Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 15/166/08. The contractual start date was in January 2017. The draft manuscript began editorial review in October 2022 and was accepted for publication in May 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Marshman et al. This work was produced by Marshman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Marshman et al.
Chapter 1 Introduction
Background
Introduction
The high prevalence and severity of dental caries adversely affect children and young people in the UK, bringing with it an economic burden. Reducing the disease, and therefore its negative impact, is a public health priority. 1–3 Dental caries is largely preventable and there are a number of successful interventions across the UK for young children, including those based on toothbrushing with fluoride toothpaste. 4,5 However, there is a lack of evidence for community-based oral health improvement programmes in older children and young people that target toothbrushing practices. A behaviour change intervention incorporating a school-based lesson together with mobile health (mHealth) technology (through mobile phones) has the potential to have a positive effect on this oral health behaviour and ultimately reduce dental disease and its sequelae.
Prevalence of dental caries in young people
Dental caries is the most prevalent non-communicable condition worldwide, with untreated caries affecting 2.4 billion people. 6 In the UK, there have been reductions in caries experience for young people reflected in the 2003 and, most recent, 2013 Child Dental Health Surveys (CDHS) which cover England, Wales and Northern Ireland. The proportion of children with teeth showing active carious lesions, dental restorations or which had been extracted due to dental caries dropped from 43% to 34% in 12-year-olds and from 56% to 46% in 15-year-olds over the 10-year period. 7 In Scotland, over a similar period, the annual National Dental Inspection Programme (NDIP) reported reductions in obvious caries experience for 12-year-olds, from 47% in 2005 to 27% in 2015, and in the most recent survey carried out in 2019, there has been a further reduction with 20% affected. 8–10
Despite these improvements across the UK, the prevalence of dental caries remains high. The overall reduction in the prevalence is positive, but potentially masks three underlying issues. First, there is a persistence in underlying oral health inequalities. The positive association between living in a deprived area and prevalence of dental caries is seen across the UK11,12 and globally. 6 A variety of indicators are used for measuring deprivation. Using eligibility for free school meals (FSM) as a measure, the 2013 CDHS found that the proportion of children with obvious caries experience was 46% among 12-year-old children eligible for FSM, compared with 30% among those not eligible for FSM. 7 Similarly, for 15-year-olds, this was 59% for those eligible for FSM compared with 43% for those not eligible. 7 In Scotland, the difference in obvious decay experience levels between those living in the most and least deprived areas, was 26.3% in 2009 reducing to 18.6% in 2019. 10
Second, although the overall prevalence has reduced, the disease is severe in those affected. The burden of disease in children can be measured using the mean number of decayed, missing and filled teeth (DMFT) per child. In 2013, for England, Wales and Northern Ireland, for children with obvious decay experience, this was 2.5 teeth per child7 and in Scotland, 2.1 teeth per child. 10
Finally, although there has been a reduction in the prevalence of dental caries in children, there is a high burden of untreated disease. The 2013 CDHS found obvious untreated decay into dentine in 19% of 12-year-olds and in 21% of 15-year-olds. 7 Similarly, in Scotland, in 2019, untreated caries into dentine was found in 8% of 12-year-olds. However, this rose to 40% who had untreated disease, when only the children with carious lesions were included. 10 COVID-19-related challenges with access to dental care will potentially increase the burden from disease for all and widen inequalities. 13,14
Impact of dental caries on young people and their families
The impact of dental caries on the lives of children and young people has been well documented, including pain15 and difficulties with eating and sleeping, with disrupted social activities and absences from school. 12,16 Families report guilt, lack of sleep and taking time off work related to their child’s carious teeth in 5- to 16-year-old children. 17 Following treatment of dental caries, there is a reduction in these impacts for both children up to the age of 16 years and their families. 16–19
In a secondary analysis of data from the 2013 CDHS, 3859 children were included to investigate the relationship between the presence/absence of severe dental caries (e.g. pulp involvement, ulceration, fistula or an abscess in at least one tooth) and seven items from the Family Impact Scale. 20 Three in 10 parents (29.5%) reported that their child’s oral health impacted on their family life. The areas most frequently affected were parents having to take time off work (15.5%), feeling stressed (14.2%), feeling guilty (10.4%) and their child needing more attention (13.4%); the least frequent impacts related to financial difficulties (2.4%), disruption in normal activities (6.1%) and disturbed sleep (7.4%).
For those children waiting for tooth extractions in hospital in the North of England, 67% of parents reported that their child had pain and 38% reported parental sleepless nights. 21 In a national health survey conducted in the USA, dental pain was reported in 32% (n = 7.5 million) of children whose parents stated that they had caries. 22 Around 50% of 12- and 15-year-olds reported toothache and 6% of 12-year-olds and 3% of 15-year-olds reported difficulty with schoolwork because of their teeth and mouth condition over the previous 3 months in the 2013 CDHS. 7
Economic impact of dental caries
Treatment of oral diseases is expensive, with NHS England spending £3.4 billion in 201423 and £3.6 billion in 2021 for adults and children in treatment costs. 24 Dental expenditure in the UK has been estimated to be around £196 per capita. 25 In 2017, hospital admissions (usually for general anaesthetic) in NHS England for tooth extractions due to dental caries, for children aged 0–19 years cost £33.0 million. 1 However, the economic costs when taking into consideration time off work and school are much greater. Worldwide, these indirect costs are estimated to be US$144 billion per year. 26
Aetiology and prevention of dental caries
Dental caries is a biofilm-mediated, diet-modulated, multifactorial, non-communicable, dynamic disease resulting in mineral loss from dental hard tissues. 27 It is determined by biological, behavioural, psychosocial and environmental factors. The main modifying factors in the development of caries are sugar consumption and the use of fluoride. 28 Therefore, prevention of dental caries can be achieved by optimising exposure to fluoride and reducing sugar consumption by improving diet. These changes can be encouraged at an individual level by professional intervention or through community- or population-based public health programmes. 29
Brushing with fluoride toothpaste is one of the most effective measures to prevent caries. 5 The Scottish multicomponent oral health programme targeted at young children has toothbrushing in schools as a major component. It was introduced as a pilot in 2006 and then delivered across all Health Boards in Scotland from 2011. Around 80% of 12- and 15-year-olds stated that they brushed their teeth twice per day in the 2013 CDHS, although this was 82% of those not eligible for FSM and 72% of those who were eligible for FSM. 30 The same survey found that only 25% of 12-year-olds and 32% of 15-year-olds were considered to have good periodontal health (plaque in no more than one sextant, no gingival inflammation, no calculus), which may demonstrate that there is less adherence to oral hygiene practices than reported. 31 This is in line with observational studies that have shown current levels of efficacy, frequency and duration of toothbrushing to be inadequate,32–34 thereby increasing the risk of caries. 35
School-based oral health promotion programmes
Community-based oral health interventions in the UK have been aimed mainly at younger children of pre-school age or in primary education. Few interventions are aimed at reducing dental caries in young people, despite adolescence being a critical transition stage where independence develops, diet begins to become self-managed and oral health behaviours change,36 with irregular toothbrushing associated with reduced fluoride exposure and resultant increased caries risk reported. 37 While national oral health promotion programmes such as Childsmile have been implemented in Scotland, and Designed to Smile has been developed in Wales, these programmes focus mainly on children under 12 years of age. Examples of current community-based interventions to improve the oral health of young people have been categorised into oral health education interventions and more complex interventions involving additional activities such as clinical prevention measures alongside the education component. 38 However, limitations of these existing programmes include the lack of use of behaviour change theory and that these interventions are not embedded as statutory content within the school curriculum, as recommended by the World Health Organization’s (WHO) Health Promoting Schools framework. 38–42
Behaviour change interventions
To reduce caries prevalence and its associated burden, young people need to adopt and/or maintain oral health behaviours, including regular toothbrushing with a fluoride toothpaste and sugar reduction. Successfully intervening to induce behaviour change, whether at an individual or community-based level, requires an understanding of these behaviours in the context of people’s lives and developing interventions to change behaviour. For example, an intervention to improve toothbrushing behaviour may be considered to require education (improving knowledge and understanding) plus persuasion (that the behaviour will produce a positive effect) and training in the necessary skills. The type of intervention required then influences the choice of the delivery method; both the setting where it is delivered, the vehicle(s) used and the timing and frequency. For example, digital interventions are recommended as appropriate vehicles for delivering behaviour change interventions directed at young people. 43
Use of mHealth for delivering health interventions
There has been increasing interest in the development and use of health interventions (including behaviour change interventions) through the vehicle of mobile phones and wearable technologies. 43,44 mHealth has been defined by the WHO45 as ‘a medical and public health practice supported by mobile devices such as mobile phones, patient monitoring devices, personal digital assistants and other devices’. mHealth is a particular category of a wider eHealth area,46 which provides innovative ways of improving the overall health. 47
Short messaging service (SMS), also known as ‘text messages’, are the most widely investigated mHealth interventions and have been used to deliver health education, promote treatment adherence in a range of conditions and in the prevention of communicable diseases. A systematic review of preventive health behaviour change text message interventions found a small but statistically significant positive effect for the impact of text messages both shortly after cessation of the intervention and also after a period of ‘no intervention’ to demonstrate whether the effects can be maintained longer term. 48 Potential moderators of effect size were considered including the duration, tailoring and targeting of the content and how text messages were used, along with other activities such as educational content. It appeared that interventions lasting 6–12 months were associated with greater effects than shorter interventions. The limitations of the component studies included insufficiently powered studies lacking the ability to detect change, short-term follow-up, failure to blind those assessing outcomes and lack of a theoretical framework to inform the behaviour change intervention. 48,49 It has been recommended that future studies ensure the intervention is developed rigorously, the text messages are appropriate for the target population in terms of their age and wording and the messages use the participant’s name.
Most studies in this field have involved adults, although more recently there have been studies involving young people. In the UK, in a 2022 Ofcom report on children’s media use, it was estimated that 9 in 10 children owned their own mobile phone by the time they reached the age of 11, and 97% of 12- to 15-year-olds used a smartphone for texting and making calls. 50 It is perhaps the ubiquity of mobile phones that explains why smartphone ownership among young people does not seem to vary by socioeconomic status,51 suggesting that mHealth interventions may suit this age group.
mHealth interventions to improve oral health
mHealth has gained popularity and has been investigated as a possible vehicle to deliver dental behaviour change interventions. Three recent systematic reviews have evaluated the effectiveness of mHealth. They cover all teledentistry (including mHealth) on oral health promotion and prevention compared with other strategies and in all ages;52 mobile applications and text messages compared with conventional oral hygiene instructions53 in adolescents, adults and mothers of young children; and text messages only in dental patients. 54 Many of the primary studies were included in more than one review.
In the review of all teledentistry, apps and text messages were found to be used most frequently. 52 This review included 18 studies, with 12 involving young people undergoing orthodontic treatment and 4 focusing on prevention. The nine studies that focused on text messaging reminders often had other parts to the intervention (education element before or during, phone calls, etc.). The meta-analysis was carried out on the basis of outcomes for those receiving all mHealth interventions. There was a demonstration of an overall reduction in plaque scores and improvement in gingival health. However, because of the combined delivery formats (apps, texts, etc.), multicomponent interventions and variable intervention delivery times as well as follow-ups, it was not clear what the exact contribution of each of the components, including text message reminders. The second53 included 15 studies with 12 involving text messaging, of which 11 studies included patients undergoing orthodontic treatment and one study of adult patients. Again, an improvement in plaque control and gingival health was found and an improvement in knowledge. This review assessed the interventions on the basis of behaviour change techniques, which were evaluated according to the Michie and colleagues’ taxonomy. 55 Most of the studies that used text messaging combined two behaviour change techniques, prompts and cues, and information about health consequences. Twelve studies involved text messages and were included in the meta-analysis, which showed improvements for the groups that received the mHealth intervention compared to the control groups. However, follow-up times were short, ranging from 4 weeks to 12 months, although the majority (13/15) were 6 months or less. The GRADE evaluation found the quality of evidence to be very low. The third review subgroup analyses again found consistently in favour of the text intervention improving oral hygiene clinical outcomes (plaque index and gingival index). The overall summary statistics were incorrectly calculated with double counting.
Although there are studies investigating text messages, the actual effect of these as individual components within multicomponent interventions is unclear. They are of variable duration and frequency, follow-up times to investigate effect have been short (usually weeks or months), none seem to have investigated dental caries as an outcome, with dental plaque levels and gingival bleeding most commonly used as indicators of behaviour change, and, less frequently, level of knowledge. In addition, few have been delivered as community-based interventions via settings such as schools when compared to interventions delivered in clinical settings to patients. All three systematic reviews found a high degree of heterogeneity, especially in plaque and gingival health outcome measures, as well as variable, short follow-up times and poor reporting quality. Recommendations were made that longer follow-up periods were needed with standardised methodologies and outcome measures.
Community-based behaviour change text message interventions
The first, and so far only, community-based text message intervention aimed at improving toothbrushing was conducted in New Zealand with unemployed people aged 18–24 years. This study investigated the Keep on Brushing (KOB) programme of weekly text messages and provision of free toothbrushes/toothpaste. 56 The intervention was underpinned by the Health Belief Model. 57 One hundred and seventy-one participants were recruited and completed a baseline survey. No important differences were noted between sex, ethnicity or age. Participants then received a series of motivational text messages over 10 weeks. To increase recruitment, participants were also given a pack containing toothpaste and a toothbrush. Self-reported toothbrushing of twice or more per day increased from 51% at baseline to 70% at week 3, 74% at week 6 and 73% at week 9; however, by week 9, only 26% of the original participants were still taking part. The authors concluded that motivational text messages improved the self-reported oral health of this hard-to-reach group and suggested that a randomised controlled trial (RCT) including a longer intervention with tailoring of the messages was needed.
Rationale
This intervention was developed based on the commissioning brief from the National Institute for Health Research (NIHR, now known as the National Institute for Health and Care Research) Health Technology Assessment (HTA) programme (HTA no. 15/166 Interventions to improve oral health in deprived young people). Reducing childhood dental caries remains a public health priority; however, there are limited interventions aimed at preventing dental caries in adolescents. There is strong evidence for the effectiveness of toothbrushing with a fluoride toothpaste in preventing dental caries. The commissioning brief requested an evaluation of a digital behaviour change intervention to promote toothbrushing based on the pilot work of the KOB study. Behaviour change to reduce sugar intake, which is essential to the development of caries, was not included in this intervention. In terms of an appropriate setting for such an intervention, the use of the school setting with an mHealth component could potentially deliver the type of behaviour change needed. Existing interventions have predominantly involved oral health education only, without being underpinned by behaviour change theory or embedded within the school curriculum.
As with any public health investment, the value of the health benefits needs to be balanced against the cost of generating them using economic evaluation. Without convincing economic information, support for any health intervention from the National Institute for Health and Care Excellence (NICE) and national public health bodies is unlikely. Consequently, any evaluation of an attempt to change toothbrushing behaviour in young people needs to be combined with a robust economic evaluation.
Aim and objectives
Aim
The aim of the Brushing RemInder 4 Good oral HealTh (BRIGHT) trial is to establish the clinical and cost effectiveness of an intervention for young people from deprived areas, delivered through a short classroom-based session (CBS) embedded in the curriculum and a series of text messages, compared to usual education and no text messages, on dental caries.
Trial objectives
Objectives of the BRIGHT trial:
-
conduct an internal pilot phase with feasibility components to:
-
tailor the intervention to young people
-
test trial processes in schools
-
assess the feasibility of within-school cluster randomisation (by year group)
-
-
investigate the effect of the intervention on caries prevalence
-
investigate the effect of the intervention on twice-daily toothbrushing, oral health-related quality of life (HRQoL) and oral health behaviours
-
investigate the cost effectiveness of the intervention
-
explore implementation, mechanisms of impact and context through a process evaluation
Chapter 2 Methods
The BRIGHT trial was designed (including target group, setting, intervention, comparator and outcomes) based on the details in the commissioning brief (HTA no. 15/166 Interventions to improve oral health in deprived young people).
Trial design
Brushing RemInder 4 Good oral HealTh is a multicentre, school-based, assessor-blinded, two-arm cluster RCT with an internal pilot phase, and embedded health economic and process evaluations. Schools with above the national average proportion of children receiving FSM were targeted to participate. Pupils in Years 7 and 8 (England and Wales) and S1 and S2 (Scotland) (i.e. age 11–12 years) in participating schools were recruited and, within each school, these year groups were randomly allocated using a 1 : 1 randomisation ratio to either the intervention or control group. The trial intervention consisted of a short CBS embedded within the school curriculum on dental health and looking after teeth, followed by twice-daily text messages to remind pupils to brush their teeth. The control arm of the trial was routine education and no text messages. The outcomes (caries prevalence, twice-daily toothbrushing, oral HRQoL and oral health behaviours) were assessed through clinical examination and questionnaires.
The trial protocol for BRIGHT has been published previously. 58,59 Figure 1 presents an overview of the trial design, including the planned follow-up time points (baseline, after the CBS, 12 weeks, 6 months, 1, 2 and 2.5 years following the lesson in the school). Final follow-up was originally planned for 3 years following CBS delivery but was amended to 2.5 years with the funder’s approval. This was to avoid the final clinical examinations coinciding with school exams during the spring/summer when school staff and space are at a premium. Two early follow-ups, at the time of the CBS and 12 weeks later, were only conducted in schools recruited during the internal pilot phase, as these data were to assess the appropriateness of the study design and inform progression to the main phase and were not required in schools that were part of the trial main phase. In addition, data collection at 2 years following CBS delivery was also only carried out in internal pilot schools. This follow-up was removed from the main phase, with the funder’s approval, when the final follow-up was amended to 2.5 years to reduce burden on schools and pupils.
FIGURE 1.
Trial design diagram. CARIES-QC, Caries Impacts and Experiences Questionnaire for Children.

Note, the descriptors of the time points for the assessments (e.g. 2.5 years) reflect the planned follow-up schedule and, in the interests of brevity and consistency, are used throughout the report when referring to the follow-ups. However, some of the actual average time intervals of follow-up varied from that planned (e.g. the actual average length of follow-up at the 2.5-year assessment was closer to 3 years, as this was unexpectedly delayed due to disruptions caused by the COVID-19 pandemic). This is elaborated on in The impact of the COVID-19 on data collection and in Results.
Figure 1 also illustrates the point at which the internal pilot phase progression criteria were reviewed to determine continuation to the main phase of the trial.
The progression criteria that were used to determine trial continuation from the internal pilot phase are listed below. These were developed based on the commissioning brief and guidance from the NIHR HTA and were pre-specified within the protocol.
-
An indication of a positive effect of the intervention on self-reported frequency of toothbrushing at 12 weeks using an 80% one-sided confidence interval (CI) approach.
-
Engagement with 80% of the number of schools required for the main phase of the trial, including obtaining agreement to participate, in principle.
-
Recruited an average of 48 pupils per year group from the 10 schools included in the internal pilot (48 was 80% of our target average recruitment of pupils per year group).
-
Minimum 80% response to questionnaires, completed by pupils.
-
Confirmation of feasibility of embedding the education component within the curriculum through discussion with school head teachers.
-
Confirmation of the feasibility of the outcome data collection methods and time points within the school year.
-
Assessment of contamination in the control group and whether feasible to undertake randomisation within schools (by year group) or whether randomisation at the school level was required, and calculation therefore of the required school sample size.
The independent Trial Steering Committee (TSC), Data Monitoring and Ethics Committee (DMEC) and the funder reviewed the progress of the internal pilot phase against the progression criteria at the relevant time point and determined that the trial should continue without major amendment. 60
Regulatory approvals and research governance
The BRIGHT trial was granted ethical approval by the East of Scotland Research Ethics Service on 14 August 2017 [Research Ethics Committee (REC) reference number 17/ES/0096; the favourable opinion letter is provided on the NIHR BRIGHT project web page]. 59 Approval was also obtained from Research and Development offices at the participating NHS sites, NHS Tayside (on 23 August 2017) and Cardiff and Vale University Health Board (26 September 2017). A summary of amendments made to the protocol is provided in Appendix 1, and a more detailed discussion regarding the changes to the protocol can also be found in Changes to the protocol. All protocol deviations/breaches were reported as necessary and are explained in Appendix 2, Table 22.
The BRIGHT trial was conducted in accordance with the requirements of the HTA programme. A TSC and a DMEC were formed and both were independently chaired. Both the TSC and the DMEC met at least once a year during the trial period. The Trial Management Group (TMG), which consisted of the Chief Investigator, Co-Principal Investigator, Regional Clinical Leads and members of the local research teams (LRTs), as well as team members from York Trials Unit (YTU) (including the Methodological Expert, Health Economist, Statistician, Senior Statistician, Trial Manager, Trial Coordinators and Trial Support Officers) and other study co-investigators, was responsible for the management of the trial and met once a month from initiation until after final data collection. A smaller group from within the TMG met every 1–2 weeks to closely monitor milestones and delivery of the trial.
The study was cosponsored by the University of Dundee and NHS Tayside from the start of the trial until 31 July 2020. From 1 August 2020 until the trial end, the sponsor was Cardiff University as one of the Chief Investigators changed institutions.
The trial was registered with the ‘International Standard Randomised Controlled Trial Number’ (ISRCTN) Registry on 10 May 2017 (ISRCTN12139369).
School recruitment
Schools in Scotland, England (West Yorkshire and South Yorkshire) and Wales were recruited to take part in this trial. A number of schools were recruited to start the trial in the 2017–8 academic year (i.e. complete pupil recruitment and baseline data collection and commence the intervention) and these formed the population for the internal pilot phase. The remaining schools were recruited to start the trial during the 2018–9 academic year.
Schools were eligible for participation if they met all the following inclusion criteria:
-
located in Scotland, England (South Yorkshire and West Yorkshire) or Wales (Cardiff, Vale of Glamorgan, Rhondda Cynon Taf and Merthyr Tydfil local authority areas)
-
were state-funded
-
had pupils attending the school who were aged 11–16 years old
-
had at least 60 pupils per year group
-
had above the national average percentage (for each devolved nation) of pupils eligible for FSM. The cut-offs used to determine eligibility were 13.2% for schools in England, 14.2% for schools in Scotland and 15.6% for schools in Wales, which were the average percentages of children eligible for FSM in state-funded secondary schools in each devolved nation in 2016 (i.e. the most recent figures available at the start of school recruitment). 61–63
Schools were not eligible to take part in the trial if they were in ‘special measures’ [i.e. judged by the Office for Standards in Education, Children’s Services and Skills (Ofsted) to be failing, or likely to fail, to provide an acceptable standard of education] or if the school was due to close.
Eligible schools were identified using the Department for Education’s register of educational establishments in England and using school data available from the Scottish and Welsh government websites. 64–66 School recruitment strategies were developed based on consultation with teachers and head teachers (particularly David Cooper from Batley Girls’ High School), researchers with experience of recruiting schools and local authorities. LRTs for each region approached schools using a variety of recruitment strategies, including: engaging with local or national organisations for schools (e.g. School Leaders Scotland and Learn Sheffield); use of professional and personal contacts of the LRT; approaching schools through contacts held by the recruiting universities and public health and education contacts at local councils; approaching academy trust chief executives; involving local school nursing teams; advertising through local authority networks; approaching eligible schools through letter, e-mail or phone call; and through head teachers recommending the trial to other schools.
Members of the LRTs met with interested schools and provided information describing what the school’s participation in the trial would involve, including the procedures for distributing participant information resources, gaining consent, delivering the CBS and collecting data at the baseline and follow-up time points. Interested schools were asked to sign an Agreement to Participate Form and a Data Sharing Agreement to confirm their involvement in the trial. At recruitment, schools were informed that they would receive £500 after baseline testing was completed and £500 after the final follow-up to cover any administrative costs associated with being involved in the trial.
Participant recruitment
Pupils
Pupils from Years 7 and 8 (England and Wales) and S1 and S2 (Scotland) were recruited from participating schools. These year groups were chosen purposefully to minimise disruption to English and Welsh General Certificate of Secondary Education (GCSE) and Scottish Qualifications Authority National 5 exam years; and also to confine final follow-up to within the school setting to avoid the need to follow participants to further education settings.
The LRTs delivered information sessions, typically within assemblies, in each school to pupils in Years 7/S1 and 8/S2. BRIGHT trial information packs were then distributed to the parents/carers of all pupils in participating classes in these year groups (in most cases this was all classes in the year) via post, or by sending them home with pupils. Information packs contained a cover letter signed by the school head teacher, a parent/carer information sheet, parent/carer opt-out form and a copy of the pupil information sheet and consent form. Parents/carers could decline their child’s participation by completing and returning the opt-out form to their child’s school within a 2-week opt-out window. Schools were requested to record which pupils had been opted out on a spreadsheet. If parents/carers did not return an opt-out form within the 2-week window, it was assumed they were happy for their child to decide themselves if they would like to participate. Parents/carers could withdraw their child at any point over the trial.
Eligible pupils, whose parents/carers had not opted them out of the trial, were subsequently invited to consent to take part in the trial. These pupils were provided with a pupil information sheet and were asked to complete a consent form if they were happy to take part. Schools were requested to do this within class or form time in a dedicated consent session and LRTs offered to deliver or facilitate these sessions. As the information session would have taken place at least 2 weeks prior to the consent session (i.e. before the parent/carer opt-out window), pupils were able to consent to take part within the consent session. Schools were requested to make additional pupil information sheets and consent forms available for any pupils who were absent on the day of the consent session or who wanted more time to consider participation.
The school, supported by the LRT, collected and checked completed consent forms and updated their spreadsheet to record which pupils had consented to take part, ensuring that a parent/carer opt-out form had not been received for each consenting pupil. Completed consent forms for pupils whose parents/carers had not opted them out were collected via courier and returned to the YTU where the trial team checked whether all consent forms had been completed correctly.
Pupils were also asked to complete a contact form in order to provide their mobile telephone number and to indicate their text message preference times and preferred name to be used in the text messages should they be in the intervention group. If they did not own their own mobile telephone or could not provide their own mobile telephone number, they were considered ineligible for participation in the trial. For the internal pilot phase, we requested for the contact form to be completed at the consent session and returned with the consent forms; in the main phase of the trial, the contact form was completed at the time of baseline data collection to allow LRTs to provide greater assistance to pupils in the completion of these forms and reduce errors.
Parents/carers of participating pupils
All parents/carers of participating pupils were invited to complete resource use questionnaires59 to provide data for the health economic evaluation. These were either posted to parents’ home addresses or sent home with the pupils from school.
Retention
Schools
Each school was asked to nominate a lead contact and member of administrative staff with whom the LRTs liaised closely throughout the trial to try to pre-empt and troubleshoot any problems and maximise retention. In addition, newsletters were issued on a yearly basis over the trial period. Within the final newsletter, delivered prior to the 2.5-year follow-up, schools were reminded that they could claim £500 at the end of the data collection period to cover any administrative costs associated with being involved in the trial.
Participants
The BRIGHT Youth Forum, run by a charity called Children and Young People’s Empowerment Project (Chilypep) whose lead (Lesley Pollard) was one of the co-applicants for BRIGHT, proposed a variety of methods to optimise retention, response rates and completion rates. These included prize draws for shopping vouchers, trial-branded merchandise or ‘freebies’ (such as pens, stickers and pencils), thank you vouchers, using the school’s house-point system to encourage engagement and having more senior school pupils as Research Champions to provide peer support.
All pupils who completed the baseline questionnaire and dental assessment were given a £10 voucher as a thank you. All pupils who completed the final follow-up questionnaire and dental assessment were given a £5 voucher as a thank you. Pupils received trial-branded merchandise such as pens during data collection activities in the trial.
All parents/carers who completed and returned parent/carer questionnaires were entered into a prize draw with the chance of winning £300 in vouchers (with one annual prize draw each for the internal pilot phase and the main phase).
Withdrawal procedure
Pupils or their parents/carers were able to fully withdraw from the BRIGHT trial (i.e. no longer be involved in any further data collection or receive any further intervention delivery) at any point over the trial by letting a member of either the LRT or dental team know (e.g. when they visited the school), telling their school or via contacting the research team at YTU. For participants who fully withdrew or could no longer be followed up (e.g. due to leaving the school), data already collected were retained and used in the analysis (based on current Health Research Authority guidance in relation to the General Data Protection Regulation).
At each data collection point, if pupils did not want to take part in the dental assessment or complete pupil questionnaires, they did not have to. However, such pupils were only fully withdrawn from BRIGHT if they explicitly stated that they did not want to take part in the trial anymore, otherwise they were approached again at the next data collection time point.
Sample size
Internal pilot phase
For the internal pilot, the sample size was calculated in order to address the following progression criterion:
-
an indication of a positive effect of the intervention on self-reported frequency of toothbrushing at 12 weeks using an 80% one-sided CI approach.
As randomisation for this trial occurred within schools at the year-group level, year groups within schools acted as the ‘clusters’. At least four clusters per arm are recommended for cluster pilot RCTs. 67 It was determined that 1200 pupils from 10 schools [equivalent to approximately 284 young people in an individually randomised trial, assuming 60 recruited pupils per year group, 20% attrition at follow-up and an intracluster correlation coefficient (ICC) of 0.02 (see justification in next section)] would be sufficient to produce an 80% one-sided CI that excluded a 5% difference in the event of a zero or negative effect of the BRIGHT intervention on self-reported toothbrushing at the 12-week follow-up, assuming 66% reported brushing twice daily in each of the two groups. 68,69 A trial of this size would also allow a participation rate of 50% and a completion rate of 80% to be estimated within a 95% CI of ± 6% and ± 5%, respectively. 70 We therefore aimed to recruit 1200 pupils across 10 schools, an average of 60 pupils per year group (Year 7/S1 and Year 8/S2) per school, into the internal pilot.
Full trial (internal pilot and main phase)
In 2013, the estimated proportion of UK 12-year-olds with caries was 34%. 7 The definition of caries here is described as ‘obvious decay experience’, which incorporates untreated decay into dentine and decay that has previously been subject to restorative treatment (fillings) or tooth extraction. Based on a systematic review of interventions to increase the frequency of toothbrushing for caries prevention, a reduction of caries prevalence of 8% might be expected. 71 An individually randomised trial powered at 90% (5% two-sided α) to detect an 8% absolute reduction, from 34% to 26%, in caries would require 1376 pupils. Few estimates of school-level ICC are available for dental data. In a previous study evaluating a behaviour change programme for preventing dental caries in primary schools, an ICC of 0.01 was used, which was estimated using their own unpublished data. 72 It was agreed that the present trial would use a more conservative ICC of 0.02.
Assuming partial contamination effects (i.e. those contaminated gain half the treatment benefits) for 27% of the control group (based on findings from the internal pilot), this trial required 42 schools in total across the internal pilot and main phase of the trial, assuming within-school (year-group level) randomisation, an average of 60 pupils per year group per school, an ICC of 0.02 and 20% attrition at follow-up. This would give 90% power (5% two-sided α) to detect an 8% absolute difference, from 34% to 26%, in the proportion of pupils with ‘obvious decay experience’. Therefore, this trial aimed to recruit a total of 42 schools and 5040 pupils across the internal pilot and main phase of the trial.
Randomisation
Allocation took place within schools by randomising schools 1 : 1 to one of two regimes: (1) pupils aged 11–12 years (Year 7 in England and Wales/S1 in Scotland) to receive the intervention and pupils aged 12–13 years (Year 8 in England and Wales/S2 in Scotland) to act as the control group; or (2) pupils aged 12–13 years (Year 8 in England and Wales/S2 in Scotland) to receive the intervention and pupils aged 11–12 years (Year 7 in England and Wales/S2 in Scotland) to act as the control group.
An allocation sequence, stratified by school using blocks of size two, was generated by an independent YTU statistician. Once all baseline assessments were complete for a school and the assessment paperwork had been received by YTU, the year groups in that school were randomised by allocating them to the next available block in the sequence in the order Year 7/S1 then Year 8/S2. The statistician then informed the relevant members of the research team of the school’s year group allocation and they disseminated this to the school.
Blinding
Given the nature of the intervention, it was not possible to blind schools or pupils to their group allocation; however, clinical examinations for the outcome assessments were performed by a trained and calibrated dentist/dental therapist who was blind to the allocation of the pupils, as far as possible. We aimed to minimise the risk of the dental assessors becoming unblinded by asking pupils not to discuss the intervention they received with the assessors. Dental staff were asked to record whether or not they were unblinded to each pupil’s randomisation group during the assessment. Researchers and trial team members, including the trial statistician and health economist, were not blinded to group allocation.
Intervention
The commissioning brief required a ‘digital behaviour change programme’ and specified a programme which ‘initiates good oral health practice followed by a series of text or other media messages to change behaviour and promote tooth brushing’. The intervention evaluated was developed according to this brief and based on the KOB study described in Chapter 1, which was referenced in the brief. 56 The KOB intervention was refined to strengthen the behaviour change techniques employed and through input from school staff and young people tailored to be appropriate for pupils aged 11–14 years in secondary schools in the UK. The intervention development drew on the Health Action Process Approach and was informed by the Behaviour Change Wheel. 73,74 The intervention consisted of two components: (1) a CBS delivered by teachers and embedded in the school’s curriculum followed by (2) a series of text messages to pupil’s mobile phones. 75 The logic and causal models for the intervention are included in Appendix 3. Further details of the refinement of the intervention are reported elsewhere. 75
Schools were requested to deliver the CBS to all classes in the year group allocated to the intervention arm, regardless of whether the pupils had completed a BRIGHT consent form. Text messages were only sent to pupils in the intervention year group who completed a BRIGHT consent form and were considered as part of the randomised sample. Year groups allocated to the control arm received routine education but neither the CBS nor the text messages.
Classroom-based session
The CBS was developed by the School of Education and Social Work at the University of Dundee and the research team to be appropriate for the curricula as part of personal, social health, and economic education (PSHE) (England) and personal and social education (Scotland and Wales). The lesson plan was developed using the curriculum guidelines for: Science Key Stage 3 (a) and 4 (b);76,77 PSHE study Key Stage 3,78 the Scottish Curriculum for excellence experiences and outcomes for both health and well-being (a) and science (b)79,80 and the Welsh Personal and Social Education framework. 81
Teachers delivered the 50-minute CBS in the school environment. The schools received a lesson plan (which outlined the learning intentions and success criteria for the lesson – see Report Supplementary Material 1) and pupil-facing materials in advance of teaching the lesson. The pupil-facing materials included a young person booklet, an effective toothbrushing video, photographs on PowerPoint slides, post-it notes (not provided by the trial team) and a young person toothbrushing factsheet (see Report Supplementary Materials 2–4). All CBS materials and resources were provided to schools as digital copies only.
The CBS contained the following elements:
-
Helping pupils establish the motivation to brush twice daily for:
-
social reasons – interpersonal considerations of having a ‘fresh and clean feeling’ when interacting with others
-
health reasons – toothbrushing prevents tooth decay and gum disease
-
appearance reasons – to stop teeth looking discoloured.
-
The literature and Patient and Public Involvement and Engagement (PPIE) activities suggested these were key motivating reasons for young people to brush their teeth.
-
Encouraging pupils to ‘own the goal’ of twice-daily toothbrushing so they want to brush twice daily for themselves, not just when parents/carers remind them.
-
Developing pupil’s toothbrushing skills and the intention to brush effectively twice daily with a fluoride toothpaste.
-
Discussing the ‘when’ and ‘where’ of toothbrushing and ways to overcome barriers to toothbrushing.
Text messages
The content of the intervention text messages used young people’s own words developed through the workshops and BRIGHT Youth Forum to remind and reinforce the messages from the CBS. The text messages were delivered to mobile phones via TextApp, a software tool developed by the Health Informatics Centre (HIC), University of Dundee. TextApp has been successfully adopted in a number of behaviour change interventions which targeted alcohol and obesity. 82,83
The message schedule (see Report Supplementary Materials 5 and 6) and any personalisation were programmed into the TextApp delivery system, which also handled replies and delivery monitoring. The minimum data set required was stored, that is, phone number, the preferred name specified by the pupil for text messages to be addressed to, each pupil’s preferred timings for the twice-daily text messages (weekdays: 7 a.m. or 7.30 a.m., and 9 p.m. or 9.30 p.m.; weekends: 8.30 a.m. or 9 a.m., and 9.30 p.m. or 10 p.m.) and any responses a pupil sent to the BRIGHT text messaging intervention number.
Text messages were triggered by YTU using TextApp for participating pupils in the intervention year group in each school shortly after the school had provided confirmation that they had delivered the CBS. When mobile phones first became widely used, people tended to change their number whenever they changed, lost or damaged their phones or switched supplier. However, it is now possible and relatively easy to keep the same number in all these cases and it is much more common for people to have the same number for many years. We therefore anticipated the loss of participants due to changes in mobile phone number being lower than in previous studies. However, to help mitigate this, participants were reminded to inform the research team of any changes to their mobile phone number by texting the dedicated BRIGHT text messaging intervention number. Reminders were also issued through the school at the time of engagement in any trial-related activity such as questionnaires and clinical examinations.
Replies received were monitored by the research team and any updates were managed through the TextApp monitoring website. When pupils wanted to stop receiving text messages, they could text STOP for free at any time. Messages were stopped as soon as reasonably possible. Messages sent to the BRIGHT text messaging intervention number were monitored for safeguarding purposes and messages could be restarted if a participant indicated that this was their wish. For participants who requested text messages to be stopped, we assumed continued participation in the trial (based on original consent and current Health Research Authority guidance in relation to the General Data Protection Regulation); therefore, we retained and used data already collected and continued to collect follow-up data.
Outcomes
Primary outcome
Caries prevalence for obvious decay experience (D4–6MFT) at 2.5 years
Caries assessments were completed using the International Caries Detection and Assessment System (ICDAS). 84 The ICDAS was used to evaluate each surface (n = 5; mesial, occlusal, distal, buccal, lingual) of a tooth [up to n = 32, though this includes four third molars (i.e. wisdom teeth) that were unlikely to have erupted in pupils of this age] via a two-digit coding system: a measure of the restorative status of each surface of the tooth (assigned one of nine numbers, 0–8, where 0 indicates a surface that has not been restored or sealed); and a measure of the extent of any carious lesion(s) present on the surface (assigned one of seven numbers, 0–6,84 where 0 indicates no carious activity). There were also four codes that could be assigned for all tooth states: 96, 97, 98 and 99. A full breakdown of the ICDAS scoring codes is provided as an appendix to the trial protocol. 59 The ICDAS scoring system also allows the components of ICDAS to be collapsed to give a DMFT equivalent (ICDAS caries codes 4–6 indicate caries into dentine) score and therefore can be compared with studies using traditional caries indices, which record decay at the level of dentine.
If both primary and permanent teeth were visible at a single site, the assessor was asked to only score the permanent tooth. The primary outcome was the presence of at least one treated or untreated carious lesion in any permanent tooth, measured at the pupil level during the 2.5-year clinical assessment using DICDAS4–6MFT where:
-
Decay was measured as carious lesions extending into dentine – ICDAS levels 4–6,84 that is, on any surface, the caries code was 4, 5 or 6, regardless of the associated restoration code. The surface and/or tooth was counted as decayed if the restoration code was 8 regardless of the caries code.
-
Missing included any tooth extracted due to caries.
-
Filled included any restoration but not an obvious pit or fissure sealant, that is, the restoration code was between 3 and 7 and the caries code was 0, 1, 2 or 3.
This was also considered as a secondary outcome at the 2-year time point for schools that were recruited during the pilot phase only (this assessment could not be conducted for schools recruited in the subsequent academic year as clinical examinations were affected by the COVID-19 pandemic).
Secondary outcomes
Caries prevalence for all carious lesions (DICDAS1–6MFT)
The presence of at least one treated or untreated carious lesion in any permanent tooth, measured using DICDAS1–6MFT where:
-
Decay was measured as any enamel or dentinal caries – ICDAS levels 1−6,4 that is, on any surface, the caries code was 1–6, regardless of the associated restoration code. The surface tooth was also counted as decayed if the restoration code was 8 regardless of the caries code.
-
Missing included any tooth extracted due to caries.
-
Filled included any restoration but not an obvious pit or fissure sealant, that is, the restoration code was between 3 and 7 and the caries code was 0 (only).
This was measured at baseline, 2 years (internal pilot only) and 2.5 years.
Number of carious teeth
The number of permanent DICDAS4–6MFT and DICDAS1–6MFT at 2 years (pilot schools only) and 2.5 years.
Frequency of toothbrushing
Pupils were asked the question ‘How often do you usually brush your teeth?’ on questionnaires at baseline, at the time of the CBS (internal pilot only) and at 12 weeks post CBS (internal pilot only), 6 months, 1 year, 2 years (internal pilot only) and 2.5 years. This is a validated question from the national CDHS 2013. 7 Self-reported toothbrushing is considered to be a reliable measure of toothbrushing behaviour85 in epidemiological studies and was the primary outcome of the KOB study mentioned in the NIHR HTA commissioning brief. 56 Response options were: ‘Never’, ‘Less than once a day’, ‘Once a day’, ‘Twice a day’, ‘Three times a day’ and ‘More than three times a day’. The categories ‘Never’ to ‘Once a day’ were combined, as were the categories ‘Twice a day’ to ‘More than three times a day’, to consider the proportion of pupils who reported brushing their teeth at least twice a day. This categorisation is based on national guidance. 86
In addition to questions on the frequency of toothbrushing, questions were included to examine the determinants of toothbrushing behaviour, specifically the motivational and volitional factors from the causal model, including self-efficacy, attitude (social norms, outcome expectancy and risk perception), intention, coping planning and action planning. Questions were adapted from those used previously in the literature and asked at baseline, 1 year, 2 years (pilot phase only) and 2.5 years. 87,88
Dental plaque and bleeding gingivae
Clinical measurement of levels of dental plaque on teeth and gingivitis followed national protocols established for dental epidemiology. 86 Plaque levels were assessed using the Turesky Modification of the Quigley Hein Plaque Index. 89,90 Plaque scores were given for all buccal (n = 14) and palatal (n = 14) surfaces of the upper arch, and buccal (n = 14) and lingual (n = 14) surfaces of the lower arch. A plaque score for the entire mouth was determined by summing the surface codes (0 = no plaque to 5 = plaque covering two-thirds or more of the crown of the tooth) and dividing this total score by the number of surfaces (a maximum of 4 × 14 = 56 surfaces) examined.
The degree of gingival inflammation was assessed using a modification of the Gingival Index of Löe91 and mean number of bleeding gingival sites per child. The gingival index was recorded using an approach that has been validated in young people as a replacement for full mouth recordings. 92 These clinical measures were carried out at baseline, 2 years (internal pilot only) and 2.5 years.
A gingival bleeding score was recorded using a periodontal probe on the buccal and lingual/palatal sites of each of the eight index teeth (16, 26, 36, 46, 12, 11, 32, 31; maxillary right and mandibular left first molars, maxillary left and mandibular right first premolars and maxillary left and mandibular right central incisors). Each index tooth scored 1 if gingival bleeding was present, 0 if not and X if the index tooth was missing.
A total bleeding score was obtained by adding the individual bleeding scores and dividing by the number of scorable sites (maximum 16, excluding missing teeth). The sum of the number of teeth associated with bleeding gingivae (i.e. bleeding present at one or both sites of the tooth) was also calculated.
These measures of plaque and gingivitis were used as surrogate outcomes to assess the impact of toothbrushing.
Health-related quality of life
Health-related quality of life was pupil reported using the Child Health Utility 9D (CHU9D),93,94 which consists of nine dimensions (worried, sad, pain, tired, annoyed, schoolwork/homework, sleep, daily routine and activities), each represented by a single question with five response options. Each response is associated with its own weighting, with participant responses to all questions combined to produce an overall HRQoL score on a scale anchored on 0 and 1 (which represent ‘death’ and ‘full health’, respectively). 94 The recall period was today/last night. This was measured at baseline, and at 1 year, 2 years (internal pilot only) and 2.5 years.
Oral health-related quality of life
Child oral HRQoL was assessed using the Caries Impacts and Experiences Questionnaire for Children (CARIES-QC),95 a measure of the impact of caries validated in children and young people aged 5–16 years, at baseline, and at years 1, 2 (internal pilot only) and 2.5. CARIES-QC contains 12 items and one global question. The items are scored on a 3-point Likert scale from 0 to 2, with a higher score indicating increased impact (possible total score range, 0–24). As CARIES-QC focuses on attributes which are not directly measurable, the raw score is only indicative of a rank along the scale. In order to use the raw score to accurately measure change, conversion to an interval level scale is required. This can be achieved by transforming the ordinal score to a logit score. 18 Both raw and interval scores were summarised at each time point to allow comparison with other studies; however, only raw scores were used in the hypothesis testing to compare the intervention and control groups at the different time points.
