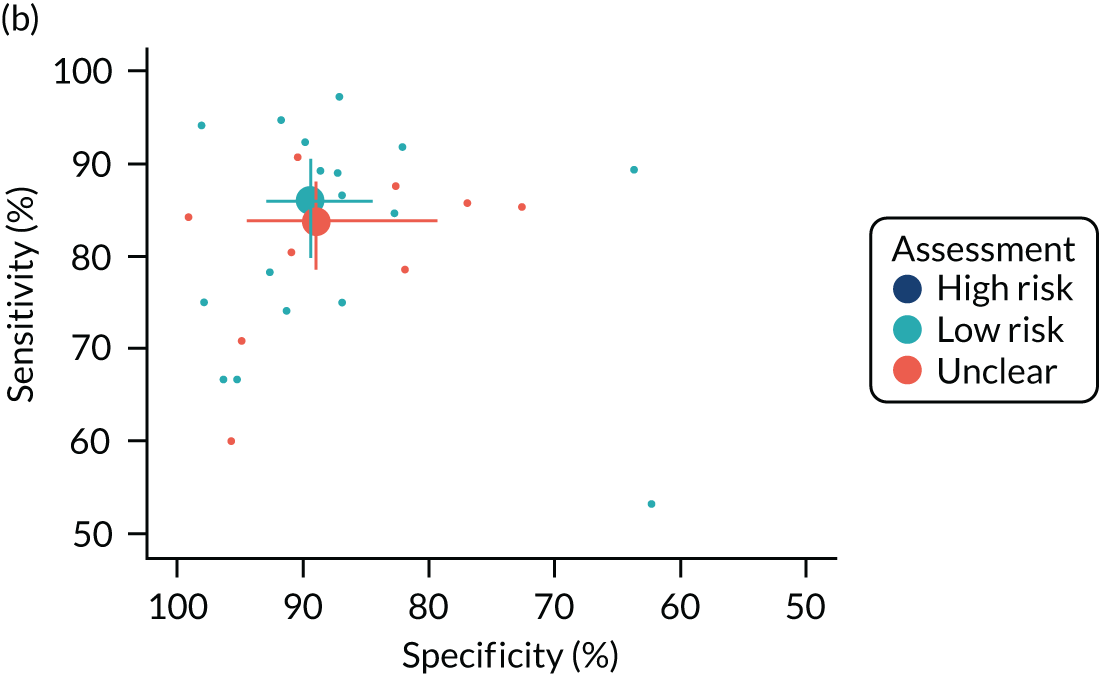


FIGURE 35.
Bivariate meta-analyses according to QUADAS-2 applicability classification: (a) index test; (b) patient selection; and (c) reference standard.
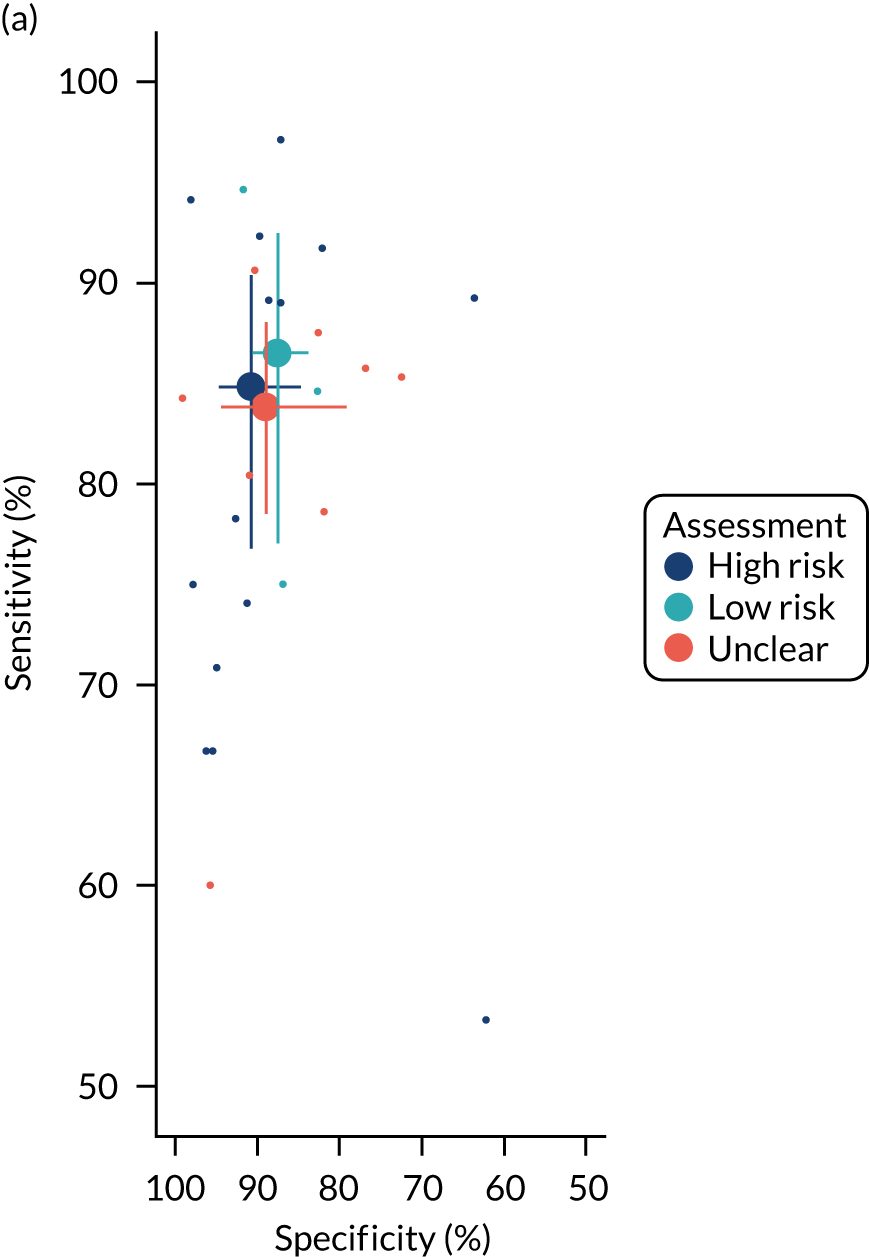


FIGURE 36.
Bivariate meta-analyses according to other factors that might cause bias: (a) blinding; (b) both tests; (c) complete data; (d) online test; (e) same exam; and (f) stable CAD.

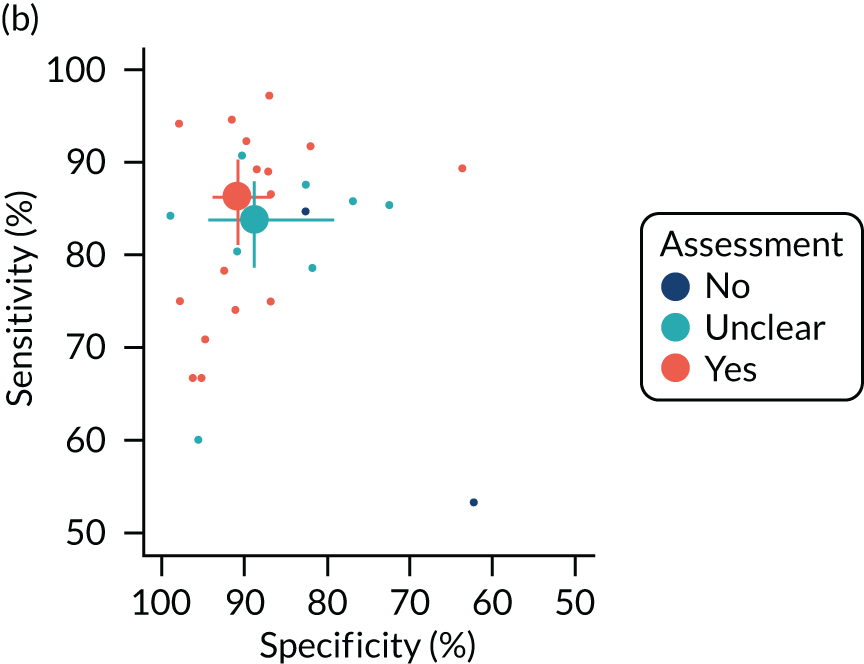
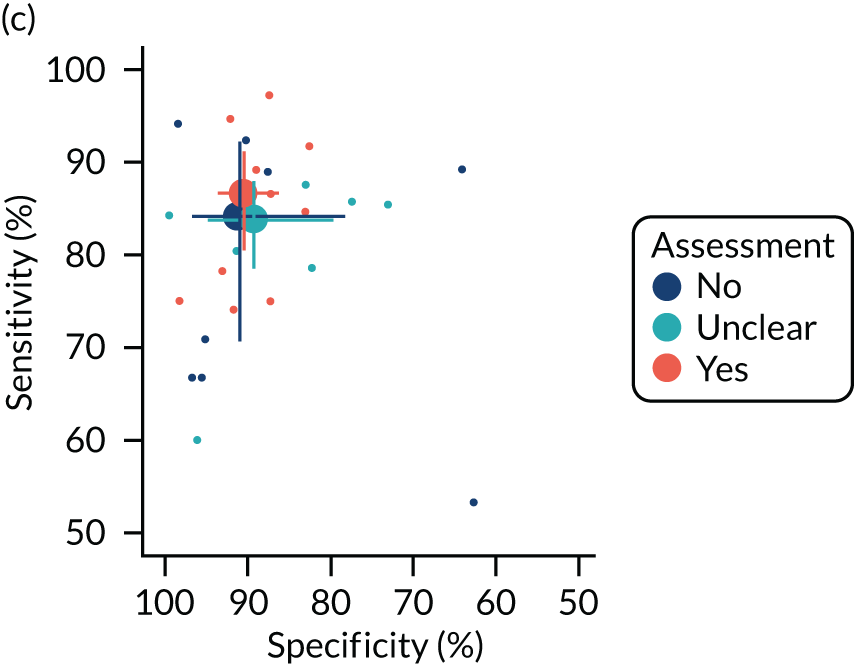


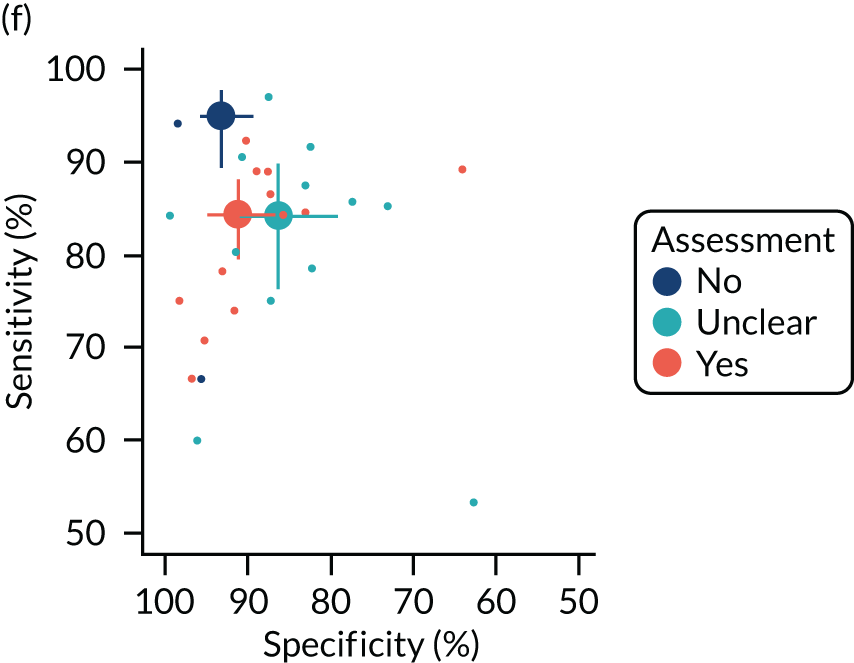
FIGURE 37.
Bivariate meta-analysis of extracted figure data.

