Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/166/05. The contractual start date was in March 2018. The draft report began editorial review in October 2019 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Vale et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the underlying health problem
Melanoma is one of the most deadly of all skin cancers. 1,2 Metastatic melanoma is a highly aggressive disease with rapid dissemination and, until recently, had a median overall survival of between 6 and 10 months once metastasis had occurred. 3 The introduction of targeted therapies and immunotherapies has improved outcomes for these patients, with median overall survival now reaching at least 2 years. 4
The worldwide incidence of melanoma is estimated to be approximately 2% of the population per annum,5 which continues to increase across the globe, with the highest incidence rates being seen in Australia, New Zealand, northern Europe and the USA. In Australia/New Zealand, the incidence has been reported as being > 33 cases per 100,000 population, followed by northern Europe (Norway, Denmark and the Netherlands) with > 25 cases per 100,000. 6 In Australia, the lifetime risk of developing melanoma is 1 in 25 for men and 1 in 34 for women.
Melanoma affects a disproportionate number of people aged < 50 years, compared with other cancers. 7 For example, 11% of all melanomas are diagnosed in those aged < 50 years, compared with 5% for other cancer types. 8,9
In the UK, 2019 figures estimate that the incidence rate has increased 134% since the 1990s, and melanoma is now the fifth most common cancer, accounting for 5% of all new cancer cases, which is on a par with the incidence in other European countries. 10 Globally, there are approximately 232,000 new cases of melanoma diagnosed annually, of which > 140,000 are in Europe. 11–13
Cutaneous melanoma is a cancer that develops from pigmented cells (melanocytes) in the skin. Melanocytes are responsible for production of the main pigment in the skin: melanin. The proportion of the darker eumelanin and lighter pheomelanin play key roles in offering protection against deoxyribonucleic acid damage induced by ultraviolet (UV) radiation. The development of melanoma may occur de novo from melanocytes, or in a stepwise manner from benign naevus to invasive melanoma. 14,15
Description of current service provision
Staging of disease
There have been great advances in the earlier detection of primary melanoma through increased public awareness, the adoption of dermatoscopic examinations and a rapid ‘2-week wait’ referral system in the UK. 16 There is also widespread belief that earlier detection of metastatic disease results in improved patient outcomes. 17 However, at present, there is no internationally accepted standardised model of follow-up of patients diagnosed with cutaneous melanoma, with wide variations in care across North America, Australia, Europe and the UK. 18
When it comes to follow-up of those treated for melanoma, disease staging and judgements on the risk of spread (metastasis) are based on the microscopic appearance and depth of the original tumour. Currently, this is based on the American Joint Committee on Cancer (AJCC) eighth edition staging criteria, which were published in 2016 and formally implemented on 1 January 2018. 19 However, as this is an evidence synthesis project, the definitions used in the 2010 seventh edition20 are also pertinent, as existing data would have based decisions on staging using this edition or earlier editions. The seventh edition included mitotic count (the number of actively dividing cells in the tumour), as this was thought to be an important prognostic feature for thin melanomas,21 but this has been dropped from the eighth edition staging guidelines because of a lack of evidence supporting its prognostic significance. The key definitions of stage I and II disease for both the seventh and eighth editions are set out in Table 1.
| TNM stage | Breslow thickness (mm) | Ulceration | Mitotic count | AJCC stage |
|---|---|---|---|---|
| AJCC – seventh edition20 | ||||
| T1 | ||||
| T1a | < 1.00 | Absent | < 1 mitosis/mm2 | IA |
| T1b | < 1.00 | Present | ≥ 1 mitosis/mm2 | IB |
| T2 | ||||
| T2a | 1.01–2.00 | Absent | N/A | IB |
| T2b | 1.01–2.00 | Present | N/A | IIA |
| T3 | ||||
| T3a | 2.01–4.00 | Absent | N/A | IIA |
| T3b | 2.01–4.00 | Present | N/A | IIB |
| T4 | ||||
| T4a | > 4.00 | Absent | N/A | IIB |
| T4b | > 4.00 | Present | N/A | IIC |
| AJCC – eighth edition19 | ||||
| T1 | ||||
| T1a | < 0.80 | Absent | Not included | IA |
| T1b | < 0.80 | Absent | Not included | IB |
| T2 | ||||
| T2a | 1.01–2.00 | Absent | Not included | IB |
| T2b | 1.01–2.00 | Present | Not included | IIA |
| T3 | ||||
| T3a | 2.01–4.00 | Absent | Not included | IIA |
| T3b | 2.01–4.00 | Present | Not included | IIB |
| T4 | ||||
| T4a | > 4.0 | Absent | Not included | IIB |
| T4b | > 4.0 | Present | Not included | IIC |
Specific changes of note between the seventh and eighth AJCC staging criteria affecting stage I melanoma criteria are as follows:
-
T1a has had the Breslow depth reduced to 0.8 mm when non-ulcerated
-
T1b is now any ulcerated tumour of < 0.8 mm Breslow depth or between 0.8 and 1.00 mm Breslow depth regardless of ulceration status
-
mitotic count has no role in the defining stage.
One further change of note is the distinction between clinical and pathological staging for non-ulcerated tumours that are between 0.8 and 1 mm. These tumours are clinical stage IB, but if they undergo a sentinel lymph node biopsy (SLNB) that is negative, the tumour is ‘downgraded’ to pathological stage IA, with associated changes in overall prognostication. This subcohort of patients is likely to represent a tiny proportion of UK patients, given that, under the National Institute for Health and Care Excellence (NICE) guidelines,16 they would not be routinely offered SLNB if their tumour is of < 1 mm Breslow depth.
The AJCC stage I disease encompasses both stages IA and IB disease and represents the thinnest tumours. At initial diagnosis, 70% of melanomas are classified as AJCC stage I. Early-stage tumours are treated by surgical excision. However, the 5-year overall survival of patients with stage I disease is only 95%. 22 Stage II disease encompasses thicker, but still localised, tumours. Stages III/IV patients have evidence of local and distant metastases; 2-year mortality is up to 82% in stage IV disease, although, with the introduction of new systemic agents, this is now falling.
Surveillance strategies
Given the relatively low rates of local or systemic recurrence, those with AJCC stage I melanoma may not need the same level of clinician follow-up as is generally recommended,23 whereas patients at higher risk following surgical treatment may benefit from more intensive future surveillance to detect recurrent or metastatic disease early. However, balanced against this is the fact that approximately 10% of patients with AJCC stage I disease develop metastases and the prognosis for these people remains poor. Currently, this results in rigorous, routine follow-up for all melanoma patients.
The potential interventions and investigations used as part of a post-surgical treatment surveillance strategy also varies in AJCC stage I patients. An important element of surveillance is education of the patient to allow them to identify any new lesions of concern or signs of recurrence. In one study by Hofmann et al. ,24 30 (24%) of the 127 patients who had a first relapse were not being formally followed up at the time that the relapse was detected. This is because they had never been in a follow-up programme, had dropped out of follow-up, or had completed the formal follow-up process. These data demonstrate the often erratic and unpredictable course of the disease. In the same study, 68% of first relapses were detected by follow-up activity.
Nevertheless, regular clinical history and examination is the mainstay of most surveillance guidelines. Again, which type of health-care practitioner (e.g. nurse, surgeon, dermatologist) undertakes the examinations varies, as does the setting of these reviews, with recommendations for either primary care-based or secondary care-based (in-hospital) appointments. Specific radiological examination of patients may also be recommended for follow-up of stage I melanoma. Routine use of imaging modalities aims to detect the development of regional and distant metastases as early as possible, even before these become clinically apparent. However, if a patient is found to have clinical evidence of metastases, a further set of imaging modalities such as ultrasonography, computerised tomography (CT) and positron emission tomography-computerised tomography (PET-CT) may be used. These methods allow targeted biopsy, when possible, of the relevant melanoma deposits to allow histopathological assessment of the tissue. In the UK, modalities such as CT and PET-CT are not currently advocated as part of the routine management and follow-up of AJCC stage I disease, and it is felt to be unlikely that this situation will change in the next 5–7 years. As others have noted,25 and described in more detail in Chapter 2, there is considerable variability of surveillance practices worldwide.
The British Association of Dermatologists (BAD) revised UK guidelines for the management of cutaneous melanoma26,27 and the more recent NICE Melanoma: Assessment and Management guideline16 advise that patients who have stage I melanoma are followed up to detect signs of recurrence after history and examination. This surveillance is undertaken as follows:
-
Patients with stage IA melanoma should be seen two to four times over a period of up to 12 months, and then discharged.
-
Patients with stage IB melanoma should be seen every 3 months for 3 years, and then every 6 months for a further 2 years.
There are no recommendations for the routine use of any radiological modality, only guidance that these can be implemented if required in symptomatic patients.
There are currently no biomarkers in routine use in any guidelines for stage I disease. Lactate dehydrogenase blood levels are validated for use in patients with evidence of metastases only. 28 There is also increased application of serum S100B, but, once again, in patients with evidence of metastatic disease only. 29
Any changes to current recommended practice would need to consider multiple components, each of which would determine the costs, effectiveness, feasibility and acceptability of an alternative strategy (Box 1).
-
Person(s) undertaking surveillance:
-
Patient, dermatologist, surgeon, primary care physician, specialist nurse, combination of practitioners.
-
-
Site of surveillance:
-
Patient’s home, primary/community care, secondary care setting.
-
-
Availability/clinical utility of prognostic risk prediction tools for further disease stratification.
-
Interval timing of review appointments.
-
Duration of overall surveillance:
-
Immediate discharge, 1 year, 5 years, 10 years, life.
-
-
Routine imaging interventions:
-
Which modalities, how often.
-
-
Assessment of clinical benefit from surveillance strategy.
-
Acceptance of any model by melanoma patients and service providers.
-
Value for money.
Current treatment options
The NICE guideline for melanoma,16 published in July 2015, recommends excision of melanomas of stages 0–II with a 0.5- to 2-cm total margin, depending on stage and histopathological assessment of the biopsy (Figure 1). Risk of disease progression is estimated based on the AJCC eighth edition staging criteria. 19 As described in the previous subsection, patients deemed to be at a low risk of disease progression are followed up at regular intervals for a period of 5 years following diagnosis, undergoing visual and physical examinations.
FIGURE 1.
The National Collaborating Centre for Cancer’s melanoma staging algorithm, including recommended use of SNLB.
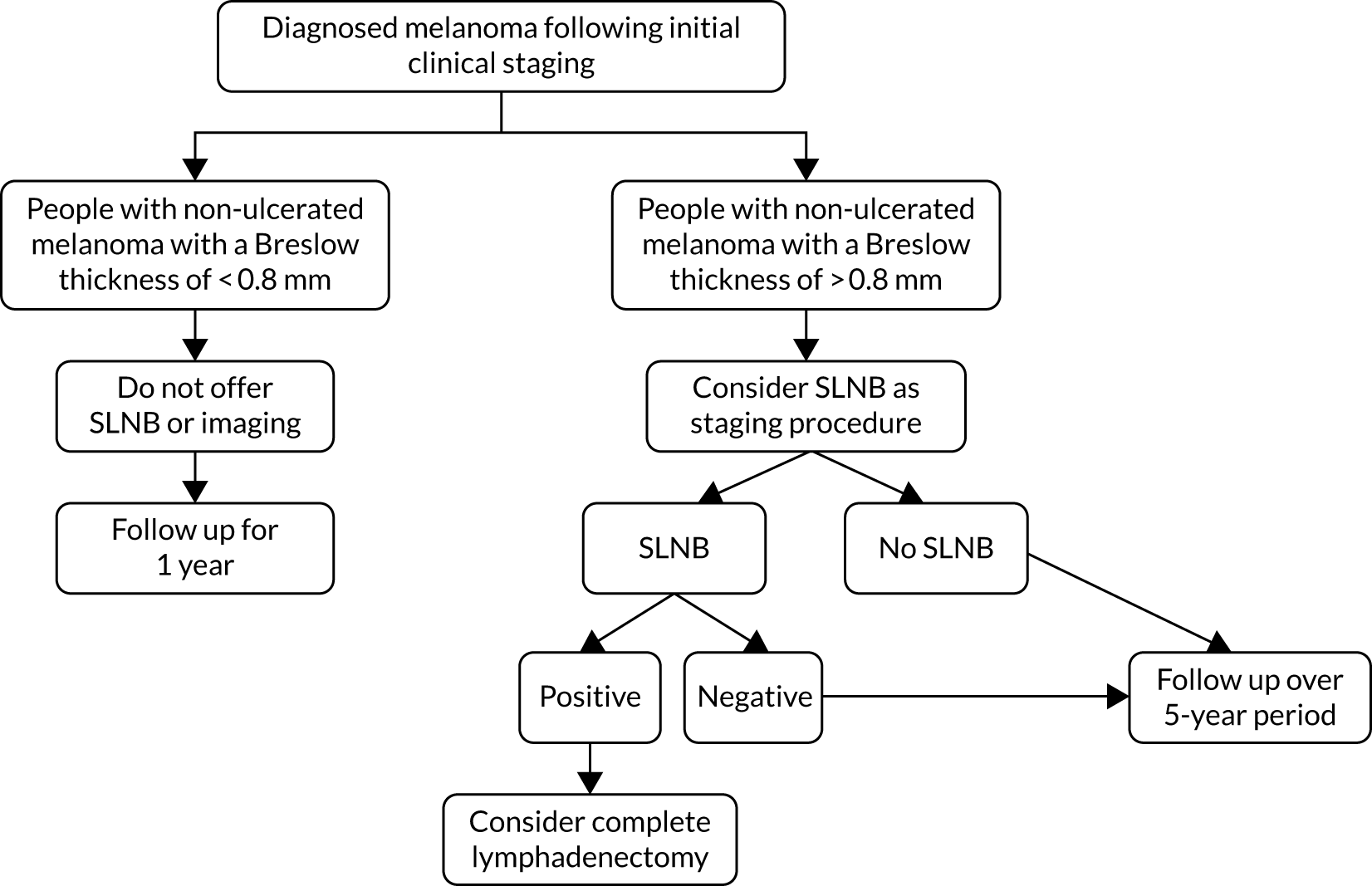
Current draft NICE guidance does not suggest using SNLB for AJCC stage IA or IB melanomas with a Breslow thickness of < 1 mm, and acknowledges that a proportion of those patients with a negative SLNB will still experience melanoma recurrence. 16 This stance is further supported by a UK consensus statement made through a multidisciplinary meeting held by the Melanoma Focus group in 2018. 30
Since 2011, there have been rapid developments in the therapeutic options available for metastatic disease, with accompanying improvements in patient-related outcomes. These developments have been so rapid that we are currently in the follow-up period for many drug trials.
Metastatic disease encompasses the following:
-
Satellite lesions – skin or subcutaneous deposits within 2 cm of the primary tumour.
-
In-transit metastases – these occur > 2 cm from the primary tumour, but before the regional lymph node.
-
Nodal micrometastases – metastatic deposits evident only following histopathological analysis of SLNB tissue or regional lymph node dissection.
-
Nodal macrometastases – metastatic deposits in regional lymph nodes that are either clinically apparent or found on histopathological assessment of regional lymph node dissection.
-
Metastases to distant skin, subcutaneous tissue, lymph nodes or other visceral sites/organs.
Localised metastatic disease is broadly distinguished based on the distance of spread and the total metastatic tumour bulk (and is based on AJCC staging criteria):
-
IIIA
-
one to three local lymph nodes with micrometastases (diagnosed on SLNB or node dissection)
-
-
IIIB
-
one to three local lymph nodes with macrometastases (clinically palpable lymph node involvement or within-node dissection)
-
in-transit metastases/satellite lesions with no metastatic lymph node involvement
-
-
IIIC
-
four or more local lymph nodes involved
-
in-transit metastases/satellite lesions with frank metastatic lymph node involvement.
-
‘In-transit metastases’ covers a wide range of clinical presentations, ranging from localised, small melanoma deposits that are easily amenable to further surgery to > 100 deposits of bulky melanoma tissue. In such cases, the clinical decisions are made based on the extent and technical feasibility of treatment.
Among the most established therapies for in-transit metastases are isolated limb perfusion (ILP) and isolated limb infusion (ILI). Both of these therapies involve the isolation of a limb’s vasculature, with the addition of an anti-tumour agent into this closed system. The aim of therapy is to allow anti-tumour concentrations of the chemotherapeutic agent, without the associated systemic side effects. Traditionally, ILP and ILI have been carried out using melphalan, but, recently, they have been carried out with the addition of tumour necrosis factor. Overall, although tumour response rates range from 64% to 93%,31,32 the median survival time post treatment is still only 2 years. 33 There is currently no suggestion that ILP/ILI can be used in localised melanomas without any evidence of frank metastatic disease.
For metastatic deposits in lymph nodes (following detection by either SLNB or nodal biopsy), the most common therapy is for a lymphadenectomy (with or without post-operative radiotherapy34) of the involved lymph node basin. This has significant morbidity attached to the procedure and it is debatable whether or not there is any benefit for patients in terms of overall melanoma survival; it is currently not recommended routinely in SLNB-positive patients. 30,35,36
Distant metastases, encompassing stage IV disease, rely on systemic therapeutic options. In recent years, a raft of new therapeutic agents have been introduced. The current standard of care in the UK is in constant flux, but remains based on NICE guidance distilled from the continually changing evidence base for systemic therapies; however, there is still variation in local practice. It is generally accepted that adjuvant therapy with immune modulators should be made available to patients with frank stage II disease, or high-risk stage IIIA disease (a deposit of melanoma of > 1 mm2 in the lymph node following SLNB), and that this is also a first-line treatment for stage IV disease. Combination therapies are also preferable for first-line use in stage IV or unresectable stage III disease.
The newer systemic agents can be categorised by their mode of action, either targeting the mitogen-activated protein kinase signalling pathway, or via immune checkpoint blockade. A multitude of clinical trials have been undertaken assessing the benefits of each group as first-line systemic therapy in patients with metastatic disease (usually AJCC IIIB and above), either as monotherapy or combined with another agent affecting the same pathway. Table 2 outlines the most influential recent clinical trials.
| Drug | Trial name | Stages enrolled | Main outcomes |
|---|---|---|---|
| Nivolumab (Opdivo, Bristol Myers Squibb, New York City, NY, USA) | CheckMate 03737 | Unresectable III, IV (second-line study in patients progressing following ipilimumab or targeted therapy) |
|
| CheckMate 06638 | IV |
|
|
| CheckMate 23839 | IIIB, IIIC, IV |
|
|
| Ipilimumab (Yervoy, Bristol Myers Squibb, New York City, NY, USA) | CheckMate 06740 | Unresectable III, IV |
|
| CheckMate 23839 | IIIB, IIIC, IV |
|
|
| Pembrolizumab (Keytruda, Merck Sharp Dohme, Kenilworth, NJ, USA) | KEYNOTE-00241 | Advanced melanoma |
|
| Vemurafenib (Zelboraf, Hoffmann La Roche, Basel, Switzerland) | COLUMBUS42 | IIIB, IIIC, IV |
|
| COMBI-v43 | IIIC, IV |
|
|
| BRIM-344 | IIIC, IV |
|
|
| Encorafenib (Braftovi, Array Biopharma Inc, Boulder, CO, USA) + binimetinib (Mektovi, Array Biopharma Inc, Boulder, CO, USA) | COLUMBUS42 | IIIB, IIIC, IV |
|
| Nivolumab + ipilimumab | CheckMate 06740 | Unresectable III, IV |
|
| Trametinib (Mekinist, Novartis, Basel, Switzerland) + dabrafenib (Tafinlar, Novartis, Basel, Switzerland) | COMBI-d45 | IIIC, IV |
|
| COMBI-AD46 | High-risk IIIA, IIIB, IIIC |
|
The vast majority of systemic agents are aimed at patients with evidence of distant disease progression. However, with the long-standing hypothesis that earlier introduction of systemic therapies may result in better response outcomes, studies have shown a benefit in introducing systemic agents before there is clinical evidence of metastasis. The 2019 NICE guidelines47 for treating stage III melanoma recommend that consideration be given to the use of two adjuvant therapies, nivolumab and pembrolizumab, in resected melanoma with evidence of lymph node involvement, including stage IIIA disease identified following SLNB. Similarly, dabrafenib and trametenib are also licensed and approved for resected stage III disease in BRAF-positive patients.
Such adjuvant regimes continue to be studied,48 but there is a need for better risk prediction of the prognosis of people treated for melanoma, especially for those with an earlier-stage disease, a significant minority of whom will experience progression. However, even for later-stage disease, a considerable number of people may receive these newer systemic regimens, but with only limited prospect of any gain. Hence, they are potentially being unnecessarily exposed to the side effects of systemic therapy with little or no overall benefit.
Description of the technologies under assessment
The technologies under assessment are alternative approaches to the surveillance of people who have been treated for AJCC stage IA or IB disease. Specifically, we will be considering strategies that vary in terms of one or more of the following, and which may include there being no organised surveillance in place:
-
person(s) undertaking surveillance
-
site of surveillance
-
availability/clinical utlity of prognostic risk prediction tools for further disease stratification
-
interval timing of review appointments
-
duration of overall surveillance.
Summary of patient engagement
In addition to drawing on wider patient and public involvement activities undertaken by members of the research team, the study team included three people who have personal experience of melanoma and who are already engaged more broadly with members of the research team in improving the care for those with melanoma. These people were included as co-applicants on the original application to the National Institute for Health Research, and they commented and advised on that application. They have been involved as the work progressed, particularly in helping shape detailed research plans during an advisory group meeting held in May 2018 and in discussions around whether or not the research is likely to meet service user needs, and how it could be best modified to do so. They and the rest of the team have also discussed, via e-mail and during an advisory group meeting held in July 2019, the results of the research. These discussions have been used to draw out key findings and the implications for patients, the public, practitioners, the NHS and further research.
Decision problem
Given that the incidence of cutaneous melanoma is increasing and the majority of people treated for melanoma have AJCC stage I disease that is seemingly low risk, there is an urgent, unmet need to identify those patients with the genuinely lowest-risk disease. Currently, a rigorous patient follow up is routinely carried out for all patients, perhaps unnecessarily straining health-care resources that are already stretched. Identifying those patients with genuinely low-risk disease and discharging them from follow-up earlier could save the NHS upwards of £22.5M over a 5-year period,49 facilitating reallocation of these resources to the smaller group of high-risk patients.
There is little evidence-based guidance on how surveillance regimens should be organised, with considerable variability internationally. Before any changes in a surveillance strategy are introduced, it is essential that any alternative is evidence based. This means that it is essential to gather and synthesise what is already known in a transparent, concise manner to help guide judgements.
Specifically, for those who have been treated for AJCC stage I disease, we want to help reduce the anguish and distress felt genuinely by truly low-risk patients who unnecessarily fear that they are at risk of metastatic disease. A systematic review by Rychetnik et al. 50 in 2013 reported that around half of melanoma patients surveyed said that follow-up appointments made them anxious (with clinically significant levels of anxiety in approximately 20% of patients), sometimes accompanied by physical symptoms that can start weeks before the appointment. Should it be shown to be safe to follow up low-risk patients less intensively, then some of this distress and anxiety could be mitigated. Conversely, a less intensive follow-up may increase anxiety that a cancer could be missed, thereby running the very real risk that detection of metastasis may be delayed.
Should a viable alternative surveillance regimen be identified, in addition to the health impacts on patients, and the service implications to the NHS, there should be a decrease in the number of follow-up appointments. Thus, there could be reductions in the time and travel costs of attending visits incurred by patients and their families, wider system effects of less time away from usual activities (the majority of people treated for melanoma are aged ≤ 50 years and many have work and carer responsibilities) and an impact on traffic pollution caused by reduced patient travel.
To address the evidence gap, this assessment includes an evidence synthesis of relevant information needed to construct and evaluate alternative surveillance strategies. It includes a set of systematic reviews addressing different aspects of the decision problem, an economic decision model to determine the most effective and cost-effective strategy, and a value-of-information analysis to help inform the direction of future research.
Aims and objectives
The aim of this research was to evaluate the effectiveness and cost-effectiveness of different surveillance strategies for patients with AJCC stage I melanoma after surgical excision of a primary cutaneous tumour.
To meet this aim, the objectives were to:
-
identify different strategies for surveillance and follow-up after surgical excision of a primary cutaneous tumour and review the evidence on their effectiveness and cost-effectiveness
-
determine the prognostic performance of risk models used to determine the prognosis and risk stratification of patients with AJCC stage I melanoma after surgical excision of a primary cutaneous tumour
-
determine the diagnostic performance of tests used in surveillance and follow-up strategies in detecting recurrence and metastatic diseases in patients with AJCC stage I melanoma after surgical excision of a primary cutaneous tumour
-
develop a decision-analytic model to estimate the effectiveness and cost-effectiveness of the surveillance and follow-up strategies after surgical excision of a primary cutaneous tumour
-
undertake a value-of-information analysis to assess the need for further primary research.
Structure of the report
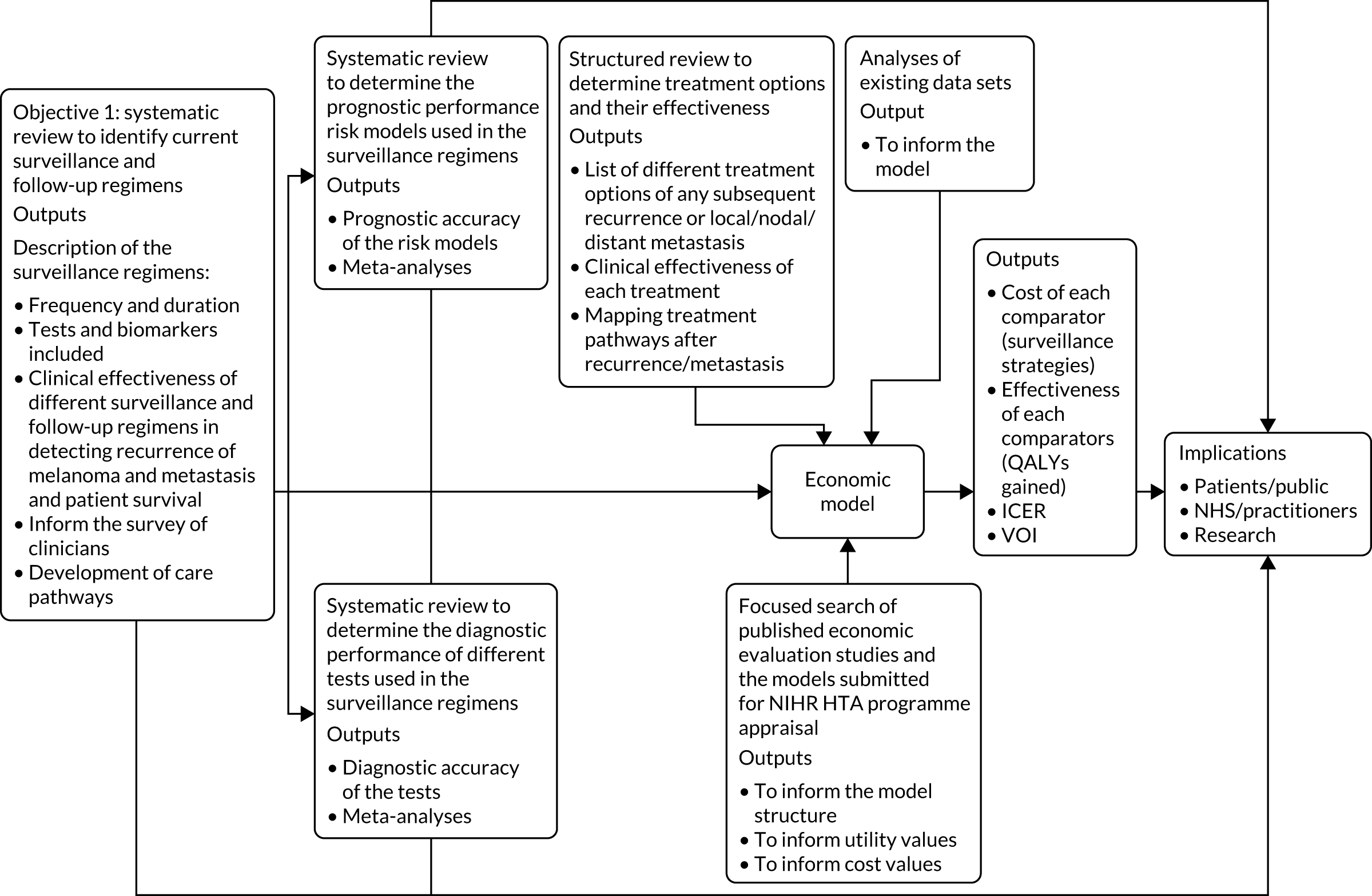
As with most health technology assessments, the work conducted has several related pieces of work. Chapter 2 puts the research in context by presenting a summary of the existing guideline recommendations for the surveillence of stage I melanoma and considering the underlying quality of those recommendations. Each of the four subsequent chapters addresses one or more objective (see Aims and objectives), with the earlier pieces of work informing later pieces of work. Objectives 1–3 are addressed using systematic review methods that are appropriate to their objectives. These systematic reviews are reported in Chapters 3–5. These reviews are then used to inform and parameterise the economic evaluation decision model reported in Chapter 6. Each of Chapters 3–6 ends with a discussion of the chapter’s findings, and an overall summary of key findings is provided in the discussion (Chapter 7), along with strengths and limitations of the work and implications for practice and for future research. A schematic for how the different elements of reseach fit together is shown in Figure 2.
FIGURE 2.
Schematic of the components of the health technology assessment. HTA, Health Technology Assessment; ICER, incremental cost-effectiveness ratio; NIHR, National Institute for Health Research; QALY, quality-adjusted life-year; VOI, value of information.
Chapter 2 Summary of existing guidelines for surveillance following treatment of stage I melanoma
Introduction
As described in Chapter 1, melanoma is a global health burden with a rising incidence. Given this, there is a need for guidelines focusing on prevention, diagnosis and further management. This chapter provides a narrative critique of the current melanoma guidelines available globally, with particular emphasis on the surveillance strategies for stage I melanoma.
The aims of this chapter are to (1) summarise the existing recommendations on surveillance for stage I melanoma and (2) consider if differences in recommendations can be explained in terms of the differences in the evidence base used, the interpretation of that evidence base or the methods adopted to develop the guideline. To provide a common basis of comparison, the Appraisal of Guidelines for Research & Evaluation II (AGREE II) was used. 51,52
For the critique of how the surveillance recommendations were developed, three domains of the AGREE II were focused on. These were ‘scope and purpose’, ‘rigour of development’ and ‘clarity of presentation’, as these were most pertinent to our aims in reviewing these guidelines. These domains are briefly described in the following paragraphs.
The scope and purpose of a guideline includes its objectives and relevant health questions, as well as the population of interest. In this domain, the health intent, interventions, target population, outcomes/benefits, as well as context/setting, should be clearly stated in the guidelines. In addition, the disease stage, associated comorbidities and appropriate comparators should be included in the guidance, and appropriate health questions defined. This domain aims to clarify the potential impact of the guidance. For instance, the NICE melanoma guidance states its aim as:
. . . the assessment and management of melanoma . . . in children, young people and adults. It aims to reduce variation in practice and improve survival.
Reproduced with permission from NICE. 16
This covers the expected outcomes (i.e. reducing variation in practice and improving survival), the target population and the health intent. To fully achieve these stated aims and support critical recommendations, there is a need for well-tailored questions to be included in the guideline.
The ‘rigour of development’ domain looks at the methodological thoroughness employed in producing a guideline. It covers the search process for supporting materials, selection criteria, description of the strengths and limitations of the body of evidence, clear description of evidence formulation process and explicit links between recommendations and supporting evidence. In addition, it appraises the review processes before publication and plans for updating the guidelines. For example, in the appendix section of its guidance, the Australian clinical practice guidelines for the diagnosis and management of melanoma53 state the stepwise process used in developing its guidelines, including the role of systematic reviews in providing key recommendations, dissemination to relevant stakeholders and plans for future updates. When available, it also offered explicit references to published literature for key recommendations.
Clarity of presentation examines how explicit the recommendations are, the provision of different management options and the ease of identifying key recommendations. Explicit guidance should be clear on the population that is affected by the recommendation, the intent of the recommendation, appropriate provisos and descriptions of alternatives, as well as being aesthetically accessible. For instance, flow charts, summary boxes or other forms of graphics may be employed in presenting the entire guideline or sets of recommendations, grouped according to relevance.
Ten melanoma guidelines were identified through grey literature searches carried out in May 2018 and updated in August 2019 (the guidelines were systematically identified as part of the searches conducted for the systematic review of surveillance strategies, reported in Chapter 3). Table 3 summarises each guideline in terms of their recommendations for surveillance following treatment for stage I melanoma.
| Guideline | Duration of follow-up | Routine investigations | Clinician undertaking surveillance |
|---|---|---|---|
|
Do not routinely offer screening investigations (including imaging and blood tests) as part of follow-upReproduced with permission from NICE.54© NICE 2015. Surveillance Proposal Consultation Document: 2019 Surveillance of Melanoma (NICE Guidelines NG14 and CSG8). Available from www.nice.org.uk/guidance/ng14/documents/surveillance-review-proposal. All rights reserved. Subject to Notice of rights (www.nice.org.uk/terms-and-conditions#notice-of-rights). NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication2015NICEhttps://www.nice.org.uk/re-using-our-content/uk-open-content-licence |
|
|
| BAD 201027 |
|
Nil |
|
| NCCN Guidelines® 201955 | AJCC stages IA and IB: every 6 months for 5 years, then yearly | Nil | Not specified |
| ESMO 201556 | Defer to national guidelines | Nil | Not specified |
| American Academy of Dermatology 201957 | Every 6–12 months for 2–5 years. At least annually thereafter | Nil | Not specified |
| Cancer Council Australia Melanoma Guidelines Working Party53 | AJCC stage I: annually up to year 10 | Nil | Follow-up with a medical professional (GP, dermatologist, surgeon or medical oncologist) |
| Dutch Working Group on Melanoma 201358 |
|
Nil | Not specified |
| German Guideline Program in Oncology 201359 |
|
AJCC stage IB:
|
Not specified |
| Swiss Cancer League 201660 |
|
AJCC stage I (T2N0):
|
Not specified |
| Brazilian guidelines 201661 | No explicit recommendations for AJCC stage I melanoma | None routinely | Not specified |
As Table 3 illustrates, there are variations between guidelines in their recommendations on surveillance. This variation exists not just for the intensity and duration of follow-up, but also over the tests that are recommended and who conducts the surveillance. In part, differences in who performs the surveillance may relate to differences in geography and national priorities, but other differences are less easily explained. By summarising the guidelines in terms of the selected AGREE II criteria, it may be possible to shed light on why guideline recommendations differ.
Summary of individual guidelines according to the selected AGREE II criteria
National Institute for Health and Care Excellence: Melanoma: Assessment and Management
This NICE guideline16 was published in 2015 to guide clinical practice for the management of melanoma in England. The process of the guideline formulation began in 2013 and the full draft was published in July 2015. There is an ongoing peer review of the guideline; the most recent report was in 2019,54 and suggested significant section updates to the guidelines.
Scope and purpose
In the introduction to the NICE melanoma guideline,16 the purpose was clearly stated as focusing on where there was differences in clinical practice. Recommendations were made on the staging and treatment of melanoma. This included the use of chemotherapy and immunotherapy to address more advanced disease.
The target populations were clearly defined and appropriate caveats were issued for specific population groups, such as children and adolescents. The health-care context was defined as appropriate for the various recommendations. As part of the guideline, tools to aid the implementation of the guideline in various settings were included in the additional tools and resources section of the guideline.
Rigour of development
The NICE guideline16 contains a chapter detailing the methodology used in the design of the guideline. In developing the NICE guideline, a systematic review of the available evidence was undertaken using the population, intervention, comparator, outcome (PICO) question format and the evidence was graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) and Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tools. The grading of the evidence for the recommendations on follow-up for stage I melanoma was very low. The NICE guideline16 was also balanced against economic evaluations on efficiency in making its recommendations. The Portable Document Format (PDF) file of the recommendation did not include explicit links to the sources of evidence, as seen in the web version. However, the PDF of the full guidance has a record of the evidence used and summarises this in the paragraphs preceding the recommendation summary box. The evidence used as the basis of recommendations around follow-up for stage I disease were mainly based on data from case series.
Clarity of presentation
The NICE guideline16 includes flow charts, with headers based on the management options, and further classifications within each header into the various stages using the AJCC classification for melanoma. The web and PDF versions of the recommendations are easy to navigate, as they contain links to other areas of the guidelines. The recommendations on the follow-up of stage I melanoma were explicit and different from other stages. For instance, in addition to the general follow-up recommendations for all people who have had melanoma, those with stage IA were recommended to have follow-up for 1 year, whereas those with stage IB–IIB/IIC with negative sentinel nodes were to be followed up for 5 years. In both groups (i.e. stage IA and stage IB–IIC with negative SLNBs), routine screening investigations were not recommended.
A clear distinction was also made between patients with stage IIC melanoma who had negative sentinel nodes and those who had no lymph node biopsied. Those with no lymph node biopsy were followed up as a stage III melanoma, with recommendations to consider surveillance imaging as part of follow-up.
British Association of Dermatologists revised UK guidelines for the management of cutaneous melanoma
The 2010 revised UK guidelines for the management of cutaneous melanoma27 are the current guidelines from the BAD, which have not been updated because of ongoing updates by NICE on melanoma management (see National Institute for Health and Care Excellence: Melanoma: Assessment and Management16). The BAD’s methodology for guideline formulation was also accredited by NHS evidence.
Scope and purpose
A multidisciplinary group was employed to agree on the best management practice for cutaneous melanoma in the UK.
Rigour of development
Although searches were carried out, based on the available evidence, it was difficult to ascertain the rigour of such searches, as some were performed by individual authors in addition to group search findings. Full details of the searches were not readily available. However, in the introductory paragraphs, search terms were listed and the use of the PubMed database was alluded to, but the inclusion or exclusion criteria were not stated. The assessment of the BAD’s guideline against the AGREE criteria was discussed in an article by Cox and Williams62 in 2003, in which it was noted that the method of formulating evidence was not usually specified in the BAD guidelines. As detailed in appendix 1 of the guideline, it used both levels of evidence (I–IV) and a five-point grading system (A, meaning ‘there is good evidence to support the use of the procedure’, to E, meaning ‘there is good evidence to support the rejection of the procedure’) to assess the quality of the evidence. The source or the process of formulation of these levels or gradings were not explicitly referenced.
For stage IA melanoma, it was recommended that patients be followed up for 1 year after treatment. For stage IIB melanoma, follow-up was recommended for 5 years. These recommendations were assessed to be a level III (evidence obtained from well-designed non-experimental descriptive studies), grade B (fair evidence to support the use of the procedure) evidence, based on their predefined grading system. However, there was only one study explicitly linked to these recommendations. 63 In terms of peer review during the guideline development phase, the pre-publication guideline was reviewed in a multidisciplinary meeting, which included lay representatives, and was further reviewed by the BAD executive before publication. 64
Clarity of presentation
Summary recommendations were clearly set out in boxes for some of the areas covered, with the use of different coloured font for main headings. As this guideline was published in a journal, its presentation was constrained by journal style. The proposed recommendations for follow-up were clearly presented and grouped according to the various stages of disease. This reduced ambiguity in the guideline, as once the skin lesion was properly staged, it was easy to choose an appropriate follow-up plan.
National Comprehensive Cancer Network guidelines, version 2.2019: Cutaneous Melanoma
This is the most recent (2019) version of the cutaneous melanoma guideline produced by the National Comprehensive Cancer Network® (NCCN). 55 The NCCN is a not-for-profit alliance of 31 member cancer centres in the USA.
Scope and purpose
The aim of the NCCN Guidelines is to support the:
. . . sequential management decisions and interventions that currently apply to 97 per cent of cancers affecting patients in the United States.
The target audience of this review was clinicians, with a separate document available on the website for patients. 65 It aims to incorporate current evidence in response to gaps identified from annual institutional reviews and external requests. There is at least a yearly update of the guidelines to address these questions or gaps in knowledge.
Rigour of development
The development of the NCCN Guidelines was based on a critical assessment of research evidence, clinical expertise and consensus agreement. Similarly, the clinical evidence was categorised based on clinical evidence and consensus among panel members. The NCCN has a four-point category system for grading evidence. These are categories 1, 2A, 2B and 3. Category 1 is a high level of evidence with uniform NCCN consensus, 2A is a lower level of evidence with uniform NCCN consensus, 2B is lower level with some NCCN consensus and 3 is any level of evidence with no NCCN consensus.
The evidence for the follow-up of stage I disease was classified as category 2A:
Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
The guideline recommended that duration of follow-up should be individualised after the initial 5-year period. In the discussion section of the guideline, the clinical evidence that was considered when making the recommendations was referenced in the text, and mainly pertained to observational and retrospective data.
Clarity of presentation
The first section of the guideline contains the summary of recommendations in an interactive chart format, which facilitates navigation along a pathway for a specific stage of the disease. The ease of navigation facilitates the clarity of the guideline, given the depth of information on treatment and follow-up. However, the links in the interactive chart meant that a reader could go around in circles when trying to identify relevant information. For this reason, the recommendations on follow-up in the guidelines are best accessed by reading the text of the guideline.
European Society of Medical Oncologists clinical practice guidelines for cutaneous melanoma
The European Society of Medical Oncologists (ESMO) produced a guideline on cutaneous melanoma in 2015. 56 ESMO comprises expert members from all over the world, although they are predominantly European. Hence, this guideline is more clinician focused than the NICE guidelines, which consider other audiences.
Scope and purpose
The precise aim of the guideline is not clearly stated in the text. However, ESMO guidelines provide evidence-based recommendations on cancer care. 67 As with the other guidelines appraised, the ESMO guideline focuses on cutaneous melanoma. The ESMO guideline is a short guideline aimed at health professionals.
Rigour of development
There is a very brief methods section describing the ESMO standard operating procedure, which allows for relevant literature to be selected by expert authors, as well as by external expert review. The literature reviews on which the guideline recommendations were based were not extensive. However, there were discussions on the sources of recommendations, with the actual recommendations summarised in a box and graded using an adaptation of the Infectious Diseases Society of America’s grading system. 68 This grading system used both levels and grades of evidence. The levels of evidence were from I to V. Evidence from at least one large randomised controlled trial (RCT) was at level I, whereas case reports and expert opinions were level V evidence. The grades of evidence ranged from A (‘Strong evidence for efficacy with a substantial clinical benefit, strongly recommended’) to E (‘Strong evidence against efficacy or for adverse outcome, never recommended’) (reproduced with permission from ESMO Guidelines Committee). 69
Unlike the previously reviewed guidelines, this guideline does not grade the evidence on follow-up for all stages of melanoma. Instead, there is a comment on the variations in the recommendation. There is no specific recommendation for the follow-up of stage I and II melanomas. Instead, the main point raised was the lack of consensus on the duration and frequency of follow-up, as well as the use of imaging in follow-up. Like the other guidelines reviewed in this section, the recommendations on follow-up are based on evidence from cohort studies (judged by the guideline developers to be based on level III evidence). The recommendations derived from this level III evidence are general to all melanoma patients, regardless of the stage of the disease. These recommendations are protection from extended UV exposure, avoidance of artificial UV radiation, and self-examination.
Clarity of presentation
This guideline is considered to be a quick and easy guide to use. Recommendations are shown in tables supported by short sections of text summarising the literature supporting the recommendations. These recommendations are, as previously mentioned, based on summaries of the evidence. In addition, an updated flow chart covering diagnosis and treatment was added in 2016. This update was produced following an electronic update procedure in place for rapid dissemination of significant breakthroughs. There are no explicit target population-based recommendations on follow-up.
American Academy of Dermatology: guidelines of care for the management of primary cutaneous melanoma
Scope and purpose
The 2019 American Academy of Dermatology (AAD) guideline is the most recent version of the 2018 guideline by the AAD for cutaneous melanoma. 57 This guideline addresses the treatment of cutaneous melanoma in children, adolescent and adult populations. As with the other guidelines considered in this chapter, it explores the role of laboratory tests and radiological tests in surveillance, as well as the appropriate duration of follow-up. The mechanism for the ongoing update is unclear, but, on the guideline scripts available on the Journal of the American Academy of Dermatology website (www.jaad.org/; accessed 3 October 2019) (published in January 2019), there was a button to click on to check for the latest updates underneath the list of authors.
Rigour of development
A systematic review of available evidence was undertaken and the evidence was graded using the three-point scale unified grading of the Strength of Recommendation Taxonomy. 70 The evidence used for each set of recommendations was discussed and referenced in supporting text. A boxed summary of the evidence was then included. However, for the surveillance of stage I melanoma, expert opinion was used in the guidance, with no explicit link to any studies.
Clarity of presentation
The guideline had few summary boxes, which affected its ease of access. The recommendations that were made, however, were clear and easy to read. The full text of each recommendation was contained in a single box and there was a link to a further box that contained the sources on which the recommendation was based, along with the level of evidence for each recommendation.
Cancer Council Australia Melanoma Guidelines Working Party
As of September 2019, the clinical practice guidance for melanoma from the Cancer Council Australia Melanoma Guidelines Working Party53 is undergoing revision, with sections being released as they are completed. The current revision began in 2014, using a ‘wiki’ web-based platform to enable rapid updates, necessitated by the rapid turnover of new evidence, and to allow for sharing of information and ease of contribution among panel/working group members. It also allowed for collaboration with the German Dermatologic Cooperative Oncology Group, which provided access to some of its systematic reviews. Most of the guideline has now been published, with only two questions under development: ‘How should melanoma in children be managed?’ and ‘How should melanocytic tumour of unknown malignant potential be managed?’ Neither of these questions are directly relevant to the aims of the research reported in this health technology assessment.
Scope and purpose
The foreword section of the guideline described a generic intent, covering the target population and benefits:
The purpose of evidence-based clinical guidelines for the management of any medical condition is to achieve early diagnosis whenever possible . . .
Although the relevant criteria stated in the AGREE II tool were present,52 these were not applied to this guideline directly, but stated as a purpose for any guideline formation.
The questions for each portion of the guideline are clearly stated. When relevant, the target population is identified and the intervention/exposure stated, as well as the expected outcomes. These questions are delineated in boxes for each section and were clear and concise. Similarly, when appropriate, the population of interest in each section is defined using either systematic or non-systematic review evidence.
Rigour of development
The guideline includes a dedicated chapter to the guideline development process, which includes a flow chart detailing the steps used in arriving at the recommendations. This chapter makes references to protocols used for the systematic review of the evidence, the grading of evidence, formulation of final drafts and content review. The plans for continued updates are incorporated into the design of the guideline.
For individual recommendations, there are explicit links to the sources of evidence, as well as due considerations of benefits, side effects and risks. It is worth noting that, in including systematic reviews from the German Dermatologic Cooperative Oncology Group, these guidelines were initially assessed for quality using the AGREE II checklist by the Australian working group.
Specific recommendations for surveillance of stage I melanoma were all grade C evidence, meaning that the ‘Body of evidence provides some support for the recommendation(s), but care should be taken in its application’53 (reproduced with permission from the Cancer Council Australia Melanoma Guidelines Working Party53). The evidence used in these recommendations was mostly derived from case series articles.
Clarity of presentation
The description of the different management options is well presented for population and practice contexts. These are grouped in header boxes for each set of recommendations. Although recommendations for each stage of melanoma are reported, this is not clearly flagged in the headers for each section. For instance, self-examination is a boxed recommendation for all stages. The next boxed recommendation is history and physical examination by a physician for stages I–III during follow-up. As with the other guidelines reviewed, radiological follow-up is not recommended for stages I–IIB.
Dutch Working Group on Melanoma
This is the 2012 national guideline produced by the Dutch Working Group on Melanoma. 58 The guideline addresses 19 questions. The appendices showing the list of questions covered are not available in English. However, a systematic review was conducted for three of the questions in the entire guideline; the remaining 16 questions had their recommendations based on studies put forward by guideline committee members.
A further revision was completed between 2014 and 2016 to answer three key questions: ‘The role of 18F-FDG-PET[fluorodeoxyglucose PET]/CT at diagnosis’,58 ‘the role of 18F-FDG-PET/CT in follow-up’58 and ‘the role of the sentinel node biopsy’58 (reproduced with permission from the Dutch Working Group on Melanoma58). This additional review was carried out by the Nederlandse Vereniging voor Nucleaire Geneeskunde (Dutch Society for Nuclear Medicine) and the Nederlandse Vereniging voor Pathologie (Dutch Association for Pathology).
Scope and purpose
The objectives of the guideline are clearly stated: ‘The intention of the document is to be a guideline for daily practice in prevention, diagnosis, treatment and follow-up of patients with a skin melanoma’58 (reproduced with permission from the Dutch Working Group on Melanoma58). The guideline is intended to cover all stages of the disease and is targeted at a health-based audience ranging from clinical staff to social workers. As with the other guidelines, this guideline covers all patient groups with melanoma.
Rigour of development
Akin to the previously discussed guidelines, the composition of the group, the methodology and the grading of evidence are all contained in the appendices of the guideline. It describes how guideline recommendations were arrived at using evidence-based and consensus agreements. This process of evidence synthesis is similar to that used by other guidelines considered in this chapter, in that expert opinion, or consensus agreements, were also incorporated. The recommendations for the follow-up of stage I melanoma are limited and are based on low-quality evidence.
Most of the recommendations on follow-up were based on other Dutch guidelines (‘Cancer Survivorship Care’ and cancer rehabilitation,71 and detection of psychosocial distress72). These earlier guidelines have not been assessed for the rigour of the development. As the appendices available online are in Dutch only, critical assessment of the grading process and the entire guideline formulation process has not been performed. Nonetheless, the follow-up recommendations are linked to some evidence, although, in some cases, it is just one source of evidence.
Clarity of presentation
The English-language version of the guideline is available as a PDF only,58 but there is a comprehensive index showing the various topics and subtopics covered. In-text, summary recommendations were tabulated following discussions on the evidence base and consensus agreements. The grading of these recommendations is included in the summary box. The language barrier made it difficult to assess the functionality of the website and additional components of the guideline. However, the English-language version makes a distinction between follow-up and aftercare in its recommendations. These are explained in simple terms and were judged to be easy to follow for all types of clinical or social care workers.
German Guideline Program in Oncology
The S3-Guideline ‘Diagnosis, Therapy and Follow-up of Melanoma’ – Short Version is the most recent (2013) English-language version of the short version of the German guideline for cutaneous melanoma. 59,73 However, there have been updates in 2015 and in 2016/17, owing to the rapid developments in the field. These updated versions are available online in German only. 73 The ongoing stated plan will be to have a live system that allows for regular updates. A new version of this guideline is planned by the end of 2019.
Scope and purpose
The focus of the guideline was on cutaneous melanoma diagnosis, management and follow-up. The guideline was aimed at clinical practitioners in the field of medical oncology, providing ‘an accepted, evidence-based decision-making aid for the selection and performance of suitable measures for diagnostics, therapy and follow-up of cutaneous melanoma.’59 Although the guideline has a clear clinician focus, there is an extended version of this guideline, as well as guidelines for patients. 73
Rigour of development
Although there is a link to the methodology used, it was difficult to assess the rigour of development because this material is not presented in English. The modified Scottish Intercollegiate Guidelines Network74 classification of evidence was combined with an agreed grading system developed by the guideline authors to assess the evidence used in the development of the guideline. A small portion of the recommendations are based on what was considered by the guideline developers to be sound clinical practice, rather than based on scientific evidence; this includes recommendations on the frequency of follow-up for stage I patients. It is recommended that, for all stages of melanoma, there should be a 10-year risk-adapted follow-up. This recommendation is based on evidence rated to be of level 1b, with a grade of B (which means a recommendation of ‘should’ be followed). For each recommendation, there are explicit links to the sources of evidence used to develop that recommendation.
Clarity of presentation
This guide is well presented, with tables and charts delineating the guideline recommendations. There is limited text in between recommendations, making it less cumbersome to locate the relevant sections. The recommendations are phrased in an unambigiuous way, making it easy to read and follow. It is also easy to understand the rational behind the recommendations by reading the text accompanying each recommendation.
Swiss Cancer League
The updated Swiss guidelines 2016 for the treatment and follow-up of cutaneous melanoma60 are an update of a 2006 guideline and were initiated as a result of advances in diagnostic capabilities and treatment options. 60
Scope and purpose
The guideline aims to provide ‘a reasonably practical guide for all physicians (general practitioners, dermatologists, surgeons, oncologists, and others) who encounter cutaneous melanoma in their daily work’. 60 Hence, the target audience is clinicians. This guideline also focuses on cutaneous melanoma. The desired outcome of the update was to ensure adequate treatment of this condition among Swiss patients.
Rigour of development
There was no mention of the methods used in gathering the evidence. Available evidence was graded using the ‘level of evidence’ classification. 75 Using this classification system, the evidence level of IV (historical cohort or case–control studies) was given to the available evidence for follow-up length of stage I melanoma. This is because these data were reported to be ‘historical’ and ‘dated’. Nonetheless, a 10-year follow-up is recommended. Links to supporting studies are placed in the body of the discussions, but are not explicitly linked to each recommendation.
Clarity of presentation
This guideline is a short review article. As with the other guidelines, the recommendations for follow-up are placed in a table and are easy to understand. The follow-up guidance is clearly differentiated by tumour stage.
Brazilian guidelines for diagnosis, treatment and follow-up of primary cutaneous melanoma – Part II
This guideline, from 2016, is a follow-up to the 2002 Brazilian guideline and is the second part of the full guideline. 61 There was a need for the update as a result of recent developments in diagnosis and treatment. This update covered 10 questions; five of these questions are covered in this second part of the guideline. The five questions were on follow-up for stage 0 and I melanoma, the role of body mapping in follow-up, the benefit of sentinel lymph node in primary melanoma and the benefits of preventative excisions of acral naevi and giant congenital naevi.
Scope and purpose
The aim of this part of the guideline is:
intended for diagnostic and therapeutic approach and follow-up of patients with suspected or confirmed diagnoses of primary [cutaneous melanoma] (PCM) with no clinical or histological evidence of metastatic disease (stages 0, I and II).
Reproduced with permission from Castro et al. 61
Rigour of development
A systematic review was employed to synthesise the evidence and the selected studies were graded based on the level of evidence on a four-point scale from A to D:Reproduced with permission from Castro et al. 61© 2016 by Anais Brasileiros de Dermatologia. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium provided the original work is properly cited2016Anais Brasileiros de Dermatologiahttps://creativecommons.org/licenses/by-nc/3.0/
Recommendations, other than those relating to duration and frequency of follow-up, are not grouped according to disease stage. A 10-year follow-up period is recommend based on grade D level of evidence (i.e. opinion without critical evaluation, based on consensus, physiological studies or animal models). There is no explicit link to the source of this recommendation.
Clarity of presentation
The recommendations for initial diagnosis and those for follow-up are lumped together. However, the guideline is strictly for non-metastatic cutaneous cancer, stages 0–II, and uses a question-and-answer format for each section. For instance, one of the questions in the article is ‘How should stages 0 and I primary cutaneous melanoma patients be followed?’61 [reproduced with permission from Castro et al. 61 © 2016 by Anais Brasileiros de Dermatologia. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and production in any medium provided the original work is properly cited], and this question makes up the section on the follow-up recommendation for stages 0–II cutaneous melanoma. Hence a thorough read of each section would be required to clearly unpick the recommendations. This approach also creates some ambiguity in interpretation of the guideline from a clinical perspective, and there is not a clear link between some recommendations and the various stages of cutaneous melanoma. This is examplified by the recommendations on the role of cutaneous mapping, which are based on risk stratification only.
Summary
This chapter has considered the recommendations on surveillance and follow-up of patients made in clinical guidelines throughout the world. In total, 10 guidelines were considered from eight countries, which were published between 2011 and 2019. The follow-up recommendations for stage I melanoma varied between the guidelines in terms of intensity and duration of follow-up, as well as what tests were recommended and who should perform the follow-up. Comparing recommendations form national bodies, some countries’ recommendations are less intensive for stage IA (e.g. Dutch Working Group on Melanoma 201358) than the NICE recommendations16,54 for the same stage, whereas other countries’ recommendations are more intensive (e.g. German Guideline Program in Oncology 201359) than the NICE recommendation for the same stage. 16 In many respects, these differences cannot be explained by differences in underlying risks. Nor can they be fully explained by differences in the methodologies that the different guidelines adopted, although there were some differences in methodology between guidelines. There were also some variations in evidence on which the recommendations were based and in the grading of available evidence. However, clearly defined questions and robust methodologies were employed for guideline development for most guidelines. A common thread for the recommendations made is that the value and strength of the recommendations were low. The limited data on which recommendations were based may well have contributed to the variations in guideline recommendations.
Arguably, even though the methdologies adopted by the different guideline developers were generally strong, recommendations have had to be made on very limited evidence. It is this unexplained variation in recommendations and the underlying evidence gap that guideline developers have faced that motivate the work in the following chapters.
Chapter 3 Systematic review to identify different surveillance and follow-up strategies for stage I melanoma patients following surgical excision
Brief overview
Surveillance strategies vary in a number of ways: by duration and frequency of contact with patients, and in terms of which practitioner sees patients, and in what type of diagnostic and prognostic tools are used. In a systematic review of surveillance strategies, all the countries that provided data on surveillance had programmes that followed patients for 5 years after treatment and recommended between one and six visits per year, in addition to recommended self-examination. 25 Self-examination is important because many (if not most) melanoma recurrences are detected by patients themselves. 76 As outlined in Chapter 2, not all countries use diagnostic imaging in surveillance visits, but many use sonography; radiography of the regional nodal basin, chest or abdomen; clinical photography; or positron emission tomography (PET), CT or magnetic resonance imaging (MRI). Some also assess a patient’s blood count and liver function.
The NICE guideline for melanoma77 recommends that, after stage IA, patients are seen by a clinician between two and four times in the first year after completion of treatment, and then discharged. After stages IB to IIB melanoma, or stage IIC melanoma with a negative SLNB, the guideline recommends that patients are followed up every 3 months for 3 years and then every 6 months for the next 2 years, after which they can be discharged. No imaging or blood tests are recommended during follow-up for either of these groups.
As described in Chapter 2, there is little consensus about the most effective and cost-effective way to follow up patients who have been treated for melanoma. Furthermore, the evidence base for the different strategies adopted is unclear. Previous studies suggest that existing guidance, which includes variation in frequency and duration of patient contact, as well as in recommended diagnostic and prognostic tools, is based on anecdotal evidence or retrospective assessment of historical cohorts. 25,76
To clarify what evidence there is to support any surveillance for stage I melanoma, a high-quality systematic review is needed. This systematic review would be used to gather and synthesise the most robust evidence about all elements of surveillance strategies for melanoma.
Research aim
The aim of this systematic review was to identify variations in strategies for surveillance and follow-up after surgical excision of AJCC stage I primary cutaneous melanomas in adults and to assess the relative effectiveness on clinical and oncological outcomes, including recurrences, metastases and survival.
Methods
This review adheres to the guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to ensure transparency of the process. 78 A protocol for the whole project of which this review is part is published on PROSPERO (CRD42018086784). 79
Search strategy
The search strategy was designed by an experienced information specialist in collaboration with the project team. The search was designed in MEDLINE [via Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands)] according to the following main concepts: [melanoma] AND [surveillance OR screening]. Database-specific thesaurus headings were used, together with title and abstract keywords. The strategy was translated to other databases (Box 2), altering thesaurus headings and search syntax as appropriate. The databases listed in Box 2 were searched during the first week of May 2018 and the search was updated on 2 July 2019.
-
MEDLINE (via Ovid), 1946 to June week 3 2019.
-
EMBASE™ (Elsevier, Amsterdam, the Netherlands; via Ovid), 1980 to week 26 2019.
-
CENTRAL [the Cochrane Library via Wiley Online Library (John Wiley & Sons, Inc., Hoboken, NJ, USA)] issue 6, 2019.
-
Cochrane Database of Systematic Reviews (the Cochrane Library via Wiley Online Library), issue 6, 2019.
-
Database of Abstracts of Reviews of Effects (the Cochrane Library via Wiley Online Library), issue 2, 2015.
-
Health Technology Assessment Database (the Cochrane Library via Wiley Online Library), issue 2, 2018.
-
NHS-EED (the Cochrane Library via Wiley Online Library) issue 2, 2015.
-
CINAHL [via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA)], 1982 to June 2019.
-
Science Citation Index [Clarivate Analytics; via the Web of Science™ (Clarivate Analytics)], 1970 to June 2019.
-
Conference Proceedings Citation Index – Science (Clarivate Analytics; via the Web of Science), 1990 to June 2019.
CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; NHS EED, NHS Economic Evaluation Database.
The search was then limited to studies published from 2011 onwards, the search date of a previously published systematic review of surveillance strategies for melanoma. 25 There were no restrictions according to language or publication status. The search strategy used in MEDLINE can be found in Appendix 1.
We did not update the systematic review authored by Cromwell et al. ;25 instead, we used it to identify publications prior to 2011. To reduce the screening burden of systematically searching from database inception, we screened all studies included in this review. 25 Full references of the review were also screened, in addition to the results of the systematic search limited to studies published from 2011 onwards.
A grey literature search plan was developed to complement this search by exploring (1) grey literature databases, (2) targeted websites and (3) reference leads from (1) and (2), with a focused attempt to locate international and national guidelines. By checking the references of international guidelines and the studies cited in a review by Cromwell et al. 25 extended out search to before the 2011 limit described above. The sources described in Box 3 were searched between 20 July 2018 and 10 September 2018.
-
OpenGrey (www.opengrey.eu/; accessed 17 April 2019, includes SIGLE, EAGLE, GreyNet).
-
ClinicalTrials.gov (accessed 17 April 2019).
-
Cancer Research UK (www.cancerresearchuk.org/about-cancer/find-a-clinical-trial; accessed 17 April 2019).
-
BAD (http://www.bad.org.uk/; accessed 17 April 2019).
-
British Skin Foundation (www.britishskinfoundation.org.uk/; accessed 17 April 2019).
EAGLE, European Association for Grey Literature Exploitation; SIGLE, System for Information on Grey Literature in Europe.
Titles and abstracts of search results were imported into EndNote [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated.
Inclusion and exclusion criteria
We based inclusion and exclusion criteria on the population, intervention, comparator, outcomes, timing and setting (PICOTS) formula, as outlined in the following sections. 80
Population
Adults aged ≥ 18 years treated for AJCC (eighth edition) stage I cutaneous melanoma [stage IA (≤ 0.8 mm thick without ulceration) or stage IB (< 0.8 mm thick, or < 1 mm thick and ulcerated skin)]. 19
Or:
Adults aged ≥ 18 years treated for AJCC (seventh edition) stage I cutaneous melanoma [stage IA (T1a ≤ 1 mm thick) or stage IB (T1b with ulceration or mitoses ≤ 1 mm thick, or T2a 1.01 to 2.00 mm thick and no ulceration)]. 20
Non-randomised studies reporting patients with varying stages of cutaneous melanoma were included if ≥ 80% cases were at stage I, as we expected data to be sparse and did not want to omit potentially relevant evidence. Studies that did not specify a patient population were initially included pending confirmation from study authors. However, none of these met the inclusion criteria; therefore all were excluded. Studies reporting the Breslow depth, for patients with tumours of ≤ 2 mm, were included if there were no data on AJCC stage. Studies that included only patients with stage II–IV melanoma were excluded.
Intervention
We included studies that had any surveillance or follow-up strategies aiming to identify further primary melanoma, local recurrence or in-transit, regional or distant metastases. These were not limited by setting or by the type of clinician undertaking the follow-up. They could include clinical evaluation, patient education, skin self-examination (SSE) or radiological examination at any frequency. We excluded studies that focused on treatment of melanoma rather than surveillance.
Comparator
Studies with any comparator that allowed for the assessment of relative clinical effectiveness were eligible for inclusion (i.e. no surveillance or an alternative strategy).
Outcomes
The following were the outcomes of interest: overall survival, progression-free or recurrence-free survival, melanoma-specific survival, detection of recurrence as a new primary tumour, in-transit metastases and locoregional metastases. This could be presented as dichotomous or time-to-event data, such as percentages, hazard ratios (HRs), risk ratios (RRs) or Kaplan–Meier plots. No restrictions were placed on how outcomes were determined or confirmed (e.g. through biopsy, histology or imaging); all study-defined outcomes were allowed.
Timing
The timing for onset of surveillance strategies was restricted to patients who were post resection of any primary cutaneous melanoma tumours. The duration of surveillance (follow-up) was determined by individual studies and interpreted accordingly.
Setting
All studies were eligible for inclusion, regardless of whether the study was conducted in primary, secondary or tertiary care. No restrictions were applied to countries of origin conducting the primary research, although the relevance to current or future UK practice was assessed.
Study designs
We included RCTs and non-randomised comparative studies, for example quasi-experimental and comparative retrospective or prospective observational studies. We also looked at guidelines that recommended strategies for surveillance of stage I melanoma so that we could search their references for eligible studies. We excluded potentially underpowered non-RCT studies (arbitrarily defined as having < 100 patients) because they are at risk of selective reporting bias and publication bias, and they lead to small-study effects with imprecision.
To minimise selection bias in non-randomised study designs, we included studies that used statistical adjustment for baseline case mix using multivariate analyses, provided that the study had at least 80% stage I patients. We expected to see variables such as age, sex, ethnicity, tumour stage and grade, histology or performance status as adjustment variables; we excluded comparative observational studies if they did not adjust for at least two of these variables.
Data collection
Selection of studies
Selection of studies that met the inclusion criteria was conducted in two stages. In the first instance, studies were exported from the EndNote library and into Rayyan (Qatar Computing Research Institute, Doha, Qatar), a web-based tool designed to aid screening and selection of studies for systematic reviews. 81 For consistency and accuracy, two sets of two reviewers initially piloted the screening process. This was done by assessing 10% of the titles and abstracts, along with some full text studies, against the prespecified inclusion and exclusion criteria. Disagreements at this stage were resolved by either discussion between the reviewers or arbitration with another member of the study team. In the second stage, studies that appeared to meet the inclusion and exclusion criteria were imported into EndNote and full-text papers obtained. When full texts were not readily available, we accessed articles via interlibrary loans. Two reviewers independently evaluated these articles and made their selection in accordance with the eligibility criteria.
Data extraction
A data extraction form was created in Microsoft Word (Microsoft Corporation, Redmond, WA, USA) in accordance with Cochrane guidelines82 and piloted on one study prior to use. After necessary adjustments, one reviewer undertook the data extraction of the included articles. The completed extraction form was checked for accuracy, completeness and consistency by a second reviewer. When stage I data were grouped with other stages of disease in an included study, we contacted the study authors, which led to us obtaining the relevant data for patients with stage I disease. We also contacted authors to obtain missing data or to clarify uncertainties. The following domains were extracted: country of origin, patient characteristics, study objectives, study design, tumour characteristics, follow-up regimens, analysis methods, risk of bias, outcomes and conclusions. An example of the data extraction form can be found in Report Supplementary Material 1.
When possible, all data extracted were those relevant to an intention-to-treat analysis, in which participants were analysed in the groups to which they were assigned. The time points at which outcomes were collected and reported were recorded.
Guidelines that recommended strategies for surveillance of stage I melanoma did not form part of the systematic review. However, to provide context, they were summarised and their conclusions are presented in Summary of review of different surveillance strategies.
Risk-of-bias assessment in included studies
We used the Cochrane Collaboration’s ‘Risk-of-bias’ tool, RoB 2.0, which uses signalling questions to assess risk-of-bias judgements. 83 The tool examines five domains graded as being at a low risk-of-bias, of some concern or at a high risk of bias, from which an overall risk-of-bias judgement can be made. The domains considered are biases resulting from the randomisation process, deviations from the intended intervention, missing outcome data, measurement of outcomes, and selection of reported results.
Measures of effect
We planned to extract the following reported measures of effects of surveillance:
-
dichotomous or binary data, odds ratios (ORs) or RRs or percentages
-
time-to-event data, HRs or Kaplan–Meier plots.
Confidence intervals for all estimates missing data
We set out to report the number (per cent) of missing data for all variables/outcomes. We did not impute missing outcome data for any of our specified outcomes.
Data analysis
We summarised the characteristics of the surveillance strategies from included studies and guidelines in a table and provided a narrative summary of these.
We planned to conduct random-effects meta-analyses to pool data for each outcome in the review. In the absence of sufficiently robust or similar studies for a meta-analysis, we carried out a summary of studies, rather than a more formal narrative synthesis, owing to a lack of evidence.
Quality of the evidence using the GRADE approach
The GRADEpro tool was used to assess the overall certainty in the body of evidence for key outcomes. 84 The GRADE approach uses the risk of bias of individual studies, along with characteristics such as the imprecision and inconsistency of their results, to produce an overall estimate in terms of whether there is high, moderate, low or very low confidence that the systematic review estimates the true effect. These data are presented as a summary of findings table (see Table 6).
Results
Number of studies identified
The searches retrieved 10,723 citations in total; 10,592 were retrieved from the electronic databases, 104 from the published systematic review25 and 27 from the grey literature and guidelines search (Figure 3). After deduplication, 6205 references remained. Following a title and abstract sift by one reviewer and two clinicians, 6134 references were excluded, resulting in 33 citations of articles and conference abstracts for full-text assessment.
FIGURE 3.
The PRISMA flow diagram for the review of surveillance strategies’ clinical effectiveness.

