Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR128617. The contractual start date was in March 2020. The draft manuscript began editorial review in January 2023 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Lansdale et al. This work was produced by Lansdale et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Lansdale et al.
Chapter 1 Introduction
Background
Neonates undergoing emergency surgery on their abdomen for problems such as necrotising enterocolitis (NEC), spontaneous intestinal perforation (SIP) or a congenital bowel obstruction frequently require stomas to be formed. While stomas can be life-saving, they pose a number of challenges, including fluid and electrolyte imbalance; local wound and skin problems; and malnutrition and growth failure. 1–3 Reversing (closing) these stomas with a second operation is therefore an essential part of the infant’s recovery. The timing of this closure is known to be variable and the best time to do it remains unclear, with conflicting evidence from published studies and reviews.
A systematic review and meta-analysis from 2017 looked at the timing of stoma closure in infants with NEC: six articles were included (n = 280 infants) comparing early stoma closure (before 8 weeks from formation) with late stoma closure (after 8 weeks). 1 The review found that total duration of parenteral nutrition (PN) was similar in infants with early versus late closure. Likewise, total length of hospital stay (pre and post stoma closure) was not influenced by timing of closure. Included studies also reported similar complication rates after stoma closure between early and late groups. It is likely this review is compromised by a high risk of bias and all studies except one were retrospective. Three of the included studies (n = 124 infants) were published in the 1980s and neonatal care has changed considerably since then. Other studies have reported conflicting results: a retrospective study from Canada (2009) compared infants who had their stoma closed within 10 weeks (n = 13) and after 10 weeks (n = 24). Infants with earlier closure had a longer postoperative duration of mechanical ventilation, longer need for PN and longer hospital stay. 4 There were no differences observed in survival rates or complications between study groups. The authors concluded that stoma closure should be deferred until at least 10 weeks after formation. The opposite conclusion was reached by a Dutch group in 2012: they retrospectively compared infants undergoing stoma closure before (n = 13) or after 6 weeks (n = 62). 2 They found no differences between the two groups in terms of postoperative adhesions, costs of hospital stay, surgical interventions and outpatient clinic visits and concluded that, after stabilisation of the patient, the stoma closure could be considered within 6 weeks. A North American study (2015) compared stoma closure before (n = 7) or after 8 weeks (n = 37) and reported no differences in PN duration and associated cholestasis, duration of mechanical ventilation, incidence of bowel adhesive obstruction, morbidity or mortality after closure. 5 Given there was no difference in the end points studied, they concluded that there is no advantage to early or late enterostomy reversal. A further retrospective review of infants (birthweight < 1000 g) with stomas (n = 55) favoured waiting for stoma closure until a minimum weight was attained. 6 Higher postoperative complications (66.7% vs. 10.8%, p < 0.001), and longer operative time, ventilation, hospital stay and PN use were reported in those < 2100 g at closure. More recently, two conference abstracts [including three UK units] reported retrospective data for preterm infants (n = 34 and n = 76). 7,8 Both reported a wide variability in time to closure (27–394 and 21–469 days). Both describe significant stoma morbidity, including stoma complications 7/34 (21%); severe growth failure 46/76 (61%) and emergency re-admission in 10% of those discharged prior to closure.
The above studies demonstrate that the current evidence base to inform optimal timing of neonatal stoma closure is of low methodological quality and conflicting in its assessment of the risk/benefit profile. The studies all have important limitations which include (1) low numbers and (2) high risk of bias owing to the retrospective and non-randomised nature of the studies, and hence the inability to account for important confounders such as disease severity, gestational age and patient comorbidities. The studies also measure different outcomes and there is no specific core outcome set available for neonatal stoma closure: data synthesis is therefore challenging. The limited data available do, however, highlight the considerable morbidity associated with stomas in infants and the marked variability in practice. Furthermore, they highlight the potential risks and benefits of early closure and hence contribute to the rationale behind the Timing of Stoma Closure in Neonates (ToSCiN) study.
Rationale
Determining the best time to close stomas in neonates is imperative as it has significant implications for:
-
infant health outcomes (short term, e.g. avoiding complications, and long term, e.g. tackling growth failure, which impacts neurodevelopment)
-
families (e.g. reduced neonatal unit stay/healthcare burden/time off work)
-
health providers (reduced costs, e.g. neonatal unit bed-days, PN use and reoperation).
In addition, reducing unwarranted variability in surgical care, such as that highlighted above, is a key priority for the NHS at present; setting standards for a more consistent approach requires a robust evidence base. 9 The most methodologically robust way to determine optimal timing of stoma closure would be through an adequately powered randomised controlled trial (RCT).
A trial of different stoma closure times in neonates is likely to be highly challenging, due to:
-
the patient group, which is characterised by marked heterogeneity of underlying disease and comorbidities
-
clinician factors such as willingness to recruit
-
parent factors such as trial acceptability.
Given that infants have stomas formed for a range of diseases (e.g. NEC, SIP, jejunoileal atresia, meconium ileus and complicated gastroschisis) and are themselves very different (e.g. premature vs. term, varying weights/sizes, with an isolated problem vs. multiple comorbidities), it is important to describe the characteristics of the population of infants that have stomas formed and closed, how many are treated each year in the UK and which groups should and could be included in a trial. This study aimed to tackle these potential challenges and hence determine if a trial comparing early and late stoma closure is feasible.
Aims
The overarching aim of the ToSCiN study was to answer the question: is it feasible to conduct a clinical trial comparing ‘early’ versus ‘late’ stoma closure in neonates?
Objectives
The specific objectives of the ToSCiN study were:
-
to establish current UK practice for stoma closure in neonates
-
to determine whether there is equipoise among clinicians (neonatal surgeons and neonatologists) and allied health professionals (specialist nurses and dietitians) over when it is best to close stomas in neonates
-
to define ‘early’ and ‘late’ stoma closure for a potential trial
-
to define a population of neonates for inclusion in a trial (in whom there is significant uncertainty over timing) and determine how many infants are eligible for inclusion
-
to establish the most appropriate design and outcome measures for a trial
-
to determine the willingness of parents, neonatal surgeons and neonatologists to include neonates in a trial that would randomise to ‘early’ or ‘late’ stoma closure and identify potential barriers to recruitment
-
to assess the suitability of using routinely collected data for gathering clinical information for a trial.
Chapter 2 Methods
The ToSCiN study used a mixed-methods approach (qualitative and quantitative methodology) and comprised three parallel workstreams.
Workstream 1: Survey of clinician and allied health professional perspectives on neonatal stoma closure
Study design and setting
An online survey of clinicians and allied health professionals from neonatal surgical units across the UK who were involved in the care of newborn infants requiring formation of a stoma.
Eligibility criteria
Inclusion criteria
Neonatologists in surgical neonatal units, neonatal surgeons, neonatal dietitians and neonatal surgical nurses.
Recruitment and sampling
Potential participants were identified via promotion through two national organisations: (1) The British Association of Paediatric Surgeons and (2) The British Association of Perinatal Medicine, and personal contacts of the study team, ensuring representative sampling (e.g. geographical area and healthcare professional type). Invitations to complete the survey were sent via e-mail. The survey was also promoted at relevant national conferences and research meetings.
Informed consent
Voluntary completion of the online survey was considered to be consent for the anonymous use of provided data for the purposes of the study.
Survey design
The survey (see Report Supplementary Material 1) was designed by the team of ToSCiN co-investigators to meet the aims of the study. The survey asked a series of questions focusing on the above key objectives for a number of different clinical scenarios. The survey asked participants to choose between options for (1) ‘early’ and ‘late’ stoma closure and (2) which groups of infants should or should not be included in a trial and sought reasoning behind these choices and whether equipoise existed. The respondent’s preferences for timing of stoma closure (in different groups), which factors were most important to them when determining when to close a stoma and barriers to achieving the perceived optimal timing were explored. Finally, the survey asked whether the respondents wanted to attend the final trial design (consensus) meeting.
Survey conduct
The survey was conducted online via LimeSurvey (LimeSurvey GmbH, Hamburg, Germany) and distributed as above by the National Perinatal Epidemiology Unit (NPEU), Oxford, UK. 10 There were automated e-mail reminders to optimise response rates over a period of 6 weeks. Further reminders used identified principal investigators (PIs) in each centre to encourage local colleagues to complete. Responses were downloaded as a spreadsheet of answers and stored on a secure server at the NPEU.
Sample size
The survey was sent to approximately 300 clinicians and a minimum response rate of 25% was anticipated, giving a sample size of 75. Specifically, a response from at least one neonatologist and one neonatal surgeon from each of the 27 UK neonatal surgical centres was targeted.
Analysis
The survey data were summarised using appropriate descriptive statistics. Numbers (with percentages) for binary and categorical variables and means [and standard deviations (SDs)], or medians (with lower and upper quartiles) for continuous variables were presented. Free-text responses were categorised to identify common themes.
Workstream 2: Parent and clinician perspectives regarding a clinical trial of neonatal stoma closure
Overall design
The aim of this workstream was to determine how clinicians and parents viewed the prospect of a clinical trial that would randomise infants to ‘early’ or ‘late’ closure (the term ‘parent’ includes ‘legal representative’ and applies for the remainder of this report). Factors that influence the timing of stoma closure and outcomes that are likely to be important in a future trial were sought through the collection of clinical data. It explored which of these factors are most important when determining when to close a neonate’s stoma.
Workstream 2 involved:
-
(2.1) an observational cohort study of neonates who had a stoma formed
-
(2.2) questionnaires for the principal clinicians (surgeon and neonatologist) caring for each infant recruited to the cohort study
-
a qualitative study incorporating: (1) focus groups with clinicians and (2) interviews with parents of neonates who had a stoma.
Setting
Workstream 2 took place in eight neonatal surgical units distributed throughout the UK: Birmingham, Bristol, Evelina (London); Chelsea and Westminster (London), Glasgow, Manchester, Alder Hey (Liverpool), and Southampton.
Recruitment process and consent
When an infant met the inclusion criteria, a member of the infant’s care team already known to the parents approached them as soon as practically possible to discuss the study. The most appropriate time for this initial approach took the infant’s clinical condition and family’s needs into account. If the parents expressed an interest in the study, a site staff member delegated to take consent discussed the study further and provided a participant information sheet (PIS) (see Report Supplementary Material 2) to provide information on participation in both an interview (Workstream 2.3) and clinical data collection (Workstream 2.1). After an appropriate time interval to allow parents to consider the provided information, informed consent was taken for inclusion in the study and this was recorded on a specific study consent form (see Report Supplementary Material 3).
Workstream 2.1: Observational cohort study
Recruitment
Inclusion criteria
Eligible infants included those having a stoma as part of emergency surgery before 44 weeks post-conceptual age.
For some aspects of the analysis, recruited infants were divided into two groups:
Group A – had stomas formed for NEC or SIP and were usually born prematurely.
Group B – had stomas formed for other diagnoses and were often born closer to term. These other diagnoses included congenital anomalies that lead to bowel obstruction (e.g. intestinal atresias, meconium ileus, complicated gastroschisis) and other acquired conditions (e.g. milk curd obstruction).
Exclusion criteria
Cases where a stoma was part of a planned treatment pathway, for example, for an anorectal malformation or Hirschsprung’s disease, were not included in this sample.
Sample size
The recruitment target was 15–20 infants in each of the above two groups (total 30–40 infants).
Data collection
Key clinical and demographic information for recruited infants was recorded prospectively. The data set comprised factors that could influence the timing of stoma closure and outcomes which are likely to be important in a future trial. These data points were developed via an iterative process by the Co-Investigator Group (CIG) using the output from Workstream 1 as a guide. The fields for this data set are provided in Report Supplementary Material 4. Data were collected at the following time points: (1) study entry (as soon as possible after stoma formation), (2) 1 week post stoma formation, (3) 6 weeks post formation (known as the ‘early intervention’ time point), (4) 12 weeks post formation (‘late intervention’ time point) and (5) after stoma closure. If an infant was discharged home, transferred or had their stoma closed prior to the 6- or 12-week time point, site staff were asked to provide clinical data dating from immediately before this occurred.
Data were held on an online custom database in a secure cloud-based clinical data management application (OpenClinica, Needham, USA), hosted in the UK by Amazon Web Services (AWS, Seattle, USA).
Workstream 2.2: Practitioner questionnaire
Data collection
The principal neonatologist and paediatric surgeon caring for each infant recruited into Workstream 2.1 were approached to complete online questionnaires. These were distributed via the OpenClinica Participate system at three time points – 1, 6 and 12 weeks post stoma formation – to explore the viewpoints of the principal neonatologist and surgeon caring for recruited infants as to whether they believed the infant they were caring for was suitable for inclusion in a trial that randomises to ‘early’ or ‘late’ stoma closure and whether, if an infant had been randomised to such a trial, they would follow the allocation (‘early’ or ‘late’) that the infant had been allocated to. The questionnaire also sought views on what clinical factors were most important in determining whether or not an eligible infant was suitable for randomisation in a trial. The questionnaires are provided in Report Supplementary Material 5. The approach of asking questions at different time points allowed changes in view on trial suitability and/or acceptability (as an infant’s clinical status changed) to be captured.
Data were held on an online custom database in a secure cloud-based clinical data management application (OpenClinica), hosted in the UK by AWS.
Workstream 2.3: Interviews and focus group
Recruitment
Inclusion criteria
Parents of premature and term infants who had a stoma in the previous 3 years (including those who did not participate in Workstream 2.1) were invited for an interview. Paediatric surgeons, neonatologists, specialist nurses, research nurses, neonatal intensive care unit staff and dietitians in participating surgical units who were involved in the treatment of infants requiring emergency stoma formation were invited to focus groups.
Exclusion criteria
Parents who did not speak English were excluded from interviews as resources were not available to offer appropriate translation services for this activity.
Sample size
For the qualitative Workstream 2.3 focus groups with practitioners, we aimed to hold six focus groups (based on the original number of expected study sites). We aimed to include approximately eight practitioners in each focus group. 11 Based on previous studies,12–14 we anticipated that 20–25 parent interviews would be needed to reach information power15 (when study aims, sample specificity and sufficient quality of interview dialogue are reached) in a varied sample of parents of neonates who had a stoma. This is the point where additional data do not lead to any new major themes during analysis and the researchers note high levels of ‘information redundancy’ during data collection. 16,17
Design and development of Workstream 2.3
The design and development of the protocol, including sample estimation, recruitment strategy, PISs (see Report Supplementary Material 2) and interview topic guide (see Report Supplementary Material 6), were informed by previous Health Technology Assessment (HTA)-funded trial feasibility research studies18–21 and early Workstream 122 and Workstream 2.1 findings. A review of previous studies relevant to the research question was conducted to develop a list of outcomes to inform outcome-related discussions with parents during interviews (see Report Supplementary Material 7).
This qualitative work stream was conducted towards the end of the 9-month data collection period of Workstream 2.1 and involved interviews with parents of infants with experience of stoma closure and focus groups with practitioners participating in Workstream 2.1.
Recruitment to parent interviews
Parents were recruited through two routes to maximise the potential sample within the active recruitment period: (1) parents of infants recruited in Workstream 2.1 were invited to participate in an interview and (2) social media adverts invited eligible parents to be interviewed.
For social media recruitment, TKM contacted charity leads or chief executive officers of existing networks (e.g. Bliss and Colostomy UK charity support groups) and asked them to post the ToSCiN study advert on their website and/or Facebook and Twitter social media pages (see Report Supplementary Material 8). The study team also posted adverts on Twitter and tagged key networks, support groups and clinicians with requests to retweet.
Consent
Parents recruited through Workstream 2.1 had already provided written consent as part of the Workstream 2.1 process. However, ED or TKM read each aspect of the ToSCiN interview-only consent form (see Report Supplementary Material 3) to parents recruited through social media, including consent for audio recording, the use of quotations in reports/dissemination of findings, storage of data and being sent a summary of the findings at the end of the study, in order for the parents to give audio-recorded verbal consent.
Staff were asked to provide written consent before the focus group began. If focus groups were held online, practitioner consent forms (see Report Supplementary Material 3) were sent to ED or TKM via e-mail either by printing out, signing and scanning a copy, or by typing their name and signature into an electronic copy. Counter-signed consent forms were returned to participants, using the same method of delivery, once the focus group had ended.
Interview conduct
Parents’ expressions of interest to participate in an interview were initially responded to in sequential order by ED and TKM. A strategy of purposive sampling was employed with the aim of maximum variation, ensuring that mothers and fathers were represented from multiple treatment centres and recruitment routes.
ED and TKM arranged convenient times for parental interviews and gave the option of telephone or Microsoft Teams/Zoom interviews. Due to COVID-19 guidelines at the time of data collection, face-to-face interviews were not possible. Parents were e-mailed an interview PIS (see Report Supplementary Material 2), the draft ToSCiN trial PIS (see Report Supplementary Material 9) and the list of potential outcomes (see Report Supplementary Material 7) to read in advance of the interview.
Interviews began with ED or TKM introducing themselves, discussing the aims of the study, providing an opportunity for questions and checking that the parent had read the information sheets and list of potential outcomes sent prior to interview. If parents had not read the draft trial information sheet or outcomes list, ED or TKM read these to them. Demographic details were then gathered. Parents’ baseline understanding of the proposed ToSCiN clinical trial and their views and experiences on the following were then reviewed and explored:
-
having a child with a stoma
-
the acceptability of a trial that would randomise infants to stoma closure at 6 and 12 weeks
-
the timing of recruitment
-
any potential barriers to or facilitators of trial participation
-
the draft trial PIS
-
whether they would hypothetically consent for their child to be in the ToSCiN trial or not
-
prioritised outcome measures.
Respondent validation11 was used to add unanticipated topics to the topic guide as interviewing and analyses progressed. After the interview, participants were sent a copy of their consent form (social media recruits only) and a thank-you letter, including a £30 Amazon voucher to thank them for their time. Researchers (ED and TKM) conducted a similar number of parent interviews.
Interviews were conducted until information power15 was reached. Interview audio files, transcripts and consent forms were retained and stored securely by the University of Liverpool.
Recruitment to practitioner focus groups
ED or TKM sent an e-mail to the PI and associate principal investigator (API) in all eight participating neonatal surgical unit study sites, inviting them to hold a face-to-face or online focus group (depending upon current COVID-19 guidelines) with practitioners from their site. The PI/API circulated the details about the purpose and anticipated duration of the focus group to appropriate practitioners (listed in the inclusion criteria above), along with a practitioner PIS (see Report Supplementary Material 2) and consent form (see Report Supplementary Material 3). ED or TKM sent a Microsoft Teams/Zoom calendar invitation link once the PI/API had selected and agreed a convenient date and time for the focus group with interested practitioners. The PI/API shared this calendar invitation link with individual practitioners at their site who were dialling into the focus group remotely.
Data collection
At the start of the focus group, ED or TKM checked that all participants had read the PIS. The focus group aims and topics to be covered were discussed, followed by an opportunity to ask questions. An online voting system, Poll Everywhere (Poll Everywhere Inc., San Francisco, CA, USA), was used alongside verbally administered questions in practitioner focus groups. This method enabled the collection of data from all practitioners present and was a means of generating statistical data from all sites alongside qualitative data from group discussions. ED and/or TKM conducted the focus groups. One led the discussion and the other administered the Poll Everywhere questions. This involved some of the key questions being presented to the group and each participant using their phone or computer to select their answer from those shown on the screen. A paper-based version of the same questions was also available for those that could not access Poll Everywhere (see Report Supplementary Material 10). An ice-breaker question was used at the beginning of each focus group to help demonstrate how the voting system would work. Practitioners were then asked to introduce themselves, their role and their involvement in stoma care. Practitioner equipoise and their views and experiences on the following topics were then explored:
-
current stoma closure practice
-
stoma closure at 6 weeks as ‘early’ and 12 weeks as ‘late’
-
potential barriers to and facilitators of trial participation
-
willingness to recruit and randomise children to the trial
-
acceptability of the trial
-
prioritised outcome measures.
Transcription
Digital audio recordings of Workstream 2.3 parent interviews and practitioner focus groups were transcribed verbatim by a professional transcription company (UK Transcription Ltd, Brighton, UK). Transcripts were checked for accuracy and all identifiable information such as family or hospital names were anonymised as the study progressed. NVivo version 12 (QSR International, Warrington, UK, 2022) was used to assist in the organisation and coding of qualitative parent interview and practitioner focus group data, while SPSS version 27 (IBM Corporation, Armonk, NY, USA) was used to assist in the organisation and coding of quantitative focus group data (practitioner closed questions). All data were processed in accordance with UK General Data Protection Regulation and the Data Protection Act 2018 (legislation.gov.uk).
Data analysis
Qualitative interview and focus group data from Workstream 2.3 were analysed interpretively and iteratively. 23,24 Analysis was informed by the work of Braun and Clarke and their guide to thematic analysis. 25–27 Thematic analysis is a method for identifying, analysing and reporting patterns (or themes) within data. The aim of utilising a thematic analysis approach28 (Table 1) was to provide accurate representation of views on trial design and acceptability. This approach allowed for themes to be identified at a semantic level (i.e. surface meanings or summaries) and at a latent level (i.e. interpretive – theorising the significance of the patterns and their broader meanings and implications). 29 Quantitative data from closed questions during practitioner focus groups were examined using descriptive statistics. Synthesis of qualitative and quantitative data drew on the constant comparative method30,31 and modified to fit with the criterion of catalytic validity, whereby findings should be relevant to future research and practice,32 that is, the design of the proposed ToSCiN RCT). The researchers (ED and TKM) led the analysis and 10% of transcripts were second coded by the qualitative lead (KW). Findings from the interviews and focus groups were fed into the design of the consensus meeting.
| Stage of analysis | Description of action |
|---|---|
| 1. Familiarisation with the data | ED (practitioners) and TKM (parents) read and re-read transcripts noting down initial ideas |
| 2. Coding across the entire data set | Transcripts were imported into NVivo Version 12 (QSR International, 2022). Initially, two data-coding frameworks were developed by ED and TKM, who led the analysis, using deductive codes identified from the study protocol and the interview/focus group topic guide. Additional data-driven codes and concepts not previously captured in the initial coding frame were identified inductively as coding continued. Ten per cent of the analysis was second coded by qualitative study lead investigator (KW). ED, TKM and KW met regularly to review, discuss and refine initial codes (practitioner and parent). Transcripts coded before new codes or subcodes were identified were revisited to ensure that the new codes were representative of the data coded under them |
| 3. Searching for themes | All codes and subcodes were interrogated by TKM and ED to search for and name themes that would provide a trustworthy account of the data |
| 4. Reviewing themes | ED and TKM compared and contrasted themes and subthemes to ensure that they accurately represented parent and practitioner narratives when themes (or subthemes) were renamed. Themes and subthemes were then reviewed by KW |
| 5. Defining and naming themes | Themes and subthemes were mapped to the study protocol and interview and focus group topic guides to identify data under these themes and subthemes that would answer the research objectives. Review of defined themes was, once again, carried out by KW |
| 6. Producing the report and finalising themes | ED, TKM and KW developed the manuscript using themes that related back to the study aims to ensure key findings and recommendations were relevant to the TOSCiN study. Qualitative and quantitative findings were synthesised. Final discussion and development of selected themes occurred during the write-up phase |
| 7. Participant validation | Parents and practitioners had opportunities to discuss, validate or disagree with the findings that were presented by ED, TKM and/or KW during study meetings and consensus meetings |
An additional analysis step was conducted to identify outcomes of importance to parents and practitioners. The number of parents who ranked each outcome as most, second or third most important was counted, and then a weighted point-based system used to determine parents’ top-prioritised outcomes; for example, a score of 3 was given to the outcomes that parents ranked most important, a score of 2 for those ranked second most important and a score of 1 for those ranked third most important.
Workstream 3: Analysis of three existing national databases
Workstream 3 design
Analyses of three existing national databases were carried out to generate quantitative data to address the following study objectives: (1) establishing current UK practice, (2) defining a population for trial inclusion and providing the number of eligible infants, (3) establishing appropriate trial design and outcome measures and (4) assessing suitability of using routinely collected data for a future trial. Analyses were guided by the results of the clinician survey in Workstream 1. The databases were not linked, and analyses proceeded separately.
Workstream 3 data sources
The National Neonatal Research Database
The National Neonatal Research Database (NNRD) holds data from all infants admitted to NHS neonatal units in England and Wales. Data are extracted from neonatal electronic health records completed by health professionals during routine clinical care. A defined data extract comprising approximately 450 items,33 the Neonatal Data Set, is transmitted quarterly to the Neonatal Data Analysis Unit (NDAU) at Imperial College London where data are cleaned and entered into the NNRD. High completeness and accuracy (> 95%) of data held in the NNRD have been confirmed by a formal comparison with those recorded in case record forms of a multicentre, randomised placebo-controlled trial. 33
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System (BAPS-CASS) is the UK’s principal data collection system studying the surgical management of a range of neonatal conditions on a population basis. 34 It has conducted a number of prospective, multicentre cohort studies over the past 10 years. Two of these were of infants with conditions that frequently require stoma formation: NEC and meconium ileus, and data from these studies were analysed for Workstream 3.
Hospital Episode Statistics
Hospital Episode Statistics – Admitted Patient Care (HES-APC) data were obtained from NHS Digital (Data Uses Register reference DARS-NIC-315419-F3W7K). HES-APC records are routinely collected statistical abstracts of inpatient hospital care occurring in NHS hospitals in England. Each HES-APC record contains dates of admission and discharge, patient characteristics, clinical diagnoses coded using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and clinical procedures coded using the OPCS Classification of Interventions and Procedures, version 4.8 (OPCS-4). The full list of data items collected in HES-APC are described in detail in the HES Data Dictionary (https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary).
Workstream 3 analyses
The National Neonatal Research Database
In our study, the NNRD was used to identify infants of all gestational ages on neonatal units who had a record of a stoma in England and Wales between 1 January 2012 and 31 December 2019. Infants recorded as having anorectal abnormalities or Hirschsprung’s disease, or who were not cared for completely in units in Wales and England and hence had missing data, were excluded from the analysis (Figure 1).
FIGURE 1.
Flowchart depicting the exclusion and data cleansing process. Neonates with a record of a stoma in situ in England and Wales, within the NNRD, 1 January 2012–31 December 2019.
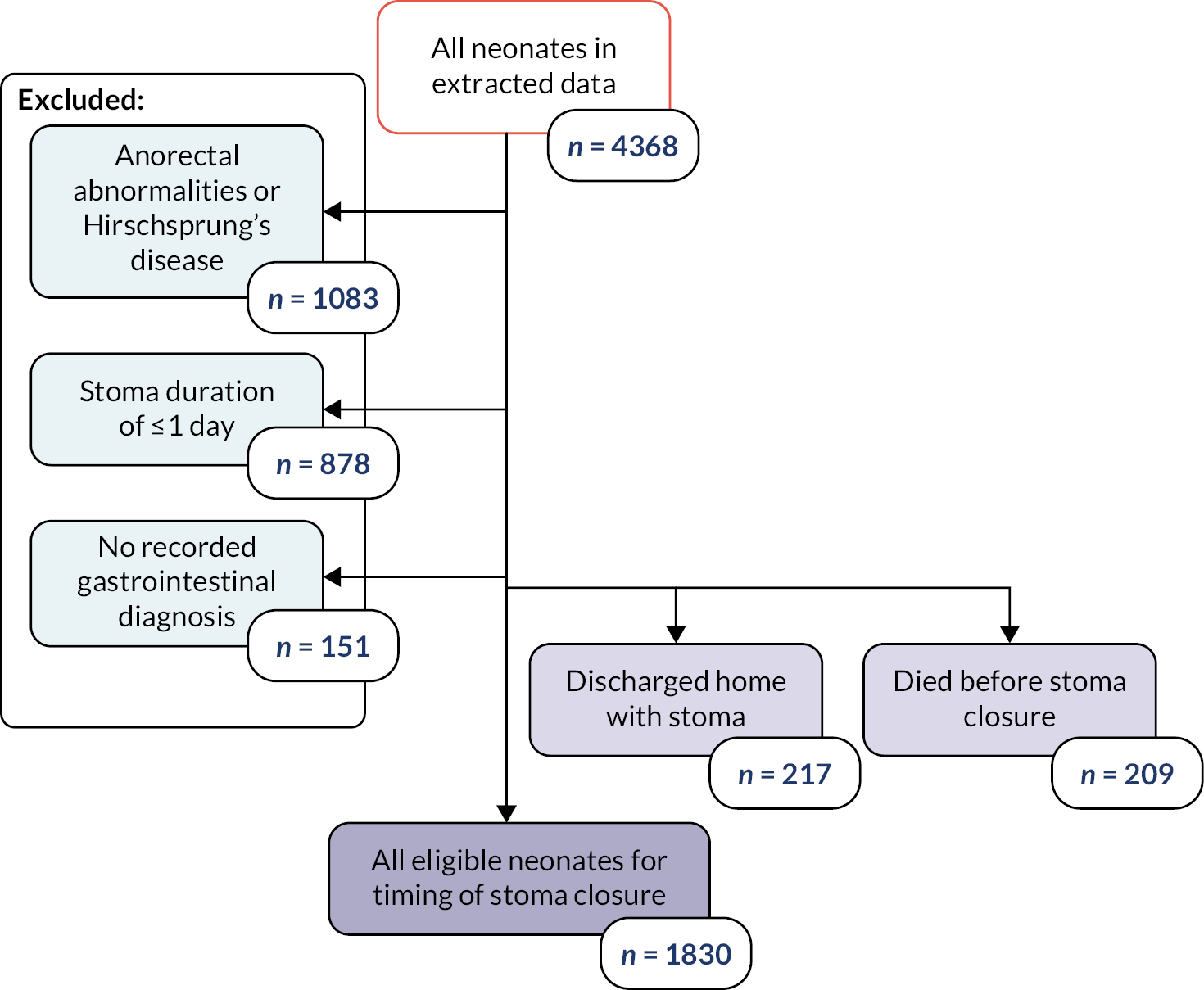
To be classed as having a stoma, infants had to:
-
have at least two daily records of having a stoma in situ
-
have a gastrointestinal diagnosis potentially related to a stoma formation, including NEC, small intestinal conditions, malrotation, volvulus and intussusception.
Neonates that were discharged home with a stoma in situ or died prior to stoma closure were analysed separately as they did not have a recorded stoma closure date in the NNRD.
We extracted patient characteristics (birthweight, birth year, sex, gestational age at birth); gastrointestinal diagnoses associated with stoma insertion; condition of infant prior to stoma closure (weight on the day prior to the day of stoma closure, receipt of inotropes, PN or respiratory support within 2 days prior to stoma closure, corticosteroids within 7 days prior to stoma closure); whether the infant was discharged to surgical centre at any point during care; stoma complications defined as codes including postoperative intestinal obstruction, postoperative wound abscess and small intestinal obstruction due to postop adhesions; and survival to discharge.
Data were summarised with counts and percentages for categorical variables, or medians [interquartile range (IQR)] for continuous variables. Furthermore, patients were subdivided into ‘early’ stoma closure, defined as ≤ 9 weeks, and ‘late’ closure, defined as > 9 weeks, based upon Workstream 1. 22
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System
The BAPS-CASS NEC study was conducted from 1 March 2013 to 28 February 2014 in 27 paediatric surgical centres in the UK and Ireland. 35 Inclusion criteria included any infant with suspected NEC where a decision for surgery was made, irrespective of whether they underwent surgery and whether the infant was subsequently found to have SIP. Infants were excluded if the diagnosis at the time of surgery was not NEC/SIP. Cases were identified by visual inspection of the bowel at surgery, at post-mortem or clinically using the Vermont-Oxford criteria. Further study details are described in Allin et al. 35 The BAPS-CASS meconium ileus study was conducted between 1 October 2012 and 30 September 2014 in 27 paediatric surgical centres in the UK and Ireland. 36 Infants were included if they had bowel obstruction caused by inspissated meconium in the terminal ileum in addition to an established diagnosis of cystic fibrosis.
Data were summarised with counts and percentages for categorical variables, means (SD) or medians (IQR) for continuous variables.
Hospital Episode Statistics
This study used a bespoke extract of all HES-APC records belonging to children aged ˂ 90 days who underwent stoma formation from 1 January 2011 to 31 December 2018, followed up for up to 1 year (using data to 31 December 2019). Stoma formation and stoma closure were defined using OPCS-4 codes (see Appendix 1). ICD-10 codes were selected to exclude infants with anorectal malformation or Hirschsprung’s and to define a NEC/SIP group for subanalysis (see Appendix 2).
Consensus meeting
The ToSCiN consensus meeting was held at the end of the study period when data collection and a preliminary analysis had been completed.
Invitations to the meeting
All professionals and parents involved in any aspect of ToSCiN were invited to the meeting. Further advertisement was via social media groups including the Bliss charity and professional networks. An online registration process was used. Attendee demographics were monitored.
Meeting format
Individuals who registered to attend the meeting were sent a study pack in advance, containing a summary of ToSCiN study findings and information about how the consensus meeting would be undertaken. An online video platform (Zoom; Zoom Video Communications, Inc., San Jose, CA, USA) was used to hold the meeting virtually. An independent, external neonatal expert with experience of similar activities chaired the meeting.
Presentation of study data
The ToSCiN consensus meeting was divided into three parts and each part focused on a specific theme. These were:
-
Theme 1: What’s important to measure? Outcomes.
-
Theme 2: What should we compare? Trial design.
-
Theme 3: Who should we include? Population.
A summary of data gathered by the three ToSCiN workstreams was presented to attendees for each of the above themes.
Consensus and voting
Facilitated, small group discussions took place (so-called ‘breakout rooms’) for each theme and these were chaired by a member of the CIG. After these small group discussions, a summary was presented to all attendees by each group chair. Following this summary, electronic voting took place using the Slido online software (Cisco Systems Inc., San Jose, CA, USA). This included some free-text questions.
Study management
Clinical Trials Unit: the NPEU Clinical Trials Unit managed the overall project, undertook the clinician survey, organised the consensus meeting and enabled data collection.
Sponsor: Manchester University NHS Foundation Trust was the nominated sponsor for the study.
Project Management Group (PMG): the study was run on a day-to-day basis by this group, which reported to the Study Steering Committee (SSC). The core PMG consisted of the Chief Investigator, the Lead for Qualitative Work, the NPEU CTU Clinical Director, the Senior Trials Manager, the Study Coordinator, the Trials Programmer and other project staff. The core PMG met every month.
Co-Investigator Group: this extended PMG met every 2 months initially and every 3–4 months subsequently. It comprised all members of the co-applicant group and the members of the core PMG in order to review progress, troubleshoot and plan strategically.
Study Steering Committee: this included an independent chair and vice-chair, two other independent members, an independent patient and public involvement (PPI) representative and the chief investigator. It was ratified by the NIHR. The SSC reviewed the progress of the study and reported on progress to the funder.
Ethical approval
The study protocol, patient information resources (including Parent Information Sheets), consent forms and other study-related documents were reviewed and approved by the NIHR HTA Programme and an NHS Research Ethics Committee (REC) with respect to scientific content and compliance with applicable research regulations involving human subjects (IRAS PROJECT ID 278331, REC Reference 20/LO/1227). The NHS REC was London (Dulwich); favourable opinion with conditions was provided on 5 January 2021 and acknowledgement that conditions had been met on 19 January 2021. Modification to the protocol and/or study-related documents that could have an impact on the conduct of the study, potential benefit to patients or patient safety were submitted as amendments to the HRA, and, where required, the NHS REC.
The study was conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964 and later revisions.
Existing database approvals
The NDAU holds UK REC approval, 16/LO/1093, and Confidential Advisory Group (CAG) approval, Ethics and Confidentiality Committee [ECC 8-05(f/2010)], to form the NNRD. Study-specific REC approval to access the NNRD was provided by London Dulwich NHS REC (20/LO/1227), and approval was obtained from all English, Scottish and Welsh neonatal units.
British Association of Paediatric Surgeons-Congenital Anomalies Surveillance System has been approved by the National Research Ethics Service Committee South Central-Oxford A (Ref: 12/SC/0416).
The linked HES-APC and mortality data have been supplied by NHS Digital with signatories Garry Coleman (NHS Digital), Richard Langley (Health and Social Care Information Centre), Sophie Baines (University of Oxford) for application ‘Epidemiological and health services research using routine NHS data: work programme of the Unit of Health Care Epidemiology, Oxford University’ and application reference number DARS-NIC-315419-F3W7K.
Chapter 3 Patient and public involvement
Introduction
This chapter presents and discusses the patient and public involvement and engagement (PPIE) activity in the ToSCiN feasibility study. Previous chapters address patient and parent participation in the observational cohort study, but it is important to distinguish between participation, involvement and engagement.
Participation refers to enrolling (with informed consent) participants with a condition of interest in a research project with a specified research protocol with the aim of answering an identified research question. Involvement is different. Involvement seeks to give individuals with lived experience a platform from which their views on a range of aspects of a research project are sought, and can be offered freely, to influence decisions in research. Finally, engagement consists of sharing of information about research with patients and the public. We aimed to both involve and engage parents of infants with a stoma in the ToSCiN feasibility study. This activity is the focus of this chapter.
Methods
A parent co-investigator who had experience of having an infant with a stoma contributed to drafting the grant proposal and was subsequently involved at several points throughout the research programme. We formed a Parental Advisory Group (PAG) of parents who had a lived experience of caring for an infant who had had a stoma during the newborn period. These parents were approached via personal contacts of the study team, and through two parent support organisations known to have members who had experience of caring for an infant with a stoma. These were the preterm birth charity Bliss, and NEC-UK – a family support organisation for families with an infant affected by NEC. Our intention was for this PAG to meet during the study to inform and advise on study activities.
We had originally intended for PAG meetings to be held face to face. However, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic that commenced in spring 2020 and the subsequent national lockdown prevented face-to-face meetings. Instead, all meetings of the PAG were held virtually via the Zoom platform. Ultimately, this enabled involvement with all those who were interested, in a way that was comfortable, acceptable and convenient for them. The PAG was convened by NH, who had experience of running a similar group in previous research. NH organised and ‘chaired’ all PAG meetings. The parent co-investigator also participated in PAG meetings when able, as it was felt she could provide additional insight into discussions of the PAG. At the outset, it was anticipated that four meetings of the PAG would be held, with timings of meetings guided by the needs of the study.
Prior to each meeting of the PAG, NH circulated to all those attending a brief summary agenda of what would be discussed at the meeting along with other documents to review in advance of the meeting if relevant for that particular meeting. Parents were typically given at least a week to review materials in advance of each meeting. Meetings lasted between 60 and 120 minutes. All participants were provided with an honorarium following their attendance, in accordance with INVOLVE guidance.
The PPIE activity with ToSCiN feasibility study was developed and is reported in accordance with GriPP guidance. Our approach addressed all the values and principles advocated by INVOLVE, of respect, support, transparency, responsiveness, fairness of opportunity and accountability.
Involved people – our Parental Advisory Group
A total of 38 parents responded to our initial request to join our PAG. From these, we requested a small amount of information regarding their experience of caring for an infant with a stoma in order to achieve a breadth of experience in the final PAG. We wished to achieve a PAG that was representative in terms of the underlying conditions suffered by babies who had a stoma and of gestational age at birth (specifically a mix of preterm and term babies), and diverse in terms of centre at which the infant had been treated and sex (mothers and fathers). We also aimed to involve parents who had a relatively recent experience of neonatal care – ideally within the previous 2 years. Following communication between NH and parent responders by e-mail and/or telephone, ultimately seven parents agreed to form the PAG. All were mothers (no fathers responded to initial request) and between them they had experienced care at six different specialist children’s hospitals. Five parents had a preterm infant who had had a stoma (four for NEC and one for an isolated perforation) and two parents had had a term infant (both were born with an anorectal malformation).
Meetings
Meetings were planned to correspond with times when the project most required the perspective of parents. Overall, throughout the study, we wished to get the PAG to contribute to the recruitment materials for the project, review interview topic guides, review proposed recruitment materials for a proposed future RCT and finally to create and review dissemination materials. Actual meeting and other activity timings, topics covered and outputs are presented in Table 2. To date, three meetings have taken place and one more is planned in order to co-produce dissemination materials with our PAG and discuss the most appropriate route for disseminating findings to parents and the public. All PAG meetings were attended by the PPI co-investigator and NH. Minutes of meetings can be seen in (see Report Supplementary Material 11).
| Date | Topic covered/activity planned | Output/learning |
|---|---|---|
| 8 June 2020 |
|
|
|
|
|
|
|
|
| 7 December 2020 |
|
|
|
|
|
|
|
|
| E-mail communication May 2021 |
|
|
| 24 November 2021 |
|
|
|
|
|
|
||
| By e-mail Spring 2022 |
|
|
Remuneration
We recognise the value that PAG members added to the ToSCiN feasibility study and remunerated them following guidelines (Mental Health Research Network and INVOLVE, 2013) and experience. Consequently, every member was given a voucher following each meeting or involvement activity to the value of £75 in the form of either a Love2Shop voucher or Amazon voucher, depending on the individual’s preference. Each PAG member was offered reimbursement of child care costs or other out-of-pocket expenses in the event that these were needed to facilitate their attendance at meetings. None claimed.
Impact
On the study
The PPI work in this project was designed to ensure the study was conducted and proceeded in a way that maintained its relevance to parents and encouraged their participation. The most substantial impact was that the PAG helped keep the study grounded in the interests and priorities of parents who have an infant with a stoma. Parents readily expressed their views about proposed study activities and regularly made helpful recommendations for improvement when the proposed activities were not in keeping with their preferences. This ranged from relatively simple yet important interventions such as amending wording on information sheets, through to more substantial redesign of study printed materials and making important observations and recommendations regarding the most appropriate timing of approach to parents for participation in the study. PAG members often spoke passionately about topics and had important and sometimes strong views based on their lived experience. They wished to express these in the interest of improving the experience of the research for parent participants. PAG members were also able to propose insight into the most important outcomes to measure in future work, bringing a slightly different perspective to those of parents who were recruited into the study, since the PAG members had lived through the experience of having an infant with a stoma rather than it being a current active concern for them. Some PAG members were also able to attend the consensus meeting in July 2022, increasing the numbers of parents who were able to participate in this important stage of the research. In the future, we hope that PAG members will contribute to information enabling dissemination of the research, including making recommendations about the most appropriate channels for dissemination. The study team hope to co-produce dissemination products with members of the PAG and in doing so make them more accessible, interesting and relevant to parents, as a direct result of their involvement.
On the researchers
The PAG and its members made a significant impression on the study team. The group was highly enjoyable to work with and always embraced involvement in every task. It was motivating to see parents who had clearly been through so much with their own infant’s experience willingly give up their time (including making time to attend meetings), and willingly express their opinions in the interest of improving the research. Researchers involved in PAG discussions further developed their skills in facilitating PPIE activities, in particular facilitating these discussions online given the enforced virtual nature of PAG meetings for this study.
The study team found the experience of working with PAG an enjoyable one from a personal perspective. While the PAG activity contributed in a material way to the study, in particular optimising its acceptability to parents, the process of working with the PAG was very rewarding. We believe this additional emotional motivation cannot be underestimated when considering the value it brings to the study and research team; yet it cannot be measured.
