Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/143/01. The contractual start date was in January 2016. The draft report began editorial review in July 2021 and was accepted for publication in December 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Kai et al. This work was produced by Kai et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Kai et al.
Chapter 1 Introduction
Heavy menstrual bleeding
Heavy menstrual bleeding (HMB) is a common problem that can significantly affect women’s lives. HMB is conventionally defined as menstrual blood loss of more than 80 ml per cycle,1 which equates to significant anaemia. 2 In clinical practice, it is the impact on a woman’s physical, emotional, social and economic quality of life that guides treatment, rather than patient-reported or objective methods of assessing menstrual blood loss.
Recent, relevant data on incidence or prevalence are scarce. A prospective population cohort that was identified through a community survey in 20023 estimated a 12-month cumulative incidence of women reporting HMB of 25% [95% confidence interval (CI) 22% to 29%]. An internet survey that took place in five European countries in 20124 found that 1225 (27%) out of 4506 women who responded reported two or more predefined HMB symptoms. A medical record analysis in the USA5 found that 1.4 million (95% CI 1.3 to 1.5 million) women per year reported abnormal uterine bleeding (International Classification of Diseases, Ninth Edition code 626, which includes HMB) in an average non-pregnant population of 56.2 million women aged 18–50 years. A review of primary care records between 2004 and 2013 in the Netherlands6 found a mean annual incidence of 9.3 per 100 person-years (95% CI 8.5 to 10.2 per 100 person-years).
Despite the high incidence and burden of HMB, many women do not seek medical help. In one European survey,4 46% of women had not sought help for HMB. Multiple influences and perceptions may act as disincentives to accessing appropriate care, including the taboo of menstruation, inability to discuss with friends and family, and poor previous experiences with general practitioners (GPs). 7 A UK hospital organisational audit8 found that one-third of women attending an outpatient gynaecological clinic had not received treatment in primary care prior to their referral, with non-white women and those with HMB alone more likely to have received no prior treatment.
Diagnosis of heavy menstrual bleeding
The identification of pathological causes of HMB enables targeted treatments and, ideally, more effective management. The aim of diagnostic tests is not only to identify structural pathology, such as endometrial polyps and uterine fibroids, which cause HMB and reduce the effectiveness of simple treatments, but also to reassure women that they do not have a serious underlying condition, such as endometrial hyperplasia or cancer. Outpatient hysteroscopy is advised over ultrasound if the patient’s clinical history suggests polyps or fibroids, unless the uterus is palpable abdominally or examination suggests a mass or is inconclusive. Otherwise, a physical examination is not recommended unless an intrauterine device is to be considered. A full blood count is advised, but no other routine blood tests are indicated unless the patient’s history suggests a coagulation disorder. 9
Initial medical treatments
The National Institute for Health and Care Excellence (NICE) issued guidelines for the treatment of HMB in 2007, at which time the Clinical effectiveness and cost-effectiveness of levonorgestrel releasing intrauterine system in primary care against standard treatment for menorrhagia trial (ECLIPSE) was recruiting participants; these guidelines were subsequently updated in 2018. Among the recommendations, NICE stated that the levonorgestrel-releasing intrauterine system (LNG-IUS) should be considered as the first-line treatment for women with no pathology, adenomyosis, or fibroids of < 3 cm. 9 NICE emphasised that, in considering initial treatments, consideration should be given to comorbidities; the presence of fibroids, adenomyosis or endometrial polyps; contraceptive need; and women’s preferences. Thus, for women who are unsuitable for or decline the LNG-IUS, non-hormonal treatments of tranexamic acid, the non-steroidal anti-inflammatory drugs (NSAIDs) mefenamic acid or the lesser used naproxen, or hormonal treatments, including the combined oral contraceptive pill or cyclical oral progestogens, should be considered.
The LNG-IUS is marketed as Mirena™ (Bayer, Reading, UK) or Levosert™ [Gideon Richter (UK) Ltd, London, UK]. The device is a T-shaped plastic rod that delivers approximately 20 µg of levonorgestrel per day, and can remain in place for 5 years. Formulations with lower doses and shorter lifespans are available but are not licensed for HMB management. The LNG-IUS is a very effective contraceptive that increases cervical mucus and thins the endometrium, which results in less menstrual bleeding. However, bleeding patterns in the first few cycles after fitting can be irregular and bothersome, and women are advised to persist for 6 months to see benefits.
Non-steroidal anti-inflammatory drugs have been reported to reduce HMB to a greater extent than placebo, but direct comparisons with hormonal treatments are lacking. 10 Tranexamic acid is associated with a greater reduction in mean blood loss and a higher likelihood of improvement in symptoms than both NSAIDs and cyclical progesterones, but the quality of evidence for these comparisons is low and very low, respectively. 11,12 The combined oral contraceptive pill dramatically normalises menstrual bleeding compared with placebo. 13 Prior to the start of the ECLIPSE trial, the evidence for the LNG-IUS as a treatment for HMB was of low quality, with nine trials reporting on 3- to 12-month follow-ups for a total of 783 women. These trials showed that the LNG-IUS resulted in a greater reduction in objective menstrual blood loss than non-hormonal and hormonal treatments, but the effects on bleeding-related quality of life were unknown. 14
The selective progesterone uptake inhibitor ulipristal acetate was licensed for pre-operative treatment of uterine fibroids and then intermittent treatment of HMB associated with fibroids, following observations of significant reductions in bleeding. 15–17 A recent clinical trial18 that compared ulipristal acetate with the LNG-IUS in women with no or small fibroids, assessing bleeding-related quality of life was initiated; however, this was terminated when drug alerts from the UK and European regulatory authorities restricted the use of ulipristal following reports that it was associated with liver injury. 19
Ablative and surgical procedures
If medical treatments fail to ameliorate the burden of HMB, ablative or surgical procedures can be considered. 9 Hysterectomy is definitive, with the complete removal of the uterus, whereas endometrial destruction uses intracavity energy sources to ablate the endometrium. Previous endometrial procedures required direct visualisation, general anaesthetic and, potentially, resection. Second-generation techniques that have been developed in the past two decades do not require hysteroscopic visualisation, use smaller-diameter instruments and can often be carried out under local anaesthetic. Systematic reviews20,21 have not demonstrated major differences between first- and second-generation endometrial ablative techniques in terms of effectiveness or satisfaction with treatment, or requirement for further surgery. Second-generation bipolar radiofrequency and microwave ablation achieve higher rates of amenorrhoea than thermal balloon ablation 12 months after treatment. 22
Some 20% of women who have endometrial ablation will ultimately need a subsequent procedure, potentially hysterectomy, for relief of their symptoms. 23,24 An individual participant data meta-analysis of randomised trials found that total hysterectomy was more clinically effective and cost-effective than endometrial ablation. 20,25 A study that compared minimally invasive hysterectomy techniques with thermal balloon or radiofrequency ablation found that hysterectomy resulted in higher quality of life and satisfaction after 2 years and significantly reduced the risk of further interventions. 26,27
The ECLIPSE trial
The ECLIPSE trial randomised 571 women presenting to primary care with HMB to treatment with either the LNG-IUS (Mirena was the only available system at that time) or usual medical treatment (tranexamic acid, mefenamic acid, combined oral contraceptive pill or progesterone alone, chosen as clinically appropriate by the general practitioner and the woman). At the trial’s outset in 2004, evidence of the clinical effectiveness of the LNG-IUS had not been well established and LNG-IUS was not yet considered to be a usual treatment for HMB in primary care. The primary outcome was the Menorrhagia Multi-Attribute Scale (MMAS) patient-reported score (ranging from 0 to 100, with lower scores indicating greater severity of HMB), which was assessed over a 2-year period. Secondary outcomes included general quality of life [measured using the Short Form questionnaire-36 items (SF-36) and EuroQol-5 Dimensions (EQ-5D) scales], sexual activity scores and ablative or surgical intervention. The time frames for comparative analyses were 2 and 5 years after randomisation, with data also collected at 6 and 12 months. The trial was designed to recruit from and fit around clinical primary care practice.
The trial included women aged 25–50 years who presented to their GP with HMB involving at least three consecutive menstrual cycles. Women were excluded if they intended to become pregnant over the following 5 years; were taking hormone replacement therapy or tamoxifen; had intermenstrual bleeding (bleeding between expected periods); experienced postcoital bleeding; had an abdominally palpable uterus equivalent in size to that at 10–12 weeks’ gestation (suggestive of fibroids) or other disorders; or had contraindications to or a preference for either the LNG-IUS or any of the usual medical treatments. Women with heavy, irregular bleeding were ineligible unless the results of endometrial biopsy were reported to be normal. No further diagnostic investigations or examinations were mandated by the protocol. All patients provided written informed consent.
The total MMAS scores improved significantly in both groups across all time points compared with baseline scores. At 2 years of follow-up, the improvement in the MMAS score was significantly higher in the LNG-IUS group than in the usual medical treatment group (mean between-group difference of 13.4 points, 95% CI 9.9 to 16.9 points; p < 0.001). 28 By 5 years of follow-up, this benefit was reduced to 3.9 points (95% CI –0.6 to 8.3; p = 0.09). 29 Women in the LNG-IUS group were nearly twice as likely as those assigned usual medical treatment to still have the LNG-IUS in place at 2 years (64% vs. 38%, respectively; p < 0.001), with these proportions dropping to 47% and 15%, respectively, by 5 years of follow-up. Within the first 2 years, 6% of women in both groups had undergone a hysterectomy, while 4% of women in the LNG-IUS group and 6% of women in the usual medical treatment group had undergone endometrial ablation. At 5 years, surgery-free survival rates remained comparable: 80% in the LNG-IUS group and 77% in the usual medical treatment group (hazard ratio 0.90, 95% CI 0.62 to 1.31; p = 0.6). There was no evidence of a difference between groups in general quality of life or sexual activity at any time point. The LNG-IUS was considered to be cost-effective over the 2- and 5-year time horizons using the method generally recommended by NICE,9 but was sensitive to alternative methods of valuing quality of life used in the trial.
Women’s perspectives on heavy menstrual bleeding
The use of ‘objective’ measures of blood loss in HMB has been questioned, given that the more subjective perception of menstrual blood loss for women may not correlate with the volume of blood loss experienced30 or semi-objective bleeding diaries. 31 There is increasing recognition of the debilitating effect of HMB on women’s physical, social, emotional and material quality of life. 32,33 Women perceiving their periods to be heavy report pain as the aspect of their HMB that bothers them the most, followed by the heaviness of bleeding, mood changes and tiredness. 34 When evaluating treatments, outcomes such as quality of life and patient satisfaction are now considered as helpful as objective measures,35 which is reflected in clinical care guidelines. 9
In the mid-1990s, the annual rate of HMB in women aged 30–49 years was 26 in every 1000 women per year in general practice;36 however this probably underestimates how many women experience the adverse effects of HMB because not all women will seek medical help. As mentioned previously, a European internet survey4 found that 27% of respondents reported HMB symptoms, but nearly half of these women had never consulted a physician. Women may be reluctant to approach their GP with their HMB problems, having felt dismissed in the past. 37 The provision of information regarding treatment options is an important challenge for primary care, where a decision aid has been shown to improve HMB-related quality of life and reduce decisional conflicts around medical and surgical treatment choices. 38 Further understanding of why women may not discuss menstrual issues with their clinician is needed. 39
Long-term data on heavy menstrual bleeding
Data on the natural history of HMB before the menopause are sparse. To our knowledge, there is no evidence on long-term outcomes following initiation of medical treatments in primary care for women presenting with HMB in this setting, other than the 5-year data from the ECLIPSE trial. 29
Other evidence on outcomes is limited, even after surgical treatment. One of the original randomised trials of the LNG-IUS,40 comparing it with hysterectomy, followed 221 out of the 236 participants up to 10 years. Overall, levels of health-related quality of life and psychosocial well-being improved during the first 5 years but diminished between 5 and 10 years, and the level of quality of life returned close to the baseline level. 40 There were no significant differences between the LNG-IUS group and the hysterectomy group. 40
In a study of health records of 216 Spanish women who had a LNG-IUS fitted between 2000 and 2003,41 68 women had their devices removed prior to 5 years, with about half of these removed because women perceived themselves as perimenopausal. Of the 129 (60%) women who completed 5 years of use, 51 had a second system inserted. 41 This is a little higher than the 47% of women allocated to receive the LNG-IUS in the ECLIPSE trial who still had the LNG-IUS in situ at 5 years. 29 Younger age (< 45 years) and severe dysmenorrhoea have been identified as factors associated with discontinuation of the LNG-IUS within 2 years, although the overall rate of discontinuation in this cohort study was 46%,42 higher than the 36% observed in the ECLIPSE trial at 2 years. An investigation of subsequent procedures following endometrial ablation and hysterectomy using data linkage in the entire Scottish health record system23 achieved a median follow-up of 6.2 and 11.6 years, respectively. A 2014 systematic review22 of clinical trials of endometrial ablation reporting outcomes beyond 2 years found only one study with a 10-year follow-up.
Rationale for long-term follow-up of the ECLIPSE cohort
Given the long natural history of HMB, treatments may be sought and taken over many years; however, evidence on the continuation or long-term effectiveness of treatments, the proportion of women who opt for surgery and the motivations for treatment choices in the longer term are lacking. Women’s and their clinicians’ decisions about medical treatments for HMB may also include changing considerations over time, such as women’s preferences for and expectations about using standard oral treatments or having an intrauterine device, contraception, when to expect menopause or anticipating surgery. Greater long-term evidence to help guide such decision-making in practice is needed.
By 5 years of follow-up, of the 571 women who participated in the original ECLIPSE study, 70 did not wish to receive further contact from the trial team and one had died. ECLIPSE trial participants had a mean age of 41.9 years [standard deviation (SD) 5.0 years] at randomisation. This cohort of women provided a unique opportunity to secure long-term data on women’s treatment trajectories for HMB, which might vary as they approach and reach the menopause.
Objectives of the project
The primary objective of this study was to assess continuation rates of medical treatments, and the rates and nature of ablative and surgical interventions, in women 10 years after initial management for HMB in primary care.
The secondary objectives were to develop a greater understanding of the natural history and treatment of HMB, in particular:
-
an assessment of whether or not initial medical treatment (the LNG-IUS or usual medical treatments) influences women’s trajectories
-
an assessment of quality of life and sexual function experienced, and evaluation of whether or not that is influenced by initial medical treatment
-
a qualitative exploration of women’s experiences of HMB and decisions about treatments or surgical interventions to provide insight into women’s choices, and what influences them, over this time period.
Chapter 2 Observational study methods
This chapter describes the methods used to address the primary objective and secondary objectives.
Design
This was an observational follow-up study of women who had participated in the ECLIPSE trial. 28 We aimed to recontact and reconsent women to obtain follow-up data after 10 years via a self-completed questionnaire.