Following this, we excluded 31 articles. The reasons for excluding the full-text papers were as follows: < 80% of participants at stage I or wrong stage (58%), and studies identified as prognostic studies (26%) diagnostic studies (10%) or prevention studies (6%). In addition, all included studies from the Cromwell et al. 25 systematic review were excluded because they were not surveillance strategies among individuals post resection of a stage I melanoma, and most included studies had a non-comparative design. 25 A list of excluded full-text articles retrieved from the literature search, with reasons for exclusion, is provided in Appendix 2.
Authors of relevant studies presenting aggregated data were contacted to provide data stratified by stage. Correspondence was received from Robinson et al. 85 and Damude et al. ;23 however, only Robinson et al. 85 provided data that fulfilled our inclusion criteria.
Characteristics of included studies
One RCT met our inclusion criteria85,86 after provision of further data by the authors. Robinson et al. 85 assessed the frequency of SSE by patient–partner dyads. The study was conducted in the USA. 85 The study included a total of 494 participants with a mean age of 55 years [standard deviation (SD) ±10 years, range 18 to > 70 years). Descriptive information of the study is presented in Table 4.
| Study (first author and year of publication) | Design | Country, setting | Intervention strategy | Control strategy | Number of patients at baseline and stage | Patient median age in years) (range) | Patients at stage I, n (%) | Duration of follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Robinson et al. 201685 | RCT, from June 2011 to April 2013 |
|
Skin self-examination with a partner after a structured skills training intervention (dyads) | Customary care of patients with partners (dyads) |
|
|
|
|
Patients with stage 0–IIB melanoma participated in the trial from June 2011 to April 2015. This was a continuation of the trial initially reported by Turrisi et al. 87 Patients in the intervention arm received a structured skills training intervention, whereas patients in the control arm received customary care.
A total of 494 patients and their partners were randomised to one of four groups. Three of the dyad groups received a structured skills training intervention in SSE, either in person, from a written workbook or via a tablet. The fourth group served as control and received treatment as usual87 and customary education. 85 Patients were seen by a dermatologist every 4 months. The primary outcome was frequency of SSE by patient–partner dyads, and the follow-up period and end point of the trial was 24 months. The secondary outcome was detection of a new or recurrent melanoma by the dyad or physician.
Patients at stages 0–IIB receiving the intervention had significantly increased SSEs with their partners at 4 months, compared with controls [mean difference 1.57, 95% confidence interval (CI) 1.29 to 1.85]. Mean differences at 12 and 24 months were lower (mean difference 0.72, 95% CI 0.39 to 1.06, and mean difference 0.94, 95% CI 0.58 to 1.30, respectively). Overall, data reported for stages 0–IIB showed that the intervention was successful in increasing SSE by patient–partner dyads, compared with controls at 24 months (mean difference 0.94, 95% CI 0.58 to 1.30; p < 0.001). We contacted the authors for data by disease stage, which were provided by them for our analyses.
The individuals undertaking surevillance (through SSE) of the outcome of interest to our review (detection of a new primary tumour, recurrent melanoma or metastases) were both the dyad and physician, with the site of surveillance being either the home or the care setting of the dermatologist (predominantly a secondary care setting). The interval timing of the review appointments with the dermatologist were 4 months; however, for surveillance by the dyad, the interval timing was dependent on their own timeline of use of SSE. The duration of follow-up for the surveillance in the trial, and thus by dermatologists, was 2 years. However, the SSE by the dyads was intended to last for life. There was no routine imaging involved in the surveillance strategy; rather, the strategy was based on a structured skills training intervention on how to self-identify plausible new or recurrent melanoma. Given that the study reported that SSE increased, it has been assumed that this surveillance stratgey is well accepted by melanoma patients. There is no large burden on health-care providers due to surveillance by SSE.
Risk-of-bias assessment of included studies
It was judged that there were ‘some concerns’ regarding the risk of bias in the study by Robinson et al. 85 Two allied papers were used to identify data pertinent to the risk-of-bias assessment. 86,87 The results are discussed in the following sections, and the judgements made using the Cochrane risk-of-bias tool, RoB 2.0,83 are shown in Table 5.
| Study (first author and year of publication) | Risk of bias | |||||
|---|---|---|---|---|---|---|
| Randomisation processa | Deviation from intended interventionsb | Missing outcome datac | Measurement of outcomesd | Selection of reported resultse | Overall bias | |
| Robinson et al.85 2016 | Some concerns | Low | Some concerns | Low | Low | Some concerns |
Bias from randomisation process
Reviewers considered the randomisation process as giving rise to some concerns. 85 There were baseline imbalances in the number of participants assigned to each of the intervention arms. The first 150 pairs were randomised to one of the three groups (workbook, in person or control), and the remaining 344 pairs were randomised to one of four groups (workbook, in person, tablet or control). 85
Bias from deviations of intended interventions
The study was judged to be at a low risk of bias. No deviations from the intended interventions were reported. However, an additional intervention (using a tablet computer) was added while recruitment was ongoing.
Bias due to missing outcome data
The study was judged to give rise to some concerns regarding incomplete outcome data. 85 This related to high and varying levels of attrition between trial arms. Reasons for non-participation were reported and there did not appear to be any notable differences between those completing the 24 months’ assessment and those lost to attrition by demographic characteristics, initial melanoma diagnoses or time since diagnosis, as reported in the study results. 85 Reasons for not attending follow-up were reported as ‘not learning anything new,’ ‘no change in pigmented lesion’ and ‘too far to travel’. Attrition reduces the ability of the study to detect a difference, should one exist.
Bias in selection of reported results
The study was assessed as having a low risk of selective reporting because the trial protocol was available as a trial registration on ClinicalTrials.gov and as a peer-reviewed manuscript. All prespecified outcomes were reported in the results.
Publication bias
We were unable to assess whether or not there was any publication bias because there was only a single study was included. However, we carried out comprehensive searches to reduce the risk of missing relevant studies.
Assessment of clinical effectiveness
Although three publications (including a conference abstract) reporting two RCTs were eligible for this systematic review,23,85,88 they did not report data for stage I patients separately. Authors were contacted to provide further data, which we obtained for stage I melanoma patients from the authors of the study by Robinson et al. 85
We were unable to conduct a meta-analysis as only one RCT met the inclusion criteria. We were unable to assess reporting biases using funnel plots or to conduct any subgroup or sensitivity analyses. Thus, data on effectiveness and safety from the included RCT were tabulated and presented in the summary of findings table (Table 6) and narratively summarised. For outcomes of interest, we have calculated and reported the magnitude of effect.
| Outcomes | Relative effect (95% CI) | Number of participants (n studies) | Quality of the evidence (GRADE) | Comments |
|---|---|---|---|---|
|
RR 0.75 (0.43 to 1.31) | 258 (1 study) | ⊕⊕⊝⊝ Low12 |
|
| Overall survival, progression or recurrence-free survival and detection of new primary melanoma, recurrence or metatstases were not reported for stage I disease by any study | ||||
| One ongoing multicentre trial also met the inclusion criteria.23 The trial was conducted in six hospitals comparing a reduced follow-up schedule with the conventional schedule in 180 patients with stage IB to IIC cutaneous melanomas over a period of 1 year. Results at 3 years are available in abstract form only and we did not receive a breakdown by stage I on request. The primary end point of this trial was patient well-being; secondary end points were development of recurrence, second primary melanoma or metastases | ||||
The primary outcome of the study by Robinson et al. 85 was the frequency of SSE by patient–partner dyads. The secondary outcome (among those post resection of stage 0–IIB melanoma) was detection of a new or recurrent melanoma by the dyad or physician. For those post resection of stage I melanoma, the population of interest in this review, data were provided by the study’s author. New primaries, recurrences or metastases were detected in 49 out of 258 (19%) patients with stage IA or IB melanomas post resection of a primary melanoma followed up for up to 24 months. Data were not split by whether the disease was a new primary or recurrence, and recurrences could be at different stages from the original primaries. The types of melanomas identified were melanoma in situ, stage IA, superficial spreading, lentigo maligna and melanomas of ≥ 0.1 mm.
There was no evidence of a difference between intervention and control arms in the proportion of patients with stage IA or IB melanomas in which a new primary or recurrence was detected in this subset (RR 0.75, 95% CI 0.43 to 1.31). However, imprecision affects our certainty of this finding and more evidence is needed to draw any conclusions.
The authors85 concluded that patients with melanoma and their partners reliably performed SSE after participating in a structured skills training programme lasting approximately 30 minutes, with reinforcement every 4 months by the study dermatologist. No conclusions were drawn by the study authors85 about how new primaries or recurrences were detected.
Discussion
Summary of review of different surveillance strategies
This review sought evidence about the relative effectiveness of surveillance and follow-up strategies to identify melanoma recurrence, new primary tumours and metastases in stage I cutaneous melanoma patients following surgical excision of the primary tumour. Only two RCTs (reported in three papers)23,85,88 were eligible and we could obtain data on stage I patients from only one of them. 85 This study suggested that an educational intervention for patients and their partners improved self-identification of new primaries, regardless of whether it was delivered in person, through a workbook or via a tablet. However, among the subset of author-provided data on patients post resection of a stage IA or IB melanoma, there was no evidence of a difference in detection of a new primary tumour, recurrence or metastases between those undergoing SSE surveillance and those receiving usual follow-up.
This evidence is of low certainty according to GRADE because of the small number of studies and limited number of available relevant outcome data;86 it is probable that the results of this review would change with the addition of new eligible studies. The certainty of the evidence was downgraded for imprecision, sparse data and a low event rate and for concerns regarding risk of bias. At present, evidence is based on just a single study85 (n = 258 participants) and the evidence is incomplete and offers only internal validity as it was set in the USA. Only one of the prespecified outcomes (new primary or tumour recurrence) in our review was reported, meaning that there are complete gaps in the evidence in this area in terms of overall survival, progression-/recurrence-free survival and detection of recurrence (see Table 6).
As stage I is the most common stage at melanoma diagnosis, it is critical to understand the most effective method of surveillance following treatment. This review demonstrates that current evidence is insufficient and uncertain, so further robust RCTs are required, measuring recurrence and metastases, in addition to overall survival, as outcomes, to establish the most effective surveillance strategy. No assessment of surveillance strategies among those post resection of a stage I melanoma using clinical review, imaging, or diagnostic biopsy as the main component of the strategy were identified.
Strengths and limitations
To our knowledge, this is the first systematic review of surveillance or follow-up strategies for AJCC stage I melanoma. The review followed procedures set out by the Cochrane Collaboration for conducting systematic reviews of RCTs and non-randomised studies, and was robust. 82 We conducted comprehensive searches of bibliographic databases and grey literature. All stages of the review, involving screening, data extraction and assessment of risk of bias, were conducted by at least two researchers, either in duplicate or by one researcher with checks by a second researcher. We assessed the risk of bias using the Cochrane Collaborations’s RoB 2.0 tool. 83 We contacted authors from both studies to provide further information on participants’ staging of melanoma and obtained data for stage I patients from one study. 85 We excluded the majority of potentially relevant primary full-text articles because they were non-comparative or did not assess surveillance strategies.
Because the single included study85 was conducted in a single country, the USA, the findings could be limited in applicability and generalisability. The assessment of risk of bias revealed an overall judgement of ‘some concerns’ due to attrition in the trial at 24 months. 85 Publication bias could not be investigated because of the number of studies identified, but the possibility should be considered as there may be studies that did not find positive results and remain unpublished.
Impact and implementations
Evidence for the effectiveness of surveillance and follow-up strategies for stage I melanoma is limited. A previous systematic review sought to identify the range of stage-specific surveillance practices for melanoma patients (any stage) and concluded that surveillance strategies vary around the world during the first 5 years post treatment. 25 Our review had narrower inclusion criteria with respect to study design and staging of melanoma. The paucity of this evidence in our review makes it difficult to make recommendations regarding the effectiveness of surveillance and follow-up strategies for stage I melanoma in the UK.
Conclusion
This review demonstrates that evidence for the effectiveness of surveillance strategies is poor for stage I melanoma patients. We were able to obtain data specific to stage I patients from only one of two included studies. This study suggested that an educational intervention encouraging SSE by patients and their partners might be promising and effective overall in increasing SSE and detection of new or recurrent disease by the patient–partner dyads. However, for patients with stage I disease, there was little evidence of benefit of the intervention, compared with control, for detecting new or recurrent disease.
The findings of this review are not wholly unexpected, given the assessment of the existing guidelines for surveillance of stage I disease presented in Chapter 2. What the work presented in both this chapter and Chapter 2 illustrates is the paucity of data on which existing strategies are based on and it raises questions as to whether or not, or how, alternative strategies may be better than current practice. The following chapters go on to consider whether or not alternative strategies for surveillance could be developed. Chapter 4 begins this process by considering the evidence base for approaches to identify those people with stage I disease who might be more at risk of recurrence and, consequently, where there may be more merit in adopting a more intensive surveillance strategy, rather than a less intensive strategy.
Chapter 4 A systematic review of the prognostic accuracy of risk models used for the prediction of recurrence, new primary tumours or metastasis for patients with American Joint Committee on Cancer stage I melanoma following surgical excision of a primary cutaneous tumour
Brief overview
A risk prediction model is a statistical tool that uses multiple predictors to estimate the absolute probability or risk that a certain outcome will occur in an individual with specific risk factors. 89 Great advances in the earlier detection of melanoma have been achieved through increased public awareness, the adoption of dermatoscopic examinations and a rapid ‘2-week wait’ referral system in the UK. 18 There is also widespread belief that earlier detection of metastatic disease results in improved overall patient outcomes. 90 At present, however, there is no internationally accepted standardised model of follow-up of patients diagnosed with AJCC stage I cutaneous melanoma, with wide variations in care across North America, Australia, Europe and the UK. 25 Although the surgical excision of a primary melanoma is effective and long established, there has been a rapid pace of change recently with the addition of earlier investigatory techniques such as SLNB91,92 and various radiological modalities,93 and a raft of advances in the treatment of metastatic disease. 94–96 However, a structured, uniformly adopted, evidence-based model of patient follow-up after initial diagnosis is lacking. Current guidelines vary across the world, with most high-income countries using anecdotal evidence and expert opinion. 53 These are usually underpinned by the assumption that earlier detection of metastatic disease results in improved overall outcome. However, they often do not take a wider, holistic view of the patient pathway to identify a model that incorporates all of the elements used in the diagnosis and management of the condition. Thus, they may fail to adequately capture physical, psychological consequences and the costs of such strategies.
Although systematic reviews of predictive models for primary cutaneous melanoma have been conducted,97,98 to date, to our knowledge, none has investigated the potential of models to predict recurrence, new primary tumours and metastases of AJCC stage I melanoma. This work seeks to systematically review and pool the evidence of the various elements that underpin an ideal model of follow-up, thus allowing others to make recommendations on future care models for AJCC stage I melanoma in the UK. With the rapid increase in melanoma rates, it is paramount that the UK develops a robust, evidence-based model of follow-up care for the majority of affected patients, namely patients with AJCC stage I disease.
Research aim
This review aimed to:
-
identify all studies of prognostic risk models for recurrence of melanoma among AJCC stage I survivors
-
determine the performance of prognostic risk models (external validation, including discrimination and calibration) used to determine the risk stratification of patients with AJCC stage I melanoma after surgical excision of primary cutaneous tumour.
Methods
The study adheres to the guidelines for the PRISMA statement to ensure transparency of the process. 78 As with the review reported in Chapter 3, details of this review are published in the study protocol published on PROSPERO (CRD42018086784). 79
For this review, we planned to assess the prognostic accuracy of the biochemical and biophysical biomarkers and risk models used (alone or in combination) for the prediction of recurrence, new primary tumours or metastasis for patients with AJCC stage I melanoma following surgical excision of primary cutaneous tumour. However, following expert advice of methodological and clinical experts, we modified the objectives to focus on the accuracy of risk prediction models only. This change was necessary to make the review feasible, as a larger number of studies may have made completion of the study impossible.
Search strategy
The search strategy was designed by an experienced information specialist, in collaboration with the project team. The search was designed in MEDLINE (via Ovid) according to the following concepts: [melanoma] AND [risk models] AND [prognosis]. A published and validated prognostic study filter was used. 99 The strategy used database-specific thesaurus headings, along with title and abstract keywords, with appropriate use of stemming for alternative word endings, alternative spelling and plurals. The search strategy was translated to the databases listed in Box 4. No restrictions were applied according to language or country.
-
MEDLINE (via Ovid).
-
EMBASE (via Ovid).
-
CENTRAL (the Cochrane Library via Wiley Online Library).
-
Health Technology Assessment Database (the Cochrane Library via Wiley Online Library).
-
CINAHL (via EBSCOhost).
-
Science Citation Index (via the Web of Science).
-
Conference Proceedings Citation Index – Science (via the Web of Science).
-
Cochrane Database of Systematic Reviews (the Cochrane Library via Wiley Online Library – to check included studies of relevant reviews).
-
Grey literature was sought using similar keywords to search various resources including, but not limited to, the following:
-
OpenGrey (www.opengrey.eu/; accessed 17 April 2019, includes SIGLE, EAGLE, GreyNet).
-
Cancer Research UK (www.cancerresearchuk.org/about-cancer/find-a-clinical-trial; accessed 17 April 2019).
-
Melanoma UK (www.melanomauk.org.uk; accessed 17 April 2019).
-
National Guideline ClearingHouse (www.guideline.gov/; accessed 17 April 2019).
-
-
Ongoing trials identified using the WHO’s ICTRP platform of trials registries. (www.who.int/trialsearch; accessed 17 April 2019).
CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; EAGLE, European Association for Grey Literature Exploitation; ICTRP, International Clinical Trials Registry Platform; SIGLE, System for Information on Grey Literature in Europe; WHO, World Health Organization.
An example of the search strategy used in MEDLINE can be found in Appendix 3.
Supplementary searches were limited based on the development and uptake in practice of SLNB in melanoma as a new way of diagnosing and managing patients. The first evidence of its routine use in some of the larger melanoma centres in the UK, as well as in routine practice in the USA, was around 2000. 100 We, therefore, limited the subsidiary search criteria to this date.
In addition to the databases and resources reported in Box 5, guidelines and other subsidiary journal content were handsearched and references of relevant publications were searched. This supplemented the structured documented searches, aiming to ensure that relevant studies were not overlooked as a result of selective, poorly or inaccurately indexed, or unindexed content.
-
MEDLINE (via Ovid), 1946 to 2 July 2019.
-
EMBASE (via Ovid), 1980 to 2 July 2019.
-
CENTRAL (the Cochrane Library via Wiley Online Library), issue 6, 2019.
-
CINAHL (via EBSCOhost), 1981 to July 2019.
-
Scopus® (Elsevier, Amsterdam, the Netherlands).
-
Conference Proceedings Citation Index – Science (via the Web of Science), 1990 to July 2019.
-
Cochrane Database of Systematic Reviews (the Cochrane Library via Wiley Online Library), issue 6, 2019.
-
Science Citation Index (via Web of Science — to include Conference Proceedings), 1990 to July 2019.
CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Inclusion and exclusion criteria
We based inclusion and exclusion criteria on the PICOTS formula, as outlined in the following sections. 80
Population
Adults aged ≥ 18 years treated for AJCC (eighth edition) stage I cutaneous melanoma [stage IA (≤ 0.8 mm thick without ulceration) or stage IB (< 0.8 mm thick, or < 1 mm thick and ulcerated skin)]. 19
Or:
Adults aged ≥ 18 years treated for AJCC (seventh edition) stage I cutaneous melanoma [stage IA (T1a ≤ 1 mm thick) or stage IB (T1b with ulceration or mitoses ≤ 1 mm thick, or T2a 1.01 to 2.00 mm thick and no ulceration)]. 20
Studies that combined patient populations (e.g. all stages of disease) were included only in cases for which it was specified that the test/data also applied to stage I cases. Studies that did not specify a patient population were included in the first instance, pending confirmation from study authors, when possible.
Types of prognostic models
We assessed all prognostic or predictive models used to predict the likelihood of recurrence (any site) or metastasis or survival in patients with stage I melanoma. 89 For a definition of a prognostic model, see Brief overview.
Outcomes
Studies were included that presented the predictive accuracy of the risk model in relation to recurrence, metastasis and survival using statistical measures:101
-
discrimination: ability to differentiate between high and low risk
-
calibration: agreement between observed and predicted risk
-
overall performance: a combination of discrimination and calibration.
Timing
The application of the model had to have been post resection of the primary cutaneous tumour. The timing will be dictated by the duration of included studies and interpreted accordingly.
Setting
All studies were eligible for inclusion, regardless of whether the study was conducted in primary, secondary, or tertiary care.
Study design
Studies were included if they:
-
used statistical methods to present or validate (external) models used to:
-
predict melanoma outcomes of interest [minimum of two predictors of outcomes, e.g. Breslow depths, location of tumour, type of recurrence (local, regional, distant), age, sex]
-
group patients based on their risk of developing such outcomes (risk prediction models).
-
-
were validated – evaluated to determine the reproducibility of a developed prediction model for the derivative sample and prevent overinterpretation of current data either:101
-
internally – data for model development and evaluation are both random samples from the same underlying population
-
externally – predictions are calculated from the previously developed model and tested in new data that are different from the development population (e.g. from another hospital).
-
Data collection
Selection of studies
The selection of studies that met the inclusion criteria was conducted in two stages. Studies were exported from the EndNote library and into Rayyan. 81 For consistency and accuracy, two sets of two reviewers initially piloted the screening process by assessing 10% of the studies based on the titles and, when available, abstracts and some full texts against the prespecified inclusion and exclusion criteria. Disagreements at this stage were resolved either by discussion between the reviewers or with arbitration from another member of the study team. In the second stage, studies that appeared to meet the inclusion and exclusion criteria were imported into EndNote and full-text papers were obtained. When full texts were not readily available, we obtained articles via interlibrary loans. Two reviewers independently screened these articles and made their selection in accordance with the eligibility criteria.
Data extraction
In pairs, four reviewers independently extracted data according to the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS). 102 The following data were extracted: source of data, participants, outcomes, predictors, model development methods, model performance and validation. The completed extraction forms were independently checked for accuracy and consistency, with any disagreements resolved through discussion or by arbitration from another member of the team. An example of this checklist is available in Report Supplementary Material 1.
Risk-of-bias assessment in included studies
Working in pairs, the risk of bias of each included paper was assessed independently by one reviewer and was checked by a second reviewer. Disagreements were resolved through discussion or with arbitration from a third member of the study team. Studies were assessed using the Prediction model Risk Of Bias ASsessment Tool (PROBAST), which addresses four domains that may influence the applicability of the prediction models (participants, predictors, outcome and analysis). 103
Missing data
We set out to report the number (per cent) of missing data for all variables/outcomes. We did not impute missing outcome data for any of our specified outcomes.
Data analysis and synthesis
We narratively synthesised the predictive performance of the prediction models by analysing the statistical measures of predictive performance. Models were assessed for discrimination, calibration and overall performance from studies providing sufficient data. The review aimed to pool the evidence of model performance by performing a meta-analysis when possible.
Quality of the evidence using GRADE approach
We had planned to assess the overall quality of evidence for key outcomes using the GRADEpro tool;84 however, it was not developed for, and the evidence suggests it performs poorly for, prediction modelling studies. 104 Given this, we decided not to use the GRADE approach.
Results
Number of studies identified
Our search identified 25,251 records from the electronic databases. After deduplication, 20,878 records remained; following screening of the titles and abstracts, 112 full texts of potentially relevant articles were retrieved for examination. The PRISMA flow diagram outlines the study selection process and the reasons for exclusion (Figure 4). A total of 11 articles reporting 11 different risk prediction models met the full inclusion criteria of this review. 105–115 A total of 101 studies were reviewed fully and excluded for the following reasons: used single prognostic factors for model development (21%), combined stages of the disease (28%) or were not validated (51%). Details of the excluded studies are presented in Appendix 4.
FIGURE 4.
The PRISMA flow diagram for the risk prediction model review.
Characteristics of included studies
A summary of the studies and patient characteristics is presented in Table 7. Eight of the studies were conducted in the USA,106–109,111,113–115 one in the UK,112 one in Australia105 and one in Italy. 110 Seven studies used a retrospective cohort design,105–109,112,115 three used a prospective design111,113,114 and one used a retrospective cohort of prospectively collected data. 110 Patient data for development and validation of these models were taken from cancer registries, AJCC melanoma databases, clinical data from patients diagnosed and treated for melanoma or a combination of one of these sources.
| Study (first author and year of publication) | Study design | Country | Statistical methods | Data source | Time period | Follow-up time | Participants |
|---|---|---|---|---|---|---|---|
| Baade et al.105 2015 | Retrospective cohort | Australia | Multivariable probit regression model | Population-based Queensland cancer registry | 1995–2008 | Up to 16 years; median 7.2 years (86.4 months) | n = 28,654
|
| Balch et al.106 2001 | Retrospective cohort | USA | Cox proportional hazards regression model | Prospective population-based databases from 13 institutions merged to form the AJCC Melanoma Database | NR |
|
|
| Cochran et al.107 2000 | Retrospective cohort (consecutive) | USA | Cox proportional hazards regression model |
Subset of John Wayne Cancer Institute Melanoma clinical database, Division of Surgical Oncology, UCLA Random sampling of patients into two equal groups: |
1980–90 | Total database: median 42.5 months (range 1–26.5 years) | n = 1042 |
| Gimotty et al.109 2004 | Retrospective cohort (consecutive) | USA | A recursive partitioning algorithm used in classification and regression tree |
Development set: population-based SEER registry Validation set: new SEER patients seen 1991–5 |
|
At least 10 years |
|
| Gimotty et al.108 2007 | Retrospective cohort | USA | Recursive partitioning to develop classification trees in development and validation sets |
|
|
|
|
| Maurichi et al.110 2014 | Retrospective cohort of prospectively collected data | Italy: six European centres | Multivariable Cox regression |
|
1996–2004 | Median follow-up:
|
N = 2243
|
| Rosenbaum et al.111 2017 | Prospective cohort. Note that data were not split into discovery and test cohorts, because disease recurred in only 63 stage IB patients | USA | Linear regression analysis, Cox proportional hazards regression model, area under the receiver operating characteristic curve |
|
August 2002–May 2014 | Median 4.4 years | N = 655
|
| Saldanha et al.112 2018 | Retrospective cohort (consecutive) | UK | Cox proportional hazards regression model and Kaplan–Meier survival plots. Models compared using Akaike information criterion |
|
|
Median 71 months (5.9 years) | N = 1329
|
AJCC (seventh edition):
|
|||||||
| Soong et al.113 2010 | Prospectively observed cohort | USA | Multivariate analysis based on the Cox regression model |
|
|
NR | N = 25,734
|
Breslow thickness (mm):
|
|||||||
|
|||||||
| Tsai et al.114 2007 | Prospective cohort study | USA | Survival tree | Registry data: AJCC population-based Melanoma Database | NR | NR | N = 13,268
|
| Vollmer and Seigler115 2001 | Retrospective cohort | USA | Cox proportional hazards regression model | University Melanoma Clinic | Late 1980 to early 1990s | Median follow-up 7.6 years | NR |
Each study presented a model that could be used to predict either recurrence, metastasis or survival. The studies concerned the development of a risk or prognostic score,107,115 a nomogram,110 the Melanoma Severity Index model,105 a novel histopathological classifier,111 an electronic prediction tool,113 a classification tree,114 prognostic trees,108,109 a model focused on adding a new predictor to an established model112 and a model used to validate AJCC staging. 106
Outcome definitions and follow-up times varied across studies. The median time of follow-up ranged from 42.5 months107 to 10.3 years. 110 All studies reported outcome measures of survival. Seven studies defined survival as patients who were alive at last follow-up or who died without evidence of melanoma. 105,106,108–110,112,113 Two studies defined survival as the number of patients who are alive after diagnosis. 107,111 The other studies did not provide a definition of survival. 114,115 Risk stratification for predicting overall survival was reported in nine studies. 106–114 Patients were reportedly grouped according to tumour thickness,106,113 ulceration status,114 melanoma-specific death or survival,107,108,110,111 growth phase lesions109 or Breslow depth. 112 Two studies measured the risk of recurrence, defined and stratified either as recurrence for individual melanoma patients at different points after initial treatment107 or as local recurrence (a recurrence in the scar at the primary site). 109 Gimotty et al. 109 classified and stratified patients with local recurrences as:
-
type 1 – with radial growth phase (RGP)
-
type 2 – with RGP and vertical growth phase
-
type 3 – without RGP.
Another study111 measured recurrence-free survival, defined as the time from diagnosis to the first recorded date of regional or distant metastases. Two studies109,110 provided outcome measures for metastasis. Gimotty et al. 109 defined metastasis as regional metastasis (in-transit dermal or subcutaneous metastases and/or nodal involvement). Maurichi et al. 110 did not provide a definition of metastasis.
Characteristics of included models
Most of the studies used regression methods for building the models. Characteristics of the models are presented in Table 8. Seven studies used the Cox proportional hazards method. 106,107,110–113,115 Two studies used a recursive partitioning algorithm used in classification and regression tree analysis. 108,109 Tsai et al. 114 used the survival tree analysis method. Baade et al. 105 used the probit regression method.
| Study (first author and year of publication) | Predictors in final model | Model performance | Validation | |||
|---|---|---|---|---|---|---|
| Discrimination | Calibration | Overall performance | Internal | External | ||
| Baade et al.105 2015 | MSI: n = 7
|
|
Not reported | Explained variation: RD2 statistic: 0.47 (95% CI 0.45 to 0.49) | Internal–external cross-validation across geographically defined subset | None |
| Balch et al.106 2001 | n = 8
|
Not reported | Not reported | Not reported | The melanoma patient data were used to validate the proposed AJCC staging system | None |
| Cochran et al.107 2000 | n = 5
|
Not reported | Not reported | Not reported | Patients randomly sampled into two equal groups: an estimation set and a test set | None |
| Gimotty et al.109 2004 | n = 11
|
|
Not reported | Not reported | New patients meeting study eligibility criteria between 1991 and April 1995 | None |
| Gimotty et al.108 2007 | n = 6
|
|
Not reported | Not reported | None | New patients |
| Maurichi et al.110 2014 | n = 8
|
Discrimination by adjusted Harrell’s c-statistic = 0.88 | Nomogram performance was assessed by calibration plot as an indicator of internal calibration | Not reported | Internal validation of nomogram by calibration of nomogram | None |
| Rosenbaum et al.111 2017 | n = 6
|
Patients classified using Youden Index of the ROC curve using digital area, conformation and baseline variables:
|
Not reported | Not reported | 10-fold cross-validation | None |
n = 3
|
||||||
| Saldanha et al.112 2018 | n = 2
|
Discrimination:
|
Calibration: perfect calibration in any validation set would be represented by a calibration slope of 1, and the slope in the validation cases was 0.88 (SE 0.12) | Not reported | None | Patients from Nottingham University Hospitals NHS Trust |
| Soong et al.113 2010 | n = 6
|
Not reported | Calibration: concordance correlation coefficients of 0.90 and 0.93 for 5- and 10-year survival rates | Not reported | None | Patients from Sydney Melanoma Unit, Australia |
| Tsai et al.114 2007 | n = 6
|
Not reported | Not reported | Measure for overall performance, captures both discrimination and calibration (Brier score). Brier score range: 0.02 at year 1 to 0.20 at year 15 for the propsed model (intergrated tree-based scheme) | Fivefold cross-validation | None |
| Vollmer and Seigler115 2001 | n = 3
|
Not reported | Not reported | Not reported | Cross-validation using data from another set by Stadelmann117 | None |
At model development stage, various methods were used across the studies to choose the variables used in the final model. Two studies used the backward procedure based on the Akaike information criterion, an estimate of the measure of the quality of available models as they relate to one another for a certain set of data. 110,112 The Akaike information criterion is used to determine what variables influence the prediction of an outcome of interest and how these variables influence the outcome by estimating several different regression models to balance the trade-offs between the complexity of a given model and its goodness of fit. 118 Two studies108,109 selected predictors based on evidence of previous validation studies and two studies107,111 performed a univariate analysis to select only the variables for which evidence of statistical significance was generated. The remaining studies did not report on the criteria used to select the candidate predictors. 105,106,113–115 The number of predictors in the studies ranged from 3115 to 11 predictors. 109 Various possible risk factors were identified, the most common being age, tumour site, tumour thickness, sex and ulceration. Other predictors identified included metastasis, mitotic rate, positive lymph node, Clark’s level, growth phase, RGP regression, microsatellites, anatomical level, presence or absence of lymphovascular invasion, tumour-infiltrating lymphocytes, histological subtype, conformation status and digital or manual area.
Risk-of-bias assessment of included studies
Table 9 summarises the risk of bias and concerns regarding applicability to the intended population and setting of the included studies. Assessments were conducted using the PROBAST. Overall, eight studies105,107,109–112,114,115 were judged to be at high risk of bias and three106,108,113 were rated as having an unclear risk of bias. Five studies106,108–110,113 were deemed to have a low risk of bias regarding applicability, another five105,111,112,114,115 were deemed to have unclear risk and one study107 was deemed to have high risk. Bias was introduced by various methods.
| Study | Risk of bias | Applicability | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | Risk of bias | Applicability | |
| Baade et al.105 2015 | – | ? | – | ? | – | + | – | – | – |
| Balch et al.106 2001 | – | ? | – | ? | – | + | – | – | – |
| Cochran et al.107 2000 | – | + | – | – | – | + | – | – | – |
| Gimotty et al.109 2004 | – | + | – | ? | ? | + | + | – | ? |
| Gimotty et al.108 2007 | – | + | – | ? | ? | + | + | – | ? |
| Maurichi et al.110 2014 | – | + | – | + | ? | + | + | – | ? |
| Rosenbaum et al.111 2017 | – | + | – | ? | ? | + | + | – | ? |
| Saldanha et al.112 2018 | – | + | – | ? | – | + | – | – | – |
| Soong et al.113 2010 | – | + | – | ? | – | + | – | – | – |
| Tsai et al.114 2007 | – | + | – | ? | – | + | – | – | – |
| Vollmer and Seigler115 2001 | – | + | – | ? | – | + | – | – | – |
Selection of participants
All studies were judged to be at a high risk of bias for this domain. All studies used existing data sources to develop or validate their models. Participant selection was based on retrospective105–110,112,115 and prospective111,113,114 cohort studies of cancer registries,105,106,108,109 AJCC databases,113,114 clinical databases,107,110,111,115 and hospital records. 112 As data are taken from existing sources, there is no information on how patients were selected and for what purpose. In model development and validation, bias may be introduced when routinely collected data are used, as opposed to data obtained from primary research. Existing data may not provide the full or accurate clinical features under investigation. 119
Two studies107,115 were also rated as being at a high risk of bias because of the particpants selected. In neither study is it clear how many participants had stage I disease, as per our review question, because very little detail was given on the participants in the study, and there was no summary of patient characteristics to judge the severity of their melanoma.
Risk of bias introduced by predictors
Nine studies107–115 were judged to have a low risk of bias for the selection of risk factors included in the final model. The risk factors considered for use in all the studies appear to be representative of the factors used primarily in melanoma. The study by Baade et al. 105 was rated as having an unclear risk of bias because there was an issue of missing data for 30% of the initial cohort. The study used a predictive mean matching multiple imputation approach for handling missing data to evaluate their effect on risk model estimation and the reliability of the predictions. This method assumes that the missing data can be replaced by the available data, as they are thought to be similar. In this case, as the data were reviewed retrospectively, it is unclear how similar the missing values and the replacement values are. Another study was also rated as having an unclear risk of bias because the information about some prognostic factors was not available consistently enough to include them in the prognostic factors analysis. 106
Risk of bias introduced by outcomes
All the studies were rated as having a high risk of bias for this domain. Follow-up times introduced bias in some of the studies. Six studies105,107,111,112,114,115 were rated as having a high risk of bias for outcome analysis because insufficient time was taken to follow up patients. The median follow-up times ranged from 3.5 years107 to 7.6 years. 115 In localised melanoma, this is too short to detect recurrence and death from melanoma; 10 years’ follow-up is generally considered sufficient for adequate patient evaluation. 113 Although the study by Soong et al. 113 reported a follow-up time ranging from 0 to 20 years, the risk of bias was minimised by identifying patients who were alive at the time of the last follow-up or who died without evidence of melanoma.
Although all model development or validation studies prespecified the outcome definition for overall survival, one of the studies110 failed to provide the definition of metastsis as an outcome. Overall, all studies were rated as having a high risk of bias because the data used were based on routinely collected data, meaning it is possible that different outcome definitions were used.
Risk of bias introduced by the analysis
All studies were rated as having an unclear risk of bias due to the analysis methods used. Six of the studies105,106,108,109,112,115 did not include all the enrolled participants in their analyses. This was because data were missing for at least 30% of the initial cohort,105 an unknown number of patients died106 or there were unexplained missing data. 108,109,112,115 Excluding some participants would likely introduce differences in the performance of a prediction model for predicting outcomes. Moons et al. 120 also suggest that determining the extent of bias in prediction models developed based on routine data can be problematic, often because of the lack of clarity in the elibility criteria. One study was rated as having an unclear risk of bias as a result of the inclusion of variables identified as significant following a univariate analysis. 111 As this method excludes risk factors that are considered non-significant for the developed model, it is likely to reduce the performance of the model and the model may not perform well in different populations. 121 The other two studies were rated as having an unclear risk of bias because they provided no information regarding the enrolment of participants, handling of missing data and whether or not complexities in the data were accounted for appropriately. 113,114
In addition to this, it was difficult to decide whether or not an appropriate sample size was used for model development and internal validation. The events per variable (EPVs) were not reported in each study and none of the studies described which method it used to determine the appropriate sample size.
Concerns regarding applicability
Concern regarding selection of participants
Four studies108–111 were rated as having low overall concern regarding applicability, five were rated as having unclear concern105,106,112–114 and two were rated as having high concern. 107,115 The four studies were rated as unclear because, although participant data were taken from routine care or cancer registries, it was unclear whether or not the participants included in the studies matched the participants in the review question, as inclusion criteria were not provided. There was high concern for the participants selected not matching the review question for two studies. 107,115 Both studies provided no information regarding the characteristics of patients or the severity of their melanoma. The five studies that were rated as having an unclear concern regarding applicabilty included participants with different stages of melanoma. Therefore, the population was not confined to patients with stage I melanoma. The proportions of stage I patients included were 85.9%,105 75.2%,113 68.2%,114 68.1%106 and 66.4%. 112 Including melanoma patients of varying stages is likely to introduce differences in the performance of a prediction model122 for predicting outcomes in early-stage versus advanced-stage melanoma.
Concern regarding assessment and timing of predictors
All studies were rated as having low concern regarding the definition, assessment and timing of the predictors. All predictors were measured using methods potentially applicable to the daily practice that is addressed by the review.
Concern regarding the applicability of the outcome determined
The applicability of seven studies105–107,112–115 was rated as being of high concern. This was due to the inclusion of patients with advanced-stage melanoma, which meant that an accurate prediction estimate for early-stage melanoma patients only was unlikely. The rest of the studies were judged to have an unclear concern in terms of outcome definition, timing and method of determination defining the outcome, as intended by the review question. This was due to the inclusion of patient data from existing records. Not enough information was provided regarding the inclusion and exclusion of patients in the registries.
Performance of prediction models
Discrimination
Six studies105,108–112 reported the discriminatory ability of the models to distinguish between patient survival, or those who have either recurrence or metastases of melanoma, and those who have not. There are several measures that can be used to quantify how well a test can accurately distinguish between patients, from low to high risk.
Three studies reported the estimates for the area under the receiver operating characteristic (AUROC), which plots sensitivity against (1 – specificity). 108,109,111 Values range from 0.5 (no discriminative ability) to 1 (perfect discriminative ability). 123 Gimotty et al. 109 reported discrimination for metastasis using the AUROC for risk groups as 0.85; a lower value for AJCC stages Ia and Ib was reported: 0.59. Gimotty et al. 108 reported the AUROC for survival from a sample of a US cancer registry, the original data set, as 0.76, and for patients seen at a hospital, the validation sample, as 0.83. Neither study reported the variability statistics for the AUROC. Rosenbaum et al. 111 reported discrimination for recurrence using the AUROC for a novel histopathological classifier as 0.733 (95% CI 0.647 to 0.818), compared with the baseline classifier alone, which was 0.635 (95% CI 0.545 to 0.724).
Three studies reported discrimination for survival data using the Harrell’s c-statistic, also known as the concordance statistic/probability. 105,110,112 This is an equivalent of and interpreted in the same way as the AUROC that is used to measure the discriminative ability of linear regression models for binary outcomes. The concordance probability is the probability that, of a randomly selected pair of patients, the patient with the shorter survival time has the higher predicted risk. 124 Baade et al. 105 reported the c-index as 0.88 (95% CI 0.88 to 0.89), Maurichi et al. 110 reported it as 0.88 and Saldanha et al. 112 reported the c-index for Leicester cases, the original data set, as 0.84, compared with 0.81 for Nottingham cases, the validation sample. Again, no variability statistics for the c-index were reported.
Baade et al. 105 also assessed the discriminatory ability of the model to predict overall survival by calculating Royston and Sauerbrei’s125 D-statistic. This statistic quantifies the observed separation between patients with low and high predicted risk, and was reported as 1.50 (95% CI 1.44 to 1.56).
Saldanha et al. 112 also reported the discrimination ability of the model using the Gönen and Heller116 k-statistic, used to evaluate the discriminatory power and predictive accuracy of non-linear statistical models. The k-statistic is an extension to time-to-event data of the AUROC, which is used to assess the discrimination of logistic regression models. It involves only the regression parameters and the covariate distribution, and is, therefore, asymptotically unbiased. 126 This is based on the reverse definition of concordance, which is the probability that, of a randomly selected pair of patients, the patient with the higher predicted risk has the shorter survival time, and has the same interpretation as the c-index. 116 Saldanha et al. 112 reported the k-statistic for Leicester cases, the original data set, as 0.78 (no variability statistics provided), compared with Nottingham cases, the validation sample, which was 0.78, demonstrating evidence of discriminatory ability and that the model fit was retained between training and validation sets.
Calibration
Calibration measures were reported in three studies. 110,112,113 This refers to a model’s accuracy of predicted risk probabilities and indicates the extent to which expected outcomes (predicted from the model) and observed outcomes agree. It is often assessed graphically by a calibration plot, with predictions on the x-axis and the actual outcome on the y-axis, whereby a perfect calibration is represented by a diagonal line on the graph with numerical values between 0 (no agreement) and 1 (perfect agreement). 101 Visual representation by Maurichi et al. 110 shows that most of the predicted values for 12-year overall survival were ≥ 0.7, suggesting that the model was well calibrated. Another study reported a calibration slope of 0.88 (p = 0.5)112 in the validation cases for predicting overall survival. The other study113 reported concordance correlation coefficients of 0.9 and 0.93 for 5- and 10-year survival rates, demonstrating high accuracy of the prediction model. Both results indicate high accuracy of the prediction models, as the predicted and actual observed survival probabilities are close to each other, given that the values of the slopes reported are closer to 1 (perfect calibration). 127
Overall performance
Two studies measured the overall performance of the developed models. 105,114 Baade et al. 105 used the the R2 measure, a statistic that indicates the percentage of the variance to measure overall model performance. The statistic ranges from 0%, when no variation is accounted for, to 100%, when all variation is accounted for. The measure of explained variation measure in the model was reported as 0.47 (95% CI 0.45 to 0.49).
Tsai et al. 114 assessed the Brier score, the result of a statistical test used to examine the accuracy of goodness of fit. 114 The score ranges from 0% for a perfect model to 0.25% for a non-informative model and a higher score means a higher inaccuracy of a prognostic classification scheme. 101 The model proposed by Tsai et al. 114 included an integrated tree-based approach for prognostic grouping of localised melanoma patients, compared with the existing AJCC melanoma staging system that used the tumour, regional lymph nodes and distant metastasis [i.e. tumour–node–metastasis (TNM)] system to classify patients. The study calculated the Brier score as a function of time for three classification schemes (all patients pooled in one group, the proposed integrated approach and the AJCC schemes) for up to 15 years; this was represented graphically. The score for all three schemes increased with time from 0% at year 1 to approximately 0.25% at year 15. However, both the AJCC and integrated schemes were shown to yield superior performance, compared with the pooled Kaplan–Meier estimates, with the integrated approach having a slightly better improvement in Brier score than the AJCC scheme. 114 These results show that the proposed integrated approach is preferred over the AJCC scheme.
Model evaluation methods
All studies in the review were validated either internally or externally. Internal validation refers to the efficiency of a model developed and evaluated from the same underlying population. 128 External validation refers to how well a model predicts an outcome in a new data set, different from the development population. 129
Eight studies105–107,109–111,114,115 reported that they internally validated their models. Four studies105,111,114,115 used four different cross-validation methods. Baade et al. 105 reported that they used the internal–external method (assessing consistency across a variety of different geographical areas in the state). However, the model was not validated against an external, independent data set. Rosenbaum et al. 111 used the 10-fold method (whereby the original sample is randomly partitioned into 10 equal-sized subsamples). Tsai et al. 114 used the fivefold method (whereby the original sample is randomly partitioned into five equal-sized subsamples). Vollmer and Seigler115 used simple cross-validation methods (splitting the data sets into training/development samples and validation samples). Cochran et al. 107 used the random split-sample method, whereas Maurichi et al. 110 validated their model based on a calibration plot by assessing the congruence of expected outcomes (predicted from the model) and observed outcomes. Gimotty et al. 109 validated their model on new patients meeting study eligibility and Balch et al. 106 validated the model using the melanoma patient data used to validate the proposed AJCC staging system.
The rest of the models108,109,112 were externally validated using the geographical validation method. Predictions were calculated from the previously developed model using the training/development population and tested in new data sets different from this population in a different geographical area. Soong et al. 113 developed their model in the USA and validated it by testing it on a data set comprising patients treated at a Sydney Melanoma Unit in Australia. Gimotty et al. 108 developed their model using data from a US population-based Surveillance, Epidemiology, and End Results cancer registry (1988–2001) and validated it using patients seen by the University of Pennsylvania’s Pigmented Lesion Group (1972–2001). Saldanha et al. 112 developed their model using data from Leicester cases diagnosed between 2004 and 2011 and validated in set of cases from Nottingham University diagnosed from 2003 to 2005 and from 2008 to 2010. All three models reported measures of model performance in both the original and validation samples.
Discussion
Summary of model performance assessment
This systematic review identified studies describing 11 different models developed for the prediction of recurrence, new primary tumours or metastasis in patients with AJCC stage I cutaneous melanoma following excision. The models differed in the predictors used depending on the outcome of interest and statistical measures used to assess model performances; therefore, it was inappropriate to quantitatively synthesise their results. The lack of consensus in the approach used to select predictors is reflected in the model development methods. Only six studies reported the criteria used. Two studies used the backward procedure, which starts by including all predictors at the beginning and then subsequently removing predictors based on predefined criteria. 110,112 This is a preferred method because it has the ability to eliminate redundant predictors. 130 Two studies used the univariate analysis method to screen variables. 107,111 This is the simplest method as it analyses one predictor at a time. However, this is likely to overestimate regression coefficients and overfit models. 131 Two studies108,109 used evidence from previous study reports to identify predictors, and the rest of the studies did not report on how predictors were selected; therefore, it is difficult to determine whether or not the methods used were appropriate.
Model performance measures were available for assessing discrimination in six studies,105,108–112 assessing calibration in three studies110,112,113 and assessing overall performance in two studies. 105,114 The area under the curve (AUC) of the six studies ranged from 0.59 to 0.88. The discriminative performance of the models is considered acceptable when the AUROC statistics and their equivalent are ≥ 0.7. 123 Not all studies reported the variability statistics for the AUC. However, those that did report variability statistics reported a value below this estimate; therefore, it is unclear if all the models could accurately discriminate between those with defined outcomes and those without. The three studies that assessed calibration measures all reported values ≥ 0.7, which is closer to 1 (perfect calibration). 110,112,113 This suggests that all three models have the ability to accurately generate predictions that are close to the observed outcomes. Two studies105,114 measured overall performance. Baade et al. 105 assessed this by assessing how well the model fits the data using the R2 statistic. Higher R2 values represent smaller differences between the observed data and the fitted values; the model by Baade et al. 105 reported an R2 of 0.47 (95% CI 0.45 to 0.49), indicating that the model explains an estimated 47% of the variation. Another study114 measured the overall performance by assessing the Brier score, a statistical test used to examine the goodness of fit. This score is useful because it simultaneously captures discrimination and calibration, and summarises the magnitude of error in the probability forecast; values range from 0.0 (total accuracy) to 1.0 (total inaccuracy). 132 The score tested the performance of an integrated tree approach, as opposed to the AJCC scheme, over a period of 15 months. Although there was little difference between the scores (all were < 0.25%), the new model presented slightly better scores, suggesting that it was the preferred model to accurately predict survival rates for individual patients with localised melanoma.
Most of the studies validated their prediction models internally using data from their development set. 105–107,109–111,114,115 Research shows that, although models validated internally may show acceptable performance, it is not guaranteed that they will produce the same results in a different group of participants. 133 A few of the models were validated using external populations from other institutes. 108,112,113
All models were rated as having a high risk of bias. The main source of bias related to the inclusion of patient data from existing databases or cancer registries. Although using existing data can be beneficial in that they are cheaper and quicker to obtain, there is a risk that information may have been collected for a particular purpose, thereby including irrelevant items and perhaps not recording outcomes of interest. Some of the studies identified as such included patients with advanced stages of melanoma; this could have distorted the predictive ability of the models in their favour.
Another source of bias was the omission of statistical analysis in the estimated predictive performance of the models. There was often insufficient or no information given regarding sample size determination. Sample size is often based on the ratio of the number of individuals with the outcome event to the number of candidate predictors (EPV). Models developed from data sets with a low EPV are likely to produce biased estimates. 120 Three studies did not provide definitions for outcome measures. 110,114,115 Having a predefined outcome reflecting a clinically significant and relevant health state limits the potential of bias. 134
Strengths and limitations
The main strength of this systematic review is that, to our knowledge, it is the first to summarise the evidence presented by prediction models for AJCC stage I melanoma. The review followed procedures documented by the Cochrane Collaboration for conducting systematic reviews, the CHARMS for extracting data, the PRISMA guidelines for reporting and the PROBAST for assessing risk of bias; therefore, it is robust. We had planned to assess the overall quality of evidence using GRADE. Although the tool has been adapted for assessing overall quality in prognostic studies, it was not found to be suitable for prediction models. 104 We conducted comprehensive searches of bibliographic databases and grey literature. All stages of the review, including screening, data extraction and the risk-of-bias assessment, were conducted by either two researchers in duplicate or by one researcher with checks by a second researcher.
As with most systematic reviews, the main limitation was the quality of the published studies. None of the studies reported having followed the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). 135 First, however, eight of the studies in the review were published between 2001 and 2014, before TRIPOD was published; therefore, they could not follow these guidelines. Second, only a small number of models were externally validated, making it difficult to determine external validity. Third, model comparisons and meta-analyses were problematic because of the variety in the predictors and statistical measures used for model performances.
Impact and implementation
The results of this systematic review highlight the relative lack of appropriate evidence levels underpinning current melanoma follow-up practice in AJCC stage I disease. As all of the studies were judged to be at high risk of bias, they cannot be recommended for estimating the probability or risk of recurrence, new primary tumours or metastases in routine clinical practice. This review identifies the need for ongoing development of risk prediction models that encompass known patient and tumoral variables in conjunction with new and developing prognostic biomarkers. These models must be developed in a way that follows biomarker development and reporting guidelines, such as TRIPOD, thus enabling appropriate critical appraisal and further assessment in the general AJCC stage I melanoma population.
The results of this review clearly outline a need for ongoing biomarker and prognostication studies and, therefore, should act as an evidence base and catalyse project development and funding. The review also demonstrates the potential impact of such studies on future follow-up guidelines and management of patients, given the relative scarcity of evidence-based practice at present.
Conclusion
This review identifies prediction models to predict the recurrence, new primary tumours and metastases in early-stage melanoma. However, they were all rated as having a high risk of bias and, therefore, cannot be recommended for use in clinical practice. The data elements most commonly used in these tools are patient demographic information or histological features of the primary tumour. 136 However, these data do not offer a wide enough scope of information to allow accurate prognostication of melanoma, which is heterogeneous in its biology and progression. Numerous biomarkers have been identified in recent years, with varying degrees of validation in clinical cohorts. 137 These offer greater potential to prognosticate at the individual patient and tumour levels, thus facilitating individualised follow-up and treatment regimens for patients.
Chapter 5 The diagnostic accuracy of high-resolution ultrasonography, fine-needle aspiration or core biopsy to detect recurrence and locoregional metastases during surveillance
Brief overview
After excision of a primary cutaneous melanoma, the disease may relapse, initially presenting as a local recurrence or satellite lesion (skin or subcutaneous lesion) within 2 cm of the primary tumour. In-transit metastasis may occur following progression through the lymphatic system with a lesion developing > 2 cm from the original site. Further progression of disease through the lymphatic system to the regional lymph nodes is known as regional recurrence. Progression to non-regional lymph nodes, then to organs and distant sites is known as metastatic melanoma. Lymph node metastases are believed to be an important prognostic factor for stage I and II melanoma patients. 138,139 Detection of metastases in the lymph nodes is initially by palpation of the affected nodes, followed by confirmation using sonography.
As described in Chapter 2, surveillance strategies for recurrence vary across countries by frequency and by the types of monitoring tests utilised. The range of approaches routinely used for detection of recurrences or new primary tumours and metastases may include clinical examinations, such as medical history, skin and lymph node examination and palpation. Further testing on suspicion of melanoma may involve imaging techniques or biopsy, such as ultrasonography of the abdomen, resected tumour scar, lymphatic drainage areas and regional lymph nodes; chest radiography; CT; MRI; PET; PET-CT; skin biopsies; and SLNB or blood tests. However, guidance published by the BAD advises that routine tests, including CT and blood investigations, are not recommended for staging of asymptomatic patients with stage I or II primary melanomas, because true-positive rates are low and false-positive rates are high. 27
In practice, ultrasonography and fine-needle aspiration cytology (FNAC) (a minimally invasive procedure) or core biopsy are used when an enlarged lymph node is detected during follow-up visits. Ultrasonography may also be used for the detection of non-enlarged metastatic lymph nodes, which may not be palpable, and partially metastatic lymph nodes. 140 Importantly, ultrasonography may distinguish between benign and malignant palpable nodes, but is unable to detect micrometastases. If micrometastases are suspected in the regional nodes, then high-resolution ultrasonography and FNAC may be used preoperatively to replace SLNB. 141,142
Sensitivities of between 5% and 89.4% have been reported for ultrasonography and/or FNAC in melanomas. 141,143–145 Specificities are higher, ranging from 84% to 100%. 146 This variability may be explained by heterogeneity between patients with melanomas of different stages in the populations of these studies. However, the findings are comparable to meta-analyses of ultrasonography and FNAC for surveillance of lymph nodes in melanoma, which report overall sensitivities and specificities of 96% and 99%, respectively,147 for ultrasonography and of 97% and 98%, respectively, for FNAC. 148
This systematic review supplements and updates these reviews by focusing on high-resolution ultrasonography for detecting satellite, in-transit and locoregional lymph node metastases during surveillance or in symptomatic patients with an initial diagnosis of stage I melanoma.
Research aim
The aim of this systematic review was to determine the diagnostic performance of high-resolution ultrasonography with or without FNAC for surveillance and follow-up to detect recurrence in patients who have had AJCC stage IA or IB melanomas surgically excised.
Specifically, the focus was on detection of:
-
local recurrence or satellite metastases within 2 cm of the surgical scar of the primary tumour
-
new primary melanomas – in-transit metastases occurring on the skin or subcutaneous layers that are > 2 cm from the primary lesion, but not beyond the regional nodal basin and lymph node metastases
-
regional recurrence in local lymph nodes
-
metastatic melanoma.
Methods
This review adheres to the guidelines for the PRISMA statement to ensure transparency of the process. 78 A protocol for the whole project, of which this review is part, is published on PROSPERO (CRD42018086784). 79
Search strategy
The search strategy was designed by an experienced information specialist, in consultation with the project team, and was based on previous scoping of the literature. The search was designed on MEDLINE (via Ovid), using a combination of controlled medical subject heading thesaurus terms, relevant keywords and text words to identify studies of early-stage, stage I and stage II cutaneous melanoma patients and diagnostic tests. The search strategy contains appropriate use of stemming for alternative word endings, alternative spellings and plurals, and was translated to conform to other bibliographic databases using the following topic outline: [melanoma] AND [ultrasound OR biopsy] AND [surveillance]. Database-specific thesaurus headings, along with title and abstract keywords, were used and translated to other databases, altering the thesaurus headings and search syntax as appropriate. Terms relating to ocular melanoma were excluded. Identification of a suitable diagnostic filter on which to limit database results was researched seeking reliability and consistency in performance. 149 The diagnostic search filter chosen and used was published, validated and adapted for use in other databases as necessary [the study developed three search strategies (A–C), from these strategy A was selected as this was the most sensitive strategy identified]. 150 The searches of the databases (see Box 5) were run on 4 April 2019 and updated on 3 July 2019. The search strategy used in MEDLINE can be found in Appendix 5. Following deduplication, publications were limited from 1998 to July 2019, as this is when SLNB became clinically utilised as a prognostic indicator. 100
A grey literature and guidelines search was conducted to identify further relevant material not retrieved by the database searches (Box 6).
-
Open Grey (www.opengrey.eu/; accessed 17 April 2019).
-
Cancer Research UK (www.cancerresearchuk.org/about-cancer/find-a-clinical-trial; accessed 17 April 2019).
-
Melanoma UK (www.melanomauk.org.uk; accessed 17 April 2019).
-
National Guideline Clearinghouse (www.guideline.gov/; accessed 17 April 2019).
-
Organisational websites: BAD, British Skin Foundation, The King’s Fund, etc.
-
Ongoing trials identified using the World Health Organization’s International Clinical Trials Registry Platform (www.who.int/trialsearch; accessed 17 April 2019).
A range of guidelines on melanoma from a range of countries were also identified, and the evidence supporting relevant recommendations relating to the diagnosis of recurrent disease was checked.
Inclusion and exclusion criteria
Population characteristics
Studies were included if they considered one of the following:
-
adults aged ≥ 18 years treated for AJCC (eighth edition) stage I cutaneous melanoma [stage IA (≤ 0.8 mm thick without ulceration) or stage IB (< 0.8 mm thick, or < 1 mm thick and ulcerated skin)]19
-
adults aged ≥ 18 years treated for AJCC (seventh edition) stage I cutaneous melanoma [stage IA (T1a ≤ 1 mm thick) or stage IB (T1b with ulceration or mitoses ≤ 1 mm thick, or T2a 1.01–2.00 mm thick and no ulceration)]. 20
If more than one study was reported by the same centre or institution, where participant populations could have been duplicated, we included either the most recent publication or the publication with the most complete participant data. Studies of patients with any stage of melanoma were included if the data for stage I disease were available independently. If the patients’ disease stage was not clear, then authors were contacted for further information.
Target condition
The target conditions were local recurrence, satellite lesions, new primary lesions, in-transit metastases, locoregional lymph node metastases or metastatic melanoma after resection of a stage I melanoma.
Index/comparator tests
The detection of local melanoma recurrence, satellite lesions, new primary lesions, in-transit or locoregional lymph node metastases, or metastatic melanoma could include the following tests independently or in combination:
-
ultrasonography of the resected tumour scar, lymphatic drainage area or regional lymph nodes
-
FNAC/fine-needle biopsy (FNB).
All index tests should be confirmed by the use of an independent reference standard.
Reference standards
In patients testing positive for local recurrence, satellite lesions, new primary lesions, in-transit metastases and/or locoregional lymph node metastases, or metastatic melanoma, the reference standard was taken to be:
-
histopathology results from excision biopsy, incision biopsy, wide local excision, punch biopsy, shave biopsy or core biopsy
-
histopathology results from lymph nodes or distant secondary sites sampled by lymph node dissection, SLNB or core biopsy
-
clinical follow-up when histopathology was not available.
Outcomes
Conventional outcomes for assessment of diagnostic test accuracy were extracted from each eligible study. These included the sensitivity, specificity, likelihood ratio, diagnostic odds ratio, false-positive rate and summary receiver operating curve (ROCs) of the index and reference tests. The data required to derive these parameters were the number of true-positive, true-negative, false-positive and false-negative cases reported in each included study for index and reference tests. If data were missing for a full 2 × 2 table, we contacted the authors of the article. Data were extracted from studies at the time point of diagnosis of lesions under consideration and before commencement of any treatments.
For indeterminate values (e.g. atypical, suspicious, probable or possible malignant lesions for which the test did not provide a clear negative or positive result, or values were missing in reported test results), the sensitivity and specificity were calculated for each scenario. 151
Settings
Studies from any country and all settings were eligible, including primary, secondary and tertiary care. Non-English-language articles were translated when resources were available.
Study designs
Primary studies eligible for inclusion were randomised trials, prospective or retrospective cohort designs, cross-sectional studies, and diagnostic case–control studies with separate diseased and non-diseased groups. Studies had to report sufficient data to enable us to construct 2 × 2 tables of diagnostic metrics. Eligible studies had to have participants receive a single index test and a reference standard, or receive more than one index test and a reference standard.
Data collection
Study selection
Titles and abstracts of articles retrieved from the search strategies were screened by three reviewers using Rayyan. 81 Studies selected by two of the three reviewers were included for review. At this stage, non-English-language papers were included from titles and abstracts. Full-text articles were obtained for included citations. If translation of full-text non-English-language papers was not possible, these papers were coded as ‘non-English studies awaiting assessment.’
Data extraction
Data were extracted from each study by one reviewer and independently verified by a second reviewer. Any disagreements were resolved by discussion between the reviewers or with arbitration from a third reviewer. Data were extracted from each included study based on the STAndards for the Reporting of Diagnostic accuracy studies (STARD) checklist. 152 The type of data extracted is outlined in Report Supplementary Material 1.
Risk of bias
The QUADAS-2 tool was used to assess the risk of bias of diagnostic accuracy studies. 153 This tool covered four domains: summarising the review question, tailoring the tool, constructing a flow diagram for the primary study, and judging bias and applicability.
Data analysis and synthesis
Data from the 2 × 2 tables were used to calculate sensitivity and specificity for each study. Individual study results were presented graphically by plotting estimates of sensitivities and specificities in forest plots. If more than one threshold was reported, data from one threshold were to be chosen to be incorporated into a meta-analysis. Meta-analysis of pairs of sensitivity and specificity values was planned using a bivariate random-effects approach. This approach would enable the calculation of summary estimates of sensitivity and specificity, while correctly dealing with the different sources of variation. 154 The planned meta-analysis was not conducted, as each study used different index tests and would be used at a different stage in the diagnostic work-up.
Sources of heterogeneity in studies
Risk factors for melanoma progression and prognosis obtained from demographic data in the studies were to be included as potential sources of patient heterogeneity between studies. A range of potential factors influencing heterogeneity may include sex, age of participants, tumour characteristics, presence of ulceration, stage of disease, site of primary tumour (trunk, lower limbs, upper limbs, head or neck, hand or foot), clinical node status at follow-up (post operative), sentinel lymph node status at follow-up (post operative) and comorbidities. Potential sources of heterogeneity in reference tests may include the experience of surgeons, the method of sampling (either cytology or biopsy) and preparation techniques of the tissue sample [e.g. fresh-frozen section (cryosection) or paraffin section]. Heterogeneity of the index tests may arise from variations in the clinical pathway, including disease stage; professionals involved (e.g. radiographers, radiologists or clinicians); experience of operators performing index and reference tests; differing frequencies of ultrasonography instruments, manufacturers and models; and tumour depth and spread, ulceration, regions of interest for ultrasonography and anatomical sites of lesions.
Heterogeneity was to be investigated in the first instance through visual examination of forest plots of sensitivities and specificities and through visual examination of the ROC plot of the raw data. Heterogeneity was also to be assessed statistically using the I2 statistic. 155 Had suitable data to meta-analyse been identified, sensitivity analyses exploring heterogeneity would have been conducted. Likewise, subgroup analyses by sample size of the study would have been conducted, as sample size is known to influence sensitivity and specificity. 156,157
Results
Number of studies identified
Afer deduplication, the electronic database searches retrieved 2226 records and a further 24 from search updates. One additional study was included from reference lists of systematic reviews. From screening these citations, 106 primary studies, including conference abstracts, were identified as potentially relevant. Full-text articles were then retrieved for review. Five studies were non-English language (written in French, Hungarian, Japanese, Russian and Spanish). Seventeen citations were conference abstracts; each was checked for journal publication of the full reports. One foreign language paper was unable to be translated. 158 Study authors of five studies that reported combined data across stages were contacted for more information. 24,159–162
Following this process, two English-language studies met the inclusion criteria and were assessed. 163,164 These reported the diagnostic accuracy of FNB163 and the diagnostic accuracy of high-frequency ultrasonography. 164 We wrote to five study authors to seek further clarification and a breakdown by stage I disease to attempt to include further data.
In addition, other than the five studies awaiting classification and one needing translation, 98 full-text studies or conference abstracts that did not meet the inclusion criteria are recorded in Appendix 6, with reasons for exclusion. Studies were excluded based on the following: no analysis by disease stage or Breslow thickness (n = 29), stage not reported (n = 22), preoperative staging (n = 13), stages combined (n = 10), insufficient patients or data (n = 5), not diagnostic accuracy study (n = 4), no relevant outcomes (n = 3), advanced stage (n = 3), no diagnostic test (n = 1), letters (n = 3), review (n = 3), treatment (n = 1) and animal study (n = 1). Studies could meet one or more of these criteria. The PRISMA flow diagram in Figure 5 outlines the study selection process and the reasons for exclusion.
FIGURE 5.
The PRISMA flow diagram for the diagnostic accuracy review. DTA, diagnostic test accuracy.

