Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 17/21/06. The contractual start date was in November 2018. The draft report began editorial review in April 2022 and was accepted for publication in January 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Scholefield et al. This work was produced by Scholefield et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Scholefield et al.
Chapter 1 Phase 1a: scoping review of literature
Introduction
This review aimed to evaluate early mobilisation and rehabilitation (ERM) within paediatric intensive care as reported within the published literature. We characterised the evidence base using a narrative synthesis approach to understand features of ERM associated with effectiveness and successful implementation within paediatric intensive care units (PICU).
Study management
The work package was led by BRS. The study management group was responsible for defining and reviewing scope of search. JYT performed searches, data extraction, risk of bias assessment, evidence synthesis and first draft of chapter. Second screening of articles, data extraction and risk of bias performed by Dr Olivia Craw, JMc and JMen. Methodological expertise provided by DM and BRS.
Objectives
Our primary objective was to summarise the types and effectiveness of ERM interventions and outcome measures delivered to children admitted to PICUs.
Our secondary objective was to thematically identify subpopulations (if any) that benefit most from ERM or experience associated adverse or clinical events, and any patterns or gaps during implementation.
Methods
Search strategy
Searches were conducted in the bibliographic databases [Excerpta Medica Database (Embase) (via OVID), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCO), MEDLINE (via OVID), PEDro, Open grey or Cochrane CENTRAL)]. Original search was from inception to 12 October 2019 and an updated search was performed 1 November 2021, using strategies that combined, where relevant, free text and index terms for:
-
children and young people (CYP);
-
admitted to paediatric critical care settings;
-
receiving early (within 7 days) rehabilitation and mobilisation.
The search strategy was developed in MEDLINE (via OVID) (see Appendix 2) and adapted for other databases.
Database of Abstracts of Reviews of Effects, National Health Service (NHS) Economic Evaluation Database and Health Technology Assessment database (all via Centre for Reviews and Dissemination) were searched for relevant systematic reviews to identify primary studies for the review. These were supplemented by relevant websites using hand-searching for mobilization-network.org and search terms for clinicaltrials.gov or Chinese clinical trial registry, checking reference lists of relevant studies, and forward citation-checking of included studies in Web of Science. We screened reference lists to identify relevant primary studies and contacted primary authors to find full texts of incomplete records.
Eligibility criteria
We included all completed studies published in English that met the following criteria:
-
Study participants: critically ill infants, children or young people aged ≤18 years, admitted to PICUs, who received an intervention described as rehabilitation or mobilisation delivered by any health professional within ≤7 days after admission. Rehabilitation or mobilisation interventions could include but were not limited to physiotherapy (PT), occupational therapy, speech and language therapy (SLT) and bundled interventions; these included ABCDEFH bundles (spontaneous awakening and breathing trials; choice of sedation and analgesia, delirium prevention, surveillance and management; early mobilisation and exercise programmes with or without adjuncts; family engagement and empowerment; proper nutrition and humanism) so long as their application was considered within the first 7 days of admission; AND
-
Outcome: at least one outcome was related to participants’ health and well-being, health service utilisation, feasibility, acceptability or intervention implementation.
-
Study design: primary research studies of any designs, with >10 participants to synthesise evidence on intervention effectiveness. Case reports, case series with ≤10 patients, qualitative studies and systematic reviews were excluded if relevant primary studies were not identified in the references. Abstracts or ongoing studies identified from clinical trial registries were used to highlight the presence of future emerging research.
Screening and selection
Records identified were imported into a bibliographic referencing software programme (EndNote X9, Thomson Reuters, San Francisco, CA, USA), and duplicates were removed. One PT researcher (JT) and one clinical academic (BS or JMen) independently screened titles and abstracts for relevance against the eligibility criteria within Rayyan systematic review software. 1 The full texts of relevant articles were obtained and assessed against the selection criteria by two reviewers independently (JT, Dr Olivia Craw). A wider range of publication types (abstracts and full texts) were selected to identify all possible lists of ERM interventions but were not analysed to summarise types of ERM. Reasons for exclusion were noted. Discrepancies were discussed via consensus meeting with a third author (JMc).
Data extraction
Data were extracted using a standardised, piloted data-extraction form in Excel. Information within the following domains was extracted:
-
Study – author, year of publication, country, study design using an algorithm for classifying studies. 2
-
Patient demographics – age, sex, admission diagnosis, the severity of illness and, comorbidity using established criteria3 or paediatric scoring tools such as Pediatric risk of mortality (PRISM III), the Pediatric logistic organ dysfunction (PELOD), the Pediatric Cerebral Performance Category (PCPC) and the Pediatric Overall Performance Category (POPC). We also considered the following prognostic factors when assessing non-randomised studies: age, sex, weight or body mass index (BMI) in percentile, baseline severity, comorbidities and admission diagnosis on the intervention.
-
Intervention details – definition of ERM, type of interventions, the volume of ERM (time-to-initiation, duration, number of sessions), implementation strategies such as safety and progression criteria, involvement of health professionals or availability of organisational support.
-
Study comparators and outcome – components of usual care, primary and secondary outcomes (where specified) and assessment time points. When outcome measures were not specified or reported, the outcomes most proximal to the health domain were considered the primary outcome.
Data were extracted (JT) and independently verified by co-authors (Dr Olivia Craw, JMc and JMen). We used all eligible studies, abstracts or full texts that reported any intervention to summarise types of interventions. We only included full-text reports where ERM was initiated within the first 7 days of admission to PICU to synthesise ERM outcomes.
Quality assessment of individual studies
Risk of bias was assessed in randomised controlled trials (RCTs) the using Cochrane Risk of Bias tool version 24 and in non-randomised studies using the Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) tool. 5 One reviewer (JT) assessed the methodological quality of studies and this was independently verified by a second (Dr Olivia Craw, JMc).
We evaluated the reporting quality of studies using the Consensus on Exercise Reporting Template (CERT). 6 Due to the nature of the study interventions included in this review, participants, providers and assessors were aware of the intervention, which can affect compliance, outcome assessment or intervention fidelity. To understand implementation, we grouped studies that provided information on different aspects of delivering ERM, such as core content of ERM, who commonly delivers it, mode, timing, frequency of delivery, and the adaptation process for tailoring ERM.
Data synthesis
A narrative synthesis of all included studies was undertaken. Due to the heterogeneous nature of paediatric populations and interventions, meta-analysis was not appropriate. All outcomes were grouped as short-term (≤6 months post-discharge) or intermediate-term (≥6 months post-discharge) outcomes.
Results
Characteristics of included studies
As shown in Figure 1, 2580 unique records were screened for relevance, and 62 relevant full-text articles were assessed for inclusion. Twenty-six of these were excluded, mainly due to the ineligibility of the study design or outcomes. Eighteen of the 36 studies that met the eligibility criteria were abstracts, and 18 had full-text reports. Most were conducted in North America,7–25 Australia,26 Belgium,27,28 Brazil,29,30 Italy,31 Japan,32–34 the Netherlands,35 Turkey36,37 and the UK. 38–41 One study was conducted across 15 countries in Europe42 (see Appendix 2, Table 32).
FIGURE 1.
PRISMA diagram.
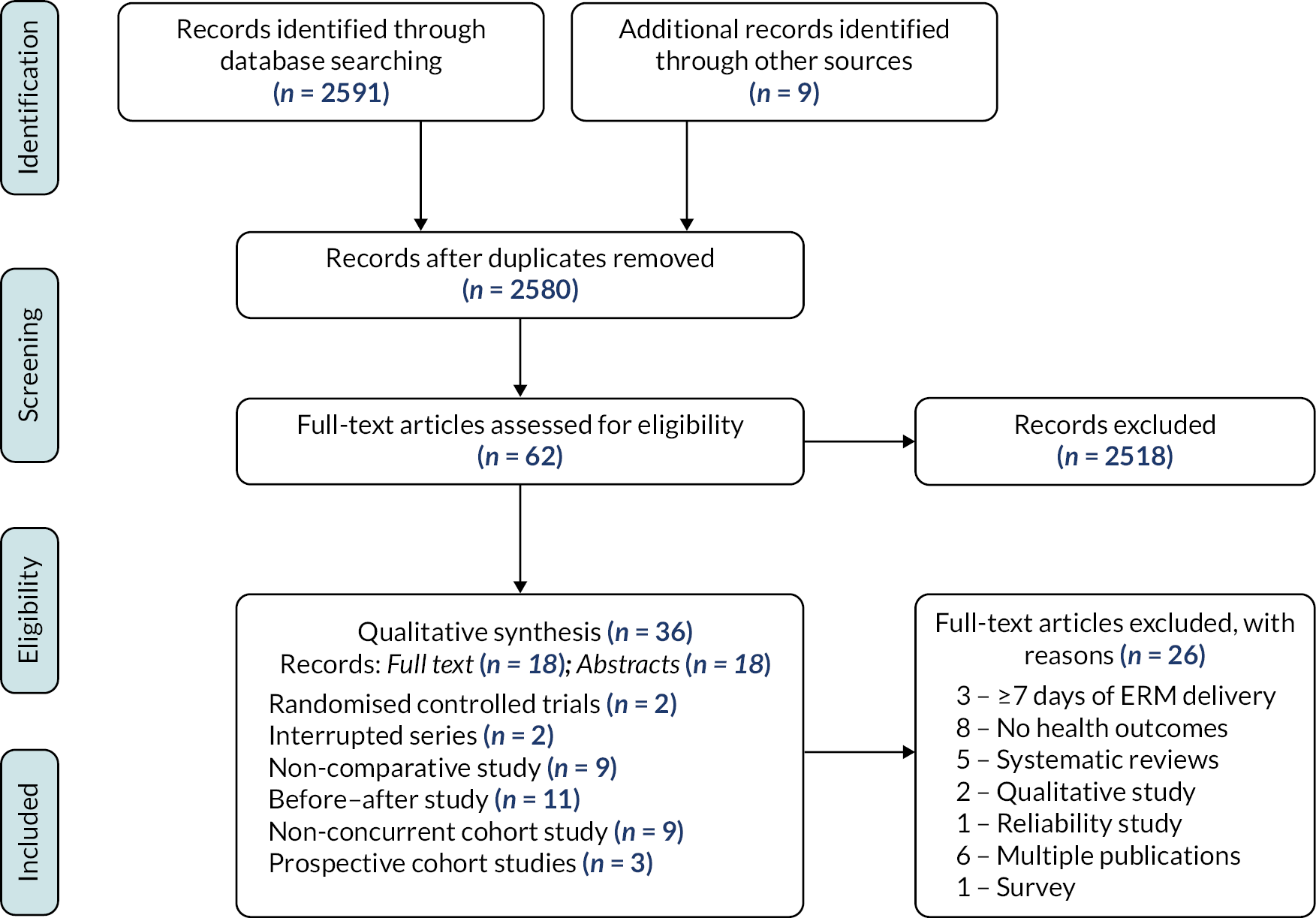
Five studies15,18,21,22,24 were conducted across multiple PICUs, and two studies15,43 used controlled designs. Three studies were prospective cohorts,24,41,42 2 were interrupted-time series,7,27 9 non-comparative studies,8,10,13,22,30,31,34,36,40 11 before–after studies9,11,12,17,23,25,32,33,35,38,44 and 9 non-concurrent cohort studies. 14,16,18,19,21,26,28,37,45
Types of interventions identified
Of the 36 studies that evaluated PICU rehabilitation, we identified two broad categories of early rehabilitation or mobilisation (ERM): non-mobility and mobility interventions. Non-mobility interventions mentioned in included studies were pain and agitation assessment,23 sleep hygiene/delirium screening,17,22,23,35 ERM screening checklist,26 cuddles,18,40 SLT8,12,15,21 and chest PT. 21 The majority were mobility interventions and included mobility goals/orders,22,23,35,40,41 out-of-bed exercises,15,17 in-bed cycling,11,19,20,46 edge-of-bed mobility,40 bed-mobility exercises,15,17,21,32 interactive boxing,7 physical therapy8,10,12–16,18,21,30,32 and OT. 8,10,12–16,18,21,30 When usual care15,20,24 was used as a comparison, it consisted of positioning,
Interventions were commonly administered from 24 to 72 hours after PICU admission. The volume of sessions varied widely, but most sessions were delivered twice daily. In some situations,32 information on initiating ERM delivery was unavailable. Five studies7,11,19,26,43 used single-component interventions. One study13 reported the number of encounters or admissions but provided no information about patient characteristics. Multicomponent interventions (12/16 studies) consisting of PT, OT and SLT were more commonly explored.
Patient and study characteristics
Out of the 36 studies that met our eligibility criteria, 18 full-text records evaluated at least one ERM outcome (as defined by the study authors) within 7 days after admission; the results reported here are for these 18 full-text publications. The study population consisted of day-old children to ≤18 years, with sample sizes of 12–722 participants. In almost all studies (n = 17/18), patients were admitted with a mixture of medical and surgical diagnoses – respiratory, neurological and cardiac conditions.
Outcomes
Among 18 studies that evaluated ERM, the feasibility and safety of ERM alongside process outcomes were the most frequent outcomes considered. See Appendix 2, Table 32.
Adverse events
Fifteen studies7,10–14,17–19,24,25,32,36,42,43 provided information on adverse events (AEs). The most common event was tachycardia/desaturation. 13,14,24,42 Three studies reported haemodynamic changes7,15,42 or tube removals. 7,10,42 Other events mentioned include pain,7 fall,7 behavioural changes,24 excessive secretions24 and discontinuation of therapy. 14,15,43
Evaluation of early rehabilitation and mobilisation interventions
Feasibility in randomised controlled designs
Only two studies used randomised controlled designs, both judged as having a moderate risk of bias. 15,43 These studies15,43 evaluated the feasibility of ERM as a primary outcome. The consent rates were 60%15 and 94%. 43 One RCT, in 58 children aged 3–17 years with brain injury, showed that physical therapy was delivered 80% of the time in the usual-care arm and 100% among patients receiving early protocolised ERM. 15 In addition, patients receiving early protocolised rehabilitation received less post-PICU rehabilitation, but there were no differences in functional or quality of life (QoL) outcomes at 6 months. 15
In a pilot randomised trial with 30 children aged 3–17 years,43 the primary end point was feasibility defined as (1) the ability to enrol at least 75% of eligible patients, (2) an accrual rate of 1–2 patients a month and (3) a 30-day follow-up of >75%. The consent rate was 94%, and the 30-day follow-up rate was 87%. The median time from randomisation to delivery of mobility PT was not different between patients in the control standard mobilisation group [2.3 hours, interquartile range (IQR) 1–20] and standard mobilisation plus in-bed cycling (2.5 hours, IQR 0.9–11 hours). In this study,43 the authors found no difference between arms (0.17, IQR −0.01–0.36) among 24/30 patients (80%) who developed new functional difficulties in PICU [Paediatric Evaluation of Disability Inventory-Computer Adaptive test (PEDI-CAT)]. In the usual-care arm 7/10 (70%) did with median of 0.4 (IQR: 0.3–0.6), compared with 17/20 (85%) in the cycling arm (median: 0.6, IQR: 0.4–0.7). Overall, only 10% of these patients fully recovered functional ability at 1 month, and mobility was the slowest to return. 43 No differences were identified for other outcomes evaluated in these studies. 15,43
Feasibility/prevalence of early rehabilitation and mobilisation in observational prospective and retrospective studies
Single-centre studies
Most studies (n = 16) reported improvements in early mobilisation rates, demonstrating the feasibility of ERM. One study completed enrolment 1 month earlier than anticipated with an 85% consent rate. 19 Another study12 reported more PT and OT ERM consultations. Betters et al. 10 also reported higher ERM consultations (median 30, IQR 29–45 minutes). Alqaqaa et al. 8 reported higher mobilisation rates among non-ventilated patients (mean difference of a day) compared to no change for mechanically ventilated patients. In another study,17 mobilisation and OT consultation rates increased, but this change was not significantly different for PT consultations.
Choong and colleagues11 showed higher lower-limb activity during in-bed cycling [mean ± standard deviation (SD) 266.47 ± 166.12] versus during non-intervention times (mean ± SD 20.94 ± 15.26, counts/20 minutes, p < 0.001) different to baseline. In another study,21 the median time to mobilisation was 2 days (IQR 1–6) compared to 1 day for non-mobility interventions (IQR 1–3). Likewise, the frequency of physical therapy improved in another study,32 with more patients achieving their rehabilitation goals.
Multicentre studies
In a multicountry study,42 the prevalence of PT- and/or OT-provided mobility was 39% [95% confidence interval (CI) 34.7 to 43.9%] and did not differ according to baseline neurodevelopmental function level (PCPC ≤ 2.22% vs. PCPC ≥ 3.26%, p = 0.331), while PT or OT consultations were higher in a different study, ordered in 68/128 (49.6%) within 2 days (1–5 days) after PICU admission. 24 The prevalence of out-of-bed mobility was nearly two-thirds (87/110; 63.5%), passive range-of-motion 13.9% and no activity 19.7%. Out-of-bed mobility was common among non-mechanically ventilated children (48/56; 85.7%) and those under 3 years (71/100; 71%), while older children (18/37; 48.6%) were commonly not actively mobilised. 24 Similarly, consultations for all admissions were increased from 25% pre-implementation to 56% (p < 0.001) post implementation,25 while consultations within 2 days increased from 34% to 67% (p < 0.001) and within 3 days, from 21% to 30% (p = 0.02) for at least one PT and/or OT mobility or 29% to 35% (p = 0.29) for mobilisations.
In another multicentre study, ~70% of children18 received at least one mobilisation intervention. For children on mechanical ventilation (MV), one-third of those <3 years and ~50% of those ≥3 years were mobilised using passive range of movement (ROM) (72%). 18 Out-of-bed mobility was achieved 70% of the time, but less frequently among mechanically ventilated children, 47% (95% CI 44% to 49%).
Secondary or clinical outcomes in observational prospective and retrospective studies
Single-centre studies
Choong et al. 19 found improved functional ability measured using PEDI among 28% and 42% of participants at 3 and 6 months. Only 22% of those with a pre-existing chronic condition and 14% with functional limitations returned to baseline levels at 6 months. In comparison, 60% of previously healthy children and 58% of children with normal baseline function regained full functional abilities. The overall mortality rate was 3/33 (9%), and 19/33 (63%) were readmitted within 6 months after PICU discharge. 19
Alqaqaa et al. 16 reported small improvements in length of stay [LOS; average LOS pre-WeeMove = 6.25 days vs. post-WeeMove LOS = 5.23 days] and time spent intubated (pre-WeeMove was 27.86 hours, post-WeeMove = 25.09 hours). 16 In contrast, Abdulsatar and co-authors7 noted that PCPC scores were worse (mean ± SD change of 1.08 ± 1.0, p = 0.02) among two-thirds of patients who received ERM. 7 There was no improvement in grip strength or physiological status, despite increased activity levels (mean ± SD, upper-limb (UL) activity pre: 9.36 ± 4.12 vs. post: 57.12 ± 46.60 counts) and higher carer satisfaction. 7
Colwell and co-authors reported higher adherence among younger patients (p = 0.04), with higher baseline severity of illness (p < 0.001) when mobilisation sessions were goal-directed (p < 0.001). However, there were no significant differences in mobilisation rates (pre: mean = 0.86 vs. post: = 0.84) or AEs 14/560 (2.5%, p = 0.18). 13 In the study by Wieczorek et al., nearly half of the children (48/100) received at least one ERM intervention by day 3 of admission, and the proportion of children receiving at least one in-bed activity increased by 18% from post intervention (p < 0.001). 17 However, there was no change in passive ROM, only an increase in active interventions (57% vs. 26%; p < 0.001), especially among children ≥3 years. There was also a slight increase from 0% (0/39) ambulation while orally intubated to 10% (4/40) post intervention. 17
Multicentre observational studies
Studies described a lower amount of mobilisation among younger patients with higher baseline disability13,17,18 or severity of illness on admission. 13,18 One study21 showed that older, less sick children admitted during the winter who were not mechanically ventilated, sedated or receiving neuromuscular blockade were more likely to receive mobility interventions. Similarly, consultations within 3 days were higher among males, older children and those with lower baseline function, without an indwelling endotracheal tube (ETT) or urinary catheter, who had adequate family support. 18
Predictors of early rehabilitation and mobilisation
Three studies evaluated predictors of ERM using multivariate analysis. 18,24,42 The adjusted odds ratio (aOR) for out-of-bed mobility was negatively associated with the presence of an ETT (aOR, 0.13; 95% CI 0.08 to 0.2), a urinary catheter (aOR, 0.28; 95% CI 0.14 to 0.57), opioid infusion (0.42; 95% CI 0.24 to 0.73) and severe baseline disability (PCPC 4 vs. 1) (aOR, 0.59; 95% CI 0.4 to 0.87). Longer PICU LOS and lower nurse-to-patient ratio (1.82; 95% CI 1.2 to 2.8) increase the odds of out-of-bed mobilisation. For children <3 years old, family presence was associated with out-of-bed mobility (aOR, 4.55; 95% CI 3.1 to 6.6) while being older predicted PT or OT (aOR, 3.1; 95% CI 2.01 to 4.79). 18 In another study,24 the presence of ETT or infusion among children ≤3 years reduced the likelihood of receiving therapist-provided out-of-bed mobility [odds ratio (OR) 3.62; 95% CI 1.49 to 8.82]. However, this improved when the family were present. 24
Ista and colleagues42 showed that older age (2.28, 95% CI 1.23 to 4.22), moderate baseline disability (defined as PCPC: 3 vs. 1) (2.12, 95% CI 1.02 to 4.56), severe baseline disability (PCPC: 4 vs. 1) (2.24, 95% CI 1.14 to 4.40), having a central venous line (CVC) in place (aOR 1.63, 95% CI 1.02 to 2.62) and family presence (aOR 5.13, 95% CI 2.55 to 10.32) increased the odds of receiving a mobility session. However, the presence of a urinary catheter reduced the chance of mobilisation (aOR 0.46, 95% CI 0.22 to 0.92). MV through an ETT (aOR 0.29, 95% CI 0.12 to 0.68), being admitted for a surgical reason (aOR 0.58, 95% CI 0.35 to 0.95) and the presence of a urinary catheter (aOR 0.39, 95% CI 0.19 to 0.81) reduced odds of out-of-bed mobility, but this improved with family presence (aOR 7.83, 95% CI 3.09 to 19.79). 42
Quality assessment
Controlled trials
Two studies15,43 randomly assigned participants into groups and were assessed using RoB v2. One study43 ensured allocation concealment using a computer-generated sequence and reported adequate sample size considerations. In the other study,43 sample size calculations were not applicable; consequently, outcomes were possibly underpowered. We did not identify attrition bias for both studies, outcomes assessment was similar at baseline, and co-interventions were similar across groups.
Prospective and retrospective studies
We used the ROBINS-I tool to assess the quality of observational studies (see Appendix 2, Table 33). Overall, most studies were judged to have a serious or critical risk of bias. Three studies (3/18)18,24,42 were judged to have a low risk of bias. Nine10,12,13,15,19–21,25,32 were judged to be at moderate risk of bias, while in four there was serious risk. 11,14,16,31 In some studies bias was judged as critical,8 or information reported was insufficient17 and could not be assessed (Figure 2).
FIGURE 2.
Pictorial presentation of quality assessment using ROBINS-I.
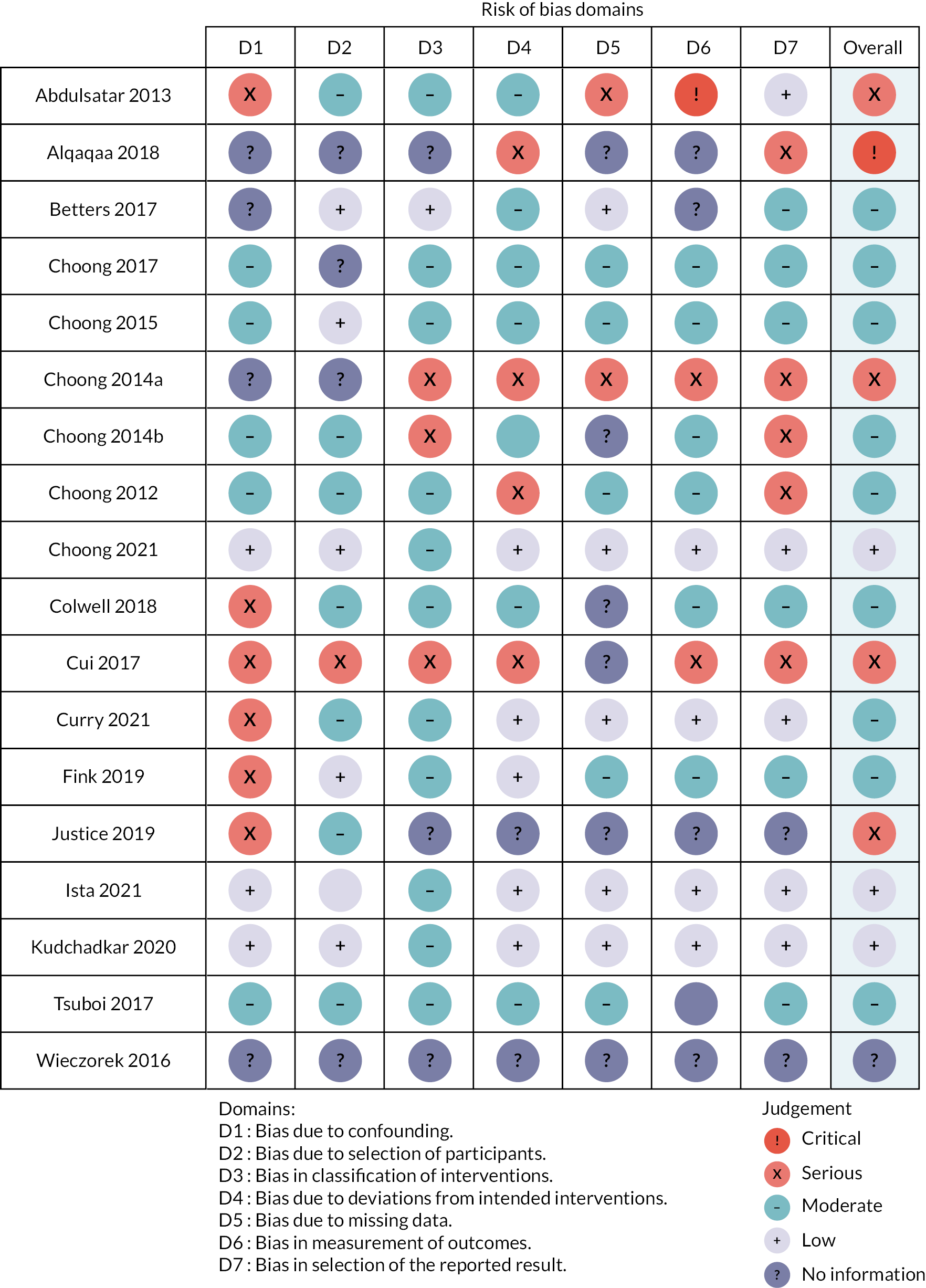
The primary reason for downgrading studies was bias due to blinding or poor consideration of baseline confounding. There was no indication of selection bias during enrolment; studies were judged as adequate except in three retrospective studies. 14,17,31 The lack of a clear definition of ERM hierarchy limited the evaluation of demonstrable effects on objective clinical outcome measures. This made it challenging to determine intervention superiority. Broadly, intervention categories of non-mobility and mobility were consistent across studies, which were judged to be at risk of misclassification bias half of the time (9/18). We assessed the impact of bias due to deviations from the intended response as adequate in most studies. In some studies,7,8,10,11,13,14,16,17,21,32 the technique for handling missing data was unclear or not reported. We did not identify any evidence of selective outcome reporting or errors due to outcome measurements. Most studies demonstrated congruence between previously defined analyses and outcomes reported. However, none of the studies published a protocol. Overall, most studies did not provide definitive evidence of the effects of the intervention. However, consistent evidence across all studies supports the feasibility of ERM as an intervention in PICU, while physical therapy was the most common intervention considered.
Quality of consensus on exercise reporting
We described techniques considered during intervention design and excluded items not relevant to this review. These items included item no. 3 (descriptions of individual or group exercises), item no. 9 (use of home equipment; discharge interventions were not considered) and item no. 12 (setting of exercise delivery; all studies were conducted in PICUs). See Appendix 2, Table 34 for details.
Quality of consensus on exercise reporting reporting
Most studies (12/18)7,10,16–18,20,21,24,25,31,32,42 provided details on the type of ERM intervention to aid replication. Some studies (4/18)18,21,25,32 provided training or engaged multidisciplinary teams (MDTs) to facilitate ERM delivery. Organisational strategies were sometimes applied across MDTs to facilitate PICU culture change. Some studies (12/18) explicitly mentioned using qualified providers such as physiotherapists, nurses or other therapists to supervise sessions and ensure intervention fidelity. 10,11,13–15,17,18,21,24,25,42,43 However, the detail provided was insufficient to explain how differences in experience levels, treatment approaches and therapists’ behaviour in these circumstances influence outcomes.
Intervention components were generally tailored and not standardised. Sometimes, it was unclear what aspects of the interventions were usual practice or complementary during ERM delivery. In most studies, information on how deviations from study protocols were handled (i.e. regression or progression) and how these events may have affected outcomes was unavailable. Personalising the volume of ERM was a common concept across studies used to improve compliance. However, since interventions were tailored to tolerance levels, we did not assess intervention fidelity. Some studies provided a detailed description of how compliance or adherence was measured. Strategies mentioned include rehabilitation sheets11,19,20,32 and electronic records during PICU ward rounds. 17,18 Two studies7,11 used an objective outcome to measure compliance – ACTi-graph accelerometers. This outcome measure can be used as a benchmark for future studies, incorporating routinely collected outcomes to increase transferability. 47 Labour-intensive or ad hoc approaches for determining adherence or compliance, such as caregiver verbal confirmation following direct observation, charts or checklists, may limit the implementation of rehabilitation and future attempts at service evaluation.
Early rehabilitation and mobilisation change techniques
No study provided explicit details about applying complex intervention theories when designing interventions, while details on the implementation processes were inconsistently reported. Therefore, it is unclear to what extent interactions between intervention volume of ERM and health systems produce positive effects. It is not impossible to envisage a situation where contextual factors such as how the intervention works, for example the presence of a local champion or an ERM enthusiast, staff turnover, population demographics, or existing PICU culture, affect outcomes, either positively or negatively. No study provided details on motivation strategies or precise details on how interventions were personalised. Safety guidelines (underpinned by clinical stability), verbal feedback and tolerance levels to determine progression were commonly used across studies. Eight studies10,14,17,18,21,24,25,42 provided information to enable replication. Hence, these studies can be used as a springboard to undertake detailed intervention mapping when designing ERM manuals. Overall, key aspects of intervention delivery were poorly reported, such as co-interventions, strategies for tailoring interventions and motivating patients.
Discussion
This review aimed to summarise evidence on the effectiveness of ERM research within PICUs. We narratively synthesised evidence to improve interpretation of effectiveness given variation in ERM implementation. We identified a broad range of activities, categorised as non-mobility and mobility interventions. Other interventions identified but not considered in this review include undefined ERM,48–50 music therapy51 and neuro-psychological training. 52 This review suggests that interdisciplinary multicomponent interventions, sometimes delivered as a bundle, are feasible, safe, acceptable and possibly beneficial to patients. The programmes mainly were designed using safety criteria, were goal-directed and tailored. Although the rate of intervention-related AE reporting across all studies was low, we cannot rule out selective reporting as none of the included studies had published their statistical analysis plan a priori.
Given the limited description of interventions, intervention manuals and process data would be essential to understand the complexity of ERM. There are also organisational factors that need to be considered when implementing ERM. What remains unclear is the number of organisational levels that should be targeted and how. Other issues to consider include mechanisms of effect (moderators – participants’ responses to and interactions with the intervention and mediators that affect intervention outcomes in unexpected ways). Administering staff training was a common feature we identified across studies to ensure consistency. However, nursing capacity required to deliver the intervention and family involvement need to be carefully considered. Family involvement is crucial when considering non-mobility ERM for children under 3 years old.
Evidence
We found that children under 3 years of age admitted to PICU tend to be given passive and active in-bed activities. Children 3 years and older were primarily involved in out-of-bed activities when this was considered safe. In addition, one study18 reported higher ERM incidence among male children, but this finding was not consistent across studies. Hence, it is unclear what interventions should be administered to these age groups and whether certain combinations of interventions are superior to those given to their older counterparts.
We found equivocal evidence suggesting that other factors such as time to admission, tube presence, MV, or sedation also influenced developmental or mobility goals. Only four studies13,18,24,42 evaluated baseline severity of illness (measured using validated tools) and comorbidities that affected clinical and functional outcomes in multivariate models. Consequently, the effect of residual confounding or chance on the estimated intervention effect remains unknown. Overall, the evidence about subgroup effectiveness was indicative of clinical pragmatism but otherwise inconclusive.
Comparison with the broader literature
Our findings reflect the evidence in previous systematic reviews of ERM interventions within adult and paediatric PICU. 53–56 The evidence emerged from North American PICU contexts, and its transferability to the UK settings remains uncertain. 38,39,46,57 Besides variation in practice, interactions within complex health systems have additional issues. As an additional complexity, due to the nature of interventions considered in this review, it is difficult to determine if intervention effects are additive, multiplicative (biologically plausible) when combined or neutralise each other and plateau.
Most outcomes of feasibility or acceptability were exploratory findings, and the strength of the evidence for objective clinical or functional outcomes remains unclear. Nevertheless, PT and OT, sometimes with SLT, were frequently implicated in the exposure-mechanism-outcome pathway. Furthermore, barriers reported reflect previous literature and support the need for activity orders.
Summary of findings to inform the paediatric early rehabilitation and mobilisation during intensive care study
-
Optimal aspects of intervention delivery – timing, content, active ingredients, dose–response relationships, progression and implementation strategies – have yet to be established.
-
Improvements in functional independence (though small and sometimes inconsistent) have not been matched with improved PICU-acquired weakness or survival measures. ERM appears safe but requires long-term studies.
-
Standardised definitions for ERM, safety and core outcome measures will improve comparability across studies. Authors should consider the effect of an intervention on core outcome sets recommended for paediatric critical care. These include four domains: global cognition, emotional, physical and overall health. Four child-specific outcomes of health-related QoL, pain, survival and communication have also been recommended. 47 Benefits so far indicate some improvement in QoL.
-
ERM has been demonstrated to be feasible and acceptable within PICU. There are still uncertainties about the effectiveness of ERM interventions; only two studies used randomised designs. The evidence uncertainty is worse for objective outcomes such as PICU LOS. Overall, non-mobility rather than mobility interventions seem to be preferred in children 3 years and younger compared to their older counterparts.
-
The lack of a well-defined ERM protocol is a significant barrier to ERM implementation. There is no clear evidence on the impact of bundles of care or behavioural interventions incorporated with ERM. We identified several studies that evaluated the feasibility of ERM in PICU, some of which report improved QoL as a longer-term outcome. However, the evidence for effectiveness is inconsistent, uncertain and needs further testing. Most studies were quality-improvement studies, which may be the best methodology for evaluating ERM within PICU until a better consensus on intervention components is achieved. As an alternative, nested controlled trials embedded within longitudinal studies or routine data collection can be used to evaluate the effectiveness of these interventions. Data from such studies might also enable mediation analysis to understand key intervention components and mechanisms of action.
Chapter 2 Phase 1b: national survey to establish standard practice
Introduction
This study involved a national electronic web-based survey for paediatric intensive care healthcare professionals to understand the context and professional perspectives of delivering early rehabilitation and mobilisation (ERM) within UK PICUs.
Study management
The work package was led by BRS. JYT co-ordinated survey responses and developed the on-line tool. The study management group provided input into survey questions and designs. The study was piloted in Birmingham and Nottingham by JYT, Emily Brush and Francesca Ryde. Statistical analysis of the full survey was undertaken by JYT, BRS, JMen, JMan and JMc. Qualitative analysis of the free-text responses was undertaken by JYT.
Aims and objectives
To explore how healthcare professionals describe ERM, identify current ERM practice and understand perceived barriers and facilitators of ERM.
Methods
A web-based survey (administered through www.smartsurvey.co.uk) was developed that included 25 questions that related to the study aims. The survey was piloted with multidisciplinary teams of health professionals (n = 40) at two PICUs to assess acceptability and comprehensiveness. Minor changes to improve question clarity were made. Pilot responses were excluded from main survey analysis.
The University of Birmingham granted institutional ethical approval on 5 February 2019 (reference ERN_18-1134). Consent was implied through survey completion.
The survey was administered using a chain-referral method. A UK Paediatric Critical Care Society Study Group (PCCS-SG) member from each UK PICU (n = 29) was contacted via e-mail and requested to identify and cascade an invitation e-mail to members of their local MDT (including at least one physiotherapist, doctor and nurse). Participating PICUs were sent a survey link between May and August 2019 to distribute. Three follow-up reminders were sent at weekly intervals to PICUs that had not responded.
Statistical analysis was performed using R version x64 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Data were analysed using descriptive statistics, with categorical responses expressed as numbers (percentage), with Likert scales (median IQR) used to express the frequency of practice or level of agreement. Ranking of perceived ERM benefits was calculated using the sum of ranked scores of respondents’ top five important benefits (five points for first, reducing to one point for fifth placed).
Free-text data from the open-ended responses were analysed using a qualitative content analysis approach. 58 Two researchers independently familiarised themselves with the data and conducted open-coding, utilising NVivo™ (QSR International, Warrington, UK) software for data management. Codes were then discussed, summarised and organised. 59,60 Anonymised, free-text quotes from respondents are used in the reporting of this analysis to add context and clarity. 61
Results
Demographics
We received responses from PCCS-SG link members in 26/29 (90%) UK PICUs. A total of 191 health-care professionals opened the survey link with 124 (65%) submitting responses, with a median of 4.5 participants (IQR 3–6) per PICU.
Most respondents were nurses (n = 34, 27%), physiotherapists (n = 28, 23%) and doctors (n = 22, 18%) (see Appendix 3, Table 36). Respondents also included occupational therapists (OT) (n = 19, 15%), play therapists (n = 7, 6%), psychologists (n = 7, 6%), dieticians (n = 6, 5%) and SLTs (n = 1, 1%). Almost three-quarters of health professionals had ≥5 years’ experience, with 48 (39%) ≥15 years.
Description of early rehabilitation and mobilisation
We invited participants to describe ERM in their own terms, with 104 (84%) responding. Participant definitions of ERM aligned to four categories, ‘Activity-focused’, ‘Tailored’, ‘Promote recovery’ and ‘Timing of ERM’ (Table 1). Overall ERM was an individualised package of graded interventions, based on an activity-focused programme, to reduce the sequelae of critical illness or injury. However, responses differed for when ERM should be initiated, often emphasising the need for individualisation.
| Category | Subcategories | Examples |
|---|---|---|
| Activity-focused ERM is activity-focused, with consideration of seating and equipment, primarily delivered by nursing and allied healthcare professionals with optimised sedation management to promote patient engagement. There is a lack of consensus on what is routine care compared with purposeful ERM activity |
Activity – mobilising, positioning, stretching | ‘ERM provided includes – passive movements of limbs, periodic change of position, use of splints on extremities and if stable in the long-term patients – sitting up in tumble form chair and mobilising out of bed.’ (Doctor, 67) ‘We aim to get the children sitting upright, either over the edge of the bed or in appropriate seating as soon as possible.’ (OT, 001) ‘Nursing staff are taught the importance of regular position changes for patient … and positioning to maintain ranges of movement and prevent foot drop.’ (Nurse, 017) |
| Core healthcare professional involvement – physiotherapist, nurse, OT | ‘We try and mobilise patients as soon as able when not invasively ventilated – physio led.’ (Doctor, 058) ‘As physiotherapists, we work with the OT and nursing staff to either re-position, sit up in bed, sit on the edge of the bed or stand whilst on the ventilators.’ (Physiotherapist, 069) |
|
| Additional healthcare professional involvement – psychology, dietician, play therapist, SLTs | ‘Speech and language therapy become involved usually when nursing or medical staff identify a need for referral.’ (Doctor, 003) ‘We have a Play Specialist on PICU, who assists with communication tools/toys.’ (Nurse, 082) |
|
| Seating and equipment | ‘Specialist seating need – linking in with OT to ensure appropriate seating available.’ (Physiotherapist, 019) ‘We have also in the past asked adult (services) to use their moto-med bike and used this with teenagers, but this can be a challenge as it is very far from PICU and it is often in use.’ (Physiotherapist, 014) |
|
| Sedation management | ‘Working with the medics to wean sedation as quickly as possible. This promotes faster ability to mobilise and progress in their rehabilitation.’ (Nurse, 034) | |
| ‘Routine care’ vs. ‘purposeful ERM package’ | ‘Some centres define ERM as passive range of movement, repositioning or providing splints. We would deem this as essential core cares rather than ERM; the large majority of our patients will have positioning and movement plan from day 1 of admission.’ (Physiotherapist, 111) | |
| Tailored Following the assessment of needs and patient and family preference, ERM activities are personalised to the individual patient |
Assessment of need | ‘We have a programme where patients are categorised to one of 3 levels. Each level provides nursing and therapy staff with activities aligned to the acuity of the patient.’ (Nurse, 040) |
| Individual preference | ‘Parents will tell us what their child enjoys doing and make suggestions, often provide toys/games from home.’ (Nurse, 076) ‘Discuss with parents what patient enjoys doing, watching etc. Discuss options regarding taking patients “out” where possible if long-term patients.’ (Nurse, 015) |
|
| Promote recovery The purpose of ERM is to normalise the PICU environment, to create an environment that addresses holistic needs, sustains or promotes development and supports recovery from critical illness |
Normalising PICU environment | ‘Provide activities they would use for their enjoyment. This enables them to be more relaxed and less aware of what is going on with/around them.’ (Play specialist, 102) ‘Being in PICU can be a frightening experience. Not only is a child/young person away from home, but also away from their usual environment, family and friends. There are unfamiliar sounds, smells, equipment and people. The play specialist can help to make the stay much more enjoyable and children/young people to cope and understand.’ (Play specialist, 055) |
| Sustain/promote child development | ‘Encouraging the patient to regain or further their development in a therapeutic fun manner.’ (Play therapist,102) | |
| Restoration/recovery | ‘Interventions aim to promote physical recovery – movement, and ability to engage in activities and psychological recovery – orientation, speech, ability to play and attend school.’ (Nurse, 021) | |
| Timing of ERM The optimal timing of ERM initiation is challenging for healthcare professionals to define, although likelihood perceived to increase with increased LOS. Influenced by the perceived stability of patients and the balance of risk to benefit |
‘Early’ poorly defined | ‘I like to think we consider it within 2–3 days we have a better idea of the patients’ PICU journey and how long they are going to stay. The reality is it’s usually longer … usually week 2 of admission if they are still on the unit.’ (Physiotherapist, 090) ‘There is no formal plan for who decides if a patient should start ERM and when …. Currently the delivery of ERM is fairly ad hoc, based on what is highlighted from daily handover each morning.’ (Physiotherapist, 083) ‘We provide ERM as a structured programme … every patient after 24 hours of admission is considered for ERM.’ (Doctor, 012) |
| Patient stability, risk and benefit | ‘Members of the PICU team with less experience of early rehab can deem ERM “unsafe” which can occasionally act as a barrier.’ (Physiotherapist, 016) ‘Usually ERM activity is not considered until patients can physiologically tolerate movement and are stable with observations.’ (Nurse, 008) |
|
| ‘Long-stay’ | ‘ERM is often thought about as a patient comes up to extubation or has failed extubation, and we feel that the reason for failure may be because of critical care weakness.’ (Physiotherapist, 031) ‘We provide very little ERM on PICU, other than for long-term patients, which is often, in my opinion, delayed.’ (Nurse, 007) |
Most respondents considered ERM to be a priority, either crucial 15 (12%), very important 67 (55%) or important 35 (29%) in the care of PICU patients (see Appendix 3, Table 37).