On the Parent Advisory Group members
We will run a reflective exercise at our final PAG meeting to understand the impact that involvement in the study has had on the parents who have been involved. We intend to use these to develop the study team’s understanding of how to best include PPIE activity in our future work and to develop the skills of the research team when organising and facilitating PPIE activity.
Discussion
This chapter reports on the PPI approach and activities that occurred over the course of the ToSCiN feasibility study, and those activities that are still to take place. Our approach to involvement was framed by the study team believing that engaging with parents and the public is the ‘right’ thing to do and also based on their experience of working with similar groups in previous research studies. The team need no convincing of the real benefits that engaging with groups such as the PAG can have on research. While the impact of the PAG on the research is difficult to formally evaluate, we believe that their involvement has improved the study, primarily from the perspective of optimising its accessibility, relevance and engagement. We believe it likely that this impact will be seen beyond the lifetime of this current project since important insights have been learnt that will be incorporated into a future trial in this patient population.
The majority of the involvement tasks requested of the PAG, for example reviewing patient information sheets, were fairly straightforward and easily comprehended. The discussion about outcomes was arguably more complex, yet easily understood by the PAG members and could therefore easily be conducted in an online setting. Had a different range of tasks had been required, it is possible that in-person meetings may have been beneficial. Clearly, there are benefits to online meetings, including avoidance of travel time, time away from home and reduced costs. These benefits should be balanced against the needs of the meeting taking place and the tasks required by the research.
Due to the longer-than-intended intervals between PAG meetings (a consequence of delays to the overall project due to the SARS-CoV-2 pandemic), the study team made certain to provide a thorough reminder of the project at the beginning of each PAG meeting. Between meetings, update e-mails were sent in an attempt to keep PAG members updated about the progress of the research (even if progress had been delayed). While it is not known whether these were necessary, given the extended timelines of the study, the study team felt this was important in maintaining the engagement of the PAG.
Notably, there were no male parent responders to the initial invitation for PAG members. While the study team would have preferred to have a mix of male/female PAG members in terms of achieving diversity, this was not achieved. We do not know if the views of the group would have been different had this diversity been achieved; yet we do not have significant concerns about the output that we have accomplished. We were, however, able to achieve some diversity in terms of geographic representation and the range of underlying conditions of which PAG members had experience. We believe that this is important for the generalisability of the output of the group.
Conclusion
We involved parents with experience of having an infant with a stoma in our research through the formation of a parent advisory group that informed and advised the main study design and processes. The various components of the study all benefitted from this programme of PPI. The group continued to be involved throughout the study, which enabled them to have the greatest impact. There remain future plans for co-producing dissemination outputs that are relevant and accessible regarding the research undertaken.
Chapter 4 Results of Workstream 1
Responses
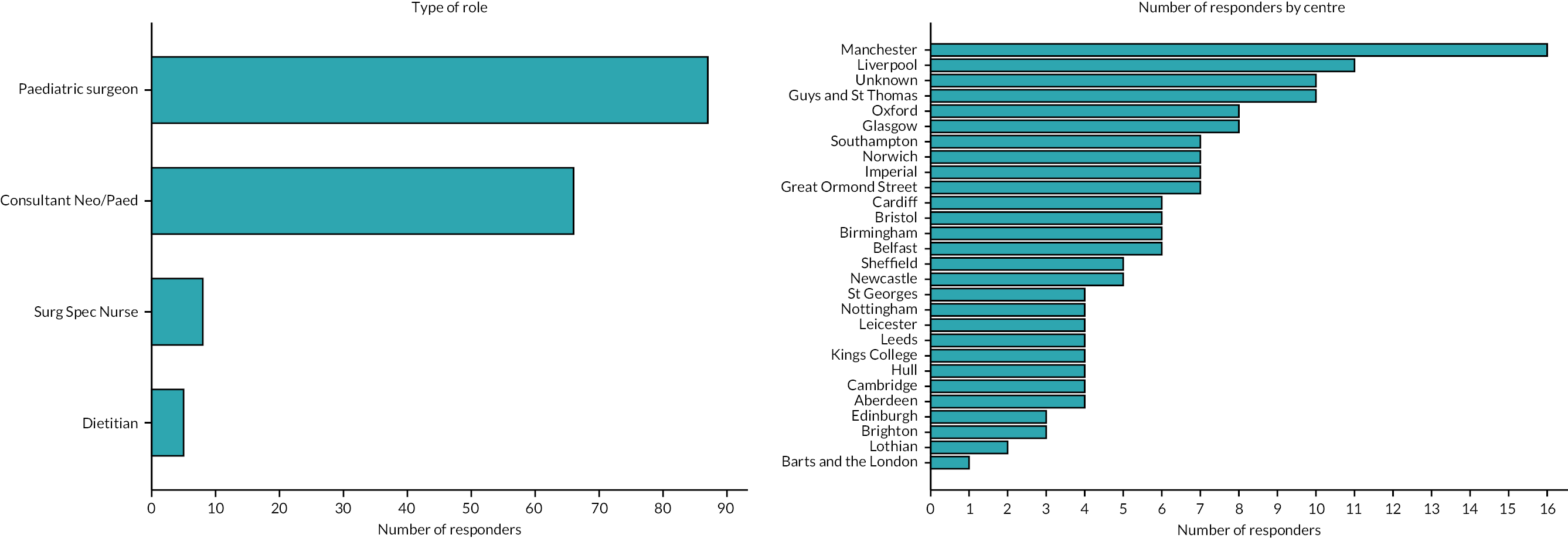
A total of 166 professionals completed the survey: 52% were paediatric surgeons, 40% neonatologists, 5% surgical nurse specialists and 3% dietitians (Figure 2). There was at least one respondent from each of the 27 UK neonatal surgical centres. Seventy-seven per cent of respondents worked in a unit with a co-located neonatal intensive care unit (NICU) and surgical centre. Individual centre response volume is detailed in Figure 2.
FIGURE 2.
Number of survey responses according to professional role (left panel) and providing centre (right panel).
Attitudes to timing of stoma closure
Without defining the terms, 47% of respondents considered themselves proponents of ‘early’ stoma closure and 28% ‘late’. Twenty-five per cent were unsure. Ninety-two per cent of units recycled stoma output through the mucous fistula: 25% routinely, 41% sometimes, 26% rarely and 8% never (two respondents did not answer).
Respondents then had four scenarios aiming to explore how gestation and stoma location, if at all, influenced their attitudes to timing of stoma closure (Table 3). Response rates to the target of stoma closure timing were 63–75% across the scenarios, with the remainder being unsure.
| Scenario 1 | ‘A premature infant born at 26 weeks’ gestation (birthweight 800 g) deteriorates clinically on day 3 of life. An isolated perforation of the distal small bowel (ileum) is found at laparotomy and a stoma and mucous fistula are formed at this level’. |
| Scenario 2 | ‘A premature infant born at 26 weeks’ gestation (birthweight 800 g) develops clinical signs of NEC at 4 weeks of age. A laparotomy confirms diffuse small bowel involvement and 50 cm of bowel is resected. A stoma and mucous fistula are formed at the level of the mid-jejunum’. |
| Scenario 3 | ‘A term infant is born with signs of distal bowel obstruction and a failure to pass meconium. “Simple” meconium ileus and a microcolon are found at laparotomy. A stoma and mucous fistula are formed in the mid-ileum’. |
| Scenario 4 | ‘A term infant is born with signs of proximal bowel obstruction and a failure to pass meconium. At laparotomy, a jejunal atresia is found. A stoma and mucous fistula are formed at the site of the atresia (mid-jejunum)’. |
In both preterm scenarios (either an ileal stoma, or a jejunal stoma with 50 cm of bowel resected), the majority of respondents would aim to close the stoma at 6 weeks of age with the median at 8 weeks. Most respondents would want to reverse the stoma before discharge. In both term infant scenarios, the expectation is that the stoma would be reversed earlier. The median target time was 4–6 weeks, and respondents’ experience was that they were closed earlier than in preterm infants. However, for term infants with an ileostomy, only 32% of respondents – compared with around 70% in the other three scenarios – would normally want the stoma closed before discharge.
Factors potentially influencing timing of stoma closure
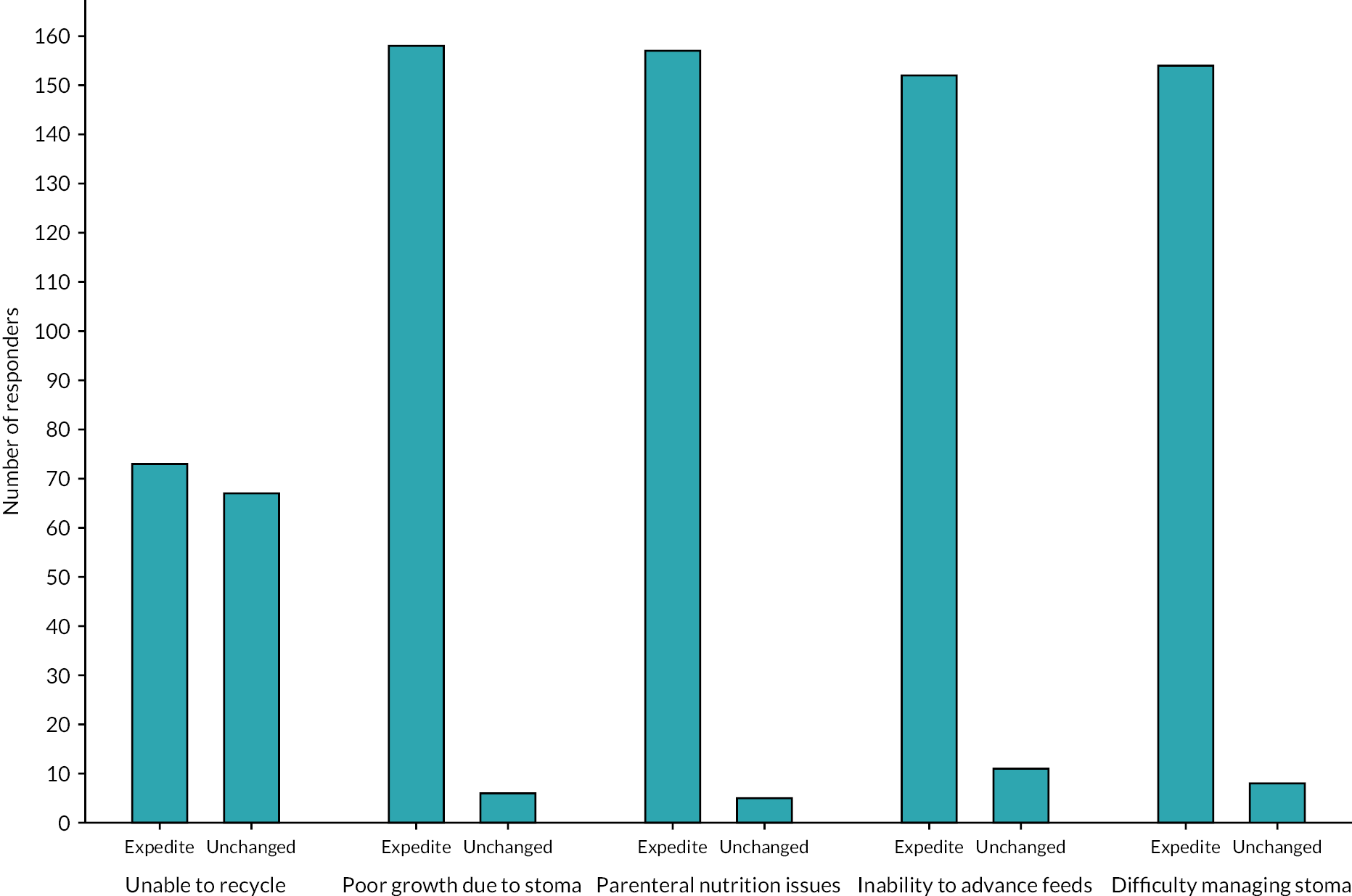
Weight was a commonly cited factor in determining the timing of stoma closure, with 41% (54 of 132 respondents) (Table 4) wanting their patients to have achieved a predefined weight: the median threshold was 2000 g (IQR 1625–2500 g) and the most commonly cited value 2500 g (Figure 3). Receiving steroids in the last week was the only additional clinical factor for which the majority of respondents (58%) would delay closure. Over 90% of respondents would want to bring forward stoma reversal if there were difficulties with growth, liver disease, line sepsis, being unable to advance feeds or managing the stoma (Figure 4).
| Factors potentially delaying reversal | Percentage of respondents who would delay | Factors potentially expediting reversal | Percentage of respondents who would expedite |
|---|---|---|---|
| Invasively ventilated but clinically stable | 40 | Unable to recycle via distal stoma | 52 |
| Non-invasively ventilated but clinically stable | 29 | Growth concerns due to stoma | 96 |
| Weight below a specified threshold | 41 | Complications of PN | 97 |
| Steroids within the previous week | 58 | Unable to advance feeds | 93 |
| Stoma complications | 95 |
FIGURE 3.
Minimum weight threshold for in those delaying stoma closure surgery until predefined weight achieved.
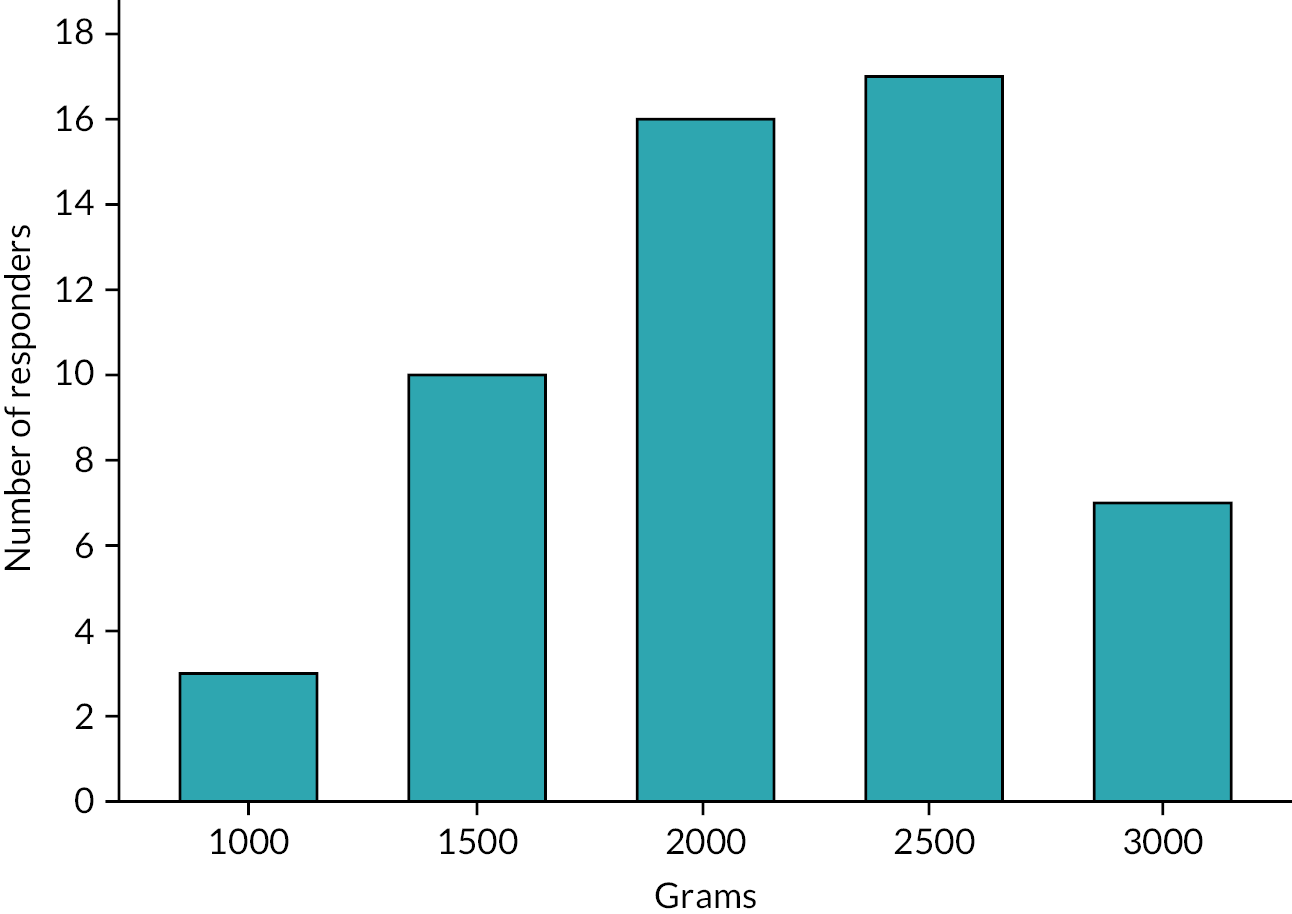
FIGURE 4.
Factors that would result in stoma closure being expedited.
Opinions on the design of a potential future trial
There were a series of questions asking about the acceptability of a future study design. At least 86% of respondents would include preterm infants with either NEC or SIP, and 72% would include term infants with an atresia, meconium ileus or malrotation (Figure 5). The preferred later time point for stoma closure was 12 weeks. For the earlier time point, over 70% of respondents selected either 4 or 6 weeks, but 6 weeks was the most commonly cited value (Figure 6).
FIGURE 5.
Infants to be included in a trial population.
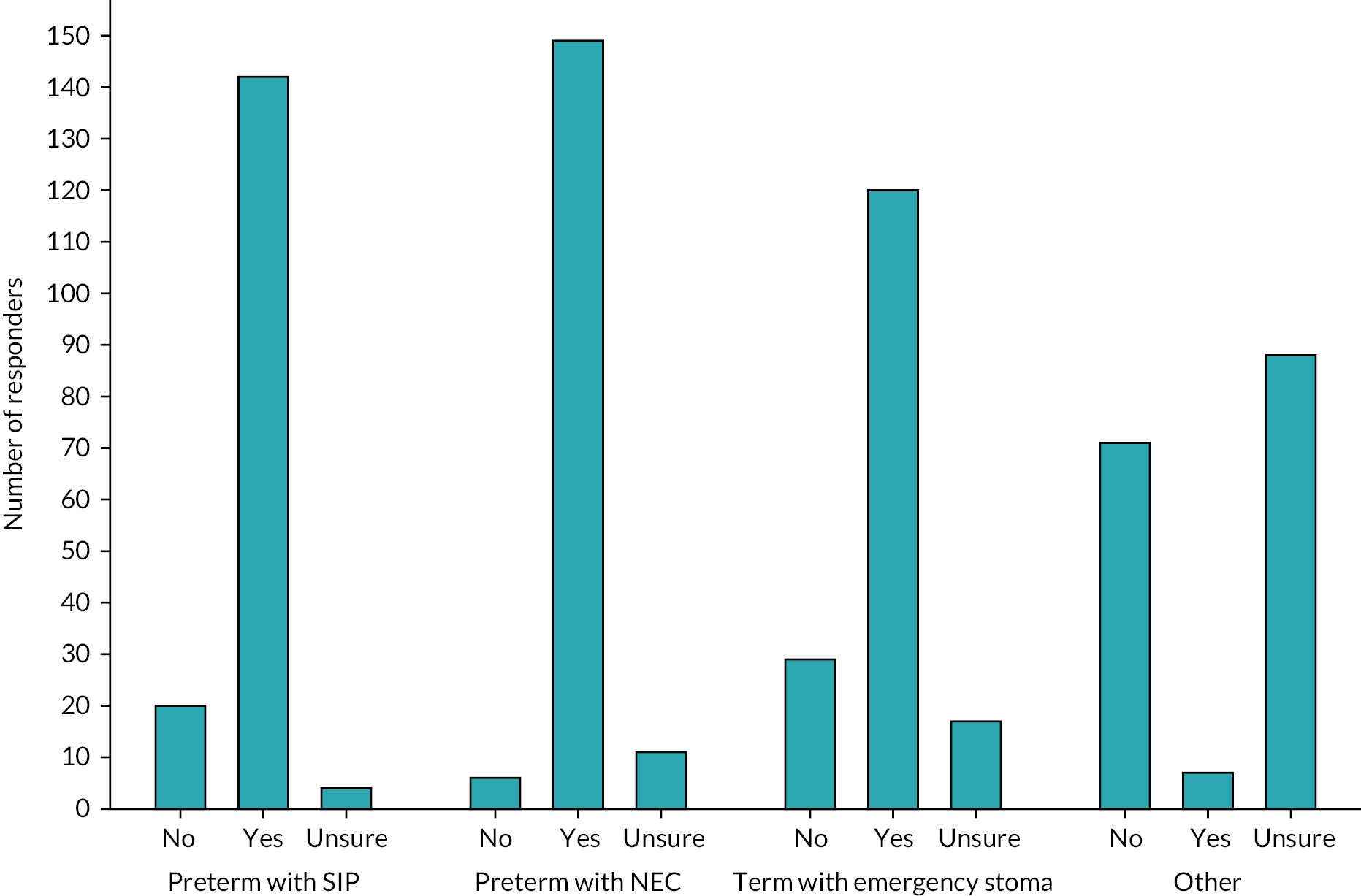
FIGURE 6.
Timing of the early and late intervention.
Trial outcomes
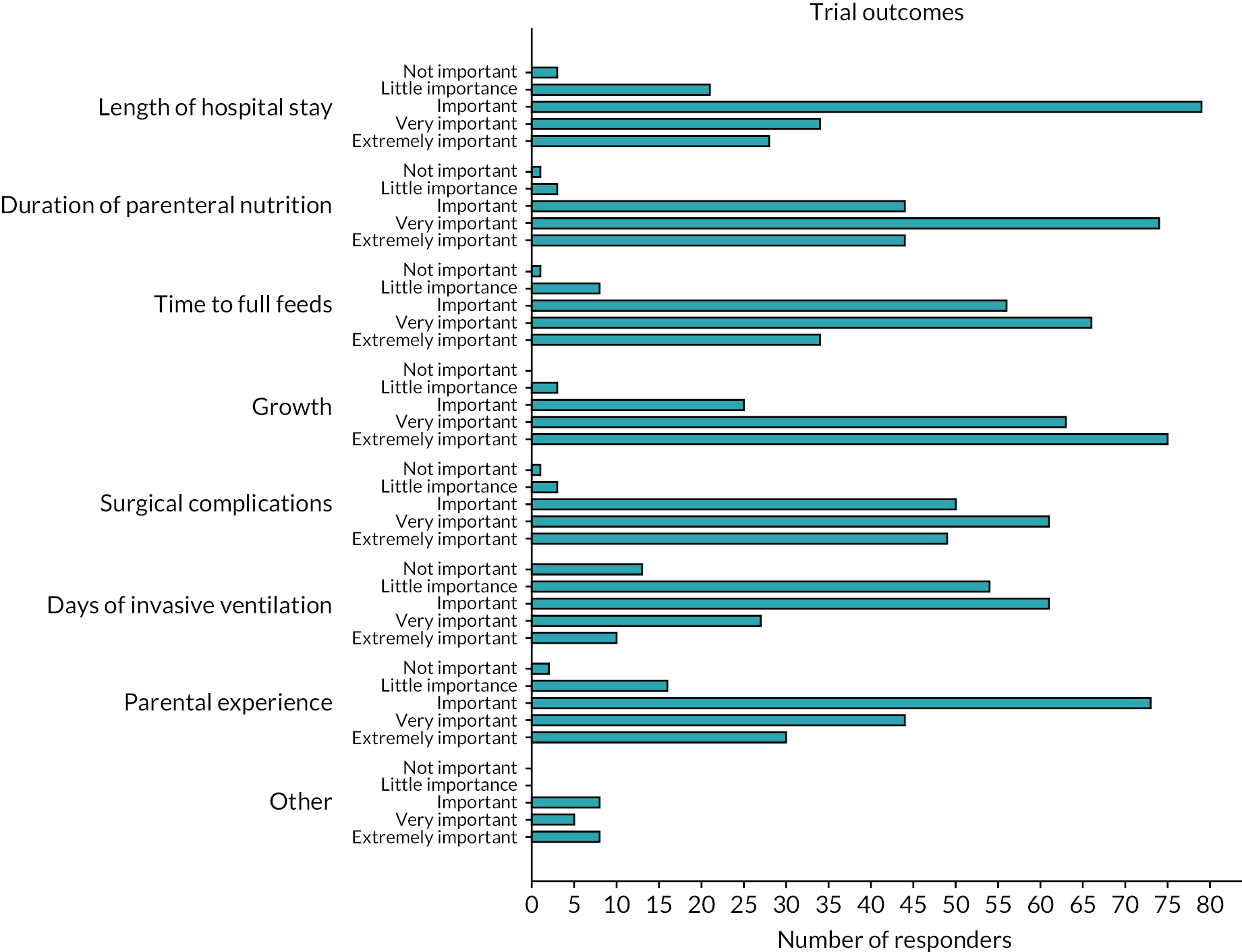
Respondents were asked to consider a wide range of trial outcomes. Growth was most commonly selected as the favoured primary outcome for a trial (Figure 7) and most commonly was indicated to be ‘extremely important’ as an outcome measure (Figure 8). Time to full feeds was the second most commonly selected primary outcome measure, followed by length of stay (LOS) and duration of PN. Respondents were also asked to state how important a number of prespecified outcomes were and they are shown in Figure 8.
FIGURE 7.
Survey respondents’ favoured primary outcome measure for a trial.
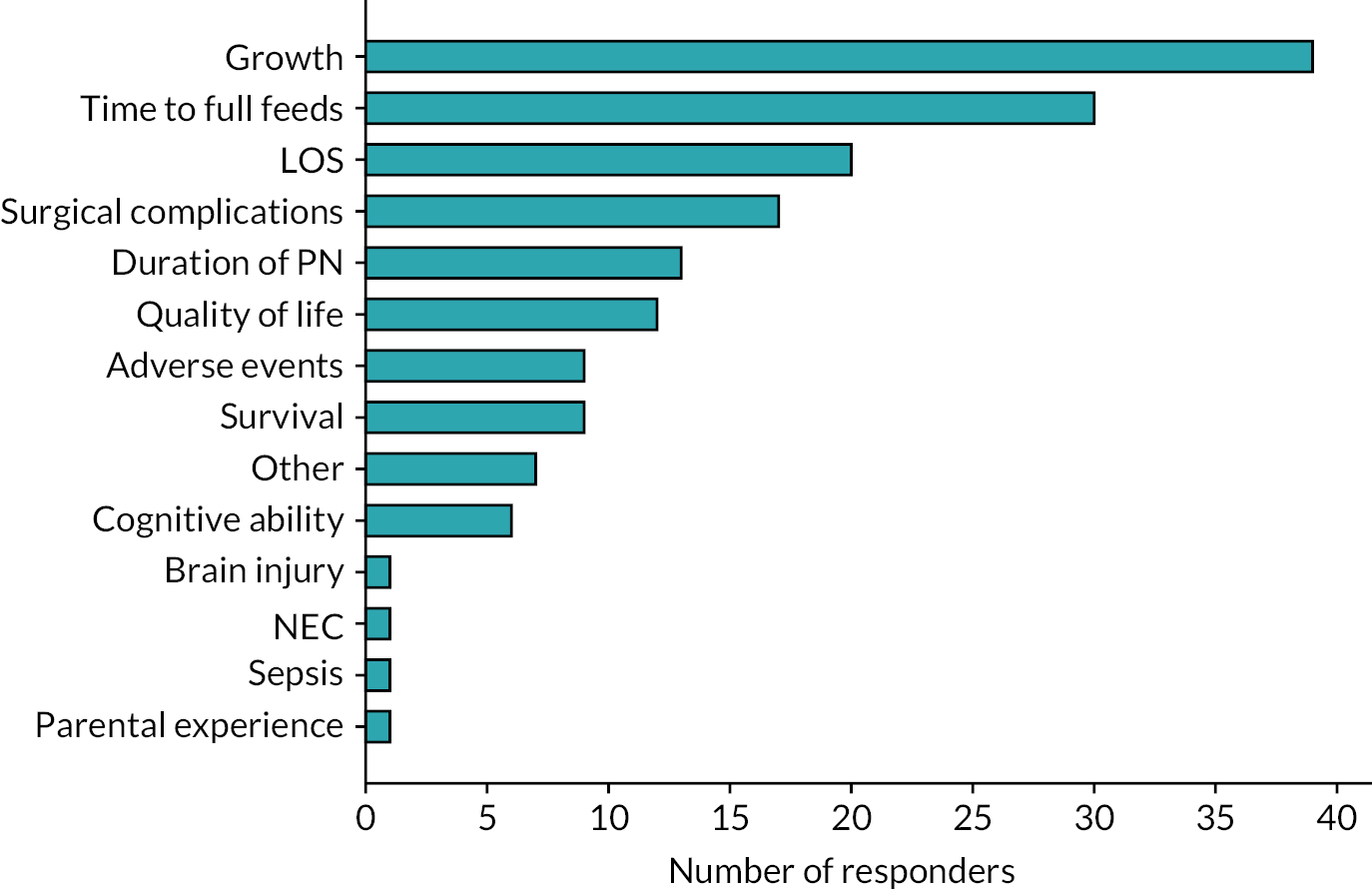
FIGURE 8.
Importance of predefined outcome measures to survey respondents.
Potential barriers to a clinical trial
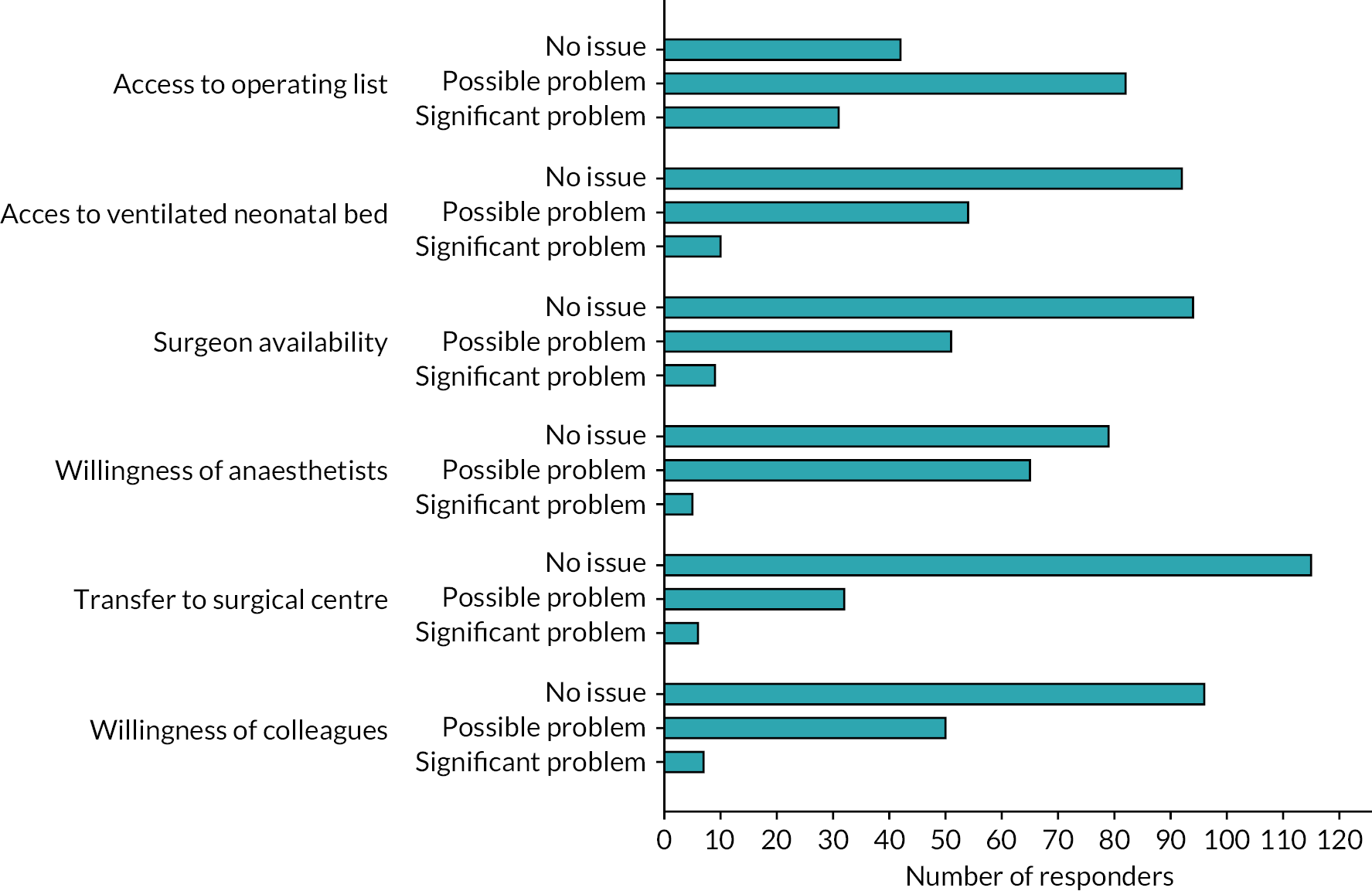
Respondents stated that, in their current practice, the typical time from decision to undertaking a stoma reversal was most commonly 1–2 weeks. Reasons for delays and potential barriers to being able to fulfil a trial treatment allocation as per protocol were then explored. The ability to access a neonatal operating list was most commonly cited as ‘likely significant problem’ and ‘may be a problem’, by 20% and 53% of respondents, respectively (Figure 9). Other factors put forward in the survey were not considered an issue by the majority of respondents.
FIGURE 9.
Significance of factors that may act as a barrier to a clinical trial.
Strengths and weaknesses
A key strength of this survey is its wide coverage and high response rate, with multidisciplinary responses from all UK neonatal surgical units. The results are therefore likely to be highly representative of current UK practice. However, as with most surveys of practice, a limitation is that respondents reported what they believe their practice to be, rather than providing data on actual practice. We attempted to mitigate against this through provision of real-world clinical scenarios, and observational data about practice are found later in the ToSCiN study.
Chapter 5 Results of Workstream 2
Introduction to Workstream 2
In this part of the ToSCiN study, we aimed to determine factors that influence the timing of stoma closure and the clinical outcomes that are likely to be important in a future trial. We also aimed to determine how clinicians and parents viewed the prospect of a clinical trial that would randomise infants to ‘early’ or ‘late’ closure. This workstream comprised three components: (2.1) an observational cohort study of neonates who had a stoma formed; (2.2) questionnaires for the principal clinicians (surgeon and neonatologist) caring for each infant recruited to the cohort study; and (2.3) a qualitative study incorporating: (1) focus groups with clinicians; and (2) interviews with parents of neonates who had a stoma.
Workstream 2.1 and 2.2: Findings from the prospective cohort study
Between 13 April 2021 and 31 January 2022, a total of 56 infants were enrolled in the study across eight different specialist neonatal surgical units in the UK. The median age at enrolment was 13.5 days (range 1–85 days) and just under half were male. The cohort comprised 37 type A (NEC/SIP) infants and 19 type B (other diagnoses) infants. Demographic details of the cohort as a whole and the two subgroups, including distribution of gestational age at birth, are shown in Table 5 and Figure 10. Although group A infants were mainly preterm, 8 of 37 (22%) were over 32 weeks’ gestation and hence outside the typical high-risk gestation range for NEC and SIP. Similarly, although type B infants were more mature, 4 of 19 (21%) were < 32 weeks' gestation and only 7 (37%) were truly term (> 37+0 weeks' gestation).
| Overall N = 56 |
Infant type Aa N = 37 |
Infant type Ba N = 19 |
||||
|---|---|---|---|---|---|---|
| Recruiting centre | ||||||
| Alder Hey Hospital, Liverpool | 6 | (10.7) | 1 | (2.7) | 5 | (26.3) |
| Birmingham Children’s Hospital | 4 | (7.1) | 3 | (8.1) | 1 | (5.3) |
| Bristol Royal Hospital for Children | 11 | (19.6) | 7 | (18.9) | 4 | (21.1) |
| Chelsea and Westminster Hospital | 3 | (5.4) | 3 | (8.1) | 0 | (-) |
| Evelina London Children’s Hospital | 7 | (12.5) | 6 | (16.2) | 1 | (5.3) |
| Royal Hospital for Children, Glasgow | 5 | (8.9) | 2 | (5.4) | 3 | (15.8) |
| Royal Manchester Children’s Hospital | 13 | (23.2) | 10 | (27.0) | 3 | (15.8) |
| Southampton General Hospital | 7 | (12.5) | 5 | (13.5) | 2 | (10.5) |
| Age at enrolment (days) | ||||||
| Mean (SD) | 20.5 | (17.3) | 23.2 | (18.4) | 15.4 | (14.0) |
| Median (IQR) | 13.5 | (9.0–26.0) | 16 | (12.0–28.0) | 10 | (7.0–22.0) |
| Minimum, maximum | 1, 85 | 5, 85 | 1, 54 | |||
| Missing | 0 | 0 | 0 | |||
| Gestational age at birth | ||||||
| 22+0–24+6 | 14 | (25.0) | 13 | (35.1) | 1 | (5.3) |
| 25+0–27+6 | 16 | (28.6) | 14 | (37.8) | 2 | (10.5) |
| 28+0–31+6 | 3 | (5.4) | 2 | (5.4) | 1 | (5.3) |
| 32+0–36+6 | 11 | (19.6) | 3 | (8.1) | 8 | (42.1) |
| 37+0–40+6 | 9 | (16.1) | 3 | (8.1) | 6 | (31.6) |
| 41+0–44+6 | 3 | (5.4) | 2 | (5.4) | 1 | (5.3) |
| Missing | 0 | 0 | 0 | |||
| Infant sex | ||||||
| Male | 24 | (42.9) | 17 | (46.0) | 7 | (36.8) |
| Female | 32 | (57.1) | 20 | (54.0) | 12 | (63.2) |
| Indeterminate | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 0 | 0 | 0 | |||
| Birthweight (g) | ||||||
| Median (IQR) | 961 | (632–2373) | 782 | (600–1190) | 2720 | (1415–3280) |
| Minimum, maximum | 415, 3962 | 415, 3962 | 478, 3840 | |||
| Missing | 0 | 0 | 0 | |||
| One of a multiple pregnancy | 11 | (19.6) | 9 | (24.3) | 2 | (10.5) |
| Missing | 0 | 0 | ||||
FIGURE 10.
Bar chart for gestational age at birth (completed weeks).
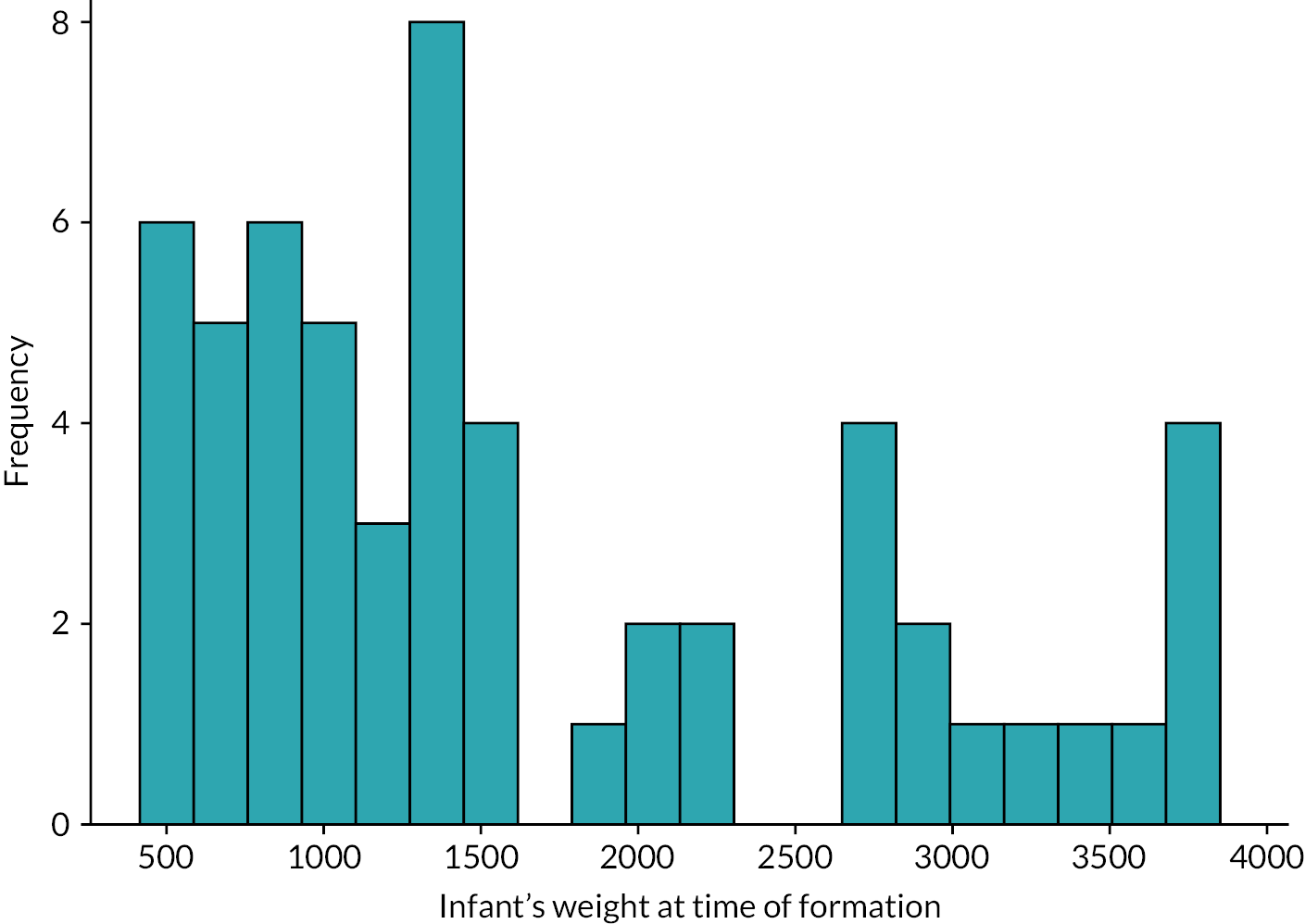
The primary diagnoses requiring stoma formation, age and weight at stoma formation are shown in Table 6 and Figures 11 and 12. The majority of stomas were made in the small bowel (86% in the ileum, 9% in the jejunum) with just three infants (5%) having a colostomy.
| Overall N = 56 |
Infant type Aa N = 37 |
Infant type Ba N = 19 |
||||
|---|---|---|---|---|---|---|
| Age at stoma formation (days) | ||||||
| Median (IQR) | 8.0 | (5.0–16.5) | 9 | (7.0–18.0) | 5 | (2.0–12.0) |
| Minimum, maximum | 1, 80 | 2, 80 | 1, 50 | |||
| Missing | 0 | 0 | 0 | |||
| Weight at stoma formation (g) | ||||||
| Median (IQR) | 1342 | (874–2440) | 1025 | (760–1405) | 2720 | (1560–3280) |
| Minimum, maximum | 415, 3851 | 415, 3851 | 465, 3840 | |||
| Missing | 0 | 0 | 0 | |||
| Primary diagnosis requiring stoma formation | ||||||
| NEC | 20 | (35.7) | 20 | (54.0) | 0 | (-) |
| SIP | 17 | (30.4) | 17 | (46.0) | 0 | (-) |
| Meconium ileus | 6 | (10.7) | 0 | (-) | 6 | (31.6) |
| Gastroschisis | 1 | (1.8) | 0 | (-) | 1 | (5.3) |
| Intestinal atresia | 5 | (8.9) | 0 | (-) | 5 | (26.3) |
| Volvulus | 2 | (3.6) | 0 | (-) | 2 | (10.5) |
| Milk curd obstruction | 2 | (3.6) | 0 | (-) | 2 | (10.5) |
| Otherb | 3 | (5.4) | 0 | (-) | 3 | (15.8) |
| Missing | 0 | 0 | 0 | |||
| Any comorbidities making randomisation inappropriate | 1 | (1.8) | 1 | (2.7) | 0 | (-) |
| Missing | 0 | 0 | 0 | |||
| Details of comorbidities (not mutually exclusive) Known major syndrome or genetic disorder | 0 | 0 | 0 | |||
| Complex cardiac comorbidity including requirement for surgeryc | 1 | 1 | 0 | |||
| Other major congenital anomaly | ||||||
| Palliative care pathway for other reason | 0 | 0 | 0 | |||
| Other | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Operative findings present (not mutually exclusive) | ||||||
| Presence of perforation | 35 | (62.5) | 29 | (78.4) | 6 | (31.6) |
| Missing | 0 | 0 | 0 | |||
| Signs of NEC | 21 | (37.5) | 21 | (56.8) | 0 | (-) |
| Localised | 13 | 13 | 0 | |||
| Diffuse | 4 | 4 | 0 | |||
| Multifocal | 4 | 4 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Evidence of residual NEC | ||||||
| Distal to stoma | 5 | (8.9) | 5 | (13.5) | 0 | (-) |
| Missing | 0 | 0 | 0 | |||
| Type of resection performed (not mutually exclusive) | ||||||
| Small bowel | 40 | (71.4) | 26 | (70.3) | 14 | (73.7) |
| Ileocaecal valve | 8 | (14.3) | 7 | (18.9) | 1 | (5.3) |
| Colon | 12 | (21.4) | 9 | (24.3) | 3 | (15.8) |
| Ascending | 8 | 7 | 1 | |||
| Transverse | 5 | 4 | 1 | |||
| Descending | 3 | 3 | 0 | |||
| Sigmoid | 3 | 2 | 1 | |||
| Rectum | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| None | 7 | (12.5) | 4 | (10.8) | 3 | (15.8) |
| Missing | 0 | 0 | 0 | |||
| Site of active stoma | ||||||
| Duodenum | 0 | (-) | 0 | (-) | 0 | (-) |
| Jejunum | 5 | (8.9) | 4 | (10.8) | 1 | (5.3) |
| Ileum | 48 | (85.7) | 31 | (83.8) | 17 | (89.5) |
| Colon | 3 | (5.4) | 2 | (5.4) | 1 | (5.3) |
| Missing | 0 | 0 | 0 | |||
| Length of small bowel from DJ flexure to active stoma (cm) | ||||||
| Median (IQR) | 52.5 | (40.0–90.0) | 40 | (35.0–45.0) | 90 | (60.0–95.0) |
| Minimum, maximum | 5, 120 | 5, 70 | 29, 120 | |||
| Not measured | 31 | 22 | 9 | |||
| Not applicable (stoma site is duodenum or colon) | 3 | 2 | 1 | |||
| Missing | 4 | 4 | 0 | |||
| Total length of small bowel remaining (cm) | ||||||
| Median (IQR) | 77 | (60–100) | 63 | (60–77) | 95 | (75–100) |
| Minimum, maximum | 15, 130 | 15, 85 | 44, 130 | |||
| Not measured | 40 | 27 | 13 | |||
| Missing | 5 | 5 | 0 | |||
FIGURE 11.
