Notes
Article history
This issue of the Health Technology Assessment journal series contains a project commissioned/managed by Better Methods, Better Research (BMBR). The Medical Research Council (MRC) is working with NIHR to deliver the single joint health strategy, which includes BMBR as part of the delivery model. This was launched as the Methodology Research Programme (MRP) in 2008. MRC is lead funding partner for MRP and part of this programme is the joint MRC–NIHR funding panel ‘The Methodology Research Programme Panel’.
To strengthen the evidence base for health research, BMBR aims to ensure optimal research methods are being used to advance biomedical, health and care focused research, policy and delivery. In addition to the MRC and NIHR funding partners, the BMBR takes into account the needs of other stakeholders including the devolved administrations, industry R&D, and regulatory/ advisory agencies and other public bodies. The BMBR funds investigator-led and needs-led research proposals from across the UK that develops and delivers ways to improve the research methods being used by others. In addition to the standard MRC terms and conditions on governance of good research practice, projects commissions/ managed by BMBR are expected to provide a detailed report on the research findings and may publish the findings in the Health Technology Assessment, if supported by NIHR funds.
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Parker et al. This work was produced by Parker et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Parker et al.
Introduction
What is covered in this report?
This report focuses on randomised Studies Within A Trial (SWATs) testing the effectiveness of recruitment and retention strategies. The structure of this report begins by presenting the background to the PROMoting THE Use of Studies Within A Trial (PROMETHEUS) programme (see Chapter 1). In Chapter 2, we present the methods used in PROMETHEUS, followed by the results (see Chapter 3) and then the discussion of the programme (see Chapter 4).
Subsequent chapters focus on the lessons learnt from PROMETHEUS and how this can inform future work focused on SWATs. This includes lessons learnt for; patient and public involvement (PPI) (see Chapter 5), the development of SWATs and its funding (see Chapter 6), and ethical and other governance approvals (see Chapter 7). In Chapter 8, we focus on different approaches to implementing SWATs, using case studies of standalone SWATs, as well as case studies of co-ordinated SWATs, undertaken in a co-ordinated way across multiple host trials simultaneously. Chapter 9 reports lessons learnt for analysing SWATs, and Chapter 10 on lessons learnt for reporting SWATs.
At each point, we provide an outline of key points to consider, using specific examples of work from the PROMETHEUS programme to illustrate our point. Wherever relevant, we highlight what different stakeholder groups such as trial management teams, PPI partners, trial oversight committees, those overseeing trial governance, statisticians, journals, and peer reviewers can learn from the PROMETHEUS experience.
Finally, we discuss recommendations for future research practice, direction and support (see Chapter 11), and then present conclusions (see Chapter 12).
Who should use this report?
This report has been written for anyone with an interest in using randomised controlled trials to inform evidence-based health and social care. Lessons learnt that we detail in this report will be of use to those interested in how the design and delivery of future trials can be improved to benefit patient health and social care. This includes individuals and organisations involved in funding trials; those who design, plan and undertake trials such as Chief Investigators and Trial Managers; trial oversight committees; those providing ethical and other regulatory approvals; patient and public partners in trials; trial infrastructure organisations supporting trial delivery such as the UK Clinical Research Networks (CRNs), Trials Units and the Research Design Service. The report will be of use to those recruiting and retaining trial participants, as well as those interested in trials methodology.
The report has been written for those with an understanding of trials who are new to SWATs; those with some experience of SWATs who want to learn more; and those with SWAT experience who may wish to use our experience to consider possible methodological innovations of their own. This report is also written for journal editors publishing SWATs, as well as those undertaking peer reviews of SWATs.
Chapter 1 Background to the PROMETHEUS programme
The importance of randomised controlled trials
Randomised controlled trials (‘trials’) attempt to improve the health and care of populations and minimise the potentially harmful effects of treatments and interventions. Trials are undertaken on the basis that there are uncertainties about the effects of treatments, and such treatments are tested to adequately reduce these uncertainties. Consequently, trials are usually accepted as the foundation for evidence-based practice,1 the most complete paradigm for delivering safe and effective health and care for patients and citizens. Globally in 2022, there were more than 400,000 trials being undertaken across 220 countries. 2 The UK National Institute for Health and Care Research (NIHR) invested £250 million on research, predominantly in the form of trials, in 2019/2020, and £373 million in 2020–1. 3,4 To be efficient, trials need to recruit large numbers of participants quickly – often in excess of 1000 participants. 5 To have external validity, a generalisable sample needs to be recruited from all relevant patient and population groups, and to avoid post-randomisation selection bias, retention needs to be kept as high as possible.
Recruitment and retention difficulties in trials
Despite the importance of trials, they are susceptible to both poor participant recruitment and retention. As a consequence of this, many trials fail to recruit to both time and budget, often with lower participant retention than anticipated. 5 The cost of poor participant recruitment can be huge,6 contributing to significant research waste. 7,8 Furthermore, poor recruitment has a detrimental impact on mortality and morbidity, as this increases the time to the implementation of effective care and treatments. An analysis of the randomised evaluation of COVID-19 therapy (RECOVERY) trial, which recruited only 10% of eligible patients, showed that had recruitment been 50% or more of eligible patients, there would have been a significant improvement in lives saved due to earlier reporting. 9 Similarly, poor participant retention, which is often overshadowed by the research focus of poor participant recruitment,10 can also be detrimental to the success of a study. Poor retention may lead to the underpowering of a study and cause the estimates of an intervention’s effect to be biased. 11 Aligned with this, a priority-setting exercise involving 85% of UK Clinical Trials Units (CTUs) placed recruitment and retention as the top two priorities for methodological research,12 cementing the need to identify and implement tools within trials that help to improve both.
Study Within A Trial methodology
To date, there has been a distinct lack of robust evidence to inform trial design and conduct, including for recruitment and retention. However, there is an increasing international movement to improve the efficiency and successful delivery of trials by using robust research methods. 13–15 These robust studies use methods that are embedded within real ‘host’ trials and are referred to as ‘SWATs’. A SWAT has been defined as a: ‘self-contained study that has been embedded within a host trial with the aim of evaluating or exploring alternative ways of delivering or organising a particular trial process’. 15
Treweek et al. (2018)15 outline several key features of a SWAT. Firstly, SWATs aim to resolve key uncertainties around trial processes. Secondly, a SWAT is embedded within a host trial, but it should have a formal protocol, in the same way as the host trial. Thirdly, a SWAT must not affect the scientific integrity of the host trial, its rationale or outcome measures. Fourthly, a SWAT can be either tested in a single trial or embedded across multiple host trials in a co-ordinated way. Finally, SWATs provide evidence to inform the design and conduct of future trials; however, they may also produce evidence to inform decisions about the host trial.
SWATs can adopt a range of different methods including qualitative,16,17 mixed methods18 and before-and-after designs. 19 SWATs can be used to understand and refine implementation processes. 20 SWATs can also be non-randomised21 or randomised. 22
Addressing recruitment and retention difficulties using SWATs
Randomised SWATs are the most rigorous methods for evaluating the effectiveness of strategies for improving participant recruitment and retention in trials. 23 Two Cochrane reviews identified 150 SWATs of strategies to increase recruitment and/or retention in trials;23,24 however, effective, evidence-based strategies are rare and where evaluations exist, they tend to occur in the context of a single trial, meaning that they usually have limited statistical power and their effects across different trial contexts are unclear. 23 The most recently published Cochrane review on retention interventions concluded that there was no high-certainty evidence for any of the evaluated strategies, as assessed by Grading of Recommendations, Assessment, Development and Evaluations (GRADE). 24
Prior to the PROMETHEUS programme, the Medical Research Council (MRC) START programme was launched25,26 – a feasibility study that successfully developed the conceptual, methodological and logistical framework to improve recruitment through the embedding of two recruitment strategies in 12 host trials in primary care and developed reporting guidelines for embedded trials. 25,27 In addition, since 2014, the Health Research Board – Trials Methodology Research Network (HRB-TMRN) Ireland has supported and funded Irish researchers to conduct methodological studies to improve the efficient conduct of future trials including SWATs, offering funding of up to €25,000 for evaluations. 28 More recently, the NIHR Health Technology Assessment (HTA) programme is encouraging applications to embed at least one high-quality SWAT with funding of up to £10,000 per host trial. 29 The TRials Engagement in Children and Adolescents (TRECA) study, also funded by the NIHR, aimed to develop multimedia interventions to improve the quality of decision-making about recruitment to trials involving children and young people with long-term conditions and subsequently tested them using SWATs. 30
Aims and objectives
The PROMETHEUS programme aimed to build on the work of these previous initiatives by making the embedding of recruitment and retention SWATs within host trials standard practice across multiple CTUs, working to at least double the number of SWATs produced by the MRC START project, given a similar level of funding. This was to be achieved through pump-priming and facilitating the start of at least 25 SWATs within 30 months. The ultimate aim was to make the inclusion of SWATs routine practice when conducting a trial in a CTU.
Chapter 2 PROMETHEUS methods
PROMETHEUS preparatory work
Prior to the initiation of the PROMETHEUS programme, a network of 10 CTUs, 1 primary care research centre in the UK and the HRB-TMRN in the Republic of Ireland was established, each of whom committed to embedding either a randomised controlled recruitment and/or retention SWAT within at least two host trials.
Promising recruitment and retention strategies (which had some evidence of benefit but with substantial uncertainty) were identified from a variety of sources. These sources included Cochrane systematic reviews,23,31 the SWAT Repository Store,32 and the priorities of recruitment and retention strategies identified by CTUs. 33 In addition, the PRioRiTy list of top 10 unanswered questions on trial recruitment, for which there is no current evidence, was also reviewed. 34 A PPI panel was also convened to highlight the top priority strategies for evaluation (see Chapter 5).
The identified strategies were then prioritised if they met one or more of the following criteria:
-
Strategies that had already been evaluated with results published in peer-reviewed journal publications.
-
Strategies currently under evaluation.
-
Easy to implement strategies, that is those requiring little additional resource (input or cost) from the host trial, as assessed by the PROMETHEUS investigators. Such strategies might not have a large impact; however, they might still make a useful contribution to the evidence base, based on marginal gains and cost effectiveness.
-
Had the potential to significantly impact participant retention or recruitment (which are often the more challenging, expensive strategies to implement).
-
Strategies identified by host trial teams as suitable for testing in a SWAT within their trial.
Prioritisation decisions were made through group discussion and consensus. These priorities formed an initial strategy priority list of seven recruitment and eight retention strategies (Table 1). The priority of these strategies was reassessed and rearranged accordingly throughout the programme, based on emerging SWAT evidence.
| Recruitment strategies |
|---|
| Retention strategies |
|
|
Eligibility criteria
Host trials
To be eligible for PROMETHEUS funding, host trials were required to meet the following criteria:
-
Registered or eligible for registration on the UK CRN Portfolio. 35
-
In the planning phase, recruiting or following up participants, or be in the process of applying for ethics permission (i.e. any trial stage bar the point of trial closure).
-
Willing to apply for ethics permission or amendment to undertake at least one SWAT of a recruitment or retention strategy.
-
Willing to randomise and deliver the recruitment or retention strategy according to a shared protocol.
-
Willing to share data with the PROMETHEUS team including patient-level data to allow individual patient-level meta-analysis.
-
Willing to use or register their SWAT on the Northern Ireland Network for Trials Methodology Research SWAT Repository, a free-to-use online database of ongoing SWATs if the strategy being evaluated is not already registered. 32
-
Able to provide evidence of funding for the host trial (such as a letter from the funder).
SWAT interventions
PROMETHEUS prioritised a broad list of recruitment and retention strategies that could be evaluated (see Table 1); however, host trials could also evaluate their own strategies if they wished to. Support included assistance in writing SWAT protocols; provision of templates and guidance in achieving Research Ethics Committee (REC) and Health Research Authority (HRA) approvals; guidance on writing and submitting SWATs for publication.
PROMETHEUS programme outcomes
PROMETHEUS was designed to analyse the effectiveness of recruitment and retention interventions within the context of a single host trial, as well as across a range of different trials through the synthesis of similar SWATs results.
Sample size
The programme aimed to implement at least 25 SWATs across eligible host trials. Generally, as the sample size of SWATs are driven by the size of the host trial, single SWATs are often underpowered; a known and accepted attribute of most SWATs is that while sample size calculations may be made, formal power calculations are not required. 36 Instead, the SWAT’s sample size is usually driven by the host trial. Therefore, it was always planned that, where appropriate, SWAT intervention results were to be aggregated. To reduce study heterogeneity, studies were encouraged to follow common SWAT protocols to promote homogeneity in interventions in readiness for pooling data (see Chapter 9). This aggregation of SWATs was intended to help with the detection of small differences and provide evidence as to whether results might generalise to a range of contexts, providing evidence with greater external validity.
Host trial recruitment
Eligible host trials were largely identified and recruited through a combination of the programme collaborators, advertisement on the University of York Trials Unit (YTU) web page, e-mails to all registered UK CTUs and conference presentations such as at the International Clinical Trials Methodology Conference 2019. Following their identification, trial teams were provided with a PROMETHEUS Information Sheet, invited to submit an expression of interest form, and apply for funding from the programme for up to £5000 per SWAT embedded in their host trial. The funding application consisted of submitting a SWAT protocol, including a project timetable and an outline of costs. To support the development of the SWAT protocols, host trial teams were provided with the opportunity to meet with PROMETHEUS team members for support to determine an appropriate recruitment and/or retention strategy.
Two independent members of the PROMETHEUS programme peer-reviewed each host trial application and protocol to ensure methodologically robust replicable research was planned. Reviewers were asked to report their peer review comments and scores using a Peer Review Assessment Form, which was adapted from the peer review form used by the HRB-TMRN. 28 Peer reviewers were asked to comment on the following:
-
eligibility;
-
priority and scientific quality;
-
costings; and
-
overall rating of the application.
Randomisation
Typically, randomisation was completed by the host trial team and therefore the methods of randomisation, allocation concealment, and implementation were applied at the level of the host trial.
Blinding
Often it is deemed that SWATs do not require individuals to provide participation consent due to them being generally low risk, rarely imposing additional burden on a participant and due to potential risks of confusing the participant as to what they are consenting to. 23 As such, participants were not blind to their received intervention [e.g. a thank-you card or short message service (SMS)], but they were unaware of their participation in the SWAT.
Data collection methods
Studies Within A Trial data were collected in line with both the relevant SWAT and host trial protocols. The PROMETHEUS team provided trial teams with support to determine appropriate data collection methods where required. The funded teams were asked to provide the PROMETHEUS team with the data for each SWAT, with the aim of collating these in pooled analyses.
Statistical methods
To enable pooling of findings, a standardised framework was established, similar to that of MRC START. 25,27 Template protocols with standard outcomes and a template Statistical Analysis Plan (SAP) were designed. Trial teams were encouraged to analyse the outcomes in terms of absolute differences in recruitment and retention rates, as appropriate and to provide a cost per recruited or retained participant where possible. The primary outcome measure was compared across intervention and control arms using logistic regression. For SWATs of retention, teams also looked at elements such as: time to, and completeness of, and responses to follow-up outcome measures. Where possible, odds ratios from multiple SWATs on the same or similar interventions were combined using a random-effects meta-analysis, ideally using a one-stage approach if the individual patient-level data were available.
Data monitoring
A Project Management Group (PMG) was established to oversee the management of the SWATs work consisting of the project Statistician, Research Fellows, and other co-applicants, and was chaired by the Chief Investigator (author DJT). The role of the PMG was to monitor all aspects of the conduct and progress of the study. The PMG met quarterly by teleconference, with annual face-to-face meetings where feasible, meeting more frequently when there was a need to monitor the programme’s progress more closely.
Harms
Due to the nature of the SWAT strategies, no strategy was likely to be responsible for any health-related adverse events. Therefore, adverse event reporting and review remained with the host trial teams with data not being collected for the PROMETHEUS programme.
Auditing
The PROMETHEUS team maintained contact with each of the host trials throughout the duration of their SWAT conduct, requesting and routinely monitoring their SWAT progress.
Research ethics approval
Each individual SWAT obtained approval from the host trial’s REC, and institutional governance committees as needed. This approval was sought as part of the initial study application or as an amendment, depending on the status of the study at the time of SWAT implementation and/or the nature of the SWAT. Careful consideration was given, during the application as to whether informed consent was required for SWAT participation. Most SWATs also required approval from National Health Service (NHS) site Research and Development (R&D) departments, in line with HRA procedure, prior to implementation. Given that participants may not have provided informed consent for SWAT participation, patient identifiable information was held by the host trial and not released as part of the SWAT data set.
Consent or assent
Participants were not informed of their involvement, as it was not appropriate and may have affected the host trial outcomes by resentful demoralisation. 37 Providing the SWAT did not involve knowingly withholding pertinent information about participant involvement within it, and involvement was non-invasive, it was deemed appropriate that patients were not informed about the SWAT and informed consent for involvement was not obtained.
Confidentiality
All SWAT data transferred to the PROMETHEUS team via trial teams was done anonymously; for instance, by removing identifiers such as date of birth (e.g. simply putting age) and participant identity number (e.g. hospital number) and then randomly sorting the data. This ensured that it would not be possible to re-identify participants in the data set, in line with General Data Protection Regulation (GDPR) requirements with individual participants being identified by their host trial identification number only, or linked SWAT identification number if the host trial identification were removed. All electronic records were stored on a secure server.
Access to data
PROMETHEUS access to the data for each individual SWAT was obtained via signed data-sharing agreements in line with the principles for access to, and use of, MRC-funded research data report,38 negotiated at the outset. Ongoing access to the data depended on agreement with the individual host trial teams, with agreements about authorship and dissemination of results from the individual studies and the combined data set. Subject to the consent of the host trial teams, primary data were made available in an anonymised format suitable for release in the public domain.
Chapter 3 PROMETHEUS results
Host trials funded, numbers of recruitment Studies Within A Trial funded, number of retention Studies Within A Trial funded
In total, the PROMETHEUS programme supported 42 SWATs, which were implemented within 31 host trials, across 13 CTUs and Research Centres (Figure 1).
FIGURE 1.
Flow chart of SWATs funded by the PROMETHEUS programme.
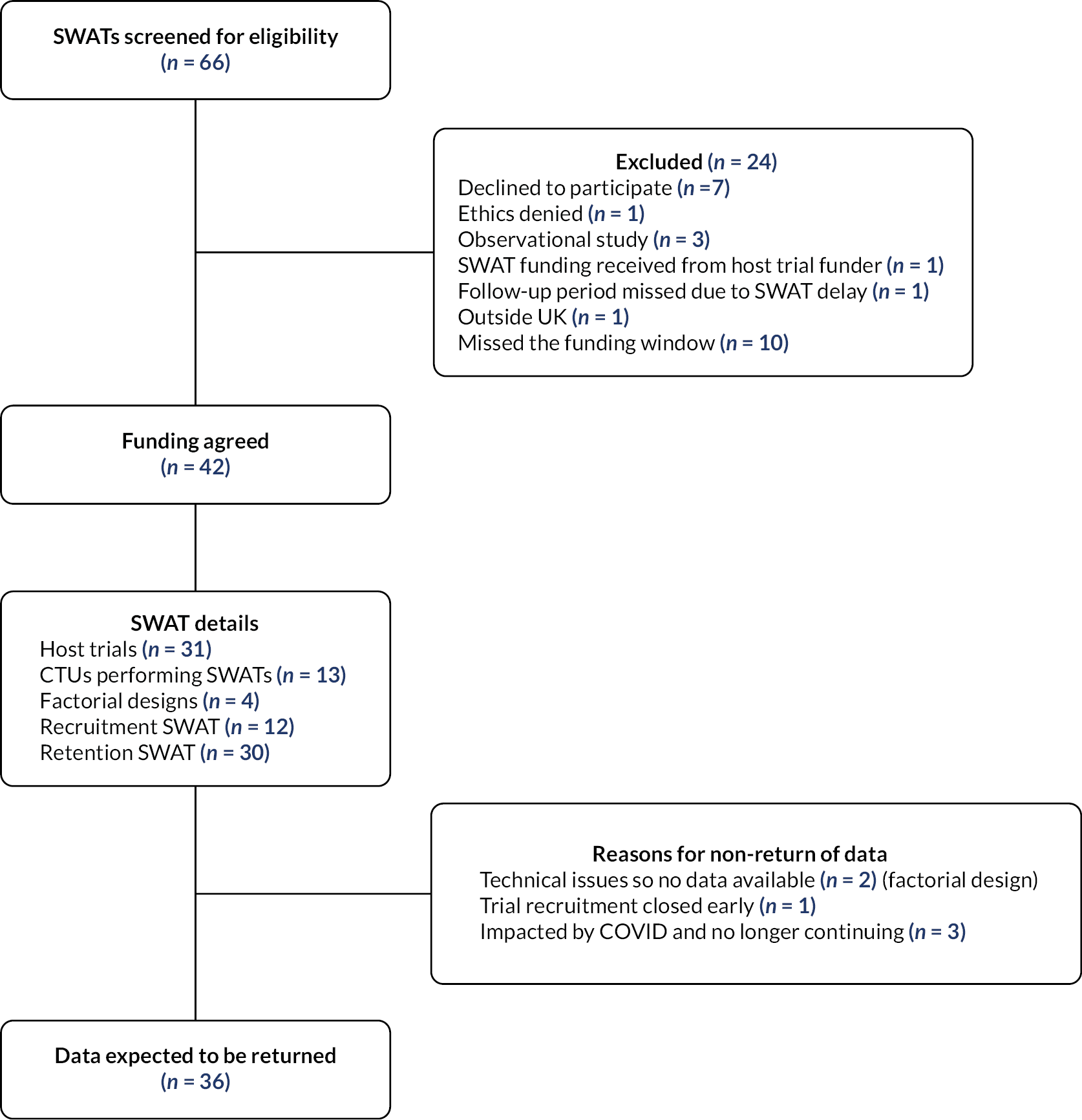
Collectively these 31 host trials spanned 17 research areas (Table 2; see Appendix 1 for a full list of the funded host trials and their characteristics). It is important to note that not all participants in host trials were available for nor included in the SWATs. Five of the host trials implemented more than 1 SWAT, a further 4 implemented a factorial design SWAT and 12 were involved in the co-ordinated evaluation of 2 SWAT strategies.
| Research area | Host trial (sample size)a | Number of host trials | Number of SWATs |
|---|---|---|---|
| Surgical | ACTIVE (334) DISCb (710) L1FE (600) ProFHER-2b (380) START: REACTS (221) UK FROST (503) C-Gall (430) MAGIC (1650) PUrE (1044) |
9 | 11 |
| Fall reduction | OTISb (1299) SSHeW (4400) |
2 | 3 |
| Orthopaedic (rehabilitation) | ARTISAN (478) KReBSb (2600) |
2 | 3 |
| Respiratory | CLEARb (380) | 1 | 3 |
| Smoking cessation | CPIT-III (940) MiQuit-3b (692) |
2 | 3 |
| Wound care | SWHSI-2b (696) | 1 | 3 |
| Oncology (screening and treatment) | IntAct (880) POSNOCb (1900) ActWELL (552) |
3 | 3 |
| Community pharmacy | CHAMP-1b (820) | 1 | 2 |
| Physiotherapy | PEP-TALK (250) GRASP (704) |
2 | 2 |
| Primary care (signs and symptoms) | MSS3b (376) | 1 | 2 |
| Rheumatology | TOPaZ (380) WORKWELL (240) |
2 | 2 |
| Urology | FUTURE (1096) SARC (118) |
2 | 2 |
| Oral health | REFLECT (1174) | 1 | 1 |
| Gastrointestinal | IBD-BOOST (680) | 1 | 1 |
| Gynaecology | VITA (1900) | 1 | 1 |
In total, 12 of the funded SWATs evaluated recruitment strategies (see Results: findings from the funded SWATs, Tables 4 and 5) and 30 SWATs evaluated retention strategies (see Results: findings from the funded SWATs, Tables 6 and 7).
Recruitment strategies
The 12 recruitment strategies have been grouped into the recruitment research domains framework mapped by Online Resource for Research in Clinical triAls (ORRCA). 39 The six ORRCA domains covered recruitment strategies aimed at the following:
-
trial design;
-
pre-trial planning;
-
trial conduct;
-
recruitment information needs;
-
recruiter differences;
-
incentives.
Our classification resulted in the funded recruitment strategies being classified under domain D, ‘recruitment information needs’ or domain F, incentives (see Results: findings from the funded SWATs, Tables 4 and 5). Within domain D, individual recruitment strategies were either classified into subdomains as ‘D1. Researcher training needs’ (4 SWATs) or ‘D2. Participant Information Sheet and Consent Form’ (7 SWATs). The only recruitment strategy to fall under domain F was classified into the subdomain of ‘F3. Participant non-monetary incentives’.
Retention strategies
The 30 retention strategies have been grouped into the retention research domains framework mapped by ORRCA2. 40 The five ORRCA domains covered retention strategies aimed at the following:
-
data collection;
-
participants;
-
sites and site staff;
-
Central Study Management;
-
study design.
Our classification resulted in all the funded retention strategies being classified under domains A ‘Data collection’ or domain B ‘Participants’. There was only one SWAT classified under domain A, and this SWAT fell under the subdomain of ‘A1. Questionnaire design’. The remaining SWATs in domain B (Participants) were classified under the subdomains of ‘B1. Reminders and prompts’ (13 SWATs); ‘B2. Monetary incentives’ (1 SWAT); ‘B3. Non-monetary incentives’ (3 SWATs) and ‘B4. Maintaining participant engagement’ (12 SWATs).
Results were expected for 36 SWATs (see SWAT results). Six SWATs could not be completed: two SWATs encountered technical issues so had to be abandoned (factorial SWAT);41 one SWAT was embedded in a host trial that was closed early due to poor recruitment;42 one SWAT was embedded in a host trial that stopped recruitment early, having answered its question;43 and three further SWATs could not proceed due to the COVID-19 pandemic, which forced the host trial to change its mode of following-up participants. 42,44,45
We also identified an opportunity to test the methodological feasibility of a co-ordinated SWAT design, which involved the implementation of a recruitment or retention strategy within multiple pre-identified host trials simultaneously. In this co-ordinated SWAT, REC approval only needed to be obtained once to allow SWAT implementation within all of the included host trials. The results from each host trial were reported simultaneously within one publication, allowing a more rapid increase of the evidence base. This approach was tested in two SWATs; one evaluated the effect of clinician recruitment training on participant recruitment (SWAT 111),46 and the other sending of Christmas cards to participants on participant retention (SWAT 82),47 with each being implemented within four and eight host trials, respectively (see Chapter 8 for case studies of these SWATs). 48,49
SWAT costs
Individual SWATs
The average cost of funding requested for a SWAT within the PROMETHEUS programme was £2600 (range £500–5000). This figure was calculated from 29 SWATs – each element of factorial SWAT was counted separately, with the costs split between them, the co-ordinated SWATs (n = 12 separate SWATs) were not included due to their costs being unrepresentative of the cost a single SWAT, and one SWAT requested no funding after applying due to such low funds needed (approximately £300). The average cost of consumables (such as pens) was £867 (from 15 SWATs), and £1753 to cover staff time (from 13 SWATs) – it was not always possible to distinguish the separate costs from the funding applications. As many of the PROMETHEUS supported SWATs were performed by YTU, where the PROMETHEUS programme was based, it was anticipated that the costs were underestimated, that is often they did not account for staff time. When excluding YTU SWATs, the average cost rose to £3535 – with mean staff cost being £2359 (data from 17 and 9 SWATs, respectively).
During the discussions with host trial teams with regard to embedding a SWAT, it was apparent that many teams required additional support and insight regarding how long SWAT-specific tasks may take to enable them to cost activities accurately. To assist with this, example costings were developed for two individual SWATs undertaken as part of the PROMETHEUS programme, as shown in Table 3. These highlight that within different SWATs and different CTUs the associated times required may vary, depending on level of experience and infrastructure, for example, if a Trial Manager had to manually send text reminders, as opposed to an automated system, the associated time costs would be vastly different.
| Type of cost | Recruitment SWAT example | Retention SWAT example |
|---|---|---|
| Set-up (including amending protocol and documentation; setting up and testing randomisation; providing training) | 5 days of a Trial Manager’s and 3 days of a Programmer’s time | 5 days of a Trial Manager’s time |
| Activities involved in the SWAT (e.g. undertaking randomisation, implementing the intervention) | - | 15 days of a Trial Manager’s time |
| Data cleaning and analysis and write-up | 2 days of a Trial Statistician’s time | 10–15 days of a Trial Statistician’s time |
Co-ordinated SWATs
The total cost of the recruitment training SWAT was £10,668. This can be broken down into £2188.57 for staff time to prepare and deliver the training and £8479.43 for consumables, including travel costs to deliver and attend the training, venue hire and subsistence, as well as thank-you vouchers to participants for completing the follow-up questionnaires. The costs of developing the training package were not included.
The cost calculated for the Christmas card SWAT was £1306.40 – an average cost of £0.76 per card sent. This included time for staff time to prepare the cards, and for consumables such as the printing, postage, and delivery of the cards.
However, the cost estimates for both SWATs do not include the following, which need to be accounted for when planning to undertake a SWAT of this design:
-
co-ordination from a central point (i.e. to liaise with host trials about involvement and undertaking the SWAT);
-
data preparation and sharing by the host teams;
-
analysis and data cleaning;
-
write-up.