Availability of established early rehabilitation and mobilisation protocols
Respondents were asked to describe the content of established ERM protocols within their PICU. Only 12 (10%) participants from 5/26 PICUs reported having an established ERM protocol. The most common components of ERM protocols were ‘physical therapy not requiring additional equipment’ (9/12, 75%) and ‘OT interventions’ (8/12, 67%). Only 4/12 (33%) referred to play therapy or SLT, and no ERM protocol specified input from psychologists or psychiatrists.
All participants were asked about the content of non-ERM protocols in their PICU. Only 18/124 (15%) participants reported that guidance for physical or OT activities existed in other, non-ERM protocols that were used in the PICU setting (see Appendix 2, Table 35).
Recipients of early rehabilitation and mobilisation
Despite the paucity of ERM protocols, 51 (41%) respondents reported that all PICU patients ‘always’ or ‘very often’ received ERM (Table 2). ERM was reported to be more likely to be delivered to patients when PICU LOS exceeded 28 days. Patients admitted for 28 days or more were more likely (91, 75%) to ‘always’ or ‘very often’ receive ERM in comparison to only 17 (13%) of those reported to stay fewer than 3 days. Participants reported that patients with acquired brain injury (75, 60%) and severe developmental delay (54, 44%) were ‘always’ or ‘very often’ likely to receive ERM.
| By age group | Always, n, (%) |
Very often, n, (%) |
Sometimes, n, (%) |
Seldom, n, (%) |
Never, n, (%) |
Don’t know, n, (%) |
Not applicable,b n |
|---|---|---|---|---|---|---|---|
| All PICU patients (any age) | 6 (5) | 45 (36) | 48 (39) | 14 (11) | 0 (0) | 11 (9) | 0 |
| >10–18 years old | 18 (15) | 40 (32) | 43 (35) | 8 (6) | 1 (1) | 14 (11) | 0 |
| >4–10 years old | 16 (13) | 42 (34) | 41 (33) | 10 (8) | 1 (1) | 14 (11) | 0 |
| 1–4 years old | 13 (10) | 42 (34) | 39 (31) | 13 (10) | 1 (1) | 16 (13) | 0 |
| Infants and <1 year old | 6 (5) | 47 (38) | 32 (26) | 20 (16) | 3 (2) | 16 (13) | 0 |
| By LOS in PICU | |||||||
| PICU > 28 days | 43 (35) | 48 (39) | 14 (11) | 4 (3) | 0 (0) | 15 (12) | 0 |
| PICU > 7–28 days | 27 (22) | 46 (37) | 26 (21) | 8 (6) | 0 (0) | 17 (14) | 0 |
| PICU 3–7 days | 12 (10) | 32 (26) | 40 (32) | 19 (15) | 5 (4) | 16 (13) | 0 |
| PICU < 3 days | 3 (2) | 14 (11) | 39 (31) | 33 (27) | 17 (14) | 18 (15) | 0 |
| By diagnostic category | |||||||
| Acquired brain injury | 36 (29) | 39 (31) | 16 (13) | 11 (9) | 4 (3) | 18 (15) | 0 |
| Severe developmental delay | 11 (9) | 43 (35) | 39 (31) | 12 (10) | 3 (2) | 16 (13) | 0 |
| Cancer | 11 (9) | 24 (19) | 36 (29) | 14 (11) | 4 (3) | 35 (28) | 0 |
| Pre-existing physical morbidity | 9 (7) | 38 (31) | 44 (35) | 12 (10) | 4 (3) | 17 (14) | 0 |
| Mechanically ventilated | 9 (7) | 38 (31) | 39 (31) | 18 (15) | 5 (4) | 15 (12) | 0 |
| Congenital heart disease | 9 (7) | 24 (19) | 39 (31) | 15 (12) | 6 (5) | 31 (25) | 0 |
| Respiratory illness | 7 (6) | 35 (28) | 39 (31) | 22 (18) | 3 (2) | 18 (15) | 0 |
| Sepsis | 7 (6) | 28 (23) | 44 (35) | 20 (16) | 5 (4) | 20 (16) | 0 |
| Multiorgan failure | 5 (4) | 14 (11) | 32 (26) | 35 (28) | 12 (10) | 26 (21) | 0 |
| Mechanically supported (e.g. extracorporeal life support) | 4 (3) | 8 (6) | 14 (11) | 13 (10) | 47 (38) | 38 (31) | 0 |
| Healthcare professional team members and parent or family member(s) involvement in ERM (when applicableb) | |||||||
| Physiotherapist | 86 (70) | 27 (22) | 4 (3) | 2 (2) | 0 (0) | 4 (3) | 1 |
| Nurse | 54 (44) | 49 (40) | 9 (7) | 5 (4) | 0 (0) | 6 (5) | 1 |
| Parent or family member | 36 (29) | 56 (46) | 19 (15) | 7 (6) | 1 (1) | 4 (3) | 1 |
| OT | 23 (19) | 34 (29) | 29 (24) | 20 (17) | 6 (5) | 7 (6) | 5 |
| Doctor | 22 (18) | 20 (16) | 26 (21) | 30 (24) | 13 (11) | 12 (10) | 1 |
| Play therapist | 12 (10) | 24 (21) | 39 (34) | 26 (23) | 9 (8) | 5 (4) | 9 |
| Dietician | 10 (8) | 20 (17) | 12 (10) | 29 (24) | 37 (31) | 13 (11) | 3 |
| SLT | 4 (3) | 11 (9) | 40 (33) | 35 (29) | 20 (17) | 10 (8) | 4 |
| Psychologist | 3 (3) | 11 (10) | 24 (23) | 32 (30) | 30 (28) | 6 (6) | 18 |
Perceived benefits of early rehabilitation and mobilisation
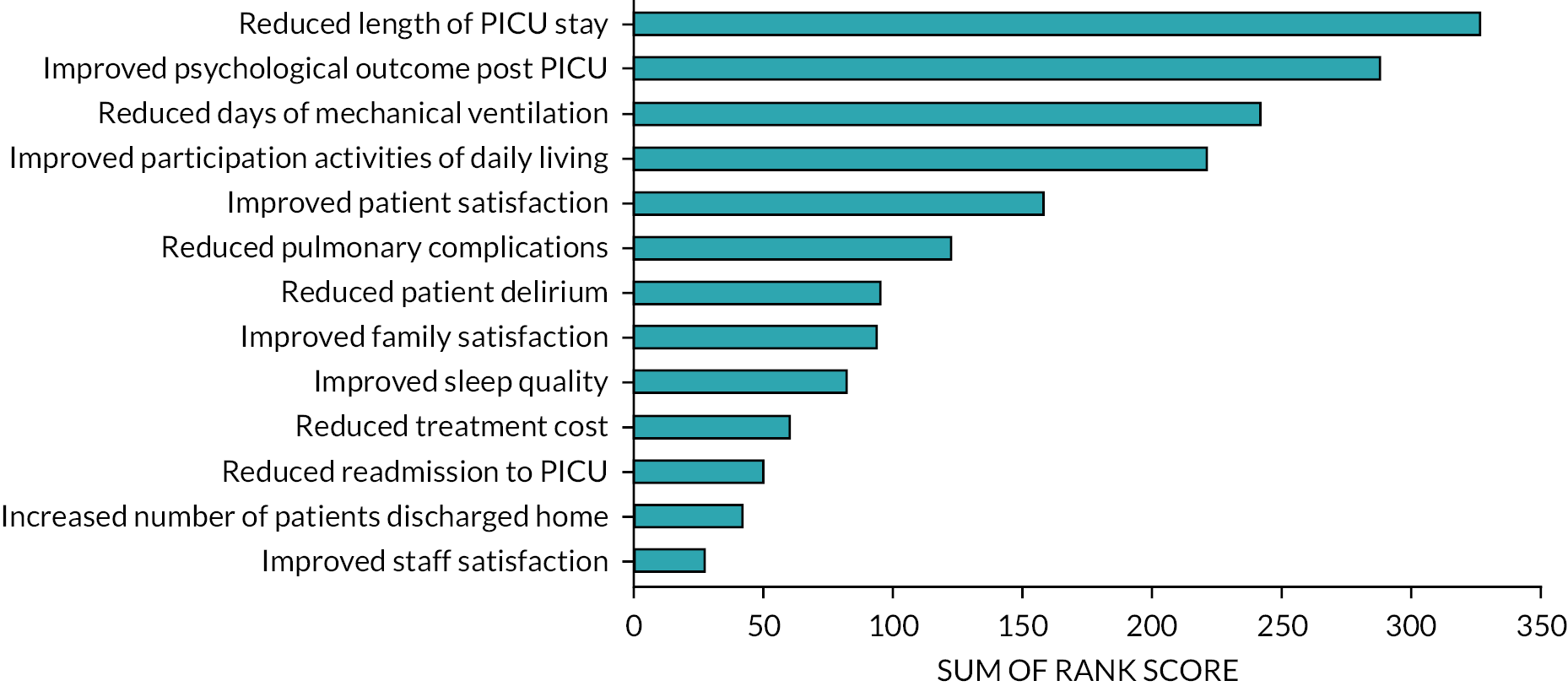
Participants ranked the 5 most important potential benefits of ERM out of 13 options (Figure 3). The most important outcomes identified were: (1) reduced PICU LOS, (2) improved psychological outcomes for patients after PICU, (3) reduced days of MV, (4) improved participation in activities of daily living and (5) improved patient satisfaction.
FIGURE 3.
Perceived benefits of ERM. Ranking of participants’ potential top five perceived benefits of delivering ERM within PICUs. Sum of rank score: ranking of top five (1–5) (first-placed rank scored five points to fifth placed scored one point). Ranked scores of 121/124 (98%) participants.
Initiation and delivery of early rehabilitation and mobilisation
The decision for ERM initiation was perceived by respondents to be primarily led by physiotherapists (96, 77%), doctors (92, 74%) and bedside nurses (64, 52%). Parents were felt to initiate ERM by only 24 (19%) of respondents (see Appendix 3, Table 38).
Factors that influenced ERM initiation included patient stability (69, 56%) and LOS, specifically within 3 days of admission (31, 25%). Five (4%) respondents reported they would not consider ERM at all. The influence of perceived clinical stability is demonstrated in respondents’ free-text comments:
We are involved as early as required depending on the child/young person medical stability and their rehabilitation needs.
(OT, 033)
Usually, ERM activity is not considered until patients can physiologically tolerate movement and are cardio-vascularly stable.
(Nurse, 008)
Assessment of patient stability and tolerance of ERM were less well described. Most respondents (98, 79%) provided subjective cues or informal clinical criteria. These included monitoring of vital signs, physiological changes, observation of behavioural changes and documentation of AEs.
Physiotherapists (113, 92%), nurses (103, 84%) and parents or family members (92, 75%) were ‘always’ or ‘very often’ involved in the ongoing delivery of ERM, with less frequent input from other members of the MDTs (see Table 2).
Barriers to early rehabilitation and mobilisation implementation
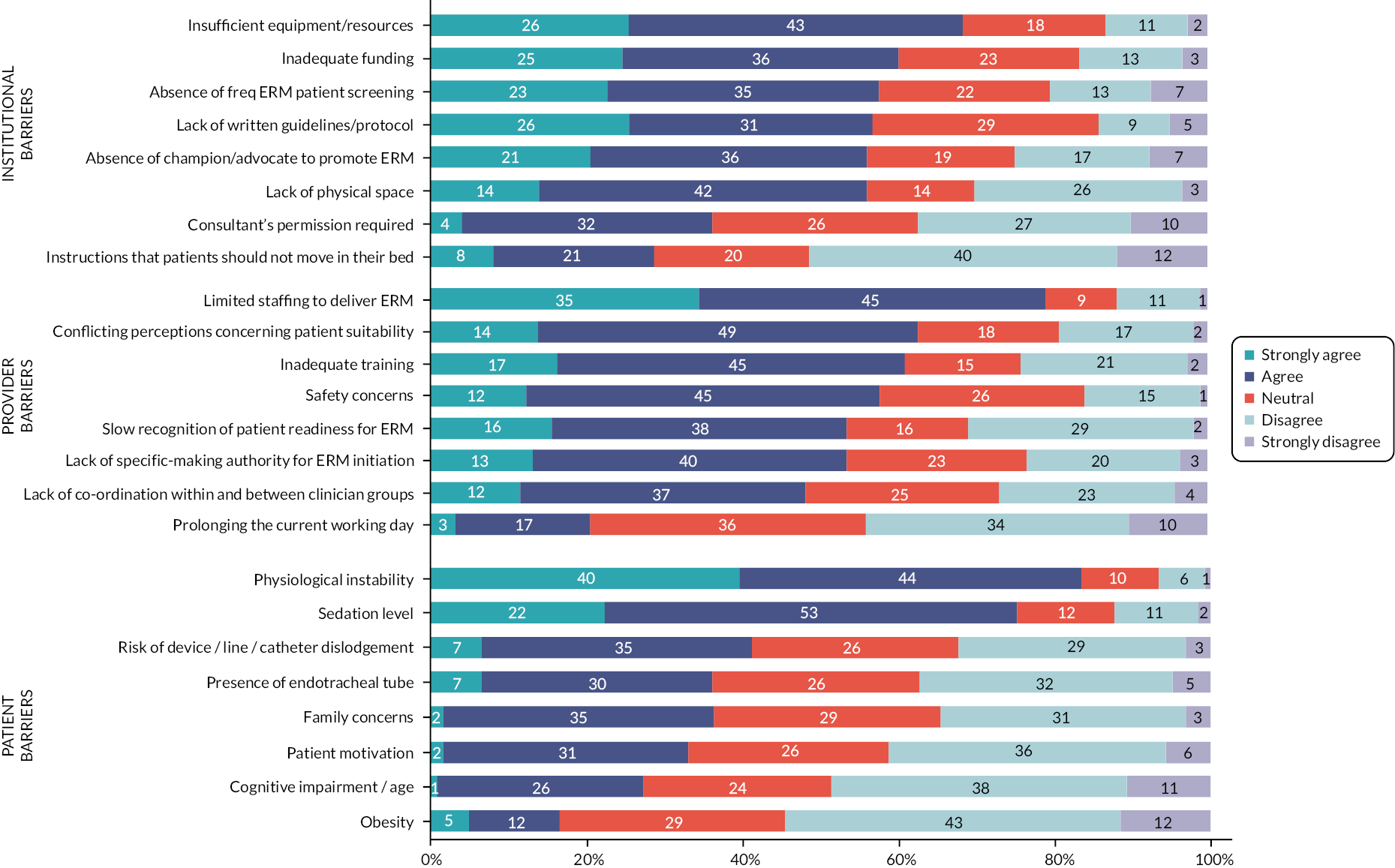
Figure 4 presents the perceived barriers of ERM. The most significant factors identified as barriers at the institutional levels were insufficient resources/equipment (83, 69%) and inadequate funding (73, 61%). Participants provided examples of resources having to be shared across organisations or having to be specially ordered to deliver ERM to patients.
FIGURE 4.
Perceived barriers of ERM.
All equipment shared with the whole therapy department at present, therefore dependent on availability.
(OT, 010)
Most PICUs had access to standard lifting 22/26 (85%) and specialist static seating equipment 25/26 (96%). However, bedside or in-bed cycling machines were only available in 10 (38%) of PICUs (see Appendix 3, Table 39).
A lack of established protocols (69, 57%), ERM champions (68, 57%), space (68, 56%) and robust patient-screening processes (63, 58%) were also issues identified by respondents.
Limited staffing was the most frequently reported barrier to ERM being delivered (101, 79%). Approximately half of the respondents agreed issues such as training, patient safety, lack of decision-making authority and delays in recognition of patients’ ERM needs were barriers to ERM initiation. However, only 25 (21%) identified that the impact of ERM potentially prolonging the working day was a barrier.
At the patient level, the two most frequently reported barriers to delivering ERM were physiological instability (101, 81%) and sedation (91, 73%).
Institutional, patients and provider barriers to ERM
Table 2 shows the percentage of responses for the categories strongly agree, agree, neutral, disagree and strongly disagree. Responses ranked on the cumulative score of percentage ‘strongly agree and agree’.
Summary of findings to inform the PERMIT study
-
This national survey of healthcare practitioners (HCPs) from UK PICUs identified the importance of ERM as an intervention which participants believe can improve the physical, psychological and cognitive recovery of critically ill or injured infants and children across all ages.
-
Our findings indicate support for ERM, but highlight uncertainty with suitability, variability with the definition of this complex intervention, variation in timing of initiating and which patient groups should receive ERM.
-
Key barriers to ERM delivery were identified (e.g. funding and staffing) and potential clinical (e.g. improved psychological outcomes) and economic (e.g. reduced PICU LOS) benefits to patients and PICUs.
-
Our results indicate uncertainty and wide variation in time to start ERM (24 hours to over 7 days), increasing agreement for ERM to be considered after longer periods on PICU, and support for the concept of ‘as early as the patient’s clinical condition allows’, which may be much longer.
-
The uncertainty of the content of ERM also adds to the challenge for healthcare professionals to appreciate when ERM could be delivered. Understandably, normal bedside nursing care (e.g. functional positioning) may be considered acceptable earlier than more advanced physical therapies requiring multiple staff (e.g. sitting a ventilated child out of bed or in-bed cycling).
-
Our survey identified that clinical stability is the most influential patient factor for initiation.
-
The reported lack of ERM protocols in most (21/26) UK PICUs reinforces a strong requirement for evidence-based standardised protocols with optimal timing, intensity, frequency and duration of ERM. There is a need for flexible protocols to allow for tailoring rather than prescription.
-
ERM was more likely to be delivered to patients admitted for >28 days, among patients with acquired brain injury or severe developmental delay across all age ranges. Most published ERM intervention studies to date have excluded patients <3 years of age. 20,15 However, this represents 60% of the UK PICU patient population,62 and this age group was as likely to receive ERM as older children in our study. Future ERM trials should include all PICU age groups to ensure ERM content and efficacy are assessed across all potential patients.
-
Our results show that within the UK NHS setting, doctors, physiotherapists and nurses have an equally significant role in the decision to initiate ERM.
-
Nurses’ and parent’s roles are also important in both initiation and delivery of ERM. In our study, 91% felt ‘involved’ in delivery of ERM. However, parents were reported to be the least likely group to initiate ERM (19%), although becoming influential in its ongoing delivery.
-
The key barriers to ERM practice were (1) at institutional level: insufficient resources, equipment and funding; (2) at provider level: limited staffing, training, protocols and slow recognition of readiness for ERM; and (3) at patient level: physiological instability, risk of ETT dislodgement and amount of sedation.
Chapter 3 Phase 1c: observational study
Introduction
This chapter describes the observational study to ascertain current ERM practices, as well as barriers and facilitators to ERM delivery, within the PICU setting. Following the scoping review and survey (see Chapters 1 and 2), we were interested in the concept of early ERM occurring by day 3 and over the following 7 days of PICU admission, and ERM to include the broad category of any rehabilitation or mobilisation, including both mobility and non-mobility activities. We directly observed current ERM practices within UK PICUs, identify patients who do and do not receive ERM and describe variation between PICUs and factors associated with ERM practices.
Study management
The work package was led by BRS. The study management group provided input into protocol and ethics design. JYT was study co-ordinator and piloted and developed the Research Electronic Data Capture (REDCap) database. Statistical analysis was undertaken by JYT, BRS and James Martin. Data interpretation was by BRS, JYT, JMan and JMen with input from all study management group.
Objectives
-
Observe and describe current ERM practice, including barriers and facilitators, in UK PICUs.
-
Assess the capability of UK PICUs to deliver ERM.
-
Establish and model how many/which CYP may be suitable for ERM in the PICU population using routinely collected data.
Method
Study design
A multicentre prospective observational study.
Target population/setting
All CYP (0 to <16 years) admitted to PICU and remaining on PICU by 9 a.m. on day 3 after PICU admission were eligible to participate. The exclusion criteria included a local decision by PI or treating clinical team not to include patients (e.g. receiving end-of-life care) and parents or guardians who choose to opt out. The broad study inclusion criteria allowed for the observation of all types of patients admitted for PICU care (e.g. planned and unplanned admissions), and all age ranges.
Site and patient selection
The Paediatric Early Rehabilitation and Mobilisation during InTensive care (PERMIT) study was an observational study conducted in 15 UK PICUs across two 21-day periods: (1) 26 November–16 December 2019 and (2) 14 January–3 February 2020. PICUs were identified from the PERMIT survey (see Chapter 2). The PICUs selected were of varying sizes (n = 6 large: >800 admissions/year, n = 5 medium: 500–800 admissions/year, n = 3 small: <500 admissions/year) and reported ERM activity of differing levels in the survey.
This study was conducted in two separate time periods to maximise efficiency, overcome recruitment hurdles and meet the target. Ten sites recruited and collected data during period 1, with a further five sites in period 2. Patients were observed on study day 1–14 with a further week to complete follow-up (study day 15–21). Individual patient data collection and observations took place for up to 7 days after patients were recruited or until PICU discharge, whichever was sooner.
Recruitment/enrolment
The study protocol, manual of operation, checklists and case report forms (CRFs) provided details on the study procedures. Staff at participating sites received remote training on research conduct before, and ongoing support sessions during, the study period.
Research staff screened patients admitted to PICU for the PERMIT study using a bespoke study screening log. The daily screening process ensured patients becoming eligible (e.g. the day before their third day) were identified. Designated research co-ordinators entered data on ERM activities recorded by clinical staff in clinical notes on the study proformas. Data were transferred to a secure electronic database (REDCap™), with scanned copies uploaded for data validation. Data were pseudo-anonymised at the local site before secure transfer to the PERMIT trials office.
Consent
As the study was observational, Regional Ethics Committee (REC) approved data collection without seeking prior consent from parents/legal representatives. In addition, this avoided unnecessary burden for parents/legal guardians in approaching consent during a very sensitive time. Information about the study was provided to all eligible patients’ families and was displayed within public areas of participating PICUs. This explained the study to parents, family, friends and children who were able to make autonomous decisions. Parents/legal guardians were able to opt the child’s data out of the study at any time and were aware that the future care their child would receive would not be affected.
Study procedure and data collection
Site staff collected demographic data on the third day of admission. Clinical and ERM data were collected from enrolment until discharge at the end of the study period. Data were collected twice, between 09.00 and 10.00 and between 14.00 and 15.00, each day.
Patient-level data
Patient characteristics collected at PICU admission included age (in categories); reason for admission; primary diagnosis; the severity of illness using Pediatric Index of Mortality (PIM3) score, with clinical function being assessed at baseline (pre-PICU state) and admission via the PCPC and POPC, which scores from 1 to 6 (1: normal, 2: mild disability, 3: moderate disability, 4: severe disability, 5: vegetative state or coma and 6: death). 63 PICU LOS was also recorded.
Clinical data
Data collected during PICU stay included healthcare interventions; requirement for MV; sedation and level of consciousness; presence of delirium; critical care interventions; indicators of physiological status; and individual patient PICU resource use. We calculated PELOD score (PELOD-2),64 which is a measure to describe the severity of organ dysfunction/illness in critically ill CYP, daily at 9 a.m.
Observed early rehabilitation and mobilisation active interaction
Clinical staff performing ERM activities were instructed to record the planned and delivered ERM activity duration in medical records. A research nurse or co-ordinator used these data to complete the bespoke active interaction CRF, which was submitted to the PERMIT study office. CRFs were completed hourly between 9 a.m. and 5 p.m. by the local site research nurse. A retrospective review of clinical case records of ERM activities that occurred overnight was carried out, with CRFs completed accordingly. Overnight ERM interventions were defined as the time from the end of the observed active interaction period 17.01 until 08.59 before the start of the next period.
We defined a priori ERM as (1) any ERM activity, (2) any mobility ERM activity and (3) mobility activity out of bed, informed by scoping review and survey (see full breakdown of ERM activities, ERM group and level of ERM detailed in Appendix 3, Table 40). We excluded chest PT, tracheal tube suctioning and routine nursing ‘cares’ (such as mouth and eye cleaning).
Patients were defined as receiving any ERM intervention if any ERM was recorded on study day. Details on interventions such as type of ERM activity (mobility, non-mobility, out-of-bed, passive, active and psychological) and safety events (such as changes in heart rate, oxygen saturation, removal of tubes or falls) were recorded.
Primary outcome
-
the delivery of any ERM activity on day 3 post admission (study day 1).
Secondary outcomes
-
the delivery of any ERM activity on days 4–10 post admission (study day 1–7);
-
the delivery of ERM involving any mobility activity and out-of-bed mobility ERM on days 3–10;
-
the number, type and duration (e.g. dose) of ERM delivered on each day;
-
predictive factors related to the delivery of ERM on day 3 post admission.
Data analysis
We reviewed data for errors: missing data, duplicated records and outliers. Extreme values were set to missing if they were deemed impossible, based on their validity range. Continuous variables were reported as mean and SD or median and IQR based on data distribution. Categorical variables were described in numbers and/or percentages.
The prevalence and scope of ERM were described as the proportion of patients provided with any ‘active interaction’ of any ERM on day 3 post admission. Proportions of eligible patients receiving ERM interventions were analysed for each day. Rate ratios of patients receiving an ERM intervention during the study period were analysed using a Poisson regression model.
Cumulative prevalence for each day in PICU after day 3 up to day 10 post admission was calculated. Cumulative proportion of patients receiving ERM interventions as per prespecified categories during the study period was described graphically using Kaplan–Meier estimation and event rate plots.
We undertook further analysis to understand potential predictive factors associated with ERM and the incidence of ERM. We performed multivariable logistic regression to evaluate predictive factors of ERM provided on day 3. Factors of interest were established following the PERMIT survey and expert group consensus. These included age; baseline PCPC score; unplanned versus emergency admission; ventilation status; requirement of vasoactive infusion, sedative infusion, or neuromuscular blocking drugs; presence of urinary catheter, CVC or arterial line; family member present or participating in ERM activity; and presence of PICU protocol.
We calculated level of mobility activity using a modified progression score previously described in the EU-PACK (European Prevalence of Acute Rehabilitation for Kids in the PICU) study42 – Level 1: passive ROM, 2: sitting and exercise in bed, 3: sitting edge of bed, 4: held by parent or nurse (cuddle), 5: transfer to chair, 6: mat play, 7: standing, 8: walking in room/PICU, 9: walking out of PICU (see Appendix 3, Table 40). Further post hoc categorisation of ERM activities as enrichment, passive and active activities was also applied.
Adverse events rates were calculated per ERM activity. For zero rate observed AEs, the upper 95% CI are presented using Hanley’s formula. 65
Patient and public involvement and engagement
For an overview of the approach to patient and public involvement and engagement (PPIE) adopted throughout the study, please see Chapter 8. With this element of the study, we explored the potential consent model, especially the acceptability of an ‘opt out’ approach to consent. There was a study recruiting at the time on PICU with an ‘opt out’ approach – the Sedation AND Weaning In Children trial (SANDWICH) trial. 66 At the time, the SANDWICH trial had recruited over 700 patients at Birmingham Children’s Hospital (BCH), with only three families choosing to opt out of their child’s data being collected. It was therefore regarded as a highly successful approach to consenting.
For the SANDWICH trial, parents were given a leaflet outlining that data collection was taking place on data routinely collected as part of ‘normal care’ which goes to the national PICU audit. 67 They were told that the SANDWICH team would have access to some of this information. In addition, the research team would also collect information from the child’s medical notes and charts. All the data were anonymised and there were no interventions (at the individual level), just data collection. Parents were not asked for their informed consent. They received the Participant Information Sheet (PIS). If they did not want to take part, they then spoke to the clinical staff, who informed the research staff, and this was documented as an ‘opt out’.
We spoke to families who had received the information to ask how they had experienced the process of approach for the SANDWICH study. In addition, we approached families who were participating in other research studies known to the PPIE lead (JMen) and whose children had recently been discharged from hospital. We spoke to six parents of four children (aged 0.3–6 years) who had experienced one or more PICU admission(s) and had experience of their child being recruited to research. We also spoke to three young people (aged 17–20) naive to PICU to participate as PPIE participants. Different models of consent were discussed and where there was no intervention then an opt-out model of consent was universally popular (Table 3). We also discussed the PISs and poster to inform parents about the observational study with them and they suggested a number of changes to the language, graphics and layout of them.
| Aspect | PPIE feedback | Impact/changes made |
|---|---|---|
| Consent model | Opt-out consent acceptable Informed consent model also acceptable but adds burden at a difficult time |
Opt-out consent approach used. Well received with no negative feedback from the 15 sites that participated and no queries or amendments from REC |
| Participant-facing information | Language: current draft was understandable and clear but few suggestions to change phrasing and shorten sentence length Graphics: would like to see pictures of what was meant by early rehabilitation activities and feedback about the selection of figures used Layout: need larger font, better spacing, figures to break up text |
PIS and poster both amended. No negative feedback from the REC or the 15 sites that participated. Used as a template for Phase 3 work |
Results
Eligibility and enrolment
A total of 169 patients were enrolled into the study from 15 PICUs, with each PICU enrolling a median (IQR) of 10.0 (9.5–15.3) patients.
During the 14-day enrolment period, the median census on each PICUs was 10.5 patients (IQR 7.0–17.0; range 3–26). Overall, there was a median (IQR) of 1 patient (0–2) eligible per PICU per study day of whom 1 patient (0–1) was enrolled into the study per PICU per study day. This identified 203/2447 (8.7%) patients within each PICU eligible, of whom 158/203 (77.8%) were enrolled over the 14-day enrolment period (enrolment data were missing from 1 unit which recruited 11 patients). Ineligible patients had either not reached day 3 or had already reached day 4 or greater on day of screening. Table 4 shows PICU patient census, eligibility and enrolment proportion. During days 8–14, some sites reported not enrolling as they had already reached their target of 10 patients. Enrolment rate in study days 1–7 was 98/108 [90.7% (95% CI 83.6% to 95.4%)].
| Study day | Patients in PICUa | Eligible, n |
Eligible (%) | Enrolled, n |
Enrolled (%) |
|---|---|---|---|---|---|
| 1 | 174 | 11 | 6 | 11 | 100 |
| 2 | 183 | 15 | 8 | 14 | 93 |
| 3 | 183 | 14 | 8 | 12 | 86 |
| 4 | 183 | 20 | 11 | 18 | 90 |
| 5 | 178 | 16 | 9 | 14 | 88 |
| 6 | 171 | 15 | 9 | 14 | 93 |
| 7 | 169 | 17 | 10 | 15 | 88 |
| 8 | 189 | 16 | 8 | 13 | 81 |
| 9 | 182 | 12 | 7 | 7 | 58 |
| 10 | 183 | 19 | 10 | 13 | 68 |
| 11 | 177 | 9 | 5 | 6 | 67 |
| 12 | 163 | 15 | 9 | 9 | 60 |
| 13 | 157 | 9 | 6 | 5 | 56 |
| 14 | 155 | 15 | 10 | 7 | 47 |
Demographics
Of the 169 patients, 59.2% were male; median age was 4.5 months (IQR 1.1–37.9). The majority (81%) were <4 years with 62.7% <1 year. Only 48 (28.4%) were ambulatory prior to PICU admission (key demographics at admission are in Table 5).
| Factor | All | No ERM | Any ERM activity | No mobility ERM | Mobility ERM | Not out-of-bed | Out-of-bed ERM | Missing |
|---|---|---|---|---|---|---|---|---|
| N | 169 | 7 | 162 | 22 | 147 | 105 | 64 | |
| Age (days), median (IQR) | 135 (34–1137) | 207 (51–680) | 134 (33–1169) | 49.5 (10–283) | 183 (40–1299) | 260 (47–1169) | 92.5 (25–734.5) | 0 |
| Patient age group | ||||||||
| <1 month | 34 (20.1) | 0 (0.0) | 34 (21.0) | 6 (27.3) | 28 (19.0) | 18 (17.1) | 16 (25.0) | 0 |
| 1–3 months | 46 (27.2) | 3 (42.9) | 43 (26.5) | 7 (31.8) | 39 (26.5) | 26 (24.8) | 20 (31.3) | |
| 4–6 months | 11 (6.5) | 0 (0.0) | 11 (6.8) | 1 (4.5) | 10 (6.8) | 6 (5.7) | 5 (7.8) | |
| 7–11 months | 14 (8.3) | 2 (28.6) | 12 (7.4) | 4 (18.2) | 10 (6.8) | 9 (8.6) | 5 (7.8) | |
| 1–4 years | 31 (18.3) | 1 (14.3) | 30 (18.5) | 1 (4.5) | 30 (20.4) | 26 (24.8) | 5 (7.8) | |
| 5–8 years | 6 (3.6) | 0 (0.0) | 6 (3.7) | 0 (0.0) | 6 (4.1) | 2 (1.9) | 4 (6.3) | |
| 9–13 years | 13 (7.7) | 1 (14.3) | 12 (7.4) | 1 (4.5) | 12 (8.2) | 10 (9.5) | 3 (4.7) | |
| 13–17.9 years | 14 (8.3) | 0 (0.0) | 14 (8.6) | 2 (9.1) | 12 (8.2) | 8 (7.6) | 6 (9.4) | |
| Ethnicity | ||||||||
| White | 93 (55.0) | 5 (71.4) | 88 (54.3) | 14 (63.6) | 79 (53.7) | 56 (53.3) | 37 (57.8) | 22 |
| Mixed | 4 (2.4) | 0 (0.0) | 4 (2.5) | 0 (0.0) | 4 (2.7) | 2 (1.9) | 2 (3.1) | |
| Asian | 18 (10.7) | 0 (0.0) | 18 (11.1) | 0 (0.0) | 18 (12.2) | 11 (10.5) | 7 (10.9) | |
| Black | 12 (7.1) | 2 (28.6) | 10 (6.2) | 3 (13.6) | 9 (6.1) | 10 (9.5) | 2 (3.1) | |
| Other | 2 (1.2) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 2 (1.4) | 2 (1.9) | 0 (0.0) | |
| Not stated | 18 (10.7) | 0 (0.0) | 18 (11.1) | 1 (4.5) | 17 (11.6) | 11 (10.5) | 7 (10.9) | |
| Reason for admission | ||||||||
| Post surgery: neurology | 5 (3.0) | 0 (0.0) | 5 (3.1) | 0 (0.0) | 5 (3.4) | 4 (3.8) | 1 (1.6) | 1 |
| Post cardiac surgery | 16 (9.5) | 1 (14.3) | 15 (9.3) | 5 (22.7) | 11 (7.5) | 9 (8.6) | 7 (10.9) | |
| Other surgery | 21 (12.4) | 0 (0.0) | 21 (13.0) | 1 (4.5) | 20 (13.6) | 13 (12.4) | 8 (12.5) | |
| Haematology/oncology | 4 (2.4) | 1 (14.3) | 3 (1.9) | 1 (4.5) | 3 (2.0) | 2 (1.9) | 2 (3.1) | |
| Cardiac (medical) | 16 (9.5) | 0 (0.0) | 16 (9.9) | 1 (4.5) | 15 (10.2) | 7 (6.7) | 9 (14.1) | |
| Infectious | 5 (3.0) | 0 (0.0) | 5 (3.1) | 0 (0.0) | 5 (3.4) | 3 (2.9) | 2 (3.1) | |
| Neurology | 9 (5.3) | 1 (14.3) | 8 (4.9) | 3 (13.6) | 6 (4.1) | 6 (5.7) | 3 (4.7) | |
| Renal | 2 (1.2) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 2 (1.4) | 1 (1.0) | 1 (1.6) | |
| Respiratory | 80 (47.3) | 4 (57.1) | 76 (46.9) | 10 (45.5) | 70 (47.6) | 51 (48.6) | 29 (45.3) | |
| Trauma | 1 (0.6) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (0.7) | 1 (1.0) | 0 (0.0) | |
| Other medical | 9 (5.3) | 0 (0.0) | 9 (5.6) | 1 (4.5) | 8 (5.4) | 7 (6.7) | 2 (3.1) | |
| POPC | ||||||||
| 1. Good | 85 (50.3) | 5 (71.4) | 80 (49.4) | 13 (59.1) | 72 (49.0) | 49 (46.7) | 36 (56.3) | 1 |
| 2. Mild disability | 41 (24.3) | 1 (14.3) | 40 (24.7) | 6 (27.3) | 35 (23.8) | 23 (21.9) | 18 (28.1) | |
| 3. Moderate disability | 20 (11.8) | 0 (0.0) | 20 (12.3) | 0 (0.0) | 20 (13.6) | 12 (11.4) | 8 (12.5) | |
| 4. Severe disability | 21 (12.4) | 1 (14.3) | 20 (12.3) | 2 (9.1) | 19 (12.9) | 19 (18.1) | 2 (3.1) | |
| 5. Coma/vegetative state | 1 (0.6) | 0 (0.0) | 1 (0.6) | 1 (4.5) | 0 (0.0) | 1 (1.0) | 0 (0.0) | |
| PCPC | ||||||||
| 1. Good | 113 (66.9) | 6 (85.7) | 107 (66.0) | 16 (72.7) | 97 (66.0) | 64 (61.0) | 49 (76.6) | 3 |
| 2. Mild disability | 21 (12.4) | 0 (0.0) | 21 (13.0) | 2 (9.1) | 19 (12.9) | 11 (10.5) | 10 (15.6) | |
| 3. Moderate disability | 14 (8.3) | 0 (0.0) | 14 (8.6) | 0 (0.0) | 14 (9.5) | 11 (10.5) | 3 (4.7) | |
| 4. Severe disability | 17 (10.1) | 1 (14.3) | 16 (9.9) | 2 (9.1) | 15 (10.2) | 16 (15.2) | 1 (1.6) | |
| 5. Coma/vegetative state | 1 (0.6) | 0 (0.0) | 1 (0.6) | 1 (4.5) | 0 (0.0) | 1 (1.0) | 0 (0.0) | |
The most common admission diagnosis was bronchiolitis in 55 (32.5%). Eighty-four (49.7%) were admitted from another hospital, requiring retrieval into PICU. There were 127 (75.1%) emergency admissions, 150 (89.3%) required invasive ventilation at admission, 5 (3%) extracorporeal membrane oxygenation (ECMO) and 60 (35.5%) required cubicle isolation. Prior to admission, 66% had a PCPC score of 1 or 2 and 50% a POPC score 1 or 2, indicating a high proportion with moderate to severe disability pre-PICU. Admission predicted probability of mortality, as measured by PIM3, was median 1.2% (0.5–4.4%) and 68 (40.2%) children were enrolled in a PICU with an existing ERM protocol (as reported in the PERMIT survey – Chapter 2).
Patient clinical status on day 3 of admission (study day 1)
Between PICU admission and study day 1, 12 (7.1%) had surgery, 1 had a cardiac arrest. Most (119; 70%) remained ventilated by an ETT, 40 (23.7%) required vasoactive infusions and 3 remained on ECMO (Table 6).
| Factor n(%) or median (IQR) |
All | No ERM | Any ERM activity | No mobility ERM | Mobility ERM activity | Not out-of-bed | Out-of-bed ERM activity | Missing |
|---|---|---|---|---|---|---|---|---|
| n = | 169 | 7 | 162 | 23 | 146 | 105 | 64 | |
| PELOD 2 score median (IQR) | 4 (2–6) (n = 169) | 5 (1.5–6) (n = 8) | 4 (2–6) (n = 161) | 5 (4–7) (n = 23) | 4 (1–5) (n = 146) | 5 (3–6) (n = 105) | 2.5 (0–5) (n = 64) | |
| Type of ventilation | ||||||||
| No oxygen support | 19 (11.2) | 1 (12.5) | 18 (11.2) | 2 (8.7) | 17 (11.6) | 6 (5.7) | 13 (20.3) | |
| High-frequency oscillator | 5 (3.0) | 0 (0.0) | 5 (3.1) | 1 (4.3) | 4 (2.7) | 5 (4.8) | 0 (0.0) | |
| Conventional ventilation | 114 (67.5) | 6 (75.0) | 108 (67.1) | 19 (82.6) | 95 (65.1) | 86 (81.9) | 28 (43.8) | |
| Non-invasive (CPAP/BiPAP) | 15 (8.9) | 1 (12.5) | 14 (8.7) | 1 (4.3) | 14 (9.6) | 6 (5.7) | 9 (14.1) | |
| High-flow oxygen | 10 (5.9) | 0 (0.0) | 10 (6.2) | 0 (0.0) | 10 (6.8) | 1 (1.0) | 9 (14.1) | |
| Supplemental oxygen only | 6 (3.6) | 0 (0.0) | 6 (3.7) | 0 (0.0) | 6 (4.1) | 1 (1.0) | 5 (7.8) | |
| Vasoactive infusions | 40 (23.7) | 3 (37.5) | 37 (23.0) | 7 (30.4) | 33 (22.6) | 27 (25.7) | 13 (20.3) | 1 |
| Neuromuscular blocking drugs | 26 (15.4) | 4 (50.0) | 22 (13.7) | 8 (34.8) | 18 (12.3) | 23 (21.9) | 3 (4.7) | 1 |
| Sedation medication | 108 (63.9) | 6 (75.0) | 102 (63.4) | 20 (87.0) | 88 (60.3) | 82 (78.1) | 26 (40.6) | 1 |
| Screened for delirium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| ETT | ||||||||
| Oral | 82 (48.5) | 3 (37.5) | 79 (49.1) | 12 (52.2) | 70 (47.9) | 63 (60.0) | 19 (29.7) | |
| Nasal | 44 (26.0) | 3 (37.5) | 41 (25.5) | 8 (34.8) | 36 (24.7) | 30 (28.6) | 14 (21.9) | |
| No tube | 43 (25.4) | 2 (25.0) | 41 (25.5) | 3 (13.0) | 40 (27.4) | 12 (11.4) | 31 (48.4) | |
| Central venous line | 103 (60.9) | 4 (50.0) | 99 (61.5) | 13 (56.5) | 90 (61.6) | 72 (68.6) | 31 (48.4) | 1 |
| Arterial line | 72 (42.6) | 5 (62.5) | 67 (41.6) | 15 (65.2) | 57 (39.0) | 57 (54.3) | 15 (23.4) | 2 |
| Haemodialysis catheter | 7 (4.1) | 0 (0.0) | 7 (4.3) | 1 (4.3) | 6 (4.1) | 5 (4.8) | 2 (3.1) | 2 |
| Extracorporeal membrane oxygenation | 3 (1.8) | 2 (28.6) | 1 (0.6) | 3 (13.0) | 0 (0.0) | 3 (2.9) | 0 (0.0) | 2 |
| Urinary catheter | 103 (60.9) | 5 (62.5) | 98 (60.9) | 16 (69.6) | 87 (59.6) | 83 (79.0) | 20 (31.3) | 2 |
| Surgical drain | 8 (4.7) | 1 (12.5) | 7 (4.3) | 2 (8.7) | 6 (4.1) | 5 (4.8) | 3 (4.7) | 12 |
| Chest tube | 15 (8.9) | 2 (25.0) | 13 (8.1) | 6 (26.1) | 9 (6.2) | 11 (10.5) | 4 (6.3) | 11 |
| Intracranial pressure monitor | 4 (2.4) | 0 (0.0) | 4 (2.5) | 0 (0.0) | 4 (2.7) | 3 (2.9) | 1 (1.6) | 2 |
Sedative medications were used in 108 (63.9%); 102 on opiates, 40 on benzodiazepines and 26 (15.3%) were on neuromuscular blocking drugs. In patients who could be assessed and on sedative drugs, comfort B sedation score was median (IQR) 12 (11–14), and 12 (12–15) if not receiving sedation. No patient was screened for delirium in any PICU. PELOD2 severity of illness median score was 4 (IQR 2–6). Central venous access was used in 103 (60.9%), arterial access in 72 (42.6%), and 103 (60.9%) had a urinary catheter. Pressure ulcers were reported in 6 (3.6%) patients.