Histogram of age at stoma formation.
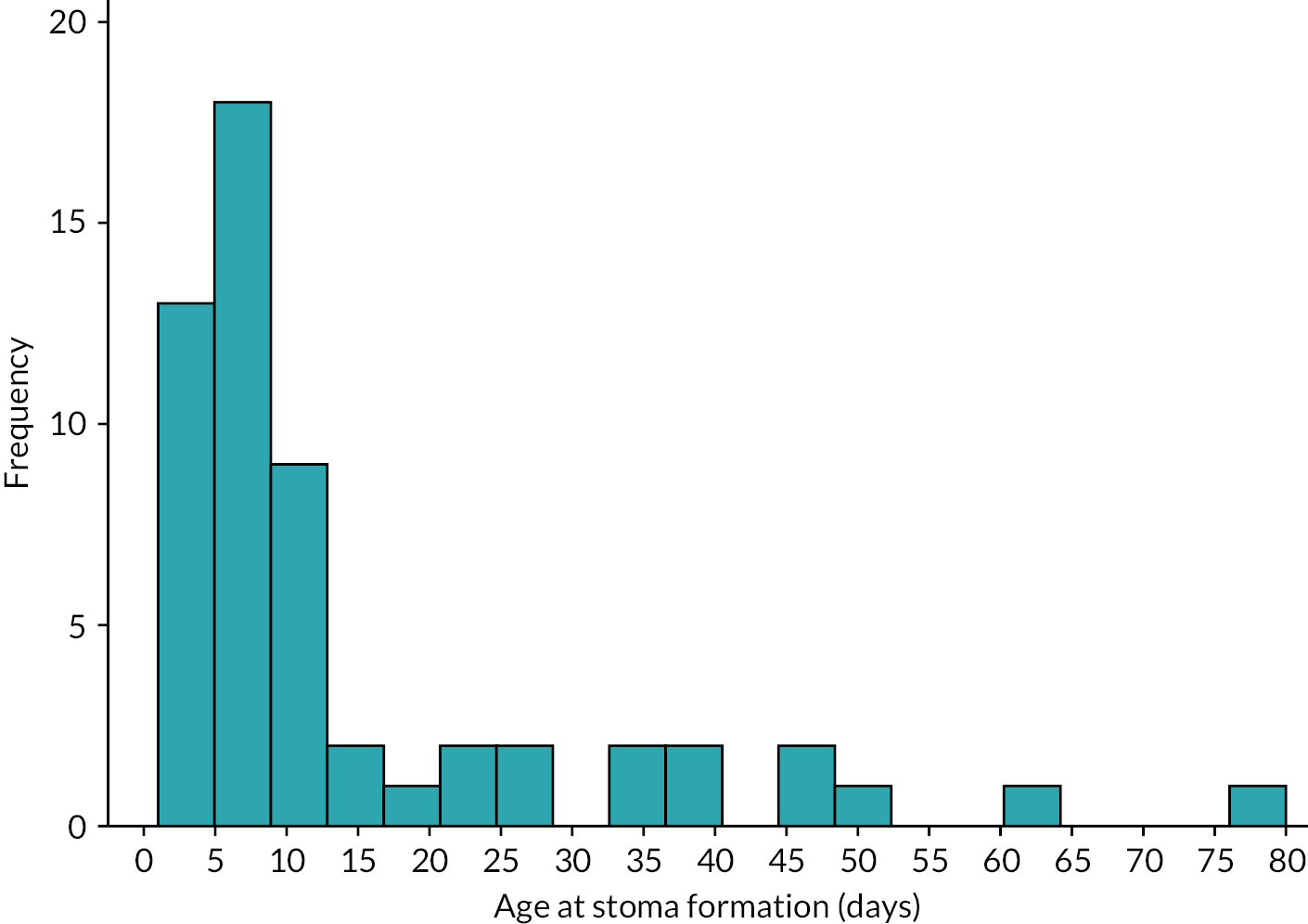
FIGURE 12.
Histogram of infant’s weight at time of stoma formation.
Among the 37 type A infants, 20 (54%) had NEC and 17 (46%) had SIP. Overall, nearly 80% (29/37) were found to have an intestinal perforation at the time of initial surgery. The majority of infants (33/37) had an intestinal resection, the most frequent site of which was the small bowel. The site of stoma formation was most frequently in the small bowel, and the length of small bowel from duodenojejunal (DJ) flexure to site of stoma was median 40 cm. The total residual small bowel length was median 63 cm. Once a stoma had been formed, five infants had evidence of residual NEC distal to their stoma. Full details including distribution of these variables are shown in Table 6. Just one type A infant was felt to be inappropriate for randomisation at the point of enrolment in this study in a future hypothetical RCT of timing of stoma closure. This was an infant with complex congenital cardiac disease that would require surgery, and the surgeon preferred to delay restoration of intestinal continuity until after repair of the congenital cardiac disease.
Among 19 type B infants, the most frequent diagnosis requiring stoma formation was meconium ileus followed by intestinal atresia. Other diagnoses in this group are shown in Table 6 (note that infants with a condition that would result in a planned timing of stoma formation such as Hirschsprung’s disease and anorectal malformation were specifically excluded from this study). Six of these infants were found to have a perforation at the time of initial surgery and 16 had an intestinal resection, most frequently involving the small bowel. The site of the stoma was in the small bowel in 18 of the 19 infants at median 90 cm from DJ flexure. No type B infant was thought to be unsuitable for enrolment in a future hypothetical RCT of timing of stoma formation on the basis of comorbidities.
One-week time point
A range of clinical data were captured at 1 week following stoma formation, since this is the point in time previously identified at which enrolment into a hypothetical RCT of timing of stoma formation might be considered (Table 7). If an infant was particularly unstable or remained critically unwell 1 week following stoma formation, then it may not be feasible to recruit at that point. Just under one-third of infants (n = 18) were mechanically ventilated 1 week post stoma formation, including eight who were on high-frequency oscillator ventilation and six who were receiving inhaled nitric oxide. All but two infants who were mechanically ventilated were type A infants. Overall, eight infants were receiving inotropic support (seven type A, one type B) and just under one-quarter (n = 13) had received blood products in the preceding 24 hours (12 type A, one type B).
| Overall N = 56 |
Infant type Aa N = 37 |
Infant type Ba N = 19 |
||||
|---|---|---|---|---|---|---|
| Time of form completion since stoma (days) | ||||||
| Median (IQR) | 7 | (7–8) | 7 | (7–9) | 7 | (7–8) |
| Minimum, maximum | 4, 18 | 4, 18 | 6, 13 | |||
| Missing | 0 | 0 | 0 | |||
| Current weight (g) | ||||||
| Median (IQR) | 1448 | (927–2515) | 1180 | (860–1698) | 2750 | (1850–3233) |
| Minimum, maximum | 540, 4110 | 540, 4110 | 540, 3820 | |||
| Missing | 0 | 0 | 0 | |||
| Current level of respiratory support (highest level of day) | ||||||
| None | 21 | (37.5) | 7 | (18.9) | 14 | (73.7) |
| Non-invasive support | 9 | (16.1) | 6 | (16.2) | 3 | (15.8) |
| Mechanical ventilation | 18 | (32.1) | 16 | (43.2) | 2 | (10.5) |
| High-frequency oscillatory ventilation | 8 | (14.3) | 8 | (21.6) | 0 | (-) |
| Missing | 0 | 0 | 0 | |||
| Receiving nitric oxide | 6 | (23.1) | 6 | (25.0) | 0 | (-) |
| Not applicableb | 30 | 13 | 17 | |||
| Missing | 0 | 0 | 0 | |||
| Number of days of respiratory support since stoma formation | ||||||
| Non-invasive support | ||||||
| Median (IQR) | 0 | (0–1) | 0 | (0–2) | 0 | (0–0) |
| Minimum, maximum | 0, 5 | 0, 5 | 0, 3 | |||
| Missing | 0 | 0 | 0 | |||
| Mechanical ventilation | ||||||
| Median (IQR) | 5 | (2–7) | 6 | (4–7) | 2 | (0–5) |
| Minimum, maximum | 0, 18 | 1, 18 | 0, 8 | |||
| Missing | 0 | 0 | 0 | |||
| High-frequency oscillatory ventilation | ||||||
| Median (IQR) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) |
| Minimum, maximum | 0, 7 | 0, 7 | 0, 0 | |||
| Missing | 0 | 0 | 0 | |||
| Currently receiving inotropic support | 8 | (14.3) | 7 | (18.9) | 1 | (5.3) |
| Missing | 0 | 0 | 0 | |||
| Receiving blood products in the last 24 hours | 13 | (23.2) | 12 | (32.4) | 1 | (5.3) |
| Missing | 0 | 0 | 0 | |||
| Products received (not mutually exclusive) | ||||||
| Packed red cells | 11 | 10 | 1 | |||
| Fresh-frozen plasma | 1 | 1 | 0 | |||
| Cryoprecipitate | 0 | 0 | 0 | |||
| Missing | 1 | 1 | 0 | |||
At this putative randomisation time point (1 week following stoma formation), surgeons stated they would be willing to randomise 31 infants (59%) into a future hypothetical RCT of timing of stoma formation [19 type A (56% of all type A), 12 type B (63% of all type B)], would not be willing to randomise 20 infants (13 type A, 7 type B) and two infants had died. At the same time point, neonatologists stated they would be willing to randomise 37 infants (74% of total) of which 25 were type A (76% of all type A) and 12 type B (71% of type B), would not be willing to randomise 7 (3 type A, 4 type B), 3 infants had died and 3 had been transferred elsewhere (slightly different numbers of transfers and deaths between surgeons and neonatologists owing to pragmatic nature of data collection, e.g. differing timings of response). Reasons for clinician unwillingness to randomise at 1 week following stoma formation are shown in Tables 8 and 9 for each type of infant.
| Overall N = 56 |
Type A infantsa N = 37 |
Type B infantsa N = 19 |
||||
|---|---|---|---|---|---|---|
| Randomisation time point (1 week post stoma formation) | ||||||
| Willing to randomise to stoma closure at ≤ 6 vs. 12 weeks post stoma formation | ||||||
| Yes | 31 | (58.5) | 19 | (55.9) | 12 | (63.2) |
| No | 20 | (37.7) | 13 | (38.2) | 7 | (36.8) |
| Infant died | 2 | (3.8) | 2 | (5.9) | 0 | (-) |
| Unable to say as infant transferred | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 3 | 3 | 0 | |||
| Significant factors in decision if not suitable for inclusion in a trial (not mutually exclusive) | ||||||
| Condition prior to stoma | 14 | 10 | 4 | |||
| Too small and/or too premature | 10 | 8 | 2 | |||
| Medical comorbidity (e.g. cardiac) | 2 | 1 | 1 | |||
| Other | 2 | 1 | 1 | |||
| Underlying disease process requiring a stoma | 9 | 5 | 4 | |||
| Diagnostic uncertainty | 1 | 1 | 0 | |||
| Distal disease | 2 | 2 | 0 | |||
| Other | 6 | 2 | 4 | |||
| Current clinical status | 10 | 7 | 3 | |||
| Logistical reasons | 0 | 0 | 0 | |||
| Social/family reasons | 0 | 0 | 0 | |||
| Other | 1 | 1 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Early closure time point (6 weeks post stoma formation) | ||||||
| Willing to follow trial allocation of stoma closure at ≤ 6 weeks post stoma formation | ||||||
| Yes | 17 | (33.3) | 11 | (34.4) | 6 | (31.6) |
| No | 25 | (49.0) | 18 | (56.3) | 7 | (36.8) |
| Stoma already closed | 9 | (17.7) | 3 | (9.4) | 6 | (31.6) |
| Infant died | 0 | (-) | 0 | (-) | 0 | (-) |
| Unable to say as infant transferred | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 5 | 5 | 0 | |||
| Logistically possible to close stoma, if willing to follow trial allocation of early closure | ||||||
| Yes | 12 | (70.6) | 8 | (72.7) | 4 | (66.7) |
| No | 5 | (29.4) | 3 | (27.3) | 2 | (33.3) |
| Reason if no (not mutually exclusive) | ||||||
| Theatre list | 5 | 3 | 2 | |||
| Cot availability | 1 | 0 | 1 | |||
| Surgeon availability | 0 | 0 | 0 | |||
| Anaesthetist availability | 1 | 0 | 1 | |||
| Other | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Reasons for not following trial allocation of early closure (not mutually exclusive) | ||||||
| Respiratory status | 7 | 6 | 1 | |||
| Cardiovascular status | 4 | 4 | 0 | |||
| Growth/nutrition/stoma status | 6 | 3 | 3 | |||
| Social/family reasons | 1 | 1 | 0 | |||
| Logistical, e.g. lack of list space | 2 | 0 | 2 | |||
| Size specifically mentioned | 9 | 8 | 1 | |||
| Diagnostic uncertainty/awaiting further tests | 3 | 3 | 0 | |||
| Other | 5 | 2 | 3 | |||
| Missing | 9 | 8 | 1 | |||
| Late closure time point (12 weeks post stoma formation) | ||||||
| Willing to follow trial allocation of stoma closure at 12 weeks post stoma formation | ||||||
| Yes | 28 | (53.9) | 16 | (48.5) | 12 | (63.2) |
| No | 24 | (46.2) | 17 | (51.5) | 7 | (36.8) |
| Infant died | 0 | (-) | 0 | (-) | 0 | (-) |
| Unable to say as infant transferred | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 4 | 4 | 0 | |||
| Logistically possible to close stoma, if willing to follow trial allocation of late closure | ||||||
| Yes | 22 | (78.6) | 14 | (87.5) | 8 | (66.7) |
| No | 6 | (21.4) | 2 | (12.5) | 4 | (33.3) |
| Reason if no (not mutually exclusive) | ||||||
| Theatre list | 5 | 2 | 3 | |||
| Cot availability | 0 | 0 | 0 | |||
| Surgeon availability | 1 | 1 | 0 | |||
| Anaesthetist availability | 0 | 0 | 0 | |||
| Other | 1 | 0 | 1 | |||
| Missing | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Reasons for not following trial allocation of late closure | ||||||
| Required earlier closure | 12 | 8 | 4 | |||
| Unsuitable for closure | 9 | 7 | 2 | |||
| Other | 3 | 2 | 1 | |||
| Missing | 0 | 0 | 0 | |||
| If unsuitable for closure or other reason given | (n = 12) | (n = 9) | (n = 3) | |||
| Reasons for not following trial allocation of late closure (not mutually exclusive) | ||||||
| Respiratory status | 3 | 2 | 1 | |||
| Cardiovascular status | 2 | 2 | 0 | |||
| Growth/nutrition/stoma status | 3 | 1 | 2 | |||
| Social/family reasons | 1 | 0 | 1 | |||
| Other | 5 | 5 | 0 | |||
| Missing | 1 | 1 | 0 | |||
| Overall N = 56 |
Type A infantsa N = 37 |
Type B infantsa N = 19 |
||||
|---|---|---|---|---|---|---|
| Randomisation time point (1 week post stoma formation) | ||||||
| Willing to randomise to stoma closure at ≤ 6 vs. 12 weeks post stoma formation | ||||||
| Yes | 37 | (74.0) | 25 | (75.8) | 12 | (70.6) |
| No | 7 | (14.0) | 3 | (9.1) | 4 | (23.5) |
| Infant died | 3 | (6.0) | 3 | (9.1) | 0 | (-) |
| Unable to say as infant transferred | 3 | (6.0) | 2 | (6.1) | 1 | (5.9) |
| Missing | 6 | 4 | 2 | |||
| Significant factors in decision if not suitable for inclusion in a trial (not mutually exclusive) | ||||||
| Condition prior to stoma | 4 | 2 | 2 | |||
| Too small and/or too premature | 1 | 1 | 0 | |||
| Medical comorbidity (e.g. cardiac) | 1 | 1 | 0 | |||
| Other | 2 | 0 | 2 | |||
| Underlying disease process requiring a stoma | 3 | 1 | 2 | |||
| Diagnostic uncertainty | 0 | 0 | 0 | |||
| Distal disease | 1 | 0 | 1 | |||
| Other | 2 | 1 | 1 | |||
| Current clinical status | 3 | 2 | 1 | |||
| Logistical reasons | 0 | 0 | 0 | |||
| Social/family reasons | 0 | 0 | 0 | |||
| Other | 1 | 0 | 1 | |||
| Missing | 0 | 0 | 0 | |||
| Early closure time point (6 weeks post stoma formation) | ||||||
| Willing to follow trial allocation of stoma closure at ≤ 6 weeks post stoma formation | ||||||
| Yes | 31 | (63.3) | 21 | (67.7) | 10 | (55.6) |
| No | 8 | (16.3) | 4 | (12.9) | 4 | (22.2) |
| Stoma already closed | 6 | (12.2) | 3 | (9.7) | 3 | (16.7) |
| Infant died | 0 | (-) | 0 | (-) | 0 | (-) |
| Unable to say as infant transferred | 4 | (8.2) | 3 | (9.7) | 1 | (5.6) |
| Missing | 7 | 6 | 1 | |||
| Logistically possible to close stoma, if willing to follow trial allocation of early closure | ||||||
| Yes | 28 | (90.3) | 20 | (95.2) | 8 | (80.0) |
| No | 3 | (9.7) | 1 | (4.8) | 2 | (20.0) |
| Reason if no (not mutually exclusive) | ||||||
| Theatre list | 2 | 1 | 1 | |||
| Cot availability | 1 | 0 | 1 | |||
| Surgeon availability | 0 | 0 | 0 | |||
| Anaesthetist availability | 0 | 0 | 0 | |||
| Other | 1 | 0 | 1 | |||
| Missing | 0 | 0 | 0 | |||
| Missing | 0 | 0 | 0 | |||
| Reasons for not following trial allocation of early closure (not mutually exclusive) | ||||||
| Respiratory status | 2 | 2 | 0 | |||
| Cardiovascular status | 2 | 2 | 0 | |||
| Growth/nutrition/stoma status | 2 | 1 | 1 | |||
| Social/family reasons | 0 | 0 | 0 | |||
| Other | 7 | 4 | 3 | |||
| Missing | 1 | 0 | 1 | |||
Key reasons for surgeons being unwilling to randomise at this time point were that the infant was too small or premature (n = 10), and other underlying disease process (n = 6). However, there was evidence of variation in the attitude of surgeons to randomise at this 1-week time point in relation to gestational age and size. Of the 31 infants that surgeons indicated they would be willing to randomise, half (n = 16) were born at < 28 weeks' gestation and 6 at < 25 weeks. Of the 20 that surgeons said they would not be willing to randomise, similar proportions were born at < 28 weeks (n = 11) or < 25 weeks (n = 7, Table 10). Thus, there may be variation among surgeons regarding attitude to the concept of randomising the least mature infants at this 1-week time point; some would be willing and some would not. It is possible that actual size may influence this attitude given that overall those that surgeons were unwilling to randomise appeared slightly lighter [1285 g (790–2225) vs. 1650 g (1029–2810), see Table 9], but of note, the minimum weight of an infant that was deemed suitable for randomisation was 570 g (see Table 10).
| Willing to randomisea | Willing to follow trial allocation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early closure time pointb | Late closure time pointc | |||||||||||
| Yes (n = 31) |
No (n = 20) |
Yes (n = 17) |
No (n = 25) |
Yes (n = 28) |
No (n = 24) |
|||||||
| Gestational age at birth | ||||||||||||
| 22+0–24+6 | 6 | 7 | 2 | 9 | 7 | 4 | ||||||
| 25+0–27+6 | 10 | 4 | 6 | 7 | 6 | 9 | ||||||
| 28+0–31+6 | 2 | 1 | 0 | 1 | 2 | 1 | ||||||
| 32+0–36+6 | 5 | 5 | 4 | 3 | 6 | 5 | ||||||
| 37+0–40+6 | 6 | 2 | 5 | 4 | 5 | 4 | ||||||
| 41+0–44+6 | 2 | 1 | 0 | 1 | 2 | 1 | ||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Age at stoma formation (days) | ||||||||||||
| Median (IQR) | 8 | (4–26) | 8 | (5–12) | 9 | (6–15) | 8 | (5–18) | 7 | (4–21) | 9 | (6–15) |
| Minimum, maximum | 1, 63 | 1, 45 | 2, 50 | 1, 63 | 1, 63 | 1, 50 | ||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Current weightd | ||||||||||||
| Median (IQR) | 1650 | (1029–2810) | 1285 | (790–2225) | 2090 | (1710–2663) | 1609 | (1150–2955) | 2566 | (1992–3840) | 2530 | (2240–3620) |
| Minimum, maximum | 570, 3845 | 540, 4110 | 1023, 3349 | 810, 4150 | 1170, 5480 | 1700, 5060 | ||||||
| Missing | 0 | 0 | 0 | 1 | 3 | 7 | ||||||
At 1 week following stoma formation, there was consensus between surgeon and neonatologist that 22/56 (46%) infants overall were suitable for enrolment (49% of type A infants and 41% of type B infants), consensus that 3 (6%) were not suitable for enrolment and no consensus in 16 (33%) (Table 11).
| Overall N = 56 |
Type A infantsa N = 37 |
Type B infantsa N = 19 |
||||
|---|---|---|---|---|---|---|
| Randomisation time point (1 week post stoma formation) | ||||||
| Willing to randomise to stoma closure at ≤ 6 vs. 12 weeks post stoma formation | ||||||
| Consensus – yes | 22 | (45.8) | 15 | (48.4) | 7 | (41.2) |
| Consensus – no | 3 | (6.3) | 1 | (3.2) | 2 | (11.8) |
| No consensus | 16 | (33.3) | 9 | (29.0) | 7 | (41.2) |
| At least one healthcare professional (HCP) unable to say | 7 | (14.6) | 6 | (19.4) | 1 | (5.9) |
| Missing | 8 | 6 | 2 | |||
| Early closure time point (6 weeks post stoma formation) | ||||||
| Willing to follow trial allocation of stoma closure at ≤ 6 weeks post stoma formation | ||||||
| Consensus – yes | 9 | (18.8) | 5 | (16.7) | 4 | (22.2) |
| Consensus – no | 5 | (10.4) | 2 | (6.7) | 3 | (16.7) |
| No consensus | 19 | (39.6) | 15 | (50.0) | 4 | (22.2) |
| At least one HCP unable to say/stoma closed | 15 | (31.3) | 8 | (26.7) | 7 | (38.9) |
| Missing | 8 | 7 | 1 | |||
| Late closure time point (12 weeks post stoma formation) | ||||||
| Willing to follow trial allocation of stoma closure at 12 weeks post stoma formation | ||||||
| Consensus – yes | 11 | (22.9) | 7 | (23.3) | 4 | (22.2) |
| Consensus – no | 8 | (16.7) | 5 | (16.7) | 3 | (16.7) |
| No consensus | 24 | (50.0) | 15 | (50.0) | 9 | (50.0) |
| At least one HCP unable to say | 5 | (10.4) | 3 | (10.0) | 2 | (11.1) |
| Missing | 8 | 7 | 1 | |||
Six-week time point
At the 6 weeks following stoma formation time point (Tables 12 and 13), data were available for 54 of 56 infants (data missing for 2) of whom 9 (17%) were mechanically ventilated (8 type A, 1 type B), 1 was receiving inotropes (type A infant) and 2 had received steroids in the preceding week (both type A). Further details of respiratory interventions between 1 and 6 weeks following stoma formation are shown in the tables. Fourteen infants (25%) were receiving antibiotics at the 6-week time point and five had had a positive blood culture in the previous 2 weeks, but none was being treated for a further episode of NEC. Weight in type A infants at 6 weeks following stoma formation was median 1710 g (range 795–4460 g) and in type B infants 3127 g (830–4410 g). Three infants were reported to have generalised oedema. Encouragingly, 80% were reported to have gained weight in the past week (13% lost weight, 7% no change). Stoma-related complications were reported in one-third (similar between type A and type B), stoma output was < 20 ml/kg/day in 86% and refeeding (recycling of stoma output) was being performed in just over one-quarter.
| Infant’s condition at early time point N = 56 |
Infant’s condition at late time point N = 56 |
|||
|---|---|---|---|---|
| Time of form completion since stoma (days) | ||||
| Median (IQR) | 45 | (42–48) | 85 | (84–90) |
| Minimum, maximum | 14, 66 | 42, 103 | ||
| Missing | 2 | 13 | ||
| Respiratory support | ||||
| Current level of respiratory support (highest level of day) | ||||
| None | 28 | (51.9) | 24 | (57.1) |
| Non-invasive support | 16 | (29.6) | 14 | (33.3) |
| Mechanical ventilation | 9 | (16.7) | 4 | (9.5) |
| High-frequency oscillatory ventilation | 1 | (1.9) | 0 | (-) |
| Missing | 2 | 14 | ||
| Received nitric oxide | 3 | (30.0) | 1 | (25.0) |
| Not applicable (respiratory support none or non-invasive) | 44 | 38 | ||
| Missing | 2 | 14 | ||
| Number of days of respiratory support since stoma formation | ||||
| Non-invasive support | ||||
| Median (IQR) | 4 | (0–18) | 29 | (0–46) |
| Minimum, maximum | 0, 41 | 0, 79 | ||
| Missing | 2 | 14 | ||
| Mechanical ventilation | ||||
| Median (IQR) | 8 | (2, 22) | 8 | (2, 25) |
| Minimum, maximum | 0, 43 | 0, 73 | ||
| Missing | 2 | 14 | ||
| High-frequency oscillatory ventilation | ||||
| Median (IQR) | 0 | (0, 0) | 0 | (0, 0) |
| Minimum, maximum | 0, 24 | 0, 26 | ||
| Missing | 2 | 14 | ||
| Postnatal steroids for chronic lung disease received in last week | 2 | (3.7) | 1 | (2.4) |
| Missing | 2 | 14 | ||
| Cardiovascular status | ||||
| Currently receiving inotropic support | 1 | (1.9) | 0 | (-) |
| Missing | 2 | 14 | ||
| Fluids and nutrition | ||||
| Currently receiving PN | ||||
| Yes | 25 | (46.3) | 23 | (54.8) |
| Volume currently receiving (ml/kg/day) | ||||
| Median (IQR) | 100 | (80, 120) | 102 | (100, 120) |
| Minimum, maximum | 21, 150 | 61, 150 | ||
| Missing | 0 | 0 | ||
| No, not required | 27 | (50.0) | 17 | (40.5) |
| No, inadequate vascular access to allow it | 2 | (3.7) | 2 | (4.8) |
| Missing | 2 | 14 | ||
| Currently receiving enteral feeds | 49 | (90.7) | 37 | (88.1) |
| Missing | 2 | 14 | ||
| Volume currently receiving (ml/kg/day) | ||||
| Median (IQR) | 127 | (62–165) | 150 | (70–163) |
| Minimum, maximum | 5, 190 | 6, 240 | ||
| Missing | 1 | 0 | ||
| Current weight (g) | ||||
| Median (IQR) | 2024 | (1400–2830) | 2548 | (2100–3681) |
| Minimum, maximum | 795, 4460 | 1170, 5480 | ||
| Missing | 3 | 14 | ||
| Currently oedematous | 3 | (5.6) | 2 | (4.8) |
| Missing | 2 | 14 | ||
| Gained or lost weight in the last week | ||||
| Yes, gained weight | 42 | (79.3) | 36 | (85.7) |
| Yes, lost weight | 7 | (13.2) | 3 | (7.1) |
| No | 4 | (7.6) | 3 | (7.1) |
| Missing | 3 | 14 | ||
| Stoma average output over 5 days (ml/kg/day) | ||||
| ≤ 20 | 43 | (86.0) | 26 | (74.3) |
| > 20 | 7 | (14.0) | 9 | (25.7) |
| Median (IQR) | 15 | (10–18) | 13 | (8–22) |
| Minimum, maximum | 0, 46 | 0, 71 | ||
| Missing | 6 | 21 | ||
| Stoma recycling distally | 15 | (27.8) | 12 | (28.6) |
| Missing | 2 | 14 | ||
| Problems with stoma present (not mutually exclusive) | ||||
| Yes | 18 | (33.3) | 14 | (33.3) |
| Prolapse | 3 | 4 | ||
| Stenosis | 1 | 0 | ||
| Retraction | 1 | 0 | ||
| Leaking bags | 13 | 8 | ||
| Skin problems | 5 | 6 | ||
| None of the above | 36 | (66.7) | 28 | (66.7) |
| Missing | 2 | 14 | ||
| Infection | ||||
| Currently receiving antibiotics | 14 | (25.9) | 7 | (16.7) |
| Missing | 2 | 14 | ||
| Positive blood culture in last 2 weeks | 5 | 1 | ||
| Missing | 0 | 0 | ||
| Currently being treated for a new episode of NEC | 0 | (-) | 0 | (-) |
| Missing | 2 | 14 | ||
| Other | ||||
| Last conjugated bilirubin level (μmol/l) | ||||
| Median (IQR) | 30 | (9–62) | 35 | (14–78) |
| Minimum, maximum | 0, 168 | 0, 133 | ||
| Missing | 11 | 22 | ||
| Infant’s condition at early time point | Infant’s condition at late time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Infant type Ab N = 37 |
Infant type Bb N = 19 |
Infant type Ab N = 37 |
Infant type Bb N = 19 |
|||||
| Time of form completion since stoma formation (days) | ||||||||
| Median (IQR) | 44 | (42–48) | 45 | (43–52) | 86 | (84–90) | 85 | (84–94) |
| Minimum, maximum | 14, 66 | 27, 64 | 42, 103 | 54, 103 | ||||
| Missing | 2 | 0 | 9 | 4 | ||||
| Survival | ||||||||
| Alive | 33 (89.2) | 19 (100) | 33 (89.2) | 19 (100) | ||||
| Respiratory support | ||||||||
| Current level of respiratory support (highest level of day) | ||||||||
| None | 11 | (31.4) | 17 | (89.5) | 11 | (40.7) | 13 | (86.7) |
| Non-invasive support | 15 | (42.9) | 1 | (5.3) | 12 | (44.4) | 2 | (13.3) |
| Mechanical ventilation | 8 | (22.9) | 1 | (5.3) | 4 | (14.8) | 0 | (-) |
| High-frequency oscillatory ventilation | 1 | (2.9) | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Received nitric oxide | 3 | (33.3) | 0 | (-) | 1 | (25.0) | 0 | (-) |
| Not applicable (respiratory support none or non-invasive) | 26 | 18 | 23 | 15 | ||||
| Missing | 2 | 0 | 10 | 4 | ||||
| Number of days of respiratory support since stoma formation | ||||||||
| Non-invasive support | ||||||||
| Median (IQR) | 10 | (1–29) | 0 | (0–2) | 36 | (27–52) | 0 | (0–4) |
| Minimum, maximum | 0, 41 | 0, 30 | 0, 79 | 0, 69 | ||||
| Missing | 2 | 0 | 10 | 4 | ||||
| Mechanical ventilation | ||||||||
| Median (IQR) | 11 | (4–26) | 2 | (0–7) | 15 | (5–30) | 2 | (0–8) |
| Minimum, maximum | 1, 43 | 0, 28 | 1, 73 | 0, 32 | ||||
| Missing | 2 | 0 | 10 | 4 | ||||
| High-frequency oscillatory ventilation | ||||||||
| Median (IQR) | 0 | (0–1) | 0 | (0–0) | 0 | (0–2) | 0 | (0–0) |
| Minimum, maximum | 0, 24 | 0, 3 | 0, 26 | 0, 3 | ||||
| Missing | 2 | 0 | 10 | 4 | ||||
| Postnatal steroids for chronic lung disease received in last week | 2 | (5.7) | 0 | (-) | 1 | (3.7) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Cardiovascular status | ||||||||
| Currently receiving inotropic support | 1 | (2.9) | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Fluids and nutrition | ||||||||
| Currently receiving PN | ||||||||
| Yes | 16 | (45.7) | 9 | (47.4) | 16 | (59.3) | 7 | (46.7) |
| Volume currently receiving (ml/kg/day) | ||||||||
| Median (IQR) | 94 | (80–112) | 110 | (90–120) | 101 | (100–114) | 106 | (90–120) |
| Minimum, maximum | 47, 150 | 21, 150 | 69, 150 | 61, 150 | ||||
| Missing | 0 | 0 | 0 | 0 | ||||
| No, not required | 18 | (51.4) | 9 | (47.4) | 9 | (33.3) | 8 | (53.3) |
| No, inadequate vascular access to allow it | 1 | (2.9) | 1 | (5.3) | 2 | (7.4) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Currently receiving enteral feeds | 33 | (94.3) | 16 | (84.2) | 24 | (88.9) | 13 | (86.7) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Volume currently receiving (ml/kg/day) | ||||||||
| Median (IQR) | 122 | (58–165) | 140 | (74–158) | 94 | (65–157) | 150 | (110–180) |
| Minimum, maximum | 30, 190 | 5, 180 | 44, 200 | 6, 240 | ||||
| Missing | 1 | 0 | 0 | 0 | ||||
| Current weight (g) | ||||||||
| Median (IQR) | 1710 | (1170–2390) | 3127 | (2024–3570) | 2308 | (1992–2692) | 3900 | (2680–4450) |
| Minimum, maximum | 795, 4460 | 830, 4410 | 1170, 4460 | 1876, 5480 | ||||
| Missing | 2 | 1 | 10 | 4 | ||||
| Currently oedematous | 3 | (8.6) | 0 | (-) | 2 | (7.4) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Gained or lost weight in the last week | ||||||||
| Yes, gained weight | 28 | (80.0) | 14 | (77.8) | 22 | (81.5) | 14 | (93.3) |
| Yes, lost weight | 4 | (11.4) | 3 | (16.7) | 2 | (7.4) | 1 | (6.7) |
| No | 3 | (8.6) | 1 | (5.6) | 3 | (11.1) | 0 | (-) |
| Missing | 2 | 1 | 10 | 4 | ||||
| Stoma average output over 5 days (ml/kg/day) | ||||||||
| ≤ 20 | 29 | (87.9) | 14 | (82.4) | 19 | (76.0) | 7 | (70.0) |
| > 20 | 4 | (12.1) | 3 | (17.7) | 6 | (24.0) | 3 | (30.0) |
| Median (IQR) | 15 | (10–20) | 15 | (11–17) | 13 | (10–20) | 14 | (6–22) |
| Minimum, maximum | 0, 46 | 0, 23 | 0, 46 | 0, 71 | ||||
| Missing | 4 | 2 | 12 | 9 | ||||
| Stoma recycling distally | 7 | (20.0) | 8 | (42.1) | 6 | (22.2) | 6 | (40.0) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Problems with stoma present (not mutually exclusive) | ||||||||
| Yes | 11 | (31.4) | 7 | (36.8) | 11 | (40.7) | 3 | (20.0) |
| Prolapse | 2 | 1 | 4 | 0 | ||||
| Stenosis | 1 | 0 | 0 | 0 | ||||
| Retraction | 1 | 0 | 0 | 0 | ||||
| Leaking bags | 8 | 5 | 6 | 2 | ||||
| Skin problems | 2 | 3 | 4 | 2 | ||||
| None of the above | 24 | (68.6) | 12 | (63.2) | 16 | (59.3) | 12 | (80.0) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Infection | ||||||||
| Currently receiving antibiotics | 10 | (28.6) | 4 | (21.1) | 5 | (18.5) | 2 | (13.3) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Positive blood culture in last 2 weeks | 2 | 3 | 1 | 0 | ||||
| Missing | 0 | 0 | 0 | 0 | ||||
| Currently being treated for a new episode of NEC | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 2 | 0 | 10 | 4 | ||||
| Other | ||||||||
| Last conjugated bilirubin level (μmol/l) | ||||||||
| Mean (SD) | 48.8 | (48.1) | 30.9 | (28.7) | 59.6 | (43.9) | 26 | (25.4) |
| Median (IQR) | 31 | (9–77) | 27 | (8–39) | 45 | (28–108) | 17 | (8–37) |
| Minimum, maximum | 0, 168 | 3, 104 | 0, 133 | 0, 88 | ||||
| Missing | 9 | 2 | 16 | 6 | ||||
At the 6-week time point, surgeons said they would be willing to follow trial allocation to early closure (see Table 8) in 17/56 (33%), would not be willing to follow allocation in 25 (50%) and in 9 infants (18%, 3 type A, 6 type B) the stoma had already been closed (data missing for 5 infants). The main reasons given for not being willing to follow trial allocation were current clinical status (more detail in Table 8) and size. The majority of infants for whom surgeons said they would not be willing to follow trial allocation to closure at 6 weeks (16 of 25) were < 28 weeks' gestation at birth (see Table 10).
Overall, those for whom surgeons were not willing to follow trial allocation at this time point were also lighter (median weight 1609 vs. 2090 g). This suggests that following allocation to closure at 6 weeks may be systematically challenging for the most preterm/smallest infants. Again, however, this was not a universal opinion based on gestation and weight alone, since of the 17 infants deemed by surgeons suitable to follow an early closure allocation, 8 were born < 28 weeks' gestation and the smallest infant weighed 1023 g. For those infants in whom the surgeon would have been willing to follow trial allocation at 6 weeks, stoma closure would have been logistically possible in the majority of cases (71%, similar for type A and type B infants).
At the 6-week time point, neonatologists said they would be willing to follow a trial allocation to stoma closure at 6 weeks in 31/56 overall (similar in type A and type B), would not be willing in 8 infants (mix of type A and type B), were unable to say because the infant had either died or been transferred in 6 and in 6 infants the stoma had already been closed (see Table 9). The main reason for not being willing to follow trial allocation at this time point was ‘other’. For those deemed suitable for stoma closure at 6 weeks, neonatologists suggested it would be logistically possible to follow this allocation in 90% of cases.
Overall, at the 6-week time point, there was no consensus between surgeon and neonatologist regarding willingness to follow a trial allocation to stoma closure at this point in time, or the clinician was unable to say in the majority of cases. There was consensus to follow trial allocation in just 19% of cases (9 of 56) and consensus not to follow it in 10%.
Twelve-week time point
At 12 weeks following stoma formation, data were available for 43 infants (data missing for 13). At this time point, 10% of infants for whom data were available were mechanically ventilated (n = 4), all of whom were type A infants. One type A infant was receiving nitric oxide, none were receiving inotropes and one type A infant had received steroids within the preceding week. Seven were receiving antibiotics and one had a positive blood culture in the previous 2 weeks with none being actively treated for a new episode of NEC. The majority of infants had gained weight over the previous week with just three (two type A, one type B) having lost weight with the remainder unchanged. The majority of infants (55%) were still receiving PN (a higher number than at 6 weeks) and 12% were not receiving any enteral feed at this late time point. Stoma-related problems were present in one-third and one-quarter had stoma output of > 20 ml/kg/day. Refeeding (recycling of stoma output) was being performed in 29%.
At the 12-week time point, surgeons said they would be willing to follow a trial allocation of stoma closure at 12 weeks in 28 infants and would be unwilling to follow this trial allocation in 24. Of these 24, 12 had required earlier stoma closure and 9 (mainly type A infants) were considered unsuitable for stoma closure at this time point for a variety of mainly clinical reasons shown in Table 8. Again, most of the infants for whom the surgeon was unwilling to follow trial allocation at 12 weeks were < 28 weeks' gestation (13 of 24, see Table 10) but in similarity to the situation at 6 weeks, many infants of gestation < 28 weeks were also considered suitable for stoma closure at the late time point (13 of 28). Hence, birth at gestational age < 28 weeks in itself did not appear to influence decision-making for all surgeons. At this late time point, current weight was similar between those deemed suitable for stoma closure and those not. In the 28 infants in whom surgeons would have been willing to follow this trial allocation, stoma closure would have been logistically possible in the majority (79%), with the most frequently cited reason for stoma closure not being logistically possible being theatre list availability. Neonatologists said they would be willing to follow trial allocation to stoma closure at 12 weeks in just under 50% of infants overall and would not be willing to follow this trial allocation in 42%, of whom 9 had already had earlier closure and 11 were felt to be unsuitable for closure at that point in time. Overall, at the 12-week time point, there was consensus between surgeon and neonatologist that trial allocation to closure at 12 weeks could be followed for 11 of the 56 infants (23%) and consensus that stoma closure at 12 weeks could not be followed in 8 infants. Notably, there was no consensus between clinicians in 50% of infants.