The global item is not included in the calculation of the total score and was summarised separately. A preference-based measure was constructed using five items from the CARIES-QC. The preference weights generated from adolescents were used to calculate quality-adjusted life-years (QALYs) anchored on a 0 to 1 scale for the cost-effectiveness analysis. 96
School attendance
Impact on school attendance was measured by asking schools to provide the past and current year’s attendance record for each participating pupil at baseline, 1, 2 (internal pilot only) and 2.5 years post CBS. However, this proved difficult for schools and was only provided by a small number of them; therefore, the decision was made instead to request average school attendance at an aggregate level, by year group, for each academic year that the school had been involved in the trial as this was easier to obtain.
Other data collected
Oral health behaviours
Self-reported oral health behaviours were assessed at baseline within the pupil questionnaire using questions from the national CDHS7,86 on diet, use of dental services and other forms of fluoride use which allowed assessment of confounding.
Participants were asked at baseline to report the frequency they consumed cariogenic foods/drinks [cakes or biscuits, sweets or chocolate, cola or squash (not diet or non-sugar), fruit juices and smoothies and energy (sport) drinks (e.g. Powerade, Lucozade)]. These were scored 0 = ‘Never’ to 5 = ‘Four or more times a day’. A summary cariogenic score was calculated by summing these, dividing by the total possible score N, where N = 5 * the number of completed items, and multiplying by 100.
Availability of toothbrushes and toothpaste
Questions on toothbrush and toothpaste availability were included on pupil questionnaires at baseline and 6 months as part of the pilot phase as these resources were necessary for the implementation of the intervention.
Determinants of toothbrushing behaviours
As per the intervention’s causal model, questions on motivational and volitional factors influencing toothbrushing (self-efficacy, attitude, intention and coping and action planning) were included at all time points. Self-efficacy was assessed by the item ‘I know how to brush my teeth properly’, coping planning by ‘I have a plan of how I will make myself brush when I find myself not brushing properly’ and intention by ‘How often do you want to brush your teeth?’ A scale for attitude was generated by taking the mean of responses to the following six items: ‘If I brush my teeth twice every day, then my teeth will look clean when talking to friends’, ‘If I brush my teeth twice every day, then my teeth will be healthy’, ‘If I brush my teeth twice every day, then my teeth will feel good’, ‘If I don’t brush my teeth twice every day, I risk getting tooth decay’, ‘If I don’t brush my teeth twice every day, I risk my teeth looking dirty’ and ‘If I don’t brush my teeth twice every day, I might have bad breath’. A scale for action planning was generated by taking the mean of the items ‘I know where and when I will brush my teeth in the morning’ and ‘I know where and when I will brush my teeth in the evening’. All items had responses 1 = Not true at all, 2 = Not true, 3 = True and 4 = Definitely true, except for the intention item, which had six responses ranging from ‘Never’ to ‘More than three times a day’.
Contamination
A question, adapted from the national CDHS, was used in the trial to estimate contamination in the control group and was collected from all pupils at 12 weeks and 6 months, during the pilot phase of the trial only. Pupils were asked ‘Have you received helpful information about how to keep your teeth and mouth healthy from any of these places?’ with 10 possible sources listed, including ‘a lesson in school’, ‘friends in another year group’ and ‘text messages’. Data from this question were used to investigate the likely impact of contamination of the intervention in the control group to help determine the feasibility and efficiency of continuing with within-school randomisation for the rest of the trial, rather than switching to randomisation at the level of the school. This was considered as part of the review of progression criteria following completion of the internal pilot (see Appendix 6 for further details).
Orthodontic appliances
As part of the 2.5-year dental assessment, dental teams recorded whether the pupil was wearing a fixed or removable orthodontic appliance. This allowed identification of instances where a fixed orthodontic appliance was worn during the assessment, which may affect the ability to record the ICDAS codes, and confirmed that pupils wearing removable orthodontic appliances were asked to take them out during the examination.
Data collection
Data were collected via dental assessments, pupil-completed questionnaires and parent/carer-completed questionnaires. 59 In addition, schools provided sociodemographic and school attendance data electronically, and information about participating schools was collected from publicly available sources. Procedures for data collection are outlined below. Data collection time points are outlined in the trial design diagram in Figure 1 and in Table 1.
| Baseline | CBS (internal pilot only)a | Between CBS and 12 weeks (internal pilot only)a | 6 months | 1 year | 2 years (internal pilot only) | 2.5 years | |
|---|---|---|---|---|---|---|---|
| Dental assessment | |||||||
| Caries (ICDAS) | ✓ | ✓ | ✓ | ||||
| Dental plaque score | ✓ | ✓ | ✓ | ||||
| Gingival bleeding score | ✓ | ✓ | ✓ | ||||
| Pupil questionnaire measures | |||||||
| Self-reported toothbrushing frequency and factors influencing toothbrushing behaviour 13 questions |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Oral HRQoL (CARIES-QC) 13 questions |
✓ | ✓ | ✓ | ✓ | |||
| HRQoL (CHU9D) 9 questions |
✓ | ✓ | ✓ | ✓ | |||
| Toothbrush/paste availability 2 questions |
✓ | ✓ | |||||
| Oral health behaviours 5 questions |
✓ | ||||||
| Contamination 1 question |
✓ | ✓ | |||||
| Parent/carer questionnaires | |||||||
| NHS resource use | ✓ | ✓ | ✓ | ✓ | |||
| Pupil demographics | |||||||
| FSM eligibility | ✓ | ||||||
| Sex | ✓ | ||||||
| School attendance | ✓ | ✓ | ✓ | ✓ | |||
| Deprivation | ✓ | ||||||
Dental assessments
Dental assessments were carried out in participating schools, under standard dental epidemiological data collection conditions, at baseline, 2 (internal pilot only) and 2.5 years following CBS delivery. LRTs each put together dental teams, consisting of at least a dentist/dental therapist and dental nurse, for each dental assessment time point. Each child’s dental assessment consisted of a caries assessment, taking around 10 minutes, and a plaque and gingivitis assessment, taking around 5 minutes. All dental assessment data were recorded on paper case report forms. 59
Training and calibration
Prior to the start of baseline data collection for the internal pilot, a hands-on training and calibration event was run in a school with an experienced dental epidemiologist for all dental team members. Prior to baseline data collection for the main phase, another training and calibration event was held for new dental team members and update training/recalibration was provided for those who attended the initial training and calibration event. Update training/recalibration was also provided for all dental team members prior to the 2-year follow-up (for the internal pilot) and prior to the 2.5-year follow-up. Details of the training and calibration can be found as an appendix to the trial protocol, and in Appendix 4. 59
Reproducibility
At each dental assessment time point, where time constraints allowed, dental teams completed a second ICDAS assessment for 5% of participating pupils at each school to assess intra- and interexaminer reproducibility. For plaque and gingivitis assessments, the initial examination disturbs plaque and probing and can increase susceptibility to gingival bleeding so, as is standard practice, there was no reproducibility measured for these outcomes.
Pupil questionnaires
The time points for pupil questionnaire completion are given in Table 1. At dental assessment time points [baseline, 2 years (internal pilot only) and 2.5 years post CBS delivery], LRTs requested pupils to complete the pupil questionnaire either before or just after completing their dental assessment and were on hand to encourage questionnaire completion and answer any queries. At other follow-up time points, pupil questionnaires were distributed and collected by school staff or LRTs, and schools were requested to allow pupils approximately 10 minutes of class time for questionnaires to be completed. School staff and LRT members recorded reasons for non-completion of questionnaires. All pupil questionnaires were completed on paper case report forms59 and were returned to YTU via courier.
The measures included within the pupil questionnaire at each time point are summarised in Table 1. More details about the measures are provided in the Outcomes section and copies of each questionnaire can be found on the NIHR project web page. 59
Parent/carer questionnaires
For the health economic evaluation, parents/carers of participating pupils were asked to complete a resource use questionnaire at baseline, 1, 2 (internal pilot only) and 2.5 years post CBS delivery. Parent/carer questionnaires59 were handed to pupils at the time they completed their pupil questionnaire (apart from the baseline time point for the internal pilot) and they were asked to take these home to their parents/carers to complete. The questionnaire was enclosed in an envelope along with a cover letter and a freepost envelope, which parents/carers were requested to use to return the completed questionnaire via post to YTU. Approximately 2 weeks after parent/carer questionnaires were distributed (excluding the baseline time point for the internal pilot), schools were provided with a second copy of the questionnaire to distribute in order to try to maximise response rates. For the internal pilot, baseline parent/carer questionnaires,59 along with a cover letter and freepost envelope, were posted to parents/carers who had returned a consent form and shared their name and address so questionnaires could be posted directly to them.
Sociodemographic characteristics and school attendance data of participants
At baseline, schools were also asked to complete and return an encrypted spreadsheet to YTU, via the University of York DropOff secure data transfer service, in order to provide the following for each consenting pupil: sex, year group, form group, current eligibility for FSM, past and current year’s school attendance, and home postcode [to facilitate data linkage for the economic evaluation and for calculation of Index of Multiple Deprivation (IMD) scores]. Participating schools were also asked to provide the past or current year’s attendance record for each participating pupil at 1, 2 (internal pilot only) and 2.5 years post CBS. Average year group attendance rates were also requested after final data collection for all previous academic years within which the BRIGHT trial had been active.
School-level data were also captured at recruitment, from publicly available sources, on the proportion of children eligible for FSM. 64,97–99 Publicly available school inspection ratings (i.e. Ofsted for schools in England, Estyn for schools in Wales and Education Scotland for schools in Scotland) were also obtained. 64,100,101
Adverse events, suspected pathology and child safeguarding
Due to the nature of participant involvement, no serious adverse events or adverse events were anticipated that would be unexpected and related to being in the trial. However, a procedure was described in the protocol58 to capture and report any complications associated with the trial raised by participants, parents/carers or dental assessors. Any suspected serious pathologies were also recorded and a procedure put in place to enable a referral to a relevant dental service. A procedure was also established to deal with child safeguarding issues to ensure the young person’s school and parent/carer could be informed and any further action taken.
Statistical analysis
Analyses were conducted in STATA v17 (StataCorp, 4905 Lakeway Drive, College Station, TX). Outcomes were analysed collectively after follow-up had been completed in all schools. Data from pupils recruited during the internal pilot and the main trial phase were combined for analysis. Analyses followed the principles of available case intention to treat (ITT) with participant’s outcomes analysed according to their original, randomised group, where data were available, irrespective of deviations based on non-compliance. Statistical tests were two-sided at the 5% significance level and 95% CIs were used.
A Consolidated Standards of Reporting Trials (CONSORT) diagram depicts the flow of schools and pupils through the trial. The number of schools and pupils approached, eligible and randomised are presented, with reasons for non-participation at each stage provided where known.
Characteristics of the participating schools are presented to provide the context to the intervention delivery. All participant baseline data are summarised descriptively by randomised group both as randomised and as analysed in the primary analysis (the available case population). No formal statistical comparisons were undertaken on baseline data. Continuous measures are reported as means and standard deviations (SDs) and categorical data as counts and percentages.
Participants were free to withdraw from the intervention (intervention group only) and/or from data collection at any point. For participants in the intervention group, they could request at any time that their text messages cease by replying ‘STOP’. Pupil withdrawals are summarised by type and randomised group.
Intervention implementation is summarised, including whether, when and to whom the schools delivered the CBS and number of text messages sent and delivered. Time to final text message delivered is presented on a Kaplan–Meier curve.
The number and percentage of completed follow-ups are presented by randomised group and according to the time point for each type of follow-up. Reasons for missing follow-up are provided, where known (e.g. absent from school on day of assessment, no longer at the school, declined). The time of the completion of follow-up from the time of the CBS at the school is summarised.
Data summaries
All data collected at follow-up (both dental and from the pupil questionnaires) are summarised by randomised group and overall. This includes the primary and secondary outcomes that were formally analysed, and also data not included in hypothesis tests such as toothbrush/paste availability, influences on toothbrushing and contamination. The following pupil-level dental outcomes are summarised descriptively for each time point:
-
presence of obvious decay experience in at least one permanent tooth as measured by DICDAS4–6MFT
-
total number of permanent teeth assessed
-
total number of DICDAS4–6MFT
-
number of teeth extracted due to caries
-
number of decayed permanent teeth based on ICDAS 4–6 definition, that is, number of teeth whose highest surface caries severity code is 4–6 or restoration code is 8
-
number of filled permanent teeth based on ICDAS 4–6 definition, that is, number of teeth whose lowest surface caries severity code is 0–3 and the restoration code is 3–7
-
presence of decay experience in at least one permanent tooth as measured by DICDAS1–6MFT
-
total number of DICDAS1–6MFT
-
number of decayed permanent teeth based on ICDAS 1–6 definition, that is, number of teeth whose highest surface caries severity code is 1–6 or restoration code is 8
-
number of filled permanent teeth based on ICDAS 1–6 definition, that is, number of teeth whose lowest surface caries severity code is 0 and the restoration code is 3–7
-
dental plaque score
-
gingival bleeding score
-
number of teeth where there were sites with gingival bleeding
-
presence of an orthodontic appliance, by type, and for pupils with a removable orthodontic appliance, whether this was removed during the assessment
-
whether blinding of the dental assessor to the child’s group allocation was maintained.
We also summarise the following, based on comparing baseline and 2.5-year dental assessments:
-
the number (%) of pupils who moved from ‘caries negative’ at baseline to ‘caries positive’ at 2.5 years (for both DICDAS1–6MFT and DICDAS4–6MFT definitions)
-
the number (%) of pupils who developed new carious lesions over this time, defined by an increase in total number of DICDAS1–6MFT/DICDAS4–6MFT
-
mean caries increment using the individual surface scores to do the calculations (dmft_surface_1@2.5 years – dmft_surface_1@baseline), where the individual surface scores are 1 (positive for caries, based on DICDAS1–6MFT/DICDAS4–6MFT definitions) or 0 otherwise. Therefore, increments for each surface can take values of −1, 0 and 1, where −1 indicates reversal from carious to sound, 0 no change in surface status and 1 change from sound to carious surface. A maximum of 120 surfaces were assessed per pupil. The total caries increment was calculated as the sum of these individual increments, treating reversals in the following three different ways:
-
a net caries increment on a surface level – leave the reversals as is when calculating the caries increment
-
a crude caries increment on a surface level – summing only the positive increments and ignoring any reversals
-
consider that the negative reversals have been erroneously coded and replace instances where the total caries increment (all surfaces combined) was negative with a zero increment.
-
Changes from the protocol
In the published trial protocol, the proposed method of analysis of dental outcomes involved regression models that accounted for repeated measures, that is, the dental outcomes at 2 and 2.5 years. Similarly, for self-reported twice-daily toothbrushing, a repeated measures binary logistic model incorporating 6 months, 1, 2 and 2.5 years was planned (not including earlier time points since these were for pilot schools only). However, a subsequent trial amendment removed the 2-year dental assessment for main trial schools, and, at this point, it was clear that very few main trial schools had been able to provide data at 1 year due to school closures caused by the COVID-19 pandemic. Therefore, a repeated measures approach was deemed no longer appropriate nor necessary, and it was detailed in the statistical analysis plan (SAP) (reviewed and approved by the TMG, TSC and DMEC prior to the 2.5-year data collection) that analyses would largely take the form of separate (cross-sectional) regression models for the outcomes at relevant time points, as detailed below. The principle and output of the two approaches are the same, to provide a comparison of the outcome at a specific time point between groups, so this does not reflect a fundamental change in the primary end-point analysis.
Primary analysis
The primary analysis compared the proportion of pupils with any presence of obvious decay experience measured by DICDAS4–6MFT at 2.5 years in any permanent tooth, between the intervention and control groups using a binary logistic mixed-effect model. The model adjusted for school year (Year 7/S1 or Year 8/S2) and number of DICDAS4–6MFT (excluding primary teeth) at baseline as fixed effects and school as a random effect. The adjusted odds ratio (OR) from the model was estimated with a 95% CI and p-value. The predicted probabilities in each group and the adjusted risk difference and 95% CI are also presented, so these can be compared with the probabilities assumed in the sample size calculation. This analysis was based on the available case population, including participants who completed both baseline and 2.5-year dental assessments, and had at least one permanent tooth (as opposed to all primary teeth, which was very unlikely). Analysis of the primary outcome was checked and verified by a second statistician.
Sensitivity analyses
Year group as a random effect
A sensitivity analysis was conducted by repeating the primary analysis model including year group as a random effect nested within school to assess the impact of this level of clustering.
Missing data
Pupils were excluded from the primary analysis if they did not complete the dental assessment at both baseline and 2.5 years. We aimed to complete dental assessments in schools as close to the intended due date as possible, provided conditions relating to the COVID-19 pandemic allowed, but in some cases, dental assessments were missing for entire schools. Baseline characteristics of those included and excluded from the primary analysis were compared. We ran a logistic regression to determine if there were any statistically significant associations between baseline variables (allocation, age, sex, FSM status, school year, number of DICDAS4–6MFT, twice-daily toothbrushing, CARIES-QC score, cariogenic score, plaque index and bleeding score) and missingness. Any baseline variables observed as statistically significantly associated with missingness (p < 0.05) were included as a covariate in the primary analysis model in a sensitivity analysis.
Timing of follow-up
All attempts were made to conduct dental assessments as close to their due date as possible, but some were early or late. Follow-up assessments were particularly affected by disruptions and school closures caused by the COVID-19 pandemic. The duration of follow-up is summarised by randomised group and time point. The primary analysis used all data, but a sensitivity analysis excluded pupils whose dental assessments were completed outside of 3 months either side of the average length of follow-up for the 2.5-year time point.
Complier-average causal effect analysis
A two-stage complier-average causal effect (CACE) analysis was performed for the primary outcome using an instrumental variable (IV) approach with randomised group as the IV. Compliance with the intervention was defined at the pupil level in three ways as:
-
Binary (yes/no) – pupil attended the CBS. The pupil was assumed to have attended the CBS if their school provided a register for attendance and they were indicated to have attended. If the school indicated they delivered the CBS but did not provide a register, it was assumed all participating children in the year group allocated to receive the intervention attended the session.
-
Binary (yes/no) – pupil attended the CBS (definition as above) and received at least 50% (n = 7) of the text messages per week for the first 12 weeks (based on HIC data).
-
Continuous – total number of text messages received (based on HIC data).
Subgroup analyses
Deprivation indices
The initial plan was to conduct subgroup analyses taking into account data on deprivation. It was intended that variables for eligibility for FSM (yes/no) and IMD decile (continuous variable) would be added to the primary analysis model, as well as an interaction with randomised group. However, due to the differences in how IMD indices are measured in England, Scotland and Wales, and the level of missing data for these variables, the subgroup analysis based on IMD was not conducted. A subgroup analysis based on FSM status was conducted.
Baseline caries
A subgroup analysis was conducted to evaluate the interaction between the randomised group and the total number of DICDAS4–6MFT in permanent teeth at baseline.
Pilot or main trial school
The average timing of the 2.5-year follow-up was considered likely to be lower among schools recruited in the pilot phase than schools recruited in the subsequent academic year. To assess the impact of this, we conducted a subgroup analysis for the primary outcome in which we repeated the primary analysis including an indicator variable for whether the school was in the pilot or main trial phase of the trial, and an interaction between this factor and the treatment group.
Analysis of secondary outcomes
Caries prevalence for all carious lesions (DICDAS1–6MFT) at 2.5 years
The presence of at least one treated or untreated carious lesion in any permanent tooth, measured at 2.5 years using DICDAS1–6MFT, was analysed as described for the primary outcome.
Number of carious teeth at 2.5 years
The number of permanent teeth with obvious decay experience (DICDAS4–6MFT) measured at 2.5 years was analysed via a mixed-effect negative binomial regression model including school year and number of DICDAS4–6MFT at baseline as fixed effects and school as a random effect. Length of follow-up (in months from baseline to 2.5-year dental assessment) was accounted for as an exposure variable. The adjusted incidence rate ratio (IRR) for the treatment effect was estimated with associated 95% CI and p-value.
The number of permanent teeth with any treated or untreated carious lesions measured at 2.5 years using DICDAS1–6MFT was analysed similarly.
Self-reported frequency of toothbrushing
Self-reported twice-daily toothbrushing at 6 months and 2.5 years was compared between the two groups using separate mixed-effect logistic regression models, adjusting for school year and an indicator for twice-daily brushing at baseline as fixed effects, and school as a random effect. The adjusted OR for the intervention effect was estimated for each model with associated 95% CI and p-value.
Dental plaque, gingival bleeding scores and Caries Impacts and Experiences Questionnaire for Children at 2.5 years
The plaque score was analysed via a mixed-effect linear regression model, adjusting for baseline plaque score and school year, with school as a random effect. The adjusted mean difference in score between the intervention and control groups was estimated with associated 95% CI and p-value. Gingival bleeding and CARIES-QC scores were similarly analysed. Model assumptions were checked using a Q–Q plot to assess the normality of residuals and a scatter plot to assess the scedasticity.
Number of teeth with sites where there was gingival bleeding was analysed using a mixed-effect negative binomial regression model adjusting for school year and number of teeth with bleeding gingivae at baseline, with school as a random effect.
School attendance
Attendance data are summarised by year of follow-up in the trial and by allocation.
Exploratory analysis of pilot trial schools only
Since some of the follow-ups only took place in schools recruited during the internal pilot phase (CBS, 12 weeks, 2 years) or mainly in these schools (at 1 year when few main trial schools responded), analyses, as described above, were replicated but using repeated measures models incorporating all post-randomisation time points and restricted to pupils in pilot schools only. Mixed-effect models were used including allocation, time point, allocation by time point interaction and other baseline covariates as described in the previous sections. School and participant were included as random effects to account for the clustering by school and repeated measures per participant, respectively. Estimates of effect at each time point are provided with a 95% CI and p-value.
Reproducibility
Cohen’s kappa coefficient and percentage agreement were used to measure the intrarater (when the same dentist assessed the child) and inter-rater (when the child was assessed by two different dentists) reliability for the presence of carious lesions for the pupils who were re-examined at each dental assessment time point.
Adverse events, suspected pathology and child safeguarding
The number and type of adverse events, suspected serious pathology and safeguarding issues are summarised by trial arm.
Economic analysis plan
Overview
A cost–utility analysis was conducted in line with current recommendations from NICE. 102 In particular, an NHS and Personal Social Services perspective was adopted for costs, and health benefits were quantified using QALYs. The value of undertaking longer-term cost-effectiveness modelling was assessed, with an appropriate model structure having been identified through a literature review and consultation with experts.
Quality-adjusted life-years were estimated via the trapezium rule using the CHU9D that was reported at baseline and annually thereafter. The CHU9D was valued using the UK tariff values. 94 NHS resource use was measured for each participant at baseline and annually up to 2.5 years. This included all medication costs (e.g. antibiotics) and visits to dental practices for treatment and health services (e.g. dental admission for a general anaesthetic) using the parent/carer resource use questionnaire.
The detailed methods were set out in a health economics analysis plan (HEAP). 59 The HEAP was developed in advance of the health economics analysis being undertaken. The main components of the HEAP relating to the measurement of resource use, unit costs and analysis are summarised below. The items of resource associated with the intervention, together with their source(s) used for costing, are shown in Table 2.
| Item | Source |
|---|---|
| Classroom-based, face-to-face lesson | Trial protocol, information from schools and trial team |
| Text messages | Information from HIC and trial team |
| Messaging infrastructure | Information from HIC and trial team |
| Medication | Parent/carer questionnaire |
| Dental visits and treatments | Parent/carer questionnaire (all regions), administrative data (Scotland only) |
| Type of dental visit | Administrative data (Scotland only) |
| Dental treatments | Trial dental assessments |
| Costs to parents/carers | Parent/carer questionnaire |
There were multiple sources for data relating to dental treatments/visits, and after reviewing the quality of the data sources, it was decided that the trial dental assessment data should be used to estimate resource use in the primary analysis. Sensitivity analyses were to be undertaken using the parent/carer questionnaire data if imputation was deemed appropriate. A subgroup analysis was planned for Scotland using administrative data from Public Health Scotland to take advantage of the availability of those data and recognise the potential impacts of a different dental fee schedule. As the administrative data were not obtained in time for this report, this subgroup analysis will be reported separately.
In order to generate costs from the dental assessment data for the primary analysis, two steps were required. First, the translation of clinical findings to associated procedures (e.g. a missing permanent tooth implied an extraction has taken place); this process is reasonably uncontentious. Second, the translation of procedures into numbers of visits generated a lot more uncertainty. However, after discussion with dentists who have worked in general practice, it was felt that a simple approach could be adopted. By recognising that children do not normally have more than two extractions or four restorations at any visit, we calculated the number of visits as such:
-
In the case of a child having had x extractions, the number of visits for treatment was x/2, rounded up to the next whole number.
-
In the case of a child having had y restorations, the number of visits for treatment was y/4, rounded up to the next whole number.
An alternative approach that used parent/carer questionnaire data was undertaken in a sensitivity analysis.
Unit costs
The unit costs to be used are shown in Table 3 and are at 2020–1 price levels, which represents the most recent year for which earlier costs can be adjusted to using the NHS Cost Inflation Index (NHSCII). Costs derived from previous years were inflated to this level using the NHSCII taken from Unit Costs and Health and Social Care. The individual unit costs for different items of treatment in Scotland were obtained from weighted costs by activities presented in the Statement of Dental Remuneration (SDR) items of service claims for 2019–20 uplifted to 2020–1 prices above. Patient charges used in the sensitivity analyses (Table 4) were obtained from SDR Amendment No. 155 at 2020–1 prices. The full list of treatments for Scotland is presented in Appendix 5, Table 23.
| Item | Value | Source/notes |
|---|---|---|
| Text messages | 4.68p | Cost per participant Price available to the study |
| Band 1 – check-up and simple treatment, e.g. examination, X-rays and prevention advice | £24.90 | Relevant only to England/Wales and based on 1 UDA |
| Band 2 – mid-range treatments, e.g. fillings, extractions and root canal work | £74.70 | Relevant only to England/Wales and based on 3 UDAs |
| Band 3 – includes complex treatments, e.g. crowns, dentures and bridges | £298.80 | Relevant only to England/Wales and based on 12 UDAs |
| Scottish dental costs | Various | Dental statistics – fees and treatments 2021 www.publichealthscotland.scot/publications/dental-statistics-fees-and-treatments/dental-statistics-fees-and-treatments-statistics-as-at-march-2021/ Patient charges based on SDR (No. 155, February 2022, www.scottishdental.org/professionals/statement-of-dental-remuneration/) – See Appendix 5, Table 23 |
| Medications (for NHS and private expenditure) | Various | Prescription costs analysis (2020–1, www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-202021).
|
| Item | Value | Source/notes |
|---|---|---|
| Cost per UDA for societal perspective (2018–9) | £47.04 | A cost including patient charges for the societal perspective of £44.65 per UDA was uplifted 5.36% to 2020–1 price levels using the NHSCII for pay and prices |
| Patient costs for Scotland | Various | See Appendix 5, Table 23 |
| Day case (England and Wales) | £2029 | Activity weighted average of elective day case episodes for dental healthcare resource groups in relation to patients aged under 18 years requiring general anaesthesia. Calculated using National References Costs (2020–1) |
| CBS | £1.85 | This is the cost per participant incurred by schools to deliver the intervention and included in the societal analysis |
| Travel costs and production losses associated with dental treatments | Various | Calculated as the mean of costs derived from all dental visits captured on the patient questionnaires:
|
The derivation of English Unit of Dental Activity (UDA) costs used NHS data pertaining to payments to dental practices in England 2018–9. While more recent data were available, the impact of the COVID-19 pandemic was such that dental activity for 2019–20 and 2020–1 were not thought to be appropriate. We started by identifying data on 8581 practices, for which UDAs and Net Payment to Dental Contract were available (www.nhsbsa.nhs.uk/dental-data/nhs-payments-dentists). Practices that also had non-zero units of orthodontic activity were excluded, to produce 6899 practices. The Net Payment includes several adjustments relating to performance and other activities, and so we have excluded these. Cost per UDA, net of patient charges were calculated for the NHS perspective, and a cost including patient charges for the societal perspective was also calculated. This produced costs of £23.63 per UDA and £44.65 per UDA, respectively. These were then uplifted 5.36% to 2020–1 price levels using the NHSCII for pay and prices.
Analytical approach
Both a within-trial analysis and a longer-term model-based analysis were planned. However, if there were lasting clinical effects, it was deemed that the trial data would be sufficient to assess the cost and health impacts of the intervention. This assessment was based on the presence of two clinical outcomes at 2.5 years that are associated with long-term patient benefits: the primary outcome measure (DICDAS4–6MFT) and the secondary outcome of frequency of self-reported toothbrushing. However, so that we were not too restrictive on the assessment of an observed effect being due to random variation, we used a p-value of 0.1 for our assessment, rather than 0.05.
The within-trial analysis followed published recommendations103 and was undertaken within STATA v17. All costs and benefits were discounted at the rate of 3.5% in line with the NICE reference case. Incremental costs and QALYs were estimated using multilevel modelling to take into account the hierarchical structure of the data. Models for costs and QALYs were estimated separately using maximum likelihood estimation. For costs, bias-corrected and accelerated bootstrapping with 1000 replications was used. 104 For QALYs, normal-based 95% CIs were estimated via regression models with a dummy variable describing the randomised group, a random effect associated with schools adjusting for relevant baseline characteristics. We ran logistic regressions to assess whether any baseline variables (allocation, age, sex, FSM status, number of DICDAS4–6MFT and cariogenic score) were statistically significant predictors of missingness of costs and QALYs. Missing data were imputed using multiple imputation, following published recommendations for economic evaluations. 105
Sensitivity analysis
Deterministic sensitivity analyses were carried out to test the impact of data, assumptions and analysis methods on results. The following deterministic sensitivity analyses were undertaken:
-
A societal perspective was taken with respect to costs that included costs of intervention delivery to schools, private expenditure and production losses related to dental treatment/problems. Time taken away from school was also measured but was not valued.
-
Utilities generated by the Caries Impacts and Experiences Questionnaire for Children – Utility Measure (CARIES-QC-U), which is a condition-specific utility measure generated from the CARIES-QC. 96
-
Alternative time horizons of 5 and 20 years (for model-based analyses that include a long-term effect).
-
Use of parent/carer questionnaire responses for an ITT analysis (with or without imputation, as appropriate).
Probabilistic sensitivity analysis was planned for both the within-trial analysis and the model-based analysis.
Subgroup analysis
Two sets of subgroup analyses were undertaken. The first set provides consistency with the SAP; subgroup analyses were undertaken in relation to eligibility for FSM at baseline and pilot versus main trial schools. The second provides information based on consistent costs of dental treatment, by splitting the full sample into Scotland and ‘Other’. Analyses were undertaken by separately estimating cost effectiveness in each of the subgroups (e.g. Scottish schools vs. all other schools) using multilevel modelling as above. The subgroup analysis based on eligibility of FSM was also undertaken at the school level, with two groups of schools being defined by having a proportion of children eligible for FSM either above or below the median value across the 41 schools (which was 22%).
Incremental analysis
Incremental analysis was undertaken using the results of the within-trial regression analyses and/or the model-based probabilistic sensitivity analysis. Results were plotted on the cost-effectiveness planes with associated cost-effectiveness acceptability curves.
Process evaluation
A mixed-method process evaluation was conducted, and the findings will be reported in the relevant sections of the Results (see Chapters 3 and 5) according to whether they are quantitative or qualitative results. The process evaluation was designed according to the Medical Research Council guidance on complex interventions106 and outlines three essential interacting components: implementation (the process through which interventions are delivered, and what is delivered in practice), mechanisms of impact (how participants interact with intervention mechanisms to generate change) and context (contextual factors potentially shaping intervention outcomes).
The process evaluation aimed to explore the BRIGHT intervention from the perspective of those involved including pupils, members of school staff and stakeholders to understand how and why the BRIGHT intervention was implemented and to contribute to the interpretation of the outcome evaluation results. The process evaluation included the following objectives:
-
Assess and document the implementation of the intervention. Implementation includes a combination of fidelity (the quality of the intervention delivered), reach (who participated), dose delivered and dose received, and adaptations (whether elements of the intervention were modified for a better contextual fit).
-
Explore the acceptability of the BRIGHT intervention from the perspective of those involved: pupils, school staff and key stakeholders, including the sustainability of the intervention and potential dissemination of the BRIGHT intervention.
Intervention implementation (objective 1)
For the BRIGHT trial, fidelity was operationalised as the degree to which the two components of the intervention (CBS and follow-up text messages) were carried out according to the protocol. For the CBS, it included aspects such as date of delivery, duration and timing, any adaptations of the lesson to fit with PSHE education and school curriculum and whether the CBS was structured as one session or broken down over more than one session. For text messages, it involved assessing whether twice-daily text messages were delivered to pupils as intended.
The component dose includes ‘dose delivered’ and ‘dose received’. ‘Dose delivered’ can be defined as the number or amount of intended units of each intervention or each component delivered or provided, whereas ‘dose received’ refers to the extent to which participants actively engage with, interact with, are receptive to and/or use materials or recommended resources. 107 In the BRIGHT trial, the dose delivered was defined as the number of CBS and text messages sent. Dose received was defined as the number of pupils who attended the CBS and the number of text messages received. Reach was assessed in terms of the extent to which the intervention was reaching the target population according to the eligibility criteria, particularly in terms of FSM status. Data collection on intervention implementation involved asking schools to confirm they had delivered the CBS and to whom by providing a delivery date and pupil attendance details.
Text message dosage (the number of texts sent to participants) was collected from recording information via the TextApp software. Start date of text messages, all messages sent and any replies received via the TextApp software were logged and audited in the underlying database with date and time stamps. Similarly, delivery receipts were recorded with date and time when the phone network provider acknowledged successful delivery of the message. Unfortunately, there was no facility to confirm if messages were read. If the network did not receive a successful delivery receipt within 24 hours, the message was considered to be undelivered and would not be resent. Undelivered messages could occur if the mobile phone was switched off, out of signal or the number was no longer in use. These function logs were used to determine the following metrics:
-
number of sent text messages per participant
-
number of text messages undelivered per participant
-
the number of pupils texting back STOP and when this occurred
-
total number of replies to text messages
-
the number of replies per participant/per message sent
-
timings between message delivered and reply
-
number of participants who reported a change of telephone number.
Intervention acceptability (objective 2)
Intervention acceptability was explored through interviews with pupils, school staff and key stakeholders involved in health and education policy.
Recruitment and consent
The sample for the qualitative component was drawn from the three BRIGHT trial sites: England, Scotland and Wales, and included six schools.
Pupils
Pupils, who had not explicitly withdrawn from the trial, were selected from the intervention arm of the BRIGHT trial and invited to participate. Pupils were identified from BRIGHT trial records by means of purposive maximum variation sampling using the variables of year group, sex, age and regional location.
Participant documentation was developed with input from young people to inform pupils about the BRIGHT qualitative study. Schools distributed the documentation for pupils to take home. This included a participant information sheet, a reply slip and a parents/carers cover letter to inform them that their child was being invited to participate in the interviews. The focus groups were then arranged through the school for those pupils who expressed an interest by returning the reply slip. Before beginning the focus group, the researcher obtained written consent from all participants. There were no dropouts by those who had registered interest. Recruitment continued until no new themes were observed.
School staff
Members of school staff who may provide an insight on the process of the BRIGHT intervention were invited to participate. This included those involved in the delivery of the CBS such as PSHE education teachers, those in leadership teams such as the Head of Year and any other staff members involved in the intervention such as the school nurse. Schools distributed study documentation to eligible staff members and interviews were then arranged with those who expressed an interest. 108
Key stakeholders
Key stakeholders with positions of responsibility in health or education policy were invited to provide an insight on the current context and to share their views. These were identified and contacted through professional networks of the research team. Both members of school staff and key stakeholders were offered the option of a face-to-face or telephone interview. Before beginning the interviews, the researcher obtained written consent for face-to-face interviews and verbal consent for telephone interviews.
Data collection
Pupils
The focus groups took place at school and were facilitated by experienced qualitative researchers (SE, HL, RJ, MR and ZM) with different academic backgrounds, including dentistry and social science. In addition, four of the focus groups were facilitated by two peer mentors who were young people from the BRIGHT Youth Forum run by Chilypep. Field notes were made after the interview and used to provide additional context to the analytical process.
Six focus groups were conducted with 50 pupils (25 girls and 25 boys) aged 11–13 years in the intervention arm from 6 secondary schools across the UK (3 England, 2 Wales and one Scotland). The focus groups lasted, on average, 45 minutes.
School staff
Semistructured interviews were conducted with 12 members of school staff. Four interviews were face to face and conducted at school and eight via telephone. These included teachers (n = 6), learning managers (n = 2) and those in senior leadership roles (n = 4). Interviews lasted, on average, 20 minutes.
Key stakeholders
Semistructured interviews were conducted with six key stakeholders. All interviews were conducted via telephone. Participants included a Chief Dental Officer (n = 1), an Oral Health Promotion Lead (n = 1), Dental Public Health Consultants (n = 2), a Local Authority Commissioner (n = 1) and a stakeholder involved in education policy (n = 1). Interviews lasted, on average, 25 minutes.
All interviews were informed by the process evaluation framework. 106 Interviews with pupils and school staff were additionally informed by the theory of framework acceptability. 109
Interviews continued with each group of participants (pupils, staff and key stakeholders) until data saturation was reached, hence the sample size. All interviews were audio-recorded and subsequently transcribed verbatim and anonymised. Interview participants (pupils and staff) received a £10 shopping voucher to thank them for participating. Data collection took place between June 2019 and November 2019.
Data analysis
The framework approach,110 a matrix-based method for the analysis of qualitative data, was employed for data analysis. This involved the following stages: familiarisation, identifying initial themes, labelling the data, sorting the data by theme and synthesising the data. The data were primarily analysed by two experienced doctoral researchers. This involved reading and re-reading the transcripts, independently identifying initial themes and independently and systematically coding transcripts line by line using an a priori thematic coding framework. This framework was developed from several sources: familiarisation with the interview transcripts; the theoretical framework of acceptability;109 and research team discussion. A pragmatic approach was adopted that drew on both deductive and inductive processes, enabling the exploration of a priori themes identified from the literature search and allowing new themes to be identified. Any discrepancies were resolved through discussion. In addition, any relevant field notes taken were used to help interpret the data.
Qualitative data were handled through the software NVivo Version 12 QSR International, providing data management and retrieval facilities to support analysis and write-up. The NVivo retrieval facilities allowed the researchers to remain connected to the original raw data facilitating the verification of conclusions throughout the refinement stages. Further refinement was undertaken by one of the postdoctoral researchers and any discrepancies were discussed and resolved through discussion. This process strengthened inter-rater reliability and credibility and thus ensured the trustworthiness of the data analysis.
Patient and public involvement and engagement in the BRIGHT trial
Young people, teachers, parents/carers and lay people were involved throughout the trial design and conduct, with plans for involvement in the dissemination.
The trial design was developed following surveys and workshops involving 319 pupils aged 11–16 years from deprived areas. The survey suggested that the majority of pupils owned their own mobile phones and also obtained information on preferred timing, content and duration of the text message intervention. The survey also found that many pupils considered existing ways of delivering oral health messages to be ‘boring’ or ‘annoying’. Views obtained from 10 parents/carers also suggested that the intervention needed to be developed with input from pupils. As a result, workshops were conducted at the beginning of the trial to refine the intervention. The survey also found that pupils were very keen to know who would have access to their phone number and who would be sending the text messages. To address this, as part of the recruitment process, an introductory session to the BRIGHT trial was developed, with input from a youth advisor, and was delivered by a member of the LRT in assemblies at least two weeks prior to consent being sought from pupils to participate in the trial.
Young people were also involved throughout the research through a BRIGHT Youth Forum run by Chilypep. The forum received training from Chilypep and resources were allocated to remunerate their time and expenses based on guidance from INVOLVE at the time. 111 Chilypep gained the views of the BRIGHT Youth Forum of the draft text messages and these were refined as a result. Chilypep then piloted the text schedule with a further nine young people and held a group discussion on ways the content of the messages could be improved. The comments from this pilot informed the text messages used in the pilot phase of the trial. Towards the end of the main phase of the trial, the BRIGHT Youth Forum informed the decision on the timing of the final intervention text message and the wording of that message.
The BRIGHT Youth Forum were involved in developing participant information and data collection resources, including the design of the trial logo, questionnaires, participant information sheet, consent form and an information letter that detailed changes to the protocol and the impact of the COVID-19 pandemic. The BRIGHT Youth Forum also assisted in the design of the process evaluation topic guides and conducted some peer-to-peer facilitation of the focus groups of pupils in schools, with support from Chilypep youth workers. Three members of the BRIGHT Youth Forum were involved in facilitating the focus group interviews to address the power imbalance usually experienced when adult researchers interview children and young people. The three BRIGHT Youth Forum members were provided with training from the Chilypep youth worker (Emma Manser), the research associate (Sarab El-Yousfi) and the lead principal investigator for PPIE (Zoe Marshman). Chilypep also advised the trial team on recruitment in deprived areas, ways to promote questionnaire completion and dissemination to young people.
The BRIGHT Youth Forum commented on the CBS activity sheets as these were amended and updated for future use. They have agreed to advise on the further development of the dissemination plan and contribute to the wording of the document sent to schools, which will also be uploaded to the website summarising the trial’s findings.
The views of teachers and school staff were also sought, and this influenced the development of the intervention in terms of avoiding impact on GCSEs, ways to improve engagement of hard-to-reach pupils and delivering and quality assurance of the CBS.
There were three lay members on the independent TSC. Pre-meeting briefings were held with the PPIE members of the TSC to enable their perspective to feed into the TSC more effectively. Their involvement included discussing the original protocol, process evaluation plan, the SAP, changes to the protocol and impact of COVID-19, HEAP and dissemination plan. They appreciated the challenges of undertaking this research in schools and welcomed regular updates on our progress. A head teacher at one of the participating schools was also invited to every TMG meeting.
Changes to the protocol
Protocol amendments are outlined in Appendix 1, Table 21. Within this section, key changes to the protocol are explained.
Sample size
The sample size was revised prior to the main phase of the trial, following the progression criteria review point. This is because we planned for the worst-case scenario in the original sample size calculation by assuming the less efficient design of randomisation at the school level (between-schools), rather than at the year-group level (within-schools). Assuming between-school randomisation, an ICC of 0.02, an average of 60 participating pupils per year group (120 per school), and allowing for 20% attrition, we originally estimated that 48 schools would be required in total (5760 pupils). Within-school randomisation was piloted in the internal pilot with 10 schools in order to address the following relevant progression criteria:
-
assessment of contamination in the control group and whether feasible to undertake randomisation within schools (by year group) or whether randomisation at the school level will be required, and calculation therefore of the required school sample size.
Following the progression criteria review point, within-school randomisation (by year group) was considered feasible and the sample size was revised to assume within-school randomisation and partial contamination effects (i.e. those contaminated gain half the intervention benefits) for 27% of the control sample (based on findings from the internal pilot, see Appendix 6 for more detail). The other assumptions for the sample size remained the same (i.e. an average of 60 pupils per year group, an ICC of 0.02 and 20% attrition at follow-up). With these assumptions, we calculated that 42 schools were required in total across the internal pilot and main phase of the trial. This would provide 90% power (5% two-sided α) to detect an 8% absolute reduction, from 34% to 26%, in the proportion of pupils with ‘obvious decay experience’ (the primary outcome).
While the revised sample size still assumed an average of 60 pupils per year group, the average number of pupils randomised per year group in the internal pilot was lower than this (49 pupils). Within the internal pilot, we were able to calculate an estimate of the participation rate for the number of pupils approached: an average of 121 pupils per year group were invited to partake in the trial, and 49 (40%) were randomised. Based on this participation rate of 40% and considering the size of the schools that had expressed an interest in taking part in the main phase, we were satisfied that we could achieve an average of 60 recruited pupils per year group in the main phase of the trial by approaching a larger pool of pupils in each year group (i.e. by inviting, on average, at least 150 pupils per year group).
Parent/carer consent for questionnaires
For the internal pilot, following the return of pupil consent forms, schools were asked to provide parents/carers of children who had consented with an additional information sheet and consent form regarding the completion of parent/carer questionnaires. 59 Parents/carers were requested to complete and return the consent form to the YTU trial team, using the provided freepost envelope, if they were happy to complete parent/carer questionnaires and to share their name and address so questionnaires could be posted directly to them. However, an amendment was made prior to the main phase of the trial to no longer complete this process. This was because the return rate of parent/carer consent forms for questionnaire completion was low and the process of distributing information sheets and consent forms was a burden on schools. Instead, for the main phase of the trial, and for all subsequent time points for the internal pilot, schools distributed parent/carer questionnaires via pupils (rather than the trial team posting these to parents/carers). 59 Parents/carers were provided with a cover letter explaining what the data would be used for and a freepost envelope to return the questionnaire if they were happy to complete it.
Final follow-up time point
The original planned final follow-up was at 3 years following CBS delivery, but it was amended to 2.5 years with funder approval. This was to avoid the final clinical examinations coinciding with school exams during the spring/summer when school staff and space are at a premium.