Results: findings from the funded SWATs
Results of recruitment SWATs
Twelve recruitment SWATs were funded within nine host trials. Six of the SWATs had published findings (Table 4) and six of the SWATs were ongoing at the time of writing this manuscript (Table 5). The published SWATs consisted of only two publications: a 2 × 2 factorial SWAT50 and a co-ordinated SWAT involving four host trials, which tested the feasibility of staff training to improve participant recruitment to surgical RCTs. 51 There was no evidence of a significant difference in recruitment rates in any of the strategies tested. However, the staff training SWAT found that it was feasible to randomise sites across four surgical trials in a co-ordinated SWAT design. This SWAT also reported that in the intervention group, there was evidence of increased staff confidence when pre- and post-training scores were compared – but had a limited sample size and so requires further replications.
| Number of SWATs | Host trial acronym | SWAT domain | Brief SWAT description | SWAT outcomes | Published SWAT results |
|---|---|---|---|---|---|
| 4 | DISC52 IntAct53 ProFHER-254 START:REACTS55 |
D1. Researcher training needs | Staff training to improve participant recruitment into surgical randomised trials (SWAT 111)46 | Primary: The feasibility of recruiting sites across multiple surgical trials in a co-ordinated way. | Parker et al. (2022):51 Four RCTs (33%) comprising 91 sites participated. Of these, 29 sites agreed to participate (32%) and were randomised to intervention (15 sites, 29 staff) or control (14 sites, 29 staff). Research nurses attended and found the training to be acceptable; no surgeons attended. In the intervention group, there was evidence of increased confidence when pre- and post-training scores were compared (mean difference in change 1.42; 95% CI 0.56, 2.27; p = 0.002). There was no effect on recruitment rate. |
Secondary:
|
|||||
| 2 | CLEAR56 | D2. PIS and Consent Form | 2 × 2 factorial SWAT design: Participant invitation letter with personal wet signature vs. generic signature (variant of SWAT 3)57 Participant study invitation including a generic doctor–patient photograph vs. including no photograph (SWAT 53)58 |
Primary: Proportion of invited patients who joined the trial. Secondary: Proportion of patients retained in the trial. |
Anand et al. (2022):50 368 letters were given to potential participants in the CLEAR trial and 121 (33%) joined. Proportions for each randomised group were generic signature and no photograph: 38% (33/88); generic signature and photograph: 32% (28/88); wet-ink personal signature and no photograph: 29% (26/91); wet-ink personal signature and photograph: 34% (34/101). There was no evidence of a significant difference in recruitment between those receiving the patient invitation letter containing a wet-ink vs. generic signature (OR: 0.86, 95% CI: 0.55 to 1.32, p = 0.49) or photograph vs. no photograph (OR: 0.99, 95% CI 0.64 to 1.53, p = 0.97). Retention was similar for the wet-ink and generic signature groups (OR: 1.20, 95% CI 0.35 to 4.16, p = 0.77) but significantly better when a photograph was used (OR: 5.40, 95% CI 1.12 to 26.15, p = 0.04), based on two withdrawals in the photograph group vs. nine in the no photograph group). |
| Number of SWATs | Host trial acronym | SWAT domain | SWAT question | SWAT outcomes | Expected completion data and details of delay if applicable |
|---|---|---|---|---|---|
| 1 | IBD-BOOST59 | D2. PIS and Consent Form | Brief PIS provided in addition to a standard-length PIS (SWAT 137)60 | Primary: Recruitment rate | Completion date is expected to be December 2022. This SWAT had a delay in site set-up due to COVID-19. |
Secondary:
|
|||||
| 2 | MSS361 | D2. PIS and Consent Form F3. Participant non-monetary incentives |
2 × 2 factorial design: Brief PIS provided in addition to a standard-length PIS (SWAT 137) Inclusion or not of a trial logo branded pen (SWAT 137)60 |
Primary: Recruitment rate | Recruitment was delayed due to COVID-19; however, data collection is now complete. The SWAT is undergoing analysis and is being prepared for publication. |
Secondary:
|
|||||
| 1 | POSNOC62 | D2. PIS and Consent Form | Addition of a pictorial aid to the PIL (SWAT 102)63 | Primary: Proportion of patients randomised to the trial. | Data collection is complete. |
| 1 | SARC64 | D2. PIS and Consent Form | Optimised PIS vs. conventional PIS (SWAT 101) | Primary: Proportion of patients who consent to take part in the interventional trial. Secondary: Qualitative outcomes assessing the impact/value of the PIL in the decision-making. |
Data collection completed in May 2022; in analysis stage. |
| 1 | SWHSI-265 | D2. PIS and Consent Form | Inclusion of an infographic provided in addition to a standard PIL (SWAT 119)66 | Primary: Recruitment rate | The SWAT was expected to finish in December 2022. |
Secondary:
|
Results of retention SWATs
Thirty SWATs testing retention strategies were funded in 25 host trials. At the time of writing, 21 SWATs had concluded and been published, including two 2 × 2 factorial SWATs,67,68 and the co-ordinated SWAT consisting of eight trials49 (Table 6). Three SWATs were still ongoing (Table 7). Unfortunately, six further SWATs were stopped early (Table 8) and will produce no results: one host trial stopped early prior to reaching its recruitment target,43 a 2 × 2 factorial SWAT of text messaging strategies encountered a significant system fault with the text messaging software (two SWATs),41 and three SWATs could not proceed due to the COVID-19 pandemic. 42,44,45
| Number of SWATs | Host trial acronym | SWAT domain | Brief SWAT description | SWAT outcomes | Published SWAT results |
|---|---|---|---|---|---|
| 1 | PEP-TALK69 | A1. Questionnaire design | Printing the primary outcome measure on pink paper vs. on white paper (SWAT 110)70 | Primary: Host trial primary outcome measure completion. | Ooms et al. (2022):71 176 participants were randomised: 88 received pink paper, 88 white paper. Host trial primary outcome measures were returned by 84.1% (74/88 participants) in the pink paper group and by 90.9% (80/88 participants) in the white paper group [risk ratio, 0.92 (95% CI 0.80, 1.06); p = 0.24]. Reminders were sent to 48.9% (43/88 participants) in the pink paper group and to 30.7% (27/88 participants) in the white paper group [risk ratio 1.59 (95% CI 1.09, 2.33); p = 0.01]. No other results were statistically significant. |
Secondary:
|
|||||
| 1 | UK FROST72 | B1. Reminders and prompts | Timing of text message prompts (reminder received prior to questionnaire arrival or 4 days later) (SWAT 44)73 | Primary: Proportion of participants who returned a valid questionnaire. Secondary: A systematic review was undertaken to identify other embedded trials to perform a meta-analysis. |
Partha Sarathy et al. (2020):74 In the pre-notification arm, 122/135 (90.4%) participants returned a valid questionnaire compared with 119/134 (88.8%) in the post-notification arm (difference of −1.6%; 95% CI of difference: −8.9%, 5.7%). There was no difference in time to response (HR = 1.04; 95% CI 0.80 to 1.34) or need for additional reminders (OR = 0.71; 95% CI 0.43 to 1.17). When combined with two RCTs in a meta-analysis, no difference in response rates between groups, in relation to reminders, was observed (OR = 0.78 95% CI 0.42 to 1.45). |
| 1 | GRASP75 | B1. Reminders and prompts | Personalised text message vs. a standard text message (SWAT 35)76 | Primary: Questionnaire response rate at 6 months. | Cureton et al. (2021):77 618 participants were randomised to a personalised (n = 309) or standard (n = 309) text message. The overall questionnaire response rate was 87% (n = 537/618); 90% (n = 277/309) of participants responded in the personalised text message group compared to 84% (n = 260/309) in the standard text message group (RR 1.07; 95% CI 1.00 to 1.13). Participants randomised to receive the personalised text message were more likely to return their initial postal questionnaire than those who received the standard text message (n = 185/309; 60% vs. n = 160/309; 52%) (RR 1.16; 95% CI 1.00 to 1.33); this represents an absolute percentage difference between intervention groups of 8%. Post hoc subgroup analysis showed that males under 65 years were the group most likely to return their initial questionnaire if they received a personalised text message. |
Secondary:
|
|||||
| 2 | KReBs78 | B1. Reminders and prompts | Two separate SWATs: | Primary: The proportion of 12-month questionnaires returned. | Mitchell et al. (2020):80 1465 participants were included in the SWAT. In the personalised group, 644/723 (89.1%) of participants returned a questionnaire, compared to 654/742 (88.1%) in the non-personalised group. The absolute difference in return rate was 0.9% (95% CI −2.3% to 4.2%; p = 0.57). There was no evidence of a difference between the groups in the likelihood of returning a questionnaire (OR 1.09; 95% CI 0.79 to 1.51; p = 0.61), the likelihood of returning a complete questionnaire (OR 1.11; 95% CI 0.82 to 1.51; p = 0.50) nor in time to return (HR 1.05; 95% CI 0.94 to 1.17; p = 0.40). Mitchell et al. (2021):81 2305 participants were randomised into the SWAT. In the pen group, 1020/1145 (89.1%) of participants returned a questionnaire, compared to 982/1147 (85.6%) in the no pen group. The absolute difference in questionnaire return rate was 3.5% (95% CI 0.8% to 6.2%; p = 0.01). There were statistically significant differences in questionnaire return rate (OR 1.36; 95% CI 1.06 to 1.74; p = 0.02), questionnaire completion rate (OR 1.40; 95% CI 1.11 to 1.78; p < 0.01) and time to questionnaire return (HR 1.17; 95% CI 1.07 to 1.27; p < 0.01) favouring the pen group. |
Secondary:
|
|||||
| 2 | MiQuit-382 | B1. Reminders and prompts | 2 × 2 factorial SWAT design: Personalised text message vs. a standard text message (SWAT 35)76 Timing of text message prompts (SWAT 44)73 |
Primary: Completion rate of questionnaire via telephone. | Coleman et al. (2021):67 194 participants were randomised into the SWAT; 50 to personalised early text, 47 to personalised late text, 50 to non-personalised early text, and 47 to non-personalised late text. There was no evidence that the timing of the text message (early: 1 week before; or late: 1 day before) had an effect on any of the outcomes. There was evidence that a personalised text would result in fewer completions via telephone compared with a non-personalised text (adjusted OR 0.44, 95% CI 0.22 to 0.87, p = 0.02). However, there was no evidence to show that personalisation or not was better for any of the secondary outcomes. |
Secondary:
|
|||||
| 1 | ActWELL83 | B1. Reminders and prompts | Sending pre-notification cards to trial participants before outcome measurement (SWAT 76)84 | Primary: Number of trial participants who complete the outcome measurement (i.e. are retained). Secondary: Cost per participant retained. |
Treweek et al. (2021):85 558 participants were included in the SWAT. Of the 274 women sent a card, 231 attended the primary outcome visit (84.3%) compared to 230/284 (81.0%) for those not receiving a card. Sending a pre-notification card may result in a slight increase in attendance at a face-to-face primary outcome measurement visit at 1 year: risk difference = 3.3% (95% CI = −3.0% to 9.6%). This is GRADE low-certainty evidence. The direct cost of producing and sending the cards was £192 GBP (€213 EUR; US$ 260), or £21.33 (€23.55; $28.77) per additional retained participant). |
| 1 | WORKWELL86 | B1. Reminders and prompts | Pre-notification (SWAT 86)87 (retention) | Primary: Valid response for the primary outcome (yes/no). | Sutton et al. (2022):88 244 trial participants took part in the SWAT. Among those sent a pre-reminder, 100/121 (83%) provided a valid response for the WORKWELL primary outcome, compared to 97/123 (79%) of those not sent a pre-reminder. The estimated adjusted odds ratio was 1.28 (95% CI 0.67 to 2.42), with a risk difference of 3.8% (95% CI −6.1% to 13.6%), favouring the pre-reminder. The estimated intervention cost per additional participant retained was £53.42, and the total cost per additional participant retained was £46.52. |
Secondary:
|
|||||
| 2 | OTIS89 | B3. Non-monetary incentives | 2 × 2 factorial design: Pen incentive to enhance retention in a randomised trial (SWAT 92)79 Social incentive text cover letter sent with a postal follow-up questionnaire (SWAT 144)90 |
Primary: Proportion of who returned the questionnaire. | James et al. (2021):68 12-month questionnaire response rate was 721 out of 755 (95.5%). Neither the pen nor social incentive cover letter had a statistically significant effect on response rate: pen 95.2% vs. no pen 95.8%, adjusted OR 0.90 (95% CI 0.45 to 1.80; p = 0.77); social incentive cover letter 95.2% vs. no social incentive cover letter 95.8%, adjusted OR 0.84 (95% CI 0.42 to 1.69, p = 0.63). No statistically significant differences were observed between either of the intervention groups on time to response, need for a reminder or completeness. Therefore, neither intervention was cost-effective. |
Secondary:
|
|||||
| 1 | SSHeW91 | B3. Non-monetary incentives | Pen incentive to enhance retention in a randomised trial (SWAT 92)79 | Primary: Proportion of participants who return questionnaire. | Cunningham-Burley et al. (2020):92 1466 SSHEW trial participants were randomised into the SWAT. In total, 13 withdrew from the host trial before they were due to be sent their follow-up questionnaire, 728 participants received a pen with their questionnaire, and 725 did not receive a pen. A questionnaire was returned from 67.7% of the pen group and 64.7% of the group who did not receive a pen. There was no significant difference in return rates between the two groups (OR 1.15, 95% CI 0.92 to 1.43, p = 0.22), nor level of completeness of the questionnaires (AMD −0.01, 95% CI 0.06 to 0.05, p = 0.77). There was weak evidence of a reduction in the proportion of participants requiring a reminder and in time to response in the pen group. |
Secondary:
|
|||||
| 8 | C-GALL93 CPIT-III94 DISC52 FUTURE95 ProFHER-254 PUrE-RCT96 REFLECT97 SWHSI-265 |
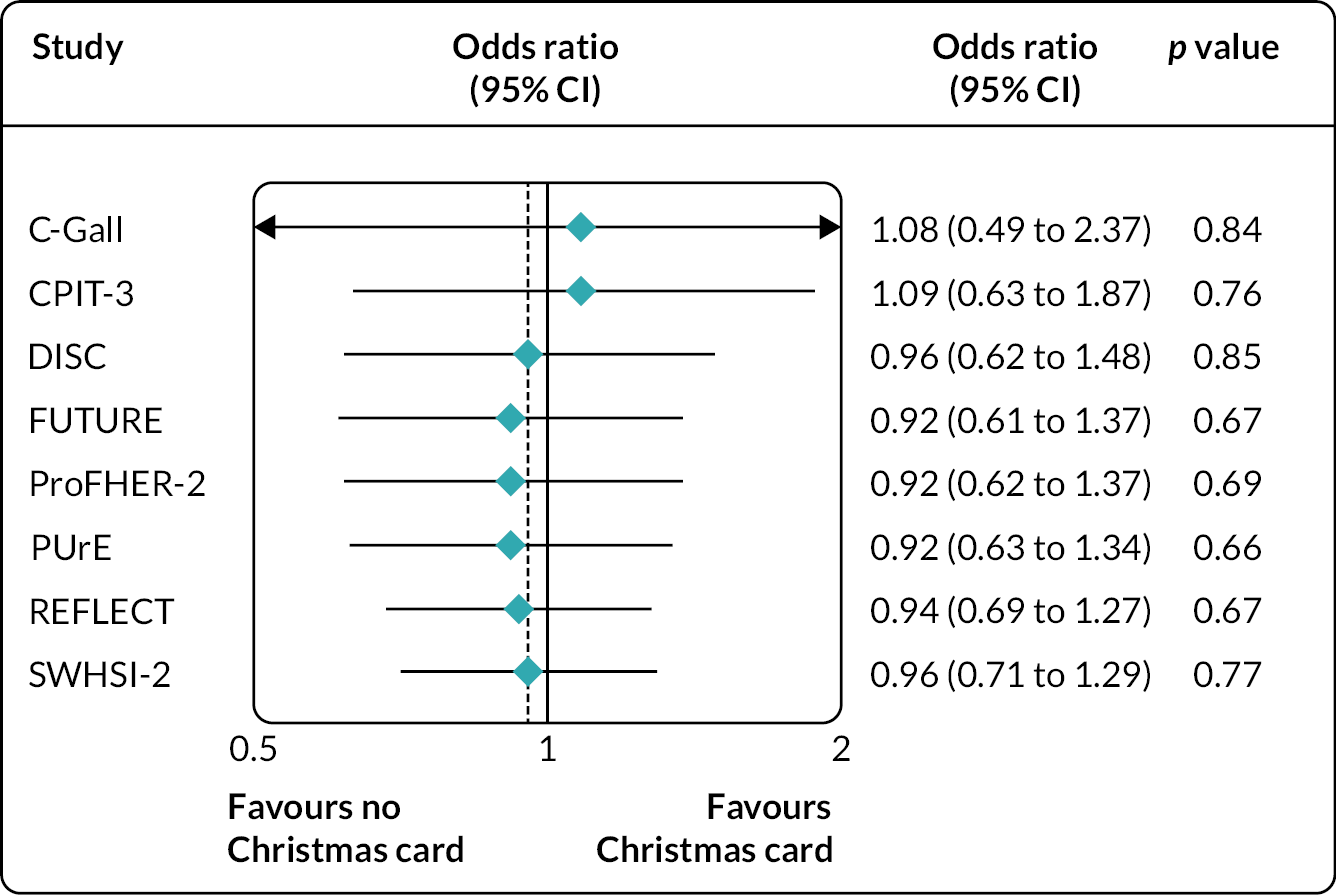
B4. Maintaining participant engagement | Sending Christmas cards to trial participants (SWAT 82)47 | Primary: Proportion of participants completing their next follow-up. | Coleman et al. (2021):49 8 host trials were recruited. 1469 participants (age 16–94 years; 70% (n = 1033) female; 96% (813/847) white ethnicity) across the eight host trials were involved in the analysis (cut short owing to COVID-19). No evidence was found of a difference in retention rate between the two arms for any of the host trials when analysed separately or when the results were combined [85.3% (639/749) for cards vs. 85.4% (615/720) for no card; odds ratio 0.96, 95% CI 0.71 to 1.29; p = 0.77]. |
Secondary:
|
|||||
| 1 | CLEAR56 | B4. Maintaining participant engagement | Combined in a three-arm SWAT: Thank-you note or card after each study visit (SWAT 54)98 Personalisation (not including patient’s name, electronically signed vs. personalised handwritten name and wet-ink signature) |
Primary: Proportion of participants who remain in the study. Secondary: Time that participants remain in the study before they withdraw. |
Anand (2021)99 (interim analysis only): 141 participants were randomised into the SWAT (generic thank-you card, n = 48; personalised thank-you card, n = 46; no thank-you card, n = 47). Of these, 67 patients had completed all five visits at the time of the interim analysis. This analysis only uses data for the 11 patients who voluntarily withdrew following randomisation. Proportions withdrawing: personalised thank-you card = 8.7%, generic thank-you card = 8.3%, No thank-you card = 6.4% (Total = 7.8%). Generic thank-you card vs. Personalised thank-you card OR 0.95 (95% CI 0.22 to 4.07, p = 0.9498); Generic thank-you card vs. No thank-you card OR 1.33 (95% CI, 0.28 to 6.31, p = 0.7168); Personalised thank-you card vs. No thank-you card, OR 1.40 (95% CI 0.29 to 6.62, p = 0.6737); Card vs. No card, OR 1.36 (95% CI −0.34 to 5.40, p = 0.6581). No significant differences were found for any of these four comparisons. |
| Number of SWATs | Host trial acronym | SWAT domain | SWAT question | SWAT outcomes | Expected completion data and details of delay if applicable |
|---|---|---|---|---|---|
| 1 | ARTISAN100 | B4. Maintaining participant engagement | Courtesy telephone calls vs. postcards to trial participants following enrolment (SWAT 121)101 | Primary: Proportion of participants returning questionnaire by post at the 6 months. | In progress Data collection ongoing, expected to be completed in September 2022. |
Secondary:
|
|||||
| 1 | MAGIC102 | B1. Reminders and prompts | Personalised text message vs. a standard text message (SWAT 35)76 | Primary: Questionnaire response rate at 6 months. | In progress. Host trial stopped recruitment due to COVID-19, recommencing early 2022. Anticipated to finish recruitment 31 July 2023. |
Secondary:
|
|||||
| 1 | SWHSI-265 | B4. Maintaining participant engagement | Thank-you card following each study visit (SWAT 119)103 | Primary: Proportion of participants who complete the 6-month questionnaire. | In progress. The SWAT paused for 3 months due to COVID-19 and recommenced July 2020. The SWAT is expected to finish at the end of 2023. |
Secondary:
|
| Number of SWATs | Host trial acronym | SWAT domain | SWAT question | SWAT outcomes | Details of reasons the SWAT will not be completed |
|---|---|---|---|---|---|
| 2 | CHAMP-141 | B1. Reminders and prompts | 2 × 2 factorial SWAT design: Personalised text message vs. a standard text message (SWAT 35)76 Timing of text message prompts (SWAT 44)73 |
Primary: Questionnaire completion rate. | During the course of undertaking this SWAT, there occurred a system error with the text messaging software, resulting in a high proportion of the messages not being sent to participants as planned, so this SWAT could not proceed. |
Secondary:
|
|||||
| 1 | ACTIVE45 | B1. Reminders and prompts | Text message reminder which participants can respond to, compared with a ‘no reply’ text message on questionnaire response rates (SWAT 109)104 | Primary: Proportion of questionnaires completed at the 3-month follow-up. | Due to COVID-19 the SWAT is not continuing. |
Secondary:
|
|||||
| 1 | TOPaZ44 | B1. Reminders and prompts | Pre-notification cards to trial participants (SWAT 76)84 (retention) | Primary: Number of trial participants who complete the outcome measurement Secondary: Cost per participant retained. |
This SWAT was unable to continue due to COVID-19. |
| 1 | VITA43 | B2. Monetary incentives | Conditional financial incentives vs. unconditional financial incentives105 | Primary: The number of participants who complete the primary outcome at 2-week follow-up. Secondary: The number of reminders sent for each group. |
Recruitment in this host trial stopped prior to reaching the target of 1900 participants on recommendation from the DMC and TSC after a planned review of the results indicated that the research question had been answered. Therefore, the SWAT could not proceed. |
| 1 | L1FE42 | B4. Maintaining participant engagement | Telephone calls or postcards to trial participants (SWAT 114)106 | Primary: Proportions of participants who complete and return the questionnaire at 6 weeks, 12 weeks and 6-month time points. | The host trial was terminated early by the funder, so the SWAT was abandoned. |
Secondary:
|
Retention strategies demonstrating evidence of effectiveness
The published SWATs reported the following evidence of effectiveness for the strategies evaluated:
Reminders and prompts
For pre-notification of trial participants, SWAT 76 found that sending a pre-notification card may result in a slight increase in attendance at a face-to-face primary outcome measurement visit at 1-year: risk difference = 3.3% [95% confidence interval (CI) = −3.0% to 9.6%]. 85 SWAT 86 compared sending a pre-notification letter or e-mail before sending a self-report questionnaire, versus no pre-notification on retention rates (valid response for the host trial primary outcome). This SWAT found that of those sent a pre-notification, 100/121 (83%) provided a valid response for the host trial primary outcome, compared to 97/123 (79%) of those not sent a pre-reminder. The estimated adjusted odds ratio was 1.28 (95% CI 0.67 to 2.42), with a risk difference of 3.8% (95% CI −6.1% to 13.6%), favouring the pre-notification. The estimated intervention cost per additional participant retained was £53.42, and the total cost per additional participant retained was £46.52. 88
For the text messaging of trial participants, SWAT 35 found that participants randomised to receive a personalised text message were more likely to return their initial postal questionnaire than those who received a standard text message (n = 185/309; 60% vs. n = 160/309; 52%) (RR 1.16; 95% CI 1.00 to 1.33); this represents an absolute percentage difference between intervention groups of 8%. Post hoc subgroup analysis showed that males under 65 years were the group most likely to return their initial questionnaire if they received a personalised text message. 77 Another evaluation of SWAT 35 showed that when comparing personalised text messages versus non-personalised text messages, there was evidence that a personalised text would result in fewer completions via telephone compared with a non-personalised text (adjusted OR 0.44; 95% CI 0.22 to 0.87; p = 0.02). 67
Non-monetary incentives
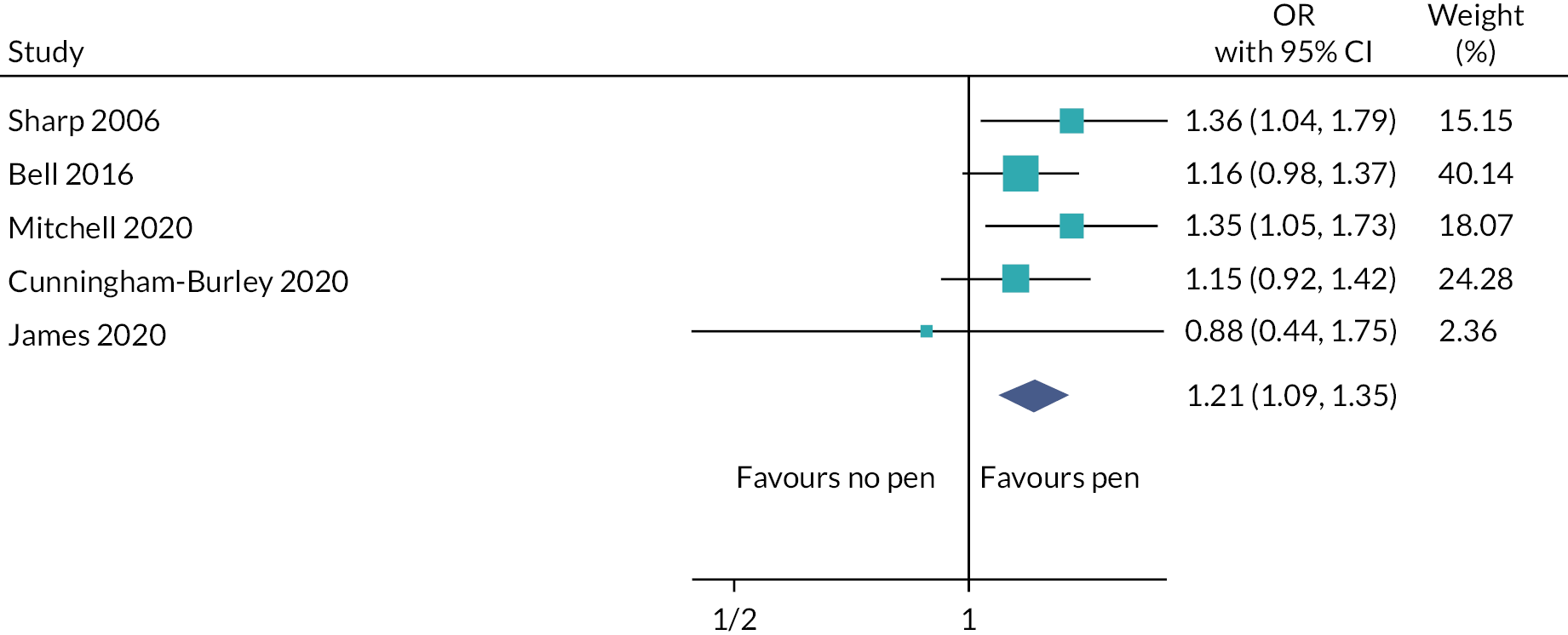
In the evaluations of the pen strategy, SWAT 92 reported that when participants were randomised to receive a pen compared to no pen with their postal questionnaire, there were statistically significant differences in questionnaire return rate (OR 1.36; 95% CI 1.06 to 1.74; p = 0.02), questionnaire completion rate (OR 1.40; 95% CI 1.11 to 1.78; p < 0.01) and time to questionnaire return [hazard ratio (HR) 1.17; 95% CI 1.07 to 1.27; p < 0.01] favouring the pen group. 81 However, James et al., also testing SWAT 92, found no statistically significant effect on response rate: pen 95.2% versus no pen 95.8%, adjusted OR 0.90 (95% CI 0.45 to 1.80; p = 0.77). 68 SWAT 37 found no statistically significant difference in return rates when participants were sent a pen with their postal questionnaire compared to not being sent a pen (OR 1.15, 95% CI 0.92 to 1.43, p = 0.22), nor level of completeness of the questionnaires [adjusted mean difference (AMD) −0.01; 95% CI 0.06 to 0.05; p = 0.77]. 92 However, there was weak evidence of a difference, in favour of the pen group, in both time to return (median time to return 15 vs. 18 days; HR 1.12; 95% CI 0.98 to 1.27; p = 0.09) and in the proportion of participants requiring a reminder (OR 0.83; 95% CI 0.68 to 1.02; p = 0.08).
In Chapter 8, we present a meta-analysis combining the results of the PROMETHEUS pen SWATs with other published SWATs of this strategy (see Case study of individual SWATs: pens for retention). The pooled effect combining all SWATs indicated that including a trial pen with a questionnaire probably increases retention and response rate (pooled OR 1.21; 95% CI 1.09 to 1.35). 107
Retention strategies demonstrating a lack of effectiveness, or with the potential to harm retention rates
There was no evidence of a significant positive difference in retention rates in any of the other 25 strategies tested. This includes the co-ordinated SWAT testing the effectiveness of sending Christmas cards on retention rates; no evidence was found of a difference in retention rate between the two arms for any of the host trials when analysed separately, nor when the results were combined. 49
One SWAT identified that there was a potential harmful impact on retention from one of the strategies evaluated. SWAT 110 reported that printing the trial primary outcome on pink paper does not increase data return, when compared to printing the primary outcome on white paper [risk ratio 0.92 (95% CI 0.80 to 1.06); p = 0.24]. This SWAT also showed some evidence that it potentially decreases response and is more burdensome to collect postal data by increasing the necessity for reminders, with reminders sent to 48.9% (43/88 participants) in the pink paper group and in 30.7% (27/88 participants) in the white paper group [risk ratio 1.59 (95% CI 1.09 to 2.33); p = 0.01]. 71
Chapter 4 Discussion of the PROMETHEUS programme
Summary of main findings from PROMETHEUS
The PROMETHEUS programme successfully embedded 42 SWATs within 31 host RCTs, exceeding the original target of 25 SWATs. This is the biggest single effort to generate SWAT evidence in the world, representing a substantial increase in the global methodological evidence base. The PROMETHEUS-funded SWATs represent an increase of 18% (12/68) more SWATs to the Cochrane systematic review of recruitment strategies,23 and 79% (30/38) more SWATs to the Cochrane systematic review of retention strategies. 24
The main findings from the PROMETHEUS SWATs reported to date show that for recruitment, there was no evidence of a significant difference in recruitment rates in any of the strategies tested. For retention, we found that pre-notification of trial participants by sending a card may result in a slight increase in attendance at a face-to-face primary outcome measurement visit; and that sending a pre-notification letter or e-mail before sending a self-report questionnaire by post increases response rates, compared with no pre-notification. We also found that participants randomised to receive a personalised text message were more likely to return their initial postal questionnaire than those who received a non-personalised text message; and that men aged under 65 years were most likely to return their initial questionnaire if they received a personalised text message. Another SWAT comparing personalised text messages versus non-personalised text messages, found evidence that a personalised text would result in fewer telephone follow-ups, which were more resource intensive. When participants were randomised to receive a pen compared to no pen with their postal questionnaire, combined results from PROMETHEUS together with other published SWATs found that including a pen probably increases retention and response rate (pooled OR 1.21, 95% CI 1.09 to 1.35). One SWAT showed evidence of an adverse impact on retention when printing the trial primary outcome on pink paper compared to printing the primary outcome on white paper; this showed a decreased response, and it was also more burdensome to collect postal data as it increased the need for reminders. The other strategies tested showed no evidence of a significant difference in retention rates.
This was possible to fund more SWATs than initially anticipated due to the majority of the SWATs costing less than the proposed funding limit, with the mean SWAT cost being £3535; less than the proposed £5000. We have shown that typically SWATs can cost less than the £10,000 SWAT funding made available by the HTA. Therefore, PROMETHEUS is currently the largest programme of work to act as a central co-ordination point to offer both funding and practical support for the embedding of SWATs, independently contributing the largest amount of evidence to the recruitment and retention strategy evidence base. These SWATs were collectively implemented within a large number of host trials across multiple CTUs, demonstrating the wide reach of the programme. The programme’s success confirms the feasibility of implementing methodological research within a vast range of research areas when appropriate resource and infrastructure support is made available. It also confirms the feasibility and acceptability among trial teams of conducting co-ordinated SWATs, enabling a more rapid evaluation of recruitment and retention strategies.
As host trial teams were often inexperienced in conducting and implementing SWATs, the PROMETHEUS programme acted as an invaluable co-ordination point, providing teams with the confidence, knowledge and resources to do so. Furthermore, the programme identified that the lack of SWAT funding was often a barrier to implementation; a concept which was reinforced as the number of conducted SWATs increased following the introduction of PROMETHEUS funding. However, despite providing both funding and guidance, the encouragement of external researchers to conduct a SWAT proved difficult; with the majority of the SWATs being conducted by both YTU (18 SWATs, 43%) and the CTUs which PROMETHEUS co-applicants were associated with (16 SWATs, 38%). It is important to place this seeming lack of poor external engagement in context, given that the original PROMETHEUS focus was to support our collaborating network of CTUs and centres, who had already committed to starting at least two SWATs.
Strengths
Five of the host trials implemented more than one SWAT, and a further four implemented a factorial design SWAT, allowing for the assessment of two SWATs in a co-ordinated way. This finding demonstrates that, within an individual host trial, there is often a capacity to address more than one SWAT question, either separately (such as testing a strategy aimed at recruitment during the recruitment phase; and then testing a retention strategy during the follow-up phase) or simultaneously, using a factorial design. This suggests that there is capacity to significantly speed up and strengthen the evidence base through teams undertaking more than one SWAT in their trials where relevant. Another pathway to speeding up and strengthening the evidence base is through the co-ordinated SWAT approach that we pioneered, where the same recruitment or retention strategy is tested across multiple host trials simultaneously. Thus, our work highlights that given finite resources, robust evidence can be generated at speed in two key ways: by doing more SWATs in the same host trial, or by testing the same SWAT question across different host trials in a co-ordinated way.
Aside from the provision of SWAT funding, the work undertaken within PROMETHEUS also presents added value through working with CTUs, which are the key centres in the UK supporting the delivery of trials, to support the uptake of SWATs. Uniquely, PROMETHEUS focused on generating evidence for trial recruitment as well as retention, both of which are crucial for the successful delivery of trials, but which until PROMETHEUS had not been addressed together. Despite similar funding as other programmes such as MRC START (which funded 12 SWATs) and TRECA (which funded 6), PROMETHEUS has been able to generate more than three and seven times the number of SWATs, respectively, adding substantial, much-needed evidence to support trial process decision-making.
Limitations
Our experience of five funded SWATs failing to complete highlights the reality of SWATs being entirely subject to the fortunes of the host trials; if the host trial stops for any reason or changes its mode of follow-up, it places the SWAT in jeopardy. One study commenced the SWAT but technical errors in the software associated with the intervention (text messaging) resulted in a significant proportion of participants not receiving the intervention and so the SWAT had to be terminated. For two other SWATs recruitment to the host trial was stopped prematurely (one on direction of the associated oversight committees; one by the Funder) and so planned retention SWATs could not proceed. Finally, although we had awarded funding to all of our host trials prior to the COVID-19 pandemic proliferating in the UK in March 2020, the widespread societal changes that this forced resulted in the recruitment pathway of many of the PROMETHEUS host trials either pausing or altering their study pathways. As a result, 2 SWATs did not proceed, and 10 others remain in progress at the time of writing this report. We will work with the host trial teams of the ongoing SWATs to ensure their findings appropriately contribute to the evidence base.
Further to this, the funded SWATs evaluated a wide range of recruitment and retention strategies. While this led to an overall increase in the evidence base, the evidence generated did not allow for enough replications to have been conducted to conclude the effectiveness of all SWAT strategies evaluated via meta-analyses. This limited our ability to answer most SWAT questions definitively. Despite this, significant progress has been made with some SWATs (e.g. pens for retention) for which only a limited number of further replications in specific populations (male populations, younger adult populations) are now required to reach a definitive evaluation of strategy effectiveness. The development of the Trial Forge SWAT Network should enable additional replications to be completed more rapidly to fully answer these SWAT questions.