| Study | Data extracted from figures | Data extracted from text/tables | ||||
|---|---|---|---|---|---|---|
| n | Sensitivity (%) | Specificity (%) | n | Sensitivity (%) | Specificity (%) | |
| Cliff and Ong (2019)16 | 39 | 87.50 | 82.61 | |||
| Cortés et al. (2019)17 | 15 | 100.00 | 100.00 | 11 | 100.00 | 100.00 |
| Emori et al. (2018)20 | 143 | 93.90 | 88.52 | 150 | 93.59 | 84.72 |
| Emori et al. (2018)21 | 100 | 97.10 | 87.10 | |||
| FAVOR II China: Xu et al. (2017)52 | 252 | 90.70 | 86.75 | 328 | 94.64 | 91.67 |
| FAVOR II Europe–Japan: Westra et al. (2018)50 | 238 | 85.06 | 85.43 | 317 | 86.54 | 86.85 |
| FAVOR pilot: Tu et al. (2016)46 | 84 | 74.07 | 91.23 | |||
| Hamaya et al. (2019)23 | 136 | 94.20 | 73.13 | |||
| Hwang et al. (2019)24 | 274 | 83.46 | 89.80 | 358 | 92.31 | 89.77 |
| Kajita et al. (2019)27 | 28 | 60.00 | 95.65 | |||
| Kameyama et al. (2016)28 | 24 | 80.00 | 88.89 | 25 | 78.57 | 81.82 |
| Kanno et al. (2019)29 | 381 | 86.29 | 71.84 | 504 | 85.29 | 72.56 |
| Kirigaya et al. (2019)31 | 89 | 79.17 | 90.24 | 95 | 80.39 | 90.91 |
| Kołtowski et al. (2018)32 | 205 | 84.04 | 83.78 | 306 | 89.23 | 63.64 |
| Liontou et al. (2019)34 | 72 | 90.00 | 76.19 | |||
| Liu et al. (2017)35 | 40 | 85.71 | 76.92 | |||
| Mejia-Renteria et al. (2019)37 | 278 | 86.29 | 87.66 | 300 | 88.97 | 87.20 |
| Sato et al. (2018)39 | 64 | 90.91 | 90.32 | 63 | 90.63 | 90.32 |
| Smit et al. (2019)40 | 240 | 63.38 | 94.67 | 320 | 69.23 | 92.14 |
| Spitaleri et al. (2018)41 | 42 | 94.12 | 96.00 | 45 | 93.75 | 93.10 |
| Stähli et al. (2019)42 | 267 | 73.44 | 97.54 | 516 | 75.00 | 97.84 |
| SYNTAX II: Asano et al. (2019)15 | 809 | 73.73 | 73.93 | |||
| Ties et al. (2018)43 | 101 | 66.67 | 96.25 | |||
| Toi et al. (2018)44 | 34 | 68.75 | 66.67 | |||
| Tu et al. (2014)45 | 78 | 76.92 | 90.38 | 77 | 78.26 | 92.59 |
| van Rosendael et al. (2017)48 | 26 | 100.00 | 75.00 | |||
| Watari et al. (2019)49 | 150 | 84.62 | 82.65 | |||
| WIFI II: Westra et al. (2018)51 | 206 | 73.75 | 82.54 | 240 | 75.00 | 86.84 |
| Yazaki et al. (2017)53 | 129 | 89.19 | 88.04 | 151 | 89.13 | 88.57 |
| Ziubryte et al. (2019)54 | 69 | 84.21 | 100.00 | |||
FIGURE 38.
Fractional flow reserve and QFR data showing QFR grey zone between 0.78 and 0.84.
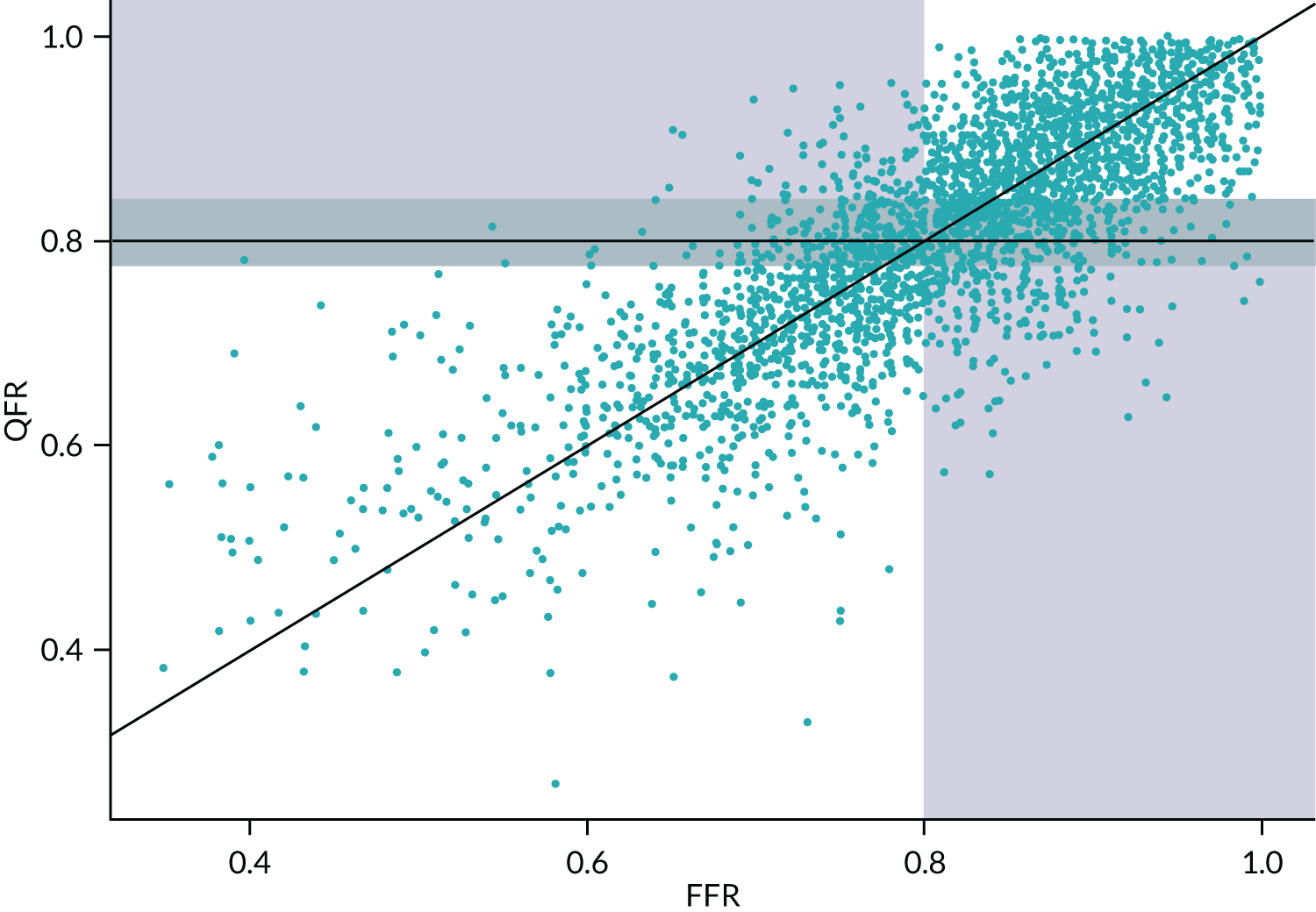
FIGURE 39.
Difference between FFR and QFR values in the grey zone: (a) FN; (b) FP; (c) TN; and (d) TP.
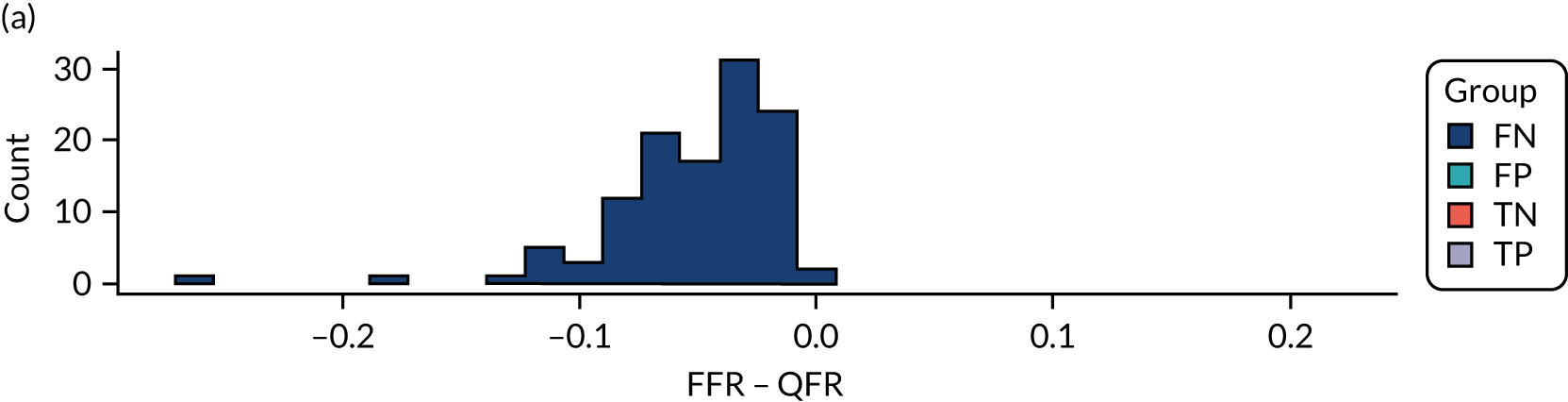



FIGURE 40.
Diagnostic accuracy of QFR with and without using the grey zone.

| Sensitivity (%) | Specificity (%) | Grey-zone threshold | |
|---|---|---|---|
| Lower | Upper | ||
| 90 | 90 | 0.78 | 0.82 |
| 90 | 95 | 0.75 | 0.82 |
| 95 | 90 | 0.78 | 0.85 |
| 95 | 95 | 0.75 | 0.85 |
FIGURE 41.
Diagnostic meta-analysis using FFR/QFR thresholds of 0.75 and 0.80.