Changes to the wording of the primary and secondary outcomes
The primary outcome was initially described as the incidence of carious lesions in permanent teeth, measured using DMFT where decay is measured as caries into dentine (ICDAS 4–6). The word ‘incidence’ was felt, by independent members of the TSC and DMEC, to be ambiguous in its meaning, so language was clarified throughout the protocol to make the primary outcome and relevant secondary outcomes clear, using the words ‘prevalence’ and ‘presence’ instead. This also served to make clear that the outcomes were looking at the prevalence of caries at each time point, rather than the development of new caries from baseline, which was how some members of the TSC and DMEC thought ‘incidence’ could be interpreted. More detailed definitions of DMFT were also added to the protocol in relation to the primary and secondary outcomes.
Previously, the incidence of enamel carious lesions in permanent teeth, measured using DICDAS1–3MFT, was included within the protocol as a secondary outcome. This secondary outcome was incorrectly specified and was subsequently corrected to clarify that caries prevalence for all carious lesions will be reported, defined as the presence of at least one treated or untreated carious lesion of any severity (DICDAS1–6MFT, as opposed to just DICDAS1–3MFT) in any permanent tooth at 2 (internal pilot only) and 2.5 years.
The number of permanent teeth with any treated or untreated carious lesions (using the DICDAS1–6MFT) and caries into dentine (DICDAS4–6MFT) at 2 (internal pilot only) and 2.5 years was added as a secondary outcome. Although previously referred to in the data analysis section of the protocol (and listed as a secondary outcome in the grant proposal), this secondary outcome was omitted in error in the lists of secondary outcomes.
Finally, oral health behaviours, toothbrush/paste availability, contamination and intervention compliance were previously listed as secondary outcomes, but were reclassified as ‘other collected measures’ instead, as they were collected for the purposes of describing the sample, confounding factors and compliance rather than strictly as secondary outcomes.
The impact of the COVID-19 pandemic on data collection
Final follow-up data collection for the internal pilot and main phase of the trial (planned for collection at approximately 2.5 years post CBS) was delayed as a consequence of COVID-19-related suspension of data collection activities during the pandemic. It actually ended up being an average of about 3 years, as originally planned, due to these unforeseen delays. The protocol was amended to include the following measures to mitigate any potential risk during the dental examinations and questionnaire completion:
-
All participating institutions conducted necessary risk assessments.
-
Pupils and their parents/carers were sent an updated information leaflet to remind them of upcoming data collection, and that they could withdraw from the dental assessments and/or questionnaire completion if they so wished.
-
The LRTs asked schools to identify any pupils who would be classed as clinically extremely vulnerable (high risk) or clinically vulnerable (moderate risk), so they would not be invited to participate in the dental assessments (however, they were asked via schools to complete the pupil questionnaires).
-
All school visits followed current government, research and school guidance on measures to reduce risk (e.g. social distancing, use of appropriate personal protective equipment and use of COVID-19 screening questionnaire, as required).
Where it was infeasible to conduct dental examinations at participating schools due to the restrictions of the COVID-19 pandemic (e.g. dental teams not allowed into the school), schools were asked to just distribute the pupil and parent/carer questionnaires. Where this happened, but the schools later allowed dental teams into schools for the dental assessments, non-responders were asked to complete their pupil questionnaire during the dental assessments.
Chapter 3 Results: quantitative
Recruitment
The flow of schools and pupils through the BRIGHT trial is summarised in the CONSORT diagram presented in Figure 2. School and pupil recruitment for the trial started in 2017 and finished in 2019.
FIGURE 2.
Consolidated standards of reporting trials flow diagram illustrating the flow of schools and pupils through the trial. a, Approximate numbers, based on data available on the number of state-funded secondary schools in Scotland,98 England (South and West Yorkshire)64 and Wales (Cardiff, Vale of Glamorgan, Rhondda Cynon Taf, and Merthyr Tydfil local authorities)112 in 2016. b, Approximate numbers, based on data available on the percentage of pupils eligible for FSM in state-funded secondary schools in Scotland,98 England64 (South and West Yorkshire) and target local authorities in Wales97 in 2016. As outlined in the main text, FSM data from 2017 were also used to determine eligibility for some schools in England and Scotland. Due to the availability of school-level data and the expanse of the target regions, it was also not feasible to calculate the number of schools excluded due to not meeting other inclusion criteria (e.g. the criteria of at least 60 pupils per year group) and due to meeting the exclusion criteria. c, Forty-seven schools were actually recruited, but two of the recruited internal pilot schools in Wales were due to merge in the 2018–9 academic year (single sex schools merged into two mixed sex schools) and therefore are considered as one school for the purposes of the trial (i.e. the same year group allocations were applied to both schools). d, Years 7 and 8 were approached in schools in England and Wales and S1 and S2 were approached in schools in Scotland.
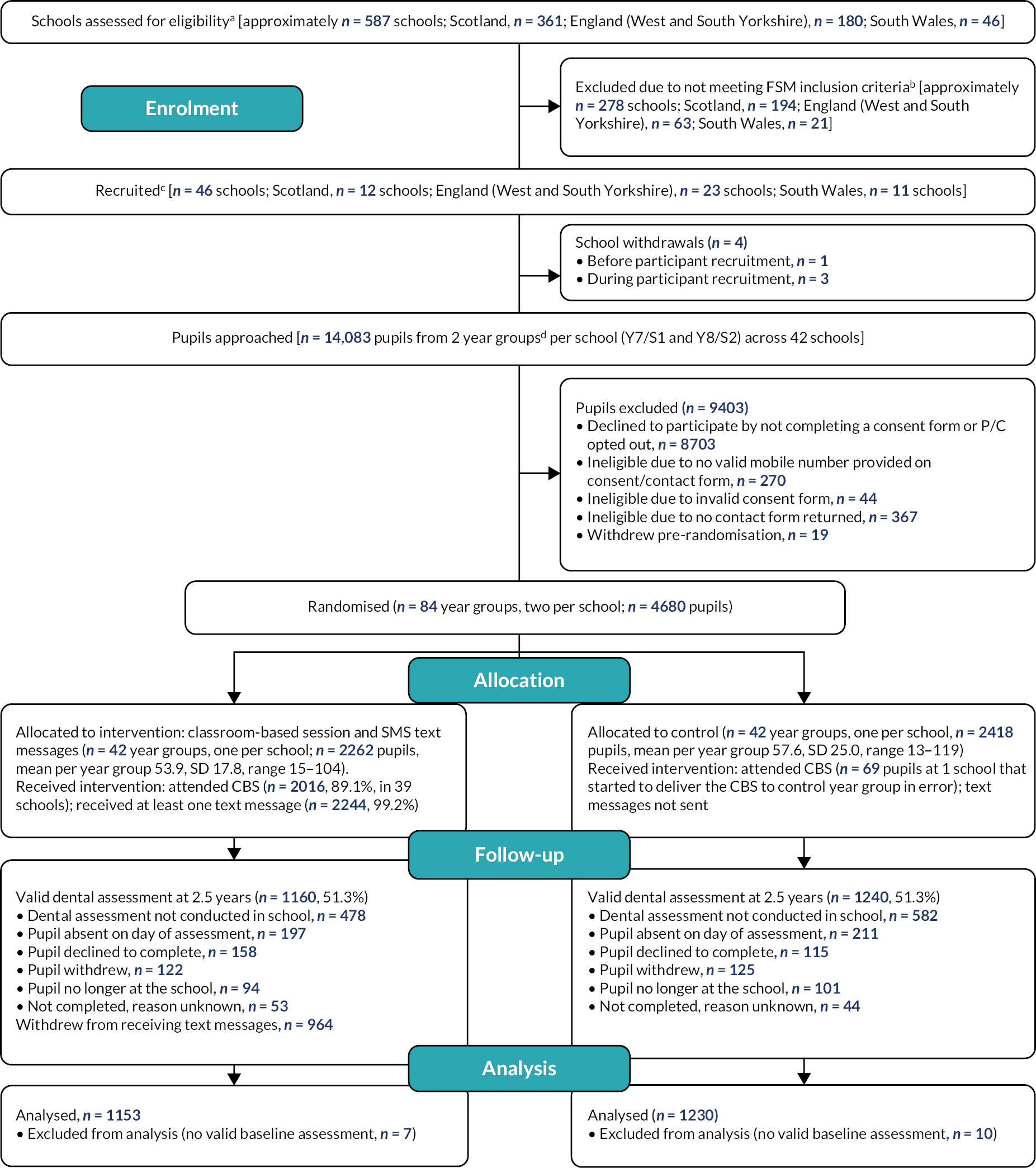
School recruitment
Approximate numbers of schools assessed for eligibility in each region, via use of publicly available data, are provided in Figure 2 [i.e. all state-funded schools in Scotland, England (South and West Yorkshire) and Wales (Cardiff local authority, Vale of Glamorgan local authority, Rhondda Cynon Taf local authority and Merthyr Tydfil local authority)].
Figure 2 also presents the approximate number of schools excluded due to having a lower proportion of pupils eligible for FSM than the average for that devolved nation. Due to the availability of school-level data and the expanse of the regions eligible to participate, it was not feasible to calculate the number of schools excluded due to not meeting other inclusion criteria (e.g. having at least 60 pupils per year group) or due to meeting the exclusion criteria. LRTs checked school eligibility where they could before directly approaching individual schools and checked the eligibility of each school that expressed interest in participating regions against the school inclusion and exclusion criteria. Due to the recruitment methods used in some regions, which reached an unknown number of schools (e.g. advertising through local authority networks and through local or national organisations for schools, such as School Leaders Scotland), it also was not possible to calculate the number of schools approached across all regions.
In total, 47 schools were recruited into the trial (i.e. a school representative signed an Agreement to Participate Form to confirm their involvement). For the internal pilot, 11 schools were recruited (Scotland, n = 3; England, n = 4; Wales, n = 4); however, two of the schools recruited in Wales were due to merge in the 2018–9 academic year (1 year post CBS delivery; two single sex schools merged into two mixed sex schools under one executive head) and therefore were considered as one school for the purposes of the trial (i.e. they were randomised as one school) and hereafter in this report. For the main phase, 36 schools were recruited (Scotland, n = 9; England, n = 19; Wales, n = 8). As shown in Figure 2, four schools withdrew pre randomisation (one school in England withdrew after signing the Agreement to Participate Form, but before starting participant recruitment; three schools in Wales withdrew during participant recruitment). In total, and in line with the sample size calculation, 42 schools were randomised; 10 were randomised during the internal pilot phase (Scotland, n = 3; England, n = 4; Wales, n = 3) and 32 during the main phase of the trial (Scotland, n = 9; England, n = 18; Wales, n = 5).
Participant recruitment
As shown in Figure 2, pupil recruitment was completed in 42 schools. Of the 14,083 pupils approached, 4699 (33.4%) consented and were eligible (i.e. they provided a valid mobile phone number) and were asked to complete baseline data collection. However, 19 participants withdrew pre randomisation (e.g. due to changing their mind) and so a total of 4680 pupils were included in the randomised sample (intervention, n = 2262; control, n = 2418). This was 92.9% of our target of 5040. The average number of pupils recruited per school was 111.4 (SD 35.9, median 107, range 46–189) and per year group was 55.7 (SD 21.6, median 53, range 13–119).
Randomisation for the BRIGHT trial occurred shortly after baseline data collection at the year-group level (i.e. at each school, 1 year group was randomised to the intervention group and the other to the control group), rather than at the pupil level. All consenting and eligible pupils (who provided a valid mobile number; excluding those that withdrew before randomisation) were considered as part of the randomised sample of pupils, regardless of whether baseline data were collected for them. A small number of randomised pupils did not complete the baseline dental assessment and did not provide any pupil questionnaire data (n = 47, 1.0%). In addition, seven participants completed the dental assessment but not the pupil questionnaire (0.15%) and eight completed the pupil questionnaire but not the dental assessment (0.17%).
School characteristics
Contextual information on school characteristics was collected. According to data collected in 2016 from the school census (England and Wales) and from the School Healthy Living Survey (Scotland)64,97,98 (i.e. the most recently available data at the start of school recruitment), the average proportion of pupils eligible for FSM among randomised schools was 24.2% (SD 7.1, range 11.4–43.0; Scotland: mean 20.7%, SD 5.2, range 14.5–32.7; England: mean 24.3%, SD 7.2, range 11.4–38.0; Wales: mean 28.6%, SD 7.3, range 20.6–43.0). For one school in England, the proportion of pupils eligible for FSM in 2016 was below the cut-off used to determine eligibility for the trial (13.2% for England; 14.2% for Scotland; 15.6% for Wales). This is because for schools in England, data from the 2017 school census were also used to determine eligibility for the main phase of the trial, including for the school in question. According to school census data collected in 2017,64 the average proportion of pupils eligible for FSM among randomised schools in England was 24.4% (SD 7.0, range 12.6–40.8). Using the 2017 data, one school did not meet the cut-off in pupils eligible for FSM but did meet the cut-off using the 2016 data. For one school in Scotland, FSM data were not available from 2016 (as the school formed in 2016 from merging two schools), but FSM data were available for the school from 2017 and were therefore used to determine eligibility. According to data from the 2017 School Healthy Living Survey,99 the average proportion of pupils eligible for FSM among schools in Scotland was 21.6% (SD 5.5, range 14.9–33.6).
Of the 22 participating schools in England, Ofsted ratings for the most recent inspection completed up to 2018 (i.e. the start of the intervention delivery period for the trial) were available for 18 schools. 64 Eight schools were rated as ‘requires improvement’, five schools were rated as ‘good’ and five schools were rated as ‘outstanding’. The four remaining schools had recently opened (either converted to an academy or moved to a new academy trust) and had not had an inspection by 2018. Two of the predecessor schools were rated as ‘good’, one as ‘requires improvement’ and one was in ‘special measures’. 64 For the nine participating schools in Wales (counting the merged schools separately), the latest Estyn monitoring report prior to 2018 was identified (these reports were published between 2013 and 2018). 100 One of the reports provided an overall judgement on the school’s current performance and on its prospects for improvement using a four-point scale (excellent, good, adequate and unsatisfactory) and was graded as ‘excellent’. The other reports provided a judgement of the progress made in respect of the key issues for action identified following the school’s most recent visit from Estyn. Three had made ‘sufficient progress’ and five ‘good progress’. Inspection data are not provided for the schools in Scotland as Education Scotland inspection reports for the participating schools were not publicly or readily available.
Baseline characteristics of randomised pupils
Baseline data for the 4680 randomised pupils are presented in Tables 5–8. A baseline pupil questionnaire was at least partially completed for 4626 randomised participants (98.8%; intervention, n = 2234, 98.8%; control, n = 2391, 98.9%); hence, there were some missing data. Reasons for incomplete baseline were absent from school on day of baseline data collection (n = 29); declined to complete questionnaire (n = 13); no longer at the school (n = 4); and unknown (n = 8).
The average age of pupils at recruitment was 12.7 years (SD 0.6) and 54.2% (n = 2538) were female (see Table 5). Overall, 21.9% (n = 1025) of pupils were eligible for FSM. The average decile of deprivation for pupils in England, Scotland and Wales was 3.1, 4.4 and 3.3, respectively.
| Characteristics | Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) |
|---|---|---|---|
| Year, n (%) | |||
| 7/S1 | 1045 (46.2) | 1578 (65.3) | 2623 (56.0) |
| 8/S2 | 1217 (53.8) | 840 (34.7) | 2057 (44.0) |
| Age, mean (SD) | 12.8 (0.7) | 12.6 (0.6) | 12.7 (0.6) |
| Sex, n (%) | |||
| Female | 1217 (53.8) | 1320 (54.6) | 2537 (54.2) |
| Male | 1045 (46.2) | 1097 (45.4) | 2142 (45.8) |
| Rather not say | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| Eligible for FSM, n (%) | |||
| Yes | 512 (22.6) | 513 (21.2) | 1025 (21.9) |
| No | 1674 (74.0) | 1809 (74.8) | 3483 (74.4) |
| Missing | 76 (3.4) | 96 (4.0) | 172 (3.7) |
| % pupil attendance in the previous academic year to the one in which they were recruited, mean (SD) | 95.4 (6.3) | 95.1 (6.6) | 95.3 (6.4) |
| % pupil attendance in the academic year in which they were recruited up to the point of recruitment, mean (SD) | 95.9 (5.7) | 95.8 (5.9) | 95.9 (5.8) |
| England IMD decile, mean (SD) | 3.0 (2.3) | 3.2 (2.5) | 3.1 (2.4) |
| Scottish IMD decile, mean (SD) | 4.5 (2.8) | 4.3 (3.0) | 4.4 (2.9) |
| Welsh IMD decile, mean (SD) | 3.1 (2.3) | 3.4 (2.2) | 3.3 (2.2) |
| Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) | |
|---|---|---|---|
| How satisfied are you with the appearance of your teeth?/How do you feel about the way your teeth look?, n (%) | |||
| Very satisfied/happy | 336 (14.9) | 380 (15.7) | 716 (15.3) |
| Satisfied/a bit happy | 754 (33.3) | 788 (32.6) | 1542 (32.9) |
| Neither satisfied/happy nor dissatisfied/unhappy | 651 (28.8) | 666 (27.5) | 1317 (28.1) |
| Dissatisfied/a bit unhappy | 376 (16.6) | 432 (17.9) | 808 (17.3) |
| Very dissatisfied/unhappy | 109 (4.8) | 116 (4.8) | 225 (4.8) |
| Missing | 36 (1.6) | 36 (1.5) | 72 (1.5) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 36 (1.6) | 37 (1.5) | 73 (1.6) |
| Three times a day | 134 (5.9) | 158 (6.5) | 292 (6.2) |
| Twice a day | 1587 (70.2) | 1679 (69.4) | 3266 (69.8) |
| Once a day | 418 (18.5) | 439 (18.2) | 857 (18.3) |
| < once a day | 49 (2.2) | 67 (2.8) | 116 (2.5) |
| Never | 6 (0.3) | 6 (0.2) | 12 (0.3) |
| Missing | 32 (1.4) | 32 (1.3) | 64 (1.4) |
| CARIES-QC raw score, mean (SD) | 3.7 (3.6) | 3.7 (3.5) | 3.7 (3.5) |
| CARIES-QC interval score, mean (SD) | 5.7 (3.6) | 5.7 (3.5) | 5.7 (3.5) |
| CARIES-QC global question – How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 1229 (54.3) | 1300 (53.8) | 2529 (54.0) |
| A bit | 914 (40.4) | 1001 (41.4) | 1915 (40.9) |
| A lot | 84 (3.7) | 83 (3.4) | 167 (3.6) |
| Missing | 35 (1.5) | 34 (1.4) | 69 (1.5) |
| Do you usually go to the dentist?, n (%) | |||
| For a check-up | 1881 (83.2) | 2001 (82.8) | 3882 (82.9) |
| Only when I have trouble with my teeth | 315 (13.9) | 330 (13.6) | 645 (13.8) |
| I have never been to the dentist | 31 (1.4) | 47 (1.9) | 78 (1.7) |
| Missing | 35 (1.5) | 40 (1.7) | 75 (1.6) |
| Over the last year, have you regularly used any of the following products to look after your teeth or mouth?, n (%) | |||
| Toothbrush (non-electric) | 1708 (75.5) | 1806 (74.7) | 3514 (75.1) |
| Electric/battery-operated toothbrush | 1218 (53.8) | 1321 (54.6) | 2539 (54.3) |
| Toothpaste | 2198 (97.2) | 2346 (97.0) | 4544 (97.1) |
| Mouthwash | 1506 (66.6) | 1575 (65.1) | 3081 (65.8) |
| Dental floss | 629 (27.8) | 633 (26.2) | 1262 (27.0) |
| Sugar-free or dental chewing gum | 693 (30.6) | 766 (31.7) | 1459 (31.2) |
| Other | 138 (6.1) | 151 (6.2) | 289 (6.2) |
| Do you have your own toothbrush?, n (%) | |||
| Yes, I have my own toothbrush | 2216 (98.0) | 2373 (98.1) | 4589 (98.1) |
| No, I share one | 10 (0.4) | 7 (0.3) | 17 (0.4) |
| No, I do not have a toothbrush | 5 (0.2) | 1 (0.0) | 6 (0.1) |
| Missing | 31 (1.4) | 37 (1.5) | 68 (1.5) |
| Do you have a toothpaste you can use?, n (%) | |||
| There is always a toothpaste I can use | 2172 (96.0) | 2318 (95.9) | 4490 (95.9) |
| There is sometimes a toothpaste I can use | 50 (2.2) | 51 (2.1) | 101 (2.2) |
| There is no toothpaste I can use | 6 (0.3) | 10 (0.4) | 16 (0.3) |
| Missing | 34 (1.5) | 39 (1.6) | 73 (1.6) |
| Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.4 (0.6) | 3.4 (0.6) | 3.4 (0.6) |
| Attitudes, mean (SD) | 3.2 (0.4) | 3.2 (0.4) | 3.2 (0.4) |
| Coping planning, mean (SD) | 2.7 (0.7) | 2.7 (0.7) | 2.7 (0.7) |
| Action planning, mean (SD) | 3.3 (0.6) | 3.3 (0.6) | 3.3 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 129 (5.7) | 138 (5.7) | 267 (5.7) |
| Three times a day | 443 (19.6) | 470 (19.4) | 913 (19.5) |
| Twice a day | 1488 (65.8) | 1591 (65.8) | 3079 (65.8) |
| Once a day | 132 (5.8) | 148 (6.1) | 280 (6.0) |
| < once a day | 16 (0.7) | 20 (0.8) | 36 (0.8) |
| Never | 19 (0.8) | 16 (0.7) | 35 (0.7) |
| Missing | 35 (1.5) | 35 (1.4) | 70 (1.5) |
The mean cariogenic score was 39.5 (SD 16.9) out of 100 (Table 8). Over two-thirds of pupils reported eating cakes or biscuits at least once a day on average (n = 3234, 69.1%), and a similar proportion with sweets or chocolate (n = 3291, 70.3%). More than half drank sugary soft drinks (n = 2578, 55.1%) or fruit juice/smoothies (n = 2702, 57.7%) at least once a day, and a quarter (n = 1197, 25.6%) energy/sports drinks. Summary of consumption of non-cariogenic food and drinks at baseline is summarised in Appendix 7, Table 27.
| Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) | |
|---|---|---|---|
| How many times do you usually eat? | |||
| Cakes or biscuits, n (%) | |||
| 4 +/day | 114 (5.0) | 93 (3.8) | 207 (4.4) |
| 3/day | 215 (9.5) | 215 (8.9) | 430 (9.2) |
| 2/day | 598 (26.4) | 625 (25.8) | 1223 (26.1) |
| 1/day | 652 (28.8) | 722 (29.9) | 1374 (29.4) |
| < 1/day | 562 (24.8) | 618 (25.6) | 1180 (25.2) |
| Never | 64 (2.8) | 85 (3.5) | 149 (3.2) |
| Missing | 57 (2.5) | 60 (2.5) | 117 (2.5) |
| Sweets or chocolate, n (%) | |||
| 4 +/day | 161 (7.1) | 167 (6.9) | 328 (7.0) |
| 3/day | 321 (14.2) | 271 (11.2) | 592 (12.6) |
| 2/day | 543 (24.0) | 547 (22.6) | 1090 (23.3) |
| 1/day | 577 (25.5) | 704 (29.1) | 1281 (27.4) |
| < 1/day | 545 (24.1) | 604 (25.0) | 1149 (24.6) |
| Never | 55 (2.4) | 67 (2.8) | 122 (2.6) |
| Missing | 60 (2.7) | 58 (2.4) | 118 (2.5) |
| How many times do you usually drink? | |||
| Soft drinks that contain sugar, n (%) | |||
| 4 +/day | 127 (5.6) | 138 (5.7) | 265 (5.7) |
| 3/day | 185 (8.2) | 217 (9.0) | 402 (8.6) |
| 2/day | 354 (15.6) | 397 (16.4) | 751 (16.0) |
| 1/day | 572 (25.3) | 588 (24.3) | 1160 (24.8) |
| < 1/day | 724 (32.0) | 746 (30.9) | 1470 (31.4) |
| Never | 256 (11.3) | 278 (11.5) | 534 (11.4) |
| Missing | 44 (1.9) | 54 (2.2) | 98 (2.1) |
| Energy/sports drinks, n (%) | |||
| 4 +/day | 65 (2.9) | 71 (2.9) | 136 (2.9) |
| 3/day | 84 (3.7) | 87 (3.6) | 171 (3.7) |
| 2/day | 146 (6.5) | 150 (6.2) | 296 (6.3) |
| 1/day | 304 (13.4) | 290 (12.0) | 594 (12.7) |
| < 1/day | 798 (35.3) | 830 (34.3) | 1628 (34.8) |
| Never | 813 (35.9) | 942 (39.0) | 1755 (37.5) |
| Missing | 52 (2.3) | 48 (2.0) | 100 (2.1) |
| Fruit juices and smoothies, n (%) | |||
| 4 +/day | 195 (8.6) | 240 (9.9) | 435 (9.3) |
| 3/day | 274 (12.1) | 286 (11.8) | 560 (12.0) |
| 2/day | 374 (16.5) | 386 (16.0) | 760 (16.2) |
| 1/day | 478 (21.1) | 469 (19.4) | 947 (20.2) |
| < 1/day | 568 (25.1) | 620 (25.6) | 1188 (25.4) |
| Never | 332 (14.7) | 376 (15.6) | 708 (15.1) |
| Missing | 41 (1.8) | 41 (1.7) | 82 (1.8) |
| Cariogenic score, mean (SD) | 39.9 (17.1) | 39.2 (16.7) | 39.5 (16.9) |
The intervention and control groups were well balanced for all pupil characteristics, except for a difference in the distribution of year groups, with more Year 8/S2 groups allocated to the intervention group than the control (intervention, n = 1217, 53.8%; control, n = 840, 34.7%) (Table 5). This is a chance difference resulting from the randomisation, but since year group is included in the primary analysis as a fixed effect, this imbalance is controlled for.
Baseline dental data
A baseline dental assessment was at least partially completed for 4625 randomised participants (98.8%; intervention 2233, 98.7%; control 2392, 98.9%). Reasons for missing data are absent from school on day of baseline data collection (n = 29); declined to complete questionnaire (n = 12); no longer at the school (n = 4); and unknown (n = 10).
Dental data are summarised for those with a valid baseline dental assessment in Table 9. Just over a third of pupils assessed had evidence of obvious decay experience indicated by the presence of DICDAS4–6MFT in at least one permanent tooth (n = 1603, 34.7%; intervention 769, 34.4%; control 834, 34.9%), and nearly two-thirds had at least one treated or untreated carious lesion in any permanent tooth as indicated by DICDAS1–6MFT (n = 2929, 63.3%; intervention 1430, 64.0%; control 1499, 62.7%).
| Variable | Intervention (n = 2233) | Control (n = 2392) | Total (n = 4625) |
|---|---|---|---|
| Presence of DICDAS4–6MFT, n (%) | 769 (34.4) | 834 (34.9) | 1603 (34.7) |
| Number of permanent teeth assessed for ICDAS per pupil | |||
| Mean (SD) | 30.8 (2.5) | 30.5 (2.7) | 30.6 (2.6) |
| Median (IQR) | 32.0 (31.0–32.0) | 32.0 (30.0–32.0) | 32.0 (30.0–32.0) |
| Number of DICDAS4–6MFT per pupil | |||
| Mean (SD) | 0.76 (1.40) | 0.77 (1.35) | 0.76 (1.37) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 4–6), mean (SD) | 0.24 (0.75) | 0.29 (0.78) | 0.27 (0.77) |
| M: teeth extracted due to caries, mean (SD) | 0.11 (0.60) | 0.07 (0.44) | 0.09 (0.52) |
| F: filled teeth (ICDAS 4–6), mean (SD) | 0.41 (0.93) | 0.40 (0.90) | 0.40 (0.91) |
| Presence of DICDAS1–6MFT, n (%) | 1430 (64.0) | 1499 (62.7) | 2929 (63.3) |
| Number of DICDAS1–6MFT per pupil | |||
| Mean (SD) | 2.15 (2.53) | 2.11 (2.57) | 2.13 (2.55) |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 1–6), mean (SD) | 1.75 (2.29) | 1.75 (2.34) | 1.75 (2.32) |
| M: teeth extracted due to caries, mean (SD) | 0.11 (0.60) | 0.07 (0.44) | 0.09 (0.52) |
| F: filled teeth (ICDAS 1–6), mean (SD) | 0.29 (0.74) | 0.29 (0.72) | 0.29 (0.73) |
| Plaque score, mean (SD) | 0.93 (0.67) | 0.84 (0.63) | 0.89 (0.65) |
| Gingival bleeding score, mean (SD) | 0.13 (0.17) | 0.13 (0.17) | 0.13 (0.17) |
| Number of teeth with bleeding gingivae per pupil | |||
| Mean (SD) | 1.79 (2.05) | 1.79 (2.04) | 1.79 (2.04) |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
The proportion with untreated decay in at least one tooth was 58.0% for all caries (ICDAS 1–6) and 15.8% for caries into dentine (ICDAS 4–6). Among those with the presence of DICDAS4–6MFT in at least one permanent tooth, the mean number of DICDAS4–6MFT was 2.2 (SD 1.5, median 2) and DICDAS1–6MFT was 4.1 (SD 2.7, median 4).
Baseline dental data were similar for the intervention and control groups.
Outcome data
Pupil follow-up finished in March 2022. All data collected at follow-up (both dental and from the pupil questionnaires) are summarised by randomised group and time point in Appendix 8, Tables 28–42, with timing of follow-up and reasons for non-completion provided.
No schools formally withdrew from the evaluation following randomisation. In total, 663 pupils (14.2%; intervention 315, 13.9%; control 348, 14.4%) withdrew from follow-up over the course of the trial. The most common reason was that the pupil was no longer at the participating school (n = 487, 73.5%). Follow-up could not be completed in all schools at all time points, largely due to disruptions caused by the COVID-19 pandemic (see Appendix 8, Tables 28 and 29).
Follow-up
At the 2.5-year follow-up, 2400 randomised pupils (51.3%; intervention 1160, 51.3%; control 1240, 51.3%) had a valid dental assessment. This was completed an average of 35.9 months (SD 3.2, range 26.7–45.9) after the baseline dental assessment. Reasons for missing data are (percentages out of 2280 missing): dental assessment not conducted in the school [n = 1060 pupils (46.5%) from seven schools], absent from school on day of data collection (n = 408, 17.9%); declined to complete questionnaire (n = 273, 12.0%); withdrew from trial prior to data collection time point (n = 247, 10.8%), no longer at the school (n = 195, 8.6%); and unknown (n = 97, 4.3%).
Primary analysis
Of the 2400 pupils with a valid dental assessment at 2.5 years, 1052 (43.8%; intervention 518, 44.7%; control 534, 43.1%) had obvious decay experience in at least one permanent tooth.
A total of 2383 randomised pupils (50.9%; intervention 1153, 51.0%; control 1230, 50.9%) had a valid dental assessment at both baseline and 2.5 years. Baseline characteristics and dental assessment data for this ‘as analysed’ population are provided in Appendix 9, Tables 43–47. No notable differences between the populations as randomised and as analysed were observed in pupil-reported data. In the baseline dental data, the proportion of pupils with evidence of obvious decay experience indicated by presence of DICDAS4–6MFT in at least one permanent tooth was lower in the analysed population (29.4% compared to 34.9%), as was the proportion with at least one treated or untreated carious lesion in any permanent tooth as indicated by DICDAS1–6MFT (58.5% vs. 63.3%).
The 2383 pupils included in the primary analysis were from 33 schools, and there was a mean of 36.6 pupils per year group (SD 17.2).
Among these 2383 pupils, 1043 (43.8%; intervention 514, 44.6%; control 529, 43.0%) had obvious decay experience in at least one permanent tooth at the final follow-up (Table 10). There was no evidence of a difference between the intervention and control groups (OR 1.04, 95% CI 0.85 to 1.26, p = 0.72). The predicted probabilities from the model were 44.2% (95% CI 40.7 to 47.6) in the intervention group and 43.5% (95% CI 40.1 to 46.9) in the control group (adjusted risk difference 0.6, 95% CI −2.8 to 4.1).
| Dental variable | Intervention (n = 1153) | Control (n = 1230) | Total (n = 2383) | Treatment effecta (95% CI) p-value |
|---|---|---|---|---|
| Primary outcome – presence of DICDAS4–6MFT, n (%) | 514 (44.6) | 529 (43.0) | 1043 (43.8) | OR 1.04 (0.85 to 1.26) p = 0.72 |
| Number of permanent teeth assessed for ICDAS per pupil | ||||
| Mean (SD) | 31.7 (1.2) | 31.8 (0.9) | 31.7 (1.1) | – |
| Median (IQR) | 32.0 (32.0–32.0) | 32.0 (32.0–32.0) | 32.0 (32.0–32.0) | – |
| Secondary outcome – number of DICDAS4–6MFT per pupil | ||||
| Mean (SD) | 1.08 (1.72) | 1.20 (2.07) | 1.14 (1.91) | IRR 0.96 (0.85 to 1.07) p = 0.45 |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | – |
| Number of: | ||||
| D: decayed teeth (ICDAS 4–6), mean (SD) | 0.48 (1.16) | 0.53 (1.31) | 0.51 (1.24) | – |
| M: teeth extracted due to caries, mean (SD) | 0.10 (0.50) | 0.10 (0.50) | 0.10 (0.50) | – |
| F: filled teeth (ICDAS 4–6), mean (SD) | 0.50 (1.01) | 0.57 (1.22) | 0.54 (1.12) | – |
| Secondary outcome – presence of DICDAS1–6MFT, n (%) | 717 (62.2) | 746 (60.7) | 1463 (61.4) | OR 1.05 (0.86 to 1.28) p = 0.64 |
| Secondary outcome – number of DICDAS1–6MFT per pupil | ||||
| Mean (SD) | 2.37 (3.02) | 2.47 (3.27) | 2.42 (3.15) | IRR 0.98 (0.89 to 1.08) p = 0.65 |
| Median (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–4.0) | 1.0 (0.0–4.0) | |
| Number of: | ||||
| D: decayed teeth (ICDAS 1–6), mean (SD) | 1.89 (2.75) | 1.90 (2.88) | 1.90 (2.82) | – |
| M: teeth extracted due to caries, mean (SD) | 0.10 (0.50) | 0.10 (0.50) | 0.10 (0.50) | – |
| F: filled teeth (ICDAS 1–6), mean (SD) | 0.39 (0.80) | 0.47 (1.07) | 0.43 (0.95) | – |
| Secondary outcome – plaque score, mean (SD) | 0.90 (0.69) | 0.87 (0.70) | 0.88 (0.69) | AMD −0.02 (−0.07 to 0.02) p = 0.31 |
| Secondary outcome – gingival bleeding score, mean (SD) | 0.13 (0.18) | 0.14 (0.20) | 0.14 (0.19) | AMD 0.92 (0.85 to 1.00) p = 0.05 |
| Number of teeth with bleeding gingivae per pupil | ||||
| Mean (SD) | 1.54 (1.93) | 1.63 (2.07) | 1.59 (2.00) | – |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | |
| Presence of a fixed orthodontic appliance, n (%) | 132 (10.7) | 111 (9.6) | 243 (10.2) | – |
| Presence of a removable orthodontic appliance, n (%) | 18 (1.6) | 16 (1.3) | 34 (1.4) | – |
| If yes, was removable appliance removed during assessment?, n (%) | 11 (61.1) | 9 (56.2) | 20 (58.8) | – |
| Unblinding of the dental assessor, n (%) | 3 (0.2) | 3 (0.3) | 6 (0.3) | – |
| Caries (DICDAS4–6MFT) increment from baseline, mean (SD) | ||||
| Net caries increment | 0.33 (3.46) | 0.51 (3.76) | 0.43 (3.62) | – |
| Crude caries increments | 1.17 (2.40) | 1.40 (3.22) | 1.29 (2.86) | |
| Net caries increment curtailed at 0 | 0.92 (2.20) | 1.14 (2.97) | 1.04 (2.63) | |
| Caries (DICDAS1–6MFT) increment from baseline, mean (SD) | ||||
| Net caries increment | 0.11 (4.54) | 0.35 (4.73) | 0.23 (4.64) | – |
| Crude caries increments | 2.08 (3.22) | 2.34 (3.99) | 2.21 (3.64) | |
| Net caries increment curtailed at 0 | 1.35 (2.79) | 1.56 (3.46) | 1.46 (3.15) | |
The ICC associated with school from this model was 0.05 (95% CI 0.02 to 0.10).
At the final follow-up, for those pupils who had at least one DICDAS4–6MFT, the mean number of DICDAS4–6MFT was 2.6 (SD 2.1, median 2) and the mean number of DICDAS1–6MFT was 4.5 (SD 3.3, median 4). The proportion with untreated decay in at least one tooth was 52.9% for all caries (ICDAS 1–6) and 24.5% for caries into dentine (ICDAS 4–6).
Just under a fifth of the pupils assessed at both time points moved from having no caries into dentine at baseline to having caries into dentine at 2.5 years (438/2383, 18.4%; intervention 18.5%; control 18.3%), and 14.2% from being negative on DICDAS1–6MFT at baseline to positive at 2.5 years (n = 339; intervention 13.9%; control 14.6%). Just under a third of the pupils developed at least one new DICDAS4–6MFT (756/2383, 31.7%; intervention 32.5%; control 31.0%), and 38.3% (n = 913) developed new DICDAS1–6MFT (intervention 37.9%; control 38.7%). For each calculation of caries increment between baseline and follow-up, the mean score is higher in the control group than intervention (Table 10).
Sensitivity analyses
Year group as a random effect
Year group was included in the primary analysis model as a random effect nested within school, which produced virtually identical results to the primary analysis (OR 1.04, 95% CI 0.85 to 1.26, p = 0.72).
Missing data
Several statistically significant associations between baseline variables and missingness (in terms of inclusion in the primary analysis model) were observed. The following were all associated with an increased likelihood of having missing data:
-
females [missing 1331/2537 (52.5%) vs. males 966/2143 (45.1%); OR 1.47, 95% CI 1.24 to 1.73, p < 0.001]
-
pupils eligible for FSM [missing 565/1025 (55.1%) vs. non-FSM 1659/3483 (47.6%); OR 1.59, 1.32 to 1.91, p < 0.001]
-
pupils in Year 8 at recruitment/Year 11 during the dental assessment [missing 1054/2057 (51.2%) vs. Year 7 1243/2623 (47.4%); OR 1.70, 95% CI 1.26 to 2.30, p < 0.001]
-
more DICDAS4–6MFT at baseline (OR associated with a one unit increase in score 1.13, 95% CI 1.07 to 1.20, p < 0.001)
-
higher cariogenic score (OR associated with a one unit increase in score 1.006, 95% CI 1.002 to 1.011, p = 0.01)
-
higher plaque index score (OR associated with a one unit increase in score 1.31, 95% CI 1.10 to 1.55, p = 0.002).
When these variables were included in the primary analysis model (school year and number of DICDAS4–6MFT at baseline are already covariates in the model), the OR for the effect of the intervention was 1.01 (95% CI 0.83 to 1.24, p = 0.91), which is similar to the primary result, providing additional evidence that the data were missing at random.
Timing of follow-up
The average time interval between the baseline and 2.5-year dental assessment was 36 months. Among the pupils with a valid dental assessment at both time points whose final assessment was completed between 33 and 39 months after their baseline, the treatment effect was very similar to the primary analysis (OR 1.02, 95% CI 0.78 to 1.33, p = 0.87).
Intervention implementation and complier-average causal effect analysis
Classroom-based session attendance
In total, 39 out of the 42 participating schools confirmed that they had held the CBS. One school confirmed that they did not deliver the CBS and no response was received from the remaining two; therefore, we assumed these schools did not deliver the session. All but one school delivered the CBS in a single session; one school decided to deliver the CBS over three lessons, rather than one, over a week for timetabling reasons. Among the 39 schools, the only/initial CBS took place a median of 26 days (range 7–87) after the letter was sent to the school informing them of the allocations for their year groups.
Registers of attendance at the CBS were received from 30 schools. For the remaining schools that confirmed they held a CBS, we assumed that all randomised pupils in the intervention year group attended the session. For the school that delivered three CBS lessons, we only classed the pupil as attending if they attended all three sessions. Therefore, in total, 2016 (89.1%) of randomised pupils in the intervention group attended the CBS. However, this is likely to be a slight overestimation as not all pupils in the intervention year groups for which a register was not received would have attended the session. Indeed, we know that two out of the eight classes in the intervention year group for one school did not receive the CBS. We failed to obtain the CBS registers from that school despite multiple reminders.
One school started to deliver the session to the wrong year group in error (i.e. delivered to the year group allocated to control). This was due to a communication error at the school. This led to some partial contamination in the control group, but it is not clear how much of the CBS was delivered (as this was the school that delivered the CBS over three sessions eventually to the correct year group) nor to whom, as registers were not requested for the control group. Therefore, we conservatively assumed that all control pupils in this school received the full CBS (n = 69).
Text messages
Text messages were triggered to commence for pupils in the intervention year group of a school as soon as the school provided confirmation that they had delivered the CBS. For schools that did not confirm they had delivered the CBS, a date was chosen for participants to start receiving the text messages. Text messages were sent to 2258 (99.8%) of the 2262 pupils randomised to the intervention group. The other four withdrew shortly after the date of the CBS at their schools and so their messages were not commenced. The first message was sent a median of 5 days (range −19 to 168) after the CBS (or imputed) date at the school. Participants were sent text messages until they requested them to stop or until 12 July 2020. The text messages should have been available for participants to continue to receive or restart after July 2020. However, on 12 July 2020, HIC moved to a new text messenger provider platform and in December 2020, they informed the BRIGHT trial team that < 2% of the text messages from 12 July 2020 had been delivered successfully. At that stage, 1368 (60.5%) participants had not withdrawn or texted STOP and would have been eligible to receive the messages. A decision was made to cease text messages. For these summaries, we have assumed that no messages were sent after 12 July 2020.
A total of 962 intervention participants (42.5%) withdrew from receiving the text messages, a median of 2.8 months after they commenced (range 1 day to 30 months). Time to intervention withdrawal is depicted in the Kaplan–Meier curve below (Figure 3). Participants were sent messages for between 0 and 127 weeks (approximately 30 months, mean 53.4 weeks, SD 35.4, median 62). This equated to between 1 and 1708 text messages (mean 694.5, SD 468.9, median 789). On average, 71.4% of the text messages sent to a participant were successfully delivered.
FIGURE 3.
Kaplan–Meier survivor curve of time to intervention withdrawal.

Text message response content
Although participants were told that any replies sent back to the text message number were not monitored, some participants sent replies. Of the 2258 participants in the intervention group who were sent text messages, 8461 text responses were received from 1388 participants (61.5%), with between 1 (n = 360) and 585 (n = 1) responses received per participant (mean 6.1, SD 18.4, median 3, mode 1), resulting in a highly skewed distribution with three participants responsible for 929 responses (11.0%), all of which conveyed affirmative messages, for example, ‘OK’, ‘yes’, etc.
Excluding 1289 STOP (or equivalent) and 48 START (or equivalent) messages, there were a total of 7124 SMS responses received. These were categorised as follows: agreement to brush their teeth or confirmation they had already brushed their teeth, for example, ‘done’, ‘I know’, ‘OK’, ‘yes’ (n = 4252, 59.3%); uncategorisable as not relevant or nonsensical (n = 1485, 20.7%); ‘positive’ messages, for example ‘thanks’, ‘hello’, ‘bye’ (n = 454, 6.3%); disagreement with action of toothbrushing, for example ‘no’, ‘forgot’ (n = 435, 6.1%); negative or expletive messages (n = 175, 2.4%); questions relating to toothbrushing or oral health (n = 152, 2.1%); and requests relating to the process of receiving the texts (n = 171, 2.4%) – these were further grouped as indicating the participant did not know who was sending the text (n = 72, 1.0%); change of phone number (n = 53, 0.7%); and change of timing of messages (n = 46, 0.6%). A small number of replies led to a safeguarding concern being raised, as detailed in Adverse events, suspected pathology and child safeguarding.
For the CACE analysis, we calculated the number of pupils who attended the CBS session and received at least 50% (n ≥ 7) of their messages per week for the first 12 weeks. This criterion was met for just under half the intervention participants (n = 1093; 48.3%).
Complier-average causal effect analysis
The CACE estimate of the treatment effect based on attending the CBS session was similar to the ITT estimate (OR 1.05, 95% CI 0.85 to 1.31, p = 0.64). The CACE estimate for attending the CBS session and having at least 50% of their messages delivered per week for the first 12 weeks was also similar (OR 1.07, 95% CI 0.72 to 1.59, p = 0.74). For the continuous compliance variable of number of text messages sent, the CACE estimate was OR 1.00 (95% CI 0.999 to 1.001, p = 0.93), which indicates that for every additional text message, there was no evidence of a decrease in likelihood of having a carious lesion.
