Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/84/10. The contractual start date was in March 2015. The draft report began editorial review in March 2020 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Bruce et al. This work was produced by Bruce et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Bruce et al.
Chapter 1 Introduction
Background
Breast cancer is the most common cancer in women in the UK, with over 55,000 new cases diagnosed each year. 1 Breast cancer incidence has increased by 20% since the early 1990s. 1 Despite increasing incidence, survival rates have improved dramatically as a result of advances in early diagnosis and treatment. 1 Breast cancer survival has doubled in the UK over the last 40 years; now, nearly 8 in 10 women (78%) treated for invasive breast cancer survive for ≥ 10 years. 1 Treatments are complex and can be toxic, causing side effects that persist in the long term. There is increased recognition of the benefits of providing supportive care for people living with and beyond cancer treatment. 2
Surgical treatment of breast cancer
Surgery is the mainstay of treatment for breast cancer, supplemented with chemotherapy, radiotherapy and biotherapy, with or without reconstruction surgery. 3 Treatment decisions are based on clinical criteria, tumour stage, lymphatic spread and patient preference. Surgery to the breast consists of either mastectomy or breast-conserving surgery (BCS), with newer oncoplastic conserving procedures increasingly being used. 4 Breast-conserving procedures, such as lumpectomy or wide local excision, combined with whole-breast radiotherapy, aim to achieve disease control with minimal morbidity. These breast-conserving treatments have been demonstrated to be as effective as mastectomy in increasing long-term overall survival in patients with early breast cancer. 5,6 Conservative surgery followed by radiation therapy allows for the preservation of the breast, which can improve patient quality of life (QoL) and satisfaction with treatment. 7 Sentinel lymph node biopsy (SLNB) has largely replaced axillary lymph node dissection (ALND) for disease staging, and also reduced the need for extensive axillary node clearance (ANC). There is good evidence that 10-year survival among women receiving SLNB only is equivalent to that among women receiving ANC after SLNB. 8,9
Treatment-related side effects
Although largely curative, breast cancer treatments have negative sequelae. Surgery and radiotherapy can affect the upper body, especially the shoulder joint and upper limb, causing restricted shoulder range of movement (ROM), impaired strength and functional limitations. Arm morbidity has been strongly associated with the extent of axillary node surgery. Although arm lymphoedema can affect up to 20% of women, systematic reviews report higher rates of lymphoedema after ALND than after SLNB up to 2 years after surgery [20%, 95% confidence interval (CI) 14% to 28%, n = 18 studies, n = 3599 participants, vs. 6%, 95% CI 4% to 9%, n = 18 studies, n = 3583 participants]. 10,11
A systematic review11 of upper limb problems after surgery and radiotherapy (32 observational studies) reported prevalence estimates for restricted shoulder ROM (up to 67%), arm weakness (< 28%) and shoulder/arm pain (< 68%). Prevalence estimates vary widely, in part because of differences in definitions, methods of measurement and timing of postoperative follow-up. Other common postoperative complications include wound infection, seroma and axillary web syndrome (cording) and chronic pain. 11,12
A nationwide Danish study13 of 2500 women undergoing breast cancer surgery found that over one-third of women reported persistent pain and half reported sensory disturbances up to 7 years after treatment. Persistent upper limb dysfunction and pain are debilitating, affecting sleep quality, QoL and physical and emotional function. These enduring adverse sequelae of cancer treatment are burdensome and associated with increased health-care utilisation.
Risk factors for persistent post-treatment complications
Research has examined patient- and treatment-related risk factors associated with upper body problems after breast cancer treatment. 11,14,15 Women undergoing mastectomy have higher odds of postoperative shoulder restriction than those undergoing BCS [odds ratio (OR) 5.67, 95% CI 1.03 to 31.2]. 11 More invasive axillary surgery is associated with greater impairments of abduction ROM and strength than SLNB, up to 7 years post treatment. 16 Radiotherapy to the axilla or chest wall, compared with no radiotherapy, slightly increases the odds of shoulder ROM restriction (pooled OR 1.67, 95% CI 0.98 to 2.86) and lymphoedema (pooled OR 1.46, 95% CI 1.16 to 1.84). 11 A higher body mass index (BMI) was found to be an independent risk factor for shoulder external rotation problems up to 7 years after treatment. 16 Higher BMI (overweight or obese) is also a known risk factor for lymphoedema10 and for development of chronic post-surgical pain (six studies, pooled OR 1.34, 95% CI 1.08 to 1.67). 17
Evidence for the effect of exercise on shoulder dysfunction
A Cochrane systematic review,12 published in 2010 [24 randomised controlled trials (RCTs), 2132 participants], reported that exercise and/or physiotherapy may help to prevent shoulder and arm morbidity after breast cancer treatment. This review12 found that physiotherapy, compared with usual care or control, improved shoulder flexion only within the first 2 weeks and at 3 and 6 months postoperatively. The timing of starting postoperative physiotherapy may also be important for shoulder ROM and upper limb function. Early exercise, started on the first postoperative day, was beneficial in improving flexion and abduction at 1 week postoperatively, and flexion at 4–6 weeks postoperatively, when compared with delayed exercise (exercise that started after the fourth postoperative day). 12
A more recent systematic review,14 published in 2015 (18 RCTs, 2389 participants), compared different exercise modalities (multifactorial therapy, passive mobilisations, stretching and exercise therapy) and the timing of application. 14 The overall findings were similar, suggesting that early exercise improved upper arm ROM in the short and long term after breast cancer treatment. However, exercising in the first postoperative week also increased the risk of greater wound drainage volume and seroma formation. 14 Regarding physiotherapy modalities, adding stretching to an exercise programme may improve postoperative ROM. 14
Although these reviews suggest that physiotherapy may prevent postoperative shoulder problems, the majority of trials conducted to date are small, methodologically weak and with short-term follow-up. Many trials investigated exercise delay prescription until after completion of adjuvant therapy. 12 Few fully report details of prescribed regimes; hence there is a lack of knowledge regarding the optimum content, frequency, intensity, timing or safety of exercise prescription. Another limitation is the exclusion of patients with existing shoulder problems, the very population who may benefit the most from targeted postoperative support. 18
Rationale for the PRevention Of Shoulder ProblEms tRial
We designed the PRevention Of Shoulder ProblEms tRial (PROSPER) to address the evidence gap and to investigate whether or not an early supervised exercise programme, compared with usual care, could prevent musculoskeletal shoulder conditions in patients undergoing treatment for breast cancer. This research was commissioned in 2013–14 by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, with specifications to design an exercise intervention for women identified as being at higher risk of developing shoulder problems as a consequence of their breast cancer treatment. At the time of funding, the UK National Institute for Health and Care Excellence (NICE) recommended that all breast cancer patients should be provided with instructions on functional exercises to start doing from the first postoperative day. Each breast cancer centre should have written local guidelines for postoperative physiotherapy, but NICE recommended that patients be referred to physiotherapy services only if they experienced persistent shoulder restrictions after cancer treatment. 3
Literature update
We reviewed literature to identify new trials investigating exercise after breast cancer surgery published since the commissioned call in 2014. We sought RCTs comparing exercise and/or physiotherapy with standard or usual care (i.e. no active intervention), regardless of the type of outcome. Our search strategies were adapted from previous systematic reviews12,14 and applied to MEDLINE (via Ovid), EMBASE (via Ovid), PEDro (Physiotherapy Evidence Database) and LILACS (Latin American and Caribbean Health Sciences Literature). We also searched for trials registered on the World Health Organization (WHO) search portal, the European Union clinical trials register and www.clinicaltrials.gov (US National Library of Medicine). We searched for citations published from 1 January 2014 to 10 December 2019. Of 439 potentially eligible studies identified, after screening titles and abstracts, 12 trials were included, eight of which were published trials and four of which were registered as ongoing. One trial reported ROM and grip strength data across two separate publications. 19,20
Recent evidence: physiotherapy compared with usual care
Of eight published trials, four were pilot RCTs and all studies were single centred with small sample sizes (mean 79 participants), although one trial recruited 153 participants. 21 Type of exercise varied and included aquatic-based,22 aerobic23 and resisted exercises. 19–21,24–26 Interventions were delivered either in the clinic setting19–23,25,26 or using an online interface. 24 Exercise programmes varied widely in terms of duration and frequency, ranging from 3 to 9 months (Table 1). 23,26 Outcomes also varied, but the most commonly reported were health-related quality of life (HRQoL), function and lymphoedema. Five studies reported improvements favouring the intervention group for the majority of outcomes (n = 427 participants). Three studies reported no differences between groups for function19 (n = 59 participants), lymphoedema22 (n = 29 participants) or limb volume25 (n = 35 participants).
| First author | Country (design) | Sample size (n) | Participants | Intervention | Comparison | Primary outcome | Findings |
|---|---|---|---|---|---|---|---|
| Casla23 | Spain (RCT) |
94 I: 47 C: 47 |
Stages I–III breast cancer 1 month to 3 years post completion of RT or chemotherapy |
Resisted and aerobic supervised exercise Frequency Twice per week for 12 weeks plus dietary counselling (three sessions) |
No intervention | Cardiorespiratory capacity: VO2 max at 12 weeks and 6 months |
Improvement in VO2 max at 12 weeks in favour of exercise Only the exercise group reassessed at 6 months: maintained improvements from 12 weeks |
| Galiano-Castillo24 | Spain (RCT) |
81 I: 40 C: 41 |
Stages I–III breast cancer Completed adjuvant therapy |
Tailored exercise programme using an online interface Frequency Three times per week for 8 weeks; 90 minutes for each session |
Information about exercise only | QoL: EORTC QLQ-C30 at 8 weeks and 6 months | Difference in QoL at 8 weeks and 6 months in favour of exercise |
| Ibrahim19,20 | Canada (Pilot RCT) |
59 I: 29 C: 30 |
Stages I-III breast cancer Younger women aged 18–45 years, scheduled for adjuvant therapy |
Progressive exercises Frequency 6 weeks, with an optional additional 6 weeks |
Advice on healthy lifestyle and exercise |
Function: DASH score at 18 months post radiotherapy19 ROM: goniometer at 18 months post radiotherapy20 |
No differences between groups for function19 The intervention group had better ROM at 6 months, but it was not sustained at 12 and 18 months20 |
| Johansson22 | Sweden (pilot RCT) |
29 I: 15 C: 14 |
Unilateral breast cancer and lymphoedema for at least 6 months |
Aquatic physiotherapy Frequency Three times per week for 8 weeks; 30-minute sessions |
Instructions to continue exercises if any | Lymphoedema: volume, bioimpedance and tissue water at end of 8-week intervention | No differences in lymphoedema at 8 weeks |
| Leal25 | Brazil (RCT) |
35 I: 17 C:18 |
Women undergoing breast cancer surgery and RT | Supervised exercise | No intervention | Volume: circumference | No differences observed at 8 weeks post therapy |
| Rafn26 | Canada (Pilot RCT) |
41 I: 21 C: 20 |
Women scheduled for breast surgery |
Patients assessed at 3, 6 and 9 months after surgery. Physiotherapy started because of restricted ROM, weakness or lymphoedema Usual care also provided |
Usual care: three sessions at 3, 6 and 9 months post surgery. Information on nutrition, stress and fatigue management |
Arm morbidity: ROM – goniometer. Strength: hand-held dynamometer and handgrip dynamometer Volume: circumference Function: QuickDASH Pain: NRS QoL: FACT-B4 |
At 12 months, control group had complex arm morbidity compared with intervention group |
| Yuste Sánchez21 | Spain (non-randomised comparative study) |
153 I: 76 C: 77 |
Stages I and II breast cancer, unilateral surgery with ALND |
Exercises plus MLD Frequency Three times per week for 3 weeks, plus information on treatment, morbidity and behavioural change |
Information on treatment, morbidity and behavioural change | QoL: EORTC QLQ-C30 + EORTC QLQ-BR23 at 3 weeks, and 3, 6 and 12 months |
Differences in physical and social dimensions only at 3 and 6 months The intervention group showed greater improvement |
Forthcoming studies: registered trials
At the time of writing, we found four registered trials, all overdue for reporting, from Spain (n = 90 participants27 and n = 84 participants28), Brazil (n = 38 participants29) and the USA (n = 568 participants30) (Table 2). These trials have different primary outcomes and postoperative follow-up points: ROM at 1 month,28 pain and fatigue after 7 weeks of exercise sessions,29 functional capacity at 12 months27 and presence of lymphoedema at 18 months. 30 The American lymphoedema trial30 has provided interim data on the clinicaltrials.gov website suggesting early benefit on lymphoedema outcomes; final results are pending. We present an overview of findings regarding the content and safety of exercise interventions in Chapter 3, which describes the development of the PROSPER exercise intervention.
| First author | Country | Estimated completion date | Target sample size (n) | Participants | Intervention | Comparator | Primary outcome |
|---|---|---|---|---|---|---|---|
| de Santana29 | Brazil | December 2018 | 38 | Women with mastectomy, without drain within 4 months of surgery |
Supervised exercises Frequency Three times per week for 50 minutes, 20 sessions (7 weeks) |
Information leaflet. Patients to have face-to-face sessions once per week, in which the physiotherapist will help patients with exercise and load progression; exercises are performed at home three times per week | Pain (NRS) at 7 weeks |
| Gomez27 | Spain | February 2017 | 90 | Women with breast cancer, completed at least 6 months before, with persistent fatigue | Supervised physiotherapy and information about healthy habits and exercise following breast cancer treatment | Patients instructed to practice exercises of their preference and received information about healthy habits and exercise following breast cancer treatment | Functional capacity: 6-minute walking test at 12 months |
| Paskett30 | USA | December 2018 | 568 |
Stages I–III breast cancer Women, newly diagnosed with or without neoadjuvant, surgery and/or RT |
Supervised physiotherapy and information on lymphoedema | Information on lymphoedema only | Lymphoedema events at 18 months |
| Sánchez28 | Spain | August 2019 | 84 | Women with SLNB | Supervised six sessions of physiotherapy over 4 weeks | Information on adequate recovery | ROM at 4 weeks |
Aims and objectives of PROSPER
The overall aim of PROSPER was to investigate whether or not an early supervised exercise programme compared with best practice usual care was clinically effective and cost-effective for women at high risk of shoulder problems after breast cancer treatment on outcomes of upper limb function, complications and QoL.
The study objectives were to:
-
develop and refine a complex intervention of physiotherapy-led exercises, incorporating behavioural strategies, for women at risk of developing musculoskeletal problems after breast cancer treatment
-
assess the acceptability of the structured exercise programme and outcome measures, to optimise participant recruitment and refine trial processes during a 6-month internal pilot phase
-
use findings from the internal pilot phase to undertake a definitive, full RCT in approximately 15 UK NHS breast cancer centres.
A health economic analysis and a qualitative substudy were embedded within the trial. Qualitative research was undertaken throughout to inform intervention development and gain insight into the experiences of both women and physiotherapists taking part in trial interventions.
Overview of report
The report is structured across seven subsequent chapters. We present the methods and describe intervention development and trial results, followed by separate chapters reporting the qualitative findings and the health economic evaluation. Finally, we present an overarching discussion and conclusion.
Chapter 2 Methods
Trial design and setting
This trial was a two-arm, pragmatic RCT with an internal pilot study, an embedded qualitative evaluation and a parallel economic analysis. A detailed description of the trial protocol has been published. 31 The trial was undertaken in secondary care settings, in breast cancer centres within NHS trusts across England.
Participants
Inclusion criteria
Those eligible to participate were women aged ≥ 18 years who had been diagnosed with primary breast cancer scheduled for surgical excision and were willing and able to comply with the study protocol. All participants provided signed, informed consent. Only patients considered at high risk of developing shoulder problems were eligible to participate, defined in accordance prespecified PROSPER criteria.
Definition of high risk of shoulder problems
We specified high-risk criteria for the purpose of the trial. Participants deemed at higher risk of developing shoulder problems were defined in accordance with one or more of the following criteria: planned ANC, planned radiotherapy to the axilla or supraclavicular nodes, shoulder problems before breast cancer treatment, obesity (defined as a BMI of ≥ 30 kg/m2) or any subsequent axillary surgery related to the primary surgery. Existing shoulder problems were defined as a history of shoulder surgery, shoulder trauma injury (fracture or shoulder dislocation), frozen shoulder, osteoarthritis or rheumatoid arthritis affecting the shoulder, non-specific shoulder pain, stiffness or decreased function. Decreased function was assessed using simple screening questions: ‘Can you do any of the following without problems? (a) wash your hair, (b) wash your back, (c) reach up to a high shelf?’. Participants who could not undertake one or more of these activities were eligible to be invited to take part in the trial.
Eligibility for late entry (within 6 weeks of surgery)
The decision about the need for radiotherapy is often taken after surgery, after pathological confirmation of tumour size, grade, histology and margin status. Patients who were ineligible at the time of primary surgery but who were later informed of the need for axillary and/or supraclavicular nodes radiotherapy within 6 weeks of surgery were eligible to participate. Six weeks was selected as the cut-off point for commencement of physiotherapy treatment.
Exclusion criteria
Men were excluded, as were women known to be undergoing immediate breast reconstruction surgery at the time of recruitment, women undergoing SLNB without other high-risk criteria, women undergoing bilateral breast surgery and those with known metastatic disease at the time of recruitment.
Participant recruitment and consent
Patients were identified from multidisciplinary cancer team meetings and preoperative oncology and radiotherapy clinics. Screening was undertaken by a member of the clinical team (a specialist breast nurse, surgeon, research nurse or facilitator trained in PROSPER screening and recruitment procedures). Eligible patients were given a patient information sheet while attending an oncology clinic and were given at least 24 hours to consider participation. In the case of those interested and willing to participate, written informed consent was obtained by the delegated site investigator after discussion and clarification of any queries. We sought consent for multiple levels of access to medical data, including medical records and routine data held by NHS Digital. At the time of trial launch, the wording of consent forms was appropriate for access to Hospital Episode Statistics (HES) data and approved by the Ethics Committee and relevant monitoring committees.
Trial setting and prespecified requirements
The trial was undertaken in secondary care settings in England, in NHS trusts with specialist breast oncology services. Any centre could take part as long as hospital physiotherapy services had the capacity to treat participants for the trial duration. We specified that a minimum of two physiotherapists per site attend intervention training. Any hospital providing routine preoperative or postoperative physiotherapy for non-reconstructive breast surgery could not take part. We also screened usual-care practices at hospitals expressing an interest in taking part. A specification was the agreement of centres to provide PROSPER usual-care leaflets for all trial participants.
Allocation sequence generation and randomisation
The unit of randomisation was the individual participant. Three stratification variables were used: the centre, whether it was the participant’s first or repeat surgery and whether or not the participant had been informed of the need for radiotherapy within 6 weeks of surgery. These stratification variables accounted for late-entry participants and for those having multiple surgical procedures, which can increase the risk of postoperative complications. We used a computer-generated randomisation algorithm held and controlled centrally within the Warwick Clinical Trials Unit (WCTU) by an independent programmer. Trial participants were registered after screening checks, then randomised to treatment. Treatment allocation was coded and unavailable to the trial management team.
Blinding
We adhered to the Consolidating Standards of Reporting Clinical Trials (CONSORT) statement. 32 Owing to the nature of the exercise intervention, it was not possible to blind participants or physiotherapists delivering the intervention. Data entry staff were unaware of treatment allocation, and we undertook data cleaning blind to treatment allocation. Senior members of the research team and the statistical team were blind to treatment allocation for the duration of the trial. Final statistical analysis was undertaken by a statistician independent of the core trial team.
Trial interventions
Full details of trial interventions are described in Chapter 3. In summary, all participants received best-practice usual care consisting of two information leaflets describing postoperative exercises and advice for recovery after surgery. 33,34 The control group participants received no further intervention other than usual clinical care. Participants randomised to the exercise intervention were then referred to physiotherapy. We designed a new exercise programme that was underpinned with evidence and co-developed with cancer rehabilitation specialists and breast cancer survivors. The newly developed intervention was a physiotherapist-led, individualised, structured exercise programme comprising shoulder-specific exercises, behavioural change strategies and physical activity. 35 Three face-to-face appointments with a trained physiotherapist were scheduled at specific postoperative time points, with up to three optional appointments at any time up to 12 months after randomisation. An overview of the PROSPER intervention is detailed in Figure 1.
FIGURE 1.
Overview of trial interventions.
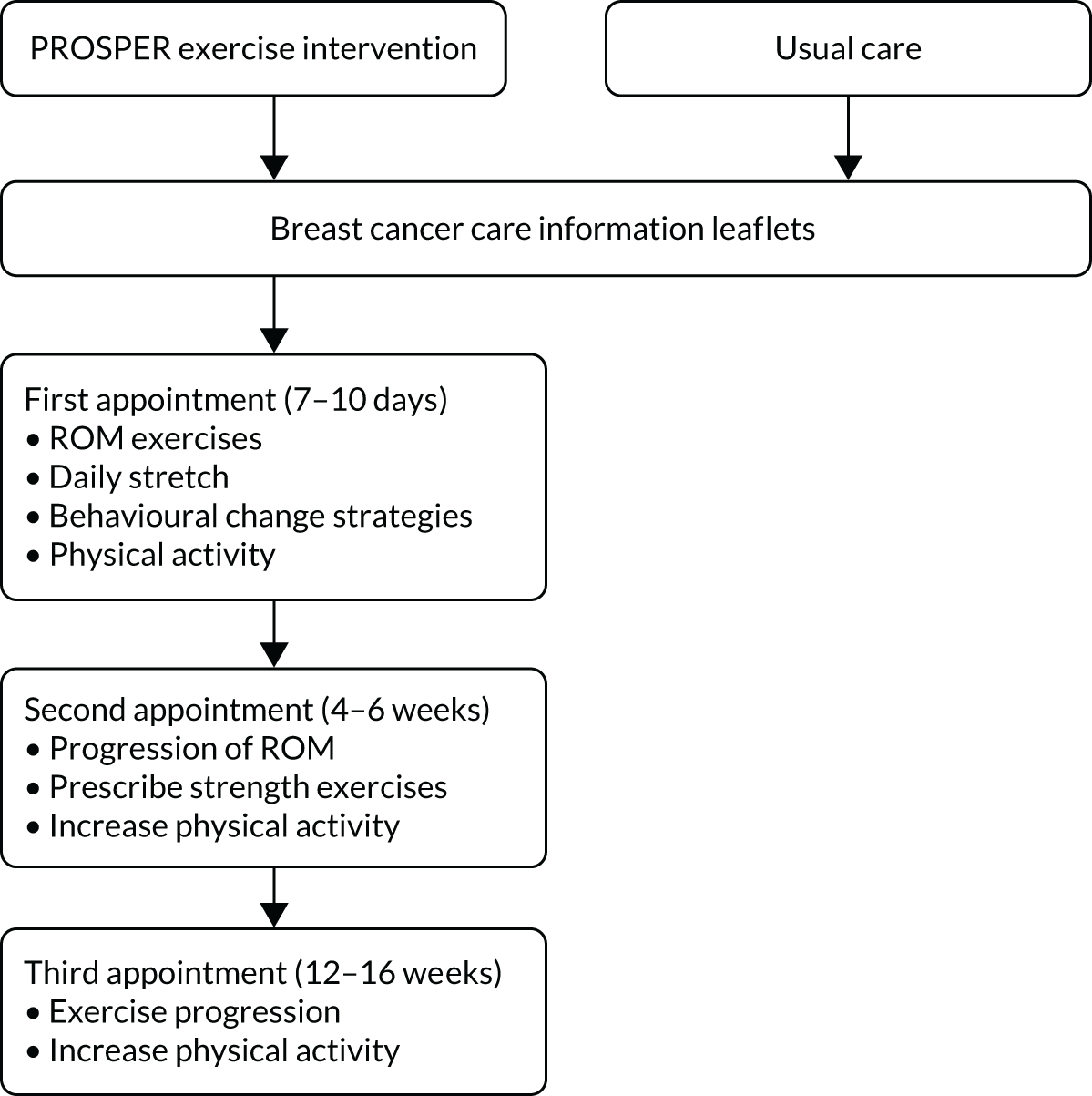
Co-interventions
No restrictions were placed with regard to referral to other health-care providers or private physiotherapy during the trial. At trial closure, participants continued with their usual health care.
Data collection
Baseline data
Baseline data were collected after participants provided informed consent. Baseline questionnaire booklets were given to participants preoperatively either to complete in clinic or to take home and return by post to the study office. Late entry trial participants completed booklets after surgery. Descriptive data included age, height, weight, marital status, education level, employment, handedness, ethnicity and self-reported comorbidity. Questionnaires also included all patient-reported outcome measures, described in Outcomes. We gathered data on planned clinical treatment at recruitment. Follow-up data were collected by postal questionnaire.
Outcomes
Primary outcome: function of upper limb
The primary outcome was upper limb function at 12 months measured using the Disabilities of the Arm, Shoulder and Hand (DASH) scale. 36 Breast cancer treatments mainly affect the axilla and shoulder region, but can also affect the arm and hand, leading to functional problems with daily activities, such as dressing, writing, opening or closing jars and lifting and/or holding shopping bags. 37 We opted to use the 30-item DASH scale to measure upper limb function rather than the shorter Quick-DASH or a shoulder-specific assessment tool. The DASH scores range from 0 (no disability) to 100 (most severe disability). 36 The DASH questionnaire includes 21 items on function, five items on symptoms (pain, activity-related pain, tingling, stiffness and weakness) and three items on social/role function. There is good evidence that the DASH scale can detect change in function over time and detect clinically important differences between groups. 38 The DASH scale has also been used in observational studies39 and clinical trials of breast cancer populations. 18,40,41 An overview of outcomes is provided in Table 3.
| Outcome | Timing | Instrument, description of outcome |
|---|---|---|
| Function | Baseline, 6 months, 12 months | Primary outcome: upper-limb function – DASH 30 items (no difficulty, mild, moderate, severe difficulty, unable to do). Total score 0 (no difficulty) to 100 (extreme difficulty) |
| Function subscales | Baseline, 6 months, 12 months | Secondary outcomes: AL 17 items, I 6 items and PR 5-item subscale using DASH 30 items,a modified from Dixon et al.42 |
| Wound related | 6 weeks only | Wound healing, self-reported and doctor-diagnosed SSI |
| Pain in breast and armpit (acute, chronic, neuropathic) | Baseline, 6 weeks, 6 months, 12 months | Pain on movement and at rest in last week, NRS. Pain intensity 0 (no pain) to 10 (pain as bad as can imagine). Single NRS at other time points. Neuropathic pain: DN4 – seven-item descriptive scale; score of ≥ 4 indicative of neuropathic pain. FACT-B4. Arm symptom scale from 0 (not at all) to 4 (very much). Symptoms: arm swollen or tender, movement is painful, poor range of arm movements, arm numbness, arm stiffness |
| Lymphoedema | Baseline, 6 weeks, 6 months, 12 months | LBCQ: arm feels heavy and arm looks swollen, previous week. Presence of both is indicative of lymphoedema |
| Physical activity | Baseline, 6 weeks, 6 months, 12 months | PASE: two activity items: walking in home or garden and strenuous sport/recreational activity, in the past week. How many hours per day on average |
| Confidence in activity | Baseline, 6 weeks, 6 months, 12 months |
Confidence in return to usual activities and regular physical activity in future NRS: score 0 (not confident at all) to 10 (very confident) |
| HRQoL | Baseline, 6, 12 months |
EQ-5D-5L + VAS: 5-item score converted into a single summary score (–0.594 to 1). A score of 1 indicates maximum HRQoL. VAS numerical 0 to 100, maximum health SF-12: HRQoL: 12 items, score 0 to 100, higher score indicates better HRQoL |
| Health-care resource use | 6, 12 months | Self-report and routine HES data for APC and outpatient activity for years 2015–16, 2016–17, 2017–18 |
Secondary outcomes
DASH subscales (baseline, 6 months and 12 months)
The DASH scale has been used to generate three health outcome subscores for activity limitations, impairment and participation restriction, as per the WHO International Classification of Functioning Disability and Health taxonomy. 42,43 DASH subscores were measured at baseline, at 6 months and at 12 months.
Health-related quality of life (baseline, 6 months and 12 months)
We used the Short Form questionnaire-12 items (SF-12)44 to measure physical function, engagement in usual activities and mental functioning. The physical health composite scale (PCS) and mental health composite scale (MCS) allow comparison with national norms and to cancer populations. We also used the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) questionnaire, a standardised measure of self-reported HRQoL that includes five domains of mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 45
Acute and chronic postoperative pain (baseline, 6 weeks, 6 months and 12 months)
We measured treatment-related complications, including pain intensity, pain character and neuropathic pain at multiple time points. We measured acute postoperative pain using an 11-point numerical rating scale (NRS) from 0 (no pain) to 10 (pain as bad as you can imagine) for pain at rest and movement-evoked pain at 6 weeks postoperatively, as per recommended international guidance for postoperative pain assessment. 46 A single pain NRS was used to capture chronic pain at 6 and 12 months. We examined proportions with none/mild (0–3 NRS) and moderate/severe intensity pain (≥ 4 NRS). We used the Douleur Neuropathique (DN4)47 seven-item scale, validated for postal use, to capture neuropathic pain at 6 weeks, 6 months and 12 months.
Wound-related outcomes: surgical site infection (6 weeks)
We measured wound-related outcomes, including patient-reported wound healing and surgical site infection (SSI), at 6 weeks: questions included whether or not a doctor or nurse had diagnosed a wound infection, whether or not the participant thought that they had had a wound infection and whether or not the participant had been prescribed antibiotics. Any other postoperative complication could be reported using free text.
Lymphoedema (baseline, 6 weeks, 6 months and 12 months)
We also used the Functional Assessment of Cancer Therapy – Breast, version 4 (FACT-B4), five-item subscale48 to capture symptoms of arm disability: higher scores indicate greater arm disability or morbidity (scale range 0–20). We assessed patient-reported symptoms of lymphoedema using the validated Lymphoedema and Breast Cancer Questionnaire (LBCQ). 49 Two items (i.e. arm swelling and arm heaviness) from the full LBCQ are predictive of arm swelling of at least 2 cm change in arm circumference. 49 Objective measurement, such as optoelectronic limb volumeters (perometry), was not available across all NHS breast cancer units and thus was not feasible to use in this multicentre trial. Patient-reported symptoms are more meaningful to patients and these self-reported LBQC items have been used in other large-scale international trials investigating morbidity outcomes after axillary treatment (NIHR-funded POSNOC50 and ATNEC51). Items included ‘my arm feels heavy’ and ‘my arm/hand looks swollen’ on the side on which surgery had been carried out in the previous week. We accepted a positive response to both questions as indicative of self-reported lymphoedema.
Physical activity (baseline, 6 weeks, 6 months and 12 months)
We collected physical activity data using selected items from the Physical Activity Scale for the Elderly (PASE)52 for activity in the previous week: walking outside the home and sport/recreational activities, with average hours per day for each. The term ‘yard’ was replaced with garden to align with UK terminology.
Health-care resource use (over 12 months)
Health-care resource use was captured for health economic analyses, using self-report. This is described further in Chapter 6. We obtained HES data from NHS Digital for three financial years, from 2015 to 2018. Data sets included admitted patient care and outpatient activity.
Data collection
Follow-up data were collected at 6 weeks, 6 months and 12 months postoperatively. We used postal questionnaires for all patient-reported outcomes with an explanatory cover letter. Questionnaire booklets were professionally printed, in colour, with a freephone number on the front page and a free-text section on the final page. Draft versions were modified after pilot testing and feedback from women treated for breast cancer. Core outcome data were collected by telephone if no response was received after one postal reminder. Clinical data on all surgeries and adjuvant treatment delivered over the study duration were gathered from medical records after completion of the 12-month follow-up.
Process evaluation
We measured a range of process evaluation indicators relating to intervention uptake and delivery. Data included time from randomisation to first appointment, participant uptake of the exercise intervention, number of contacts with physiotherapists and number of quality control (QC) assessments. We defined adherence to or compliance with the prescribed exercise programme as a participant having three or more contacts with the physiotherapist. Those having one or two contacts only were defined as partial compliers. We calculated ‘strength and work capacity’ for ROM and strength exercises, defined as the product of repetitions and sets prescribed at each appointment. The terminology ‘work capacity’ is used throughout to denote ‘strength and work capacity exercises’. For strength exercises, this was calculated using the product of resistance for Therabands [Paterson Medical, Cascade Healthcare Solutions, Tukwila, WA, USA (1.1 kg, 1.7 kg or 2.7 kg)] by repetitions and sets prescribed from the second appointment onwards. Theraband length was standardised, although physiotherapists spent time with each participant to demonstrate how to use the band correctly to ensure that the band length was suitable and to check their technique. If necessary, the bands could be shortened by adjusting the grip.
Monitoring of intervention delivery
Physiotherapists completed treatment logs to record information on attendance, exercise prescription, shoulder ROM, muscle strength, lymphoedema, wound healing, pain intensity and confidence in exercising for every participant. Completed treatment logs were returned to the co-ordinating centre after participants were discharged from physiotherapy. Chapter 3 describes quality assessment procedures for intervention fidelity.
Data management
Questionnaires were entered manually by the research team into a bespoke database, designed by the WCTU programming team. All data were checked for range, outliers, data missingness and date discrepancies. Any identified anomaly was checked against original data sources for rectification.
Data analyses
Sample size calculation
The target sample size for the trial was 350 patients, allocated in a 1 : 1 ratio. This calculation was based on data from a Dutch trial41 comparing the effects of a leaflet only with an exercise intervention, started 2 weeks postoperatively in 30 women having breast surgery and ALND (n = 15 per group). The authors reported a between-group difference of 7 points on the DASH scale at 6 months [mean 21.6 points in the control group; mean 14.6 points in the intervention group; pooled standard deviation (SD) of 19.5 points at baseline and a standardised mean difference (SMD) 0.36 points] after a 3-month exercise intervention. 41 At 80% power and significance level of < 0.05 points, this yielded a target total sample of 242 participants. Accounting for therapist effects, with up to nine patients per therapist in the intervention group, an intracluster coefficient (ICC) of 0.01, yielding a design effect of 1.05, gave a target of 256 patients. The ICC estimate of 0.01 was based on previous experience of exercise interventions in a range of musculoskeletal trials. 53 We anticipated very little therapist effect but, in the eventuality of lower therapist effects, we would have greater power with the given numbers to detect the same difference. We considered a loss to follow-up of 25% because the complexity and challenges of cancer treatment increase the risk of attrition over time. This higher percentage of loss to follow-up allows the detection of smaller effects.
We considered the recommended minimally clinically important difference (MCID) for the DASH scale. 38 Observational studies of rheumatology and orthopaedic populations suggested that the MCID is 10 points (95% CI 5 to 15 points) and that between-group difference for trials using the DASH scale should be set at 10. 38 However, this fails to account for many of the eventualities that occur in pragmatic trials, notably that there is not a no-treatment control, and that some members of the control group may be exposed by serendipity to an intervention of similar intensity, particularly in a high-risk population. Previous studies also used shorter time frames for follow-up, assessing change in function from weeks to several months. 38,41 Therefore, we powered the trial to detect a 7-point difference between groups at 12 months.
Statistical analyses
Statistical analyses were carried out using Stata® version 15 (StataCorp LP, College Station, TX, USA). All statistical tests were two-sided and performed at the 5% significance level. We undertook two levels of analysis: using intention to treat (ITT), as per CONSORT guidelines,32 and complier-average causal effect (CACE). 54 We reviewed other examples53,55 of CACE analyses from physiotherapy trials to inform our definition of compliance with exercise. We found variation in the reporting of compliance or adherence with exercise because definitions depend on the content and duration of interventions being evaluated, for example defined as completing half of six recommended sessions53 or exploration of different thresholds. 55 For PROSPER, we defined ‘complete’ compliance with the intervention as three or more sessions with the physiotherapist. This was the specified minimum number of recommended contacts that would ensure that all elements of the exercise programme were introduced and progressed. We defined non-compliance as none or fewer than three physiotherapy sessions.
Descriptive analysis
All baseline demographic and pre-randomisation clinical measures were summarised by treatment allocation. Continuous data were summarised using mean, SD, median and range values. Categorical data were summarised by number and proportion (%) by treatment group. For both types of data, CIs were also specified.
Primary analysis
The primary analysis compared the DASH score at 12 months between usual care and the exercise intervention. The clustering effect was assessed prior to data analysis and was found to be negligible. For this reason, the primary outcome was assessed using ordinary linear regression. In each case, we summarised the mean DASH change score from baseline to 6 and 12 months respectively, by treatment group and for differences between treatment groups using unadjusted and adjusted estimates. We adjusted for baseline scores, age, type of breast surgery (BCS vs. mastectomy), type of axillary surgery (ANC vs. SNLB), radiotherapy (yes/no) and chemotherapy (yes/no). For the primary analyses, a post hoc sensitivity analysis was undertaken to assess the impact of adjusting for age only at baseline, given that participants had not completed adjuvant therapy on recruitment. Mean changes and 95% CIs were plotted graphically to assess change over 12 months.
Missing data
We followed the DASH scoring manual, which specifies that a score cannot be calculated if there are more than three missing items. As a sensitivity analysis, the impact of the missing data was assessed using multiple imputation. Two data sets were used for the statistical analysis, observed and imputed. The observed data set comprised all observed data, including follow-up, with missing values. Impact of data missingness was assessed using multiple imputation. Missing data due to participants or health professionals incorrectly leaving fields blank (invalidly missing) were examined further to assess whether multiple imputation was viable: missing completely at random, missing at random or not missing at random. For normally distributed data, we used multiple imputation methods. 56 These were carried out using the imputation by chained equations (ICE) procedure. 57 The imputed ITT data set was used for sensitivity analyses.
Subgroup analyses
We prespecified subgroup analyses to examine differences in baseline DASH scores depending on previous clinical history. We anticipated that women reporting a history of shoulder problems were more likely to have arm disability than those without a history of shoulder problems, although this would be reflected in DASH scores at baseline. This was explored and reported.
Sensitivity analyses
Various sensitivity analyses were planned: first by comparing high- and low-volume recruiting centres to examine the impact on clustering effect and to analyse high-volume centres to examine therapist effect. We also explored time of follow-up from randomisation. As this was a surgical trial, we anticipated variation in follow-up in relation to timing of surgery. Most participants will have a short time period between randomisation and surgery, except for late-entry participants informed of the need for radiotherapy postoperatively. We assessed differences between date of randomisation and date of surgery by treatment group, but these were negligible. As the clustering effect was also negligible, the first two sensitivity analyses were discarded.
Adverse event reporting
An adverse event (AE) was defined as any untoward medical occurrence in a participant that did not necessarily have a causal relationship with this intervention. We defined serious adverse events (SAEs) as an untoward occurrence that resulted in death, was life-threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was considered medically significant. Expected common postoperative AEs included superficial and deep SSIs, seroma, bruising/haematoma and drain site infections. Some muscle soreness was to be expected after stretching and strengthening exercises. We recorded postoperative events, but they were not considered serious unless they arose as a direct consequence of the trial intervention. All AE data were reviewed by independent monitoring committees. We compared the number of AEs and SAEs by treatment group using chi-squared tests or Fisher’s exact test, with odds ratios (ORs) and 95% CIs.
Withdrawals
There were different levels of withdrawal within the trial: (1) withdrawals from treatment (exercise intervention), (2) withdrawals from postal questionnaires but with permission for all other data collected to be used, (3) withdrawal of approval for access to routine hospital data (HES) and (4) complete withdrawal of all data. The level of withdrawal from follow-up was explored by treatment group.
All planned analyses and template tables were detailed in the statistical analysis plan and reviewed and approved by the Data Monitoring and Ethics Committee (DMEC) prior to any final statistical analysis. One amendment was made to the statistical analysis plan after publication of the protocol to adjust analyses for baseline DASH scores.