| Study | Number of patients, vessel (lesion) (n) | Unit of analysis | Sensitivity, % (95% CI) | Specificity, % (95% CI) | AUC (95% CI) | Correlation | ICA cut-off point, % | FFR cut-off point |
|---|---|---|---|---|---|---|---|---|
| FAVOR II China and FAVOR II Europe–Japan: Ding et al. (2019)62 | 576 (645) | Vessel | 47.1 (40.5 to 53.6) | 74.4 (70.2 to 78.6) | 0.66 (0.62 to 0.71) | r = 0.59 | ≥ 50 | ≤ 0.80 |
| Kim et al. (2016)63 | 463 (724) | Vessel | 78 (72 to 82) | 48 (43 to 53) | NR | r = 0.49 | ≥ 50 | < 0.80 |
| Mejia-Renteria et al. (2017),64 conference abstract | 196 (246) | Vessel | 82 | 41 | 0.67 (0.61 to 0.74) | r = –0.39 | ≥ 50 | ≤ 0.80 |
| DAN-NICAD: Serj-Hansen et al. (2020)65 | 176 (232) | Patient | NR | NR | NR | ρ = 0.30 | ≥ 50 | ≤ 0.80 |
Appendix 5 Further narrative synthesis results
| Study | Population | Design | Test | Unit of analysis | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV (%) | NPV (%) | AUC (95% CI) | Correlation | MD (FFR-QFR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WIFI prototype study: Andersen et al. (2017),14 conference abstract |
|
Prospective | QAngio XA 3D/QFR (prototype) | Patient | 0.64 (0.48 to 0.77) | 0.8 (0.66 to 0.89) | 74.0 | 71.0 | 0.77 (0.67 to 0.87) | NR | –0.02 (0.12) |
| Retrospective reanalysis | QAngio XA 3D/QFR (modified prototype) | Patient | 0.66 (0.51 to 0.79) | 0.86 (0.73 to 0.93) | 81.0 | 74.0 | 0.87 (0.79 to 0.94) | NR | 0.0 (0.07) | ||
| NR | ICA (3D DS) | Patient | NR | NR | NR | NR | 0.75 (0.65 to 0.85) | NR | NR | ||
| Neylon et al. (2016),38 conference abstract |
|
Retrospective | QAngio XA 3D/QFR | Lesion | NR | NR | NR | NR | 0.78 (0.67 to 0.96) | NR | –0.01 (NR) |
| Goto et al. (2019),22 conference abstract |
|
Retrospective | QAngio XA 3D/QFR | NR | 84.8 | 68.2 | 84.8 | 68.2 | 0.82 (0.71 to 0.93) | r = 0.578 | NR |
| Kleczyński et al. (2019)33 |
|
Retrospective | QAngio XA 3D/QFR | Vessel | 91.8 | 97.3 | NR | NR | 0.98 (0.94 to 1.00) | NR | NR |
| Mehta et al. (2019),36 conference abstract |
|
Retrospective | QAngio XA 3D/QFR | Lesion | 84 | 92 | 76 | 95 | 0.94 | r = 0.801 | NR |
| Van Diemen et al. (2019),47 conference abstract |
|
Retrospective | QAngio XA 3D/QFR | Vessel | fQFR: 76 (59 to 89); cQFR: 71 (53 to 86) | fQFR: 94 (88 to 98); cQFR: 93 (86 to 97) | fQFR: 79 (64 to 89); cQFR: 74 (59 to 85) | fQFR: 93 (88 to 96); cQFR: 92 (86 to 95) | NR | NR | NR |
| Study | Population | Design | Test | Unit of analysis | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | Correlation | MD (FFR-QFR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kołtowski et al. (2018)32 | Stable CAD; 268 patients; 306 lesions | Retrospective | vQFR | Lesion | 90.5 | 69.7 | 68.8 | 90.8 | 0.900 | r = 0.78 | 0.03 (0.07) |
| lQFR | Lesion | 48.6 | 96.5 | 91.1 | 71.7 | 0.822 | r = 0.7 | –0.06 (0.07) | |||
| iQFR | Lesion | 83.8 | 86.6 | 82.2 | 87.9 | 0.936 | r = 0.85 | –0.002 (0.054) | |||
| FAVOR pilot: Tu et al. (2016)46 | 73 patients; 84 vessels | Retrospective | aQFR | Patient | 78.0 | 89.0 | 80.0 | 88.0 | 0.90 (0.81 to 0.96) | NR | NR |
| aQFR | Vessel | 78.0 | 91.0 | 81.0 | 90.0 | 0.91 (0.83 to 0.96) | r = 0.72 | –0.001 (0.065) | |||
| van Rosendael et al. (2017)48 | 15 vessels; non-acute CAD | Retrospective | aQFR (observer 1) | Vessel | 100 | 92.9 | 50 | 100 | NR | NR | 0.01 (0.04) |
| aQFR (observer 1) | Vessel | 100 | 92.3 | 50 | 100 | NR | NR | NR |
| Study | Subgroup | n a | Test | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | Correlation |
|---|---|---|---|---|---|---|---|---|---|
| Kanno et al. (2019)29 | High IMR | 155 patients | cQFR | 96.7 | 64.2 | 63.0 | 96.8 | NR | NR |
| Kanno et al. (2019)29 | Low IMR | 349 patients | cQFR | 81.5 | 77.2 | 78.8 | 80.0 | NR | NR |
| Mejia-Renteria et al. (2019)37,57 | High IMR | 83 vessels | cQFR | 86 | 69 | 67 | 87 | 0.88 (NR) | 0.77 |
| Mejia-Renteria et al. (2019)37,57 | Low IMR | 217 vessels | cQFR | 90 | 94 | 93 | 92 | 0.96 | 0.86 |
| Stähli et al. (2019)42,60 | Small vessel disease (≤ 2.8 mm reference diameter) | 225 vessels | cQFR | 80.0 | 98.5 | 94.6 | 94.0 | 0.89 (0.85 to 0.93) | r = 0.84 |
| Stähli et al. (2019)42 | Small vessel disease (≤ 2.8 mm reference diameter) | 225 vessels | fQFR | 73.9 | 96.6 | 87.3 | 92.1 | NR | r = 0.83 |
| Stähli et al. (2019)42 | No small vessel disease | 154 vessels | cQFR | 65.7 | 97.2 | 79.3 | 94.5 | 0.81 (0.76 to 0.86) | r = 0.77 |
| Stähli et al. (2019)42 | No small vessel disease | 154 vessels | fQFR | 68.6 | 96.7 | 77.4 | 94.9 | NR | r = 0.74 |
| WIFI II: Westra et al. (2018)51 | Multiple lesions | 81 lesions | QAngio | NR | NR | NR | NR | NR | MD = 0.01 ± 0.09 |
| WIFI II: Westra et al. (2018)51 | Single lesion | 174 lesions | QAngio | NR | NR | NR | NR | NR | MD = 0.01 ± 0.07 |
| FAVOR II China: Xu et al. (2017)52 | DS 40–80% | 273 patients | QAngio | 92.2 | 92.3 | 82.6 | 96.8 | NR | NR |
| FAVOR II China: Xu et al. (2017)52 | DS 40–80% | 272 patients | ICA (2D DS) | 54.5 | 60.0 | 50.5 | 22.9 | NR | NR |
| Emori et al. (2018)21 | LADb | 63 vessels | cQFR | 95.0 | 83.0 | 93.0 | 88.0 | NR | NR |
| Emori et al. (2018)21 | Non-LADb | 37 vessels | cQFR | 100.0 | 92.0 | 96.0 | 100.0 | NR | NR |
| Study | Subgroup | n | Test | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | Correlation |
|---|---|---|---|---|---|---|---|---|---|
| Smit et al. (2019)58 | Diabetic | 66 patients | QAngio (NS) | 75.0 | 95.0 | 90.0 | 97.0 | NR | NR |
| Non-diabetic | 193 patients | QAngio (NS) | 69.0 | 88.0 | 75.0 | 85.0 | NR | NR | |
| Diabetic | 82 vessels | QAngio (NS) | 71.0 | 95.0 | 85.0 | 89.0 | 0.91 (0.84 to 0.99) | r = 0.74 | |
| Non-diabetic | 238 vessels | QAngio (NS) | 69.0 | 91.0 | 74.0 | 88.0 | 0.93 (0.89 to 0.96) | r = 0.83 | |
| Stähli et al. (2019)42 | Diabetes | 98 patients | QAngio (NS) | NR | NR | NR | NR | 0.84 (0.76 to 0.90) | r = 0.82 |
| Stähli et al. (2019)42 | No diabetes | 338 patients | QAngio (NS) | NR | NR | NR | NR | 0.87 (0.83 to 0.90) | r = 0.81 |
| Kołtowski et al. (2018)32 | Diabetes | 21 patients/vessels | QAngio (NS) | NR | NR | NR | NR | NR | MD = –0.059 ± 0.07 |
| Kołtowski et al. (2018)32 | No diabetes | 173 patients/vessels | QAngio (NS) | NR | NR | NR | NR | NR | MD = –0.027 ± 0.074 |
| Emori et al. (2018)20 | Prior MI | 75 patients/vessels | fQFR | 94.0 | 62.0 | 69.4 | 92.3 | 0.90 (0.81 to 0.95) | r = 0.84 |
| Emori et al. (2018)20 | Prior MI | 75 patients/vessels | cQFR | 92.0 | 82.0 | 82.5 | 91.4 | 0.93 (0.86 to 0.97) | r = 0.88 |
| Emori et al. (2018)20 | No prior MI | 75 patients/vessels | fQFR | 98.0 | 73.0 | 82.0 | 96.0 | 0.97 (0.93 to 0.99) | r = 0.91 |
| Emori et al. (2018)20 | No prior MI | 75 patients/vessels | cQFR | 95.0 | 88.0 | 90.9 | 93.5 | 0.97 (0.93 to 0.99) | r = 0.94 |
| Hwang et al. (2019)24 | Stable CADa | 253 vessels | fQFR | 90.1 | 89.5 | 82.8 | 94.2 | 0.946 | r = 0.86 |
| Hwang et al. (2019)24 | MI non-culprita | 105 vessels | fQFR | 96.2 | 90.6 | 90.9 | 96.0 | 0.967 | r = 0.88 |
| Kołtowski et al. (2018)32 | CKDa | 32 patients | QAngio (NS) | NR | NR | NR | NR | 0.67 (0.46 to 0.88) | r = 0.63 |
| Kołtowski et al. (2018)32 | No CKDa | 170 patients | QAngio (NS) | NR | NR | NR | NR | 0.89 (0.84 to 0.94) | r = 0.79 |
| Variable | Study | |||
|---|---|---|---|---|
| FAVOR II Europe–Japan: Westra et al. (2018)50 | Mejia-Renteria et al. (2018,57 201937) | WIFI II: Westra et al. (2018)51 | SYNTAX II: Asano et al. (2019)15 | |
| ACS/acute MI (not previous MI) | OR 3.97 (95% CI 1.78 to 8.86) | |||
| Adenosine route | No | |||
| Age | No | No | No | |
| Bifurcation/trifurcation | OR 1.81 (95% CI 1.10 to 2.98) | |||
| BMI | No | No | ||
| Chronic total inclusion (main vessel) | No | |||
| Diabetes | OR 2.88 (95% CI 1.30 to 6.43) | No | ||
| DS | No | No | No | |
| Ejection fraction | ||||
| eGFR | ||||
| FFR | No | r = –1.17 (SE 0.53) | ||
| Hypertension | No | |||
| IMR | OR 1.05 (95% CI 1.02 to 1.08) | |||
| LAD | No | |||
| Left circumflex artery | No | |||
| Left marginal artery | No | |||
| Lesion length | No | |||
| Lesion location | ||||
| LM/LCA/left main coronary artery | ||||
| MI history/previous MI | No | |||
| MLD | No | |||
| Multivessel disease | No | |||
| Pa | No | |||
| Previous PCI | No | |||
| Proximal/mid-segment location | No | |||
| Right coronary artery | ||||
| Reference diameter | No | |||
| Sex | No | No | No | |
| Side branch location | OR 2.07 (95% CI 1.14 to 3.76) | |||
| Small vessel | OR 1.67 (95% CI 1.14 to 2.44) | |||
| Smoker | No | No | ||
| Vessel | No | No | ||
| Study | Failed/excluded, n | % | Unit | Reasons (n or %) |
|---|---|---|---|---|
| QAngio (prospective studies) | ||||
| FAVOR II Europe–Japan: Westra et al. (2018)50 | 57 | 17 | Patient | Lesion > 90% and no additional lesions with FFR measurement (8), no lesions > 30% apart (6), AF (1), acute MI (1), ostial right coronary artery (1), stepdown (1), protocol violation (7), FFR not measured (2), overlap (1), poor image quality (3), projection < 25% apart (1), technical issue (1), drift (9), damping (15). The authors stated that bifurcation lesions were excluded but numbers NR |
| Toi et al. (2018),44 conference abstract | NR | NR | NR | NR |
| van Rosendael et al. (2017)48 | NR | NR | NR | NR |
| Watari et al. (2019)49 | 11 | 7 | Vessel | Non-optimal angiographic projections for QFR analysis (9) or incomplete pressure wire measurement (2) |
| WIFI prototype study: Andersen (2017),14 conference abstract | NR | NR | NR | NR |
| FAVOR II China: Xu et al. (2017)52 | 29 | 9 | Patients | Two enrolled patients [three vessels, including data for QFR (2), poor image quality (1)]. Withdrew informed consent (4), atrial fibrillation during coronary angiography (1), total occlusion lesion (1), lesion DS < 30% or > 90% in all vessels (9), ineligible for diagnostic intervention or FFR examination (12) |
| WIFI II: Westra et al. (2018)51 | 190 | 52 | Patient |
