Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/58/18. The contractual start date was in September 2016. The draft report began editorial review in October 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Cigarette smoking is one of the leading causes of early death both in the UK and worldwide. 1,2 Although smoking is now down to fewer than 1 in 6 adults (14.4%) in the UK, this still equates to approximately 7.35 million people in the population. 3 In 2017, 77,800 deaths were estimated to be attributable to smoking in England, representing 16% of all deaths and 33% of deaths from conditions that can be caused by smoking. 3 The cost of smoking to the NHS has been estimated at between approximately £2.6B and £5B per year,4,5 with the total cost to society in England estimated at approximately £12.9B per year. 6
Description of the interventions under assessment
Smoking cessation medicines and electronic cigarettes
National Institute for Health and Care Excellence (NICE) public health guidance recommends the use of three medicines, varenicline, bupropion and nicotine replacement therapy (NRT), as aids to quitting smoking in the UK. 7 Varenicline is a partial agonist selective for alpha-4 beta-2 nicotinic receptor subtypes. It binds to these receptors, causing a dopamine release, albeit less than that from smoking, while simultaneously blocking the action of nicotine itself. 8,9 Therefore, it acts to both limit the reward experienced by smoking and counteract the withdrawal symptoms experienced during smoking cessation attempts that result from low dopamine release in the absence of nicotine. Varenicline was approved as a prescription smoking cessation aid in 2006 by the US Food and Drug Administration (FDA) as Chantix (Pfizer Inc., Mission, KS, USA), and by the European Medicines Agency (EMA) as Champix (Pfizer Europe MA EEIG, Brussels, Belgium). It was recommended by NICE in July 2007 as an option for smokers who had expressed a desire to quit smoking as part of a programme of behavioural support. 10,11
Bupropion, or Zyban (GlaxoSmithKline, Brentford, UK), was licensed by the FDA in 1997 and by the Medicines and Healthcare products Regulatory Agency (MHRA) in June 2000 as a stop-smoking medicine. It is also used off-licence in the UK and in the USA as an antidepressant. 12 It has both dopaminergic and adrenergic actions and also appears to be an antagonist at the nicotinic acetylcholine receptor. 13 The drug promotes smoking cessation by blocking nicotine effects, relieving withdrawal symptoms,14,15 or, in its antidepressant role, by blocking the neuronal reuptake of dopamine and noradrenaline, thereby reducing low mood. 16
Nicotine replacement therapy (NRT) refers to products used to assist with a quit attempt by delivering nicotine to satisfy tobacco cravings and preventing withdrawal symptoms. NRT can come in fast-acting forms, such as gum, lozenge, spray, inhalator and tablet, or as slow-acting patches, and are often used in combination, to deliver varying doses of nicotine based on one’s level of tobacco dependence, as standard care in the UK.
In late 2015, the MHRA approved the use of the first electronic cigarette, British American Tobacco’s ‘e-Voke’, as a smoking cessation medicine. 17 Electronic cigarettes (also known as e-cigarettes, e-cigs, electronic nicotine delivery systems or vapes) are battery-powered devices that heat a liquid, typically containing nicotine, flavourings and additives, to generate an aerosol or a ‘vapour’, which the user then inhales. 18 However, e-Voke’s development was terminated before the product could come to market. Although no e-cigarettes are currently licensed as medicines, NICE guidance recognises that e-cigarettes may help people to quit smoking cigarettes. 7,19 An independent expert review of e-cigarettes published by Public Health England in 201520 advised that e-cigarettes should be considered as an option for smokers who have failed to quit smoking by other methods. The report’s statement that e-cigarettes are 95% safer than tobacco smoking remains controversial. An updated report published in 2018 recommended improved access to e-cigarettes for people in disadvantaged groups and the importance of facilitating the regulation of some e-cigarettes as medicines via the MHRA. 21
Changes in prescribing patterns
The number of prescriptions of all smoking cessation medicines has shown an overall reduction over the past 10 years, which may reflect the decrease in smoking prevalence and/or the increased use of e-cigarettes. Prescription data from 2018/19 show 740,000 total prescriptions of smoking cessation medicines, with 396,000 prescriptions of NRT (note that NRT is also available over the counter without a prescription), 24,000 prescriptions of bupropion and 320,000 prescriptions of varenicline. 3 In 2018, there were an estimated 3.2 million adult users of e-cigarettes in Great Britain. 22 Notably, the number of prescription items of varenicline dispensed in England decreased by 51% from a peak of 987,000 prescriptions in 2011 to 489,000 prescriptions in 2016,23 possibly reflecting ongoing fears among prescribers and patients about varenicline’s neuropsychiatric safety as a result of the safety warnings on varenicline’s product labelling during that time (see Adverse events).
Effectiveness
All of the currently licensed smoking cessation medicines have been shown to improve people’s chances of quitting smoking compared with placebo. 24–27 Varenicline has been shown to be the most clinically effective monotherapy for long-term smoking abstinence (i.e. > 6 months). 25 However, combination NRT has been shown to be just as effective as varenicline as an aid to quitting smoking. 25
Adverse events
Concerns have been raised about the safety of smoking cessation medicines, particularly with respect to the neuropsychiatric safety of varenicline and the cardiovascular safety of varenicline and NRT. There are emerging concerns about the safety of e-cigarettes. Severe safety warnings about a potential increased risk of serious neuropsychiatric adverse events (AEs) (depression, suicidal ideation and suicidal behaviour) in patients prescribed varenicline have previously been issued by regulatory agencies. 28,29 A black-box warning, the FDA’s most serious safety warning, was placed on varenicline’s product labelling between 2009 and 2016. 30,31 These safety warnings were based on spontaneous reports to the MHRA’s Yellow Card Scheme in the UK and the FDA’s Adverse Events Reporting System in the USA.
Previous research into the neuropsychiatric safety of varenicline has provided inconsistent findings, adding to the debate. 32 In April 2016, the results of the EAGLES trial,33 a randomised controlled trial (RCT), were published. This study randomised 8144 smokers to receive varenicline, transdermal NRT patch, bupropion or placebo. The trial’s findings provided evidence suggesting that neither varenicline nor bupropion were associated with an increase in neuropsychiatric AEs relative to nicotine patch or placebo. Subsequently, the EMA lifted the warning about possible suicidal risks from varenicline in April 2016,34 which was followed by the FDA’s decision to remove the black-box warnings on varenicline’s labelling in December 2016. 35 In terms of neuropsychiatric events, bupropion use has been specifically associated with an elevated risk of seizures. 36 However, a review by Hughes et al. 26 determined that, despite reported events, seizures remained rare, as the average rate was lower than the 1 : 1000 estimated risk reported in the product’s safety information.
Previous systematic reviews comparing varenicline with placebo have reported inconsistent findings regarding varenicline’s cardiovascular safety. 37,38 Mills et al. 39 conducted a network meta-analysis (NMA) to investigate the comparative safety of varenicline, bupropion and NRT for cardiovascular events, including major adverse cardiovascular events (MACEs) such as cardiovascular death, non-fatal myocardial infarction and non-fatal stroke. Although the authors found no clear evidence that varenicline or bupropion use was associated with an elevated risk of any cardiovascular events, NRT use was associated with an increased risk of events. This finding was driven by lower-risk events, typically tachycardia or arrhythmia. However, NRT use was not associated with an increased risk of experiencing a MACE. 39 Similarly, based on the EAGLES trial and a 28-week extension that followed a subset of 4595 participants, Benowitz et al. 40 found inconclusive evidence that varenicline, bupropion or NRT increased the risk of MACEs.
Safety concerns about e-cigarettes include the risks associated with the devices being manufactured to variable standards, the risks of specific flavouring components, potentially harmful constituents found in the vapour and uncertainty about the long-term health impact on e-cigarette users. 41–43 More recently, there have been reports of an outbreak of lung injury associated with e-cigarette use in the USA, with seven confirmed deaths. 44 A similar outbreak has not been observed in the UK. However, in general, there is limited research and a lack of evidence regarding the safety of e-cigarettes compared with licensed smoking cessation medicines. Nonetheless, concerns about safety have led to a wide variety of regulatory decisions regarding the sale and use of e-cigarettes worldwide. Whereas e-cigarettes are widely available for sale as consumer products in the UK, their use is restricted in several countries. 45 Some countries (India, Uruguay, Jordan and Saudi Arabia) have banned the devices; in Thailand, possessing the devices can result in a 10-year prison conviction. 45,46
Reasons for conducting this review
The ongoing debate regarding the safety of drugs for smoking cessation may be a result of the inconsistent research findings in this area. 32 Studies without control groups (those using AE reporting data and case studies)47–49 have reported increased neuropsychiatric risks of varenicline and bupropion, whereas studies with control groups (observational cohort studies, RCTs, and systematic reviews of RCTs) have reported the opposite, and found inconclusive evidence of an increased risk of severe outcomes in patients prescribed these medicines. 50–56 Although large RCTs such as the EAGLES trial (the largest RCT comparing the neuropsychiatric safety of smoking cessation medicines) provide better evidence than non-randomised studies, even its sample size, and thereby its statistical power, was limited relative to that of much larger observational cohort studies. 53,57
There have also been inconsistent findings regarding the cardiovascular safety of these medicines. A 2011 meta-analysis38 of 14 trials reported an increased risk of serious adverse cardiovascular events. However, a larger meta-analysis37 published the following year found no significant increase in serious cardiovascular AEs associated with varenicline use. The largest meta-analysis39 of cardiovascular safety to date found no clear evidence that varenicline or NRT were associated with major adverse cardiovascular events. Bupropion was shown to be protective. However, NRT was associated with an elevated risk of less serious AEs, including tachycardia and palpitations.
To date, studies have focused mainly on comparing the safety of varenicline monotherapy with placebo. 50–52,58 However, making comparisons with other smoking cessation drugs is likely to be of greater relevance to patients, prescribers and regulators. Additionally, in the UK, although e-cigarettes are not licensed medicines, they are also used in smoking cessation, and, given their popularity, it is important to review their safety and effectiveness as smoking cessation aids. 22 Updated cost-effectiveness analyses of these medicines in UK settings will also be conducted to inform the overall risk–benefit evaluation of the different smoking cessation medicines and to determine which treatment represents the best ‘value for money’ to the NHS.
Clinical trials in this area have the following limitations:
-
Relatively few smoking cessation trials compare medicines against each other or in combination, which can be addressed using NMA to estimate the comparative effectiveness and safety of medicines tested against a common comparator (placebo).
-
Safety reporting varies greatly across trials.
The limitations of previous synthesis research in this area are as follows:
-
There have been no comprehensive reviews of the neuropsychiatric safety of the smoking cessation medicines in relation to each other, as existing reviews mainly compare monotherapies with placebo.
-
Previous reviews have failed to comprehensively investigate the safety of smoking cessation medicines in a NMA by not including data from all RCTs irrespective of their duration. As AEs may occur within hours or days of starting treatment,59 the previous NMAs that excluded RCTs of < 6 months58,60 may have failed to capture AEs reported in shorter-duration trials.
-
There is a lack of sufficient data for nodes in the previous neuropsychiatric safety NMAs. 58,60
-
None of the previously published NMAs has examined combined therapies of smoking cessation medicines,25,58,60 not currently licensed for use in the UK, although the effectiveness and safety of combined treatments are increasingly being examined in trials.
-
No recent cost-effectiveness analyses have fully accounted for AEs in order to determine which UK-licensed smoking cessation medicine is estimated to be the most cost-effective in UK settings.
The limitations of previous cost-effectiveness analyses in this area are as follows:
-
No previous cost-effectiveness analysis could be identified that compared the full range of available pharmacological interventions, comparing the standard licenced interventions with combination therapies and e-cigarettes.
-
No previous cost-effectiveness analysis has incorporated safety outcomes.
-
Only one previous study has compared the cost-effectiveness of e-cigarettes with that of NRT61 but, to our knowledge, no previous study has assessed the cost-effectiveness of e-cigarettes compared with all other interventions available in the UK.
There is, therefore, a need for an updated and comprehensive review of the evidence for the safety and effectiveness of licensed smoking cessation medicines and electronic cigarettes to allow patients, prescribers and regulators to make informed decisions about treatment choice and to establish the cost-effectiveness of these treatments in UK settings.
Chapter 2 Research questions
Objectives of the evidence review
Our specific objectives were:
-
to perform a comprehensive systematic review and NMA of the clinical effectiveness and safety of varenicline, bupropion, NRT and e-cigarettes as monotherapies and combination therapies in relation to each other, placebo or usual care
-
to adapt a published economic model to incorporate the disutilities and costs resulting from AEs in order to estimate the cost-effectiveness of monotherapy and combination therapies of smoking cessation medicines and e-cigarettes in the context of the NHS and primary care settings in the UK
-
where sufficient data are available, to explore the following subgroups in the NMA: those with psychiatric illness, those with comorbid conditions, heavy smokers (defined as people who smoke > 20 cigarettes per day), smokeless-tobacco users and smokers not willing to quit.
Chapter 3 Review methods: assessment of clinical effectiveness and safety
Introduction
We conducted systematic reviews with NMAs of:
-
effectiveness of smoking cessation medicines and e-cigarettes using RCTs
-
safety of smoking cessation medicines and e-cigarettes using RCTs and non-randomised (observational) studies.
We undertook these reviews in accordance with the Centre for Reviews and Dissemination (CRD) guidelines for undertaking systematic reviews62 and the Cochrane Handbook for Systematic Reviews of Interventions63 (as updated online during 2011: www.cochrane-handbook.org; accessed September 2019). We prospectively registered the reviews in the PROSPERO (international prospective register of systematic reviews) database (www.crd.york.ac.uk/prospero; accessed September 2019), with registration number CRD42016041302. A protocol of the review has also been published as a journal article. 64
Eligibility criteria
Study designs
For the review of studies reporting effectiveness, we included RCTs with duration of ≥ 6 months (≥ 22 weeks) in any setting, including, but not limited to, primary care practices, hospitals, including inpatient and outpatient clinics, universities, workplace clinics, nursing or residential homes. Trials with two or more study arms were included in the effectiveness analyses, whereas crossover trials, non-randomised trials, quasi-randomised trials, large factorial studies and interrupted time series analyses were excluded.
For the review of studies examining safety, RCTs of any duration were included in addition to non-randomised (observational) studies with control groups. Uncontrolled observational studies (e.g. case reports and case series) were excluded, as were large factorial studies.
Participants
In both reviews, we included smokers aged ≥ 18 years of all ethnicities using UK-licensed smoking cessation therapies and/or electronic cigarettes. This included adult smokers accessing local authority stop-smoking services. We also included smokeless-tobacco users irrespective of whether or not they smoked. We excluded studies with participants aged < 18 years, as varenicline, bupropion and electronic cigarettes are licensed for use only in adults in the UK. Non-smoking populations were excluded, as were pregnant and breastfeeding women, as varenicline and bupropion are not licensed for use in these groups in the UK.
Interventions and comparators
Three smoking cessation medicines were the focus of all reviews, varenicline, bupropion and nicotine replacement therapy (NRT), as monotherapies as well as in combination treatments (e.g. varenicline combined with NRT, varenicline combined with bupropion and bupropion combined with NRT). We also assessed e-cigarette monotherapies as e-cigarettes are used in smoking cessation, although they are not licensed medicines in the UK. For NRT, combinations of different formulations given concurrently, for example patch and gum, were also included. Different dosages of treatments were also examined, classified into low, standard and high, as described below. The dose categories for active interventions were determined using the British National Formulary (BNF)65 and the MHRA public assessment report for the ‘e-Voke’66 (Table 1).
| Treatment (formulation) | Low dose | Standard dose | High dose |
|---|---|---|---|
| Bupropion (oral extended-release tablets) | < 150 mg b.i.d. | 150 mg b.i.d. | > 150 mg b.i.d. |
| Varenicline (tablets) | < 1 mg b.i.d. | 1 mg b.i.d. | > 1 mg b.i.d. |
| E-cigarette (electronic inhaler, five cartridges/day) | 10 mg | 15 mg | |
| NRT | |||
| NRT patch (16 hours) | < 15 mg | 15 mg | > 15 mg |
| NRT patch (24 hours) | < 14 mg | 14 mg | > 14 mg |
| NRT gum (15/day) | 2 mg | 4 mg | |
| NRT nasal spray (2 sprays/hour, 64/day) | 0.5 mg | ||
| NRT mouth spray (4 sprays/hour, 64/day) | 1 mg | ||
| NRT lozenge (1 lozenge/1–2 hours, 15/day) | < 2 mg | 2 mg | 4 mg |
| NRT sublingual tablet (2 mg/tablet, 40/day) | 1/hour | 2/hour | |
| NRT inhalator | 10 mg (12/day) | 15 mg (6/day) |
We also identified two additional NRT treatments: NRT combination, whereby two or more NRT products were administered in combination in a single arm, and NRT choice, whereby participants were given a choice of NRT products they could select to use. The dosage for NRT combination was indicated based on the highest dose among assigned products, while the dosage for NRT choice was indicated only when dosages for every offered product were reported.
We excluded trial arms of interventions in which patients could receive more than one intervention but where these were undefined (i.e. ‘mixed’ rather than ‘combination’ interventions). We also excluded alternative and complementary therapies (e.g. hypnotherapy, acupuncture, aromatherapy and herbal therapies).
As the reviews were conducted to inform NMAs, we determined the comparator interventions to ensure that they would provide information on the relative effectiveness/safety of the interventions of interest. Comparators were chosen based on the possibility of informing indirect evidence on the relative effectiveness of the interventions; and on the ‘distance’ of these comparators from our interventions of interest in the network, which relates to the likely increase in precision in the estimates of relative effectiveness and safety. We defined the following comparators:
-
placebo (reference comparator for the NMAs)
-
no drug treatment (including brief advice)
-
usual care
-
waitlist.
Where psychotherapies were included in each arm of a study (e.g. studies of a pharmacological treatment plus psychotherapy vs. psychotherapy alone), these studies were included and were analysed as pharmacological treatment compared with no drug treatment, under the assumption of no interaction between pharmacological and psychotherapies when given together. Where psychotherapies were given as an adjunct to pharmacological treatments, but not in all arms of the study (e.g. studies of pharmacological treatment plus psychotherapy vs. usual care), these studies were included in the base case analysed as pharmacological treatment compared with no drug treatment, and the impact of the addition of psychotherapy was estimated using meta-regression. We assessed the sensitivity of our findings to excluding such studies in a sensitivity analysis. Although the efficacy of psychotherapies was not the focus of this review, studies in which psychotherapy was used as a comparator (e.g. studies of pharmacological treatment vs. psychotherapy) could potentially provide useful indirect evidence for estimates between pharmacological therapies. However, only four trials were identified in which this could have been possible and the psychotherapies used were very different across these studies, making such a comparison unreliable. We, therefore, did not include psychotherapy as a comparator in the NMA.
Outcomes of interest
Effectiveness
We only included bioverified events in our main analyses reported at ≥ 6 months’ follow-up. We used Cochrane definitions for all outcomes. 68 Our primary effectiveness outcome was continuous (or sustained) abstinence defined as avoidance of all tobacco use since the quit day until the time the assessment was made, occasionally allowing for lapses where specified. Secondary effectiveness outcomes included:
-
Prolonged abstinence – a measure of cessation that typically allows a ‘grace period’ following the quit date (usually of about 2 weeks) to allow for slips/lapses during the first few days when the effect of treatment may still be emerging.
-
Any abstinence – an outcome where we included abstinence by any definition reported at 6 months. Where studies reported more than one cessation outcome, we preferred continuous/sustained abstinence, followed by prolonged abstinence, 30-day point prevalence abstinence (PPA), 7-day PPA and any other abstinence.
-
7-day PPA – a measure of cessation based on behaviour over a 7-day period.
Safety
The primary composite safety outcome was serious adverse events (SAEs), defined as events that resulted in death, were life-threatening, required hospitalisation or resulted in significant disability. 69 We also recorded hospitalisation, treatment discontinuation and withdrawal from study as a result of AEs.
Furthermore, we sought data on the following outcome categories.
-
Cardiovascular outcomes:
-
Secondary composite outcome – MACEs, including cardiovascular death, non-fatal myocardial infarction (excluding unstable angina), and fatal and non-fatal stroke. 70
-
Tertiary outcomes – arrhythmias, congestive heart failure, unstable angina, palpitations, thromboembolism (deep-vein thrombosis or pulmonary embolism), and transient ischaemic attack.
-
-
Neuropsychiatric outcomes:
-
Secondary composite outcome – major adverse neuropsychiatric events (MANEs), comprising suicide, attempted suicide, suicidal ideation, depression and seizures. 51
-
Tertiary outcomes – abnormal dreams, aggression, anxiety, insomnia, irritability, sleep disorders and somnolence.
-
-
Other outcomes: chronic obstructive pulmonary disease (COPD), dry mouth, fatigue, headache, nausea, pruritus, skin rash and all-cause death.
For the systematic review of RCTs, primary and secondary composite outcomes and the most frequent other outcomes were addressed in NMAs, whereas the remaining outcomes were reported in tables. Conversely, the systematic review of non-randomised studies retrieved a much smaller number of interventions; therefore, we decided to combine safety outcomes from randomised and non-randomised evidence in a sensitivity analysis. Outcomes reported in observational studies were presented in tables.
Identification of evidence
Search strategy
We searched the following databases: MEDLINE, EMBASE™ (Elsevier, Amsterdam, the Netherlands), PsycInfo® (American Psychological Association, Washington, DC, USA), Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA), ClinicalTrials.gov and the Cochrane databases including the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects (DARE; updated until March 2015), the Cochrane Central Register of Controlled Trials (CENTRAL), the NHS Economic Evaluation Database (NHS EED) and the Health Technology Assessment (HTA) database. Searches were conducted with the help of information specialists and did not include any language restrictions. Non-English-language articles were reviewed by native speakers before a full translation was obtained. We also manually searched the reference lists of relevant research articles and previous reviews and communicated with authors to identify unpublished information.
To identify studies for the effectiveness NMAs, the search strategies from recent Cochrane reviews24,26,71,72 were used to create an updated strategy to identify more recent trials for inclusion in the current study in addition to trials identified by past reviews. To identify studies for the safety NMAs, we built on the basic search strategy included in the cardiovascular NMA by Mills et al. 39 Searches for non-randomised studies were not date-limited. We completed our original searches on 16 March 2017 and our update searches were completed on 19 February 2019. For the search terms we used for our MEDLINE searches, see Appendix 1.
Assessing relevance and inclusion
Search results were uploaded to Covidence,73 which we used to screen abstracts and full texts and to resolve disagreements. Three reviewers independently screened abstracts to determine whether or not full-text reports should be obtained. The same reviewers independently identified eligible full-text reports for inclusion. Each record was screened by at least two reviewers at each stage. Discrepancies were resolved by reaching consensus among reviewers.
Data extraction
Data for included studies were extracted by one reviewer and checked by co-reviewers. Information was collected on study design (duration of treatment, description of allocation concealment and blinding), study participants (country, region and population studied), baseline characteristics (e.g. ethnicity, sex and smoking history), intervention and comparison groups (including the smoking cessation intervention, whether or not there was cotreatment, dosage and formulation), our predefined primary and secondary outcomes of interest including measures of effectiveness and safety outcomes, losses to follow-up and study sponsor. In the event of missing data, we contacted authors by e-mail to ask for original data. Authors of all identified studies with randomised controlled designs were contacted to verify the accuracy of the extracted data and/or to provide safety data.
Assessment of risk of bias in included trials
For all studies, the Cochrane tool for assessing the risk of bias74 was used to determine whether there was a high, low or unclear risk of bias in the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. An overall risk of bias was also determined by selecting the highest rating of bias across domains, with the exception of selective outcome reporting. Reviewers independently assessed the risk of bias in each of the trials. Discrepancies were resolved by referring to the original publication and reaching consensus among reviewers. To aid with the risk-of-bias assessment, study authors of RCTs were contacted to obtain study protocols and additional information that may not have been published.
Selection of data for analysis
Intervention definitions
To perform NMAs, we had to allocate each intervention group in each trial to a category, with each intervention category forming a ‘node’ in the network. We defined intervention nodes according to the type and intensity of treatment and/or NRT received.
Quantitative synthesis (including network meta-analysis)
We performed a NMA for each outcome. NMA is a methodology that enables the quantitative integration of a collection of primary studies by pooling evidence from all intervention comparisons considered in those primary studies. Results for each pairwise comparison combine both the direct evidence, based on the head-to-head intervention comparisons made in primary studies, and the indirect evidence, which refers to the intervention comparisons inferred from the network via comparator interventions. 75,76 NMA thus enables an estimation of relative intervention effect estimates for every pair of interventions, regardless of whether or not they have been compared directly in a RCT. It also enables the inference of the ranking of treatments for a given outcome.
To be included in the analyses, studies were required to report the proportion of events for each arm (or enough information to calculate it manually), and to report at least one event in one of the arms for the outcome analysed. We considered three different NMA models:
-
Full interaction model – this is the standard NMA model in which each different combination of drug type, drug intensity, NRT type and NRT intensity is considered as a separate intervention.
-
Fixed-class model – this model assumes that the interventions can be grouped into classes, with treatment effects in the same class assumed to be identical. 77 We defined classes according to type of treatment and delivery.
-
Random-class model – this model also groups treatments into classes, but treatment effects in the same class are now assumed to be centred around a class mean effect with between-treatment variability within class. 77 This model yields both treatment and class effects. We defined classes according to type of treatment and delivery.
We chose between different models using the posterior mean deviance as an indicator of model fit and the deviance information criterion (DIC) as a measure of parsimony (with a preference for lower values of each). We created network plots to provide visual images of the data structure in each analysed outcome. The node sizes of the network plots are proportional to the number of patients randomised to each intervention, whereas the thickness of the edges (lines) is proportional to the number of patients contributing to that comparison. Therefore, the edges in the network plots connect interventions for which direct evidence was available. We plotted the networks to illustrate the data structure for each analysed outcome. Interventions not included in the analysis have their names written between square brackets. Interventions were excluded if they were disconnected from the main network or if they caused convergence problems in the statistical estimation (typically owing to small numbers, with zero events in one or more arms of a study). Our NMAs treat data as binomial, modelling the number of events out of the total number of participants (number randomised) using a logistic model. 77 For each outcome, we defined the denominator for each group using the intention-to-treat principle (i.e. the number of randomised patients as the denominator of the formula, irrespective of attrition). Where outcome data were presented for multiple time points, we took the longest period of follow-up. For some effectiveness outcomes (sustained and prolonged abstinence), we included only observations with a minimum follow-up period of 24 weeks.
We excluded studies that had insufficient information about the numbers of events per arm (i.e. the number of events was not reported and it was not possible to calculate this using the available information in the paper) and we also excluded studies with no events in any arm from the analyses. Where there were events in at least one arm of a trial but no events in one or more other arms, we added 0.5 events to all cells in the 2 × 2 table for that trial. 78
Owing to the anticipation of heterogeneity,79 we took a random-effects approach to the meta-analyses, assuming a common heterogeneity variance across all comparisons. 75 We conducted the statistical analyses within a Bayesian framework using OpenBUGS (version 3.2.3; Andrew Thomas, OpenBUGS Foundation; Cambridge), simulating two Markov chains with 30,000 iterations for each chain (plus 15,000 burning iterations). We monitored the treatment effects, between-study and between-treatment (within-class) standard deviation (SD). Furthermore, we examined ranking of classes of monotherapies or combinations of therapies by estimating the probability that each intervention is best, second best, and so on, across safety and effectiveness outcomes. We included only standard doses, except for e-cigarettes.
We assessed convergence of the Markov chains by using the potential scale reduction factor80 and examining the history and autocorrelation plots for each estimated parameter. We appraised goodness of fit by calculating the posterior mean residual deviance, whereby smaller values indicate better-fitting models and values close to the number of unconstrained parameters indicate a well-fitting model. Comparisons of models were made using the DIC. 81 The DIC penalises the posterior mean residual deviance (a measure of model fit) by the effective number of parameters in the model (as measure of complexity) and can, therefore, be viewed as a trade-off between the fit and complexity of the model. Smaller values are preferred, with differences of three or more considered meaningful. When two models fit the data similarly, we interpreted results from the most parsimonious model.
The validity of NMA depends on the assumption that there is no effect modification of the pairwise intervention effects or that the prevalence of effect modifiers is similar in the different studies. This key assumption has been referred to variously as exchangeability, transitivity, similarity and consistency. 80,82–84 We examined the tenability of the consistency assumption for different networks by comparing the posterior mean residual deviance, DIC, and between-study SD for the NMA model that assumes consistency with an inconsistency model that relaxes this assumption (an unrelated mean effects model). 85 When both direct and NMA effect estimates were available and differed (up to the second decimal place of the standard error), we used both to back-calculate the indirect estimates, while making the assumption that the NMA estimates (from the consistency model) are the result of a weighted average of normally distributed direct estimates (from the unrelated mean effects model) and the indirect estimates. A local measure of inconsistency for a specific comparison can be obtained by comparing the direct and indirect estimates for that comparison. Note that for many comparisons there was either only direct evidence or only indirect evidence, so that the NMA estimates correspond to one of these.
Further analyses
We performed meta-regression86 to explore the influence of several covariates as potential effect modifiers for the primary effectiveness and safety outcomes, namely:
-
Dependence – we combined average scores of several scales at the arm level with a hierarchy of preferred measurements. Specifically, we used scores on the Fagerström Test for Nicotine Dependence87 in preference to the Fagerström Tolerance Questionnaire,88 with Heaviness of Smoking Index89 as a third alternative. The average scores from each arm were standardised to make the numbers comparable across different scales.
-
Funding source – industry compared with non-industry sponsorship.
-
Counselling – interventions that included pharmacological treatment plus counselling compared with pharmacological treatment alone.
-
Type of placebo – for placebo arms, we examined the influence of including a drug placebo (alone or combined with NRT placebo) in comparison with NRT-only placebo arms.
-
Duration of treatment in each arm (in weeks).
-
Studies including samples in which all (or most) participants had one or more current psychiatric condition (e.g. depression, schizophrenia, bipolar disorder or substance misuse), compared with other studies.
-
Studies including samples in which all (or most) participants had one or more of the 17 comorbidities specified by the Charlson Comorbidity Index,90 compared with other studies.
-
Studies in which patients were not required to make a quit attempt, compared with other studies.
-
Studies focused on smokeless-tobacco consumers, compared with other studies.
-
Studies in which patients were heavy smokers, compared with other studies. We defined samples of heavy smokers as those in which the average of smoked cigarettes was > 20 per day.
-
Publication year.
Furthermore, we performed sensitivity analyses excluding different subsets of studies assessed as being at high risk of bias on any domain, and also a sensitivity analysis excluding studies that compared a pharmacological intervention plus counselling with control (where counselling was not given in each arm). Threshold analysis, a form of sensitivity analysis, was also performed to determine the robustness of our treatment recommendations for the primary effectiveness and safety outcomes to changes in the evidence provided by the individual studies. 91–93 Threshold analysis determines how much the evidence could change for any reason, such as bias or random error, before the treatment recommendation changes, and describes this using a set of thresholds. These thresholds can be compared with judgements of the plausible magnitude of potential biases and with estimates of uncertainty [e.g. confidence intervals (CIs)]. In this manner, we may have more confidence in conclusions that are shown to be robust, and can appropriately acknowledge where conclusions are shown to be sensitive to plausible biases or uncertainty in the evidence.
Chapter 4 Economic evaluation methods: assessment of cost-effectiveness
Introduction
The economic evaluation aimed to compare the cost-effectiveness of pharmacological treatments to aid smoking cessation, including NRT and e-cigarettes. The population considered in the decision was smokers in the UK aged ≥ 18 years who were motivated to quit smoking. The treatments compared were those included in the NMA on sustained abstinence (see Chapter 5). The NMA showed that it was important to distinguish between doses of the treatments (see Table 1), but that the mode of administration of NRT was not an effect modifier (based on model fit; see Appendix 5). We did not include treatments when the dose was not specified. The following treatments were included in the economic evaluation:
-
NRT at low, standard and high dose
-
bupropion at low and standard dose
-
varenicline at low and standard dose
-
e-cigarette at low and high dose
-
bupropion standard dose plus NRT high dose
-
varenicline low dose plus NRT standard dose
-
varenicline standard dose plus NRT standard dose
-
varenicline standard dose plus NRT high dose
-
varenicline standard plus bupropion standard dose.
Combination treatments (varenicline or bupropion in combination with NRT at any dose) are not currently licensed as smoking cessation treatments in the UK. We include these in the base case but exclude them from a sensitivity analysis. Standard practice in the NHS is to offer smokers attempting to quit NRT a dose based on their level of cigarette use, including combinations of NRT modes of delivery (e.g. patch and gum). We use NRT standard dose as the reference treatment for comparison in the cost-effectiveness analysis. We do not include waitlist, no treatment, or placebo in the economic evaluation because these would not be used in standard care. Evidence from studies including those comparators is included in the NMA and contributes indirectly to the estimates between the active treatments that we include in our economic evaluation.
The perspective taken is that of the NHS for costs, and health effects on the individual for outcomes, in line with NICE guidance. 94 A lifetime time horizon was taken, using a cohort simulation model to predict costs and utilities over a participant’s lifetime.
Methods
Model description
The model structure is based on the ‘Sheffield model’ used in a recent Health Technology Assessment report58 on the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation. This in turn was based on the Benefits of Smoking Cessation on Outcomes (BENESCO) model, which was adapted from the Health Economic Consequences of Smoking (HECOS) model used by the World Health Organization European Partnership Project to reduce tobacco dependence. 95 The BENESCO model is an existing and widely used economic model that has previously been applied to model the effects of smoking cessation interventions in the UK, the USA, Germany, France, Belgium, the Netherlands, Finland, Sweden and the Republic of Korea. 96–105
A cohort simulation model is used for smokers making a one-time smoking cessation attempt. Smoking status, morbidity and mortality are simulated over a lifetime (until the age of 100 years) to calculate the costs and benefits of smoking cessation strategies from the perspective of the health-care payer. The model uses an annual cycle length. UK estimates are used to determine the percentage of the initial cohort that are male or female, their age (18–34, 35–64 or 65–100 years) and their underlying health conditions [lung cancer, COPD, coronary heart disease (CHD) and stroke].
Every cohort member begins in the smoker state and at the end of the first year a percentage of the cohort will have quit smoking, with this proportion dependent on the efficacy of the cessation aid treatment they receive. No further quit attempts are modelled. It is, therefore, assumed that those who fail to quit will remain smokers until death.
There is a possibility that quitters may relapse and start smoking again in future years. This possibility decreases as time since cessation increases, with the risk of relapse being highest in the four model cycles following cessation (recent quitters). After four cycles without relapse, recent quitters become long-run quitters and the annual relapse rate is lower in the next five cycles, and lower still in subsequent cycles, with this underlying relapse rate continuing for the duration of the model. It is assumed that the probability of relapse at any stage is the same regardless of which treatment is used to aid cessation.
At the end of each year, the cohort is distributed into smokers, quitters and relapsed smokers (Figure 1). Within these broad smoking states, cohort members can have no current morbidity or one of the following smoking-related morbidities: lung cancer, COPD, CHD, stroke or an asthma exacerbation. These health states correspond to the smoking-related diseases that cause the greatest morbidity, mortality and cost. It is assumed that a person can be in one of these health states at a time only. When a person dies, they are removed from the model. The probability of moving to a new health state at the end of each cycle depends on current health state, smoking status, age and sex.
FIGURE 1.
Transitions between smoking states. Note that death can occur from any state.