Early rehabilitation and mobilisation prevalence
On the first day of PERMIT study (day 3 post-PICU admission) overall 162/169 (95.9%) received at least one ERM activity. A mobility ERM activity was delivered to 147/169 (87.0%) of which an out-of-bed mobility ERM was delivered to 64/169 patients (37.9%).
Figure 5 shows the reduction in number of patients remaining in PICU following enrolment to PERMIT and the prevalence for (1) any ERM, (2) mobility ERM and (3) out-of-bed ERM mobility. Of note, by day 7 post PICU admission, half of patients enrolled into PERMIT had been discharged and by day 9, 113 (67%) had been discharged and 2 (1%) had died.
FIGURE 5.
Prevalence of ERM for each study day in PICU.

The rate of receiving ERM during the PERMIT study period, analysed using a Poisson regression model, did not change across the study period. We did not identify a significant trend with rate ratios of (1) any ERM: 0.98 (95% CI 0.92 to 1.05, p = 0.57), (2) mobility ERM: 0.97 (95% CI 0.93 to 1.01, p = 0.14) and (3) out-of-bed mobility 0.97 (95% CI 0.93 to 1.01; p = 0.14).
Cumulative probability of early rehabilitation and mobilisation
Figure 6a shows that while over 95% of enrolled patients were observed to have received an ERM intervention on day 1 of the study period, mobility ERM and out-of-bed ERM were less frequent (see Figure 6b and c). However, by day 6 of the study, 98% of patients had received a mobility ERM and 80% (see Figure 6b) of patients had received an out-of-bed ERM at some point during their observed period (see Figure 6c).
FIGURE 6.
Cumulative probability of (a) any ERM, (b) mobility ERM and (c) out-of-bed ERM.
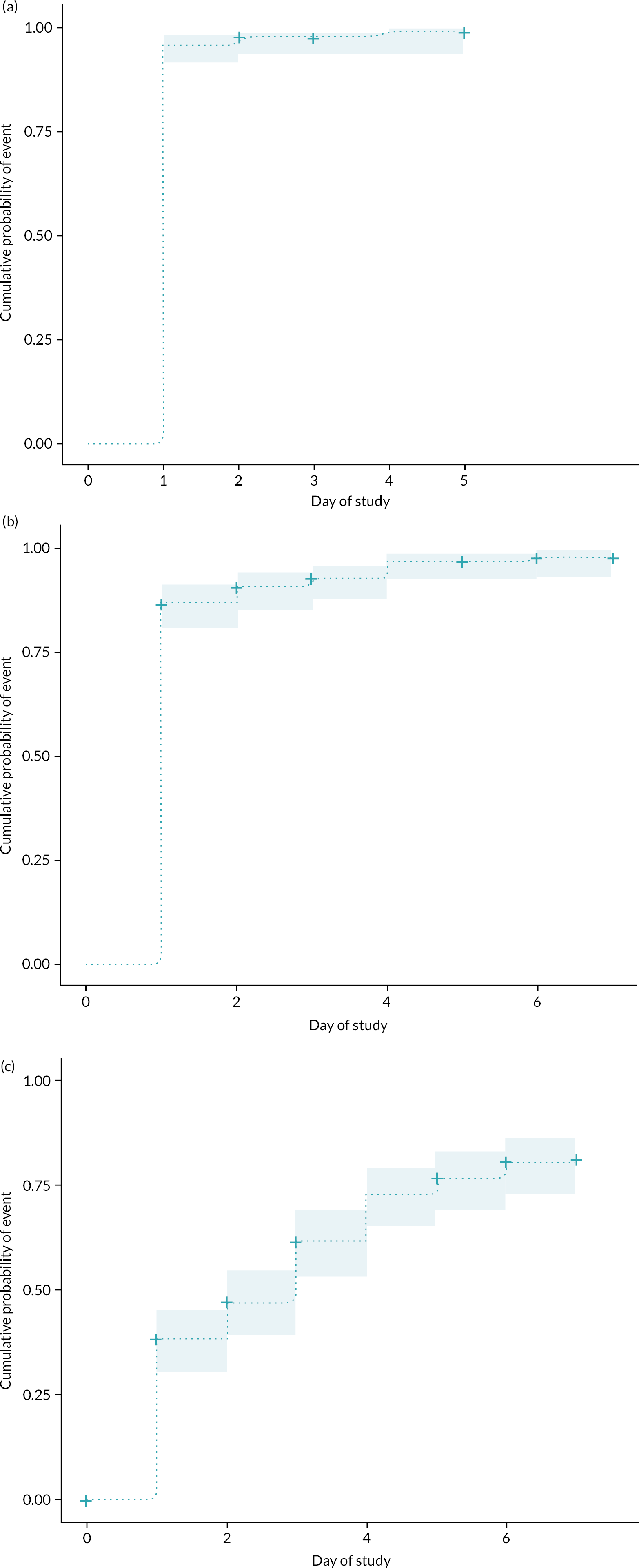
Description of early rehabilitation and mobilisation
In total, 3696 ERM episodes capturing 4978 ERM activities occurred during 729 patient days. On the first study day (day 3 post PICU admission), 169 patients received 977 ERM episodes and 1302 ERM activities [median IQR 7 (4–10) ERM activities per patient].
Analysing the whole study period, positioning (which incorporated the mobility element) (1205/4978; 24%) and non-mobility positioning (1177/4978; 23.6%) were the most frequent activities (Figure 7). Active mobility was less frequent: the majority were active movement (e.g. rolling, active ROM) and sitting up in bed or transfer out of bed to chair or mat. No in-bed cycling was reported throughout the observation period.
FIGURE 7.
All ERM activities ranked by active, enrichment and passive categories.

Table 5 compares the baseline characteristics of patients receiving any ERM, mobility ERM or out-of-bed ERM and those who did not on the first study day. Patients receiving mobility ERM tended to be older that those who did not; however, the opposite was seen in out-of-bed mobility, where younger patients (especially <3 months) were more likely to receive mobility out of bed (e.g. cuddles). We identified no difference across ethnicity groups or diagnostic admission group in the proportion of patients receiving each category of ERM. We did identify that patients with moderate (PCPC 3) or severe disability (PCPC 4) received less out-of-bed mobility.
Table 6 compares the clinical status of patients with ERM type on the first study day. Patients receiving or not receiving any ERM or mobility ERM were similar in respect to the majority of measured clinical status factors. The only differences were between patients receiving out-of-bed mobility or not. There were more patients receiving invasive ventilation (high-frequency oscillation and conventional ventilation), use of neuromuscular blocking drugs, sedative drugs and with additional lines or tubes (e.g. central venous catheter, arterial line, urinary catheter and chest tubes) in the group not receiving out-of-bed mobility. This was also reflected in the higher PELOD2 organ dysfunction score for patients not receiving out-of-bed ERM [5 (IQR 3–6)] versus those who did receive [2.5 (IQR 0–5)].
Mobility activities
Using the ranking scale described by Ista et al. 42 on the first day of study 147/169 (87%) had a mobility ERM activity. The ranked activities are shown in Table 7. Most were either passive ROM or cuddles. Only 29 (17%) involved ranked 5–9 activities (transferring out of bed, mat play, standing or walking).
| Rank | Description | Number (%) of patients |
|---|---|---|
| 1 | Passive ROM | 38 (25.9) |
| 2 | Sitting in bed | 21 (14.3) |
| 4 | Held/cuddle | 59 (40.1) |
| 5 | Transfer to chair | 17 (11.6) |
| 6 | Mat play | 2 (1.4) |
| 7 | Standing | 4 (2.7) |
| 8 | Walking in room | 6 (4.0) |
| 9 | Walking out of room | 0 (0) |
Staffing and parental involvement in early rehabilitation and mobilisation
We examined staffing and parent data across all ERM episodes (which may have involved a combination of activities). As shown in Figure 8, registered nursing staff were involved with the majority of ERM episodes (3127/3696, 85%). Parents/guardians were present for 1614/3696 (44%) and participated in delivering 1372/3696 (37%). Physiotherapists delivered only a small proportion of ERM episodes (361/3696; 10%).
FIGURE 8.
Number of parents, guardians or healthcare professionals involved in ERM episodes.
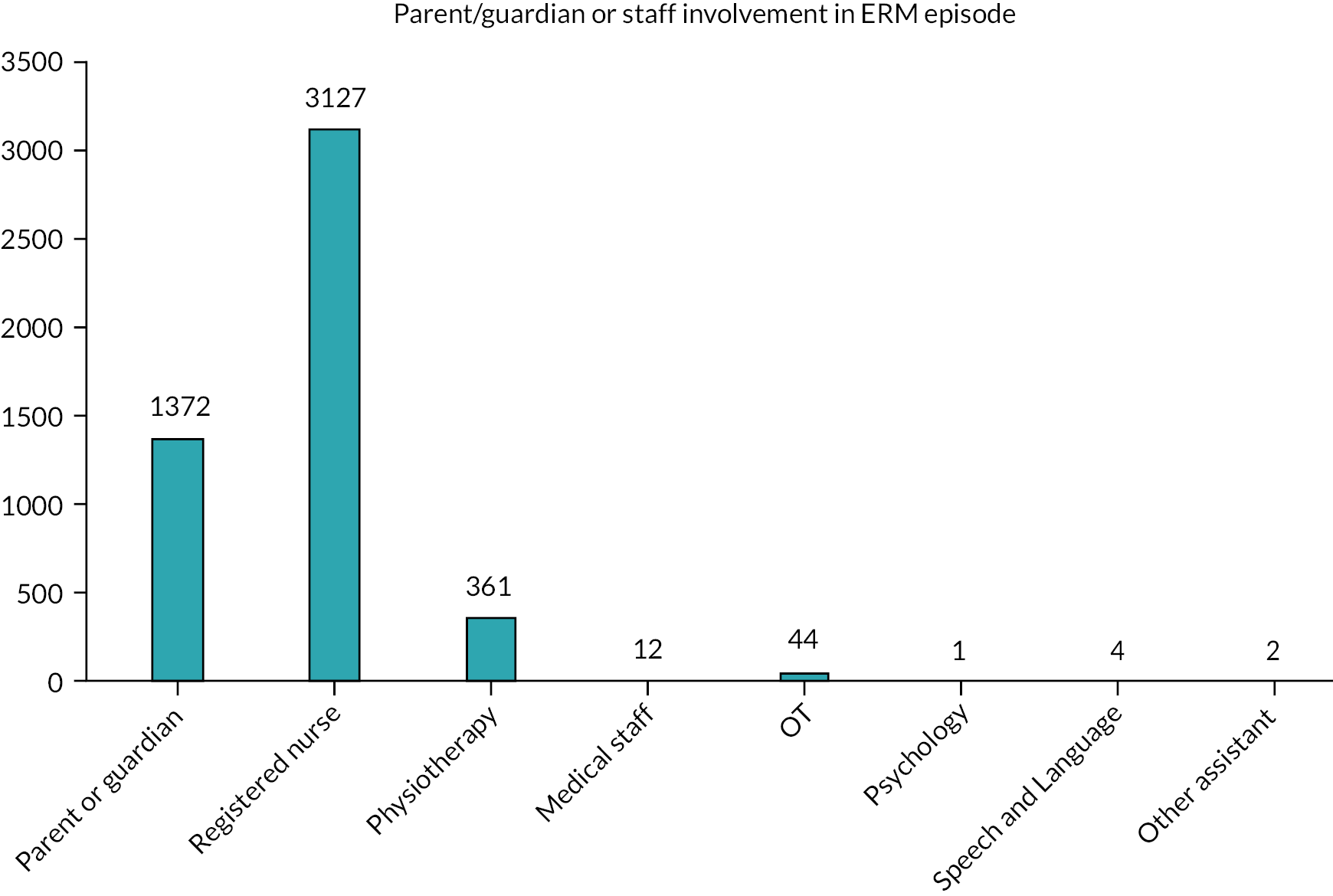
For individual patients, on the first study day, only 57/162 (35%) of patients received at least one ERM episode delivered by a physiotherapist. Across the duration of the study period this increased to 95/162 (59%) of patients having the input of a physiotherapist for at least one ERM activity. While the majority of ERM episodes were delivered by registered nursing staff or parents, without specialist therapy input there is a risk that ERM activity quality may be low (e.g. not targeted at the patient acuity or developmental age).
Timing and duration of early rehabilitation and mobilisation
ERM episodes were commenced throughout the 24-hour period of PICU with 1855/3730 (49.7%) occurring between 09.00 and 17.00 (Figure 9). The median duration of ERM episode was 15 (IQR 10–30) minutes and 37% of ERM episodes were of short duration (1–14 minutes) (Figure 10).
FIGURE 9.
Histogram of ERM start time.
FIGURE 10.
Duration of ERM episode (data available n = 3410).
As the professional group present at the bedside 24/7, nursing staff delivered 85% of ERM activities and 48% of these activities were commenced outside ‘9 a.m. to 5 p.m. office hours’ and emphasise the need to view ERM as a 24/7 intervention and not just an activity performed during office hours by physiotherapists. Less than 10% of ERM episodes delivered by a physiotherapist were outside 9 a.m. to 5 p.m..
Predictive factors associated with out-of-bed mobility
We created a multivariable logistic regression model to explore predictors of interest for out-of-bed ERM on study day 1 (day 3 of PICU) (Figure 11). Factors associated with a decreased odds of out-of-bed mobility included pre-PICU severe disability (PCPC score 4 vs. 1) [OR 0.09 (95% CI 0.01 to 0.61)], invasive ventilation [OR 0.23 (95% CI 0.06 to 0.83)] and presence of a urinary catheter [OR 0.16 (95% CI 0.16 to 0.42)]. The only statistically significant factor associated with increased odds of out-of-bed mobility was the presence of a parent or guardian and/or their involvement in the ERM activity [OR 13.46 (95% CI 1.05 to 172.7)], although the CI was wide. Of note, a vasoactive infusion was associated with increased out-of-bed mobility [OR 1.85 (95% CI 0.5 to 6.82)] and use of neuromuscular blocking drugs was associated with decreased out-of-bed mobility [OR 0.33 (95% CI 0.08 to 1.4)]; however, both of their CIs crossed 1. We were unable to create models for any ERM or mobility ERM because of the high rate of positive events for both on study day 1.
FIGURE 11.
Forest plot of odds of out-of-bed mobility on study day 1 by key factors.

Adverse events
There were 106 recorded AEs during 78 separate ERM episodes (Table 8). Overall proportion of ERM activities with an AE was 106/3696 (2.87%; 95% CI 2.35 to 3.45), which equates to 1 in 35 (95% CI 1 in 29 to 1 in 43) ERM activities. The most frequent reported event was desaturation in 38 (1.03%; 1 in 97) of ERM activities and discomfort of patient or tiredness in 18 (0.49%; 1 in 205). There was only one ETT tube dislodged, and seven other tubes dislodged, during the entire study period. Overall ERM delivery was safe, with a very low rate of AEs.
| Adverse events | Number | Per cent of ERM activities (95% CI) |
1 in x rate |
|---|---|---|---|
| Desaturation (>15% decrease from baseline) | 38 | 1.03 | 97 |
| Discomfort of patient or tiredness | 18 | 0.49 | 205 |
| Clinically significant change in heart rate | 14 | 0.38 | 264 |
| Clinically significant increase in O2 requirement | 8 | 0.22 | 462 |
| Line/tube dislodgement or removal | 7 | 0.19 | 528 |
| Clinically significant change in blood pressure | 5 | 0.14 | 739 |
| Clinically significant increase in end-tidal CO2 | 5 | 0.14 | 739 |
| Other tube removal | 3 | 0.08 | 1232 |
| Asynchrony with ventilator | 3 | 0.08 | 1232 |
| Clinically significant change in respiratory rate | 2 | 0.05 | 1848 |
| Dislodgement/unplanned removal ETT | 1 | 0.03 | 3696 |
| Pain | 1 | 0.03 | 3696 |
| Arrhythmia | 1 | 0.03 | 3696 |
| Fall | 0 | 0.00 | 11,088a |
| Cardiac arrest or CPR | 0 | 0.00 | 11,088a |
| Changes in mental status | 0 | 0.00 | 11,088a |
| Total: Any AE | 106 | 2.87 (2.4 to 3.5) | 35 (29 to 43) |
Summary of findings to inform the PERMIT study
-
Paediatric intensive care units were able to enrol 90% of eligible patients into the observational study during week 1, using an opt-out consent model.
-
All participating UK PICUs delivered some form of ERM to patients. However, the range of ERM delivered is broad, with most of the time and resources delivered to basic patient positioning, family holding of patients and active movement in bed.
-
ERM, using its broadest definition, is delivered to nearly all PICU patients on day 3 after admission and throughout their PICU stay. We did not identify a change in the prevalence of ERM delivery across study days. However, mobility and especially out-of-bed mobility was less frequent.
-
We identified some barriers and modifiable factors associated with delivery of out-of-bed mobility (e.g. invasive ventilation, presence of urinary catheter, pre-existing severe disability and parental presence and involvement in ERM). Strategies to address these could improve rates of out-of-bed mobility.
-
The presence of an existing ERM protocol neither increased nor decreased the odds of receiving out-of-bed mobility.
-
Reported safety profile of patients receiving ERM is very good. There is a very low level of incidents, safety events, or physiological effects reported during ERM delivery.
-
Important information regarding PICU practice was obtained. Delirium screening is universally absent in participating PICUs. Nursing staff and parents deliver the majority of ERM activities throughout the 24-hour period. Physiotherapists only delivered 10% of ERM, although 59% of patients received at least one PT delivered ERM episode at some point. There was minimal input from other medical, therapy, or support staff reported. Any ERM manual or intervention plan will need to utilise available staff.
Chapter 4 Phase 2: developing early rehabilitation and mobilisation intervention
Introduction
Early rehabilitation and mobilisation in PICU, like most rehabilitation interventions, is a complex intervention, in relation to the diverse actions, reasoning, and resources embedded in enacting ERM in intensive care, as well as the diversity of PICU contexts. Following the Medical Research Council (MRC) guidance on developing complex interventions,68–70 we anticipated that our intervention development work would involve learning from and adapting elements of existing ERM interventions, as they are already in use in some PICU settings internationally and a few sites in the UK. In this chapter, we show how we used the results from our survey, observational study and scoping review (see Chapters 1, 2 and 3), together with a range of other evidence and expertise, to design the ERM intervention manual, called the PERMIT manual (Figure 12). The intervention manual is a detailed prototype specifying the content of ERM for diverse patient populations and setting out how ERM can be implemented in varied paediatric intensive care settings. The feasibility and acceptability of the manual, and core elements of clinical trial designs for evaluating ERM, were subsequently explored across three PICUs (see Chapter 5).
FIGURE 12.
Overview of the intervention development process.
Study management
Phase 2 was co-led by co-applicants JMc, RF and TR (the core intervention development team). Two research associates (Dr Laura Cutler and Dr Olivia Craw, Faculty of Medical Sciences, Newcastle University) supported the organisation of the workshops and literature-based work. The study management group provided input into protocol, ethics application design and data interpretation.
Important changes to protocol
The PERMIT protocol planned a Phase 2a, Health Research Association (HRA) approved, workshop with parents and children. In discussion with and approval of National Institute for Healthand Care Research (NIHR) Health Technology Assessment (HTA) funder, this subphase was not started due to the impact of the COVID19 pandemic.
Methods and results
In developing the proposed ERM intervention, alongside the MRC frameworks we also drew on a range of conceptual resources from implementation science – notably Normalisation Process Theory (NPT),71,72 Theoretical Framework of Acceptability (TFA),73 Expert Recommendations for Implementing Change (ERIC),74 behaviour change Theory and Technique Tool75 – as well as the Person-Based Approach (PBA) to intervention development. 76 PBA emphasises:
prioritising and incorporating user perspectives wherever possible, while ensuring the intervention retains all the elements that theory and evidence suggest will be effective. 77
In line with PBA, the development of our ERM intervention prototype(s) has been intimately shaped by the involvement of key target users of the manual – multidisciplinary clinical stakeholders. Throughout the development process, we worked with clinical stakeholders within the wider PERMIT research team as well as healthcare professionals across the UK and international PICU clinicians. However, due to the evolving COVID context, planned workshops with parents/carers and CYP were not possible.
As outlined in Figure 12, the intervention development process initially involved establishing key messages and areas of uncertainty from our survey, observational study, and scoping review. We then explored the potential dimensions of the intervention with clinical stakeholders and iteratively refined prototypes of the intervention manual in relationship to evidential, practice-based and theoretical ideas.
Patient and public involvement and engagement
For an overview of the approach to PPIE adopted throughout the study, please see Chapter 8. Within this intervention development element of the study, PPIE work was only conducted to inform the Research Ethics Submission. We had planned to undertake PPIE work with parents with direct experience of involvement in PICU research studies in relation to discussing potential trial designs as well as patient-centred outcomes, but the research suspension imposed in response to directives to prioritise COVID-related research78 created a challenge. As outlined in Chapter 8, suspension of research recruitment reduced the number of studies which were open and recruiting and reduced researchers’ contact with families. Additionally, there were no pre-established local or national groups of parents with experiences of PICU. This created challenges for the PPIE lead to speak to parents with experience of being recruited to research. We decided to delay that element of the PPIE work until the feasibility study in Phase 3.
Formal reflection – establishing core issues
Purpose: to formally describe and synthesise the key messages, assumptions and uncertainties that emerged from our prior Phase 1 work to guide our intervention development work.
Methods: we reviewed the results of the survey, observational study and scoping review (see Chapters 1, 2 and 3), and undertook discussions with the wider PERMIT research team. We further refined and conceptualised the core findings of this process by aligning them to elements of the template for intervention description and replication (TIDieR) checklist. 79
Results: as outlined in Table 9, people see the potential value of ERM for the diverse patient populations within PICU and are willing to support its (safe) delivery but are uncertain how best to deliver it. The current evidence base and analysis of existing practice demonstrate that ERM is defined and enacted in multiple ways, ranging from more formal referral processes (directing patients only to specific allied health professionals), to a range of mobilisation and non-mobilisation activities, to being seen as encompassing everyday standards of good practice in PICU care. Core questions remain unresolved in relation to the conceptualisation of ERM, the pragmatics of operationalisation (e.g. how ‘early’ should ERM be delivered), and how best to measure the delivery and potential impact of ERM.
| What is the underpinning rationale, theory or goal of ERM? |
|
| Which patient populations are eligible for ERM? |
|
| When is ERM initiated? |
|
| What processes and materials are involved in ERM? |
|
| Where is ERM delivered? |
|
| How much ERM is delivered? |
|
| How is ERM tailored? |
|
| Who delivers ERM? |
|
| How is ERM delivered safely? |
|
| What other factors influence implementation of ERM? |
|
| What are the proposed outcomes of ERM? |
|
User development – generating guiding principles for the intervention
Purpose: to explore clinical stakeholders’ views and experiences of ERM for different populations, including feasible and acceptable content, delivery, implementation and important outcomes of ERM.
Methods: we took forward the key messages and uncertainties from Phase 1 into interactive workshops with UK NHS healthcare professionals and international ERM experts. The workshops received ethical approval from Newcastle University (NU Reference 14224/2018). We also planned to conduct workshops and interviews with parents and CYP in Spring 2020. These received HRA approval (IRAS 270791) but were unable to proceed as NHS Research and Development were not allowing non-COVID research at the time.
Sampling and recruitment: we recruited a multidisciplinary group of NHS healthcare professionals with diverse experience of ERM in PICU settings. We recruited from those who had participated in the Phase 1 survey and agreed to be approached about further work. They were approached via e-mail and a total of 18 professionals from 5 PICUs were included – 3 consultant doctors, 3 senior nurses, 5 physiotherapists (including 2 clinical specialists), 5 OTs (including 1 clinical specialist), 1 dietician and 1 play specialist. We also recruited a multidisciplinary group of international experts leading research and quality improvement on ERM in paediatric and adult intensive care settings. We recruited individuals who are active in the ERM community, identified from reviewing recent published work and from the team’s clinical and research networks. These people were approached via e-mail and a total of three were included from Europe and the USA – a senior clinical academic doctor, a senior clinical academic nurse and a senior physiotherapist.
Data collection: we undertook three face-to-face workshops with NHS healthcare professionals and one online workshop with international experts. Initial workshop plans were designed to explore the key reflections from Phase 1 and examples of existing ERM protocols. Plans evolved through findings from prior workshops shaping the focus of the next. The workshops were audio recorded and transcribed and facilitators recorded contemporaneous fieldnotes. The workshops lasted for between 90 minutes and 2 hours.
Data analysis: we drew on standard procedures from rigorous qualitative analysis80 including coding, constant comparison, memoing and team debrief sessions. The core intervention development team (JMc, RF, TR) reviewed, discussed, organised and summarised the analytic work. Following ideas from PBA,76 we focused on generating ‘guiding principles’ in relation to:
-
Intervention design objectives – what an ERM manual must do to address the needs of target users and enhance engagement.
-
Key features of the intervention – how these design objectives may be achieved in practice.
These guiding principles were critically discussed and revised with the wider PERMIT research team.
Results: as outlined in Table 10 ERM can be conceptualised as patients engaging in progressive movement and mobility in the context of individually meaningful and purposeful activities and a supportive environment that promotes familiar and orientating daily routines. Several discrete interventions can be legitimately conceptualised as either closely related to or a core part of ERM and these topics are being investigated in their own right (e.g. sedation, ventilation, delirium, nutrition and mental health). 66,81–84 A specific focus on progressive movement and mobility within PERMIT would add to the current research and further advance the overall goal of improving PICU care. Clinical stakeholders perceive that ERM is relevant for most patients and can be actively considered from the second morning of admission for those anticipated to still be on the unit the following day. However, clinicians especially require safety guidelines for delivering ERM to the most severely ill and complex patients. Factors influencing the implementation of ERM vary according to unit, team, individual clinician and individual patient. Therefore, both ERM activities and ERM implementation support need to balance flexibility (so they can be highly tailored) and specificity (so they can be differentiated from usual care, implemented and evaluated). Clinicians’ views on the potential impact of ERM seem to converge on a small cluster of key clinical, functional, psychological, family-related, QoL and economic outcomes.
| Design objectives – what an ERM intervention must do to address the needs of target users and enhance engagement | Key features – how ERM design objectives may be achieved in practice |
|---|---|
| What is the underpinning rationale, theory, or goal of ERM? | |
|
|
|
|
|
|
|
|
|
|
| Which patient populations are eligible for ERM? | |
|
|
|
|
|
|
|
|
|
|
| When is ERM initiated? | |
|
|
|
|
|
|
| What processes and materials are involved in ERM? | |
|
|
|
|
|
|
| Where is ERM delivered? | |
|
|
| How much ERM is delivered? | |
|
|
| How is ERM tailored? | |
|
|
| Who delivers ERM? | |
|
|
|
|
| How is ERM delivered safely? | |
|
|
| What other factors influence implementation of ERM? | |
|
|
| What are the proposed outcomes of ERM? | |
|
|
Literature-based work – developing a prototype intervention manual
After the workshops, we conducted a range of literature-based work to identify available concepts, tools and resources to develop and shape the form and content of a prototype ERM intervention manual. This work took place alongside formal and informal meetings with clinical members of the wider PERMIT research team (see user development – reported below) and had an iterative relationship.
Reviewing existing ERM interventions
Purpose: to review existing ERM interventions and compare them to our ERM guiding principles.
Methods: we used a rapid pragmatic approach to identify, characterise and understand existing PICU ERM interventions and compared the intervention components to the guiding principles we developed. We identified existing interventions by hand-searching the literature, through topic experts in the wider research team and from the Phase 1b survey (see Chapter 2). We focused on coding, memoing and team debrief sessions.
Results: we explored ERM systematic reviews and practice recommendations55,85,86 along with six UK paediatric ERM protocols provided by participants in the Phase 1 survey, some of which had been adapted from the PICU Up! early mobilisation programme in the United States. 17 Three key elements emerged:
-
Focus on enabling progressive movement and mobility in diverse patient groups – several existing interventions involve matching ERM activity levels to a patient’s acuity levels, as opposed to focusing on one specific piece of ERM equipment. Many also focus on delivering ERM to diverse population groups over one type of patient. These approaches echo those in the guiding principles. The interventions also offer a range of tried-and-tested materials (e.g. checklists and safety thresholds) that can be used or adapted.
-
Lack of specificity around key activities – existing practice recommendations specify progressive movement and mobility activities; however, this specificity is often lacking in locally adapted ERM protocols that appear to rely more on tacit knowledge of staff and have been expanded to include elements of usual care. Broad concepts such as neurodevelopmental play and activities of daily have been included as ERM activities; however, the guiding principles outline that PICU teams need practical specification of all core ERM concepts. Existing interventions consistently emphasise the importance of developmentally appropriate and individualised ERM; however, more practical guidance is required on how to operationalise these principles in the context of multidisciplinary delivery of ERM.
-
Implementation support is missing – existing interventions offer very little guidance or support on how to introduce and embed ERM into a specific setting. The guiding principles outline how clinical stakeholders want and need support with initial implementation work, from getting initial staff buy-in to sustaining ERM over time.
Current ERM interventions offered a useful and important base. However, implementation issues need to be considered as early as possible to improve the design and sustainability of ERM interventions and reduce the chance of implementation failure. 87 Two of the core implementation issues in ERM are making sure that adequate support and guidance are offered in the day-to-day delivery of ERM activities and that all activities are both flexible (to enable tailoring for individual units and patients) and specific (to enable differentiation from usual care, implementation, and evaluation).
Specifying the bedside bundle
Purpose: to specify the essential clinical materials needed to plan and deliver ERM for individual patients.
Methods: we used a rapid pragmatic approach to identify, characterise and understand existing PICU ERM clinical materials. We identified existing materials by hand-searching the literature, through topic experts in the wider research team and from the Phase 1b survey (see Chapter 2). We focused on coding, memoing and team debrief sessions.
Results: we identified existing clinical materials that were tried-and-tested for delivering ERM in different PICU contexts and could be adapted to achieve key design objectives within the guiding principles. These were patient acuity levels, ERM activity levels, safety and tolerance criteria and a daily ERM flowchart. They were further developed and specified to form the first part of a prototype ERM intervention manual – the beside bundle (Table 11).
| Bedside bundle The essential clinical materials needed to do ERM with individual patients |
Implementation toolkit A step-by-step guide to putting the bedside bundle into practice across a PICU |
Training and support materials Flexible resources for tailoring the bedside bundle and implementation toolkit to an individual unit |
|---|---|---|
| Patient Acuity Levels – a table displaying four levels of patient acuity ranging from the lowest (level 1) to the highest (level 4) level of clinical stability. Levels are based on specific clinical parameters related to the central nervous system, cardiovascular system, respiratory system and other factors (e.g. lines, tubes etc.). | PICU preparation: |
|
|
||
| ERM Activity Levels – a table displaying four increasingly challenging levels of ERM activity. ERM activity levels focus on patients engaging in progressive movement and mobility in the context of individually meaningful and purposeful activities and a supportive environment that promotes familiar and orientating daily routines. |
|
|
| Pause and Re-assess Criteria – a list of clinical parameters that should be monitored before, during and after ERM activities. The criteria should be personalised based on the clinical team’s judgement and discretion about acceptable deviations from the individual patient’s baseline status. |
|
|
| Daily ERM Flowchart – a graphic used during ward rounds to prompt key actions for each patient every day 2, including: screen for ERM eligibility, assign patient acuity level, assign ERM activity level, select specific ERM activities, decide who is leading the ERM and how the parent/carer will be involved, and feedback from previous day’s ERM activities. |
|
|
| Patient delivery: |
|
|
|
||
|
|
Patient acuity levels
Several existing ERM interventions already set out patient acuity levels that describe the full spectrum of clinical stability across diverse patient populations ranging from the least to the most stable. The levels are based on central nervous, cardiovascular and respiratory system parameters, as well as other factors such as the presence of lines or tubes. They are then linked to corresponding levels of ERM activities that may be safe and appropriate. This approach was pioneered in paediatric intensive care by the PICU Up! programme17 and has been further adapted within UK PICU settings. We compared and contrasted existing patient acuity levels along with specific contraindications, exclusions, precautions and eligibility criteria from other ERM interventions identified in our literature-based work (reported above).
Based on the existing interventions, we drafted four levels of patient acuity that were further refined by clinical members of the wider PERMIT research team (see user development – reported below). We developed comprehensive guidance for the patient acuity levels that includes an explanation of each clinical parameter and a description of how it should be considered in shaping ERM delivery, including for the most severely ill and complex patients. The guidance encourages tailoring of the patient acuity levels to fit in with practice in a particular unit. For example, it sets out how users can remove clinical parameters that are not relevant to their patient population, add clinical parameters that are particularly relevant, or change parameters to fit in with local practice standards.
ERM activity levels
We set out to specify the three core elements in our conceptualisation of ERM (see Table 10) – ‘progressive movement and mobility’ carried out within a ‘supportive environment that promotes familiar and orientating daily routines’ and delivered with the context of ‘individually meaningful and purposeful activities that into account the patient’s age, developmental level and reason for admission’. We extracted practical examples of these elements from the ERM interventions identified in our literature-based work (reported above) and supplemented them with examples from the International Classification of Functioning Disability and Health88 and adult ERM literature. 89–93 We were mindful of identifying examples that related to the more severely ill and complex patients for whom adaptive positioning in bed and gentle sensory experiences would be most relevant. We were also mindful of the very young age and early developmental level of many paediatric patients and so we drew on examples from developmental care interventions, neonatal therapy and early intervention, specifically about protecting sleep, developmentally supportive activities of daily living, infant positioning, family-centred care and controlling light and noise in the environment. 94–96
The core intervention development team compared, contrasted and further specified the examples, removing duplicates and prioritising examples more closely aligned to the key design objectives within the guiding principles. Following the tried-and-tested structure of the PICU Up! programme,17 we arranged this content into four levels of ERM activities to correspond with our four levels of patient acuity. The activity levels were then further refined by clinical members of the wider PERMIT research team (see user development – reported below).
Activity level 1 focuses on positioning the patient to maintain ROM, avoid triggering of maladaptive tonic neurological reflexes, particularly in children with pre-existing neuro-disability, prevent pressure ulcers and other complications of immobility, and optimise respiratory and gut function. Level 2 focuses on assisting the patient to practise different activities. Level 3 focuses on progressing to more challenging activities. Level 4 focuses on preparing the patient for transition off the unit by regaining as much of their pre-admission levels of independence as possible. The levels are progressively more challenging for the patient and each level includes a minimum recommended dosage of ERM activities. Across the levels, there is an emphasis on orientating the patient to themselves, others, place, and time, and on protecting their sleep. We also developed comprehensive guidance for the activity levels that includes:
-
An overall explanation of the activity levels and how ERM has been conceptualised in the PERMIT intervention manual.
-
An explanation of each of the four activity levels, including the main aim of each level, a description of the type of patient for whom each level is usually appropriate, guidance on specific activities within each level, and suggestions for who may need to lead or be involved in delivering activities at each level (e.g. allied health professional, nurse, parent/carer).
-
How to select meaningful and purposeful ERM activities for an individual patient, considering their acuity, pre-admission characteristics (e.g. age, pre-existing developmental level, pre-existing impairments or limitations, favourite toys and activities), the ERM confidence and skills of the multidisciplinary team at that point in time, and parent/carer intuition about what their child enjoys, dislikes, and may be able to tolerate.
-
How to progress patients to more challenging activities within a specific level as well as progressing up through the four activity levels.
As with the patient acuity levels, the guidance encourages tailoring of the activity levels to fit in with practice in an individual unit. For example, it sets out how users can specify who should lead certain ERM activities depending on the staff groups that are usually available and users can add specific activities that are available in their unit or add/remove activities they especially do/do not want to be considered for certain patient populations or levels of acuity.
Safety and tolerance criteria
Our literature-based work (reported above) highlighted a range of tried-and-tested safety checklists used just before initiating ERM activities and safety criteria used during activities to monitor patient tolerance and guide decisions about whether ERM should be paused, altered, continued or stopped. These clinical materials include key indicators of cardiorespiratory and central nervous system instability, pain or discomfort, and concern for the integrity of critical lines and tubes. We compared and contrasted safety and tolerance criteria identified through our literature-based work and supplemented these with examples from adult ERM literature. 53,97,98
Through discussion between the core intervention development team and clinical members of the wider PERMIT research team, we initially considered and ruled out three approaches to specifying safety and tolerance criteria:
-
Define explicit safety thresholds by age and illness category. Age-related normal values for cardiovascular, respiratory and central nervous system parameters are widely established. However, in a PICU setting many patients’ observations will be outside normal thresholds and a priori specification of thresholds in all scenarios would not be feasible given the heterogeneity of the clinical population.
-
Use a ‘triggered alarm’ approach. Bedside nurses frequently review and adjust alarm limits for oxygen saturation, heart rate, arterial blood pressure monitoring etc. However, false alarms are common (e.g. brief, insignificant perturbations, particularly in more awake patients) and therefore strict application of a triggered alarm approach may not be feasible.
-
Define thresholds in terms of percentage change relative to a patient’s baseline (e.g. change in heart rate of >20%). While this approach to individualising safety and tolerance criteria is attractive, it may not be feasible to calculate a meaningful and useful baseline. For example, clinicians do not routinely calculate an average heart rate over the last 4 hours and to do so before initiating ERM activities may be burdensome and open to discretion about how to interpret periods of elevated heart rate during procedures over those 4 hours.
We determined that, as part of a routine risk assessment conducted just before ERM activities are carried out, clinicians should use their judgement to prospectively define individualised safety and tolerance criteria that are meaningful and useful for a given patient [e.g. acceptable upper and lower thresholds for heart rate, respiratory rate, saturation, intracranial pressure (ICP)]. If observations exceed these limits the ERM activity should be paused and the appropriateness of further activities should be re-assessed. Our rationale was that clinicians have intuitions of acceptable bounds on physiological parameters for a given patient, based on their routine observations to date, knowledge of the patient’s condition etc. Patient acuity level can change rapidly during the day and between procedures. Therefore, clinicians are accustomed to reviewing the appropriateness of any intervention before it is carried out and monitoring how the patient is responding throughout and afterwards. The same principle may apply to ERM. In the ERM intervention manual, we set out the safety and tolerance (‘pause and re-assess’) criteria that clinicians should prospectively define and monitor for individual patients before, during and after ERM activities. The manual also includes guidance on incorporating the criteria into a unit’s existing documentation and processes and provides a practical example of implementation in one unit (Birmingham Women and Children’s NHS Foundation Trust) for clinicians to use as a model.
ERM daily flowchart
Having specified the essential clinical materials needed to deliver ERM, we set out to identify an approach to planning and organising delivery on a daily basis across a PICU. In line with the guiding principles, we needed a mechanism for prompting routine screening of all patients across the unit for ERM eligibility, ensuring timely initiation of ERM on the second morning of admission for patients anticipated to still be on the unit the following day, assigning acuity levels, selecting meaningful and purposeful ERM activities for individual patients, deciding who would support each patient’s ERM on a given day and how parents/carers would be involved, and feeding back on the patient’s tolerance of ERM to inform subsequent team decision-making.
Our literature-based work identified an existing ERM intervention – MOVE4WARD – that included a tried-and-tested daily flowchart used in the morning ward round to plan and organise ERM delivery. MOVE4WARD is the ERM programme at Birmingham Women and Children’s NHS Foundation Trust and was adapted from the PICU Up! programme. 17 We further specified the MOVE4WARD daily flowchart and developed guidance on how to use it and tailor it to fit in with practice in a particular unit. For example, we encourage users to change the design and layout, insert their own language and terminology for local processes and documentation, and add more detail such as the specific people who will be carrying out certain tasks.
Specifying the implementation toolkit
Purpose: to specify the second part of a prototype ERM intervention manual – a step-by-step guide to putting ERM into practice across a unit.
Methods: we used a rapid pragmatic approach to identify, characterise, and understand literature focusing on the implementation of ERM interventions in PICU settings and conceptualised the core findings in relation to implementation theories.
Search strategy and study selection: we included all the papers from the Phase 1 scoping review as well as those that were excluded on the basis of sample size or study design. We updated the searches to identify more recent work in paediatric intensive care. We also identified published systematic reviews, meta-analyses, and scoping reviews of ERM in adult intensive care.
Data extraction: we extracted information on determinants (i.e. barriers and facilitators) to introducing, embedding, and sustaining implementation of ERM as well as strategies used to overcome those identified determinants.
Analysis: we coded the determinants against theoretical concepts from NPT71,72 and TFA73 and strategies against those identified by ERIC,74 and the behaviour change Theory and Technique Tool. 75 We reviewed and discussed the findings. Through an iterative process, we developed a step-by-step toolkit and related resources to support tailored implementation of ERM within individual and diverse units. This was further refined by clinical members of the wider PERMIT research team (see user development – reported below).
