Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/98/01. The contractual start date was in April 2019. The final report began editorial review in October 2022 and was accepted for publication in March 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Ingram et al. This work was produced by Ingram et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Ingram et al.
Chapter 1 Introduction
Background
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that can have a profound impact on quality of life due to pain, suppuration of pus and scarring. 1,2 It is characterised by inflammatory nodules, abscesses and skin tunnels occurring in flexural skin regions such as the axilla, groin and perineum, often leading to scarring. 3 At least 1% of the UK population is affected;4,5 however, the prevalence may be higher due to undiagnosed cases resulting from a typical diagnostic delay of 7 years. 6 Onset of HS is usually at or soon after puberty, so HS affects young adults, with major impact on relationships and careers, producing cumulative life course impairment. 1 The aetiology of HS remains uncertain, with genetics, microbiological, immune dysregulation, lifestyle and endocrine factors thought to contribute. 7
The management goals for HS are to prevent and treat flares of inflammatory skin lesions and the avoidance of scarring. Medical (drug) treatments for HS are intended to improve and prevent disease flares, while surgery and other non-drug therapies are required to treat scarring once it has occurred. Holistic management of HS therefore requires integration of medical and surgical treatment pathways. 8
Existing evidence
A 2015 Cochrane review of interventions for HS found a relative lack of randomised controlled trials (RCTs), identifying only 12 RCTs involving a total of 615 participants, despite HS being such a common condition. 9,10 Since 2015, few RCTs have been performed and so the UK, European and North American guidelines for HS management continue to rely on expert consensus to a large extent. 8,11–13 Trials of biological therapies for HS sponsored by the pharmaceutical industry are now under way; however, the evidence base for current HS systemic treatments continues to rely mainly on retrospective case series/cohort evidence.
Surveys investigating current HS management in the UK, undertaken to inform the Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS), demonstrated substantial variation in HS care among dermatologists, surgeons and general practitioners (GPs). 14–16 It is likely that the variance produces inequalities of care and poorer outcomes for some people with HS depending on their UK location.
Rationale for the Treatment of Hidradenitis Suppurativa Evaluation Study
Prioritisation of the research question
A James Lind Alliance Priority Setting Partnership (PSP) for HS produced a top 10 list of research questions in 2014, following a prioritisation exercise involving people with HS and their clinicians. 17 THESEUS was designed to improve the evidence base for several of the top 10 priorities including: what is the most effective and safe group of oral treatments in treating HS (ranked number one priority); what is the impact of HS and the treatments on people with HS (ranked third) and what is the best surgical procedure to perform in treating HS (ranked sixth).
The design of THESEUS was also guided by the funding call from the UK National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme, which posed the question: ‘What are the best management options for HS when first line treatments fail?’
The aims of THESEUS are to understand how HS treatments are currently used in the UK and to inform the design of future RCTs in HS.
Introduction of deroofing and laser treatment for hidradenitis suppurativa into the UK
Deroofing is a tissue-sparing procedure, usually performed under local anaesthetic, which removes the subcutaneous linear channels known as skin tunnels (also known as sinus tracts or fistulae) that form in HS. 18 It is routinely performed in some countries, such as the Netherlands and USA, but is rarely performed in the UK. The advantage of deroofing compared with conventional surgery is that blunt probing of tunnels ensures that all subcutaneous branches are removed while avoiding excision of unaffected skin. By including deroofing as one of the treatment options within THESEUS, the intention was to upskill several centres in the UK to perform deroofing for HS as part of the IDEAL Collaborative’s stage 2b of surgical innovation.
Several small RCTs suggest benefit from laser treatment targeting the hair follicle in HS. Access to laser treatment for HS is limited in the UK, with seemingly unwarranted variation. This is despite it being included in some HS management guidelines. 8 Including laser therapy as an intervention within THESEUS would help to understand the desirability of this treatment to patients, upskill recruiting sites ready for any future RCT involving laser therapy for HS and gather initial data on likely treatment effectiveness.
Validation of HiSTORIC core outcomes set instruments
A systematic review of outcome measure instruments (OMIs) included in the HS Cochrane review demonstrated substantial heterogeneity, with 30 different OMIs used in the 12 RCTs. 19 This finding led to the creation of the Hidradenitis SuppuraTiva cORe outcomes set International Collaboration (HiSTORIC), with the aim of developing a core outcomes set for HS. 20 The six domains of the set have been established by consensus and HiSTORIC is now developing and validating OMIs for each domain. 21,22 THESEUS has the opportunity to contribute to HiSTORIC OMI validation, in particular providing evidence for feasibility and interpretation of the OMIs, including evaluation of minimum important difference.
Nested process evaluation studies
Qualitative studies were nested within THESEUS to gain a deeper understanding of the processes involved in HS clinical trials and to inform the design of future RCTs. The aims were as follows:
-
to characterise current conventional surgical procedures and document best practice for laser and deroofing interventions;
-
to understand the factors influencing choice of intervention from the perspectives of patients and clinicians;
-
to identify barriers and facilitators to recruitment into future RCTs.
Consensus workshop
To inform the design of future HS RCTs, the final aim of THESEUS was to host a consensus workshop attended by study participants, HS patient advocates, clinicians, methodologists and researchers. Objectives of the workshop were to identify the highest priorities for future HS RCTs in terms of participants, interventions, comparators and outcomes.
Chapter 2 Methods for the THESEUS observation cohort study
Study design
The THESEUS study was a prospective observational cohort study, with a nested process evaluation, of individuals living with HS, for which the protocol has been published. 23 Study participants were patients receiving treatment for their HS recruited from secondary care. Following recruitment, and after undergoing a clinical examination and baseline data collection, participants were asked to indicate their willingness to receive the five treatment options detailed below and to rank them from most preferred to least preferred, with the help of a THESEUS treatment decision grid (see Chapter 6). The participant’s clinician provided guidance regarding treatment eligibility and together the participant and their clinician agreed to the final treatment selection. This was a pragmatic, non-randomised study with the aim to understand and explore current practices around HS management and care pathways for those living with HS and to inform the design of future HS RCTs. Described below are the primary and secondary objectives and the outcome measures.
The THESEUS study was informed by surveys of patients (n = 358), dermatologists (n = 57), plastic and general surgeons (n = 225) and GPs (n = 133). 14–16 The surveys provided insight into current HS treatment pathways, gaps in treatment provision and willingness of respondents to take part in a HS clinical trial.
Patient and public involvement (THESEUS patient research partners)
A patient research partner (PRP) and founder of the HS Trust patient support group was a co-applicant for the THESEUS grant application. A further two PRPs joined the THESEUS study management group (SMG), attending regular meetings and contributing to all aspects of the conduct of the study. The THESEUS PRPs also made substantial contributions to the management and implementation of the THESEUS consensus workshop, through preworkshop results dissemination, facilitation of group discussions involving study participants and collating feedback from the discussions. Another PRP was a member of the study steering group.
Ethical approval and governance
The Wales Research Ethics Committee 4 provided ethical approval for THESEUS on 26 September 2019, reference number 19/WA/0263. Cardiff University acted as sponsor for the study. All sites received local research and development approvals. Prospective trial registration on the ISRCTN Registry was obtained on 9 August 2019 (ISRCTN69985145). Study oversight was provided by a combined study steering committee and independent data monitoring committee. There were four independent members of the committee: a chairperson experienced in the conduct of clinical trials, an academic, a biostatistician and a patient representative. The study was conducted in accordance with the Research Governance Framework for Health and Social Care, principles of good clinical practice, General Data Protection Regulation and Cardiff University Centre of Trials Research standard operating procedures.
THESEUS study interventions
Participants recruited into the study had to be eligible and willing to receive at least one of the five THESEUS interventions offered: (1) oral doxycycline; (2) oral clindamycin and rifampicin; (3) laser treatment; (4) deroofing of skin tunnels; and (5) conventional surgery. The choice and dose of the THESEUS treatments were informed by the results of the stakeholder surveys.
If the participant’s first treatment choice carried a long waiting time, the participant was offered an alternative treatment to cover the interim period, based on a joint decision between the clinician and the participant. Using one of the study treatments in the interim period was preferred; however, other treatments were permitted, depending on clinician judgement. Participants could opt to switch treatments to another THESEUS intervention, or a combination of interventions, once they had been on their chosen intervention for 6 months.
Option 1: oral doxycycline
Oral doxycycline was offered at a dose of 200 mg once daily.
Option 2: oral clindamycin and rifampicin
Oral clindamycin and rifampicin were each taken at a dose of 300 mg twice daily as a combined course for 10 weeks initially, with the option to continue up to 6 months. Prior to commencing treatment, participants were required to have safety blood tests (full blood count, renal function, liver function) at baseline and repeated 4 weeks after starting the treatment, as per usual care.
Option 3: laser treatment
Laser treatment targeting the hair follicle was specified in the protocol with Nd-YAG laser (skin types 2–6) or alexandrite/diode laser (skin types 1–3). This treatment option was scheduled to be administered on four occasions, each 1 month apart.
Laser hair removal treatment was performed by healthcare professionals (HCPs) trained and certified in the use of medical lasers. Training in laser treatment is already formalised as part of medical laser training and certification was required for practitioners to be insurable. THESEUS did not provide study-specific training in laser treatment; however, a laser protocol was provided.
Option 4: deroofing of skin tunnels
Deroofing of skin tunnels was carried out using electrocautery and details of the procedure performed were documented in a clinical report form.
A protocol and training video was developed by the study team to guide HCPs (including dermatologists, plastic surgeons and other surgeons) through the deroofing procedure. HCPs wishing to use the procedure HCPs were invited to attend an in-person training event. The training video and an information video for participants were made available on the publicly accessible THESEUS study website (https://www.cardiff.ac.uk/centre-for-trials-research/research/studies-and-trials/view/theseus).
Deroofing procedures were performed under local anaesthetic in most cases and could be repeated if required. Details of the procedure were recorded in a clinical report form in each case. The total area treated at one time was limited by the volume of local anaesthetic needed and expected degree of impairment of activities of daily living during recovery. Wound healing took place by secondary intention healing over a period of a few weeks.
Option 5: conventional surgery
Participants selecting the conventional surgery option were assessed as to the most appropriate excision margins (narrow or wide). Skin closure following excision could also vary depending on which method the clinician felt most appropriate.
No formal training was provided for conventional surgical options because one of the objectives of THESEUS was to document current practice and assess any variability. A protocol was provided to surgeons, which contained some basic parameters, allowing for wide variation in practice. The surgical technique used for each procedure was documented using an online questionnaire.
Study objectives and outcome measures
Study objectives
The primary objective of the THESEUS study was to understand how HS treatments are currently used and to inform the design of future HS RCTs.
The secondary objectives of the study were to determine the feasibility of recruiting individuals with HS; to test the feasibility and responsiveness of OMIs; to understand current patient pathways and what influences patients’ and clinicians’ treatment choices; to fully characterise the study interventions (dose of medication, type of surgical techniques used); and to explore consensus-agreed recommendations for future RCT study designs.
Outcome measures
The primary outcome of THESEUS was to determine the proportion of participants who were eligible, and hypothetically willing, to use the different THESEUS treatment options.
The secondary outcomes included the proportion of participants choosing each of the study interventions, with reasons for their choices; the proportion of participants who switch treatments, with reasons for switch; study treatment fidelity; the loss to follow-up rates during the study; treatment efficacy outcome estimates after 6 months of follow-up, and to inform OMI responsiveness.
Setting and participants
Site selection
Clinical sites were selected for participant recruitment based on their clinical services and experience in HS management. Sites with the following expertise were selected: (1) those that offered a multidisciplinary team (MDT) approach integrating HS medical and surgical care; (2) sites with experience in HS surgery; (3) dermatology departments that were experienced in HS medical management. Additionally, study sites had to offer at least four of the five THESEUS interventions.
Clinical site set-up
Between November 2019 and May 2021, 11 secondary care sites were identified to carry out participant recruitment. Ten sites actually opened to recruitment as one site had to be withdrawn because it was unable to provide capacity and capability approvals following the restart period during the COVID-19 pandemic. Site set-up and recruitment were staggered due to the pandemic, and not all 10 sites were open to recruitment simultaneously. Dermatologists or surgeons took the role of principal investigator in 9 of the 10 sites, and a GP working in a dermatology department undertook the role in 1 site. The local investigating team comprised research nurses, dermatologists, surgeons, specialist nurses and trial co-ordinators. Training was delivered to the principal investigators and the site research team in one site set-up session, usually via teleconference. THESEUS was a low-risk study following usual clinical practice. A risk-based approach to study monitoring was adopted and outlined in the study risk assessment document. The study was monitored centrally and there were no preplanned site monitoring visits.
Participant eligibility
Individuals could be included in the study if they met all of the inclusion criteria and none of the exclusion criteria. Individuals had to be at least 18 years of age with active HS of any severity and not adequately controlled by current treatment; their HS diagnosis had to meet the disease definition (i.e. a lifetime history of at least five flexural skin boils or two flexural skin boils in the past 6 months) and the disease had to be confirmed by a recruiting clinician with experience of HS care. Individuals also had to be eligible and willing to receive at least one of the five THESEUS study interventions.
Individuals were excluded if they were unable or unwilling to provide informed consent and if they were pregnant or breastfeeding. Most of the study questionnaires were only validated in English, so individuals who were not sufficiently fluent in English were also excluded.
Participant recruitment
Assessment of eligibility was undertaken by a medically qualified clinician. Informed consent to take part in the study was obtained by an appropriately trained local researcher at the study site. After completing the consent process, baseline data collection was undertaken.
The research team recommended that the recruitment/baseline appointment should be carried out in person; however, for patients who were well known to the local investigating team, the recruitment/baseline appointment could be conducted remotely via telephone or videoconference. Patients who were not known to local investigators were required to attend a recruiting clinic in-person to ensure study eligibility and assess disease severity. Exceptions to in-person attendance could be made if the patient’s HS skin involvement was limited to non-intimate sites and could be assessed remotely. The inclusion of remote appointments was included in the study protocol as an amendment during the first wave of the COVID-19 pandemic.
Data collection
Data were collected with the participant at the hospital-based clinic. Data were also collected with the participant over the telephone or via video- or teleconferencing if preferred. Data were collected electronically via a bespoke Cardiff Centre for Trials Research-built online database, with paper copies as a backup. All data collected on paper were later entered electronically into the database by local researchers at the site. Data were added to the database using a secure electronic device.
The database had in-built ranges, checks and validation rules, with incomplete fields and data outliers flagged at the time of entry. Data queries and missing data were referred back to the site. Once participants had completed data collection at the relevant time point the data manager would note completion of the data collection on a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet. The schedule of interventions and assessments is shown in Table 1.
| Review number | –1 | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Planned month | –1 | Baseline | 3 | 6 | 9 | 12 |
| Screening | X | X | ||||
| Eligibility assessment | X | X | ||||
| Demographics and consent | X | |||||
| Clinical examination including Hurley stage | X | X | X | X | X | |
| Interventions for which participant is potentially eligible | X | |||||
| Intervention received, with reasons for choice (including treatments switched after baseline) | X | X | X | X | X | |
| Outcomes | ||||||
| Hidradenitis Suppurativa Quality of Life (HiSQOL) | X | X | X | X | X | |
| Dermatology Life Quality Index (DLQI) | X | X | X | X | X | |
| European Quality of Life 5 dimension 5 level questionnaire | X | X | X | X | X | |
| Pain numerical rating scale (NRS) | X | X | X | X | X | |
| Pain score (via text message) | 12 weeks from start of intervention | |||||
| Need for dressings | X | X | X | X | X | |
| Fatigue Severity Scale (FSS) | X | X | X | X | X | |
| Patient Global Assessment (PtGA) | X | X | X | X | X | |
| Anchor question for change in severity | X | X | X | X | ||
| Flare frequency | X | X | X | X | X | |
| Assessment of HS physical signs | X | X | X | X | X | |
| Adverse effects of study treatment | X | X | X | X | ||
| Treatment fidelity | X | X | X | X | ||
| End-of-study questionnaire (participants and clinicians) | X | |||||
| Surgeon questionnaires/pro forma | After each surgery | |||||
| Structured interview (subset of participants) | Single interview | |||||
| Consensus workshop (subset of participants, clinicians and researchers) | Single workshop | |||||
While the majority of clinical data and questionnaire data were added to the bespoke online database, THESEUS also used the ‘Online Surveys’ platform (https://www.onlinesurveys.ac.uk) to enter and hold the THESEUS clinician questionnaire data. Data were manually entered into the platform by the THESEUS clinician at the time of data collection. Additionally, a telecommunications provider (Esendex, Commify UK Limited, Nottingham, UK) was used to send text messages to the participants asking for their daily pain scores. Data returned from participants via text messages were stored on Esendex servers. All data, including sensitive and personal data, were handled in accordance with the General Data Protection Regulation 2016.
Data were extracted from all databases on completion of data cleaning and supplied to the statistician or qualitative researchers for analysis. As per Cardiff University’s procedures, data will be retained for 15 years following study closure.
Baseline review
Baseline data and contact details were collected immediately following participant recruitment.
The baseline appointment entailed a clinical examination to assess baseline severity of disease (measured by Hurley staging and refined Hurley staging). 24,25 The participant’s smoking status, body mass index (BMI) and other demographics were recorded. Details of their past medication history and history of surgery relating specifically to their HS were collected.
The participant was asked to complete questionnaires about their HS and its impact on functioning and quality of life. Outcome measures included the six core domains recommended by the HiSTORIC core outcomes set initiative for HS (pain, HS-specific quality of life, global assessment, disease progression, physical signs and symptoms)22 as measured by pain numerical rating scale (NRS), HS quality of life questionnaire (HiSQOL),26 Patient Global Assessment (PtGA),27 number of patient-reported HS flares, a count of inflammatory HS lesions, the use of dressings and fatigue severity. 28 Dermatology life quality index (DLQI)29 and general health-related quality of life [EuroQoL 5 dimension 5 level (EQ5D-5L)] questionnaires were also administered. The baseline appointment concluded with a clinical assessment of participant eligibility for each of the THESEUS treatments, with the participant finally choosing a THESEUS treatment that they were eligible to receive (and available at the recruiting site) in consultation with their clinician.
Follow-up data collection
Follow-up data collection included daily pain data returned by text message for 12 weeks after the chosen intervention was first received by the participant, as well as face-to-face or telephone follow-up review appointments at 3, 6, 9 and 12 months after the baseline appointment. Clinicians who undertook the THESEUS non-medical interventions (laser, deroofing or conventional surgery) were asked to complete a questionnaire providing details of the procedure performed in each case. Members of the local investigating team who had been involved in THESUS recruitment, data collection or procedure delivery were also asked to complete a questionnaire about their experience of carrying out the THESEUS study.
Pain score collected via text message
The feasibility of collecting daily pain data using short messaging service (SMS) text messages was trialled in the THESEUS study. When a participant commenced their treatment, or first received their procedure, they were sent a text message asking the magnitude of their current pain, using the pain 0–10 NRS instrument. The messages would be sent to the participant for up to 12 weeks. The text to the participants read:
Hello. This a text message from the THESEUS study. Please indicate the level of pain you are CURRENTLY experiencing due to your HS. The scale is from 0–10. ‘0’ means no pain and ‘10’ means pain as severe as it could be. You have until 02.00 am tomorrow morning to return today’s pain score. If you no longer wish to receive these messages please text STOP to [telephone number]
The participant receiving the messages could withdraw from participation in the text messaging by texting ‘STOP’ directly to the message. They were not charged for the withdrawal text message.
The messages were sent by Esendex, a telecommunications service provider. This was an automated process whereby Esendex was instructed to send the same message to the participant at the same time, 6 p.m., each day, with responses accepted until 2 a.m. the following day. The series of messages were triggered by the addition of data/dates into the intervention case report form within the online database. The responses from the text messages were held securely within the Esendex servers.
Once text message data collection was concluded a command was issued from the THESEUS server to query the Esendex application programming interface and request the inbound participant responses (as SMS messages).
Three-, six- and nine-month follow-up reviews
Reviews 1, 2 and 3 took place 3, 6 and 9 months after recruitment, respectively. Sites were encouraged to collect data within a window of 2 weeks either side of the intended follow-up date.
Reviews 1, 2 and 3 included a clinical examination of the disease stage using Hurley Staging24 and a HS skin lesion count, documenting the number of inflamed nodules, abscesses and draining skin tunnels. The patient reported outcome measures collected at baseline were repeated at Reviews 1, 2, and 3, as described in Table 1.
Participants were asked questions related to the THESEUS intervention they had selected. If the participant had chosen one of the THESEUS procedures (laser, deroofing, surgery), questions would focus on receipt of the procedures and whether the participant was content to continue with the course of treatment. If the participant had selected one of the THESEUS antibiotics options, then questions would centre around adherence to the chosen medication and whether they would be continuing with the treatment.
Twelve-month follow-up review
The final review (review 4) took place 12 months after recruitment. Review 4 repeated the assessments performed at the previous reviews, including information about receipt of the intervention, fidelity of procedure delivery and treatment adherence, as well as the clinician- and patient-reported outcome measures. In addition, participants were asked to complete an end-of-study questionnaire, which contained multiple choice and free text responses aimed at understanding their experience of taking part in the THESEUS study, and their recommendations around future HS-based research.
Follow-up adaptations as a result of the COVID-19 pandemic
The COVID-19 pandemic limited the capacity for THESEUS sites to follow up participants in person and so a study amendment was submitted and approved to permit remote follow-up. Remote assessment was carried out by video call or telephone call. In the case of telephone calls, participants could send photographs of skin regions affected by HS by secure e-mail. In the absence of photographs, participants were permitted to provide their own count of active HS skin lesions, supported by guidance from their investigator during the telephone call. The method of lesion count assessment was recorded in each case.
Adverse events
THESEUS was a low-risk observational study, and the adverse event reporting procedure was developed to reflect this. As such, adverse events that were not deemed to be related to any of the study interventions were not reported. Local investigators were encouraged to follow their usual processes for reporting adverse events (e.g. yellow card reporting) when required.
Adverse events that were, or could be, related to the study procedures or treatments were recorded in the THESEUS adverse event reporting form or in the Intervention case report forms at the routine review appointments. The adverse event was described in a free text box in the case report forms.
Study withdrawal
Participants could withdraw from any aspect of the study, at any time, without giving an explanation. If a participant wished to withdraw from the study a withdrawal form was available on the THESEUS online database. The participant could also withdraw from text messages directly by texting ‘STOP’. Using the online form, the participant could withdraw from the following elements of the study: THESEUS treatment(s), text message pain scores, study data collection (choice of complete or partial withdrawal), withdrawal from being contacted about the interview study and consensus workshop.
Statistical methods
Sample size
A sample size of 150 participants, permits estimation of the proportion of participants who are hypothetically willing and eligible to be randomised in a clinical study to within a 95% confidence interval (CI) of ±7%. We also wished to identify the case mix of patients for each of the possible treatment options. From our patient survey, the least favoured treatment option (13%) was minor surgical procedures. A total of 150 patients would provide us with 20 patients opting for each of the non-medical interventions, which is sufficient to explore delivery in an IDEAL 2b evaluation. The IDEAL 2b framework for the evaluation of surgical interventions outlines a process of innovation, development, exploration, assessment and long-term study. 30 Stage 2b refers to the exploration stage in evaluating new surgical techniques.
Statistical methods/analysis plan
The analysis and reporting of this study is in accordance with the Consolidated Standards of Reporting Trials (CONSORT) extension for randomised pilot and feasibility trials guidelines and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The THESEUS study was not powered to test hypotheses. Most analyses are descriptive in nature. Continuous data are reported as means and standard deviations (SDs), or medians and interquartile ranges (IQR), as appropriate, and categorical data reported as frequencies and proportions. The analyses and presentation of this study are based on the participants’ final treatment selection. All statistical analysis was carried out using Stata version 16.1 (Timberlake Consultants Ltd, Richmond upon Thames, UK).
Participation in the study (screened, eligible, recruited, withdrawals) and completeness of follow-up at each time point (3, 6, 9 and 12 months) was illustrated by a CONSORT flow diagram and table. Reasons for not being eligible and for not being recruited are described. We characterised participants recruited to the study by their demographics, clinical history and severity of HS.
The willingness of participants (participant preference) to receive each of the five treatment options was described along with the number of treatments participants were willing to receive. For individuals not willing to receive a particular intervention, reasons were reported. We characterised willingness to use treatment options, using selective baseline demographics and clinical examination data. We also examined the clinicians’ assessment of their eligibility and described the number and characteristics of individuals eligible/not eligible for each treatment option to help inform future RCTs. For the primary outcome, the willingness and eligibility data were combined for each treatment option. We also described the final treatment decision for each participant and reasons for selection. We characterised the group membership of the final intervention choice to determine the drivers of treatment choice. For participants choosing non-medical interventions, we also described where participants chose another treatment during the waiting period. Where a participant switched initial intervention within 6 months, we reported the reasons for this and explored the characteristics of switching (including intervention type, site and other baseline demographics). Treatment fidelity (concordance) was measured by self-reported adherence at each review time point. During the study period, we reported whether participants continued with and adhered to their chosen intervention or whether they switched to alternative HS interventions during the study period. Reasons for discontinuing or switching intervention were reported.
Efficacy outcome measures covering the six core domains recommended by the HiSTORIC core outcome set initiative for HS were examined:
-
HS quality of life questionnaire (HiSQOL)26 score (17 items);
-
PtGA28 – ‘In the past 7 days how much has your HS influenced your quality of life? (select one option from 0 to 10 where 10 is maximum influence)’;
-
progression of course:
-
number of flares in the last month;
-
change in disease severity – ‘Overall, has there been any change in your HS disease severity since you were last seen for the THESEUS study? Please select one option where “0” represents no change in disease severity, “–7” represents a very great deal worse, and “7” represents a very great deal better’;
-
refined Hurley stage. 5
-
-
physical signs:
-
symptoms:
-
drainage and need for dressings;
-
Fatigue Severity Scale (FSS). 28
-
-
pain NRS;
plus generic measures of quality of life:
-
DLQI score;29
-
EQ5D-5L score (5 items);
-
EQ5D health today (score 0–100).
Outcomes were described at each time point (baseline, 3, 6, 9 and 12 months). Owing to the skewed nature of the data and small numbers in each treatment group, the median and IQR was reported for each outcome. Effect over time, from baseline to 6 months, was estimated for each efficacy outcome for each treatment group again using median (IQR) change. As THESEUS is a feasibility study and not powered to detect differences between arms, a decision was made by the SMG to not perform any mixed-effect modelling to examine the effect of outcomes over time by treatment group.
The pattern of missingness of daily pain scores over the 12-week period was examined to determine whether concordance reduced over time or on specific days (proportion of valid texts received of an expected 84). We modelled the predictors of adherence using the demographic, clinical data and baseline scores using time to event analysis. We described the mean NRS score over the 12-week period and computed the standard errors of the mean scores over time to use them for the calculation of 95% CI around the mean score to produce a graphical display of the estimates over time. A generalisability theory analysis33 was performed to examine if efficient and consistent results of the NRS score are produced if different time windows such as weekly, fortnightly or monthly for the self-reported measures of NRS scale were to be used in a future study. This analysis was performed using linear mixed-effects regression model for the NRS scores within the framework of generalised linear mixed-effect modelling techniques to account for an appropriate structure of the within person correlations over time. Initially, the model included the random effects of patients, time, treatment selection, ‘reports/no reports of NRS scale’ and centres as well as their interaction terms as independent variables. In addition, the model allowed us to adjust for the fixed effects of other baseline potential confounders (such as age, gender, ethnicity, HiSQOL) if required.
Chapter 3 Prospective observational cohort results
Recruitment and follow-up rates
The first participant was recruited on 18 February 2020 and the last on 28 July 2021. Recruitment was affected by the COVID-19 pandemic and there were two substantial pauses which mirrored the two main waves of the pandemic in the UK, in the spring and summer of 2020 and the beginning of 2021 (Figure 1). The flow of participants through the trial is represented in Figure 2.
FIGURE 1.
THESEUS cumulative recruitment.

FIGURE 2.
Study flow diagram. Oral clind. and rif. = oral clindamycin and rifampicin; conventional surg. = conventional surgery.