Pilot study
A 6-month internal pilot was planned in three centres, with a target recruitment of 30 participants. We launched the pilot study in January 2016 in three breast cancer units: Coventry, Oxford and Wolverhampton. We launched the main trial after reaching recruitment targets and satisfying stop/go criteria.
Monitoring and approval
Ethics approval for the trial was granted on 20 July 2015. The first amendment, granted on 16 December 2015, was for modifications to participant materials. Amendments 2 and 3, granted on 4 April and 18 July 2016, respectively, related to the opening of new centres and transfer to Health Research Authority-regulated approvals. Amendment 4, granted on 18 April 2017, amended wording on the 12-month data collection forms. The final amendment was to add qualitative interviews with physiotherapists and was granted on 4 May 2018. We first applied for HES data in October 2017. Approval was granted by the Caldicott Guardian in April 2019 and we received data in October 2019.
Patient and public involvement
Patient representatives were involved at multiple stages throughout the trial, from grant writing and protocol refinement to intervention development and trial oversight. We sought input into the proposed intervention from breast cancer survivors attending a local community support group, described in Chapter 3. Our lead lay applicant passed away in 2017 (CH).
Trial Steering Committee
The Trial Steering Committee (TSC) was responsible for monitoring and supervising PROSPER progress. The TSC comprised independent members with expertise in oncology, physiotherapy, radiotherapy and medical statistics and one lay member (CH).
Data Monitoring and Ethics Committee
The DMEC reviewed trial progress, recruitment, protocol compliance and interim analysis of outcomes. The committee included independent experts with expertise in surgical oncology, health services research and medical statistics.
Chapter 3 Intervention development
Parts of this chapter have been reproduced or adapted with permission from Richmond et al. 35 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter presents a description of the PROSPER usual-care and exercise interventions. We developed an exercise intervention for the trial following Medical Research Council guidance for the design of complex interventions. 58 We present an overview of the evidence to inform the selection of core components and we describe the final intervention as per the Template for Intervention Development and Replication (TIDieR)59 recommendations and the Consensus on Exercise Reporting Template (CERT). 60
Control group: information leaflets
Participants allocated to the control group received best-practice usual care. At the time of trial set-up, usual NHS care was to provide newly diagnosed breast cancer patients with information leaflets describing cancer treatments and advice for postoperative recovery. 61 Specialist breast cancer nurses or oncology teams gave leaflets preoperatively. Patients undergoing non-reconstructive surgery were not routinely referred to physiotherapy services unless for a specific postoperative musculoskeletal problem. In 2005, a review61 of patient materials from 105 UK oncology departments across England was undertaken to identify usual clinical practice for postoperative shoulder mobilisation after breast cancer surgery. This survey61 found that half of the responding centres used materials published by Breast Cancer Care (now Breast Cancer Care/Breast Cancer Now; London, UK). We undertook a scoping survey of information leaflets used in breast cancer units across England and evaluated materials published by breast cancer charities. The content and design of hospital leaflets varied widely, although many were adapted or photocopied from charity materials. Most recommended restricted arm movement in the first postoperative week. We reviewed all materials, in collaboration with patient representatives and clinical rehabilitation experts, to select leaflets for best-practice usual care. We selected two well-designed, colourful leaflets produced by the charity Breast Cancer Care: Exercises After Breast Cancer Surgery33 and Your Operation and Recovery. 34 These leaflets describe exercises to do after breast surgery, lymph node removal and/or radiotherapy. Recommendations were to start upper body exercises from the first postoperative day, with instructions for warm up, basic arm exercises restricted to 90° of shoulder elevation, and a cool-down (BCC633). More advanced exercises, such as wall climbing, arm lifts and elbow pushes, were recommended from the second postoperative week onwards. The surgical recovery leaflet (BCC15134) covered generic postoperative advice and common complications, including wound infection, pain, cording, seroma and lymphoedema. These leaflets were provided to the study team free of charge by Breast Cancer Care. All participants were given a copy of each leaflet on recruitment to the trial; these were given out by the surgical oncology teams.
Overview of exercise intervention development
We designed a new exercise programme specifically for testing within PROSPER. We followed multiple steps to develop and refine the intervention before testing in the main trial. The key stages of development included:
-
an exploratory phase incorporating a literature review to identify evidence of effectiveness and harm from exercise interventions and of behaviour change techniques, a survey of postoperative materials used in the NHS, a patient and public involvement (PPI) focus group with breast cancer survivors and consultation with cancer rehabilitation experts
-
production of a draft intervention protocol for agreement at an intervention development consensus day, hosted by the WCTU, attended by 20 clinical rehabilitation experts and two breast cancer survivors
-
assessment of feasibility and acceptability of the prototype exercise programme with women attending a community-based support group for breast cancer patients, testing the intervention with 15 newly diagnosed breast cancer participants recruited to the internal pilot study from three NHS trusts
-
final refinement of the exercise programme based on patient and therapist feedback during the internal pilot study.
The final PROSPER exercise intervention was a physiotherapist-led, early, progressive, home-based postoperative programme that incorporated three components: shoulder-specific exercises, physical activity and behavioural support strategies to encourage adherence. The PROSPER programme was designed to be adaptable and flexible to allow for prolonged cancer treatment schedules and cancer-related fatigue. The evidence and rationale for inclusion of core components are given below.
Overview of evidence for exercise after breast cancer surgery
We undertook a literature review to identify systematic reviews and clinical trials investigating the effectiveness and potential adverse effects from exercise interventions for breast cancer patients. We also reviewed national and international breast cancer clinical guidelines62–64 and the types of exercises reported to be prescribed within the UK survey of physiotherapy and oncology departments. 61 We considered the content, timing, duration and setting of delivery of exercises.
A Cochrane systematic review,12 published in 2010, investigated the effectiveness of exercise interventions in preventing, minimising or improving upper limb dysfunction due to breast cancer treatment (24 trials; 2132 participants). Exercise type was broadly classified as active, active-assisted, passive, manual stretching and resistance. The review compared interventions on outcomes of ROM, strength, lymphoedema and pain. Subgroup analyses compared the timing of exercise in relation to cancer treatment (10 trials; 1304 participants) and the effect of postoperative exercise to usual care (six trials; 354 participants). Authors defined early exercise as that commencing from days 1 to 3 postoperatively; in contrast ‘delayed’ exercise was defined as starting from day 4 onwards. This definition differed from that used in the UK survey,61 which defined delayed exercise as starting after 1 week. Only one of the 24 trials in the Cochrane review was considered at low risk of bias:65 the remainder were of low to moderate quality and with small sample sizes (mean 44 participants per treatment arm). Included trials were published between 1979 and 2008 and older trials examined rehabilitation after extensive breast surgery, such as modified radical mastectomy. These procedures have since been replaced with less invasive surgeries; therefore, some rehabilitation practices, such as wearing a sling, are no longer indicated.
We summarise findings from the literature review in relation to the core components considered for the PROSPER intervention. The Cochrane review12 informed intervention development, although the findings are now outdated as other trials of exercise interventions have been published or registered since publication of the Cochrane review in 2010. To date, however, only two trials have ever been undertaken in the UK NHS: a small trial examining wound drainage, conducted in 197966 (n = 69 radical mastectomy), and a single-centre trial67 examining lymphoedema after breast surgery with ALND (n = 116).
Evidence for early postoperative exercises
Axillary surgery and radiotherapy increase the risk of shoulder ROM restrictions, in particular flexion, abduction and abduction with external rotation. 14 Breast cancer treatments can damage the lymphatic system, causing secondary lymphoedema. Patients may benefit from ROM exercises as active and active-assisted ROM exercises can improve fluid drainage and lymphatic flow. These exercise modalities activate physiological mechanisms as a result of muscle contraction, and they can also increase blood flow to the joints and soft tissues. 14,68
The Cochrane review12 found some evidence that early ROM exercises were more beneficial than delayed exercise for shoulder flexion ROM in the short term, within 4–6 weeks postoperatively [mean difference (MD) 12.12°, 95% CI 0.35° to 23.88°; I2 = 89%; three studies, 608 participants]. 12 However, there was no evidence of a difference up to 2 years after surgery (MD 3.00°, 95% CI –0.65° to 6.65°; one study, 181 participants). 12 Early exercise was beneficial for shoulder abduction ROM at 1 week (MD 11.65°, 95% CI 2.93° to 20.38°; I2 = 85%, three studies, 677 participants), with meta-analysis suggesting some increased benefit at 6 months compared with delayed exercising (MD 4.31°, 95% CI 1.38° to 7.25°; I2 = 0%; two studies, 549 participants). 12
Evidence for harm or adverse events after early postoperative exercise
Early ROM exercises, when compared with delayed exercises, did not increase the risk of seroma after surgery (OR 1.52, 95% CI 0.82 to 2.82; five studies), delay wound healing (OR 1.30, 95% CI 0.82 to 2.31; four studies), increase the number of wound aspirations (weighted mean difference 0.11, 95% CI –0.23 to 0.45; three studies), increase postoperative pain at 6 months (OR 1.87, 95% CI 0.70 to 4.96; one study) or increase lymphoedema incidence at 6 months (OR 1.24, 95% CI 0.45 to 3.41; three studies). 12 However, early ROM did increase volume of wound drainage (SMD 0.31, 95% CI 0.13 to 0.49; seven studies, 912 participants) and extended duration of wound drainage by 1 day (weighted mean difference 1.15; 95% CI 0.65 to 1.65; five studies, 725 participants) compared with delayed exercises. In a sensitivity analysis excluding older surgical trials published pre 1995, these findings did not change: early ROM increased the volume of postoperative wound drainage. 12
The single-centre UK trial,67 published since the Cochrane review,12 found that introducing ROM exercises limited to 90° shoulder elevation in the first week after ALND did not increase the risk of AEs compared with starting exercises after 1 week. 67 As programmes incorporating ROM exercises were known to be safe, we included shoulder ROM from the first week onwards while recommending restriction of shoulder ROM below 90° in the first postoperative week. Early restriction of shoulder ROM was also recommended in hospital information leaflets and cancer charity materials.
Evidence for stretching exercises
Surgery to the axilla and radiotherapy to the supraclavicular and infraclavicular nodes can lead to structural and functional problems as a result of tissue inflammation and damage. 69 Treatments can lead to tightening and contracture across the shoulder and chest area. 70 Studies report that the pectoralis muscles can atrophy after treatment. 71 Stretching exercises may contribute to remodelling injured connective tissues and may prevent negative physiological adaptations to the muscle spindles. 72,73 Stretching may prevent the shortening of muscle fibres. 74 Few trials provided details of the included exercises, but, in those that did, stretching of the pectoral muscles was most commonly reported. 65,75,76 Although study findings were largely inconclusive in terms of ROM improvement, there was no evidence to suggest that exercise regimes incorporating pectoral muscle stretching increased the risk of arm comorbidity. 65,75 Some studies found evidence of benefit when stretching was combined with other ROM exercises. 14,77 Another consideration was the arm position required for radiotherapy, which requires flexibility and adequate ROM of the shoulder joint. A minimum range of 90° of shoulder abduction and lateral rotation is required for radiotherapy targeting the lateral side of the breast and chest wall. 61 Owing to the pectoralis major insertion on the humerus, a shortened pectoralis will affect the ability to place the arm into the required position for radiotherapy. 78 Given that we aimed to recruit women already considered at risk of shoulder problems, the PROSPER exercise intervention included a daily ‘stretch and hold’ exercise for the pectoralis muscles.
Evidence for strengthening exercises
Patients undergoing cancer treatment are at an increased risk of loss of muscle mass and reduced muscle strength as a consequence of treatment-related pathophysiological changes. 79,80 Older age is also a risk factor for reduced muscle mass and strength. Muscle strength is greatest in our younger years, with maximum strength peaking at age 20–40 years. 81 Breast cancer is more common in women aged > 50 years, when 10% of muscle mass is already lost. The rate of muscle decline then accelerates,81 although this decline is thought to be, in part, due to decreasing levels of physical activity. Strengthening exercises can prevent the loss of muscle and bone mass. 82 The physiological stimulus from strengthening exercises can improve muscle mass and strength even during active treatment, as demonstrated in studies of cancer populations. 82 Targeted strength training can stimulate other changes, including improvements in insulin action, bone density and energy metabolism. 81 One systematic review found some evidence for improved HRQoL in adult cancer survivors participating in resistance training compared with usual care or alternative exercise regimes (SMD –0.17, 95% CI –0.34 to 0.00; I2 = 0%; six studies, 548 participants). 83
Another systematic review84 investigated the impact of low- to moderate-intensity resistance training on outcomes of muscle function, body composition and fatigue in cancer survivors (nine trials, 752 participants). Seven of the nine trials included breast cancer patients. 84 A meta-analysis revealed that resistance training improved upper limb muscle strength up to 1 year after cancer treatment (weighted mean difference ≥ 6.9 kg, 95% CI 4.78 to 9.03 kg; I2 = 79%; nine studies, 752 participants). 84 Improvements in lower limb strength and percentage body fat were also observed, but with weaker evidence of an improvement in cancer-related fatigue [weighted mean difference 1.86 Functional Assessment of Cancer Therapy – Fatigue, 95% CI –0.03 to 3.75; I2 = 0%; four studies, 437 participants]. 84
One trial82 included in the systematic review84 directly compared strengthening exercises to a stretching only programme for breast cancer patients (n = 106). 82 Those randomised to do strengthening exercises three times per week for 1 year maintained their bone and muscle mass throughout cancer treatment, in contrast to those following the stretching-only protocol. 82 Given the evidence for the benefits of strengthening exercises, we included progressive and individually tailored strengthening exercises to the PROSPER intervention. We specified that strengthening exercises should start only after the first postoperative month, to allow time for wound and tissue healing.
Evidence for physical activity
The American Cancer Society recommends that cancer patients should complete at least 150 minutes of moderate activity and at least two sessions of strength training per week. 85 This is in line with Department of Health and Social Care recommendations for physical activity for adults. 86 The American College of Sports Medicine recently recommended that cancer survivors should undertake aerobic and resistance training for approximately 30 minutes, for three sessions per week. 87 Physical activity during and after cancer treatment is safe, and has been associated with improved survival, reduced cancer recurrence and improvement in cancer-related side effects, with some studies reporting beneficial effects on fatigue, anxiety and depression. 83,88,89 Despite these benefits, only a small proportion of people achieve the recommended activity levels. One systematic review90 found that up to 70% of cancer survivors did not achieve the recommended activity targets. Cancer survivors are twice as likely to be physically inactive as the general population. 91
A Cochrane review,92 published since we developed the PROSPER intervention, found moderate-quality evidence that exercise during adjuvant treatment for breast cancer improved physical fitness (SMD 0.42, 95% CI 0.25 to 0.59; 15 studies, 1310 participants) and slightly reduced fatigue (SMD –0.28, 95% CI –0.41 to –0.16; 19 studies, 1698 participants), although there was weaker evidence for physical activity improving cancer-specific QoL or depression. 92 Given international recommendations to increase physical activity and evidence for improved outcomes during cancer treatment, without incurring risk of AEs, we incorporated physical activity guidance as a core component within the PROSPER intervention. 85
Evidence for behavioural change strategies
Early engagement with our lay representatives highlighted the need for a supported self-management approach to rehabilitation. National guidelines93 recommended that behaviour change strategies should be incorporated into any self-management interventions to support adherence and to achieve long-term behaviour change. 93 Adherence to any exercise intervention is essential to achieve the expected positive outcomes. However, there are numerous barriers to adherence and engagement with exercise, particularly during cancer treatment, when symptom burden can be overwhelming. 94 Psychological factors play a key role, particularly fear of cancer recurrence and fear of being active during treatment. 94–96
We referred to the NHS Health Trainer Manual,93,97 which was developed by health psychology experts in behaviour change. This practical guide summarises evidence-based strategies to promote positive health-related behavioural change. We selected the most relevant techniques to meet the needs of our patient population, while also considering demands placed on physiotherapy teams working in NHS clinics. Our aim was to increase motivation to exercise and to encourage adherence to the PROSPER programme. We recommended and trained physiotherapists in the motivational interviewing mode of communication. Motivational interviewing uses techniques to encourage compliance and participation. It has been found to be an effective technique for facilitating change in other lifestyle behaviours leading to improved health outcomes, such as weight loss and increased physical activity, and also for addressing the psychosocial needs of cancer survivors. 98 We incorporated the motivational interviewing approach into the PROSPER programme, along with other evidence-based psychological techniques. These included working with participants to set short- and long-term goals, promoting confidence to exercise, developing strategies to solve problems and reduce barriers and encouraging motivation and sustaining exercise adherence.
Intervention refinement with clinicians and patients
We refined the draft intervention to produce a long menu of exercises after discussion with 20 cancer rehabilitation specialists, upper limb physiotherapists and patient representatives who attended our 1-day consensus event at the WCTU in March 2015. We refined the menu of exercises after the consensus meeting and applied a colour-coded classification framework to each movement direction (e.g. flexion, abduction, external rotation with abduction). We described each exercise using lay-friendly terminology: for example, one shoulder abduction and external rotation movement was named ‘the woodchopper’ exercise. We developed patient manuals using colour photographs with clear instructions for each exercise. Qualitative interviews were held with seven women who had been recently treated for breast cancer and recruited from the lead hospital site, University Hospitals Coventry and Warwickshire (UHCW) NHS Trust. The women were positive about draft trial materials, preferring photographs to cartoon diagrams commonly used in NHS materials; they also preferred the term ‘physiotherapy’ to ‘exercise’ on patient-facing materials.
We also attended a community-based breast cancer support group for feedback on the almost finalised version of trial intervention materials before testing in the pilot study. This support group was attended by women with a recent breast cancer diagnosis, but also by cancer survivors who had been treated years previously. Feedback on materials was again positive, and the only recommendation was that more information on lymphoedema should be included.
Pilot study
We tested pragmatic implementation of the intervention by testing the PROSPER intervention with the first 15 women with newly diagnosed primary breast cancer recruited from three hospital sites within the pilot study. The qualitative study (see Chapter 5), with feedback from participants and physiotherapists, informed refinement of trial-related procedures and paperwork. Minor edits were made to the wording of participant materials. We reviewed treatment pathways and algorithms for the management of postoperative complications including pain, cording, wound infection, lymphoedema and cancer-related fatigue.
Overview of exercise programme
The aim of the exercise intervention was to prevent shoulder problems, caused by breast cancer treatments, by improving shoulder function through a physiotherapist-led, early, progressive, home-based postoperative exercise programme. It used behavioural strategies to encourage exercise adherence and support participants to be more physically active (see Table 5). Although several of the upper body exercises were familiar to physiotherapists and used in clinical practice, the PROSPER exercise programme was packaged as a new intervention to be prescribed and delivered as a whole. The intervention was delivered by NHS physiotherapists with musculoskeletal expertise.
Number and duration of physiotherapy contacts
The trial was designed to be pragmatic rather than explanatory; hence we tested an exercise intervention suitable for delivery in the NHS setting. All participants were advised to follow the Breast Cancer Care leaflets (usual care) in the first postoperative week. 99 For the intervention group, we then recommended a minimum of three face-to-face appointments with the trained PROSPER physiotherapist. These sessions were scheduled at specific time points after surgery: at 7–10 days, 4–6 weeks and 12–16 weeks postoperatively. These timings were selected to broadly fit around the cancer treatment pathway and expected tissue healing. Participants could also have up to three optional appointments, which could be face to face or by telephone. These appointments could be arranged at any time over the 12-month postoperative period, and physiotherapists could provide telephone support as and when needed. A maximum of six physiotherapy contacts were specified.
The first appointment was scheduled for 1 hour, to allow sufficient time for physical assessment, explanation of the programme and to prescribe stretches and ROM exercises. We recommended that the subsequent face-to-face follow-up appointments should be approximately 30-minute appointments.
Discharge from physiotherapy
Participants were discharged from physiotherapy when they had reached their long-term goals or when ROM and muscle strength in relation to their functional needs was achieved. This was determined by the physiotherapist, unless the participant decided that they no longer wanted to attend treatment sessions. We allowed more than six appointments if the services had capacity. After discharge, the participant could still contact their physiotherapist up to 12 months postoperatively.
Participant materials
Each trial participant was given an exercise folder, entitled ‘Your Exercise Folder’, which contained the full menu of exercises with colour pictures and instructions. The folder contained information on self-monitoring for postoperative complications. Each folder contained a supply of exercise diaries to record progression at home.
Recommended content of physiotherapy sessions
Table 435 summarises the initial targeted exercise dosage; all prescription details were recorded in a treatment log. All exercises are illustrated in Figure 1.
| Exercise type/category | Exercise | Frequency | Sets | Repetitions | Hold | Initial load | Progression | |
|---|---|---|---|---|---|---|---|---|
| From 7 days after surgery | ||||||||
| Warm-up | Posture check | Twice per day | 1 | 5 | 5 seconds | – | – | |
| Shoulder circles | N/A | |||||||
| Trunk twists (1–4) | 3 seconds | |||||||
| Range of movement | Daily stretch | Daily stretch and hold | Daily | 1 × 10 minutes or 2 × 5 minutes | ||||
| Forward | Clasp hand raise or forward wall slide | Twice per day | 1 | 5 | 3 seconds | – |
Step 1: increase up to 10 repetitions Step 2: if applicable, progress to next level of difficulty for the exercise |
|
| Side | Morning stretch or sideways wall slide | |||||||
| Open chest | Back broom lift or surrender | |||||||
| From 4 weeks after surgery | ||||||||
| Strength | Forward | Forward band lift or rocker (advanced only) | 2–3 times per week | 1 | 10 (minimum 8 repetitions, maximum 12 repetitions) | 3 seconds | Selected so that two repetitions are rated as 5 or 6 on modified Borg scale |
Step 1: maintain 5–6 rating on Borg scale through increasing load (from tan to red to blue Theraband tubing) Step 2: build up to three sets with 1–3 minutes’ rest between sets |
| Side | Sideways band stretch or woodchopper | |||||||
| Open chest | Overhead band stretch or front band stretch or low band row | |||||||
| Physical activity | From day 1 | Gentle | Daily | 3 | 10 minutes | – | – | Build up to 30 minutes continuous |
| From 4 weeks | Moderate | 5 times per week | – | 30 minutes | No restrictions after 12 weeks | |||
| From 12 weeks | Moderate to hard | |||||||
First physiotherapy appointment (7–10 days postoperatively)
Participants followed usual-care leaflets for the first postoperative week. At the first appointment, physiotherapists were asked to complete a short medical history; assess usual physical activity, pain intensity, wound healing, posture and active ROM; and screen for lymphoedema. Short- and long-term goals were discussed, along with barriers to and facilitators of exercise and an assessment of the participant’s confidence in their ability to do the prescribed exercises.
Participants were prescribed stretches and ROM shoulder movements above 90°. We recommended three ROM exercises that targeted the three main shoulder movements affected by breast cancer treatment (i.e. flexion, abduction and abduction with external rotation). One exercise of each movement was selected from a menu of exercises after discussion between the participant and physiotherapist. We encouraged collaborative decision-making rather than didactic prescription of exercise. The ‘daily stretch and hold’ exercise of the pectoralis muscles was also recommended for either 10 minutes once per day or 5 minutes twice per day. Participants were advised to first complete a warm-up comprising an active posture check, shoulder circles and trunk twists. If there were early signs or symptoms of lymphoedema, fist pump exercises were prescribed.
Manual therapy techniques
If the physiotherapist observed any signs of cording, we allowed two manual therapy techniques within the programme: gentle massage techniques, using effleurage and petrissage, and cording release by skin traction. We encouraged physiotherapists to teach participants how to do these techniques at home, either by themselves or with a partner, to promote self-management. We provided training and also laminated guides for physiotherapists on how to do these manual techniques.
Second face-to-face appointment (4–6 weeks postoperatively)
In the second appointment, recommended at 4–6 weeks postoperatively, the physiotherapist monitored progress, assessed wound healing, progressed ROM exercises and introduced strengthening exercises. We asked physiotherapists to repeat physical examinations and screen for postoperative complications, including cording and lymphoedema, and assess pain intensity and wound healing.
Prescription of strength exercises (4–6 weeks postoperatively)
Shoulder ROM was reassessed and isometric muscle strength was tested. Muscle strength was tested using a standardised protocol for glenohumeral flexion, abduction, and internal and external rotation. Both shoulders were assessed and given a score from 3 (can hold position with no resistance) to 5 (can hold position against maximal resistance), according to Kendall’s method. 100 The intervention included seven strengthening exercises: two flexion movements, two abduction movements and three abduction and external rotation movements (see Table 4). Selection of one strengthening exercise for each movement was made after assessment and discussion with the participant. All strengthening exercises, apart from the ‘rocker’ exercise, were performed using resistance bands (Theraband tubing). Each band was cut to 1 m length and provided at 100% elongation resistance of 1.1 kg (tan), 1.7 kg (red) and 2.6 kg (blue).
Third face-to-face appointment (12–16 weeks postoperatively)
The third appointment was scheduled for approximately 3 months after surgery. This time period was selected for exercise progression according to individual ability and final review of surgical wounds, which were expected to be fully healed. We also expected muscle adaptation to stimuli from strengthening exercises by this time. Exercises were progressed according to individual ability. The focus at 3 months was on the return to usual activities and the ability to undertake more demanding functional activities.
Establishing baseline level and progression for exercises
Range of movement and stretch exercises
At all appointments, the ROM of active scapula elevation, protraction and retraction was assessed. In addition, glenohumeral flexion, abduction, and internal and external rotation were assessed. ROM was graded as either ‘full’ or ‘restricted’ by comparing the affected (operated side) with the unaffected arm. ROM exercises could be progressed by increasing the number of sets and repetitions and/or advancing to the next difficulty level for the chosen exercise. The daily stretch was progressed by increasing time spent stretching, if needed.
Strength exercises
Strength exercises were prescribed only from 4 weeks postoperatively. Given the challenges of measuring a 1-repetition maximum (1RM) to determine the percentage to achieve an adequate stimulus to increase muscle strength, a modified Borg scale was used to define the correct level of resistance based on self-perceived effort. This 10-point scale has been validated for use in determining intensity of resistance exercises. 101 Participants were asked to perform two repetitions of each selected strength exercise using a resistance band. The target resistance was reached if the participant rated their level of exertion as 5 or 6 (‘hard’ rating) on the modified Borg scale. The initial prescription was to complete one set of 8–12 repetitions, equivalent to a resistance of 70–80% of 1RM. When the participant could perform 12 repetitions easily, progression was achieved by changing band resistance, progressing to the next difficulty level exercise(s) or by increasing repetitions and/or sets. We specified that participants should aim to build to three sets of 10 repetitions for each exercise. We recommended that strength exercises be performed two or three times per week. This was to allow rest, recovery and adaptation of muscles from overload.
General physical activity
General physical activity was encouraged during every contact. The aim was to progressively increase activity every week. Participants were encouraged to start off gently, then gradually work up to moderate or more intense activity. Physiotherapists were asked to work collaboratively with each participant to develop an individualised plan and strategies to achieve 150 minutes of moderate intensity physical activity per week. The specified amount was 30 minutes of activity, 5 days per week.
Behavioural support strategies
Behavioural support strategies were incorporated to promote confidence and encourage motivation to exercise over the longer term (Table 5). Physiotherapists were encouraged to use a motivational interviewing approach to communication to support self-management, explore any barriers to exercise and problem-solve solutions. They were trained to help each participant define their own short- and long-term upper limb and/or physical activity goals using SMART (specific, measurable, achievable, relevant and timely) principles. We provided SMART examples during intervention training, for example to complete exercises every day after breakfast (short-term goal) and to be able to play one game of tennis again by 8 months postdiagnosis (long-term goal). We prespecified that one short-term goal was to complete the PROSPER exercises daily, to achieve and link to long-term goals. This strategy of linking short- and long-term goals can improve adherence. 93 Participants were asked to rate their confidence in their ability to complete the prescribed exercises using a 10-point numerical rating scale. If the participant scored ≤ 7, the physiotherapist would explore reasons and discuss possible solutions to increase their confidence.
| Strategy | Example of activity |
|---|---|
| Collaborative goal setting | Joint setting of short- and long-term functional and social goals |
| Confidence scale | Measured using numerical rating scale 0–10. Low confidence in ability to complete prescribed exercises defined as score of < 793 |
| Implementation intentions | Where, when and which exercises will be done |
| Exercise diary | Complete diary at home, physiotherapist to review and monitor progress during contacts |
| Barriers and facilitators | Barriers (hurdles) explored and discussed during contacts |
Exercise diaries
Each participant was provided with an exercise diary to record their exercises. These served as a method of self-monitoring if they had achieved the short-term goal of completing their exercises. Exercise planning, based on concepts of implementation intentions,93 was incorporated into the exercise diary. Participants recorded the best time and place for them to do their exercises, which acted as a prompt to regular exercise. At each follow-up appointment, the physiotherapist would discuss if the participant had difficulties following the PROSPER intervention. They would explore any barriers to and facilitators of exercise and the physiotherapist would help to find solutions to facilitate adherence to the intervention.
Exercise quality control assessments
All intervention sites were visited by one of the research physiotherapists (HR/BM) to undertake QC assessments. These visits were scheduled shortly after completion of training and after the physiotherapist had undertaken one or more assessments with participants referred to the intervention group. The aim of the QC assessment was to ensure that the intervention was delivered in a standardised manner, according to the trial protocol and training. At least one appointment, either the first or follow-up appointment, was observed. It was a requirement for all physiotherapists to demonstrate competency in the intervention. We used a standardised checklist during the visit to monitor exercise prescription, to ensure safe delivery of the programme and to check that the intervention was delivered according to the intervention manual. Checks were also made of all trial-related documentation, including prescription logs, treatment forms, withdrawal forms and electronic appointment spreadsheets. Each physiotherapist received a written graded report at the end of the assessment, graded as satisfactory, minor concerns or serious concerns. Follow-up visits were arranged if any concerns were identified. The chief investigator was notified if any serious concerns were identified. The research physiotherapists provided regular supervision and support to all physiotherapists over the duration of the trial.
Chapter 4 Results
Study timeline
The first trial participant was recruited in January 2016 and recruitment continued until July 2017. Postal follow-up completed in August 2018 and NHS Digital data were received in October 2019. We made no substantial changes to interventions from pilot to main trial other than minor adaptations to physiotherapy treatment logs.
Recruiting centres
We recruited participants from 17 breast cancer units across England, from Lancashire to Cornwall. These ranged from high-volume cancers centres within major teaching hospitals to smaller units serving more rural localities. All hospitals had access to physiotherapy services. Appendix 1 provides details of participating hospitals by NHS trust. The trial recruitment graph is displayed in Report Supplementary Material 1.
Participant flow
Screening
The CONSORT flow diagram (Figure 2) illustrates the overall flow of participants through the study from screening to the 12-month follow-up. A total of 951 breast cancer patients were screened. Of these, 761 out of 951 (80%) were eligible for invitation to the study and 662 out of 761 (87% of those eligible) patients were approached by clinical teams; 99 (13%) were not approached by clinical staff owing to limited time to recruit. Of those invited, 392 out of 662 (59%) were consented and were randomised to interventions (392/761; 52% of eligible patients). Table 6 reports screening by hospital code and by treatment allocation. The mean number of participants randomised per recruiting centre was 23 (range 7–73). The main reasons for not participating in the study included lack of transport or car parking issues, having ‘too much going on’ or feeling overwhelmed with cancer treatment.
FIGURE 2.
The CONSORT flow diagram of trial participants. Adapted with permission from Bruce et al. 102 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
| Site code | Screened, n (N = 951) | Randomised, n (%)a | ||
|---|---|---|---|---|
| Usual-care group (N = 191) | Exercise group (N = 191) | Total (N = 392) | ||
| UHCW | 89 | 36 | 37 | 73 (82.0) |
| DCH | 68 | 19 | 19 | 38 (55.9) |
| OUH | 92 | 18 | 18 | 36 (39.1) |
| HH | 89 | 18 | 17 | 35 (39.3) |
| NCH | 57 | 16 | 17 | 33 (57.9) |
| CRH | 97 | 14 | 14 | 28 (28.9) |
| BCH | 58 | 11 | 12 | 23 (39.7) |
| WMH | 72 | 12 | 10 | 22 (30.6) |
| HCH | 53 | 7 | 8 | 15 (28.3) |
| MKH | 23 | 7 | 8 | 15 (65.2) |
| RCH | 35 | 7 | 8 | 15 (42.9) |
| RSH | 45 | 7 | 7 | 14 (31.1) |
| MDH | 16 | 5 | 7 | 12 (75.0) |
| BTH | 93 | 5 | 4 | 9 (9.7) |
| MPH | 10 | 6 | 3 | 9 (90.0) |
| GEH | 11 | 4 | 4 | 8 (72.7) |
| QAH | 43 | 4 | 3 | 7 (16.3) |
Recruitment
Our target sample was 350 participants; however, we extended recruitment by 1 month to replace 42 participants who either failed to return their baseline questionnaires or were randomised in error (Figure 2). Hence, our final randomised sample was 392 participants, 196 allocated to each intervention group. Of the 392 randomised, eight (2%) participants were randomised in error, as either they were booked for or they changed their mind to request bilateral breast surgery or immediate breast reconstruction after being screened as eligible (five from the usual-care group, three from the exercise group). Two participants from the exercise group withdrew at randomisation because they changed their mind or they did not want to complete questionnaires: no data were collected for these participants. Of the 392 randomised, 350 (89%) returned their baseline questionnaire (usual-care group, n = 175; exercise group, n = 175). The 32 out of 392 (8%) participants who did not return baseline questionnaires were balanced by treatment group (usual-care group, n = 16; exercise group, n = 16). Clinical- and patient-related criteria used for eligibility screening are reported in Table 7. Of those randomised, 44 out of 392 (11%) participants were recruited later in their care pathway because they were informed of the need for axillary radiotherapy postoperatively (usual-care group, n = 22; exercise group, n = 22).
| Eligibility criteriona | Number of participants (%) meeting criterion | ||
|---|---|---|---|
| Usual-care group (N = 196) | Exercise group (N = 196) | Total (N = 392) | |
| Randomised, no data collectedb | 5 (2.6) | 5 (2.6) | 10 (2.6) |
| Randomised, data collected | 191 (97.4) | 191 (97.4) | 382 (97.4) |
| Planned ANC surgery | 117 (59.7) | 114 (58.2) | 231 (58.9) |
| Planned radiotherapy to axilla/supraclavicular nodes | 22 (11.2) | 22 (11.2) | 44 (11.2) |
| BMI ≥ 30 kg/m2 | 142 (72.4) | 135 (68.9) | 277 (70.7) |
| Existing shoulder problem | 32 (16.3) | 51 (26.0) | 83 (21.2) |
Cancer treatments
We report cancer treatments for 382 out of 392 (97%) randomised participants; we did not collect treatment data for 10 participants who were withdrawn or randomised in error. Of the 382 participants, all underwent breast cancer surgery and most had adjuvant chemotherapy and/or radiotherapy over the duration of the study (Table 8). Treatment pathways were complex, with one-fifth (22%) of participants readmitted for resection of tumour margins, more invasive surgery or oncoplastic revisions over the follow-up period. Most participants had ANC surgery (322/382; 84%). Five participants had reconstruction surgery after mastectomy over the course of the 12-month follow-up period (usual-care group, n = 2; exercise group, n = 3).
| Treatmenta | Treatment group, n (%) | Total (N = 392) | |
|---|---|---|---|
| Usual care (N = 196) | Exercise (N = 196) | ||
| Randomised, no data collected | 5 | 5 | 10 |
| Randomised, treatment data collected | 191 | 191 | 382 |
| Breast surgery | |||
| BCS | 116 (60.7) | 106 (55.5) | 222 (58.1) |
| Mastectomy | 73 (38.2) | 84 (44.0) | 157 (41.1) |
| None | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Missing | 2 (1.0) | 0 (0.0) | 2 (0.5) |
| Axillary surgery | |||
| ANC | 162 (84.8) | 165 (86.4) | 327 (85.6) |
| SLNB | 26 (13.6) | 26 (13.6) | 52 (13.6) |
| None | 2 (1.0) | 0 (0.0) | 2 (0.5) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| More than two surgeries | 42 (22.0) | 40 (20.9) | 82 (21.5) |
| Radiotherapy | |||
| Yes | 166 (86.9) | 151 (79.1) | 317 (83.0) |
| No | 14 (7.3) | 26 (13.6) | 40 (10.4) |
| Missing | 11 (5.8) | 14 (7.3) | 25 (6.5) |
| Site of radiotherapyb | |||
| Breast | 114 (59.7) | 94 (49.2) | 208 (54.5) |
| Chest wall | 50 (26.2) | 57 (29.8) | 107 (28.0) |
| Axilla/supraclavicular nodes | 62 (32.5) | 51 (26.7) | 113 (29.6) |
| Radiotherapy boost given | 60 (31.4) | 44 (23.0) | 104 (27.2) |
| Chemotherapy | 118 (61.8) | 108 (56.5) | 226 (59.2) |
| Chemotherapy and radiotherapy | 111 (58.1) | 97 (50.8) | 208 (54.5) |
| ANC and axilla/supraclavicular nodes radiotherapy | 65 (34.0) | 53 (27.7) | 118 (30.9) |
Participant characteristics, completed baseline questionnaires (n = 350)
Among the 392 participants randomised, the mean age was 58.1 years (SD 12.1 years, range 28–88 years). Among the 350 out of 392 (89%) participants who returned baseline questionnaires, the mean age was similar: 58.2 (SD 11.9) years (see Table 9). For those randomised preoperatively, the median time from completion of baseline questionnaire to surgery was 5 days [interquartile range (IQR) 1–14 days]. For the later-entry participants, the median time from surgery to completion of baseline questionnaire was 9 days (IQR 4–33 days). Table 9 presents participant characteristics for those who returned completed questionnaires (n = 350 used as denominator). Overall, characteristics were well balanced across treatment groups. The majority of participants were white (321/350; 92%) and two-thirds of the sample returning questionnaires were either married or co-habiting (251/350; 72%). One-third had school-only education (112/350; 32%), although almost half of participants had some college or university education (164/350; 47%). Most participants were either full- or part-time employed (135/350; 39%) or were retired (132/350; 38%). Three-quarters of those returning baseline questionnaires had a BMI of ≥ 25 kg/m2 (277/350; 79%) and thus categorised as being overweight or obese on recruitment. Approximately one-fifth (74/350; 21%) of participants recruited had a history of or existing shoulder problems at baseline. Half of responding participants (175/350; 50%) reported one or two other comorbidities, in addition to their cancer diagnosis on recruitment (see Table 9). Of those reporting more than one health condition, the most commonly reported conditions were arthritis and mental health disorders. Nearly half of participants were physically active at baseline, reporting walking outside for 5–7 days per week (160/350; 46%), although most never took part in any strenuous sports or recreational activities (266/350; 76%) (see Table 9).