Results: Table 11 includes an overview of the implementation toolkit and related resources. We identified six steps towards ERM implementation – four geared towards preparing the PICU for ERM delivery and two geared towards actual delivery of ERM to patients. PICU preparation involves: taking stock – making an overall assessment of where the unit is currently at with ERM and deciding the next steps; building the team – bringing together a group of multidisciplinary ERM champions to lead implementation; getting buy-in – building a shared understanding across the unit of what ERM is all about; and getting ready – tailoring the bedside bundle to make it as workable as possible for the staff and systems already in place in the unit. Patient delivery involves: making it work – gradually putting the bedside bundle into practice in the unit until it is fully implemented with all patients; and keeping it going – coming together as a unit to review the bedside bundle, decide how it could be working better, and plan how ERM can be sustained over the next 6–12 months. Table 12 sets out a more detailed theoretical overview of the implementation toolkit.
| Implementation steps | What does it mean? | Theoretical processes | Why does it matter? | Theoretical processes | What’s involved? (corresponding ERIC strategies) |
|---|---|---|---|---|---|
| TAKE STOCK | Making an overall assessment of where the unit is currently at with ERM and decide the next steps to be taken. Creating a tailored action plan for delivering the bedside bundle across the unit, focusing time and effort on the areas that most need support. |
Communal appraisal (NPT) Interactional workability (NPT) |
Focusing on a tailored action plan makes ERM feel more manageable for everybody. It is harder to change practice if clinicians believe they are already doing all aspects of ERM. Therefore, it helps to specify exactly where the bedside bundle is not happening or needs to be improved. |
Burden (TFA) Differentiation (NPT) |
The manual includes a self-assessment checklist for taking stock. (Assess for readiness and identify barriers and facilitators.) From the self-assessment, the next steps for progressing ERM can be prioritised. Be specific about who will do what, with and for whom, and when. This is the action plan. (Tailor implementation strategies) |
| BUILD THE TEAM | Bringing together multidisciplinary champions to support and market ERM, drive it through and overcome any indifference or resistance. | Initiation (NPT) | Leadership from a credible, representative and organised group of multidisciplinary clinicians makes people trust ERM and feel more positive about it. ERM champions with a good understanding of the different roles, expertise and personalities in the unit can allocate tasks and responsibilities to those with the right skills. |
Affective attitude (TFA) Relational integration (NPT) Skill-set workability (NPT) |
Champions should represent the multidisciplinary team and include a lead doctor, nurse and physiotherapist as a minimum. (Identify and prepare champions) The manual includes resources to help champions prepare for presenting the evidence for ERM and persuading colleagues about its benefits and safety. (Identify and prepare champions) |
| Champions should organise weekly team meetings to plan, monitor and review ERM and support one another’s learning. (Organise clinician implementation team meetings) | |||||
| GET BUY-IN | Building a shared understanding across the unit of what ERM is all about – what the bedside bundle involves, how it is supposed to work, and what are the potential benefits and intended outcomes for patients. Achieving agreement that ERM fits in with the unit’s values of providing the best possible care. |
Communal specification (NPT) Intervention coherence (TFA) Internalisation (NPT) Ethicality (TFA) |
When clinicians buy-in to ERM, they understand how they can contribute to ERM and feel comfortable that it can be done safely. They can see where the bedside bundle is currently working well, where it is not happening, and where it needs to be improved. They are willing to make it happen. When unit managers buy-in, they make it clear that they support ERM. |
Legitimation (NPT) Affective attitude (TFA) Differentiation (NPT) Contextual integration (NPT) |
Get everybody in the unit involved in discussions about ERM to start building positive feelings and understanding. (Conduct local consensus discussions) Get people actively involved in problem-solving around ERM. For example, what are their concerns around patient safety and staffing? How do they think ERM should fit in with sedation and nutrition practices? (Facilitation) |
| Share examples of successful ERM and positive patient and parent/carer feedback. (Obtain and use patient/family feedback) Ask unit managers to make it clear that ERM is a priority and that they support implementation. (Mandate change) |
|||||
| GET READY | Tailoring the bedside bundle to make it workable for the staff and systems already in place in the unit. Specifically, this means allocating ERM tasks and responsibilities appropriately and fitting the bedside bundle into the current ways of ordering, recording, communicating, and monitoring patient care. | Enrolment (NPT) Skill-set workability (NPT) Individual specification (NPT) Systematisation (NPT) |
When clinicians understand their role in the bedside bundle and prepare for carrying out their allocated tasks and responsibilities, they feel more confident and positive about ERM. Building the bedside bundle into existing systems makes ERM feel more manageable. |
Self-efficacy (TFA) Burden (TFA) |
The manual includes guidance on tailoring the bedside bundle to an individual unit. (Promote adaptability) It is easier to deliver the bedside bundle if units are specific about who does what and when. (Revise professional roles) Consider how to use existing systems (e.g. ward round checklists, electronic records, whiteboards). (Change record systems) |
| Plan training sessions to show people how the bedside bundle works. Make sessions interactive and tailored to the needs of different clinicians. (Conduct educational meetings; Make training dynamic) | |||||
| MAKE IT WORK | Gradually putting the bedside bundle into practice until it is fully delivered with all patients. The unit tests out ERM and gets feedback on how it is working (e.g. have tasks and responsibilities been allocated appropriately, is it fitting into existing systems?). | Interactional workability (NPT) Skill-set workability (NPT) Systematisation (NPT) |
Gradual implementation provides time and space for clinicians to build confidence in their own and each other’s skills. As they gain first-hand experience of safe delivery, clinicians feel more positive and less worried about ERM. |
Self-efficacy (TFA) Relational integration (NPT) Affective attitude (TFA) Individual appraisal (NPT) |
Phase-in the bedside bundle and very gradually move to a unit-wide rollout. (Stage implementation scale-up) Consider rostering an ERM champion onto every shift to help implement the bedside bundle, solve problems, and monitor quality. (Facilitation) |
| As they gradually gain more experience, clinicians are able to give feedback about the impact of ERM on themselves, their workload, patients, and the unit overall. | Build in reminders that prompt clinicians about the bedside bundle and help them remember what exactly they need to do. (Remind clinicians) Put in place a system for getting feedback about ERM from clinicians, patients (where possible), and parents/carers. (Obtain and use patient/family feedback) |
||||
| KEEP IT GOING | Coming together as a unit to review the bedside bundle, decide how it could be working better, and plan how ERM can be sustained over the next 6–12 months. | Communal appraisal (NPT) Reconfiguration (NPT) Activation (NPT) |
Ongoing evaluation of the bedside bundle combined with comprehensive feedback for clinicians builds trust and confidence in the effectiveness of ERM. | Relational integration (NPT) Perceived effectiveness (TFA) Skill-set workability (NPT) Self-efficacy (TFA) |
Review how the unit is doing with the bedside bundle. (Purposely re-examine implementation) Provide feedback to the overall unit, clinician groups, and individuals about how ERM is going. (Audit and provide feedback) Conduct spot checks on different parts of the bedside bundle and provide feedback to the overall unit, clinician groups, and individuals. (Audit and provide feedback) |
| Staff turnover in the unit means that who is delivering the bedside bundle changes over time. Permanent staff also change as they develop their confidence and skills in ERM. Ongoing evaluation and training should consider these changes. | Plan ongoing training sessions to refresh permanent staff and show new staff how the bedside bundle works. (Conduct ongoing training) The manual includes guidance on creating a unique identity for the locally tailored bedside bundle and spreading the word about ERM through posters, newsletters, events and social media to help create a positive buzz and maintain interest. (Use mass media) |
The six steps towards implementation form a flexible framework that can enable both those units new to ERM to get started and more experienced units to renew their efforts and build on the ERM they have already been doing. The steps are not conceptualised as a linear or sequential process. We suggest in the intervention manual that it makes sense for users to start off with taking stock and thinking through their unit’s readiness for implementing ERM. Otherwise, given that implementation factors vary between units and that every PICU is at a different point in their ERM journey, we envisage that settings will focus their implementation efforts on the steps most in need of attention locally.
Reviewing outcomes
In the workshops with clinical stakeholders, we collected information about potential outcomes of ERM and incorporated these into our guiding principles (see Table 10). We planned to conduct a rapid literature review to identify related patient-centred outcome measurement tools and potentially incorporate these into the prototype ERM intervention manual. However, prior to starting the review work, we learnt that Fink et al. were developing a PICU core outcome set and core outcome measurement set – a PERMIT co-applicant had been invited to join that study’s International Steering Committee. 99 Rather than repeat this work, we reviewed the outcomes within our guiding principles against the published PICU core outcome set47 (Table 13) and developed our ERM logic model accordingly (see logic model – reported below).
| Outcomes of ERM proposed by clinical stakeholders | Corresponding global outcomes considered for the PICU COS | Included in PICU COS | Included in PICU COS – Extended | Excluded from PICU COS | Corresponding specific outcomes considered for the PICU COS | Included in PICU COS | Included in PICU COS – Extended | Excluded from PICU COS |
|---|---|---|---|---|---|---|---|---|
| Duration of MV | Healthcare utilisation | X | New technological supports (tracheostomy, gastrostomy tube, oxygen, etc.) | X | ||||
| LOS in paediatric intensive care and hospital overall | Healthcare utilisation | X | Cost of care | X | ||||
| Movement function and mobility capacity and participation at discharge relative to pre-admission | Physical function | x | Physical mobility | X | ||||
| Activities of daily living capacity and participation at discharge relative to pre-admission | Overall health | X | Activities of daily living | X | ||||
| Social function | X | Child participation | X | |||||
| Body functions – respiratory | Physical function | X | Organ function | X | ||||
| Body functions – muscle functioning/strength | Physical function | X | Medical frailty | X | ||||
| Body function – cognitive | Cognitive function | X | ||||||
| Patient psychological well-being | Emotional health and function | X | Mood and feelings | X | ||||
| Post-traumatic stress | X | |||||||
| Family psychological well-being | Family function | X | Parent/legal guardian emotional function | X | ||||
| Patient QoL | Overall health | X | Child QoL | X | ||||
| Family satisfaction with care | n/a | n/a |
User development – refining the prototype intervention manual
Purpose: to refine the prototype ERM intervention manual through exploring clinical stakeholders’ views on acceptability and feasibility.
Methods: we took forward key topics and outstanding issues and uncertainties emerging from the ERM intervention manual development process into a series of formal and informal meetings with clinical members of the wider PERMIT research team. These meetings took place alongside the literature-based work (reported above) and had an iterative relationship.
Sampling and recruitment: we worked with existing members of the wider PERMIT research team. They represented a multidisciplinary group of NHS healthcare professionals with diverse experience of ERM in PICU settings. A total of 13 professionals from three PICUs took part in the process – four consultant doctors (three paediatric intensivists and one paediatric neurologist), two senior nurses (one clinical academic and one PICU lead) and seven senior physiotherapists.
Data collection: we undertook nine online group meetings. Meeting focus evolved with findings of literature-based work and insights from prior meetings shaping the focus of the next. The core intervention development team would present materials from the bedside bundle and implementation toolkit and/or facilitate the discussion and the clinical team members would support, refine and challenge elements of the manual. Members of the intervention development team recorded contemporaneous fieldnotes. The meetings lasted for 60 minutes. We also undertook additional formal and informal smaller meetings, with specific people, to further explore or refine difficult issues, as well as to undertake ‘walk-throughs’ of manual processes and explore reactions.
Data analysis: we focused on reviewing fieldnotes, memoing and debrief sessions within the core intervention development team. We reviewed, discussed and summarised the meetings, and worked to adapt the manual in an iterative fashion focusing on maximising potential successful introduction and embedding of the intervention.
Results: Table 11 summarises the content of the PERMIT ERM intervention manual. The key feedback points that were used to refine the prototype manual and improve its overall feasibility and acceptability can be summarised as follows:
-
Introduction and orientation – tell the user what is in the manual, where to start and how to work through the different sections.
-
Layout and formatting – introduce the bedside bundle first as this is of primary importance to users. Keep the manual brief, simple and accessible by ensuring that each of the clinical materials in the bedside bundle and each of the steps in the implementation toolkit fits onto one side of A4. Minimise the burden of flipping between sections in the manual by clearly separating the bedside bundle and implementation toolkit from the lengthier training and support materials.
-
Language and tone – use labels that are already familiar and acceptable, for example a ‘checklist’ for implementing the pause and re-assess criteria evokes more confidence and positive emotion than a ‘risk assessment’. When providing instructions, ensure the tone comes across as flexible and enabling – clear and straightforward instructions may inadvertently come across as too prescriptive or authoritarian.
-
Promote and enable ownership – make it explicit how the bedside bundle builds on systems, processes and expertise that are already in place across a unit. Empower users by highlighting where the intervention manual can be tailored to fit in with local practice and actively encourage them to tailor and take ownership of the materials and resources.
-
Minimise extra burdens and manage expectations – remove unnecessary detail from the tables and flowchart within the bedside bundle. Make it clear which training and support materials are provided within the manual and clarify any materials that will need to be developed or tailored locally by the unit. Take advantage of all opportunities to build the manual into existing documentation rather than introducing new paperwork to the unit.
-
Create a positive buzz and maintain interest – consider providing branded promotional materials such as pens, stickers etc.
In addition, the wider research team identified several key topics that warranted further exploration in our subsequent feasibility study (see Chapter 5): for example, the practicalities around recording ERM intervention data within existing documentation systems and processes, the impact of different clinical perspectives about how to deliver ERM to particular patient populations such as those on ECMO, and the acceptability of our conceptualisation and specification of ERM to diverse stakeholders beyond the PERMIT research team and study participants.
Logic model – refining our understanding
Purpose: to refine a PERMIT ERM logic model.
Methods: we had already developed a preliminary ERM logic model based on current literature and the clinical expertise within the research team. We reviewed and refined our logic model in relation to findings from the manual development process, the correspondence between the outcomes within our guiding principles and the published PICU core outcome set,47 and through discussions within the wider PERMIT research team.
Results: the refined logic model is presented in Figure 13. It provides an overview of the proposed intervention components and implementation processes set out in the refined ERM intervention manual, as well as the proposed outcomes of ERM and the key contextual factors thought to influence its implementation and potential effectiveness.
FIGURE 13.
Logic model of PERMIT.

Summary of findings to inform the PERMIT study
We know that ERM is currently defined and enacted in multiple ways and that people see the potential value for the diverse patient populations within PICU and are willing to support the (safe) delivery of ERM but are uncertain how best to deliver it.
-
We developed core guiding principles around the potential shape and content of the intervention. For example, everyone in PICU, including but not limited to doctors, nurses, physiotherapists and parents, are all essential for ERM delivery – everyone should take some ownership. Also, ERM needs to be as inclusive as possible, with a focus on promoting movement and mobility as early as possible and with progressive increases over time.
-
We developed a prototype ERM manual that is focused on both the (safe) delivery of ERM for each patient as well as the introduction and embedding of an ERM approach within a PICU. Centrally, the proposed PERMIT ERM manual is informed by current evidence, experience and theory. It offers a flexible, progressive, approach to the delivery of ERM, with resources including essential clinical materials – the ‘bedside bundle’ – that consist of patient acuity levels, ERM activity levels, pause and re-assess criteria, and an ERM daily flowchart. It also includes a step-by-step guide to putting ERM into practice – the ‘implementation toolkit’ – that focuses on building ERM leadership, generating staff buy-in, making ERM workable and keeping it going over time.
Chapter 5 Phase 3: paediatric early rehabilitation and mobilisation during intensive care feasibility study – part 1 study feasibility
Introduction
Following the development of the PERMIT ERM intervention manual in Phase 2 we designed and delivered a pilot feasibility study which would allow both the assessment of the implementation of the intervention in the NHS setting and also the trial feasibility of screening, recruitment and enrolment of CYP into a study that would deliver our manualised ERM intervention. In this chapter, we present the quantitative results of the feasibility of delivering the ERM intervention to CYP. In Chapter 6 we described in detail the process evaluation of the implementation, feasibility and delivery of the ERM manual within three NHS PICUs.
Study management
The work package was co-led by BRS, JMen and FK. The study management group provided input into protocol, ethics application design and data interpretation. Sati Sahota was trial co-ordinator. JYT designed, adapted and managed REDCAP database. Statistical analysis performed by BRS, RF and James Martin.
Aim
To explore the feasibility and acceptability of implementing the intervention manual in three PICUs.
Objectives
-
Determine enrolment, recruitment and delivery of the PERMIT intervention.
-
Monitor the safety of the PERMIT intervention and related AEs.
-
Establish whether clinically important outcomes can be measured following delivery of the PERMIT intervention.
Methods
Design
A non-randomised unblinded intervention feasibility study with embedded process evaluation.
Intervention
The PERMIT intervention, as described in Chapter 4, was defined as a PICU-wide, healthcare professional-delivered intervention, aiming to promote opportunities for the delivery of ERM. The intervention included strategies to develop an organisational environment that supported the delivery of ERM, as well as ERM activities that could be tailored for each individual patient. Each PICU received the PERMIT intervention manual (see Table 12). The manual defined two key steps: preparing for the PERMIT intervention; and recruiting patients and delivering the ERM intervention. Within these two steps there were six key phases (Figure 14).
FIGURE 14.
Phase 3 feasibility study overview.

Step 1 - PICU preparation: to prepare the PICU for implementing the PERMIT intervention, a group of PERMIT champions (lead multidisciplinary healthcare professionals and managers) was formed (Phase 1 ‘build the team’). The team then led a rapid self-assessment of their PICU’s readiness to implement ERM (Phase 2 ‘take stock’). Relevant stakeholders were then brought together to participate in local discussions about ERM (Phase 3 ‘get buy-in’). The local PERMIT team reviewed, adapted and tailored the PERMIT intervention manual to suit the unique circumstances of their PICU and planned how best to incorporate ERM into local work routines (Phase 4, ‘get ready’).
Step 2 - Patient delivery: once the PICU self-assessed themselves as ready to start to deliver the PERMIT intervention to CYP, the site then progressed into Step 2. At this point, the local PERMIT team started to recruit patients to the trial, receiving ERM as defined by the manual (Phase 5 ‘make it work’). The study team reviewed the implementation process and worked with the local clinical teams to support ongoing education and training, adjust elements of the process as needed and plan for sustaining the programme beyond the end of the study (Phase 6 ‘keep it going’).
Setting
The study is based in PICUs within three NHS organisations: Birmingham Women and Children’s NHS Foundation Trust (Birmingham), King’s College Hospital NHS Foundation Trust (London) and University Hospital Southampton NHS Foundation Trust (Southampton). The PICUs were selected because of their diversity in (1) clinical expertise and the patient populations they serve (e.g. two PICUs were both cardiac and specialist PICUs, one non-cardiac but a large neurocritical care and liver transplantation programme), (2) the types of resources and staff groups they have available (e.g. allied health professionals, play specialists, rehabilitation equipment) and (3) ERM experience to date of implementing ERM within usual care (e.g. two units with minimal experience of implementing ERM and one with nationally recognised expertise in ERM).
Sampling and recruitment
Since this study was focused on feasibility and acceptability, an a priori sample size estimation was not required. Instead, our sample size was sufficient for capturing data across the diverse patient groups receiving ERM and the multidisciplinary healthcare professionals involved in implementing ERM.
Children and young people
We aimed to recruit 30 CYP (10 per unit) to receive ERM. All CYP were screened daily against the following eligibility criteria: aged 0–<16 years at the point of admission, admitted to PICU for any reason and likely to remain in the PICU on day 3 post admission. We excluded individuals where, for any reason, the local clinical team did not feel it was appropriate to include them in the study and we recorded the reasons. The majority of CYP were unable to provide informed consent or participate in an assent process (e.g. because of their very young age, levels of sedation medication). We therefore relied primarily on consent from parents/legal guardians (see below). However, where possible and appropriate, we attempted to take steps to involve CYP in decision-making and inform them about the study to the fullest level of their understanding.
Parents/legal guardians
To gain consent for delivering ERM, we approached parents/legal guardians of eligible CYP on day 1 or 2 of admission. As a minimum, they could choose to consent only to their child receiving ERM and to the accompanying data collection (i.e. clinical data about their child and intervention data about the delivery of ERM). This placed no direct burden of data collection on the parents/legal guardians themselves, offering option of participation, without any additional demands during the PICU admission. If parents declined the study then the child received ‘standard care’ in accordance with the local PICU policy. In addition, parents/legal guardians could choose to consent to completing outcome measures at two different time points – during their child’s stay in intensive care and around the time of discharge or shortly afterwards. They could agree to this when they were first approached on day 1 or 2 of admission or at a later stage during their child’s PICU stay. We also invited parents/legal guardians to take part in qualitative interviews (see Chapter 6: Process evaluation).
Data collection
We collected data about the CYP receiving ERM, the delivery of ERM to patients and the health-related outcomes of relevance to ERM. In this chapter, we report CYP clinical data, PERMIT intervention delivery data and parent/guardian outcome measurement data (Table 14). Chapter 6: Process evaluation contains details about the data collection of debrief conversations with PICU champions, interviews and survey with health professionals and interviews with parents/legal guardians.
| Target population | Measure | Data collection | Items/time required | PERMIT study step | Frequency | Pre-PICU baseline | During PICU | PICU Discharge or 30 days |
|---|---|---|---|---|---|---|---|---|
| CYP | Various | Local PERMIT Research Team | Various | 2 | Various | X | ||
| CYP | Height and weight Z-score (admission and discharge) | Local PERMIT Research Team | 2 | Twice per participant | X | |||
| Pain visual analogue scale (admission and discharge) | 1 item/1 minute | 2 | Twice per participant | X | ||||
| PELOD-2 score | Local PERMIT Research Team | 2 minutes | 2 | Daily | X | |||
| cCPAx | 5 minutes | 2 | Daily | X | X | X | ||
| BRADEN-QD | 7 items/5 minutes | 2 | Daily | X | ||||
| CAPD | 8 items/4 minutes | 2 | Daily | X | ||||
| COMFORT Behavioral Score | 8 items/3 minutes | 2 | Daily | X | ||||
| POPC and PCPC | 2 items/6 minutes | 2 | Twice per participant | Xa | X | |||
| PERMIT intervention data | Patient acuity level | Local PERMIT Research Team | 2 | Daily | X | |||
| Prescribed ERM activity level and specific ERM activities | 2 | Daily | X | |||||
| Delivered ERM activity levels and specific ERM activities (including timing, duration, number and type of staff or family member assisting) | 2 | Daily | X | |||||
| Reasons for deviating from the prescription, where relevant | 2 | Daily | X | |||||
| Use of pause and reassess criteria | 2 | Daily | X | |||||
| Safety and AEs | 2 | Daily | X | |||||
| Any intervention/manual tailoring proposed or undertaken within the site | 2 | Daily | X | |||||
| CYP | PedsQL™ Infant Scales Version 4.0 – Acute (aged: 1–23 months) OR PedsQL™ Generic Core Scales Version 4.0 – Acute (aged: 2 years+) |
Local PERMIT Research Team collect the data from parents/legal guardians | 36 items/<7 minutes 45 items/<10 minutes 21/23 items/<5 minutes |
3 | Twice per participant | Xa | X | |
| PedsQL™ Multi-dimensional Fatigue Scale Version 3.0 – Acute | 18 items/5 minutes | 3 | Twice per participant | Xa | X | |||
| Parent/legal guardian | Parent Stressor Scale: PICU | Local PERMIT Research Team | 30 items/10 minutes | 3 | Once per participant | X | ||
| EMPATHIC-30 | 30 items/<15 minutes | 3 | Once per participant | X | ||||
| PedsQL™ Family Impact Module Version 2.0 | 36 items/5 minutes | 3 | Twice per participant | Xa | X | |||
| PHQ-4 | 4 items/2 minutes | 3 | Once per participant | X | ||||
| Process evaluation data collection | ||||||||
| PERMIT champions | Weekly debrief discussion | Central PERMIT Research Team | 30 minutes | 1,2,3 | Weekly | n/a | ||
| PICU healthcare professionals | Online survey | 15 minutes | 1,2,3 | Three times per participant | n/a | Online survey | Central PERMIT Research Team | |
| Interviews | ≤60 minutes | 1, 2 or 3 | Once per participant | n/a | Interviews | Central PERMIT Research Team | ||
| Parent/legal guardian (interview) | Interview | ≤60 minutes | 3 | Once per participant | X | |||
Children and young people receiving ERM: currently, all CYP admitted to PICU have clinical data routinely recorded via the Paediatric Intensive Care Audit Network (PICANet). 67 We identified PERMIT study participants on the PICANet web-based system and conducted a data download of a pseudo-anonymised comprehensive data set pertaining to each individual CYP enrolled into the PERMIT study.
The data download included the PICANet minimum data set for each individual participant. This included demographic and socioeconomic data (participant’s date of birth, sex, ethnicity); pre-PICU health status (past medical history including underlying conditions and comorbidities); acute illness data [PIM3 (model of PIM that assesses the risk of mortality among children admitted to a PICU)]; PICU admission and discharge diagnoses; comorbidities; operations and invasive procedures performed; type of admission; PICU and hospital LOS, duration of MV, high-frequency oscillatory ventilation, ECMO, renal replacement therapy and vasopressor/inotropic support; sedative medications and days of exposure.
We also collected additional clinical data. The majority of data were collected daily from time of enrolment into the study until PICU discharge (see Table 14) and included:
-
Level of organ dysfunction: PELOD-2 score.
-
Level of physical activity: Children’s Chelsea Critical Care Physical Assessment Tool (cCPAx).
-
Skin integrity: Braden QD was completed to assess skin integrity and risk of pressure damage/injury.
-
Presence of delirium: Cornell Assessment of Pediatric Delirium (CAPD).
-
Level of sedation: The COMFORT behaviour scale (COMFORT‐B scale) sedation assessment score.
-
POPC and PCPC (these were collected at admission to reflect the child’s pre-admission status and PICU discharge only).
Data were recorded on individual patient CRFs and transferred for analysis into the REDCap database for the PERMIT study.
Paediatric early rehabilitation and mobilisation during intensive care intervention data: the Local PERMIT Research Team conducted daily screening of CYP on PICU, to calculate the number of patients fulfilling inclusion eligibility and number of patients approached, consented/declined for the study.
Once parents/legal guardians (and CYP where appropriate) had consented, the patients received the PERMIT intervention until PICU discharge. The Local PERMIT Research Team used the PERMIT CRF for each individual CYP to collect the following data about the PERMIT intervention at ward rounds, ERM intervention sessions and through discussion with local clinicians:
-
patient acuity level;
-
prescribed ERM activity level and specific ERM activities;
-
delivered ERM activity levels and specific ERM activities (including timing, duration, number and type of staff or family member assisting);
-
reasons for deviating from the prescription, where relevant;
-
use of pause and reassess criteria;
-
safety and AEs;
-
any intervention/manual tailoring proposed or undertaken within the site.
Outcome measurement tools: the Local PERMIT Research Team distributed outcome measurement tools (questionnaires) to parents/legal guardians at two time points: during their child’s PICU admission and at the point of PICU discharge or within 30 days after PICU discharge, whichever occurred first. The outcome measurement tools were administered initially as paper questionnaires then by additional methods at parents’/legal guardians’ preference. The information was entered into the PERMIT study REDCap database.
During PICU admission: parents/legal guardians provided a retrospective report based on their child’s pre-admission status (2 weeks before) by completing a QoL and a fatigue measure (1 and 2) and an assessment of the family pre-admission status (3) from the Pediatric Quality of Life Inventory (PedsQL)™ family of questionnaires:
-
PedsQL™ Infant Scales Version 4.0 – Acute (Aged: 1–23 months) OR PedsQL™ Generic Core Scales Version 4.0 – Acute (Aged: 2 years+);
-
PedsQL™ Multi-dimensional Fatigue Scale Version 3.0 – Acute;
-
The PedsQL™ Family Impact Module Version 2.0 (parent report).
At point of PICU discharge or within 30 days post admission to PICU: parents/legal guardians completed questionnaires based on their child’s current health status (1–3) and the family status (4–7):
-
PedsQL™ Infant Scales Version 4.0 – Acute (Aged: 1–23 months) OR PedsQL™ Generic Core Scales Version 4.0 – Acute (aged: 2 years+);
-
PedsQL™ Multi-dimensional Fatigue Scale Version 3.0 – Acute;
-
the PedsQL™ Family Impact Module Version 2.0 (parent assessment);
-
parent Stressor Scale;
-
the EMpowerment of PArents in The Intensive Care – 30 Item Version (EMPATHIC-30);
-
Patient Health Questionnaire-4 (PHQ-4).
Feasibility and acceptability of the questionnaires were captured through monitoring completion rates and feedback from the parent/carer interviews. This is reported in Chapter 6: Process evaluation.
Data analysis
We reviewed data for errors, missing data, duplicated records and outliers. Extreme values were set to missing if they were deemed impossible, based on their validity range. Continuous variables were reported as mean and SD or median and IQR based on data distribution. Categorical variables were described in numbers and/or percentages.
Descriptive statistics were used to summarise the PERMIT intervention data, including: success of implementation, recruitment, proportion of consented patients triaged from the acuity table, proportion of consented patients allocated an ERM intervention from the manual appropriate to their acuity level, proportion of consented patients receiving the prescribed ERM, number of AEs and proportion of patients experiencing AEs. Results are presented in text and tables with a narrative summary of findings. Stata v16 was used for all statistical analyses.
Patient and public involvement and engagement
For an overview of the approach to PPIE adopted throughout the study, please see Chapter 8. PPIE in this phase of the study focused on exploring the appropriate model of consent, refining participant-facing documents and establishing the overall acceptability of the study. We had planned to speak to parents with direct experience of involvement in research studies, but the research suspension imposed in response to directives to prioritise COVID-related research made this challenging. 100 We spoke to parents of four children (aged 1 month to 16 years) who had experienced one or more PICU admission(s) at BCH and Kings College London. One parent had experience of their child being recruited to a number of non-PICU research studies.
The concept of a staged approach to consent to help reduce the burden about deciding on all aspects of the study at once was liked by the parents (Table 15). The challenge of speaking to families so early on in the child’s PICU stay was recognised. One parent described the overwhelming emotions and the importance of reducing the demands being placed upon them:
There’s also the challenge of emotional engagement. When * first came in, there was an overwhelming, primeval pain. This developed into a rollercoaster of positive and negative emotions which over-rided my ability to absorb information ….
(PPIE parent)
| Aspect | PPIE feedback | Impact/changes made |
|---|---|---|
| Consent model | ||
| Observational data | Where you are collecting data on what is already taking place – emphasise this. Keep consent simple. If too much is asked at this stage there is a risk people will decline as they are overwhelmed. |
Two-stage consent process planned and endorsed: Level A consent: consent for the CYP to receive the PERMIT intervention, with observation and safety monitoring by the team. No additional requirements placed upon parents/CYP. This offers an option for families who want to participate in research, but feel they cannot deal with any additional requirements. |
| Level B consent: additional questionnaires and interview | Some people might feel able to consider this at the same time as Level A; some families will not. Having the option to defer decision-making about these additional aspects will help some people. |
Level B consent: offers parents/legal guardians the opportunity to provide feedback on the experience of receiving the PERMIT ERM intervention through questionnaires and an optional interview post discharge. Parents/legal guardians could consent at the same time as Level A if they felt able to, or at a later stage in PICU stay. Provides choice. |
| Perspective of those who decline | Good idea, but unsure if people will agree to consent when they’ve already declined research. | Option of an interview for those who decline to participate. |
| Patient-facing documents | ||
| Length of the PIS | All four parents commented the document was long and too ‘wordy’. | Paragraphs shortened. More subheadings to break up blocks of text. Illustrations added. |
| Understanding of what ERM interventions could entail or look like | Three parents thought useful to have illustrations/pictures to help visualise the type of interventions involved. Uncertain about use of photos but possibly might be useful for children to help them relate. |
Illustrations added, developed by the research team. Illustrations depicted a CYP receiving ERM activities e.g. sitting up in bed or mobilising. In younger child version photos of a child were used (aged 4). These were of D, daughter of our PPIE co-applicant with her consent. |
| Key information | Minimise information within PIS to ‘key information’. Avoid too much focus on data management, this is not a concern. Add this information at the end of the PIS rather than near the start (as less important from parent perspective). | Data-management section kept as brief as possible and added at the end of the PIS. |
| Infographic | Appropriate and helpful to add clarity to the text. | Infographic used in the PIS to help outline the key steps of the study and to help explain the differences between the levels of consent. |
| Acceptability of ERM research | ||
| Acceptability of PERMIT phase 3 trial | Trial is important and acceptable based on the information provided in the PIS. Concerns about how this works for children with a pre-existing plan. |
Endorsed the study and the current design. PIS emphasises that the alternative (if they decline) is ‘standard care’ for that PICU to help clarify there is a choice. Site initiation visit (SIV) material prepped to include training on how to discuss this with parents/legal guardians. |
Being able to stagger the informed-consent process until a parent was better placed to consider what was being asked of them was endorsed. Other factors that helped were having these messages clearly conveyed in the PIS and ensuring that this process was supported by someone who could provide clarification and answer questions. Parents noted that the material was written at the right level for them; however, concerns reflected the volume of information – ‘It’s like a book!’ – to read and digest. All commented on the value of figures or illustrations to help visualise the sort of interventions that would be involved.
We had also hoped to explore outcome measures and the interview schedules (for consenting parents and declining parents) but this was not possible. All four patients were inpatients on either PICU or had just been discharged to the ward. The parents we worked with were the only parents allowed with their child, so they had a lot of competing demands. It was not felt to be appropriate to provide them with multiple outcome measure questionnaires or interview schedules to review; in fact, this was not allowed in some ward areas due to COVID. The study team therefore drew on our PPIE work from other recent PICU studies that were involved in selecting questionnaires (the OCEANIC study101) and qualitative interviews (BRICC trial102) to shape the decision-making.
The study received Health Research Authority approval (ref 21/SC/0127), and was registered at https://clinicaltrials.gov/ct2/show/NCT04909762.
Results
Site implementation and recruitment to PERMIT study
Overall, three PICUs participated in the PERMIT feasibility study. The PICUs were chosen as a pragmatic sample with variation in average annual admissions, number of overall PICU staff, types of paediatric specialties and stand-alone children’s hospital, co-located adult/paediatric centre.
Sites 1 and 2 started Step 1 of the PERMIT implementation programme in early June 2021. Site 3 was delayed due to contract issues until 2 August 2021 (Figure 15). All three sites (100%) achieved the primary outcome and progressed through phases 1–4 of Step 1 (PICU preparation phase) of the PERMIT manual. Time to complete Step 1 and progress to Step 2; site 1: 12.9 weeks, site 2: 15.0 weeks, site 3: 8.4 weeks. Table 16 reports key feasibility outcomes.
FIGURE 15.
Progress of sites through Phase 3 feasibility study.

| Site 1 | Site 2 | Site 3 | All sites | |
|---|---|---|---|---|
| Implementation steps | ||||
| Date study opened | 8 June 2021 | 9 June 2021 | 2 August 2021 | |
| Date achieved step 1 | 6 September 2021 | 22 September 2021 | 30 September 2021 | Range 59–64 days |
| Days from study open | 90 days | 105 days | 59 days | |
| Date achieved Step 2 target recruitment | 29 October 2021 | 15 October 2021 | 26 November 2021 | Range 23–55 days |
| Days from start step 2 | 53 days | 23 days | 57 days | |
| Date of discharge of final recruitment | 6 December 2021 | 24 October 2021 | 26 November 2021 | Range 32–91 days |
| Days from start step 2 | 91 days | 32 days | 57 days | |
| Recruitment of CYP (and families) | ||||
| Eligible CYP patients during Step 2 period | 11 | 14 | 10 | 35 |
| Recruited CYP patients during Step 2 (% eligible) (consent A) | 10 (91%) | 11 (79%) | 10 (100%) | 31 (89%) |
| Number of families of CYP agreeing to consent B (% of total recruited) | 9/10 (90%) | 9/11 (90%) | 3/10 (30%) | 21/31 (68%) 20/30a |
| Number of families of CYP not consenting to the study but agreeing to interview (consent C) | 0/1 | 0/3 | 0/0 | 0/4 |
| Days from PIC admission to study consent med (IQR) | 2.5 (2–4) | 2 (2–2) | 3 (2–4) | 2 (2–4) |
| Days from PIC admission to 1st study day median(IQR) | 3 (2–5) | 3 (2.5–3) | 4 (3–5) | 3 (3–5) |
All three sites (100%) recruited their target of 10 patients within Step 2. Site 2 over-recruited (n = 11) as one patient was discharged from PICU a few hours after signing consent and was excluded from reporting below. Full recruitment was completed within 53, 23 and 57 days from the start of Step 2. Overall, 31/35 (89%) of eligible patients were successfully recruited. Only four families declined consent to PERMIT.
Sites completed follow-up to discharge or 30 days (whichever was sooner) of all recruited patients by 91, 32 and 57 days respectively of commencing Step 2.
Of the 31 patients who agreed to consent A (participation in data collection and to receive ERM interventions), 21/31 (68%) also consented to consent B (follow-up assessment tool collection and to be approached about an interview). One patient was discharged from PICU very soon after consent so no data were collected. Therefore, 30 patients were included in ERM assessment data.
Outcome assessment tool completion
Assessment tools were completed at baseline, during daily clinical status assessments and at follow-up (discharge or 30 days after PICU admission) (Table 17).
| Clinical status scores | Expected (n) | Actual (n) | Patients (n) | Comments |
|---|---|---|---|---|
| Baseline assessment | ||||
| POPC | 30 | 30 (100) | 30 | Scored for all patients |
| PCPC | 30 | 30 (100) | 30 | Scored for all patients |
| PedsQL Family Impact | 20 | 19 (95) | 20 | Only consent B |
| PedsQL Core 4.0 (total) | 20 | 19 (95) | 20 | Only consent B |
| Parent report for infants (ages 1–12 months) | 7 | 7 | ||
| Parent report for infants (ages 13–24 months) | 6 | 6 | ||
| Parent report for toddlers (ages 2–4) | 0 | 0 | ||
| Parent report for young children (ages 5–7) | 3 | 3 | ||
| Parent report for children (ages 8–12) | 0 | 0 | ||
| Parent report for teenagers (ages 13–18) | 3 | 3 | ||
| Young child report (ages 5–7) | 0 | 0 | ||
| Child report (ages 8–12) | 0 | 0 | ||
| Teenager report (ages 13–18) | 3 | 0 | ||
| PedsQL Multi-dimensional (total) | 7 | 7 (100) | 7 | Only consent B and patients >2 years |
| Fatigue Scale 2–4 years | 3 | 3 | 3 | |
| Fatigue Scale 5–7 years | 1 | 1 | 1 | |
| Fatigue Scale 8–12 years | 3 | 3 | 3 | |
| Fatigue Scale 13–18 years | n/a | n/a | 0 | |
| Daily clinical status scores | ||||
| Level of organ dysfunction: PELOD-2 score | 191 | 189 (99) | 30 | All patients daily |
| Level of physical activity: cCPAx. | 191 | 189 (99) | 30 | All patients daily |
| Skin integrity: Braden QD will be assessed to assess skin integrity and risk of pressure damage/injury | 191 | 175 (92) | 30 | Daily. 15 missed no research nurse |
| Presence of delirium: CAPD | 191 | 175 (92) | 30 | Daily. 15 missed no research nurse |
| Level of sedation: the COMFORT‐B scale sedation assessment score | 173 | 157 (91) | 30 | Daily. 5 on neuromuscular blocking drugs. 15 not able to assess |
| Pain score | 191 | 184 (96) | 30 | |
| Follow-up PICU discharge or 30 days assessment | ||||
| PedsQL Multi-dimensional | 7 | 4 (57) | 7 | |
| Fatigue Scale 2–4 years | 3 | 2 | ||
| Fatigue Scale 5–7 years | 1 | 1 | ||
| Fatigue Scale 8–12 years | 3 | 1 | ||
| Fatigue Scale 13–18 years | n/a | n/a | ||
| PedsQL Family Impact | 20 | 12 (60) | ||
| PedsQL Core 4.0 | 20 | 12 (60) | 20 | |
| Parent report for infants (ages 1–12 months) | 7 | 4 | ||
| Parent report for infants (ages 13–24 months) | 6 | 3 | ||
| Parent report for toddlers (ages 2–4) | 0 | 0 | ||
| Parent report for young children (ages 5–7) | 3 | 2 | ||
| Parent report for children (ages 8–12) | 0 | 1 | ||
| Parent report for teenagers (ages 13–18) | 3 | 1 | ||
| Young child report (ages 5–7) | 0 | 0 | ||
| Child report (ages 8–12) | 0 | 0 | ||
| Teenager report (ages 13–18) | 3 | 1 | ||
| PSS PICU | 20 | 12 (60) | 20 | |
| Empathic 30 | 20 | 12 (60) | 20 | |
| PHQ-4: Questionnaire for anxiety and depression | 20 | 12 (60) | 20 | |
Baseline assessment tools for PCPC and POPC were recorded in 30/30 (100%) of cases. Additional baseline forms were scored for the families consenting to consent B (n = 20). Of these, 19/20 (95%) completed the Family Impact Scale and PedsQL parental core reports, with 7/7 (100%) of those eligible completing the PedsQL Multi-dimensional Fatigue Scale.
The daily patient assessment scores and organ dysfunction scores (PELOD-2 and cCPAX) were collected in nearly all cases (see Table 17). In more than 91% of available PICU study days the CAPD, Braden QD and Comfort B scores were collected. Missing data were related to unavailability of research staff to calculate scores contemporaneously, indicating that these were not routinely collected clinical scores by bedside staff.
Follow-up outcome assessment score completion rate was much lower (12/20; 60% of patients), although these were all fully filled in. The protocol defined follow-up time occurring at point of PICU discharge or within 30 days post admission to PICU with flexibility for research staff to assess feasibility. Time from PICU admission to follow-up assessment completion was a median (IQR) of 31.5 (30–40) days. The length of time from discharge of PICU to follow up was median (IQR) 26 (13–36) days.
Demographics
The median age of the 30 patients recruited was 1.8 years (IQR 0.4–5.4) with around a third (9/30; 30%) <12 months (Table 18). The most common primary admission diagnosis was a respiratory illness (40%) and 8/30 (27%) were admitted following surgery. Prior to admission, 16/30 (53%) had a normal (PCPC 1) cerebral performance score, and 11/30 (37%) a normal (POPC 1) overall performance score with 19/30 (63%) ambulatory. This population was similar to the general population observed in Phase 1c observational study (see Chapter 3).
| Group | Med (IQR), n (%) n = 30 |
|---|---|
| Age (days) median (IQR) | 651.5 (128–1968) |
| Patient age group | |
| <1 month | 1 (3) |
| 1–3 months | 6 (20) |
| 4–6 months | 2 (7) |
| 7–11 months | 2 (7) |
| 1–4 years | 10 (33) |
| 5–8 years | 3 (10) |
| 9–13 years | 2 (7) |
| 13–17.9 years | 4 (13) |
| Sex (female) | 14 (47) |
| Ethnicity | |
| White | 23 (77) |
| Asian | 2 (7) |
| Black | 3 (10) |
| Not stated | 2 (7) |
| Source of admission | |
| Same hospital | 18 (60) |
| Reason for admission | |
| Respiratory | 12 (40) |
| Post general surgery | 5 (17) |
| Cardiac (non-surgery) | 2 (7) |
| Infectious/inflammatory | 2 (7) |
| Neurology | 2 (7) |
| Trauma | 2 (7) |
| Post cardiac surgery | 2 (7) |
| Post neurosurgery | 1 (3) |
| Other medical | 2 (3) |
| Baseline PCPC | |
| 1. Normal | 16 (53) |
| 2. Mild disability | 10 (33) |
| 3. Moderate disability | 1 (3) |
| 4. Severe disability | 3 (10) |
| Baseline POPC | |
| 1. Good | 11 (37) |
| 2. Mild disability | 13 (43) |
| 3. Moderate disability | 3 (10) |
| 4. Severe disability | 3 (10) |
| Ambulatory prior to PICU admission | 19 (63) |
| Crawling/bum shuffling | 1 (3) |
| Standing with support | 2 (7) |
| Assisted walking | 1 (3) |
| Independent walking | 14 (47) |
| Patient weight (kg), median (IQR) | 12.2 (4.7–21.6) |
| Patient height (cm), median (IQR) | 85 (58–94.3) |
| Surgery since PICU admission before start of study | 5 (17) |
On day 1 of the study (day 3 PICU stay), the median PELOD 2 score was 4 (IQR 3–6). Nearly all were on assisted ventilatory support; 23/30 (77%) invasive MV and 5/30 (10%) on non-invasive support [bilevel positive airway pressure (BIPAP), continuous positive airway pressure (CPAP) or high flow nasal cannula], 6/30 (20%) were on vasoactive infusions and 2/30 (7%) were on neuromuscular blocking drugs (Table 19).
| Category | Median (IQR), n (%) n = 30 |
|---|---|
| PELOD 2 score | 4 (3–6) |
| Airway | |
| Intubation status | |
| ETT | 22 (73) |
| Oral | 16 (57) |
| Nasal | 6 (20) |
| Tracheostomy | 1 (3) |
| No airway | 7 (23) |
| Difficult intubation | 0 |
| Breathing | |
| Ventilation status | |
| No oxygen support | 1 (3) |
| High-frequency oscillator | 0 |
| Conventional ventilation | 23 (77) |
| Non-invasive (CPAP/BiPAP) | 3 (10) |
| High-flow oxygen | 2 (7) |
| Supplemental oxygen only | 1 (3) |
| Fraction inspired O2 [median (IQR)] | 0.3 (0.25–0.45) (n = 28) |
| Circulation | |
| Blood pressure | 72 (59–80) (n = 30) |
| Blood lactate | 0.75 (0.6–1) (n = 22) |
| Vasoactive drugs (excluding milrinone) | 6 (20) |
| Milrinone | 7 (3) |
| ECMO | 0 |
| Open chest/abdomen post surgery | 1 (3) |
| Neurology | |
| Neuromuscular blocking drugs | 2 (7) |
| Previous delirium screening | 9 (30) |
| Previous positive for delirium | 4 (13) |
| CAPD score | 14.5 (9–19) (n = 26) |
| Current CAPD ‘positive’ for delirium | 16 (59) |
| Glasgow Coma Score | 10 (8–13) (n = 30) |
| Drain (chest/wound/other) | 5 (17) |
| Lines and catheters | |
| Cannular | 23 (77) |
| Arterial line | 16 (53) |
| Central venous catheter | 16 (53) |
| Haemodialysis catheter | 3 (10) |
| Nasogastric tube | 23 (77) |
| Cerebral function monitoring | 1 (3) |
| Cardiac pacing wires | 1 (3) |
| Drain (chest/wound/other) | 5 (17) |
Prior to the first study day, four patients had screened positive for delirium. On the first study day the median CAPD score was 14.5 (IQR 9–19). The standard cut-off CAPD score indicating delirium is a score of >12. Therefore, a high proportion (16/30; 59%) screened positive for delirium.