Stoma closure
Overall, stoma closure took place before the end of the data collection period in 46 of the 56 infants, 4 infants (all type A) died with a stoma in situ and 6 infants still had a stoma at the end of the data collection period. Median time between stoma formation and closure was just under 10 weeks, at median 88 days of age and median weight of 2631 g. Data for each infant type are shown in Table 14. Interestingly, the age at stoma closure and time from stoma formation to closure were quite similar between type A and type B infants. At the time when the stoma was closed, this was performed as a planned procedure in 87% of cases, but the timing was not as had been planned in six cases (five expedited, one delayed).
| Overall | Infant type Aa | Infant type Ba | ||||
|---|---|---|---|---|---|---|
| N = 56 | N = 37 | N = 19 | ||||
| Stoma closed before end of data collection | ||||||
| Yes | 46 | 29 | 17 | |||
| No | 6 | 4 | 2 | |||
| Infant died with soma in situ | 4 | 4 | 0 | |||
| Stoma closed | N = 46 | N = 29 | N = 17 | |||
| Age at stoma closure/reversal (days) | ||||||
| Mean (SD) | 93.2 | (42.5) | 95.3 | (38.9) | 89.5 | (49.2) |
| Median (IQR) | 88 | (60–118) | 90 | (63–122) | 82 | (53–97) |
| Minimum, maximum | 33, 201 | 39, 184 | 33, 201 | |||
| Missing | 0 | 0 | 0 | |||
| Time from stoma formation to stoma closure (weeks) | ||||||
| Mean (SD) | 11.2 | (6.1) | 11.2 | (5.3) | 11.2 | (7.3) |
| Median (IQR) | 9.9 | (6.7–14.1) | 9.7 | (7.1–14.1) | 10.6 | (6.0–12.3) |
| Minimum, maximum | 4.4, 28.3 | 4.7, 25.6 | 4.4, 28.3 | |||
| Missing | 0 | 0 | 0 | |||
| Weight at stoma closure/reversal (g) | ||||||
| Mean (SD) | 3258.7 | (1615.1) | 2875.4 | (1435.3) | 3912.5 | (1735.3) |
| Median (IQR) | 2631 | (2169–4322) | 2400 | (2169–2989) | 3900 | (2566–5060) |
| Minimum, maximum | 1780, 9100 | 1840, 9100 | 1780, 7450 | |||
| Missing | 0 | 0 | 0 | |||
| Stoma closure at this point planned | ||||||
| Yes | 40 | (87.0) | 25 | (86.2) | 15 | (88.2) |
| No | 6 | (13.0) | 4 | (13.8) | 2 | (11.8) |
| Expedited | 5 | 4 | 1 | |||
| Delayed | 1 | 0 | 1 | |||
| Missing | 0 | 0 | 0 | |||
| Principal reason for expedited or delayed closure | ||||||
| Clinical | 6 | (100) | 4 | (100) | 2 | (100) |
| Social/family | 0 | 0 | 0 | |||
| Logistical | 0 | 0 | 0 | |||
| Other | 0 | 0 | 0 | |||
| Not applicable (stoma planned) | 40 | 25 | 15 | |||
| Missing | 0 | 0 | 0 | |||
| Complications within 30 days of closure/reversal (not mutually exclusive) | ||||||
| Yes | 9 | (19.6) | 5 | (17.2) | 4 | (23.5) |
| Anastomotic leak | 2 | 2 | 0 | |||
| Anastomotic stricture | 0 | 0 | 0 | |||
| Local wound problems | 3 | 3 | 0 | |||
| Adhesive bowel obstruction | 0 | 0 | 0 | |||
| Unplanned return to theatre | 0 | 0 | 0 | |||
| Prolonged feed intolerance/ileus | 4 | 2 | 2 | |||
| Other | 3 | 1 | 2 | |||
| None of the above | 37 | (80.4) | 24 | (82.8) | 13 | (76.5) |
| Missing | 0 | 0 | 0 | |||
| Time to achieve full enteral feeds post stoma closure (days) | ||||||
| Median (IQR) | 11 | (5–16) | 11 | (6–15) | 8 | (5–20) |
| Minimum, maximum | 2, 140 | 2, 140 | 3, 30 | |||
| Missing | 6 | 3 | 3 | |||
| Stoma closed | N = 46 | N = 29 | N = 17 | |||
| Duration of invasive ventilation postoperatively (days) | ||||||
| Median (IQR) | 1 | (1–2) | 2 | (1–4) | 1 | (0–1) |
| Minimum, maximum | 0, 6 | 0, 6 | 0, 2 | |||
| Still ventilated | 1 | 1 | 0 | |||
| Transferred on ventilation to another trust | 0 | 0 | 0 | |||
| Missing | 1 | 0 | 1 | |||
| Outcome status 30 days post closure/reversal | ||||||
| Still an inpatient | 13 | (28.3) | 8 | (27.6) | 5 | (29.4) |
| Discharged home | 23 | (50.0) | 13 | (44.8) | 10 | (58.8) |
| Transferred to another trust | 10 | (21.7) | 8 | (27.6) | 2 | (11.8) |
| Died | 0 | (-) | 0 | (-) | 0 | (-) |
| Missing | 0 | 0 | 0 | |||
The main reason for expedited or delayed stoma closure was for clinical reasons. Following stoma closure, complications occurred within 30 days in nine infants (20%), comprising anastomotic leak in two infants (both type A), local wound problem in three (all type A) and prolonged feed intolerance or ileus in four (two type A, two type B). These are important findings: the rate of complications is prioritised highly as a trial outcome elsewhere in ToSCiN and the safety of the intervention and comparator will be a key feature in a future trial.
Full feeds were achieved at median 11 days following stoma closure (11 days in type A and 8 in type B) and infants were ventilated post stoma closure for a median of 1 day. At 30 days following stoma closure, half of infants in the study had been discharged home, just over one-quarter were still an inpatient in the same hospital and the remainder had been transferred to another hospital.
Overall numbers of infants who may be enrolled in a hypothetical future trial
Figure 13 illustrates surgeon willingness to randomise at the 1-week time point and then adhere to trial allocation at 6 and 12 weeks. We have used surgeon opinion as opposed to neonatologist opinion as through discussion we have identified that it is more likely to be surgeons rather than neonatologists who will carry the final decision to approach infants and their families about the trial and carry the decision about willingness to follow treatment allocation. Essentially, this represents the flow of a case through a hypothetical trial, enabling an understanding of how many cases in our cohort study would have been enrolled and adhered to the proposed treatment pathways (i.e. stoma closure at 6 or 12 weeks following formation). Of the 56 infants enrolled, surgeons would have been willing to randomise 31 infants (59%) to a trial. Of these 31, surgeons would have been willing to follow trial allocation to closure at 6 weeks in 13 and of these an allocation to closure at 12 weeks in just 8. Therefore, just 8 of the 56 infants would theoretically have been randomised and adhered to either treatment arm. However, surgeons subsequently indicated that they would have been willing to close the stoma at 12 weeks in 20 of these 31 overall. Thus, if the reason for not being willing to close at 6 weeks could be overcome, there may potentially be more infants for whom trial allocation would have overall been followed.
FIGURE 13.
Flowchart of surgeons’ willingness to randomise and follow trial allocation.
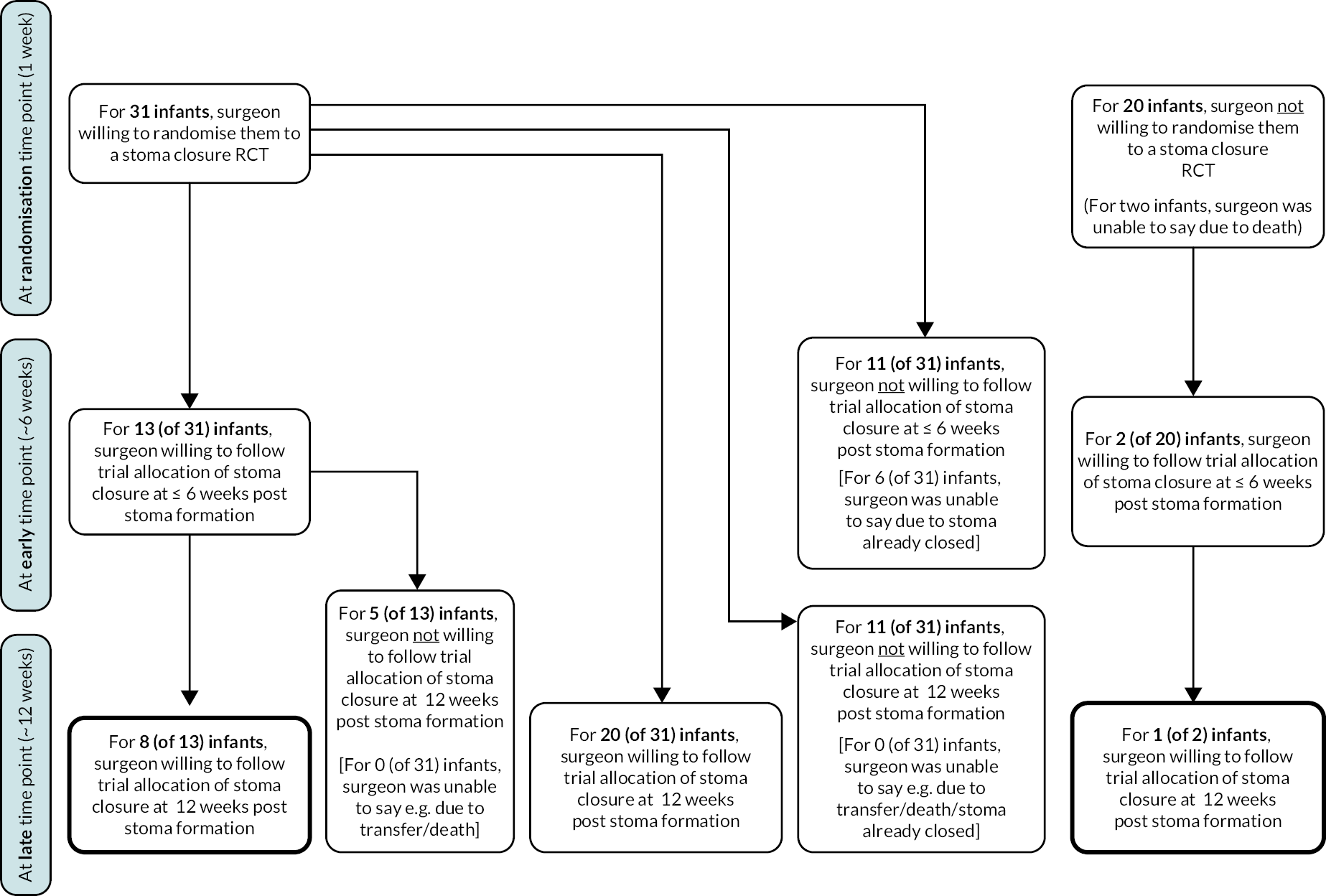
Of the 31 infants that surgeons indicated they would have been willing to randomise, there were 11 who were deemed not suitable for closure at 6 weeks and also 11 who were deemed not suitable for closure at 12 weeks (although not the same 11, with only 3 for whom surgeons would not be willing at both time points). Finally, for the 20 infants for whom the surgeon would not have been willing to randomise at 1 week, just 1 infant was felt to be suitable for stoma closure at both 6 and 12 weeks. Thus, for those infants felt unsuitable at 1 week, surgeons were unwilling to adhere to either time of stoma closure, that is, their opinion did not change. Understanding the clinical parameters of this group of infants may give some insight into those for whom this trial is not suitable since surgeons appear to lack equipoise over their inclusion. Unfortunately, however, the key reasons for not being willing to randomise at 1 week were prematurity/size or underlying disease status and there were minimal differences in these variables between infants whom surgeons would and would not have been willing to randomise. Thus, it appears that suitability for a future trial may be dependent not only on the clinical and demographic characteristics of the infant but also on the interaction of this and surgeon willingness to enrol.
Workstream 2.3: Findings from interviews and focus groups
Participants
Parent interviews
Twenty-four parents (7 fathers and 17 mothers) took part in a telephone (n = 23) or online via Zoom (n = 1) interview between July 2021 and February 2022, representing 22 infants (12 boys and 10 girls). One mother had twins and three parents were couples. Interviews lasted an average of 55 minutes (range 23–109 minutes). Fifteen parents were recruited via hospital sites and nine were recruited via social media (Figure 14). Parents were aged between 22 and 38 years old (mean = 32 years). Most parents (17/24, 71%) identified their ethnic group as being White British (n = 17; Asian or Asian British n = 4; mixed or multiple n = 1; white other n = 2). The majority of infants (n = 12; 54.5%) were born extremely preterm (< 28 weeks' gestation). Three infants (13.6%) were very preterm (between 28+1 and 31+6 weeks), two (9.1%) were moderate to late preterm (32–36+6 weeks). Only five (22.7%) infants were born at term (≥ 37 weeks). Infants had their stoma(s) formed between day 2 and 63 of their life (mean = 11 days; mode = 4 days; median = 6 days). The main reason for needing a stoma was due to perforation (n = 10; 45.5%) or NEC (n = 8; 36.4%). Other reasons given were atresia (n = 3) and meconium ileus (n = 1). Of the infants who had had their stoma(s) closed at the time of their parents’ interview, these were closed between 4 and 18 weeks after formation (mean = 9 weeks). Infants were cared for in 28 different hospital sites (n = 17 peripheral and n = 11 surgical) across the UK.
FIGURE 14.
Route of parent recruitment.
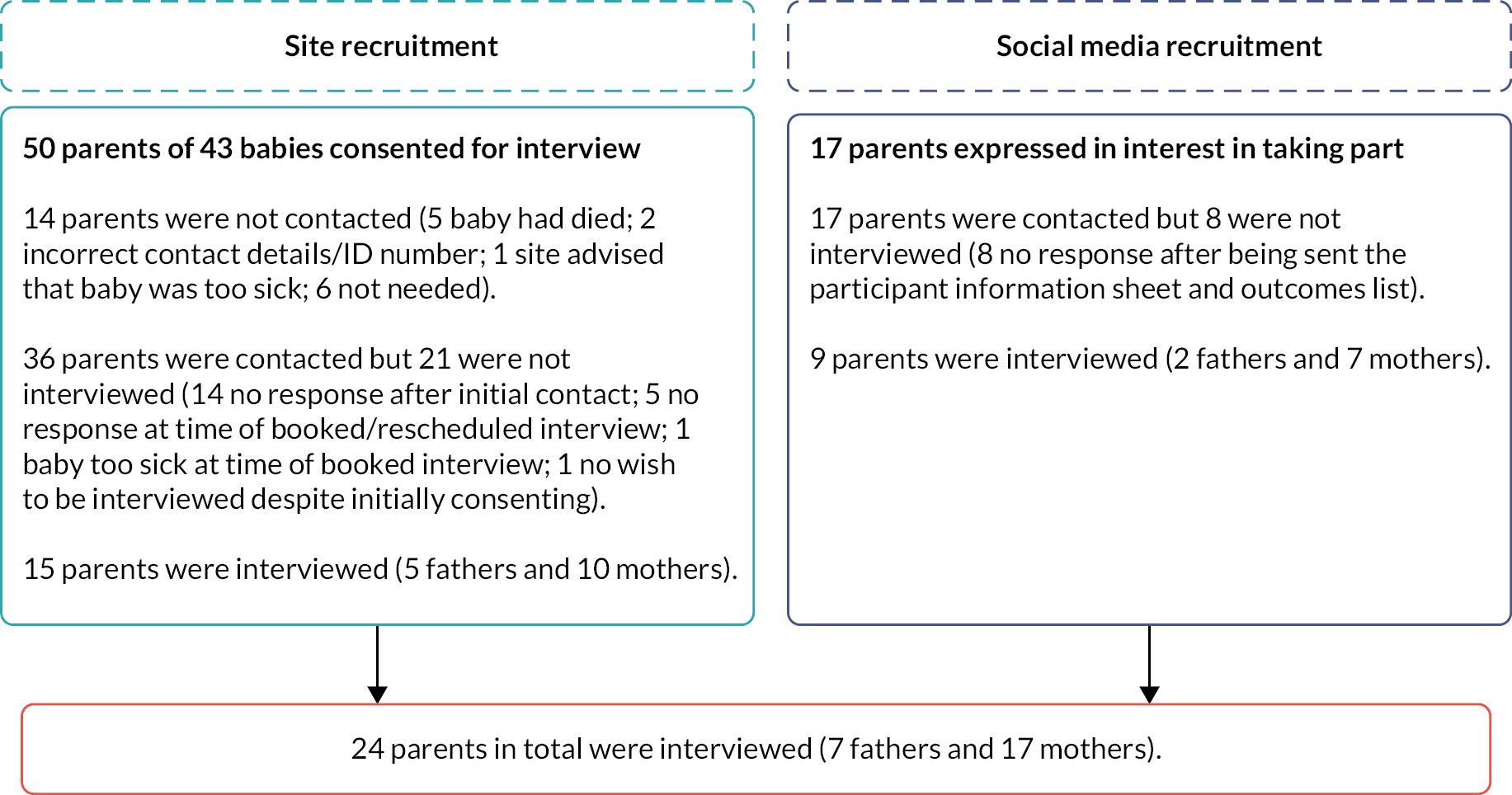
Staff focus groups
Thirty-six staff from five out of eight hospital study sites took part in one of six focus groups (five online via Teams and one face-to-face); two focus groups were conducted with staff from one study site. Participants included 14 (39%) surgeons, 10 (28%) consultant neonatologists, 6 (17%) neonatal surgical nurses, 2 (6%) research nurses, 1 (3%) dietitian and 3 (8%) ‘other’ roles (anaesthetist, haematologist, nurse). Focus groups lasted on average 56 minutes (range 25–93 minutes).
Factors that influence the timing of stoma closure
At the start of their interview, parents were asked to give an outline of how and why their child needed intensive care/stoma surgery. They were also asked questions around the care and closure of their child’s stoma such as: ‘When was the stoma closure surgery first discussed?’ and ‘Were the doctors waiting for anything before they were happy for your child to have the stoma closure surgery?’
Similarly, at the start of the focus groups, staff were asked about their current stoma closure practice and how they choose when to close a stoma.
Despite reported variance in practice, staff consistently took into consideration four different factors: primarily, how well the infant is holistically and stoma-related factors, followed by organisational and family factors, as summarised by this staff member:
The higher the stoma on the GI tract, the earlier I would want to close it. The more preterm the baby, probably the longer I would want to wait, but, again, it probably comes back to where it [the stoma] is in the gut. And then comorbidities come into it… So, what is the rest of the baby like? Is the baby on a ventilator? Or, actually, is it one of these little babies who is, kind of, struggling to survive? … So, the more comorbidities might put you off and think, ‘Actually, the stoma is the least of its [the baby’s] worries…' They are the bits of information that I would put into the [stoma closure] decision-making algorithm. Then it also comes down to the family. You know, what do they think? Some families find it really difficult. They do not want to look… after a baby with a stoma versus a nappy… Are there complications of the stoma? … Is the stoma easy to look after, practically? Or is it one of these stomas that bleeds, prolapses, retracts and, basically, people are pulling their hair out saying, ‘This stoma is a nightmare, every day!’
(P04, FG4)
The four factors highlighted by staff were also reflected in the parents’ accounts of their infant’s stoma journey. Each of these four factors will now be discussed.
Infant-related factors that influence the timing of stoma closure
The first factor taken into consideration when determining the timing of stoma closure is how well the infant is viewed to be overall. Parents and staff repeatedly used the phrase ‘it all depends on the baby’ (P18, mother, site). This was especially important when the infant had been born preterm, as the number of associated problems with their early birth can lead them to be very ‘up and down’ (P4, mother, site; P9, mother, social media). Other infant-related factors that are taken into consideration when deciding when to close a stoma included: ‘the overall size of the baby, the gestational age, comorbidities, their ventilation status, if they recovered well after the operation’ (P04, FG1); ‘growth’ (P04, FG5); ‘the amount of feeds the baby’ can tolerate (P06, FG1); if ‘they have made it onto enteral feeds’ (P05, FG3); and ‘liver function’ (P02, FG4):
It’s completely – it’s all dependent on … how big they [the infants] were when they were born, how old they were when they were born, gestational age, corrective age, other comorbidities, kind of respiratory factors.
(P02, FG2)
Other infant-related factors that staff and parents observed influence the timing of stoma closure were any ‘acute changing condition [such as] line sepsis, … [which] means that acutely they [infants] are not in a fit state for an anaesthetic or intervention’ (P01, FG3):
[Infant] had meningitis at one stage. Until he had fully recovered, the doctors weren’t willing to even discuss with the surgeons when they could go down [for stoma closure surgery]. It was other complications rather than anything to do with the bowel.
(P7, mother, social media)
Stoma-related factors that influence the timing of stoma closure
Once the infant has been evaluated as a whole, stoma-related factors are taken into consideration. The first stoma-related factor identified by staff and parents that influences the timing of stoma closure is the placement of the stoma. Whether the stoma is distal or proximal ‘would probably be the key one’ (P01, FG1) because ‘high up’ (P23, mother, social media) stomas meant there was not ‘much bowel to absorb the milk’ (P5, mother, social media), which impacted weight gain and growth: ‘they’re unlikely to grow with it [a high stoma] and it might be more challenging to manage’ (P04, FG1). This therefore increases the likelihood of an earlier stoma closure.
The cause of the stoma and status of the bowel were the second stoma-related factor to influence the timing of stoma closure: The infant’s ‘bowel [needs time] to settle and just repair … [from] NEC [necrotising enterocolitis]’ (P23, mother, social media) and the bowel needs ‘time to grow … [to] have a decent length to connect up to’ (P10, father, social media).
High stoma output and stoma prolapse were the third stoma-related factor to influence the timing of stoma closure. Infants can lose ‘salt’ (P3, mother, site) and ‘too many nutrients’ (P6, mother, site) if stoma output is high. ‘The constant leaking of the stoma’ (P16, father, site) that results from a high stoma output or ‘way too much of it [the stoma] outside’ (P5, mother, social media) can result in infants having ‘severe stoma sores … he was in so much pain … he had morphine for them’ (P14, mother, social media) and parents ‘really struggling to bag the stoma and maintain and preserve the integrity of the skin around it … [that] was exposed to the faeces’ (P5, mother, social media). Furthermore, ‘all the washing that goes with that, changing her, keeping her happy…. [creates] a lot of pressure’ for parents (P3, mother, site).
It was clear that there is a need to balance looking ‘at the baby in the whole’ (P04, FG1) (the holistic condition of the infant) with limiting any negative side effects of the stoma:
We tended to use early stoma closure to avoid the negative consequences of having a stoma rather than the positive consequences of closing it. So, in other words, if the baby is doing fine and growing, we have taken a very relaxed view by saying, ‘We will get to it when we can’.
(P01, FG3)
Logistical and organisational factors that influence the timing of stoma closure
Although secondary to infant- and stoma-related factors, logistical and organisational factors such as the surgeon, bed and theatre availability were influential factors for the timing of stoma closure. The usual practice of the clinicians/hospital site performing the closure, the patient and family population at surgical and peripheral hospitals and the quality of stoma care at peripheral hospitals were also acknowledged to influence the timing of stoma closure.
If the stoma closure is a ‘non-emergency surgery’ (P01, FG2), logistical factors such as ‘the availability of the surgeon’ (P6, mother, site), ‘the beds and the theatre times’ (P05, FG3) and the need for the infant to be transferred from another hospital can influence the timing of stoma closure:
Sometimes having been, I will say ‘pushed’ in inverted commas, to close stomas earlier than perhaps I would have otherwise done, for bed availability or [if a list] becomes available, and sometimes having to delay stoma closure – than I would normally do as well, for those reasons.
(P03, FG3)
When we went in for that appointment, he [the surgeon] said, ‘Everything is great, and it’s just about co-ordinating everyone to be in theatre at the right time’ … He said it’s a big job, sorting it out.
(P3, mother, site)
The usual practice of the clinicians/hospital site performing the closure also impacted upon staff views about optimal timing of stoma closure. In some hospitals, it was considered standard practice to close a stoma at around 6 weeks (adjusting for gestational age) and in others, if the stoma was not problematic, a later closure is standard (see following section for more details). However, it was highlighted that clinicians within the same hospital may have different opinions about the best time for stoma closure:
They [clinicians] were so worried about her ability to cope with it [stoma closure surgery] … That was always the surgeon’s concern is that if they tried to operate and she wasn’t coping then it would put her back even further … I appreciated that quite difficult discussion that was going on. It was a healthy argument but it genuinely was an argument between the consultants and the surgeons at one point as to what should happen. Eventually I guess they compromised a little bit, well, the consultants won and they got them to agree to do it.
(P24, mother, social media)
I just think, from a nursing point of view, it is very, at the moment, surgeon dependent. So, if I know a stoma has been made by X, I think, ‘That will stay there for ages’ or if I know it has been made by Y, I can think, ‘It is more likely to be closed a bit earlier’.
(P06, FG4)
Different hospitals had different patient and family populations based on their location and whether they were a general hospital or a specialised hospital:
Yes, and we are skewed by the fact that we see a disproportionately large number of very, very small babies and a disproportionate amount of NEC … Our catchment area has a lot of non-white families, a lot of immigrant families. There’s a lot of deprivation where we are.
(P01, FG1)
The mothers who’ve had multiple previous preterms, they come here.
(P03, FG1)
Linked to the placement of the stoma discussed above was the fact that different staff and hospital sites had varying approaches to feeding and nutrition, with some encouraging refeeding and others not:
We have started being much more successful at refeeding these babies down their distal limb, and so we probably have a bit less of a pressure from every single patient that has got a high stoma absolutely needing a stoma closure early on, because I think the nursing type care of refeeding has massively improved in recent times, so we don’t have the same totally not thriving baby with a high stoma. It is not as common as it used to be.
(P02, FG3)
The timing of stoma closure was also influenced by ‘whether or not … [a] local [peripheral] unit would be happy to have a baby with a stoma … another unit may not be familiar with how to do that and may not be able to provide that care’ (P06, FG5) and the quality of stoma care available in peripheral hospitals, as this was regarded as being variable and, in some cases, appeared to be reliant upon parents to deliver the stoma care. Consequently, stoma closure may be prioritised so that an infant can be discharged to a peripheral hospital without the need for stoma care. Several parents (unprompted) said that they did not want their infant to be discharged to a peripheral hospital because they felt that the staff there lacked the required knowledge and experience of neonatal stoma care and because they had built a trusting relationship with the staff in the specialist hospital:
It was just getting harder and harder to manage because her dad and I were doing all the stoma care, because the nurses at [hospital] SCBU don’t really deal with stomas very often, and naturally, they’re [stomas are] all quite different and behave differently. There isn’t a ‘one-size-fits-all’ for stoma bags and things like that, and the care plans are all very different.
(P5, mother, social media)
You feel like you’ve built up trust. You’ve built up a relationship. You trust those doctors and nurses to know your child and what they need.
(P7, mother, interview)
The practice of discharging clinically well infants’ home with their stoma was also variable and depended upon whether or not this was the standard practice of the hospital and whether discharge home was acceptable for parents. Staff reported mixed views and practices regarding family stoma care. Although some felt it best to discharge clinically well infants ‘home with a stoma’ (P02, FG3) to allow their parents to ‘have a period of time at home managing the baby, managing the stoma, and then come back into hospital at a later date, when the baby is just a bit more robust’ (P05, FG3), others focused on the stress that sending an infant home with a stoma might cause the family, particularly if the stoma is problematic and there is limited community support. Staff reported taking into consideration how the family respond to the stoma in general, home circumstances, distance from the hospital and family quality of life when determining the timing of closure of non-problematic stomas:
I suppose the additional thing is social things like how well will the family cope with a stoma, what is the circumstance at home and that sort of thing? That is often a decision.
(P01, FG3)
We do alter, sometimes, their path of care, depending on where they are. If we have got a parent that is really struggling to visit, we will try and get them back to their peripheral unit, maybe, as quickly as we can. So, do we move their treatment forward? … I mean, we will have some parents who can only visit every other day, or every few days. Now, for them, if they have got other children at home, that is miles and miles and miles away, they are going to want to get their baby either local or home.
(P06, FG4)
Family factors that influence the timing of stoma closure
Some parents spoke about the practical, mental health, social, emotional and financial impacts of having an infant in hospital with a stoma for their quality of life. Parents (mostly fathers as mothers tended to stay in hospital with their infant) had to travel from 30 to 120 minutes each way to see their infant, which took its toll on all aspects of parent and family life:
We just had to share, because we had three children here [at home] as well. So, one of us had to – Well, I say one of us, it was never me, I was with the baby all the time. But yes, he was … with the children, and then he was backwards and forwards to the hospital every single day. There was not a single day where he didn’t come. So yes, it was so hard.
(P18, mother, site)
I think we were spending about £1,000 on petrol or something we calculated, which you just do then. You make it work, don’t you? But there was a huge financial impact for us a family.
(P24, mother, social media)
When considering their infant’s possible discharge home with a stoma, some parents said that they wanted ‘to be involved’ (P9, mother, social media) and ‘were doing all the stoma care’ (P5, mother, social media) and ‘recycling’ (P11, mother, site) ‘and doing a better job of it, basically … [than] the nurses at [hospital] SCBU … just because we were with her every day and we were very used to it’ (P5, mother, social media). One mother explained that ‘there was a big push on integrated family care [at the hospital her baby was in], so me feeling like I was able to look after her. I was trained on how to change the stoma bag and clean it and do all of that’ (P24, mother, social media).
On the other hand, five parents, unprompted, said that they would ‘not [be] happy to take baby in that form and shape home. It is not an easy job’ (P12, father, site), corroborating the concerns of staff about how the family’s response to the stoma in general impacts the timing of stoma closure and indicating that there could be issues if infants are discharged home with a stoma:
I felt better if she had around-the-clock care … She had around-the-clock care [in hospital] … If I was at home, I feel like I would have driven myself crazy.
(P23, mother, social media)
My partner and I have spoken about what would we have done if we had taken her home with her stomas and we both agreed that it might have been a bit much to manage. As much as we did want to go home, we wanted to go home in such a way where we knew what we were doing and we knew that we could do it to the level that [Infant’s name] needed.
(P22, father, social media)
Furthermore, some parents spoke about ‘a lot of … mums [on the ward] … [who] didn’t ever feel comfortable with it [their infant’s stoma, and] … were pretty horrified and repulsed and scared that the child was going to have it for the rest of their lives’ (P7, mother, social media) and fathers who ‘still can’t look at’ their infant’s stoma now (P9, mother, social media). This resulted in mainly mothers ‘doing all the cares’ (P3, mother, site).
Two mothers found that ‘everything was fine’ (P2, mother, site) and it was ‘really easy [to care for their infant with a stoma at home]. We had a stoma nurse come out a few times to the house to check we were okay. We got the supplies regularly through [anonymised]’ (P15, mother, site). However, other mothers said that ‘by the end of it [their time at home with their infant who still had a stoma], I just wanted it [the stoma] closed because of the lack of [adequate] training in the surgical hospital … [and lack of access to both family and professional] neonatal stoma support in peripheral hospitals and at home’ (P14, mother, social media). Some mothers reported that they felt ‘a lot of pressure … [and found it] quite stressful’ (P3, mother, site) and ‘really hard work’ (P14, mother, social media) ‘to keep … [their infant] healthy’ at home (P3, mother, site), supporting staff concerns about the stress that sending an infant home with a stoma might cause the family, particularly if there is limited community support, and indicating potential barriers to trial success if infants are discharged home with a stoma:
We had nothing like [stoma care nurses or community nurses at home]. We had nothing like that. The only thing that we had of support was we got a phone call once, from the stoma team. Actually, twice they called; they called me twice. And they only asked if we’ve got enough stock. And that was the stoma nurse; that’s it. That’s all they asked. And we got one call once from the consultant who actually did the surgery, and he was only asking about the general care of the stoma, and if everything was okay. That’s about it. There was no physical help, you know, post-discharge, in regards to helping us with leaky stoma, or putting stoma [bags] on.
(P16, father, site)
We [both parents] don’t think … we had enough training [in the surgical hospital]. Once we got [to peripheral hospital], no disrespect to them, but they weren’t used to babies like [Infant’s name] … We did kind of feel like we were muddling along, even in hospital, a little bit. Then when we got home you did have stoma nurses visiting you, but, again, they were adult stoma nurses. I think it’s very different, isn’t it, from an adult to a child?
(P14, mother, social media)
Views of 6 weeks as early and 12 weeks as late
Workstream 1 (see Chapter 4) established that the two most common time points for stoma closure are 6 and 12 weeks. These were then set as the proposed ‘early’ and ‘late’ arms of the trial, respectively. Staff were asked if they agreed with our definitions of ‘early’ and ‘late’ closure.
Parents and staff both raised concerns over the use of early and late terminology, with one parent saying ‘that the words early and late are quite emotive’ (P7, mother, social media). This terminology made parents question, ‘Well, why are they getting it early?’ (P15, mother, site) and, on the other hand, ‘Late means … I felt like it was a failure. “Oh, she is not well enough!”’ (P24, mother, social media).
Staff from all sites considered 6 weeks to be ‘routine, or normal’ (P01, FG2), unless the infant was extremely premature, in which case 6 weeks would be considered very early. However, there was disagreement on whether 12 weeks is ‘late’ based on the current practice at their hospital. Staff at some sites considered 12 weeks or more as standard: ‘I would struggle to call 12 weeks late’ (P03, FG3), while staff at other sites would consider 8 weeks as ‘late’: ‘That’s a very uncommon occurrence … Six weeks for us is standard and then late for us would be eight’ (P02, FG1). Consequently, staff at some sites viewed 12 weeks as ‘very late’. Some staff and parents found it difficult to see past this emotive terminology, which appeared to influence their perceptions of the proposed trial.
Exploring the definition of early and late stoma closure made some staff question what the standard practice was at their own site: ‘Do we know what our current average is?’ (P05, FG3). Others were surprised that there is a difference in the timing of stoma closure across hospitals: ‘They do them all early? … They must have loads of beds’ (P03, FG4). The lack of awareness of variance in the practice of stoma closure and the corresponding justifications for these differences consistently impacted on potential trial equipoise (see following sections). For context, three sites closed stomas at around 6 weeks, one site closed stomas nearer to 12 weeks and the other site closed according to each infant’s individual need, with one staff member saying that they were ‘surprised to hear other places sticking to such stringency’ (P02, FG6).
Acceptability of stoma closure at 6 weeks as a trial arm
After exploring their experiences of current stoma closure practice, parents and staff were asked: ‘How acceptable do you feel it is to close a stoma at six weeks as part of a trial?’ For staff, this question was initially asked using the Poll Everywhere software and then discussed.
It initially appeared that most parents would be ‘100% for fixing them early’ (P3, mother, site) as they felt that closing an infant’s stoma at 6 weeks was ‘completely viable’ (P11, mother, site) and would be ‘a very good idea’ (P3, mother, site). They ‘could not see the problem in doing it at 6 weeks’ (P8, mother, social media). Indeed, nine parents said that they would prefer for their infant to have/have had their stoma closed at 6 weeks, as one mother said: ‘Just to make sure then he had time to get back on track with everything else, with the feeding and about healing and everything. Get him home as soon as possible, basically (Laughter)’ (P11, mother, site).
The majority of staff also viewed the 6-week arm as acceptable, with 97% rating it ‘acceptable or very acceptable’ (67% acceptable; 30% very acceptable) and only 3% rating it as ‘unacceptable’. This acceptability was based on 6 weeks being commonly viewed as a standard time point for stoma closure. Those who did not rate it ‘very acceptable’ noted that: ‘You could argue that acceptable is the right answer for a trial, because if it was very acceptable then we should just be doing them all early, right?’ (P03, FG4).
Staff and parents then went on to note that this acceptability was dependent on a ‘clearly defined exclusion criteria’ (P01, FG1) for the trial and clinician autonomy regarding whether ‘the child is well enough’ (P13, father, site):
Nobody wants to be forced into doing an operation on a baby who, genuinely, it is not safe to do it, but I am sure the study protocol would take account of that and allow clinician discretion in terms of going ahead.
(P05, FG3)
Whether or not it was acceptable for an infant to be recruited to the 6-week arm was also based on their gestational age: ‘I simply wouldn’t recruit a 24-weeker’ (P01, FG1); ‘the reason why they’ve ended up with the stoma in the first place’ (P2, mother, site); any acute condition(s) that they might need immediate closure for; or a clear need for delay.
Acceptability of stoma closure at 12 weeks as a trial arm
Parents and staff were then asked about the acceptability of closing a stoma at 12 weeks as part of a trial. Twenty-three out of 24 parents felt that it would be acceptable, as this would give the infant (especially those born prematurely) time to grow bigger, heavier and stronger and, consequently, they felt that their infant’s stoma closure operation would be ‘safer’ (P6, mother, site):
The longer you can leave it, the bigger they can grow … nothing is a bother to the bag, to the stoma. If there is no issue to the actual stoma itself and they are coping then let them grow. Just let them grow.
(P9, mother, social media)
Because he was so premature, he was very, very small, and they wanted to make sure that he was in a healthy enough position to be able to cope with the anaesthetic and going back in for surgery again.
(P13, father, site)
In addition, parents noted that the 12-week arm would give their infant’s bowel time to rest and grow, increasing the chance of a positive outcome for the infant’s long-term quality of life:
From a child’s point of view, you’d have an optimum length of bowel. You’ve obviously given the lower part more time to rest and recover … Obviously you need it [the bowel] to be in a good condition to be able to get it back together in the first place. But equally, you need it to be a certain length [for the infant] to have a quality of life.
(P10, father, social media)
This time frame was also in line with many parents’ experiences of their infant’s stoma closure. Of the 16 infants who had had their stomas closed at the time of their parent’s interview (range 4–18 weeks; mean = 9 weeks, mode = 11 weeks, median = 9 weeks), 7 infants had their stoma closed at between 11 and 18 weeks (5 of these infants were extremely preterm, 1 was very preterm and 1 was term) (Table 15).
| Timing of stoma closure (weeks) | Gestation |
|---|---|
| 4 | Term (37+ weeks) |
| 5 | Moderate to late preterm (32–37 weeks) |
| 6 | Extremely preterm (< 28 weeks) |
| 6 | Extremely preterm (< 28 weeks) |
| 7 | Extremely preterm (< 28 weeks) |
| 7 | Moderate to late preterm (32–37 weeks) |
| 8 | Extremely preterm (< 28 weeks) |
| 9 | Very preterm (28–32 weeks) |
| 9 | Extremely preterm (< 28 weeks) |
| 11 | Extremely preterm (< 28 weeks) |
| 11 | Extremely preterm (< 28 weeks) |
| 11 | Term (37+ weeks) |
| 12 | Extremely preterm (< 28 weeks) |
| 13 | Very preterm (28–32 weeks) |
| 14 | Extremely preterm (< 28 weeks) |
| 18 | Extremely preterm (< 28 weeks) |
However, although 23 out of 24 parents reported stoma closure at 12 weeks to be acceptable, parents did go on to report concerns that, as well as increasing the length of hospital stay, the 12-week arm could increase the risk of infection, negatively impact upon the integrity of the infant’s skin and have a detrimental impact on the infant’s development:
There certainly were babies on the unit with us, maybe the later prem babies or even term babies that end up with a stoma for some reason who are just hanging around waiting for their stoma to be reversed … there was no point in sending them home to bring them back as an outpatient. But nevertheless, that was all they were waiting for … Had I been one of those parents that was just waiting for the closure so we can go home, I would have definitely found that quite difficult.
(P7, mother, social media)
I imagine that if there are some parents that have got a child that wasn’t as early gestation as [Infant’s name] was and they got given the ‘12 week’ one, they might then question – ‘Well, could that be changed?’ – if it was going to impact them coming home.
(P11, mother, site)
Staff perceptions of the acceptability of the 12-week arm were split, with n = 17 (53%) rating it ‘acceptable’ or ‘very acceptable’ and n = 15 (47%) rating it as ‘unacceptable’. This split was influenced by the current practice within their site for non-problematic stomas. Three out of five sites (sites 2, 3 and 5) where they commonly closed stomas after 12 weeks considered this time frame as ‘acceptable’ and felt that it would actually be more acceptable if the arm was ≥ 12 weeks: ‘I think [what] is probably stopping me from saying very acceptable because my impression, like we were saying, is that we are later than that’ (P05, FG3) (Figure 15).
FIGURE 15.
Staff perceptions by site on the acceptability of closing a stoma at 12 weeks.
Staff, particularly those in focus groups 1 and 2 (site 1) and 5 (site 4) who considered stoma closure after 6 weeks as ‘very late’, in turn viewed 12 weeks as unacceptable. Rather than stoma closure at 12 weeks being viewed as standard practice for some sites, they viewed it as meaning that some infants in the trial would have a stoma for an ‘unnecessary’ additional 6 weeks. They had concerns about the impact of extra time in hospital on the infant, the family and the hospital:
I think it would be extremely difficult to talk to parents about getting them to wait until 12 weeks when I think, quite clearly, as a group, we seem to be very comfortable with six weeks … I think it’s just the idea of a child sitting there with a stoma that we want to close at six weeks, but the trial says, ‘No, you’ve got to wait until 12 weeks,’ so you’ve got to sit on the unit with TPN [total parenteral nutrition] running for another six weeks seems unnecessary. There are some children, as we’ve already said, that 12 weeks is perfectly acceptable, but to do it as a trial, I think I would go with the clinical individual status rather than what I was told to do.
(P03, FG5)
Yes, the parents, because potentially extending their stay for a clinical trial, I feel is … something that really would need to be thought about. Especially when you’re almost thinking about doubling the length of time that we would normally close the stoma.
(P03, FG2)
Indeed, one parent spoke about finding it difficult waiting an additional 6 weeks while a theatre slot was co-ordinated for their child’s stoma closure:
It has been quite tough, then waiting six weeks for an operation that could help with a lot of things … [it was] just a slot to coordinate everyone for theatre, that’s it.
(P3, mother, site)
It was evident that there would be a tendency to not discharge an infant with a stoma from these sites and that these ‘additional’ 6 weeks were viewed by staff as having a negative impact on infants’ health and development and family quality of life.
The impact of an extended hospital stay on infants’ health and development
Many staff spoke about the need to ‘look at the baby as a whole’ (P01, FG1) and said that they ‘would be unhappy about leaving [infants] for three months with a stoma … we wouldn’t want to repatriate them. We wouldn’t want to send them home, so they’d’ (P01, FG1) ‘probably … end up having to be in hospital’ (P06, FG5). Staff highlighted ‘lots and lots of reasons why it’s a bad idea to keep a baby in [hospital] longer than it needs to’ (P06, FG5). Issues such as ‘poor’ (P04, FG5) or ‘faltering growth’ (P01, FG1; P02, FG6) and delayed development were discussed, as were long-term issues with establishing oral feeding:
They [infants] definitely have delayed development, being in hospital. So, we have very limited physio or child play therapists. We don’t have those within the neonatal unit. And my other thought is, really, if the baby’s not feeding particularly well, it may actually impact on long-term feeding, as well, for the baby, the longer we delay, to establish normal feeding.