| Characteristics | Usual-care group (N = 175) | Exercise group (N = 175) | Total (N = 350) |
|---|---|---|---|
| Age (years), mean (SD) | 57.9 (11.7) | 58.4 (12.1) | 58.2 (11.9) |
| Age band (years), n (%) | |||
| < 50 | 49 (28.0) | 48 (27.4) | 97 (27.7) |
| 50–70 | 98 (56.0) | 97 (55.4) | 195 (55.7) |
| > 70 | 28 (16.0) | 30 (17.2) | 58 (16.6) |
| BMI (kg/m2), mean (SD) | 29.9 (6.7) | 29.9 (7.1) | 29.9 (6.9) |
| Missing | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| BMI (kg/m2), n (%) | |||
| < 25 | 44 (25.1) | 50 (28.6) | 94 (26.9) |
| 25–≤ 30 | 48 (27.4) | 42 (24.0) | 90 (25.7) |
| ≥ 30 | 79 (45.1) | 82 (46.9) | 161 (46.0) |
| Missing | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| Marital status, n (%) | |||
| Single | 18 (10.3) | 15 (8.6) | 33 (9.4) |
| Married/co-habiting | 127 (72.3) | 124 (70.9) | 251 (71.7) |
| Divorced/separated | 16 (9.1) | 20 (11.4) | 36 (10.3) |
| Widowed | 14 (8.0) | 14 (8.0) | 28 (8.0) |
| Missing | 0 (0.0) | 2 (1.1) | 2 (0.6) |
| Education, n (%) | |||
| School only | 54 (30.9) | 58 (33.1) | 112 (32.0) |
| Work qualification | 36 (20.6) | 35 (20.0) | 71 (20.3) |
| College/university, non-degree | 49 (28.0) | 43 (24.6) | 92 (26.3) |
| College/university, degree | 35 (20.0) | 37 (21.1) | 72 (20.6) |
| Missing | 1 (0.6) | 2 (1.1) | 3 (0.9) |
| Employment status, n (%) | |||
| Working full-time | 40 (22.9) | 45 (25.7) | 85 (24.3) |
| Working part-time | 25 (14.3) | 25 (14.3) | 50 (14.3) |
| Retired | 67 (38.3) | 65 (37.1) | 132 (37.7) |
| Housewife, mother/carer | 16 (9.1) | 6 (3.4) | 22 (6.3) |
| Illness/disability | 11 (6.3) | 19 (10.9) | 30 (8.6) |
| Self-employed | 6 (3.4) | 10 (5.7) | 16 (4.6) |
| Other | 5 (2.9) | 4 (2.3) | 9 (2.6) |
| Missing | 5 (2.9) | 1 (0.6) | 6 (1.7) |
| Ethnicity, n (%) | |||
| White | 159 (90.9) | 162 (92.6) | 321 (91.7) |
| Indian/Pakistani/Bangladeshi | 12 (6.9) | 5 (2.9) | 17 (4.9) |
| Other | 3 (1.7) | 7 (4.0) | 10 (2.9) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Comorbidities (type), n (%) | |||
| Heart problems | 20 (11.4) | 32 (18.3) | 52 (14.9) |
| Arthritis | 52 (29.7) | 45 (25.7) | 98 (28.0) |
| Mental health problems | 44 (25.1) | 35 (20.0) | 79 (22.6) |
| Diabetes | 17 (9.7) | 18 (10.3) | 35 (10.0) |
| Back problems | 40 (22.9) | 40 (22.9) | 80 (22.9) |
| Lung problems | 19 (10.9) | 16 (9.1) | 35 (10.0) |
| Migraine/headaches | 25 (14.3) | 20 (11.4) | 45 (12.9) |
| Irritable bowel syndrome | 21 (12.0) | 24 (13.7) | 45 (12.9) |
| Other gastrointestinal problem | 5 (2.9) | 7 (4.0) | 12 (3.4) |
| Any other condition | 12 (6.9) | 9 (5.1) | 20 (5.7) |
| Comorbidities (number), n (%) | |||
| None | 47 (26.9) | 47 (26.9) | 94 (26.9) |
| 1 or 2 | 86 (49.1) | 90 (51.4) | 176 (50.3) |
| ≥ 3 | 42 (24.0) | 38 (21.7) | 80 (22.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Outside walking, days per week | |||
| Never or seldom (1–2) | 38 (21.7) | 46 (26.3) | 84 (24.0) |
| Sometimes (3–4) | 51 (29.1) | 54 (30.9) | 105 (30.0) |
| Often (5–7) | 85 (48.6) | 75 (42.9) | 160 (45.7) |
| Missing | 1 (0.6) | 0 | 1 (0.3) |
| Outside walking, hours per day | |||
| < 1 | 45 (25.7) | 38 (21.7) | 83 (23.7) |
| 1–< 2 | 53 (30.3) | 54 (30.9) | 107 (30.6) |
| 2–4 | 45 (25.7) | 48 (27.4) | 93 (26.6) |
| > 4 | 31 (17.7) | 33 (18.9) | 64 (18.3) |
| Missing | 1 (0.6) | 2 (1.1) | 3 (0.9) |
| Strenuous sport/recreation, days per week | |||
| Never | 134 (76.6) | 132 (75.4) | 266 (76.0) |
| Seldom (1–2) | 26 (14.9) | 25 (14.3) | 51 (14.6) |
| Sometimes/often (≥ 3) | 13 (7.4) | 15 (8.6) | 28 (8.0) |
| Missing | 2 (1.1) | 3 (1.7) | 5 (1.4) |
| Strenuous activity, hours | |||
| No strenuous activity | 136 (77.7) | 135 (77.1) | 271 (77.4) |
| < 1 hour | 13 (7.4) | 17 (9.7) | 30 (8.6) |
| > 1 hour | 26 (14.9) | 23 (13.1) | 49 (14.0) |
| Handedness, n (%) | |||
| Right-handed | 157 (89.7) | 168 (96.0) | 325 (92.8) |
| Left-handed | 17 (9.7) | 6 (3.4) | 23 (6.6) |
| Missing | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Any shoulder problem, n (%) | |||
| Yes | 29 (16.6) | 45 (25.7) | 74 (21.1) |
| No | 120 (68.6) | 105 (60.0) | 225 (64.3) |
| Missing | 26 (14.8) | 25 (14.3) | 51 (14.6) |
| Upper limb function | |||
| DASH score, mean (SD) | 18.2 (19.8) | 19.5 (21.2) | 18.8 (20.5) |
| DASH score, median (IQR) | 11.7 (1.7–30.0) | 12.5 (2.5–30.8) | 12.3 (1.7–30.2) |
| Missing, n (%) | 4 (2.3) | 8 (4.6) | 12 (3.4) |
| Neuropathic pain, DN4 | |||
| No pain, n (%) | 95 (54.3) | 89 (50.9) | 184 (52.6) |
| ≤ 3 non-neuropathic pain, n (%) | 57 (32.6) | 63 (36.0) | 120 (34.3) |
| > 3 non-neuropathic pain, n (%) | 17 (9.7) | 16 (9.1) | 33 (9.4) |
| Missing, n (%) | 6 (3.4) | 7 (4.0) | 13 (3.7) |
| Pain intensity NRS, mean (SD) | 1.9 (2.5) | 1.9 (2.4) | 1.9 (2.4) |
| Missing, n (%) | 6 (3.4) | 13 (7.4) | 19 (5.4) |
| FACT-B4, mean (SD) | 2.7 (4.0) | 3.1 (4.2) | 2.9 (4.1) |
| Missing, n (%) | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Lymphoedema, LBCQ, n (%) | |||
| Arm feels heavy (yes) | 38 (21.7) | 43 (24.6) | 81 (23.1) |
| Arm looks swollen (yes) | 27 (15.4) | 25 (14.3) | 52 (14.9) |
| Not heavy and swollen (neither) | 152 (86.9) | 148 (84.6) | 300 (85.7) |
| Arm heavy and swollen (both) | 20 (11.4) | 17 (9.7) | 37 (10.6) |
| Missing | 3 (1.7) | 10 (5.7) | 13 (3.7) |
| HRQoL | |||
| EQ-5D-5L, mean (SD) | 0.67 (0.22) | 0.68 (0.20) | 0.67 (0.2) |
| Missing, n (%) | 18 (10.3) | 16 (9.1) | 34 (9.7) |
| SF-12 PCS, mean (SD) | 47.6 (11.6) | 46.8 (11.6) | 47.2 (11.6) |
| Missing, n (%) | 8 (4.6) | 7 (4.0) | 15 (4.3) |
| SF-12 MCS, mean (SD) | 44.7 (11.7) | 46.8 (10.6) | 45.8 (11.2) |
| Missing, n (%) | 8 (4.6) | 7 (4.0) | 15 (4.3) |
| Confidence | |||
| Return to usual activities, mean (SD) | 7.5 (2.5) | 8.1 (2.3) | 7.8 (2.4) |
| Missing, n (%) | 2 (1.1) | 0 (0.0) | 2 (0.6) |
| Return to physical activity, mean (SD) | 7.5 (2.3) | 8.0 (2.3) | 7.7 (2.3) |
| Missing, n (%) | 2 (1.1) | 0 (0.0) | 2 (0.6) |
Participant baseline outcome measures
We present a summary of baseline outcome measures in Table 9. All outcome measures were similar across treatment groups at baseline as expected, owing to randomisation, and there was no difference in mean DASH score at baseline (mean 18.8; SD 20.5). In relation to the sample size, we anticipated that our pooled SD for the DASH score would be 19.5 at baseline. Of those returning baseline questionnaires, completion of the DASH scale was good (338/350; 97%); this equated to 338/392 (86%) of those randomised (Table 10). Approximately 10% (36/350) women reported some arm heaviness and swelling, indicative of lymphoedema symptoms on recruitment, possibly as a consequence of preoperative biopsy or investigative procedures. A similar proportion reported preoperative neuropathic pain in the area of breast or armpit (33/350; 9%). QoL scores were comparable at baseline, with mental health scores being slightly lower than physical health scores on recruitment.
| Scale | Number (%) of participants with complete data | |||||
|---|---|---|---|---|---|---|
| At baseline | At 6 months | At 12 months | ||||
| Usual-care group (N = 175) | Exercise group (N = 175) | Usual-care group (N = 133) | Exercise group (N = 145) | Usual-care group (N = 139) | Exercise group (N = 135) | |
| DASH | ||||||
| Completeda | 171 (97.7) | 167 (95.4) | 125 (94.0) | 134 (92.4) | 138 (99.3) | 132 (97.8) |
| Missing ≥ 4 | 4 (2.3) | 8 (4.6) | 8 (6.0) | 11 (7.6) | 1 (0.7) | 3 (2.2) |
| DASH-I | ||||||
| Completed | 170 (97.1) | 172 (98.3) | 125 (94.0) | 141 (97.2) | 137 (98.6) | 133 (98.5) |
| Missing | 5 (2.9) | 3 (1.7) | 8 (6.0) | 4 (2.8) | 2 (1.4) | 2 (1.5) |
| DASH-AL | ||||||
| Completed | 168 (96.0) | 170 (97.1) | 122 (91.7) | 132 (91.0) | 135 (97.1) | 130 (96.3) |
| Missing | 7 (4.0) | 5 (2.9) | 10 (7.5) | 13 (9.0) | 4 (2.9) | 5 (3.7) |
| DASH-PR | ||||||
| Completed | 170 (97.1) | 159 (90.9) | 124 (93.2) | 132 (91.0) | 133 (95.7) | 126 (93.3) |
| Missing | 5 (2.9) | 16 (9.1) | 9 (6.8) | 13 (9.0) | 6 (4.3) | 9 (6.7) |
Outcomes and analyses
Participant follow-up
Postal response return rates were 87% (303/350 at 6 weeks), 79% at 6 months (278/350) and 78% at 12 months (274/350). As a proportion of all randomised participants, this equated to 303/392 (77%), 278/392 (71%) and 274/392 (70%) at 6 weeks, 6 months and 12 months, respectively. Response rates were similar by treatment allocation, as detailed in the CONSORT diagram (Figure 2). Median time to return questionnaires was also similar by group. The median time from randomisation to return of the 6-week questionnaire was 1.8 months (IQR 1.6–2.3 months), with a median of 6.6 months (IQR 6.3–7.2 months) and 12.5 months (IQR 12.3–13.3 months) for the return of 6- and 12-month questionnaires, respectively.
Withdrawals and loss to follow-up
Withdrawals and loss to follow-up were similar across treatment groups over the duration of the trial (Figure 2). We were notified of five deaths over the follow-up period (5/392; 1%), two in the usual-care group and three in the treatment group. Common reasons for withdrawal from the trial were ongoing cancer treatment burden, cancer recurrence or the fact that participants did not want to complete questionnaires. There were no differences in the number of participants excluded from analysis because of withdrawal, death or non-response by treatment group (58/196, usual-care group; 56/196, exercise group; Figure 2).
Primary outcome: DASH scale completion
We examined completion of the DASH questionnaire at each follow-up time point (Table 10). Of those returning questionnaires, missingness of four or more items was low across each time point: 3% at baseline, 7% at 6 months and 2% at 12 months of those returning questionnaires. The questions most commonly omitted by participants were difficulty with ability to wash or blow dry hair and the question relating to difficulty with sexual activities. Women reported they had hair loss from chemotherapy around the 6-month follow-up time point.
Comparison of randomised sample with analysed sample
We report the descriptive characteristics of the randomised sample to those returning data at 12 months (Table 11). Those who remained in the trial and who returned 12-month data were slightly older than those who were randomised. There were no differences between groups in terms of marital status, clinical treatment given over 12 months, BMI or self-reported comorbidity. Mean DASH scores, pain intensity scores, rates of neuropathic pain, lymphoedema, arm symptoms and HRQoL scores differed between the randomised sample and those providing 12-month follow-up data, although this is partly explained by comparing outcomes before and after cancer treatment, thus comparing preoperative data with post-treatment data.
| Characteristic | Randomised sample | Sample providing 12-month primary outcome data | ||
|---|---|---|---|---|
| Usual-care group (N = 196) | Exercise group (N = 196) | Usual-care group (N = 139) | Exercise group (N = 135) | |
| Age (years), mean (SD) | 57.8 (12.0) | 58.4 (12.2) | 58.6 (11.2) | 59.7 (12.1) |
| Randomised, with treatment data | N = 175 | N = 175 | N = 139 | N = 135 |
| ANC surgery, n (%) | 162 (92.5) | 165 (94.3) | 115 (82.7) | 116 (85.9) |
| Radiotherapy, n (%) | 166 (94.9) | 151 (86.3) | 124 (89.2) | 112 (83.0) |
| BMI (kg/m2), mean (SD) | 30.6 (7.2) | 29.9 (6.9) | 29.7 (6.4) | 29.2 (6.4) |
| Marital status, n (%) | ||||
| Single | 18 (10.3) | 15 (8.6) | 13 (9.4) | 12 (8.9) |
| Married/co-habiting | 127 (72.3) | 124 (70.9) | 98 (70.5) | 91 (67.4) |
| Divorced/separated | 16 (9.1) | 20 (11.4) | 11 (7.9) | 13 (9.6) |
| Widowed | 14 (8.0) | 14 (8.0) | 11 (7.9) | 10 (7.4) |
| Comorbidity, n (%) | ||||
| None | 47 (26.9) | 47 (26.9) | 34 (24.5) | 35 (25.9) |
| 1 or 2 | 86 (49.1) | 89 (50.9) | 66 (47.5) | 64 (47.4) |
| ≥ 3 | 42 (24) | 39 (22.2) | 33 (23.7) | 28 (20.7) |
| Walking outside, days per week, n (%) | ||||
| Never/seldom (1–2) | 38 (21.7) | 46 (26.3) | 24 (17.3) | 20 (14.8) |
| Sometimes (3–4) | 51 (29.1) | 54 (30.9) | 36 (25.9) | 34 (25.2) |
| Often (5–7) | 85 (48.6) | 75 (42.8) | 77 (55.4) | 81 (60.0) |
| Strenuous sport/recreation, days per week | ||||
| Never/seldom (1–2) | 160 (91.4) | 157 (89.7) | 125 (90.0) | 113 (83.7) |
| Sometimes/often (≥ 3) | 13 (7.4) | 15 (8.6) | 13 (9.4) | 20 (14.8) |
| Upper limb function | ||||
| DASH score, mean (SD) | 18.2 (19.8) | 19.5 (21.2) | 23.7 (22.9) | 16.3 (17.6) |
| DASH score, median (IQR) | 11.7 (1.7–30.0) | 12.5 (2.5–30.8) | 16.3 (4.2–35.3) | 9.5 (3.5–23.3) |
| DN4, n (%) | ||||
| ≤ 3 non-neuropathic pain | 57 (32.6) | 63 (36.0) | 75 (54.0) | 82 (60.7) |
| > 3 neuropathic pain | 17 (9.7) | 16 (9.1) | 32 (23.0) | 22 (16.3) |
| Pain intensity, mean (SD) NRS | 1.9 (2.5) | 1.9 (2.4) | 2.6 (2.4) | 1.9 (2.0) |
| FACT-B4, mean (SD) | 2.7 (4.0) | 3.1 (4.2) | 5.4 (5.2) | 3.4 (4.0) |
| Lymphoedema, n (%) | ||||
| Arm heavy and swollen | 20 (11.4) | 17 (9.7) | 36 (25.9) | 33 (24.4) |
| HRQoL | ||||
| EQ-5D-5L, mean (SD) | 0.67 (0.22) | 0.68 (0.20) | 0.63 (0.22) | 0.71 (0.21) |
| SF-12 PCS, mean (SD) | 47.6 (11.6) | 46.8 (11.6) | 43.8 (11.5) | 48.1 (10.0) |
| SF-12 MCS, mean (SD) | 44.7 (11.7) | 46.8 (10.6) | 46.6 (11.2) | 48.7 (10.0) |
Primary outcome: upper limb function
For the primary ITT analysis, we found evidence of a difference in upper limb function at 12 months, with those randomised to the exercise programme having better upper limb function scores than those in the usual-care group (Table 12). The unadjusted mean difference in DASH scores for usual care versus exercise was –7.34 (95% CI –12.23 to –2.44; p = 0.003) and the adjusted mean difference was –7.81 (95% CI –12.44 to –3.17; p = 0.001). Given that the primary outcome data were skewed, we also examined non-parametric models, with similar findings of an improvement in the exercise group compared with usual care for both unadjusted (median –4.89, 95% CI –7.84 to –1.58); p = 0.002) and adjusted models (median –7.87, 95% CI –12.01 to –2.66; p = 0.01).
| Outcome | Usual care group (N = 196) | Exercise group (N = 196) | Unadjusted MD (95% CI) | p-value | Adjusted MDa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Primary outcome: upper limb function at 12 months, ITT analysis | ||||||
| n | 138 | 132 | –7.34 (–12.23 to –2.44) | 0.001 | –7.81 (–12.44 to –3.17) | 0.001 |
| Mean (SD) | 23.7 (22.9) | 16.3 (17.6) | ||||
| Primary outcome: change in upper limb function, baseline to 12 months, ITT analysis | ||||||
| n | 130 | 117 | 7.98 (3.03 to 12.92) | 0.002 | 7.81 (3.17 to 12.44) | 0.001 |
| Mean (SD) | –5.3 (19.7) | 2.6 (19.7) | ||||
| Secondary outcome: upper limb function at 6 months | ||||||
| n | 125 | 134 | –2.76 (–7.31 to 1.79) | 0.23 | –4.60 (–8.90 to –0.30) | 0.04 |
| Mean (SD) | 20.8 (20.1) | 18.0 (17.1) | ||||
| Secondary outcome: change in upper limb function, baseline to 6 months | ||||||
| n | 118 | 121 | 5.96 (1.23 to 10.70) | 0.01 | 4.60 (0.31 to 8.90) | 0.04 |
| Mean (SD) | –5.3 (19.4) | 0.7 (17.8) | ||||
We also examined recovery trajectory in upper limb function over time (see Table 12). At 6 months, DASH scores had improved from baseline in the exercise group but declined in those receiving usual care, resulting in a statistically significant difference in the mean change from baseline to 6 months between groups, for both unadjusted and adjusted comparisons (adjusted MD 4.60, 95% CI 0.31 to 8.90; p = 0.04) (see Table 12).
Primary outcome: complier–average causal effect analysis
Using our compliance definition of at least three physiotherapy contacts, 143 out of 196 participants (73%) fully complied with the intervention. For the CACE analysis, we found a statistically significant difference in upper limb function at 12 months by compliance status (adjusted MD –8.74, 95% CI –13.71 to –3.77; p = < 0.001) (Table 13).
| Analysis | MD (95% CI) | p-value |
|---|---|---|
| ITT | ||
| Unadjusted | –7.34 (–12.23 to –2.44) | 0.001 |
| Adjusted | –7.81 (–12.44 to –3.17) | 0.001 |
| CACE | ||
| Unadjusted | –8.35 (–13.84 to –2.85) | 0.003 |
| Adjusted | –8.74 (–13.71 to –3.77) | < 0.001 |
We also carried out multiple imputation as detailed in the statistical analysis plan. Owing to low missingness, the imputation chained equation procedure was not valid for implementation. We replaced this with imputation of the total DASH score, where some items were missing, to assess the impact. The results for multiple imputation were similar to those presented for the CACE (see Table 13).
Secondary outcomes
Upper limb function: impairment, activity limitations and participation restriction
We observed a difference in DASH activity limitations at 12 months, with higher activity limitation scores in the usual-care group relative to the exercise group (adjusted MD –8.04, 95% CI –12.93 to –3.14; p = 0.001) (Table 14). For those receiving usual care, activity limitations were worse at 12 months than they were at baseline, indicating greater limitation over time. Similarly, DASH participation restriction and impairment scores differed between groups at 12 months: participation restrictions (adjusted MD –5.77, 95% CI –10.67 to –0.88; p = 0.02) and impairment increased over time in those randomised to usual care compared with those randomised to exercise (adjusted MD –7.15, 95% CI –13.19 to –1.11; p = 0.02). We examined non-parametric models, and this did not change the strength or direction of findings. We also found differences in activity limitations, but not in impairment or participation restrictions, between groups at 6 months.
| Subdomain | Mean (SD) score | Unadjusted MD (95% CI) | p-value | Adjusteda MD (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Usual-care group (N = 196) | Exercise group(N = 196) | |||||
| 6 months | ||||||
| DASH-AL | 20.0 (20.8) | 16.5 (17.8) | –3.47 (–8.25 to 1.31) | 0.15 | –5.21 (–9.78 to –0.63) | 0.03 |
| DASH-PR | 19.7 (21.4) | 16.1 (17.2) | –3.60 (–8.37 to 1.16) | 0.14 | –4.25 (–8.81 to 0.31) | 0.07 |
| DASH-I | 23.2 (20.3) | 20.9 (17.7) | –2.24 (–6.83 to 2.36) | 0.34 | –2.94 (–7.77 to 1.88) | 0.23 |
| 12 months | ||||||
| DASH-AL | 22.6 (23.3) | 15.4 (18.2) | –7.23 (–12.30 to –2.17) | 0.005 | –8.04 (–12.93 to –3.14) | 0.001 |
| DASH-PR | 19.0 (22.4) | 14.9 (20.2) | –6.08 (–11.28 to –0.88) | 0.02 | –5.77 (–10.67 to –0.88) | 0.02 |
| DASH-I | 26.8 (24.5) | 19.1 (19.6) | –7.65 (–13.00 to –2.33) | 0.005 | –7.15 (–13.19 to –1.11) | 0.02 |
Wound outcomes at 6 weeks
Of those returning postal questionnaires at 6 weeks (303/392; 77%), most participants reported that their surgical wounds had fully healed (248/303; 82%) (Table 15). One-quarter of participants reported having a wound infection diagnosed by a doctor or nurse. Level of agreement was high between doctor/nurse diagnosed and patient-diagnosed SSIs, although as these were self-reported, assessments were not independent of each other. Overall, 121 women used free-text boxes to describe other postoperative complications (usual-care group, n = 61; exercise group, n = 60) at the 6-week follow-up. The most frequently reported complication was wound seroma (64/303; 21%), which was described as bothersome and often requiring multiple hospital visits for drainage. Of the reported seromas, there were no differences by treatment group (see Table 15). Nine women described symptoms indicative of cording syndrome in the axilla at 6 weeks (usual-care group, n = 3; exercise group, n = 6). Other complications included pain, numbness, wound infection, haematoma or combinations of these.
| Outcome | Number (%) of participants | ||
|---|---|---|---|
| Usual-care group (N = 150) | Exercise group (N = 153) | Total (N = 303) | |
| Wound fully healed | 122 (81.3) | 126 (82.4) | 248 (81.8) |
| Doctor-diagnosed SSI | 40 (26.7) | 38 (24.8) | 78 (25.7) |
| Patient-reported SSI | 40 (26.7) | 39 (25.5) | 80 (26.4) |
| Antibiotics prescribed for SSI | 47 (31.3) | 40 (26.1) | 87 (28.7) |
| Any other complication after surgery | 58 (38.7) | 61 (39.9) | 119 (39.3) |
| Wound seroma | 31 (20.7) | 33 (21.6) | 64 (21.1) |
Postoperative pain outcomes
Among responders at the 6-week follow-up, mean scores for acute postoperative pain intensity in the area of the breast and armpit while at rest were higher for those randomised to usual care than for those randomised to the exercise group (adjusted MD –0.58, 95% CI –1.09 to –0.07; p = 0.03) (Table 16). All models were adjusted for baseline preoperative pain scores. We also observed differences between treatment groups in movement-evoked postoperative pain scores at 6 weeks, with those in the exercise group reporting lower pain scores than those in the usual-care group (adjusted MD –0.55, 95% CI –1.10 to –0.01; p = 0.05). At 6 months, there were no differences in mean pain scores. At 12 months, mean pain intensity scores were again lower for those randomised to exercise than those randomised to usual care (adjusted MD –0.71, 95% CI –1.23 to –0.14; p = 0.02). Pain data were negatively skewed; hence, we examined non-parametric models, with similar findings of differences between treatment groups at 12 months (median NRS 2.0 usual care vs. 1.0 exercise; Mann–Whitney test p = 0.02).
| Outcome | Usual-care group | Exercise group | Unadjusted estimate (95% CI) | p-value | Adjusted estimate (95% CI) | p-value |
|---|---|---|---|---|---|---|
| FACT-B4 | ||||||
| 6 weeks | N = 150 | N = 153 | ||||
| Mean (SD) score | 4.5 (4.4) | 4.1 (3.8) | –0.37 (–1.32 to 0.56) | 0.44 | –0.48 (–1.40 to 0.43) | 0.30 |
| Missing, n | 1 | 3 | ||||
| 6 months | N = 133 | N = 145 | ||||
| Mean (SD) score | 4.7 (4.4) | 3.4 (3.4) | –1.06 (–1.99 to –0.13) | 0.03 | –1.11 (–2.01 to –0.21) | 0.02 |
| Missing, n | 0 | 0 | ||||
| 12 months | N = 139 | N = 135 | ||||
| Mean (SD) score | 5.4 (5.2) | 3.4 (4.0) | –1.99 (–3.10 to –0.88) | 0.001 | –2.02 (–3.11 to –0.93) | 0.001 |
| Missing, n | 1 | 1 | ||||
| Pain, NRS | ||||||
| 6 weeks | N = 150 | N = 153 | ||||
| Pain at rest, mean (SD) score | 2.2 (2.5) | 1.6 (1.9) | –0.54 (–1.05 to –0.03) | 0.04 | –0.58 (–1.09 to –0.07) | 0.03 |
| Pain on movement, mean (SD) score | 2.6 (2.6) | 2.1 (2.1) | –0.48 (–1.01 to 0.05) | 0.07 | –0.55 (–1.10 to –0.01) | 0.04 |
| Missing, n | 0 | 1 | ||||
| 6 months | N = 133 | N = 145 | ||||
| Mean (SD) score | 2.2 (2.3) | 2.0 (2.1) | –0.20 (–0.72 to 0.32) | 0.45 | –0.17 (–0.70 to 0.35) | 0.52 |
| Missing, n | 0 | 0 | ||||
| 12 months | N = 139 | N = 135 | ||||
| Mean (SD) score | 2.6 (2.4) | 1.9 (2.0) | –0.72 (–1.25 to –0.17) | 0.008 | –0.68 (–1.23 to –0.12) | 0.02 |
| Missing, n | 0 | 1 | ||||
| Pain severity, NRS | ||||||
| 6 weeks | N = 150 | N = 153 | ||||
| None–mild (score 0–3), n (%) | 104 (69.3) | 124 (81.1) | 1.96 (1.14 to 3.35) | 0.01 | 1.90 (1.02 to 3.52) | 0.04 |
| Moderate–severe (score 4–10), n (%) | 46 (30.7) | 28 (18.3) | ||||
| Missing, n | 0 | 1 | ||||
| 6 months | N = 133 | N = 145 | ||||
| None–mild (score 0–3), n (%) | 103 (77.4) | 120 (82.8) | 1.40 (0.77 to 2.53) | 0.27 | 1.42 (0.72 to 2.84) | 0.31 |
| Moderate–severe (score 4–10), n (%) | 30 (22.6) | 25 (17.2) | ||||
| Missing, n | 0 | 0 | ||||
| 12 months | N = 139 | N = 135 | ||||
| None–mild (score 0–3), n (%) | 96 (69.1) | 113 (83.7) | 2.30 (1.29 to 4.11) | 0.005 | 2.41 (1.24 to 4.70) | 0.01 |
| Moderate–severe (4–10), n (%) | 43 (30.9) | 22 (16.2) | ||||
| Missing, n | 0 | 0 | ||||
| Neuropathic pain | ||||||
| 6 weeks | N = 150 | N = 153 | ||||
| Pain free, n (%) | 81 (54.0) | 89 (58.2) | ||||
| Non-neuropathic pain, n (%) | 48 (32.0) | 38 (24.8) | 0.69 (0.34 to 1.43) | 0.32 | 0.73 (0.22 to 2.45) | 0.61 |
| Neuropathic pain, n (%) | 21 (14.0) | 24 (15.7) | ||||
| Missing, n | 0 | 2 | ||||
| 6 months | N = 133 | N = 145 | ||||
| Pain free | 28 (21.0) | 28 (19.3) | ||||
| Non-neuropathic pain, n (%) | 72 (54.1) | 88 (60.7) | 1.36 (0.74 to 2.52) | 0.32 | 1.64 (0.63 to 4.23) | 0.31 |
| Neuropathic pain, n (%) | 29 (21.8) | 26 (17.9) | ||||
| Missing, n | 4 | 3 | ||||
| 12 months | N = 139 | N = 135 | ||||
| Pain free, n (%) | 30 (21.6) | 28 (20.7) | ||||
| Non-neuropathic pain, n (%) | 75 (54.0) | 82 (60.7) | 1.59 (0.85 to 2.98) | 0.15 | 1.29 (0.45 to 3.69) | 0.64 |
| Neuropathic pain, n (%) | 32 (23.2) | 22 (16.3) | ||||
| Missing, n | 2 | 3 | ||||
| Lymphoedema | ||||||
| 6 weeks | N = 150 | N = 153 | ||||
| No heaviness or swelling, n (%) | 129 (86.0) | 128 (83.7) | 1.11 (0.58 to 2.13) | 0.76 | 1.07 (0.52 to 2.24) | 0.85 |
| Arm heavy and swollen, n (%) | 20 (13.3) | 22 (14.4) | ||||
| Missing, n | 1 | 3 | ||||
| 6 months | N = 133 | N = 145 | ||||
| No heaviness or swelling, n (%) | 101 (75.9) | 114 (78.6) | 0.80 (0.45 to 1.42) | 0.45 | 0.82 (0.43 to 1.56) | 0.55 |
| Arm heavy and swollen, n (%) | 32 (24.1) | 29 (20.0) | ||||
| Missing, n | 0 | 2 | ||||
| 12 months | N = 139 | N = 135 | ||||
| No heaviness or swelling, n (%) | 101 (72.7) | 101 (74.8) | 0.92 (0.53 to 1.58) | 0.76 | 1.17 (0.62 to 2.23) | 0.62 |
| Arm heavy and swollen, n (%) | 36 (25.9) | 33 (24.4) | ||||
| Missing | 2 | 1 | ||||
Those randomised to usual care were twice as likely as those in the exercise group to report moderate to severe pain (NRS ≥ 4) at 6 weeks (adjusted OR 1.90, 95% CI 1.02 to 3.52) and at 12 months (adjusted OR 2.41, 95% CI 1.24 to 4.70) (see Table 16). There were no differences in the odds of moderate to severe pain by group at 6 months.
We also observed group differences in arm symptoms, with higher FACT-B4 scores, indicating more symptoms, in the usual-care group than in the exercise group at 6 months (FACT-B4 adjusted MD –1.06, 95% CI –1.99 to –0.13; p = 0.02) and 12 months (adjusted MD –1.99, 95% CI –3.10 to –0.88; p = 0.001). However, despite differences in pain intensity and arm-related symptoms, we found no difference in rates of neuropathic pain over time (see Table 16). Although at 12 months rates of neuropathic pain were higher in those randomised to usual care (32/139; 23%) than in those randomised to exercise (22/135; 16%), this difference was not statistically significant (adjusted OR 1.29, 95% CI 0.45 to 3.69; p = 0.64).
Lymphoedema symptoms
We found no differences in the proportion of women reporting lymphoedema symptoms at baseline, 6 weeks, 6 months or 12 months. Among those responding to postal questionnaires, exercise did not increase the risk of arm swelling or feelings of arm heaviness over the 12-month follow-up period (see Table 16).
Physical activity
We report descriptive data for physical activity by treatment group and follow-up time point in Table 17. Of those responding at 6 weeks, 119 out of 150 (79%) usual-care participants reported walking outside ≥ 3 days per week, compared with 132 out of 153 (86%) of those allocated to exercise. At 6 months, the proportion of responders who reported that they were active, in terms of walking outside regularly, was slightly lower than at baseline and 6 weeks, (67% of usual-care group responders and 79% and exercise group responders) (see Table 17). By 12 months, activity rates had increased again, with most participants walking outside for at least 3 days per week [usual-care, 113/139 (81%), vs. exercise, 115/135 (85%)]. Very few reported taking part in strenuous sport or recreational activity at 6 weeks (14%), although this proportion increased over time, with one-third of responders reporting taking part in strenuous sports by 12 months (usual care, 30%; exercise, 32%) (Table 18).
| Physical activity | Number (%) of participants | |
|---|---|---|
| Usual-care group | Exercise group | |
| 6 weeks | ||
| Number of participants | 150 | 153 |
| Days walking per week | ||
| Never/seldom (1 or 2) | 28 (18.7) | 20 (13.1) |
| Sometimes (3 or 4) | 36 (24.0) | 36 (23.5) |
| Often (5–7) | 83 (55.3) | 96 (62.7) |
| Missing | 3 (2.0) | 1 (0.7) |
| Hours/day walking in past week | ||
| < 1 | 43 (28.7) | 55 (35.9) |
| 1–2 | 54 (36.0) | 52 (34.0) |
| > 2 | 50 (33.3) | 46 (30.1) |
| Missing | 3 (2.0) | 0 |
| 6 months | ||
| Number of participants | 133 | 145 |
| Days walking per week | ||
| Never/seldom (1 or 2) | 27 (20.3) | 20 (13.8) |
| Sometimes (3 or 4) | 33 (24.8) | 46 (31.7) |
| Often (5–7) | 68 (51.1) | 75 (51.7) |
| Missing | 5 (3.8) | 4 (2.8) |
| Hours/day walking in past week | ||
| < 1 | 27 (20.3) | 35 (24.1) |
| 1–2 | 42 (29.7) | 43 (29.7) |
| > 2 | 59 (44.4) | 64 (44.1) |
| Missing | 5 (3.8) | 3 (2.1) |
| 12 months | ||
| Number of participants | 139 | 135 |
| Days walking per week | ||
| Never/seldom (1 or 2) | 24 (17.3) | 20 (14.8) |
| Sometimes (3 or 4) | 36 (25.9) | 34 (25.2) |
| Often (5–7) | 77 (55.4) | 81 (60.0) |
| Missing | 2 (1.4) | 0 (0.0) |
| Hours/day walking in past week | ||
| < 1 | 32 (23.0) | 26 (19.3) |
| 1–2 | 34 (24.5) | 40 (29.6) |
| > 2 | 72 (51.8) | 68 (50.4) |
| Missing | 1 (0.7) | 1 (0.7) |
| Strenuous activity | Number (%) of participants | |
|---|---|---|
| Usual-care group | Exercise group | |
| 6 weeks | ||
| Number of participants | 150 | 153 |
| How often | ||
| Never | 126 (84.0) | 130 (85.0) |
| Any | 21 (14.0) | 22 (14.4) |
| Missing | 3 (2.0) | 1 (0.7) |
| Hours per session | ||
| None | 126 (84.0) | 130 (85.0) |
| < 2 | 17 (11.3) | 21 (13.7) |
| ≥ 2 | 4 (2.7) | 1 (0.7) |
| 6 months | ||
| Number of participants | 133 | 145 |
| How often | ||
| Never | 96 (72.2) | 110 (75.9) |
| Any | 32 (24.1) | 31 (21.4) |
| Missing | 5 (3.8) | 4 (2.8) |
| Hours per session | ||
| None | 101 (75.9) | 115 (79.3) |
| < 2 | 25 (18.8) | 25 (17.2) |
| ≥ 2 | 7 (5.3) | 5 (3.5) |
| 12 months | ||
| Number of participants | 139 | 135 |
| How often | ||
| Never | 96 (69.1) | 90 (66.7) |
| Any | 42 (30.2) | 43 (31.9) |
| Missing | 1 (0.7) | 2 (1.4) |
| Hours per session | ||
| None | 100 (71.9) | 92 (68.2) |
| < 2 | 31 (22.3) | 35 (25.9) |
| ≥ 2 | 8 (5.8) | 8 (5.9) |
Confidence in return to usual activity
Confidence scores in ability to return to usual activities and to regular physical activity were consistently higher among those participants randomised to exercise than those randomised to usual care, across all time points (Table 19). All models were adjusted for baseline confidence score.
| Return to activity | Usual-care group | Exercise group | Unadjusted estimate MD (95% CI) | p-value | Adjusteda estimate MD (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 6 weeks | ||||||
| Number of participants | 150 | 153 | ||||
| Usual activities, mean (SD) | 8.2 (2.4) | 8.8 (1.9) | 0.59 (0.10 to 1.07) | 0.02 | 0.32 (–0.16 to 0.81) | 0.19 |
| Missing, n | 3 | 0 | ||||
| Regular physical activity, mean (SD) | 7.6 (2.6) | 8.5 (1.8) | 0.87 (0.34 to 1.41) | 0.002 | 0.67 (0.13 to 1.20) | 0.02 |
| Missing, n | 1 | 1 | ||||
| 6 months | ||||||
| Number of participants | 133 | 145 | ||||
| Usual activities, mean (SD) | 7.9 (2.2) | 8.6 (1.7) | 0.77 (0.27 to 1.26) | 0.002 | 0.68 (0.22 to 1.14) | 0.004 |
| Missing, n | 18 | 16 | ||||
| Regular physical activity, mean (SD) | 7.7 (2.2) | 8.3 (2.0) | 0.62 (0.09 to 1.14) | 0.02 | 0.57 (0.07 to 1.09) | 0.03 |
| Missing, n | 18 | 16 | ||||
| 12 months | ||||||
| Number of participants | 139 | 135 | ||||
| Usual activities, mean (SD) | 7.6 (2.6) | 8.5 (1.8) | 0.87 (0.34 to 1.41) | 0.002 | 0.67 (0.13 to 1.20) | 0.02 |
| Missing, n | 1 | 1 | ||||
| Regular physical activity, mean (SD) | 7.4 (2.7) | 8.3 (2.1) | 0.87 (0.30 to 1.45) | 0.003 | 0.73 (0.17 to 1.30) | 0.01 |
| Missing, n | 1 | 1 | ||||
Health-related quality of life over time by intervention
We assessed HRQoL over time by treatment group. Findings for EQ-5D-5L HRQoL outcomes are presented in Chapter 6. Table 20 presents SF-12 scores and missingness for PCS and MCS scores. Among participants returning 12-month questionnaires (274/392; 70%), item missingness for the SF-12 was low (17/274; 6%). We found differences in mean physical HRQoL scores at 6 and 12 months, with higher physical HRQoL scores observed in the exercise group than in the usual-care group (adjusted MD at 12 months, 4.84; 95% CI 2.06 to 7.63; p < 0.001). In the usual-care group, mean scores for physical HRQoL were lower at both 6 and 12 months than baseline scores (mean PCS score of 47.6 at baseline vs. PCS score of 43.2 and 43.8 at 6 and 12 months, respectively). We found no differences in mental HRQoL scores between groups at 6 or 12 months.
| SF-12 domain | Usual-care group | Exercise group | Unadjusted estimate (95% CI) | p-value | Adjusteda estimate (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 6 months | ||||||
| Number of participants | 133 | 145 | ||||
| PCS mean (SD) | 43.2 (11.2) | 45.9 (9.5) | 2.73 (0.21 to 5.25) | 0.03 | 2.73 (0.24 to 5.21) | 0.03 |
| MCS mean (SD) | 45.9 (11.1) | 48.0 (9.8) | 2.11 (–0.42 to 4.64) | 0.10 | 2.12 (–0.37 to 4.61) | 0.09 |
| Missing, n (%) | 5 (3.8) | 9 (6.2) | ||||
| 12 months | ||||||
| Number of participants | 139 | 135 | ||||
| PCS mean (SD) | 43.8 (11.5) | 48.1 (10.0) | 4.30 (1.63 to 6.97) | 0.002 | 4.39 (1.74 to 7.04) | < 0.001 |
| MCS mean (SD) | 46.6 (11.2) | 48.7 (10.0) | 2.10 (–0.51 to 4.71) | 0.11 | 1.99 (–0.58 to 4.57) | 0.13 |
| Missing, n (%) | 7 (5.0) | 10 (7.4) | ||||
Serious adverse events and adverse events
No SAEs were reported. Six AEs were reported by physiotherapists treating participants in the exercise group. Of the six events notified to the study team, four [one SSI, one drain fall-out during exercise necessitating reinsertion in accident and emergency, one late-onset haematoma and some neck pain in one participant with known cervical spondylosis] did not result in the participant stopping exercising. Two participants experiencing AEs withdrew from treatment: one with a seroma that caused discomfort while exercising and one awaiting review for calcific tendinosis who felt that postoperative activity and exercises aggravated her existing condition.