It is assumed that a restricted hierarchy exists in which subjects can enter the CHD or stroke health state and subsequently transit to the COPD or lung cancer state. However, because of the irreversible nature of COPD and lung cancer, once subjects enter these health states, they stay there until they transition to death. An asthma exacerbation or exacerbations can occur from the no current morbidity health state only and are assumed to resolve within 1 year.
The original BENESCO model did not consider the AEs of treatment, but we have incorporated these as a probability of experiencing depression or fatal/non-fatal self-harm in the first year of treatment. We included these aspects of MANEs because we were able to identify cohort data sources for these outcomes with which to estimate the baseline probability of event on NRT standard. Depression and non-fatal self-harm are represented by a one-off disutility and cost, whereas fatal self-harm results in death. These are applied in the first year only because it is assumed that depression or self-harm would lead to discontinuation of treatment. We did not include cardiovascular treatment-related AEs in our model. This is because cardiovascular events are already included in the model as a consequence of smoking. Any differences observed between treatment groups in the RCTs in terms of cardiovascular events are more likely to be a result of having successfully quit or not (and subsequent reduction in risk), which is already captured in the model, or a side effect of the treatment. It is not possible to distinguish between these two causes from the RCT evidence, and we, therefore, could not estimate treatment-related adverse cardiovascular events.
All other health states are associated with utility and cost values, as detailed later. Therefore, cohort members accumulate costs and health outcomes each cycle until death. Future costs and benefits were discounted at a rate of 3.5% per annum. 94
Model inputs
In this section, we describe the evidence sources used for each parameter in the model. Evidence sources were identified as follows. First, we looked for updates to the evidence sources used in the Sheffield model58 by searching for studies that cited these sources and carrying out targeted searches on PubMed and EMBASE. Where alternative or more recent evidence sources were available, we considered these and made a decision about which evidence sources to use based on sample size and relevance to a contemporary UK population.
When searching for data on prevalence, we concentrated on large routine data sources such as the Health Survey for England. For incidence and relative risks (RRs), we searched for prospective cohorts (if RCT evidence was not available/not possible). Where no preferable evidence sources could be identified, we used the same data as used in the Sheffield model.
The assumed characteristics of the initial cohort
The distribution of the cohort across sex and age categories at the start of the model was designed to reflect the distribution of smokers in the UK. The proportion of male and female adults, and the mortality risk in each of the three age categories was determined from general population data. 106,107 Smoking prevalence data108 were applied to these data to calculate the distribution across age and sex groups for a representative sample of 10,000 UK smokers (see Appendix 2, Table 18).
The prevalence of smoking-related diseases in the smoking cohort was estimated from various literature sources on the prevalence of each disease in the general UK population and risk ratios of these diseases in smokers (see Table 19). The most recent data source identified on the prevalence of COPD and asthma in the UK was an online report from the British Lung Foundation109 based on data from 12.6 million patients in The Health Improvement Network (THIN) database, a UK general practice database that contains anonymised longitudinal patient records from over 500 practices (about 6% of the population). It reports the number of people ever diagnosed with COPD and asthma per 100,000 people, by age group, in 2012. This is updated from the estimate used in the Sheffield model, which was taken from a 2000 paper by Soriano et al. ,110 who used the General Practice Research Database to calculate the prevalence of COPD in the UK from January 1990 to December 1997. The British Lung Foundation reports an increased prevalence of COPD compared with Soriano et al. (e.g. new estimate of 7% in females aged > 65 years as opposed to 2%). This may be because of a difference in the number of elderly people included in the studies, although the British Lung Foundation estimate was thought to be a more representative estimate of COPD in the current population of those aged ≥ 65 years. 109,110
The prevalence of lung cancer was taken from a paper by Maddams et al. ,111 who used data from cancer registries in the UK to provide prevalence estimates by sex and age for 2008. This was updated from a 2003 paper by Forman et al. ,112 who used UK cancer registries to provide estimates for 1992. The estimates were relatively similar (e.g. new estimate of 0.3% in females aged ≥ 65 years compared with 0.24%).
History of CHD prevalence was taken from the 2016 Health Survey for England,113 which is one in a series of annual surveys designed to measure health and health-related behaviours in adults and children living in private households in England. In 2016, interviews were completed with 8011 adults. This was updated from an estimate taken from the 2005 Office for National Statistics (ONS) General Household Survey and was, again, higher (12% in females aged ≥ 65 years compared with 5.9%).
The source for prevalence of stroke history was Bhatnagar et al. ,114 who obtained 2013 prevalence data from the Clinical Practice Research Datalink Global Initiative for Chronic Obstructive Lung Disease (GOLD) database, which collates records from a widely used general practice software system and covers approximately 8.8% of the UK population. This was updated from a 2004 report from Asthma UK and was, again, higher (11% in females aged ≥ 65 years compared with 5.3%).
Relative risks for the prevalence of each disease in smokers relative to never-smokers were taken from the Statistics on Smoking, England – 2017115 report for COPD, lung cancer, CHD and stroke (see Table 37), and from Cassino et al. 116 for asthma. These estimates were used to calculate the expected number of cases in the cohort of smokers using the formulae shown in Appendix 3. The data are reproduced in Appendix 2, Table 20.
Transition probabilities
The annual incidence of disease was estimated by age and sex categories for smokers, recent quitters and long-run quitters. These values relied on estimates used in the Sheffield model58 (which, in turn, used estimates from a previous manufacturer’s single technology assessment submission to NICE117) as no preferable evidence could be identified for COPD, CHD, stroke or asthma. For lung cancer, the 2016 ONS release (updating the 2005 release used in the Sheffield model) was identified,118 which reports directly age-standardised rates per 100,000 population of newly diagnosed cases of cancer in England. These estimates showed that the incidence of lung cancer did not greatly increase between 2003 and 2016 (see Table 21). For simplicity, we, therefore, assumed that all incidence estimates were the same as those reported in the Sheffield model.
Appendix 2, Tables 22–25 show the estimates of the annual incidences of diseases for the general population, smokers, recent quitters and long-run quitters, respectively. These were obtained using the same method as for prevalence (see Appendix 3), assuming that the RRs of incidence in smokers, short-run and recent quitters relative to never-smokers were the same as the RRs of prevalence (see Table 37). 115,116
In accordance with previous BENESCO models, the RRs in recent quitters relative to never-smokers were assumed to be equal to the RRs for current smokers at year 1 for each disease. The RRs for COPD, stroke and asthma exacerbations are reduced when the smoker has quit for at least 1 year. The RRs in long-term quitters compared with never-smokers are assumed to be equal to the RRs in never-smokers after the smoker has quit smoking for > 5 years. Lung cancer has been approached differently: lung cancer risk for long-term quitters is kept equal to the risk in recent quitters. Although there is evidence that quitting smoking does reduce the risk of developing lung cancer, the risk does not return to that of non-smokers. 119
Mortality
Annual mortality probability by condition, excluding asthma, was estimated using the British Heart Foundation’s published total numbers of deaths in the UK in 2016 in each age group,120 which are based on general population data. These numbers were used as the numerator, with the denominator as the number of prevalent cases in the UK calculated using the population and prevalence estimates for 2016 (see Tables 18 and 19).
It was assumed that no additional mortality was associated with asthma exacerbation. Mortality for chronic diseases, COPD and lung cancer is the probability of death from these diseases given the disease is present. Mortality from acute events, CHD and stroke is the probability of a fatal event that differs by smoking status, age and sex.
Appendix 2, Tables 26–29 show the disease-specific mortality estimates for the general population, smokers, recent quitters and long-run quitters. The same RRs for smokers, short-run and recent quitters relative to never-smokers that were used for prevalence and incidence of diseases were also used to generate absolute probabilities of mortality. The probability of smoking-related mortality is equivalent or lower for recent quitters compared with smokers, and for long-run quitters relative to recent quitters. The exception is lung cancer, for which the mortality risk is the same regardless of smoking status.
Relapse rates
Hawkins et al. 121 used British Household Panel Survey data to look at smokers who quit, but then relapsed. These data were used to calculate the annual relapse probability for short-run quitters (people for whom it had been < 5 years since they quit) and long-run quitters (people who had quit smoking for > 5 years but < 10 years). The annual relapse probability ≥ 10 years post cessation was based on a study by Krall et al. ,122 which followed 483 men for up to 35 years.
The probabilities of relapse that were used in the model are shown in Appendix 2, Table 30. Uncertainty around relapse rates is modelled as a beta distribution using event data from the original studies.
Costs
Costs included in the model related to health states and intervention costs. Owing to a lack of recent or relevant UK data, the mean costs of COPD and lung cancer estimated by the Irish Health Information and Quality Authority (HIQA) in its 2017 report on interventions for smoking cessation123 were used in the model. This report used Irish data on the total annual spending divided by the total number of people with a diagnosis of each disease. The total direct costs to the Irish health service of inpatient and day-case treatment were estimated from the Hospital Inpatient Enquiry database for 2015, which is based on 2014 prices. The annual primary care and medication costs of COPD were estimated using 2014 Primary Care Reimbursement Service data from Ireland on the total costs of adrenergic and other drugs for obstructive airway diseases. The primary care and medication costs of lung cancer were estimated from a report on European cardiovascular disease statistics published by the European Society of Cardiology in 2012. 124 These costs were converted from euros to Great British pounds and inflated to 2019 prices using HM Revenue & Customs monthly exchange rates for February 2019. 125
This resulted in a total annual cost of COPD of £1468, an increase from the £971 used in the Sheffield model based on a paper by Britton. 126 The annual cost of lung cancer (£5429) is lower than the estimate used in the Sheffield model (£6524), which was based on Flack et al. 127
The cost of CHD was estimated from the British Heart Foundation’s cardiovascular disease statistics reported in 2014. 128 These data are taken from analysis of commissioning expenditure in the UK (the programme budgeting data return). These estimates are based on the price paid for specific activities and services purchased from health-care providers for each region. The annual cost of CHD estimated (£1460) is higher than the £1163 used in the Sheffield model based on McMurray et al. 129
The source used for the cost of stroke was Xu et al. ,130 who developed an individual patient simulation model to estimate health and social care costs at 1 and 5 years after stroke. The results were estimated using data on all patients with stroke included in Sentinel Stroke National Audit Programme (the national stroke register of England, Wales and Northern Ireland) from April 2015 to March 2016 (n = 84,184). The annual cost of stroke estimated (£1460) is lower than the £5484 used in the Sheffield model based on Simpson et al. ,131 who calculated the cost of a dependent/independent state due to stroke using NHS reference costs for ‘non-transient stroke or cerebrovascular accident, nervous system infections of encephalopathy’ long-stay/short-stay non-elective inpatients.
Tan et al. 132 documented asthma costs over time for asthma patients who were enrolled in an asthma care programme in Singapore, using a 10-year longitudinal data set. The study population comprised different cohorts of 939 asthma patients entering the programme at different times during 2004–13. Ten-year average annual asthma costs were estimated as £341 per patient. The main drivers of costs were asthma medications and consultation fees. This is lower than the £1162 used in the Sheffield model based on Hoskins et al. ,133 which was a retrospective cohort analysis of a representative data set of 12,203 patients with asthma in the UK over a 1-year period. The estimate from Tan et al. was preferred as it was thought to be more reflective of mild asthma, which is what the majority of adults will have.
The cost associated with depression (£340) was taken from a paper by Hunter et al. 134 Here, a weighted average annual cost per UK patient to treat depression was calculated by multiplying the proportion of patients who access each type of treatment by the average annual cost of the treatment. The cost associated with self-harm was taken from a paper by Tsiachristas et al. ,135 who estimated hospital resource use and care costs for all patients presenting with self-harm to the John Radcliffe Hospital (Oxford, UK) between 1 April 2013 and 31 March 2014.
Uncertainty around cost estimates was incorporated into the probabilistic analysis. In the absence of data, the SDs for COPD and lung cancer were assumed to be 10% of the mean estimate, and the standard errors were calculated using this figure, along with the number of people on which the mean estimate was based. As it was not possible to identify the number of people on whom the depression and CHD cost estimates were based, the standard error in this case was assumed to be 10% of the mean. These data were assumed to follow a gamma distribution. 136 All costs have been inflated to 2019 prices using HM Revenue & Customs monthly exchange rates for February 2019. 125
Appendix 2, Table 31 details the source, summary estimates and distributions used for the health state costs employed in the model.
Intervention costs comprised the cost of the interventions alone. It was assumed that, although counselling and other health professional support are likely to occur, the cost of these is likely to be the same or very similar across interventions, thus not having an impact on the relative cost–utility. These costs were, therefore, excluded from the economic analysis.
Data from the BNF on dosage and pricing are used to calculate the costs of varenicline, bupropion and NRT. 137 For varenicline, the cost of treatment is the cost of a starter pack covering the first 2 weeks of tapered treatment (£27.30) plus the cost of 10 weeks at full dose (5 × £27.30), giving £163.80 in total. The cost of low-dose varenicline is assumed to be the same, as the BNF states the same price for both 1 mg and 0.5 mg (500 µg) tablets.
Bupropion was costed as 150 mg daily for 6 days and then 150 mg twice daily for 7–9 weeks at a cost of £83.52. 137 The cost of low-dose bupropion is assumed to be £62.54 based on a dose of one tablet per day for an average of 13 weeks.
Similarly, for NRT, standard treatment is assumed to be a high-strength patch daily for 6–8 weeks, followed by the medium-strength patch for 2 weeks and then the low-strength patch for the final 2 weeks, at a cost of £105.65. 137 The cost of NRT low is assumed to be £83.84, based on 4 weeks of 10-mg/16-hour patches and 4 weeks of 5-mg/16-hour patches. The cost of NRT high is estimated as £77.46, based on a 4-week supply of 21 mg NicoDerm CQ (GlaxoSmithKline plc, Brentford, UK) transdermal patches followed by 2 weeks at 14 mg and 2 weeks at 7 mg.
E-cigarettes are not medically licensed in the UK. 138 The HIQA report123 costed a 12-week supply of e-cigarettes (e-cigarette + 3.55 ml liquid per day, including a replacement atomiser in months 2 and 3) as €93.80, based on Liber et al. 139 This is equivalent to approximately £82. 125
The costs of all interventions including combinations of interventions are shown in Appendix 2, Table 32.
Utilities associated with health states
Baseline utility for smokers with no current comorbidity was taken from the general population utility profile estimated by Ara and Brazier140 using 2003 and 2006 Health Survey for England data (see Table 33). These data are a function of age and sex and are based on random samples of the population living in private households in England. A total of 26,679 participants were asked to complete the EuroQol-5 Dimensions (EQ-5D) questionnaire (a commonly used questionnaire to describe and value health), and preference-based health state utility values were estimated from the weights obtained using time trade-off valuations. Health state utility was determined by multiplying baseline utility by age by an estimate of the impact of the disease.
Disease-specific utility values for smoking-related diseases were estimated from the literature. For lung cancer utility, two sources were identified. Jang et al. 141 measured EQ-5D scores in 172 consecutive outpatients with non-small-cell lung cancer attending a major Canadian cancer centre outpatient clinic and estimated a mean utility of 0.76 (95% CI –0.7 to 0.78). A more recent paper by Bertranou et al. 142 derived a similar utility value for progressed non-small-cell lung cancer from EQ-5D patient-level data collected in two lung cancer treatment trials, AURA2 (n = 199) and IMPRESS (n = 265) (0.72, SD 0.029). The progressed disease utility was given by the mid-point of the two studies. The source used in our model is that estimated by Bertranou et al. 142 (0.72) owing to the larger sample size. This is higher than the estimate used in the Sheffield model (0.5), which was taken from Trippoli et al. ,143 who measured quality of life in 95 patients with non-small-cell lung cancer from 15 Italian hospitals.
For utility associated with COPD, the source was Pickard et al. ,144 who synthesised the literature on the validity and reliability of EQ-5D use in studies of asthma and COPD, and estimated EQ-5D utility scores associated with stage of disease. The authors found eight studies that recorded EQ-5D scores ranging from 0.52 (SD 0.16) to 0.84 (SD 0.15) for patients with COPD. Sufficient studies in COPD were available to calculate pooled mean utility scores according to GOLD stage, which categorises COPD severity in four stages, from very mild to very severe. The utilities estimated were 0.74 for stage I (95% CI 0.62 to 0.87), 0.74 for stage II (95% CI 0.66 to 0.83), 0.69 for stage III (95% CI 0.60 to 0.78) and 0.61 for stage IV (95% CI 0.44 to 0.77) (most severe). The utility used was that for stage II (moderate disease) as this should capture the mid-point of severities. This could have been estimated by calculating a weighted average based on patient numbers in each stage, but it was assumed that the utility for moderate disease would adequately reflect a mix of mild to severe. This is higher than the estimate used in the Sheffield model (0.63), which was based on Spencer et al. ,145 who derived utility values from 283 patients with COPD who took part in the 1996 Health Survey for England.
The source used for utility associated with CHD was Stevanović et al. 146 This estimate (0.76) was based on a multivariate meta-analysis of preference-based quality-of-life values from 40 studies representing over 30,575 patients with CHD. For this utility, the Sheffield model cited Hay and Sterling,147 who sourced their utilities from the Beaver Dam Health Outcomes Study (a longitudinal cohort study of health status and health-related quality of life in a random sample of 1356 US adults). The average CHD utility (0.77) was a weighted average of myocardial infarction and angina utilities and was similar to that found in Stevanović et al.
Utility associated with stroke was estimated from Haacke et al. ,148 who assessed health-related quality of life (HRQoL) in 77 patients who had experienced an ischaemic stroke, a transient ischaemic attack or a haemorrhagic stroke. The mean EQ-5D value was 0.73 (SD 0.32). This replaces the estimate used in the Sheffield model (0.62), which was taken from Tengs and Lin,149 who carried out a systematic search to identify 20 articles reporting 53 unique quality-of-life weights for stroke and pooled these using a hierarchical linear model. The estimate from Haacke et al. 148 was preferred as it was from a more recent study and was thought to more accurately reflect the specific disutility associated with stroke. 148
For utility associated with second stroke, an estimate (0.48) was sourced from Ara and Brazier,140 who looked at EQ-5D data collected in Health Survey for England from individuals who reported a history of more than one cardiovascular condition. For utility associated with second stroke, the Sheffield model cited Gage et al. ,150 who elicited preferences from 69 volunteers at the Veterans Affairs Palo Alto Health Care System and Stanford University who had atrial fibrillation. Twenty of the volunteers had previously had a stroke. This paper estimated a utility value of 0.12.
Lloyd et al. 151 reported the impact of asthma exacerbations on health-related quality of life and health utility in patients with moderate to severe asthma in the UK. Prospective data regarding health-related quality of life were collected from 112 patients at four asthma centres across the UK using the EQ-5D at two time points. The EQ-5D utility estimated was 0.57 (SD 0.27) for patients with an exacerbation that required oral steroids. For utility associated with asthma, the Sheffield model cited Szende et al. 152 In this study, 228 consecutive adult outpatients and inpatients at four sites in Hungary completed the EQ-5D questionnaire. Patients had to have been diagnosed and already treated for asthma, and were involved in the study at their outpatient visit or during their hospital stay. The utility value estimated for poorly controlled asthma is 0.52.
The utility associated with depression [0.58, standard error (SE) 0.015] was taken from a paper by Hunter et al. ,134 who calculated a score for depressed patients using a weighted average from four UK trials. 153–156 The utility associated with self-harm came from a paper by Byford et al. ,157 in which baseline EQ-5D was collected in 480 patients with a history of recurrent deliberate self-harm.
Intervention effectiveness
The absolute probabilities of cessation at 1 year for interventions were generated by combining the results of the NMA (see Chapter 5) on sustained abstinence with an estimate of response on NRT estimated from Taylor et al. 158 This was a prospective cohort study of electronic medical records from 654 general practices in England in the UK’s Clinical Practice Research Datalink, including 287,079 patients who were prescribed smoking cessation medications during the study period. Of these, 149,526 patients prescribed NRT were eligible for analysis. At 1 year, 21.2% (31,695/149,526) of those prescribed NRT had quit smoking.
The mean probability of 1-year sustained abstinence with all treatments, and 95% credible intervals (CrIs), are shown in Appendix 2, Table 34. The results of the NMA suggested that varenicline low plus NRT standard and varenicline standard plus NRT standard have the highest absolute probability of sustained abstinence, followed by e-cigarette low/varenicline plus bupropion standard/e-cigarette high. Note that the absolute probabilities are derived from the NMA estimates, which are correlated because they are jointly estimated from a single model.
The absolute probabilities of depression at 1 year for interventions were generated by combining the results of the NMA on MANE (see Chapter 6) with an estimate of depression on NRT standard estimated from Kotz et al. 57 This was a retrospective cohort study using data from patients included in the validated QResearch database (www.qresearch.org), which holds data from 753 NHS general practices across England. Patients who were prescribed smoking cessation medications during the study period were identified and followed for 6 months. Of these, 106,759 patients prescribed NRT were eligible for analysis and 8274 reported suffering from depression. This gave a probability of 7%. The mean probabilities of depression for all treatments and 95% CrIs are shown in Appendix 2, Table 35. As no data were available on the other interventions, assumptions had to be made about their relative level of harm. It was, therefore, assumed that NRT low and e-cigarette low have the same level of harm as NRT standard, e-cigarette high has the same level of harm as NRT high, bupropion low has the same level of harm as bupropion standard, and varenicline low plus NRT standard has the same level of harm as varenicline standard plus NRT standard.
The absolute probabilities of self-harm at 1 year for interventions were also generated by combining the results of the NMA on MANE with an estimate of self-harm on NRT estimated from Kotz et al. 57 A total of 540 of the patients in this study reported self-harm, giving a probability of 0.5%. The mean probability of self-harm for all treatments for which data were available is shown in Appendix 2, Table 36.
Cost-effectiveness analysis methods
We conduct a probabilistic analysis in which uncertainty in the model inputs is captured by simulating 5000 times from the assumed distributions described in the previous section, using Monte Carlo simulation performed in Microsoft Excel® version 1908 (Microsoft Corporation, Redmond, WA, USA). The absolute probabilities of abstinence, depression and self-harm were estimated using Bayesian inference, computed using Markov chain Monte Carlo simulation in OpenBUGS (URL: www.openbugs.net). Simulated samples for the model were drawn from 60,000 Markov chain Monte Carlo samples from the posterior distributions, taken from OpenBUGS and read into Microsoft Excel. Care was taken to preserve correlations from the Markov chain Monte Carlo.
We report mean lifetime costs and quality-adjusted life-years (QALYs) for each treatment option. Incremental cost-effectiveness ratios (ICERs), interpreted as the additional expected cost per additional unit gain in QALY for one treatment compared with another, are computed by first ordering treatments by increasing expected cost and then removing treatments that are dominated or extendedly dominated (i.e. have a higher expected cost and lower expected utility than another intervention). ICERs are then calculated for each non-dominated treatment relative to the previous (lower expected cost) non-dominated treatment, where:
For each treatment we also computed net benefit for a given willingness to pay per additional QALY, λ (cost per QALY gained ratio), where net benefit is defined as:
‘Net benefit’ represents the value of a treatment in monetary terms by scaling both QALYs and use of resources to costs. 159 Averaging the net benefit over the probabilistic simulation samples gives the expected net benefit. The intervention with the highest expected net benefit (the optimal intervention) at any willingness-to-pay threshold, λ, can be calculated. We present the expected net benefit for λ = £20,000.
We also plot cost-effectiveness acceptability curves (CEACs), which present the uncertainty in the optimal treatment by plotting the probability that each treatment is the most cost-effective (has the highest net benefit) against the willingness to pay per QALY.
We also present uncertainty between these interventions using rank-o-grams that show the distribution of the probabilities that each treatment is optimal, second, third and so on for each of the 14 treatments, at a willingness-to-pay threshold of £20,000 per QALY. The x-axis reports each of the possible ranks, for which position 1 means that the intervention is optimal. The y-axis shows the probability that each treatment has been ranked at each of the possible positions and, therefore, fully encapsulates the uncertainty in the intervention rankings. The peaks in the rank-o-gram plots show the most likely rank of a given treatment. Flat lines indicate a high degree of uncertainty for the ranking of that treatment type. We also explore how uncertainty in the model inputs impacts on the treatment considered to be optimal using value-of-information methods. 160 This method is also useful in guiding research recommendations as it can estimate the value of a future trial. The expected value of perfect information (EVPI) measures the value (in terms of net benefit) of eliminating all uncertainty in model inputs. The expected value of partial perfect information (EVPPI) measures the value (in terms of net benefit) of eliminating uncertainty in some of the model inputs. This allows us to identify which model inputs are the key drivers of decision uncertainty. Therefore, it follows that it is these areas in which further research might be most beneficial. EVPI and EVPPI are computed per person for the threshold of willingness to pay per QALY of £20,000. Population-level EVPI and EVPPI are also calculated, given an estimated number of smokers attempting to quit in England of 274,021. 3 The lifetime of a treatment represents the time until it becomes obsolete or goes out of use, for example by being superseded by a new intervention. We assume a lifetime of T = 1 year and 5 years, respectively, discounted at 3.5%. The Sheffield Accelerated Value of Information web application161 was used to compute EVPPI for subsets of parameters. 162
We also a present one sensitivity analysis in which the impact of depression and self-harm in the model is removed, so the results are driven by abstinence from smoking alone, and another limiting the analysis to UK-licensed treatments.
Chapter 5 Clinical results: effectiveness
Included studies
Study selection
The results of our search strategy are summarised in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram in Figure 2. Our original searches identified 345 studies for inclusion and our update searches identified an additional 18 studies for inclusion, resulting in a total of 363 studies reported on one or more effectiveness outcomes that are described below. 19,33,69,70,163–511 For the purpose of our analyses, the EAGLES study33 was treated as two studies, where Anthenelli 2016a33 included the four study arms from the non-psychiatric cohort and Athenelli 2016b33 included the four arms from the psychiatric cohort. A list of records excluded at full-text screening (those that did not meet randomised or non-randomised inclusion criteria for effectiveness or safety analyses) and their reasons for exclusion are presented in Report Supplementary Material 1.
FIGURE 2.
The PRISMA flow diagram for effectiveness study records. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
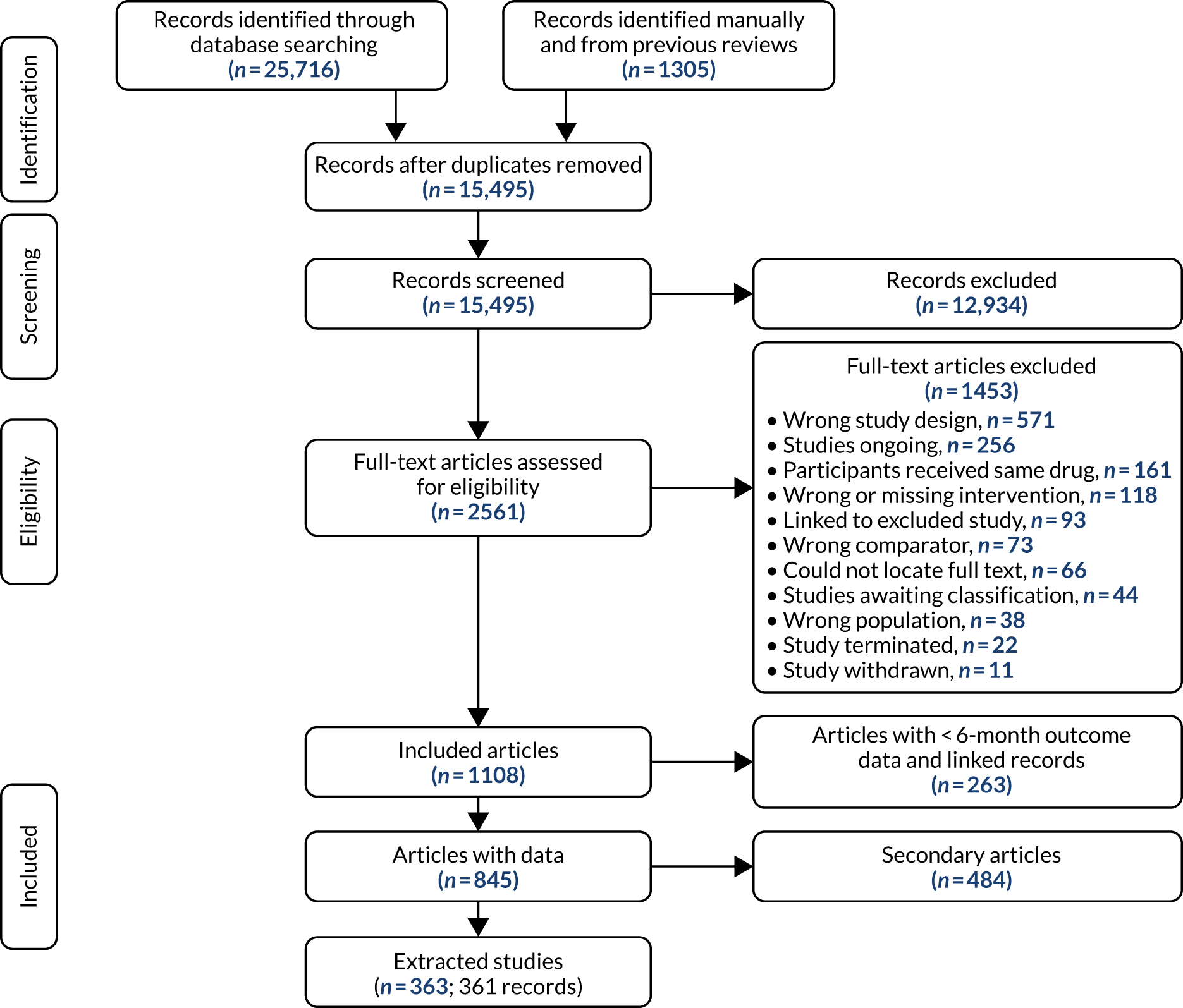
Study characteristics
The number of participants randomised across the 363 trials ranged from 15 to 7354, with a total of 201,045 participants. Trials were conducted across six continents, with 208 trials in the USA, 29 trials in the UK, and 27 multicountry trials. Trials were conducted in several settings, including medical centres and facilities, academic and research centres, universities, community centres, low-income or subsidised housing neighbourhoods, hospitals, pharmacies, clinics (e.g. smoking cessation, dental, urology, methadone, surgical), primary care (general, family and private practices), over the counter, dispensaries, schools, companies and workplaces, Navy ships and over the telephone, by mail or online.
Study duration ranged from 24 to 754 weeks, with the duration of drug treatment ranging from 2 to 104 weeks. One hundred and twenty-one trials were industry sponsored and 122 trials were publicly registered online. We included 24 trials in which smokers were unwilling or not necessarily motivated to quit and 15 trials of smokeless-tobacco users. Twenty-six trials recruited smokers with comorbidities as specified by the Charlson Comorbidity Index,90 16 trials recruited smokers with current or a history of psychiatric conditions and 17 trials recruited smokers with current or a history of drug- or alcohol-related conditions.
The mean age of trial participants ranged from 27.1 to 62 years and the percentage of female participants (in studies that did not exclusively recruit male or female participants) ranged from 0.3% to 81%. Study populations ranged between ethnicities (e.g. white/Caucasian, African American, Latino/Hispanic, Asian, Indigenous Maori), types of tobacco use (e.g. smokeless tobacco, spit tobacco, cigars, waterpipes) and heaviness of smoking. Studies included smokers who were hospital inpatients or outpatients (including smokers scheduled for surgery), smokers with human immunodeficiency virus (HIV), smokers under criminal justice supervision, smokers with substance misuse, smokers with psychiatric conditions, smokers who were health-care professionals, smokers who were active or former armed forces and their family members (e.g. veterans, Navy, National Guard), smokers with prior quit attempts or who had recently relapsed, smokers who were cancer patients or prone to cancer or cancer survivors, female smokers concerned about weight, smokers from low-income or subsidised housing neighbourhoods, smokers with tuberculosis and smokers with asthma. Study-level characteristics of included trials can be found in tables in Report Supplementary Material 2.
Risk of bias in included studies
Ratings ranged from low to high risk of bias and an overall risk-of-bias domain was rated by selecting the highest rating of bias across domains, with the exception of selective outcome reporting (as this domain was usually rated as unclear as a result of inaccessibility of trial protocols and limited trial registration). Risk-of-bias ratings by trial and summarised across studies are presented in Report Supplementary Material 3 and Appendix 4, Figure 34, respectively.
Random sequence generation
Few trials were rated as high risk of bias for random sequence generation, with 45% rated as being at low risk of bias, 53% rated as being at unclear risk of bias and only 2% rated as being at high risk of bias.
Allocation concealment
As was the case for random sequence generation, very few trials (3%) were rated as being at high risk of bias for allocation concealment; 38% of trials were rated as being at low risk of bias and 59% were rated as being at unclear risk of bias.
Blinding of participants and personnel
Blinding of participants and personnel saw nearly one-third of studies rated at each level; 31% of trials were rated as being at low risk of bias, 39% were rated as being at unclear risk of bias and 30% were rated as being at high risk of bias. This was because of a number of trials in which drugs were delivered open label without any blinding.
Blinding of outcome assessment
Nearly half of trials (48%) were rated as being at unclear risk of bias for blinding of outcome assessment domains, with 37% of the remaining trials rated as being at low risk of bias and 15% rated as being at high risk of bias.
Incomplete outcome data
More than half (62%) of trials were rated as being at low risk of bias for incomplete outcome data domain, as many studies used intention-to-treat analyses, and loss to follow-up was either low and/or similar among trial arms. Twenty-nine per cent of trials were rated as being at unclear risk of bias and only 9% were rated as being at high risk of bias.
Selective reporting
As previously mentioned, most (76%) trials were rated as being at unclear risk of bias for selective reporting due to a lack of study protocols or public trial registrations. Of the remaining 24%, 23% of trials were rated as being at low risk of bias and only 1% were rated as being at high risk of bias.
Other bias
Most trials (93%) were rated as being at low risk of bias for the other bias domain, with 4% rated as being at unclear risk of bias and 3% rated as being at high risk of bias.
Overall bias
Finally, ratings for our overall risk of bias domain indicated that 13% of trials were rated as being at low risk of bias, 47% of trials were rated as being at unclear risk of bias and 40% of trials as being at high risk of bias.
Results on clinical effectiveness
We performed NMA on four bioverified effectiveness outcomes: sustained (or continuous) abstinence, prolonged abstinence, any abstinence at 6 months, and 7-day PPA. We fitted a standard (full interaction) NMA model as well as fixed- and random-class NMA models for each outcome. Based on the model fit indices (see Appendix 5), we focused on fixed-class NMA models. A list of treatments delivered in the trials included in effectiveness analyses and their frequency is reported in Appendix 3, Table 38. In this chapter, we present results for each outcome based on a fixed-class NMA model, with additional results for other models provided in Appendix 5. The results are presented as median odds ratios (ORs) alongside 95% CrIs. A summary of results across outcomes is provided at the end of the effectiveness results in the form of a rank-o-gram.
Sustained abstinence
Our primary effectiveness outcome, sustained abstinence at a follow-up of at least 24 weeks, was reported in 171 studies with a total of 90,443 patients, of which 161 (86,884 patients) studies compared two or more of the treatment classes of interest. The network of treatments for this outcome is displayed in Figure 3, where thicker edges represent comparisons with a larger number of randomised patients. Similarly, interventions with a larger number of randomised patients have larger circles. Most interventions were compared with placebo in the primary studies, although arms with no drug treatment or with usual care were also used as comparators in some studies, and some direct comparisons between different drug types are also available. One study comparing varenicline standard plus NRT gum standard (114/245 patients quit) with varenicline low plus NRT gum standard (111/240 patients quit) was disconnected from the network at the treatment level (see Figure 37) but not at the class level (see Figure 3); hence, we excluded that study when comparing the different models (see Table 39) but were able to include it in the analyses discussed in this section (which report results at the class level).
FIGURE 3.
Network plot for sustained abstinence at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Figure 4 displays the results for the fixed-class NMA model based on 161 studies with placebo as a comparator. There was evidence that smokers randomised to usual care were less likely to quit than those who received placebo (OR 0.49, 95% CrI 0.33 to 0.71), whereas smokers randomised to no drug treatment were more likely to quit than those who received placebo (OR 1.28, 95% CrI 1.01 to 1.83). Moreover, there was evidence that smokers receiving NRT standard (OR 2.01, 95% CrI 1.68 to 2.41) and NRT high (OR 2.32, 95% CrI 1.88 to 2.86) were more likely to quit than those randomised to placebo. Most interventions were more effective than placebo, including bupropion low (OR 1.75, 95% CrI 1.03 to 3.00), bupropion standard (OR 1.73, 95% CrI 1.43 to 2.10), varenicline low (OR 1.79, 95% CrI 1.07 to 2.97), varenicline standard (OR 2.83, 95% CrI 2.34 to 3.39), e-cigarette high (OR 3.22, 95% CrI 1.63 to 6.36), varenicline low plus NRT standard (OR 5.70, 95% CrI 1.57 to 21.12), varenicline standard plus NRT standard (OR 5.75, 95% CrI 2.27 to 14.88), varenicline standard plus NRT high (OR 2.34, 95% CrI 1.12 to 4.90) and varenicline standard plus bupropion standard (OR 3.25, 95% CrI 1.35 to 7.92). There was weak evidence for the effectiveness of e-cigarette low compared with placebo (OR 3.22, 95% CrI 0.97 to 12.55).
FIGURE 4.
Forest plot with results of the fixed-class NMA model for sustained abstinence. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
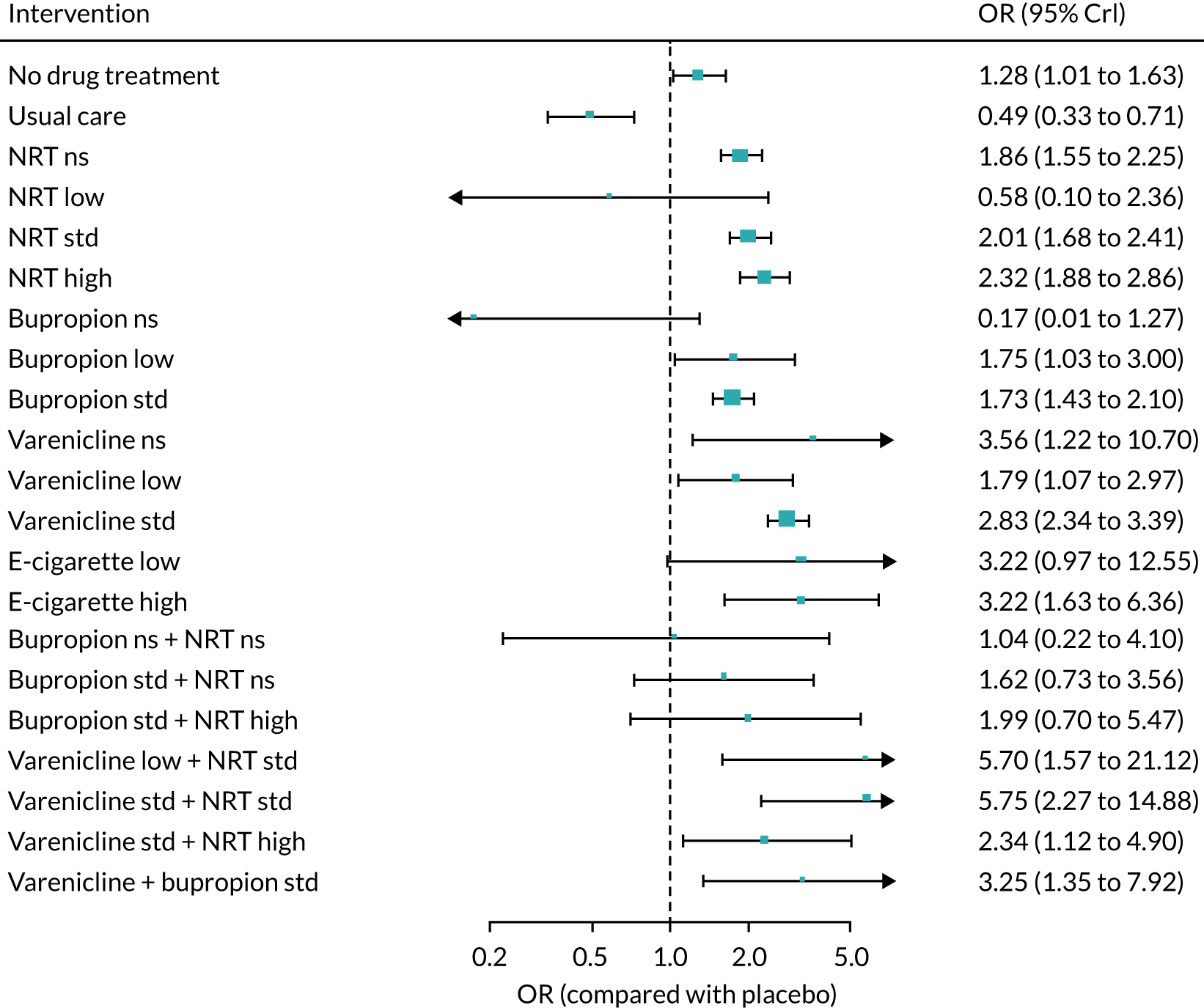
Table 2 presents the class effect estimates with placebo as comparator obtained from the NMA (last column) alongside the estimates obtained from direct and indirect evidence. Direct evidence was available for most monotherapies, whereas comparisons of combinations of interventions with placebo largely relied on indirect evidence only. Most effect estimates are above 1, suggesting that the interventions helped smokers to reach sustained abstinence more frequently than placebo. Where there was enough information to back-calculate indirect evidence and compare it with direct evidence, the results show some potentially inconsistent results, with examples where direct evidence shows a less beneficial effect for the experimental drug than the indirect evidence (e.g. bupropion low vs. placebo) and also the opposite (e.g. varenicline standard plus bupropion standard vs. placebo).
| Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) | |
|---|---|---|---|
| No drug treatment | 0.70 (0.08 to 4.01) | 1.30 (1.02 to 1.64) | 1.28 (1.01 to 1.63) |
| Usual care | 0.81 (0.38 to 1.72) | 0.41 (0.27 to 0.63) | 0.49 (0.33 to 0.71) |
| NRT not specified | 1.86 (1.55 to 2.25) | – | 1.86 (1.55 to 2.25) |
| NRT low | – | 0.58 (0.10 to 2.36) | 0.58 (0.10 to 2.36) |
| NRT standard | 2.01 (1.68 to 2.41) | – | 2.01 (1.68 to 2.41) |
| NRT high | 2.32 (1.88 to 2.86) | – | 2.32 (1.88 to 2.86) |
| Bupropion not specified | – | 0.17 (0.01 to 1.27) | 0.17 (0.01 to 1.27) |
| Bupropion low | 0.96 (0.44 to 2.05) | 3.04 (1.46 to 6.33) | 1.75 (1.03 to 3.00) |
| Bupropion standard | 1.73 (1.43 to 2.10) | – | 1.73 (1.43 to 2.10) |
| Varenicline not specified | 3.56 (1.22 to 10.7) | – | 3.56 (1.22 to 10.7) |
| Varenicline low | 1.79 (1.07 to 2.97) | – | 1.79 (1.07 to 2.97) |
| Varenicline standard | 2.83 (2.34 to 3.39) | – | 2.83 (2.34 to 3.39) |
| E-cigarette low | 3.22 (0.97 to 12.6) | – | 3.22 (0.97 to 12.6) |
| E-cigarette high | 2.46 (0.86 to 7.03) | 3.89 (1.63 to 9.28) | 3.22 (1.63 to 6.36) |
| Bupropion not specified plus NRT not specified | – | 1.04 (0.22 to 4.10) | 1.04 (0.22 to 4.10) |
| Bupropion standard plus NRT not specified | – | 1.62 (0.73 to 3.56) | 1.62 (0.73 to 3.56) |
| Bupropion standard plus NRT high | – | 1.99 (0.70 to 5.47) | 1.99 (0.70 to 5.47) |
| Varenicline low plus NRT standard | – | 5.70 (1.57 to 21.1) | 5.70 (1.57 to 21.1) |
| Varenicline standard plus NRT standard | – | 5.75 (2.27 to 14.9) | 5.75 (2.27 to 14.9) |
| Varenicline standard plus NRT high | – | 2.34 (1.12 to 4.90) | 2.34 (1.12 to 4.90) |
| Varenicline standard plus bupropion standard | 3.42 (1.39 to 8.67) | 1.88 (0.09 to 39.9) | 3.25 (1.35 to 7.92) |
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 5, Table 39). The pairwise comparisons between interventions for this outcome are presented in Table 3. Most of the effect estimates were informed by indirect evidence only, and the results were consistent when both direct and indirect evidence were available. There was evidence that smokers randomised to varenicline standard plus NRT standard were more likely to achieve sustained abstinence than those receiving NRT standard (OR 2.87, 95% CrI 1.11 to 7.49) or bupropion standard (OR 3.34, 95% CrI 1.28 to 8.65). The results also suggest higher odds of abstinence with varenicline standard compared with NRT standard (OR 1.40, 95% CrI 1.10 to 1.78) or bupropion standard (OR 1.63, 95% CrI 1.27 to 2.07). Furthermore, there was weak evidence that e-cigarette high might increase the odds of sustained abstinence compared with bupropion standard (OR 1.86, 95% CrI 0.92 to 3.73).
| Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) | |
|---|---|---|---|
| Bupropion standard vs. NRT standard | 0.70 (0.17 to 2.83) | 0.87 (0.67 to 1.12) | 0.86 (0.67 to 1.11) |
| Varenicline standard vs. NRT standard | – | 1.40 (1.10 to 1.78) | 1.40 (1.10 to 1.78) |
| E-cigarette low vs. NRT standard | – | 1.60 (0.48 to 6.30) | 1.60 (0.48 to 6.30) |
| E-cigarette high vs. NRT standard | – | 1.60 (0.80 to 3.20) | 1.60 (0.80 to 3.20) |
| Varenicline standard plus NRT standard vs. NRT standard | – | 2.87 (1.11 to 7.49) | 2.87 (1.11 to 7.49) |
| Varenicline standard plus bupropion standard vs. NRT standard | – | 1.61 (0.66 to 3.98) | 1.61 (0.66 to 3.98) |
| Varenicline standard vs. bupropion standard | – | 1.63 (1.27 to 2.07) | 1.63 (1.27 to 2.07) |
| E-cigarette low vs. bupropion standard | – | 1.85 (0.55 to 7.29) | 1.85 (0.55 to 7.29) |
| E-cigarette high vs. bupropion standard | – | 1.86 (0.92 to 3.73) | 1.86 (0.92 to 3.73) |
| Varenicline standard plus NRT standard vs. bupropion standard | – | 3.34 (1.28 to 8.65) | 3.34 (1.28 to 8.65) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 1.87 (0.76 to 4.59) | 1.87 (0.76 to 4.59) |
| E-cigarette low vs. varenicline standard | – | 1.14 (0.34 to 4.47) | 1.14 (0.34 to 4.47) |
| E-cigarette high vs. varenicline standard | – | 1.14 (0.57 to 2.30) | 1.14 (0.57 to 2.30) |
| Varenicline standard plus NRT standard vs. varenicline standard | 2.05 (0.82 to 5.17) | – | 2.05 (0.82 to 5.17) |
| Varenicline standard plus bupropion standard vs. varenicline standard | – | 1.15 (0.48 to 2.76) | 1.15 (0.48 to 2.76) |
| E-cigarette high vs. e-cigarette low | – | 1.00 (0.22 to 4.03) | 1.00 (0.22 to 4.03) |
| Varenicline standard plus NRT standard vs. e-cigarette low | – | 1.80 (0.34 to 8.39) | 1.80 (0.34 to 8.39) |
| Varenicline standard plus bupropion standard vs. e-cigarette low | – | 1.01 (0.20 to 4.41) | 1.01 (0.20 to 4.41) |
| Varenicline standard plus NRT standard vs. e-cigarette high | – | 1.79 (0.57 to 5.71) | 1.79 (0.57 to 5.71) |
| Varenicline standard plus bupropion standard vs. e-cigarette high | – | 1.00 (0.33 to 3.08) | 1.00 (0.33 to 3.08) |
| Varenicline standard plus bupropion standard vs. varenicline standard plus NRT standard | – | 0.56 (0.16 to 1.98) | 0.56 (0.16 to 1.98) |
Estimates of absolute probabilities of sustained abstinence for each intervention can be obtained by applying the relative effects in Table 3 to an assumed cessation rate on NRT standard (estimates shown in Appendix 2, Table 34).
With regard to effect modifiers, there was evidence of effect modification as a function of counselling, with interventions that included counselling being associated with a higher proportion of smokers achieving sustained abstinence (additional log-OR was 0.86, 95% CrI 0.45 to 1.27; see Figure 45). We also found evidence of effect modification as a function of dependence, with higher odds of sustained abstinence among participants with higher average dependence scores (additional log-OR 0.23, 95% CrI 0.02 to 0.43; see Figure 47). We found inconclusive evidence of effect modification according to industry sponsorship, type of placebo, treatment duration, comorbidities, willingness to quit, smokeless tobacco, smoking level or publication year (see Figures 42, 44, 48 and 53). A sensitivity analysis excluding studies at high risk of bias yielded the same findings reported in this section for active interventions, although with wider intervals for most effect estimates, and particularly for e-cigarettes and treatment combinations (see Appendix 5, Figure 40). However, the results from this analysis suggested no difference between usual care and placebo (OR 1.16, 95% CrI 0.64 to 2.12). We observed the same trends in a further sensitivity analysis where we excluded studies comparing pharmacological with non-pharmacological interventions (the estimate of the OR comparing usual care with placebo was 1.03, 95% CrI 0.57 to 1.86; see Figure 41). Within this sensitivity analysis, we found inconclusive evidence of effect modification when pharmacological interventions were given with counselling, with a trend towards a synergistic effect (more effective than would be expected based on the sum of the pharmacological and counselling effects alone) (additional log-OR of 0.16, 95% CrI –0.05 to 0.37; see Figure 46).
Threshold analysis
Varenicline standard plus NRT standard had the highest estimated odds of sustained abstinence and was the first-ranked treatment in our analyses. Figure 5 shows the results of the threshold analysis for sustained abstinence, focusing on a selection of the eight treatment classes considered most relevant (and examined in the rank-o-grams presented later in this chapter). Threshold analysis determines how much the evidence could change before the first-ranked treatment changes. Each row in Figure 5 corresponds to a single study estimate, and displays the estimate (log-OR) and 95% CI from that study, along with the invariant interval (shaded bar). Any changes to the study estimate that lie within the invariant interval will not affect the first-ranked treatment. Changes that pass the thresholds at either end of the invariant interval will result in a new first-ranked treatment, which are shown as numeric treatment codes at either side of the invariant interval.
FIGURE 5.
Threshold analysis results for sustained abstinence, sorted by size of threshold (smallest to largest). Only studies with thresholds of < 10 log-OR are shown, for brevity. Treatment codes are (1) placebo, (7) NRT standard, (11) bupropion standard, (14) varenicline standard, (17) e-cigarette low, (18) e-cigarette high, (26) varenicline standard plus NRT Standard and (28) varenicline plus bupropion standard. For a full list, see Appendix 6. Bold study labels and red shaded invariant intervals show where a 95% CI crosses the corresponding threshold, indicating sensitivity to the level of uncertainty in this estimate. AC, allocation concealment; BOA, blinding of outcome assessment; BPP, blinding of participants and personnel; IOD, incomplete outcome data; NT, no threshold; OB, other bias; RSG, random sequence generation; SR, selective reporting. ‘+’, low risk of bias; ‘?’, unclear risk of bias; ‘–’, high risk of bias. 19,33,69,172,173,192,198,202,207,239,252,294,306,351,392,454,473,476 This figure is reproduced with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Figure 5 also shows the risk-of-bias judgements (see Report Supplementary Material 3). The smallest threshold is 0.44 (on the log-odds scale) for the Cinciripini et al. 207 study estimate of varenicline standard plus bupropion standard versus placebo; if the log-OR of this estimate changed from 1.63 to 2.07 (= 1.63 + 0.44) or higher in favour of varenicline standard plus bupropion standard, then varenicline standard plus bupropion standard would become the first-ranked treatment. This study was rated as being at low risk of bias; however, the upper end of the 95% CI for the study estimate crosses this threshold, meaning that the first-place ranking is sensitive to the level of uncertainty in this study estimate. The second smallest threshold is –0.47 (on the log-odds scale) for the Koegelenberg et al. 351 estimate of varenicline standard plus NRT standard versus varenicline standard; if the log-OR of this estimate changes from 0.71 to 0.24 (= 0.71 – 0.47) or lower, in favour of varenicline standard, then e-cigarette low would become the first-ranked treatment. This study was rated as being at unclear risk of bias, and it may be judged whether or not any bias due to inadequate random sequence generation or allocation concealment would lead to such an overestimation of treatment effects. There is no threshold in the other direction for this study estimate, indicated by ‘NT’ in the invariant interval; no amount of change to this estimate in favour of varenicline standard plus NRT would change the first-ranked treatment. Only one other study (Caponnetto et al. ;202 e-cigarette low vs. placebo) has a threshold < 0.7 log-OR (equivalent to a factor of 2 on the OR scale); the threshold is 0.61 in the direction favouring e-cigarette low, at which point e-cigarette low would be the first-ranked treatment. The upper end of the 95% CI for the study estimate crosses this threshold, meaning that the first-place ranking is sensitive to the level of uncertainty in this study estimate. Only another two studies19,192 have thresholds < 3 log-OR (equivalent to a factor of 20 on the OR scale). Bullen et al. 192 is rated as being at low risk of bias, but Hajek et al. 19 is rated as being at high or unclear risk of bias for blinding. To change the first-ranked treatment (to e-cigarette high), the Hajek et al. 19 estimate of e-cigarette high versus NRT not specified would have to underestimate the true OR by a factor of 2.6. The remaining 212 study estimates have even larger thresholds, and it is unlikely that any potential biases could plausibly change these estimates by such an amount to affect the first-place ranking.
Overall, the first-place ranking of varenicline standard plus NRT standard appears relatively robust. However, there is some sensitivity to the level of uncertainty and potential biases in the evidence, which could lead to varenicline plus bupropion standard, e-cigarette low or e-cigarette high being ranked first for sustained abstinence.
Prolonged abstinence
Prolonged abstinence was also restricted to measurements with a follow-up of at least 24 weeks. It was reported in 19 studies (4434 patients), with 17 studies (3512 patients) including at least one relevant comparison. Appendix 5, Figure 54 presents the structure of this network, sparser than the previous one but still connected both at (a) the treatment and (b) the class level. Placebo was, again, the main comparator across studies, although some direct comparisons between different drug types are also available. We excluded one study comparing NRT gum standard (8/79 patients quit) with no drug treatment (2/82 patients quit), which was disconnected from the main network both at the treatment and at the class levels, and one study comparing bupropion low (1/9 patients quit) with placebo (0/9 patients quit) because of small numbers that caused convergence problems in the models.
Therefore, the NMA for this outcome was based on 15 studies. Results with placebo as a comparator are presented in Appendix 5, Figure 55. There was evidence that smokers treated with bupropion standard (OR 2.34, 95% CrI 1.46 to 3.86), varenicline standard (OR 3.63, 95% CrI 2.23 to 6.36) and varenicline standard plus bupropion standard (OR 4.76, 95% CrI 2.48 to 10.10) were more likely to achieve prolonged abstinence than those who received placebo.
Appendix 5, Table 40 gives the NMA results with placebo as comparator alongside effect estimates from direct and/or indirect evidence, where available. Most estimates are above 1, which suggests that smokers assigned to each of the drugs examined were more likely to achieve prolonged abstinence than those receiving placebo. As would be expected, CrIs around the NMA effect estimates are typically narrower than those obtained using either direct or indirect evidence in isolation.
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 5, Table 42 and Figures 55 and 56). Pairwise comparisons between active interventions, mostly obtained through indirect evidence, are displayed in Appendix 5, Table 41. There was inconclusive evidence that bupropion standard, varenicline standard, and varenicline standard plus bupropion standard differed from each other in the odds of achieving prolonged abstinence.
Any abstinence
Any abstinence was restricted to measurements with a follow-up of at least 22 weeks. A total of 216 studies (99,630 smokers) reported on it, with 196 (91,667 smokers) including at least one relevant comparison. The structure of the network at the treatment level is displayed in Appendix 5, Figure 58 and at the class level in Figure 6.
FIGURE 6.
Network plot for any abstinence at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Results for the fixed-class random-effects NMA with placebo as a comparator are presented in Figure 7. Compared with placebo, there was evidence that smokers randomised to no drug treatment were more likely to achieve any abstinence (OR 1.48, 95% CrI 1.19 to 1.86), whereas smokers receiving usual care were less likely to do so (OR 0.66, 95% CrI 0.46 to 0.96). With regard to active interventions, smokers allocated to NRT not specified (OR 1.86, 95% CrI 1.57 to 2.20), NRT standard (OR 2.03, 95% CrI 1.72 to 2.44), NRT high (OR 2.46, 95% CrI 2.03 to 2.94), bupropion low (OR 2.89, 95% CrI 1.34 to 6.23), bupropion standard (OR 1.84, 95% CrI 1.57 to 2.16), varenicline not specified (OR 4.06, 95% CrI 1.04 to 11.90), varenicline standard (OR 2.69, 95% CrI 2.27 to 3.19), e-cigarette low (OR 3.29, 95% CrI 1.13 to 10.80), e-cigarette high (OR 2.77, 95% CrI 1.01 to 7.69), bupropion low plus NRT high (OR 5.75, 95% CrI 1.79 to 19.10), bupropion standard plus NRT not specified (OR 2.10, 95% CrI 1.22 to 3.60), bupropion standard plus NRT high (OR 2.56, 95% CrI 1.60 to 4.14), varenicline standard plus NRT standard (OR 5.53, 95% CrI 2.12 to 14.40), varenicline standard plus NRT high (OR 2.36, 95% CrI 1.12 to 4.90), and varenicline standard plus bupropion standard (OR 3.56, 95% CrI 1.84 to 6.89) had higher odds of any abstinence than those receiving placebo.
FIGURE 7.
Forest plot with results of the fixed-class NMA model for any abstinence. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
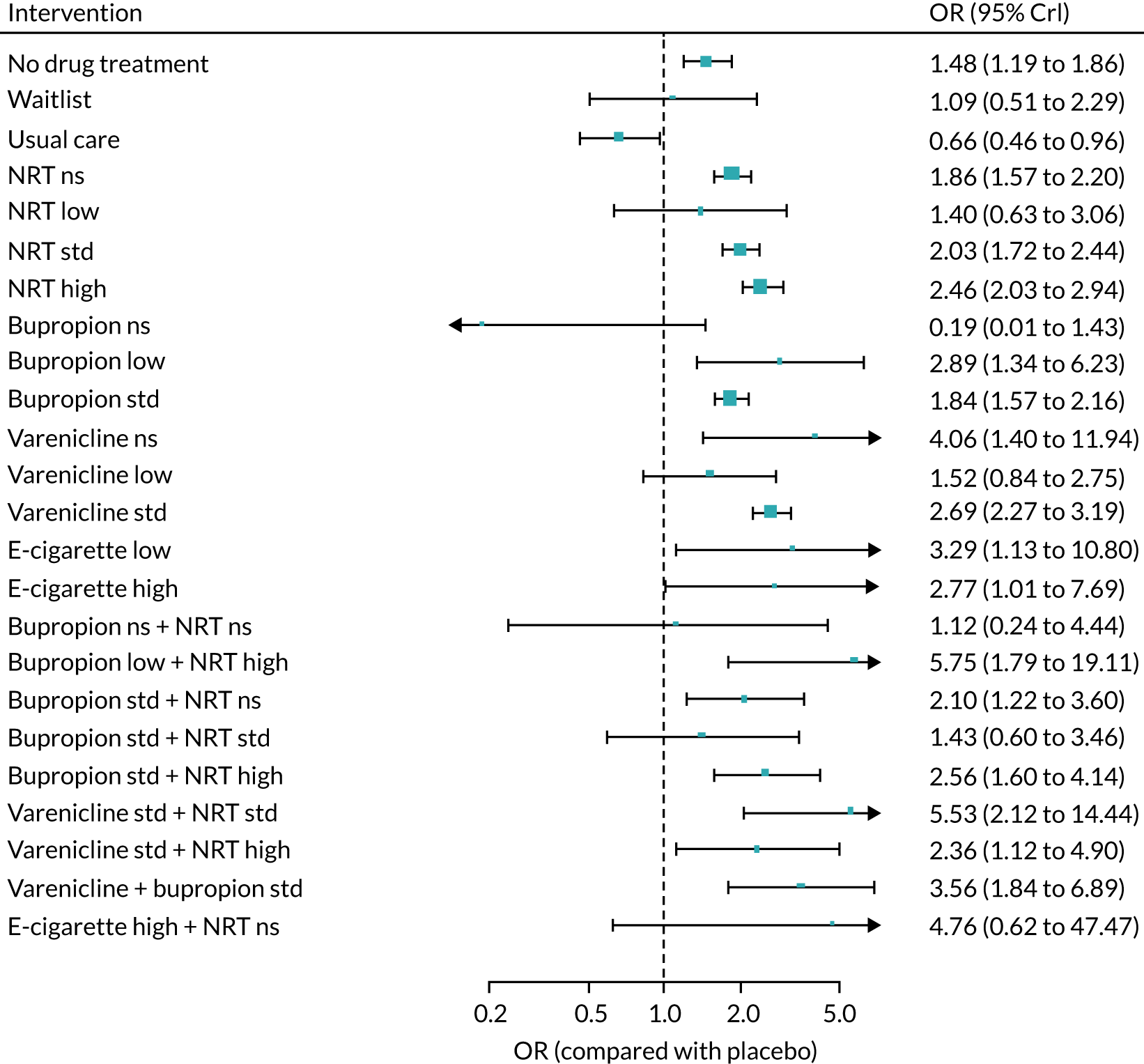
Appendix 5, Table 43 presents the class effect estimates with placebo as comparator obtained from the NMA and direct and indirect evidence. Direct evidence was available for most monotherapies, whereas comparisons of combinations of interventions compared with placebo largely relied on indirect evidence only. Most effect estimates are above 1, suggesting that the interventions helped smokers to reach sustained abstinence more frequently than placebo.
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 5, Table 45 and Figures 59 and 60). Where there was enough information to back-calculate indirect evidence and compare it with direct evidence, the results were largely consistent, although there were instances when direct evidence showed a less beneficial effect for the experimental drug than the indirect evidence (e.g. e-cigarette low vs. placebo).
The pairwise comparisons between experimental interventions for this outcome are presented in Appendix 5, Table 44. Most effect estimates were informed by indirect evidence only, and the results were consistent when both direct and indirect evidence were available. There was evidence that smokers randomised to varenicline standard were more likely to achieve any abstinence than those allocated to NRT standard (OR 1.32, 95% CrI 1.05 to 1.65) and bupropion standard (OR 1.46, 95% CrI 1.18 to 1.81). Furthermore, varenicline standard plus NRT standard led to higher odds of abstinence than NRT standard alone (OR 2.70, 95% CrI 1.02 to 7.13), bupropion standard (OR 2.99, 95% CrI 1.13 to 7.88) and standard doses of bupropion and NRT combined (OR 3.83, 95% CrI 1.05 to 14.00). Last, there was weak evidence that the combination of varenicline standard plus bupropion standard was more effective than bupropion standard alone (OR 1.93, 95% CrI 0.98 to 3.79).
Seven-day point prevalence abstinence
Point prevalence abstinence at 1 week was reported in 139 studies (55,724 patients), with 122 studies (48,110 patients) including at least one relevant comparison. Appendix 5, Figure 61 presents the structure of this network at the treatment level and Figure 8 presents the structure of this network at the class level.
FIGURE 8.
Network plot for 7-day PPA at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
The NMA for this outcome was based on 122 studies. Results with placebo as a comparator are presented in Figure 9. There was evidence that smokers randomised to NRT not specified (OR 1.75, 95% CrI 1.48 to 2.08), NRT standard (OR 1.58, 95% CrI 1.21 to 2.12) and NRT high (OR 1.99, 95% CrI 1.67 to 2.39) were more likely to remain abstinent for a full week than those receiving placebo. Similarly, smokers treated with bupropion standard (OR 1.67, 95% CrI 1.48 to 1.88), varenicline not specified (OR 2.56, 95% CrI 1.21 to 5.42), varenicline standard (OR 2.14, 95% CrI 1.86 to 2.46), bupropion low plus NRT high (OR 4.76, 95% CrI 1.82 to 12.70), bupropion standard plus NRT high (OR 2.16, 95% CrI 1.57 to 2.97), varenicline standard plus NRT standard (OR 4.01, 95% CrI 2.16 to 7.54) and varenicline standard plus bupropion standard (OR 2.29, 95% CrI 1.48 to 3.56) reached 7-day PPA more often than those taking placebo. There was weak evidence that varenicline low was more effective than placebo (OR 1.75, 95% CrI 0.97 to 3.13).
FIGURE 9.
Forest plot with results of the fixed-class NMA model for PPA. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Appendix 5, Table 46 presents the NMA results with placebo as comparator alongside effect estimates from direct and/or indirect evidence, where available. Most estimates are above 1, which suggests that smokers assigned to each of the drugs examined were more likely to remain abstinent for a full week than those receiving placebo. Nonetheless, intervals around effect estimates obtained from direct and indirect evidence tend to be wider than those obtained when integrating both in the NMA, which reflects the gain in precision when using the latter approach. A comparison between direct and indirect evidence reveals largely consistent results, although examples can be found where direct evidence suggests a less beneficial effect of the experimental intervention (e.g. varenicline low vs. placebo) and also the opposite (NRT high vs. placebo).
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 5, Table 48 and Figures 62 and 63). Pairwise comparisons between active interventions, mostly obtained through indirect evidence, are displayed in Appendix 5, Table 47. Smokers allocated to varenicline standard achieved the target more often than those treated with NRT standard (OR 1.35, 95% CrI 0.99 to 1.82) or bupropion standard (OR 1.28, 95% CrI 1.08 to 1.53). Furthermore, there was strong evidence that the combination of varenicline standard plus NRT standard led to higher odds of abstinence than NRT standard alone (OR 2.54, 95% CrI 1.28 to 4.98), bupropion standard (OR 2.42, 95% CrI 1.28 to 4.57) and varenicline standard alone (OR 1.88, 95% CrI 1.02 to 3.46).
Ranking of interventions
Table 4 presents ranks for a selection of classes according to the primary effectiveness outcome, namely sustained abstinence. Varenicline standard plus NRT standard yielded the highest probability of being the most effective intervention (0.61, mean rank 1.67), followed by e-cigarette low (0.19, mean rank 3.61), varenicline standard plus bupropion standard (0.12, mean rank 3.42) and e-cigarette high (0.08, mean rank 3.32). Conversely, there was strong evidence that placebo had the lowest rank among the eight shortlisted interventions, with a mean rank of 7.97.
| Intervention | Pr(best) | Mean rank |
|---|---|---|
| Placebo | 0 | 7.97 |
| NRT standard | 0 | 5.64 |
| Bupropion standard | 0 | 6.57 |
| Varenicline standard | 0 | 3.80 |
| E-cigarette low | 0.19 | 3.61 |
| E-cigarette high | 0.08 | 3.32 |
| Varenicline standard plus NRT standard | 0.61 | 1.67 |
| Varenicline standard plus bupropion standard | 0.12 | 3.42 |
Figure 10 is a rank-o-gram displaying the ranking of the same interventions across the four effectiveness outcomes examined in our NMA models. Varenicline standard plus NRT standard showed a high probability to be ranked best or second-best intervention for all of them (note that there was no information for the effect of this drug combination on prolonged abstinence). Furthermore, varenicline standard plus bupropion standard yielded the highest probability to be ranked as the best intervention for prolonged abstinence, although there was higher uncertainty about its ranking for the other outcomes. Moreover, varenicline standard showed highest probabilities of being ranked second to fourth best for the different outcomes, whereas e-cigarettes presented a more uncertain ranking profile. Last, placebo was ranked as the least effective intervention for all outcomes.
FIGURE 10.
Rank-o-gram of interventions across effectiveness outcomes. Rank of (a) placebo; (b) NRT standard; (c) bupropion standard; (d) varenicline standard; (e) e-cigarette low; (f) e-cigarette high; (g) bupropion standard plus NRT standard; (h) varenicline standard plus NRT standard; and (i) varenicline standard plus bupropion standard. Std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Chapter 6 Clinical results: safety
Included studies
Randomised evidence
Study selection
The results of our search strategy are summarised in Figure 11. Our original searches identified 335 studies for inclusion and our update searches identified an additional 20 studies for inclusion, resulting in a total of 355 studies19,33,40,69,70,164–166,169–171,173–180,184–186,190,192,193,196–198,200–203,205–208,212,216–218,220,221,223–226,228–231,233–252,255,257–259,261–263,265–268,271–275,277–279,281–283,286–288,295–297,300–303,307–311,313,315,318–331,336–340,343–345,347,349–352,354,356,357,359,361–366,371,373–378,380,381,385–387,390,391,393,395–397,399,401,403,404,406,408,410,411,413–416,418–422,424–427,429–436,438–440,442–450,453–458,460,463–476,478–482,484,487,488,490–495,497–506,509,511–604 that reported on one or more safety outcomes; these are described in the following sections. For the purpose of our analyses, the EAGLES study33 was treated as two studies, where Anthenelli 2016a33 included the four study arms from the non-psychiatric cohort and Anthenelli 2016b33 included the four arms from the psychiatric cohort. A list of records excluded at full-text screening (that did not meet randomised or non-randomised inclusion criteria for effectiveness or safety analyses) and the reasons for their exclusion are presented in Report Supplementary Material 1.
FIGURE 11.
The PRISMA flow diagram for randomised safety study records. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
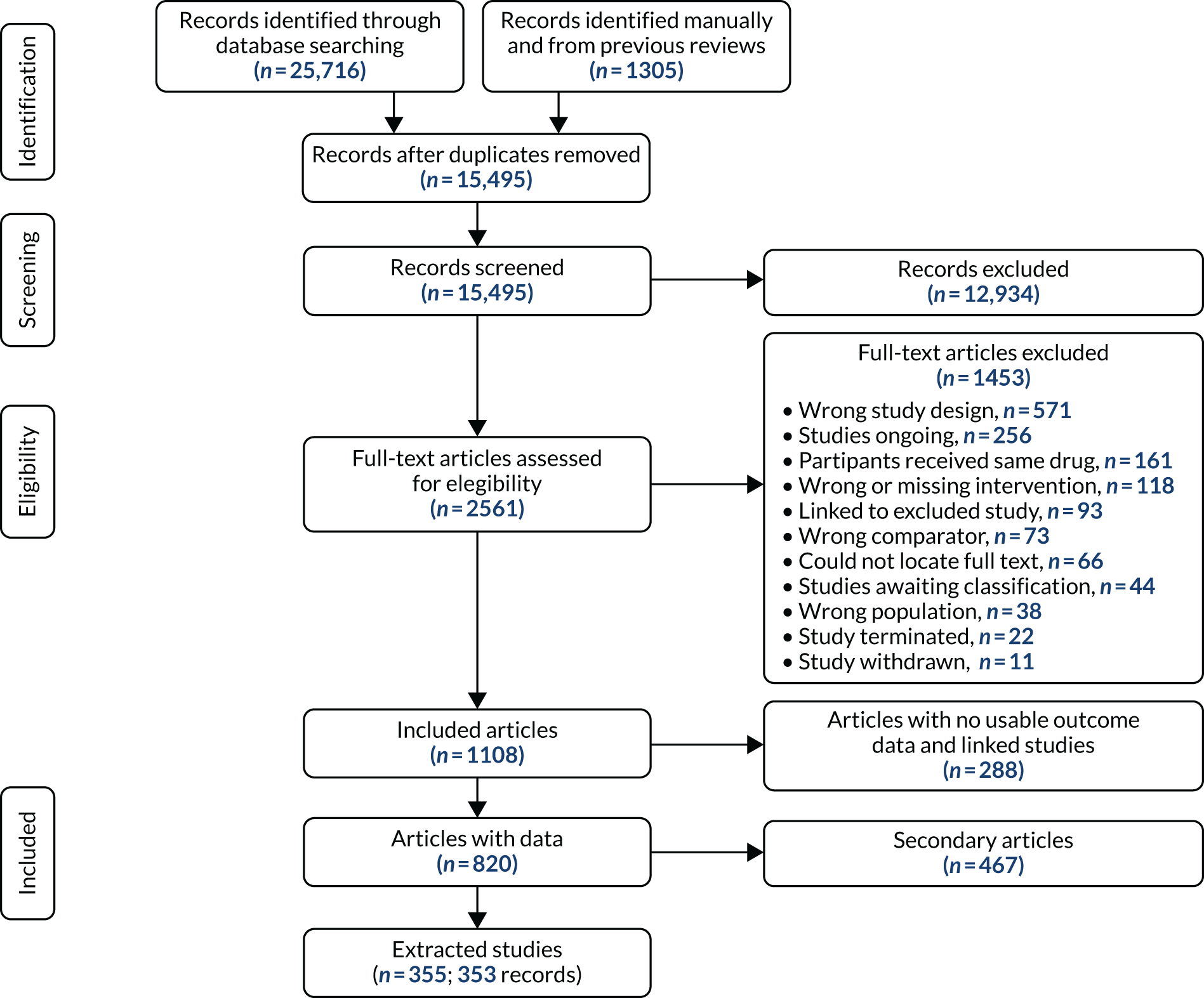
Study characteristics
The number of participants randomised across the 355 trials ranged from 5 to 5887, with a total of 159,101 participants. Trials were conducted across six continents, with 211 trials in the USA, 34 trials in the UK, and 31 multicountry trials. Trials were conducted in several settings, including medical centres and facilities, academic and research centres, universities, community centres, subsidised housing neighbourhoods, hospitals, pharmacies, clinics (e.g. smoking cessation, dental, substance misuse, HIV), primary care (general, family and private practices), over-the-counter, companies and workplaces, over the telephone and by mail.
Trial duration ranged from 0.14 (1 day/single session) to 754 weeks and duration of drug treatment ranged from 0.07 (half a day) to 104 weeks. One hundred and forty trials were industry sponsored and 154 trials were publicly registered online. We included 48 trials in which smokers were unwilling or not necessarily motivated to quit and 13 trials of smokeless-tobacco users. Twenty-three trials recruited smokers with comorbidities as specified by the Charlson Comorbidity Index,90 29 trials recruited smokers with current or a history of psychiatric conditions and 25 trials recruited smokers with current or a history of drug- or alcohol-related conditions.
The mean age of trial participants ranged from 28.4 to 62.8 years and the percentage of female participants (in studies that did not exclusively recruit male or female participants) ranged from 0.3% to 79%. Study populations ranged between ethnicities (e.g. white/Caucasian, African American, Asian, Indigenous Maori), types of tobacco use (e.g. smokeless tobacco, spit tobacco, cigars, waterpipes) and heaviness of smoking. Studies included smokers who were hospital inpatients or outpatients (including smokers scheduled for surgery), smokers with HIV, smokers with substance misuse, smokers with psychiatric conditions, smokers who were health-care professionals, smokers who were active or former armed forces and their family members (e.g. veterans, National Guard), smokers with previous quit attempts or who had recently relapsed, smokers who were cancer patients or survivors, female smokers concerned about weight, smokers from low-income or subsidised housing neighbourhoods, smokers with tuberculosis and smokers with asthma. Study-level characteristics can be found in tables in Report Supplementary Material 4.
Non-randomised evidence
Study selection
The results of our search strategy are summarised in Figure 12. Our original searches identified 48 studies for inclusion and our update searches identified an additional five studies for inclusion, resulting in a total of 53 studies53–55,57,605–653 that reported on one or more safety outcomes; these are described in the following sections. A list of records excluded at full-text screening (i.e. they did not meet randomised or non-randomised inclusion criteria for effectiveness or safety analyses) and the reasons for their exclusion are presented in Report Supplementary Material 1.
FIGURE 12.
The PRISMA flow diagram for non-randomised safety study records.

Study characteristics
The number of participants randomised across the 53 studies ranged from 32 to 7,917,436, with a total of 8,783,403 participants. Study designs included case–control, population cohort, retrospective cohort, prospective cohort and quasi-randomised. Studies were conducted across five continents, with 19 studies in the USA, seven studies in the UK, and no multicountry studies. Studies were conducted in several settings, including hospitals, primary care (e.g. GP practices), clinics (e.g. community, smoking cessation, tobacco dependence, surgical preoperative), academic, drug monitoring and medical centres. Observational studies used a range of databases, including the Clinical Practice Research Datalink (CPRD) (formerly the General Practice Research Database), New York City Fire Department, Bureau of Medical Services, Medicaid, Military Health System Data Repository, nationwide registries (National Prescription Registry, National Patient Registry), and were conducted over the telephone and online.
Study duration ranged from 4 to 208 weeks and the duration of drug treatment ranged from 1.26 to 208 weeks. Three studies were industry sponsored and three studies were publicly registered online. We included two studies in which smokers were not necessarily motivated to quit and no studies of smokeless-tobacco users. Five studies recruited smokers with comorbidities as specified by the Charlson Comorbidity Index,90 five studies recruited smokers with current or a history of psychiatric conditions and two studies recruited smokers with current or a history of drug- or alcohol-related conditions.
The mean age of study participants ranged from 37.9 to 58.3 years and the percentage of female participants (in studies that did not exclusively recruit male or female participants) ranged from 1% to 61.6%. Studies included smokers who were hospital inpatients or outpatients (including smokers scheduled for surgery), smokers with HIV, smokers with substance misuse, smokers with psychiatric conditions, smokers who were veterans, New York City Fire Department employees and their household family members who smoked, smokers with previous quit attempts, smokers who were critically ill and smokers who were Medicare patients. Study-level characteristics can be found in tables in Report Supplementary Material 6.
Risk of bias in included studies
Randomised evidence
Ratings ranged from low to high risk of bias, and an overall risk of bias domain was rated by selecting the highest rating of bias across domains, with the exception of selective outcome reporting (as this domain was usually rated as unclear owing to inaccessibility of study protocols and limited trial registration). Risk-of-bias ratings by study and summarised across studies are presented in Report Supplementary Material 5 and Appendix 4, Figure 35, respectively.
Random sequence generation
Few trials were rated as being at high risk of bias for random sequence generation, with only 1% rated as being at high risk of bias. Conversely, over half (52%) of trials were rated as being at low risk of bias and the remaining 47% of trials were rated as being at unclear risk of bias.
Allocation concealment
As with random sequence generation, only 2% of trials were rated as being at high risk of bias for allocation concealment, whereas 42% were rated as being at low risk of bias and over half (56%) of trials were rated as being at unclear risk of bias.
Blinding of participants and personnel
Blinding of participants and personnel was rated as being at high risk of bias in 22% of trials, largely as a result of trials where drugs were delivered open label without any blinding. Ratings for the remaining trials were similarly split between low risk of bias (39%) and unclear risk of bias (39%).
Blinding of outcome assessment
Almost half (45%) of trials were rated as being at unclear risk of bias for blinding of outcome assessment domains, with 43% of trials rated as being at low risk of bias and 12% of trials rated as being at unclear risk of bias.
Incomplete outcome data
More than half (69%) of the trials were rated as being at low risk of bias for the incomplete outcome data domain, as loss to follow-up was either low or similar among trial arms. The remining trials were rated as being at unclear risk of bias (21%) or high risk of bias (10%).
Selective reporting
As previously mentioned, most (66%) of trials were rated as being at unclear risk of bias for selective reporting owing to a lack of study protocols or public trial registrations. One-third of studies (33%) were rated as being at low risk of bias and only 1% of trials were rated as being at high risk of bias.
Other bias
Nearly all (95%) trials were rated as being at low risk of bias for the other bias domain, with only 3% rated as being at unclear risk of bias and 2% rated as being at high risk of bias.
Overall bias
Finally, ratings for our overall risk of bias domain indicated that 16% of trials were rated as being at low risk of bias, 51% of trials were rated as being at unclear risk of bias and 33% of trials as being at high risk of bias.
Non-randomised evidence
Ratings ranged from low to high risk of bias and an overall risk-of-bias domain was rated by selecting the highest rating of bias across domains, with the exception of selective outcome reporting (as this domain was usually rated as unclear owing to inaccessibility of study protocols and limited study registration). Risk-of-bias ratings by study and summarised across studies are presented in Report Supplementary Material 7 and Appendix 4, Figure 36, respectively.
Given the non-randomised study designs, nearly all studies were rated as at high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment domains. This was as a result of the studies not randomising participants and commonly not concealing the allocation to study arms, and in most studies blinding was not used. Most studies (81%) of studies were rated as unclear for the incomplete outcome data domain, as the number of participants followed up was either not reported or not applicable in the study design. As previously mentioned, most (94%) of the studies were rated as unclear for selective reporting and 92% of studies were rated as low for the other bias domain. Finally, ratings for our overall risk of bias domain indicated that 100% of studies were rated as being at high risk as a result of the ratings for most studies across the first four domains.
Results on safety
We performed NMA on three safety outcomes: SAEs, MACEs and MANEs. We fitted a standard (full interaction) NMA model as well as fixed- and random-class NMA models for each outcome. Based on the model fit indices (see Appendix 7), we focused on fixed-class NMA models. Appendix 7, Tables 49 and 50 provide a list of the treatments delivered in the randomised trials and non-randomised studies included in safety analyses and their frequency, respectively. In this chapter we present results for each outcome based on a fixed-class NMA model; for additional results using other models, see Appendix 7. Results are presented as median ORs alongside 95% CrIs. A summary of results across outcomes is provided at the end of this chapter in the form of a rank-o-gram.
Serious adverse events
Randomised evidence only
Our primary safety outcome, SAEs, was reported in 111 studies with a total of 63,927 patients, of which 101 studies (58,318 patients) compared two or more of the treatment classes of interest. We excluded one study from all analyses [varenicline not specified (3/160 patients with event) vs. placebo (0/160 patients with event)] because of small event numbers causing convergence problems. Furthermore, two studies (bupropion standard plus NRT inhalator vs. usual care, and varenicline standard plus NRT gum standard vs. varenicline low plus NRT gum standard) were disconnected at the treatment level (see Figure 64), but connected to the main network at the class level (Figure 13). We excluded both studies when comparing the different models (see Table 51), but we were able to include them in the analyses discussed in this section (which report results at the class level).
FIGURE 13.
Network plot for SAEs at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Figure 14 displays results for the fixed-class NMA model based on 100 studies with placebo as a comparator. There was evidence that bupropion standard (OR 1.27, 95% CrI 1.04 to 1.58) increased the odds of SAEs compared with placebo.
FIGURE 14.
Forest plot with results of the fixed-class NMA model for SAEs. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Table 5 presents the class effect estimates with placebo as comparator obtained from the NMA (final column) alongside the estimates obtained from direct and indirect evidence. Direct evidence was available for most monotherapies, whereas comparisons of most combinations of interventions with placebo were obtained largely through indirect evidence only. Evidence for some of the main interventions (e.g. bupropion standard and varenicline standard) was informed by large trials that compared these drugs against placebo, so that indirect evidence did not add anything to the NMA results. When both direct and indirect evidence was available, results were mostly consistent, although there were some instances in which direct evidence suggested a larger increase in the odds of a SAE for the experimental drug (e.g. e-cigarette high vs. placebo).
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| No drug treatment | – | 1.11 (0.56 to 2.29) | 1.11 (0.56 to 2.29) |
| Usual care | – | 0.28 (0.02 to 2.20) | 0.28 (0.02 to 2.20) |
| NRT not specified | 1.19 (0.86 to 1.62) | 0.72 (0.31 to 1.68) | 1.12 (0.79 to 1.49) |
| NRT standard | 1.11 (0.74 to 1.68) | 1.37 (0.38 to 4.96) | 1.13 (0.76 to 1.67) |
| NRT high | 1.11 (0.77 to 1.58) | 1.40 (0.87 to 2.27) | 1.21 (0.89 to 1.63) |
| Bupropion low | 0.26 (0.03 to 1.52) | 0.37 (0.01 to 9.36) | 0.21 (0.01 to 1.57) |
| Bupropion standard | 1.27 (1.04 to 1.58) | – | 1.27 (1.04 to 1.58) |
| Varenicline low | 1.12 (0.54 to 2.29) | 1.62 (0.15 to 17.6) | 1.07 (0.40 to 2.29) |
| Varenicline standard | 1.09 (0.91 to 1.34) | – | 1.09 (0.91 to 1.34) |
| E-cigarette low | – | 7.24 (0.46 to 3262) | 7.24 (0.46 to 3262) |
| E-cigarette high | 1.99 (0.90 to 4.44) | 1.44 (0.61 to 3.42) | 1.72 (0.77 to 2.89) |
| Bupropion low plus NRT high | – | 1.19 (0.24 to 7.46) | 1.19 (0.24 to 7.46) |
| Bupropion standard plus NRT not specified | – | 0.49 (0.03 to 3.35) | 0.49 (0.03 to 3.35) |
| Bupropion standard plus NRT high | 0.73 (0.05 to 4.18) | 1.57 (0.47 to 5.28) | 1.31 (0.49 to 3.67) |
| Varenicline low plus NRT standard | – | 6.62 (0.09 to 4964) | 6.62 (0.09 to 4964) |
| Varenicline standard plus NRT standard | – | 1.38 (0.33 to 9.78) | 1.38 (0.33 to 9.78) |
| Varenicline standard plus NRT high | – | 1.11 (0.29 to 3.49) | 1.11 (0.29 to 3.49) |
| Varenicline standard plus bupropion standard | 2.51 (0.73 to 9.49) | 1.48 (0.57 to 3.84) | 1.79 (0.86 to 4.06) |
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 7, Table 51 and Figures 66 and 67). The pairwise comparisons between active interventions for this outcome are presented in Table 6. Most effect estimates were informed by indirect evidence only. As a consequence of this, and also the small event rates reported, effects were imprecisely estimated and all intervals included the null.
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| Bupropion standard vs. NRT standard | – | 1.12 (0.72 to 1.80) | 1.12 (0.72 to 1.80) |
| Varenicline standard vs. NRT standard | – | 0.98 (0.62 to 1.50) | 0.98 (0.62 to 1.50) |
| E-cigarette low vs. NRT standard | – | 6.48 (0.39 to 3087) | 6.48 (0.39 to 3087) |
| E-cigarette high vs. NRT standard | – | 1.54 (0.58 to 2.90) | 1.54 (0.58 to 2.90) |
| Varenicline standard plus NRT standard vs. NRT standard | – | 1.23 (0.28 to 8.39) | 1.23 (0.28 to 8.39) |
| Varenicline standard plus bupropion standard vs. NRT standard | – | 1.60 (0.69 to 3.82) | 1.60 (0.69 to 3.82) |
| Varenicline standard vs. bupropion standard | – | 0.86 (0.67 to 1.11) | 0.86 (0.67 to 1.11) |
| E-cigarette low vs. bupropion standard | – | 5.75 (0.37 to 2419) | 5.75 (0.37 to 2419) |
| E-cigarette high vs. bupropion standard | – | 1.34 (0.69 to 2.28) | 1.34 (0.69 to 2.28) |
| Varenicline standard plus NRT standard vs. bupropion standard | – | 1.10 (0.25 to 8.09) | 1.10 (0.25 to 8.09) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 1.39 (0.67 to 3.20) | 1.39 (0.67 to 3.20) |
| E-cigarette low vs. varenicline standard | – | 6.36 (0.42 to 2879) | 6.36 (0.42 to 2879) |
| E-cigarette high vs. varenicline standard | – | 1.56 (0.74 to 2.67) | 1.56 (0.74 to 2.67) |
| Varenicline standard plus NRT standard vs. varenicline standard | 1.28 (0.30 to 7.77) | – | 1.28 (0.30 to 7.77) |
| Varenicline standard plus bupropion standard vs. varenicline standard | 1.24 (0.49 to 3.34) | 2.55 (0.78 to 8.31) | 1.64 (0.80 to 3.59) |
| E-cigarette high vs. e-cigarette low | – | 0.23 (0.00 to 3.47) | 0.23 (0.00 to 3.47) |
| Varenicline standard plus NRT standard vs. e-cigarette low | – | 0.21 (0.00 to 5.40) | 0.21 (0.00 to 5.40) |
| Varenicline standard plus bupropion standard vs. e-cigarette low | – | 0.27 (0.00 to 4.28) | 0.27 (0.00 to 4.28) |
| Varenicline standard plus NRT standard vs. e-cigarette high | – | 0.83 (0.17 to 5.52) | 0.83 (0.17 to 5.52) |
| Varenicline standard plus bupropion standard vs. e-cigarette high | – | 1.07 (0.42 to 3.31) | 1.07 (0.42 to 3.31) |
| Varenicline standard plus bupropion standard vs. varenicline standard plus NRT standard | – | 1.33 (0.19 to 6.31) | 1.33 (0.19 to 6.31) |
With regard to effect modifiers, there was inconclusive evidence of effect modification according to industry sponsorship, type of placebo, treatment duration, counselling, dependence score, comorbid samples, studies in which patients were not required to be willing to quit, smokeless-tobacco users, analyses restricted to samples of heavy smokers, and publication year (see Figures 69–80); sensitivity analysis excluding studies at high risk of bias yielded the same findings reported in this section, although with wider intervals for most effect estimates (see Figure 68; full results provided in Appendix 7). A sensitivity analysis excluding studies that compared pharmacological interventions with psychological interventions (that were not given on all arms of the study) was very similar to that in the main analysis (see Appendix 7).
Threshold analysis
The results of the threshold analyses for the first- and last-ranked treatments for SAEs are shown in Figures 15 and 16. The first-ranked treatment is placebo and the last-ranked is e-cigarette low. Both figures also include the risk-of-bias judgements from Report Supplementary Material 5.
FIGURE 15.
Threshold analysis results for SAEs (first-ranked treatment), sorted by size of threshold (smallest to largest). Only studies with thresholds < 3 log-OR are shown, for brevity. Treatment codes are (1) placebo, (7) NRT standard, (11) bupropion standard, (14) varenicline standard, (17) e-cigarette low, (18) e-cigarette high, (26) varenicline standard plus NRT standard and (28) varenicline plus bupropion standard (for a full list, see Appendix 6). Bold study labels and red shaded invariant intervals show where a 95% CI crosses the corresponding threshold, indicating sensitivity to the level of uncertainty in this estimate. AC, allocation concealment; BOA, blinding of outcome assessment; BPP, blinding of participants and personnel; IOD, incomplete outcome data; OB, other bias; RSG, random sequence generation; SR, selective reporting. ‘+’, low risk of bias; ‘–’, high risk of bias; ‘?’, unclear risk of bias. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

FIGURE 16.
Threshold analysis results for SAEs (last-ranked treatment), sorted by size of threshold (smallest to largest). Only studies with thresholds < 3 log-OR are shown, for brevity. Treatment codes are (1) placebo, (7) NRT standard, (11) bupropion standard, (14) varenicline standard, (17) e-cigarette low, (18) e-cigarette high, (26) varenicline standard plus NRT standard and (28) varenicline plus bupropion standard (for a full list, see Appendix 6). Bold study labels and red shaded invariant intervals show where a 95% CI crosses the corresponding threshold, indicating sensitivity to the level of uncertainty in this estimate. AC, allocation concealment; BOA, blinding of outcome assessment; BPP, blinding of participants and personnel; IOD, incomplete outcome data; OB, other bias; NT, no threshold; RSG, random sequence generation; SR, selective reporting. ‘+’, low risk of bias; ‘–’, high risk of bias; ‘?’, unclear risk of bias. This figure is reproduced with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
The first-place ranking (of placebo) is very sensitive to the level of uncertainty in the evidence, as shown by the number of study estimates with 95% CIs that cross the corresponding threshold. At each of these thresholds, the new first-ranked treatment is NRT standard, varenicline standard plus NRT standard, e-cigarette high, e-cigarette low, or varenicline plus bupropion standard. Five studies351,386,415,444,468 have thresholds < 0.7 log-OR (equivalent to a factor of 2 on the OR scale), and, of these, only one468 is rated as being at low risk of bias.
The last-place ranking (of e-cigarette low) is sensitive to the level of uncertainty from only one study525 (e-cigarette low vs. no drug treatment). The first-place ranking is also sensitive to the level of uncertainty in this study estimate. If the estimate were to change by –0.80 log-OR from 1.32 to 0.52 in favour of e-cigarette low, then e-cigarette low would no longer be ranked last (replaced by varenicline plus bupropion standard), and if the estimate were to change only a little more to 0.24, then e-cigarette low would be ranked first for SAEs. Owing to the high level of uncertainty in this study estimate and the resulting wide CI, both of these are plausible owing to sampling error. Cravo et al. 525 has the smallest threshold for the last-place ranking (–0.80 log-OR, equivalent to a factor of 2.2 on the OR scale) and was judged to be at high risk of bias owing to inadequate blinding and other bias. The remaining thresholds for the other 122 study estimates are equivalent to a factor of ≥ 8 on the OR scale, which may be larger than any plausible biases.
Overall, the first- and last-place rankings for SAEs are not very robust: both are sensitive to the level of uncertainty in the data, and there may be plausible biases in studies at high or unclear risk of bias that could result in a change of first- or last-place ranking. The Cravo et al. 525 study is notably influential for both first- and last-place rankings, displays high levels of uncertainty and is rated as being at high risk of bias. The last-place ranking is likely to be robust to plausible biases in all other studies.
Incorporating non-randomised evidence
Only one non-randomised study632 reported one or more SAEs. This was a three-arm study comparing e-cigarette standard (14 events in 343 patients), dual smoking (10 events in 319 patients) and no drug treatment (14 events in 693 patients). The network plots at the treatment level and at the class level, combining randomised and non-randomised evidence for this outcome, are presented in Appendix 7, Figure 65 and Figure 17, respectively.
FIGURE 17.
Network plot for SAEs, incorporating non-randomised evidence at class level. Ns, not specified; std, standard.

The statistical integration was identical to that restricted to randomised evidence, except for the addition of the non-randomised study. The results for the fixed-class random-effects NMA, based on 102 studies and 59,673 smokers, are displayed in Figure 18. Comparison with Figure 14 suggests that, although only one study was added, the effect estimates changed substantially, now suggesting that varenicline standard (OR 0.63, 95% CrI 0.45 to 0.64) and e-cigarette low (OR 0.01, 95% CrI 0.00 to 0.15) might lead to lower odds of SAEs than placebo. The notable influence of the study added might be because of its contribution of 38 events to a network focused on a rare outcome. Furthermore, the non-randomised study, conducted by Manzoli et al. ,632 found that no drug treatment might be the safest of the three interventions compared. Given that no drug treatment is one of the main comparators in the network, this finding from a single study is likely to have an impact on the effect estimates for many interventions. In summary, this analysis illustrates the difficulties of drawing solid conclusions about the relative safety of different smoking interventions from the currently available evidence on SAEs.
FIGURE 18.
Forest plot displaying the NMA results for SAEs, combining randomised and non-randomised evidence. Ns, not specified; std, standard.