(P01, FG5)
Staff also described how they are ‘very keen to get the long lines out and to get these babies fed as quickly as possible’ (P06, FG5). They articulated concerns about infants randomised to the 12-week arm having a ‘longer time with the central line’ (P06, FG1) and infants who would otherwise be ‘nutritionally independent’ (P01, FG2) being dependent ‘on parental nutrition …, [because of the] associated risk of infection’ (P04, FG5) and ‘sepsis’ (P06, FG1), which can impact infants’ cognitive development:
Then if we’re on long-term TPN [total parenteral nutrition] … the risk of infection with having lines in, long lines in to administer the TPN, and the risk of white matter injury if they do get septic with the line in.
(P06, FG5)
I would worry about the risk of sepsis, the length of time and line management. One is trying to keep a long line in, or a central line in, in order to deliver parenteral nutrition. It’s slightly different to say that one [an infant that] might have a late closure, if a baby is nutritionally independent than the late closure for a baby who’s dependent through the entirety of that time upon a central line.
(P01, FG2)
Other staff were concerned that babies randomised to the 12-week arm of the trial would have to undergo additional interventions because they are going to have to ‘change their central line, because that line is not going to last three months’.
(P04, FG1)
The above reasons appeared to make some staff uncomfortable about justifying the trial to parents and caused concerns about dropout:
I think it’s something to say, they’re not ready ventilation-wise, it will be very difficult, or if they become septic, or there’s some other issue, but just to say, ‘Well they’ve been randomised so there’s no –’ I wonder if that’s the point parents may back out.
(P02, FG2)
The impact of an extended hospital stay on family quality of life
In addition to having concerns about the impact of an extended hospital stay on infants randomised to the 12-week arm of the trial, staff from all focus groups had concerns about the practical, psychological, emotional and financial impact on parents and the wider family. Staff were worried that ‘it would be extremely difficult to talk to parents about getting them to wait until 12 weeks’ for their infant’s stoma to be closed (P04, FG5) because they would potentially be ‘offering that … they’ll be in hospital for another month’ (P03, FG1), raising concerns about recruitment.
Staff were also concerned about ‘the … massive … psychological impact on the parents … [by] knowingly increasing the amount of time they’re here [at the hospital] for’ (P03, FG2). They spoke about parents comparing their infant’s stoma journey to those of other infants on the ward, and this increasing the likelihood of them withdrawing their infant from the 12-week arm of the trial:
By seeing other children who haven’t been a part of that trial, or other children within the trial, having stomas closed at a different time point and seeing different consequences. Parents talk to each other, and so my worry would be that parents in the delayed arm may withdraw their consent, negate the benefit of the trial, and therefore not help us answer the question which we want answered.
(P01, FG2)
The financial burden to parents of having to travel long distances to the hospital was highlighted, with many living ‘at least three hours away’ (P01, FG2), and how this separation and an infant’s extended hospitalisation can also have a ‘big’ (P01, FG5) impact on the wider family, including siblings and grandparents:
Parents have to travel to our units. So, many parents come from faraway, they’re separated from their families. At the moment, with COVID, the grandparents aren’t allowed to visit – they’re not seeing their grandchild.
(P06, FG5)
We don’t have an outreach service, so that would be very difficult … parents have to travel a long way. We do have facilities on-site for parents to stay here so they can be close to the baby and involved in care, but … that’s really hard on the dynamics of the family at home, not only grandparents but the older siblings, as well.
(P01, FG5)
Staff from sites that typically close stomas earlier (FG1/FG2, FG4, FG5) questioned the proposed 6- and 12-week timing of stoma closure for the trial. They said that ‘the 12 weeks, to me, does not make sense’ (P07, FG4) and wanted ‘to hear somebody explain to me what they think the benefits of waiting are, and to allay my fears of burden to the parents, the cost on the service and the relative risk to the baby’ (P01, FG2). However, four parents (three of whom had premature infants) said that they would actually prefer their infant’s stoma to have been closed at 12 weeks with the hindsight of knowledge that they now have:
If I would have been approached before … I would have thought it’s better to do it early. I would have thought if it’s [the stoma is] closed early, this means that they are doing well. As it is now [with the hindsight of knowledge], it would explain that, actually, it’s more likely that they’re not doing well and losing more nutrients, so that has to be closed earlier. But if they’re doing well, it’s better to do it later.
(P6, mother, site)
Furthermore, several parents implied that stoma closure at 12 weeks would not create delays to going home or concerns about providing stoma care at home for infants who are premature because they would still be in hospital anyway:
[Going home] was never discussed with me as an option actually for one anyway. They never had that in mind for [Infant’s name] … because they said, ‘She’s not going to be ready to go [home] before her due date anyway, just because of how early she was’.
(P24, mother, social media)
We knew he was going to be in for at least 12 weeks anyway, so for us, personally, it wouldn’t have been an impact to us if it [stoma closure] had waited until he was 12 weeks, really … for us, personally, it wouldn’t have impacted our hospital stay, so it wouldn’t have been an issue for us. We would have been happy to go ahead because, at the end of the day, it’s not something that would have made any impact to him, really, and possibly helped out future babies.
(P11, mother, site)
The impact of an extended hospital stay on each hospital
Another impact of having babies hospitalised for longer than they need clinically was bed blocking. Staff spoke about the ‘knock-on effect … [of] delayed closure [for bed blocking] … [By] keeping that baby at [hospital name] means that we are declining another baby and cannot offer them treatment’ (P06, FG5).
Some staff were worried about the trial ‘putting a bigger burden on … resources’ (P01, FG2) and said that their unit did not have ‘the resources for keeping … [infants who have] … to wait six weeks longer in hospital’ (P05, FG5).
Overall acceptability of the proposed ToSCiN trial
Having discussed current practice and the proposed trial arms, parents and staff were then asked: ‘Overall, do you think the ToSCiN trial is acceptable to conduct?’ The majority of parents and staff (n = 28/31, 90%) considered the proposed ToSCiN trial acceptable to conduct. Both groups thought that the research question is important and may improve infant outcomes: ‘if it helps any baby in the future’ (P18, mother, site) and ‘if it helps the NHS and it helps other mothers and dads that go through what we went through’ (P15, mother, site). Some parents said that the trial could assist in the standardisation of practice:
I think it’s important to have good systems in place and where nationally people go by the same kind of rules rather than it being different from trust to trust. I think that if we can help that outcome where everyone has a straight forward, knows what happens, then that can only be a good thing.
(P15, mother, site)
The fact that both 6 and 12 weeks are standard practice in the UK appeared to make both arms acceptable when this information was conveyed to parents. Indeed, the more awareness that staff and parents had of this variance in practice, the more acceptable the trial appeared to be viewed:
If there are units who are doing that [closing at 12 weeks], then I would say yes, it’s acceptable.
(P04, FG1)
The doctors … were unsure themselves, when they close it. They said to me, ‘You know, it depends. It’s 50/50. Some people say it’s best to keep it longer, so we have a full recovery of the descending colon. And other people say that no, it’s best to close the stoma as soon as possible, and have an active descending colon’. So, yes, they said, you know, it differs, the thought process of which one’s better and which one’s worse. But they said according to their information, the longer you leave it the better, for adaptation of the higher colon, the small intestine, and of course, to repair the descending colon, as well, to let it relax.
(P16, father, site)
The underlining certainty from both parents and staff that the infant’s clinical need will supersede any clinical trial, that is, if the stoma needs to be closed outside of the randomised arm, underpins the acceptability of the ToSCiN study.
Barriers to trial participation and success
Even though most staff (n = 24/31, 77.4%) answered that the 6- versus 12-week ToSCiN trial would be practically possible to conduct when asked ‘Given everything that you’ve discussed so far today, overall, do you think the ToSCiN trial is practically possible to conduct?’, the concerns raised about potential infant-related, stoma-related, logistical and organisational and family barriers to trial participation and success appeared to contradict this.
As mentioned in the subsections of the Factors that influence the timing of stoma closure section, staff ultimately wanted the flexibility to ‘go with the clinical individual status [of the infant] rather than what I was told to do’ (FG5, P03). They felt that stoma-related factors and the infant’s condition as a whole should be the main factor to influence the timing of stoma closure because there is ‘too much dichotomy in … every baby less than 44 weeks post conceptual age who gets a stoma … and too many factors’ (P01, FG1) (see ‘Infant-related factors that influence the timing of stoma closure’ and ‘Stoma-related factors that influence the timing of stoma closure’ sections).
While this wish for clinician autonomy in of itself is not problematic with regard to conducting a trial, the agreement that there is a substantial proportion of infants that staff believe would benefit from ‘early’ (high/problematic stomas) or ‘late’ (preterm infants or non-problematic stomas) closure may lead to a high proportion of crossover between arms and protocol deviations:
I think if this went to a RCT I could envisage an awful lot of protocol violations mandated by clinical need … there are lots of reasons why you’d want to close a stoma early for if you have got faltering growth and having continued with parental nutrition because of that. Then that just places the baby at an increased risk, and that’s another of our indications to get stomas closed.
(P02, FG6)
Staff recognised that the differences between these two groups of infants could create a risk of bias and selection of only the infants who are well enough to be randomised to the 12-week arm of the trial:
I think that kind of … then biases your patients, because you are then selecting ones who are doing well, and you then think, ‘Okay. This is a good one that can be pushed to 12 weeks.’ Then you are not really randomising. It’s picking and choosing them.
(P03, FG6)
In addition, they would be reluctant to recruit extremely preterm infants in case they are randomised to the 6-week arm:
If I knew that they [extremely premature infants] might be randomised to closure at six weeks, … I simply wouldn’t recruit … that 24-weeker… I would already have only selected the ones [infants] around 29 to 30 whatever weeks when I operated so I’d know that that would then put the [infants to almost term by the time they had the closure surgery].
(P01, FG1)
Staff said they were ‘just not convinced I would operate around 30 weeks. You kind of think, “Really? Just for an elective kind of operation?”’ (P04, FG4). This reluctance was often underpinned by experience of poor outcomes:
We’re a centre for extreme prems, so we’ve got 22-weekers … We did a couple of early closures in our extreme prems who were only 26 weeks who have run into trouble and died. I’ve now been quite cautious about closing [stomas] under 30 weeks.
(P02, FG1)
Staff also had concerns about recruiting some infants to the 12-week arm, because their infant would potentially have ‘to wait six weeks longer in hospital’ (P05, FG5). Some parents questioned the ethicality of the study, especially for infants randomised to the 12-week arm of the trial because ‘discomfort for the baby and parents could have been reduced weeks before’ (P12, father, site). They felt that it was an ‘absolute priority’ (P24, mother, social media) that the timing of stoma closure be driven by ‘the clinical states of the children’ (P24, mother, social media). They were concerned which arm ‘would be best because sometimes early could be too early and late could be too late or it could still be too early’ (P2, mother, site). The terminology of ‘early’ and ‘late’ stoma closure appeared to exacerbate some of these concerns.
As mentioned earlier in the section Factors that influence the timing of stoma closure, the impact of an extended hospital stay on the infant, the family (including siblings and grandparents) and the hospital caused concern for staff, who questioned whether parents would ‘back out’ (P02, FG2) of the trial if their child was randomised to the 12-week arm, with an extended hospital stay or discharge to a peripheral hospital or home with a stoma being viewed as another barrier to trial participation and success.
Other barriers that staff envisaged could be detrimental to the success of the ToSCiN trial were theatre list, bed and staff availability to be able to adhere to ‘the [allocated] stoma closure times’ (P05, FG1). There were concerns that ‘by the time theatre space is organised … [the trial] will, by definition, have more overlap between the two groups [6 weeks and 12 weeks]’ (P01, FG2). As such, staff were worried about ‘merging … arms [of the trial] if theatre is delayed for some reason [for those in the 6-week arm] … [and it is] slightly early for the other group [the 12-week arm’ (P01, FG2).
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the potential ToSCiN trial were discussed throughout the focus groups. Staff argued for and against having broad or selective inclusion criteria. They highlighted that the population of infants who are included and excluded from the trial will impact the usability of findings and the feasibility and acceptability of the study, as also found in the ToSCiN study consensus meeting (see Chapter 7). However, the dichotomy in infants caused challenges in deciding which infants to include or exclude because ‘it’s talking about apples and pears’ (P06, FG1), ‘melons and pineapples’ (P01, FG1).
Staff were happier to recruit term infants with a low stoma ‘who would be amenable to either early or late closure’ (P07, FG4). Some felt that high-stoma infants should be excluded from the ToSCiN trial ‘because they are going to have a planned journey’ (P07, FG4), as should infants with a high stoma output who have a higher chance of needing an earlier stoma closure and not being able to wait potentially 12 weeks for their stoma closure (FG5, P06).
Even staff from hospitals who typically closed stomas earlier were cautious about the inclusion of extremely preterm infants and believed that stoma closure at ‘six weeks is probably very early, rather than just early … [for the] extremely low birthweight, extremely premature babies’ (P02, FG1). When considering the inclusion and exclusion criteria, in addition to whether the infant is preterm or term, the position and reason for the stoma were the other two most important considerations in whether infants should be included or excluded in the ToSCiN trial:
So, number one, where in the GI tract is the stoma? So, is it a jejunostomy or is it a distal colostomy? I think that, honestly, is probably the most important [factor to consider]. Number two is, is it a preterm baby or is it a term baby? I think that is, probably, a secondary, kind of, consideration. Then number three, why have you made the stoma? So, what is the diagnosis? Because there is a big difference between necrotising enterocolitis or spontaneous perforation or anorectal malformations. Are they in this study?
(P04, FG4)
Extremely preterm infants with SIP are the infants that staff were most anxious about, especially when randomised to the 6-week stoma closure arm of the trial, because they might not ‘be well enough’ (P02, FG6) due to their prematurity and other comorbidities. For this reason, some staff suggested setting the ‘inclusion criteria up for a baby … over 28 weeks’ (P01, FG1). Others were concerned about the inclusion of infants with NEC who may have ‘no gut left, so it is just inappropriate. And you cannot make that decision pre-operatively’ (P08, FG4), but said that ‘you would not just exclude them right off, I guess you would put them all in to consider, but then you would maybe, at two weeks or four weeks then go, “Is this person actually randomisable?”’ (P04, FG4).
It was suggested that using ‘corrected gestational age’ (P01, FG2) may help alleviate some of the concerns of including extremely premature infants. Alternatively, it was suggested having ‘two trials, one for preterm babies and one for term babies’ (P07, FG4) or a trial for infants who are ‘off PN … so, the enterally autonomous patient … [where] you are considering closing … [the stoma] before discharge or just discharging with a stoma’ (P03, FG4) and a trial for infants who are ‘PN dependent’ (P04, FG4).
Facilitators to enhance recruitment and retention in a full ToSCiN trial
Having discussed the barriers to the trial, staff and parents spoke about a number of facilitators and suggested recommendations to enhance recruitment and retention in the ToSCiN trial.
Timing of randomisation and approach
Parents and staff both highlighted the need to wait to randomise until the infant was stable after surgery. Parents said that the timing of the recruitment approach should definitely not be ‘in those first couple of days [after surgery, when] obviously, it was pretty stressful’ (P1, mother, site). Almost a third of parents (n = 7) described having situational incapacity, meaning that they could not have made decisions about their infant taking part in the trial because their infant ‘was very poorly, actually [and] may not survive’ (P6, mother, site) so they ‘wouldn’t have had the headspace to have even thought about [their infant taking part]’ (P1, mother, site). Parents said that they ‘genuinely wouldn’t even have been able to process that conversation when … [their infant] was in intensive care’ (P24, mother, social media) and that they ‘were like emotional wrecks’ (P18, mother, site). One mother described how ‘it’s only become apparent in the last couple of weeks that I’ve been on autopilot and, sort of, survival mode for quite a while. I think I was barely functioning around that time’ (P4, mother, site). One mother said that she would prefer the opt-out approach to consent, to remove this decision-making process in ‘a high intense, worrying situation … [where she] genuinely wouldn’t even have been able to process that conversation when she [her infant] was in intensive care’:
I’m really behind research and trials and the importance of that, and that’s why we got to where we got with [Infant’s name], so we need to ‘pay it forward’, because of our mental state at that time I wouldn’t have been able to cope with it … I don’t know how this would ever work ethically, but I genuinely would feel that I would have rather her be entered into trials and not known about it.
(P24, mother, social media)
More than a third of parents said that it is vital that parents are not approached about the trial until ‘the conversation is starting to be around planning for that recovery rather than survival, which is often what the intensive care conversations are about. I feel very strongly about that as a minimum’ (P24, mother, social media). Staff also raised the necessity of time to assess possible survival before conversations with parents of premature infants take place:
I mean it’s also really tricky. With an extreme preterm who is having a stoma formed due to NEC. A third to half of those babies are not going to survive discharge. We are quite careful with families not to be getting too far ahead of ourselves when actually you know there is an awful lot of hurdles being approached in the next few days or weeks with those babies. It’s a bit paradoxical that approach to be then making these six-week, three-month plans ahead when you know just from statistics that a third to half of your babies are not going to survive that long.
(P02, FG6)
Most parents recommended that parents should not be approached until at least 1–2 weeks after the stoma formation surgery, once they have ‘gotten used to seeing the stoma, understanding how it worked, things like that – Once it was working properly and she had the bag on’ (P2, mother, site). That is, any approach should be made once the baby is stable, around 1–2 weeks post stoma formation when parents have somewhat emotionally recovered from their baby’s stoma formation (and any other) surgery. The preference for both parents and staff was then to recruit as soon as possible. Waiting too long could increase the chances of selection bias:
On an intention-to-treat basis you almost, I think, want to make this randomisation decision as early as possible. Otherwise, even subjectively, you will end up selecting out a group of patients who, come four weeks later, you have got more information about them.
(P07, FG4)
In addition, parents would be less likely to have expectations surrounding stoma closure that may affect their willingness to consent to the study:
It’s a very difficult time when you’ve just had the stoma put in. I don’t know how easy it would be to work closely with the medical units, but I think, certainly, it would be a good idea to get in there before anybody gives a time frame. Because in our case, if somebody gave us two weeks, we would think, ‘Ah, right, this is going to be done and dusted in two weeks.’ Then, if someone comes along and says, ‘Would you like to take part in this research, and it will be six weeks or 12 weeks?’ we’d be like, ‘Err, no. No thanks!’
(P5, mother, social media)
This rough 2-week time frame is the same that parents recruited to the ToSCiN observation study experienced and both parents and recruiters reflected that this was appropriate:
It has got to be two weeks then, doesn’t it? Because you have got to demonstrate survivability, have enough time to let the parents adjust to the whole situation, but then have time to practically plan closure, but also allow them to compute the consent to be involved, which sometimes might take a week or two.
(P04, FG4)
One parent suggested that consultants broach the idea of the ToSCiN trial to parents at their baby’s stoma formation follow-up appointment, to increase acceptability about being approached:
Just once you have had that follow-up with the consultant, to say how the surgery has gone … Maybe during that wrap-up, introduce the idea of the trial. Say, ‘One of my colleagues may want to speak to you in the next couple of days, to explain this trial, to help future babies,’ and all this kind of stuff. Maybe to broach the idea to start with in that follow-up with the consultant, and then have someone explain it fully in a couple of days, once you have had time to process what’s going on.
(P19, father, site)
Another parent felt that there is never a right time to approach parents who have a baby in NICU and that assessing the emotional wellness of parents is vital for informing the timing of approach:
Obviously, a parent that has a NICU baby … I think that there’s not really a right time. There’s not really a right time to provide that information because you can’t take the fact that the baby is in there and the parents, the mum or dad are going through so many emotions. I honestly don’t think that there’s a right time. I think if you need to ask somebody about a clinical trial, then maybe just assess that parent, like ask a nurse on the day, ‘How is the parent feeling today?’ If they’re in quite a positive mood, that would probably be the right time. I think if the mum is having a mental breakdown or is having a guilty day because they feel like crap because they’ve had to leave their baby for the 10th time, that’s probably the wrong time to [approach].
(P23, mother, social media)
Once parents had been approached and given the information about the ToSCiN trial, a quarter of parents said that they would need 2 days to: ‘digest the information’ (P7, mother, social media) and ‘talk to my husband, talk to any professional that I want to, like the paediatricians … surgeons’ (P14, mother, social media), ‘a nurse or someone that we trusted’ (P7, mother, social media) to make the decision about whether to consent to their baby taking part in the trial.
The participant information sheet
During their interview, after confirming that parents had had a chance to look at the draft PIS, parents were asked questions around its clarity, any suggestions for its improvement and their understanding of what the trial is aiming to do.
All parents could accurately articulate the purpose of the proposed trial: ‘You want to find out whether early or late closure is best’ (P7, mother, social media), ‘what could be the risks and benefits between these two closures’ (P12, father, site) and ‘what’s the better success rate for when it gets reversed?’ (P21, mother, site).
Parents stated that the PIS was ‘perfect … very clear’ (P3, mother, site), ‘straightforward’ (P15, mother, site), ‘to the point. It’s not too much to read’ (P24, mother, social media), yet ‘that it covers all bases’ (P13, father, site). While 11 parents did not suggest any edits to the PIS, others made recommendations that may enhance recruitment and retention:
Changing the ‘emotive’ (P7, mother, social media) terminology of ‘early’ (6 weeks) and ‘late’ (12 weeks) stoma closure.
Highlight that differences in the timing of stoma closure are already happening: ‘probably hammer it home that hospitals do do both options, so it’s not like they’re being a guinea pig – “Both of these things do happen”’ (P5, mother, social media).
Include a subsection that says that all infants’ needs will come first, above the trial: ‘to highlight it on the key points. To say, “No stress, it is baby-led. If your child needs earlier or later and they have already been allocated to a group, then your clinician will do what they deem to be most beneficial for your baby”’ (P19, father, site).
Highlight that there will be no ‘additional intervention or invasive checks’ (P7, mother social media) for the baby as part of the trial.
Provide a verbal explanation of what is included in the PIS or ‘just explain the summary’ (P20, mother, site) to parents.
Include a section about ‘what the risks [of the study] are’ (P7, mother, social media).
Having background knowledge about the trial and knowing that there are differences in standard practice for the timing of stoma closure between different hospitals and, indeed, different clinicians within the same hospital and knowing that the clinical status of the baby takes priority over the trial supported acceptance of the trial for parents and staff:
I think as long as everything is explained properly, like with the research programme it’s been explained really, really, well. As long as it’s explained properly and the risks and what happens in stages, so if the baby isn’t ready then it’ll be pushed back, I think that’d be fine as long as it’s explained properly.
(P2, mother, site)
I think to do it well, people, they need to have … a good bit of understanding as to the background of the study … how – the clinicians that propose the study, why has it come about like that? So, I guess understanding about the differences in practice currently across the UK.
(FG3, P05)
Staff training
One of the biggest facilitators of trial success will ‘need to be equipoise’ (P05, FG3). As previously discussed, many of the staff were unaware of the difference in practice across hospitals regarding the timing of stoma closure. Some staff were not clear about the average timings of closure within their own hospital. It was these staff that were not in equipoise and therefore had reservations about the study.
If a study around the timing of stoma closure goes ahead, a substantial part of the site training should be dedicated to explaining what standard practice is and why these differences occur across and within hospitals:
[Just because] some units close it at six weeks just because that is what they have always done, that is going to appal the other centres … So, actually, a bit of showing that nobody seems to know the answer is going to be really important.
(P08, FG4)
The other thing, just in terms of clinician buy-in, if you like, to the study is, I guess, education in the run-up … because we are probably all very biased for thinking that we do the right thing. ‘Our centre does it the best, and so whatever we do is definitely the right thing to do.’ … So, the literature around how there is no right or wrong answer would be good to share with clinicians.
(P05, FG3)
Staff were interested to know ‘how the parents have been responding’ to the acceptability of the proposed trial (P01, FG2). As part of the training package, staff would also value receiving feedback around parent acceptability because ‘if there was informed consent from the parents, then ultimately it [the trial] is a decision with our support. With everything else that we said, it is acceptable, but how acceptable it is to parents is the challenge’ (P01, FG2).
The site study training package was also seen as key to staff buy-in: ‘It’s persuading surgeons as well as the parents’ (P05, FG5) ‘that it [the trial] is a good idea. It really is going to need a lot of discussion and encouragement’ (P08, FG4). Findings from this feasibility study should therefore be included in site-training packages.
Parent stoma care training
As previously mentioned, one mother felt that she and her husband did not have enough stoma care training in the surgical hospital and felt like they ‘were muddling along, even in hospital, a little bit’ (P14, mother, social media) (see Family factors that influence the timing of stoma closure). The consequence was that this mother found ‘it was really hard work and by the end of it I just wanted it [the stoma] closed’ (P14, mother, social media) because of the lack of training in the surgical hospital and the neonatal stoma support in peripheral hospitals and at home. A father said that his wife still struggled with stoma care despite receiving stoma training:
We struggled with the stoma training, my wife really struggled with this. Even though she’s like – you know, she’s educated, right? She really struggled with the training, and how to change the bag, and how to cut into the sides for the actual hole for the outlet … Even though she was trained; trained in the hospital.
(P16, father, site)
Alternatively, some parents described ‘doing a better job of’ (P5, mother, social media) stoma care than some nurses:
It’s exhausting for a mother to have to come and see that … the nurses, [despite] over 50% of the babies … having stomas, [so] it’s not a new thing for them, [have] not done [the stoma care] correctly … It’s leaking quite frequently because of the way that they place it. And the frustration from my side where I come to see her [baby] and it’s every time the wrong way, so I’m having to educate each nurse because it’s a different nurse each time.
(P21, mother, site)
Therefore, working ‘very closely with the family’ (P01, FG5) and ‘good stoma training’ (P02, FG6) will be essential for parents whose infants are randomised into the 12-week arm of the trial and are clinically well enough to be discharged home while they wait for closure.
Furthermore, it ‘is very important’ that a ‘good explanation’ is given to parents about the stoma and how it works and that their baby is not in pain in the stoma area:
They explained the stoma.
They just explained what it was and how it worked and said, ‘The skin on the outside of it is like your inner lip, so like the inside of your lip. That is what it is like. And also, that is what it feels like for them’. And we have been told it doesn’t really have any nerve endings. They can’t really feel it. And I think that as a parent that is quite nice to know, because you don’t want to know your baby is in pain. Do you know what I mean? And they can’t speak out for you, so you have got all your trust in the medical team to tell you your baby is not in pain, because you are none the wiser.
(P9, mother, social media)
Logistical support
Providing designated research nurse time was seen as imperative for the success of the trial:
I don’t know how heavy the data collection side of things is going to be, but without research nurse support, then it is difficult to do big studies in a unit that is busy like ours, because clinician time is precious. Certainly, from experience of another study that is running at the moment … they have been absolutely invaluable.
(P05, FG3)
As was ring-fenced theatre time:
The most straightforward two things, I guess, are … the support that would be offered in terms of data collection, etc., and certainly, from a surgical perspective, access to theatres because we are working in a time where everything is having to be prioritised. So, each case that we book has to almost go through a committee to justify being done. So, I think, yes, support for the trial itself and theatre accessibility would certainly help.
(P03, FG3)
The main site one, currently it has to be access to theatre … We have contributed to another trial that involved a small surgical procedure, and we were able to get slightly ring-fenced slots on various lists for that. So, I guess we could explore that if and when the time came, about whether we could get agreement from senior management to have some way of accessing an extra slot, for example, to do these cases at a set time, but we would have to explore that.
(P01, FG3)
As mentioned above, ‘good enough outreach’ (P01, FG4) in ‘local hospitals’ (P06, FG5) and ‘nice stoma support at home, in the community’ (P01, FG4) will support acceptance of the trial, especially for infants randomised to the 12-week arm of the trial (see Acceptability of stoma closure at 12 weeks as a trial arm, The impact of an extended hospital stay for infants’ health and development and The impact of an extended hospital stay for family quality of life).
Outcome measures
Outcomes of importance to parents
Supplementary materials include the list of outcomes and accompanying descriptive text sent to parents prior to the interview. Towards the end of the interview, a definition of each outcome was read to parents, including an explanation about why it is important to explore parents’ perspectives about important outcomes (Box 1).
As we have discussed, in the ToSCiN trial, we want to find out when is the best time to close a stoma to improve outcomes for babies.
To do this, we will collect information on (read through outcomes list sent prior to interview). By collecting information on these main things, we hope to find out which should be used in the future. These are called outcome measures.
However, these outcomes have come from research papers and do not really give us much information on how children or families feel, or what is important to them. It is important that we include outcome measures that matter to children and their families.
We then asked parents, ‘Thinking about your experience of your child being admitted to NICU – what would you hope the stoma closure surgery would do to help your child?’ Most parents hoped that the stoma closure surgery would help their child to have a ‘functioning bowel’, that is, ‘be able to poo and eat food … like everybody else’ (P13, father, site) and that their ‘body [would be able to] take in the nutrients and the fat from [their] milk’ (‘Feeding and nutrition’) (P8, mother, social media).
From our team’s previous work exploring parents’ prioritised outcomes for trials,18–20,37,38 we were aware that parents sometimes find this a difficult question to conceptualise and answer. Therefore, all parents were then asked, ‘In general, what would you be looking for as an indicator that your child was getting better?’ Responses centred around three main outcomes: their child would have a ‘perfectly working bowel’ (‘Functioning bowel’) (P23, mother, social media) and no ‘trouble … pooing from … [their] bum’ (P2, mother, site), would be able to ‘feed orally’ (‘Feeding and nutrition’) (P24, mother, social media) and would ‘gain weight’ (P5, mother, social media; P8, mother, social media) and ‘grow’ (P9, mother, social media; P10, father, social media; P20, mother, site; P24, mother, social media) (‘Growth, feeding and weight gain’). Other important indicators of their child getting better mentioned by some parents were that their child was not ‘a pale colour’ (P16, father, site) because their ‘colour was not okay’ (P21, mother, site) when they were so ill; that their baby would be more responsive (P13, father, site) and that their child would be able to wear clothes for the first time (P14, mother, social media; P21, mother, site; P24, mother, social media).
When directly questioned about the provided outcomes list, parents overall felt that it was ‘pretty extensive’ (P2, mother, site) and that all of the outcome measures ‘are really important. I didn’t actually look at that and think, “Why is that on there?”’ (P24, mother, social media), although a number of additional outcome measures were suggested for inclusion such as: functioning bowel, parent and family quality of life (rather than just mothers’ quality of life) and stoma-related outcomes such as skin integrity (as mentioned in ‘Stoma-related factors that influence the timing of stoma closure’ earlier):
Obviously having a poo will be a big event, I think that is quite important, just knowing that she can do it by herself. Obviously, she has never, ever done that before.
(P3, mother, site)
There is nothing actually mentioned about parents or siblings or anything like that, so something added about them as well.
(P12, father, site)
Child quality of life was described to include longer-term social outcomes for the baby:
Can he just go out and have lunch round a friend’s house without him then shitting himself two minutes later because it passed through him too quick …? Or, if he wants to go for a sleepover and it’s like, ‘Can you hook me up to my bag of goodies every hour?’ or whatever. That’s what I’d say by quality of life. Just having a normal dietary requirement … Just the same cares in life as a same age person.
(P10, father, social media)
The researcher then repeated back the outcome measures identified by the individual throughout the interview discussion. Parents were then asked to rank their identified outcomes in order of importance for the proposed ToSCiN trial.
Findings from all outcomes data were then combined to determine the top-prioritised outcome by parents (Table 16). ‘Functioning bowel’ (an outcome not previously highlighted), ‘Weight and growth’ and ‘Feeding’ were the top three outcomes of importance to parents, followed by ‘Child’s quality of life’. See Report Supplementary Material 12 for parent details of ranking, the total number of parents who ranked each outcome and the weighted score for each outcome.
| Parent outcomes – ranked and weighted | Staff outcomes – unranked and not weighted |
|---|---|
|
|
Outcomes of importance to staff
Due to time constraints and the planned consensus meeting, we did not aim to achieve a ranked list of outcomes from staff at this stage. Rather, we wished to establish which outcome measures were of importance to them and why. Staff often raised possible outcome measures in relation to other aspects of trial design, reflecting that it is difficult to talk about one without the context of the other. Towards the end of the focus groups, using Poll Everywhere, staff responded to the question ‘What do you think should be the primary outcome measure for the ToSCiN trial?’ and we used any remaining time to explore their responses. Staff responses overlapped with those prioritised by parents, focusing on the importance of growth and nutrition (see Table 16).
Staff highlighted that growth and nutrition have direct immediate impacts and, in turn, impact on many subsequent outcomes such as neurological development at 2 years:
I am interested in growth, and the jury is out a bit, but I would like to believe that better nutrition and better growth does lead to better neurodevelopment. So therefore, by my own rationale, neurodevelopment outcomes should be a thing which you look at. But it just … needs a lot of babies.
(P03, FG4)
Some staff also placed importance on the number of surgical complications as a primary or secondary outcome, as a difference in this would also affect their future decision-making:
It has got to be the two, those have got to be your two outcomes, major surgical complication, and then something about nutrition and PN. I think your neurodevelopmental thing is important, but I think it is further down the list, in my view.
(P07, FG4)
It has got to be surgical complications, because if you think of the people who shy away from early stoma closure in our department, they are shying away because they are terrified that there is going to be a third and a fourth operation. So, you have got to convince those people that maybe there will not be, and that is why I think surgical complications becomes your number one outcome, followed closely by nutrition. Because it is the surgeons you are trying to change, it is their behaviour, because they want us – They are trying to change our behaviour, right?
(P04, FG4)
Conclusion and recommendations
Our interview and focus group findings suggest there was support for a RCT of the ToSCiN and, for this reason, parents and staff found the proposed trial to be acceptable. As a cohort there was equipoise, with parents and staff providing support for each arm. However, there was not always individual equipoise. Some parents and staff had clear arm preference and had concerns about the alternative arm (this was true for both arms). With the exception of extremely preterm infants, staff stated that they would be willing to randomise infants to the trial and parents would be hypothetically willing to consent to their baby taking part. However, due to the concerns each group raised, our findings suggest that using a 6- versus 12-week trial arm design may result in high dropout rates or arm crossover. A clear message from participants was that decisions about when to close a stoma should be ‘baby-led’, with clinical need, logistical factors and potential burden on families taking precedence over trial arm allocation.
Overall, the 6-week arm was perceived to be logistically and ethically acceptable by staff because, again, with the exception of extremely preterm infants, this was the standard practice of most units. Most parents preferred the 6-week arm even when many had experienced later stoma closures for their own baby (at least 11 weeks). The 12-week arm of the proposed trial did not appear to be feasible for staff from the sites who aim to close stomas ‘early’ due to the logistical issues surrounding this, such as bed and stoma support availability.
Findings related to the population for trial inclusion and trial design and outcome measures from this work stream were fed into presentations for and discussions at the consensus meeting (see Chapter 7) to determine optimal trial design. For example:
-
Inclusion criteria: There were concerns about including extremely preterm infants in the 6-week arm, although it was viewed as important to include extremely preterm infants in this arm if the trial is to be useful in practice.
-
The trial arms appeared more feasible if under 7 weeks and over 11 weeks, adjusting for gestational age.
-
Randomisation should take place at approximately 2 weeks (or 1 week for term infants) post stoma formation, when the baby is stable but before conversations about the timing of stoma closure have taken place.
-
Outcome measures of importance include functioning bowel (new outcome), weight, growth and time to enteral feeds.
Further recommendations generated from this workstream that may improve views on acceptability and clinical equipoise within a trial into the ToSCiN include:
-
use of neutral terminology such as ‘time point one’/‘closure at 6 weeks’ or ‘time point two’/‘closure at 12 weeks’, rather than ‘early’ or ‘late’
-
comprehensive site training to ensure equipoise and surgeon buy-in:
-
highlighting the differences in standard practice for the timing of stoma closure that are already happening in different hospitals and with different clinicians within the same hospital
-
using the findings from this ToSCiN study to reassure staff that, in the main, parents were supportive of the proposed trial
-
-
designated research nurse time
-
ring-fenced theatre time.
Strengths and weaknesses
A key strength of Workstream 2.3 is the variance in sample, which represented multiple time points, perspectives and aetiologies. Parents were recruited at the time planned for this hypothetical future trial and those with the hindsight of experience. Their children had multiple morbidities reflecting the main patient courses reported in Workstream 2.1. There was variance with the parents as they were a mix of genders and ethnic backgrounds. We therefore feel we have got the most representative sample possible given the diverse causes of stoma and possible comorbidities of this population. In addition, we obtained insight into this area from a range of staff from sites with competing views on the best time to close stomas which aligns with the statistical data collected.
Chapter 6 Results of Workstream 3
National Neonatal Research Database
A total of 512,964 neonates were born in England and Wales in 2012–9 and registered on the NNRD. Out of this, 4368 neonates had a daily record of stoma in situ. After data exclusion, 1830 neonates were included in our timing of stoma closure analysis. Thousand five hundred and forty-three neonates had a diagnosis of NEC and 287 did not (labelled as having other malformations). List of the gastrointestinal diagnosis is shown in Table 17.
| List of gastrointestinal diagnosis | Number of neonates | % of neonates | |
|---|---|---|---|
| NEC | 1543 | 84.3 | |
| Other malformations (n = 287)a |
Gastroschisis | 66 | 3.6 |
| Meconium ileus | 53 | 2.9 | |
| Volvulus | 44 | 2.4 | |
| Duodenal atresia/stenosis | 21 | 1.1 | |
| Ileal/jejunal atresia | 63 | 3.4 | |
| Small intestine atresia/absence/stenosis/obstruction | 108 | 5.9 | |
| Malrotation/intussusception of intestine | 14 | 0.8 | |
The median (IQR) postnatal age (days) for stoma formation for neonates with NEC was 18 (9–37) and 5 (3–11) for neonates with other malformations, and the median (IQR) postnatal age (days) for stoma closure was 88 (64–112) and 45 (25–72) for other malformations, respectively. The median birthweights for neonates with NEC were 860 g, and 2440 g for neonates with other malformations. Neonates with NEC were also born more preterm than neonates with other malformations, with a median gestational age of 26 and 35 weeks, respectively.
For the group of neonates with NEC, 4.5% of these received inotropes, 58.1% received PN, 41% needed non-invasive ventilation and 13.3% received invasive ventilation in the 2 days prior to stoma closure, while 3.8% received steroids in the 7 days before closure.
Lower proportions of neonates with other malformations were receiving received inotropes, corticosteroids, non-invasive or invasive ventilation prior to stoma formation (Table 18).
| NEC/perforation (n = 1543) | Other malformations (n = 287) | |
|---|---|---|
| Birthweight (g) (median, IQR) | 860 (695–1190) | 2440 (1430–2980) |
| Gestation age at birth (weeks) (median, IQR) | 26 (25–29) | 35 (31–37) |
| Weight at stoma formation (g) (median, IQR) | 1150 (820–1657) | 2310 (1540–3135) |
| Age at stoma formation (days) (median, IQR) | 18 (9–37) | 5 (3–11) |
| Weight at stoma closure (g) (median, IQR) | 2550 (2060–3125) | 3150 (2576–3796) |
| Age at stoma closure (days) (median, IQR) | 88 (64–112) | 45 (25–72) |
| Duration of stoma in situ (days) (median, IQR) | 60 (41–83) | 37 (17–55) |
| Inotropes 2 days pre closure (%, n) | 4.5 (69) | 2.1 (6) |
| Parental nutrition 2 days pre stoma closure (%, n) | 58.1 (897) | 65.2 (187) |
| Ventilation 2 days pre closure (%, n) | ||
| None | 45.3 (699) | 67.6 (194) |
| Non-invasive | 41.0 (633) | 16.0 (46) |
| Invasive | 13.3 (204) | 16.4 (47) |
| Missing | 0.6 (10) | 0 (0) |
| Steroids in last 7 days (%, n) pre stoma closure | 3.8 (60) | 1.4 (4) |
The median (IQR) duration of stoma was 57 (36–80) days for all neonates, 60 (41–38) days for neonates with NEC and 37 (17–55) days for neonates without NEC. Histograms depicting the distribution of stoma closure timings are shown in Figure 16.
FIGURE 16.
Histograms depicting stoma closure timings for (a) all neonates, (b) neonates with NEC and (c) other malformations.
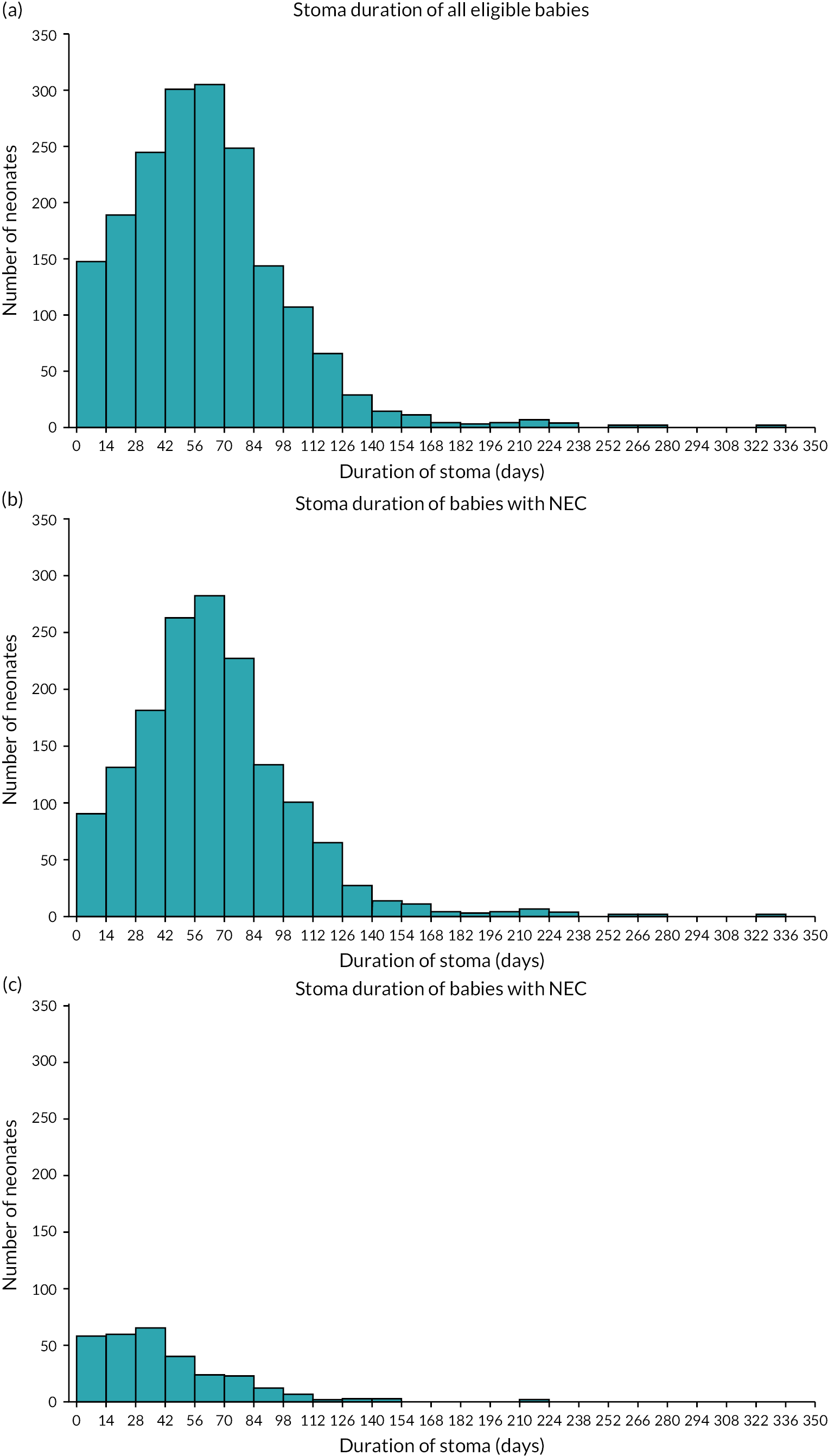
Two hundred and seventeen neonates were analysed separately since they were discharged from the unit prior to stoma closure: 178 neonates with NEC and 39 with other malformations. The recorded median length of stoma duration before discharge was 49 days for the NEC group and 28 days for other malformations. Data describing stoma closure were not available for these babies as the NNRD only holds data for babies receiving care in neonatal units, and stoma closure following neonatal discharge occurs in paediatric surgical settings in the UK.