Study oversight and information governance
The original ECLIPSE trial was sponsored by the University of Birmingham and was formally closed as a clinical trial of an investigational product on 15 June 2015. The observational study protocol was granted a favourable ethics opinion by the London-Chelsea Multicentre Research Ethics Committee (17/LO/1876), with the University of Nottingham as the study sponsor. A data-sharing agreement was put in place to transfer the ECLIPSE trial participants’ names and contact details from the University of Birmingham to the University of Nottingham for the purposes of recontacting and reconsenting women for further data collection. Reconsent included confirmation that the participants were aware and accepted that any new information that they provided would be linked to the original trial data they had provided, or had been provided by their GP, for the ECLIPSE trial.
Amendments were made to the data-sharing agreement: first to enable the transfer of additional identifiable data (i.e. date of birth and NHS number) to assist in recontacting the original participants and, second, to enable the transfer of identifiable, linked trial data for reconsented participants and to provide anonymised trial data for those remaining participants who had either been uncontactable or declined to participate in further follow-up. These amendments were required in 2018 at the time of the implementation of the European Union General Data Protection Regulation in the UK. Lack of clarity around the regulations caused significant delays in agreeing the scope of the transfer and in executing the amended agreement. This subsequently caused a delay in being able to locate and reconsent a proportion of trial participants whose contact details had changed since their last contact with the ECLIPSE trial 5 years before or earlier. Data were transferred via a secure cloud server, with access granted to a sole person at each institution and the password for transferred files exchanged in a separate e-mail.
Population
The ECLIPSE trial randomised 571 women between 25 and 50 years of age who presented to their GP with HMB involving at least three consecutive menstrual cycles. Women were excluded if they intended to become pregnant over the next 5 years; were taking hormone replacement therapy or tamoxifen (Soltamox™, Rosemont Pharmaceuticals, Leeds, UK); had intermenstrual or postcoital bleeding; had findings suggestive of fibroids (abdominally palpable uterus equivalent in size to that at 10–12 weeks’ gestation) or other disorders, or had contraindications to or a preference for either the LNG-IUS or usual medical treatments. Women with heavy, irregular bleeding were ineligible unless the results of an endometrial biopsy were reported to be normal. All trial participants provided written informed consent prior to randomisation, enabling contact to be made for 10 years following trial entry. The demographic and medical profiles of the original trial population have been reported previously. 28
Recontact and reconsent
Although all women in the original ECLIPSE trial had provided their consent to be contacted for questionnaire follow-up at 10 years, further confirmation of their consent was required for this observational study, as the ECLIPSE trial had formally ended after the 5-year data were reported.
Women were contacted by mail at the last known address recorded in the ECLIPSE trial database. They received study information, a consent form, the follow-up questionnaire (with the option to complete this online if preferred) and contact details for the University of Nottingham research team. If there was no response to the postal invitation, attempts were made to contact the participant using their last known telephone number and/or e-mail address if this was available in the ECLIPSE database. The research team also contacted women’s previously recorded general practice for confirmation of details if the participant was still registered with that practice. If women did not respond to the study pack mailed to their previously known address or attempts to contact via telephone call and/or e-mail, and it was confirmed that they were no longer registered at their previously recorded practice, for example owing to relocation, they were considered lost to follow-up.
Once recontact, reconsent and data collection were complete, the University of Nottingham team securely provided the University of Birmingham with a list of participants who had consented to their previous ECLIPSE trial data being shared. The original trial data manager securely transferred consenting participants’ full trial data with linking identifiers, and anonymised data for the remainder of participants, to the University of Nottingham.
Anticipated size of the cohort
The original ECLIPSE trial randomised 571 participants. At 5 years, 70 women had withdrawn consent to be contacted, one had died and 424 (74%) had returned questionnaires. After receipt of the original trial participants’ contact details and further clarification, a potential maximum of 490 women were available to be approached. We set a target of collecting 10-year data from 276 women, equating to 65% of the 424 women who provided data at 5 years post randomisation. Our target anticipated further loss to follow-up owing to the length of time elapsed since previous contact, relocation, non-completion of the questionnaire or death.
Data collection from general practices
Originally, we had proposed data collection by manual extraction of data from patients’ GP records on surgical interventions and medical treatments for HMB, by either practice staff or the study research team. Initially, 34 general practices from the original ECLIPSE trial responded and agreed to participate in data extraction. Of these general practices, 16 practices had a total of 25 women who also reconfirmed consent to data extraction from their GP records at 10 years. These practices returned extracted data for these 25 women on study-specific pro formas, which were compared with their corresponding questionnaire data. We conducted an interim assessment of the value that additional GP record data extraction might add to the data returned by women on their postal questionnaires. The completeness and accuracy of the questionnaire data self-reporting medical and surgical treatments were reviewed independently by two researchers and assessed as very high compared with the GP records. Further data extraction from GP records was thus deemed unnecessary unless questionnaire data subsequently received from each participant were incomplete, and the participant and corresponding GP practice consented to this process. No further GP record extraction was conducted for the remaining 181 women who returned questionnaires.
Early cessation owing to the COVID-19 pandemic
A total of 206 women had provided reconsent and returned completed 10-year follow-up data by 31 March 2020 (mail, n = 200; online, n = 6). This represented 75% of our intended total study target of 276 completed questionnaires. The advent of the global COVID-19 pandemic, and the formal UK lockdown from 23 March 2020, caused further attempts to contact non-responders beyond this time to be ceased early. It was considered unlikely that the remaining ‘hard-to-reach’ participants would prioritise completion of the questionnaires at that time. In any case, we would be unable to access questionnaires returned to the trial team office. This view was also informed and supported by our two patient and public involvement (PPI) advisors, who agreed that we should stop further attempts to contact non-responders.
Original trial interventions
The allocation method of the ECLIPSE trial interventions and the distribution of the treatments used have been previously reported. 28,29 Consenting women were randomly assigned to either the LNG-IUS or usual medical treatment, which included oral tranexamic acid, mefenamic acid, norethisterone, a combined oestrogen–progestogen or progesterone-only oral contraceptive pill (any formulation), or medroxyprogesterone acetate injection. The usual medical treatments were chosen by the clinician and patient on the basis of any contraceptive needs or the desire to avoid hormonal treatment. 9,43 In line with real-life practice, treatments could be changed (from one usual medical treatment to another, from the LNG-IUS to a usual medical treatment, or from a usual medical treatment to the LNG-IUS) or could be discontinued because of a perceived lack of benefit, side effects, a change in the need for contraception, referral for endometrial ablation or hysterectomy, or any other reasons in accordance with usual clinical practice. 9,43
Outcome measures
As the primary outcomes for this observational study, data were collected directly from women on the use of treatments for HMB and the surgical interventions of hysterectomy and endometrial ablation. Data on the changes in treatment or cessation of treatment were also collected. The following other outcome measures were used as previously reported. 29 Generic quality of life was assessed using the SF-36, version 2 [with scores ranging from 0 (severely affected) to 100 (not affected)], the EQ-5D descriptive system [with scores ranging from −0.59 (health state worse than death) to 100 (perfect health state)] and the EQ-5D visual analogue scale [with scores ranging from 0 (worst health state imaginable) to 100 (most perfect health state imaginable)]. The validated Sexual Activity Questionnaire (SAQ) was used to measure pleasure [with scores ranging from 0 (lowest level) to 18 (highest level)], discomfort [with scores ranging from 0 (greatest) to 6 (none)] and frequency (assessed as an ordinal response relative to perceived usual activity). 44
The primary outcome measure in the original trial was the patient-reported, condition-specific MMAS at 2 years of follow-up. 45,46 The MMAS is designed to measure the effect of HMB on six domains of daily life (practical difficulties, social life, psychological health, physical health, work and daily routine, and family life and relationships). Summary scores, which range from 0 (severely affected) to 100 (not affected), were assessed. The MMAS has a high degree of reliability and internal consistency,45 has good content and construct validity,47,48 is responsive38,49 and is acceptable to respondents. 42,46,49,50 The MMAS seeks responses in relation to current HMB, so was anticipated to be not relevant to the vast majority of women at this 10-year follow-up and was presented as an optional section of the questionnaire to complete.
Statistical analysis
The study sample of women completing 10 years of follow-up was compared with all other women in the original trial cohort (those declining when recontacted or not responding to the recontact invitation). The proportions of women of different ethnicity, with and without menorrhagia and randomised to different types of treatment were compared using the chi-squared test. Age in years, body mass index (BMI), blood pressure and questionnaire scores (SF-36, EQ-5D, MMAS and SAQ) in the groups were compared using either Student’s t-test for normally distributed variables or the Mann–Whitney U-test.
In deriving the SF-36 score, and in contrast to the original ECLIPSE trial, the SF-36 Health Survey manual and interpretation guide for missing data was followed. 51 If information was partially missing but over half of the questions in a domain were answered, the average score of the responses was used. If more than half of the questions were missing, the score was classed as missing for that participant. The same approach was used for missing question responses in the SAQ.
Further analyses were conducted on women consenting to the 10-year follow-up. This group was split into two subgroups according to their initial randomised treatment allocation. Characteristics and questionnaire scores at baseline and at 10 years of follow-up were compared using the same approach as above. Changes between baseline and the 10-year follow-up were assessed using the paired t-test. Changes over the 10-year period between groups were examined using an unpaired t-test. To compare surgical intervention rates in women allocated to different treatments, we used the log-rank test for equality of survival functions and presented the estimates using Kaplan–Meier survival plots.
Patient and public involvement
Two PPI advisors, both of whom were women with direct personal health experience of the area of enquiry and of a similar age to the target study participants, were recruited following open advertisements to regional public-facing PPI research networks. They provided feedback at the outset of this follow-up study on the study objectives and study protocol, including procedures for approaching women, and reviewed all information and study materials for participants, as well as the qualitative interview schedule, for acceptability and appropriateness prior to submission for ethics approval.
During the study, the two PPI advisors met with project team members in regular (4- to 6-monthly) meetings with formal agendas to develop and gain PPI contributions, and with regular iterative communication by e-mail between meetings to comment on study updates or issues as they arose. They contributed valuable ideas on how to enhance the appeal of study participation to women and informed strategies to increase recruitment to follow-up, and reviewed the utility of information from health records. They also provided helpful insights and advice on the feasibility and early cessation of attempts to follow up women at the advent of the COVID-19 pandemic.
The PPI advisors read and discussed findings from early qualitative interviews and suggested additional topic prompts for interviews. They subsequently contributed to the review and interpretation of the observational follow-up study and qualitative results. This included helpfully commenting on a summary of preliminary findings prior to its use for respondent validation (member checking) with participants in the qualitative study. In relation to dissemination, our PPI advisors have informed and actively contributed to our plans after publication, including the production of a video podcast on findings and sharing results with women’s organisations and on social media. Their input on a whole study summary has been sought prior to our planned dissemination to study participants. They also provided input to the Plain language summary.
Chapter 3 Observational study results
This chapter reports the results of the observational study.
Flow and characteristics of the original ECLIPSE trial and observational study participants
The number of women available to be contacted from the original ECLIPSE trial and the number who were reconsented and provided data (hereafter referred to as responders) are shown in Figure 1. Those who had withdrawn from the ECLIPSE trial, who were not able to be recontacted for the observational study or who did not provide 10-year observational data are described as not having been followed up at 10 years.
FIGURE 1.
Flow of participants from the original ECLIPSE trial to the observational study. Navy, participants in original ECLIPSE trial; aqua, participants in current observational follow up study.
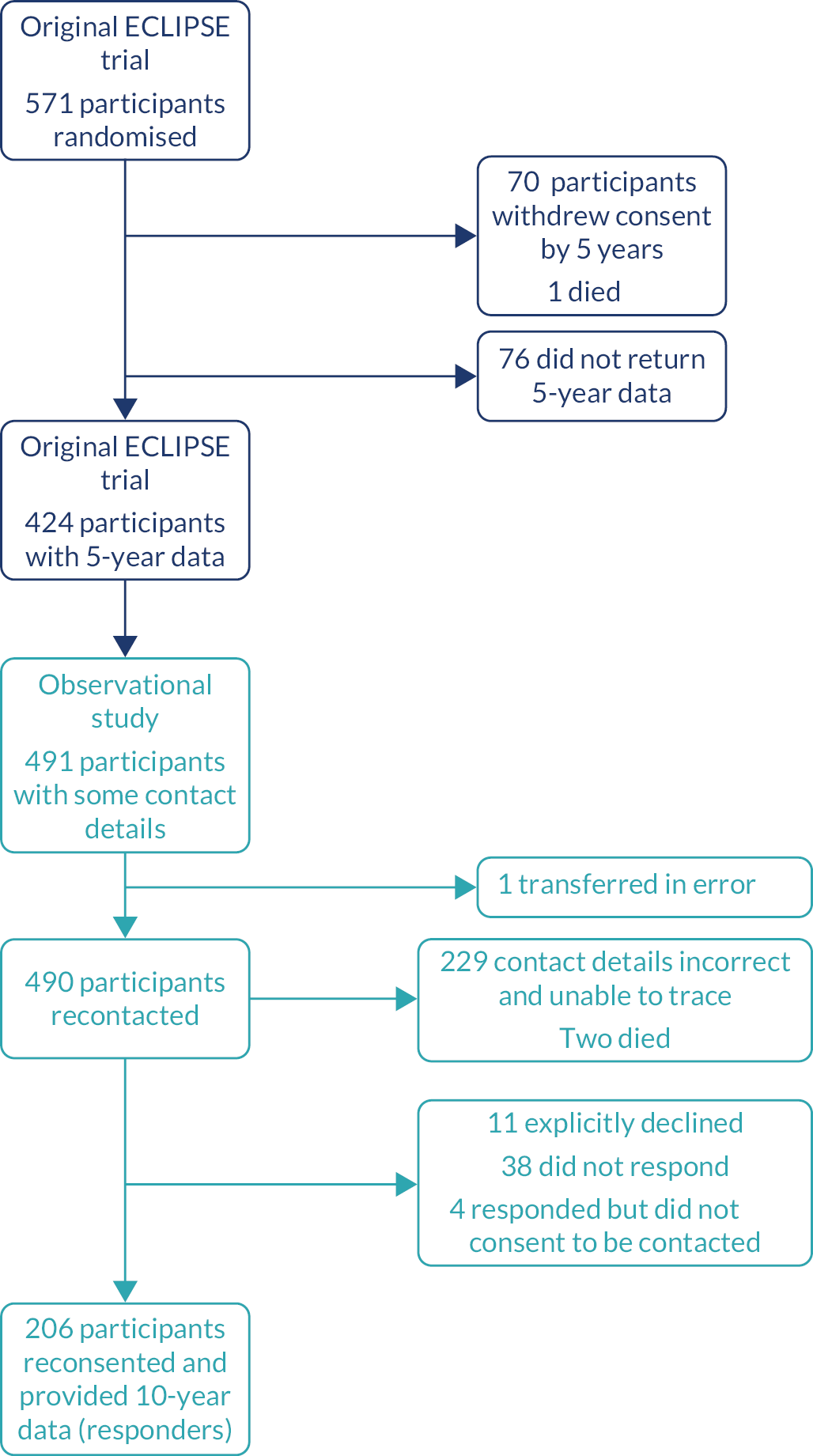
The baseline (prior to randomisation) characteristics of the reconsented responding women and those who were not followed up are presented in Table 1. Responders were very similar to those women not followed up, with an average age of 41.9 and 41.1 years, respectively, and the two groups did not differ in their initial symptoms and presentations of HMB. They had similar BMI and blood pressure, with slightly more people of white ethnicity among responders than in the group that were not followed up.