Major adverse cardiovascular events
Randomised evidence only
A total of 49 studies (38,329 patients) reported MACEs, with 44 studies (36,231 patients) including at least one relevant comparison. The structure of this network is presented at the treatment (see Figure 81) and at the class (Figure 19) level. We discarded three studies from all analyses owing to small numbers causing convergence problems, namely one study comparing varenicline not specified (1/160 patients with event) with placebo (0/160 patients with event), one study comparing bupropion standard plus NRT inhalator not specified (0/267 patients with event) with usual care (1/271 patients with event) and one study comparing NRT not specified (0/61 patients with event) with usual care (5/61 patients with event). Furthermore, one further study comparing NRT gum standard (25/3923 patients with event) with usual care (12/1964 patients with event) was disconnected from the network and, hence, was excluded from any further analyses.
FIGURE 19.
Network plot for major adverse cardiovascular events at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
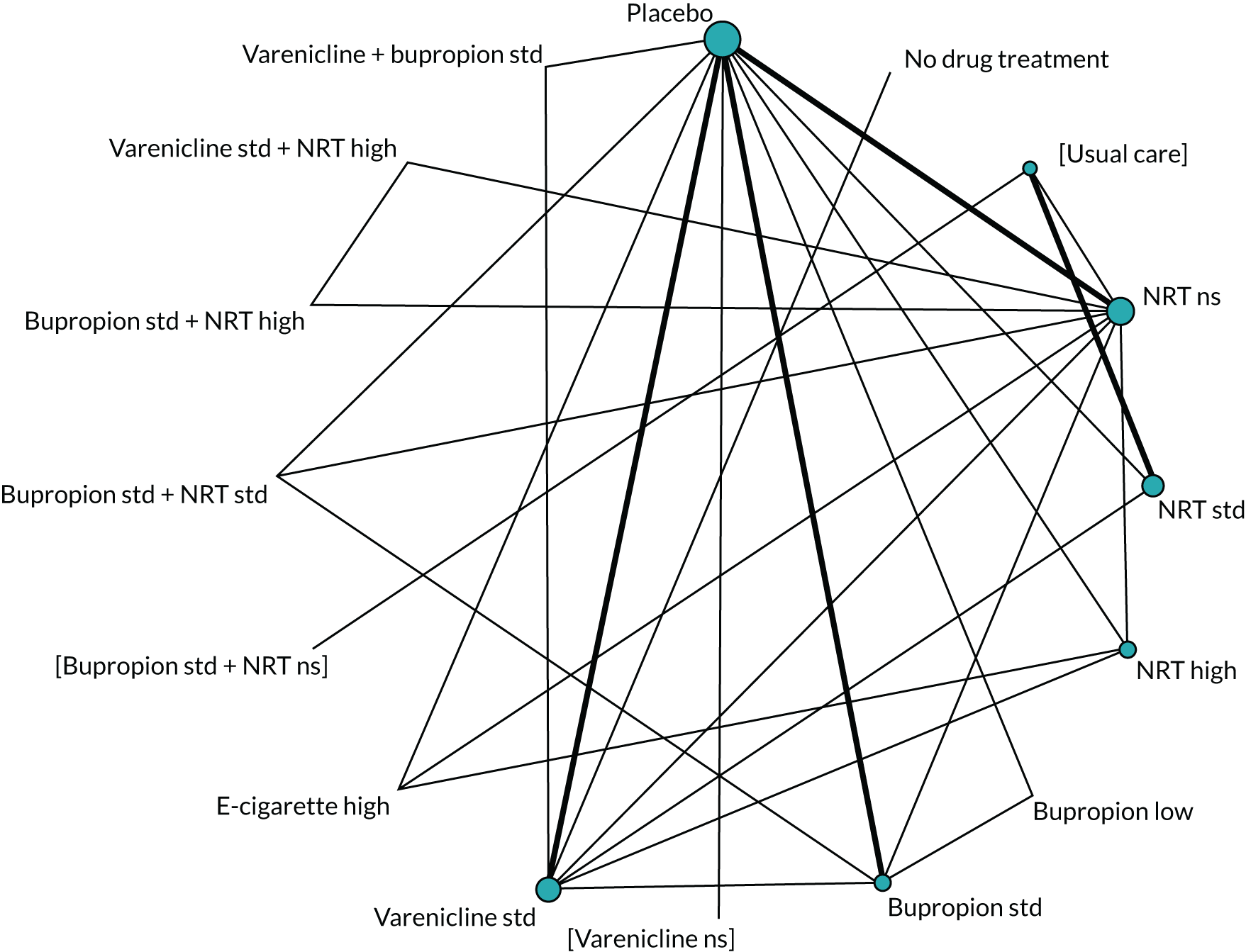
Therefore, the NMA for this outcome was based on 41 studies. The results with placebo as a comparator are presented in Figure 20. Owing to the small numbers of events reported across studies, all effect estimates show very wide intervals and, hence, it was not possible to identify differences among any pair of interventions.
FIGURE 20.
Forest plot with results of the fixed-class NMA model for MACEs. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
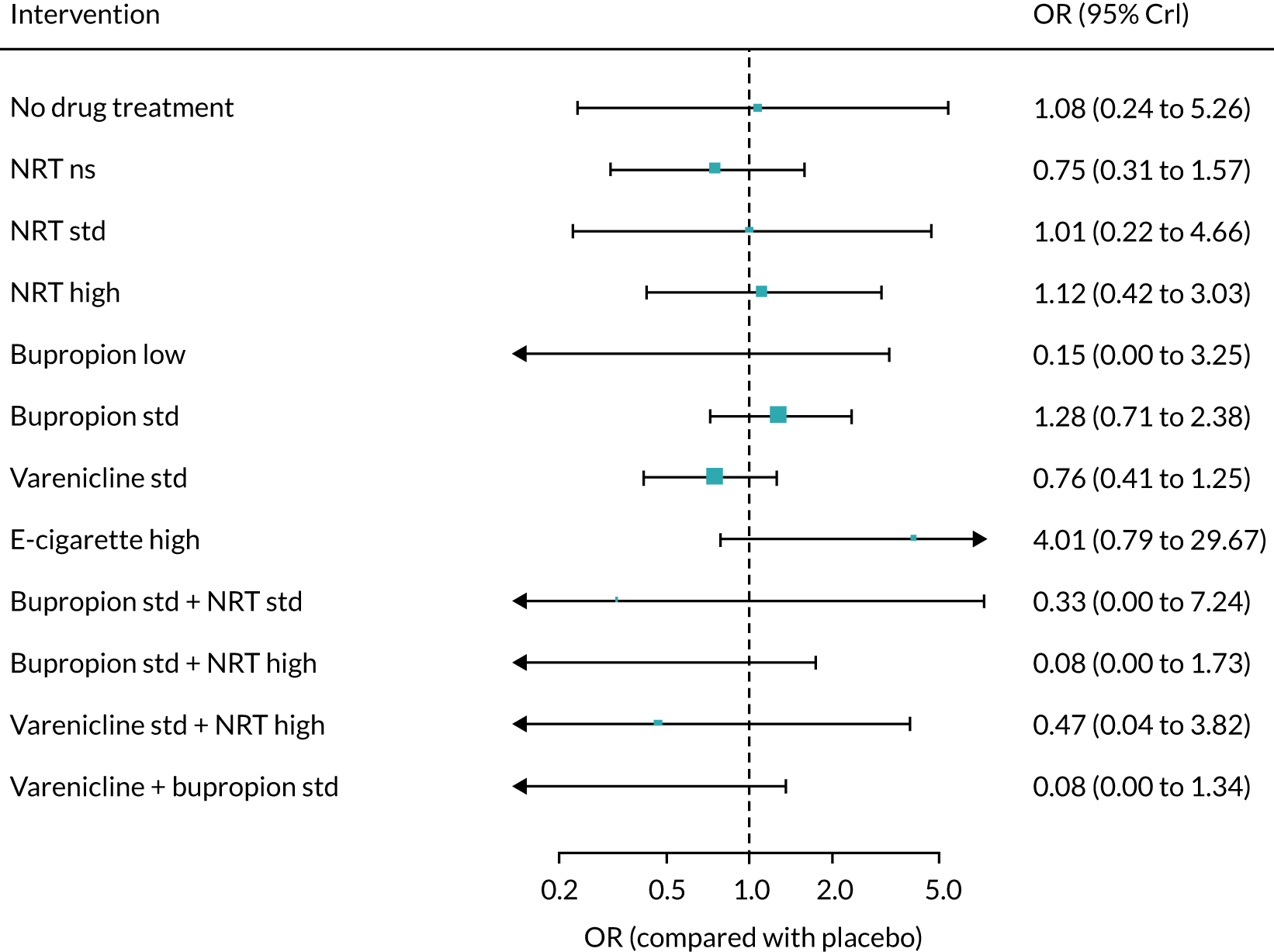
Table 7 presents the NMA results with placebo as comparator alongside effect estimates from direct and/or indirect evidence, where available. Although intervals were wide and always included the null, NMA estimates were generally more precise when both direct and indirect evidence were available.
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| No drug treatment | – | 1.08 (0.24 to 5.26) | 1.08 (0.24 to 5.26) |
| NRT not specified | 0.82 (0.30 to 2.03) | 0.61 (0.15 to 2.52) | 0.75 (0.31 to 1.57) |
| NRT standard | 1.28 (0.21 to 8.67) | 0.57 (0.03 to 9.47) | 1.01 (0.22 to 4.66) |
| NRT high | 0.44 (0.11 to 1.75) | 2.98 (0.71 to 12.5) | 1.12 (0.42 to 3.03) |
| Bupropion low | 0.15 (0.00 to 3.25) | – | 0.15 (0.00 to 3.25) |
| Bupropion standard | 1.31 (0.68 to 2.48) | 0.95 (0.08 to 11.0) | 1.28 (0.71 to 2.36) |
| Varenicline standard | 0.76 (0.41 to 1.25) | – | 0.76 (0.41 to 1.25) |
| E-cigarette high | 2.61 (0.44 to 28.22) | 26.8 (0.39 to 1860) | 4.01 (0.79 to 29.7) |
| Bupropion standard plus NRT standard | 0.33 (0.00 to 7.24) | – | 0.33 (0.00 to 7.24) |
| Bupropion standard plus NRT high | – | 0.08 (0.00 to 1.73) | 0.08 (0.00 to 1.73) |
| Varenicline standard plus NRT high | – | 0.47 (0.04 to 3.82) | 0.47 (0.04 to 3.82) |
| Varenicline standard plus bupropion standard | 0.08 (0.00 to 1.34) | – | 0.08 (0.00 to 1.34) |
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 7, Table 52 and Figures 83 and 84). The pairwise comparisons between active interventions presented in Table 8 were almost entirely obtained through indirect evidence only. All effect estimates had wide intervals including the null, and some effects were very imprecisely estimated. There was inconclusive evidence of effect modification based on comorbidities (see Figure 85) and smoking level (see Figure 86).
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| Bupropion standard vs. NRT standard | – | 1.26 (0.26 to 6.15) | 1.26 (0.26 to 6.15) |
| Varenicline standard vs. NRT standard | 0.99 (0.03 to 49.3) | 0.68 (0.12 to 3.84) | 0.73 (0.15 to 3.39) |
| E-cigarette high vs. NRT standard | – | 4.35 (0.42 to 43.1) | 4.35 (0.42 to 43.1) |
| Bupropion standard plus NRT standard vs. NRT standard | – | 0.33 (0.00 to 9.83) | 0.33 (0.00 to 9.83) |
| Varenicline standard plus bupropion standard vs. NRT standard | – | 0.07 (0.00 to 1.96) | 0.07 (0.00 to 1.96) |
| Varenicline standard vs. bupropion standard | – | 0.58 (0.27 to 1.19) | 0.58 (0.27 to 1.19) |
| E-cigarette high vs. bupropion standard | – | 3.17 (0.57 to 25.3) | 3.17 (0.57 to 25.3) |
| Bupropion standard plus NRT standard vs. bupropion standard | – | 0.26 (0.00 to 5.50) | 0.26 (0.00 to 5.50) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 0.06 (0.00 to 1.14) | 0.06 (0.00 to 1.14) |
| E-cigarette high vs. varenicline standard | – | 5.51 (1.05 to 40.1) | 5.51 (1.05 to 40.1) |
| Bupropion standard plus NRT standard vs. varenicline standard | – | 0.44 (0.00 to 10.4) | 0.44 (0.00 to 10.4) |
| Varenicline standard plus bupropion standard vs. varenicline standard | – | 0.11 (0.00 to 1.88) | 0.11 (0.00 to 1.88) |
| Bupropion standard plus NRT standard vs. e-cigarette high | – | 0.07 (0.00 to 2.97) | 0.07 (0.00 to 2.97) |
| Varenicline standard plus bupropion standard vs. e-cigarette high | – | 0.02 (0.00 to 0.55) | 0.02 (0.00 to 0.55) |
| Varenicline standard plus bupropion standard vs. bupropion standard plus NRT standard | – | 0.20 (0.00 to 567) | 0.20 (0.00 to 567) |
Incorporating non-randomised evidence
A total of 10 non-randomised studies reported at least one major adverse cardiovascular event. The treatments examined in these studies, alongside the arm sizes and events per arm, are listed in Table 9. Furthermore, the network plots resulting from combining randomised and non-randomised evidence for this outcome are displayed in Appendix 7, Figure 82 (treatment level) and Figure 21 (class level). The disparity between sizes of the nodes for different treatments and classes stems from the very large numbers of smokers enrolled in some non-randomised studies (see Table 9).
| Study (first author and year) | Treatment | Arm size | Number of events |
|---|---|---|---|
| Manzoli 2015632 | Dual smoking | 319 | 2 |
| Manzoli 2015632 | E-cigarette standard | 343 | 4 |
| Manzoli 2015632 | No drug treatment | 693 | 2 |
| Davies 2015609 | Varenicline not specified | 41,742 | 531 |
| Davies 2015609 | NRT choice not specified | 84,976 | 160 |
| Ferketich 2013615 | NRT combination high | 110 | 1 |
| Ferketich 2013615 | Varenicline standard | 118 | 0 |
| Kotz 2017629 | Bupropion not specified | 350 | 155 |
| Kotz 2017629 | Varenicline not specified | 3574 | 3 |
| Kotz 2017629 | NRT not specified | 10,426 | 34 |
| Kotz 201557 | Bupropion not specified | 6557 | 2148 |
| Kotz 201557 | Varenicline not specified | 51,450 | 52 |
| Kotz 201557 | NRT not specified | 106,759 | 594 |
| Woolf 2012651 | NRT choice not specified | 184 | 8 |
| Woolf 2012651 | No drug treatment | 479 | 23 |
| Svanström 2012648 | Varenicline not specified | 17,926 | 16 |
| Svanström 2012648 | Bupropion not specified | 17,926 | 21 |
| Graham 2014618 | Bupropion standard | 14,133 | 216 |
| Graham 2014618 | Varenicline not specified | 74,824 | 44 |
| Panos 2010637 | No drug treatment | 113 | 3 |
| Panos 2010637 | NRT patch (24 hours) not specified | 114 | 6 |
| Deniz 2016611 | Bupropion not specified | 47 | 1 |
| Deniz 2016611 | Varenicline not specified | 94 | 0 |
FIGURE 21.
Network plot for major adverse cardiovascular events (including randomised and non-randomised studies) at class level. Ns, not specified; std, standard.

The combination of both types of evidence enabled the inclusion of 54 studies in the NMA, as the incorporation of 10 non-randomised studies also made it possible to include some randomised trials that had been excluded from the main analyses owing to small numbers causing convergence problems. The results of this combined analysis, based on a fixed-class random-effects model, are presented in Figure 22 and suggest that, even with the additional studies, substantial uncertainty remains about the relative safety of the different treatment classes for this outcome.
FIGURE 22.
Forest plot with results for major adverse cardiovascular events (combining randomised and non-randomised evidence). Ns, not specified; std, standard.

Major adverse neuropsychiatric events
Randomised evidence only
Major adverse neuropsychiatric events were reported in 75 studies (42,088 patients), with 73 studies (41,483 patients) including at least one relevant comparison. The structure of this network is presented at the treatment (see Figure 87) and the class (Figure 23) levels, which show that placebo (main comparator), NRT not specified, bupropion standard, and varenicline standard were the best represented interventions. One study comparing bupropion standard plus NRT inhalator not specified (1/267 patients with event) with usual care (1/271 patients with event) was disconnected from the treatment network and, hence, we excluded it from the model comparisons (see Table 53), although we were able to include it in the analyses reported in this section. Moreover, we excluded two studies from all analyses owing to small numbers causing convergence problems. One of these excluded studies compared NRT nasal spray standard (1/506 patients with event) with placebo (0/255 patients with event), while the other one compared e-cigarette high (2/440 patients with event) with NRT choice not specified (0/448 patients with event).
FIGURE 23.
Network plot for major adverse neuropsychiatric events at class level. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

The NMA for this outcome was based on 71 studies. The results with placebo as a comparator are presented in Figure 24 and show wide intervals around the effect estimates owing to small numbers. There was weak evidence that patients randomised to no drug treatment (OR 0.27, 95% CrI 0.05 to 1.05) and strong evidence that those randomised to waitlist (OR 0.03, 95% CrI 0.00 to 0.44) were less likely to report MANEs than those allocated to placebo. Regarding active treatments, there was evidence that patients who received NRT not specified (OR 0.60, 95% CrI 0.36 to 0.89), bupropion standard (OR 0.67, 95% CrI 0.47 to 0.91), bupropion standard plus NRT high (OR 0.21, 95% CrI 0.03 to 0.92) were less likely to report MANEs than those treated with placebo. There was weak evidence for patients randomised to varenicline standard plus bupropion standard (OR 0.15, 95% CrI 0.01 to 1.12) compared with placebo.
FIGURE 24.
Forest plot with results of the fixed-class NMA model for major adverse neuropsychiatric events. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
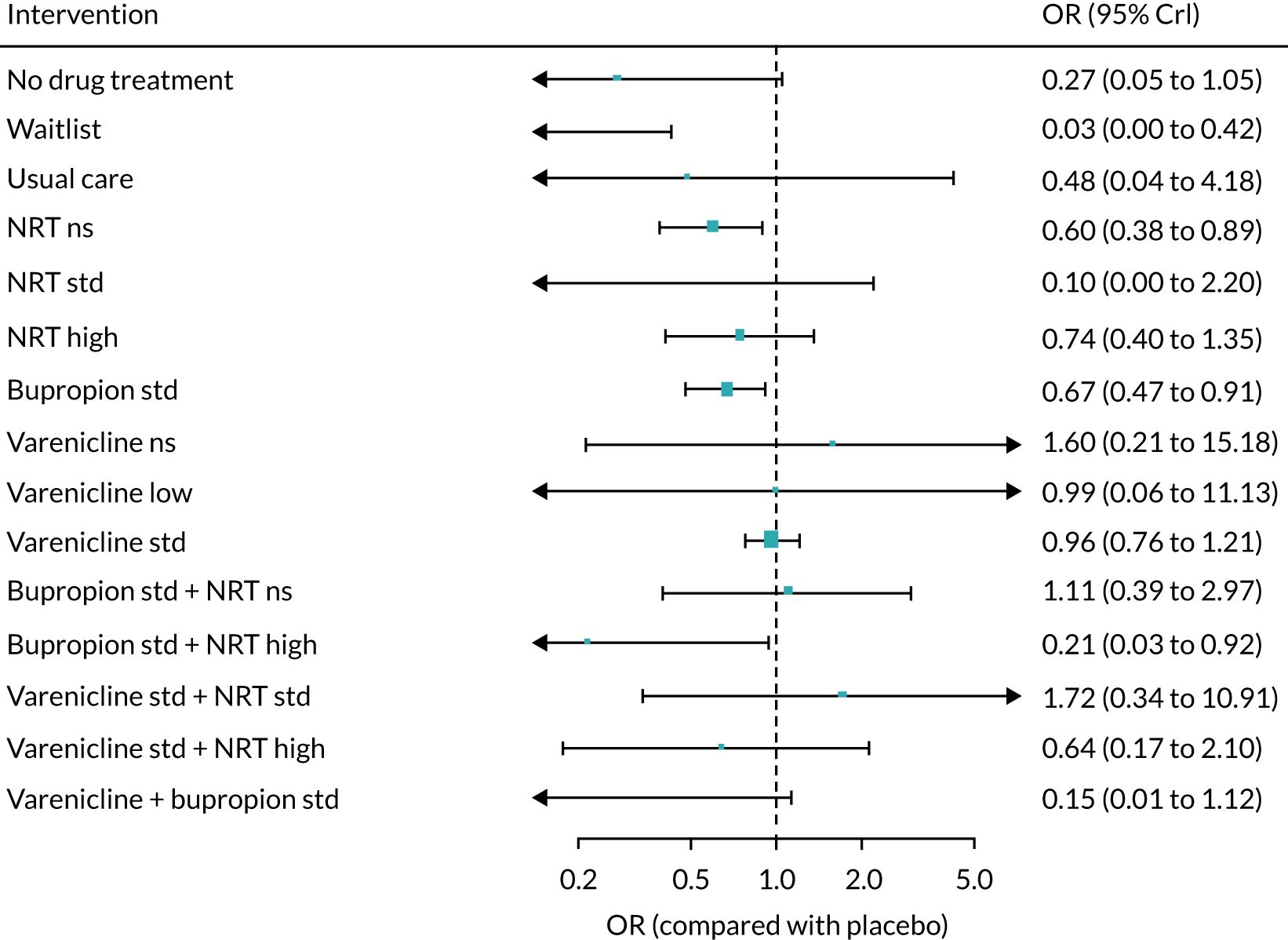
Table 10 presents the NMA results with placebo as comparator alongside effect estimates from direct and/or indirect evidence where available. Most comparisons were informed by direct or indirect evidence only, and, where back-calculation of indirect evidence was possible, this led to very imprecise and uninformative estimates.
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| No drug treatment | – | 0.27 (0.05 to 1.05) | 0.27 (0.05 to 1.05) |
| Waitlist | 0.03 (0.00 to 0.42) | – | 0.03 (0.00 to 0.42) |
| Usual care | – | 0.48 (0.04 to 4.18) | 0.48 (0.04 to 4.18) |
| NRT not specified | 0.73 (0.49 to 1.09) | 5.16 (1.43 to 18.7) | 0.60 (0.38 to 0.89) |
| NRT standard | 0.12 (0.00 to 1.93) | 0.04 (0.00 to 5506) | 0.10 (0.00 to 2.20) |
| NRT high | 0.73 (0.38 to 1.38) | 0.80 (0.14 to 4.70) | 0.74 (0.40 to 1.35) |
| Bupropion standard | 0.62 (0.44 to 0.82) | 0.47 (0.25 to 0.87) | 0.67 (0.47 to 0.91) |
| Varenicline not specified | 1.60 (0.21 to 15.2) | – | 1.60 (0.21 to 15.2) |
| Varenicline low | 0.99 (0.06 to 11.1) | – | 0.99 (0.06 to 11.1) |
| Varenicline standard | 0.96 (0.76 to 1.21) | – | 0.96 (0.76 to 1.21) |
| Bupropion standard plus NRT not specified | 2.39 (0.17 to 22.7) | 0.94 (0.31 to 2.83) | 1.11 (0.39 to 2.97) |
| Bupropion standard plus NRT high | – | 0.21 (0.03 to 0.92) | 0.21 (0.03 to 0.92) |
| Varenicline standard plus NRT standard | – | 1.72 (0.34 to 10.9) | 1.72 (0.34 to 10.9) |
| Varenicline standard plus NRT high | – | 0.64 (0.17 to 2.10) | 0.64 (0.17 to 2.10) |
| Varenicline standard plus bupropion standard | 0.06 (0.00 to 1.05) | 0.23 (0.01 to 5.97) | 0.15 (0.01 to 1.12) |
There was no statistical evidence of inconsistency based on model fit statistics (see Appendix 7, Table 53 and Figures 89 and 90). Pairwise comparisons between active interventions are displayed in Table 11. Although most effect estimates were imprecisely estimated owing to small numbers, there was evidence of increased odds of MANEs for smokers randomised to varenicline standard compared with those allocated to bupropion standard (OR 1.43, 95% CrI 1.02 to 2.09). There was inconclusive evidence of effect modification based on psychiatric comorbidities (see Figure 91).
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| Bupropion standard vs. NRT standard | – | 6.55 (0.29 to 3523) | 6.55 (0.29 to 3523) |
| Varenicline standard vs. NRT standard | – | 9.43 (0.43 to 4989) | 9.43 (0.43 to 4989) |
| Varenicline standard plus NRT standard vs. NRT standard | – | 18.52 (0.49 to 10,312) | 18.52 (0.49 to 10,312) |
| Varenicline standard plus bupropion standard vs. NRT standard | – | 1.45 (0.02 to 950) | 1.45 (0.02 to 950) |
| Varenicline standard vs. bupropion standard | – | 1.43 (1.02 to 2.09) | 1.43 (1.02 to 2.09) |
| Varenicline standard plus NRT standard vs. bupropion standard | – | 2.58 (0.49 to 16.6) | 2.58 (0.49 to 16.6) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 0.22 (0.01 to 1.73) | 0.22 (0.01 to 1.73) |
| Varenicline standard plus NRT standard vs. varenicline standard | 1.80 (0.35 to 11.2) | – | 1.80 (0.35 to 11.2) |
| Varenicline standard plus bupropion standard vs. varenicline standard | 0.22 (0.00 to 5.43) | 0.12 (0.00 to 3.20) | 0.15 (0.01 to 1.16) |
| Varenicline standard plus bupropion standard vs. varenicline standard plus NRT standard | – | 0.08 (0.00 to 1.19) | 0.08 (0.00 to 1.19) |
Incorporating non-randomised evidence
A total of 16 non-randomised studies reported one or more major adverse neuropsychiatric events. The treatments compared in those studies, the arm sizes and the event counts are presented in Table 12, whereas the network plots combining both types of evidence at the treatment level and class level are displayed in Appendix 7, Figure 88 and Figure 25, respectively.
| Study (first author and year) | Treatment | Arm size | Number of events |
|---|---|---|---|
| Cunningham 2016608 | Varenicline not specified | 11,774 | 6 |
| Cunningham 2016608 | NRT patch (24 hours) not specified | 23,548 | 12 |
| Dhelaria 2012612 | Varenicline not specified | 171 | 2 |
| Dhelaria 2012612 | NRT not specified | 200 | 0 |
| Ferketich 2013615 | NRT combination high | 110 | 0 |
| Ferketich 2013615 | Varenicline standard | 118 | 1 |
| Kotz 2017629 | NRT not specified | 10,426 | 722 |
| Kotz 2017629 | Bupropion not specified | 350 | 18 |
| Kotz 2017629 | Varenicline not specified | 3574 | 176 |
| Jiménez-Ruiz 2017624 | NRT combination high | 215 | 0 |
| Jiménez-Ruiz 2017624 | Varenicline standard | 134 | 1 |
| Hodgkin 2013619 | NRT choice not specified | 236 | 0 |
| Hodgkin 2013619 | Bupropion not specified plus NRT choice not specified | 162 | 3 |
| Hodgkin 2013619 | Varenicline not specified plus NRT choice not specified | 16 | 2 |
| Jiménez Ruiz 2012623 | NRT choice not specified | 233 | 5 |
| Jiménez Ruiz 2012623 | Bupropion standard plus NRT choice not specified | 45 | 1 |
| Jiménez Ruiz 2012623 | Varenicline standard plus NRT choice not specified | 190 | 6 |
| Kaduri 2015625 | Varenicline not specified | 98 | 10 |
| Kaduri 2015625 | NRT choice not specified | 98 | 8 |
| Kotz 201557 | NRT not specified | 106,759 | 8814 |
| Kotz 201557 | Bupropion not specified | 6557 | 377 |
| Kotz 201557 | Varenicline not specified | 51,450 | 2514 |
| Thomas 201353 | NRT not specified | 81,545 | 874 |
| Thomas 201353 | Bupropion not specified | 6741 | 44 |
| Thomas 201353 | Varenicline not specified | 31,260 | 276 |
| Gunnell 200954 | NRT choice not specified | 63,265 | 1824 |
| Gunnell 200954 | Bupropion not specified | 6422 | 162 |
| Gunnell 200954 | Varenicline not specified | 10,973 | 297 |
| Garcia-Portilla 2016616 | NRT patch (24 hours) not specified | 36 | 0 |
| Garcia-Portilla 2016616 | Varenicline standard | 39 | 1 |
| Koçak 2015627 | Varenicline not specified | 206 | 4 |
| Koçak 2015627 | Bupropion not specified | 137 | 2 |
| Koçak 2015627 | NRT patch (24 hours) not specified | 112 | 0 |
| Stapleton 2008646 | NRT choice not specified | 204 | 2 |
| Stapleton 2008646 | Varenicline not specified | 208 | 10 |
| Pasternak 2013638 | Varenicline not specified | 59,790 | 4 |
| Pasternak 2013638 | Bupropion not specified | 17,936 | 1 |
| Shiltz 2012645 | Bupropion standard plus NRT patch (24 hours) standard | 121 | 18 |
| Shiltz 2012645 | Varenicline standard plus bupropion standard | 204 | 12 |
| Shiltz 2012645 | Varenicline standard | 164 | 19 |
FIGURE 25.
Network plot for major adverse neuropsychiatric events (combining randomised and non-randomised evidence) at class level. Ns, not specified; std, standard.

We fitted a fixed-class random-effects NMA model combining randomised and non-randomised evidence for this outcome, which led to the inclusion of 89 studies in total. The results displayed in Figure 26 suggest that bupropion standard (OR 0.68, 95% CrI 0.50 to 0.91), bupropion standard plus NRT high (OR 0.23, 95% CrI 0.04 to 0.93) and varenicline standard plus bupropion standard (OR 0.36, 95% CrI 0.15 to 0.89) are associated with lower odds of events than placebo. Some of the effect estimates were imprecisely estimated (e.g. e-cigarette high vs. placebo), and there was also one extreme result for varenicline not specified plus NRT not specified, stemming from a single small study in which 2 out of 16 smokers treated with this combination reported an event (see Table 12).
FIGURE 26.
Forest plot of NMA results for major adverse neuropsychiatric events (combining randomised and non-randomised evidence). Ns, not specified; std, standard.

Ranking of interventions
Table 13 presents ranks for a selection of classes according to the primary safety outcome, SAEs. Placebo yielded the largest probability of being ranked as the best intervention for reducing the odds of SAEs (0.33) and also showed the lowest mean rank (1.97). Varenicline standard plus NRT standard showed the next largest probability to be ranked best (0.31), but the mean rank for this intervention (4.41) suggests substantial uncertainty about its ranking. On the other hand, the highest mean ranks were obtained by e-cigarette low (6.97), varenicline standard plus bupropion standard (5.94), and e-cigarette high (5.73), suggesting that these interventions were least likely to be ranked highly for reducing the occurrence of SAEs.
| Intervention | Pr(best) | Mean rank |
|---|---|---|
| Placebo | 0.33 | 1.97 |
| NRT standard | 0.17 | 3.4 |
| Bupropion standard | 0 | 4.5 |
| Varenicline standard | 0.06 | 3.08 |
| E-cigarette low | 0.07 | 6.97 |
| E-cigarette high | 0.04 | 5.73 |
| Varenicline standard plus NRT standard | 0.31 | 4.41 |
| Varenicline standard plus bupropion standard | 0.03 | 5.94 |
Figure 27 is a rank-o-gram displaying the ranking of eight selected intervention classes across the three safety outcomes examined in our NMA models. Placebo was most likely to be ranked best or second best out of eight interventions for SAEs, with lower rankings for MACEs (4/7) and MANEs (5/7). NRT standard was also most likely to be ranked among the best two interventions to reduce the odds of SAEs, with uncertain rankings for the other adverse outcomes. However, these findings may not be robust because of the uncertainty associated with SAE estimates. The rankings for MACEs suggest that bupropion standard plus NRT standard, and varenicline standard plus bupropion standard might be the safest interventions, although we note that these rankings are based on rather imprecise effect estimates (see Figure 20). The latter statement also applies to MANEs (see Figure 24).
FIGURE 27.
Rank-o-gram of interventions across safety outcomes. Std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Tertiary and other outcomes
We now present the results for tertiary and other outcomes. The results of NMAs for nausea, headache, dry mouth and skin rash are presented using RCTs identified in the original search. Tables for all other AEs of interest are presented in Report Supplementary Material 8 for events reported in RCTs and in Report Supplementary Material 9 for events reported in non-randomised studies.
Nausea
The data set for nausea comprised 168 studies, with a total of 398 arms and 72,875 patients. Appendix 8, Figure 92, shows the structure of this network at the treatment level. The graph shows a large number of interventions that were mostly connected and had placebo as the main comparator. One study comparing varenicline standard plus NRT gum standard (17/245 patients with event) with varenicline low plus NRT gum standard (13/240 patients with event) was disconnected from the network and, therefore, was excluded from the analyses. Furthermore, to avoid convergence problems, we excluded one study comparing bupropion low (9/169 patients with event) with no treatment (0/36 patients with event) and another study comparing e-cigarette high plus NRT patch (24 hours) not specified (2/20 patients with event) with NRT not specified (0/20 patients with event). Last, to avoid computational problems, we excluded the varenicline standard arm (24 events in 16 patients) from a three-arm trial that also examined no treatment (0 events in 16 patients) and NRT combined not specified (11 events in 16 patients).
The NMA results are presented in Appendix 8, Figure 93 (standard NMA results are presented in Appendix 8, Figure 94). There was evidence that patients randomised to no treatment were less likely to report nausea than those receiving placebo (OR 0.18, 95% CrI 0.08 to 0.39). Conversely, those allocated to bupropion standard (OR 1.62, 95% CrI 1.30 to 2.05), varenicline standard (OR 3.86, 95% CrI 3.25 to 4.62), varenicline high (OR 19.11, 95% CrI 5.75 to 64.07), varenicline standard plus NRT standard (OR 5.21, 95% CrI 2.32 to 11.7), varenicline plus bupropion standard (OR 2.92, 95% CrI 1.49 to 5.64), and NRT high (OR 1.82, 95% CrI 1.01 to 3.46) were more likely to report nausea than those in the placebo group. There was weak evidence for those allocated to varenicline low (OR 1.43, 95% CrI 0.95 to 2.18), bupropion standard plus NRT high (OR 1.95, 95% CrI 0.95 to 4.01), varenicline standard plus NRT high (OR 2.01, 95% CrI 0.91 to 4.44), NRT standard (OR 1.45, 95% CrI 0.92 to 2.29) and e-cigarette (OR 2.48 95% CrI 0.61 to 12.18).
Headache
The data set for headache comprised 152 studies, with a total of 356 arms and 67,956 patients. Appendix 8, Figure 95 presents the structure of the network for headache. Two studies were disconnected from the network and, therefore, were excluded from the analyses: one study comparing varenicline standard plus NRT gum standard (5/245 patients with event) with varenicline low plus NRT gum standard (2/240 patients with event), and another study comparing bupropion standard plus NRT Inhalator not specified (34/267 patients with event) with usual care (13/271 patients with event). Furthermore, we excluded three studies owing to small numbers causing convergence problems: one study comparing e-cigarette high plus NRT patch (24 hours) not specified (1/20 patients with event) with NRT not specified (0/20 patients with event), one study comparing NRT inhalator standard (1/145 patients with event) with placebo (0/141 patients with event), and another study comparing NRT patch (24 hours) standard (1/40 patients with event) with placebo (0/40 patients with event).
The NMA results for this outcome are presented in Appendix 8, Figure 96 (standard NMA results are presented in Appendix 8, Figure 97). There was evidence that patients randomised to no treatment reported headache less frequently than those receiving placebo (OR 0.26, 95% CrI 0.13 to 0.48), Furthermore, there was inconclusive evidence of any differences between patients randomised to any of the experimental drugs and those in the placebo group.
Dry mouth
The data set for dry mouth comprised 88 studies, with a total of 216 arms and 40,721 patients. Appendix 8, Figure 98 shows the structure of this network, which is smaller than the ones presented before for other tertiary outcomes. One study comparing bupropion standard plus NRT inhalator not specified (14/267 patients with event) with usual care (0/272 patients with event) was disconnected from the main network and, therefore, was excluded from the analyses. Moreover, to avoid convergence problems, we also excluded one study comparing e-cigarette low (8/306 patients with event) with no treatment (0/102 patients with event). Last, to avoid computational problems we excluded the varenicline standard arm (36 events in 16 patients) from a three-arm trial that also examined no treatment (0 events in 16 patients) and NRT combined not specified (5 events in 16 patients).
The NMA results for dry mouth are displayed in Appendix 8, Figure 99 (standard NMA results are presented in Appendix 8, Figure 100). We found strong evidence that smokers allocated to the no treatment group (OR 0.05, 95% CrI 0.00 to 0.36) and weak evidence that those allocated to waitlist (OR 0.23, 95% CrI 0.03 to 1.12) were less likely to report dry mouth problems than those receiving placebo. Conversely, there was evidence that smokers randomised to bupropion standard (OR 1.92, 95% CrI 1.58 to 2.34), bupropion standard plus NRT high (OR 1.99, 95% CrI 1.20 to 3.42), and varenicline plus bupropion standard (OR 2.44, 95% CrI 1.28 to 4.66) were more prone to dry mouth than those receiving placebo. There was weak evidence that smokers randomised to bupropion low were more likely to experience dry mouth than those randomised to placebo (OR 1.65, 95% CrI 0.90 to 3.03).
Skin rash
The data set for skin rash comprised 43 studies, with a total of 103 arms and 16,147 patients. Appendix 8, Figure 101 shows that this outcome was reported less often than the previous outcomes. To avoid convergence problems, we excluded one study comparing NRT gum high (2/54 patients with event) with NRT gum standard (0/162 patients with event) and another study comparing e-cigarette low (6/306 patients with event) with no treatment (0/102 patients with event).
The NMA results for this outcome are presented in Appendix 8, Figure 102 (standard NMA results are presented in Appendix 8, Figure 103). We found evidence that patients receiving no drug treatment were less likely to suffer from skin rash than those allocated to placebo (OR 0.03, 95% CrI 0.00 to 0.65). Conversely, patients randomised to bupropion standard were more likely to report skin rash problems than those in the placebo group (OR 2.23, 95% CrI 1.06 to 4.76).
Chapter 7 Results: cost-effectiveness
Table 14 shows the primary results of the probabilistic analysis. The expected (average) total discounted costs and QALYs for all interventions are reported, which represent the estimated average costs and benefits (allowing for length and quality of life) per smoker, having accounted for uncertainty in the inputs to the economic model. This analysis includes disutilities and costs related to depression and self-harm. Interventions are ordered by increasing expected total cost, with NRT low having the lowest expected total cost and varenicline standard plus NRT standard having the highest expected total cost. E-cigarette low has the highest expected QALYs, followed by varenicline standard plus bupropion standard, and varenicline standard plus NRT standard. NRT low has the lowest expected QALYs.
| Treatment | Total costs (£) | Total QALYs | ICER (£) | ENB (£) |
|---|---|---|---|---|
| NRT low | 10,259 | 10.934 | 0 | |
| E-cigarette low | 10,279 | 11.290 | 56 | 7085 |
| Bupropion low | 10,283 | 11.038 | Dominated | 2056 |
| NRT standard | 10,292 | 11.119 | Dominated | 3663 |
| Bupropion standard | 10,304 | 11.033 | Dominated | 1937 |
| NRT high | 10,309 | 11.092 | Dominated | 3092 |
| E-cigarette high | 10,319 | 11.189 | Dominated | 5036 |
| Bupropion standard plus NRT high | 10,346 | 11.128 | Dominated | 3786 |
| Varenicline standard | 10,413 | 11.127 | Dominated | 3697 |
| Varenicline standard plus bupropion standard | 10,437 | 11.281 | Dominated | 6756 |
| Varenicline low | 10,440 | 10.959 | Dominated | 308 |
| Varenicline standard plus NRT high | 10,467 | 11.117 | Dominated | 3440 |
| Varenicline low plus NRT standard | 10,587 | 11.273 | Dominated | 6454 |
| Varenicline standard plus NRT standard | 10,587 | 11.280 | Dominated | 6591 |
We prefer interventions with lower costs and higher QALYs. Any intervention that has a higher expected cost and lower expected QALYs than another intervention is said to be dominated. As can be seen in Table 14, all treatments apart from NRT low are dominated by e-cigarette low, which is more effective, in terms of increased utility, and less expensive than the other interventions. If the funder is not willing to pay £56 per QALY, then NRT low is estimated to be most cost-effective. If the funder is willing to pay ≥ £56 per QALY, then e-cigarette low is estimated to be most cost-effective.
If the payer is willing to pay up to £20,000 per QALY, e-cigarette low has the highest expected net benefit (£7085), followed by varenicline standard plus bupropion standard (£6756), and varenicline standard plus NRT standard (£6591).
We present the uncertainty surrounding the cost-effectiveness of the various interventions using a CEAC (Figure 28), which plots the probability that each intervention is the most cost-effective at a given willingness-to-pay threshold. Only interventions with a probability of being the optimal treatment of > 10% at any willingness-to-pay value are plotted.
FIGURE 28.
Cost-effectiveness acceptability curve. Probability that treatment is optimal plotted against different willingness-to-pay per unit increase in utility (ceiling ratio). Based on 5000 Monte Carlo simulations. Std, standard.

Figure 28 shows that, at any willingness-to-pay value, e-cigarette low has the highest probability of being cost-effective, followed by varenicline low plus NRT standard. At any threshold above £20,000, the probability of e-cigarette low being the most cost-effective intervention is never > 30%, indicating a high degree of uncertainty about the optimal intervention.
The rank-o-grams presented in Figure 29 further demonstrate the uncertainty in the results. The lines are relatively flat for most interventions, showing that there is no strong probability that they will be the most or least cost-effective at a willingness-to-pay threshold of £20,000 per QALY. The exception is NRT low, which shows a clear probability that it is among the least cost-effective interventions if the payer is willing to pay £20,000 per QALY. There is a similar trend for bupropion low, bupropion standard and varenicline low, which have higher probabilities of being among the worst interventions than being among the best. The reverse trend is seen for e-cigarette low, e-cigarette high, varenicline low plus NRT standard, varenicline standard plus NRT standard and varenicline plus bupropion standard.
FIGURE 29.
Rank-o-grams showing the probability that each intervention is ranked first, second, etc. based on net benefit at a willingness-to-pay threshold of £20,000 per QALY. Std, standard.
Value-of-information analysis
Table 15 shows the results of the value-of-information analyses for the base-case model at a willingness to-pay per QALY threshold of £20,000. EVPI estimates the most the funder would be prepared to pay to eliminate uncertainty in the model input parameters. EVPI is helpful for understanding whether or not future research may potentially be of value. The per-quitter EVPI is £3645 and the population EVPI, representing all of the smokers attempting to quit in England, is £999M over a 1-year time horizon and £4994M over a 5-year time horizon. These values are substantial and suggest that future research studies to reduce parameter uncertainty in the model would be valuable, as the decision is clearly sensitive to uncertainty in the model inputs.
| Model parameter subsets | EVPPI per smoker attempting to quit (£) | 1-year population EVPPI (£M to 1 decimal place) | 5-year population EVPPI (£M to 1 decimal place) |
|---|---|---|---|
| All (EVPI) | 3645 | 998.8 | 4994.0 |
| All costs | 1216 | 333.3 | 1666.7 |
| All utilities | 947 | 259.4 | 1297.1 |
| All costs and utilities | 1415 | 387.9 | 1939.2 |
| All abstinence probabilities | 3053 | 836.7 | 4182.4 |
| All depression and self-harm probabilities | 1654 | 453.1 | 2265.7 |
| E-cigarette low vs. varenicline standard plus NRT standard (probabilities, costs and utilities) | 2342 | 641.8 | 3209.0 |
| E-cigarette low vs. varenicline standard plus NRT standard (probabilities only) | 1676 | 459.3 | 2296.7 |
Expected value of partial perfect information (EVPPI) estimates the most that the funder would be prepared to pay to eliminate uncertainty in a specific subset of model input parameters. Comparing EVPPI for different parameters allows us to identify the subsets of model inputs to which the decision is most sensitive. This can indicate where future research efforts may be invested most effectively. There is a high value per smoker in reducing uncertainty in all of the abstinence probabilities (£3053) but less of a value in reducing uncertainty in all of the AEs probabilities (£1654). EVPPI is marginally higher for cost parameters (£1216) than for utility parameters (£947).
We explored the potential value of a new trial comparing the two interventions with the highest expected net benefit, e-cigarette low and varenicline standard plus bupropion standard, which would provide information on the effectiveness of the interventions, costs and utilities. This gives a per-quitter EVPPI of £2342 and a population EVPPI of £642M over a 1-year time horizon and £3869M over a 5-year time horizon. Restricting to the collection of intervention effects only would reduce this value marginally to £1676, suggesting that a large trial, conducted well and adequately powered, may be a cost-effective area of future research, but that it may be most important to collect information on probabilities of abstinence and AEs. In particular, a trial comparing e-cigarettes with an active comparator such as varenicline standard plus NRT standard or NRT standard is likely to be a cost-effective investment.
Sensitivity analysis with results based on abstinence alone
Table 16 shows the primary results of the sensitivity analysis when the impact of depression and self-harm is removed from the model. In this case, bupropion low has the lowest expected total cost. Varenicline standard plus NRT standard, again, has the highest expected total cost. Varenicline standard plus NRT standard has the highest expected QALYs, followed by varenicline low plus NRT standard. NRT low has the lowest expected QALYs.
| Treatment | Total costs (£) | Total QALYs | ICER (£) | ENB (£) |
|---|---|---|---|---|
| Bupropion low | 10,219 | 11.135 | 3159 | |
| NRT low | 10,231 | 10.977 | Dominated | 0 |
| NRT high | 10,238 | 11.198 | Extendedly dominated | 4400 |
| Bupropion standard | 10,240 | 11.130 | Dominated | 3041 |
| E-cigarette high | 10,248 | 11.295 | Extendedly dominated | 6335 |
| E-cigarette low | 10,250 | 11.332 | 159 | 7072 |
| NRT standard | 10,264 | 11.162 | Dominated | 3657 |
| Bupropion standard plus NRT high | 10,319 | 11.168 | Dominated | 3721 |
| Varenicline low | 10,320 | 11.138 | Dominated | 3120 |
| Varenicline standard | 10,327 | 11.254 | Dominated | 5434 |
| Varenicline standard plus NRT high | 10,402 | 11.214 | Dominated | 4556 |
| Varenicline plus bupropion standard | 10,415 | 11.314 | Dominated | 6558 |
| Varenicline low plus NRT standard | 10,446 | 11.476 | Extendedly dominated | 9759 |
| Varenicline standard plus NRT standard | 10,447 | 11.483 | 1302 | 9895 |
An intervention is said to be ‘extendedly dominated’ if a mix of two interventions can provide the same QALYs at a lower cost. As can be seen in Table 16, all treatments apart from NRT high, e-cigarette high, e-cigarette low, varenicline low plus NRT standard and varenicline standard plus NRT standard are dominated by bupropion low, which is more effective, in terms of increased utility, and less expensive than the other interventions.
The interventions on the efficiency frontier (i.e. those that are not dominated or extendedly dominated) are NRT low, e-cigarette low and varenicline standard plus NRT standard. If the payer is not willing to pay £159 per QALY, then bupropion low is estimated to be most cost-effective. If the payer is willing to pay between £159 and £1302 per QALY, then e-cigarette low is estimated to be most cost-effective, and if the willingness to pay per QALY is above £1302, then varenicline standard plus NRT standard is estimated to be most cost-effective.
At a willingness-to-pay threshold of £20,000, varenicline standard plus NRT standard has the highest expected net benefit (£9895), followed by varenicline low plus NRT standard (£9759).
We present the uncertainty surrounding the cost-effectiveness of the various interventions, using a CEAC (Figure 30). Only those interventions with a probability of being the optimal treatment of more than 10% at any willingness-to-pay value are plotted. Figure 30 shows that, at any willingness-to-pay value, varenicline low plus NRT standard has the highest probability of being cost-effective, followed by varenicline standard plus NRT standard. At any threshold above £20,000, the probability of any intervention being the most cost-effective intervention is never > 40%, again, indicating a degree of uncertainty in the optimal intervention.
FIGURE 30.
Cost-effectiveness acceptability curve. Probability that treatment is optimal plotted against different willingness-to-pay per unit increase in utility (ceiling ratio). Based on 5000 Monte Carlo simulations. Sensitivity analysis based on abstinence alone. Std, standard.

The rank-o-grams are presented in Figure 31.
FIGURE 31.
Rank-o-grams showing the probability that each intervention is ranked first, second, etc. based on net benefit at a willingness-to-pay threshold of £20,000 per QALY. Sensitivity analysis based on abstinence alone. Std, standard.