Characteristics of included studies
One of the two included studies was conducted in Australia,163 and the other in Germany. 164 The two studies considered different diagnostic tools used at different points in the care pathway. The characteristics of each study are presented in Table 10.
| Study (first author and year of publication), country and design | Index test and definitions | Reference test and definitions | Number of patients at baseline | Age (years) | FNB or ultrasonography of patients at stage I | Duration of follow-up | Outcome |
|---|---|---|---|---|---|---|---|
|
FNB with results read and reported by cytopathologists |
|
|
Range: 10 to ≥ 81 |
|
≥ 6 months | TP, FN, TS, FS, FP, TN, sentinel node |
Test categories: positive, suspicious or negative for metastatic melanoma
|
|||||||
|
|
|
|
|
|
|
TP, FN, FP, TN |
Doubrovsky et al. 163 evaluated the diagnostic accuracy of FNB, which is analagous to FNAC, to detect metastatic melanoma. They used a retrospective cohort study design, with data collected between January 1992 and December 2002 from the Royal Alfred Hospital in Sydney, NSW, Australia. The sample comprised 1582 confirmed FNBs at melanoma stages I–IV. A total of 323 confirmed cases (20%, 323/1582) were included in an analysis of stage I disease. Males accounted for 63% of participants and ages ranged from 10 to ≥ 81 years. Diagnostic accuracy was evaluated by AJCC stage, the anatomical sites of lesions, use of immunostaining, year, sex, age, FNB attempts, needle size, presence of necrosis, pathologist case load, primary Breslow thickness, primary lesion ulceration status, primary lesion mitotic rate, histological subtype and predominant subtype.
Krüger et al. 164 evaluated the diagnostic accuracy of high-resolution ultrasonography of the lymph nodes to detect locoregional lymph node metastases. A prospective cohort design was used, with data collected prospectively between 2004 and 2008 from the Dermatology Department in Göttingen, Germany. Participants recruited had stage I–IV disease (AJCC 2002), and a median age of 58 years. Forty-eight per cent were male. The diagnostic accuracy of combined clinical and sonographic examinations was evaluated in 433 patients. The sample was composed of 1314 investigations in patients with melanoma stages I–IV. A total of 669 investigations (51%, 669/1314) were included in an analysis of stage I disease. An average of three paired investigations (clinical and sonographic) were performed for each participant on the same day. With respect to stage I disease, we estimated that 223 participants received 669 paired investigations. Diagnostic accuracy was evaluated by AJCC stage, melanoma subtype, lymph node dissection status and lymph node surgery before investigation.
Description of index tests
In the study by Doubrovsky et al. ,163 FNBs were performed by the reporting cytopathologist or a supervised trainee pathologist. A hollow bore needle was inserted directly into the lesion (once localised and stabilised) and the sample was retrieved. The specimen was then transferred directly onto glass slides. One slide was stained with Diff-Quik (MICROPTIC S.L, Barcelona, Spain) and the other stained using the Papanicolaou staining method. Residual material was retained for subsequent testing if necessary. Air-dried slides were assessed by a cytopathologist immediately after the sampling and staining procedure.
Krüger et al. 164 performed high-resolution ultrasonography using a 7.5- to 10.0-MHz real-time scanner. Paired, non-blinded clinical and ultrasonography investigations were performed for each patient. Each clinical examination included a medical history, physical examination, and inspection and palpation of the primary tumour scar. Scanning of the regional lymph nodes was standardised, with investigations performed in longitudinal and transverse sections. The morphological criteria of the lymph nodes (including size, shape, echogenicity of the centre and cortex) were evaluated. No information was reported on the expertise of staff who conducted these tests.
Interpretation of index tests
Doubrovsky et al. 163 looked at FNAC alone as an index test. FNB findings were categorised by Doubrovsky et al. 163 as positive, suspicious or negative for metastatic melanoma. Samples classified as suspicious for metastatic melanoma were composed of cells from unclassified/unspecified malignancies, or cases categorised as suspicious for melanoma that had a small number of atypical cells, poorly preserved cells or cells lacking specific features of melanoma. When verified, these were reclassified as either true suspicious or false suspicious.
Krüger et al. 164 looked at ultrasonography alone as an index test. Ultrasonography findings were considered suspicious for malignancy by Krüger et al. 164 when at least one of the following criteria applied: the Solbiati165/Vasallo166 index was < 2, the whole lymph node structure had a predominance of low echogenicity, the lymph node centre had low echogenicity, or there were asymmetrical regions with low echogenicity in the lymph node margin.
Description of reference tests
In the study detailed by Doubrovsky et al. ,163 histopathological evaluation of the excised lesion was the reference test used to confirm metastasis. When histological material was not available, follow-up was used as the reference test.
In the study detailed by Krüger et al. ,164 lymph nodes were removed by excisional biopsy for histopathological assessment of lymph node sections. 164,167 The definitive reference test was histopathology of the excised lymph node, but, in some cases, fine-needle aspiration was also performed under sonographic guidance. 164 When clinical and ultrasonography tests were negative, follow-up was used as a further reference test.
Interpretation of reference tests
When evaluating the diagnostic performance of FNB, Doubrovsky et al. 163 used two reference standards. Histopathology was the reference standard in 1120 of the 1582 cases (71% of cases) and follow-up for ≥ 6 months was the reference standard in the remainder (n = 462, 29% of cases). Follow-up was relied on as the reference standard when histopathology was not appropriate. 163 Instances when histopathology was indicative of metastatic melanoma were not described in the study report.
When evaluating the diagnostic performance of clinical examination and lymph node ultrasonography, Krüger et al. 164 also used two reference standards. The reference standards were the removal of lymph nodes by excisional biopsy for histopathology of lymph node sections,164 or follow-up among those identified as clinically negative to identify false-negative cases as follows:
-
negative on sonography based on clinically suspicious lesions subsequently proven to be malignant by histopathology or
-
negative based on lack of clinical suspicion and sonography (not histologically testable owing to lack of suspicious region/mass), with metastases identified during follow-up.
In the study by Krüger et al. ,164 reference standard techniques and instances when histopathology was indicative of the target condition were not reported with clarity. 164
Risk of bias
Risk-of-bias assessments were performed using the QUADAS-2 tool across four domains. 153 The results of this are summarised in Table 11.
| Risk-of-bias domain (corresponding subchapter) | Study (first author and year of publication) | |||
|---|---|---|---|---|
| Doubrovsky et al.163 2008 | Krüger et al.164 2011 | |||
| Risk of bias | Applicability concerns | Risk of bias | Applicability concerns | |
| Patient selectiona (5.4.3.1) | Low | Low | Low | Low |
| Index testb (5.4.3.2) | Low | Low | Low | Low |
| Reference standardc (5.4.3.3–5.4.3.4) | High | Low | High | Low |
| Flow and timingd (5.4.3.5–5.4.3.6) | High | Low | High | Low |
Methods of patient selection
The risk of patient selection bias was considered to be low for each study, although consecutive recruitment was reported for Doubrovsky et al. 163 only. Patients were identified over specified times either retrospectively163 or prospectively. 164 There were no concerns regarding the included patients and settings not matching the review question in either study (both studies were deemed to have a low risk of bias regarding applicability).
The index test and how it was conducted and interpreted
The conduct of the index test was judged as being at low risk of bias in the study completed by Doubrovsky et al. 163 because sampling and interpretation of the index test was completed while blinded to the reference standard outcome. The reading of the index test (cytology slide interpretation) was completed by the cytopathologists who performed the FNB and prepared the cytology slides. 163 However, this is standard practice and unlikely to have promoted bias.
The conduct of the index test in the study completed by Krüger et al. 164 was judged to be at low risk of bias as ultrasonography interpretation was completed while blinded to the reference standard. Furthermore, although sonographers completing the test made judgement on the outcome of the test (standard practice), prespecified thresholds for ultrasonography were outlined a priori and followed. 164
Describe the reference standard and how it was conducted and interpreted
In the study by Doubrovsky et al. ,163 classification of the reference standard (histopathology following surgical resection) for the target condition was adequate and blinded appropriately. Krüger et al. 164 used histopathology as the reference test, but did not clearly report the technique used.
There were no concerns over whether or not the reference standards were identifying the target condition as defined by the review question (both studies were deemed to have a low risk of bias regarding applicability). 163,164 However, both studies used clinical follow-up as a second reference standard; although this is, realistically, unavoidable among those with no evidence of the target condition after index testing, it is a less reliable reference test, and its use based on the index test outcome may introduce differential verification bias. 163,164 For both studies, the definition used for a metastasis-free interval is likely to strongly affect the number of false-negative results, and the specificity of the investigations. 163,164 Given this, both studies were deemed to have a high risk of bias.
Flow and timing
The time interval between index and reference tests was not reported in the study by Krüger et al. ,164 but could have been long for those undergoing clinical follow-up. However, the study did adhere to the melanoma guidelines of the German Dermatological Society. 167 All 669 investigations conducted in patients with stage I melanoma were accounted for in the results. 164 SLNB or complete lymph node dissection was conducted in 272 out of 433 (63%) patients (stage of disease not reported) before clinical and sonographic follow-up at a mean interval of 166 days (± 101 days). 167 This surgery is unlikely to have been offered to patients with stage IA melanoma. Clinically negative investigations in a separate subgroup of the Krüger et al. 164 study were evaluated separately and not reported in the results; for this reason, this domain was judged to have a high risk of bias.
In the Doubrovsky et al. 163 study, clinically suspicious lesions were detected by palpation or by imaging techniques. These were then investigated by FNB. No other intermediate interventions were reported. 163 Of the whole study sample reported by Doubrovsky et al. ,163 462 individuals had clinical follow-up as the reference standard.
The time interval between index and references tests in Doubrovsky et al. 163 was within the same day. However, not all patients received the reference standard, as some were followed up clinically; for these individuals, the time interval could have been long. For these reasons, this domain was judged to have a high risk of bias. 163
Diagnostic accuracy
A meta-analysis was not conducted as there was only one study for each index test. The results of the studies by Doubrovsky et al. 163 and Krüger et al. 164 for diagnostic accuracy of FNB and ultrasonography, respectively, are shown in Table 12. Forest plots are also presented for the sensitivity and specificity of the index tests for the two studies (Figures 6 and 7).
| Study (first author and year of publication) | AJCC stage I melanoma | Follow-up | True positive (n) | False negative (n) | True suspicious (n) | False suspicious (n) | False positive (n) | True negative (n) | Sensitivity (95% CI) | Specificity (95% CI) | Likelihood ratio (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||||||
| Doubrovsky et al.163 2008; FNB | Base case
|
|
150 | 9 | 0 | 0 | 9 | 155 | 0.94 (0.90 to 0.97) | 0.95 (0.90 to 0.97) | 17.2 (9.1 to 32.5) | 0.06 (0.03 to 0.11) |
| Excludes suspicious histopathology, n = 295 | As above | 128 | 9 | 22 | 6 | 3 | 155 | 0.93 (0.88 to 0.97) | 0.98 (0.95 to 1.00) | 49.2 (16.0 to 151) | 0.07 (0.04 to 0.13) | |
| Krüger et al.164 2011; clinical and sonography | Base case
|
Mean ± SD (days): 363.0 ± 318.4 | 1 | 0 | – | – | 3 | 220 | 1.00 (0.03 to 1.00) | 0.99 (0.96 to 0.99) | 74 (24 to 229) | 0.00 |
|
Unit = paired investigations n = 669 |
As above | 1 | 0 | – | – | 9 | 659 | 1.00 (0.03 to 1.00) | 0.99 (0.97 to 0.99) | 74 (39 to 142) | 0.00 | |
FIGURE 6.
Forest plot of sensitivity and specificity of FNB for detection of stage I melanoma recurrence. 163 FN, false negative; FP, false positive; TN, true negative; TP, true positive.

FIGURE 7.
Forest plot of sensitivity and specificity of ultrasonography for detection of stage I melanoma recurrence. 164 FN, false negative; FP, false positive; TN, true negative; TP, true positive.

Fine-needle biopsy
Doubrovsky et al. 163 excluded suspicious findings, but stated which were true suspicious and which were false suspicious. 163 We assumed that test results classified as true suspicious by Doubrovsky et al. 163 were true positives and, similarly, that false-suspicious test results were false positives. 163 Sensitivity was estimated as 0.94 (95% CI 0.90 to 0.97) and specificity as 0.95 (95% CI 0.90 to 0.97). When excluding suspicious findings from the analysis, the results were similar (see Figure 6).
Ultrasonography
In Krüger et al. ,164 the unit of analysis was the number of investigations in 433 patients (an average of three investigations per patient). To estimate results at a patient level, true-positive, false-positive, false-negative and true-negative results were divided by three. Krüger et al. 164 reported 217 stage I patients in the sample; this approximation was equivalent to 224 patients. For sensitivity, both the investigation and the patient-level analysis were similar, with very wide 95% CIs (see Figure 7). This result was expected, as only one true positive (and no false negatives) occurred in the sample. Specificity was high in both analyses.
Discussion
Summary of findings
This systematic review sought to estimate the diagnostic accuracy of ultrasonography and FNAC procedures, routinely used to detect recurrence and metastases during follow-up of patients initially diagnosed with stage I cutaneous melanoma. Comprehensive literature searches were performed in a range of bibliographic databases from 1998 to July 2019, and were complemented by grey literature searches for guidelines and other potentially relevant literature. Searches were restricted to 1998, which coincided with the introduction of SLNB assessment following melanoma diagnosis in some centres internationally. Despite this extensive searching, only two studies met the inclusion criteria. As SLNB involves the assessment of an initial lymph node basin, and often the complete removal of all associated nodes when a positive SLNB is identified, this would probably have an impact on the routine clinician examination findings of patients, as well as the potential choice and application of further investigation modalities in such patients. By limiting the searches to dates after this change in routine management, a more appropriate and contemporaneous assessment of the evidence could be made.
The two identified studies considered different tests (lymph node ultrasonography and FNB). 163,164 The findings reported for diagnostic accuracy of FNB in patients who were diagnosed initially with stage I melanoma were comparable to those reported for patients at stages II–IV. 163 Doubrovsky et al. 163 reported that the sensitivity was positively correlated with the following factors: the use of immunostaining (currently widely accepted168); if the cytopathologists had performed > 500 FNBs; the case mix, especially in patients presenting with ulcerated primary melanomas; and lesions located in the skin and subcutis. False-negative findings were reported to be associated with masses located in the axillary lymph nodes and when sampling required more than one needle pass.
The diagnostic accuracy of ultrasonography for stages II–IV was reported to be comparable to that for stage I. Krüger et al. 164 found that the sensitivity and specificity varied between melanoma subtypes (e.g. superficial or nodular).
In line with Cochrane guidance82 on the use of narrative summary of findings tables, we did not include one for the systematic review of diagnostic accuracy of high-resolution ultrasonography, fine-needle aspiration or core biopsy to detect local recurrence, satellite lesions, new primary lesions, in-transit metastases, locoregional lymph node metastases or metastatic melanoma during surveillance. We did not calculate pooled results in this review; instead we presented the results of a single study. As a result, a narrative SoF table would not include any certainty of evidence judgements; thus, we felt that it was an unnecessary addition. Furthermore, heterogeneity is expected in diagnostic test accuracy reviews; therefore, with just one study included, by reporting on each test, we cannot make any inferences from the results presented.
Comparison of findings with other reviews
Four reviews retrieved by our searches had investigated the diagnostic accuracy of high-resolution ultrasonography or FNAC/core biopsy. None of the reviews conducted analyses by disease stage. Hall et al. 148 identified 10 studies published between 1980 and 2007 for inclusion. The review reported summary estimates for both palpation and ultrasonography-guided FNAC together as a sensitivity of 0.97 (95% CI 0.95 to 0.98) and a specificity of 0.98 (95% CI 0.98 to 1.00). The positive likelihood ratio was 58 (95% CI 23 to 139). These data are similar to those reported by Doubrovsky et al. 163 on FNB in stage I patients.
A second review summarised the diagnostic accuracy of different imaging techniques for primary staging and surveillance of lymph nodes and distant metastases. 147 This review included 74 studies, of which 21 considered ultrasonography. Ultrasonography was reported at primary staging and surveillance. Twenty-one studies that considered ultrasonography and which were published between 1990 and 2007 met inclusion criteria for the review. The mean QUADAS-2 score across the 21 included studies was 5.8, with a SD of ± 2.5, from a potential score of 14. Ultrasonography during surveillance imaging had a median sensitivity of 0.96 [95% credible interval (CrI) 0.85 to 0.99] and median specificity of 0.99 (95% CrI 0.95 to 1.00). Although no analysis was conducted by disease stage in this review, the results were similar to those reported by Krüger et al. 164
Another review compared ultrasonography and palpation to detect nodal invasion in patients with stage I–III melanoma. 169 Twelve studies published between 1997 and 2003 met the inclusion criteria. Positive likelihood ratios were 41.9 (95% CI 29 to 75) for ultrasonography and 4.55 (95% CI 2 to 18) for palpation. Negative likelihood ratios were 0.024 (95% CI 0.01 to 0.03) for ultrasonography and 0.22 (95% CI 0.06 to 0.31) for palpation.
A Cochrane review of ultrasonography and other imaging techniques for staging and re-staging of adults with cutaneous melanoma, at any stage of initial disease, included 11 studies. 170 It reported a summary sensitivity for ultrasonography alone of 0.35 (95% CI 0.17 to 0.59) and a specificity of 0.94 (95% CI 0.86 to 0 98) for detection of regional lymph node metastases before SLNB. A combination of pre-SLNB ultrasonography with FNAC reduced sensitivity to 0.18 (95% CI 0.036 to 0.57) and increased specificity to 1.00 (95% CI 0.99 to 1.00). These findings are at odds with those reported by Xing et al. 147 and those reported by Krüger et al. 164
Overall, it is likely that differences in sensitivity between the reviews may be due to heterogeneity between studies of mixed populations, for example patients at different stages of disease and variations in case mix of melanoma subtypes.
Strengths and limitations
This review sought to systematically identify all relevant studies looking at the diagnostic performance of tests used to detect recurrance after treatment for stage I melanoma. It followed a prespecified protocol and conducted rigorous searches. The methods for the review correspond to best-practice recommendations.
The review is limited in a number of respects. First, it considered only two diagnostic tests. The rationale for this was based on clincial opinion about which tests might be viable for the NHS to consider, but it also took into account judgements about which tests would not be relevant; for example, sophisicated imaging was excluded for the review. Although these tests are not recommended by many guidelines currently, whether or not those recommendations should change has not been assessed (although recent surveillance reviews by NICE suggest not, at least for stage I disease). 54
The dates of the searches were limited from 1998 to July 2019. This date was chosen because this is when SLNB became clinically utilised as a prognostic indicator. 100 However, it is possible that relevant studies could have been published before this date. The applicability of the findings of any such study to current practice is unclear, as staging criteria have changed several times since 1998.
The key limitation of this review is the lack of recent evidence on the diagnostic performance of ultrasonography and FNB in the target population. Planned meta-analyses were not possible as the two studies included investigated different index tests. Furthermore, Krüger et al. 164 identified only one participant initially treated for stage I disease as a true positive. Consequently, the CI for sensitivity was very wide (95% CI 0.03 to 1.00), indicating that the study is non-informative.
Conclusion
Few data were found on the diagnostic accuracy of FNAC or high-frequency ultrasonography for detecting recurrence or metastasis in patients initially diagnosed with stage I melanoma. This may be, in part, because the natural history of AJCC stage I melanoma results in relatively few patients developing metastatic spread of the condition, thereby limiting the scope for analysis and the relative need for research, compared with higher-risk groups. The data applicable to stage I disease that are available do not provide strong evidence to support the use of these tests in this target patient group. However, the consistency of findings with other reviews (often looking at a wider population) provides some reassurance.
Chapter 6 Economic evaluation
Introduction and aim
This chapter presents the methods and results of a cost-effectiveness analysis based on an economic model. This economic evaluation aims to compare alternative surveillance strategies provided by the NHS for people who have been treated for AJCC stage IA and IB melanoma in the UK.
Methods
Development of a cost-effectiveness model
The development of the cost-effectiveness model started with a targeted review of the literature (see Appendix 7), which identified 15 economic evaluation studies of potential relevance. 24,171–184 None of these directly addressed the study question posed here. Therefore, a de novo model was developed to compare potential surveillance strategies provided by the NHS.
Using the information gathered from the systematic reviews reported in Chapters 3–5 and extensive dialogue with the clinical members of the study team, we developed a Markov microsimulation model to evaluate the cost-effectiveness of plausible surveillance options based on differing clinical specialty, interval between visits and duration of follow-up. We used the most relevant UK epidemiological statistics and best available data from the literature to populate the model, reflecting the clinical reality of care for these patients. This included the probability of self-detection, ‘false alarms’ that result in unscheduled emergency appointments and a pathway of care that included investigational diagnosis and any subsequent treatment.
Model overview
The cost-effectiveness of surveillance strategies for stage I melanoma patients was assessed using a Markov microsimulation model. A microsimulation model is a form of economic modelling whereby individuals are simulated through the model one by one, rather than as a cohort. Individual results are then stored and the experience of the cohort is obtained by aggregating the individual results. 185 Markov health states are used to simply describe a patient’s health status. In cancer models, disease progression will occur and the progression of the disease is described by a set of health states, typically of increasing severity. These states are commonly described as tunnel states. The minimum period of time (defined as the cycle length) that a person can be in a state before moving to another state was taken as 1 month. A 1-month cycle length was chosen as surveillance strategies were based on monthly surveillance interval differences.
The decision to use a microsimulation model as opposed to a simpler cohort-based Markov model186 was based on the properties of a microsimulation. In a microsimulation, the memorylessness restrictions of a cohort-based Markov model can be overcome. Building the Markov microsimulation model in TreeAge Pro 2019 R1.0 (TreeAge Software Inc., Williamstown, MA, USA) enabled us to keep track of the treatment history, time since last treatment and recurrence of simulated patients, which all have an impact on the cost and effect estimates. In a cohort-based Markov model, movement from a given state is not affected by how the person arrived in that state (the model has no memory of prior events or care).
The microsimulation model describes the care pathway of individuals from the point when they received treatment (i.e. wide local excision) for stage I melanoma. The model allows for alternative surveillance strategies to be compared. The model seeks to estimate their longer-term (i.e. lifetime) costs and consequences, including those that might arise from any subsequent melanoma diagnosis, be it for new or recurrent disease. Surveillance strategies were compared based on lifetime costs and health outcomes, expressed in terms of quality-adjusted life-years (QALYs). These data were then used to estimate cost-effectiveness. The costs were estimated in Great British pounds from an NHS and Personal Social Service perspective for the financial year 2017–18. As both costs and QALYs were estimated over a lifetime time horizon, they were discounted using a 3.5% annual discount rate, as per NICE’s reference case. 187
We used anonymised individual data from 160 early-stage patients obtained from a local hospital (University Hospital of North Durham, ethics approval: NREC 19/NE/004) to populate the basic demographic characteristics (mean age 56 years, range 17–98 years; ratio of males to females 36% : 64%). Stage I patients (ratio of stage IA to stage IB was 76% : 24%) had up to 8 years’ follow-up on recurrence (15%) and mortality (melanoma-specific mortality over that time was 6% and all-cause mortality was 17%). Any additional melanoma skin cancer statistics were taken from the Cancer Research UK website. 188
Model structure
Once discharged into follow-up care, a patient enters into the model as disease free. Surveillance is captured as an event undertaken at discrete time intervals. At any given point in time, an individual is in one of four Markov states: disease free, recurrence (diagnosed stage IA–IV), death from melanoma or death from other causes. There is a chance that, when a recurrence occurs and is not detected in the monthly cycle, disease progression will occur. This is depicted as a tunnel state in Figure 8. The detailed model structure is shown in Appendix 8.
FIGURE 8.
Description of a simplified version of the Markov microsimulation model.

