Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR131717. The protocol was agreed in November 2020. The assessment report began editorial review in June 2021 and was accepted for publication in April 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Westwood et al. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Westwood et al.
Chapter 1 Objective
Sections of this report are reproduced from Westwood et al. 1 © NICE 2020 Diagnostic Assessment Report Commissioned by the NIHR HTA Programme on behalf of the National Institute for Health and Care Excellence – Protocol. Available from www.nice.org.uk/guidance/dg44/documents/final-protocol. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
The overall objective of this project was to provide an update to the National Institute for Health and Care Excellence (NICE) diagnostics guidance (DG) on tauroselcholic [75selenium] acid (SeHCAT™) (GE Healthcare, Chicago, IL, USA) testing for the investigation of diarrhoea among adults with diarrhoea-predominant irritable bowel syndrome (IBS-D) or Crohn’s disease without ileal resection (DG7), published in November 2012. 2 This update report summarises the current evidence on the clinical effectiveness and cost-effectiveness of SeHCAT for investigating bile acid diarrhoea (BAD) and the measurement of bile acid pool loss in adults referred to secondary care for the investigation of chronic diarrhoea with an unknown cause, functional diarrhoea (FD), or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD). This update also considered SeHCAT for the investigation of possible secondary BAD among adults with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection.
To address the stated objective, the following research questions were defined.
-
What are the effects of a care pathway that includes a SeHCAT test, compared with no SeHCAT test, in terms of clinical symptoms, other relevant health outcomes and costs among adults with chronic diarrhoea, in the specified populations?
-
Does the result of a SeHCAT test predict response to treatment with bile acid sequestrants (BASs) among adults with chronic diarrhoea, in the specified populations?
-
What is the cost-effectiveness of including a SeHCAT test in the diagnostic pathway for the investigation of chronic diarrhoea, in the specified populations?
Chapter 2 Background and definition of the decision problem
Population
This assessment considers SeHCAT for the assessment of adults referred to secondary care for the investigation of chronic diarrhoea with an unknown cause, FD, or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD).
Bile acid diarrhoea is a form of chronic diarrhoea in which the recycling of bile acids in the body is not functioning properly. Bile acids are produced by the liver and stored in the gallbladder until they are released into the small bowel to aid digestion. Usually, bile acids are reabsorbed into the liver in the final section of the small bowel. If they are not reabsorbed, or the body produces more bile acid than can be reabsorbed, excess amounts of bile travel from the small bowel to the colon, stimulate salt and water secretion and bowel movements, and result in diarrhoea.
Symptoms of BAD may include explosive, smelly or watery diarrhoea, urgency in going to the toilet, abdominal pain, swelling or bloating and faecal incontinence.
The most common form of BAD is caused by overproduction of bile acid in people with no physical damage to the bile acid recycling system. This primary form of BAD is often missed as a cause of chronic diarrhoea. Because of the similarity in symptoms between BAD and both IBS-D and FD, BAD may be misdiagnosed. The actual cause of diarrhoea in up to 30% of people with suspected IBS-D or FD may be BAD. 3
Bile acid diarrhoea can also appear as a secondary condition after the small bowel or another part of the bile acid recycling system has been damaged by disease, surgery or other clinical interventions (e.g. pelvic radiotherapy or chemotherapy).
This assessment also considered SeHCAT for the investigation of possible secondary BAD among adults with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection.
Intervention technology
Tauroselcholic [75selenium] acid is a radiopharmaceutical capsule that is indicated for use in the investigation of bile acid malabsorption (BAM) and measurement of bile acid pool loss. It may also be used in assessing ileal function, in the investigation of inflammatory bowel disease (IBD) and chronic diarrhoea and in the study of enterohepatic circulation (these uses are outside the current scope). SeHCAT is manufactured by GE Healthcare.
The SeHCAT test is used to measure how well the body absorbs bile acids. The radiopharmaceutical capsule contains 75selenium (a gamma emitter) and a synthetic bile acid (tauroselcholic acid). When swallowed, SeHCAT is absorbed by the body like a natural bile acid. It can be detected in the body using a gamma camera.