Sensitivity analysis with only UK-licensed interventions included
Table 17 shows the primary results of the sensitivity analysis including only interventions that are licensed in the UK (NRT low, standard and high, bupropion low and standard, and varenicline low and standard). In this case, NRT low has the lowest expected total cost and varenicline low has the highest expected total cost. Varenicline standard has the highest expected QALYs, followed by NRT standard. NRT low has the lowest expected QALYs.
| Treatment | Total costs (£) | Total QALYs | ICER (£) | ENB (£) |
|---|---|---|---|---|
| NRT low | 10,259 | 10.934 | 0 | |
| Bupropion low | 10,283 | 11.038 | Extendedly dominated | 2056 |
| NRT standard | 10,292 | 11.119 | 32 | 3663 |
| Bupropion standard | 10,304 | 11.033 | Dominated | 1937 |
| NRT high | 10,309 | 11.092 | Dominated | 3092 |
| Varenicline standard | 10,413 | 11.127 | 15,665 | 3697 |
| Varenicline low | 10,440 | 10.959 | Dominated | 308 |
As can be seen in Table 17, all treatments apart from NRT low, bupropion low and NRT standard are dominated by varenicline standard, which is more effective, in terms of increased utility, and less expensive than the other interventions. Bupropion low is extendedly dominated by NRT standard.
The interventions on the efficiency frontier are NRT low, NRT standard and varenicline standard. If the payer is not willing to pay £32 per QALY, then NRT low is estimated to be most cost-effective. At a willingness to pay per QALY above £32, but below £15,665, NRT standard is estimated to be most cost-effective. At a willingness to pay per QALY above £15,665, varenicline standard is estimated to be most cost-effective.
At a willingness-to-pay threshold of £20,000, varenicline standard has the highest expected net benefit (£3697), followed by NRT standard (£3663).
We present the uncertainty surrounding the cost-effectiveness of the various interventions using a CEAC (Figure 32). Only those interventions with a probability of being the optimal treatment of more than 10% at any willingness-to-pay value are plotted. Figure 32 shows that, at any willingness-to-pay value above £5000, NRT standard has the highest probability of being cost-effective, followed by varenicline standard.
FIGURE 32.
Probability treatment is optimal plotted against different willingness-to-pay per unit increase in utility (ceiling ratio). Based on 5000 Monte Carlo simulations. Sensitivity analysis based on licensed interventions. Std, standard.
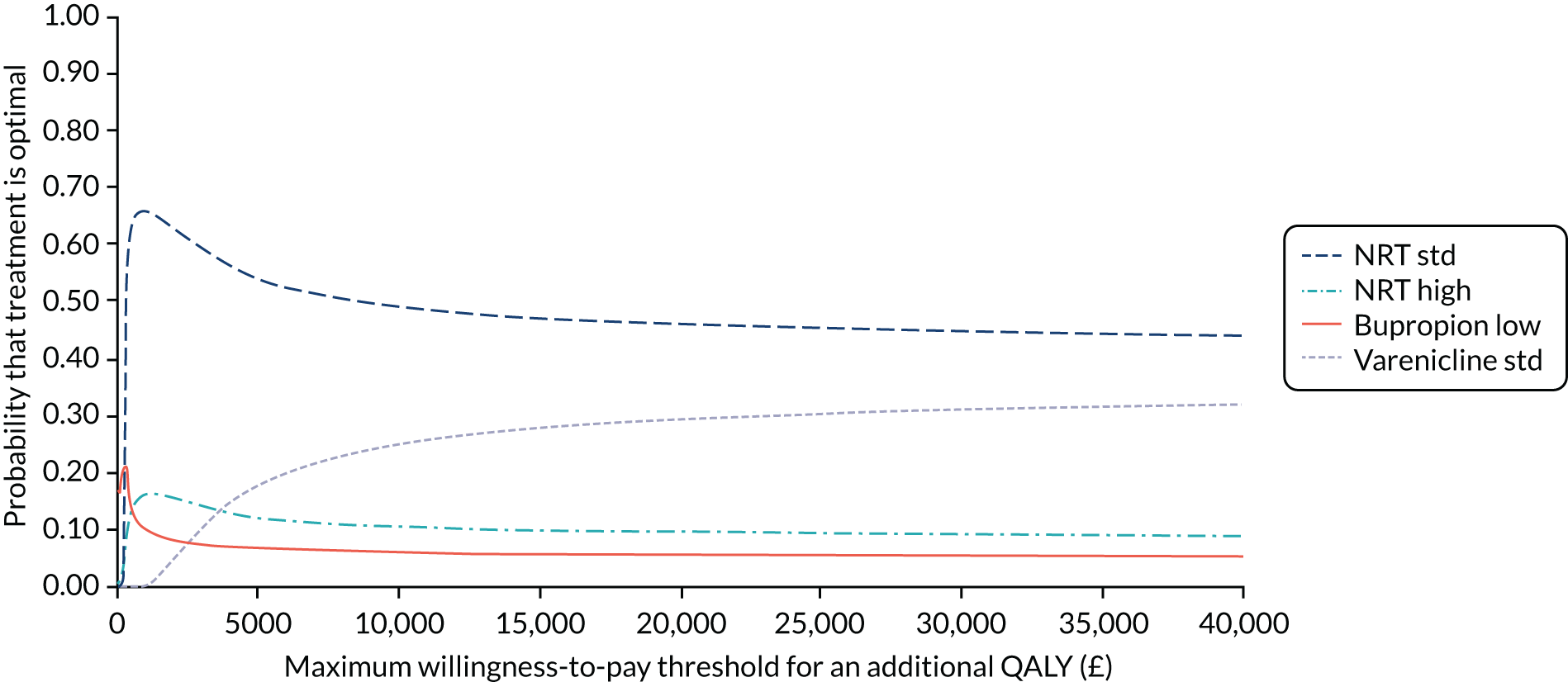
The rank-o-grams are presented in Figure 33. These show that, at a willingness-to-pay value of £20,000, NRT standard and varenicline standard have the highest probabilities of being the most cost-effective treatment. NRT low and varenicline low have very low probabilities of being the most cost-effective.
FIGURE 33.
Rank-o-grams showing the probability that each intervention is ranked first, second, etc. based on net benefit at a willingness-to-pay threshold of £20,000 per QALY. Sensitivity analysis based on licensed interventions only. Std, standard.
Chapter 8 Discussion and conclusions
Key findings
The main findings for the clinical effectiveness, safety and cost-effectiveness analyses are summarised in the following sections.
Key findings of the effectiveness network meta-analysis
We performed a systematic review and NMA to investigate the effectiveness of UK-licensed smoking cessation medicines and e-cigarettes for smoking cessation. We included 363 trials that reported on one or more effectiveness outcomes, involving 201,045 participants, that took place internationally across a range of settings. We found that only 13% of trials were rated as being at low risk of bias, with 40% rated as being at high risk of bias.
We found evidence that most monotherapies and combination treatments were more effective than placebo at helping participants achieve sustained abstinence. The three most effective treatments compared with placebo were varenicline standard plus NRT standard (OR 5.75, 95% CrI 2.27 to 14.88), varenicline low plus NRT standard (OR 5.70, 95% CrI 1.57 to 21.12) and e-cigarette low (OR 3.22, 95% CrI 0.97 to 12.55). Smokers randomised to varenicline standard plus NRT standard were more likely to achieve sustained abstinence than participants receiving NRT standard or bupropion standard. We also found that varenicline standard resulted in higher odds of sustained abstinence than NRT standard or bupropion standard, and weak evidence that e-cigarette high may increase the odds compared with bupropion standard. As combination therapies are currently not licensed in the UK, when limiting our findings to interventions that are licensed in the UK (NRT low, standard and high, bupropion low and standard, and varenicline low and standard), varenicline standard (OR 2.83, 95% CrI 2.34 to 3.39), NRT high (OR 2.32, 95% CrI 1.88 to 2.86), and NRT standard (OR 2.01, 95% CrI 1.68 to 2.41) were the three most effective treatments compared with placebo at helping participants achieve sustained abstinence.
The results of our threshold analyses confirmed that varenicline standard plus NRT standard’s first-place ranking (offering the highest estimated odds of sustained abstinence) was relatively robust. However, uncertainty or potential biases in a small number of studies could lead to one of varenicline standard plus bupropion standard, e-cigarette low, or e-cigarette high being ranked first. Finally, we found evidence of effect modification, whereby interventions delivered with counselling were associated with a higher proportion of smokers achieving sustained abstinence than those same interventions delivered without counselling, and we also found a higher OR of sustained abstinence among participants who had higher average dependence scores.
The results for the secondary effectiveness outcomes were largely similar to those for sustained abstinence. Although reported in fewer studies and for fewer interventions, we found evidence that smokers treated with NRT high, bupropion standard, varenicline standard and varenicline standard plus bupropion standard were more likely to achieve prolonged abstinence than those using placebo. Bioverified prolonged abstinence data at ≥ 6 months for e-cigarette or varenicline standard plus NRT standard were not available. When considering pairwise comparisons between interventions, there was inconclusive evidence that bupropion standard, varenicline standard and varenicline standard plus bupropion standard differed from each other in the odds of resulting in prolonged abstinence.
For our ‘any abstinence’ outcome, as for sustained abstinence, we found that most interventions were more effective than placebo at helping participants abstain from smoking, including e-cigarette at low and high doses. The three most effective treatments compared with placebo were bupropion low plus NRT high, varenicline standard plus NRT standard and varenicline not specified. For ‘any abstinence’, our NMA indicated that smokers randomised to varenicline standard were more likely to achieve abstinence than those allocated to NRT standard or bupropion standard. We also found that varenicline standard plus NRT standard led to higher odds of abstinence than NRT standard, bupropion standard, and bupropion standard plus NRT standard, while varenicline standard plus bupropion standard led to higher odds of abstinence than bupropion standard alone.
Finally, there was evidence that a number of interventions were more effective than placebo at attaining 7-day PPA, including e-cigarette high. The three most effective treatments compared with placebo, were bupropion low plus NRT high, varenicline standard plus NRT standard and varenicline not specified. In terms of 7-day PPA, our NMA indicated that smokers allocated to varenicline standard achieved abstinence more often than those using NRT standard or bupropion standard. We also found that varenicline standard plus NRT standard led to higher odds of abstinence than NRT standard, bupropion standard or varenicline standard.
Ranking the interventions based on smokers attaining sustained abstinence, varenicline standard plus NRT standard had the highest probability of being ranked first, followed by e-cigarette low, varenicline standard plus bupropion standard, and e-cigarette high, with placebo in last place. Based on our rank-o-grams, ranking the interventions across primary and secondary effectiveness outcomes, varenicline standard plus NRT standard showed a high probability of being ranked as the best or second-best intervention for all outcomes, with the exception of prolonged abstinence, for which there were no data. Varenicline standard plus bupropion standard had the highest probability of being ranked as best for prolonged abstinence, but its rankings for other outcomes were less certain. Finally, varenicline standard showed high probabilities of being ranked second- to fourth-best across outcomes, while e-cigarette rankings were uncertain, and placebo was consistently ranked last.
Comparison with other studies
Our findings are largely comparable to those of previous NMAs. 25,123 We found evidence that nearly all identified doses of smoking cessation medicines increased the probability of sustained abstinence compared with placebo. An exception to this is bupropion plus NRT; a health technology assessment (HTA) report of smoking cessation interventions by the HIQA123 found evidence that this treatment improved the likelihood of cessation (from the quit date or PPA) compared with placebo (control), whereas we saw this result for the ‘any abstinence’ and 7-day PPA outcomes only. Similar to our findings, previous NMAs also found evidence that varenicline increased the chance of cessation compared with bupropion and with NRT, while not finding evidence of a difference in likelihood of quitting between bupropion and NRT. 25,123 Findings were also consistent for varenicline plus NRT, which showed improved probability of quitting compared with bupropion and with NRT. However, although the HIQA HTA found evidence that varenicline plus bupropion was more effective than bupropion or NRT delivered as monotherapies, we did not. 123 Nonetheless, the results of the ranking of treatments for smoking cessation were similar across NMAs. 25,123
Key findings of the safety network meta-analysis
A systematic review and NMA were performed to investigate the safety of UK-licensed smoking cessation medicines and electronic cigarettes. We included 355 trials that reported on one or more safety outcomes involving 159,101 participants, and 53 observational studies involving 8,783,403 participants that took place internationally across a range of settings. We found that only 16% of trials were rated as being at low risk of bias, while one-third (33%) were rated as being at high risk of bias. All observational studies were rated as being at high risk of bias owing to their non-randomised nature.
There was evidence that, compared with placebo, bupropion standard increased the odds of experiencing SAEs (OR 1.27, 95% CrI 1.04 to 1.58). The results of our threshold analyses indicated that the first- and last-place rankings were very sensitive to the level of uncertainty and risk of bias in the evidence. Unlike our effectiveness analyses, we found inconclusive evidence of effect modification on the likelihood of experiencing a SAE. Placebo yielded the largest probability of being ranked the best intervention for reducing the odds of experiencing a SAE; however, the first-place ranking could change to NRT standard, varenicline standard plus NRT standard, e-cigarette high, e-cigarette low, or varenicline plus bupropion standard simply owing to sampling error. NRT standard was also the most likely to be ranked among the best two interventions for reducing the odds of SAEs. The last-place ranking is held by e-cigarette low, but this is very sensitive to the high level of uncertainty in the single study by Cravo et al. 525 Changes to the estimate could result in e-cigarette low being replaced by varenicline standard plus bupropion standard in last place or see e-cigarette low replace placebo in first place, both of which are plausible owing to sampling error. Therefore, the first- and last-place rankings for SAEs are not very robust, as they are sensitive to levels of uncertainty in the data and plausible biases in at-risk studies that could alter the rankings. Although only one observational study632 reported one or more SAE, incorporating this study into our analyses resulted in the effect estimates changing substantially, suggesting that varencline standard and e-cigarette low might lead to lower odds of experiencing a SAE than placebo. This may be a result of the observational study adding a substantial number of events to a network of what was otherwise a rare outcome.
Regarding secondary outcomes, we could not find any differences between interventions for MACEs owing to the rarity of events reported across studies, resulting in effect estimates with very wide CIs. This did not change with the addition of 10 observational studies to our analyses; there is substantial uncertainty regarding the relative cardiovascular safety of the treatments. For MANEs, there was evidence that smokers receiving NRT not specified, bupropion standard, bupropion standard plus NRT high or varenicline standard plus bupropion standard were less likely to report MANEs than smokers treated with placebo. In pairwise comparisons between interventions, there was evidence of increased odds of MANEs among smokers randomised to varenicline standard compared with those using bupropion standard. Although 16 observational studies reported one or more MANEs, our analyses incorporating these studies produced similar results to that of the randomised evidence. We found that bupropion standard, bupropion standard plus NRT high and varenicline standard plus bupropion standard were associated with lower odds of experiencing a MANE than placebo. Whereas placebo and varenicline standard plus NRT standard yielded the largest probabilities of being ranked the best interventions to reduce the odds of SAEs, e-cigarette low, varenicline standard plus bupropion standard and e-cigarette high were the least likely to be ranked highly. Based on our rank-o-grams, ranking the interventions across primary and secondary safety outcomes, placebo and NRT standard were most likely to be ranked among the best interventions for reducing the odds of experiencing SAEs, but were ranked lower for MACEs and MANEs. Bupropion standard plus NRT standard, and varenicline standard plus bupropion standard may be the safest interventions in terms of MACEs and MANEs, but these rankings were based on imprecise effect estimates.
We conducted random-effects NMAs at the class level for a number of our tertiary and other safety outcomes based on data from studies identified from our initial searches ending in March 2017. Compared with smokers randomised to placebo, we found evidence of increased odds of experiencing nausea among smokers allocated to bupropion standard, varenicline standard, varenicline high, varenicline standard plus NRT standard, varencline standard plus bupropion standard, NRT not specified, NRT high and bupropion standard plus NRT not specified. However, we did not find any evidence of a difference between interventions in the odds of experiencing headache. We found evidence of increased odds of experiencing dry mouth among smokers using bupropion standard, bupropion standard plus NRT high, varenicline standard plus bupropion standard and bupropion standard plus NRT not specified compared with those allocated to placebo. Finally, we found that smokers randomised to bupropion standard had higher odds of experiencing skin rash than smokers allocated to placebo.
Comparison with other studies
The finding of our NMA of MACEs mirrors that of Mills et al. ,39 as we also did not find evidence that any smoking cessation increased the likelihood of experiencing a MACE compared with placebo or with each other. As pairwise comparisons between active interventions were almost entirely based on indirect evidence only, and because MACEs were uncommon, it was very difficult to effectively compare treatments with each other. Other NMAs25,123 only summarised safety data from previous reviews and did not analyse them; this study is the first, to our knowledge, to conduct a NMA of SAE, MANE and other AE data. Although the HIQA HTA123 reported that its two included trials of e-cigarettes did not report SAEs linked to their use, we included the SAEs reported by Bullen et al. ,192 as we included all events whether or not study authors attributed them to the use of the medication. The authors of Cochrane’s review of electronic cigarettes similarly chose to consider SAEs that were deemed related to e-cigarette use only, however, the occurrence of other AEs that we have presented in our NMA in outcome tables were reported in Report Supplementary Materials 8 and 9. 72
Key findings of the cost-effectiveness analysis
This analysis has shown that, in the base case, e-cigarette low appears to be the most cost-effective intervention at any willingness to pay per QALY value above £56. However, these findings are uncertain, with no intervention having more than a 40% chance of being the most cost-effective intervention at a willingness to pay per QALY above £5000. When the impact of the safety outcomes of depression and self-harm are excluded, varenicline standard plus NRT standard and varenicline low plus NRT standard are the most cost-effective interventions. There is, again, considerable uncertainty about the optimal intervention. When the analysis is limited to interventions that are licensed in the UK, varenicline standard is the most cost-effective intervention at any willingness-to-pay value above £15,665, followed by NRT standard. Value-of-information analyses indicated that a trial comparing e-cigarettes with an active comparator such as varenicline standard and bupropion standard or NRT standard is likely to be a cost-effective investment.
Comparison with other studies
No previous cost-effectiveness analysis could be identified that compared a similar range of interventions, compared the standard licensed interventions with combination therapies and e-cigarettes, or incorporated safety outcomes. A recent systematic review of cost-effectiveness analyses123 identified four studies98,104,654,655 published in the last 10 years that compared varenicline, bupropion or NRT with each other or with standard of care. All but one of these studies655 also used the BENESCO model, but did not adjust to account for safety outcomes. The studies consistently found varenicline to be the most cost-effective intervention. A report by Leaviss et al. 58 compared the cost-effectiveness of varenicline and cytisine in a UK setting, also using the BENESCO model. This study found cytisine to be the most cost-effective intervention; however, cytisine was beyond the scope of this review. Our results show that, although the varenicline combination treatments dominate the other interventions, among the treatments licenced in the UK, NRT standard is the most cost-effective.
One previous study61 was identified that compared the cost-effectiveness of e-cigarettes with that of NRT in stop smoking services in England. Similar to our study, in which an ICER of £56 was calculated for e-cigarette low compared with NRT low, this previous study found an ICER of £65 per QALY gained by using e-cigarettes as a smoking cessation aid, in comparison with NRT. This suggests strong evidence of the cost-effectiveness of e-cigarettes compared with NRT; however, to our knowledge, our study is the first to assess the cost-effectiveness of e-cigarettes compared with all other interventions in the UK.
Strengths and limitations
Strengths and limitations of the effectiveness and safety network meta-analyses
Strengths
This is the first NMA of SAEs, MANEs and other AEs associated with smoking cessation medicines, and the second to analyse MACEs. Whereas a previous NMA suffered from insufficient data for AE outcomes,25 our decision to include RCTs of any duration and the inclusion of observational studies allowed us to utilise as many data as possible to create our networks (although this also brings some limitations; see Limitations). Most NMAs25,123 failed to include RCTs of less than 6 months’ duration for AEs, based on their inclusion criteria for analysing effectiveness outcomes. Given that AEs can occur within a short time after treatment has started, previous reviews would have excluded several of the studies that we included in our safety analyses. We also benefited from the publication of some large studies since the start of our study that made important contributions to our analyses, such as the EAGLES trial,33 and several electronic cigarette studies, including a large trial by Hajek et al. 19 Therefore, these decisions allowed us to include and analyse data from more participants in more studies reporting AEs than any previous NMA of these licensed medicines. 25,39,123 A significant strength of this study is the inclusion of combined therapies of smoking cessation medicines, as most reviews have only included monotherapies and combination NRT. This proved to be a crucial decision, as our study has found combined therapies, notably varenicline standard plus NRT standard, to be among both the most effective and cost-effective treatments for smoking cessation. Although we only found evidence of effect modification for the inclusion of counselling with smoking cessation treatment, the size of our study allowed us to investigate the influence of several important covariates as potential effect modifiers for our primary outcomes. Additionally, this is the first NMA to compare medicines stratified by dosage. This allowed us to more specifically identify how dose affected a medicine’s effectiveness and safety across outcomes, revealing differential effects by dose that would otherwise have been lost. This includes the investigation of data for each licensed form of NRT by dose in our full interaction models. We also reported effectiveness across multiple specific cessation outcomes rather than using the approach in past NMAs of authors using the most rigorous definition of abstinence available. Finally, this study was the first NMA of smoking cessation medicines to use threshold analysis. 91,92 This technique allowed us to visualise thresholds of how much the evidence could change before our recommendation based on the ranking of our treatments would change, and helps readers to understand the robustness of our treatment recommendations.
Limitations
Despite the large number of studies we were able to include in our review, there were still limitations in the data available. Primary and secondary safety outcomes included rare events, which limited the ability of analyses to draw firm conclusions. Pairwise comparisons between active interventions were almost exclusively informed by indirect evidence and were affected by the small number of events, resulting in imprecisely estimated effects and wide intervals, often including the null. Additionally, there were instances of extreme results based on the findings of a single or very few studies, which may be particularly problematic when attempting to draw conclusions about the safety of e-cigarettes (e.g. Cravo et al. 525). For the SAE, MACE, and MANE outcomes, we conducted a NMA that incorporated both RCT and non-randomised evidence, as well as a NMA restricted to RCTs. Although including non-randomised evidence increases the precision of the estimates, this comes with a risk of introducing bias in the resulting estimates. Non-randomised evidence is vulnerable to bias by confounding, and there was no attempt to adjust for this (which would have required the availability of individual participant data). Identifying methods to combine RCT and non-randomised evidence while adjusting for bias using individual patient data is an area for further research.
Network meta-analysis (like any pairwise meta-analysis) makes the assumption that the included studies do not differ in the distribution of factors that might moderate the relative treatment effects (effect modifiers). This assumption can be assessed statistically by checking for evidence of heterogeneity (different effects across studies making the same comparison) and by checking for evidence of inconsistency (different effects from studies providing direct and indirect estimates). There was a moderate level of heterogeneity in all of our NMAs (as has also been found in previous meta-analyses and NMAs in this field). We explored a range of covariates to explain the heterogeneity in meta-regression analyses, and did not find any evidence of effect modification other than using counselling alongside pharmacological treatments. We made an assumption that the effect of counselling is additive when given together with a pharmacotherapy, which is a potential limitation of our findings. It may be that there is a synergistic (or even antagonistic) effect of counselling when it is used together with pharmacotherapies. We explored this in a sensitivity analysis and found some evidence to support a synergistic effect. Future research to explore this potential synergistic effect of smoking cessation medicines being used together with counselling would be of value. There may be other important effect modifiers that we have not included, for example changes in practice over time. However, although absolute cessation rates are expected to change with time, this will be the case for all study arms, and we found no evidence that year of publication was an effect modifier. We did not find any statistical evidence of inconsistency for any of the outcomes. An inspection of direct and indirect estimates, where both can be calculated, shows that in general there is good overlap of the credible intervals for the direct and indirect estimates, although there are some differences in the point estimates. We conclude that, for each outcomes, there is evidence of effect modification that manifests as heterogeneity between estimates from studies on the same comparison, but no systematic differences between direct and indirect estimates (over and above the heterogeneity seen across the entire network of evidence).
Unlike previous NMAs, we opted to use only bioverified cessation data, as most studies in our review reported one or more bioverified outcomes. However, it is possible that more data would have been included in our networks had we adopted the approaches used in previous projects and included self-reported cessation data. Finally, despite extensive efforts, we were unable to obtain safety data for industry-funded trials from pharmaceutical companies. Although we hoped to include as many safety data as possible from these trials, our findings are limited to those events reported in publications. Finally, we were unable to include and analyse craving and withdrawal data, as these were rarely reported across included studies and the outcomes were assessed using a variety of measures and scales that made summarising or analysing these data impossible.
Strengths and limitations of the cost-effectiveness analysis
This analysis has improved on previous analyses of smoking cessation interventions, as it has taken into account not only effectiveness in terms of abstinence from smoking but also potential AEs of treatment (depression and self-harm). The effects of the interventions on each of these outcomes have been informed by NMAs that have shown that, although the combination of varenicline (low or standard) plus NRT standard gives the highest probability of continuous abstinence at 1 year, this is slightly offset by the association of this combination with a higher probability of depression and self-harm than the other interventions. This has led to e-cigarette low being slightly more cost-effective (the intervention with the highest expected net benefit) than varenicline (low or standard) plus NRT standard, although these results are very uncertain.
In terms of limitations, no comparative evidence on subsequent quit attempts in these treatments could be identified in the literature. The model, therefore, assumes that no further attempts to quit are made and that those who fail to quit remain smokers until death. In reality, people often make several quit attempts before they are successful and despite a failed attempt a person may have moved forward towards their future successful attempt. We would expect our qualitative findings to be robust to this as long as the likelihood of a successful subsequent quit attempt does not depend on the treatment used for the index quit attempt.
Another data limitation is the assumption that the risk ratios of developing or dying from smoking-related diseases in current smokers and former smokers compared with non-smokers (incidence and mortality) are equal to the risk ratios of having smoking-related diseases (prevalence). We considered this to be a reasonable assumption given that no alternative sources of information on the relative incidence or mortality from these diseases within the relevant age and sex categories could be identified. Longitudinal studies measuring these outcomes for the different smoking categories would be useful to test this assumption.
This distribution of the cohort across sex and age categories at the start of the model was designed to reflect the distribution of smokers in the UK. One issue is that this is not necessarily the same as the distribution of smokers making a quit attempt. Another issue is that data availability meant that this cohort needed to be grouped into quite broad age categories (18–34, 35–64 and ≥ 65 years) that were assigned the same prevalence, incidence and probability of mortality from diseases. It is likely, therefore, that greater variation exists in these categories than is being accounted for. A study measuring patient characteristics of those seeking treatment to make a quit attempt would be useful to update the model to better reflect the population of interest.
As e-cigarettes are not medically licensed in the UK, it is difficult to estimate a prescribing cost if they were to be prescribed on the NHS. The best evidence we could find on this was from the HIQA HTA,123 which costed a 12-week supply of e-cigarettes at €93.80. Current high-street/internet prices may be considerably lower than this. However, it is unclear whether the NHS would be able to access these lower prices if e-cigarettes were made available on the NHS. If a lower price could be accessed, this could only have the impact of increasing the cost-effectiveness of e-cigarettes compared with the other interventions.
In addition, as no data were available, assumptions had to made about the relative effectiveness of several interventions for the outcomes of depression and self-harm. It was assumed that (1) NRT low and e-cigarette low have the same effect as NRT standard, (2) e-cigarette high has the same effect as NRT high, (3) bupropion low has the same effect as bupropion standard, and (4) varenicline low plus NRT standard has the same effect as varenicline standard plus NRT standard. The assumption that NRT and e-cigarettes have the same impact on psychological outcomes is reasonable as the active ingredient is the same in both (nicotine). Although a higher dose of bupropion or varenicline may increase the probability of depression or self-harm, no evidence was available to inform this. A study comparing the impact of different doses of these interventions on psychological outcomes would be useful to inform the model.
We did not explicitly model treatment discontinuation, although it should be noted that the RCTs included in the NMA will have included outcomes for patients who did not adhere to treatment, and the treatment costs are likely to be incurred regardless of discontinuation. A final limitation was a lack of available data to explore the cost-effectiveness of these interventions in subgroups such as those with psychiatric illness, heavy smokers or smokers not willing to quit.
Conclusions
Implications for practice
Our findings suggest that combined therapies of smoking cessation medicines are among the most effective, safe and cost-effective treatment options for smokers. Although combination NRT is commonly prescribed, combined therapy of NRT delivered alongside varenicline at standard doses (currently unlicensed) was shown to be the most effective treatment for most cessation outcomes. Using combined therapies instead of monotherapy treatments may offer smokers a better chance of successfully quitting smoking over both short and long periods of time. We also found that interventions that included counselling were more effective at helping smokers to quit and should be considered when planning a cessation attempt. Although the use of bupropion standard may increase the odds of SAEs compared with placebo, we did not find strong evidence of any other negative associations between medicines and SAEs, MACEs or MANEs relative to placebo. Although electronic cigarettes showed promise as cessation tools, their safety profile remains uncertain and no existing model of the devices has been licensed as a medicine. This study has used the most up-to-date information to give an estimate of the most cost-effective intervention for smoking cessation in the UK today. This analysis has shown that, in the base case, e-cigarette low, varenicline standard plus NRT standard, and varenicline standard plus bupropion standard appear to be the most cost-effective interventions. When the impact of the safety outcomes of depression and self-harm is excluded, varenicline standard plus NRT standard and varenicline low plus NRT standard are the most cost-effective interventions. These results should be taken with the caveat of substantial uncertainty, however, with no intervention having a probability of being the most cost-effective of > 30% at any willingness-to-pay per QALY threshold above £20,000.
Recommendations for research
Based on the findings of this study, we propose several recommendations.
First, given the relatively small number of studies rated as being at low risk of bias overall (including recent publications), we recommend that study authors ensure complete and accurate reporting of their study methodology. Most domains were predominantly rated as unclear risk of bias owing to a lack of detailed description of study procedures. Although some of these ambiguities were resolved following contact with corresponding authors, details pertinent to assessments of bias should be reported in the main body or supplemental material of publications. We also urge those designing future studies to think carefully about potential biases when designing their studies.
Second, there were also large discrepancies in the completeness of safety reporting. A significant number of trials did not report any safety data at all, while those that did varied substantially in their reporting. This included not providing definitions of what they considered to be a SAE, not providing details about SAEs, not reporting AEs by study arm, and a wide array of reported events with seemingly no consistency across studies (e.g. a study recording events of nausea or headache vs. another study recording events of dry mouth, headache and abnormal dreams). There may be scope for the creation of a core outcome set for safety outcomes for studies of smoking cessation medicines to ensure systematic recording and reporting of AEs, as this information is of importance to patients, practitioners and policy-makers.
Third, although we included non-randomised evidence in our safety analyses to increase the precision of our estimates, the use of non-randomised data may introduce bias. Further research should explore methods for combining randomised and non-randomised data to most effectively incorporate all of the available safety evidence.
Fourth, there have been few published trials or observational studies of electronic cigarettes with control groups. Although our findings suggest that e-cigarettes show promise for smoking cessation, the limited amount of evidence available results in uncertainty about their safety profile and how they compare with licensed medicines. Although e-cigarettes are regulated by The Tobacco and Related Products Regulations 2016,656 no available e-cigarette devices are licensed as a smoking cessation medicine at present. Medicinal e-cigarettes would need to meet the standards for consumer e-cigarettes as well as any additional requirements needed to meet efficacy, safety and quality criteria under medicines regulation. 657 We recommend that researchers continue to investigate the use of e-cigarettes for smoking cessation, particularly with respect to long-term effectiveness and safety outcomes, preferably in studies with active interventions as comparators. Our value-of-information analysis suggested that a large adequately powered and well-conducted trial comparing e-cigarettes with an active comparator such as varenicline standard plus NRT standard or NRT standard is likely to be a cost-effective use of resources.
Finally, although it was not the focus of this report, we found in our NMA that combining counselling and pharmacological treatments increased cessation rates compared with pharmacological treatment alone. Further research to explore the clinical effectiveness and cost-effectiveness of combination pharmacological and psychological interventions that account for AEs are likely to be of value.
Chapter 9 Patient and public involvement
A lay summary of this project was reviewed by participants of the UK Centre for Tobacco and Alcohol Studies (UKCTAS) smokers’ panel and was presented to members of the Elizabeth Blackwell Institute’s Public Advisory Group. Originally set up in 2008, the UKCTAS smokers’ panel consists of active smokers and recent quitters who meet two or three times per year in Nottingham. The panel meets regularly to discuss tobacco, tobacco policy, approaches to smoking cessation and new developments in tobacco harm reduction. Panel members also serve as lay advisers on research applications submitted from UKCTAS universities, which involves commenting on study information sheets, consent forms and data collection instruments (literature review protocols, data management plans and other key documents); commenting on press releases and communication plans; participating in bespoke meetings to develop or advise on new studies; and other forms of engagement as appropriate. The Elizabeth Blackwell Institute’s Public Advisory Group comprises key stakeholders in public engagement and health and social care as well as representatives of patient and public involvement groups linked to research projects at the University of Bristol. The Public Advisory Group works with the Elizabeth Blackwell Institute to ensure excellent engagement across the research life cycle. The group meets a few times per year, and researchers across the University of Bristol are invited to share their research with the group and receive feedback. During the course of the project, we interviewed vapers from the UKCTAS smokers’ panel for input on our outcomes and planned analyses. We also presented preliminary findings based on data from studies identified in our original searches to the Elizabeth Blackwell Institute’s Patient Advisory Panel for feedback and suggestions for further analyses.
Acknowledgements
We are grateful to Professor David Gunnell for help with preparing the original project proposal and grant submission, and to Sarah Dawson and Cath Borwick for help with designing and running search strategies.
Contributions of authors
Kyla H Thomas (https://orcid.org/0000-0001-5418-4034) (Consultant Senior Lecturer in Public Health Medicine) co-conceived the project, led the grant application, planned the data extraction and statistical analyses, led the project, contributed to analyses (screening and checking data extraction, assessment of risk of bias) and planning of statistical analyses, drafted sections of the report and finalised the report.
Michael N Dalili (https://orcid.org/0000-0002-6687-5374) (Senior Research Associate in Public Health) screened, extracted and checked data, assessed risk of bias, contributed to drafting the report, including preparation of tables and figures, and reviewed the final report.
José A López-López (https://orcid.org/0000-0002-9655-3616) (Research Fellow in Medical Statistics) undertook the statistical analyses of clinical effectiveness and safety and drafted relevant parts of the report.
Edna Keeney (https://orcid.org/0000-0002-4763-8891) (Senior Research Associate in Statistical and Health Economic Modelling) contributed to the cost-effectiveness analysis and drafting relevant chapters, and reviewed the final report.
David Phillippo (https://orcid.org/0000-0003-2672-7841) (Research Associate in Evidence Synthesis) carried out the threshold analyses and reviewed the final report.
Marcus R Munafò (https://orcid.org/0000-0002-4049-993X) (Professor of Biological Psychology) provided critical insight and reviewed the final report.
Matt Stevenson (https://orcid.org/0000-0002-3099-9877) (Professor of Health Technology Assessment) advised on the cost-effectiveness modelling and reviewed the final report.
Deborah M Caldwell (https://orcid.org/0000-0001-8014-7480) (Senior Lecturer in Public Health) co-conceived the project, contributed to the grant application, contributed to the systematic review and planning of NMAs and reviewed the final report.
Nicky J Welton (https://orcid.org/0000-0003-2198-3205) (Professor in Statistical and Health Economic Modelling) co-conceived the project, contributed to the grant application, provided statistical advice, made critical comments that helped in the interpretation of the results, planned and oversaw the cost-effectiveness analysis, helped in writing sections of the report and reviewed the final report.
Data-sharing statement
All study data will be available from the corresponding author on request, once papers reporting the study findings have been published. Corresponding author’s contact details: Population Health Sciences, Bristol Medical School, University of Bristol.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323-9. https://doi.org/10.1136/bmj.321.7257.323.
- Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014;370:60-8. https://doi.org/10.1056/NEJMra1308383.
- NHS Digital . Statistics on Smoking – England, 2019 2019.
- Public Health England . Cost of Smoking to the NHS in England: 2015 2017.
- Allender S, Balakrishnan R, Scarborough P, Webster P, Rayner M. The burden of smoking-related ill health in the UK. Tob Control 2009;18:262-7. https://doi.org/10.1136/tc.2008.026294.
- Action on Smoking and Health (ASH) . The Economics of Tobacco 2017.
- National Institute for Health and Care Excellence . Stop Smoking Interventions and Services 2018.
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005;48:3474-7. https://doi.org/10.1021/jm050069n.
- Sands S, Brooks P, Chambers L, Coe J, Liu Y, Rollema H. A New Therapy for Smoking Cessation: Varenicline, a Selective Nicotinic Receptor Partial Agonist [SYM10C] n.d.
- Raw M, McNeill A, Arnott D. Varenicline: Guidance for Health Professionals on a New Prescription-only Stop Smoking Medication. London: Action on Smoking and Health; 2006.
- National Institute for Health and Care Excellence . Varenicline for Smoking Cessation 2007.
- Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016;6:99-144. https://doi.org/10.1177/2045125316629071.
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther 1999;288:88-92.
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 2003;168:347-58. https://doi.org/10.1007/s00213-003-1445-7.
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197:371-7. https://doi.org/10.1007/s00213-007-1041-3.
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend 2002;67:219-23. https://doi.org/10.1016/S0376-8716(02)00067-4.
- Medicines and Healthcare products Regulatory Agency . Public Assessment Report. E-Voke 10mg and 15mg Electronic Inhaler (PL 42601 0003-4) 2015 n.d. https://mhraproductsprod.blob.core.windows.net/docs-20200330/56f25daab2a2968139bc37075e194d1a5f12b33f (accessed 23 November 2020).
- Action on Smoking and Health (ASH) . Briefing: Electronic Cigarettes 2018.
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629-37. https://doi.org/10.1056/NEJMoa1808779.
- McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H. E-Cigarettes: An Evidence Update – A Report Commissioned by Public Health England 2015.
- McNeill A, Brose LS, Calder R, Bauld L, Robson D. Evidence Review of Ecigarettes and Heated Tobacco Products 2018 – A Report Commissioned by Public Health England 2018.
- Action on Smoking and Health (ASH) . Use of E-Cigarettes (Vapourisers) Among Adults in Great Britain 2018.
- Prescribing and Primary Care team Health and Social Care Information Centre . Prescription Cost Analysis, England, 2013 2014. https://files.digital.nhs.uk/publicationimport/pub13xxx/pub13887/pres-cost-anal-eng-2013-rep.pdf (accessed 8 December 2020).
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016;5. https://doi.org/10.1002/14651858.CD006103.pub7.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;5. https://doi.org/10.1002/14651858.CD009329.pub2.
- Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD000031.pub4.
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2018;5. https://doi.org/10.1002/14651858.CD000146.pub5.
- Medicines and Healthcare products Regulatory Agency . Varenicline: adverse psychiatric reactions, including depression. Drug Saf Update 2008;2:2-3.
- US Food and Drug Administration . Information for Healthcare Professionals: Varenicline (Marketed As Chantix) and Bupropion (Marketed As Zyban, Wellbutrin and Generics) 2009;2. www.natap.org/2009/newsUpdates/070209_01.htm (accessed 8 December 2020).
- US Food and Drug Administration . FDA Drug Safety Communication: FDA Revises Description of Mental Health Side Effects of the Stop-Smoking Medicines Chantix (Varenicline) and Zyban (Bupropion) to Reflect Clinical Trial Findings 2016. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking (accessed 8 December 2020).
- US Food and Drug Administration . Public Health Advisory: FDA Requires New Boxed Warnings for the Smoking Cessation Drugs Chantix and Zyban 2009. http://wayback.archive-it.org/7993/20170112005513/http:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm169988.htm (accessed 8 December 2020).
- Harrison-Woolrych M. Mental health effects of varenicline. BMJ 2015;350. https://doi.org/10.1136/bmj.h1168.
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. https://doi.org/10.1016/S0140-6736(16)30272-0.
- European Medicines Agency (EMA) . Champix Procedural Steps Taken and Scientific Information After the Authorization 2016. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Procedural_steps_taken_and_scientific_information_after_authorisation/human/000699/WC500025256.pdf.
- US Food and Drug Administration . FDA Drug Safety Communication: FDA Revises Description of Mental Health Side Effects of the Stop-Smoking Medicines Chantix (Varenicline) and Zyban (Bupropion) to Reflect Clinical Trial Findings 2016. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking.
- Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry 2007;62:345-54. https://doi.org/10.1016/j.biopsych.2006.09.023.
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ 2012;344. https://doi.org/10.1136/bmj.e2856.
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ 2011;183:1359-66. https://doi.org/10.1503/cmaj.110218.
- Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation 2014;129:28-41. https://doi.org/10.1161/CIRCULATIONAHA.113.003961.
- Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Intern Med 2018;178:622-31. https://doi.org/10.1001/jamainternmed.2018.0397.
- BMA Board of Science . E-Cigarettes: Balancing Risks and Opportunities 2018.
- Royal College of Physicians . Nicotine Without Smoke: Tobacco Harm Reduction 2016.
- WHO Framework Convention on Tobacco Control . Electronic Nicotine Delivery Systems and Electronic Non-Nicotine Delivery Systems (ENDS ENNDS) 2016.
- Centers for Disease Control and Prevention . Outbreak of Lung Injury Associated With E-Cigarette Use, or Vaping 2019. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
- Global Tobacco Control . Country Laws Regulation E-Cigarettes: A Policy Scan 2019. www.globaltobaccocontrol.org/e-cigarette/country-laws/view (accessed 23 November 2020).
- UK Government . Foreign Travel Advice – Thailand 2019. www.gov.uk/foreign-travel-advice/thailand/local-laws-and-customs (accessed 23 November 2020).
- Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0027016 (accessed 23 November 2020).
- Harrison-Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: an intensive postmarketing prospective cohort study in New Zealand. Drug Saf 2011;34:763-72. https://doi.org/10.2165/11594450-000000000-00000.
- Kasliwal R, Wilton LV, Shakir SA. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription-event monitoring study. Drug Saf 2009;32:499-507. https://doi.org/10.2165/00002018-200932060-00006.
- Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf 2010;33:289-301. https://doi.org/10.2165/11319180-000000000-00000.
- Thomas KH, Martin RM, Knipe DW, Higgins JP, Gunnell D. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ 2015;350. https://doi.org/10.1136/bmj.h1109.
- Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry 2013;170:1460-7. https://doi.org/10.1176/appi.ajp.2013.12121599.
- Thomas KH, Martin RM, Davies NM, Metcalfe C, Windmeijer F, Gunnell D. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ 2013;347. https://doi.org/10.1136/bmj.f5704.
- Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ 2009;339. https://doi.org/10.1136/bmj.b3805.
- Meyer TE, Taylor LG, Xie S, Graham DJ, Mosholder AD, Williams JR, et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction 2013;108:203-10. https://doi.org/10.1111/j.1360-0443.2012.04024.x.
- Campbell AR, Anderson KD. Mental health stability in veterans with posttraumatic stress disorder receiving varenicline. Am J Health Syst Pharm 2010;67:1832-7. https://doi.org/10.2146/ajhp100196.
- Kotz D, Viechtbauer W, Simpson C, van Schayck OC, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med 2015;3:761-8. https://doi.org/10.1016/S2213-2600(15)00320-3.
- Leaviss J, Sullivan W, Ren S, Everson-Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? A systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18330.
- Strom BL, Strom BL, Kimmel SE, Hennessy S. Textbook of Pharmacoepidemiology. Chichester: John Wiley & Sons, Inc.; 2013.
- Canadian Collaboration for Drug Safety, Effectiveness and Network Meta-Analysis . A Systematic Review and Network Meta-Analysis of Pharmacologic Interventions for Smoking Cessation 2014.
- Li J, Hajek P, Pesola F, Wu Q, Phillips-Waller A, Przulj D, et al. Cost-effectiveness of e-cigarettes compared with nicotine replacement therapy in stop smoking services in England (TEC study): a randomized controlled trial. Addiction 2020;115:507-17. https://doi.org/10.1111/add.14829.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Inc.; 2008.
- Thomas KH, Caldwell D, Dalili MN, Gunnell D, Munafò MR, Stevenson M, et al. How do smoking cessation medicines compare with respect to their neuropsychiatric safety? A protocol for a systematic review, network meta-analysis and cost-effectiveness analysis. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015414.
- National Institute for Health and Care Excellence . British National Formulary (BNF) 2016. https://bnf.nice.org.uk/.
- Medicines and Healthcare products Regulatory Agency . Public Assessment Report. E-Voke 10mg and 15mg Electronic Inhaler (PL 42601 0003-4). Secondary Public Assessment Report. E-Voke 10mg and 15mg Electronic Inhaler (PL 42601 0003-4) 2015.
- Thomas KH, Dalili MN, López-López JA, Keeney E, Phillippo DM, Munafò MR, et al. Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta-analysis of randomized controlled trials [published online ahead of print 11 October 2021]. Addiction 2021. https://doi.org/10.1111/add.15675.
- Cochrane Tobacco Addiction Group . Glossary n.d. https://tobacco.cochrane.org/resources/glossary.
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther 2007;29:1040-56. https://doi.org/10.1016/j.clinthera.2007.06.012.
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic posttraumatic stress disorder. J Clin Psychopharmacol 2001;21:94-8. https://doi.org/10.1097/00004714-200102000-00017.
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD000146.pub4.
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD010216.pub3.
- Veritas Health Innovation . Covidence Systematic Review Software n.d. www.covidence.org/.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. https://doi.org/10.1002/sim.1875.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. https://doi.org/10.1136/bmj.331.7521.897.
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-analysis for Decision-making. Hoboken, NJ: John Wiley & Sons, Inc.; 2018.
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004;23:1351-75. https://doi.org/10.1002/sim.1761.
- Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350. https://doi.org/10.1136/bmj.h1551.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. https://doi.org/10.1177/0272989X12458724.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B 2002;64:583-616. https://doi.org/10.1111/1467-9868.00353.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. https://doi.org/10.1002/sim.3767.
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. https://doi.org/10.1177/0962280207080643.
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7387.472.
- Dias S, Welton, NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials 2011. www.nicedsu.org.uk.
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411. https://doi.org/10.1002/sim.4780140406.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 1978;3:235-41. https://doi.org/10.1016/0306-4603(78)90024-2.
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989;84:791-9. https://doi.org/10.1111/j.1360-0443.1989.tb03059.x.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19. https://doi.org/10.1016/0895-4356(92)90133-8.
- Phillippo DM, Dias S, Ades AE, Didelez V, Welton NJ. Sensitivity of treatment recommendations to bias in network meta-analysis. J R Stat Soc Ser A Stat Soc 2018;181:843-67. https://doi.org/10.1111/rssa.12341.
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses threshold analysis in guideline development. Ann Intern Med 2019;170:538-46. https://doi.org/10.7326/M18-3542.
- Caldwell DM, Ades AE, Dias S, Watkins S, Li T, Taske N, et al. A threshold analysis assessed the credibility of conclusions from network meta-analysis. J Clin Epidemiol 2016;80:68-76. https://doi.org/10.1016/j.jclinepi.2016.07.003.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Orme ME, Hogue SL, Kennedy LM, Paine AC, Godfrey C. Development of the health and economic consequences of smoking interactive model. Tob Control 2001;10:55-61. https://doi.org/10.1136/tc.10.1.55.
- Annemans L, Nackaerts K, Bartsch P, Prignot J, Marbaix S. Cost effectiveness of varenicline in Belgium, compared with bupropion, nicotine replacement therapy, brief counselling and unaided smoking cessation: a BENESCO Markov cost-effectiveness analysis. Clin Drug Investig 2009;29:655-65. https://doi.org/10.2165/11317730-000000000-00000.
- Hoogendoorn M, Welsing P, Rutten-van Mölken MP. Cost-effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Curr Med Res Opin 2008;24:51-6. https://doi.org/10.1185/030079908x242917.
- Linden K, Jormanainen V, Linna M, Sintonen H, Wilson K, Kotomäki T. Cost effectiveness of varenicline versus bupropion and unaided cessation for smoking cessation in a cohort of Finnish adult smokers. Curr Med Res Opin 2010;26:549-60. https://doi.org/10.1185/03007990903542666.
- Bae JY, Kim CH, Lee EK. Evaluation of cost-utility of varenicline compared with existing smoking cessation therapies in South Korea. Value Health 2009;12:70-3. https://doi.org/10.1111/j.1524-4733.2009.00631.x.
- Bolin K, Mörk AC, Wilson K. Smoking-cessation therapy using varenicline: the cost-utility of an additional 12-week course of varenicline for the maintenance of smoking abstinence. J Eval Clin Pract 2009;15:478-85. https://doi.org/10.1111/j.1365-2753.2008.01045.x.
- Bolin K, Wilson K, Benhaddi H, de Nigris E, Marbaix S, Mork AC, et al. Cost-effectiveness of varenicline compared with nicotine patches for smoking cessation – results from four European countries. Eur J Public Health 2009;19:650-4. https://doi.org/10.1093/eurpub/ckp075.
- Bolin K, Mörk AC, Willers S, Lindgren B. Varenicline as compared to bupropion in smoking-cessation therapy – cost-utility results for Sweden 2003. Respir Med 2008;102:699-710. https://doi.org/10.1016/j.rmed.2007.12.018.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US adult smokers. PharmacoEconomics 2008;26:497-511. https://doi.org/10.2165/00019053-200826060-00004.
- Knight C, Howard P, Baker CL, Marton JP. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health 2010;13:209-14. https://doi.org/10.1111/j.1524-4733.2009.00672.x.
- Vemer P, Rutten-van Mölken MP. Crossing borders: factors affecting differences in cost-effectiveness of smoking cessation interventions between European countries. Value Health 2010;13:230-41. https://doi.org/10.1111/j.1524-4733.2009.00612.x.
- ONS . Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2016 2017.
- ONS . Deaths by Single Year of Age, UK, 2016 2016.
- Unrod M, Simmons VN, Sutton SK, Cummings KM, Celestino P, Craig BM, et al. Relapse-prevention booklets as an adjunct to a tobacco quitline: a randomized controlled effectiveness trial. Nicotine Tob Res 2016;18:298-305. https://doi.org/10.1093/ntr/ntv079.
- British Lung Foundation . Chronic Obstructive Pulmonary Disease Statistics n.d. https://statistics.blf.org.uk/copd.
- Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000;55:789-94. https://doi.org/10.1136/thorax.55.9.789.
- Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, et al. Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer 2009;101:541-7. https://doi.org/10.1038/sj.bjc.6605148.
- Forman D, Stockton D, Møller H, Quinn M, Babb P, De Angelis R, et al. Cancer prevalence in the UK: results from the EUROPREVAL study. Ann Oncol 2003;14:648-54. https://doi.org/10.1093/annonc/mdg169.
- NHS Digital . Health Survey for England, 2016. Adult Health Trends 2016.
- Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015;101:1182-9. https://doi.org/10.1136/heartjnl-2015-307516.
- NHS Digital . Statistics on Smoking, England – 2017 2017.
- Cassino C, Ito K, Bader I, Ciotoli C, Thurston G, Reibman J. Cigarette smoking and ozone-associated emergency department use for asthma by adults in New York City. Am J Respir Crit Care Med 1999;159:1773-9. https://doi.org/10.1164/ajrccm.159.6.9809042.
- Pfizer Ltd . Manufacturer’s Submission for NICE STA of Varenicline for Smoking Cessation 2007.
- King A, Broggio J. Cancer Registration Statistics, England: 2016, 2018. Newport: ONS; 2018.
- Brennan P, Crispo A, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, Lissowska J, et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am J Epidemiol 2006;164:1233-41. https://doi.org/10.1093/aje/kwj340.
- British Heart Foundation . CVD Statistics Compendium 2018.
- Hawkins J, Hollingworth W, Campbell R. Long-term smoking relapse: a study using the British Household Panel Survey. Nicotine Tob Res 2010;12:1228-35. https://doi.org/10.1093/ntr/ntq175.
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 2002;4:95-100. https://doi.org/10.1080/14622200110098428.
- Health Information and Quality Authority (HIQA) . Health Technology Assessment (HTA) of Smoking Cessation Interventions 2017.
- Nichols M, Townsend N, Luengo-Fernandez R, Leal J, Gray A, Scarborough P, et al. European Cardiovascular Disease Statistics 2012 2012.
- HM Revenue & Customs . February 2019: Monthly Exchange Rates 2019.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med 2003;97:71-9. https://doi.org/10.1016/S0954-6111(03)80027-6.
- Flack S, Taylor M, Trueman PJ. Cost-effectiveness of Interventions for Smoking Cessation. York: York Health Economics Consortium; 2007.
- British Heart Foundation . Cardiovascular Disease Statistics 2014 2014.
- McMurray J, Hart W. Rhodes G. An Evaluation of the Cost of Heart Failure to the National Health Service in the UK 1993;6:99-110.
- Xu XM, Vestesson E, Paley L, Desikan A, Wonderling D, Hoffman A, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J 2018;3:82-91. https://doi.org/10.1177/2396987317746516.
- Simpson E, Stevenson M, Scope A, Poku E, Minton J, Evans P. Echocardiography in newly diagnosed atrial fibrillation patients: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17360.
- Tan NC, Nguyen HV, Lye WK, Sankari U, Nadkarni NV. Trends and predictors of asthma costs: results from a 10-year longitudinal study. Eur Respir J 2016;47:801-9. https://doi.org/10.1183/13993003.00188-2015.
- Hoskins G, McCowan C, Neville RG, Thomas GE, Smith B, Silverman S. Risk factors and costs associated with an asthma attack. Thorax 2000;55:19-24. https://doi.org/10.1136/thorax.55.1.19.
- Hunter RM, Nazareth I, Morris S, King M. Modelling the cost-effectiveness of preventing major depression in general practice patients. Psychol Med 2014;44:1381-90. https://doi.org/10.1017/S0033291713002067.
- Tsiachristas A, McDaid D, Casey D, Brand F, Leal J, Park AL, et al. General hospital costs in England of medical and psychiatric care for patients who self-harm: a retrospective analysis. Lancet Psychiatry 2017;4:759-67. https://doi.org/10.1016/S2215-0366(17)30367-X.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2018.
- Hartmann-Boyce J, Begh R, Aveyard P. Electronic cigarettes for smoking cessation. BMJ 2018;360. https://doi.org/10.1136/bmj.j5543.
- Liber AC, Drope JM, Stoklosa M. Combustible cigarettes cost less to use than e-cigarettes: global evidence and tax policy implications. Tob Control 2017;26:158-63. https://doi.org/10.1136/tobaccocontrol-2015-052874.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Jang RW, Isogai PK, Mittmann N, Bradbury PA, Shepherd FA, Feld R, et al. Derivation of utility values from European Organization for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol 2010;5:1953-7. https://doi.org/10.1097/JTO.0b013e3181f77a6a.
- Bertranou E, Bodnar C, Dansk V, Greystoke A, Large S, Dyer M. Cost-effectiveness of osimertinib in the UK for advanced EGFR-T790M non-small cell lung cancer. J Med Econ 2018;21:113-21. https://doi.org/10.1080/13696998.2017.1377718.
- Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. PharmacoEconomics 2001;19:855-63. https://doi.org/10.2165/00019053-200119080-00007.
- Pickard AS, Wilke C, Jung E, Patel S, Stavem K, Lee TA. Use of a preference-based measure of health (EQ-5D) in COPD and asthma. Respir Med 2008;102:519-36. https://doi.org/10.1016/j.rmed.2007.11.016.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. PharmacoEconomics 2005;23:619-37. https://doi.org/10.2165/00019053-200523060-00008.
- Stevanović J, Pechlivanoglou P, Kampinga MA, Krabbe PF, Postma MJ. Multivariate meta-analysis of preference-based quality of life values in coronary heart disease. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0152030.
- Hay JW, Sterling KL. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. PharmacoEconomics 2005;23:133-41. https://doi.org/10.2165/00019053-200523020-00005.
- Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193-8. https://doi.org/10.1161/01.STR.0000196990.69412.fb.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. PharmacoEconomics 2003;21:191-200. https://doi.org/10.2165/00019053-200321030-00004.
- Gage BF, Cardinalli AB, Owens DK. Cost-effectiveness of preference-based antithrombotic therapy for patients with nonvalvular atrial fibrillation. Stroke 1998;29:1083-91. https://doi.org/10.1161/01.str.29.6.1083.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7. https://doi.org/10.3132/pcrj.2007.00002.
- Szende A, Svensson K, Ståhl E, Mészáros A, Berta GY. Psychometric and utility-based measures of health status of asthmatic patients with different disease control level. PharmacoEconomics 2004;22:537-47. https://doi.org/10.2165/00019053-200422080-00005.
- Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al. A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9160.
- Kendrick T, Peveler R, Longworth L, Baldwin D, Moore M, Chatwin J, et al. Cost-effectiveness and cost-utility of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine: randomised controlled trial. Br J Psychiatry 2006;188:337-45. https://doi.org/10.1192/bjp.188.4.337.
- Mann R, Gilbody S, Richards D. Putting the ‘Q’ in depression QALYs: a comparison of utility measurement using EQ-5D and SF-6D health related quality of life measures. Soc Psychiatry Psychiatr Epidemiol 2009;44:569-78. https://doi.org/10.1007/s00127-008-0463-5.
- Hollinghurst S, Peters TJ, Kaur S, Wiles N, Lewis G, Kessler D. Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry 2010;197:297-304. https://doi.org/10.1192/bjp.bp.109.073080.
- Byford S, Knapp M, Greenshields J, Ukoumunne OC, Jones V, Thompson S, et al. Cost-effectiveness of brief cognitive behaviour therapy versus treatment as usual in recurrent deliberate self-harm: a decision-making approach. Psychol Med 2003;33:977-86. https://doi.org/10.1017/s0033291703008183.
- Taylor GMJ, Taylor AE, Thomas KH, Jones T, Martin RM, Munafò MR, et al. The effectiveness of varenicline versus nicotine replacement therapy on long-term smoking cessation in primary care: a prospective cohort study of electronic medical records. Int J Epidemiol 2017;46:1948-57. https://doi.org/10.1093/ije/dyx109.
- York Health Economics Consortium . Net Monetary Benefit 2016. https://yhec.co.uk/glossary/net-monetary-benefit/ (accessed September 2019).
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. https://doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- University of Sheffield . Sheffield Accelerated Value of Information 2015.
- Rasmussen CE, Williams CKI. Gaussian Processes for Machine Learning. Cambridge, MA: MIT Press; 2006.
- Abdullah AS, Hedley AJ, Chan SS, Lam TH. A randomized controlled trial of two different lengths of nicotine replacement therapy for smoking cessation. Biomed Res Int 2013;2013. https://doi.org/10.1155/2013/961751.
- Abelin T, Buehler A, Müller P, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989;1:7-10. https://doi.org/10.1016/S0140-6736(89)91671-1.
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002;288:468-74. https://doi.org/10.1001/jama.288.4.468.
- Ahluwalia JS, McNagny SE, Clark WS. Smoking cessation among inner-city African Americans using the nicotine transdermal patch. J Gen Intern Med 1998;13:1-8. https://doi.org/10.1046/j.1525-1497.1998.00001.x.
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction 2006;101:883-91. https://doi.org/10.1111/j.1360-0443.2006.01461.x.
- Anderson CM, Cummins SE, Kohatsu ND, Gamst AC, Zhu SH. Incentives and patches for Medicaid smokers: an RCT. Am J Prev Med 2018;55:138-47. https://doi.org/10.1016/j.amepre.2018.07.015.
- Andrews JO, Mueller M, Dooley M, Newman SD, Magwood GS, Tingen MS. Effect of a smoking cessation intervention for women in subsidized neighborhoods: a randomized controlled trial. Prev Med 2016;90:170-6. https://doi.org/10.1016/j.ypmed.2016.07.008.
- Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med 2013;159:390-40. https://doi.org/10.7326/0003-4819-159-6-201309170-00005.
- Areechon W, Punnotok J. Smoking cessation through the use of nicotine chewing gum: a double-blind trial in Thailand. Clin Ther 1988;10:183-6.
- Aryanpur M, Hosseini M, Masjedi MR, Mortaz E, Tabarsi P, Soori H, et al. A randomized controlled trial of smoking cessation methods in patients newly-diagnosed with pulmonary tuberculosis. BMC Infect Dis 2016;16. https://doi.org/10.1186/s12879-016-1727-4.
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 2008;63:717-24. https://doi.org/10.1136/thx.2007.090647.
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction 2004;99:1206-18. https://doi.org/10.1111/j.1360-0443.2004.00814.x.
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. Am J Psychiatry 2006;163:1934-42. https://doi.org/10.1176/ajp.2006.163.11.1934.
- Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA 2016;315:371-9. https://doi.org/10.1001/jama.2015.19284.
- Baldassarri SR, Bernstein SL, Chupp GL, Slade MD, Fucito LM, Toll BA. Electronic cigarettes for adults with tobacco dependence enrolled in a tobacco treatment program: a pilot study. Addict Behav 2018;80:1-5. https://doi.org/10.1016/j.addbeh.2017.11.033.
- Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clin Pharmacol Ther 2005;78:689-96. https://doi.org/10.1016/j.clpt.2005.08.019.
- Bernstein SL, D’Onofrio G, Rosner J, O’Malley S, Makuch R, Busch S, et al. Successful tobacco dependence treatment in low-income emergency department patients: a randomized trial. Ann Emerg Med 2015;66:140-7. https://doi.org/10.1016/j.annemergmed.2015.03.030.
- Binnie VI, McHugh S, Jenkins W, Borland W, Macpherson LM. A randomised controlled trial of a smoking cessation intervention delivered by dental hygienists: a feasibility study. BMC Oral Health 2007;7. https://doi.org/10.1186/1472-6831-7-5.
- Bjurlin MA, Cohn MR, Kim DY, Freeman VL, Lombardo L, Hurley SD, et al. Brief smoking cessation intervention: a prospective trial in the urology setting. J Urol 2013;189:1843-9. https://doi.org/10.1016/j.juro.2012.11.075.
- Blöndal T. Controlled trial of nicotine polacrilex gum with supportive measures. Arch Intern Med 1989;149:1818-21. https://doi.org/10.1001/archinte.1989.00390080080018.
- Blöndal T, Franzon M, Westin A. A double-blind randomized trial of nicotine nasal spray as an aid in smoking cessation. Eur Respir J 1997;10:1585-90. https://doi.org/10.1183/09031936.97.10071585.
- Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ 1999;318:285-8. https://doi.org/10.1136/bmj.318.7179.285.
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized, double-blind, placebo-controlled trial. Arch Intern Med 2000;160:3128-34. https://doi.org/10.1001/archinte.160.20.3128.
- Bolliger CT, Issa JS, Posadas-Valay R, Safwat T, Abreu P, Correia EA, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin Ther 2011;33:465-77. https://doi.org/10.1016/j.clinthera.2011.04.013.
- Bolliger CT, van Biljon X, Axelsson A. A nicotine mouth spray for smoking cessation: a pilot study of preference, safety and efficacy. Respiration 2007;74:196-201. https://doi.org/10.1159/000097136.
- Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ 2000;321:329-33. https://doi.org/10.1136/bmj.321.7257.329.
- Bonevski B, Twyman L, Paul C, D’Este C, West R, Siahpush M, et al. Smoking cessation intervention delivered by social service organisations for a diverse population of Australian disadvantaged smokers: a pragmatic randomised controlled trial. Prev Med 2018;112:38-44. https://doi.org/10.1016/j.ypmed.2018.04.005.
- Boyle RG. Smokeless tobacco cessation with nicotine replacement: a randomized clinical trial. Diss Abstr Int 1992;54.
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res 2007;9:721-30. https://doi.org/10.1080/14622200701416955.
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382:1629-37. https://doi.org/10.1016/S0140-6736(13)61842-5.
- Bullen C, Howe C, Lin RB, Grigg M, Laugesen M, McRobbie H, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction 2010;105:1474-83. https://doi.org/10.1111/j.1360-0443.2010.02989.x.
- Burns RJ, Rothman AJ, Fu SS, Lindgren B, Vock DM, Joseph AM. Longitudinal care improves cessation in smokers who do not initially respond to treatment by increasing cessation self-efficacy, satisfaction, and readiness to quit: a mediated moderation analysis. Ann Behav Med 2016;50:58-69. https://doi.org/10.1007/s12160-015-9732-1.
- Caldwell AL, Tingen MS, Nguyen JT, Andrews JO, Heath J, Waller JL, et al. Parental smoking cessation: impacting children’s tobacco smoke exposure in the home. Pediatrics 2018;141:S96-10. https://doi.org/10.1542/peds.2017-1026M.
- Caldwell BO, Adamson SJ, Crane J. Combination rapid-acting nicotine mouth spray and nicotine patch therapy in smoking cessation. Nicotine Tob Res 2014;16:1356-64. https://doi.org/10.1093/ntr/ntu084.
- Caldwell BO, Crane J. Combination nicotine metered dose inhaler and nicotine patch for smoking cessation: a randomized controlled trial. Nicotine Tob Res 2016;18:1944-51. https://doi.org/10.1093/ntr/ntw093.
- Campbell IA. Comparison of 4 methods of smoking withdrawal in patients with smoking related diseases. Br Med J 1983;286:595-7. https://doi.org/10.1136/bmj.286.6365.595.
- Campbell IA, Lyons E, Prescott RJ. Stopping smoking. Do nicotine chewing-gum and postal encouragement add to doctors’ advice. Practitioner 1987;231:114-17.
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respir Med 1991;85:155-7. https://doi.org/10.1016/S0954-6111(06)80295-7.
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking-related diseases: a placebo-controlled study. Respir Med 1996;90:47-51. https://doi.org/10.1016/S0954-6111(96)90244-9.
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0066317.
- Carson KV, Smith BJ, Brinn MP, Peters MJ, Fitridge R, Koblar SA, et al. Safety of varenicline tartrate and counseling versus counseling alone for smoking cessation: a randomized controlled trial for inpatients (STOP study). Nicotine Tob Res 2014;16:1495-502. https://doi.org/10.1093/ntr/ntu112.
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106:1155-63. https://doi.org/10.1111/j.1360-0443.2011.03363.x.
- Chengappa KN, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2014;75:765-72. https://doi.org/10.4088/JCP.13m08756.
- Cinciripini PM, Cinciripini LG, Wallfisch A, Haque W, Van Vunakis H. Behavior therapy and the transdermal nicotine patch: effects on cessation outcome, affect, and coping. J Consult Clin Psychol 1996;64:314-23. https://doi.org/10.1037//0022-006x.64.2.314.
- Cinciripini PM, Minnix JA, Green CE, Robinson JD, Engelmann JM, Versace F, et al. An RCT with the combination of varenicline and bupropion for smoking cessation: clinical implications for front line use [published online ahead of print April 21 2018]. Addiction 2018. https://doi.org/10.1111/add.14250.
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013;70:522-33. https://doi.org/10.1001/jamapsychiatry.2013.678.
- Clavel F, Benhamou S, Company-Huertas A, Flamant R. Helping people to stop smoking: randomised comparison of groups being treated with acupuncture and nicotine gum with control group. Br Med J 1985;291:1538-9. https://doi.org/10.1136/bmj.291.6508.1538.
- Clavelchapelon F, Paoletti C, Benhamou S. A randomized 2x2 factorial design to evaluate different smoking cessation methods. Rev Epidemiol Sante Publique 1992;40:187-90.
- Collins BN, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine Tob Res 2004;6:27-3. https://doi.org/10.1080/14622200310001656830.
- Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, et al. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction 2009;104:1588-96. https://doi.org/10.1111/j.1360-0443.2009.02624.x.
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Alcohol and tobacco cessation in alcohol-dependent smokers: analysis of real-time reports. Psychol Addict Behav 2007;21:277-86. https://doi.org/10.1037/0893-164X.21.3.277.
- Cooper TV, Klesges RC, Debon MW, Zbikowski SM, Johnson KC, Clemens LH. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addict Behav 2005;30:61-75. https://doi.org/10.1016/j.addbeh.2004.04.013.
- Cooperman NA, Lu SE, Richter KP, Bernstein SL, Williams JM. Pilot study of a tailored smoking cessation intervention for individuals in treatment for opioid dependence. Nicotine Tob Res 2018;20:1152-6. https://doi.org/10.1093/ntr/ntx189.
- Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction 2007;102:1292-302. https://doi.org/10.1111/j.1360-0443.2007.01887.x.
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst 2012;104:290-8. https://doi.org/10.1093/jnci/djr513.
- Croghan GA, Sloan JA, Croghan IT, Novotny P, Hurt RD, DeKrey WL, et al. Comparison of nicotine patch alone versus nicotine nasal spray alone versus a combination for treating smokers: a minimal intervention, randomized multicenter trial in a nonspecialized setting. Nicotine Tob Res 2003;5:181-7. https://doi.org/10.1080/1462220031000073252.
- Cropsey KL, Clark CB, Zhang X, Hendricks PS, Jardin BF, Lahti AC. Race and medication adherence moderate cessation outcomes in criminal justice smokers. Am J Prev Med 2015;49:335-44. https://doi.org/10.1016/j.amepre.2015.03.014.
- Cummings KM, Hyland A, Carlin-Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line. J Public Health Manag Pract 2011;17:E16-23. https://doi.org/10.1097/PHH.0b013e3182113871.
- Cummins SE, Gamst AC, Brandstein K, Seymann GB, Klonoff-Cohen H, Kirby CA, et al. Helping hospitalized smokers: a factorial RCT of nicotine patches and counseling. Am J Prev Med 2016;51:578-86. https://doi.org/10.1016/j.amepre.2016.06.021.
- Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Effect of Mailing nicotine patches on tobacco cessation among adult smokers: a randomized clinical trial. JAMA Intern Med 2016;176:184-90. https://doi.org/10.1001/jamainternmed.2015.7792.
- Dale LC, Ebbert JO, Glover ED, Croghan IT, Schroeder DR, Severson HH, et al. Bupropion SR for the treatment of smokeless tobacco use. Drug Alcohol Depend 2007;90:56-63. https://doi.org/10.1016/j.drugalcdep.2007.02.008.
- Dale LC, Ebbert JO, Schroeder DR, Croghan IT, Rasmussen DF, Trautman JA, et al. Bupropion for the treatment of nicotine dependence in spit tobacco users: a pilot study. Nicotine Tob Res 2002;4:267-74. https://doi.org/10.1080/14622200210153821.
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA 1995;274:1353-8. https://doi.org/10.1001/jama.1995.03530170033028.
- Dalsgareth OJ, Hansen NC, Søes-Petersen U, Evald T, Høegholm A, Barber J, et al. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res 2004;6:55-61. https://doi.org/10.1080/14622200310001656867.
- Daughton D, Susman J, Sitorius M, Belenky S, Millatmal T, Nowak R, et al. Transdermal nicotine therapy and primary care. Importance of counseling, demographic, and participant selection factors on 1-year quit rates. The Nebraska Primary Practice Smoking Cessation Trial Group. Arch Fam Med 1998;7:425-30. https://doi.org/10.1001/archfami.7.5.425.
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post-quit day. Prev Med 1999;28:113-18. https://doi.org/10.1006/pmed.1998.0391.
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effect of transdermal nicotine delivery as an adjunct to low-intervention smoking cessation therapy. A randomized, placebo-controlled, double-blind study. Arch Intern Med 1991;151:749-52. https://doi.org/10.1001/archinte.1991.00400040091020.
- Dautzenberg B, Ruff F, Vaucher M, Maillon P, Jacob N, Kienzler JL, et al. First demonstration of the good efficacy/safety ratio of Nicotinell® 1mg lozenge (NL 1mg), a new form of nicotine substitution, by randomised clinical trial. Eur Respiratory J 2001;18.
- Davidson M, Epstein M, Burt R, Schaefer C, Whitworth G, McDonald A. Efficacy and safety of an over-the-counter transdermal nicotine patch as an aid for smoking cessation. Arch Fam Med 1998;7:569-74. https://doi.org/10.1001/archfami.7.6.569.
- de Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M. Project Impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. J Subst Abuse Treat 2012;43:322-30. https://doi.org/10.1016/j.jsat.2012.01.004.
- Dogar O, Zahid R, Mansoor S, Kanaan M, Ahluwalia JS, Jawad M, et al. Varenicline versus placebo for waterpipe smoking cessation: a double-blind randomized controlled trial. Addiction 2018;113:2290-9. https://doi.org/10.1111/add.14430.
- Ebbert J, Croghan I, Hurt R, Schroeder D, Hays J. Varenicline for smoking cessation in light smokers. Nicotine Tob Res 2016;18:2031-5. https://doi.org/10.1093/ntr/ntw123.
- Ebbert JO, Croghan IT, Schroeder DR, Hurt RD. A randomized phase II clinical trial of high-dose nicotine patch therapy for smokeless tobacco users. Nicotine Tob Res 2013;15:2037-44. https://doi.org/10.1093/ntr/ntt097.
- Ebbert JO, Croghan IT, Severson HH, Schroeder DR, Hays JT. A pilot study of the efficacy of varenicline for the treatment of smokeless tobacco users in Midwestern United States. Nicotine Tob Res 2011;13:820-6. https://doi.org/10.1093/ntr/ntr078.
- Ebbert JO, Dale LC, Patten CA, Croghan IT, Schroeder DR, Moyer TP, et al. Effect of high-dose nicotine patch therapy on tobacco withdrawal symptoms among smokeless tobacco users. Nicotine Tob Res 2007;9:43-52. https://doi.org/10.1080/14622200601078285.
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA 2014;311:155-63. https://doi.org/10.1001/jama.2013.283185.
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313:687-94. https://doi.org/10.1001/jama.2015.280.
- Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A randomized clinical trial of nicotine lozenge for smokeless tobacco use. Nicotine Tob Res 2009;11:1415-23. https://doi.org/10.1093/ntr/ntp154.
- Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A pilot study of mailed nicotine lozenges with assisted self-help for the treatment of smokeless tobacco users. Addict Behav 2010;35:522-5. https://doi.org/10.1016/j.addbeh.2009.12.020.
- Ehrsam RE, Bühler A, Müller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch. Schweiz Rundsch Med Prax 1991;80:145-50.
- Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol 2013;61:524-32. https://doi.org/10.1016/j.jacc.2012.08.1030.
- Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016;133:21-30. https://doi.org/10.1161/CIRCULATIONAHA.115.019634.
- Ellerbeck EF, Nollen N, Hutcheson TD, Phadnis M, Fitzgerald SA, Vacek J, et al. Effect of long-term nicotine replacement therapy vs standard smoking cessation for smokers with chronic lung disease: a randomized clinical trial. JAMA Netw Open 2018;1. https://doi.org/10.1001/jamanetworkopen.2018.1843.
- Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. J Clin Psychopharmacol 2002;22:487-95. https://doi.org/10.1097/00004714-200210000-00008.
- Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Arch Intern Med 2009;169:1028-34. https://doi.org/10.1001/archinternmed.2009.12.
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol 2007;27:380-6. https://doi.org/10.1097/01.jcp.0b013e3180ca86fa.
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CM, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol 2005;25:218-25. https://doi.org/10.1097/01.jcp.0000162802.54076.18.
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 2014;311:145-54. https://doi.org/10.1001/jama.2013.285113.
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob Res 2001;3:397-403. https://doi.org/10.1080/14622200110073920.
- Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ 2010;341. https://doi.org/10.1136/bmj.c6549.
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. J Behav Med 1982;5:343-51. https://doi.org/10.1007/BF00846161.
- Fagerström KO. Effects of nicotine chewing gum and follow-up appointments in physician-based smoking cessation. Prev Med 1984;13:517-27. https://doi.org/10.1016/0091-7435(84)90020-3.
- Fee WM, Stewart MJ. A controlled trial of nicotine chewing gum in a smoking withdrawal clinic. Practitioner 1982;226:148-51.
- Fernandez Arias IG, Garcia-Vera MP, Sanz J. The more psychology, the better: the efficacy of smoking cessation treatment using intensive cognitive-behavioral therapy versus a combination of nicotine patches plus intensive or less intensive cognitive-behavioral therapy: first prize of the 20th ‘Rafael Burgaleta’ Applied Psychology Awards 2013. Clinic Salud 2014;25:1-10.
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in non depressed smokers. J Addictive Dis 1994;13.
- Ferry LH, Robbins AS, Scariati PD, Masterson A, Abbey DE, Burchette RJ. Enhancement of smoking cessation using the antidepressant bupropion hydrochloride. Circulation 1992;86:I-671.
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524-33. https://doi.org/10.1378/chest.105.2.524.
- Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: an effectiveness study. Prev Med 2004;38:412-20. https://doi.org/10.1016/j.ypmed.2003.11.002.
- Fortmann SP, Killen JD. Nicotine gum and self-help behavioral treatment for smoking relapse prevention: results from a trial using population-based recruitment. J Consult Clin Psychol 1995;63:460-8. https://doi.org/10.1037//0022-006x.63.3.460.
- Fossati R, Apolone G, Negri E, Compagnoni A, La Vecchia C, Mangano S, et al. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care. Arch Intern Med 2007;167:1791-7. https://doi.org/10.1001/archinte.167.16.1791.
- Fouz-Rosón N, Montemayor-Rubio T, Almadana-Pacheco V, Montserrat-García S, Gómez-Bastero AP, Romero-Muñoz C, et al. Effect of 0.5 mg versus 1 mg varenicline for smoking cessation: a randomized controlled trial. Addiction 2017;112:1610-19. https://doi.org/10.1111/add.13855.
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. J Psychoactive Drugs 2007;39:487-97. https://doi.org/10.1080/02791072.2007.10399888.
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. J Subst Abuse Treat 2009;37:247-55. https://doi.org/10.1016/j.jsat.2009.01.006.
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine Tob Res 2000;2:53-6. https://doi.org/10.1080/14622200050011303.
- George TP, Vessicchio JC, Sacco KA, Weinberger AH, Dudas MM, Allen TM, et al. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry 2008;63:1092-6. https://doi.org/10.1016/j.biopsych.2007.11.002.
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry 2002;52:53-61. https://doi.org/10.1016/S0006-3223(02)01339-2.
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, et al. Acceptance-based treatment for smoking cessation. Behav Ther 2004;35:689-705. https://doi.org/10.1016/S0005-7894(04)80015-7.
- Gilbert JR, Wilson DM, Best JA, Taylor DW, Lindsay EA, Singer J, et al. Smoking cessation in primary care. A randomized controlled trial of nicotine-bearing chewing gum. J Fam Pract 1989;28:49-55.
- Ginsberg D, Hall SM, Rosinski M. Partner support, psychological treatment, and nicotine gum in smoking treatment: an incremental study. Int J Addict 1992;27:503-14. https://doi.org/10.3109/10826089209063465.
- Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double-blind study. Croat Med J 2003;44:219-24.
- Glavas D, Rumboldt Z. Smoking cessation using the transdermal nicotine system. Lijec Vjesn 2003;125:8-12.
- GlaxoSmithKline . Efficacy and Safety Study of Nicotine Mint Lozenge (2mg and 4mg) in Smoking Cessation 2009. https://ClinicalTrials.gov/show/NCT00985985.
- Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, et al. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine Tob Res 2002;4:441-50. https://doi.org/10.1080/1462220021000018443.
- Goldstein MG, Niaura R, Follick MJ, Abrams DB. Effects of behavioral skills training and schedule of nicotine gum administration on smoking cessation. Am J Psychiatry 1989;146:56-60. https://doi.org/10.1176/ajp.146.1.56.
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng LJ, McRae TD, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clin Pharmacol Ther 2014;96:390-6. https://doi.org/10.1038/clpt.2014.124.
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55. https://doi.org/10.1001/jama.296.1.47.
- Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther 2001;69:438-44. https://doi.org/10.1067/mcp.2001.115750.
- Górecka D, Bednarek M, Nowiński A, Puścińska E, Goljan-Geremek A, Zieliński J. Effect of treatment for nicotine dependence in patients with COPD. Pneumonol Alergol Pol 2003;71:411-17. https://doi.org/10.1136/bmj.311.7001.363.
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ 1995;311:363-6.
- Graham AL, Papandonatos GD, Cha S, Erar B, Amato MS, Cobb NK, et al. Improving adherence to smoking cessation treatment: intervention effects in a web-based randomized trial. Nicotine Tob Res 2017;19:324-32. https://doi.org/10.1093/ntr/ntw282.
- Grant KM, Kelley SS, Smith LM, Agrawal S, Meyer JR, Romberger DJ. Bupropion and nicotine patch as smoking cessation aids in alcoholics. Alcohol 2007;41:381-91. https://doi.org/10.1016/j.alcohol.2007.03.011.
- Gross J, Johnson J, Sigler L, Stitzer ML. Dose effects of nicotine gum. Addict Behav 1995;20:371-81. https://doi.org/10.1016/0306-4603(94)00078-D.
- Haas JS, Linder JA, Park ER, Gonzalez I, Rigotti NA, Klinger EV, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Intern Med 2015;175:218-26. https://doi.org/10.1001/jamainternmed.2014.6674.
- Haggsträm FM, Chatkin JM, Sussenbach-Vaz E, Cesari DH, Fam CF, Fritscher CC. A controlled trial of nortriptyline, sustained-release bupropion and placebo for smoking cessation: preliminary results. Pulm Pharmacol Ther 2006;19:205-9. https://doi.org/10.1016/j.pupt.2005.05.003.
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. Am J Public Health 2011;101:2349-56. https://doi.org/10.2105/AJPH.2010.300084.
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009;104:1043-52. https://doi.org/10.1111/j.1360-0443.2009.02548.x.
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry 2002;59:930-6. https://doi.org/10.1001/archpsyc.59.10.930.
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol 1996;64:1003-9. https://doi.org/10.1037//0022-006x.64.5.1003.
- Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health 2006;96:1808-14. https://doi.org/10.2105/AJPH.2005.080382.
- Hall SM, Tunstall C, Rugg D, Jones RT, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. J Consult Clin Psychol 1985;53:256-8. https://doi.org/10.1037//0022-006x.53.2.256.
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. J Consult Clin Psychol 1987;55:603-5. https://doi.org/10.1037/0022-006X.55.4.603.
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med 2018;378:2302-10. https://doi.org/10.1056/NEJMsa1715757.
- Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax 2002;57:715-18. https://doi.org/10.1136/thorax.57.8.715.
- Hanioka T, Ojima M, Tanaka H, Naito M, Hamajima N, Matsuse R. Intensive smoking-cessation intervention in the dental setting. J Dent Res 2010;89:66-70. https://doi.org/10.1177/0022034509350867.
- Harackiewicz JM, Blair LW, Sansone C, Epstein JA, Stuchell RN. Nicotine gum and self-help manuals in smoking cessation: an evaluation in a medical context. Addict Behav 1988;13:319-30. https://doi.org/10.1016/0306-4603(88)90038-X.
- Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of behavioral and pharmacological treatment on smokeless tobacco users. J Consult Clin Psychol 1996;64:153-61. https://doi.org/10.1037//0022-006x.64.1.153.
- Hatsukami DK, Grillo M, Boyle R, Allen S, Jensen J, Bliss R, et al. Treatment of spit tobacco users with transdermal nicotine system and mint snuff. J Consult Clin Psychol 2000;68:241-9. https://doi.org/10.1037/0022-006X.68.2.241.
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. Am J Med 2004;116:151-7. https://doi.org/10.1016/j.amjmed.2003.07.018.
- Hays JT, Croghan IT, Schroeder DR, Offord KP, Hurt RD, Wolter TD, et al. Over-the-counter nicotine patch therapy for smoking cessation: results from randomized, double-blind, placebo-controlled, and open label trials. Am J Public Health 1999;89:1701-7. https://doi.org/10.2105/AJPH.89.11.1701.
- Hays JT, Hurt RD, Decker PA, Croghan IT, Offord KP, Patten CA. A randomized, controlled trial of bupropion sustained-release for preventing tobacco relapse in recovering alcoholics. Nicotine Tob Res 2009;11:859-67. https://doi.org/10.1093/ntr/ntp077.
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med 2001;135:423-33. https://doi.org/10.7326/0003-4819-135-6-200109180-00011.
- Herrera N, Franco R, Herrera L, Partidas A, Rolando R, Fagerström KO. Nicotine gum, 2 and 4 mg, for nicotine dependence. A double-blind placebo-controlled trial within a behavior modification support program. Chest 1995;108:447-51. https://doi.org/10.1378/chest.108.2.447.