Patients’ total LOS on PICU from admission was a median (IQR) of 5 (1–13) days. Following recruitment to PERMIT, the number of study days per study participant was median (IQR) 2 (1–7) days and patients were potentially available to receive ERM on 191 study days. Site 1 had the largest number of ERM study days (n = 108) compared to site 2 (n = 42) and site 3 (n = 41) as a result of a longer LOS for patients at site 1 (Table 20).
| Site 1 | Site 2 | Site 3 | All | |
|---|---|---|---|---|
| Number of patients recruited | 10 | 10 | 11 | 31 |
| Number of patients included in analysis | 10 | 10 | 10 | 30 |
| Total ERM patient days | 108 | 42 | 41 | 191 |
| ERM patient days per patient (Med IQR) | 5 (1–13) | 1.5 (0.5–4.5) | 2.5 (2–5) | 2 (1–7) |
| Total days received any ERM | 89/108 (82) | 21/42 (50) | 30 (73) | 140/191 (73) |
| Total ERM episodes | 234 | 64 | 30 | 328 |
| Total ERM activities | 609 | 96 | 120 | 825 |
| Number of ward rounds observed (% ERM days) | 106 (98) | 27 (68) | 41 (100) | 174 (91) |
| Number (%) of ward rounds acuity scored | 100/106 (94) | 25/27 (93) | 36/41 (88) | 161/174 (93) |
| Acuity level at time of scoring (of n = 161 scored) | ||||
| 1 (Most sick) | 2 | 8 | 2 | 12 |
| 2 | 2 | 1 | 8 | 11 |
| 3 | 39 | 13 | 15 | 67 |
| 4 (Least sick) | 57 | 3 | 11 | 71 |
| Acuity level of patients (Med IQR) n = 161 | 4 (3–4) | 3 (1–3) | 3 (2–4) | 3 (3–4) |
| ERM activity level documented | 100/106 (94) | 23/27 (85) | 36/41 (88) | 159/174 (91) |
| ERM activity planned | 80/106 (75) | 21/27 (78) | 36/41 (88) | 137/174 (79) |
| Total ERM prescriptions | 111 | 22 | 36 | 169 |
| ERM prescriptions per patient per day | 6 (4–7) | 2(1–3) | 5 (4–6) | 5 (3–7) range 0–16 |
| Documented in ERM prescription booklet | 77 (76) | 21/21 (100) | 36/36 (100) | 133/137 (97) |
| Document in medical records/daily activities | 93 (89) | 11/27 (41) | 38/41 (93) | 146/173 |
| Researcher present on ward round | 54 (51) | 11 (41) | 26 (66) | 91/156 (58) |
| Level of support from research team | (n = 159) | |||
| No prompt/support needed | 19 | 24 | 3 | 46 (29) |
| Prompt/encouragement given | 37 | 0 | 13 | 50 (31) |
| Prompt and explanation needed | 22 | 0 | 14 | 36 (23) |
| Lots of support/education needed | 22 | 0 | 5 | 27 (17) |
| Risk assessment needed | 50/107 (47) | 11/21 (52) | 14/33 (42) | 75/161 (47) |
| Parents present for ERM episodes | 200/234 (85) | 37/64 (58) | 26/30 (87) | 263/328 (80) |
| Parents/staff involved in ERM episodes | ||||
| Parents | 173 | 33 | 26 | 232 (71) |
| Nurses | 185 | 22 | 28 | 235 (72) |
| Physios | 10 | 14 | 17 | 41 (13) |
| OT | 0 | 9 | 0 | 9 (3) |
| Speech and language | 0 | 2 | 0 | 2 (6) |
| Others | 0 | 3 | 0 | 3 (1) |
| Medics | 0 | 0 | 0 | 0 |
Number of early rehabilitation and mobilisation activities prescribed per children and young people following patient acuity screening
Of the 191 available study days, 174 (91%) had a morning ward round observed. During the ward round the patient acuity was recorded on 161/174 (93%) occasions. The median (IQR) acuity level (level 1, most unwell to level 4 least, unwell) for study patients was 3 (3–4); only 23/161 (14%) were level 1 or 2 (i.e. most sick). A corresponding ERM activity level for the patient was documented for 159/174 (91%) and an ERM activity prescribed on 137/174 (79%) occasions. PERMIT local researchers were present at the time of the ward round on over half of occasions (58%), providing a range of levels of support (from 17% ‘lots of support’ to 29% ‘no support’) to the ward round clinical team.
In total, 825 ERM activities were delivered within 328 ERM episodes on 140/191 (73%) of patient study days across the three study sites.
Type of early rehabilitation and mobilisation
On study day 1, 26/30 (87%) patients had an ERM activity prescribed and 22/30 (73%) had an ERM episode delivered. Of the eight patients who did not receive ERM, five had an ERM prescription for an activity. One of the four patients not prescribed ERM received an ERM activity on study day 1. Across the first 7 days of the study (Figure 16), the rate of receiving a mobility ERM was similar to receiving any ERM. The proportion of patients receiving out-of-bed mobility gradually increased across the study period. For illustration, Appendix 28 shows the range of ERM activities prescribed and the corresponding proportion that were delivered on study day 1. All sites prescribed a wide range of ERM with a good conversion rate from prescribed to delivered for passive, enrichment and active mobility ERM activities.
FIGURE 16.
Prevalence of ERM delivered on study day 1 by any, mobility and out-of-bed categories.

Duration of early rehabilitation and mobilisation
Times were recorded at the start and end of ERM episodes. Recording was variable in the database, with some documented episodes as a single ERM activity, others recorded as multiple ERM activities, or continuous delivery of ERM making interpretation difficult.
Appendix 29 shows the distribution of recorded durations of ERM: 36/266 (14%) recorded times were <15 minutes duration and 61/266 (23%) 15–29 minutes. The median duration was 30 (IQR 15–75) minutes, which was longer that the median time observed in the Phase 1c observational study of 15 minutes.
Safety and adverse event rates during an early rehabilitation and mobilisation activity
The ‘pause and assess’ criteria embedded within the ERM manual for safe deliver and re-assessment of patients before and during an ERM activity were used in 21/330 (6%) of ERM episodes across 13/30 (43%) patients (Table 21). The most frequent categories were desaturation limit exceeded (5/21; 24%) and agitation, anxiety and distress (4/21; 19%).
| Category | Proportion of ERM episodes n (%) n = 330 |
|---|---|
| Desaturation limits exceeded | 5 (2) |
| Agitation, anxiety or distress | 4 (1) |
| Pain or discomfort | 3 (1) |
| Parent, care or patient refusal | 3 (1) |
| Heart rate deviation | 2 (1) |
| Blood-pressure deviation | 1 (1) |
| Increase work of breathing | 1 (1) |
| Concerns for airway integrity | 1 (1) |
| Concern for lines | 1 (1) |
| ICP/CPP targets exceeded | 0 |
| New arrhythmia | 0 |
| Increased respiratory support | 0 |
| Ventilator asynchrony (mild) | 0 |
| Concern for wound or skin | 0 |
| Fall | 0 |
| Total | 21/330 (6)a |
Only 2/328 (0.6%) of ERM activities in two patients were associated with an AE as specified in the study protocol (Table 22). The first was a nasogastric tube dislodged during an ERM episode, but not related to the activity and with mild severity impact on the patient. The second was a blood-pressure change, possibly related to ERM activity, with mild impact on the patient.
| AE category | Site 1 | Site 2 | Site 3 | All sites |
|---|---|---|---|---|
| Safety and AEs by site | ||||
| Total AEs | 0 | 1 | 1 | 2 |
| Total severe AEs | 0 | 0 | 0 | 0 |
| Severe AEs per CYP | n/a | n/a | n/a | n/a |
Summary of findings to inform the PERMIT study
Overall we successfully and safely introduced a multidisciplinary delivered, PICU wide, early rehabilitation programme using the PERMIT manual to three UK PICUs. All sites implemented the PERMIT programme as described in the PERMIT manual requirement within 8–15 weeks to reach Step 2 to allow patient recruitment.
-
A diverse group of PICU patients were recruited on day 3 of PICU admission which represented the wider population of patients identified in Phase 1 observational study.
-
All sites successfully recruited the 10-patient target.
-
All patients had an acuity level scored and these were repeated on 84% of ward rounds. The acuity level was correctly linked to ERM activity prescription and then subsequently to ERM activity delivered. The level of activity was broadly representative of the acuity level. However, the manual description of progression of activity levels over time was difficult to translate to bedside clinical decision-making and data-recording (e.g. demonstration of increased dose of standing, or mat play).
-
All sites modified a local ‘menu’ of ERM activities specific for each acuity level to allow wider choice of ERM prescriptions.
-
A large number of potentially clinically relevant patient outcomes were measured and recorded successfully through validated tools. Baseline and daily tools were recorded over 90% by research and bedside clinical staff and the REDCap database.
-
Clinical trial data integration with PICANet existing audit network data collection was successful and allowed reduction in trial data collection.
-
All patients received ERM activities safely using the pause and assess criteria with only two trial reported AEs and no severe AEs. The rate of physiological deterioration (e.g. desaturation limit exceeded) was lower than the self-reported AEs in the Phase 1 observational study. None adversely affect the patient, as assessed by the bedside clinical team.
-
Although the feasibility study did not aim to do a pre-post implementation assessment on ERM delivery, we did identify that ERM episode durations overall were longer, increasing from a median of 15 minutes in Phase 1 to 30 minutes in Phase 3. In addition, a higher proportion of ERM activities were ‘active’ compared to ‘passive’ or ‘enrichment’ and PT delivered ERM increased from 59% of patients in phase 1 to 93% of patients in Phase 3.
A future trial of ERM therefore appears to be feasible; however, there are a number of recommendations (see Tables 27, 28 and 29). Key messages include:
-
A future trial would need to plan for at least a 12-week (3-month) implementation training programme to work through the key components of implementing the PERMIT manual.
-
The provision of adequate resources for research data entry and research co-ordinator and regular research team presence on ward rounds appear important to retain the quality and accuracy of data collection.
-
Automated integration with existing PICU clinical information systems and workflows would allow additional improvement to data collection.
-
We also identified a high rate of delirium when research staff completed the CAPD screening. Previously, PICUs did not screen for delirium automatically (0% of the 15 PICUs in Phase 1c observational study). Delirium screening is a key component of the wider ABCDEF intensive care unit (ICU) liberation bundle. 103 Training and implementation of delirium screening and further investigation of modifiable factors and potential treatments should remain a priority for NIHR funders. Future PERMIT trials should include delirium screening as a key component of the PERMIT manual.
Chapter 6 Phase 3: paediatric early rehabilitation and mobilisation during intensive care feasibility study – part 2 process evaluation
Introduction
Alongside the PERMIT non-randomised implementation feasibility study, we undertook a process evaluation focused on the feasibility and acceptability of ERM and study processes. The implementation evaluation was informed by NPT. NPT identifies factors that promote and inhibit the routine incorporation of complex interventions into everyday practice. It also explains how these interventions work, looking not only at early implementation, but beyond this to the point where an intervention becomes so embedded into routine practice that it ‘disappears’ from view (i.e. it is normalised). (Text adapted from ‘Normalisation process theory: a framework for developing, evaluating and implementing complex interventions’ by Murray et al. , under CC BY 4.0 licence http://creativecommons.org/licenses/by/4.0/). 87
Study management
The work package was co-led by BRS, JMen and FK. The study management group provided input into protocol, ethics application design and data interpretation. Sati Sahota was trial co-ordinator. JMen led the process evaluation, with methodological expertise by TR. Qualitative analysis was performed by JMen, TR and Natalie Read.
Methods
We undertook a mixed-methods process evaluation across the three PICU sites. Evaluation of the implementation process was conducted through five key components:
-
The weekly debriefs with PERMIT champions from each of the three PICUs.
-
Survey of wider PICU healthcare professionals conducted at three time points at each of the three sites throughout the 5-month study period [normalisation measure development (NoMAD) survey – implementation evaluation tool104].
-
Qualitative feedback from interviews with healthcare professionals from all three PICUs.
-
Qualitative feedback from interviews with parents from all three PICUs.
-
Completion rates of the parent-completed questionnaires (to review feasibility and acceptability).
Sampling and recruitment
We invited two groups of health professionals to take part in feasibility and acceptability work: firstly, ERM champions, those people identified, as part of the PERMIT intervention, as leading the implementation of ERM in the unit and secondly PICU multidisciplinary health professionals involved in any aspect of preparing for or delivering ERM across the unit. Each site aimed to recruit 2–5 ERM champions to take part in weekly debrief conversations (20 debriefs per site; n = 60 total). PICU health professionals were invited to take part in a survey at three time points, aiming for 30 in total per site (n = 90) and consenting staff could also volunteer for a qualitative interview aiming for four or five participants per site (n = 12–15). Parents/legal guardians of CYP recruited to receive the PERMIT intervention were also asked about completing optional outcome measurement tools and an interview. This included those who consented for their child to participate in the PERMIT study (aiming for a sample size of 12–15) and those who declined their child’s participation (aiming for one participant per site, n = 3). The study received Health Research Authority approval (ref 21/SC/0127).
Data collection
Weekly debriefs were conducted with ERM champions from each site, either in person or remotely (telephone/video conference). They were recorded in contemporaneous fieldnotes. Qualitative interviews with PICU health professionals and parents were conducted in person or remotely (telephone/video conference/face to face) and were audio-recorded, intelligently transcribed. 105 and edited to ensure respondents’ anonymity. Finally, PICU health professionals also completed a brief online survey at three time points (beginning, middle and end of study period).
Debrief conversations, interviews and survey with health professionals focused on exploring implementation progress, determinants and key learning; this happened throughout the study period. Interviews with parents focused on exploring acceptability and feasibility of PERMIT intervention and study processes; this happened towards the end of PICU admission or following PICU discharge (up to 30 days post admission to PICU).
Initial topic guides for debrief and health professionals and parent interviews were designed, drawing on prior literature, experience, NPT71,72 and TFA. 73 They evolved during the course of data collection, allowing for tailoring and gradual integration of a variety of follow-up issues and topics. The survey was based on the NoMAD Questionnaire, a 20-item self-report instrument104 (see Appendix 3, Table 41 for NoMAD survey). The survey was distributed by the local PERMIT champion team through internal e-mail lists, and survey participants had the option to consent to be contacted about an interview with a member of the central PERMIT study team. To ensure both the surveys and interviews captured diverse views across the three units, local teams were encouraged to promote the survey widely to promote different professional groups to engage and participate (criterion-based recruitment). 106 Although data saturation is aspired to within qualitative research, that is when no new themes are emerging,107 we recognised this would not be achievable within the time scales of this feasibility study.
Data analysis
Notes from the debriefs were imported into NVivo 12 and deductively coded and analysed using a qualitative content analysis approach of systematic coding and categorising. 58 Two researchers (JM, NR) independently familiarised themselves with the data and conducted open-coding, utilising NVIVO™ software for data management. Codes were then discussed and organised into higher-order subcategories and categories. 59,60 In relevant sections of the paper free-text quotes from respondents are reported to add clarity, staying close to the original meanings and context. 61
The interviews were analysed using a thematic analysis approach. This approach was taken because thematic analysis allows for the identification of common threads that extend across an entire interview or set of interviews. 108 It also values the detailed and nuanced account of data, particularly emphasising the context. 60 Analysis was informed by the work of Braun and Clarke,109 with NVivo™ software used to assist in the organisation and coding of data. The Framework approach was then used to facilitate systematic, rigorous and transparent data management. 110,111
The survey data were analysed descriptively. Three questions explored: (1) how familiar ERM felt, (2) if ERM was a ‘normal’ part of work and (3) if it will become a normal part of work – responses were on a scale of 0–10 (e.g. 0 very new up to 10 completely familiar). The remaining 20 questions required a Likert-scale response (strongly disagree, disagree, neutral, agree, strongly disagree). Responses were allocated a score −2 (strongly disagree) to +2 (strongly agree) for interpretation; median scores for each question are presented.
Results
Study participants
We undertook 48 debrief sessions with ERM champions (Table 23). Despite recruiting well to the champion role at each site, the debrief calls were attended consistently by the same people. Debrief attendees included nurses (n = 2), medical representatives (n = 3), physiotherapists (n = 2), OT (n = 1) and clinical research nurses (n = 3).
| Site 1 | Site 2 | Site 3 | Total | ||
|---|---|---|---|---|---|
| ERM champion debriefs | No. champions | 22 | 11 | 12 | 45 |
| Attended debriefs | 20/20 | 17/20 | 11/11a | 48 | |
| Attendees at debrief (Mean) | 3.7 | 2 | 1 | 2.2 | |
| Participant interviews | Health professionals | 7 | 4 | 2 | 13 |
| Parents | 7 | 6 | 1 | 14 | |
| PICU health professionals survey | Health professionals | 47 | 34 | 37 | 118 |
We received survey responses from 118 health professionals across the MDT. The largest response group (66/118; 56%) were registered nurses and overall most had been in PICU 3 years or longer (88/118; 75%) (Table 24).
| Category | Site 1 N = 47 |
Site 2 N = 37 |
Site 3 N = 34 |
Total N = 118 |
|---|---|---|---|---|
| Profession | ||||
| Nurse | 27 | 23 | 16 | 66 |
| Medic | 14 | 4 | 5 | 23 |
| Physiotherapist | 3 | 5 | 7 | 15 |
| Advanced nurse practitioner | 0 | 4 | 0 | 4 |
| OT | 1 | 0 | 3 | 4 |
| SLT | 0 | 0 | 2 | 2 |
| Clinical skills worker | 1 | 0 | 0 | 1 |
| Pharmacist | 1 | 0 | 0 | 1 |
| Research nurse | 0 | 1 | 0 | 1 |
| Speech therapist | 0 | 0 | 1 | 1 |
| Years of experience in PICU | ||||
| >1 year | 3 | 1 | 3 | 7 |
| 1–2 years | 11 | 3 | 9 | 23 |
| 3–5 years | 10 | 7 | 9 | 26 |
| 6–10 years | 4 | 9 | 7 | 20 |
| 11–15 years | 5 | 9 | 3 | 17 |
| >15 years | 14 | 8 | 3 | 25 |
We interviewed 13 health professionals, including medical representatives (n = 4), PICU physiotherapists (n = 3) and one physiotherapist from a different team (provided on-call cover), advanced nurse practitioners (ANP) (n = 2), one OT, one bedside registered nurse and one team leader registered nurse.
In total, 21 parents consented to participate with the optional questionnaires and the interview, with 15 parents interviewed. Of the six who did not participate, two subsequently declined the interview when contacted, one was only in the study for a few hours so did not feel they could offer any insight and the second child had been re-admitted to hospital so parents did not feel able to participate at the time. One child died before discharge from hospital and three parents did not respond when contacted, after a median of three attempts to contact them. One parent was interviewed but then withdrew therefore interviews from 14 parents were included in the analysis. Interviews took place between November and December 2021 and were conducted in a variety of ways, subject to parental preference. Two were conducted face to face at BCH, five took place on Zoom and seven took place over the telephone. The final analysis included mothers (n = 12) and fathers (n = 2). All of the parents had experience of their child having been intubated and mechanically ventilated during their admission to PICU, and 12 were parents of a child admitted as an unplanned admission.
Pre-paediatric early rehabilitation and mobilisation during intensive care context – questions of capacity
The PERMIT study was introduced into three PICU sites. Across all the sites, potential issues around capacity, especially in relation to levels of MDT staffing, as well as priorities, in relation to place of ERM in ongoing care, were present (Table 25). All sites reflected that although ERM was part of care, they ‘didn’t do it actively or in a structured way’ (Site Two: Medic interview). Patients with defined pathways – neurology patients, those classified as ‘long stay’, infants and patients who were elective and had a more predictable recovery – were more likely to receive ERM. Delivery did not often occur ‘early’ and was undertaken inconsistently:
quite limited in terms of what was being done on the unit. There were elements of, I guess, what you could classify as ERM starting to emerge, but I think the timing was definitely at a later point of the child’s stay.
(Site Three: Physio interview)
| Workforce contexts | Organisational contexts |
|---|---|
Bedside nurses
|
Unit bed capacity
|
Nursing workforce
|
Patient acuity
|
Specialist services (such as SLT, OT)
|
Culture surrounding ERM
|
MDT
|
ERM programmes
|
ERM funding
|
|
Additional challenges of 2021
|
In addition, it was more likely to occur on weekdays, in office hours, and interventions were likely to be ‘low level’ and often movement-focused. In Site 1, an ERM programme had been launched in 2019, with good leadership and good staff engagement. However, the programme had floundered – with maternity leave, staff turnover and service pressures – and in 2021 there was little awareness of the programme. The champions felt that the feasibility study would be an opportunity to reinvigorate an ERM programme and secure staff buy-in. Neither of the other two sites had a pre-existing ERM programme, although they both reported being very enthusiastic about the idea of commencing one.
Introducing PERMIT – (re)organising the work
Throughout the whole study period all three units experienced huge service demand, running significantly over capacity with reduced staffing. In addition, alongside the impact of COVID-19, there were unanticipated challenges or competing demands. At Site 1 this included the move of the whole PICU to a temporary location. The sites progressed through the first four phases at different paces (see Figure 15). Due to the prior history of an ERM programme, Site 1 progressed through initial phases quickly, with more time spent on Phase 3 – supporting staff engagement and training – and Phase 4 – adapting the manual and planning how to effectively deliver the ERM intervention and the trial at their site. Site 2, in contrast, spent more time on Phases 1 and 2. There was less focus on education and training and the specifics of where and how discussions about ERM took place, and more on the logistical factors associated with running the research study and capturing the data. Site 3 experienced a significant delay (9 weeks) in commencing the study, due to local site approval issues. This delay was not only frustrating for a site enthusiastic to start staff training and education, but also added pressure about the feasibility of recruiting 10 patients within an 11-week period. As a result, the site spent relatively little time in each of the phases, with the site starting to screen and recruit by week 16, only 1 week after Site 2 and only 3 weeks after Site 1.
To embed ERM, all sites realised – often more strongly in retrospect – the fundamental importance of ‘education, education, education! … I think that’s the key thing I think to try and make it work’ (Site One: Physio interview). Staff training and education were central to conveying information to all staff on the PICU, supporting them to understand the key principles of the ERM programme and build experience and confidence in delivering ERM. Ongoing education and teaching within formal contexts (e.g. organised study days), alongside informal, ad hoc moments (e.g. at bedside) and being ‘more opportunistic with teaching when clinical’ (Site Two: Debrief) were needed to support delivery of ERM. A refined, considered, educational strategy was introduced in Site 1:
Clear education plan, trainers are being trained and there is a training log planned to keep track of who has had what training.
(Site One: Debrief)
This strategy focused on identifying what key information staff needed to understand, how many people needed training and a plan for how to train them.
There were challenges to providing the education and training sites that were felt to be required to achieve adequate staff commitment, confidence and skills. A core factor was the short trial period – 20 weeks from start to finish, less for Site 3, due to delays. At Site 2, someone commented that ‘the speed at which we had to put it in place and the speed at which we had to then recruit I don’t think gave us time to grow the roots enough’ (Site Two: ANP interview). At this site an additional challenge emerged, with educational resources having to be directed towards getting staff trained as quickly as possible on a new ventilator. Sites felt that the absence of any pre-prepared education and training materials accompanying the manual had been a challenge throughout the 5-month study period. As a result, Site 1 shared training material (which was then localised and rebranded) as well as access to a core trainer to support training and education at the other sites.
Delivering PERMIT – integrating early rehabilitation and mobilisation
Despite all the challenges associated with education and the speed with which the study was rolled out, PERMIT was seen by health professionals and parents as worthwhile (Table 26). All three sites reported positively about how the ERM programme cultivated multidisciplinary working, helped with clarification of roles and helped with elements about leadership and staff buy-in around ERM. MDT work was recognised as being central to delivering the desired level of ERM. This meant embracing multidisciplinary working to optimise service provision:
There are not enough hours in the day for a therapist to deliver the amount of ERM they’d like to be seeing going on. … It needs to become an MDT-based focus … everybody’s role. Therapists can’t be the only ones delivering this.
(Site Three: Physio interview)
| NPT construct | Site 1 | Site 2 | Site 3 |
|---|---|---|---|
| Coherence: do people make sense of ERM? | |||
| Understanding of the purpose of ERM | Good. Expanded beyond ‘movement’ to become more holistic | Unclear within the study team. Unclear about wider PICU. Clear for parents | Good within site champions. Unclear about wider PICU. Clear for parents |
| Understanding of the roles of staff in relation to ERM delivery | Developed over the study. Some differences in views of what was ERM vs. ‘good nursing care’. Some concerns about professional boundaries emerged | Staff clear about roles. Also, clear no capacity to do ‘extra’ | Staff clear about roles, but lack of engagement from nursing. Also, clear no capacity to do ‘extra’ |
| Understanding of the study within clinical team | Moderate, but supported by research team | Limited, but supported by research team where possible | Unclear, limited (research team) resources to support |
| Understanding of the study within parents | Generally good on purpose and possible impact | Generally, on purpose and possible impact | Generally good on purpose and possible impact |
| Understanding of the impact of ERM on staff’s work | Good, but felt already at capacity | Good, but felt already at capacity | Good, but felt already at capacity |
| Potential value and worth of ERM programme | Good and worthwhile. Staff some concerns about sustainability without additional resources | Good and worthwhile. Staff concerned not possible to continue without additional resources once study ends. | Potentially good and worthwhile. Champions aware not possible to continue without additional resources once study ends. |
| Cognitive participation: do people get involved with providing ERM and stay committed? | |||
| Were there key people driving ERM forward? | Good leadership, MDT involvement and good attendance at debrief | Physio and research nurses predominantly. Debrief attendance minimal | Good leadership from physio but limited other engagement. Debrief attendance minimal |
| Is ERM seen as a legitimate part of staff role? | Yes: research team Yes: clinical nurses/physios Uncertainty for medical staff about exact role Yes: parents |
Yes: research team (funded) Yes: physios Uncertainty from some other staff Yes: parents |
No: research team (none available) Yes: physios Uncertainty from some other staff Yes: parents |
| Did staff get involved and incorporate ERM into practice? | ERM rolled out to all patients as standard care. ERM delivery reported (mostly low level) | Only patients recruited to PERMIT received ERM. Unclear extent ERM delivered | Only patients recruited to PERMIT received ERM. Unclear extent ERM delivered |
| Are the team motivated to support ERM over time? | Yes, but concerned as already experienced one failed attempt to embed an ERM programme | Yes, but will not take a programme any further until further resources funded | Yes, but recognise they need time and resources to re-launch a programme |
| Are parents motivated to support ERM over time? | With support parents are willing and motivated to work with healthcare professionals to deliver | With support parents are willing and motivated to work with healthcare professionals to deliver | With support parents are willing and motivated to work with healthcare professionals to deliver |
| Collective action: do people make ERM work in practice? | |||
| Did participation with the study require additional staff training? | Yes, underestimated. Tried initially to train all staff, then scaled back | Yes, targeted to staff caring for study participants | Yes, targeted to staff caring for study participants |
| Was ERM operationalisable? | Yes | Unclear | Yes |
|
Change difficult, but ↑ | Unclear | Unclear |
|
↑ with time | Unclear | Unclear |
|
↑ with time | Unclear | Unclear |
|
↑, but not fully conducted | Unclear | ↑, but not fully conducted |
|
Supported and encouraged | Supported and encouraged | Supported and encouraged |
|
Completed by bedside staff and research nurses | Completed by research nurses. Bedside staff struggled with paper documents | Completed by PI/champion – found very challenging. No research nurse support |
| Reflexive monitoring: do people evaluate ERM and PERMIT as worthwhile? | |||
| Was ERM evaluated as worthwhile? | Yes: feel ERM shifted to being seen as part of routine care. Positive impact for child and family | Yes: not sustainable without additional resources and nursing leadership. Positive impact for child and family | Yes: considering innovative methods to sustain. Positive impact for child and family |
| Acceptability of PERMIT study data collection? | Yes: because there was research nurse support | No: too much burden on clinical staff because it did not integrate with IT system | No: too much burden on PI/champion. No research nurse support |
| Acceptability of PERMIT outcome measurement tools for staff? | Yes: number of questionnaires a concern but they were short | Some concerns: re time points and length for families | Yes: but fewer families consented (3/10) |
| Acceptability of PERMIT outcome measurement tools for parents? | Yes: but not always perceived as relevant to their child | Yes: but not always perceived as relevant to their child | Yes: but not always perceived as relevant to their child |
| Overall acceptability of the study for staff | Good | Mixed – good | Good |
| Overall acceptability of the study for parents | Good: increased parents’ confidence, sense of purpose and satisfaction. Some saw direct benefit for child. Saw positive impact on staff behaviour and prioritisation |
Good: increased parents’ confidence, sense of purpose and satisfaction. Some saw direct benefit for child. Saw positive impact on staff behaviour and prioritisation |
Good: increased parents’ confidence, sense of purpose and satisfaction. Some saw direct benefit for child. Saw positive impact on staff behaviour and prioritisation |
| Is a future study viewed as worthwhile by staff? | Yes: staff concerns reflect workload and resources | Yes: staff feel need to address nursing and medical leadership, workload and resources | Yes: staff feel need to address leadership, workload and resources |
| Is a future study viewed as worthwhile by parents? | Yes: further research is important | Yes: further research is important | Yes: further research is important |
In particular, the role of the bedside nurse was recognised as vital to support delivery. The challenge was about raising the profile of ERM as a priority in nurses’ daily work so ERM was no longer seen as just a desirable component, but an essential aspect of care provision. At moments, people struggled with whether to prioritise supporting colleagues:
Nursing staff report feeling guilty that they’re doing nice things with patients – play, music etc. when the rest of the staff are very busy and struggling with unit demands.
(Site One: Debrief)
However, exposure to ERM over time, especially seeing ERM in action on a unit, could help in the process of it becoming embedded as ‘standard care’.
Exposure was, in part, challenging, however, because there were differences across sites to the extent that PERMIT was rolled out. At Site 1, ERM following the PERMIT manual – that is assessment of acuity, activity planning, risk assessments and family involvement – was regarded as the standard for all patients. At the other sites, only patients recruited to PERMIT received ERM as per the manual. Although there was variety in the use of the bedside bundle, there was recognition of the value to support ERM delivery and a shift in the traditional mind-set of a patient needing to be stable to be eligible to receive ERM. In practice, exposure emerged in a variety of ways, be that through strategies in place to promote staff buy-in and enhance involvement over time:
there’s posters going up, conversation in the coffee room taking place. Physio colleagues are more active in reminding clinicians at the bed space, or at least reminding the nurses to ask doctors doing the ward rounds … lots of general awareness that early rehab and mobilisation is actually important for the patients.
(Site One: Medic interview)
In and through taking part in PERMIT, staff exposure to ERM increased over the study period. Ward rounds also offered a space in which to undertake ERM discussions, at the very least in relation to whether a patient may be eligible for the study, as well identifying activities of rehabilitation for those recruited, if not for others outside PERMIT. Additionally, positive family feedback about involvement with ERM, when shared with staff, increased exposure and understanding of potential impact.
Over time, health professionals understood that ERM was important for the physical and psychological recovery of the CYP, as well as the psychological well-being of parents/carers supporting their involvement in their child’s care. For example, one site noted that:
Parents are engaged and involved, really keen on the ERM process. Parents are filling out the activity booklet and adding lots of detail. Seems to give them a focus and a process to be involved in.
(Site Three: Debrief)
All the sites reported that PERMIT has supported parental involvement. Feedback from parents was reported as ‘overwhelmingly positive and everyone’s saying how important it was within their journey’ (Site Three: Physio interview). All parents commented that there had been benefits to participating. This seemed to span from the emphasis the programme placed on parental involvement to the purpose that the programme provided. However, parental involvement in activity selection varied, with some referring to paperwork to help guide choices, while for others it appeared to be more led by the health-care professionals, whereas other parents reported not seeing a list or feeling actively involved in activity selection. Engagement in process was clearly enabling for parents:
It was quite nice to know, “Oh we can move onto the next stage” or you know, “Oh look there’s a new activity on the list, oh I hadn’t thought about doing that activity with him”. Or realising that sitting in the bed is a milestone almost. You know, it’s a new activity. As a parent you can see that as a milestone of their recovery.
(Site Two: Parent interview)
Overall, most of the activities were undertaken while their child was ‘in bed’. The most commonly reported activities were cuddles, reading, singing, talking, touch or massage and stretches, which matched recorded activities completed by the bedside staff. The impression was that most parents felt the activities were tailored and appropriate for the stage the child was at.
Many parents reported feeling more confident in becoming involved in supporting ERM, that for them ‘I think it was so empowering’ (Site One: Parent interview). Parents reflected on how sick their child had seemed to them initially, but once they were familiar with what ERM involved and how they could be involved it gave them a sense of purpose:
But it also gives us something to do to feel useful because you’re in a situation where it’s one to one care. They know exactly what they’re doing, we’ve got no idea. We can’t help do anything in ICU, but you’re just looking at your child and you can’t do anything. But being able to have a structure as to what we can do to help, all of the activities that we could do … it meant that we could do something to actively help his recovery.
(Site Two: Parent interview)
Parents perceived that being recruited to the study provided guidance to staff about activity assessment and selection and also encouraged them to consider the child holistically. They also felt more confident leaving their child to have a much-needed break because they trusted that staff would also follow the programme. Some saw a direct benefit to their child. Others were less sure or queried whether it was fully relevant to their child because they were an infant, but still saw the study as worthwhile. For many the main positive impact for their child was the contact the programme encouraged with parents. This spanned all aspects of care, particularly cuddles. Parents recognised how central they were to their child and their presence was therefore important to help support their child. However, outside the context of PICU delivery, support and guidance for delivering ERM were lacking. Several families reported that leaving PICU is only the first stage in recovery and more needed to be done to support them with ERM beyond PICU discharge.
Delivering PERMIT – integrating trial processes
Having access to research delivery support was central to supporting recruitment, data collection and data entry. This reduced demand on bedside staff as well as freeing up the site team for education and ERM delivery. Sites 1 and 2 worked with research nurses – with Site 1 having access to a clinical research nurse from Stage 4 – whereas at Site 3 did not. At Site 3 the lead champion took on all the workload associated with implementing the study and evaluating ERM delivery and they noted that:
The research side is very time consuming. The data collection and research collection are the biggest barrier to doing the ERM, the data collection, the consent process, the questionnaires – it’s all so time-consuming.
(Site Three: Debrief)
All three sites commented on the large volume of work associated with conducting the PERMIT study. A research team presence, ideally to cover all days and times of the week, was seen as essential. All parents also provided positive feedback about the role and presence of the research team during recruitment: their professionalism, the sensitivity with the timing of discussions and their ability to respond to questions.
Overall, the families were positive about the study recruitment process. Two parents referred to the challenge of written leaflets for parents with additional needs. As ERM was rendered as the ‘usual’ standard of care within the units – by the PIS and research team – many parents had low expectations about the potential risk of participation:
My main question was about if there was anything extra that was going to be done, anything invasive, and if it wasn’t then I didn’t see any reason why I wouldn’t consent to it.
(Site One: Parent interview)
For the majority the perception of ‘minimal’ to ‘no’ risk meant that they found this a relatively simple decision. The choice of a staged consent model was significantly influenced by other studies within the PICU environment66,102 and the PPIE work conducted within the PERMIT study (see Chapter 8). Of the 30 children recruited to the trial, 21 of the parents/legal guardians consented to participate in the ‘additional’ aspects of the PERMIT study (70% consent rate). Seven families (33%) consented in a staged approach, with 14 (67%) happy to consent to all parts in one go. Some parents felt signing up in stages had been really helpful for them, not overloading them at a difficult time. There was also a sense from some families that because ERM was standard practice on a PICU that an opt-out consent approach where families did not have to actively consent or could be asked at a later stage could be acceptable.
Introducing and embedding the study paperwork was challenging at times. This was only produced as physical documents and Site 2 did not have time or capacity to adapt it to their electronic patient management system, so
[h]aving the documentation all on paper created resistance from staff, they like everything to be done on the computer. This was perceived as a lot more work to have to do.
(Site Two)
Easy access to documents at the bedside helped support and sustain study work. Sites also had some concerns about the outcome measurement tools provided to families, in part tied to the logistics of managing the process – with uncertainty about the time points required and the challenge of getting these completed. They also were uncertain about the volume of questionnaires to be provided to families. However, on the whole the questionnaires were viewed as acceptable by parents. Negative comments reflected that some questions were inappropriate for their child in terms of their ability to do the developmental outcomes (mostly for the parents of neonates), length and the slightly repetitive nature of some questions.
Embedding ERM post-PERMIT and PERMIT trial – (continuing) questions of capacity
As part of Phase 4 work, a debrief was held 2 months after the study had closed to recruitment. Despite huge enthusiasm about the potential for ERM to continue after PERMIT closed, all ERM activity related to the programme had stopped at Sites 2 and 3. However, at Site 1, even in the contexts of increased PICU workloads and COVID creating challenges for staffing, they still felt that ERM had shifted to being seen as part of routine care. They noted how ‘the research has increased frequency and awareness of ERM’ (Site One: Debrief), with most patients now having their acuity assessed daily and staff considering what ERM could be conducted, even if it was not always possible to undertake the planned activities. Bedside nurses appeared to have increased confidence in initiating and conducting ERM, with increased awareness of what each MDT member could contribute to ERM.
Core issues to embedding ERM after PERMIT remained, with Site 3 reflecting that
the challenges have taught us so much! … Need increased presence and visibility of an ICU specific therapy team to fully integrate into ICU. We learned also that therapy doesn’t need to be so structured and planned, we can do it in so many ways.
(Site Three: Debrief)
They felt they would ‘review, revamp and re-launch!’ with more time factored in for the programme to embed, and that the ‘minimum time to make this really embed would be 12 months’ (Site Three: Debrief). All three sites felt that additional resources were required to support (ongoing) staff education and training; that visibility, experience and (positive) feedback around multidisciplinary ERM working was key, as well nursing staff taking ownership being central to embedding.
The NoMAD survey results align with the way that sites, over time, began to realise the complexity of the work of introducing and embedding an ERM programme. Between time point one and two there was an increase in ERM familiarity through initial engagement, with a realisation that ERM was currently part of normal practice and would become part of normal practice (see Appendix 3, Figure 20). However, by the final and third survey the level decreased back to baseline.
A similar temporal pattern was seen in four NPT items: around questions of the legitimacy of ERM being a part of their role (Q15); being open to working with colleagues in new ways to use ERM (Q16); and notably, continuing to support ERM (Q17) and that sufficient resources are available to support ERM (Q23) (see Appendix 3, Figure 21). However, across all time points there was strong (and stable) agreement about seeing the potential value of ERM in their work (Q13) and strong (and stable) disagreement across all time points that ERM disrupts working relationship (Q19).
Summary of findings to inform PERMIT study
Although there were many challenges to implementation of the PERMIT intervention within the context of busy PICUs during a global pandemic, there was significant multidisciplinary input and support from parents/carers for ERM to be standardised within clinical practice. A future trial was therefore viewed as acceptable and we identified a number of recommendations for a future trial (Tables 27, 28 and 29). Key messages include:
-
Within a future trial, ERM needs to be embedded as standard routine practice for all CYP within the PICU. This would also enable an opt-out informed consent to be utilised and potentially reduce some of the challenges that parents outlined in relation to consent processes.
-
Research delivery support is vital to successfully complete the trial processes, but the research team cannot lead the implementation of a programme or it will not fully embed.
-
Bedside nurses are ideally placed to help deliver ERM but this workforce is already stretched. Currently ERM is not in their priorities and is seen as desirable, rather than essential. Adequately resourced PICU therapists will be required to support implementation and delivery.
-
There is no spare capacity to deliver ‘extra’ ERM within current resources.
-
ERM is seen as important for the physical and psychological recovery of the CYP, but is also important for the psychological well-being of parents/carers supporting their involvement in their child’s care.
-
Being involved with ERM is seen as hugely important for parents/carers. Further consideration of how we provide training and support for parents to facilitate this involvement is needed.
| Source of information | Debrief | Staff int. | Parent int. | Trial data | |
|---|---|---|---|---|---|
| ERM |
|
✓ | ✓ | ✓ | |
|
✓ | ✓ | ✓ | ||
| Population |
|
✓ | |||
|
✓ | ✓ | ✓ | ||
|
✓ | ||||
| Parental consent |
|
✓ | ✓ | ||
|
✓ | ✓ | |||
|
✓ | ||||
|
✓ | ||||
| Parent information |
|
✓ | |||
|
✓ | ||||
|
✓ | ||||
| Study outcome measures |
|
✓ | ✓ | ✓ | ✓ |
|
✓ | ✓ | |||
|
✓ | ✓ | ✓ | ||
|
✓ | ||||
|
✓ | ✓ | |||
|
✓ |
| Source of information | Debrief | Staff int. | Parent int. | Trial data | |
|---|---|---|---|---|---|
| ERM launch |
|
✓ | ✓ | ||
|
✓ | ✓ | |||
| ERM staff education and training |
|
✓ | ✓ | ||
|
✓ | ||||
|
✓ | ||||
|
✓ | ✓ | |||
|
✓ | ||||
| Acuity assessment |
|
✓ | ✓ | ✓ | |
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | |||
|
✓ | ✓ | ✓ | ||
| Activity-selection tool |
|
✓ | ✓ | ✓ | ✓ |
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | ✓ | ✓ | |
|
✓ | ✓ | |||
|
✓ | ✓ | ✓ | ||
| Risk-assessment tools |
|
✓ | |||
|
✓ | ✓ | |||
|
✓ | ||||
|
✓ | ||||
| ERM delivery |
|
✓ | ✓ | ||
|
✓ | ✓ | |||
|
✓ | ✓ | ✓ | ✓ | |
|
✓ | ✓ | ✓ | ✓ | |
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | ✓ | ✓ | |
|
✓ | ✓ |
| Source of information | Debrief | Staff int. | Parent int. | Trial data | |
|---|---|---|---|---|---|
| Safety |
|
✓ | ✓ | ✓ | |
|
✓ | ✓ | |||
|
✓ | ||||
| Staff feedback and comments |
|
✓ | ✓ | ||
|
✓ | ||||
|
✓ | ✓ | |||
| CYP and parent/carer feedback |
|
✓ | ✓ | ||
|
✓ | ✓ |
Chapter 7 Phase 4: consensus and future recommendations
Introduction
To inform the design of a definitive trial of ERM intervention within UK PICUs we consolidated the Phase 1–3 PERMIT study findings and presented them to key stakeholders. In addition, we developed a preliminary proposal for the design of a definitive effectiveness trial, incorporating the key feasibility points from Phase 3.