A total of 291 patients with HS were assessed for eligibility, with 151 (51.9%) recruited into the study over a period of 18 months; 81 (27.8%) patients were ineligible, 59 (20.3%) were eligible but not recruited. Reasons for patients’ ineligibility and for not taking part when eligible are reported in Table 2. Of the 151 recruited, one participant’s data were removed due to a lack of consent (and did not complete baseline). One individual completed baseline data collection twice; in the first instance they withdrew soon after choosing their intervention but were re-recruited and their latest data retained for analysis. The number of participants included in this study was 149, 51.2% of those assessed for eligibility. Follow-up rates (participants with at least one field recorded at review) at 3, 6, 9 and 12 months were 89% (n = 132), 83% (n = 123), 70% (n = 104) and 42% (n = 63), respectively, of those recruited. The 12-month follow-up rate was affected by recruitment delays that prevented complete follow-up of some participants due to closure of the study to adhere to study timelines; this accounted for 26 of the 35 participants who were lost to follow-up. Seventeen withdrawals were observed, two from the doxycycline arm, three from the clindamycin and rifampicin arm, eight from laser treatment, one from deroofing and three from conventional surgery.
| Patients n (%) | |
|---|---|
| Patients ineligible: | 81 (27.8)a |
| 0 of the 5 study interventions appropriate | 27 (33.3) |
| HS adequately controlled by current treatment | 18 (22.2) |
| Diagnosis did not meet disease definition | 13 (16.0) |
| Unable or unwilling to give informed consent | 10 (12.3) |
| Pregnant or breastfeeding | 8 (9.9) |
| Not fluent in English | 3 (3.7) |
| Age <18 years with active HS of any severity | 2 (2.5) |
| Patients eligible but not recruited: | 59 (20.3)a |
| Declined to take part | 46 (78.0) |
| Unable to arrange appointment/uncontactable | 13 (22.0) |
| Eligible participants recruited | 151 (51.9)a |
| Participant data removed due to lack of consent | 1 (0.3)a |
| Patients who were eligible and recruited: participated | 150 (51.5)a |
| Individual re-recruited after initial withdrawal (excluded initial record) | 1 |
| Participants included in the analysis at baseline | 149 (51.2)a |
| Participants followed up at:b | |
| 3 months | 132 (88.6) |
| 6 months | 123 (82.6) |
| 9 months | 104 (69.8) |
| 12 months | 65 (43.6) |
| Did not complete due to shortened follow-up period | 23 (15.4) |
Study sites
Ten sites across the UK recruited 149 participants, including one site in Scotland and one in Wales (Table 3). Two sites, Salford and Sussex, were initiated towards the end of the recruitment period. A total of 64 of the participants were recruited from six dermatology-led sites, 50 were recruited from two surgery-led sites and 35 from two sites that already had an integrated medical and surgical HS MDT approach. Six of the sites offered laser treatment.
| Sites | Participants recruited (n) |
|---|---|
| Dermatology-led: | 64 |
| NHS Forth Valley | 15 |
| Oxford University Hospitala | 16 |
| Newcastle Upon Tyne Hospitals NHS Foundation Trusta | 14 |
| Barnsley Hospital NHS Foundation Trust | 5 |
| Salford NHS Foundation Trust | 4 |
| University Hospital Sussex | 10 |
| Surgery-led: | 50 |
| Buckinghamshire Healthcare NHS Trusta | 37 |
| Mid Essex Hospital Servicesa | 13 |
| Pre-established multidisciplinary service: | 35 |
| Guy’s and St Thomas NHS Foundation Trusta | 10 |
| Cardiff and Vale University Health Boarda | 25 |
Baseline characteristics
The baseline characteristics of the participants recruited to the study by their demographics, clinical history, medications and severity of HS are reported in Table 4. Participants were on average 36 years (range: 18.2–67.1 years), 81% were female, and 86% had a raised BMI. Just over 20% had non-white ethnicity and Fitzpatrick skin photo type from IV to VI. Two-thirds of the participants were either current (43%) or ex-smokers (22%), and smokers on average smoked 10 cigarettes per day. There was balanced representation across the quintiles of deprivation apart from a lower proportion in the least deprived quintile.
| Demographics | Descriptive statistics |
|---|---|
| Age (years) Mean (SD) | 36.1 (10.5) |
| Sex: Female n (%) | 121 (81.2) |
| Ethnic group or background n (%) | |
| White | 118 (79.7) |
| Mixed/Multiple ethnic groups | 8 (5.4) |
| Asian/Asian British | 9 (6.1) |
| Black/African/Caribbean/Black British | 11 (7.4) |
| Other ethnic background | 2 (1.4) |
| Fitzpatrick scale n (%) | |
| I – very fair; always burns, cannot tan | 17 (11.5) |
| II – fair; usually burns, sometimes tans | 50 (33.8) |
| III – medium; sometimes burns, usually tans | 46 (31.1) |
| IV – olive; rarely burns, always tans | 13 (8.8) |
| V – brown; rarely burns, tans easily | 16 (10.8) |
| VI – dark brown; never burns, always tans | 6 (4.1) |
| BMI (kg/m2) | N = 143 |
| BMI mean (SD) | 33.0 (7.9) |
| Healthy weight (BMI ≥ 18.5 to 24.9 kg/m2), n (%) | 20 (14.0) |
| Overweight (BMI ≥ 25.0 to 29.9 kg/m2), n (%) | 40 (28.0) |
| Obese (BMI ≥ 30.0 to 39.9 kg/m2), n (%) | 54 (37.8) |
| Severely obese (BMI ≥ 40 kg/m2), n (%) | 29 (20.3) |
| Index of Multiple Deprivation (IMD) quintiles n (%): | |
| 5----- least deprived | 15 (10.1) |
| 4---- | 29 (19.5) |
| 3--- | 31 (20.8) |
| 2-- | 37 (24.8) |
| 1- most deprived | 37 (24.8) |
| Type of study site n (%): | |
| Dermatology-led (6 sites) | 64 (43.0) |
| Surgery-led (2 sites) | 50 (33.5) |
| Pre-established multidisciplinary service (2 sites) | 35 (23.5) |
| Smoking n (%): | |
| Non-smoker | 53 (35.8) |
| Ex-smoker | 32 (21.6) |
| Current smoker | 63 (42.6) |
| For smokers, number cigarettes smoked per day, median (IQR) | 10.0 (5.0 to 11.0) |
| Nicotine replacement therapy n (%) | 21 (14.3) |
In the previous 12 months, around two-thirds of participants had previously been treated by a GP or dermatologist (70.1% and 64.6%, respectively) with just under one-third being treated by a surgeon (30.6%), while 20% reported seeing a doctor in the emergency department. The groin and axilla were the most common skin regions affected. On average, participants had four inflammatory nodules, one abscess and one draining or inflamed skin tunnel at baseline. Hurley stage at baseline was I (mild) in 13%, II (moderate) in 68% and III (severe) in 19% of the participants. Components of the refined Hurley stage are listed in Table 5, the greatest proportion of participants being stage IIC (30%), with a range from IA to III.
| Baseline variables | Descriptive statisticsa | |
|---|---|---|
| Clinical history | ||
| Participants’ HS recently treated by n (%): | ||
| GP | 103 | (70.1) |
| Dermatologist | 95 | (64.6) |
| Surgeon | 45 | (30.6) |
| Doctor in emergency department | 29 | (19.7) |
| Nurse (community/primary care) | 29 | (19.7) |
| Anybody else (others) | 12 | (8.1) |
| Severity of HS | ||
| Skin region affected: n (%) | ||
| Axilla | 102 | (68.5) |
| Groin | 114 | (76.5) |
| Perineum | 47 | (31.8) |
| Buttocks | 58 | (38.9) |
| Chest | 46 | (30.9) |
| Other | 45 | (30.4) |
| Total number of inflammatory nodules, median (IQR) | 4 | (1.0–8.5) |
| Total number of abscesses, median (IQR) | 1 | (0–3) |
| Total number of draining or inflamed skin tunnels, median (IQR) | 1 | (0–2) |
| IHS4,a median (IQR) | 11 | (4–21) |
| Number of HS flares in the last month, median (IQR) | 4 | (2–10) |
| Drainage of pus, blood, other fluid due to HS,b median (IQR) | 3.5 | (0–6) |
| Magnitude of skin odour,b median (IQR) | 3.5 | (0–7) |
| Hurley stage (most severely affected region) n (%) | ||
| H-I: mild; individual, non-scarring lesions | 19 | (12.8) |
| H-II: moderate; multiple scarring lesions separated by normal skin | 102 | (68.5) |
| H-III: severe; lesions coalescing into inflammatory plaques | 28 | (18.8) |
| Skin lesions fixed in location or migratory, n (%): | ||
| Fixed | 94 | (63.5) |
| Migratory | 54 | (36.5) |
| Draining skin tunnels due to HS present in any skin region n (%) | 86 | (58.1) |
| Three or more body regions with draining skin tunnels n (%) | 27 | (18.1) |
| Skin regions across body with at least 1% interconnected draining tunnels, n (%) | 15 | (10.1) |
| Refined Hurley stage for HS severity, n (%): | ||
| Hurley IA | 13 | (8.7) |
| Hurley IB | 32 | (21.5) |
| Hurley IC | 18 | (12.1) |
| Hurley IIA | 12 | (8.1) |
| Hurley IIB | 14 | (9.4) |
| Hurley IIC | 45 | (30.2) |
| Hurley III | 15 | (10.1) |
| How lesion count was assessed for the purposes of this review, n (%)c | ||
| By a health professional in person | 47 | (69.1) |
| By the patient self-reported | 21 | (30.9) |
Patients were not required to discontinue current HS therapy before entering the study.
Table 6 reports recent HS interventions received prior to study entry. In the case of non-biological medical therapies, including topical and oral therapy, results relate to the 1-month period prior to THESEUS recruitment. For biological therapy, details are provided for the preceding 3-month period and for surgical and laser therapy information was collected for the previous 12-month period.
| Baseline variables | Descriptive statisticsa | |
|---|---|---|
| Topical and oral therapy in previous month: | ||
| Chlorhexidine solution | 48 | 32.2 |
| Antiseptic | 37 | 25.0 |
| Clindamycin 1% | 29 | 19.5 |
| Other antibiotic | 11 | 7.4 |
| Corticosteroid | 9 | 6.1 |
| Non-biological treatment in previous month: | ||
| Tetracycline | 39 | 26.4 |
| Clindamycin | 19 | 12.8 |
| Rifampicin | 16 | 10.8 |
| Other oral antibiotic | 47 | 32.0 |
| Dapsone | 1 | 0.7 |
| Isotretinoin | 3 | 2.0 |
| Metformin | 13 | 8.7 |
| Spironolactone | 4 | 2.7 |
| Prednisolone | 2 | 1.3 |
| Zinc | 7 | 4.7 |
| Non-steroidal anti-inflammatory drug | 50 | 34.0 |
| Paracetamol | 94 | 63.5 |
| Codeine | 35 | 23.6 |
| Morphine | 5 | 3.4 |
| Oral contraceptive | 17 | 11.6 |
| Finasteride | 1 | 0.7 |
| Botulinum toxin injections | 1 | 0.7 |
| Steroid injection into acute HS lesion(s) | 1 | 0.7 |
| Phototherapy | 1 | 0.7 |
| IV antibiotic | 5 | 3.4 |
| Other HS treatment | 8 | 5.4 |
| Biological medication use in previous 3 months: | ||
| Adalimumab | 9 | 6.0 |
| Etanercept | 1 | 0.7 |
| Infliximab | 1 | 0.7 |
| Anakinra | 1 | 0.7 |
| Other biologic | 1 | 0.7 |
| Incision and drainage under local anaesthetic | 23 | 15.6 |
| Lesion removed surgically and wound closed with stitches | 11 | 7.5 |
| Skin tunnel laid open and allowed to heal naturally | 15 | 10.2 |
| Wider area of skin removed | 4 | 2.7 |
| Method of wound healing: | ||
| Skin graft | 1 | 0.7 |
| Secondary intention | 3 | 2.1 |
| Laser treatmentb | 4 | 2.8 |
| Other surgical treatmentb | 5 | 3.4 |
Participants’ willingness to use the different THESEUS treatment options
Participants were asked to report their willingness to receive the five available treatment options, while clinicians assessed their eligibility for each of the treatment options (Table 7). Participant willingness to receive treatment was highest for laser (79.2%), deroofing of skin tunnels (66.4%) and conventional surgery (64.2%), and lower for the oral antibiotic treatments. The most common reason for unwillingness to receive oral doxycycline and oral clindamycin and rifampicin was ‘had the treatment before and was not effective’ (see Table 7). Some participants were unwilling to receive laser or deroofing due to anticipation of insufficient benefit. Some 41% of patients ranked laser treatment as the most preferred followed by 21% for deroofing treatment.
| Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Willingness | ||||||||||
| Participant willing to receive treatment | 63 | (42.3) | 76 | (51.0) | 118 | (79.2) | 99 | (66.4) | 95 | (63.8) |
| Reasons for unwillingness: | ||||||||||
| Will not provide enough benefit | 14 | (9.4) | 12 | (8.1) | 18 | (12.1) | 23 | (15.4) | 19 | (12.8) |
| Potential side effects/complications | 11 | (7.4) | 12 | (8.1) | 1 | (0.7) | 5 | (3.4) | 13 | (8.8) |
| Had this before – not effective | 40 | (26.8) | 29 | (19.5) | 1 | (0.7) | 4 | (2.7) | 3 | (2.0) |
| Had this before – experienced side effects | 15 | (10.1) | 14 | (9.4) | 1 | (0.7) | 0 | (0.0) | 0 | (0.0) |
| Information from other sources | 0 | (0.0) | 0 | (0.0) | 1 | (0.7) | 1 | (0.7) | 2 | (1.3) |
| Other reason | 6 | (4.0) | 6 | (4.0) | 9 | (6.0) | 17 | (11.4) | 16 | (10.7) |
| Patient ranked 1 (most preferred) | 17 | (14.3) | 19 | (15.8) | 52 | (40.6) | 26 | (20.8) | 15 | (12.0) |
| Clinician assessed eligibility | ||||||||||
| Clinically appropriate | 88 | (59.5) | 96 | (64.4) | 89 | (59.7) | 100 | (67.1) | 94 | (63.1) |
| Eligible but treatment not available at the site | na | na | 22 | (14.8) | na | na | ||||
Clinician-assessed eligibility for the different THESEUS treatment options
Overall, clinician-assessed eligibility was highest for laser treatment, with a total of 74.5% of participants being suitable, factoring in 14.8% in whom laser was clinically appropriate but not available at the study centre (see Table 7). The second highest proportion was for deroofing, with 67% of participants deemed eligible for the treatment.
Primary outcome: participants who are eligible, and hypothetically willing, to use the different THESEUS treatment options
Table 8 provides details of the THESEUS primary outcome, the proportion of participants who were both clinically eligible and willing to receive the intervention. The highest proportion was for laser treatment (68.5%) followed by deroofing (57.7%) and then conventional surgery (53.7%).
| Primary outcome: patients willing and eligible for study interventiona | Patients | |
|---|---|---|
| n | % | |
| Doxycycline | 55 | 36.9 |
| Clindamycin and rifampicin | 65 | 43.6 |
| Laser | 102 | 68.5 |
| Deroofing | 86 | 57.7 |
| Conventional surgery | 80 | 53.7 |
Secondary outcome: participants’ final intervention choice
The participants’ final intervention choice is provided in Table 9. Laser was the most frequently chosen intervention, followed by deroofing, the oral antibiotic options and then conventional surgery. The most frequent reason for the intervention choice (59%) was ‘My doctor recommended it’, followed by ‘I wanted to try something new’ (19% of participants; Table 10). For both antibiotic options and also for deroofing, the main reason underpinning the participant’s choice was that their doctor had recommended it, while for laser, 27% wanted to try something new (see Table 10). Some 33% chose conventional surgery because they had received it before, while 17% based their choice on the information read on the THESEUS website. Participants’ first ranked treatment preference was often the same as their final treatment choice, ranging from 70% for doxycycline to 92% for conventional surgery (see Table 8).
| Final intervention choice,a n (%) | |||||
|---|---|---|---|---|---|
| Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery | |
| Patients, n (%) | 23 (15.4) | 23 (15.4) | 56 (37.6) | 35 (23.5) | 12 (8.1) |
| Patients’ ranking of treatment | |||||
| 1 = most preferred | 16 (70%) | 19 (83%) | 51 (91%) | 25 (71%) | 11 (92%) |
| 2 | 1 (2%) | 1 (3%) | |||
| 3 | 1 (4%) | 1 (3%) | |||
| 4 | 1 (4%) | 3 (13%) | |||
| 5 = least preferred | |||||
| Missing | 5 (22%) | 1 (4%) | 4 (7%) | 8 (23%) | 1 (8%) |
| Reason for deciding on the final treatment | Final choice of treatment, n (%) | ||||
|---|---|---|---|---|---|
| Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery | |
| n = 23 | n = 22 | n = 55 | n = 35 | n = 12 | |
| My doctor recommended it | 15 (65.2) | 15 (68.2) | 27 (49.1) | 27 (77.1) | 3 (25.0) |
| I wanted to try something new | 5 (21.7) | 5 (22.7) | 15 (27.3) | 2 (5.7) | 1 (8.3) |
| I’ve used it before | 1 (4.4) | 1 (4.6) | 0 | 0 | 4 (33.3) |
| Based on | |||||
| Information read in THESEUS information sheet | 2 (8.7) | 0 | 5 (9.1) | 2 (5.7) | 0 |
| Information read on website(s) | 0 | 0 | 1 (1.8) | 1 (2.9) | 2 (16.7) |
| Information read in THESEUS decision grid | 0 | 1 (4.6) | 1 (1.8) | 0 | 0 |
| My preferred option was not available | 0 | 0 | 1 (1.8) | 1 (2.9) | 0 |
| Other reason | 0 | 0 | 5 (9.1) | 2 (5.7) | 2 (16.7) |
Characterisation of ineligibility to receive the intervention options
Table 11 describes the number and characteristics of individuals not eligible for each treatment option to understand clinicians’ treatment choices and why individuals were not suitable, to inform a future trial’s eligibility criteria and target group. Individuals with migratory skin lesions were more likely to be deemed as ineligible for both deroofing and conventional surgery compared with those with fixed skin lesions as were those without skin tunnels. Age was a factor for non-eligibility for laser treatment, with older participants more likely to be unsuitable (average age = 41.2 years) compared with eligible patients aged 33.3 years on average (see Table 11). Older patients were slightly more likely to be eligible for oral antibiotics (doxycycline and clindamycin and rifampicin). Female patients were more likely to be ineligible for oral doxycycline and clindamycin and rifampicin (19 and 17 percentage points higher than male patients, respectively) and laser treatment (10 percentage points higher than male patients). Sex does not appear to drive differences in deroofing eligibility. Participants with obesity/severe obesity were more likely to be ineligible for deroofing (43%/41%, respectively). Participants of other ethnic background were more likely to be eligible for oral antibiotics or laser intervention.
| Baseline characteristics | Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery | |
|---|---|---|---|---|---|---|
| Ineligible to receive intervention | 60 (40.5) | 52 (35.1) | 38 (25.5) | 49 (32.9) | 55 (36.9) | |
| Eligible to receive intervention | 88 (59.5) | 96 (64.9) | 89 (59.7) | 100 (67.1) | 94 (63.1) | |
| Participant eligible but intervention not available | na | na | 22 (14.8) | na | na | |
| Age (years) Mean (SD) | N = 148 | |||||
| Ineligible | 33.4 (8.4) | 34.0 (10.0) | 41.2 (12.5) | 36.8 (11.9) | 35.4 (10.2) | |
| Eligible | 38.0 (11.5) | 37.3 (10.7) | 33.3 (8.7) | 35.8 (9.8) | 36.5 (10.7) | |
| Participant eligible but intervention not available | na | na | 38.6 (10.0) | na | na | |
| Ineligible for intervention, n (%) | ||||||
| Sex: | ||||||
| Male | 28 | 7 (25.0) | 6 (21.4) | 5 (17.9) | 9 (32.1) | 9 (32.1) |
| Female | 120 | 53 (44.2) | 46 (38.3) | 33 (27.3) | 40 (33.1) | 46 (38.0) |
| BMI: | ||||||
| Healthy weight | 20 | 11 (55.0) | 7 (35.0) | 9 (45.0) | 7 (35.0) | 7 (35.0) |
| Overweight | 40 | 14 (35.0) | 14 (35.0) | 8 (20.0) | 10 (25.0) | 12 (30.0) |
| Obese | 54 | 23 (42.6) | 19 (35.2) | 10 (18.5) | 19 (35.2) | 23 (42.6) |
| Severely obese | 29 | 10/28 (35.7) | 9/28 (32.1) | 11 (37.9) | 13 (44.8) | 12 (41.4) |
| Smoking status: | ||||||
| Non-smoker | 53 | 22 (41.5) | 17 (32.1) | 14 (26.4) | 19 (35.8) | 19 (35.8) |
| Current smoker | 62 | 24 (38.7) | 21 (33.9) | 16 (25.4) | 18 (28.6) | 21 (33.3) |
| Ex-smoker | 32 | 14 (43.8) | 14 (43.8) | 8 (25.0) | 12 (37.5) | 14 (43.8) |
| Ethnicity: | ||||||
| White | 118 | 49/117 (41.9) | 46/117 (39.3) | 34 (28.8) | 38 (32.2) | 43 (36.4) |
| Other ethnic background | 30 | 11 (30.4) | 6 (20.0) | 4 (13.3) | 11 (36.7) | 12 (40.0) |
| IMD quintiles | ||||||
| 5----- least deprived | 15 | 4 (46.7) | 8 (53.3) | 4 (26.7) | 3 (20.0) | 5 (33.3) |
| 4---- | 29 | 13 (44.8) | 10 (34.5) | 6 (20.7) | 4 (13.8) | 13 (44.8) |
| 3--- | 31 | 9 (29.0) | 9 (29.0) | 10 (32.3) | 12 (38.7) | 10 (32.3) |
| 2-- | 36 | 13 (36.1) | 13 (36.1) | 8 (21.6) | 12 (32.4) | 12 (32.4) |
| 1- most deprived | 37 | 18 (48.6) | 12 (32.4) | 10 (27.0) | 18 (48.6) | 15 (40.5) |
| Skin lesions: | ||||||
| Fixed | 93 | 40 (43.0) | 34 (36.6) | 27 (28.7) | 26 (27.7) | 30 (31.9) |
| Migratory | 54 | 20 (37.0) | 18 (33.3) | 11 (20.4) | 23 (42.6) | 24 (44.4) |
| Skin tunnels present: | ||||||
| No | 62 | 24 (38.7) | 26 (41.9) | 13 (21.0) | 27 (43.5) | 27 (43.5) |
| Yes, n (%) | 85 | 35 (41.2) | 26 (30.6) | 25 (29.1) | 21 (24.4) | 27 (31.4) |
| Hurley stage for HS, n (%): | ||||||
| H-I: mild | 19 | 4 (21.1) | 8 (42.1) | 5 (26.3) | 8 (42.1) | 8 (42.1) |
| H-II: moderate | 102 | 47 (46.1) | 34 (33.3) | 29 (28.4) | 34 (33.3) | 39 (38.2) |
| H-III: severe | 27 | 9 (33.3) | 10 (37.0) | 4 (14.3) | 7 (25.0) | 8 (28.6) |
Characterisation of participants’ willingness to receive interventions
Table 12 characterises participant willingness to receive each intervention in terms of demographic and disease factors. Those willing to receive antibiotics were older than those who were unwilling, while younger people were more likely to be willing to undergo laser treatment. As expected, participants with skin tunnels and fixed skin lesions were more willing to receive deroofing, while participants with migratory lesions were more willing to receive laser treatment. Severe HS predicted willingness to receive laser, deroofing or conventional surgery.
| Baseline characteristics | Total | Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery |
|---|---|---|---|---|---|---|
| Patients willing to receive treatment, n (%) | 149 | 63 (42.3) | 76 (51.0) | 118 (79.2) | 99 (66.4) | 95 (64.2) |
| Age (years), mean (SD): | ||||||
| Willing | 39.5 (10.8) | 38.1 (10.7) | 35.0 (10.2) | 36.0 (10.5) | 36.4 (10.6) | |
| Not willing | 33.6 (9.6) | 34.0 (9.9) | 40.2 (10.6) | 36.3 (10.7) | 35.6 (10.6) | |
| Sex: | ||||||
| Male | 28 | 17 (60.7) | 20 (71.4) | 22 (78.6) | 18 (64.3) | 19 (67.9) |
| Female | 121 | 46 (38.0) | 56 (46.3) | 96 (79.3) | 81 (66.9) | 76 (62.8) |
| BMI (kg/m2): | ||||||
| Healthy weight | 20 | 4 (20.0) | 11(55.0) | 17 (85.0) | 14 (70.0) | 14 (70.0) |
| Overweight | 40 | 20 (50.0) | 21 (52.5) | 32 (80.0) | 25 (62.5) | 23 (57.5) |
| Obese | 54 | 24 (44.4) | 29 (53.7) | 44 (81.5) | 35 (64.8) | 36 (66.7) |
| Severely obese | 29 | 12 (41.4) | 13 (44.8) | 19 (65.5) | 19 (65.5) | 17 (58.6) |
| Smoking status: | ||||||
| Non-smoker | 53 | 18 (34.0) | 28 (52.8) | 42 (79.3) | 32 (60.4) | 35 (66.0) |
| Current smoker | 63 | 32 (50.8) | 31 (49.2) | 50 (79.4) | 50 (79.4) | 42 (66.7) |
| Ex-smoker | 32 | 13 (40.6) | 17 (53.1) | 25 (78.1) | 17 (53.1) | 18 (56.3) |
| Ethnicity: | ||||||
| White | 118 | 53 (44.9) | 60 (50.9) | 92 (78.0) | 82 (69.5) | 76 (64.4) |
| Other ethnic backgrounda | 30 | 10 (33.3) | 15 (50.0) | 25 (83.3) | 16 (53.3) | 18 (60.0) |
| IMD quintiles | ||||||
| 5----- least deprived | 15 | 5 (33.3) | 4 (26.7) | 14 (93.3) | 11 (73.3) | 12 (80) |
| 4---- | 29 | 10 (34.5) | 12 (41.4) | 24 (82.8) | 23 (79.3) | 20 (69.0) |
| 3--- | 31 | 18 (58.1) | 21 (67.7) | 26 (83.9) | 19 (61.3) | 19 (61.3) |
| 2-- | 37 | 11 (29.7) | 18 (48.7) | 26 (70.3) | 24 (64.9) | 22 (59.5) |
| 1- most deprived | 37 | 19 (51.4) | 21 (56.8) | 28 (75.7) | 22 (59.5) | 22 (59.5) |
| Skin lesions: | ||||||
| Fixed in one region | 94 | 37 (39.4) | 46 (48.9) | 70 (74.5) | 67 (71.3) | 63 (67.0) |
| Migratory | 54 | 26 (48.2) | 30 (55.6) | 47 (87.0) | 32 (59.3) | 32 (59.3) |
| Skin tunnels present: | ||||||
| No | 62 | 30 (48.4) | 36 (58.1) | 53 (85.5) | 34 (54.8) | 35 (56.5) |
| Yes | 86 | 33 (38.4) | 39 (45.4) | 64 (74.4) | 65 (75.6) | 59 (68.6) |
| Hurley stage for HS: | ||||||
| H-I: mild | 19 | 10 (52.6) | 10 (52.6) | 15 (79.0) | 11 (57.9) | 12 (63.2) |
| H-II: moderate | 102 | 41 (40.2) | 53 (52.0) | 78 (76.5) | 68 (66.7) | 62 (60.8) |
| H-III: severe | 28 | 12 (42.9) | 13 (46.4) | 25 (89.3) | 20 (71.4) | 21 (75.0) |
Characterisation of participants by final treatment choice
Regarding participants’ final treatment choice, a higher proportion of female patients chose laser and deroofing (Table 13). Laser was favoured by a younger group of patients while doxycycline was selected by older patients. Current smokers favoured doxycycline and deroofing over other treatments while a higher proportion from ethnic minority backgrounds favoured laser treatment.
| Baseline characteristics | Final treatment choice | ||||
|---|---|---|---|---|---|
| Doxycycline | Clindamycin and rifampicin | Laser | Deroofing | Conventional surgery | |
| n (%) | n = 23 | n = 23 | n = 56 | n = 35 | n = 12 |
| Sex | |||||
| Male | 8 (34.8) | 5 (21.7) | 7 (12.5) | 5 (14.3) | 3 (25.0) |
| Female | 15 (65.2) | 18 (78.3) | 49 (87.5) | 30 (85.7) | 9 (75.0) |
| Age (years), mean (SD) | 40.2 (13.2) | 38.1 (10.4) | 33.1 (8.9) | 37.0 (10.7) | 36.2(8.8) |
| BMI groups | |||||
| Healthy weight | 2 (8.7) | 5 (21.7) | 6 (11.1) | 7 (21.9) | 0 (0.0) |
| Overweight | 4 (17.4) | 4 (17.4) | 19 (35.2) | 9 (28.1) | 4 (36.4) |
| Obese | 11 (47.8) | 9 (39.1) | 18 (33.3) | 10 (31.3) | 6 (54.5) |
| Severely obese | 6 (26.1) | 5 (21.7) | 11 (20.4) | 6 (18.8) | 1 (9.1) |
| Smoking | |||||
| Non-smoker | 5 (21.7) | 9 (39.1) | 26 (47.3) | 10 (28.6) | 3 (25.0) |
| Current smoker | 14 (60.9) | 9 (39.1) | 15 (27.3) | 20 (57.1) | 5 (41.7) |
| Ex-smoker | 4 (17.4) | 5 (21.7) | 14 (25.5) | 5 (14.3) | 4 (33.3) |
| Ethnicity | |||||
| White | 21 (91.3) | 18 (78.3) | 40 (72.7) | 29 (82.9) | 10 (83.3) |
| Other ethnic backgrounda | 2 (8.7) | 5 (21.7) | 16 (27.3) | 6 (17.1) | 2 (16.7) |
| IMD quintiles | |||||
| 5----- Least deprived | 3 (13.0) | 1 (4.4) | 6 (10.7) | 4 (11.4) | 1 (8.3) |
| 4---- | 4 (17.4) | 5 (21.7) | 10 (17.9) | 9 (25.7) | 1 (8.3) |
| 3--- | 7 (30.4) | 4 (17.4) | 14 (25.0) | 4 (11.4) | 2 (16.7) |
| 2-- | 5 (21.7) | 6 (26.1) | 10 (17.9) | 11 (31.4) | 5 (41.7) |
| 1- Most deprived | 4 (17.4) | 7 (30.4) | 16 (28.6) | 7 (20.0) | 3 (25.0) |
| Skin lesions | |||||
| Fixed in one region | 11 (47.8) | 14 (60.9) | 37 (67.3) | 26 (74.3) | 6 (50.0) |
| Migratory | 12 (52.2) | 9 (39.1) | 19 (32.7) | 9 (25.7) | 6 (50.0) |
| Skin tunnels present (% yes) | |||||
| No | |||||
| Yes | 12 (52.2) | 12 (52.2) | 24 (43.6) | 29 (82.9) | 9 (75.0) |
| Hurley stage for HS | |||||
| H-I: mild; individual | 5 (21.7) | 2 (8.7) | 7 (12.5) | 4 (11.4) | 1 (8.3) |
| H-II: moderate; multi | 14 (60.9) | 20 (87.0) | 38 (67.9) | 24 (68.6) | 6 (50.0) |
| H-III: severe; lesion | 4 (17.4) | 1 (4.4) | 11 (19.6) | 7 (20.0) | 5 (41.7) |
Treatment fidelity
We summarised the treatment fidelity for each treatment option in Tables 14–18. Regarding fidelity to doxycycline, at the 3-month review, 52% of participants were still receiving doxycycline, a proportion which was maintained at 6 months and dropped to 26% after 9 months (Table 14). At 9 months, 65% did not provide fidelity data which could indicate that use of doxycycline had ceased. However, the expectation would be for patients to continue with doxycycline if it was still effective and well tolerated. The most common participant reasons for treatment discontinuation were lack of effectiveness, opting to try an alternative intervention, and adverse effects.