Sensitivity analyses
Various sensitivity analyses were planned to compare high- and low-volume recruiting centres to examine the impact on clustering effect and to consider high-volume centres to examine therapist effect. We also examined differences between date of randomisation and date of surgery by treatment group. We found that the clustering effect was negligible; therefore, these sensitivity analyses were discarded. We observed a difference between those sites that returned data in the proportion of participants reporting a history of shoulder problems at recruitment. Sensitivity analyses explored the impact of adjusting the primary analyses for history of shoulder problems; there was no change in the direction or strength of observed effect of the primary outcome at 12 months (adjusted MD DASH score –6.79, 95% CI –12.05 to –1.53; p = 0.01). Findings were similar for non-parametric sensitivity analyses. As the primary analyses adjusted for baseline DASH score, there was a risk of over-adjustment given the correlation between upper arm function and self-reported shoulder problems.
Process evaluation
Staff trained
Between December 2015 and March 2017, a total of 44 physiotherapists were trained to deliver the PROSPER intervention. In April 2016, we hosted an intervention training day at WCTU, attended by 14 physiotherapists. We trained the remaining 30 physiotherapists in 15 training sessions delivered at other venues, most commonly in NHS trust facilities. At least two physiotherapists were trained for each of the 17 recruiting sites. Eight refresher training sessions were delivered. None of the participating physiotherapists routinely treated breast cancer, although all were specialists in musculoskeletal rehabilitation. Intervention training comprised three core components: (1) an overview of breast cancer treatments and common complications, and an introduction to PROSPER; (2) details of the exercise intervention, how to complete assessments and prescribe exercises, behavioural training and management of postoperative complications; and (3) completion of trial-related paperwork, including safety reporting and good clinical practice. Each physiotherapist received a detailed intervention manual. 103
Quality control assessments
Of the 44 physiotherapists trained, 36 (82%) treated at least one trial participant. QC assessments were undertaken with 16 out of 17 centres delivering treatment. One physiotherapy department referred only three participants, who were all treated and discharged before the QC visit was completed. One physiotherapist received a ‘minor concern’ report during the QC visit and a reassessment visit was arranged within 3 weeks. These minor concerns were resolved after further therapist training and support.
Uptake and adherence to exercise intervention
Among those randomised to exercise, 181 out of 196 (92%) participants attended at least one appointment with a PROSPER physiotherapist. Ten participants were randomised to exercise but did not attend the exercise intervention (10/392; 3%). Of those who did attend, 38 out of 181 (21%) attended either one or two appointments, thus partially complying with the recommended programme (Table 21). The remainder (143/181; 79%) attended three or more appointments, thus fully complying with the recommended exercise programme (see Table 22). This equates to 73% (143/196) of all participants randomised to exercise (ITT). Although we recommended up to six physiotherapy contacts over the 12-month follow-up period, four participants (4/196; 2%) exceeded this, having up to eight (three participants) or nine sessions (one participant). These additional contacts were to support women with ongoing, protracted, treatment-related problems.
| Contacts with physiotherapist | Number (%) of participants (N = 196) | Median (IQR)a weeks |
|---|---|---|
| Withdrawn at randomisation | 5 (2.6) | 0 |
| Did not attend | 10 (5.1) | 0 |
| 1 or 2 only | 38 (19.4) | 4 (3–7) |
| 3 sessions | 58 (29.6) | 12 (8–15) |
| ≥ 4 sessions | 85 (43.4) | 15 (12–19) |
| Physiotherapy appointment | Number of participants attending | Appointment duration (minutes) | |
|---|---|---|---|
| Mean (SD) | Median (IQR) | ||
| First | 181 | 57.5 (13.6) | 60 (50–60) |
| Second | 163 | 37.9 (13.1) | 35 (30–45) |
| Third | 143 | 33.3 (15.2) | 30 (25–40) |
| Fourth | 85 | 30.7 (13.1) | 30 (25–40) |
| Fifth | 48 | 29.1 (9.5) | 30 (25–30) |
| Sixth | 19 | 30.9 (12.7) | 30 (25–35) |
Time from surgery to exercise intervention
Median time from surgery to the first physiotherapist appointment was 9 days (IQR 8–10 days), thus within the recommended time frame for the programme. Median time from the first to the second physiotherapy appointments was 21 days (IQR 18–28 days) and median time from the second to third appointments was 38 days (IQR 19–56 days) (see Table 21).
Type of physiotherapy contact
Physiotherapists had a total of 622 contacts with the 181 participants who attended physiotherapy (mean 3.7, median 3). The majority of contacts were face to face (603/622; 97%) and the remainder by telephone (19/622; 3%). We had partially completed physiotherapy treatment logs for contacts with 11 participants from one site (11/181 participants; 6%).
Length of physiotherapy appointments
Physiotherapists spent approximately 1 hour during the first appointment with trial participants, as per the recommended exercise programme (Table 22). For the second and third face-to-face appointments, these lasted on average 35 minutes per therapy session (see Table 22). For those participants who had more than three appointments, the mean appointment duration was very similar to those having two or three face-to-face contacts (mean 30 minutes).
Exercise progression over time: range of movement
We assessed progression in ROM by comparing exercise difficulty at each physiotherapy session. We analysed exercises according to movement direction: forward/flexion (clasp hand raise and forward wall slide exercises), abduction/sideways (morning stretch and sideways wall slide), and abduction and external rotation exercises/open chest (back broom lift and surrender). We present ROM data for compliers and partial compliers with the exercise progression in Table 23. We also present ROM data by movement direction by number of participants across up to six appointments in Figures 3–6. We calculated work capacity for ROM by calculating the product of total sets and repetitions, as recorded by physiotherapists at each contact.
| Movement direction | Compliers, mean (SD) work capacitya | MD (95% CI) | p-value | Partial compliers, mean work (SD)a | MD (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| First appointment (N = 152) | Second appointment (N = 150) | Third appointment (N = 147) | First appointment (N = 32) | Second appointment (N = 19) | |||||
| Flexion | 10.1 (4.7) | 10.3 (5.4) | 10.3 (6.2) | 0.17 (–1.45 to 1.14) | 0.80 | 10.2 (2.9) | 10.0 (2.8) | 0.16 (–1.56 to 1.87) | 0.86 |
| Abduction | 9.7 (4.6) | 10.1 (5.3) | 10.3 (6.2) | 0.59 (–1.89 to 0.70) | 0.37 | 8.6 (3.4) | 8.4 (3.3) | 0.17 (–1.80 to 2.15) | 0.86 |
| Abduction and external rotation | 9.7 (4.3) | 9.8 (4.9) | 9.6 (5.6) | –0.07 (–1.12 to 1.25) | 0.91 | 9.2 (3.6) | 8.9 (2.6) | 0.27 (–1.65 to 2.19) | 0.78 |
FIGURE 3.
Range of movement progression: flexion exercises.
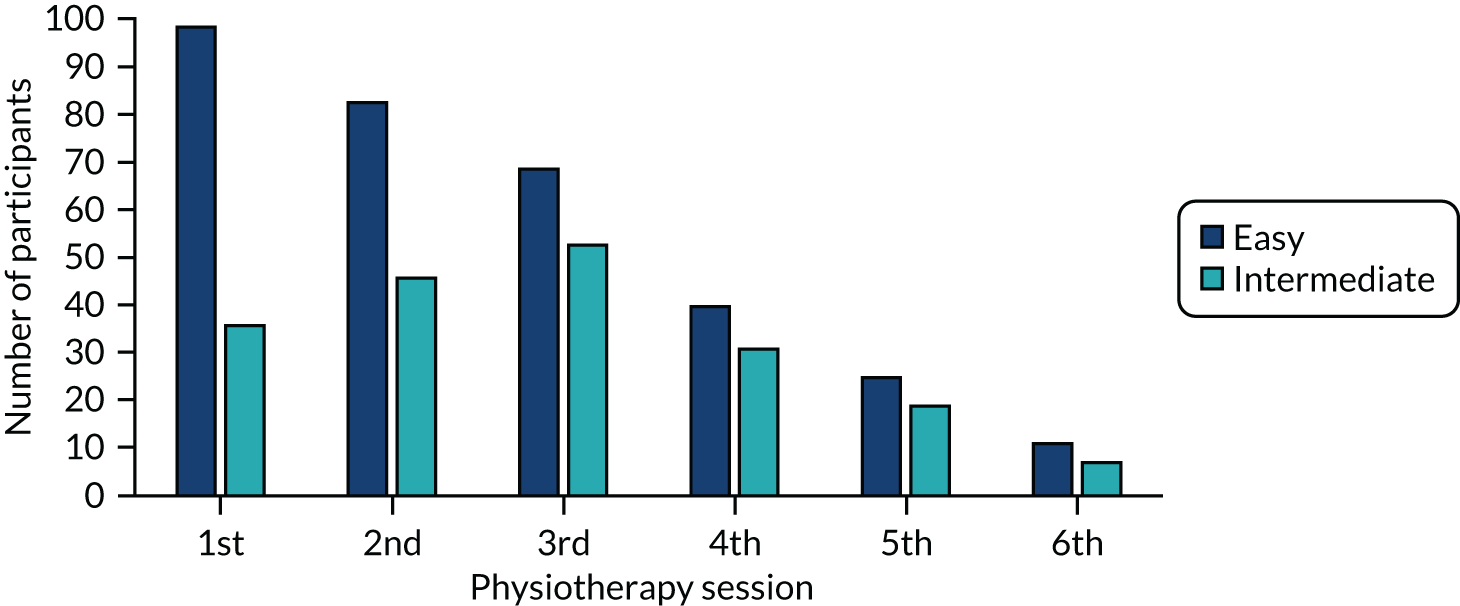
FIGURE 4.
Range of movement progression: abduction exercises.
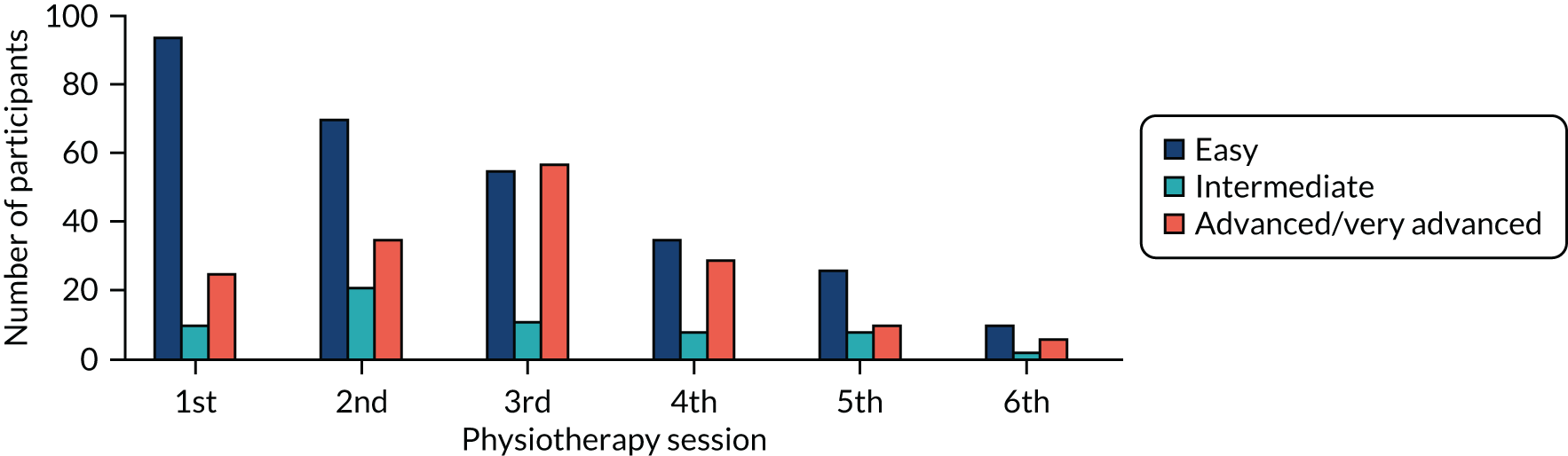
FIGURE 5.
Range of movement progression: abduction and external rotation exercises.
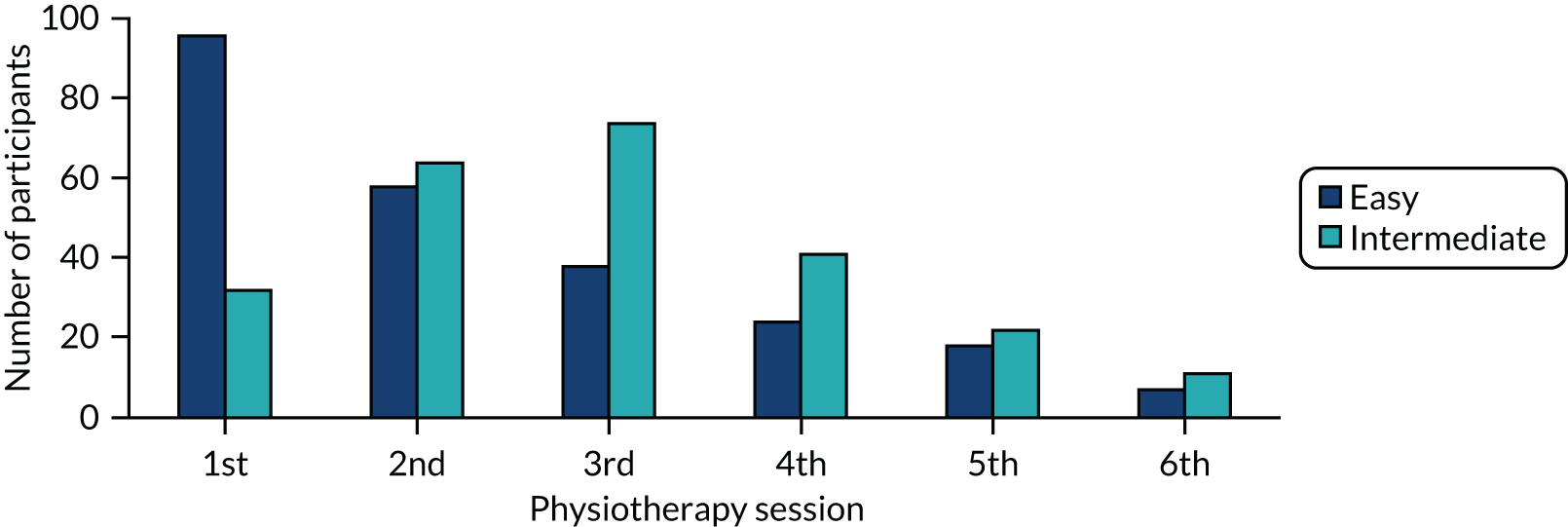
FIGURE 6.
Mean work capacity by movement direction in exercise compliers (n = 143).
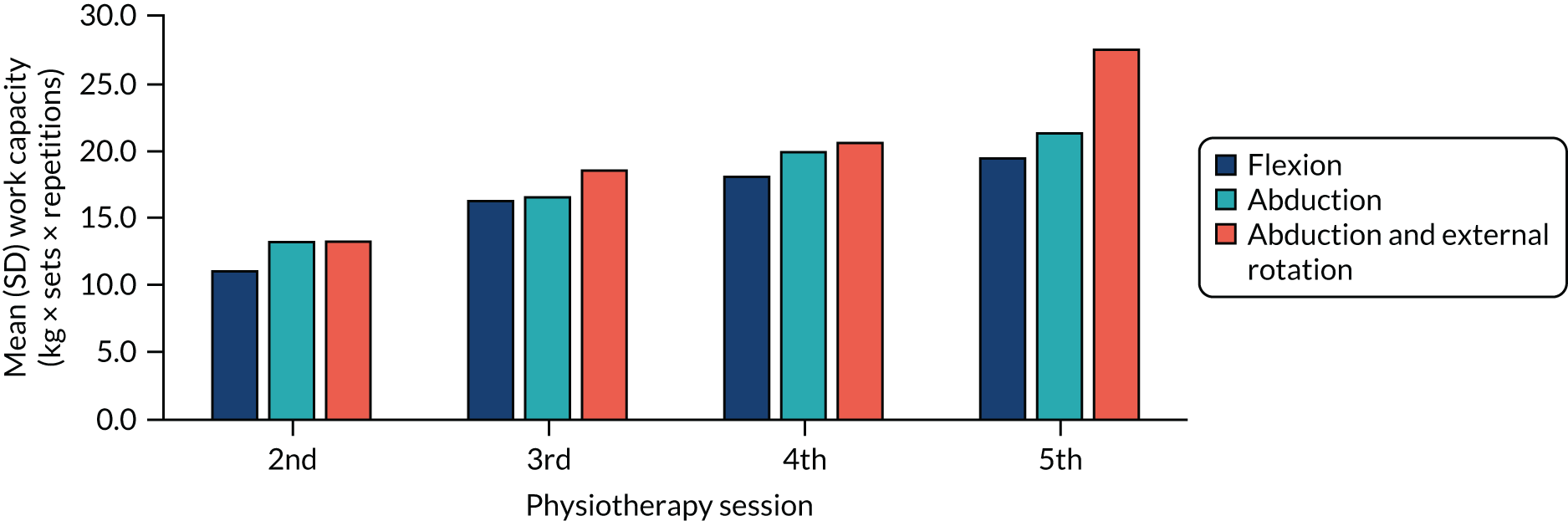
At the first appointment, most participants (approximately 65%) started off at the easiest level for all ROM exercises for flexion, abduction, and abduction and external rotation movements (Appendix 2). We observed ROM progression across the second and third appointments: by the third appointment, fewer than half of participants were still doing ‘easy’ exercises for flexion, abduction, and abduction with external rotation (27%) (see Figures 3–5). For abduction ROM exercises, participants progressed to advanced or very advanced ROM movements (Figure 5). We observed a small increase in mean (SD) work capacity for flexion and abduction exercises, but this was not statistically significant (Table 23).
Progression over time: strength exercises
Strength exercises were prescribed only from 1 month postoperatively, corresponding to the second physiotherapy appointment. We examined strength progression over time by calculating mean (SD) work capacity (resistance × repetitions × sets), as presented in Table 24 and Figure 6. We observed an increase in mean strength over time among exercise compliers for each of the movement directions: flexion (t-test, p = 0.003), abduction (t-test, p = 0.01) and abduction and external rotation (t-test, p = 0.004). As strength was prescribed from the second appointment, we had data for one time point only for partial compliers (n = 19 participants). Although this was a very small sample, the partial compliers had good baseline strength compared with the full compliers who attended the second appointment of their programme (Table 24).
| Movement direction | Mean (SD) work capacity,a compliers | p-valueb | Mean (SD) work capacity,a partial compliers | ||
|---|---|---|---|---|---|
| Second appointment (N = 152) | Third appointment (N = 138) | Fourth appointment (N = 81) | Second appointment (N = 19) | ||
| Flexion | 11.1 (14.9) | 16.4 (19.1) | 18.2 (19.1) | 0.003 | 12.2 (13.1) |
| Abduction | 13.3 (17.9) | 16.7 (20.0) | 20.0 (21.4) | 0.01 | 12.6 (12.4) |
| Abduction and external rotation | 13.3 (17.0) | 18.7 (20.1) | 20.7 (19.7) | 0.004 | 14.7 (15.0) |
Figure 7 is a graphical representation of strength progression by Therabands prescribed across follow-up appointments. For each of the three movement directions, most participants were started on either the tan (1.1 kg resistance) or red bands (1.7 kg). By the third appointment, a large proportion of participants had progressed to the more advanced blue bands (2.6 kg). In the case of those attending four physiotherapy appointments (85/181; 47%), the blue band was the most commonly prescribed band across all three movement directions (Figures 6 and 7).
FIGURE 7.
Progression over time of band strength for abduction exercises among exercise compliers (n = 143).
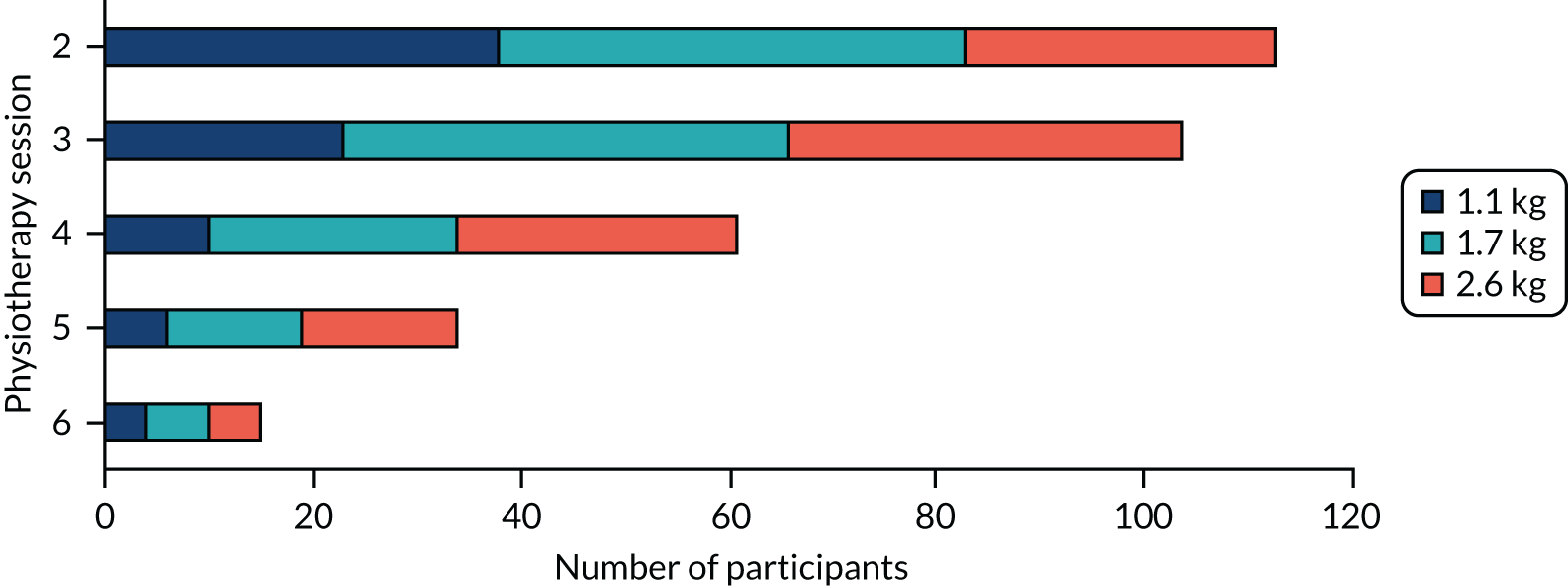
Self-reported adherence with arm or exercises
Finally, all participants were asked at follow-up whether they had done arm or shoulder exercises in the previous week (Table 25). Among postal responders, the proportion of participants doing shoulder-specific exercises was higher in the exercise group than in the usual-care group both at 6 weeks (exercise group 97% vs. usual-care group 90%) and at 6 months (exercise group 79% vs. usual-care group 62%), but there was no apparent difference at 12 months (exercise group 75% vs. usual-care group 72%) (Table 25). At the 6- and 12-month follow-ups, we asked all participants whether or not they felt that doing arm/shoulder exercises had helped with their recovery. At 6 months, the proportion responding positively was higher in the exercise group (118/145; 81%) than in the usual-care group (91/133; 68%) but by the 12-month follow-up the proportions were similar (exercise group 79% vs. usual-care group 74%) (Table 25).
| Question | Number (%) of participants | |||||
|---|---|---|---|---|---|---|
| 6 weeks’ follow-up | 6 months’ follow-up | 12 months’ follow-up | ||||
| Usual-care group (N = 150) | Exercise group (N = 153) | Usual-care group (N = 133) | Exercise group (N = 145) | Usual-care group (N = 138) | Exercise group (N = 134) | |
| Have you done arm or shoulder exercises in the last week? | ||||||
| Yes, n (%) | 135 (90.0) | 148 (96.7) | 82 (61.7) | 114 (78.6) | 99 (71.7) | 101 (75.4) |
| No, n (%) | 12 (8.0) | 5 (3.3) | 34 (25.6) | 15 (10.3) | 38 (27.5) | 32 (23.9) |
| Missing, n (%) | 3 (2.0) | 0 (0.0) | 17 (12.8) | 16 (11.0) | 1 (0.7) | 1 (0.7) |
| If yes, how often?, n (%) | ||||||
| Every day, n (%) | 108 (72.0) | 137 (89.5) | 50 (37.6) | 67 (46.2) | 58 (42.0) | 44 (32.8) |
| Every few days, n (%) | 23 (15.3) | 8 (5.2) | 26 (19.5) | 39 (26.9) | 34 (24.6) | 44 (32.8) |
| Once a week, n (%) | 3 (2.0) | 2 (1.3) | 5 (3.8) | 7 (4.8) | 7 (5.1) | 13 (9.7) |
| Missing, n (%) | 16 (10.7) | 6 (3.9) | 52 (39.1) | 32 (22.1) | 39 (28.3) | 33 (24.6) |
| Do you think these arm and shoulder exercises are helping your recovery?, n (%) | ||||||
| Yes, n (%) | – | – | 91 (68.4) | 118 (81.4) | 102 (73.9) | 106 (79.1) |
| No, n (%) | – | – | 13 (9.8) | 6 (4.1) | 18 (13.0) | 14 (10.4) |
| Missing, n (%) | – | – | 29 (21.8) | 21 (14.5) | 18 (13.0) | 14 (10.4) |
Chapter 5 Qualitative study
Introduction
Qualitative research was undertaken in parallel with the trial to inform recruitment processes and the refinement of intervention materials, and to understand experiences of the trial interventions from the perspective of both participants and physiotherapists. Qualitative research can be used alongside RCTs to achieve varying objectives, such as informing recruitment processes, developing interventions that are acceptable and appropriate to the population of interest (and thus more likely to be taken up) and understanding the context in which a complex intervention is introduced. 104 In this study, qualitative research was important to refine aspects of the intervention and trial processes, and to understand the experiences of receiving and delivering the complex intervention.
This chapter reports on the qualitative research we conducted. First, we undertook pre-pilot interviews with seven newly diagnosed breast cancer patients and two breast care nurses (BCNs) to refine our procedures to approach and consent newly diagnosed patients. We also received early feedback on draft information materials before launching the internal pilot study. We then completed interviews with 10 participants randomised to usual care and 10 randomised to the exercise intervention to understand experiences of each trial intervention. We aimed to interview a sample of patients who declined participation in the trial: one patient agreed to interview. Finally, interviews were completed with 11 physiotherapists to investigate experiences of delivering the intervention. Selected findings from the qualitative research have been submitted for publication. 105
Aims of qualitative study
The aims of the qualitative study were to:
-
understand patients’ and BCNs’ perspectives of the trial materials and processes to refine these materials and processes
-
understand the acceptability of the exercise intervention to participants
-
explore how the trial interventions affected experiences of recovery after cancer treatment
-
investigate the experiences of physiotherapists delivering the exercise intervention
-
explore participants’ and physiotherapists’ perspectives on issues related to the PROSPER programme to inform future plans for implementation.
Methodology
Philosophical framework and methodology
This study was underpinned by critical realism. 106 This perspective recognises the existence of multiple interpretations of a phenomenon while acknowledging that these interpretations are based in a reality. For example, pain and physical rehabilitation are real phenomena, but the meaning and experience of these phenomena are mediated by sociocultural factors and vary between individuals. We used a qualitative approach, enabling exploration, depth and understanding of experiences, thus taking an interpretive ‘sense-making’ approach rather than a hypothesis-testing or confirmatory approach. We opted for semistructured interviews and reflexive thematic analysis (see Data analysis). 107
Sampling for qualitative study
Pre-pilot interviews
We used opportunistic sampling at one breast cancer unit (UHCW) before the site was opened to recruitment to recruit newly diagnosed breast cancer patients. We asked BCNs to identify women who were potentially eligible to participate in PROSPER. These interviews explored women’s views on being approached to take part in a study and views regarding draft trial materials. Interviews were conducted until data saturation was achieved. A dyad interview was held with two specialist BCNs at this site to explore their experiences of, and perspectives on, approaching participants.
Main trial interviews
On recruitment to the trial, all participants were asked if they were willing to be approached for an interview at a later date; written, signed consent was recorded. This served as the sampling frame for all subsequent trial participant interviews. Women in the intervention group were contacted for interview after discharge from physiotherapy to avoid contamination bias. We had intended to sample purposively based on participant characteristics (i.e. age, ethnicity, socioeconomic background, geographical location and employment status); however, qualitative interviews were undertaken after discharge from physiotherapy. Therefore, we did not wait until the end of intervention delivery to sample from a large pool of all participants, but rather used convenience sampling, approaching participants from different sites as they were discharged from physiotherapy.
After the completion and analysis of seven intervention group interviews, we then sought ethics approval to interview a sample of usual-care participants to compare experiences of recovery. We wanted to explore whether or not similar themes would emerge across the two intervention groups to ensure that we had identified and explored the impact of the intervention. We used theoretical sampling, and sampled usual-care group participants with a date of primary surgery close to the date of surgery for intervention participants. This was to ensure that participants were similar in terms of duration since their surgery to more accurately compare experiences of postoperative recovery by allowing them to reflect over a similar length of time. For the physiotherapist interviews, we purposively sampled from high- and low-recruiting centres, aiming for 10 interviewees. During the pilot study only, patients who were eligible to participate but who declined were asked, when appropriate, if they would be willing to participate in a short interview to discuss reasons for non-participation.
Recruitment and consent procedures
Pre-pilot interviews
Newly diagnosed patients were approached by the BCN team; those who consented to have their contact details passed to a study researcher were then telephoned to further explain the purpose of the interview and to arrange an appointment (SR). Written consent for these interviews was obtained by post.
Main trial interviews
We used the PROSPER study database to identify trial participants who provided consent on recruitment. The researcher telephoned each participant to explain the purpose of the interview and ask if they were still interested. An information sheet and separate qualitative study consent form were then mailed. Interviews were scheduled after receipt of signed consent forms. As the aim was to explore experiences of postoperative recovery, we ensured that participants were at similar stages of postoperative follow-up, based on date of primary surgery. Physiotherapists were informed of the interview study by the study team. Physiotherapists were approached individually by the researcher via e-mail or telephone, and given an information sheet and consent form.
Data collection
Interview schedules were developed by the research team, based on the aims of the study and informed by the relevant literature. All pre-pilot and trial participant interviews were conducted at the participant’s home or by telephone, decided by mutual agreement between the participant and researcher. Physiotherapist and BCN interviews were conducted at the place of work. The researcher took copies of trial materials to prompt discussion. All interviews were audio-recorded, transcribed verbatim and anonymised. Ethics permission was obtained as per Chapter 2. All interviews were conducted sensitively by a researcher experienced in interviewing cancer patients (SR), recognising that cancer patients may be at a heightened level of distress and taking care to avoid causing additional distress. 108
Data analysis
Interview transcripts were checked for accuracy and anonymity and then entered into NVivo 11 (QSR International, Warrington, UK). We used the same approach to analyse each data set (pre-pilot and main trial) and each data set was analysed independently. The team had initially planned to use framework analysis, which can be useful when there is a team of researchers conducting analysis and when findings are being generated to inform policy and practice. 109 Ultimately, we opted for a thematic analysis to enable us to explore topics that were pre-identified (such as acceptability and issues for wider implementation) but also to develop themes and subthemes based within participants’ responses. We wanted to make central the views of women with breast cancer and their caregivers (physiotherapists). Reflexive thematic analysis is a form of thematic analysis that emphasises the researcher’s role in knowledge production and awareness of philosophical and theoretical assumptions informing decisions made during analysis. 107 A thematic analysis was conducted, which involved three phases. 107,110,111 First, initial transcripts were closely coded to identify common themes. This informed future interviews and also the decision to include usual-care group participants as a comparative group. On completion of all interviews, transcripts were then coded using themes generated from the first phase as a flexible guideline, allowing the adaption and addition of new themes according to the data. Finally, umbrella nodes were printed, allowing analysis to be conducted by hand to identify connections between nodes and within themes. The research team met regularly to discuss the evolving analysis and findings (SR/JB/HR/BM). 112
For the main trial interviews, we agreed that data saturation was reached after seven interviews with intervention group participants (final sample, n = 10). After seven interviews, we sought ethics approval to interview usual-care participants. Our interpretation of ‘saturation’ was that there were enough data to flesh out each of the identified categories and themes. 107 The same analytical approach was used for the pre-pilot, participant and physiotherapist interviews.
Rigour was assessed using Lincoln and Guba’s113 conceptualisation of trustworthiness. Sophie Rees collected the data and was immersed in the data during analysis. Sophie Rees reflected on her positionality throughout analysis. Quotations are provided to illustrate themes.
Overview of interviewees
Pre-pilot interviews
The BCNs approached 11 female patients, who agreed to have their details passed to the researcher. Two declined at the point of telephone contact, one because of time pressures and one because she felt too upset. Two other women were uncontactable. One woman who had recently completed breast cancer treatment approached the research team directly, expressing an interest in being involved. Seven women were interviewed, all of white British identity (Table 1). Two experienced BCNs from one breast cancer unit were interviewed to explore their views of the trial protocol, recruitment plan and draft intervention materials.
Main trial interviews
The characteristics of trial participants included in the substudy are shown in Table 26. All those interviewed from the exercise intervention had been discharged from physiotherapy at the time of their interview. Only one patient who declined to take part in the trial agreed to be interviewed. Dyad interviews were conducted with physiotherapists; a dyad comprised two physiotherapists working at the same hospital site. One interview was undertaken with an individual physiotherapist. A total of six interviews were carried out with 11 physiotherapists. All participants were female and had treated between 1 and 16 trial participants (mean 6.7, median 5).
| Characteristic | Pre-pilot (N = 7) | Main trial treatment allocation | |
|---|---|---|---|
| Exercise (N = 10) | Usual care (N = 10) | ||
| Age, mean (range), years | 54 (27–73) | 51 (28–69) | 60 (44–79) |
| Age bands (years), n | |||
| < 40 | 1 | 5 | 2 |
| 40–59 | 3 | 3 | 3 |
| 60–69 | 3 | 2 | 3 |
| ≥ 70 | 0 | 0 | 2 |
| Months since surgery (range) | N/A | 7 (3–11) | 7 (3–12) |
| Ethnicity, n | |||
| White | 7 | 9 | 10 |
| Mixed | 0 | 1 | 0 |
| Treatment,a n | |||
| Mastectomy | 4 | 4 | 3 |
| BCS | 3 | 6 | 7 |
| ANC | 6 | 10 | 8 |
| SNLB | 2 | 3 | 4 |
| Chemotherapy | – | 9 | 7 |
| Radiotherapy | – | 9 | 10 |
Findings
An overview of the qualitative objectives and the key findings are summarised in Table 27.
| Objective | Method/timing | Participants | Key findings |
|---|---|---|---|
| Refine trial materials and recruitment processes | Pre-pilot interviews |
Patients (not trial participants) BCNs |
Acceptable to approach women for recruitment before surgery, liked emphasis on getting back to their everyday lives, changes made to title of folder to use ‘physiotherapy’ instead of ‘exercise’, patients expressed willingness to be randomised. BCNs advised on timing to approach women for invitation to participate, advised on clinical pathways |
| Explore acceptability of trial interventions | Post-treatment interviews | Intervention trial participants | Participants found intervention acceptable and described characteristics of the intervention that motivated them to adhere over time |
| Explore how intervention/usual care shaped experience of recovery | Post-treatment interviews; physiotherapist interviews | Intervention and control group trial participants; PROSPER physiotherapists | Intervention helped participants regain a sense of control at a time of high uncertainty, physiotherapists felt that they could reassure patients about postoperative movement, and control group participants expressed sense of ‘living with’ their upper limb symptoms rather than being able to improve them |
| Investigate physiotherapists’ experiences of delivering the intervention | Physiotherapist interviews | PROSPER physiotherapists | Longer protected appointment time and focus on shared decision-making, goals, etc., made physiotherapists feel that they were delivering an optimal intervention |
| Explore perspectives on future implementation in NHS | Physiotherapist interviews | PROSPER physiotherapists | Identified potential challenges for wider implementation across NHS, for example need for longer clinic appointment time, provision of emotional support for physiotherapists |
Pre-pilot interviews
Recruitment
Willingness to be randomised and timing of approach
We recognised that we were approaching vulnerable patients and wanted to ensure that recruitment and consent procedures were acceptable and appropriate. All women interviewed expressed a willingness to be randomised when the process was explained to them in the interviews. Most women felt that the time they were approached about the study was acceptable. One participant who was approached after having been given the news that the cancer had spread to her lymph nodes felt that this was an unacceptable time to be approached, and had agreed only because she, as a retired nurse, appreciated the value of research:
In a lot of cases the time that I was approached would perhaps not be a good time, but because I was receptive to it y’know and have a very good relationship with the breast nurse . . . [but] I was quite shocked.
Interviewee 6
Eligibility criteria
The BCNs indicated that they would not be keen to use BMI-related criteria as the only criteria to screen for entry to the trial, as they felt that the criteria were impractical and potentially insensitive risk factors. They were also unconvinced of its credibility as a risk factor:
I think it’s hard to say ‘You’re bigger so you’re at higher risk’ . . . Weight doesn’t tend to show shoulder issues I’ve never come across [that].
BCN1
Obstacles and facilitators of recruitment
The BCNs felt comfortable about approaching women about PROSPER, as they were used to talking to cancer patients in a sensitive manner about taking part in research studies. They did anticipate problems with recruiting patients who lived further afield, who were elderly or who had young children, because of time commitments and travel for physiotherapy appointments. Interviewees felt that it should be made clear to women that they are at risk of experiencing shoulder problems, and that this would aid recruitment:
When you’re going in for breast surgery you don’t realise . . . maybe they think ‘Oh y’know what’s my shoulder or my arms got to do with it?’.
Interviewee 3
Women felt that it should be made clear to potential participants that they would have their own physiotherapist, and that the study was to research postoperative recovery and mobility:
Having your own physiotherapist to help you through these exercises for your benefit, there’s something quite uh I can’t think of the right word, nice isn’t good enough but . . .
Mmm, it’s quite appealing?
Appealing, yes . . . Physiotherapy definitely is the word you need, the key word.
Although the term ‘exercise’ was not felt to be negative, participants felt that promoting the study using the terms ‘recovery’ and ‘physiotherapy’ rather than ‘exercise’ might be viewed more positively. As a result of feedback, we relabelled the patient folder ‘Your Physiotherapy Folder’.