Lesion > 90% and no FFR measurement (51), no confirmed lesion with DS > 30% (86), FFR not measured (34), drift (4), dampening (5), no sign of adenosine effect (1), other angiographic or procedural criteria (9) Lesions excluded owing to unsuccessful QFR computation (15), comprising overlap at the lesion segment of interest (6), excessive foreshortening in stenotic segments (7), insufficient contrast flow quality (1) and inability to contour a tight stenosis because of poor contrast filling (1) |
| QAngio (retrospective studies) | ||||
| Cliff and Ong (2019),16 conference abstract | NR | NR | NR | NR |
| Cortés et al. (2019)17 | 134 | 92 | Patient | Suboptimal angiographic images (46, of which 13 were primary, 33 staged procedure), retrospective (88) |
| Emori et al. (2018)20 | 13 | 8 | Patient | Incomplete CAG (9), ostial lesions (3), collateral donor artery (1) |
| Emori et al. (2018)21 | 6 | 6 | Patient | Incomplete coronary angiography (4), ostial lesion (1), collateral donor artery (1) |
| FAVOR pilot: Tu et al. (2016)46 | 15 | 17 | Patient | Excessive overlap of vessels (5), incomplete data (3), excessive pressure wire drift (3), noisy angiograms (2), < 25° apart (1), no sign of induced hyperaemia (1) |
| Goto et al. (2019),22 conference abstract | NR | NR | NR | NR |
| Hamaya et al. (2019)23 | 140 | 20 | Patient | Small (< 2 mm) right coronary artery left circumflex coronary artery (52), arrhythmia during ICA (32), ineligible coronary anatomy (98), insufficient image quality (10) |
| Hwang et al. (2019)24 | 127 | 35 | Vessel | Calibration failure (49), ostium lesion (35), insufficient projection (25), tortuous vessel (7), overlapped vessel (7), inadequate contrast filling (3), no iFR (1) |
| Ishihara et al. (2019),25 conference abstract | NR | NR | NR | NR |
| Jin et al. (2019),26 conference abstract | 20 | 20 | Patients | Unsuitable coronary anatomy, invalid FFR measurements, poor image quality and lack of two projections ≥ 25° apart (20 total) |
| Kajita (2019),27 conference abstract | NR | NR | NR | NR |
| Kameyama et al. (2016),28 conference abstract | 9 | 26 | Vessel | Poor angiographic images (9) |
| Kanno et al. (2019)29 | NR | NR | NR | NR |
| Kanno et al. (2019)30 | NR | NR | NR | NR |
| Kirigaya et al. (2019),31 conference abstract | NR | NR | NR | NR |
| Kleczyński et al. (2019)33 | NR | NR | NR | NR |
| Kołtowski et al. (2018)32 | 551 | 64 | Lesion | Lack of proper angiographic projections (299). Other reasons: bifurcation lesion (43), AF (41), vessel overlap/shortening (37), low image quality (28), ostial lesion (26), tandem lesion (21), collateral (18), bypass grafting (15) |
| Liontou et al. (2019)34 | 124 | 61 | Vessels | History of CABG, ostial left main or ostial right coronary artery lesions, occlusive restenosis, bioresorbable scaffolds, incompatibility of angiographic images (90). Lack of at least two angiographic projections > 25° apart, severe vessel tortuosity and/or overlap limiting QFR analysis (34) |
| Liu et al. (2017),35 conference abstract | NR | NR | NR | NR |
| Mehta et al. (2019),36 conference abstract | NR | NR | NR | NR |
| Mejia-Renteria et al. (2019)37 | 101 | 26 | Vessel | Ostial in left main or right coronary artery (10), grafted target vessels (2), inadequate projections (28), significant overlapping (17), inadequate ICA quality (19), resting haemodynamic data not available (6), contrast filling not optimal for TIMI frame count analysis (19) |
| Neylon et al. (2016),38 conference abstract | NR | NR | NR | NR |
| Sato et al. (2018),39 conference abstract | NR | NR | NR | NR |
| Smit et al. (2019)40 | 52 | 13 | Vessels | Insufficient image quality (17), presence of a coronary stent (4), excessive overlap and/or foreshortening of coronary arteries (18), absence of angiographic views with projection angles > 25° apart (6), ostial stenosis (5) or aneurysm (2) |
| Spitaleri et al. (2018)41 | 31 | 41 | Patients | No successful PCI on culprit lesion (3), diffuse disease in non-culprit vessel (8), severe tortuosity (2), vessel < 2.5 mm (2), operator preferred not to perform FFR (16) |
| Stähli et al. (2019)42 | 43 | 9 | Patients | Lack of two projections 25° apart (24), insufficient image quality (12), vessel overlap/shortening (10), low-contrast filling (7), technical issues (4), plus 14 patients with incomplete FFR measurement |
| SYNTAX II: Asano et al. (2019)15 | 341 | 29 | Vessel | No two appropriate projections (311), lumen diameter < 2.0 mm (12), ostial lesion near aorta (4), other (14) |
| Ties et al. (2018)43 | 232 | 70 | Vessel | Lack of basic requirements (200), insufficient image quality (17), inappropriate reference diameter function (8), overlap/foreshortening (5), no perpendicularity (1), true bifurcation (1) |
| Tu et al. (2014)45 | 3 | 4 | Vessel | Too much overlap or foreshortening (> 90%), insufficient image quality, mean pressure of the guiding catheter or blood haematocrit value not documented |
| Van Diemen et al. (2019),47 conference abstract | 266 | 48 | Vessels | No FFR (72). QFR analysis succeeded in 286 (52%) of the remaining 552 arteries. No further details reported |
| WIFI II: Westra et al. (2018)51 | 190 | 52 | Patient |
Lesion > 90% and no FFR measurement (51), no confirmed lesion > 30% (86), FFR not measured (34), drift (4), dampening (5), no sign of adenosine effect (1), other angiographic or procedural criteria (9) Lesions excluded owing to unsuccessful QFR computation (15), comprising overlap at the lesion segment of interest (6), excessive foreshortening in stenotic segments (7), insufficient contrast flow quality (1), and inability to contour a tight stenosis because of poor contrast filling (1) |
| Yazaki et al. (2017)53 | 20 | 12 | Vessel | Lacking two optimal angiographic projections at least 25° apart, overlapping vessels, no preferred references in proximal or distal vessels, insufficient contrast, target lesion at ostium of left or right artery |
| Ziubryte et al. (2019),54 conference abstract | NR | NR | NR | NR |
| CAAS vFFR | ||||
| FAST-EXTEND: Daemen et al. (2019)18 | NR | NR | NR | NR |
| FAST: Masdjedi et al. 202056 [CAAS vFFR] | 170 | 63 | Patients | Inadequate pressure waveform (25), STEMI (16), bypass graft (3), left main lesion (2), collaterals (5), lack of two adequate orthogonal views > 30° (58), overlap or foreshortening (35), no invasive blood pressure available (15), unknown position of FFR pressure wire (11) |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | 405 (pre and post PCI) | 65 | Lesions | Unavailability of at least two angiographic projections (172; 42.5%), table movement while acquiring angiographic images (104; 25.7%), angiography pixel/resolution incompatibility (61; 15%), < 30° angle between projections (26; 6.4%), multiple reasons (21; 5.2%), other (calibration issues, missing SID value, CABG, occluded vessel, total 21; 5.2%) |
| Study | Population | Outcomes |
|---|---|---|
| Spitaleri et al. (2018)41 |
|
POCE (5 years):
|
| Hamaya et al. (2019)23 |
|
MACE (median 2.2 years):
|
| Kanno et al. (2019),30 conference abstract |
|
MACE (4 years):
|
| Study | Grey zone | Diagnostic accuracy of grey-zone strategy (QFR vs. FFR) | Percentage of adenosine/FFR procedures avoided |
|---|---|---|---|
| FAVOR II Europe–Japan: Westra et al. (2018)50 | 0.77–0.86 | Sensitivity and specificity > 95% | 64 |
| Kanno et al. (2019),29 conference abstract | 0.73–0.84 | PPV and NPV > 90% | 52 |
| Mejia-Renteria et al. (2019),37 Lauri et al. (2018)171 | 0.74–0.84 | > 95% agreement | 59 |
| Smit et al. (2019)40 | 0.77–0.86 | Sensitivity: 95%; specificity: 92.5% | 61 |
| WIFI II Westra et al. (2018)51 | 0.78–0.87 | Sensitivity and specificity > 90% | 68 |
| 0.71–0.90 | Sensitivity and specificity > 95% | 42 |
| Study | Index test | Number of observations/observers blinding | Results |
|---|---|---|---|
| Cortés et al. (2019)17 | QAngio XA 3D/QFR (prototype) | 20 selected patients assessed by two analysts. No blinding reported |
|
| FAST: Masdjedi et al. (2020)56 [CAAS vFFR] | CAAS vFFR | 100 vessels assessed independently by two blinded analysts |
|
| Hwang et al. (2019)24 | QAngio XA 3D/QFR 1.2 | 30 randomly selected vessels assessed independently by two analysts |
|
| Ishihara et al. (2019),25 conference abstract | QAngio (no further details) | 100 vessels (94 patients) assessed independently by three analysts twice |
|
| Jin et al. (2019),26 conference abstract | QAngio XA 3D/QFR; CAAS vFFR | 101 vessels independently analysed by two analysts |
|
| Kajita et al. (2019),27 conference abstract (data from Hideo-Kajita et al.159) | QAngio XA 3D/QFR 1.0 | 34 vessels analysed by two analysts |
|
| Kleczyński et al. (2019)33 | QAngio XA 3D/QFR 2.1.12.2 | NR |
|
| Tu et al. (2014)45 | QAngio XA 3D/QFR 1.0 | 10 randomly selected vessels assessed independently by two blinded analysts |
|
| Study | Index test | Number of observations/observers | Results |
|---|---|---|---|
| Cliff and Ong (2019),16 conference abstract | QAngio XA 3D/QFR | 17 anonymised lesions reanalysed after 2 weeks | MD = 0.01 (0.05) |
| Cortés et al. (2019)17 | QAngio XA 3D/QFR (prototype) | 20 lesions assessed by two independent analysts reanalysed oncea | r = 0.958 (95% CI 0.877 to 0.984) |
| Ishihara et al. (2019),25 conference abstract | QAngio (no further details) | 100 vessels (94 patients) assessed independently by three analysts twice |
ICC per rater: 1 – 0.695 (95% CI 0.579 to 0.784) 2 – 0.820 (95% CI 0.733 to 0.879) 3 – 0.479 (95% CI 0.313 to 0.617) Average of all three raters: ICC 0.806 (95% CI 0.711 to 0.869) Intrarater reliability of measurements: ICC = 0.428 |
| Jin et al. (2019),26 conference abstract | QAngio XA 3D/QFR; CAAS vFFR | 101 vessels assessed by two independent analysts reanalysed oncea |
cQFR: MD 0.009 ± 0.053; p = 0.230 fQFR: MD 0.016 ± 0.060; p = 0.066 vFFR: MD 0.008 ± 0.040; p = 0.175 |
| Kajita et al. (2019),27 conference abstract (data from Hideo-Kajita et al.159) | QAngio XA 3D/QFR 1.0 | 34 vessels reanalysed after 1 week |