Another 209 neonates were also analysed separately because they died prior to stoma closure: 197 neonates with NEC and 12 with other malformations. The median duration of stoma prior to death was 16 days. These results can be seen in Table 19.
| NEC/perforation | Other malformations | ||
|---|---|---|---|
| Discharged prior to stoma closure | Number of neonates (n) | N = 178 | N = 39 |
| Duration of stoma prior to discharge (median, IQR) | 49 (33–80) | 28 (17–49) | |
| Gestation age at birth (median, IQR) | 29 (26–33) | 36 (30–38) | |
| Birthweight in g (median, IQR) | 1110 (823–1790) | 2160 (1135–3090) | |
| Death prior to stoma closure | Number of neonates (n) | 197 | 12 |
| Duration of stoma prior to death (median, IQR) | 16 (7–40) | 16 (4–39) | |
| Gestation age at birth (median, IQR) | 25 (24–27) | 26 (24–34) | |
| Birthweight in g (median, IQR) | 717 (610–870) | 720 (585–1935) |
Neonates with NEC were further subdivided as having ‘early’ or ‘late’ stoma closure depending on whether closure occurred before or after 9 weeks stoma duration; this was based upon data from a national survey of practice (Workstream 1). Eight hundred and fifteen neonates were categorised as ‘early’ stoma closure, and 728 were categorised as ‘late’ stoma closure; clinical characteristics of neonates by timing of stoma closure is shown in Table 20.
| Characteristics of neonates with NEC | Early ≤ 9 weeks (n = 815) | Later > 9 weeks (n = 728) |
|---|---|---|
| Male sex (%, n) | 60.0 (489) | 63.2 (460) |
| Gestation (median, IQR) | 28 (25–31) | 25 (24–27) |
| Birthweight (g) | 986 (761–1486) | 764 (650–938) |
| Age at stoma formation (days) | 22 (9–43) | 15 (9–31) |
| Weight at stoma formation (g) | 1450 (1040–1983) | 916 (745–1200) |
| Age at stoma closure (days) | 66 (49–84) | 108 (92–129) |
| Weight at stoma closure (g) | 2330 (1857–2850) | 2767 (2320–3344) |
| Duration of stoma | 42 (25–53) | 85 (73–105) |
| Inotropes 2 days pre closure (%, n) | 4.9 (40) | 3.7 (27) |
| Parental nutrition 2 days pre stoma closure (%, n) | 61.1 (498) | 54.8 (399) |
| Ventilation 2 days pre closure (%, n) | ||
| None | 47.6 (388) | 42.3 (308) |
| Non-invasive | 36.9 (301) | 45.6 (332) |
| Invasive | 15.0 (122) | 11.3 (82) |
| Missing | 0.5 (4) | 0.8 (6) |
| Steroids in last 7 days (%, n) pre stoma closure | 2.7 (22) | 5.2 (38) |
Neonates with NEC were also subdivided into groups based on gestational age at birth: < 28 weeks (extremely preterm), 28–31 weeks (very preterm), ≥ 32 weeks (late preterm and term). Histograms depicting the distribution of stoma closure timings are shown in Figure 17.
FIGURE 17.
Histograms depicting stoma closure timings for neonates with NEC born at gestational ages. (a) < 28 weeks, (b) 28–31 weeks and (c) ≥ 32 weeks.
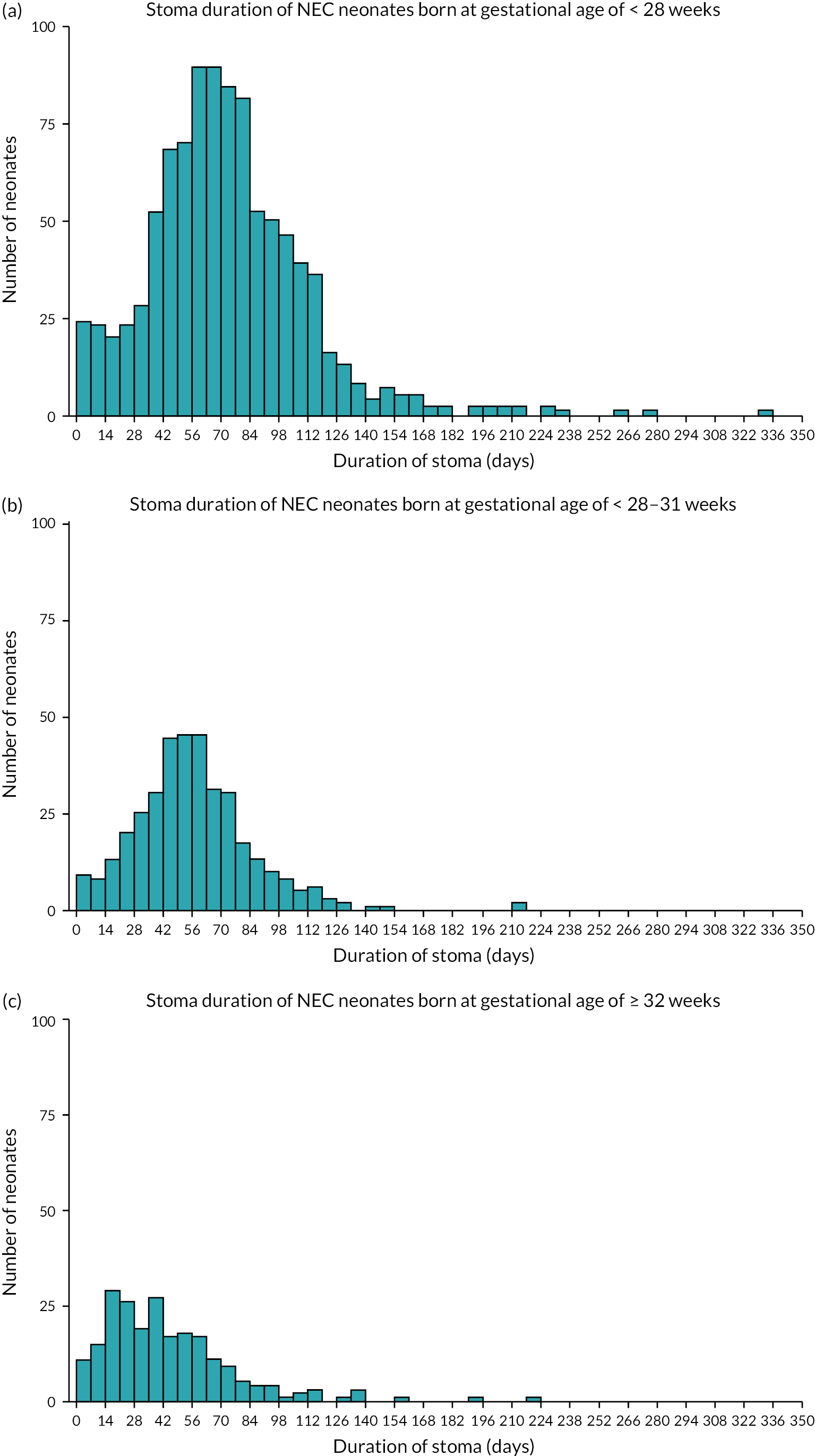
Strengths and limitations of the National Neonatal Research Database
The strengths of this study include analysing a large cohort using data entered in real time during clinical practice. This study describes neonates born over a period of 8 years covering most neonatal units in England and Wales, making it the largest study done so far on stoma closure in the neonatal period. The large population allows for a better depiction of clinical practice regardless of geographical variation and, being a database study, negates the element of bias inherent with cohort studies.
However, the NNRD is not without its limitations. Due to being a retrospective database study, it was difficult to extract data for neonates transferred to units that do not submit to the NNRD. Similarly, missing data could not be cross-checked with clinical notes; hence, we had to exclude 151 neonates that had no record of a gastrointestinal diagnosis consistent with requiring a stoma. These cases may represent erroneous data entry indicating a stoma was in situ. Another key limitation of the data from the NNRD is that many stand-alone paediatric units that perform surgery do not contribute data; hence, stoma formation and closure undertaken in these settings will not be captured, potentially underestimating the incidence of stoma formation and closure in the neonatal period. However, these data are captured through HES-APC and BAPS-CASS data sources, hence the pooling of data for this study.
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System necrotising enterocolitis study
There were 256 infants who had NEC/SIP (23 with SIP); 163 of these infants had a stoma. The mean age at stoma formation was 17 days and for closure it was about 3 months (94 days). Eighty-one infants underwent stoma closure and the median (IQR) time from stoma formation to closure was 63 days (41–130 days) (Figure 18). Characteristics of infants with early stoma closure (≤ 9 weeks) and later stoma closure (> 9 weeks) are shown in Table 21.
| Characteristics | Total (N = 81) |
Early ≤ 9 weeks (n = 42) |
Later > 9 weeks (n = 39) |
|---|---|---|---|
| Male sex (n, %) | 50 (61.7) | 24 (57.1) | 26 (66.7) |
| Gestation (weeks) (median, IQR) | 27 (25–30) | 26 (25–28) | 28 (25–31) |
| Birthweight (g) (median, IQR) | 950 (745–1375) | 925 (685–1294) | 1000 (780–1600) |
| Age at stoma formation (days) (median, IQR) | 17 (8–33) | 20 (10–37) | 12 (8–27) |
| Weight at stoma formation (g) | N/A | N/A | N/A |
| Age at stoma closure (days) (median, IQR) | 94 (60–154) | 61 (43–81) | 155 (118–195) |
| Weight at stoma closure (g) | N/A | N/A | N/A |
| Local stoma complications (n, %) | 14 (17.3) | 12 (28.6) | 2 (5.1) |
| Bowel length DJ to stoma, cma (median, IQR) | 51.5 (35–70) | 47.5 (35 –62) | 57.5 (37–85) |
| Receiving TPN at 28 days (n, %) | 48 (60.8) | 28 (68.3) | 20 (52.6) |
| Feed started at 28 days (n, %) | 75 (94.9) | 38 (92.7) | 37 (97.4) |
FIGURE 18.
Timing of stoma closure among NEC infants in BAPS-CASS.
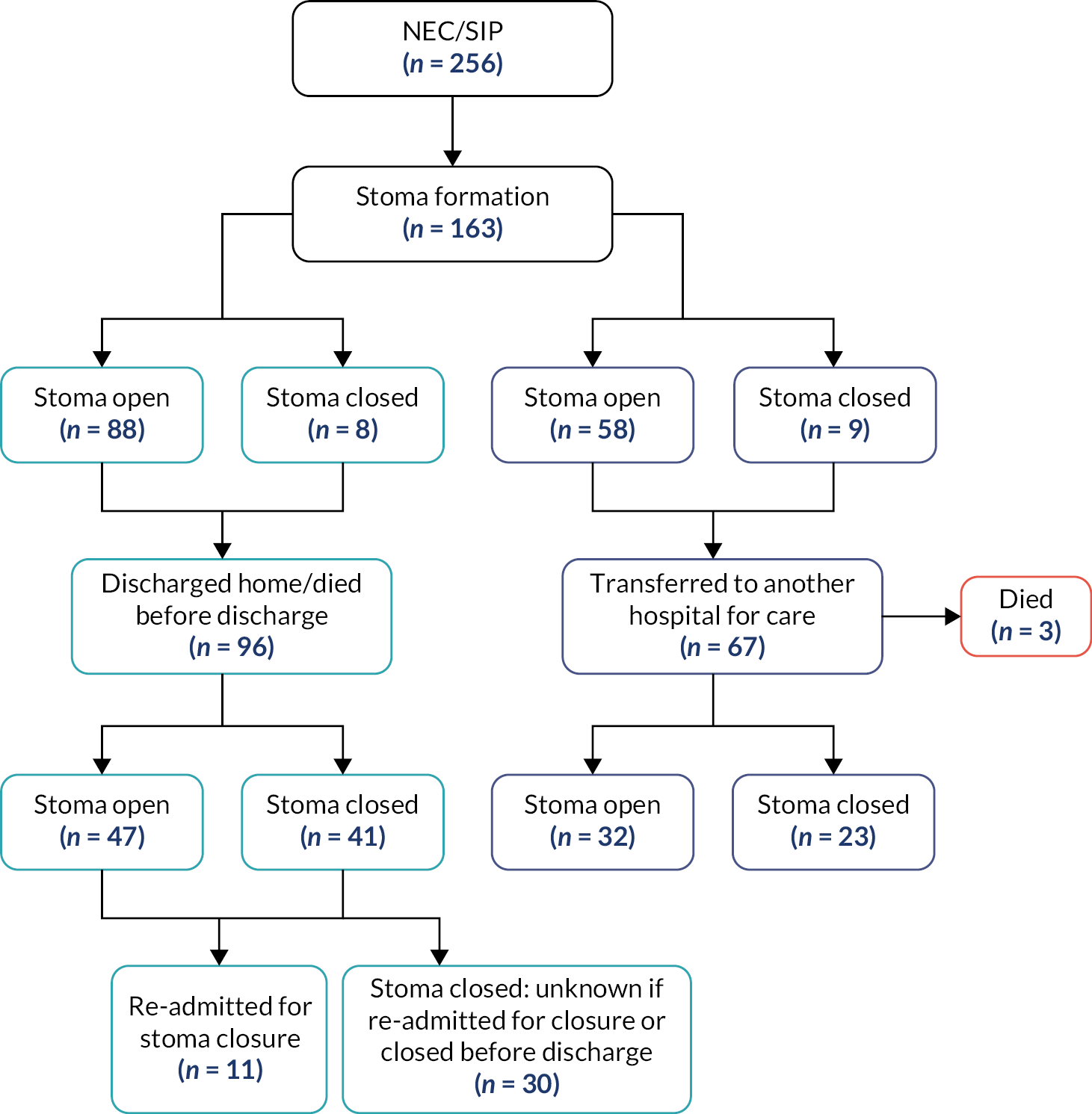
Thirty-seven (22.7%) infants died during the study period; the majority (n = 30) of these deaths occurred within 28 days after surgery.
Sixty-seven infants were transferred to another hospital for further care and 96 infants were either discharged home or died before discharge. Forty-nine out of 96 infants who were not transferred underwent surgery for stoma closure (7 of these were prior to discharge and 1 before death within 28 days of surgery). Thirty-two out of 67 infants who were transferred to another hospital had surgery for stoma closure (9 of these were before they were transferred) (Figure 19). Data for LOS were available for 36 infants (28 who were discharged home and 8 who were transferred) where the median (IQR) was 64 (39.5–79) days. Infants who were discharged home or transferred to another hospital prior to stoma closure were re-admitted for closure at around 5 months of age (158 days, Table 22).
| Indication for stoma | NEC/intestinal perforation |
|---|---|
| Male sex (%, n) | 24 (70.6) |
| Gestation (median, IQR) | 28 (26–32) |
| Birthweight (g) (median, IQR) | 1086.5 (800–1692) |
| Age at stoma formation (days) (median, IQR) | 12 (8–33) |
| Weight at stoma formation (g) | N/A |
| Age at discharge (days) (median, IQR) | 69 (45–84) |
| Age when re-admitted for closure (days) (median, IQR) | 158 (104–195) |
FIGURE 19.
Flowchart of NEC/SIP infants – stoma formation and closure. Note: 34/96 infants who were not transferred died; however, due to missing discharge dates, it is unclear whether they died before or after discharge.
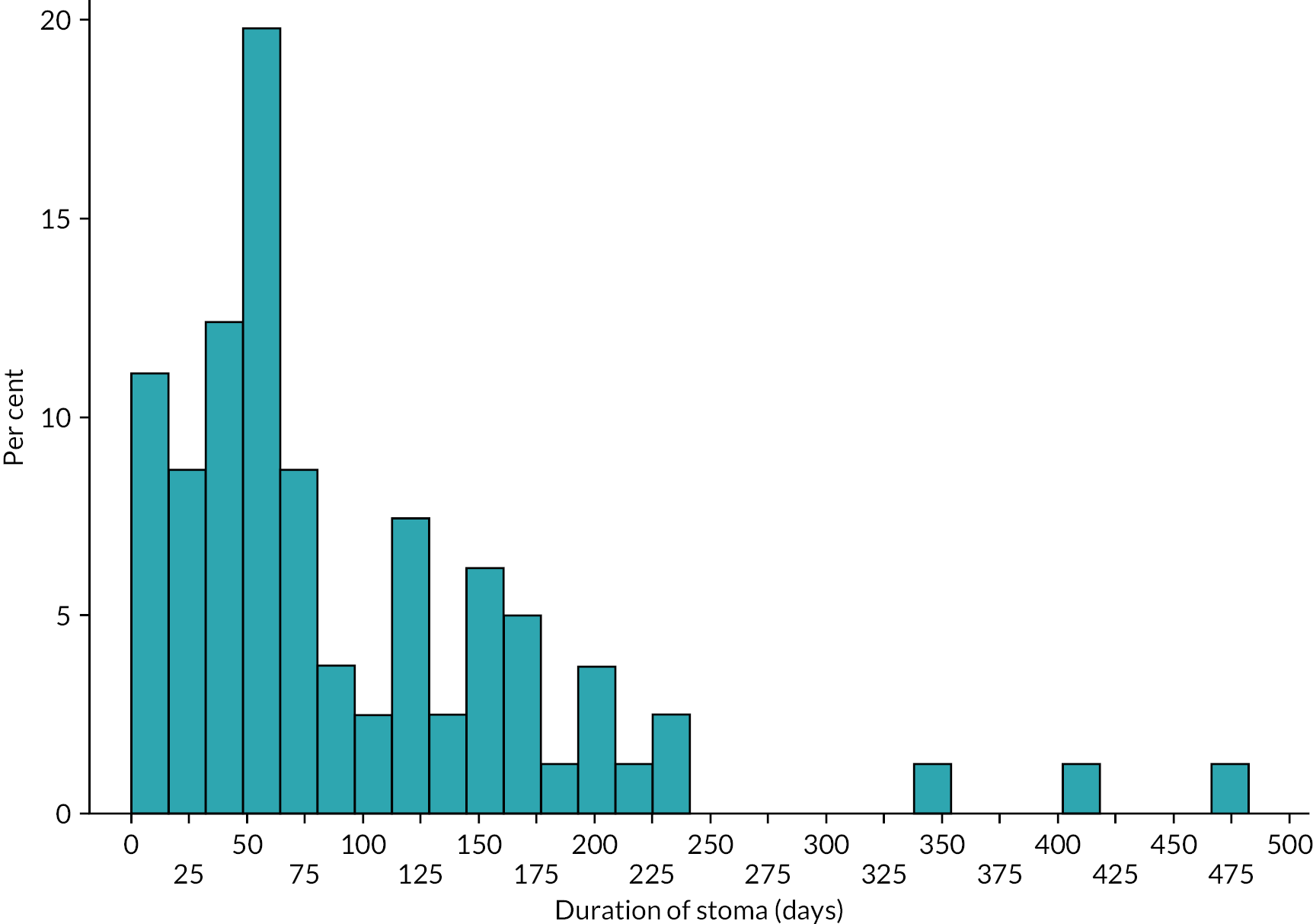
Fourteen infants had stoma-related complications post surgery, of which 10 occurred within 28 days after surgery. Sixteen infants underwent additional surgical procedures for their stoma. At 1 year post surgery, three infants were still receiving PN support.
Meconium ileus
The British Association of Paediatric Surgeons Congenital Anomalies Surveillance System meconium ileus study
The other BAPS-CASS study available for analysis that included data on infants with stomas captured infants with meconium ileus. Fifty-six infants had meconium ileus of whom 21 had stomas formed. Twenty infants had their stomas closed during the study period [median time to stoma closure (IQR): 51 (36–106) days] (Figure 20). For nine infants, this was before discharge from the hospital. The median (IQR) LOS was 46.5 (23–71.5) days. No infant died during the study period. Nine infants had stoma-related complications within 28 days post surgery and an additional four infants had complications by 1-year post surgery. One infant underwent surgery to form a second stoma. Enteral feeds were started for all infants within 28 days after surgery and 11 infants progressed to full enteral feeds within 28 days post surgery. Median (IQR) days from stoma formation to full enteral feeds for those who were fed within the first 28 days was 12.5 (7–17). At 1 year post surgery, all infants were receiving full enteral feeds.
FIGURE 20.
Timing of stoma closure among meconium ileus infants in BAPS-CASS.
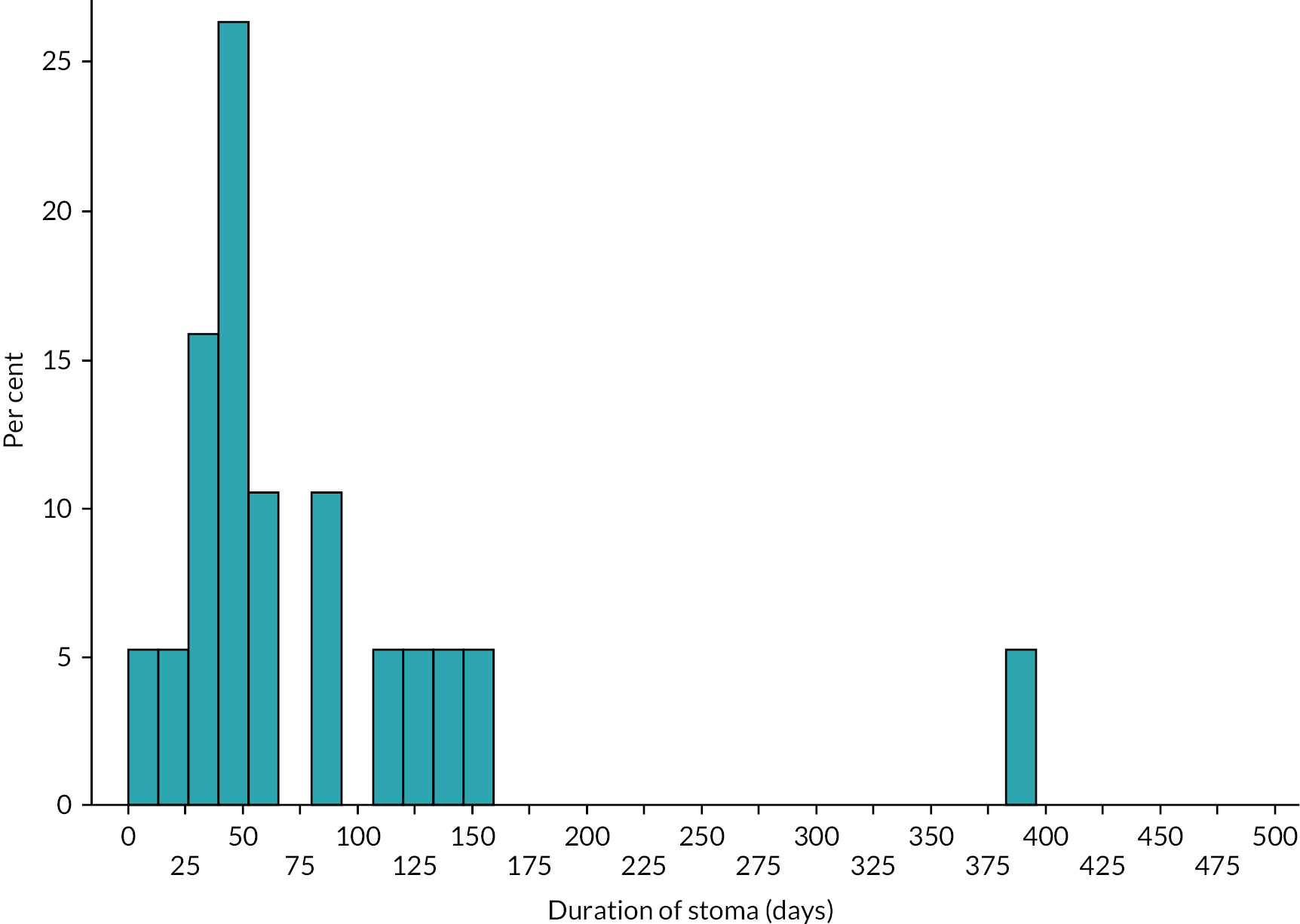
Strengths and limitations of British Association of Paediatric Surgeons Congenital Anomalies Surveillance System
The strength of this study was that for both NEC and meconium ileus data were obtained from population-based prospective cohort studies of infants with these conditions in the UK and Ireland; this enabled generalisability of the findings and minimisation of biases inherent in retrospective studies. Another strength is the collection of data on feeds and stoma complications at 28 days and 1 year post surgery. However, a limitation is the small sample size for infants who have undergone stoma, especially for meconium ileus and for some of the comparisons for NEC. Another limitation is the absence of data for weight of infant at stoma formation and closure.
Hospital Episode Statistics – Admitted Patient Care
Between 1 January 2011 and 31 December 2018, there were 3541 infants aged < 90 days who had a stoma formation procedure (annual mean: 443). After exclusion of anorectal malformations and Hirschsprung’s disease, the total number was 2477 (mean per year: 310), of whom 1537 (mean per year: 192) had a diagnosis of NEC/SIP without congenital malformation and 940 (mean per year: 118) did not (Figure 21).
FIGURE 21.
Number of infants aged < 90 days who underwent stoma formation, English national HES 2011–8. a, Excluding congenital malformations.
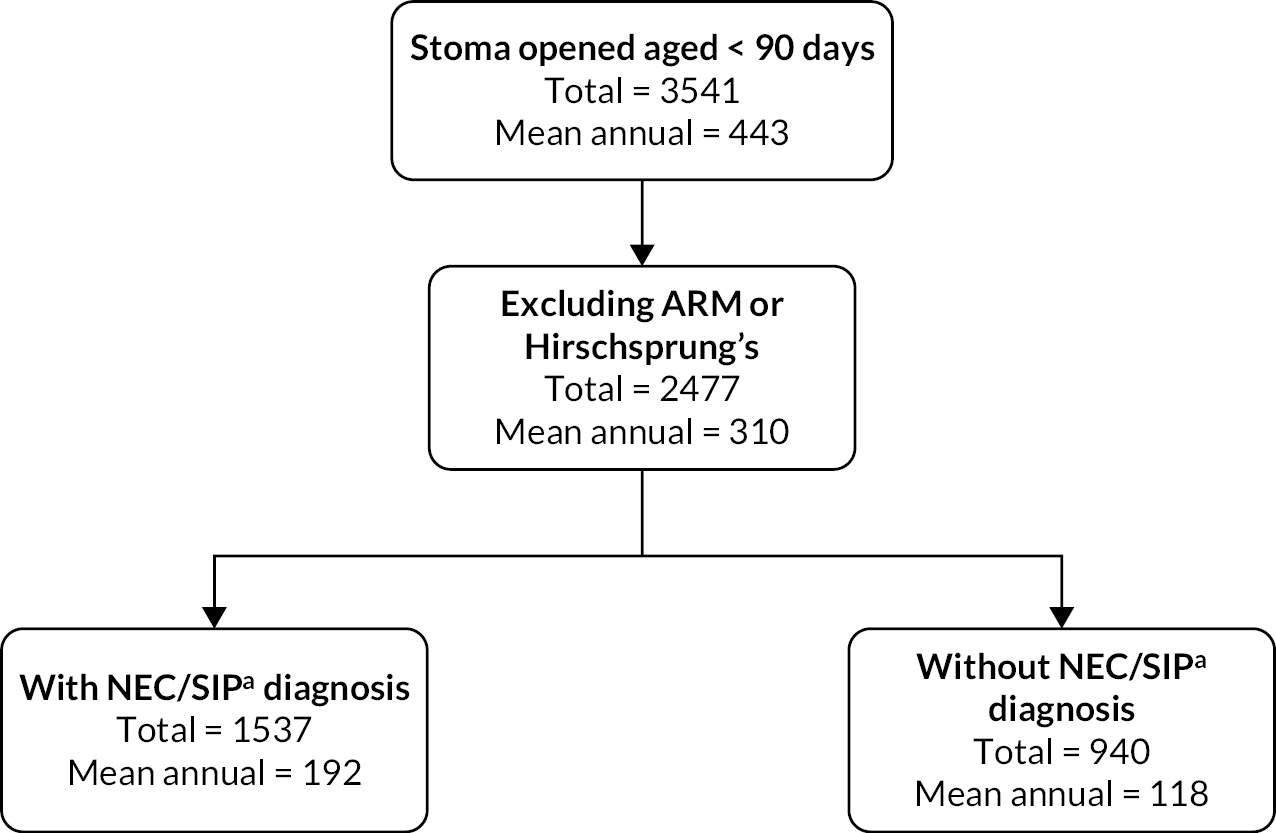
The characteristics and outcomes of the study population (total and in subgroups with and without NEC/SIP diagnosis) are shown in Table 23.
| Total (N = 2477) | % of totala | NEC/SIP (N = 1537) | % of totala | Other (N = 940) | % of totala | ||
|---|---|---|---|---|---|---|---|
| Year | 2011 | 306 | 12.4 | 192 | 12.5 | 114 | 12.1 |
| 2012 | 310 | 12.5 | 203 | 13.2 | 107 | 11.4 | |
| 2013 | 289 | 11.7 | 173 | 11.3 | 116 | 12.3 | |
| 2014 | 327 | 13.2 | 195 | 12.7 | 132 | 14.0 | |
| 2015 | 311 | 12.6 | 194 | 12.6 | 117 | 12.5 | |
| 2016 | 279 | 11.3 | 174 | 11.3 | 105 | 11.2 | |
| 2017 | 312 | 12.6 | 187 | 12.2 | 125 | 13.3 | |
| 2018 | 343 | 13.8 | 219 | 14.3 | 124 | 13.2 | |
| Sex | Male | 1476 | 59.6 | 952 | 61.9 | 524 | 55.7 |
| Female | 1001 | 40.4 | 585 | 38.1 | 416 | 44.3 | |
| Gestational age (completed weeks) | 22 | 1 | 0.1 | 1 | 0.1 | 0 | 0.0 |
| 23 | 78 | 5 | 70 | 7.3 | 8 | 1.3 | |
| 24 | 157 | 10.1 | 138 | 14.5 | 19 | 3.2 | |
| 25 | 165 | 10.6 | 146 | 15.3 | 19 | 3.2 | |
| 26 | 119 | 7.7 | 94 | 9.8 | 25 | 4.2 | |
| 27 | 120 | 7.7 | 98 | 10.3 | 22 | 3.7 | |
| 28 | 105 | 6.8 | 87 | 9.1 | 18 | 3.0 | |
| 29 | 60 | 3.9 | 47 | 4.9 | 13 | 2.2 | |
| 30 | 55 | 3.5 | 40 | 4.2 | 15 | 2.5 | |
| 31 | 47 | 3 | 28 | 2.9 | 19 | 3.2 | |
| 32 | 57 | 3.7 | 38 | 4 | 19 | 3.2 | |
| 33 | 31 | 2 | 18 | 1.9 | 13 | 2.2 | |
| 34 | 60 | 3.9 | 19 | 2 | 41 | 6.9 | |
| 35 | 61 | 3.9 | 16 | 1.7 | 45 | 7.6 | |
| 36 | 90 | 5.8 | 19 | 2 | 71 | 11.9 | |
| 37 | 94 | 6.1 | 20 | 2.1 | 74 | 12.4 | |
| 38 | 82 | 5.3 | 27 | 2.8 | 55 | 9.2 | |
| 39 | 74 | 4.8 | 25 | 2.6 | 49 | 8.2 | |
| 40 | 59 | 3.8 | 15 | 1.6 | 44 | 7.4 | |
| 41 | 34 | 2.2 | 8 | 0.8 | 26 | 4.4 | |
| 42 | 2 | 0.1 | 1 | 0.1 | 1 | 0.2 | |
| Missing | 926 | 37.4 | 582 | 37.9 | 36.6 | ||
| Birthweight | 400–999 | 667 | 43 | 558 | 58.4 | 109 | 18.3 |
| 1000–1499 | 218 | 14.1 | 182 | 19.1 | 36 | 6.0 | |
| 1500–2499 | 249 | 16.1 | 105 | 11 | 144 | 24.2 | |
| 2500–4999 | 417 | 26.9 | 110 | 11.5 | 307 | 51.5 | |
| Missing | 926 | 37.4 | 582 | 37.9 | 344 | 36.6 | |
| Stoma closure | Closed | 1763 | 71.2 | 1078 | 70.1 | 685 | 72.9 |
| Discharged alive | Before stoma formation | 212 | 8.6 | 84 | 5.5 | 128 | 13.6 |
| Between formation and closure | 840 | 33.9 | 472 | 30.1 | 368 | 39.1 | |
| After closure | 987 | 39.8 | 635 | 41.3 | 352 | 37.4 | |
| Death | Before closure | 404 | 16.3 | 307 | 20 | 97 | 10.3 |
| Before age 1 | 466 | 18.8 | 355 | 23.1 | 111 | 11.8 |
In total, 466 (18.8%) patients died in infancy; the majority (n = 404) of these occurred before stoma closure. Among the subgroup of patients with NEC/SIP, 355 patients (23.1%) died in infancy (307 before stoma closure). Among the subgroup of patients without NEC/SIP, 111 patients (11.8%) died in infancy (97 before stoma closure).
For the hospital spells in which the stoma was formed, median LOS (including any inter-hospital transfers) was 76 (IQR 32–127) days overall and 82 (IQR 36–131) days among those who did not die during the spell; in the subgroup of patients with NEC/SIP, median LOS was 84 (IQR 37–129) days overall and 92 (IQR 43–136) days among those who did not die; in the subgroup of patients without NEC/SIP, median LOS was 65 (IQR 28–119) days overall and 67 (IQR 28–123) days among those who did not die.
The frequency distribution of time from stoma formation to closure is shown in Figure 22. Figure 23 shows the cumulative percentage over time of infants whose stoma was closed. In patients overall, the median number of days to closure was 77 (IQR 52–117); in patients with NEC/SIP, the median was 78 (IQR 55–112); in patients without NEC/SIP, the median was 74 (IQR 45–124). When those discharged with a stoma in situ were excluded, the median time to closure was 61 (IQR 45–84) days overall, days (IQR 49–87) days in those with NEC/SIP and 51 (IQR 37–73.5) days in those without NEC/SIP.
FIGURE 22.
Frequency distribution of time (in days) from stoma formation to closure in infants whose stoma was closed within 1 year.
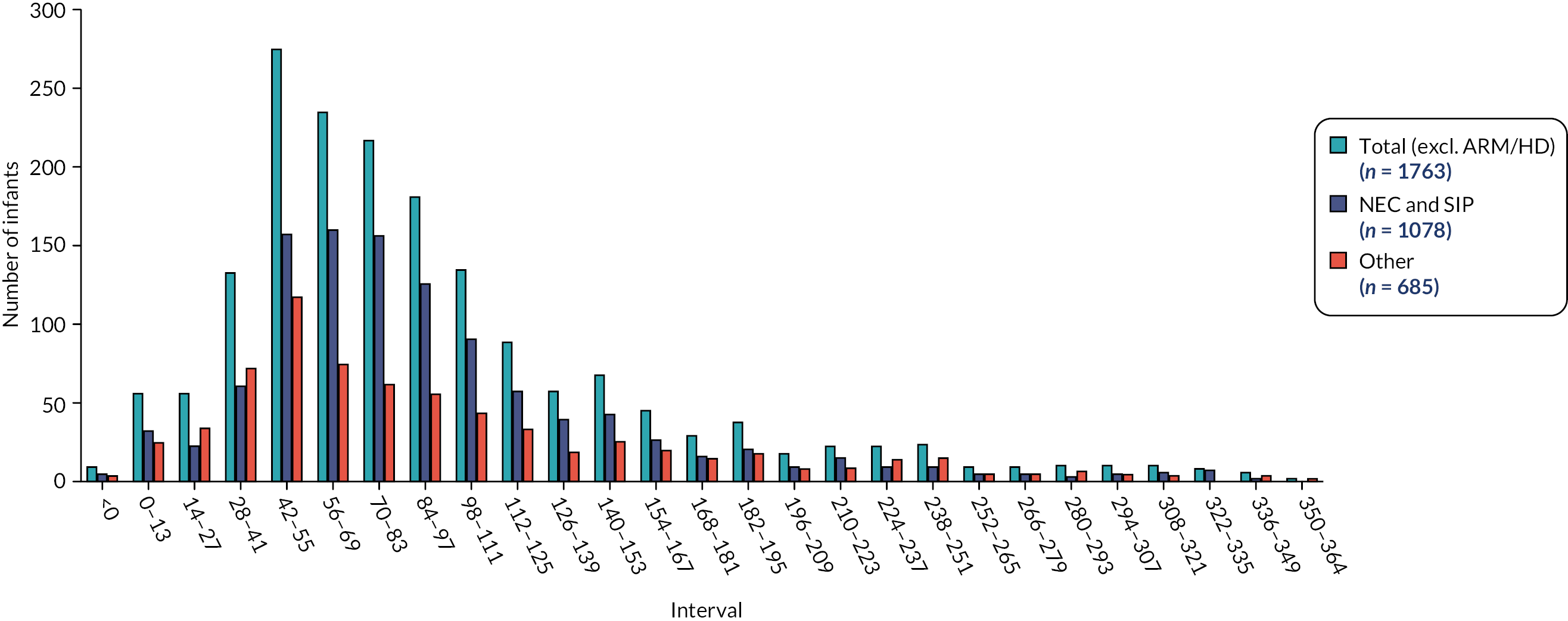
FIGURE 23.
Cumulative percentage over time of infants whose stoma was closed within 1 year.
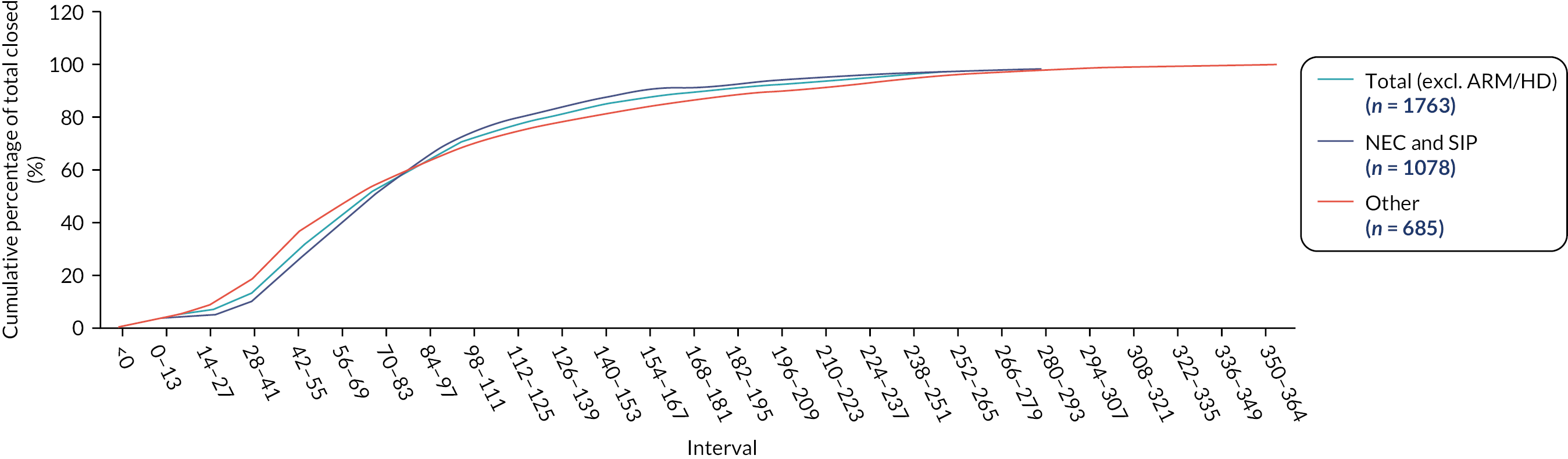
Strengths and limitations of the Hospital Episode Statistics – Admitted Patient Care data set
Strengths of the HES-APC data include coverage of every NHS hospital admission, irrespective of cause, in the whole of England over multiple years. Since the HES-APC records contained anonymised patient IDs, each patient could be traced over time through their hospital records. The HES-APC were also linked to civil registrations (mortality) data to enable additional capturing of any deaths, however few, that may have occurred without hospitalisation. The data are recorded prospectively, which avoids biases inherent in retrospective studies. The diagnostic coding (ICD-10) enables algorithmic phenotyping of the causes for stoma formation (e.g. identifying patients with NEC/SIP but excluding those with congenital malformations). Each new operative procedure is detailed according to the OPCS-4 and recorded with an exact date, enabling precise ascertainment of the time interval from stoma formation to stoma closure. Limitations of HES-APC include a high percentage of missing data on patient characteristics such as birthweight and gestational age at birth and the absence of other detailed patient characteristics such as bowel length, total parenteral nutrition (TPN), enteral feeds and weight at stoma closure.
Chapter 7 Consensus meeting
Attendance
The consensus meeting was attended online by 52 individuals from a range of health professional and non-professional backgrounds. The roles of attendees are shown in Figure 24.
FIGURE 24.
Roles of consensus meeting attendees.