Aids to encourage adherence
The study free telephone number was welcomed by women, but the idea of text message reminders was considered intrusive and unnecessary:
I get text messages from my dentist, from my GP [general practitioner], from the hospital [. . .] it’s a little intrusive.
Interviewee 6
The women thought that some patients may find exercise difficult owing to lack of motivation, the physical effects of treatment and the effort required. However, they felt that the support provided to women should ease this.
Intervention materials
Information
The PROSPER intervention folder contained information regarding upper limb symptoms. We showed patients the draft trial materials and women thought that the exercise intervention folder was straightforward and easy to understand. Women spoke about being given information from the hospital that had not been translated from ‘medical speak’. These information packs about postoperative recovery were often described as being of poor quality:
I just basically had a piece of A4 paper folded with just y’know an arm on there and an arrow pointing [. . .]
Interviewee 7
They appreciated the information about scar massage and what strange sensations they might experience. They felt that surgeons were preoccupied with the cosmetic appearance of surgery and medical professionals in general focused on clinical outcomes, but women themselves were more concerned about how surgery would affect their everyday lives. They viewed the trial exercise materials (patient folder) and the physiotherapy intervention as a positive step towards redressing this balance.
Appearance of materials
Participants liked the photographs of the exercises and preferred images of a ‘real woman’ to drawn illustrations. Women who were more interested in sport and exercise thought that some exercises might be tricky for those less familiar with exercise, but this was not reflected in the interviews with women who described themselves as less sporty; all felt that a physiotherapist would help explain any exercises they were unsure about.
Participant exercise diaries:
The exercise diaries were received positively, but women asked that we ensured that physiotherapists were understanding and sympathetic about the fact that some women would have chemotherapy after their surgery and would have fatigue:
If you’re feeling absolutely rubbish y’know and you just can’t face doing anything and all you’re doing is staying in bed then that’s fair enough but as long as they let you know.
Interviewee 7
Key findings from the pre-pilot study
Women wanted clear information that was easy to follow and made sense to them. We renamed patient materials and opted not to use text reminders for appointments as a result of the interview findings. Participants expressed a willingness to be randomised and felt that the information and intervention materials were acceptable and understandable.
Main trial interviews
We aimed to interview a sample of women who chose not to take part in the trial; however, only one woman agreed to this. In her interview, car parking and treatment burden were cited as reasons for not agreeing to participate in the trial.
Exercise intervention: acceptability and experiences
We interviewed 10 participants in each trial group and 11 physiotherapists who delivered the intervention. In this section, we describe the experiences of those taking part in the exercise intervention, considering trial participants’ and physiotherapists’ perspectives in parallel, supplemented with direct quotations. We also compare the experiences of participants across both intervention groups. We generated three themes. Theme 1, ‘healing’, encapsulated the responses women and physiotherapists gave when talking about what benefits they gained from the intervention, and how it shaped experiences of recovery. Theme 2, ‘being a “perfect” physiotherapist’, refers to the physiotherapists’ views of delivering the intervention. Theme 3, ‘meeting the needs of breast cancer patients’, describes issues raised about the wider implementation of the exercise intervention in the NHS setting and the challenges and opportunities that may bring.
Theme 1: healing
Our thematic analysis of the patient–participants’ experiences and perspectives of recovery, and physiotherapists’ experiences of delivering the exercise intervention, resulted in an overarching theme of ‘healing’ with subthemes of ‘reassurance’, ‘making progress’, ‘helping myself’ and ‘looking ahead’. These subthemes focus on patients’ experiences of recovery and how these were shaped by the intervention.
Reassurance from intervention physiotherapists
In the acute postoperative period, all participants described feeling afraid to move their upper body, and felt unable to follow the exercises within the Breast Cancer Care information leaflet:33,34
It’s quite tender . . . you don’t feel like you ought to be doing it . . . you feel like it’s too soon . . . I was aching so much that I just thought ‘I just can’t do this’.
Qualitative respondent (QR)24 (usual-care group participant)
However, the physiotherapists could reassure women in the intervention group that they were capable and that it was safe to move and that sensations such as stiffness were normal and not worrying:
The, er, physiotherapist was there to suggest, to help . . . was able to tell you whether you were doing things right or wrong or how things were going within your body.
QR09 (intervention group participant)
I think it also prevented sort of fear avoidance . . . seeing them at quite an early stage kind of let us discuss with them what was normal and kind of discussed any fears that they had.
PT03 (physiotherapist)
The physiotherapists felt strongly that this reassurance translated into greater confidence in general for the patient about her well-being:
What do you think they get out of coming to see you?
Confidence to actually move . . . confidence to look after themselves, that they can do things.
Some people it completely changed their kind of outlook on what they could achieve you know, um after the surgery some people came in and were very negative because they couldn’t move their arm and they had all this other stuff going on and they just wanted to be able to do normal tasks . . . so um it was really encouraging for me to see like you’d given them a new lease of life or like a new hopefulness about what they could achieve in the future.
PT08 (physiotherapist)
Making progress: a motivator to adhere
Another major theme was that of ‘making progress’ for those in the exercise programme. This was related to experiencing the increase in difficulty of the exercises prescribed by the physiotherapist, and also feeling the improvement in their bodies, for example in terms of how far they could stretch or reach. For some women, this was a powerful way of reclaiming some sense of autonomy and control of their body, which had been stripped away by cancer:
You could kind of measure it yourself and assess it yourself because you knew how far you could get your group up.
QR08 (intervention group participant)
You saw results and sometimes with your cancer . . . you don’t see results until the end ‘til they say ‘You’re all clear’ you are just going through awful, awful, awful praying and hoping . . . But it is a really positive thing to think ‘Oh something is getting better’.
QR12 (intervention group participant)
This measurable and tangible progress was identified by both patients and physiotherapists as a motivator for women to continue with their exercises:
When we would do the exercises and when we would move the kind of categories in the folder that was given . . . that made me feel good and made me want to kind of continue.
QR13 (intervention group participant)
I think they were all quite happy when they went onto strengthening exercises it meant they were progressing.
PT03 (physiotherapist)
The progression element of the PROSPER programme made it much more fulfilling and rewarding, particularly in the context of cancer treatment, which does not have a sense of progression.
Helping myself: a motivator to adhere
During breast cancer treatment, patients reported feeling at the mercy of their health-care team, having to surrender control of their body while passively receiving treatment. 114–116 As one usual-care group participant said, being a cancer patient is like being ‘a professional waiter, you just sit and wait, and you just let everyone do what they’re doing’ (QR23). Those interviewed noted that the exercise intervention gave them something that they could proactively do for themselves. They could measurably perceive their progress in relation to strength and movement, which may have helped to restore a sense of bodily autonomy:
I think it was more than the exercise. I think it was because you were doing something, because so much of um cancer care is being done to you . . . It was just quite nice to have something proactive for you to do rather than just turn up and have the drugs.
QR12 (intervention group participant)
That was the biggest thing was that they felt that they were doing something for themselves to try and help their arm with the cancer that we weren’t always doing things to them, they had like the confidence to do it for themselves.
PT02 (physiotherapist)
Another feature was that women could choose which exercises they felt most confident and happy doing. The physiotherapists also felt that this patient-centred approach added to the sense of ownership and control:
It gave them an element of control over the exercises didn’t it and a lot of patients also said to me that it gave them something to focus on other than feeling helpless.
PT04 (physiotherapist)
Progress was not a major theme in the usual-care group interviews. Apart from a few highly motivated individuals who spoke about pushing through their pain and even inventing their own exercises to do, most control participants spoke about ‘living with’ problems and waiting for them to get better:
If you sit around too long or if you are not, um, doing anything if you are not moving around you will get stiff, it still gets stiff now but you just have to deal with it.
QR19 (usual-care group participant)
Lifting up now and I can feel the stretching down that left-hand side, but, um, you know I don’t know, I suppose it’s had trauma . . . they said you know it takes a good 12 months for your tissue to settle down after surgery.
QR15 (usual-care group participant)
Motivators to stick to the exercises for this group instead included wanting to be a ‘good’ patient and doing as one was told:
Just the fact that the hospital gave them you and, you know, they know what they’re talking about. You do it because you’ve been told to.
QR23 (usual-care group participant)
This was in stark contrast to the sense of self-determination and control that those in the exercise group described. This suggested that the PROSPER exercise intervention encouraged a sense of empowerment for patients, at a time when they may otherwise feel considerably disempowered.
Looking ahead
A number of the exercise group participants said that they were still performing the exercises after finishing the structured programme if they felt tight or sore. They appreciated having gained this knowledge, which they felt they could continue to use when they experienced any upper limb symptoms:
It’s a nice thing to fall back on when I haven’t and I think ‘Oh this feels a bit tight’ then it’s like ‘Right’ get your act into gear and then do it and it does straight away it loosens it.
QR12 (age 55, intervention group participant)
Now I’m just doing the massage for lymphoedema and exercises only if I feel the problem . . . For example if, if I feel the problem to reach the shelf I’m taking [the] band and I might warm it up just do the exercises with the elastic band exactly for this movement.
QR10 (age 50, intervention group participant)
Participants continued to draw on knowledge gained from the exercise intervention to alleviate any ongoing problems with tightness and stiffness. Some were continuing their strength training, or planned to:
I still go to the gym and there’s a really nice instructor there and he’s set me a new, um, what do you call it, programme [for] strengthening.
QR12 (age 55, intervention group participant)
In a couple of months or so, I would like to kind of start using weights so that I can strengthen my groups . . . It’s kind of like building up the strength that I was building towards whilst I was doing the [PROSPER] exercises before.
QR13 (age 28, intervention group participant)
The exercise intervention was a starting point for some women to continue with their strength and mobility training, especially as treatment ended and life began to resume its normal structure and temporality. These exercise group participants appeared to feel quite confident in managing this in the future. They felt assured that continuing with such activities would help them, and that they would know how to continue or where to seek help if they needed it. This was again in contrast to the control group participants, who, as described above, did not feel that they could do much about their ongoing pain and stiffness.
Theme 2: being a ‘perfect’ physiotherapist
Another major theme identified from physiotherapist interviews was that of being the ‘perfect’ physiotherapist. Physiotherapists reflected on how the trial intervention compared with their usual practice and implications for providing care:
It’s almost like it made you be the perfect physio and the perfect way you should treat patients but you don’t always have time to do that.
PT03 (physiotherapist)
We did have extra time for these patients because we were allocated that time so it was nice to be able to treat them properly . . . That sounds awful but we don’t get the time to do it.
PT09 (physiotherapist)
Physiotherapists felt that they had no time to provide such a high standard of care to routine patients when some had experienced treating women post breast cancer surgery who struggled with mobility, pain and psychological issues many years after diagnosis:
We get people coming in about 2 years later and they’ve never touched their scar, they never saw a physio, they’re stiff, their scar’s horrible, they’ve got awful myofascial trigger points and tightness . . . I’m like, ‘Has nobody talked to you?’ ‘Nah I just got given a leaflet but nobody told me to massage it nobody told me it was OK to stretch it’. They still think 2 years down the line they’re going to hurt themselves if they overstretch so if you get them in at the early stage then it’s just better . . . I had a lady who had a mastectomy it was 3 years later she never went back to work, she never went back to any exercise, she never touched her scar, her mental well-being was like absolutely awful when I first started seeing her because she just didn’t even know that she could have her life back.
PT01 (physiotherapist)
Physiotherapists connected this to the broader organisation of the NHS, and described the need to provide preventative care and allocate resources where they are most needed:
I think we work too much reactive in the NHS don’t we and I think a direction to move in is work in prevention rather than cure.
PT02 (physiotherapist)
Physiotherapists felt that they were providing an important service to participants, and there were specific characteristics of the exercise intervention that facilitated this, namely having more time and the emphasis on shared goals and shared decision-making, both of which encouraged adherence to the programme.
Theme 3: delivering physiotherapy after or alongside breast cancer treatment
This theme reports physiotherapists’ views on delivering a new physiotherapist-led exercise programme for breast cancer patients.
Meeting the needs of breast cancer patients
Adjuvant treatment such as chemotherapy interfered with the patients’ ability to maintain the exercise programme:
A patient would come in for their first appointment and probably just post surgery and most of them were quite positive had quite a lot of goals . . . they’d start their chemotherapy and then it was a whole different ball game because it was just kind of managing their fatigue and we struggled to get people back in for appointments . . . that was the difficult bit wasn’t, that’s a new experience for me I’ve not really treated people kind of in the middle of chemotherapy.
PT02 (physiotherapist)
After stopping the exercises when they became unwell, participants reported that it was physically more difficult to restart the programme, and so it may be helpful for physiotherapists to intervene at this particular point to encourage and motivate patients to continue.
The physiotherapists felt that it was more difficult to meet breast cancer patients’ emotional support needs in a curtained cubicle within an open-plan space, where patients potentially felt more vulnerable:
If I was to launch a service based on this intervention I would try and get a private treatment room ’cause we’re working in curtained cubicles a lot of the time and I felt that didn’t set the tone, I think if you’re asking someone to take their bra off then and you can feel y’know curtains move with the best will in the world, not move open necessarily but you have that sense of, ‘Oh it’s just a piece of material between me and goodness knows who’.
PT10 (physiotherapist)
It was also suggested in two interviews that a woman would be better able to understand the meaning of losing a breast, and that this was important for being able to engage in the emotional and physical work of treating the patient:
They would probably connect better with a female and I was surprised how much women wanted to talk to me about their connection with their breasts so for a lot of them they felt like that was their femininity or that was um a connection to their womanhood and so I think most guys couldn’t relate to how that feels so I could get where they were coming from.
PT08 (physiotherapist)
Physiotherapists’ time, skills, and organisational integration
Although having extra time made the physiotherapists feel that they were providing high-quality care, some expressed doubts about the practicalities of the implementation of the PROSPER intervention as part of routine clinical care given current time restrictions on appointments:
I would say giving them the choice of exercise is time-consuming, which you wouldn’t have in real life, you wouldn’t have the time.
PT09 (physiotherapist)
All the physiotherapists reported providing emotional support to the participants:
I am a person who cries quite easily so I was like, ‘OK I need to keep things under control myself because I am the professional’.
PT08 (physiotherapist)
Although they felt used to providing some form of emotional support to patients, there were particular challenges in relation to this patient group owing to the acute context of cancer treatment; for example, patients were dealing with the fear of dying from breast cancer. This was in contrast to their usual caseload, which often involved caring for musculoskeletal patients with chronic conditions.
The physiotherapists felt that were they to work routinely with these patients, they would need emotional support themselves:
If we were permanent members of staff in oncology you would be given some . . . de-briefing or kind of decompression but we were never offered that . . . both of us have had very close relatives die because of cancer . . . nobody considered that at all.
PT06 (physiotherapist)
Cording, lymphoedema and seroma were postoperative complications that were unfamiliar to a number of the physiotherapists until they took part in the trial, and they expressed a need for further training about breast cancer, its treatments and the specific complications that they might see:
We are MSK [musculoskeletal] physios and we know what a tight shoulder is and we know how to get it moving, so actually the assessment and the exercises wasn’t so much of a worry, but patients occasionally asked me a question that maybe I couldn’t answer . . .
PT03 (physiotherapist)
Physiotherapists also felt disconnected from the surgical or oncology teams; this left them unsure about delivering certain treatments and also unaware of the patients’ adjuvant treatment:
I think it does need to be a multidisciplinary approach and because we’re not involved with them it makes it a little bit difficult [to know] whether we should or shouldn’t be doing those interventions.
PT03 (physiotherapist)
I sometimes found it difficult to ask about things like chemo, radiotherapy and repeat surgeries because I almost felt like it was something that I should know . . . I find that, I feel a bit uncomfortable about that, that I think they come in, and expect, and that’s what I’d want as a health-care professional I want them to know what’s going on I shouldn’t have to tell you when I am having my chemo or this is happening.
PT05 (physiotherapist)
Discussion of qualitative findings
The pre-pilot interviews were valuable in informing the refinement of our trial materials and recruitment and consent procedures. Women interviewed expressed a willingness to be randomised. On the whole, they felt that they were approached about research sensitively and at the right time, and the BCNs felt comfortable approaching patients, although expressed some concerns around using high BMI as a criterion for entry to the study. Useful findings were that women preferred the photographs of women doing exercises, suggested that we change the folder title from ‘Your Exercise Folder’ to ‘Your Physiotherapy Folder’ and indicated that SMS text reminders to do the exercises would have been unwelcomed and excessive. These findings informed procedures for the main trial.
Both participants and physiotherapists identified a need for the exercise intervention and recognised the benefits it offered to patients, both physical and psychological. We found from our data from the main trial interviews that exercise group participants felt that they were benefiting both physically and mentally. This demonstrated the acceptability of an individual supported exercise intervention to both patients and physiotherapists. We included all stakeholders in the study, and gained multiple perspectives on the same issues, allowing us to identify themes that were present across all groups.
As well as improvement in their mobility and strength, patients described physical pleasure from doing the exercises, which was a further motivator to sustain the exercise programme. Beyond this, patients and physiotherapists also recognised emotional and psychological benefits. These included providing cancer patients with the confidence to move and a feeling of control over part of their treatment, and the fact that they could see tangible progress. This appeared to restore patients’ sense of autonomy over their bodies, and improved their well-being as they felt less disempowered and hopeless. The exercise intervention gave them something to focus on and a way of helping themselves throughout their cancer treatment, an essentially disempowering experience. Uncertainty has been identified as a feature of the experience of cancer. 108,117 Being reassured by a physiotherapist that they were improving cut through the uncertainty surrounding cancer and its treatment.
Previous research has also shown that being diagnosed with a serious illness such as cancer can cause an individual to lose trust in their bodily knowledge, as they no longer feel confident that they know what is happening within their bodies. 118–120 Kinesiophobia is associated with lymphoedema and greater pain intensity. 121,122 Physiotherapists could reassure patients that their bodily sensations were normal and they were safe to push themselves physically in ways that they would otherwise have been unsure about. Through the physiotherapy they not only felt more cared for, but they also felt more in tune with their bodies, and they began to trust their bodily knowledge and gain confidence. Their progress was a joint enterprise between the patient and physiotherapist. The encouragement motivated them to adhere to the programme. The interview data suggested that the role of the physiotherapist in affirming this progress and confidence was crucial. Physiotherapists provided invaluable emotional support, as patients unburdened on them and shared their fears about the future and their bodies. This echoed existing research findings, which demonstrated that patients may feel increased empowerment when participating in physical activity during active cancer treatment. 96,123 Participants receiving usual care in our study did not express this sense of empowerment or progress towards improvement. Participating in a group activity can be a way of forgetting about the illness. 124 Our study illustrated that this can also be true for individual, home-based exercise interventions.
Our interviews with physiotherapists highlighted differences between experiences of treating the trial participants versus their usual experiences of treating women who are referred at a much later stage, often when problems have become chronic. These women were referred for therapy after struggling with mobility and pain, affecting their mental and physical well-being. The experience of managing referred patients was in contrast to the trial, in which physiotherapists expressed satisfaction in being able to take preventative action against such future problems for the trial participants. We echo calls for a more proactive model of health care provision for this patient group;125 we also identified a need to improve physiotherapists’ confidence in supporting breast cancer patients.
The interviews highlighted potential issues for the future implementation of the intervention. The intervention should be delivered in a private walled room, ideally with a specially trained, female physiotherapist who is well integrated into the multidisciplinary oncology team. The format of materials provided in the trial were well received and may have helped to motivate patients, but these may need modification for successful translation into routine NHS care. The patient diary was reported as a useful prompt to do their exercises, and having photographs of ‘real’ women doing the exercises was helpful. Longer appointments with physiotherapists, creating shared goals and making shared decisions about exercises were viewed as the most important ingredients in the successful delivery of the intervention. Some physiotherapists reported emotional distress owing to the patients’ distress or because of their own experiences of cancer. Health-care professionals caring for oncology patients should be given the opportunity of debriefing and emotional support.
Future research should evaluate any wider implementation of PROSPER, to understand the challenges associated and the resources required to achieve the positive experiences identified in our study. Research could also further explore obstacles to participating in an individual home-based supported exercise programme after breast cancer surgery, and particular emphasis could be placed on underserved groups, such as minority ethnic women.
Strengths and limitations
One major strength was the triangulation of data from multiple groups, which allowed exploration of different perspectives. However, we accept that those interviewed may be a highly motivated group, and it is possible we did not capture challenges that less motivated women may have experienced. We expect our findings to be representative of breast cancer patients willing to participate in research studies. Our sample was overwhelmingly white, with only one non-white participant. Findings thus may not reflect the experiences of women from ethnic minority backgrounds. However, our interviewed sample were, proportionately, similar in ethnic background (5% non-white) compared with all participants recruited to the trial (8% identified as non-white). Although we aimed to interview a sample of women who chose not to take part in the trial, only one woman agreed to this, limiting our ability to draw conclusions. We cannot accurately estimate how many women were offered/invited to an interview to explore reasons for not taking part in the trial; nevertheless, BCNs reported travel burden, car parking and ‘feeling overloaded’ as common reasons for decline. The majority of women approached agreed to take part in the trial and a high proportion expressed a willingness to be interviewed. We used convenience sampling to approach participants soon after they were discharged from physiotherapy, which may have resulted in a lack of diversity among participants. Time from randomisation was similar between the two groups of intervention and usual-care group participants; however, the usual-care group sample received the BCC leaflet, which was only relevant for first 2 weeks and so they sometimes found it difficult to recall their experiences of that time.
In conclusion, the exercise intervention was highly acceptable to both trial participants and physiotherapists and was viewed to benefit both physical and mental health. The findings suggest that implementation in routine NHS care would be welcomed, although there may be challenges in implementation related to the time and resources required.
Chapter 6 Health economics
Overview of health economic analysis
We conducted a within-trial economic evaluation to estimate the cost-effectiveness of the PROSPER exercise programme compared with usual care after breast cancer surgery. The primary health economic analysis took the form of a cost–utility analysis, expressed in terms of cost per quality-adjusted life-year (QALY) gained and incremental net monetary benefit. The analysis adopted the ITT principle. In line with NICE guidance,126 the analysis was based on an NHS and Personal Social Services (PSS) perspective. The price year adopted for the analysis was 2015, which was when the trial intervention materials were developed. The health economic analysis used a 12-month time horizon and consequently no discounting of costs or outcomes was required. Multiple imputation was used to address missing data. Hierarchical linear models were used to analyse the single cost and QALY end points, whereas a hierarchical net benefit regression framework was used to jointly examine costs and consequences. Uncertainty around cost-effectiveness was characterised through the use of net benefit plots and cost-effectiveness acceptability curves (CEACs), in addition to multiple sensitivity analyses.
Aim
We aimed to estimate the cost-effectiveness of the PROSPER exercise intervention compared with usual care.
Methods
Data collection overview
To conduct the economic evaluation, it was necessary to capture information on costs and consequences. Intervention costs were captured using a combination of methods including case report forms (CRFs), an adapted Client Service Receipt Inventory (CSRI) at 6 months’ and 12 months’ follow-up, and intervention delivery data collected by physiotherapists and the trial team. The EQ-5D-5L127 was completed at baseline and the 6-month and 12-month follow-up. Utility values derived from the EQ-5D-5L were used to calculate QALYs for the primary analysis. For use in the sensitivity analysis, we also collected secondary care use data from NHS Digital. Data on inpatient hospital spells and outpatient attendances over the duration of the trial were sourced from HES data for financial years 2015–16, 2016–17 and 2017–18.
Costs: identifying resource use and costs
The costs within the analysis were divided into four components:
-
direct intervention costs (e.g. physiotherapy time and patient materials)
-
broader health-care/PSS costs (e.g. attendance at pain clinic)
-
wider costs (e.g. informal care)
-
set-up costs (e.g. intervention training costs).
The primary analysis adopted an NHS and PSS perspective and was concerned with the costs of delivering the intervention within an NHS setting. Thus, the primary analysis was concerned only with the direct intervention costs and the broader health-care and PSS costs. Set-up costs and wider costs were considered within the secondary analysis. This section first outlines the delivery costs and then set-up and training costs.
Direct intervention costs
Direct intervention costs were the costs associated with the introduction of the intervention compared with the usual-care group. All participants received usual care, which involved a 5-minute contact with a specialist BCN who provided usual-care leaflets (BCC633 and BCC15134). In addition to leaflets, the intervention group then received a physiotherapist-led exercise programme. Resource use was captured prospectively alongside the trial and we summarise the collection of resource use components in Table 28.
| Resource type | Resource use | Unit cost source |
|---|---|---|
| Usual care | ||
| BCC6 leaflet | 1 per participant | Trial team |
| BCC151 leaflet | 1 per participant | Trial team |
| Nurse time to explain information | 5 minutes per participant | PSSRU |
| Exercise intervention | ||
| BCC6 leaflet | 1 per participant | Trial team |
| BCC151 leaflet | 1 per participant | Trial team |
| Nurse time to explain information | 5 minutes per participant | PSSRU |
| Patient exercise planner | 1 per participant | Trial team |
| Your Exercise manual | 1 per participant | Trial team |
| Physiotherapist preparation time | Treatment log | PSSRU |
| Physiotherapist appointment (length) | Treatment log | PSSRU |
| Equipment | CSRI/exercise log | NHS Supply chain |
| Contacts between appointments | Treatment log | PSSRU |
Broader health-care costs
Health-care resource use was captured primarily through section D of the CRF at 6 and 12 months (Table 29). Data on health-care use were collected for inpatient care, outpatient care, community health care, medication and equipment provided. HES data were obtained for 242 patients who had reached 12 months from randomisation by the end of the 2017–18 financial year, for use in secondary analysis. The resource use data collected within the CRFs were the primary source of cost data within the trial. Other wider costs considered within secondary analyses included out-of-pocket costs, privately purchased equipment and private health-care costs. A further analysis included set-up costs, which included resource use associated with training physiotherapists.
| Resource type | Questiona | Unit cost source |
|---|---|---|
| Broader health-care resource use | ||
| Inpatient and day hospital care | D1 and D2 | NHS reference costs128 |
| Outpatient care | D3 and D4 | NHS reference costs128 |
| Community health care | D5 | NHS reference costs/PSSRU129 |
| Medication | D7 | NHS prescription cost analysis |
| Equipment | D8 | NHS supply chain130 |
| Other wider resource use | ||
| Wider health care | D9 | Stated within CRF |
| Employment impacts | D10 | Income lost stated within CRF |
| Private health care | D6 | Stated within CRF |
| Intervention set-up resource use | ||
| Trainers’ time – trained on site | Trial team | PSSRU129 |
| Trainers’ time – centrally trained | Trial team | PSSRU129 |
| Trainees’ attendance | Time and travel questionnaire | PSSRU129 |
Outcomes
In line with NICE guidelines,126 QALYs were the primary outcome for the economic evaluation.
Estimating quality-adjusted life-years
We used QALYs within the health economic evaluation, as per national recommendations. 126 QALYs combine quantity and QoL into a single metric. To calculate QALYs, it was necessary to obtain health state values for trial participants over multiple time points. We used the EQ-5D-5L, a five-dimension measure of HRQoL recommended by NICE. 126,127 There are value sets, also referred to as tariff values, that allow the calculation of utility values associated with each and every state generated by the EQ-5D-5L measure. 131 At the time of writing, NICE preferred the use of the van Hout et al. 132 algorithm;132 hence this value set was used to calculate utility values.
Health states were measured prospectively using the EQ-5D-5L at three time points: baseline, 6 months and 12 months. Health state values as measured by the EQ-5D-5L were combined with time to calculate QALYs by calculating the area under the curve using the trapezium rule. 133 This method assumes that the health states reported at each time point were linearly interpolated. Participants who died during follow-up were given an EQ-5D-5L score of zero at subsequent follow-ups beyond the date of death.
Secondary health economic outcome
The primary clinical outcome was the DASH upper limb disability questionnaire. 36 A secondary analysis used this clinical outcome measure to consider the cost per DASH point associated with the exercise intervention.
Missing data and multiple imputation
Although resource use/cost component data are presented in their raw form, for the cost-effectiveness analysis that combined multiple cost components and multiple EQ-5D-5L scores across time points, multiple imputation was necessary to avoid the pitfalls associated with complete-case analysis with substantial missing data. Multiple imputation avoids many of the issues encountered with simpler imputation methods (e.g. last observation carried forward), which have been criticised for underestimating uncertainty. 134 Missing data were assumed to be missing at random. To maximise the use of available data, multiple imputation was conducted at the component level (e.g. for each health-care cost variable and EQ-5D-5L) at each time point. Costs and EQ-5D-5L scores were imputed jointly using chained equations and predictive mean matching; the imputation model included age, ethnicity, marital status, employment status and recruiting site as co-variates. For 15 participants lacking covariate data, these were dropped from the multiple imputation analysis. Given that approximately 30–35% of data were missing for each cost component, a total of 35 imputations were calculated to produce 35 complete data sets. Multiple imputation procedures were conducted in Stata 16.
Analyses of resource use, cost and quality-adjusted life-years
Resource use between trial groups was examined using standard statistical methods: descriptively and using t-tests for continuous variables and chi-squared tests for categorical variables. Regression models using the multiple imputation data were used to examine the impact of the intervention on the single cost and QALY end points. The data were hierarchical, that is we expected participants within each site to be more similar to each other than to individuals in different sites. Consequently, multilevel linear models that accounted for clustering by including random-effect parameters were used to estimate end points. Following recommendations, it was necessary to adjust for baseline differences between the two groups when examining differences in QALYs. 126 Consequently, the baseline EQ-5D-5L score was included within the incremental analysis of QALYs as a covariate.
Estimating cost-effectiveness
To examine cost-effectiveness, it was necessary to jointly assess the incremental costs and incremental effects. In its most simple form, an incremental cost-effectiveness ratio (ICER) was presented. An ICER was calculated as follows:
Although ICERs provide a point estimate for cost-effectiveness, by themselves they do not characterise the uncertainty that may surround them. ICERs are estimated through the analysis of sample data, which may be subject to large variability, and there are inherent difficulties associated with characterising uncertainty around ratios, for example when the CI of the ICER overlaps zero and when the effect size approaches zero. Consequently, the net benefit approach is favoured for assessing the cost-effectiveness and characterising uncertainty within this analysis.
Net benefit regression framework
The net benefit regression framework was chosen as it has several strengths: (1) it transforms the cost/QALY data from a ratio into a continuous variable, allowing for easier manipulation while often normalising the data; (2) by combining costs and outcomes, it can seamlessly account for correlation between the two end points; (3) it allows easy control for baseline and covariate imbalances;135 (4) it can correct for clustering using a multilevel framework; (5) it effectively deals with uncertainty around the decision-makers’ willingness to pay (WTP) for the health outcome of interest; (6) it facilitates the generation of CEACs to present decision uncertainty; and (7) it is relatively straightforward to implement in Stata using multiple imputation data.
Characterising uncertainty
It is important to present uncertainty in a manner that decision-makers can easily interpret. CEACs are a graphical representation of the probability that an intervention is cost-effective at different levels of WTP. NICE recommends that WTP thresholds of £20,000 and £30,000 per QALY are included in the CEAC when assessing uncertainty. 126 For a range of WTP thresholds, including those specified by NICE, CEACs were created to characterise uncertainty within cost-effectiveness estimates.
Sensitivity analyses
A number of sensitivity analyses were conducted to examine the uncertainty surrounding trial results. These sensitivity analyses included:
-
Complete case analysis. This analysis considered only complete cases.
-
Cost per DASH point. Should the intervention group be associated with higher costs than the usual-care group, then the costs per DASH point were to be estimated.
-
Costing from a societal perspective. In this sensitivity analysis, wider societal costs were included within the cost-effectiveness analysis. This included NHS health costs, private costs and over the counter (OTC) medication.
-
Incorporating training within the evaluation. Site staff were trained both centrally and at hospital sites, this analysis used a conservative approach whereby it was assumed each site was trained separately, with up to two trial staff undertaking training for 4 hours at each hospital site.
-
Excluding high-cost cancer health-care use. This analysis limited costs to intervention costs, community care costs, outpatient physiotherapy, outpatient pain clinics, outpatient complementary therapies/exercise facilities and analgesics.
-
Using HES cost data instead of CSRI data for hospital costs. This sensitivity analysis re-ran the primary analysis for the 242 participants with 12 months of complete data post randomisation, prior to the HES cut-off date (31 March 2018) and used HES data for costing hospital costs instead of CSRI inpatient and outpatient data. As these hospital data are obtained centrally, we assumed that these data were complete. Inpatient spells during the study and other hospital-based care costs were estimated by linking hospital episode data with Health Resource Groups, using the Reference Cost Grouper software136 and then costed using NHS reference costs. 128
Results
Resource use: NHS and Personal Social Services resources
Health-care resource use by type and quantity of resource for both trial groups at 6 and 12 months, along with statistical tests for difference between the two groups, is reported in Tables 30 and 31.
| Resource use | Breast cancer related | Non-breast cancer related | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Usual-care group | Exercise group | p-valuea | Total | Usual-care group | Exercise group | p-valuea | |
| Inpatient, 6 months | ||||||||
| Any contact: no, n | 149 | 76 | 73 | 241 | 118 | 123 | ||
| Any contact: yes, n | 125 | 55 | 70 | 0.25 | 30 | 14 | 16 | 0.81 |
| Mean, days | 1.84 | 1.98 | 1.71 | 0.55 | 0.29 | 0.34 | 0.25 | 0.71 |
| Median, days | 0 | 0 | 0 | N/A | 0 | 0 | 0 | N/A |
| Mean cost (SD), £ | 1453 (2270.74) | 1451 (208.88) | 1454 (183.51) | 0.99 | 102.88 (529.91) | 98.46 (478.76) | 107.13 (576.65) | 0.89 |
| Inpatient, 12 months | ||||||||
| Any contact: no, n | 245 | 121 | 124 | 351 | 117 | 234 | ||
| Any contact: yes, n | 23 | 15 | 8 | 0.15 | 42 | 13 | 29 | 0.60 |
| Mean, days | 0.40 | 0.72 | 0.07 | 0.037b | 0.25 | 0.22 | 0.28 | 0.67 |
| Median, days | 0 | 0 | 0 | N/A | 0 | 0 | 0 | N/A |
| Mean cost (SD), £ | 145.02 (610.36) | 209.15 (761.20) | 79.43 (393.92) | 0.08 | 118.87 (515.38) | 111.94 (420.20) | 125.74 (596.61) | 0.83 |
| Outpatient, 6 months | ||||||||
| Any contact: no, n | 16 | 11 | 5 | 196 | 98 | 98 | ||
| Any contact: yes, n | 258 | 120 | 138 | 0.08 | 73 | 32 | 41 | 0.37 |
| Mean, contacts | 17.90 | 18.55 | 17.90 | 0.35 | 0.97 | 0.62 | 1.29 | 0.023c |
| Median, contacts | 17 | 16 | 18 | N/A | 0 | 0 | 0 | N/A |
| Mean cost (SD), £ | 2669.64 (1821.39) | 2567.48 (1882.44) | 2765.65 (1763.81) | 0.38 | 145.05 (361.02) | 102.53 (288.80) | 183.81 (413.39) | 0.07 |
| Outpatient, 12 months | ||||||||
| Any contact: no, n | 64 | 32 | 32 | 176 | 89 | 87 | ||
| Any contact: yes, n | 206 | 105 | 101 | 0.89 | 91 | 48 | 43 | 0.74 |
| Mean, contacts | 7.79 | 8.26 | 7.30 | 0.47 | 1.36 | 1.12 | 1.62 | 0.47 |
| Median, contacts | 3 | 2.5 | 3 | N/A | 0 | 0 | 0 | N/A |
| Mean cost (SD), £ | 855.45 (1285.35) | 849.66 (1262.56) | 861.19 (1312.70) | 0.94 | 203.61 (798.58) | 169.16 (346.70) | 240.29 (1091.70) | 0.48 |
| Resource use | Total | Usual-care group | Exercise group | p-valuea |
|---|---|---|---|---|
| Community and social care, 6 months | ||||
| Any contact: no, n | 52 | 24 | 28 | 0.07 |
| Any contact: yes, n | 218 | 105 | 113 | |
| Mean, contacts | 5.61 | 6.49 | 4.81 | 0.08 |
| Median, contacts | 3 | 3 | 2 | N/A |
| Mean cost (SD), £ | 305.15 (436.96) | 345.12 (473.34) | 267.61 (397.94) | 0.16 |
| Community and social care, 12 months | ||||
| Any contact: no, n | 75 | 34 | 41 | 0.27 |
| Any contact: yes, n | 193 | 102 | 91 | |
| Mean, contacts | 3.02 | 3.05 | 2.99 | 0.92 |
| Median, contacts | 1 | 2 | 1 | N/A |
| Mean cost (SD), £ | 162.10 (232.66) | 163.25 (202.51) | 160.97 (259.54) | 0.94 |
| Special equipment resource use, 6 months | ||||
| Any use: no, n | 150 | 70 | 80 | 0.69 |
| Any use: yes, n | 114 | 56 | 58 | |
| Mean cost (SD), NHS, £ | 26.12 (48.95) | 25.64 (50.00) | 26.56 (48.16) | 0.88 |
| Mean cost (SD), private, £ | 48.29 (334.65) | 32.13 (142.14) | 63.04 (442.77) | 0.46 |
| Special equipment resource use, 12 months | ||||
| Any use: no, n | 157 | 76 | 81 | 0.29 |
| Any use: yes, n | 109 | 60 | 49 | |
| Mean cost (SD), NHS, £ | 18.29 (41.87) | 17.87 (41.90) | 18.72 (42.00) | 0.87 |
| Mean cost (SD), private, £ | 49.59 (337.88) | 75.81 (524.81) | 22.36 (68.03) | 0.25 |
| Private treatment resource use, 6 months | ||||
| Any use: no, n | 242 | 113 | 129 | 0.38 |
| Any use: yes, n | 25 | 14 | 11 | |
| Mean cost (SD), £ | 29.40 (191.92) | 27.56 (170.75) | 30.49 (203.87) | 0.90 |
| Private treatment resource use, 12 months | ||||
| Any use: no, n | 231 | 113 | 118 | 0.54 |
| Any use: yes, n | 31 | 17 | 14 | |
| Mean cost (SD), £ | 36.00 (203.94) | 44.57 (241.60) | 28.36 (163.69) | 0.52 |
| Medication resource use, 6 months | ||||
| NHS mean cost (SD), £ | 1886.29 (6384.83) | 1874.38 (5819.52) | 1897.40 (6889.87) | 0.97 |
| OTC mean cost (SD), £ | 12.20 (44.20) | 13.04 (36.89) | 11.42 (50.17) | 0.76 |
| Medication resource use, 12 months | ||||
| NHS mean cost (SD), £ | 1504.86 (5547.08) | 1991.46 (6584.38) | 1018.25 (4236.03) | 0.15 |
| OTC mean cost (SD), £ | 14.18 (72.34) | 12.11 (47.29) | 16.24 (90.90) | 0.65 |
| Other wider costs, 6 months | ||||
| Any use: no, n | 126 | 57 | 69 | 0.44 |
| Any use: yes, n | 136 | 68 | 68 | |
| Mean wider cost (SD), £ | 129.92 (443.11) | 126.87 (285.13) | 132.61 (443.11) | 0.91 |
| Other wider costs, 12 months | ||||
| Any use: no, n | 169 | 86 | 83 | 0.79 |
| Any use: yes, n | 95 | 50 | 45 | |
| Mean wider cost (SD), £ | 84.48 (450.42) | 57.04 (232.47) | 112.39 (595.32) | 0.34 |
Inpatient resource use
Inpatient resource use was separated into breast cancer-related and non-breast cancer-related stays. We observed a decrease in resources used between 6 months and 12 months, with the proportion of inpatient contacts falling in both groups, and mean inpatient days falling from 1.8 to 0.4 for breast cancer-related inpatient stays, and from 0.3 to 0.2 for non-breast cancer inpatient stays. At 6 months there was no difference in resource use between the two groups for either breast cancer stays or non-breast cancer stays. There was no difference in the number reporting breast cancer-related inpatient stays (p = 0.25) or non-breast cancer-related inpatient stays (p = 0.81) by trial arm, and this was reflected in costs, with very little difference between the two groups (p = 0.89). At 12 months, there was no difference in contacts with inpatient services between breast cancer stays (p = 0.15) and non-breast cancer stays (p = 0.60). When considering mean breast cancer inpatient days, the number of days per person was lower in the intervention group than in the usual-care group (0.07 vs. 0.72 days, respectively; p = 0.04). Considering multiple testing and the very few non-zero values, this finding should be treated with caution. This is reflected in the relatively small and non-significant cost difference between the two groups at this time point.