fQFR: R2 = 0.91 cQFR: R2 = 0.94 DS: R2 = 0.76 |
| Kleczyński et al. (2019)33 | QAngio XA 3D/QFR 2.1.12.2 | NR | ICC: 0.991 (95% CI 0.988 to 0.993) |
| Tu et al. (2014)45 | QAngio XA 3D/QFR 1.0 | 10 randomly selected vessels reanalysed after 1 week | Mean 0.00 (SD 0.03) |
| Ziubryte et al. (2019),54 conference abstract | QAngio XA 3D/QFR | 69 lesions measured three times with 3-day intervals between measurements | r = 0.997 (p < 0.001) |
| Study | Failed/excluded, n | % | Unit | Reasons (n or %) |
|---|---|---|---|---|
| QAngio (prospective studies) | ||||
| FAVOR II Europe–Japan: Westra et al. (2018)50 | 57 | 17 | Patient | Lesion > 90% and no additional lesions with FFR measurement (8), no lesions > 30% apart (6), AF (1), acute MI (1), ostial right coronary artery (1), stepdown (1), protocol violation (7), FFR not measured (2), overlap (1), poor image quality (3), projection < 25% apart (1), technical issue (1), drift (9), damping (15). The authors stated that bifurcation lesions were excluded but numbers were not reported |
| Toi et al. (2018),44 conference abstract | NR | NR | NR | NR |
| van Rosendael et al. (2017)48 | NR | NR | NR | NR |
| Watari et al. (2019)49 | 11 | 7 | Vessel | Non-optimal angiographic projections for QFR analysis (9) or incomplete pressure wire measurement (2) |
| WIFI prototype study: Andersen et al. (2017),14 conference abstract | NR | NR | NR | NR |
| FAVOR II China: Xu et al. (2017)52 | 29 | 9 | Patients | Two enrolled patients [three vessels, including data for QFR (2), poor image quality (1)]. Withdrew informed consent (4), atrial fibrillation during coronary angiography (1), total occlusion lesion (1), lesion DS < 30% or > 90% in all vessels (9), ineligible for diagnostic intervention or FFR examination (12) |
| WIFI II: Westra et al. (2018)51 | 190 | 52 | Patient |
Lesion > 90% and no FFR measurement (51), no confirmed lesion with DS > 30% (86), FFR not measured (34), drift (4), dampening (5), no sign of adenosine effect (1), other angiographic or procedural criteria (9) Lesions excluded owing to unsuccessful QFR computation (15), including: overlap at the lesion segment of interest (6), excessive foreshortening in stenotic segments (7), insufficient contrast flow quality (1) and inability to contour a tight stenosis because of poor contrast filling (1) |
| QAngio (retrospective studies) | ||||
| Cliff and Ong (2019),16 conference abstract | NR | NR | NR | NR |
| Cortés et al. (2019)17 | 134 | 92 | Patient | Suboptimal angiographic images (46, of which 13 were primary, 33 staged procedure), retrospective (88) |
| Emori et al. (2018)20 | 13 | 8 | Patient | Incomplete CAG (9), ostial lesions (3), collateral donor artery (1) |
| Emori et al. (2018)21 | 6 | 6 | Patient | Incomplete coronary angiography (4), ostial lesion (1), collateral donor artery (1) |
| FAVOR pilot: Tu et al. (2016)46 | 15 | 17 | Patient | Excessive overlap of vessels (5), incomplete data (3), excessive pressure wire drift (3), noisy angiograms (2), < 25° apart (1), no sign induced hypermia (1) |
| Goto et al. (2019),22 conference abstract | NR | NR | NR | NR |
| Hamaya et al. (2019)23 | 140 | 20 | Patient | Small (< 2 mm) right coronary artery or left circumflex coronary artery (52), arrhythmia during ICA (32), ineligible coronary anatomy (98), insufficient image quality (10) |
| Hwang et al. (2019)24 | 127 | 35 | Vessel | Calibration failure (49), ostium lesion (35), insufficient projection (25), tortuous vessel (7), overlapped vessel (7), inadequate contrast filling (3), no iFR (1) |
| Ishihara et al. (2019),25 conference abstract | NR | NR | NR | NR |
| Jin et al. (2019),26 conference abstract | 20 | 20 | Patients | Unsuitable coronary anatomy, invalid FFR measurements, poor image quality and lack of two projections ≥ 25° apart (20 total) |
| Kajita et al. (2019),27 conference abstract | NR | NR | NR | NR |
| Kameyama et al. (2016),28 conference abstract | 9 | 26 | Vessel | Poor angiographic images (9) |
| Kanno et al. (2019)29 | NR | NR | NR | NR |
| Kanno et al. (2019)30 | NR | NR | NR | NR |
| Kirigaya et al. (2019),31 conference abstract | NR | NR | NR | NR |
| Kleczyński et al. (2019)33 | NR | NR | NR | NR |
| Kołtowski et al. (2018)32 | 551 | 64 | Lesion | Lack of proper angiographic projections (299). Other reasons: bifurcation lesion (43), AF (41), vessel overlap/shortening (37), low image quality (28), ostial lesion (26), tandem lesion (21), collateral (18), bypass grafting (15) |
| Liontou et al. (2019)34 | 124 | 61 | Vessels | History of CABG, ostial left main or ostial right coronary artery lesions, occlusive restenosis, bioresorbable scaffolds, incompatibility of angiographic images (90). Lack of at least two angiographic projections > 25° apart, severe vessel tortuosity and/or overlap limiting QFR analysis (34) |
| Liu et al. (2017),35 conference abstract | NR | NR | NR | NR |
| Mehta et al. (2019),36 conference abstract | NR | NR | NR | NR |
| Mejia-Renteria et al. (2019)37 | 101 | 26 | Vessel | Ostial in LM or right coronary artery (10), grafted target vessels (2), inadequate projections (28), significant overlapping (17), inadequate ICA quality (19), resting haemodynamic data no available (6) contrast filling not optimal for TIMI frame count analysis (19) |
| Neylon et al. (2016),38 conference abstract | NR | NR | NR | NR |
| Sato et al. (2018),39 conference abstract | NR | NR | NR | NR |
| Smit et al. (2019)40 | 52 | 13 | Vessels | Insufficient image quality (17), presence of a coronary stent (4), excessive overlap and/or foreshortening of coronary arteries (18), absence of angiographic views with projection angles > 25° apart (6), ostial stenosis (5) or aneurysm (2) |
| Spitaleri et al. (2018)41 | 31 | 41 | Patients | No successful PCI on culprit lesion (3), diffuse disease in non-culprit vessel (8), severe tortuosity (2), vessel < 2.5 mm (2), operator preferred not to perform FFR (16) |
| Stähli et al. (2019)42 | 43 | 9 | Patients | Lack of two projections 25° apart (24), insufficient image quality (12), vessel overlap/shortening (10), low-contrast filling (7), technical issues (4), plus 14 patients with incomplete FFR measurement |
| SYNTAX II: Asano et al. (2019)15 | 341 | 29 | Vessel | No two appropriate projections (311), lumen diameter < 2.0 mm (12), ostial lesion near aorta (4), other (14) |
| Ties et al. (2018)43 | 232 | 70 | Vessel | Lack of basic requirements (200), insufficient image quality (17), inappropriate reference diameter function (8), overlap/foreshortening (5), no perpendicularity (1), true bifurcation (1) |
| Tu et al. (2014)45 | 3 | 4 | Vessel | Too much overlap or foreshortening (> 90%), insufficient image quality, mean pressure of the guiding catheter or blood haematocrit value not documented |
| Van Diemen et al. (2019),47 conference abstract | 266 | 48 | Vessels | No FFR (72). QFR analysis succeeded in 286 (52%) of the remaining 552 arteries. No further details reported |
| WIFI II: Westra et al. (2018)51 | 190 | 52 | Patient |
Lesion > 90% and no FFR measurement (51), no confirmed lesion > 30% (86), FFR not measured (34), drift (4), dampening (5), no sign of adenosine effect (1), other angiographic or procedural criteria (9) Lesions excluded owing to unsuccessful QFR computation (15), including: overlap at the lesion segment of interest (6), excessive foreshortening in stenotic segments (7), insufficient contrast flow quality (1) and inability to contour a tight stenosis because of poor contrast filling (1) |
| Yazaki et al. (2017)53 | 20 | 12 | Vessel | Lacking two optimal angiographic projections at least 25° apart, overlapping vessels, no preferred references in proximal or distal vessels, insufficient contrast, target lesion at ostium of left or right artery |
| Ziubryte et al. (2019),54 conference abstract | NR | NR | NR | NR |
| CAAS vFFR | ||||
| FAST-EXTEND: Daemen et al. (2019)18 | NR | NR | NR | NR |
| FAST: Masdjedi et al. (2020)56 [CAAS vFFR] | 170 | 63 | Patients | Inadequate pressure waveform (25), STEMI (16), bypass graft (3), left main lesion (2), collaterals (5), lack of two adequate orthogonal views > 30° (58), overlap or foreshortening (35), no invasive blood pressure available (15), unknown position of FFR pressure wire (11) |
| ILUMIEN I: Ely Pizzato et al. (2019)19 | 405 (pre and post PCI) | 65 | Lesions | Unavailability of at least two angiographic projections (172, 42.5%), table movement while acquiring angiographic images (104, 25.7%), angiography pixel/resolution incompatibility (61, 15%), < 30° angle between projections (26, 6.4%), multiple reasons (21, 5.2%), other (21, 5.2%) |
| Study | Design | Population | Index test | Data acquisition duration |
|---|---|---|---|---|
| WIFI prototype: Andersen et al. (2017),14 conference abstracta | Prospective/retrospective | n = 93; unselected, consecutive population referred to FFR | QAngio (prototype) |
|
| Kołtowski et al. (2018)32 | Retrospective | n = 306 lesions (268 patients); stable CAD, intermediate stenosis | QAngio XA 3D/QFR (model NR) |
|
| Tu et al. (2014)45 | Retrospective | n = 77 vessels (68 patients) | QAngio XA 3D/QFR 1.0 |
|
| FAVOR II China: Xu et al. (2017)52 | Prospective | n = 330 vessels (306 patients) | QAngio (AngioPlus) |
|
| Yazaki et al. (2017)53 | Retrospective | n = 151 vessels (142 patients) | QAngio XA 3D/QFR |
|
| FAVOR II Europe–Japan: Westra et al. (2018)50 | Prospective | n = 295 lesions | QAngio XA 3D/QFR |
|
Appendix 6 Further simulation study results
FIGURE 42.
Example simulation MACEs. The upper-left shaded region shows the FNs where QFR > 0.8 but FFR ≤ 0.8; the lower-right shaded region shows the FPs where QFR ≤ 0.8 but FFR > 0.8.

FIGURE 43.
Simulation study: MACEs prevented.
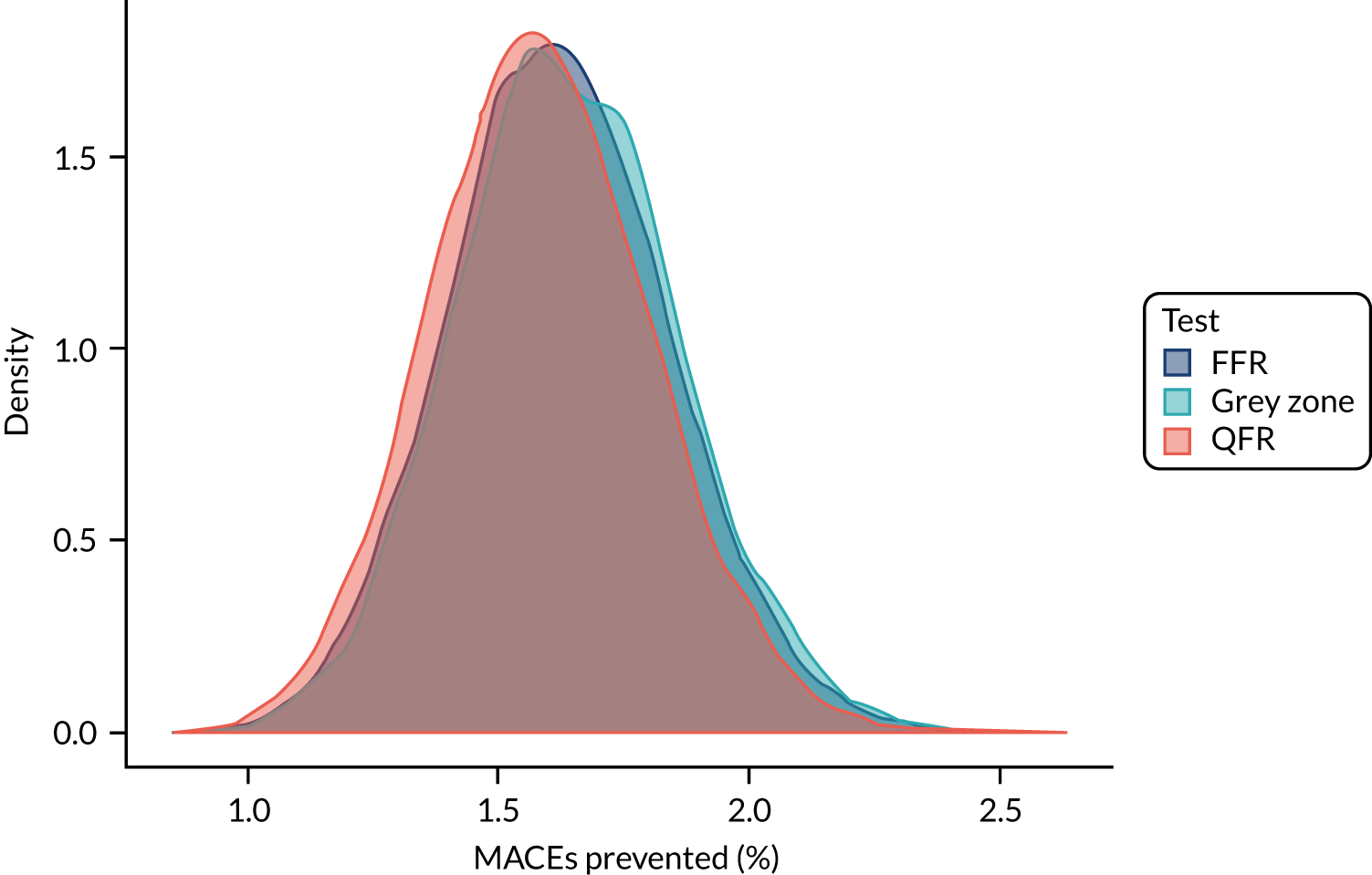
FIGURE 44.
Simulation study: MACEs not prevented.
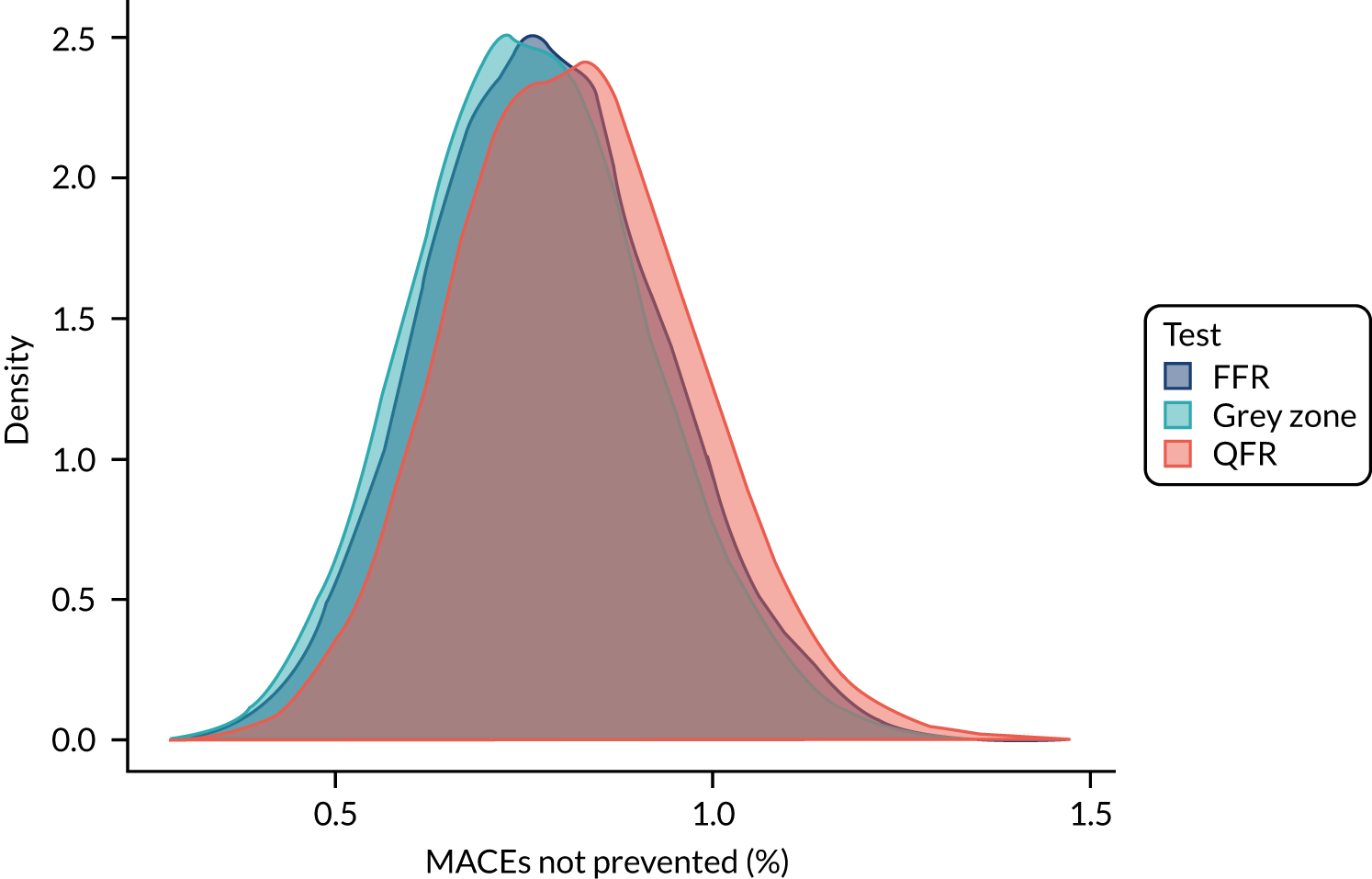
FIGURE 45.