Subgroup analyses
Eligible for free school meals
Of the pupils included in the primary analysis, 460 (19.3%) were eligible for FSM, 1824 (76.5%) were not, and data were missing for 99 (4.2%). Among those who were and were not eligible for FSM, the proportion who reported brushing their teeth at least twice a day at baseline was 71.6% and 80.6%, respectively, and 73.6% and 81.5% at 2.5 years. At 2.5 years, the proportion of FSM pupils with at least one DICDAS4–6MFT was 54.8% and 41.3% among non-FSM pupils.
There was a statistically significant interaction between randomised group and FSM status (p = 0.04) for the primary outcome, providing evidence that the treatment effect was different between pupils who were and were not eligible for FSM; this was a qualitative interaction. The OR for the intervention among pupils with FSM status was 0.69 (95% CI 0.44 to 1.08, p = 0.10; predicted proportions 46.8% and 53.7% in intervention and control groups, respectively), demonstrating a benefit, whereas there was a negative effect among non-FSM pupils (OR 1.17, 95% CI 0.93 to 1.46, p = 0.18; predicted proportions 44.0% and 41.4% in intervention and control groups, respectively).
Baseline caries
There was no evidence of an interaction between randomised group and total number of DICDAS4–6MFT in permanent teeth at baseline for the primary outcome (interaction effect p = 0.87).
Pilot or main trial school
There was no evidence of an interaction between randomised group and whether the pupil attended a school that was recruited in the pilot or main trial phases of the trial for the primary outcome (interaction effect p = 0.81).
Secondary analysis
Caries prevalence for all carious lesions (DICDAS1–6MFT) at 2.5 years
Of the 2400 randomised pupils with a valid dental assessment at the final follow-up, 1474 (61.4%; intervention 723, 62.3%; control 751, 60.6%) had at least one treated or untreated carious lesion in any permanent tooth as indicated by DICDAS1–6MFT.
Of the 2383 randomised pupils with a valid dental assessment at both time points, 1463 (61.4%; intervention 717, 62.2%; control 746, 60.7%) had at least one DICDAS1–6MFT at the final follow-up (Table 10). There was no evidence of a difference in this outcome between the intervention and control groups (OR 1.05, 95% CI 0.86 to 1.28, p = 0.64). The predicted probabilities from the model were 61.3% (95% CI 57.1 to 65.5) in the intervention group and 60.4% (95% CI 56.3 to 64.6) in the control group (adjusted risk difference 0.8, 95% CI −2.6 to 4.3).
For those pupils who had at least one DICDAS1–6MFT recorded at the final dental assessment, the mean number of DICDAS4–6MFT was 1.9 (SD 2.1, median 1) and the mean number of DICDAS1–6MFT was 3.9 (SD 3.2, median 3).
Number of carious teeth at 2.5 years
At the 2.5-year follow-up, the mean number of DICDAS4–6MFT per pupil was 1.08 (SD 1.72, median 0) in the intervention group and 1.20 (SD 2.07, median 0) in the control group (Table 10). There was no evidence of a difference in this outcome between the groups (IRR 0.96, 95% CI 0.85 to 1.07, p = 0.45), nor in the number of DICDAS1–6MFT (intervention mean 2.37, SD 3.02, median 1; control mean 2.47, SD 3.27, median 1; IRR 0.98, 95% CI 0.89 to 1.08, p = 0.65).
Frequency of self-reported toothbrushing
At baseline, 4616 (98.6%; intervention 2230, 98.6%; control 2386, 98.7%) provided a response to the question asking how frequently they brushed their teeth. Of these, 3631 (78.7%; intervention 1757, 78.8%; control 1874, 78.5%) responded that they brushed their teeth at least twice a day.
At 6 months, 3177 (67.9%; intervention 1480, 65.4%; control 1697, 70.2%) provided a response to the question asking how frequently they brushed their teeth. Of these, 2696 (84.9%; intervention 1287, 87.0%; control 1409, 83.0%) responded that they brushed their teeth at least twice a day.
Of the 3154 randomised pupils with a valid response at both time points, 2674 (84.8%; intervention 1275, 86.9%; control 1399, 83.0%) responded that they brushed their teeth at least twice a day. There was evidence that pupils in the intervention group were more likely to report brushing their teeth at least twice a day compared to the control group, at the 6-month time point (OR 1.30, 95% CI 1.03 to 1.63, p = 0.03).
At the 2.5-year follow-up, 2638 (56.4%; intervention 1285, 56.8%; control 1353, 56.0%) provided a response to the question asking how frequently they brushed their teeth. Of these, 2121 (80.4%; intervention 1039, 80.9%; control 1082, 80.0%) responded that they brushed their teeth at least twice a day.
Of the 2616 randomised pupils with a valid response at both time points, 2105 (80.5%; intervention 1033, 81.0%; control 1072, 79.9%) responded that they brushed their teeth at least twice a day. There was no evidence that the intervention group were more likely to report brushing their teeth at least twice a day compared to the control group, at the final follow-up (OR 1.05, 95% CI 0.84 to 1.30, p = 0.69).
Dental plaque and gingival bleeding scores, and Caries Impacts and Experiences Questionnaire for Children at 2.5 years
At the 2.5-year follow-up, the mean plaque score was 0.90 (SD 0.69, n = 1159) in the intervention group and 0.87 (SD 0.70, n = 1240) in the control group (Table 10). There was no evidence of a difference between the two groups in plaque score (adjusted mean difference −0.02, 95% CI −0.07 to 0.02, p = 0.31).
At the 2.5-year follow-up, the mean gingival bleeding score was 0.13 (SD 0.18, n = 1157) in the intervention group and 0.14 (SD 0.20, n = 1239) in the control group (Table 10). Visual inspection of the Q–Q plot of the residuals from the initial model demonstrated deviations from normality. Therefore, the outcome was log transformed and the model rerun, which resulted in a small improvement in the normality of the residuals. In this model, there was borderline evidence of a difference in gingival bleeding score between the two groups (0.92, 95% CI 0.85 to 1.00, p = 0.05). These estimates are interpreted such that the intervention group is predicted to have a mean gingival bleeding score 0.92 times that of the control group.
At the 2.5-year follow-up, the mean CARIES-QC score was 2.79 (SD 2.97, n = 1279) in the intervention group and 2.95 (SD 3.22, n = 1347) in the control group. The residuals from the untransformed model showed evidence of deviation from normality; therefore, the outcome was log transformed. Responses to the global CARIES-QC item at 2.5 years are reported in Appendix 8, Table 41 and are very similar between the two groups. Two-thirds of pupils reported that their teeth were ‘not at all’ a problem (intervention 67.5%, control 68.4%), just under a third that their teeth were ‘a bit’ of a problem (intervention 29.9%, control 29.3%), and only a small number reported that their teeth were ‘a lot’ of a problem (intervention 2.6%, control 2.2%).
There was no evidence of a difference between the two groups in CARIES-QC score (adjusted mean difference 1.00, 95% CI 0.94 to 1.06, p = 0.89). As above, the coefficients from the model were exponentiated such that the interpretation is that the intervention group has a predicted mean CARIES-QC score 1 time that of the control group.
Child health utility 9D
At baseline, the mean CHU9D scores were 0.91 (SD 0.09, n = 2221) and 0.91 (SD 0.09, n = 2366) in the intervention and control groups, respectively. At the 2.5-year follow-up, the mean CHU9D score was 0.89 (SD 0.10) in both the intervention group (n = 1277) and in the control group (n = 1341).
School attendance
Some pupil-level school attendance data were received from schools. From the date of the CBS at the school to the 1-year time point, the average attendance was 93.4% (SD 7.4, n = 510) in the intervention group and 93.9% (SD 6.5, n = 540) in the control. Between the 1- and 2-year time points, the average attendance was 94.0% (SD 8.0, n = 172) in the intervention group and 95.1% (SD 6.9, n = 175) in the control (these data were collected at the 2-year assessment and so were only requested from schools recruited during the internal pilot).
Due to the difficulties in schools providing pupil-level attendance data, they were asked instead to provide average school attendance at an aggregate level, by year group, for each academic year that the school had been involved in the trial (Table 11). Some limitations of these data are that they relate to the whole year group and not just the trial participants, that they are for the school academic years, which do not correlate exactly with years of follow-up in the trial, and that they were not provided by all participating schools. In addition, these data should be interpreted in the context that the COVID-19 pandemic led to an increase in school closures and absences from 2019 to 20 onwards. Indeed, average attendance was lower in these years than earlier years, for example, the average attendance was 84.1% among pilot schools and 88.4% among main trial schools in the academic year 2020–1, while it was 91.6% and 92.9%, respectively, in 2018–9, which was unaffected by the pandemic. Across all schools, the average attendance reported decreased for each year the schools were involved in the trial, but figures were similar between the intervention and control year groups.
| School attendance, % | Intervention (n = 42) | Control (n = 42) | Overall (n = 84) |
|---|---|---|---|
| Pilot | |||
N, mean (SD) |
|||
| Academic year of recruitment, 2017–8 | 6, 93.4 (1.0) | 6, 92.5 (2.1) | 12, 93.0 (1.6) |
| Academic year in which the year 1 FU was conducted, 2018–9 | 7, 92.3 (1.6) | 7, 91.0 (2.1) | 14, 91.6 (2.0) |
| Academic year in which the year 2 FU was conducted, 2019–20 | 7, 89.6 (2.6) | 7, 89.3 (2.5) | 14, 89.4 (2.4) |
| Academic year in which the year 2.5 FU was conducted, 2020–1 | 7, 84.0 (8.4) | 7, 84.1 (7.4) | 14, 84.1 (7.6) |
| Main | |||
|
N , mean (SD) |
|||
| Academic year of recruitment, 2018–9 | 22, 92.5 (3.4) | 22, 93.3 (2.6) | 44, 92.9 (3.0) |
| Academic year in which the year 1 FU was conducted, 2019–20 | 22, 89.9 (4.8) | 22, 90.8 (4.3) | 44, 90.4 (4.5) |
| Academic year in which the year 2 FU was conducted, 2020–1 | 22, 88.2 (5.8) | 22, 88.6 (5.1) | 44, 88.4 (5.4) |
| Academic year in which the year 2.5 FU was conducted, 2021–2 | 22, 85.8 (7.1) | 22, 86.8 (6.2) | 44, 86.3 (6.6) |
| Overall | |||
|
N , mean (SD) |
|||
| Academic year of recruitment | 28, 92.7 (3.0) | 28, 93.1 (2.5) | 56, 92.9 (2.8) |
| Academic year in which the year 1 FU was conducted | 29, 90.5 (4.3) | 29, 90.8 (3.8) | 58, 90.7 (4.0) |
| Academic year in which the year 2 FU was conducted | 29, 88.6 (5.2) | 29, 88.8 (4.5) | 58, 88.7 (4.8) |
| Academic year in which the year 2.5 FU was conducted | 29, 85.4 (7.3) | 29, 86.1 (6.5) | 58, 85.8 (6.9) |
Exploratory repeated measures analysis among schools recruited during the pilot phase
Results from the longitudinal analysis among the pupils recruited in the pilot phase of the trial are presented in Table 12. These indicate that there is evidence of increased risk of caries (DICDAS4–6MFT and DICDAS1–6MFT) at the 2-year time point among the intervention group relative to the control (OR 1.81, 95% CI 1.17 to 2.79, p = 0.01 and 1.96, 95% CI 1.14 to 3.36, p = 0.01), but there is no evidence of a difference at the later follow-up time point (p = 0.86 and p = 0.97, respectively). There is no evidence of a difference in the number of DICDAS4–6MFT or DICDAS1–6MFT, or the CARIES-QC score between the groups at any time point. There is some indication that the prevalence of reported twice-daily toothbrushing is increased in the intervention group relative to control at the earlier time points of 6 months and 1 and 2 years (OR 1.39, 2.06 and 1.82, respectively), but these are not statistically significant in this population (though in the full sample, the 6-month effect was significant, the OR was similar at 1.30, but the CI was narrower due to the larger sample size) and the effect is virtually null at the final follow-up. There is evidence that dental plaque levels and gingival bleeding scores were reduced in the intervention group relative to control at the 2.5-year follow-up with an absolute mean difference of −0.13 (95% CI −0.25 to 0.00, p = 0.05) in plaque score and a relative mean difference of 0.79 (95% CI 0.65 to 0.95, p = 0.01) in bleeding score, but there is no difference at the 2-year time point, nor in number of index teeth with gingival bleeding at either time point.
| Outcome and time point | Intervention group | Control group | ||
|---|---|---|---|---|
| Presence of D ICDAS4–6 MFT | Predicted probability (95% CI) | Predicted probability (95% CI) | OR (95% CI)a | p -value |
| 2 years | 0.48 (0.43 to 0.53) | 0.40 (0.35 to 0.45) | 1.81 (1.17 to 2.79) | 0.01 |
| 2.5 years | 0.45 (0.39 to 0.51) | 0.46 (0.39 to 0.53) | 0.96 (0.60 to 1.53) | 0.86 |
| Presence of D ICDAS1–6 MFT | Predicted probability (95% CI) | Predicted probability (95% CI) | OR (95% CI) | p -value |
| 2 years | 0.71 (0.66 to 0.76) | 0.62 (0.57 to 0.68) | 1.96 (1.14 to 3.36) | 0.01 |
| 2.5 years | 0.68 (0.62 to 0.73) | 0.68 (0.61 to 0.74) | 1.01 (0.54 to 1.90) | 0.97 |
| Number of D ICDAS4–6 MFT | Predicted incidence rate (95% CI) | Predicted incidence rate (95% CI) | IRR (95% CI) | p -value |
| 2 years | 2.05 (1.32 to 2.79) | 1.85 (1.17 to 2.53) | 1.11 (0.85 to 1.45) | 0.45 |
| 2.5 years | 2.14 (1.38 to 2.90) | 2.34 (1.48 to 3.20) | 0.91 (0.70 to 1.20) | 0.52 |
| Number of D ICDAS1–6 MFT | Predicted incidence rate (95% CI) | Predicted incidence rate (95% CI) | IRR (95% CI) | p -value |
| 2 years | 3.47 (2.66 to 4.28) | 2.87 (2.17 to 3.57) | 1.21 (0.97 to 1.50) | 0.09 |
| 2.5 years | 3.33 (2.56 to 4.09) | 3.38 (2.55 to 4.21) | 0.98 (0.79 to 1.23) | 0.89 |
| Twice-daily toothbrushing | Predicted probability (95% CI) | Predicted probability (95% CI) | OR (95% CI) | p -value |
| 6 months | 0.88 (0.85 to 0.91) | 0.86 (0.83 to 0.89) | 1.39 (0.64 to 2.98) | 0.40 |
| 1 year | 0.90 (0.87 to 0.93) | 0.85 (0.82 to 0.89) | 2.06 (0.95 to 4.45) | 0.07 |
| 2 years | 0.88 (0.85 to 0.91) | 0.84 (0.81 to 0.88) | 1.82 (0.88 to 3.77) | 0.11 |
| 2.5 years | 0.84 (0.80 to 0.88) | 0.84 (0.80 to 0.88) | 0.98 (0.48 to 2.02) | 0.96 |
| CARIES-QC | Predicted mean (95% CI) | Predicted mean (95% CI) | Mean difference (95% CI) b | p -value |
| 1 year | 3.20 (2.84 to 3.56) | 2.97 (2.59 to 3.34) | 1.06 (0.94 to 1.20) | 0.33 |
| 2 years | 2.72 (2.43 to 3.01) | 2.69 (2.39 to 3.00) | 0.99 (0.88 to 1.11) | 0.81 |
| 2.5 years | 2.68 (2.35 to 3.02) | 2.67 (2.30 to 3.04) | 1.05 (0.92 to 1.20) | 0.48 |
| Plaque score | Predicted mean (95% CI) | Predicted mean (95% CI) | Mean difference (95% CI) | p -value |
| 2 years | 0.86 (0.66 to 1.06) | 0.90 (0.70 to 1.10) | −0.05 (−0.13 to 0.04) | 0.29 |
| 2.5 years | 0.86 (0.65 to 1.06) | 0.98 (0.77 to 1.19) | −0.13 (−0.25 to −0.00) | 0.05 |
| Gingival bleeding score | Predicted mean (95% CI) | Predicted mean (95% CI) | Mean difference (95% CI) b | p -value |
| 2 years | 0.10 (0.06 to 0.14) | 0.10 (0.06 to 0.14) | 0.95 (0.82 to 1.10) | 0.52 |
| 2.5 years | 0.08 (0.04 to 0.12) | 0.13 (0.08 to 0.17) | 0.79 (0.65 to 0.95) | 0.01 |
| Number of index teeth with gingival bleeding | Predicted incidence rate (95% CI) | Predicted incidence rate (95% CI) | IRR (95% CI) | p -value |
| 2 years | 1.65 (0.40 to 2.89) | 1.68 (0.41 to 2.95) | 0.98 (0.80 to 1.21) | 0.87 |
| 2.5 years | 1.45 (0.32 to 2.57) | 1.75 (0.41 to 3.09) | 0.83 (0.63 to 1.08) | 0.17 |
Reproducibility
Table 13 presents the percentage agreement and Cohen’s kappa for the inter- and intrarater reliability for the dental second checks for the primary and secondary outcome across time.
| Outcome and time point | Inter-rater reliability | Intrarater reliability | ||||
|---|---|---|---|---|---|---|
| No. of observations | Percentage agreement | Cohen’s kappa statistic (95% CI) | No. of observations | Percentage agreement | Cohen’s kappa statistic (95% CI) | |
| Primary outcome – presence of DICDAS4–6MFT | ||||||
| Baseline | 50 | 82.0 | 0.54 (0.28 to 0.81) | 141 | 90.1 | 0.78 (0.67 to 0.89) |
| 2 years | 4 | – | – | 33 | 93.9 | 0.88 (0.71 to 1.00) |
| 2.5 years | 85 | 84.7 | 0.69 (0.54 to 0.85) | 70 | 95.7 | 0.91 (0.81 to 1.00) |
| Secondary outcome – presence of DICDAS1–6MFT | ||||||
| Baseline | 50 | 76.0 | 0.50 (0.26 to 0.74) | 141 | 87.9 | 0.74 (0.63 to 0.86) |
| 2 years | 4 | – | – | 33 | 93.9 | 0.84 (0.61 to 1.00) |
| 2.5 years | 85 | 74.1 | 0.31 (0.08 to 0.53) | 70 | 94.3 | 0.89 (0.78 to 0.99) |
Second checks were performed for 191 pupils at baseline; second checks at baseline were only conducted in schools recruited during the main phase of the trial. This is 5.3% of the 3594 valid dental assessments conducted with main trial pupils at baseline. Nearly three-quarters (n = 141, 73.8%) of the second checks were performed by the same dentist who conducted the initial assessment for the pupil and a quarter (n = 50, 26.2%) were performed by a different dentist.
Second checks were performed for 37 pupils at 2 years; second checks at 2 years were only conducted in schools recruited during the internal pilot. This is 5.5% of the 667 valid dental assessments conducted with pilot trial pupils at 2 years. Most (n = 33, 89.2%) of the second checks were performed by the same dentist who conducted the initial assessment for the pupil, while 4 (10.8%) were performed by a different dentist (this sample size was too low to allow calculation of inter-rater reliability at this time point).
Second checks were performed for 155 pupils at 2.5 years; this is 6.5% of the 2400 valid dental assessments conducted at 2.5 years. Nearly half (n = 75, 45.2%) of the second checks were performed by the same dentist who conducted the initial assessment for the pupil and the rest (n = 85, 54.8%) were performed by a different dentist.
The percentage agreement and kappa statistics were higher for intrarater reliability than inter-rater reliability. For the primary outcome, the percentage agreement ranged from 80.0% to 84.7% and the kappa statistic from 0.54 to 0.69 for inter-rater reliability across the different time points, and from 90.1% to 95.7% and 0.78 to 0.91, respectively, for intrarater reliability. These figures were slightly lower for the secondary outcome. Nonetheless, moderate to strong reliability was observed throughout. 113
Adverse events, suspected pathology and child safeguarding
One non-serious adverse event was recorded during the trial, which was deemed possibly related and unexpected, for a pupil in the control group. The pupil reported that their brace had been broken during a dental assessment for the trial and the dentist they saw for regular care advised that they should not continue to receive trial dental examinations. The pupil was withdrawn from the trial and the event was resolved with no further action.
No suspected serious pathologies were identified during dental assessments.
Fifteen safeguarding issues arose during the course of the trial. Thirteen of these related to a pupil free-text response to a trial text message (n = 12, all intervention group) or a comment left on a pupil questionnaire (n = 1, intervention group) which caused concern, and one safeguarding issue related to concerns about a pupil’s welfare raised by a dental assessor (control group). All of these were passed on to the school to be discussed with the pupil and/or their parent/carer(s) as appropriate and were resolved with no further action required. One issue was raised via a letter sent to YTU from a pupil who was not identified as being a participant in the trial by name and who attended a school that was not a participating BRIGHT school. The letter indicated the region the child was from but no further identifiable information about them. The letter was sent to the LRT lead who contacted social services. The pupil was identified and their school’s safeguarding lead confirmed they would follow up as per the school’s safeguarding policy. It was not clear how the pupil became aware of the BRIGHT team contact details. Full details of these events are provided in Appendix 10, Table 48.
Chapter 4 Results: economic evaluation
This chapter presents the results from the economic evaluation. In line with the HEAP, an assessment was made relating to the need for longer-term modelling based on the results of the primary outcome measure (DICDAS4–6MFT) and the secondary outcome of frequency of toothbrushing. This indicated that longer-term modelling was not warranted. Consequently, the results shown here relate only to the within-trial analysis.
Missing data
Missing data for costs and CHU9D were a matter for concern with rates of missing data for QALYs being 45% and 44% in the control and intervention group, respectively, and 49% for total costs in both groups (Table 14).
| Variable | Missing values % | Mean | SD | Range | |
|---|---|---|---|---|---|
| Control | Intervention | ||||
| Outcome variables for HRQoL | |||||
| CHU9D at baseline | 2 | 2 | 0.910 | 0.086 | 0.397–1 |
| CHU9D at 1-year follow-up | 73 | 72 | 0.886 | 0.108 | 0.385–1 |
| CHU9D at 2-year follow-up | 86 | 85 | 0.910 | 0.087 | 0.422–1 |
| CHU9D at 2.5-year follow-up | 45 | 44 | 0.893 | 0.096 | 0.326–1 |
| Outcomes for cost effectiveness | |||||
| Total discounted QALYs over 2.5 years | 45 | 44 | 2.204 | 0.176 | 1.204–2.441 |
| Total discounted costs over 2.5 years | 49 | 49 | £53.80 | 52.56 | £0.14–661.29 |
From the logistic regressions, cariogenic score, DMFT at baseline, sex, age, proportion of FSM and baseline utility were found to be significant predictors of missing QALYs ruling out the assumption that data were missing completely at random. Imputation, based on the assumption of the data being missing at random, was considered to be the best approach, and so multiple imputation of total costs and QALYs was used for the primary analysis (as set out in the HEAP). Multiple imputation of total costs and QALYs was based on the significant predictors of missingness.
Descriptive analysis
Actual resource use is described in Table 15 and shows minor differences in the mean number of visits for the various treatments (restorations, extractions and crowns) between the intervention and the control group. The mean visits for restorations and extractions were lower in the intervention group, but the difference was not statistically significant at the 2.5-year follow-up. The proportion of participants with zero visits for restorations, extractions and crowns was 78%, 96% and 99%, respectively.
| Item | Control (n = 1230) | Intervention (n = 1153) | Difference | |
|---|---|---|---|---|
| Visits for restorations | Mean | 0.223 | 0.221 | −0.002 |
| 0 visits (n) | 966 | 900 | −66 | |
| 1 visit (n) | 255 | 251 | −4 | |
| 2 visits (n) | 7 | 0 | −7 | |
| 3 visits (n) | 2 | 0 | −2 | |
| Visits for extractions | Mean | 0.043 | 0.038 | 0.005 |
| 0 visits (n) | 1183 | 1113 | −70 | |
| 1 visit (n) | 41 | 37 | −4 | |
| 2 visits (n) | 6 | 2 | −2 | |
| 3 visits (n) | 0 | 1 | 1 | |
| Number of visits for crowns | Mean | 0.008 | 0.009 | 0.001 |
| 0 visits (n) | 1220 | 1145 | ||
| 1 visit (n) | 10 | 6 | −4 | |
| 2 visits (n) | 0 | 2 | 2 |
Table 16 reports the mean and standard error (SE) costs and QALYs used in the primary analysis, prior to their inclusion in the regression analysis to account for baseline differences in prognostic factors. The cost of the intervention, in terms of the costs of text messaging, was £32.53. There were minor and non-statistically significant differences in the cost of dental treatments between the two groups. The difference in total discounted costs at 2.5 years was significantly higher in the intervention group (p < 0.001). The differences in utilities generated from CHU9D were lower in the intervention group at all time points except at Year 1; however, none of the differences were statistically significant.
| Costs per participant | Control mean (SE) |
Intervention mean (SE) |
Mean difference (95% CI) |
p-value |
|---|---|---|---|---|
| Primary analysis with imputed costs and QALYs | ||||
| Intervention costs | – | 32.53 (0.462) (n = 2258) |
32.53 (31.66 to 33.41) |
< 0.001 |
| Dental treatment costs | 20.73 (1.296) (n = 1230) |
21.02 (1.379) (n = 1153) |
0.29 (−3.42 to 3.99) |
0.88 |
| Total discounted costs at 2.5 years (imputed) | 23.04 (0.753) (n = 2329) |
55.33 (0.842) (n = 2194) |
32.28 (30.07 to 34.49) |
< 0.001 |
| Utilities and QALYs | ||||
| CHU9D scores at baseline | 0.910 (0.002) (n = 2366) |
0.909 (0.002) | −0.001 (−0.006 to 0.004) |
0.72 |
| CHU9D scores at 1 year | 0.886 (0.004) (n = 648) |
0.891(0.004) (n = 644) |
0.004 (−0.008 to 0.164) |
0.47 |
| CHU9D scores at 2 years | 0.910 (0.004) (n = 328) |
0.905 (0.005) (n = 348) |
−0.006 (−0.020 to 0.008) |
0.43 |
| CHU9D scores at 2.5 years | 0.893 (0.003) (n = 1341) |
0.892 (0.003) | −0.001 (−0.009 to 0.006) |
0.71 |
| Total discounted QALY at 2.5 years (imputed) | 2.196 (0.003) (n = 2322) |
2.193 (0.004) (n = 2193) |
−0.001 (−0.014 to 0.013) |
0.90 |
Primary analysis
The regression analysis of total costs estimated the intervention as having higher mean costs of £1.02 (95% CI −£1.29 to £3.29), as shown in Table 17. The estimated difference in QALYs was also very small with a mean of −0.003 (95% CI −0.009 to 0.002) favouring the control group. As such, the intervention is said to be dominated by the control group (with an incremental cost-effectiveness ratio of £340 for every QALY lost).
| Mean difference | SE | 95% CI | |
|---|---|---|---|
| Total cost (£) | 1.02 | 1.16 | −1.29 to 3.23 |
| QALYs | −0.003 | 0.003 | −0.009 to 0.002 |
| ICER | £340 per QALY lost | ||
| Probability that the intervention is cost-effective | 7% | ||
Examination of the cost-effectiveness plane in Figure 4 reflects these results with the mean incremental costs and QALY estimates being very close to the origin. There is relatively little uncertainty relating to the incremental costs, yet the 95% confidence ellipse extends into all four quadrants. The cost-effectiveness acceptability curve associated with this is shown in Figure 5 and estimates that the probability that the intervention is cost-effective is only 7% at the £20,000 threshold, which is commonly used by NICE in the UK for decision-making.
FIGURE 4.
Cost-effectiveness plane for primary analysis.

FIGURE 5.
Cost-effectiveness acceptability curve for primary analysis.
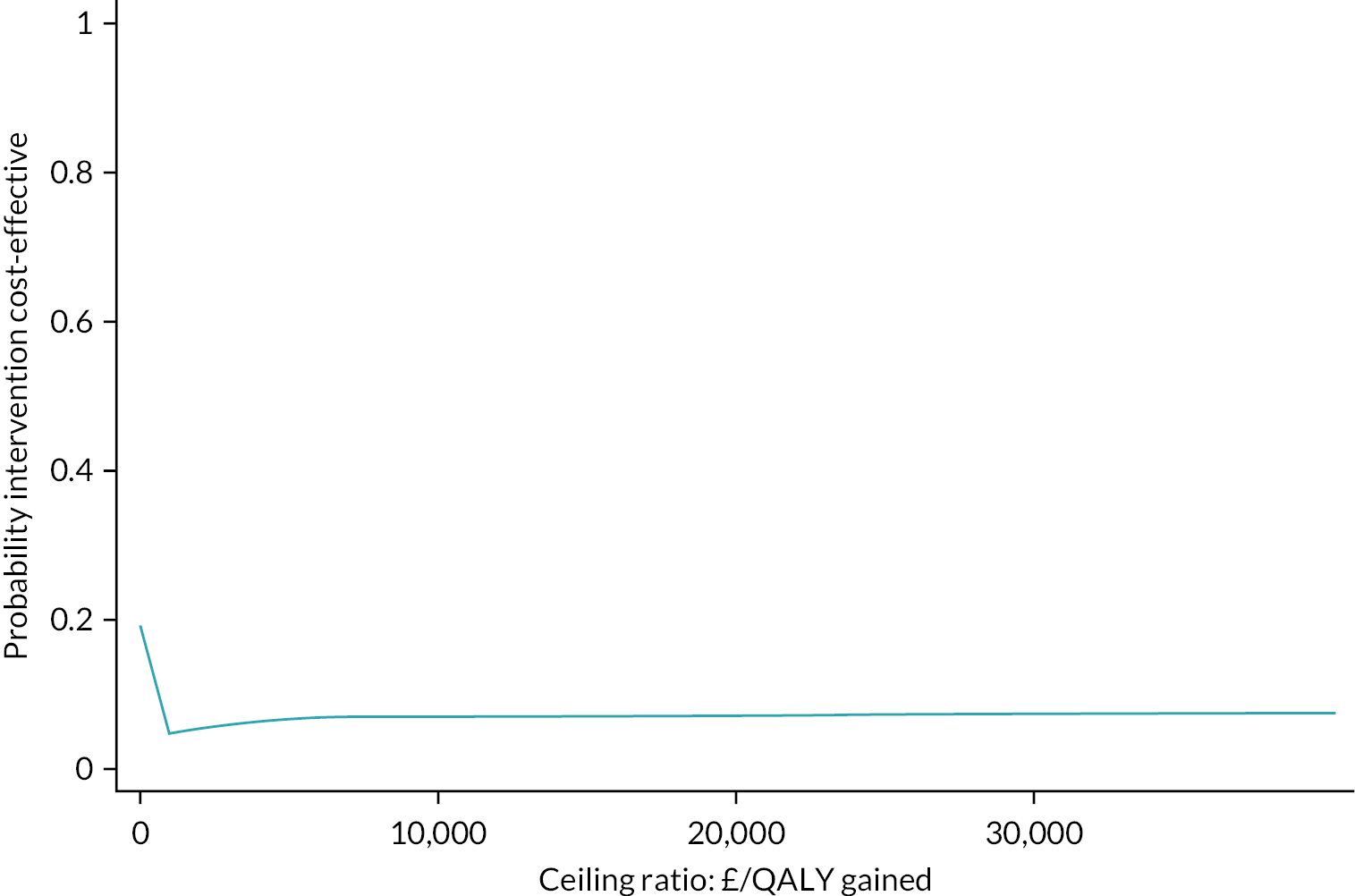
Sensitivity analyses
The sensitivity analyses (Table 18) show that the results of the primary analysis are robust if we do not undertake multiple imputation of total costs and QALYs; incremental costs and QALYs are still small and the intervention is still dominated. When QALYs are computed using CARIES-QC-U, there are very small QALY gains in favour of the intervention group and there are very small cost savings (the incremental costs change because we control for baseline utility generated by CARIES-QC-U). This produces an ICER of £79 per QALY gained and an associated probability of the intervention being cost-effective of 96%. When societal costs are considered, which include those to the education sector associated with delivering the intervention, costs to families and lost productivity, very little changes from the primary analysis. When parent-reported data are used to cost dental treatments, incremental costs and QALYs are both negative and small, yielding a small probability of the intervention being cost-effective (28%). In all analyses, the incremental costs and QALYs are not significant at the 5% level.
| Mean difference | SE | 95% CI | |
|---|---|---|---|
| I. ITT – no imputation | |||
| Total cost (£) | 1.67 | 2.06 | −2.31 to 5.43 |
| QALYs | −0.003 | 0.005 | −0.012 to 0.007 |
| ICER | £631 per QALY lost | ||
| Probability that the intervention is cost-effective | 26% | ||
| II. Using QALYs generated from CARIES-QC-U (multiple imputation) | |||
| Total cost (£) | −£1.12 | 1.18 | −1.31 to 3.42 |
| QALYs | 0.014 | 0.010 | −0.005 to 0.033 |
| ICER | −£79 per QALY gained | ||
| Probability that the intervention is cost-effective | 96% | ||
| III. Societal perspective (multiple imputation) | |||
| Total cost (£) | 0.20 | 0.89 | −1.33 to 2.21 |
| QALYs | −0.003 | 0.003 | −0.008 to 0.003 |
| ICER | £79 per QALY lost | ||
| Probability that the intervention is cost-effective | 14% | ||
| IV. Societal perspective using self-reported resource use data from the parent/carer (n = 2039) | |||
| Total cost (£) | −5.75 | 16.7 | −42.16 to 25.11 |
| QALYs | −0.004 | 0.007 | −0.017 to 0.009 |
| ICER | £1560 per QALY lost | ||
| Probability that the intervention is cost-effective | 28% | ||
Subgroup analyses
The subgroup analyses (Table 19), which use the methods employed for the primary analysis, suggest that there are no differences between Scotland versus England and Wales with the probability of the intervention being cost-effective remaining 6% in both cases. When we examined subgroups defined by the proportion of pupils eligible for FSM, a positive but not significant (p = 0.83) QALY gain was observed in those schools with higher levels of pupils with FSM. This produces an ICER of £2254 per QALY gained and a probability of the intervention being cost-effective of 60%. We also observed a QALY gain for pilot schools which leads to the intervention having an ICER of £3049 per QALY gained in pilot schools (with an 84% chance of it being cost-effective).
| Mean difference | SE | 95% CI | ||
|---|---|---|---|---|
| Schools with higher proportion of pupils eligible FSM vs. schools with lower proportions of pupils eligible for FSM (above and below median) | ||||
| Schools with higher proportion of eligible FSM (n = 2357) Intervention (n = 1143) Control (n = 1214) |
Total cost (£) | 1.83 | 2.07 | −2.21 to 5.72 |
| QALYs | 0.001 | 0.004 | −0.007 to 0.008 | |
| ICER | £2254 per QALY gained | |||
| Probability that the intervention is cost-effective | 60% | |||
| Schools with lower proportion of eligible FSM (n = 2076) Intervention (n = 1013) Control (n = 1063) |
Total cost (£) | −0.41 | 1.00 | −2.47 to 1.34 |
| QALYs | −0.008 | 0.004 | −0.017 to −0.000 | |
| ICER | −£49 per QALY lost | |||
| Probability that the intervention is cost-effective | 8% | |||
| Scotland vs. England and Wales | ||||
| Scotland (n = 1279) Intervention (n = 571) Control (n = 708) |
Total cost (£) | 11.86 | 1.66 | 8.68 to 5.26 |
| QALYs | −0.002 | 0.004 | −0.010 to 0.007 | |
| ICER | £6681 per QALY lost | |||
| Probability that the intervention is cost-effective | 6% | |||
| England and Wales (n = 3222) Intervention (n = 1613) Control (n = 1609) |
Total cost (£) | 10.35 | 1.79 | 7.18 to 14.16 |
| QALYs | −0.004 | 0.004 | −0.011 to 0.003 | |
| ICER | £2411 per QALY lost | |||
| Probability that the intervention is cost-effective | 6% | |||
| Pilot vs. main schools | ||||
| Pilot schools (n = 1012) Intervention (n = 528) Control (n = 484) |
Total cost (£) | 17.19 | 3.60 | 12.06 to 26.86 |
| QALYs | 0.006 | 0.006 | −0.005 to 0.017 | |
| ICER | £3049 per QALY gained | |||
| Probability that the intervention is cost-effective | 84% | |||
| Main schools (n = 3489) Intervention (n = 1656) Control (n = 1833) |
Total cost (£) | 1.64 | 1.23 | −0.76 to 4.03 |
| QALYs | −0.007 | 0.003 | −0.013 to 0.001 | |
| ICER | £235 per QALY lost | |||
| Probability that the intervention is cost-effective | 5% | |||
Chapter 5 Results: qualitative
Some text in this chapter has been reproduced from Elyousfi et al. 108 This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter reports the results of the qualitative element of the mixed-method process evaluation. Participants’ views and experiences of the intervention were explored to address two key objectives: assessing intervention implementation and acceptability. The results have been presented according to the process evaluation components assessed (e.g. dose, adaptations), and the theoretical framework of acceptability and identified themes. Given the BRIGHT intervention is a two-component intervention, it was imperative to understand how participants perceived each component in addition to the intervention as a whole.
Throughout this chapter, quotes are presented using the following nomenclature. For quotes from staff participants, the school and participant identification number are indicated in brackets; for example, ‘(School staff: 37:1)’ represents a quote from a member of school staff at a school assigned the school identification number ‘37’ where the member of staff was assigned a participant identification number of ‘1’. For quotes from pupil participants, a school identification number, followed by the pupil’s year group, followed by the speaker identification number are indicated in brackets; for example, ‘(Pupil: 57:Y7:PS9)’ represents a quote from a pupil attending school ‘57’, is in Year 7, and assigned as participant speaker 9 within the focus group interview. For pupil participants attending schools in Scotland, Year 7 and Year 8 are denoted as S1 and S2. Year 7/S1 includes 11- to 12-year-old pupils and year 8/S2 includes 12- to 13-year-old pupils. Quotes from stakeholder participants are presented with the abbreviation ‘STP’ followed by the participant identification number in brackets, for example, ‘(STP03)’.
The objectives and procedure for the process evaluation are described in Chapter 2.
Objective 1. This objective has mainly been addressed in the quantitative section, Chapter 3. This section reports the qualitative findings which further expand on the implementation and mechanisms of impact of the intervention pertaining to the fidelity, dose, reach and any adaptations to the intervention. The quantitative data represent the ‘dose delivered’; however, the qualitative data indicate that this does not equate to the ‘dose received’ regarding both components of the intervention. This is a critical finding in terms of understanding the mechanisms of impact of the intervention. For example, regarding CBS attendance, some pupils may have been counted as in attendance if the school used a daily attendance register rather than a register taken during the CBS. Indeed, some pupils reported missing the CBS lesson due to other ongoing activities, for instance, sports day.
It was all during the sports day thing when we had to go to assembly and I wasn’t part of it.
Pupil: 62:Y8:PS3
As for the text messages, only the participants who directly requested, from the BRIGHT trial team, through texting back ‘STOP’ were recorded as no longer receiving texts. However, some pupils reported muting and blocking the texts after some time and thus in effect did not receive them despite them being delivered. There was no method available to record this. Therefore, the actual dosage of texts received, rather than delivered, and for how long each individual participant continued to receive the texts is unclear.
I got frustrated there, I kept it for a few weeks and then I blocked it after.
Pupil: 37:Y7:PS7
As part of the process evaluation, it was important to establish whether there had been any adaptations to the intervention and to explore whether they improved the contextual fit or compromised the mechanisms of impact of the intervention. Indeed, some adaptations to the CBS were identified. These included changes to the method of delivery and the CBS content. For the BRIGHT intervention, the lesson was designed to be delivered as a CBS; however, some staff members reported delivering it as part of a whole year assembly due to the logistics involved, particularly if they did not have a dedicated PSHE education lesson.
I think it’s between 80 and 90, so I had all those students in front of me … It’s the only way that we could make it work in our timetable, because obviously requesting an hour off from lesson is not easily accommodatable given that we’re focused on one-year group, they’re not all in the same lesson at once…We don’t have PSHE lessons.
School staff: 75:1
In addition, some staff members felt that some amendments to the content of the lesson plan were required either to better suit their cohort of pupils or to fill in the duration of the lesson.
I tried to keep to it as much as possible, but I did like just try and introduce a few other structures … I think I put those pictures from Jeremy Kyle into … So it was those sorts of ideas just so I could point out what we were talking about and why. It was mainly just a few more pictures and ways that the kids were going to work with what we were doing.
School staff: 37:1
So, staff were having to fill in that gap, and they felt like things like the videos were a bit sort of childish for our boys. So, they found their own videos.
School staff: 39:2
Objective 2. The following section reports the acceptability of the BRIGHT intervention from the perspective of those involved: pupils, school staff and key stakeholders.
Overall, the intervention was found to be acceptable and pupil participants reported that the lesson provided oral health knowledge and the text messages reinforced the need for twice-daily toothbrushing. Some pupils described the text messages as ‘annoying’; nevertheless, they were perceived as helpful brushing reminders.
The lessons help you understand. And they text you. So it gets in your head. They get annoying. Then you have to start doing that. That’s fun.
Pupil: 16:S1:PS3
Yeah. Now we’ve learned it, how to do it and like quite properly.
Pupil: 57:Y7:PS5
Similarly, staff participants found the intervention to be acceptable and reported the lesson as ‘all in all, it worked fairly well in terms of pupils, fairly easy to follow, easy to deliver’. (School staff: 78:1).
Likewise, the intervention was well accepted by stakeholders with positions of responsibility in health or education policy. Stakeholders saw the value of the intervention, especially as it aimed to engage and improve the oral health of young people, a particularly overlooked and difficult cohort.
… it’s not the adult, it’s not the child, it’s kind of there, so in some ways that’s really good that you’re focusing on that cohort I think teenager years are often overlooked, I think perhaps because it’s quite a difficult cohort to undertake that intervention and for them to take it seriously, but absolutely worthwhile doing.
STP01
Using the theoretical framework of acceptability,109 five dimensions were identified: affective attitude, perceived effectiveness, burden, ethicality and self-efficacy. While some themes were identified across the participant groups, others were exclusive. Several themes were identified under the dimensions of affective attitude and perceived effectiveness (Table 20). From the data collected, the theoretical framework of acceptability domains of opportunity costs and intervention coherence were not presented. Participants did not mention missing out any opportunities as a result of their participation in the intervention. Similarly, there was no indication that participants were aware of any coherence or indeed incoherence of the intervention, albeit the understanding of the mechanisms of impact of the intervention was not directly probed during the interviews.
| CBS | |
|---|---|
| Construct | Theme |
| Affective attitude and perceived effectiveness |
|
| Self-efficacy |
|
| Ethicality |
|
| Burden |
|
| Text messages | |
| Construct | Theme |
| Affective attitude and perceived effectiveness |
|
| Ethicality |
|
The following section provides further detail regarding the acceptability of the components of the intervention, CBS and text messages, and is presented according to the theoretical framework of acceptability constructs outlined in Table 20.
Classroom-based session
The theoretical framework of acceptability109 dimensions identified for participants acceptability of the lesson included affective attitude, perceived effectiveness, self-efficacy, ethicality and burden.
Affective attitude and perceived effectiveness
The dimensions of affective attitude and perceived effectiveness have been presented jointly due to some overlap in the themes presented. Overall, both staff and pupils found the lesson acceptable. Staff reported that it was appropriate and had gone well.
It was good. It was very thorough. I would say that it was a success … Yes, for year sevens and eights, I would say it was appropriate definitely.
School staff: 33:1
Pupils described the lesson as informative and reported being more interested in oral health as a result, particularly regarding the consequences of poor oral hygiene and the importance of brushing their teeth.
I didn’t know you had to brush in two minutes so I used to do it a minute but now I do it for two minutes.
Pupil: 37:Y7:PS7
Like what would happen if I didn’t brush my teeth every day and I didn’t used to be like interested in that before.
Pupil: 75:Y8:PS8
Pupils provided mixed responses regarding the delivery of the lesson. Some reported the lesson ‘was covered well’ (Pupil: 37:Y7:PS10) and they found it interesting and fun. Others, however, found the lesson a bit long, and had become bored and disengaged by the end.
They’ve done it in a way that it was interesting like fun. But then, like it wouldn’t be too boring, but like you’re learning about something that you don’t know you want to learn about. But then when you’re doing it, you actually enjoy doing it.
Pupil: 16:S1:PS3
At the end it got very boring … because it’d been like really long.
Pupil: 57:Y7:PS6
In terms of engagement, the content of the lesson was deemed appropriate by some staff participants and consequently pupils ‘were very engaged with it’ (School staff: 33:1), while others found that for their pupil cohort, it was not engaging enough.