| Review 1, 3-month follow-up, n (%) | Review 2, 6-month follow-up, n (%) | Review 3, 9-month follow-up, n (%) | Review 4, 12-month follow-up, n (%) | |
|---|---|---|---|---|
| Final treatment choice at baseline review = doxycycline | 23 | 23 | 23 | 23 |
| Still receiving doxycycline? | ||||
| Yes | 12 (52.2) | 13 (56.5) | 6 (26.1) | 4 (17.4) |
| If yes, self-reported adherence | ||||
| Very well | 8 | 10 | 3 | 3 |
| Somewhat well | 4 | 2 | 2 | 1 |
| Not at all | – | 1 | 1 | – |
| No | 8 (34.8) | 5 (21.7) | 2 (8.7) | 1 (4.3) |
| If no, reason why? | ||||
| Participant chose different treatmenta | 2 | 1 | 0 | 0 |
| Clinician chose different treatmenta | 4 | 1 | 1 | 0 |
| Treatment delay | 0 | 0 | 0 | 0 |
| Missing | 2 | 3 | 1 | 1 |
| Missing/No review (na) | 3 (13.0) | 5 (21.7) | 15 (65.2) | 18 (78.3) |
| Continuing with doxycycline? | ||||
| Yes | 12 (52.2) | 8 (34.8) | 6 (26.1) | 4 (17.4) |
| No | 8 (34.8) | 10 (43.5) | 2 (8.7) | 1 (4.3) |
| If no, reason why?b | ||||
| Opted to try alternative treatment | 4 | 4 | 0 | 0 |
| Did not find treatment effective | 5 | 5 | 0 | 0 |
| Adverse effects | 5 | 3 | 0 | 0 |
| Other reason | 2 | 5 | 1 | 1 |
| Missing/No review (na) | 3 (13.0) | 5 (21.7) | 15 (27.7) | 18 (78.3) |
Of the 23 participants that chose clindamycin and rifampicin, at 3 months post recruitment 30% were still receiving treatment, all of whom self-reported that they were adhering somewhat or very well to therapy (Table 15). In some cases, treatment discontinuation was due to the review being after the scheduled finish for the 10 weeks of therapy, while adverse effects and treatment ineffectiveness were also reasons for not continuing with the intervention.
| Review 1, 3-month follow-up, n (%) | Review 2, 6-month follow-up, n (%) | Review 3, 9-month follow-up, n (%) | Review 4, 12-month follow-up, n (%) | |
|---|---|---|---|---|
| Final treatment choice at baseline review = clindamycin and rifampicin | 23 | 23 | 23 | 23 |
| Still receiving clindamycin and rifampicin? | ||||
| Yes | 7 (30.4) | 1 (4.3) | 1 (4.3) | 0 |
| If yes, self-reported adherence: | ||||
| Very well | 5 | 1 | 1 | - |
| Somewhat well | 2 | 0 | 0 | - |
| Not at all | 0 | 0 | 0 | - |
| No | 12 (52.2) | 10 (43.5) | 9 (39.1) | 4 (17.4) |
| If no, reason why? | ||||
| Participant chose different treatmenta | 2 | 2 | 1 | 2 |
| Clinician chose different treatmenta | 4 | 5 | 4 | 0 |
| Treatment delay | 0 | 0 | 0 | 0 |
| Missing | 2 | 3 | 4 | 2 |
| Missing/No review (na) | 4 (17.4) | 12 (52.2) | 13 (56.5) | 19 (82.6) |
| Continuing with clindamycin and rifampicin? | ||||
| Yes | 7 (36.8) | 7 (58.3) | 3 (30.0) | 1 (25.0) |
| No | 12 (63.2) | 5 (41.7) | 7 (70.0) | 3 (75.0) |
| If no, reason why?b | ||||
| Opted to try alternative treatment | 4 | 1 | 3 | 5 |
| Did not find treatment effective | 5 | 0 | 2 | 1 |
| Adverse effects | 5 | 0 | 3 | 1 |
| Other reason | 2 | 5 | 5 | 5 |
| Missing/No review (na) | 4 | 11 | 13 | 19 |
Of the 56 participants who chose laser as their intervention, only 43% started treatment (received at least one treatment session) at the 3-month review (Table 16). This relates to delays receiving treatment due to waiting times that were exacerbated by the COVID-19 pandemic, with THESEUS being an observational study. The figure rose to 64% by 6 months, 77% at 9 months and 79% by 12 months. Treatment delay was reported by 23, 8, 4 and 3 participants at 3, 6, 9 and 12 months, respectively. Only three reports of switching the treatment were observed at follow-ups.
| Review 1, 3-month follow-up, n (%) | Review 2, 6-month follow-up, n (%) | Review 3, 9-months follow-up, n (%) | Review 4, 12-month follow-up, n (%) | |
|---|---|---|---|---|
| Final treatment choice at baseline review = laser | 56 | 56 | 56 | 56 |
| Did the participant receive first laser treatment? | ||||
| Yes | 21 (37.5) | 31 (55.4) | 36 (64.3) | 39 (69.6) |
| Partially | 3 (5.4) | 5 (8.9) | 7 (12.5) | 5 (8.9) |
| Missing/No review (na) | 8 (14.2) | 11 (19.6) | 9 (16.1) | 9 (10.7) |
| No | 24 (42.9) | 9 (16.1) | 4 (7.1) | 3 (5.4) |
| If no, reason why? | ||||
| Participant chose different treatmenta | ||||
| Clinician chose different treatmenta | ||||
| Treatment delay | 23 | 8 | 4 | 3 |
| Participant did not attend their procedure appointment | 1 | 1 | 0 | 0 |
| Continuing with laser treatment? | ||||
| Yes | 48 (85.7) | 43 (76.8) | 31 (55.4) | 14 (25.0) |
| No | 0 | 2 | 0 | 3 |
| If no, reason why?b | ||||
| Opted to try alternative treatment | 0 | 1 | 0 | 0 |
| Other reason | 0 | 1 | 0 | 1 |
| Time between recruitment and the first procedure (days), median (IQR) | N = 59 105 (53–172) |
|||
Of the 35 participants who chose deroofing as their final treatment, only 25% received their procedure after 3 months of follow-up (Table 17). Some 43% reported receipt of their first deroofing at 6 months of follow-up, 54% after 9 months and 63% after 12 months. The majority of participants who provided follow-up information preferred to continue deroofing. Deroofing delay was reported by 21, 14, 8 and 5 participants at 3, 6, 9 and 12 months, respectively. During follow-up, there were only three reports of switching treatment to a different intervention.
| Review 1, 3-month follow-up, n (%) | Review 2, 6-month follow-up, n (%) | Review 3, 9-month follow-up, n (%) | Review 4, 12-month follow-up, n (%) | |
|---|---|---|---|---|
| Final treatment choice at baseline review = deroofing | 35 | 35 | 35 | 35 |
| Did the participant receive first deroofing? | ||||
| Yes | 9 (25.7) | 15 (42.9) | 19 (54.3) | 22 (62.9) |
| Missing/No review (na) | 5 (14.3) | 6 (17.1) | 7 (20.0) | 7 (20.0) |
| No | 21 (60.0) | 14 (40.0) | 9 (25.7) | 6 (17.1) |
| If no, reason why? | ||||
| Participant chose different treatmenta | 0 | 0 | 0 | 0 |
| Clinician chose different treatmenta | 0 | 0 | 1 | 1 |
| Treatment delay | 21 | 14 | 8 | 5 |
| Participant did not attend their procedure appointment | 0 | 0 | 0 | 0 |
| Continuing with deroofing treatment? | ||||
| Yes | 28 (80.0) | 24 (68.5) | 17 (48.6) | 12 (34.3) |
| No | 1 | 2 | 2 | 4 |
| If no, reason why?b | ||||
| Opted to try alternative treatment | 1 | 1 | 0 | 1 |
| Other reason | 2 | 3 | 3 | 1 |
| Time between recruitment and the first procedure [days, median (IQR)] | N = 31 116 (71–245) |
|||
Conventional surgery was substantially delayed by the COVID-19 pandemic and only one participant reported receiving surgery by 3 months (Table 18) and six (50%) by the end of the study. Delay in surgery was reported by eight and five participants at the 3- and 6-month follow-up reviews, respectively. Overall, three participants chose to switch to an alternative intervention.
| Review 1, 3-month follow-up, n (%) | Review 2, 6-month follow-up, n (%) | Review 3, 9-month follow-up, n (%) | Review 4, 12-month follow-up, n (%) | |
|---|---|---|---|---|
| Final treatment choice at baseline review = conventional surgery | 12 | 12 | 12 | 12 |
| Did the participant receive first surgery? | ||||
| Yes | 1 (8.3) | 3 (25.0) | 6 (50.0) | 6 (50.0) |
| Missing/No review (na) | 3 (25.0) | 3 (25.0) | 6 (50.0) | 6 (50.0) |
| No | 8 (66.7) | 6 (50.0) | 0 | 0 |
| If no, reason why? | ||||
| Participant chose different treatmenta | 0 | 0 | 0 | 0 |
| Clinician chose different treatmenta | 0 | 1 | 1 | 1 |
| Treatment delay | 8 | 5 | ||
| Participant did not attend their procedure appointment | ||||
| Continuing with conventional surgery? | ||||
| Yes | 8 (66.7) | 7 (58.3) | 1 (8.3) | 1 (8.3) |
| No | 1 | 2 | 2 | |
| If no, reason why?b | ||||
| Opted to try alternative treatment | 1 | 2 | 0 | 0 |
| Other reason | 0 | 1 | 1 | 0 |
| Time between recruitment and the first procedure (days), median (IQR) | N = 8 175 (129.5–214) |
|||
Efficacy outcome estimates
Clinical outcomes over time have been described using medians alongside IQRs at each time point (baseline, 3, 6 and 9 months) and by each treatment group (Table 19). Interpretation of the change in outcome measures over time was hindered by the fact that individuals especially in the surgical interventions may not have received their treatment by the 6-month review. For example, of the 56 participants who chose laser surgery, 32 (57.1%) had received their first treatment before the 6-month review, 9 (16.1%) had not and 7 (12.5%) had received partial treatment (8 missing information by 6 months). Of the 36 that chose deroofing, 15 (41.7%) had received their first treatment by the 6-month review and 14 (38.9%) had not (7 missing information by 6 months). Of the 12 that chose conventional surgery, 3 (25.0%) had received their procedure by the 6-month review and 6 (50.0%) had not (3 missing information by 6 months).
| Baseline | Review 1, 3-month follow-up | Review 2, 6-month follow-up | Review 3, 9-month follow-up | Review 4, 12-month follow-up | Change, median (IQR) (baseline to 6 months) | |
|---|---|---|---|---|---|---|
| DLQI score: sum of the responses to all 10 DLQI items ranging from 0 to 30, with a higher score corresponding to worse quality of life | ||||||
| Doxycycline | n = 23 | n = 20 | n = 19 | n = 13 | n = 7 | n = 19 |
| 6.0 (4.0, 13.0) | 3.5 (1.5, 8.0) | 4.0 (1.0, 7.0) | 4.0 (3.0, 11.0) | 3.0 (2.0, 7.0) | –2.0 (–6.0, 0.0) | |
| Clindamycin and rifampicin | n = 23 | n = 18 | 18 | n = 15 | n = 10 | n = 18 |
| 14.0 (9.0, 18.0) | 10.5 (4.0, 13.0) | 8.5 (2.0, 12.0) | 12.0 (4.0, 16.0) | 6.0 (4.0, 15.0) | –6.5 (–1.0, −2.0) | |
| Laser | n = 56 | n = 45 | n = 43 | n = 41 | n = 19 | n = 43 |
| 15.0 (10.5, 9.0) | 11.0 (7.0, 16.0) | 11.0 (4.0, 15.0) | 10.0 (6.0, 19.0) | 6.0 (4.0, 12.0) | –3.0 (–11.0, 0.0) | |
| Deroofing | n = 35 | n = 32 | n = 31 | n = 27 | n = 23 | n = 31 |
| 12.0 (8.0, 18.0) | 11.5 (5.5, 16.0) | 10.0 (3.0, 18.0) | 7.0 (2.0, 16.0) | 7.0 (2.0, 13.0) | 0.0 (–7.0, 2.0) | |
| Conventional surgery | n = 12 | n = 11 | n = 8 | n = 7 | n = 4 | n = 8 |
| 16.0 (10.0, 21.0) | 13.0 (8.0, 22.0) | 11.5 (10.5, 6.5) | 15.0 (7.0, 8.0) | 12.5 (6.5, 4.0) | –2.5 (–9.0, 0.0) | |
| EQ5D-5L score: health-related quality of life index where 1 = best possible health, through 0 = death to –0.59 = worse than death | ||||||
| Doxycycline | n = 21 | n = 20 | n = 18 | n = 13 | n = 7 | n = 17 |
| 0.71 (0.50, 0.90) | 0.80 (0.50, 0.90) | 0.80 (0.70, 0.90) | 0.72 (0.50, 0.80) | 0.77 (0.20, 1.00) | 0 (–0.18, 0.15) | |
| Clindamycin and rifampicin | n = 23 | n = 17 | n = 16 | n = 14 | n = 9 | n = 16 |
| 0.69 (0.50, 0.90) | 0.77 (0.50, 0.80) | 0.67 (0.50, 0.90) | 0.72 (0.60, 0.80) | 0.80 (0.60, 1.00) | 0.06 (–0.16, 0.33) | |
| Laser | n = 50 | n = 34 | n = 40 | n = 36 | n = 17 | n = 38 |
| 0.69 (0.50, 0.80) | 0.70 (0.50, 0.80) | 0.69 (0.50, 0.80) | 0.70 (0.60, 0.80) | 0.72 (0.60, 0.80) | 0 (–0.12, 0.10) | |
| Deroofing | n = 33 | n = 31 | n = 31 | n = 27 | n = 22 | n = 30 |
| 0.64 (0.60, 0.80) | 0.70 (0.60, 0.80) | 0.73 (0.60, 0.80) | 0.83 (0.50, 1.00) | 0.77 (0.60, 0.90) | 0 (–0.12, 0.16) | |
| Conventional surgery | n = 9 | n = 10 | n = 7 | n = 6 | n = 4 | n = 5 |
| 0.65 (0.60, 0.90) | 0.60 (0.40, 0.80) | 0.70 (0.40, 0.80) | 0.65 (0.20, 0.80) | 0.66 (0.60, 1.0) | –0.24 (–0.29, 0.01) | |
| EuroQol-Visual Analogue Scale: visual analogue scale where 0 = worst imaginable health to 100 = best imaginable health | ||||||
| Doxycycline | n = 21 | n = 20 | n = 19 | n = 13 | n = 7 | n = 17 |
| 50 (40.0, 90.0) | 80.0 (60.0, 90.5) | 75.0 (51.0, 90.0) | 90.0 (51.0, 95.0) | 76.0 (40.0, 90.0) | 0 (–9, 15) | |
| Clindamycin and rifampicin | n = 23 | n = 17 | n = 16 | n = 14 | n = 9 | n = 16 |
| 70.0 (50.0, 80.0) | 60.0 (37.0, 70.0) | 60.0 (40.0, 77.5) | 63.5 (60.0, 80.0) | 75.0 (55.0, 83.0) | –7.5 (–10.5, 2.5) | |
| Laser | n = 50 | n = 36 | n = 41 | n = 36 | n = 19 | n = 39 |
| 60.0 (50.0, 80.0) | 60.0 (48.0, 80.0) | 65.0 (45.0, 80.0) | 37.0 (27.0, 52.0) | 27.5 (21.0, 41.0) | 0.0 (–13.0, 10.0) | |
| Deroofing | n = 32 | n = 31 | n = 31 | n = 27 | n = 22 | n = 29 |
| 72.5 (50.0, 81.0) | 75.0 (51.0, 85.0) | 70.0 (60.0, 90.0) | 75.0 (54.0, 85.0) | 72.5 (65.0, 85.0) | 0.0 (–7.0, 6.0) | |
| Conventional surgery | n = 9 | n = 10 | n = 7 | n = 6 | n = 4 | n = 6 |
| 70.0 (60.0, 80.0) | 75.0 (50.0, 80.0) | 60.0 (50.0, 80.0) | 74.5 (60.0, 90.0) | 62.5 (27.0, 81.5) | –10.0 (–15.0, 0.0) | |
| FSS score: 9 items scored on a Likert scale (1 = strongly disagree; 7 = strongly agree). The higher the score, the greater the severity of fatigue and the negative effect on the person’s activities. | ||||||
| Doxycycline | n = 23 | n = 20 | n = 19 | n = 13 | n = 7 | n = 19 |
| 43.0 (25.0, 54.0) | 33.5 (17.5, 54.0) | 43.0 (22.0, 52.0) | 41.0 (27.0, 53.0) | 41.0 (21.0, 59.0) | 0.0 (–7.0, 5.0) | |
| Clindamycin and rifampicin | n = 23 | n = 18 | n = 18 | n = 14 | n = 10 | n = 18 |
| 42.0 (32.0, 52.0) | 41.0 (27.0, 52.0) | 41.0 (35.0, 50.0) | 44.0 (37.0, 51.0) | 50.0 (45.0, 52.0) | –3.0 (–9.0, 5.0) | |
| Laser | n = 56 | n = 47 | n = 43 | n = 41 | n = 18 | n = 43 |
| 35.5 (24.5, 51.0) | 37.0 (26.0, 51.0) | 34.0 (22.0, 53.0) | 37.0 (27.0, 52.0) | 27.5 (21.0, 41.0) | 0.0 (–6.0, 8.0) | |
| Deroofing | n = 35 | n = 33 | n = 31 | n = 27 | n = 23 | n = 31 |
| 35.0 (27.0, 49.0) | 39.0 (23.0, 50.0) | 31.0 (26.0, 51.0) | 29.0 (23.0, 52.0) | 31.0 (23.0, 51.0) | 0.0 (–7.0, 10.0) | |
| Conventional surgery | n = 12 | n = 10 | n = 8 | n = 8 | n = 4 | n = 8 |
| 37.5 (29.0, 45.0) | 37.0 (23.0, 52.0) | 44.0 (22.5, 61.0) | 37.5 (34.0, 49.5) | 36.5 (27.0, 46.0) | 10.0 (–9.0, 18.5) | |
| HiSQOL: ranges from 0 = best to 68 = worst where a higher score indicates more severe impact on health-related quality of life | ||||||
| Doxycycline | n = 22 | n = 20 | n = 18 | n = 13 | n = 7 | n = 17 |
| 26.5 (6.0, 40.0) | 11.5 (5.5, 27.0) | 12.0 (6.5, 17.0) | 10.0 (5.0, 26.0) | 8.0 (2.0, 31.0) | 0.0 (–16.0, 7.0) | |
| Clindamycin and rifampicin | n = 23 | n = 19 | n = 18 | n = 15 | n = 10 | n = 18 |
| 34.0 (18.0, 42.0) | 23.0 (9.0, 33.0) | 18.0 (7.0, 31.0) | 27.0 (9.0, 36.0) | 12.0 (10.0, 34.0) | –14.0 (–23.0, –3.0) | |
| Laser | n = 56 | n = 47 | n = 43 | n = 41 | n = 18 | n = 43 |
| 32.0 (18.0, 40.0) | 27.0 (11.0, 44.0) | 26.0 (10.0, 37.0) | 22.0 (10.0, 37.0) | 15.0 (6.0, 27.0) | –4.0 (–14.0, 2.0) | |
| Deroofing | n = 35 |
n = 32 | n = 30 | n = 27 | n = 23 | n = 30 |
| 31.0 (11.0, 38.0) | 24 (12.5, 42.0) | 15.0 (8.0, 46.0) | 20.0 (2.0, 36.0) | 13.0 (2.0, 24.0) | –1.0 (–6.0, 3.0) | |
| Conventional surgery | n = 12 | n = 11 | n = 7 | n = 8 | n = 4 | n = 7 |
| 31.0 (11.5, 52.5) | 38.0 (17.0, 48.0) | 22.0 (16.0, 48.0) | 38.5 (23.5, 43.5) | 29.5 (12.5, 46.5) | –4.0 (–18.0, 6.0) | |
| Abscess and inflammatory nodule count: total count of abscesses and inflammatory nodules: higher count indicates more abscesses and/or nodules | ||||||
| Doxycycline | n = 23 | n = 20 | n = 19 | n = 13 | n = 7 | n = 19 |
| 4.0 (1.0, 8.0) | 2.0 (1.0, 7.5) | 2.0 (0.0, 5.0) | 3.0 (1.0, 6.0) | 3.0 (0.0, 12.0) | 0.0 (–5.0, 2.0) | |
| Clindamycin and rifampicin | n = 23 | n = 20 | n = 18 | n = 15 | n = 10 | n = 18 |
| 3.0 (2.0, 9.0) | 2.0 (1.0, 6.5) | 2.0 (1.0, 5.0) | 2.0 (1.0, 8.0) | 3.0 (1.0, 5.0) | –2.0 (–5.0, 1.0) | |
| Laser | n = 56 | n = 47 | n = 44 | n = 39 | n = 18 | n = 44 |
| 8.0 (3.0, 15.0) | 6.0 (2.0, 11.0) | 4.0 (2.0, 9.5) | 6.0 (2.0, 10.0) | 4.5 (2.0, 10.0) | –2.0 (–6.5, 0.5) | |
| Deroofing | n = 35 | n = 33 | n = 31 | n = 27 | n = 23 | n = 31 |
| 4.0 (1.0, 7.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 8.0) | 2.0 (0.0, 8.0) | 2.0 (1.0, 8.0) | 0.0 (–4.0, 2.0) | |
| Conventional surgery | n = 12 | n = 11 | n = 7 | n = 7 | n = 4 | n = 7 |
| 6.5 (2.5, 14.0) | 13.0 (9.0, 21.0) | 11.0 (3.0, 16.0) | 18.0 (13.0, 24.0) | 6.0 (2.5, 14.0) | –4.0 (–7.0, 3.0) | |
| IHS4: a continuous score that assigns different weights to different lesion types; inflammatory nodules (1 point), abscesses (2 points) and draining tunnels (4 points) across all body locations; higher count indicates higher severity. | ||||||
| Doxycycline | n = 22 | n = 20 | n = 19 | n = 13 | n = 7 | n = 19 |
| 7.0 (3.0, 14.0) | 6.0 (1.0, 11.0) | 4.0 (1.0, 8.0) | 4.0 (1.0, 13.0) | 3.0 (0.0, 22.0) | –3.0 (–10.0, 3.0) | |
| Clindamycin and rifampicin | n = 23 | n = 20 | n = 18 | n = 15 | n = 10 | n = 18 |
| 11.0 (4.0, 16.0) | 5.0 (1.0, 18.5) | 6.0 (3.0, 10.0) | 6.0 (1.0, 18.0) | 3.5 (1.0, 14.0) | –4.5 (–13.0, 3.0) | |
| Laser | n = 55 | n = 47 | n = 44 | n = 39 | n = 18 | n = 43 |
| 15.0 (5.0, 22.0) | 13.0 (5.0, 23.0) | 8.5 (3.0, 20.0) | 10.0 (2.0, 19.0) | 10.5 (2.0, 20.0) | –2.0 (–10.0, 2.0) | |
| Deroofing | n = 35 | n = 33 | n = 30 | n = 27 | n = 23 | n = 30 |
| 10.0 (4.0, 16.0) | 6.0 (3.0, 15.0) | 8.5 (3.0, 19.0) | 6.0 (0.0, 20.0) | 5.0 (1.0, 19.0) | –0.5 (–5.0, 4.0) | |
| Conventional surgery | n = 1222.5 | n = 11 | n = 7 | n = 7 | n = 4 | n = 7 |
| (5.5, 36.0) | 26.0 (17.0, 37.0) | 28.0 (3.0, 34.0) | 27.0 (26.0, 64.0) | 17.5 (2.5, 37.0) | 0.0 (–54.0, 7.0) | |
| Pain Score on NRS: score range: 0 = no pain to 10 = worst pain | ||||||
| Doxycycline | n = 23 | n = 20 | n = 19 | n = 13 | n = 7 | n = 19 |
| 2.0 (1.0, 6.0) | 1.0 (0.0, 5.5) | 2.0 (0.0, 5.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 8.0) | 0.0 (–1.0, 1.0) | |
| Clindamycin and rifampicin | n = 23 | n = 19 | n = 18 | n = 15 | n = 10 | n = 18 |
| 4.0 (1.0, 6.0) | 2.0 (0.0, 5.0) | 6.0 (2.0, 7.0) | 3.0 (1.0, 8.0) | 3.0 (0.0, 6.0) | 1.0 (–3.0, 3.0) | |
| Laser | n = 56 | n = 47 | n = 45 | n = 40 | n = 19 | n = 45 |
| 4.0 (2.0, 6.0) | 4.0 (1.0, 7.0) | 5.0 (2.0, 6.0) | 5.0 (1.5, 7.0) | 4.0 (1.0, 5.0) | 0.0 (–2.0, 2.0) | |
| Deroofing | n = 35 | n = 30 | n = 31 | n = 27 | n = 23 | n = 31 |
| 3.0 (2.0, 6.0) | 3.5 (1.0, 6.0) | 4.0 (1.0, 7.0) | 3.0 (0.0, 6.0) | 3.0 (0.0, 6.0) | 0.0 (–2.0, 3.0) | |
| Conventional surgery | n = 12 | n = 11 | n = 8 | n = 8 | n = 4 | n = 8 |
| 6.0 (2.0, 8.5) | 7.0 (4.0, 7.0) | 4.0 (2.0, 6.0) | 5.0 (4.0, 7.5) | 3.5 (1.5, 5.0) | –2.5 (–5.5, 2.0) | |
| Patient Global Health Assessment: ‘In the past 7 days how much has your HS influenced your quality of life?’ answers on a 5-point Likert scale: not at all (0), slightly, moderately, very much, or extremely (4) | ||||||
| Doxycycline | n = 21 | n = 20 | n = 19 | n = 13 | n = 7 | n = 17 |
| 2.0 (1.0, 3.0) | 1.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 1.0 (0.0, 2.0) | 0.0 (0.0, 4.0) | –1.0 (–1.0, 0.0) | |
| Clindamycin and rifampicin | n = 22 | n = 19 | n = 18 | n = 15 | n = 10 | n = 17 |
| 2.5 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | –1.0 (–2.0, 0.0) | |
| Laser | n = 46 | n = 47 | n = 45 | n = 40 | n = 19 | n = 34 |
| 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 1.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 1.0 (0.0, 1.0) | –0.5 (–1.0, 0.0) | |
| Deroofing | n = 26 | n = 31 | n = 30 | n = 27 | n = 23 | n = 23 |
| 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.0 (–1.0, 1.0) | |
| Conventional surgery | n = 9 | n = 10 | n = 8 | n = 8 | n = 4 | n = 5 |
| 1.0 (0.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.5) | 2.0 (1.0, 2.5) | 1.5 (0.5, 3.0) | 1.0 (–2.0, 2.0) | |
| Hidradenitis Suppurativa Clinical Response: n (%), 50% or more reduction in inflammatory lesion count and no increase in abscesses or draining tunnels between each review and baseline | ||||||
| Doxycycline | – | 9 (45.0) | 6 (31.6) | 3 (23.1) | 3 (42.9) | – |
| Clindamycin and rifampicin | – | 7 (35.0) | 6 (33.3) | 5 (33.3) | 3 (25.0) | – |
| Laser | – | 9 (19.6) | 11 (33.3) | 9 (23.7) | 10 (52.6) | – |
| Deroofing | – | 11 (33.3) | 11 (36.7) | 13 (48.1) | 11 (47.8) | – |
| Conventional surgery | – | 1 (9.1) | 1 (14.3) | 0 (0.0) | 1 (20.0) | – |
| Hurley staging: n (%) | ||||||
| H-I | 19 (12.8) | 18 (14.1) | 20 (17.2) | 18 (18.2) | 14 (23.0) | – |
| H-II | 102 (68.5) | 81 (63.3) | 77 (66.4) | 60 (60.6) | 34 (55.7) | – |
| H-III | 28 (18.8) | 29 (22.7) | 19 (16.4) | 21 (21.2) | 13 (21.3) | – |
| Refined Hurley staging:a n (%) | ||||||
| Hurley IA | 13 (8.7) | 27 (21.1) | – | 22 (22.2) | 29 (46.8) | – |
| Hurley IB | 32 (21.5) | 15 (11.7) | – | 14 (14.1) | 2 (3.2) | – |
| Hurley IC | 18 (12.1) | 8 (6.3) | – | 7 (7.1) | 3 (4.8) | – |
| Hurley IIA | 12 (8.1) | 6 (4.7) | – | 6 (6.1) | 0 (0.0) | – |
| Hurley IIB | 14 (9.4) | 12 (9.4) | – | 5 (5.1) | 5 (8.1) | – |
| Hurley IIC | 45 (30.2) | 39 (30.5) | – | 33 (33.3) | 18 (29.0) | – |
| Hurley III | 15 (10.1) | 21 (16.4) | – | 12 (12.1) | 5 (8.1) | – |
Assessment of the feasibility of collecting pain scores
Consent to participate in the daily pain NRS text message element of THESEUS was received from 146 (98.0%) participants. The text messages were initiated in 110 participants, with 100 returning at least one text message response. During the text messages period (84 days), 4898 daily messages from 100 patients (86% female, 80% white, on average 37 years) were received (Figure 3). Responses to the text messages service reduced over time; the median time of concordance was week 5 (day 36) and concordance at the end of the period (day 84) was only 20%.