Simulation study: MACE caused by revascularisation.
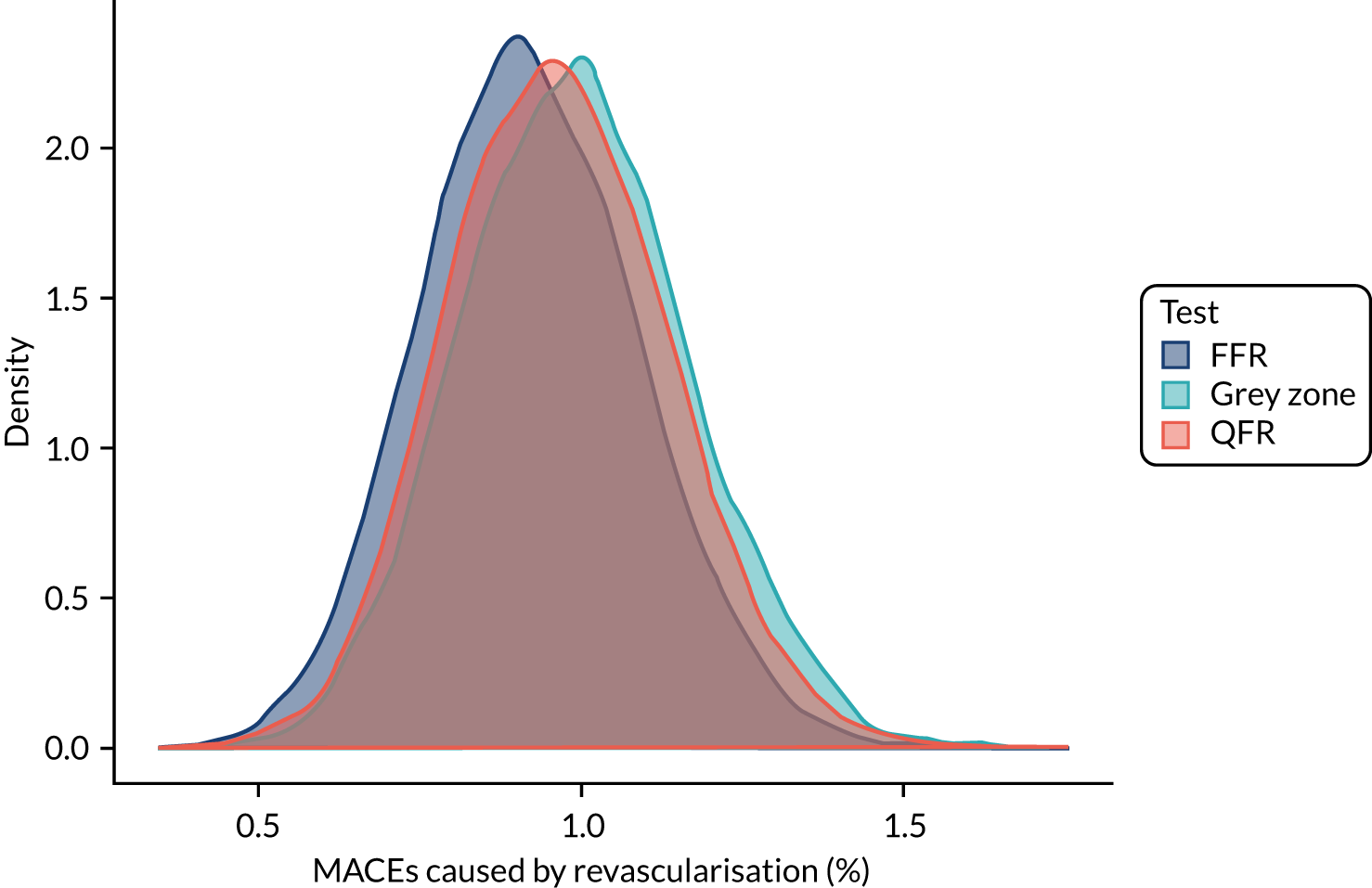
Appendix 7 Review of decision models evaluating invasive coronary angiography
Search strategies
QAngio literature: cost-effectiveness 18 October 2019
Database search strategies
Database searches were carried out to identify cost-effectiveness studies in which ICA (alone and/or with FFR) was one of the interventions under comparison.
Databases searched: EconLit (via Ovid), EMBASE (via Ovid), HTA database (via CRD), MEDLINE, NHS EED (via CRD).
Total number of records identified: 1740.
Total number of records identified after deduplication in EndNote X.9.2 (Clarivate Analytics) bibliographic software: 1264.
EconLit (via Ovid)
Search date: 18 October 2019.
Date range searched: 1886 to 3 October 2019.
Records retrieved: 2.
Search strategy
1 coronary angiograph$.mp. (2).
EMBASE (via Ovid)
Search date: 18 October 2019.
Date range searched: 1974 to 17 October 2019.
Records retrieved: 858.
Search strategy
-
*Coronary Angiography/ (3060)
-
coronary angiography.ti,ab. (50,830)
-
1 or 2 (51,869)
-
Economics/ (234,606)
-
Cost/ (57,396)
-
exp Health Economics/ (818,928)
-
Budget/ (27,893)
-
budget*.ti,ab,kw. (37,185)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw. (268,523)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (382,513)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw. (216,418)
-
(value adj2 (money or monetary)).ti,ab,kw. (3124)
-
Statistical Model/ (157,315)
-
economic model*.ab,kw. (4585)
-
Probability/ (97,263)
-
markov.ti,ab,kw. (27,735)
-
monte carlo method/ (37,601)
-
monte carlo.ti,ab,kw. (46,807)
-
Decision Theory/ (1711)
-
Decision Tree/ (11,762)
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kw. (31,964)
-
or/4-21 (1,560,379)
-
3 and 22 (1858)
-
limit 23 to yr = “2000 -Current” (1596)
-
limit 24 to embase (858).
Health Technology Assessment database
Searched via the CRD’s website (URL: www.crd.york.ac.uk/CRDWeb).
Search date: 18 October 2019.
Date range searched: inception to 1 October 2019.
Records identified: 62.
Search strategy
MEDLINE (via Ovid)
Search date: 18 October 2019.
Records identified: 665.
Date range searched: 1946 to 17 October 2019.
Search strategy
-
*Coronary Angiography/ (18,583)
-
coronary angiography.ti,ab. (30,052)
-
1 or 2 (41,395)
-
Economics/ (27,093)
-
exp “Costs and Cost Analysis”/ (229,217)
-
Economics, Nursing/ (3994)
-
Economics, Medical/ (9035)
-
Economics, Pharmaceutical/ (2894)
-
exp Economics, Hospital/ (23,942)
-
Economics, Dental/ (1908)
-
exp “Fees and Charges”/ (29,932)
-
exp Budgets/ (13,585)
-
budget*.ti,ab,kf. (28,234)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. (218,635)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (272,429)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. (152,531)
-
(value adj2 (money or monetary)).ti,ab,kf. (2256)
-
exp models, economic/ (14,432)
-
economic model*.ab,kf. (3128)
-
markov chains/ (13,736)
-
markov.ti,ab,kf. (21,125)
-
monte carlo method/ (27,269)
-
monte carlo.ti,ab,kf. (46,880)
-
exp Decision Theory/ (11,629)
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf. (22,091)
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 (696,909)
-
3 and 26 (855)
-
limit 27 to yr = “2000 -Current” (665).
NHS Economic Evaluation Database
Searched via CRD website (URL: www.crd.york.ac.uk/CRDWeb).
Search date: 18 October 2019.
Date range searched: inception to 1 October 2019.
Records identified: 161.