| Characteristic | All women followed up at 10 years (N = 206) | All women not followed up at 10 years (N = 365) |
|---|---|---|
| Age | ||
| Mean (SD) (years) | 41.9 (4.9) | 41.1 (5.4) |
| Aged ≥ 35 years, n (%) | 188 (91) | 324 (89) |
| Ethnicity, n (%) | ||
| White | 178 (86) | 293 (80) |
| Asian | 11 (5) | 40 (11) |
| Black | 9 (4) | 21 (6) |
| Other | 8 (4) | 11 (3) |
| BMI | ||
| Mean (SD) (kg/m2) | 29.4 (6.4) | 29.1 (6.4) |
| ≥ 25 kg/m2, n (%) | 146 (71) | 255 (70) |
| Blood pressure (mmHg), mean (SD) | ||
| Systolic | 129.7 (17.0) | 128.5 (16.3) |
| Diastolic | 78.8 (10.2) | 78.7 (10.5) |
| Presentation to primary care for HMB, n (%) | ||
| Initial | 157 (76) | 279 (76) |
| Subsequent | 49 (24) | 86 (24) |
| Duration more than 1 year | 164 (80) | 296 (81) |
| Menstrual pain | 151 (73) | 273 (75) |
| Contraception requirement | 35 (17) | 75 (21) |
| Copper or non-hormonal coil | 7 (3) | 12 (3) |
| Treatment at randomisation, n (%) | ||
| LNG-IUS | 110 (53) | 175 (48) |
| Usual medical treatments | 96 (47) | 190 (52) |
Allocation to different treatments was balanced across both groups of women: 110 out of 206 (53%) responders and 175 out of 365 (48%) women not followed up were allocated to the LNG-IUS. The two groups also had similar baseline quality-of-life scores according to the SF-36 and EQ-5D, with no domains showing a statistically significant difference (Table 2). Average MMAS scores at baseline were slightly higher for women responding at 10 years than for those not followed up (42.8 vs. 39.7, respectively), and the difference was not statistically significant. The baseline SAQ was completed by 166 out of 206 (81%) responders and 248 out of 365 (68%) not followed up at 10 years, with average scores for the pleasure and discomfort domains slightly lower for responders; however, again, the differences were not statistically significant.
| Questionnaire item | Mean score (SD), n | |
|---|---|---|
| All women followed up at 10 years | All women not followed up at 10 years | |
| SF-36 | ||
| Physical functioning | 82.5 (19.4), 205 | 76.2 (24.6), 339 |
| Physical role | 71.7 (24.3), 205 | 69.6 (26.2), 340 |
| Emotional role | 72.0 (24.9), 204 | 70.2 (26.6), 339 |
| Social functioning | 65.7 (23.7), 205 | 61.9 (26.0), 342 |
| Mental health | 60.7 (19.6), 205 | 59.1 (19.5), 340 |
| Energy and vitality | 40.8 (21.9), 205 | 40.7 (20.9), 340 |
| Pain | 48.5 (22.6), 205 | 45.6 (22.3), 342 |
| Perception of general health | 62.2 (21.8), 205 | 60.2 (21.7), 342 |
| EQ-5D | ||
| Descriptive system | 0.769 (0.228), 206 | 0.714 (0.276), 340 |
| Visual analogue scale | 71.6 (18.9), 185 | 69.0 (19.7), 311 |
| SAQ | ||
| Pleasure | 10.5 (5.0), 166 | 11.1 (4.9), 248 |
| Discomfort | 4.8 (1.4), 166 | 4.5 (1.7), 248 |
| Menorrhagia Multi-Attribute Score | 42.8 (19.4), 12 | 39.7 (21.8), 240 |
Outcomes at up to 10 years’ follow-up
Table 3 shows the characteristics of all responders and by initial treatment allocation.
| Characteristic | All responders (N = 206) | Allocated to LNG-IUS (N = 110) | Allocated to usual medical treatment (N = 96) |
|---|---|---|---|
| Mean (SD) age at baseline (years) | 41.9 (4.9) | 41.9 (4.9) | 42.0 (5.0) |
| Mean (SD) age at response to 10-year follow-up (years) | 53.7 (5.1) | 53.7 (5.0) | 53.7 (5.2) |
| Ethnicity: white, n (%) | 178 (86) | 90 (82) | 88 (92) |
At the time of completing the 10-year follow-up questionnaire, 106 (51%) women had reached menopause (defined for the responders as having experienced no menstrual bleeding for at least 1 year) and 34 (17%) had undergone a hysterectomy, as shown in Table 4. Of the postmenopausal women, 28 (14%) were taking menopausal hormone therapy. Of those women still menstruating, 12 (6%) were still experiencing HMB and did not consider themselves to be menopausal.
| Trial allocation, n (%) | |||
|---|---|---|---|
| All responders (N = 206), n (%) | LNG-IUS (N = 110) | Usual medical treatment (N = 96) | |
| Menstrual status and reported treatments for HMB | |||
| Premenopausal | 32 (16) | 16 (15) | 16 (17) |
| Postmenopausal | 106 (51) | 54 (49) | 52 (54) |
| Undergone hysterectomy | 34 (17) | 18 (16) | 16 (17) |
| Perimenopausal or uncertain | 32 (16) | 21 (19) | 11 (11) |
| Missing | 2 (1) | 1 (1) | 1 (1) |
| Using menopausal hormone therapy | 28 (14) | 16 (15) | 12 (13) |
| Still experiencing HMB | 12 (6) | 6 (5) | 6 (6) |
| Using the LNG-IUS at response to 10-year follow-up | 56 (28) | 38 (35) | 18 (19) |
| Classes of treatments used between 5 and 10 years | |||
| LNG-IUS | 67 (33) | 47 (43) | 20 (21) |
| Usual medical treatment | 29 (14) | 10 (9) | 19 (20) |
| LNG-IUS and usual medical treatment | 21 (10) | 11 (10) | 10 (10) |
| None | 89 (43) | 42 (38) | 47 (49) |
| Standard medical treatments used between 5 and 10 years | |||
| Tranexamic acid | 24 (12) | 7 (6) | 17 (18) |
| Mefenamic acid | 6 (3) | 3 (3) | 3 (3) |
| Norethisterone | 13 (6) | 4 (4) | 9 (9) |
| Desogestrel | 3 (1) | 0 | 3 (3) |
| Oral contraceptives | 8 (4) | 3 (3) | 5 (5) |
| Medroxyprogesterone acetate injection | 1 (< 1) | 1 (< 1) | 0 |
| Naproxen | 1 (< 1) | 0 | 1 (< 1) |
| Surgical intervention for HMB | |||
| Hysterectomy | 34 (17) | 18 (16) | 16 (17) |
| Endometrial ablation | 26 (13) | 10 (9) | 16 (17) |
Overall, 56 (28%) women reported that they were using the LNG-IUS at the time of their response to the 10-year follow-up, and the proportion was higher in those initially allocated to the LNG-IUS (35%) than in the group allocated to usual medical treatment (19%). Between 5 and 10 years of follow-up, a substantial proportion of women reported not taking treatment for HMB. However, 88 (43%) women used the LNG-IUS (67 women used only the LNG-IUS and 21 used the LNG-IUS in combination with usual medical treatment). The proportion using the LNG-IUS, alone or in combination, was higher for women initially allocated to the LNG-IUS than for women allocated to usual medical treatment: 58 out of 110 women (53%) and 30 out of 96 women (31%), respectively. Tranexamic acid was the most frequently used of the usual medical treatments, with 24 (12%) responding women using this over the 5- to 10-year follow-up period. Table 4 shows the reported treatments by original randomised allocation. There were no statistically significant differences in treatments between the two randomised groups for any menopausal or treatment category.
Table 5 shows the distributions of scores for the two generic quality-of-life questionnaires and the SAQ for all responders and by the original allocation at 10 years after randomisation. There were no statistically significant differences between the randomised groups in any domain of the three questionnaires. Only 13 respondents, 12 of whom described their bleeding as heavy, completed the MMAS questionnaire; therefore, distributions were not calculated and groups were not compared. The SAQ was completed by 116 of the 206 responding women, indicating that at least 56% of women were sexually active.
| Questionnaire item | All responders, mean score (SD), n | Trial allocation, mean score (SD), n | |
|---|---|---|---|
| LNG-IUS | Usual medical treatment | ||
| SF-36 | |||
| Physical functioning | 80.2 (26.2), 205 | 81.4 (24.9), 110 | 78.8 (27.7), 95 |
| Physical role | 78.4 (28.6), 204 | 80.1 (26.2), 109 | 76.4 (31.1), 95 |
| Emotional role | 79.4 (27.5), 204 | 79.3 (26.4), 109 | 79.5 (28.9), 95 |
| Social functioning | 74.7 (25.8), 206 | 75.5 (25.2), 110 | 73.8 (26.6), 96 |
| Mental health | 68.6 (21.5), 205 | 68.1 (21.1), 110 | 69.2 (22.0), 95 |
| Energy and vitality | 48.9 (10.2), 205 | 48.3 (8.8), 110 | 49.5 (11.6), 95 |
| Pain | 63.4 (24.8), 206 | 64.3 (23.9), 110 | 62.4 (25.9), 96 |
| Perception of general health | 55.4 (9.6), 206 | 55.9 (10.3), 110 | 54.9 (8.7), 95 |
| EQ-5D | |||
| Descriptive system | 0.748 (0.266), 204 | 0.757 (0.249), 110 | 0.736 (0.286), 94 |
| Visual analogue scale | 73.4 (20.7), 176 | 74.9 (19.8), 93 | 71.8 (21.6), 83 |
| SAQ | |||
| Pleasure | 11.2 (4.6), 116 | 11.5 (4.6), 62 | 10.9 (4.6), 54 |
| Discomfort | 2.01 (1.99), 116 | 2.19 (2.09), 62 | 1.80 (1.87), 54 |
Table 6 presents scores for these three questionnaires by randomised group at baseline and at 10-year follow-up, comprising women who completed questionnaires at both time points. There were improvements over time in SF-36 scores in all domains exception general health perception. These improvements occurred in both groups, with small and statistically insignificant differences between the groups. Changes over time for the EQ-5D scores were very small and, again, no differences were seen between the original allocation groups. Of the 206 women, 40 were not in an intimate relationship and 116 reported via the SAQ that they were sexually active. There was a clear deterioration in the discomfort domain of the SAQ, with no evidence of a difference between the allocation groups, but no changes were seen in the pleasure domain.