In the microsimulation model, each individual has a chance (probability) of recurrence, metastasis and occurrence of new primary tumours in a given monthly cycle. In the model, any future melanoma diagnosis can be self-detected in a cycle by an individual/partner. This will result in an unscheduled emergency visit. There is also a chance that the emergency visit is a ‘false alarm’ and that the patient does not have melanoma. If the individual was scheduled for a surveillance visit that month, there is a chance that the attending clinician will detect the melanoma. There is also a chance that a clinician will fail to diagnose a melanoma if one is present.
For all suspicious lesions, the diagnostic process starts with a history and physical examination with dermoscopy by a clinician (note that, although it is recommended by NICE that all examinations should use dermoscopy, anecdotally there is varied uptake across different specialist settings). The use of subsequent investigations is determined by the findings of the prior investigations (i.e. if a local excision biopsy is positive, then SLNB will be performed; if SLNB is positive, then CT is performed).
If a cancer is present but is not detected during a 1-month cycle, then the cancer may progress. If this occurs, then, in the next cycle of the model, the individual moves into a tunnel health state. The tunnel state itself describes the AJCC staging of melanoma.
The whole process described here for the movement of an individual through the model is then repeated for each individual in the cohort, thereby generating individual life histories of the population modelled.
Plausible strategies
The development of the model strategies was based on an iterative consultation process with the local clinical expert team. Potentially thousands of strategies are possible based on the possible service provision over a patient lifetime. 25 We eliminated non-realistic strategies, such as those involving general practitioners (GPs), who do not have the skills, confidence or capacity to provide this service in the UK, let alone the support of specialist organisations (Dr Timothy Cunliffe, chair-elect, Primary Care Dermatology Society, November 2018, personal communication). Furthermore, it was established that:
-
diagnostic imaging would not be considered as part of the surveillance regimen because the evidence from the USA189 and the systematic review reported in Chapter 5 did not support its use
-
prognostic risk models had not been suitably validated to form part of a plausible surveillance strategy (see Chapter 4).
The three variables used to define variations between surveillance strategies were clinical specialty, surveillance intervals and duration of follow-up. In calculating the number of strategies for both stage IA and IB disease, different combinations of the above stratifying variables were used. Initially, 400 strategies were defined for stage IA and 600 for stage IB. The clinical team reviewed these strategies iteratively, and those considered as implausible were excluded from further consideration. Following this process, the number of plausible strategies was reduced to 75 strategies for stage IA and 75 for stage IB. To complete the strategy selection process, we added 12 additional low-resource strategies that might be options for people who had initially been treated for stage IB disease. Thus, for stage IB, a total of 87 strategies were included in the final model.
The clinical specialty options for conducting the surveillance are as follows: dermatologist; surgeon; and specialist dermatological nurse, also generically referred to as clinical nurse specialist (CNS) in the NHS. The realistic frequency of follow-up considered was follow-up every 3, 4, 6 and 12 months. The durations of follow-up considered for stage IA were 1, 2, 5 and 10 years. For stage IB, the durations of follow-up considered were 1, 3, 5, 10 and 20 years. We compared all strategies with current practice, based on that which has been recommended by the NICE 2015 guidelines16 and the BAD 2010 revised UK guidelines27 for the management of cutaneous melanoma. Both the NICE16 and BAD27 guidelines advise that patients who have stage I melanoma are followed up to detect signs of recurrence after history and examination. The NICE guidelines16 recommend that patients with stage IA melanoma should be seen two to four times over 12 months following initial treatment, then discharged if no sign of recurrence or metastasis is found. Patients with stage IB melanoma should be seen every 3 months for 3 years, then every 6 months up to 5 years following initial treatment. At the end of that period, they should then be discharged if no sign of recurrence or metastasis is found.
Model inputs
To provide estimates of relative cost-effectiveness, the model required estimated values for a range of different types of parameters. We aimed to use the best available values derived in a systematic and reproducible manner to avoid bias caused by the distorted and selected use of data. 190 We focused on identifying the most relevant data to the decision problem, which is the comparison of alternative surveillance regimens after treatment (i.e. local excision) for stage I melanoma.
We assembled the different types of data required for the economic model from analyses of existing data sets, a series of systematic reviews and focused searches for specific types of data (see Appendix 9). In brief, the broad types of data required to populate the economic model were as follows:
-
patient behaviour with regards to surveillance and follow-up (e.g. self-detection/self-diagnosis and ‘false alarms’ resulting in emergency visits)
-
clinical pathway once diagnosed with melanoma
-
the performance of different regimens (e.g. clinical examinations) in terms of diagnostic accuracy (see Diagnostic accuracy)
-
the prevalence, incidence and risk of progression of the disease, risk of recurrence [i.e. its epidemiology and natural history (see Natural history of melanoma)]
-
resource use and unit costs required to estimate the costs of alternative surveillance regimens; the specific parameters and methods used to provide estimates that are relevant to the UK context (see Resource use and unit costs)
-
health-state utilities (see Health utilities).
Patient behaviour
We based estimates of patient behaviour on data from the literature and on advice from local clinical experts. For example, the probability of self-diagnosis was based on the MELanoma Follow-Up (MELFO) study. 23 This study reported that 8 out of 17 (48%) recurrences among 180 patients in the 1-year time-frame were identified by self-diagnosis. Based on advice from the clinical experts in the study team, a value of 60% was used in the base-case scenario in the NHS setting based on their experiences. The probability of a ‘false alarm’ emergency visit occurring was based on an earlier Dutch study, which reported that almost 80% of patients (538/699) with a Breslow thickness of < 1 mm reported more frequent follow-up visits than the guideline recommends. 191 The clinical experts in the study team thought that an emergency visit would occur for between 80% and 90% of patients in a given year in their clinic; therefore, a yearly value of 85% was used. Annual probabilities of self-diagnosis and ‘false alarm’ emergency visits were converted into a monthly probability.
Clinical pathway
The 2015 NICE guidelines,16 along with work from Wilson et al. ,182 was the starting basis of the clinical pathway modelled. As acknowledged in NICE’s decision to update its guidelines,16,192 new evidence has been identified that may change current recommendations. A plausible care pathway was modelled that used SLNB for staging; if positive, lymph node dissection was performed. The model did not include the ultrasonography-guided fine-needle aspiration option (because of lack of evidence; see Chapter 5), but did include the cost of treating advanced-stage disease with newer systematic targeted therapies and immunotherapies (Dr Janine Graham, South Tees Hospitals NHS Foundation Trust, June 2019, personal communication; see Appendix 9, Table 24, for list of clinical parameter values).
Epidemiology
National melanoma epidemiology statistics, such as summary stage of melanoma incidence and mean age at stage I diagnosis, were obtained from the National Cancer Registration and Analysis Service (NCRAS), which is now part of Public Health England (PHE) (NCRAS enquiries, February 2019, personal communication). The data on the natural history of melanoma were derived by expert elicitation among clinical experts from the UK. 193 The mean age of people diagnosed with AJCC stage I melanoma from 2000 to 2017 was 57.9 years (Charlotte Eversfield, PHE, NCRAS, personal communication).
How each set of data and the values used in the model were derived is described in more detail in the following sections.
Diagnostic accuracy
Diagnostic accuracy of practitioners
A Cochrane systematic review identified studies that described the accuracy of clinicians in identifying melanoma. 170 None of the studies identified in this review was from the UK. As part of the work conducted for the economic evaluation reported in this chapter, a meta-analysis of the diagnostic performance of different categories of staff was conducted using data from studies included in the Cochrane review. For dermatologists, data were pooled from 11 studies, all of which came from mainland Europe. 194–204 This gave a mean sensitivity of 0.875 (95% CI 0.784 to 0.931) and specificity of 0.893 (95% CI 0.792 to 0.949). For surgical oncologists, two studies (both from Italy) were pooled and gave a mean sensitivity of 0.886 (95% CI 0.795 to 0.940) and specificity of 0.734 (95% CI 0.688 to 0.775). 205,206 For specialist dermatological nurse or cancer nurse specialist, the only equivalent data were from a US study based on only eight physician assistants. 207 In that study, health-care professionals reviewed 65 dermoscopic images each; the physician assistants had a mean sensitivity of 0.800 (95% CI 0.590 to 0.930) and specificity of 0.470 (95% CI 0.320 to 0.640) (see Appendix 9, Table 31–33, for diagnostic accuracy values).
Diagnostic accuracy of staging disease
The sensitivity and specificity of local biopsy were obtained from a study that aimed to investigate how accurate and reproducible the results are of pathologists’ diagnosis of melanocytic skin lesions. 208 A total of 240 skin biopsy cases were grouped into sets of 36 or 48 and were assessed by a randomised sample of US pathologists from 10 US states on two different occasions within a period of at least 8 months. The results of the paper indicate that 82.8% (95% CI 81.0% to 84.5%) of melanocytic skin biopsy diagnoses would have their diagnosis verified if reviewed by a consensus reference panel of experienced pathologists.
The accompanying review of the clinical evidence for the NICE guideline was the source of data for staging of melanoma. 16 For patients, the sensitivity of SLNB in identifying micrometastatic nodal/regional disease was estimated to be 86.6% (95% CI 84.6% to 88.4%), based on 47 studies with 19,607 data points. Specificity was 100%, as was reported in the review conducted for the NICE guideline. For advanced-stage disease, a meta-analysis assessed the clinical utility of various diagnostic tests for staging and surveillance of melanoma patients. 147 The 2015 NICE guideline recommended that CT staging be offered to people with stage III or suspected stage IV melanoma. 16 According to the meta-analyses, the median sensitivity of CT was 51% (95% CrI 24% to 76%) and specificity was 69% (95% CrI 30% to 92%) for staging distant metastasis. The corresponding CrIs were used in the model as beta distributions (see Appendix 9, Tables 34–36, for diagnostic accuracy values). 147
Natural history of melanoma
Disease progression/transition probabilities
The natural history of undetected or untreated melanoma is unknown. 193 To get an estimate of the progression of melanoma within a certain time frame, expert elicitation was required. A novel approach to elicit expert opinion on the rate of progression from each stage to any other stage was developed and piloted on 14 clinical experts from the UK and Australia/New Zealand. 193 Participants were asked for their beliefs about the probability of progression from each of the starting stages stated (i.e. in situ to stage IV) to any other stage and death. Questions were asked in the format of: ‘Imagine a cohort of 100 patients with stage X undiagnosed and hence untreated disease. After 6 months, the patients may be in any of the following stages’. Experts assigned probabilities using the quantile method, whereby median and upper and lower 95% CrIs were elicited. Wilson et al. 193 fitted a modified Connor–Mosimann (mCM) distribution to the elicited quantities from each expert. The mCM distribution is a generalisation of the Dirichlet distribution, which defines a multinomial distribution. The median value was used with a Dirichlet distribution fitted of the UK experts’ opinions (Table 13). Because the probabilities derived reflected a 6-month cycle, they had to be converted to monthly probabilities for the purposes of the model. Furthermore, although the derived probabilities included transition to death, it was decided to use the ORs of death from a different study by the same author (see Mortality rates). 182 Given this adjustment, the sum of the probabilities for each stage was < 1, and thus had to be rescaled to ensure that they summed to 1.
| From stage | To stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIC | IIIA | IIIB | IIIC | IV | Death | |
| IA | 0.714 | 0.209 | 0.022 | 0.000 | 0.000 | 0.000 | 0.055 | 0.000 | 0.000 | – |
| IB | 0.732 | 0.093 | 0.062 | 0.041 | 0.052 | 0.000 | 0.021 | 0.000 | – | |
| IIA | 0.617 | 0.191 | 0.074 | 0.074 | 0.011 | 0.021 | 0.011 | – | ||
| IIB | 0.477 | 0.205 | 0.159 | 0.102 | 0.057 | 0.000 | – | |||
| IIC | 0.416 | 0.225 | 0.157 | 0.124 | 0.079 | – | ||||
| IIIA | 0.654 | 0.198 | 0.099 | 0.049 | – | |||||
| IIB | 0.365 | 0.435 | 0.200 | – | ||||||
| IIIC | 0.549 | 0.451 | – | |||||||
| IV | 0.450 | |||||||||
| Death | ||||||||||
Recurrence probabilities
Ideally, melanoma incidence data from the NCRAS/PHE would be used as a surrogate to find the recurrence probability in England/UK. However, the NCRAS/PHE data are based on the summary stage information (i.e. AJCC stage I–IV) of all patients diagnosed by Clinical Commissioning Groups (CCGs) in England. The Durham cohort did not record the stage of recurrence adequately. Therefore, more granular AJCC stage I–III incidence information (stages IA, IB, IIA, IIB, etc.) is needed for the model. A large registry-based German study (n = 33,384),209 which is likely to be similar to English patient cohorts in terms of characteristics of individuals, was used for this purpose. From the German study, the proportion of recurrences was 43.9% for AJCC stage IA, 25.3% for stage IB, 12.5% for stage IIA, 7.7% for stage IIB, 2.6% for stage IIC, 1.0% for stage IIIA and 3.0% for stage IV.
Recurrence probabilities over time for AJCC stages IA–IIC were obtained from a different study, conducted in Australia by Turner et al. 210 The study authors analysed 2298 patient records for the development of recurrence and new primary melanoma up to 10 years. In this paper,210 Kaplan–Meier curves showing time to recurrence for localised melanoma were able to be digitised using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer; accessed 23 April 2020). Various points along the curve were chosen and the co-ordinates of those points were extracted. These were then used to calculate the lamda and gamma parameters of the Weibull distribution for AJCC stage IA. The two parameters were then used to calculate the transition probabilities (tp) for recurrence using the following equation:
where:
-
t is time (measured in terms of the number of cycles; each cycle is equivalent to 1 month)
-
λ is the scale parameter, which describes the probability that melanoma recurs for an individual, given that he/she is recurrence free during the current time period
-
γ is the shape parameter, which describes the hazard function of Weibull function for the survival time
-
µ is the length of the Markov cycle.
Moreover, for the calculation of the baseline transition probability, the following formula was used, in which µ is the length of the Markov cycle:
Recurrence rates for the remaining melanoma stages were computed as a function of the probability of recurrence of stage IA and the distribution of the hazard ratio of each stage up to stage IIC reported in the study. By using the CIs presented in the paper, the corresponding standard errors (SEs) were calculated, which, along with the hazard ratios, were used as Dirichlet distributions in the model (see Appendix 9, Tables 26–30, for recurrence values). Because it was not possible to source recurrence ratios for AJCC stages III and IV, it was assumed that the recurrence rates for these two stages are similar to those of stage IIC. Given the very poor prognosis for these stages, and the non-curative nature of treatments, this was not expected to cause any meaningful limitation to the model. Depending on the stage of the recurrence in the model, the monthly probabilities were selected (e.g. stage IA: 0.0022 or 0.22%; and stage IB: 0.0046 or 0.46%).
Mortality rates
Age-specific, all-cause mortality rates were derived from general population mortality statistics reported in national life tables from the Office for National Statistics. 211 The melanoma-specific risks of death for each stage, as reported by Wilson et al. ,182 were used and these were expressed as ORs. These mortality ORs are based on the original US-based AJCC Staging Database (n = 17,600) (see Appendix 9, Tables 24 and 25). 90
Resource use and unit costs
The costs of surveillance were broken down into the following cost categories: scheduled surveillance (e.g. consultation time), further invasive tests (e.g. local biopsy, SLNB) and treatment (e.g. targeted therapy and immunotherapy).
We derived data on the costs incurred for the different surveillance regimens and their consequences from routine data sources, such as the NHS reference costs. 212 Table 14 shows the cost estimates used in the economic model. The costs of drug treatment, targeted therapy [trametinib in combination with dabrafenib, NICE Technology Appraisal (TA) 396213] for AJCC stage III and immunotherapy for AJCC stage IV disease (nivolumab in combination with ipilimumab, NICE TA400214) were obtained from the British National Formulary as per current clinical treatment regimen. 215 Gamma distributions were assigned to cost values for stages III and IV treatments (see Appendix 9, Table 38).
| Description | Mean cost (£) | Source |
|---|---|---|
| Clinical specialty | ||
| CNS | 89 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Surgeon | 97 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Dermatologist | 108 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Diagnostic investigations | ||
| Local biopsy | 387a | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Wide local excision | 218 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| CT | 106 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Surgical costs | ||
| Radical lymph node dissection | 1599 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| SLNB | 1599 | Reference Costs 2017/18: Highlights, Analysis and Introduction to the Data 212 |
| Treatment | ||
Stage III, per month (targeted therapy):
|
10,400 | Based on local clinical expert opinion. This is the average monthly cost of dabrafenib (£5600) + trametinib (£4800) |
Stage IV, per month (immunotherapy):
|
10,326 | Based on local clinical expert opinion. This is the average monthly cost based on 12 months of combination treatment with ipilimumab (£15,000/vial of 200 mg/40 ml) + nivolumab (£2663/vial of 240 mg/24 ml) |
Health utilities
As melanoma and its treatment affects not only survival, but also quality of life, a focused search of the literature and other relevant sources [e.g. the Sheffield School of Health and Related Research Health Utilities Database (ScHARRHUD) and the Health Economics Research Centre (HERC), Oxford, database of mapping studies] was initiated (see Appendix 9, Table 37). However, in 2018, a systematic review and meta-analysis of melanoma utility weights based on 33 studies was published. 216 This review included studies from Australia, Europe and North America, and sought to define post-treatment utilities by stage with time of data collection component up to 3 months, 3–12 months and > 12 months. The synthesis of health-state utilities is controversial. Although it aims to generate a more accurate estimate of the mean health-state utility and the associated uncertainty, a formal synthesis may not be meaningful considering the variability in measures [e.g. EuroQol-5 Dimensions (EQ-5D) vs. Short Form questionnaire-6 Dimensions], valuation method, types of anchors used, the country where the valuation was done and who provided the preference weights. 217
As no relevant utility values derived from a UK population were available, the published values from the meta-analysis were used in the economic model (Table 15). It is further assumed that individuals in the ‘disease-free’ state have a health-state utility value of full health (utility score = 1), as per Wilson et al. 182 These are values without age adjustment. These values were chosen for the model as they are published in the literature and are consistent across all strategies. Beta distributions were assigned to utility values in the base-case analysis.
| Time on treatment (months) | Utility estimates (95% CI) |
|---|---|
| Disease free | 1.000 |
| AJCC stage I/II | |
| Day 1 to 3 months | 0.772 (0.753 to 0.790) |
| 3–12 months | 0.852 (0.844 to 0.860) |
| > 12 months | 0.857 (0.850 to 0.865) |
| AJCC stage III/IV | |
| Day 1 to 3 months | 0.803 (0.783 to 0.823) |
| 3–12 months | 0.797 (0.786 to 0.809) |
| > 12 months | 0.848 (0.787 to 0.910) |
| AJCC stage IV | |
| Day 1 to 3 months | 0.653 (0.621 to 0.685) |
| 3–12 months | 0.831 (0.808 to 0.855) |
| > 12 months | 0.833 (0.820 to 0.847) |
Main modelling assumptions
This section provides a brief summary of the key assumptions made when developing the economic model. It was assumed that the time interval between initial surgery and any subsequent event/treatment is 1 month. No utility decrements associated with undertaking any of the tests (local biopsy, SLNB, etc.) or treatments (immunotherapies have severe adverse effects) were incorporated in the model. It was assumed that the delay in recurrence/new primary diagnosis would eventually be captured by self-detection as advanced disease symptoms become evident. It was also assumed that individuals attend all of their scheduled follow-up appointments, that the initial surgical excision is curative and that individuals enter the model as disease free. Recurrence rates for stages IIIA, IIIB, IIIC and IV were assumed to be similar to the recurrence rates for stage IIC. Estimates of melanoma-specific risk of death for each stage were taken from Wilson et al. 182 It was assumed that patients received systemic treatment until treatment failure or death (Dr Janine Graham, personal communication). In the model, it was assumed that patients were BRAF positive and received dabrafenib and trametinib targeted therapy. The literature suggests that 50% of metastatic melanomas are BRAF positive. 218 If patients were BRAF negative, they would probably receive immunotherapy (e.g. ipilimumab). 219 The assumption that stages III and IV are now treated with new (expensive) therapies is captured in the model and the assumed costs are approximations. Finally, the ‘no-surveillance’ option was not considered in the model, as it was deemed to be unacceptable to patients by the advisory board.
Incremental cost-effectiveness analysis
Base-case analysis
The joint estimates of costs and effects were combined in an incremental analysis and presented in terms of the mean incremental cost-effectiveness ratio (ICER) for each comparator. The ICERs were calculated as the difference in costs divided by the difference in effects (QALYs) between treatment options. The ICERs were calculated for each successive alternative, from the least costly to the most costly. To help identify the optimal approach, the net monetary benefit (NMB) framework was used, whereby the NMB for a given strategy is equal to the accrued QALYs multiplied by the ceiling ratio (λ) of willingness to pay (WTP) per QALY, minus the strategy costs:
A value of £20,000, which is typically used by NICE as a threshold to inform judgements on cost-effectiveness, was placed on the ceiling ratio. 220 The threshold means that NICE is prepared to pay £20,000 for each extra QALY gained.
Measures of variance for the joint incremental costs and effects were obtained using Monte Carlo simulation in the probabilistic sensitivity analysis (PSA) (see Probabilistic sensitivity analysis) and presented graphically using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). 221
Sensitivity analyses
Both deterministic and probabilistic sensitivity analyses were used to explore parameter uncertainty surrounding estimates of cost-effectiveness. Threshold analyses were used to explore further considerations around the main parameters of interest.
Deterministic sensitivity analyses
Deterministic sensitivity analysis is a method that can be used to investigate the sensitivity of the results to variations in a specific input parameter or set of parameters. The parameters of most interest were manually changed (across a plausible range) and the results were analysed to determine to what extent the change affects the output values.
Given that microsimulation models are computationally burdensome, and owing to the large number of strategies, deterministic analysis also helped exclude strategies that were deemed never to be cost-effective. Therefore, many of the model parameters were subject to one-way sensitivity analyses, using hypothetical increases or decreases, to determine the key drivers of the model results. Deterministic sensitivity analyses were also carried out to test for the effect of assumptions and variability.
Probabilistic sensitivity analysis
When available, data were also entered into the model as distributions to more fully incorporate the uncertainty around parameter values so that a PSA could be undertaken. In decision modelling, many of the parameter values are often estimated with a degree of uncertainty. The probabilistic distribution for each parameter was defined by considering the mean, SE and anticipated shape of the distribution. The PSA was run with 1000 simulations for each individual and CEACs were produced to identify the probability of the different strategies being cost-effective across a range of WTP thresholds. Estimation of costs and QALYs were calculated as the expectation over the joint distribution of the parameters.
One-way sensitivity and scenario analyses
One-way sensitivity analyses can indicate the effect of parameter values on the expected net benefit, but not the overall decision on what strategy the NHS should approve. A key feature in the model is the role of self-diagnosis (self-detection). In their paper, Turner et al. 210 reported that self-detection rates for recurrence ranged from as low as 15%222 up to 90%. 223 For new primaries, the literature suggests plausible self-detection rates as low as 5%222 and as high as 50%. 224 The aforementioned studies do not explicitly mention a time period over which this self-detection occurs. Therefore, additional analyses are warranted to determine if optimal strategies, in terms of NMB, change in response to changes in self-detection rates.
The nature of recurrence of early-stage disease in the base-case analysis was taken from an Australian study. 210 It is likely that the natural history of melanoma is similar around the world, irrespective of geography. However, there is uncertainty in the estimate and additional analyses are warranted to determine if there is a threshold when, in terms of NMB, optimal strategies change in response to changes in recurrence rates.
The use of a meta-analysis for utility values estimate is controversial. 217 As the model is set up in a way that recurrences are detected ranging from stage IA to IV, the utility values are likely to have a plausible range that is greater than the range in the base case. This sensitivity analysis assessed whether or not the relative efficiency of strategies changed when alternative utility values were used.
From an NHS health-care management perspective, the role of specialist dermatological nurses in melanoma surveillance of stage I patients is worth exploring further. This is to ascertain what level of diagnostic accuracy they need to have to be considered a viable strategy, compared with the other strategies considered.
Even though a validated prognostic risk model was not identified in the systematic review, reported in Chapter 4, the hypothetical scenario of a prognostic approach (be that as a risk model, a biomarker test, or a combination of the two) is explored here. As evidence emerges in this field about such technologies, it is plausible that this may lead to further plausible surveillance strategies that could be potentially cost-effective.
Value-of-information analysis
A critical role of the PSA was that it facilitated the estimation of the value of further research. Therefore, in addition to exploring the cost-effectiveness of different follow-up strategies for individuals with stages IA and IB melanoma, the main uncertainties were quantified to inform any future direction of research. This was explored through the expected value of partial perfect information (EVPPI) analysis. EVPPI analysis can be used to estimate the expected value of removing the uncertainty of specific parameters or groups of parameters in a given model. It can be used to help guide decisions on where future research should be directed to obtain more precise and reliable estimates of specific parameters. 225 EVPPI places an upper value on conducting further research on a specific area of information. It indicates the maximum gain that could be obtained by removing all uncertainty in that area. In reality, any piece of future research would remove only part of that uncertainty, but the approach can still help indicate where the most gain could be made from further research.
To calculate the population EVPPI, the individual EVPPI is first calculated by the model. The individual EVPPI is then multiplied by the size of the population that will be affected by the information over the anticipated lifetime of the technology. For the estimation of the size of the population, the annual prevalence (the number of patients affected by the decision each year) of stage I disease is needed. The assumption is that the annual incidence of stage I melanoma is, in essence, the number of patients affected by the decision each year. In 2017, 8555 patients were diagnosed with stage I disease. 226 The proportion (63% for IA vs. 37% for IB) was provided by Leiter et al. 209 in their work based in Germany.
Two-level simulations were conducted to estimate the EVPPI. The first level happened in the microsimulation by randomly selecting individuals of different age and sex from the individual-level data. Then, each selected individual was simulated 10,000 times (PSA) and values for the parameters were selected from the prespecified distributions. Results from the PSA were then used in the Sheffield Accelerated Value of Information (SAVI) tool to estimate the EVPPI. 227
Four groups of parameters were considered in the EVPPI analysis:
-
health utility values
-
diagnostic accuracy of health-care professional to detect a melanoma
-
probability of transitioning between stages
-
recurrence of melanoma.
Model validation
Model validation was achieved by clinical expert concurrence, by a review by modelling colleagues at Newcastle University and by being transparent in reporting. As laid out in a review on validating cost-effectiveness models, ‘internal validity’ related to input parameters, whereas ‘descriptive validity’ of a clinical pathway requires analysts to make trade-offs between accuracy, complexity and fulfilling the purpose of the model. 228 For this project, the objective was to compare surveillance strategies for stage I disease, rather than capturing the complex and evolving management of advanced-stage disease.
Results
Base-case analysis results
Given the computational burden of running the model with 10,000 PSA simulations over 87 strategies, the model was first run deterministically to identify 20 strategies that were considered to have the most promise of being cost-effective for the stage IA model. This was repeated for the stage IB model. These strategies were then used in the base-case analysis.
Base-case analysis: stage IA
Monte Carlo simulation was performed to obtain probabilistic estimates of the cost-effectiveness of the different surveillance strategies, compared with NICE’s recommended follow-up schedule. The 20 strategies included in the analysis are presented in Table 16.
| Strategy | Duration of follow-up | Intervals of follow-up each year | Health-care professional undertaking screening |
|---|---|---|---|
| 1–NICE | 1 year | Every 3 months for 1 year | Dermatologist |
| 4 | 10 years | Every 3 months for 1 year and every 6 months thereafter | Dermatologist |
| 7 | 10 years | Every 3 months the first year and every 12 months thereafter | Dermatologist |
| 14 | 10 years | Every 4 months the first year and every 12 months thereafter | Dermatologist |
| 15 | 1 year | Every 6 months for 1 year | Dermatologist |
| 16 | 3 years | Every 6 months for 3 years | Dermatologist |
| 19 | 3 years | Every 6 months for 1 year and every 12 months thereafter | Dermatologist |
| 21 | 10 years | Every 6 months for 1 year and every 12 months thereafter | Dermatologist |
| 22 | 1 year | Once for 1 year | Dermatologist |
| 23 | 3 years | Every 12 months for 3 years | Dermatologist |
| 29 | 10 years | Every 3 months for 1 year and every 6 months thereafter | Surgeon |
| 32 | 10 years | Every 3 months for 1 year and every 12 months thereafter | Surgeon |
| 37 | 3 years | Every 4 months for 1 year and every 12 months thereafter | Surgeon |
| 39 | 10 years | Every 4 months for 1 year and every 12 months thereafter | Surgeon |
| 40 | 1 year | Every 6 months for 1 year | Surgeon |
| 41 | 3 years | Every 6 months for 3 years | Surgeon |
| 44 | 3 years | Every 6 months for 1 year and every 12 months thereafter | Surgeon |
| 46 | 10 years | Every 6 months for 1 year and every 12 months thereafter | Surgeon |
| 47 | 1 year | Once for 1 year | Surgeon |
| 48 | 3 years | Every 12 months for 3 years | Surgeon |
Table 17 and Figure 9 (which excludes strategies that are, on average, dominated) report the results of the base-case analyses for the average patient treated for stage IA melanoma. Results are reported in terms of total and incremental costs and effectiveness, incremental cost per QALY (ICER) and NMB (were society’s WTP for a QALY (λ) = £20,000 per QALY), alongside the probability that each strategy would have the highest NMB for different threshold values used by NICE.
| Strategya | Cost (£) | Incremental cost (£) | QALY | Incremental QALY | ICER (£) (Δcost/ΔQALY) | NMB (£) | Probability of being cost-effective for different threshold values for society’s WTP for a QALY (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | £50,000 | |||||||
| 22 | 8476 | 14.74 | 286,321 | 13.2 | 9.7 | 6.2 | |||
| 15 | 8824 | 348 | 14.75 | 0.01 | 33,367 (extendedly dominatedb) | 286,181 | 7.1 | 5.6 | 4.1 |
| 23 | 8874 | 50 | 14.73 | –0.02 | Absolutely dominatedc | 285,820 | 10.8 | 9.5 | 7.6 |
| 19 | 9215 | 391 | 14.77 | 0.02 | 25,894 d | 286,153 | 9.8 | 8.4 | 6.7 |
| 1-NICE | 9336 | 121 | 14.76 | –0.00 | Absolutely dominatedc | 285,952 | 12.4 | 10.3 | 8.2 |
| 16 | 9613 | 399 | 14.77 | 0.00 | 199,677(extendedly dominatedb) | 285,794 | 7.2 | 6.8 | 6.0 |
| 21 | 10,254 | 641 | 14.76 | –0.01 | Absolutely dominatedc | 284,952 | 8.3 | 8.2 | 7.3 |
| 14 | 10,494 | 881 | 14.76 | –0.01 | Absolutely dominatedc | 284,747 | 11.3 | 11.4 | 10.4 |
| 7 | 10,768 | 1154 | 14.78 | 0.01 | 103,890 e | 284,899 | 7.8 | 7.9 | 7.1 |
| 4 | 12,222 | 1455 | 14.78 | 0.00 | 1,462,441 (extendedly dominatedb) | 283,464 | 6.3 | 7.6 | 8.1 |
| 47 | 13,909 | 1687 | 14.74 | –0.04 | Absolutely dominatedc | 280,893 | 1.7 | 2.7 | 3.6 |
| 40 | 14,427 | 2205 | 14.75 | –0.04 | Absolutely dominatedc | 280,522 | 0.1 | 0.8 | 2.2 |
| 48 | 14,579 | 2356 | 14.74 | –0.05 | Absolutely dominatedc | 280,173 | 0.7 | 1.4 | 2.2 |
| 44 | 15,072 | 2850 | 14.76 | –0.03 | Absolutely dominatedc | 280,089 | 0.6 | 1.5 | 3.0 |
| 37 | 15,518 | 3296 | 14.76 | –0.02 | Absolutely dominatedc | 279,749 | 0.7 | 1.5 | 2.6 |
| 41 | 15,754 | 3532 | 14.76 | –0.02 | Absolutely dominatedc | 279,501 | 0.3 | 0.8 | 1.7 |
| 46 | 16,923 | 4701 | 14.77 | –0.02 | Absolutely dominatedc | 278,449 | 0.5 | 1.2 | 3.2 |
| 39 | 17,367 | 5145 | 14.78 | –0.00 | Absolutely dominatedc | 278,227 | 0.6 | 2.1 | 4.0 |
| 32 | 17,765 | 5543 | 14.78 | 0.00 | 10,081,791 (extendedly dominatedb) | 277,932 | 0.3 | 1.9 | 3.6 |
| 29 | 20,319 | 2554 | 14.79 | 0.01 | 1,335,810 f | 275,490 | 0.3 | 0.7 | 2.2 |
FIGURE 9.
The CEAC for stage IA melanoma (dominated strategies excluded). See Table 16 for strategy description.

For stage IA, over a lifetime time horizon, none of the strategies involving a specialist dermatological nurse is likely to be considered cost-effective. It is worth noting that, overall, all strategies for stage IA produced very similar QALYs. This might be expected, given the relatively low chance of recurrence or metastasis and the high rate of self-diagnosis (it has been assumed that 60% of all melanomas are self-diagnosed within 12 months). Therefore, the lifetime costs are the main driver for evaluating surveillance strategies.
Strategy 22, which is follow-up once at 12 months by a dermatologist, is, on average, the least costly surveillance strategy (£8476 per person) and generates 14.74 QALYs. This strategy also has the highest NMB (£286,321) at a WTP threshold of £20,000 per QALY. Should the NHS wish to make a decision on cost alone, then this strategy has approximately a 60% chance of being considered the most cost-effective. As society’s willingness for a QALY increases, other, more effective, strategies become increasingly cost-effective. At a £20,000 threshold for society’s WTP for a QALY, strategy 22 has almost a 13% probability of being considered cost-effective. At the same threshold, three other strategies have a probability of > 10% of being cost-effective. These are strategy 23 (follow-up every 12 months for 3 years), strategy 1 (the NICE-recommended strategy: every 3 months for 1 year) and strategy 14 (surveillance every 4 months for the first year and then every 12 months for the next 9 years). Two strategies have a probability of 8–10% of being cost-effective: strategy 19 (every 6 months for 1 year and every 12 months for the next 2 years) and strategy 21 (every 6 months for 1 year and every 12 months for the next 9 years).
Given that 20 strategies are being compared, should each strategy be equally likely to be considered cost-effective, it would be expected that each strategy would have only a 5% chance of being considered cost-effective. Several of the dermatology-based strategies (including the NICE-recommended strategy) have probabilities greater than this, and four strategies have probabilities of > 10%. These four include strategies that are both less intensive and more intensive than the strategy recommended by NICE.
Base-case analyses: stage IB
The 20 strategies included in the analysis for stage IB disease are presented in Table 18. As with strategies for stage IA, strategies involving the specialist dermatological nurse were judged very unlikely to be considered cost-effective and were not included in the base-case analysis.
| Strategy | Duration of follow-up | Intervals of follow-up each year | Health-care professional undertaking screening |
|---|---|---|---|
| 1–NICE | 5 years | Every 3 months for the first 3 years and every 6 months thereafter | Dermatologist |
| 2 | 3 years | Every 3 months for 3 years | Dermatologist |
| 4 | 20 years | Every 3 months for the first 3 years and every 6 months thereafter | Dermatologist |
| 5 | 5 years | Every 3 months for the first 3 years and every 12 months thereafter | Dermatologist |
| 8 | 3 years | Every 4 months for 3 years | Dermatologist |
| 9 | 5 years | Every 4 months for the first 3 years and every 6 months thereafter | Dermatologist |
| 11 | 20 years | Every 4 months for the first 3 years and every 6 months thereafter | Dermatologist |
| 15 | 3 years | Every 6 months for 3 years | Dermatologist |
| 18 | 20 years | Every 6 months for 20 years | Dermatologist |
| 23 | 5 years | Every 12 months for 5 years | Dermatologist |
| 25 | 20 years | Every 12 months for 20 years | Dermatologist |
| 29 | 20 years | Every 3 months for the first 3 years and every 6 months thereafter | Surgeon |
| 77 | 1 year | Once for 1 year | Dermatologist |
| 78 | 1 year | Once for 1 year | Surgeon |
| 80 | 2 years | Every 12 months for 2 years | Dermatologist |
| 81 | 2 years | Every 12 months for 2 years | Surgeon |
| 82 | 2 years | Every 6 months for 2 years | Surgeon |
| 83 | 2 years | Every 6 months for 2 years | Dermatologist |
| 86 | 1 year | Every 6 months for 1 year | Dermatologist |
| 87 | 1 year | Every 6 months for 1 year | Surgeon |
Table 19 and Figure 10 report the results of the base-case analyses for stage IB melanoma patients. None of the strategies involving a surgeon conducting the surveillance had a probability of > 1% of being cost-effective across any of the values for society’s WTP for a QALY. The strategy recommended by NICE (strategy 1, surveillance by a dermatologist every 3 months for the first 3 years and then every 6 months thereafter for the next 2 years) also never had a probability of > 6% of being cost-effective over any of the values for society’s WTP for a QALY. As with the analysis for stage IA disease, given that 20 strategies are being compared, should each strategy be equally likely to be considered cost-effective, it would be expected that each strategy would have only a 5% chance of being considered cost-effective.
| Strategya | Cost (£) | Incremental cost (£) | QALY | Incremental QALY | ICER (£) (Δcost/ΔQALY) | NMB (£) | Probability of being cost-effective for different threshold values for society’s WTP for a QALY (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | £50,000 | |||||||
| 77 | 9536 | 14.57 | 0.00 | 281,945 | 12.9 | 9.7 | 6.8 | ||
| 80 | 9833 | 297 | 14.61 | 0.04 | 7818 | 282,408 | 9.8 | 7.9 | 6.2 |
| 86 | 9971 | 141 | 14.62 | 0.00 | 47,383 (extendedly dominatedb) | 282,326 | 10.4 | 8.4 | 6.0 |
| 23 | 10,320 | 346 | 14.60 | –0.01 | Absolutely dominatedc | 281,727 | 8.6 | 7.7 | 6.1 |
| 83 | 10,475 | 501 | 14.63 | 0.02 | 31,172 | 282,146 | 6.2 | 5.2 | 4.0 |
| 15 | 10,696 | 221 | 14.62 | –0.01 | Absolutely dominatedc | 281,642 | 4.5 | 3.6 | 3.2 |
| 8 | 11,420 | 945 | 14.61 | –0.02 | Absolutely dominatedc | 280,811 | 6.5 | 5.7 | 5.0 |
| 25 | 11,910 | 1435 | 14.63 | –0.01 | Absolutely dominatedc | 280,600 | 7.9 | 7.7 | 7.1 |
| 9 | 12,158 | 1683 | 14.62 | –0.01 | Absolutely dominatedc | 280,332 | 6.5 | 6.8 | 7.1 |
| 2 | 12,187 | 1712 | 14.64 | 0.01 | 175,956 (extendedly dominatedb) | 280,629 | 3.6 | 3.6 | 4.0 |
| 5 | 12,485 | 297 | 14.64 | –0.01 | Absolutely dominatedc | 280,317 | 3.6 | 4.1 | 4.4 |
| 1–NICE | 12,748 | 561 | 14.64 | 0.00 | 3,079,045 (extendedly dominatedb) | 280,072 | 4.1 | 4.9 | 5.5 |
| 18 | 14,607 | 1858 | 14.65 | 0.01 | 139,038 (extendedly dominatedb) | 278,480 | 5.5 | 7.1 | 8.1 |
| 78 | 14,970 | 363 | 14.59 | –0.06 | Absolutely dominatedc | 276,838 | 0.2 | 1.6 | 2.8 |
| 11 | 15,352 | 745 | 14.65 | –0.00 | Absolutely dominatedc | 277,657 | 4.7 | 6.1 | 7.5 |
| 81 | 15,391 | 784 | 14.62 | –0.04 | Absolutely dominatedc | 276,926 | 0.7 | 1.2 | 2.0 |
| 87 | 15,552 | 946 | 14.62 | –0.04 | Absolutely dominatedc | 276,748 | 0.8 | 1.5 | 2.3 |
| 4 | 16,045 | 1438 | 14.69 | 0.03 | 45,598 | 277,673 | 2.8 | 4.6 | 6.2 |
| 82 | 16,353 | 309 | 14.64 | –0.05 | Absolutely dominatedc | 276,400 | 0.7 | 1.8 | 2.6 |
| 29 | 25,871 | 9826 | 14.69 | –0.00 | Absolutely dominatedc | 267,840 | 0.0 | 0.8 | 3.1 |
FIGURE 10.
The CEAC for stage IB strategies (dominated strategies excluded). See Table 18 for strategy description.

For stage IB, over a lifetime time horizon, strategy 77 (follow-up once for 1 year by a dermatologist) is, on average, the least costly strategy (£9536) and generates 14.57 QALYs. Should society not be willing to pay for additional QALYs, then this strategy has a 55% chance of being cost-effective. As society’s WTP for a QALY increases, the probability that strategy 77 would be considered cost-effective falls, but strategy 77 has the highest probability of being cost-effective (12.9%) when society is willing to pay £20,000 per QALY. At the same threshold value, only one other strategy considered had a probability of being cost-effective of > 10%: strategy 86 (surveillance by a dermatologist every 6 months for 1 year). It is worth noting that, at a WTP threshold of £20,000 per QALY, strategy 80 (surveillance by a dermatologist every 12 months for 2 years) had the highest NMB and an ICER of £7818 per QALY gained, compared with strategy 77. Three further strategies had a probability of being cost-effective of between 7% and 10%: strategy 80, strategy 23 (surveillance by a dermatologist every 12 months for 5 years) and strategy 25 (surveillance by a dermatologist every 12 months for 20 years). With the exception of strategy 25, all of the aforementioned strategies are less intensive than the NICE-recommended strategy. Strategy 25 has a longer follow-up duration (20 years), with patients followed up annually.
Sensitivity analyses results
The results of the PSA base case best capture the uncertainty in the decision problem. The one-way sensitivity can help indicate the effect of parameter values on the expected NMB of each comparator strategy (see Appendix 10).
Keeping all other parameters at their base-case values, the impact of the probability of self-diagnosis on the NMB was examined. The model uses a yearly probability of 60%, which was transformed to a monthly probability (0.074 or 7%). A number of sensitivity analyses were run for lower and higher values of self-diagnosis. Strategies with a probability of > 1% of being cost-effective at a WTP of £20,000 were chosen for stages IA and IB. Results are presented in Figures 11 and 12 in terms of NMB, and in Appendix 10, Tables 39 and 40, in terms of cost, effectiveness, ICER and NMB of each strategy. Figure 11 and Appendix 10, Table 39, report the results for stage IA; Figure 12 and Appendix 10, Table 40, report the results for stage IB. The results indicate that, without self-diagnosis, more intensive and longer surveillance strategies for stage IA (strategies 21, 14, 7 and 4) and stage IB (strategies 25, 18, 11 and 4) produce a fraction more QALYs (< 0.2 over the estimated patient’s average life expectancy) and are more expensive (stage IA: £2000–5000; stage IB: £4000–10,000). At higher monthly probabilities of self-diagnosis (≥ 0.35, or 35%), the benefits of surveillance are questionable as there is such little gain in QALYs. Thus, more costs are incurred from the continuous surveillance without any substantial gain in QALYs.
FIGURE 11.
One-way sensitivity analysis of monthly probability of self-diagnosis for stage IA patients.
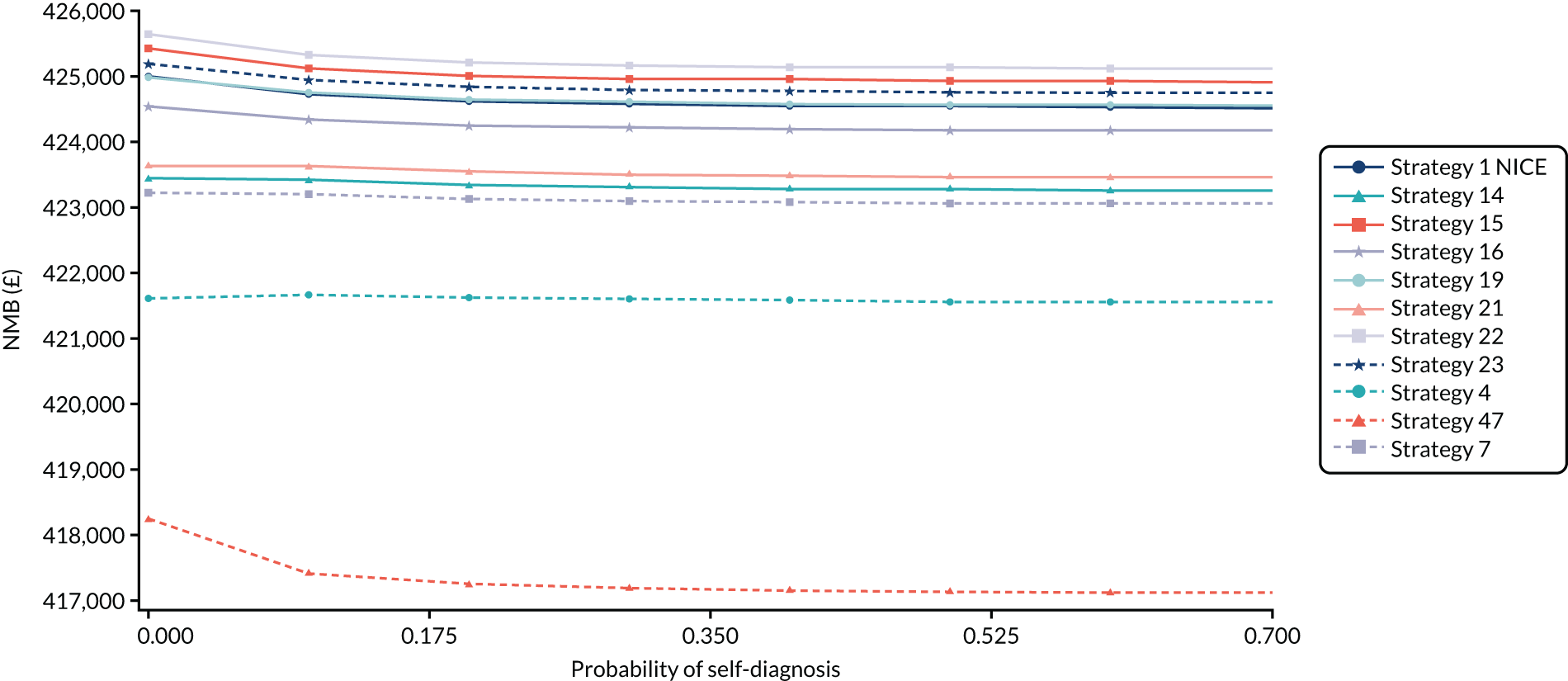
FIGURE 12.
One-way sensitivity analysis of monthly probability of self-diagnosis for stage IB patients.
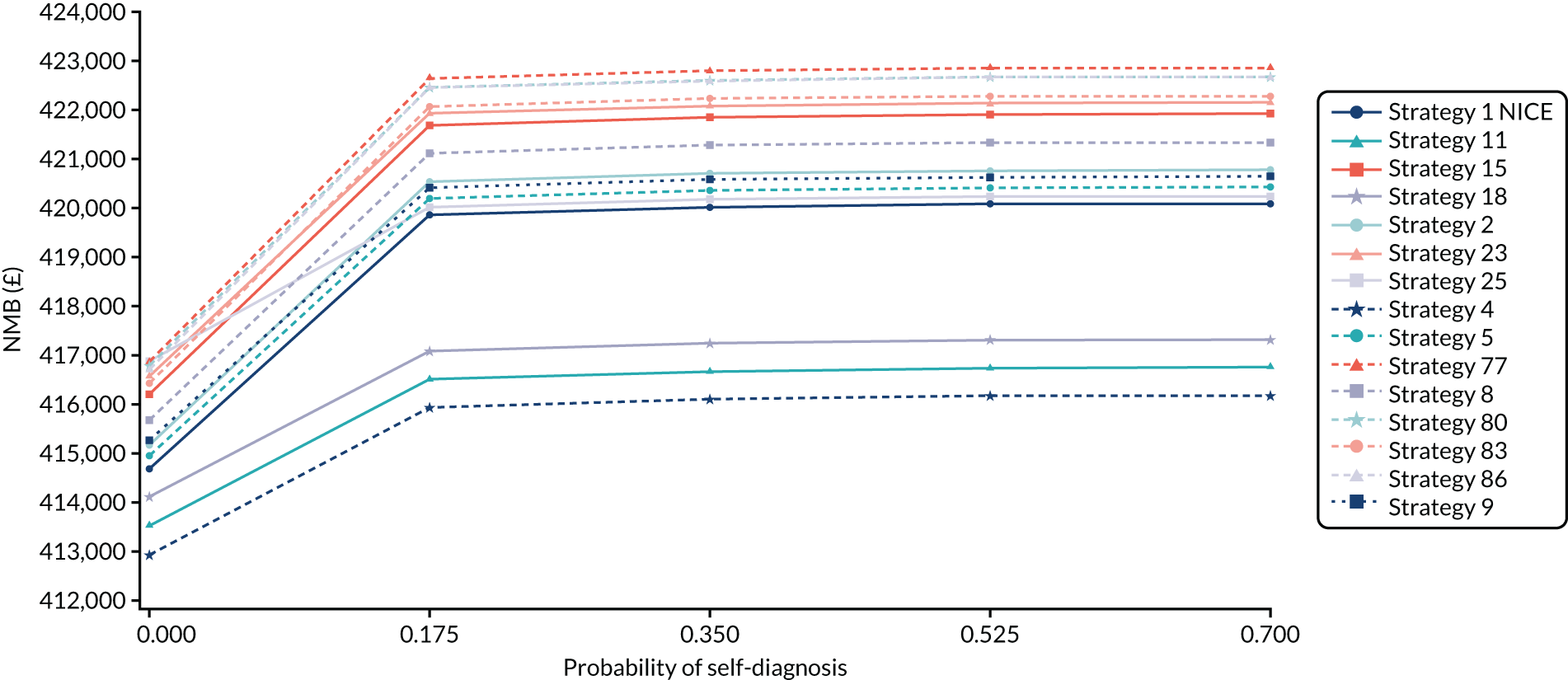
Similar patterns are observed when the probabilities of recurrence are varied and all other parameters are kept at their base-case values. Results are presented in Figures 13 and 14 and in Tables 19 and 20 for stages IA and IB, respectively (further results are reported in Appendix 10, Tables 41 and 42). At no or very low monthly probability of recurrence, more intensive and longer strategies for stage IA (strategies 21, 14, 7 and 4) and stage IB (strategies 25, 1-NICE, 18, 11 and 4) are more costly without producing any additional QALYs. As monthly recurrence probabilities increase, QALYs tend to drop (as would be expected because there are reductions in quality of life and survival). When monthly recurrence probability reaches ≥ 0.29, effectiveness between high and low resource use surveillance strategies, in terms of QALYs gained, is low (e.g. for stage IB, for strategy 11, there are 19.49 QALYs, and for strategy 77, there are 19.45 QALYs). Although the benefits of surveillance are faster detection of the recurrence, the actual gain in QALYs will be minimal.
FIGURE 13.
One-way sensitivity analysis of monthly probability of recurrence for stage IA patients.

FIGURE 14.
One-way sensitivity analysis of monthly probability of recurrence for stage IB patients.