We found a greater interest and conduct of SWATs evaluating retention, rather than recruitment strategies. This may be due to retention SWATs being potentially less challenging to undertake than recruitment SWATs, as logistically, there is likely to be more time to introduce a retention strategy when there are multiple follow-up time points. Recruitment SWATs may also present additional challenges, such as the inaccurate perception that multiple levels of consent are required from individuals being approached to enter a trial (e.g. consent to be invited into the trial, consent to be included in the SWAT, and consent to be enrolled into the trial). Alternatively, there may also be greater time pressure associated with the embedding of a recruitment rather than retention SWAT, due to the additional tasks of site set-up. Lessons learnt from the PROMETHEUS programme are also discussed by Clark et al. (2022). 108
As all the funded SWATs were also conducted within the UK, the SWAT evidence generated through PROMETHEUS is not necessarily applicable to populations within other countries, limiting the reach of the programme.
We included only randomised host trials, which may have limited our ability to identify variation in effectiveness of recruitment and retention strategies across different research contexts. However, we believe that focusing on randomised controlled trials in this particular programme was appropriate, since trials are particularly difficult to recruit to, with randomisation and strong treatment preferences leading to issues around both clinician and participant equipoise, something not experienced in non-randomised studies. A direction for future research is to include non-randomised studies as host studies. We also focused only on randomised SWATs. Non-randomised SWATs such as qualitative studies, observational studies, and surveys have a substantial role to play in improving the evidence base for recruitment and retention. One strong way that non-randomised SWATs can help with the evidence base is through process evaluations, which might include interviews to elicit participants’ experiences of being exposed to a recruitment strategy, as well as the experiences of patients who decline the invitation to participate in a trial. This will be crucial to include in future SWATs to ensure both effectiveness in terms of recruitment and retention but also acceptability for participants.
Comparison with existing programmes of SWAT research: Medical Research Council START and TRECA
Building on and improving on the SWAT work initiated by MRC START, which established the feasibility of testing recruitment strategies across multiple host trials,25,26 as well as TRECA,30 which aimed to undertake a programme of SWATs in trials recruiting children and young people, PROMETHEUS has successfully undertaken more SWATs at a faster pace and successfully disseminated findings. However, PROMETHEUS faced challenges that were similar to those in MRC START, such as a large proportion of the SWATs being undertaken by researchers linked with the PROMETHEUS team. There is a range of reasons for the success of PROMETHEUS, which includes prior learning on SWATs gained from MRC START and other work undertaken by its collaborators, the financial support to undertake SWATs, as well as the support mechanism provided to host trial teams from a well-established registered CTU. This suggests that speed and efficiency for undertaking SWATs can be improved further, but it is important that the momentum and skill set of staff are maintained.
Patient and public involvement
Patient and public involvement was an important part of this programme, both for the overarching PROMETHEUS programme and for the SWATs undertaken. We actively involved PPI members in identifying, prioritising and implementing the SWATs, and we have dedicated Chapter 5 to outline the PPI undertaken, as well as lessons learnt for the trials community, and PPI partners.
Equality, diversity and inclusion
Increasing the diversity of trial participants is fundamental to increase participation rates in trials. An ambition in testing recruitment and retention strategies is not only to increase the numbers of people taking part in trials, but also helping to recruit participants that better reflect those that might benefit from the trial results, or to avoid harm. Our SAP reflected this and for each host trial funded, we asked that wherever possible, demographic data on the age, gender and ethnicity of participants should be recorded. The importance of capturing these data can be illustrated in the getting it right: addressing shoulder pain (GRASP) SWAT, which tested personalised versus, non-personalised text messages. 77 In this SWAT, post hoc subgroup analysis showed that males under 65 years were the group most likely to return their initial questionnaire if they received a personalised text message. Capturing such data in all SWATs will also allow subsequent future patient-level meta-analysis to be undertaken, and differences in effect between different populations to be assessed. In time, there may also be a need for testing of population-specific SWATs if there is evidence of differential effects in distinct population groups.
Conclusions
PROMETHEUS originally aimed to fund 25 SWATs and supported 42 SWATs, generating the single biggest body of SWAT activity in the world. When SWAT funding was made available, we found that many teams embedded SWATs into their research. The mean cost of each SWAT was £3535. In addition to funding SWATs, PROMETHEUS successfully demonstrated the methodological feasibility of undertaking co-ordinated SWATs, a powerful new tool with the potential to rapidly accelerate the evidence base for recruiting and retaining trial participants. In addition to money, the co-ordination PROMETHEUS provided was crucial to increasing the recruitment and retention evidence base.
The PROMETHEUS SWATs reported to date found that there was no evidence of a significant difference in recruitment rates in any of the strategies tested. For the retention strategies, we found evidence of effectiveness for sending pre-notification cards, letters and e-mails to trial participants; that personalised text messages were more effective than non-personalised text messages, especially for men aged under 65 years; that sending a pen compared to no pen with postal questionnaires was more effective, although this result was not consistent as another SWAT found no difference. One SWAT comparing pink versus white paper for printing the primary outcome showed evidence of a decreased response in the pink paper group, and that it was also more burdensome to collect postal data in this group. We found no evidence of a significant difference in retention rates for any of the other strategies tested. More replications of the recruitment and retention strategies funded by the PROMETHEUS programme are required to provide definitive evidence for these strategies.
In the chapters to follow, we reflect on lessons learnt from undertaking the PROMETHEUS programme.
Chapter 5 Lessons learnt for patient and public involvement
Chapter overview
PPI is important for developing and evaluating interventions aimed at recruiting and retaining patients in RCTs. In this section, we report the PPI in PROMETHEUS using the guidance for reporting involvement of patients and the public 2 (GRIPP2) Reporting Checklist109 and reflect on the lessons learnt for involving PPI partners in SWAT research.
Defining patient and public involvement in PROMETHEUS
The traditional aim of PPI is to involve end users in the research process. Given the methodological focus of PROMETHEUS, we defined ‘patients and the public’ broadly, to include those who would potentially be impacted by the recruitment and retention strategies, such as patients, potential patients, carers and members of the public.
We acknowledge the importance of stakeholders from the trial community who may be involved in undertaking SWATs, recruiting participants, or applying the evidence from SWATs, such as principal investigators, statisticians, trial managers and staff based within CTUs and other centres undertaking rigorous RCTs. However, we consider the contributions of these stakeholders as separate from PPI.
Aims
The aims of the PPI in PROMETHEUS were to:
-
Inform the overarching prioritisation of the recruitment and retention strategies that host trial teams were funded to undertake.
-
Inform how individual host trial teams could select, adopt and/or adapt specific recruitment or retention strategies for their specific patient population and recruitment/retention contexts.
Patient and public involvement: methods
Patient and public involvement input prior to funding being awarded
A significant amount of PPI underpinned the PROMETHEUS programme. MRC START, which PROMETHEUS builds on, included significant public involvement in developing recruitment interventions and the methodological frameworks around recruitment SWATs. 25 Five members of the PROMETHEUS team (authors Devane, Torgerson, Gillies, Galvin and Treweek) undertook two James Lind Alliance priority setting exercises: one for recruitment34 and the other for retention strategies110 with stakeholders including members of the public, clinicians and researchers; these priority lists were used to inform the prioritisation of the recruitment and retention strategies used in PROMETHEUS. A number of the existing recruitment and retention strategies registered on the SWAT Repository involved patients and the public in their development;111,112 or were led by the host trial’s PPI group. 113,114
Patient and public involvement in prioritisation of strategies to be funded
We hosted a SWAT public involvement panel to ensure the project met the needs of and gained input from patients and the public. We advertised for members through PPI forums and our existing contacts. The panel comprised five men and women with a range of health conditions, and diverse experiences of being involved as PPI members in specific trials, prior involvement in methodological research (such as MRC START) as well as part of research funding panels. Panel members were provided with a ‘Lay Summary’ of the PROMETHEUS programme, along with a ‘Remit and Role Description’, which outlined the role and provided practical information such as how expenses and payments would be made, and who to contact for further information. We organised a ‘Priority setting meeting’, ahead of which we circulated an agenda and a briefing document providing an initial draft outline of a priority list of recruitment and retention strategies that we wanted to fund as part of PROMETHEUS, and the methods that we had used to identify this initial priority list (see Chapter 2). We also specifically asked members of the PPI panel to think of any strategies that they additionally wanted to prioritise to be tested. During this meeting, PPI members commented on the initial priority list and each member ranked the strategies, listing their preferred strategy first, and their next preferred option second. Suggestions made by PPI panel members for additional SWATs not on the initial priority list included thanking participants for taking part in trials, which could include offering ‘thank you’ gifts, and including a person’s name in trial correspondence. We provided a ‘PPI Feedback form’ for panel members to provide feedback on the meeting and how they felt about their ability to contribute.
Patient and public involvement input into individual SWATs
To ensure ongoing PPI, we requested that each host trial consulted with patients and public members involved in their RCTs as part of the implementation process for each SWAT. This gained bespoke advice relevant to the context and population of each host trial. Additionally, where individual host trial teams undertaking funded SWATs lacked access to PPI members, we sought PPI input from the PROMETHEUS PPI panel on their behalf.
Patient and public involvement: results
Patient and public involvement in prioritisation of strategies to be funded
We developed a final priority list which the PPI group agreed with (see Chapter 2). These strategies were listed and presented in the order of priority selected by the PPI panel. The PROMETHEUS priority list was presented on our programme website, included in the information for funding applicants, and highlighted in the funding peer reviewer form to ensure that reviewers prioritised funding applications that were proposed to evaluate the SWATs. Overall, the feedback from PPI panel members was very positive, with members saying that they were encouraged to and felt able to contribute to the meeting discussion, that their ideas were listened to by other members of the group, and that their views were valued in the forum.
Patient and public involvement input into individual Studies Within A Trial
Promoting the use of SWAT, PPI panel members provided additional input into the implementation of two funded SWATs. They reviewed participant information material in a SWAT which assessed the effectiveness of a brief participant information leaflet (PIL) versus standard length PIL on participant recruitment rates; and a theoretically informed cover letter which aimed to improve response rates to follow-up postal questionnaires. For the Christmas card SWAT, we consulted a PPI group consisting of six members from the Health Services Research Unit PPI Partnership at CHaRT (University of Aberdeen) on the design and content of the card. The group was asked to provide input and comments on the following: the design of the card (two designs were presented); the wording inside the card; and to give feedback on any other aspect. Most of the group members agreed on the card that was ultimately used in all the host trials (please refer to Appendix 2 to view the card) and expressed that the card was acceptable. Other host trials consulted their own PPI groups on the specific SWATs funded as part of PROMETHEUS; however, we did not specifically ask for feedback, so we were unable to report the results of these PPI activities.
Patient and public involvement: discussion and conclusions
There was significant PPI in PROMETHEUS from its earliest stages to prioritisation of the strategies to be funded, to implementation of the funded SWATs. Involving patients and members of the public in methodological research can be challenging, as methodological research is often several steps removed from direct patient care. 115 However, we were able to successfully incorporate PPI in a way that members felt able to contribute to and found rewarding.
Patient and public involvement: reflections and critical perspective
Because of the methodological focus of PROMETHEUS, our PPI panel members were more ‘experienced’, and had had prior involvement in trials, and some also had prior involvement in methodological research. While this allowed our group to quickly understand and appreciate the aims, objectives and methods involved in PROMETHEUS, and to contribute in a way that was meaningful and rewarding, this also meant that we may have excluded more ‘naïve’ PPI members who could potentially have brought novel perspectives to the process. However, on the whole, we felt that having panel members who had prior experience was more likely to have benefitted rather than hindered our programme.
Lessons learnt for involving patient and public involvement partners in SWAT research
-
Trial teams should include PPI when planning SWATs: In the same way that PPI is expected for the main trial, PPI should be an important consideration when planning the SWAT and should include those who would potentially be impacted by the recruitment or retention strategy being evaluated.
-
Depending on the nature of the SWAT, PPI members can include potential or enrolled trial participants.
-
Helping to identify novel or adapt existing strategies: PPI partners can help trial teams to identify novel or untested strategies to be evaluated. In PROMETHEUS, we asked PPI members to consider potential strategies that they would be interested in getting tested; a strategy suggested in response to this, which was to thank trial participants, led to the development of SWAT 119, which is testing the effectiveness of a thank-you card following each study visit. Where a strategy has been tested in a different population, format or context, PPI partners could be particularly valuable in assisting trial teams to adapt that strategy to be tested within their particular trial.
-
Accessing PPI partners: For SWAT strategies targeting participants or potential participants, the first port of call should be to approach the PPI members that informed the host trial. For strategies targeting staff, such as a recruitment training course for site staff, the key activity would be to approach staff undertaking the recruitment at sites.
Chapter 6 Lessons learnt: SWAT development and funding
Chapter overview
In this chapter, we outline how SWAT strategies should be prioritised or selected, as well as key elements to consider when designing a SWAT.
Selecting which strategy to test in a SWAT: the importance of prioritisation
When a decision has been made to include a SWAT within a host trial, the next step is to determine the most suitable SWAT to undertake. To do this, the PROMETHEUS group found that there were several areas where research teams wanted support, information, and clarity:
-
to have clear SWAT research priorities;
-
identifying an intervention suited to a host trial population and trial type;
-
communication as to when no further SWAT replications are necessary;
-
transparent costs of SWATs;
-
SWAT interventions for low budgets.
This would ensure efficient resource use and maximum research output.
The PROMETHEUS priorities were based on SWATs that could be undertaken with funding of £5000 or less. For further information on how the PROMETHEUS priorities were identified, please refer to Chapter 2.
While the PROMETHEUS programme identified a list of priorities for this work (see Chapter 2), there remains a need for continually updated research priorities to allow researchers to address the questions relevant at that time. Collaboration is essential when undertaking SWATs. The priority list needs to be developed and finalised in collaboration with working groups that are involved in SWAT and methodology work such as Trial Forge116 and those involved in the Cochrane reviews. 23,24 PROMETHEUS has already proactively collaborated with Trial Forge and the MRC NIHR Trial Methodology Research Partnership (TMRP) to begin this work. 117 Together with Trial Forge we have set up the Trial Forge SWAT Network118 and have utilised membership of the TMRP Recruitment and Retention working groups to promote priorities for SWAT work. In addition to a priority list, to ensure research output is maximised, establishing a real-time method to communicate SWAT priorities to researchers is a recommended future development. The Cochrane reviews on recruitment and retention strategies23,24 are updated every few years and so establishing a more rapid method would enable the research gaps to be identified and addressed. Additionally, we identified that there is a need to work with research teams to help establish SWAT priorities based on their experiences as to what is important with their research areas, settings, and populations. Currently, some of the research priorities that have been identified by both the PRioRITY34,110 and PROMETHEUS groups are not suitable for all trial teams and therefore teams have expressed a reluctance to embed them. One such example is the use of SMS for participant retention. Many teams already use SMS and so do not want to undertake a SWAT testing SMS versus no SMS, which the Cochrane review24 states currently requires further evaluations. This further highlights the importance of working with teams undertaking methodological research to identify SWATs that are acceptable, thus increasing the likelihood of SWATs being undertaken and the findings implemented. There is also a changing landscape that trialists need to be mindful of, for example, SWATs relating to postal follow-up of participants are becoming less relevant with moves towards electronic data collection.
To ensure that SWATs are undertaken in areas of need, it is essential that there is a priority list that demonstrates not only which SWATs are needed but in which trial populations. This will prevent unnecessary SWAT replications from being undertaken and ensure resources are directed to areas that are needed to increase the evidence base. For example, there is high-certainty evidence that pens are an effective strategy for older females as a retention strategy but only moderate-certainty evidence in a younger population and males. 118 Therefore, it is recommended that if additional replications are undertaken, they are done so within these populations.
There is also a need for creating more awareness around embedding a SWAT which ‘de-adopts’ a strategy that has already been planned and budgeted for within a host trial, but further evidence is needed for evidence of its effectiveness, for example, newsletters. These are often sent to sites and participants so these could be tested as part of a low-cost SWAT. Additionally, in-person site initiation visits could be compared to remote site initiation visits and if site initiation visits are ‘standard’ practice then such a SWAT would save money. 119
SWATs prioritisation, GRADE and pragmatism
When considering SWAT priorities there is a need for pragmatism; the question needs to be asked as to whether additional replications are actually necessary. It is not possible to complete a SWAT within every population, each replication requires resources, and these resources could be directed to an area where there is still large uncertainty around a strategy.
When assessing the SWAT evidence base GRADE is used to rate the quality of evidence and grade the strength of recommendations. 120 The GRADE domains for rating a judgement down are based on five different criteria: risk of bias, imprecision, inconsistency, indirectness and publication bias, and result in a classification of either very low, low, moderate or high. Currently, the PROMETHEUS team has collaborated with Trial Forge and assessed SWAT evidence to create Trial Forge evidence packs118 that break down recommendations by population according to the GRADE assessments (see the ‘support teams to undertake SWATs: Trial Forge Evidence packs’ section and Figure 2). There is, however, a trade-off between high certainty and directing resources to maximise output and so it is suggested that we may have to accept that we do not have ‘high certainty’ for all populations and a pragmatic consensus will need to be made as to whether further SWAT replications are recommended.
FIGURE 2.
Summary example in Trial Forge evidence packs, used with kind permission from Trial Forge. 118
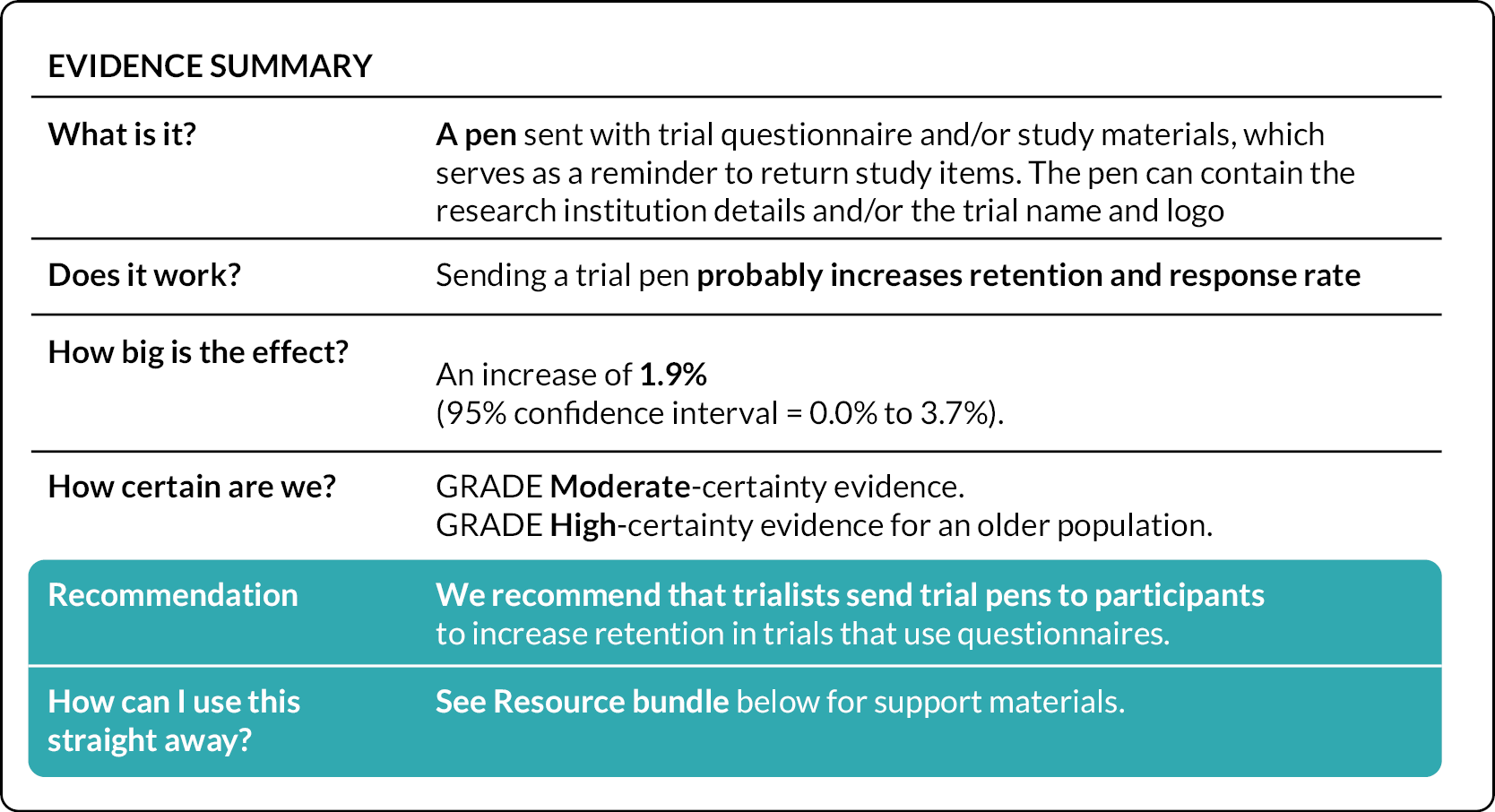
Supporting teams to undertake SWATs: Trial Forge evidence packs and an information repository
Trial Forge107 have developed a number of Trial Forge evidence packs. 118 These packs provide trialists with information on how many SWAT replications have been undertaken and the evidence base to date, including a summary of GRADE. Figure 2 is an example summary found in a Trial Forge evidence pack. Additionally, they provide teams with practical implementation advice on how to undertake and embed the specific SWAT. These packs enable research teams to make an informed decision – feedback from researchers that have used them is that they are a useful additional resource to support teams.
To further aid teams in selecting a SWAT, a repository of information could be developed to share necessary information on a freely accessible public platform. This would include not just protocols which are currently found on the Northern Ireland SWAT store,32 but additional helpful documents to aid trial teams such as those detailed in Table 9. Currently, steps are being made to progress a repository in collaboration with the Trial Forge SWAT Network. 121 This development will likely take several months to ensure that it is detailed and piloted, and a way to operationalise it presently and in the future is identified. Both the PROMETHEUS and Trial Forge initiatives will lead this and report on the progress via their websites and social media channels.
|
Design choices: cluster randomised versus individual randomised
In planning a SWAT, an important consideration is whether to randomise at the individual-level (e.g. the participant or potential participant) or at the cluster level (e.g. recruiters or hospitals). If the recruitment or retention strategy is focusing on participants, potential participants, or their proxies, an individual-level randomised design may be the most efficient and appropriate randomisation method. However, for logistical reasons cluster randomisation, perhaps by study site, might be more feasible. On the other hand, if the recruitment or retention strategy affects individual participants or potential participants, but is mainly happening at a different level, such as whether training trial recruiters improve recruitment rates then a cluster design may be more appropriate (see Chapter 8 for case study). Cluster randomisation is often used to minimise ‘contamination’ between intervention and control participants. 119 For example, in a SWAT where the intervention is to train research nurses recruiting participants on how to improve trial recruitment, it would be difficult to undertake individual patient randomisation within hospital sites (i.e. intervention patients are recruited using the training method; control patients recruited using standard practice) as nurses who receive the recruitment training might use some of the training received to recruit control patients. Nurses within the same hospital site might also share the recruitment training information (advertently or inadvertently) with colleagues recruiting control patients, who may also change their usual recruitment practice, leading to contamination. This contamination might result in dilution bias and consequently lead to type II error (i.e. erroneously concluding there is no effect when the intervention is effective), which cluster randomisation would guard against.
The design of the host trial, as well as the recruitment methods, clinical context and patient population of the host trial, can influence the decision on whether to use individual or cluster randomisation. If the host trial is a cluster randomised trial, the SWAT could use the same clusters as the host trial (although the trial clusters would be randomised separately for the SWAT stratified by the host trial’s cluster status so that half the intervention and half the control clusters would receive the SWAT).
For many SWATs, the decision about whether to randomise at the individual or cluster level is also influenced by the practicalities and ease of implementing the SWAT, which often focuses on minimising the burden on the staff delivering the SWAT. For instance, a retention trial of different letters, or incentives, such as pens, may use a cluster design for practical reasons to avoid centres having to individually randomise follow-up letters to different conditions. The MRC-funded START programme, which tested 2 recruitment strategies across 12 host trials anticipated that some host trial teams would prefer to use cluster allocation of the START recruitment interventions, to ease the logistical burden on the trial, as well as to reduce the risk of possible contamination. 25 This is also the approach we took in PROMETHEUS, so where individual randomisation was likely to be a challenge or burdensome to those undertaking the SWAT, we suggested to applicants that they consider cluster randomisation. Table 10 outlines examples of individual and cluster randomised designs in SWATs.
| Types of individual randomisation | Reference |
|---|---|
| Types of cluster randomisation | Reference |
| Potential participants are randomised: such as inviting them into a trial, either face-to-face or through written invitation. | See Parker et al. (2018)49,122 |
| Existing trial participants are randomised. | See Chapter 8 Christmas card SWAT, and Coleman et al. (2021)49 |
| Factorial randomisation: such as a 2 × 2 factorial SWAT where participants due their follow-up questionnaire are randomised to be sent: a pen; a social incentive text cover letter; both; or neither. | See James et al. (2021)68 |
| Zelen design: only those randomised to the intervention group are asked for consent. | See Fowell et al. (2006)123 |
| Recruiters are randomised: such as individual GPs, surgeons, research nurses or lay advocates. | See Larkey et al. (2002)124 |
| Recruiting sites are randomised: such as general practices, hospitals or community-based mental health teams. | See Chapter 8 Training SWAT, and Parker et al. (2022)51 |
| Time periods are randomised: such as a week or month of recruitment. | See Sheridan et al. (2020)125 |
| Matched pairs (typically recruiting sites) are randomised together: such as similar-sized hospitals or mental health teams. This can use the same cluster pairs as the host trial. | See Hughes-Morley et al. (2016)126 |
For statistical considerations of SWATs, please see Chapter 9.
Funding for SWATs
There are limited funding streams available for methodological work both in the UK and internationally. PROMETHEUS, funded by the MRC UK and NIHR as part of the MRC-NIHR Methodology Research programme was able to offer teams funding, lack of which can be a limiting factor to researchers undertaking a SWAT. 36 In Chapter 3 we outline the average cost of a SWAT funded through PROMETHEUS. Prior to PROMETHEUS, the UK MRC funded the MRC START programme, which tested the feasibility of undertaking SWATs in a co-ordinated way. 25
Some funding agencies have engaged with the need for SWATs to improve trial conduct. These include the Irish HRB which has a programme of funding SWATs up to €25,000 and the UK’s NIHR HTA programme that offers UK research teams up to £10,000 to embed a SWAT4 and up to £30,000 to embed Studies Within A Project (SWAPs) across a number of their funding streams for multiple long-term conditions. 5 Our experience with PROMETHEUS suggests that this level of funding should be suitable for many different SWAT interventions to be embedded within host trials. However, within the PROMETHEUS programme lower cost and easier SWATs were deliberately chosen by the PROMETHEUS Trial Management Group (TMG) as priorities. Additionally, the NIHR through its Health and Social Care Delivery Research (HSDR) and Fellowship streams has also funded SWATs. 30,126
Lessons learnt for trial teams and methodologists undertaking Study Within A Trial research
-
When setting SWAT research priorities, methodologists should provide as much information as possible so that teams are able to make informed decisions. They should consider developing strategies to aid teams to identify and select suitable SWAT interventions for their individual host trials. SWAT priorities need to be clearly communicated to trial teams and funders.
-
We would recommend that the priorities are presented alongside estimated costs, resource use (including the time needed to undertake the SWAT) and, if possible, a protocol. This was a strategy that we undertook during the PROMETHEUS programme, and feedback received from trial teams was that this was helpful.
-
The allocation units of the host trial that can be randomised for the SWAT consist not only trial participants but also sites, recruiting staff and time periods. The decision on the best allocation unit will be informed by a combination of what is most appropriate methodologically, and what is practical and acceptable to the host trial team, PPI members of the host trial team and staff undertaking recruitment activities.
Lessons learnt for funders
-
Trial teams want to know that they were undertaking a SWAT that is necessary and relevant to increase the evidence base. This will ensure efficient resource use and maximum research output. More funding is needed to support the development of these types of resources.
-
When applying for funding, trial teams need to indicate whether the question they are addressing is a priority SWAT question, as well as provide a clear rationale for selecting that particular question.
Chapter 7 Lessons learnt for governance approvals for SWATs
Chapter overview
In this chapter, we discuss our experience of obtaining governance approvals for SWATs, including approvals needed from the host trial Sponsor, trial oversight committees, REC and HRA approvals. We outline Key Learning for Sponsors, oversight committees, Trial Management and for journals and peer reviewers. Additionally, we consider the operational ethical issues in relation to embedding SWATS at scale, rather than an empirical analysis of the conceptual ethical issues.
Governance approvals
Studies Within A Trial are designed to be embedded within a host trial with no implication on its integrity, rationale or outcomes15 and, as a result, the obtaining of governance approvals should, in theory, be relatively simple. Despite this, many difficulties including those similar to obtaining approvals for large multi-centre studies continue to prevail. 127–129 These include, but are not limited to, the need for patient consent and/or information regarding the SWAT, study power and a misunderstanding or lack of understanding with regard to the methodology such as the need for replication. These difficulties pertain at every level of study approval from oversight committees and patient advisory groups, through to Sponsors and governance bodies.
Throughout the PROMETHEUS programme, a range of queries around the approvals process for SWATs were raised. This chapter outlines the queries noted at each level of approval, details some potential ongoing developments in this regard, and outlines suggestions for each group involved in the approvals process for a SWAT.
Sponsor approval
In the case of individual SWATs, sponsorship usually rests with the host trial Sponsor. Many of the PROMETHEUS SWATs were conducted through YTU and therefore were sponsored by the University of York; a Sponsor who has for a long time supported SWATs and other methodological work and so has an understanding of the nuances associated with these. As a result, obtaining Sponsor approval of individual SWATs was generally straightforward. SWAT naïve Sponsors however often raised queries with regard to the methodology or processes for adding the SWAT to the host trial approval. For example, Sponsors have asked for the SWAT to be added to the main study protocol, thus resulting in a substantial amendment and the addition of additional information on the SWAT to participant-facing materials which has potential to dilute the SWAT (see Ethics/HRA approvals).
On the converse, the fact that SWATs are usually methodological, rather than clinical, posed difficulties for another sponsorship arrangement. The host trial was sponsored by an NHS Clinical Commissioning Group (CCG), with the SWAT being undertaken by study team members based at the local university. The CCG was keen that the university assume sponsorship for the SWAT, which posed difficulties for amendment submission given two Sponsors cannot be detailed in a single application. In the end, the CCG agreed to be Sponsor.
For co-ordinated SWATs, sponsorship arrangements can be more complicated given the number of host trials involved and that many, if not all, may have different Sponsors. For the two co-ordinated SWATs undertaken within the PROMETHEUS programme (see Chapter 8 for case studies), sponsorship was, ultimately, held by the institution associated with the SWAT co-ordination (in both cases the University of York). In the instance of the Christmas Card SWAT (see Chapter 8 for case studies), the original proposed Sponsor did not understand SWAT methodology and so was unwilling to approve this. The SWAT was only delivered successfully when sponsorship was undertaken by a Sponsor with an understanding of SWATs. As a result, there is undoubtedly potential for difficulties with obtaining Sponsor agreement and approval for co-ordinated SWATs. It may be difficult to get agreement for sponsorship where the proposed SWAT Sponsor is an academic organisation with no existing association with the host trials, or where multiple Sponsors are associated with the host trials, but they are unwilling to take sole oversight of the methodological component.
Oversight committee approvals (Trial Management Group/Trial Steering Committee/Data Monitoring Committee)
Throughout the PROMETHEUS programme, there was little difficulty in obtaining support for SWATs from host trial oversight committees. TMGs were generally happy to support the inclusion of a SWAT if the Chief Investigator and Trial Manager proposed this. Furthermore, where the trial management team was supportive of undertaking a SWAT then the TSC and/or Data Monitoring Committee (DMC) were happy to support their inclusion in the host trial. As part of routine study oversight, it is suggested that the TSC particularly should have oversight of SWAT progress given their remit to endorse TMG actions130 and provide overall study supervision. 131 The general low-risk nature of SWATs oversight from the DMC may not be warranted, although this is dependent on the nature of the host trial and SWAT, and DMC agreement.
Ethics/Health Research Authority approval
Many research teams have reported barriers when gaining ethical approval with extensive queries being made during the approvals process. This was particularly frustrating for trialists when the application was for a replication SWAT which had already received ethical approval in another host trial.