Outpatient resource use
Similar to inpatient use, we observed a fall in the number outpatient contacts from 6 to 12 months (Table 30). The proportion of participants reporting breast cancer outpatient contacts fell in both groups, with the mean number of breast cancer contacts falling from 18 to 8. However, non-breast cancer contacts remained relatively steady, with an average of one contact per participant at each time point. Regarding the proportion of participants having breast cancer contacts, there were no differences between the two groups at 6 months (p = 0.08) or 12 months (p = 0.89). In terms of the proportion having non-breast cancer contacts, there were no differences at 6 months (p = 0.37) or 12 months (p = 0.74). There was, however, a statistically significant difference (p = 0.02) in non-breast cancer contacts at 6 months, with the intervention group having, on average, 1.3 contacts, compared with 0.6 contacts in the usual-care group. Again, multiple testing should be considered when interpreting this result. There were no differences at 12 months. In terms of cost, there was no significant differences between the two groups at either of the two time points for breast cancer and non-breast cancer outpatient contacts.
Community care resource use
Regarding use of community care resources, the proportion reporting contacts fell over the 6–12 months time period (Table 31). Likewise, the mean number of contacts fell from six to three over this time period. However, there were no between-group differences in the proportion of community care contacts at 6 months (p = 0.07) or 12 months (p = 0.27). Likewise, there were no between-group differences in number of community contacts at 6 (p = 0.08) or 12 months (p = 0.92). There were no differences in cost between the two groups at either time point (p = 0.16 and p = 0.94).
Special equipment resource use: NHS/Personal Social Services provided
There was no difference in the number of individuals receiving specialist equipment/accessories (Table 31). When focusing on equipment provided by the NHS and PSS, there were no difference in terms of cost of equipment between the two groups, with there being, on average, less than a £1 cost difference to the NHS between the two groups at both time points.
Medication resource use: NHS provided
Given the severity and burden of illness of trial participants, it is unsurprising to find high levels of cost associated with medication use per participant (Table 31). Focusing on NHS medication, as opposed to OTC, high mean costs were observed at both 6 months and 12 months. At 6 months, there were negligible cost differences between the intervention group and the usual-care group (p = 0.97). At 12 months, although there was a large cost difference (≈£900) between the two groups, this was not statistically significant (p = 0.15). This discrepancy appeared to be due to a small number of participants within the usual-care group receiving high-cost cancer drugs.
Direct intervention resource use
The direct costs related to the intervention were based on physiotherapy logs for participants who had attended physiotherapy. Participants had a mean of four contacts with physiotherapist and the mean length of appointment was approximately 40 minutes. The addition of physiotherapy administrative time added 8 minutes to this consultation (Table 32). There were a number of other small intervention-related costs, including an exercise manual and planner given to each intervention participant. Therabands were also given after 1 month postoperatively. All participants received cancer rehabilitation leaflets during a short preoperative session with a breast cancer nurse (Tables 32 and 33). Compared with other health-care costs, these costs were relatively minor, with total intervention costs coming to just over £100 per intervention participant.
| Physiotherapy resource use | Costs |
|---|---|
| Physiotherapy participant contacts | |
| Mean (SD) | 3.83 (1.59) |
| Minimum, maximum | 1.00, 9.00 |
| Appointment duration, minutes | |
| Mean (SD) | 39.86 (17.24) |
| Minimum, maximum | 0.00, 120.00 |
| Appointment duration including admin, minutes | |
| Mean (SD) | 48.20 (18.88) |
| Minimum, maximum | 0.00, 120.00 |
| Physiotherapy appointment costs | |
| Mean (SD) cost, £ | 102.56 (44.37) |
| 95% CI | 95.88 to 109.24 |
| Intervention | Quantity | Unit cost (£) | Cost (£) |
|---|---|---|---|
| All participants | |||
| BCC6 leaflet | 13 pages | 0.15 | 1.95 |
| BCC151 leaflet | 32 pages | 0.15 | 4.80 |
| Nurse time, minutes | 5 per participant | 52 | 4.33 |
| Additional cost to all participants | 11.08 | ||
| Exercise intervention only | |||
| Participant manual | 1 manual | 4.40 | 4.40 |
| Additional sheets | 7 pages | 0.15 | 1.05 |
| Physiotherapist manual | 100 pages | 0.15 | 15.00 |
| Physiotherapist manual folder | 1 | 1.00 | 1.00 |
| Therabands | 600 m | 960.98 | 5.03 |
| Additional cost to intervention participants only | 26.48 | ||
Other resource use: non-NHS/non-Personal Social Services
Resource use: special equipment – privately obtained
There were some private costs associated with purchasing specialist equipment (Table 31). Across the trial population, these remained relatively constant at the 6- and 12-month time points. There were no statistically significant differences between treatment groups in terms of private costs at the 6- or 12-month time point.
Resource use: medication – over the counter
Compared with NHS provided medication, the costs associated with OTC medications were relatively minor, accounting for only £12 per person at 6 months and £14 per person at 12 months (Table 31). There was no difference in OTC medication costs at 6 months or 12 months between the two trial groups.
Resource use: private treatment
Only a small number of participants reported using private treatment (Table 31). Costs per participant across both groups were relatively low (£29 at 6 months and £36 at 12 months), with no differences between trial groups.
Resource use: other wider costs
From 6 to 12 months, other wider costs fell across both trial groups (Table 31). There was no difference in the proportion of participants in each trial group reporting wider costs at 6 months (p = 0.44) or 12 months (p = 0.79). Likewise, there were no differences in the costs reported at each of the time points (p = 0.91 and p = 0.34, respectively).
Resource use: impact on employment
Reported impacts on employment are presented in Table 34. Across both groups, there was an increase in the number and proportion of participants able to work at 12 months compared with 6 months, although this was not a statistically significant difference. There were no differences between groups in terms of the numbers who reported taking time off work, the number of days of work missed or lost income as a result of missing work.
| Outcome | Total | Usual-care group | Exercise group | p-valuea |
|---|---|---|---|---|
| Able to work | ||||
| 6 months | ||||
| No | 187 | 92 | 95 | 0.51 |
| Yes | 81 | 36 | 45 | |
| 12 months | ||||
| No | 153 | 78 | 75 | 0.95 |
| Yes | 111 | 57 | 54 | |
| Time off work | ||||
| 6 months | ||||
| No | 108 | 53 | 55 | 0.73 |
| Yes | 107 | 50 | 57 | |
| 12 months | ||||
| No | 156 | 78 | 78 | 0.48 |
| Yes | 69 | 38 | 31 | |
| Number of days off work | ||||
| 6 months | ||||
| Mean (SD) | 20.41 (45.87) | 20.53 (45.07) | 20.30 (46.94) | 0.98 |
| 95% CI | 13.01 to 27.81 | 10.09 to 30.97 | 9.58 to 31.03 | |
| 12 months | ||||
| Mean (SD) | 7.79 (28.97) | 5.69 (24.42) | 9.86 (32.86) | 0.33 |
| 95% CI | 3.63 to 11.95 | 0.69 to 10.69 | 3.17 to 16.56 | |
| Lost income (£) | ||||
| 6 months | ||||
| Mean (SD) | 1522.29 (4430.92) | 1647.58 (5474.56) | 1395.56 (3061.21) | 0.71 |
| 95% CI | 861.21 to 2183.37 | 487.63 to 2807.53 | 743.13 to 2048.00 | |
| 12 months | ||||
| Mean (SD) | 1176.84 (4294.67) | 1343.17 (5146.94) | 1003.65 (3192.93) | 0.58 |
| 95% CI | 574.94 to 1778.73 | 327.10 to 2359.24 | 360.13 to 1647.17 | |
Health-care cost components
Direct intervention costs
The mean cost of physiotherapy appointments for those in the intervention group was £103 (Table 32). Both trial groups received information leaflets; however, these contributed very little to cost. For the intervention group, there were other small costs, such as a personalised exercise planner and manual, manuals for the physiotherapist (Table 33) and Therabands; these costs were again relatively small (£26). The total direct incremental cost associated with the intervention compared with the usual-care group was £129.
Inpatient costs
In both trial groups, the costs associated with inpatient breast cancer care were significant (Table 35). Total breast cancer-related inpatient costs per person were slightly (£69) higher in the usual-care group than in the exercise group, but the difference was not statistically significant (p = 0.84). Non-breast cancer-related inpatient costs were slightly (£49) higher in the exercise group than in the usual-care group but, again, the difference was not statistically significant (p = 0.59).
| Costs | Mean (SD), £ | 95% CI | Mean difference | p-valuea |
|---|---|---|---|---|
| Inpatient | ||||
| Breast cancer related | ||||
| Usual care | 1707.20 (2748.50) | 1201.71 to 2212.68 | –69.06 | 0.84 |
| Exercise | 1638.14 (2345.37) | 1210.54 to 2065.73 | ||
| Non-breast cancer related | ||||
| Usual care | 149.46 (433.81) | 68.60 to 230.31 | 48.98 | 0.59 |
| Exercise | 198.44 (852.21) | 38.14 to 358.74 | ||
| Outpatient | ||||
| Breast cancer related | ||||
| Usual care | 3637.72 (2494.08) | 3150.28 to 4125.16 | –20.29 | 0.95 |
| Exercise | 3617.43 (2606.48) | 3115.46 to 4119.41 | ||
| Non-breast cancer related | ||||
| Usual care | 239.24 (466.88) | 151.01 to 327.46 | 216.69 | 0.088 |
| Exercise | 455.93 (1239.94) | 221.61 to 690.24 | ||
| Community care | ||||
| Usual care | 530.56 (584.26) | 417.50 to 643.63 | –70.44 | 0.38 |
| Exercise | 460.12 (583.61) | 347.73 to 572.52 | ||
| Medication | ||||
| Usual care | 3211.05 (9508.71) | 1527.71 to 4894.90 | –334.68 | 0.79 |
| Exercise | 2876.37 (10 to 278.99) | 1092.68 to 4660.06 | ||
| OTC medication | ||||
| Usual care | 26.88 (65.34) | 15.31 to 38.44 | 0.27 | 0.99 |
| Exercise | 27.14 (140.77) | 2.71 to 51.57 | ||
| NHS equipment | ||||
| Usual care | 45.62 (80.64) | 30.52 to 60.72 | 3.16 | 0.77 |
| Exercise | 48.78 (80.23) | 33.90 to 63.67 | ||
| Wider equipment costs | ||||
| Usual care | 121.78 (601.79) | 9.10 to 234.46 | –28.9 | 0.70 |
| Exercise | 92.89 (530.82) | –5.61 to 191.38 | ||
| Private care | ||||
| Usual care | 44.57 (241.60) | 1.80 to 87.34 | –12.53 | 0.519 |
| Exercise | 28.36 (163.69) | 1.01 to 55.71 | ||
| Other wider costs | ||||
| Usual care | 147.44 (320.27) | 81.48 to 213.40 | 114.29 | 0.23 |
| Exercise | 261.73 (865.30) | 85.46 to 438.00 | ||
| Lost income | ||||
| Usual care | 3408.41 (11,867.76) | 557.46 to 6259.35 | ||
| Exercise | 2362.28 (5319.57) | 999.87 to 3724.68 | 1046.13 | 0.26 |
Outpatient costs
The total costs associated with breast cancer-related outpatient care was over £3600 per person in both groups (Table 35). This dwarfed the costs associated with non-breast cancer-related outpatient visits. There was minimal difference between the two groups in terms of total cost (£20). By comparison, non-breast cancer outpatient contacts were relatively low in both the usual-care (£239) and exercise (£455) groups. Although the cost per person for non-breast cancer contacts was £217 higher in the exercise group than in the usual-care group, the difference was not statistically significant.
Community care costs
The total community care costs were relatively small compared with the outpatient care costs, with the average participant costing £531 in the usual-care group and £460 in the intervention group (Table 35). Again, there were no significant differences between the two groups in terms of the cost of community care accrued (p = 0.38).
Medication
Medication was the second biggest driver of cost (Table 35). The costs associated with medication were high in both groups (≈£3000 per person). Although the cost per person of medication was £335 lower in the exercise group than in the usual-care group, the difference was not significant because in both groups costs varied considerably between individuals (p = 0.79). The costs of OTC medication were minimal compared with those of prescribed medication and were almost identical, at only £27 per person in both groups (p = 0.99).
Equipment
There were few reported costs related to specialist equipment provided by the NHS (Table 35). There was very little difference between the two groups in terms of NHS equipment costs. The intervention group reported slightly lower (–£29) privately purchased equipment costs than the usual-care group (p = 0.7).
Other non-NHS/non-Personal Social Services costs
Private health care costs are shown in (Table 35). The cost of private health care was slightly lower (–£13) in the exercise group than in the usual-care group but the difference was not statistically significant. Wider costs were higher in the exercise group than in the usual-care group (+£114), but once more the difference was not statistically significant (p = 0.23). Both groups reported significant loss of income as a result of time off work, although lost income per person was over £1000 lower in the exercise group than in the usual-care group. However, there was high missingness for this self-report variable and, given the large variability in this variable, the difference was not statistically significant (p = 0.26).
Utility values by time point
Health utility at each time point is reported in Table 36 and Figure 8. At baseline, there was a very slight imbalance between the two groups, with the usual-care group having a mean utility score of 0.666 and the exercise group a score of 0.683. The period from baseline to 6 months was associated with a small decrease in health utility in both groups, with the mean utility score falling to 0.648 in the usual-care group and to 0.673 in the exercise group. Between 6 months and 12 months the utility scores diverged, with that in the exercise group increasing to 0.705 while, in contrast, the score in the usual-care group fell to 0.633. Thus, by 12 months, utility scores had improved compared with baseline in the exercise group, but in the usual-care group were worse than at baseline. The utility scores that use the multiple imputation data show a very similar picture. Imputed utility scores at all time points are nearly identical to the complete case data; however, uncertainty surrounding those estimates is reduced, as is reflected in the slightly narrower CIs.
| Time point | EQ-5D-5L utility score | |||||
|---|---|---|---|---|---|---|
| Usual-care group | Exercise group | |||||
| Mean | 95% CI | SE | Mean | 95% CI | SE | |
| Imputed data (multiple imputation) | ||||||
| Baseline | 0.665 | 0.630 to 0.700 | 0.018 | 0.685 | 0.651 to 0.719 | 0.017 |
| 6 months | 0.636 | 0.598 to 0.674 | 0.019 | 0.673 | 0.644 to 0.701 | 0.014 |
| 12 months | 0.626 | 0.592 to 0.660 | 0.017 | 0.693 | 0.658 to 0.728 | 0.018 |
| Complete cases | ||||||
| Baseline | 0.666 | 0.633 to 0.699 | 0.018 | 0.683 | 0.651 to 0.719 | 0.015 |
| 6 months | 0.648 | 0.611 to 0.685 | 0.019 | 0.673 | 0.643 to 0.702 | 0.015 |
| 12 months | 0.633 | 0.597 to 0.669 | 0.018 | 0.705 | 0.670 to 0.741 | 0.018 |
FIGURE 8.
The EQ-5D-5L profile by group (multiple imputation data).
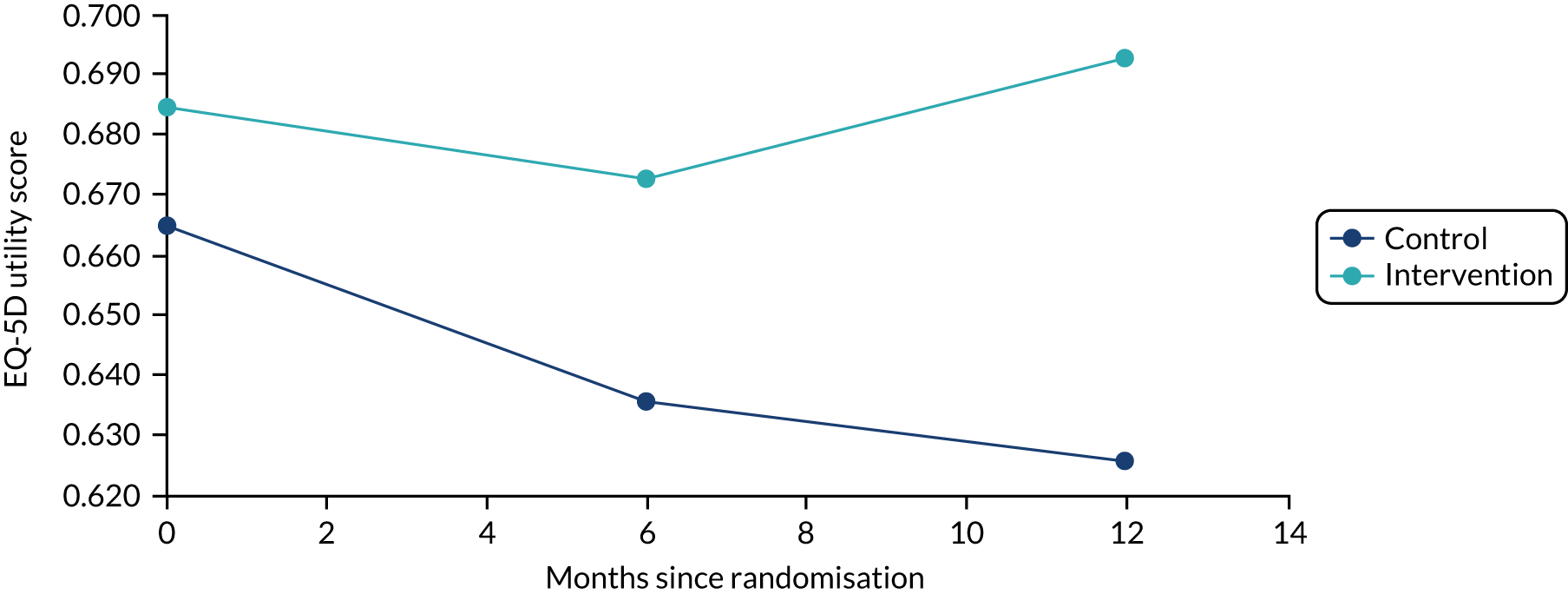
Analysis of costs
Table 37 presents the incremental costs associated with intervention resulting from the multilevel regression of NHS and PSS costs. There was an incremental cost difference of –£387 (95% CI –£2491 to £1718) in favour of the exercise group. This represents a cost saving. However, the wide CI shows that there is a high degree of uncertainty surrounding this estimate. The cost difference using the complete-case analysis also shows exercise to be cheaper than usual care; however, the difference is slightly smaller (–£258.56). Reflecting the large numbers of missing data in the complete-case analysis, the uncertainty (95% CI –£3609 to £3092) surrounding this estimate is much greater than in the multiple imputation data set.
| Analysis | MD | SE | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| Imputed data | |||||
| Intervention | –386.78 | 1073.48 | –0.36 | 0.72 | –2491.18 to 1717.62 |
| Complete case (n = 158) | |||||
| Intervention | –258.56 | 1696.19 | –0.15 | 0.88 | –3609.01 to 3091.90 |
Analysis of quality-adjusted life-years
The analysis of QALYs is shown in Table 38. Using the multiple imputation data and controlling for baseline imbalance, the intervention group accrued 0.029 (95% CI 0.001 to 0.056) more QALYs than the usual-care group. This is a statistically significant increase (p = 0.04). The results of the complete-case analysis reflect the multiple imputation results, with the intervention group accruing 0.030 QALYs (95% CI 0.002 to 0.059), a statistically significant difference (p = 0.04).
Cost-effectiveness analysis
To examine cost-effectiveness, incremental costs and QALYs were analysed simultaneously. From the analysis of costs and QALYs it is evident that exercise dominated usual care. The results combining costs and QALYs within a net benefit framework are shown in Figures 9 and 10. As seen in Figure 9, net benefit was positive at all levels of WTP including zero; this reflects the domination of exercise over usual care. That is, even if we are not willing to pay any money for health gains, the intervention group still provides a greater net benefit to society because of the lower health-related costs. As can be seen by the lower 95% CI for net benefit being below zero, there is uncertainty surrounding the results. This aligns with the previous finding of a high degree of uncertainty surrounding the incremental cost estimate.
FIGURE 9.
Primary analysis: net benefit by WTP.
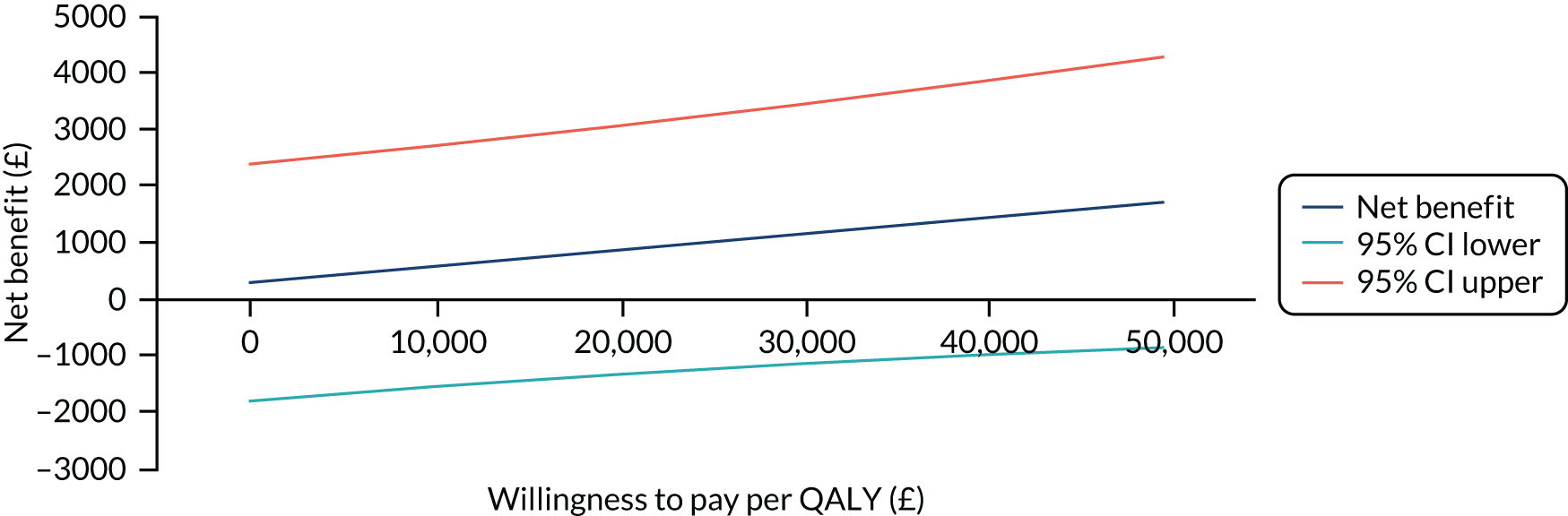
FIGURE 10.
Primary analysis: cost-effectiveness acceptability curve.
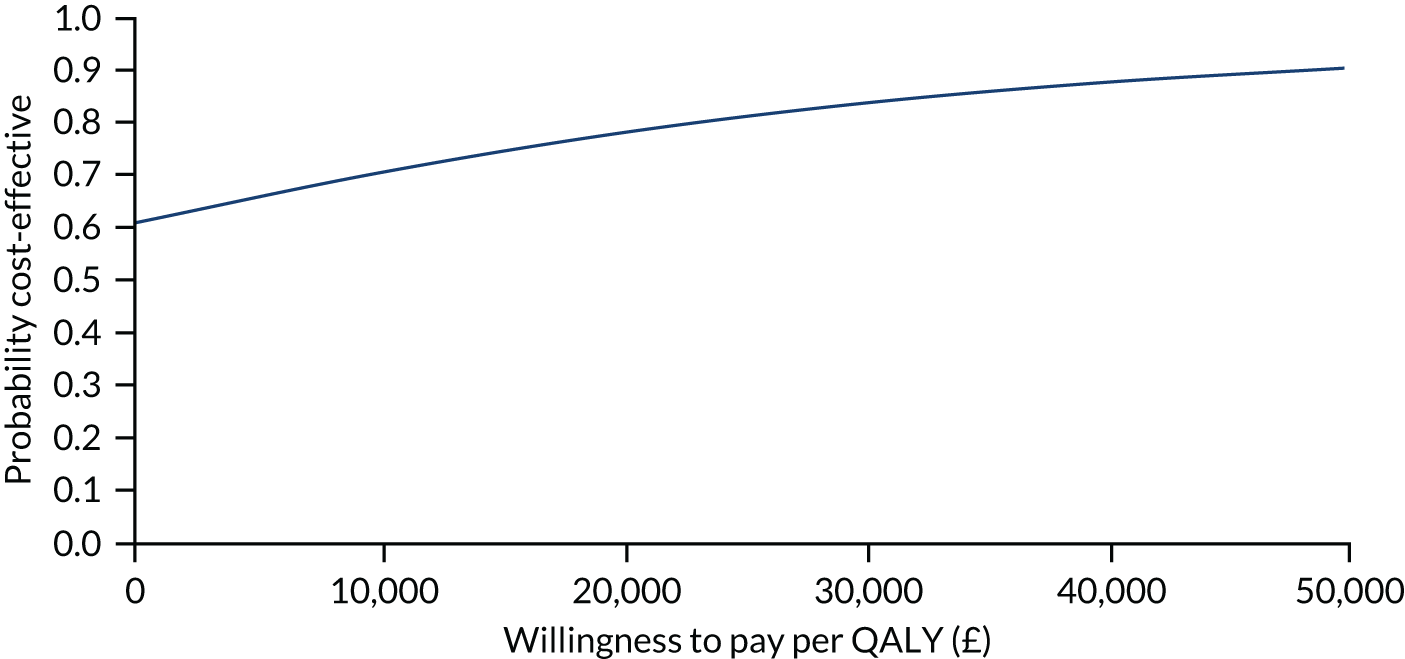
To examine the levels of uncertainty around the results, a CEAC (presented in Figure 10) was created. This shows the probability that the intervention is cost-effective at different levels of WTP for QALYs. Even at a WTP of £0 there is still a 61% chance that exercise is more cost-effective than usual care. The CEAC is upwards-sloping because of the positive coefficient associated with incremental QALYs in the intervention group. That is, as the WTP for health benefits increases, so does the probability that the intervention is cost-effective. At the NICE-specified WTP threshold values of £20,000 per QALY and £30,000 per QALY, there is, respectively, a 78% and 84% probability that exercise is more cost-effective than usual care. Given that EQ-5D-5L utility scores were diverging at the final time point it is reasonable to conclude that this probability would increase if the time horizon were extended beyond the trial, as the exercise group would continue to accrue more QALYs than the usual-care group.
Secondary analyses
Secondary analysis 1: complete-case analysis
Secondary analysis 1 considered the cost-effectiveness results using the complete-case data. The results are shown in Appendix 3 in the form of a net benefit chart (Figure 11) and a CEAC (Figure 12). Again, the complete-case analysis provided supporting evidence for cost-effectiveness, with there being a 65% chance exercise is the more cost-effective option at a WTP of £20,000 per QALY, rising to 68% at a WTP of £30,000 per QALY.
Secondary analysis 2: cost per DASH point
As reported in Chapter 4 and in Table 12, the exercise intervention was associated with improved DASH scores and lower costs. Given this, exercise dominated usual care and so calculating a cost per DASH point was deemed unnecessary because of the problems associated with interpreting a negative ICER.
Secondary analysis 3: including societal costs
Secondary analysis 3 considered the impact of broadening the costing perspective from NHS and PSS to a societal perspective. This included other private health-care costs, private equipment purchases, OTC medication and other costs. Income losses were omitted owing to the lack of data for this variable. In terms of cost-effectiveness, this further strengthens the case for cost-effectiveness, with the intervention continuing to dominate usual care. The results of the cost-effectiveness analysis are presented in Appendix 3, Figures 13 and 14. As can be seen from the CEAC, the intervention at a threshold of £20,000 per QALY has an 83% chance of being more cost-effective than usual care when costed from a societal perspective.
Secondary analysis 4: including training cost
Across the 17 sites, a total of 312 hours of training time were accounted for, including the time of the trainers. The inclusion of these costs led to an increase in costs per participant in the exercise group of £55.54. As shown in Appendix 3, Figures 15 and 16, the inclusion of training costs had very little impact on the results of the cost-effectiveness analysis. In this analysis the probability of the intervention being cost-effective at a cost-per-QALY threshold of £20,000 falls marginally, to 76.8%.
Secondary analysis 5: excluding high-cost cancer treatment
This analysis considered a narrower costing perspective limited to those costs that are most likely to be affected by shoulder problems, such as upper limb stiffness and pain, rather than cancer more generally. This led to much lower cost estimates, with the mean costs falling to £732 (95% CI £649 to £815) per person. In this analysis, the intervention was associated with an increased cost per person of £106 (95% CI –£49 to £262). As shown in Appendix 3, Figures 17 and 18, at this limited cost perspective the probability of the intervention being cost-effective at a cost-per-QALY threshold of £20,000 per QALY increases to 97%. This reflects the low costs and reduced uncertainty around cost-estimates within this analysis while QALYs remain the same.
Secondary analysis 6: using Hospital Episode Statistics costs for hospital care
This analysis used HES data to calculate hospital costs instead of CSRI data. Given the timescales involved in obtaining HES data within the trial timeline, it was possible to obtain full 12-month data for only 242 (62%) of the recruited participants. According to this analysis, costs were slightly higher in the exercise group (£166), with a great deal of uncertainty surrounding the estimate (95% CI –£3849 to £4181). This is reflected in the CEAC shown in Appendix 3, Figure 19, in which the CEAC, while maintaining similar overall shape to the primary analysis, has shifted downwards slightly. This reflects the increased costs and smaller sample size, which manifest in increased levels of uncertainty. However, there remains a 62% chance that the intervention is more cost-effective than usual care at a threshold of £30,000 per QALY.
Discussion
This economic evaluation examined the costs and outcomes associated with the PROSPER exercise intervention in comparison with usual care. A multilevel net benefit regression framework was used to assess the cost-effectiveness of the intervention and to estimate the uncertainty surrounding the results. The results found that the exercise intervention was cost-effective compared with usual care, with the exercise intervention in the primary analysis having a 78% chance of being the more cost-effective option at the NICE cost-effectiveness threshold of £20,000 per QALY. 126 The results were robust to a range of sensitivity analyses. Given that the EQ-5D-5L utility scores were diverging at the final time point, it is reasonable to assume that these estimates are conservative. This is reinforced by secondary analysis 5, which found that there was a 97% chance of cost-effectiveness when excluding likely non-attributable costs (e.g. high-cost cancer treatments and inpatient surgery), which drove much of the uncertainty around the cost estimates in the other analyses.
There were a number of challenges in conducting this economic analysis. First, the hierarchical nature of the data resulting from being a cluster RCT provided methodological challenges. To account for this, a multilevel net benefit framework that adjusted for baseline differences in addition to clustering was used. Although the number of missing EQ-5D-5L data as relatively small, a significant number of data for health-care usage were missing, as is common within trials. To address this, multiple imputation was used to make the most of available data while retaining uncertainty. Although the cost-effectiveness estimates were favourable, there was a large uncertainty surrounding incremental cost estimates. This is probably because of the high cost and variable nature of breast cancer treatments, whereby certain cancer treatments unrelated to the rehabilitation of the shoulder post surgery account for the vast majority of costs. Consequently, to focus on costs that are more likely to be attributable to shoulder pain and its rehabilitation, we conducted a secondary analysis that included only those costs that might plausibly be related to shoulder pain and discomfort. In this analysis, there was much less uncertainty around cost estimates, which resulted in a very high probability of the intervention being cost-effective (97%).
This chapter has reported the results for the trial-based analysis. A limitation to this is that the EQ-5D-5L utility scores had not converged by the final time point. Given that participants in the exercise group were still in a better health state at the final time point than those in the usual-care group, and costs were incurred largely upfront, it is likely that the strength of evidence for cost-effectiveness would be stronger still if longer-term follow-up was conducted. This is an avenue for future research and as part of this study we are conducting an additional beyond-trial time point outcome data collection. A further limitation was that linear interpolation was specified as the method for calculating QALYs, as the time between each follow-up was significant and trajectories may not follow a linear pattern. Given the prolonged nature of treatment in this cohort, we, however, felt that this was the best approximation with the data we had. Finally, there is still debate about the validity of the EQ-5D-5L. 137 At the inception of the study this measure was recommended by NICE126 and hence was chosen to ‘futureproof’ results. The use of the three-level version, however, may have given slightly different results. Given the difference in QALYs between the two groups, we do not anticipate that this would have meaningfully changed the results.
Chapter 7 Discussion
Study findings and key messages
To our knowledge, this is the first large-scale, multicentre, pragmatic, definitive trial to investigate the clinical effectiveness and cost-effectiveness of an early structured exercise programme for women at higher risk of shoulder problems after breast cancer surgery. We delivered the trial in an NHS setting, across 17 breast cancer centres within secondary care services. Treatments were delivered by physiotherapists independent of multidisciplinary oncology teams. We aimed to provide evidence of whether or not early postoperative physiotherapy, currently not offered to women undergoing non-reconstructive breast surgery, is a clinically effective and cost-effective use of NHS resources.
We found evidence that the exercise programme led to greater intermediate and longer-term benefits on upper limb function, postoperative pain, arm symptoms and QoL than information leaflets only. Exercises started within 10 days of surgery were not associated with increased risk of wound-related AEs at 6 weeks or chronic AEs, such as lymphoedema, over 1 year. Overall, our results suggest that physiotherapy-supported exercise is a cost-effective intervention for women undergoing invasive cancer treatments, particularly treatments targeting the axilla, which can increase the risk of shoulder problems.
Key findings from the trial are discussed below. We consider issues relating to recruitment, uptake and retention, and also potential threats to the internal and external validity of the study. We also consider the characteristics of trial participants, the implications of losses to follow-up, and intervention adherence and fidelity. We then discuss our findings in relation to the current literature. Finally, we consider the clinical implications of our findings on recommendations for breast cancer care.
Recruitment uptake
A total of 951 patients were screened in the clinical setting and, of these, 190 (20%) were deemed ineligible for the trial. The main reasons for ineligibility were not being at higher risk of shoulder problems and opting for immediate breast reconstruction surgery. Current UK guidance62 now recommends that all women undergoing mastectomy should be offered either immediate or delayed breast reconstruction and rates of immediate implant-based reconstruction surgery have doubled in England since 2005. 138 Of those eligible to participate, a proportion of women were given study materials but had limited time to consider the information (43/761; 6%). Recruitment staff did not always record the specific reason for non-participation (77/761; 10%). Time from diagnostic testing of breast cancer to surgery can be rapid, within weeks, as per recommended NHS cancer referral and treatment guidelines. 3 Evidence from the US139 suggests that fewer than 1 in 20 cancer patients approached to take part in clinical trials are actually enrolled, although a recent meta-analysis suggested uptake to cancer clinical trials was nearer 8%. 140 Enrolment and uptake into prevention trials and trials testing exercise interventions can present additional challenges, such as the discounting of future perceived benefits,141 particularly when faced with a distressing diagnosis. Other known barriers to participation in cancer clinical trials include structural (e.g. access), clinical and attitudinal (physician and patient) factors. 140
Of those eligible and approached, 270 out of 761 (35%) women declined to participate. Before starting PROSPER, we considered the findings of a single-centre RCT67 conducted in the NHS in England, in which two exercise programmes were compared after ANC surgery. The authors reported low recruitment, with 64% of 345 women invited declining participation. 67 Timing of invitation was cited as a key issue, with women being distressed or more concerned with their immediate cancer treatment or pending surgery when approached preoperatively. 67 These authors recommended approaching women to participate in research after breast cancer surgery.
For PROSPER, we incorporated pre-pilot interviews with patients and BCNs specifically to inform our recruitment procedures and development of trial materials. Based on the literature and interview findings, we opted to screen and recruit women preoperatively. Most breast cancer surgery is undertaken as short-stay or day-case surgery, as per the NHS 23-hour ambulatory care model; thus, postoperative recruitment would be challenging. Consequently, uptake to PROSPER was good, with 59% (392/662) of women actually approached by clinical/research staff about the study agreeing to take part. Our materials explained equipoise, but also that, if allocated to the exercise intervention, participants would then be supported by a trained physiotherapist with flexible appointments arranged around routine clinical follow-ups.
Participant retention
Although uptake was good, eight participants were randomised in error and two allocated to the exercise group declined when notified of their treatment allocation. In all cases, errors in randomisation or declining to participate occurred on the day of randomisation and no further data were collected. An additional 8% of randomised participants were lost because they did not return their baseline questionnaires, and thus the baseline DASH scores required for treatment group comparison over time could not be calculated. These losses mostly occurred during the internal pilot study, when procedures were being refined. We extended recruitment by 1 month to achieve the required sample size of 350. We analysed data according to the ITT principle of allocated treatment, irrespective of subsequent non-compliance, and without imputation. Imputation for data missingness did not change the strength or direction of our estimates. Unlike other surgical trials, we did not undertake a modified ITT142 (excluding the 10 randomisations in error and those withdrawn at point of randomisation) or a per-protocol analysis, although the compliance-based approach does provide a realistic estimate of those who complied with treatment.
Response rates to follow-up postal questionnaires were lower than predicted, increasing the risk of attrition bias. We predicted a 25% loss to follow-up at 1 year, based on other clinical trials investigating exercise interventions. 67 However, we observed a slightly higher loss to follow-up, with 70% (274/392) of our randomised sample returning final 12-month questionnaires (although this equated to 78% of those returning baseline data). Despite losses, the trial was sufficiently powered, as the required sample size was calculated to be 256 participants at 12 months. Rates of withdrawal were comparable by treatment group, and reasons for withdrawing were mostly cited as treatment burden, including being informed of a cancer recurrence, or that women did not want to complete questionnaires during adjuvant treatment.
Risk screening criteria
Only women at high risk of developing shoulder problems after breast cancer treatment were eligible to take part in the study. We developed our own criteria, based on the published literature, to determine future risk of developing shoulder problems. Screening criteria were applied preoperatively, based on planned cancer treatment pathways. Over half of participants (59%) were booked for ANC surgery when recruited, although actual cancer treatments delivered after randomisation differed from preoperative treatment plans. A total of 327 out of 392 (83%) of women underwent ANC, thus we are confident that we recruited those who were, indeed, at higher risk of developing shoulder problems. One-third of women had both ANC and radiotherapy to the axilla/supraclavicular area, regardless of other entry criteria. A high proportion (75%) of women were screened as being overweight or obese, having a BMI of ≥ 25 kg/m2. Preoperative height and weight were recorded at preoperative clinics and used by clinical teams for risk screening. We also captured patient-reported height and weight from baseline questionnaires. Patient-reported BMI was marginally lower than clinically recorded data (72% overweight/obese). Cancer treatment covered a wide spectrum and different combinations of modalities, including repeated surgeries, adjuvant chemotherapy, radiotherapy and hormonal therapy. Although treatment modality and sociodemographic variables were well balanced across treatment groups at baseline, we noted differences between planned and actual cancer treatments delivered over the 12-month follow-up. For example, a higher proportion of women in the exercise group underwent mastectomy (44%) than those randomised to usual care (38%), and thus those in the exercise group were more likely to have more extensive breast surgery and less likely to have breast-conserving procedures with adjuvant therapy. We adjusted for cancer treatment in all analyses. We also noted differences in proportions of participants reporting a history of shoulder problems, although this was not reflected in baseline DASH scores. We did not stratify allocation by risk criteria; therefore, some random variation is expected. Nevertheless, we undertook sensitivity analyses to explore this further, but adjustment for self-reported shoulder problems did not change effect estimates.