| Questionnaire item | Mean baseline score for responders (95% CI) | Mean 10-year follow-up score for responders (95% CI) | Mean difference between groups over 10 years (95% CI); p-value | Mean change within group (95% CI), p-value | |||
|---|---|---|---|---|---|---|---|
| LNG-IUS | Usual medical treatment | LNG-IUS | Usual medical treatment | LNG-IUS | Usual medical treatment | ||
| SF-36 | |||||||
| Physical functioning | 84.0 (81.5 to 86.5) | 80.7 (77.8 to 83.6) | 81.2 (78.2 to 84.2) | 78.8 (75.5 to 82.1) | –0.9 (–4.4 to 2.6); 0.786 | –2.8 (–5.7 to 0.2); 0.220 | –1.9 (–4.9 to 1.1); 0.409 |
| Physical role | 74.0 (71.0 to 76.9) | 69.1 (65.9 to 72.2) | 79.9 (76.8 to 83.0) | 76.4 (72.9 to 79.9) | –1.3 (–5.4 to 2.8); 0.760 | 6.0 (2.7 to 9.3); 0.038 | 7.3 (3.7 to 10.9); 0.034 |
| Emotional role | 72.4 (69.4 to 75.5) | 71.2 (68.1 to 74.4) | 79.8 (76.8 to 82.9) | 79.5 (76.1 to 82.8) | –0.8 (–4.9 to 3.2); 0.844 | 7.4 (4.2 to 10.6); 0.007 | 8.2 (4.6 to 11.9); 0.018 |
| Social functioning | 67.2 (64.4 to 70.0) | 64.1 (60.9 to 67.3) | 75.2 (72.2 to 78.3) | 73.8 (70.6 to 77.1) | –1.7 (–5.6 to 2.2); 0.661 | 8.0 (5.0 to 11.0); < 0.001 | 9.8 (6.2 to 13.3); 0.004 |
| Mental health | 61.7 (59.0 to 64.4) | 60.0 (57.3 to 62.8) | 68.1 (65.3 to 70.9) | 69.2 (66.2 to 72.1) | –2.8 (–6.1 to 0.5); 0.331 | 6.3 (3.7 to 9.0); < 0.001 | 9.1 (6.2 to 12.1); < 0.001 |
| Energy and vitality | 41.6 (38.8 to 44.4) | 40.0 (37.0 to 43.0) | 48.3 (46.5 to 50.1) | 49.5 (47.4 to 51.7) | –2.8 (–6.4 to 0.7); 0.392 | 6.7 (3.8 to 9.6); 0.003 | 9.5 (6.5 to 12.6); < 0.001 |
| Pain | 49.0 (46.1 to 51.9) | 47.9 (44.9 to 50.9) | 64.1 (61.2 to 67.1) | 62.4 (59.2 to 65.6) | 0.7 (–3.2 to 4.5); 0.866 | 15.1 (12.0 to 18.3); < 0.001 | 14.5 (11.1 to 17.8); < 0.001 |
| Perception of general health | 63.5 (60.7 to 66.3) | 60.7 (57.7 to 63.6) | 56.0 (54.0 to 57.9) | 54.9 (53.0 to 56.7) | –1.8 (–5.2 to 1.7); 0.564 | –7.5 (–10.4 to –4.7); < 0.001 | –5.8 (–8.7 to –2.8); 0.011 |
| EQ-5D | |||||||
| Descriptive system | 0.78 (0.50 to 1.07) | 0.75 (0.44 to 1.06) | 0.76 (0.46 to 1.06) | 0.74 (0.40 to 1.07) | –0.01 (–0.39 to 0.37); 0.782 | –0.03 (–0.33 to 0.28); 0.270 | –0.02 (–0.36 to 0.33); 0.607 |
| Visual analogue scale | 73.5 (70.7 to 76.3) | 70.3 (67.3 to 73.3) | 76.2 (73.5 to 78.9) | 72.3 (69.3 to 75.4) | 0.7 (–2.9 to 4.3); 0.832 | 2.8 (–0.2 to 5.7); 0.214 | 2.0 (–1.1 to 5.2); 0.442 |
| SAQ | |||||||
| Pleasure | 11.8 (10.3 to 13.3) | 10.4 (8.6 to 12.1) | 11.3 (9.7 to 12.8) | 10.9 (9.3 to 12.5) | –1.1 (–3.2 to 1.0); 0.323 | –0.5 (–2.3 to 1.2); 0.487 | 0.6 (–1.2 to 2.3); 0.482 |
| Discomfort | 4.6 (3.8 to 5.5) | 5.0 (4.1 to 5.8) | 2.3 (1.3 to 3.4) | 1.7 (0.8 to 2.7) | 0.9 (–0.5 to 2.3); 0.075 | –2.3 (–3.5 to –1.1); < 0.001 | –3.2 (–4.4 to –2.1); < 0.001 |
Surgical interventions
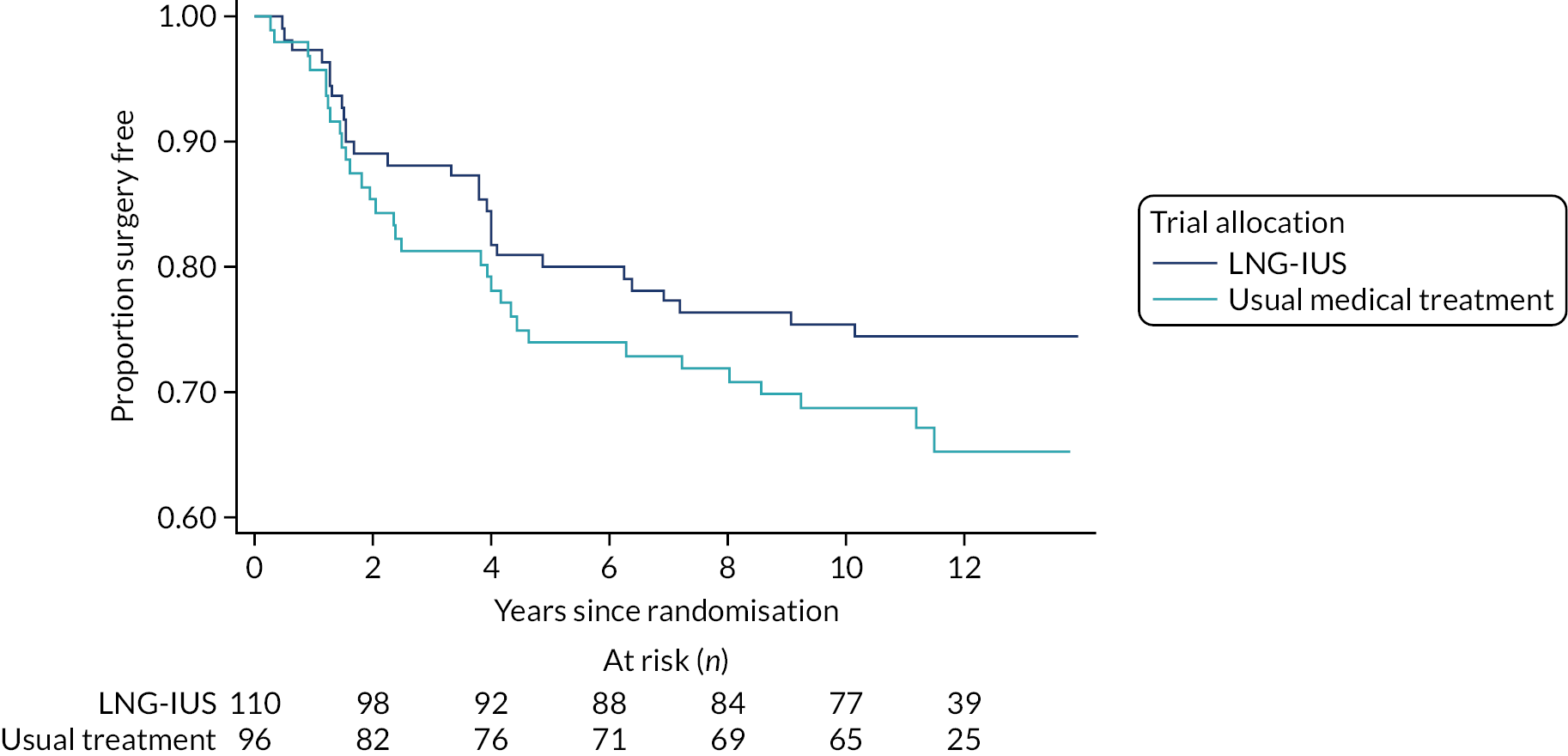
Over the 10-year follow-up period, 60 out of 206 (29%) women had received a surgical intervention, either hysterectomy (n = 34, 16.5%) or endometrial ablation (n = 26, 12.6%), as reported in Table 4. No woman had undergone both procedures and no woman who had undergone a surgical procedure reported HMB at 10 years. The cumulative rate of surgery was slightly lower among women initially allocated to LNG-IUS (28/110 women, 25%) than among those allocated to usual medical treatment (32/96 women, 33%) in the ECLIPSE trial. Considering the opposite outcome (i.e. the surgery-free rate), including all data collected over a median follow-up time of 11.2 years, the cumulative surgery-free rate was 74% for LNG-IUS and 65% for usual medical treatment (Figure 2), and the difference was not statistically significant (hazard ratio 0.73, 95% CI 0.44 to 1.21; p = 0.22).
FIGURE 2.
Surgery-free time for all responders by original ECLIPSE trial allocations.
Chapter 4 Qualitative study
Objectives
As noted in Chapter 1, most evidence on the treatment of HMB has focused on the effectiveness of medical treatments or surgery in reducing menstrual blood loss,9 and more recently on reducing the impact of HMB on quality of life. 28,29 However, less is known about women’s qualitative experiences of HMB in relation to their treatment over time, which may involve trajectories of many years. In this qualitative study, we approached women who 10 years previously had presented to primary care with HMB and had been initiated on treatment. The aim was to explore women’s treatment ‘journeys’ and experiences of HMB in order to contextualise the results on 10-year outcomes reported in Chapter 3 and to enhance the utility of findings for application in clinical practice. The qualitative study aimed to explore women’s experiences of and decisions about HMB treatments or surgical interventions to provide insight into their choices and what influenced them over this extended time period.
Methods
Sampling
A purposeful sample was selected from women participating in the 10-year follow-up study described in Chapter 2. We used women’s responses on their self-reported questionnaires, including free-text comments, to select a sample with diverse demographic characteristics and a range of reported treatment trajectories. The sample included women of differing age, ethnicity, socioeconomic status [based on the Index of Multiple Deprivation (IMD) derived from their current postcode using https://imd-by-postcode.opendatacommunities.org (accessed 16 July 2021)], self-reported menopausal status and HMB status and receiving different treatments (i.e. single medical treatment, single surgical treatment or multiple surgical and medical treatments).
Recruitment and consent
The consent form to take part in the 10-year observational study included a section to indicate the participant’s interest in an optional interview. Participants selected for an interview were sent a postal invitation and a participant information sheet for the qualitative study, and were asked to return a separate consent form for the interview using the freepost envelope provided. We aimed to recruit a purposeful sample of up to 30–40 women. Once the consent form was received, the research assistant (BD) contacted the participant by telephone to schedule either a telephone or a face-to-face interview, according to their preference, and at a convenient time for the participant. Consent was further gained verbally at the beginning of the interview. During the COVID-19 pandemic, we were unable to offer face-to-face interviews or gain written consent, and an ethics and protocol amendment was approved to gain consent solely verbally to allow interviews to continue by telephone (in the period April to December 2020).
Data generation and analysis
All semistructured telephone and face-to-face interviews were conducted by a female researcher (BD). Interviews were audio-recorded and transcribed verbatim. The interviewer encouraged participants to speak freely about their experiences, and followed broad topic prompts that were developed initially with the help of two study PPI advisors and reviewed and refined after early interviews. Questions aimed to explore women’s experiences of and reflections about their HMB, treatment trajectory, the influences on treatment decisions, and the impact of HMB. The topic guide was reviewed by two study PPI advisors and refined in response to their comments (see Appendix 1).
The researcher made contemporaneous field notes during the interviews, noting issues arising and reflections immediately after each interview. Coding of interview transcripts was aided by application of NVivo (QSR International, Warrington, UK) software, with the field researcher and a senior researcher with a background in clinical primary care each identifying emerging themes from the data52 and then developing these together. This was aided by contemporaneous reflexive notes from the interviews to clarify contextual or other issues. Data generation and analysis were iterative, each informing the other, with further purposeful sampling of women and data generation used to extend and challenge earlier data and interpretation. This tested the integrity and credibility of the analysis until no new themes emerged, suggesting saturation.
Member checking
To check and potentially further refine the interpretation of data, all interviewee participants were invited to review and comment on a summary of preliminary findings from the analysis of interviews in a process of member checking. 53 Study PPI advisors commented on and helped refine the readability of the summary prior to its circulation to participants, and also reviewed and commented on the findings themselves.
Findings
Purposeful sample
A total of 145 out of 206 (70%) women responding in the observational follow-up study returned their consent form to be contacted for potential interview over a 17-month qualitative study recruitment period (August 2019 to December 2020). This enabled incremental selection for sampling from a large and diverse range of participants willing to share their experience. Ultimately, a purposeful sample of 36 women was interviewed, including women with a range of ages, social diversity and educational background, and who had varied treatment experiences and trajectories. The characteristics of these women are summarised in Table 7.
| Characteristic | Purposeful sample (N = 36) |
|---|---|
| Age (years) | |
| Range | 41–61 |
| Median | 55 |
| Mode | 60 |
| Mean | 54 |
| Self-defined ethnicity, n (%) | |
| White British/English | 30 (83) |
| South Asian | 2 (6) |
| Mixed white/African | 1 (3) |
| Black British/Caribbean | 3 (8) |
| Highest formal educational attainment, n (%) | |
| No qualification | 5 (14) |
| GCSE or equivalent | 13 (36) |
| NVQ3/A Level or equivalent | 8 (22) |
| Undergraduate degree | 6 (17) |
| Postgraduate degree or higher | 4 (11) |
| Treatment, n (%) | |
| Single medical treatment | 14 (39) |
| Still using coil | 4 (29) |
| No longer using coil | 6 (43) |
| Never had coil | 4 (29) |
| Single surgical treatment | 5 (14) |
| No longer using coil | 2 (40) |
| Never had coil | 3 (60) |
| Multiple surgical and medical treatments | 15 (42) |
| Single medical treatment still using coil | 4 (27) |
| Single medical treatment no longer using coil | 10 (67) |
| Single medical treatment never had coil | 1 (7) |
| No treatment used between 5 and 10 years | 2 (6) |
The sample selected had a similar age range, IMD and broad ethnic distribution to all women expressing interest in being interviewed (n = 145), and also to all women completing the 10-year follow-up (n = 206) (Table 8). The current study sample also had a similar ethnic distribution to that of the whole original trial cohort (n = 571) reported at baseline28 (82% white, 9% Asian, 5% black and 3% mixed/other).
| Characteristic | Purposeful sample chosen (N = 36) | Consenting and willing to be interviewed (N = 145) | All responders at 10-year follow-up (N = 206) |
|---|---|---|---|
| Age (years) | |||
| Range | 41–61 | 40–61 | 40–63 |
| Median | 55 | 55 | 55 |
| Mode | 60 | 55 | 56 |
| Mean | 54 | 54 | 54 |
| Ethnicity, n (%) | |||
| White British | 30 (83) | 134 (92) | 185 (90) |
| Othera | 6 (17) | 11 (8) | 21 (10) |
| IMD category,b n (%) | |||
| Unknown | 0 (0) | 2 (1) | 4 (2) |
| 1–3 | 16 (44) | 49 (34) | 68 (33) |
| 4–7 | 13 (36) | 53 (37) | 80 (39) |
| 8–10 | 7 (19) | 41 (28) | 54 (26) |
Influences on experience and treatment decisions for heavy menstrual bleeding over time
Quality of interactions and relationship with health-care professionals
Women’s experience of health-care interactions and their relationship with clinicians was a principal influence on their choices regarding and experience of treatment for HMB over time. However, over half those interviewed reported consistently positive experiences of their initial and subsequent health-care interactions, even if this resulted in them receiving multiple treatments. This is because they trusted their GP or gynaecologist and felt fully informed about all of their options and realistic expectations were set about the likelihood of success for each intervention in response to how their HMB had improved or remained problematic. Women’s accounts underlined the powerful influence of clinicians’ communication in this context and the value of joint decision-making in discussing what may work best for women as individuals:
I felt he included me in any decisions he was making and he sort of didn’t say this is what you must do, he said how about if we try this and see how you get on? . . . Yes, trusted him 100%.
N016, 55 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
They would say we don’t know if it will work [name of GP] you know but it is an option, give it a try . . . once you don’t trust your doctor it is a bit well where do you go and what do you do?
N181, 55 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS
By contrast, some women with negative experiences of health care, characterised by less communication or information-sharing, felt that they were denied the treatment that they may have preferred throughout the course that their HMB had taken. These women did not feel that they had a say in treatment decisions or had felt less informed or uninformed about their options or the treatment that had been given at different stages:
I feel as if the hysterectomy could have been discussed a bit more, I knew why I was having it because of the bleeding erm but I didn’t realise the . . . the overall effects of it . . . I think maybe that could have been explained a bit more because none of that was explained. Whether I would have still had it [hysterectomy] or not I don’t know . . . because when you are having bleeding like that you just want it to stop. Erm so I do think it would have been nice to know sort of what . . . you know what could have happened . . . I don’t know what [other] options there are.
N012, 56 years, no longer experiencing HMB; LNG-IUS, then hysterectomy
Women could be concerned that they were not being taken seriously or were being ‘fobbed off’. They felt that their HMB may not be considered a legitimate problem that justified medical treatment or was not recognised for its emotional impact:
[Doctors] just kept trying to say it is nothing and just kind of fobbing me off . . . it is almost as if they don’t understand the gravity of it and the seriousness of it . . . But it does, it affects your life, doesn’t it?
N301, 45 years, still experiencing HMB; contraceptive pill, then LNG-IUS
Sometimes . . . they are like a mechanic, they go in and they fix something and then leave it again, they don’t think about the emotional aspects or the physical things.
N238, 57 years, no longer experiencing HMB; LNG-IUS
Some speculated that their GP had not referred them to secondary care because of concerns about cost. Others perceived that some clinicians, particularly those who were male, may deny them treatment. They reported often having to push for something to be undertaken when their HMB was not improving:
. . . I felt sometimes they used to just say ‘oh have these tablets and you will be all right’ but I wasn’t – and I did go back and I don’t like to be a nuisance, but I just couldn’t understand why they just didn’t . . . give me a hysterectomy . . . because I didn’t want any more [children].