- Heydari G, Talischi F, Batmanghelidj E, Pajooh M, Boroomand A, Zamani M, et al. Dual addictions, parallel treatments: nicotine replacement therapy for patients receiving methadone treatment in the Islamic Republic of Iran. East Mediterr Health J 2014;19:25-31. https://doi.org/10.26719/2013.19.Supp3.S25.
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis 2012;16:268-72. https://doi.org/10.5588/ijtld.11.0183.
- Hilberink SR, Jacobs JE, Breteler MH, de Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: impact after 1 year of two complex interventions. Patient Educ Couns 2011;83:120-4. https://doi.org/10.1016/j.pec.2010.04.009.
- Hilleman DE, Mohiuddin SM, Delcore MG. Comparison of fixed-dose transdermal nicotine, tapered-dose transdermal nicotine, and buspirone in smoking cessation. J Clin Pharmacol 1994;34:222-4. https://doi.org/10.1002/j.1552-4604.1994.tb03989.x.
- Hjalmarson A, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation. A randomized, placebo-controlled, double-blind study. Arch Intern Med 1994;154:2567-72. https://doi.org/10.1001/archinte.1994.00420220059007.
- Hjalmarson A, Nilsson F, Sjöström L, Wiklund O. The nicotine inhaler in smoking cessation. Arch Intern Med 1997;157:1721-8. https://doi.org/10.1001/archinte.1997.00440360143016.
- Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo-controlled, double-blind study. JAMA 1984;252:2835-8. https://doi.org/10.1001/jama.252.20.2835.
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control 2007;16:i53-9. https://doi.org/10.1136/tc.2006.019794.
- Holt S, Timu-Parata C, Ryder-Lewis S, Weatherall M, Beasley R. Efficacy of bupropion in the indigenous Maori population in New Zealand. Thorax 2005;60:120-3. https://doi.org/10.1136/thx.2004.030239.
- Dale Horst W, Klein MW, Williams D, Werder SF. Extended use of nicotine replacement therapy to maintain smoking cessation in persons with schizophrenia. Neuropsychiatr Dis Treat 2005;1:349-55.
- Howard-Pitney B, Killen JD, Fortmann SP. Quitting chew: results from a randomized trial using nicotine patches. Exp Clin Psychopharmacol 1999;7:362-71. https://doi.org/10.1037/1064-1297.7.4.362.
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self-control method. Pharmacopsychiatry 1988;21:461-2. https://doi.org/10.1055/s-2007-1017054.
- Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotines reinforcing, withdrawal-suppression and self-reported effects. J Pharmacol Exp Ther 1990;252:1175-83.
- Hughes JR, Gust SW, Keenan RM, Fenwick JW, Healey ML. Nicotine vs. placebo gum in general medical practice. JAMA 1989;261:1300-5. https://doi.org/10.1001/jama.1989.03420090064032.
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, et al. Are higher doses of nicotine replacement more effective for smoking cessation?. Nicotine Tob Res 1999;1:169-74. https://doi.org/10.1080/14622299050011281.
- Hughes JR, Novy P, Hatsukami DK, Jensen J, Callas PW. Efficacy of nicotine patch in smokers with a history of alcoholism. Alcohol Clin Exp Res 2003;27:946-54. https://doi.org/10.1097/01.ALC.0000071742.86555.4D.
- Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res 2011;13:955-64. https://doi.org/10.1093/ntr/ntr103.
- Hughes JR, Solomon LJ, Livingston AE, Callas PW, Peters EN. A randomized, controlled trial of NRT-aided gradual vs. abrupt cessation in smokers actively trying to quit. Drug Alcohol Depend 2010;111:105-13. https://doi.org/10.1016/j.drugalcdep.2010.04.007.
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA 1994;271:595-600. https://doi.org/10.1001/jama.1994.03510320035026.
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol 2003;21:914-20. https://doi.org/10.1200/JCO.2003.08.160.
- Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine-replacement therapy with use of a transdermal nicotine patch – a randomized double-blind placebo-controlled trial. Mayo Clin Proc 1990;65:1529-37. https://doi.org/10.1016/S0025-6196(12)62186-7.
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-202. https://doi.org/10.1056/NEJM199710233371703.
- Hurt RT, Ebbert JO, Croghan IT, Schroeder DR, Hurt RD, Hays JT. Varenicline for tobacco-dependence treatment in alcohol-dependent smokers: a randomized controlled trial. Drug Alcohol Depend 2018;184:12-7. https://doi.org/10.1016/j.drugalcdep.2017.11.017.
- Imperial Cancer Research Fund General Practice Research Group . Randomised trial of nicotine patches in general practice: results at one year. BMJ 1994;308:1476-7. https://doi.org/10.1136/bmj.308.6942.1476.
- Ikonomidis I, Marinou M, Vlastos D, Kourea K, Andreadou I, Liarakos N, et al. Effects of varenicline and nicotine replacement therapy on arterial elasticity, endothelial glycocalyx and oxidative stress during a 3-month smoking cessation program. Atherosclerosis 2017;262:123-30. https://doi.org/10.1016/j.atherosclerosis.2017.05.012.
- Jamrozik K, Fowler G, Vessey M, Wald N. Placebo controlled trial of nicotine chewing gum in general practice. Br Med J 1984;289:794-7. https://doi.org/10.1136/bmj.289.6448.794.
- Jarvis MJ, Raw M, Russell MA, Feyerabend C. Randomised controlled trial of nicotine chewing-gum. Br Med J 1982;285:537-40. https://doi.org/10.1136/bmj.285.6341.537.
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. The effect of nicotine, silver acetate, and placebo chewing gum on the cessation of smoking. The influence of smoking type and nicotine dependence. Int J Addict 1991;26:1223-31. https://doi.org/10.3109/10826089109062156.
- Johns D. Randomised controlled trial comparing nicotine replacement therapy (NRT) and counselling on smoking cessation in patients prone to lung cancer using bed font micro-smokerlyzer. Support Care Cancer 2017;25:21-266.
- Johns D. Randomised controlled trial comparing varenicline plus counselling and brief counselling alone on smoking cessation in patients prone to lung cancer using carbon monoxide moniter. Support Care Cancer 2017;25:21-266.
- Johns DA. Randomised controlled trial comparing nicotine replacement therapy (NRT) plus brief counselling and brief counselling alone on smoking cessation in patients prone to lung cancer using a breath carbon monoxide (CO) monitor. Ann Oncol 2016;27. https://doi.org/10.1093/annonc/mdw600.003.
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63. https://doi.org/10.1001/jama.296.1.56.
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91. https://doi.org/10.1056/NEJM199903043400903.
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274:1347-52. https://doi.org/10.1001/jama.1995.03530170027027.
- Joseph AM, Fu SS, Lindgren B, Rothman AJ, Kodl M, Lando H, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med 2011;171:1894-900. https://doi.org/10.1001/archinternmed.2011.500.
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med 1996;335:1792-8. https://doi.org/10.1056/NEJM199612123352402.
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. J Stud Alcohol 2004;65:681-91. https://doi.org/10.15288/jsa.2004.65.681.
- Joyce GF, Niaura R, Maglione M, Mongoven J, Larson-Rotter C, Coan J, et al. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Serv Res 2008;43:2106-23. https://doi.org/10.1111/j.1475-6773.2008.00891.x.
- Kalman D, Herz L, Monti P, Kahler CW, Mooney M, Rodrigues S, et al. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double-blind, placebo-controlled study. Drug Alcohol Depend 2011;118:111-18. https://doi.org/10.1016/j.drugalcdep.2011.03.005.
- Kalman D, Kahler CW, Garvey AJ, Monti PM. High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes. J Subst Abuse Treat 2006;30:213-17. https://doi.org/10.1016/j.jsat.2006.01.001.
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy?. Exp Clin Psychopharmacol 1999;7:226-33. https://doi.org/10.1037/1064-1297.7.3.226.
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self-help video for cigarette smoking cessation. J Consult Clin Psychol 1997;65:663-72. https://doi.org/10.1037/0022-006x.65.4.663.
- Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol 2006;74:286-94. https://doi.org/10.1037/0022-006X.74.2.286.
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. J Consult Clin Psychol 1990;58:85-92. https://doi.org/10.1037//0022-006x.58.1.85.
- Killen JD, Maccoby N, Taylor CB. Nicotine gum and self-regulation training in smoking relapse prevention. Behav Ther 1984;15:234-48. https://doi.org/10.1016/S0005-7894(84)80026-X.
- Klesges RC, Ebbert JO, Talcott GW, Thomas F, Richey PA, Womack C, et al. Efficacy of a tobacco quitline in active duty military and TRICARE beneficiaries: a randomized trial. Mil Med 2015;180:917-25. https://doi.org/10.7205/MILMED-D-14-00513.
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O’Brien JA, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 2014;312:155-61. https://doi.org/10.1001/jama.2014.7195.
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med 1995;24:41-7. https://doi.org/10.1006/pmed.1995.1006.
- Kornitzer M, Kittel F, Dramaix M, Bourdoux P. A double blind study of 2 mg versus 4 mg nicotine-gum in an industrial setting. J Psychosom Res 1987;31:171-6. https://doi.org/10.1016/0022-3999(87)90073-0.
- Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT-supported smoking reduction. Nicotine Tob Res 2002;4.
- Krupski L, Cummings KM, Hyland A, Mahoney MC, Toll BA, Carpenter MJ, et al. Cost and effectiveness of combination nicotine replacement therapy among heavy smokers contacting a quitline. J Smoking Cessation 2016;11:50-9. https://doi.org/10.1017/jsc.2014.15.
- Lee SM, Landry J, Jones PM, Buhrmann O, Morley-Forster P. The effectiveness of a perioperative smoking cessation program: a randomized clinical trial. Anesth Analg 2013;117:605-13. https://doi.org/10.1213/ANE.0b013e318298a6b0.
- Lee SM, Tenney R, Wallace AW, Arjomandi M. E-cigarettes versus nicotine patches for perioperative smoking cessation: a pilot randomized trial. Peer J 2018;6. https://doi.org/10.7717/peerj.5609.
- Leischow SJ, Muramoto ML, Cook GN, Merikle EP, Castellini SM, Otte PS. OTC nicotine patch: effectiveness alone and with brief physician intervention. Am J Health Behav 1999;23:61-9. https://doi.org/10.5993/AJHB.23.1.7.
- Leischow SJ, Nilsson F, Franzon M, Hill A, Otte P, Merikle EP. Efficacy of the nicotine inhaler as an adjunct to smoking cessation. Am J Health Behav 1996;20:364-71.
- Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the nicotine inhaler for smoking cessation in an OTC setting. Am J Health Behav 2004;28:291-30. https://doi.org/10.5993/ajhb.28.4.1.
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med 2004;140:426-33. https://doi.org/10.7326/0003-4819-140-6-200403160-00009.
- Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2015;3:131-8. https://doi.org/10.1016/S2213-2600(14)70294-2.
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch Intern Med 2010;170:543-50. https://doi.org/10.1001/archinternmed.2010.33.
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: a randomized clinical trial. Prev Med 1998;27:296-303. https://doi.org/10.1006/pmed.1998.0266.
- Littlewood RA, Claus ED, Wilcox CE, Mickey J, Arenella PB, Bryan AD, et al. Moderators of smoking cessation outcomes in a randomized-controlled trial of varenicline versus placebo. Psychopharmacology 2017;234:3417-29. https://doi.org/10.1007/s00213-017-4721-7.
- Llivina TS, Tuya DM, Quintana JG, Torres CI, Barrera EC, Saez CM, et al. Smoking cessation – effectiveness of nicotine containing chewing gum – a double-blind trial. Med Clin 1988;90:646-50.
- Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction 2009;104:1891-900. https://doi.org/10.1111/j.1360-0443.2009.02623.x.
- Malcolm RE, Sillett RW, Turner JA, Ball KP. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology 1980;70:295-6. https://doi.org/10.1007/BF00427889.
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J Consult Clin Psychol 1997;65:190-4. https://doi.org/10.1037/0022-006x.65.1.190.
- McAfee TA, Bush T, Deprey TM, Mahoney LD, Zbikowski SM, Fellows JL, et al. Nicotine patches and uninsured quitline callers: a randomized trial of two versus eight weeks. Am J Prev Med 2008;35:103-10. https://doi.org/10.1016/j.amepre.2008.04.017.
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res 2008;10:717-29. https://doi.org/10.1080/14622200801968343.
- McGovern PG, Lando HA. An assessment of nicotine gum as an adjunct to freedom from smoking cessation clinics. Addict Behav 1992;17:137-47. https://doi.org/10.1016/0306-4603(92)90018-Q.
- Mercié P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV 2018;5:e126-35. https://doi.org/10.1016/S2352-3018(18)30002-X.
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax 2003;58:484-8. https://doi.org/10.1136/thorax.58.6.484.
- Mori T, Shimao T, Yulchiro G, Namiki M, Hyachi T. A Clinical Trial of Nicotine Chewing Gum for Smoking Cessation n.d.
- Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: a randomised controlled trial. Anaesthesia 2004;59:1053-8. https://doi.org/10.1111/j.1365-2044.2004.03943.x.
- Myung SK, Seo HG, Park S, Kim Y, Kim DJ, Lee DH, et al. Sociodemographic and smoking behavioral predictors associated with smoking cessation according to follow-up periods: a randomized, double-blind, placebo-controlled trial of transdermal nicotine patches. J Korean Med Sci 2007;22:1065-70. https://doi.org/10.3346/jkms.2007.22.6.1065.
- Nahvi S, Ning Y, Segal KS, Richter KP, Arnsten JH. Varenicline efficacy and safety among methadone maintained smokers: a randomized placebo-controlled trial. Addiction 2014;109:1554-63. https://doi.org/10.1111/add.12631.
- Nakamura M, Saito J, Oshima A, Miyamoto M, Matushita A, Endo S. Effect of nicotine chewing gun in smoking cessation classes. Global War 1990:665-7.
- Fox Chase Cancer Center, National Cancer Institute . Counseling and Nicotine Replacement Therapy in Helping Adult Smokers Quit Smoking 2006. https://ClinicalTrials.gov/show/NCT00365508.
- Pfizer . Long-Term Varenicline Treatment for Smoking Cessation 2009. https://ClinicalTrials.gov/show/NCT00828113.
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking-cessation advice?. Fam Pract Res J 1992;12:263-70.
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction 1999;94:685-95. https://doi.org/10.1046/j.1360-0443.1999.9456856.x.
- Niaura R, Goldstein MG, Abrams DB. Matching high- and low-dependence smokers to self-help treatment with or without nicotine replacement. Prev Med 1994;23:70-7. https://doi.org/10.1006/pmed.1994.1010.
- Niaura R, Hays JT, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin 2008;24:1931-41. https://doi.org/10.1185/03007990802177523.
- Nides M, Danielsson T, Saunders F, Perfekt R, Kapikian R, Solla J, et al. Efficacy and safety of a nicotine mouth spray for smoking cessation; a randomized, multicenter, controlled study in a naturalistic setting. Nicotine Tob Res 2018;18. https://doi.org/10.1093/ntr/nty246.
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-8. https://doi.org/10.1001/archinte.166.15.1561.
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician-delivered smoking interventions: a randomized clinical trial. J Gen Intern Med 1991;6:1-8. https://doi.org/10.1007/BF02599381.
- Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav 2007;34:43-54. https://doi.org/10.1177/1090198106288046.
- Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav 2007;32:296-309. https://doi.org/10.1016/j.addbeh.2006.04.004.
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7. https://doi.org/10.1001/archinte.166.15.1571.
- Ortega F, Vellisco A, Márquez E, López-Campos JL, Rodríguez A, de los Ángeles Sánchez M, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients. Arch Bronconeumol 2011;47:3-9. https://doi.org/10.1016/j.arbres.2010.07.007.
- Pack QR, Jorenby DE, Fiore MC, Jackson T, Weston P, Piper ME, et al. A comparison of the nicotine lozenge and nicotine gum: an effectiveness randomized controlled trial. WMJ 2008;107:237-43.
- Page AR, Walters DJ, Schlegel RP, Best JA. Smoking cessation in family practice: the effects of advice and nicotine chewing gum prescription. Addict Behav 1986;11:443-6. https://doi.org/10.1016/0306-4603(86)90025-0.
- Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J 1996;9:643-51. https://doi.org/10.1183/09031936.96.09040643.
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double-blind, placebo-controlled study of transdermal nicotine patch for smoking cessation. J Formos Med Assoc 1998;97:547-51.
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, et al. a randomized controlled trial of an optimized smoking treatment delivered in primary care. Ann Behav Med 2018;52:854-64. https://doi.org/10.1093/abm/kax059.
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res 2007;9:947-54. https://doi.org/10.1080/14622200701540820.
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry 2009;66:1253-62. https://doi.org/10.1001/archgenpsychiatry.2009.142.
- Pirie PL, McBride CM, Hellerstedt W, Jeffery RW, Hatsukami D, Allen S, et al. Smoking cessation in women concerned about weight. Am J Public Health 1992;82:1238-43. https://doi.org/10.2105/AJPH.82.9.1238.
- Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med 2011;171:1055-60. https://doi.org/10.1001/archinternmed.2011.72.
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addict Behav 2007;32:1416-32. https://doi.org/10.1016/j.addbeh.2006.10.005.
- Puska P, Bjŏrkqvist S, Koskela K. Nicotine-containing chewing gum in smoking cessation: a double blind trial with half year follow-up. Addict Behav 1979;4:141-6. https://doi.org/10.1016/0306-4603(79)90048-0.
- Puska P, Korhonen HJ, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation – a clinical trial in North Karelia. Tob Control 1995;4:231-5. https://doi.org/10.1136/tc.4.3.231.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994;272:1497-505. https://doi.org/10.1001/jama.1994.03520190043033.
- Buchkremer G, Bents H, Horstmann M, Opitz K, Tölle R. Combination of behavioral smoking cessation with transdermal nicotine substitution. Addict Behav 1989;14:229-38. https://doi.org/10.1016/0306-4603(89)90054-3.
- Buchkremer G, Minneker E. Efficiency of multimodal smoking cessation therapy combining transdermal nicotine substitution with behavioral therapy. Methods Find Exp Clin Pharmacol 1989;11:215-18.
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med 2011;171:1901-7. https://doi.org/10.1001/archinternmed.2011.492.
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Granero Fernández EJ, Vila Coll MA, Estruch Riba J. Double-blind study of the efficacy of nicotine chewing gum for smoking cessation in the primary care setting. Aten Primaria 1989;6:719-26.
- Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0172-8.
- Ratner PA, Johnson JL, Richardson CG, Bottorff JL, Moffat B, Mackay M, et al. Efficacy of a smoking-cessation intervention for elective-surgical patients. Res Nurs Health 2004;27:148-61. https://doi.org/10.1002/nur.20017.
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat 2008;35:68-77. https://doi.org/10.1016/j.jsat.2007.08.010.
- Reid R, Pipe A, Higginson L, Johnson K, D’Angelo MS, Cooke D, et al. Stepped care approach to smoking cessation in patients hospitalized for coronary artery disease. J Cardiopulm Rehabil 2003;23:176-82. https://doi.org/10.1097/00008483-200305000-00003.
- Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res 2012;14:343-50. https://doi.org/10.1093/ntr/ntr220.
- Rennard SI, Glover ED, Leischow S, Daughton DM, Glover PN, Muramoto M, et al. Efficacy of the nicotine inhaler in smoking reduction: a double-blind, randomized trial. Nicotine Tob Res 2006;8:555-64. https://doi.org/10.1080/14622200600789916.
- Richmond RL, Harris K, Dealmeidaneto A. The transdermal nicotine patch – results of a randomized placebo-controlled trial. Med J Aust 1994;161:130-5. https://doi.org/10.5694/j.1326-5377.1994.tb127344.x.
- Richmond RL, Makinson RJ, Kehoe LA, Giugni AA, Webster IW. One-year evaluation of three smoking cessation interventions administered by general practitioners. Addict Behav 1993;18:187-99. https://doi.org/10.1016/0306-4603(93)90049-F.
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010;121:221-9. https://doi.org/10.1161/CIRCULATIONAHA.109.869008.
- Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med 2006;119:1080-7. https://doi.org/10.1016/j.amjmed.2006.04.024.
- Rodríguez-Artalejo F, Lafuente Urdinguio P, Guallar-Castillón P, Garteizaurrekoa Dublang P, Sáinz Martínez O, Díez Azcárate JI, et al. One year effectiveness of an individualised smoking cessation intervention at the workplace: a randomised controlled trial. Occup Environ Med 2003;60:358-63. https://doi.org/10.1136/oem.60.5.358.
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, et al. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction 2017;112:1808-20. https://doi.org/10.1111/add.13861.
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am J Psychiatry 2013;170:860-7. https://doi.org/10.1176/appi.ajp.2013.12070919.
- Roto P, Ojala A, Sundman K, Jokinen K, Peltomakl R. Nicotine gum and withdrawal from smoking. Suomen Laakarllehtl 1987;36:3445-8.
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, et al. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Ther Adv Respir Dis 2009;3:279-87. https://doi.org/10.1177/1753465809350653.
- Russell MAH, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general-practitioners advice against smoking. Br Med J 1983;287:1782-5. https://doi.org/10.1136/bmj.287.6407.1782.
- Sachs DP, Säwe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Arch Intern Med 1993;153:1881-90. https://doi.org/10.1001/archinte.1993.00410160041003.
- Sadr Azodi O, Lindström D, Adami J, Tønnesen H, Nåsell H, Gilljam H, et al. The efficacy of a smoking cessation programme in patients undergoing elective surgery: a randomised clinical trial. Anaesthesia 2009;64:259-65. https://doi.org/10.1111/j.1365-2044.2008.05758.x.
- Schauffler HH, McMenamin S, Olson K, Boyce-Smith G, Rideout JA, Kamil J. Variations in treatment benefits influence smoking cessation: results of a randomised controlled trial. Tob Control 2001;10:175-80. https://doi.org/10.1136/tc.10.2.175.
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive-behavioral therapy for smoking cessation in women. Nicotine Tob Res 2007;9:699-70. https://doi.org/10.1080/14622200701365335.
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo-controlled, double-blind trial. Addict Behav 1983;8:253-61. https://doi.org/10.1016/0306-4603(83)90020-5.
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, et al. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction 1995;90:1671-82. https://doi.org/10.1046/j.1360-0443.1995.901216719.x.
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double-blind, placebo-controlled trial. Addiction 1996;91:1293-306. https://doi.org/10.1111/j.1360-0443.1996.tb03616.x.
- Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao NC, et al. A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: efficacy, safety, and adherence. Psycho-Oncology 2019;28:561-9. https://doi.org/10.1002/pon.4978.
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med 2015;175:504-11. https://doi.org/10.1001/jamainternmed.2014.8313.
- Schnoll RA, Martinez E, Tatum KL, Weber DM, Kuzla N, Glass M, et al. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes Control 2010;21:811-20. https://doi.org/10.1007/s10552-010-9507-8.
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med 2010;152:144-51. https://doi.org/10.7326/0003-4819-152-3-201002020-00005.
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes Control 1991;2:239-46. https://doi.org/10.1007/BF00052140.
- Selby P, Brands B, Stepner N. Retreatment With ZYban SR: 52 Week Follow-up of a Canadian Multicentre Trial (POS3-63) n.d.
- Bozkurt Zincir S, Zincir S, Kaymak E, Aydin Sunbul E. Comparison of the effectiveness of varenicline, extended-release bupropion and nicotine replacement therapy on the success and the maintenance of a smoking cessation program. Bull Clin Psychopharmacol 2013;23:224-30. https://doi.org/10.5455/bcp.20130313045037.
- Severson HH, Danher BG, Ebbert JO, van Meter N, Lichtenstein E, Widdop C, et al. Randomized trial of nicotine lozenges and phone counseling for smokeless tobacco cessation. Nicotine Tob Res 2015;17:309-15. https://doi.org/10.1093/ntr/ntu145.
- Sharifirad GR, Eslami AA, Charkazi A, Mostafavi F, Shahnazi H. The effect of individual counseling, line follow-up, and free nicotine replacement therapy on smoking cessation in the samples of Iranian smokers: examination of transtheoretical model. J Res Med Sci 2012;17:1128-36.
- Sharma SK, Mohan A, Singh AD, Mishra H, Jhanjee S, Pandey RM, et al. Impact of nicotine replacement therapy as an adjunct to anti-tuberculosis treatment and behaviour change counselling in newly diagnosed pulmonary tuberculosis patients: an open-label, randomised controlled trial. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-26990-5.
- Sherman SE, Aldana I, Estrada M, York L. Comparing the tolerability and effectiveness of two treatment regimens in a smoking clinic. Mil Med 2008;173:550-4. https://doi.org/10.7205/milmed.173.6.550.
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med 2002;162:1267-76. https://doi.org/10.1001/archinte.162.11.1267.
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. Am J Prev Med 2009;36:96-104.e1. https://doi.org/10.1016/j.amepre.2008.09.039.
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, et al. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann Intern Med 2013;158:667-75. https://doi.org/10.7326/0003-4819-158-9-201305070-00006.
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Arch Intern Med 2004;164:1797-803. https://doi.org/10.1001/archinte.164.16.1797.
- Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine Tob Res 2009;11:663-9. https://doi.org/10.1093/ntr/ntp047.
- Simon JA, Solkowitz SN, Carmody TP, Browner WS. Smoking cessation after surgery. A randomized trial. Arch Intern Med 1997;157:1371-6. https://doi.org/10.1001/archinte.1997.00440330111013.
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med 2009;169:2148-55. https://doi.org/10.1001/archinternmed.2009.426.
- Solomon LJ, Marcy TW, Howe KD, Skelly JM, Reinier K, Flynn BS. Does extended proactive telephone support increase smoking cessation among low-income women using nicotine patches?. Prev Med 2005;40:306-13. https://doi.org/10.1016/j.ypmed.2004.06.005.
- Solomon LJ, Scharoun GM, Flynn BS, Secker-Walker RH, Sepinwall D. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Prev Med 2000;31:68-74. https://doi.org/10.1006/pmed.2000.0683.
- Sønderskov J, Olsen J, Sabroe S, Meillier L, Overvad K. Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark. Am J Epidemiol 1997;145:309-18. https://doi.org/10.1093/oxfordjournals.aje.a009107.
- Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, et al. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 2013;108:2193-201. https://doi.org/10.1111/add.12304.
- Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995;90:31-42. https://doi.org/10.1046/j.1360-0443.1995.901316.x.
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial. Drug Alcohol Depend 2013;133:486-93. https://doi.org/10.1016/j.drugalcdep.2013.07.005.
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med 2009;150:447-54. https://doi.org/10.7326/0003-4819-150-7-200904070-00004.
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addict Behav 2011;36:1127-32. https://doi.org/10.1016/j.addbeh.2011.07.002.
- Stockings EA, Bowman JA, Baker AL, Terry M, Clancy R, Wye PM, et al. 2014 World Congress Abstracts, 3–6 December 2014, Melbourne VIC, Australia. Asia Pac J Clin Oncol 2014:1-263.
- Sutherland G, Stapleton JA, Russell MA, Jarvis MJ, Hajek P, Belcher M, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324-9. https://doi.org/10.1016/0140-6736(92)91403-U.
- Sutton S, Hallett R. Smoking intervention in the workplace using videotapes and nicotine chewing gum. Prev Med 1988;17:48-59. https://doi.org/10.1016/0091-7435(88)90071-0.
- Sutton S, Hallett R. Randomized trial of brief individual treatment for smoking using nicotine chewing gum in a workplace setting. Am J Public Health 1987;77:1210-11. https://doi.org/10.2105/AJPH.77.9.1210.
- Swanson NA, Burroughs CC, Long MA, Lee RW. Controlled trial for smoking cessation in a Navy shipboard population using nicotine patch, sustained-release buproprion, or both. Mil Med 2003;168:830-4. https://doi.org/10.1093/milmed/168.10.830.
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001;357:1571-5. https://doi.org/10.1016/S0140-6736(00)04724-3.
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 2011;139:591-9. https://doi.org/10.1378/chest.10-0865.
- Thomsen T, Tønnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, et al. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine Tob Res 2010;12:1118-24. https://doi.org/10.1093/ntr/ntq158.
- Tønnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, et al. Two and four mg nicotine chewing gum and group counselling in smoking cessation: an open, randomized, controlled trial with a 22 month follow-up. Addict Behav 1988;13:17-2. https://doi.org/10.1016/0306-4603(88)90021-4.
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J 2012;40:548-54. https://doi.org/10.1183/09031936.00155811.
- Tønnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334-42. https://doi.org/10.1378/chest.130.2.334.
- Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. Eur Respir J 2000;16:717-22. https://doi.org/10.1034/j.1399-3003.2000.16d25.x.
- Tønnesen P, Nørregaard J, Mikkelsen K, Jørgensen S, Nilsson F. A double-blind trial of a nicotine inhaler for smoking cessation. JAMA 1993;269:1268-71. https://doi.org/10.1001/jama.1993.03500100066029.
- Tønnesen P, Nørregaard J, Simonsen K, Säwe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med 1991;325:311-15. https://doi.org/10.1056/NEJM199108013250503.
- Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J 1999;13:238-46. https://doi.org/10.1034/j.1399-3003.1999.13b04.x.
- Tønnesen P, Tonstad S, Hjalmarson A, Lebargy F, Van Spiegel PI, Hider A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med 2003;254:184-92. https://doi.org/10.1046/j.1365-2796.2003.01185.x.
- Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J 2003;24:946-55. https://doi.org/10.1016/S0195-668X(03)00003-4.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296:64-71. https://doi.org/10.1001/jama.296.1.64.
- Tromeur C, Le Mao R, Couturand F. Effect of varenicline on smoking cessation in COPD patients recovering from exacerbation: a randomized trial. Fundam Clin Pharmacol 2018;32.
- Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB, et al. A randomized, placebo-controlled trial of varenicline, a selective α4β2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 2007;29:1027-39. https://doi.org/10.1016/j.clinthera.2007.06.011.
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study). Circ J 2010;74:771-8. https://doi.org/10.1253/circj.CJ-09-0803.
- Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo-controlled, prospective study. Basic Clin Pharmacol Toxicol 2016;119:78-84. https://doi.org/10.1111/bcpt.12548.
- Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0626-2.
- University of Michigan, National Heart, Lung and Blood Institute . A Randomized Trial of Internet Access to Nicotine Patches 2011. https://ClinicalTrials.gov/show/NCT00534404.
- Urdapilleta-Herrera E, Pina-Rosales MF, Vargas-Rojas MI, Ramirez-Venegas A, Sansores-Martinez R. Bupropion together with cognitive-conductual therapy (CBT) is an alternative for a long-term abstinence of smoking. Eur Respiratory J 2013;42.
- Uyar M, Filiz A, Bayram N, Elbek O, Herken H, Topcu A, et al. A randomized trial of smoking cessation. Medication versus motivation. Saudi Med J 2007;28:922-6.
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. J Pharmacy Pract Res 2002;32:57-62. https://doi.org/10.1002/jppr200232157.
- Villa RS, Alvarez ABD, Hermida JRF. Effectiveness of a multicomponent program to quit smoking with and without nicotine chewing gum. Psicologia Conductual 1999;7:107-18.
- Wagena EJ, Knipschild PG, Huibers MJH, Wouters EFM, Schayck CPR. Efficacy of bupropion and nortriptyline for smoking cessation among people who are at risk for or have chronic obstructive pulmonary disease: results from a randomized, placebo-controlled trial. Nicotine Tob Res 2005;7:683-4. https://doi.org/10.1001/archinte.165.19.2286.
- Wallström M, Nilsson F, Hirsch JM. A randomized, double-blind, placebo-controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction 2000;95:1161-71. https://doi.org/10.1046/j.1360-0443.2000.95811613.x.
- Walsh MM, Hilton JF, Masouredis CM, Gee L, Chesney MA, Ernster VL. Smokeless tobacco cessation intervention for college athletes: results after 1 year. Am J Public Health 1999;89:228-34. https://doi.org/10.2105/AJPH.89.2.228.
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology 2009;14:384-92. https://doi.org/10.1111/j.1440-1843.2008.01476.x.
- Ward KD, Asfar T, Al Ali R, Rastam S, Weg MW, Eissenberg T, et al. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction 2013;108:394-403. https://doi.org/10.1111/j.1360-0443.2012.04048.x.
- Weaver K, Kaplan S, Urbanic J, Case D, Zbikowski S, Dakhil C, et al. Incorporating evidence-based smoking cessation into community oncology practices: feasibility and preliminary efficacy of an enhanced quitline-based smoking cessation intervention for cancer survivors. Psycho-Oncology 2015;24.
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction 2003;98:1395-402. https://doi.org/10.1046/j.1360-0443.2003.00489.x.
- Westergaard CG, Porsbjerg C, Backer V. The effect of varenicline on smoking cessation in a group of young asthma patients. Respir Med 2015;109:1416-22. https://doi.org/10.1016/j.rmed.2015.07.017.
- Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Arch Intern Med 1993;153:1917-23. https://doi.org/10.1001/archinte.1993.00410160087008.
- Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. J Assoc Nurses AIDS Care 2000;11:37-44. https://doi.org/10.1016/S1055-3290(06)60353-1.
- Wiggers LC, Smets EM, Oort FJ, Peters RJ, Storm-Versloot MN, Vermeulen H, et al. The effect of a minimal intervention strategy in addition to nicotine replacement therapy to support smoking cessation in cardiovascular outpatients: a randomized clinical trial. Eur J Cardiovasc Prev Rehabil 2006;13:931-7. https://doi.org/10.1097/hjr.0b013e328010f263.
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012;73:654-60. https://doi.org/10.4088/JCP.11m07522.
- Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin 2007;23:793-801. https://doi.org/10.1185/030079907x182185.
- Wilson JS, Fitzsimons D, Bradbury I, Stuart Elborn J. Does additional support by nurses enhance the effect of a brief smoking cessation intervention in people with moderate to severe chronic obstructive pulmonary disease? A randomised controlled trial. Int J Nurs Stud 2008;45:508-17. https://doi.org/10.1016/j.ijnurstu.2006.10.001.
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, Penn P, et al. A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers. J Clin Psychiatry 2014;75:336-43. https://doi.org/10.4088/JCP.13m08449.
- Wiratmoko MR, Yunus F, Susanto AD, Ginting TT, Kekalih A. Efficacy of varenicline, an nicotinic acetylcholine receptor partial agonist, vs. placebo for smoking cessation: a randomized controlled trial. Respirology 2013;18.
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care – a randomized controlled trial of bupropione, nicotine replacements, CBT and a minimal intervention. Int J Methods Psychiatr Res 2011;20:28-39. https://doi.org/10.1002/mpr.328.
- Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction 1999;94:1227-37. https://doi.org/10.1046/j.1360-0443.1999.948122713.x.
- Wong J, Abrishami A, Riazi S, Siddiqui N, You-Ten E, Korman J, et al. A perioperative smoking cessation intervention with varenicline, counseling, and fax referral to a telephone quitline versus a brief intervention: a randomized controlled trial. Anesth Analg 2017;125:571-9. https://doi.org/10.1213/ANE.0000000000001894.
- Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, et al. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial. Anesthesiology 2012;117:755-64. https://doi.org/10.1097/ALN.0b013e3182698b42.
- Yang DX, Gu CJ, Ni L, Li N, Li QY, Zhou JP. [Assessment of efficacy of medication combined with WeChat platform for quitting smoking in patients with chronic obstructive pulmonary disease]. J Shanghai Jiaotong University (Med Sci) 2016;36:385-9.
- Yuhongxia L. The compliance of vanrenicline usage and the smoking abstinence rate via mobile phone text messaging combine with varenicline: a single-blind, randomised control trial. Respirology 2011;16:46-7.
- Zellweger JP, Boelcskei PL, Carrozzi L, Sepper R, Sweet R, Hider AZ. Bupropion SR vs. placebo for smoking cessation in health care professionals. Am J Health Behav 2005;29:240-9. https://doi.org/10.5993/ajhb.29.3.5.
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J Consult Clin Psychol 1992;60:943-52. https://doi.org/10.1037//0022-006x.60.6.943.
- Zernig G, Wallner R, Grohs U, Kriechbaum N, Kemmler G, Saria A. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction 2008;103:2024-31. https://doi.org/10.1111/j.1360-0443.2008.02348.x.
- Allen MH, Debanné M, Lazignac C, Adam E, Dickinson LM, Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 2011;168:395-9. https://doi.org/10.1176/appi.ajp.2010.10040569.
- Ihara H. Diagnosis and treatment for head and neck cancer-virus association and characterization. Nippon Jibiinkoka Gakkai Kaiho 2013;116:1064-6. https://doi.org/10.3950/jibiinkoka.116.1064.
- Austin AJ, Duka T, Rusted J, Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology 2014;231:3771-85. https://doi.org/10.1007/s00213-014-3512-7.
- Bernstein SL, Bijur P, Cooperman N, Jearld S, Arnsten JH, Moadel A, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Acad Emerg Med 2011;18:575-83. https://doi.org/10.1111/j.1553-2712.2011.01097.x.
- Bernstein SL, Rosner J, Toll B. A multicomponent intervention including texting to promote tobacco abstinence in emergency department smokers: a pilot study. Acad Emerg Med 2016;23:803-8. https://doi.org/10.1111/acem.12990.
- Bloch B, Reshef A, Cohen T, Tafla A, Gathas S, Israel S, et al. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Res 2010;175:38-42. https://doi.org/10.1016/j.psychres.2008.12.015.
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology 2011;218:391-403. https://doi.org/10.1007/s00213-011-2327-z.
- Brantmark B, Ohlin P, Westling H. Nicotine-containing chewing gum as an anti-smoking aid. Psychopharmacologia 1973;31:191-200. https://doi.org/10.1007/BF00422509.
- Burstein AH, Fullerton T, Clark DJ, Faessel HM. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. J Clin Pharmacol 2006;46:1234-40. https://doi.org/10.1177/0091270006291837.
- Carpenter MJ, Heckman BW, Wahlquist AE, Wagener TL, Goniewicz ML, Gray KM, et al. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Biomarkers Prev 2017;26:1795-803. https://doi.org/10.1158/1055-9965.EPI-17-0460.
- Chen HK, Lan TH, Wu BJ. A double-blind randomized clinical trial of different doses of transdermal nicotine patch for smoking reduction and cessation in long-term hospitalized schizophrenic patients. Eur Arch Psychiatry Clin Neurosci 2013;263:75-82. https://doi.org/10.1007/s00406-012-0338-3.
- Christen AG, McDonald JL, Olson BL, Drook CA, Stookey GK. Efficacy of nicotine chewing gum in facilitating smoking cessation. J Am Dent Assoc 1984;108:594-7. https://doi.org/10.14219/jada.archive.1984.0387.
- Cosci F, Ibrahim HM, Nannini A, Schruers K. Experimental study on the effects of anxiety sensitivity and somatosensory amplification on the response to the 35% CO(2) challenge in abstinent smokers. Exp Clin Psychopharmacol 2015;23:464-76. https://doi.org/10.1037/pha0000048.
- Cravo AS, Bush J, Sharma G, Savioz R, Martin C, Craige S, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol 2016;81:1-14. https://doi.org/10.1016/j.yrtph.2016.10.003.
- de Jong B, Schuppers AS, Kruisdijk-Gerritsen A, Arbouw MEL, van den Oever HLA, van Zanten ARH. The safety and efficacy of nicotine replacement therapy in the intensive care unit: a randomised controlled pilot study. Ann Intensive Care 2018;8. https://doi.org/10.1186/s13613-018-0399-1.
- Du D, Borders J, Selmani A, Waverczak W. A pilot study to investigate the efficacy of nicotine oral soluble film, lozenge and gum in relief of acute smoking cue-provoked craving for cigarette in low dependence smokers. J Smoking Cessation 2015;10:87-95. https://doi.org/10.1017/jsc.2014.5.
- Fatemi SH, Yousefi MK, Kneeland RE, Liesch SB, Folsom TD, Thuras PD. Antismoking and potential antipsychotic effects of varenicline in subjects with schizophrenia or schizoaffective disorder: a double-blind placebo and bupropion-controlled study. Schizophr Res 2013;146:376-8. https://doi.org/10.1016/j.schres.2013.02.015.
- Foulds J, Stapleton J, Hayward M, Russell MA, Feyerabend C, Fleming T, et al. Transdermal nicotine patches with low-intensity support to aid smoking cessation in outpatients in a general hospital. A placebo-controlled trial. Arch Fam Med 1993;2:417-23. https://doi.org/10.1001/archfami.2.4.417.
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 2011;215:655-63. https://doi.org/10.1007/s00213-010-2160-9.
- Garza D, Murphy M, Tseng LJ, Riordan HJ, Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol Psychiatry 2011;69:1075-82. https://doi.org/10.1016/j.biopsych.2010.12.005.
- GlaxoSmithKline . Provoked Craving Relief Study by NRT 2011. https://ClinicalTrials.gov/show/NCT01476202.
- Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A comparison of sustained-release bupropion and placebo for smokeless tobacco cessation. Am J Health Behav 2002;26:386-93. https://doi.org/10.5993/ajhb.26.5.7.
- Gray KM, McClure EA, Baker NL, Hartwell KJ, Carpenter MJ, Saladin ME. An exploratory short-term double-blind randomized trial of varenicline versus nicotine patch for smoking cessation in women. Addiction 2015;110:1027-34. https://doi.org/10.1111/add.12895.
- Haarmann H, Gossler A, Herrmann P, Bonev S, Nguyen XP, Hasenfuß G, et al. Effects of varenicline on sympatho-vagal balance and cue reactivity during smoking withdrawal: a randomised placebo-controlled trial. Tob Induc Dis 2016;14. https://doi.org/10.1186/s12971-016-0091-x.
- Hajek P, McRobbie H, Myers Smith K, Phillips A, Cornwall D, Dhanji AR. Increasing varenicline dose in smokers who do not respond to the standard dosage: a randomized clinical trial. JAMA Intern Med 2015;175:266-71. https://doi.org/10.1001/jamainternmed.2014.6916.
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med 2011;171:770-7. https://doi.org/10.1001/archinternmed.2011.138.
- Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-140.
- Hawk LW, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther 2012;91:172-80. https://doi.org/10.1038/clpt.2011.317.
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse. Addict Biol 2019;24:765-76. https://doi.org/10.1111/adb.12664.
- Heydari G. Poster presentations from the World Congress of Cardiology Scientific Sessions 2012. Circulation 2012;125. https://doi.org/10.1161/CIR.0b013e31824fcdb3.
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 2011;68:1195-206. https://doi.org/10.1001/archgenpsychiatry.2011.83.
- Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, Goelz P, et al. A double-blind placebo-controlled randomized trial of varenicline for smokeless tobacco dependence in India. Nicotine Tob Res 2014;16:50-7. https://doi.org/10.1093/ntr/ntt115.
- Jennings C, Kotseva K, De Bacquer D, Hoes A, de Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. Eur Heart J 2014;35:1411-20. https://doi.org/10.1093/eurheartj/ehu051.
- Karam-Hage M, Strobbe S, Robinson JD, Brower KJ. Bupropion-SR for smoking cessation in early recovery from alcohol dependence: a placebo-controlled, double-blind pilot study. Am J Drug Alcohol Abuse 2011;37:487-90. https://doi.org/10.3109/00952990.2011.598591.
- Kupecz D, Prochazka A. A comparison of nicotine delivery systems in a multimodality smoking cessation program. Nurse Pract 1996;21. https://doi.org/10.1097/00006205-199602000-00006.
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, et al. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. J Clin Psychopharmacol 1994;14:41-9. https://doi.org/10.1097/00004714-199402000-00006.
- Li J, Zhang T-l, Wang B, Li X-w. An efficacy analysis of bupropion for smoking cessation in schizophrenia. Zhongguo Xinyao Yu Linchuang Zazhi 2009;28:231-4.
- Lindson-Hawley N, Coleman T, Docherty G, Hajek P, Lewis S, Lycett D, et al. Nicotine patch preloading for smoking cessation (the preloading trial): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-296.
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 2013;7:277-86. https://doi.org/10.1097/ADM.0b013e31829623f4.
- Marsh HS, Dresler CM, Choi JH, Targett DA, Gamble ML, Strahs KR. Safety profile of a nicotine lozenge compared with that of nicotine gum in adult smokers with underlying medical conditions: a 12-week, randomized, open-label study. Clin Ther 2005;27:1571-87. https://doi.org/10.1016/j.clinthera.2005.10.008.
- Masiero M, Lucchiari C, Mazzocco K, Veronesi G, Maisonneuve P, Jemos C, et al. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob Res 2019;21:119-26. https://doi.org/10.1093/ntr/nty047.
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res 2013;15:139-48. https://doi.org/10.1093/ntr/nts101.
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 2009;66:185-90. https://doi.org/10.1016/j.biopsych.2009.01.029.
- Merz PG, Kellerstanislawski B, Huber T, Woodcock BG, Rietbrock N. Transdermal nicotine in smoking cessation and involvement of nonspecific influences. Int J Clin Pharmacol Therapeutics 1993;31:476-82.
- Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Ajagbe TO, Batki SL. Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo-controlled pilot trial. J Clin Psychopharmacol 2013;33:243-7. https://doi.org/10.1097/JCP.0b013e3182870551.
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology 2012;223:299-306. https://doi.org/10.1007/s00213-012-2717-x.
- Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002;359:114-17. https://doi.org/10.1016/S0140-6736(02)07369-5.
- Mulligan SC, Masterson JG, Devane JG, Kelly JG. Clinical and pharmacokinetic properties of a transdermal nicotine patch. Clin Pharmacol Ther 1990;47:331-7. https://doi.org/10.1038/clpt.1990.36.
- ClinicalTrials.gov . Trial of Nicotine Nasal Spray As an Aid for Smoking Cessation in Schizophrenia 2009. https://ClinicalTrials.gov/show/NCT01010477.
- Duke University . Supplemental Nicotine Administration for Smoking Cessation in Posttraumatic Stress Disorder (PTSD) 2009.
- Nides M, Shanga GM, Bishop A, Becker WD. Nicotine lozenges in the relief of behaviorally provoked craving. Am J Health Behav 2018;42:69-80. https://doi.org/10.5993/AJHB.42.3.7.
- Olson LC, Hong D, Conell-Price JS, Cheng S, Flood P. A transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose-ranging study. Anesth Analg 2009;109:1987-91. https://doi.org/10.1213/ANE.0b013e3181bd1612.
- O’Malley S, Zweben A, Fucito L, Wu R, Piepmeier M, Ockert D, et al. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry 2018;75:129-38. https://doi.org/10.1001/jamapsychiatry.2017.3544.
- Pei H, Zhang LJ, Zeng LM, Yu F. Effect of preoperative smoking intervention on postoperative complications of total hip replacement. Chin J Evidence-Based Med 2014;14:399-403.
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O’Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend 2013;133:754-8. https://doi.org/10.1016/j.drugalcdep.2013.06.019.
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict 2010;19:401-8. https://doi.org/10.1111/j.1521-0391.2010.00066.x.
- Ponciano-Rodriguez G, Paez-Martinez N, Villa-Romero A, Gutierrez-Grobe Y, Mendez-Sanchez N. Early changes in the components of the metabolic syndrome in a group of smokers after tobacco cessation. Metab Syndr Relat Disord 2014;12:242-50. https://doi.org/10.1089/met.2014.0007.
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology 2014;231:3843-53. https://doi.org/10.1007/s00213-014-3519-0.
- Rennard S, Daughton D, Cheney R, Thompson A, Miles R, Windle J, et al. Nicotine replacement therapy for patients with coronary-artery disease. Arch Intern Med 1994;154:989-95. https://doi.org/10.1001/archinte.1994.00420090063007.
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry 2014;171:1199-205. https://doi.org/10.1176/appi.ajp.2014.13050595.
- Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits highly dependent smokers in an adaptive smoking cessation paradigm. Nicotine Tob Res 2017;19:999-1002. https://doi.org/10.1093/ntr/ntw283.
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res 2009;11:1067-75. https://doi.org/10.1093/ntr/ntp103.
- Rose JE, Levin ED, Behm FM, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clin Pharmacol Ther 1990;47:323-30. https://doi.org/10.1038/clpt.1990.35.
- Rungruanghiranya S, Ekpanyaskul C, Hattapornsawan Y, Tundulawessa Y. Effect of nicotine polyestex gum on smoking cessation and quality of life. J Med Assoc Thai 2008;91:1656-62.
- Rutgers, The State University of New Jersey, Pfizer . Varenicline-Aided Cigarette Reduction in Smokers Not Ready to Quit 2011. https://ClinicalTrials.gov/show/NCT01308736.
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 2014;231:3799-807. https://doi.org/10.1007/s00213-014-3518-1.
- Schnoll RA, Wileyto EP, Leone FT, Tyndale RF, Benowitz NL. High dose transdermal nicotine for fast metabolizers of nicotine: a proof of concept placebo-controlled trial. Nicotine Tob Res 2013;15:348-54. https://doi.org/10.1093/ntr/nts129.
- Schuurmans MM, Diacon AH, van Biljon X, Bolliger CT. Effect of pre-treatment with nicotine patch on withdrawal symptoms and abstinence rates in smokers subsequently quitting with the nicotine patch: a randomized controlled trial. Addiction 2004;99:634-40. https://doi.org/10.1111/j.1360-0443.2004.00711.x.
- Sheng LX, Tang YL, Jiang ZN, Yao CH, Gao JY, Xu GZ, et al. Sustained-release bupropion for smoking cessation in a Chinese sample: a double-blind, placebo-controlled, randomized trial. Nicotine Tob Res 2013;15:320-5. https://doi.org/10.1093/ntr/nts124.
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol 2006;74:276-85. https://doi.org/10.1037/0022-006X.74.2.276.
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology 2012;37:660-8. https://doi.org/10.1038/npp.2011.238.
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend 2008;96:222-32. https://doi.org/10.1016/j.drugalcdep.2008.03.010.
- Singh P, Kumar R. Assessment of the effectiveness of sustained release bupropion and intensive physician advice in smoking cessation. Lung India 2010;27:11-8. https://doi.org/10.4103/0970-2113.59262.
- Smith RC, Amiaz R, Si TM, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia: a double-blind randomized trial. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0143490.
- Sorensen LT, Jorgensen T. Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Colorectal Dis 2003;5:347-52. https://doi.org/10.1046/j.1463-1318.2003.00450.x.
- Southern Illinois University Carbondale, National Institute on Drug Abuse . Nicotine Replacement Therapy (NRT) and Bupropion Mechanisms of Effectiveness in Smokers 2005. https://ClinicalTrials.gov/show/NCT01048944.
- Stanford University, Pfizer . Varenicline In-Patient Study 2011. https://ClinicalTrials.gov/show/NCT01413516.
- Stapleton JA, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo-controlled trial. Addiction 2011;106:824-32. https://doi.org/10.1111/j.1360-0443.2010.03274.x.
- Steinberg ML, Lu SE, Williams JM. Varenicline for smoking reduction in smokers not yet ready to quit: a double-blind, proof-of-concept randomized clinical trial. Addict Behav 2018;84:20-6. https://doi.org/10.1016/j.addbeh.2018.03.026.
- Thorsteinsson HS, Gillin JC, Patten CA, Golshan S, Sutton LD, Drummond S, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology 2001;24:350-8. https://doi.org/10.1016/S0893-133X(00)00217-7.
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology 2011;217:279-87. https://doi.org/10.1007/s00213-011-2282-8.
- Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res 2016;18:1937-43. https://doi.org/10.1093/ntr/ntw017.
- Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther 1998;12:239-44. https://doi.org/10.1023/a:1007757530765.
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage-based therapies in a population-based effectiveness trial. J Consult Clin Psychol 2006;74:1162-72. https://doi.org/10.1037/0022-006X.74.6.1162.
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J Addict Med 2016;10:166-73. https://doi.org/10.1097/ADM.0000000000000208.
- Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR. Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients. Anesthesiology 2005;102:1138-46. https://doi.org/10.1097/00000542-200506000-00013.
- Weinberger AH, Vessicchio JC, Sacco KA, Creeden CL, Chengappa KN, George TP. A preliminary study of sustained-release bupropion for smoking cessation in bipolar disorder. J Clin Psychopharmacol 2008;28:584-7. https://doi.org/10.1097/JCP.0b013e318184ba3c.
- Weiner E, Ball MP, Buchholz AS, Gold JM, Evins AE, McMahon RP, et al. Bupropion sustained release added to group support for smoking cessation in schizophrenia: a new randomized trial and a meta-analysis. J Clin Psychiatry 2012;73:95-102. https://doi.org/10.4088/JCP.10m06143gre.
- Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res 2011;129:94-5. https://doi.org/10.1016/j.schres.2011.02.003.
- Whelton H, Kingston R, O’Mullane D, Nilsson F. Randomized controlled trial to evaluate tooth stain reduction with nicotine replacement gum during a smoking cessation program. BMC Oral Health 2012;12. https://doi.org/10.1186/1472-6831-12-13.
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre-operative surgical patients: randomised controlled trial. Anaesthesia 2005;60:172-9. https://doi.org/10.1111/j.1365-2044.2004.04070.x.
- Yale University, National Institute on Alcohol Abuse and Alcoholism . The Effect of Varenicline (Chantix) and Bupropion (Zyban) on Smoking Lapse Behavior 2007. https://ClinicalTrials.gov/show/NCT00580853.
- Zhu J. The Effectiveness of Bupropion SR on Depressive Symptoms in Smokers: Self-Reports, EEG, and Individual Differences 2015.
- Barrueco M, Otero MJ, Palomo L, Jiménez-Ruiz C, Torrecilla M, Romero P, et al. Adverse effects of pharmacological therapy for nicotine addiction in smokers following a smoking cessation program. Nicotine Tob Res 2005;7:335-42. https://doi.org/10.1080/14622200500124768.
- Bars MP, Banauch GI, Appel D, Andreachi M, Mouren P, Kelly KJ, et al. ‘Tobacco Free With FDNY’: the New York City Fire Department World Trade Center Tobacco Cessation Study. Chest 2006;129:979-87. https://doi.org/10.1378/chest.129.4.979.
- Cartin-Ceba R, Warner DO, Hays JT, Afessa B. Nicotine replacement therapy in critically ill patients: a prospective observational cohort study. Crit Care Med 2011;39:1635-40. https://doi.org/10.1097/CCM.0b013e31821867b8.
- Cunningham FE, Hur K, Dong D, Miller DR, Zhang R, Wei X, et al. A comparison of neuropsychiatric adverse events during early treatment with varenicline or a nicotine patch. Addiction 2016;111:1283-92. https://doi.org/10.1111/add.13329.
- Davies NM, Taylor G, Taylor AE, Thomas KH, Windmeijer F, Martin RM, et al. What are the effects of varenicline compared with nicotine replacement therapy on long-term smoking cessation and clinically important outcomes? Protocol for a prospective cohort study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009665.
- Demir T, Tutluoğlu B, Koç N, Bilgin L. One-year follow up results of Smoking Cessation Outpatient Clinic. Tuberk Toraks 2004;52:63-8.
- Deniz S, Emre JC, Ozdemir O, Baysak A. Two-years follow-up results of a smoking cessation clinic in a state hospital. Eurasian J Pulmonol 2016;18:80-4. https://doi.org/10.5152/ejp.2016.69885.
- Dhelaria RK, Friderici J, Wu K, Gupta E, Khan C, Rothberg MB. Effectiveness of varenicline for smoking cessation at 2 urban academic health centers. Eur J Intern Med 2012;23:461-4. https://doi.org/10.1016/j.ejim.2012.03.017.
- Dollerup J, Vestbo J, Murray-Thomas T, Kaplan A, Martin RJ, Pizzichini E, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol 2017;9:231-43. https://doi.org/10.2147/CLEP.S127775.
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res 2009;11:572-6. https://doi.org/10.1093/ntr/ntp042.
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, et al. Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine Tob Res 2013;15:247-54. https://doi.org/10.1093/ntr/nts121.
- Garcia-Portilla MP, Garcia-Alvarez L, Sarramea F, Galvan G, Diaz-Mesa E, Bobes-Bascaran T, et al. It is feasible and effective to help patients with severe mental disorders to quit smoking: an ecological pragmatic clinical trial with transdermal nicotine patches and varenicline. Schizophr Res 2016;176:272-80. https://doi.org/10.1016/j.schres.2016.05.011.
- Gomez-Bastero A, Almadana V, Romero C, Luque E, Vega A, Montserrat S, et al. Differences between the recommended and real dose in a smoking cessation practice. Eur Respir J 2012;40.
- Graham DJ, By K, McKean S, Mosholder A, Kornegay C, Racoosin JA, et al. Cardiovascular and mortality risks in older Medicare patients treated with varenicline or bupropion for smoking cessation: an observational cohort study. Pharmacoepidemiol Drug Saf 2014;23:1205-12. https://doi.org/10.1002/pds.3678.
- Hodgkin JE, Sachs DP, Swan GE, Jack LM, Titus BL, Waldron SJ, et al. Outcomes from a patient-centered residential treatment plan for tobacco dependence. Mayo Clin Proc 2013;88:970-6. https://doi.org/10.1016/j.mayocp.2013.05.027.
- Hsueh KC, Hsueh SC, Chou MY, Pan LF, Tu MS, McEwen A, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology 2014;231:2819-23. https://doi.org/10.1007/s00213-014-3482-9.
- Hsueh SC, Hsueh KC, Chou MY, Tu MS. A comparison of the effectiveness of varenicline and transdermal nicotine patch in outpatients following a standardized smoking cessation program in Southern Taiwan. Eval Health Prof 2015;38:115-25. https://doi.org/10.1177/0163278712466868.
- Jiménez Ruiz CA, Cisneros C, Perelló Bosch O, Barruero Ferrero M, Hernández Mezquita MA, Solano Reina S. Individual treatment of smoking addiction. Results using 2 and 4 mg nicotine gum. Arch Bronconeumol 2000;36:129-32. https://doi.org/10.1016/S0300-2896(15)30197-6.
- Jiménez Ruiz CA, Ramos Pinedo A, Cicero Guerrero A, Mayayo Ulibarri M, Cristobal Fernández M, Lopez Gonzalez G. Characteristics of COPD smokers and effectiveness and safety of smoking cessation medications. Nicotine Tob Res 2012;14:1035-9. https://doi.org/10.1093/ntr/nts001.
- Jiménez-Ruiz CA, Pascual-Lledó JF, Cícero-Guerrero A, Cristóbal-Fernández M, Mayayo-Ulibarri M, Villar-Laguna C. Effectiveness and safety of varenicline and nicotine replacement therapy among mental health patients: a retrospective cohort study [published online ahead of print December 21 2017]. Pulmonology 2017. https://doi.org/10.1016/j.rppnen.2017.10.008.
- Kaduri P, Voci S, Zawertailo L, Chaiton M, McKenzie K, Selby P. Real-world effectiveness of varenicline versus nicotine replacement therapy in patients with and without psychiatric disorders. J Addict Med 2015;9:169-76. https://doi.org/10.1097/ADM.0000000000000111.
- Kerr A, McVey JT, Wood AM, Van Haren F. Safety of nicotine replacement therapy in critically ill smokers: a retrospective cohort study. Anaesth Intensive Care 2016;44:758-61. https://doi.org/10.1177/0310057X1604400621.
- Koçak ND, Eren A, Boğa S, Aktürk ÜA, Öztürk ÜA, Arınç S, et al. Relapse rate and factors related to relapse in a 1-year follow-up of subjects participating in a smoking cessation program. Respir Care 2015;60:1796-803. https://doi.org/10.4187/respcare.03883.
- Korzeniowska K, Cieślewicz A, Jabłecka A. Safety of nicotine addiction treatment. Prz Lek 2013;70:839-41.
- Kotz D, Viechtbauer W, Simpson CR, van Schayck OCP, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline and bupropion in smokers with chronic obstructive pulmonary disease. Thorax 2017;72:905-11. https://doi.org/10.1136/thoraxjnl-2017-210067.
- Lee AH, Afessa B. The association of nicotine replacement therapy with mortality in a medical intensive care unit. Crit Care Med 2007;35:1517-21. https://doi.org/10.1097/01.CCM.0000266537.86437.38.
- Sicras Mainar A, Navarro Artieda R, Díaz Cerezo S, Martí Sánchez B, Sanz De Burgoa V. Abstinence rates with varenicline compared to bupropion and nicotine replacement therapy for quitting smoking in primary care. Aten Primaria 2011;43:482-9. https://doi.org/10.1016/j.aprim.2010.09.010.
- Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, Gualano MR, et al. Electronic cigarettes efficacy and safety at 12 months: cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0129443.
- Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol 2005;95:976-8. https://doi.org/10.1016/j.amjcard.2004.12.039.
- Molero Y, Lichtenstein P, Zetterqvist J, Gumpert CH, Fazel S. Varenicline and risk of psychiatric conditions, suicidal behaviour, criminal offending, and transport accidents and offences: population based cohort study. BMJ 2015;350. https://doi.org/10.1136/bmj.h2388.
- Orsel O, Orsel S, Alpar S, Uçar N, Sipit T, Kurt B. The comparison of nicotine replacement therapy and behavioral education in smoking cessation: a study of naturalistic follow-up. Tuberk Toraks 2005;53:354-61.
- Ossip DJ, Abrams SM, Mahoney MC, Sall D, Cummings KM. Adverse effects with use of nicotine replacement therapy among quitline clients. Nicotine Tob Res 2009;11:408-17. https://doi.org/10.1093/ntr/ntp005.
- Panos NG, Tesoro EP, Kim KS, Mucksavage JJ. Outcomes associated with transdermal nicotine replacement therapy in a neurosurgery intensive care unit. Am J Health Syst Pharm 2010;67:1357-61. https://doi.org/10.2146/ajhp090402.
- Pasternak B, Svanström H, Hviid A. Use of varenicline versus bupropion and risk of psychiatric adverse events. Addiction 2013;108:1336-43. https://doi.org/10.1111/add.12165.
- Peña P, Zagolin M, Acuña M, Navarrete S, Bustamante P, Suárez C, et al. Results of a multidisciplinary program to quit smoking. Rev Med Chil 2013;141:345-52. https://doi.org/10.4067/S0034-98872013000300010.
- Politis A, Ioannidis V, Gourgoulianis KI, Daniil Z, Hatzoglou C. Effects of varenicline therapy in combination with advanced behavioral support on smoking cessation and quality of life in inpatients with acute exacerbation of COPD, bronchial asthma, or community-acquired pneumonia: a prospective, open-label, preference-based, 52-week, follow-up trial. Chron Respir Dis 2018;15:146-56. https://doi.org/10.1177/1479972317740128.
- Postolache P, Cojocaru DC, Olaru M, Todea D, Nemes RM. Pharmacotherapy for Smoking Cessation – The Experience of a Smoking Cessation Center n.d. https://doi.org/10.1109/EHB.2013.6707332.
- Roth MT, Andrus MR, Westman EC. Outcomes from an outpatient smoking-cessation clinic. Pharmacotherapy 2005;25:279-88. https://doi.org/10.1592/phco.25.2.279.56957.
- Sachs R, Wild TC, Thomas L, Hammal F, Finegan BA. Smoking cessation interventions in the pre-admission clinic: assessing two approaches. Can J Anaesth 2012;59:662-9. https://doi.org/10.1007/s12630-012-9716-6.
- Saxon AJ, Baer JS, Davis TM, Sloan KL, Malte CA, Fitzgibbons K, et al. Smoking cessation treatment among dually diagnosed individuals: preliminary evaluation of different pharmacotherapies. Nicotine Tob Res 2003;5:589-96. https://doi.org/10.1080/1462220031000118702.
- Shiltz D, Paniagua A, Hastings JE. A retrospective comparison of varenicline monotherapy versus the combination of varenicline and bupropion or bupropion and nicotine patches in a VA tobacco cessation clinic. J Smoking Cessation 2012;6:65-73. https://doi.org/10.1375/jsc.6.1.65.
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction 2008;103:146-54. https://doi.org/10.1111/j.1360-0443.2007.02083.x.
- Steinberg MB, Bover MT, Richardson DL, Schmelzer AC, Williams JM, Foulds J. Abstinence and psychological distress in co-morbid smokers using various pharmacotherapies. Drug Alcohol Depend 2011;114:77-81. https://doi.org/10.1016/j.drugalcdep.2010.06.022.
- Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ 2012;345. https://doi.org/10.1136/bmj.e7176.
- Williams JH, Jones TE. Smoking cessation post-discharge following nicotine replacement therapy use during an inpatient admission. Intern Med J 2012;42:154-9. https://doi.org/10.1111/j.1445-5994.2011.02442.x.
- Wolfenden L, Wiggers J, Campbell E, Knight J. Pilot of a preoperative smoking cessation intervention incorporating post-discharge support from a Quitline. Health Promot J Austr 2008;19:158-60. https://doi.org/10.1071/he08158.
- Woolf KJ, Zabad MN, Post JM, McNitt S, Williams GC, Bisognano JD. Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol 2012;110:968-70. https://doi.org/10.1016/j.amjcard.2012.05.028.
- Xiao D, Zhong N, Bai C, Xiu Q, Xie C, Hu D, et al. Nicotine gum or patch treatment for smoking cessation and smoking reduction: a multi-centre study in Chinese physicians. Front Med 2014;8:84-90. https://doi.org/10.1007/s11684-014-0311-9.
- Xu Z, Fujiwara R, Fucito L, Bernstein S, Hsia HC. 30(th) Annual Meeting of the Wound Healing Society SAWC-Spring/WHS Joint Meeting: Charlotte Convention Center, Charlotte, North Carolina, USA, April 25–29, 2018. Wound Repair Regen 2018;26:A1-42. https://doi.org/10.1111/wrr.12622.
- von Wartburg M, Raymond V, Paradis PE. The long-term cost-effectiveness of varenicline (12-week standard course and 12 + 12-week extended course) vs. other smoking cessation strategies in Canada. Int J Clin Pract 2014;68:639-46. https://doi.org/10.1111/ijcp.12363.
- Athanasakis K, Igoumenidis M, Karampli E, Vitsou E, Sykara G, Kyriopoulos J. Cost-effectiveness of varenicline versus bupropion, nicotine-replacement therapy, and unaided cessation in Greece. Clin Ther 2012;34:1803-14. https://doi.org/10.1016/j.clinthera.2012.07.002.
- Great Britain . The Tobacco and Related Products Regulations 2016 2016. www.legislation.gov.uk/uksi/2016/507/contents.
- Medicines and Healthcare products Regulatory Agency . Guidance: Licensing Procedure for Electronic Cigarettes As Medicines 2017. www.gov.uk/guidance/licensing-procedure-for-electronic-cigarettes-as-medicines (accessed 23 November 2020).
- Office for National Statistics (ONS) . Registrations of Cancer Diagnosed in 2003, England 2005.
- British Heart Foundation . Coronary Heart Disease Statistics Fact Sheet 2006.
- Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HA. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford Myocardial Infarction Incidence Study Group. Heart 1998;80:40-4. https://doi.org/10.1136/hrt.80.1.40.
- ONS . Health Statistics Quarterly, No. 12 2001.
- Asthma UK . Where Do We Stand?: Asthma in the UK Today 2004.
- Health Pricing Office (HPO) . Hospital Inpatient Enquiry (HIPE) Database 2016.
- Primary Care Reimbursement Service (PCRS) . Statistical Analysis of Claims and Payments 2014. 2014.
Appendix 1 MEDLINE search strategies
MEDLINE search strategy for randomised controlled trials
-
Smoking/ (134,671)
-
Tobacco Smoking/ (397)
-
Tobacco/ (29,151)
-
Nicotine/ (24,376)
-
Tobacco Products/ (3005)
-
Smoking Cessation/ (26,370)
-
“Tobacco Use Cessation”/ (1045)
-
“Tobacco Use Disorder”/ (10,555)
-
(smoking or smoker*).ti,ab,kf. (231,897)
-
(tobacco* or cigar* or cigarette* or nicotine).ti,ab,kf. (164,203)
-
or/1-10 (353,307)
-
Bupropion/ (2887)
-
Varenicline/ (1147)
-
Nicotinic Agonists/ (6990)
-
(NRT or nicotine replacement).ti,ab,kf. (3901)
-
bupropion.ti,ab,kf. (4038)
-
(amfebutamone or quomen or wellbutrin or zyban or zyntabac).ti,ab,kf. (201)
-
varenicline.ti,ab,kf. (1578)
-
(champix or tabex or chantix).ti,ab,kf. (125)
-
(nicotine adj2 (patch* or gum* or nasal spray* or lozenge* or tablet* or sublingual* or inhal* or replacement or chewing or polac* or transdermal* or product*)).ti,ab,kf. (5818)
-
(nicorette or niquitin or nicotinell).ti,ab,kf. (110)
-
(nicotinic adj3 agonist*).ti,ab,kf. (2237)
-
(benzazepine* adj2 derivative*).ti,ab,kf. (84)
-
nicotinic receptor partial agonist*.ti,ab,kf. (58)
-
or/12-24 (17,839)
-
Electronic Nicotine Delivery Systems/ (2174)
-
Vaping/ (237)
-
(electr* adj2 (cig* or nicotine or device*)).ti,ab,kf. (17,861)
-
(ecig* or e-cig*).ti,ab,kf. (2982)
-
(nicotine adj4 (electr* or ENDS or aerosol*)).ti,ab,kf. (1023)
-
(vape or vaper or vapers or vaping or vapor or vapour).ti,ab,kf. (42,208)
-
or/26-31 (60,589)
-
((smoking or tobacco) adj5 (cessation or ceas* or quit* or stop* or giv* or prevent* or abstain* or abstin* or control*)).ti,kf. (18,876)
-
randomized controlled trial.pt. (475,058)
-
controlled clinical trial.pt. (92,883)
-
pragmatic clinical trial.pt. (951)
-
clinical trial.pt. (514,264)
-
clinical trial/ or clinical trial, phase i/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or controlled clinical trial/ (575,942)
-
Random Allocation/ (97,344)
-
randomized controlled trial/ (475,058)
-
pragmatic clinical trial/ (951)
-
Double-Blind Method/ (149,187)
-
Single-Blind Method/ (26,163)
-
Placebos/ (34,201)
-
((clin* or randomi?ed) adj5 trial*).ti,ab,kf. (586,919)
-
((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab,kf. (163,772)
-
placebo*.ti,ab,kf. (202,178)
-
control groups/ (1604)
-
randomi?ation.ti,ab,kf. (32,653)
-
randomly.ab. (304,261)
-
(random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. (429,094)
-
drug therapy.fs. (2,077,773)
-
trial.ti,ab,kf. (534,702)
-
groups.ab. (1,874,309)
-
(control* adj3 (trial* or study or studies)).ab,ti. (470,250)
-
((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. (222,854)
-
(quasi adj (experimental or random$)).ti,ab. (15,129)
-
((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab. (5264)
-
or/34-58 (4,771,652)
-
11 and (25 or 32) and 59 (6074)
-
(animals not (humans and animals)).sh. (4,506,319)
-
60 not 61 (5269)
-
(2017* or 2018*).yr,dp,dt,ep,ez. (2,712,640)
-
62 and 63 (740)
-
(2018* or 2019*).yr,dp,dt,ep,ez. (1,556,937)
-
62 and 65 (438).
MEDLINE search strategy for non-randomised studies
-
Smoking/ (134,503)
-
Tobacco Smoking/ (424)
-
Tobacco/ (29,125)
-
Nicotine/ (24,327)
-
Tobacco Products/ (3020)
-
Smoking Cessation/ (26,263)
-
“Tobacco Use Cessation”/ (1039)
-
“Tobacco Use Disorder”/ (10,482)
-
(smoking or smoker*).ti,ab,kf. (231,830)
-
(tobacco* or cigar* or cigarette* or nicotine).ti,ab,kf. (164,354)
-
or/1-10 (353,421)
-
Bupropion/ (2875)
-
Varenicline/ (1143)
-
Nicotinic Agonists/ (6977)
-
(NRT or nicotine replacement).ti,ab,kf. (3893)
-
bupropion.ti,ab,kf. (4026)
-
(amfebutamone or quomen or wellbutrin or zyban or zyntabac).ti,ab,kf. (200)
-
varenicline.ti,ab,kf. (1574)
-
(champix or tabex or chantix).ti,ab,kf. (125)
-
(nicotine adj2 (patch* or gum* or nasal spray* or lozenge* or tablet* or sublingual* or inhal* or replacement or chewing or polac* or transdermal* or product*)).ti,ab,kf. (5809)
-
(nicorette or niquitin or nicotinell).ti,ab,kf. (110)
-
(nicotinic adj3 agonist*).ti,ab,kf. (2235)
-
(benzazepine* adj2 derivative*).ti,ab,kf. (84)
-
nicotinic receptor partial agonist*.ti,ab,kf. (58)
-
or/12-24 (17,812)
-
Electronic Nicotine Delivery Systems/ (2189)
-
Vaping/ (243)
-
(electr* adj2 (cig* or nicotine or device*)).ti,ab,kf. (17,929)
-
(ecig* or e-cig*).ti,ab,kf. (3005)
-
(nicotine adj4 (electr* or ENDS or aerosol*)).ti,ab,kf. (1026)
-
(vape or vaper or vapers or vaping or vapor or vapour).ti,ab,kf. (42,034)
-
or/26-31 (60,491)
-
((smoking or tobacco) adj5 (cessation or ceas* or quit* or stop* or giv* or prevent* or abstain* or abstin* or control*)).ti,kf. (18,825)
-
epidemiologic studies/ or case-control studies/ or cross-sectional studies/ or cohort studies/ or follow-up studies/ or longitudinal studies/ or prospective studies/ or retrospective studies/ (2,244,426)
-
((epidemiologic or prospective or retrospective or cross-sectional or case control* or cohort or longitudinal or followup or follow-up) adj3 (study or studies)).ti,ab,kf. (1,011,257)
-
(cross-sectional or follow-up or followup or longitudinal or prospective or retrospective or observational or population).ti. (563,430)
-
cohort?.ti,ab,kf. (497,828)
-
(case control* or case series).ti,ab,kf. (179,847)
-
or/34-38 (2,928,405)
-
(dream* or nightmare? or aggression or aggressive* or anxiety or anxious or (angina or arrhythmia* or cardiac or cardiovascular or coronary or myocardi* or heart failure? or heart attack? or isch?emi*) or COPD or chronic obstructive pulmonary disease or death? or mortalit* or (depression or depressive) or dry mouth or fatigue or headache? or migraine? or hospitali* or (insomnia* or sleep disorder* or somnolence) or irritability or irritable or (nausea or vomiting) or palpitations or pruritus or seizure* or rash or (stroke or strokes or thrombo* or thrombus or emboli* or VTE or DVT or TIA or bleed* or h?emorrhag*) or suicid* or parasuicid* or selfharm* or self-harm* or selfinjur* or self-injur*).mp. (5,115,897)
-
exp Hypersensitivity/ (327,542)
-
Drug Interactions/ or exp "drug-related side effects and adverse reactions"/ (187,530)
-
exp Product Surveillance, Postmarketing/ (14,019)
-
adverse effects.fs. (1,622,477)
-
drug effects.fs. (2,835,326)
-
complications.fs. (1,875,364)
-
poisoning.fs. (64,263)
-
toxicity.fs. (399,562)
-
chemically induced.fs. (559,975)
-
(safe* or adverse or tolerability or toxicity or toxic or adrs or adr or tolerance or tolerat* or harm or harms or harmful or complication* or drug? interaction? or hypersensitiv* or hyper-sensitiv*).ti,kf. (650,849)
-
((adverse adj2 (event? or react*)) or side effect* or treatment emergent or undesirable effect*).ti,ab,kf. (417,393)
-
((discontinu* or withdr*) adj3 (study or treatment) adj3 due to).ti,ab,kf. (2430)
-
or/40-52 (9,947,953)
-
(11 and (25 or 32)) or 33 (31,504)
-
(2017* or 2018* or 2019*).yr,dp,dt,ep,ez. (2,836,508)
-
39 and 53 and 54 and 55 (405).
Appendix 2 Inputs into the economic model
| Model parameter | Source | Age and sex category | |||||
|---|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | ||
| General population (n) | ONS 2016107 | 7,459,224 | 12,446,202 | 5,359,995 | 7,288,586 | 12,759,446 | 6,454,090 |
| Smoking prevalence (% of population) | ONS 2016107 | 22.48 | 18.75 | 8.80 | 17.85 | 15.06 | 7.80 |
| Risk of all-cause mortality in the general population (annual probability of all-cause mortality) (%) | ONS 2016107 | 0.07 | 0.40 | 4.40 | 0.03 | 0.26 | 4.13 |
| Disease | Data source | n | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |||
| COPD | British Lung Foundation 2012109 | 12.6 million | 0.0 | 1.0 | 7.0 | 0.0 | 1.0 | 7.0 |
| Lung cancer | Maddams et al. 2009111 | 7.7 million | 0.0 | 0.1 | 0.7 | 0.0 | 0.1 | 0.3 |
| History of CHD | Health Survey for England 2016113 | 8011 | 0.2 | 4.5 | 20.9 | 0.4 | 1.9 | 12.0 |
| History of stroke | Bhatnagar et al. 2015114 | 35 million | 0.1 | 1.8 | 10.6 | 0.1 | 1.4 | 8.4 |
| Asthma exacerbation | British Lung Foundation 2012109 | 12.6 million | 19.0 | 12.0 | 11.0 | 19.0 | 12.0 | 11.0 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.0 | 2.1 | 12.1 | 0.0 | 2.3 | 15.2 |
| Lung cancer | 0.0 | 0.3 | 2.5 | 0.0 | 0.2 | 1.3 |
| History of CHD | 0.2 | 9.1 | 29.0 | 0.4 | 4.6 | 18.6 |
| History of stroke | 0.1 | 4.4 | 17.8 | 0.1 | 4.0 | 14.6 |
| Asthma exacerbation | 24.8 | 12.1 | 12.1 | 25.2 | 12.2 | 12.1 |
| Original source | Age and sex category | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| ONS (2005b) Registrations of Cancer Diagnosed in 2003658 | 0 | 0.05 | 0.4 | 0 | 0.03 | 0.2 |
| Cancer Registration Statistics, England, 2016 (First Release) ONS118 | 0 | 0.04 | 0.4 | 0 | 0.04 | 0.3 |
| Disease | Data source | n | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |||
| COPD | Pfizer 2007117 | UK population | 0.00 | 0.01 | 0.30 | 0.00 | 0.01 | 0.20 |
| Lung cancer | ONS 2005658 | Population of England | 0.00 | 0.04 | 0.40 | 0.00 | 0.04 | 0.33 |
| CHD (first non-fatal event) | British Heart Foundation 2006659 | 151,000 | 0.00 | 0.08 | 0.80 | 0.00 | 0.02 | 0.60 |
| CHD (any non-fatal event) | Volmink et al. 1998660 | 568,800 | 0.00 | 0.12 | 1.40 | 0.00 | 0.03 | 0.90 |
| Stroke (first non-fatal event) | ONS 2001661 | UK population | 0.00 | 0.15 | 0.65 | 0.00 | 0.10 | 0.60 |
| Stroke (any non-fatal event) | ONS 2001661 | UK population | 0.00 | 0.20 | 1.00 | 0.00 | 0.14 | 1.00 |
| Asthma | Asthma UK662 | 0.06 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 | |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.00 | 0.02 | 0.52 | 0.00 | 0.02 | 0.44 |
| Lung cancer | 0.00 | 0.13 | 1.35 | 0.00 | 0.14 | 1.34 |
| CHD (first non-fatal event) | 0.00 | 0.16 | 1.11 | 0.00 | 0.05 | 0.93 |
| Stroke (first non-fatal event) | 0.00 | 0.36 | 1.09 | 0.00 | 0.28 | 1.04 |
| CHD (any non-fatal event) | 0.00 | 0.24 | 1.94 | 0.00 | 0.07 | 1.40 |
| Asthma | 0.08 | 0.05 | 0.07 | 0.08 | 0.05 | 0.06 |
| Stroke (any non-fatal event) | 0.00 | 0.49 | 1.68 | 0.00 | 0.40 | 1.73 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.00 | 0.02 | 0.47 | 0.00 | 0.02 | 0.43 |
| Lung cancer | 0.00 | 0.05 | 0.50 | 0.00 | 0.05 | 0.48 |
| CHD (first non-fatal event) | 0.00 | 0.09 | 0.83 | 0.00 | 0.02 | 0.64 |
| Stroke (first non-fatal event) | 0.00 | 0.11 | 0.63 | 0.00 | 0.08 | 0.61 |
| CHD (any non-fatal event) | 0.00 | 0.13 | 1.46 | 0.00 | 0.03 | 0.96 |
| Asthma | 0.06 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 |
| Stroke (any non-fatal event) | 0.00 | 0.14 | 0.97 | 0.00 | 0.11 | 1.02 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.000 | 0.001 | 0.030 | 0.000 | 0.002 | 0.036 |
| Lung cancer | 0.00 | 0.05 | 0.50 | 0.00 | 0.05 | 0.48 |
| CHD (first non-fatal event) | 0.000 | 0.048 | 0.693 | 0.000 | 0.012 | 0.533 |
| Stroke (first non-fatal event) | 0.000 | 0.097 | 0.575 | 0.000 | 0.062 | 0.533 |
| CHD (any non-fatal event) | 0.000 | 0.072 | 1.213 | 0.000 | 0.018 | 0.799 |
| Asthma | 0.055 | 0.050 | 0.059 | 0.056 | 0.050 | 0.050 |
| Stroke (any non-fatal event) | 0.000 | 0.130 | 0.885 | 0.000 | 0.087 | 0.889 |
| Disease | Data source | n | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |||
| COPD | BHF 2018120 | UK population | 0.00 | 1.45 | 4.22 | 0.00 | 1.26 | 3.63 |
| Lung cancer | BHF 2018120 | UK population | 0.00 | 29.30 | 48.80 | 0.00 | 23.80 | 34.30 |
| Stroke (first non-fatal event) | Assumption. The same split between first event and all events is assumed for first/subsequent for mortality as for incidence | UK population | 0.41 | 0.51 | 1.78 | 0.14 | 0.58 | 2.74 |
| Stroke (any non-fatal event) | BHF 2018120 | UK population | 0.60 | 0.68 | 2.74 | 0.20 | 0.81 | 4.57 |
| CHD (first non-fatal event) | Assumption. The same split between first event and all events as used in a previous manufacturer's STA submission117 is assumed for first/subsequent | UK population | 0.53 | 0.92 | 1.71 | 0.06 | 0.88 | 2.23 |
| CHD (any non-fatal event) | BHF 2018120 | UK population | 0.70 | 1.37 | 2.90 | 0.08 | 0.88 | 3.05 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.00 | 3.05 | 7.28 | 0.00 | 2.88 | 7.89 |
| Lung cancer | 0.00 | 29.30 | 48.80 | 0.00 | 23.80 | 34.30 |
| CHD (first non-fatal event) | 0.53 | 1.85 | 2.37 | 0.06 | 2.14 | 3.46 |
| CHD (any non-fatal event) | 0.70 | 2.76 | 4.02 | 0.08 | 2.14 | 4.74 |
| Stroke (first non-fatal event) | 0.41 | 1.24 | 3.00 | 0.14 | 1.64 | 4.75 |
| Stroke (any non-fatal event) | 0.60 | 1.65 | 4.61 | 0.20 | 2.29 | 7.92 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.00 | 2.79 | 6.65 | 0.00 | 2.81 | 7.71 |
| Lung cancer | 0.00 | 29.30 | 48.80 | 0.00 | 23.80 | 34.30 |
| CHD (first non-fatal event) | 0.53 | 0.99 | 1.78 | 0.06 | 0.98 | 2.37 |
| Stroke (first non-fatal event) | 0.41 | 0.36 | 1.73 | 0.14 | 0.47 | 2.80 |
| CHD (any non-fatal event) | 0.70 | 0.99 | 2.51 | 0.08 | 0.98 | 3.25 |
| Stroke (any non-fatal event) | 0.60 | 0.48 | 2.67 | 0.20 | 0.66 | 4.67 |
| Disease | Age and sex category (%) | |||||
|---|---|---|---|---|---|---|
| Male aged 18–34 years | Male aged 35–64 years | Male aged ≥ 65 years | Female aged 18–34 years | Female aged 35–64 years | Female aged ≥ 65 years | |
| COPD | 0.0 | 0.2 | 0.4 | 0.0 | 0.2 | 0.7 |
| Lung cancer | 0.00 | 29.30 | 48.80 | 0.00 | 23.80 | 34.30 |
| CHD (first non-fatal event) | 0.5 | 0.6 | 1.5 | 0.1 | 0.5 | 2.0 |
| Stroke (first non-fatal event) | 0.4 | 0.3 | 1.6 | 0.1 | 0.4 | 2.4 |
| CHD (any non-fatal event) | 0.7 | 0.8 | 2.5 | 0.1 | 0.5 | 2.7 |
| Stroke (any non-fatal event) | 0.6 | 0.4 | 2.4 | 0.2 | 0.5 | 4.1 |
| Data | Original source | Mean probability | 95% CI | Distribution over relapse category time period |
|---|---|---|---|---|
| Annual relapse probability, > 1 and < 5 years post cessation (time period 4 years) | Hawkins et al. 2010121 | 0.1291 | 0.1174 to 0.1414 | Beta(395, 35) |
| Annual relapse probability, ≥ 5 and < 10 years post cessation (time period 5 years) | Hawkins et al. 2010121 | 0.0331 | 0.0230 to 0.0452 | Beta(33, 180) |
| Annual relapse probability, > 10 years post cessation (time period 26 years) | Krall et al. 2002122 | 0.0009 | 0.0004 to 0.0015 | Beta(9, 360) |
| Data | Original source | n | Mean cost from paper (currency) | 95% CrI (currency) | Cost for model (£) | Distribution |
|---|---|---|---|---|---|---|
| COPD | Hospital Inpatient Enquiry Database 2015.663 Cost per prevalent case of inpatient and day case treatment | 73,901 | 868 (euros) | 664 to 1097 (euros) | 1468 (exchange rate £1 = €1.14)125 | Gamma (7,390,100, 0.0002) |
| Primary Care Reimbursement Service 2014.664 Cost per prevalent case of primary care treatment | 73,657 | 662 (euros) | 504 to 831 (euros) | |||
| Total of inpatient and primary care | 1530 (euros) | |||||
| Lung cancer | Hospital Inpatient Enquiry Database 2015.663 Cost per prevalent case of inpatient and day-case treatment | 4666 | 5107 (euros) | 3915 to 6499 (euros) | 5429 (exchange rate £1 = €1.14)125 | Gamma (466,600, 0.01) |
| Primary Care Reimbursement Service 2014.664 Cost per prevalent case of primary care treatment | 4666 | 555 (euros) | 423 to 698 (euros) | |||
| Total of inpatient and primary care | 5662 (euros) | |||||
| CHD (non-fatal event) | British Heart Foundation. 2014128 | 1323 (GBP) | 1460 | Gamma (100, 14.60) | ||
| Stroke (non-fatal event) | Xu et al. 2018130 | 84,184 | 13,452 at 1 year (GBP) | 13,788 | Gamma (8,418,400, 0.002) | |
| Asthma exacerbation | Tan et al. 2016132 | 939 | 341 (GBP) | SE 12.94 (GBP) | 367 | Gamma (805, 0.46) |
| Depression | Hunter et al. 2014134 | 340.35 (GBP) | 395 | Gamma (100, 3.95) | ||
| Self-harm | Tsiachristas et al. 2017135 | 1140 | 809 (GBP) | SE 26.78 (GBP) | 850 | Gamma (1007, 0.84) |
| Treatment | Assumed mean cost (£) | Source | Assumption |
|---|---|---|---|
| NRT low | 83.84 | BNF137 | 4 weeks of 10-mg/16-hour patches and 4 weeks of 5-mg/16-hour patches |
| NRT standard | 105.65 | BNF137 | High-strength patch daily for 6–8 weeks, followed by medium-strength patch for 2 weeks, and low-strength patch for final 2 weeks |
| NRT high | 77.46 | BNF137 | 4-week supply of 21 mg NicoDerm transdermal patches, followed by 2 weeks of 14 mg and 2 weeks of 7 mg |
| Bupropion low | 62.64 | BNF137 | One 150-mg tablet a day for an average of 13 weeks |
| Bupropion standard | 83.52 | BNF137 | 150 mg daily for 6 days, then 150 mg twice daily for a period of treatment of 7–9 weeks |
| Varenicline low | 163.80 | BNF137 | 0.5 mg once daily for 3 days, increased to 0.5 mg twice daily for 4 days, then 1 mg twice daily for 11 weeks |
| Varenicline standard | 163.80 | BNF137 | 1 mg once daily for 3 days, increased to 1 mg twice daily for 4 days, then 1 mg twice daily for 11 weeks |
| E-cigarette | 82 | Liber et al. 2017139 | 12-week supply of e-cigarettes (e-cigarette + 3.55 ml liquid per day including a replacement atomiser in months 2 and 3) |
| Bupropion standard plus NRT high | 160.98 | BNF137 | |
| Varenicline low plus NRT standard | 269.45 | BNF137 | |
| Varenicline standard plus NRT standard | 269.45 | BNF137 | |
| Varenicline standard plus NRT high | 241.26 | BNF137 | |
| Varenicline standard plus bupropion standard | 247.32 | BNF137 |
| Health state | Utility source | n | Mean utility | SE |
|---|---|---|---|---|
| NCM, male, 18–34 years | Ara and Brazier 2010140 | 26,679 | 0.94 | |
| NCM, male, 35–64 years | Ara and Brazier 2010140 | 26,679 | 0.88 | |
| NCM, male, 65–100 years | Ara and Brazier 2010140 | 26,679 | 0.72 | |
| NCM, female, 18–34 years | Ara and Brazier 2010140 | 26,679 | 0.92 | |
| NCM, female, 35–64 years | Ara and Brazier 2010140 | 26,679 | 0.86 | |
| NCM, female, 65–100 years | Ara and Brazier 2010140 | 26,679 | 0.70 | |
| Lung cancer | Bertranou et al. 2018142 | 464 | 0.72 | 0.001 |
| COPD | Pickard et al. 2008144 | 0.69 | 0.043 | |
| CHD | Stevanović et al. 2016146 | 30,575 | 0.76 | 0.01 |
| Stroke (first event) | Haacke et al. 2006148 | 77 | 0.73 | 0.036 |
| Stroke (second event) | Ara and Brazier 2010140 | 18 | 0.48 | 0.087 |
| Asthma exacerbation | Lloyd et al. 2007151 | 112 | 0.57 | 0.026 |
| Depression | Hunter et al. 2014134 | 0.58 | 0.015 | |
| Self-harm | Byford et al. 2003157 | 480 | 0.50 | 0.016 |
| Treatment | 1-year continuous abstinence probability | ||
|---|---|---|---|
| Mean | 2.5% | 97.5% | |
| Varenicline low plus NRT standard | 0.43 | 0.17 | 0.73 |
| Varenicline standard plus NRT standard | 0.43 | 0.23 | 0.66 |
| E-cigarette low | 0.32 | 0.12 | 0.63 |
| Varenicline plus bupropion standard | 0.31 | 0.15 | 0.51 |
| E-cigarette high | 0.30 | 0.18 | 0.45 |
| Varenicline standard | 0.27 | 0.23 | 0.32 |
| Varenicline standard plus NRT high | 0.24 | 0.12 | 0.38 |
| NRT high | 0.23 | 0.19 | 0.27 |
| NRT standard | 0.21 | 0.21 | 0.21 |
| Varenicline low | 0.19 | 0.12 | 0.28 |
| Bupropion standard plus NRT high | 0.19 | 0.10 | 0.32 |
| Bupropion low | 0.19 | 0.12 | 0.28 |
| Bupropion standard | 0.18 | 0.15 | 0.22 |
| NRT low | 0.07 | 0.02 | 0.16 |
| Treatment | Depression probability | ||
|---|---|---|---|
| Mean | 2.5% | 97.5% | |
| NRT standard | 0.07 | 0.00 | 0.39 |
| Bupropion standard plus NRT high | 0.08 | 0.01 | 0.22 |
| NRT not specified | 0.15 | 0.15 | 0.15 |
| Bupropion standard | 0.17 | 0.11 | 0.24 |
| Varenicline standard plus NRT high | 0.17 | 0.06 | 0.37 |
| NRT high | 0.19 | 0.10 | 0.30 |
| Varenicline standard | 0.22 | 0.16 | 0.31 |
| Varenicline low | 0.29 | 0.02 | 0.78 |
| Varenicline standard plus NRT standard | 0.36 | 0.09 | 0.77 |
| Treatment | Self-harm probability | ||
|---|---|---|---|
| Mean | 2.5% | 97.5% | |
| Varenicline plus bupropion standard | 0.004 | 0.000 | 0.018 |
| Bupropion standard plus NRT high | 0.005 | 0.001 | 0.016 |
| NRT standard | 0.006 | 0.000 | 0.036 |
| NRT not specified | 0.010 | 0.010 | 0.011 |
| Bupropion standard | 0.012 | 0.007 | 0.018 |
| Varenicline standard plus NRT high | 0.013 | 0.003 | 0.033 |
| NRT high | 0.013 | 0.006 | 0.024 |
| Varenicline standard | 0.016 | 0.011 | 0.025 |
| Varenicline low | 0.034 | 0.001 | 0.171 |
| Varenicline standard plus NRT standard | 0.042 | 0.006 | 0.158 |
| Disease | RR in smokers | RR in former smokers | RR in never-smokers |
|---|---|---|---|
| COPDa | |||
| Male aged 18–34 years | 1 | 1 | 1 |
| Male aged 35–64 years | 17.1 | 15.64 | 1 |
| Male aged ≥ 65 years | 17.1b | 15.64b | 1 |
| Female aged 18–34 years | 1 | 1 | 1 |
| Female aged 35–64 years | 12.04 | 11.77 | 1 |
| Female aged ≥ 65 years | 12.04b | 11.77b | 1 |
| Lung cancera | |||
| Male aged 18–34 years | 1 | 1 | 1 |
| Male aged 35–64 years | 23.26 | 8.7 | 1 |
| Male aged ≥ 65 years | 23.26b | 8.7b | 1 |
| Female aged 18–34 years | 1 | 1 | 1 |
| Female aged 35–64 years | 12.69 | 4.53 | 1 |
| Female aged ≥ 65 years | 12.69b | 4.53b | 1 |
| CHDc | |||
| Male aged 18–34 years | 1 | 1 | 1 |
| Male aged 35–64 years | 3.35 | 1.80 | 1 |
| Male aged ≥ 65 years | 1.60 | 1.20 | 1 |
| Female aged 18–34 years | 1 | 1 | 1 |
| Female aged 35–64 years | 4.05 | 1.85 | 1 |
| Female aged ≥ 65 years | 1.75 | 1.20 | 1 |
| Strokec | |||
| Male aged 18–34 years | 1 | 1 | 1 |
| Male aged 35–64 years | 3.75 | 1.1 | 1 |
| Male aged ≥ 65 years | 1.9 | 1.1b | 1 |
| Female aged 18–34 years | 1 | 1 | 1 |
| Female aged 35–64 years | 4.55 | 1.3 | 1 |
| Female aged ≥ 65 years | 1.95 | 1.15 | 1 |
Appendix 3 Formulae to calculate the expected number of cases of disease in the cohort of smokers
Total disease prevalence (PT) within the overall population is the weighted sum of the prevalence within the three subgroups [current smokers (PCS), former smokers (PFS) and never smokers (PNS)], with the weights being the proportion of people in each group [current smokers (πCS), former smokers (πFS) or never smokers (1 – πCS – πFS); Equation 3].
Disease prevalence within the group of current and former smokers can be expressed in terms of the prevalence among never smokers using the RR of the disease in current and former smokers (RRCS and RRFS, respectively).
Substituting these into Equation 3 gives Equation 6, which on rearrangement allows the disease prevalence among never-smokers (PNS) to be expressed in terms of the total population prevalence (PT), the proportion of people who are current smokers (πCS), former smokers (πFS), and the RR associated with being a current or former smoker (RRCS, RRFS), all of which are known (Equation 7):
Solving for PNS then allows us to calculate PFS (prevalence rate in former smokers) and PCS (prevalence rate in current smokers) using Equations 4 and 5.
Appendix 4 Risk-of-bias summary figures
FIGURE 34.
Risk-of-bias summary figure for RCTs reporting one or more effectiveness outcomes. This figure is reproduced with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
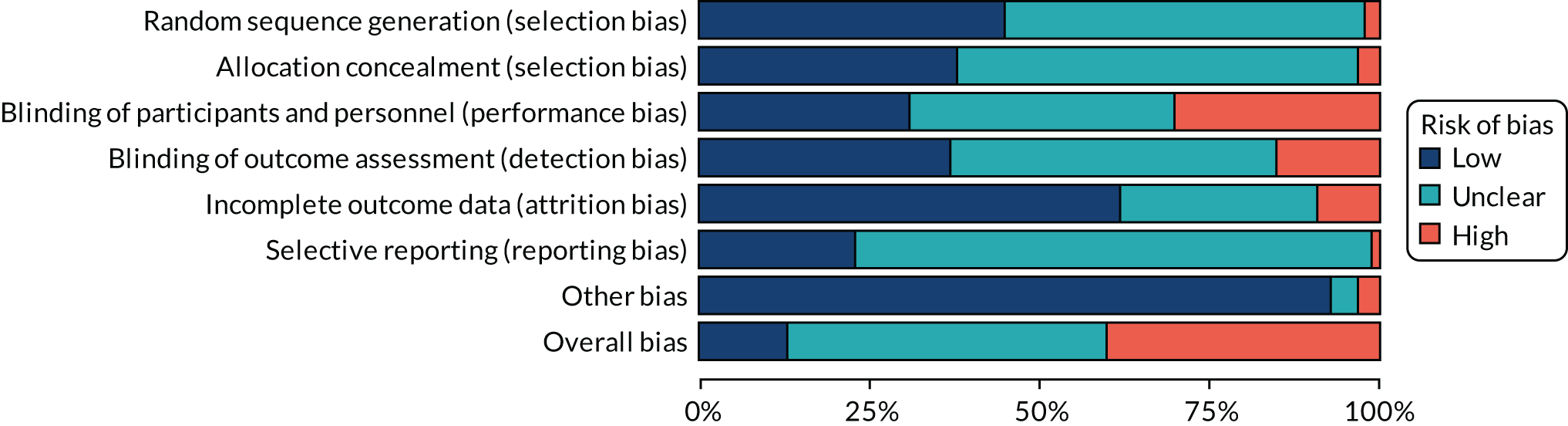
FIGURE 35.
Risk-of-bias summary figure for RCTs reporting one or more safety outcomes. This figure is reproduced with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
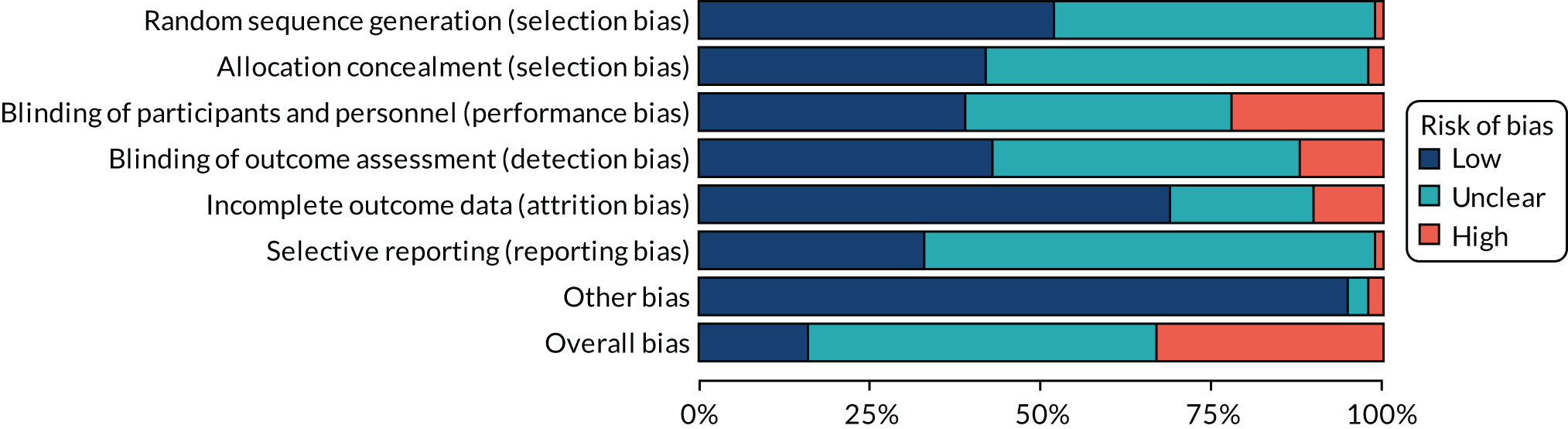
FIGURE 36.
Risk-of-bias summary figure for non-randomised studies reporting one or more safety outcomes.
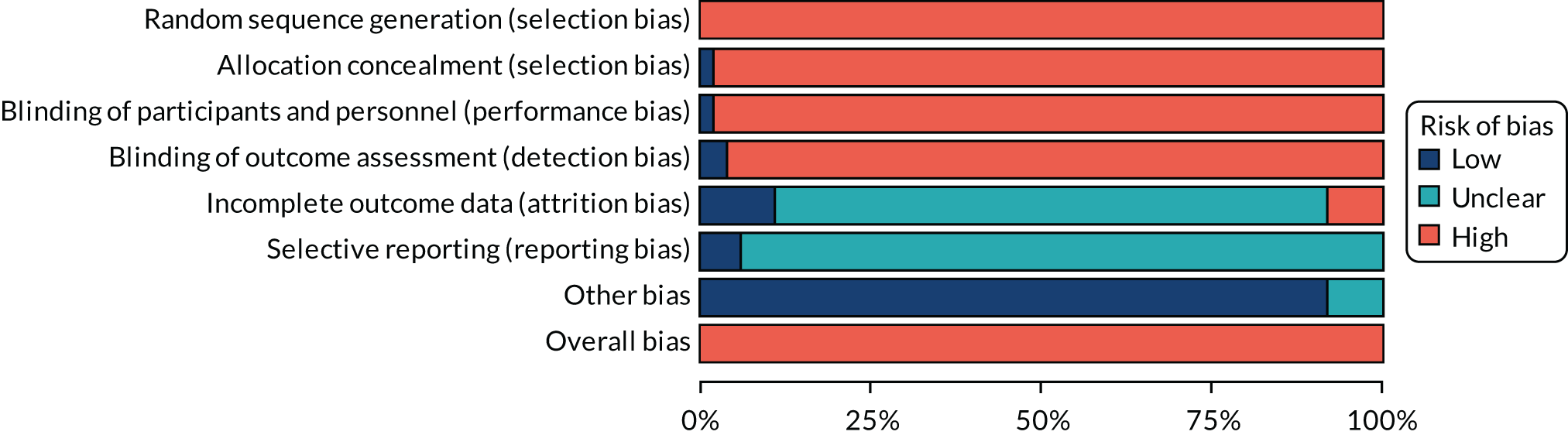
Appendix 5 Effectiveness analyses
| Treatment | Frequency |
|---|---|
| Bupropion low | 10 |
| Bupropion high plus NRT combination high | 1 |
| Bupropion not specified | 2 |
| Bupropion not specified plus NRT choice not specified | 1 |
| Bupropion not specified plus NRT patch (24 hours) not specified | 1 |
| Bupropion standard | 73 |
| Bupropion standard plus NRT choice not specified | 1 |
| Bupropion standard plus NRT combination high | 1 |
| Bupropion standard plus NRT gum high | 1 |
| Bupropion standard plus NRT gum not specified | 4 |
| Bupropion standard plus NRT gum standard | 1 |
| Bupropion standard plus NRT inhalator not specified | 1 |
| Bupropion standard plus NRT lozenge not specified | 2 |
| Bupropion standard plus NRT patch (24 hours) high | 8 |
| Bupropion standard plus NRT patch (24 hours) not specified | 2 |
| E-cigarette high | 2 |
| E-cigarette high plus NRT patch (24 hours) not specified | 1 |
| E-cigarette high | 4 |
| No drug treatment | 97 |
| NRT choice high | 1 |
| NRT choice not specified | 29 |
| NRT choice standard | 1 |
| NRT combination high | 8 |
| NRT combination not specified | 7 |
| NRT combination standard | 6 |
| NRT gum high | 13 |
| NRT gum high | 1 |
| NRT gum not specified | 14 |
| NRT gum standard | 79 |
| NRT inhalator standard | 10 |
| NRT lozenge high | 7 |
| NRT lozenge high | 1 |
| NRT lozenge not specified | 9 |
| NRT lozenge standard | 2 |
| NRT mouth spray standard | 3 |
| NRT nasal spray standard | 6 |
| NRT not specified | 55 |
| NRT patch (16 hours) high | 3 |
| NRT patch (16 hours) high | 3 |
| NRT patch (16 hours) standard | 14 |
| NRT patch (24 hours) high | 63 |
| NRT patch (24 hours) high | 1 |
| NRT patch (24 hours) not specified | 48 |
| NRT patch (24 hours) standard | 2 |
| NRT sublingual tablet not specified | 4 |
| Placebo | 210 |
| Usual care | 30 |
| Varenicline standard plus bupropion standard | 2 |
| Varenicline high | 6 |
| Varenicline high plus NRT gum standard | 1 |
| Varenicline not specified | 4 |
| Varenicline standard | 64 |
| Varenicline standard plus NRT gum standard | 1 |
| Varenicline standard plus NRT patch (16 hours) standard | 1 |
| Varenicline standard plus NRT patch (24 hours) high | 3 |
| Waitlist | 4 |
Sustained abstinence
FIGURE 37.
Network plot for sustained abstinence at treatment level. Square brackets denote disconnected interventions. Ns, not specified; std, standard.

| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 340.0 | 2194 | 0.41 (0.34 to 0.49) | – |
| Random-class model, consistency | 338.3 | 2187 | 0.40 (0.34 to 0.48) | 0.12 (0.01 to 0.32) |
| Fixed-class model, consistency | 338.5 | 2186 | 0.41 (0.34 to 0.49) | – |
| Fixed-class model, inconsistency | 353.7 | 2209 | 0.32 (0.25 to 0.40) | – |
FIGURE 38.
Forest plot with full interaction NMA model results for sustained abstinence. Ns, not specified; std, standard.

FIGURE 39.
Forest plot with random-class NMA model results for sustained abstinence. Ns, not specified; std, standard.
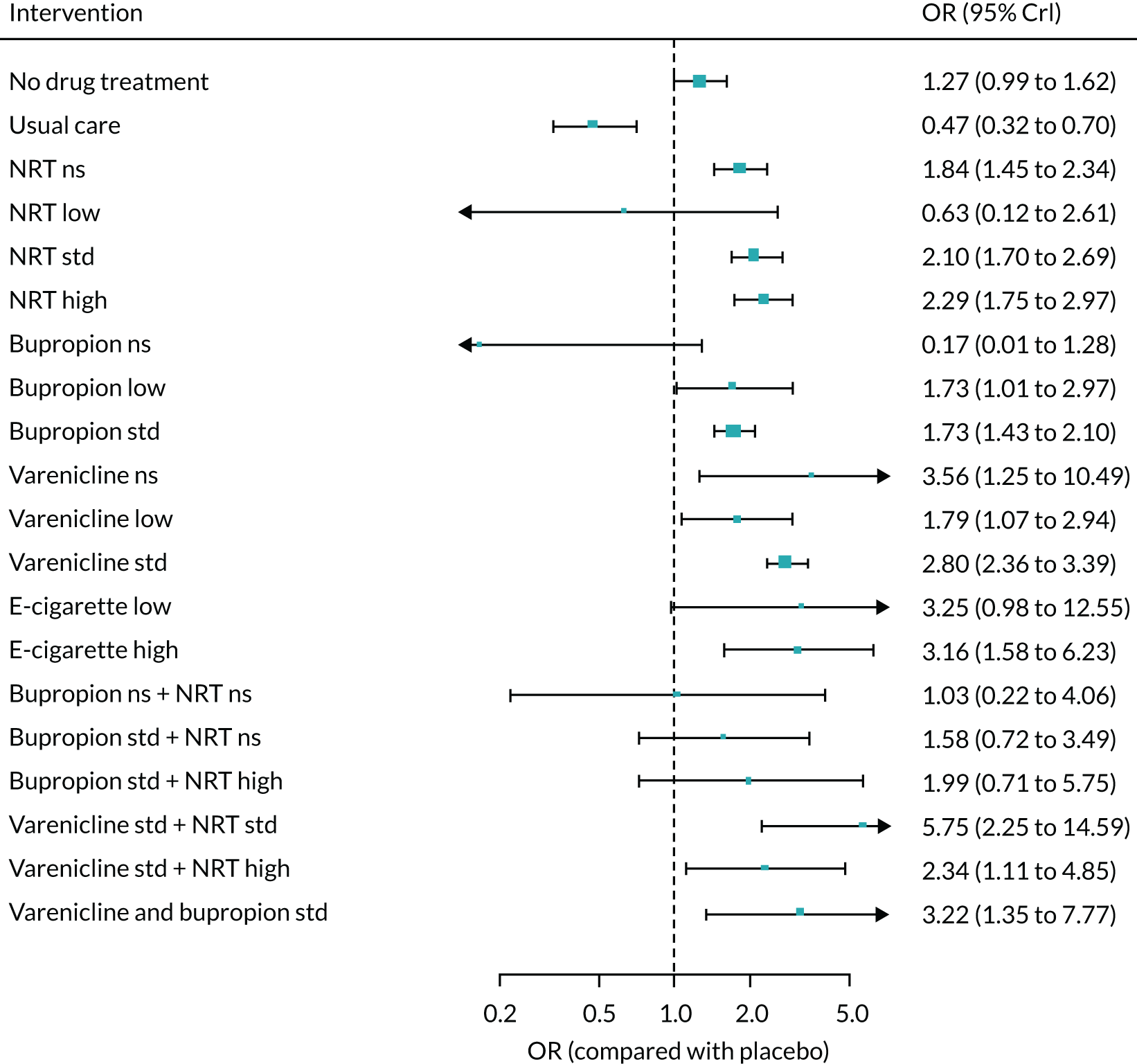
Sensitivity analyses
Analysis excluding studies at high risk of bias
This analysis was based on 111 studies. The estimate of the SDs between class effects was 0.34 (95% CrI 0.27 to 0.43).
FIGURE 40.
Forest plot with fixed-class NMA model results for sustained abstinence without studies at high risk of bias. Ns, not specified; std, standard.

Sensitivity analysis excluding studies of pharmacological treatment plus counselling (if counselling is not given in all study arms)
This analysis was based on 143 studies. The estimate of the SD between class effects was 0.31 (95% CrI 0.31 to 0.39), which is nearly identical to that for the main analysis.
FIGURE 41.
Forest plot with fixed-class NMA model results for sustained abstinence without studies that include pharmacological treatment plus counselling (unless counselling included on all arms). Ns, not specified; std, standard.
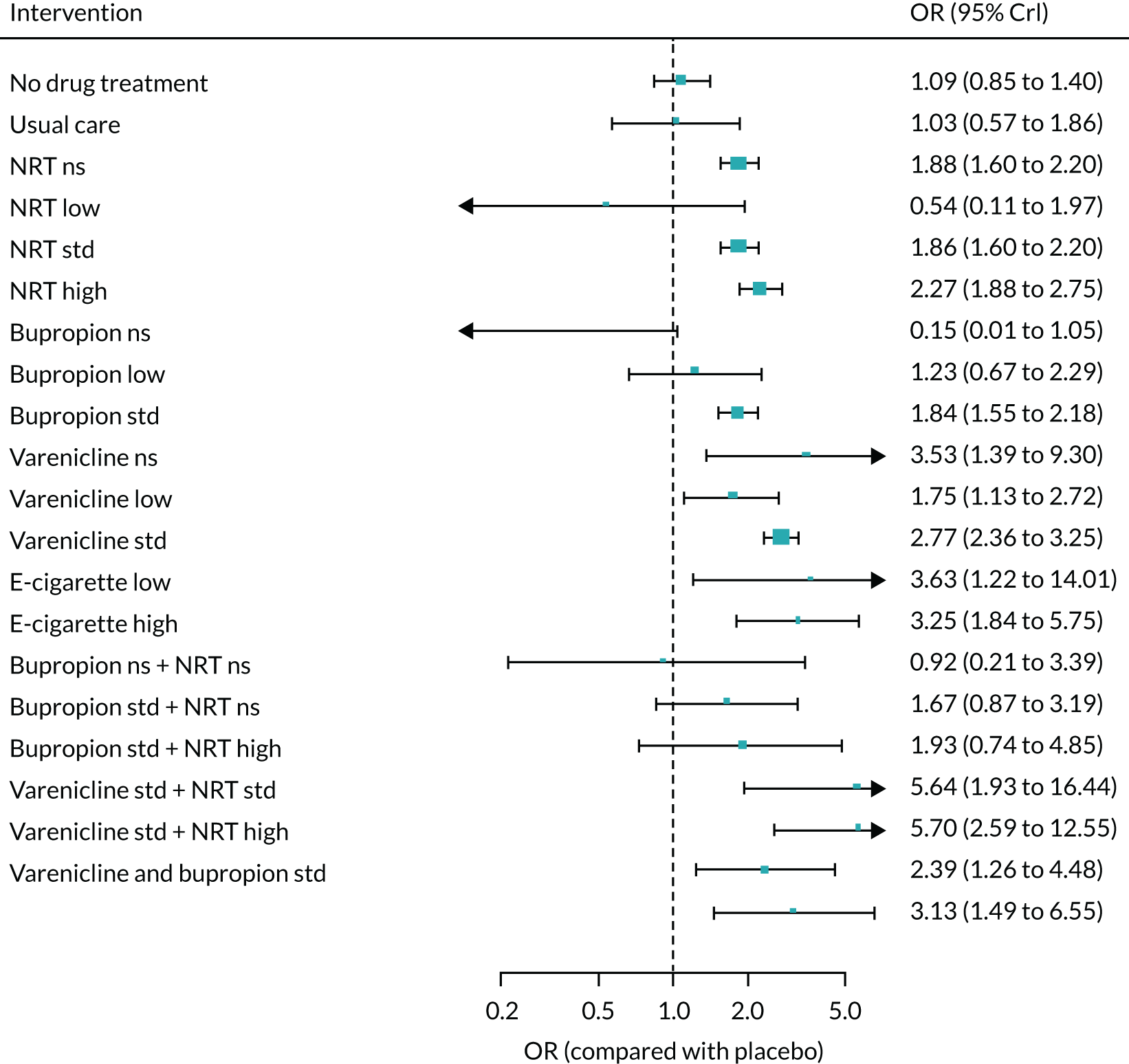
Meta-regressions
Industry sponsorship as covariate
This analysis was based on 145 studies. There was inconclusive evidence of effect modification based on industry sponsorship (B = 0.42, –197.0 to 198.3). The estimate of the SD between class effects was 0.42 (95% CrI 0.35 to 0.50).
FIGURE 42.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for sponsorship. Ns, not specified; std, standard.
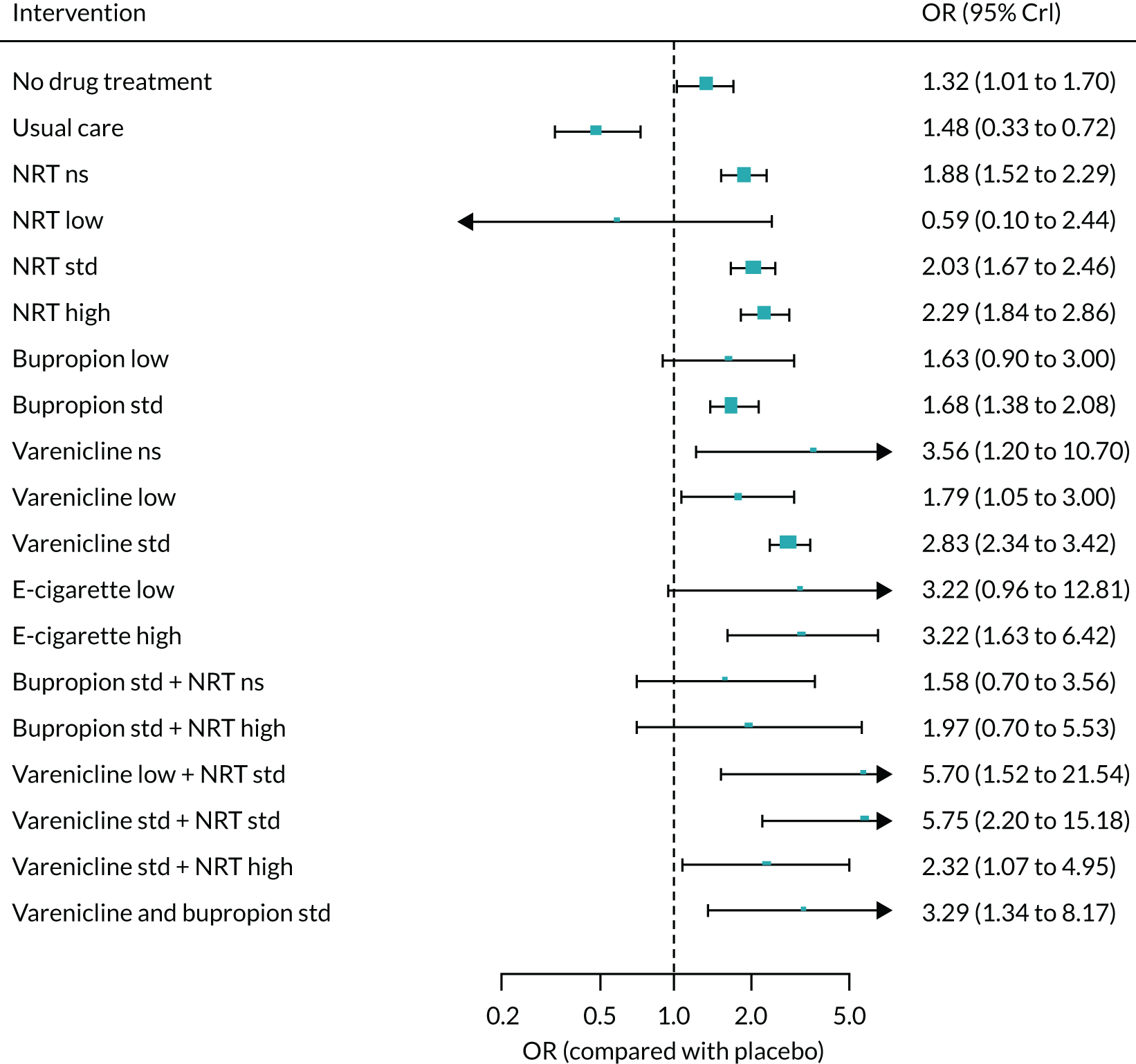
Placebo type as covariate
This analysis was based on 113 studies. There was inconclusive evidence of effect modification based on type of placebo (B = 0.34, –196.8 to 196.2). The estimate of the SD between class effects was 0.34 (95% CrI 0.26 to 0.43).
FIGURE 43.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for type of placebo. Ns, not specified; std, standard.
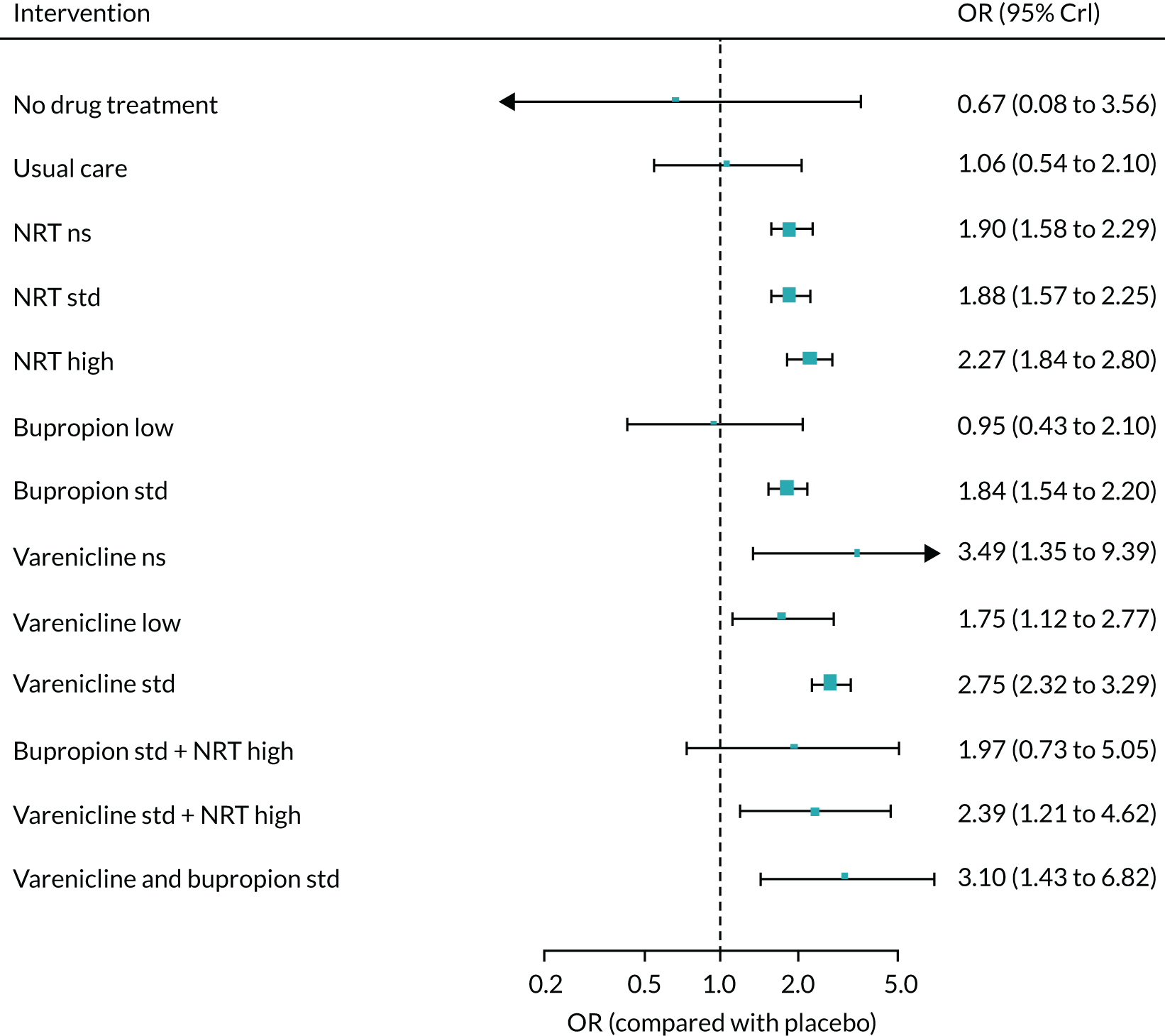
Treatment duration as covariate
This analysis was based on 150 studies. There was inconclusive evidence of effect modification as a function of treatment duration (B = –0.01, –0.05 to 0.04). The estimate of the SD between class effects was 0.32 (95% CrI 0.13 to 0.56).
FIGURE 44.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for treatment duration. Ns, not specified; std, standard.
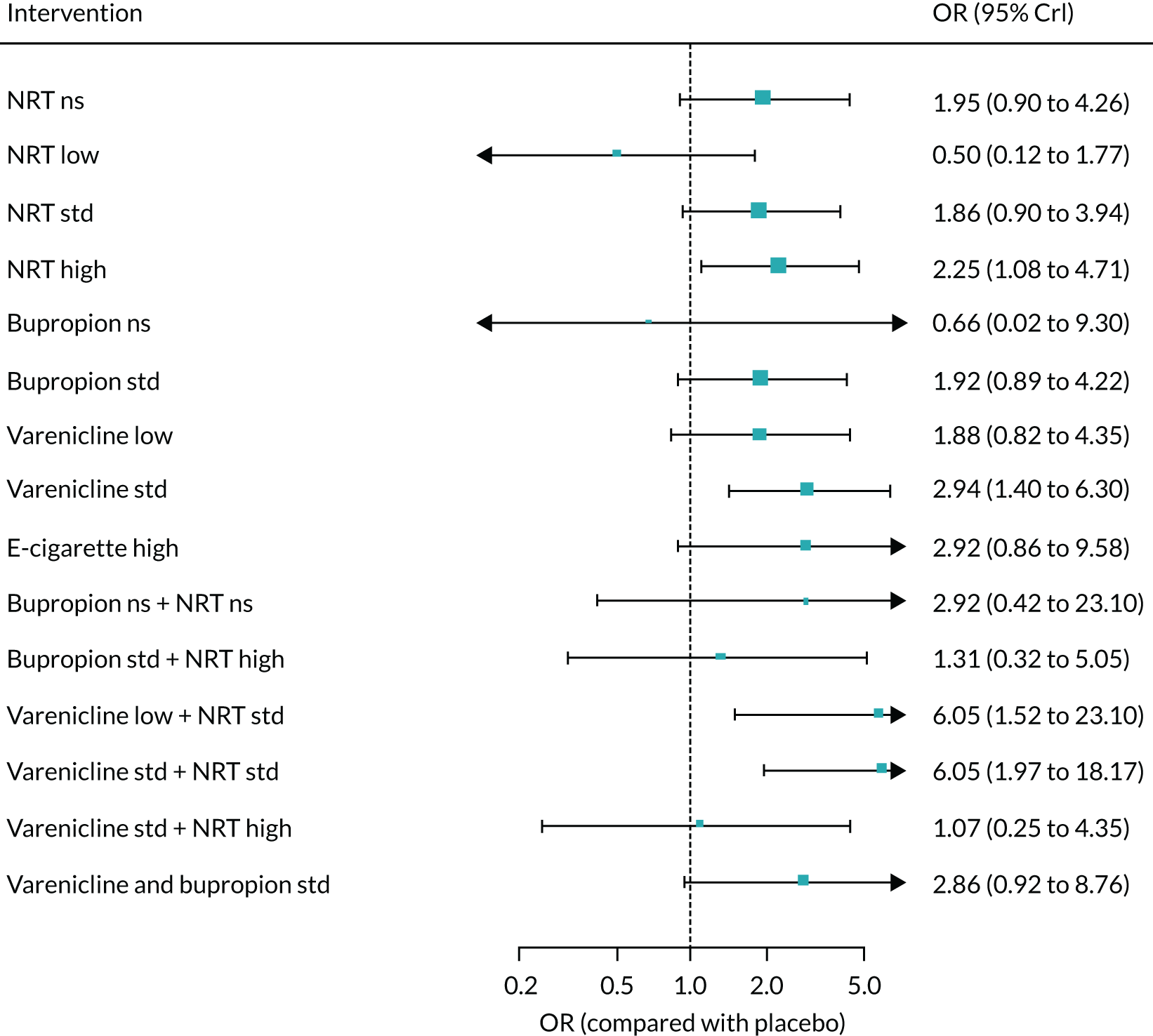
Counselling as covariate
This analysis was based on 161 studies. There was evidence of effect modification as a function of counselling, with interventions including counselling associated with a higher proportion of smokers achieving sustained abstinence (B = 0.86, 0.450 to 1.27). The estimate of the SD between class effects was 0.36 (95% CrI 0.29 to 0.44).
FIGURE 45.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for counselling. Ns, not specified; std, standard.