… it’ll be different levels of abilities in different areas … I mean, the area that we’re based in is more of a deprived area. So … it was probably better to be at that level for the students.
School staff: 33:1
… well we have boys … that play on quite high-level computer games and things like that. It wasn’t gripping enough for them.
School staff: 39:1
The setting of the delivery also appeared to impact engagement. Pupils who attended the lesson as part of a whole year assembly reported not having the opportunity to ask questions.
I didn’t really get the chance to ask why we should brush our teeth twice a day so yeah.
Pupil: 75:Y8:PS6
And I didn’t feel like I could ask questions because there was so many people.
Pupil: 75:Y8:PS7
As mentioned previously, this was an adaptation to the intervention and appears to have had a negative impact on pupil engagement.
Generally, staff found the materials and lesson activities to be suitable and appropriate. There were mixed views, however, regarding their fit with the duration of the lesson. Some felt the lesson plan was suitable, whereas others felt there was either too much or insufficient content for the time allocated.
… our lessons are 50 minutes long. Now that was a stretch to keep that going for 50 minutes … so there wasn’t enough content.
School staff: 39:1
I think the resources that were given were enough … I think with the activities that the students had to do with the time that you spent discussing … it was a nice mixture … it lasted in total about an hour. So it was a good length, I think, to engage the students.
School staff: 33:1
One element of the lesson plan (an educational animated video on toothbrushing) was described by some staff participants as ‘babyish for the year group that it was targeted at’ (School staff: 75:1). Similarly, several pupils reported finding it too childish. Some pupils felt they already knew how to brush and would have preferred if the video elaborated more and explained why toothbrushing is important.
Like it was like telling me like how to brush your teeth and stuff like that. It was like…it just felt like it wasn’t meant for our age.
Pupil: 33:Y8:PS7
The photos that were provided as part of the lesson to illustrate the consequences of inadequate toothbrushing were received positively by both pupils and staff.
Moderator: What were your favourite parts?
I enjoyed it … yeah and when they showed us the pictures of really disgusting teeth.
Pupil: 75:Y8:PS6
And they talked about facts and stuff that I found interesting.
Pupil: 75:Y8:PS11
I liked the pictures that you sent of the before and after sort of pictures that you sent, and they liked those as well.
School staff: 39:1
Pupils expressed wanting more oral information. As part of the lesson, they wanted to know more about:
Why brush your teeth twice a day.
Pupil: 75:Y8:PS6
How different toothpastes like affect your teeth and how they help them.
Pupil: 75:Y8:PS7
And what mouthwash to use as well.
Pupil: 75:Y8:PS8
They also suggested the delivery of several lessons over the pupil’s time at secondary school, rather than a one-off lesson.
… more lessons would be really helpful to tell us more about like teeth.
Pupil: 75:Y8:PS5
To improve the lesson, more visual resources such as photos and videos were recommended, particularly on the consequences of inadequate toothbrushing, with pupils regarding graphic pictures as potentially influential in behaviour change.
And like show more like stuff what could happen, you know, like more graphic pictures. It’s like what they do with the car crash isn’t it like showing like everywhere was like learning, like great graphic images so then it’s stopping dangerous driving.
Pupil: 33:Y8:PS13
There could have been more videos.
Pupil: 75:Y8:PS7
More videos.
Pupil: 75:Y8:PS4
Yeah, and less like talking about it.
Pupil: 75:Y8:PS7
Recent government guidance in England now requires oral health to be included as part of the curriculum. 114 However, at the time of intervention delivery, this was not the case, though many school staff were aware of its upcoming inclusion in the curriculum. Introducing new content that does not relate to national qualifications can be challenging. Incorporating the content covered by the CBS into the PSHE education curriculum had a positive impact on the acceptability of the oral health lesson.
… but now obviously, with the government agenda which is to prepare students to … they’ve introduced PSHE now … so, it’s gone into the curriculum, so that will allow far more flexibility to put that in because it ticks a lot of boxes for PSHE.
School staff 38:1
Indeed, embedding oral health into the curriculum was viewed, by stakeholders, as a key factor for sustainability of the intervention. Moreover, stakeholders attributed the acceptability of the intervention to its sustainability.
… it seems to be a really good programme in terms of sustainability, because if we could overcome the barrier of the phone number, and then if we can, bringing something as important as dental health into the curriculum in secondary schools.
STP03
Self-efficacy
Belief in ability to deliver the lesson was reported as an important acceptability factor. Staff members in leadership teams felt that it was important for those delivering the CBS to feel confident by having sufficient oral health knowledge and support.
Probably that lack of knowledge themselves maybe, lack of confidence in delivering it if they didn’t have that knowledge.
School staff 62:1
It’s something that we’d maybe have to look at freeing up so that maybe she could have a conference call with somebody beforehand to go through it all … maybe just be given a little bit more at our end as well … to give her the confidence in delivering it.
School staff 75:2
Ethicality
The personal beliefs and values of staff and perceiving it as ‘worthwhile’ (School staff 39:2) was a key factor for acceptability of the intervention.
… it was a good idea because we’ve got a lot of kids losing their teeth so I felt it was definitely worthwhile … one of the girls who was in the class told me … she was 12, she’d already had 8 teeth out, so that made it feel like this feels important.
School staff 37:1
Moreover, recognising oral health as an element of general health underpinned the positive interpretation, by staff members, of curriculum targets such as promoting personal hygiene.
… if we put it straight into that personal hygiene sort of framework, how you keep yourself healthy in all aspects … So, it’s about your body health, your mental health, your physical health … So, I think it all ties straight into that.
School staff 38:1
Similarly, stakeholders spoke of the significance of the personal beliefs and values of those involved in delivering the intervention. The recognition of the importance of oral health in particular was perceived as a critical acceptability factor.
I think it depends on school … if they see that as a priority and they see the benefits of such a programme they would be more than happy to do that.
STP03
Interviewer: So, possibly something that the council would commission as opposed to individual schools running it for their own children?
I can’t – schools don’t tend to see it as a priority.
STP02
Burden
A fundamental acceptability factor for staff members was the preparation time and effort required to deliver the CBS. Those delivering the lesson appreciated the resources provided and not having to prepare material for the lesson.
The resources were good … they were grateful they didn’t have to prepare anything … happy to get on board … I may have had some initial comments about whether or not they have to prepare the resources once I assured them … they just have to … review the material before the lesson and deliver it, they were happy.
School staff 62:1
However, some staff members who delivered the lesson found themselves having to print off the materials and arrange a dedicated time for the lesson which was viewed as burdensome.
… that someone just has it dropped on them as an additional extra like what I was given … obviously the printing as well, obviously that took money out of my budget that wasn’t necessarily signposted for this.
School staff 75:1
Moreover, the lack of dedicated PSHE education lessons in this particular school added logistical challenges of finding the time to incorporate something new.
We don’t have PSHE lessons. No, we teach that throughout the curriculum. So … like the logistics of getting them all together … it did work well but like I said, it did take a lot of teacher time for me to prepare it and make sure it was ready.
School staff 75:1
Stakeholders echoed the views of staff and were conscious of the intervention being perceived as burdensome in a busy school environment and reported that minimising the time and effort required by those involved in delivering the intervention was a critical factor for acceptability.
That might be issues around right attitudes, about taking on what could be deemed as extra work. It would really have to be very low in administration, schools are very driven by a lot of pressure on schools about attainment.
STP03
Text messages
The theoretical framework of acceptability109 dimensions identified for participants acceptability of the text messages included affective attitude, perceived effectiveness and ethicality.
Affective attitude and perceived effectiveness
Pupils generally found the text messages to be acceptable and useful reminders. The language used within the texts was described as appropriate; nonetheless, some pupils described them as ‘cringey’ (Pupil: 37:Y7:PS10) and ‘annoying’ (Pupil: 75:Y8:PS6) as time went on.
The language is just perfectly fine for our age group.
Pupil: 62:Y8:PS6
I think the most helpful thing … was like the reminding that like help me do that.
Pupil: 16:S1:PS5
I find it nice and good because it’s just saying about like teeth ‘Have you brushed your teeth?’… makes you think ‘Oh yeah’ and then I’ll go through my teeth. It’s definitely better than just, someone in your house still telling you to do your teeth it’s better than them … it’s more … pleasant.
Pupil: 57:Y7:PS6
Some pupils reported the texts improved their toothbrushing habits, for instance brushing twice a day and brushing at night.
It was a nice, like reminder to remind me about brushing my teeth every day anyway.
Pupil: 75:Y8:PS11
I’m not used to brushing twice a day, but it helped me to brush them twice a day.
Pupil: 75:Y8:PS7
They helped me remember in the night because I didn’t used to do it in the night but I do now.
Pupil: 75:Y8:PS6
Despite some pupils finding the text messages annoying, the perceived effectiveness of the text messages appears to have played a role in choosing to continue receiving them.
It was good but I found that the text messages were really annoying … I do like to use it as a reminder to brush your teeth.
Pupil: 33:Y8:PS15
Feeling annoyed with the text messages was attributed to their frequency and repetitiveness. Some pupils felt the messages were repetitive and came too often and ‘drains your energy after a while’ (Pupil: 33:Y8:PS8). This may have led to the pupils disengaging with the oral health messages in the texts. To improve the texts, they recommended more varied and creative messages.
Got them every single day.
Pupil: 75:Y8:PS6
Like how often they come, like not so much.
Pupil: 75:Y8:PS10
Just like they’re helpful, but they’re a bit like repetitive and like they don’t vary, is like a set five that just keeps repeating.
Pupil: 33:Y8:PS8
Maybe it should be creative, more creative with texts.
Pupil: 16:S1:PS6
In addition, some pupils found the text messages annoying as they interrupted mobile phone activities such as playing games.
When I’m playing something or like watching something it just pops up and the game just freezes when I’m like about to win so it gets me annoyed a bit.
Pupil: 57:Y7:PS4
It just keeps popping up when you’re like playing stuff.
Pupil: 57:Y7:PS5
Moreover, upon receiving a text message and realising it was not from a friend but rather from the BRIGHT trial, some pupils described feeling disappointed.
Makes you feel important like somebody’s trying to talk to you and then it’s like, no.
Pupil: 16:S1:PS7
Nonetheless, pupils still reported the texts were helpful reminders that prompted them to ‘get up and brush your teeth’ (Pupil: 57:Y7:PS5). For some pupils, however, alert fatigue of receiving texts eventually outweighed their usefulness and they reported blocking the messages as they had ‘done my head in’ (Pupil: 62:Y8:PS2), while others reported blocking them as they felt confident regarding their toothbrushing routine.
I blocked it because I know what times to do them, I just blocked it because I knew my routine.
Pupil: 37:Y7:PS10
Regarding the delivery timing of the text reminders, pupils generally found them suitable; however, for some pupils, the timings did not suit their personal circumstances. This was despite pupils being offered a choice of two set times for receiving the morning texts and two set times for the evening texts with later delivery times offered on weekends.
I think the timing was suitable for my timetable. Like I wake up a couple of minutes before got the message and then the message came and then that would usually be the time I’d go down and brush my teeth.
Pupil: 75:Y8:PS11
No, because I play football. I don’t get home sometimes till half nine.
Pupil: 16:S1:PS2
Pupils recommended having more choice of delivery times particularly on weekends and holidays where they were more likely to stay up late and sleep in.
I think on weekends and holidays, they could have been a bit later … Like I wake up around 11:00.
Pupil: 75:Y8:PS11
Furthermore, pupils suggested providing more information regarding oral health as a way of improving the text messages. In addition, they spoke of the importance of being able to interact and engage with the content delivered, and that they would appreciate the opportunity to be active receivers of information. They proposed an interactive app where they could control and set the delivery times of the reminders in addition to serving as an oral health resource with more detailed content and videos that they could access.
Like an interactive app … Like you can do stuff.
Pupil: 75:Y8:PS4
So like you can have an app with how many times you should brush your teeth regularly, and then facts about it, and then like your reminder, something like that.
Pupil: 75:Y8:PS3
Yeah, so maybe some of the things you had in there … in the lesson be in the apps that you could go back to it.
Pupil: 75:Y8:PS4
Moreover, engagement was also an important factor reported by stakeholders who spoke of the importance of engaging young people in oral health. Furthermore, they valued understanding novel ways to engage with this particular age group suggesting that the results could potentially be applicable to other interventions targeting this cohort.
… once you find a way of communicating with the teenagers, in particular, it’s like everybody wants to get on board. If you find it works, you can find an avenue for loads of stuff.
STP02
As mentioned previously, stakeholders voiced concerns regarding the sustainability of the intervention and incorporating oral health into the curriculum was seen to ensure the sustainability of the CBS. They also highlighted the importance of addressing barriers regarding the text message component such as those related to accessing and maintaining up-to-date phone numbers.
Not necessarily … in your more affluent areas, but definitely in your deprived communities, they lose the phone, they change the phone, they sell the phone.
STP02
Ethicality
Similarly to the views expressed by staff and stakeholders, personal beliefs and values regarding oral health played a key role in the acceptability of the intervention with pupils perceiving it as ‘good’ (Pupil: 57:Y7:PS5).
Well it’s good that like they want to encourage young people to like brush their teeth. So yeah … I mean like it influences them not to have like teeth like smoker’s teeth or teeth that people don’t brush properly and they’re all just like holey and yellow and black.
Pupil: 57:Y7:PS4
The personal significance of oral health led some pupils, despite being initially frustrated and stopping them, to restart receiving the text messages.
Moderator: Does anyone block the messages?
At one point I was because I got really angry with it.
Pupil: 57:Y7:PS5
Moderator: And then what made you start up again?
But it’s like something good because it reminds you to brush your teeth.
Pupil: 57:Y7:PS5
The results of this chapter report participant’s views and experiences of the intervention. This includes the acceptability of the intervention and implementation. Together with the quantitative results, the qualitative results provide a better understanding of how and what was implemented and how participants interacted with the intervention. These results will be brought together and discussed in more detail in the following chapter.
Chapter 6 Discussion
Summary of the findings
The BRIGHT trial investigated the effectiveness and cost effectiveness of a two-component behaviour change intervention (CBS and text messages) to prevent dental caries in secondary-school pupils, where there is a lack of proven effective preventive interventions. The trial was conducted during the COVID-19 pandemic which brought significant challenges for data collection and negatively impacted follow-up rates. The overall proportion of participants with any obvious decay experience (DICDAS4–6MFT), the primary outcome in this trial, was high (34.7%) at baseline, and increased at the final (2.5-year) follow-up (43.8%). These figures align with national data from children of similar ages from the national CDHS.
At the final follow-up, the proportion of participants with any obvious decay experience (DICDAS4–6MFT) was similar in the trial arms (intervention 44.7%; control 43.1%) and there was no evidence of a difference between the two groups. Similarly, there was no evidence of a difference in the prevalence of caries including enamel lesions (DICDAS1–6MFT), the number of DMFT at either threshold or using the clinical surrogate marker for toothbrushing (plaque index). There was no difference in HRQoL or oral HRQoL. However, there was some evidence of a difference in gingivitis with a lower bleeding score in the intervention group at the final follow-up, and in participant’s self-reported toothbrushing behaviour at 6 months (OR 1.30, 95% CI 1.03 to 1.63, p = 0.03); however, the effect on twice-daily toothbrushing was not sustained at the final follow-up.
The increase in the proportion of participants in the intervention group self-reporting toothbrushing at least twice per day (from 78.7% at baseline to 84.9% at 6-month follow-up) indicates a positive effect from the intervention on this behaviour. Exploratory evidence from pupils in the pilot schools indicated that this benefit may have lasted for up to 2 years; however, it did not translate into sustained behaviour change at the final follow-up and had no effect on caries prevalence at 2.5 years. In terms of self-reported twice-daily toothbrushing, there was a difference in rates for pupils who were eligible for FSM (71.6% at baseline and 73.6% at 2.5 years) and those who were not (80.6% at baseline and 81.5% at 2.5 years). There was also a difference in the proportion of pupils with at least one DICDAS4–6MFT between those eligible for FSM (54.8%) and non-FSM pupils (41.3%). The subgroup analysis considering FSM eligibility suggests a significant, qualitative interaction effect whereby the intervention appeared to be beneficial in terms of caries prevalence within pupils eligible for FSM but not for those not eligible for FSM. The potentially differential effect of the intervention depending on FSM status shows the value of research which focuses on underserved groups and suggests further research is needed to build on the NIHR INCLUDE framework.
The primary health economic analysis shows that the intervention is not cost-effective in this population as a whole. There is some evidence that it may be cost-effective in two subgroups, (1) the pilot schools and (2) schools with higher proportions of pupils eligible for FSM (with 84% and 60% of it being cost-effective, respectively). However, the incremental costs and QALYs for these subgroups remain very small and non-statistically significant. Overall, the economic analysis is weakened by high rates of missing data relating to treatment costs and QALYs.
The data collected during the process evaluation show that the BRIGHT intervention was generally implemented as intended, although some schools did not confirm delivery of the CBS. In addition, adaptations were made to both the content and method of delivery for a better contextual fit within individual schools. For example, in schools that had a longer lesson duration and those who did not have a dedicated PSHE education lesson. The text message component was also delivered largely as intended; however, there were some technical challenges experienced which resulted in all texts being stopped 30 months after the initial ones were sent at the beginning of the pilot trial. Furthermore, 42.5% of pupils formally withdrew from receiving text messages at a median of 2.8 months after they started to receive the messages and others reported blocking or muting them. Pupils, staff members and stakeholders voiced overall support for the BRIGHT intervention and appreciated what it was trying to achieve. Feedback from pupils and staff members regarding the CBS was generally positive. The resources were well received by staff members and for those delivering the lesson, it was particularly appreciated when the resources were prepared for them. The CBS was also well received by pupils; however, varying levels of engagement were reported. Some pupils found it boring towards the end and those that attended the lesson as a whole assembly were unable to engage with their teacher and ask questions. Despite pupils reporting that the text messages were generally acceptable, the findings indicate that the pupils may have experienced alert fatigue and boredom; hence, a proportion chose to no longer receive the texts.
In terms of generalisability, the target population for this trial were children in deprived areas and, to achieve this, schools where there were greater than the national average proportion of pupils eligible for FSM (13.2%, 14.2% and 15.6% for England, Scotland and Wales, respectively) were recruited. In the trial, 21.9% of pupils were eligible for FSM indicating that the target population was recruited. The results for the prevalence and severity of caries, oral health behaviours and use of dental services were similar to those found in the CDHS in 2013. In relation to key dental data, the CDHS found 77% of 12-year-olds and 81% of 15-year-olds self-reporting toothbrushing twice per day or more. For BRIGHT participants, this was 77.6% at baseline and at follow-up, when the participants were 15–16 years old, this was 80.4%. 115
Mean CARIES-QC scores were low at both baseline (intervention = 3.7; control = 3.7) and follow-up (intervention = 2.79; control = 2.95), which is expected from a population where mean DICDAS4–6MFT was less than 1 at baseline. The results are similar to those from a longitudinal study in New Zealand which used CARIES-QC and other oral HRQoL measures to evaluate the outcome of a toothbrushing programme for 10- to 13-year-olds with a baseline DICDAS4–6MFT of 2.1. Participants had similar mean CARIES-QC scores at baseline (3.5) and follow-up (2.7), which represented a moderate clinical effect from that intervention.
Toothbrushing with fluoride toothpaste
The intervention under evaluation in the BRIGHT trial was developed based on behaviour change theory with an education component and use of mHealth technology. Both the target behaviour (toothbrushing with a fluoride toothpaste) and the mode of delivery of the intervention were prescribed in the commissioned call under which this trial was funded. There is unequivocal evidence for the role of fluoride in preventing carious lesions from establishing and progressing. 5 This has been demonstrated at the micro-level of the molecular surface of the tooth, through clinical trials and at a macro-country and region-wide level. Indeed, supervised toothbrushing programmes are a core part of UK national oral health promotion programmes such as Childsmile in Scotland and Designed to Smile in Wales. Yet, in this trial, there was no difference in caries prevalence between the groups, indicating that the intervention did not achieve the desired clinical outcome of prevention of caries although improvements in the frequency of toothbrushing were reported at 6 months and potentially for up to 2 years. There are three considerations in terms of changes in the target behaviour: the ease of the behaviour to be changed, the centrality of the behaviour to the young people’s lives and the measurability of the behaviour change in terms of disease prevention.
This intervention focused on improving the frequency of toothbrushing with a fluoride toothpaste. The data from the trial suggest a high proportion of participants carried out twice-daily toothbrushing (77.6%) with a very high proportion of participants having access to a toothbrush and toothpaste and indicating high dental utility with 82.9% attending dental check-ups and using dental floss (27.0%) and sugar-free/dental gum (31.2%). These data call into question the ability of this intervention to bring about significant increases in these activities across the trial sample. As clinical proxy measures of toothbrushing were only carried out at 2 and 2.5 years (plaque levels and gingival bleeding), any change in behaviour was not captured longitudinally apart from through self-report.
There is also the question about whether the dose of the intervention achieved is sufficient to bring about any clinically meaningful improvement. Indeed, a systematic review indicates some evidence of positive effects of text message interventions on measures of toothbrushing, plaque and bleeding gingivae but the included studies only examined changes up to a maximum of 6 months. In addition, most were implemented in clinical settings, for example, with patients undergoing orthodontic treatment, rather than school-based settings. 52 One study found plaque and gingival bleeding scores were lower after 3 and 6 months in a group of patients undergoing orthodontic treatment116 who were sent text reminders (one or two message reminders and educational videos during treatment) compared to a control group. Similarly, another trial involving 22 orthodontic patients117 with an average age of 14 years found positive effects on plaque and gingival bleeding scores after 30 days. The generalisability of these findings to a wider population of young people is limited as orthodontic patients must demonstrate a good level of plaque control to be eligible for treatment and are reminded at their regular treatment visits of the need to maintain meticulous oral hygiene.
Measuring the clinical impact of toothbrushing behaviour change
Self-reported toothbrushing is considered to be a reliable measure of toothbrushing behaviour85 in epidemiological studies. The primary outcome in the BRIGHT trial was caries prevalence, with the ICDAS system used as the measure for the presence of any type of restoration or carious lesions at the tooth-surface level. In contrast to earlier scoring systems that simply scored the presence or absence of carious lesions, ICDAS allows the assessor to score lesions at different stages and has been widely used in contemporary clinical trials. This gives more sensitivity to detect change in severity of carious lesions. In addition, ICDAS allows the measurement of carious lesions confined to enamel, whereas the DMFT system is almost exclusively used at the threshold of recording only carious lesions that have reached dentine and it cannot detect the extent of carious lesions. ICDAS as an outcome measure is more sensitive. However, because carious lesions are slow to progress, often showing periods of inactivity and even reversal (when confined to enamel), detecting any change in the prevalence of the disease in a population is dependent on time for the disease to progress. If a behaviour is only changed over a short period of time, it will be difficult to detect any reduction in carious lesion progression, even at the level of enamel (where the disease begins and would be initially detected). Where enamel lesions are prevented in a group or reversed for a short time (through increased toothbrushing) if the behaviour is not sustained, the disease will continue again.
If there had been any caries reduction effect from even a relatively short-term behaviour change, this might have been seen in the secondary outcome measuring ICDAS 1–6, if enamel lesions, rather than lesions into dentine, had occurred. However, this was not seen, indicating that there was no evidence of an effect on the caries levels, even at the enamel stage by any increased toothbrushing.
Radiographs could not be used to improve the sensitivity of carious lesion detection because of the unjustifiable risk of using X-rays for research purposes only. ICDAS has been shown to be as sensitive as a dentinal carious lesion diagnostic tool as radiographs, and other supplementary measures. However, there were some constraints imposed by the clinical examinations being carried out in schools rather than in a dental clinical setting. For example, it was not possible to dry the teeth by using a triple syringe or have access to a spittoon/sink, so food debris from meals was removed by the examiner using a piece of gauze or cotton roll to wipe the teeth clean. We had initially attempted to get the young person to brush their teeth (with toothbrushes which were provided) immediately prior to the examination but there was nowhere for them to spit out, which they did not like, so this was changed to simply wiping with gauze/cotton rolls. There was no access to compressed air to dry the teeth and again, gauze and cotton rolls were used to remove the saliva film and allow the examiner to see the surface of the teeth clearly. As a full dental lamp was not available, a mobile dental lamp matching the specifications for epidemiological trials was used.
Plaque levels and gingival bleeding (indicating gingivitis as a result of plaque accumulation) are commonly used as surrogate markers for plaque removal by toothbrushing. These were measured only at 2 (for pilot school participants only) and 2.5 years. There was some evidence of a benefit in gingival bleeding scores in the intervention group compared to the control group at 2.5 years; however, this was a very modest effect. The mean bleeding scores differed by 0.01 points, and the median number of bleeding sites was 1 in both groups. There is little literature on the likely minimum clinically important differences in plaque and bleeding scores with which we can compare our results; it seems that the observed differences would be of borderline, if any, clinical significance, but more research is required to investigate clinically meaningful effects in these outcomes.
Classroom-based session
The findings suggest that staff valued the lesson and reported that the content was generally appropriate for their pupils. Factors that improved the acceptability of the CBS included its inclusion in the curriculum, having available support and having minimal preparation required. In addition, school staff appreciated having the freedom to adapt the lesson to better suit the school context such as varying capabilities for different pupil cohorts and lesson duration. However, to allow pupils to ask questions, it should be recommended that the lesson plan is delivered as a CBS as it was intended rather than an assembly.
In addition, there were some challenges with assessing the actual dose received of the CBS. Three participating schools did not confirm delivery of the CBS and from the 39 schools that confirmed delivery, only 30 provided registers of CBS attendance. It was assumed that all pupils in the intervention year group attended where registers were not provided; however, we know anecdotally that the CBS was delivered to only six of the eight intervention year classes in one of these schools, highlighting the challenges in accurately assessing the dose received. An adaptation was made by one school to deliver the lesson over three sessions rather than one, and for this school, attendance was only recorded if the pupil attended all three sessions. We therefore estimated that 2016 (89.1%) of randomised pupils in the intervention group attended the CBS; however, for the reasons outlined above, this is likely to be an overestimation. There was some contamination as one school started to deliver the CBS to the control year group (n = 69 pupils) in error, before correctly delivering the CBS to the intervention year group.
To further improve the CBS, consideration should be given to the participants’ feedback and appropriate changes made. Accordingly, the CBS lesson plan and activities should include more information on specific aspects of oral health such as explaining the reason why brushing twice a day is important and the difference between toothpastes. In addition, it was suggested that a lesson about dental health should be offered more than just once over a pupil’s time at secondary school to consolidate their learning. The addition of an optional activity should be added to the CBS plan for lessons of longer duration. However, once these changes have been made, the level of testing to date makes the lesson plan appropriate for use in schools across the UK. If a lesson was delivered to pupils who were older than the age group for which the BRIGHT lesson was developed, the content would need to be appropriate to that age group and build on the original lesson plan and activities.
Recently, specific oral health content in the curriculum has become a statutory requirement in England114 and will now be embedded within the curriculum. It is important to note that at the time of the development and delivery of the intervention, this was not the case. Being aware of the upcoming changes in policy appears to have had a positive impact on the acceptability of the lesson for school staff members in England. However, in Scotland and Wales, although general health is part of the curricula, there is less emphasis placed specifically on oral health. The lesson plan was designed based on the literature and tailored by education professionals and young people but may need to be amended if used internationally.
The addition of oral health in the curriculum also addresses the concerns raised by professional stakeholders involved in health or education policy regarding sustainability. Indeed, a lesson on oral health will no longer be considered an add-on but a mandatory part of the formal curriculum thus ensuring it is delivered as part of pupils’ education. This is critical in terms of sustainability and improving young people’s oral health, reinforced by the high levels of caries prevalence we found in the participating schools. Nevertheless, the formal curriculum is only one of the three interacting spheres of a health-promoting school as described by the WHO’s Health Promoting Schools framework. 118 The other interacting elements include promoting health through the ethos and social and/or physical environment and engaging with communities to reinforce health messages. Consequently, for behaviour change to happen, the necessary structures must be in place, such as conducive healthy environments, and enabling pupils to apply their knowledge as education alone is necessary but insufficient to support behaviour change.
Text messages
The findings of this study suggest that, overall, the text messages were acceptable to pupils and were perceived as useful brushing reminders. Other studies reporting on the acceptability of text message behaviour change interventions for young people have reported similar findings. The interventions included those aimed at improving clinic attendance, oral hygiene, physical activity and weight management, contraception use, sun-protective measures or reducing smoking and alcohol misuse. 119,120
Some pupils, however, found the text messages ‘annoying’ and described the wording as ‘cringey’, other pupils described feeling frustrated by their frequency and repetitiveness and, consequently, reported blocking or muting the messages. This is despite the messages being designed with young people during the intervention development stage. Moreover, in addition to the text messages being co-designed, a short pilot was conducted for 2 weeks involving the BRIGHT youth forum. This finding highlights the importance of exploring the perspectives of participants receiving the intervention over time. This is critical to intervention effectiveness as participant engagement needs to be maintained. Indeed, the findings of the process evaluation suggest that dose delivered did not equate to dose received, which will be discussed in more detail below. Previous studies have similarly reported boredom, annoyance, habituation (ignoring messages) and alert fatigue as challenges of mHealth interventions that potentially impact long-term engagement. 121–123 This finding has implications for the development and evaluation of future text message interventions, particularly those aimed at young people.
To further improve the acceptability of text message interventions, the following should be considered: choice of delivery times, interactivity to reduce tedium, varied and creative messages and messages that are informative rather than purely reminders. Some pupils suggested using an app instead of text messages. Indeed, text messages lack the interactivity an app can provide. Furthermore, an app could serve as an oral health resource and be used for signposting. However, using an app in isolation also has limitations. Reminders would still need to be sent twice daily, in accordance with brushing guidelines, potentially resulting in habituation and alert fatigue. Relying solely on an app may exclude those young people without smartphones, those with insufficient space/storage/data on their phone to install the app or those with older models that may be incompatible with the app or unable to make the necessary software updates,124,125 potentially increasing oral health inequalities. There is evidence suggesting oral health interventions that use a mixed approach, adopting text messages and an app, are more effective than employing either approach in isolation. 119
As part of assessing implementation fidelity of the intervention, it was important to record the dose delivered (number of CBS and text messages delivered) and as part of assessing mechanisms of impact, to record the dose received (number of pupils who attended the sessions and the number of text messages received). As mentioned in Chapter 2, a system was in place to collect this information. It is estimated that messages were sent to pupils for a median of about 14 months, and just over 70% of messages sent were recorded as being delivered. However, it was not feasible to document how many messages were blocked or muted and thus the actual dose of text messages received is unclear.
Pupils were given the option to request that messages no longer be sent to their number by texting back STOP and 42.5% of pupils did this, at a median of 2.8 months after they started. However, the focus group interviews revealed that pupils who no longer wanted to receive the messages mainly spoke of blocking or muting them instead of following the formal mechanism in place for stopping delivery of the messages. This meant that the texts were still recorded as delivered but in effect were not received or read. There was some evidence of engagement with the texts with 7124 free-text responses received (excluding the STOP and START messages). This was despite participants being told and reminded that the number was not monitored. Over 60% of these responses were positive or affirmative of compliance with the reminder to brush.
In terms of the technical feasibility of the text messages, the HIC provided the text message service for the BRIGHT trial. In early December 2020, the HIC made the BRIGHT team aware of an issue with the provision of text messages originating from July 2020 when the service provider moved to a new cloud platform; it was discovered that very few messages had been successfully sent or delivered after this time. The BRIGHT trial team were under the impression that the issue had been resolved and that messages had restarted in December 2020; however, in early February 2021, the HIC provided a further update explaining that issues with text messages were ongoing. Following discussions with the TMG, TSC and DMEC, it was decided not to restart the text messages as the hiatus had already been greater than 6 months, most participants had already received text messages for greater than 10 months (unless they had requested the messages be stopped) and that this duration was longer than in other health studies of the impact of text messages. It was agreed that restarting messages after such a long delay would be confusing for the participants.
Role of sugar consumption
The BRIGHT intervention did not tackle the other main risk factor for caries – sugar consumption. The responses to the diet questions indicate participants had a cariogenic dietary intake, particularly of sugar-sweetened beverages. Indeed, the BRIGHT Youth Forum, as part of the discussions about the dissemination of the findings of the trial, were particularly interested in the role of sugar-sweetened beverages, especially energy and sports drink consumption. However, while some data were collected at baseline on sugar consumption, there was no attempt to change sugar intake through the intervention and data on sugar consumption were not collected at any follow-up time points. Prevention of dental caries is, in part, complicated by the multiple factors that contribute to its development and their complex interplay. These factors range from biological, behavioural, cultural and social risk factors. All of these factors contribute to the development of a carious lesion, but ultimately the pathophysiological process is caused by acid production through sugar metabolism by bacteria in the biofilm and dissolution of hard tissue. Early multicountry epidemiological studies led to the belief that there was a linear relationship between increasing sugar intake and increasing dental caries;126 however, this association is now known to be more complex. An example of this is an ecological study where a global evaluation based on countries’ income and income disparities found a positive correlation between per capita sugar intake (kg/capita/year) and DMFT in low-income countries, but that for high-income countries, there was a negative relationship between per capita sugar intake and DMFT. 127 However, the study did not account for national fluoride policies, which are more often seen and well implemented in high-income countries. This mitigation of caries in high-income countries, despite high sugar intake, has been attributed to the availability of fluoride and oral health care, that is, a materialistic model rather than one based on simple social inequalities. Nevertheless, the majority of evidence supports the dose–response relationship, while it is acknowledged that this is attenuated, but not completely negated, by fluoride. 128,129 While this process can, to some extent, be reversed by use of fluorides, it is driven (biologically) by sugar and it is not known whether there is a threshold of sugar intake (frequency or amount) beyond which fluorides become ineffective. In addition, the global epidemiological studies have included fluoridated water in their considerations rather than toothbrushing with a fluoride toothpaste, the use of which requires not only availability but also the appropriate behaviours to use it.
Findings from the economic evaluation
The results of this trial’s economic evaluation showed that there are very small incremental costs and that HRQoL benefits are lower in the intervention group. This results in a situation where the intervention has a very low probability of being cost-effective at the relevant NICE funding threshold. Sensitivity analyses show that the results are robust to the use of imputation, the use of societal perspective and the use of parental recall as the data source for dental treatments. However, when QALYs are measured using utilities generated from CARIES-QC-U, the intervention is cost-effective, producing an incremental cost-effectiveness ratio much lower than the acceptable threshold. Likewise, the intervention appears to be cost-effective in schools which have relatively high proportions of pupils eligible for FSM, and in those schools that were recruited for the pilot phase.
All the economic analyses, including those showing the intervention to be cost-effective, are based on differences in costs and QALYs that are small and not statistically significant. When considered alongside the clinical results, it is tempting to conclude that the results relating to CARIES-QC-U and school subgroups are the results of random variation; however, it is important to consider other possible causes that may suggest a real effect. In relation to the use of the CARIES-QC-U to generate QALYs, it is possible that higher rates of self-reported toothbrushing may lead to differences in some specific questions and that these feed through to the utility estimates, albeit, to a degree that is not statistically significant. However, examination of the five questions from the CARIES-QC-U that are used to generate the utilities in the sensitivity analysis – ‘hurt’, ‘annoy’, ‘kept awake’, ‘hard to eat’ and ‘cried’ – suggests that toothbrushing is unlikely to have a major impact on most of these items.
The greater QALY gains seen in those schools with a greater proportion of pupils who are eligible for FSM is in line with the clinical analysis that saw an analogous effect on the primary outcome measure. This is suggestive of this economic result not being an artefact of the data; however, the incremental costs and QALYs remain very small, and statistically insignificant, even in the higher FSM group.
The results in relation to the schools in the pilot phase show bigger changes in incremental costs and QALYs, with the intervention having an 84% chance of being cost-effective. One possible reason for this greater effect could be due to the participants in the pilot phase having been studied prior to the COVID-19 pandemic; as such, participants were still attending school and following their normal routine.
It should also be recognised, too, that these findings relate to the costs and outcomes up to 2.5 years of the intervention being initiated. Longer-term modelling evaluation of effects on costs and outcomes were ruled out of the analysis based on the results of the clinical evaluation, which showed no robust evidence that such changes were possible.
The main strength of the economic analysis is the internal validity of the underlying data, with costs and QALYs being based on the trial data. The analysis was also undertaken in line with a pre-specified HEAP, from which there were only minor deviations (described in the limitations).
The major limitation is considered to be the uncertainty relating to treatment costs. Due to the low response rates for the parent/carer questionnaires, a novel approach to estimating visits for dental treatment was adopted. The validity of this approach has not been tested empirically. While we also produced results based on the parent/carer questionnaires in a sensitivity analysis, exploratory analysis showed that these results are expected to be biased due to data being missing not at random.
Measuring and valuing quality of life for children and young children presents methodological challenges. 130 In its latest guidance, NICE does not recommend specific measures of HRQoL for children and young people but recommends the use of a generic measure that has been shown to have good psychometric properties in the relevant age ranges. 131 The CHU9D, having been validated in a UK population aged 7–17 years, remains the most appropriate measure in keeping with NICE recommendations. 132 We also recognise that the ability of the CHU9D to detect changes in overall health that are related to oral health is limited. 115 An alternative approach would be to ignore the CHU9D data, thereby ignoring its associated QALYs, and generate a cost-effectiveness analysis directly based on the primary outcome measures (DICDAS4–6MFT), that is, produce an incremental cost per DICDAS4–6MFT avoided. Previous evaluations have used this approach; however, in the absence of a robust clinical effect, it is unlikely that such an analysis would be of value to decision-makers.
A subgroup analysis was planned for Scotland using the administrative data from Public Health Scotland. However, the data did not arrive in time for this report but will be analysed subsequently and published separately.
We also acknowledge that there were several deviations from the pre-specified HEAP as described in the remainder of this paragraph, but these are considered minor. First, an alternative source for the cost of day case dental surgery was identified for Scotland; while this is quite different to the original cost (see Appendix 5, Table 23), it has no impact on the primary analysis as costs related to day rate surgery are not included in that. Second, a sensitivity analysis was planned using charges for England based on 2020–1 data even though the 2018–9 data were considered more robust due to its absence of a ‘COVID-19 effect’. However, as the differences were negligible, the analysis was dropped. Third, the proposed subgroup analysis based on DICDAS4–6MFT was dropped in favour of an analysis based on the proportion of pupils eligible for FSM. This was undertaken as FSM was considered to be more relevant to potential policy-makers if they were to target the implementation of the intervention; identification of schools by DICDAS4–6MFT is not possible, whereas identification of schools based on the proportion of pupils eligible for FSM is straightforward. Finally, the proposed use of seemingly unrelated regression was abandoned after consideration of the distribution of dental treatment costs; it was felt that the highly irregular distribution was not suited to a method based on the assumption of normality and a multilevel model accounting for random effects was adopted instead.
Strengths and limitations of the BRIGHT trial
Strengths
This is one of the largest dental trials in the UK to investigate a novel approach to improving oral health of young people based on behaviour change theory. It used the gold-standard experimental design of a RCT, and successfully recruited from schools with above average proportions of pupils who are eligible for FSM. The methodology included an embedded process evaluation, rigorous health economic analysis and meaningful PPIE activities at the design stage and throughout the trial. The multidisciplinary nature of the team brought methodological experience of conducting school-based trials, with dental, public health, education and psychological expertise. The findings are likely to have implications for (1) secondary-school-based oral health promotion activities across the UK and potentially internationally and (2) the more general development and evaluation of mHealth interventions, particularly text message interventions aimed at young people. There is little information on the content, optimal frequency of or the length of time that text interventions should be sent to support health behaviour change and so this intervention was designed to be open-ended for the participants to decide on when they no longer wanted to receive them.
Limitations
The trial investigated a two-component behaviour change intervention: the CBS and the text messages. It was designed to fulfil the requirements of the commissioning brief (HTA number 15/166) which called for an evaluation of ‘a programme which initiates good oral health practice followed by a series of text or other media messages’ to improve toothbrushing in ‘deprived young people’ and, as a result, improve oral health. As required, the intervention was designed based on behaviour change theory with the two components acting together and so it was not possible to determine the relative contribution of each to the outcome.
The trial was based in schools and recruited pupils across two school year groups. During conception of the trial, consideration was given to the trial design including the unit of randomisation (school or year-group). Randomising at the school level would have minimised the potential for contamination between the intervention and control. Contamination in trials occurs when members of the control group receive the intervention. In this trial, this would have involved (but is not limited to) the school delivering the CBS to control pupils, and/or pupils in the control group independently seeking out technology that would remind them to brush their teeth twice a day. In the design where the unit of randomisation is the year group, as both the intervention and control conditions are present within each school, there is increased risk of contamination. However, a design whereby we randomised at the school level would have required a larger sample size (estimated to be at least 48 schools) relative to within-school randomisation. We decided to commence with the more efficient design of randomising year groups within schools and measure contamination during the pilot phase. This was achieved by asking schools to record who they delivered the CBS to, and asking pupils if, and from where, they had received useful messages about their teeth. We then inflated the sample size to allow for a level of assumed contamination, and it was determined that retaining this design was still more efficient than switching to randomising at the school level (which would have resulted in a larger, more expensive and time-consuming trial). In addition, the trial design was discussed with school representatives who expressed a preference for within-school randomisation, as this meant that at least one of their year groups received the intervention. Discussions regarding the trial design were held with the independent oversight committee (TSC and DMEC) including consideration of potential contamination and they supported a within-school randomisation design. However, it is possible that we did not capture all possible routes of contamination or that this was under-reported by schools and pupils. Increased contamination can dilute the observed treatment effect and so, if not properly accounted for, the trial can ultimately be underpowered to detect this smaller effect.
While the trial recruited well and achieved the target number of schools (n = 42), pupil recruitment was slightly lower, at 4680, than the target of 5040. The observed ICC for the primary outcome associated with school was 0.05, which was higher than the 0.02 assumed in the sample size calculation. Therefore, even if attrition had only reached 20%, as was allowed for in the sample size calculation, the trial would have been underpowered (~74%) to detect a difference from 34% to 26% in the primary outcome, even assuming some partial contamination.
Furthermore, follow-up data collection in secondary schools proved challenging, particularly where there were changes to key trial contacts, changes to the school leadership teams, school mergers and, for schools in England, changes to academy trust status. This was exacerbated by the length of the trial follow-up, particularly as the key trial contacts in the schools had to be changed several times. This was not only because of natural staff turnover but also because the trial contacts were often linked to the year group when the pupils were recruited and changed as the pupils progressed through the school. It is also worth noting the potential implications of these challenges on the implementation of the CBS as well as on data collection.
The biggest impact on the follow-up rate resulted from the COVID-19 pandemic, which led to a national shutdown of schools and prevented or disrupted follow-up data collection. Once schools reopened, several schools did not allow collection of any trial data, or only allowed collection of questionnaire data and not clinical assessments. Schools quoted particular pressures due to: participants in the final year of school were due to complete qualification certificates so research activities could not coincide with these periods of assessment; high levels of COVID-related staff and pupil absence; periods of local school shutdowns or remote learning; and prioritisation of flu and COVID-19 vaccine programmes. The timing of the data collection periods for the schools in the pilot and main trial phase coincided with peak infection rates of the virus particularly in the winter of 2021–2. In addition, during this time, in Scotland, many schools had policies in place which prevented visitors entering schools.
Ultimately, follow-up data collection rates in the trial were low. While the levels of missing data resulted in an underpowered trial to detect the difference assumed in the sample size calculation, ultimately the difference observed was smaller than this and not clinically significant anyway, so statistical significance was of less relevance. Due to the method of randomisation being at the year-group level, levels of missing data were similar in both groups, which lessens the chance for differential attrition and selection bias; however, differences in schools that completed follow-up and those that did not may limit the generalisability of the trial results.
The huge disruption to the lives of young people in terms of routine, schooling and home life caused by the pandemic will also have had an unquantifiable impact on the effect of the intervention. The aim of the intervention was to increase twice-daily toothbrushing within the young people’s normal day-to-day life. With the change in schooling for most pupils from in-person to online, instability of life and lack of routine during the pandemic, the intervention’s ability to establish toothbrushing within their daily routines and linked to specific activities at particular times of the day: waking and getting up (usually for school) and going to bed was likely to have been adversely affected.
The COVID-19 pandemic rendered in-person training and calibration impossible as of March 2020. To mitigate this, online training with one-to-one calibration in-person by a previously trained and experienced assessor was developed. In addition, pandemic restrictions meant examining dentists joining the trial team after March 2020 were trained in completion of the paper data collection forms online. There was significant turnover of examining dentists throughout data collection for a variety of reasons, and instances when dentists could not attend school visits due to COVID-19 infection or were reallocated to other duties because of the pandemic. This required significant allocation of staff, time and financial resources, ad hoc, to ensure school visits to facilitate data collection could proceed as planned in light of unforeseen examiner absence.