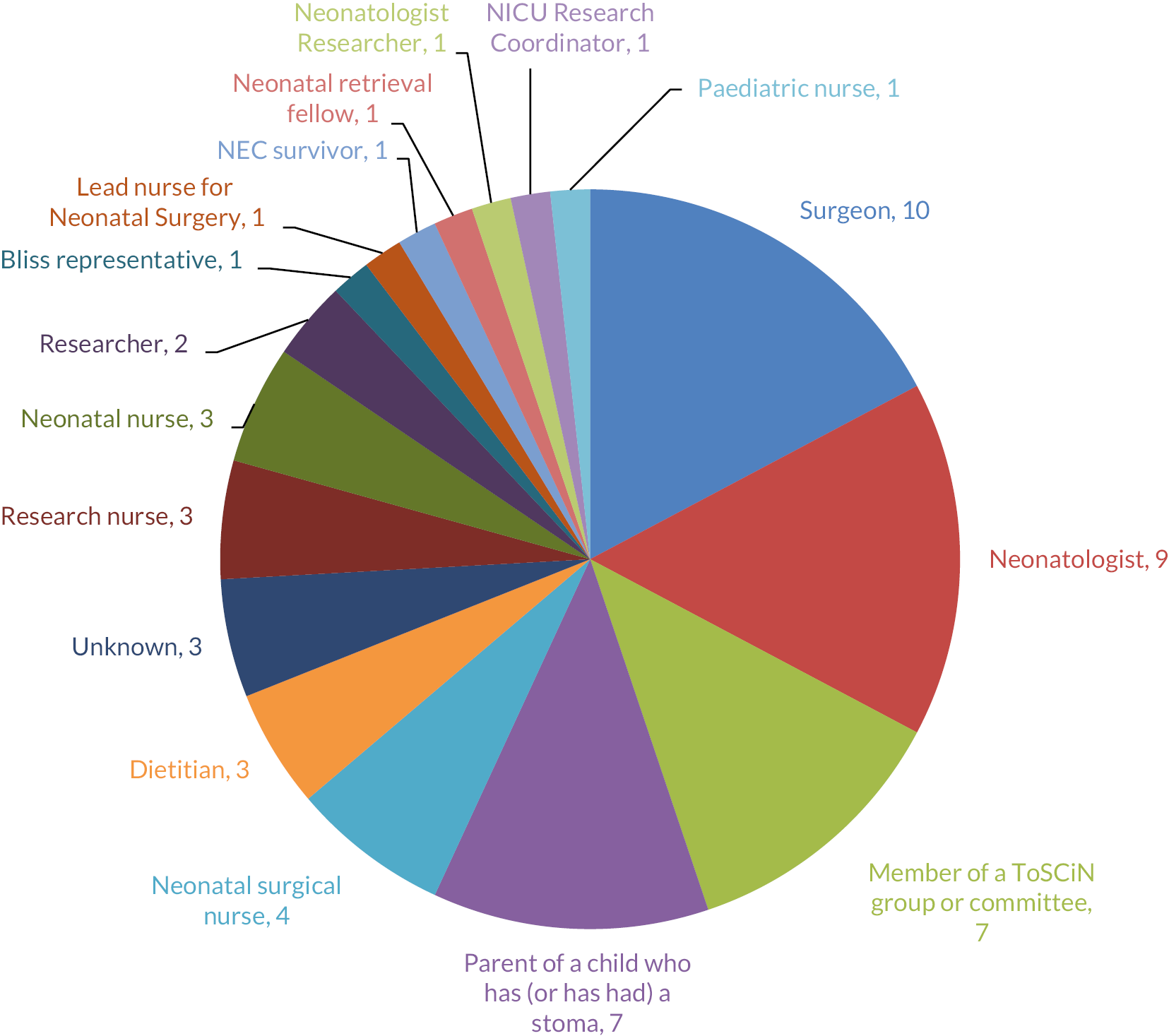
Meeting conduct
After an introduction to the ToSCiN study from the chairperson, a summary of findings for each of the three meeting themes was presented. After each presentation, breakout groups were convened to discuss the findings and the facilitator for each group fed back a summary of discussions to all attendees. Voting was then undertaken.
Summary of small group discussions
Theme 1: What’s important to measure? Outcomes
Key outcomes were felt to be:
-
growth
-
quality of life (of both infant and family/parents)
-
length of hospital stay
-
neurodevelopment
-
long-term outlook/lack of future complications.
People generally felt that one of these should be the primary outcome (with growth and LOS receiving the most votes) (see Appendix 3, Table 26 and Figure 25), but it was recognised that some of them (e.g. quality of life) may be difficult to measure or power a trial with. It was agreed that these outcomes are all to some extent proxies for parents’ end goal of having a thriving, healthy infant who goes on to lead a normal life. They are to some extent interlinked/could act as proxies for each other and this should be kept in mind.
The group also considered what outcome would convince clinicians to change their practice. Should this then be the primary outcome?
Other recommended secondary outcomes included bacterial infection (sepsis), liver dysfunction, central venous catheter complications, refeeding and recycling, ability to feed/establishment of oral feeding/time of total PN and feed tolerance (see Appendix 3, Table 27 and Figure 26).
Theme 2: What should we compare? Trial design
The importance of flexibility around the time points was consistently emphasised; a trial comparing closure at (rather than before/after) specific time points was felt to be unfeasible.
It was also agreed that the research question must be clearly defined; what are we trying to compare and under which criteria?
Parents prefer to wait for ‘their’ surgeon to be the one to close their child’s stoma, which is a potential additional difficulty when trying to adhere to arms.
It was felt that gestational age and/or weight should be taken into account at randomisation; potentially, a minimal gestational age; alternatively, qualifying the ‘early’ time point with a caveat related to gestational age (e.g. ‘close at six weeks or when infant has reached a certain post-menstrual age’); possibly a stratified trial of preterms and non-preterms. At the voting stage, various ideas around qualifying the time point based on gestational age, or treating extremely preterm infants differently, were proposed (see Appendix 3, Table 28, Figure 27 and Box 2).
One group proposed a trial that compares stoma closure in infants born at 28 weeks' gestational age or less when they reach the 32-week gestational age point versus 38–40-week gestational age point; that is, a criterion based on age, not time from formation. This idea was added to the voting list and 27% of attendees chose it as their preferred design.
It was also noted that timing of randomisation should be considered: in particular, would it be possible to put together a list of parameters that influence decision to close a stoma, identify infants ready for closure using this and then randomise at that point to ‘closure now’ or ‘closure later?’.
Randomisation to a later closure time may have capacity and cost implications for sites, particularly if it will prevent infants going home; parents may be unhappy about taking infants home with a stoma if community support is not available.
Education on equipoise will likely be necessary.
The design of 6 weeks versus expectant management (or an alternative, ‘less than seven weeks vs. expectant management’) was generally felt to be the most pragmatic, but people agreed that it would present challenges:
-
Too many differences in practice in the expectant management arm.
-
Not enough separation between arms (to mitigate this, there could be a restriction on the expectant management arm, e.g. no sooner than 12 weeks after stoma formation).
-
How to prevent the expectant management arm from being influenced by the outcomes of infants not randomised to the study?
-
Parents may prefer the expectant management arm because it would be seen as more clinician-led, and thus be unhappy about their infant being allocated to the other arm.
Theme 3: Who should we include? Population
A sizeable majority felt that eligibility criteria should be as broad as possible, with appropriate subgroup analyses carried out at the end of the trial (see Appendix 3, Table 29, Figure 28 and Box 3).
However, some people advocated exclusion of term infants: it was commented that clinicians would be more interested in results as they pertained to preterms; if term infants were included, equipoise could be more of an issue (see Appendix 3, Table 30 and Box 4).
During the voting, some attendees recommended exclusion of infants with particularly complicated conditions or significant congenital abnormalities.
Inclusion of extremely preterm infants could be made easier by having some treatment criterion around gestational age as discussed in theme 2.
There could be additional criteria around level of sickness (e.g. low weight, level of respiratory support, inotropes) or other ‘get-out clauses’ whereby the early allocation would not need to be followed. Most people felt that very sick or very preterm infants should not be excluded entirely but would need to be treated differently/have their clinical condition taken into account in some way when deciding whether to follow the allocated arm. Infants unlikely to survive should not be recruited.
There was general support for minimisation or stratification by term/preterm.
A trial will involve asking clinicians to go beyond their comfort zone and be less cautious; this should be taken into account in training and presentation of the trial to potential sites (but generational shift may help).
Voting and free-text comments
Full details of voting results and free-text comments submitted by attendees are provided in Appendix 3.
Chapter 8 Discussion and conclusions
Principal findings
The ToSCiN study examined the feasibility of a definitive RCT comparing ‘early’ and ‘late’ stoma closure in neonates by: (1) surveying those professionals routinely looking after infants with stomas (Workstream 1), (2) prospectively studying neonates with new stomas (Workstream 2.1 and Workstream 2.2), (3) exploring the views of parents and health professionals regarding a potential RCT (Workstream 2.3) and (4) analysing existing national databases of neonates with stomas (Workstream 3). Finally, a consensus meeting involving a range of stakeholders was carried out towards the end of the above processes.
We found that a randomised trial of early compared with late stoma closure in neonates is feasible and identified the following components as being critical to a successful future trial:
-
comparison of closure at 6 weeks versus expectant management
-
comparison that accounts for completed gestational age, rather than solely duration of stoma.
We identified the potential population and outcomes for such a trial and established that a sufficient population exists in the UK.
We also identified that a trial comparing early and late closure at rigidly defined time points (e.g. closure at 6 compared with 12 weeks) would not be feasible.
The objectives and relevant study findings are listed below.
Establish current UK practice for stoma closure in neonates
Data regarding current UK practice were gathered in the national survey (Workstream 1) and database analyses (Workstream 3). Further data were also collected about current practice in Workstream 2.1, but from a smaller group of infants when compared with the national cohorts in the databases. Finally, further insight was gained from focus groups in Workstream 2.3. Although we identified a wide variation in the timing of neonatal stoma closure across the UK, there are consistent infant-level factors that influence the timing of closure, such as corrected gestation and weight. This supports the feasibility of a trial that accounts for such infant-level factors.
With regard to the key question of any RCT, timing of stoma closure, the three national data sets demonstrated that closure for infants with NEC/SIP (the largest patient group) is performed at an average of approximately 2 months post formation. Median stoma durations in the three databases were as follows: BAPS-CASS 63 days; NNRD 60 days and HES-APC 78 days (66 days when those discharged home with a stoma were excluded). For infants with stomas for reasons other than NEC/SIP, closure was usually done earlier: in the NNRD 45 days; in HES-APC 74 days (51 days when those discharged home with a stoma were excluded); in BAPS-CASS, stomas in infants with meconium ileus were closed at 51 days.
However, we found evidence of variability in UK practice, both with regard to the time interval to stoma closure and whether this was done before or after the infant was discharged home. This was evident from both observational data and self-reported practice. The interval from stoma formation to closure varied in national data sets (see Figures 16, 17, 19, 20 and 22); for example, for those infants with NEC/SIP in the BAPS-CASS and NNRD databases, the upper quartiles were 130 and 83 days, and the lower quartile was 41 in both cases. We found this time interval was consistently influenced by patient factors such as gestation and birthweight, with more preterm and smaller infants waiting longer for surgery (see Tables 20 and 21). However, our survey and focus group findings indicate that even for similar patients, individual centres and surgeons will choose different timings of stoma closure (Table 24) and different preferences for closing stomas before or after discharge home, with one scenario (term infant with meconium ileus) attracting the following responses: 46% before discharge; 32% after discharge; and 22% unsure. The qualitative research (Workstream 2.3) also found evidence of variability between professionals within providing surgical centres; for example, one parent stated, ‘it genuinely was an argument between consultants and the surgeons as to what should happen’, and a nurse described their experience of different surgeons choosing to close stomas at differing time points. We also identified additional patient-specific factors that influence decisions around timing and account for some of the additional observed variability; these are discussed later in this section.
| Scenario 1 Preterm ileal |
Scenario 2 Preterm jejunal |
Scenario 3 Term ileal |
Scenario 4 Term jejunal |
||
|---|---|---|---|---|---|
| Observed practice weeks [median (IQR)] | Earliest | 6 (4–6) |
6 (4–8) |
4 (4–6) |
4 (3–6) |
| Latest | 20 (12–30) |
20 (12–26) |
16 (12–24) |
12 (8–16) |
|
| Target closure weeks [median (IQR)] | 8 (6–12) |
8 (6–10) |
4 (6–8) |
6 (4–6) |
|
| Relation to discharge (% of respondents) | Before | 70 | 76 | 32 | 72 |
| After | 15 | 9 | 46 | 12 | |
| Unsure | 14 | 15 | 22 | 16 |
An insight into intercentre variability was also provided by the focus groups (Workstream 2.3) where professionals described the approach taken by their centre alongside limited awareness of different approaches taken elsewhere. Three sites stated they aim for early closure (around 6 weeks), two did not have specific time frames and one preferred longer intervals to closure (around 12 weeks). This variability between providing centres also extended to whether or not they would discharge an infant home with a stoma, with some staff happy to do this but others having concerns. Furthermore, there were differences in whether surgical centres could transfer infants back to more local centres (closer to parental homes) with a stoma in situ, with some local units able to care for these infants but others unable to do this. This has important implications for families including travel and financial burden, and for surgical centres which have to shepherd finite resources such as neonatal cot availability. Within a trial, the decision and timing on whether or not to close a stoma have to take these factors into account.
Looking at the decision-making behind timing of stoma closure in more detail, professionals reported common themes in the survey (Workstream 1) that influenced whether this should be expedited or delayed.
Common reasons for bringing closure forward were:
-
growth failure and PN dependence (including liver disease)
-
high stoma output
-
peristomal issues, for example, wound problems and leaking bags
-
social issues
-
vascular access problems.
Common reasons for delaying stoma closure were:
-
thriving with stoma and enterally autonomous
-
comorbidities (not optimised for surgery)
-
underlying gut pathology/surgical technical concerns
-
difficulty accessing operating lists.
The findings from the qualitative research in Workstream 2.3 correlated extremely closely with the survey findings. All centres stated that decisions about timing of stoma closure were ‘baby-led’ and hence there was significant variance in closure timing at all sites. When parents and professionals identified factors that influenced closure timing, the themes were essentially the same as those in Workstream 1 listed above.
Interestingly, the above finding that infants with stoma problems and growth require earlier closure is also supported by our observational data. In the BAPS-CASS national cohort (Workstream 3), infants who underwent stoma closure earlier (< 9 weeks) had a higher rate of local stoma complications and were more likely to still be requiring TPN at 28 days than those closed after 9 weeks.
In summary, while the timing of neonatal stoma closure varies in national data sets and there is evidence of intercentre and intersurgeon variability from survey data and focus groups, there are highly consistent, key factors that influence when stomas are closed on a case-by-case basis.
Determine whether there is equipoise among clinicians and allied health professionals over when it is best to close stomas in neonates.
Equipoise among clinicians and allied health professionals was tested in the survey (Workstream 1), focus groups (Workstream 2.3) and consensus meeting. Strong evidence of individual equipoise was found in the results of the survey as demonstrated by high levels of uncertainty: around one in three respondents were unsure what the best target time was to close the infant’s stoma in each clinical scenario (range 29–37% across four scenarios). In these scenarios, further uncertainty was expressed about whether the infant’s stoma should be closed before or after discharge home (range 14–22%). These data demonstrate there is genuine ambiguity about the optimal timing of stoma closure among a large group of professionals and indicates there is equipoise among this group. In keeping with this, support for a trial was also expressed in the free-text comments in the survey.
Equipoise was also considered in the survey when potential barriers to a clinical trial were explored through free-text comments: 16/166 (9.6%) respondents made comments judged to include concerns about a lack of equipoise among professionals. An example of one such comment is: ‘some surgeons may not feel there is equipoise. What would be the study design and opt-outs? So, if a baby was randomised to late (e.g. > 12 weeks), but the clinical team wanted to perform early, then how would you cope with protocol violations?’ Other comments related to hypothetical lack of equipoise among other professional groups, for example, ‘convincing surgeons of equipoise is the argument’. These concerns about lack of equipoise were only raised by < 10% of respondents. This is in keeping with many feasibility studies, as it is exceptionally rare for a clinical trial to find equipoise from all health professionals.
Staff focus group discussions and parental interviews (Workstream 2.3) found further equipoise and support for a trial, with parents and staff providing support for each arm. As expected, individual concerns were raised about a lack of equipoise in some clinical situations, with some parents and staff having clear trial arm preference and concerns about the alternative arm. Interestingly, it was found that staff who were not aware of differing practice around the UK and differing timings of closure tended to lack equipoise. For this reason, we have highlighted the need to ensure staff training at trial sites should a RCT take place, highlighting the existing differences in practice around stoma closure between different hospitals and clinicians within hospitals.
The key determinants of equipoise, and which overlapped with trial acceptability, were the population to be included in a trial and the definition of ‘early’ versus ‘late’ used (the intervention and comparator). While there was a consistent theme of uncertainty among professionals, strong views were also expressed about clear lack of equipoise for certain infants. An example of a comment made about this is, ‘the case definition of early vs. late, if extremes are used e.g. (in my view, 3wks for early, 12 weeks in late) would impact on recruitment as there would be lack of equipoise and concern regarding safety of early stoma closure’.
Strongly expressed views from healthcare professionals and parents were that a RCT comparing early and late stoma closure: (1) was warranted, (2) was an important research question to answer and (3) may improve outcomes for other infants in the future. Two quotes from parents highlight this: ‘if it helps any baby in the future’ and ‘if it helps the NHS and it helps other mothers and dads that go through what we went through’.
Define ‘early’ and ‘late’ stoma closure for a potential trial
Defining ‘early’ and ‘late’ stoma closure as intervention and comparator for a potential trial was a key objective of the ToSCiN study. This was challenging due to: (1) a wide variety of viewpoints among professionals and parents, (2) these time points being dependent on the populations of infants included in the trial and (3) the challenge of achieving a trial design that was acceptable to all stakeholders to ensure good recruitment and minimise crossover, while retaining a meaningful difference or ‘clear water’ between the trial arms. Acceptable comparator arms for a future trial were 6 weeks after stoma formation (but infants must have reached 32 corrected gestational weeks) and expectant management guided by the clinical team – and avoiding the term ‘late’ stoma closure.
Initial findings from the survey (Workstream 1) indicated that the time points favoured most frequently by professionals for comparison were 6 weeks as the ‘early’ intervention and 12 weeks for the ‘late’ comparator. These were therefore used to guide data collection in Workstream 2.1/2.2 and to frame discussions in the qualitative work in Workstream 2.3. During this qualitative work, it became apparent that the terms ‘early’ and ‘late’ (originally provided in the HTA commissioning brief and hence used thereafter) were not acceptable to professionals or parents. Individuals consistently expressed they felt the term ‘late’ in particular had negative implications and indicated that the later time point was inferior. We therefore explored using different terminology at the consensus meeting and among the CIG: alternative names for the comparator suggested included ‘later’, ‘standard management’ and ‘expectant management’. Another alternative would be to use neutral terminology such as ‘time point one’ or ‘closure at 6 weeks’.
When the time points of 6 and 12 weeks were discussed at the focus groups, parent interviews and consensus meeting, there were several concerns raised. These included: (1) a lack of flexibility, that is, the time points were too ‘rigid’, (2) the late comparator may be too late for many infants that otherwise would have had their stoma closed earlier if they had not been in a trial (including the need to stay in hospital and use other resources because of this), (3) some extremely premature infants would not be suitable for closure at 6 weeks and hence allowances may have to be made for gestation and/or birthweight and (4) parents preferred to know their surgeon had decided what was best for their individual infant. Among these listed concerns, the timing of the late intervention seemed to pose the greatest difficulty: parents questioned whether prolonging a stoma to meet trial requirements rather than the infant’s needs was ethical and expressed concern that this could lead to a situation whereby the ‘discomfort for the baby and parents could have been reduced weeks before’ if they had been outside the trial. These concerns were discussed in depth at the consensus meeting and alternative trial designs proposed by members of the CIG and attendees of the meeting. It was recognised that all potential trial designs had pros and cons and attempts to mitigate some of the concerns listed above could pose further problems. If, for example, there was less ‘rigidity’ around the timing of the closure stipulated (by using either a range of times for each arm or one of the arms being ‘standard practice’), this would likely result in less of a difference between trial arms. This could then result in an absence of ‘clear water’ between the groups being compared and hence a lack of clinically significant difference. Similarly, if stratification of the intervention was done based on gestation or birthweight (i.e. different intervention for more premature infants), this would pose challenges for statistical analysis and result in larger sample sizes.
The proposed trial designs were put forward for voting at the consensus meeting and the majority (58%) of attendees favoured a comparison whereby the intervention (the ‘early’ time point) was closure at 6 weeks after formation and the comparator (the ‘late’ time point) was expectant management (closure when the infant’s doctors choose for it to be done). Discussions around this option acknowledged it would provide the flexibility in the comparator to reassure parents and professionals that infants were not waiting too long for closure. It does not, however, address the problem of including infants who are extremely premature and may often be deemed to be unsuitable for closure at 6 weeks. Furthermore, the expectant management arm could result in a number of infants’ stomas being closed shortly after 6 weeks and hence the ‘lack of clear water’ described above. Further solutions to these issues that were proposed at the consensus meeting included basing the timing of interventions on corrected gestational age rather than stoma duration and specifying a minimum period of ‘clear water’. With the above issues in mind, the CIG also discussed the possibility of a trial comparing closure at 6 weeks post formation (but infants must have reached 32 weeks' gestation) with expectant management, could also specify a caveat for the comparator to ensure ‘clear water’, for example, for stoma closure no sooner than 8 weeks after formation.
Define a population of neonates for inclusion in a trial (in whom there is significant uncertainty over timing) and determine how many infants are eligible for inclusion.
The population for inclusion in a trial was explored in the survey (Workstream 1), focus groups and interviews (Workstream 2.3) and consensus meeting. There were felt to be advantages and disadvantages of both broader and narrower populations to be included in the trial, that is, having more or less restrictive inclusion criteria. Too restrictive a trial population risks poor external validity and findings that will not be generalisable to the larger, real-world group of newborn infants who require a temporary stoma39 and will then miss an important opportunity to answer the question of when the best time is to close stomas in this larger group. Furthermore, restrictive inclusion criteria may lead to problems with recruiting enough infants to reach the sample size required. Conversely, including a wider range of infants could result in the trial population’s background characteristics being too varied and risk heterogeneity of treatment effect, that is, factors beyond the intervention itself (e.g. gestation or medical comorbidities) modifying the measured effect. If heterogeneity of treatment effect was significant in a trial of stoma closure timing, there is a risk of poor internal validity and any measured treatment effect being biased by systematic error.
From the conception of the ToSCiN study, we planned to exclude all infants with a planned treatment pathway including a stoma: two principal groups of neonates, those with Hirschsprung’s disease and those with anorectal malformations. This is because in these groups, the timing of the stoma closure depends on when they have definitive treatment (surgery) for their underlying pathology and therefore is not subject to the same clinical equipoise.
In relation to a trial population, after excluding infants with Hirschsprung’s disease and anorectal malformations, the findings of the survey, focus groups, interviews and consensus meeting were consistent: while concerns were expressed about including certain groups of infants (namely extremely premature infants), the majority of stakeholders favoured including all neonates with stomas. When voting took place at the consensus meeting, 83% of respondents felt that all infants should be included. This echoed findings of the survey (Workstream 1) where 120/166 (72%) of respondents felt that term infants should be included and 149/166 (90%) felt that preterms with NEC should be included.
The number of eligible neonates in these potential trial populations were assessed using the three national data sets. While direct comparison of these data sets is complicated by differences in coverage (NNRD covers neonatal unit admissions in England and Wales, HES-APC covers all NHS admissions in England and the BAPS-CASS cohort included infants with a decision for surgery from UK and Ireland), a reasonably consistent picture can be put together. The number of potentially eligible infants with a stoma for NEC/SIP was 150–200/year: with 163 in 1 year in the BAPS-CASS cohort and mean annual volume of 193 (NNRD) and 192 (HES-APC). The smaller number of infants in BAPS-CASS is somewhat surprising, given this should have the largest population coverage. It seems likely, however, that one reason for this may be the strict inclusion criteria for this study, compared with the other two data sets where routine data collection based on coding is employed. The definitions used to define the SIP group, in particular, are likely to result in other causes of intestinal perforation being included in this group for NNRD and HES-APC (highlighting the limitations of coding in large routine data sets). A further point to consider is the number of deaths prior to stoma closure in this high-risk group of infants: in the NNRD cohort, 197/1543 (13%) died prior to stoma closure. These data will be helpful for future sample size calculations.
Determining the number of potentially eligible infants with pathologies other than NEC/SIP is more complex owing to the large numbers and variable coding of congenital and acquired conditions that infants may require a stoma. There was a mean of 118 infants per year in the HES-APC data set with a stoma that did not have NEC/SIP or a stoma with a planned treatment pathway (anorectal malformation and Hirschsprung’s disease). The number of such infants identified annually through the NNRD was considerably lower at 36. We believe this is because the NNRD only holds data on infants who are receiving care in a neonatal unit and therefore will not capture those who undergo surgery outside of a neonatal unit, for example, infants discharged home after birth (usually born closer to term), who will routinely be admitted to specialist surgical units at children’s hospitals (rather than neonatal units) with problems that require stoma formation. For these reasons, we feel that the number of infants with stomas formed for reasons other than NEC/SIP is more accurately represented by the HES-APC data. It is reassuring that this ratio of approximately 2 : 1 infants with stomas for NEC/SIP to other infants with stomas was consistent with the ratio we observed in the cohort study (Workstream 2.1/2.2) where of 56 infants recruited 37 had NEC/SIP and 19 had other pathologies leading to stoma formation.
In conclusion, there would be approximately 300 UK infants with stomas formed for NEC/SIP or other pathologies (excluding Hirschsprung’s or anorectal malformations) eligible for inclusion in a trial per annum. The exact number would depend on what inclusion criteria were used and whether the whole of the UK was included. The number of infants available for inclusion at individual centres will vary considerably (largely dependent on the size of the unique population served by the centre) and this will need to be considered when choosing which centres to include in a trial. Larger centres with a higher neonatal surgery activity will allow more efficient trial recruitment: information on activity in order to guide centre selection is available via HES-APC data for England. 40
Establish the most appropriate design and outcome measures for a trial
When considering trial design, we used the PICO (population, intervention, comparator and outcome) format to provide a structure to this design. In the above paragraphs, we have discussed the population for a trial and the definition of what ‘early’ and ‘late’ stoma closure should be (the intervention and comparator for a trial). In this section, we will therefore focus on possible trial outcomes. Outcomes are what should be measured in a research study (trial) to find out whether a treatment is effective; in this case, whether ‘early’ or ‘late’ stoma closure is better. Outcomes need to be important to patients (and their families), healthcare professionals, researchers, healthcare providers and policy-makers. In this study, we sought to determine which outcomes were most important by surveying healthcare professionals looking after infants with stomas (Workstream 1 and focus groups) and by discussing them with parents/families (Workstream 2.3). We also used the consensus meeting to discuss the findings from the above discussions and reach a final consensus of which outcomes were most important. Finally, we involved research methodology experts in the CIG and SSC to discuss the practicalities of using these outcomes in a trial. We were able to achieve consensus from relevant stakeholder groups (parents, healthcare professionals and methodologists) on appropriate primary and secondary outcomes for a trial examining timing of stoma closure.
The top five outcomes from the clinician survey and parent interviews are summarised in Table 25: for the practitioner focus groups, seven are listed as these were unranked and not weighted.
| Clinician survey (Workstream 1) | Practitioner focus groups (Workstream 2.3)a | Parent interviews (Workstream 2.3) |
|---|---|---|
| Growth | Growth | Functioning bowel |
| Time to full feeds | Time to full feeds | Feeding and nutrition |
| LOS | Neurodevelopment at 2 years | Weight gain (growth) |
| Surgical complications | Number of surgical complications | Child quality of life |
| Duration of PN | Parent quality of life | No further surgical procedures/complications |
| Infection | ||
| Survival |
Interestingly, most of the clinician outcomes (perhaps with the exception of growth) are relatively easy to measure and, thus, if they were a primary outcome, could be used in sample size (power) calculations. While growth is vitally important for infants, measuring ‘good-quality’ growth can be challenging; simple increases in weight, for example, can represent oedema and increases in body water rather than true growth and there is considerable debate about what represents an optimal trajectory of growth for preterm infants. 41 While there are more complex methods to measure compositional growth in infants more accurately, these are unlikely to be practical on a day-to-day basis or be considered important by parents and we believe that while growth is an important outcome in a trial such as this, it is unlikely to make a good primary outcome. Perhaps unsurprisingly, there is overlap between the healthcare professional focus groups and the clinician survey, with three of five outcomes being the same. The two added outcomes concerning quality of life and development were more holistic in nature when compared with the five in the clinician survey which tended to be more ‘traditionally’ medical. On the whole, outcomes identified by parents in the interviews tend to be more holistic than those specified from the survey and to represent how the infant ‘functions’ after their surgery. This pattern of differing prioritisation of trial outcomes between different stakeholders (e.g. between patients and professionals) is well reported and highlights the necessity of involving patients/families in trial design and feasibility research such as ToSCiN. 42
The findings from these three data sources were presented to the consensus meeting and discussed at length. A further round of voting was carried out including all meeting attendees. When the primary outcome measure was considered in isolation, two outcomes received the majority of votes (see Appendix 3):
-
weight gain/growth 38%
-
length of hospital stay 32%.
Six further outcomes received far fewer votes (< 9% of delegates).
When delegates were asked to prioritise three further outcomes, the following received a vote from at least 25% of delegates (see Appendix 3):
-
weight gain/growth
-
infant’s quality of life
-
length of hospital stay
-
neurodevelopment
-
time to full feeds
-
surgical complications
-
infant’s bowel function.
This process of asking different stakeholders their opinions on outcomes in the survey, focus groups and parent interviews and then feeding the results back in a consensus meeting ensured that all voices were heard and the range of important outcomes gathered. Once these results were discussed at the consensus meeting and put into further context of a feasible trial, the results of the voting demonstrated that there was relative consistency among stakeholders regarding what should be measured in a trial. There was an understanding that the primary outcomes must be practical in terms of powering the sample size, but that secondary outcomes could be more varied and include things such as quality of life that would require more complex questionnaires, for example. The above list is therefore a good balance of outcomes that are important to all stakeholders and feasible to measure in a trial.
An important further consideration when deciding on the outcomes of a trial is how the trial results will influence clinical practice going forward. All else being equal, the presence of a stoma is largely considered to be undesirable for infants, parents, health professionals and health-resource providers. With this in mind, the task of a trial is to determine whether or not it is better to close these stomas earlier: hence, on a background of demonstrable variation in practice, it is important the trial tackles what factors currently stop professionals closing stomas earlier. We have shown above that the clinical decision-making for neonatal stoma closure is complex and the reasoning for not closing a stoma earlier can be influenced by patient-specific factors, logistical and organisational factors and family factors: the design (and principally the outcomes) of any trial must therefore cover all of these aspects of care.
A further aspect of trial design is the timing of when randomisation takes place. Although we did not formally assess this in the ToSCiN study, the effects of different timings were considered by the investigator group during elements of the study. While delaying randomisation could pose methodological challenges in terms of narrowing the pool of infants eligible for inclusion and introduce an element of selection bias, it may have the advantage of mitigating some of the difficulties in recruiting very sick and premature infants. These infants are often critically unwell postoperatively and face an uncertain future; their parents are likely to be upset and understandably preoccupied by their child’s condition: randomisation to a trial of different stoma closure timings would therefore seem inappropriate if undertaken too early after the initial operation. Our qualitative study demonstrated that there was a very clear message that parents (and staff) felt strongly that trial enrolment and randomisation should be delayed for one or 2 weeks after stoma formation and done at a time of relative stability for the infant, when the conversation had moved from survival of the infant to planning the infant’s recovery.
Determine the willingness of parents, neonatal surgeons and neonatologists to include neonates in a trial that would randomise to ‘early’ or ‘late’ stoma closure and identify potential barriers to recruitment.
The willingness of stakeholders to include neonates in a trial was assessed in the different workstreams of ToSCiN. After discussions around trial design, parents were specifically asked about the overall acceptability of a trial during interviews (Workstream 2.3); they also contributed to the discussions at the consensus meeting. It is important to note that discussions during parent interviews were centred around the initial proposal of 6 versus 12 weeks as trial arms (as discussed earlier). Although these rigid time points were considered less than optimal by stakeholders in later qualitative work, interestingly, there was strong support for a trial using these from a large proportion of parents. A key factor in this acceptability was the fact that parents could be told the two trial arms were currently standard practice in parts of the UK and hence the two possible treatments could be seen as being safe and valid. Unsurprisingly, it was clear that parents placed a high degree of importance on the trial not being ‘too experimental’ for their infant. There was also evidence that parents understood both trial arms could have pros and cons, for example, noting that early closure could allow their child to get home sooner but that later closure may allow them to grow first and get stronger prior to surgery. From these initial observations, it seemed as though parents understood that both trial arms were valid treatment options and that there was equipoise among the cohort of parents involved.
It was also clear that parents required reassurance of the certainty that their infant’s clinical need would supersede any clinical trial, for example, if the stoma needed to be closed outside of the randomised arm. The acceptability of a trial therefore seemed to be dependent on the infant being able to cross over trial arms if their clinician felt strongly that this was in their best interest. This theme – treatment being dictated by the clinical condition of the infant and the decisions of their doctors, rather than the trial allocation – was common to many of the conversations with parents, being described by one parent, for example, as an ‘absolute priority’. In summary, while parents almost universally expressed support and a willingness to participate in a trial, there were some caveats to this; contradictory findings around parental equipoise highlight the need to ensure clear explanation of treatment arms being standard practice during recruitment to any future trial. Furthermore, many of the above concerns are likely to be tackled by a revised trial design with less rigid time points.
The willingness of surgeons and neonatologists to randomise to a trial was assessed directly for a series of neonates with a newly formed stoma currently under their care (Workstream 2.2). The question about willingness to randomise was asked around 1 week post stoma formation to the primary consultant neonatologist and surgeon caring for each infant recruited. While this was a hypothetical question given that no trial was in operation, it was framed around a ‘real-life’ case and hence the professional had detailed insight into the nuances of each case. Overall, 31/56 (59%) of infants were deemed suitable by neonatal surgeons for randomisation in a trial of 6- versus 12-week stoma closure (see Table 8). This figure was higher for neonatologists at 37/56 (74%) (see Table 9). When individual cases were reviewed, agreement between professionals that an infant was suitable for randomisation was reached in 23/56 (48%) of cases (see Table 11). Given that neonatologists were more willing to randomise (37 vs. 31 infants), it would be interesting to consider if surgeons would be more willing if they were aware that their neonatologist was in favour of including an infant in a trial. This issue could be considered and addressed in the education phase of a future trial.
Of the 31 infants deemed suitable for randomisation by surgeons, a closure of the stoma at 6 weeks according to a protocol was deemed appropriate in only 13 (see Figure 13). Interestingly, a high proportion of those infants felt to be unsuitable for closure at 6 weeks by surgeons were extremely premature [16/25 (64%) < 28 weeks at birth, see Table 10]. Modification of the intervention to include a caveat of the infant having to have reached at least 32 weeks' gestation, therefore, seems likely to reduce the number of infants ‘lost’ from a trial due to issues around the early timing of the intervention. Reviewing HES-APC data (see Table 23) allows approximation of how many infants will be included in this caveat: while approximately 25% of infants with stomas are born before 26 weeks, not all of this group will be < 26 weeks at the time of stoma formation and hence the proportion will be smaller (hence < 12.5% assuming 1 : 1 randomisation ‘early’ vs. ‘late’ closure). Although only 8 of the 13 deemed appropriate for closure at 6 weeks were then deemed appropriate for closure at 12 weeks, there was evidence of infants initially deemed ineligible at 6 weeks becoming eligible at 12 weeks (20 of the 31 deemed suitable for randomisation) (see Figure 13). It therefore seems that if acceptability of the early intervention can be improved, there are likely to be a far larger number of infants that would follow the protocol of a trial and fulfil either treatment allocation. Our proposal for the design of a trial (provided later) takes account of these factors.
Further insight into the willingness of professionals to randomise infants to a trial was gained in the focus groups of Workstream 2.3. The findings here were somewhat similar to those from the interviews with parents: there was again broad support from staff as a group for a trial (24/31, 77% answered that a trial would be practically possible) but also concerns were expressed. Again, concerns centred around specific clinical scenarios when staff members would strongly favour one trial arm over the other and no longer have personal equipoise. These scenarios followed the previously described pattern of (1) infant-specific factors, for example, not randomising extremely premature infants, (2) stoma-specific factors, for example, very proximal, high output stomas needing closing early, (3) organisational factors, for example, prolonged hospital stay in the later arm resulting impacting resources and (4) family factors, for example, the impact of longer hospital stay on families and quality of life.
In summary, the evidence around willingness of parents and professionals to randomise infants to a trial of early versus late stoma closure is conflicting: while there is broad support and signs of equipoise (discussed earlier), there are common areas of concern about a trial that are raised by both groups. We believe these concerns can be overcome by modifying trial design from that originally put forward to parents and professionals, by (1) allowing the most premature infants to have reached 32 weeks and (2) providing flexibility for the late comparator.
Assess the suitability of using routinely collected data for gathering clinical information for a trial
The use of routinely collected data for collecting data in a trial was assessed indirectly by reviewing the reliability of data captured in the electronic patient record widely used in UK neonatal units (Badger EPR and Badger Summary Care systems, Clevermed Ltd., Edinburgh, UK) as part of clinical care, and held in the NNRD. This was done by reviewing how reliably the data field for ‘presence of stoma’ was completed in the neonatal record that then populates the NNRD database. We found a high level of inconsistency here, with this data point being completed on some days but not others. Evidence for this inconsistency is provided by 878/4368 (20%) infants having had this data field completed only once and hence having an apparent stoma duration of ≤ 1 day. Given it seems highly implausible that an infant would truly have a stoma for ≤ 1 day, it appears that this data field is completed with insufficient precision to be relied upon in a clinical trial. Cross-validation with other data fields within the NNRD allowed for identification of infants with stomas, which was validated by two other data sources (BAPS-CASS and HES-APC) – indicating that such data have potential for use in future research.
A further limitation of using routinely recorded neonatal data is that a high proportion of infants with stomas are cared for on units that do not contribute data to the NNRD, for example, paediatric surgical centres in stand-alone children’s hospitals; this was particularly pronounced for infants that had a stoma for conditions other than NEC/SIP. Current routine data collection for UK neonates therefore seems to lack the accuracy to be used in a trial of early versus late stoma closure as the sole data source.
Interpretation
Interpreting the findings of the ToSCiN study to reach firm conclusions is challenging, owing to the often-conflicting findings and differing perspectives provided by key stakeholders. Overall, we find that a randomised trial of early compared with late stoma closure in neonates is feasible but would require a modified design compared with that laid out originally by the HTA commissioned brief. This is so that any trial adequately addresses the principal challenge we identified: a ‘baby-led’ narrative that comes through very strongly from all voices. Parents and professionals lack equipoise in certain scenarios and believe the optimal treatment course is already known for certain infants in that scenario; hence, the need to retain flexibility of treatment course in the best interest of the infant (rather than adhere to trial protocols). However, this lack of personal equipoise is not exclusive to ToSCiN and similar themes have been overcome in many trials in the past, including in other complex trials of surgical interventions. 43,44 Communicating randomisation and equipoise is known to be challenging; there is evidence that recruiters often have difficulty in presenting treatments equally: effective training of staff at study sites is therefore key and specific recruitment interventions have been shown to be effective in assisting with this. 45 Our findings in Workstream 2.3 give valuable insight into the factors that such training could address for a trial of early versus late stoma closure in neonates.
Other challenges are: (1) concerns about the inclusion of extremely premature infants, (2) concerns about infants waiting too long for stoma closure if randomised to the ‘late’ comparator arm and (3) logistical arrangements for closing a stoma at the time dictated by trial allocation. These challenges are eminently addressable though, by adapting the design of any trial by: (1) incorporating a degree of flexibility into the trial design (e.g. using ‘expectant management’ as the comparator), (2) making allowances for certain groups (e.g. having a higher corrected gestational age limit for timing of stoma closure for extremely premature infants in any trial), (3) ensuring parents and health professionals are aware that both trial arms are currently standard practice and hence valid treatment options and (4) providing resources and training to ensure trial allocations to be followed.
We consistently found that the question ‘when is it best to close stomas in newborn infants?’ is an important one, with strong support from all stakeholders for a trial. The efficient recruitment of families into the ToSCiN study further demonstrates that parents of infants with stomas are keen to participate in such research. The extensive feasibility work undertaken in ToSCiN, the findings of which are summarised earlier in this chapter, allows us to make recommendations below on the design of such a trial to ensure it (1) answers the above question, (2) measures outcomes which are important to all stakeholders and (3) is acceptable to parents and health professionals.
Strengths and limitations
A key strength of our survey (Workstream 1) was its wide coverage, with multidisciplinary responses from all UK neonatal surgical units. As with most surveys of practice, a limitation is that respondents reported what they believe their practice to be, rather than providing data on actual clinical cases. We attempted to mitigate against this through provision of real-world clinical scenarios: accurate observational data about practice were gathered in other areas of the ToSCiN study. Further evidence of ToSCiN gathering perspectives from a wide range of stakeholders can be found in the range of attendees of the focus groups and study consensus meeting. The focus groups took place around the UK and are likely to be a reasonably good representation. They involved professionals from a good range of disciplines, although nurses were relatively under-represented in the survey and focus groups.
The data collection in Workstreams 2.1 and 2.2 had a number of strengths: (1) it mimicked a trial as much as possible by studying real patients in real time (rather than using clinical scenarios and gathering data retrospectively) and (2) it collected data at different time points in order to take changes in perspective (as the infant’s condition changed) into account. Potential limitations of this part of ToSCiN include: (1) it only provided a ‘snapshot’ and hence will not be able to demonstrate any changes in practice and (2) it may have sampled the most research-active and -supporting surgeons, hence genuine equipoise may be lower across the UK. Furthermore, while we aimed to collect clinical data at 6 and 12 weeks post stoma formation, variation in practice and a tendency for infants not to remain in the same unit with a stoma for 12 weeks limited our ability to collect complete data at these time points. We acknowledge that the requirement to exclude non-English-speaking families could lead to some bias: unfortunately, this was necessary due to the practical challenges of specialist qualitative researchers conducting interviews in multiple languages.
A further strength was that ToSCiN had strong PPI from conception right the way through to the consensus meeting and included the input of a PAG in many important areas. Direct parental interviews made up a large proportion of the ToSCiN study and hence parental voices were heard throughout the process.
Key research recommendations
A trial addressing when the best time is to close a stoma in a neonate is feasible and is important to families and health professionals: there is strong support across stakeholder groups to carry out a trial to answer this question.
Although the initial proposal from the HTA of a trial of early versus late stoma closure would not be feasible in a rigid form, for example, closure at 6 versus 12 weeks, other designs would be feasible. These could incorporate the following components:
-
a trial of closure at 6 weeks versus expectant management
-
a trial with timings based on completed gestational age, rather than duration of stoma
-
a trial of closure at 6 weeks (but once 32 weeks post conceptual age has been reached) versus expectant management (± specification for > 8 weeks).
In order to optimise a trial, we recommend the following practical steps:
-
Involve higher volume neonatal surgical centres for efficient recruitment.
-
Ensure trial staff at each centre are highly trained regarding current standard practice and equipoise.
-
Approach parents 1–2 weeks after stoma formation.
-
Provide resources to centres to permit stoma closure as per the trial protocol, for example, for ring-fenced operating theatre time and possibility of extended hospital stay.
We recommend the following PICO as a starting point to inform the design of a future trial:
-
Population: neonates with stomas, excluding those with a stoma as part of a fixed treatment pathway, for example, anorectal malformations and Hirschsprung’s disease.
-
Intervention: stoma closure at 6 weeks for babies born > 26 weeks' gestation; and for those born 22–26 weeks' gestation, stoma closure on reaching 32 weeks post-conceptual age.