Barriers to approval reported by trials participating in the PROMETHEUS programme included ethics committees being concerned that participants would not explicitly be asked to give consent to take part in the SWAT and that participants would not be informed about the SWAT. From a methodological perspective, pursuing consent for a recruitment or retention SWAT may dilute SWAT intervention effects through the Hawthorne effect and/or resentful demoralisation. Recruitment SWATs themselves due to their nature could be very confusing for potential participants as it would not be clear whether they were being asked for consent to take part in the SWAT or the host trial itself, and in the case of recruitment SWATs the participant may not yet have been approached about the host trial. Similarly, for retention SWATs, there is potential for confusion if additional consent is sought. As a result, it may therefore be preferable to not request explicit consent for SWAT participation, although, in accordance with Good Clinical Practice and Research Ethics, this should be considered on an individual basis to ensure participants’ interests and rights are protected. It may therefore be appropriate to discuss the need, or not, for consent with the study PPI group or other relevant stakeholders while reviewing other elements of the SWAT and to reflect on this discussion, and the agreed consensus, in the submission to the REC.
Furthermore, existing consent arrangements of the host trial may provide appropriate consent for SWAT inclusion. For example, if the host trial has obtained consent to contact a participant by a range of methods (e.g. postal, e-mail, telephone, SMS), then this would cover SWAT interventions such as newsletters, different cover letters, or SMS reminders. Furthermore, many trials routinely now obtain consent to contact for future research opportunities which would also cover SWAT inclusion for both recruitment and retention interventions.
Where notification and consent of participants regarding the SWAT are not deemed to be required, this can be challenging to explain to a REC and so the PROMETHEUS team was frequently asked for advice. 132 We suggest that study teams should document explicitly what will happen as part of the SWAT, when this will occur, and which elements comprise host trial procedures and which comprise SWAT processes. 132 It may also be useful to reflect on the lack of current evidence in relation to recruitment or retention, the need to improve research efficiency, and to note any precedent set by similar SWATs which have been approved and conducted. 132
In addition to the concerns around informed consent, there was also concern that SWATs are underpowered and additional information was requested to explain how SWATs were analysed and the need for replications and evidence synthesis (see Chapter 9).
In many instances, such issues were often resolved when sufficient information and justification was provided, and adding PPI input to these justifications would undoubtedly allay concerns further. Despite this, there was however one instance where the ethical committee refused approval of the SWAT. The ethics committee viewed the question proposed by the SWAT (different information in two cover letters with a follow up questionnaire) as valuable, however, deemed that as this question could be considered for any study that this should be undertaken as a new non-interventional research study and not within the confines of the host trial. Despite the host trial team providing additional information on SWAT methodology, support of their oversight committees, lack of impact on the research sites and links to the PROMETHEUS programme, it was not possible to change their view on this and so the SWAT did not commence.
Where SWATs are low risk, and supported by PPI input, a risk-based approach may be relevant to ensure streamlined activity. It is worth noting that most if not all the SWAT strategies and the SWATs themselves undertaken within PROMETHEUS are low risk to host trial participants. In PROMETHEUS we have generally tested strategies that are already being used in trials, but without any real evidence regarding their effectiveness. As a consequence, it is likely that one of the key issues related to risk is the need to blind host trial participants to their participation in the SWAT, in order to avoid (or at least reduce) information (outcome) bias. This would be beneficial for the individual SWAT and for the wider research community; if there were fewer barriers, more SWATs could and perhaps would be undertaken which would increase the evidence base. In the case of recruitment and retention, this would lead to more effective strategies which would result in more efficient research being undertaken and so prevent research waste, which itself becomes an ethical issue when it results in future patients receiving effective treatments more slowly (or prolongs harmful treatment).
During the course of the PROMETHEUS programme, HRA also identified this barrier. As a result, they sought collaboration from both the PROMETHEUS group and Trial Forge107 to develop a more efficient streamlined approvals process for SWATs. The proposed revised procedure would result in one team holding overall permission for the overarching SWAT evaluation with a specific protocol to be embedded by other host trials. This approach is similar to the approvals process used for the Christmas card SWAT49 where an overarching approval was granted for the SWAT which covered all host trials involved. As detailed in Table 11 the proposed HRA process differs slightly as, depending on the type of SWAT, a graded approach to ethical approval is required.
| Group | Description | REC approval required | UK approvals and amendments |
|---|---|---|---|
| 1 | The activities targeted by the SWAT are not of a type that needs to be described in detail in a host trial protocol (and so have not required previous approval) but are operational or management activities generally done by the central trial team. For example, randomising the host trial chief investigator to visit some recruiting sites and not others to see if this improves recruitment. | The overarching SWAT does not need REC approval, nor are amendments to host trial REC approvals required. | The overarching SWAT would need study-wide review. UK amendment is not required for individual host trials evaluating the SWAT. |
| 2 | The SWAT interventions affect participants but affect a process that does not need to be described in detail in a host trial protocol (and so have not required previous approval). These interventions are provided to the research team via operational or instruction documents, rather than a protocol, which are not reviewed by the REC. However, in some cases these interventions are also described in the protocol. Where this is the case, the intervention remains a Group 2 SWAT due to the low risk and unobtrusive nature of the intervention. For example, randomising whether trial participants are sent a greetings card or not to see if this improves retention. |
The overarching SWAT needs REC approval. Where the host trial protocol does not detail the process targeted by the SWAT, no amendments to host trial REC approval required. Where the process targeted by the SWAT is described in the host trial protocol, an individual SWAT that changes the process should be submitted to the host trial REC as a substantial amendment. |
The overarching SWAT would need study-wide review. A non-substantial amendment to the host trial for UK approval should be submitted. Where the process targeted by the SWAT is described in the host trial protocol, an individual SWAT that changes the process should be submitted as a substantial amendment to the host trial. |
| 3 | These SWAT interventions change the design of the host trial, change procedures undertaken by participants, or the information received by participants. For example, randomising whether potential participants receive a standard information sheet or one designed by PPI members. | The overarching SWAT needs REC approval. Amendments to host trial REC approvals will be required and will be submitted as a substantial amendment to the REC that approved the host trial. |
The overarching SWAT would need study-wide review. A substantial amendment to the host should be submitted. |
In Group 1, no additional ethical approval would be required, however, HRA study-wide review would still be required. The HRA have deemed this to be appropriate on the basis that these SWATs do not involve or impact directly on participants. Examples of such SWATs include randomising sites to different methods of study training. 46,119
The HRA propose that Group 2 SWATs would be those which may be participant focused but are deemed to be very low risk, modest interventions, for example sending a festive greetings card or newsletter to improve retention (Coleman et al. 49, Mitchell et al. 153). Such overarching SWATs would only require ethical approval and HRA study-wide review required, should the process targeted be detailed in approved study documentation.
Ethical and HRA review would be required for any Group 3 SWAT due to the impacts this would have on trial procedures or information provided to participants, for example use of alternative information sheets or cover letters. 68,133 These types of strategies are still low risk to participants, but they impact on direct participant-facing materials, which always require ethical approval, so such a review would simply be in line with current review processes. 68,134
Where required, these approvals may be gained at the start of the trial or as a substantial amendment if the trial has commenced. See Appendix 3 for further details of this.
Conclusion
The success of obtaining approvals of SWATs undertaken within the PROMETHEUS programme has varied, largely depending on the knowledge and understanding of this methodology. In the main, difficulties have been relatively minor, centring around delays to obtaining approvals, and the associated hassle for busy trialists of responding to ethical queries. There have however been a couple of more substantial issues where sponsorship or ethical approval was not provided. In such instances, this stands to limit the development of the evidence base and so increase or sustain existing levels of research waste.
First and foremost, the key learning for all stakeholders involved in SWAT approvals is developing a better understanding and awareness of the importance of such methodological research, and the nuances of SWATs versus multicentre trials. The co-ordinated SWAT approach proposed by the HRA is a step in the right direction and should over time help to improve and simplify the undertaking of SWATs moving forward.
In addition, there are specific learning points for individual stakeholder groups:
Lessons learnt for Sponsors
-
The changes proposed by the HRA to SWAT approvals should be communicated clearly and applied consistently when made publicly available.
Lessons learnt for oversight committees
-
TMG members should encourage and support the undertaking of SWATs within their trials.
-
TSCs should routinely review SWAT progress. Whether a DMC review is required is dependent on the host trial and SWAT.
Lessons learnt for trial teams and methodologists undertaking SWAT research
-
The input of PPI members with regard to the need for SWAT information and/or consent should be discussed during review of intervention design and content.
-
The HRA approach to SWAT approvals should be utilised wherever possible. Where SWATs are prospectively registered with the SWAT Repository, consideration should also be given to obtaining approvals to enable building of the evidence base simply and effectively.
Lessons learnt for journals and reviewers
-
Reviewers should consider the need to query informed consent with authors on the basis that this may not be feasible or appropriate for a SWAT. Transparent reporting (see Chapter 10) should help to facilitate this.
Chapter 8 Lessons learnt for implementing SWATs
Chapter overview
In this chapter, we describe our experience of implementing SWATs, and present case studies of different approaches to implementing SWATs using a case study of individual, standalone SWATs testing the effectiveness of pens for retention; and case studies of co-ordinated SWATs undertaken – one testing a training course for staff recruiting trial participants, and the other testing the effectiveness of sending Christmas cards to participants on retention rates. This chapter also highlights the pros and cons of standalone and co-ordinated SWATs.
Introduction
Due to the sample size of SWATs often being constrained to the size of the host trial, SWATs should be designed for replication and, ultimately, to be meta-analysed together with other similar SWATs of the same strategies. This provides the statistical power needed to identify small yet meaningful differences and ensure generalisability across different patient groups. However, when undertaking a meta-analysis, careful consideration needs to be given about when it is appropriate to combine data from SWATs in different patient populations, health conditions, and trial contexts. 15
Studies Within A Trial can be undertaken in one of two ways: individually and co-ordinated.
Individual Studies Within A Trial
Individual SWATs can be undertaken by individual host trial teams on an ad hoc basis, based on the interests and needs of the trial team. Individual SWATs independently test the same or similar interventions, within host trials at a time or opportunity that is convenient to the host trial. Multiple evaluations over an extended period may therefore be required in order to reach a definitive answer.
Individual SWATs can also be linked, such as in the MRC START programme, which developed an optimised PIL intervention and a multimedia information intervention; testing them across multiple host trials in SWATs over several years. 25,26 The TRECA study similarly aimed to embed the use of multimedia information resources into six host trials in the UK in a co-ordinated way. 30 In both MRC START and TRECA, each host trial had a linked but separate SWAT protocol, a separate ethical approval, and analysis, with the aim of undertaking a meta-analysis to determine the effectiveness. A hybrid approach to individual SWATs can be undertaken, with SWATs initially being conducted on an ad hoc basis, and later individual linked SWATs might be conducted to provide a definitive answer to the research question. The case study of individual SWATs of pens for retention in this chapter is an example of such a hybrid approach.
Advantages of individual Studies Within A Trial
The main advantage of individual SWATs is that these can be easier to implement than co-ordinated SWATs. There is no reliance on all host trials being able and ready to embed the SWAT at the same time and so trialists can implement a SWAT at a time which best suits their trial, as was the case with the pens for improving retention SWATs.
Individual SWATs also have the benefit of enabling the targeting of specific groups. The majority of SWATs in this case study were undertaken in trials with an older, female population; however, in order to further fill the evidence base, additional, individual replications of this SWAT are required in younger and/or male populations.
They also enable intervention modifications as required or preferred by the host trial. For example, in this case study, SWATs tested the use of a trial or university branded pen, as per the individual trial’s choosing. Other options included unbranded pens made from recycled materials or with limited plastic components to reduce carbon footprint, although these were not tested here.
Individual SWATs can take a long time to produce enough evidence to determine whether a strategy is effective or ineffective, and there is a clear economy of effort associated with co-ordinated SWATs. It is however also possible to derive sufficient data to boost, if not complete the evidence base, with individual SWATs. Three of the five pens for recruitment SWATs were completed individually via the PROMETHEUS programme which demonstrates that targeted effort to support can provide sufficient additional data to boost the evidence base and support more informed trial process decisions.
Disadvantages of individual Studies Within A Trial
Due to the sample size of SWATs often being constrained to the size of the host trial, sample size or power calculations are often not undertaken for individual SWATs, which has led to criticisms about the lack of power calculations and also, generally, being underpowered. 36 Even with replication of individual SWATs, it will take a long time to produce enough evidence to determine whether a strategy is effective or ineffective. Of the 137 strategies identified in the Cochrane systematic reviews of recruitment and retention strategies, only three strategies have robust evidence of their effectiveness. 23 Both Cochrane reviews called for more ‘depth’ to the evidence base, to allow more replication SWATs to develop the evidence more quickly for particular strategies. However, this can take a long time. For the pen SWATs, the first was reported by Sharp in 2006, and it was not until 14 years later in 2020 that there were sufficient data to determine the effectiveness of using pens as a retention strategy. There is still a need for further evidence in certain populations (younger people, and men), so this case study highlights that generating evidence in an ad hoc way using individual SWATs can be slow.
Case study of individual Study Within A Trial: pens for retention
Background
Postal questionnaires can be susceptible to low response rates. These are frequently used in trials to collect patient-reported outcomes, and so it is important to use effective strategies to maximise response rates.
The Cochrane review by Brueton et al. (2013)31 found that non-monetary incentives (e.g. pens, certificates or other similar tokens) with postal questionnaires had no clear effect in improving response rates or retention. The review however grouped all non-monetary incentives together and so individual strategies within this grouping may subsequently prove to be effective.
Including a pen with a questionnaire, as a non-monetary incentive, has been suggested as a low-cost intervention that may improve response rates. In the context of trial retention, firstly providing a pen with a questionnaire facilitates completion of this. Secondly, the inclusion of a pen acts as an acknowledgement of the participant’s involvement in the study, and as a reminder, which may make the recipient more likely to complete the accompanying questionnaire. Thirdly, the theoretical basis of reciprocation, results in participants feeling obliged to respond to this positive behaviour with the same in return. 134–137
Prior to the PROMETHEUS programme commencing, there was some evidence (two SWATs)138,139 to suggest that this may be an effective strategy in improving questionnaire response rates and reducing the number of reminders required. Given the existing evidence, and its match to PRioRiTY II110 priority six, this strategy was selected as a medium-priority question for the PROMETHEUS programme.
Aims and objectives
The individual SWATs were designed to evaluate the effects of providing a pen with a follow-up questionnaire on retention rates.
Results from the series of pen SWATs undertaken prior to or within the PROMETHEUS programme were then combined via meta-analysis by the Trial Forge Initiative in order to determine a definitive answer as to the effectiveness of pens for retention.
Methods
Individual SWAT methods
All pen retention SWATs undertaken in PROMETHEUS followed a core protocol. The only difference was that two SWATs68,92 included additional secondary outcomes and associated analyses.
Participants, interventions and outcomes
Table 12 summarises the participants, interventions and outcomes associated with the individual pen SWATs.
| Participants | Any participant in the host trial who was due to be sent a follow-up questionnaire at the study’s primary outcome time point was included in the SWAT substudy. Participants who withdrew from host trial follow-up before their follow-up questionnaire was due or those who had received their follow-up questionnaire prior to the start of the pen substudy were excluded. |
| Intervention | A pen printed with either the trial or university logo. |
| Control | Standard practice for the host trial, i.e. no pen. |
| Outcomes (Primary) | The proportion of participants in each group who return the questionnaire. |
| Outcomes (Secondary) |
|
Cunningham-Burley92 and James68 also assessed additional secondary outcomes
|
Randomisation and blinding
In each SWAT, participants were allocated using simple randomisation 1 : 1 to either intervention or control. A statistician who was independent of the questionnaire mailing activity generated the randomisation sequence.
Participants were not informed of and so could not provide informed consent for their participation in the SWATs. Given the nature of the intervention, it was however not possible to blind the participants to receipt, or not, of a pen with the questionnaire nor was it possible to blind research staff implementing the SWAT.
Sample size
Given their nature, the sample size for each SWAT was determined by the number of participants due to receive their postal questionnaire at the primary outcome time point within the host trial.
Analyses
For each SWAT, data were analysed using the version of Stata current at the time of analysis, on an intention-to-treat (ITT) basis, using two-sided tests at the 5% significance level.
Primary outcome
-
The proportion of participants in each group who returned the questionnaire was compared using logistic regression.
-
As the SWAT by James et al. 68 was factorial (social incentive cover letter, pen), the interaction term was also assessed in the logistic regression model.
Secondary outcomes
-
The difference in time to response between the two groups was compared by a Cox proportional hazards model.
-
Completeness of response between the two groups was compared using a linear regression model68,92 or logistic regression. 81
-
Requirement for a reminder mailing was compared using logistic regression.
-
The difference in costs between the intervention and control groups including direct and indirect costs.
Each trial adjusted the analysis models for main trial allocation. In addition, James adjusted for age and gender,68 and Mitchell for trial site. 81
Study Within A Trial meta-analysis methods
A meta-analysis of all relevant published SWATs was undertaken by the Trial Forge initiative in 2020 as part of their evidence pack series. 118
Results of PROMETHEUS SWATs testing the use of pens for retention
In total, 4550 participants were randomised into pen SWATs funded by the PROMETHEUS programme: 2276 to receive the intervention, and 2274 to receive the control. Fifty participants either withdrew or were deceased before the SWATs could be implemented leaving 4500 participants included in the analyses: 2249 intervention and 2251 control. The majority of participants were female (67.1%), and the average age was 64 years.
The results of the individual studies are reported separately. 68,81,92
Comparison with existing literature
Prior to the PROMETHEUS programme two trials, consisting of 8512 participants, reported within a 10-year period,138,139 suggested that when pooled, as shown in Figure 3, the inclusion of a pen with a follow-up questionnaire may be an effective strategy for improving response rates (pooled odds ratio 1.21; 95% CI 1.05 to 1.40; p = 0.01).
When the results of Cunningham-Burley,92 James68 and Mitchell81 were added to the meta-analysis,136 this contributed a further 4500 participants (an increase of 52.8%). The pooled effect as shown in Figure 4 indicated that including a trial pen with a questionnaire probably increases retention and response rate (pooled OR 1.21; 95% CI 1.09 to 1.35). 118 The evidence when combined has moderate-certainty GRADE evidence overall, but high GRADE evidence for an older population.
Cost effectiveness
The cost of retaining one additional participant due to inclusion of a pen with a questionnaire mailing is £25, which is cost-effective if based on the cost of enrolling an average participant in a NIHR-funded trial being £2200. 118
Discussion
Over 14 years, five individual SWATs have been undertaken sequentially to ascertain whether the inclusion of a pen with questionnaire mailings is an effective retention strategy. 68,81,92,138,139 Four of the five SWATs were conducted within a 4-year period (2016–20),68,81,92,138 and three of these were conducted within the PROMETHEUS programme. 68,81,92
The pooled estimate of effect for all five pen SWATs for retention showed an increase in retention of 1.9% (95% CI 0.0 to 3.7%). 140 Depending on population demographics, there is moderate- or high-certainty evidence in these estimates and so the recommendation is that trialists should include pens to increase retention rates in trials that use postal questionnaires. The bulk of replications undertaken were in female and older populations and so evidence for younger and male populations should be the focus of any further replications.
The costs associated with the inclusion of a pen for retention were estimated, using generic trials information, to be around £25 per additional participant retained. 140 Cunningham-Burley et al. identified a cost of £10.56 per additional participant retained,92 while others have found lower rates, for example a cost of $6.98 (approximately £5.60) for an additional participant to be retained. 140 While these costs are not inconsequential, the intervention is relatively low cost compared to other retention interventions, and in a large randomised controlled trial, these costs may well be possible to accommodate.
Co-ordinated Studies Within A Trial
Simultaneous SWATs are also a type of co-ordinated SWAT, but they are undertaken across multiple host trials at the same time. Simultaneous SWATs have one protocol for all the host trials, one ethical approval, one analysis and a write-up.
Advantages of co-ordinated Studies Within A Trial
A co-ordinated SWAT usually provides a much larger sample size than individual SWATs. They speed up the process of generating evidence and allow high-quality evidence to be generated at scale for one research question of interest. Due to their scale, it may be possible to embed the SWAT within enough host trials to allow for sufficient evidence to reach a definitive answer in one overarching evaluation. As the same protocol is standardised across the different host trials and patient populations, data can be pooled in a meta-analysis with greater certainty. They are much faster than undertaking the same number of individual SWATs and then undertaking a meta-analysis. They are also much more efficient, typically requiring just one ethical and regulatory approval for all the SWATs, with one central co-ordination point which also reduces the burden on host trial teams. They also benefit from an economy of scale, as well as having one central team instead of multiple host trial teams planning, undertaking, analysing and reporting the SWAT. They can be a useful methodology for testing more complex strategies that may be more challenging and/or costly for individual host trial teams, such as delivering a training course to trial recruiters.
Disadvantages of co-ordinated Studies Within A Trial
There are possible disadvantages to undertaking co-ordinated SWATs. They require co-ordination from a central point, which includes recruiting host trial teams. This requires a network or infrastructure to exist, to allow host trials to be recruited. In the UK, this has been possible through the infrastructure network established by the NIHR, particularly through the CRNs, through the speciality groups such as the Surgical Speciality Group, and through the CTU Network.
Due to their novelty and complexity, it may be more difficult to obtain regulatory approvals than just for a single SWAT. As part of PROMETHEUS, we have worked with the HRA in the UK to develop new regulatory approval processes for SWATs, which aim to make it easier for simultaneous SWATs to be approved. Chapter 7 outlines this in detail, and Appendix 3 provides a copy of the draft HRA guidance on SWATs.
There are also logistical challenges. It may be difficult to set up a co-ordinated SWAT across multiple host trial teams and trials units. For example, all the host trial teams must be ready for the recruitment/retention strategy to be embedded in the SWAT at a similar time. Delivering the SWAT in multiple host trials may also limit flexibility to deliver the SWAT strategy; for example, being restricted to the extent to which the SWAT strategy may be adapted to the host trial, and if the SWAT involves a strategy such as attending a training course, the dates of the training may be limited. Another challenge is that routinely collected data in the participating host trials may differ. Where host trial teams are external to the SWAT co-ordinating team, it may be difficult to obtain the SWAT data from host trials. Ways to address this are to establish clear timelines for transfer of data; identify during the planning stages how long it is likely to take to clean and securely transfer the data once generated; identify who will clean and transfer the data; and make sufficient funds clearly available to the host trial team to cover the costs of data preparation and sharing.
Case studies of co-ordinated Studies Within A Trial
In PROMETHEUS, we undertook two simultaneous SWATs, which we present here as case studies. The first co-ordinated SWAT focused on recruitment and was a feasibility study of staff training to improve participant recruitment into surgical trials and was embedded across four host trials. 51 The second was a retention SWAT, which tested the effectiveness of sending Christmas cards to trial participants on retention rates, across eight host trials, which was published as part of the BMJ Christmas edition. 49
Case study – staff training to improve participant recruitment into surgical randomised controlled trials: a feasibility Study Within A Trial across four host randomised controlled trials simultaneously
Background
The training of trial recruiters has been identified as the top priority topic for recruitment research according to the Directors of UK CTUs,33 and has been highlighted in a James Lind Alliance priority setting exercise for recruitment research. 34 A systematic review of recruiter training showed that training programmes were well received, and they increased recruiters’ self-confidence. 141 However, only 3 of the 17 studies included were RCTs. A more recent systematic review looking at the effectiveness of staff training on recruitment concluded that further work on developing a substantial evidence base around the effectiveness of education and training interventions for recruiters to trials is required. 142 As a result, there is a lack of evidence on the effectiveness of staff training to improve recruitment.
Aims and objectives
To test the feasibility of undertaking a co-ordinated SWAT to train staff who recruit participants into surgical trials, by assessing key uncertainties around recruitment, randomisation, intervention delivery and data collection.
Methods
Host trial and participant recruitment
Surgical trials recruiting or likely to be recruiting participants to the host trials in UK hospitals in April 2019 were invited to take part in this SWAT. To be eligible, host trials had to be undertaking face-to-face recruitment. Staff (surgeons and nurses) recruiting participants to the host trials were asked to express interest in attending the training.
SWAT intervention
The 1-day training course for staff recruiting participants into surgical trials was developed by The University of Bristol’s QuinteT team (Qualitative research integrated within Trials). 143 The training aims to share experiences, raise awareness of the hidden challenges of recruitment and equip attendees with strategies to optimise recruitment and informed consent. All staff members involved in recruiting participants at each hospital site were invited to attend a 1-day training course relevant to their profession (one date was offered to surgeons, and one to research nurses). Academic researchers within the University of Bristol’s QuinteT group delivered the training. The 1-day training was supplemented with the GRANULE (Generating surgical recruiters to randomised trials) online e-learning course (https://learn.nihr.ac.uk/course/view.php?id=385), which was e-mailed to all sites in the intervention arm after the course, or in place of if staff were unable to attend.
SWAT control group
The control group did not receive the recruitment training.
Outcomes
The primary outcome of interest was the ability to recruit multiple surgical trials simultaneously. To be considered feasible, we needed to enrol a minimum of two host trials. Secondary outcomes were:
-
Recruitment: Numbers of recruiting sites and recruiting staff from each site enrolling into the SWAT.
-
Randomisation: Number of sites recruited, number of sites randomised and any reasons for sites dropping out after recruitment.
-
Intervention delivery: Number of intervention training course groups initiated, staff attendance at training in the intervention group, number of participants per group, any reasons for non-attendance or failures in intervention delivery.
-
Acceptability of the training.
-
Staff confidence in discussing trial recruitment with potential participants immediately before, immediately after and at 1–3 months post training.
-
Participant screening and recruitment rate (defined as the proportion of eligible participants who gave their consent and were randomised into the host trial 6 months following delivery of the course).
Randomisation
Randomisation was performed separately for each host trial at the cluster (i.e. recruiting hospital site) level. On expressing willingness to participate in the training, recruiting sites within each host trial were randomised to be offered the training workshop (intervention group) or no training (control group) on a 1 : 1 basis. A computer-generated randomisation schedule was generated using permuted blocks, stratified by recruiting trial.
Data collection
Online questionnaires were distributed to participants using Qualtrics (QualtricsXM, Provo, UT, USA). Participants were asked to complete questionnaires 1 month before the workshop (all participants), immediately after the workshop (intervention group only) and 1–3 months after the training (all participants).
Statistical analysis
Statistical analyses were undertaken on an ITT basis, using a 5% significance level. Baseline data are reported descriptively by SWAT group, using counts and percentages for categorical data, and mean and standard deviation for continuous data. The paired t-test was used to compare pre- and post-training workshop responses, and follow-ups are reported as mean and standard deviation by arm. A linear regression model adjusting for baseline score and allocation was run on each of the 11 questions to compare the self-confidence at follow-up between the arms. Recruitment rate and the number of patients screened post training were compared using linear regression adjusting for host trial and SWAT intervention.
Results
Feasibility of recruiting host trials
We identified 12 host trials that were eligible to participate in the SWAT and 4 trials (33%) comprising 91 sites participated. Recruitment of surgical host trials occurred as planned, and we recruited four host trials in a 6-month period.
Details of the host trials can be found in Table 13.
| Trial | Area | Interventions | Number of sites | Target sample size | Primary outcome (time point) |
|---|---|---|---|---|---|
| DISC | Dupuytren’s contracture in adults over 18 | Injection of collagenase or surgery (≥ 18 years old) | 30 | 710 | Patient Evaluation Measure (2 years) |
| IntAct | Rectal cancer in adults over 18 | IFA, or white light endoscopic surgery | 25 (14 UK) | 880 | Clinical anastomotic leak rate within 90 days post operation (1 year) |
| PROFHER-2 | Acute 3- and 4-part fractures of the proximal humerus in patients aged over 65 years | Reverse shoulder arthroplasty, hemi arthroplasty or non-surgical treatment | 40 | 380 | Oxford Shoulder Score (2 years) |
| START: REACTS |
Rotator cuff tears that cannot be repaired | Arthroscopic debridement or arthroscopic debridement with the InSpace balloon | 16 | 212 | Constant–Murley score collected 12 months after surgery. |
Feasibility of recruiting sites, participants and randomisation
Across the four host trials, 29 sites agreed to participate (32%) and were randomised to intervention (15 sites, 29 staff) or control (14 sites, 29 staff). The 58 recruiting staff who enrolled into the SWAT were mostly research nurses (58.6% control and 41.4% intervention) and surgeons (31.0% in both groups). We found it feasible to recruit sites and recruiting staff.
Feasibility of training course
There were 29 staff in the intervention group who expressed an interest in attending the workshop, and of these, 11 (37.9%) attended. Those who attended the workshop were all female (100%), predominantly research nurses (81.8%) and the modal age group was 40–49 years (45.5%). Only one surgeon agreed to attend the workshop, which resulted in the surgeons’ workshop being cancelled. We initiated just one workshop – for research nurses, which was relatively well attended, so the training course initiation and attendance was partially feasible.
Acceptability of the training course
Research nurses who attended the workshop felt positively about the format of the course with a mean score of 9.3 [0 = very poor, 10 = excellent; standard deviation (SD) 1.0]. Participants felt they ‘learnt a lot’ with a mean score of 9.2 (SD 0.9). They also felt that the workshop would make ‘a lot of difference’ to their future recruitment practices, with a mean score of 8.7 (SD 0.9).
Impact of recruitment training on self-confidence and awareness
When comparing pre–post scores in the intervention group, participants rated their confidence in discussing recruitment higher after the workshop with an increase in mean score of 1.45 (95% CI 0.70 to 2.21, p = 0.002). They were also more confident about overcoming recruitment challenges with an increase in the mean score of 2.18 (95% CI 1.19 to 3.17, p = 0.001). However, the change in their awareness of the recruitment challenges was not statistically significant with an increase in score of 0.45 (95% CI −1.06 to 1.97, p = 0.52).
Impact of training on screening and recruitment rates
At baseline, the number of patients approached and/or recruited was similar across both groups (Table 14). At 6 months post training, there was no evidence of a difference in screening between sites randomised to the intervention versus sites allocated to control (coefficient −0.35, 95% CI −7.84 to 7.15, p = 0.92). Over the 6 months post training, the average eligible to recruited conversion rate across the participating studies was 58%; it was 55% in the intervention group and 63% in the control group. Linear regression of sites (63%) with screening activity post training identified there was no evidence of a difference in recruitment rate between the intervention and control groups (coefficient −0.07, 95% CI −0.43 to 0.29, p = 0.66).
| Summary | Control | Intervention | Total | |||
|---|---|---|---|---|---|---|
| Baseline (n = 22) | Follow-up (n = 21) | Baseline (n = 13) | Follow-up (n = 16) | Baseline (n = 35) | Follow-up (n = 37) | |
| Approached | ||||||
| 0 | 2 (9.1) | 9 (42.9) | 0 (0.0) | 7 (43.8) | 2 (5.7) | 16 (43.2) |
| 1–3 | 11 (50.0) | 1 (4.8) | 8 (61.5) | 0 (0.0) | 19 (54.3) | 1 (2.7) |
| 4–6 | 5 (22.7) | 5 (23.8) | 5 (38.5) | 5 (31.2) | 19 (54.3) | 10 (27.0) |
| 7–10 | 3 (13.6) | 5 (23.8) | 0 (0.0) | 2 (12.5) | 3 (8.6) | 7 (18.9) |
| > 10 | 1 (4.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (4.8) | 0 (0.0) | 2 (12.5) | 0 (0.0) | 3 (8.1) |
| Recruited | ||||||
| 0 | 3 (13.6) | 0 (0.0) | 1 (7.7) | 1 (6.3) | 4 (11.4) | 1 (2.7) |
| 1–3 | 12 (54.6) | 11 (52.4) | 8 (61.5) | 9 (56.3) | 20 (57.1) | 20 (54.1) |
| 4–6 | 6 (27.3) | 6 (28.6) | 3 (23.1) | 3 (18.8) | 9 (25.7) | 9 (24.3) |
| 7–10 | 1 (4.6) | 3 (14.3) | 0 (0.0) | 1 (6.3) | 1 (2.9) | 4 (10.8) |
| > 10 | 0 (0.0) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (4.8) | 0 (0.0) | 2 (12.5) | 0 (0.0) | 3 (8.1) |
Discussion
This SWAT demonstrated the feasibility of recruiting multiple surgical host trials to undertake a co-ordinated SWAT to evaluate the effectiveness of a staff training course. We found that there was no evidence of a difference in the number of patients screened between the intervention and control groups. Equally, there was no evidence of a difference in the number of eligible patients recruited and enrolled into the host trials between the two groups.
Undertaking a co-ordinated evaluation across multiple host trials can significantly speed up generating the evidence base for recruiting participants into trials and, consequently, improving trial efficiency and reducing research waste. However, this is only the case if key personnel attend training.