Study management
The work package was led by BRS. JMen led the PPIE component. Rebecca Wooley and Karla Hemmings (BCTU trial statisticians) performed sample size calculation. RF co-ordinated PICANet data analysis. The study management group participated in consensus conference and reviewed the future trial proposal.
Aims
This study aimed to:
-
provide parent perspectives on the results to ensure clarity in the summary of results for health-care professionals;
-
provide parent perspectives on the summary of results for parents of children who were PERMIT trial participants;
-
refine messages to share with parents/legal guardians who were not study participants;
-
obtain feedback on the importance of a future trial of ERM and the outcome measures we should include in a future trial;
-
propose a preliminary design of a future trial.
Method
This study consisted of three parts: (1) parent consensus meeting; (2) HCP meeting; and (3) study management group trial design.
Parent consensus meeting
We invited all parent/family member participants (n = 17) who consented and provided contact e-mail address details in Phase 3 to a virtual meeting (via Zoom) in January 2022. Invitations were sent in December 2021 and a reminder invitation in January 2022. The meeting included a presentation by BS (study design, rationale and key quantitative results), Natalie Read (results from site debriefs) and JMen (key results from healthcare professional and parent interviews).
The video presentation (30 minutes duration) was recorded, uploaded to a private web-based video-housing channel and a summarised leaflet with key study findings (pdf format) was created.
Following the meeting all parents/family members were sent the slides and video link with a short online questionnaire relating to feasibility and acceptability. No sensitive or identifiable information was collected. Implied consent was indicated on completion and submission of the online questionnaire.
The questionnaire asked:
-
whether they found it useful to see the study results;
-
appropriateness of an opt-out consent in future study;
-
which outcome measures should be included in a future study (ranked according to importance);
-
the amount of ERM a child received (dose);
-
the type of ERM activities a child received;
-
the length of time spent on PICU;
-
the length of time on a ventilator;
-
the LOS in hospital;
-
physical measure such as muscle strength;
-
an outcome reflecting longer recovery (e.g. return to nursery/school);
-
-
other suggestions of what should be measured (free-text response);
-
whether a future trial of ERM was required.
Healthcare practitioner consensus meeting
We planned to hold an online HCP consensus meeting to discuss a proposed future trial design. However, additional COVID pandemic restrictions and NHS workforce pressures in January 2022 resulted in the cancellation of this meeting, with a plan to reschedule in May 2022 during the national PCCS-SG annual investigators meeting.
Study management group trial design
The study management group met on three occasions to review the findings of the Phase 3 feasibility study and discuss the design and structure of the future trial. Additional methodological expertise was obtained from Birmingham Clinical Trials Unit statisticians and study team at PICANet. Meetings were conducted over Zoom and, following the verbal consent of all attendees, recorded for comprehensive notes to be made to inform trial development.
Results
Parent consensus meeting
An invitation to attend the parent consensus meeting was sent to 17 participants in the Phase 3 feasibility study with the recorded materials. No parents/legal guardians (n = 0/17, 0%) attended the live virtual meeting. However, four parents/legal guardians responded to the online survey. All respondents (n = 4) found it useful to see the key study findings and agreed that an opt-out model of consent was appropriate for a future study.
The outcome measures were rated as follows.
All four respondents rated as very important:
-
the amount of ERM a child received (dose).
In addition, they ranked from highest to lowest the following outcomes:
-
the type of ERM activities a child received (highest ranked);
-
the length of time spent on PICU;
-
the length of time on a ventilator;
-
the LOS in hospital;
-
physical measure such as muscle strength;
-
an outcome reflecting longer recovery, e.g. return to nursery/school (lowest ranked).
Other measures suggested in free-text response included:
For younger children/babies perhaps a measure of stress/anxiety – do erm activities such as reading help with sat [oxygen saturation] stability or overall sats [oxygen saturation] vs. children who do not receive ERM. E.g.: we continued to read night-time stories to try and maintain a degree of normality as well as comfort.
(Parent C)
Two parents replied Yes: further research on ERM was required and two said No: reasons being that they felt the evidence was conclusive:
Probably not, the benefits are obvious and the feedback is conclusive I would say. (Parent D) I guess to get this to be a standard of care you will need more evidence it works. But with the positive feedback from parents I personally think enough research has been done – it provides a positive environment for all.
(Parent C)
Impact for future paediatric early rehabilitation and mobilisation during intensive care trial
Parent satisfaction is extremely high surrounding ERM and contributed to two parents feeling that from their perspective further research was not required. However, there was recognition that for this to become a standard of care for all patients this would require more evidence, with further work focusing on identification of outcome measures relevant to patients and parents/legal guardians.
Study management group trial design proposal
The study management group, with trial methodologist input, met, reviewed all the PERMIT study findings and discussed an overall proposal for a future trial design.
Importance of research question and professional support for early rehabilitation and mobilisation
There remains a clear need to improve the morbidity and mortality of children requiring care within PICU after PICU critical surgery, illness or injury. We identified evidence from our scoping review that ERM is safe, feasible and, in combination with the adult literature, has the potential to be effective at improving patients’ recovery from critical illness; however, further trials are needed. We identified significant HCP enthusiasm, across the multidisciplinary groups within PICU, regarding the role and potential of ERM intervention. The Phase 1b survey demonstrated wide community buy-in for future studies, the Phase 1c observational study had a high level of interest, investment of time and participation by bedside nursing staff and therapists, and the speed and number of PERMIT champions recruited in Phase 3 was very reassuring. Establishing ERM as a credible PICU intervention through a future definitive study is clearly a high priority with NHS staff.
Intervention
The development of the PERMIT manual and the successful, safe, implementation of the manual within the PERMIT Phase 3 feasibility study has confirmed that a complex, multidisciplinary, PICU-wide ERM intervention can be evaluated. The PERMIT manual includes a six-step implementation guide, learning and training resources, and ongoing site support from ERM experts. It incorporates daily acuity scoring, activity-level guidance and a pause and assess safety structure, which are modified to local resources, environment and staffing structure. Through research staff investment, debriefs and local site involvement, we have established the initial implementation of the manual takes approximately 12 weeks. This time period should be integrated into any implementation training phase in a trial.
Trial design and consent model
As with other trials in PICUs, the most powerful trial would use a stepped-wedged cluster design. Not only would this increase the power of the trial, it would also mean that all PICUs involved would benefit from training in the intervention. This design has worked well in PICU previously; the HTA-funded SANDWICH trial66 of a sedation and ventilator liberation protocol intervention recruited 8843 patients across 18 UK PICUs over 18 months.
Opt-out consent is the preferred model of consent. This was successful in the Phase 1 observational study and strongly recommend in the Phase 3 process evaluation, interviews and PPIE work. This consent model would work within a cluster RCT design and implementation of the PERMIT manual and ERM delivery across the whole PICU.
Population
The PERMIT programme of research has clearly shown that elements of ERM are delivered in some form to nearly all patients in PICU, including the sickest patients on maximal organ support. Therefore, the whole diverse spectrum of PICU patients should have the opportunity to benefit from ERM early in their PICU care pathway and for as long as is required. However, for ERM to impact a measurable patient-related outcome it would be logical that a period of exposure to the ERM intervention is required. Therefore, patients with short stays in PICU (e.g. <48 hours) are unlikely to gain measurable benefit. Our approach of including patients on day 3 of PICU in the Phase 1c observational study and Phase 3 feasibility study allowed identification of this patient group. Trial inclusion criteria should allow selection of these patients for assessment of efficacy of ERM, although the ERM intervention could and ideally should be delivered to every patient within the PICU to ensure maximal embedding of the ERM manual and processes with staff. We therefore recommend that a future trial population includes critically ill/injured patients of all ages (0–<18 years) admitted to PICU. They would be identified and enrolled in the study early in their PICU stay (e.g. within 72 hours after PICU admission). This would allow the interventions in the PERMIT manual to be commenced in patients who could receive an adequate amount of ERM, which may lead to measurable benefit. Patients would be excluded by parents/guardians opting out, or clinicians’ decision that ERM or trial inclusion is inappropriate (e.g. anticipated death in PICU, or planned palliative care and withdrawal of life-sustaining therapies). We recognise that ERM activities may be important and recommended end-of-life interventions in this population (e.g. part of pain relief, enrichment to surroundings patient and family PICU experience); however, these would require different patient-reported outcomes or to be delivered outside of a trial setting.
Comparison
The comparison (control arm) would be standard of care before implementation of the PERMIT manual. We have established that even PICUs with an existing ERM programme can benefit and improve with the PERMIT manual and programme (e.g. Site 1 in the Phase 3 feasibility study). The ability for the PERMIT manual to work alongside and enhance, refine and develop existing programmes would allow increased recruitment of PICU sites to a future study. The research programme and process evaluation are also attractive to PICU sites as additional resources would be available.
Outcomes
Through PPIE and parent/family feedback, the amount and dose of ERM were identified as the most important outcome measure. However, we identified in Phases 1 and 3 that it is very difficult to quantify ERM dose due to (1) a heterogeneous patient population (e.g. 0–<18 years of age, developmental stages, size, ambulatory status), and (2) diverse types of ERM activities delivered. Inclusion of health-related QoL outcome measures is recommended in PICU studies47 and is felt important by healthcare professionals and parents/carers; however, the burden on families to complete these tools was clear in Phase 3. Therefore, a pragmatic measure of improvement in critical illness or injury recovery would be required, with accompanying intervention fidelity assessment (i.e. measurement of increased ERM dose delivery at the site and patient levels). Potentially suitable outcomes include length of MV and LOS in PICU (or days free of ventilation/PICU). The advantages to length of MV as an outcome is that it has a clearly defined beginning and end point (e.g. successful extubation without the need for re-intubation). It is also the primary outcome measure in the ongoing pilot, stepped-wedge trial of ERM in the USA PICU UP! study, which would allow direct comparison. 112 The disadvantage is that this will exclude up to 35% of PICU patients with critical illness not requiring ventilation. LOS, as an outcome measure, would allow all patients admitted to PICU to be included in a trial. However, the disadvantage is that the end point is less precise. Discharge from PICU can be to any of step-down to high-dependency unit, ward location, other hospital PICU, neonatal intensive care, home, palliative care facility. The timing of this discharge can be affected by additional factors external to improvement in critical illness and injury (e.g. bed availability on the ward) and is therefore subject to measurement inaccuracy, which may affect trial findings.
With no definitive primary outcome choice, we present a draft proposal based on LOS and length of ventilation (LOV). We need to explore this area further.
Sample size
Approximately 20,000 patients are admitted to PICU each year, of whom around 65% receive invasive MV at admission. The eligible population, using LOV as an outcome, would come from the patients who are on MV on day 3 and stay in ICU for an additional 4 days (this gives us the population who are both most likely to benefit and can receive at least a minimal amount of ERM). The UK national PICANET data report that approximately 19% of patients admitted fulfil these criteria, so 2470 patients will be eligible per year. 67 Allowing for ~5% attrition, 2350 patients will be available for analysis. This equates to approximately seven eligible participants per 1-month period per cluster.
There is a total of 26 suitable PICUs across the UK. Provisional interest in participation in a full PERMIT study is high, and it is reasonable to assume that 21 PICUs would want to participate, which could also include the three sites in the feasibility study. This trial design requires that all participating PICUs begin the control phase of the trial when the data-collection period begins. Further analysis will be required to accurately estimate required parameters for a stepped-wedge cluster randomised trial including the intracluster coefficient (ICC) and cluster autocorrelation coefficient (CAC). Provisional data from BCU PICU provided a mean length of time on MV as 19.3 days for those in PICU more than 7 days, with a SD of 28.4. A reduction of 1.5 days on MV is considered to be meaningful to patients and parents. The SANDWICH trial66 estimated the ICC to be 0.005. Personal correspondence from the USA PICU Up! trial and SANDWICH trial66 leaders suggests that the CAC may be close to 1.
Our proposed design is in shown in Figure 17. Similar to the SANDWICH trial protocol (adapted under CC BY 4.0 license http://creativecommons.org/licenses/by/4.0/),113 there will be an initial 3-month period of baseline data collection during which the PICU will not be exposed to the intervention. Following this, every 3 months, three sites will be randomly selected to transition to the intervention. This will start a 3-month training period of the intervention. As we cannot assume that the PICUs are exposed or not exposed to the intervention during the training, no patients will be recruited through this 3-month period. Once a PICU crosses over to the intervention, it will remain exposed to the intervention for the remaining duration of the study. There will be a final 12-month period during which all PICUs will be fully exposed after the last PICU has transitioned to the intervention. This 12-month period is to ensure that the ERM programme is fully embedded following implementation, which is a key requirement expressed by clinicians to be part of PERMIT and was not achieved in the feasibility study. Assuming an average of seven patients per 1-month period, and total follow-up period of 36 months, 80% power would be achieved. This would equate to a sample size of 4851 participants.
FIGURE 17.
PERMIT trial design cluster, stepped-wedge.

If LOS is used as primary outcome this increases the eligible population to 3800 (19% of 20,000) (Table 30). Allowing for 5% attrition provides a population size of approximately 3600 patients and approximately 11 participants per 1-month period per cluster. The same data from BCH PICU provide a mean estimate of LOS to be 23.7 with a SD of 31.0. Using the same design matrix as for length of time on MV, and an ICC of 0.02 estimated from the PICANet data, the sample size of 7623 would provide over 80% power to detect a 1.5 reduction in LOS.
| Year | Total admission | LOS 3–<7 days | LOS 7+ | All patient LOS 3+ |
|---|---|---|---|---|
| 2017 | 19,869 | 4673 (23.5%) | 3842 (19.3%) | 8515 (42.9%) |
| 2018 | 20,172 | 4825 (23.9%) | 3977 (19.7%) | 8792 (43.6%) |
| 2019 | 20,383 | 4855 (23.8%) | 3878 (19.0%) | 8733 (42.9%) |
There will be an initial 3-month pre-implementation block for 21 clusters (e.g. PICUs), stepping into a 3-month training block (staggered for 3 clusters at a time) with 12 months or more post-implementation period.
A further sample size calculation will be performed using the whole PICANET data set with LOV outcome for UK population. At the time of report submission these data were not available because of a cyber-incident on the Leeds University server.
Additional consideration
Chapter 7 concludes with a list of trial elements which will be important to incorporate into a future trial design (see Tables 27, 28 and 29).
In addition, further evaluation of the sample size and primary outcome will be required to justify the size and scale of a definitive trial. We acknowledge that plausible effect size for the primary outcome will also need to be estimated in an internal or external pilot phase of a future study.
Important considerations are needed on the potential confounders which may occur in a large pragmatic trial. The risk of education contamination bias, for example retaining separation between arms of the trial, is important. The huge investment of staff time, resources and effort that we identified during the Phase 3 feasibility study would be replicated in a definitive trial in each PICU. The risk of this occurring outside of the conduct of the trial and at the correct step in the design we believe is low. However, we acknowledge that PICUs in the UK have implemented ERM programmes in various forms, but we also recognise that those PICUs have struggled with maintaining programmes, without well-theorised implementation programmes like PERMIT (e.g. Birmingham and the MOVE4WARD programme).
Finally, the PERMIT feasibility study was conducted with a limited budget and although we provided research support staff funding for data collection and trial conduct at each site, we did not provide resources for any additional ERM delivery by therapists or nursing staff. In addition, we did not undertake an economic evaluation or involve a health economist in the PERMIT feasibility study as per the original commissioning brief. In a future trial, additional NHS support costs will be needed for adequate staff support of both the implementation and the delivery of ERM within clinical care, as identified in Chapter 6: Process evaluation.
Summary of findings to inform paediatric early rehabilitation and mobilisation during intensive care study
-
The PERMIT manual is a complex intervention and is best assessed through a clustered stepped-wedge RCT in PICU.
-
Following consultation, a draft proposal for the clustered stepped-wedge RCT in PICU is proposed.
-
There is strong support for a definitive efficacy trial of ERM from HCPs. Feedback from participants for the PERMIT study, although limited in number, also supports a future trial and suggests important design and measurement elements.
-
The primary outcome of LOV is a pragmatic compromise on measurable PICU outcome and likely accurate measure of improvement in critical illness recovery. Further consensus work in developing the primary outcome will be required with the UK PCCS-SG and trialist prior to a definitive study proposal.
Chapter 8 Paediatric early rehabilitation and mobilisation during intensive care study approach to patient and public involvement and engagement
Introduction
Designing and conducting research within the PICU context are recognised to be extremely challenging. 114 The PERMIT study team therefore set out from the outset to ensure the study was designed in collaboration with parents of a child who has experienced a PICU admission. We needed co-applicants who really understood the perspective of what it was like to have a child admitted to PICU and the role rehabilitation and mobilisation could play in recovery. SL and her daughter Darcy (D) agreed to join the team. They have direct experience of PICU and with JMen (our PPIE lead) can ensure PPIE has been woven through the study, building on information gained through each phase.
Study management
The PPIE work throughout Phases 1, 2, 3 and 4 was led by JMen and SL (our PPIE co-applicant). The study management group inputted into protocol designs, ethics application scope and drafting of the PPIE report.
Overview of patient and public involvement and engagement work
-
In Phase 1 our PPIE co-applicant endorsed the importance of understanding current practice surrounding ERM through a survey of practice with healthcare professionals and understanding the published literature. The area that was felt to require focus was PPIE work to support the design and conduct of the observational study.
-
In Phase 2 the focus was on the development of an Early Rehabilitation and Mobilisation Manual: the intervention for the Phase 3 feasibility study. PPIE activity was envisaged to be about co-designing the manual and identifying relevant trial outcome measures. PPIE work was conducted to inform the Research Ethics Submission. Unfortunately, following ethics review and approval, all non-COVID-related research was suspended. Following discussion with the NIHR HTA funder, it was agreed that some elements of Phase 2 would not be conducted and modifications to the Phase 3 feasibility study design were made to include some components of the aims and objectives from Phase 2 as well as related PPIE activity.
-
In Phase 3 PPIE work was therefore essential to ensure the trial was feasible and acceptable. Activities focused on the trial design, conduct and duration and the appropriate model for informed consent, as well as all the study-related patient-facing material.
-
In Phase 4 the purpose is to draw together all the key messages from the three phases and develop consensus about a future ERM RCT. PPIE elements to this are to ensure that the key messages from the Phase 3 work are correct and endorsed by parents/legal guardians.
Objectives for each phase are outlined in Table 31.
| Phase 1: to understand current practice surrounding ERM | Phase 2: to develop an ERM manual in conjunction with CYP and parents | Phase 3: to develop a feasibility trial of ERM | Phase 4: consensus work |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
Our approach to patient and public involvement and engagement
There are three key domains to conducting patient and public involvement (PPIE) work – planning, supporting and recording and evaluating. 115
Planning patient and public involvement and engagement – developing a plan for patient participation, involvement and engagement in a trial
The study team adopted an approach based on consultation, where people are asked for their views, which are then used to inform decision-making. 116 Although this is regarded as the lowest rung of the patient form of involvement117 this was felt to be appropriate given the nature of the research context and the specific remit of the NIHR funding. The team were keen to contribute to the knowledge base surrounding PPIE within the PICU context118 and highlight the impact of PPIE at each stage. A plan was therefore made, although this had to be adapted significantly due to COVID.
Planning patient and public involvement and engagement – identifying parent contributors
Researchers are advised to approach patients and public through formal patient groups, charities, community groups, national directories such as ‘People in Research’ or Health and Social Care patient advisory panels. 119 Partnership with parents who are in a similar situation to potential study participants is vital to ensure that important aspects of the research question have been considered. 120 However, there is recognition that there can be challenges with identifying appropriate contributors. 121,122 The concept of ‘similar situation’ is challenging in the PICU context as the service provision is for all CYP aged 0–<18 years and there is a broad case mix of underlying diseases and diagnosis. 123 In addition, the PERMIT team faced a challenge in accessing a formal established group. There were no pre-established groups in existence within the Clinical Research Network (CRN), the affiliated Trusts for the research (Birmingham Women’s and Children’s NHS Foundation Trust and Southampton Children’s Hospital), and none within the two sites’ PICUs. There were also no national groups of parents with experience of PICU established,118,124 a situation which continues to the current day. In these circumstances identifying people through personal contact or recommendation is therefore acceptable. 119 Our PPIE co-applicant SL and her daughter both felt that it was important that we made efforts to engage with parents who had experience of a child being admitted to PICU. Families without this experience might not understand the extreme emotions parents experience during the first few days of a PICU admission. This approach is supported by other national PICU studies. 120,125–127
Planning patient and public involvement and engagement – impact of COVID
The plan to recruit to a PERMIT-specific parent advisory group made up of parents who had experience of their child being in a PICU was then compounded by COVID. The PERMIT team were re-deployed to clinical work, contact with families was hindered through the wearing of personal protective equipment (PPE) impairing communication and there were the additional challenges of restricted visiting and parental exclusion if parents were symptomatic. 128
In addition, there was an added challenge to speaking to parents with experience of deciding about research participation in PICU because of research suspension. Despite fewer children with COVID-19 admitted to hospital and even fewer admitted to ICUs, PICUs experienced the same disruption to clinical research as adult ICUs. Seventy-five per cent of PICU/ICUs internationally suspended recruitment to some if not all research during 2020. 100 Suspension of research recruitment reduced the number of studies which were open and recruiting and reduced researchers contact with families. This created additional challenges for the PPIE lead to speak to parents with experience of being recruited to research.
There were therefore challenges to the number of parents we could engage with for PPIE. INVOLVE (2012)129 recommend a minimum of two PPIE participants and there is growing recognition that the available number of participants with the relevant experience may be low. 119 We therefore did not set a specific number of PPIE participants, but aimed to involve parents with as varied experience as possible.
Planning patient and public involvement and engagement – children and young people
Conducting PPIE work with CYP was also challenging. A previous review had identified that few PICU studies had managed to conduct PPIE work with CYP with experience of PICU. 118 Although there are excellent Young People’s Advisory Groups available through the NIHR130 and locally131 their membership at the time included no CYP with experience of a PICU admission. Previous studies have consulted with siblings of CYP who have had experience of PICU132,133 However, COVID meant there were no sibling visitors allowed on site within the hospital (still the situation currently) and the YPAG groups were suspended for a period of time.
The decision was therefore made to conduct ‘standalone’ PPIE – identifying and liaising with parents/carers as required for each of the phases. The team also had extensive wider PPIE and qualitative research experience from related studies which were drawn on. Pre-COVID efforts were made to engage with CYP and siblings where possible but this was not possible from 2020 onwards.
One of the concerns of the study, especially pertinent given the additional challenges of COVID, was to ensure that we facilitated parents/legal guardians to have the opportunity to shape and influence research. In a study which was designed to be relevant to all patients admitted to PICU it was vital that we considered equality, diversity and inclusivity in our PPIE. Study materials were specifically developed with graphics to help those for whom English was not their first language, those with dyslexia and those with lower literacy levels. PPIE was also conducted across more than one site to ensure there was representation of parents from a wide geographical area as well as families who lived locally in Birmingham and London. A variety of methods were used to assist with families’ engagement. Face-to-face introductions helped to explain that this was to inform research design, rather than to participate in research. Short information leaflets were provided about what was required. Parents could provide feedback face to face with the PPIE lead or a member of the local research team or by writing responses and returning them in a stamped addressed envelope. PPIE activity could happen within the hospital or within the home environment (if it coincided with other research activity).
Planning patient and public involvement and engagement – allocating appropriate costs
Historically PPIE participants have not always received any payment for their time and involvement; however, this payment is now recognised as good practice. 134 All PPIE activities were costed in line with NIHR (2018) guidance on reward and recognition for public contributors and participants appropriately rewarded. 135
Planning patient and public involvement and engagement – managing the expectations of public contributors
The PERMIT PPIE co-applicant and the parent representative on the Trial Steering Committee were prepared for their roles with clear guidance on the role and what was required. PPIE participants were provided with information about the consultation work by the staff member approaching them. The expectations of their role were identified and as they were involved with ‘standalone’ PPIE there was no ongoing commitment.
Supporting patient participation, involvement and engagement
For patients new to a PPIE role, support to develop their abilities and confidence to express their views and question researchers may be relevant. In order to support our parent co-applicant and the parent representative recruited to the Study Oversight Group both were offered access to appropriate training via a number of resources. Materials supplied included guidance on involvement in PPIE,136 links to local resources within the West Midlands CRN and on-line training available through NIHR. 137 In addition, SL attended a national one-day UK symposium about PICU outcomes and rehabilitation (2017) with members of the PERMIT team in order to help her understand the wider perspective.
There are many PPIE tasks where training is not necessary, where a different perspective or experience of a healthcare condition is the required expertise. 119,134 In line with this PPIE participants within PERMIT did not receive any formal training.
Recording and evaluating patient participation, involvement and engagement
While there is consensus that PPIE has considerable potential to benefit clinical trials, there has been little formal evaluation of its impact. 115 None of the PICU studies in a UK review provided an objective measurement of the impact of PPIE. 118 The PERMIT team were therefore keen to create a clear picture of when and where PPIE happened, what impact this had on the trial, the nature of PPIE activities and the impact of the activity on the trial design and conduct. 138 This is captured in the report above, with specific examples of what changed as a result of consultation detailed below.
Impact of patient and public involvement and engagement: the PERMIT team collaborators
Researchers who conduct PPIE often report on the experiential learning and positive impact it has for them as a researcher. 139 As a research team we would therefore like to report the positive impact PPIE had for us as a team. Despite all the challenges encountered, particularly due to COVID, we felt that that we were able to engage with parents and obtain meaningful insights which made an impact on the design and conduct of the study; particularly the models of consent utilised in Phases 1 and 3. We would also like to acknowledge the impact for SL and D, our co-applicants. They have linked up with PICU, BCH, to help with all things ERM (outside of the PERMIT study). From featuring on posters promoting ERM on the unit, to pictures on online training material, to reviewing local documents such as an information leaflet about ERM for elective surgical patients, they have both taken an active role. D is now in secondary school. She recently contacted the PPIE lead to provide some feedback about the leaflet and expressed how she was wondering if there was anything else she could do to help promote rehabilitation and help other children:
Being as I had some experience with the same things other may go through, I was talking to my mum about maybe coming to PICU to help others, if its allowed? I’d really like to help other children.
(D)
We are now working with SL and D to develop videos and guides to help guide children, parents and family members about ERM activities. In addition, D has joined the local Young Person’s Advisory Group (YPAG) and is contributing to their wider work within the Trust.
Chapter 9 Equality, diversity and inclusivity
Background
The NIHR strategy Best Research for Best Health (2021) highlights inclusion within the five key operating principles, from ensuring there is opportunity for everyone to participate in research through to providing opportunity for researchers from different disciplines, specialisms, geographies and backgrounds to develop and progress a career in research. Within this chapter we will demonstrate how the study team considered equality, diversity and inclusion within the PERMIT study team, PPIE participants and PERMIT study participants, both patients and parents.
PERMIT study team
The PERMIT study team was assembled to reflect expertise on the subject of rehabilitation and mobilisation and paediatric intensive care. The study team was composed of eight females, eight males. Ten of the study team were affiliated to eight NHS organisations, in a range of different geographical areas. These organisations had a wide variety of service users from a wide range of socioeconomic backgrounds. Early rehabilitation and mobilisation is an intervention which requires MDT involvement and the study team represented this with three paediatric intensivists (BS/KM/NP), two consultant paediatric neurologists (FK/RF), two physiotherapists (JT/MG), two registered nurses (JMan/JMen), an OT (JMc) and a psychologist (GC). From 2018 when the PERMIT study funding was awarded more junior members of the PERMIT team were supported with their career progression and development opportunities. JT was successfully appointed to a PhD, University of Birmingham (2020). JMc completed her PhD and was successfully appointed to Lecturer at the University of Salford. JMen was initially responsible for PPIE but went on to become second author on the national survey paper and was supported to lead the process evaluation within Phase 3.
Patient and public involvement and engagement
In Chapter 7 we report in full on our approach to PPIE. In total our PPIE participants included mothers (n = 6), fathers (n = 4), and one sibling of infants and children who had experienced an admission to PICU and young people naive to PICU (n = 2). The current age of the child admitted to PICU (n = 9) ranged from 1 month to <16 years of age and the reason for admission ranged from single organ failure (respiratory) in children who had been previously fit and well, through to children with multiple health conditions and rare diseases. All the families we engaged with could speak and read English and felt able to contribute to PPIE activities, although it was not the first language for at least three parents. Three participants identified as Asian and one parent as black, although parental ethnicity of PPIE participants was not fully reported. Parents of children admitted to two NHS organisations – Kings College Hospital, London, and Birmingham Women’s and Children’s NHS Foundation Trust – were approached about PPIE engagement. Both organisations have a local population, as well as families referred to the centre due to specialist services. Both perspectives were represented by participants with our PPIE work.
PERMIT study participants
Question 1: who should my trial results apply to?
Children and young people
Early rehabilitation and mobilisation is an intervention which is believed to be useful for all patients admitted to PICU. It was therefore important for the feasibility study to be as inclusive as possible, including infants and children of all ages 0–<16 years, admitted for PICU care either electively or as an emergency. The only exclusion criteria were if the parents/legal guardians chose to opt out (Phase 1) or declined consent (Phase 3) or if there was a local decision by the clinical team/principal investigator not to include the patient. CYP are recognised to be an under-served group with respect to research opportunities140 and recruitment of critically ill CYP to research is low. 141 Fewer than 1 in 100 admissions to PICU globally are recruited to a clinical trial, compared to 1 in 10 adults in adult ICU,142 and a recent review identified that <1% of patients undergoing cardiac surgery are currently recruited to research. 143 The PERMIT study therefore set out to be inclusive, opening the observational study in 50% of all UK PICUs (n = 15) to ensure participants from across the UK had the opportunity to participate. All CYP admitted to participating PICUs for over 48 hours were then eligible for inclusion. This ensured that some of the most vulnerable patients, for example CYP with cognitive impairments, learning and physical disabilities and multiple health conditions, were eligible for participation.
We recruited 169 participants in the Phase 1 observational study and 30 participants were recruited to the Phase 3 study. The ethnicities of CYP recruited across the two phases of the study were: white 116 (79%), Asian 20 (14%), black 15 (10%), mixed 4 (3%), other 2, not stated 20 (14%). When this is compared to the data collected by one study67 of patients admitted to PICU (2018–20) we can see that the ethnic diversity of the study participants’ is representative of the ethnicity of all UK/Republic of Ireland PICU admissions: white: 75%, Asian 12.5%, black 5.4%, mixed 3.6%, other 3.5%. Overall there were no differences in our planned trial population and those who successfully participated.
Parents
All parents of children admitted to PICU and approached for the feasibility study (Phase 3) were also offered the opportunity to be study participants and provide feedback about their child and family experience through questionnaires (21/30) and an additional optional interview (n = 14 participated). There were no exclusion criteria, although we were unable to provide translated versions of the validated questionnaires and we did not have the option to interview participants in any language other than English. In addition, we sought to hear the voice of parents who declined their child’s participation in the study. This perspective is seldom heard and the four families which this applied to were all offered the opportunity. Unfortunately, this was declined by all four families.
Are the groups identified in Question 1 likely to respond to the treatment in different ways?
A number of previous ERM studies have excluded patients <3 years of age,20,15 even though 60% of UK PICU admissions are <36 months. 62 The PERMIT Phase 3 study team was therefore keen to ensure that families of infants were offered the opportunity of participation. This was successfully achieved across the three study sites with 9/30 (30%) participants under 12 months of age. We were also concerned to ensure that the study captured patients at risk of or experiencing multiple organ failure. Twenty-seven per cent (n = 8) of patients were admitted following surgery, including two patients who had undergone cardiac surgery. Other admission reasons included trauma (n = 2), infectious/inflammatory (n = 2) and neurosurgery (n = 1). In addition, 63% of recruited patients had an underlying disability (mild-severe) as indicated by a baseline POPC score. We therefore feel that opportunities to participate in PERMIT were offered equitably and ensured that under-served CYP and families had an opportunity to participate in research.
Will my trial intervention and/or comparator make it harder for any of the groups identified in Question 1 to engage with the intervention and/or comparator?
Evidence has shown that admission to PICU is extremely stressful for parents and many find it difficult to consider research during the initial few days. 141 Our concern was that this would then serve as a barrier to families who were approached about the study for a full informed consent. Our PPIE work indicated that an opt-out consent approach would be appropriate in Phase 1 (observational only), which was confirmed by 100% (n = 169) of parents choosing for their child’s data to be included in the study. In Phase 3 an informed consent approach was required; however, steps were taken to help parents to understand each step of the study and reduce the stress associated with participation. Study participants endorsed this approach and, importantly, helped provide vital insight into the low perception of risk associated with a future trial. A future trial could therefore proceed with an opt-out consent, which helps facilitate research engagement for a wider range of patients and families.
Will the way I have planned and designed my trial make it harder for any of the groups identified in Question 1 to consider taking part?
We worked hard with our PPIE participants to design the trial and the patient-facing information as clearly as possible given the challenging situation eligible families were in. We also provided thorough training within the site initiation visit (SIV) with sites to discuss the importance of a sensitive approach to families and the value of the staged informed consent approach. We view the relatively low decline rate of 4/34 (12%) as testament to the clear participant information sheets and support of the research delivery teams. One aspect where we were mindful of ensuring choice was in the method of completing outcome measures. We did not want to digitally discriminate or disadvantage families so offered outcome measures in a number of different formats, including paper, online and completed with a researcher by phone.
Reflections and areas for improvement
One of the challenges for future research is about developing study information to help make research more accessible. Families suggested access to information leaflets on YouTube and opportunities to learn more about research in advance of a PICU admission when there is more time to consider what is involved. We also recognise that CYP want to be more involved with decision-making about research, but with the complications of assent while intubated and sedated we need to do more work to consider how we can better prepare those coming for an elective admission.
Upon reflection we realise that to demonstrate evidence about addressing equality, diversity and inclusion, we need to identify and measure relevant metrics. In our PPIE work we were cautious about collecting ‘unnecessary data’ and did not record or ask parents for information such as their ethnicity, age, employment status, number of other children and their own health status, all of which help to demonstrate that under-served communities are being included. In the future we will adopt a more standardised way of capturing these data so we can ensure a wider variety of perspectives are captured.
Chapter 10 Discussion and conclusions
Summary of main findings
Phase 1a: scoping review
In our scoping review of paediatric ERM literature, we identified that optimal aspects of ERM intervention delivery, timing, content, active ingredients, dose–response relationships, progression and implementation strategies have yet to be established. Multicomponent ‘non-mobility’ and ‘mobility’ ERM interventions were feasible and safe and most involved physical therapy, OT and SLT.
Children under 3 years old were more likely to receive interventions such as cuddles or in-bed mobilisation, whereas non-ventilated children or those aged 3 years and older were more likely to receive mobility interventions involving physical therapy or OT. Importantly, family involvement appeared crucial, and the lack of a well-defined ERM protocol was a significant barrier to ERM implementation. With no clear evidence on the impact of bundles of care or behavioural interventions incorporated within ERM, further research in this area is essential.
Phase 1b: survey of current practice
This national survey of HCPs from UK PICUs identified the importance of ERM as an intervention which participants believe can improve the physical, psychological and cognitive recovery of critically ill or injured infants and children across all ages. Our findings indicated support for ERM, but highlight uncertainty with suitability, variability with the definition of this complex intervention, variation in timing of initiating and which patient groups should receive ERM. Similar to our scoping review, the reported lack of ERM protocols in most (21/26) UK PICUs reinforced a strong requirement for evidence-based standardised protocols with optimal timing, intensity, frequency and duration of ERM. There is a need for flexible protocols to allow for tailoring rather than prescription. Key barriers to ERM delivery were identified (e.g. funding and staffing) and potential clinical (e.g. improved psychological outcomes) and economic (e.g. reduced PICU LOS) benefits to patients and PICUs.
Phase 1c: observational study of early rehabilitation and mobilisation practice
We observed ERM practice in 169 patients across 15 PICUs who reached 9 a.m. on day 3 after PICU admission in our 14-day observation period. Ninety per cent of eligible patients were enrolled using an opt-out consent model. On the first study day (day 3 after PICU admission) 162/169 (96%) of patients received an ERM activity; 87% involved a mobility and 38% an out-of-bed mobility. The rate of ERM activities for patients remained constant across the subsequent 7 days of their PICU admission (or until PICU discharge).
Over the observation period, 3696 ERM episodes delivered 4978 ERM activities across all PICUs. Most were delivered by a registered nurse or parent/family member. Positioning with and without mobility elements accounted for nearly half of all ERM activities. A wide range of ERM activities were reported, but were more likely to be passive or enrichment activities rather than active ERM. ‘Cuddles’ by a family member/nursing staff was the most frequent out-of-bed activity. We identified that family presence significantly increased out-of-bed ERM, although MV, presence of a urinary catheter and pre-existing severe developmental delay were associated with not receiving out-of-bed ERM. Presence of an ERM protocol did not impact the chance of out-of-bed mobility. However, ERM was delivered to nearly all patients, including those of all ages, admission diagnosis and with the highest level of organ dysfunction or organ support.
ERM was delivered safely with a low (<3%) reported rate of AEs per ERM activity. Most AEs did not require any corrective intervention. Of concern was that delirium screening was universally absent in study-participating PICUs and this requires attention. Nursing staff and parents delivered the majority of ERM activities throughout the 24 hours period. Physiotherapists only delivered 10% of ERM although 59% of patients received at least one PT-delivered ERM episode at some point. There was minimal input from other medical, therapy, or support staff reported. Any ERM manual or intervention plan will need to be designed to utilise available staff or require significant increased resources to support them.
Phase 2: paediatric early rehabilitation and mobilisation during intensive care manual development
Our synthesis of the key messages, assumptions and uncertainties that emerged from our prior Phase 1 work showed that ERM is currently defined and enacted in multiple ways. Importantly, people see the potential value for the diverse patient populations within PICU and are willing to support the safe delivery of ERM but are uncertain how best to deliver it. Our three face-to-face workshops with NHS HCPs (n = 18) and one online workshop with international experts (n = 3) helped generate some core guiding principles around the potential shape and content of the intervention. For example, everyone in PICU (doctors, nurses, physiotherapists, and parents) is essential for ERM delivery – everyone should take some ownership. Also, ERM needs to be as inclusive as possible, with a focus on promoting movement and mobility as early as possible and with progressive increases over time. Our review of existing ERM protocols and discussions with healthcare professionals enabled us to develop a prototype ERM manual that was focused both on the safe delivery of ERM for each patient, and on the introduction and embedding of an ERM approach within a PICU. The manual was informed by current evidence, experience, and theory. It offered a flexible, progressive, approach to the delivery of ERM, with resources including essential clinical materials – the ‘bedside bundle’ – that consist of an ERM daily flowchart, patient acuity levels, ERM activity levels, and pause and re-assess criteria. It also included a step-by-step guide to putting ERM into practice – the ‘implementation toolkit’ – that focused on building ERM leadership, generating staff buy-in, making ERM workable, and keeping it going over time.
Phase 3: feasibility trial and implementing evaluation of non-randomised early rehabilitation and mobilisation intervention
All three sites implemented the PERMIT programme as described in the manual. The families were positive about the study recruitment process and all sites successfully recruited the 10-patient target. To achieve ERM delivery, all patients (1) had an acuity level scored (and repeated on 84% of ward rounds) and (2) had an acuity level correctly linked to an ERM activity prescription and then subsequently to a delivered ERM activity. The level of activity was broadly representative of the acuity level.
Other key findings were that a large number of clinically relevant patient outcomes were measured through validated tools and all patients received ERM activities safely using the pause and assess criteria with only two trial reported AEs and no severe AEs. ERM was important for the physical and psychological recovery of the CYP, as well as the psychological well-being of parents/carers supporting their involvement in their child’s care. PERMIT was seen by health professionals and parents as worthwhile, feasible and acceptable. Finally, having access to research delivery support was central to support recruitment, data collection and data entry.
Phase 4: consensus study and trial design
With input from PPIE, parent/family members and multidisciplinary members of the study management group we reviewed and refined the findings from Phases 1, 2 and 3 of PERMIT. We confirmed that a future PERMIT ERM clinical trial was necessary and likely to be feasible with consideration of additional trial design elements. The most suitable trial design for the complex intervention is a clustered stepped-wedge randomised control trial within PICUs across the NHS. The primary outcome requires further consideration. LOV (or days free from ventilation) is a pragmatic compromise on measurable PICU outcome and a likely accurate measure of improvement in critical illness recovery. It would also allow comparison of the PERMIT manual and intervention with the only other similar PICU-wide ERM intervention study, the PICU UP! programme, ongoing in the USA. 112 Further consensus work in developing the primary outcome will be required with the UK PCCS-SG and trialists prior to a definitive study proposal. Any future trial will require health economic evaluation in addition to assessment of patient-related outcomes.
Strengths and limitations
Phase 1a: scoping review
We conducted a comprehensive search using broad inclusion criteria, but the risk of publication bias remains. We also acknowledge the inherent limitations of vote-counting used to determine effective ERM interventions since RCTs included in this review were not adequately powered, and findings may not be clinically relevant.
The majority of studies included in this review had design flaws, and varied in the consistency and reporting of interventions or outcomes evaluated. No between-group mean/SDs or effect with precision intervals were ever reported. These outcomes might have different implications on the strength of the evidence when the cost effectiveness of ERM is considered. Lastly, most studies did not provide information about intervention delivery to understand active drivers of successful implementation. Consequently, inferences drawn are based solely on explanatory findings.
Phase 1b: survey
The strength of this survey was an inclusive representation of 90% of UK PICUs and views from the wider MDT. A limitation was the use of a non-validated questionnaire. None or partial responses may indicate poor engagement in the ERM topic, and as with all self-reported surveys, responses indicate reported rather than necessarily actual clinical practice. Finally, the findings represent the views of UK NHS staff and may not be generalisable to other healthcare settings.
Phase 1c: observational study
We observed over half of all UK PICUs, geographically diverse, with varying population characteristics, PICU size, case mix and staffing structures during the two observation periods. However, the types of PICUs may not be generalisable to other UK or non-UK PICUs. To focus observations on early ERM, but to avoid very short-stay PICU patients (e.g. <48 hours), we started observation at 9 a.m. on the third day after PICU admission. ERM practice and delivery to patients in the first 72 hours of PICU stay may also be important in guaranteeing dose and efficacy of intervention. In addition, we stopped observation after 7 days of study observation (day 10 of PICU) or at discharge. Thirty-two per cent (54/169) of our cohort continued to stay in PICU and longer-term PICU care and use of ERM may also be important. Also, ERM may have a role in step-down high-dependency care or ward areas.
We acknowledge the potential risk of observer bias and Hawthorne effect in our study design. ERM activities were listed on the bedside activities document to allow ease of recording. Presence of the documentation or the known process of observation may have affected the amount of ERM delivered to patients during the observation period (e.g. increased number, duration or variety of ERM activities, stimulated by the bedside information).
ERM activities were recorded in the medical records by bedside clinical staff. Research staff extracted this information for our PERMIT CRFs. AEs were self-reported by the clinical team without independent confirmation and therefore may have under- or overestimated the frequency of events or relatedness to the ERM activity; however, the rates identified closely matched those extracted in the scoping review.
We identified a very high rate of ERM activity on study day 1 using the inclusive definition of any ERM. This limited any ability to perform the planned logistic regression modelling to explore patient-, site- or ERM-related factors. In our out-of-bed logistic regression model, due to the small number of patients per site, we were unable to use site as a random effect. Further exploratory analysis of factors affecting different subtypes of ERM are planned and may highlight wider variation in practice across sites.