N004, 55 years, no longer experiencing HMB; tranexamic acid
I did look around [information] and I had asked . . . would I be able to have that? And it was always, ‘loads of women have fibroids, you just have to kind of put up with it’.
N307, 44 years, no longer experiencing HMB; tranexamic acid, subsequent fibroids identified and hysteroscopic procedure, then declined LNG-IUS, then had a hysterectomy
Women also reported their disappointment when there was a lack of communication between primary and secondary care or when they had experienced less continuity of care from their general practice, with attendant consequences for their treatment:
I don’t know, he was just sort of like ‘you’re being dismissed’ . . . I just think if I had had [my] proper doctor [GP] I probably would have had the Mirena coil put back in.
N139, 60 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS, then tranexamic acid
Women’s life transitions and concerns
Women reflected on other factors and motivations affecting their treatment for HMB and their related decisions. This included changing considerations in their lives or health concerns. Available treatment choices for HMB in relation to fertility had a major impact on women who experienced HMB, particularly when younger and anticipating trying to start a family or wanting to retain the future option to do so. Oral contraception or tranexamic acid could be continued for a readily reversible or no contraceptive effect, respectively, and for other reasons such as familiarity with the treatment, even though its effects on HMB might be partial and so oral treatment was continued rather than subsequently considering the LNG-IUS. This similarly influenced decisions to avoid or delay endometrial ablation. In others not wishing to have children, progression through oral medical treatment and then the LNG-IUS treatment were successful and contrary to their expectations that surgery would be needed:
I had decided not to have children, I thought it would be so much easier if I had a hysterectomy or something.
N242, 50 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS successful
When other medical treatments had not helped, more invasive intervention by hysterectomy was also not deemed feasible for some women owing to the impact on their life from having surgery, although the increasing availability of less invasive endometrial ablative procedures made this more possible:
They did give me the option of the hysterectomy, but they said I would be out of action for 6 weeks and I thought how can I not drive the kids to school for 6 weeks?
N019, 49 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS, then endometrial ablation
Health concerns about some medical treatment options manifested in two main ways, with some women wanting to avoid hormone-based treatments, or women favouring surgery over medical treatments to remove concerns about future sinister risks, such as cancer:
I was originally on the pill to start with but because my mum and dad both had cancer that always made me very reluctant to continue anything hormone based.
N213, 55 years, no longer experiencing HMB; LNG-IUS, then fibroids removed
There would be no site for ovarian cancer or anything like that, so I had the whole lot taken away.
N228, 58 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
Women described how the influences on their treatment decisions changed as they approached menopause. This could include persevering with medical treatments even if these had less effect on their HMB than desired, or opting for endometrial ablation after the failure of oral treatments or the LNG-IUS as they waited for natural menopause to occur:
I think at that point I realised my next step was a hysterectomy. [But] I kept thinking at that point I was getting nearer what I thought would be the menopause.
N462, 59 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS, then endometrial ablation
Several women reported retaining their LNG-IUS for fear that their HMB might return or using this as a form of menopausal hormone replacement therapy. Others wanted to avoid surgical intervention by hysterectomy, perceiving that this may cause them to enter menopause too early, and this further influenced some to opt for less invasive endometrial ablation:
Mirena . . . I still kept with it because I just was so nervous about going back to my life [with HMB] because I didn’t know I was kind of reaching the menopause . . . I was so frightened about going back to what I used to have.
N285, 54 years, no longer experiencing HMB; LNG-IUS
It is overdue [Mirena] it must be about 2 or 3 years overdue [being removed]. It is not affecting me in any way and [name of doctor] said [in relation to HRT] it would reduce the amount of hormone it is putting in you.
N242, 50 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS retained after menopause
I refused [hysterectomy] because I didn’t want to go into the menopause in my thirties at all and I am glad that I didn’t do that, it was just [had] my second daughter . . . it just seemed quite an extreme intervention.
N301, 45 years, still experiencing HMB; contraceptive pill, then LNG-IUS, declined hysterectomy in mid-30s (not clear how this was discussed by clinician), unable to have endometrial ablation
Shared experience with others
Mothers, friends or other family members often influenced women’s decisions and helped inform their preference for treatment that they expressed to clinicians. This could reflect others’ beliefs or others sharing their positive or negative experiences of treatments for their HMB, shaping participants’ treatment seeking, subsequent choices and trajectories accordingly:
I have spoken to my mum about it and she was like ‘no take it out [Mirena], you shouldn’t have these things sort of stuck inside you anyway’ or whatever so erm I had it taken out.
N202, 52 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS (removed), then hysterectomy
My mum had . . . a hysterectomy and . . . was absolutely fine so I thought if there is something in there that shouldn’t be in there anyway . . .
N206, 53 years, no longer experiencing HMB; mefenamic acid and tranexamic acid, then endometrial ablation
Although such shared experiences could raise women’s awareness of different treatments, there was some recognition that individual women may respond differently and that, as medical understanding of HMB and treatments changed, some information from parents or peers may be outdated. Some participants had a good understanding of this, whereas others’ knowledge still drew on their parents’ generation and the use of hysterectomy as a ‘first’ treatment for HMB:
I asked if I could go on the pill because I had heard that would help . . . my sister . . . had quite a lot of information and she tried a few different things herself.
N127, 43 years, no longer experiencing HMB; mefenamic acid, then contraceptive implant
. . . I said to my eldest daughter see if the doctor will give you norethisterone . . . everybody you know their bodies are different, what has worked for me might not work for my daughter you know what I mean?
N214, 56 years, no longer experiencing HMB; endometrial ablation unsuccessful, following this discussed LNG-IUS but contraindicated, managed with norethisterone
Some women did not have relationships with family members or friends through which they could gain menstruation knowledge through shared experience, or did not feel comfortable talking about HMB with them. They felt that support networks, including social media, would have helped them to be more aware that HMB is a problem. Many women who were interviewed did not routinely use the internet as a resource when their HMB became troublesome but now reflected on the increasing benefits of online sources for gaining menstruation knowledge and awareness of treatment choices to make more fully informed decisions.
To further illustrate the variety of women’s experiences of HMB and their ‘treatment journeys’ over time, four women’s case stories are presented in Box 1.
This woman’s problems with HMB started when she was 39 years old. She described her periods as very heavy and prolonged. She felt very ill during menstruation and had to change the ‘biggest size Tampax [Procter & Gamble, Cincinnati, OH, USA]’ every hour.
Her mood and confidence were affected by her HMB and she adjusted life to fit around her periods. She was unable to do certain activities, such as longer walks, when menstruating. Her work was affected and she felt vulnerable as a result: ‘. . . didn’t feel like I could talk to my boss even though she was female as she may think I’m dirty, and I’m the cleanest person in the world, she may have wanted to get rid of me’.
She had felt that her HMB was ‘normal’ and to be expected with getting older. She spent 4 years enduring her HMB before she approached her GP, saying she had ‘just got on with it’, and did not talk to friends. She reflected that at that time she would have benefited from a support group or forum ‘where women can have opportunity to get together to meet other people with same experiences’.
Presenting to her GP with HMB, at 43 years old, she was recruited into the ECLIPSE trial and had a LNG-IUS inserted. She felt having the LNG-IUS ‘gave me my life back’. She still had periods, but they were a lot lighter and more manageable for her.
The LNG-IUS was removed and replaced 5 years later, but following referral to a urologist she was advised that it could be contributing to her urinary problems and should be removed. She did not do so for over a year as she was very afraid that her HMB may return.
At 51 years old, with a blood test confirming that she was in menopause, she had the LNG-IUS removed and experienced no further HMB. She also felt that the LNG-IUS had benefits hormonally before this.
Reflecting on her experiences at the outset, she had not been aware that effective treatment for HMB was available. She did not talk about her HMB because of the societal taboo and hopes that greater awareness can be raised to ‘help defuse the taboo and get people talking’.
Multiple medical treatments N429, 48 years, no longer experiencing HMB; mefenamic acid, then contraceptive pill, then repeated LNG-IUS.This woman’s menstruation started at the age of 9 years, with HMB problems developing in her early twenties. This interfered with her everyday life, with embarrassing and unexpected flooding of her clothes, including in public settings. Her periods were unrelenting, sometimes lasting up to 35 days. She became severely anaemic, with extreme tiredness. She had to stop being a blood donor. She would isolate herself on the days when she was bleeding but would otherwise carry additional supplies of sanitary protection and clothes when she had activities she could not avoid.
She had HMB for 3 or 4 years before she consulted her GP in her late twenties. She tried mefenamic acid initially but experienced side effects and was referred to a gynaecologist, who replaced this with the progesterone-only contraceptive pill. This did not alleviate her HMB. She then had a LNG-IUS inserted and had irregular bleeding. This was not heavy to her, ‘what everyone else would consider normal on a heavy day’, and she experienced the LNG-IUS as effective for 18 months.
She had a 6-month break from the LNG-IUS to see if her HMB was still happening, and it did return. She, thus, had a second LNG-IUS inserted, which she found ‘brilliant, lasted for full 5 years’. She then experienced some breakthrough bleeding and investigations showed small fibroids. The LNG-IUS was embedded and removing it necessitated a surgical procedure.
She had a third LNG-IUS inserted some months later, with further breakthrough bleeding (and normal investigations), and this was replaced with a fourth LNG-IUS 5 years later. Despite the problems, especially with her second LNG-IUS, she has found using the LNG-IUS over several years to be effective for her. Overall, she reports having a positive experience of her health care, feeling that she had always been well informed by her GP about what treatments were available. This meant that she ‘knew I wasn’t being annoying and bothering her [GP]’.
She was concerned about the general lack of awareness of HMB. She suggested inserting leaflets in tampon packs to ask ‘are you experiencing heavy periods?’, so that women may know that this is not normal and that something can be done if necessary. She wanted boys to be educated about this too, starting at school age, to reduce the taboo and so that they understood what women may experience and could be supportive.
Medical and surgical treatments N345, 58 years, no longer experiencing HMB; tablets ‘over the counter’, then LNG-IUS, then fibroids identified, then endometrial ablation, then hysterectomy.This woman did not experience HMB until her late forties. As she got older her periods were heavier, progressively more painful and more prolonged than her usual cycle, to the point that they became ‘unmanageable’. HMB at work became debilitating; she worked as a teacher and was fearful of an embarrassing flooding incident, causing her high levels of anxiety. She felt very unwell physically and would frequently vomit and pass very large blood clots. Her mood was affected; she would feel irritated and snappy, affecting those around her. She would actively avoid making plans when she knew she would be menstruating and found that holidays were ruined because of unexpected HMB. Her husband was seriously ill, which made her feel guilty about seeking medical help, so she ‘normalised’ her HMB, reflecting ‘I thought it may just go away’, and used over-the-counter tablets from the pharmacy to help.
She waited until she was aged 49 years to see her GP following a flooding incident on a friend’s sofa, about which she was mortified. Recruited to the ECLIPSE trial, she had a LNG-IUS inserted, which did not help her HMB and it ‘came out on its own’. She continued to have ‘horribly big blood clots that scared me a bit’.
Fibroids were identified after investigations. She then had an endometrial ablation, which also did not improve her HMB. She subsequently opted for vaginal hysterectomy and was relieved that ‘something had been done’.
She had a male GP and felt she ‘had to satisfy him there wasn’t something I was moaning about’. She had felt embarrassed and shame about her HMB, which she felt came from her upbringing and led to her not talking to anyone about it and normalising her problem: ‘I didn’t perceive myself to be unwell’ or having a ‘medical condition’.
Still experiencing HMB N301, 45 years, still experiencing HMB; contraceptive pill, then LNG-IUS, declined hysterectomy in mid-30s, unable to have endometrial ablation.This woman described having always had heavy periods since her teens, with her HMB becoming problematic after having her second and final child in her early thirties. Her periods were very heavy, and she used tampons and thick sanitary pads simultaneously, changing them every couple of hours, to conceal her menstruation. She experienced terrible pain, shakes and sweating, at which times she ‘couldn’t function in any way’ and would be sent home from work. She was found to be anaemic after presenting to her GP with tiredness.
Family relations were affected by the hormonal fluctuation and mood swings she experienced with her HMB. She described feeling paranoid about her HMB, carrying precautionary supplies, checking seats and wearing dark clothes. She felt ‘mortified, humiliated and actually upset’ after some instances of flooding. Her menstruation was usually regular so she would plan her activities around her HMB but on occasion would ‘let friends down’.
She sought help from her GP when she was aged 34 years because her HMB was affecting her life, and she entered the ECLIPSE trial. She initially tried an oral contraceptive pill, which did not help. She then had a LNG-IUS inserted, which she was happy to try because she had heard positive reports from friends. However, her bleeding continued and also became irregular, which was unusual for her and prevented her being able to manage her HMB by knowing when to expect her period. She did not feel that her LNG-IUS had been inserted properly, and after some months of HMB continuing she had this removed and was referred to gynaecology.
She was offered a hysterectomy after her LNG-IUS was removed but was concerned about entering the menopause in her mid-thirties, and is glad that she declined. It was not clear how hysterectomy was explained at this time. She was not offered other options and so ‘just managed’ with tablets and precautions.
Around 3 to 4 years later, colleagues told her about endometrial ablation and how this had worked for them. Her GP referred her for this but the procedure was deemed unsuitable because of the position of her womb. She has, thus, had to manage her HMB in her mid-forties with tablets and precautions, and still feels significantly affected by her HMB.
She has experienced her health care as negative. She did not feel that her problem was taken seriously and has ‘felt I had to push’ to try treatments to help.
Impact of heavy menstrual bleeding and its negotiation
The impacts of HMB on women were often profound and debilitating across multiple aspects of their daily lives. Women recognised their subjective individual experiences of ‘heavy’ menstrual bleeding, and that these may be different in different women. However, their reported experiences had much in common. Participants described how their menstruation commonly included episodes of flooding and the unpleasant release of clots. They described precautions that they would take to manage or conceal their volume of blood loss. They highlighted the economic burden of needing large amounts of sanitary products, and the toll of soiling bed linen, underwear and clothing:
To me mine is really heavy but you might think yours is heavy and it is not as much as mine.
N266, 61 years, no longer experiencing HMB; mefenamic acid, then removal of polyp and LNG-IUS inserted
The clots that had come out were so big that they thought it was a miscarriage and I came home and I was just really upset and it was literally pouring out of me like a tap . . . you wouldn’t always get a warning.
N202, 52 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
Having to throw clothes away because there was so much blood on them . . . wearing two pads and a tampon when I went to bed at night and then I would get up and it would be completely flooded.