FIGURE 15.
One-way sensitivity analysis for utility values for stage IA patients.
FIGURE 16.
One-way sensitivity analysis for utility values for stage IB patients.

| Parameter | Per person EVPPI (£) | EVPPI for England per year (£) | EVPPI for England over 20 years (£) |
|---|---|---|---|
| Probabilities of transitioning between stages | 3422 | 19,024,352 | 380,487,047 |
| Diagnostic accuracy | 2483 | 13,804,052 | 276,081,046 |
| Health utility values | 864 | 4,803,343 | 96,066,864 |
| Recurrence of melanoma | 224 | 1,245,311 | 24,906,224 |
In the case of utility, Figures 15 and 16 show that, as long as the utility value of the recurrence is > 0.1 for stage IA and IB patients, then the NMBs of all the surveillance strategies are positive. The most important utility values are related to stage I, as approximately 70% of all disease is diagnosed when the cancer is stage I. 226 Without data to the contrary, it is likely that most second primary melanoma diagnosis or first primary recurrences are also diagnosed at stage I.
Role of specialist dermatological nurse in surveillance
One of the research questions at the start of this analysis was whether or not specialist dermatological nurses/CNSs have a role to play in surveillance. From the initial analyses presented in the base-case analyses, none of the nurse strategies was cost-effective; therefore, these strategies were not included in the PSA. To explore the reason why they were not cost-effective, additional analyses, based on the diagnostic accuracy of the strategies, were performed (i.e. the ability of dermatological nurses to correctly diagnose whether or not a recurrence has occurred). Given that the specificity and sensitivity of nurses were obtained from a single study, which assessed the performance of eight ‘physician assistants’ in rating 173 dermoscopic images of skin lesions with known histological diagnosis,207 sensitivity analysis explored the impact on the cost-effectiveness of changes in the sensitivity and specificity of diagnosing recurrences in the nurse-based strategies. One-way sensitivity analysis showed that, at low values of specificity, nurse strategies are not expected to be cost-effective. This is because individuals are referred for further tests instead of being discharged. However, as specificity increases, nurses become more cost-effective, compared with alternative strategies. When the value of specificity reaches 0.876 for stage IA and 0.878 for stage IB, nurses become as cost-effective as dermatologists (Figures 17 and 18).
FIGURE 17.
One-way sensitivity analysis of specificity of specialist dermatological nurses for stage IA patients.

FIGURE 18.
One-way sensitivity analysis of specificity of specialist dermatological nurses for stage IB patients.

Potential role of prognostic test
A hypothetical scenario in which a validated prognostic test, be it a risk factor-based model or a biomarker or a combination of both, is available to the NHS (cost: £250) was added to the model to compare surveillance strategies. It was assumed that the test has a sensitivity of 0.8 and a specificity of 0.8 in identifying high- and low-risk patients. It was also assumed that the ‘true’ prevalence of recurrence over a lifetime is 20%, based on an Australian study. 210 Those identified as being at high risk received the recommended NICE strategy and those identified as being at low risk received the strategy that was identified in the base-case analysis as being cost-effective (stage IA: strategy 22; stage IB: strategy 77). This hypothetical prognostic test strategy was compared with a few strategies, including the current NICE strategy for stage IA and IB patients.
The results of the costs, effectiveness, ICER and NMB are presented in Appendix 10, Table 43. In this simplified analysis, the least costly option had the highest NMB for stage IB patients. However, the prognostic test strategy had the highest NMB in stage IA patients and performed slightly better than the NICE strategy in both subgroups. When the associated CEACs are taken into account, the NICE strategy is likely to be the most cost-effective strategy at WTP thresholds of > £12,000 for stage IA and £26,000 for stage IB (Figures 19 and 20).
FIGURE 19.
The CEAC for prognostic test strategy for stage IA patients (only three strategies included).

FIGURE 20.
The CEAC for prognostic test strategy for stage IB patients (only four strategies included).