Phase 2: manual development
A strength of this study was the multiple sources of evidence we used to develop the ERM intervention manual: evidence from a wide range of existing studies, concepts, tools and resources, as well as the experience of diverse practitioners and topic experts. A further strength was that the manual was informed by a range of theories of implementation. However, given the COVID context during the study, we were unable to conduct workshops and interviews with parents and CYP with PICU experience, so we were not at all successful in using their direct experience to shape the ideas within the manual. The work of learning from parents’ experiences was instead undertaken through the process evaluation work in Phase 3.
Phase 3: part 1 feasibility study
A strength of this study was the phased introduction and embedding of a novel intervention (the PERMIT manual) alongside a set of novel trial processes. A further strength was the engagement of staff within and across sites, including those who championed and led the ERM programme, those who delivered ERM, as well as those other staff at the sites not directly involved in bedside ERM delivery. This enabled us to introduce an ERM programme at pace, enrol planned numbers of patients and parents, demonstrate data collection is feasible and the ERM is delivered safely. We were not successful in obtaining timely local site approvals at one site, which meant that the introduction of the ERM programme was delayed. As a result, this site spent relatively little time in each of the phases. Also, when research staff were not available at sites – notably on ward rounds – we did lose some quality and accuracy in some areas of data collection. A health economic evaluation was removed from the feasibility study after the request of the HTA board. Full health economic evaluation and support in a future study are required.
Phase 3: part 2 process evaluation
A strength of our data is the spread of data across methods, as well as across sites and participants. We obtained a comprehensive account of the difficulties of introduction and embedding across the sites. A further strength was the multidisciplinary respondents from each site – including those who championed and led the introduction, those who delivered ERM, as well as those other staff at the sites not directly involved in bedside ERM delivery. This enabled us to document the range of perspectives on problems and tensions around the evolving context and delivery across the sites. Parental accounts were helpful in clarifying trial processes, as well as providing insight into their perspectives of delivering ERM, and were essential to the highlighting of elements to change in future. We were not successful in recruiting parents who had refused consent to the main study for an interview to explore their reasons for declining.
Phase 4: consensus and trial development
The short duration of the Phase 4 study and continued impact of COVID pandemic on PICU health-care staff and parents and families who participated in PERMIT limited the scope of consultation and feedback. Further work with PPIE groups and the PICU clinical community is required to evaluate the definitive primary outcome. This will include additional sample size calculations with more extensive PICANet data after overcoming the cyber-incident at the University of Leeds.
Conclusions and summary of key research recommendations
Early rehabilitation and mobilisation is a complex intervention requiring institutional, departmental and multidisciplinary involvement. We have demonstrated that implementation of the PERMIT manual is acceptable, feasible and can deliver ERM safely to critically unwell and injured infants and CYP within PICUs. Further research in a definitive trial with economic assessment and demonstration of improvement in patient-related outcomes is justified and required.
Implications for healthcare practice
This is a feasibility study and thus has no direct implications for healthcare practice at this stage.
Acknowledgements
We would like to sincerely thank all the infants, children, young people, parents, guardians and families who took part in the PERMIT study. Without their support, generosity and dedication to this study and to informing future research, during an extremely difficult and challenging period of their lives, the PERMIT study would not have succeeded.
In addition, we would like to wholeheartedly thank all of the healthcare practitioners, research staff and managers across all the NHS trusts, higher-education authorities and NIHR organisations involved in PERMIT. They have worked tirelessly to set up and deliver safely all the phases of the PERMIT study through the most exceptional times experienced by the NHS as a result of the COVID pandemic.
Below we list key members of those teams.
Contributions of authors
Barnaby R Scholefield (https://orcid.org/0000-0002-6198-4985) (Chief Investigator, NIHR Clinician Scientist and paediatric intensive care (PICU) physician, University of Birmingham) conceived the study, original grant application and protocols and led Phases 1 and 4, and co-led Phase 3 of PERMIT. Wrote the first draft final report, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Julie C Menzies (https://orcid.org/0000-0003-2080-3364) (Nurse Researcher, PICU and NIHR 70@70 Senior Nurse Research Leader, Birmingham Women’s and Children’s NHS Foundation Trust) led the PPIE throughout the study, contributed to the analysis of the Phase 1 survey, contributed to the research delivery of Phase 3 and led the Phase 3 process evaluation. Critically reviewed, analysed and edited full report and was a co-investigator.
Jennifer McAnuff (https://orcid.org/0000-0002-1636-0049) (Research Fellow, Northumbria University and paediatric occupational therapist) co-designed and led the Phase 2 intervention development study, led all Phase 2 data collection and analysis, co-wrote the ethics application for Phase 3, led the write-up of Chapter 4 and was a co-investigator.
Jacqueline Y Thompson (https://orcid.org/0000-0002-9775-361X) (Research Fellow, University of Birmingham) trial co-ordinator and data manager for PERMIT observational study, designed and led the scoping review, co-ordinated and analysed Phase 1 survey. Database designer and manager Phase 1 observational and Phase 3 feasibility study. Wrote the first drafts of Chapters 1 and 2.
Joseph C Manning (https://orcid.org/0000-0002-6077-4169) (NIHR ICA Clinical Lecturer, Clinical Associate Professor in CYP and Families Nursing, University of Nottingham) critically reviewed, analysed and edited full report, member of the Phase 1 and Phase 3 study core-development team and was a co-investigator.
Richard G Feltbower (https://orcid.org/0000-0002-1728-9408) (Principal Investigator for PICANet, Senior Epidemiology, University of Leeds) critically reviewed, analysed and edited full report, statistical expertise and was a co-investigator.
Michelle Geary (https://orcid.org/0000-0002-2092-6113) (Divisional therapy lead and Specialist children’s physiotherapist within neurology and neurorehabilitation Southampton children’s Hospital) critically reviewed, analysed and edited full report and was a co-investigator.
Sophie Lockley, Patient and Public Involvement and Engagement (PPIE) representative and was a co-applicant.
Kevin P Morris (https://orcid.org/0000-0001-8911-6306) (Consultant in Paediatric Intensive Care, Birmingham Women’s and Children’s NHS Foundation Trust) critically reviewed, analysed and edited full report and was a co-investigator.
David Moore (https://orcid.org/0000-0002-4163-4080) (Associate Professor of Evidence Synthesis, University of Birmingham) provided methodological guidance and contributed to the literature review and was a co-investigator.
Nazima Pathan (https://orcid.org/0000-0001-7656-9453) (Consultant in Paediatric Intensive Care, Addenbrookes, University of Cambridge) critically reviewed, analysed and edited full report and was a co-investigator.
Fenella Kirkham (https://orcid.org/0000-0002-2443-7958) (Professor of Paediatric Neurology, UCL and Southampton children’s Hospital), co-lead for Phase 3, co-wrote the ethics application for Phase 3, critically reviewed, analysed and edited full report and was a co-investigator.
Robert Forsyth (https://orcid.org/0000-0002-5657-4180) (Consultant in Paediatric Neurology, University of Newcastle), co-lead for Phase 2, critically reviewed, analysed and edited full report and was a co-investigator.
Tim Rapley (https://orcid.org/0000-0003-4836-4279) (Professor of Applied Healthcare Research, Northumbria University), co-lead for Phase 2, provided expert guidance on normative process theory analysis in Phase 3. Critically reviewed, analysed and edited full report and was a co-investigator.
Additional acknowledgement
We would like to thank all members of the Trial Steering Committee and the Data Monitoring and Ethics Committee for their support and guidance of the PERMIT study (see Appendix 1).
Ms Faaria Hussain (Project Manager, University or Birmingham) lead project manager for PERMIT study. Darcy (PPIE representative) for her support and invaluable input to all the PPIE elements with her mum, Sophie Lockley (PPIE co-applicant). Ms Francessca Ryde and Ms Emily Brush (BMedSci students, University of Birmingham) for their development and piloting of the Phase 1b survey. Dr James Martin (Biostatistician, University of Birmingham) and Dr Hari Krishnan (Consultant in Paediatric Intensive Care, Birmingham Women’s and Children’s NHS Foundation Trust) for statistical analysis support in Phase 1c observational study and Phase 3 feasibility study. Dr Laura Cutler and Dr Olivia Craw (Research Assistants, University of Newcastle) for the conduct of the Phase 2 manual development and Hari Krishnan Statistical support Phases 1 and 3. Mrs Hannah Child, Ms Natalie Hemming and Mr Louis Skevington-Postles (physiotherapists) for their expert guidance and input into the Phase 2 PERMIT manual development. Ms Natalie Read (Research Coordinator, Birmingham Women and Children’s Hospital NHS Foundation Trust) Phase 3 qualitative research and PERIT debriefs. Mrs Hannah Child for her contribution localising education material and responding to site training requirements during Phase 3. Dr Rebecca Woolley and Prof Karla Hemmings (University of Birmingham Clinical Trials Unit) for methodological support for the future trial design Phase 4 PERMIT. Dr Sapna Kudchadkar (Johns Hopkins, Baltimore, USA) and Dr Karen Choong (McMaster University, Ontario, Canada), international experts in paediatric ERM who provided mentorship, review and guidance to the chief investigator and PERMIT team. Paediatric Critical Care Society Study Group (PCCS-SG) members and members of the Paediatric Intensive Care Units and Principal Investigators for their contribution to the development of the PERMIT study. Mr Martin Simpson Scott (NIHR HTA Research programme manager) for his guidance and support throughout the whole PERMIT study.
Ethics committee
We offer our thanks to the following ethics committees for their review of the individual phases of the PERMIT study:
-
Phase 1b survey: University of Birmingham, 5 February 2019: Ref: BMS_1819_03.
-
Phase 1c observational study: REC approval: 2 September 2019. East of Scotland Research Ethics Service. HRA ref: 19/ES/0102.
-
Phase 2 manual development workshop: healthcare professionals: Newcastle University, 1 September 2019: Ref 14224/2018. Parents, children and young people: REC approval: 28 February 2020 19/LO/1987 (substudy stopped because of COVID pandemic).
-
Phase 3 feasibility study: REC approval: 26 April 2021. Berkshire Research Ethics Service. HRA ref: 21/SC/0127.
We thank and acknowledge the support of the following organisations, representatives and principal investigators who participated in PERMIT study:
Phase 1b survey
Addenbrooke’s Cambridge, Dr Nazima Pathan; Alder Hey Children’s NHS Foundation Trust, Liverpool, Dr Petr Jirasek; Birmingham Women and Children’s Hospital NHS Foundation Trust, Dr Barnaby Scholefield; Bristol Royal Hospital for Children, Kate Baptiste; Evelina London Children’s Hospital, Dr Miriam Fine-Goulden; Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Dr Deborah Cross; GOSH CICU, Great Ormond Street Hospital for Children NHS Foundation Trust, Emma Shkurka; GOSH PICU, Great Ormond Street Hospital for Children NHS Foundation Trust, Dr Peter Sidgwick; Great North Children’s Hospital, Newcastle, Dr Rachel Agbeko; Kings College Hospital NHS Foundation Trust, Dr Bogdana Zoica; Leeds Teaching Hospitals NHS Trust, St. James’s University Hospital, Dr Sian Cooper; Noah’s Ark Children’s Hospital for Wales, Dr Siva Oruganti; Nottingham Children’s Hospital, Queen’s Medical Centre, Dr Georgina Harlow; Oxford University Hospitals, Dr Deirdre O’Shea; Royal Belfast Hospital for sick children, located in Royal Victoria Hospital, Dr Julie Richardson; Royal Hospital for Children Glasgow, Dr Richard Levin; Royal Hospital for Sick Children in Edinburgh, Dr Tsz Yan and Dr Milly Lo; Royal Manchester Children’s Hospital, Dr Ravishankar Nagaraj; Sheffield Children’s Hospital NHS Foundation Trust, Dr Rum Thomas; Southampton Children’s Hospital, Katy Morton; St Georges Hospital, London, Dr Soumendu Manna; St Mary’s Hospital, London, Dr Tom Bycroft; University Hospital Leicester, Dr Sanjiv Nichani; Glenfield Hospital, Dr Sanjiv Nichani; University Hospital North Midlands, Dr Kanaris Constantinos; South Tees Hospitals NHS Foundation Trust.
Phase 1c observational study
Addenbrookes Hospital, Cambridge, Dr Nazima Pathan; Alder Hey Children’s NHS Foundation Trust, Dr Kent Thorburn; Birmingham Women and Children’s Hospital NHS Foundation Trust, Dr Julie Menzies; Great Ormond Street CICU, Dr Aparna Hoskote and Great Ormond Street Hospital PICU, Dr Sophie Skellett; Nottingham Children’s Hospital, Dr Patrick Davies; Oxford University Hospitals, Dr James Weitz, Dr Avishay Sarfatti; Royal Hospital for Children Glasgow, Dr Richard Levin; Royal Manchester Children’s Dr Rachel Barber and Dr Ravishankar Nagaraj; Southampton Children’s Hospital, Dr John Pappachan; St Mary’s Hospital, Imperial College Healthcare NHS Trust, Dr David Inwald; University Hospital Leicester. Dr Simon Robinson; Glenfield Hospital, Leicester, Dr Peter Barry; Leeds Teaching Hospitals NHS Trust, Dr Sian Cooper; Royal Hospital for Sick Children, Edinburgh, Dr Milly Lo.
Phase 3 feasibility study
Kings College Hospital, London: Bogdana Zoica (PI), Louis Skevington-Postles (Co-PI), Daleep Meena, Christina Balnta, Pam D’Silva, Kerry Murphy, Tamsin Jones, Stacy Rice. Southampton Children’s Hospital: Dr Kim Sykes (PI), Jessica Mason, Hannah Clarke, Amber Cook, John Pappachan, Donna Austin, Nicola Etherington, Tracey Curtis, Hannah White, Caitlin Oxford, Verity Penner, Jenny Pond. Birmingham Women and Children’s Hospital NHS Foundation Trust: Julie Menzies (Co-PI), Will Tremlett (Co-PI), Natalie Read, Helen Winmill, Clare Burt, Hannah Child, Kirsty Stanley, Katie Ingram, Lydia Ashton, Lizzie Greensall, Navjyot Jabbak, Ben Harris, Harriet Payne, Sarah Bundy, Natalie Hemming, Chelsea Watson, Chiedza Chikurunhe.
Acknowledging the use of patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016;5.
- Seo H-J, Kim SY, Lee YJ, Jang B-H, Park J-E, Sheen S-S, et al. A newly developed tool for classifying study designs in systematic reviews of interventions and exposures showed substantial reliability and validity. J Clin Epidemiol 2016;70:200-5.
- Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68-74.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366.
- Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res Ed) 2016;355.
- Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on Exercise Reporting Template (CERT): explanation and elaboration statement. Br J Sports Med 2016;50:1428-37.
- Abdulsatar F, Walker RG, Timmons BW, Choong K. ‘Wii-Hab’ in critically ill children: a pilot trial. J Pediatr Rehabil Med 2013;6:193-204.
- Alqaqaa Y, Herbsman J, Folks T, O’Donnell S, Klien D, Seilikoff L, et al. Early mobilization in the pediatric intensive care unit. Crit Care Med 2018;46.
- Ames S, Fink E, Alessi L, Chrisman M. Implementation of Early Mobility Guidelines in a PICU: Evaluation of Adherence and Outcomes 2019;47.
- Betters KA, Hebbar KB, Farthing D, Griego B, Easley T, Turman H, et al. Development and implementation of an early mobility program for mechanically ventilated pediatric patients. J Crit Care 2017;41:303-8.
- Choong K, Chacon M, Walker R, Al-Harbi S, Clark H, Al-Mahr G, et al. Early rehabilitation in critically ill children: a pilot study. Pediatr Crit Care Med 2014;15.
- Choong K, Tran N, Clark H, Cupido C, Corsi DJ. Acute rehabilitation in critically ill children. J Pediatr Intensive Care 2012;1:183-92.
- Colwell BRL, Williams CN, Kelly SP, Ibsen LM. Mobilization therapy in the pediatric intensive care unit: a multidisciplinary quality improvement initiative. Am J Crit Care 2018;27:194-203.
- Cui LR, LaPorte M, Civitello M, Stanger M, Orringer M, Casey F, et al. Physical and occupational therapy utilization in a pediatric intensive care unit. J Crit Care 2017;40:15-20.
- Fink EL, Beers SR, Houtrow AJ, Richichi R, Burns C, Doughty L, et al. PICU-Rehabilitation Study Group . Early protocolized versus usual care rehabilitation for pediatric neurocritical care patients: a randomized controlled trial. Pediatr Crit Care Med 2019;20:540-50.
- Justice L, Malone K, Simsic J. Impact of a quality improvement intervention to promote early mobilization (WeeMove) in the pediatric CTICU. World J Pediatr Congenit Heart Surg 2019;10.
- Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU up!: impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2016;17:e559-66.
- Kudchadkar SR, Nelliot A, Awojoodu R, Vaidya D, Traube C, Walker T, et al. Prevalence of Acute Rehabilitation for Kids in the PICU (PARK-PICU) Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Physical rehabilitation in critically ill children: a multicenter point prevalence study in the United States. Crit Care Med 2020;48:634-44.
- Choong K, Al-Harbi S, Siu K, Wong K, Cheng J, Baird B, et al. Functional recovery following critical illness in children: the ‘wee-cover’ pilot study. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2015;16:310-8.
- Choong K, Awladthani S, Khawaji A, Clark H, Borhan A, Cheng J, et al. Early exercise in critically ill youth and children, a preliminary evaluation: the wEECYCLE pilot trial. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2017;18:e546-54.
- Choong K, Foster G, Fraser DD, Hutchison JS, Joffe AR, Jouvet PA, et al. Canadian Critical Care Trials Group . Acute rehabilitation practices in critically ill children: a multicenter study. Pediatr Crit Care Med 2014;15:e270-9.
- Alfarsi A, Awladthani S, Cupido C, Kho M, Krasevich K, Nanji J, et al. Liberation from PICU-acquired complications: bi-center implementation study. Pediatr Crit Care Med 2021;22.
- Kothari H, Panicucci T, Houston A, Cifra C, Badheka A. PADGO! a quality improvement intervention to improve measures of ICU liberation. Crit Care Med 2021;49.
- Choong K, Zorko D, Awojoodu R, Ducharme-Crevier L, Fontela P, Lee L, et al. Prevalence of acute rehabilitation for kids in the PICU: a Canadian multicenter point prevalence study (PARK-PICU Canada). Pediatr Crit Care Med 2021;22:68-9.
- Curry A, Brown A, Williams S, Schwartzberg S, Parkhurst L, Butrum-Sullivan J, et al. Impact of an early mobility program in the hematologic oncologic PICU setting. Crit Care Med 2021;49.
- Ganu S, Keeley S, Muir C, Maki K, Allan K, Tsiros M, et al. Early mobilisation in critically ill children: does routine patient screening change practice?. Pediatr Crit Care Med 2021;22.
- Moerman D, Derycke E, Bednarek P, Hickmann C, Houtekie L, Haenecour A, et al. Feasibility and Safety of Early Mobilization in Critically Ill Children 2018;8.
- Roeseler J, Sottiaux T, Beduneau G, Bialais E, Bradai N, Castelain V, et al. Management of early mobilisation (including electrostimulation) in adult and pediatric patients in the intensive care unit. [French]. Reanimation 2013;22:207-18.
- Remondini R, Valerio N, Barcellos PG, do Prado C, Santos E. Early Mobilization in Children Underinvasive and Noninvasive Positive Pressure Ventilation: Description and Preliminary Results of a New Protocol 2012.
- Redivo J, Nelliot A, Colleti Junior J, Kudchadkar S. Physical rehabilitation in Brazilian pediatric ICUS: a multicenter point prevalence study. Pediatr Crit Care Med 2021;22:61-.
- Carlisi E, Pavese C, Mandrini S, Carenzio G, Dalla Toffola E. Early rehabilitative treatment for pediatric acute disseminated encephalomyelitis: case report. Eur J Phys Rehabil Med 2015;51:341-3.
- Tsuboi N, Nozaki H, Ishida Y, Kanazawa I, Inamoto M, Hayashi K, et al. Early mobilization after pediatric liver transplantation. J Pediatr Intensive Care 2017;6:199-205.
- Takaada K, Kikuchi N, Sashika H. Effectiveness of early phase rehabilitation for pediatric and adolescent traumatic brain injury patients in the critical care and emergency unit. Brain Inj 2012;26.
- Amao R, Imamura T, Sawada Y, Endo S, Ozaki S, Okamura K, et al. Experiences with aggressive cardiac rehabilitation in pediatric patients receiving mechanical circulatory supports. Int Heart J 2016;57:769-72.
- Van Den Adel T, Hoekstra S, Steenhorst J, De Heer M, Floor P, Ista E, et al. Quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care Med 2021;22.
- Coskun-Benlidayi I, Basaran S, Gul-Mert G, Guzel R. Early rehabilitation of a child with intensive care unit acquired weakness secondary to membranoproliferative glomerulonephritis: a case report. Turk J Pediatr 2015;57:422-5.
- Ergene T, Karadibak D. Early physiotherapy program in pediatric liver transplant recipients. Fizyoterapi Rehabilitasyon 2018;29.
- Sargent S, Sharp K, Hills J, Johnstone B. Paediatric In-bed Cycling: A Safety and Feasibility Evaluation in Intensive Care 2017;5.
- McGibbon L, Johnstone B, Hills J, Sharp K. Mobilising within the Paediatric Intensive Care Unit – A Service Evaluation 2017;5.
- Carter L, Woyda J, Shkurka E. GOSH Go! a service evaluation investigating early rehabilitation on cardiac intensive care (CICU). Arch Dis Child 2020;105.
- Wood A, Khan U, Piper I, Van Dijke M, Lenathen C, Reilly L, et al. Sparking paediatric activity in critical care Edinburgh (S.P.A.C.E): how staff perceptions and current practice shape the development of a novel early mobilisation programme. Pediatr Crit Care Med 2021;22.
- Ista E, Scholefield B, Manning J, Harth I, Gawronski O, Bartkowska-Sniatkowska A, et al. Physical rehabilitation in critically ill children: a European point prevalence study. Pediatr Crit Care Med 2021;22.
- Choong K, Awladthani S, Khawaji A, Clark H, Borhan A, Cheng J, et al. Canadian Critical Care Trials Group . Early exercise in critically ill youth and children, a preliminary evaluation: the wEECYCLE pilot trial. Pediatr Crit Care Med 2017;18:e546-54.
- Remondini R, Valerio N, Barcellos PG, Do Prado C, Santos E. Early Mobilization in Children under Invasive and Noninvasive Positive Pressure Ventilation: Description and Preliminary Results of a New Protocol 2012;185.
- McGibbon L, Johnstone B, Hills J, Sharp K. Mobilising within the paediatric intensive care unit-a service evaluation 2017;5.
- Child H, Scholefield B, Menzies J. An early evaluation of the utility of the MOTOmed supine bike in a UK paediatric intensive care (PIC). Pediatr Crit Care Med 2018;19.
- Fink EL, Maddux AB, Pinto N, Sorenson S, Notterman D, Dean JM, et al. Pediatric Outcomes STudies after PICU (POST-PICU) Investigators of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) . A core outcome set for pediatric critical care. Crit Care Med 2020;48:1819-28.
- Jedrzejewska A, Dobosiewicz K, Ickowicz I, Majka W, Flak M, Szota M, et al. [Rehabilitation of children after craniocerebral injuries with particular attention paid to the reflex stimulation Powiertowski’s method. Preliminary study]. Wiad Lek 2009;62:3-10.
- Tepas JJ, Leaphart CL, Pieper P, Beaulieu CL, Spierre LR, Tuten JD, et al. The effect of delay in rehabilitation on outcome of severe traumatic brain injury. J Pediatr Surg 2009;44:368-72.
- Joyce CL, Taipe C, Sobin B, Spadaro M, Gutwirth B, Elgin L, et al. Provider beliefs regarding early mobilization in the pediatric intensive care unit. J Pediatr Nurs 2018;38:15-9.
- Hatem TP, Lira PI, Mattos SS. The therapeutic effects of music in children following cardiac surgery. J Pediatr 2006;82:186-92.
- Melchers P, Maluck A, Suhr L, Scholten S, Lehmkuhl G. An early onset rehabilitation program for children and adolescents after Traumatic Brain Injury (TBI): methods and first results. Restor Neurol Neurosci 1999;14:153-60.
- Cameron S, Ball I, Cepinskas G, Choong K, Doherty TJ, Ellis CG, et al. Early mobilization in the critical care unit: a review of adult and pediatric literature. J Crit Care 2015;30:664-72.
- Connolly B, O’Neill B, Salisbury L, Blackwood B. Enhanced Recovery After Critical Illness Programme Group . Physical rehabilitation interventions for adult patients during critical illness: an overview of systematic reviews. Thorax 2016;71:881-90.
- Cuello-Garcia CA, Mai SHC, Simpson R, Al-Harbi S, Choong K. Early mobilization in critically ill children: a systematic review. J Pediatr 2018;203:25-33.e6.
- Walker T, Kudchadkar SR. Early mobility in the pediatric intensive care unit: can we move on?. J Pediatr 2018;203:10-2.
- Hills J, Sharp K, Johnstone B. Move on ventilation early (MOVE): an early mobility quality improvement initiative. Pediatr Crit Care Med 2018;19.
- Mayring P. Qualitative content analysis. Forum Qualitative Sozialforschung Forum: Qual Soc Res 2000;1. http://nbn-resolving.de/urn:nbn:de:0114-fqs0002204 (accessed September 2020).
- Bengtsson M. How to plan and perform a qualitative study using content analysis. NursingPlus Open 2016;2:8-14.
- Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398-405.
- Burnard P. A method of analysing interview transcripts in qualitative research. Nurse Educ Today 1991;11:461-6.
- PICANet . Paediatric Intensive Care Audit Network National Annual Report 2016–2018 2019. www.picanet.org.uk/annual-reporting-and-publications/ (accessed 15 September 2020).
- Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616-20.
- Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP) . PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med 2013;41:1761-73.
- Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983;249:1743-5.
- Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, et al. SANDWICH Collaborators . Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA 2021;326:401-10.
- PICANet 2022. www.picanet.org.uk/wp-content/uploads/sites/25/2022/01/PICANet-2021-Annual-Report_v1.0-13Jan2022-2.pdf (accessed 28 March 2022).
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ (Clin Res Ed) 2021;374.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ (Clin Res Ed) 2008;337.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ (Clin Res Ed) 2000;321:694-6.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17.
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10.
- Johnston M, Carey RN, Connell Bohlen LE, Johnston DW, Rothman AJ, de Bruin M, et al. Development of an online tool for linking behavior change techniques and mechanisms of action based on triangulation of findings from literature synthesis and expert consensus. Transl Behav Med 2020;11:1049-65.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17.
- Bradbury K, Morton K, Band R, van Woezik A, Grist R, McManus RJ, et al. Using the person-based approach to optimise a digital intervention for the management of hypertension. PLOS ONE 2018;13.
- Duffett M, Cook DJ, Strong G, Lee JH, Kho ME. The effect of COVID-19 on critical care research during the first year of the pandemic: a prospective longitudinal multinational survey. MedRxiv 2021. https://doi.org/10.1101/2020.10.21.20216945.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Rapley T. Qualitative Research. London: Sage; 2011.
- O’Connor G, Marino LV, Tume LN, Stewart A, Gates S, Lanigan J, et al. Research priorities for pediatric intensive care nutrition within the United Kingdom: a National Institute of Health Research James Lind Alliance Priority Setting Partnership. Crit Care Explor 2022;4.
- Semple D, Howlett MM, Strawbridge JD, Breatnach CV, Hayden JC. A systematic review and pooled prevalence of delirium in critically ill children. Crit Care Med 2022;50:317-28.
- Ista E, Nydahl P. Delirium in adult and paediatric ICU patients: what is the way forward?. Nurs Crit Care 2021;26:147-9.
- Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in children – the PICS-p framework. Pediatr Crit Care Med 2018;19:298-300.
- Piva TC, Ferrari RS, Schaan CW. Early mobilization protocols for critically ill pediatric patients: systematic review. Rev Bras Ter Intensiva 2019;31:248-57.
- Choong K, Canci F, Clark H, Hopkins RO, Kudchadkar SR, Lati J, et al. Practice Recommendations for early mobilization in critically ill children. J Pediatr Intensive Care 2018;7:14-26.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8.
- World Health Organization . International Classification of Functioning, Disability and Health: ICF 2001.
- Clarissa C, Salisbury L, Rodgers S, Kean S. Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care 2019;7.
- Amidei C. Mobilisation in critical care: a concept analysis. Intensive Crit Care Nurs 2012;28:73-81.
- Weinreich M, Herman J, Dickason S, Mayo H. Occupational therapy in the intensive care unit: a systematic review. Occup Ther Health Care 2017;31:205-13.
- Costigan FA, Duffett M, Harris JE, Baptiste S, Kho ME. Occupational therapy in the ICU: a scoping review of 221 documents. Crit Care Med 2019;47:e1014-21.
- Bittencourt ES, Moreira PS, Paixão GM, Cardoso MM. The role of the occupational therapist in the Intensive Care Unit: a systematic review. Cad Bras Ter Ocup 2021;29:1-21.
- Lavallée A, De Clifford-Faugère G, Garcia C, Fernandez Oviedo AN, Héon M, Aita M. Part 1: narrative overview of developmental care interventions for the preterm newborn. J Neonatal Nurs 2019;25:3-8.
- Khurana S, Kane AE, Brown SE, Tarver T, Dusing SC. Effect of neonatal therapy on the motor, cognitive, and behavioral development of infants born preterm: a systematic review. Dev Med Child Neurol 2020;62:684-92.
- Royal College of Occupational Therapists . Occupational Therapy in Neonatal Services and Early Intervention - Practice Guideline 2017. https://www.rcot.co.uk/practice-resources/rcot-publications/downloads/neonatal-services (accessed 15 September 2020).
- Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care 2014;18.
- Sakai T, Hoshino C, Okawa A, Wakabayashi K, Shigemitsu H. The safety and effect of early mobilization in the intensive care unit according to cancellation criteria. Prog Rehabil Med 2020;5.
- Fink EL, Jarvis JM, Maddux AB, Pinto N, Galyean P, Olson LM, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Long-term Outcomes Subgroup Investigators, and Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) . Development of a core outcome set for pediatric critical care outcomes research. Contemp Clin Trials 2020;91.
- Duffett M, Cook DJ, Strong G, Binf M, Hau Lee J, Kho ME. The pre-print sever for health sciences. The effect of COVID-19 on critical care research during the first year of the pandemic: A prospective longitudinal multinational survey. MedRxiv 2020. https://doi.org/10.1101/2020.10.21.20216945.
- Manning JC, Latour JM, Curley MAQ, Draper ES, Jilani T, Quinlan PR, et al. OCEANIC Study Investigators . Study protocol for a multicentre longitudinal mixed methods study to explore the Outcomes of ChildrEn and fAmilies in the first year after paediatric Intensive Care: the OCEANIC study. BMJ Open 2020;10.
- Drury NE, Menzies JC, Taylor CJ, Jones TJ, Lavis AC. Understanding parents’ decision-making on participation in clinical trials in children’s heart surgery: a qualitative study. BMJ Open 2021;11.
- Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med 2017;45:321-30.
- Finch TL, Girling M, May CR, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: part 2 - validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18.
- Gale N, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health. BMC Med Res Methodol 2013;13:1-8.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44.
- Green J, Thorogood N. Qualitative Methods for Health Research. Thousand Oaks: Sage; 2009.
- DeSantis L, Ugarriza DN. The concept of theme as used in qualitative nursing research. West J Nurs Res 2000;22:351-72.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Gale N, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health. BMC Med Res Methodol 2013;13.
- Ritchie J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Kudchadkar SR. 2022. https://clinicaltrials.gov/ct2/show/NCT03860168 (accessed 1 April 2022).
- Blackwood B, Agus A, Boyle R, Clarke M, Hemming K, Jordan J, et al. Paediatric Intensive Care Society Study Group (PICS-SG) . Sedation AND Weaning In Children (SANDWICH): protocol for a cluster randomised stepped wedge trial. BMJ Open 2019;9.
- Chiche JD, Angus DC. Testing protocols in the intensive care unit: complex trials of complex interventions for complex patients. JAMA 2008;299:693-5.
- Bagley HJ, Short H, Harman NL, Hickey HR, Gamble CL, Woolfall K, et al. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials – a work in progress. Res Involv Engagem 2016;2.
- Hanley B, Bradburn J, Barnes M, Evans C, Goodare H, Kelson M, et al. Involving the Public in NHS, Public Health and Social Care Research: Briefing Notes for Researchers. Hampshire: INVOLVE; 2004.
- Forbat L, Hubbard G, Kearney N. Patient and public involvement: models and muddles. J Clin Nurs 2008;18:2547-54.
- Menzies J, Morris K, Duncan H, Marriott J. Patient and public involvement in Paediatric Intensive Care research: considerations, challenges and facilitating factors. Res Invol Engagem 2016;2. https://researchinvolvement.biomedcentral.com/articles/10.1186/s40900-016-0046-7#citeas (accessed 15 September 2020).
- Hoddinott P, Pollock A, O’Cathain A, Boyer I, Taylor J, MacDonald C, et al. How to incorporate patient and public perspectives into the design and conduct of research. F1000Research 2018;7.
- Bioethics NC. Children and Clinical Research: Ethical Issues. London: Nuffield Council on Bioethics; 2015.
- Conroy E, Harman N, Lane JA, Lewis SC, Murray G, Norrie J, et al. Trial Steering Committees in randomised controlled trials: a survey of registered clinical trials units to establish current practice and experiences. Clin Trials 2015;12:664-76.
- Dudley L, Gamble C, Allam A, Bell P, Buck D, Goodare H, et al. A little more conversation please? Qualitative study of researchers’ and patients’ interview accounts of training for patient and public involvement in clinical trials. Trials 2015;16.
- Kleiber N, Tromp K, Mooii M, van de Vathorst S, Tibboel D, de Wildt S. Ethics of drug research in the Pediatric Intensive Care Unit. Paediatr Drugs 2015;17:43-5.
- Tume L, Preston J, Blackwood B. Parents’ and young people’s involvement in designing a trial of ventilator weaning. Nurs Crit Care 2015;21:e10-18.
- CONNECT Advisory Group . The CONNECT Study. Research Without Prior Consent (Deferred Consent) in Trials Investigating the Emergency Treatment of Critically Ill Children: CONNECT Study Guidance 2015.
- Woolfall K, Frith L, Dawson A, Gamble C, Lyttle MD. Fifteen-minute consultation: an evidence based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Arch Dis Child Educ Pract 2016;101:49-53.
- Lyttle M, O’Sullivan R, Hartshorn S, Bevan C, Cleugh F, Maconochie I, et al. LETTER Pediatric Emergency Research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child 2014:1-2.
- Menzies JC, Owen S, Read N, Fox S, Tooke C, Winmill H. COVID-19: challenges and opportunities for research nursing and nursing research on paediatric intensive care. Nurs Crit Care 2020;25:321-3.
- INVOLVE . Briefing Notes for Researchers 2012.
- NIHR . Generation R Young People Improving Research: 2013 Meeting Report 2014.
- Birmingham Women’s and Children’s NHS Foundation Trust . Young Persons’ Advisory Group 2022. https://bwc.nhs.uk/ypag/ (accessed 28 March 2022).
- Manning JC, Latour JM, Curley MAQ, Draper ES, Jilani T, Quinlan PR, et al. OCEANIC Study Investigators . Study protocol for a multicentre longitudinal mixed methods study to explore the Outcomes of ChildrEn and fAmilies in the first year after paediatric Intensive Care: the OCEANIC study. BMJ Open 2020;10.
- Menzies JC. Thesis: Designing and Conducting Feasible and Acceptable Pharmacokinetic Research in Critically Ill Children: A Mixed Methods Study 2018.
- South A, Hanley B, Gafos M, Cromarty B, Stephens R, Sturgeon K, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: findings from ten case studies. Trials 2016;17.
- NIHR 2018. www.nihr.ac.uk/documents/reward-and-recognition-for-public-contributors-a-guide-to-the-payment-of-fees-and-expenses/12248 (accessed 16 February 2022).
- University of Oxford . Guide for Researchers Working With Patient and Public Involvement (PPI) Contributors 2017.
- NIHR 2022. www.invo.org.uk/resource-centre/learning-and-development/public-reviewing-with-the-national-institute-for-health-research-nihr/ (accessed 28 February 2022).
- Buck D, Gamble C, Dudley L, Preston J, Hanley B, Williamson P, et al. EPIC Patient Advisory Group and EPIC Patient Advisory Group . From plans to actions in patient and public involvement: qualitative study of documented plans and the accounts of researchers and patients sampled from a cohort of clinical trials. BMJ Open 2014;4.
- Staley K. ‘Is it worth doing? Measuring the impact of patient and public involvement in research’. Rese Invol Engagem 2015;1.
- NIHR 2020. www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (accessed 28 March 2022).
- Kanthimathinathan H, Scholefield BR. Dilemmas in undertaking research in paediatric intensive care. Arch Dis Child 2014;99:1043-9.
- Choong K, Dufett M, Cook DJ, Randolph AG. The impact of clinical trials conducted by research networks in pediatric critical care. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2016;17:837-44.
- Drury NE, Patel AJ, Oswald NK, Chong CR, Stickley J, Baron DJ, et al. Randomized controlled trials in children’s heart surgery in the 21st century: a systematic review. Eur J Cardiothorac Surg 2018;53:724-31.
Appendix 1
Trial oversight committee
An independent trial oversight committee was appointed by the NIHR in keeping with standard structure and definitions. The Trial Steering Committee was responsible for overall supervision on behalf of the Sponsor and Funder, and ensured that it was conducted in accordance with the rigorous standards set out in the Department of Health’s Research Governance Framework for Health and Social Care and the Guidelines for Good Clinical Practice. The Trial Steering Committee comprised the Chief Investigator plus independent members (including independent PPIE representatives).
Dr Shane Tibby (Consultant in PICU), Chair, Clinician, Trialist.
Prof Mark Peters (Professor of Paediatric Intensive Care), Clinician, Trialist.
Dr Kerry Woolfall (Senior Lecturer Health Services Research), Qualitative Researcher.
Ms Suzanne Dottin-Payne (Parent representative), PPIE representative.
Prof Jim Lewsey (Professor of Medical Statistics), Statistician.
Data monitoring and ethics committee (DMEC)
An independent DMEC was appointed by the NIHR in keeping with standard structure and definitions. An independent DMEC monitored recruitment and retention, adherence with the intervention and patient safety.
Prof Bronagh Blackwood (Professor), Chair, Clinician, Trialist.
Dr ClionaMcDowell (Senior Statistician), Statistics.
Dr Siva Oruganti (Consultant in PICU), Clinician.
Appendix 2
Scoping review search strategy
MEDLINE
Database: Ovid MEDLINE(R) and In-Process and Other Non-Indexed Citations < 1946 to 12 December 2019 (repeated 1 November 2021).