We found it challenging to engage surgeons to attend the face-to-face training, with only one surgeon being available to attend the training. As a result, a key cohort of recruiting staff was not represented which may have limited the findings. Delivering the co-ordinated SWAT in four host trials limited our ability to be flexible with the training dates, which may have made it more difficult for surgeons to have sufficient notice to fit this around their clinical commitments. There was an online component to the course with GRANULE, which was a reduced version of the face-to-face course, to supplement the training. Fully online courses may afford an opportunity to maximise attendance by removing the need to travel. Given the increasing use of remote, video-conferencing meetings and training technologies arising from the COVID-19 pandemic, this is now even more pertinent and therefore the medium by which training is delivered should be an important consideration for future studies.
Recruiting host trials for this co-ordinated SWAT required significant logistical organisation and demanded a central co-ordination point. The process for recruiting host trial teams typically involved an initial approach to the Chief Investigator (CI). If the CI was interested, then they would discuss the SWAT with their team to obtain buy-in, including from the TMG and Trial Manager. If there was buy-in, then there were further discussions about how the SWAT would align with the host trial, how sites would be approached, and about other logistical and methodological issues. This alignment was not always possible, and one host trial was not recruited because although interested in the training SWAT, they had different ideas about how they wanted the SWAT to be delivered in their trial. For instance, they wanted significantly more training dates for both nurses and surgeons than we were able to offer and wanted outcomes to be followed up over their entire recruitment phase of 3 years, which we were unable to do for resource reasons.
Case study – Bah humbug! Association between sending Christmas cards to trial participants and trial retention: randomised Study Within A Trial conducted simultaneously across eight host trials
Background
Poor participant retention can adversely impact study validity. 24,33,36 Christmas cards are often used to encourage participants to continue participation in a trial. A survey of UK-registered CTUs found that 40% had used Christmas cards as a retention intervention. 33 However, there is no evidence that providing Christmas cards improves retention. For those trials that use resources (both time and money) to send Christmas cards, there is a potential opportunity cost in that these resources might be better invested in other potentially more effective interventions. On the other hand, there could be a case for routine use of this incentive if there was evidence to support it.
One way to evaluate the effectiveness of Christmas cards on retention rates, and allow for rapid collection of evidence, would be to plan to do the same co-ordinated SWAT in several host trials at the same time.
Aims and objectives
To determine the effectiveness of sending Christmas cards to participants in randomised controlled trials to increase retention rate at follow-ups and to explore the feasibility of doing co-ordinated SWAT across multiple host trials simultaneously.
Methods
Design
Randomised co-ordinated SWAT conducted across eight host trials.
Setting
Eight randomised controlled trials researching various areas including surgery and smoking cessation.
Participants and sample size
Three thousand two hundred and twenty-three trial participants who were still due at least one follow-up from their host randomised controlled trial.
Intervention and comparator
Participants in the intervention group received a Christmas card sent by post. Participants in the control group did not receive a Christmas card.
Primary outcome
Proportion of participants completing their next follow-up (retention rate) within their host randomised controlled trial.
Randomisation and blinding
Participants were randomised in a 1 : 1 ratio (separately by each host trial) to either receive a Christmas card in mid-December 2019 or not receive a card.
Statistical analysis
Intention-to-treat analysis was undertaken, using a 5% significance level. Analysis models adjusted for the host trial allocation and the SWAT allocation and were run for each host trial separately. The results of the individual trials were combined using a random-effects meta-analysis. The primary analysis compared the retention rate between the two arms using a logistic regression model. The average cost per card sent was calculated using staff time for preparing the cards, and costs for printing and postage. Assumptions for CO2 emissions calculation were made, based on previous research were applied which assumes the card weighs 10 g, is printed on recycled paper and posted and recycled in the UK. 144
Results
Host trials and participants
Table 15 provides a description of the host trials involved. One thousand four hundred and sixty-nine (1469) participants [age 16–94 years; 70% (n = 1033) female; 96% (813/847) white ethnicity] across the eight host randomised controlled trials were involved in the analysis (cut short owing to COVID-19).
| Acronym | Area | Interventions | Follow-up method | Target sample size | Primary outcome |
|---|---|---|---|---|---|
| C-Gall | Surgery – gallbladder | Laparoscopic cholecystectomy or conservative management. | Postal | 430 | Short Form-36 at 18 months. |
| CPIT-3 | Smoking in pregnancy | Both groups receive smoking cessation service support and contingent shopping vouchers. Intervention group receives additional shopping vouchers (up to £400). | Telephone | 940 | Self-reported abstinence from smoking for 8 weeks. |
| DISC | Surgery/ Drug – hand |
Injection of collagenase or surgery. | Clinic/postal | 710 | Patient evaluation measure at 1 year. |
| FUTURE | Female bladder weakness | Urodynamics plus comprehensive clinical assessment or comprehensive clinical assessment only. | Postal | 1096 | Patient Global Impression of Improvement at 15 months. |
| ProFHER-2 | Surgery – shoulder | Reverse shoulder arthroplasty or hemi-arthroplasty or non-surgical. | Clinic/Postal | 380 | Oxford Shoulder Score at 2 years |
| PUrE | Surgery – kidney | Extracorporeal shockwave lithotripsy or percutaneous nephrolithotomy or flexible ureterorenoscopy with laser lithotripsy. | Postal | 1044 | EQ-5D-5L at 12 weeks |
| REFLECT | Dental | Prescription of 5000 ppm fluoride toothpaste or usual care. | Postal | 1174 | Proportion of participants receiving dental care due to caries at 36 months. |
| SWHSI-2 | Wound healing | Negative pressure wound therapy or usual care (normal dressing). | Postal | 696 | Time (days) to wound healing. |
Impact of sending Christmas cards on retention rates
No evidence was found of a difference in retention rate between the two arms for any of the host trials when analysed separately or when the results were combined [85.3% (639/749) for cards versus 85.4% (615/720) for no card; odds ratio 0.96 (95% CI 0.71 to 1.29; p = 0.77)]. A cumulative meta-analysis of the results can be seen in Figure 5.
Costs and carbon footprint
The cost of this intervention was £0.76 (€0.91; $1.02) per participant, and it will have a carbon footprint of approximately 140 g CO2 equivalent per card. One benefit of this approach was the need to only submit one ethics application.
Discussion
This co-ordinated SWAT investigated the effectiveness of sending a Christmas card to trial participants on retention rates across eight host trials and showed that sending a Christmas card to trial participants does not increase the retention rate.
This study showed that a co-ordinated SWAT can be embedded successfully by multiple host trials simultaneously. Not only does this increase the speed at which evidence can be accumulated, it has the additional benefit of allowing only one ethics application to be submitted to cover all the work, which will decrease the burden on individual trial teams.
When this co-ordinated SWAT was initially planned, it was hoped to involve more than 10 trials, and over 10,000 participants to ensure that the question could be answered by this one evaluation. For various reasons, the evaluation was delayed, and some host trials could no longer embed the SWAT which reduced the sample size.
This SWAT was successfully implemented across eight host trials, from two CTUs in the UK, at the same time. This is one of the first instances where a SWAT evaluation has been undertaken in this way, and its success should influence other researchers to consider undertaking simultaneous SWATs in the future, to allow answers to the methodological questions that SWATs pose to be obtained more quickly.
Conclusions
In the PROMETHEUS programme, we have demonstrated that it is feasible to adopt different approaches to undertake SWATs. Host trial teams can undertake individual SWATs focusing on their patient populations and recruitment context to add to the wider evidence base, which in time can be meta-analysed with similar SWATs to provide definitive evidence on the effectiveness of a particular strategy. An alternative is the co-ordinated SWAT, which potentially offers a much more efficient methodology than individual SWATs, requiring just one regulatory approval for all the SWATs through one central co-ordination point, providing economies of scale to significantly speed up evidence generation.
Both individual and co-ordinated SWATs have their advantages and disadvantages, and depending on the situation, one approach may be more appropriate than the other. Indeed, either approach may be suitable in some instances, depending on the preferences of the research team and the resources available to them (such as resources to recruit host trials and co-ordinate the SWAT), and the methods and interventions being used by the potential host trials. Strategies that are specific to the methods or interventions being used in a host trial, or are highly adapted to the host trial, such as offering a free yoga class to participants to enhance retention,145 or a patient and family co-developed participant information,146 may be more suited to an individual SWAT approach. Strategies that are more widely used, such as sending Christmas cards, text message reminders and financial incentives all lend themselves well to being tested using a co-ordinated SWAT approach. A mixture of both approaches may be used to provide a definitive answer to a question. For instance, a simultaneous SWAT was successfully undertaken for the recruitment training course to assess feasibility; however, a conclusion on the effectiveness could not be reached. A future evaluation of this training could take a co-ordinated SWAT approach, or a trial team may wish to undertake an individual evaluation by randomising its recruiting sites to receive the training or not.
Lessons learnt for trial teams and methodologists undertaking SWAT research
-
Embedding a co-ordinated SWAT in multiple host trials simultaneously, using the same SWAT protocol, has been shown to be a feasible, efficient way to scale up and speed up evidence generation using SWATs. This approach should be used more often to build an evidence base to support the selection of recruitment and retention strategies. This SWAT design could be utilised for other methodological aspects such as protocol/medication compliance strategies, and is suitable within most, if not all, research fields.
-
Teams wishing to undertake a co-ordinated SWAT should plan this carefully. Significant co-ordination is required to develop a common SWAT protocol that adequately covers all potential host trials, recruit host trials, apply for overarching ethical approvals, prepare and deploy the SWAT intervention and collect outcome data.
-
For most trial teams, individual SWATs remain the most convenient method for testing the effectiveness of strategies for their trial population or trial team; however, where resources allow, a co-ordinated SWAT approach should be considered as it has the potential to provide definitive answers more rapidly and are more efficient.
Lessons learnt for funding bodies
-
Funding bodies should fund SWATs, particularly co-ordinated SWATs as they are potentially efficient and are likely to provide robust evidence at speed, which in turn can help to reduce research waste.
Chapter 9 Lessons learnt for statistical considerations
Chapter overview
Randomised SWATs are like any other trial and as such, the statistical considerations for SWATs are similar to those for a typical trial. Although constrained within the context of the host trial, a SWAT should still follow principles for rigorous analysis, to ensure that the findings are considered reliable. Within this chapter, sample size, analyses and costing considerations when undertaking a SWAT will be detailed, alongside examples of our learnings from the PROMETHEUS programme.
Sample size considerations
It is well known that the sample size for a SWAT is constrained by the host trial. That is, for a recruitment SWAT the maximum number of participants will be the number approached to participate in the host trial, and for a retention SWAT, it is the number who are randomised into the host trial. However, SWATs are not necessarily implemented from the start of a trial, they are often embedded mid-way through recruitment/retention, and as such their sample size is reduced.
Typically, in UK trials funded by NIHR, recruitment of eligible patients is found to be between 50% and 88%, with retention seen to be between 80% and 97%. 5 If a SWAT was powered in a similar way to typical trials, with 80% or 90% power (using a two-sided 5% significance level), the host trial would need to be between 1438 and 26,256 participants, depending on the initial rates seen to detect a difference between 2% and 4% (Table 16). With the average target sample size of 1122 for trials published in the HTA Journal,147 sufficiently powering these assessments using one trial alone would usually be infeasible. Furthermore, should a SWAT have been undertaken using cluster randomisation, the number of participants required would be larger due to the likely correlation of the participants within the clusters. For example, in MRC START, the minimum sample size for cluster SWATs was appropriately inflated to consider cluster allocations, assuming an intraclass correlation of 0.02 in line with estimates from community studies. 25,26 Thus, multiple replications of a single SWAT evaluation are typically needed, where the results from each are combined to produce a more reliable result.
| Difference (%) | 80% power | 90% power |
|---|---|---|
| 50–52 | 19,614 | 26,256 |
| 50–54 | 4892 | 6550 |
| 60–62 | 18,672 | 24,996 |
| 60–64 | 4618 | 6182 |
| 70–72 | 16,158 | 21,632 |
| 70–74 | 3950 | 5288 |
| 80–82 | 12,076 | 16,166 |
| 80–84 | 2890 | 3868 |
| 90–92 | 6424 | 8598 |
| 90–94 | 1438 | 1924 |
Although it may seem inefficient to need to undertake multiple evaluations of the same strategy, this is actually a beneficial approach. Firstly, this allows for the generalisability of the results – Treweek et al. (2020)148 suggest that to conclude further SWAT evaluations are no longer a priority, it must have been tested in a wide range of contexts. If it were possible to conduct a large enough SWAT in one host trial for example, the findings would only be directly applicable to that specific trial population, so there is a need for replication in further host trials with a different patient population to ensure the findings are externally valid. This allows for the effectiveness of the strategy as a whole to be determined. As such the PROMETHEUS programme planned to fund several implementations of the same SWAT – for instance, the Christmas Card SWAT – to allow for the results to be combined within a meta-analysis. 47 This allowed us to conclude that Christmas cards are inefficient at increasing retention in an adult population, as it has been evaluated within a range of different host trials, over different research areas and a range of patient populations, all at once. 49
Analysis considerations
The approach for the analysis should use ‘intention to treat’. Indeed, it is more usual to use ‘pure intention treat analysis’ in a SWAT than is often the case with the host trials. This is because the primary outcome in a SWAT is recruitment or retention, so there is usually no missing data problem that afflicts most other forms of trials.
As with any analysis undertaken within trials, the analysis of a SWAT should be pre-planned, and detailed with a SAP. This could be within the main SAP for the trial, or as a separate stand-alone document – the choice may depend upon at what point of the trial the SWAT is being undertaken. This SAP should detail the data that are to be collected (i.e. the source of the data), the planned outcomes and the statistical methods to analyse each of the outcomes – similar to any SAP. Within the PROMETHEUS programme we created a template SAP for the host trial teams to adapt for their SWATs.
Typically, for a recruitment SWAT, the primary outcome will be the proportion of eligible participants consenting to be randomised. Although this is not necessarily always the case as was demonstrated in the ‘training’ SWAT which was a feasibility SWAT (see Chapter 8, case studies of simultaneous SWATs), and the primary outcome was measures of recruitment confidence, while actual recruitment and randomisation rates were secondary measures. But it is always important to report actual recruitment rates to enable all SWATs to be included in a future meta-analysis.
Similarly, for a retention SWAT, the primary outcome will most likely be the proportion of participants who are retained. The definition of a retained participant may vary; it could be those who have completed a follow-up, attended a clinical visit, or another way, but this should be explicitly stated. Ideally, however, one measure should include the number of participants that provided primary outcome data to, again, allow a meta-analysis. Equally, whether all participants, or only those participants that were due to be followed-up (i.e. excluding those who withdrew prior to the follow-up), are to be included should be detailed.
For both types of SWATs, a logistic regression model is an appropriate analysis model. For retention SWATs this model should be adjusted for the host trial allocation and any stratification variables that were included within the SWAT randomisation. The SWAT design should be taken into account when analysing; for instance, if it was a cluster-randomised SWAT the clustering should be accounted for in the analysis.
Thought should be given as to whether it is appropriate to undertake a sensitivity analysis, where those participants who did not receive the SWAT allocation are excluded (for instance, if there is a text message sending failure) – though in most instances this is not required.
Secondary outcomes may include time to events, which would be most appropriately analysed with a Cox proportional hazard model, or count data, such as the number of reminders required, which may require a Poisson or negative binomial regression model, although for each analysis thought should be given to whether the relevant assumptions are met, and planned analysis adjusted to be suitable (i.e. using a zero-inflated model).
Once several evaluations of a SWAT have been undertaken, a meta-analysis should be performed to combine the results. Typically, it would be expected that each SWAT would be analysed and reported within its own right, thus a two-stage meta-analysis approach, in which the results from each SWAT are combined, would be used. Should it be possible to obtain the individual data from each trial, a one-stage approach could be considered, though there are typically no additional benefits to this approach apart from considering interactions with pre-specified covariates (e.g. age, gender) and ensuring consistency in model adjustments. A cumulative meta-analysis can be useful in determining if the results appear to have converged, which can be used to help judge if another evaluation is required. 145 When planning a replication of a SWAT, thought should be given to the design and approach taken to ensure that the replications are similar. For instance, using similar wording if evaluating a message-based intervention, or consistent timing, so that the results of the meta-analysis are reliable. The same can be said about the definition of outcomes, is a questionnaire considered complete if it has been returned, if all the questions were answered, or if the primary outcome was completed? These differences can impact the results and should be consistent across SWAT replications wherever possible.
Meta-analysis
A key element of accumulating any evidence for trials is to combine similar trials in a meta-analysis. For SWATs, this is particularly important as, unlike a host trial, they are rarely large enough on their own to demonstrate any difference as being statistically significant. Consequently, for individual SWATs we have a high risk of a type II error, erroneously concluding there is no important difference when there is. To deal with this important issue we have to rely on combining our SWATs. Typically, this is done using a random-effects meta-analysis, as we cannot assume that the effect size will be the same across all SWATs – especially when undertaken in various contexts. In this programme of work, we planned to undertake a one-stage meta-analysis, as the individual patient-level data were to be provided to the team, as part of the funding agreement. However, typically it would not be possible to access this level of data, and thus a two-stage approach, where the estimates of effect from each SWAT are combined is most appropriate. We, therefore, would encourage researchers to undertake a cumulative meta-analysis if there are similar published SWATs available – this recommendation is in line with that provided previously. 145
Economics and costings
Economic evaluations alongside SWATs aim to assess the costs of the strategies that were being evaluated. Despite the very high costs associated with poor recruitment and retention in trials, there has been very little attention paid to the economic aspects of SWATs. It is not enough to simply identify effective recruitment or retention strategies; we need to identify which strategies are also cost-effective.
Within the PROMETHEUS programme, we encouraged host trials to undertake a cost analysis. To do this, we suggested they collect costs both in terms of resources required (for instance, the cost of printing cards), and associated staff time (such as preparing the cards for postage). The importance here is to highlight the additional costs that are being acquired by implementing the strategy, as opposed to the standard treatment. This allows a trial team to determine if, for their trial, the cost would be feasible. For instance, within the Christmas card SWAT we determined that the cost per card sent was on average £0.76 – so if you were to use this strategy in a trial of 1000 participants, it would be a direct cost of £760. However, this analysis can then be taken one step further to allow for an assessment of cost per additional participant recruited or retained – where the total associated cost is divided by the additional participants recruited/retained. However, in the Christmas card example, it was not found to be an effective strategy, and as such no additional participants were retained, so even a relatively small finance cost (such as £760), would be considered wasted resources when assessing retention. In some instances, the information from these evaluations from the PROMETHEUS-funded SWATs have helped inform Trial Forge Evidence packs,136 to allow trial teams to easily determine if the associated costs are worth the potential benefits.
There is additional work that can be done in this area. For instance, for a retention SWAT there may be additional cost benefits if the number of reminders sent also is decreased by the strategy, as this may lead to less paper and postage costs. However, to date, this level of in-depth economic evaluation has not been undertaken within SWAT research, and the area is of growing interest.
Conclusions
SWATs are generally straightforward to analyse. We should use an ITT approach with a pre-specified SAP. Power calculations are generally not required as the sample sizes are constrained by the size of the host trial. The prospect of each individual SWAT being included in a future meta-analysis should always be at the forefront of the researcher’s mind when the analysis is undertaken. Consequently, all the numbers of patients randomised should be reported, and the numbers who respond (i.e. for retention SWAT the number who provided the primary outcome) and the number who did not, and for recruitment, the numbers who were randomised into the host trial.
Lessons learnt for trial teams and methodologists undertaking Study Within A Trial research
-
Generally, the analyses are relatively straightforward and are much less complex than the standard analysis for the host trial.
-
A SWAT SAP should be produced before the analysis as per the standard practice in trial analysis.
-
It is not necessary, generally, to undertake a power calculation because each SWAT is constrained by the size of the existing host trial. The lack of statistical power in most SWATs to show the small, but important, differences in retention and recruitment should be addressed by combining SWATs in a meta-analysis. Consequently, researchers are encouraged when writing up their SWAT to include a cumulative meta-analysis of similar SWATs.
Lessons learnt for funders
-
The analysis of SWATs is generally a much simpler procedure compared with the usual clinical trial analysis. Consequently, the impact on funding for statistical input is relatively light.
Chapter 10 Lessons learnt for reporting Studies Within A Trial
Chapter overview
Research teams participating in the PROMETHEUS programme have noted challenges in reporting SWATs. These include accessing funding, identifying a journal that will publish a SWAT and having resources and dedicated time available to analyse and write up a SWAT publication. This chapter outlines these barriers and identifies progress made by the PROMETHEUS programme in mitigating these.
The need for transparent reporting of Studies Within A Trial
The need for transparent reporting of clinical trials is well known, and the same applies to SWATs. Given that SWATs are designed to be meta-analysed together with similar evaluations of the same strategies, the need for transparent and timely reporting is arguably more crucial, particularly given the limited evidence base around effective recruitment and retention strategies. Without this, the evidence base will not be increased rapidly, and accurate SWAT priorities cannot be recommended. The findings of a SWAT may be detailed within the host trial publication or in a standalone dedicated publication.
Reporting Studies Within A Trial
Concise, clear and structured reporting of SWATs in a timely manner is crucial to building the evidence base. Given these methodological studies utilise a randomised controlled trial approach, many trialists may use and adapt the CONSORT guidance when reporting SWATs. However, there are also guidelines, developed by Madurasinghe et al. (2016),27 primarily for the reporting of embedded recruitment trials, which offer a useful starting point for the structuring of both recruitment and retention SWAT-related publications. Noting the use of both guidelines in the reporting of SWATs, the PROMETHEUS team, in conjunction with Trial Forge, have developed concise SWAT reporting guidelines (see Appendix 4). The guidelines developed by the PROMETHEUS team are designed to be methodologically robust and encourage GRADE adherence, which is crucial to aiding evidence synthesis and so deriving definitive evidence. These have recently been piloted by those writing SWAT publications,80,81,92,149,150 and once feedback has been received the final iteration of the guidance will be submitted for publication.
Studies Within A Trial may be published within the host trial publication, which offers benefits in obtaining publication in a potentially high-impact journal. There are, however, disadvantages to this approach, particularly where the SWAT is recruitment focused and the host trial has a long follow-up period, thus resulting in delays to making the SWAT results publicly available. This, therefore, limits the prompt inclusion of SWAT findings in evidence synthesis, and this may be further exacerbated by the fact that the host trial publication may not note the SWAT in relevant parameters to allow it to be identified in a search strategy focusing specifically on SWAT methodology. Indeed, publishing the SWAT separately means that ‘SWAT’ can be included either in the title of the paper or the list of keywords to facilitate identification for future systematic reviews and meta-analyses. Furthermore, where SWATs are submitted as standalone publications, these offer an opportunity for early career researchers to obtain first-author publications. It also allows members of the host trial team to obtain peer-reviewed publications before the host trial is completed. For instance, the SCOOP trial of screening for osteoporosis published three SWATs of retention interventions,134,151,152 which were completed and published before the main SCOOP trial results publication. There is, however, a dearth of methodological journals, which would be an ideal resource for the publication of SWATs, and so population or condition specific journals may often be approached. From the experience of PROMETHEUS, these journals often have limited interest and may reduce the subsequent acceptance of SWAT publications.
Reporting platforms such as F1000Research153 can therefore be a simpler, and quicker, alternative to the traditional journal. For example, this platform undertakes pre-publication checks on submission, and once approved the publication is made available on the platform with open peer review and revision following accordingly, which means the publication is available promptly. The costs of platform publication are often calculated on the basis of word count and so may also offer an affordable alternative for open-access publication, although it should be noted that since the PROMETHEUS programme commenced costs for platform publications have increased. Alternatively, a SWAT might be reported in a preprint server while under review by a conventional journal.
Identifying peer reviewers
Once a journal has accepted the SWAT for review, an additional barrier is the difficulty in finding suitable peer reviewers to review SWAT publications. This has resulted in substantial delays in the submission of SWATs until the journal finds a peer reviewer. This not only increases the length of publication time, but it also slows down advances in the evidence base and could result in teams continuing to undertake strategies which have no effect or a negative effect on their trials.
The small pool of reviewers is due to several reasons. Firstly, some journals have strict criteria for those who are eligible for being a reviewer (e.g. the need to hold a PhD). Secondly, SWATs are a niche area and so many SWAT authors have collaborated that conflict of interests are commonplace, further reducing the pool.
Due to the difficulties observed in PROMETHEUS in identifying potential reviewers, a database of potential peer reviewers has been developed. Currently, this is not publicly available due to data protection and GDPR regulations,154 although it is hoped that this may be available in the future once permission has been sought from individuals.
Many journals require reviewers to list reviewing preferences or classifications which could be beneficial to journal editors in identifying and allocating publications for review. It is therefore useful for journals to include ‘SWATs’, ‘Trials Methodology’, ‘Embedded Trials’, or other similar fields to reviewer classification fields, and for those with an interest in this methodology to ensure that they have included this in their preferences.
Peer review feedback
As noted previously, SWATs are often constrained to the size of the host trial and so formal sample size calculations are often not completed. Often this leads to peer-review feedback, specifically noting the need for a sample size calculation and sample size justifications based on power criteria.
This may be as a result of the difficulties in obtaining appropriate peer review (see above) and so the additional use of methodological classifications may ensure that future reviewers are SWAT experienced rather than perhaps just trials experienced. Where reviewers have limited SWAT experience, it may be beneficial to provide additional guidance on the characteristics of a SWAT and what is expected to be reported methodologically, to assist with mitigating unnecessary queries.
Conclusions
As SWATs are designed to be meta-analysed, the need for transparent and timely reporting is necessary to develop the evidence base, find definitive conclusions on effective (or ineffective) strategies, and identify further SWAT priorities.
During the PROMETHEUS programme progress has been made with improving the reporting of SWATs, including guideline development, identification of publication routes and improving peer review. In addition, the publication platform F1000 has set up a SWAT Collection,153 and Research Methods in Medicine and Health Sciences155 is currently developing a SWAT special edition. From these developments, the following key learning points are suggested for relevant stakeholders:
Lessons learnt for trial teams and methodologists undertaking Study Within A Trial research
-
SWAT-specific reporting guidelines should be used to ensure that sufficient detail is provided to support GRADE evaluation of SWAT interventions.
-
Trialists should be encouraged to include SWAT or methodology classifications in their journal reviewer profiles if they have an interest in this methodology and are willing to provide reviews.
Lessons learnt for journals and reviewers
-
When selecting reviewers, SWAT and methodology classifications should be used where possible.
-
Journals should consider potential flexibility to reviewer credentials for niche areas such as SWATs; for example, a PhD can be substituted with relevant experience in the area.
Chapter 11 Recommendations for future research practice, direction and support
Chapter overview
Below we outline a range of recommendations for stakeholders such as funders, trial teams who are planning and undertaking recruitment and retention activities and for trial methodologists interested in SWATs.
Recommendations
Recommendations for funders
-
All trial funders should contribute to the effort to improve the efficiency of trials. Funders should encourage the teams that they fund to undertake SWATs.
-
Funding streams specifically designed to support SWATs must be made available to trial teams to continue building the trial process evidence base, for recruitment and retention as well as for other stages of the trial design and delivery process. This includes funding streams for undertaking specific SWATs, as well as infrastructure funding to support CTUs and other centres to undertake co-ordination activities that will support the design, conduct, reporting and implementation of SWATs and their findings to inform the work of the NIHR, the MRC and other funders.
-
The PROMETHEUS programme has demonstrated that co-ordination of activity remains crucial to the delivery of SWATs. A central, national co-ordination point that provides hands-on support needs to continue and funding should be allocated for this. Additionally, CTUs should identify a lead for SWATs to support SWAT activities and evidence-based trial conduct within the CTU, as well as a link with others undertaking SWATs elsewhere to share best practice. The funding for both central and CTU based support should be ongoing, in the same way as commissioned NIHR research and developing NICE guidance.
-
SWAT priorities need to be identified and communicated clearly to funders, and funders should use these priorities to inform their funding decisions.
-
Funders should develop a mechanism to promote SWAT questions that have been identified as a priority during the application process as a strategy to increase the evidence base for trial conduct using SWATs.
-
The mean cost of funding requested for a standalone SWAT within PROMETHEUS was £3535 (range £500–5000). The co-ordinated SWATs cost £10,668 (training SWAT), and £1306.40 (Christmas cards); however, these did not include costs for central co-ordination, data preparation and sharing by the host trial teams, data cleaning, analysis and write-up. These costs suggest that the £10,000 being offered by the NIHR for trial teams to include a SWAT should be sufficient for most planned SWATs. However, there may be occasions where trial teams may wish to test strategies that may be more expensive.
-
When applying for funding, trial teams should be asked to indicate whether the question they are addressing is a priority SWAT question, and to provide a clear rationale for selecting that particular question.
-
If teams are unable to undertake a SWAT, funders should ask that recruitment and retention methods are clearly reported to support the evidence base.
Recommendations for Sponsors
-
While most Sponsors did not raise any issues or difficulties with SWATs being undertaken within their trials, our experience suggests that there is a need for clear, easily accessible information about the nature of SWATs, as well as the role of the funder in supporting SWATs.
-
Any future changes proposed by the HRA to the approvals process need to be communicated clearly and applied consistently to each SWAT.
Recommendations for involving patient and public involvement partners in Study Within A Trial research
-
PPI should be considered when undertaking a SWAT, in the same way PPI work is expected to be undertaken in the main trial.
-
PPI partners should be involved to develop novel and untested recruitment and retention strategies, as well as adapting existing strategies to the context of their specific host trial and the population being enrolled.
-
PPI work can extend to professional stakeholders such as surgeons or research nurses who are intrinsic to site recruitment.
Recommendations for oversight committees
-
Our experience suggests that TMGs play a key role in decisions about whether a SWAT is undertaken and continued in the host trial or not. TMG members should encourage the uptake of SWATs in their trials. While the findings of SWATs may not always directly inform their host trial, the findings of SWATs undertaken during the early phase of the trial (such as during the pilot phase), may inform the decisions about which strategies should be used later, such as in the main trial.
-
TSCs should review the SWAT activity and progress, in the same way that they review substudies in a trial.
-
DMC review is dependent on the specific host trial and SWAT strategy being evaluated.
Recommendations for journals and reviewers
-
Journal peer reviewer profiles should be updated to include methodological interests and expertise, to support evidence of suitability to undertake a peer review for a SWAT publication.
-
When selecting peer reviewers, the SWAT and methodology interests as registered by reviewers should be used where possible.
-
SWATs are a niche area and so to increase the pool of reviewers, journals should consider being more flexible when assessing reviewer credentials to review a SWAT, such as allowing relevant experience in place of a PhD.
-
Reviewers should be advised that informed consent from participants is often not obtained when undertaking a SWAT, and that it is not necessary to query this with authors.
-
Robust and transparent reporting is necessary, that is compliant with CONSORT.
Recommendations for trial teams and methodologists undertaking Study Within A Trial research
-
There remains a need for continually updated research priorities to allow researchers to address the questions relevant at that time.
-
When SWAT priorities are set, methodologists need to provide as much information as possible to enable teams to make informed decisions about evaluating the priorities set.
-
Further work is needed to help teams identify suitable SWAT strategies for their host trials.
-
SWAT priorities need to be communicated clearly and consistently to trial teams.
Recommendation for trial conduct and using Study Within A Trial evidence
-
As the evidence base develops for effective and cost-effective recruitment and retention strategies, it will become increasingly important for trial teams to use this evidence base to inform their recruitment and retention activities. Trial teams need to actively engage with the evidence base to inform their practice, including using evidence from systematic reviews, and from web-based resources such as Trial Forge. 107 Funders will need to actively support the trials they fund to use evidence-informed recruitment and retention strategies.
Recommendations for future research
-
There remains a substantial need for more high-quality SWAT evidence and so Chief Investigators should be encouraged to consider the embedding of a SWAT at the funding stage. Further work is therefore needed to increase the awareness of the methodological importance of SWAT research with research teams and to develop engagement strategies to increase SWAT activity.
-
Future research needs to focus on identifying whether further replications are needed for existing evidence. If so, the gaps in the evidence base should be targeted. More co-ordination and replication of SWAT evaluations are encouraged.
-
A ‘real-time’ and dynamic communication strategy including a clear cost and resource breakdown for each suggested SWAT should be developed. This will alleviate the burden to trials teams to begin costing exercises and enables them to make an informed decision more quickly as to whether they can embed a given SWAT.
-
There is a need to aid teams to identify and select a suitable SWAT for their host trial populations. Pragmatic decisions on which SWAT may be appropriate and feasible to include should be taken as required. A mechanism to communicate SWAT research priorities is needed, and this information needs to be readily accessible for all trialists to refer to.