In these illustrated examples (see Figures 19 and 20), only a few of the possible strategies were compared in this analysis (compared with the 20 strategies in the base-case analysis). Furthermore, the way in which the prognostic test is incorporated into surveillance strategy in this sensitivity analysis may not be optimal. Both these points will have an effect on what is deemed cost-effective. What these results illustrate is that, other things being equal, increases to the sensitivity and specificity of the prognostic test (or reductions in the cost of the prognostic test) could markedly change which strategy is considered cost-effective (and may lead to additional strategies being considered cost-effective). If a prognostic test that can acceptably differentiate stage IA and IB into high and low risk of recurrence were to become available in the future at a reasonable price, then the recommended surveillance strategy may change.
Value-of-information analysis
The usefulness of a value-of-information/expected value of perfect information (EVPI) analysis is in calculating a maximum reasonable price for information. Additional evidence is valuable because it can improve patient outcomes by resolving existing uncertainty about the cost-effectiveness of the interventions available, thereby informing treatment choices for subsequent patients. 229 It also allows for the comparison of the cost of uncertainty with the cost of obtaining additional evidence.
The overall EVPI for IA is £5545 per person affected by the decision, and the population EVPI is £30,824,655 per year in England. The overall EVPI for stage IB is £6339 per person affected by the decision, and the population EVPI is £20,256,750 per year in England. We assume that the population EVPI exceeds the expected cost of research and consider the EVPPI for groups of parameters for which it is likely that a new study (or studies) would be informative for the whole group, rather than for individual parameters.
The parameters that were considered to contribute most significantly to EVPPI estimates for both stage IA and IB by the SAVI tool were probabilities of transitioning between stages (natural history), diagnostic accuracy, health utility values and recurrence of melanoma on the overall decision uncertainty in the economic evaluation model. These parameters are potentially relevant for the design of future research, as well as for broader policy questions. Table 20 reports the EVPPI for the four groups of parameters, with the overall decision EVPI at a threshold of £20,000 per QALY. The EVPPI associated with probabilities of transitioning between stages is relatively high for both stage IA (see Table 20) and stage IB (Table 21). This research is more likely to take the form of an epidemiological study. Other parameters, such as diagnostic accuracy and utility values, require other forms of study design.
| Parameters | Per person EVPPI (£) | EVPPI for England per year (£) | EVPPI for England over 20 years (£) |
|---|---|---|---|
| Probabilities of transitioning between stages | 4109 | 22,843,677 | 456,873,547 |
| Diagnostic accuracy | 3013 | 9,628,267 | 192,565,350 |
| Health utility values | 1371 | 4,381,133 | 87,622,667 |
| Recurrence of melanoma | 617 | 1,971,670 | 39,433,396 |
For an NHS decision-maker, the EVPPI for England per year is based on the number of patients affected by the decision each year. This was assumed to be equal to the annual incidence of stage I melanoma. In 2017, 8555 patients were diagnosed with stage I melanoma. 226 The split in IA and IB patients (63% : 37%) was taken from incident data from a German Registry data set. 209 EVPI is expressed for the total population of patients who stand to benefit from additional information over the expected lifetime of the technology. The EVPPI over an arbitrary 20-year time horizon is presented.
Discussion
This chapter presented the methods and results of a cost-effectiveness analysis that compared alternative surveillance strategies for people who have been treated for AJCC stage IA and IB melanoma by the NHS in the UK. The paucity and limitations of existing evidence from English/UK data sources resulted in many data values from various international sources being used in the economic model. Therefore, the evidence on cost-effectiveness should be treated cautiously, in part because of the inherent problems in combining data from multiple sources.
Summary of the cost-effectiveness analysis
The base-case PSAs economic model presented in this report compared 20 different follow-up surveillance strategies for stage IA melanoma and 20 different strategies for stage IB melanoma. Cumulative costs and QALYs were compared over a lifetime time horizon, with the results suggesting that a less intensive follow-up strategy for both stages IA and IB has a higher NMB than the surveillance strategy recommended in the NICE guidelines. The results indicate that, for the strategies examined, the main difference is in the costs of each strategy, and there are only minimal differences observed in QALYs between strategies. Strategy 22 for stage IA and strategy 77 for stage IB are the least costly options. However, in the base-case analyses, only restricted strategies were analysed and, by omitting relevant comparators, an underestimation of ICERs may have occurred. 230 However, the NMB statistic highlights the similarity of all evaluated strategies.
The reported one-way sensitivity analyses carried out on the base-case model results suggest that altering the probability of self-diagnosis did not make a major impact on the NMB. Recurrence rates are estimated to be 0.22% for stage IA and 0.46% for stage IB per month and, even if all patients had a recurrence, the analysed surveillance strategies would still have a positive NMB. This suggests that surveillance is worthwhile.
There is uncertainty in the appropriate utility values to use in the base-case analysis. In the one-way sensitivity analysis, varying utility values associated with recurrence between zero and one was explored. As utility values associated with recurrence increase, so does the NMB. It is likely that most recurrences are going to be stage I and utility values are likely to be > 0.7. However, the analysis is not able to distinguish between strategies and, therefore, is not as informative to decision-makers.
Strategies with dermatological nurses/CNSs as the main health-care professional performing the screening were the least cost-effective, despite their lower resource use cost. This was because their diagnostic accuracy and, most importantly, their specificity was lower than those of dermatologists/surgeons. The consequence of this is that patients who are initially seen by the dermatological nurse/CNS accrue further costs because of the additional diagnostic tests performed.
A hypothetical prognostic test is likely to result in some savings, relative to the current NICE strategy, and may have a role in surveillance. Further work is warranted to identify a relevant and viable prognostic test and how surveillance strategies may be altered to accommodate such a test in a cost-effective manner. The analysis reported in this chapter has been partial with respect to how surveillance strategies may be altered to accommodate a prognostic test; further work would be warranted if and when a viable prognostic test becomes available.
Value-of-information analyses, and specifically EVPPI, were also carried out on the base-case analysis. This analysis sought to estimate the value of removing uncertainty around particular parameters or groups of parameters in the model. The results indicate that further research is valuable and warranted in removing uncertainty around the diagnostic accuracy of health-care professionals, the probability of transitioning between stages, health-state utility values and the recurrence of melanoma to make a confident decision to change current NICE guidelines.
Strengths and limitations
The main strength of the economic evaluation is that a de novo model has been created to answer the research question from the perspective of the NHS. The research team has attempted to use rigorous and systematic methods to obtain parameter inputs into the economic evaluation. These were then assembled in the economic model, whose structure was informed by detailed discussions with the clinical members of the research team. One of the most important challenges faced when conducting this economic evaluation was incorporating patient behaviour. A unique feature of this model is that it considers self-diagnosis by patients and the heightened anxiety melanoma survivors experience that result in ‘false-alarm’ appointments.
A number of limitations in the economic evaluation need to be acknowledged. With respect to patient characteristics, the subtype of melanoma was not accounted for in the model. The location of the primary site of the melanoma was available in the Durham cohort, but was not utilised in the model. Accordingly, it was assumed that recurrence is independent of primary location of melanoma. Recent Australian data suggested that head and neck location of the primary tumour had the highest rate of 2-year recurrence, compared with primary tumours occurring on the trunk (HR 1.67, 95% CI 1.01 to 2.67), with upper and lower limbs having lower HRs than the trunk area. 231
The model was based on current NICE guidelines; given the complex nature of the model, the assessment of structural uncertainty (i.e. whether or not relevant processes are reflected in the model and whether or not the model reflects reality) was considered with respect to the number of follow-up regimens considered, including the frequency and duration of follow-up, and who completes that follow-up. Other areas of structural uncertainty were not addressed. Few data were available for many of the model parameters. What data were available were not ideally suited to the research question being addressed. For example, health-state ‘utilities’ values are based on patients with first primary melanoma diagnosis, rather than recurrence. Utility values are required to estimate QALYs. In this analysis, data were taken from a meta-analysis of utility values, which could be questioned. 216 As highlighted in an editorial,232 instruments or questionnaires used should be sensitive to the domains of quality of life that are likely to change as a result of the disease, the routine treatment or a targeted intervention. It is unclear whether or not the EQ-5D is sufficiently sensitive to changes in some of the domains of quality of life that might occur. For example, a European study233 reported that over half the patients with melanoma in their study reported anxiety. However, the EuroQol-5 Dimensions, five-level version, instrument was somewhat insensitive to detecting this. 233
Given the limitations of the data, the base-case and sensitivity analyses were not able to provide a strong basis on which the NHS could draw robust conclusions about what surveillance strategy would be best for the UK to adopt. Instead, ‘best bets’ that would be worthy of further consideration were identified.
Impact and implications
The impact of the work presented in this chapter is important for patients, practitioners, the NHS and for researchers. One key feature of melanoma surveillance is the important role of patients (and their partners) in detecting changes in their moles, and thus detecting recurrences or new primary melanomas. This suggests that patient education is important. The evidence suggests that dermatologists have the highest accuracy in detecting melanomas. For nurses to be considered the main health-care provider in surveillance, further work would be needed to determine and, if necessary, support the development of their diagnostic performance. Should their diagnostic performance be less than dermatologists, then training may be worthwhile. This is because, other things being equal, nurses providing surveillance would be less costly than dermatologists and it may be relatively easier to increase the cadre of nurses able to provide surveillance, rather than increasing the numbers of dermatologists.
Although no viable stratification approach above AJCC staging has thus far been identified, should one be developed, then the initial results suggest that there may be merit in further research to identify how it could be used to refine surveillance in the optimal way.
Chapter 7 Discussion
Summary of findings
Cutaneous melanoma is a cancer developing from melanocytes, which are the pigment-producing cells in the skin. It is one of the most deadly of all skin cancers, with metastatic disease being highly aggressive. 1,2 Until the introduction of targeted therapies and immunotherapies, median overall survival was between 6 and 10 months once metastasis had occurred. 3 In the UK, it is the leading cause of cancer-related death among people aged 20–35 years.
The incidence of melanoma is increasing worldwide; currently, approximately 2% of the population develop melanoma each year. 5 In the UK, there are approximately 17,000 cases of melanoma per year; the incidence rate has increased by 134% since the 1990s, and melanoma now makes up 5% of new cancer cases. Reflecting the impact on mortality, melanoma affects a disproportionate number of people aged < 50 years compared with other cancers. 7
Primary melanomas are staged according to the AJCC staging criteria and the focus of this research has been on AJCC stage I melanomas. Although these tumours have the lowest mortality risk (at approximately 14% over 10 years) compared with other stages of the disease, approximately 10,500 of the 17,000 melanomas occurring each year are stage I. Thus, for the majority of people who develop stage I melanoma, surgical treatment is effective. However, for a sizable minority of people, the disease recurs. Hence, follow-up and surveillance of those initially treated for stage I melanoma is required.
How surveillance is organised for stage I disease varies considerably between countries. In the Netherlands, following excision of stage IA disease, individuals receive a one-off appointment 1 month after diagnosis. 58 In contrast, the German guidelines recommend that individuals treated for stage IA disease receive a follow-up appointment every 6 months for the first 3 years and are then followed up annually for a further 7 years. 59 The current NICE guideline recommendation for stage IA lies between these two, with follow-up visits recommended every 3–6 months for 1 year. 16 The 2019 surveillance report for NICE suggested no change to recommendations for surveillance of stage I disease. 54 The differences in recommendations for surveillance between guidelines of stage I melanomas (summarised in Chapter 2) partly reflect the fact that the evidence base is from non-randomised and anecdotal evidence, along with expert opinion. Recommendations are based on the assumption that earlier detection of metastatic disease results in improved overall outcome, but often do not consider the potential physical, psychological and economic costs of these regimens.
With the increasing melanoma rates, the pressures on the NHS will increase. One approach to managing these pressures is to reconsider how surveillance is organised for those treated for stage I disease. To begin this, consideration must first be given to what is known to form judgements as to whether or not the current evidence base is sufficient to justify changes in practices and, if it is, what is the effectiveness and cost-effectiveness of alternative ways of organising surveillance.
This evidence synthesis work has attempted to do this. It started by systematically reviewing the existing evidence base on the relative effectiveness of surveillance and follow-up strategies following surgical excision of a stage I tumour. Only one RCT (reported in two papers) was eligible. 85 The one study71 for which data were available suggested that an educational intervention for patients and their partners improved self-identification of new primary melanomas (rather than locoregional metastatic disease). Even if the data were available from both studies, quantitative synthesis would not be possible, as they employed different follow-up strategies. Furthermore, because the study was not conducted in the UK, the applicability of the findings to the UK may be limited and the evidence from this systematic review was judged to be of low certainty234 because of the few data available. The findings from this systematic review are similar to those found by Cromwell et al. 25
To address the limitations of the empirical evidence, an evidence synthesis approach was used to construct a comparison of alternative surveillance strategies following treatment for stage I melanoma. As part of this evidence synthesis, a systematic review of the prognostic accuracy of risk prediction models to predict recurrence, new primary tumours and metastases was conducted. The purpose of this review was to identify if there was any evidence that might enable surveillance to be stratified by risk. Such stratification could enable low-risk patients to safely receive less intensive surveillance than higher-risk patients. This review identified 11 different risk prediction models, with the number of predictors per model ranging from 3 to 11. The models differed in the predictors used, the outcomes of interest and the statistical measures used to assess model performance. Consequently, no quantitative synthesis of their results was performed. None of the identified risk prediction models has undergone rigorous validation. The data elements most commonly used in these models are patient demographic information or histological features of the primary tumour. 136 Overall, the identified evidence did not allow accurate prognostication of melanoma.
Any surveillance strategy would also require that further disease can be accurately diagnosed if it occurs. For this reason, a systematic review was conducted that explored the diagnostic test accuracy of FNB and ultrasonography to detect recurrence and locoregional metastases during follow-up of stage I melanoma. Despite extensive searching, only two studies assessing different index tests met the inclusion criteria. One considered FNB163 and was judged to be at high risk of bias and one assessed ultrasonography164 and was also considered to be at high risk of bias. The findings reported for diagnostic accuracy of FNB in patients who were diagnosed initially as having stage I melanoma were comparable to those reported at stages II–IV. 163 They were also similar to those reported in another systematic review on the subject. 148 Similarly, the diagnostic performance of ultrasonography estimated by Kruger et al. 164 was similar to that reported by two different reviews, even though no analysis was conducted by disease stage. 147,170
No existing economic evaluation that directly addressed the study question was identified. Therefore, using the information gathered from the systematic reviews and discussions among the study team, a Markov microsimulation model was developed. The economic evaluation compared alternative surveillance strategies for people who have been treated for AJCC stage IA and IB melanoma provided by the NHS in the UK.
Initially, 400 surveillance strategies were defined for stage IA and 600 for stage IB. The clinical team reviewed these and implausible strategies were excluded from further consideration. Initial modelling focused on 75 strategies for stage IA and 87 strategies for stage IB. Surveillance strategies varied in terms of who performed the surveillance (dermatologist, surgeon or cancer nurse specialist), frequency of follow-up (i.e. every 3, 4, 6 or 12 months) and duration of follow-up (for stage IA, follow-up was 1, 3, 5 or 10 years, and for stage IB, follow-up was 1, 3, 5, 10 or 20 years).
This initial modelling showed that strategies involving a cancer nurse specialist were highly unlikely to be cost-effective. The reason for this is that, although the cost of using cancer nurse specialists to provide surveillance was low, the data available suggested that cancer nurse specialists were not good at identifying people without disease. Hence, a considerable number of individuals were referred on for further (costly) investigation. Nevertheless, the evidence on the diagnostic performance of cancer nurse specialists was poor. However, this suggests that training cancer nurse specialists to accurately diagnose recurrent melanoma could potentially increase capacity within the NHS to undertake surveillance. A sensitivity analysis showed that, should it be possible to do this so that the diagnostic performance of cancer nurse specialists approached that estimated for dermatologists, strategies involving cancer nurse specialists might be cost-effective. Currently, training support for cancer nurse specialists in this role is limited, but the results of the analysis suggest that work to develop training for these staff may be worthwhile.
Following the initial modelling, 20 strategies for stage IA and 20 for stage IB disease were selected based on relevance to the NHS, the potential to be cost-effective and the ability to extrapolate findings to similar strategies that were not modelled. For each stage of the disease, the current NICE recommendations for surveillance were modelled. The results of these analyses showed that there were only very small differences in QALYs between strategies, with the main differences being in costs. This was primarily because rates of recurrence were expected to be comparatively low (approximately 14% of people who have been treated for stage I experience a recurrence over 10 years).
For stage IA patients, the strategy of follow-up once for 1 year by a dermatologist was, on average, the least costly and most effective. For stage IB, the strategy of follow-up once for 1 year by a dermatologist was, on average, the least costly and least effective. If all strategies were equally likely to be cost-effective, then each strategy would have a 5% chance of being considered cost-effective at any given threshold. For both stages IA and IB, the strategy of follow-up once for 1 year by a dermatologist was the most likely to be considered cost-effective at a £20,000 threshold per QALY (13% and 12.9% for stages IA and IB, respectively). For stage IA disease, the NICE strategy has a ≈ 9% chance of being considered cost-effective at the same threshold. For stage IB, the NICE strategy (surveillance by a dermatologist every 3 months for the first 3 years and then every 6 months for the next 2 years) never has a probability of > 6% of being cost-effective over any of the values for society’s WTP for a QALY.
Overall, no strategy clearly stands out as superior, unless the NHS was to make a decision between the strategies based solely on cost (follow-up once for 1 year by a dermatologist had a > 50% chance of being less costly for both stages IA and IB disease). This suggests that, based on the reviews and economic modelling, there are no firm grounds to suggest changing current NICE recommendations. However, the analyses also indicate that there are plausible strategies that could represent a more efficient use of NHS resources for both stages IA and IB. These strategies include ones both less intensive and more intensive than the strategy recommended by NICE. This suggests that further research to explore these strategies may be worthwhile.
In the model, a high rate of self-diagnosis of recurrence was assumed (60% of all recurrences were assumed to be self-diagnosed within 12 months). This high rate of self-diagnosis restricts the gains that any surveillance strategy could provide. The evidence supporting the self-diagnosis rate is weak, and rates of self-diagnosis could be much lower. When modelling the impact of much lower rates of self-diagnosis, those strategies that are more intensive than the NICE-recommended strategies become more likely to be considered cost-effective. However, the gains in QALYs are small, primarily because the underlying rates of recurrence are low for stage I melanomas. For some groups, rates of self-diagnosis may be low235 (e.g. males aged > 50 years, those with a darker skin colour, patients living alone); a valid question is whether or not it would be worth investing in melanoma awareness campaigns designed to increase the rates of self-diagnosis.
A hypothetical scenario in which a validated prognostic test, be it a risk factor-based model, a biomarker or a combination of both, is available to the NHS was also modelled. As Chapter 4 shows, no good risk prediction model currently exists. However, there is considerable interest in developing tools to help with prognostication. Indeed, initial scoping searches for prognostic biomarkers for melanoma conducted as part of the review of risk prediction models identified several tens of thousands of potentially relevant titles and abstracts. Such tools offer the potential to focus surveillance resources on those most likely to experience a recurrence or metastasis, while allowing low-risk individuals to be either discharged or followed up in a far less intensive manner. The analyses conducted illustrated the potential scope for such tests, especially for the higher-risk stage IB patients, provided the costs of the test were modest (we modelled a cost per test of £250). The analysis also illustrated that much more thought could go into how a surveillance strategy could be adapted to incorporate such a test. This is because the analyses conducted may not have optimised the use of such a test. However, work in this area is of less importance until a viable tool becomes close to being validated for use.
Uncertainty about the assessment
Key uncertainties for the assessment relate to the paucity of data available for evidence synthesis, few of which related to the latest version of the AJCC staging. The various clinical guidelines when making recommendations on surveillance reflect this, as did the review of different surveillance strategies. Considerable efforts were made in the three reviews reported (see Chapters 3–5) to identify relevant data and all used relevant tools to assess the quality of the evidence identified that was relevant to the questions posed by that review.
The review of different surveillance strategies identified just one comparative study. 85 Given the very large number of potential strategies that could be considered for surveillance, the evidence base is sparse. However, although there is a need for well-designed studies in this area comparing alternative strategies, it is currently unclear which strategies should be compared. The economic evaluation identified a very large number of potential strategies, of which a small number might be worthy of further consideration. Further work would be needed to develop strategies that might be relevant to trial further. As a surveillance strategy is a complex intervention containing several components, such development and evaluation work should be placed in the context of the Medical Research Council’s framework for complex interventions. 236
The current evidence synthesis provides no evidence that any possible alternative surveillance strategies should include a tool to help identify those at low and those at high risk of recurrence and progression. A number of risk prediction models were identified, but none met the recommendations set out by TRIPOD. 135 Only a small number of models were externally validated, limiting judgements on their applicability to the NHS. However, considerable research efforts are ongoing internationally to develop tools that could be used to identify those at low risk and those at high risk of recurrence and progression. The economic modelling showed that, should a tool be developed (even with only modest performance of 0.80 sensitivity and specificity), further work to determine whether or not and how to integrate such a tool into a surveillance strategy is necessary.
There was also considerable uncertainty about how and for whom a diagnosis of recurrent disease might be made. With respect to diagnostic tests, based on clinical judgement, the focus was on FNB and ultrasonography to detect melanomas. The uncertainty surrounding the performance of different types of practitioners (as discussed earlier in this chapter for cancer nurse specialists) was one aspect of this uncertainty, but few data were available for the performance of the specified tests for stage I melanoma. The data identified appeared to be consistent with those drawn from other reviews, which did not differentiate by stage. 147,169,170
The uncertainties that remain after the reviews are compounded in the economic model, as the economic model is a further level of evidence synthesis. In addition, the economic model itself may not have fully captured the complexity of melanoma follow-up. Although considerable efforts were used to develop a model that reflected the disease process and UK practice, inevitably, simplifications were made in the model structure. Furthermore, although a very large number of surveillance strategies were considered in the base-case analysis, only a restricted number of strategies were analysed. Although interpretation of this restricted number was not straightforward, by omitting relevant comparators, ICERs may have been underestimated230 and probabilities of a given strategy being cost-effective may have been misspecified. Furthermore, the economic evaluation considered NHS costs only. Costs to patients and their families of accessing surveillance and treatment were omitted. Such costs may be disproportionately felt by those least able to bear these costs. Other aspects of patient outcomes that might not be adequately captured by the EQ-5D and the utilities included in the model were also omitted. These include the psychological effects of surveillance and diagnosis and treatment.
Given the uncertainties, the economic evaluation sought to identify the areas where further research would be worthwhile. For this, it estimated the EVPI and the EVPPI. These analyses relate to the value of information for the model (so if the model is misspecified, then the EVPI and EVPPI will be misspecified). Furthermore, the EVPI and EVPPI represent the total value of removing all uncertainty. In reality, any study could remove only some of this uncertainty. Nevertheless, the EVPI and EVPPI show that there could be considerable value in removing uncertainty overall, and specifically in research, to improve estimates for the probabilities of transitioning between stages for both stages IA and IB. There may be less (but still considerable) value in research around improving estimates of diagnostic accuracy and for utility values.
Chapter 8 Conclusions
Based on the results of the evidence synthesis and economic evaluation, the following implications for practice and research can be drawn out.
Implications for practice
-
Few data were available specific to surveillance of people after treatment for melanoma. Furthermore, few data were available for key components of a surveillance strategy that could be used to model alternative strategies. Therefore, the results are imprecise and considerable uncertainty exists.
-
There is insufficient evidence to recommend any changes to the current guidelines produced by NICE with respect to surveillance. For stage IA disease, there are plausible surveillance strategies that may perform better than the current recommendation of clinical follow-up by a dermatologist every 3–6 months for 1 year. However, the NICE strategy still performs comparatively well compared with these. For stage IB disease, the NICE-recommended strategy of follow-up every 3 months for 3 years then every 6 months for a further 2 years performs poorly compared with other strategies considered, but there is currently insufficient evidence to support any changes.
-
Surveillance strategies whereby the clinical follow-up is conducted by a cancer nurse specialist may ease pressure on dermatologists. However, methods to enhance their diagnostic performance may be needed, as the current limited evidence base suggests that their ability to correctly identify who has or does not have a recurrence is lower than that of dermatologists.
-
Encouraging and supporting patients in making accurate self-diagnosis of recurrence in stage I disease may reduce the need for any active surveillance strategy for those initially treated for stage I disease.
Implications for research
It is tempting to recommend that a RCT should be conducted to compare alternative surveillance strategies. However, a surveillance strategy is a complex intervention and research should first establish what sensible comparators should be used against current practice. What an appropriate comparator would be may vary between stages IA and IB disease, and establishing this requires improved evidence on:
-
How disease in patients with stage I disease develops over time especially as defined by the latest AJCC staging criteria. The economic modelling shows that both the incidence of recurrent and metastatic disease over time and how the disease progresses are important. Such data would inform whether or not a more or less intensive surveillance strategy than the ones recommended by NICE for stage IA or IB should be considered. The value-of-information analysis suggested that this is where there would be the greatest value in removing all uncertainty. Potential research techniques may include constructing cohorts relying on routine data collection.
-
How well recurrent and metastatic disease is diagnosed. This may also include research designed to improve the diagnostic performance of particular practitioner groups in the clinical workforce, especially when their increased use may alleviate current or impending capacity constraints in the availability of dermatologists. Study designs may include behaviour change studies designed to improve the diagnostic performance of practitioners.
-
Low-cost tools that can better stratify patients into low or high risk of future recurrence and metastasis. This would help develop better stratified follow-up, allowing some of the ≈ 85% of patients treated for stage I melanoma based on the AJCC’s seventh edition classification who do not develop further disease to be safely discharged more quickly. It would also allow more focused use of scarce resources for those at higher risks.
-
Identifying patients’ preferences for alternative methods of surveillance, the impacts on health-related quality of life of surveillance (or no surveillance) and the longer-term consequences of melanoma.
Acknowledgements
We would like to thank the following people:
-
Kirsty Laing for contributing to the literature searches for Chapter 2
-
those who assisted with the review reported in Chapters 3 and 4 – Ryan Kenny and Hosein Shabaninejad, and Akvile Stoniute for taking part in the updating of the screening for reviews
-
Professor J Robinson for providing data for the subset of stage I melanoma patients for use in the review of surveillance strategies
-
those who assisted with the review reported in Chapter 5 – Theophile Birgirumurame for translating a French article, Cristina Fernandez-Garcia for taking part in the translation for one Spanish paper, Svetlana Glinianaia for translating a Russian article for Melanoma Review and Jac Dinnes for providing timely assistance in providing specialist diagnostic accuracy data
-
Ed Wilson for providing timely assistance in model development and data gathering for Review 3
-
Eugenie Johnson for providing administrative support to the project
-
Janice Legge for taking part in the administrative support
-
Dr Janine Graham for providing expert opinions related to oncological elements of the study.
Contributions of authors
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Professor of Health Economics) was responsible for management of the study and contributed to the conception of the study and the conduct of all elements.
Patience Kunonga (https://orcid.org/0000-0002-6193-1365) (Systematic Reviewer) assisted in design of the surveillance review with Margaret Astin and was responsible for the design of the review for risk prediction models.
Diarmuid Coughlan (https://orcid.org/0000-0002-5348-3750) (Health Economist) and Vasileios Kontogiannis (https://orcid.org/0000-0002-9823-992X) (Health Economist) assisted in the design of the economic evaluation and were equally responsible for the conduct and reporting of the economic evaluation.
Margaret Astin (https://orcid.org/0000-0002-3482-5567) (Systematic Reviewer) was responsible for the protocol and conduct of the diagnostic accuracy review and assisted with the surveillance and prediction models reviews, from data extraction to reporting of study results.
Fiona Beyer (https://orcid.org/0000-0002-6396-3467) (Senior Research Associate – Evidence Synthesis) provided methodological expertise and advice for the systematic review element of the study, and was responsible for developing searches for the surveillance review.
Catherine Richmond (https://orcid.org/0000-0002-2940-5197) (Information Specialist) was responsible for developing searches for the review of diagnostic accuracy, managing update searches and reference management.
Dor Wilson (https://orcid.org/0000-0002-3034-001X) (Information Specialist) was responsible for developing the searches for the review of risk prediction models.
Dalvir Bajwa (https://orcid.org/0000-0002-0965-7012) (Dermatologist) designed and collected the data from the patient cohort.
Mehdi Javanbakht (https://orcid.org/0000-0002-8661-8439) (Health Economist) contributed to the conception, study and design of the economic evaluation.
Andrew Bryant (https://orcid.org/0000-0003-4351-8865) (Statistician) contributed to the systematic reviews of risk prediction models and diagnostic tests.
Wanwuri Akor (https://orcid.org/0000-0001-9291-3816) (Health Services Researcher) conducted the structured review of clinical guideline recommendations on surveillance following treatment for stage I melanoma.
Dawn Craig (https://orcid.org/0000-0002-5808-0096) (Professor of Evidence Synthesis) advised on the conduct of the study.
Penny Lovat (https://orcid.org/0000-0002-6657-6825) (Professor of Cellular Dermatology and Oncology), Marie Labus (https://orcid.org/0000-0002-8339-7423) (Business Development Manager), Batoul Nasr (https://orcid.org/0000-0002-4670-0171) (Consultant Dermatologist) and Timothy Cunliffe (https://orcid.org/0000-0001-6081-5891) (Consultant Dermatologist) contributed to the conception of the study and advised on the conduct of the study.
Helena Hinde (https://orcid.org/0000-0003-0057-6758) (Dermatology Cancer Nurse Specialist) advised on the conduct of the study.
Mohamed Shawgi (https://orcid.org/0000-0002-1021-8439) (Consultant Radiologist) advised on the conduct of the diagnostic accuracy review.
Daniel Saleh (https://orcid.org/0000-0003-4849-6933) (Consultant Plastic Surgeon) advised on the conduct of the study.
Pam Royle (https://orcid.org/0000-0001-8033-6556) (Patient Representative), Paul Steward (https://orcid.org/0000-0002-5460-231X) (Patient Representative) and Rachel Lucas (https://orcid.org/0000-0002-3612-6495) (Patient Representative) contributed to the conception of the study and advised on the conduct of the study.
Robert Ellis (https://orcid.org/0000-0001-6305-2121) (Principal Investigator, Consultant Dermatologist) contributed to the conception of the study and the conduct of all elements.
All authors contributed to the interpretation of the results and the writing/editing of the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Matthews NH, Li WQ, Qureshi AA, Weinstock MA, Cho E, Ward WH, et al. Cutaneous Melanoma: Etiology and Therapy. Brisbane, QLD: Codon Publications; 2017.
- American Cancer Society . Cancer Facts and Figures 2018 n.d. www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html (accessed 8 October 2019).
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34. https://doi.org/10.1200/JCO.2007.12.7837.
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463-82. https://doi.org/10.1038/nrclinonc.2017.43.
- de Vries E, Sierra M, Piñeros M, Loria D, Forman D. The burden of cutaneous melanoma and status of preventive measures in Central and South America. Cancer Epidemiol 2016;44:100-9. https://doi.org/10.1016/j.canep.2016.02.005.
- World Cancer Research Fund International . Skin Cancer Statistics 2018. www.wcrf.org/dietandcancer/cancer-trends/skin-cancer-statistics (accessed 8 August 2019).
- Cancer Research UK. Skin Cancer: Risks and Causes 2017. www.cancerresearchuk.org/about-cancer/skin-cancer/risks-causes (accessed 8 August 2019).
- Ballantine KR, Watson H, Macfarlane S, Winstanley M, Corbett RP, Spearing R, et al. Small numbers, big challenges: adolescent and young adult cancer incidence and survival in New Zealand. J Adolesc Young Adult Oncol 2017;6:277-85. https://doi.org/10.1089/jayao.2016.0074.
- Watson M, Geller AC, Tucker MA, Guy GP, Weinstock MA. Melanoma burden and recent trends among non-Hispanic whites aged 15–49 years, United States. Prev Med 2016;91:294-8. https://doi.org/10.1016/j.ypmed.2016.08.032.
- Cancer Research UK . Melanoma Skin Cancer Incidence Statistics 2019. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer/incidence#ref-0 (accessed 8 August 2019).
- International Agency for Research on Cancer . Cancer Today 2018. http://gco.iarc.fr/today/home (accessed 8 August 2019).
- Miiskin . Facts About Melanoma and Other Skin Cancers 2019. https://miiskin.com/melanoma-skin-cancer-facts/ (accessed 8 August 2019).
- Melanoma Patient Network Europe . Melanoma – The Facts 2013. www.melanomapatientnetworkeu.org/melanoma.html (accessed 8 August 2019).
- Colebatch AJ, Scolyer RA. Trajectories of premalignancy during the journey from melanocyte to melanoma. Pathology 2018;50:16-23. https://doi.org/10.1016/j.pathol.2017.09.002.
- Miller AJ, Mihm MC. Melanoma. N Engl J Med 2006;355:51-65. https://doi.org/10.1056/NEJMra052166.
- National Institute for Health and Care Excellence . Melanoma: Assessment and Management 2015. www.nice.org.uk/guidance/ng14 (accessed 17 July 2019).
- Voss RK, Woods TN, Cromwell KD, Nelson KC, Cormier JN. Improving outcomes in patients with melanoma: strategies to ensure an early diagnosis. Patient Relat Outcome Meas 2015;6:229-42. https://doi.org/10.2147/PROM.S69351.
- Watts CG, Dieng M, Morton RL, Mann GJ, Menzies SW, Cust AE. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol 2015;172:33-47. https://doi.org/10.1111/bjd.13403.
- American Joint Committee on Cancer . AJCC Cancer Staging Manual 2016.
- American Joint Committee on Cancer . AJCC Cancer Staging Manual 2010.
- Ribero S, Argenziano G, Lallas A, Moscarella E, Benati E, Raucci M, et al. Dermoscopic features predicting the presence of mitoses in thin melanoma. J Dermatol Sci 2017;86:158-61. https://doi.org/10.1016/j.jdermsci.2017.01.013.
- Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe – a systematic review of the literature. Clin Epidemiol 2016;8:109-22. https://doi.org/10.2147/CLEP.S99021.
- Damude S, Hoekstra-Weebers JE, Francken AB, Ter Meulen S, Bastiaannet E, Hoekstra HJ. The MELFO-Study: prospective, randomized, clinical trial for the evaluation of a stage-adjusted reduced follow-up schedule in cutaneous melanoma patients-results after 1 year. Ann Surg Oncol 2016;23:2762-71. https://doi.org/10.1245/s10434-016-5263-7.
- Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients – monocenter evaluation of methods, costs and patient survival. Br J Cancer 2002;87:151-7. https://doi.org/10.1038/sj.bjc.6600428.
- Cromwell KD, Ross MI, Xing Y, Gershenwald JE, Royal RE, Lucci A, et al. Variability in melanoma post-treatment surveillance practices by country and physician specialty: a systematic review. Melanoma Res 2012;22:376-85. https://doi.org/10.1097/CMR.0b013e328357d796.
- Marsden JR, Newton-Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J Plast Reconstr Aesthet Surg 2010;63:1401-19. https://doi.org/10.1016/j.bjps.2010.07.006.
- Marsden JR, Newton-Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol 2010;163:238-56. https://doi.org/10.1111/j.1365-2133.2010.09883.x.
- Weinstein D, Leininger J, Hamby C, Safai B. Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol 2014;7:13-24.
- Guo HB, Stoffel-Wagner B, Bierwirth T, Mezger J, Klingmüller D. Clinical significance of serum S100 in metastatic malignant melanoma. Eur J Cancer 1995;31:924-8. https://doi.org/10.1016/0959-8049(95)00087-9.
- Melanoma Focus . The Current Role of Sentinel Lymph Node Biopsy in the Management of Cutaneous Melanoma – a UK Consensus Statement 2019. https://melanomafocus.com/wp-content/uploads/2019/01/SNB-Consensus-Final-1.pdf (accessed 8 August 2019).
- Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006;24:4196-201. https://doi.org/10.1200/JCO.2005.05.5152.
- Madu MF, Deken MM, van der Hage JA, Jóźwiak K, Wouters MWJM, van Akkooi ACJ. Isolated limb perfusion for melanoma is safe and effective in elderly patients. Ann Surg Oncol 2017;24:1997-2005. https://doi.org/10.1245/s10434-017-5803-9.
- Grünhagen DJ, Verhoef C. Isolated limb perfusion for stage III melanoma: does it still have a role in the present era of effective systemic therapy?. Oncology 2016;30:1045-52.
- Keenan LG, O’Sullivan S, Glynn A, Higgins M, Flavin A, Brennan S. Clinical review of treatment outcomes and patterns of failure with adjuvant radiotherapy in node-positive malignant melanoma. J Med Imaging Radiat Oncol 2017;61:258-62. https://doi.org/10.1111/1754-9485.12536.
- Liu JB, Bilimoria KY. Weighing the value of completion nodal dissection for melanoma. J Surg Oncol 2016;114:281-7. https://doi.org/10.1002/jso.24273.
- Raigani S, Cohen S, Boland GM. The role of surgery for melanoma in an era of effective systemic therapy. Curr Oncol Rep 2017;19. https://doi.org/10.1007/s11912-017-0575-8.
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. https://doi.org/10.1016/S1470-2045(15)70076-8.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30.
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824-35. https://doi.org/10.1056/NEJMoa1709030.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23-34. https://doi.org/10.1056/NEJMoa1504030.
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. https://doi.org/10.1016/S1470-2045(15)00083-2.
- Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603-15. https://doi.org/10.1016/S1470-2045(18)30142-6.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. https://doi.org/10.1056/NEJMoa1412690.
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. https://doi.org/10.1056/NEJMoa1103782.
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. https://doi.org/10.1056/NEJMoa1406037.
- Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017;377:1813-23. https://doi.org/10.1056/NEJMoa1708539.
- National Institute for Health and Care Excellence . NICE Pathways – Melanoma Overview 2019. https://pathways.nice.org.uk/pathways/melanoma (accessed 8 August 2019).
- D’Aniello C, Perri F, Scarpati GDV, Pepa CD, Pisconti S, Montesarchio V, et al. Melanoma adjuvant treatment: current insight and clinical features. Curr Cancer Drug Targets 2018;18:442-56. https://doi.org/10.2174/1568009617666170208163714.
- Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health 2014;36:140-8. https://doi.org/10.1093/pubmed/fdt032.
- Rychetnik L, McCaffery K, Morton R, Irwig L. Psychosocial aspects of post-treatment follow-up for stage I/II melanoma: a systematic review of the literature (provisional abstract). Psycho-Oncology 2013;22:721-36. https://doi.org/10.1002/pon.3060.
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol 2010;63:1308-11. https://doi.org/10.1016/j.jclinepi.2010.07.001.
- Appraisal of Guidelines for Research and Evaluation (AGREE) Research Trust . AGREE Enterprise Website 2014. www.agreetrust.org/ (accessed 11 September 2019).
- Barbour A, Millward M, Morton R, Saw R. Cancer Council Australia Melanoma Guidelines Working Party . Clinical Practice Guidelines for the Diagnosis and Management of Melanoma 2018. https://wiki.cancer.org.au/australia/Guidelines:Melanoma (accessed 8 August 2019).
- National Institute for Health and Care Excellence . Surveillance Proposal Consultation Document: 2019 Surveillance of Melanoma (NICE Guidelines NG14 and CSG8) 2019. www.nice.org.uk/guidance/ng14/documents/surveillance-review-proposal (accessed 3 October 2019).
- National Comprehensive Cancer Network . Cutaneous Melanoma 2019. www.nccn.org/professionals/physician_gls/default.aspx#melanoma (accessed 2 October 2019).
- Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U. ESMO Guidelines Committee . Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v126-32. https://doi.org/10.1093/annonc/mdv297.
- Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol 2019;80:208-50. https://doi.org/10.1016/j.jaad.2018.08.055.
- Dutch Working Group on Melanoma . Melanoma Guideline 2012 2013. www.oncoline.nl/uploaded/docs/melanoom/201208_vertaling%20Richtlijn%20melanoom%20def.pdf (accessed 1 October 2019).
- Pflugfelder A, Kochs C, Blum A, Capellaro M, Czeschik C, Dettenborn T, et al. S3-guideline ‘diagnosis, therapy and follow-up of melanoma’ – short version. J Dtsch Dermatol Ges 2013;11:563-602. https://doi.org/10.1111/ddg.12044.
- Dummer R, Siano M, Hunger RE, Lindenblatt N, Braun R, Michielin O, et al. The updated Swiss guidelines 2016 for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly 2016;146. https://doi.org/10.4414/smw.2016.14279.
- Castro LG, Bakos RM, Duprat Neto JP, Bittencourt FV, Di Giacomo TH, Serpa SS, et al. Brazilian guidelines for diagnosis, treatment and follow-up of primary cutaneous melanoma – Part II. An Bras Dermatol 2016;91:49-58. https://doi.org/10.1590/abd1806-4841.20164715.
- Cox NH, Williams HC. The British Association of Dermatologists therapeutic guidelines: can we AGREE?. Br J Dermatol 2003;148:621-5. https://doi.org/10.1046/j.1365-2133.2003.05241.x.
- Einwachter-Thompson J, MacKie RM. An evidence base for reconsidering current follow-up guidelines for patients with cutaneous melanoma less than 0.5 mm thick at diagnosis. Br J Dermatol 2008;159:337-41. https://doi.org/10.1111/j.1365-2133.2008.08641.x.
- Griffiths CE. The British Association of Dermatologists guidelines for the management of skin disease. Br J Dermatol 1999;141:396-7. https://doi.org/10.1046/j.1365-2133.1999.3029a.x.
- National Comprehensive Cancer Network . About the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) n.d. www.nccn.org/professionals/ (accessed 26 August 2020).
- National Comprehensive Cancer Network . NCCN Categories of Evidence and Consensus n.d. www.nccn.org/guidelines/guidelines-process/development-and-update-of-guidelines (accessed 26 August 2020).
- ESMO . ESMO Clinical Practice Guidelines: Melanoma n.d. www.esmo.org/guidelines/melanoma (accessed 12 December 2020).
- Dykewicz CA. Centers for Disease Control and Prevention (U.S.) . Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33:139-44. https://doi.org/10.1086/321805.
- ESMO Guidelines Committee . Standard Operating Procedures (SOPs) Instructions for Authors and Templates for Standard ESMO Clinical Practice Guidelines (CPGs) and ESMO-MCBS Scores 2019. www.esmo.org/content/download/77789/1426712/file/ESMO-Clinical-Practice-Guidelines-Standard-Operating-Procedures.pdf (accessed 1 October 2019).
- Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract 2004;17:59-67. https://doi.org/10.3122/jabfm.17.1.59.
- Association of Community Cancer Centers . Cancer Survivorship Care 2011. www.oncoline.nl/cancer-survivorship-care (accessed 1 October 2019).
- Association of Community Cancer Centers . Cancer Screening for Psychosocial Distress 2011. www.oncoline.nl/screening-for-psychosocial-distress (accessed 1 October 2019).
- German Guideline Program in Oncology . S3-Leitlinie Zur Diagnostik, Therapie and Nachsorge Des Melanoms 2018. www.leitlinienprogramm-onkologie.de/leitlinien/melanom/ (accessed 8 August 2019).
- Scottish Intercollegiate Guidelines Network . SIGN 50: A Guideline Developer’s Handbook 2015. www.sign.ac.uk/sign-50.html (accessed 2 October 2019).
- Oxman AD, Sackett DL, Guyatt GH. Users’ guides to the medical literature. I. How to get started. The Evidence-Based Medicine Working Group. JAMA 1993;270:2093-5. https://doi.org/10.1001/jama.1993.03510170083036.
- Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol 2005;6:608-21. https://doi.org/10.1016/S1470-2045(05)70283-7.
- National Institute for Health and Care Excellence . Melanoma: Assessment and Management. 1.9 Follow-up After Treatment for Melanoma 2015. www.nice.org.uk/guidance/ng14/chapter/1-recommendations#follow-up-after-treatment-for-melanoma-2 (accessed 8 August 2019).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
- Javanbakht M, Ellis R, Vale L, Lovat P, Bryant A, Kunonga P, et al. Optimal Surveillance Strategies for AJCC Stage I Cutaneous Melanoma Post Primary Tumour Excision: An Evidence Synthesis and Economic Evaluation 2019. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018086784 (accessed 23 May 2019).
- Whitlock EP, Lopez SA, Chang S, Helfand M, Eder M, Floyd N. AHRQ series paper 3: identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the effective health-care program. J Clin Epidemiol 2010;63:491-50. https://doi.org/10.1016/j.jclinepi.2009.03.008.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0384-4.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 [updated September 2020]. London: Cochrane; 2020.
- Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. Cochrane Methods. Cochrane Database of Systematic Reviews 2016, Issue 10 (Suppl 1). Hoboken, NJ: John Wiley & Sons, Inc.; 2016.
- McMaster University, Evidence Prime Inc . GRADEpro GDT: GRADEpro Guideline Development Tool n.d. https://gradepro.org/ (accessed 8 August 2019).
- Robinson JK, Wayne JD, Martini MC, Hultgren BA, Mallett KA, Turrisi R. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol 2016;152:979-85. https://doi.org/10.1001/jamadermatol.2016.1985.
- Robinson JK, Gaber R, Hultgren B, Eilers S, Blatt H, Stapleton J, et al. Skin self-examination education for early detection of melanoma: a randomized controlled trial of Internet, workbook, and in-person interventions. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.2883.
- Turrisi R, Hultgren B, Mallett KA, Martini M, Robinson JK. Comparison of efficacy of differing partner-assisted skin examination interventions for melanoma patients: a randomized clinical trial. JAMA Dermatol 2015;151:945-51. https://doi.org/10.1001/jamadermatol.2015.0690.
- Deckers E, Hoekstra-Weebers J, Damude S, Francken A, Ter Meulen S, Bastiaannet E, et al. The MELFO-Study: a multi-center prospective randomized clinical trial on the effects of a reduced stage-adjusted follow-up schedule on cutaneous melanoma IB–IIC patients: results after 3-years. Ann Surg Oncol 2018;25. https://doi.org/10.1245/s10434-019-07825-7.
- Moons KG, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012;98:691-8. https://doi.org/10.1136/heartjnl-2011-301247.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. https://doi.org/10.1200/JCO.2009.23.4799.
- Bigby M, Popescu C. Sentinel lymph node biopsy in melanoma. BMJ 2015;351. https://doi.org/10.1136/bmj.h5940.
- Ferrara G, Partenzi A, Filosa A. Sentinel node biopsy in melanoma: a short update. Dermatopathology 2018;5:21-5. https://doi.org/10.1159/000484892.
- Botar-Jid CM, Cosgarea R, Bolboacă SD, Şenilă SC, Lenghel LM, Rogojan L, et al. Assessment of cutaneous melanoma by use of very high-frequency ultrasound and real-time elastography. AJR Am J Roentgenol 2016;206:699-704. https://doi.org/10.2214/AJR.15.15182.
- Albertini MR. The age of enlightenment in melanoma immunotherapy. J Immunother Cancer 2018;6. https://doi.org/10.1186/s40425-018-0397-8.
- Lugowska I, Teterycz P, Rutkowski P. Immunotherapy of melanoma. Contemp Oncol 2018;22:61-7. https://doi.org/10.5114/wo.2018.73889.
- Koppolu V, Rekha Vasigala VK. Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J Cancer Res Ther 2018;14:1167-75. https://doi.org/10.4103/jcrt.JCRT_1290_16.
- Usher-Smith JA, Emery J, Kassianos AP, Walter FM. Risk prediction models for melanoma: a systematic review. Cancer Epidemiol Biomarkers Prev 2014;23:1450-63. https://doi.org/10.1158/1055-9965.EPI-14-0295.
- Vuong K, McGeechan K, Armstrong BK, Cust AE. Risk prediction models for incident primary cutaneous melanoma: a systematic review. JAMA Dermatol 2014;150:434-44. https://doi.org/10.1001/jamadermatol.2013.8890.
- Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG, et al. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0032844.
- Thomson DR, Rughani MG, Kuo R, Cassell OCS. Sentinel node biopsy status is strongly predictive of survival in cutaneous melanoma: extended follow-up of Oxford patients from 1998 to 2014. J Plast Reconstr Aesthet Surg 2017;70:1397-403. https://doi.org/10.1016/j.bjps.2017.05.025.
- Steyerberg EW. Clinical Prediction Models. New York, NY: Springer Publishing Company; 2009.
- Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001744.
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies (Prediction model Risk Of Bias ASsessment Tool). Ann Intern Med 2019;170:51-8. https://doi.org/10.7326/M18-1376.
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350. https://doi.org/10.1136/bmj.h870.
- Baade PD, Royston P, Youl PH, Weinstock MA, Geller A, Aitken JF. Prognostic survival model for people diagnosed with invasive cutaneous melanoma. BMC Cancer 2015;15. https://doi.org/10.1186/s12885-015-1024-4.
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622-34. https://doi.org/10.1200/JCO.2001.19.16.3622.
- Cochran AJ, Elashoff D, Morton DL, Elashoff R. Individualized prognosis for melanoma patients. Hum Pathol 2000;31:327-31. https://doi.org/10.1016/S0046-8177(00)80246-4.
- Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 2007;25:1129-34. https://doi.org/10.1200/JCO.2006.08.1463.
- Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol 2004;22:3668-76. https://doi.org/10.1200/JCO.2004.12.015.
- Maurichi A, Miceli R, Camerini T, Mariani L, Patuzzo R, Ruggeri R, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol 2014;32:2479-85. https://doi.org/10.1200/JCO.2013.54.2340.
- Rosenbaum BE, Schafer CN, Han SW, Osman I, Zhong H, Brinster N. Computer-assisted measurement of primary tumor area is prognostic of recurrence-free survival in stage IB melanoma patients. Mod Pathol 2017;30:1402-10. https://doi.org/10.1038/modpathol.2017.64.
- Saldanha G, Yarrow J, Pancholi J, Flatman K, Teo KW, Elsheik S, et al. Breslow density is a novel prognostic feature that adds value to melanoma staging. Am J Surg Pathol 2018;42:715-25. https://doi.org/10.1097/PAS.0000000000001034.
- Soong SJ, Ding S, Coit D, Balch CM, Gershenwald JE, Thompson JF, et al. AJCC Melanoma Task Force . Predicting survival outcome of localized melanoma: an electronic prediction tool based on the AJCC Melanoma Database. Ann Surg Oncol 2010;17:2006-14. https://doi.org/10.1245/s10434-010-1050-z.
- Tsai CA, Chen DT, Chen JJ, Balch CM, Thompson JF, Soong SJ. An integrated tree-based classification approach to prognostic grouping with application to localized melanoma patients. J Biopharm Stat 2007;17:445-60. https://doi.org/10.1080/10543400701199585.
- Vollmer RT, Seigler HF. Using a continuous transformation of the Breslow thickness for prognosis in cutaneous melanoma. Am J Clin Pathol 2001;115:205-12. https://doi.org/10.1309/WAVR-560R-NU5E-4Q96.
- Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005;92:965-70. https://doi.org/10.1093/biomet/92.4.965.
- Stadelmann WK, Rapaport DP, Soong SJ, Reintgen DS, Buzaid AC, Balch CM, et al. Cutaneous Melanoma. St Louis, MO: Quality Medical Publishing; 1998.
- Burnham K, Anderson D. Model Selection and Multi-model Inference: A Practical Information-Theoretic Approach. New York, NY: Springer-Verlag; 2002.
- Deeny SR, Steventon A. Making sense of the shadows: priorities for creating a learning healthcare system based on routinely collected data. BMJ Qual Saf 2015;24:505-15. https://doi.org/10.1136/bmjqs-2015-004278.
- Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1-W33. https://doi.org/10.7326/M18-1377.
- Shipe ME, Deppen SA, Farjah F, Grogan EL. Developing prediction models for clinical use using logistic regression: an overview. J Thorac Dis 2019;11. https://doi.org/10.21037/jtd.2019.01.25.
- Riley RD, Ensor J, Snell KI, Debray TP, Altman DG, Moons KG, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ 2016;353. https://doi.org/10.1136/bmj.i3140.
- Debray TP, Koffijberg H, Nieboer D, Vergouwe Y, Steyerberg EW, Moons KG. Meta-analysis and aggregation of multiple published prediction models. Stat Med 2014;33:2341-62. https://doi.org/10.1002/sim.6080.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128-38. https://doi.org/10.1097/EDE.0b013e3181c30fb2.
- Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med 2004;23:723-48. https://doi.org/10.1002/sim.1621.
- Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-33.
- Austin PC, Steyerberg EW. Graphical assessment of internal and external calibration of logistic regression models by using LOESS smoothers. Stat Med 2014;33:517-35. https://doi.org/10.1002/sim.5941.
- Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54:774-81. https://doi.org/10.1016/S0895-4356(01)00341-9.
- Debray TP, Vergouwe Y, Koffijberg H, Nieboer D, Steyerberg EW, Moons KG. A new framework to enhance the interpretation of external validation studies of clinical prediction models. J Clin Epidemiol 2015;68:279-89. https://doi.org/10.1016/j.jclinepi.2014.06.018.
- Hendriksen JM, Geersing GJ, Moons KG, de Groot JA. Diagnostic and prognostic prediction models. J Thromb Haemost 2013;11:129-41. https://doi.org/10.1111/jth.12262.
- Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009;338. https://doi.org/10.1136/bmj.b604.
- Assel M, Sjoberg DD, Vickers AJ. The Brier score does not evaluate the clinical utility of diagnostic tests or prediction models. Diagn Progn Res 2017;1. https://doi.org/10.1186/s41512-017-0020-3.
- Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338. https://doi.org/10.1136/bmj.b605.
- Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009;338. https://doi.org/10.1136/bmj.b606.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med 2015;13. https://doi.org/10.1186/s12916-014-0241-z.
- Ankeny JS, Labadie B, Luke J, Hsueh E, Messina J, Zager JS. Review of diagnostic, prognostic, and predictive biomarkers in melanoma. Clin Exp Metastasis 2018;35:487-93. https://doi.org/10.1007/s10585-018-9892-z.
- Hyams DM, Cook RW, Buzaid AC. Identification of risk in cutaneous melanoma patients: Prognostic and predictive markers. J Surg Oncol 2019;119:175-86. https://doi.org/10.1002/jso.25319.
- Sartore L, Papanikolaou GE, Biancari F, Mazzoleni F. Prognostic factors of cutaneous melanoma in relation to metastasis at the sentinel lymph node: a case-controlled study. Int J Surg 2008;6:205-9. https://doi.org/10.1016/j.ijsu.2008.03.003.
- Kettlewell S, Moyes C, Bray C, Soutar D, MacKay A, Byrne D, et al. Value of sentinel node status as a prognostic factor in melanoma: prospective observational study. BMJ 2006;332. https://doi.org/10.1136/bmj.38849.680509.AE.
- Catalano O, Siani A. Cutaneous melanoma: role of ultrasound in the assessment of locoregional spread. Curr Probl Diagn Radiol 2010;39:30-6. https://doi.org/10.1067/j.cpradiol.2009.04.001.
- Voit CA, van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Schmitz PI, Sterry W, et al. Rotterdam criteria for sentinel node (SN) tumor burden and the accuracy of ultrasound (US)-guided fine-needle aspiration cytology (FNAC): can US-guided FNAC replace SN staging in patients with melanoma?. J Clin Oncol 2009;27:4994-5000. https://doi.org/10.1200/JCO.2008.19.0033.
- Voit C, Van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Kowalczyk K, Roewert JC, et al. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. J Clin Oncol 2010;28:847-52. https://doi.org/10.1200/JCO.2009.25.7428.
- Bossi MC, Sanvito S, Lovati E, De Fiori E, Testori A, Bellomi M. Role of high resolution color-Doppler US of the sentinel node in patients with stage I melanoma. Radiol Med 2001;102:357-62.
- Hocevar M, Bracko M, Pogacnik A, Vidergar-Kralj B, Besic N, Zgajnar J, et al. The role of preoperative ultrasonography in reducing the number of sentinel lymph node procedures in melanoma. Melanoma Res 2004;14:533-6. https://doi.org/10.1097/00008390-200412000-00015.
- van Rijk MC, Teertstra HJ, Peterse JL, Nieweg OE, Olmos RA, Hoefnagel CA, et al. Ultrasonography and fine-needle aspiration cytology in the preoperative evaluation of melanoma patients eligible for sentinel node biopsy. Ann Surg Oncol 2006;13:1511-16. https://doi.org/10.1245/s10434-006-9106-9.
- Ulrich J, van Akkooi AJ, Eggermont AM, Voit C. New developments in melanoma: utility of ultrasound imaging (initial staging, follow-up and pre-SLNB). Expert Rev Anticancer Ther 2011;11:1693-701. https://doi.org/10.1586/era.11.115.
- Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst 2011;103:129-42. https://doi.org/10.1093/jnci/djq455.
- Hall BJ, Schmidt RL, Sharma RR, Layfield LJ. Fine-needle aspiration cytology for the diagnosis of metastatic melanoma: systematic review and meta-analysis. Am J Clin Pathol 2013;140:635-42. https://doi.org/10.1309/AJCPWSDDHLLW40WI.
- Beynon R, Leeflang MM, McDonald S, Eisinga A, Mitchell RL, Whiting P, et al. Search strategies to identify diagnostic accuracy studies in MEDLINE and EMBASE. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.MR000022.pub3.
- Vincent S, Greenley S, Beaven O. Clinical Evidence diagnosis: Developing a sensitive search strategy to retrieve diagnostic studies on deep vein thrombosis: a pragmatic approach. Health Info Libr J 2003;20:150-9. https://doi.org/10.1046/j.1365-2532.2003.00427.x.
- Simel DL, Feussner JR, DeLong ER, Matchar DB. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making 1987;7:107-14. https://doi.org/10.1177/0272989X8700700208.
- Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012799.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. https://doi.org/10.1002/sim.1186.
- Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 2014;48:193-204. https://doi.org/10.1016/j.jbi.2014.02.013.
- Bujang MA, Adnan TH. Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diagn Res 2016;10:YE01-YE06. https://doi.org/10.7860/JCDR/2016/18129.8744.
- Pánczél G, Liszkay G, Borbola K, Balatoni T, Hunyadi J. The importance of fine needle aspiration cytology in the management of recurrent and metastatic melanoma. Orv Hetil 2012;153:1419-23. https://doi.org/10.1556/OH.2012.29434.
- Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma: results of a prospective study of 1288 patients. Cancer 2000;88:2534-9. https://doi.org/10.1002/1097-0142(20000601)88:11<2534::AID-CNCR15>3.0.CO;2-2.
- Dalle S, Paulin C, Lapras V, Balme B, Ronger-Savle S, Thomas L. Fine-needle aspiration biopsy with ultrasound guidance in patients with malignant melanoma and palpable lymph nodes. Br J Dermatol 2006;155:552-6. https://doi.org/10.1111/j.1365-2133.2006.07361.x.
- Hayes AJ, Moskovic E, O’Meara K, Smith HG, Pope RJE, Larkin J, et al. Prospective cohort study of ultrasound surveillance of regional lymph nodes in patients with intermediate-risk cutaneous melanoma. Br J Surg 2019;106:729-34. https://doi.org/10.1002/bjs.11112.
- Rubaltelli L, Beltrame V, Tregnaghi A, Scagliori E, Frigo AC, Stramare R. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR Am J Roentgenol 2011;196:W8-12. https://doi.org/10.2214/AJR.10.4711.
- Doubrovsky A, Scolyer RA, Murali R, McKenzie PR, Watson GF, Lee CS, et al. Diagnostic accuracy of fine needle biopsy for metastatic melanoma and its implications for patient management. Ann Surg Oncol 2008;15:323-32. https://doi.org/10.1245/s10434-006-9341-0.
- Krüger U, Kretschmer L, Thoms KM, Padeken M, Peter Bertsch H, Schön MP, et al. Lymph node ultrasound during melanoma follow-up significantly improves metastasis detection compared with clinical examination alone: a study on 433 patients. Melanoma Res 2011;21:457-63. https://doi.org/10.1097/CMR.0b013e328348dad3.
- Solbiati L, Rizzatto G, Bellotti E. High Resolution Sonography of Cervical Lymph Nodes in Head and Neck Cancer: Criteria for Differentiation of Reactive Versus Malignant Lymph Nodes n.d.
- Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high resolution US. Radiology 1992;183:215-20. https://doi.org/10.1148/radiology.183.1.1549675.
- Garbe C, Schadendorf D, Stolz W, Volkenandt M, Reinhold U, Kortmann RD, et al. Short German guidelines: malignant melanoma. J Dtsch Dermatol Ges 2008;6:9-14. https://doi.org/10.1111/j.1610-0387.2008.06711.x.
- Knackstedt T, Knackstedt RW, Couto R, Gastman B. Malignant melanoma: diagnostic and management update. Plast Reconstr Surg 2018;142:202e-216e. https://doi.org/10.1097/PRS.0000000000004571.
- Bafounta ML, Beauchet A, Chagnon S, Saiag P. Ultrasonography or palpation for detection of melanoma nodal invasion: a meta-analysis. Lancet Oncol 2004;5:673-80. https://doi.org/10.1016/S1470-2045(04)01609-2.
- Dinnes J, Ferrante di Ruffano L, Takwoingi Y, Cheung ST, Nathan P, Matin RN, et al. Ultrasound, CT, MRI, or PET-CT for staging and re-staging of adults with cutaneous melanoma. Cochrane Database Syst Rev 2019;7. https://doi.org/10.1002/14651858.CD012806.pub2.
- Mooney MM, Mettlin C, Michalek AM, Petrelli NJ, Kraybill WG. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pulmonary recurrences: a cost-effectiveness analysis. Cancer 1997;80:1052-64.
- Bassères N, Grob JJ, Richard MA, Thirion X, Zarour H, Noe C, et al. Cost-effectiveness of surveillance of stage I melanoma. A retrospective appraisal based on a 10-year experience in a dermatology department in France. Dermatology 1995;191:199-203. https://doi.org/10.1159/000246546.
- Hengge UR, Wallerand A, Stutzki A, Kockel N. Cost-effectiveness of reduced follow-up in malignant melanoma. J Dtsch Dermatol Ges 2007;5:898-907. https://doi.org/10.1111/j.1610-0387.2007.06454.x.
- Morton RL, Howard K, Thompson JF. The cost-effectiveness of sentinel node biopsy in patients with intermediate thickness primary cutaneous melanoma. Ann Surg Oncol 2009;16:929-40. https://doi.org/10.1245/s10434-008-0164-z.
- Serra-Arbeloa P, Rabines-Juárez ÁO, Álvarez-Ruiz MS, Guillén-Grima F. Sentinel node biopsy in patients with primary cutaneous melanoma of any thickness: a cost-effectiveness analysis. Surg Oncol 2016;25:205-11. https://doi.org/10.1016/j.suronc.2016.05.020.
- Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: a cost-effectiveness analysis. J Am Acad Dermatol 1999;41:738-45. https://doi.org/10.1016/S0190-9622(99)70010-1.
- Losina E, Walensky RP, Geller A, Beddingfield FC, Wolf LL, Gilchrest BA, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol 2007;143:21-8. https://doi.org/10.1001/archderm.143.1.21.
- Krug B, Crott R, Roch I, Lonneux M, Beguin C, Baurain JF, et al. Cost-effectiveness analysis of FDG PET-CT in the management of pulmonary metastases from malignant melanoma. Acta Oncol 2010;49:192-200. https://doi.org/10.3109/02841860903440254.
- Kansal AR, Shaul AJ, Stern S, Busam K, Doucet CA, Chalfin DB. Cost-effectiveness of a FISH assay for the diagnosis of melanoma in the USA. Expert Rev Pharmacoecon Outcomes Res 2013;13:371-80. https://doi.org/10.1586/erp.13.22.
- Gordon LG, Brynes J, Baade PD, Neale RE, Whiteman DC, Youl PH, et al. Cost-effectiveness analysis of a skin awareness intervention for early detection of skin cancer targeting men older than 50 years. Value Health 2017;20:593-601. https://doi.org/10.1016/j.jval.2016.12.017.
- Kurtz J, Beasley GM, Agnese D, Kendra K, Olencki TE, Terando A, et al. Surveillance strategies in the follow-up of melanoma patients: too much or not enough?. J Surg Res 2017;214:32-7. https://doi.org/10.1016/j.jss.2017.02.070.
- Wilson ECF, Usher-Smith JA, Emery J, Corrie P, Walter FM. A modeling study of the cost-effectiveness of a risk-stratified surveillance program for melanoma in the United Kingdom. Value Health 2018;21:658-68. https://doi.org/10.1016/j.jval.2017.11.009.
- Wilson EC, Emery JD, Kinmonth AL, Prevost AT, Morris HC, Humphrys E, et al. The cost-effectiveness of a novel SIAscopic diagnostic aid for the management of pigmented skin lesions in primary care: a decision-analytic model. Value Health 2013;16:356-66. https://doi.org/10.1016/j.jval.2012.12.008.
- Edwards SJ, Mavranezouli I, Osei-Assibey G, Marceniuk G, Wakefield V, Karner C. VivaScope® 1500 and 3000 systems for detecting and monitoring skin lesions: a systematic review and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20580.
- York Health Economics Consortium . Micro-simulation 2016. www.yhec.co.uk/glossary/micro-simulation/ (accessed 2 October 2019).
- York Health Economics Consortium . Markov Model 2016. www.yhec.co.uk/glossary/markov-model/ (accessed 2 October 2019).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 2 October 2019).
- Cancer Research UK . Melanoma Skin Cancer Statistics 2019. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer#heading-One (accessed 23 May 2019).
- Rueth NM, Xing Y, Chiang YJ, Cromwell KD, Ross MI, Lee JE, et al. Is surveillance imaging effective for detecting surgically treatable recurrences in patients with melanoma? A comparative analysis of stage-specific surveillance strategies. Ann Surg 2014;259:1215-22. https://doi.org/10.1097/SLA.0000000000000233.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Holterhues C, van de Poll-Franse LV, de Vries E, Neumann HA, Nijsten TE. Melanoma patients receive more follow-up care than current guideline recommendations: a study of 546 patients from the general Dutch population. J Eur Acad Dermatol Venereol 2012;26:1389-95. https://doi.org/10.1111/j.1468-3083.2011.04297.x.
- National Institute for Health and Care Excellence . Improving Outcomes for People With Skin Tumours Including Melanoma 2006. www.nice.org.uk/guidance/csg8 (accessed 2 October 2019).
- Wilson ECF, Usher-Smith JA, Emery J, Corrie PG, Walter FM. Expert elicitation of multinomial probabilities for decision-analytic modeling: an application to rates of disease progression in undiagnosed and untreated melanoma. Value Health 2018;21:669-76. https://doi.org/10.1016/j.jval.2017.10.009.
- Ahnlide I, Bjellerup M, Nilsson F, Nielsen K. Validity of ABCD rule of dermoscopy in clinical practice. Acta Derm Venereol 2016;96:367-72. https://doi.org/10.2340/00015555-2239.
- Bauer P, Cristofolini P, Boi S, Burroni M, Dell’Eva G, Micciolo R, et al. Digital epiluminescence microscopy: usefulness in the differential diagnosis of cutaneous pigmentary lesions. A statistical comparison between visual and computer inspection. Melanoma Res 2000;10:345-9. https://doi.org/10.1097/00008390-200008000-00005.
- Carli P, de Giorgi V, Salvini C, Mannone F, Chiarugi A. The gold standard for photographing pigmented skin lesions for diagnostic purposes: contact versus distant imaging. Skin Res Technol 2002;8:255-9. https://doi.org/10.1034/j.1600-0846.2002.00335.x.
- Carli P, De Giorgi V, Donati E, Pestelli E, Giannotti B. Epiluminescence microscopy reduces the risk of removing clinically atypical, but histologically common, melanocytic lesions. G Ital Dermatol Venereol 1994;129:599-605.
- Dreiseitl S, Binder M, Hable K, Kittler H. Computer versus human diagnosis of melanoma: evaluation of the feasibility of an automated diagnostic system in a prospective clinical trial. Melanoma Res 2009;19:180-4. https://doi.org/10.1097/CMR.0b013e32832a1e41.
- Gokdemir A, Guler OM, Bek Y, Aydin F, Senturk N, Canturk T. Dermoscopic and histopathological correlation in melanocytic and non-melanocytic lesions. Turkiye Klinikleri Dermatoloji 2011;21:7-16.
- Haenssle HA, Korpas B, Hansen-Hagge C, Buhl T, Kaune KM, Rosenberger A, et al. Seven-point checklist for dermatoscopy: performance during 10 years of prospective surveillance of patients at increased melanoma risk. J Am Acad Dermatol 2010;62:785-93. https://doi.org/10.1016/j.jaad.2009.08.049.
- Morales-Callaghan AM, Castrodeza-Sanz J, Martínez-García G, Peral-Martínez I, Miranda-Romero A. Correlation between clinical, dermatoscopic, and histopathologic variables in atypical melanocytic nevi. Actas Dermosifiliogr 2008;99:380-9. https://doi.org/10.1016/S0001-7310(08)74697-0.
- Nachbar F, Stolz W, Merkle T, Cognetta AB, Vogt T, Landthaler M, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol 1994;30:551-9. https://doi.org/10.1016/S0190-9622(94)70061-3.
- Soyer HP, Smolle J, Leitinger G, Rieger E, Kerl H. Diagnostic reliability of dermoscopic criteria for detecting malignant melanoma. Dermatology 1995;190:25-30. https://doi.org/10.1159/000246629.
- Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol 2009;129:131-8. https://doi.org/10.1038/jid.2008.193.
- Bono A, Bartoli C, Baldi M, Tomatis S, Bifulco C, Santinami M. Clinical and dermatoscopic diagnosis of small pigmented skin lesions. Eur J Dermatol 2002;12:573-6.
- Bono A, Bartoli C, Cascinelli N, Lualdi M, Maurichi A, Moglia D, et al. Melanoma detection. A prospective study comparing diagnosis with the naked eye, dermatoscopy and telespectrophotometry. Dermatology 2002;205:362-6. https://doi.org/10.1159/000066436.
- Ferris LK, Harkes JA, Gilbert B, Winger DG, Golubets K, Akilov O, et al. Computer-aided classification of melanocytic lesions using dermoscopic images. J Am Acad Dermatol 2015;73:769-76. https://doi.org/10.1016/j.jaad.2015.07.028.
- Elmore JG, Barnhill RL, Elder DE, Longton GM, Pepe MS, Reisch LM, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ 2017;357. https://doi.org/10.1136/bmj.j2813.
- Leiter U, Buettner PG, Eigentler TK, Bröcker EB, Voit C, Gollnick H, et al. Hazard rates for recurrent and secondary cutaneous melanoma: an analysis of 33,384 patients in the German Central Malignant Melanoma Registry. J Am Acad Dermatol 2012;66:37-45. https://doi.org/10.1016/j.jaad.2010.09.772.
- Turner RM, Bell KJ, Morton RL, Hayen A, Francken AB, Howard K, et al. Optimizing the frequency of follow-up visits for patients treated for localized primary cutaneous melanoma. J Clin Oncol 2011;29:4641-6. https://doi.org/10.1200/JCO.2010.34.2956.
- Office for National Statistics . National Life Tables: UK 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 27 August 2019).
- Department of Health and Social Care . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed 27 August 2019).
- National Institute for Health and Care Excellence . Trametinib in Combination With Dabrafenib for Treating Unresectable or Metastatic Melanoma 2016. www.nice.org.uk/guidance/ta396/ (accessed 8 August 2019).
- National Institute for Health and Care Excellence . Nivolumab in Combination With Ipilimumab for Treating Advanced Melanoma 2016. www.nice.org.uk/guidance/ta400/ (accessed 3 October 2019).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 3 October 2019).
- Tran AD, Fogarty G, Nowak AK, Espinoza D, Rowbotham N, Stockler MR, et al. A systematic review and meta-analysis of utility estimates in melanoma. Br J Dermatol 2018;178:384-93. https://doi.org/10.1111/bjd.16098.
- Brazier J, Ara R, Azzabi I, Busschbach J, Chevrou-Séverac H, Crawford B, et al. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2019;22:267-75. https://doi.org/10.1016/j.jval.2019.01.004.
- Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med 2012;10. https://doi.org/10.1186/1479-5876-10-85.
- National institute for Health and Care Excellence . Nivolumab for Treating Advanced (Unresectable or Metastatic) Melanoma 2016. www.nice.org.uk/guidance/ta384/ (accessed 3 October 2019).
- Dakin H, Devlin N, Feng Y, Rice N, O’Neill P, Parkin D. The influence of cost-effectiveness and other factors on NICE decisions. Health Econ 2015;24:1256-71. https://doi.org/10.1002/hec.3086.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Garbe C. A rational approach to the follow-up of melanoma patients. Recent Results Cancer Res 2002;160:205-15. https://doi.org/10.1007/978-3-642-59410-6_24.
- Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based?. Ann Surg 1995;221:566-9. https://doi.org/10.1097/00000658-199505000-00014.
- Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF. Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Ann Surg Oncol 2007;14:1924-33. https://doi.org/10.1245/s10434-007-9347-2.
- Coyle D, Oakley J. Estimating the expected value of partial perfect information: a review of methods. Eur J Health Econ 2008;9:251-9. https://doi.org/10.1007/s10198-007-0069-y.
- National Cancer Registration and Analysis Service . Survival by Stage 2019. www.ncin.org.uk/publications/survival_by_stage (accessed 25 September 2019).
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- McCabe C, Dixon S. Testing the validity of cost-effectiveness models. PharmacoEconomics 2000;17:501-13. https://doi.org/10.2165/00019053-200017050-00007.
- Drummond M. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- O’Mahony JF, Naber SK, Normand C, Sharp L, O’Leary JJ, de Kok IM. Beware of kinked frontiers: a systematic review of the choice of comparator strategies in cost-effectiveness analyses of human papillomavirus testing in cervical screening. Value Health 2015;18:1138-51. https://doi.org/10.1016/j.jval.2015.09.2939.
- von Schuckmann LA, Hughes MCB, Ghiasvand R, Malt M, van der Pols JC, Beesley VL, et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol 2019;155:688-93. https://doi.org/10.1001/jamadermatol.2019.0440.
- Morton RL. Essential inputs for studies of cost-effectiveness analysis in melanoma. Br J Dermatol 2014;171:1294-5. https://doi.org/10.1111/bjd.13455.
- Tromme I, Devleesschauwer B, Beutels P, Richez P, Leroy A, Baurain JF, et al. Health-related quality of life in patients with melanoma expressed as utilities and disability weights. Br J Dermatol 2014;171:1443-50. https://doi.org/10.1111/bjd.13262.
- Schünemann H, Brożek J, Guyatt G, Oxman A. The GRADE Working Group . GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations 2013. https://gdt.gradepro.org/app/handbook/handbook.html (accessed 25 February 2019).
- Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol 2014;70:748-62. https://doi.org/10.1016/j.jaad.2013.11.038.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Haenssle HA, Korpas B, Hansen-Hagge C, Buhl T, Kaune KM, Johnsen S, et al. Selection of patients for long-term surveillance with digital dermoscopy by assessment of melanoma risk factors. Arch Dermatol 2010;146:257-64. https://doi.org/10.1001/archdermatol.2009.370.
- Tufts Medical Center . CEA Registry 2018. www.cevr.tuftsmedicalcenter.org/databases/cea-registry (accessed 15 August 2019).
- Health Economics Research Centre . HERC Database of Mapping Studies 2019. www.herc.ox.ac.uk/downloads/herc-database-of-mapping-studies (accessed 15 August 2019).
- National Institute for Health Research . PROSPERO: International Prospective Register of Systematic Reviews 2019. www.crd.york.ac.uk/PROSPERO (accessed 15 August 2019).
- Canadian Agency for Drugs and Technologies in Health . Strings Attached: CADTH Database Search Filters 2016. www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#health (accessed 29th August 2019).
Appendix 1 The MEDLINE strategy for the systematic review of surveillance strategies
MEDLINE (via Ovid)
Date range searched: 1946 to April week 4 2018.
Date searched: 4 April 2018.
| # | Searches | Results (n) |
|---|---|---|
| 1 | Melanoma/or Hutchinson’s Melanotic Freckle/or Melanoma, Amelanotic/ | 77,620 |
| 2 | melanoma*.ti,ab,kf. | 93,966 |
| 3 | ((skin or freckle* or lentigo* or lentigin*) adj3 (melanotic or tumor* or tumour* or cancer* or maligna*)).ti,ab,kf. | 29,969 |
| 4 | (cutaneous adj3 (tumor* or tumour* or cancer*)).ti,ab,kf. | 3679 |
| 5 | dubreuilh.ti,ab,kf. | 41 |
| 6 | or/1-5 | 130,861 |
| 7 | Public Health Surveillance/ | 1907 |
| 8 | Population Surveillance/ | 54,425 |
| 9 | Mass Screening/ | 93,615 |
| 10 | Aftercare/ | 7802 |
| 11 | exp Genetic Testing/ | 40,228 |
| 12 | or/7-11 | 194,425 |
| 13 | 6 and 12 | 1201 |
| 14 | Melanoma/pc [Prevention & Control] | 1697 |
| 15 | Hutchinson’s Melanotic Freckle/pc [Prevention & Control] | 3 |
| 16 | Melanoma, Amelanotic/pc [Prevention & Control] | 3 |
| 17 | ((melanoma* or melanotic or skin cancer*) adj6 (monitor* or surveill* or follow up or followup or screen* or posttreatment or post treatment or after care or aftercare or check up or checkup or examin*)).ti,ab. | 5206 |
| 18 | or/14-17 | 6646 |
| 19 | 13 or 18 | 7254 |
| 20 | limit 19 to (humans and yr = ‘2011 -Current’) | 2182 |
| 21 | (comment or editorial or letter or news).pt. | 1,652,061 |
| 22 | 20 not 21 | 2050 |
Search update carried out on 2 July 2019, added to the end of the search strategy described above.
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date range searched: 1946 to 1 July 2019.
Date searched: 2 July 2019.
| # | Searches | Results (n) |
|---|---|---|
| 22 | 20 not 21 | 2409 |
| 23 | (201803* or 201804* or 201805* or 201806* or 201807* or 201808* or 201809* or 20181* or 2019*).ed. | 1,355,420 |
| 24 | 2019*.dp. | 601,134 |
| 25 | 23 or 24 | 1,818,467 |
| 26 | 22 and 25 | 434 |
Appendix 2 Systematic review of surveillance strategies: excluded studies
| Study | Reason(s) for exclusion |
|---|---|
| Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–75 | Insufficient stage I participants |
| Auckland R, Wassell P, Hall S, Nicolson MC, Murchie P. Exploring patterns of recurrent melanoma in Northeast Scotland to inform the introduction a digital self-examination intervention. BMC Dermatol 2014;14:4 | Insufficient stage I participants |
| Balamurugan A, Rees JR, Kosary C, Rim SH, Li J, Stewart SL. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol 2011;65(Suppl. 5):69–77 | Stage not reported |
| Chen J, Xu Y, Zhou Y, Wang Y, Zhu H, Shi Y. Prognostic role of sentinel lymph node biopsy for patients with cutaneous melanoma: a retrospective study of surveillance, epidemiology, and end-result population-based data. Oncotarget 2016;7:45671–45677 | Prognostic study |
| Chen T, Fallah M, Försti A, Kharazmi E, Sundquist K, Hemminki K. Risk of next melanoma in patients with familial and sporadic melanoma by number of previous melanomas. JAMA Dermatol 2015;151:607–15 | Stage not reported |
| Czajkowska Z, Hall NC, Sewitch M, Wang B, Körner A. The role of patient education and physician support in self-efficacy for skin self-examination among patients with melanoma. Patient Educ Couns 2017;100:1505–10 | Less than 80% of participants at stage I |
| Danielsen M, Højgaard L, Kjær A, Fischer BM. Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging 2013;4:17–28 | Systematic review of melanoma stages II–III |
| Dika E, Chessa MA, Veronesi G, Ravaioli GM, Fanti PA, Ribero S, et al. A single institute experience on melanoma prognosis: a long term follow-up. G Ital Dermatol Venereol 2018;12:12 | No surveillance and insufficient stage I participants |
| de Vries M, Speijers MJ, Bastiaannet E, Plukker JT, Brouwers AH, van Ginkel RJ, et al. Long-term follow-up reveals that ulceration and sentinel lymph node status are the strongest predictors for survival in patients with primary cutaneous melanoma. Eur J Surg Oncol 2011;37:681–7 | Prognostic study |
| Glenn BA, Chen KL, Chang LC, Lin T, Bastani R. Skin examination practices among melanoma survivors and their children. J Cancer Educ 2017;32:335–43 | Stage not reported |
| Jones MS, Torisu-Itakura H, Flaherty DC, Schoellhammer HF, Lee J, Sim MS, Faries MB. Second primary melanoma: risk factors, histopathologic features, survival, and implications for follow-up. Am Surg 2016;82:1009–13 | Less than 80% of participants at stage I |
| Lee HJ, Jin H, You HS, Shim WH, Kim JM, Kim GW, et al. Various dermatoses what the patients with cutaneous melanoma had anxiety for the recurrence during postoperative surveillance. Ann Dermatol 2017;29:433–7 | Insufficient stage I participants |
| Memari N, Hayen A, Bell KJ, Rychetnik L, Morton RL, McCaffery K, et al. How often do patients with localized melanoma attend follow-up at a specialist center? Ann Surg Oncol 2015;22:S1164–71 | Less than 80% of participants at stage I |
| Nahar VK, Allison Ford M, Brodell RT, Boyas JF, Jacks SK, Biviji-Sharma R, et al. Skin cancer prevention practices among malignant melanoma survivors: a systematic review. J Cancer Res Clin Oncol 2016;142:1273–83 | Systematic review of prevention strategies of survivors |
| Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol 2015;72:794–800 | All stages of invasive melanomas |
| Ribero S, Podlipnik S, Osella-Abate S, Sportoletti-Baduel E, Manubens E, Barreiro A, et al. Ultrasound-based follow-up does not increase survival in early-stage melanoma patients: a comparative cohort study. Eur J Cancer 2017;85:59–66 | Less than 80% of participants at stage I |
| Rodríguez VM, Berwick M, Hay JL. Communication about melanoma and risk reduction after melanoma diagnosis. Psycho-Oncology 2017;26:2142–8 | Prevention strategies |
| Rohren EM. PET/computed tomography and patient outcomes in melanoma. PET Clin 2015;10:243–54 | Diagnostic study |
| Saiag P, Aegerter P, Vitoux D, Lebbé C, Wolkenstein P, Dupin N, et al. Prognostic Value of 25-hydroxyvitamin D3 levels at diagnosis and during follow-up in melanoma patients. J Natl Cancer Inst 2015;107:djv264 | Prognostic study |
| Salerni G, Lovatto L, Carrera C, Puig S, Malvehy J. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol 2011;147:549–55 | Less than 80% of participants at stage I |
| Sanlorenzo M, Ribero S, Osella-Abate S, Zugna D, Marenco F, Macripo G, et al. Prognostic differences across sexes in melanoma patients: what has changed from the past? Melanoma Res 2014;24:568–76 | Prognostic study |
| Sjoestroem C, Khosravi S, Cheng Y, Safaee Ardekani G, Martinka M, Li G. DLC1 expression is reduced in human cutaneous melanoma and correlates with patient survival. Mod Pathol 2014;27:1203–11 | Prognostic study |
| Solivetti FM, Elia F, Graceffa D, Di Carlo A. Ultrasound morphology of inguinal lymph nodes may not herald an associated pathology. J Exp Clin Cancer Res 2012;31:88 | Diagnostic study |
| Turner RM, Bell KJ, Morton RL, Hayen A, Francken AB, Howard K, et al. Optimizing the frequency of follow-up visits for patients treated for localized primary cutaneous melanoma. J Clin Oncol 2011;29:4641–6 | Less than 80% of participants at stage I |
| Vensby PH, Schmidt G, Kjær A, Fischer BM. The value of FDG PET/CT for follow-up of patients with melanoma: a retrospective analysis. Am J Nucl Med Mol Imaging 2017;7:255–62 | Less than 80% of participants at stage I |
| Wolf A, Kvint S, Chachoua A, Pavlick A, Wilson M, Donahue B, et al. Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J Neurosurg 2018;128:23–31 | Advanced stage of disease |
| Wu CE, Hsieh CH, Chang CJ, Yeh JT, Kuo TT, Yang CH, et al. Prognostic factors for Taiwanese patients with cutaneous melanoma undergoing sentinel lymph node biopsy. J Formos Med Assoc 2015;114:415–21 | Prognostic study |
| Xing Y, Cromwell KD, Cormier JN. Review of diagnostic imaging modalities for the surveillance of melanoma patients. Dermatol Res Pract 2012;2012:941921 | Diagnostic study |
| Youl PH, Soyer HP, Baade PD, Marshall AL, Finch L, Janda M. Can skin cancer prevention and early detection be improved via mobile phone text messaging? A randomised, attention control trial. Prev Med 2015;71:50–6 | Stage not reported |
| Zhang G, Cheng Y, Chen G, Tang Y, Ardekani G, Rotte A, et al. Loss of tumor suppressors KAI1 and p27 identifies a unique subgroup of primary melanoma patients with poor prognosis. Oncotarget 2015;6:23026–35 | Prognostic study |
| Zörnig I, Halama N, Lorenzo Bermejo J, Ziegelmeier C, Dickes E, Migdoll A, et al. Prognostic significance of spontaneous antibody responses against tumor-associated antigens in malignant melanoma patients. Int J Cancer 2015;136:138–51 | Prognostic study |
Appendix 3 The MEDLINE strategy for the systematic review of risk prediction models
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date range searched: 1946 to 15 July 2019.
Date of original search: September 2018.
Date of updated search: 16 July 2019.
| # | Searches | Results (n) |
|---|---|---|
| 1 | (Validat* or Predict* or Rule* or (Predict* and (Outcome* or Risk* or Model*)) or ((History or Variable* or Criteria or Scor* or Characteristic* or Finding* or Factor*) and (Predict* or Model* or Decision* or Identif* or Prognos*))).mp. or (Decision*.mp. and ((Model* or Clinical*).mp. or Logistic Models/)) or (Prognostic and (History or Variable* or Criteria or Scor* or Characteristic* or Finding* or Factor* or Model*)).ti,ab. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 4,600,871 |
| 2 | Stratification.mp. or ROC Curve/or Discrimination.mp. or Discriminate.mp. or c-statistic.mp. or c statistic.mp. or Area under the curve.mp. or AUC.mp. or Calibration.mp. or Indices.mp. or Algorithm.mp. or Multivariable.mp. or Prognosis.mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 1,450,061 |
| 3 | 1 or 2 | 5,285,509 |
| 4 | *melanoma/ | 65,466 |
| 5 | *skin neoplasms/ | 99,710 |
| 6 | melanoma.kw. | 7937 |
| 7 | (malignant adj3 melanoma*).ti,ab. | 27,244 |
| 8 | (tumo* adj5 (mole or melanoma)).ti,ab. | 12,160 |
| 9 | 4 or 5 or 6 or 7 or 8 | 149,902 |
| 10 | (((prognos* or melanoma) adj5 surviv*) or factor*).ti,ab. | 3,091,009 |
| 11 | (metas* or advance* or recur* or relaps* or invasive or second* or disseminat*).ti,ab. | 3,355,846 |
| 12 | (distant metastases or local recurren*).ti,ab. | 45,561 |
| 13 | 10 or 11 or 12 | 5,901,577 |
| 14 | exp comment/or exp letter/or exp editorial/ | 1,731,981 |
| 15 | exp animals/not exp humans/ | 4,595,710 |
| 16 | (animal or mouse).mp. or mice.ti,ab. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 2,072,446 |
| 17 | exp review/ | 2,504,343 |
| 18 | exp case reports/or case report*.ti,ab. | 2,103,512 |
| 19 | or/14-18 | 10,922,034 |
| 20 | (3 and 9 and 13) not 19 | 13,880 |
| 21 | (201808* or 201809* or 20181* or 2019*).ed. or 2019*.dp. | 1,423,830 |
| 22 | 20 and 21 | 1023 |
Appendix 4 Systematic review of risk prediction models: excluded studies
| Study | Reason(s) for exclusion |
|---|---|
| Arce PM, Camilon PR, Stokes WA, Nguyen SA, Lentsch EJ. Is sex an independent prognostic factor in cutaneous head and neck melanoma? Laryngoscope 2014;124:1363–7 | One factor |
| Ariyan C, Brady MS, Gönen M, Busam K, Coit D. Positive nonsentinel node status predicts mortality in patients with cutaneous melanoma. Ann Surg Oncol 2009;16:186–90 | One factor |
| Avilés-Izquierdo JA, Lázaro-Ochaita P. [Sentinel node biopsy as a prognostic factor in cutaneous melanoma.] Actas Dermosifiliogr 2009;100:486–92 | One factor |
| Byrom L, Dasgupta P, Youlden D, Baade P, Green A, Khosrotehrani K. Melanoma prognosis differs according to sex: an Australian population study. Australas J Dermatol 2014;55:9–10 | One factor |
| Cadili A, Dabbs K. Predictors of sentinel lymph node metastasis in melanoma. Can J Surg 2010;53:32–6 | Stage not reported |
| Callender GG, Egger ME, Burton AL, Scoggins CR, Ross MI, Stromberg AJ, et al. Prognostic implications of anatomic location of primary cutaneous melanoma of 1 mm or thicker. Am J Surg 2011;202:659–64 | No validation |
| Callender GG, Gershenwald JE, Egger ME, Scoggins CR, Martin RC, Schacherer CW, et al. A novel and accurate computer model of melanoma prognosis for patients staged by sentinel lymph node biopsy: comparison with the American Joint Committee on Cancer model. J Am Coll Surg 2012;214:608–17 | Excludes stage I |
| Cascinelli N, Bombardieri E, Bufalino R, Camerini T, Carbone A, Clemente C, et al. Sentinel and nonsentinel node status in stage IB and II melanoma patients: two-step prognostic indicators of survival. J Clin Oncol 2006;24:4464–71 | No validation |
| Chen G, Chen Y, Zhang Z, Martinka M, Li G. Reduced Tip60 expression as a predictive biomarker for advanced melanoma and patient outcome. Cancer Res 2011;71:2103 | One factor |
| Chen N, Gong J, Chen X, Xu M, Huang Y, Wang L, et al. Cytokeratin expression in malignant melanoma: potential application of in-situ hybridization analysis of mRNA. Melanoma Res 2009;19:87–93 | One factor |
| Cheng Y, Zhou Y. BRAF protein expression as a prognostic marker for thin melanomas. J Invest Dermatol 2014;134:S131 | Stages mixed |
| Cook RW, Covington KR, Monzon FA. Continued evaluation of a 31-gene expression profile to predict metastasis in an expanded cohort of 782 cutaneous melanoma patients. Pigment Cell Melanoma Res 2017;30:e73 | Stage I and II |
| Crocetti E, Mangone L, Lo Scocco G, Carli P. Prognostic variables and prognostic groups for malignant melanoma. The information from Cox and classification and regression trees analysis: an Italian population-based study. Melanoma Res 2006;16:429–33 | Stages mixed |
| Cymerman RM, Wang K, Murzaku EC, Penn LA, Osman I, Shao Y, et al. De novo versus nevus-associated melanomas: Differences in associations with prognostic indicators and survival. J Clin Oncol 2015;33:9025 | No validation |
| Emmett MS, Symonds KE, Rigby H, Cook MG, Price R, Metcalfe C, et al. Prediction of melanoma metastasis by the Shields index based on lymphatic vessel density. BMC Cancer 2010;10:208 | Stage not reported |
| Fang S, Wang Y, Chun YS, Liu H, Ross MI, Gershenwald JE, et al. Association of common genetic polymorphisms with melanoma patient IL-12p40 blood levels, risk, and outcomes. J Invest Dermatol 2015;135:2266–72 | Stage I and II |
| Fang S, Wang Y, Chun YS, Liu H, Ross MI, Gershenwald JE, et al. The relationship between blood IL-12p40 level and melanoma progression. Int J Cancer 2015;136:1874–80 | Single prognostic factor |
| Fang S, Wang Y, Sui D, Liu H, Ross MI, Gershenwald JE, et al. C-reactive protein as a marker of melanoma progression. J Clin Oncol 2015;33:1389–96 | No validation |
| Findeisen P, Zapatka M, Peccerella T, Matzk H, Neumaier M, Schadendorf D, Ugurel S. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol 2009;27:2199–208 | Stages mixed |
| Garnier JP, Letellier S, Cassinat B, Lebbé C, Kerob D, Baccard M, et al. Clinical value of combined determination of plasma L-DOPA/tyrosine ratio, S100B, MIA and LDH in melanoma. Eur J Cancer 2007;43:816–21 | No validation |
| Gerami P, Jewell SS, Pouryazdanparast P, Wayne JD, Haghighat Z, Busam KJ, et al. Copy number gains in 11q13 and 8q24 [corrected] are highly linked to prognosis in cutaneous malignant melanoma. J Mol Diagn 2011;13:352–8 | No validation |
| Gillgren P, Brattström G, Frisell J, Persson JO, Ringborg U, Hansson J. Effect of primary site on prognosis in patients with cutaneous malignant melanoma. A study using a new model to analyse anatomical locations. Melanoma Res 2005;15:125–32 | No validation |
| Gimotty P, Guerry D, VanBelle P, Montone K, Guerra M, Hwang W, et al. Ki67 as a prognostic biomarker for patients with vertical growth phase (VGP) melanomas. J Clin Oncol 2009;27:9043 | Stage I and II |
| Gomez GV, de Oliveira C, Rinck-Junior JA, de Moraes AM, Lourenço GJ, Lima CS. XPC (A2920C), XPF (T30028C), TP53 (Arg72Pro), and GSTP1 (Ile105Val) polymorphisms in prognosis of cutaneous melanoma. Tumour Biol 2016;37:3163–71 | No validation |
| Governa M, Dorizzi RM, Gatti S, Tambuscio A, Minic J, Barisoni D. Is increased serum S-100 protein concentration a marker of metastasis in malignant melanoma? A four-year experience report. Eur J Plast Surg 2005;28:17–20 | No validation |
| Henry L, Lavabre-Bertrand T, Douche T, Uttenweiler-Joseph S, Fabbro-Peray P, Monsarrat B, et al. Diagnostic value and prognostic significance of plasmatic proteasome level in patients with melanoma. Exp Dermatol 2010;19:1054–9 | No validation |
| Henry L, Fabre C, Guiraud I, Bastide S, Fabbro-Peray P, Martinez J, et al. Clinical use of p-proteasome in discriminating metastatic melanoma patients: comparative study with LDH, MIA and S100B protein. Int J Cancer 2013;133:142–8 | One factor |
| Hoon DS, Bostick P, Kuo C, Okamoto T, Wang HJ, Elashoff R, Morton DL. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res 2000;60:2253–7 | No validation |
| Hsueh EC, DeBloom JR, Lee J, Sussman JJ, Covington KR, Middlebrook B, et al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J Hematol Oncol 2017;10:152 | All stages in analysis by class |
| Karagiannis P, Villanova F, Josephs DH, Correa I, Van Hemelrijck M, Hobbs C, et al. IgG4: a new tool to predict the risk of disease progression in melanoma. Cancer Immunol Res 2016;4:A009 | No validation |
| Kashani-Sabet M, Nosrati M, Miller JR, Sagebiel RW, Leong SPL, Lesniak A, et al. Prospective validation of molecular prognostic markers in cutaneous melanoma: a correlative analysis of E1690. Clin Cancer Res 2017;23:6888–92 | Stages not reported |
| Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, et al. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res 2011;17:2417–25 | Stage I and II |
| Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, et al. Age and sex are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008;112:1795–804 | Not validated |
| Leiter U, Buettner PG, Eigentler TK, Garbe C. Prognostic factors of thin cutaneous melanoma: an analysis of the central malignant melanoma registry of the german dermatological society. J Clin Oncol 2004;22:3660–7 | No validation |
| Li N, Diao Z, Huang X, Niu Y, Liu T, Liu ZP, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep 2017;7:2970 | Single prognostic factor |
| Liu J, Li R, Zhou X, Zhang J, Luo R. [Multivariate regression analysis of the biomarkers and clinical characteristics in the prognosis of malignant melanoma.] Nan Fang Yi Ke Da Xue Xue Bao 2012;32:847–53 | Stages mixed |
| Lyth J, Mikiver R, Nielsen K, Isaksson K, Ingvar C. Prognostic instrument for survival outcome in melanoma patients: based on data from the population-based Swedish Melanoma Register. Eur J Cancer 2016;59:171–8 | No independent validation |
| Mitchell B, Leone D, Feller K, Menon S, Bondzie P, Yang S, et al. Protein expression of the chemokine receptor CXCR4 and its ligand CXCL12 in primary cutaneous melanoma – Biomarkers of potential utility? Hum Pathol 2014;45:2094–100 | No validation |
| Mocellin S, Pasquali S, Rossi CR, Nitti D. Validation of the prognostic value of lymph node ratio in patients with cutaneous melanoma: a population-based study of 8,177 cases. Surgery 2011;150:83–90 | All stages |
| Murali R, Shaw HM, Lai K, McCarthy SW, Quinn MJ, Stretch JR, et al. Prognostic factors in cutaneous desmoplastic melanoma. Cancer 2010;116:4130–8 | No validation |
| Murtas D, Piras F, Minerba L, Ugalde J, Floris C, Maxia C, et al. Nuclear 8-hydroxy-2’-deoxyguanosine as survival biomarker in patients with cutaneous melanoma. Oncol Rep 2010;23:329–35 | One factor |
| Naffouje R, Salti GI. The role of microphthalmia transcription factor (Mitf) in prediction of distant metastases in cutaneous melanoma. J Clin Oncol 2016;34:9564 | No validation |
| Naffouje SA, Naffouje R, Chen J, Salti GI. Validation and enhancement of the clinicopathological melanoma nomogram via incorporation of a molecular marker in the primary tumor. Anticancer Res 2016;36:6603–10 | Stages mixed |
| Nagore E, Heidenreich B, Garcia-Casado Z, Requena C, Kumar R. TERT promoter mutations in melanoma survival. Pigment Cell Melanoma Res 2017;30:124 | No validation |
| Nagore Enguídanos E, Oliver Martínez V, Botella Estrada R, Insa Mollá A, Fortea Baixauli JM. [Prognostic factors of localized malignant melanoma: study of 639 patients.] Med Clin 2005;124:361–7 | No validation |
| Nsengimana J, Laye J, Filia A, Walker C, Jewell R, Van den Oord JJ, et al. Independent replication of a melanoma subtype gene signature and evaluation of its prognostic value and biological correlates in a population cohort. Oncotarget 2015;6:11683–93 | Stage not reported |
| Oliveira C, Rinck JA, Lourenço GJ, Moraes AM, Lima CSP. Polymorphisms in the apoptosis pathway and prognosis in cutaneous melanoma. J Clin Oncol 2014;32(Suppl. 1):9084 | No validation |
| Ortega-Bernal D, Rangel-Escareno C, Arechaga-Ocampo E, Gonzalez-De La Rosa CH. Biomarkers for staging melanoma, a search at transcriptome level. Cancer Res 2018;78:2252 | No validation |
| Ostmeier H, Fuchs B, Otto F, Mawick R, Lippold A, Krieg V, Suter L. Prognostic immunohistochemical markers of primary human melanomas. Br J Dermatol 2001;145:203–9 | No validation |
| Ozao-Choy J, Nelson DW, Hiles J, Stern S, Yoon JL, Sim MS, Faries MB. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol 2017;116:337–43 | No validation |
| Pacifico MD, Grover R, Richman PI, Daley FM, Buffa F, Wilson GD. CD44v3 levels in primary cutaneous melanoma are predictive of prognosis: assessment by the use of tissue microarray. Int J Cancer 2006;118:1460–4 | No validation |
| Palmieri G, Ascierto PA, Perrone F, Satriano SM, Ottaiano A, Daponte A, et al. Prognostic value of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction. J Clin Oncol 2003;21:767–73 | No validation |
| Passos Lima CS, Gomez GVB, Oliveira C, Lourenço GJ, Rinck JA, Moraes AM. XPC, XPF, TP53 and GSTP1 polymorphisms in prognosis of cutaneous melanoma patients. J Clin Oncol 2015;33:9038 | No validation |
| Pizzichetta MA, Massi D, Mandalà M, Queirolo P, Stanganelli I, De Giorgi V, et al. Clinicopathological predictors of recurrence in nodular and superficial spreading cutaneous melanoma: a multivariate analysis of 214 cases. J Transl Med 2017;15:227 | No validation |
| Ponti G, Pollio A, Cesinaro AM, Pellacani G, Magnoni C, Seidenari S. Value and prognostic significance of mitotic rate in a retrospective series of pT1 cutaneous malignant melanoma patients. Cancer Epidemiol 2012;36:303–5 | No validation |
| Poukka M, Bykachev A, Siiskonen H, Tyynelä-Korhonen K, Auvinen P, Pasonen-Seppänen S, Sironen R. Decreased expression of hyaluronan synthase 1 and 2 associates with poor prognosis in cutaneous melanoma. BMC Cancer 2016;16:313 | No validation |
| Ramsden AJ, Grover R, Chana J, Tulley P, Sanders R, Wilson GD. A prospective analysis of c-myc oncoprotein levels as a prognostic marker in malignant melanoma. J Plast Reconstr Aesthet Surg 2007;60:626–30 | One factor |
| Rangel J, Nosrati M, Torabian S, Shaikh L, Leong SP, Haqq C, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer 2008;112:144–50 | Single prognostic factor |
| Rangel J, Torabian S, Shaikh L, Nosrati M, Baehner FL, Haqq C, et al. Prognostic significance of nuclear receptor coactivator-3 overexpression in primary cutaneous melanoma. J Clin Oncol 2006;24:4565–9 | Single prognostic factor |
| Rashed H, Bamford M, Flatman K, Teo KWW, Saldanha G. Breslow density as an independent prognostic indicator in cutaneous malignant melanoma. J Pathol 2016;240:S47 | No validation |
| Reschke M, Mihic-Probst D, van der Horst EH, Knyazev P, Wild PJ, Hutterer M, et al. HER3 is a determinant for poor prognosis in melanoma. Clin Cancer Res 2008;14:5188–97 | One factor |
| Rex J, Paradelo C, Mangas C, Hilari JM, Fernandez-Figueras MT, Fraile M, et al. Single-institution experience in the management of patients with clinical stage I and II cutaneous melanoma: results of sentinel lymph node biopsy in 240 cases. Dermatol Surg 2005;31:1385–93 | No validation |
| Gould Rothberg BE, Berger AJ, Molinaro AM, Subtil A, Krauthammer MO, Camp RL, et al. Melanoma prognostic model using tissue microarrays and genetic algorithms. J Clin Oncol 2009;27:5772–80 | Stage II |
| Rotte A, Bhandaru M, Cheng Y, Sjoestroem C, Martinka M, Li G. Decreased expression of nuclear p300 is associated with disease progression and worse prognosis of melanoma patients. PLOS ONE 2013;8:e75405 | Stage I and II |
| Rotte A, Martinka M, Li G. MMP2 expression is a prognostic marker for primary melanoma patients. Cell Oncol 2012;35:207–16 | Single prognostic factor |
| Rowe C, Tang F, Hughes MC, Rodero M, Malt M, Lambie D, et al. Prognostic value of nomograms incorporating biomarkers vs. sentinel node status in patients with stage IB and II melanoma. Australas J Dermatol 2016;57:3 | Stages mixed |
| Roxanis I, Chow J. Cellular cohesion as a prognostic factor in malignant melanoma: a retrospective study with up to 12 years follow-up. Mod Pathol 2010;23:502–10 | One factor |
| Sabel MS, Liu Y, Griffith KA, He J, Xie X, Lubman DM. Clinical utility of serum autoantibodies detected by protein microarray in melanoma. Int J Proteomics 2011;2011:413742 | No validation |
| Sanlorenzo M, Ribero S, Osella-Abate S, Zugna D, Marenco F, Macripo G, et al. Prognostic differences across sexes in melanoma patients: what has changed from the past? Melanoma Res 2014;24:568–76 | No validation |
| Schäfer A, Emmert S, Kruppa J, Schubert S, Tzvetkov M, Mössner R, et al. No association of vitamin D metabolism-related polymorphisms and melanoma risk as well as melanoma prognosis: a case-control study. Arch Dermatol Res 2012;304:353–61 | No results by stage |
| Schmidt H, Johansen JS, Sjoegren P, Christensen IJ, Sorensen BS, Fode K, et al. Serum YKL-40 predicts relapse-free and overall survival in patients with American Joint Committee on Cancer stage I and II melanoma. J Clin Oncol 2006;24:798–804 | No validation |
| Shi Q, Liu H, Li C, Wang Y, Liu Z, Amos C, et al. Genetic variants in the Wnt pathway genes NFATC1 and PLCB1 predict melanoma survival. J Invest Dermatol 2016;136:S37 | No validation |
| Shourkaei SMJ, Wani AA, Martinka M, Li G. Prognostic significance of nuclear Sox4 expression in cutaneous melanoma and its role in cell migration. Cancer Res 2010;70:2243 | Single prognostic factor |
| Silva S, Cox A, Teare D, Bradford J, Brock I, Connley D, et al. Copy-number profiles from circulating cell-free DNA as a potential biomarker in melanoma. Eur J Surg Oncol 2018;44:S26–S7 | No validation |
| Sjoestroem C, Khosravi S, Cheng Y, Safaee Ardekani G, Martinka M, Li G. DLC1 expression is reduced in human cutaneous melanoma and correlates with patient survival. Mod Pathol 2014;27:1203–11 | No validation |
| Stiegel E, Xiong D, Ya J, Funchain P, Isakov R, Gastman B, Vij A. Prognostic value of sentinel lymph node biopsy according to Breslow thickness for cutaneous melanoma. J Am Acad Dermatol 2018;78:942–8 | No validation |
| Stokes WA, Lentsch EJ. Age is an independent poor prognostic factor in cutaneous head and neck melanoma. Laryngoscope 2014;124:462–5 | One factor |
| Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res 2000;6:1845–53 | No validation |
| Tchernev G, Orfanos CE. Downregulation of cell cycle modulators p21, p27, p53, Rb and proapoptotic Bcl-2-related proteins Bax and Bak in cutaneous melanoma is associated with worse patient prognosis: preliminary findings. J Cutan Pathol 2007;34:247–56 | Stage II |
| Thies A, Mangold U, Moll I, Schumacher U. PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol 2001;195:537–42 | No validation |
| Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 2013;31:4252–9 | No validation |
| Thomas NE, Kricker A, Waxweiler WT, Dillon PM, Busman KJ, From L, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol 2014;150:1306–314 | No validation |
| Thompson JF, Soong SJ, Balch CM, Gershenwald JE, Ding S, Coit DG, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 2011;29:2199–205 | No validation |
| Väisänen AH, Kallioinen M, Turpeenniemi-Hujanen T. Comparison of the prognostic value of matrix metalloproteinases 2 and 9 in cutaneous melanoma. Hum Pathol 2008;39:377–85 | No validation |
| van Akkooi ACJ, Rutkowski P, Van Der Ploeg IM, Voit C, Robert C, Hoekstra HJ, et al. Excellent long-term survival of patients with minimal sentinel node tumor burden (< 0.1 mm) according to Rotterdam Criteria: a study of the EORTC melanoma group. Eur J Cancer Supp 2009;7:576–7 | No validation |
| van der Ploeg AP, van Akkooi AC, Rutkowski P, Nowecki ZI, Michej W, Mitra A, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol 2011;29:2206–14 | No validation |
| Vergilis IJ, Szarek M, Ferrone S, Reynolds SR. Presence and prognostic significance of melanoma-associated antigens CYT-MAA and HMW-MAA in serum of patients with melanoma. J Invest Dermatol 2005;125:526–31 | Excludes stage I |
| Vollmer RT, Seigler HF. A model for pretest probability of lymph node metastasis from cutaneous Melanoma. Am J Clin Pathol 2000;114:875–9 | No results by stage |
| Vuylsteke RJ, van Leeuwen PA, Statius Muller MG, Gietema HA, Kragt DR, Meijer S. Clinical outcome of stage I/II melanoma patients after selective sentinel lymph node dissection: long-term follow-up results. J Clin Oncol 2003;21:1057–65 | No validation |
| Wan X, Liu R, Li Z. The prognostic value of HRAS mRNA expression in cutaneous melanoma. Biomed Res Int 2017;2017:5356737 | No validation |
| Wang K, Zhang ZW. Expression of miR-203 is decreased and associated with the prognosis of melanoma patients. Int J Clin Exp Pathol 2015;8:13249–54 | Single prognostic factor |
| Wang Q, Wang X, Liang Q, Wang S, Xiwen L, Pan F, et al. Distinct prognostic value of mRNA expression of guanylate-binding protein genes in skin cutaneous melanoma. Oncol Lett 2018;15:7914–22 | Stage not reported |
| Wang Q, Wang X, Liang Q, Wang S, Liao X, Li D, Pan F. Prognostic value of dynactin mRNA expression in cutaneous melanoma. Med Sci Monit 2018;24:3752–63 | Stage I and II |
| Weinlich G, Bitterlich W, Mayr V, Fritsch PO, Zelger B. Metallothionein-overexpression as a prognostic factor for progression and survival in melanoma. A prospective study on 520 patients. Br J Dermatol 2003;149:535–41 | Single prognostic factor |
| Weinlich G, Eisendle K, Hassler E, Baltaci M, Fritsch PO, Zelger B. Metallothionein – overexpression as a highly significant prognostic factor in melanoma: a prospective study on 1270 patients. Br J Cancer 2006;94:835–41 | Single prognostic factor |
| White RL, Ayers GD, Stell VH, Ding S, Gershenwald JE, Salo JC, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol 2011;18:3593–600 | No validation |
| Xing Y, Badgwell BD, Ross MI, Gershenwald JE, Lee JE, Mansfield PF, et al. Lymph node ratio predicts disease-specific survival in melanoma patients. Cancer 2009;115:2505–13 | No validation |
| Yuan H, Liu H, Liu Z, Zhu D, Amos CI, Fang S, et al. Genetic variants in Hippo pathway genes YAP1, TEAD1 and TEAD4 are associated with melanoma-specific survival. Int J Cancer 2015;137:638–45 | Stage I/II combined |
| Yun SJ, Gimotty PA, Hwang WT, Dawson P, Van Belle P, Elder DE, et al. High lymphatic vessel density and lymphatic invasion underlie the adverse prognostic effect of radial growth phase regression in melanoma. Am J Surg Pathol 2011;35:235–42 | No validation |
| Zhang Z, Chen G, Cheng Y, Martinka M, Li G. Prognostic significance of RUNX3 expression in human melanoma. Cancer 2011;117:2719–27 | No results by stage |
| Zietek M, Donizy P, Leskiewicz M, Kaczorowski M, Kozyra C, Wojnar A, et al. ALCAM overexpression in primary tumour predicts shorter overall survival in cutaneous malignant melanoma patients. Eur J Surg Oncol 2014;40:S149 | Stage not reported |
Appendix 5 Example search strategy for diagnostic accuracy review
| # | Searches | Results (n) |
|---|---|---|
| Melanoma | ||
| 1 | exp melanoma/or melanoma*.ti,ab,kw,kf. | 124,381 |
| 2 | amelanotic.ti,ab,kw,kf. | 1956 |
| 3 | ((lentigo* or lentigin*) adj3 (tumo?r* or cancer* or maligna* or n?evus)).ti,ab,kw,kf. | 1323 |
| 4 | or/1-3 | 124,778 |
| 5 | (ocular or uveal or iris or cornea or eye or choroidal or ciliary or intraocular).ti. | 160,371 |
| 6 | 4 not 5 | 117,550 |
| Diagnostic method | ||
| 7 | exp ultrasonography, doppler/or exp ultrasonography, interventional/ | 89,434 |
| 8 | ultrasound.ti,ab,kw,kf. | 224,479 |
| 9 | ultrason*.ti,ab,kw,kf. | 154,906 |
| 10 | exp image guided biopsy/ | 4564 |
| 11 | exp Biopsy, Fine-Needle/ | 14,316 |
| 12 | (“fine needle biopsy” or FNB).ti,ab,kw,kf. | 2199 |
| 13 | (“fine needle aspirat*” or FNA).ti,ab,kw,kf. | 29,692 |
| 14 | (“fine needle aspiration cytology” or FNAC).ti,ab,kw,kf. | 8227 |
| 15 | exp Biopsy, Needle/ | 62,995 |
| 16 | “core biopsy”.ti,ab,kw,kf. | 3971 |
| 17 | or/7-16 | 455,777 |
| Diagnostic test filter | ||
| 18 | exp “sensitivity and specificity”/ | 544,996 |
| 19 | (sensitivity or specificity or accuracy).tw. | 1,228,698 |
| 20 | ((predictive adj3 value$) or (roc adj curve$)).tw. | 123,584 |
| 21 | ((false adj positiv$) or false negativ$).tw. | 71,980 |
| 22 | ((observer adj variation$) or (likelihood adj3 ratio$)).tw. | 15,344 |
| 23 | likelihood function/ | 20,943 |
| 24 | exp mass screening/ | 119,419 |
| 25 | diagnosis, differential/or exp Diagnostic errors/ | 535,465 |
| 26 | di.xs. or du.fs. | 3,322,576 |
| 27 | or/18-26 | 4,663,491 |
| Follow up/surveillance terms | ||
| 28 | follow up.ti,ab,kw,kf. | 872,434 |
| 29 | surveillance.ti,ab,kw,kf. | 159,164 |
| 30 | monitor*.ti,ab,kw,kf. | 729,895 |
| 31 | exp Neoplasm Recurrence, Local/di, dg, pc [Diagnosis, Diagnostic Imaging, Prevention & Control] | 21,379 |
| 32 | exp Recurrence/di, dg, pc [Diagnosis, Diagnostic Imaging, Prevention & Control] | 8 |
| 33 | recur*.ti,ab,kw,kf. | 538,847 |
| 34 | exp Neoplasm Metastasis/di, dg, pc [Diagnosis, Diagnostic Imaging, Prevention & Control] | 8124 |
| 35 | metast*.ti,ab,kw,kf. | 462,364 |
| 36 | exp Aftercare/ | 177,538 |
| 37 | or/28-36 | 2,591,386 |
| 38 | 6 and 17 and 27 and 37 | 928 |
| 39 | exp animals/not humans.sh. | 4,548,295 |
| 40 | 38 not 39 | 904 |
Appendix 6 Diagnostic accuracy review: excluded studies
| Study | Reason(s) for exclusion |
|---|---|
| Ben Lakhdar A, Ilie M, Tomasic G, Chami L, Robert C, Vielh P. Benefits of ultrasound-guided fine needle aspirationcytology before lymph node biopsy in melanoma patients. Virchows Arch 2011;459:S10 | Stage not reported (conference abstract) |
| Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma: results of a prospective study of 1288 patients. Cancer 2000;88:2534–9 | No analysis by disease stage or Breslow depth |
| Blum A, Schmid-Wendtner MH, Mauss-Kiefer V, Eberle JY, Kuchelmeister C, Dill-Müller D. Ultrasound mapping of lymph node and subcutaneous metastases in patients with cutaneous melanoma: results of a prospective multicenter study. Dermatology 2006;212:47–52 | Not diagnostic accuracy: ultrasonography mapping |
| Brountzos EN, Panagiotou IE, Bafaloukos DI, Kelekis DA. Ultrasonographic detection of regional lymph node metastases in patients with intermediate or thick malignant melanoma. Oncol Rep 2003;10:505–10 | No analysis by disease stage or Breslow depth |
| Calvo López MJ, Vallejos Roca E, Muñoz Alcántara I, Navarro Díaz F, García Palacios MV. [Ultrasonographic and power Doppler appearance of locoregional metastases from cutaneous melanoma.] Radiologia 2008;50:483–8 | Stage not reported (Spanish) |
| Caudron A, Chassine AF, Arnault JP, Dadban A, Chaby G, Lok C. Elastography as a new screening tool for metastatic lymph nodes in patients monitored for melanoma. Melanoma Res 2011;1:e32–e3 | No analysis by disease stage or Breslow depth |
| Chai CY, Zager JS, Szabunio MM, Marzban SS, Chau A, Rossi RM, et al. Preoperative ultrasound is not useful for identifying nodal metastasis in melanoma patients undergoing sentinel node biopsy: preoperative ultrasound in clinically node-negative melanoma. Ann Surg Oncol 2012;19:1100–6 | Pre-operative staging |
| Dalle S, Paulin C, Lapras V, Balme B, Ronger-Savle S, Thomas L. Fine-needle aspiration biopsy with ultrasound guidance in patients with malignant melanoma and palpable lymph nodes. Br J Dermatol 2006;155:552–6 | No analysis by disease stage or Breslow depth |
| Fakhry N, Tessonnier L, Cohen F, Gras R, Grob JJ, Giovanni A, et al. Management of cervical lymph node recurrence of melanoma of the head and neck. Rev Laryngol Otol Rhinol 2009;130:211–14 | No analysis by disease stage or Breslow depth |
| Galanzha EI, Menyaev YA, Yadem AC, Sarimollaoglu M, Juratli MA, Nedosekin DA, et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med 2019;11:eaat5857 | Advanced stage |
| Georgieva M, Prantl L, Utpatel K, Wiesinger I, Stroszczynski C, Jung F, Jung EM. Diagnostic performance of ultrasound strain elastography for differentiation of malignant breast lesions. Clin Hemorheol Microcirc 2019;71:237–47 | Insufficient patients |
| Hayes AJ, Moskovic E, O’Meara K, Smith HG, Pope RJE, Larkin J, Thomas JM. Prospective cohort study of ultrasound surveillance of regional lymph nodes in patients with intermediate-risk cutaneous melanoma. Br J Surg 2019;106:729–34 | Stages I to II |
| Herceg GH, Bracic I, Kusacic-Kuna S, Mutvar A, Antulov J, Herceg D. Introduction of US-guided FNAC in preoperative staging prior to sentinel lymph node biopsy: benefit for patients with cutaneous melanoma. Nuklearmedizin 2014;53:A132 | Pre-operative staging (conference abstract) |
| Heřman J, Sedláčková Z, Fürst T, Vachutka J, Salzman R, Vomáčka J, Heřman M. The role of ultrasound and shear-wave elastography in evaluation of cervical lymph nodes. Biomed Res Int 2019;2019:4318251 | Insufficient patients |
| Hinz T, Hoeller T, Wenzel J, Bieber T, Schmid-Wendtner MH. Real-time tissue elastography as promising diagnostic tool for diagnosis of lymph node metastases in patients with malignant melanoma: a prospective single-center experience. Dermatology 2013;226:81–90 | No analysis by disease stage or Breslow depth |
| Hinz T, Wilsmann-Theis D, Buchner A, Wenzel J, Wendtner CM, Bieber T, et al. High-resolution ultrasound combined with power Doppler sonography can reduce the number of sentinel lymph node biopsies in cutaneous melanoma. Dermatology 2011;222:180–8 | Pre-operative staging |
| Hocevar M, Bracko M, Pogacnik A, Vidergar-Kralj B, Besic N, Zgajnar J, Music MM. The role of preoperative ultrasonography in reducing the number of sentinel lymph node procedures in melanoma. Melanoma Res 2004;14:533–6 | No analysis by disease stage or Breslow depth |
| Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients – monocenter evaluation of methods, costs and patient survival. Br J Cancer 2002;87:151–7 | Stages I–II |
| Horvatic Herceg G, Bracic I, Kusacic-Kuna S, Herceg D, Mutvar A, Dodig D. Ultrasound and US-guided FNAC can reduce the number of sentinel lymph node biopsies in cutaneous melanoma. Eur J Nucl Med Mol Imaging 2012;39:S591 | Stages I–II (conference abstract) |
| Kaushik V, Baloch Z, Jones L, Gupta P. Diagnostic utility of ultrasound-guided fine needle aspiration (USG-FNA) in head and neck lesions detected by positron emission tomography (PET) scan. J Am Soc Cytopathol 2012;1:S72 | Stage not reported (conference abstract) |
| Kiyohara Y, Tsuchiya T, Nakajima M, Adachi M, Shimizu M, Hatano M, et al. Evaluation of color and power Doppler images in malignant melanoma. Ultrasound Med Biol 2000;26:A184 | Stage not reported (conference abstract) |
| Klebl FH, Gelbmann CM, Lammert I, Bogenrieder T, Stolz W, Schölmerich J, Schlottmann K. [Detection of lymph node metastases of malignant melanoma by palpation and ultrasound.] Med Klin 2003;98:783–7 | No analysis by disease stage or Breslow depth (German) |
| Kunte C, Schuh T, Eberle JY, Baumert J, Konz B, Volkenandt M, et al. The use of high-resolution ultrasonography for preoperative detection of metastases in sentinel lymph nodes of patients with cutaneous melanoma. Dermatol Surg 2009;35:1757–65 | Pre-operative staging (German) |
| Lassau N, Koscielny S, Avril MF, Margulis A, Duvillard P, De Baere T, et al. Prognostic value of angiogenesis evaluated with high-frequency and color Doppler sonography for preoperative assessment of melanomas. AJR Am J Roentgenol 2002;178:1547–51 | Pre-operative staging |
| Lassau N, Chami L, Peronneau P. [Imaging of melanoma: accuracy of ultrasonography before and after contrast injection for diagnostic and early evaluation of treatments.] Bull Cancer 2007;94:93–8 | Stage not reported (French) |
| Layfield LJ. Diagnosis and work-up of malignant melanoma in the age of fine needle aspiration and molecular testing. Eur Oncol Haematol 2014;10:58–61 | Review |
| Lean CL, Bourne R, Thompson JF, Scolyer RA, Stretch J, Li LX, et al. Rapid detection of metastatic melanoma in lymph nodes using proton magnetic resonance spectroscopy of fine needle aspiration biopsy specimens. Melanoma Res 2003;13:259–61 | Stage not reported |
| Levang J, Manzoni P, Puyraveau M, Sarlieve P, Puzenat E, Humbert P, et al. The value of contrast-enhanced ultrasonography in the detection of liver metastases in the follow-up of patients with stages III and IV melanoma. Arch Dermatol Res 2007;299:282 | Stage III–IV (conference abstract) |
| Lussier C, Klijanienko J, Vielh P. Fine-needle aspiration of metastatic nonlymphomatous tumors to the major salivary glands: a clinicopathologic study of 40 cases cytologically diagnosed and histologically correlated. Cancer 2000;90:350–6 | Stage not reported |
| Machet L, Nemeth-Normand F, Giraudeau B, Perrinaud A, Tiguemounine J, Ayoub J, et al. Is ultrasound lymph node examination superior to clinical examination in melanoma follow-up? A monocentre cohort study of 373 patients. Br J Dermatol 2005;152:66–70 | No analysis by disease stage or Breslow depth |
| Machet L, Vaillant L, Lorette G. Follow-up of excision of cutaneous melanoma: sentinel node biopsy, lymph node ultrasound or clinical surveillance alone? Ann Dermatol Venereol 2005;132:941–4 | Editorial |
| Marone U, Catalano O, Caracò C, Anniciello AM, Sandomenico F, Di Monta G, et al. Can high-resolution ultrasound avoid the sentinel lymph-node biopsy procedure in the staging process of patients with stage I-II cutaneous melanoma? Ultraschall Med 2012;33:E179–85 | Pre-operative staging |
| Metzger S, Dohmen BM, Breuninger H, Rassner G, Flerlbeck G. Sensitivity and specificity of 18FDG-PET in the staging diagnosis of patients with high-risk melanomas in comparison with sonography and CT. Z Hautkr 2000;75:465 | Stage not reported |
| Moehrle M, Blum A, Rassner G, Juenger M. Lymph node metastases of cutaneous melanoma: diagnosis by B-scan and color Doppler sonography. J Am Acad Dermatol 1999;41:703–9 | Stage not reported |
| Molajo A, Powell B. Ultrasound guided FNAC of sentinel nodes in melanoma. Which patients may be suitable? J Dtsch Dermatol Ges 2013;11:36 | Not diagnostic accuracy of ultrasonography (conference abstract) |
| Murali R, Doubrovsky A, Watson GF, McKenzie PR, Lee CS, McLeod DJ, et al. Diagnosis of metastatic melanoma by fine-needle biopsy: analysis of 2,204 cases. Am J Clin Pathol 2007;127:385–97 | Stage not reported |
| Nasuti JF, Yu G, Boudousquie A, Gupta P. Diagnostic value of lymph node fine needle aspiration cytology: an institutional experience of 387 cases observed over a 5-year period. Cytopathology 2000;11:18–31 | Stage not reported |
| Nazarian LN, Alexander AA, Kurtz AB, Capuzzi DM, Rawool NM, Gilbert KR, Mastrangelo MJ. Superficial melanoma metastases: appearances on gray-scale and color Doppler sonography. AJR Am J Roentgenol 1998;170:459–63 | Stage not reported |
| Oehr P, Stegemann G, Steen K, Ruhlmann J. The value of FDG-PET whole body imaging, conventional imaging, and serum S-100 determinations in metastatic malignant melanoma. Clin Lab 1999;45:523–8 | No analysis by disease stage or Breslow depth: ultrasonography results not reported |
| Ogata D, Uematsu T, Yoshikawa S, Kiyohara Y. Accuracy of real-time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): a pilot study. Int J Clin Oncol 2014;19:716–21 | Stage not reported |
| Olmedo D, Brotons-Seguí M, Del Toro C, González M, Requena C, Traves V, et al. Use of lymph node ultrasound prior to sentinel lymph node biopsy in 384 patients with melanoma: a cost-effectiveness analysis. Actas Dermosifiliogr 2017;108:931–8 | Stage not reported |
| Olson MT, Novak A, Kirby J, Shahid H, Boonyaarunnate T, Ali SZ. Cytotechnologist-attended on-site evaluation of adequacy for metastatic disease involving bone and soft tissue. Acta Cytol 2013;57:550–6 | Stage not reported |
| Olszanski AJ. mutation testing and adjuvant systemic therapy in cutaneous melanoma. J Natl Compr Canc Netw 2019;17:615–17 | Treatment |
| Oude Ophuis CMC, Koppert LB, de Monyé C, van Deurzen CHM, Koljenović S, van Akkooi ACJ, et al. Gamma probe and ultrasound guided fine needle aspiration cytology of the sentinel node (GULF) trial – overview of the literature, pilot and study protocol. BMC Cancer 2017;17:258 | No analysis by disease stage or Breslow depth |
| Oude Ophuis CMC, Verhoef C, Grünhagen DJ, Siegel P, Schoengen A, Röwert-Huber J, et al. Long-term results of ultrasound guided fine needle aspiration cytology in conjunction with sentinel node biopsy support step-wise approach in melanoma. Eur J Surg Oncol 2017;43:1509–16 | Not diagnostic study: protocol |
| Panagiotou IE, Brountzos EN, Bafaloukos D, Tsavaris N, Mylonakis N, Karabelis A, et al. Evaluation of imaging studies at the initial staging and during follow-up of patients with local-regional malignant melanoma. JBUON 2001;6:411–4 | Initial stage not reported: advanced stage |
| Pánczél G, Liszkay G, Borbola K, Balatoni T, Hunyadi J. [The importance of fine needle aspiration cytology in the management of recurrent and metastatic melanoma.] Orv Hetil 2012;153:1419–23 | Hungarian translation required |
| Papadopoulos O, Konofaos P, Georgoulakis J, Chrisostomidis C, Tsantoulas Z, Kostopoulos E, et al. The role of ThinPrep cytology in the investigation of SLN status in patients with cutaneous melanoma. Surg Oncol 2007;16:121–9 | No analysis by disease stage or Breslow depth |
| Pilko G, Zgajnar J, Music M, Hocevar M. Lower tumour burden and better overall survival in melanoma patients with regional lymph node metastases and negative preoperative ultrasound. Radiol Oncol 2012;46:60–8 | No relevant outcomes |
| Porcellato I, Brachelente C, De Paolis L, Menchetti L, Silvestri S, Sforna M, et al. FoxP3 and IDO in canine melanocytic tumors. Vet Pathol 2019;56:189–99 | Animals |
| Proebstle T, Schwurzer-Voit M, Sterry W, Knop J, Voit C. Detection of regional melanoma metastases by ultrasound B-scan, cytology or tyrosinase RT-PCR of fine needle aspirates. J Invest Dermatol 1999;113:514 | Stage not reported (conference abstract) |
| Radzhabova ZA, Barchuk AS, Kostromina EV, Anisimov VV. [The detection of early regional metastases in patients with skin melanoma by dopplerography.] Vestn Khir Im I I Grek 2009;168:50–3 | Stage not reported (Russian) |
| Ribero S, Podlipnik S, Osella-Abate S, Sportoletti-Baduel E, Manubens E, Barreiro A, et al. Ultrasound-based follow-up does not increase survival in early-stage melanoma patients: a comparative cohort study. Eur J Cancer 2017;85:59–66 | No relevant outcomes |
| Rodrigues LKE, Leong SPL, Ljung BM, Sagebiel RW, Burnside N, William Hu TL, et al. Fine needle aspiration in the diagnosis of metastatic melanoma. J Am Acad Dermatol 2000;42:735–40 | Stage not reported |
| Rossi CR, Scagnet B, Vecchiato A, Mocellin S, Pilati P, Foletto M, et al. Sentinel node biopsy and ultrasound scanning in cutaneous melanoma: clinical and technical considerations. Eur J Cancer 2000;36:895–900 | Pre-operative staging |
| Rubaltelli L, Beltrame V, Scagliori E, Bezzon E, Frigo AC, Rastrelli M, Stramare R. Potential use of contrast-enhanced ultrasound (CEUS) in the detection of metastatic superficial lymph nodes in melanoma patients. Ultraschall Med 2014;35:67–71 | No analysis by disease stage or Breslow depth |
| Rubaltelli L, Beltrame V, Tregnaghi A, Scagliori E, Frigo AC, Stramare R. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR Am J Roentgenol 2011;196:W8–12 | Stages I–II |
| Rue Nielsen K, Klyver H, Hougaard Chakera A, Nedergaard L, Hesse B, Bachmann Nielsen M. Sentinel node detection in melanomas using contrast-enhanced ultrasound. Acta Radiol 2009;50:412–17 | No relevant outcomes |
| Saiag P, Bernard M, Beauchet A, Bafounta ML, Bourgault-Villada I, Chagnon S. Ultrasonography using simple diagnostic criteria vs. palpation for the detection of regional lymph node metastases of melanoma. Arch Dermatol 2005;141:183–9 | No analysis by disease stage or Breslow depth |
| Saiag P, Lebbe C, Basset-Seguin N, Wolkenstein P, Dupin N, Descamps V, et al. Role of lymph-node ultrasonography (US) in the follow-up of melanoma patients to detect nodal recurrence after sentinel lymph node biopsy (SNLB): a prospective cohort study. Melanoma Res 2010;20:e31 | Stage not reported (conference abstract) |
| Saiag P, Lebbe C, Seguin NB, Wolkenstein P, Dupin N, Descamps V, et al. Role of lymph-node ultrasonography (US) in the follow-up of melanoma patients to detect nodal recurrence after sentinel lymph node biopsy (SNLB): a prospective cohort study. J Clin Oncol 2010;28:1 | Stage not reported (conference abstract) |
| Samimi M, Perrinaud A, Naouri M, Maruani A, Perrodeau E, Vaillant L, Machet L. High-resolution ultrasonography assists the differential diagnosis of blue naevi and cutaneous metastases of melanoma. Br J Dermatol 2010;163:550–6 | No analysis by disease stage |
| Sanki A, Uren RF, Moncrieff M, Tran KL, Scolyer RA, Lin HY, et al. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J Clin Oncol 2009;27:5614–9 | Pre-operative staging; no analysis by disease stage at recurrence |
| Sasaki Y, Kanki H, Nagano T, Nishigori C, Fukuoka K, Kumagai S. Assessment of sentinel lymph nodes identified by lymphoscintigraphy, compared histopathology to ultrasonography. Skin Res 2008;7:586–92 | Insufficient patients (Japanese) |
| Schmid-Wendtner MH, Dill-Müller D, Baumert J, Wagner A, Eberle J, Tilgen W, Plewig G. Lymph node metastases in patients with cutaneous melanoma: improvements in diagnosis by signal-enhanced color Doppler sonography. Melanoma Res 2004;14:269–76 | No analysis by disease stage or Breslow depth |
| Schmid-Wendtner MH, Paerschke G, Baumert J, Plewig G, Volkenandt M. Value of ultrasonography compared with physical examination for the detection of locoregional metastases in patients with cutaneous melanoma. Melanoma Res 2003;13:183–8 | No analysis by disease stage or Breslow depth |
| Schmid-Wendtner MH, Partscht K, Korting HC, Volkenandt M. Improved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast-enhanced color Doppler sonography. Arch Dermatol 2002;138:491–7 | No analysis by disease stage or Breslow depth |
| Sijan G, Kozarski J, Stefanović D, Lalković M, Milićević S, Stanković G. [Ultrasonographic findings validity in the identification of metastatic regional lymph nodes in patients with cutaneous melanoma.] Vojnosanit Pregl 2010;67:25–31 | Advanced stage (Croatian) |
| Šijan G, Kozarski J, Stepić N, Milojević S, Stefanović D, Tatomirović Ž, et al. Validity of ultrasound-guided aspiration needle biopsy in the diagnosis of micrometastases in sentinel lymph nodes in patients with cutaneous melanoma. Vojnosanit Pregl 2016;73:934–40 | Pre-operative staging of lymph node |
| Solivetti FM, Desiderio F, Guerrisi A, Bonadies A, Maini CL, Di Filippo S, et al. HF ultrasound vs PET-CT and telethermography in the diagnosis of In-transit metastases from melanoma: a prospective study and review of the literature. J Exp Clin Cancer Res 2014;33:96 | Pre-operative staging |
| Solivetti FM, Di Luca Sidozzi A, Pirozzi G, Coscarella G, Brigida R, Eibenshutz L. Sonographic evaluation of clinically occult in-transit and satellite metastases from cutaneous malignant melanoma. Radiol Med 2006;111:702–8 | No analysis by disease stage or Breslow depth |
| Solivetti FM, Elia F, Graceffa D, Di Carlo A. Ultrasound morphology of inguinal lymph nodes may not herald an associated pathology. J Exp Clin Cancer Res 2012;31:88 | Pre-operative staging |
| Solivetti FM, Elia F, Santaguida MG, Guerrisi A, Visca P, Cercato MC, Di Carlo A. The role of ultrasound and ultrasound-guided fine needle aspiration biopsy of lymph nodes in patients with skin tumours. Radiol Oncol 2014;48:29–34 | No analysis by disease stage or Breslow depth |
| Teng E, Sue GR, Sawh-Martinez R, Nishikawa S, Ariyan S, Natarajan A, Narayan D. Scalp melanoma and in-transit metastases: a retrospective case-controlled study. Am Surg 2014;80:1272–4 | No diagnostic tests reported |
| Ternov NK, Lambine TL, Wagenblast ALH, Clasen-Linde E, Oturai PS, Klyver H, et al. Targeted ultrasound and fine-needle aspiration cytology for sentinel node diagnostics in early-stage melanoma: a validation study. Melanoma Res 2018;28:319–25 | Pre-operative staging |
| Testori A, Lazzaro G, Baldini F, Tosti G, Mosconi M, Lovati E, et al. The role of ultrasound of sentinel nodes in the pre- and post-operative evaluation of stage I melanoma patients. Melanoma Res 2005;15:191–8 | Insufficient diagnostic data at recurrence |
| Testori A, Rastrelli M, De Fiori E, Soteldo J, Della Vigna P, Trifirò G, et al. Radio-guided ultrasound lymph node localization: feasibility of a new technique for localizing and excising nonpalpable lymph nodes ultrasound suspicious for melanoma metastases. Melanoma Res 2010;20:197–202 | Stage not reported |
| Thompson JF, Haydu LE, Sanki A, Uren RF. Ultrasound assessment of lymph nodes in the management of early-stage melanoma J Surg Oncol 2011;104:354–60 | Review |
| Tombesi P, Tassinari D, Sartori S. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR Am J Roentgenol 2011;197:W371 | Letter |
| Tsimpaki T, Beis E, Othmer V, Grabbe S, Tuttenberg A. The role of preoperative ultrasound as an auxiliary tool for the detection of nodal micro- and macrometastasis in melanoma patients undergoing sentinel lymph node biopsy: a retrospective analysis. J Dtsch Dermatol Ges 2017;15:57–8 | Stage not reported (poster) |
| Ulrich J, van Akkooi AC, Eggermont AM, Voit CA. [Sonographic criteria for diagnosing sentinel node metastases in melanoma patients.] Ultraschall Med 2015;36:149–53 | No analysis by disease stage or Breslow depth |
| Uren RF, Howman-Giles R, Thompson JF, Shaw HM, Roberts JM, Bernard E, McCarthy WH. High-resolution ultrasound to diagnose melanoma metastases in patients with clinically palpable lymph nodes. Australas Radiol 1999;43:148–52 | No analysis by disease stage or Breslow depth |
| Ustün M, Risberg B, Davidson B, Berner A. Cystic change in metastatic lymph nodes: a common diagnostic pitfall in fine-needle aspiration cytology. Diagn Cytopathol 2002;27:387–92 | No analysis by disease stage or Breslow depth |
| Val-Bernal JF, Martino M, Yllera E, Romay F, Sánchez-Ares M, Nallib IA. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of hilar and mediastinal lymph node metastases of melanoma. Turk Patoloji Derg 2019;35:92–101 | Insufficient number of stage I patients |
| van Akkooi ACJ, Siegel P, Gooskens S, Schoengen A, Sterry W, Eggermont AM, et al. Use of preoperative ultrasound (US)-guided fine needle aspiration cytology (FNAC) to identify positive sentinel nodes (SN) in melanoma. J Clin Oncol Conf 2013;31:e20035 | Stage I–II (conference abstract) |
| van Akkooi ACJ, Siegel P, Schoengen A, Roewert-Huber J, Eggermont AM, Voit CA. Long-term results of ultrasound (US)-guided fine needle aspiration cytology (FNAC) in conjunction with sentinel node biopsy (SNB) to support step-wise approach in melanoma. J Clin Oncol Conf 2015;33:9067 | Stage I–II (conference abstract) |
| Vensby PH, Schmidt G, Kjær A, Fischer BM. The value of FDG PET/CT for follow-up of patients with melanoma: a retrospective analysis. Am J Nucl Med Mol Imaging 2017;7:255–62 | No analysis by disease stage or Breslow depth |
| Voit C, Kron M, Schäfer G, Schoengen A, Audring H, Lukowsky A, et al. Ultrasound-guided fine needle aspiration cytology prior to sentinel lymph node biopsy in melanoma patients. Ann Surg Oncol 2006;13:1682–9 | No analysis by disease stage or Breslow depth |
| Voit C, Mayer T, Kron M, Schoengen A, Sterry W, Weber L, Proebstle TM. Efficacy of ultrasound B-scan compared with physical examination in follow-up of melanoma patients. Cancer 2001;91:2409–16 | No analysis by disease stage or Breslow depth |
| Voit C, Mayer T, Proebstle TM, Weber L, Kron M, Krupienski M, et al. Ultrasound-guided fine-needle aspiration cytology in the early detection of melanoma metastases. Cancer 2000;90:186–93 | No analysis by disease stage or Breslow depth |
| Voit C, Van Akkooi ACJ, Schaefer G, Schoengen A, Sterry W, Eggermont AMM. Early ultrasound criteria drive sensitivity for detection of sentinel node metastases in melanoma patients: a prospective study in 800 patients. Pigment Cell Melanoma Res 2010;23:984 | Stages I-II (conference abstract) |
| Voit CA, Gooskens S, Van Akkooi ACJ, Eggermont AMM. Ultrasound (US) – guided fine needle aspiration cytology (FNAC) of the sentinel node (SN) in 1000 consecutive melanoma patients. Eur J Cancer 2013;2:S855 | Stage I-II (conference abstract) |
| Voit CA, Oude Ophuis CM, Ulrich J, van Akkooi AC, Eggermont AM. Ultrasound of the sentinel node in melanoma patients: echo-free island is a discriminatory morphologic feature for node positivity. Melanoma Res 2016;26:267–71 | No analysis by disease stage or Breslow depth |
| Voit CA, van Akkooi AC, Schaefer-Hesterberg G, Schoengen A, Sterry W, Eggermont AM. Correlation of ultrasound criteria for detection of melanoma metastases in the sentinel lymph node (SN) with tumor burden and survival. J Clin Oncol 2009;27:9015 | Initial staging – Rotterdam criteria |
| Voit CA, van Akkooi ACJ, Catalano O, Eggermont AMM. Pre-SN ultrasound-FNAC can be sensitive for lymph node metastases in melanoma patients if performed with the use of the Berlin criteria. Ann Surg Oncol 2017;24:661–2 | Letter |
| Voit CA, Van Akkooi ACJ, Siegel P, Schoengen A, Sterry W, Eggermont AMM. High sensitivity rate of ultrasound (US) guided fine needle aspiration cytology (FNAC) using the Berlin morphology criteria for lymph node metastases significantly reduces need for surgical sentinel node (SN) staging in melanoma. Skin Res Technol 2012;19:e574–e5 | Stage I–II (conference abstract) |
| Voit CA, Van Akkooi ACJ, Siegel P, Sterry W, Schoengen A, Schaefer-Hesterberg G, et al. Ultrasound (US)-guided fine-needle aspiration cytology (FNAC) for the prediction of sentinel node (SN) metastases and its effect on the nomogram for melanoma patients. J Clin Oncol Conf 2011;29:8850 | No analysis by disease stage or Breslow depth (conference abstract) |
| Voit CA, van Akkooi AJ, Schäfer-Hesterberg G, Sterry W, Eggermont AM. The value of preoperative ultrasound (after lymphoscintigraphy) in conjunction with pre-sentinel lymph node biopsy fine-needle aspiration outweighs the usage of ultrasound alone in conjunction with lymphoscintigraphy: the need for an algorithm. Melanoma Res 2010;20:357–9 | Letter |
Appendix 7 Targeted economic search
| Objectives and research questions | |
|---|---|
| Primary objective | To review primary health economic evaluation for surveillance strategies for stage I melanoma post primary tumour excision |
| Studies to include | |
| Study designs |
|
| Population |
|
| Interventions |
|
| Comparators |
|
| Language | Studies with abstracts in the English language, but full-text published in a language other than English, will be evaluated; if local expertise is available, they will be included |
| Publication time frame | Studies published from start of database to present will be included in this review in order to retrieve evidence from all the available data. No restriction on publication period will be applied |
| Data sources | |
| Databases |
|
| Information to extract (indicative list only. Outcomes to be finalised prior to data extraction after identification of all included studies) | |
| Study details |
|
| Population characteristics |
|
| Basic modelling methodologies |
|
| Model structure and key data sources/risk equations |
|
| Outcomes |
|
Search strategy and number of hits for different searched databases
Dates searched: 29 March 2018 and 18 April 2018.
| Number | Search term | Facet | Results (n) |
|---|---|---|---|
| NHS Economic Evaluation Database | |||
| 1 | MELANOMA | Disease | 321 |
| 2 | SKIN TUMO(U)R | 1 | |
| 3 | CUTANEOUS TUMO(U)R | 0 | |
| 4 | SKIN CANCER | 97 | |
| 5 | #1 AND Surveillance | Follow-up | 12 |
| 6 | #1 AND Monitoring | 14 | |
| 7 | #1 AND Follow-up | 92 | |
| 8 | #1 AND Screening | 24 | |
| 9 | #1 AND Management | 42 | |
| 10 | #4 AND Surveillance | 4 | |
| 11 | #4 AND Monitoring | 4 | |
| 12 | #4 AND Follow-up | 33 | |
| 13 | #4 AND Screening | 19 | |
| 14 | #4 AND Management | 14 | |
| 15 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 | Final numbers | 260 |
| Cost-effectiveness Analysis Registry | |||
| 1 | MELANOMA | Disease | 33 |
| 2 | SKIN TUMO(U)R | 0 | |
| 3 | CUTANEOUS TUMO(U)R | 0 | |
| 4 | SKIN CANCER | 9 | |
| 13 | or/1-4 | Final numbers | 42 |
MEDLINE (via Ovid) without revisions
Date range searched: 1996 to April week 2 2018.
| Number | Search term | Facet | Results (n) |
|---|---|---|---|
| 1 | exp Melanoma/ | Disease | 52,542 |
| 2 | melanoma$.tw. | 62,394 | |
| 3 | (maligna$ adj1 lentigo$).tw. | 623 | |
| 4 | (hutchinson$ adj1 (freckle$ or melano$)).tw. | 10 | |
| 5 | dubreuilh.tw. | 8 | |
| 6 | Melanoma/ | 508 | |
| 7 | or/1-6 | 70,232 | |
| 8 | (follow-up or “follow up” or followup).tw. | Follow-up | 589,929 |
| 9 | (check-up*1 or check up*1).tw. | 5394 | |
| 10 | surveillance.tw. | 105,813 | |
| 11 | exp Aftercare/ | 110,607 | |
| 12 | (aftercare or after-care).tw. | 1974 | |
| 13 | ((post-treatment or posttreatment) adj1 evaluation*).tw. | 330 | |
| 14 | ((post-treatment or posttreatment) adj1 care).tw. | 83 | |
| 15 | ((post-treatment or posttreatment) adj1 monitoring).tw. | 117 | |
| 16 | ((post-treatment or posttreatment) adj1 surveillance).tw. | 237 | |
| 17 | or/8-16 | 789,181 | |
| 18 | 7 and 17 | 6269 | |
| 19 | exp models, economic/ | Economics | 11,826 |
| 20 | *models, theoretical/ | 44,201 | |
| 21 | *models, organizational/ | 5210 | |
| 22 | Markov chains/ | 11,638 | |
| 23 | monte carlo method/ | 22,458 | |
| 24 | exp decision theory | 8779 | |
| 25 | (Markov* or monte carlo).ti, ab. | 34,389 | |
| 26 | econom* model*.ti.ab | 2302 | |
| 27 | (decision* adj2 (tree* or analy* or model*)).ti,ab. | 13,341 | |
| 28 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 | 117,975 | |
| 29 | 18 and 28 | Final Numbers | 29 |
| 30 | limit 29 to yr=”2015 -Current” | 9 |
EMBASE
Date range searched: 1996 to 2018 week 16.
| Number | Search term | Facet | Results (n) |
|---|---|---|---|
| 1 | exp Melanoma/ | Disease | 110,054 |
| 2 | melanoma$.tw. | 109,004 | |
| 3 | (maligna$ adj1 lentigo$).tw. | 1068 | |
| 4 | (hutchinson$ adj1 (freckle$ or melano$)).tw. | 17 | |
| 5 | dubreuilh.tw. | 19 | |
| 6 | Melanoma/ | 917 | |
| 7 | or/1-6 | 135,649 | |
| 8 | (follow-up or “follow up” or followup).tw. | Follow-up | 1,144,022 |
| 9 | (check-up*1 or check up*1).tw. | 9880 | |
| 10 | surveillance.tw. | 178,560 | |
| 11 | exp Aftercare/ | 1,195,286 | |
| 12 | (aftercare or after-care).tw. | 3395 | |
| 13 | ((post-treatment or posttreatment) adj1 evaluation*).tw. | 634 | |
| 14 | ((post-treatment or posttreatment) adj1 care).tw. | 177 | |
| 15 | ((post-treatment or posttreatment) adj1 monitoring).tw. | 199 | |
| 16 | ((post-treatment or posttreatment) adj1 surveillance).tw. | 499 | |
| 17 | or/8-16 | 1,626,817 | |
| 18 | 7 and 17 | 16,537 | |
| 19 | statistical model/ | Economics | 142,384 |
| 20 | exp economic aspect/ | 1,155,950 | |
| 21 | 19 and 20 | 19,009 | |
| 22 | *theoretical model/ | 23,005 | |
| 23 | *nonbiological model/ | 4027 | |
| 24 | stochastic model/ | 9777 | |
| 25 | decision theory/ | 1303 | |
| 26 | decision tree/ | 8931 | |
| 27 | monte carlo method/ | 32,115 | |
| 28 | (Markov* or monte carlo).ti,ab. | 54,952 | |
| 29 | econom* model*.ti,ab. | 4413 | |
| 30 | (decision* adj2 (tree* or analy* or model*)).ti,ab. | 23,730 | |
| 31 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 | 1,371,121 | |
| 32 | 18 and 31 | Final Numbers | 756 |
| 33 | limit 32 to yr=“2015 -Current” | 289 |
The total number of studies retrieved by each individual database is provided in Table 23.
| Database | Retrieved (n) |
|---|---|
| NHS Economic Evaluation Database | 260 |
| Cost-effectiveness Analysis Registry | 42 |
| MEDLINE | 9 |
| EMBASE | 289 |
| Total | 590 |
After reviewing titles and abstracts, 15 studies were fully reviewed to see if any model could be adapted to answer the research question and inform the structure of the model. No study was judged as being suitable for adaptation as the structure of the model would need to be malleable to patient behaviour, such as self-diagnosis and false alarms.
Appendix 8 Model structure
A simplified version of the Markov model is shown in Figure 6. This appendix presents the detailed structure of this model.
With no recurrence, patients will end up back in the disease-free health cycle for the next cycle (Figure 21). However, there is a chance that patients will have a ‘false alarm’ that results in an ‘E Visit: Emergency visit’ to their local dermatologist. There is a chance that, after a history and physical examination, a local biopsy would be taken and a false-positive result will ensue, which may result in a SLNB. All patients return to the disease-free health state in the Markov model.
FIGURE 21.
Disease-free health state: no recurrence. +ve, positive; –ve, negative; E visit, Emergency visit; FN, False negative; HP, health and physical examination; SNB, sentinel node biopsy; spec, specificity.