N307, 44 years, no longer experiencing HMB; tranexamic acid, subsequent fibroids removed, then declined LNG-IUS, then hysterectomy
Some women were able to minimise the impact of HMB by planning social events and activities around their regular cycle, often managing this through avoidant behaviours, although this isolation was also upsetting. However, a higher proportion of the women interviewed did not have a regular cycle, which would lead them to cancel plans at the last minute, to the detriment of their social relationships, or having the inconvenience of being prepared to manage the bleeding.
Intimate relationships suffered in the context of HMB owing to the common experience of a lack of libido and prolonged or heavy bleeding itself preventing sexual activity. Some women attributed the breakdown of their relationships to HMB. Women also experienced mood swings or premenstrual tension in relation to their HMB, which further impacted their relationships, both intimate relationships with a partner and relationships with family and friends, creating associated guilt:
I got divorced when my daughter was eight and . . . our sex life sort of dwindled because of all of this [HMB] so I mean . . . I think relationships were affected . . . it sort of did away with my libido really.
N123, 55 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
The impact of HMB on women’s working lives could be far-reaching. Women spoke of enduring embarrassment and stigma when experiencing episodes of flooding. They experienced anxiety and felt pressure to conceal their menstruation in the workplace. In some cases, women attributed the loss of their employment to their HMB because they had needed to take sickness absence on multiple occasions:
Yes, I used to just ring in sick and not . . . I couldn’t go in in the end I was just like erm . . . lost my job really.
N004, 55 years, no longer experiencing HMB; tranexamic acid
I couldn’t go to work some days because I was just flooding . . . and I found myself making excuses because I had a male manager and I didn’t want to tell him what the problem was you know and then I started to think ‘oh God I am going to lose my job if I carry on like this’, it really affected me.
N123, 55 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
Pain was commonly a comorbid issue for women with HMB, further undermining their ability to undertake usual daily activities. They experienced aching and cramping, shaking, and feeling tired and drained. Multiple impacts of HMB took a toll on women’s emotional well-being. Women reported regularly feeling anxious and low and lacking confidence, often having to rely on someone else to fulfil the daily activities that could not be avoided, such as food shopping and school runs, but then feeling like a burden. Overwhelmingly, women reported feeling anxiety, embarrassment and shame about HMB across the whole spectrum of life, including work, social activities, relationships or daily activities, and in the context of the societal taboo and stigma surrounding HMB. Some participants themselves, despite actively volunteering to discuss their menstruation for the study, avoided certain terminologies relating to their HMB, suggesting embarrassment:
I was just mortified, it always made me really anxious I would get very tearful erm I think because I was just scared.
N181, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS
I did worry you know that it might smell, or you know it is just that have you leaked through, can anyone see you know? I tended to sit on something you know because I was always embarrassed as to like oh my god have I gone through?
N483, 61 years, no longer experiencing HMB; tranexamic acid
Unsurprisingly, women had concerns about the volume of blood loss with HMB and its implications, commonly experiencing iron-deficiency anaemia and related hair loss. This sometimes preceded recognition that their HMB was the cause. Women reported a low awareness of HMB and that it was not taken seriously because it related to menstruation:
To be fair I think if anybody else had bled for that long a time they would take them in and like transfuse them. Because it is coming from that part of your body, nobody cares really do they?
N429, 48 years, no longer experiencing HMB; mefenamic acid, then contraceptive pill, then LNG-IUS
My hair was coming out in clumps and stuff and then he told me I have got it because you’re severely anaemic . . .
N202, 52 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
Perception and normalisation of heavy menstrual bleeding
Discussing their perceptions of HMB, some women spoke of starting from a position of a lack of menstruation knowledge and awareness because HMB had not been spoken about in their childhood, or created embarrassment with their parents, and this continued into their adulthood and subsequent relationships. Generational embarrassment and not talking about HMB could reduce the likelihood that it would be recognised as a problem that might be treatable, and women did not proactively seek help:
I didn’t feel like I could talk to my mum about it . . . I thought that was normal . . . I didn’t know there was something I could do about it.
N127, 43 years, no longer experiencing HMB; mefenamic acid, then contraceptive implant
I just spent all of my life putting up with it instead of doing something about it. I am of that generation . . . it is sort of put up and shut up.
N266, 61 years, no longer experiencing HMB; mefenamic acid, then polyp removal and LNG-IUS inserted
Most women who were interviewed had tended to normalise the impact of their HMB, perceiving that this problem happened to everyone and ‘one just had to get on with it’ as ‘a woman’s problem’:
You think ‘oh it can’t be that bad . . . I am sure it will get better’, you know ‘oh really do I want to bother them with this?’.
N056, 41 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS, then endometrial ablation
I just thought this is normal, you know, I have just got to stick with it until the menopause [laughing], it never really occurred to me to try and seek help.
N191, 60 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS
Recognition of heavy menstrual bleeding as a problem
Women moved to recognising that they had a problem in several common ways. These included their partners noticing the impact that HMB was having on their life or sharing their experience with friends and colleagues, who encouraged them to seek help. When their life with HMB, sometimes after several years, became unmanageable or embarrassing, or after they had had traumatic experiences of flooding (particularly when this occurred in a public setting, in the workplace or out at a social event), they sought help from their GP:
I think when it started causing problems with going to work and I started talking to people and they said ‘oh no that shouldn’t be happening’ and I suddenly realised well maybe I better go and sort this out.
N123, 55 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
it just got me down erm but it was . . . probably 4 or 5 years before I kind of did anything you know about it.
N051, 60 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
I didn’t really come to the conclusion . . . that really you shouldn’t have to accept it until I was in my forties. I just became more confident . . . it is affecting my life.
N246, 46 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
Understanding and making sense of heavy menstrual bleeding
A common frustration for many women was the apparent lack of a medical or pathological explanation for their HMB. They pondered ‘why me?’. Almost half of the women who were interviewed did not recall having investigations to determine any cause of their HMB or noted that investigations were conducted only after the failure of the first or second treatment attempt. Women had previously been assessed in primary care and were eligible for initiation of treatments in the ECLIPSE study if pathology such as fibroids had been excluded clinically. Over time, those women who did later have problems identified, such as the development of polyps and fibroids, were able to make better sense of their HMB and subsequent decisions about treatment:
I went to have another gynaecologist and they found these polyps so they sort of scraped my womb a little bit more and then . . . the past 2 years I have not had a period at all.
N019, 49 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS, then endometrial procedure
I had no problem whatsoever after I had the fibroids removed . . . I don’t know erm whether they knew about my fibroids [before] and then they kept doing other stuff [treatment] . . . but I just felt it was a slow procedure [process] and I think to myself they would have got me in sooner to have the operation, I wouldn’t have had that accident like I did.
N036, 60 years, no longer experiencing HMB; contraceptive pill, then tranexamic acid, then fibroids removal
Some women had been advised to lose weight to help their HMB or before they tried a treatment. One participant described doing this and losing 5 stone, but the HMB had persisted and, subsequently, fibroids were identified:
[I was told] that I am a bit overweight and if I lost weight maybe that would solve the problem.
N307, 44 years, no longer experiencing HMB; tranexamic acid, subsequent fibroids identified and treated, then declined LNG-IUS, then had hysterectomy
In making sense of their HMB, women also referred to getting older and approaching menopause. Some delayed seeking help because they thought that they would soon undergo menopause. Other women attributed their HMB to childbirth or an inherited cause:
I have always had heavy periods but after the birth of my only child it got a lot worse.
N123, 55 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
. . . [L]ike my daughter and granddaughter are going through it now, it must be a family thing because my mum had an emergency hysterectomy at 44 because she was haemorrhaging, you know I and sort of started around the same time.
N214, 56 years, no longer experiencing HMB; endometrial ablation, discussed LNG-IUS but contraindicated, managed with norethisterone
Taboo and stigma of heavy menstrual bleeding
Women pointed out that menstruation and HMB remains a taboo subject. They pointed out that HMB is rarely spoken about openly, or publicly portrayed, reinforcing people’s embarrassment and the taboo around the issue, contributing to wider lack of awareness and knowledge that it can be helped:
It is silly really because you know half the population of the world you know have a period and it is . . . I don’t know why it is such a taboo still.
N012, 56 years, no longer experiencing HMB; LNG-IUS, then hysterectomy
I remember my mum having the discussion with me about periods and she just handed me a little book and said ‘read that’ . . . If you’re not discussing it with your mum and your sister, the prospect of discussing it with friends or perfect strangers is just, you know it is never going to happen is it?
N191, 60 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS
Taboo and stigma were often linked to embarrassment when men were present in any scenario; this could be in a work setting, relationship or health-care encounter with a clinician. Participants thought that men should be more aware and understanding of HMB’s impact on women and how this could help:
To start with my GP was quite an elderly gentleman and he was lovely but I don’t think I was probably as open with him erm as maybe I have been with ladies.
N181, 55 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS
All of my superiors [at work] were men . . . so that made it uncomfortable . . . then your reporting line is men, that makes it harder, that upsets me . . . I don’t want to have to discuss that with them because it is embarrassing for me.
N202, 52 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS, then hysterectomy
Respondents referred to the taboo of HMB being reinforced by its relative absence in conversation, in the media or on television. However, there was a common feeling that a generational change is under way, with more women, including older women, working. They felt the development and availability of more diverse sanitary products for today’s generation of women and advances in treatments for HMB would also help:
I think I was cringing with embarrassment the first TV [television] advert I saw for sanitary towels and tampons and things like that . . . [laughing] but they have helped to change people’s attitudes towards these things and now you don’t think twice do you?
N191, 60 years, no longer experiencing HMB; contraceptive pill, then LNG-IUS
They are definitely are bringing it up more and more in our workplace because I think we have got so many more women working later and later in life as well . . . there is a lot more women now in high positions.
N213, 55 years, no longer experiencing HMB; LNG-IUS, then fibroids removed
In addition to the greater availability of information and access to growing knowledge and awareness from online media and platforms, women considered it important to avoid passing on the taboo about menstruation and HMB to their own children. They sought to be more open and unembarrassed than when they were younger and experiencing HMB:
A lot of people suffer erm alone really and without talking about it and I think it . . . I don’t know why it is such a taboo now because nowadays everything is so out in the open.
N123, 55 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
Challenging stigma of heavy menstrual bleeding to improve treatment experience
In relation to improving treatment experience, women made a range of suggestions to challenge the taboo and stigma of HMB. These focused on raising awareness of the problem and, in particular, ensuring that women do not suffer in silence by being empowered to seek medical help, including when any earlier treatment is not helping:
Perhaps more information about because people . . . I think women just suffer . . . just keep persevering with your doctor and keep pushing because you shouldn’t have to suffer like that every month, it is awful.
N242, 50 years, no longer experiencing HMB; mefenamic and tranexamic acid, then LNG-IUS
. . . [I]t [treatment] only happened later in life when I started pushing for it . . . before then, I never really got an answer . . . and I had mentioned the heavy periods over the years, but I have never really complained about it so whether it was because I wasn’t pushy enough . . .
N246, 46 years, no longer experiencing HMB; tranexamic acid, then LNG-IUS
Women further advocated wider initiatives across society to get people talking about HMB, for example education in schools for boys and girls, as well as in the workplace and in the media:
More education . . . if you’re talking openly about these sorts of things at home and then talking openly about them at school then it becomes normal and it is OK it is not embarrassing to talk about it because everybody is talking about it . . .
N227, 54 years, still experiencing HMB; mefenamic and tranexamic acid
Coronation Street, Emmerdale [ITV Studios, London] . . . bring the storyline in you know for heavy menstrual bleeding and the process that you know a woman goes through.
N012, 56 years, no longer experiencing HMB; LNG-IUS, then hysterectomy
Is there something that we can put in the [tampon] packet . . . that talks about what is normal and for people to be able to access [help] readily?
N429, 48 years, no longer experiencing HMB; mefenamic acid, then contraceptive pill, then LNG-IUS
Respondent validation
Respondent validation took place during the second wave of the COVID-19 pandemic in the first quarter of 2021. Member-checking was undertaken of responses received from 17 women among the purposeful sample, along with their comments on the summary of preliminary findings from analysis. These confirmed and affirmed interpretation as true to participants’ experiences. No reflections contesting or additional to the findings presented were made. Examples of women’s comments are provided below:
I have just read the summary and it is brilliant. You have hit on every point of what it feels like to have HMB – and the taboo subject. It needs to be put in the spotlight. Thank you all for doing this it hopefully will help women with HMB in the future.
N139
Just read it through. All sounds good to me. I can see my lived experience in the comments.
N429
I really have nothing further to add. It has been some time since I suffered symptoms. I appreciate the work you are all doing to help others overcome the challenges they face in this area.
N202
I’m happy with the outcome [summary] of your work, its good, you have many points of view covering a lot of different experiences. Thank you for letting me give my input.
N012
It was a great study enjoyed doing it – just to let you know I’ve now gone through the change and everything is great, thank you.
N214
I agree with these and found the results interesting and was more than happy to take part. Raising awareness on this taboo should hopefully make talking about, and seeking help easier for women in the future.
N227
I have read the summary and I’m happy with it. For me, it is an accurate representation of how I felt. Thank you so much.
N238
Chapter 5 Discussion
Principal findings
The observational follow-up study of women from the original ECLIPSE trial shows that medical treatments for women with HMB can be initiated in primary care with improvement in quality of life 10 years later, and with high likelihood of avoiding surgery over this period. Our findings indicate that, among such women, who typically present with HMB in their early forties, half reach the menopause in the ensuing decade and over 40% may be expected to cease medical treatments during this time. However, a similar proportion (43%) continue to use the LNG-IUS alone or in combination with other oral treatments, and almost 30% will still be using the LNG-IUS after 10 years.
Relatively low rates of surgical intervention were sustained at 29% after 10 years, modestly increasing from those at 5 (20%) and 2 (10%) years after commencing treatment in primary care. 28,29 Women initially treated with the LNG-IUS were slightly less likely to need surgical intervention than those initially treated with standard medical treatments; however, this difference was not statistically significant. There were improvements over time in generic quality-of-life scores in both women who were initially allocated to the LNG-IUS and women who were allocated to usual medical treatment, with small and statistically insignificant differences between the two original groups.
The qualitative study explored women’s experiences of HMB and their treatment journeys over the decade following their presentation with HMB in primary care. This found that women’s HMB was debilitating, with wide-ranging impacts on their quality of life, from tiredness, pain, embarrassment and anxiety to economic burden and compromising their work, social activities, emotional well-being, and intimate relationships. Women had often normalised their HMB experience, reflecting wider societal and generational taboos about menstruation, and a low awareness of HMB as a treatable problem. Commonly, women had been affected by HMB for several years before they sought medical advice from their GP, when its effects on their lives had worsened, or after talking to friends.