The limitations of the process evaluation include the potential for response bias from some participants as a result of the vouchers given to thank them for their time in participating in the focus group. Nonetheless, the data predominantly captured participants’ likes and dislikes of the intervention. It was not possible to gain more information from the participants who after receiving text messages for several weeks requested for them to stop as they could not be purposively sampled in order to maintain anonymity of participants. However, the reasons could be similar to those reported for blocking or muting the texts as a result of the frequency and repetitiveness, and consequent alert fatigue. Another limitation acknowledged is the challenges in assessing implementation fidelity as a result of insufficient data regarding the dose of the intervention.
The duration of this and other caries clinical trials
The primary outcome in this trial was dental caries and was measured clinically as per the commissioning brief. The protocol for this trial planned for the final clinical examination to be conducted 36 months after the baseline examination. This is traditionally regarded as the gold standard duration for a dental caries clinical trial that does not use radiographic examination to supplement the detection of carious lesions.
A consensus statement on clinical trial duration following an international workshop concluded that a 2- to 3-year period was sufficient to balance efficient trial design and disease progression. 133 On average, the time from baseline to final clinical examination was 35.9 months (this ranged from 26.7 to 45.9 months due to the pandemic). This is of sufficient duration for carious lesion progression and development. While work has been reported which suggests a duration of less than 2 years is acceptable when testing dental products,134,135 the conditions pertaining are not applicable to this pragmatic study.
It has also been suggested that proxy outcome measures such as determining changes in the oral microbiome and metabolome might remove the need for waiting 24 or 36 months, until the disease has shown enough signs of progress to be measured with clinical examination. 136 Currently, such proxy measures of caries clinical activity are insufficiently advanced for use in pragmatic clinical trials such as BRIGHT, although some, such as salivary lactic acid, are under active investigation. 137 Further, surrogate variables have been considered unacceptable as primary end points in caries clinical trials. 133
This intervention was designed to reduce dental caries through behaviour change to increase toothbrushing, and self-report toothbrushing was one the secondary outcomes. This is subject to reporting bias as most of the young people will know that the recommendation is to brush twice per day and this is reinforced in the CBS. The use of technology enhanced data collection methods, such as toothbrushes with built-in sensors that can record or send information centrally on when they are used, for how long and even how effectively, might be helpful. We looked at using these in the BRIGHT trial, but the cost was very high and there was concern that the use of electronic toothbrushes available at the time might themselves influence behaviour. However, it might now be possible to use a small sensor attached to a normal toothbrush to allow monitoring of its use and generate more accurate data.
Assessors were trained and calibrated at the tooth surface level using the ICDAS two-digit scoring system to build on International Caries Classification and Management System (ICCMS)-based training and maximise examiner sensitivity to the spectrum of caries presentation. For the analyses and primary outcome, the codes were collapsed and analysed at tooth level. Training and calibration of examining dentists presented challenges throughout the trial as new assessors had to be brought in and COVID-19 reassignment of duties and mitigations compounded this. Resource allocation during training updates and training of new assessors was significant as there was considerable turnover of examining dentists/dental therapists throughout data collection for a variety of reasons. Every new examining dentist/dental therapist joining the trial was subject to a programme of training (including through ICCMS e-learning and live online training) and calibration with experienced members of the trial team. An additional difficulty was related to the paucity of guidance or available literature on calibration levels that should be ideally reached associated with the ICDAS scoring system.
Recruitment and follow-up
Strategies to support recruitment of schools were assessed through the pilot trial with learning carried forward to help recruit schools for the main trial. Barriers to recruitment included competing demands within schools, changes in leadership and structures within schools and cold e-mail approaches being rarely successful. Successful strategies for recruitment included use of personal connections to make first contact with the schools, using a snowballing approach by asking participating schools to recommend others, making use of regional networks and events, and approaching schools that had already taken part in similar research studies. Similarly, successful retention strategies for the schools involved: creating and maintaining a close working relationship with schools and individual contacts within them, flexibility in offering administrative support on site and remotely (to suit individual school’s preferences), offering to contribute to school careers fairs and science events and issuing a yearly newsletter to schools taking part in the pilot.
Recruitment of young people followed a similar process of learning through the pilot trial and then focusing on the successful strategies in the main trial plus input from the BRIGHT Youth Forum. These included raising the BRIGHT profile within the school environment through assemblies with members of the LRTs attending and engaging senior members of the school to emphasise the importance of them participating as this influenced young people to consider participation. Of the 14,083 pupils approached, 4699 (33.4%) were eligible and consented to participate. As shown in Figure 2, there was a high proportion of pupils who declined to consent or who were not eligible. The literature suggests a range of pupil recruitment rates for school-based trials which vary depending on the target population, process for approaching and consenting pupils or parents/carers and the topic of the trial. 138–141 There are several potential reasons for the low pupil recruitment including: (1) insufficient interest in taking part in research that required completion of several forms and questionnaires; (2) worries about having a dental assessment conducted in school due to dental fear or embarrassment; and (3) concerns about revealing or remembering their mobile phone number.
Young people appeared to be grateful for the thank you voucher but being entered into a prize draw appeared to be less appealing. Having the BRIGHT team members visibly present in schools when undertaking baseline data collection and being available to answer queries about the trial; working with school staff to smooth pathways for recruitment and data collection; and continuing to recruit young people during baseline data collection periods within schools all seemed to aid recruitment of young people.
Carrying out the clinical dental data collection involved challenges prior to the COVID-19 pandemic and strategies that supported the process included having appointment lists available for the research teams and using ‘runners’ to collect young people from their classroom – sometimes this was a member of staff and sometimes a designated pupil, but also tannoy systems were used in some schools. Having an administrator from the team onsite to facilitate further recruitment or to locate missing pupils, while managing the paperwork and vouchers, was found to be essential. The time for the appointments was reduced by having young people complete questionnaires while waiting for their dental assessment and this allowed researchers to support, for example, in cases of reading difficulty. Keeping this time short was considered important by schools as for some data collection time points, young people in the trial were at the age of preparing for key exams and schools wanted to reduce young people’s time out of learning environments.
Ethical considerations
Collection of clinical data at dental examinations in schools was conducted in line with NDIP and CDHS guidance. 9,86 The BRIGHT trial was considered low risk, as defined by the National Statement on Ethical Conduct in Human Research, with no risk of harm to participants psychologically, socially, financially or culturally. 142 Participant discomfort during the dental examinations was the only anticipated risk. However, a single adverse event was reported, deemed ‘possibly related and unexpected’.
Text messages were sent twice daily to participants’ phones, by the University of Dundee’s HIC secure data management services. Participants’ phone numbers were linked with a unique participant ID only. Although participants could choose to stop and start the messages, they were informed via text message that the phone number was not monitored and there would be no reply. Despite this, some of the participants (61%) still sent replies other than, ‘START’ or ‘STOP’. Of these, the majority of responses (66%) were positive, often affirming intention to brush. However, management of participants who used the response element to raise concerns about their safety or welfare was considered and safeguarding protocols to investigate and manage these were developed. Of the 15 safeguarding concerns raised throughout data collection, 87% (n = 13) were about the content of text message replies. These were followed up by the trial team and managed in line with trial safeguarding protocols. Future trials based on mHealth interventions might anticipate significant responses to an electronic intervention and consider the staff and cost resources required to ensure duty of care to participants. Otherwise, consideration should be given to whether participants should be able to respond.
If a large-scale text-based or similar intervention was to be implemented, where users were able to respond in any way, staffing and economic costs to provide a safeguarding monitoring service should be carefully considered. Sporadic monitoring with ad hoc responses is likely to be unsustainable.
Patient and public involvement and engagement
The PPIE in the BRIGHT trial was described in Chapter 2 with young people, teachers, parents/carers and lay people involved throughout the trial design and conduct, with plans for involvement in the dissemination. The aim of the PPIE was to ensure the intervention was appropriate, to maximise recruitment and retention, and capture authentic views on the acceptability of the intervention. One of the strengths of the PPIE was the involvement of the BRIGHT Youth Forum run by Chilypep. Chilypep were allocated appropriate remuneration for them to work alongside the research team and BRIGHT Youth Forum at key stages of the trial. The peer-to-peer facilitation of the pupil focus groups by members of the BRIGHT Youth Forum was an innovative approach to PPIE not previously used in dental trials. Plans are in place for dissemination to ensure the findings of the trial are communicated to participants and young people in an appropriate way to reflect their views and interests.
The pre-TSC meetings held with the lay members of the TSC were very successful to enable their perspective to feed into the TSC more effectively. Although the text message schedule was discussed at length with the BRIGHT Youth Forum and was piloted for 2 weeks and then refined, the results of the qualitative components of the process evaluation suggest more extensive piloting may have been warranted to reduce the repetitiveness of the messages used. The reporting of PPIE meets the requirements of the Guidance for Reporting Involvement of Patients and Public 2 checklist. 143
Equality, diversity and inclusion
Principles of equality, diversity and inclusion have been embedded throughout the BRIGHT trial. The rationale for the trial itself came from an evidence gap about effective oral health promotion programmes for secondary-school children, particularly those children living in deprived areas who are traditionally an underserved group in research. In terms of participant representation, this was optimised by including schools with above average proportion of pupils eligible for FSM across England and two of the devolved nations. This approach was successful in reaching the target population, when we consider that the average proportion of pupils eligible for FSM among randomised schools was 24.2%, and with 21.9% of trial participants being eligible for FSM. This participant representation was achieved through PPIE, engagement of co-applicants and advisors, and intensive work by the research team to ensure participation was made as easy as possible for schools and pupils alike. This included bespoke approaches for each school based on their individual communication and contact requirements. The clinical and cost-effectiveness findings relating to pupils who received FSM, compared to non-FSM pupils, support further research in this field. From a methodological perspective, the use of the Indices of Multiple Deprivation across the devolved nations would warrant further development.
As mentioned in Strengths of the trial, the multidisciplinary nature of the team and strong PPIE helped to ensure an inclusive approach as possible was taken. The involvement of peer-to-peer facilitation of focus groups was particularly innovative. The list of staff acknowledged also demonstrate the involvement of teams across the UK, including junior and more senior colleagues in each LRT. Upon reflection, guidance to ensure Equality, Diversity and Inclusion has advanced significantly since the BRIGHT trial commenced which will ensure future trials extend the implementation of these principles further.
Summary of trial aim and objectives
This trial fulfilled its aim and objectives. The internal pilot phase was successfully completed and the feasibility components found to be appropriate for participant recruitment and data collection in the schools. The within-school, by-year, cluster randomisation was also found to be an appropriate method to allow the trial to be carried out. The intervention (CBS and text messages) had no effect on caries prevalence over 2.5 years and was not cost-effective. There was a 4% increase in twice-daily toothbrushing in the intervention group at 6 months but no effect on oral HRQoL.
The intervention was designed to change behaviour, but it is unclear just how effective a single lesson and text messages might be in sustaining behaviour change. The desired outcome, reduction in caries, is also quite distant from the improvements in toothbrushing behaviours reported particularly as participants had a cariogenic diet, an already high self-reported toothbrushing rate and other positive oral health behaviours. Together, these may not have allowed an intervention of this kind to translate into caries reduction, even with some evidence of increasing toothbrushing rates.
Conclusions
-
At 2.5 years, there was no evidence that the two-component intervention (CBS and text messages) had been clinically effective or cost-effective.
-
There was a short-term effect from the intervention indicated by a positive effect on toothbrushing behaviour at 6 months in the intervention group.
-
Over three-quarters of participants reported that they brushed their teeth at least twice per day and yet the proportion of participants with obvious decay experience was high at baseline and increased by almost 9.1% over 2.5 years.
-
The high rates of reported toothbrushing may have negatively affected any increase in toothbrushing rates being translated downstream into reduced caries experience.
-
Schools with higher proportions of FSM were targeted and although the intervention did not show benefit across all pupils in the intervention group, there was evidence of a difference (reduction) in obvious decay in pupils who received FSM, compared to non-FSM pupils, indicating that the intervention may have benefitted the most socioeconomically disadvantaged pupils. The positive QALY gain found for schools with higher levels of pupils eligible for FSM than schools with lower levels of FSM eligibility corroborates this.
-
The intervention was delivered as planned, generally implemented as intended and overall, pupils, staff members and stakeholders felt the intervention was acceptable.
-
There may be some clinical benefit to targeting an intervention for toothbrushing to the most socioeconomically deprived pupils but that a behaviour change intervention would need to be regularly reinforced. There is a need to look at other interventions to reduce caries rates in young people of secondary-school age.
Implications for practice
While there is little evidence from this trial to support the implementation of the two-component intervention (classroom-based lesson followed by SMS text reminders), the classroom-based lesson was codeveloped with young people and found to be acceptable when tested with teachers and young people in schools. With oral and dental health being part of the general health school curriculum across the UK, the BRIGHT Trial’s available and tested classroom-based lesson should be adopted for use as a standard part of the curriculum to allow schools to meet this learning outcome.
Recommendations for research
-
Development of behaviour change interventions to improve the oral health of young people targeting toothbrushing with fluoride toothpaste and for sugar reduction.
-
Improving understanding of the negating effect of sugar consumption on toothbrushing with fluoride toothpaste benefit in young people.
-
Investigations of differences in frequency of toothbrushing and FSM status to inform future targeting of similar interventions. Future research should build on the NIHR INCLUDE framework to include more underserved groups in research.
-
Developing understanding of the mechanism of impact of the intervention on toothbrushing behaviour.
-
Development of approaches to the evaluation of text message interventions including duration, frequency of messages, interactivity, dose and reach. In addition, with multicomponent interventions, further research is needed into how the component parts interact.
-
Investigating whether recording toothbrushing behaviours, such as with haptic toothbrushes, might help identify barriers and facilitators to toothbrushing.
Additional information
Contributions of authors
Zoe Marshman (https://orcid.org/0000-0003-0943-9637) (Professor of Dental Public Health, Co-Principal Investigator, Clinical Lead South Yorkshire) took overall responsibility for the trial and writing this report.
Hannah Ainsworth (https://orcid.org/0000-0001-8461-2183) (Research Fellow, Trial Manager) was responsible for day-to-day trial management and contributed to reviewing the report.
Caroline Fairhurst (https://orcid.org/0000-0003-0547-462X) (Senior Research Fellow – Statistician, co-applicant) wrote the statistical analysis plan and conducted the statistical analysis and contributed to writing and reviewing the report.
Katie Whiteside (https://orcid.org/0000-0002-7610-5429) (Research Fellow, Trial Coordinator) contributed to day-to-day trial co-ordination, data collection and to writing and reviewing the report.
Debbie Sykes (https://orcid.org/0000-0002-5726-9110) (Research Fellow, Trial Coordinator) contributed to day-to-day trial co-ordination, data collection and to writing and reviewing the report.
Anju Keetharuth (https://orcid.org/0000-0001-8889-6806) (Health Economist) co-wrote the health economics plan and performed the economic evaluation and contributed to writing and reviewing the report.
Sarab El Yousfi (https://orcid.org/0000-0001-9295-9953) (Research Associate) contributed to the process evaluation, quantitative and qualitative data collection and analysis and to writing and reviewing the report.
Emma Turner (https://orcid.org/0000-0001-8173-0248) (Trial Support Officer) supported day-to-day trial co-ordination and data collection and contributed to writing and reviewing the report.
Peter F Day (https://orcid.org/0000-0001-9711-9638) (Professor and Consultant in Paediatric Dentistry, Clinical Lead West Yorkshire, co-applicant) contributed to the design of the study, data collection and to reviewing the report.
Ivor G Chestnutt (https://orcid.org/0000-0002-9228-800X) (Professor of Dental Public Health, Clinical Lead South Wales, co-applicant) contributed to the design of the study, data collection and to writing and reviewing the report.
Simon Dixon (https://orcid.org/0000-0001-7394-7009) (Professor of Health Economics, Health Economist) co-wrote the health economics plan, performed the economic evaluation and contributed to writing and reviewing the report.
Ian Kellar (https://orcid.org/0000-0003-1608-5216) (Associate Professor of Health Psychology, Behaviour Change Lead, co-applicant) contributed to the design of the intervention and to reviewing the report.
Fiona Gilchrist (https://orcid.org/0000-0002-0418-6274) (Senior Lecturer in Paediatric Dentistry, co-applicant) contributed to data collection and analysis of questionnaire data and to writing and reviewing the report.
Mark Robertson (https://orcid.org/0000-0002-0005-3044) (Clinical Lecturer in Paediatric Dentistry, co-applicant) contributed to data collection, training/calibration for clinical examinations and to writing and reviewing the report.
Sue Pavitt (https://orcid.org/0000-0001-7447-440X) (Professor of Translational and Applied Health Research, Clinical Lead West Yorkshire, co-applicant) contributed to the design of the study, data collection and to reviewing the report.
Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor of Trials and Statistics, Co-Director of York Trials Unit, co-applicant) advised on the design and conduct of the trial and the statistical analysis and contributed to reviewing the report.
Donna Dey (https://orcid.org/0000-0002-0985-1210) (Education Lead, co-applicant) contributed to the design of the intervention, recruitment of schools and contributed to reviewing the report.
David Torgerson (https://orcid.org/0000-0002-1667-4275) (Co-Director of York Trials Unit, Methodological Expert, co-applicant) advised on the design and conduct of the trial and contributed to reviewing the report.
Lesley Pollard (https://orcid.org/0000-0002-0818-6749) (Children and Young People’s Empowerment Project, Trial Advisory Group member, co-applicant) was responsible for the link with Chilypep and the BRIGHT Youth Forum and contributed to reviewing the report.
Emma Manser (https://orcid.org/0000-0001-8236-3128) (Children and Young People’s Empowerment Project) led the co-ordination of the BRIGHT Youth Forum on behalf of Chilypep and contributed to reviewing the report.
Nassar Seifo (https://orcid.org/0000-0001-9748-5216) (Research Assistant) contributed to data collection and to writing and reviewing the report.
Mariana Araujo (https://orcid.org/0000-0002-4022-2105) (Research Assistant) contributed to data collection, training and calibration for clinical examinations and to writing and reviewing the report.
Waraf Al-Yaseen (https://orcid.org/0000-0002-7071-5074) (Research Assistant) contributed to data collection and to writing and reviewing the report.
Claire Jones (https://orcid.org/0000-0001-6136-7111) (Senior Software Engineer, co-applicant) led the team who developed and managed the software tool used to deliver the intervention text messages and contributed to reviewing the report.
Kate Hicks (https://orcid.org/0000-0002-6424-0164) (Research Fellow, Trial Coordinator) contributed to day-to-day trial co-ordination, data collection and to reviewing the report.
Kathryn Rowles (https://orcid.org/0000-0001-8465-2337) (Clinical Lecturer in Paediatric Dentistry) analysed the text message replies from intervention participants and contributed to reviewing the report.
Nicola Innes (https://orcid.org/0000-0002-9984-0012) (Professor of Paediatric Dentistry; Chief Investigator) took overall responsibility for the trial and writing this report.
Acknowledgments
The authors would like to thank all of the school staff, pupils and parents/carers who took part in the research, particularly for their commitment to working with us to find ways to support the trial and collect follow-up data during the difficult COVID restrictions. We would like to acknowledge the help of the BRIGHT Youth Forum and Children and Young People’s Empowerment Project (Chilypep) for their contributions to the design of the intervention and conduct of the trial and for their involvement and engagement, giving us young people’s views, throughout the trial, and mHealth for contributing to the design of the classroom-based session component of the intervention.
We are also grateful to the independent members of the Trial Steering Committee for their expert advice, guidance and support throughout the trial: Professor David Conway (Chair; Professor of Dental Public Health, University of Glasgow), Dr John Morris (Senior Lecturer in Dental Public Health, University of Birmingham), Professor Marjon Van der Pol (Professor of Health Economics, University of Aberdeen), Professor Tanya Walsh (Professor of Healthcare Evaluation, Statistician, University of Manchester), as well as Maria Clark (Patient Public Involvement Representative), Margaret Ogden (Patient Public Involvement Representative) and Irene Soulsby (Patient Public Involvement Representative).
Our thanks also goes to the independent members of the Data Monitoring and Ethics Committee for their expert advice, support and guidance throughout the trial: Dr Ben Styles (Chair; Head of Education Trials Unit, The National Foundation for Educational Research), Dr Christopher Vernazza (Clinical Senior Lecturer, Newcastle University) and Vicky Ryan (Lecturer in Biostatistics, Newcastle University).
We would like to thank the following members of the local research teams/dental teams, who are not listed as authors, for their hard work with schools: Hiba Al-Diwani (Dentist, West Yorkshire), Ayna Beden (Administrative Assistant, University of Leeds), James Bird (Clinical Fellow, South Yorkshire), Hazel Braid (Trial Administrator, University of Dundee), Lori Brown (Trial Administrator, University of Dundee), Heather D’Apice (Dental Team Support, South Yorkshire), Katy D’Apice (Research Dental Nurse, South Yorkshire), Chris Deery (Dental Epidemiologist, University of Sheffield), Louise Dell’Amico (Research Dental Nurse, West Yorkshire), Adele Dickinson (Dental Team, South Yorkshire), Joanne Ellis (Dental Team Support, South Yorkshire), Yasmen Elsadek (Dental Team, West Yorkshire), Joanne Fielding (Local Research Team Support, West Yorkshire), Helen Gledhill (Dentist, West Yorkshire), Rebecca Grewcock (Local Research Team support, Scotland), Iain Hay (Dentist, Scotland), Noor Jebur (Local Research Team, West Yorkshire), Louise Jones (Dental Nurse, South Wales), Susan Long (Trial Administrator, University of Dundee), Minnie Lyons-Coleman (Dentist, West Yorkshire), Joanna Mitchell (Research Dentist, West Yorkshire), Jane Morgan (Dentist, South Wales), Nuria Navarro-Coy (DenCTRU Operations and Strategic Manager, University of Leeds), Anna Nielsen (Research Dental Nurse, West Yorkshire), Fiona Sotir (Paediatric Dentist, South Yorkshire), Carron Paige (Research Dentist, West Yorkshire), Emma Pengelly (Clinical Lead Secretary, Cardiff University), Victoria Pickering (Clinical Lead Secretary, University of Dundee), Catherine Porter (Project Manager, University of Leeds), Daniela Raggio (Dental Team, Scotland), Helen Rogers (Local Research Team Support, South Yorkshire), Natalie Simpson (Dentist, South Wales), Lucy Slater (Dentist, West Yorkshire), Rebecca Smith (Administrative Assistant, University of Leeds), Annalea Staples (Dental Therapist, West Yorkshire), Louise Taylor (Dentist, Scotland), Fiona Thompson (Dental Nurse, Scotland), Heather Thompson (Research Assistant, West Yorkshire), Carolyn Tsang (Dental Team, South Yorkshire), Eleanor Webber (Trial Coordinator and PA to Prof Nicola Innes, Cardiff University), Julie Ann Williams (Dental Team, South Wales) and Rhianwen Williams (Dental Nurse, South Wales).
Our thanks also goes to the following staff from York Trials Unit, University of York, not listed as authors, for their contribution to this trial: Matthew Bailey (Software Development Team Lead), Sally Baker (York Trials Unit Administrative Manager), Sharon Eiles (Data Assistant), Louise Elliott (Trial Coordinator), Maddy Elliott (Data Administrator), Wendy Ellis (Data Assistant), Mirza Fazlic (Data Administrator), Imogen Fountain (Trial Support Officer), David Goodge (Data Administrator), Andrew Haynes (Trial Support Officer), Rachel Herdsman (Assistant Data Manager), Sue Heslop (Data Administrator), Jobie Kirkwood (Senior Web Developer), Heather Leggett (Research Fellow), Amy Marshall (Research Data Administrator), Tanya Pawson (Data Manager – Software Development), Dan Pearson (Data Manager – Design), Lyn Robinson-Smith (Trial Manager), Julie Ross (Data Administrator), Val Wadsworth (Lead Data Manager), Mo Wakefield (Data Administrator) and J Ward (Web Developer).
Our thanks also goes to the following other contributors: Emma Marshman (Freelance Medical Writer) who contributed to reviewing and editing the final report; Alicia Ridout (Digital Technology engagement), who provided support with intervention development; and Katherine Stevens (Health Economist, co-applicant, University of Sheffield).
Data-sharing statement
All data requests should be submitted to the chief investigator, Professor Nicola Innes, for consideration. Access to anonymised data may be granted following review.
Ethics statement
The BRIGHT trial was granted ethical approval by the East of Scotland Research Ethics Service on 14 August 2017 (REC reference number 17/ES/0096); the favourable opinion letter is provided on the NIHR BRIGHT project web page [National Institute for Health and Care Research. BRIGHT Trial: Brushing RemInder 4 Good Oral HealTh: The Clinical and Cost-Effectiveness of a Short Messaging Service Behaviour Change Programme to Improve the Oral Health of Young People Living in Deprived Areas. NIHR Funding and Awards. 2021. URL: www.fundingawards.nihr.ac.uk/award/15/166/08 (accessed 12 April 2021)]. Approval was also obtained from Research and Development offices at the participating NHS sites, NHS Tayside (on 23 August 2017) and Cardiff and Vale University Health Board (26 September 2017).
The Trial was registered with the International Standard Randomised Controlled Trial Register with the reference number ISRCTN12139369 and was funded by the National Institute for Health Research Health Technology Assessment Programme.
Information governance statement
All of the information collected was kept strictly confidential and held in accordance with the principles of the Data Protection Act 2018 (Great Britain. Data Protection Act 2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
University of York and Cardiff University both hold the role of data controllers; only the University of York holds the full trial data set and is responsible for secure archiving and data deletion.
Data Processing responsibilities were held in each of the Local Research Teams in Scotland (University of Dundee and Tayside Medical Science Centre), Wales (Cardiff University and Cardiff and Vale University Health Board), West Yorkshire (University of Leeds) and South Yorkshire (University of Sheffield); clear instructions were provided by the University of York for each stage to ensure data were processed suitably. All researchers working on the project completed Good Clinical Practice training and work to YTU and/or Tayside medical Science Centre and/or Cardiff University standard operating procedures.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/JQTA2103.
Primary conflicts of interest: Zoe Marshman, Caroline Fairhurst, Peter F Day, Ivor G Chestnutt, Ian Kellar, Fiona Gilchrist, Sue Pavitt, Catherine Hewitt, Donna Dey, David Torgerson, Lesley Pollard, Claire Jones and Nicola Innes were co-investigators on the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) Programme award for the BRIGHT trial (award ID: 15/166/08) and declare funding received by their institutions. Anju Keetharuth and Simon Dixon also declare funding made to their institutions from this award. Catherine Hewitt declares the roles of Deputy Chair and member of the NIHR HTA Commissioning Committee (1 November 2015–30 September 2022), as well as the following memberships: HTA Commissioning Sub-board (EOI; 1 April 2016–31 March 2017), NIHR CTU Standing Advisory Committee (1 May 2020–1 May 2024), HTA Post-funding Committee teleconference (POC members to attend; 1 February 2020–31 January 2023), HTA Funding Committee Policy Group (formerly CSG; 1 February 2020–31 January 2023), and HTA Fast Track Funding Committee (June 2021). Fiona Gilchrist declares membership of the HTA PCCPI Panel (1 January 2018–31 December 2018) and HTA Prioritisation Committee C (Mental health, women and children’s health; 1 January 2018–31 January 2019). Nicola Innes declares being a member of the Scottish Dental Clinical Effectiveness Group. Nicola Innes also declares other funding received from the NIHR HTA, Health and Care Research Wales, Tenovus Scotland (Glasgow, UK), internal institution awards from University of Dundee (Dundee, UK) and Cardiff University (Cardiff, UK). Nicola Innes also declares honoraria for work as an external assessor at Hong Kong University and the University of Malaysia. Sue Pavitt declares holding the role of National Lead of the NIHR Clinical Research Network National Oral and Dental Specialty Group (2019–present), membership on the Medical Research Council and NIHR Efficacy and Mechanism Evaluation (EME) Board (2012–8), membership on the EME Funding Committee Sub-group Remit and Comp (2015–7), membership on the NIHR Clinical Trials Unit Standing Advisory Board (2014–9), the role of Career Development Fellowship Chair for MS Society UK The Expert Review Network, and the role of Data Monitoring Ethics Committee Chair for a NIHR Research for Patient Benefit (RfPB) trial. Sue Pavitt also declares other grants awarded from the NIHR, including the NIHR HTA Programme, the NIHR Efficacy and Mechanism Evaluation (EME) Programme and the NIHR Clinical Research Network (CRN), as well as from Health Education England, MS Society UK (London, UK), and Leeds Hospital Charities (Leeds, UK). Peter F Day also declares other grants or contracts from the NIHR, Better Start Bradford (Bradford, UK), Health Education England, The Borrow Foundation (Waterlooville, UK), British Orthodontic Society (London, UK), European Orthodontic Society (London, UK), and White Rose University Consortium (York, UK). Peter F Day also declares institutional royalties or licenses and consulting fees from Oral-B, as well as institutional and personal honoraria from Oral B for giving lectures. Waraf Al-Yaseen declares funding from the Oral and Dental Research Trust (Birmingham, UK).
Publication
Innes N, Fairhurst C, Whiteside K, Ainsworth H, Sykes D, El Yousfi S et al. Behaviour change intervention for toothbrushing (lesson and text messages) to prevent dental caries in secondary school pupils: The BRIGHT randomized control trial. Community Dent Oral Epidemiol 2024;00:1–10. https://doi.org/10.1111/cdoe.12940
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Public Health England . Health Matters: Child Dental Health 2017. www.gov.uk/government/publications/health-matters-child-dental-health/health-matters-child-dental-health (accessed 15 June 2021).
- Welsh Government . The Oral Health and Dental Services Response 2018. https://gov.wales/sites/default/files/publications/2019-03/the-oral-health-and-dental-services-response.pdf (accessed 11 September 2022).
- NHS England . Outcome of 2022 23 Dental Contract Negotiations 2022. www.england.nhs.uk/wp-content/uploads/2022/07/B1802_First-stage-of-dental-reform-letter_190722.pdf (accessed 11 September 2022).
- Macpherson LM, Rodgers J, Conway DI. Childsmile after 10 years part 2: programme development, implementation and evaluation. Dent Update 2019;46:238-46. https://doi.org/10.12968/denu.2019.46.3.238.
- Walsh T, Worthington HV, Glenny AM, Marinho VCC, Jeroncic A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst Rev 2019;3. https://doi.org/10.1002/14651858.CD007868.pub3.
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res 2015;94. https://doi.org/10.1177/0022034515573272.
- Pitts NB, Chadwick B, Anderson T. Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children. England, Wales and Northern Ireland. Health and Social Care Information Centre; 2015.
- Merrett MCW, Goold S, Jones CM, Levin KA, McCall DR, Macpherson LMD, et al. National Dental Inspection Programme of Scotland: Report of the 2005 Survey of P7 Children. Scottish Dental Epidemiological Co-ordinating Committee; 2005.
- ISD Scotland . National Dental Inspection Programme (NDIP) 2015: Report of the 2015 Detailed National Dental Inspection Programme of Primary 7 Children and the Basic Inspection of Primary 1 and Primary 7 Children 2015. https://ndip.scottishdental.org/ndip-reports/ (accessed 8 February 2023).
- ISD Scotland . National Dental Inspection Programme (NDIP) 2019: Report of the 2019 Detailed Inspection Programme of Primary 7 Children and the Basic Inspection of Primary 1 and Primary 7 Children 2019. https://ndip.scottishdental.org/ndip-reports/ (accessed 8 February 2023).
- Jackson SL, Vann WF, Kotch JB, Pahel BT, Lee JY. Impact of poor oral health on children’s school attendance and performance. Am J Public Health 2011;101:1900-6. https://doi.org/10.2105/AJPH.2010.200915.
- Gilchrist F, Marshman Z, Deery C, Rodd HD. The impact of dental caries on children and young people: what they have to say?. Int J Paediatr Dent 2015;25:327-38. https://doi.org/10.1111/ipd.12186.
- Stennett M, Tsakos G. The impact of the COVID-19 pandemic on oral health inequalities and access to oral healthcare in England. Br Dent J 2022;232:109-14. https://doi.org/10.1038/s41415-021-3718-0.
- Adam M. Chart of the Week: How Has the Pandemic Affected Inequalities in Tooth Extractions for Children? 2022. www.nuffieldtrust.org.uk/resource/chart-of-the-week-how-has-the-pandemic-affected-inequalities-in-tooth-extractions-for-children (accessed 11 September 2022).
- Krisdapong S, Prasertsom P, Rattanarangsima K, Sheiham A. School absence due to toothache associated with sociodemographic factors, dental caries status, and oral health-related quality of life in 12- and 15-year-old Thai children. J Public Health Dent 2013;73:321-8. https://doi.org/10.1111/jphd.12030.
- Knapp R, Gilchrist F, Rodd HD, Marshman Z. Change in children’s oral health-related quality of life following dental treatment under general anaesthesia for the management of dental caries: a systematic review. Int J Paediatr Dent 2017;27:302-12. https://doi.org/10.1111/ipd.12259.
- Knapp R, Marshman Z, Gilchrist F, Rodd H. The impact of dental caries and its treatment under general anaesthetic on children and their families. Eur Arch Paediatr Dent 2021;22:567-74. https://doi.org/10.1007/s40368-020-00591-1.
- Gilchrist F, Rodd HD, Deery C, Marshman Z. Development and evaluation of CARIES-QC: a caries-specific measure of quality of life for children. BMC Oral Health 2018;18. https://doi.org/10.1186/s12903-018-0662-8.
- Paisi M, Plessas A, Pampaka D, Burns L, Witton R. Effect of treating carious teeth on children’s and adolescents’ anthropometric outcomes: a systematic review of randomised controlled trials. Community Dent Health 2020;37:32-8. https://doi.org/10.1922/CDH_4611Paisi07.
- Abed R, Bernabe E, Sabbah W. Family impacts of severe dental caries among children in the United Kingdom. Int J Environ Res Public Health 2020;17. https://doi.org/10.3390/ijerph17010109.
- Public Health England . Hospital Tooth Extractions of 0 to 19 Year Olds 2021. www.gov.uk/government/publications/hospital-tooth-extractions-of-0-to-19-year-olds (accessed 5 August 2022).
- Lewis C, Stout J. Toothache in US children. Arch Pediatr Adolesc Med 2010;164:1059-63. https://doi.org/10.1001/archpediatrics.2010.206.
- NHS England . Improving Dental Care and Oral Health – A Call to Action 2017. www.england.nhs.uk/2014/02/improve-dental-cta/.
- GOV.UK . Adult Oral Health: Applying All Our Health 2022. www.gov.uk/government/publications/adult-oral-health-applying-all-our-health/adult-oral-health-applying-all-our-health (accessed 11 September 2022).
- Righolt AJ, Jevdjevic M, Marcenes W, Listl S. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J Dent Res 2018;97:501-7. https://doi.org/10.1177/0022034517750572.
- Listl S, Galloway J, Mossey PA, Marcenes W. Global economic impact of dental diseases. J Dent Res 2015;94:1355-61. https://doi.org/10.1177/0022034515602879.
- Pitts NB, Twetman S, Fisher J, Marsh PD. Understanding dental caries as a non-communicable disease. Br Dent J 2021;231:749-53. https://doi.org/10.1038/s41415-021-3775-4.
- Scottish Dental Clinical Effectiveness Programme . Prevention and Management of Dental Caries in Children: Dental Clinical Guidance 2018. www.sdcep.org.uk/published-guidance/caries-in-children/ (accessed 8 February 2023).
- Innes NP, Chu CH, Fontana M, Lo EC, Thomson WM, Uribe S, et al. A century of change towards prevention and minimal intervention in cariology. J Dent Res 2019;98:611-7. https://doi.org/10.1177/0022034519837252.
- Tsakos G, Hill K, Chadwick B, Anderson T. Children’s Dental Health Survey 2013. Report 1: Attitudes, Behaviours and Children’s Dental Health. England, Wales and Northern Ireland, 2013 2015. https://digital.nhs.uk/data-and-information/publications/statistical/children-s-dental-health-survey/child-dental-health-survey-2013-england-wales-and-northern-ireland (accessed 15 June 2021).
- White D, Steele J, Fuller E. Children’s Dental Health Survey 2013. Report 3: Good Oral Health in Children. England, Wales and Northern Ireland 2015. https://digital.nhs.uk/data-and-information/publications/statistical/children-s-dental-health-survey/child-dental-health-survey-2013-england-wales-and-northern-ireland (accessed 8 February 2023).
- Verrips GH, Kalsbeek H, Van Woerkum CM, Koelen M, Kok-Weimar TL. Correlates of toothbrushing in preschool children by their parents in four ethnic groups in The Netherlands. Community Dent Health 1994;11:233-9.
- Zeedyk MS, Longbottom C, Pitts NB. Tooth-brushing practices of parents and toddlers: a study of home-based videotaped sessions. Caries Res 2005;39:27-33. https://doi.org/10.1159/000081653.
- White DJ, Kozak KM, Gibb R, Dunavent J, Klukowska M, Sagel PA. A 24-hour dental plaque prevention study with a stannous fluoride dentifrice containing hexametaphosphate. J Contemp Dent Pract 2006;7:1-11.
- Pine CM, Adair PM, Nicoll AD, Burnside G, Petersen PE, Beighton D, et al. International comparisons of health inequalities in childhood dental caries. Community Dent Health 2004;21:121-30.
- American Academy of Pediatric Dentistry . Guideline on adolescent oral health care. Pediatr Dent 2015;37:151-8.
- Skinner J, Johnson G, Blinkhorn A, Byun R. Factors associated with dental caries experience and oral health status among New South Wales adolescents. Aust N Z J Public Health 2014;38:485-9. https://doi.org/10.1111/1753-6405.12245.
- Tsai C, Raphael S, Agnew C, McDonald G, Irving M. Health promotion interventions to improve oral health of adolescents: a systematic review and meta-analysis. Community Dent Oral Epidemiol 2020;48:549-60. https://doi.org/10.1111/cdoe.12567.
- Webb T, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res 2010;12. https://doi.org/10.2196/jmir.1376.
- Calderon SJ, Mallory C. A systematic review of oral health behavior research in American adolescents. J Sch Nurs 2014;30:396-403. https://doi.org/10.1177/1059840514544034.
- Albino J, Tiwari T. Preventing childhood caries: a review of recent behavioral research. J Dent Res 2016;95:35-42. https://doi.org/10.1177/0022034515609034.
- Kay E, Vascott D, Hocking A, Nield H, Dorr C, Barrett H. A review of approaches for dental practice teams for promoting oral health. Community Dent Oral Epidemiol 2016;44:313-30. https://doi.org/10.1111/cdoe.12220.
- Public Health England . Achieving Behaviour Change. A Guide for Local Government and Partners 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/875385/PHEBI_Achieving_Behaviour_Change_Local_Government.pdf (accessed 11 August 2022).
- Mohr DC, Schueller SM, Montague E, Burns MN, Rashidi P. The behavioral intervention technology model: an integrated conceptual and technological framework for eHealth and mHealth interventions. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3077.
- WHO Global Observatory for eHealth . MHealth: New Horizons for Health through Mobile Technologies: Second Global Survey on EHealth 2011. https://apps.who.int/iris/handle/10665/44607 (accessed 13 February 2023).
- Nilsen W, Kumar S, Shar A, Varoquiers C, Wiley T, Riley WT, et al. Advancing the science of mHealth. J Health Commun 2012;17:5-10. https://doi.org/10.1080/10810730.2012.677394.
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med 2009;36:165-73. https://doi.org/10.1016/j.amepre.2008.09.040.
- Armanasco AA, Miller YD, Fjeldsoe BS, Marshall AL. Preventive health behavior change text message interventions: a meta-analysis. Am J Prev Med 2017;52:391-402. https://doi.org/10.1016/j.amepre.2016.10.042.
- Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 2010;32:56-69. https://doi.org/10.1093/epirev/mxq004.
- Ofcom . Children and Parents: Media Use and Attitudes Report 2022 2022. www.ofcom.org.uk/research-and-data/media-literacy-research/childrens/children-and-parents-media-use-and-attitudes-report-2022 (accessed 13 February 2023).
- Ofcom . Children and Parents: Media Use and Attitudes Report 2017 2017. www.ofcom.org.uk/__data/assets/pdf_file/0020/108182/children-parents-media-use-attitudes-2017.pdf (accessed 15 June 2021).
- Fernández CE, Maturana CA, Coloma SI, Carrasco-Labra A, Giacaman RA. Teledentistry and mHealth for promotion and prevention of oral health: a systematic review and meta-analysis. J Dent Res 2021;100:914-27. https://doi.org/10.1177/00220345211003828.
- Toniazzo MP, Nodari D, Muniz FWMG, Weidlich P. Effect of mHealth in improving oral hygiene: a systematic review with meta-analysis. J Clin Periodontol 2019;46:297-309. https://doi.org/10.1111/jcpe.13083.
- Lima IFP, de Andrade Vieira W, de Macedo Bernardino I, Costa PA, Lima APB, Pithon MM, et al. Influence of reminder therapy for controlling bacterial plaque in patients undergoing orthodontic treatment: a systematic review and meta-analysis. Angle Orthod 2018;88:483-93. https://doi.org/10.2319/111117-770.1.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The Behavior Change Technique Taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Schluter P, Lee M, Hamilton G, Coe G, Messer-Perkins H, Smith B. Keep on brushing: a longitudinal study of motivational text messaging in young adults aged 18–24 years receiving work and income support. J Public Health Dent 2015;75:118-25. https://doi.org/10.1111/jphd.12079.
- Champion VL, Skinner CS. Health Behavior and Health Education – Theory, Research and Practice. Hoboken, NJ: Jossey-Bass/Wiley; 2008.
- Marshman Z, Ainsworth H, Chestnutt IG, Day PF, Dey D, El Yousfi S, et al. Brushing RemInder 4 Good oral HealTh (BRIGHT) trial: does an SMS behaviour change programme with a classroom-based session improve the oral health of young people living in deprived areas? A study protocol of a randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3538-6.
- National Institute for Health and Care Research . BRIGHT Trial: Brushing RemInder 4 Good Oral HealTh: The Clinical and Cost-Effectiveness of a Short Messaging Service Behaviour Change Programme to Improve the Oral Health of Young People Living in Deprived Areas 2021. www.fundingawards.nihr.ac.uk/award/15/166/08 (accessed 12 April 2021).
- Ainsworth H, Marshman Z, Whiteside K, Sykes D, Fairhurst C, Turner E, et al. Set up and assessment of progression criteria for internal pilots: the Brushing RemInder 4 Good oral HealTh (BRIGHT) trial example. Pilot Feasibility Stud 2023;9. https://doi.org/10.1186/s40814-023-01243-z.
- Department for Education . Schools, Pupils and Their Characteristics: January 2016 2016. www.gov.uk/government/statistics/schools-pupils-and-their-characteristics-january-2016 (accessed 18 June 2021).
- Welsh Government . School Census Results, 2016 2016. https://statswales.gov.wales/Catalogue/Education-and-Skills/Schools-and-Teachers/Schools-Census/Pupil-Level-Annual-School-Census/Provision-of-Meals-and-Milk/pupilseligibleforfreeschoolmeals-by-school-year (accessed 28 June 2017).
- Scottish Government . Summary Statistics for Attainment, Leaver Destinations and Healthy Living, No.6: 2016 Edition 2016. http://www.gov.scot/Topics/Statistics/Browse/School-Education/SchoolMealsDatasets/schmeals2016 (accessed 31 March 2017).
- GOV.UK . Get Information about Schools 2021. www.get-information-schools.service.gov.uk/ (accessed 18 June 2021).
- StatsWales . Pupil Level Annual School Census (PLASC) 2021. https://statswales.gov.wales/Catalogue/Education-and-Skills/Schools-and-Teachers/Schools-Census/Pupil-Level-Annual-School-Census (accessed 16 June 2021).
- Scottish Government . School Education Statistics 2020. www.gov.scot/collections/school-education-statistics/ (accessed 18 June 2021).
- Murray DM. Design and Analysis of Group-randomized Trials. Oxford: Oxford University Press; 1998.
- White DA, Chadwick BL, Nuttall NM, Chestnutt IG, Steele JG. Oral health habits amongst children in the United Kingdom in 2003. Br Dent J 2006;200:487-91. https://doi.org/10.1038/sj.bdj.4813523.
- Cocks K, Torgerson DJ. Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol 2013;66:197-201. https://doi.org/10.1016/j.jclinepi.2012.09.002.
- Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31:180-91. https://doi.org/10.1002/nur.20247.
- Twetman S, Axelsson S, Dahlgren H, Holm A-K, Källestål C, Lagerlöf F, et al. Caries-preventive effect of fluoride toothpaste: a systematic review. Acta Odontol Scand 2003;61:347-55. https://doi.org/10.1080/00016350310007590.