-
exp Pediatrics/or Paediatric.mp. (106506);
-
Paediatrics.mp. (7305);
-
Pediatric.mp. (276918);
-
1 or 2 or 3 (356323);
-
Intensive Care Units.mp. or exp Intensive Care Units/(91849);
-
Critical Illness.mp. or exp Critical Illness/(31374);
-
Critical Care.mp. or exp Critical Care/(73300);
-
(critical* adj3 (ill* or care*)).tw. (71316);
-
intensive care.tw. (132554);
-
critical care.tw. (25491);
-
icu.ab,ti. (50876);
-
‘intensive care’.ab,ti. (132554);
-
(critical* adj3 (ill* or care)).ab,ti. (70706);
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 (243400);
-
4 and 14 (23482);
-
Physical Therapy.mp. or exp Physical Therapy/(48742);
-
Physical Therapy Modalities.mp. or exp Physical Therapy Modalities/(147898);
-
Exercise Therapy.mp. or exp Exercise Therapy/or Exercise Movement Techniques/(50084);
-
Occupational Therapy.mp. or exp OT/(16730);
-
exp Rehabilitation/or rehabilitation.mp. (504279);
-
physiotherapy.mp. (18150);
-
Early Ambulation.mp. or exp Early Ambulation/(3460);
-
Early Mobilization.mp. or Early Mobilisation/(4999);
-
Chest PT.mp. or exp Chest PT/(802);
-
(therap* adj3 (physical* or exercise* or occupation* or respiratory or music or animal)).ab,ti. (50992);
-
((cycle or bicycle) adj1 ergomet*).ab,ti. (11390);
-
((bed or ‘daily living’) adj3 activity).ab,ti. (2394);
-
‘physical therapy’.ab,ti. (16266);
-
‘Physical Therapy Modalities’.ab,ti. (134);
-
16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 (553008);
-
(Early or earlier or accelerat* or acute or immediate*).mp. (3288276);
-
15 and 30 and 31 (228).
| Author, year, design, centre, country | Population (n, inclusion/exclusion criteria); |
Intervention (definition of ERM, content) | Primary/co-primary and secondary outcome measures | Other findings |
|---|---|---|---|---|
| Study design | Timing | |||
| ≥24 hours of admission | ||||
| Choong 2014a11 Prospective pilot study Single centre Canada |
N = 33 CYP aged 1–18 years admitted with a minimum of 24-hour PICU stay Exclusion criteria Patients cardiorespiratory instability (e.g. unstable airway, progressive shock), an adequate baseline level of function, imminent death, and contraindication to mobilisation |
Single-component intervention In-bed cycling: Ex N’Flex EF-300 for children 3–7 years and MOTOmed Letto2 for patients 8–17 years Day 1: Minimum 10 (safety/tolerance) to 20 minutes Day 2: 20 minutes × 2 days Usual care: Physical therapy Patient intolerance, i.e. pain and/or discomfort, and safety (tube or catheter dislodgement) |
Primary/co-primary outcome(s) Physiological/clinical: cardiorespiratory response via physiological measurements at baseline, during and after ERM Degree of UL and LL extremity movement using Actigraph GT3X accelerometers attached to the participants’ wrists and ankles Methodological: feasibility, safety (AEs such as cardiorespiratory instability, tube dislodgment, pain, discomfort or injury) @24-hours |
Increase in in-bed cycling (mean ± SD: 266.47 ± 166.12) vs. during non-intervention times (mean ± SD: 20.94 ± 15.26, counts/20 minutes, p < 0.001) different to baseline had no impact on UL activity (mean ± SD: 27.3 ± 12.55 vs. 51.32 ± 23.68 counts/20 minutes, p < 0.05) |
| Curry 202125 Quality improvement project Quaternary care, paediatric onco-critical care unit, Single centre USA |
Pre-implementation period, N = 294 Post-implementation period, N = 272 Children ages 1 day to 21 years who required ICU or step-down admission were eligible for early mobility. Exclusion criteria included patients with an open chest or abdomen, unstable fractures, or with provider-placed medical orders specifying otherwise. A retrospective review of the medical records for all critical care and step-down admissions from January 2019 through June 2020 was performed; divided into two 9-month periods: pre-BRAVE implementation (January–September 2019) and post-BRAVE implementation (October 2019–June 2020) Early mobility activity was defined as any activity intended to maintain or restore musculoskeletal strength and function performed within 72 h of admission to the ICU |
Multicomponent interventions ABCDEF ICU liberation bundle (spontaneous awakening and breathing trials; choice of sedation and analgesia, delirium prevention, surveillance and management; early mobilisation and exercise programmes with or without adjuncts; family engagement and empowerment) Early mobilisation – within 72 hours of admission from baseline of 21% to 80% within 9 months of implementation delirium. A positive delirium screen was defined as a CAPD score ≥9 Activities could be passive or active; included in-bed and out-of-bed interventions given by rehabilitation therapists, nursing staff or family members/caregivers. Level of activity was discussed during daily medical rounds, determined by patient’s stability and the amount of medical support required, and documented |
Primary/co-primary outcome(s) Physiological/clinical: cardiorespiratory response via physiologic measurements at baseline, during and after ERM Methodological: proportion of patients with a physician- or AP consult orders for OT and/or PT within 72 hours of admission, who received an early mobility activity provided by rehabilitative services, and the percentage of positive delirium screens after 24 hours of ICU admission Early mobility outcomes were evaluated in patients with LOS >48 hours and all LOS. Secondary outcome measures: type of early mobilisation activities performed, perceived and identified barriers to early mobility, deferral and AEs during rehabilitation interventions, and doses of sedative and analgesic infusions |
Positive delirium screen in 43% of patients pre-implementation and 37% post-implementation time frame (p = 0.46). Patients who received at least one out-of-bed activity with PT/OT within 72 hours of admission increased (16–29%, p < 0.001) Number of activities deferred increased because of caregiver refusal, conflict with a diagnostic test or procedure, and staff concern about the patient’s clinical status. 47/51 (92%) of staff reported positive impact on their patients and caregivers due to early mobility. 33 (65%) identified a collaborative approach as helpful. 43/51 (84%) reported moderate or full support from PICU during implementation, and 35/51 (69%) felt that they could prioritise and actively incorporate mobility as part of their patients’ daily plan |
| ≥48 hours PICU admission | ||||
| Abdulsatar 20137 Prospective single-arm RCT Single centre |
N = 12 CYP aged 3–18 years admitted minimum 48-hour PICU stay. Exclusion criteria Anticipated death or withholding life-sustaining therapy, physical inability to mobilise, cardio-respiratory instability, language barrier and inability to comprehend instructions or perform the intervention (i.e. deep sedation or severe cognitive) or functional disability, poor POPC and PCPC |
Single-component intervention Interactive virtual reality game using Nintendo Wii Boxing for at least 10 minutes twice a day for 2 days Not defined. Probably at 48 hours PICU admission |
Primary/co-primary outcome(s) Methodological: feasibility and safety over 8 months Secondary outcome(s) Clinical/PROMs: vital signs; UL activity measured using Actigraph GT3X accelerometers; Muscle strength using the dynamometer; Caregiver and participant satisfaction using self-administered questionnaire with a 7-point Likert scale |
Clinical outcomes were worse (mean ± SD change of 1.08 ± 1.0, p = 0.02) among two-thirds of patients who received ERM, although no activity-related AEs were reported No improvement in grip strength or physiological status, despite increased activity levels (mean ± SD, UL activity pre: 9.36 ± 4.12 vs. post: 57.12 ± 46.60 counts) and higher carer satisfaction |
| Choong 201720 Pilot RCT – randomised 2 : 1 Single centre Canada |
N = 30 CYP aged 3–17 years, admitted to 12-bed tertiary care, medical-surgical PICU Immobilised at time of screening and predicted at least an additional 48-hour PICU stay Exclusion criteria:
|
Single-component intervention In-bed cycling: paediatric cycle ergometers 30 minutes/day, 5×/week + PT until functional independence achieved for at least 2 days or max. of 7 days of cycling Control: Usual care PT or OT in-bed cycling for 30 minutes/day |
Primary/co-primary outcome(s) Physiological/clinical/PROMs: PICU-acquired morbidities, duration of MV, PICU and hospital LOS, 30-day mortality Functional outcome measured by PEDI-CAT speedy version at baseline, PICU discharge and 1-month post PICU discharge Methodological: feasibility (enrolment of 75% of eligible patients, accrual rate of >75% and 30-day follow-up rate of >75%) Safety/AEs related to mobilisation |
No difference between arms (0.17, IQR: −0.01–0.36) among 24/30 patients (80%) who developed new functional difficulties in PICU (PEDI-CAT). In the usual-care arm 7/10 (70%) with median of 0.4 (IQR: 0.3–0.6), and 17/20 (85%) in the cycling arm (median: 0.6, IQR: 0.4–0.7) 10% of patients recovered functional ability at 1 month, and mobility was the slowest outcome to return |
| Fink 201943 RCT Multicentre USA |
N = 58 CYP aged 3–17 years admitted with acute diagnoses: traumatic brain injury, cardiac arrest, stroke, brain mass (e.g. tumour, arteriovascular malformation), or CNS infection/inflammation Minimum 48-hour PICU stay were enrolled ≤72 hours of admission, English-speaking parents/guardians, expected PICU stay ≥2 days Exclusion criteria Children with a do not resuscitate status, PCPC score 4–5 prior to diagnosis, or imminent death within 24 hours |
Multicomponent interventions Early assessment and rehabilitation including PT, OT, SLP or usual care Intervention: PT, OT and SLT consultations placed within 72 hours of PICU admission, n = 26 Usual care: PT, OT and/or SLT consultations per the treating team, n = 32 |
Primary outcome(s) Methodological: feasibility and safety of PICU-based PT, OT and SLT therapies Secondary outcome(s) Physiological/clinical/PROMs: physical, cognitive, emotional, family functioning, QoL, 6-month hospital discharge, PCPC and POPC and FSS, VABS scores Methodological: timing of PT initiation, OT, SLT consultation Process: Family disposition at hospital discharge and PICU and hospital LOS |
PT was delivered 80% of the time in the usual-care arm and 100% of the time among patients receiving early protocolised ERM Patients receiving early protocolised rehabilitation had less post-PICU rehabilitation |
| Prospective cohorts | ≥48 hours of admission | |||
| Choong 201519 Prospective observational study Single centre Canada |
Age: over 12 months – 17 years. with developing functional skills, presence of at least 1 organ dysfunction on admission, limited mobility or bed rest during the first 48 hours of PICU admission, a minimum 48 hour PICU LOS Exclusion criteria Children directly transferred from a neonatal PICU prior to ever being discharged home, patients already mobilising well or at baseline functional status at the time of screening/or caregivers with an English language barrier and prior enrolment |
Single-component intervention In-bed cycling |
Primary outcome(s) Methodological: feasibility (protocol violation, withdrawal and follow-up rates) Secondary outcome(s) Clinical: POPC, PCPC, ventilator-free days, mortality, PICU and hospital LOS, and immobility morbidities (new-onset joint contractures, pressure ulcers and PICU-AW) Functional outcome (FSS), task performance measured by CAS. Factors influencing participation in mobility and self-care tasks in children ≥ 6 months to 7.5 years Age-appropriate exercise tolerance (>4 years) at hospital discharge, 3 and 6 months discharge Process: parental or caregiver stress measured via PSI at 3 months post discharge |
ERM was feasible. At 3 and 6 months, functional ability measured using PEDI improved among 28% and 42% of the study cohort, respectively. 22% of those with a pre-existing chronic condition and 14% with functional limitations returned to baseline levels at 6 months. 60% of previously healthy children and 58% of children with normal baseline function regained full functional abilities. Moreover, the overall mortality rate was 3/33 (9%), and almost a third of patients (63%) were readmitted within 6 months of PICU discharge |
| Prospective cohorts | ≥72 hours admission | |||
| Choong 202124 cross-sectional, 2-day point prevalence study Multicentre Canada |
N = 128 patients across 16 PICU’s; day 1 N = 9 patients across 11 PICU’s; day 2 Patients with a PICU LOS (LOS) ≥72 hours as of 7 am on each point prevalence day were included PICUs were eligible to participate if they: |
PT and OT documented assessments in 72 hours of PICU admission. Mobility events were collected prospectively: (1) types and timing of mobility; (2) providers or assistance with mobility; (3) barriers to mobilisation |
Primary outcome(s) Process: prevalence and nature of mobilisation practices in critically ill children while in the PICU Secondary outcome(s) |
Highest level of mobility (HLM) achieved in children with ETTs in situ were most commonly not actively mobilised (31/50; 62.0% patient-days) but were mobilised out-of-bed on 18/50 patient-days (36%) Out-of-bed mobility (48/56; 85.7% patient-days) was the most common in non-MV patients Out-of-bed mobility (being held by a parent or PICU staff (37/87; 42.5%)) was the most common activity under 3 years (71/100; 71%), whereas no active mobility predominated in older children (18/37; 48.6% patient-days) |
| (1) cared for mechanically ventilated infants and children and (2) were located in a distinct physical space dedicated to paediatric patients. Institutional review board approval was obtained at all sites with a waiver of informed consent for the collection of de-identified data |
(4) potential AEs Mobility was defined as any activity involving physical movement of patient, except routine care and was categorised as follows: (1) no active mobility (2) in-bed mobility (3) edge of bed mobility (4) out-of-bed mobility |
Process: factors associated with out-of-bed mobilisation and rehabilitation therapist-provided mobility, the rate of potential AEs, and perceived barriers to mobilisation Rehabilitation therapists were physiotherapists (PT) or occupational therapists (OT) Therapist-provided mobility was identified when a patient received ≥1 mobility event facilitated by a therapist on the study day | Nurses (286/387; 73.9%) commonly facilitated mobility alone, with family or other PICU staff and family facilitated 188 mobility events (48.6%). Children under 3 years were most commonly held (36/71; 50.7%) or were transferred from bed to chair (22/71; 31.0%). Children ≥3 years participated in transfers (6/16; 37.5%), pre-ambulation (4/16; 25.0%), and ambulation activities (3/16; 18.8%) | |
| Barriers were cardiovascular instability (n = 18; 14.9%), excessive sedation (n = 13; 10.7%), medical contraindication (n = 13; 10.7%), postoperative restrictions (n = 13; 10.7%) and lack of supportive personnel/equipment (5.0%) and invasive MV (4.1%) | ||||
| Cui 201714 Prospective study Single centre USA |
Children between ages 2 weeks and 18 years, PICU LOS ≥3 days over 3 months | Multicomponent intervention PT, OT screening with the goal of formulating a rehabilitation plan within 72 hours of admission, followed by referral when indicated |
Primary outcome(s) Process: PICU PT/OT consultation and treatment consultation timing, therapy type and duration and reasons for deferral Secondary outcome(s) Process: factors associated with PT/OT and patient outcome |
Feasibility and safety of interventions were demonstrated |
| Ista 202142 Multicentre across 15 countries in Europe Netherlands, France, Spain, Germany, Italy, UK |
N = 722 Two-day cross-sectional point prevalence study (29 May 2018, and 6 November 2018) PICUs from European countries were eligible to participate if they met the following criteria: (1) provide care for mechanically ventilated infants and children and (2) located in a distinct physical space within a paediatric hospital |
Records of PT and OT consultation and treatment documentation for the first 72 hours of PICU admission were abstracted Out-of-bed mobility was defined as ‘a transfer from bed to chair, held by family or staff, mat play, standing, or walking’ Activities such as passive motion, sitting in bed, and bath were defined as in-bed mobility Mobility events were any single activity or clustered activities involving the child’s physical movement, except for routine care. Barriers and potential safety events were selected from a pre-specified list |
Primary outcome(s) Process: ‘therapist-provided mobility’, defined as at least one mobility event performed by a PT or OT on the study day Secondary outcome(s) Process: out-of-bed mobility, barriers to mobilisation and potential safety events |
78% of children <3 years received out-of-bed mobility compared to 22% of patient’s ≥3 years (p < 0.001). Non-MV patients were more likely than MV patients to achieve out-of-bed mobility (70% vs. 30%; p < 0.001). MV did not receive more therapist-provided mobility than those who were not (42% vs. 36%; p = 0.181) |
| Out-of-bed activity was being held by a parent or a nurse (n = 519), bed-to-chair transfer without standing (n = 120) and mat play (n = 51) Patients ≥3 years received more PT- and/or OT-provided mobility, 50% (68/137) compared with age <3 years, 35% (111/319) (p = 0.003) Nurses (n = 584, 46%) provided mobilisation alone; with family (17%) or with PT or OT (6%); and family alone (16%) | ||||
| Mobilisation events did not occur on 115/456 patient-days (25%) in MV patients (75 of 115 patient days, 65%). Among MV children, passive range of motion (age <3, 23%; age ≥3, 23%) and being held by family/nurse (age <3, 27%; age ≥3, 22%) were common. Non-MV children held by family/nurse (44%); while bed-to-chair transfer was 17% in ≥2-year-olds | ||||
| Barriers were cardiovascular instability (n = 47, 10%), over sedation (n = 39, 9%), and medical contraindication (n = 37, 8%) | ||||
| Kudchadkar 202018 Cross-sectional point prevalence study Multicentre study USA |
N = 82 US PICUs on 2 days (the 9 November 2017, and the 12 February 2018) (1) PICU LOS ≥ 72 hours at 7 am on each point prevalence day (2) mechanically ventilated infants and children and (3) located in PICU |
Multicomponent interventions PT and OT consultation and treatment documentation for the first 72 hours of PICU admission |
Primary outcome(s) Process: PT mobility of ≥1 mobility event performed by PT/OT on the study day. Secondary outcome(s) Process: mobilisation rates, type and timing of mobility events, barriers and facilitators of mobilisation, AEs |
Among children who did not require MV, children <3 years old were frequently cuddled (51%) by family or nurses, while older children were commonly ambulated (29%) |
| Tsuboi 201732 Prospective pre-post study Single centre Japan |
N = 57 (23 before EM and 34 following EM) CYP ≤16 years who received liver transplantation |
Multicomponent interventions MDT approach to PICU ERM: PT; bed mobility activities (e.g. bridging, rolling, lying to sitting) 72 hours after transplantation |
Primary outcome(s) Process: PT consultations @ 72 hours after transplantation Secondary outcome(s) Clinical/PROMs: Functional mobility, intubation length, PICU or hospital LOS, mortality Methodological: safety (AEs from ERM) |
Increase in frequency of physical therapy, with more patients achieving their rehabilitation goals. Proportion of patients who received physical therapy pre- and post-ERM periods increased, 11 and 33, respectively. More patients achieve post-intervention goals at 72 hours post-surgery (pre-ERM: 0 vs. post-ERM: 22), irrespective of baseline status and pre-operative hospitalisation |
| Prospective cohorts | Undefined timing of admission | |||
| Colwell 201813 Prospective before-after study Single centre (cardiac and medical-surgical unit) USA |
PICU patients on admission, supported by vasoactive infusions, invasive respiratory support and ECMO, and did not exceed practitioner-ordered activity limitations | Multicomponent interventions Goal-directed mobilisation protocol (PT and OT) over 9 months (3 months implementation period and 6 months post implementation) |
Primary outcome(s) Process: bi-weekly mean mobilisation ratio Secondary outcome(s) Methodological: safety (AEs), adherence, protocol deviation Process: barriers to mobilisation |
Higher adherence among younger patients (p = 0.04), with higher baseline severity of illness (p < 0.001) when mobilisation sessions were goal-directed (p < 0.001) There was no difference in mobilisation rates (pre: p = 0.86 vs. post: p = 0.84) or AEs (2.5%, p = 0.18) between groups |
| Retrospective cohorts | ≥24 hours of admission | |||
| Choong 201212 Single centre Observational pre- and post-study Canada |
N = 91 CYP aged 0–17 years admitted with a minimum 24-hour PICU stay. |
Multicomponent interventions PT, OT, SLP |
Primary/Co-primary outcome(s) Process: proportion and nature of rehabilitation – type, timing and frequency Secondary outcome(s) Process: barriers to PT mobilisation, clinical outcomes of non-mobility/PT, morbidities due to immobility AEs due to PT |
ERM was frequently delivered when evaluated using PT and OT consultations |
| Retrospective cohorts | ≥48 hours of admission | |||
| Alqaqaa 20178 Retrospective study Single centre USA |
N = 29 CYP 2 months to 18 years, requiring invasive and non-invasive MV regardless of patient interface except for nasal cannula and flow support Exclusion criteria Severe disability, coma or vegetative state and brain death |
Multicomponent interventions Algorithm to identify patients suitable for mobilisation (PT, OT, SLP) + training on benefits of mobilisation + education on safety techniques + family advisors’ feedback |
Primary outcome(s) Process: mobilisation rate Secondary outcome(s) Physiological/clinical/PROMs: PICU and hospital LOS, FSS Methodological: safety (incidence rates of AEs) Process: discharge disposition |
Higher mobilisation rates among non-ventilated patients (mean difference of about a day) than no change for mechanically ventilated patients No safety concerns or AEs were identified |
| Betters 201710 Retrospective study Single centre (36-bed medical-surgical PICU) USA |
Consecutive review of admission records of patients who receive early mobilisation (EM) in the PICU between December 2013 and October 2016 Screening to formulate rehabilitation plan within 72 hours of admission, with a referral when indicated Exclusion criteria High-frequency oscillator ventilation, critical airway, deep sedation, neuromuscular blockade, unstable traumatic brain injury, reduced inspired oxygen (FiO2), spinal precautions, haemodynamic instability |
Multicomponent interventions PT, OT (PT and OT co-ordinate with the patient’s nurse, SLP and RT to schedule an optimal time to mobilise the patient) EM sessions last from 10 minutes to an hour |
Primary outcome(s) Process: ERM consultation Secondary outcome(s) Methodological: safety (AEs during EM) |
Higher ERM consultations (median duration 30, IQR: 29–45 minutes) with no major AEs |
| Choong 2014b21 Multicentre Retrospective study Canada |
N = 600 CYP aged 0–17 years. Minimum 24-hour PICU stay in tertiary PICUs |
Multicomponent interventions PT, OT, SLT. Bed mobility activities (e.g. bridging, rolling, lying to sitting); strengthening exercises |
Primary outcome(s) Process: nature and timing of PICU rehabilitation practices Secondary outcome(s) Process: predictors of mobilisation and time to mobilisation |
Children who were older, healthy, admitted during the winter, not mechanically ventilated, receiving neuromuscular blockade or sedatives were more likely to receive mobility therapy |
| Justice 201916 Retrospective pre- and post-study Single centre USA |
Comparison of data from January 2015 to December 2016 (pre-WeeMove) to January 2017 to post-WeeMove for all patients admitted to the CTICU for >48 hours ERM defined as activity (active or passive) within 48 hours of critical illness Exclusion criteria: High frequency of activity was contraindicated |
Multicomponent interventions PT, OT |
Primary outcome(s) Process: OT and PT consultations Secondary outcome(s) Physiological/ clinical/PROMs: PICU LOS Methodological: safety (AEs) Process: staff barriers |
Small improvements in PICU length-of-stay (average LOS pre-WeeMove = 6.25 days vs. post-WeeMove LOS = 5.23 days), and time spent intubating patients (pre-WeeMove was 27.86 hours, post-WeeMove = 25.09 hours) |
| Retrospective cohorts | ≥72 hours of admission | |||
| Wieczorek 201617 Observational pre-post study USA |
N = 200 (100 pre-intervention and 100 post intervention) CYP aged 1 day to 17 years admitted with minimum 72-hour PICU stay Retrospective review: July–August 2014 (pre-implementation phase) and July–August 2015 (post implementation) medical-surgical unit Exclusion criteria: ECMO, open chest or abdomen, unstable fractures, or medical orders specifying alternate activities |
Multicomponent interventions Implementation of a tiered early mobilisation programme. Sleep hygiene promotion and routine delirium screening for all children Increasing mobility levels from level 1 (repositioning) to level 3 (sitting, out of bed activities or ambulation) |
Primary outcome(s) Process: OT/PT consultations; number/types of mobilisation activities on PICU Day 3 Secondary outcome(s) Methodological: adherence (number and reasons activities were stopped), safety (mobilisation-related AEs, inadvertent extubation or line removal) Process: barriers to activities |
Nearly half of the children (48/100) received at least one ERM intervention by day 3 of admission, and the proportion of children receiving at least one in-bed activity increased by 18% (p < 0.001) There was no change in passive mobilisation rates, only an increase in active interventions (26% vs. 57%; p < 0.001), especially among children ≥3 years. There was also a slight increase in the number of children orally intubated post intervention, facilitating ambulation |
| Studies/signalling questions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Abdulsatar 20137 | Serious | Moderate | Moderate | Moderate | Serious | Critical | Low | Moderate | Favours intervention |
| 2. | Alqaqaa 20188 | No information | No information | No information | Serious | No information | No information | Serious | Critical | Unpredictable |
| 3. | Betters 201710 | No information | Low | Low | Moderate | Low | No information | Moderate | Moderate | Favours intervention |
| 4. | Choong 201720 | Moderate | No information | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Towards null |
| 5. | Choong 201519 | Moderate | Low | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Towards null |
| 6. | Choong 2014a11 | No information | No information | Serious | Serious | Serious | Serious | Serious | Serious | Away from null |
| 7. | Choong 2014b21 | Moderate | Moderate | Serious | Moderate | No information | Moderate | Serious | Moderate | Unpredictable |
| 8. | Choong 201212 | Moderate | Moderate | Moderate | Serious | Moderate | Moderate | Serious | Moderate | Unpredictable |
| 9. | Choong 202124 | Low | Low | Moderate | Low | Low | Low | Low | Low | Unpredictable |
| 10. | Colwell 201813 | Serious | Moderate | Moderate | Moderate | No information | Moderate | Moderate | Moderate | Unpredictable |
| 11. | Cui 201714 | Serious | Serious | Serious | Serious | No information | Serious | Serious | Moderate | Unpredictable |
| 12. | Curry 202125 | Serious | Moderate | Moderate | Low | Low | Low | Low | Moderate | Unpredictable |
| 13. | Fink 201943 | Serious | Low | Moderate | Low | Moderate | Moderate | Moderate | Moderate | Unpredictable |
| 14. | Ista 202142 | Low | Low | Moderate | Low | Low | Low | Low | Low | Unpredictable |
| 15. | Justice 201916 | Serious | Moderate | No information | No information | No information | No information | No information | Serious | Unpredictable |
| 16. | Kudchadkar 202018 | Low | Low | Moderate | Low | Low | Low | Low | Low | Unpredictable |
| 17. | Tsuboi 201732 | Moderate | Moderate | Moderate | Moderate | Moderate | No information | Moderate | Moderate | Unpredictable |
| 18. | Wieczorek 201617 | No information | No information | No information | No information | No information | No information | No information | No information | Unpredictable |
| Study | WHAT: materials 1 | WHO: provider 2 | DELIVERY: 4 | DELIVERY: 5 | DELIVERY: 6 |
DELIVERY: 7a | DELIVERY: 7b | DELIVERY: 8 | DELIVERY: 10 | DELIVERY: 11 | DOSAGE: 13 | TAILORING: 14a | TAILORING: 14b | TAILORING: 15 | HOW WELL: 16a | HOW WELL: 16B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdulsatar 20137 | Yes | No | Yes | Yes | No | No | No | No | No | Yes | Yes | No | No | Yes | Yes | Yes |
| Alqaqaa 20188 | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No |
| Betters 201710 | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Choong 201720 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Choong 201519 | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No |
| Choong 2014a11 | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No |
| Choong 2014b21 | Yes | Yes | No | Yes | No | Yes | No | No | No | No | No | Yes | Yes | No | Yes | No |
| Choong 201212 | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Choong 202124 | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes |
| Colwell 201813 | No | Yes | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No |
| Cui 201714 | No | Yes | Yes | Yes | No | No | No | Yes | No | Yes | No | No | No | No | No | No |
| Curry 202124 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Fink 201943 | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Ista 202142 | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes |
| Justice 201916 | Yes | No | No | No | No | No | Yes | No | No | No | No | Yes | No | No | Yes | No |
| Kudchadkar 202018 | Yes | Yes | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| Tsuboi 201732 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | No |
| Wieczorek 201617 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No |
Appendix 3
Additional tables and figures
| Items | Within an ERM protocol (n = 12 respondents) Yes n (%) |
Within a non-ERM protocol (n = 124 respondents) Yes n (%) |
|---|---|---|
| Physical therapy requiring additional equipment | 9 (75) | 18 (15) |
| OT interventions | 9 (75) | 18 (15) |
| Physical therapy not requiring additional equipment | 8 (67) | 17 (14) |
| SLT interventions | 4 (33) | 12 (10) |
| Psychology interventions | 0 (0) | 8 (6) |
| Delirium screening | 0 (0) | 1 (1) |
| Professional group | n (%)a |
| Nurse | 34 (27) |
| Physiotherapist | 28 (23) |
| Medical doctor (consultant) | 22 (18) |
| OT | 19 (15) |
| Play therapist | 7 (6) |
| Psychologist | 7 (6) |
| Dietician | 6 (5) |
| SLT | 1 (1) |
| Years of experience | n (%)a |
| <1 year | 7 (6) |
| 1–<5 years | 27 (22) |
| 5–<10 years | 30 (24) |
| 10–<15 years | 14 (11) |
| 15–<20 years | 33 (27) |
| More than 20 years | 15 (12) |
| Current view of ERM in PICU | n (%)a |
|---|---|
| Crucial, should be the top priority in the care of PICU patients | 15 (12) |
| Very important, should be a priority in the care of PICU patients | 67 (55) |
| Important, should be a priority in the care of PICU patients | 35 (29) |
| Somewhat important, should be considered in the care of PICU patients | 4 (3) |
| Not of great importance, clinicians should bear it in mind in the care of PICU patients | 0 (0) |
| Of minimal importance to the care of PICU patients | 0 (0) |
| Of no importance to the care of the PICU patients | 0 (0) |
| Professional or family group | Yes n (%) |
|---|---|
| Physiotherapist | 96 (77) |
| Physicians | 92 (74) |
| Bedside nurse | 64 (52) |
| Senior nurse | 58 (47) |
| Other members of the medical team | 55 (44) |
| OT | 37 (30) |
| Parent or family member | 24 (19) |
| ERM equipment available in each PICU | n (%) |
|---|---|
| Specialist static seating | 25 (96) |
| Portable ventilators | 23 (88) |
| Mobile lifts | 22 (85) |
| Tilt table | 22 (85) |
| Bed with full chair position | 18 (69) |
| Specialist wheelchair | 18 (69) |
| Bed with Trendelenburg features | 13 (50) |
| Patient rolling walker | 11 (42) |
| Bedside cycle or in bed cycle | 10 (38) |
| Ceiling lifts | 8 (31) |
| Speciality bed with continuous side-to-side rotation | 8 (31) |
| Bed with retractable footboard | 7 (27) |
| Bed with chair egress exit out the foot of the bed | 5 (19) |
| Transcutaneous electrical nerve stimulation | 3 (12) |
| Description | Broad group | Enrichment, passive, active mobility group | Any ERM | Mobility ERM | Out-of-bed mobility ERM | Mobility ranking |
|---|---|---|---|---|---|---|
| 1. Cuddles (in bed)/comfort holding | CUDDLES | Enrichment | X | X | 4 Held by parent or nurse | |
| 2. Cuddles out of bed/kangaroo care | CUDDLES | Enrichment | X | X | X | 4 Held by parent or nurse |
| 3. Sibling support, e.g. watching TV/iPad together | FAMILY SUPPORT | Enrichment | X | |||
| 4. Reading a story/singing | MUSIC THERAPY | Enrichment | X | |||
| 5. Entry in patient diary | PSYCHOLOGICAL SUPPORT | Enrichment | X | |||
| 6. Communication using communication tools | PSYCHOLOGICAL SUPPORT | Enrichment | X | |||
| 7. Music therapy, e.g. listening to music or singing to a child | MUSIC THERAPY | Enrichment | X | |||
| 8. Massage therapy | SENSORY STIMULATION | Enrichment | X | |||
| 9. Counselling/psychological support | PSYCHOLOGICAL SUPPORT | Enrichment | X | |||
| 10. Pet therapy | PSYCHOLOGICAL SUPPORT | Enrichment | X | |||
| 11. Engagement in activities of daily living (age-appropriate) | ENGAGEMENT IN DAILY ACTIVITIES | Active mobility | X | |||
| 12. Orientation to self/place/time of day | SENSORY STIMULATION | Enrichment | X | |||
| 13. Established daily routine, e.g. timetable | SENSORY STIMULATION | Enrichment | X | |||
| 14. Day/night routine encouraged, e.g. eye mask/earplugs/headphones used to promote sleep at night | SENSORY STIMULATION | Enrichment | X | |||
| 15. Positioning for tone | POSITIONING – NM | Passive | X | |||
| 16. Neonatal positioning | POSITIONING – NM | Passive | X | |||
| 17. Positioning for development or functional benefit | POSITIONING – MOB | Passive | X | X | 1 Passive ROM | |
| 18, Splints/braces – application of | POSITIONING – NM | Passive | X | |||
| 19. Sitting up in bed (30–90°) | SITTING | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 20. Sitting on the edge of bed | SITTING | Active mobility | X | X | 3 Sitting edge of bed | |
| 21. Sitting in a chair | SITTING | Active mobility | X | X | X | 5 Transfer bed to chair |
| 22. Sliding board transfer | TRANSFERS | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 23. Sit to stand (bed to chair) | STANDING | Active mobility | X | X | X | 5 Transfer bed to chair |
| 24. Step transfer | TRANSFERS | Active mobility | X | X | X | 7 Standing |
| 25. Stand transfer | TRANSFERS | Active mobility | X | X | X | 7 Standing |
| 26. Lift|57. (1) One person|58. (2) Two-person | TRANSFERS | Active mobility | X | X | X | 5 Transfer bed to chair |
| 27. Hoist transfer | TRANSFERS | Active mobility | X | X | X | 5 Transfer bed to chair |
| 28. Sensory stimulation via touch/textures/visual | SENSORY STIMULATION | Active mobility | X | |||
| 29. Floor/mat play | DEVELOPMENTAL PLAY – MOB | Active mobility | X | X | X | 6 Mat play |
| 30. Developmental play: supine, side-lying, prone, sitting | DEVELOPMENTAL PLAY – MOB | Active mobility | X | X | X | 6 Mat play |
| 31. Play (e.g. messy play, painting, finger painting, etc.) | DEVELOPMENTAL PLAY – MOB | Active mobility | X | X | 0 | 2 Sitting and exercises in bed |
| 32. Interactive virtual reality, e.g. games, goggles | DEVELOPMENTAL PLAY – NM | Enrichment | X | X | ||
| 33. School teacher to bedside | SCHOOL | Enrichment | X | |||
| 34. Passive ROM | RANGE OF MOVEMENT | Passive | X | X | 1 Passive ROM | |
| 35. Active ROM (movement following instructions) | RANGE OF MOVEMENT | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 36. Active assisted ROM (assisted movement following instructions) | RANGE OF MOVEMENT | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 37. Rolling – active or facilitated | RANGE OF MOVEMENT | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 38. Stretching exercises in bed | RANGE OF MOVEMENT | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 39. Strengthening exercises (age-appropriate bed exercises such as bridging, straight-leg raises, etc.) | EXERCISES IN BED | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 40. Strengthening exercises – out of bed, e.g. squatting | EXERCISES OUT OF BED | Active mobility | X | X | X | 6 Mat play |
| 41. MOTOmed or cycling in bed | IN-BED CYCLING | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 42. Physio-directed exercise programme (comprehensive) | PHYSIOTHERAPY | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 43. Standing (next to bed with or without support) | STANDING | Active mobility | X | X | X | 7 Standing |
| 44. Standing frame | STANDING | Active mobility | X | X | X | 7 Standing |
| 45. (1) Walking with support/aid. (2) Walking unsupported | AMBULATING | Active mobility | X | X | X | 8 Walking in room/unit |
| 46. (1) Mobilising (≤1 m) (2). Mobilising (≤5 m). (3) Mobilising (>5 m) | AMBULATING | Active mobility | X | X | X | 8 Walking in room/unit |
| 47. Marching on the spot | AMBULATING | Active mobility | X | X | X | 8 Walking in room/unit |
| 48. School | SCHOOL | Active mobility | X | |||
| 49. Physio. dept. e.g. gym session | PHYSIOTHERAPY | Active mobility | X | X | X | 9 Walking off unit |
| 50. Trip/outing, e.g. chapel, canteen/restaurant | AMBULATING | Active mobility | X | X | X | 9 Walking off unit |
| 51. Mobilising | AMBULATING | Active mobility | X | X | 2 Sitting and exercises in bed | |
| 52. Bath/shower/toilet | ENGAGEMENT IN DAILY ACTIVITIES | Active mobility | X | X | U Uncategorised | |
| 53. Other exercises out of bed | EXERCISES OUT OF BED | Active mobility | X | X | U Uncategorised | |
| 54. SLT (speech & lang. therapy), e.g. tastes, development of communication tools (i.e. using letters/words/typing in tablets) and swallow assessment | SENSORY STIMULATION | Enrichment | X | |||
| 55. FES or NMES | SENSORY STIMULATION | Passive | X | X |
FIGURE 18.
ERM prescribed and delivered activities on study day 1.

FIGURE 19.
Number of ERM episodes by duration.
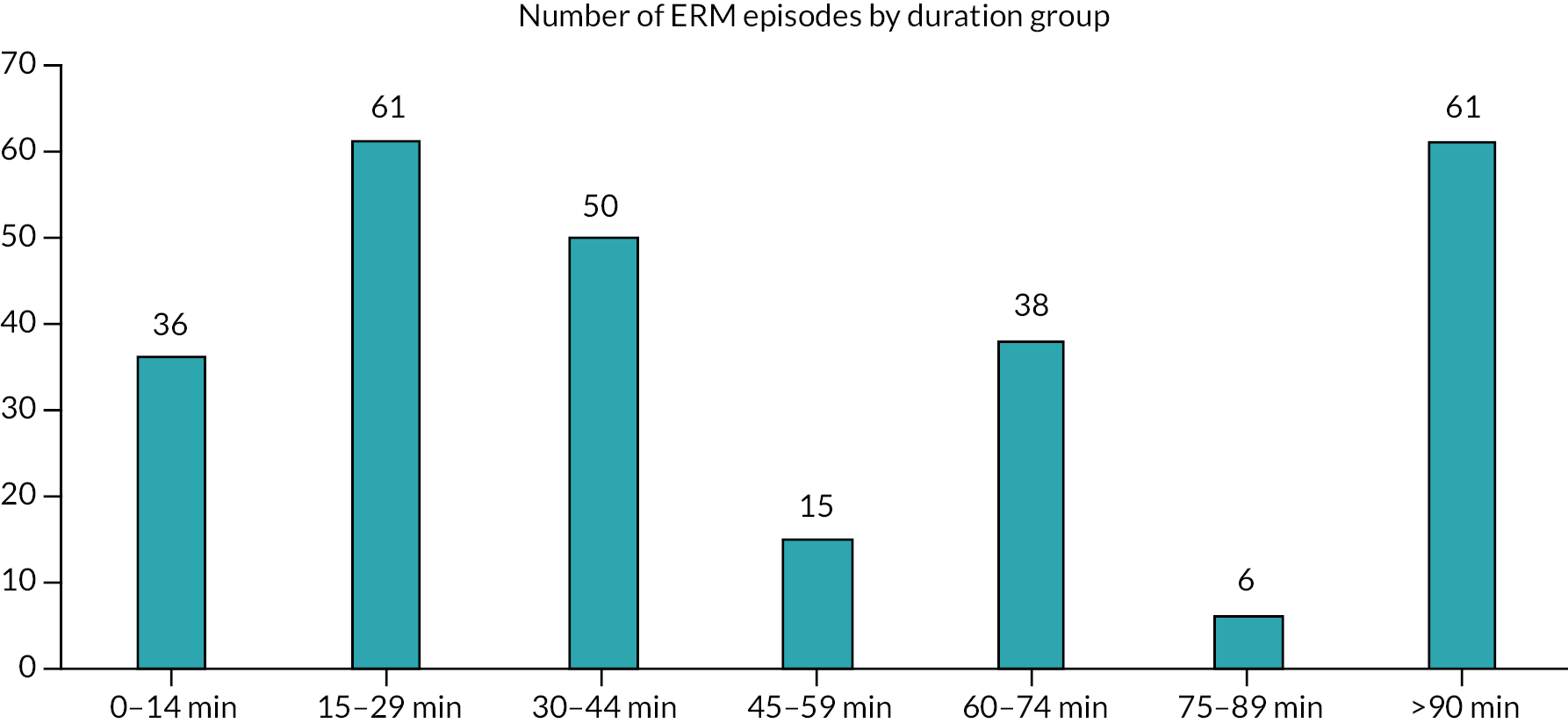
FIGURE 20.
NoMAD reports of familiarity and normality of ERM in practice across first, second and third surveys.
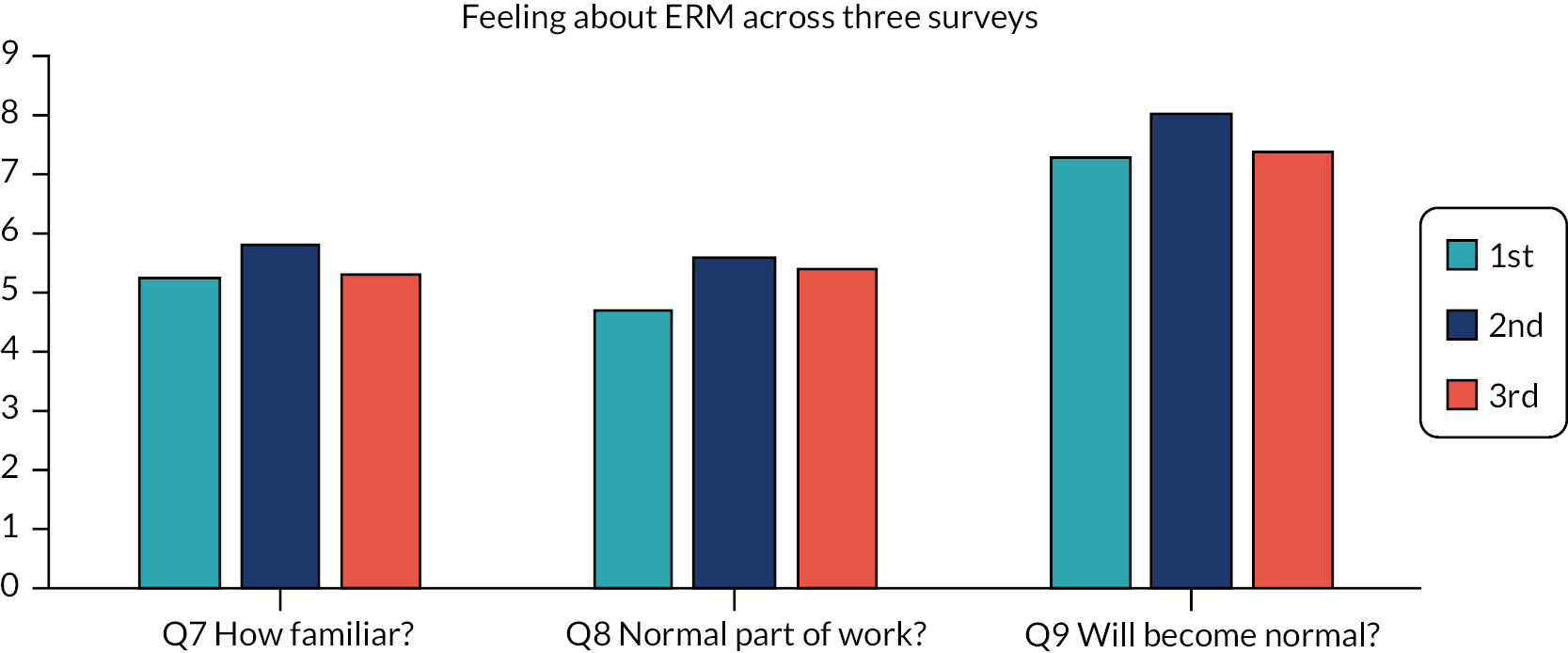
FIGURE 21.
Radar plot of overall (median) score for questions (see Table 41) for all participants across three survey time points.
| Q7. When you undertake ERM, how familiar does it feel? |
| Q8. Do you feel ERM is currently a normal part of your work? |
| Q9. Do you feel ERM will become a normal part of your work? |
| Q10. I can see how ERM differs from usual ways of working |
| Q11. Staff in this organisation have a shared understanding of the purpose of ERM |
| Q12. I understand how ERM affects the nature of my own work |
| Q13. I can see the potential value of ERM for my work |
| Q14. There are key people who drive ERM forward and to get others involved |
| Q15. I believe that participating in ERM is a legitimate part of my role |
| Q16. I am open to working with colleagues in new ways to use ERM |
| Q17. I will continue to support ERM |
| Q18. I can easily integrate ERM into my existing work |
| Q19. ERM disrupts working relationships |
| Q20. I have confidence in other people’s ability to use ERM |
| Q21. Work is assigned to those with skills appropriate to ERM |
| Q22. Sufficient training is provided to enable staff to implement ERM |
| Q23. Sufficient resources are available to support ERM |
| Q24. Management adequately supports ERM |
| Q25. I am aware of reports about the effects of ERM |
| Q26. The staff agree ERM is worthwhile |
| Q27. I value the effects ERM has had on my work |
| Q28. Feedback about ERM can be used to improve it in the future |
| Q29. I can modify how I work with ERM |
List of abbreviations
- AE
- adverse event
- aOR
- adjusted odds ratio
- BCH
- Birmingham Children’s Hospital
- BIPAP
- bilevel positive airway pressure
- CAC
- cluster autocorrelation
- CAPD
- Cornell assessment of paediatric delirium
- CERT
- consensus on exercise reporting template
- CI
- confidence interval
- CINAHL
- cumulative index to nursing and allied health literature
- COVID
- coronavirus disease
- CPAP
- continuous positive airway pressure
- CRF
- case report form
- CRN
- clinical research networks
- CVC
- central venous line
- CYP
- children and young people
- ECMO
- extracorporeal membrane oxygenation
- EMPATHIC
- empowerment of parents in the intensive care
- ERIC
- expert recommendations for implementing change
- ERM
- early rehabilitation and mobilisation
- ETT
- endotracheal tube
- EU-PACK
- European Prevalence of Acute Rehabilitation for Kids in the PICU
- HCP
- healthcare practitioners
- HRA
- Health Research Association
- ICC
- intracluster correlations
- ICU
- intensive care unit
- IQR
- interquartile range
- LOS
- length of stay
- LOV
- length of ventilation
- MRC
- Medical Research Council
- MDT
- multidisciplinary team
- MED
- median
- MV
- mechanical ventilation
- NIHR
- National Institute for Health and Care Research
- NoMAD
- normalisation measure development
- NPT
- normalisation process theory
- OR
- odds ratio
- OT
- occupational therapist
- PBA
- person-based approach
- PCPC
- paediatric cerebral performance category
- PedsQL
- Pediatric Quality of Life Inventory
- PEDI-CAT
- paediatric evaluation of disability inventory-computer adaptive test
- PELOD
- pediatric logistic organ dysfunction
- PERMIT
- paediatric early rehabilitation and mobilisation during intensive care
- PHQ-4
- Patient Health Questionnaire-4
- PCCS-SG
- paediatric critical care society – study group
- PICU-AW
- paediatric intensive care acquired weakness
- PIS
- patient information sheet
- POPC
- paediatric overall performance category
- PPIE
- patient and public involvement and engagement
- PRISM
- paediatric risk of mortality
- PT
- physiotherapy
- QoL
- quality of life
- REC
- Regional Ethics Committee
- REDCAP
- research electronic data capture
- ROBINS-I
- risk of bias in non-randomised studies – of interventions
- ROM
- range of movement
- SANDWICH
- sedation and weaning in children trial
- SIV
- site initiation visit
- SLT
- speech and language therapist
- TFA
- theoretical framework of acceptability