Women’s individual responses to treatments varied, with the LNG-IUS, oral treatments or endometrial ablation working for some but not others. However, women’s treatment journeys were shaped most by their perceptions and feelings about the quality of health-care interactions with clinicians. These were positively experienced when women felt that their problem was acknowledged, at presentation or at subsequent stages if their HMB was not improving, they had a relationship of trust with health professionals, and when they felt fully informed and involved in discussing what may work best for them as individuals. Less successful or negative experiences of treatment for HMB followed poor communication by professionals, with women feeling unheard, dismissed or not taken seriously and disempowered from seeking effective treatment. Other influences on decisions about treatment for HMB over time included considerations in relation to fertility, health concerns, and views on approaching menopause or avoiding premature menopause, in addition to experiences of treatments that others had shared with them.
Strengths and limitations
This research has enabled the follow-up of women beyond 5 years after presentation to ascertain 10-year outcomes on surgical interventions and long-term rates of treatment use for HMB. To our knowledge, this has been assessed in the primary care context for the first time, and with women from the largest trial available of medical treatments for HMB. 28,32 We achieved responses from 206 women, 36% of the original trial population and 42% of those whom we could potentially recontact after 10 years. Although this number was smaller than anticipated owing to difficulties during the COVID-19 pandemic, these long-term data have not, to our knowledge, been reported before, nor at this scale for women with HMB. Women who were successfully contacted and reconsented and who provided these follow-up data were very similar at presentation, both demographically and clinically, to those women who were not followed up, lending confidence to the generalisability of the trajectories reported.
Outcomes have been assessed over a decade, an appropriate time period given the chronic nature of HMB, and using the same instruments as the original ECLIPSE trial to enable longitudinal analysis. Given the proportion of participants who had changed or ceased their original allocated treatments by 5 years, it was anticipated that comparisons at 10 years between the original randomised groups would have limited ability to demonstrate a difference for the participant-reported quality of life instruments. Furthermore, a large proportion of women had, as expected, stopped having periods, owing to either the menopause or surgical treatment, meaning that few women were able to report on the original primary outcome measure, the MMAS. Nevertheless, we have, to our knowledge, been able for the first time to illustrate the proportion of women progressing to surgical intervention by initial medical treatment.
The 10-year data reported are from women recruited to and randomised in the original ECLIPSE trial as described, and should be regarded in this context. These were women from the general population who presented to their GP with HMB, and who were assessed as clinically appropriate for and who chose to have medical treatments in the community. To our knowledge, long-term data on the natural history of women with HMB who do not seek medical help or treatment, and their quality of life over 10 years, are not available for comparison.
We proposed to collect data on treatment and surgical interventions directly from patients’ GP records, but initial cross-checking against participants’ own reported questionnaire data suggested that this process did not add value. As GP practices then became inaccessible to researchers during the COVID-19 pandemic, missing data on treatment or surgical intervention are possible but probably limited. More specific detail on timings of women ceasing or changing medical treatments would have been helpful and may potentially have been more available from GP records in the 5- to 10-year follow-up period. However, GP records of oral medication prescriptions may not reliably indicate if and when treatments were used, ceased or restarted, as such oral medications may be kept and used in an ad hoc way in real life. Some of these oral medications may also be obtained from online pharmacy services and, therefore, are not recorded by GPs. Similarly, the LNG-IUS used subsequently by some women may have been obtained through sexual health services and not routinely recorded by GPs. Women’s own knowledge and reporting of whether or not they had a LNG-IUS in situ, their use of other oral medical treatments, or of having surgery, is likely to be accurate and was most realistically achievable.
Unlike the original ECLIPSE trial analyses, missing responses on the SF-36 and SAQ were estimated from the available data, maximising the number of responses but creating small, insignificant differences in the baseline scores between these analyses and the original ECLIPSE trial. As anticipated, only a small proportion of women were menstruating and able to complete the MMAS and so comparisons were unfeasible at the 10-year time point.
To our knowledge, the qualitative investigation offers the first exploration of women’s experience of HMB and its treatment in the longer term: after presentation in primary care through to menopause. Strengths of this research include data generation with a purposeful sample that was socially diverse, engaging women with a wide range of differing treatment experiences and trajectories. Purposeful selection was directly informed by prior knowledge of individual women’s 10-year questionnaire responses, and further benefited from the unusually large number of women who were willing to be interviewed.
It is, however, recognised that qualitative findings must be interpreted with regard to the selected sample as described. Nevertheless, the sample is reported in some detail, which may aid assessment of the relevance of these findings beyond this study’s context. We also note that women participating in interviews were demographically similar to women in the wider follow-up study.
Women were interviewed by a female, non-clinical, researcher, which was appropriate to the area of enquiry and is likely to have facilitated women’s engagement in sharing their experiences fully. Analysis of data was developed by two researchers of different disciplinary backgrounds. A process of validation with respondents themselves was also undertaken, confirming the interpretation of their views and experiences as described.
Relation to other studies
This study extends findings at 5 years, showing outcomes a decade after commencing medical treatment in primary care. To our knowledge, there have been very few previous studies with follow-up beyond 5 years, and none in which treatments were initiated in primary care. Long-term data from women with HMB who do not seek medical help or treatment, and on their quality of life over 10 years, are not available for comparison with natural history. Such data would arguably be unfeasible or challenging to obtain reliably or prospectively.
In the long-term follow-up of a randomised study comparing the LNG-IUS with hysterectomy, in the LNG-IUS group of 119 women, 55 (46%) had had a hysterectomy, 44 (37%) were still using the LNG-IUS, one had had endometrial ablation and 18 were not using the LNG-IUS. 40 The higher rate of hysterectomy can be attributed to the original design of the trial, which recruited women from a hysterectomy waiting list and 24 of the 55 hysterectomies occurred in the first year of follow-up. Women allocated to the LNG-IUS in this study had a mean age at randomisation of 43 years and their scores on the SF-36 general health and physical functioning domains were very similar to the baseline scores of ECLIPSE participants. However, the number of SF-36 domains that had regressed to baseline scores by 10 years was greater in this previous study than in our study, in which all but the physical functioning scale showed sustained and statistically significant improvements. Reference values for SF-36 are known to decrease with increasing age, particularly in the general health and physical functioning domains,54 so the reduction in scores we observed is to be expected.
As our starting point was initial medical treatment, we had too few women who had had endometrial ablation to determine rate of subsequent procedures, so we cannot compare our data to previous findings of around 20% of women needing further surgery. 23
There are no recent UK data to suggest a change in patterns of treatments for HMB. Danish data between 1996 and 2017 show increasing use of the LNG-IUS and less use of oral tranexamic acid. 55 The incidence of hysterectomy decreased from 3.1 to 2.1 per 1000 person-years (p < 0.001), whereas that of endometrial ablations increased from 0.7 to 1.3 per 1000 person-years (p < 0.001). 55
Women we interviewed were as concerned with the wide-ranging effects of HMB on their physical and emotional health and quality of life as with HMB in itself. This is strongly consistent with earlier qualitative work. 32,34,56–58 Similarly, the concealment of and taboo surrounding menstruation and women’s normalisation of HMB and managing it without seeking advice have been recurrent themes in previous studies,59,60 as found here. It is concerning that our findings still echo work up to two decades earlier, including that women with HMB may feel dismissed by clinicians. 58,60–62 The concealment of and taboo surrounding menstruation contribute to low awareness and lack of open discussion about HMB in a reinforcing cycle39 of delaying or not seeking help. 4 It is striking that these issues for women remain current despite advances in medical treatment and surgical interventions for HMB. A recent systematic review to identify barriers to seeking care among women with a range of abnormal menstrual symptoms, including HMB, further highlighted the taboo of menstruation and poor experiences with GPs. 7 There is an absence of published qualitative evidence on GPs’ perspectives in this context. Further interrogation of factors influencing women’s and different clinicians’ decision-making about use of subsequent treatments or interventions might be undertaken, potentially including direct observation of health-care consultations. However, the current qualitative work adds some new insights into women’s experiences and influences on treatment decisions, and how they make sense of HMB over time. The need for greater availability of high-quality information about HMB and its treatment, while recognising the influences of family and peers on women’s expectations, is also underlined.
Implications for practice
The original trial recruited women in the community from the general population who had HMB that was affecting their lives; who chose to present to their GP with this problem; and who were clinically assessed as appropriate for, and wanted to have, medical treatment. This assessment and the range of medical treatments used in the trial (the LNG-IUS or other standard medical treatments) reflected real-life practice and the range of treatment choices available to women of any age and their GP in this setting. This remains consistent with current NICE guidance on treatment options for HMB. 9
The sustained low rates of progression to surgical intervention and the general improvement in quality of life now observed, 10 years from women’s initial presentation, underline the importance and value of initiating medical management of women’s HMB in primary care, which is where and when most women seek help from health services. Avoiding referrals to secondary care are therefore likely to reduce operative intervention rates. The findings provide helpful empirical information for women and GPs on what to expect in the longer term from starting treatments for HMB and to inform related individual clinical and patient decision-making for this common chronic episodic condition affecting women’s health. This includes women’s chances of surgery and of continuing or gradually ceasing medical treatments and an accurate estimate of 10-year retention of the LNG-IUS. Wider public awareness is also needed to encourage women to seek help for HMB if it is affecting their lives, as they are likely to benefit from medical treatments commenced in the community setting.
Despite previous research, tackling the enduring taboo and stigma of menstruation and HMB remains a major challenge for society, public and health policy in relation to improving women’s health care and treatment experience. As women interviewed underlined, wider strategies and initiatives for raising awareness are needed, including through media and entertainment, in the workplace, education in schools, online support and information with sanitary products. More specific patient informational resources and education to support women’s decision-making about HMB are also needed. Women should be aware that assessment and treatment of HMB can be helpful and be empowered to seek it.
Like others in society, health professionals may contribute to social taboo and low awareness about HMB by not asking about, or identifying women with, HMB. GPs, for example, might more routinely ask women about their experience with menstruation. GPs, and other primary care practitioners, should be cognisant of the considerable challenges that women with HMB face prior to seeking help: overcoming taboo, normalising and tolerating HMB and its physical, emotional and social effects, and fear of being dismissed or their problems not being taken seriously. In the context of clinical practice, the qualitative findings emphasise the importance and value to women of patient-centred interaction and communication.
In addition to managing HMB itself,63 health professionals should actively explore and acknowledge the wider impacts of HMB on women’s lives, and respond to these appropriately. This might involve helping women feel heard and listened to, being empathic, and offering support for anxiety, mood or challenges at work or in relationships. A clear explanation of HMB should be offered to achieve a shared understanding with women that this is either considered benign HMB with no clinical pathology suspected64 or alternatively HMB that may require investigation if history or examination suggests fibroids, polyps or endometrial pathology (e.g. persistent intermenstrual bleeding). 9
Women will value attention to good communication, use of appropriate information and shared decision-making about treatments for their HMB tailored to individual contexts, noting other influences for women and their changing needs or circumstances. For example, women may have differing treatment expectations or preferences depending on their age and requirements for contraception or fertility, and as they near the end of their menstruating lives. Ongoing care for women with HMB should also ensure clinical willingness to appropriately continue to review women’s responses and progress, their working diagnosis, or the need for further investigation or different treatment or surgical options over time. For example, this should include counselling those women considering removal or renewal of the LNG-IUS at 5 years that they may continue to benefit and avoid surgery. This approach is likely to positively affect the quality of women’s care experience and satisfaction with treatment.
Key points for further research
The are no immediate new research questions arising from the findings of this observational study. Research recommendations in the wider sphere of management of HMB are listed here but in no implied priority order as they encompass quantitative, qualitative and methodology perspectives:
-
The NICE guidelines recommend further randomised trials to determine the effectiveness of the progesterone-only pill, injectable progestogens or progestogen implants compared with the LNG-IUS or combined oral contraceptive pill. 16
-
The few recently licensed treatments for HMB either are limited to short-term use [up to 2 years in the case of the oral gonadotrophin-releasing hormone antagonist elagolix (Orilissa®; AbbVie, North Chicago, IL, USA) for bleeding associated with uterine fibroids] or, in the case of ulipristal acetate, have a limited indication owing to the potential risk of severe liver injury. Long-term evaluations of these compared with the LNG-IUS would be challenging.
-
Repurposed drugs with known safety profiles may emerge as efficacious, as in the case of low-dose dexamethasone,65 and warrant comparison with the currently recommended treatments.
-
Development of measures reflective of wider and long-term experience of HMB are needed, as existing HMB-specific quality-of-life measure such as the MMAS are limited by ceiling effects and reduced content validity when treatments such as the LNG-IUS or ulipristal acetate are used.
-
Further qualitative research might investigate the perspectives of health professionals, in particular GPs and nurse practitioners and practice nurses in primary care, alongside gynaecologists, to understand challenges they may experience seeing women with HMB and perceptions of how care may be enhanced.
-
Economic models that capture the costs and effects of pharmacological and surgical treatments for HMB across the whole of a women’s menstrual lifetime could be developed.
Conclusions
The study provides a helpful new indication of the expected proportions of women continuing to use or not use treatments for HMB, or progressing to surgical intervention, and of the significant proportion of women using the LNG-IUS after a decade. Medical treatments for women with HMB can be initiated in primary care with low rates of surgical intervention and improvement in quality of life observed 10 years later. The study supports NICE recommendations9 and confirms that many women with HMB do not require surgery as there are less invasive and acceptable alternatives.
Over half of the women interviewed had positive experiences of treatment, but the challenges others experienced provide clear messages for improving women’s health-care experience and treatment journeys. There is a need for greater availability of high-quality information about HMB and its treatment, while recognising the influences of family and peers on women’s expectations. Tackling the still enduring taboo and stigma of menstruation and HMB, with wider societal and educational strategies for raising awareness, is also underlined. Clinicians should be aware of the considerable challenges women with HMB experience at presentation and subsequently as different treatments are reviewed and tried, and the importance and value of patient-centred communication to women in this context.
Acknowledgements
The authors acknowledge the contribution of the ECLIPSE Trial Collaboration [listed in Gupta JK, Daniels JP, Middleton LJ, Pattison HM, Prileszky G, Roberts TE, et al. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system in primary care against standard treatment for menorrhagia: the ECLIPSE trial. Health Technol Assess 2015;19(88)]. The authors dedicate this project to the memory of Professor Helen Pattison (1954–2021).
We thank all women participating in the study for their time and commitment to this research. We thank our two PPI advisors Claire Mann and Farah Jamil for their valuable contributions, advice and support throughout the study, described and presented in this report, including helping to revise the Plain language summary. We thank Pamela Pepper for her administrative support and help with bibliographic referencing in producing this report. We thank the three anonymous peer reviewers for helpful comments on this report.
Contributions of authors
Joe Kai (https://orcid.org/0000-0001-9040-9384) (Professor of Primary Care): conceptualisation, writing (original draft), writing (review and editing), project supervision, investigation, analysis and funding acquisition.
Brittany Dutton (https://orcid.org/0000-0001-5146-4911) (Research Assistant): writing (original draft), writing (review and editing), investigation, formal analysis (see Chapter 4) and project administration.