FIGURE 3.
Line graph of the daily response rates of participants over 12 weeks (84 days).

The baseline characteristics of the subsample were similar to the overall sample. The mean pain scores fluctuated over time with day 1: 4.24 (95% CI 3.68 to 4.80), dropping to day 14: 2.77 (95% CI 2.13 to 3.41), day 36: 2.79 (95% CI 2.10 to 3.48), while it was lowest during the last week (day 80), 2.23 (95% CI 1.36 to 3.09; Figure 4).
FIGURE 4.
Line graph of the daily mean pain scores over 12 weeks (84 days).

The mixed-effect models showed a significant decrease of the pain scores over time [with a decrease in pain score of 0.035 points per day (95% CI 0.027 to 0.044) (p < 0.0001; Table 20; see Figure 4)]. A higher consistency was observed in the model for daily pain scores in the first 14 and 28 days, intraclass correlation (ICC) 0.85 (95% CI 0.81 to 0.89), ICC 0.79 (95% CI 0.74 to 0.84), respectively. For the whole period of 84 days, the ICC was 0.76 (0.70 to 0.81). In mixed-effect models for the weekly pain scores, better consistency was for the initial 6-week pain scores (ICC 0.69, 95% CI 0.60 to 0.77). The ICC for the monthly pain scores was 0.71 (95% CI 0.58 to 0.81). The daily pain score was significantly associated with baseline HiSQOL and the number of abscesses.
| Baseline variables | Coefficient (95% CI) | p-values |
|---|---|---|
| Days post text initiation | –0.035 (–0.044 to –0.027) | < 0.0001 |
| Square of the days | 0.0004 (0.00027 to 0.00044) | < 0.0001 |
| HiSQOL | 0.05 (0.03 to 0.08) | < 0.0001 |
| Number of abscesses | 0.15 (0.04 to 0.26) | 0.007 |
Safety analysis
A total of 37 adverse events were recorded during the study period from 29 participants, of which all were deemed as relevant. There were no serious adverse events. Table 21 shows the adverse events subdivided by intervention and timing during the follow-up period.
| Treatment option (adverse effect) | Review 1, 3-month follow-up (n) | Review 2, 6-month follow-up (n) | Review 3, 9-month follow-up (n) | Review 4, 12-month follow-up (n) | Total adverse events (total patients) (n) |
|---|---|---|---|---|---|
| Oral doxycycline | 4 | 3 | 1 | 0 | 8 (8) |
| Gastrointestinal | 4 | 3 | 1 | 0 | 8 |
| Oral clindamycin and rifampicin | 6 | 2 | 4 | 1 | 13 (11) |
| High temperature | 1 | 0 | 0 | 0 | 1 |
| Gastrointestinal | 2 | 1 | 3 | 1 | 7 |
| Elevated liver enzymes | 0 | 0 | 1 | 0 | 1 |
| Menorrhagia | 1 | 0 | 0 | 0 | 1 |
| Skin rash | 1 | 0 | 0 | 0 | 1 |
| Worsening of condition | 1 | 1 | 0 | 0 | 2 |
| Deroofing | 3 | 4 | 2 | 3 | 12 (9) |
| Wound inflammation | 1 | 2 | 1 | 1 | 5 |
| Wound infection | 0 | 0 | 0 | 1 | 1 |
| Worsening of condition | 2 | 2 | 1 | 1 | 6 |
| Laser | 0 | 2 | 1 | 0 | 3 (3) |
| Wound inflammation | 0 | 0 | 1 | 0 | 1 |
| Worsening of condition | 0 | 2 | 0 | 0 | 2 |
| Surgery | 0 | 1 | 0 | 0 | 1 (1) |
| Wound infection | 0 | 1 | 0 | 0 | 1 |
| Total | 13 | 12 | 8 | 4 | 37 |
Chapter 4 Qualitative evaluation
Nested qualitative study of patient and healthcare staff perspectives
Introduction
A systematic review from 2021 found that no qualitative studies of the experiences of people with HS had been conducted in the UK. 34 The review found studies in Ireland, USA, Denmark and Israel. 34 Most studies focused on the impact of symptoms and psychosocial adjustment. Studies suggest there are widespread physical, psychological and social impacts of HS on an individual, and that stigmatisation that leads to shame is a key factor driving distress and coping strategies. 35 To a more limited extent, studies also investigated patient perspectives of health care and treatment for HS, which together indicate that HS patients’ needs are not being met by healthcare systems. 34 As the THESEUS study was broadly reflective of UK current practice and treatment pathways, it was an ideal setting to explore patient and healthcare staff perspectives of HS care and treatment within the UK. This can help us better understand patient experiences of current treatments and inform the design of future clinical trials.
Study aims and objectives
Aim
-
to inform future clinical trials in HS.
Objectives
-
to understand influences on treatment choices from both a patient and clinician perspective.
-
to understand barriers and facilitators to recruitment into future clinical studies of HS treatments.
Methods
Qualitative approach
This is an interview study nested within a broader clinical piece of research. It is a pragmatic interview study driven by the need for recommendations for future research.
Patient interviews
Participant selection
The sample was taken from the group of THESEUS cohort study participants who agreed to be contacted about the interview study using a purposeful sampling framework (Table 22).
| Characteristic | Sampling aim |
|---|---|
| Treatment arm | 20% doxycycline 20% clindamycin and rifamycin 20% laser 20% deroofing 20% conventional surgery From at least three sites for each treatment arm |
| Age | 60% < 40 years 40% ≥ 40 years |
| Sex | 30% male 70% female |
| Ethnicity | 75% white 25% other ethnic groups |
| Site | As many sites included in the THESEUS cohort study as possible |
Study procedure
Semistructured interviews took place via telephone and were audio recorded. At the start of the interview, consent was taken verbally. The interviews ranged from approximately 30 minutes to 75 minutes, with most taking around 60 minutes. A topic guide covered: (1) treatment experiences prior to the study; (2) treatment experiences during the study; and (3) experiences of taking part in the research study. A debrief at the end of the interview advised participants where to go if the interview had raised any concerns or questions about their care.
Research team and reflexivity
The interviewer was a female psychologist and postgraduate researcher (LH) who had previous training and experience in qualitative research and was also supervised by an experienced qualitative researcher (PL) with regular opportunities to debrief. The interviewer had no previous involvement with participants prior to the interview study. As the interviews were being conducted by the THESEUS study team, participants were regularly encouraged to share both positive and negative experiences of the study. Findings were discussed periodically with the multidisciplinary SMG to adapt design as necessary (e.g. agreed to adapt the topic guide to explore the impact of COVID-19).
Analysis
Interviews were transcribed verbatim and managed in NVivo 12 (QSR International, Warrington, UK). 36 A prespecified thematic framework was used to code data, and the framework was refined during the coding process to capture pertinent, unanticipated topics. This coding was then reviewed by the interviewer and sections of coded data were reviewed by a second author (PL) and refinements were discussed. The interviewer then looked at framework matrices to understand and interpret the data, which resulted in three final themes. Findings were discussed periodically between LH and PL during the analysis process. The three themes were then presented alongside illustrative data to a wider group of authors to view the findings from different methodological, clinical and patient perspectives (KT, JI, AB and CM). These discussions helped to develop key learning points for future HS research and clinical practice and fed into the THESEUS consensus workshop discussions (see Chapter 7).
Sample size
We aimed to recruit up to 50 participants taking part in THESEUS for interview, but data collection was deemed sufficient to answer research questions earlier than anticipated.
Staff interviews
Participant selection
We aimed to interview up to 10 site staff (one per site) involved in delivering THESEUS. They could be involved in delivering any aspect of the study at any point. We did not include members of the SMG. A purposeful sampling approach was used to speak to individuals with different roles. The end-of-study questionnaire for site staff also contained open-text questions.
Study procedure
Interview participants were contacted by e-mail or telephone by the interviewer (LH or PL) to arrange a time and date. Participants were then sent the participant information sheet and a form containing all the consent statements (via e-mail) and asked to complete this ahead of the interview. Interviews ranged from approximately 20 to 40 minutes. The interview was semistructured.
Analysis
Findings were mapped to the same three broad themes as the patient data to aid comparison across data sources, but subthemes were tailored using a more inductive approach.
Open text from end-of-study questionnaires
The THESEUS participants and staff were able to submit open-text responses (questions in Appendix 1) as part of end-of-study questionnaires that were mapped to the same frameworks indicated above.
Results
Participant characteristics
Thirty-five patient interviews took place between December 2020 and October 2021. Twenty-five (71%) participants were female. Participant ages ranged from 19 to 67 years. Those under 40 years accounted for 69% (n = 24) of the sample. Participants described their ethnicity as white (n = 23, 66%), mixed/multiple ethnic groups (n = 2, 6%), Asian/Asian British (n = 5, 14%) and black/African/Caribbean/Black British (n = 4, 11%). One participant did not state their ethnicity. Treatment choices were doxycycline (n = 6), clindamycin and rifampicin (n = 7), laser (n = 9), deroofing (n = 7) and conventional surgery (n = 6). At the time of interview, not all had received their baseline treatment choice (laser n = 2, deroofing n = 1, conventional surgery n = 4). Participants were from eight different sites.
Eight staff interviews took place between January and March 2022. We interviewed people with a range of roles and each from different sites.
There were 61 patient responses and 26 healthcare staff who provided open-text responses to analyse in the end-of-study questionnaires.
Framework matrix
The final framework matrices are in Appendix 2. The patient interview matrix includes 67 codes that were ordered hierarchically into three levels (codes, subcodes and further subcodes). The main codes were: (1) beliefs and experiences; (2) study treatment experiences; and (3) HS research experiences. ‘Beliefs and experiences’ covered beliefs about HS, beliefs about HS treatments and experiences seeking treatment. ‘Study treatment experiences’ covered reasons for treatment choice, treatment processes and satisfaction with treatment. ‘HS research experiences’ covered reflections on the study and recommendations for future research. The staff interview matrix includes 17 codes that were hierarchically coded into two levels (codes and subcodes). The three main codes were adjuncts to the three main codes in the patient interviews.
Final themes
Through a process of charting and mapping, final themes were developed that provide an interpretation of how the data answer our research questions.
Theme 1: treatment choices
Doxycycline
Doxycycline was typically preferred when individuals had limited experiences with HS (e.g. had not tried many treatments for HS). It was described as a ‘starting point’ and less invasive than surgical options.
So, start you on that one first and obviously when I go back [ … ] I will say, not that one, so then we will look at the other options.
(Participant 25, white male)
Sometimes there had been experience with other treatment options, but it was not perceived as the correct time for them to have surgery (e.g. still healing, not required for symptoms).
No, that’s what we both agreed was right at the time, mine’s not quite an advanced HS. I’ve had a few where they’ve had to be removed via surgery, but other than that I’ve been quite stable over the last 15 years of it.
(Participant 9, male, white)
Some information was new to individuals, and it was via interactions with HCPs that they had developed these perceptions of the treatment. Some were interested in the laser treatment, but it was not available at their site, or their doctor had encouraged them to start with the tablets first.
Clindamycin and rifamycin
Previous non-favourable experience of taking other tablets, including doxycycline and other similar drugs, led people to believe that they would not work for them again and so had a strong preference to try something new.
It was using the treatment grid and basically the first one was doxycycline, which is basically the same as lymecycline, which I’d been taking, which didn’t really seem to make an awful lot of difference either. So, we ruled that one out because I’d already been on that one. That’s why we went for the second one, because I thought there’s no point in wasting time doing something I’ve already taken that’s not really been effective. So, that’s why I chose the clindamycin and rifampicin.
(Participant 7, female, white)
Some were concerned about using medication (or taking more medication), particularly in the long term, but it was considered a necessary trade-off to stop symptoms. Reasons it was chosen over other options are that it was less invasive, participants had a lack of familiarity with other treatment options, concerns about what other options would entail or other options (e.g. laser, deroofing) not being available at their site.
Participant 6, female, white: I don’t think the deroofing would help.
Interviewer: Okay can you say why you think that’s the case?
Participant 6: I don’t understand what deroofing is. Sorry. […] I’m not a hundred per cent sure what it is or what it does or what it means.
The treatment grid was referenced when deciding not to ‘jump’ to surgery and give the tablets a try first. Sometimes patient initial treatment preferences were overridden by the treatment choice reported as what the doctor felt was most appropriate.
Yes, it’s not like I chose, she said this might be the way to go and I thought I’ll go with it, because I’d asked about surgery for my groin and she wants to try this first, because obviously it’s a very invasive treatment to get surgery. But because I had such positive result I’m quite pro surgery, I know it’s going to sound terrible, but I just go with what she said.
(Participant 15, female, white)
Laser
Laser was the most popular choice within the cohort. Past experiences left individuals unsatisfied by other options. Some felt that tablets had not worked for them, did not want them long term, had caused side effects they could not tolerate or worked to some extent but were looking for something to improve beyond what they could offer.
The two tablets, I mean for me in my head I was just like I have tried tablets already and I know what that means. That’s not really a long-term solution, I can’t just take tablets for my whole life.
(Participant 32, female, black/African/Caribbean/Black British)
One person ruled out tablets due to wanting to get pregnant. There was sometimes a push away from surgery if that had not gone well for individuals in the past or they were viewing it as more invasive.
It was perceived as a preventative measure as hair removal potentially prevents future boils, and many favoured this as a solution that was addressing the cause. It was also known to some that it was ‘new’ to the NHS, and so it had the added draw of being a chance to try a new treatment that was usually unavailable.
And the laser had just become available on the NHS for this, because obviously it is an infection of the hair follicle, so if can stop the hair follicle from growing, it’s hoping we can stop the boils. That’s why he is going for the main bits where they are really, really bad at the moment.
(Participant 27, female, white)
Some of the reasons for preferencing laser came from doctors or information provided within the study.
Not much to say it was just the [unclear 0:33:20.1] your two surgeries didn’t work I don’t know if a third surgery is going to help you let’s try a laser treatment to you know try and get rid of the hairs that may be causing the inflammation because it’s got HS has got to do with sweat glands so if we limit the what’s called the inflamed hairs it could also limit the amount of legions that show up. Which was a good sort of theory so I was like okay I’ll go with that it’s not too invasive sort of thing and hopefully over time it would reduce, if not reduce [unclear 0:33:57.5] which me sounds good so yeah, that’s how we came to that sort of conclusion.
(Participant 20, male, black/African/Caribbean/Black British)
Deroofing
Some had heard of deroofing previously, but often HCPs and study information were the primary sources of information. For those that had seen other sources of information, there was an experience where online information made it seem highly effective, whereas another where online forums made it seem like a ‘temporary fix’.
There were concerns about deroofing, and surgery more generally, but it was considered a necessary ‘last resort’. Reasons were that medication did not work effectively enough for them, caused them unwanted side effects and they had concerns about long-term use. One person preferred deroofing over laser due to previous facial hair laser removal resulting in bumps in their skin.
I mean I am kind of limited because I have never really tried, I tried one of them, I know it’s some sort of cycline on the list but it doesn’t work for me and for me laser is a no-no.
(Participant 29, female, ethnicity not reported)
There were initial preferences for other treatments, particularly laser and conventional surgery, but a HCP had advised them to use deroofing, citing that laser would not remove the HS, only the hair follicles, and that deroofing is a shorter, simpler procedure than conventional surgery. Often this came with a promise to consider other options in the future.
I saw that the laser sounds good, I don’t know why I thought that. Then after speaking to the consultant the sort of said, well it’s not the best option because it just removes the hair follicles, it doesn’t remove the HS itself. They said that deroofing would be a better option, so I said, okay I’ll for deroofing.
(Participant 18, male, white)
Others did not realise that they had a choice or did not feel like they had a choice, and that they had to go with the HCP’s opinion, and others said they worked it out together with their professional. Some people wanted guidance from their professional due to finding it a difficult decision with limited information.
I did yeah, but he said it’s quite a disfiguring operation, so the deroofing would be best to start off, and then later on down the line if I was really adamant about wanting the other surgery then he would consider it. […] I didn’t have any choice, I felt like I had to go with it.
(Participant 2, female, Asian/Asian British)
Conventional surgery
Conventional surgery was the least popular choice within the cohort. Some had previous experiences of using it and felt they knew it worked and knew the process, so wanted to stick with it. It was often chosen because other options were not considered appropriate. Of particular interest is why it was chosen over deroofing as the alternative surgical option. Reasons given were that deroofing was only appropriate for HS that appears in the same place each time, seemed to be deeper so they felt may be riskier, the video was scary and they did not like the idea of being awake for the procedure.
I Googled that video and that was horrendous. […] This person was awake on the surgery bed, admittedly probably had anaesthetic, like local anaesthetic, so that’s why they were awake. There was the smell of burning skin when you’re awake, how can anybody go through that. I said to the plastic surgeon I was please do not ever advise for me to have deroofing, I really don’t think I could do that unless I was asleep.
(Participant 21, female, white)
For some, conventional surgery was chosen alongside other options (e.g. having deroofing on different areas, having laser first or having medications as well). HCPs’ advice on which was more appropriate informed choices.
Model of treatment choice process
There were patterns across all participants in how treatment choices were reached (Figure 5). Individuals had experiences and knowledge (e.g. past treatments, healthcare interactions, online information) that shaped their beliefs about HS and HS treatments. These could be described as push and pull factors, indicated by the upwards and downwards arrows, that helped individuals choose between treatments. Beliefs informed preferences, which informed treatment choice.
FIGURE 5.
Model of treatment choice process in THESEUS study.
However, study interactions could also influence choice. In some cases, there was strengthening or creating of beliefs and preferences via information shared from the HCP and study materials. For example, participants perceived a hierarchy between the THESEUS treatments, which was likely created or reinforced by study materials and communication with the study team (e.g. the study treatment grid, see Chapter 6).
I guess I’m going by the grid, so it’s kind of like the oral treatments first, then it’s laser hair removal, then it’s like the wall surgery type things. So, what I think I will do is probably just go through them and see what works best. Obviously the major surgery being the last resort, rather than jumping. If I feel a need to jump then obviously I will.
(Participant 7, female, Asian British)
In other cases, they directed treatment choice away from initial preferences. HCPs could have a very influential position, with some patients reporting that they went along with what the professional thought was the best option for them. However, there were others who felt they were left to make the final decision. Some patients felt this was too much responsibility or a particularly hard decision for them and had hoped for more HCP guidance.
I might have, if this hadn’t been an alternative, if she’d said, oh you have to go to laser surgery, you have to. I would have done that. I think I’m going to go with my doctor, I’m no specialist in this field. I just have the disease.
(Participant 15, female, white)
Staff perspectives on treatment choice
Some staff were particularly interested in certain treatments included in the study. Preferences among their patient population were also noticed. Laser was appealing as a new treatment avenue not currently available in routine NHS care and the low risk. Deroofing was described as a new approach for treating HS, but staff undertaking the surgical procedures had experiences of using similar approaches. Some centres noted that a lot of patients had already tried antibiotics and so were not keen to take these, whereas others found they were the most appropriate treatments for new patients and individuals worried about surgery. Some staff described a process of guiding a patient’s treatment choice based on eligibility criteria and what they felt was most suitable clinically.
So, when a lot of these patients come in to dermatology they’ve usually tried antibiotic treatments and they’re looking for something more really, and they’ve tried the antibiotics, and I did find when they chose what intervention they wanted based on what we told them and what we’ve informed them, they were very, very keen on trying perhaps the laser, depending on obviously how severe their condition is, but they were very interested in it because I think it’s not offered on the NHS at the moment, so that was one treatment we found that they were particularly interested in.
(Staff member 4)
It was noted that other than the new availability of some treatments, the study had little effect on prescribing practices, as it fit naturally with current care pathways. However, regular care often uses medical and surgical treatments, and treatment would vary over time due to flaring nature of the condition, rather than choose between them. It was noted by one prescriber that it perhaps encouraged use of doxycycline instead of lymecycline. Practices were also commonly prescribing adalimumab, which was not part of the THESUES framework.
They kind of fit in with what we would have been doing clinically anyway, so I didn’t find them difficult to choose, they were the natural choices.
(Staff member 1)
Theme 2: treatment experience
Antibiotics
Few pretreatment issues were reported, although some reported barriers accessing tablets (waiting for a prescription or not having access to them when not at home). There were also difficulties in taking the tablets (e.g. remembering to take them) and people often adjusted when they took them to help them remember and to limit adverse effects (e.g. take with food, not before bed). Some people experienced adverse effects, whereas others did not. Upset stomach or diarrhoea were reported for doxycycline and stomach issues, diarrhoea, orange urine, pain in ear, impact on menstruation were reported for clindamycin and rifamycin.
I struggled at first. It was like you’ve got to take two of this tablet, one of this tablet and then another one of them tablets and two of them tablets and it’s a lot to try and remember every day and then the side effects of the two different tablets in your body threw me for six.
(Participant 6, female, white)
Some continued to use the antibiotics despite side effects and often noticed that the adverse effects improved with time, whereas others stopped using them, often advised by their doctor. Adverse effects impacted people’s work or were considered only manageable as they were working from home. Many people experienced an improvement in their HS while taking the tablets, although for some this was not maintained once stopping treatment. Others did not feel that there was a noticeable difference in their HS.
So, I was maybe missing one of the doses or I was going for a few days without taking them, because I just wasn’t timing it right to get the whole empty stomach thing happening. I noticed I was starting to get a bit of pain coming back, so starting to feel, so before I get a flare up I can feel it inside, sort of like a pulling, like a tension. I know when I start to feel that, that I’m going to start to get a flare up, it must be like something coming in the surface. When I hadn’t been taking the tablets properly I started to get that sort of – it was really weak but I could feel that sort of happening. I’ve been back on the tablets like I should have been for a good five, six days now and that’s gone. So, it’s definitely preferable from the side effects to tablets to the HS. Again, because I’m working from home and everything just now, any sort of side effects are manageable, but if it was normal times, it might be a bit different. But for what I’ve got just now, it’s manageable.
(Participant 24, female, white)
For some people a review was planned for after the course had ended, but others did not know what would happen once the course had come to an end.
Laser, deroofing and conventional surgery
For some there were significant delays to receiving laser/surgical options. The COVID-19 pandemic was often recognised as a contributing factor to these delays, but it could still be frustrating. Ahead of procedures, people reported feeling nervous about pain during the procedure and concern about wound healing after the procedure.
Oh I was constantly anxious, is it going to hurt? Is it going to work? Like what’s the lady going to be like doing it, it was all just loads of stuff. I got really, really bad anxiety and it was just a mixture of everything.
(Participant 27, female, white)
For laser, people described the experience as not being as painful as expected and there was little to no healing time required. Some had been concerned about pigmentation, due to having dark skin or due to it being on a noticeable part of their body, but this had not been experienced.
No I thought I might be a bit red and stuff but there was none of that. It wasn’t painful afterwards I was still like able to do normal things as well, I haven’t had any issues there. No, it’s been fine since. […] But I mean it hasn’t really, in terms of the actual pigmentation, like I haven’t noticed it on my skin. So yes, that’s okay for me now.
(Participant 26, female, Asian/Asian British)
Some had noticed drastic changes in their HS after the first or second treatment. There were concerns that four sets of treatments would not be enough to rid of all the hair in the area and that future treatments would be required, but this would not be available on the NHS.
After the first two treatments, as weird as it sounds, I felt a hell of a lot more comfortable from where the old then scar tissue and that was, where it used to flare up the worst. Yeah, it seemed to calm it down even my wife at the time said the area seemed a lot less angry and red than what it was so, since the laser treatment it’s worked wonders personally.
(Participant 34, male, white)
Healing times for deroofing were variable. For the surgery, people described not feeling any pain until after the anaesthetic wore off. Some people were pleased with the results of their deroofing and were surprised how effective it had been and how easy the healing process had been.
No and it hasn’t been, it hasn’t been so invasive like other surgeries that I’ve had you know it’s, I don’t feel as if I’ve had anything done but I’m not having any problems anymore with the two areas that they’ve done which was always you know there wasn’t a day that it wasn’t sort of enlarged and leaking but at the moment I’m going to touch but it seems fine.
(Participant 1, female, white)
Some were not satisfied, which seemed to be because the procedure had not been done as it should be, because they felt that all the HS had not been successfully removed or that their wounds were more challenging to deal with than the HS itself.
Because it was cut underneath along the line of the tunnel and the underneath part was scraped out and the skin left on. Meanwhile deroofing is meant to take out, it’s a tissue saving surgery, so it’s meant to take out the skin and scrape out whatever is in there, it’s fills back nicely. But the skin was left over this one and it started getting infected right from the third day. It’s healed now, well it hasn’t healed completely it’s still not healed inside because the whole idea is for it to heal from inside out, but because the skin was still on top of it, it was over-granulating and it was healing from the outside first.
(Participant 13, female, black/African/Caribbean/Black British)
We have little information on conventional surgery experiences due to delays in procedures taking place.
Staff perspectives on treatment experience
Laser and surgical procedures were not always available routinely by the teams within THESEUS, and so expertise had to be outsourced and buy-in from other specialties and departments was needed to conduct the study, which was sometimes challenging. Deroofing was reported as mostly straightforward to conduct. However, there were a few concerns reported, such as one surgeon wishing they had the opportunity for loop diathermy technical training and one surgeon warning of the potential harm of deroofing done without expertise if the sinus tract is very deep. Some thought that dermatologists could be trained to deliver this technique, but perhaps they might require more training needs. The intensity of deroofing wound care was also raised. As was the psychological care patients needed alongside medical and surgical treatments (e.g. weight management, smoking cessation, pain management, individual complexities/issues).
One has to be slightly careful with this technique, in that it’s like using a hot wire on butter, and some patients with HS have got very deep sinus tracks that get into levels of anatomy where a hot wire through butter isn’t really the effect you want to have.
(Staff member 7)
It was noted that one patient had to stop laser due to a flare up and risk of taking the medication for the flare at the same time as laser.
Positive experiences of laser or deroofing treatments within the study had led staff to want to continue them beyond the study, but many cited commissioning challenges were likely to prevent this change in practice.
Theme 3: research processes
Why people take part in hidradenitis suppurativa research
People recognised benefits both for themselves and for others with HS; as one person described it, ‘a win–win’. Some people wanted to learn more about their own condition. Some wanted the findings of the study to raise awareness among others. Some felt by taking part they could help find better treatments/a cure for others. While as a research team we are aware that people may cite socially acceptable responses for participating, there also seemed to be drivers relating to own challenging experiences with HS, and a belief that other people with HS might not feel able to, or do not have a diagnosis, to be able to take part in a study. People also talked about feeling ‘selected’ for this opportunity. Sometimes family members would encourage individuals to take part.
So, if I can help find a cure, I don’t mind being the guinea pig, so to speak, that’s, to me, it’s not really an issue. I’d rather try, like, I’ve got nieces and I’d hate for my nieces to go through what I had to go through and if I’m one of the people who can try things, why not?
(Participant 11, female, mixed/multiple ethnic groups)
Another reason for taking part was to gain access to treatment or support. Reasons reported were beliefs it would help them access surgical treatments quicker, access to laser that was not routinely available, beliefs it would provide extra support and time from NHS staff.
Because I found out that people who take part in the study get a bit more attention and I just felt like I needed the kind of extra attention, because the infection was really, really bad and I didn’t feel well […] My sister is a doctor and she said if I joined this study then I would probably get the extra attention from the doctors.
(Participant 31, male, black/African/Caribbean/Black British)
There were others that took part due to a reassurance that it would not influence their treatment or if they felt that the treatment as part of the study would be of minimal risk to them. People felt the study was a chance to do something about their HS and that taking action was better than doing nothing.
Many people were willing to take part in future research, often for the same reasons why they took part in THESEUS, but also some cited satisfaction with their experience in THESEUS.
I just think that I have had massive benefit from this one so I would definitely be mining to look for another one, I would be interested in knowing more about another one, but obviously it would depend on what the treatment options were but yes I would certainly consider it.
(Participant 30, female, white)
Study procedures
Interactions with clinical care
Few reported concerns about impact on clinical care. Initial concerns were being able to use existing treatments, attend existing dermatology appointments and building a relationship with a new team. Some were not always clear which treatments and appointments were THESEUS related, which is probably reflective of how THESEUS was designed to be embedded within existing NHS clinical care pathways.