Counselling as covariate (excluding pharma vs. psychiatric studies)
This analysis was based on 143 studies. There was inconclusive evidence of effect modification as a function of counselling (B = 0.16, –0.05 to 0.37). The estimate of the SD between class effects was 0.31 (95% CrI 0.24 to 0.39).
FIGURE 46.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for counselling (excluding pharma vs. psychiatric studies). Ns, not specified; std, standard.
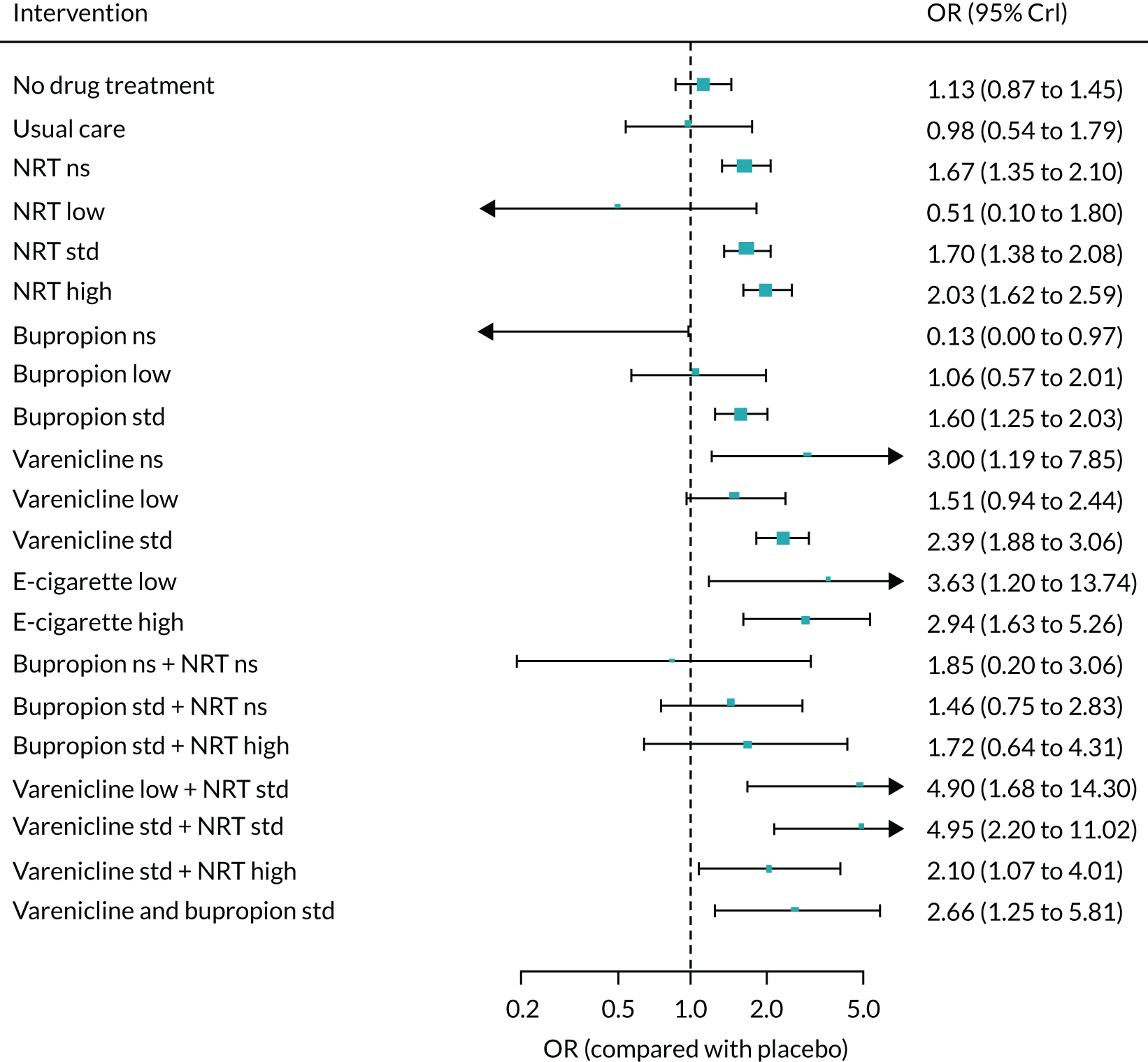
Dependence as covariate
This analysis was based on 94 studies. There was evidence of effect modification as a function of dependence, with higher odds of quitting among smokers with higher dependence scores (B = 0.23, 0.02 to 0.43). The estimate of the SD between class effects was 0.35 (95% CrI 0.27 to 0.44).
FIGURE 47.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for dependence. Ns, not specified; std, standard.
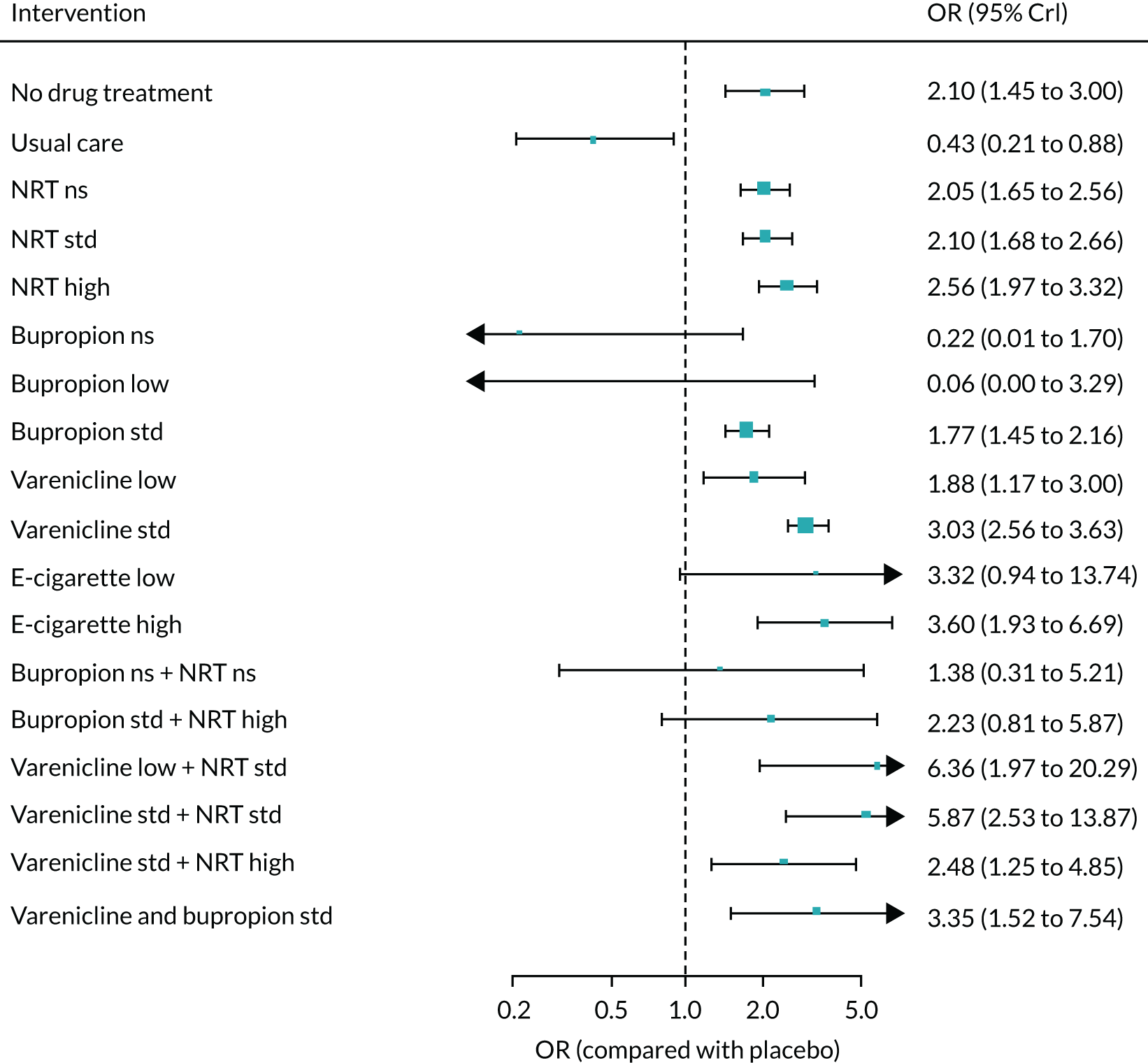
Comorbidities as covariate
This analysis was based on 161 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = 0.18, –195.6 to 196.0). The estimate of the SD between class effects was 0.41 (95% CrI 0.34 to 0.49).
FIGURE 48.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for comorbidities. Ns, not specified; std, standard.

Psychiatric comorbidities as covariate
This analysis was based on 161 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = 0.18, –195.6 to –196.0). The estimate of the SD between class effects was 0.41 (95% CrI 0.34 to 0.49).
FIGURE 49.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for psychiatric comorbidities. Ns, not specified; std, standard.
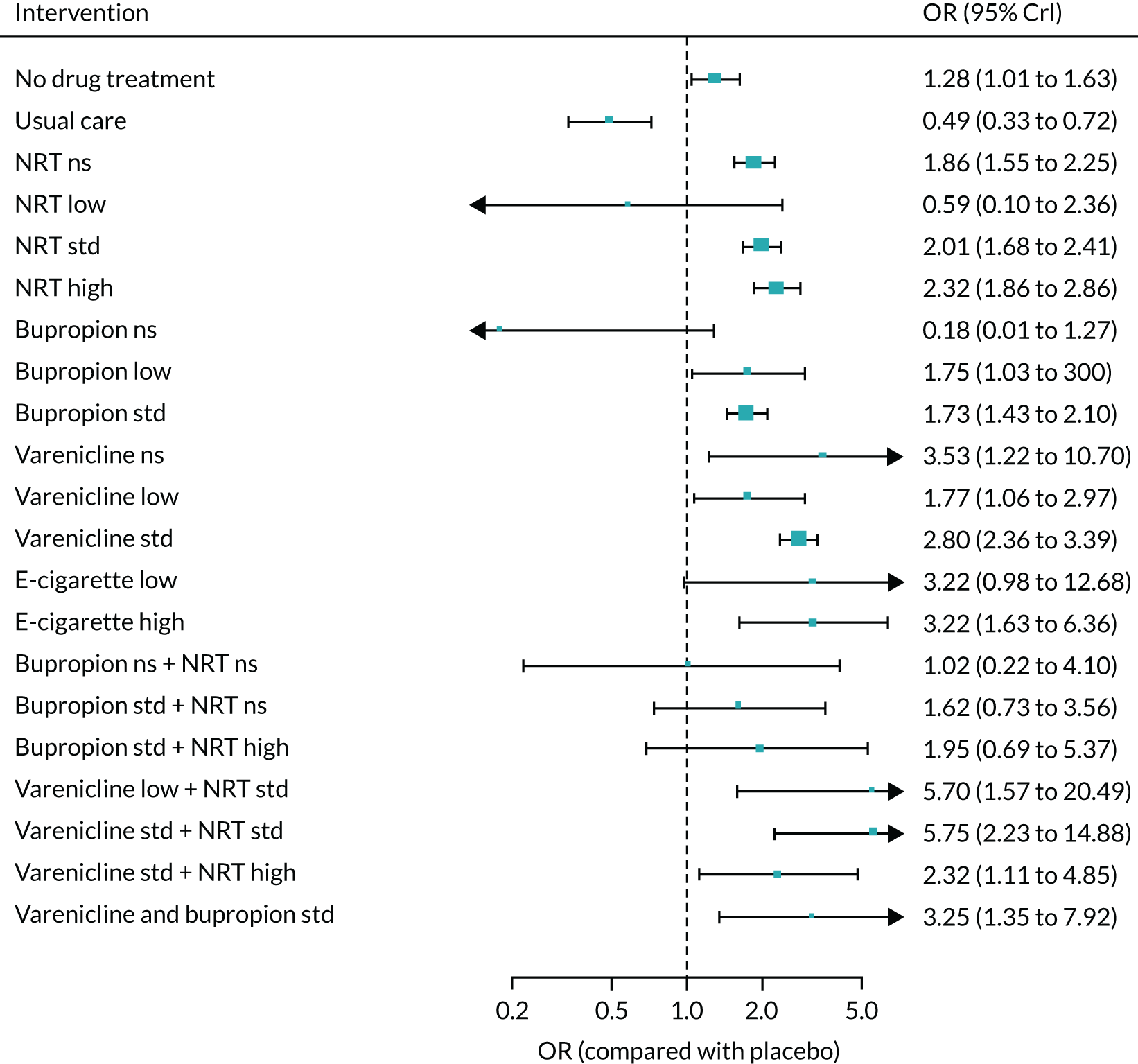
Requirement for patients to be willing to quit as covariate
This analysis was based on 161 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = 0.18, –195.6 to –196.0). The estimate of the SD between class effects was 0.41 (95% CrI 0.34 to 0.49).
FIGURE 50.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for willingness to quit. Ns, not specified; std, standard.

Smokeless tobacco as covariate
This analysis was based on 161 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = 0.18, –195.6 to –196.0). The estimate of the SD between class effects was 0.41 (95% CrI 0.34 to 0.49).
FIGURE 51.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for use of smokeless tobacco. Ns, not specified; std, standard.

Smoking level as covariate
This analysis was based on 108 studies. There was inconclusive evidence of effect modification as a function of smoking level (B = –0.06, –0.21 to 0.33). The estimate of the SD between class effects was 0.29 (95% CrI 0.33 to 0.37).
FIGURE 52.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for smoking level. Ns, not specified; std, standard.

Publication year as covariate
This analysis was based on 161 studies. There was inconclusive evidence of effect modification based on publication year (B = 0.14, –195.6 to 196.9). The estimate of the SD between class effects was 0.40 (95% CrI 0.34 to 0.48).
FIGURE 53.
Forest plot with fixed-class NMA model results for sustained abstinence adjusted for publication year. Ns, not specified; std, standard.

Prolonged abstinence
FIGURE 54.
Network plots for prolonged abstinence at (a) treatment and (b) class level. Squared brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

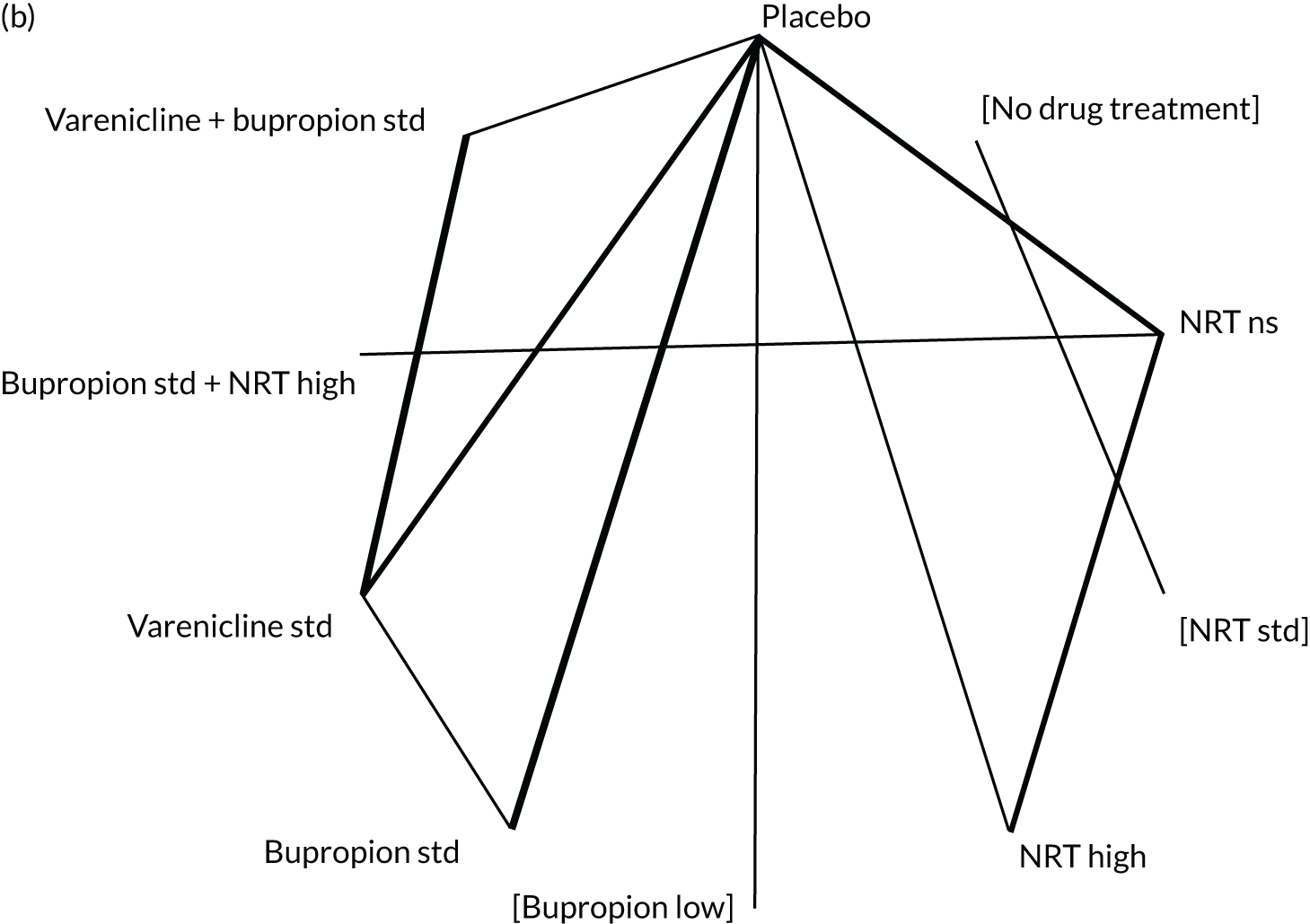
FIGURE 55.
Forest plot with results of the fixed-class NMA model for prolonged abstinence. Ns, not specified; std, standard. This figure has been adapted with permission from Thomas et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
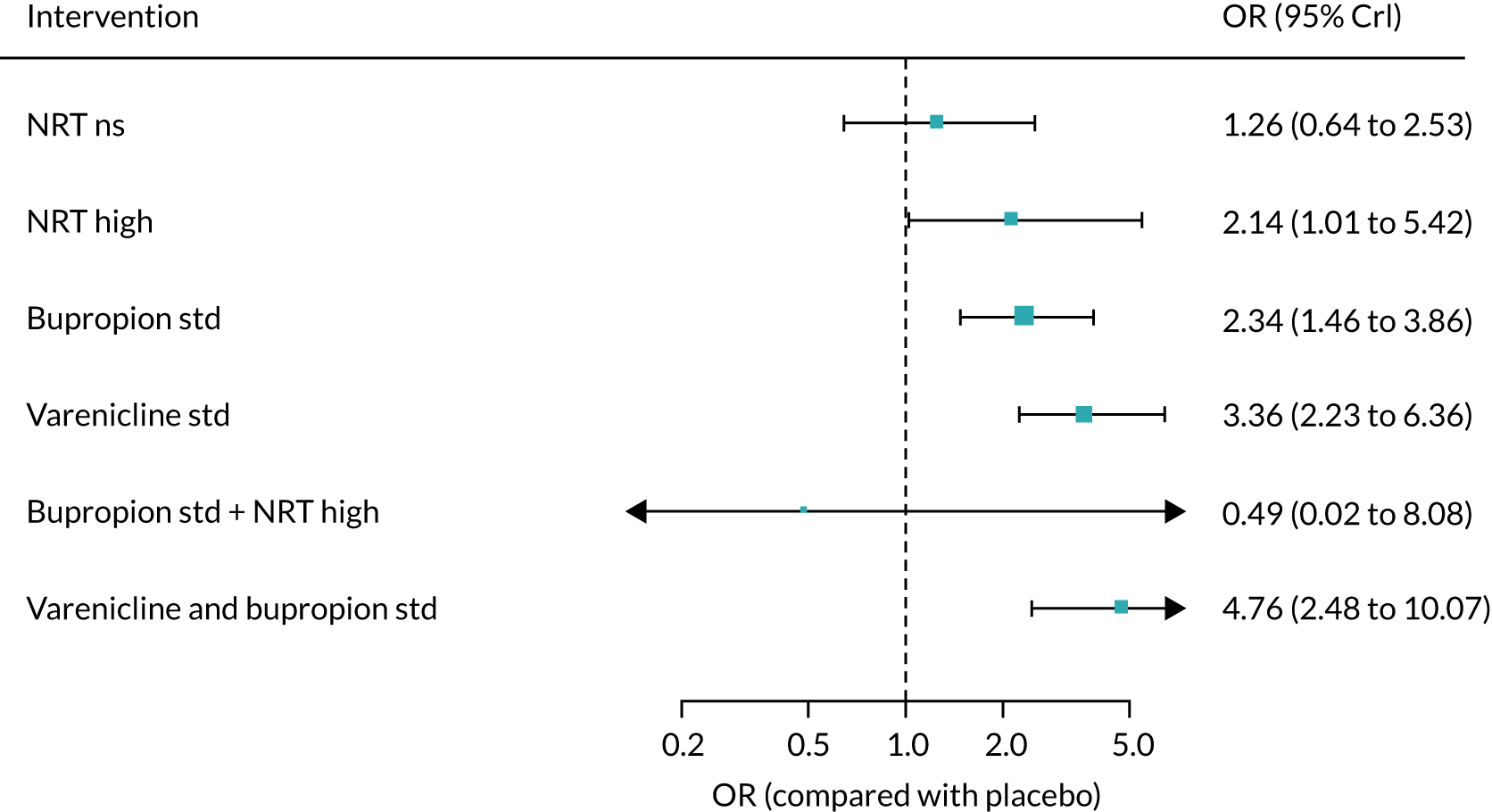
| Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) | |
|---|---|---|---|
| NRT not specified | 1.31 (0.53 to 3.29) | 1.18 (0.38 to 3.67) | 1.26 (0.64 to 2.53) |
| NRT high | 2.18 (0.43 to 11.0) | 2.12 (0.79 to 5.69) | 2.14 (1.01 to 5.42) |
| Bupropion standard | 2.39 (1.31 to 4.39) | 2.26 (1.03 to 4.95) | 2.34 (1.46 to 3.86) |
| Varenicline standard | 3.67 (1.93 to 7.17) | 3.56 (1.42 to 8.94) | 3.63 (2.23 to 6.36) |
| Bupropion standard plus NRT high | – | 0.49 (0.02 to 8.08) | 0.49 (0.02 to 8.08) |
| Varenicline standard plus bupropion standard | 5.05 (1.75 to 24.1) | 4.64 (2.00 to 10.8) | 4.76 (2.48 to 10.1) |
| Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) | |
|---|---|---|---|
| Varenicline standard vs. bupropion standard | – | 1.57 (0.86 to 2.92) | 1.57 (0.86 to 2.92) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 2.03 (0.97 to 4.61) | 2.03 (0.97 to 4.61) |
| Varenicline standard plus bupropion standard vs. varenicline standard | 1.39 (0.49 to 3.94) | 1.28 (0.71 to 2.33) | 1.31 (0.80 to 2.26) |
| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 30.0 | 182.8 | 0.18 (0.01 to 0.69) | – |
| Random-class model, consistency | 29.5 | 182.6 | 0.18 (0.01 to 0.7) | 1.33 (0.07 to 4.58) |
| Fixed-class model, consistency | 32.0 | 182.9 | 0.18 (0.01 to 0.71) | – |
| Fixed-class model, inconsistency | 32.2 | 186.7 | 0.30 (0.01 to 1.05) | – |
FIGURE 56.
Forest plot with full interaction NMA model results for prolonged abstinence. Ns, not specified; std, standard.

FIGURE 57.
Forest plot with random-class NMA model results for prolonged abstinence. Ns, not specified; std, standard.
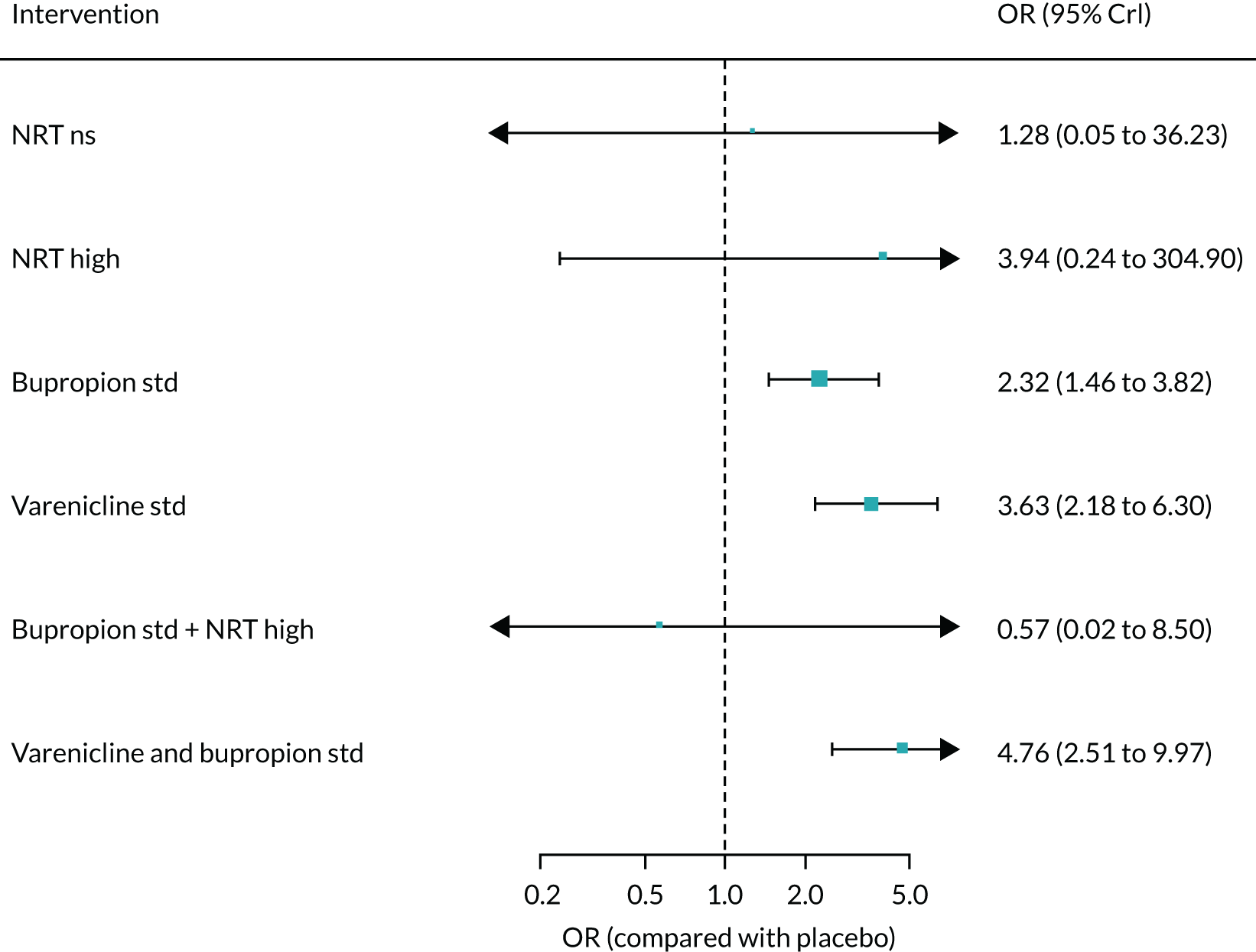
FIGURE 58.
Network plot for any abstinence at treatment level. Ns, not specified; std, standard.

| Intervention | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| No drug treatment | 1.01 (0.58 to 1.75) | 1.61 (1.24 to 2.09) | 1.48 (1.19 to 1.86) |
| Waitlist | – | 1.09 (0.51 to 2.29) | 1.09 (0.51 to 2.29) |
| Usual care | 0.93 (0.47 to 1.82) | 0.58 (0.37 to 0.90) | 0.66 (0.46 to 0.96) |
| NRT not specified | 1.93 (1.60 to 2.36) | 1.57 (1.05 to 2.35) | 1.86 (1.57 to 2.20) |
| NRT low | 1.51 (0.61 to 3.86) | 1.17 (0.26 to 5.20) | 1.40 (0.63 to 3.06) |
| NRT standard | 2.03 (1.72 to 2.44) | – | 2.03 (1.72 to 2.44) |
| NRT high | 2.39 (1.92 to 2.97) | 2.84 (1.77 to 4.54) | 2.46 (2.03 to 2.94) |
| Bupropion not specified | – | 0.19 (0.01 to 1.43) | 0.19 (0.01 to 1.43) |
| Bupropion low | 5.58 (0.15 to 3041) | 2.84 (1.31 to 6.15) | 2.89 (1.34 to 6.23) |
| Bupropion standard | 1.84 (1.57 to 2.16) | – | 1.84 (1.57 to 2.16) |
| Varenicline not specified | 4.06 (1.40 to 11.9) | – | 4.06 (1.40 to 11.9) |
| Varenicline low | 1.52 (0.84 to 2.75) | – | 1.52 (0.84 to 2.75) |
| Varenicline standard | 2.69 (2.27 to 3.19) | – | 2.69 (2.27 to 3.19) |
| E-cigarette low | 2.51 (0.78 to 9.12) | 11.1 (0.81 to 153) | 3.29 (1.13 to 10.8) |
| E-cigarette high | 2.64 (0.88 to 7.85) | 3.79 (0.24 to 59.1) | 2.77 (1.01 to 7.69) |
| Bupropion not specified plus NRT not specified | – | 1.12 (0.24 to 4.44) | 1.12 (0.24 to 4.44) |
| Bupropion low plus NRT high | – | 5.75 (1.79 to 19.1) | 5.75 (1.79 to 19.1) |
| Bupropion standard plus NRT not specified | 1.88 (0.87 to 4.06) | 2.36 (1.07 to 5.18) | 2.10 (1.22 to 3.60) |
| Bupropion standard plus NRT standard | 1.48 (0.61 to 3.63) | 0.74 (0.01 to 51.7) | 1.43 (0.60 to 3.46) |
| Bupropion standard plus NRT high | 2.48 (1.39 to 4.48) | 2.70 (1.23 to 5.91) | 2.56 (1.60 to 4.14) |
| Varenicline standard plus NRT standard | – | 5.53 (2.12 to 14.4) | 5.53 (2.12 to 14.4) |
| Varenicline standard plus NRT high | – | 2.36 (1.12 to 4.90) | 2.36 (1.12 to 4.90) |
| Varenicline standard plus bupropion standard | 3.32 (1.28 to 8.94) | 3.78 (1.52 to 9.38) | 3.56 (1.84 to 6.89) |
| E-cigarette high plus NRT not specified | – | 4.76 (0.62 to 47.8) | 4.76 (0.62 to 47.8) |
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| Bupropion standard vs. NRT standard | – | 0.90 (0.72 to 1.13) | 0.90 (0.72 to 1.13) |
| Varenicline standard vs. NRT standard | – | 1.32 (1.05 to 1.65) | 1.32 (1.05 to 1.65) |
| E-cigarette low vs. NRT standard | – | 1.60 (0.55 to 5.38) | 1.60 (0.55 to 5.38) |
| E-cigarette high vs. NRT standard | – | 1.35 (0.49 to 3.76) | 1.35 (0.49 to 3.76) |
| Bupropion standard plus NRT standard vs. NRT standard | – | 0.71 (0.29 to 1.72) | 0.71 (0.29 to 1.72) |
| Varenicline standard plus NRT standard vs. NRT standard | – | 2.70 (1.02 to 7.13) | 2.70 (1.02 to 7.13) |
| Varenicline standard plus bupropion standard vs. NRT standard | – | 1.75 (0.88 to 3.45) | 1.75 (0.88 to 3.45) |
| Varenicline standard vs. bupropion standard | – | 1.46 (1.18 to 1.81) | 1.46 (1.18 to 1.81) |
| E-cigarette low vs. bupropion standard | – | 1.78 (0.61 to 5.95) | 1.78 (0.61 to 5.95) |
| E-cigarette high vs. bupropion standard | – | 1.50 (0.54 to 4.19) | 1.50 (0.54 to 4.19) |
| Bupropion standard plus NRT standard vs. bupropion standard | – | 0.78 (0.33 to 1.89) | 0.78 (0.33 to 1.89) |
| Varenicline standard plus NRT standard vs. bupropion standard | – | 2.99 (1.13 to 7.88) | 2.99 (1.13 to 7.88) |
| Varenicline standard plus bupropion standard vs. bupropion standard | – | 1.93 (0.98 to 3.79) | 1.93 (0.98 to 3.79) |
| E-cigarette low vs. varenicline standard | – | 1.22 (0.42 to 4.07) | 1.22 (0.42 to 4.07) |
| E-cigarette high vs. varenicline standard | – | 1.03 (0.37 to 2.86) | 1.03 (0.37 to 2.86) |
| Bupropion standard plus NRT standard vs. varenicline standard | – | 0.53 (0.22 to 1.30) | 0.53 (0.22 to 1.30) |
| Varenicline standard plus NRT standard vs. varenicline standard | 2.05 (0.80 to 5.25) | – | 2.05 (0.80 to 5.25) |
| Varenicline standard plus bupropion standard vs. varenicline standard | 1.50 (0.70 to 3.27) | 0.95 (0.28 to 3.21) | 1.32 (0.69 to 2.52) |
| E-cigarette high vs. e-cigarette low | – | 0.84 (0.18 to 3.69) | 0.84 (0.18 to 3.69) |
| Bupropion standard plus NRT standard vs. e-cigarette low | – | 0.44 (0.10 to 1.74) | 0.44 (0.10 to 1.74) |
| Varenicline standard plus NRT standard vs. e-cigarette low | – | 1.67 (0.37 to 6.99) | 1.67 (0.37 to 6.99) |
| Varenicline standard plus bupropion standard vs. e-cigarette low | – | 1.08 (0.28 to 3.82) | 1.08 (0.28 to 3.82) |
| Bupropion standard plus NRT standard vs. e-cigarette high | – | 0.52 (0.14 to 1.98) | 0.52 (0.14 to 1.98) |
| Varenicline standard plus NRT standard vs. e-cigarette high | – | 2.00 (0.49 to 8.02) | 2.00 (0.49 to 8.02) |
| Varenicline standard plus bupropion standard vs. e-cigarette high | – | 1.29 (0.38 to 4.27) | 1.29 (0.38 to 4.27) |
| Varenicline standard plus NRT standard vs. bupropion standard plus NRT standard | – | 3.83 (1.05 to 14.0) | 3.83 (1.05 to 14.0) |
| Varenicline standard plus bupropion standard vs. bupropion standard plus NRT standard | – | 2.48 (0.83 to 7.34) | 2.48 (0.83 to 7.34) |
| Varenicline standard plus bupropion standard vs. varenicline standard plus NRT standard | – | 0.65 (0.21 to 2.04) | 0.65 (0.21 to 2.04) |
| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 428.6 | 2720 | 0.42 (0.35 to 0.50) | – |
| Random-class model, consistency | 426.7 | 2711 | 0.14 (0.35 to 0.48) | 0.14 (0.01 to 0.31) |
| Fixed-class model, consistency | 426.1 | 2710 | 0.42 (0.36 to 0.50) | – |
| Fixed-class model, inconsistency | 443.9 | 2723 | 0.34 (0.27 to 0.41) | – |
FIGURE 59.
Forest plot with full interaction NMA model results for any abstinence. Ns, not specified; std, standard.
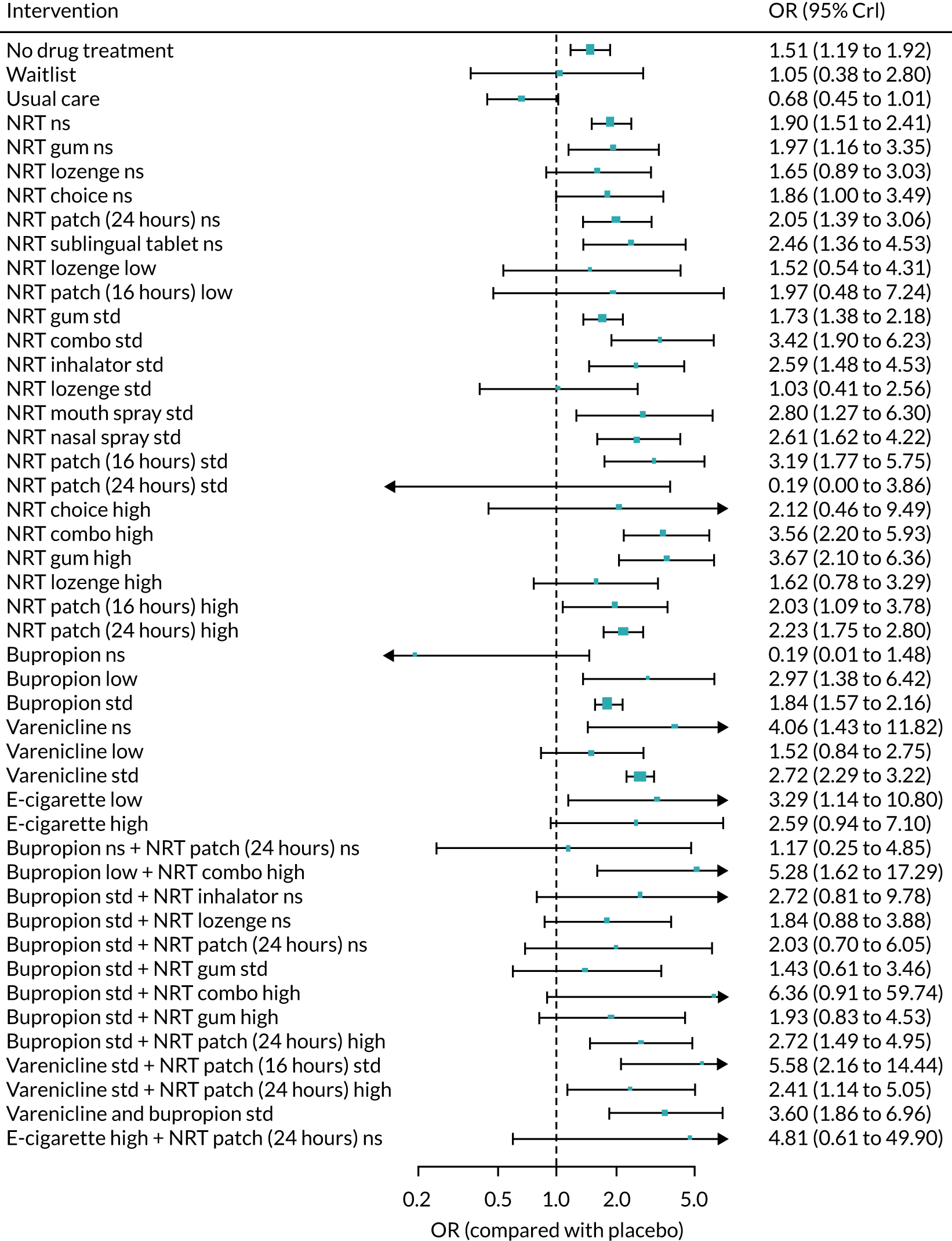
FIGURE 60.
Forest plot with random-class NMA model results for any abstinence. Ns, not specified; std, standard.
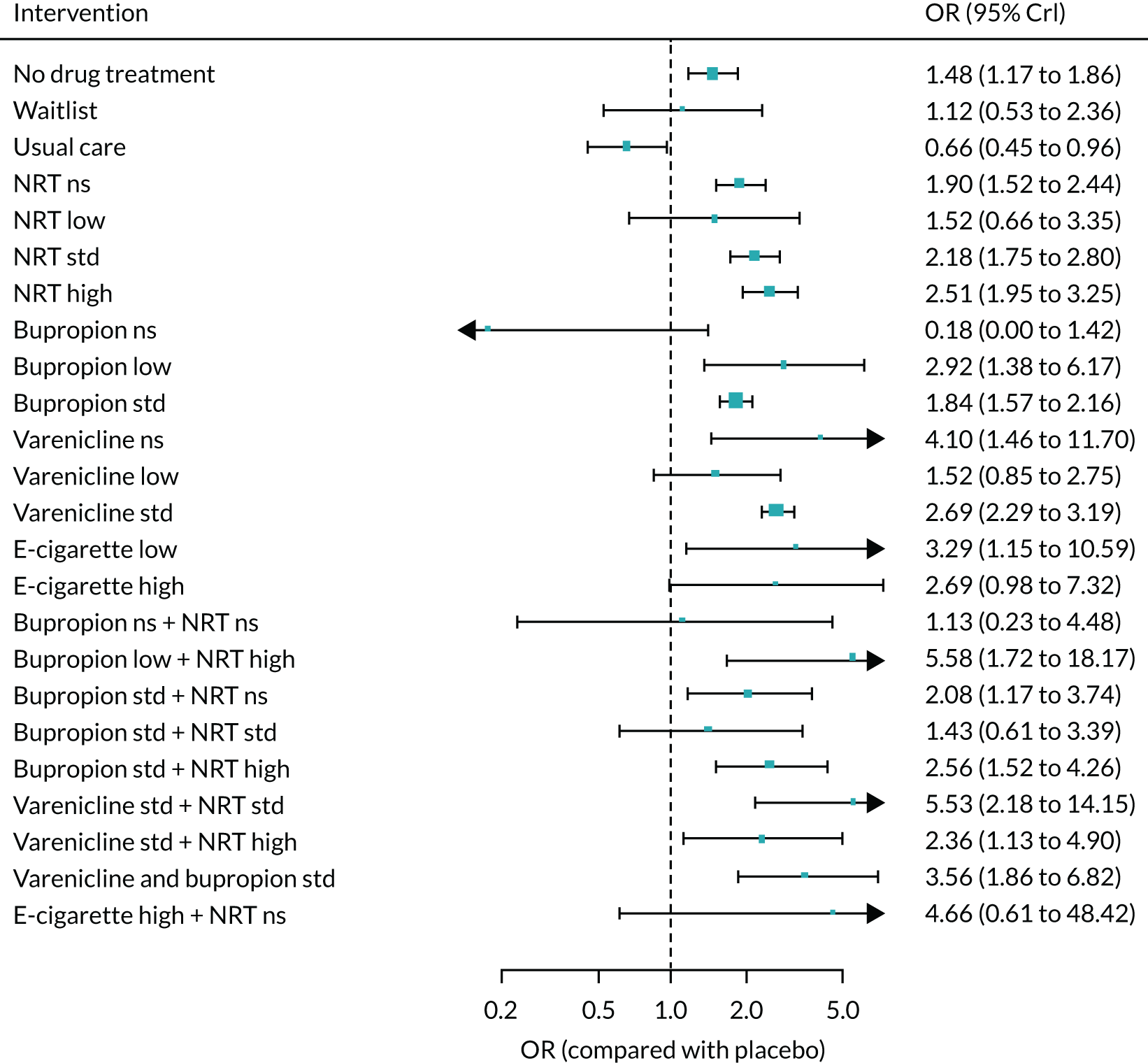
Seven-day point prevalence abstinence
FIGURE 61.
Network plot for 7-day PPA at treatment level. Ns, not specified; std, standard.

| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| No drug treatment | 1.02 (0.64 to 1.62) | 1.18 (0.87 to 1.61) | 1.13 (0.87 to 1.45) |
| Waitlist | – | 0.98 (0.53 to 1.79) | 0.98 (0.53 to 1.79) |
| Usual care | 0.84 (0.15 to 3.32) | 1.10 (0.67 to 1.80) | 1.07 (0.66 to 1.72) |
| NRT not specified | 1.60 (1.22 to 2.12) | 1.87 (1.48 to 2.35) | 1.75 (1.48 to 2.08) |
| NRT low | – | 1.48 (0.70 to 3.13) | 1.48 (0.70 to 3.13) |
| NRT standard | 1.58 (1.21 to 2.12) | – | 1.58 (1.21 to 2.12) |
| NRT high | 2.20 (1.73 to 2.80) | 1.75 (1.34 to 2.29) | 1.99 (1.67 to 2.39) |
| Bupropion low | 1.21 (0.78 to 1.90) | – | 1.21 (0.78 to 1.90) |
| Bupropion standard | 1.67 (1.48 to 1.88) | – | 1.67 (1.48 to 1.88) |
| Varenicline not specified | 2.56 (1.21 to 5.42) | – | 2.56 (1.21 to 5.42) |
| Varenicline low | 1.70 (0.90 to 3.19) | 2.18 (0.40 to 11.8) | 1.75 (0.97 to 3.13) |
| Varenicline standard | 2.14 (1.86 to 2.46) | – | 2.14 (1.86 to 2.46) |
| Bupropion low plus NRT high | – | 4.76 (1.82 to 12.7) | 4.76 (1.82 to 12.7) |
| Bupropion standard plus NRT not specified | 1.77 (1.01 to 3.13) | 1.87 (1.29 to 2.73) | 1.84 (1.35 to 2.53) |
| Bupropion standard plus NRT high | 2.46 (1.57 to 3.90) | 1.91 (1.24 to 2.96) | 2.16 (1.57 to 2.97) |
| Varenicline standard plus NRT standard | – | 4.01 (2.16 to 7.54) | 4.01 (2.16 to 7.54) |
| Varenicline standard plus NRT high | – | 2.14 (0.91 to 4.85) | 2.14 (0.91 to 4.85) |
| Varenicline plus bupropion standard | 1.75 (0.90 to 3.53) | 2.79 (1.58 to 4.90) | 2.29 (1.48 to 3.56) |
| E-cigarette high plus NRT not specified | – | 4.10 (0.63 to 37.7) | 4.10 (0.63 to 37.7) |
| Comparison | Direct evidence, OR (95% CrI) | Indirect evidence, OR (95% CrI) | NMA, OR (95% CrI) |
|---|---|---|---|
| Bupropion standard vs. NRT standard | – | 1.05 (0.78 to 1.41) | 1.05 (0.78 to 1.41) |
| Varenicline standard vs. NRT standard | – | 1.35 (0.99 to 1.82) | 1.35 (0.99 to 1.82) |
| Varenicline standard plus NRT standard vs. NRT standard | – | 2.54 (1.28 to 4.98) | 2.54 (1.28 to 4.98) |
| Varenicline plus bupropion standard vs. NRT standard | – | 1.44 (0.86 to 2.42) | 1.44 (0.86 to 2.42) |
| Varenicline standard vs. bupropion standard | – | 1.28 (1.08 to 1.53) | 1.28 (1.08 to 1.53) |
| Varenicline standard plus NRT standard vs. bupropion standard | – | 2.42 (1.28 to 4.57) | 2.42 (1.28 to 4.57) |
| Varenicline plus bupropion standard vs. bupropion standard | – | 1.38 (0.88 to 2.17) | 1.38 (0.88 to 2.17) |
| Varenicline standard plus NRT standard vs. varenicline standard | 1.88 (1.02 to 3.46) | – | 1.88 (1.02 to 3.46) |
| Varenicline plus bupropion standard vs. varenicline standard | 1.40 (0.79 to 2.49) | 0.81 (0.44 to 1.46) | 1.07 (0.70 to 1.63) |
| Varenicline plus bupropion standard vs. varenicline standard plus NRT standard | – | 0.57 (0.27 to 1.20) | 0.57 (0.27 to 1.20) |
| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 272.3 | 1672 | 0.23 (0.14 to 0.33) | – |
| Random-class model, consistency | 275.0 | 1663 | 0.22 (0.13 to 0.31) | 0.12 (0.01 to 0.34) |
| Fixed-class model, consistency | 274.8 | 1662 | 0.23 (0.15 to 0.32) | – |
| Fixed-class model, inconsistency | 276.0 | 1671 | 0.21 (0.12 to 0.31) | – |
FIGURE 62.
Forest plot with full interaction NMA model results for 7-day PPA. Ns, not specified; std, standard.
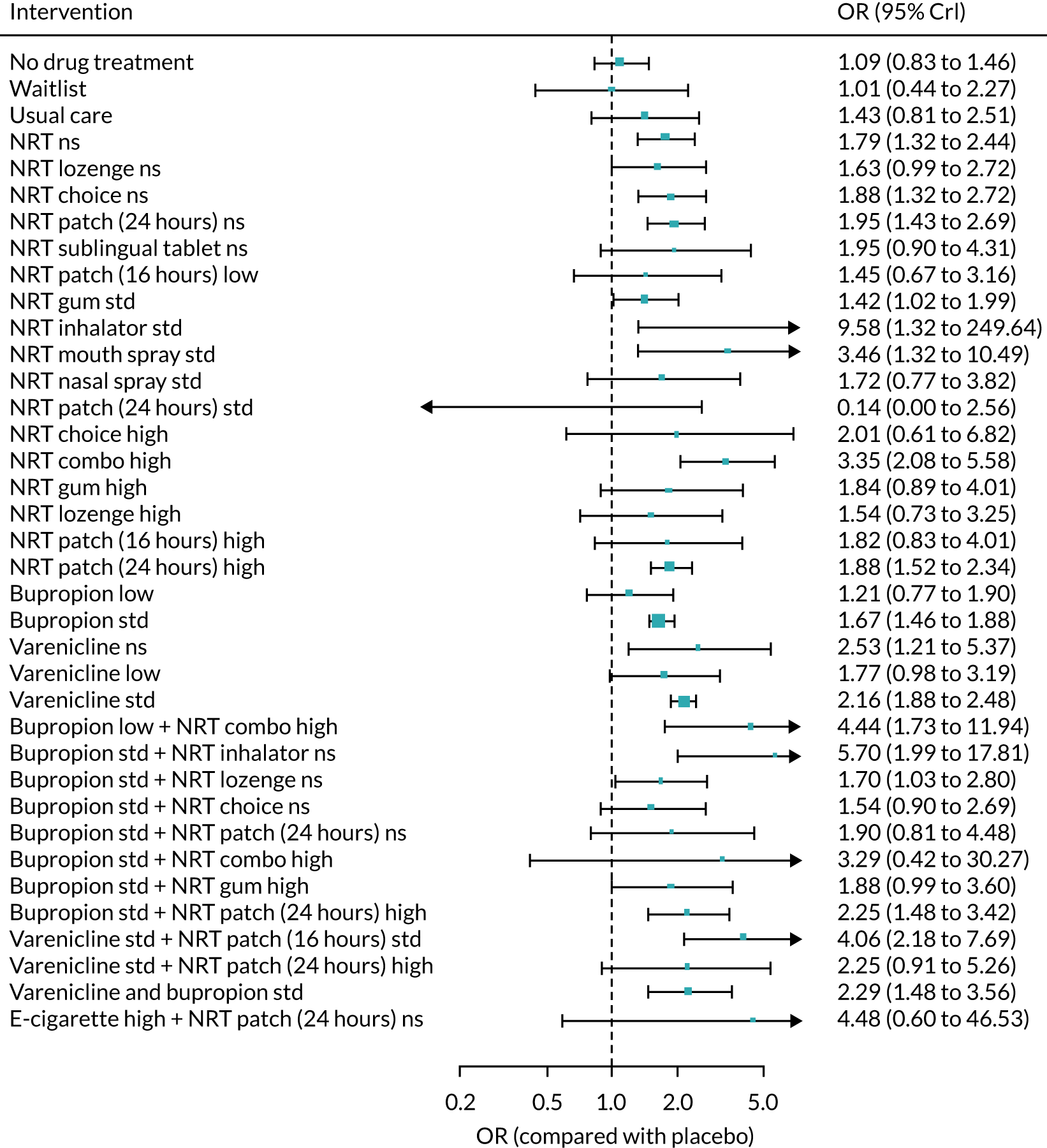
FIGURE 63.
Forest plot with random-class NMA model results for 7-day PPA. Ns, not specified; std, standard.

Appendix 6 Threshold analyses
List of treatment codes
Treatment classes in bold are the subset used for ranking and threshold analysis.
-
Placebo.
-
No drug treatment.
-
Waitlist.
-
Usual care.
-
NRT not specified.
-
NRT low.
-
NRT standard.
-
NRT high.
-
Bupropion not specified.
-
Bupropion low.
-
Bupropion standard.
-
Varenicline not specified.
-
Varenicline low.
-
Varenicline standard.
-
Varenicline high.
-
E-cigarette not specified.
-
E-cigarette low.
-
E-cigarette high.
-
Bupropion not specified plus NRT not specified.
-
Bupropion low plus NRT high.
-
Bupropion standard plus NRT not specified.
-
Bupropion standard plus NRT standard.
-
Bupropion standard plus NRT high.
-
Varenicline low plus NRT standard.
-
Null.
-
Varenicline standard plus NRT standard.
-
Varenicline standard plus NRT high.
-
Varenicline plus bupropion standard.
-
E-cigarette high plus NRT not specified.
Appendix 7 Primary and secondary safety outcome analyses
| Treatment | Frequency |
|---|---|
| Bupropion low | 9 |
| Bupropion low plus NRT combination high | 1 |
| Bupropion not specified plus NRT choice not specified | 1 |
| Bupropion standard | 79 |
| Bupropion standard plus NRT choice not specified | 1 |
| Bupropion standard plus NRT combination high | 1 |
| Bupropion standard plus NRT gum not specified | 4 |
| Bupropion standard plus NRT gum standard | 1 |
| Bupropion standard plus NRT inhalator not specified | 1 |
| Bupropion standard plus NRT lozenge not specified | 2 |
| Bupropion standard plus NRT patch (24 hours) high | 8 |
| E-cigarette high | 3 |
| E-cigarette high plus NRT patch (24 hours) not specified | 1 |
| E-cigarette low | 5 |
| E-cigarette not specified | 1 |
| No drug treatment | 40 |
| NRT choice not specified | 20 |
| NRT choice standard | 1 |
| NRT combination high | 7 |
| NRT combination not specified | 9 |
| NRT combination standard | 6 |
| NRT gum high | 10 |
| NRT gum not specified | 8 |
| NRT gum standard | 28 |
| NRT inhalator standard | 7 |
| NRT lozenge high | 8 |
| NRT lozenge low | 2 |
| NRT lozenge not specified | 9 |
| NRT lozenge standard | 4 |
| NRT mouth spray standard | 2 |
| NRT nasal spray not specified | 1 |
| NRT nasal spray standard | 6 |
| NRT not specified | 52 |
| NRT patch (16 hours) high | 1 |
| NRT patch (16 hours) low | 5 |
| NRT patch (16 hours) not specified | 1 |
| NRT patch (16 hours) standard | 15 |
| NRT patch (24 hours) high | 61 |
| NRT patch (24 hours) low | 1 |
| NRT patch (24 hours) not specified | 30 |
| NRT patch (24 hours) standard | 1 |
| NRT sublingual tablet not specified | 4 |
| Placebo | 244 |
| Usual care | 23 |
| Varenicline standard plus bupropion standard | 4 |
| Varenicline high | 1 |
| Varenicline low | 13 |
| Varenicline low plus NRT gum standard | 1 |
| Varenicline not specified | 2 |
| Varenicline standard | 91 |
| Varenicline standard plus NRT gum standard | 1 |
| Varenicline standard plus NRT patch (16 hours) standard | 2 |
| Varenicline standard plus NRT patch (24 hours) high | 3 |
| Waitlist | 2 |
| Treatment | Frequency |
|---|---|
| Bupropion low | 2 |
| Bupropion not specified plus NRT choice not specified | 2 |
| Bupropion not specified | 12 |
| Bupropion standard plus NRT choice not specified | 2 |
| Bupropion standard plus NRT patch (24 hours) not specified | 1 |
| Bupropion standard plus NRT patch (24 hours) standard | 1 |
| Bupropion standard | 4 |
| Dual use standard | 1 |
| E-cigarette standard | 1 |
| No treatment | 17 |
| NRT choice not specified | 12 |
| NRT combination high | 2 |
| NRT combination not specified | 4 |
| NRT gum high | 2 |
| NRT gum not specified | 2 |
| NRT gum standard | 2 |
| NRT inhalator low | 1 |
| NRT inhalator not specified | 1 |
| NRT lozenge not specified | 1 |
| NRT not specified | 8 |
| NRT patch (16 hours) standard | 2 |
| NRT patch (24 hours) not specified | 15 |
| Usual care | 1 |
| Varenicline low | 1 |
| Varenicline not specified plus NRT choice not specified | 1 |
| Varenicline not specified | 20 |
| Varenicline standard plus bupropion standard | 1 |
| Varenicline standard plus NRT choice not specified | 1 |
| Varenicline standard plus NRT combination not specified | 1 |
| Varenicline standard | 8 |
Serious adverse events
FIGURE 64.
Network plot for SAEs at treatment level. Square brackets denote interventions that were excluded from the NMA. Ns, not specified; std, standard.

Incorporating non-randomised evidence
FIGURE 65.
Network plot for SAEs incorporating non-randomised evidence at treatment level. Ns, not specified; std, standard.
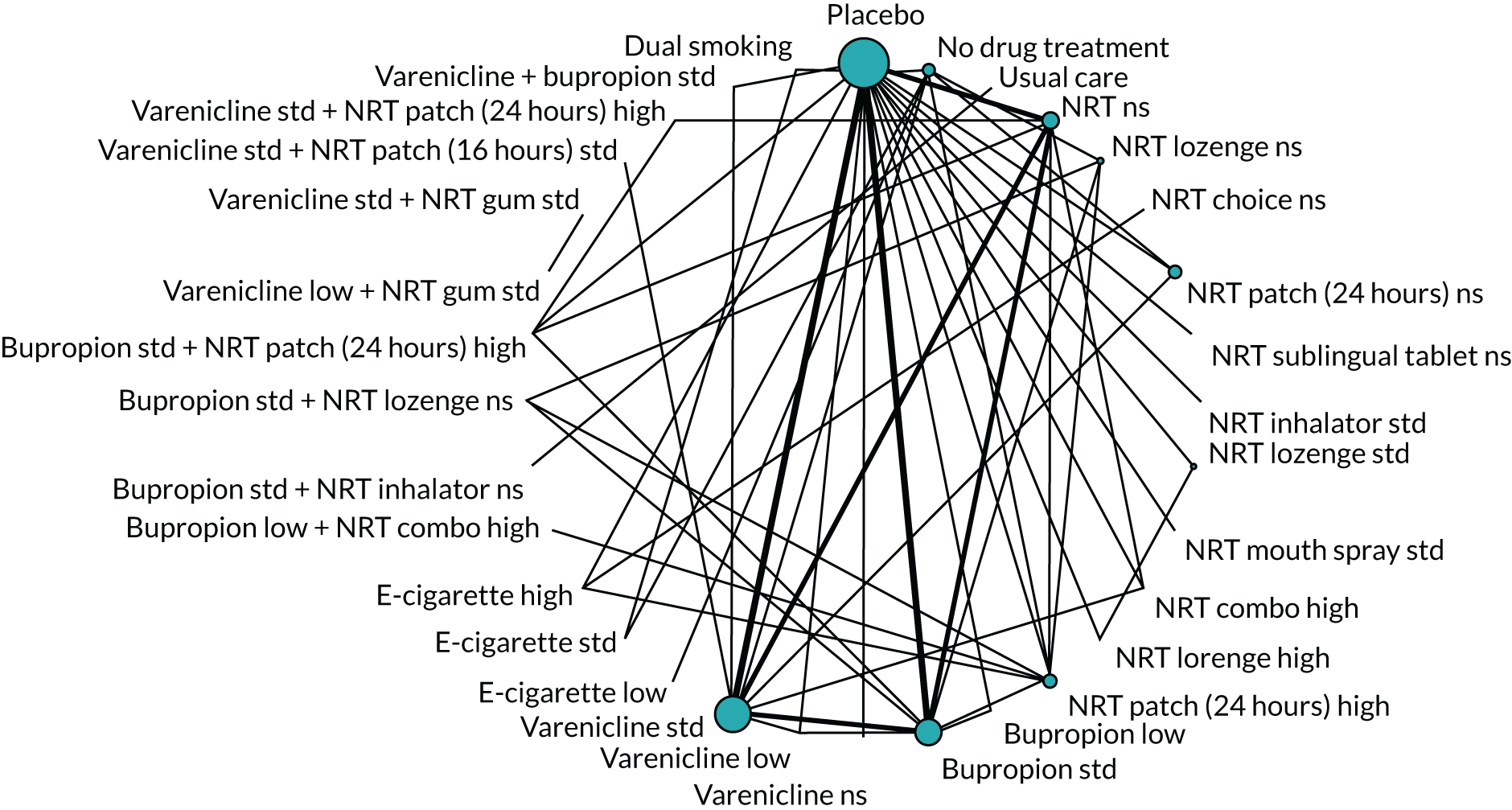
| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 207.1 | 1021 | 0.09 (0.01 to 0.29) | – |
| Random-class model, consistency | 205.6 | 1014 | 0.07 (0 to 0.28) | 0.2 (0.01 to 0.68) |
| Fixed-class model, consistency | 205.8 | 1012 | 0.09 (0 to 0.28) | – |
| Fixed-class model, inconsistency | 211.1 | 1027 | 0.09 (0.01 to 0.29) | – |
FIGURE 66.
Forest plot with full interaction NMA model results for SAEs. Ns, not specified; std, standard.

FIGURE 67.
Forest plot with random-class NMA model results for SAEs. Ns, not specified; std, standard.

Sensitivity analyses
Analysis excluding studies at high risk of bias
This analysis was based on 76 studies. The estimate of the SD between class effects was 0.11 (0.01, 0.33).
FIGURE 68.
Forest plot with fixed-class NMA model results for SAEs without studies at high risk of bias. Ns, not specified; std, standard.
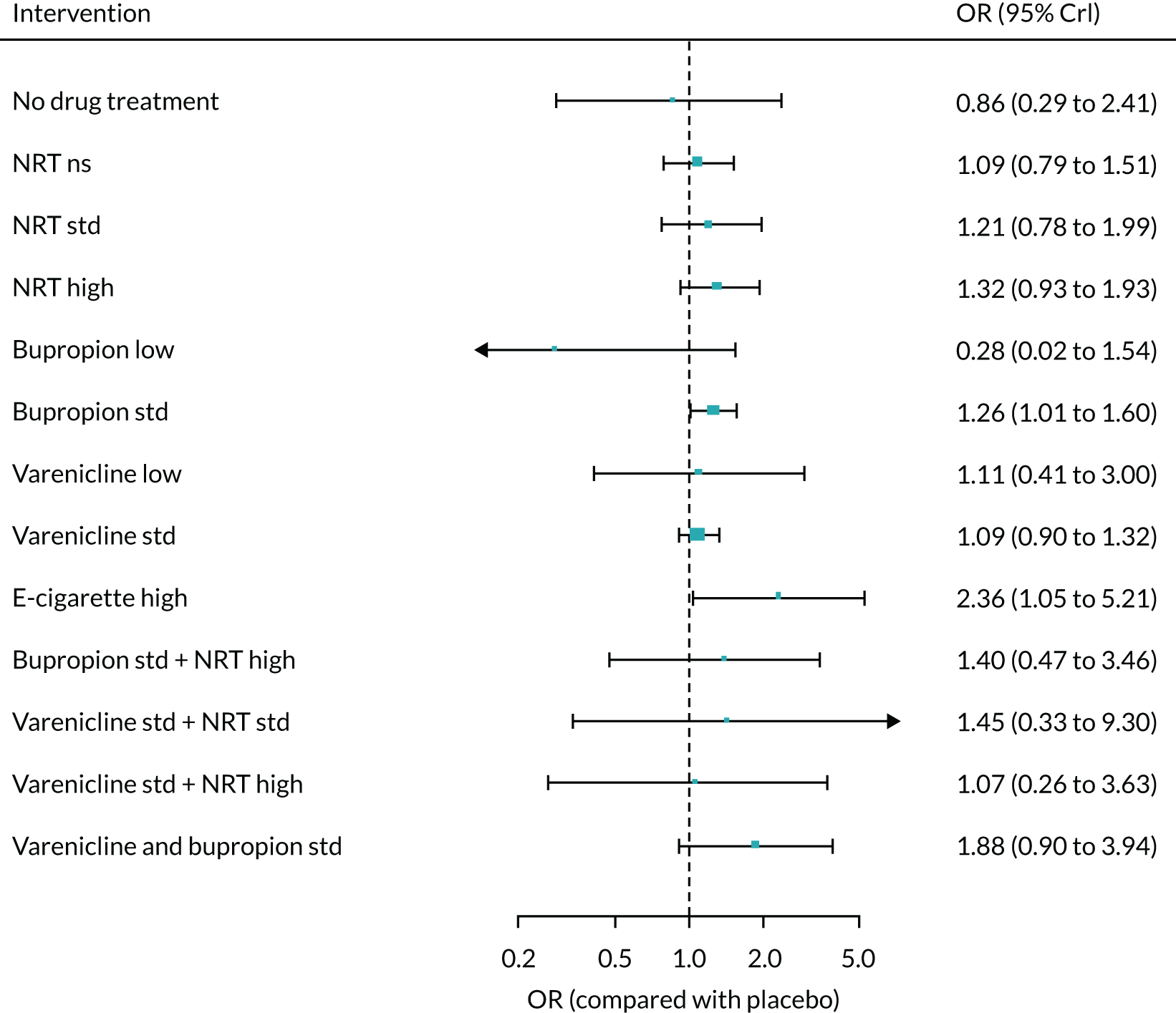
Sensitivity analysis excluding studies of pharmacological treatment plus counselling (if counselling is not given in all study arms)
This analysis was based on 97 studies. The estimate of the SD between class effects was 0.09 (0.01, 0.27), which is nearly identical to that for the main analysis.
FIGURE 69.
Forest plot with fixed-class NMA model results for SAEs without studies that include pharmacological treatment plus counselling (unless counselling included in all arms). Ns, not specified; std, standard.
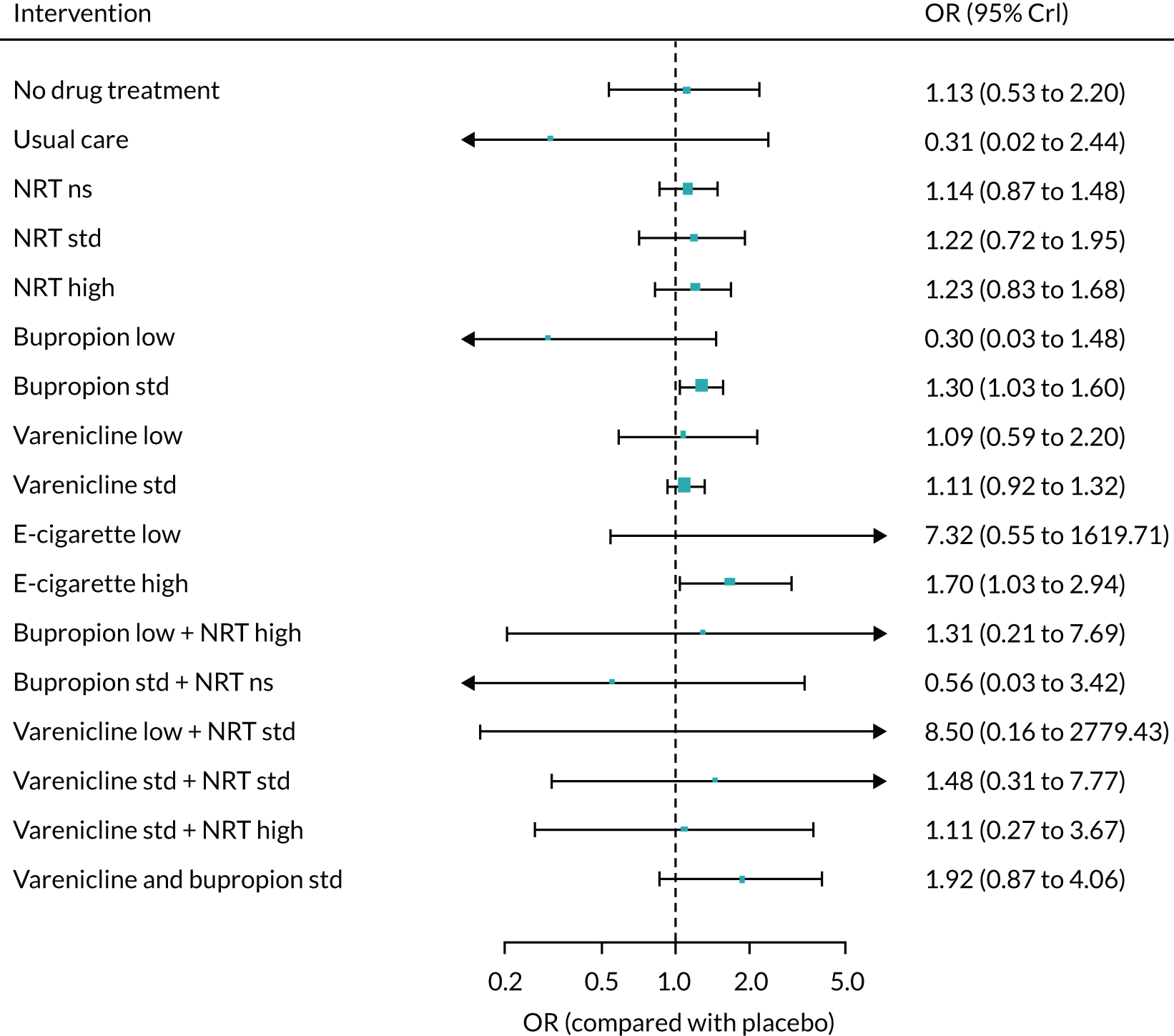
Meta-regressions
Industry sponsorship as covariate
This analysis was based on 96 studies. There was inconclusive evidence of effect modification based on industry sponsorship (B = –0.87, –195.4 to 197.5). The estimate of the SD between class effects was 0.09 (0.00, 0.29).
FIGURE 70.
Forest plot with fixed-class NMA model results for SAEs adjusted for sponsorship. Ns, not specified; std, standard.