Almost one-quarter of women (22%) underwent repeat surgery; this reoperation rate is similar to that reported across 156 NHS trusts for women having breast conserving procedures over a 3-year period (20%; 95% CI 19.6% to 20.3%), with higher repeat surgery rates (30%) noted after invasive cancer surgery. 138 Within PROSPER, reoperations were mostly revisions of tumour resection margins undertaken as an additional day-case procedure. Five women opted for a delayed breast reconstruction. No adjustment was undertaken for revisional surgeries, as these were equally distributed by treatment group and all statistical analyses were adjusted based on the most invasive breast and axillary procedure over follow-up. For intervention participants already following the exercise programme, physiotherapists advised them to restart exercises from the 7th postoperative day after revisional surgery.
Participant characteristics
Our recruited sample had a mean age of 58 years and were, thus, representative of newly diagnosed breast cancer patients. 1 Over one-quarter (28%) of women were < 50 years. Among our sample returning baseline questionnaires, participants were predominantly white (92%), although we had fair representation from the black and ethnic minority population (8%) relative to national English statistics (UK Census data: 86% white). 143 As described, most women were overweight/obese and were relatively inactive in the week prior to recruitment. Three-quarters (76%) reported not taking part in any physical activities or sport, such as dancing, swimming, jogging, cycling or tennis. We are confident, therefore, that we did not recruit a sample of highly active women who exercised regularly. This may be important when considering generalisability and wider implementation of the intervention, as well as the longer-term potential health benefits from promoting physical activity. Other studies have shown that physical activity declines after a breast cancer diagnosis and during treatment. 144 Women receiving adjuvant treatment are least likely to remain physically active; one large cohort study found that physical activity halved in those receiving both radiotherapy and chemotherapy. 145
Despite being relatively inactive in the week before recruitment, our participant sample were confident that they would return to usual activities and/or regular physical activity in the future, after cancer treatment. Confidence scores were higher in the exercise group than in the usual-care group across all postoperative time points. Some argue that the time of diagnosis is the window of opportunity to identify and motivate sedentary patients with breast (and colon) cancer, to target motivational support and sustain lifestyle changes. 146 Having confidence in ability to return to future activity is therefore an important consideration for encouraging behaviour change.
We found differences in rates of outdoor walking between groups over time, with higher rates of activity in the exercise group than in the usual-care group at 6 weeks and 6 months, but rates were broadly similar at 1 year. Light and moderate physical activity, as encouraged within the PROSPER exercise programme, has been associated with improved QoL. 147 Interestingly, at the 1-year follow-up, the majority of women reported that the arm and shoulder exercises had helped their recovery, suggesting that participants in both treatment groups attributed at least some of their recovery to exercise. We can assume that those in the exercise programme undertook their prescribed exercises, but we cannot know what exercises those in the usual-care group were following. Although women in the usual-care group perceived their exercises as being helpful to their recovery, these did not have the same impact on their upper limb disability (as measured by the DASH scale) as the structured, supported programme.
Health-related quality of life
Our sample had lower QoL scores on recruitment than population normative scores for UK females aged 45–64 years [SF-12 PCS 49.1 (SD 10.6); MCS 51.4 (SD 9.8)]. 148 We observed lower scores in our sample for mental health at recruitment than at 6 and 12 months and also when compared with population normative scores for women < 45 years and > 64 years. 148 These lower scores at baseline may reflect diagnosis-related distress and pending cancer surgery. 149 QoL scores were lower at the 6-month follow-up than at 1 year, reflecting the impact of ongoing adjuvant treatment. Among postal responders, item missingness was low for SF-12 data at baseline (4%), 6 months (5%) and 12 months (6%). We followed validated scoring guidelines for all standardised measures. Comparison of complete-case and imputed data for EQ-5D-5L scores used in the cost-effectiveness analysis did not change estimates. At 12 months, the exercise group had significantly better physical QoL than women receiving usual care.
Exercise intervention: uptake and adherence
Usual NHS postoperative care for non-reconstructive breast surgery is written information about exercise, and referrals are made to physiotherapy only when a problem has been identified. We designed an exercise programme cognisant of busy physiotherapy clinics and what could reasonably be offered within the NHS. The exercise programme was planned to start after the first postoperative week and continue throughout cancer treatment. Uptake of the intervention was excellent, with 181 out of 196 (92%) of those randomised attending at least one appointment with their physiotherapist (95% of referred). Despite the known barriers to exercise during cancer treatment, adherence to the programme was good. Twenty per cent of participants either withdrew or were discharged after two appointments, and the remainder completed three or more physiotherapy sessions (143/191; 75%). The adherence rate was higher than those reported in other trials testing exercise intervention with patients with breast cancer. 150,151 We recommended that women continue with shoulder-specific exercises and physical activity for up to 12 months, despite the fact that symptom burden, such as fatigue and other side effects caused by chemotherapy, may overwhelm the motivation to exercise. 146
All participants were advised to restrict arm movements for the first postoperative week to avoid the risk of increasing wound drainage. 12 There is debate around the optimal timing to start exercise in relation to cancer surgery. 152 Our findings show that unrestricted ROM shoulder exercises started at 7–10 days postoperatively did not increase the risk of self-reported wound-related AEs at 6 weeks. Physiotherapists notified the study team of six adverse events (6/191; 3%), although four of the six women who experienced postoperative events continued with the exercise programme. We did not objectively measure postoperative wound drainage (volume or duration), as this would have required additional hospital clinic visits. For women requiring additional surgery after starting the exercise programme, physiotherapists advised that they restart the programme from the 7th postoperative day. The intervention was designed such that face-to-face appointments were strategically planned to allow controlled, supervised progression of exercise difficulty while dovetailing with routine oncological follow-up. We used the frequency, intensity, time and type (FITT) principle to prescribe and tailor the exercise programme. The programme was adaptable and flexible, and thus could be adjusted in accordance with individual needs, preferences and cancer treatments.
Comparison of findings with other studies
We found evidence of improved upper limb function, lower intensity acute pain, reduced chronic pain, fewer arm symptoms and improved QoL in the exercise group over 1 year. We found statistically significant differences between groups for the DASH score at 6 and 12 months; the mean difference at 12 months was greater than the estimate specified a priori as the MCID. We defined MCID as a 7-point difference and our primary and sensitivity analyses confirmed this difference. Other studies38 suggest that slightly larger differences are clinically meaningful but in relation to those with other chronic conditions receiving different interventions with shorter follow-up time periods. We specified 7 points to account for the pragmatic nature of the trial and the longer duration of follow-up of over 1 year. This was also to account for the prolonged and complex cancer treatment pathways after primary surgery. We are confident of some benefit from our programme, with CIs suggesting evidence of benefit in total DASH score, and also for impairment, activity limitations and participant restriction subscores, although CIs were wider for subscores.
The primary source of evidence for exercise after breast cancer treatment remains the Cochrane review,12 which is now out of date given that at least 10 RCTs have since been registered or completed, as per our literature review update. Our findings are similar to findings from some small trials comparing exercise programmes to either non-active or information-only control groups. 14 For example, the small Dutch trial41 that we used for our sample size calculation compared a shoulder exercise programme with information leaflets on outcomes at 3 and 6 months in 30 women. The authors reported improved ROM and strength and lower pain scores in the exercise group, with a similar difference in DASH scores (unadjusted MD –9.0; p = 0.03; n = 30 participants) at 6 months after exercise41 to that observed in PROSPER (adjusted MD –8.74; p = 0.001; n = 235 CACE analysis).
Other trials have recruited patients to exercise postoperatively, either during or months after completion of adjuvant therapy. 12 ROM exercises are important to maintain shoulder mobility and to avoid common problems related to axillary surgery, such as axillary web syndrome. We found evidence of progression in ROM exercise difficulty, but this was not captured in the work capacity outcome. Participants increased the difficulty of exercises over subsequent appointments but maintained the same number of repetitions and sets. We observed improvements in strength over time using our composite measure of work capacity (incorporating band resistance) among intervention compliers. Strength exercises are important to maintain muscle strength and mass during cancer treatments; chemotherapy can reduce upper limb strength by as much as 16%. 80 A recent systematic review,153 based on several small trials with short-term follow-up, concluded that there was low-level evidence regarding the effectiveness of range of motion and muscle strength exercises to improve arm function. Our findings suggest that an early, structured, progressive exercise programme is beneficial for improving upper arm function in those who are at highest risk of shoulder problems and these findings are clinically relevant.
Intervention fidelity
Intervention fidelity is an important consideration for complex intervention trials. Multicentre trials delivering complex interventions are more vulnerable than studies testing simple interventions, being at greater risk of not being implemented as intended. 154 We developed clear treatment pathways and protocols throughout the first year, thus developing and testing the exercise intervention, incorporating multidisciplinary expert and patient input to co-design a programme suitable for delivery within the NHS. Some intervention elements were novel for physiotherapy staff, for example behavioural change strategies and co-decision of exercise prescription to encourage adherence. Although participating physiotherapists were experts in the management of musculoskeletal conditions, none was formally embedded within oncology services. Each physiotherapist completed training and achieved the required competence before training certificates were signed. Quality of training delivered was assessed and adapted during the internal pilot study. We provided clear supportive materials (e.g. laminated prompt sheets for motivational interviewing, ROM, strength assessment etc.). We did not undertake video recordings of participant consultations, as this may have affected adherence, particularly given the sensitive nature of consultations. Qualitative interviews revealed that some women became emotional during appointments, particularly in the earlier postoperative assessments, when adjusting to their new body image. We are confident, through our quality assurance procedures, that physiotherapists adhered to and prescribed the recommended programme. We are also confident that our intervention is ‘futureproof’, given that women underwent contemporaneous cancer treatment for those requiring invasive axillary treatment.
Qualitative findings
We included the qualitative study to explore insights and perspectives from patients and health-care providers taking part in the trial. Women described feeling motivated to comply with the exercise programme because they felt that it contributed to their overall well-being and recovery. Women also reported that it was something that they could achieve themselves during cancer treatment, thus having control over their own body rather than passively receiving cancer treatments. Physiotherapists described the exercise programme as rewarding; they enjoyed providing support and encouragement to women and reassuring them that it was safe to move their arm after surgery. Other positives described by physiotherapists included the longer appointment sessions and emphasis on patient choice and joint decision-making. Several issues for future implementation within the NHS setting were identified: the need for a private space for appointments, access to emotional support for physiotherapists and integration of physiotherapy expertise within the multidisciplinary oncology team.
Strengths of the study
The strengths of the study included the methodological rigour, excellent uptake of exercise and good adherence to interventions over time. Clinical data confirmed that most of our recruited sample had axillary surgery or radiotherapy treatment; only 14% were recruited for other risk factors (obesity or shoulder problems only). Although we cannot fully eliminate the risk of selection bias, we believe that the risk of bias was low, as randomisation was stratified by site and we avoided the use of permuted blocks. A major strength was the effort invested in the production of a well-designed programme with high-quality intervention materials packaged as a deliverable, stand-alone treatment programme. The intervention was delivered by NHS staff with the aim of testing the programme in an everyday clinical setting. We carefully tracked treatment referrals and monitored compliance. We incorporated an embedded processes valuation with qualitative interviews to better understand the challenges and acceptability of our interventions from the perspectives of both patients and physiotherapists. For the health economic analyses, data were triangulated from multiple sources: self-report and clinical records and routine national statistics via HES data. We undertook sensitivity analyses and also explored the impact of imputation for missingness, but these analyses did not change our findings.
Limitations
The main limitation of the trial was the loss to follow-up over study duration, being 5% higher than predicted. Although disappointing, losses to follow-up were equally distributed by treatment allocation; therefore, we are confident that systematic error (attrition bias) from disproportionate losses did not occur. Drop-out mostly occurred between 6 weeks and 6 months during ongoing cancer treatments, with minimal loss thereafter (2%). We compared the characteristics of the randomised sample with those of participants remaining in the trial at 12 months and found no differences in marital status, comorbidity, body weight or cancer treatments. However, younger women were more likely to withdraw, possibly because of family or work commitments. We could postulate that younger women are possibly more physically active in general or less in need of a supportive exercise intervention throughout cancer treatment. However, evidence from many studies suggests that younger women are more likely to experience more problems with functional limitations,155 delayed return to work, declines in physical activity156 and chronic post-surgical pain. 13,15 Nevertheless, strategies to encourage adherence to exercise during periods of intensive treatment, chemotherapy in particular, should be explored. Despite losses, the trial study was adequately powered to be definitive and we completed all prespecified statistical analyses. We made one amendment to the statistical analysis plan after protocol publication to adjust for baseline values. We observed consistency in findings across a range of patient-reported outcomes, all of which support rejection of the null hypothesis. Another limitation was that we used self-report indicators for the secondary outcome of lymphoedema, rather than objective perometry, although our primary focus was to assess patient-reported upper limb function as per the commissioned brief.
Cost-effectiveness findings
Exercise was found to be more cost-effective than usual care, with the exercise intervention having a 78% chance of being the more cost-effective option at the NICE-recommended cost-effectiveness threshold of £20,000 per QALY, and an 84% chance at £30,000 per QALY. These results were robust to a range of sensitivity analyses. The intervention itself was relatively cheap to implement, at an additional £129 per person, and was associated with lower health-care costs and improved HRQoL. As QoL utility scores were diverging at the 12-month follow-up, we conclude that these estimates are conservative as benefits may accrue beyond the end of the trial.
Patient and public involvement
We included lay members in our external committees and at all stages throughout the trial. We included PPI in the design stage and during early intervention development. A patient dissemination event was planned at the University of Warwick to feed back study findings to trial participants. This was postponed because of coronavirus but will be rearranged in due course.
Chapter 8 Conclusion
This high-quality, multicentre RCT recruited 392 women at high risk of developing shoulder problems after breast cancer treatment from 17 breast cancer centres in England. We found that an early, structured exercise programme improved upper limb function, pain reduction and QoL at 1 year after breast cancer surgery compared with usual care. Supported exercise started from the first postoperative week was safe; no SAEs were reported and exercise did not increase the risk of surgical site infections or lymphoedema. Exercise was relatively cheap to implement (£129 per participant) and associated with lower health-care costs and improved HRQoL. Thus, our economic analyses found that exercise was cost-effective relative to usual care.
We conclude that for women undergoing invasive axillary procedures or who are at risk of developing shoulder and upper limb related problems after non-reconstructive breast surgery, this trial provided robust evidence that referral for early physiotherapy can lead to improvements in upper limb function and QoL at 1 year.
Future research should evaluate wider implementation of the PROSPER exercise programme in clinical practice for those at highest risk of shoulder problems.
Acknowledgements
Trial Management Group
Professor Julie Bruce (chief investigator).
Professor Alastair Thompson (co-applicant, lead surgeon).
Professor Stavros Petrou (co-applicant, lead health economist).
Professor Ranjit Lall (co-applicant, lead statistician).
Professor Sallie Lamb (co-applicant).
Dr Esther Williamson (co-applicant).
Mrs Emma Padfield (trial manager/senior project manager).
Mrs Lauren Betteley (trial manager).
Mr Pankaj Mistry (trial statistician).
Dr Alastair Canaway (health economist).
Dr Sophie Rees (research fellow).
Dr Helen Richmond (research fellow/physiotherapist).
Dr Bruno Mazuquin (research fellow/physiotherapist).
Ms Clare Lait (research fellow/physiotherapist).
Mrs Loraine Chowdhury (trial administrator).
Mr Craig Turner (trial administrator).
Mr Phil Moss (data entry clerk).
Mrs Marie van Laar (patient representative).
Miss Raghavan Vidya (breast surgeon), Royal Wolverhampton NHS Trust.
Ms Jane Prewit (University of Warwick sponsor).
Ms Ceri Jones (University Hospitals Coventry and Warwickshire co-sponsor).
Miss Abigail Tomlins (oncoplastic breast surgeon).
Trial Steering Committee
Professor Adele Francis (deceased).
Professor Stephen Duffy (independent).
Dr Anna Kirby (independent).
Dr Karen Robb (independent).
Professor Julie Bruce (chief investigator).
Professor Ranjit Lall (statistician).
Professor Sallie Lamb (observer).
Mrs Emma Padfield (trial manager/senior project manager).
Data Monitoring and Ethics Committee
Professor Malcolm Reed (independent chairperson).
Dr Rhian Gabe (independent).
Dr Matthew Maddocks (independent).
Statisticians
Professor Ranjit Lall (lead).
Mr Pankaj Mistry (research assistant).
Dr Anower Hossain (research associate).
Ms Katie Booth (research associate).
Health economists
Dr Alastair Canaway (research fellow).
Professor Stavros Petrou (lead).
Dr Kamran Khan (research assistant).
Core trial team
Warwick Clinical Trials Unit
J Bruce, B Mazuquin, S Rees, L Chowdhury, P Mistry, A Canaway, R Lall, H Richmond, C Lait and E Padfield.
Data Programming Team
H Adjei, C Muthiah, P Bell and A Willis.
University of Oxford
S Lamb, E Williamson, C Srikesavan, M Newman and J Moser.
Surgical oncologists
Professor Alastair Thompson, Baylor College of Medicine (USA).
Miss Raghavan Vidya, Royal Wolverhampton NHS Trust.
Mrs Katherina McEvoy, University Coventry and Warwickshire NHS Trust.
Miss Abigail Tomlins, University Coventry and Warwickshire NHS Trust.
Principal investigators by NHS site
Birmingham City Hospital, Sandwell and West Birmingham Hospitals NHS Trust (Miss Fiona Hoar).
Chesterfield Royal Hospital NHS Foundation Trust (Miss Iman Azmy).
Dorset County Hospital NHS Foundation Trust (Dr Caroline Osborne).
George Eliot Hospital NHS Trust (Mr Kishore Makam).
Hereford County Hospital, Wye Valley NHS Trust (Miss Jill Donnelly).
Hillingdon Hospitals NHS Foundation Trust (Mr Ekambaran Babu).
John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust (Ms Pankaj Roy).
Macclesfield District General Hospital, East Cheshire NHS Trust (Mrs Nicola Van der Ploeg).
Milton Keynes University Hospital NHS Foundation Trust (Ms Rachel Soulsby).
Musgrove Park Hospital, Taunton & Somerset NHS Foundation Trust (Miss Amanda Thorne).
New Cross Hospital, Royal Wolverhampton NHS Trust (Mr Tapan Sircar).
Portsmouth Hospitals (Mrs Maria Noblet) and Solent NHS Trust (Ms Emma McLoughlin).
Royal Blackburn Hospital, East Lancashire Hospitals NHS Trust (Miss Jane McNicholas).
Royal Cornwall Hospitals NHS Trust (Ms Sadie Mitchell).
Royal Stoke University Hospitals, North Midlands NHS Trust (Mr Soni Soumian).
University Hospital Coventry & Warwickshire NHS Trust (Miss Abigail Tomlins).
Walsall Manor Hospital, Walsall Healthcare NHS Trust (Mrs Lynda Wagstaff).
Physiotherapists by hospital site
Birmingham City Hospital: Carolyn Casey, Yogita Stokes, Sarah Calloway, Emma Fletcher and Hannah Brown.
Chesterfield Royal Hospital: Carolyn Lindsay and Sarah Wright.
Dorset County Hospital: Clare Ruston and Rebecca Bower.
George Eliot Hospital: Nicola Layte and Julie Barratt.
Hereford County Hospital: Kim Jones and Hannah Thomas.
Hillingdon Hospital: Lisa Graves and Donna Pilgrim.
John Radcliffe Hospital: Lucy Ridgeway, Naomi Ekers and Anna McMullen.
Macclesfield Hospital: Anoosheh Redfern and Annabel Allen.
Milton Keynes Hospital: Chrissie Edley, Rosie Conway and Jo Pitts.
Musgrove Park Hospital: Alex Chisholm, Sarah Woodhill, Nicky Clarke and Sarah West.
New Cross Hospital: Anna Simpson, Michelle Evans, Tina Hadley-Burrows and Sharon Rudge.
Portsmouth and Solent Hospitals: Emma McLoughlin, Alexandra Stephenson and Jo Burke.
Royal Blackburn Teaching Hospital: Emily McNeill and Sarah Greenwood.
Royal Cornwall Hospital: Deanne De Beer, Sally Kennedy and Donna Dungey,
Royal Stoke Hospital: Clare Johnson, Amanda Heath, Gabi Evans, Lisa Beadle and Rachel Scarisbrick.
University Hospital Coventry & Warwickshire: Catherine Hegarty, Elizabeth Abbey (deceased) and Jenny Holt.
Walsall Hospital: Stephanie Rook, Sophie Horne and John O’Regan.
Intervention development
We are very grateful to the experts who contributed to the Exercise Intervention Development Day hosted at WCTU in March 2015: Elizabeth Fort (nee Abbey), Catherine Hegarty, Lyn Ankcorn, Jocelyn Choyce, Alison Jelly, Wendy Leonard, Nicola Lidstone, Kelvin Marshall, Kat Tunnicliffe, Sally Winterbourne, Jo Dixey, Leah Dalby, Karen Wilkinson, Clare Lait, Meredith Newman, Jane Moser, Cynthia Srikesavan, Esther Williamson, Helen Richmond and Lauren Betteley. We thank Janet Lowe and Susie Henning for reviewing draft versions the physiotherapy manual.
We extend grateful thanks to study participants and to the physiotherapists delivering the PROSPER intervention.
Thanks are due to Mrs Emma Padfield and Mrs Loraine Chowdhury for providing administrative support throughout. We also thank members of the Coventry Breast Cancer Support Group and our patient representatives: Dr Catherine Harkin (deceased), Mrs Marie van Laar and Lyn Ankcorn.
Contributions of authors
Professor Julie Bruce (https://orcid.org/0000-0002-8462-7999) (Chief Investigator) co-developed interventions, was a member of the Trial Management Group (TMG) and the TSC, and was responsible for writing and reviewing the report.
Dr Bruno Mazuquin (https://orcid.org/0000-0003-1566-9551) (Research Physiotherapist) was a member of the TMG and was co-responsible for the delivery of the exercise intervention and writing and reviewing the report.
Mr Pankaj Mistry (https://orcid.org/0000-0003-0351-3090) (Research Fellow, Trial Statistician) was a member of the TMG and was responsible for the statistical analysis of the trial and writing and reviewing the report.
Dr Sophie Rees (https://orcid.org/0000-0003-4399-2049) (Research Fellow, Qualitative) was a member of the TMG and was responsible for the qualitative substudy and writing and reviewing the report.
Dr Alastair Canaway (https://orcid.org/0000-0002-4270-6808) (Research Fellow, Health Economics) was a member of the TMG and was responsible for the health economic analysis and writing and reviewing the report.
Dr Anower Hossain (https://orcid.org/0000-0002-1708-9940) (Research Associate, Statistics) was responsible for the statistical analysis and writing and reviewing the report.
Dr Esther Williamson (https://orcid.org/0000-0003-0638-0406) (Research Fellow) was a member of the TMG, co-developed the intervention, and contributed to writing and reviewing the report.
Mrs Emma J Padfield (https://orcid.org/0000-0003-4832-9609) (Trial Manager/Senior Project Manager) was a member of the TMG and was responsible for trial management and writing and reviewing the report.
Professor Ranjit Lall (https://orcid.org/0000-0003-1737-2866) (Professor of Clinical Trials, Lead Statistician) was a member of the TMG and was responsible for study design, oversight of statistical analysis, and writing and reviewing the report.
Dr Helen Richmond (https://orcid.org/0000-0002-5561-016X) (Research Fellow/Physiotherapist) was a member of the TMG, co-developed the intervention and the training and delivery of the exercise intervention, and was responsible for writing and reviewing the report.
Mrs Loraine Chowdhury (https://orcid.org/0000-0002-0339-2332) (Data Entry Clerk) was a member of the TMG and contributed to trial administration and reviewing of report.
Mrs Clare Lait (https://orcid.org/0000-0002-2998-3528) (Physiotherapist) was a member of the TMG, co-developed intervention, and contributed to the training and delivery of the exercise intervention and writing and reviewing the report.
Professor Stavros Petrou (https://orcid.org/0000-0003-3121-6050) (Lead Health Economist) was a member of the TMG and was responsible for oversight of the health economics analysis and writing and reviewing the report.
Ms Katie Booth (https://orcid.org/0000-0001-8530-2566) (Research Associate) provided statistical support and contributed to writing and reviewing the report.
Professor Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Trauma Rehabilitation) was a member of the TMG and contributed to intervention development and writing and reviewing the report.
Miss Raghavan Vidya (https://orcid.org/0000-0002-6129-2444) (Breast Surgeon, Principal Investigator) was a member of the TMG and contributed to writing and reviewing the report.
Professor Alastair M Thompson (https://orcid.org/0000-0003-0604-7267) (Lead Surgical Oncology Advisor) contributed to writing and reviewing the report.
All co-applicants contributed to PROSPER study design and protocol development.
Publications
Bruce J, Williamson E, Lait C, Richmond H, Betteley L, Lall R, et al. Randomised controlled trial of exercise to prevent shoulder problems in women undergoing breast cancer treatment: study protocol for the prevention of shoulder problems trial (UK PROSPER). BMJ Open 2018;8:e019078.
Richmond H, Lait C, Srikesavan C, Williamson E, Moser J, Newman M, et al. Development of an exercise intervention for the prevention of musculoskeletal shoulder problems after breast cancer treatment: the prevention of shoulder problems trial (UK PROSPER). BMC Health Serv Res 2018;18:463.
Khan KA, Mazuquin B, Canaway A, Petrou S, Bruce J. Systematic review of economic evaluations of exercise and physiotherapy for patients treated for breast cancer. Breast Cancer Res Treat 2019;176:37–52.
Bruce J, Mazuquin B, Canaway A, Hossain A, Williamson E, Mistry P, et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. BMJ 2021;375:e066542.
Mazuquin B, Sunemi MMO, E Silva MPP, Sarian LOZ, Williamson E, Bruce J. Current physical therapy care of patients undergoing breast reconstruction for breast cancer: a survey of practice in the United Kingdom and Brazil. Braz J Phys Ther 2021;25:175–185.
Rees S, Mazuquin B, Richmond H, Williamson E, Bruce J, UK Prosper Study Group. Role of physiotherapy in supporting recovery from breast cancer treatment: a qualitative study embedded within the UK PROSPER trial. BMJ Open 2021;11:e040116.
Data-sharing statement
All requests for data should be sent to the corresponding author. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Breast Cancer Statistics 2019 2019. www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/survival/ (accessed 11 November 2019).
- Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer 2011;105:1-4. https://doi.org/10.1038/bjc.2011.416.
- National Institute for Health and Care Excellence (NICE) . Early and Locally Advanced Breast Cancer: Diagnosis and Management 2018.
- Jonczyk MM, Jean J, Graham R, Chatterjee A. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173:267-74. https://doi.org/10.1007/s10549-018-5018-1.
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. https://doi.org/10.1056/NEJMoa022152.
- van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. https://doi.org/10.1093/jnci/92.14.1143.
- Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36:1938-43. https://doi.org/10.1016/S0959-8049(00)00197-0.
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-60. https://doi.org/10.1093/jnci/djj158.
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. https://doi.org/10.1016/S1470-2045(10)70207-2.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. https://doi.org/10.1016/S1470-2045(13)70076-7.
- Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat 2008;110:19-37. https://doi.org/10.1007/s10549-007-9710-9.
- McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev 2010;6. https://doi.org/10.1002/14651858.CD005211.pub2.
- Mejdahl MK, Andersen KG, Gärtner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ 2013;346. https://doi.org/10.1136/bmj.f1865.
- De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil 2015;96:1140-53. https://doi.org/10.1016/j.apmr.2015.01.006.
- Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain 2014;155:232-43. https://doi.org/10.1016/j.pain.2013.09.028.
- Kootstra JJ, Dijkstra PU, Rietman H, de Vries J, Baas P, Geertzen JH, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat 2013;139:125-34. https://doi.org/10.1007/s10549-013-2509-y.
- Leysen L, Beckwée D, Nijs J, Pas R, Bilterys T, Vermeir S, et al. Risk factors of pain in breast cancer survivors: a systematic review and meta-analysis. Support Care Cancer 2017;25:3607-43. https://doi.org/10.1007/s00520-017-3824-3.
- Zengin Alpozgen A, Razak Ozdincler A, Karanlik H, Yaman Agaoglu F, Narin AN. Effectiveness of Pilates-based exercises on upper extremity disorders related with breast cancer treatment. Eur J Cancer Care 2017;26. https://doi.org/10.1111/ecc.12532.
- Ibrahim M, Muanza T, Smirnow N, Sateren W, Fournier B, Kavan P, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv 2017;11:791-9. https://doi.org/10.1007/s11764-017-0617-0.
- Ibrahim M, Muanza T, Smirnow N, Sateren W, Fournier B, Kavan P, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer 2018;18:e55-e64. https://doi.org/10.1016/j.clbc.2017.06.007.
- Yuste Sánchez MJ, Lacomba MT, Sánchez BS, Merino DP, da Costa SP, Téllez EC, et al. Health related quality of life improvement in breast cancer patients: secondary outcome from a simple blinded, randomised clinical trial. Breast 2015;24:75-81. https://doi.org/10.1016/j.breast.2014.11.012.
- Johansson K, Hayes S, Speck RM, Schmitz KH. Water-based exercise for patients with chronic arm lymphedema: a randomized controlled pilot trial. Am J Phys Med Rehabil 2013;92:312-19. https://doi.org/10.1097/PHM.0b013e318278b0e8.
- Casla S, López-Tarruella S, Jerez Y, Marquez-Rodas I, Galvão DA, Newton RU, et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: a randomized controlled trial. Breast Cancer Res Treat 2015;153:371-82. https://doi.org/10.1007/s10549-015-3541-x.
- Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, Ariza-García A, Díaz-Rodríguez L, Del-Moral-Ávila R, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 2016;122:3166-74. https://doi.org/10.1002/cncr.30172.
- Leal NF, Oliveira HF, Carrara HH. Supervised physical therapy in women treated with radiotherapy for breast cancer. Rev Lat Am Enfermagem 2016;24. https://doi.org/10.1590/1518-8345.0702.2755.
- Rafn BS, Hung S, Hoens AM, McNeely ML, Singh CA, Kwan W, et al. Prospective surveillance and targeted physiotherapy for arm morbidity after breast cancer surgery: a pilot randomized controlled trial. Clin Rehabil 2018;32:811-26. https://doi.org/10.1177/0269215518757292.
- Gomez VP. Therapeutic Exercise in Cancer-Related Fatigue in Women After Breast Cancer Treatment 2016. https://ClinicalTrials.gov/show/NCT02828189 (accessed 22 April 2021).
- Sanchez EMM. Effectiveness of an Early Healthcare Program and Post-Operative Management of the Upper Limb in Women With Breast Cancer 2017. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373924 (accessed 15 September 2020).
- de Santana JM. Effect of Individual or Collective Kinesiotherapy on Pain and Oncological Fatigue n.d. http://ensaiosclinicos.gov.br/rg/RBR-3m7fdv/ (accessed 5 November 2020).
- Paskett E. Education With or Without Exercise and Counseling in Preventing Lymphedema in Women With Stage I, Stage II, or Stage III Breast Cancer Who Are Undergoing Axillary Lymph Node Dissection 2006. https://ClinicalTrials.gov/show/NCT00376597 (accessed 7 February 2019).
- Bruce J, Williamson E, Lait C, Richmond H, Betteley L, Lall R, et al. Randomised controlled trial of exercise to prevent shoulder problems in women undergoing breast cancer treatment: study protocol for the prevention of shoulder problems trial (UK PROSPER). BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019078.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Breast Cancer Care . Exercises After Breast Cancer Surgery 2013.
- Breast Cancer Care . Your Operation and Recovery 2013.
- Richmond H, Lait C, Srikesavan C, Williamson E, Moser J, Newman M, et al. Development of an exercise intervention for the prevention of musculoskeletal shoulder problems after breast cancer treatment: the prevention of shoulder problems trial (UK PROSPER). BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3280-x.
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected.] The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29:602-8. https://doi.org/10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
- Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005;92:225-30. https://doi.org/10.1038/sj.bjc.6602304.
- Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am 2013;38:641-9. https://doi.org/10.1016/j.jhsa.2012.12.032.
- Harrington S, Michener LA, Kendig T, Miale S, George SZ. Patient-reported upper extremity outcome measures used in breast cancer survivors: a systematic review. Arch Phys Med Rehabil 2014;95:153-62. https://doi.org/10.1016/j.apmr.2013.07.022.
- Lee SA, Kang JY, Kim YD, An AR, Kim SW, Kim YS, et al. Effects of a scapula-oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clin Rehabil 2010;24:600-13. https://doi.org/10.1177/0269215510362324.
- Beurskens CH, van Uden CJ, Strobbe LJ, Oostendorp RA, Wobbes T. The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer 2007;7. https://doi.org/10.1186/1471-2407-7-166.
- Dixon D, Johnston M, McQueen M, Court-Brown C. The Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH) can measure the impairment, activity limitations and participation restriction constructs from the International Classification of Functioning, Disability and Health (ICF). BMC Musculoskelet Disord 2008;9. https://doi.org/10.1186/1471-2474-9-114.
- World Health Organization . International Classification of Functioning, Disability and Health 2001.
- QualityMetric . An Abridged Version of the SF-36v2® Health Survey That Uses 12 Items to Measure Functional Health and Well-Being from the Patient’s Point of View n.d. www.qualitymetric.com/%20health-surveys-old/the-sf-12v2-health-survey (accessed 18 December 2021).
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. Pain 2011;152:1734-9. https://doi.org/10.1016/j.pain.2011.02.008.
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380-7. https://doi.org/10.1016/j.pain.2007.08.013.
- Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat 2001;68:273-82. https://doi.org/10.1023/a:1012278023233.
- Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003;52:370-9. https://doi.org/10.1097/00006199-200311000-00004.
- Goyal A, Dodwell D. POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol 2015;27:692-5. https://doi.org/10.1016/j.clon.2015.07.005.
- National Institute for Health Research . ATNEC – Axillary Management in T1-3NM0 Breast Cancer Patients With Needle Biopsy Proven Nodal Metastases at Presentation After Neoadjuvant Chemotherapy 2019. https://fundingawards.nihr.ac.uk/award/NIHR128311 (accessed 19 February 2020).
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol 1999;52:643-51. https://doi.org/10.1016/S0895-4356(99)00049-9.
- Knox CR, Lall R, Hansen Z, Lamb SE. Treatment compliance and effectiveness of a cognitive behavioural intervention for low back pain: a complier average causal effect approach to the BeST data set. BMC Musculoskelet Disord 2014;15. https://doi.org/10.1186/1471-2474-15-17.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. https://doi.org/10.1191/0962280205sm403oa.
- Williams MA, Williamson EM, Heine PJ, Nichols V, Glover MJ, Dritsaki M, et al. Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19190.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: John Wiley & Sons; 1987.
- Royston P. Multiple imputation of missing values: update of ice. The Stata Journal 2005;5:527-36. https://doi.org/10.1177/1536867X0500500404.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Slade SC, Dionne CE, Underwood M, Buchbinder R, Beck B, Bennell K, et al. Consensus on Exercise Reporting Template (CERT): Modified Delphi Study. Phys Ther 2016;96:1514-24. https://doi.org/10.2522/ptj.20150668.
- Todd J, Topping A. A survey of written information on the use of post-operative exercises after breast cancer surgery. Physiotherapy 2005;91:87-93. https://doi.org/10.1016/j.physio.2004.09.022.
- National Institute for Health and Care Excellence (NICE) . Early and Locally Advanced Breast Cancer: Diagnosis and Treatment 2009.
- Harris SR, Hugi MR, Olivotto IA, Levine M. Steering Committee for Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ 2001;164:191-9.
- Harris SR, Schmitz KH, Campbell KL, McNeely ML. Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals. Cancer 2012;118:2312-24. https://doi.org/10.1002/cncr.27461.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat 2007;102:313-21. https://doi.org/10.1007/s10549-006-9339-0.
- Flew TJ. Wound drainage following radical mastectomy: the effect of restriction of shoulder movement. Br J Surg 1979;66:302-5. https://doi.org/10.1002/bjs.1800660503.
- Todd J, Scally A, Dodwell D, Horgan K, Topping A. A randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy 2008;94:265-73. https://doi.org/10.1016/j.physio.2008.09.005.
- Lane K, Worsley D, McKenzie D. Exercise and the lymphatic system: implications for breast cancer survivors. Sports Medicine 2005;35:461-71. https://doi.org/10.2165/00007256-200535060-00001.
- Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin 2016;66:43-7. https://doi.org/10.3322/caac.21319.
- Stubblefield MD, Keole N. Upper body pain and functional disorders in patients with breast cancer. PM R 2014;6:170-83. https://doi.org/10.1016/j.pmrj.2013.08.605.
- Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat 2007;106:19-27. https://doi.org/10.1007/s10549-006-9466-7.
- Berrueta L, Muskaj I, Olenich S, Butler T, Badger GJ, Colas RA, et al. Stretching impacts inflammation resolution in connective tissue. J Cell Physiol 2016;231:1621-7. https://doi.org/10.1002/jcp.25263.
- Bouffard NA, Cutroneo KR, Badger GJ, White SL, Buttolph TR, Ehrlich HP, et al. Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models. J Cell Physiol 2008;214:389-95. https://doi.org/10.1002/jcp.21209.
- Apostolopoulos N, Metsios GS, Flouris AD, Koutedakis Y, Wyon MA. The relevance of stretch intensity and position – a systematic review. Front Psychol 2015;6. https://doi.org/10.3389/fpsyg.2015.01128.
- Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat 2012;133:667-76. https://doi.org/10.1007/s10549-012-1964-1.
- Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol 2005;44:449-57. https://doi.org/10.1080/02841860510029905.
- Pace do Amaral MT, Freire de Oliveira MM, Ferreira Nde O, Guimarães RV, Sarian LO, Gurgel MS. Manual therapy associated with upper limb exercises vs. exercises alone for shoulder rehabilitation in postoperative breast cancer. Physiother Theory Pract 2012;28:299-306. https://doi.org/10.3109/09593985.2011.604709.
- Kapanen M, Laaksomaa M, Skyttä T, Haltamo M, Pehkonen J, Lehtonen T, et al. Residual position errors of lymph node surrogates in breast cancer adjuvant radiotherapy: comparison of two arm fixation devices and the effect of arm position correction. Med Dosim 2016;41:47-52. https://doi.org/10.1016/j.meddos.2015.08.001.
- Kubo Y, Naito T, Mori K, Osawa G, Aruga E. Skeletal muscle loss and prognosis of breast cancer patients. Support Care Cancer 2017;25:2221-7. https://doi.org/10.1007/s00520-017-3628-5.
- Klassen O, Schmidt ME, Ulrich CM, . Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle 2017;8:305-16. https://doi.org/10.1002/jcsm.12165.
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510-30. https://doi.org/10.1249/MSS.0b013e3181a0c95c.
- Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat 2011;127:447-56. https://doi.org/10.1007/s10549-011-1444-z.
- Cramp F, James A, Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer 2010;18:1367-76. https://doi.org/10.1007/s00520-010-0904-z.
- Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 2013;45:2080-90. https://doi.org/10.1249/MSS.0b013e31829a3b63.
- Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30-67. https://doi.org/10.3322/caac.20140.
- Department of Health and Social Care . Start Active, Stay Active: Report on Physical Activity in the UK 2011.
- Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 2019;51:2391-402. https://doi.org/10.1249/MSS.0000000000002117.
- Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: an overview. World J Clin Oncol 2014;5:406-11. https://doi.org/10.5306/wjco.v5.i3.406.
- Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:815-40. https://doi.org/10.1093/jnci/djs207.
- Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med 2009;43:32-8. https://doi.org/10.1136/bjsm.2008.053843.
- Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health 2018;6:e1077-e1086. https://doi.org/10.1016/S2214-109X(18)30357-7.
- Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD005001.pub3.
- National Institute for Health and Care Excellence (NICE) . Behaviour Change: Individual Approaches 2014.
- Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho-Oncology 2018;27:713-24. https://doi.org/10.1002/pon.4612.
- Henriksson A, Arving C, Johansson B, Igelström H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns 2016;99:1220-6. https://doi.org/10.1016/j.pec.2016.01.019.
- Husebø AM, Karlsen B, Allan H, Søreide JA, Bru E. Factors perceived to influence exercise adherence in women with breast cancer participating in an exercise programme during adjuvant chemotherapy: a focus group study. J Clin Nurs 2015;24:500-10. https://doi.org/10.1111/jocn.12633.
- Michie S, Rumsey N, Fussell A, Hardeman W, Johnston M, Newman S, et al. Improving Health: Changing Behaviour. NHS Health Trainer Handbook. Manual Guide. London: NHS; 2008.
- Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Educ Couns 2016;99:1099-105. https://doi.org/10.1016/j.pec.2016.02.003.
- Breast Cancer Care . Breast Cancer Campaign n.d. www.breastcancercare.org.uk/ (accessed 1 March 2015).
- Kendall FP, McCreary EK. Muscle Testing and Function. Baltimore, MD: Williams & Wilkins; 1993.
- Buckley JP, Borg GAV. Borg’s scales in strength training; from theory to practice in young and older adults. Appl Physiol Nutr Metab 2011;36:682-92. https://doi.org/10.1139/h11-078.
- Bruce J, Mazuquin B, Canaway A, Hossain A, Williamson E, Mistry P, et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. BMJ 2021;375. https://doi.org/10.1136/bmj-2021-066542.
- Bruce J, Richmond H, Lait C, Srikesavan C, Moser J, Newman M, et al. PROSPER Physiotherapist Manual 2015. https://wrap.warwick.ac.uk/144049 (accessed 5 November 2020).
- O’Cathain A. A Practical Guide to Using Qualitative Research with Randomized Controlled Trials. Oxford: Oxford University Press; 2018.
- Rees S, Mazuquin B, Richmond H, Williamson E, Bruce J. UK PROSPER Study Group . Role of physiotherapy in supporting recovery from breast cancer treatment: a qualitative study embedded within the UK PROSPER trial. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-040116.
- Fletcher AJ. Applying critical realism in qualitative research: methodology meets method. Int J Soc Res Methodol 2017;20. https://doi.org/10.1080/13645579.2016.1144401.
- Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exercise Health 2019;11. https://doi.org/10.1080/2159676X.2019.1628806.
- Rees S. ‘Am I really gonna go sixty years without getting cancer again?’ Uncertainty and liminality in young women’s accounts of living with a history of breast cancer. Health 2017;21:241-58. https://doi.org/10.1177/1363459316677628.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge, Taylor & Francis; 1994.
- Guest G, MacQueen KM, Namey EE. Applied Thematic Analysis. Thousand Oaks, CA: SAGE Publications Ltd; 2012.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: SAGE Publications Ltd; 1998.
- Charmaz K. Constructing Grounded Theory. London: SAGE Publications Ltd; 2014.
- Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Directions for Program Evaluation 1986;1986:73-84. https://doi.org/10.1002/ev.1427.
- Bury J, Littlewood C. Rotator cuff disorders: a survey of current (2016) UK physiotherapy practice. Shoulder Elbow 2018;10:52-61. https://doi.org/10.1177/1758573217717103.
- Dunn J, Steginga SK. Young women’s experience of breast cancer: defining young and identifying concerns. Psychooncology 2000;9:137-46. https://doi.org/10.1002/(SICI)1099-1611(200003/04)9:2<137::AID-PON442>3.0.CO;2-0.
- Thomas-MacLean R. Memories of treatment: the immediacy of breast cancer. Qual 2004;14:628-43. https://doi.org/10.1177/1049732304263658.
- Halliday LE, Boughton MA, Kerridge I. Mothering and self-othering: the impact of uncertain reproductive capability in young women after hematological malignancy. Health Care Women Int 2014;35:249-65. https://doi.org/10.1080/07399332.2013.770005.
- Crouch M, McKenzie HS. Social realities of loss and suffering following mastectomy. Health 2000;4:196-215. https://doi.org/10.1177/136345930000400204.
- Rees S. No one scans you and says ‘you’re alright now’: the experience of embodied risk for young women living with a history of breast cancer. Health Risk Soc 2018;20:312-24. https://doi.org/10.1080/13698575.2018.1539468.
- Williams SJ. The vicissitudes of embodiment across the chronic illness trajectory. Body &Amp; Society 1996;2:23-47. https://doi.org/10.1177/1357034X96002002002.
- Gencay Can A, Can SS, Eksioglu E, . Is kinesiophobia associated with lymphedema, upper extremity function, and psychological morbidity in breast cancer survivors?. Turk J Phys Med Rehabil 2019;65:139-46. https://doi.org/10.5606/tftrd.2019.2585.
- Martinez-Calderon J, Struyf F, Meeus M, Luque-Suarez A. The association between pain beliefs and pain intensity and/or disability in people with shoulder pain: a systematic review. Musculoskelet Sci Pract 2018;37:29-57. https://doi.org/10.1016/j.msksp.2018.06.010.
- Crane-Okada R, Kiger H, Anderson NL, Carroll-Johnson RM, Sugerman F, Shapiro SL, et al. Participant perceptions of a mindful movement program for older women with breast cancer: focus group results. Cancer Nurs 2012;35:E1-10. https://doi.org/10.1097/NCC.0b013e31822539c5.
- Browall M, Mijwel S, Rundqvist H, . Physical activity during and after adjuvant treatment for breast cancer: an integrative review of women’s experiences. Integr Cancer Ther 2018;17:16-30. https://doi.org/10.1177/1534735416683807.
- Levangie PK, Santasier AM, Stout NL, Pfalzer L. A qualitative assessment of upper quarter dysfunction reported by physical therapists treated for breast cancer or treating breast cancer sequelae. Support Care Cancer 2011;19:1367-78. https://doi.org/10.1007/s00520-010-0959-x.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2015.
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2017 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2017 (accessed 18 December 2021).
- NHS Supply Chain . NHS Supply Chain n.d. www.supplychain.nhs.uk (accessed 18 December 2021).
- Devlin N, Shah K, Feng Y, . Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- van Hout B, Janssen MF, Feng YS, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Olsen MK, Stechuchak KM, Edinger JD, Ulmer CS, Woolson RF. Move over LOCF: principled methods for handling missing data in sleep disorder trials. Sleep Med 2012;13:123-32. https://doi.org/10.1016/j.sleep.2011.09.007.
- Hoch JS, Hay A, Isaranuwatchai W, Thavorn K, Leighl NB, Tu D, et al. Advantages of the net benefit regression framework for trial-based economic evaluations of cancer treatments: an example from the Canadian Cancer Trials Group CO.17 trial. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-5779-x.
- NHS Digital . Costing – HRG4+ 2016 17 Reference Costs Grouper 2020. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper (accessed 21 February 2020).
- Sampson C. Bad Reasons Not to Use the EQ-5D-5L 2018. https://aheblog.com/2018/03/23/bad-reasons-not-to-use-the-eq-5d-5l/ (accessed 12 October 2020).
- Jeevan R, Mennie JC, Mohanna PN, O’Donoghue JM, Rainsbury RM, Cromwell DA. National trends and regional variation in immediate breast reconstruction rates. Br J Surg 2016;103:1147-56. https://doi.org/10.1002/bjs.10161.
- Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book 2016;35:185-98. https://doi.org/10.1200/EDBK_156686.
- Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 2019;111:245-55. https://doi.org/10.1093/jnci/djy221.
- Adams RN, Mosher CE, Blair CK, Snyder DC, Sloane R, Demark-Wahnefried W. Cancer survivors’ uptake and adherence in diet and exercise intervention trials: an integrative data analysis. Cancer 2015;121:77-83. https://doi.org/10.1002/cncr.28978.
- Costa ML, Achten J, Wagland S, Marian IR, Maredza M, Schlüssel MM, et al. Plaster cast versus functional bracing for Achilles tendon rupture: the UKSTAR RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24080.
- GOV.UK . Population of England and Wales n.d. https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest (accessed 18 December 2021).
- Jung GH, Kim JH, Chung MS. Changes in weight, body composition, and physical activity among patients with breast cancer under adjuvant chemotherapy. Eur J Oncol Nurs 2020;44. https://doi.org/10.1016/j.ejon.2019.101680.
- Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003;97:1746-57. https://doi.org/10.1002/cncr.11227.
- Møller T, Lillelund C, Andersen C, Ejlertsen B, Nørgaard L, Christensen KB, et al. At cancer diagnosis: a ‘window of opportunity’ for behavioural change towards physical activity. A randomised feasibility study in patients with colon and breast cancer. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003556.
- Sylvester BD, Ahmed R, Amireault S, Sabiston CM. Changes in light-, moderate-, and vigorous-intensity physical activity and changes in depressive symptoms in breast cancer survivors: a prospective observational study. Support Care Cancer 2017;25:3305-12. https://doi.org/10.1007/s00520-017-3745-1.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. https://doi.org/10.1016/S0895-4356(98)00109-7.
- Cho YU, Lee BG, Kim SH. Coping style at diagnosis and its association with subsequent health-related quality of life in women with breast cancer: a 3-year follow-up study. Eur J Oncol Nurs 2020;45. https://doi.org/10.1016/j.ejon.2020.101726.
- Lund LW, Ammitzbøll G, Hansen DG, Andersen EAW, Dalton SO. Adherence to a long-term progressive resistance training program, combining supervised and home-based exercise for breast cancer patients during adjuvant treatment. Acta Oncol 2019;58:650-7. https://doi.org/10.1080/0284186X.2018.1560497.
- Arem H, Sorkin M, Cartmel B, Fiellin M, Capozza S, Harrigan M, et al. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the Hormones and Physical Exercise (HOPE) study. J Cancer Surviv 2016;10:654-62. https://doi.org/10.1007/s11764-015-0511-6.
- McNeely ML, Binkley JM, Pusic AL, Campbell KL, Gabram S, Soballe PW. A prospective model of care for breast cancer rehabilitation: postoperative and postreconstructive issues. Cancer 2012;118:2226-36. https://doi.org/10.1002/cncr.27468.
- Ribeiro IL, Moreira RFC, Ferrari AV, Alburquerque-Sendín F, Camargo PR, Salvini TF. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: a systematic review of randomized controlled trials. Clin Rehabil 2019;33:1876-86. https://doi.org/10.1177/0269215519873026.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-40.
- Schmidt ME, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: the role of treatment-related side effects and potential impact on quality of life. Eur J Cancer Care 2019;28. https://doi.org/10.1111/ecc.13051.
- Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv 2010;4:119-27. https://doi.org/10.1007/s11764-009-0113-2.
Appendix 1 Recruitment centre by hospital and NHS trust
| Code | Hospital and trust |
|---|---|
| BCH | City Hospital, Sandwell and West Birmingham NHS Trust |
| BTH | Burnley General Teaching Hospital, East Lancashire Hospitals NHS Trust |
| CRH | Chesterfield Hospital, Chesterfield Royal Hospital NHS Foundation Trust |
| DCH | Dorset County Hospital, Dorset NHS Foundation Trust |
| GEH | George Eliot Hospital NHS Trust |
| HCH | Hereford County Hospital, Wye Valley NHS Trust |
| HH | Hillingdon Hospital, The Hillingdon Hospitals NHS Foundation Trust |
| MDH | Macclesfield District General Hospital, East Cheshire NHS Trust |
| MKH | Milton Keynes University Hospital NHS Foundation Trust |
| MPH | Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust |
| NCH | New Cross Hospital, The Royal Wolverhampton NHS Trust |
| OUH | Churchill Hospital, Oxford University Hospital NHS Foundation Trust |
| QAH | Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust |
| RCH | St Michael’s Hospital, Royal Cornwall Hospitals NHS Trust |
| RSH | Royal Stoke Hospital, University Hospitals of North Midlands NHS Trust |
| UHC | University Hospital Coventry, Coventry and Warwickshire NHS Trust |
| WMH | Manor Hospital, Walsall Healthcare NHS Trust |
Appendix 2 Range of movement exercises by intervention compliance
| Exercises | Intervention compliance, n (%) | |||||
|---|---|---|---|---|---|---|
| Compliers | Partial compliers | |||||
| First appointment | Second appointment | Third appointment | Fourth appointment | First appointment | Second appointment | |
| Flexion | ||||||
| Number of participants | 152 | 150 | 147 | 86 | 32 | 19 |
| Easy | 99 (65.1) | 83 (55.3) | 69 (46.9) | 40 (46.5) | 29 (90.6) | 11 (57.9) |
| Intermediate | 36 (23.7) | 46 (30.7) | 53 (36.1) | 31 (38.0) | 3 (9.4) | 7 (36.8) |
| Missing | 17 (11.2) | 21 (14.0) | 25 (17.0) | 15 (17.4) | 0 (0.0) | 1 (5.3) |
| Abduction | ||||||
| Number of participants | 148 | 148 | 148 | 88 | 32 | 19 |
| Easy | 94 (63.5) | 70 (47.3) | 55 (37.2) | 35 (39.8) | 27 (84.4) | 11 (57.9) |
| Intermediate | 10 (6.8) | 21 (14.2) | 11 (7.4) | 8 (9.1) | 0 (0.0) | 3 (15.8) |
| Advanced | 15 (10.1) | 17 (11.5) | 34 (23.0) | 17 (19.3) | 1 (3.1) | 1 (5.3) |
| Very advanced | 10 (6.8) | 18 (12.2) | 23 (15.5) | 12 (13.6) | 0 (0.0) | 2 (10.5) |
| Missing | 19 (12.8) | 22 (14.9) | 25 (16.9) | 16 (18.2) | 4 (12.5) | 2 (10.5) |
| Abduction and external rotation | ||||||
| Number of participants | 146 | 144 | 140 | 82 | 32 | 19 |
| Easy | 96 (65.8) | 58 (40.3) | 38 (27.1) | 24 (29.3) | 22 (68.8) | 5 (26.3) |
| Intermediate | 32 (21.9) | 64 (44.4) | 74 (52.9) | 41 (50.0) | 7 (21.9) | 11 (57.9) |
| Missing | 18 (12.3) | 22 (15.3) | 28 (20.0) | 17 (20.7) | 3 (9.4) | 3 (15.8) |
Appendix 3 Health economics
FIGURE 11.
Secondary analysis 1: complete-case analysis.
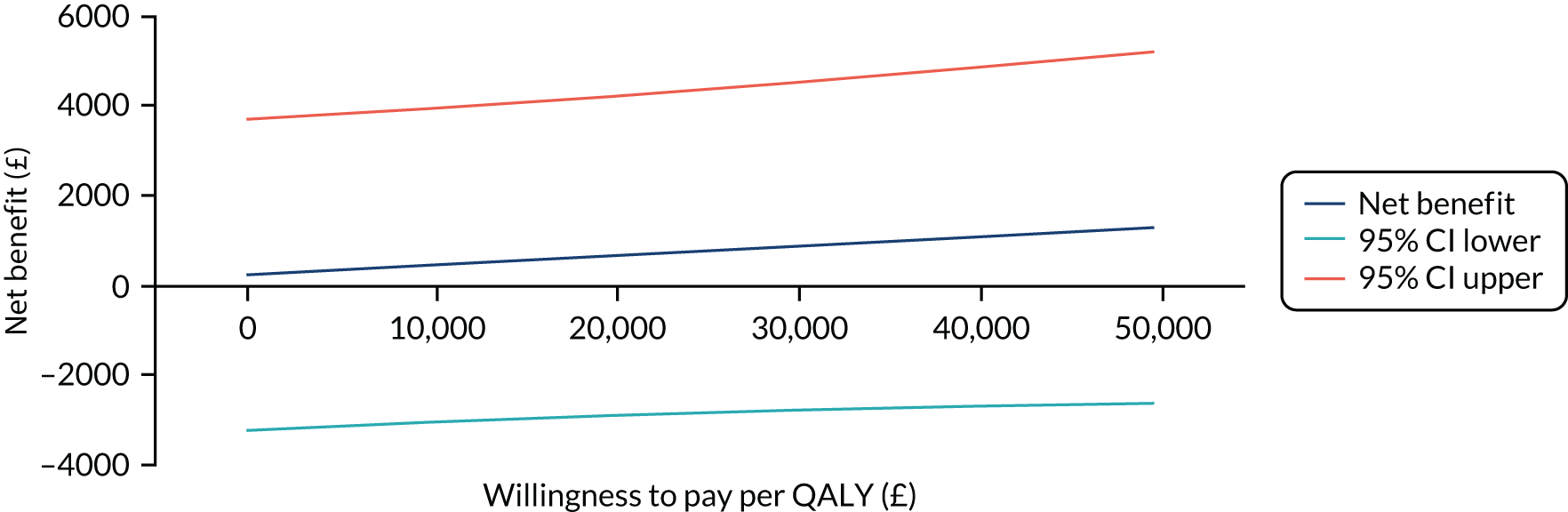
FIGURE 12.
Secondary analysis 1: complete-case analysis CEAC.
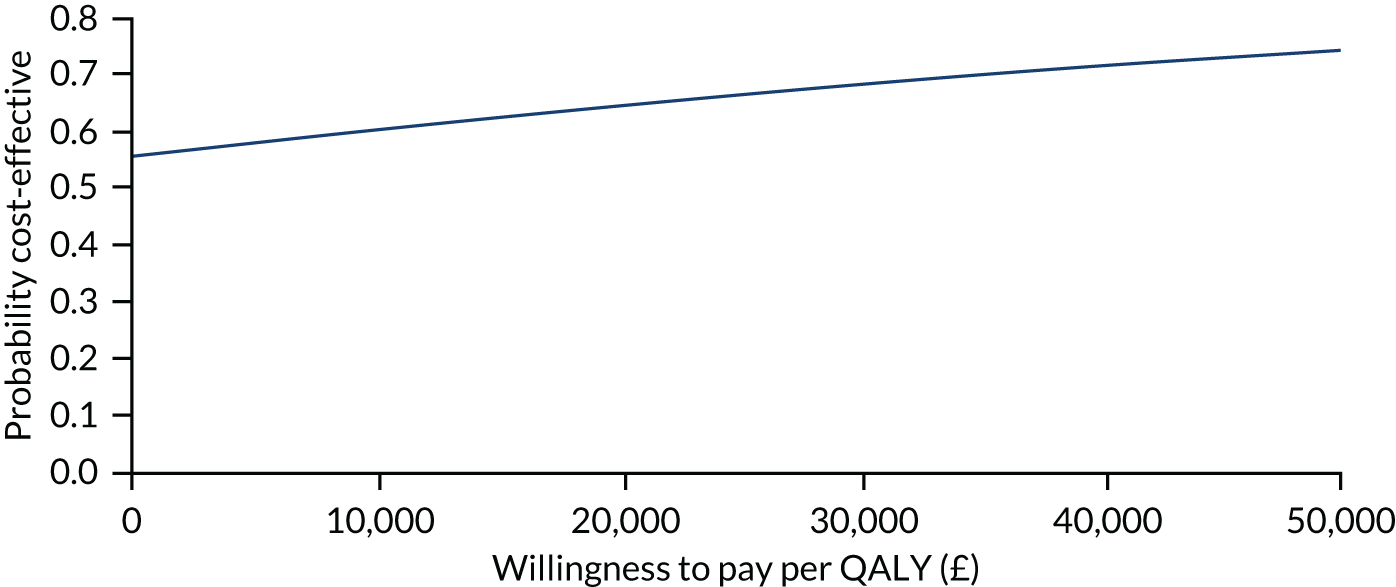
FIGURE 13.
Sensitivity analysis 3: including societal costs, CEAC.
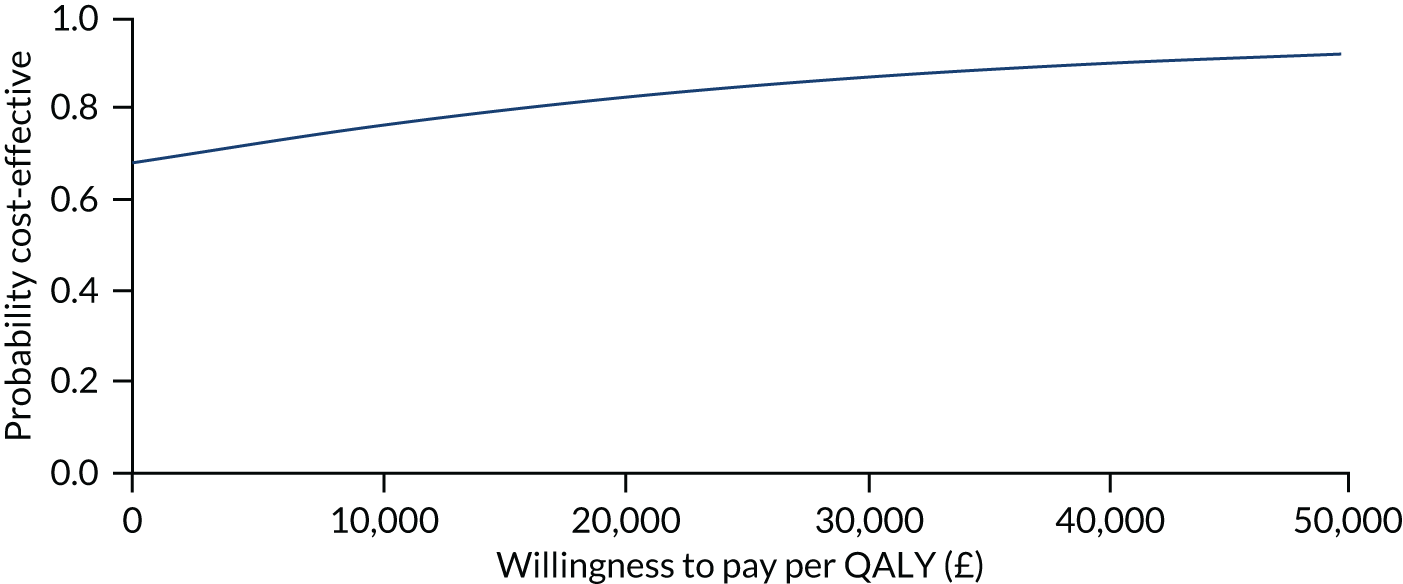
FIGURE 14.
Sensitivity analysis 3: including societal costs, net benefit by WTP.
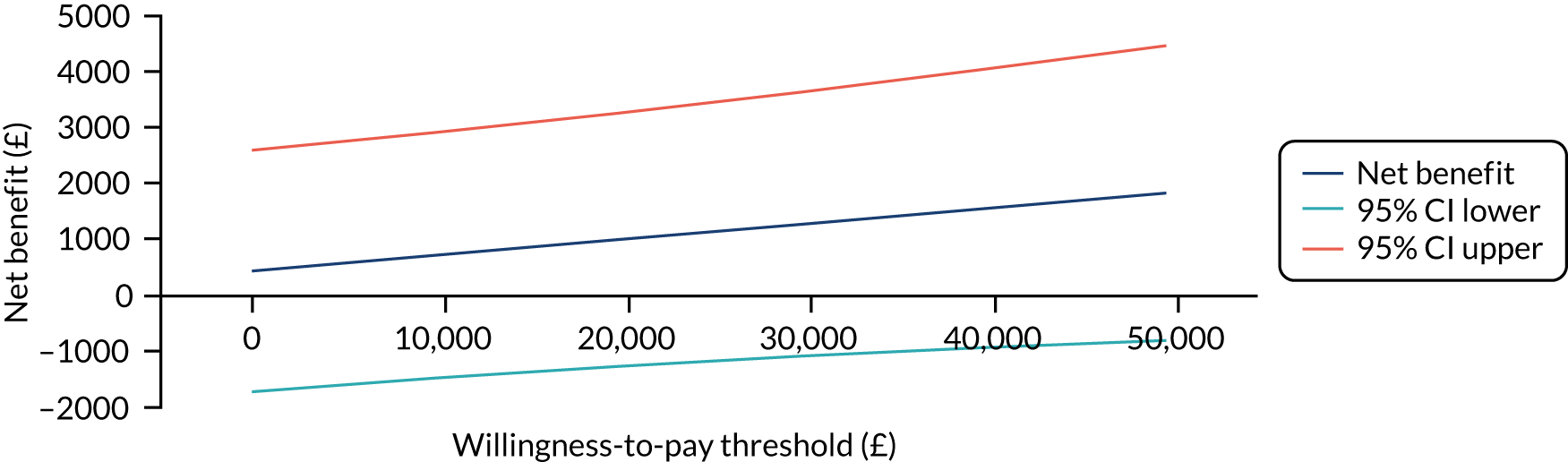
FIGURE 15.
Sensitivity analysis 4: including training costs, CEAC.
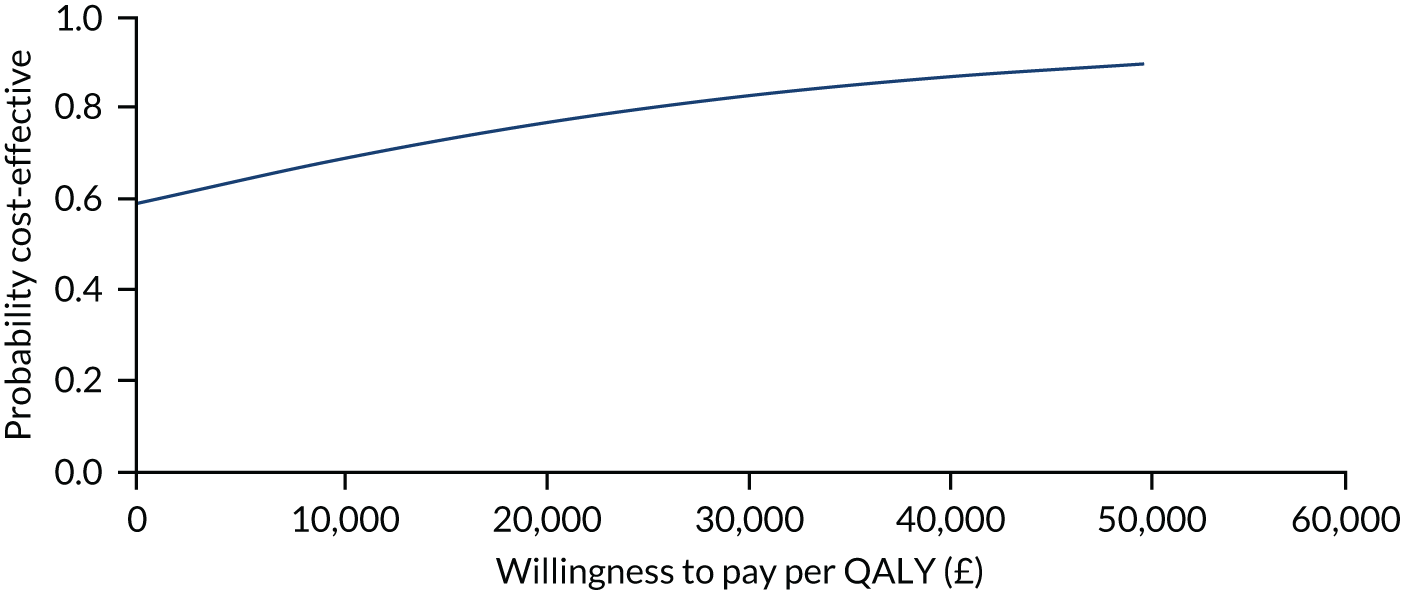
FIGURE 16.
Sensitivity analysis 4: including training costs, net benefit analysis.
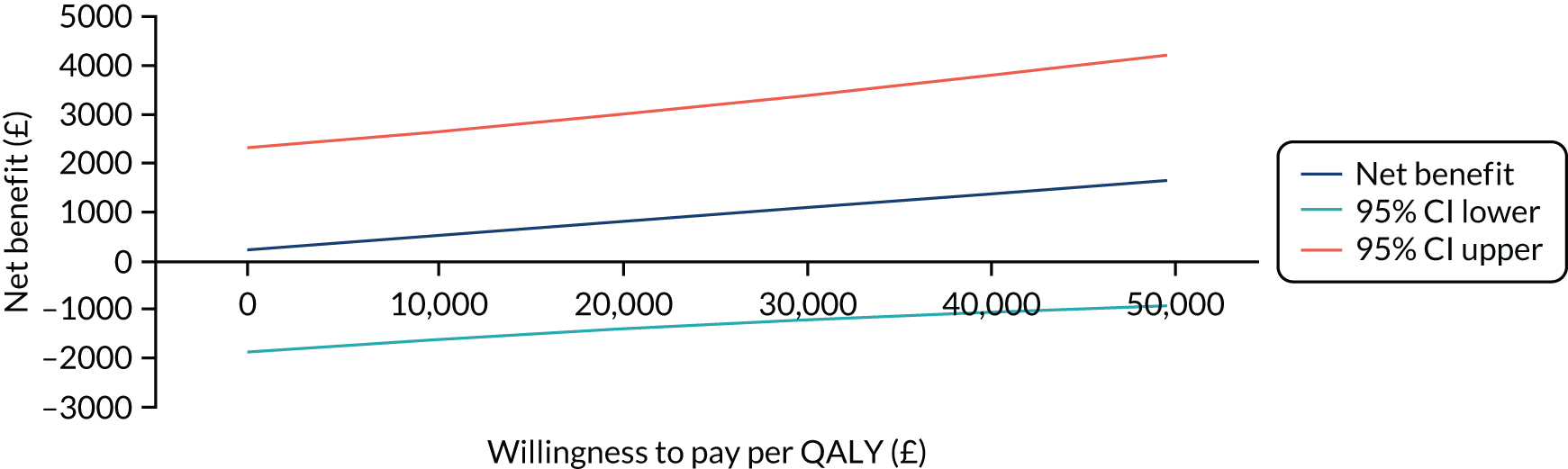
FIGURE 17.
Sensitivity analysis 5: excluding high costs cancer costs, CEAC.
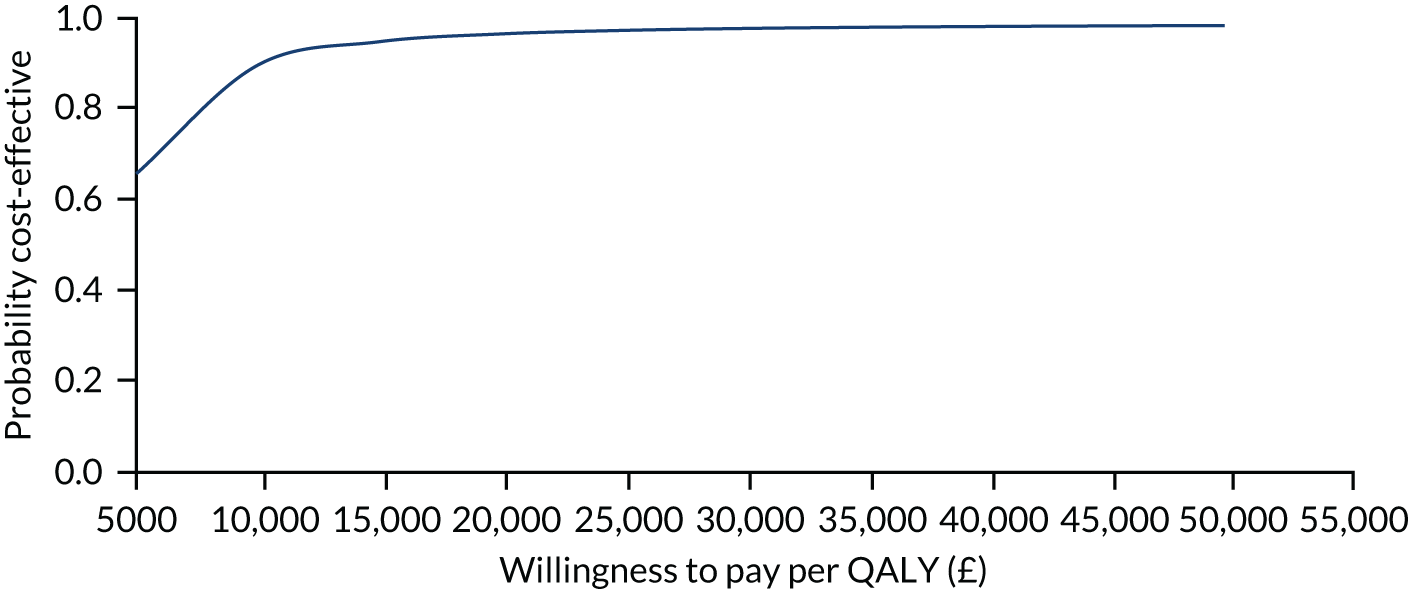
FIGURE 18.
Sensitivity analysis 5: excluding high costs cancer costs, net benefit by WTP.
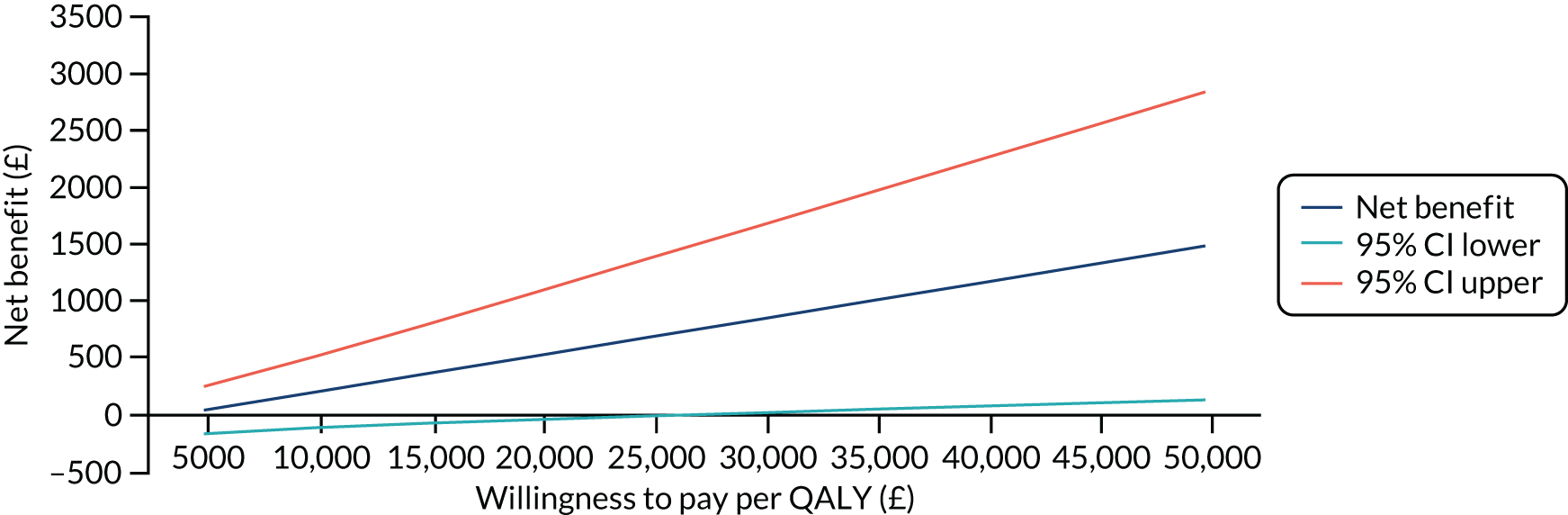
FIGURE 19.
Sensitivity analysis 6: using HES data for hospital costs, CEAC.
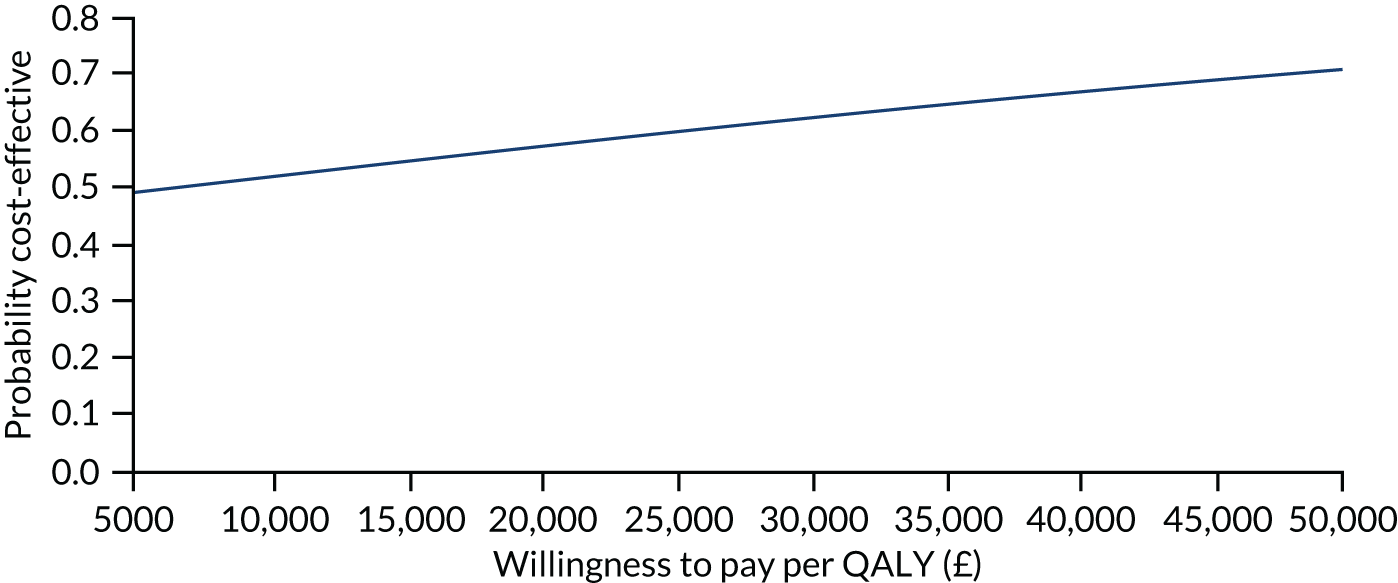
List of abbreviations
- 1RM
- 1-repetition maximum
- AE
- adverse event
- ALND
- axillary lymph node dissection
- ANC
- axillary node clearance
- BCN
- breast care nurse
- BCS
- breast-conserving surgery
- BMI
- body mass index
- CACE
- complier-average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidating Standards of Reporting Clinical Trials
- CRF
- case report form
- CSRI
- Client Service Receipt Inventory
- DASH
- Disabilities of the Arm, Shoulder and Hand
- DMEC
- Data Monitoring and Ethics Committee
- DN4
- Douleur Neuropathique
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FACT-B4
- Functional Assessment of Cancer Therapy – Breast, version 4
- HES
- Hospital Episode Statistics
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intracluster coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LBCQ
- Lymphoedema and Breast Cancer Questionnaire
- MCID
- minimally clinically important difference
- MCS
- mental health composite scale
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRS
- numerical rating scale
- OR
- odds ratio
- OTC
- over the counter
- PASE
- Physical Activity Scale for the Elderly
- PCS
- physical health composite scale
- PPI
- patient and public involvement
- PROSPER
- PRevention Of Shoulder ProblEms tRial
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QC
- quality control
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROM
- range of movement
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short-Form questionnaire-12 items
- SLNB
- sentinel lymph node biopsy
- SMART
- specific, measurable, achievable, relevant and timely
- SMD
- standardised mean difference
- SSI
- surgical site infection
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UHCW
- University Hospitals Coventry and Warwickshire
- WCTU
- Warwick Clinical Trials Unit
- WHO
- World Health Organization
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JKNZ2003).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