-
Our findings demonstrate that within an individual host trial, there is often a capacity to address more than one SWAT question, either separately, or simultaneously using a factorial design. This suggests that there is capacity to significantly speed up and strengthen the evidence base through teams undertaking more than one SWAT in their trials where relevant.
-
For certain strategies, co-ordinated SWATs should be encouraged. This method could be used to rapidly replicate SWAT evaluations to plug the evidence gap, as well as to evaluate more complex recruitment and retention strategies that may be more challenging to undertake using individual SWATs. Material should be developed to advise teams on how to undertake co-ordinated SWATs, as well as a method of networking to enable teams to promote their simultaneous SWAT and collaborate.
-
As the evidence base develops, it will become increasingly important for trialists to utilise the evidence base in a systematic way to identify both effective and ineffective strategies to inform their practice. Future work should therefore consider issues around the dissemination and implementation of SWATs and develop guidance to enable the wider trials community to undertake, report and adopt the findings of SWATs. Implementation science, the study of methods to promote the uptake of evidence-based practice, could be used to inform any such future work. Funders can also help by questioning strategies proposed by trial teams that are known to be either ineffective or not cost-effective.
-
Improving the knowledge of the potential ‘harms’ from implementing interventions that have no evidence of benefit is an important next step to help improve uptake.
-
While establishing the effectiveness of recruitment or retention strategies is important, the high costs of research waste and limited public finance means that cost considerations around SWATs are just as important. With only one retention strategy having high GRADE certainty of cost effectiveness, we encourage trial teams to undertake streamlined economic evaluations alongside all their SWATs in the future, for strategies shown to be effective, as well as those that are ineffective. For cost effectiveness, trial teams should look to report the cost per additional participant recruited or retained (i.e. the incremental cost-effectiveness ratio). Value of information analyses can help determine the need for further SWAT evidence where several SWATs already exist.
-
Many trial teams wish to contribute to developing the evidence base by undertaking non-randomised SWATs. Future work that informs the development of guidance for undertaking non-randomised SWATs would be helpful.
-
There has been limited assessment currently on the views of participants on individual SWAT interventions. Future work using process evaluations to better understand the acceptability of and impact of SWATs on participants would therefore be useful to better understanding the effectiveness of SWATs.
-
Working with trial teams to develop engagement strategies and training to undertake SWATs would be beneficial. Audience-specific guidance should be developed to support SWAT research. We suggest building on the successfully executed and attended ‘PROMETHEUS hosted webinars’ as well as undertaking research with teams to identify what training or support they require.
-
Trial teams have expressed they want to undertake SWATs that are important and necessary to increase the evidence base. Collaboration with funders, working groups involved in priority setting, and trial teams are needed to develop a mechanism to communicate this dynamic and evolving information once priority SWATs have been identified.
-
Work is needed to identify the barriers that teams have when undertaking a SWAT, and strategies and solutions for addressing these barriers should be identified and implemented.
-
Continued and proactive collaboration is needed with working groups to enable networking, and collaboration with teams undertaking SWAT research.
-
Reporting guidance is needed to support teams when writing publications to ensure sufficient information is included so that GRADE evaluation can be undertaken.
Chapter 12 Overall conclusions
In this manuscript, we have reported the design, methodology and results of the PROMETHEUS programme. We have used the lessons learnt from undertaking PROMETHEUS to highlight key practical lessons for a range of stakeholders in planning, designing, undertaking and reporting SWATs. We have used our experience from PROMETHEUS to make a range of recommendations for stakeholders. Given the PROMETHEUS programme was the first of its kind, this enabled a range of observations on the co-ordination and conduct of SWATs to be identified which have been presented here.
PROMETHEUS originally aimed to fund 25 SWATs and supported 42 SWATs, generating the single biggest body of SWAT activity in the world. When SWAT funding was made available, we found that many teams embedded SWATs into their research. Having a central point of contact that co-ordinated SWAT activity alongside providing funding has been key in determining the success of PROMETHEUS and will continue to be so to increase the SWAT evidence base. We have used our experience of undertaking PROMETHEUS to provide practical guidance and examples for undertaking SWATs.
The PROMETHEUS SWATs reported to date found that there was no evidence of a significant difference in recruitment rates in any of the strategies tested. For the retention strategies, we found evidence that pre-notification of trial participants by sending a card may result in a slight increase in attendance at a face-to-face primary outcome measurement visit; and that sending a pre-notification letter or e-mail before sending a self-report questionnaire increases response rates, compared with no pre-notification. We also found that participants randomised to receive a personalised text message were more likely to return their initial postal questionnaire than those who received a non-personalised text message, and that men aged under 65 years were the group most likely to return their initial questionnaire if they received a personalised text message. Another SWAT found that when comparing personalised text messages versus non-personalised text messages, there was evidence that a personalised text would result in fewer telephone follow-ups that were more resource intensive. Combined results from PROMETHEUS together with other published SWATs found that including a pen probably increases retention and response rate, compared to not sending a pen with postal questionnaires. One SWAT showed evidence of an adverse impact on retention when printing the trial primary outcome on pink paper compared to printing the primary outcome on white paper; this showed a decreased response and was also more burdensome to collect postal data as it increased the need for reminders. There was no evidence of a significant difference in retention rates in any of the other strategies tested. More replications of these SWATs are required.
The PROMETHEUS programme has substantially increased the number of high-quality SWATs undertaken and has identified a range of areas where further development is warranted. The conduct of 42 SWATs during this time enabled the identification of key aspects of undertaking SWAT that require further development and co-ordination. This is essential to ensure that SWATs continue to be undertaken with sufficient replications available to enable meta-analyses to be performed to provide conclusive findings. This will lead to an increase in the evidence base, enabling the identification of effective, ineffective, or harmful recruitment and retention strategies to support trialists to design and undertake efficient research.
There is a need to develop a strategy to aid teams to identify a suitable SWAT for their host trial populations and a mechanism to communicate SWAT research priorities. Ongoing work is needed to increase the awareness of the methodological importance of SWAT research with research teams and develop engagement strategies to increase SWAT activity. Continued collaboration with the HRA is also necessary to refine the SWAT approvals process.
The mean cost of each SWAT was £3535. In addition to funding SWATs, PROMETHEUS successfully demonstrated the methodological feasibility of undertaking co-ordinated SWATs, a powerful new tool with the potential to rapidly accelerate the evidence base for recruiting and retaining trial participants. In addition to the financial support, the co-ordination PROMETHEUS provided was crucial to increasing the recruitment and retention evidence base.
Following the initial success of PROMETHEUS, additional funding was received from the NIHR (award ID: NIHR132547) to extend PROMETHEUS and continue work to investigate how teams can be better supported to implement recruitment and retention SWATs. This allowed us to develop the ‘Trial Forge SWAT Network’, currently consisting of 30 CTUs and trials centres in the UK, the Republic of Ireland, Iran and Australia. The Trial Forge SWAT Network will provide ongoing networking and dissemination opportunities. The International Trial Forge SWAT Network has been well received by teams and will offer opportunities to share, disseminate and increase methodological research. We found webinars to be an effective tool to communicate methodological research and propose that these continue to be utilised in the future.
We have used our experience of undertaking PROMETHEUS to provide practical guidance and examples for undertaking SWATs, as well as make recommendations for all the key stakeholders involved in the conduct and delivery of SWATs. Collectively, these developments should enable and encourage teams to undertake SWATs, to further develop the evidence base and, ultimately, to prevent research waste by improving trial recruitment and retention.
Acknowledgements
Firstly, we thank all the PROMETHEUS host trial teams and clinicians that recruited and followed up participants for this programme, as well as the participants in all the host trials for making this research possible.
We thank YTU – a NIHR supported UK Clinical Research Collaboration-registered CTU – for hosting this methodological work. Our particular thanks to Sally Baker and Emma-Louise Rose Brooks at YTU for their support in managing the administrative aspects of the PROMETHEUS programme.
We are grateful to the Health Services Research Unit (HSRU), core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate, for their support, especially with the initiation of the Christmas card co-ordinated SWAT.
We would also like to thank the HRB-TMRN in the Republic of Ireland for kindly sharing materials, such as the ‘peer review assessment form’, which we adapted for PROMETHEUS.
We extend our thanks to The University of Bristol’s team (Qualitative research integrated within Trials) QuinteT, who developed the recruitment training course that we tested as a co-ordinated SWAT. The training was funded by the MRC Network of Hubs for Trials Methodology Research (MR/L004933/1-R53) and supported by the MRC ConDuCT-II Hub (Collaboration and innovation for Difficult and Complex randomised controlled Trials In Invasive procedures – MR/K025643/1).
We thank Professor Paul Brocklehurst, who was a co-applicant on the original MRC funded application, for his contribution to the PROMETHEUS programme.
We thank the PROMETHEUS PPI members: Christine Allmark, Phillip Bell, Ailsa Donnelly, Judith Hogg and Kay Walker. Additionally, we would like to thank the HSRU PPI Group who contributed to the Christmas card design for the Christmas card co-ordinated SWAT, as well as the PPI groups of all the host trials for their feedback and contributions on the SWATs.
Contributions of authors
Adwoa Parker (https://orcid.org/0000-0002-2880-3935) (Assistant Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, project administration, drafted the initial version of the manuscript, and read and approved the final version.
Catherine Arundel (https://orcid.org/0000-0003-0512-4339) (Research Fellow) contributed to study conceptualisation, study methodology and investigation, project administration, drafted the initial version of the manuscript, and read and approved the final version.
Laura Clark (https://orcid.org/0000-0001-9227-5447) (Research Fellow) contributed to study investigation, project administration, drafted the initial version of the manuscript, and read and approved the final version.
Elizabeth Coleman (https://orcid.org/0000-0003-4210-1865) (Statistician) contributed to study methodology and investigation, curated the data, carried out analysis and prepared the results for publication, drafted the initial version of the manuscript, and read and approved the final version.
Laura Doherty (https://orcid.org/0000-0001-5310-5808) (Trial Support Officer) contributed to study investigation, project administration, drafted the initial version of the manuscript, and read and approved the final version.
Catherine Elizabeth Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, analysis, and preparation of the results, drafting of the manuscript, and read and approved the final version of the manuscript.
David Beard (https://orcid.org/0000-0001-7884-6389) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Peter Bower (https://orcid.org/0000-0001-9558-3349) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Cindy Cooper (https://orcid.org/0000-0002-2995-5447) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Lucy Culliford (https://orcid.org/0000-0002-9255-6617) (Senior Research Fellow) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Declan Devane (https://orcid.org/0000-0002-9393-7075) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Richard Emsley (https://orcid.org/0000-0002-1218-675X) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Sandra Eldridge (https://orcid.org/0000-0001-5638-2317) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Sandra Galvin (https://orcid.org/0000-0003-3923-2506) (Programme Manager) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Katie Gillies (https://orcid.org/0000-0001-7890-2854) (Reader) contributed to study methodology and investigation, and read and approved the final version of the manuscript.
Alan Montgomery (https://orcid.org/0000-0003-0450-1606) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
Christopher J Sutton (https://orcid.org/0000-0002-6406-1318) (Senior Lecturer in Clinical Trial Statistics) contributed to study methodology and investigation, and read and approved the final version of the manuscript.
Shaun Treweek (https://orcid.org/0000-0002-7239-7241) (Professor) contributed to study conceptualisation, funding acquisition, study methodology and investigation, and read and approved the final version of the manuscript.
David J Torgerson (https://orcid.org/0000-0002-1667-4275) (Professor) conceptualised the study, led on funding acquisition, contributed to study methodology, investigation, drafting the manuscript, provided overall supervision of the programme, and read and approved the final version.
Study registrations
All SWATs in the PROMETHEUS programme had to be registered with the Northern Ireland Network for Trials Methodology Research SWAT Repository.
Funding
This award was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme (NIHR award ref: 13/55/80) and is published in full in Health Technology Assessment; Vol. 28, No. 2. See the NIHR Funding and Awards website for further award information.
Ethics statement
Required ethical approvals for all of the PROMETHEUS host trials were obtained separately by the host trial teams, and the SWATs undertaken were approved as part of the initial ethical application for the host trial, or through the submission of an amendment to the Research Ethics Committee. The following approvals for the SWATs were obtained:
-
ACTIVE: NRES Committee Yorkshire and The Humber – Bradford Leeds (ref.: 18/YH/0014).
-
ActWELL: East of Scotland Research Ethics Service REC 1 (ref.: 17/ES/0073).
-
CHAMP-1: South West – Frenchay Research Ethics Committee (ref.: 19/SW/0082).
-
CLEAR: North East – Tyne and Wear South Research Ethics Committee (ref.: 17/NE/0339).
-
GRASP: Berkshire B Research Ethics Committee, substantive amendment to the host trial (ref.: 16/SC/0508).
-
IBD-BOOST: London – Surrey Research Ethics Committee (ref.: 19/LO/0750).
-
KReBs (pen SWAT): North East – Newcastle and North Tyneside on (ref.: 16/NE/0400; amendment 2).
-
KReBs (text message SWAT): North East – Newcastle and North Tyneside on (ref.: 16/NE/0400; amendment number (16/NE/0400/AM14).
-
L1FE: London – Harrow Research Ethics Committee (ref.: 19/LO/0555).
-
MiQuit: East Midlands–Nottingham 1 Research Ethics Committee (refs: 13/EM/0427 and 17/EM/0327).
-
MSS3: North West – Greater Manchester Central Research Ethics Committee (ref.: 18/NW/0422).
-
OTIS: NHS West of Scotland Research Ethics Committee 3 (ref.: 16/WS/0154).
-
PEP-TALK: South Central Oxford B (ref.: 18/SC/0423).
-
PONOC: National Research Ethics Service Committee East Midlands – Nottingham 2 (ref.: 13/EM/0459).
-
SARC: West of Scotland REC 1 (ref.: 19/WS/0087).
-
SSHeW: Department of Health Sciences Research Ethics Committee at the University of York and the Health Research Authority (ref.: HSRGC/2016/187/A).
-
SWHSI-2: Leeds East Research Ethics Committee (ref.: 19-YH-0054).
-
TOPaZ: East of Scotland Research Ethics Service (EoSRES) (ref.: 16/ES/0110).
-
UK-FROST: North East – Newcastle and North Tyneside 2 Ethics Committee, (ref.: 14/NE/1176). Substantial Amendment 1 – REC Favourable Opinion 2 February 2015.
-
VITA: London – Harrow Research Ethics Committee on 9 September 2017 (ref.: 17/LO/1245).
-
WORKWELL: West Midlands – Solihull Research Ethics Committee (ref.: 18/WM/0327).
The ethical approval for the co-ordinated recruitment training SWAT across all the host trials was obtained from the University of York’s Department of Health Sciences Research Governance Committee. No further approvals were needed as this was classed as an evaluation of a teaching workshop (ref.: 76/76).
The ethical approval for the co-ordinated Christmas card SWAT across all the host trials was approved by the Yorkshire and the Humber – Leeds West Research Ethics Committee (ref.: 19/YH/0349). A single application was submitted to allow the SWAT to be embedded within all the host trials.
Data-sharing statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional funding received
YTU receives core funding from the NIHR CTU Support Funding. Following the initial success of PROMETHEUS, YTU received additional funding to continue some of the work of PROMETHEUS, through an institutional award from the NIHR CTU Infrastructure Support (NIHR132547). The Health Services Research Unit (HSRU) is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Each host trial received its own funding, as follows:
-
Cancer Research UK, the Chief Scientist Office, Scottish Government, Health and Social Care Public Health Agency Northern Ireland, Chest Heart and Stroke Northern Ireland, The Lullaby Trust, Public Health Agency Northern Ireland, and the Scottish Cot Death Trust: CPIT-III.
-
Medical Research Council and National Institute for Health and Care Research – Efficacy and Mechanism Evaluation: STARTS:REACTS, TOPaZ.
-
Cancer Research UK and National Institute for Health Research: MiQuit.
-
National Institute for Health and care Research – HTA programme: ARTISAN, C-Gall, CLEAR, DISC, FUTURE, GRASP, L1FE, MAGIC, ProFHER-2, PUrE, REFLECT and SWHSI-2, UK FROST, VITA.
-
National Institute for Health and care Research – Programme Grants for Applied Research: IBD-BOOST.
-
National Institute for Health Research – Health and Social Care Delivery Research: MSS3.
-
National Institute for Health Research – Public Health Research Programme: SSHeW.
-
The Scottish Government: ActWELL.
-
Versus Arthritis: WORKWELL.
Author KG’s time was funded by the Chief Scientist Office of the Scottish Government’s Health and Social Care Directorate (CZU/3/3). Author RE’s time was funded by NIHR Research Professorship (NIHR300051) and the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. Author APs time was part-funded by the Wellcome Trust (ref: 204829) through the Centre for Future Health (CFH) at the University of York, which partially supported the delivery of the recruitment training SWAT.
Publications
-
Coleman E, Arundel C, Clark L, Doherty L, Gillies K, Hewitt C, et al. Bah humbug! Association between sending Christmas cards to trial participants and trial retention: randomised study within a trial conducted simultaneously across eight host trials. BMJ (Clin Res Ed) 2021;375:e067742. https://doi.org/10.1136/bmj-2021-067742
-
Parker A, Mills N, Rooshenas L, Jepson M, Donovan JL, Arundel C, et al. Staff training to improve participant recruitment into surgical randomised controlled trials: a feasibility study embedded within four randomised controlled trials. In International Clinical Trials Methodology Conference. Brighton, 2019, p. PS8A. BMC.
-
Arundel CE, Clark L, Coleman E, Doherty L, Parker A, Torgerson DJ, et al. Challenges and solutions to the implementation of Studies Within A Trial (SWATs): the experiences of the PROMETHEUS programme. Res Health Sci Med 2022;4(0):16–23. https://doi.org/10.1177/26320843221106949
-
Parker A, Arundel C, Mills N, Rooshenas L, Jepson M, Donovan JL, et al. Staff training to improve participant recruitment into surgical randomised controlled trials: a feasibility study embedded within four randomised controlled trials. Res Meth Med Health Sci 2022;4(0):2–15. https://doi.org/10.1177/26320843221106950
-
Clark L, Arundel C, Coleman E, Doherty L, Parker A, Hewitt C, et al. The PROMoting THE USE of SWATs (PROMETHEUS) Programme: lessons learnt and future developments for SWATs. Res Meth Med Health Sci 2022;3(4):100–6. https://doi.org/10.1177/26320843221089632
-
Treweek S, Gallant S, Anderson AS. SWAT 76 evaluation: randomised evaluation of sending pre-notification cards to trial participants before a face-to-face primary outcome measurement to increase attendance. F1000Research 2021;10:84. https://doi.org/10.12688/f1000research.50890.2
-
Cureton L, Marian IR, Barber VS, Parker A, Torgerson DJ, Hopewell S. Randomised Study Within A Trial (SWAT) to evaluate personalised versus standard text message prompts for increasing trial participant response to postal questionnaires (PROMPTS). Trials 2021;22(1):1–10. https://doi.org/10.21203/rs.3.rs-262194/v1
-
Mitchell AS, Cook L, Dean A, Fairhurst C, Northgraves M, Torgerson DJ, Reed M. Using pens as an incentive for questionnaire return in an orthopaedic trial: an embedded randomised controlled retention trial. F1000Research 2021;9:321. https://doi.org/10.12688/f1000research.23018.2
-
Mitchell AS, Cook L, Dean A, Fairhurst C, Northgraves M, Torgerson DJ, Reed M. An embedded randomised controlled retention trial of personalised text messages compared to non-personalised text messages in an orthopaedic setting. F1000Research 2020;9:591. https://doi.org/10.12688/f1000research.24244.1
-
Coleman E, Whitmore R, Clark LK, Daykin K, Clark M. Pre-notification and personalisation of text-messages to retain participants in a smoking cessation pregnancy RCT: an embedded randomised factorial trial. F1000Research 2021;10:637. https://doi.org/10.12688/f1000research.51964.1
-
James S, Parker A, Cockayne S, Rodgers S, Fairhurst C, Torgerson DJ, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled Study Within A Trial. F1000Research 2020;9:623. https://doi.org/10.12688/f1000research.23767.2
-
Cunningham-Burley R, Roche J, Fairhurst C, Cockayne S, Hewitt C, Iles-Smith H, Torgerson DJ; SSHeW Study Team. Enclosing a pen to improve response rate to postal questionnaire: an embedded randomised controlled trial. F1000Research 2020;9:577. https://doi.org/10.12688/f1000research.23651.1
-
Sarathy PP, Kottam L, Parker A, Brealey S, Coleman E, Keding A, et al. Timing of electronic reminders did not improve trial participant questionnaire response: a randomized trial and meta-analyses. J Clin Epidemiol 2020;122:70–7. https://doi.org/10.1016/j.jclinepi.2020.03.001
Disclaimers
This report presents independent research funded under a MRC–NIHR partnership. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312:71-2.
- ClinicalTrials.gov . Trends, Charts, and Maps 2022. https://clinicaltrials.gov/ct2/resources/trends (accessed 27 May 2022).
- National Institute for Health and Care Research . Annual Report 2019 2020 2021. www.nihr.ac.uk/documents/about-us/our-contribution-to-research/research-performance/NIHR_Annual_Report_19_20.pdf (accessed 27 May 2022).
- National Institute for Health and Care Research . Annual Report 2020 2021 2022. www.nihr.ac.uk/documents/annual-report-20202021/30206 (accessed 27 May 2022).
- Jacques RM, Ahmed R, Harper J, Ranjan A, Saeed I, Simpson RM, et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) Journals Library (1997–2020). BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2021-059230.
- Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med J Assoc Am Med Coll 2011;86:1360-6. https://doi.org/10.1097/ACM.0b013e3182306440.
- Salman RA-S, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.
- Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: Who’s listening?. Lancet (London, England) 2016;387:1573-86. https://doi.org/10.1016/s0140-6736(15)00307-4.
- Knowlson C, Torgerson DJ. Effects of rapid recruitment and dissemination on Covid-19 mortality: the RECOVERY trial. F1000Research 2020;9.
- Treweek S, Gillies K. Trial retention: it’s time for Cinderella to go to the ball 2017. https://blogs.biomedcentral.com/on-medicine/2017/09/01/trial-retention-its-time-for-cinderella-to-go-to-the-ball/ (accessed 27 May 2022).
- Walsh M, Devereaux PJ, Sackett DL. Clinician trialist rounds: 28. When RCT participants are lost to follow-up. Part 1: Why even a few can matter. Clin Trials 2015;12:537-9. https://doi.org/10.1177/1740774515597702.
- Smith CT, Hickey H, Clarke M, Blazeby J, Williamson P. The trials methodological research agenda: results from a priority setting exercise. Trials 2014;15:1-7.
- Clarke M, Savage G, Maguire L, McAneney H. The SWAT (Study Within A Trial) programme; embedding trials to improve the methodological design and conduct of future research. Trials 2015;16.
- Anonymous . Education section – Studies Within A Trial (SWAT). J Evid Based Med 2012;5:44-5. https://doi.org/10.1111/j.1756-5391.2012.01169.x.
- Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial Forge Guidance 1: What is a Study Within A Trial (SWAT)?. Trials 2018;19. https://doi.org/10.1186/s13063-018-2535-5.
- Dwyer CP, Moses A, Rogers FM, Casey D, Joyce R, Hynes SM. A qualitative investigation of reasoning behind decisions to decline participation in a research intervention: a Study-Within-A-Trial. J Health Psychol 2021;28:374-87. https://doi.org/10.1177/13591053211037736.
- Allan S, Mcleod H, Bradstreet S, Bell I, Whitehill H, Wilson-Kay A, et al. Perspectives of trial staff on the barriers to recruitment in a digital intervention for psychosis and how to work around them: qualitative Study Within A Trial. JMIR Hum Factors 2021;8.
- Racine E, Hurley C, Cheung A, Sinnott C, Matvienko-Sikar K, Baumgartner C, et al. Participants’ perspectives and preferences on clinical trial result dissemination: the TRUST Thyroid Trial experience. HRB Open Res 2018;1. https://doi.org/10.12688/hrbopenres.12817.2.
- Clarke M, Maguire L. SWAT Store: Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2015.
- Ahmed S, Airlie J, Clegg A, Copsey B, Cundill B, Forster A, et al. A new opportunity for enhancing trial efficiency: Can we investigate intervention implementation processes within trials using SWAT (Study Within A Trial) methodology?. Res Meth Med Health Sci 2022;3:66-73. https://doi.org/10.1177/26320843221080734.
- Hurley C. SWAT 38: cost implications of conducting a risk assessment prior to developing a monitoring plan for a multicentre clinical trial. SWAT Store: Northern Ireland Network for Trials Methodology Research 2016.
- Fairhurst C, Roche J, Bissell L, Hewitt C, Hugill-Jones J, Howsam J, et al. A 2 × 2 randomised factorial SWAT of the use of a pen and small, financial incentive to improve recruitment in a randomised controlled trial of yoga for older adults with multimorbidity [version 2; peer review: 2 approved]. F1000Research 2022;10. https://doi.org/10.12688/f1000research.52164.2.
- Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev 2018;2. https://doi.org/10.1002/14651858.MR000013.pub6.
- Gillies K, Kearney A, Keenan C, Treweek S, Hudson J, Brueton VC, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2021;3. https://doi.org/10.1002/14651858.MR000032.pub3.
- Rick J, Graffy J, Knapp P, Small N, Collier DJ, Eldridge S, et al. Systematic techniques for assisting recruitment to trials (START): study protocol for embedded, randomized controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-407.
- Madurasinghe VW, Bower P, Eldridge S, Collier D, Graffy J, Treweek S, et al. Can we achieve better recruitment by providing better information? Meta-analysis of ‘Studies Within A Trial’ (SWATs) of optimised participant information sheets. BMC Med 2021;19. https://doi.org/10.1186/s12916-021-02086-2.
- Madurasinghe VW. Sandra Eldridge on behalf of MRC START Group and Gordon Forbes on behalf of the START Expert Consensus Group . Sandra Eldridge on behalf of MRCSG, Gordon Forbes on behalf of the SECG. Guidelines for reporting embedded recruitment trials. Trials 2016;17. https://doi.org/10.1186/s13063-015-1126-y.
- Health Research Board – Trials Methodology Research Network . Study Within A Trial (SWAT) 2022. www.hrb-tmrn.ie/research-and-innovation/funding-opportunities/studies-within-a-trial-swats/ (accessed 27 May 2022).
- National Institute for Health and Care Research . Studies Within A Trial (SWAT) and Studies Within A Review (SWAR) 2022. www.nihr.ac.uk/documents/studies-within-a-trial-swat/21512 (accessed 27 May 2022).
- Martin-Kerry JM, Bower P, Young B, Graffy J, Sheridan R, Watt IS, et al. Developing and evaluating multimedia information resources to improve engagement of children, adolescents and their parents with trials (TRECA study). Study Protocol for a series of linked randomised controlled trials. Trials 2017;18. https://doi.org/10.1186/s13063-017-1962-z.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12:1-187. https://doi.org/10.1002/14651858.MR000032.pub2.
- SWAT Store. Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2022.
- Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-399.
- Healy P, Galvin S, Williamson PR, Treweek S, Whiting C, Maeso B, et al. Identifying trial recruitment uncertainties using a James Lind Alliance Priority Setting Partnership – the PRioRiTy (Prioritising Recruitment in Randomised Trials) study. Trials 2018;19. https://doi.org/10.1186/s13063-018-2544-4.
- National Institute for Health and Care Research . The NIHR Clinical Research Network (CRN) Portfolio of Studies Consists of Research Studies That Are Eligible for Support from the NIHR CRN in England. CRN Portfolio 2022. www.nihr.ac.uk/researchers/collaborations-services-and-support-for-your-research/run-your-study/crn-portfolio.htm (accessed 28 May 2022).
- Adamson J, Hewitt CE, Torgerson DJ. Producing better evidence on how to improve randomised controlled trials. BMJ 2015;351. https://doi.org/10.1136/bmj.h4923.
- Cook TD, Campbell DT, Day A. Quasi-Experimentation: Design & Analysis Issues for Field Settings. Houghton Mifflin Boston; 1979.
- Medical Research Council . MRC Policy and Guidance on Sharing of Research Data from Population and Patient Studies. Medical Research Council 2017. www.ukri.org/wp-content/uploads/2021/08/MRC-0208212-MRC-policy-and-guidance-on-sharing-of-research-data-from-population-and-patient-studies-Word-version-v01.02.pdf (accessed 28 May 2022).
- Kearney A, Harman NL, Rosala-Hallas A, Beecher C, Blazeby JM, Bower P, et al. Development of an online resource for recruitment research in clinical trials to organise and map current literature. Clin Trials 2018;15:533-42. https://doi.org/10.1177/1740774518796156.
- Kearney A, Ashford PA, Butlin L, Conway T, Cragg WJ, Devane D, et al. Developing an online, searchable database to systematically map and organise current literature on retention research (ORRCA2). Clin Trials 2022;19:71-80. https://doi.org/10.1177/17407745211053803.
- Stewart D, van Dongen A, Watson M, Mandefield L, Atkin K, Dhital R, et al. A pilot cluster randomised trial of the Medicines and Alcohol Consultation (MAC): an intervention to discuss alcohol use in community pharmacy medicine review services. BMC Health Serv Res 2020;20. https://doi.org/10.1186/s12913-020-05797-z.
- Cook L. Surgical versus conservative treatment of LC1 pelvic fractures in the elderly. ISRCTN Registry 2019. www.isrctn.com/ISRCTN16478561 (accessed 28 May 2022).
- Armstrong-Buisseret L, Brittain C, Kai J, David M, Anstey Watkins J, Ozolins M, et al. Lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT. Health Technol Assess 2022;26:1-170. https://doi.org/10.3310/zzkh4176.
- Ralston SH. Treatment of osteogenesis imperfecta with parathyroid hormone and zoledronic acid. ISRCTN Registry 2016. https://doi.org/10.1186/ISRCTN15313991.
- Flett L, Adamson J, Barron E, Brealey S, Corbacho B, Costa ML, et al. A multicentre, randomized, parallel group, superiority study to compare the clinical effectiveness and cost-effectiveness of external frame versus internal locking plate for complete articular pilon fracture fixation in adults. Bone Jt Open 2021;2:150-63. https://doi.org/10.1302/2633-1462.23.BJO-2020-0178.
- Parker A, Mills N, Rooshenas L, Jepson M, Donovan JL, Arundel C, et al. SWAT 111: staff training to improve participant recruitment into surgical randomised trials. SWAT Store: Northern Ireland Network for Trials Methodology Research 2019. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,949591,en.pdf (accessed 27 May 2022).
- Treweek S, Gillies K, Innes K, MacLennan G. SWAT Store: Northern Ireland Network for Trial Methodology Research. Belfast: Queen’s University; 2017.
- Parker A, Mills N, Rooshenas L, Jepson M, Donovan JL, Arundel C, et al. Staff training to improve participant recruitment into surgical randomised controlled trials: a feasibility study embedded within four randomised controlled trials n.d.
- Coleman E, Arundel C, Clark L, Doherty L, Gillies K, Hewitt C, et al. Bah humbug! Association between sending Christmas cards to trial participants and trial retention: randomised Study Within A Trial conducted simultaneously across eight host trials. BMJ (Clin Res Ed) 2021;375. https://doi.org/10.1136/bmj-2021-067742.
- Anand R, Bradley J, O’Neill B, McAuley D, Clarke M. A randomised trial of the effects on recruitment and retention of including a wet-ink signature and photograph in the patient invitation letter for a clinical trial: results from a Study Within A Trial (SWAT 3 and SWAT 53) [version 1; peer review: awaiting peer review]. F1000Research 2022;11. https://doi.org/10.12688/f1000research.75339.1.
- Parker A, Arundel C, Mills N, Rooshenas L, Jepson M, Donovan JL, et al. Staff training to improve participant recruitment into surgical randomised controlled trials: a feasibility Study Within A Trial (SWAT) across four host trials simultaneously. Res Meth Med Health Sci 2023;4:2-15. https://doi.org/10.1177/26320843221106950.
- Dias J, Arundel C, Tharmanathan P, Keding A, Welch C, Corbacho B, et al. Dupuytren’s interventions surgery versus collagenase (DISC) trial: study protocol for a pragmatic, two-arm parallel-group, non-inferiority randomised controlled trial. Trials 2021;22. https://doi.org/10.1186/s13063-021-05595-w.
- Armstrong G, Croft J, Corrigan N, Brown JM, Goh V, Quirke P, et al. IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis 2018;20:O226-34. https://doi.org/10.1111/codi.14257.
- Tharmanathan P, Arundel C. Effectiveness and cost-effectiveness of reverse shoulder arthroplasty versus hemiarthroplasty versus non-surgical care for acute 3 and 4 part fractures of the proximal humerus in patients aged over 65 years – the PROFHER-2 randomised trial. ISRCTN Registry 2018.