A SeHCAT test involves two outpatient appointments in the nuclear medicine department of a hospital. During the first appointment, the patient swallows a SeHCAT capsule and then waits for up to 3 hours before a baseline scan is taken. A follow-up scan is taken on day 7, after the first appointment. It may be considered reasonable to stop any antidiarrhoeal medication for the duration of the test, as there is a possibility that this may interfere with the test result.
The result of the test is given as the proportion of SeHCAT remaining in the body after 7 days. To calculate the result, the amount of radioactivity detected in the follow-up scan is divided by the amount of radioactivity detected in the baseline scan. A diagnosis of BAD is usually made when ≤ 15% of SeHCAT remains in the body. SeHCAT results are on a continuous scale; hence, the threshold used for a positive result can vary. However, retention values of > 20% are not usually considered to be indicative of BAD, although values of 15–20% may sometimes be considered ‘borderline’ (clinical opinion of specialist committee members). SeHCAT results are also sometimes used to grade the severity of BAD:
-
Retention values from 10% to 15% indicate mild BAD.
-
Retention values from 5% to 10% indicate moderate BAD.
-
Retention values from 0% to 5% indicate severe BAD.
In current clinical practice, the cut-off point for a positive SeHCAT result may vary. A prospective survey, conducted in 2014/15 and published in 2016, of SeHCAT provision and practice in the UK included 38 centres and 1036 patients. Participating NHS centres were recruited through direct mailing and notices on the websites of professional societies; patients referred for a SeHCAT test with a clinical suspicion of BAD because of chronic diarrhoea without a known cause were eligible for inclusion. The survey found that > 50% used their own criteria for defining a positive SeHCAT result. 4
There are no alternative technologies currently in routine use in the NHS in England.
Comparator
The comparators for this technology appraisal are as follows:
-
no SeHCAT testing and no treatment with BAS
-
no SeHCAT testing and trial of treatment with BAS.
Care pathway
Diagnostic assessment
The initial investigation of patients with chronic diarrhoea should involve history-taking, an assessment of clinical symptoms and signs to exclude cancer, as indicated in NICE guideline 12, Suspected Cancer: Recognition and Referral. 5 The initial clinical assessment should also include blood and stool tests to exclude anaemia, coeliac disease, infection and inflammation, as recommended in the British Society of Gastroenterology (BSG) clinical guidelines. 3 The BSG guidelines position SeHCAT testing as part of secondary clinical assessment, following initial assessment/investigations to exclude coeliac disease (coeliac serology and upper gastrointestinal endoscopy and biopsy in people with suspected coeliac disease), common infections (stool examination for Clostridium difficile, ova, cysts and parasites) and colorectal cancer (colonoscopy in people with altered bowel habit and rectal bleeding, and faecal immunochemical testing to guide priority investigations in people with lower gastrointestinal symptoms and no rectal bleeding). 3
The BSG guidelines list BAD among the ‘common disorders’ to be investigated as part of secondary clinical assessment and state that a positive diagnosis of BAD should be made either using SeHCAT testing or by measuring the serum bile acid precursor 7-alpha-hydroxy-4-cholesten-3-one, depending on local availability. 3 The BSG guidelines also state that ‘there is insufficient evidence to recommend use of an empirical trial of treatment for bile acid diarrhoea rather than making a positive diagnosis’. 3 Referral to secondary care is required for investigation and diagnosis of BAD.
NICE clinical guideline 61, Irritable Bowel Syndrome in Adults: Diagnosis and Management,6 recommends considering a diagnosis of irritable bowel syndrome (IBS) for patients with abdominal pain or discomfort that is either relieved by defecation or associated with altered bowel frequency or stool form when the initial investigations are normal and at least two of the following symptoms are present: altered stool passage (straining, urgency, incomplete evacuation); abdominal bloating (more common in women than men), distension, tension or hardness; symptoms worsened by eating; and passage of mucus. The guideline also states that further tests such as colonoscopy or imaging are not necessary to confirm an IBS diagnosis. 6 Investigation of BAD may be useful among patients previously diagnosed with IBS-D; however, NICE clinical guideline 61 does not currently include any recommendations on the investigation of BAD. 6
Investigation of BAD may also be considered when diarrhoea persists, regardless of conventional treatment, in those conditions with which it may appear as a secondary condition. When chronic diarrhoea appears after ileal resection (removal of the terminal part of the small bowel to treat Crohn’s disease), BAD is so common (> 95% of cases)7 that a diagnostic test before treatment may not be considered necessary.