-
Comparator: expectant management with stoma closure undertaken when the clinical team determines is best for the infant.
-
Outcomes: weight gain/growth or length of hospital stay should be the primary outcome measure.
Implications for health care/practice
As a feasibility study, this project has no direct implications for health care or practice.
Equality, diversity and inclusion
Participant representation was optimised by ensuring the population included in ToSCiN was sampled from across the UK. This applied to both infants recruited in the cohort study and parents and professionals involved in qualitative aspects of the study. Firstly, the survey in Workstream 1 was distributed to professionals in all neonatal surgical centres across the UK using existing, inclusive networks. Secondly, the recruiting centres for the cohort study, parental interviews and focus groups were distributed across England and Scotland: these specialist centres serve populations that are diverse in their geography, for example, urban versus rural, ethnicity and socioeconomic status. Finally, parents were also recruited via social media: this allowed further diversity outside of the eight recruiting centres.
The ToSCiN research team was brought together from professional organisations across the UK and was diverse in terms of: (1) professional background and expertise; (2) geographical base; and (3) ethnicity. The study also had high levels of PPI as discussed in Chapter 3.
Finally, the ToSCiN study included groups that have previously been reported to be under-represented in research, including: (1) age extremes (neonates), (2) those with rare diseases (congenital malformations), (3) those unable to consent for themselves (children); and (4) those with severe illness (neonates in intensive care).
Additional information
Contributions of authors
Nick Lansdale (https://orcid.org/0000-0002-0054-9250) (Consultant Paediatric Surgeon) as Chief Investigator, conceived and designed the study, contributed to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the final report.
Kerry Woolfall (https://orcid.org/0000-0002-5726-5304) (Reader in Health Research Methodology) designed and led Workstream 2.3, contributed to the acquisition, analysis and interpretation of the Workstream 2.3 data and drafted and critically reviewed the final report.
Elizabeth Deja (https://orcid.org/0000-0002-3626-4927) (Research Associate, Psychology) Workstream 2.3 Research Associate, carried out data collection, analysis and write-up of Workstream 2.3 sections of the report, was accountable for the accuracy and integrity of Workstream 2.3 aspects of the work, and performed a critical review of the final full report.
Tracy Mitchell (https://orcid.org/0000-0003-0014-8016) (Research Associate, Methodologist) Workstream 2.3 Research Associate, carried out data collection, analysis and write-up of Workstream 2.3 sections of the report, was accountable for the accuracy and integrity of Workstream 2.3 aspects of the work, and performed a critical review of the final full report.
Graciaa Singhal (https://orcid.org/0000-0001-6729-5622) (Medical Student) contributed to the design, data acquisition, analysis and interpretation of national data held from the National Neonatal Research Database; drafted the section of the final report relating to Workstream 3.
Raphael Goldacre (https://orcid.org/0000-0002-7393-1880) (Epidemiologist) contributed to the design, data acquisition, analysis, interpretation and write-up of the national Hospital Episode Statistics (HES) aspect of Workstream 3.
Rema Ramakrishnan (https://orcid.org/0000-0002-6784-8319) (Senior Statistician) carried out analysis and write-up of BAPS-CASS data for Workstream 3.
Nigel Hall (https://orcid.org/0000-0001-8570-9374) (Consultant Paediatric and Neonatal Surgeon, Associate Professor of Paediatric Surgery) contributed to study design, data acquisition, analysis and interpretation of the data, drafted and critically reviewed the final report and convened and chaired the Parental Advisory Group.
Cheryl Battersby (https://orcid.org/0000-0002-2898-553X) (Clinical Senior Lecturer in Neonatal Medicine) contributed to study design, data acquisition, analysis and interpretation of national data held from the National Neonatal Research Database; drafted the section of the final report relating to Workstream 3.
Chris Gale (https://orcid.org/0000-0003-0707-876X) (Professor of Neonatal Medicine) contributed to conception and design, data acquisition, analysis and interpretation of national data held from the National Neonatal Research Database; drafted and critically reviewed the final report.
Gareth Penman (https://orcid.org/0000-0001-8792-1248) (Consultant Neonatologist) contributed to study design, data acquisition, analysis and interpretation of the data, drafted and critically reviewed the final report.
Marian Knight (https://orcid.org/0000-0002-1984-4575) (Professor of Maternal and Child Population Health) contributed to the design of the study, acquisition, analysis and interpretation of the data and drafted and critically reviewed the final report.
Kayleigh Stanbury (https://orcid.org/0000-0002-8726-2411) (NPEU CTU Head of Operations, Trial Management) contributed to study design, study conduct and oversight, an interpretation of the data and critically reviewed the final report.
Madeleine Hurd (https://orcid.org/0000-0002-2797-2358) (ToSCiN Study Co-ordinator and NPEU CTU Data Manager, Trial Management) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the final report.
David Murray (https://orcid.org/0000-0001-9010-2905) (NPEU CTU Senior Trials Programmer) carried out programming/data management and case report form development for the study.
Louise Linsell (https://orcid.org/0000-0003-3205-6511) (NPEU CTU Senior Statistician) contributed to the design of the study, analysis and interpretation of the data and critically reviewed the final report.
Pollyanna Hardy (https://orcid.org/0000-0003-2937-8368) (NPEU CTU Director and Statistician) contributed to the data acquisition, undertook the data analysis and interpretation of data for Workstreams 2.1 and 2.2 and critically reviewed the final report.
Acknowledgements
We wish to thank the NIHR Health Technology Assessment programme for funding this trial. We also wish to thank all the patients and staff from the sites that participated in the trial.
Research staff at participating sites
We acknowledge that there have been many other individuals who made a contribution within the participating sites. It is impossible to thank everyone personally; however, we would like to thank the following research staff:
Alder Hey Children’s Hospital – Miss Harriet Corbett, Mr Varun Hathiramani, Ms Rachael Dore, Ms Dawn Jones, Ms Shelley Mayor and Ms Laura O’Malley.
Birmingham Children’s Hospital – Mr Ingo Jester, Ms Tracey Hill, Ms Louise Lawrence, Ms Monica Mitchell and Ms Tara Roome.
Bristol Royal Hospital for Children – Ms Clare Skerritt, Miss Catherine Bradshaw and Ms Kate Burns.
Chelsea and Westminster Hospital – Mr Simon Clarke, Mr Alexander Macdonald, Mrs Izabela Andrzejewska and Prof Chris Gale.
Evelina London Children’s Hospital – Mr Iain Yardley, Ms Verity Haffenden, Ms Helen Broomfield, Ms Charlotte Holbrook, Ms Kate Khoo, Mr Hemanshoo Thakkar and Ms Natalie Vallant.
Royal Hospital for Sick Children, Glasgow – Mr Timothy Bradnock, Ms Aisha Tahira, Mr David Colvin, Ms Margaret Reeves and Dr Judith Simpson.
Liverpool Women’s Hospital (continuing care site) – Dr Richard Hutchinson.
The Manchester Centre for Neonatal Surgery (Royal Manchester Children’s Hospital and Saint Mary’s Hospital) – Dr Gareth Penman, Ms Nicola Booth, Ms Karen Dockery, Ms Clare Jennings, Dr Ajit Mahaveer, Mr Aiden Moore and Ms Mirsada Paskali.
Southampton General Hospital – Mr Nigel Hall, Ms Clara Chong, Mrs Sharon Cave, Ms Philippa Crowley, Ms Laura Herbert, Ms Hannah Wells and Dr Aneurin Young.
Project Management Group
Ms Christina Cole (NPEU CTU Senior Trials Manager), Dr Elizabeth Deja, Ms Pollyanna Hardy, Ms Madeleine Hurd, Mr Nick Lansdale, Assoc. Prof Louise Linsell, Dr Tracy K Mitchell, Mr David Murray, Assoc. Prof Charles Roehr (NPEU CTU Clinical Director and Honorary Professor of Neonatology and Perinatal Research), Ms Kayleigh Stanbury, Dr Kerry Woolfall.
Co-investigators Group
Dr Cheryl Battersby, Ms Christina Cole, Dr Elizabeth Deja, Prof Chris Gale, Mr Raphael Goldacre, Mr Nigel Hall, Ms Pollyanna Hardy, Ms Madeleine Hurd, Prof Marian Knight, Mr Nick Lansdale, Assoc. Prof Louise Linsell (until September 2021), Dr Tracy K Mitchell, Mr David Murray, Dr Gareth Penman, Assoc. Prof Charles Roehr, Ms Kayleigh Stanbury, Dr Kerry Woolfall.
Study Steering Committee
Dr Vincent Kirkbride (independent, Chairperson), Mr David Drake (independent, Vice-Chair), Mr Frank Shipley (independent, PPI representative), Mr Mike Bradburn (independent), Ms Saleema Rex (independent), Mr Nick Lansdale (non-independent), Ms Pollyanna Hardy (observer), Ms Kayleigh Stanbury (observer), Dr Kerry Woolfall (observer).
Other contributors
Ms Lucy Flanagan (Paediatric Nurse, Co-applicant and PPI representative).
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Ethics statement
An application was submitted to the HRA on 23 October 2020 and received full approval on 8 February 2021 (Integrated Research Application System reference number 278331, Research Ethics Committee reference number 20/LO/1227).
Information governance statement
Manchester University NHS Foundation Trust is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, Manchester University NHS Foundation Trust is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://research.cmft.nhs.uk/getting-involved/gdpr-and-research.
For Workstream 3 of ToSCiN, under the Data Protection legislation, Manchester University NHS Foundation Trust is the Data Processor; Imperial College London is the Data Controller and we process personal data in accordance with their instructions. You can find out more about how they handle personal data, including how to exercise your individual rights and the contact details for Imperial College London’s Data Protection Officer at www.imperial.ac.uk/neonatal-data-analysis-unit/neonatal-data-analysis-unit/information-for-patients-and-carers and www.imperial.ac.uk/admin-services/secretariat/information-governance/data-protection/contact-us.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/JFBC1893.
Primary conflicts of interest: Cheryl Battersby is supported by a NIHR Advanced Fellowship and is Deputy Chair of the NIHR Prioritisation committee. Marian Knight is Programme Director for the NIHR Research for Patient Benefit Programme. Pollyanna Hardy is a committee member on the NIHR HTA Commissioning Board. Chris Gale is chair of the NIHR Research for Patient Benefit London Regional Advisory Committee.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Zani A, Lauriti G, Li Q, Pierro A. The timing of stoma closure in infants with necrotizing enterocolitis: a systematic review and meta-analysis. Eur J Pediatr Surg 2017;27:7-11.
- Struijs MC, Poley MJ, Meeussen CJHM, Madern GC, Tibboel D, Keijzer R. Late vs early ostomy closure for necrotizing enterocolitis: analysis of adhesion formation, resource consumption, and costs. J Pediatr Surg 2012;47:658-64.
- Bethell G, Kenny S, Corbett H. Enterostomy-related complications and growth following reversal in infants. Arch Dis Child Fetal Neonatal Ed 2017;102:F230-4.
- Al-Hudhaif J, Phillips S, Gholum S, Puligandla PP, Flageole H. The timing of enterostomy reversal after necrotizing enterocolitis. J Pediatr Surg 2009;44:924-7.
- Veenstra M, Nagappala K, Danielson L, Klein M. Timing of ostomy reversal in neonates with necrotizing enterocolitis. Eur J Pediatr Surg 2015;25:231-5.
- Yang HB, Han JW, Youn JK, Oh C, Kim HY, Jung SE. The optimal timing of enterostomy closure in extremely low birth weight patients for acute abdomen. Sci Rep 2018;8.
- Harris L, Smee N, Bradnock T, Simpson J, Granger C. G217(P) The burden of stoma-related complications in preterm infants. Arch Dis Child 2018;103:A89-A.
- Chong C, van Druten J, Briars G, Eaton S, Clarke P, Tsang T, et al. Neonates living with enterostomy following necrotising enterocolitis are at high risk of becoming severely underweight. Eur J Pediatr 2019;178:1875-81.
- Kenny S. Paediatric General Surgery and Urology: GIRFT Programme National Specialty Report 2021. https://gettingitrightfirsttime.co.uk/surgical_specialties/paediatric-general-surgery-and-urology/ (accessed December 2022).
- The National Perinatal Epidemiology Unit, University of Oxford n.d. www.npeu.ox.ac.uk/about (accessed December 2022).
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2.
- Woolfall K, Frith L, Gamble C, Gilbert R, Mok Q, Young B. CONNECT advisory group . How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 2015;5.
- O’Hara CB, Canter RR, Mouncey PR, Carter A, Jones N, Nadel S, et al. A qualitative feasibility study to inform a randomised controlled trial of fluid bolus therapy in septic shock. Arch Dis Child 2018;103:28-32.
- Woolfall K, Frith L, Gamble C, Young B. How experience makes a difference: practitioners’ views on the use of deferred consent in paediatric and neonatal emergency care trials. BMC Med Ethics 2013;14.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907.
- Baker SE, Edwards R. National Centre for Research Methods (NCRM). How Many Qualitative Interviews Is Enough? 2012. https://eprints.ncrm.ac.uk/id/eprint/2273/ (accessed February 2024).
- Peters MJ, Khan I, Woolfall K, Deja E, Mouncey PR, Wulff J, et al. Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT. Health Technol Assess 2019;23:1-148.
- Inwald D, Canter RR, Woolfall K, O’Hara CB, Mouncey PR, Zenasni Z, et al. Restricted fluid bolus versus current practice in children with septic shock: the FiSh feasibility study and pilot RCT. Health Technol Assess 2018;22:1-106.
- Tume LN, Woolfall K, Arch B, Roper L, Deja E, Jones AP, et al. Routine gastric residual volume measurement to guide enteral feeding in mechanically ventilated infants and children: the GASTRIC feasibility study. Health Technol Assess 2020;24:1-120.
- Gupta JK, Daniels JP, Middleton LJ, Pattison HM, Prileszky G, Roberts TE, et al. ECLIPSE Collaborative Group . A randomised controlled trial of the clinical effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system in primary care against standard treatment for menorrhagia: the ECLIPSE trial. Health Technol Assess 2015;19:1-118.
- Ducey J, Kennedy AM, Linsell L, Woolfall K, Hall NJ, Gale C, et al. Timing of neonatal stoma closure: a survey of health professional perspectives and current practice. Arch Dis Child Fetal Neonatal Ed 2021;107:448-50.
- Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. London: SAGE Publications Ltd; 1996.
- Silverman D. Interpreting Qualitative Data: Methods for Analysing Talk, Text, and Interaction. London: SAGE Publications Ltd; 2006.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Byrne D. A worked example of Braun and Clarke’s approach to reflexive thematic analysis. Qual Quant 2021;56:1391-412.
- Braun V, Clarke V. What can ‘thematic analysis’ offer health and wellbeing researchers?. Int J Qual Stud Health Well-Being 2014;9.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA; London: SAGE Publications Ltd; 1998.
- Patton MQ. Qualitative Research and Evaluation Methods. London: SAGE Publications Ltd; 2002.
- Strauss AL, Corbin JM. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Thoery. Thousand Oaks: SAGE Publications Ltd; 1998.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45.
- Stiles WB. Evaluating qualitative research. Evid Based Ment Health 1999;2:99-101.
- Battersby C, Statnikov Y, Santhakumaran S, Gray D, Modi N, Costeloe K. UK Neonatal Collaborative and Medicines for Neonates Investigator Group . The United Kingdom National Neonatal Research Database: a validation study. PLOS ONE 2018;13.
- British Association of Paediatric Surgeons Congenital Anomalies Surveillance System (BAPS-CASS) n.d. www.npeu.ox.ac.uk/baps-cass (accessed December 2022).
- Allin B, Long AM, Gupta A, Knight M, Lakhoo K. British Association of Paediatric Surgeons Congenital Anomalies Surveillance System Necrotising Enterocolitis Collaboration . A UK wide cohort study describing management and outcomes for infants with surgical Necrotising Enterocolitis. Sci Rep 2017;7.
- Long AM, Jones IH, Knight M, McNally J. BAPS-CASS . Early management of meconium ileus in infants with cystic fibrosis: a prospective population cohort study. J Pediatr Surg 2021;56:1287-92.
- Woolfall K, O’Hara C, Deja E, Canter R, Khan I, Mouncey P, et al. Parents’ prioritised outcomes for trials investigating treatments for paediatric severe infection: a qualitative synthesis. Arch Dis Child 2019;104:1077-82.
- Tume LN, Arch B, Woolfall K, Roper L, Deja E, Jones AP, et al. Determining optimal outcome measures in a trial investigating no routine gastric residual volume measurement in critically ill children. JPEN J Parenter Enteral Nutr 2020;45:79-86.
- Averitt AJ, Weng C, Ryan P, Perotte A. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med 2020;3.
- Lansdale N, Goldacre R, Wilkinson DJ, Bower P. Balancing quality and equity of access in specialist neonatal surgery: implications of the GIRFT report. Br J Surg 2022;109:1017-8.
- Pereira-da-Silva L, Virella D, Fusch C. Nutritional assessment in preterm infants: a practical approach in the NICU. Nutrients 2019;11.
- Treweek S, Miyakoda V, Burke D, Shiely F. Getting it wrong most of the time? Comparing trialists’ choice of primary outcome with what patients and health professionals want. Trials 2022;23.
- Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. ProtecT study group . Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109-18.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17.
Appendix 1
| OPCS-4 code | Description |
|---|---|
| Stoma formation: | |
| G60.1 | Creation of jejunostomy |
| G60.2 | Refashioning of jejunostomy |
| G74.1 | Creation of continent ileostomy |
| G74.2 | Creation of temporary ileostomy |
| G74.3 | Creation of defunctioning ileostomy |
| G75.1 | Refashioning of ileostomy |
| G75.2 | Repair of prolapse of ileostomy |
| G75.4 | Dilation of ileostomy |
| G75.5 | Reduction of prolapse of ileostomy |
| G75.6 | Resiting of ileostomy |
| H04.1 | Panproctocolectomy and ileostomy |
| H05.2 | Total colectomy and ileostomy and creation of rectal fistula HFQ |
| H05.3 | Total colectomy and ileostomy NEC |
| H06.4 | Extended right hemicolectomy and ileostomy HFQ |
| H07.4 | Right hemicolectomy and ileostomy HFQ |
| H08.4 | Transverse colectomy and ileostomy HFQ |
| H09.4 | Left hemicolectomy and ileostomy HFQ |
| H10.4 | Sigmoid colectomy and ileostomy HFQ |
| H11.4 | Colectomy and ileostomy NEC |
| H15.1 | Loop colostomy |
| H15.2 | End colostomy |
| H15.3 | Refashioning of colostomy |
| H15.5 | Dilation of colostomy |
| H15.6 | Reduction of prolapse of colostomy |
| H15.7 | Percutaneous endoscopic sigmoid colostomy |
| H32.1 | Resiting of colostomy |
| H33.1 | Abdominoperineal excision of rectum and end colostomy |
| Y51.3 | Approach to organ through ileostomy |
| Y51.4 | Approach to organ through colostomy |
| Stoma closure: | |
| G60.3 | Closure of jejunostomy |
| G73.3 | Resection of ileostomy |
| G75.3 | Closure of ileostomy |
| H15.4 | Closure of colostomy |
Appendix 2
| To exclude infants with anorectal malformation or Hirschsprung’s (in any diagnosis position): | |
| With: | |
| Q431 | Hirschsprung’s disease OR |
| Q423 | Cong absence atresia and stenosis anus without fistula OR |
| Q422 | Cong absence atresia and stenosis anus with fistula OR |
| Q437 | Persistent cloaca |
| To define a NEC/SIP group for subanalysis using the following ICD-10 codes (in any diagnosis position): | |
| With: | |
| P77 | Necrotising enterocolitis OR |
| P780 | Perinatal intestinal perforation OR |
| K631 | Perforation of intestine (nontraumatic) |
| And without: | |
| Q793 | Gastroschisis OR |
| Q391 | Atresia of oesophagus with tracheo-oesophageal fistula |
| Q439 | Congenital malformation of intestine, unspecified OR |
| P760 | Meconium plug syndrome OR |
| Q438 | Other specified congenital malformations of intestine OR |
| Q411 | Congenital absence, atresia and stenosis of jejunum OR |
| Q412 | Congenital absence, atresia and stenosis of ileum OR |
| E841 | Cystic fibrosis with intestinal manifestations OR |
| P75 | Meconium ileus in cystic fibrosis OR |
| Q433 | Congenital malformations of intestinal fixation |
Appendix 3
Voting and free-text comments from consensus meeting.
Theme 1
| Total votes = 34 | ||
|---|---|---|
| Outcome | Number of votes | % of attendees |
| Baby’s weight gain/growth | 13 | 38.2 |
| How long the baby stays in hospital | 11 | 32.4 |
| Baby’s quality of life | 3 | 8.8 |
| How well the baby’s bowel functions | 2 | 5.9 |
| Neurodevelopment (how well the baby’s brain develops) | 2 | 5.9 |
| Nutrition | 1 | 2.9 |
| How long it takes the baby to get to full feeds | 1 | 2.9 |
| Whether the baby survives | 1 | 2.9 |
| How many days of total PN the baby receives | 0 | 0.0 |
| Whether the baby has surgical complications | 0 | 0.0 |
| Whether the baby requires further surgery | 0 | 0.0 |
| How many days the child has a central line | 0 | 0.0 |
| How many days the baby needs invasive ventilation | 0 | 0.0 |
| Parent’s experience/quality of life | 0 | 0.0 |
| Baby’s skin integrity/healing | 0 | 0.0 |
FIGURE 25.
Results of vote on primary outcome for a trial of stoma closure.
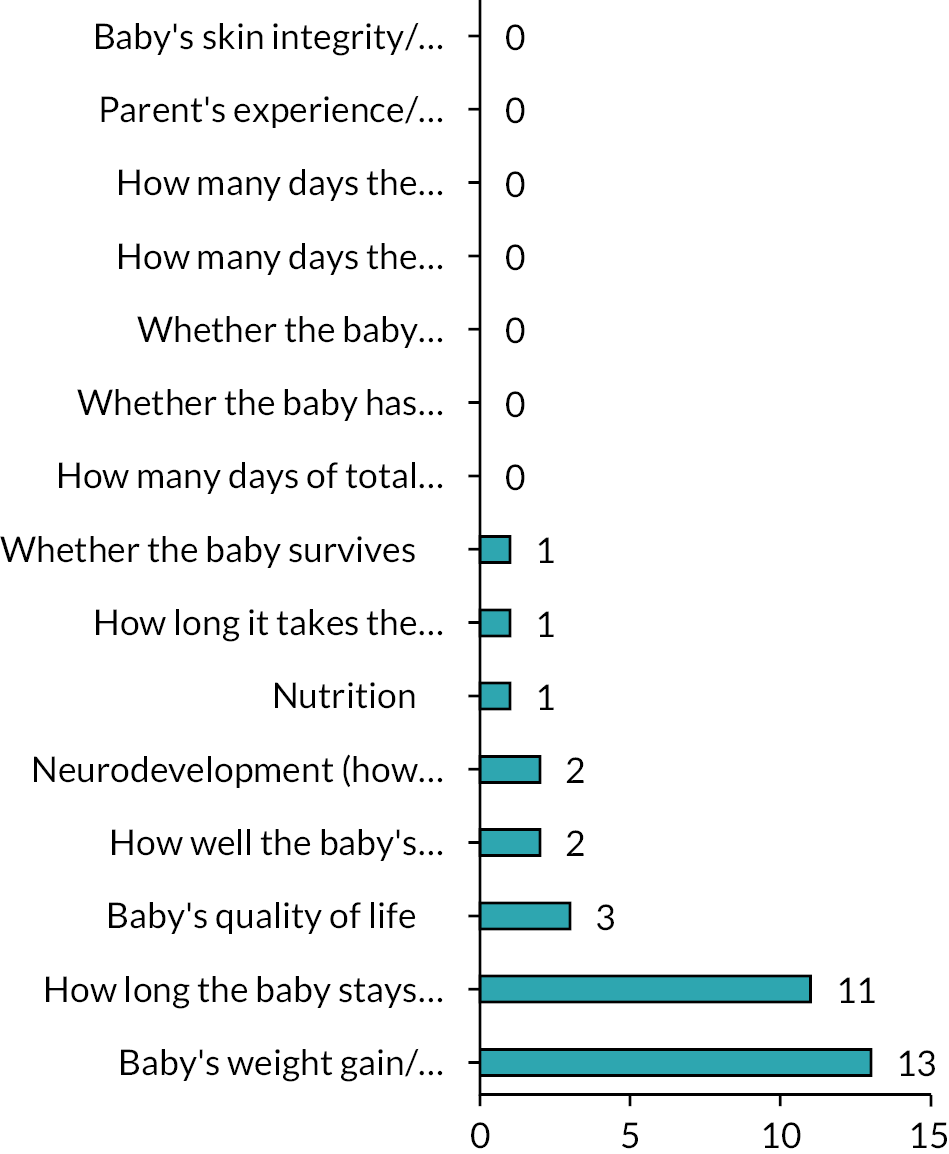
| Total votes = 36 | ||
|---|---|---|
| Outcome | Number of votes | % of attendees |
| Baby’s weight gain/growth | 19 | 52.8 |
| Baby’s quality of life | 17 | 47.2 |
| How long the baby stays in hospital | 14 | 38.9 |
| Neurodevelopment (how well the baby’s brain develops) | 12 | 33.3 |
| How long it takes the baby to get to full feeds | 10 | 27.8 |
| Whether the baby has surgical complications | 10 | 27.8 |
| How well the baby’s bowel functions | 9 | 25.0 |
| Parent’s experience/quality of life | 6 | 16.7 |
| How many days of total PN the baby receives | 5 | 13.9 |
| Whether the baby requires further surgery | 4 | 11.1 |
| How many days the child has a central line | 1 | 2.8 |
| Whether the baby survives | 1 | 2.8 |
| Nutrition | 0 | 0.0 |
| How many days the baby needs invasive ventilation | 0 | 0.0 |
| Baby’s skin integrity/healing | 0 | 0.0 |
FIGURE 26.
Results of vote on three other outcomes to be measured in a trial of stoma closure.
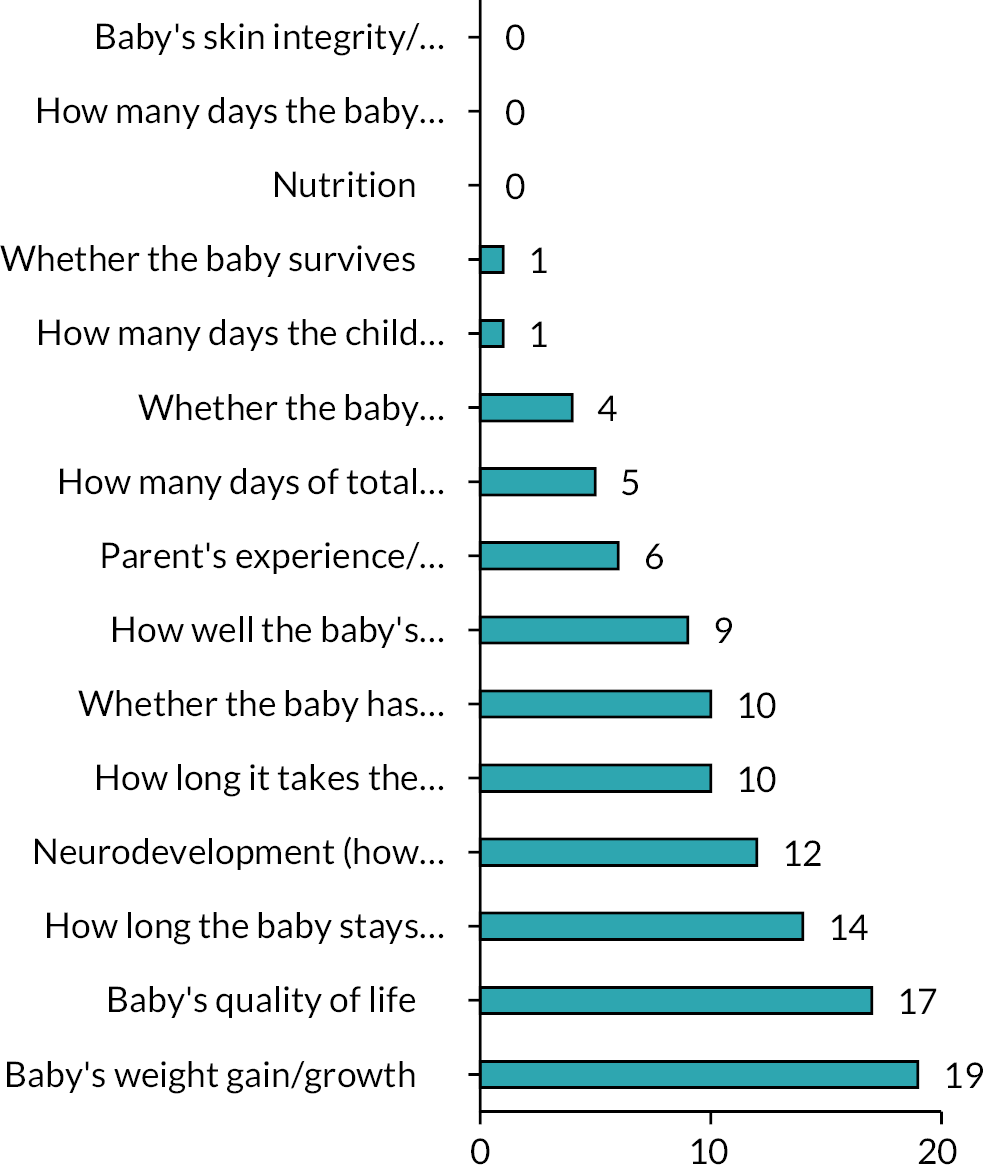
Theme 2
| Total votes = 33 | ||
|---|---|---|
| Design | Number of votes | % of attendees |
| Comparing closure at 6 weeks after the stoma is made with expectant management (the stoma is closed when the baby’s doctors choose) | 19 | 57.6 |
| Comparing 32 vs. 38–40 weeks in extremely premature babies (< 28 weeks) only | 9 | 27.3 |
| Comparing closure at < 7 weeks after the stoma is made with closure more than 12 weeks after the stoma is made | 4 | 12.1 |
| Comparing closure at 6 weeks after the stoma is made with closure at 12 weeks after | 1 | 3.0 |
FIGURE 27.
Results of vote of design for a trial of stoma closure.
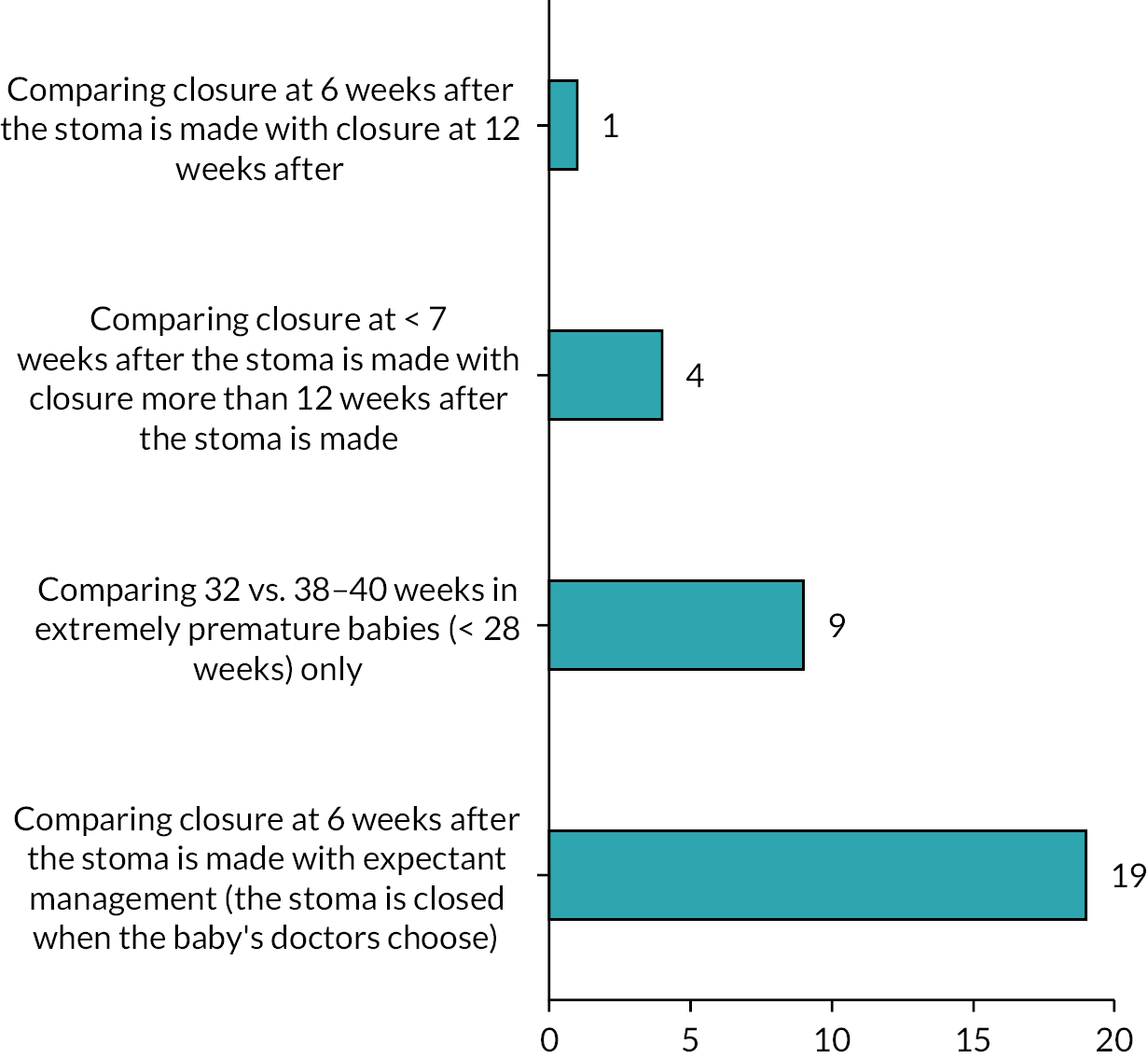
If you have another idea, please type it here!
‘Early before term, late at 8 weeks post term’
‘6 weeks vs. expected management but a secondary one for tiny babies based on gestational age’
‘The 6 weeks “early” timepoint should include a condition to add a minimum gestational age for the most preterm babies. I would choose after 6 weeks or at minimum corrected GA of 32 weeks. The late time point could be either current practice or “term corrected” – e.g. > 36 weeks’
‘Stratified sample’
‘Only enrol > 26 weeks neonates’
‘Subgroup analysis of extreme preterms’
‘Definitely something with corrected gestational age. But still a set time, e.g. 6 weeks post stoma or 34 weeks corrected gestation’
‘Stratification by gestational age’
‘At < 6 weeks and a corrected gestational age of > 32 weeks’
‘Considerations for gestational age important, i.e. very preterm infancy and some clinical parameters’
‘6 weeks or less vs. expectant management (but minimum of 12 weeks)’
‘< 6 weeks (but have to have reached 32 weeks) vs. expectant’
‘< 6 weeks but not before 32 weeks cga at time of closure vs. > 12 weeks’
‘The discussion about a stratified sample seams key to make this feasible and useful’
‘6 weeks or under vs. “expected management”’
‘Apply minimal PMA to early closure’
Theme 3
| Total votes = 29 | ||
|---|---|---|
| Population | Number of votes | % of attendees |
| All babies | 24 | 82.8 |
| Only babies who have a diagnosis of NEC/SIP | 5 | 17.2 |
| Only babies who are diagnosed with something that is not NEC/SIP | 0 | 0.0 |
FIGURE 28.
Results of vote on population for a trial of stoma closure.
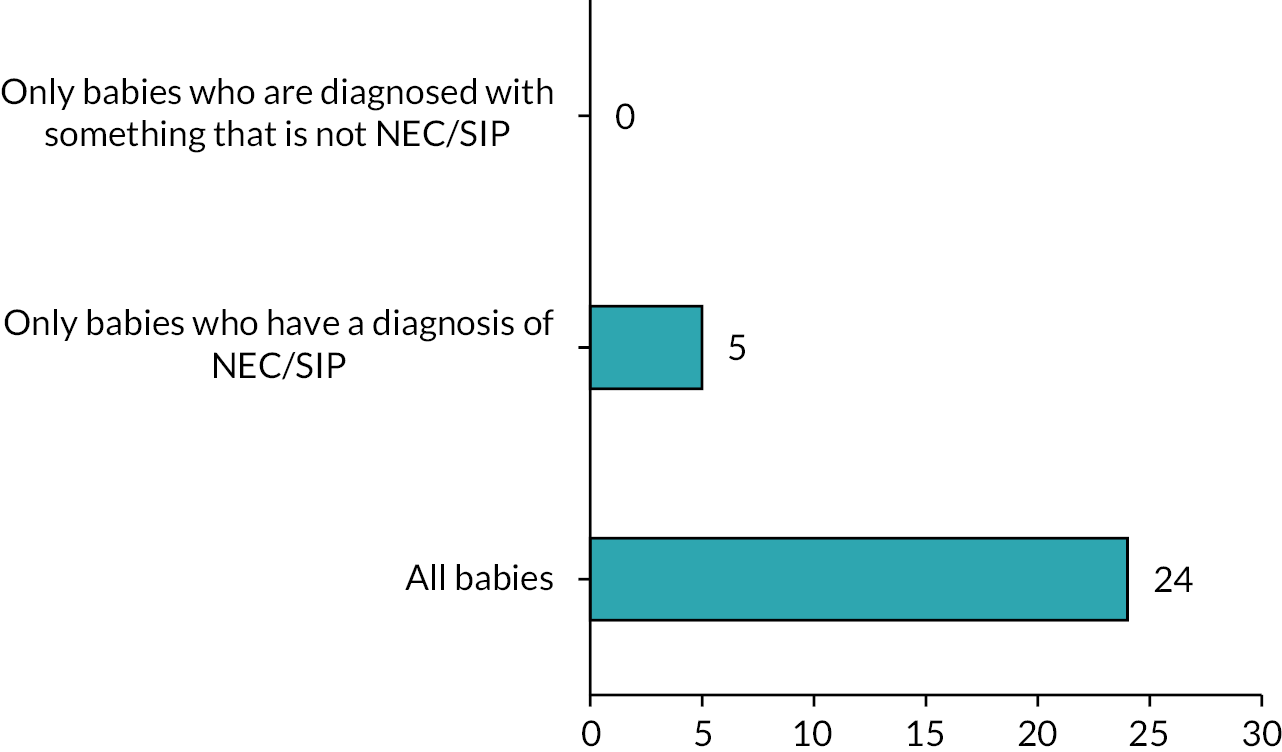
Are there other babies you think we should include, or do you have anything else to add about who to include in a trial?
‘It is important to include all babies so there is research for individuals who are considered as rare and medical staff have the best evidence to treat them’.
‘Babies who not stable cardiovascularly, i.e. still very high risk due to underlying congenital heart disease’
‘The key interest group seems to be preterms so results should be focused here; if term babies skew results’.
‘If including all babies would require stratification of the groups, e.g. NEC babies management and outcomes will be very different to term atresias for example so this would affect results’
‘I think the decision of who to exclude, or even include, will also be dependent on the time point of randomisation. May also need to consider preterm only babies’.
‘Study design would be best if both arms of study have equal numbers of SIP and NEC in both groups and maybe site of stoma, i.e. high/low’
‘Cardiac babies should be included (even though they will be in PICU). Need to stratify so groups comparable, e.g. diagnosis, position of stoma’
‘Perhaps exclude term infants and just have those < 37 weeks’.
‘Stoma location, cause of surgery, babies sent home with stomas, meconium ileus’ ‘Include every baby who might need a stoma’
‘Should have wide inclusion criteria’
| Total votes = 29 | ||
|---|---|---|
| Response | Number of votes | % of attendees |
| Yes: extremely premature babies, e.g. 24 weeks and below | 2 | 6.9 |
| Yes: babies who are extremely unwell at the time of stoma formation | 2 | 6.9 |
| Yes: some other kind of baby | 8 | 27.6 |
| No | 17 | 58.6 |
If you said that there are other types of babies that should be excluded from a trial from the start, please say which types of babies these are – or if you have anything else to add about who we should exclude.
‘From a parent point of view even if their child is very sick, it helps them to get value from a difficult situation by helping other babies. Please consider this when people are recruited’
‘Giving a period of time (e.g. 2 weeks post stoma formation) prior to randomisation would allow some time for things to settle (e.g. need for follow-up op if NEC and stabilisation time post op). This would allow time for clinicians to decide where their equipoise lies for each baby prior, including whether early or late closure is appropriate for them, prior to entering them into the trial, and perhaps reduce risk of crossover’.
‘Babies < 24 weeks are physiologically very different to older babies. Would their trajectory be expected to be the same as other babies?’
‘Term infants not sure how valuable data would be re outcomes for early vs. late closure in this group’
‘No as long as there is a minimal gestation at closure as discussed earlier, e.g. reached 32’
‘Term babies with planned pathways’
‘Babies with complex distal pathologies that need to be addressed (and will therefore affect timing)’
‘Infants with congenital anomalies that have a consistent agreed pathway (e.g. anorectal malformation). Infants where early stoma closure likely to be essential (e.g. high stomas)’
‘I would exclude: cardiac NEC as they will rarely be suitable for early closure, gastroschisis, complex meconium ileus, atresias with significant dilatation (as they may need tapered/more complex subsequent surgery and this may lead to further complications)’
‘Cardiac babies, stoma undertaken for palliative care, term babies with planned pathways’
‘Significant genetic/syndrome abnormalities – would be difficult to relate outcomes like growth or neurodevelopment to stoma closure’
‘I would favour excluding gastroschisis as I think their time to full feeds and LOS will not be to do with presence of a stoma’
‘Possibly extreme high stoma/PN associated complications requiring early closure’
‘Staged surgical treatment (anorectal malformations, HD etc.)’
‘Do not exclude anyone at point of randomisation’
List of abbreviations
- API
- associate principal investigator
- AWS
- Amazon Web Services
- BAPS-CASS
- British Association of Paediatric Surgeons Congenital Anomalies Surveillance System
- CIG
- Co-Investigator Group
- DJ
- duodenojejunal
- GDPR
- General Data Protection Regulation
- HCP
- healthcare professional
- HES-APC
- Hospital Episode Statistics – Admitted Patient Care
- HTA
- Health Technology Assessment
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IQR
- interquartile range
- LOS
- length of stay
- NDAU
- Neonatal Data Analysis Unit
- NEC
- necrotising enterocolitis
- NICU
- neonatal intensive care unit
- NNRD
- National Neonatal Research Database
- NPEU
- National Perinatal Epidemiology Unit
- PAG
- Parental Advisory Group
- PICO
- population, intervention, comparator and outcome
- PIs
- principal investigators
- PIS
- participant information sheet
- PMG
- Project Management Group
- PN
- parenteral nutrition
- PPI
- patient and public involvement
- PPIE
- patient and public involvement and engagement
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- SD
- standard deviation
- SIP
- spontaneous intestinal perforation
- SSC
- Study Steering Committee
- ToSCiN
- Timing of Stoma Closure in Neonates
- TPN
- total parenteral nutrition
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JFBC1893).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