Search results and excluded studies
| Database | Number of records retrieved before deduplication | Number of records after deduplication |
|---|---|---|
| MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE | 665 | 660 |
| EMBASE (via Ovid) | 858 | 468 |
| EconLit (via Ovid) | 2 | 1 |
| NHS EED | 161 | 87 |
| HTA | 62 | 48 |
| Total in EndNote | 1748 | 1264 |
| Study | Reason for rejection |
|---|---|
| Bosch et al. (2005) | Different patient population (acute coronary syndrome) |
| Goehler et al. (2011) | Different patient population (acute chest pain) and does not include long-term outcomes |
| Kent et al. (2013) | Different patient population (NSTEMI) |
| Nam et al. (2015) | Different patient population (NSTEMI) |
Studies included in the review and extracted data
| Study, country | Testing (and management) strategies | Patient population (base case where clearly stated) | Model type | Health states | Key assumptions/comments |
|---|---|---|---|---|---|
| Amemiya and Takao (2009),69 Brazil |
|
|
|
|
|
| Bertoldi et al. (2016),68 Brazil |
|
|
|
|
|
| Boldt et al. (2013),78 Germany |
|
|
|
|
|
| Burgers et al. (2017);71 Westwood et al. (2013),79 UK |
|
|
|
|
|
| Espinosa and Annapragada (2013),76 USA |
|
|
|
|
|
| Fearon et al. (2003),87 USA |
|
|
|
|
|
| Genders et al. (2009),83 UK, USA, the Netherlands |
|
|
|
|
|
| Genders et al. (2015),74 UK, USA, the Netherlands |
|
|
|
|
|
| Goeree et al. (2013),75 Canada |
|
|
|
|
|
| Hayashino et al. (2004),88 USA |
|
|
|
|
|
| Hernandez and Vale (2007);85 Mowatt et al. (2004),86 UK |
|
|
|
|
|
| Kreisz et al. (2009),84 Australia |
|
|
|
|
|
| Ladapo et al. (2009),82 USA |
|
|
|
|
|
| Lee et al. (2015),73 the Republic of Korea |
|
|
|
|
|
| Min et al. (2010),81 USA |
|
|
|
|
|
| Min et al. (2017),70 USA |
|
|
|
|
|
| Pletscher et al. (2016),72 Switzerland |
|
|
|
||
|
|||||
| Priest et al., (2011),80 USA |
|
|
|
|
|
| Walker et al. (2011),77 UK |
|
|
|
|
|
| Disease status | Annual rate of MACE | Treatment receiveda | ||
|---|---|---|---|---|
| No treatment | Treatment | |||
| First year | Subsequent years | |||
| Normal coronary arteries | 0.0008 | 0.0008 | 0.0008 | RF |
| Mild CAD | 0.025 | 0.025 | 0.025 | RF |
| Moderate CAD | ||||
| No inducible ischaemia | 0.025 | 0.025 | 0.025 | RF |
| Mild inducible ischaemia | 0.246 [rate × (1/HR)] | 0.172 | 0.071 | RF plus OMT |
| Severe CAD | ||||
| Mild inducible ischaemia | 0.246 [rate × (1/HR)] | 0.172 | 0.071 | RF plus OMT |
| Severe inducible ischaemia | 0.157 [rate × (1/HR)] | 0.110 | 0.043 | RF plus OMT plus PCI |
| Three-vessel disease/LM | ||||
| Mild inducible ischaemia | 0.137 [rate × (1/HR)] | 0.096 | 0.031 | RF plus OMT plus CABG |
| Severe inducible ischaemia | 0.137 [rate × (1/HR)] | 0.096 | 0.031 | RF plus OMT plus CABG |
| Disease status | No treatment | Treatment | Treatment receiveda | ||||
|---|---|---|---|---|---|---|---|
| First year | Subsequent years | ||||||
| Male | Female | Male | Female | Male | Female | ||
| Normal coronary arteries | 0.851 | 0.824 | 0.851 | 0.824 | 0.851 | 0.824 | RF |
| Mild CAD | 0.851 | 0.824 | 0.851 | 0.824 | 0.851 | 0.824 | RF |
| Moderate CAD | |||||||
| No inducible ischaemia | 0.851 | 0.824 | 0.851 | 0.824 | 0.851 | 0.824 | RF |
| Mild inducible ischaemia | 0.699 | 0.677 | 0.734 | 0.711 | 0.749 | 0.726 | RF plus OMT |
| Severe CAD | |||||||
| Mild inducible ischaemia | 0.699 | 0.677 | 0.734 | 0.711 | 0.749 | 0.726 | RF plus OMT |
| Severe inducible ischaemia | 0.699 | 0.677 | 0.740 | 0.716 | 0.760 | 0.736 | RF plus OMT plus PCI |
| Three-vessel/LM | |||||||
| Mild inducible ischaemia | 0.659 | 0.638 | 0.740 | 0.716 | 0.820 | 0.794 | RF plus OMT plus CABG |
| Severe inducible ischaemia | 0.659 | 0.638 | 0.740 | 0.716 | 0.820 | 0.794 | RF plus OMT plus CABG |
| Disease status | Medication use (%) | |||
|---|---|---|---|---|
| Platelet inhibitor: aspirin (80 mg per day) | Statin: simvastatin (40 mg per day) | Nitrate: isosorbide mononitrate (60 mg per day) | ACE inhibitor: enalapril (20 mg per day) | |
| Baseline | 48 | 22 | 0 | 0 |
| No CAD | 12 | 17 | 1 | 7 |
| Mild CAD | 32 | 31 | 5 | 11 |
| Moderate CAD without inducible ischaemia | 73 | 72 | 11 | 27 |
| OMTa | 95 | 92 | 61 | 62 |
| PCI plus OMTa | 95 | 93 | 47 | 64 |
| CABG plus OMTa | 83 | 86 | 8b | 53 |
Appendix 8 Supplemental data used to inform the cost-effectiveness analysis
| Centre | Number of centres | ICA procedures | Source | |
|---|---|---|---|---|
| Total number | Average number per centrea | |||
| NHS interventional centre | 98 | 205,085 | 2093 | BCIS audit returns98 |
| Diagnostic-only centre | 60 | 35,017 | 584 | |
| Total | 158 | 240,102 | 1520 | |
| Serious procedural complication | aORBITA97 (N = 95), n (%) | RIPCORD101 (N = 200), n (%) | IRIS-FFR registry (N = 8633), n (%) |
|---|---|---|---|
| Major bleeding | 1 (1.05) | – | – |
| Converted to PCI for procedural complication | 4 (4.21) | – | – |
| Pulmonary oedema | 1 (1.05) | – | – |
| Vessel occlusion | – | 1 (0.5) | – |
| Deep-vein thrombosis | – | 1 (0.5) | – |
| Conduction disturbance requiring treatment | – | – | 3 (0.03) |
| Bronchospasm | – | – | 2 (0.02) |
| Coronary dissection | – | 1 (0.5) | 3 (0.03) |
| Ventricular arrhythmiab | – | 1 (0.5) | 2 (0.02) |
| Thrombus formation | – | – | 1 (0.01) |
FIGURE 46.
Baseline risk of MACE by FFR value in the first year after FFR measurement.
| Study | Region | Main inclusion criteria | N | Revascularisation procedure vs. OMT* | Follow-up (years) | Primary end points | Main findings |
|---|---|---|---|---|---|---|---|
| DEFER (2001)109 | Europe, Asia | SCAD, > 50% DS, FFR ≥ 0.75, no evidence of reversible ischaemia by non-invasive testing in previous 2 months | 325 | PCI for a FFR ≥ 0.75, performance group; PCI for a FFR < 0.75, reference group; OMT for a FFR ≥ 0.75, deferral group | 2 |
|
|
| TIME (2001)106 | Switzerland | SCAD, aged ≥ 75 years, CCSC ≥ 2 despite treatment with at least two antianginal agents | 305 | PCI, CABG, OMT | 1 |
|
|
| MASS II (2007, 2006)107,127,187 | Brazil | SCAD, ≥ 70% proximal multivessel stenosis and documented ischaemia | 611 | PCI, CABG, OMT | 10 |
|
|
| COURAGE (2007, 2008)91,128 | North America | SCAD, ≥ 70% in ≥ 1 proximal epicardial coronary artery and evidence of myocardial ischaemia or at least one coronary stenosis of ≥ 80% and classic angina | 2287 | PCI, OMT | 4.6 |
|
|
| JSAP (2008)111 | Japan | SCAD, ≥ 75% coronary stenosis | 384 | PCI, OMT | 3.3 |
|
|
| BARI 2D (2009, 2011)110,129,188 | North and South America, Europe | SCAD plus type 2 diabetes, ≥ 50% DS of a major epicardial coronary artery with positive stress test or ≥ 70% stenosis of a major epicardial coronary artery and classic angina | 2368 | PCI, CABG, OMT | 5 |
|
|
| FAME II (2014)108,130 | Europe, North America | SCAD, ≥ 50% DS, FFR < 0.8 | 888 | FFR-guided PCI for a FFR ≤ 0.8, PCI group; OMT for a FFR > 0.8, OMT group | 2 |
|
|
| ORBITA (2018)97 | UK | SCAD, ≥ 70 DS single-vessel, FFR, iFR | 200 | PCI, OMT | 6 weeks |
|
|
| ISCHEMIA (2019)95,131,189,190 | USA | SCAD, ≥ 50% DS of a major epicardial coronary artery with positive stress test or ≥ 70% stenosis of a proximal or mid-vessel | 5179 | PCI (74% of revascularisation group), CABG (26% of revascularisation group), OMT | 3.2 |
|
| Procedure | Mean QALY loss (95% CI) | Source |
|---|---|---|
| ICA | 0 | Assumed to cancel across strategies |
| FFR/iFR | 0.0056 (0.0051 to 0.0062) | Assumed the same as for PCI |
| PCI | 0.0056 (0.0051 to 0.0062) | Bagust et al. (2006)126 |
| CABG | 0.033 (0.031 to 0.035) | Bagust et al. (2006)126 |
| Voucher | Software licence feea,b | Training and certification fee for up to four members of staffa | ||
|---|---|---|---|---|
| Euro | GBP | Euro | GBP | |
| 10 patients | 5000 | 4314.75 | 3500 | 3020.33 |
| 50 patients | 25,000 | 21,573.75 | 3500 | 3020.33 |
| 100 patients | 49,000 | 42,284.55 | Included in the licence fee | |
| Staff | Staff numbers | Time (hours) | Unit costa (£) | Source | Total cost (£) | |
|---|---|---|---|---|---|---|
| Training | Certification | |||||
| Cardiologist | 1 | 0.5 | – | 109 | Consultant: surgical; PSSRU (2019)137 | 54.50 |
| Cardiac physiologist | 3 | 5 | 1.5 | 37 | Allied professional (band 5);b PSSRU (2019)137 | 721.50 |
| Total | 4 | 16.5 | 4.5 | – | – | 776.00 |
| Cost element | Total cost (£) | Cost per patient tested (£) |
|---|---|---|
| Software licence fee | 84,569.10 | 422.85 |
| Training and certification fee | – | – |
| Training and certification staff costs | 1552.00 | 7.76 |
| Total | 86,121.10 | 430.61 |
| Pricing model | Software licence feea | Annual maintenance feea | Training feea,b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Euro | GBP | Conditions | Euro | GBP | Starting from | Platform | Euro | GBP | |
| 1 | 37,000 | 31,929 | Perpetual licence | 5500 | 4746 | Year 2 | e-learning | – | – |
| 2 | 200 | 173 | Per patient | 3500 | 3020 | Year 1 | Webex | 250 | 216 |
| On site | 2500 | 2157 | |||||||
| Staff | Staff numbers | Time (hours) | Unit costa (£) | Source | Total cost (£) | ||
|---|---|---|---|---|---|---|---|
| Webex or e-learning | On site | Webex or e-learning | On site | ||||
| Cardiologist | 1 | 2 | 4 | 109 | Consultant: surgical, PSSRU 2019137 | 218 | 436 |
| Cardiac physiologist | 3 | 2 | 4 | 37 | Allied professional (band 5b), PSSRU 2019137 | 222 | 444 |
| Total | 4 | 8 | 16 | – | – | 440 | 880 |
| Cost element | Total cost (£) | Cost per patient testeda (£) | |
|---|---|---|---|
| Webex or e-learning | On-site | ||
| Software licence fee | 31,929.15 | 31,929.15 | 159.65 |
| Training fee | 215.74 | 2157.38 | 5.93 |
| Staff training costs | 440.00 | 880.00 | 6.60 |
| Maintenance cost | – | – | – |
| Total | 32,584.89 | 34,966.53 | 172.18 |
FIGURE 47.
Comparison of QAngio XA 3D/QFR and CAAS vFFR test cost at different throughputs.