Communication
Generally, communication with the study team was a positive experience where participants felt respected, supported, a personal touch, the research processes were clearly explained and felt they could stop the study at any time. Where issues arose, it was related to a lack of regular contact or an inability to contact the team at key moments (e.g. not informing of cancelled appointments or when having adverse effects with treatments). Some had initial concerns about taking part that were eased due to the study team being informative, not ‘pushy’ and coming across as trustworthy.
When I went to the meeting in the hospital with a lady from THESEUS, I did say that it wouldn’t affect what I get off my dermatologist and everything, I was a bit worried that they’d stop the injections and I’d have to do what you tell me to do. […] Because I asked her on the day, and she said I’d still have my appointments with my dermatologist every month and nothing would change.
(Participant 3, female, white)
Generally, it was reported that the written communication given by the study team was clear, although many could not remember the information in detail or were sure of the purpose of the research at the time of interview. It was often remarked that it was a lot of information to read, and some found it overwhelming. For some, it was made harder to read it all as they felt they had to read it quickly within an appointment or in a busy waiting room. Some reported preferring not to read all the information.
It was okay, I think it was a bit difficult just because we were in a waiting room reading it and stuff. It was quite busy trying to then look out for being called back in. So, I think that was probably a bit difficult, because there was so much paperwork to go through and there was quite a lot of ticking boxes. Which I don’t mind, but I guess it’s just there was quite a lot of a paperwork to take in and read through.
(Participant 7, female, Asian British)
Attending appointments and accessing treatments
One of the main challenges reported was delay in treatment appointments. The impact seemed greatest when it was a long delay, lack of communication about the delay, cancellation was last minute or their condition was worsening. Delays were attributed to the COVID-19 pandemic to some extent. The pandemic also meant that some participants could not or did not want to attend appointments at the hospital at certain times, could not have visitors with them at appointments or patient transport altered as could only take one patient at a time. How far away people lived in relation to the hospital was also a factor in how easy it was for them to take part. People reported valuing having flexibility in appointment times.
Remote appointments
There were a range of experiences of having all appointments face to face, all on the telephone or a mixture of both and it was not always clear if participants referred to THESEUS study or general healthcare appointments. Recruiting sites played a role in how appointments were delivered, with participants from at least two sites only being offered the face-to-face option.
Perceptions of the usefulness of remote appointments varied between participants. Some people valued that they did not need to travel if they were only going to be answering questions. Some also reported it as a more personal touch than the alternative of answering questions online or on a paper form.
I suppose because a lot of it is more convenient now anyway because of the whole telephone conversations in the sense that I wouldn’t have to go somewhere, you know what I mean? Like I wouldn’t have to drive to like a university to sit down and have this talk or something, so because a lot of it can be done over the phone I was pretty happy to take part.
(Participant 29, female, ethnicity not reported)
Others reported concerns around examining the HS as photographs could not fully capture the lesions or they could not explain the HS fully using language.
I found them a little bit different from the face to face. You know when they are face to face they can see me and look at my skin condition but on the phone sometimes I find I have a language problem if I am speaking to my English doctor, then I have a language problem about explaining my… Well when they look at me they know what they are looking at, but that’s why I found it a little bit different, the phone and seeing the doctor.
(Participant 28, female, Asian/Asian British)
Remote appointments could also present logistical challenges including landline and broadband access, ringing a family member rather than patient’s number, participant expecting a telephone call while the team were expecting them to attend the clinic. Some spoke about uncertainty about whether their upcoming appointment would be face to face or not.
The COVID-19 pandemic had an impact on people’s experience and perception relating to remote appointments. Some were nervous to attend the hospital, particularly if they had bad experiences of COVID-19 infections or were concerned about their risk with other health factors. Some were concerned about being a burden on the healthcare system and were unsure if they were able to see a doctor. Some had face-to-face appointments cancelled or rearranged because of COVID-19.
It would mean I would have to get a train down and with the current situation with COVID I said that I’m not happy with doing that after being as ill as I was with COVID. I don’t want to put myself at any risk. So, they agreed to a telephone consultation, however the doctor didn’t call it was someone else from her team that called asking why I wasn’t in clinic. I explained, so that was probably just down to communication and disorganisation really.
(Participant 7, female, Asian British)
Photographs of hidradenitis suppurativa
Photographs were not a part of THESEUS study procedures, and not everyone had experienced taking photos of their HS, but some had them taken by medical photographers when they had treatments and others had taken them themselves for their own use or to share with HCPs.
Some people had a strong emotional reaction to the idea of photos of their HS, with it mostly being described by individuals as being embarrassing. One person described the idea of taking photographs of their HS as being ‘disgusting’. Other participants were less bothered by the idea, and willing to do it if necessary. One person described it as being just an armpit. Photos were particularly viewed as embarrassing or inappropriate when it was photos of the groin area.
For those who were embarrassed, there was only one person who preferred to take and send a photo themselves, whereas most people were concerned about the security of this. Concerns were around the permanency of the photos and the potential for error in sending them online.
Right, I’m going to be honest, some are on my groin and my bottom, my vagina, they are everywhere right. […] It’s like that’s there forever they can look back on that. I know that sounds funny, but it’s just a permanent record of it. I really wouldn’t feel comfortable. I’ve taken a picture of the ones under my arms and that was fine.
(Participant 15, female, white)
Photos were seen to have benefits in self-tracking, such as showing to your doctor or seeing the physical changes over time. One person described photos not being a full picture of their HS due to the need to feel the skin as the HS was not visible on the surface of the skin. Some people talked about the challenges of taking photos themselves. One person described not capturing all the lesions, just the main ones they could see. Some people had family members who could take some of the photos for them, but others did not.
It depends really, so yes. I think when I tell you guys I’ve got about 15–20 lesions or whatever you call them. They are in a mix place, mixing where I can take photos of them technically. But wherever they are, when they are there, I don’t know even know half the time. Like yesterday a new one came up and another one disappeared. So, taking photos of them, when I have to show it and all that, I actually just show the main ones, which is pretty easy for me to take photos of.
(Participant 19, male, Asian/Asian British)
Measurement of research outcomes
Daily texts
Communication about when will receive
People reported that the THESEUS study’s daily text messages stopped without warning. This made people worry that the study had ended, or that there were problems with the study. It was suggested that people were informed when to expect the texts to end. One person had expected the texts to start when the study began, but (most likely because they had not had their treatment yet) the texts had not started. One person reported texts stopped for a short while (approx. 1 week) and restarted and they did not know why this had occurred.
I just got used to doing it for so long but it just stopped. There was nothing to say that, I wasn’t told how long I would have to do it for or when it would stop or anything like that, it just stopped. So I felt a bit worried that the whole study had stopped kind of thing.
(Participant 27, female, white)
Benefits of completing
Texts were quick and simple to complete. People became accustomed to filling out the daily texts and noted they arrived at 6 p.m. daily, and some missed it when it was gone. Tracking pain was beneficial to understand changes over time. Some were reassured that someone was checking in.
Fine nothing major, I’ve been getting texts every day to see how my pain level is. It’s made me realise how hard going it’s been. You just kind of go along and do things, because the first time the doctor asked me I was like, I’m not sure. How to you score that because you just live with it.
(Participant 15, female, white)
Difficulties completing
Some people forgot to complete the texts sometimes. Responding straightaway was a strategy people used to remember to send them. Some people going through a difficult period in their life, one for non-HS related reasons and another with surgery recovery issues, meant that they stopped completing the texts or found them frustrating as a reminder of the pain. Some were unsure if their responses were helpful as they were continually scoring a ‘0’ or ‘1’ (‘0’ means no pain and ‘10’ means pain as severe as it could be). There was uncertainty in responding if it is about pain in general or HS-specific pain, and how to respond if pain had changed throughout the day.
I was literally giving them the same score, because after 3 days of the tablets it had all cleared up, so I was texting her number one every day. I thought well there’s no point in this, so I just asked them to stop them.
(Participant 5, female, white)
Clinic questionnaires
Content
Questions were personal or sensitive in content (e.g. sex life, relationships), and while difficult for some people, generally it was considered appropriate and relevant for the study. Some complaints were that the questions were boring, common sense, vague or a tick box, so did not capture details they felt were important. One person described the smell being hard to answer because bandages covered the smell. It was not always clear to participants if they should answer questions in relation to the effects of their HS or in relation to their general health, and one person said the study nurse did not know either (same challenge reported for daily texts).
It’s sort of like personal questions relating to like your sex life, say, I think that’s obviously quite a personal thing, but I think it helps people to understand how much it does affect stuff like that, so I don’t think it’s not an unreasonable thing to ask, to enlighten people as to how difficult this can really be.
(Participant 2, female, Asian/Asian British)
Length
Many reported that the questions were lengthy and repetitive. This could be frustrating if people did not understand why they were being asked the questions.
I remember the questions just being pretty boring and – So, [unclear 00:26:14] I don’t mind and I think it’s also me remembering things which was basically a bit trickier. But then they were just asking how many flare ups I have, where and kind of repeated itself as well. And I was like this isn’t helping me much.
(Participant 19, male, Asian/Asian British)
Timing
There were concerns that questionnaires might not be capturing important changes in the HS. Some people described feeling the timeframe that the questions asked about were not appropriate (i.e. too short or averaging over a period of variability missing the ups and downs) and other people felt the questionnaires were not asked frequently enough. Some were concerned about wasting time if their answers were low/unchanged.
I don’t really feel like you guys will be able to gather all the data relating to my condition as well as you could, because I only had two separate times, and that was before my surgery, and after my surgery, I feel like if they were done more frequently, you’d get a better understanding of what it’s like to live with long-term, if that makes sense?
(Participant 2, female, Asian/Asian British)
Role of study team
Some filled out questionnaires themselves, but other people talked about experiences of filling out questionnaires via a conversation with a healthcare provider, and there were comments that this showed care and also felt less burdensome.
Fine, I have not really had to do anything, this is the first thing. There was a few forms to fill out in the beginning which I didn’t really have to do, my dermatologist did them with me sat there and I just answered the questions and she clicked the buttons, that was nice and easy.
(Participant 32, female, black/African/Caribbean/Black British)
Randomisation in a future trial
Participants can be broadly categorised as: (1) not willing to be randomised; (2) willing to be randomised under certain conditions; and (3) willing to be randomised. a
People adverse to randomisation tended to believe this is because everyone’s HS is too different. They do not want a trial-and-error approach to their treatment, and instead want treatment that is chosen because it is the best fit for them. ‘Random’ was viewed to be the opposite of a treatment choice that is carefully selected, not been tried already and likely to work. Linked to the belief that treatment needs to be tailored, random was also viewed as being at odds with the view that HS is a complicated disease where treatments needed to be carefully considered.
But a randomisation won’t give me that, right? Because randomisation would be random, not what’s best and what I haven’t done and what could possibly work.
(Participant 19, male, Asian/Asian British)
There were some people who expressed concerns about randomisation but would take part if certain conditions were met. These included if they thought all options within the study could be beneficial for them, were not harmful for them (e.g. safe if they were to get pregnant or did not interfere with their other health conditions) or were all new options for them to try.
Some said they may enter the study but be disappointed about not getting the treatment they had hoped for. Some people expressed that they would need to feel they could drop out if they were not happy with their option or their option was not working for them.
Mmm, I think I would probably be a bit more wary doing that. Just because I wouldn’t want to jump to surgery or something if it wasn’t necessary. But if it was different things like maybe different topical things or antibiotic or things like that, I would be quite happy to try it or the laser treatment. But if it was maybe just jumping to surgery or something, I don’t know if I would be inclined to do that. […] If there was that other one and it was different options, as long as there was an option to decline and say- so if I got matched with a treatment I wasn’t comfortable with, I could decline it. That would be fine.
(Participant 24, female, white)
One person did suggest that although they had expressed willingness to have certain treatments within the THESEUS study, in reality they would have dropped out if they had been asked to take some of those options.
It depends like, for me I would say no because although I said I would consider the laser, sorry the deroofing I knew in the back of my head as soon as I had the other option to the other one there is no way I would do the other. So, obviously some other people might be different and think, oh yeah I am not actually fussed either or but I know for a fact I was fussed that’s why I made the choice to have this one and not the others.
(Participant 35, male, ethnicity not reported)
Some people felt that trial and error is needed, and part of the process, and were therefore willing to take part in a randomised study to access help and options to improve their condition, even if this meant not receiving their preferred option. This was often expressed as a ‘willingness to try anything’ to get rid of HS symptoms. Some people also expressed a desire to support research that would seek answers that themselves and others with HS are looking for.
Like I said earlier I’d will be willing to try anything and if it does help. If I try something and it doesn’t work, then at least you know. It’s just getting things out there again isn’t it. It’s trying to find the best treatment. If you don’t have people willing to participate then how are you supposed to get the research and the answers that we are all kind of looking for.
(Participant 16, female, white)
Staff perspectives on research experiences
The study procedures were often reported as straightforward, but there were some complaints about the database and the time-consuming, repetitive nature of the case report forms. Timely communication with study co-ordinating centre was reported, although a few unanswered queries raised during interviews. It was also noted by one participant that keeping communication to ‘essential’ was helpful. There was a strain on individual staff members time, and a research nurse or clinical fellow who can do a large amount of the study tasks as opposed to the consultants were seen as a good set-up for NHS research. It was clear that there were passionate individuals that made THESEUS happen in a challenging climate (including COVID-19 disruption, waiting list backlogs and busy staff schedules).
Basically there’s not really enough time or money to do all the things that we want to do, and that includes research, so the more that can be given to the dedicated research nurses, the better, so then the smoother it is, the more flexibility there is for patient visits, all that kind of thing.
(Staff member 2)
Staff were generally supportive of future research, and felt that it was necessary to meet needs of HS patients. There is a desire for more licensed treatment options so that more options can be offered to patients. There was a recognition that HS treatment may need to be different for subgroups/individuals.
Some of the next steps for future trials suggested were head-to-head biologicals, deroofing alongside biologicals compared with biologicals alone, long-term use of antibiotics compared with earlier introduction of biologicsal, dapsone, further evidence for laser or deroofing if they show promise. Aspects of deroofing research mentioned were use of general anaesthetic, wound care approaches and comparisons with conventional surgery. There was also a need for evidence around lifestyle factors and psychological support. The cost of interventions as well as their effectiveness was noted as important for NHS commissioning.
Randomised studies were considered more challenging for recruitment, particularly for surgical treatments, but experience of recruiting for randomised studies, thoughtful design (i.e. short wait if taking placebo or standard therapy) and the right combination of treatments included, meant staff members felt it could be feasible. Large long-term observational studies were also suggested.
The HS community was seen as a relatively young and motivated group that is interested in participating in research, but as many are of working age, work commitments were also noted as a challenge. There was also a need to be flexible to meet different patient needs, as it is a population that might be requiring extra support, especially during flare-ups and travel distances for care. There was also a concern about ensuring equitable and fair access to take part in research across different locations rather than just specialist centres.
Discussion
This nested qualitative study interviewed a selection of THESEUS participants and staff with a primary aim to inform the design of future HS trials. How treatment choices were made, experiences of treatments and experiences of research processes have been explored. This insight has helped us generate some key learning points for future clinical trials in HS (see Box 1).
-
Previous experience of treatments might be a bigger driver for treatment choice than disease severity.
-
Trials will need to present treatment options as being comparable treatments for the target population, rather than in a hierarchical fashion, as was used in the THESEUS trial, to better reflect clinical equipoise. Guidance for staff to explain the treatments comparably and explain randomisation would be helpful.
-
Depending on the treatments being compared, blinding of participants or HCPs to the treatment allocation might not be possible due to differences in treatment experiences and adverse effects.
-
Treatment experiences from THESEUS can be shared in patient materials for future clinical trials to help better prepare participants.
-
Studies should be flexible to site and participant requirements where possible. Participants should be given a choice of face-to-face or remote appointments where possible and consider how to minimise impact on study/work commitments of participants. Funding for dedicated research nurse/study staff and integration into sites’ current practices would also minimise burden for the site.
-
Not all participants felt comfortable in sending photos for remote appointments, particularly for HS affecting the groin and genital regions. If a future trial incorporated photos, the security of image transfer and storage would need to be carefully communicated to participants.
-
Participants should have a warning of when and for how long they will be completing research outcome measures, in particular when text messages will end and the timing of clinic visits.
-
Participants should be given an explanation of how the OMIs will be used and why they are completing them. For example, it would be helpful to explain why it is important to complete the questions even if responses have not changed since the last measurement.
-
Participants should be given clear guidance if they are answering questions specifically about HS or in general (e.g. pain from HS or pain in general).
-
Participants valued the ‘personal touch’ associated with study participation, particularly interactions with the study team.
The findings of the qualitative study are broadly consistent with the cohort study findings (see Chapter 3) and provide the underlying reasons. The cohort study found laser was the treatment most patients were willing to have (79%). The qualitative findings explain that there was a combination of push (reasons for not wanting the other treatments available) and pull (wanting to try new treatment that might prevent future flare-ups) factors. The cohort study highlighted that individuals might not take doxycycline or clindamycin and rifampicin because they had taken before and found them to be ineffective (27% doxycycline, 20% clindamycin and rifampicin) or had adverse effects (10% doxycycline, 9% clindamycin and rifampicin). This was largely reflected in the qualitative findings with participants having issues with these treatments previously not being keen to try them again. The cohort study also found that the most common reason for final treatment choice was based on clinician recommendation. This mirrors how in the qualitative study treatment choices were often influenced by study interactions, both via the materials and information or recommendations from the doctor.
The participant interviews offer additional insights into participant willingness to receive the THESEUS interventions. The cohort study found that patient’s first-ranked treatment preference was often the same as their final treatment choice (70–92% across the treatment groups). The interviews provide insight that when providing their willingness to receive the interventions, participants could be taking into account their doctor’s recommendation rather than basing their answer solely on their personal preference.
The cohort study found that there were some challenges with the daily text messages in terms of low uptake (100/151) and low completion rates. The qualitative study provides explanatory insights. Some people reported not being sure if it was appropriate to keep responding if their result had not changed, and others talked about struggling to remember to reply if they were busy when the text arrived. However, there was a subgroup of individuals who enjoyed the texts and found they could adapt them easily into their routine, which is also mirrored in the quantitative findings.
Comparison to other literature
Other studies have looked at the impact of HS, but none have been primarily focused on HS treatments in the way that THESEUS has been. To summarise what the qualitative literature already tells us about HS treatment, our previously conducted systematic review found studies that described the experiences of seeking treatment, learning to manage the condition, concerns about treatment and the burden of treatment. 34 The concerns and burdens of treatment from previous literature were broadly reflected in results from THESEUS, including concerns about adverse effects from medications, and THESEUS highlights participant concerns about long-term effects of medications. 22,37,38 Previous studies also highlight concerns about effectiveness and treatments not working, which was mirrored in THESEUS. 22,26,37,39–42 The burden on time taken to apply treatments and wound dressings and self-care was also reflected in THESEUS. Previous work highlighted issues connected to understanding there is no cure for HS and accepting the chronic nature of the condition. 35,40,42,43 Perceptions of chronicity and curability seem likely to have influenced treatment choices within THESEUS. For example, some individuals reported not wanting further antibiotics as that was not seen as a long-term solution.
Strengths and limitations
The purposeful sampling approach ensured we spoke to participants across a wide range of participant characteristics, reflective of the overall THESEUS participant demographics. An MDT approach to analysis in which the perspectives of patients, HCPs, clinical trialists, psychologists and qualitative researchers are incorporated helps to ensure the output is meaningful for future trials and clinical practice. A limitation was the timing of the interviews. Not all had received their treatment at the time of the interview due to COVID-19 related delays, and so only limited information could be gathered about experiences of some treatments (particularly conventional surgery). Some of the content discussed could also be hard for people to recall (e.g. experiences of baseline measurements and recruitment were often a few months ago, which may have produced recall bias). More depth of understanding could have been obtained by conducting longer interviews with fewer subjects; however, we chose purposively to sample from across a range of demographics and treatments to inform future HS trials. Another limitation was that the information available for each treatment was not consistent across the treatment arms. For example, a video of the deroofing procedure was made available for participants to view, but this was not available for other interventions. Based on the interview findings suggesting study materials influenced treatment choice, future RCTs should ensure that information is equally available for all study treatments.
Conclusion
This qualitative study provides some key learning for how future HS clinical trials should be conducted to optimise patient experience and trial design.
Chapter 5 Characterising surgical and laser procedures
Introduction
The THESEUS study provided treatment protocols for laser, deroofing and conventional surgery treatments within the study. In line with the IDEAL 2b framework, this cohort study provided a setting to explore which aspects of these treatments could be standardised for future RCTs. 44
Aims
The aim was to characterise current conventional surgical procedures and document best practice for laser and deroofing interventions.
Methods
Data collection on procedures
Treatment protocols produced for THESEUS treatments can be found in Appendix 3.
Site staff undertaking procedures were asked to complete a proforma that described aspects of their procedure. These included before, during and after operative procedures in line with the protocol.
Analysis was conducted in STATA. Analysis was exploratory in nature and provided descriptive summaries of the procedures undertook as part of THESEUS.
Data fidelity
The analysis raised several data cleaning challenges that we were not always able to check with sites ahead of this report due to time constraints. Decisions made by the team for how to handle these data are outlined in Appendix 4 and the data should be interpreted with some caution where assumptions or rules have been created for handling the data. As THESEUS is predominantly a feasibility study, the data collection challenges also offer helpful information to inform how surgical procedure data are collected in any future studies.
Creation of training videos for future studies
It was originally conceived that a proportion of THESEUS procedures would be videoed, which would allow us to identify areas of best practice to create training videos. However, it was identified that a training video for deroofing was required for sites within the THESEUS study, so the deroofing training video was created ahead of the study procedures and the HCP version has been viewed more than 1 million times (https://www.cardiff.ac.uk/centre-for-trials-research/research/studies-and-trials/view/theseus). There were challenges with some sites producing videos of laser procedures due to safety concerns, but plans are under way to produce a laser training video.
Results
Laser
A total of 196 laser procedures were analysed across 56 participants. The number of laser treatments per participant is shown in Figure 6. The protocol stated that individuals should receive at least four sessions but procedure numbers for participants varied from one to nine within the data set.
FIGURE 6.
Number of laser treatments per participant.
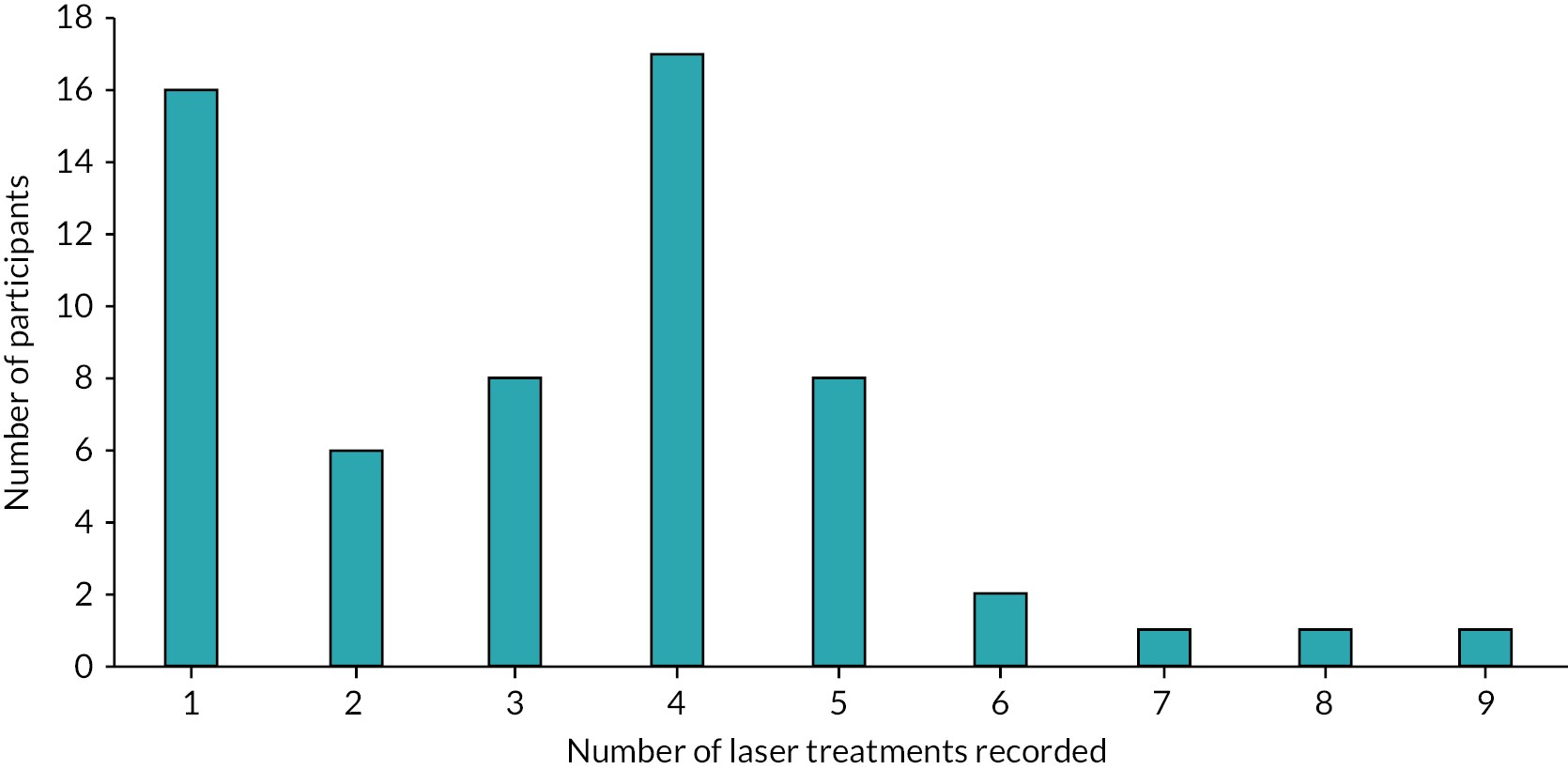
The treatment protocol stated that follow-up laser procedures should be conducted within 28 days since the last laser treatment unless there is a reason relating to patient fitness or clinical discretion not to do so. The data returned suggested that follow-up procedures were not always conducted within the 28-day window. Reasons reported were often attributed to participant choice or ‘other’ reason (COVID-19 related, feeling unwell, appointment times and availability, life commitments or holidays, not attending or cancelling appointments or in one case was an extra treatment). In only one case, it was attributed to clinical advice, as the patient had COVID-19 at the last appointment.
A total of 74 (37.76%) procedures were reported as involving treatment fields being marked and photographer prior to the treatment session; 114 (58.16%) did not mark and photograph treatment fields and 8 (4.08%) were missing.