- Metcalfe A, Gemperle Mannion E, Parsons H, Brown J, Parsons N, Fox J, et al. Protocol for a randomised controlled trial of Subacromial spacer for Tears Affecting Rotator cuff Tendons: a Randomised, Efficient, Adaptive Clinical Trial in Surgery (START:REACTS). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-036829.
- Bradley JM, Anand R, O’Neill B, Ferguson K, Clarke M, Carroll M, et al. CLEAR study group . A 2 × 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3766-9.
- Maguire L, Clarke M. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2014.
- Anand R, Green N. SWAT 53: including a photograph on the invitation letter for a prospective study. SWAT Store: Northern Ireland Network for Trial Methodology Research 2017. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,758669,en.pdf (accessed 27 May 2022).
- Norton C, Syred J, Kerry S, Artom M, Sweeney L, Hart A, et al. Supported online self-management versus care as usual for symptoms of fatigue, pain and urgency/incontinence in adults with inflammatory bowel disease (IBD-BOOST): study protocol for a randomised controlled trial. Trials 2021;22. https://doi.org/10.1186/s13063-021-05466-4.
- White D, Parker A, Torgerson D. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2018.
- Mooney C. Multiple Symptoms Study 3. ISRCTN Registry 2022. https://doi.org/10.1186/ISRCTN57050216.
- Goyal A, Mann GB, Fallowfield L, Duley L, Reed M, Dodwell D, et al. POSNOC Trialists . POSNOC – POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2021-054365.
- Montgomery A, Brittain C, Khan S, Lewis M, Tan W, Goyal A, et al. Belfast: Queen’s University; 2019.
- Johnson G, Tabner A, Fakis A, Sherman R, Chester V, Bedford E, et al. Salbutamol for analgesia in renal colic: study protocol for a prospective, randomised, placebo-controlled phase II trial (SARC). Trials 2022;23. https://doi.org/10.1186/s13063-022-06225-9.
- Chetter I, Arundel C, Martin BC, Hewitt C, Fairhurst C, Joshi K, et al. Negative pressure wound therapy versus usual care for surgical wounds healing by secondary intention (SWHSI-2 trial): study protocol for a pragmatic, multicentre, cross-surgical specialty, randomised controlled trial. Trials 2021;22. https://doi.org/10.1186/s13063-021-05662-2.
- McCaffery J, Arundel C, Chetter I, Fairhurst C, Joshi K, Mott A, et al. The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2019.
- Coleman E, Whitemore R, Clark L, Daykin K, Clark M. Pre-notification and personalisation of text-messages to retain participants in a smoking cessation pregnancy RCT: an embedded randomised factorial trial [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research 2021;10. https://doi.org/10.12688/f1000research.51964.1.
- James S, Parker A, Cockayne S, Rodgers S, Fairhurst C, Torgerson D, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled Study Within A Trial [version 2; peer review: 2 approved]. F1000Research 2021;9. https://doi.org/10.12688/f1000research.23767.2.
- Smith TO, Parsons S, Fordham B, Ooms A, Dutton S, Hing C, et al. PEP-TALK Trial Collaborators . Behaviour change physiotherapy intervention to increase physical activity following hip and knee replacement (PEP-TALK): study protocol for a pragmatic randomised controlled trial. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-035014.
- Ooms A. SWAT Store: Northern Ireland Network for Trial Methodology Research. Belfast: Queen’s University; 2019.
- Ooms A, Parsons S, Dutton S, Garrett A, Fordham B, Hing C, et al. SWAT 110: printing the primary outcomE on Pink PapER versus standard paper to increase participant engagement to postal questionnaires (PEPPER). Res Meth Med Health Sci 2022;3:49-54.
- Rangan A, Brealey SD, Keding A, Corbacho B, Northgraves M, Kottam L, et al. Management of adults with primary frozen shoulder in secondary care (UK FROST): a multicentre, pragmatic, three-arm, superiority randomised clinical trial. Lancet 2020;396:977-89. https://doi.org/10.1016/S0140-6736(20)31965-6.
- Hughes-Morley A, Brealey S, Keding A, Torgerson D, Hewitt C, Bailey M, et al. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2022.
- Partha Sarathy P, Kottam L, Parker A, Brealey S, Coleman E, Keding A, et al. Timing of electronic reminders did not improve trial participant questionnaire response: a randomized trial and meta-analyses. J Clin Epidemiol 2020;122:70-7. https://doi.org/10.1016/j.jclinepi.2020.03.001.
- Hopewell S, Keene DJ, Maia Schlüssel M, Dritsaki M, Dutton S, Carr A, et al. Clinical and cost-effectiveness of progressive exercise compared with best practice advice, with or without corticosteroid injection, for the treatment of rotator cuff disorders: protocol for a 2 × 2 factorial randomised controlled trial (the GRASP trial). BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-018004.
- Hughes-Morley A, Torgerson D. The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2015.
- Cureton L, Marian IR, Barber VS, Parker A, Torgerson DJ, Hopewell S. Randomised Study Within A Trial (SWAT) to evaluate personalised versus standard text message prompts for increasing trial participant response to postal questionnaires (PROMPTS). Trials 2021;22. https://doi.org/10.1186/s13063-021-05452-w.
- Cook L, Northgraves MJ, Fairhurst C, Ronaldson S, Torgerson DJ, Kent J, et al. Knee Replacement Bandaging Study (KReBS) evaluating the effect of a two-layer compression bandage system on knee function following total knee arthroplasty: study protocol for a randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3344-1.
- Tew G, Tilbrook H, Paul S-A, Howe L, Parker A, Bell K. SWAT Store: Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2019.
- Mitchell A, Cook L, Dean A, Fairhurst C, Northgraves M, Torgerson D, et al. An embedded randomised controlled retention trial of personalised text messages compared to non-personalised text messages in an orthopaedic setting [version 1; peer review: 1 approved]. F1000Research 2020;9. https://doi.org/10.12688/f1000research.24244.1.
- Mitchell A, Cook L, Dean A, Fairhurst C, Northgraves M, Torgerson D, et al. Using pens as an incentive for questionnaire return in an orthopaedic trial: an embedded randomised controlled retention trial [version 2; peer review: 1 approved, 1 approved with reservations]. F1000Research 2021;9. https://doi.org/10.12688/f1000research.23018.2.
- Whitemore R, Leonardi-Bee J, Naughton F, Sutton S, Cooper S, Parrott S, et al. Effectiveness and cost-effectiveness of a tailored text-message programme (MiQuit) for smoking cessation in pregnancy: study protocol for a randomised controlled trial (RCT) and meta-analysis. Trials 2019;20. https://doi.org/10.1186/s13063-019-3341-4.
- Anderson AS, Craigie AM, Gallant S, McAdam C, Macaskill EJ, Mutrie N, et al. Randomised controlled trial to assess the impact of a lifestyle intervention (ActWELL) in women invited to NHS breast screening. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-024136.
- Treweek S, Anderson A, Gallant S. The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2022.
- Treweek S, Gallant S, Anderson A. SWAT 76 evaluation: randomised evaluation of sending pre-notification cards to trial participants before a face-to-face primary outcome measurement to increase attendance [version 2; peer review: 2 approved]. F1000Research 2021;10. https://doi.org/10.12688/f1000research.50890.2.
- Hammond A, Sutton C, Cotterill S, Woodbridge S, O’Brien R, Radford K, et al. The effect on work presenteeism of job retention vocational rehabilitation compared to a written self-help work advice pack for employed people with inflammatory arthritis: protocol for a multi-centre randomised controlled trial (the WORKWELL trial). BMC Musculoskelet Disord 2020;21. https://doi.org/10.1186/s12891-020-03619-1.
- Sutton C, Cotterill S, Forshaw D, Rhodes S, Hammond A. SWAT Store: Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2018.
- Sutton CJ, Cotterill S, Forshaw D, Rhodes S, Haig A, Hammond A. Randomised evaluation of pre-notification of trial participants before self-report outcome data collection to improve retention: SWAT86. Res Meth Med Health Sci 2022;3:107-15. https://doi.org/10.1177/26320843221098427.
- Cockayne S, Pighills A, Adamson J, Fairhurst C, Drummond A, Hewitt C, et al. OTIS Study . Can occupational therapist-led home environmental assessment prevent falls in older people? A modified cohort randomised controlled trial protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022488.
- McDaid C, Cook L, James S, Ahkter Z, Podmore D. Belfast: Queen’s University; 2019.
- Cockayne S, Fairhurst C, Frost G, Hewitt C, Liddle M, Zand M, et al. SSHeW Study . SSHeW study protocol: Does slip resistant footwear reduce slips among healthcare workers? A randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-026023.
- Cunningham-Burley R, Roche J, Fairhurst C, Cockayne S, Hewitt C, Iles-Smith H, et al. SSHeW Study Team . Enclosing a pen to improve response rate to postal questionnaire: an embedded randomised controlled trial. F1000Research 2020;9. https://doi.org/10.12688/f1000research.23651.1.
- Ahmed I, Innes K, Brazzelli M, Gillies K, Newlands R, Avenell A, et al. Protocol for a randomised controlled trial comparing laparoscopic cholecystectomy with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones (C-Gall trial). BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-039781.
- Sinclair L, McFadden M, Tilbrook H, Mitchell A, Keding A, Watson J, et al. CPIT III Local Research Teams . The smoking cessation in pregnancy incentives trial (CPIT): study protocol for a phase III randomised controlled trial. Trials 2020;21. https://doi.org/10.1186/s13063-019-4042-8.
- Abdel-Fattah M, Chapple C, Guerrero K, Dixon S, Cotterill N, Ward K, et al. Female Urgency, Trial of Urodynamics as Routine Evaluation (FUTURE study): a superiority randomised clinical trial to evaluate the effectiveness and cost-effectiveness of invasive urodynamic investigations in management of women with refractory overactive bladder symptoms. Trials 2021;22. https://doi.org/10.1186/s13063-021-05661-3.
- McClinton S, Starr K, Thomas R, MacLennan G, Lam T, Hernandez R, et al. The clinical and cost effectiveness of surgical interventions for stones in the lower pole of the kidney: the percutaneous nephrolithotomy, flexible ureterorenoscopy and extracorporeal shockwave lithotripsy for lower pole kidney stones randomised controlled trial (PUrE RCT) protocol. Trials 2020;21. https://doi.org/10.1186/s13063-020-04326-x.
- Tickle M, Ricketts DJN, Duncan A, O’Malley L, Donaldson PM, Clarkson JE, et al. Protocol for a randomised controlled trial to evaluate the effectiveness and cost benefit of prescribing high dose fluoride toothpaste in preventing and treating dental caries in high-risk older adults (reflect trial). BMC Oral Health 2019;19. https://doi.org/10.1186/s12903-019-0749-x.
- Anand R. SWAT Store: Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2017.
- Anand R. Novel Methods Within a Randomised Controlled Trial in Respiratory Disease. Belfast: Queen’s University; 2021.
- Kearney RS, Dhanjal G, Parsons N, Ellard D, Parsons H, Haque A, et al. Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN (ARTISAN): protocol for a multicentre randomised controlled trial. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-040623.
- Dhanjal G. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2019.
- Hyslop M. Melatonin for Anxiety prior to General anaesthesia In Children (MAGIC). ISRCTN Registry 2019. https://doi.org/10.1186/ISRCTN18296119.
- Arundel C, Chetter I, Fairhurst C, Joshi K, McCaffery J, Mott A, et al. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2019.
- Parker A, Brealey S, Sharma H, Welch C, Keding A, Flett L, et al. SWAT Store: The Northern Ireland Network for Trials Methodology Research. Belfast: Queen’s University; 2022.
- Bedford L, Young B, Kendrick D, Vedhara K, Gallant S. Northern Ireland Network for Trial Methodology Research. Belfast: Queen’s University; 2013.
- Cook L, Backhouse M, McDaid C, Rex S, Parker A. SWAT Store: Northern Ireland Network for Trial Methodology Research. Belfast: Queen’s University; 2019.
- Trial Forge 2022. www.trialforge.org/ (accessed 2 June 2022).
- Clark L, Arundel C, Coleman E, Doherty L, Parker A, Hewitt C, et al. The PROMoting THE USE of SWATs (PROMETHEUS) programme: lessons learnt and future developments for SWATs. Res Meth Med Health Sci 2022;3:100-6. https://doi.org/10.1177/26320843221089.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ (Clin Res Ed) 2017;358. https://doi.org/10.1136/bmj.j3453.
- Brunsdon D, Biesty L, Brocklehurst P, Brueton V, Devane D, Elliott J, et al. What are the most important unanswered research questions in trial retention? A James Lind Alliance Priority Setting Partnership: the PRioRiTy II (Prioritising Retention in Randomised Trials) study. Trials 2019;20. https://doi.org/10.1186/s13063-019-3687-7.
- Dwyer C, Hynes S. SWAT 124: exploring the impact of ineligibility on individuals expressing interest in a trial aimed at improving daily functioning regarding perceptions of self, research and likelihood of future participation: a PPI-infused qualitative SWAT. Northern Ireland Network for Trials Methodology Research 2020.
- Hughes-Morley A. SWAT 26: improving trial recruitment with a leaflet advertising patient and public involvement. Northern Ireland Network for Trials Methodology Research 2014.
- ROMIO Study PPI Group . SWAT 146: adding ‘Withdrawal Slips’ within follow up reminders for questionnaires. Northern Ireland Network for Trials Methodology Research 2019.
- Morrissey E, Casey B, Power R. The D1 Now Young Adult Panel . SWAT 133: branded gift and letter from PPI group to enhance questionnaire response rate in a randomised trial. Northern Ireland Network for Trials Methodology Research 2020.
- Stewart DC, Bower P, Woolfall K, Gillies K. Actively Involving Patients Public With Trials Methodology Research (TMR): Report of Workshop 2020.
- Treweek S, Altman DG, Bower P, Campbell M, Chalmers I, Cotton S, et al. Making randomised trials more efficient: report of the first meeting to discuss the Trial Forge platform. Trials 2015;16. https://doi.org/10.1186/s13063-015-0776-0.
- MRC-NIHR . MRC-NIHR Trials Methodology Research Partnership (TMRP) 2021. www.methodologyhubs.mrc.ac.uk/about/tmrp/ (accessed 18 October 2021).
- Trial Forge . Trial Forge Evidence Packs 2021. www.trialforge.org/resource-category/evidence-pack/ (accessed 8 October 2021).
- Jefferson L, Fairhurst C, Brealey S, Coleman E, Cook L, Hewitt C, et al. Remote or on-site visits were feasible for the initial setup meetings with hospitals in a multicenter surgical trial: an embedded randomized trial. J Clin Epidemiol 2018;100:13-21.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Trial Forge . Trial Forge SWAT Network 2021. www.trialforge.org/2021/06/swat_network/ (accessed September 2021).
- Parker A, Knapp P, Treweek S, Madhurasinghe V, Littleford R, Gallant S, et al. The effect of optimised patient information materials on recruitment in a lung cancer screening trial: an embedded randomised recruitment trial. Trials 2018;19:1-8.
- Fowell A, Johnstone R, Finlay I, Russell D, Russell I. Design of trials with dying patients: a feasibility study of cluster randomisation versus randomised consent. Palliat Med 2006;20:799-804. https://doi.org/10.1177/0269216306072554.
- Larkey LK, Staten LK, Ritenbaugh C, Hall RA, Buller DB, Bassford T, et al. Recruitment of Hispanic women to the Women’s Health Initiative the case of Embajadoras in Arizona. Control Clin Trials 2002;23:289-98.
- Sheridan R, Knapp P, Bower P, Madurasinghe V, Broadbent DM, Awoyale L, et al. Patient recruitment to a diabetic retinopathy screening trial through optimised patient information materials: an embedded Study Within A Trial (SWAT). F1000Research 2020;9.
- Hughes-Morley A, Hann M, Fraser C, Meade O, Lovell K, Young B, et al. The impact of advertising patient and public involvement on trial recruitment: embedded cluster randomised recruitment trial. Trials 2016;17:1-13. https://doi.org/10.1186/s13063-016-1718-1.
- Torgerson DJ, Dumville JC. Ethics review in research. BMJ 2004;328. https://doi.org/10.1136/bmj.328.7441.710-a.
- Mallick AA, O’Callaghan FJ. Research governance delays for a multicentre non-interventional study. J R Soc Med 2009;102:195-8. https://doi.org/10.1258/jrsm.2009.080397.
- Thompson AGH, France EF. One stop or full stop? The continuing challenges for researchers despite the new streamlined NHS research governance process. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-124.
- Harman NL, Conroy EJ, Lewis SC, Murray G, Norrie J, Sydes MR, et al. Exploring the role and function of trial steering committees: results of an expert panel meeting. Trials 2015;16. https://doi.org/10.1186/s13063-015-1125-z.
- Medical Research Council . MRC Guidelines for Good Clinical Practice in Clinical Trials: Medical Research Council 1998.
- Arundel C. Challenges and solutions to the implementation of Studies Within A Trial (SWATs): the experiences of the PROMETHEUS programme. Res Methods Med Health Sci 2023;4:16-23. https://doi.org/10.1177/26320843221106949.
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1797-7.
- Gouldner AW. The norm of reciprocity: a preliminary statement. Am Soc Rev 1960;25:161-78. https://doi.org/10.2307/2092623.
- Cialdini RB, Vincent JE, Lewis SK, Catalan J, Wheeler D, Darby BL. Reciprocal concessions procedure for inducing compliance: the door-in-the-face technique. J Pers Soc Psychol 1975;31:206-15. https://doi.org/10.1037/h0076284.
- Regan DT. Effects of a favor and liking on compliance. J Exp Soc Psychol 1971;7:627-39. https://doi.org/10.1016/0022-1031(71)90025-4.
- Falk A, Fischbacher U. A theory of reciprocity. Games Econ Behav 2006;54:293-315.
- Bell K, Clark L, Fairhurst C, Mitchell N, Lenaghan E, Blacklock J, et al. SCOOP Study Team . Enclosing a pen reduced time to response to questionnaire mailings. J Clin Epidemiol 2016;74:144-50. https://doi.org/10.1016/j.jclinepi.2015.12.004.
- Sharp L, Cochran C, Cotton SC, Gray NM, Gallagher ME. TOMBOLA Group . Enclosing a pen with a postal questionnaire can significantly increase the response rate. J Clin Epidemiol 2006;59:747-54. https://doi.org/10.1016/j.jclinepi.2005.10.014.
- Gkekas A, Evans A, Parker A, Ronaldson S, Torgerson D. A systematic review of economic evaluations alongside Studies Within A Trial (SWATs) for improving recruitment and retention in RCTs. Res Meth Med Health Sci 2023;4:94-112.
- Townsend D, Mills N, Savović J, Donovan JL. A systematic review of training programmes for recruiters to randomised controlled trials. Trials 2015;16. https://doi.org/10.1186/s13063-015-0908-6.
- Delaney H, Devane D, Hunter A, Hennessy M, Parker A, Murphy L, et al. Limited evidence exists on the effectiveness of education and training interventions on trial recruitment; a systematic review. J Clin Epidemiol 2019;113:75-82. https://doi.org/10.1016/j.jclinepi.2019.05.013.
- Mills N, Gaunt D, Blazeby JM, Elliott D, Husbands S, Holding P, et al. Training health professionals to recruit into challenging randomized controlled trials improved confidence: the development of the QuinteT randomized controlled trial recruitment training intervention. J Clin Epidemiol 2018;95:34-4. https://doi.org/10.1016/j.jclinepi.2017.11.015.
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239-41.
- Tew G, Tilbrook H. Belfast: Queen’s University; 2018.
- O’Neill L, Knapp P, Doyle S, Guinan E, Hussey J. SWAT 100: patient and family co-developed participant information to improve recruitment rates, retention, and patient understanding of a randomised trial. Northern Ireland Network for Trials Methodology Research 2018.
- Rothwell JC, Julious SA, Cooper CL. A study of target effect sizes in randomised controlled trials published in the Health Technology Assessment journal. Trials 2018;19. https://doi.org/10.1186/s13063-018-2886-y.
- Treweek S, Bevan S, Bower P, Briel M, Campbell M, Christie J, et al. Trial Forge Guidance 2: how to decide if a further Study Within A Trial (SWAT) is needed. Trials 2020;21. https://doi.org/10.1186/s13063-019-3980-5.
- Arundel C, Coleman E, Fairhurst C, Peckham E, Bailey D, Gilbody S. The effectiveness of a contingent financial incentive to improve trial follow up; a randomised Study Within A Trial (SWAT). F1000Research 2019;8. https://doi.org/10.12688/f1000research.21059.2.
- Cochrane A, Welch C, Fairhurst C, Cockayne S, Torgerson DJ. OTIS Study Group . An evaluation of a personalised text message reminder compared to a standard text message on postal questionnaire response rates: an embedded randomised controlled trial. F1000Research 2020;9. https://doi.org/10.12688/f1000research.22361.1.
- Mitchell N, Hewitt CE, Lenaghan E, Platt E, Shepstone L, Torgerson DJ. SCOOP Study Team . Prior notification of trial participants by newsletter increased response rates: a randomized controlled trial. J Clin Epidemiol 2012;65:1348-52. https://doi.org/10.1016/j.jclinepi.2012.05.008.
- Mitchell N, Hewitt CE, Torgerson DJ. SCOOP Trial Group . A controlled trial of envelope colour for increasing response rates in older women. Aging Clin Exp Res 2011;23:236-40. https://doi.org/10.1007/bf03337749.
- F1000Research . Studies Within A Trial (SWAT) collection. F1000Research 2022. https://f1000research.com/collections/swat/about-this-collection (accessed 2 June 2022).
- UK Government legislation . Data Protection Act 2018. legislation.gov.Uk: The National Archives 2018. www.legislation.gov.uk/ukpga/2018/12/contents/enacted (accessed 2 June 2022).
- SAGE Journals . Research methods in medicine & health sciences. SAGE J 2022. https://journals.sagepub.com/home/rmm (accessed 2 June 2022).
- Adamson J, Hewitt CE, Torgerson DJ. Producing better evidence on how to improve randomised controlled trials. BMJ 2015;351.
- ISRCTN . ISRCTN Registry 2020. www.isrctn.com (accessed 28 May 2022).
- ClinicalTrials.gov . ClinicalTrials.Gov. Bethesda, MD: National Library of Medicine (US) 2022. https://clinicaltrials.gov/ct2/resources/trends (accessed 27 May 2022).
Appendix 1 List of funded host trials and Studies Within A Trial
PROMETHEUS funded host trials and their characteristics:
-
ACTWELL: A randomised control trial to assess the impact of a lifestyle intervention (ActWELL) in women invited to NHS breast screening. ISRCTN11057518 (Date registered: 15 February 2017).
-
ACTIVE: External frame versus internal locking plate for articular pilon fracture fixation: a multicentre randomised controlled trial. ISRCTN98152560 (Date registered: 26 February 2018).
-
ARTISAN: Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN (ARTISAN) – a multicentre RCT. ISRCTN63184243 (Date registered: 3 September 2018).
-
CHAMP-1: a multicentre, cluster randomised controlled, open pilot trial to establish the feasibility of conducting a large-scale study comparing an intervention discussing alcohol within routine medication consultations with usual care in community pharmacies. ISRCTN57447996 (Date registered: 19 June 2019).
-
C-GALL: A randomised controlled trial comparing laparoscopic cholecystectomy with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones. ISRCTN55215960 (Date registered: 27 May 2016).
-
CLEAR: A 2 × 2 factorial randomised open label trial to determine the clinical and cost effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in bronchiectasis. ISRCTN89040295 (Date Registered: 25 June 2018).
-
CPIT III: The smoking cessation in pregnancy incentives trial. ISRCTN15236311 (Date registered: 13 October 2017).
-
DISC: Dupuytren’s interventions surgery versus collagenase. ISRCTN18254597 (Date registered: 4 April 2017).
-
FUTURE: Female Urgency, Trial of Urodynamics as Routine Evaluation; a superiority randomised clinical trial to evaluate the effectiveness and cost effectiveness of invasive urodynamic investigations in management of women with refractory overactive bladder (OAB) symptoms. ISRCTN63268739 (Date registered: 14 September 2017).
-
GRASP: GRASP: Getting it Right: Addressing Shoulder Pain. ISRCTN16539266 (Date registered: 13 July 2016).
-
IBD BOOST: Living well with inflammatory bowel disease: optimising management of symptoms of fatigue, abdominal pain, and faecal urgency/incontinence via tailored online self-management: the IBD-BOOST programme. ISRCTN71618461 (Date registered: 9 September 2019).
-
IntAct: Intraoperative Fluorescence Angiography to Prevent Anastomotic Leak in Rectal Cancer Surgery. ISRCTN13334746 (Date registered: 2 May 2017).
-
KReBs: A Randomised Controlled Trial of the effect of a Two-layer Compression Bandage System on Knee Function following Total Knee Arthroplasty. ISRCTN87127065 (Date registered: 20 February 2017).
-
L1FE: Surgical versus conservative treatment of LC1 pelvic fractures in the elderly. ISRCTN16478561 (Date registered: 1 April 2019).
-
MAGIC: Melatonin for Anxiety prior to General anaesthesia In Children. ISRCTN18296119 (Date registered: 8 January 2019).
-
MIQUIT 3: RCT and meta-analysis testing effectiveness and cost-effectiveness of a tailored text message programme (MiQuit) for smoking cessation in pregnancy. ClinicalTrials.gov Identifier: NCT03231553 (ate registered: 27 July 2017).
-
MSS3: Multiple Symptoms Study 3: pragmatic trial of a community based clinic for patients with persistent (medically unexplained) physical symptoms. ISRCTN57050216 (Date registered: 24 September 2018).
-
OTIS: Does Occupational Therapist led environmental assessment and modification reduce falls among high risk older people. ISRCTN22202133 (Date registered: 20 June 2016).
-
PEP-TALK: A behaviour change physiotherapy intervention to increase physical activity following hip and knee replacement: a pragmatic phase III randomised controlled trial. ISRCTN29770908 (Date registered: 23 October 2018).
-
POSNOC: Positive sentinel node: adjuvant therapy alone versus adjuvant therapy plus clearance or axillary radiotherapy. ISRCTN54765244 (Date registered 25 February 2014).
-
PROFHER 2: Effectiveness and cost-effectiveness of reverse shoulder arthroplasty versus hemiarthroplasty versus non-surgical care for acute 3 and 4 part fractures of the proximal humerus in patients aged over 65 years – the PROFHER-2 randomised trial. ISRCTN76296703 (Date registered: 5 April 2018).
-
PUrE RCT: PurE: percutaneous nephrolithotomy, flexible ureterorenoscopy and extracorporeal shockwave lithotripsy for lower pole kidney stones. ISRCTN98970319 (Date registered: 11 November 2015).
-
REFLECT: A randomised controlled trial to evaluate the cost effectiveness of prescribing high concentration fluoride toothpaste to prevent tooth decay in older adults. ISRCTN11992428 (Date registered: 2 June 20017).
-
SARC: Salbutamol for analgesia in renal colic: a prospective, randomised, placebo-controlled Phase II trial. ISRCTN14552440 (Date registered: 1 July 2019).
-
SSHEW: The SSHeW study – Stopping slips among healthcare workers: a research study about slip resistant footwear in the NHS workplace. ISRCTN33051393 (Date registered: 14 March 2017).
-
START:REACTS: Sub-acromial spacer for Tears Affecting Rotator cuff Tendons: a Randomised, Efficient, Adaptive Clinical Trial in Surgery. ISRCTN17825590 (Date registered: 5 March 2018).
-
SWHSI-2: Surgical Wounds Healing By Secondary Intention – 2. ISRCTN26277546 (Date registered: 15 February 2019).
-
TOPAZ: The TOPaZ Trial – Treatment of Osteogenesis Imperfecta with Parathyroid hormone and Zoledronic acid. ClinicalTrials.gov Identifier: NCT03735537 (Date registered: 8 November 2018).
-
UK FROST: United Kingdom Frozen Shoulder Trial (UK FroST). ISRCTN48804508 (Date registered: 18 July 2014).
-
VITA: Lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT. ISRCTN14161293 (Date registered: 18 September 2017).