The use of SeHCAT in current clinical practice appears to vary, with some studies indicating that imaging tests and invasive investigations such as colonoscopy are often performed before SeHCAT. 4,8,9 Multiple interactions with different clinicians over many years often take place before BAD is investigated. 10
The manufacturer advises that SeHCAT testing is currently available at 85 hospitals across 74 out of 225 NHS acute trusts in England (data from August 2020). 11 According to the 2018/19 NHS National Cost Collection data,12 the trusts in which SeHCAT testing is available perform about 10,000 SeHCAT tests per year. The number of tests performed across trusts varies widely, ranging from < 50 tests per year to > 500 tests per year.
Management/treatment
The symptoms of BAD are most often controlled with BAS medication. BASs bind to bile acids in the small bowel and prevent them from irritating the colon; they may also slow transit time. The treatment may be long term.
There are currently three BASs available for use in the UK NHS: colestyramine, colestipol and colesevelam. Colestyramine and colestipol come in powder or granule form and colesevelam comes in tablet form. Use of both colestipol and colesevelam for BAD is currently off-label (NICE13). BASs can be difficult to tolerate: constipation and flatulence are commonly reported adverse events, some people find the taste and texture of colestyramine and colestipol very unpleasant and some patients have reported weight gain or weight loss. Increases in dose, addition of antidiarrhoea medication or changes in diet may also be needed to achieve adequate symptom control. Long-term use of colestyramine has been associated with reduced vitamin and folate levels. 14 However, 1–2 years of colestipol use has been reported to have no effect on vitamin A or folic acid levels, and only a small effect on vitamin D levels. 14 Colesevelam was not associated with significant reductions in the absorption of vitamins A, D, E or K in studies of up to 1 year. 14 Guidelines made no recommendation about routine monitoring of fat-soluble vitamins during long-term BAS therapy, while noting that approved product labels recommend supplementation of vitamins A, D and K only if deficiency occurs. 14
In current practice, in some UK secondary care settings (reported by specialist committee members), BAS treatment of BAD is started without a diagnostic test being performed (trial of treatment). The estimated time taken to ascertain the effectiveness of trial of treatment was between 4 and 12 weeks (clinical opinion of specialist committee members).
Chapter 3 Assessment of clinical effectiveness
The systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care,15 the NICE Diagnostics Assessment Programme manual16 and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 17 Data extraction tables for studies included in our previous Diagnostic Assessment Report (DAR),18 conducted to support the development of DG7,2 were used as a starting point for this report. Public input on the definition of the decision problem and research questions was received during the scoping phase of this project, through the lay members of the NICE Diagnostics Advisory Committee and Assessment Subgroup. Stakeholder comments were also sought, through NICE, on the draft and final report.
Systematic review methods
Search strategy
Search strategies used in the original report were updated in line with the NICE final scope. 11 Search strategies were based on target condition and intervention, as recommended in the CRD guidance for undertaking reviews in health care and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 15,17,19,20
Searches were undertaken to identify studies of SeHCAT in the diagnosis of BAD. The search strategies combined relevant search terms comprising indexed keywords [e.g. medical subject headings (MeSH) and Emtree] and free-text terms; strategies were developed specifically for each database and the keywords adapted according to the configuration of each database. Only studies conducted with humans were sought. Searches were not limited by language or publication status (unpublished or published). The original 2011 strategies were adapted to incorporate changes to the preferred terminology and search methods for each resource. Owing to the time elapsed, some resources were no longer available, but additional resources were searched to maintain completeness.