Table 23 shows which areas of body were treated, with axilla and groin being the most commonly treated areas. ‘Other’ was mostly reported within abdomen, pubic, legs and jaw areas.
| Area of body | Treated, n (%) | Not treated, n (%) | Missing, n (%) |
|---|---|---|---|
| Axilla (right) | 121 (61.73) | 74 (37.76) | 1 (0.51) |
| Axilla (left) | 117 (59.69) | 78 (39.8) | 1 (0.51) |
| Groin (right) | 140 (71.43) | 54 (27.55) | 2 (1.02) |
| Groin (left) | 142 (72.45) | 52 (26.53) | 2 (1.02) |
| Perineum | 18 (9.18) | 174 (88.78) | 4 (2.04) |
| Buttocks (right) | 12 (6.12) | 179 (91.33) | 5 (2.55) |
| Buttocks (left) | 12 (6.12) | 179 (91.33) | 5 (2.55) |
| Chest (right) | 7 (3.57) | 184 (93.88) | 5 (2.55) |
| Chest (left) | 9 (4.59) | 182 (92.86) | 5 (2.55) |
| Other | 70 (35.71) | 122 (62.24) | 4 (2.04) |
Analgesia options used are reported in Table 24. The open-text responses of ‘other’ indicate that for 138 no analgesia was used, as per the treatment protocol, but the data collection form had not provided a response category of ‘none’. The other category also included open-text entries such as gels, ice, cold sprays and so on, and these adjuncts provide both skin cooling and a degree of analgesia. Skin cooling methods are also reported in Table 24. Ice, cold sprays and other methods were reported. Open-text responses revealed that some were using cooling methods built into the laser machine (e.g. air cooler – Cryo 6, Zimmer MedizinSystems, Irvine, CA, USA). Type of laser used is also presented in Table 24, with alexandrite being the most common laser used. Intense pulsed light (IPL) was used for one-third of treatments, which provides hair removal but is not itself a laser treatment and was not originally intended via the THESEUS study. In one case, as well as receiving hair removal laser treatment, a participant also received pulsed dye laser and CO2 laser which do not provide targeted hair removal.
| N (%) | |
|---|---|
| Analgesia options | |
| Topical analgesia | 5 (2.55) |
| Oral analgesia | 1 (0.51) |
| Local anaesthetic | 8 (4.08) |
| Topical analgesia and other | 1 (0.51) |
| Missing | 3 (1.53) |
| Other | 178 (90.82) |
| Skin cooling methods used | |
| Yes | 183 (93.37) |
| No | 2 (1.02) |
| Missing | 11 (5.61) |
| Type of laser used | |
| Alexandrite | 87 (44.39) |
| Nd:YAG | 28 (14.29) |
| Alexandrite and Nd:YAG (Elite) | 10 (5.10) |
| Other, types specified below | |
| IPL | 70 (35.71) |
| Pulsed dye laser on left breast and left groin only. CO2 laser left axilla only. | 1 (0.51) |
Table 25 shows the number of background and lesion pulses reported for each body area treated. Owing to database errors, missing data and not applicable (i.e. body area not treated) data were not always correctly coded and so have been combined.
| Background | Lesions | |||||||
|---|---|---|---|---|---|---|---|---|
| Single, n (%) | Double, n (%) | Triple, n (%) | Missing data or not applicable n (%) | Single, n (%) | Double, n (%) | Triple, n (%) | Missing data or not applicable n (%) | |
| Axilla (right) | 45 (22.96) | 43 (21.94) | 8 (4.08) | 100 (51.02) | 43 (21.94) | 41 (20.92) | 8 (4.08) | 104 (53.06) |
| Axilla (left) | 47 (23.98) | 37 (18.88) | 7 (3.57) | 105 (53.57) | 44 (22.45) | 34 (17.35) | 7 (3.57) | 111 (56.63) |
| Groin (right) | 60 (30.61) | 44 (22.45) | 9 (4.59) | 83 (42.35) | 57 (29.08) | 43 (21.94) | 9 (4.59) | 87 (44.39) |
| Groin (left) | 59 (30.10) | 46 (23.47) | 10 (5.10) | 81 (41.33) | 56 (28.57) | 43 (21.94) | 10 (5.10) | 87 (44.39) |
| Perineum | 11 (5.61) | 3 (1.53) | 4 (2.04) | 178 (90.82) | 11 (5.61) | 3 (1.53) | 4 (2.04) | 178 (90.82) |
| Buttocks (right) | 8 (4.08) | 1 (0.51) | - | 187 (95.41) | 8 (4.08) | - | - | 188 (95.92) |
| Buttocks (left) | 8 (4.08) | - | - | 188 (95.92) | 8 (4.08) | - | - | 188 (95.92) |
| Chest (right) | 3 (1.53) | - | - | 193 (98.47) | 3 (1.53) | - | - | 193 (98.47) |
| Chest (left) | 4 (2.04) | - | - | 192 (97.96) | 3 (1.53) | - | - | 193 (98.47) |
| Other | 20 (10.20) | 25 (12.76) | 5 (2.55) | 146 (74.49) | 19 (9.69) | 23 (11.73) | 5 (2.55) | 149 (76.02) |
Deroofing
A total of 41 deroofing procedures were included in the analysis. There were 30 participants included, but some had more than one procedure. Table 26 shows which area of the body was treated, with axilla and groin being the most commonly treated areas.
| Area of body treated | n (%) |
|---|---|
| Axilla (right) | 8 (19.51) |
| Axilla (left) | 12 (29.27) |
| Groin (right) | 7 (17.07) |
| Groin (left) | 6 (14.63) |
| Buttocks (right) | 1 (2.44) |
| Chest (right) | 1 (2.44) |
| Chest (left) | 1 (2.44) |
| Other – lower abdomen (left) | 1 (2.44) |
| Other – left thigh | 1 (2.44) |
| Other – mons pubis | 1 (2.44) |
| Other – posterior neck | 2 (4.88) |
The preparations for deroofing are described in Table 27.
| N (%) | |
|---|---|
| How was skin prepared for deroofing | |
| Alcohol-based solution of chlorhexidine | 12 (29.27) |
| Aqueous solution of chlorhexidine | 27 (65.85) |
| Aqueous solution of povidone-iodine | 1 (2.44) |
| Other – Aqueous Tisept | 1 (2.44) |
| Hair removal perioperatively | |
| Removed using clippers | 1 (2.44) |
| Hair not removed | 39 (95.12) |
| Missing | 1 (2.44) |
Figure 7 shows the number of skin tunnels present in the region undergoing treatment mapped next to the number of skin tunnels reported as successfully explored with a blunt probe across procedures.
FIGURE 7.
Number of skin tunnels in regions undergoing treatments.
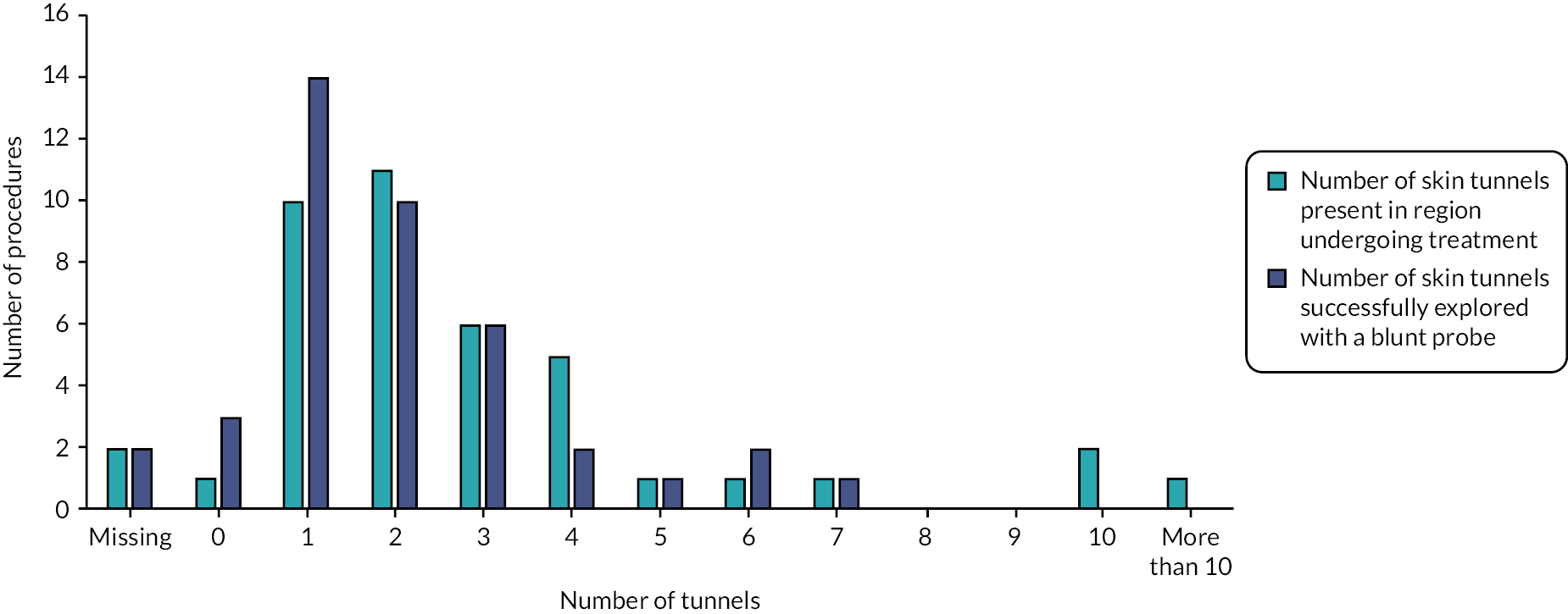
Table 28 describes what instruments were used for incision and Table 29 describes incision and dissection procedures.
| Instrument used for incision | N (%) |
|---|---|
| Loop tip diathermy | 12 (29.27) |
| Spatula tip diathermy | 3 (7.32) |
| Needle tip diathermy | 21 (51.22) |
| Other – both loop tip and needle tip diathermy | 4 (9.76) |
| Other – blade | 1 (2.44) |
| Yes, n (%) | No, n (%) | Missing, n (%) | |
|---|---|---|---|
| Incision extended into subcutaneous tissue within or around lesion | 15 (36.59) | 25 (60.98) | 1 (2.44) |
| Subcutaneous tissues and deeper tissues dissected | 8 (19.51) | 32 (78.05) | 1 (2.44) |
| Hyfrecation of base of skin tunnel performed | 35 (85.37) | 5 (12.20) | 1 (2.44) |
| Removal of a margin of normal tissue undertaken | 11 (26.83) | 29 (70.73) | 1 (2.44) |
The majority used monopolar diathermy (39/41), but one reported bipolar diathermy (1/41). Wounds were mostly left to heal by secondary intention (40/41), but one reported using sutures. Table 30 describes the type of wound dressings that were used.
| Yes | No | |
|---|---|---|
| Non-adherent dressing applied to wound | 20 (48.78) | 21 (51.22) |
| Aliginate/hydrofibre dressing | 26 (63.41) | 15 (36.59) |
| Foam dressing | 1 (2.44) | 40 (97.56) |
| Hydrocolloid dressing | 3 (7.32) | 38 (92.68) |
| Negative pressure wound therapy | - | 41 (100) |
| Othera | 7 (17.07) | 34 (82.93) |
| Drain inserted into the excised wound or donor site | - | 41 (100) |
Adaptions were reported in six procedures. A description of the adaptions made is described below:
-
On two occasions, a disposable diathermy loop was used instead of reusable as this was equipment available at the site. The disposable loop stopped working during procedure, so it was completed with needle tip diathermy.
-
On two occasions, reduced diathermy settings for hyfrecation of base was used; the diathermy tip broke during the procedure so the operation was completed with a scalpel.
-
On two occasions, a hyfrecator was used rather than diathermy.
The duration of procedures is illustrated in Figure 8 and operator confidence that skin tunnels were fully treated in affected region is reported in Figure 9.
FIGURE 8.
Duration of deroofing procedures.
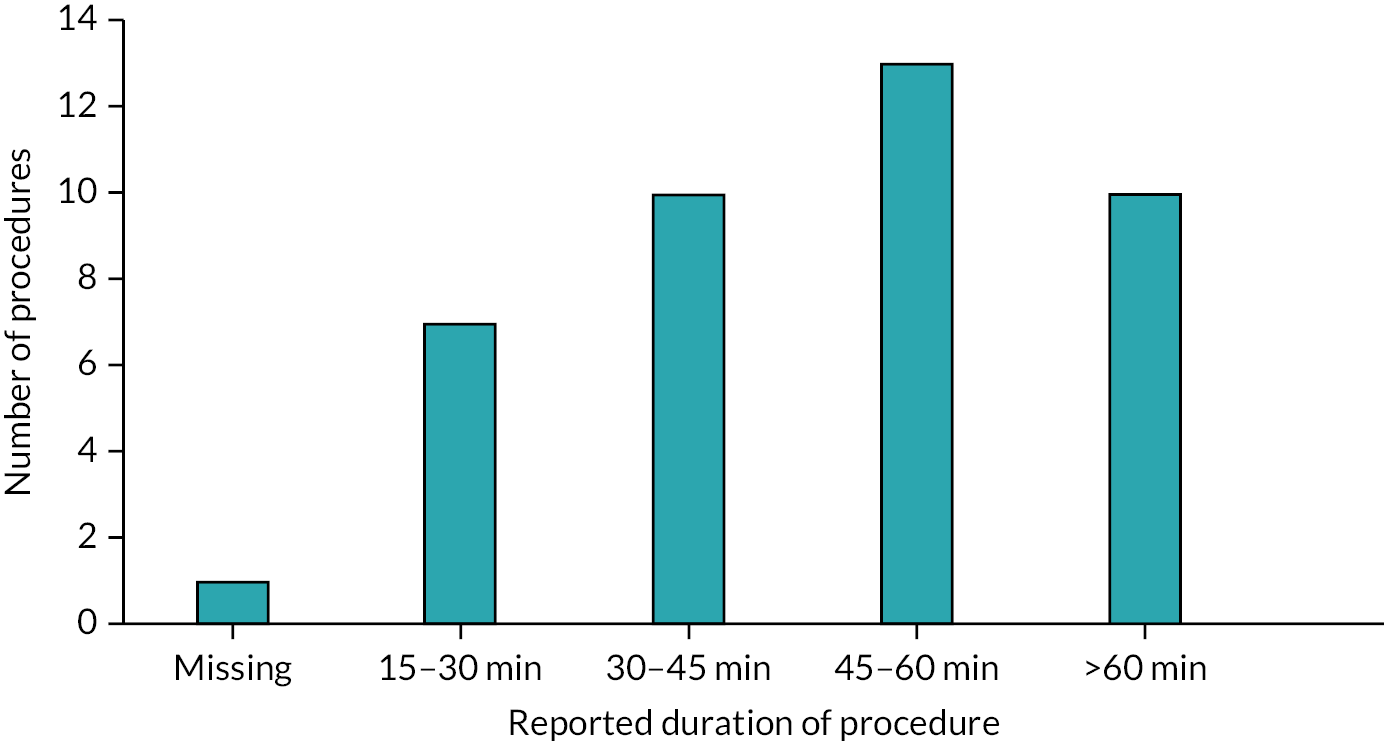
FIGURE 9.
Confidence that tunnels were fully treated.
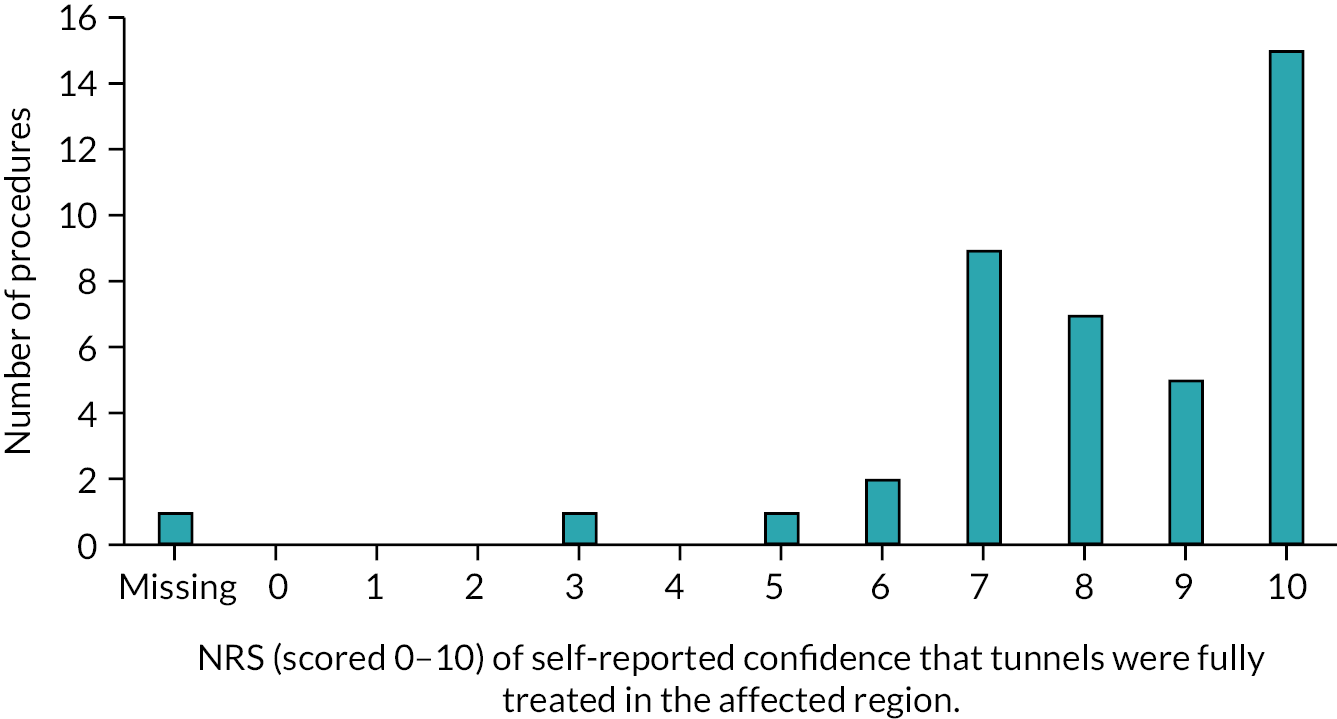
Key learning for future studies
While we seek to document best practice in deroofing and laser treatment, there are some elements of the procedures where best practice remains unknown, and the challenge for future studies will be to decide where trials should be more explanatory in nature, requiring specific protocols to be adhered to, compared with a more pragmatic approach permitting variation in practice that reflects the study sites’ preferences. Below, we summarise our key learning from the observations within THESEUS.
Laser recommendations for future trials
-
Pragmatic trials might allow for variation in number of laser treatments, whereas explanatory trials might require tighter instructions than THESEUS to reach recommended number of laser treatments. The cost implications of number of laser treatments should be considered in designing the trial.
-
A more flexible time window (e.g. 4–6 weeks) for next treatment might be more appropriate than ‘within 28 days’ used for THESEUS.
-
Photography presents challenges, is probably not essential and could be left as optional for most future studies.
-
Variation in skin cooling methods was observed and could be left for sites to decide.
-
IPL was used in one-third of treatments (n = 71) in deviation from the protocol because, while it targets the hair follicle, it is a light rather than a laser treatment. There was also variation in laser used, reflecting skin type and laser availability. Future studies of a more pragmatic nature might allow variation in laser/light treatment targeting the hair follicle.
-
Lack of standardisation of number of pulses for background and lesions. Future studies could more prominently emphasise standardisation (which, in THESEUS, was one pulse for background and two pulses for lesions).
Deroofing recommendations for future trials
-
Variation in preparations for surgery that could remain flexible, allowing site and patient preference in future studies.
-
Variation in instrument used and challenges using certain equipment point to future trials being pragmatic and allowing flexibility where possible. More explanatory trials might need tighter standardisation.
-
Variations in procedures to be encouraged in future trials depending on patient need. For example, identification of scarring or lesions of different depths may require clinically different approaches.
-
Secondary intention healing is the standardised approach to wound healing, while future trials could permit variation in wound dressings to permit participant and recruiting site choice.
-
We did not collect data on the type of anaesthetic used in THESEUS as it was assumed that the procedure would be carried out under local anaesthetic, but informal feedback was received that some were undertaken using general anaesthetic. Use of general anaesthetic may reflect procedures that were longer in duration or treatment of multiple lesions exceeding local anaesthetic dose limits. Future trials may wish to be pragmatic in allowing different types of anaesthetics dependent on patient and site preferences. Qualitative data also supports that some patients prefer either local or general anaesthetic. A future trial including deroofing as a rescue therapy for acute flares may wish to restrict to local anaesthetic to avoid the need for an operating theatre setting.
Chapter 6 Patient and public involvement
Background
Despite a UK prevalence of HS of at least 1%,4 the current availability of only one licensed therapy demonstrates a relative lack of high-quality clinical trial evidence to inform patient care. Consequently, there is a large unmet need for more HS trials and patients frequently report dissatisfaction with their care. 2 The solution is to design trials that matter to patients, following the priorities highlighted in the James Lind Alliance PSP17 and to coproduce them with HS patient advocates.
Involvement of patients and the public was particularly important for THESEUS because the study’s primary objective was to inform the design of future RCTs, including feasibility of recruitment, based on the NIHR HTA programme funding brief. 45 We wished to include as many facilitators to recruitment as possible, while minimising potential recruitment barriers. The NIHR HTA is a strong supporter of public involvement in research as active contributors and this was mirrored by THESEUS in every aspect, beginning with the grant application. 45 PPI in THESEUS was informed by the principles of the Public Involvement Impact Assessment Framework46 and the NIHR’s Involve Patients guidance. 47
Aims
The aim of this chapter is to report the impact that PPI had in the planning, design, delivery and dissemination of THESEUS.
Study design and management
The THESEUS grant proposal and study design were informed by an online survey completed by 358 HS patients and carers, providing responses within a short window of a few weeks. Four PPI representatives took part in the discussion from which the detailed study design took shape and the president of the HS Trust UK advocacy organisation was a co-applicant for the grant. 45
Patients wished to have a choice of interventions in THESEUS and to receive information about each of them, even if they may not be eligible for a particular intervention, which led to creation of a ‘decision grid’ participant facing document for THESEUS (see later in this chapter). In keeping with the results of the PSP, they requested that both medical and surgical options should be included as interventions. Based on specific PPI feedback, chlorhexidine 4% solution as a daily shower wash was removed as a cotreatment from the doxycycline intervention arm to avoid perpetuating the misconception that HS is a condition linked to poor personal hygiene. This decision was also in keeping with a lack of evidence to support use of topical antiseptics in HS. 10 Recruitment strategies for THESEUS were also altered following input from PPI partners, who recommended that potential participants should not be identified from GP records because direct contact in this manner would be too intrusive.
The choice of OMIs included in THESEUS was strongly influenced by PPI representatives in THESEUS and the HiSTORIC. It is noteworthy that nearly 50% of HiSTORIC’s membership is composed of patients and their carers. All the patient reported OMIs agreed by HiSTORIC were included in THESEUS, including pain NRS, HiSQOL, PtGA and drainage NRS, to contribute to their validation and comprehensively capture patient-reported outcome efficacy data.
The COVID-19 pandemic led to THESEUS switching to remote follow-up for some participants for whom face-to-face assessment was not possible at the time. Three options for lesion assessments were considered: (1) video consultation; (2) telephone consultation supported by photographs sent by secure e-mail; and (3) telephone consultation supported by a patient count of their lesions. The THESEUS PPI representatives made the decision that flexibility was most important and that THESEUS should offer all three options and record the method used and the reasons for the choice. They anticipated that participants in the study may have data storage security concerns regarding the use of video consultations.
Both the THESEUS SMG and the combined independent data monitoring and study steering committee benefitted from inclusion of PPI representation (Figure 10). The SMG meetings were held at lunchtime to enable the two patient representatives to attend, and the PPI agenda item was scheduled first to allow patients to return to work. During the recruitment phase of THESEUS when less frequent PPI input was needed, PPI issues were stored up and discussed at a patient-focused SMG meeting once every 3 months.
FIGURE 10.
Photo from study kick-off meeting.

Input from PPI partners was also facilitated by the UK Dermatology Clinical Trials Network, which supported THESEUS and has an extensive track record in nurturing PPI involvement.
Participant-facing materials
The PPI representatives on the SMG committee reviewed all the participant-facing documentation used in THESEUS. One specific change requested was to add a section covering pregnancy and breastfeeding to the participant information sheet.
Creation of a decision grid covering each of the THESEUS interventions was encouraged by PPI representatives (Table 31). They recommended that the order of the interventions in the first draft of the grid should be changed to follow the order in which they were likely to be used in clinical practice.
| What does it involve? | Is the treatment suitable for me? | Potential benefits of treatment | Possible adverse effects/problems | When can I start treatment? | For how long will I be on the treatment? | |
|---|---|---|---|---|---|---|
| Doxycycline | Taking 2 doxycycline capsule by mouth each morning | Designed for multiple affected regions; usually the first tablet treatment considered | Reduction in inflammation (pus, pain); no effect on scarring | Stomach upset such as pain and loose motions; a few people may be more sensitive to sunlight | Immediately | 6 months |
| Clindamycin and rifampicin | Taking 2 clindamycin and 1 rifampicin capsule by mouth each morning and evening; a blood test is needed before starting treatment and after 4 weeks | Designed for multiple affected regions | Reduction in inflammation (pus, pain); no effect on scarring | Orange urine and tears; stomach upset such as pain and diarrhoea; increase in liver blood tests, interference with some hormonal contraception and other medications – your doctor will advise | Immediately | 10 weeks initially, with the option to continue or restart |
| Laser | A laser will be passed across affected areas of skin; you will wear protective eyewear during the procedure; pain relief can be taken before treatment | For 1 or a few active skin regions; choice of laser depends on your skin pigmentation (a suitable laser may not be available at your centre) | Reduction in inflammation (pus, pain) in the short term and possibly longer | Local skin pain during procedure and for a few days afterwards; permanent change in skin colour (lighter or darker) may occur; there is a small chance of scarring or infection | Depends on waiting list at your centre | At least 4 treatments will be given, each 1 month apart |
| Deroofing | After a pain killing local anaesthetic injection, the roofs of individual skin tunnels are removed, the wound base heals naturally, covered by a dressing | For individual skin tunnels and areas of scarring | Aims to stop discharge of pus from areas of scarring that often flare in the same place | Local skin pain during procedure and for a few days afterwards; localised scarring, wound infection; duration of wound healing depends on area of skin removed and can take several weeks with daily dressing changes; new lesions are not prevented | Depends on waiting list at your centre | Usually 1 treatment is enough, however deroofing can be performed again |
| Surgical excision | Varies from removal of small areas of skin under local anaesthetic, closed with stitches, to large areas under general anaesthetic, healed with dressings/skin graft/flap | Usually treats only 1 skin region at a time to avoid too many skin wounds in one go | Removal of areas of active disease, scarring and, in some cases, surrounding skin at risk of flaring later | Local skin pain during procedure and after; scarring, bleeding, wound infection; rarely nerve damage, problems with general anaesthetic; duration of wound healing varies and can take several months with daily dressing changes; skin grafts and flaps may not be successful | Depends on waiting list at your centre | Small procedures can be repeated, larger ones are allowed to heal before any further surgery |
Care was taken to minimise participant inconvenience in receiving daily text messages asking for their pain score for 12 weeks, while optimising the response rate. The patient researchers recommended that text messages should be sent at the same time each day to provide routine and that 6 p.m. was the ideal time, to avoid clashing with work and parenting duties. They asked that the window for responses should be extended to 1 a.m. because they were aware that some patients prefer to delay sending a response until a quieter time. The PPI representatives suggested the addition of an ‘opt out’ response for the daily messages, in case they became too onerous for a participant.
A patient with HS kindly contributed to the deroofing video provided to support the introduction of the deroofing procedure (Figure 11). The PPI representatives advised creating two versions, one for patients and one for clinicians, differing only in the technical level of language used for the voice over. Interestingly, the clinician video has received many more views, nearly 1 million, compared with 3600 for the patient video, suggesting that patients mainly saw the video as being intended for clinicians.
FIGURE 11.
Screenshot from deroofing video.

Consensus workshop
Planning for the THESEUS consensus workshop required extensive PPI involvement and Table 32 details the impact of these discussions on the arrangements. In particular, PRPs recommended two additional virtual meetings for patients, run by the PRPs themselves, to prepare for the workshop. The aim was to support and empower patients to contribute to the workshop by provision of a glossary of technical terms, helping them to assimilate the study results and emphasising where their input could make a difference to THESEUS outputs.
| Issue raised by PRPs | Corresponding impact on workshop design |
|---|---|
| Patients attending workshop need to understand the aims and the brief for their involvement in advance | Two PRP-led teleconference pre-meetings were arranged to provide the briefing in advance, including aims and terminology and also allowing selection of participants |
| Some patients may not be able to attend in person, due to health issues, COVID-19 concerns and other commitments | One of the three breakout groups at the workshop was online only, allowing participants to attend via a videoconferencing link |
| Self-employed participants would lose income by attending the workshop | Patient participants at the workshop could claim a financial allowance at the Involving People daily rate if they wished, or the equivalent in vouchers |
| Patient participants may find it daunting to share their personal experiences with other patients and clinicians/researchers | Ground rules were created to ensure that all attendees were aware of their responsibilities not to share details outside the workshop |
| Insufficient patients in each breakout group could reduce the confidence of participants and decrease the patient voice | At least three patients were members of each breakout group, including one patient from the SMG, to provide support for other patients, for example, asking for clarification when needed |
| Patients attending virtually may wish not to use their camera/display their full name on the screen | Patients attending via videoconference could switch off their camera/not display their full name. First names were used for in-person badges |
Dissemination of THESEUS results
Following discussion with PRPs, it was decided to host a combined for trial participants, clinicians and researchers. The PPI representatives provided input into the presentation to ensure it was accessible to patients and encouraged patient input during the meeting, with several questions being offered by trial participants. Importantly, one of the two preworkshop patient-led virtual meetings took place just prior to the dissemination meeting, to allow the PPI representatives to prepare patients for the technical elements of the meeting, including provision of a glossary of expected terms.
Ensuring continuing communication with trial participants following the dissemination meeting and consensus workshop was an important consideration, particularly because further HS clinical trials are planned building on the foundations from THESEUS. PRPs supported the use of plain language summaries and infographics to summarise the results of THESEUS publications, and these will be created as part of the paper writing process. The HS Trust website is not currently being updated and so the THESEUS study website (currently https://www.cardiff.ac.uk/centre-for-trials-research/research/studies-and-trials/view/theseus) will be maintained indefinitely to provide a platform to disseminate the study outputs.
Reflections from patient advocates in THESEUS team
‘Being part of the THESEUS study has been insightful and empowering. It is refreshing to have been involved from the beginning and not consulted on an end product. The SMG Committee has been welcoming and accommodating of other commitments and made sure the frequency and timing of meetings were enablers.
During discussions the patient view was always encouraged and where there was a difference of opinion, the view of lived experience took precedence. As an example, subjective language used in study materials was adjusted in line with the patient voice because what is acceptable to a clinician may not be understandable to patients or could potentially cause them discomfort. We were mindful that some people in the trial would be new to their diagnosis so choosing our wording for patient information was an important role for the patient advocates to ensure we didn’t exclude potential participants at the initial stages of the study. We were able to translate terminology to make it accessible from a patient community perspective and to show how THESEUS could lay the groundwork to improve future HS care in the UK.
Leading workshops to prepare other PPI representatives ahead of the consensus workshop was a valuable opportunity to build the confidence of those new to patient research and advocacy and also for us to focus on where we feel there are gaps in the demographics of HS PRPs to encourage further participation.
I would like to think our input as patients has meant that access to the study and retention improved. As my first experience on a study of this nature, I have learnt a lot which I know will improve my contributions in future research into HS. From a patient perspective, it gives hope to us as individuals and a community that the research to support people living with HS is steeped in our experience and our hopes for the future.’
Discussion
Patient and public involvement in THESEUS has been critical to the success of the study. The foundations for THESEUS itself relied on patient involvement, in terms of the HS PSP and HiSTORIC’s core outcome set for HS. PRPs were embedded in study development at every stage, including the grant application, study design planning, study oversight, adaptation to challenges posed by the pandemic and finally the consensus workshop and results dissemination meeting. Coproduction with patients ensured that THESEUS met its primary objective to inform the design of future HS RCTs. It has also created a community of HS patient researchers and actively engaged trial participants who can help to promote and potentially participate in the HS RCTs that are intended to follow on from THESEUS.