- Pine C, Adair P, Robinson L, Burnside G, Moynihan P, Wade W, et al. The BBaRTS Healthy Teeth Behaviour Change Programme for preventing dental caries in primary school children: study protocol for a cluster randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1226-3.
- Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol 2008;57:1-29. https://doi.org/10.1111/j.1464-0597.2007.00325.x.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. London: Silverback Publishing; 2014.
- Marshman Z, El-Yousfi S, Kellar I, Dey D, Robertson M, Day P, et al. Development of a secondary school-based digital behaviour change intervention to improve tooth brushing. BMC Oral Health 2021;21. https://doi.org/10.1186/s12903-021-01907-3.
- Department for Education . Science Programmes of Study: Key Stage 3 National Curriculum in England 2013. www.gov.uk/government/publications/national-curriculum-in-england-science-programmes-of-study (accessed 12 April 2021).
- Department for Education . Science Programmes of Study: Key Stage 4 National Curriculum in England 2014. www.gov.uk/government/publications/national-curriculum-in-england-science-programmes-of-study (accessed 12 April 2021).
- PSHE Association . PSHE Education Programme of Study (Key Stage 3) (online) 2014. https://pshe-association.org.uk/key-stage-3-5.
- Learning and Teaching Scotland . Curriculum for Excellence, Health and Wellbeing: Experiences and Outcomes (online). Education Scotland 2009. https://education.gov.scot/media/5p4dvqvm/health-and-wellbeing-eo.pdf (accessed 12 April 2021).
- Learning and Teaching Scotland . Curriculum for Excellence, Science: Experiences and Outcomes (online) 2009. https://education.gov.scot/curriculum-for-excellence/curriculum-for-excellence-documents/experiences-and-outcomes/ (accessed 12 April 2021).
- Welsh Assembly Government . Personal and Social Education Framework for 7–19 Year Olds in Wales (online) 2008. https://hwb.gov.wales/curriculum-2008/key-stages-2-to-4/personal-and-social-education-framework-for-7-to-19-year-olds-in-wales (accessed 12 April 2021).
- Crombie IK, Irvine L, Williams B, Sniehotta FF, Petrie D, Jones C, et al. Texting to Reduce Alcohol Misuse (TRAM): main findings from a randomized controlled trial of a text message intervention to reduce binge drinking among disadvantaged men. Addiction 2018;113:1609-18. https://doi.org/10.1111/add.14229.
- Dombrowski SU, McDonald M, Van Der Pol M, Grindle M, Avenell A, Carroll P, et al. Game of Stones: feasibility randomised controlled trial of how to engage men with obesity in text message and incentive interventions for weight loss. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-032653.
- Pitts N. ‘ICDAS’ – an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health 2004;21:193-8.
- Gil GS, Morikava FS, Santin GC, Pintarelli TP, Fraiz FC, Ferreira FM. Reliability of self-reported toothbrushing frequency as an indicator for the assessment of oral hygiene in epidemiological research on caries in adolescents: a cross-sectional study. BMC Med Res Methodol 2015;15:1-7. https://doi.org/10.1186/s12874-015-0002-5.
- Anderson T, Thomas C, Ryan R, Dennes M, Fuller E. Children’s Dental Health Survey 2013. Technical Report. England, Wales and Northern Island 2015. https://digital.nhs.uk/data-and-information/publications/statistical/children-s-dental-health-survey/child-dental-health-survey-2013-england-wales-and-northern-ireland (accessed 25 January 2021).
- Gholami M, Knoll N, Schwarzer R. A brief self-regulatory intervention increases dental flossing in adolescent girls. Int J Behav Med 2015;22:645-51. https://doi.org/10.1007/s12529-014-9459-6.
- Kamalikhah T, Mazllomi Mahmood abad S, Khalighinejad N, Rahmati-Najarkolaei F. Dental flossing behaviour and its determinants among students in a suburb area of Tehran–Iran: using transtheoretical model. Int J Dent Hyg 2017;15:106-12. https://doi.org/10.1111/idh.12154.
- Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc 1962;65:26-9. https://doi.org/10.14219/jada.archive.1962.0184.
- Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol 1970;41:41-3. https://doi.org/10.1902/jop.1970.41.1.41.
- Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 1963;21:533-51.
- Goldberg P, Matsson L, Anderson H. Partial recording of gingivitis and dental plaque in children of different ages and in young adults. Community Dent Oral Epidemiol 1985;13:44-6. https://doi.org/10.1111/j.1600-0528.1985.tb00419.x.
- Stevens M, Hainsworth KR, Weisman SJ, Layde PM. PedsQL Performance in the Pediatric Emergency Department: Can We use HRQOL for Acute Care Outcomes?(FR). In Quality of Life Research 2012;20:96-.
- Stevens K. Valuation of the child health utility 9D index. PharmacoEcon 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Gilchrist F. Development of a Child-centred, Caries-specific Seasure of Oral Health-related Quality of Life. Sheffield: University of Sheffield; 2015.
- Rogers HJ, Sagabiel J, Marshman Z, Rodd HD, Rowen D. Adolescent valuation of CARIES-QC-U: a child-centred preference-based measure of dental caries. Health Qual Life Outcomes 2022;20:1-15. https://doi.org/10.1186/s12955-022-01918-w.
- StatsWales . Pupils Eligible for Free School Meals by School 2020. https://statswales.gov.wales/Catalogue/Education-and-Skills/Schools-and-Teachers/Schools-Census/Pupil-Level-Annual-School-Census/Provision-of-Meals-and-Milk/pupilseligibleforfreeschoolmeals-by-school-year (accessed 18 June 2021).
- Scottish Government . School Meals Dataset Set 2016 2016. http://www.gov.scot/Topics/Statistics/Browse/School-Education/SchoolMealsDatasets/schmeals2016 (accessed 28 June 2017).
- Scottish Government . School Meals Dataset Set 2017 2017. www.gov.scot/Topics/Statistics/Browse/School-Education/SchoolMealsDatasets/schmeals2017 (accessed 18 August 2022).
- Estyn . Inspection 2022. www.estyn.gov.wales/inspection (accessed 19 September 2022).
- Education Scotland . Inspection Reports 2022. https://education.gov.scot/education-scotland/inspection-reports/?orderBy=dateDescending (accessed 10 October 2022).
- Guide to the Methods of Technology Appraisal 2022. London: NICE; 2022.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- El Alili M, van Dongen JM, Goldfeld KS, Heymans MW, van Tulder MW, Bosmans JE. Taking the analysis of trial-based economic evaluations to the next level: the importance of accounting for clustering. PharmacoEcon 2020;38:1247-61. https://doi.org/10.1007/s40273-020-00946-y.
- Faria R, Gome M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. San Francisco: Jossey-Bass; 2002.
- Elyousfi S, Innes N, Leggett H, Ainsworth H, Chestnutt IG, Day P, et al. Acceptability of the Brushing RemInder 4 Good oral HealTh (BRIGHT) trial intervention: a qualitative study of perspectives of young people and school staff. BMC Oral Health 2022;22:1-12. https://doi.org/10.1186/s12903-022-02073-w.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:1-13. https://doi.org/10.1186/s12913-017-2031-8.
- Ritchie J, Lewis J, Nicholls CM, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2013.
- INVOLVE . Payment for Involvement: A Guide for Making Payments to Members of the Public Actively Involved in NHS, Public Health and Social Care Research 2010. www.invo.org.uk/wp-content/uploads/documents/INVOLVEPayment%20Guiderev2012.pdf (accessed 4 August 2022).
- StatsWales . Schools by Local Authority, Region and Type of School 2016. https://statswales.gov.wales/Catalogue/Education-and-Skills/Schools-and-Teachers/Schools-Census/Pupil-Level-Annual-School-Census/Schools/schools-by-localauthorityregion-type (accessed 15 August 2022).
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica 2012;22:276-82.
- Department for Education . Changes to Personal, Social, Health and Economic (PSHE) and Relationships and Sex Education (RSE). GOV.UK 2019. www.gov.uk/government/publications/changes-to-personal-social-health-and-economic-pshe-and-relationships-and-sex-education-rse (accessed 23 November 2021).
- Foster Page LA, Thomson WM, Marshman Z, Stevens KJ. The potential of the Child Health Utility 9D Index as an outcome measure for child dental health. BMC Oral Health 2014;14:1-7. https://doi.org/10.1186/1472-6831-14-90.
- Baherimoghadam T, Naseri N, Hamedani S, Nikmehr S, Mokhtar M. Influence of multimedia reminders on oral hygiene status during removable orthodontic treatment: a randomized controlled trial. J Orthod Sci 2022;11. https://doi.org/10.4103/jos.jos_193_21.
- Santos JB, Pereira LC, Neves JG, Pithon MM, Santamaria M. Can text messages encourage flossing among orthodontic patients?. Angle Orthod 2021;91:650-5. https://doi.org/10.2319/012121-61.1.
- World Health Organization . Promoting Health through Schools: Report of a WHO Expert Committee on Comprehensive School Health Education and Promotion 1997.
- Badawy SM, Kuhns LM. Texting and mobile phone app interventions for improving adherence to preventive behavior in adolescents: a systematic review. JMIR MHealth UHealth 2017;5. https://doi.org/10.2196/mhealth.6837.
- MacDougall S, Jerrott S, Clark S, Campbell LA, Murphy A, Wozney L. Text message interventions in adolescent mental health and addiction services: scoping review. JMIR Ment Health 2021;8. https://doi.org/10.2196/16508.
- Kocielnik R, Hsieh G. Send me a different message: utilizing cognitive space to create engaging message triggers 2017:2193-207.
- Muench F, Baumel A. More than a text message: dismantling digital triggers to curate behavior change in patient-centered health interventions. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.7463.
- Loescher LJ, Rains SA, Kramer SS, Akers C, Moussa R. A systematic review of interventions to enhance healthy lifestyle behaviors in adolescents delivered via mobile phone text messaging. Am J Health Promot AJHP 2018;32:865-79. https://doi.org/10.1177/0890117116675785.
- Peng W, Kanthawala S, Yuan S, Hussain SA. A qualitative study of user perceptions of mobile health apps. BMC Public Health 2016;16:1-11. https://doi.org/10.1186/s12889-016-3808-0.
- Ramsey A, Lord S, Torrey J, Marsch L, Lardiere M. Paving the way to successful implementation: identifying key barriers to use of technology-based therapeutic tools for behavioral health care. J Behav Health Serv Res 2016;43:54-70. https://doi.org/10.1007/s11414-014-9436-5.
- Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr 2004;7:245-50. https://doi.org/10.1079/phn2003592.
- Masood M, Masood Y, Newton T. Impact of national income and inequality on sugar and caries relationship. Caries Res 2012;46:581-8. https://doi.org/10.1159/000342170.
- Moores CJ, Kelly SM, Moynihan PJ. Systematic review of the effect on caries of sugars intake: ten-year update. J Dent Res 2022;101:1034-45. https://doi.org/10.1177/00220345221082918.
- Ha DH, Spencer AJ, Moynihan P, Thomson WM, Do LG. Excess risk of dental caries from higher free sugars intake combined with low exposure to water fluoridation. J Dent Res 2021;100:1243-50. https://doi.org/10.1177/00220345211007747.
- Rowen D, Rivero-Arias O, Devlin N, Ratcliffe J. Review of valuation methods of preference-based measures of health for economic evaluation in child and adolescent populations: where are we now and where are we going?. PharmacoEcon 2020;38:325-40. https://doi.org/10.1007/s40273-019-00873-7.
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual. Process and Methods [PMG36] 2022. www.nice.org.uk/process/pmg36 (accessed 8 February 2023).
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Pitts NB, Stamm JW. International Consensus Workshop on Caries Clinical Trials (ICW-CCT)—final consensus statements: agreeing where the evidence leads. J Dent Res 2004;83:125-8. https://doi.org/10.1177/154405910408301s27.
- Chesters RK, Pitts NB, Matuliene G, Kvedariene A, Huntington E, Bendinskaite R, et al. An abbreviated caries clinical trial design validated over 24 months. J Dent Res 2002;81:637-40. https://doi.org/10.1177/154405910208100912.
- Ellwood RP, Goma J, Pretty IA. Caries clinical trial methods for the assessment of oral care products in the 21st century. Adv Dent Res 2012;24:32-5. https://doi.org/10.1177/0022034512449464.
- da Costa Rosa T, de Almeida Neves A, Azcarate-Peril MA, Divaris K, Wu D, Cho H, et al. The bacterial microbiome and metabolome in caries progression and arrest. J Oral Microbiol 2021;13. https://doi.org/10.1080/20002297.2021.1886748.
- National Institute for Health and Care Research (NIHR) . Elucidating the MechaNism of Action of Behavioural Changes on Child TOoth Decay in the Randomised Controlled CHOICE Trial a Role for Salivary Lactic Acid? (ENCOURAGE) 2023. https://fundingawards.nihr.ac.uk/award/NIHR151317 (accessed 8 February 2023).
- Sutherland R, Ying Ooi J, Finch M, Yoong SL, Nathan N, Wrigley J, et al. A cluster randomised controlled trial of a secondary school intervention to reduce intake of sugar-sweetened beverages: mid-intervention impact of switchURsip environmental strategies. Health Promot J Aust Off J Aust Assoc Health Promot Prof 2022;33:176-86. https://doi.org/10.1002/hpja.469.
- Perry Y, Werner-Seidler A, Calear A, Mackinnon A, King C, Scott J, et al. Preventing depression in final year secondary students: school-based randomized controlled trial. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8241.
- Glazebrook C, Mullen N, McWilliams L, Batty M, Hogarth L, Macdonald I, et al. Factors influencing recruitment to a school-based trial of a targeted intervention to promote exercise self-efficacy in children with risk factors for obesity. Pediatr Res 2011;70:375-. https://doi.org/10.1038/pr.2011.600.
- Lohan M, Aventin A, Maguire L, Curran R, McDowell C, Agus A, et al. Increasing boys’ and girls’ intention to avoid teenage pregnancy: a cluster randomised control feasibility trial of an interactive video drama based intervention in post-primary schools in Northern Ireland. Public Health Res 2017;5:1-344. https://doi.org/10.3310/phr05010.
- National Health and Medical Research Council . 2007 National Statement on Ethical Conduct in Human Research 2007. www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018 (accessed 13 February 2023).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Conner M, Grogan S, Simms-Ellis R, Flett K, Sykes-Muskett B, Cowap L, et al. Evidence that an intervention weakens the relationship between adolescent electronic cigarette use and tobacco smoking: a 24-month prospective study. Tob Control 2020;29:425-31. https://doi.org/10.1136/tobaccocontrol-2018-054905.
Appendix 1 Ethical and protocol amendments
| Amendment no./date | Amendment type | Summary of changes | Protocol version |
|---|---|---|---|
| N/A | N/A | Version submitted with original ethics application | V1_20170705 |
| N/A | N/A | Change to method of sending information about the study to parent/carers; change to procedure in cases of suspected serious pathology; addition of procedure for suspected child safeguarding issues; correction of minor typographic errors. Version approved when the trial was granted a favourable ethical opinion. |
V2_20170802 |
| AM01/17 October 2017 | Substantial | Changes to the wording regarding the criteria for progression amended to outline that criteria will be considered holistically, addition of use of data linkage for cost-effectiveness analysis, clarification of which team members will be blinded, addition of sending opt-out forms to parent/carers via the pupils, correction of minor typographic errors. | V3_20171004 |
| AM02/19 November 2017 | Non-substantial | Minor changes to baseline pupil, dental and parent/carer CRFs to clarify processes and correct inconsistencies. | V3_20171004 |
| AM03/9 April 2018 | Substantial | Amendment of the timings of follow-up due to delays in recruitment of some schools and the necessity for FU2 questionnaires for progression criteria. | V4_20180406 |
| AM04/23 July 2018 | Substantial | Changes to protocol to reflect changes to study procedures ahead of moving to main trial: sample size recalculation, changes to pupil consent procedure, changes to the parent/carer questionnaire procedure, addition of parent/carer prize draw for those who return a questionnaire, removal of pupil prize draw. | V5.0_20180718 |
| AM05/29 August 2018 | Non-substantial | Minor typographical changes to protocol – change to wording of data management and confidentiality section around the joint data controllers | V5.1_20180823 |
| AM06/26 September 2018 | Non-substantial | Wording amended in protocol to clarify process of sending out parent/carer questionnaire and reminder 2 weeks later; clarification of time points that the parent/carer follow-up questionnaires will be sent; minor change to wording in cost-effectiveness section around pupil consent. | V5.2_20180914 |
| AM07/1 May 2019 | Substantial | Addition of the qualitative process evaluation methods and procedures (see Appendix 3 of the protocol). Submission of qualitative paperwork (information sheet, consent form and interview topic guides). Clarification of the type of adverse events/serious adverse events that will be reported; clarification that the incidence of carious lesions will be collected and analysed as a secondary outcome. | V6_20190415 |
| AM08/9 December 2019 | Substantial | Changes to the BRIGHT protocol in relation to changes to the wording of the primary and secondary outcomes. Also, submission of amended dental assessment CRF to include question on wearing orthodontic brace. | V7_20191204 |
| AM09/7 April 2020 | Non-substantial | Temporary halt to all data collection activities due to COVID-19. | V7_20191204 |
| AM10/16 June 2020 | Substantial | Changes to timing of the primary end point from 3 years to ‘approximately 2.5 years’ for logistical reasons. | V8.0_20200527 |
| AM11/14 October 2020 | Substantial | Change in protocol to record new sponsor, data controller and insurance details following Chief Investigator’s move from University of Dundee to Cardiff University. | V9.0_20200826 |
| AM12/15 December 2020 | Substantial | Change to protocol to outline COVID-19 safety measures ahead of final follow-ups/dental assessments. Participant and parent/carer information leaflet to confirm change of sponsor/data controllers as well as measures taken to mitigate COVID-19 risks during final follow-up. | V10.0_20201202 |
| AM13/3 March 2021 | Non-notifiable | Minor changes to the parent/carer resource use CRF cover letter and reminder letter. | V10.0_20201202 |
| AM14/29 April 2021 | Non-notifiable | Correcting minor typographical errors in the parent/carer cover letters and the dental assessment CRF. | V10.0_20201202 |
| AM15/29 April 2021 | Non-substantial | Restart of data collection activities following temporary halt due to COVID-19. | V10.0_20201202 |
| AM16/2 July 2021 | Non-notifiable | Updating the BRIGHT logo banners on CRFs. | V10.0_20201202 |
| AM17/2 July 2021 | Non-substantial | Updates to protocol including BRIGHT staff listed in protocol, the logo banner, clarification that parent/carer reminder letters can be sent via e-mail, and wording around reporting of serious Good Clinical Practice or protocol breaches. | V10.1_20210623 |
| AM18/3 December 2021 | Non-substantial | Minor amendment to protocol including clarification about distribution of pupil questionnaires during final follow-ups in cases where dental assessments are completed at a later date to questionnaires, the addition of a table to show the changes made to the protocol since the original ethics approval and an update to BRIGHT staff listed in the protocol. | V10.2 20211020 |
Appendix 2 Protocol deviations/breaches
| Breach number (file note number) | Description |
|---|---|
| i | The school started to deliver the intervention to the wrong year group. The CBS was partially delivered to Year 8 rather than Year 7. Year 7 had been randomised to receive the intervention. The school had been informed correctly of the allocation and it was confirmed the problem occurred due to a communication error within the school. The issue was discussed immediately with the TMG and delivery of the intervention to Year 8 was stopped (a third of the CBS had been delivered) and the intervention was delivered in full to Year 7, as per the randomisation allocation. Partial contamination of the control group was taken into account at the analysis stage. Measures were put in place to reduce the possibility of this happening again. The LRT informed the YTU that the incorrect criteria had been used and immediate action was taken to start using the correct criteria. For analysis, scores were transposed based on worst-case scenario approach. |
| ii | Baseline dental assessments had started in Scotland when the LRT made the local PI aware that they had not been using the correct criteria to score for plaque. This affected two schools in Scotland. |
| iii | Young person requested their text messages to stop and subsequently asked for them to be restarted. For participant 710003, texts were stopped following their initial stop request. Participant then requested texts be restarted. Texts were subsequently restarted in order to fulfil participants’ wishes. An amendment AM03 was submitted to clarify this would be the approach taken in any future cases. N.B. For participant 110102, there was a delay to restarting while the amendment was approved. |
| iv | Two young people were consented, had baseline dental assessments and completed the young person baseline questionnaires in error. During the baseline data collection week, the LRT were recruiting additional young people, whose parents/carers had not returned an opt-out form, from school years S1 and S2. The two young people who were seen in error were in S3 which meant they were not eligible to take part in BRIGHT as they were not in S1 or S2 and therefore their parents/carers had not received a parent/carer information pack about BRIGHT. The LRT contacted the YTU during baseline data collection week as soon as they realised the error. The two young people were withdrawn from the study and the parents/carers were informed by letter of what had occurred. The parents were informed that the consent forms would be shredded in 2 weeks unless they requested to see the consent form before then. All other case report forms completed during the baseline assessments for the two young people were shredded and their details were removed from the Trial Management System. Measures were put in place to reduce the possibility of this happening again. |
| 001 | All baseline CRFs, including young person questionnaires, parent/carer questionnaires and dental assessment CRFs, were submitted for REC and R&D approval. It was also made clear in the protocol which questions would be included in each CRF at each follow-up point. However, final copies of young person FU1 questionnaires were not submitted to REC and R&D before use in six schools. Use of follow-up questionnaires was immediately suspended in other schools. An amendment was swiftly submitted including final copies of all follow-up CRFs to be used in the pilot trial and approved by REC and R&D. |
| 002 | Baseline dental assessments for 35 young people were completed but were later deemed to be ineligible to take part in the trial. Of these, 32 also completed the baseline young person questionnaire. Parent/carer baseline resource use questionnaires were also sent home with these ineligible young people; seven have been completed and returned. Figures updated 25 February 2020: When deleting processed CRFs data for these participants and locating the paper versions of any paper CRFs received/annotating them to explain that they were not processed as the participant was ineligible, it was identified that two young people had not completed the CRFs as they had declined. This therefore means 33 young people completed baseline dental assessments but were later deemed to be ineligible. Of these, 31 also completed the baseline young person questionnaire. This update in figures did not affect the number of parent/carer baseline resource use questionnaires completed and returned. |
| 003 | Consent forms for a training and calibration event were not taken away and securely stored by the trial team but were left at the school where they had been placed in confidential waste by the school. |
| 004 | Intervention fidelity – undelivered SMS messages since 12 July 2020 and decision not to restart messages. |
Appendix 3 Logic and causal model of the intervention evaluated in the BRIGHT trial
To provide a clear description of the intervention and its causal assumptions, a logic and a causal model were developed to represent the processes for the intervention and the outcomes it aimed to achieve.
FIGURE 6.
Logic model.
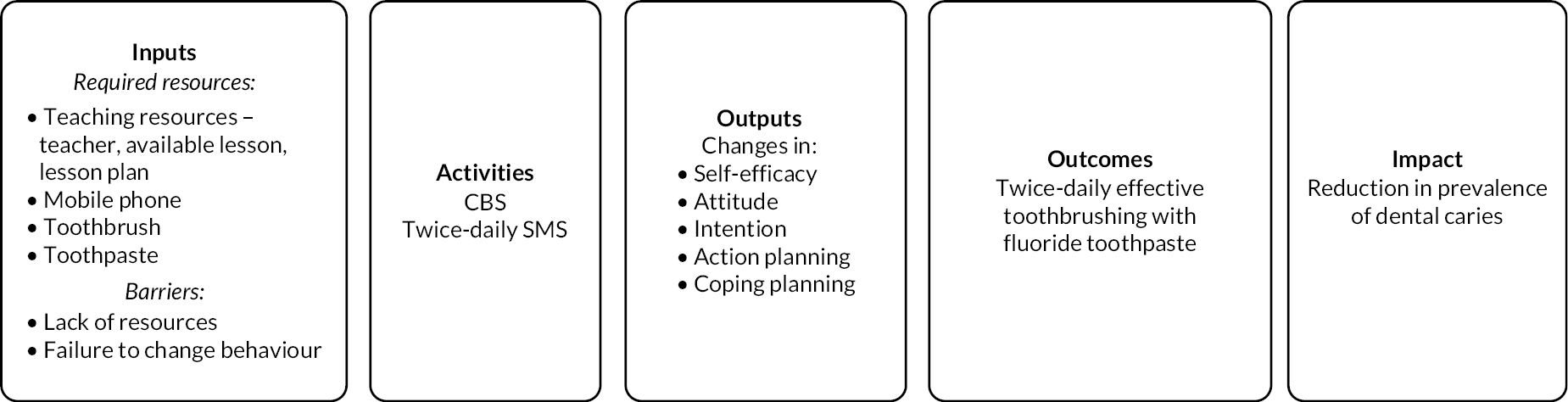
FIGURE 7.
Causal model.
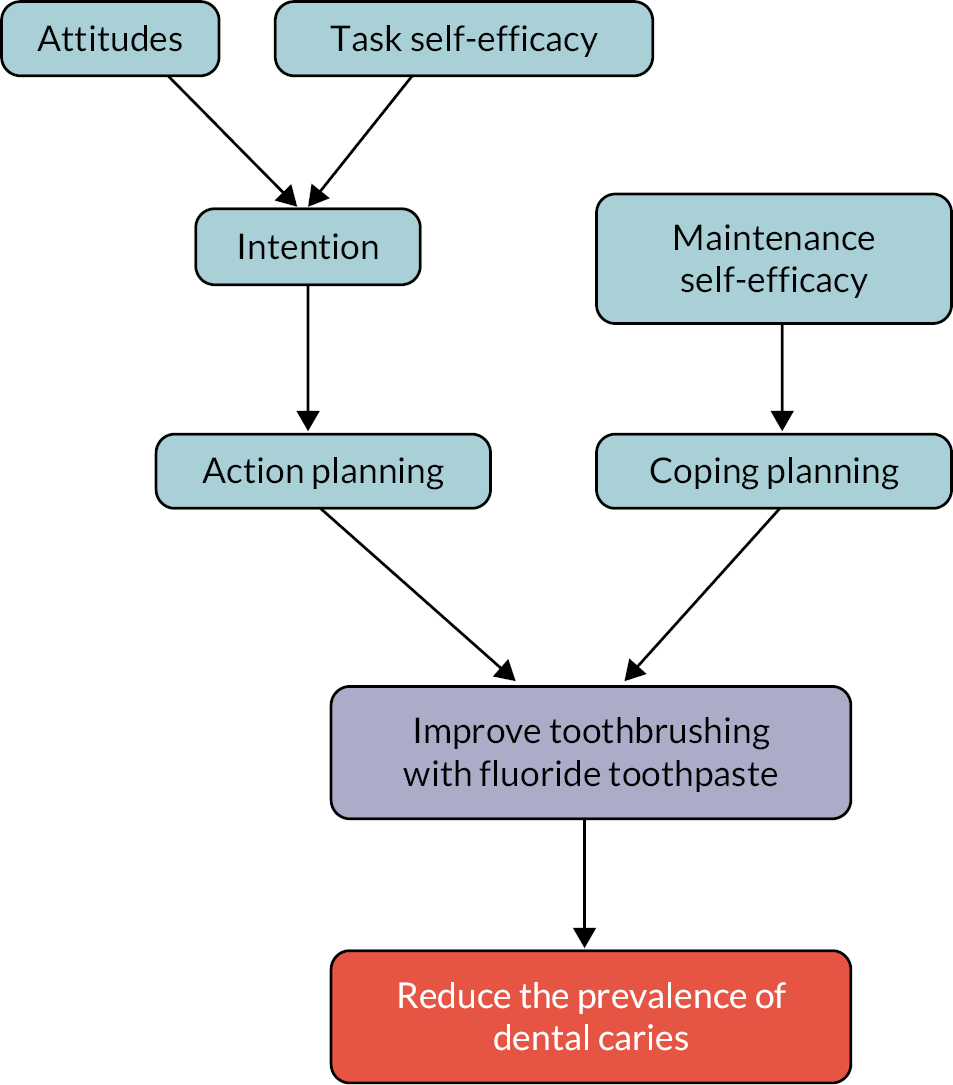
Appendix 4 Training and calibration intra- and inter-rater reliability data
Prior to the start of baseline data collection for the internal pilot, a hands-on training and calibration event was run in a school with an experienced dental epidemiologist for all dental team members. This involved six teams of dental professionals, each consisting of a Dentist/Dental therapist and a Dental Nurse/Community Dental Officer/Dental Practitioner. Six pupils were involved in this training and calibration session. The morning session was used for training, whereby the dental teams assessed children under the supervision of the clinical lead, who discussed discrepancies with scoring with the teams. In the afternoon, a calibration session was held with a different set of children. Five rounds of assessments were conducted, so each child was assessed by five of the dental teams, and their ICDAS scores recorded. From these we calculated the inter-rater reliability associated with whether the assessment indicated presence of DMFT at the ICDAS levels of 4–6 and 1–6.
Appendix 5 Full list of treatment costs for Scotland
| Description categorisation | Source | Unit cost £ | SDR item of service in Amendment No. 155 | Patient’s charge £ @ 2020–1 prices |
|---|---|---|---|---|
| Examination and report | SDR item 1a | 17.93b | 0 | |
| Fillings | SDR item 14a | 14.62 | 0 | |
| Extractions per tooth | SDR item 21a | 8.08 | 2101 | 7.76 |
| Crowns | SDR item 17a | 62.17 | Mean of 1701–1704 | 90.79 |
| Fluoride application | SDR item 44ga | 7.12 | 0701 | 7.76 |
| Root canal treatment | SDR item 15a | 90.33 | Mean of 1501–1504 | 64.15 |
| Sealant | SDR item 44fa | 9.48 | 0 | |
| X-ray | Weighted average of SDRa | 13.06 | 0 | |
| Scale and polish | Weighted average of SDRa | 15.80 | 0 | |
| Day case tariff | All treatments | 900.83 | 0 |
For Scotland, unit costs were calculated using weighted activities in the broad SDR categories. Patient charges were obtained from Amendment No. 155 based on the most appropriate SDR item of service code following discussions with clinicians.
Appendix 6 Pilot trial progression criteria review document
BRIGHT: progression criteria review
Progression criteria 1: An indication of a positive effect of the intervention on self-reported frequency of toothbrushing at FU2 using an 80% one-sided CI approach.
At FU2, 246/296 pupils (83.1%) in the intervention group and 213/272 pupils (78.3%) in the control group reported that they brushed their teeth at least twice a day (difference of 4.8 percentage points in favour of the intervention group). The likelihood of pupils brushing their teeth twice a day was compared between the intervention and control groups via a mixed-effect binary logistic model controlling for year group as a fixed-effect covariate and school as a random effect. A one-sided 80% confidence limit of 1.10 for the intervention effect was obtained from the output for the model. This limit indicates that, based on these data, we are 80% sure that the intervention group are at least 10% more likely to brush their teeth twice a day than the control group; therefore, there is an indication of a positive effect of the intervention on self-reported frequency of toothbrushing at FU2 using an 80% one-sided CI approach.
Progression criteria 2: Engagement with 80% of the number of schools required for the main trial and obtain agreement to participate, in principle. We needed 30 additional schools in the main trial and had engaged 24 (80%) (Table 24).
| Region | Pilot schools recruited | Schools potentially interested in participating in main trial | Schools definitely interested in participating in main triala | Estimated revised target | % of target engaged |
|---|---|---|---|---|---|
| Scotland | 3 | 0 | 9 | 7 | 129 |
| South Yorkshire | 2 | 2 | 2 | 8 | 50 |
| South Wales | 4b | 4 | 2 | 7 | 86 |
| West Yorkshire | 2 | 3 | 2 | 8 | 63 |
| Total | 11 | 9 | 15 | 30 | 80 |
Through regular team meetings, sharing of information between LRTs and in discussion with schools, a number of school recruitment strategies were found to be successful. Schools often had competing demands (e.g. high staff turnover, high absentee rates or competing priorities, such as a focus on improving attainment), which presented a barrier to participation, as taking part in dental research was viewed as a low priority. Also, not all schools had dedicated staff who could approve and sign the Data-Sharing Agreement (DSA) or some schools required DSAs to be signed by local authority colleagues. DSAs are now considered necessary for the nature of data sharing required in a study such as BRIGHT, and as such, this can present an additional barrier to recruitment of schools.
Successful strategies:
-
Use of personal contacts based in schools.
-
Use of school contacts held by recruiting universities.
-
Asking schools to recommend the study to other schools.
-
Engaging local or national educational organisations.
-
Making use of local authority education networks for head teachers and senior management teams.
-
Communicating with academies with several schools in local area.
-
Face-to-face meetings with school staff.
-
Approaching schools who had already taken part in similar research studies, such as the children’s dental health survey and a smoking prevention study. 144
-
Involving local school nursing teams.
-
Making contact with schools via letter from the local principal investigator.
-
Alone, making initial contact with schools via e-mail did not prove to be successful.
Progression criteria 3: recruiting an average of 48 young people per year group from the 10 schools included in the pilot trial (48 was 80% of our target average recruitment of young people per year group). The average number of young people recruited per year group was 49 (Table 25).
| Target | Randomised n (% of target) | |
|---|---|---|
| Average per year group | 60 | 49 (82) |
| Total | 1200 | 1073 (89) |
Recruitment barriers and solutions for the main trial phase:
-
In some schools, higher than expected proportions of pupils did not have a mobile telephone (meaning they did not meet eligibility criteria for participation). There was no solution possible to allow participation.
-
Pupils sometimes struggled to accurately complete the combined consent and contact form, leading to a high number of queries, which took time to resolve. For the main trial phase, the consent form and contact form were separated to streamline the consent process. The timing of completion of the contact form was changed to the time of baseline data collection, so a researcher could support completion.
-
Pupils were sometimes unable to remember their mobile telephone number and were not permitted to access their mobile telephones on school premises due to school rules. Permission was obtained from school senior leadership for the contact form to be completed with the research team as this was outside of the usual classroom situation and allowed pupils to access their mobile phone to check their phone number.
-
Pupils sometimes struggled to understand the language used in the consent forms, despite best efforts to make the consent form appropriate for pupils (e.g. some pupils did not understand the word ‘signature’). Further simplification of the forms was undertaken after the pilot phase following PPIE work. The timing was changed to allow researchers to be available on school site to support completion. The optional statements included on the consent form appeared to make completing the consent form more complicated, so the wording was changed and made non-optional.
-
Delays were experienced by other competing demands in school, such as Ofsted inspections, which led to researchers’ planned days to be onsite to support recruitment being postponed or schools’ planned sessions for pupils to complete consent forms being postponed. No solutions possible.
-
Changes to the leadership or organisation of schools led to barriers in organising planned sessions for recruitment. The LRTs endeavoured to keep in close communication with identified key contacts to mitigate the impact of this barrier.
-
A 2-week window for pupils to consent, after the parent/carer 2-week opt-out window had passed, delayed the recruitment process. To overcome this barrier, the process was changed to remove the requirement to wait 2 weeks for pupils to consent after the parent/carer opt-out window. As pupils heard about BRIGHT in an assembly before the opt-out window, and information was sent home, an additional 2-week window was deemed unnecessary.
Progression criteria 4: minimum 80% response to questionnaires.
Six of the 11 recruited schools were asked to complete FU1. This relates to 591 randomised pupils, of which 421 (71.2%) completed the questionnaire (see Table 26).
| FU1 YP questionnaire (time of CBS) | FU2 YP questionnaire (between time of CBS and 12 weeks) | |
|---|---|---|
| N completed | 421 | 523 |
| % of N randomised AND asked to complete | 71.2 | 80.1 |
Seven of the 11 recruited schools were asked to complete FU2. This relates to 653 randomised pupils, of which 523 (80.1%) completed the questionnaire.
Progression criteria 5: confirmation of feasibility of embedding the education component within the curriculum through discussion with school head teachers.
Feedback from the schools in the pilot trial suggests that although schools have different arrangements for the provision of PHSE education, there were no problems embedding the CBS into the school’s curricula. Very positive feedback was received on the quality of the lesson plan (including content, duration and level of interactivity) with some helpful comments to make minor improvements.
Progression criteria 6: confirmation of the feasibility of the outcome data collection methods and time points within the school year.
The pilot trial has demonstrated that planned outcome data collection methods are feasible, with the following points to note:
-
Strong feedback from schools to avoid examination periods – avoid data collection in the summer term.
-
Encourage schools to ensure questionnaires are completed in class time (rather than sent home).
-
Asking young people to complete questionnaires while waiting for dental assessment was successful in achieving high completion rates.
-
LRT members visiting schools in person at data collection time points aided data collection.
Progression criteria 7: assessment of contamination in the control group and whether feasible to undertake randomisation within schools (by year group) or whether randomisation at the school level will be required, and calculation therefore of the required school sample size.
Our original aim was to recruit an average of 60 pupils per year group; however, in the pilot trial, the average number randomised per year group was 49. This satisfies progression criteria 3 but was lower than the 60 we hoped to achieve. Within the pilot trial, we were able to calculate an estimate of the participation rate for the number of pupils approached: an average of 121 pupils per year group were invited to partake in the trial, and 49 (40%) were randomised. Based on this participation rate of 40% and considering the size of the schools that have expressed an interest in taking part in the main trial, we were satisfied that we could achieve an average of 60 recruited pupils per year group in the main trial by approaching a larger pool of pupils in each year group (i.e. by inviting, on average, at least 150 pupils per year group). The calculation for the main trial sample size therefore assumed that, on average, 60 pupils per year group would be randomised.
At FU2, we collected information on whether pupils had received helpful information about how to keep their teeth and mouth healthy from various sources. The sources that related to the intervention were: a lesson in school; friends in another year group; and text messages. Overall, of the 272 pupils allocated to control that provided a response to FU2, 173 (63.6%) said they had received oral health messages from at least one of: a lesson in school; friends in another year group; or text messages. This proportion is mainly driven by 159 (58.5%) pupils responding that they had received helpful oral health messages from a lesson at school. However, we are aware of only one school that provided the CBS to the usual-care year group. This was done in error. Given the wording of the question (‘Have you received helpful information about how to keep your teeth and mouth healthy from any of these places?’) it is possible that pupils have responded in relation to any point in their lives rather than just since the beginning of their participation in the trial. They may also have interpreted discussion of the BRIGHT trial in assemblies or form classes as ‘receiving helpful information about how to keep your teeth and mouth healthy’. If we consider only the pupils who said they had received oral health messages from friends in another year group and/or text messages, and those in the school where the control year received the CBS, the potential contamination rate in the control group was 27%. Even then, it is unlikely that all 27% received the full intervention effect as they are unlikely to have received the CBS and be receiving bi-daily SMS toothbrushing reminders.
Assuming partial contamination effects (i.e. those contaminated gain half the treatment benefits) for 27% of the control sample, we would require 40 schools in total across the main and (internal) pilot trials, assuming within-school (year group level randomisation), an average of 60 pupils per year group, an ICC of 0.02 and 20% attrition at follow-up. This would give us 90% power (5% two-sided α) to detect an 8% absolute reduction, from 32% to 24%, in the proportion of pupils with caries.