However, if a recurrence does occur (Figure 22), it can be stage IA to IV; this can be picked up by a scheduled screening/appointment with a health-care professional that is part of the surveillance strategy or it can be self-diagnosed (opportunistic diagnosis). The clinical pathway consists of local biopsy (B) (Figure 23), SLNB (C) (Figure 24) and CT (D) (Figure 25). If the recurrence is not picked up and the patient survives, their melanoma progresses (E) (Figure 26). Patients will re-enter the model in the next cycle, where they go through the same process. If treatment is successful, patients return to the disease-free health state.
FIGURE 22.
Recurrence health state. +ve, positive; –ve, negative; HP, health and physical examination; sens, sensitivity.
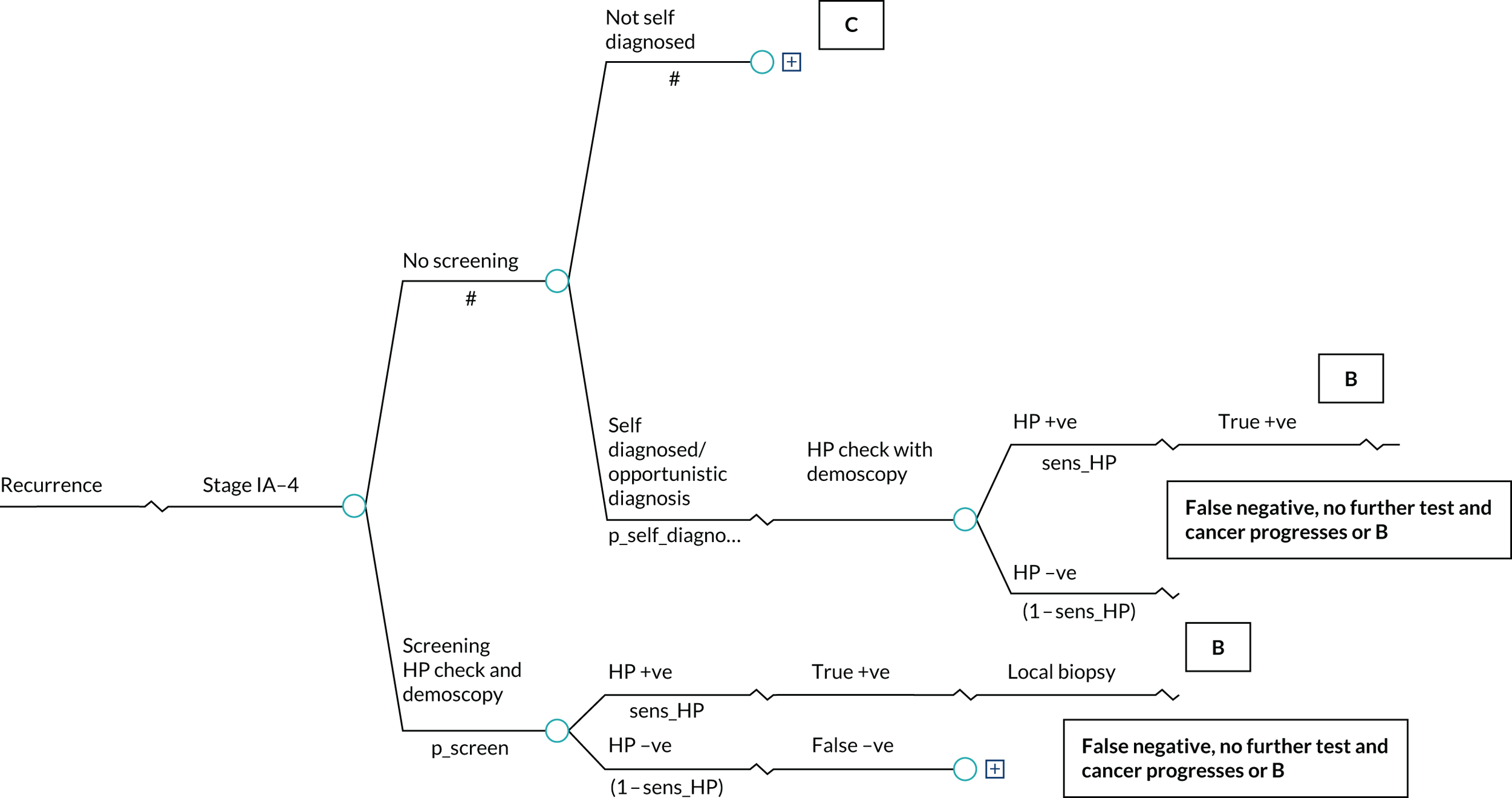
FIGURE 23.
Clinical pathway following a local biopsy. +ve, positive; –ve, negative; mort, mortality; sens, sensitivity.
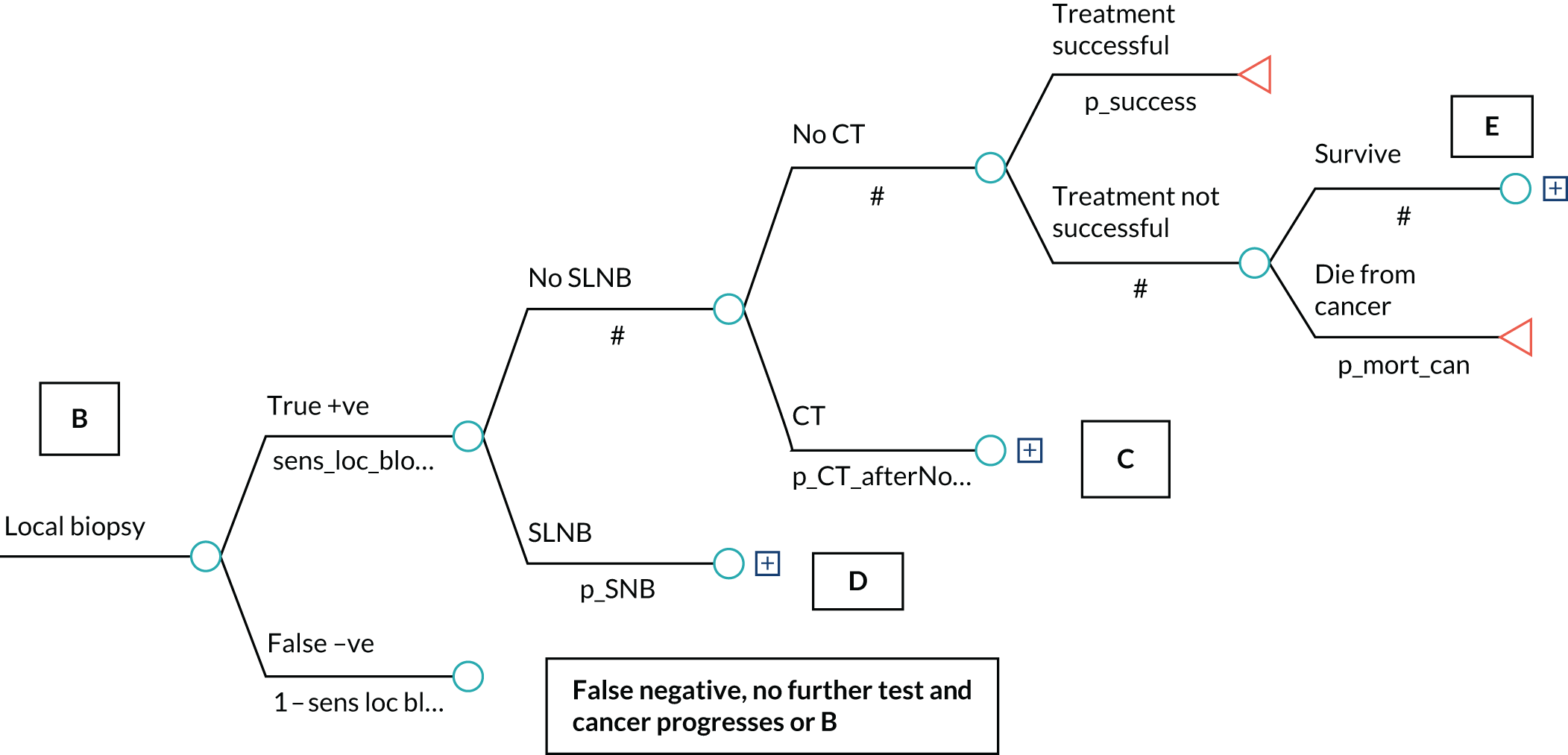
FIGURE 24.
Care pathway following a SLNB. +ve, positive; –ve, negative; FN, false negative; FP, false positive; sens, sensitivity; spec, specificity; TN, true negative; TP, true positive.