Yana Vinogradova (https://orcid.org/0000-0002-3030-5257) (Medical Statistician): statistical analysis, data curation, formal analysis (see Chapter 3) and writing (review and editing).
Nicholas Hilken (https://orcid.org/0000-0002-9553-8908) (Senior Data Manager/Database Developer): data curation and writing (review and editing)
Janesh Gupta (https://orcid.org/0000-0003-3052-8423) (Professor of Obstetrics and Gynaecology): conceptualisation, writing (review and editing), investigation and funding acquisition.
Jane Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials): conceptualisation, writing (original draft), writing (review and editing), project administration, investigation and funding acquisition.
Publications
Kai J, Dutton B, Vinogradova Y, Hilken N, Gupta J, Daniels J. Medical treatment for heavy menstrual bleeding in primary care: ten-year data from the ECLIPSE trial. Br J Gen Pract 2022;72(725):e857–64. https://doi.org/10.3399/BJGP.2022.0260
Dutton B, Kai J. Women’s experiences of heavy menstrual bleeding and medical treatment: a qualitative study in primary care. Br J Gen Pract 2023;73(729):e294–301. https://doi.org/10.3399/BJGP.2022.0460
Data-sharing statement
Data requests for 10-year data should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review. Data requests for the original ECLIPSE trial data set should be submitted to bctudatashare@bham.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss – a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand 1966;45:320-51. https://doi.org/10.3109/00016346609158455.
- Janssen CA, Scholten PC, Heintz AP. Reconsidering menorrhagia in gynecological practice. Is a 30-year-old definition still valid?. Eur J Obstet Gynecol Reprod Biol 1998;78:69-72. https://doi.org/10.1016/S0301-2115(97)00275-3.
- Shapley M, Jordan K, Croft PR. Increased vaginal bleeding: the reasons women give for consulting primary care. J Obstet Gynaecol 2003;23:48-50. https://doi.org/10.1080/0144361021000043245.
- Fraser IS, Mansour D, Breymann C, Hoffman C, Mezzacasa A, Petraglia F. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynaecol Obstet 2015;128:196-200. https://doi.org/10.1016/j.ijgo.2014.09.027.
- Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the Medical Expenditures Panel Survey. J Womens Health 2013;22:959-65. https://doi.org/10.1089/jwh.2013.4288.
- van den Brink MJ, Saaltink AL, Groenhof F, Kollen BJ, Berger MY, Lisman-van Leeuwen Y, et al. Incidence and treatment of heavy menstrual bleeding in general practice. Fam Pract 2017;34:673-8. https://doi.org/10.1093/fampra/cmx050.
- Henry C, Ekeroma A, Filoche S. Barriers to seeking consultation for abnormal uterine bleeding: systematic review of qualitative research. BMC Womens Health 2020;20. https://doi.org/10.1186/s12905-020-00986-8.
- Royal College of Obstetricians and Gynaecologists . National Heavy Menstrual Bleeding Audit, Second Annual Report 2012. www.rcog.org.uk/globalassets/documents/guidelines/research–audit/nationalhmbaudit_2ndannualreport_11.07.12_forweb.pdf (accessed 9 June 2021).
- National Institute for Health and Care Excellence (NICE) . Heavy Menstrual Bleeding: Assessment and Management 2018. www.nice.org.uk/guidance/ng88 (accessed 8 April 2021).
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev 2019;9. https://doi.org/10.1002/14651858.CD000400.pub4.
- Bryant-Smith AC, Lethaby A, Farquhar C, Hickey M. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev 2018;4. https://doi.org/10.1002/14651858.CD000249.pub2.
- Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev 2000;2. https://doi.org/10.1002/14651858.CD000249.
- Lethaby A, Wise MR, Weterings MA, Bofill Rodriguez M, Brown J. Combined hormonal contraceptives for heavy menstrual bleeding. Cochrane Database Syst Rev 2019;2. https://doi.org/10.1002/14651858.CD000154.pub3.
- Stewart A, Cummins C, Gold L, Jordan R, Phillips W. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. BJOG 2001;108:74-86. https://doi.org/10.1111/j.1471-0528.2001.00020.x.
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409-20. https://doi.org/10.1056/NEJMoa1103182.
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421-32. https://doi.org/10.1056/NEJMoa1103180.
- Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565-73.e1. https://doi.org/10.1016/j.fertnstert.2014.02.008.
- International Standard Randomised Controlled Trial Number (ISRCTN) Registry . Ulipristal Acetate Versus Conventional Management of Heavy Menstrual Bleeding (ISRCTN20426843) 2015. www.isrctn.com/ISRCTN20426843 (accessed 27 April 2022).
- Medicines and Healthcare products Regulatory Agency . Ulipristal Acetate 5mg (Esmya): Further Restrictions Due to Risk of Serious Liver Injury 2021. www.gov.uk/drug-safety-update/ulipristal-acetate-5mg-esmya-further-restrictions-due-to-risk-of-serious-liver-injury (accessed 9 June 2021).
- Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ 2010;341. https://doi.org/10.1136/bmj.c3929.
- Bofill Rodriguez M, Lethaby A, Grigore M, Brown J, Hickey M, Farquhar C. Endometrial resection and ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD001501.pub5.
- Daniels JP. The long-term outcomes of endometrial ablation in the treatment of heavy menstrual bleeding. Curr Opin Obstet Gynecol 2013;25:320-6. https://doi.org/10.1097/GCO.0b013e3283630e9c.
- Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and Mirena® for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15190.
- Bansi-Matharu L, Gurol-Urganci I, Mahmood TA, Templeton A, van der Meulen JH, Cromwell DA. Rates of subsequent surgery following endometrial ablation among English women with menorrhagia: population-based cohort study. BJOG 2013;120:1500-7. https://doi.org/10.1111/1471-0528.12319.
- Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ 2011;342. https://doi.org/10.1136/bmj.d2202.
- Cooper K, Breeman S, Scott NW, Scotland G, Clark J, Hawe J, et al. Laparoscopic supracervical hysterectomy versus endometrial ablation for women with heavy menstrual bleeding (HEALTH): a parallel-group, open-label, randomised controlled trial. Lancet 2019;394:1425-36. https://doi.org/10.1016/S0140-6736(19)31790-8.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev 2021;2. https://doi.org/10.1002/14651858.CD000329.pub4.
- Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. ECLIPSE Trial Collaborative Group . Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37. https://doi.org/10.1056/NEJMoa1204724.
- Kai J, Middleton L, Daniels J, Pattison H, Tryposkiadis K, Gupta J. ECLIPSE trial collaborative group . Usual medical treatments or levonorgestrel-IUS for women with heavy menstrual bleeding: long-term randomised pragmatic trial in primary care. Br J Gen Pract 2016;66:e861-70. https://doi.org/10.3399/bjgp16X687577.
- Bancroft J. The menstrual cycle and the well being of women. Soc Sci Med 1995;41:785-91. https://doi.org/10.1016/0277-9536(95)00045-9.
- Su S, Yang X, Su Q, Zhao Y. Prevalence and knowledge of heavy menstrual bleeding among gynecology outpatients by scanning a WeChat QR Code. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0229123.
- Matteson KA, Phipps MG, Raker CA, Cronin B, Holman L. Discussion: ‘Treatment of symptomatic uterine fibroids’ by van der Kooij et al. Am J Obstet Gynecol 2010;203:e1-6. https://doi.org/10.1016/j.ajog.2010.05.017.
- Frick KD, Clark MA, Steinwachs DM, Langenberg P, Stovall D, Munro MG, et al. STOP-DUB Research Group . Financial and quality-of-life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatment. Womens Health Issues 2009;19:70-8. https://doi.org/10.1016/j.whi.2008.07.002.
- Santer M, Wyke S, Warner P. What aspects of periods are most bothersome for women reporting heavy menstrual bleeding? Community survey and qualitative study. BMC Womens Health 2007;7. https://doi.org/10.1186/1472-6874-7-8.
- Lethaby A, Farquhar C. Treatments for heavy menstrual bleeding. BMJ 2003;327:1243-4. https://doi.org/10.1136/bmj.327.7426.1243.
- Grant C, Gallier L, Fahey T, Pearson N, Sarangi J. Management of menorrhagia in primary care – impact on referral and hysterectomy: data from the Somerset Morbidity Project. J Epidemiol Community Health 2000;54:709-13. https://doi.org/10.1136/jech.54.9.709.
- Santer M, Wyke S, Warner P. Women’s management of menstrual symptoms: findings from a postal survey and qualitative interviews. Soc Sci Med 2008;66:276-88. https://doi.org/10.1016/j.socscimed.2007.08.018.
- Protheroe J, Bower P, Chew-Graham C, Peters TJ, Fahey T. Effectiveness of a computerized decision aid in primary care on decision making and quality of life in menorrhagia: results of the MENTIP randomized controlled trial. Med Decis Making 2007;27:575-84. https://doi.org/10.1177/0272989X07306785.
- Fredericks E. Short report: How family physicians can support discussions about menstrual issues. Can Fam Physician 2014;60:e194-6.
- Heliövaara-Peippo S, Hurskainen R, Teperi J, Aalto AM, Grénman S, Halmesmäki K, et al. Quality of life and costs of levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of menorrhagia: a 10-year randomized controlled trial. Am J Obstet Gynecol 2013;209:535.e1-535.e14. https://doi.org/10.1016/j.ajog.2013.08.041.
- Lete I, del Carme Cuesta M, Marín JM, Martínez M, Bermejo A, Arina R. Acceptability of the levonorgestrel intrauterine system in the long-term treatment of heavy menstrual bleeding: how many women choose to use a second device?. Eur J Obstet Gynecol Reprod Biol 2011;154:67-70. https://doi.org/10.1016/j.ejogrb.2010.07.040.
- Beelen P, van den Brink MJ, Herman MC, Geomini PM, Duijnhoven RG, Bongers MY. Predictive factors for failure of the levonorgestrel releasing intrauterine system in women with heavy menstrual bleeding. BMC Womens Health 2021;21. https://doi.org/10.1186/s12905-021-01210-x.
- Royal College of Obstetricians and Gynaecologists (RCOG) . The Initial Management of Menorrhagia 1998.
- Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: a measure of women’s sexual functioning. Qual Life Res 1996;5:81-90. https://doi.org/10.1007/BF00435972.
- Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol 1998;105:1155-9. https://doi.org/10.1111/j.1471-0528.1998.tb09968.x.
- Pattison H, Daniels JP, Kai J, Gupta JK. The measurement properties of the menorrhagia multi-attribute quality-of-life scale: a psychometric analysis. BJOG 2011;118:1528-31. https://doi.org/10.1111/j.1471-0528.2011.03057.x.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril 2009;92:205-16. https://doi.org/10.1016/j.fertnstert.2008.04.023.
- Gibbons E, Mckintosh A, Fitzpatrick R. A Structured Review of Patient-Reported Outcome Measures for People Undergoing Elective Procedures for Benign Gynaecological Conditions of the Uterus: Report to the Department of Health 2010.
- Habiba M, Julian S, Taub N, Clark M, Rashid A, Baker R, et al. Limited role of multi-attribute utility scale and SF-36 in predicting management outcome of heavy menstrual bleeding. Eur J Obstet Gynecol Reprod Biol 2010;148:81-5. https://doi.org/10.1016/j.ejogrb.2009.09.021.
- Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD001016.pub2.
- Ware J, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 1993.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: SAGE Publications, Inc.; 2007.
- Aalto AM, Aro A, Teperi J. RAND-36 as a Measure of Health-related Quality of Life: Reliability, Construct Validity and Reference Values in the Finnish General Population. Helsinki: STAKES, Research Reports 101; 1999.
- Meaidi A, Kuhr Skals R, Alexander Gerds T, Lidegaard O, Torp-Pedersen C. Decline in Danish use of oral tranexamic acid with increasing use of the levonorgestrel-releasing intrauterine system: a nationwide drug utilization study. Contraception 2020;101:321-6. https://doi.org/10.1016/j.contraception.2019.12.013.
- O’Flynn N, Britten N. Menorrhagia in general practice – disease or illness. Soc Sci Med 2000;50:651-61. https://doi.org/10.1016/S0277-9536(99)00318-4.
- O’Flynn N, Britten N. Diagnosing menstrual disorders: a qualitative study of the approach of primary care professionals. Br J Gen Pract 2004;54:353-8.
- Garside R, Britten N, Stein K. The experience of heavy menstrual bleeding: a systematic review and meta-ethnography of qualitative studies. J Adv Nurs 2008;63:550-62. https://doi.org/10.1111/j.1365-2648.2008.04750.x.
- O’Flynn N. Menstrual symptoms: the importance of social factors in women’s experiences. Br J Gen Pract 2006;56:950-7.
- Santer M. Heavy menstrual bleeding: delivering patient-centred care. Br J Gen Pract 2008;58:151-2. https://doi.org/10.3399/bjgp08X277258.
- Byles JE, Hanrahan PF, Schofield MJ. ‘It would be good to know you’re not alone’: the health care needs of women with menstrual symptoms. Fam Pract 1997;14:249-54. https://doi.org/10.1093/fampra/14.3.249.
- Chapple A. Menorrhagia: women’s perceptions of this condition and its treatment. J Adv Nurs 1999;29:1500-6. https://doi.org/10.1046/j.1365-2648.1999.01038.x.
- Paramsothy P, Harlow SD, Greendale GA, Gold EB, Crawford SL, Elliott MR, et al. Bleeding patterns during the menopausal transition in the multi-ethnic Study of Women’s Health Across the Nation (SWAN): a prospective cohort study. BJOG 2014;121:1564-73. https://doi.org/10.1111/1471-0528.12768.
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3-13. https://doi.org/10.1016/j.ijgo.2010.11.011.
- Warner P, Whitaker LHR, Parker RA, Weir CJ, Douglas A, Hansen CH, et al. Low dose dexamethasone as treatment for women with heavy menstrual bleeding: a response-adaptive randomised placebo-controlled dose-finding parallel group trial (DexFEM). EBioMedicine 2021;69. https://doi.org/10.1016/j.ebiom.2021.103434.
Appendix 1 Qualitative interview schedule topic guide


List of abbreviations
- BMI
- body mass index
- CI
- confidence interval
- ECLIPSE
- Clinical effectiveness and cost-effectiveness of levonorgestrel releasing intrauterine system in primary care against standard treatment for menorrhagia trial
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- HMB
- heavy menstrual bleeding
- IMD
- Index of Multiple Deprivation
- LNG-IUS
- levonorgestrel-releasing intrauterine system
- MMAS
- Menorrhagia Multi-Attribute Scale
- NICE
- National Institute for Health and Care Excellence
- NSAID
- non-steroidal anti-inflammatory drug
- PPI
- patient and public involvement
- SAQ
- Sexual Activity Questionnaire
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