-
WORKWELL: A randomised controlled trial of job retention vocational rehabilitation for employed people with inflammatory arthritis: the WORKWELL trial. ISRCTN61762297 (Date registered: 13 May 2019).
| Trial | Aim | Speciality | Condition | Study design | Date of recruitment | Setting | Population | Intervention(s) | Comparator | No. of expected participants | Follow-up time points and modality | Primary outcome | Overall trial end date |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTIVE | Assess the effectiveness of external fixation vs. internal fixation for Type C Pilon fractures | Trauma and orthopaedics | Treatment of ankle fractures | 2 arm RCT | 9 March 2018–28 February 2021 | Multicentre, Secondary care, UK | Adults with Pilon C fracture | Internal fixation | External fixation | 334 | 3, 6, 12 and 24 months by postal questionnaire | DRI at 12 months post randomisation | 31 August 2022 |
| ACTWELL | Assess the impact of a lifestyle intervention in women invited to NHS breast screening | NA | Obesity | 2 arm RCT | 1 July 2017–14 August 2018 | Multicentre, UK | Women aged 50–70 years, BMI > 25 km/m | Lifestyle intervention | Usual care | 552 | 3 months by telephone 12 months by research nurses |
Change in body weight and change in physical activity at 12 months | Unclear |
| ARTISAN | Assess if a course of physiotherapy is of clear benefit when compared to a single session of advice | Trauma and orthopaedics | Traumatic anterior shoulder dislocation | 2-arm RCT | 1 November 2018–30 September 2020 | Multicentre, secondary care, UK | Adults with traumatic shoulder dislocation | A course of physiotherapy | A single advice session | 478 | 6 weeks, 3, 6 and 12 months by postal questionnaire | Oxford Shoulder Instability Score at 6 months | 30 November 2021 |
| CHAMP-1 | Incorporating discussion of alcohol within medication consultations in community pharmacy | NA | Alcohol misuse | 2-arm RCT | 24 June 2019–7 August 2019 | Multicentre, UK | Adults screened positive for unhealthy drinking on a single item alcohol screening question | Incorporate discussion of alcohol | Usual medication consultation | 820 | 2 months by telephone | Alcohol consumption at 2 months | 31 December 2022 |
| C-GALL | Conservative management and cholecystectomy in terms of patient QOL and cost effectiveness | Surgery | Gallstones | 2-arm RCT | 1 September 2016–1 August 2018 | Multicentre, Secondary care, UK | Adults aged over 18 with symptomatic uncomplicated gallstone disease | Medical management with analgesia, as required, and dietary advice | Laparoscopic cholecystectomy | 430 | 3, 9 and 18 months questionnaire | Quality of life using AUC at up to 18 months post randomisation using the SF-36 bodily pain domain | 1 October 2020 |
| CLEAR | Assess the effectiveness of hypertonic saline and carbocisteine combinations in airway clearance | Respiratory | Bronchiectasis | 2 × 2 factorial RCT | 27 June 2018–31 December 2020 | Multicentre, secondary care, UK | Adults with bronchiectasis | Saline, carbocisteine | Usual Care | Unclear | 2-, 8-, 26-, 52-week questionnaire at visits | Mean number of exacerbations over 52 weeks | 30 June 2022 |
| CPIT III | Assess the effectiveness of financial incentive to encourage pregnant smokers engaging with smoking cessation service and quite smoking | Obstetrics | Smoking cessation | 2-arm RCT | 1 February 2018–31 December 2019 | Multicentre, secondary care, UK | Pregnant women (less than 24 weeks) aged > 16 | Up to £400 depending on engagement with smoking cessation services and negative CO testing at 3 time points | Offer of smoking cessation and up to £75 on completion of trial follow-up | 940 | 34–38 weeks gestation and 6 month post partum for every participant by postal questionnaire, intervention group will be followed up face-to-face at SCS + 4 and 12 weeks post SCS | Self-reported abstinence for at least 8 weeks prior to 34–38 weeks gestation | 30 November 2020 |
| DISC | Assess the effectiveness of collagenase injections in DC | Plastic surgery | Treatment of DC | 2-arm RCT | 1 May 2017–31 October 2019 | Multicentre, secondary care, UK | Adults > 18 with DC | Collagenase injections | Limited fasciectomy surgery | 710 | 3, 6, 12 and 24 months at clinics | Patient Evaluation Measure at baseline, 3, 6, 12 and 24 months | 31 October 2021 |
| FUTURE | Whether routine urodynamics investigation and comprehensive clinical assessment significantly improves patient-reported success rates following treatment, vs. comprehensive clinical assessment only | Reproductive health and childbirth | OAB | 2 arm RCT | 1 October 2017–31 May 2020 | Multicentre, secondary care, UK | Women ≥ 18 years of age | Comprehensive clinical assessment only | Urodynamics plus comprehensive clinical assessment |
1096 | 3, 6 and 15 months by postal questionnaire | PGI-I | 30 April 2022 |
| GRASP | Assess cost effectiveness of progressive exercise in rotator cuff disorders | Musculoskeletal | Rotator cuff disorders | 2 × 2 factorial RCT | 1 February 2017–2 May 2019 | Multicentre, secondary care, UK | Adults > 18 with rotator cuff disorder | Progressive exercise programme, best practice advice session, injection | Best practice advice session | 704 | 2, 6 and 12 months by postal questionnaires | Shoulder pain and disability index | 31 August 2021 |
| IBD BOOST | Improve QoL of people with IBD by reducing symptoms | Gastroenterology | IBD | RCT | Unclear | Secondary care, UK | Patients with IBD | Online training package for nurses, and online self-management programme | Usual care | 680 | Unclear? | Condition specific quality of life at 6 months | 10 October 2022 |
| IntAct | Evaluate whether IFA decreased AL rate | Colorectal surgery | Rectal cancer | 2 arm RCT | 1 July 2017–31 August 2020 | Secondary care | Adults > 18 with rectal cancer undergoing curative elective surgery |
IFA with surgery | Surgery only | 880 | 30 and 90 days by questionnaire and clinical examination | Clinical anastomotic leak rate at 90 days | 31 May 2021 |
| KReBs | Effect of a Two-layer Compression Bandage System on knee function following total knee arthroplasty | Orthopaedics | Total knee replacement | 2-arm RCT | 1 March 2017–31 August 2018 | Multicentre, secondary care, UK | Adults having a total knee replacement | 2-layer compression bandage | Crepe bandage and synthetic wool layer | 2600 | 10 days, 4 weeks, 6- and 12-month postal questionnaire | Oxford Knee Score 12 months postoperatively | 30 April 2020 |
| L1FE | Assess the effectiveness of surgical fixation with INFIX compared to non-surgical management of LC-1 fragility fractures in older adults | Trauma and orthopaedics | Treatment of Pelvic fractures | 2-arm RCT | 9 July 2018–31 July 2019 | Multicentre, secondary care, UK | Adults > 60 with a LC-1 fracture with severe pain and reduced mobility | Surgical fixation | Non-operative management | 600 | 2 weeks by postal questionnaires or telephone interview. 4 weeks, 12 weeks and 6 months follow-up in clinic |
EQ-5D-5L at 6 months | 31 March 2023 |
| MAGIC | Assess the effectiveness of Melatonin as premedication for surgery in children | Surgery | Dental ophthalmological or ENT surgery | 2-arm RCT | 10 July 2019–15 July 2020 | Multicentre, secondary care, UK | Children aged 5–14 years undergoing dental/ophthalmological/ENT surgery | Melatonin | Midazolam | 592 | 14 days telephone follow-up | Preoperative distress using the modified Yale Preoperative Anxiety Scale | 15 July 2021 |
| MIQUIT 3 | Determine whether or not MiQuit (text-message support programme) is effective in addition to standard support for smoking cessation in pregnancy | Obstetrics | Smoking cessation | 2-arm RCT | unclear | Multicentre, UK | Pregnant women < 25 weeks gestation, aged 16 or over | Receive MiQuit text message cessation programme | Usual care | 692 | Up to 36 weeks gestation, via text messaging | Self-reported smoking abstinence | 1 January 2020 |
| MSS3 | Determine whether a 50-minute symptoms clinic is cost-effective for MUS | General practice | MUS | 2-arm RCT | 15 October 2018–31 October 2019 | Multicentre, primary care, UK | Adults aged between 18 and 69 with MUS | 50-minute symptoms clinic with 15-minute follow-ups | Usual care | 376 | 12- and 52-week postal questionnaire | PHQ-15 at 52 weeks | 31 July 2021 |
| OTIS | Assess the effectiveness of an occupational therapist led home environmental assessment and modification for high risk of falling in older people | General practice | Falls | 2-arm RCT | 1 August 2016–2 August 2018 | Multicentre, primary care, UK | Older people aged 65 and older with risk factors for falls | An occupational therapist-led home environmental assessment and a falls prevention leaflet | Usual care | 1299 | 4-, 8- and 12-month postal questionnaire | Number of falls experienced in the 12 months | 31 December 2019 |
| PEP-TALK | Assessing the effectiveness of group discussion in addition to physiotherapy on improving physical activity following hip and knee replacement. | Musculoskeletal diseases | Hip and knee replacement | 2-arm RCT | 1 January 2019–31 December 2019 | Multicentre, secondary care, UK | Adults following primary unilateral total hip or knee replacement | Group-based exercise sessions in addition to physiotherapy | Physiotherapy only | 250 | 6- and 12-month questionnaire (postal or online) | Physical activity at 6 and 12 months (using the UCLA activity scale) | 31 July 2021 |
| POSNOC | Assess the effectiveness of adjuvant therapy alone vs. adjuvant therapy plus clearance or axillary radiotherapy in women with early-stage breast cancer. | Cancer | Breast cancer | 2-arm RCT | 1 January 2014–31 August 2021 | Multicentre, secondary care, international | Women > 18 years with early breast cancer, with macro-metastasis in sentinel node biopsy. | Adjuvant therapy alone | Adjuvant therapy plus axillary treatment (usual care) | 1900 | 3, 6, 12, 24 and 36 months | Axillary recurrence at 5 years | 31 December 2023 |
| PROFHER-2 | Assess the effectiveness of reverse shoulder arthroplasty, hemi arthroplasty and non-surgical treatment for proximal humerus fractures | Trauma and orthopaedics | Treatment of proximal humeral fractures | 3-arm RCT | 1 June 2018–31 May 2021 | Multicentre, secondary care, UK | Adults > 65 with a 3–4 part humeral fracture | Reverse shoulder arthroplasty vs. hemi-arthroplasty | Conservative management | 380 | 6 months face-to-face follow-up. Postal questionnaire at 1 year and 2 years |
Oxford Shoulder Score at 6, 12 and 24 months | 31 May 2023 |
| PUrE RCT | Assess the effectiveness of three treatment options for lower kidney stones | Urology | Treatment of urolithiasis | 2-arm RCT | 1 May 2015–02 June 2019 | Multicentre, secondary care, UK | Adults > 16 with lower pole stones | FURS | Usual care (PCNL or ESWL) | 1044 | Weekly up to 12 weeks, 3 months and 12 months by questionnaires face-to-face, postal, phone or e-mail | EQ-5D-5L at 1, 2, 4, 8 and 12 weeks | 1 December 2020 |
| REFLECT | To evaluate the effectiveness and cost-benefit of GDP prescribing of 5000 ppm fluoride toothpaste and usual care compared to usual care alone in individuals 50 years and over with high risk of caries | Dentistry | Dental caries | 2-arm RCT | 10 February 2018–30 September 2019 | Multicentre, general dental practices, UK | Adults aged 50 and over | Prescription of 5000 ppm fluoride toothpaste by dentist | Usual care | 1174 | 12, 24 and 36 months by patient questionnaire; clinical assessments up to 36 months | Number and proportion of individuals requiring restoration or extraction or endodontic treatment due to caries | 30 June 2022 |
| SARC | Does salbutamol reduce the pain of kidney stones when used alongside normal pain relief? | Urological and genital diseases | Kidney stones | 2-arm RCT | 16 September 2019–1 August 2021 | Secondary care, UK | Adults with a working diagnosis of renal colic | Salbutamol | Placebo | 118 | 15-, 30-, 60- and 120-minute questionnaire during visit | Change in pain score measured using visual analogue scale at 30 minutes | 31 December 2021 |
| SSHEW | Whether wearing slip resistant shoes can reduce the number of slips, falls and injuries NHS staff have at work. | Signs and symptoms | Slips, falls and injuries | 2-arm RCT | 10 March 2017–10 January 2019 | Multicentre, secondary care, UK | NHS staff | Slip resistant shoes | Usual work footwear | 4400 | 14 weekly text messages | Self-reported slips in the workplace | 10 January 2019 |
| START:REACTS | Compare arthroscopic debridement to arthroscopic debridement with InSpace balloon on shoulder function, pain and QOL following shoulder surgery for rotator cuff tears that cannot be repaired. | Musculoskeletal diseases | Rotator cuff tears | 2-arm RCT | 1 June 2018–30 June 2020 | Multicentre, secondary care UK | Patients with irreparable rotator cuff tear | Arthroscopic debridement with the InSpace balloon | Usual care | 212 | 3-, 6- and 12-month questionnaire | Shoulder function measured with Constant–Murley score at 12 months | 31 December 2021 |
| TOPAZ | Assess effectiveness of parathyroid hormone and zoledronic acid treatment of osteogenesis imperfecta | Orthopaedics | Osteogenesis imperfecta | 2-arm RCT | Unclear | Multicentre, secondary care, UK | Adults with osteogenesis imperfecta | Teriparatide and zoledronic acid | Usual care (bisphosphonate treatment) | 380 | 12- and 24-month visits, telephone calls every 6 months | Incidental fractures (validated by X-ray or other imaging) (approx. 5 years) | 1 April 2023 |
| UK FROST | Assess effectiveness of ESP vs. MUA vs. ACR | Musculoskeletal disease | Frozen shoulder | 3-arm RCT | 1 January 2015–31 December 2017 | Multicentre, secondary care, UK | Adults with frozen shoulder | MUA or ACR with MUA | ESP | 503 | 3-, 6- and 12-month questionnaires | Oxford Shoulder Score at 12 months | 30 June 2019 |
| SWHSI-2 | Assess the effectiveness of NPWT on wound healing | General surgery | Treatment of surgical wounds | 2-arm RCT | 1 May 2019–30 April 2021 | Multicentre, Secondary care, UK | Adults > 16 with an open surgical wound | NPWT | Usual care | 696 | Weekly phone follow-up until would healing, 3 face-to-face visits after wound healing, postal questionnaires at 3, 6 and 12 months | Time to healing in days from randomisation | 30 April 2022 |
| VITA | Lactic acid gel vs. metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT | Gynaecology | Bacterial vaginosis | 2-arm RCT | 25 September 2017 to 28 June 2019 | Multicentre, secondary care, UK | Women aged 16 + with symptoms of BV plus history of one or more episodes of BV in previous 2 years | Intravaginal lactic acid gel | Oral metronidazole | 1900 | Web-based questionnaires at 2 weeks, 3 months and 6 months | Resolution of bacterial vaginosis based on participant reported resolution of symptoms at 14 days post randomisation | 28 June 2020 |
| WORKWELL | Assessing the effectiveness of work advice for people with arthritis | Musculoskeletal diseases | Inflammatory arthritis | 2-arm RCT | 18 February 2019–30 June 2020 | Multicentre, UK | Adults with inflammatory arthritis | Face-to-face contact with a WORKWELL therapist (identifying work problems and providing advice) | Self-help information pack | 240 | 6- and 12-month questionnaire | Presenteeism (work productivity) measured using Combined Work Activities Limitations Scale at 12 months | 31 January 2022 |
Appendix 2 PROMETHEUS Christmas card Study Within A Trial intervention
FIGURE 6.
PROMETHEUS Christmas card SWAT intervention.

Appendix 3 Draft Health Research Authority approvals guidance process for Studies Within A Trial
Study Within A Trial
Guidance for Study Within A Trial applicants
Note: This guidance is primarily intended for co-ordinated SWATs (see Glossary) where there is a co-ordinated evaluation in several host trials and the individual SWAT evaluations within them.
SWATs planned as a single evaluation within a particular clinical trial should look at the SWAT groups described here and, if approval is required, submit their SWAT as part of their trial submission, or as an amendment, to the ethics committee responsible for the clinical trial approval.
Key principles
SWATs are run as discreet studies but with the expectation that they will be replicated (in order to boost statistical power and assess the generalisability of the findings). Therefore, it is important to make a distinction between:
-
The overall, overarching SWAT evaluation (see Glossary) which will evaluate data from multiple SWATs which are run in multiple trials, and
-
The individual evaluations of a SWAT intervention within individual host trials, each of which will contribute data to the overarching evaluation.
The expectation is that approval will be obtained for the overarching SWAT evaluation (1) and then each individual SWAT (2) will come under the approvals obtained for the host trial; this may be from the start of the trial or as a substantial amendment after the trial has commenced. The SWAT evaluation lead and host trial team roles are shown in Figure 7.
FIGURE 7.
Study Within A Trial evaluation lead and host trial team roles.

Study Within A Trial groups
Table 18 sets out which approvals should be obtained for overarching SWATs and for individual SWATs to be included with a host trial.
| Group | Description |
|---|---|
| 1 | The activities targeted by the SWAT are not of a type that needs to be described in detail in a host trial protocol (and so have not required previous approval) but are operational or management activities generally done by the central trial team. |
Examples of this SWAT group include:
|
|

|
|
| 2 | The SWAT interventions affect participants but affect a process that does not need to be described in detail in a host trial protocol (and so have not required previous approval). These interventions are provided to the research team via operational or instruction documents, rather than a protocol, which are not reviewed by the REC. However, in some cases these interventions are also described in the protocol. Where this is the case, the intervention remains a Group 2 SWAT due to the low risk and unobtrusive nature of the intervention. |
Examples of this SWAT group include:
|
|

|
|
| 3 | These SWAT interventions change the design of the host trial, change procedures undertaken by participants, or the information received by participants. |
Examples of this SWAT group include:
|
|
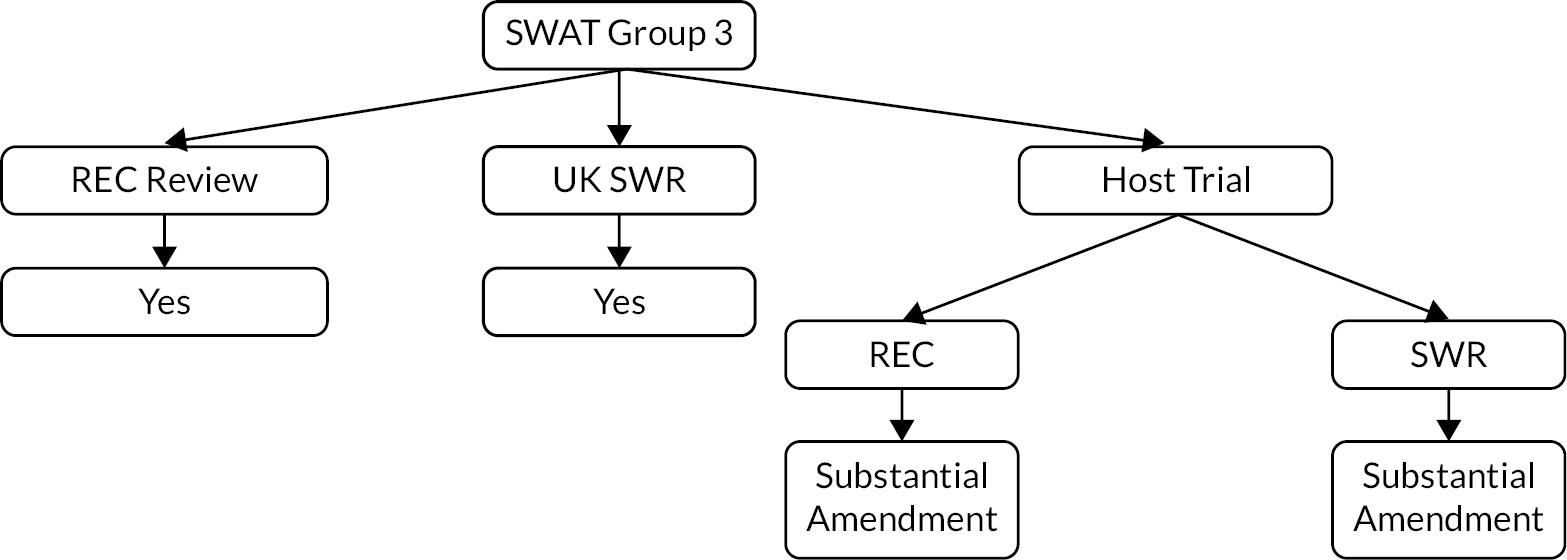
|
Research Ethics Committee review
It is the responsibility of the Sponsor to confirm the SWAT group, whether this is the overarching SWAT (SWAT Sponsor) or the individual SWATs (host trial Sponsor). It is likely that Sponsors of overarching SWATs (or a member of the overarching SWAT team working with the Sponsor) will want to contact the HRA for advice in most cases. The HRA will provide written advice identifying which group they believe the study to fall into which can then be provided to host trial Sponsors.
To request this advice, or if a Sponsor is unsure about the SWAT group, then they should contact catherine.blewett@hra.nhs.uk.
Overarching SWAT
Approval applications (SWAT Groups 2 and 3 only) will be booked directly with the SWAT REC1 (i.e. not via the e-booking system) during the initial phase and should be notified to catherine.blewett@hra.nhs.uk. These studies will likely be suitable for Proportionate Review. Overarching SWAT Group 1 applications submitted solely for the purposes of HRA Assessment will be booked directly with catherine.blewett@hra.nhs.uk.
When the overarching SWAT Sponsor knows when it plans to submit for review, they should contact catherine.blewett@hra.nhs.uk to arrange for the application to be booked. Please note, due to SWAT applications usually being reviewed by one particular REC, it may be necessary to await the next scheduled meeting for that REC.
When an individual SWAT forming part of an overarching SWAT evaluation is submitted as part of a new host trial application, it should be submitted to a single REC as two separate studies (i.e. the overarching SWAT evaluation and the host trial), making clear that the SWAT is part of the overarching SWAT evaluation. These studies may be submitted to the SWAT REC or to any other REC identified by the host trial Sponsor. The overarching SWAT Sponsor should contact catherine.blewett@hra.nhs.uk to identify the REC that they intend to submit to so that the HRA can make the necessary arrangements for review.
The covering letter which is submitted with the application should include Table 19.
| SWAT group | Choose an item |
|---|---|
| SWAT description | |
| Type of host trial | |
| Parameters (e.g. disease area, trial population, participant vulnerabilities) |
Individual Study Within A Trial evaluations done in host trials
Host trial teams doing an individual SWAT evaluation as part of an overarching SWAT only need to consider approvals for Group 2 and 3 SWATs. If needed, these may be submitted as a substantial amendment (where the host trial already has a REC favourable opinion) or alongside a new application (where the proposed host trial itself does not already have a REC favourable opinion).
When an individual SWAT is to be submitted as a substantial amendment, it should be submitted to the REC which issued the favourable opinion to the host trial. The overarching SWAT team should:
-
Complete the amendment tool in the first instance and provide this, along with other relevant documents, to the individual SWAT teams to ensure consistency in classification of the amendment and completion of the amendment tool.
-
Notify the HRA, at catherine.blewett@hra.nhs.uk which trials will be submitting amendments to incorporate the SWAT intervention, and therefore to which REC(s) the amendment(s) will be submitted.
The individual SWAT teams should then submit the amendment to the relevant REC(s) for review and notify catherine.blewett@hra.nhs.uk when they have done so.
The cover letter to the host trial REC should include the following:
-
The table which was submitted and approved for the overarching SWAT. 2
-
The integrated research application system (IRAS) ID and REC Reference of the overarching SWAT.
-
Name of the REC which issued the favourable opinion for the overarching SWAT.
-
A description of how the individual SWAT complies with the approved scope of the overarching SWAT.
An application for an individual SWAT that forms part of an overarching SWAT evaluation should not be submitted unless the overarching SWAT itself has a favourable opinion. If an application is submitted where the overarching SWAT does not yet have a favourable opinion, you will be contacted and advised that the application should be withdrawn and re-submitted after the overarching SWAT has received a favourable opinion.
Further help and guidance
More help and guidance on SWATs are available from the York Trial Forge Centre (prometheus-group@york.ac.uk), or Trial Forge more generally (http://trialforge.org; e-mail info@trialforge.org).
Glossary
| SWAT | Study Within A Trial. (These are also called ‘embedded trials’, ‘nested trials’ or ‘trials in trials’.) |
| Host trial | A clinical trial in which a SWAT is embedded. |
| SWAT intervention | The trial process intervention that is to be tested. Examples are financial incentives to improve recruitment, Christmas cards to improve retention or the format of a site initiation visit. |
| Overarching co-ordinated SWAT | A SWAT evaluation that is planned from the beginning as one that is done in many host trials at the same time. The evaluation will have a lead researcher (the SWAT evaluation lead) who will approach host trial teams to seek their involvement in the SWAT. |
| SWAT evaluation lead | The SWAT evaluation lead is responsible for developing the SWAT evaluation protocol and for obtaining overarching approval to evaluate the SWAT intervention. |
| Host trial team | The team that is embedding an evaluation of the SWAT intervention into their own trial under agreement with the evaluation lead. |
| REC | Research Ethics Committee |
| UK SWR | UK Study Wide Review |
Appendix 4 Draft Study Within A Trial reporting guidance Trial Forge Guidance (3 or 4): a template for reporting the results of randomised Studies Within A Trial
| CONSORT item to be included in publication | Additional information and example text shown in italics where possible | |
|---|---|---|
| Title and Abstract | ||
| 1a | Term ‘SWAT’ should be used in the title | SWAT registry number included if available SWAT [insert number]: [insert title of SWAT] |
| 1b | Structured summary | Structured using these headings: Background, Methods, Results, Conclusion. Details of host trial included in which the SWAT intervention was evaluated. |
| Introduction; Background and objectives | ||
| 2a | Scientific background and explanation of rationale for the SWAT. | Justify the need for the SWAT; cite systematic review evidence where appropriate Replication SWAT: Also cite previous SWAT evaluations undertaken as part of the rationale |
| 2b | Specific objectives or hypotheses for the SWAT. | State SWAT question as objective Does [insert SWAT intervention] increase/decrease [outcome] compared to [comparator] in [participants]? |
| Methods; Trial design | ||
| 3a | Description of the SWAT (such as parallel, factorial, cluster) including allocation ratio. | Describe trial design and allocation ratio A [insert number of trial arms and trial design] SWAT was undertaken with an allocation ratio of [insert allocation ratio] State where the SWAT protocol is registered The SWAT protocol can be found at [insert details of SWAT Repository link] If SWAT protocol is not registered, include as an appendix. Host trial: The host trial protocol is available at [insert details] Provide a brief description of host trial using population, intervention, comparator, outcome (PICO) format. SWAT: Use a PICO format to guide description – covered within points 4–6. If changes to the SWAT occurred: The following changes occurred once the SWAT started [insert text] |
| 3b | State changes (with reasons) to methods of SWAT following commencement. | |
| Participants | ||
| 4a | State eligibility criteria in SWAT, including differences to those from the host trial | State participant eligibility, this can be tabulated. |
| 4b | Include setting(s) and location(s) where SWAT data were collected | SWAT data were collected in the following settings/locations [insert text] |
| Interventions | ||
| 5 | Describe SWAT intervention to enable replication, include how and when interventions were administered and recruitment dates. | Briefly describe the SWAT intervention. Reference to the protocol for further details is acceptable as long as the protocol is available to the reader. |
| Outcomes | ||
| 6a | State primary and secondary outcome measure for SWAT. Include how and when they are assessed |
Primary outcome measure: [insert information including how/when assessed]. Secondary outcome measure(s): [insert information including how/when assessed]. This information can be tabulated. If appropriate: The following changes occurred once the SWAT started [insert text]. |
| 6b | Include changes (and reasons) to SWAT outcomes after commencement | |
| Sample size | ||
| 7a | How sample size was determined for the SWAT. | SWATs are often individually underpowered due to the sample size being constrained by the host trial and a reliable estimate of the effect of the SWAT intervention might depend on the aggregation of replicated SWAT evaluations. It is not expected that a formal sample size calculation will always be done. The SWAT sample size was dependent on the host trial [insert host trial details]; therefore no formal sample size calculation was performed, which is in line with SWAT methodology.15,157 [insert any reasoning for a subsample of the host trial being used – e.g. SWAT was included midway through the trial]. If interim analyses and/or stopping guidelines were planned: The following interim analyses were planned [state analyses here]. The stopping guidelines were [details here]. |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines for the SWAT. | |
| Randomisation: Sequence generation | ||
| 8a | Method used to generate the random allocation sequence for the SWAT. | Participants were randomised by [insert method with all methodological details]. |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size). | |
| Allocation concealment mechanism | ||
| 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned for the SWAT. | Allocation concealment was achieved by [insert method]. |
| Implementation | ||
| 10 | Who generated the random allocation sequence, who enrolled participants and who assigned participants to interventions for the SWAT. | Randomisation was performed by [specify centre or personnel], [specify centre or personal] enrolled participants and [specify centre or personal] assigned the participant to the SWAT intervention or comparator. |
| Blinding | ||
| 11a | If done, who was blinded to the SWAT after assignment to interventions (e.g. participants, care providers, those assessing outcomes) and how. | Explain who was blinded. Blinding was achieved by [insert details]. |
| 11b | If relevant, description of the similarity of the SWAT interventions. | |
| Statistical methods | ||
| 12a | Statistical methods used to compare groups for primary and secondary outcomes for the SWAT. | All analyses for the SWAT should be pre-planned, ideally detailed in a SWAT SAP, which might be a very short component of the SWAT registry entry. Unless detailed thoroughly and extensively in a publicly available SWAT protocol, the analysis for each outcome should be detailed in the methods of the report. Alternatively, depending on the journal, the SAP could be uploaded as supplementary material. The SAP and subsequent publication details should include the software used, the statistical methods (including significance level for hypothesis testing), and the population used for the analysis (ITT or per-protocol). |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses. | |
| Results | ||
| Participant flow | ||
| 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome for the SWAT. | Provide a participant flow diagram that includes this data. |
| 13b | For each group participating in the SWAT, losses and exclusions after randomisation, together with reasons. | |
| Recruitment | ||
| 14a | Dates defining the periods of recruitment and follow-up of the SWAT. | Participant recruitment took place between [insert dates]. If the SWAT ended or was stopped early: The SWAT stopped [recruitment/follow up] early due to [insert text]. |
| 14b | Why the SWAT ended or was stopped? | |
| Baseline data | ||
| 15 | A table showing baseline demographic and clinical characteristics for each group. | The context of the host trial for each SWAT evaluation is likely to be different and contextual information about the host trial should be provided. In addition to general information about the host trial (see ‘Methods’), we suggest a table of participant baseline characteristics for those allocated to each group of the SWAT evaluation if these details are available. |
| Numbers analysed | ||
| 16 | For each group of the SWAT, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups. | |
| Outcomes and estimation | ||
| 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% CI). | Results should be presented in tables as far as possible rather than only being presented in the body of the text. To facilitate meta-analysis, SWATs should report the actual numbers of participants in each group in the SWAT evaluation. A key element of SWAT evidence is the ability for them to be replicated and an important principle for reporting research is that new findings should be placed in the context of existing, relevant evidence. Therefore, we recommend that an updated meta-analysis is included that presents the results of the current SWAT combined with previous evaluations of the SWAT intervention. Presentation as a cumulative meta-analysis is particularly helpful because it would help to inform judgements about the need for further evaluations of a SWAT intervention.150 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended. | |
| Ancillary analyses | ||
| 18 | Results of any other analyses performed on the SWAT data, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory. | |
| Harms | ||
| 19 | All important harms or unintended effects in each group that took part in the SWAT (for specific guidance see CONSORT for harms). | |
| Discussion | ||
| Limitations | ||
| 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses for the SWAT. | |
| Generalisability | ||
| 21 | Generalisability (external validity, applicability) of the SWAT findings. | |
| Interpretation | ||
| 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence. | In addition to a general interpretation, we suggest that the report includes a brief ‘Implications for trial practice’ and ‘Implications for SWAT research’. These could make use of the cumulative meta-analysis and Trial Forge guidance154 on whether further evaluations of the intervention are warranted. |
| Other information | ||
| 23 | Registration Registration number and name of trial registry |
Include the information for both the host trial and SWAT. |
| 24 | Protocol Where the full trial protocol can be accessed, if available |
|
| 25 | Funding Sources of funding and other support (such as supply of drugs), role of funders |
|
| Additional | ||
| Data sharing | Although not part of Madurasinghe et al. 201627 or the CONSORT guidance, we suggest that authors make the data used to generate their results available as a supplementary file, or through data-sharing platforms. | |
| Registration | It is recommended that SWATs are registered on a repository to ensure all SWATs performed can be included in the evidence and base and support future replication. The following repositories are available to register SWATs: the Northern Ireland Methodology Hub’s SWAT Repository32 (this repository is for SWATs only and encourages replications of registered SWATs), the ISRCTN trial registry,156 and the Clinical Trials database.158 SWATs are usually registered in the two latter repositories as part of the host trial. |
|
List of abbreviations
- ACTIVE
- Articular Pilon Fracture Trial
- ActWELL
- a randomised controlled trial to assess the impact of a lifestyle intervention in women invited to National Health Service breast screening
- ARTISAN
- Acute Rehabilitation following Traumatic anterior shoulder dISlocAtion
- C-Gall
- a randomised controlled trial comparing laparoscopic cholecystectomy with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones
- CHAMP-1
- community pharmacy: highlighting alcohol use in medication appointments pilot study
- CLEAR
- a 2 × 2 factorial randomised open label trial to determine the CLinical and cost-Effectiveness of hypertonic saline (HTS 6%) and carbocisteine for Airway cleaRance versus usual care over 52 weeks in bronchiectasis
- CONSORT
- CONsolidated Standards Of Reporting Trials
- COVID-19
- coronavirus disease 2019
- CPIT-III
- the Cessation in Pregnancy Incentives Trial III
- CRN
- clinical research network
- CTUs
- clinical trials units
- DISC
- Dupuytren’s interventions: surgery versus collagenase trial
- DMC
- Data Monitoring Committee
- FUTURE
- female urgency, trial of urodynamics as routine evaluation
- GDPR
- General Data Protection Regulation
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluations
- GRASP
- getting it right: addressing shoulder pain
- GRIPP
- guidance for reporting involvement of patients and the public
- HRA
- Health Research Authority
- HRB-TMRN
- Health Research Board – Trials Methodology Research Network
- HSDR
- Health and Social Care Delivery Research
- HTA
- Health Technology Assessment
- IBD-BOOST
- a randomised controlled trial of supported online self-management for symptoms of fatigue, pain and urgency/incontinence in people with inflammatory bowel disease
- IntAct
- intraoperative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery
- KReBS
- knee replacement bandaging study
- L1FE
- lateral compression type-1 fracture fixation in the elderly
- MAGIC
- Melatonin for Anxiety prior to General anaesthesia In Children
- MiQuit
- the MiQuit study: feasibility trial of a computer-tailored smoking cessation intervention providing individualised written and mobile phone text message support to pregnant smokers
- MRC
- Medical Research Council
- MSS3
- Multiple Symptoms Study 3
- NIHR
- National Institute for Health and Care Research
- ORRCA
- Online Resource for Research in Clinical triAls
- OTIS
- Occupational Therapist Intervention Study
- PEP-TALK
- a study investigating whether having group discussions in addition to physiotherapy improves the amount of physical activity following hip and knee replacement
- PIL
- participant information leaflet
- PMG
- project management group
- POSNOC
- POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy
- PPI
- patient and public involvement
- PRioRiTy
- Prioritising Recruitment in Randomised Trials
- ProFHER-2
- PROximal Fracture of Humerus: Evaluation by Randomisation-2
- PROMETHEUS
- PROMoting THE Use of Studies Within A Trial
- PUrE
- Percutaneous nephrolithotomy, flexible Ureterorenoscopy and Extracorporeal shockwave lithotripsy for lower pole kidney stones
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- REFLECT
- a randomised controlled trial to evaluate the effectiveness and cost benefit of prescribing high-dose fluoride toothpaste in preventing and treating dental caries in high-risk older adults
- SAP
- statistical analysis plan
- SARC
- salbutamol for analgesia in renal colic
- SSHeW
- does slip resistant footwear reduce slips among healthcare workers? A randomised controlled trial
- START
- systematic techniques for assisting recruitment to trials
- START:REACTS
- subacromial spacer for tears affecting rotator cuff tendons: a randomised, efficient, adaptive clinical trial in surgery
- SWAT
- Study Within A Trial
- SWHSI-2
- surgical wounds healing by secondary intention – 2
- TOPaZ
- treatment of osteogenesis imperfecta with parathyroid hormone and zoledronic acid
- TRECA
- TRials Engagement in Children and Adolescents
- TMG
- Trial Management Group
- TMRP
- Trial Methodology Research Partnership
- TSC
- Trial Steering Committee
- UK FROST
- United Kingdom FROzen Shoulder Trial
- VITA
- lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over
- WORKWELL
- a randomised controlled trial of job retention vocational rehabilitation for employed people with inflammatory arthritis
- YTU
- York Trials Unit