FIGURE 25.
Care pathway following CT. +ve, positive; –ve, negative; FN, false negative; FP, false positive; sens, sensitivity; spec, specificity; TN, true negative; TP, true positive.
FIGURE 26.
Disease pathway for disease progression.

Surveillance strategies were compared based on the ‘p_screen’ variable, with associated sensitivity and specificity, and costs, based on the health-care professional involved in the screening. Each surveillance strategy is captured by a ‘follow-up duration’ variable and a ‘follow-up interval’ that contributes to lifetime costs and health outcomes expressed in terms of QALYs.
Appendix 9 Clinical parameter values
The clinical parameters used in the model are presented in Table 24 as fixed values without any distributions assigned.
| Parameter | Description | Value | Source |
|---|---|---|---|
| p_CT_afterNo_SNB | Probability of receiving a CT scan after no SNB | 0.9 | Expert opinion |
| p_CT_post_Neg_SNB | Probability of receiving a CT scan after negative SNB | 0.9 | Expert opinion |
| p_CT_post_posi_SNB | Probability of receiving a CT scan after positive SNB | 1 | Expert opinion |
| p_CT_s4 | Probability of receiving a CT scan after stage IV diagnosis | 1 | Expert opinion |
| p_false_self_diagnose | Probability of a false (diagnosis) after self-diagnosis | probtoprob(0.85; 1/12) 0.1462 | Holterhues et al.191 2016 and expert opinion |
| p_go_visit_E_SD | Probability of seeking treatment emergently after self-diagnosis | 1 | Expert opinion |
| p_loc_biopsy_HP_positive | Probability of having a further test after HP positive | 1 | Expert opinion |
| p_no_test_post_negativeHP | Probability of no test after negative HP | 1 | Expert opinion |
| p_self_diagnosed | Probability of self-diagnosis of melanoma | probtoprob(0.6; 1/12) 0.073 | Damude et al.23 2016 and expert opinion |
| p_SNB | Probability of SNB after wider excision | 0.95 | Expert opinion |
| p_SNB_self_FN | Probability of SNB after a false negative from a self-diagnosis | 0.9 | Expert opinion |
| p_success_s1 | Probability of treatment success after stage I | 0.95 | Expert opinion |
| p_success_s2 | Probability of treatment success after stage II | 0.85 | Expert opinion |
| p_success_s3 | Probability of treatment success after stage III | 0.6 | Expert opinion |
| p_success_s4 | Probability of treatment success after stage IV | 0.4 | Expert opinion |
Monthly probability values of p_false_self_diagnose and p_self_diagnosed were created from the TreeAge function probtoprob, which converts a probability into a rate, multiplies the rate by the given multiplier and converts this back to a probability.
Mortality values
The monthly probability values of mortality from melanoma are presented in Table 25. These values are based on calculations from Wilson et al. 182 The OR of survival was a function of disease stage at diagnosis. This model assumed that stage IA disease has no impact on overall survival, then the annual probability of death is calculated as the age/sex baseline rate for the general population, adjusted for the OR.
| Stage | OR | Value in model (monthly) |
|---|---|---|
| IA | – | 1.700 × 10–5 |
| IB | 4.261 | 7.243 × 10–5 |
| IIA | 12.250 | 2.082 × 10–4 |
| IIB | 21.000 | 3.569 × 10–4 |
| IIC | 41.741 | 7.091 × 10–4 |
| IIIA | 13.821 | 2.349 × 10–4 |
| IIIB | 32.667 | 5.551 × 10–4 |
| IIIC | 67.667 | 0.0011 |
| IV | 312.104 | 0.0053 |
The UK age/sex background annual mortality probabilities are presented in Table 26. These values were converted to monthly probabilities using the TreeAge function probtoprob.
| Age (index) | Male | Female |
|---|---|---|
| 0 | 0.004276 | 0.00349 |
| 1 | 2.71E–04 | 2.31E–04 |
| 2 | 1.54E–04 | 1.40E–04 |
| 3 | 1.20E–04 | 9.70E–05 |
| 4 | 1.02E–04 | 8.30E–05 |
| 5 | 9.20E–05 | 6.90E–05 |
| 6 | 7.80E–05 | 7.30E–05 |
| 7 | 8.30E–05 | 6.80E–05 |
| 8 | 7.00E–05 | 6.20E–05 |
| 9 | 7.80E–05 | 6.00E–05 |
| 10 | 7.80E–05 | 5.10E–05 |
| 11 | 9.10E–05 | 7.30E–05 |
| 12 | 9.50E–05 | 7.40E–05 |
| 13 | 1.06E–04 | 9.10E–05 |
| 14 | 1.18E–04 | 1.07E–04 |
| 15 | 1.70E–04 | 1.26E–04 |
| 16 | 2.20E–04 | 1.51E–04 |
| 17 | 3.02E–04 | 1.53E–04 |
| 18 | 3.99E–04 | 2.09E–04 |
| 19 | 4.39E–04 | 2.00E–04 |
| 20 | 4.67E–04 | 2.04E–04 |
| 21 | 4.96E–04 | 2.11E–04 |
| 22 | 4.94E–04 | 2.04E–04 |
| 23 | 5.29E–04 | 2.26E–04 |
| 24 | 5.35E–04 | 2.15E–04 |
| 25 | 6.20E–04 | 2.42E–04 |
| 26 | 5.73E–04 | 2.68E–04 |
| 27 | 6.14E–04 | 2.77E–04 |
| 28 | 6.79E–04 | 3.09E–04 |
| 29 | 6.84E–04 | 3.32E–04 |
| 30 | 7.25E–04 | 3.75E–04 |
| 31 | 7.69E–04 | 4.01E–04 |
| 32 | 9.27E–04 | 4.79E–04 |
| 33 | 8.88E–04 | 5.00E–04 |
| 34 | 9.77E–04 | 5.08E–04 |
| 35 | 0.001026 | 5.88E–04 |
| 36 | 0.001161 | 6.60E–04 |
| 37 | 0.001191 | 7.30E–04 |
| 38 | 0.001241 | 7.30E–04 |
| 39 | 0.001393 | 8.41E–04 |
| 40 | 0.001501 | 9.30E–04 |
| 41 | 0.001713 | 9.76E–04 |
| 42 | 0.001811 | 0.001076 |
| 43 | 0.001987 | 0.001144 |
| 44 | 0.002096 | 0.001294 |
| 45 | 0.002202 | 0.001441 |
| 46 | 0.00237 | 0.001528 |
| 47 | 0.002741 | 0.001655 |
| 48 | 0.002797 | 0.001776 |
| 49 | 0.003107 | 0.001919 |
| 50 | 0.003402 | 0.002142 |
| 51 | 0.003501 | 0.002349 |
| 52 | 0.003813 | 0.002576 |
| 53 | 0.003968 | 0.002807 |
| 54 | 0.004408 | 0.003014 |
| 55 | 0.004923 | 0.00329 |
| 56 | 0.005467 | 0.003611 |
| 57 | 0.005868 | 0.003861 |
| 58 | 0.006371 | 0.004228 |
| 59 | 0.007031 | 0.004764 |
| 60 | 0.007955 | 0.005166 |
| 61 | 0.008614 | 0.00562 |
| 62 | 0.009324 | 0.006282 |
| 63 | 0.010484 | 0.006855 |
| 64 | 0.011447 | 0.007317 |
| 65 | 0.012244 | 0.007878 |
| 66 | 0.013496 | 0.008883 |
| 67 | 0.014599 | 0.009608 |
| 68 | 0.015607 | 0.010351 |
| 69 | 0.017228 | 0.011515 |
| 70 | 0.018837 | 0.01267 |
| 71 | 0.021307 | 0.01424 |
| 72 | 0.023546 | 0.015891 |
| 73 | 0.026015 | 0.017933 |
| 74 | 0.029585 | 0.019667 |
| 75 | 0.032946 | 0.022235 |
| 76 | 0.036425 | 0.025461 |
| 77 | 0.03986 | 0.027457 |
| 78 | 0.044087 | 0.03139 |
| 79 | 0.049207 | 0.03462 |
| 80 | 0.055222 | 0.039684 |
| 81 | 0.06139 | 0.044619 |
| 82 | 0.069292 | 0.051081 |
| 83 | 0.078533 | 0.058425 |
| 84 | 0.087689 | 0.06669 |
| 85 | 0.098188 | 0.074956 |
| 86 | 0.109512 | 0.08545 |
| 87 | 0.122389 | 0.096451 |
| 88 | 0.137063 | 0.109963 |
| 89 | 0.151054 | 0.123634 |
| 90 | 0.168744 | 0.139389 |
| 91 | 0.186002 | 0.154579 |
| 92 | 0.201643 | 0.171622 |
| 93 | 0.220694 | 0.188373 |
| 94 | 0.243034 | 0.209568 |
| 95 | 0.26757 | 0.233693 |
| 96 | 0.28755 | 0.253124 |
| 97 | 0.305291 | 0.268228 |
| 98 | 0.313561 | 0.283968 |
| 99 | 0.348595 | 0.31482 |
| 100 | 0.385655 | 0.340913 |
Recurrence values
Given that recurrence data are not collected at a registry level, the next best alternative would be to use melanoma incidence data from the NCRAS/PHE as a surrogate to find the recurrence probability in England/the UK. However, NCRAS/PHE data are based on the summary stage information (i.e. AJCC stage I–IV) of all patients diagnosed by CCGs in England, as presented in Table 27.
| Stage | Total | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ‘X’ unknown | |
| 21,737 | 6068 | 1764 | 794 | 6560 | 36,923 |
Stage ‘X’ indicates that the full TNM stage group is unknown to the NCRAS (but not necessarily to the treating clinicians). Among other reasons, this may be because of a data quality problem (missing data), because of patient mortality before staging was completed or because it was clinically inappropriate to fully stage the patient. Table 28 presents the known melanoma diagnosis.
| Stage, n (%) | Total (n) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 21,737 (72) | 6068 (20) | 1764 (6) | 794 (3) | 30,363 |
A large registry-based German study of 33,384 patients recorded by the Central Malignant Melanoma Registry of the German Society of Dermatology between 1976 and 2007 with stage I–III initial diagnosis is presented in Table 29. 209 These values are then scaled to 100% to include stage IV from NCRAS. Unfortunately, this paper provided rate of recurrence-free survival by summary stage and did not provide information to calculate the follow-up recurrence probability over time per AJCC staging criteria.
| Stage at primary diagnosis | CMMR (n = 33,384) (%) | Total per summary stage (%) | Substage proportions (%) | Scaled to 100% (%) |
|---|---|---|---|---|
| IA | 45.3 | I = 71.4 | 45.3/71.4 = 63.4 | 43.9 |
| IB | 26.1 | 26.1/71.4 = 36.5 | 25.3 | |
| IIA | 12.9 | II = 23.5 | 12.9/23.5 = 54.9 | 12.5 |
| IIB | 7.9 | 7.9/23.5 = 33.6 | 7.7 | |
| IIC | 2.7 | 2.7/23.5 = 11.5 | 2.6 | |
| IIIA | 1.0 | III = 5.0 | 1.0/5.0 = 20.0 | 1.0 |
| IIIB | 3.6 | 3.6/5.0 = 72.0 | 3.5 | |
| IIIC | 0.4 | 0.4/5.0 = 8.0 | 0.4 | |
| IV (NCRAS) | 3.0 | 3.0 |
Recurrence probabilities over time for AJCC stages IA–IIC were obtained from a different study conducted in Australia by Turner et al. 210 The study authors analysed 2298 patient records for the development of recurrence and new primary melanoma up to 10 years. Kaplan–Meier curves in this paper showing time to recurrence for localised melanoma were able to be digitised using WebPlotDigitizer. Various points along the curve were chosen and the co-ordinates of those points were extracted.
These points were then used to calculate the lamda and gamma parameters of the Weibull distribution for AJCC stage IA. The two parameters were then used to calculate the transition probabilities (tp) for recurrence using Equation 4:
where:
-
t is time (measured in terms of the number of cycles; each cycle is equivalent to 1 month)
-
λ is the scale parameter, which describes the probability that an individual experiences recurrence, given that he/she is recurrence free during the current time period
-
γ is the shape parameter, which describes the hazard function of Weibull function for the survival time
-
µ is the length of the Markov cycle.
Moreover, for the calculation of the baseline transition probability, Equation 5 was used:
where µ is the length of the Markov cycle.
Recurrence rates for the remaining melanoma stages were computed as a function of the probability of recurrence of stage IA disease and the distribution of the hazard ratio of each stage up to stage IIC reported in the study.
By using the CIs presented in the paper by Turner et al. ,210 the corresponding SEs were calculated (Table 30), which, along with the hazard ratios, were used as gamma distributions in the model.
| Stage | Follow-up | |||||
|---|---|---|---|---|---|---|
| 5 years | 10 years | |||||
| HR | 95% CI | SE | HR | 95% CI | SE | |
| IA | 1.00 | 1.00 | ||||
| IB | 2.02 | 1.54 to 2.65 | 0.28 | 2.10 | 1.15 to 3.86 | 0.69 |
| IIA | 4.32 | 3.22 to 5.81 | 0.66 | 2.43 | 1.13 to 5.25 | 1.05 |
| IIB | 6.10 | 4.54 to 8.20 | 0.93 | 2.98 | 1.33 to 6.67 | 1.36 |
| IIC | 7.09 | 5.15 to 9.76 | 1.18 | 3.95 | 1.59 to 9.84 | 2.10 |
| IIIA | 7.09 | 5.15 to 9.76 | 1.18 | 3.95 | 1.59 to 9.84 | 2.10 |
| IIIB | 7.09 | 5.15 to 9.76 | 1.18 | 3.95 | 1.59 to 9.84 | 2.10 |
| IIIC | 7.09 | 5.15 to 9.76 | 1.18 | 3.95 | 1.59 to 9.84 | 2.10 |
| IV | 7.09 | 5.15 to 9.76 | 1.18 | 3.95 | 1.59 to 9.84 | 2.10 |
Because it was not possible to source recurrence rates for AJCC stages III and IV, it was assumed that the recurrence rates for these two stages are similar to those of stage IIC. Depending on the stage of the recurrence in the model, the monthly probabilities were selected (Table 31).
| Stage | Variable name | Probability of recurrence or new primary |
|---|---|---|
| IA | p_recur_s1a | 0.0022 |
| IB | p_recur_s1b = p_recur_s1a*dist_hr_recu_1b | 0.0046 |
| IIA | p_recur_s2a = p_recur_s1a*dist_hr_recu_2a | 0.0095 |
| IIB | p_recur_s2b = p_recur_s1a*dist_hr_recu_2b | 0.0134 |
| IIC | p_recur_s2c = p_recur_s1a*dist_hr_recu_2c | 0.0156 |
| IIIA | p_recur_s3a = p_recur_s1a*dist_hr_recu_2c | 0.0156 |
| IIIB | p_recur_s3b = p_recur_s1a*dist_hr_recu_2c | 0.0156 |
| IIIC | p_recur_s3c = p_recur_s1a*dist_hr_recu_2c | 0.0156 |
| IV | p_recur_4 = p_recur_s1a*dist_hr_recu_2c | 0.0156 |
Diagnostic accuracy
The diagnostic accuracy statistics used in the model are based on the pooled statistics (meta-analysis) taken from a Cochrane systematic review. 170 It was assumed that the diagnostic accuracy of the health-care professional should be based on visual inspection, plus the use of dermoscopy on real patients. The studies of those physicians deemed to have ‘high’ experience of invasive melanoma or atypical intraepidermal melanocytic variants were selected. The studies identified were then retrieved and physicians classified as either ‘surgical oncologists’ or ‘dermatologists’ (Tables 32 and 33). For dermatological nurse specialists, only one study in the Cochrane review was from the USA and it was based on ‘physician assistant’ (Table 34). A beta distribution of the sensitivity and specificity was then used in the base-case PSA.
| Country | Study (first author and year of publication) | True positive (n) | False positive (n) | False negative (n) | True negative (n) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| Italy | Bono et al.206 2002 | 60 | 63 | 6 | 184 | 0.91 (0.81 to 0.97) | 0.74 (0.69 to 0.80) |
| Italy | Bono et al.205 2002 | 10 | 42 | 3 | 106 | 0.77 (0.46 to 1.95) | 0.72 (0.64 to 0.79) |
| Pooled | 0.886 (0.795 to 0.94); SE 0.036 | 0.734 (0.688 to 0.775); SE 0.022 |
| Country | Study (first author and year of publication) | True positive (n) | False positive (n) | False negative (n) | True negative (n) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| Sweden | Ahnlide et al.194 2016 | 34 | 23 | 12 | 240 | 0.74 (0.59 to 0.86) | 0.91 (0.87 to 0.94) |
| Italy | Bauer et al.195 2000 | 33 | 10 | 9 | 263 | 0.79 (0.63 to 0.90) | 0.96 (0.93 to 0.98) |
| Italy | Carli et al.197 1994 | 5 | 28 | 0 | 35 | 1.00 (0.48 to 1.00) | 0.56 (0.42 to 0.68) |
| Italy | Carli et al.196 2002 | 53 | 9 | 1 | 193 | 0.98 (0.90 to 1.00) | 0.96 (0.92 to 0.98) |
| Austria | Dreiseitl et al.198 2009 | 26 | 121 | 1 | 310 | 0.96 (0.81 to 1.00) | 0.72 (0.67 to 0.76) |
| Turkey | Gokdemir et al.199 2011 | 12 | 25 | 1 | 410 | 0.92 (0.64 to 1.00) | 0.94 (0.92 to 0.96) |
| Italy (Modena) | Guitera et al.204 2009 | 68 | 83 | 11 | 33 | 0.86 (0.76 to 0.93) | 0.28 (0.20 to 0.38) |
| Germany | Haenssle et al.200 2010 | 32 | 146 | 8 | 8263 | 0.80 (0.64 to 0.91) | 0.98 (0.98 to 0.99) |
| Germany | Haenssle et al.237 2010 | 47 | 228 | 40 | 2373 | 0.54 (0.43 to 0.65) | 0.91 (0.90 to 0.92) |
| Spain | Morales-Callaghan et al.201 2008 | 4 | 6 | 2 | 188 | 0.67 (0.22 to 0.96) | 0.97 (0.93 to 0.99) |
| Germany and USA | Nachbar et al.202 1994 | 64 | 11 | 5 | 114 | 0.93 (0.84 to 0.98) | 0.91 (0.85 to 0.96) |
| Austria | Soyer et al.203 1995 | 61 | 17 | 4 | 77 | 0.94 (0.85 to 0.98) | 0.82 (0.73 to 0.89) |
| Pooled | 0.875 (0.784 to 0.931); SE 0.037 | 0.893 (0.792 to 0.949); SE 0.038 |
| Country | Study (first author and year of publication) | True positive (n) | False positive (n) | False negative (n) | True negative (n) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| USA | Ferris et al.207 2015 | 20 | 21 | 5 | 19 | 0.80 (0.59 to 0.93); SE 0.086 | 0.47 (0.32 to 0.64); SE 0.081 |
In the clinical pathway, once a health-care professional deems the mole/skin to be suspicious, a local biopsy is taken and sent to a pathologist for confirmation. The sensitivity and specificity of local biopsy were derived from a study that aimed to investigate how accurate and reproducible the results are of pathologists’ diagnoses of melanocytic skin lesions. 208 The results of the paper indicate that 82.8% (95% CI 81.0% to 84.5%) of melanocytic skin biopsy diagnoses would have their diagnosis verified if reviewed by a consensus reference panel of experienced pathologists. In the model, the sensitivity and specificity were assumed based on this study (Table 35).
| Local biopsy | Mean | 95% CI; SE |
|---|---|---|
| Sensitivity (assumed) | 0.828 | 0.810 to 0.845; SE 0.0089 |
| Specificity (assumed) | 0.50 | SE 0.05 |
For regional disease staging, the accompanying systematic review of the clinical evidence for the NICE guideline was the source of data for the staging of melanoma. 16 The sensitivity of SLNB in identifying micrometastatic nodal/regional disease for patients was estimated to be 86.6% (95% CI 84.6% to 88.4%), based on 47 studies with 19,607 data points. Specificity was 100%, as in the review (Table 36).
| Stage | n studies (n data points) | Prevalence (%) | Sensitivity (95% CI) (%) | Specificity (%) |
|---|---|---|---|---|
| Any | 47 (19,607) | 9–41 | 86.6 (84.6 to 88.4) | 100 |
For advanced staging, further diagnostic tests were used, including ultrasonography, CT, PET and a combination of both (PET-CT). The 2015 NICE guideline16 recommended that CT staging be offered to people with stage III or suspected stage IV melanoma. According to a meta-analysis147 of staging of distant metastasis, median sensitivity of CT scan was 51% (95% CrI 24% to 76%) and specificity was 69% (95% CrI 30% to 92%). These median estimates, along with the corresponding CrIs, were used in the model as beta distributions.
Health-state utilities
An initial search was conducted (in September 2018) in PubMed, Tufts Cost-Effectiveness Analysis Registry238 and other relevant sources (e.g. ScHARRHUD, the HERC database of mapping studies239 and PROSPERO240). However, once the most appropriate systematic review and meta-analysis relevant paper was identified, the search was truncated. 216
Search filters developed and maintained by the Canadian Agency for Drugs and Technologies in Health Information Services Filters Working Group were used. 241
PubMed
Health utilities/quality of life
“Value of Life” [mh] OR Quality of Life[mh] OR quality of life[tiab] OR Quality-Adjusted Life years[mh] OR quality adjusted life[tiab] OR qaly*[tiab] OR qald*[tiab] OR qale*[tiab] OR qtime*[tiab] OR life year[tiab] OR life years[tiab] OR disability adjusted life[tiab] OR daly*[tiab] OR sf36[tiab] OR sf 36[tiab] OR short form 36[tiab] OR shortform 36[tiab] OR short form36[tiab] OR shortform36[tiab] OR sf6[tiab] OR sf 6[tiab] OR short form 6[tiab] OR sf6d[tiab] OR sf 6d[tiab] OR short form 6d[tiab] OR sf8[tiab] OR sf 8[tiab] OR short form 8[tiab] OR sf12[tiab] OR sf 12[tiab] OR short form 12[tiab] OR sf16[tiab] OR sf 16[tiab] OR sf20[tiab] OR sf 20[tiab] OR short form 20[tiab] OR hql[tiab] OR hqol[tiab] OR h qol[tiab] OR hrqol[tiab] OR hr qol[tiab] OR hye[tiab] OR hyes[tiab] OR healthy year equivalent*[tiab] OR healthy years equivalent*[tiab] OR pqol[tiab] OR qls[tiab] OR quality of well being[tiab] OR index of wellbeing[tiab] OR qwb[tiab] OR nottingham health profile*[tiab] OR sickness impact profile[tiab] OR health status indicators[mh] OR health utilit*[tiab] OR health status[tiab] OR disutilit*[tiab] OR rosser[tiab] OR willingness to pay[tiab] OR standard gamble*[tiab] OR time trade off[tiab] OR time tradeoff[tiab] OR tto[tiab] OR hui[tiab] OR hui1[tiab] OR hui2[tiab] OR hui3[tiab] OR eq[tiab] OR euroqol[tiab] OR euro qol[tiab] OR eq5d[tiab] OR eq 5d[tiab] OR euroqual[tiab] OR euro qual[tiab] OR duke health profile[tiab] OR functional status questionnaire[tiab] OR dartmouth coop functional health assessment*[tiab] OR (utilit*[tiab] AND (valu*[tiab] OR measur*[tiab] OR health[tiab] OR life[tiab] OR estimat*[tiab] OR elicit*[tiab] OR disease[tiab] OR score*[tiab] OR weight[tiab])) OR (preference*[tiab] AND (valu*[tiab] OR measur*[tiab] OR health[tiab] OR life[tiab] OR estimat*[tiab] OR elicit*[tiab] OR disease[tiab] OR score*[tiab] OR instrument[tiab] OR instruments[tiab])).
AND
Disease
Melanoma OR skin tumor OR skin tumour OR cutaneous tumor OR cutaneous tumour OR skin cancer.
Using this search strategy, > 7000 hits were recorded (Table 37). However, once the most appropriate systematic review and meta-analysis relevant paper was identified, the search was truncated. 216
| Search facet | Results (n) |
|---|---|
| Health utilities/quality of life | 806,807 |
| Disease | 195,615 |
| Final numbers | 7676 |
Treatment costs
In all cases, patients undergo biopsy excision (at which point the disease is staged according to AJCC guidelines), followed by definitive surgery known as wide local excision. Patients with stage IA or stage IB disease undergo no further treatment. Patients with stage IIA or higher disease undergo SLNB and patients with stage IIB or higher disease undergo CT. Patients with a positive SLNB undergo follow-up surgery for lymph node involvement, comprising pre-operative CT and radical lymph node basin dissection. Patients with stage III disease receive systemic therapy. Patients with stage IV disease undergo surgery for removal of localised metastases and combination immunotherapy (Table 38). Gamma distributions were assigned to cost values for stage III and IV treatments.
| Initial treatment | Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIC | IIIA | IIIB | IIIC | IV | |
| Biopsy excision | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Definitive surgery (wide local excision) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Investigations | |||||||||
| CT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| SLNB (carried out at same time as definitive surgery) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Follow-up surgery for positive lymph nodes | |||||||||
| Pre-operative CT | ✓ | ✓ | ✓ | ||||||
| Radical lymph node dissection | ✓ | ✓ | ✓ | ||||||
| Metastatic disease | |||||||||
| Surgical removal of localised metastases | ✓ | ||||||||
| Targeted therapy (combination of dabrafenib + trametinib) | ✓ | ✓ | ✓ | ||||||
| Immunotherapy (combination of ipilimumab + nivolumab) | ✓ | ||||||||
Appendix 10 Sensitivity analyses: detailed results
Self-diagnosis is an important parameter to capture in the model. In one-way sensitivity analysis, with all values at their base case, there is little difference in terms of NMB; this is because of the low probability of recurrence. At the extreme, where no patient in the model self-diagnosed, strategies 22 (stage IA) and 77 (stage IB) have the highest NMB. When every recurrence is detected by self-diagnosis, there is no benefit in surveillance, as can be seen by the high ICERs. Tables 39 and 40 report the results of the one-way sensitivity analyses of self-diagnosis for stages IA and IB, respectively.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALY) | Incremental effectiveness (QALY) | ICER (£/QALY) | NMB (£) |
|---|---|---|---|---|---|---|
| Probability of self-diagnosis per month = 0 | ||||||
| 22 | 9895 | 21.78 | 425,637 | |||
| 15 | 10,133 | 238 | 21.78 | 0.00 | 244,139 | 425,418 |
| 23 | 10,456 | 323 | 21.78 | 0.00 | 73,394 | 425,184 |
| 1 (NICE) | 10,590 | 135 | 21.78 | 0.00 | Dominated | 424,990 |
| 19 | 10,681 | 226 | 21.78 | 0.00 | 355,202 | 424,971 |
| 16 | 11,166 | 485 | 21.79 | 0.02 | 172,490 | 424,542 |
| 21 | 12,452 | 1286 | 21.80 | 0.00 | 68,455 | 423,632 |
| 14 | 12,660 | 208 | 21.80 | 0.00 | 1,586,856 | 423,426 |
| 7 | 12,867 | 207 | 21.80 | 0.00 | 1,872,254 | 423,221 |
| 4 | 14,607 | 1740 | 21.81 | 0.00 | 280,098 | 421,605 |
| Probability of self-diagnosis per month = 0.35 | ||||||
| 22 | 12,611 | 0 | 21.89 | 0.00 | 425,144 | |
| 15 | 12,811 | 199 | 21.89 | 0.00 | 49,462,070 | 424,944 |
| 23 | 12,977 | 166 | 21.89 | 0.00 | 26,478,336 | 424,778 |
| 19 | 13,176 | 199 | 21.89 | 0.00 | 5,2041,509 | 424,579 |
| 1 (NICE) | 13,210 | 33 | 21.89 | 0.00 | Dominated | 424,546 |
| 16 | 13,555 | 379 | 21.89 | 0.00 | 44,940,623 | 424,201 |
| 21 | 14,273 | 718 | 21.89 | 0.00 | 31,383,914 | 423,483 |
| 14 | 14,472 | 199 | 21.89 | 0.00 | 60,603,316 | 423,284 |
| 7 | 14,671 | 199 | 21.89 | 0.00 | 66,783,439 | 423,085 |
| 4 | 16,186 | 1515 | 21.89 | 0.00 | 48,874,025 | 421,570 |
| Probability of self-diagnosis per month = 0.70 | ||||||
| 22 | 12,687 | 21.89 | 425,100 | |||
| 15 | 12,886 | 199 | 21.89 | 0.00 | 256,032,538 | 424,902 |
| 23 | 13,052 | 166 | 21.89 | 0.00 | 348,679,760 | 424,736 |
| 19 | 13,250 | 199 | 21.89 | 0.00 | 257,377,027 | 424,537 |
| 1 (NICE) | 13,283 | 33 | 21.89 | 0.00 | 209,731,828 | 424,504 |
| 16 | 13,628 | 345 | 21.89 | 0.00 | 310,586,952 | 424,160 |
| 21 | 14,345 | 717 | 21.89 | 0.00 | 290,721,349 | 423,442 |
| 14 | 14,544 | 199 | 21.89 | 0.00 | 280,009,293 | 423,244 |
| 7 | 14,743 | 199 | 21.89 | 0.00 | 289,522,912 | 423,045 |
| 4 | 16,254 | 1511 | 21.89 | 0.00 | 323,644,663 | 421,534 |
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALY) | Incremental effectiveness (QALY) | ICER (£/QALY) | NMB (£) |
|---|---|---|---|---|---|---|
| Probability of self-diagnosis per month = 0 | ||||||
| 77 | 9197 | 0 | 21.30 | 0.00 | 0 | 416,868 |
| 86 | 9473 | 276 | 21.31 | 0.01 | 54,335 | 416,693 |
| 80 | 9564 | 91 | 21.32 | 0.01 | 11,511 | 416,761 |
| 83 | 10,130 | 566 | 21.33 | 0.01 | 48,071 | 416,431 |
| 23 | 10,700 | 570 | 21.36 | 0.04 | 15,888 | 416,578 |
| 15 | 10,784 | 84 | 21.35 | –0.01 | Dominated | 416,200 |
| 8 | 11,529 | 829 | 21.36 | 0.00 | Dominated | 415,684 |
| 2 | 12,217 | 1518 | 21.37 | 0.00 | 357,121 | 415,145 |
| 9 | 12,678 | 461 | 21.40 | 0.03 | 16,535 | 415,242 |
| 5 | 12,812 | 134 | 21.39 | –0.01 | Dominated | 414,951 |
| 1 (NICE) | 13,312 | 634 | 21.40 | 0.00 | 193,317 | 414,674 |
| 25 | 14,770 | 1458 | 21.58 | 0.18 | 7967 | 416,876 |
| 18 | 18,185 | 3415 | 21.62 | 0.03 | 102,892 | 414,124 |
| 11 | 18,795 | 610 | 21.62 | 0.00 | 1,224,590 | 413,524 |
| 4 | 19,395 | 600 | 21.62 | 0.00 | 1,748,100 | 412,931 |
| Probability of self-diagnosis per month = 0.35 | ||||||
| 77 | 14,535 | 21.87 | 422,792 | |||
| 80 | 14,721 | 186 | 21.87 | 0.00 | 4,184,463 | 422,606 |
| 86 | 14,735 | 14 | 21.87 | 0.00 | Dominated | 422,592 |
| 83 | 15,114 | 393 | 21.87 | 0.00 | 5,512,013 | 422,215 |
| 23 | 15,242 | 128 | 21.87 | 0.00 | 2,579,499 | 422,088 |
| 15 | 15,481 | 238 | 21.87 | 0.00 | 9,204,223 | 421,850 |
| 8 | 16,060 | 579 | 21.87 | 0.00 | 6,814,888 | 421,272 |
| 2 | 16,639 | 579 | 21.87 | 0.00 | 8,145,629 | 420,695 |
| 9 | 16,755 | 116 | 21.87 | 0.00 | 1,908,787 | 420,580 |
| 5 | 16,980 | 225 | 21.87 | 0.00 | 21,786,488 | 420,355 |
| 25 | 17,166 | 186 | 21.87 | 0.00 | 533,666 | 420,176 |
| 1 (NICE) | 17,334 | 168 | 21.87 | 0.00 | Dominated | 420,002 |
| 18 | 20,094 | 2928 | 21.87 | 0.00 | 4,847,788 | 417,260 |
| 11 | 20,673 | 579 | 21.87 | 0.00 | 7,317,353 | 416,683 |
| 4 | 21,252 | 579 | 21.87 | 0.00 | 8,725,665 | 416,105 |
| Probability of self-diagnosis per month = 0.70 | ||||||
| 77 | 14,718 | 21.88 | 422,861 | |||
| 80 | 14,903 | 185 | 21.88 | 0.00 | 33,226,479 | 422,677 |
| 86 | 14,916 | 13 | 21.88 | 0.00 | 12,594,062 | 422,663 |
| 83 | 15,292 | 376 | 21.88 | 0.00 | 32,684,368 | 422,287 |
| 23 | 15,421 | 129 | 21.88 | 0.00 | 70,535,481 | 422,159 |
| 15 | 15,656 | 235 | 21.88 | 0.00 | 28,997,710 | 421,924 |
| 8 | 16,231 | 575 | 21.88 | 0.00 | 35,696,084 | 421,349 |
| 2 | 16,807 | 575 | 21.88 | 0.00 | 38,065,537 | 420,774 |
| 9 | 16,923 | 116 | 21.88 | 0.00 | 40,943,451 | 420,658 |
| 5 | 17,146 | 223 | 21.88 | 0.00 | 35,271,500 | 420,434 |
| 25 | 17,339 | 193 | 21.88 | 0.00 | 7,692,907 | 420,242 |
| 1 (NICE) | 17,498 | 159 | 21.88 | 0.00 | Dominated | 420,083 |
| 18 | 20,252 | 2913 | 21.88 | 0.00 | 31,305,095 | 417,331 |
| 11 | 20,827 | 575 | 21.88 | 0.00 | 35,954,371 | 416,757 |
| 4 | 21,402 | 575 | 21.88 | 0.00 | 38,343,472 | 416,182 |
Recurrence is the driving parameter that needs to be captured in the model. In one-way sensitivity analysis, with all values set at their base-case value, it can be seen that strategies 22 (stage IA) and 77 (stage IB) have the highest NMB (Tables 41 and 42).
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALY) | Incremental effectiveness (QALY) | ICER (£/QALY) | NMB (£) |
|---|---|---|---|---|---|---|
| Probability of recurrence per month = 0.00 | ||||||
| 22 | 10,664 | 21.89 | 427,152 | |||
| 15 | 10,863 | 199 | 21.89 | 0.00 | Dominated | 426,953 |
| 23 | 11,029 | 365 | 21.89 | 0.00 | Dominated | 426,787 |
| 19 | 11,228 | 565 | 21.89 | 0.00 | Dominated | 426,588 |
| 1 (NICE) | 11,261 | 598 | 21.89 | 0.00 | Dominated | 426,555 |
| 16 | 11,607 | 943 | 21.89 | 0.00 | Dominated | 426,209 |
| 21 | 12,325 | 1661 | 21.89 | 0.00 | Dominated | 425,491 |
| 14 | 12,524 | 1860 | 21.89 | 0.00 | Dominated | 425,292 |
| 7 | 12,724 | 2060 | 21.89 | 0.00 | Dominated | 425,093 |
| 4 | 14,237 | 3573 | 21.89 | 0.00 | Dominated | 423,579 |
| Probability of recurrence per month = 0.14 | ||||||
| 22 | 85,732 | 21.09 | 336,109 | |||
| 15 | 87,982 | 729 | 21.09 | 0.00 | Dominated | 333,864 |
| 23 | 93,327 | 3777 | 21.09 | 0.00 | Dominated | 330,148 |
| 1 (NICE) | 93,346 | 20 | 21.09 | 0.00 | Dominated | 330,132 |
| 19 | 95,599 | 2273 | 21.09 | 0.00 | Dominated | 327,919 |
| 16 | 101,055 | 5456 | 21.09 | 0.00 | Dominated | 322,543 |
| 21 | 113,879 | 12,824 | 21.10 | 0.00 | 5,724,843 | 309,974 |
| 14 | 115,855 | 1976 | 21.10 | 0.00 | 8,807,771 | 308,033 |
| 7 | 117,642 | 1787 | 21.10 | 0.00 | Dominated | 306,275 |
| 4 | 139,469 | 21,827 | 21.10 | 0.00 | 7,576,950 | 284,776 |
| Probability of recurrence per month = 0.29 | ||||||
| 22 | 90,527 | 21.06 | 330,643 | |||
| 15 | 93,823 | 2127 | 21.06 | 0.00 | Dominated | 327,352 |
| 23 | 97,222 | 2176 | 21.06 | 0.00 | Dominated | 323,958 |
| 1 (NICE) | 99,565 | 1093 | 21.06 | 0.00 | Dominated | 321,617 |
| 19 | 100,517 | 2045 | 21.06 | 0.00 | Dominated | 320,667 |
| 16 | 107,163 | 2298 | 21.06 | 0.00 | Dominated | 314,030 |
| 21 | 120,590 | 12,033 | 21.06 | 0.00 | 11,407,236 | 300,633 |
| 14 | 123,570 | 1433 | 21.06 | 0.00 | Dominated | 297,655 |
| 7 | 126,330 | 1148 | 21.06 | 0.00 | Dominated | 294,900 |
| 4 | 152,887 | 24,879 | 21.06 | 0.00 | 14,663,184 | 268,386 |
| Probability of recurrence per month = 0.86 | ||||||
| 22 | 93,856 | 0 | 21.03 | 326,837 | ||
| 15 | 97,741 | 2967 | 21.03 | 0.00 | Dominated | 322,953 |
| 23 | 100,982 | 2288 | 21.03 | 0.00 | Dominated | 319,715 |
| 19 | 104,867 | 2905 | 21.03 | 0.00 | Dominated | 315,831 |
| 1 (NICE) | 105,506 | 3543 | 21.03 | 0.00 | Dominated | 315,193 |
| 16 | 112,244 | 2443 | 21.04 | 0.00 | Dominated | 308,458 |
| 21 | 126,228 | 12,904 | 21.04 | 0.00 | 31,148,004 | 294,486 |
| 14 | 130,113 | 2683 | 21.04 | 0.00 | Dominated | 290,602 |
| 7 | 133,992 | 2643 | 21.04 | 0.00 | Dominated | 286,725 |
| 4 | 163,485 | 28,223 | 21.04 | 0.00 | 38,672,722 | 257,250 |
| Probability of recurrence per month = 1.0 | ||||||
| 22 | 94,101 | 21.03 | 326,556 | |||
| 15 | 98,004 | 3002 | 21.03 | 0.00 | Dominated | 322,655 |
| 23 | 101,258 | 2321 | 21.03 | 0.00 | Dominated | 319,403 |
| 19 | 105,161 | 2941 | 21.03 | 0.00 | Dominated | 315,502 |
| 1 (NICE) | 105,809 | 3590 | 21.03 | 0.00 | Dominated | 314,854 |
| 16 | 112,570 | 2479 | 21.03 | 0.00 | Dominated | 308,096 |
| 21 | 126,614 | 12,987 | 21.03 | 0.00 | 35,499,571 | 294,062 |
| 14 | 130,517 | 2725 | 21.03 | 0.00 | Dominated | 290,161 |
| 7 | 134,419 | 2692 | 21.03 | 0.00 | Dominated | 286,260 |
| 4 | 164,041 | 28,377 | 21.03 | 0.00 | 43,776,631 | 256,655 |
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALY) | Incremental effectiveness (QALY) | ICER (£/QALY) | NMB (£) |
|---|---|---|---|---|---|---|
| Probability of recurrence per month = 0.00 | ||||||
| 77 | 10,664 | 21.89 | 427,152 | |||
| 80 | 10,850 | 186 | 21.89 | 0.00 | Dominated | 426,966 |
| 86 | 10,863 | 199 | 21.89 | 0.00 | Dominated | 426,953 |
| 83 | 11,241 | 577 | 21.89 | 0.00 | Dominated | 426,575 |
| 23 | 11,370 | 706 | 21.89 | 0.00 | Dominated | 426,446 |
| 15 | 11,607 | 943 | 21.89 | 0.00 | Dominated | 426,209 |
| 8 | 12,184 | 1520 | 21.89 | 0.00 | Dominated | 425,632 |
| 2 | 12,762 | 2098 | 21.89 | 0.00 | Dominated | 425,055 |
| 9 | 12,878 | 2214 | 21.89 | 0.00 | Dominated | 424,938 |
| 5 | 13,103 | 2439 | 21.89 | 0.00 | Dominated | 424,714 |
| 25 | 13,293 | 2630 | 21.89 | 0.00 | Dominated | 424,523 |
| 1 (NICE) | 13,456 | 2792 | 21.89 | 0.00 | Dominated | 424,361 |
| 18 | 16,215 | 5551 | 21.89 | 0.00 | Dominated | 421,601 |
| 11 | 16,792 | 6128 | 21.89 | 0.00 | Dominated | 421,024 |
| 4 | 17,370 | 6706 | 21.89 | 0.00 | Dominated | 420,446 |
| Probability of recurrence per month = 0.29 | ||||||
| 77 | 83,849 | 19.45 | 305,105 | |||
| 86 | 87,146 | 2208 | 19.45 | 0.00 | Dominated | 301,823 |
| 80 | 87,253 | 2314 | 19.45 | 0.00 | Dominated | 301,718 |
| 83 | 93,929 | 5546 | 19.45 | 0.00 | 10,154,431 | 295,072 |
| 23 | 96,771 | 1610 | 19.45 | 0.00 | 22,053,492 | 292,253 |
| 15 | 100,479 | 3708 | 19.45 | 0.00 | 7,168,015 | 288,555 |
| 8 | 109,631 | 9152 | 19.45 | 0.01 | 4,262,455 | 279,446 |
| 2 | 118,106 | 8475 | 19.46 | 0.00 | 4,264,035 | 271,011 |
| 9 | 122,044 | 3938 | 19.46 | 0.00 | 2,612,543 | 267,103 |
| 5 | 124,337 | 2293 | 19.46 | 0.00 | 7,351,939 | 264,816 |
| 1 (NICE) | 130,510 | 6173 | 19.46 | 0.00 | 3,689,587 | 258,677 |
| 25 | 131,498 | 988 | 19.47 | 0.01 | 89,416 | 257,910 |
| 18 | 182,194 | 50,697 | 19.49 | 0.02 | 2,364,881 | 207,642 |
| 11 | 191,345 | 9151 | 19.49 | 0.00 | 4,258,565 | 198,535 |
| 4 | 199,818 | 8473 | 19.50 | 0.01 | 4,260,292 | 190,102 |
| Probability of recurrence per month = 0.43 | ||||||
| 77 | 85,331 | 19.42 | 303,055 | |||
| 80 | 88,845 | 2542 | 19.42 | 0.00 | Dominated | 299,552 |
| 86 | 89,018 | 2715 | 19.42 | 0.00 | Dominated | 299,379 |
| 83 | 96,132 | 6279 | 19.42 | 0.00 | 13,633,220 | 292,289 |
| 23 | 98,669 | 1448 | 19.42 | 0.00 | Dominated | 289,767 |
| 15 | 102,998 | 5778 | 19.42 | 0.00 | 13,211,340 | 285,446 |
| 8 | 113,330 | 10,332 | 19.42 | 0.00 | 6,288,715 | 275,147 |
| 2 | 123,180 | 9850 | 19.43 | 0.01 | 6,261,490 | 265,328 |
| 9 | 126,352 | 3172 | 19.43 | 0.00 | 3,522,177 | 262,174 |
| 5 | 129,613 | 3261 | 19.43 | 0.00 | 8,854,734 | 258,920 |
| 25 | 134,481 | 4867 | 19.44 | 0.01 | 580,263 | 254,221 |
| 1 (NICE) | 136,201 | 1721 | 19.43 | –0.01 | Dominated | 252,356 |
| 18 | 188,584 | 54,104 | 19.45 | 0.02 | 3,527,071 | 200,424 |
| 11 | 198,923 | 10,338 | 19.45 | 0.00 | 6,283,914 | 190,119 |
| 4 | 208,778 | 9855 | 19.45 | 0.00 | 6,256,797 | 180,295 |
| Probability of recurrence per month = 0.86 | ||||||
| 77 | 86,839 | 19.39 | 300,966 | |||
| 80 | 90,460 | 2770 | 19.39 | 0.00 | Dominated | 297,351 |
| 86 | 90,725 | 3034 | 19.39 | 0.00 | Dominated | 297,087 |
| 83 | 98,096 | 6486 | 19.39 | 0.00 | 24,721,481 | 289,728 |
| 23 | 100,582 | 1536 | 19.39 | 0.00 | Dominated | 287,250 |
| 15 | 105,210 | 6164 | 19.39 | 0.00 | 24,988,057 | 282,627 |
| 8 | 116,463 | 11,253 | 19.39 | 0.00 | 12,137,137 | 271,392 |
| 2 | 127,699 | 11,237 | 19.39 | 0.00 | 12,000,331 | 260,175 |
| 9 | 129,954 | 2254 | 19.39 | 0.00 | 5,864,334 | 257,928 |
| 5 | 134,327 | 4373 | 19.39 | 0.00 | 15,127,386 | 253,560 |
| 25 | 137,448 | 3121 | 19.40 | 0.01 | 744,399 | 250,523 |
| 1 (NICE) | 141,191 | 3743 | 19.40 | 0.00 | Dominated | 246,709 |
| 18 | 193,797 | 56,349 | 19.41 | 0.01 | 6,909,043 | 194,337 |
| 11 | 205,054 | 11,257 | 19.41 | 0.00 | 12,132,357 | 183,099 |
| 4 | 216,295 | 11,241 | 19.41 | 0.00 | 11,995,711 | 171,876 |
| Probability of recurrence per month = 1.00 | ||||||
| 77 | 87,059 | 19.39 | 300,662 | |||
| 80 | 90,696 | 2803 | 19.39 | 0.00 | Dominated | 297,031 |
| 86 | 90,961 | 3069 | 19.39 | 0.00 | Dominated | 296,765 |
| 83 | 98,364 | 6536 | 19.39 | 0.00 | 28,314,236 | 289,373 |
| 23 | 100,861 | 1567 | 19.39 | 0.00 | Dominated | 286,883 |
| 15 | 105,509 | 6215 | 19.39 | 0.00 | 28,631,462 | 282,240 |
| 8 | 116,812 | 11,304 | 19.39 | 0.00 | 14,008,860 | 270,952 |
| 2 | 128,117 | 11,304 | 19.39 | 0.00 | 13,827,533 | 259,664 |
| 9 | 130,361 | 2245 | 19.39 | 0.00 | 6,806,023 | 257,426 |
| 5 | 134,772 | 4411 | 19.39 | 0.00 | 17,294,387 | 253,020 |
| 25 | 137,881 | 3108 | 19.39 | 0.00 | 860,915 | 249,984 |
| 1 (NICE) | 141,666 | 3785 | 19.39 | 0.00 | Dominated | 246,138 |
| 18 | 194,464 | 56,584 | 19.40 | 0.01 | 8,009,114 | 193,542 |
| 11 | 205,772 | 11,307 | 19.40 | 0.00 | 14,004,157 | 182,250 |
| 4 | 217,080 | 11,308 | 19.40 | 0.00 | 13,823,009 | 170,959 |
Hypothetical prognostic test
The hypothetical prognostic test produced the results in Table 43 in terms of cost, effectiveness, ICERs and NMB.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALY) | Incremental effectiveness (QALY) | ICER (£/QALY) | NMB (£) |
|---|---|---|---|---|---|---|
| Stage IA | ||||||
| 22 | 8456 | 14.72 | 285,985 | |||
| Prognostic | 8618 | 162 | 14.74 | 0.02 | 12,344 | 286,086 |
| 1 (NICE) | 9277 | 658 | 14.74 | 0.00 | 146,685 | 285,517 |
| Stage IB | ||||||
| 77 | 9457 | 14.56 | 281,823 | |||
| Prognostic | 10,099 | 642 | 14.57 | 0.01 | 77,015 | 281,348 |
| 23 | 10,235 | 136 | 14.58 | 0.01 | 11,273 | 281,453 |
| 1 (NICE) | 12,606 | 2371 | 14.60 | 0.02 | 158,111 | 279,347 |
List of abbreviations
- AGREE II
- Appraisal of Guidelines for Research & Evaluation II
- AJCC
- American Joint Committee on Cancer
- AUC
- area under the curve
- AUROC
- area under the receiver operating characteristic
- BAD
- British Association of Dermatologists
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CHARMS
- CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies
- CI
- confidence interval
- CNS
- clinical nurse specialist
- CrI
- credible interval
- CT
- computerised tomography
- EPV
- event per variable
- EQ-5D
- EuroQol-5 Dimensions
- ESMO
- European Society of Medical Oncologists
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FNAC
- fine-needle aspiration cytology
- FNB
- fine-needle biopsy
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HERC
- Health Economics Research Centre
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- ILI
- isolated limb infusion
- ILP
- isolated limb perfusion
- mCM
- modified Connor–Mosimann
- MRI
- magnetic resonance imaging
- NCCN®
- National Comprehensive Cancer Network®
- NCRAS
- National Cancer Registration and Analysis Service
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OR
- odds ratio
- Portable Document Format
- PET
- positron emission tomography
- PET-CT
- positron emission tomography-computerised tomography
- PHE
- Public Health England
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST
- Prediction model Risk Of Bias ASsessment Tool
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies-2
- RCT
- randomised controlled trial
- RGP
- radial growth phase
- ROC
- receiver operating curve
- RR
- risk ratio
- SAVI
- Sheffield Accelerated Value of Information
- ScHARRHUD
- School of Health and Related Research Health Utilities Database
- SD
- standard deviation
- SE
- standard error
- SLNB
- sentinel lymph node biopsy
- SSE
- skin self-examination
- TA
- technology appraisal
- TNM
- tumour node metastasis
- TRIPOD
- Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis
- UV
- ultraviolet
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25640).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