Conclusion
The NIHR-funded THESEUS study was fortunate to benefit from strong patient and public contributions in every aspect of the trial design and delivery process. The PPI culminated in the THESEUS study workshop, which ensured coproduction between patients, clinicians and researchers of the top RCT designs to take forward in future publicly funded HS trials. THESEUS has created a continuing link with the HS patient community in the UK to support delivery of these much-needed RCTs.
Chapter 7 Consensus workshop
Introduction
The final stage of THESEUS was planned as a multistakeholder consensus meeting to present the main findings from the study to inform future trial design recommendations.
This chapter extends and refines research priorities established previously in a HS PSP. 17
Aims and objectives
Aim
-
to achieve consensus among key stakeholders over priority research questions for future HS trials.
Objectives
-
to identify up to three specific research questions suitable for future RCTs.
-
to agree key elements of trial design with a focus on the interventions to compare.
Methods
The consensus workshop used nominal group technique and consisted of structured small group interaction and plenary sessions. Iterative scoring and discussion of the research trials under consideration encouraged consensus. 48
A maximum of 40 stakeholders were sought for the workshop, including study participants, site investigators (dermatologists, surgeons and nurses) and the THESEUS SMG (including PPI representatives and researchers). Efforts were taken to establish broad representation for the meeting and to ensure a balance of patient, researcher and clinician participants.
The 1-day consensus meeting employed a hybrid design permitting both in-person and online participation. The meeting focused upon a prepared list of trials each comparing two interventions. This list was finalised by the THESEUS SMG and was informed by the previous prioritisation exercise17 and current clinical guidelines, and by provisional analysis of THESEUS study data. Figure 12 lists the trials included for consideration in the consensus meeting.
FIGURE 12.
Original long list of trials.

The meeting had three distinct phases:
-
pre-meeting scoring – online survey;
-
workshop small group discussion, ranking and plenary discussion; and
-
impact assessment of prioritised trials – small group discussion, plenary and online voting.
Pre-meeting scoring – online survey
Prior to the meeting, all potential workshop participants were invited to complete an online survey, providing a starting point for the workshop and allowing those not able to attend to contribute to the prioritisation process. The survey involved selecting ‘your top three’ from the predefined list of HS trials. The online survey was delivered via Microsoft Forms (Microsoft Corporation, Redmond, WA, USA) and simple descriptive statistics were produced detailing the number of times each trial had been selected as a ‘top three trial’.
Workshop small group discussion, ranking and plenary discussion
At the beginning of the workshop, the in-person and online delegates received a presentation summarising the results of the pre-meeting survey, as well as the main THESEUS results relating to participant willingness to receive treatment and eligibility. Participants were then split into break-out groups to discuss the trials and identify their preferred trials.
Groups included at least one clinician, one researcher and two people with lived experience of HS. One break-out group included only participants who were participating remotely. Each group was facilitated by two members of the THESEUS team, who acted as impartial facilitators of the discussion.
In the break-out groups, participants were invited in a round-robin fashion to identify their preferred trials and to explain their choice. Discussion within the groups subsequently considered popular trials and the reasons for their popularity. The subsequent plenary session considered which trials were favoured by different stakeholder groups, such as people with HS or clinicians; in these cases, discussion sought to demonstrate why a trial might be more important to a particular stakeholder. The break-out groups were then asked to agree a small number of preferred trials and at the next plenary session the preferences were compared to identify similarities and differences between the groups’ choices and the underlying reasons. At this point, any trial not prioritised was rejected to streamline continuing discussions.
Impact assessment of prioritised trials – small group discussion, plenary and online voting
Break-out groups were asked to reflect upon the short-list of preferred trials – considering which might have the greatest impact, change clinical practice or make most difference to people living with HS. Following discussion, each group was asked to rank the trials according to these criteria. Rankings were shared during a subsequent plenary session. Following these discussions participants were asked to vote anonymously via an online platform for the two trials which they considered should be prioritised above others. The votes were collated and the final top-three list of priority HS trials was established.
Results
Participants
Twenty-three participants completed the online, preworkshop survey; Table 33 presents the demographics of this sample.
| Respondents (n) | |
|---|---|
| Gender: | |
| Male | 5 |
| Female | 18 |
| Stakeholder group: | |
| HCP | 10 |
| Persons with HS | 12 |
| Researcher | 1 |
| Severity of HS (for persons with HS): | |
| No longer a problem | 1 |
| Mild | 2 |
| Moderate | 7 |
| Severe | 2 |
A total of 30 individuals participated in the workshop, including 7 HCPs, 10 researchers and 13 people living with HS. Fourteen of these joined online via the Zoom platform [(Zoom Video Communications, San Jose, CA, USA), including seven people with HS]; some of those who joined online were not present for the entire meeting, joining for the morning or afternoon session.
Pre-meeting online survey
Participants were allowed to select up to three trials and, in total, 68 selections were made, with one participant making only 2 selections. Every trial option was selected at least once. The most frequent selections were laser (hair removal) compared with clindamycin and rifampicin, early intervention with treatment usually used later in care pathway (e.g. biologicals) compared with doxycycline, and clindamycin and rifampicin plus laser (hair removal) compared with clindamycin and rifampicin alone (Figure 13).
FIGURE 13.
Pre-meeting online surveys responses. B: higher dose of standard medical treatment (e.g. doxycycline, clindamycin and rifampicin or other tablet treatment) compared with standard/lower dose; D: clindamycin and rifampicin + laser (hair removal) compared with clindamycin and rifampicin alone; F: early intervention with treatment usually used later in care pathway (e.g. biologicals) compared with doxycycline; J: adalimumab (or biosimilar) compared with clindamycin and rifampicin; K: adalimumab (or biosimilar) + laser (hair removal) compared with adalimumab (or biosimilar) alone; L: adalimumab (or biosimilar) + deroofing of skin tunnels compared with adalimumab (or biosimilar) alone.
Preferred trials
Small group discussion identified factors that participants felt to be important in prioritising future HS research. Discussion often focused upon the perspective of people living with HS, including the availability of treatments in a geographical region, as well as the person’s lived experience of a particular treatment. Interventions not previously available were often judged to be more appealing and more important to include in future HS trials.
There was consensus that future HS research should be available to a broad spectrum of people living with HS, irrespective of their disease stage or geographical location. Early intervention to prevent disease progression was emphasised. People with lived experience of HS supported the element of choice that was available in THESEUS, making study participation more attractive.
Biologicals were regarded positively for inclusion in future research, although people with lived experience of HS were aware that these drugs may be viewed with caution and considered a treatment of last resort. These impressions would need to be negotiated to ensure the acceptability of any future research which includes biologicals.
Table 34 presents the small group ranking of the trial options, with laser, deroofing and biological treatments favoured. As part of the ranking process, the small groups also suggested merging some trial options to broaden their reach and inclusivity and offered new trials based upon the treatment options under consideration. Group 1 suggested merging options K (adalimumab or biosimilar + laser hair removal compared with adalimumab or biosimilar alone) and L (adalimumab or biosimilar + deroofing of skin tunnels compared with adalimumab or biosimilar alone); they also proposed a new laser treatment-focused trial (laser + clindamycin and rifampicin vs. laser alone). Group 2 proposed two additional deroofing trials and group 3 suggested one additional deroofing trial.
| Rank: | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| 1 | F – early intervention with treatment usually used later in care pathway (e.g. biologicals) vs. doxycycline | D – clindamycin and rifampicin + laser hair removal vs. clindamycin and rifampicin alone | C – laser hair removal vs. clindamycin and rifampicin |
| 2 | New proposal: laser + clindamycin and rifampicin vs. laser | L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone | K – adalimumab or biosimilar + laser (hair removal) vs. adalimumab or biosimilar alone |
| 3 | K – adalimumab or biosimilar + laser hair removal vs. adalimumab or biosimilar alone and L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone |
F – early intervention with treatment usually used later in care pathway (e.g. biologicals) vs. doxycycline | E – deroofing of skin tunnels vs. local excision surgery |
| Other mentions | E – deroofing of skin tunnels vs. local excision surgery | New proposal: deroofing + doxycycline vs. deroofing | |
| New proposal: laser vs. deroofing | Discussion also recognised value in options F, B, D, H, I |
Review of the small group rankings led to further discussion and refinement of the trial options during a plenary session. Options C (laser vs. clindamycin and rifampicin) and D (clindamycin and rifampicin + laser vs. clindamycin and rifampicin alone) were combined to form a new option (option M – clindamycin and rifampicin + laser vs. laser alone). Option F (early intervention usually used later in treatment care pathway) was considered an overarching principle that could be incorporated into any of the trial designs and was excluded from further consideration as an intervention itself. Table 35 shows the status of all trial options considered and highlights those taken forward for further consideration and scoring.
| Excluded – not selected | Excluded – revised | Included for further consideration |
|---|---|---|
| A – clindamycin and rifampicin compared to doxycycline | C – laser (hair removal) versus clindamycin and rifampicin | E – deroofing of skin tunnels compared to local excision surgery |
| B – higher dose of standard medical treatment (e.g. doxycycline, clindamycin and rifampicin or other tablet treatment) vs. standard/lower dose | D – clindamycin and rifampicin + laser hair removal vs. clindamycin and rifampicin alone | K – adalimumab or biosimilar + laser hair removal vs. adalimumab (or biosimilar) alone |
| G – metformin vs. doxycycline | F – early intervention with treatment usually used later in care pathway (e.g. biologicals) vs. doxycycline | L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone |
| H – spironolactone vs. doxycycline | M – clindamycin and rifampicin + laser vs. laser alone (new) | |
| I – metformin vs. clindamycin and rifampicin | ||
| J – adalimumab or biosimilar vs. clindamycin and rifampicin |
Prioritised trials
Small group ranking of the remaining trials demonstrated a growing consensus for trials including laser as a combination treatment. Table 36 shows the small group ranking of the preferred trials and the newly proposed comparisons that address uncertainties about laser treatment in HS.
| Rank | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| 1 | M – clindamycin and rifampicin + laser vs. laser alone | K – adalimumab or biosimilar + laser hair removal vs. adalimumab or biosimilar alone | K – adalimumab or biosimilar + laser hair removal vs. adalimumab or biosimilar alone |
| 2 | K – adalimumab or biosimilar + laser hair removal vs. adalimumab or biosimilar alone | L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone | E – deroofing of skin tunnels vs. local excision surgery |
| 3 | E – deroofing of skin tunnels vs. local excision surgery | M – clindamycin and rifampicin + laser vs. laser alone | M – clindamycin and rifampicin + laser vs. laser alone |
| 4 | L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone | E – deroofing of skin tunnels vs. local excision surgery | L – adalimumab or biosimilar + deroofing of skin tunnels vs. adalimumab or biosimilar alone |
| Additional suggestions | Laser vs. no laser | Laser + drug vs. drug versus laser | Laser vs. doxycycline |
| Laser vs. standard of care | Laser + doxycycline vs. laser alone |
To close the workshop, 28 participants completed the online voting, including 13 people with HS, 8 researchers and 7 HCPs. Of the 50 responses, 2 participants selected prioritised trials and 6 selected a single prioritised trial. The top three options from the voting were: option K with 25 votes (50% of the votes cast), option M with 16 votes (32%) and option E with 8 votes (16%; Figure 14). Nearly all participants across the stakeholder groups included option K in their selection; 75% of votes for option E (6/8) were from individuals living with HS. Figure 15 presents a consensus prioritised top three for future HS trials.
FIGURE 14.
Consensus vote responses.
FIGURE 15.
Prioritised trials for future HS research.
Discussion
Through an iterative process of small group and plenary discussion, scoring and ranking the workshop established a clear stakeholder consensus to guide the future direction of HS research – with trials of laser, biological treatments and deroofing recommended. 49 The workshop extended the James Lind Alliance HS PSP, offering specific trial comparisons to operationalise the general areas of priority previously identified. 17
In its delivery, the workshop also offered further insight about the appeal and acceptability of future HS trial research. Improving access to new treatments, both geographically and to different patient groups, was seen as an important factor by workshop participants, especially those living with HS. This helps to explain the priority offered to deroofing, laser hair removal and biological treatments. The workshop also discussed the value of HS trials improving service delivery both for chronic disease management and treatment of acute flares.
Regarding limitations of the workshop, it is possible that the people living with HS who attended may not have been typical of the broader HS population; however, the group included several patient advocates and a broad range of disease severity and previous treatment experiences. It might also have been productive to include more HCPs from primary care, as only one GP was present for the workshop. The hybrid meeting allowed participants to take part without the inconvenience or cost of travel, although online participants may have limited contributions compared with those physically attending the workshop. It should also be noted that those who joined online were mostly people with lived experience of HS and at times the online group did not include a HCP participant.
In conclusion, the priority offered to laser treatment throughout the workshop, with options C, D, F and M all viewed positively, provides support for laser to feature in future HS trials. The different laser options supported, including combination treatment, could be investigated in a triple-arm trial, although this would require a larger study. Adaptive trial designs could also incorporate more than two interventions. Support for a trial of deroofing compared with local excision surgery offers the opportunity for a study investigating the management of acute HS flares, which is an important evidence gap, as highlighted in the HS PSP. 17
Chapter 8 Discussion
Main findings
THESEUS was a prospective observational cohort study with a nested process evaluation and end-of-study consensus workshop designed to lay the foundations for future RCTs of HS treatments. 50 The study was intended to be as inclusive as possible, by offering five interventions including both medical and non-medical interventions suitable for people with a wide range of HS severities and treatment experiences. Laser treatment was the intervention with the highest proportion (69%) of participants who were eligible and hypothetically willing to receive treatment, followed by deroofing (58%), conventional surgery (54%), the combination of oral clindamycin and rifampicin (44%) and finally doxycycline (37%). The final intervention choice was based on shared decision-making between the participant and their clinician, reflecting regular clinical practice, and mirrored the primary outcome of participant willingness and clinician eligibility. Both the cohort study and nested qualitative study results demonstrated that participant willingness to receive treatment and their final intervention choice could be strongly influenced by their clinician. ‘My doctor recommended it’ was the most common reason (59%) given by participants for their final choice of intervention. Participant interviews also demonstrated the influence of THESEUS prestudy information in the final treatment choice; for example, the left to right arrangement of interventions in the study decision grid indicated a sequence of treatments to some patients. Previous treatment, including lack of efficacy and adverse effects, was a push factor while the novelty of laser treatment and deroofing offered by THESEUS was a pull factor in influencing treatment choice. Availability of the interventions also influenced treatment selection, with laser treatment offered by only 6 of the 10 recruiting sites.
Characterisation of study interventions focused on the laser and deroofing procedures, as novel interventions for HS in the UK. It was originally intended to document variation in conventional surgery, but there were insufficient procedures performed to provide meaningful results, in part due to the COVID-19 pandemic reducing surgical capacity. The high uptake of deroofing may also have reduced the number of participants opting for conventional surgery. There was variation in the modality of laser treatment, either Nd-YAG or alexandrite laser, as recommended in the procedure protocol to permit participants with different skin phototypes to be safely treated. IPL was used in one-third of the treatments, which was not originally intended; however, it is also a treatment that targets the hair follicle.
Deroofing proved to be a popular intervention among both clinicians and participants, reflected by the THESEUS study video (https://www.cardiff.ac.uk/centre-for-trials-research/research/studies-and-trials/view/theseus) being viewed more than 1 million times. Deroofing proved straightforward for centres to adopt, both those led by plastic surgery and dermatology departments. There was some variation in practice regarding the instrument used for incision, with needle tip diathermy being used more often than loop tip diathermy. However, identification of skin tunnels by blunt probing and removal of the tunnel roof with secondary intention healing were highly conserved, with only one report of sutures being used to close the wound.
Recruitment was highly influenced by the COVID-19 pandemic, with the first UK wave of the pandemic delaying recruitment by 4 months and the second wave in the winter of 2020–21 slowing recruitment for a further 3 months. Outside of these periods, a recruitment rate of 15–20 participants per month was achieved and the recruitment target of 150 participants was reached due to the dedication of THESEUS recruitment sites and a highly motivated HS patient population.
Purposive selection of recruitment sites from a range of dermatology- and plastic surgery-led sites, and sites offering MDT medical and surgical care probably helped to balance recruitment into the medical and non-medical arms of THESEUS. Baseline demographics of participants are in keeping with the HS secondary care population, with an average age of 36 years, 81% female, 20% black, Asian or Caribbean participants, two-thirds current or ex-smokers and 86% with a raised BMI.
Loss to follow-up rates were 11% after 3 months and 17% after 6 months, increasing to 56% after 12 months, in part due to follow-up being truncated for some participants due to pandemic-related recruitment delays. Efforts were made to mitigate for the effects of the COVID-19 pandemic on follow-up visits, with a protocol amendment approved to permit remote assessment. THESEUS PRP input underpinned the decision to offer flexibility where possible in terms of use of video call or telephone call and provision of photographs for the lesion count assessments versus participants being asked to count their own HS lesions. Participant feedback from the interview study confirmed that a flexible approach is valued, particularly when lesions are in intimate skin regions. Interview feedback noted that remote follow-up was more convenient, but participants continued to appreciate face-to-face review with study staff.
Assessment of oral antibiotic treatment fidelity found that only 52% of participants who selected doxycycline were still receiving treatment after 3 months, with lack of effectiveness, participant preference and adverse effects cited as underlying reasons. Continuation of clindamycin and rifampicin at the 3-month review was affected by the standard course being 10 weeks initially, with an option to continue treatment, reflected by only 30% still receiving treatment after 12 weeks. THESEUS could not mandate the timing of the non-medical interventions as a non-randomised study and so treatment delays were common, with only 43% and 26% of participants commencing laser therapy and deroofing respectively at the 3-month review. Treatment switching was uncommon, however, with only five participants switching from laser treatment and nine switching from deroofing, in part because other HS treatment could be provided in the interim.
Delayed access to non-medical interventions limits interpretation of clinical efficacy data and THESEUS was a non-randomised study that was not powered to provide robust comparative treatment efficacy data. Efficacy data at 3 months for the doxycycline arm of the study demonstrates modest improvements in median IHS4 score from 7 to 6, DLQI score from 6 to 3.5 points, HiSQOL score from 26.5 to 11.5 and pain NRS from 2 to 1. Small effect sizes are in part due to doxycycline being selected for those with relatively low baseline disease severity. The corresponding score changes for oral clindamycin and rifampicin at 3 months were median changes in IHS4 score from 11 to 5 points, DLQI score from 14 to 10.5 points, HiSQOL score from 34 to 23 and pain NRS from 4 to 2.
In contributing to HiSTORIC core outcome set development for HS, the feasibility of collecting daily skin pain intensity scores by text message for 12 weeks after the chosen intervention commenced was examined. The rationale was that pain scores due to intermittent HS flares can fluctuate greatly from day to day. Consent was obtained from 146 participants and text messages were initiated in 110, the difference largely being due to a data entry issue in which investigators needed to go back to the baseline pages of the database to enter the date that a procedure was initiated to trigger the messages. Overall, 100 participants returned at least one text message. Daily responses reduced over time and the median duration of concordance was 36 days. A higher consistency of completion was observed in the first 14 and 28 days. Therefore, one option in a future study would be to collect daily scores remotely for short periods of time within the study period, linked to a scheduled in-person review. Feedback from participant interviews demonstrated some challenges to daily pain scores including uncertainty about what type of pain was relevant to the score, forgetting to reply, and frustration if sending the same score each day. Nevertheless, many participants valued the opportunity to provide daily feedback.
Relevance to the wider literature
While all five of the interventions offered by THESEUS are recognised treatments for HS, featuring in widely used guidelines,8,12,13 the evidence base for each remains relatively limited. 10 Oral tetracyclines are standard first-line treatment for mild to moderate HS; however, RCT evidence remains limited to a single small trial conducted more than 20 years ago using OMIs that have now been superseded. 51 Of the 24 participants assigned to oral tetracycline in the RCT, 8 (33%) dropped out of the study after 3 months, compared with 48% discontinuing doxycycline by this point in THESEUS. There are no published prospective RCTs examining the combination of clindamycin and rifampicin in HS. The highest level of evidence is provided by a prospective cohort study52 with 103 participants in which the median IHS4 score reduced from 13 to 6, very similar to THESEUS data, and 16% discontinued treatment due to adverse effects, compared with 22% in THESEUS.
Laser and light treatment studies targeting the hair follicle in HS have often employed a within participant side-to-side treatment design,53,54 preventing use of patient reported OMIs to evaluate treatment outcome. A within-participant RCT with 36 HS patients who received four Nd-YAG treatments at 6-week intervals used blinded outcome assessment and ensured that the non-treated side was shaved by the participant prior to evaluations to improve blinding. 55 There was a significant improvement in the number of inflammatory lesions on the treated side compared with the non-treated side 1 month after the treatment course (–2.5 lesions vs. –1.3 lesions, respectively, p = 0.017) but the change was not significant 3 months later. Several small within-participant RCTs have suggested benefit of IPL in HS. 54,56 The largest RCT with IPL involving 88 participants used IPL in combination with radiofrequency treatment and clindamycin 1% solution compared with clindamycin solution alone. 57 The unit of analysis was at the patient level. The change in IHS4 score for combination therapy given every 2 weeks was –7.2 after 16 weeks, compared with –1.8 for the control group (p < 0.001).
Deroofing evidence is provided in retrospective case series. Loop diathermy treatment of 88 HS lesions with median follow-up of 34 months demonstrated no recurrence in 83%. 18 The authors emphasised the importance of blunt probing of tunnels, which was reflected in THESEUS, with 90% of deroofing operators having a self-reported confidence of at least 7 of 10 that they had fully identified the extent of the tunnels requiring treatment. One aid for tunnel identification not employed by THESEUS is ultrasound-guided deroofing, recently reported as a helpful adjunct. 58 It is difficult to comment on conventional surgery from THESEUS results as so few participants received this treatment option. The literature suggests that secondary intention healing offers superior wound healing compared with other closure methods, but there is a lack of RCTs to confirm the observation, the closest being a prospective case series of wide excision of 20 axillae, with patients assigned on an alternate basis to grafting or secondary intention healing. 59
All of the HiSTORiC six core domains22 were measured in THESEUS, but in some cases there are not yet consensus-agreed OMIs. Pain NRS is established to measure the pain domain; however, consensus regarding current compared with maximum or average pain has not been established, as well as frequency of measurement. 60 The HiSTORiC-endorsed HiSQOL26 and PtGA27 instruments were included, while a fatigue instrument was borrowed from another setting. 28
Strengths and limitations
The prospective observational cohort design of THESEUS allowed participants to select from a wide range of medical and non-medical interventions for HS, guided by their clinicians, mirroring clinical practice. Purposive enrolment of both dermatology- and plastic surgery-led sites ensured sufficient uptake of each intervention, with the exception of conventional surgery, a result which is helpful in itself in terms of participants and their clinicians favouring deroofing over traditional surgical techniques. Use of HiSTORIC OMIs provided robust outcomes assessment, although interpretation of efficacy data was limited by variation in waiting times for procedures, in the context that THESEUS did not mandate timing of procedures, with a fixed schedule required for the follow up reviews.
There is a lack of prospective cohort data for change in HS disease severity over time and THESEUS provides much-needed evidence during a 12-month period. Under treatment, overall disease progression was largely prevented, with the proportion of participants with Hurley III, severe disease, being 19%, 16% and 21% at baseline, 6 and 12 months, respectively.
Variation in delivery of the laser and deroofing procedures was carefully documented within THESEUS and the important elements of deroofing had a high rate of fidelity. Receipt of non-laser IPL treatment within the laser arm of the study added unintended variation.
Use of text messages to receive a daily pain NRS score proved to be a secure and reliable method to measure the pain domain. During data cleaning it was noted that it would be ideal to restrict data entry to an integer between 0 and 10 to ensure that all responses could be clearly interpreted.
Recruitment rates were likely affected by the COVID-19 pandemic and this limits use of THESEUS data to predict recruitment rates for future RCTs. Nevertheless, it is anticipated that recruitment should be more in keeping with rates achieved during THESEUS recruitment periods that were relatively unaffected by COVID-19.
Another limitation of THESEUS is that it did not include a biological intervention as one of its treatment options. Adalimumab, the only licensed therapy for HS based on PIONEER study data,61 could have been included, but this would have increased the costs of study delivery. In recent times, a pipeline of biological therapies under development for HS has emerged,62,63 but none was included in THESEUS. It was anticipated that development of biological and new small-molecule therapies for HS will be led by the pharmaceutical industry and so THESEUS sought to increase the evidence base for non-biological therapies.
Generalisability
The baseline demographics of THESEUS participants are in keeping with secondary care HS patients, including a slightly higher proportion of non-white participants than the UK average. Baseline disease severity again reflects the HS secondary care population, with two-thirds having moderate disease, 19% severe disease and 13% mild disease, mirroring other cohorts. 64
The five interventions used in THESEUS are suitable for the full range of HS severity as intended. There was some variation in technique for the deroofing procedure, including use of instruments other than loop diathermy. This should not have affected outcomes because use of a scalpel or scissors for deroofing is well recognised. 65 Experience from THESEUS suggests that permitting variation in the instrument used for deroofing will promote uptake of the procedure. Inclusion of IPL within the laser treatment arm was not intended; however, IPL is a recognised method for hair removal treatment. It is expected to produce the same, or similar outcome in HS-affected tissue, and is supported by trials of IPL in HS. 54,56
Conclusions
Implications for health care
By offering medical and non-medical interventions, THESEUS was intended to encourage a MDT approach which is recommended to optimise HS care11 and this has been achieved by centres participating in the study. Integration of medical and non-medical therapy was possible for both dermatology-led and plastic surgery-led recruitment centres.
The training and equipment provided by THESEUS has led to establishment of deroofing as a surgical treatment option in the UK, bringing HS care in the UK in line with other countries. Interest in deroofing is demonstrated by more than 1 million views of the THESEUS deroofing video and by deroofing being the second highest choice in THESEUS in terms of eligibility and participant willingness to receive treatment. While laser and light hair removal treatment was already available in the UK, it was almost never used for HS therapy and THESEUS has shown it can be provided as a treatment option within existing healthcare infrastructure.
Use of HiSTORIC-developed and other recognised OMIs for HS within THESEUS has familiarised 10 centres in the UK with well-validated tools to measure HS severity and monitor patient progress. Several of the OMIs are suitable for routine clinical care, for example HiSQOL can be given to patients to complete in the waiting area before their appointment.
Implications for research
The design of THESEUS was intended to provide the foundations for future HS RCTs. The ten THESEUS sites were spread across the UK and are well placed to be recruiting centres in future HS trials. The sites are familiar with HS OMIs and have established laser and deroofing interventions for HS, should these be taken forward in a future RCT.
The qualitative study nested within THESEUS provided multiple insights to incorporate into future trials (see Chapter 5). A RCT with an active comparator will need to ensure equipoise for participants and clinicians and provide equivalent information, for example study videos, for each intervention. Flexibility should be offered where possible in terms of face-to-face or remote appointments and participant concerns regarding security of image transfer and storage indicates it may be better to avoid clinical photographs as part of virtual trial reviews. The number of OMIs should be minimised and an explanation provided regarding their use, particularly in terms of the frequency of study visits and when remote assessments are scheduled to finish.
If laser treatment is incorporated into a future trial, a decision will be required whether to allow IPL within the treatment arm as a non-laser therapy also targeting the hair follicle. The decision will depend on availability of Nd-YAG and alexandrite lasers at potential recruitment sites and whether the study is located towards the explanatory or pragmatic ends of the RCT spectrum. Some flexibility is probably permissible in terms of the timing of laser treatments 4 to 6 weeks apart; however, the number of pulses for lesions and background skin should be mandated and carefully reinforced. If deroofing is taken forward in a RCT, variation in cutting method could be permitted; however, it may be necessary to specify local anaesthetic only, particularly if deroofing is intended in an acute setting.
The THESEUS consensus workshop has identified a set of RCT designs to prioritise, several of which include combination therapy, building on the 2021 Safety and Efficacy of Adalimumab for Hidradenitis Suppurativa Peri-Surgically study publication comparing wide excision surgery and adalimumab to wide excision surgery alone. 66 In particular, the combination of laser treatment and medical therapy could be compared with laser treatment, or medical therapy, potentially in a multiarm study. Deroofing was also prioritised for future trials, and this could be compared with narrow margin excision, either for chronic lesions or in the setting of treatment of acute flares for which the evidence base is very limited.
Acknowledgements
Contribution of authors
John R Ingram (https://orcid.org/0000-0002-5257-1142) (Clinical Reader and Consultant Dermatologist) contributed to the conception and overall design of the study, implementation and interpretation of the work. He contributed to the writing of the abstract, scientific summary, introduction and discussion sections of the report, critical review of the whole report and final approval of the report submission.
Janine Bates (https://orcid.org/0000-0003-3610-2415) (Research Associate) was the trial manager, contributing to the trial implementation and contributed to the critical review of the final report. She also co-ordinated the compilation and approval of the final report.
Rebecca Cannings-John (https://orcid.org/0000-0001-5235-6517) (Principal Research Fellow in Statistics) was a co-investigator and the senior study statistician. She contributed to the study design, conducted the statistical data analysis, writing the results sections, critical review of the report and final approval of the report.
Fiona Collier (https://orcid.org/0000-0001-5271-9170) (GP and GP with Special Interest in Dermatology, retired) was a co-investigator and also a principal investigator at one of the recruiting sites. She contributed to the conception and overall design of the study, implementation and interpretation of the work.
Angela Gibbons (https://orcid.org/0000-0001-5285-4954) was a PPI representative on the study and contributed to the grant application, study design, study oversight the review of all participant-facing documentation, planning of the consensus workshop and dissemination meeting and delivery of two PPI-led meetings.