Searches for studies on economic evaluations, costs and health-related quality of life (HRQoL) were also conducted (see Chapter 4, Identifying and reviewing published cost-effectiveness studies, for further details). To ensure that no relevant studies were missed, the results of the clinical effectiveness searches were also screened for records relevant to the cost-effectiveness evaluation, and all cost-effectiveness results, including guideline searches, were screened for studies relevant to the clinical effectiveness section.
The following databases were searched for relevant studies from inception:
-
MEDLINE (Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, from 1946 to 30 November 2020
-
EMBASE (Ovid), from 1974 to 25 November 2020
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley), up to November 2020, issue 11
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley), up to November 2020, issue 11
-
Database of Abstracts of Reviews of Effects (DARE) (CRD), up to March 2015
-
Health Technology Assessment (HTA) database (CRD), up to March 2018
-
Science Citation Index (SCI) (Web of Science), up to 27 November 2020
-
KSR Evidence (https://ksrevidence.com/), up to 1 December 2020
-
Latin American and Caribbean Health Sciences Literature (LILACS) (https://lilacs.bvsalud.org/en/), up to 27 November 2020
-
National Institute for Health and Care Research (NIHR) HTA programme, up to 26 November 2020
-
PROSPERO (international prospective register of systematic reviews) (www.crd.york.ac.uk/prospero/), up to 26 November 2020.
Completed and ongoing trials were identified by searches of the following resources:
-
National Institutes of Health Clinical Trials database (www.clinicaltrials.gov/), up to 26 November 2020
-
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/), up to 2 December 2020
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/), up to 2 December 2020.
Conference abstracts and proceedings were identified in a three-stage approach, conducted as follows:
-
The main Ovid EMBASE search strategy was employed to include conference abstracts and proceedings.
-
A second set of tailored searches was conducted on:
-
Northern Light Life Sciences Conference Abstracts (Ovid), from 2010 to December 2020 week 46
-
Conference Proceedings Citation Index – Science (CPCI-S) (Web of Science), from 1990 to 30 November 2020
-
-
In addition, the 2020 United European Gastroenterology Week proceedings (not currently covered by EMBASE, Northern Light or CPCI-S) were searched manually.
Additional searches
An additional targeted search for trial of treatment with BASs for IBS/Crohn’s disease was performed on the following databases:
-
MEDLINE (Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, from 1946 to 17 February 2021
-
EMBASE (Ovid), from 1974 to 17 February 2021.
This additional search was conducted with the primary aim of identifying additional studies to inform the cost-effectiveness modelling; search results were screened as part of the main clinical effectiveness searches.
All identified references were downloaded to EndNote X20 software [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] for further assessment and handling. These references were imported into the existing project library and deduplicated against the 2011 search results. All search results (both clinical effectiveness and economics) were screened for all areas of interest. Rigorous records were maintained as part of the searching process. Individual records within the EndNote reference library were tagged with search information, including the name of the searcher, date searched, database name and host, strategy name and iteration.
The main EMBASE search strategy for each set of searches was independently peer reviewed by a second information specialist, using the evidence based checklist for the peer review of electronic search strategies (PRESS-EBC). 21,22 References in retrieved articles were checked for additional studies. Full search strategies are provided in Appendix 1.
Inclusion and exclusion criteria
Participants
The study populations eligible for inclusion were adults (aged ≥ 18 years) referred to a gastroenterology clinic for the investigation and diagnosis of possible BAD, who had previously undergone primary clinical assessment/investigations (as recommended in the BSG guidelines3) to exclude coeliac disease (coeliac serology and upper gastrointestinal endoscopy and biopsy in people with suspected coeliac disease), common infections (stool examination for C. difficile, ova, cysts and parasites) and colorectal cancer (colonoscopy in people with altered bowel habit and rectal bleeding, and faecal immunochemical testing to guide priority investigations in people with lower gastrointestinal symptoms and no rectal bleeding).
Given the paucity of evidence identified, studies that did not fully report prior investigations, or studies in which prior investigations did not match those specified previously, have been included; full details of prior investigations (when reported) are provided in Appendix 2, Table 54.
As detailed previously, this assessment focused on two specific populations:
-
adults presenting with chronic diarrhoea with unknown cause or FD, or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD)
-
adults presenting with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection (i.e. people with suspected secondary BAD).