Placebo type as covariate
This analysis was based on 84 studies. There was inconclusive evidence of effect modification based on type of placebo (B = –0.23, –195.1 to 196.5). The estimate of the SD between class effects was 0.12 (0.01, 0.33).
FIGURE 71.
Forest plot with fixed-class NMA model results for SAEs adjusted for type of placebo. Ns, not specified; std, standard.
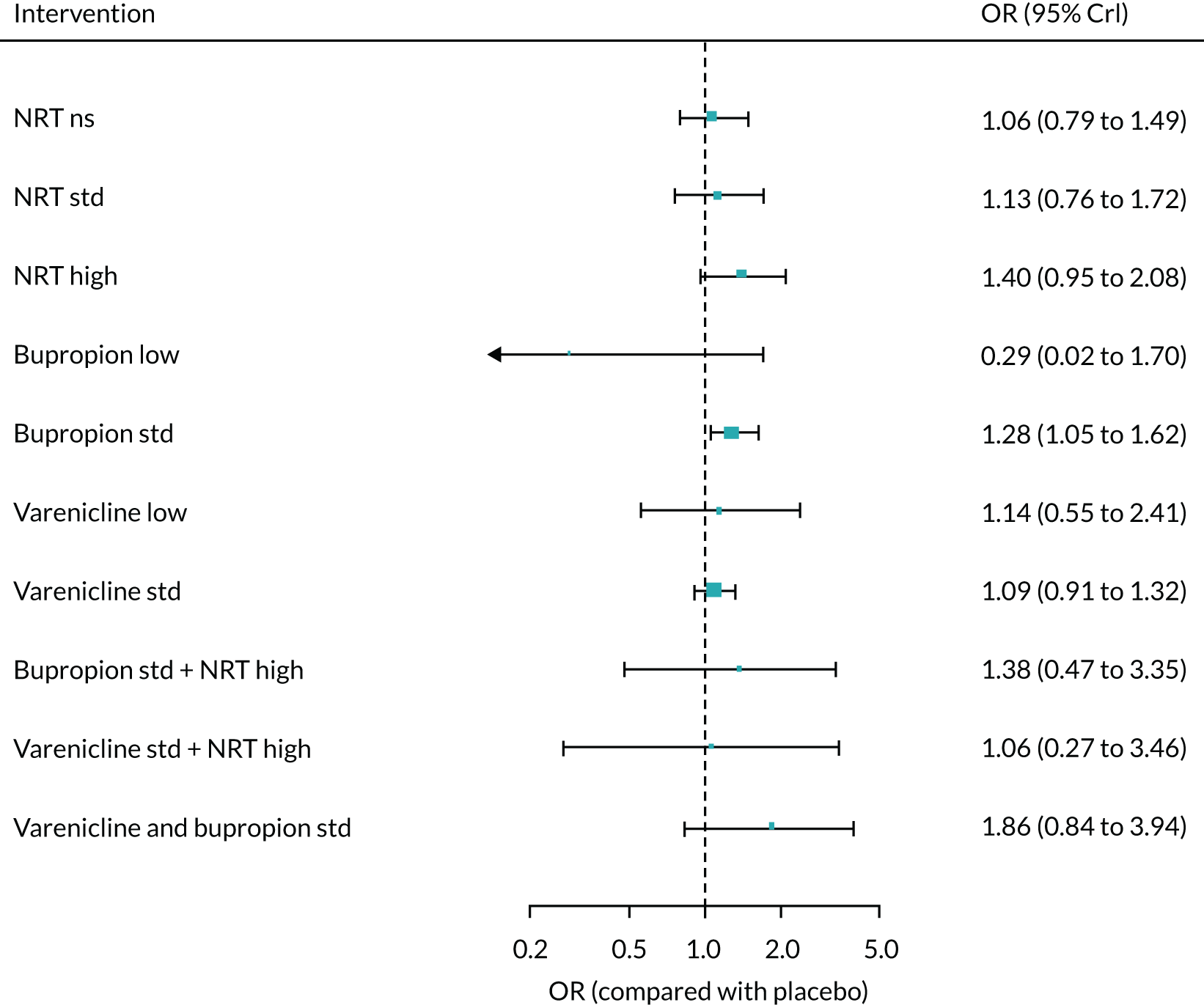
Treatment duration as covariate
This analysis was based on 98 studies. There was inconclusive evidence of effect modification as a function of treatment duration (B = 0.03, –0.04 to 0.10). The estimate of the SD between class effects was 0.16 (0.01, 0.58).
FIGURE 72.
Forest plot with fixed-class NMA model results for SAEs adjusted for treatment duration. Ns, not specified; std, standard.

Counselling as covariate
This analysis was based on 101 studies. There was inconclusive evidence of effect modification as a function of counselling (B = –0.26, –2.29 to 1.75). The estimate of the SD between class effects was 0.11 (0, 0.28).
FIGURE 73.
Forest plot with fixed-class NMA model results for SAEs adjusted for counselling. Ns, not specified; std, standard.

Dependence as covariate
This analysis was based on 70 studies. There was inconclusive evidence of effect modification as a function of dependence (B = 0.12, –0.28 to 0.47). The estimate of the SD between class effects was 0.09 (0.01, 0.31).
FIGURE 74.
Forest plot with fixed-class NMA model results for SAEs adjusted for dependence. Ns, not specified; std, standard.

Comorbidities as covariate
This analysis was based on 101 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = –0.24, –196.8 to 195.1). The estimate of the SD between class effects was 0.09 (0.01, 0.28).
FIGURE 75.
Forest plot with fixed-class NMA model results for SAEs adjusted for comorbidities. Ns, not specified; std, standard.
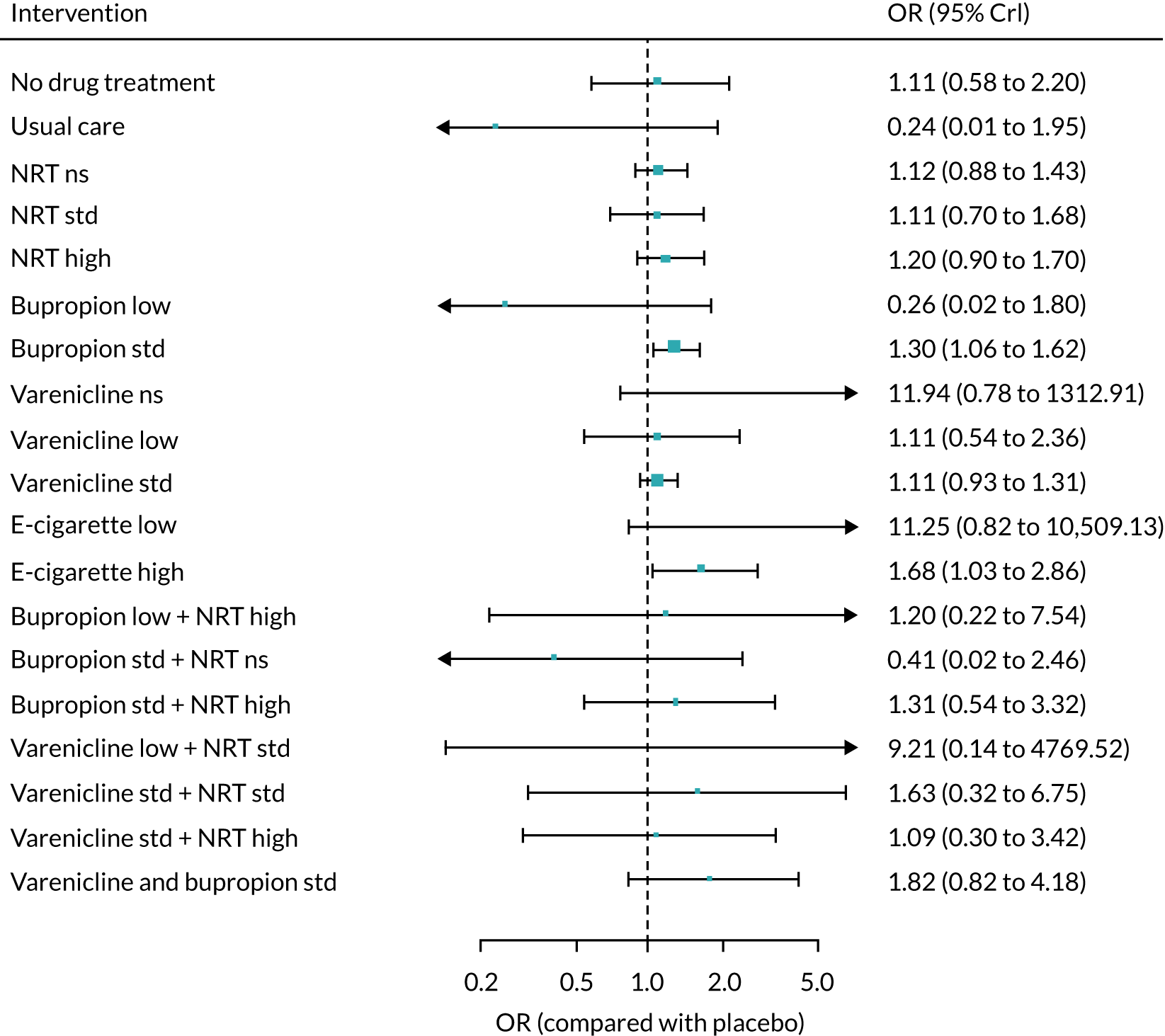
Psychiatric comorbidities as covariate
This analysis was based on 101 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = –0.24, –196.8 to 195.1). The estimate of the SD between class effects was 0.09 (0.01, 0.28).
FIGURE 76.
Forest plot with fixed-class NMA model results for SAEs adjusted for psychiatric comorbidities. Ns, not specified; std, standard.
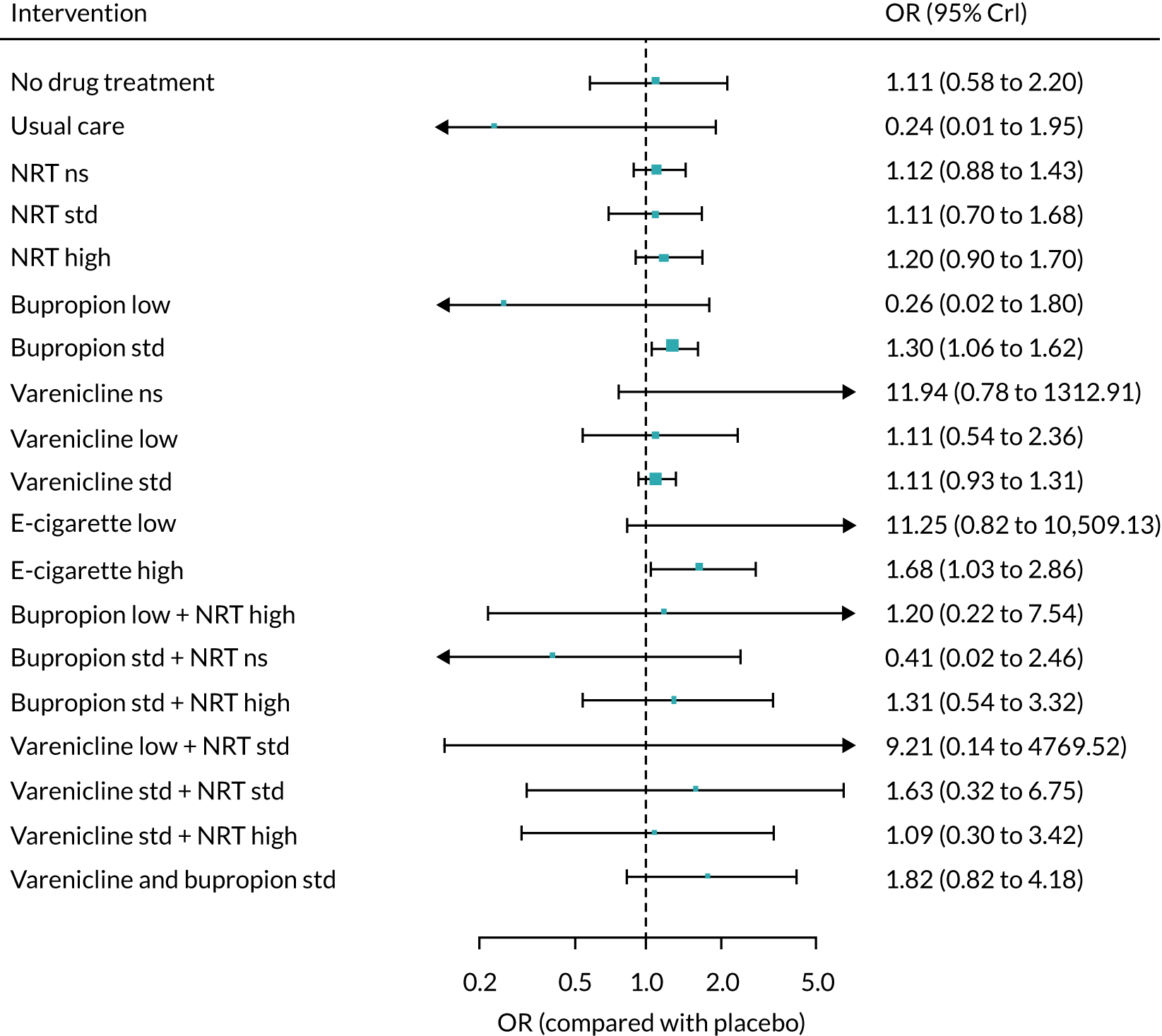
Willingness to quit as covariate
This analysis was based on 101 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = –0.24, –196.8 to 195.1). The estimate of the SD between class effects was 0.09 (0.01, 0.28).
FIGURE 77.
Forest plot with fixed-class NMA model results for SAEs adjusted for willingness to quit. Ns, not specified; std, standard.
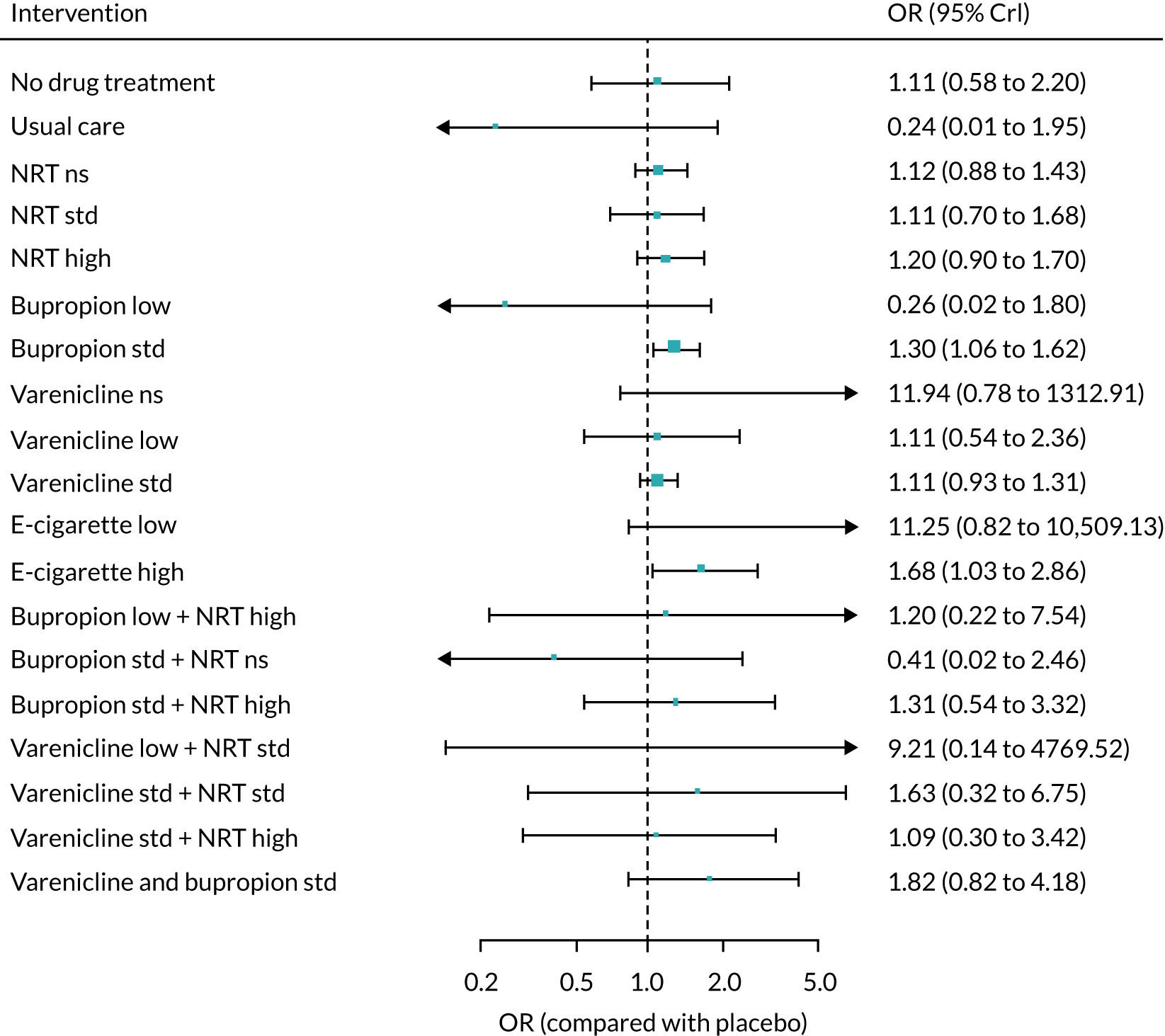
Smokeless tobacco as covariate
This analysis was based on 101 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = –0.24, –196.8 to 195.1). The estimate of the SD between class effects was 0.09 (0.01, 0.28).
FIGURE 78.
Forest plot with fixed-class NMA model results for SAEs adjusted for smokeless tobacco. Ns, not specified; std, standard.
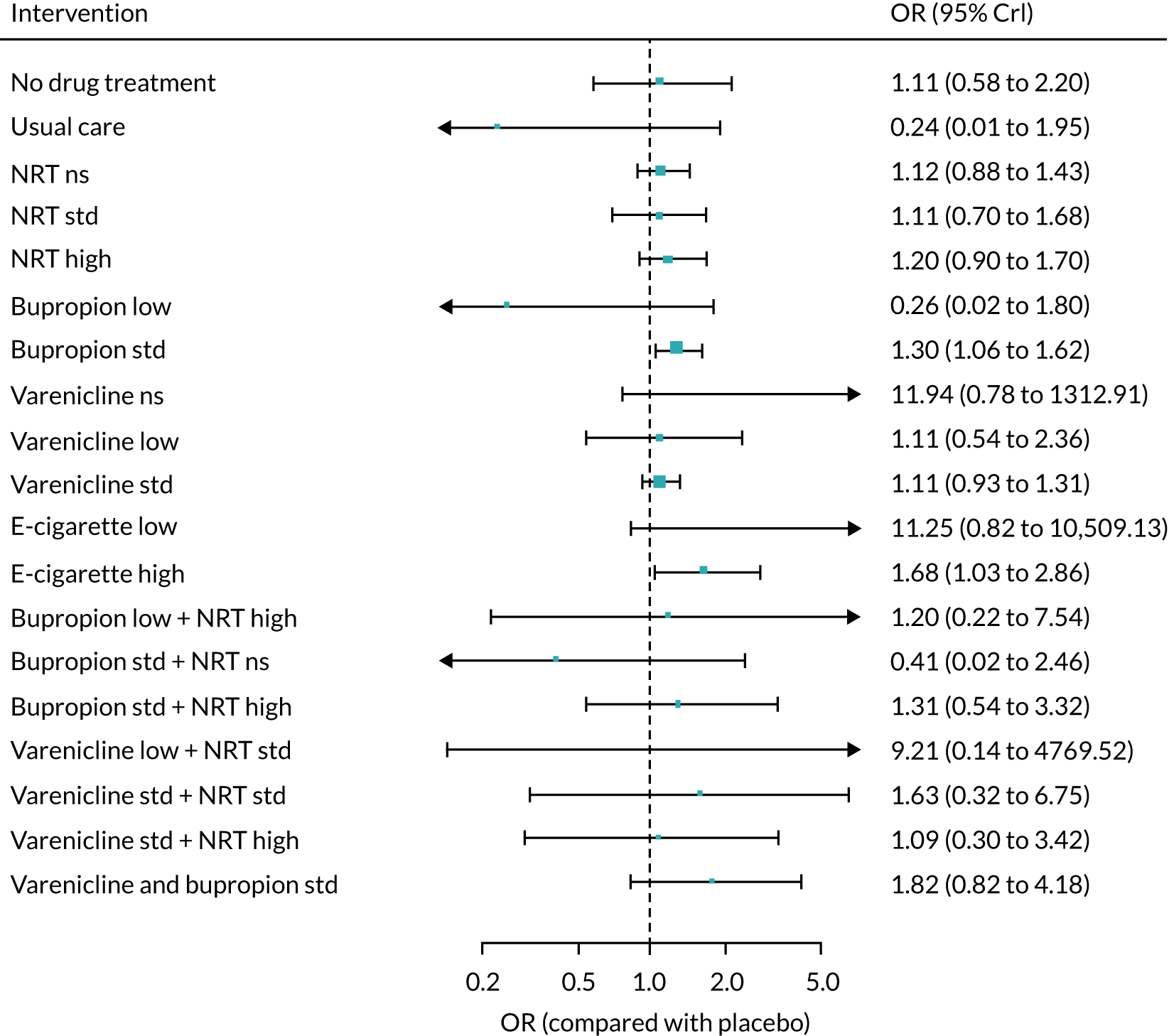
Smoking level as covariate
This analysis was based on 78 studies. There was inconclusive evidence of effect modification as a function of the covariate (B = 0.01, –0.53 to 0.48). The estimate of the SD between class effects was 0.09 (0, 0.30).
FIGURE 79.
Forest plot with fixed-class NMA model results for SAEs adjusted for smoking level. Ns, not specified; std, standard.

Publication year as covariate
This analysis was based on 96 studies. There was inconclusive evidence of effect modification based on publication year (B = 0.14, –196.2 to 194.9). The estimate of the SD between class effects was 0.41 (0.01, 0.29).
FIGURE 80.
Forest plot with fixed-class NMA model results for SAEs adjusted for publication year. Ns, not specified; std, standard.

Major adverse cardiovascular events
FIGURE 81.
Network plot for major adverse cardiovascular events at treatment level. Square brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard.
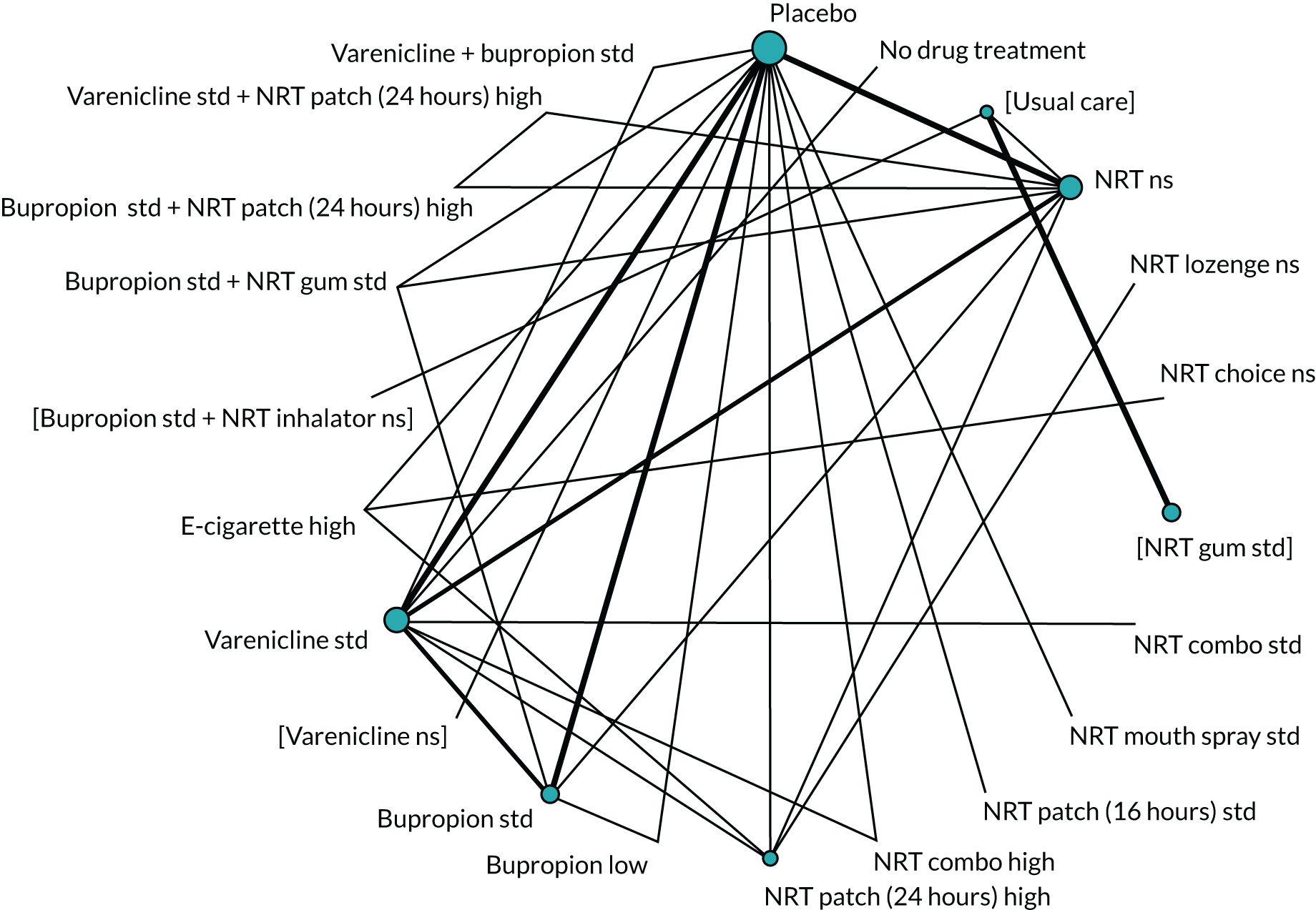
FIGURE 82.
Network plot for major adverse cardiovascular events (including randomised and non-randomised studies) at treatment level. Ns, not specified; std, standard.

| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 82.49 | 341.1 | 0.26 (0.01 to 0.82) | – |
| Random-class model, consistency | 80.3 | 366.7 | 0.26 (0.02 to 0.79) | 0.54 (0.02 to 2.75) |
| Fixed-class model, consistency | 79.51 | 334 | 0.23 (0.01 to 0.73) | – |
| Fixed-class model, inconsistency | 80.61 | 338.3 | 0.22 (0.01 to 0.72) | – |
FIGURE 83.
Forest plot with full interaction NMA model results for major adverse cardiovascular events. Ns, not specified; std, standard.

FIGURE 84.
Forest plot with random-class NMA model results for major adverse cardiovascular events. Ns, not specified; std, standard.

Meta-regressions
Comorbidities as covariate
This analysis was based on 40 studies. There was inconclusive evidence of effect modification based on comorbidities (B = –1.01, –197.3 to 195). The estimate of the SD between class effects was 0.23 (0.02, 0.76).
FIGURE 85.
Forest plot with fixed-class NMA model results for MACE adjusted for comorbidities. Ns, not specified; std, standard.
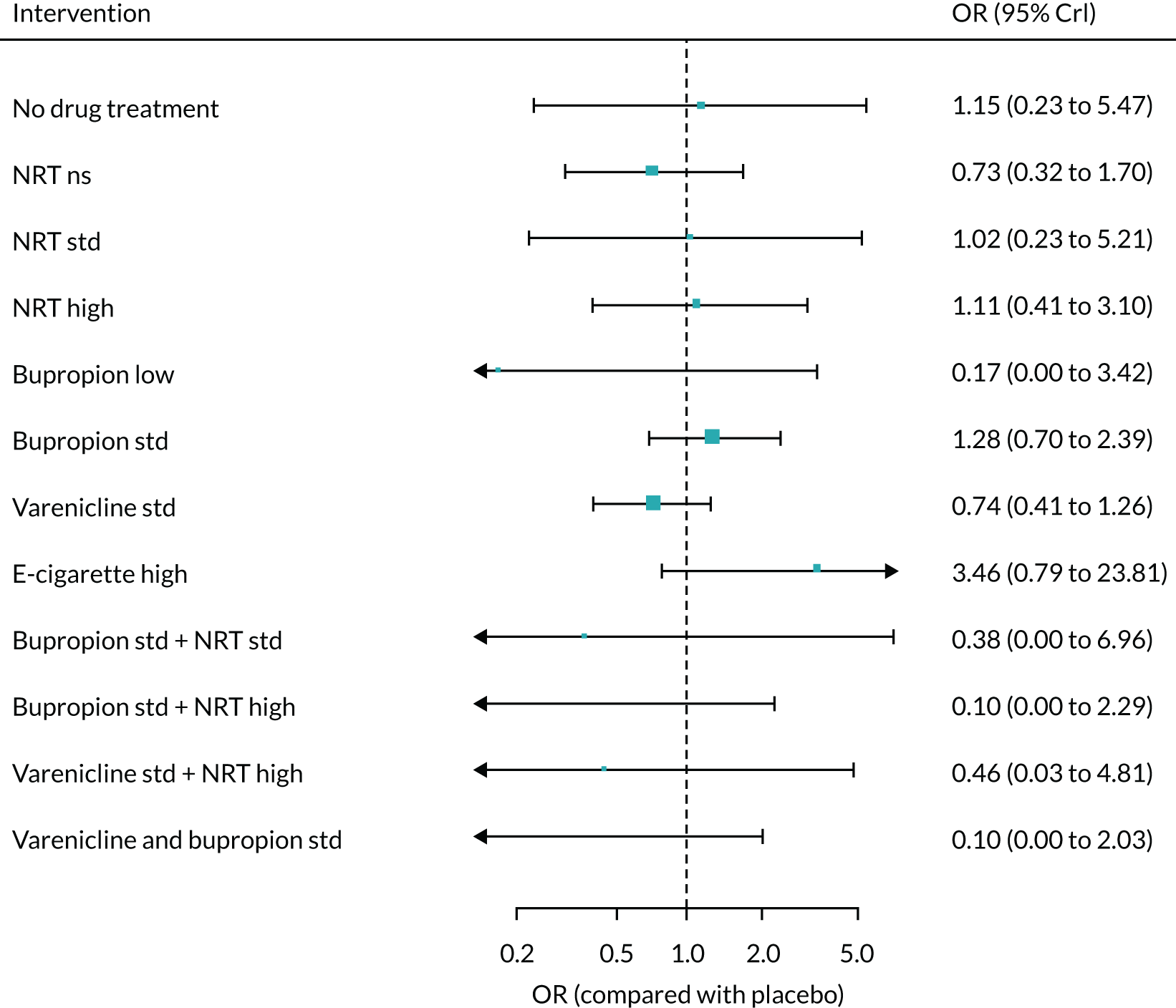
Smoking level as covariate
This analysis was based on 33 studies. There was inconclusive evidence of effect modification based on smoking level (B = –0.29, –6.12 to 3). The estimate of the SD between class effects was 0.37 (0.02, 1.06).
FIGURE 86.
Forest plot with fixed-class NMA model results for MACE adjusted for smoking level. Std, standard.
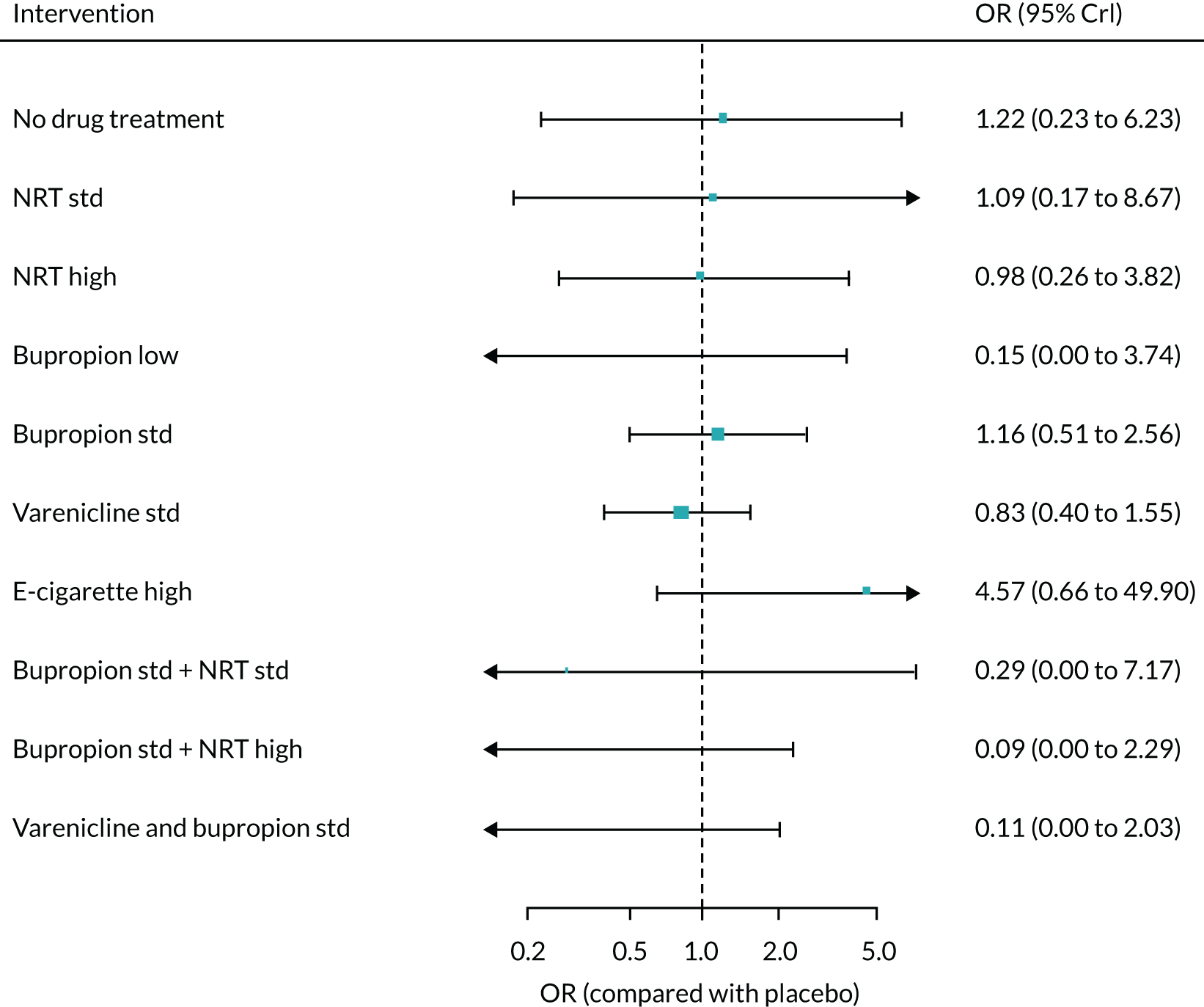
Major adverse neuropsychiatric events
FIGURE 87.
Network plot for major adverse neuropsychiatric events at treatment level. Ns, not specified; std, standard.

FIGURE 88.
Network plot for major adverse neuropsychiatric events (combining randomised and non-randomised evidence) at treatment level. Ns, not specified; std, standard.
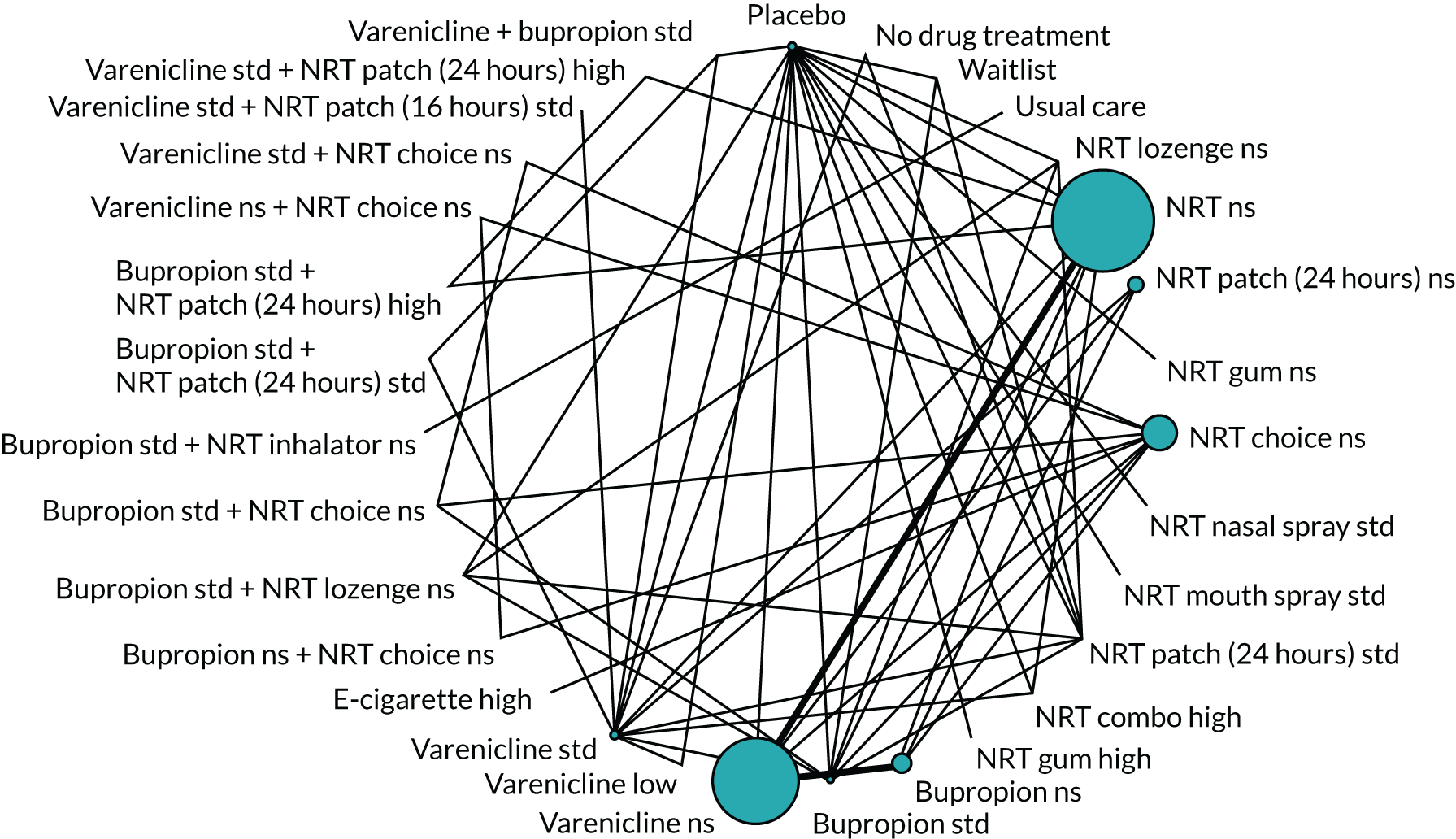
| Model | Residual deviance | DIC | SDd (95% CrI) | SDD (95% CrI) |
|---|---|---|---|---|
| Full interaction model, consistency | 154.2 | 717.5 | 0.15 (0.01 to 0.44) | – |
| Random-class model, consistency | 153.9 | 717.1 | 0.17 (0.01 to 0.45) | 0.92 (0.18 to 2.32) |
| Fixed-class model, consistency | 154.4 | 717.5 | 0.33 (0.05 to 0.60) | – |
| Fixed-class model, inconsistency | 158.2 | 722.5 | 0.18 (0.02 to 0.47) | – |
FIGURE 89.
Forest plot with full interaction NMA model results for major adverse neuropsychiatric events. Ns, not specified; std, standard.
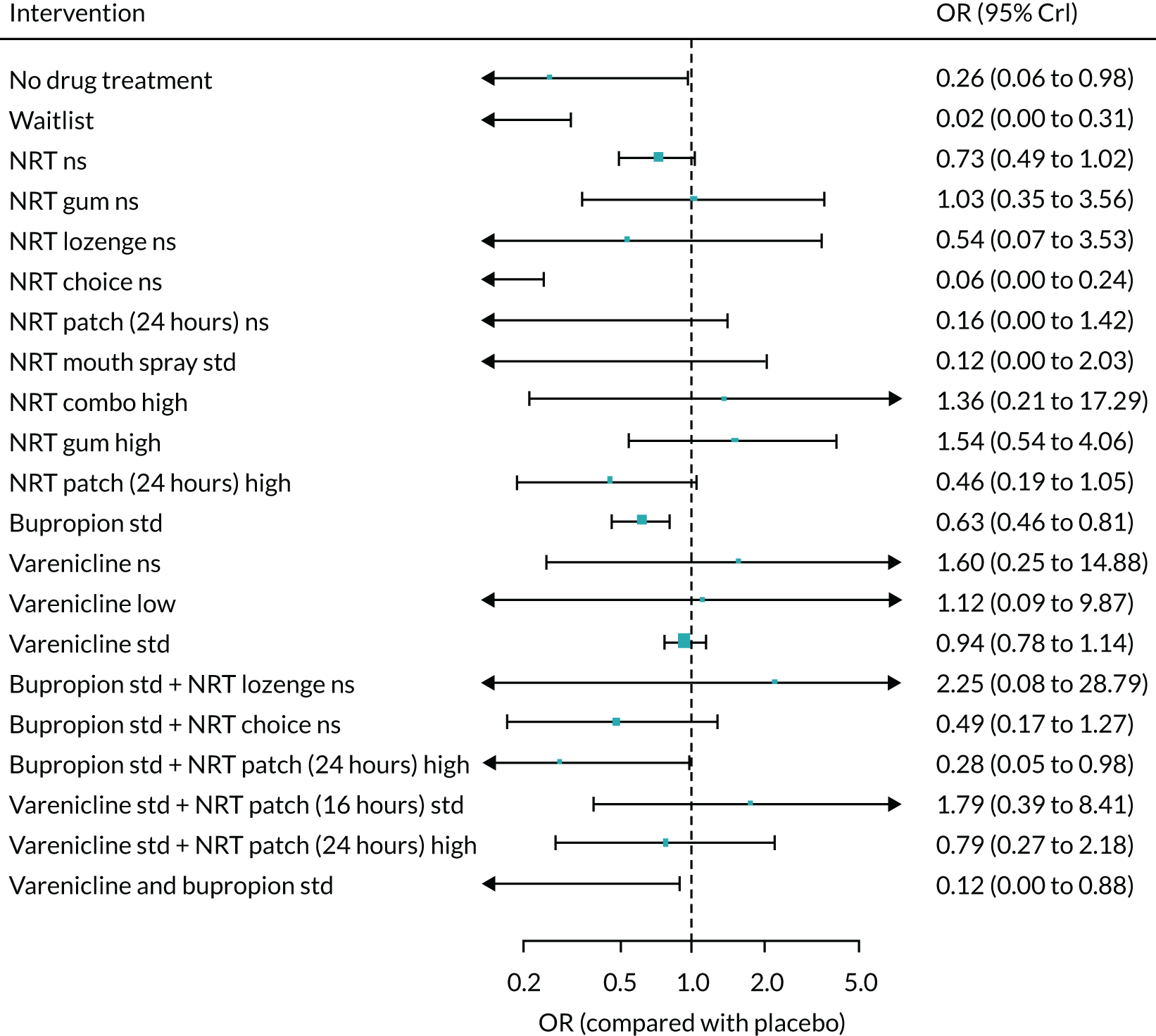
FIGURE 90.
Forest plot with random-class NMA model results for major adverse neuropsychiatric events. Ns, not specified; std, standard.

Meta-regressions
Psychiatric comorbidities as covariate
This analysis was based on 71 studies. There was inconclusive evidence of effect modification based on psychiatric comorbidities (B = 0.38, –196 to 195.2). The estimate of the SD between class effects was 0.33 (0.04, 0.61).
FIGURE 91.
Forest plot with fixed-class NMA model results for MANE adjusted for psychiatric comorbidities. Ns, not specified; std, standard.

Appendix 8 Tertiary and other safety outcome analyses
FIGURE 92.
Network plot for nausea at treatment level. Square brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard.

FIGURE 93.
Random-class NMA results for nausea. Ns, not specified; std, standard.

FIGURE 94.
Standard NMA results for nausea. Ns, not specified; std, standard.

FIGURE 95.
Network plot for headache at treatment level. Square brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard.

FIGURE 96.
Random-class NMA results for headache. Ns, not specified; std, standard.
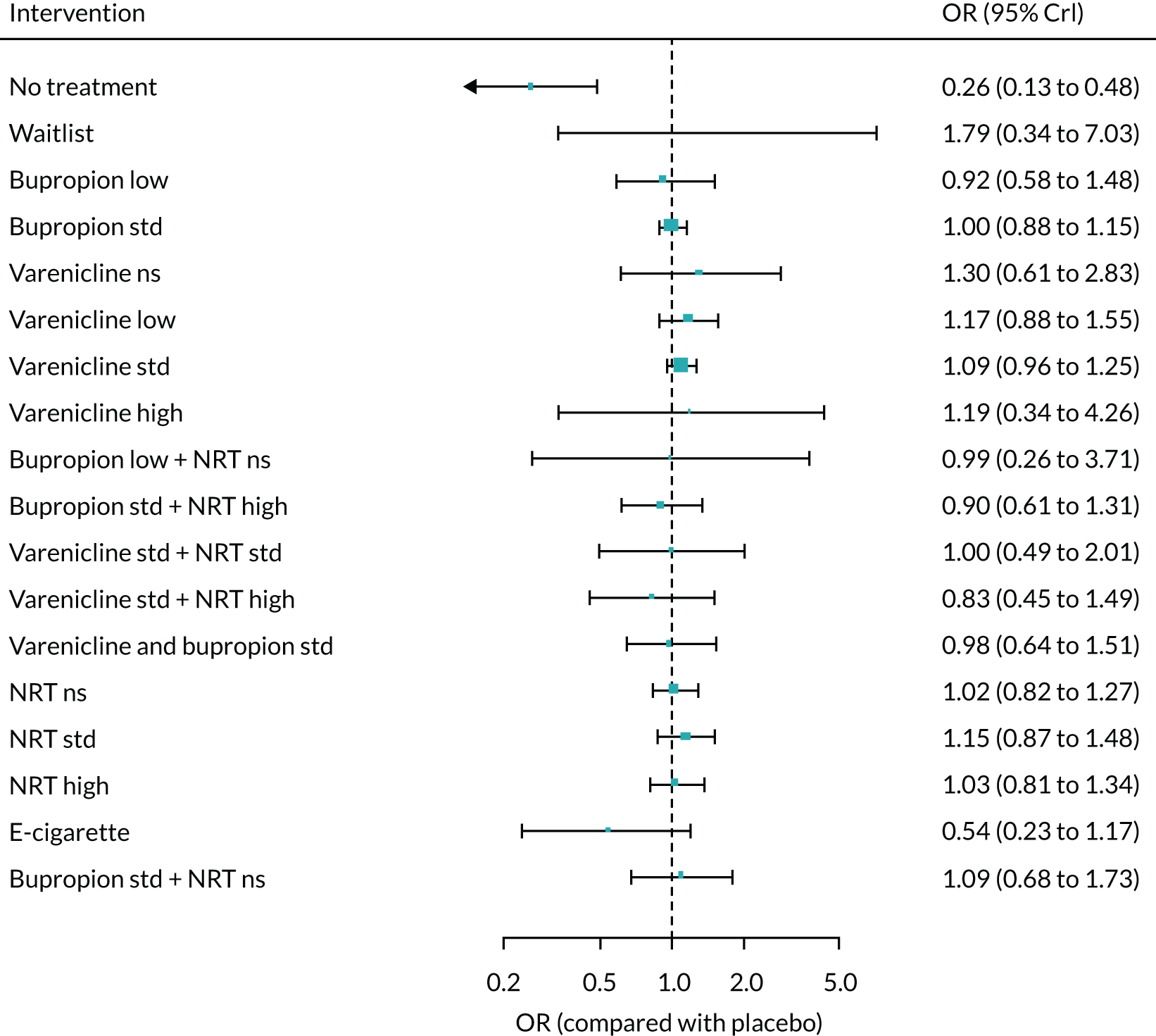
FIGURE 97.
Standard NMA results for headache. Ns, not specified; std, standard.
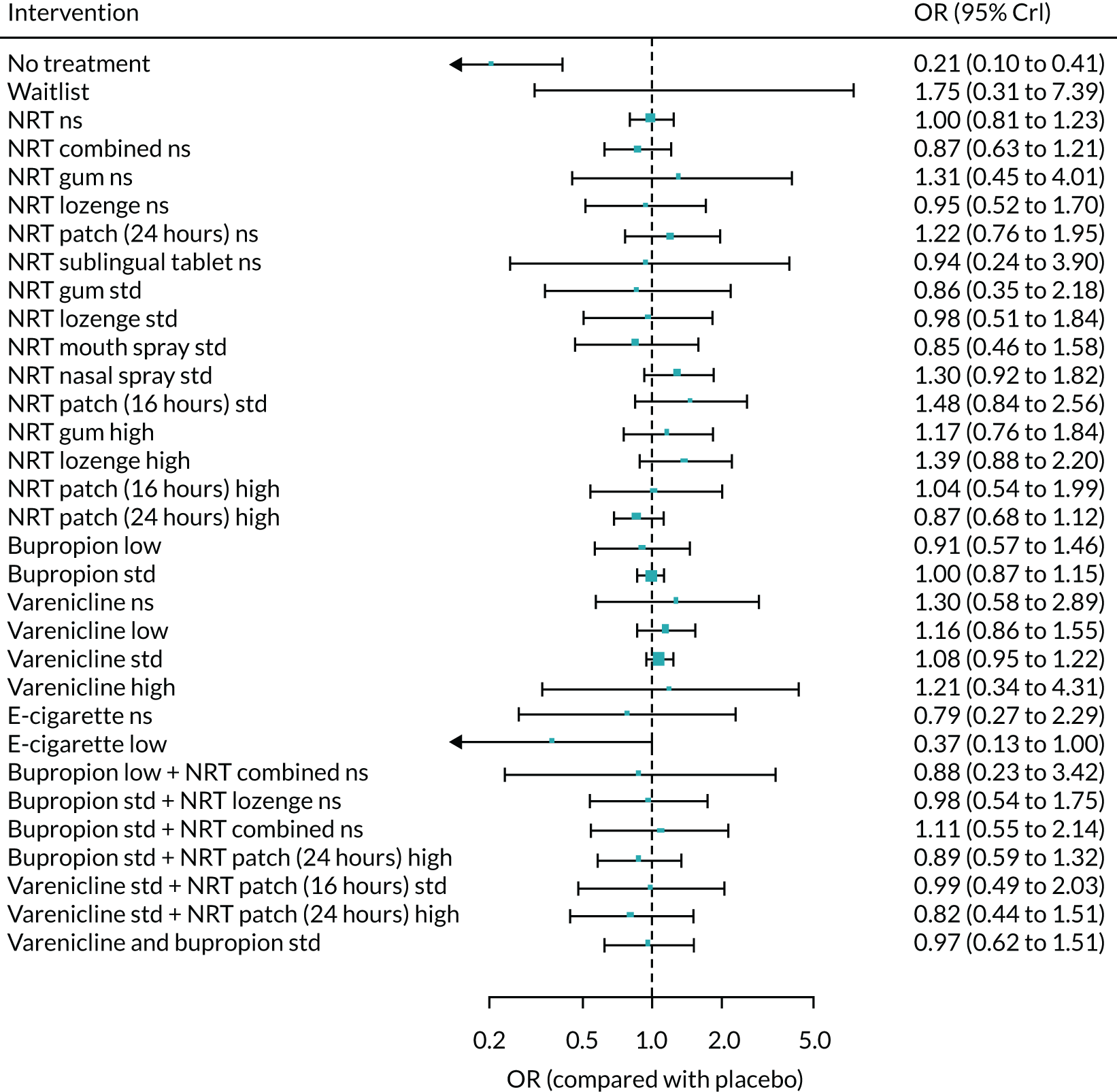
FIGURE 98.
Network plot for dry mouth at treatment level. Square brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard.
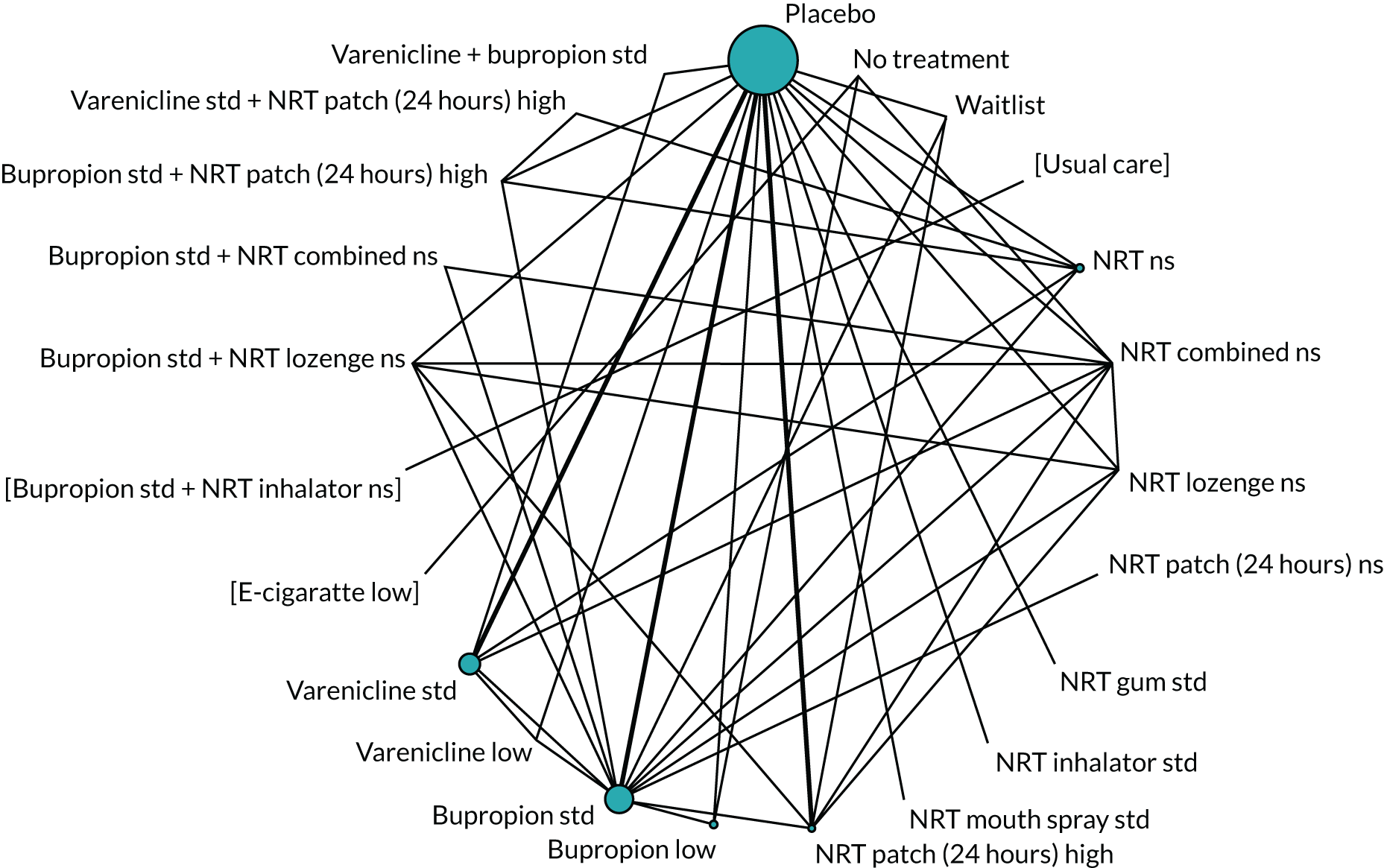
FIGURE 99.
Random-class NMA results for dry mouth. Ns, not specified; std, standard.

FIGURE 100.
Standard NMA results for dry mouth. Ns, not specified; std, standard.

FIGURE 101.
Network plot for skin rash at treatment level. Square brackets denote interventions that were not included in the NMA. Ns, not specified; std, standard.
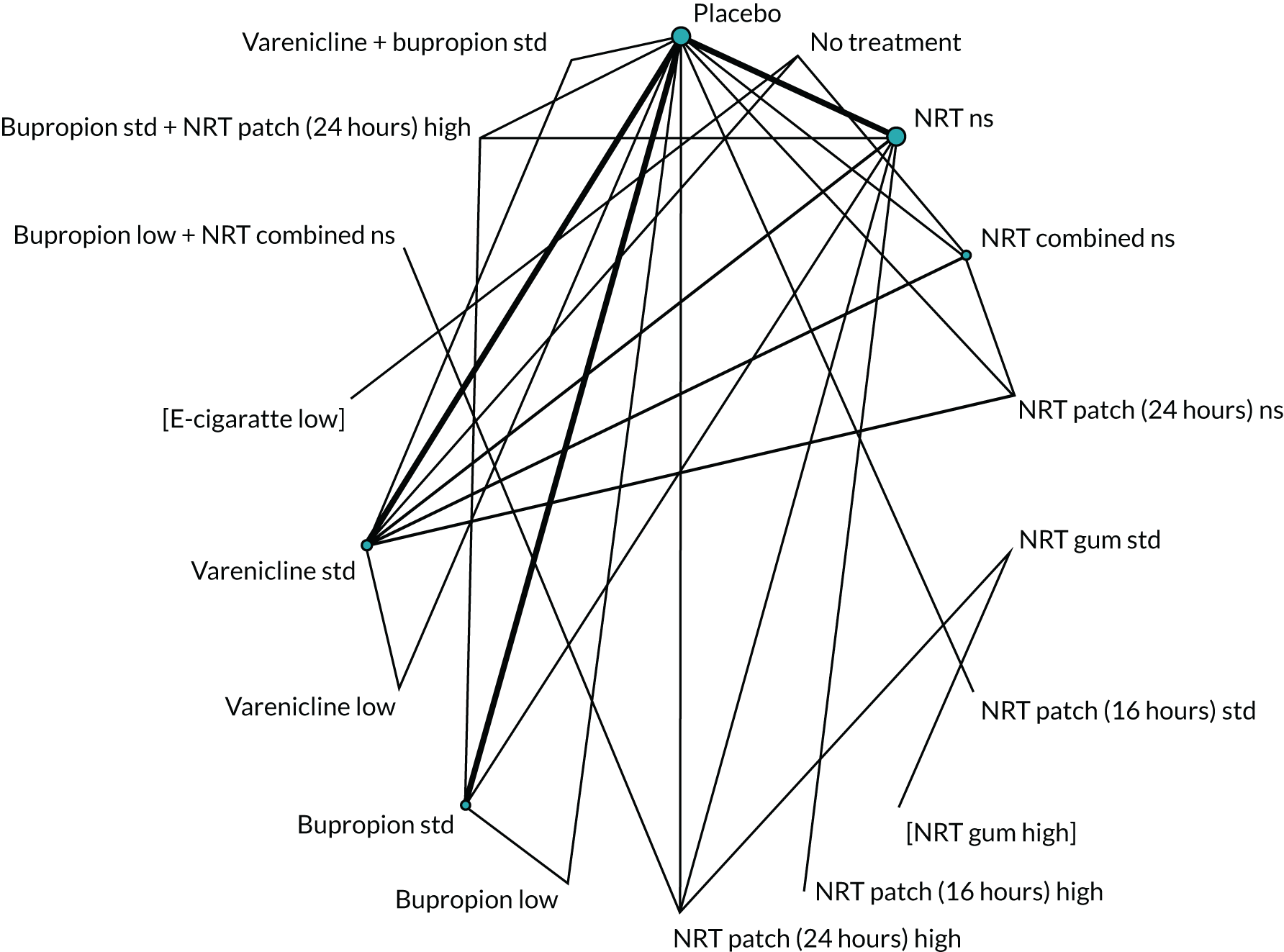
FIGURE 102.
Random-class NMA results for skin rash. Ns, not specified; std, standard.

FIGURE 103.
Standard NMA results for skin rash. Ns, not specified; std, standard.
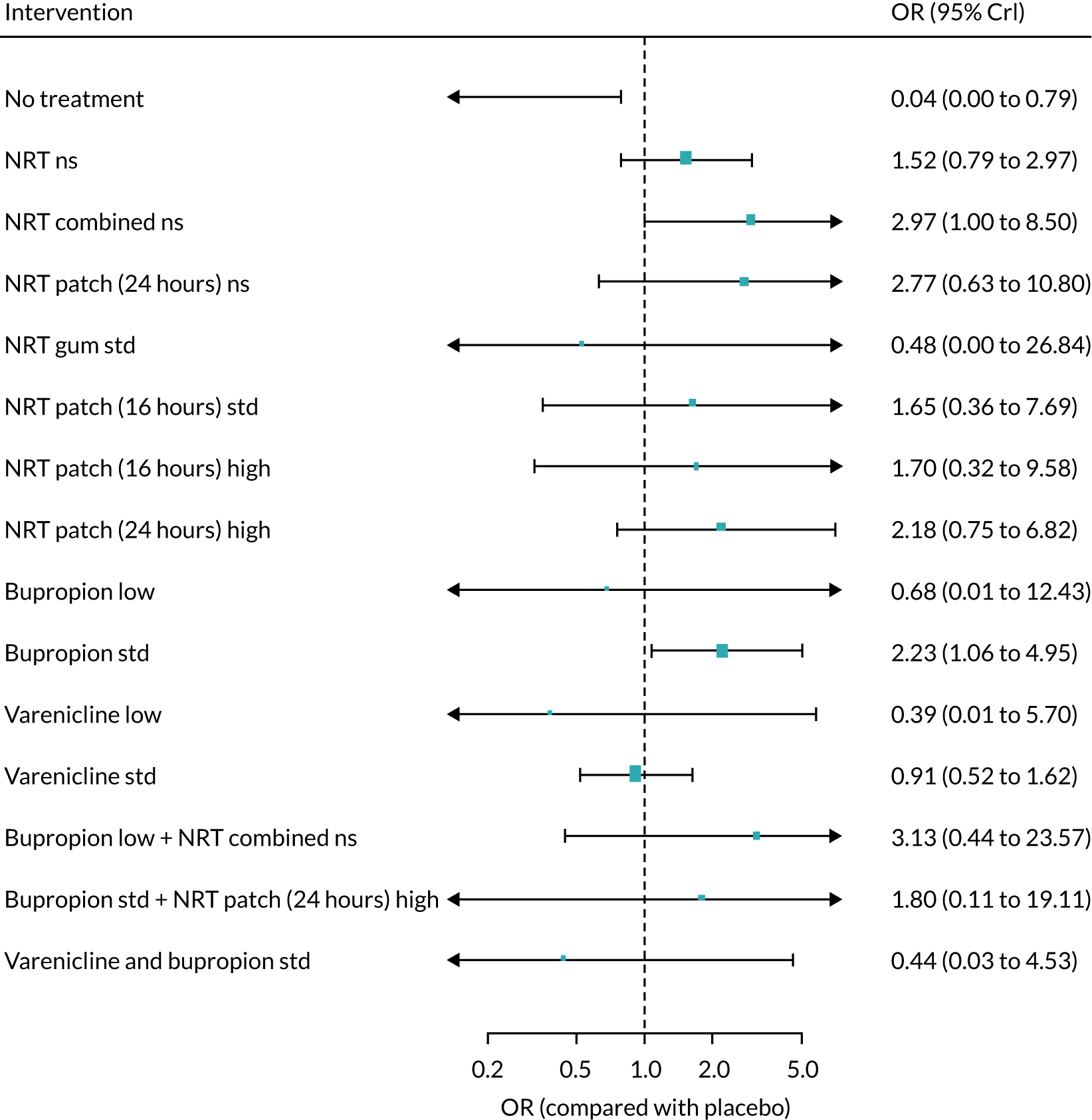
List of abbreviations
- AE
- adverse event
- BENESCO
- Benefits of Smoking Cessation on Outcomes
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPRD
- Clinical Practice Research Datalink
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- DIC
- deviance information criterion
- EMA
- European Medicines Agency
- ENB
- expected net benefit
- ENDS
- electronic nicotine delivery systems
- EQ-5D
- EuroQol-5 Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect partial information
- FDA
- Food and Drug Administration
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- HIQA
- Health Information Quality Authority
- HIV
- human immunodeficiency virus
- HRQoL
- health-related quality of life
- HTA
- health technology assessment
- ICER
- incremental cost-effectiveness ratio
- MACE
- major adverse cardiovascular event
- MANE
- major adverse neuropsychiatric event
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NRT
- nicotine replacement therapy
- ONS
- Office for National Statistics
- OR
- odds ratio
- PPA
- point prevalence abstinence
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- UKCTAS
- UK Centre for Tobacco and Alcohol Studies
Notes
-
Study characteristics for RCTs reporting effectiveness outcomes
-
Risk-of-bias ratings for RCTs reporting effectiveness outcomes
-
Study characteristics for non-randomised studies reporting safety outcomes
-
Risk-of-bias ratings for non-randomised studies reporting safety outcomes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25590).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