| Cost category | Currency codes | Unit cost (£) |
|---|---|---|
| PCIa | EY40A–D and EY41A–D, across all HRG codes | 3005.07 |
| PCI as day case | EY40A–D and EY41A–D, day case | 2178.95 |
| CABGa | ED26A–C, ED27A–C and ED28A–C, across all HRG codes | 10,898.58 |
| Procedural complication | Rate (%) | Source | Unit cost (£) | Currency codes/assumptions |
|---|---|---|---|---|
| Coronary dissection | 0.03 | IRIS13 | 3005.07 | Activity-weighted average of the PCI currency codes (EY40A–D and EY41A–D, across all HRG codes codes). It is assumed that PCI is required to repair coronary dissection. This cost is incurred only if patients do not subsequently undergo PCI as a result of testing positive for significant stenosis |
| Ventricular arrhythmia | 0.02 | IRIS13 | 974.90 | Activity-weighted average of the arrhythmia or conduction disorders currency codes (EB07A–E, across all HRG codes) |
| Conduction disturbance requiring treatment | 0.03 | IRIS13 | 974.90 | Activity-weighted average of the arrhythmia or conduction disorders currency codes (EB07A–E, across all HRG codes) |
| Thrombus formation | 0.01 | IRIS13 | 928.12 | Assumed average of unit cost of other complications, excluding coronary dissection |
| Bronchospasm | 0.02 | IRIS13 | 834.57 | Activity-weighted average of the asthma without interventions currency codes (DZ15N, P–R, across all HRG codes) |
| Death | 0.015 | Fearon et al. (2003)87 | 0.00 | Assumption |
| Health state/clinical event | Cost (£) | Source |
|---|---|---|
| No event | 0.00 | Assumption |
| MI | 2317.53 | Activity-weighted average of HRG codes for actual or suspected MI (EB10A–E), across all HRG codes (NHS reference costs 2017/18)138 |
| Post MI | 0.00 | Assumption |
| Unplanned revascularisation | 4812.23 | Activity-weighted average of HRG codes for PCI and CABG (EY40A–D, EY41A–D, ED26A–C, ED27A–C and ED28A–C), non-elective long stays – NHS reference costs 2017/18138 |
| Post unplanned revascularisation | 0.00 | Assumption |
| Cardiovascular death | 0.00 | Assumption |
| Other-cause death | 0.00 | Assumption |
| Parameter | Values | Source/assumptions | Probabilistic model set-up |
|---|---|---|---|
| Patient characteristics | |||
| Age | 64 | IRIS-FFR13 | NA |
| Proportion of male individuals | 0.72 | IRIS-FFR13 | NA |
| Proportion with clinically significant stenosis (i.e. FFR < 0.8) | 0.402 | Recreated individual level FFR measurements in the QAngio diagnostic accuracy studies (cQFR and non-specified QFR mode) | Calculated from each 5000 bootstrapped samples of the joint FFR and QFR distribution |
| Number of patients with stenosis of uncertain clinical significance | |||
| Annual throughput | 200 | BCIS audit returns98 and clinical opinion | NA |
| Diagnostic accuracy | |||
| FFR/iFR | |||
| Sensitivity | 100% | Assumption | NA |
| Specificity | 100% | ||
| ICA | |||
| Sensitivity | 62.61% | Bivariate meta-analysis (see Chapter 3, Meta-analysis of extracted figure data for two-dimensional invasive coronary angiography) | Multivariate log-normal distribution fitted to log-odds sensitivity and specificity |
| Specificity | 61.59% | ||
| QAngio | |||
| Sensitivity | 84.34% | Bivariate meta-analysis (see Table 3) for combined cQFR and non-specified QFR mode | Multivariate log-normal distribution fitted to log-odds sensitivity and specificity |
| Specificity | 89.80% | ||
| CAAS vFRR | |||
| Sensitivity | 97.00% | FAST-EXTEND18 | Independent beta distributions fitted to the diagnostic accuracy 2 × 2 tables
|
| Specificity | 74.00% | ||
| Joint probability of FFR and QFR | |||
| QFR < 0.78 and FFR ≤ 0.80 | 0.744 | Recreated individual level FFR and QFR measurements in the QAngio diagnostic accuracy studies (cQFR and non-specified QFR mode) | Calculated from each 5000 bootstrapped samples of the joint FFR and QFR distribution |
| QFR 0.78–0.84 and FFR ≤ 0.80 | 0.188 | ||
| QFR ≥ 0.84 and FFR ≤ 0.80 | 0.069 | ||
| QFR < 0.78 and FFR > 0.80 | 0.095 | ||
| QFR 0.78–0.84 and FFR > 0.80 | 0.212 | ||
| QFR ≥ 0.84 and FFR > 0.80 | 0.693 | ||
| Procedural adverse events | |||
| FFR/iFR complications | |||
| Conduction disturbance requiring treatment | 0.03% | IRIS-FFR13 | Beta distribution: α = 3, β = 8630 |
| Bronchospasm | 0.02% | Beta distribution: α = 2, β = 8631 | |
| Coronary dissection | 0.03% | Beta distribution: α = 3, β = 8630 | |
| Ventricular arrhythmia | 0.02% | Beta distribution: α = 1, β = 8631 | |
| Thrombus formation | 0.01% | Beta distribution: α = 1, β = 8632 | |
| Death | 0.015% | Fearon et al., 200387 | NA |
| Revascularisation complications | |||
| PCI death | 0.17% | 2019 NCAP Annual Report 103 | NA |
| CABG death | 0.99% | ACS 2019 summary report102 | NA |
| Revascularisation death | 0.277% | Calculated | NA |
| Proportion per revascularisation procedure | |||
| PCI | 87% | BCIS audit returns98 | NA |
| CABG | 13% | NA | |
| Clinical event rates | |||
| Non-cardiovascular mortality | Age and sex dependent | ONS mortality data120,121 | NA |
| Baseline MACE rates | Dependent on the underlying FFR distribution | IRIS-FFR13 | The underlying FFR distribution was calculated from each 5000 bootstrapped samples of the joint FFR and QFR (or DS, where applicable) distribution |
| HR of revascularisation on MACE rates | 1.0 | Assumption of no treatment effect | NA |
| HRQoL | |||
| Procedural disutility | |||
| ICA | 0.0000 | Assumption | NA |
| FFR/iFR | 0.0056 | Assumed same as PCI | Gamma distribution: mean 0.0056, SE 0.003 |
| PCI | 0.0056 | Bagust et al. (2006)126 | Gamma distribution: mean 0.0056, SE 0.003 |
| CABG | 0.033 | Bagust et al. (2006)126 | Gamma distribution: mean 0.033, SE 0.001 |
| Revascularisation | 0.0092 | Calculated | NA |
| Baseline utility in the prognostic model | Age and sex dependent | Calculated based on Ara and Brazier (2010)134 and Nishi et al. (2018)130 | Beta distribution fitted to reference group baseline utility: mean 0.821, SE 0.0112 |
| Utility increments | |||
| FN | 0.015 | Nishi et al. (2018)130 | Beta distribution: mean 0.015, SE 0.0094 |
| TN | 0.000 | Calculated based on Nishi et al. (2018)130 and the underlying distribution of FFR | NA |
| FP | 0.000 | NA | |
| TP | 0.000 |
The underlying FFR distribution was calculated from each 5000 bootstrapped samples of the joint FFR and QFR (or DS where applicable) distribution Beta distributions were fitted to the utility increment by FFR category (see Table 11) |
|
| Health states and clinical events disutility | |||
| MI | 0.0626 | Sullivan et al. (2011)135 | Gamma distribution: mean 0.0626, SE 0.0132 |
| Post-MI | 0.0368 | Sullivan et al. (2011)135 | Gamma distribution: mean 0.0368, SE 0.0257 |
| Unplanned revascularisation | 0.0091 | Calculated | NA |
| Costs | |||
| Tests (per patient tested) | |||
| Qangio XA | £430.61 | Calculated | NA |
| CAAS vFFR | £172.18 | Calculated | NA |
| FFR/iFR | £436.80 | NHS reference costs 2017/18138 and uprated to 2018/19 costs137 | NA |
| ICA | £0 | Assumption | NA |
| Optimal medication treatment (annual cost) | |||
| OMT only | £163.63 | Calculated based on Nishi et al. (2018)130 COURAGE,91 SYNTAX59 and the BNF141 | NA |
| OMT only (FN) | £168.68 | NA | |
| OMT in addition to PCI | £150.10 | NA | |
| OMT in addition to CABG | £126.27 | NA | |
| OMT in addition to revascularisation | £147.00 | Calculated | NA |
| Interventional procedures | |||
| PCI | £3005.07 | NHS reference costs 2017/18138 and uprated to 2018/19 costs137 | NA |
| CABG | £10,898.58 | NA | |
| Revascularisation | £4031.22 | Calculated | NA |
| Treatment of revascularisation complications | |||
| Coronary dissection | £3005.07 | NHS reference costs 2017/18138 and uprated to 2018/19 costs137 | NA |
| Ventricular arrhythmia | £974.90 | NA | |
| Conduction disturbance requiring treatment | £974.90 | NA | |
| Bronchospasm | £928.12 | NA | |
| Thrombus formation | £834.57 | Assumed average of unit cost of other complications, excluding coronary dissection | NA |
| Death | £0.00 | Assumption | NA |
| Health states and clinical events costs | |||
| No event | £0.00 | Assumption | NA |
| MI | £2317.53 | NHS reference costs 2017/18138 and uprated to 2018/19 costs137 | NA |
| Unplanned revascularisation | £4812.23 | NA | |
| Post MI | £0.00 | Assumption | NA |
| Post unplanned revascularisation | £0.00 | Assumption | NA |
| Death (cardiovascular and other causes) | £0.00 | Assumption | NA |
Appendix 9 Deterministic results of the cost-effectiveness analysis
| Scenario | Strategy | ||||
|---|---|---|---|---|---|
| 1. ICA alone | 2. ICA plus FFR | 3. ICA plus QFR | 4. ICA plus QFR plus confirmatory FFR | 5. vFFR | |
| Base case | 0.029 | 0.020 | 0.016 | 0.016 | |
| 1 | 0.029 | 0.019 | 0.016 | 0.016 | |
| 2 | 0.029 | 0.023 | 0.016 | 0.016 | |
| 3a | 0.008 | ||||
| 4 | 0.029 | 0.020 | 0.016 | –0.031 | |
| 5 | 0.029 | 0.020 | 0.016 | 0.024 | |
| 6 | 0.029 | 0.020 | 0.016 | 0.034 | |
| 7 | 0.018 | 0.010 | 0.006 | 0.005 | |
| 8 | 0.048 | 0.036 | |||
| 9 | 0.029 | 0.020 | 0.016 | 0.016 | |
| 10 | 0.061 | 0.039 | 0.042 | 0.042 | |
| 11 | 0.032 | 0.023 | 0.019 | 0.020 | |
| 12 | 0.033 | 0.023 | 0.019 | 0.020 | |
| 13 | 0.033 | 0.023 | 0.020 | 0.021 | |
| 14 | 0.026 | 0.021 | 0.017 | 0.008 | |
| 15 | 0.025 | 0.017 | 0.014 | 0.018 | |
| 16 | 0.033 | 0.025 | 0.019 | 0.012 | |
| 17 | 0.004 | 0.006 | –0.004 | –0.011 | |
| 18 | –0.010 | –0.002 | –0.015 | –0.025 | |
| 19 | 0.002 | 0.021 | 0.011 | 0.016 | |
| 20 | 0.030 | 0.020 | 0.016 | 0.016 | |
| 21 | 0.026 | 0.020 | 0.016 | 0.016 | |
| 22 | 0.025 | 0.020 | 0.016 | 0.016 | |
| 23 | –0.060 | 0.020 | –0.002 | 0.016 | |
| 24 | –0.060 | 0.020 | –0.002 | 0.021 | |
Glossary
- CAAS® vFFR®
- Non-invasive imaging technology produced by Pie Medical Imaging BV (Maastricht, the Netherlands).
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False negative
- Incorrect negative test result: number of diseased persons with a negative test result.
- False positive
- Incorrect positive test result: number of non-diseased persons with a positive test result.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Markov model
- An analytic method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- A statistical technique used to combine the results of two or more studies and obtain a combined estimate of effect.
- Metaregression
- A statistical technique used to explore the relationship between study characteristics and study results.
- Negative predictive value
- Proportion of patients who tested negative on the test that do not have the condition of interest.
- Opportunity cost
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Percutaneous coronary intervention
- A non-surgical procedure that uses a small structure called a stent to open up blood vessels in the heart that have been narrowed by plaque build-up.
- Positive predictive value
- Proportion of patients who tested positive on the test that have the condition of interest.
- QAngio® XA 3D/QFR®
- Non-invasive imaging software produced by Medis Medical Imaging Systems BV (Leiden, the Netherlands).
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test against which the index test is compared.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True negative
- Correct negative test result: number of non-diseased persons with a negative test result.
- True positive
- Correct positive test result: number of diseased persons with a positive test result.
List of abbreviations
- 2D
- two-dimensional
- 3D
- three-dimensional
- ACE
- angiotensin-converting enzyme
- aQFR
- adenosine–flow quantitative flow ratio
- AUC
- area under the curve
- BARI 2D
- Bypass Angioplasty Revascularization Investigation 2 Diabetes
- BCIS
- British Cardiovascular Intervention Society
- BNF
- British National Formulary
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CCS
- Canadian Cardiovascular Society
- CCTA
- coronary computed tomography angiography
- CE-MARC
- Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease
- CI
- confidence interval
- CKD
- chronic kidney disease
- cQFR
- contrast-flow quantitative flow ratio
- CRD
- Centre for Reviews and Dissemination
- DASI
- Duke Activity Score Index
- DEFER
- DEFERral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis
- DOR
- diagnostic odds ratio
- DS
- diameter stenosis
- EAG
- External Assessment Group
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EUROPA
- EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery
- FAME
- Fractional flow reserve versus Angiography for Multivessel Evaluation
- FAST
- Fast Assessment of STenosis severity
- FAVOR
- Functional Assessment by Virtual Online Reconstruction
- FFR
- fractional flow reserve
- FN
- false negative
- FP
- false positive
- fQFR
- fixed-flow quantitative flow ratio
- GBP
- Great British pounds
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSROC
- hierarchical summary receiver operating characteristic
- HTA
- Health Technology Assessment
- ICA
- invasive coronary angiography
- ICC
- intraclass correlation
- ICER
- incremental cost-effectiveness ratio
- iFR
- instantaneous wave-free ratio
- IMR
- index of microcirculatory resistance
- INHB
- incremental net health benefit
- IPD
- individual participant data
- iQFR
- index quantitative flow ratio
- ISCHEMIA
- International Study of Comparative Health Effectiveness With Medical and Invasive Approaches
- JSAP
- Japanese Stable Angina Pectoris
- LAD
- left anterior descending
- lQFR
- lesion quantitative flow ratio
- MACE
- major adverse cardiac event
- MASS II
- Medicine, Angioplasty, or Surgery Study II
- MD
- mean difference
- MI
- myocardial infarction
- MPS
- myocardial perfusion scintigraphy
- MR
- magnetic resonance
- NCL
- non-culprit lesion
- NHB
- net health benefit
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- NSTEMI
- non-ST segment elevation myocardial infarction
- OMT
- optimal medical treatment
- OR
- odds ratio
- ORBITA
- Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina
- PCI
- percutaneous coronary intervention
- POCE
- patient-oriented cardiac event
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PTCA
- percutaneous transluminal coronary angioplasty
- QALY
- quality-adjusted life-year
- QCA
- quantitative coronary arteriography
- QFR
- quantitative flow ratio
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies-2
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- SAQ
- Seattle Angina Questionnaire
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SPECT
- single-photon emission computed tomography
- STEMI
- ST segment elevation myocardial infarction
- TIME
- Trial of Invasive versus Medical therapy in the Elderly
- TN
- true negative
- TP
- true positive
- vFFR
- vessel fractional flow reserve
- vQFR
- vessel quantitative flow ratio
- WIFI
- wire-free invasive functional imaging