Ceri Harris (https://orcid.org/0000-0002-0462-7789) was a PPI representative on the study and contributed to the grant application, study design, study oversight the review of all participant-facing documentation, planning of the consensus workshop and dissemination meeting and delivery of two PPI-led meetings.
Kerenza Hood (https://orcid.org/0000-0002-5268-8631) (Professor in Statistics and Director of Centre for Trials Research) was a co-investigator and contributed to the overall trial design, and implementation, supervised the statistical analysis and final approval to the report.
Laura Howells (https://orcid.org/0000-0003-4157-7394) (Research Fellow and Health Psychologist) conducted the qualitative interviews, the analysis of the nested studies, contributed to the interpretation of the work, writing of the nested study chapters, the critical review of the whole report and final approval of the report submission.
Rachel Howes (https://orcid.org/0000-0002-6120-8433) (Specialist Registrar in Burns, Plastics and Reconstructive Surgery) was a co-investigator. She contributed to the conception and overall design of the study, implementation and interpretation of the work and contributed to the critical review of the whole report and final approval of the report submission.
Paul Leighton (https://orcid.org/0000-0001-5208-0274) (Associate Professor of Applied Health Services Research) was a co-investigator. He contributed to the conception and overall design of the study, implementation and interpretation of the work. Contributed to the writing of the qualitative chapters and critical review of the whole report and final approval of the report submission.
Muhammad Riaz (https://orcid.org/0000-0002-5512-1745) (Research Associate in Statistics) helped in cleaning the data, prepared the data for the analysis in Stata 17, conducted the statistical data analysis, wrote the results sections, critically reviewed the report and approved the final report.
Jeremy Rodrigues (https://orcid.org/0000-0002-9347-5026) (Associate Professor and NIHR Postdoctoral Fellow, Consultant Hand and Plastic Surgeon) was a co-investigator and also a principal investigator at one of the recruiting sites. He contributed to the conception and overall design of the study, implementation and interpretation of the work and contributed to the critical review of the whole report and final approval of the report submission.
Helen Stanton (https://orcid.org/0000-0003-0197-3667) (Data Manager) contributed to the trial implementation, acquisition and interpretation of data, critical review of the report and final approval of the report submission.
Kim Thomas (https://orcid.org/0000-0001-7785-7465) (Professor of Applied Dermatology Research, clinical trials) was a co-investigator. She contributed to the conception and overall design of the study, implementation, and interpretation of the work. Contributed to the writing of the nested substudy chapters, the critical review of the whole report and final approval of the report submission.
Emma Thomas-Jones (https://orcid.org/0000-0001-7716-2786) (Principal Research Fellow) was a co-investigator and the senior trials manager contributing to the trial design and implementation, critical review of the report and final approval of the report submission.
Other members of the trial team
In addition to the authors, the THESEUS study team comprised the following, who we would like to thank and acknowledge for their contribution to the trial implementation and management: Judith Evans, Research Administrator, and Oliver Cumming for information systems support.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Available data can be obtained from the corresponding author. Access to anonymised data may be granted following review.
Information governance statement
Cardiff University is committed to handling all personal information in line with the UK Data Protection Act 2018 and the General Data Protection Regulation (EU GDPR) 2016/679. Under the data protection legislation, Cardiff University is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here (https://www.cardiff.ac.uk/public-information/policies-and-procedures/data-protection/research-participants-data-protection-notice).
Ethical approval and governance
The Wales Research Ethics Committee 4 provided ethical approval for THESEUS on 26 September 2019, reference number 19/WA/0263. Cardiff University acted as sponsor for the study. All sites received local research and development approvals. Prospective trial registration on the ISRCTN registry was obtained on 9 August 2019 (ISRCTN69985145). Study oversight was provided by a combined Study Steering Committee and Independent Data Monitoring Committee. There were four independent members of the committee: a chairperson experienced in the conduct of clinical trials, an academic, a biostatistician and a patient representative. The study was conducted in accordance with the Research Governance Framework for Health and Social Care, principles of Good Clinical Practice, the General Data Protection Regulation and Cardiff University Centre of Trials Research standard operating procedures.
Department of Health disclaimer
This report presents independent research commissioned by the NIHR. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, Medical Research Council (MRC), Central Commissioning Facility (CCF), NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC), the HTA programme or the Department of Health. This report presents independent research commissioned by the NIHR. The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, CCF, NETSCC, the HTA programme or the Department of Health.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
Publication
Ingram JR, Bates J, Cannings-John R, et al. Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS): a prospective cohort study. Br J Dermatol 2023 Oct 12:ljad388. https://doi.org/10.1093/bjd/Ijad388
References
- Matusiak Ł. Profound consequences of hidradenitis suppurativa: a review. Br J Dermatol 2020;183:e171-e7.
- Garg A, Neuren E, Cha D, Kirby JS, Ingram JR, Jemec GBE, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020;82:366-76.
- Ingram JR. Hidradenitis suppurativa: an update. Clin Med (Lond) 2016;16:70-3.
- Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018;178.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol 2020;183:990-8.
- Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015;173:1546-9.
- Zouboulis CC, Benhadou F, Byrd AS, Chandran NS, Giamarellos-Bourboulis EJ, Fabbrocini G, et al. What causes hidradenitis suppurativa?- 15 years after. Exp Dermatol 2020;29:1154-70.
- Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619-44.
- Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, et al. Interventions for hidradenitis suppurativa. Cochr Datab Syst Rev 2015;2015.
- Ingram JR. Interventions for hidradenitis suppurativa: updated summary of an original Cochrane review. JAMA Dermatol 2017;153:458-9.
- Ingram JR, Collier F, Brown D, Burton T, Burton J, Chin MF, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol 2019;180:1009-17.
- Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 2019;81:76-90.
- Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol 2019;81:91-101.
- Hasan SB, Ingram JR. What has changed in the UK management of hidradenitis suppurativa from 2014 to 2019?. Br J Dermatol 2020;183:973-5.
- Howes R, Ingram JR, Thomas KS, Collier F, Rodrigues JN. THESEUS Survey collaborator group . The surgical management of hidradenitis suppurativa in the United Kingdom: a national survey of care pathways informing the THESEUS study. J Plast Reconstr Aesthet Surg 2022;75:240-7.
- Collier F, Howes R, Rodrigues J, Thomas KS, Leighton P, Ingram JR. Primary care management of hidradenitis suppurativa: a cross-sectional survey of UK GPs. BJGP Open 2021;5.
- Ingram JR, Abbott R, Ghazavi M, Alexandroff AB, McPhee M, Burton T, et al. The hidradenitis suppurativa priority setting partnership. Br J Dermatol 2014;171:1422-7.
- van Der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol 2010;63:475-80.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol 2016;175:263-72.
- Thorlacius L, Ingram JR, Garg A, Villumsen B, Esmann S, Kirby JS, et al. Protocol for the development of a core domain set for hidradenitis suppurativa trial outcomes. BMJ Open 2017;7.
- Thorlacius L, Garg A, Ingram JR, Villumsen B, Theut Riis P, Gottlieb AB, et al. Towards global consensus on core outcomes for hidradenitis suppurativa research: an update from the HISTORIC consensus meetings I and II. Br J Dermatol 2018;178:715-21.
- Thorlacius L, Ingram JR, Villumsen B, Esmann S, Kirby JS, Gottlieb AB, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol 2018;179:642-50.
- Bates J, Stanton H, Cannings-John R, . Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS): protocol for a prospective cohort study. BMJ Open 2022;12.
- Hurley H. Dermatologic Surgery. New York: Marcel Dekker; 1989.
- Horváth B, Janse IC, Blok JL, Driessen RJB, Boer J, Mekkes JR, et al. Hurley staging refined: a proposal by the Dutch hidradenitis suppurativa expert group. Acta Derm Venereol 2017;97:412-3.
- Kirby JS, Thorlacius L, Villumsen B, Ingram JR, Garg A, Christensen KB, et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020;183:340-8.
- Kirby JS, Hereford B, Thorlacius L, Villumsen B, Ingram JR, Garg A, et al. Validation of global item for assessing impact on quality of life of patients with hidradenitis suppurativa. Br J Dermatol 2021;184:681-7.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-3.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6.
- Pennell CP, Hirst AD, Campbell WB, Sood A, Agha RA, Barkun JST, et al. Practical guide to the idea, development and exploration stages of the IDEAL framework and recommendations. Br J Surg 2016;103:607-15.
- Kimball AB, Jemec GBE, Yang M, Kageleiry A, Signorovitch JE, Okun MM, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014;171:1434-42.
- Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017;177:1401-9.
- Briesch AM, Swaminathan H, Welsh M, Chafouleas SM. Generalizability theory: a practical guide to study design, implementation, and interpretation. J School Psychol 2014;52:13-35.
- Howells L, Lancaster N, McPhee M, Bundy C, Ingram J, Leighton P, et al. Thematic synthesis of the experiences of people with hidradenitis suppurativa: a systematic review. Br J Dermatol 2021;185:921-34.
- Keary E, Hevey D, Tobin A. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol 2020;182:342-7.
- Ritchie J, Spencer L. Analyzing Qualitative Data. London, UK: Routledge; 2002.
- Thorlacius L, Esmann S, Miller I, Vinding G, Jemec GB. Development of HiSQOL: a hidradenitis suppurativa-specific quality of life instrument. Skin Appendage Disord 2019;5:221-9.
- Sisic M, Kirby JS, Boyal S, Plant L, McLellan C, Tan J. Development of a quality-of-life measure for hidradenitis suppurativa. J Cutan Med Surg 2017;21:152-5.
- Kirby JS, Sisic M, Tan J. Exploring coping strategies for patients with hidradenitis suppurativa. JAMA Dermatol 2016;152:1166-7.
- Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011;91:328-32.
- Shukla N, Paul M, Halley M, Lowes M, Hester V, Aguilar C, et al. Identifying barriers to care and research in hidradenitis suppurativa: findings from a patient engagement event. Br J Dermatol 2020;182:1490-2.
- Fisher S, Jehassi A, Ziv M. Hidradenitis suppurativa on Facebook: thematic and content analyses of patient support group. Arch Dermatol Res 2020;312:421-6.
- Kimball AB, Sundaram M, Banderas B, Foley C, Shields AL. Development and initial psychometric evaluation of patient-reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J Dermatol Treat 2018;29:152-64.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12.
- NIHR . Funding Opportunities n.d. https://www.nihr.ac.uk/researchers/funding-opportunities (accessed 2 July 2023).
- Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect 2004;7:209-20.
- NIHR . Involve Patients n.d. https://www.nihr.ac.uk/researchers/funding-opportunities (accessed 2 July 2023).
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al. Consensus development methods, and their use in clinical guideline development. HTA 1998;2:i-iv.
- Leighton P, Howells L, Bates J, . Research priorities in the management of hidradenitis suppurativa. Br J Dermatol 2023;189:343-5. https://doi.org/10.1093/bjd/ljad152.
- Ingram JR, Bates J, Cannings-John R, . Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS): a prospective cohort study. Br J Dermatol 2023.
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998;39:971-4.
- van Straalen KR, Tzellos T, Guillem P, Benhadou F, Cuenca-Barrales C, Daxhelet M, et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: results of a prospective European cohort study. J Am Acad Dermatol 2021;85:369-78.
- Tierney E, Mahmoud BH, Hexsel C, Ozog D, Hamzavi I. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg 2009;35:1188-98.
- Highton L, Chan WY, Khwaja N, Laitung JK. Treatment of hidradenitis suppurativa with intense pulsed light: a prospective study. Plast Reconstr Surg 2011;128:459-65.
- Naouri M, Maruani A, Lagrange S, Cogrel O, Servy A, Collet Vilette AM, et al. Treatment of hidradenitis suppurativa using a long-pulsed hair removal neodymium:yttrium-aluminium–garnet laser: a multicenter, prospective, randomized, intraindividual, comparative trial. J Am Acad Dermatol 2021;84:203-5.
- Andersen PL, Riis PT, Thorlacius L, Sigsgaard V, Nielsen CW, Chafranska L, et al. Intense pulsed light treatment for hidradenitis suppurativa: a within-person randomized controlled trial. Eur J Dermatol 2020;30:723-9.
- Schultheis M, Staubach P, Nikolakis G, Grabbe S, Ruckes C, Von Stebut E, et al. LAight® therapy significantly enhances treatment efficacy of 16 weeks of topical clindamycin solution in Hurley I and II hidradenitis suppurativa: results from period A of RELIEVE, a multicenter randomized, controlled trial. Dermatology 2022;238:476-86.
- Der Sarkissian S, Frew JW. Ultrasound-guided de-roofing of epithelialised tunnels of hidradenitis suppurativa. Australas J Dermatol 2021;62:360-3.
- Morgan WP, Harding KG, Hughes LE. A comparison of skin grafting and healing by granulation, following axillary excision for hidradenitis suppurativa. Ann R Coll Surg Engl 1983;65:235-6.
- Hasan SB, Gendra R, James J, Morris D, Orenstein LAV, Ingram JR. Pain measurement in painful skin conditions and rheumatoid arthritis randomized controlled trials: a scoping review to inform pain measurement in hidradenitis suppurativa. Br J Dermatol 2022;187:846-54.
- Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016;375:422-34.
- Casseres RG, Prussick L, Zancanaro P, Rothstein B, Joshipura D, Saraiya A, et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: results of an open-label trial. J Am Acad Dermatol 2020;82:1524-6.
- Glatt S, Jemec GBE, Forman S, Sayed C, Schmieder G, Weisman J, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol 2021;157:1279-88.
- Ingram JR, Bettoli V, Espy JI, Kokolakis G, Martorell A, Villani AP, et al. Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. J Eur Acad Dermatol Venereol 2022;36:1597-605.
- Ravi S, Miles JA, Steele C, Christiansen MK, Sayed CJ. Patient impressions and outcomes after clinic-based hidradenitis suppurativa surgery. JAMA Dermatol 2022;158:132-41.
- Bechara FG, Podda M, Prens EP, Horvath B, Giamarellos-Bourboulis EJ, Alavi A, et al. Efficacy and safety of adalimumab in conjunction with surgery in moderate to severe hidradenitis suppurativa: the SHARPS randomized clinical trial. JAMA Surg 2021;156:1001-9.
Appendix 1 Open-text questions in end-of-study questionnaires
Patient end-of-study questionnaire
|
|
|
|
Healthcare staff end-of-study questionnaire
|
|
|
|
|
|
Appendix 2 Framework Matrices
Patient interview framework
| 1. Beliefs and experiences |
| 1.1 Beliefs about HS |
| 1.1.1 Consequences |
| 1.1.2 Timeline |
| 1.1.3 Control |
| 1.1.3.1 Personal control |
| 1.1.4.1 Treatment control |
| 1.1.4 Identity |
| 1.1.5 Concern |
| 1.1.6 Understanding and coherence |
| 1.1.7 Emotional response |
| 1.1.8 Cause |
| 1.2 Beliefs about antibiotics for HS |
| 1.2.1 Concerns |
| 1.2.2 Individual fit |
| 1.2.3 Effectiveness |
| 1.2.4 Necessity |
| 1.2.5 Other |
| 1.3 Beliefs about laser and surgery for HS |
| 1.3.1 Concerns |
| 1.3.2 Individual fit |
| 1.3.3 Effectiveness |
| 1.3.4 Necessity |
| 1.3.5 Other |
| 1.4 Seeking treatment |
| 1.4.1 Clinician and health-care system |
| 1.4.2 Diagnosis and access to treatment |
| 1.4.3 Choice |
| 1.4.4 Willing to try anything |
| 1.4.5 Other |
| Other |
| 2. Study treatment experiences |
| 2.1 Reasons for treatment choice |
| 2.1.1 Perceptions or knowledge of treatments |
| 2.1.2 The role of previous experiences of treatment |
| 2.1.3 The importance of doctor’s recommendation |
| 2.1.4 Recommendation from other HS patients |
| 2.1.5 Other |
| 2.2 Treatment process (focus on practical elements, acceptability and feasibility) |
| 2.2.1 Experiences pre-treatment |
| 2.2.2 Experiences during treatment |
| 2.2.3 Experiences post-treatment |
| 2.3 Satisfaction with treatment |
| 2.3.1 Comparison with other treatments |
| 2.3.2 Comparison with expectations |
| 2.3.3 Difficulties or side effects experienced |
| 2.3.4 Belief about long-term effects of the treatment |
| 2.3.5 Using treatment in the future |
| 2.3.6 Other |
| 3. HS research experiences |
| 3.1 Reflections on the study |
| 3.1.1 Reasons for taking part in study |
| 3.1.10 COVID-19 specific challenges |
| 3.1.11 Other |
| 3.1.2 Interaction between research and clinical care and concerns |
| 3.1.3 Difficulties or frustrations with research process |
| 3.1.4 Understanding of the research |
| 3.1.5 Measurement (daily texts and questionnaire visits) |
| 3.1.6 Remote appointments |
| 3.1.7 Photos |
| 3.1.8 Antibiotic specific challenges |
| 3.1.9 Surgery specific challenges |
| 3.2 Recommendations for future research |
| 3.2.1 How patients feel about including their treatment arm in future research |
| 3.2.2 How patients feel about randomisation in future research |
| 3.2.3 Reasons patients would take part in future research and what they would like to see in future research |
| 3.2.4 Practical suggestions for improvement |
Staff interview framework
| 1. Treatment choices – HCP beliefs and past experiences |
| 1.1 THESEUS treatment fit with current care |
| 1.2 Views on patient preferences |
| 2. HCP treatment experiences within THESEUS |
| 2.1 Training needs |
| 2.2 Concerns or problems |
| 2.3 Wound care |
| 2.3 Outcomes and using in the future |
| 3. HCP THESEUS research experiences |
| 3.1 Reflections on the study |
| 3.1.1 Communication with study team |
| 3.1.2 Procedures and logistics |
| 3.1.3 COVID-19 impact |
| 3.1.4 HS patient factors |
| 3.2 Reflections on future studies |
| 3.2.1 Need for studies – unmet patient needs |
| 3.2.2 Future study designs |
Appendix 3 Protocols
Laser protocol
| Component/step of intervention | Type of standardisation | Conditions relating to standardisation | Flexibility |
|---|---|---|---|
| Number of treatments | Mandated | Minimum of four treatments; limited only by patient tolerability of treatment | Within boundaries described |
| Interval of treatments | Mandated | Minimum of 4 weeks between treatments; delay > 4 weeks between treatments permitted for patient fitness reasons (e.g. due to acute infection of regions to be treated or patient systemic illness); or at clinical discretion (e.g. pigmentation of treated areas); reasons for delay to be documented | Within boundaries described |
| Pain control options | Optional | Clinical discretion to be used for individual patients; aim to maximise tolerability of treatment and minimise patient discomfort; options include: topicals, local anaesthetic infiltration, oral analgesia, sedation | Totally flexible – methods used must be documented |
| Prohibited | General anaesthetic | Exactly as described | |
| Cooling options | Optional | Based on local equipment availability; clinical discretion to be used for each individual patient; aim to maximise tolerability of treatment and minimise patient discomfort | Totally flexible – methods used must be documented |
| Laser choice | Optional | Fitzpatrick type I–IV: alexandrite (755 nm) or diode (810 nm) | Totally flexible – reasons for laser choice must be documented |
| Fitzpatrick type II–VI: Nd:YAG (1064 nm) | |||
| Field of treatment | Mandated | Patients to be treated in 1 or more affected regions; regions include: axilla, inframammary, groin, natal cleft, buttocks |
Within boundaries described |
| Fluence | Mandated | Fluence to be documented for each treatment | Totally flexible |
| Pulse duration | Mandated | Pulse duration to be documented for each treatment | Totally flexible |
| Spot size | Mandated | Spot size to be documented for each treatment | Totally flexible |
| Number of pulses | Mandated | Background field: single pulse | Exactly as described |
| Nodules: double pulse | |||
| Post-treatment care | Optional | Dressings: any dressing type may be applied as required | Totally flexible |
| Analgesia: topical or oral analgesia options may be used | Within boundaries described | ||
| Photography | Mandated | Treatment session 1: each field of treatment must be marked with white pencil or pen and photographed before treatment is given | Exactly as described |
| Optional | Treatment sessions 2+: each field of treatment can be photographed at the start of each session | Within boundaries described | |
| Mandated | Follow up appointment: Each field of treatment must be photographed at the follow up appointment | Exactly as described | |
| Documentation of adverse effects | Mandated | All adverse effects of treatment, and any reasons (clinical, logistical or patient choice) for discontinuing treatment must be documented | Exactly as described |
| Changes to protocol between sessions | Mandated | Any alterations of the treatment delivered between sessions must be documented, with reasons for this alteration | Exactly as described |
Deroofing protocol
| Component/step of intervention | Specifics of step | Type of standardisation | Description | Conditions | Limits |
|---|---|---|---|---|---|
| Before incision | Perioperative skin preparation | Mandatory | Skin preparation with surgeon’s routine antimicrobial preparation – alcoholic or aqueous chlorhexidine or povidone-iodine | All cases; full flexibility with choice of antimicrobial agent | Boundaries: must use antimicrobial agent |
| Preoperative laser hair removal within current course of treatment | Prohibited | Preoperative laser hair removal | All cases | Exactly as described | |
| Perioperative hair removal with clippers or blade | Optional | Hair removal will be performed at the discretion of the surgeon | When either surgery, dressing or healing believed to be impaired without hair removal | No boundaries | |
| Incision and access | Deroofing incision | Mandatory | Incision only partially through full thickness of diseased skin and not extending into subcutaneous tissue, either within lesion, or around lesion, using diathermy | All cases | Boundaries: must use a diathermy for incision |
| Dissection | Dissection of subcutaneous tissues and deeper structures | Prohibited | Dissection of subcutaneous tissues and deeper structures | All cases | Exactly as described |
| Resection | Removal of tissue implicated in hidradenitis suppurativa only, and surrounding tissue only if required to contour the area | Optional | Resection of skin and/or subcutaneous tissue, leaving base of sinus/lesion in situ may be performed if required, largely to ensure appropriate contour of area | As required depending on the nature and distribution of the lesion | Boundaries: must not involve the surgical removal of any margin around the diseased tissue, in contrast to surgery, in which excision of disease tissue and a margin of normal tissue will be performed |
| Haemostasis | intraoperative haemostasis | Optional | Use of diathermy (monopolar or bipolar), or alternative techniques such as adrenaline-soaked swabs | All cases | Boundaries: choice and use at surgeon’s discretion |
| Reconstruction | Reconstructive surgical procedure | Prohibited | Use of reconstructive surgery techniques to close the surgical wound | All cases | Exactly as described |
| Closure | Closure of wound by using sutures or other closure devices to appose wounds edges, or to inset or secure a graft or flap, or application of negative pressure wound therapy to an open wound | Prohibited | Sutures (absorbable or non-absorbable), staple or other closure device, including negative pressure wound therapy | All cases | Exactly as described |
| After skin closure | Application of conventional dressings to a wound/graft/flap, or application of negative pressure wound therapy to wound | Optional | Application of conventional dressings to wound/graft/flap, or application of negative pressure wound therapy to a wound | All cases | Boundaries: choice and use at surgeon’s discretion |
| Insertion of adjunct | Insertion of drain in the excised wound or donor site | Prohibited | Placement of a surgical drain into the wound or donor site, with or without the application of suction (excluding negative pressure wound therapy devices – covered above already) | All cases | Exactly as described |
| Intraoperative diagnosis | Not applicable | ||||
| Other | Not applicable |
Surgery protocol
| Component/Step of intervention | Specifics of step | Type of Standardisation | Description | Conditions | Limits |
|---|---|---|---|---|---|
| Before incision | Perioperative skin preparation | Mandatory | Skin preparation with surgeon’s routine antimicrobial preparation – alcoholic or aqueous chlorhexidine or povidone-iodine | All cases; full flexibility with choice of antimicrobial agent |
Boundaries: must use antimicrobial agent |
| Preoperative laser hair removal within current course of treatment | Prohibited | Preoperative laser hair removal | All cases | Exactly as described | |
| Perioperative hair removal with clippers or blade | Optional | Hair removal will be performed at the discretion of the surgeon | When either surgery, dressing or healing believed to be impaired without hair removal | No boundaries | |
| Incision and access | Skin incision | Mandatory | Incision through full thickness of skin to subcutaneous tissue, either within lesion or around lesion, using scalpel or diathermy | All cases | Boundaries: must use a generally accepted technique for incising skin |
| Dissection | Dissection of subcutaneous tissues and deeper structures | Optional | Dissection of subcutaneous tissues and deeper structures | As required depending on strategic intent of surgery, anatomical site and extent | No boundaries |
| Resection | Removal of tissue implicated in hidradenitis suppurativa, with a surrounding margin of macroscopically ‘normal’ tissue | Mandatory | Resection of skin and/or subcutaneous tissue, including excision of the lesion or lesions en bloc, with some surrounding normal tissue | All cases | Boundaries: must involve the surgical removal of tissue and some surrounding area, in contrast to deroofing, in which excision of disease tissue may be incomplete, and without a margin of normal tissue |
| Haemostasis | Intraoperative haemostasis | Optional | Use of diathermy (monopolar or bipolar), or alternative techniques such as adrenaline-soaked swabs | All cases | Boundaries: choice and use at surgeon’s discretion |
| Reconstruction | Split skin graft, or full-thickness skin graft, or flap of any kind | Optional | Use of reconstructive surgery techniques to close the surgical wound | All cases | Boundaries: choice and use at surgeon’s discretion |
| Closure | Closure of wound by using sutures or other closure devices to appose wounds edges, or to inset or secure a graft or flap, or application of negative pressure wound therapy to an open wound Alternative approaches such as leaving the wound open and applying conventional dressings may be used |
Optional | Sutures (absorbable or non-absorbable), staples or other closure device, including negative pressure wound therapy | All cases | Boundaries: choice and use at surgeon’s discretion |
| After skin closure | Application of conventional dressings to a wound/graft/flap, or application of negative pressure wound therapy to wound | Optional | Application of conventional dressings to wound/graft/flap, or application of negative pressure wound therapy to a wound | All cases | Boundaries: choice and use at surgeon’s discretion |
| Insertion of adjunct | Insertion of drain in the excised wound or donor site | Optional | Placement of a surgical drain into the wound or donor site, with or without the application of suction (excluding negative pressure wound therapy devices – covered above already) | All cases | Boundaries: choice and use at surgeon’s discretion |
| Intraoperative diagnosis | Not applicable | ||||
| Other | Not applicable |
Appendix 4 Surgical data fidelity – handling of data cleaning issues
| Data cleaning issue | How data were handled for analysis |
|---|---|
| Laser data set | |
| Entry with only participant ID and two other variables entered | Assume entered in error and dropped from analysis |
| There were seven instances where data were entered for the same participant on the same day | This was considered to be duplication of data. The entry considered to be less complete was dropped from analysis. This was confirmed with site in one instance |
| Concerns about fidelity of the data entered about whether treatment schedule was being conducted to expected treatment schedule due to the number of first treatments reported not matching the number of participants in the data set | Decided it was not necessary to quantitatively report these data. Have reported narratively and qualitatively for the open text responses on why treatment schedules were not met |
| Concerns about fidelity of the data entered about analgesia following a site query confirming that ‘gel’ reported as used was not an analgesia for one procedure | Changed ‘gel’ to ‘none’ for site where this was confirmed. For other data have noted within the results that reports of ‘other’ types of analgesia may in fact be considered by some to be defined as analgesia |
| Data on fluence, pulse duration and spot size very variable | Data on fluence, pulse duration and spot size were collected, but are not reported due to clinically led decision that they provided an aggregation of heterogeneous data that cannot be clinically interpreted and could be misleading |
| Deroofing data set | |
| There were two instances where data was entered for the same participant on the same day | This was considered to be duplication of data. For one instance, the entry considered to be less complete was dropped from analysis. For another instance, the data were exactly the same but difference in ‘number of skin tunnels present in the region undergoing treatment’ and ‘number of skin tunnels successfully explored with a blunt probe’ so these data were considered as ‘missing’ in the analysis |
| Two instances with only participant ID and no other data | Assumed error and dropped from the analysis |
| Conventional surgery | |
| Of 11 entries, 2 provided no data other than participant ID and date, and 6 others did not report type of surgery | The conventional surgery data were considered difficult to interpret due to missing data and low sample size, and so there was a clinically led decision not to report these data within the report beyond type of surgery conducted where this information was available |
List of abbreviations
- BMI
- body mass index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DLQI
- Dermatology Life Quality Index
- EQ5D-5L
- EuroQoL 5 dimension 5 level
- FSS
- Fatigue Severity Scale
- GP
- general practitioners
- HCP
- healthcare professional
- HiSQOL
- Hidradenitis Suppurativa Quality of Life score
- HiSTORIC
- Hidradenitis SuppuraTiva cORe outcomes set International Collaboration
- HS
- hidradenitis suppurativa
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation
- IHS4
- International Hidradenitis Suppurativa Severity Score System
- IPL
- intense pulsed light
- IQR
- interquartile range
- MDT
- multidisciplinary team
- Nd-YAG
- neodymium-doped yttrium aluminium garnet
- NIHR
- National Institute for Health and Care Research
- NRS
- numerical rating scale
- OMI
- outcome measure instrument
- PPI
- patient and public involvement
- PRP
- patient research partner
- PSP
- priority setting partnership
- PtGA
- Patient Global Assessment
- RCT
- randomised controlled trial
- SD
- standard deviation
- SMG
- study management group
- THESEUS
- Treatment of Hidradenitis Suppurativa Evaluation Study