Appendix 7 Additional baseline data summaries
| Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) | |
|---|---|---|---|
| How many times do you usually eat | |||
| Fruit, n (%) | |||
| 4 +/day | 307 (13.6) | 335 (13.9) | 642 (13.7) |
| 3/day | 497 (22.0) | 584 (24.2) | 1081 (23.1) |
| 2/day | 689 (30.5) | 668 (27.6) | 1357 (29.0) |
| 1/day | 488 (21.6) | 491 (20.3) | 979 (20.9) |
| < 1/day | 191 (8.4) | 224 (9.3) | 415 (8.9) |
| Never | 56 (2.5) | 69 (2.9) | 125 (2.7) |
| Missing | 34 (1.5) | 47 (1.9) | 81 (1.7) |
| How many times do you usually drink | |||
| Diet coke or other non-sugar drinks, n (%) | |||
| 4 +/day | 117 (5.2) | 116 (4.8) | 233 (5.0) |
| 3/day | 132 (5.8) | 161 (6.7) | 293 (6.3) |
| 2/day | 320 (14.1) | 331 (13.7) | 651 (13.9) |
| 1/day | 472 (20.9) | 461 (19.1) | 933 (19.9) |
| < 1/day | 837 (37.0) | 870 (36.0) | 1707 (36.5) |
| Never | 339 (15.0) | 427 (17.7) | 766 (16.4) |
| Missing | 45 (2.0) | 52 (2.2) | 97 (2.1) |
| Water, n (%) | |||
| 4 +/day | 1065 (47.1) | 1139 (47.1) | 2204 (47.1) |
| 3/day | 504 (22.3) | 550 (22.7) | 1054 (22.5) |
| 2/day | 318 (14.1) | 335 (13.9) | 653 (14.0) |
| 1/day | 179 (7.9) | 154 (6.4) | 333 (7.1) |
| < 1/day | 91 (4.0) | 113 (4.7) | 204 (4.4) |
| Never | 66 (2.9) | 83 (3.4) | 149 (3.2) |
| Missing | 39 (1.7) | 44 (1.8) | 83 (1.8) |
Appendix 8 Follow-up data summaries
| Time point | Schools recruited during pilot phase | Schools recruited during main trial phase | Total | |||
|---|---|---|---|---|---|---|
| No. of schools (n = 10) | Duration of follow-up, median (range) | No. of schools (n = 32) | Duration of follow-up, median (range) | No. of schools (n = 42) | Duration of follow-up, median (range) | |
| CBS | 6a | 2.0 days (0.0–66.0) | N/A | N/A | 6 | 2.0 days (0.0–66.0) |
| 12 weeks | 9b | 9.7 weeks (0.0 15.9) | N/A | N/A | 9 | 9.7 weeks (0.0–15.9) |
| 6 months | 10 | 7.3 months (5.4–8.8) | 31c | 7.0 months (3.5–10.3) | 41 | 7.0 months (3.5–10.3) |
| 1 year | 10 | 13.1 months (12.0–16.9) | 9d | 12.1 months (11.2–12.9) | 19 | 12.5 months (11.2–16.9) |
| 2 years | 8e | 23.7 months (20.5–25.4) | N/A | N/A | 8 | 23.7 months (20.5–25.4) |
| 2.5 years | 10 | 38.8 months (34.9–48.5) | 27f | 32.3 months (28.1–36.6) | 37 | 33.1 months (28.1–48.5) |
| Time point | Status, n (%) | Pilot | Main | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 563) | Control (n = 510) | Overall (n = 1073) | Intervention (n = 1699) | Control (n = 1908) | Overall (n = 3607) | Intervention (n = 2262) | Control (n = 2418) | Overall (n = 4680) | ||
| Baseline | Completed | 542 (96.3) | 489 (95.9) | 1031 (96.1) | 1692 (99.6) | 1903 (99.7) | 3595 (99.7) | 2234 (98.8) | 2392 (98.9) | 4626 (98.8) |
| Absent | 15 (2.7) | 14 (2.7) | 29 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (0.7) | 14 (0.6) | 29 (0.6) | |
| No longer at the school | 2 (0.4) | 2 (0.4) | 4 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 2 (0.1) | 4 (0.1) | |
| Declined to complete | 2 (0.4) | 5 (1.0) | 7 (0.7) | 3 (0.2) | 3 (0.2) | 6 (0.2) | 5 (0.2) | 8 (0.3) | 13 (0.3) | |
| Unknown | 2 (0.4) | 0 (0.0) | 2 (0.2) | 4 (0.2) | 2 (0.1) | 6 (0.2) | 6 (0.3) | 2 (0.1) | 8 (0.2) | |
| CBSa | Completed | 253 (44.9) | 168 (32.9) | 421 (39.2) | ||||||
| Absent | 5 (0.9) | 5 (1.0) | 10 (0.9) | |||||||
| No longer at the school | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Declined to complete | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Unknown | 68 (12.1) | 91 (17.8) | 159 (14.8) | |||||||
| Withdrawn | 0 (0.0) | 1 (0.2) | 1 (0.1) | |||||||
| Data collection not undertaken in their school | 237 (42.1) | 245 (48.0) | 482 (44.9) | |||||||
| 12 weeksb | Completed | 359 (63.8) | 310 (60.8) | 669 (62.3) | ||||||
| Absent | 8 (1.4) | 18 (3.5) | 26 (2.4) | |||||||
| No longer at the school | 5 (0.9) | 2 (0.4) | 7 (0.7) | |||||||
| Declined to complete | 0 (0.0) | 3 (0.6) | 3 (0.3) | |||||||
| Unknown | 116 (20.6) | 113 (22.2) | 229 (21.3) | |||||||
| Withdrawn | 3 (0.5) | 6 (1.2) | 9 (0.8) | |||||||
| Data collection not undertaken in their school | 72 (12.8) | 58 (11.4) | 130 (12.1) | |||||||
| 6 monthsc | Completed | 302 (53.6) | 327 (64.1) | 629 (58.6) | 1179 (69.4) | 1371 (71.9) | 2550 (70.7) | 1481 (65.5) | 1698 (70.2) | 3179 (67.9) |
| Absent | 17 (3.0) | 9 (1.8) | 26 (2.4) | 85 (5.0) | 87 (4.6) | 172 (4.8) | 102 (4.5) | 96 (4.0) | 198 (4.2) | |
| No longer at the school | 8 (1.4) | 3 (0.6) | 11 (1.0) | 19 (1.1) | 32 (1.7) | 51 (1.4) | 27 (1.2) | 35 (1.4) | 62 (1.3) | |
| Declined to complete | 0 (0.0) | 3 (0.6) | 3 (0.3) | 9 (0.5) | 14 (0.7) | 23 (0.6) | 9 (0.4) | 17 (0.7) | 26 (0.6) | |
| Unknown | 229 (40.7) | 158 (31.0) | 387 (36.1) | 354 (20.8) | 351 (18.4) | 705 (19.5) | 583 (25.8) | 509 (21.1) | 1092 (23.3) | |
| Withdrawn | 7 (1.2) | 10 (2.0) | 17 (1.6) | 12 (0.7) | 16 (0.8) | 28 (0.8) | 19 (0.8) | 26 (1.1) | 45 (1.0) | |
| Data collection not undertaken in their school | 0 (0.0) | 0 (0.0) | 0 (0.0) | 41 (2.4) | 37 (1.9) | 78 (2.2) | 41 (1.8) | 37 (1.5) | 78 (1.7) | |
| 1 yeard | Completed | 350 (62.2) | 320 (62.7) | 670 (62.4) | 306 (18.0) | 338 (17.7) | 644 (17.9) | 656 (29.0) | 658 (27.2) | 1314 (28.1) |
| Absent | 11 (2.0) | 27 (5.3) | 38 (3.5) | 9 (0.5) | 5 (0.3) | 14 (0.4) | 20 (0.9) | 32 (1.3) | 52 (1.1) | |
| No longer at the school | 15(2.7) | 7 (1.4) | 22 (2.1) | 10 (0.6) | 5 (0.3) | 15 (0.4) | 25 (1.1) | 12 (0.5) | 37 (0.8) | |
| Declined to complete | 5 (0.9) | 2 (0.4) | 7 (0.7) | 2 (0.1) | 3 (0.2) | 5 (0.1) | 7 (0.3) | 5 (0.2) | 12 (0.3) | |
| Unknown | 165 (29.3) | 140 (27.5) | 305 (28.4) | 130 (7.7) | 127 (6.7) | 257 (7.1) | 295 (13.0) | 267 (11.0) | 562 (12.0) | |
| Withdrawn | 17 (3.0) | 14 (2.7) | 31 (2.9) | 9 (0.5) | 14 (0.7) | 23 (0.6) | 26 (1.1) | 28 (1.2) | 54 (1.2) | |
| Data collection not undertaken in their school | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1233 (72.6) | 1416 (74.2) | 2649 (73.4) | 1233 (54.5) | 1416 (58.6) | 2649 (56.6) | |
| 2 yearse | Completed | 351 (62.3) | 331 (64.9) | 682 (63.6) | ||||||
| Absent | 27 (4.8) | 39 (7.6) | 66 (6.2) | |||||||
| No longer at the school | 11 (2.0) | 7 (1.4) | 18 (1.7) | |||||||
| Declined to complete | 22 (3.9) | 21 (4.1) | 43 (4.0) | |||||||
| Unknown | 4 (0.7) | 4 (0.8) | 8 (0.7) | |||||||
| Withdrawn | 37 (6.6) | 26 (5.1) | 63 (5.9) | |||||||
| Data collection not undertaken in their school | 111 (19.7) | 82 (16.1) | 193 (18.0) | |||||||
| 2.5 yearsf | Completed | 327 (58.1) | 270 (52.9) | 597 (55.6) | 960 (56.5) | 1086 (56.9) | 2046 (56.7) | 1287 (56.9) | 1356 (56.1) | 2643 (56.5) |
| Absent | 70 (12.4) | 64 (12.5) | 134 (12.5) | 142 (8.4) | 155 (8.1) | 297 (8.2) | 212 (9.4) | 219 (9.1) | 431 (9.2) | |
| No longer at the school | 16 (2.8) | 23 (4.5) | 39 (3.6) | 79 (4.6) | 85 (4.5) | 164 (4.5) | 95 (4.2) | 108 (4.5) | 203 (4.3) | |
| Declined to complete | 12 (2.1) | 8 (1.6) | 20 (1.9) | 78 (4.6) | 74 (3.9) | 152 (4.2) | 90 (4.0) | 82 (3.4) | 172 (3.7) | |
| Unknown | 47 (8.3) | 72 (14.1) | 119 (11.1) | 100 (5.9) | 51 (2.7) | 151 (4.2) | 147 (6.5) | 123 (5.1) | 270 (5.8) | |
| Withdrawn | 91 (16.2) | 73 (14.3) | 164 (15.3) | 73 (4.3) | 82 (4.3) | 155 (4.3) | 164 (7.3) | 155 (6.4) | 319 (6.8) | |
| Data collection not undertaken in their school | 0 (0.0) | 0 (0.0) | 0 (0.0) | 267 (15.7) | 375 (19.7) | 642 (17.8) | 267 (11.8) | 375 (15.5) | 642 (13.7) | |
| Intervention (n = 326) | Control (n = 265) | Overall (n = 591) | |
|---|---|---|---|
| How satisfied are you with the appearance of your teeth?, n (%) | |||
| Very satisfied | 32 (9.8) | 20 (7.5) | 52 (8.8) |
| Satisfied | 109 (33.4) | 70 (26.4) | 179 (30.3) |
| Neither satisfied nor dissatisfied | 84 (25.8) | 55 (20.8) | 139 (23.5) |
| Dissatisfied | 22 (6.7) | 17 (6.4) | 39 (6.6) |
| Very dissatisfied | 6 (1.8) | 6 (2.3) | 12 (2.0) |
| Missing | 73 (22.4) | 97 (36.6) | 170 (28.8) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 3 (0.9) | 4 (1.5) | 7 (1.2) |
| Three times a day | 22 (6.7) | 16 (6.0) | 38 (6.4) |
| Twice a day | 200 (61.3) | 122 (46.0) | 322 (54.5) |
| Once a day | 24 (7.4) | 22 (8.3) | 46 (7.8) |
| < once a day | 4 (1.2) | 4 (1.5) | 8 (1.4) |
| Never | 0(0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 73 (22.4) | 97 (36.6) | 170 (28.8) |
| How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 165 (50.6) | 101 (38.1) | 266 (45.0) |
| A bit | 84 (25.8) | 60 (22.6) | 144 (24.4) |
| A lot | 4 (1.2) | 7 (2.6) | 11 (1.9) |
| Missing | 73 (22.4) | 97 (36.6) | 170 (28.8) |
| Intervention (n = 326) | Control (n = 265) | Overall (n = 591) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.6 (0.5) | 3.4 (0.7) | 3.5 (0.6) |
| Attitudes, mean (SD) | 3.4 (0.4) | 3.3 (0.4) | 3.4 (0.4) |
| Coping planning, mean (SD) | 2.9 (0.7) | 2.8 (0.8) | 2.9 (0.7) |
| Action planning, mean (SD) | 3.4 (0.5) | 3.3 (0.6) | 3.4 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 8 (2.5) | 11 (4.2) | 19 (3.2) |
| Three times a day | 43 (13.2) | 38 (14.3) | 81 (13.7) |
| Twice a day | 181 (55.5) | 104 (39.2) | 285 (48.2) |
| Once a day | 18 (5.5) | 8 (3.0) | 26 (4.4) |
| < once a day | 3 (0.9) | 3 (1.1) | 6 (1.0) |
| Never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 73 (22.4) | 101 (38.1) | 174 (29.4) |
| Intervention (n = 491) | Control (n = 452) | Overall (n = 943) | |
|---|---|---|---|
| How satisfied are you with the appearance of your teeth?, n (%) | |||
| Very satisfied | 50 (10.2) | 32 (7.1) | 82 (8.7) |
| Satisfied | 139 (28.3) | 114 (25.2) | 253 (26.8) |
| Neither satisfied nor dissatisfied | 115 (23.4) | 108 (23.9) | 223 (23.6) |
| Dissatisfied | 38 (7.7) | 44 (9.7) | 82 (8.7) |
| Very dissatisfied | 16 (3.3) | 10 (2.2) | 26 (2.8) |
| Missing | 133 (27.1) | 144 (31.9) | 277 (29.4) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 16 (3.3) | 3 (0.7) | 19 (2.0) |
| Three times a day | 28 (5.7) | 27 (6.0) | 55 (5.8) |
| Twice a day | 273 (55.6) | 242 (53.5) | 515 (54.6) |
| Once a day | 38 (7.7) | 35 (7.7) | 73 (7.7) |
| < once a day | 3 (0.6) | 3 (0.7) | 6 (0.6) |
| Never | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Missing | 132 (26.9) | 142 (31.4) | 274 (29.1) |
| How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 229 (46.6) | 184 (40.7) | 413 (43.8) |
| A bit | 117 (23.8) | 111 (24.6) | 228 (24.2) |
| A lot | 13 (2.6) | 15 (3.3) | 28 (3.0) |
| Missing | 132 (26.9) | 142 (31.4) | 274 (29.1) |
| Intervention (n = 491) | Control (n = 452) | Overall (n = 943) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.6 (0.5) | 3.4 (0.6) | 3.5 (0.6) |
| Attitudes, mean (SD) | 3.4 (0.5) | 3.3 (0.5) | 3.4 (0.5) |
| Coping planning, mean (SD) | 3.0 (0.8) | 2.7 (0.8) | 2.8 (0.8) |
| Action planning, mean (SD) | 3.5 (0.6) | 3.3 (0.6) | 3.4 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 26 (5.3) | 16 (3.5) | 42 (4.5) |
| Three times a day | 55 (11.2) | 63 (13.9) | 118 (12.5) |
| Twice a day | 259 (52.7) | 212 (46.9) | 471 (49.9) |
| Once a day | 17 (3.5) | 15 (3.3) | 32 (3.4) |
| < once a day | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Never | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Missing | 133 (27.1) | 142 (31.4) | 275 (29.2) |
| Intervention (n = 1481) | Control (n = 1698) | Overall (n = 3179) | |
|---|---|---|---|
| How do you feel about the way your teeth look?, n (%) | |||
| Very happy | 366 (24.7) | 361 (21.3) | 727 (22.9) |
| A bit happy | 490 (33.1) | 574 (33.8) | 1064 (33.5) |
| Neither happy nor unhappy | 380 (25.7) | 418 (24.6) | 798 (25.1) |
| A bit unhappy | 171 (11.5) | 246 (14.5) | 417 (13.1) |
| Very unhappy | 73 (4.9) | 97 (5.7) | 170 (5.3) |
| Missing | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 29 (2.0) | 26 (1.5) | 55 (1.7) |
| Three times a day | 121 (8.2) | 125 (7.4) | 246 (7.7) |
| Twice a day | 1137 (76.8) | 1258 (74.1) | 2395 (75.3) |
| Once a day | 170 (11.5) | 264 (15.5) | 434 (13.7) |
| < once a day | 21 (1.4) | 21 (1.2) | 42 (1.3) |
| Never | 2 (0.1) | 3 (0.2) | 5 (0.2) |
| Missing | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Do you have your own toothbrush?, n (%) | |||
| Yes, I have my own toothbrush | 1461 (98.6) | 1670 (98.4) | 3131 (98.5) |
| No, I share one | 11 (0.7) | 11 (0.6) | 22 (0.7) |
| No, I do not have a toothbrush | 1 (0.1) | 4 (0.2) | 5 (0.2) |
| Missing | 8 (0.5) | 13 (0.8) | 21 (0.7) |
| Do you have toothpaste you can use?, n (%) | |||
| There is always toothpaste I can use | 1427 (96.4) | 1638 (96.5) | 3065 (96.4) |
| There is sometimes toothpaste I can use | 37 (2.5) | 40 (2.4) | 77 (2.4) |
| There is no toothpaste I can use | 7 (0.5) | 7 (0.4) | 14 (0.4) |
| Missing | 10 (0.7) | 13 (0.8) | 23 (0.7) |
| Intervention (n = 1481) | Control (n = 1698) | Overall (n = 3179) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) |
| Attitudes, mean (SD) | 3.3 (0.5) | 3.2 (0.4) | 3.3 (0.4) |
| Coping planning, mean (SD) | 2.8 (0.8) | 2.7 (0.8) | 2.8 (0.8) |
| Action planning, mean (SD) | 3.4 (0.6) | 3.4 (0.6) | 3.4 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 84 (5.7) | 91 (5.4) | 175 (5.5) |
| Three times a day | 283 (19.1) | 328 (19.3) | 611 (19.2) |
| Twice a day | 1031 (69.6) | 1154 (68.0) | 2185 (68.7) |
| Once a day | 57 (3.8) | 88 (5.2) | 145 (4.6) |
| < once a day | 5 (0.3) | 4 (0.2) | 9 (0.3) |
| Never | 14 (0.9) | 17 (1.0) | 31 (1.0) |
| Missing | 7 (0.5) | 16 (0.9) | 23 (0.7) |
| Intervention (n = 658) | Control (n = 656) | Overall (n = 1314) | |
|---|---|---|---|
| How do you feel about the way your teeth look? n (%) | |||
| Very happy | 170 (25.9) | 137 (20.8) | 307 (23.4) |
| A bit happy | 206 (31.4) | 200 (30.4) | 406 (30.9) |
| Neither happy nor unhappy | 149 (22.7) | 170 (25.8) | 319 (24.3) |
| A bit unhappy | 93 (14.2) | 107 (16.3) | 200 (15.2) |
| Very unhappy | 35 (5.3) | 40 (6.1) | 75 (5.7) |
| Missing | 3 (0.5) | 4 (0.6) | 7 (0.5) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 14 (2.1) | 13 (2.0) | 27 (2.1) |
| Three times a day | 58 (8.8) | 43 (6.5) | 101 (7.7) |
| Twice a day | 511 (77.9) | 496 (75.4) | 1007 (76.6) |
| Once a day | 62 (9.5) | 93 (14.1) | 155 (11.8) |
| < once a day | 9 (1.4) | 7 (1.1) | 16 (1.2) |
| Never | 0 (0.0) | 3 (0.5) | 3 (0.2) |
| Missing | 2 (0.3) | 3 (0.5) | 5 (0.4) |
| CARIES-QC raw score, mean (SD) | 3.1 (3.7) | 3.2 (3.5) | 3.2 (3.6) |
| CARIES-QC interval score, mean (SD) | 4.8 (3.8) | 5.1 (3.6) | 5.0 (3.7) |
| CARIES-QC global question – How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 441 (67.2) | 432 (65.7) | 873 (66.4) |
| A bit | 186 (28.4) | 207 (31.5) | 393 (29.9) |
| A lot | 28 (4.3) | 15 (2.3) | 43 (3.3) |
| Missing | 1 (0.2) | 4 (0.6) | 5 (0.4) |
| Intervention (n = 658) | Control (n = 656) | Overall (n = 1314) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.6 (0.6) | 3.5 (0.5) | 3.6 (0.5) |
| Attitudes, mean (SD) | 3.4 (0.5) | 3.3 (0.4) | 3.3 (0.5) |
| Coping planning, mean (SD) | 2.8 (0.8) | 2.7 (0.8) | 2.8 (0.8) |
| Action planning, mean (SD) | 3.5 (0.6) | 3.4 (0.6) | 3.5 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 30 (4.6) | 32 (4.9) | 62 (4.7) |
| Three times a day | 134 (20.4) | 131 (19.9) | 265 (20.2) |
| Twice a day | 460 (70.1) | 456 (69.3) | 916 (69.7) |
| Once a day | 26 (4.0) | 23 (3.5) | 49 (3.7) |
| < once a day | 2 (0.3) | 2 (0.3) | 4 (0.3) |
| Never | 2 (0.3) | 8 (1.2) | 10 (0.8) |
| Missing | 2 (0.3) | 6 (0.9) | 8 (0.6) |
| Intervention (n = 351) | Control (n = 331) | Overall (n = 682) | |
|---|---|---|---|
| How do you feel about the way your teeth look?, n (%) | |||
| Very happy | 83 (23.6) | 66 (19.9) | 149 (21.8) |
| A bit happy | 116 (33.0) | 119 (36.0) | 235 (34.5) |
| Neither happy nor unhappy | 88 (25.1) | 83 (25.1) | 171 (25.1) |
| A bit unhappy | 54 (15.4) | 49 (14.8) | 103 (15.1) |
| Very unhappy | 10 (2.8) | 12 (3.6) | 22 (3.2) |
| Missing | 0 (0.0) | 2 (0.6) | 2 (0.3) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 4 (1.1) | 2 (0.6) | 6 (0.9) |
| Three times a day | 17 (4.8) | 22 (6.6) | 39 (5.7) |
| Twice a day | 291 (82.9) | 247 (74.6) | 538 (78.9) |
| Once a day | 35 (10.0) | 53 (16.0) | 88 (12.9) |
| < once a day | 4 (1.1) | 5 (1.5) | 9 (1.3) |
| Never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 2 (0.6) | 2 (0.3) |
| CARIES-QC raw score, mean (SD) | 2.8 (3.3) | 2.7 (2.9) | 2.7 (3.1) |
| CARIES-QC interval score, mean (SD) | 4.5 (3.6) | 4.6 (3.3) | 4.5 (3.4) |
| CARIES-QC global question – How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 244 (69.5) | 240 (72.5) | 484 (71.0) |
| A bit | 99 (28.2) | 86 (26.0) | 185 (27.1) |
| A lot | 8 (2.3) | 3 (0.9) | 11 (1.6) |
| Missing | 0 (0.0) | 2 (0.6) | 2 (0.3) |
| Intervention (n = 351) | Control (n = 331) | Overall (n = 682) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.5 (0.5) | 3.4 (0.5) | 3.5 (0.5) |
| Attitudes, mean (SD) | 3.4 (0.4) | 3.3 (0.4) | 3.4 (0.4) |
| Coping planning, mean (SD) | 2.8 (0.7) | 2.7 (0.8) | 2.8 (0.7) |
| Action planning, mean (SD) | 3.5 (0.6) | 3.4 (0.6) | 3.5 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 13 (3.7) | 4 (1.2) | 17 (2.5) |
| Three times a day | 57 (16.2) | 64 (19.3) | 121 (17.7) |
| Twice a day | 267 (76.1) | 247 (74.6) | 514 (75.4) |
| Once a day | 12 (3.4) | 16 (4.8) | 28 (4.1) |
| < once a day | 2 (0.6) | 0 (0.0) | 2 (0.3) |
| Never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dental metric | Intervention (n = 338) | Control (n = 329) | Total (n = 667) |
|---|---|---|---|
| Primary outcome – presence of DICDAS4–6MFT–n (%) | 159 (47.0) | 133 (40.4) | 292 (43.8) |
| Number of permanent teeth assessed for ICDAS per pupil | |||
| Mean (SD) | 31.3 (1.4) | 31.6 (1.1) | 31.5 (1.3) |
| Median (IQR) | 32.0 (32.0–32.0) | 32.0 (32.0–32.0) | 32.0 (32.0–32.0) |
| Number of DICDAS4–6MFT per pupil | |||
| Mean (SD) | 1.18 (1.71) | 1.05 (1.73) | 1.12 (1.72) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 4–6)–mean (SD) | 0.42 (0.88) | 0.37 (0.95) | 0.39 (0.91) |
| M: teeth extracted due to caries–mean (SD) | 0.07 (0.40) | 0.08 (0.47) | 0.07 (0.44) |
| F: filled teeth (ICDAS 4–6)–mean (SD) | 0.69 (1.28) | 0.60 (1.12) | 0.65 (1.20) |
| Secondary outcome – presence of DICDAS1–6MFT–n (%) | 244 (72.2) | 209 (63.5) | 453 (67.9) |
| Number of DICDAS1–6MFT per pupil | |||
| Mean (SD) | 2.91 (3.25) | 2.54 (3.06) | 2.73 (3.16) |
| Median (IQR) | 2.0 (0.0–5.0) | 2.0 (0.0–4.0) | 2.0 (0.0–4.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 1–6)–mean (SD) | 2.33 (2.98) | 2.04 (2.73) | 2.19 (2.86) |
| M: teeth extracted due to caries–mean (SD) | 0.07 (0.40) | 0.08 (0.47) | 0.07 (0.44) |
| F: filled teeth (ICDAS 1–6)–mean (SD) | 0.51 (1.08) | 0.42 (0.87) | 0.47 (0.98) |
| Plaque index – mean (SD) | 0.90 (0.70) | 0.88 (0.65) | 0.89 (0.67) |
| Bleeding score – mean (SD) | 0.12 (0.16) | 0.10 (0.14) | 0.11 (0.15) |
| Number of bleeding gingivae per pupil | |||
| Mean (SD) | 1.62 (2.00) | 1.45 (1.85) | 1.54 (1.93) |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) |
| Unblinding of the dental assessor – n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intervention (n = 1287) | Control (n = 1356) | Overall (n = 2643) | |
|---|---|---|---|
| How do you feel about the way your teeth look?, n (%) | |||
| Very happy | 266 (20.7) | 233 (17.2) | 499 (18.9) |
| A bit happy | 415 (32.2) | 434 (32.0) | 849 (32.1) |
| Neither happy nor unhappy | 315 (24.5) | 360 (26.5) | 675 (25.5) |
| A bit unhappy | 212 (16.5) | 240 (17.7) | 452 (17.1) |
| Very unhappy | 76 (5.9) | 81 (6.0) | 157 (5.9) |
| Missing | 3 (0.2) | 8 (0.6) | 11 (0.4) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 5 (0.4) | 10 (0.7) | 15 (0.6) |
| Three times a day | 73 (5.7) | 61 (4.5) | 134 (5.1) |
| Twice a day | 961 (74.7) | 1011 (74.6) | 1972 (74.6) |
| Once a day | 225 (17.5) | 238 (17.6) | 463 (17.5) |
| < once a day | 21 (1.6) | 32 (2.4) | 53 (2.0) |
| Never | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Missing | 2 (0.2) | 3 (0.2) | 5 (0.2) |
| CARIES-QC raw score, mean (SD) | 2.8 (3.0) | 2.9 (3.2) | 2.9 (3.1) |
| CARIES-QC interval score, mean (SD) | 4.7 (3.3) | 4.9 (3.4) | 4.8 (3.4) |
| CARIES-QC global question – How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 867 (67.4) | 926 (68.3) | 1793 (67.8) |
| A bit | 384 (29.8) | 397 (29.3) | 781 (29.5) |
| A lot | 34 (2.6) | 30 (2.2) | 64 (2.4) |
| Missing | 2 (0.2) | 3 (0.2) | 5 (0.2) |
| Intervention (n = 1287) | Control (n = 1356) | Overall (n = 2643) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.5 (0.6) | 3.4 (0.6) | 3.4 (0.6) |
| Attitudes, mean (SD) | 3.3 (0.4) | 3.3 (0.4) | 3.3 (0.4) |
| Coping planning, mean (SD) | 2.7 (0.7) | 2.7 (0.7) | 2.7 (0.7) |
| Action planning, mean (SD) | 3.4 (0.6) | 3.4 (0.6) | 3.4 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 32 (2.5) | 28 (2.1) | 60 (2.3) |
| Three times a day | 217 (16.9) | 205 (15.1) | 422 (16.0) |
| Twice a day | 954 (74.1) | 1028 (75.8) | 1982 (75.0) |
| Once a day | 68 (5.3) | 78 (5.8) | 146 (5.5) |
| < once a day | 6 (0.5) | 6 (0.4) | 12 (0.5) |
| Never | 5 (0.4) | 4 (0.3) | 9 (0.3) |
| Missing | 5 (0.4) | 7 (0.5) | 12 (0.5) |
Appendix 9 Baseline characteristics and dental assessment data tables of pupils as included in the primary analysis
| Characteristics | Intervention (n = 1153) | Control (n = 1230) | Overall (n = 2383) |
|---|---|---|---|
| Year, n (%) | |||
| 7/S1 | 614 (53.3) | 766 (62.3) | 1380 (57.9) |
| 8/S2 | 539 (46.7) | 464 (37.7) | 1003 (42.1) |
| Age, mean (SD) | 12.6 (0.6) | 12.6 (0.6) | 12.6 (0.6) |
| Sex, n (%) | |||
| Female | 578 (50.1) | 628 (51.1) | 1206 (50.6) |
| Male | 575 (49.9) | 602 (48.9) | 1177 (49.4) |
| Rather not say | |||
| Eligible for FSM, n (%) | |||
| Yes | 236 (20.5) | 224 (18.2) | 460 (19.3) |
| No | 871 (75.5) | 953 (77.5) | 1824 (76.5) |
| Missing | 46 (4.0) | 53 (4.3) | 99 (4.2) |
| % attendance in the academic year in which pupil was enrolled, mean (SD) | 97.1 (4.4) | 96.7 (5.0) | 96.9 (4.7) |
| IMD decile, mean (SD) (England only) | 3.2 (2.4) | 3.4 (2.5) | 3.3 (2.5) |
| Scottish IMD decile, mean (SD) (Scotland only) | 5.3 (2.8) | 4.9 (3.2) | 5.1 (3.0) |
| Welsh IMD decile, mean (SD) (Wales only) | 3.0 (2.4) | 3.5 (2.3) | 3.3 (2.3) |
| Intervention (n = 1153) | Control (n = 1230) | Overall (n = 2383) | |
|---|---|---|---|
| How satisfied are you with the appearance of your teeth?/How do you feel about the way your teeth look?, n (%)a | |||
| Very satisfied/happy | 181 (15.7) | 213 (17.3) | 394 (16.5) |
| Satisfied/a bit happy | 402 (34.9) | 423 (34.4) | 825 (34.6) |
| Neither satisfied/happy nor dissatisfied/unhappy | 341 (29.6) | 325 (26.4) | 666 (27.9) |
| Dissatisfied/a bit unhappy | 180 (15.6) | 214 (17.4) | 394 (16.5) |
| Very dissatisfied/unhappy | 44 (3.8) | 51 (4.1) | 95 (4.0) |
| Missing | 5 (0.4) | 4 (0.3) | 9 (0.4) |
| How often do you usually brush your teeth?, n (%) | |||
| > three times a day | 18 (1.6) | 19 (1.5) | 37 (1.6) |
| Three times a day | 62 (5.4) | 72 (5.9) | 134 (5.6) |
| Twice a day | 830 (72.0) | 872 (70.9) | 1702 (71.4) |
| Once a day | 214 (18.6) | 234 (19.0) | 448 (18.8) |
| < once a day | 23 (2.0) | 28 (2.3) | 51 (2.1) |
| Never | 2 (0.2) | 1 (0.1) | 3 (0.1) |
| Missing | 4 (0.3) | 4 (0.3) | 8 (0.3) |
| CARIES-QC raw score, mean (SD) | 3.6 (3.5) | 3.6 (3.4) | 3.6 (3.4) |
| CARIES-QC interval score, mean (SD) | 5.7 (3.5) | 5.6 (3.4) | 5.6 (3.5) |
| CARIES-QC global question – How much of a problem are your teeth for you?, n (%) | |||
| Not at all | 649 (56.3) | 688 (55.9) | 1337 (56.1) |
| A bit | 459 (39.8) | 493 (40.1) | 952 (39.9) |
| A lot | 39 (3.4) | 42 (3.4) | 81 (3.4) |
| Missing | 6 (0.5) | 7 (0.6) | 13 (0.5) |
| Do you usually go to the dentist?, n (%) | |||
| For a check up | 974 (84.5) | 1031 (83.8) | 2005 (84.1) |
| Only when I have trouble with my teeth | 154 (13.4) | 162 (13.2) | 316 (13.3) |
| I have never been to the dentist | 18 (1.6) | 28 (2.3) | 46 (1.9) |
| Missing | 7 (0.6) | 9 (0.7) | 16 (0.7) |
| Over the last year, have you regularly used any of the following products to look after your teeth or mouth?, n (%) | |||
| Toothbrush (non-electric) | 892 (77.4) | 908 (73.8) | 1800 (75.5) |
| Electric/battery-operated toothbrush | 616 (53.4) | 677 (55.0) | 1293 (54.3) |
| Toothpaste | 1134 (98.4) | 1209 (98.3) | 2343 (98.3) |
| Mouthwash | 752 (65.2) | 790 (64.2) | 1542 (64.7) |
| Dental floss | 295 (25.6) | 312 (25.4) | 607 (25.5) |
| Sugar free or dental chewing gum | 341 (29.6) | 374 (30.4) | 715 (30.0) |
| Other | 70 (6.1) | 78 (6.3) | 148 (6.2) |
| Do you have your own toothbrush?, n (%) | |||
| Yes, I have my own toothbrush | 1144 (99.2) | 1221 (99.3) | 2365 (99.2) |
| No, I share one | 3 (0.3) | 2 (0.2) | 5 (0.2) |
| No, I do not have a toothbrush | 2 (0.2) | 1 (0.1) | 3 (0.1) |
| Missing | 4 (0.3) | 6 (0.5) | 10 (0.4) |
| Do you have toothpaste you can use?, n (%) | |||
| There is always toothpaste I can use | 1120 (97.1) | 1196 (97.2) | 2316 (97.2) |
| There is sometimes toothpaste I can use | 25 (2.2) | 22 (1.8) | 47 (2.0) |
| There is no toothpaste I can use | 2 (0.2) | 3 (0.2) | 5 (0.2) |
| Missing | 6 (0.5) | 9 (0.7) | 15 (0.6) |
| Intervention (n = 1153) | Control (n = 1230) | Overall (n = 2383) | |
|---|---|---|---|
| Task self-efficacy, mean (SD) | 3.4 (0.6) | 3.4 (0.6) | 3.4 (0.6) |
| Attitudes, mean (SD) | 3.2 (0.4) | 3.2 (0.4) | 3.2 (0.4) |
| Coping planning, mean (SD) | 2.7 (0.7) | 2.8 (0.7) | 2.8 (0.7) |
| Action planning, mean (SD) | 3.3 (0.6) | 3.3 (0.6) | 3.3 (0.6) |
| Intention (How often do you want to brush your teeth?), n (%) | |||
| > three times a day | 58 (5.0) | 62 (5.0) | 120 (5.0) |
| Three times a day | 211 (18.3) | 236 (19.2) | 447 (18.8) |
| Twice a day | 798 (69.2) | 837 (68.0) | 1635 (68.6) |
| Once a day | 64 (5.6) | 75 (6.1) | 139 (5.8) |
| < once a day | 8 (0.7) | 8 (0.7) | 16 (0.7) |
| Never | 7 (0.6) | 6 (0.5) | 13 (0.5) |
| Missing | 7 (0.6) | 6 (0.5) | 13 (0.5) |
| Intervention (n = 1153) | Control (n = 1230) | Overall (n = 2383) | |
|---|---|---|---|
| Non-cariogenic food and drinks | |||
| How many times do you usually eat | |||
| Fruit, n (%) | |||
| 4 +/day | 169 (14.7) | 174 (14.1) | 343 (14.4) |
| 3/day | 260 (22.5) | 308 (25.0) | 568 (23.8) |
| 2/day | 357 (31.0) | 352 (28.6) | 709 (29.8) |
| 1/day | 239 (20.7) | 254 (20.7) | 493 (20.7) |
| < 1/day | 95 (8.2) | 106 (8.6) | 201 (8.4) |
| Never | 29 (2.5) | 29 (2.4) | 58 (2.4) |
| Missing | 4 (0.3) | 7 (0.6) | 11 (0.5) |
| How many times do you usually drink | |||
| Diet coke or other non-sugar drinks, n (%) | |||
| 4 +/day | 57 (4.9) | 58 (4.7) | 115 (4.8) |
| 3/day | 61 (5.3) | 77 (6.3) | 138 (5.8) |
| 2/day | 158 (13.7) | 154 (12.5) | 312 (13.1) |
| 1/day | 247 (21.4) | 231 (18.8) | 478 (20.1) |
| < 1/day | 461 (40.0) | 462 (37.6) | 923 (38.7) |
| Never | 163 (14.1) | 232 (18.9) | 395 (16.6) |
| Missing | 6 (0.5) | 16 (1.3) | 22 (0.9) |
| Water, n (%) | |||
| 4 +/day | 555 (48.1) | 609 (49.5) | 1164 (48.8) |
| 3/day | 249 (21.6) | 281 (22.8) | 530 (22.2) |
| 2/day | 184 (16.0) | 168 (13.7) | 352 (14.8) |
| 1/day | 83 (7.2) | 74 (6.0) | 157 (6.6) |
| < 1/day | 48 (4.2) | 53 (4.3) | 101 (4.2) |
| Never | 27 (2.3) | 34 (2.8) | 61 (2.6) |
| Missing | 7 (0.6) | 11 (0.9) | 18 (0.8) |
| Cariogenic food and drinks | |||
| How many times do you usually eat | |||
| Cakes or biscuits, n (%) | |||
| 4 +/day | 49 (4.2) | 40 (3.3) | 89 (3.7) |
| 3/day | 103 (8.9) | 120 (9.8) | 223 (9.4) |
| 2/day | 303 (26.3) | 313 (25.4) | 616 (25.8) |
| 1/day | 354 (30.7) | 377 (30.7) | 731 (30.7) |
| < 1/day | 294 (25.5) | 322 (26.2) | 616 (25.8) |
| Never | 34 (2.9) | 43 (3.5) | 77 (3.2) |
| Missing | 16 (1.4) | 15 (1.2) | 31 (1.3) |
| Sweets or chocolate, n (%) | |||
| 4 +/day | 66 (5.7) | 76 (6.2) | 142 (6.0) |
| 3/day | 162 (14.1) | 137 (11.1) | 299 (12.5) |
| 2/day | 270 (23.4) | 236 (19.2) | 506 (21.2) |
| 1/day | 310 (26.9) | 392 (31.9) | 702 (29.5) |
| < 1/day | 300 (26.0) | 336 (27.3) | 636 (26.7) |
| Never | 29 (2.5) | 37 (3.0) | 66 (2.8) |
| Missing | 16 (1.4) | 16 (1.3) | 32 (1.3) |
| How many times do you usually drink | |||
| Soft drinks that contain sugar, n (%) | |||
| 4 +/day | 43 (3.7) | 61 (5.0) | 104 (4.4) |
| 3/day | 83 (7.2) | 102 (8.3) | 185 (7.8) |
| 2/day | 164 (14.2) | 190 (15.4) | 354 (14.9) |
| 1/day | 331 (28.7) | 301 (24.5) | 632 (26.5) |
| < 1/day | 392 (34.0) | 402 (32.7) | 794 (33.3) |
| Never | 132 (11.4) | 158 (12.8) | 290 (12.2) |
| Missing | 8 (0.7) | 16 (1.3) | 24 (1.0) |
| Energy/sports drinks, n (%) | |||
| 4 +/day | 22 (1.9) | 21 (1.7) | 43 (1.8) |
| 3/day | 35 (3.0) | 37 (3.0) | 72 (3.0) |
| 2/day | 48 (4.2) | 56 (4.6) | 104 (4.4) |
| 1/day | 158 (13.7) | 133 (10.8) | 291 (12.2) |
| < 1/day | 434 (37.6) | 447 (36.3) | 881 (37.0) |
| Never | 444 (38.5) | 522 (42.4) | 966 (40.5) |
| Missing | 12 (1.0) | 14 (1.1) | 26 (1.1) |
| Fruit juices and smoothies, n (%) | |||
| 4 +/day | 92 (8.0) | 126 (10.2) | 218 (9.1) |
| 3/day | 147 (12.7) | 139 (11.3) | 286 (12.0) |
| 2/day | 202 (17.5) | 205 (16.7) | 407 (17.1) |
| 1/day | 259 (22.5) | 255 (20.7) | 514 (21.6) |
| < 1/day | 296 (25.7) | 316 (25.7) | 612 (25.7) |
| Never | 152 (13.2) | 181 (14.7) | 333 (14.0) |
| Missing | 5 (0.4) | 8 (0.7) | 13 (0.5) |
| Cariogenic score, mean (SD) | 38.5 (15.9) | 37.9 (16.3) | 38.2 (16.1) |
| Dental metric | Intervention (n = 1153) | Control (n = 1230) | Total (n = 2383) |
|---|---|---|---|
| Presence of DICDAS4–6MFT–n (%) | 345 (29.9) | 355 (28.9) | 700 (29.4) |
| Number of permanent teeth assessed for ICDAS | |||
| Mean (SD) | 30.5 (2.8) | 30.4 (2.8) | 30.4 (2.8) |
| Median (IQR) | 32.0 (30.0–32.0) | 32.0 (30.0–32.0) | 32.0 (30.0–32.0) |
| Total number of DICDAS4–6MFT | |||
| Mean (SD) | 0.60 (1.20) | 0.61 (1.23) | 0.61 (1.22) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 4–6)–mean (SD) | 0.18 (0.60) | 0.23 (0.72) | 0.21 (0.67) |
| M: teeth extracted due to caries–mean (SD) | 0.09 (0.58) | 0.05 (0.38) | 0.07 (0.49) |
| F: filled teeth (ICDAS 4–6)–mean (SD) | 0.33 (0.80) | 0.34 (0.84) | 0.33 (0.82) |
| Presence of DICDAS1–6MFT–n (%) | 687 (59.6) | 706 (57.4) | 1393 (58.5) |
| Total number of DICDAS1–6MFT | |||
| Mean (SD) | 1.89 (2.32) | 1.85 (2.50) | 1.87 (2.41) |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| Number of: | |||
| D: decayed teeth (ICDAS 1–6)–mean (SD) | 1.56 (2.10) | 1.56 (2.31) | 1.56 (2.21) |
| M: teeth extracted due to caries–mean (SD) | 0.09 (0.58) | 0.05 (0.38) | 0.07 (0.49) |
| F: filled teeth (ICDAS 1–6)–mean (SD) | 0.23 (0.64) | 0.24 (0.68) | 0.24 (0.66) |
| Plaque index – mean (SD) | 1.01 (0.66) | 0.87 (0.61) | 0.94 (0.64) |
| Bleeding score – mean (SD) | 0.12 (0.17) | 0.11 (0.15) | 0.12 (0.16) |
| Number of teeth with bleeding gingivae | |||
| Mean (SD) | 1.68 (2.02) | 1.57 (1.90) | 1.63 (1.96) |
| Median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
Appendix 10 Safeguarding events
| Region | Date reported to CI/YTU | Description | Action taken | Outcome |
|---|---|---|---|---|
| Scotland | 23 April 2018 | Participant responded to a BRIGHT text message with the words ‘Get some help’ at 10.25 a.m. on 23 April 2018. This message was preceded by a message saying ‘Stop it’ on the same day and it was followed by a further text saying ‘stop’ on the same day(s). | CI and Co-Principal investigator were alerted to this response and the appropriate school was contacted. The school contact was asked to meet with the participant to ensure their well-being. The school subsequently contacted the BRIGHT LRT, by phone, to advise that the teacher had met with the participant and spoken with them. The participant is absolutely fine and advised the teacher that the message was sent as a joke. | Event resolved – no further action required. |
| South Yorkshire | 9 March 2018 | Participant responded to a BRIGHT text message with the words ‘Mi mams dead’ at 9 p.m. on 8 March 2018. | CI and Co-Principal investigator were alerted to this response and the appropriate school was contacted. The school contact advised that they would discuss this with the participant, and subsequently advised that this was not factually accurate. Co-Principal Investigator (and clinical lead for this region) asked the school contact to feedback any learning points for the BRIGHT trial. | Event resolved – no further action required. |
| South Yorkshire | 27 September 2018 | Concerning response/s to BRIGHT SMS | ZM contacted school, school not concerned but agreed to follow up with participant. | Event resolved – no further action required. |
| South Yorkshire | 27 September 2018 | Concerning response/s to BRIGHT SMS | ZM contacted school, school not concerned but agreed to follow up with participant. | Event resolved – no further action required. |
| South Wales | 16 July 2018 | Concerning response(s) to BRIGHT SMS | NI contacted school, school discussed with participant. | Event resolved – no further action required. |
| West Yorkshire | 27 November 2018 | Dental Assessor concerned about child’s welfare | Dental Assessor raised concern about the participant with the school contact, who notified the Year Manager and was intending to inform the School Safeguarding lead. Dental Assessor followed this up with the school after 2 weeks to ensure the school safeguarding lead had been informed. Dental Assessor offered for the school safeguarding lead to contact her directly if needed. | Event resolved – no further action required. |
| West Yorkshire | 20 February 2019 | Concerning response/s to BRIGHT SMS | WY LRT informed and followed up with school 8 March 2019 (not done sooner due to half term and then combined with planned face-to-face visit to school). School discussed with pupil and any pupil concerns addressed. | Event resolved – no further action required. |
| South Wales | 1 May 2019 | Concerning response(s) to BRIGHT SMS | SW LRT informed and followed up with school 9 May 2019. School discussed with pupil, and young person reminded they can opt out by replying ‘STOP’. | Event resolved – no further action required. |
| West Yorkshire | 3 May 2019 | Concerning response(s) to BRIGHT SMS | WY LRT informed and followed up with school 3 May 2019. School staff discussed response with young person. | Event resolved – no further action required. |
| Scotland | 20 May 2019 | Concerning response(s) to BRIGHT SMS | S LRT informed and followed up with school 23 May 2019. Designated safeguarding lead of school confirmed response would be discussed with parents of young person. | Event resolved – no further action required. |
| South Wales | 12 June 2019 | Concerning comment on young person CRF | SW LRT informed and asked to follow up with school 12 June 2019. | Event resolved – no further action required. |
| West Yorkshire | 15 July 2019 | Concerning response(s) to BRIGHT SMS | WY LRT informed and followed up with school 16 July 2019. School staff discussed response with parent of young person. | Event resolved – no further action required. |
| Scotland | 3 September 2019 | Concerning response(s) to BRIGHT SMS | S LRT informed and followed up with school 3 September 2019. School teacher to discuss responses with young person. | Event resolved – no further action required. |
| West Yorkshire | 24 June 2020 | Concerning response(s) to BRIGHT SMS | ZM contacted the school safeguarding lead who confirmed they would discuss responses with young person. | Event resolved – no further action required. |
| West Yorkshire | 1 July 2022 | Letter received at YTU sent in BRIGHT freepost envelope. | Received letter in freepost envelope on 1 July 2022. Letter did not contain identifiable information so unable to identify YP who sent the letter (checked TMS). Sent password-protected copy of letter and partially completed Safeguarding form to NI, MR, ZM, HA on 4 July 2022. ZM contacted PD who called Leeds Social Services. Named person known to social services and name of school was provided. ZM telephoned school’s safeguarding lead who confirmed they would let ZM know if any further actions are required. UoY Safeguarding Team notified by e-mail on 6 July 2022 that safeguarding concern was raised and concern has been reported to organisation with primary safeguarding responsibility. Physical letter filed in cabinet with BRIGHT consent forms. | Event resolved – no further action required. |
List of abbreviations
- BRIGHT
- Brushing RemInder 4 Good oral HealTh
- CACE
- complier-average causal effect
- CARIES-QC
- Caries Impacts and Experiences Questionnaire for Children
- CARIES-QC-U
- Caries Impacts and Experiences Questionnaire for Children – Utility Measure
- CBS
- classroom-based session
- CDHS
- Child Dental Health Survey
- Chilypep
- Children and Young People’s Empowerment Project
- CHU9D
- Child Health Utility 9D
- CONSORT
- Consolidated Standards of Reporting Trials
- DMEC
- Data Monitoring and Ethics Committee
- DMFT
- decayed, missing and filled teeth
- FSM
- free school meals
- GCSE
- General Certificate of Secondary Education
- GRIPP
- Guidance for Reporting Involvement of Patients and Public
- HEAP
- health economics analysis plan
- HIC
- Health Informatics Centre
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICCMS
- International Caries Classification and Management System
- ICDAS
- International Caries Detection and Assessment System
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IRR
- incidence rate ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- IV
- instrumental variable
- KOB
- keep on brushing
- LRT
- local research team
- mHealth
- mobile health
- NDIP
- National Dental Inspection Programme
- NHSCII
- NHS Cost Inflation Index
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- Ofsted
- Office for Standards in Education, Children’s Services and Skills
- OR
- odds ratio
- PPIE
- Patient and Public Involvement and Engagement
- PSHE
- personal, social, health and economic
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAP
- statistical analysis plan
- SD
- standard deviation
- SDR
- Statement of Dental Remuneration
- SE
- standard error
- SMS
- short messaging service
- STP
- stakeholder participant
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UDA
- Unit of Dental Activity
- YTU
- York Trials Unit
Notes
-
BRIGHT lesson plan
-
BRIGHT young person booklet
-
BRIGHT photos for lesson plan
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JQTA2103).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