Setting
The setting was secondary care.
Intervention (index test)
The intervention was the SeHCAT test.
Comparators
For the purposes of cost-effectiveness modelling, the comparators used in this assessment were as follows:
-
no SeHCAT testing and no treatment with BASs
-
no SeHCAT testing and trial of treatment with BASs.
Outcomes
Studies reporting any of the following outcomes were included:
-
effect of testing on treatment plan (e.g. surgical or medical management, or further testing)
-
effect of testing on clinical outcome (e.g. morbidity and adverse events)
-
effect of testing on adherence to treatment
-
prognosis – the ability of the test (SeHCAT) result to predict clinical outcome (i.e. response to treatment)
-
predictive accuracy – sensitivity and specificity of the SeHCAT test for the prediction of treatment response
-
acceptability of tests to patients, or surrogate measures of acceptability (e.g. waiting time and associated anxiety)
-
adverse events associated with testing (e.g. pain/discomfort experienced during the procedure and waiting times before results)
-
HRQoL.
Study design
The following types of study were eligible for inclusion:
-
randomised controlled trials (RCTs), non-randomised clinical controlled trials (CCTs) or observational comparative studies in which clinical or treatment planning outcomes were compared between participants who received SeHCAT testing and those who did not
-
RCTs, CCTs or observational comparative studies in which all participants received SeHCAT testing and clinical outcomes were compared between treatment decisions based on different definitions of a positive SeHCAT result (different diagnostic thresholds)
-
observational studies in which all participants received SeHCAT testing, and clinical or treatment planning outcomes were compared between patients with positive SeHCAT results and those with negative SeHCAT results
-
observational studies that reported the results of multivariable regression modelling with response to treatment with BAS as the dependent variable and index test result (continuous or categorical) as an independent variable [included studies should control adequately for potential confounders (e.g. age, gender, comorbidities)]
-
predictive accuracy studies that reported sufficient data to support the calculation of the sensitivity and specificity of SeHCAT to predict response to treatment with BAS (i.e. studies that reported the outcome of treatment with BAS for both patients with a positive SeHCAT test and those with a negative SeHCAT test).
Studies using any reported threshold for a positive SeHCAT test and any reported definition of response to treatment were eligible for inclusion.
No new studies, of the higher-level study designs described previously, were identified. Therefore, studies that reported treatment outcome only for those participants with a positive SeHCAT result [i.e. sufficient data to calculate positive predictive value (PPV) only] were included.
Studies that were included in our previous DAR,18 conducted to support the development of DG7,2 and which met the aforementioned inclusion criteria, were also included in this review.
Exclusion criteria
The following study/publication types were excluded:
-
preclinical and animal
-
reviews, editorials and opinion pieces
-
case reports
-
studies reporting only technical aspects of the test, or image quality.
Inclusion screening and data extraction
Two reviewers (MW and ER or Gill Worthy) independently screened the titles and abstracts of all reports identified by searches; any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full-text screening stage are presented in Appendix 4, Table 56.
Studies cited in materials provided by the manufacturer of the SeHCAT test (GE Healthcare Ltd) were first checked against the project reference database, in EndNote X20; any studies not already identified by our searches were screened for inclusion following the process described previously.
When available/applicable, data were extracted on the following: study design/details; participant characteristics; previous investigations; details of the application of the SeHCAT test (e.g. threshold used to define a positive test result); details of any treatments received for BAD (e.g. BAS used and dosing regimen, and any concomitant treatments such as diet or loperamide); any information about intolerance to, or discontinuation of, BASs; and the definition of response to treatment, including duration of follow-up and outcomes (as defined in Chapter 4, Identifying and reviewing published cost-effectiveness studies). Data were extracted by one reviewer (MW or ER) using data extraction forms based on those used for the original systematic review,18 which was conducted to support the development of DG7. 2 A second reviewer (MW or ER) checked data extraction and any disagreements were resolved by consensus or discussion with a third reviewer (NA). Full data extraction tables are provided in Appendix 2, Tables 54 and 55.
Quality assessment
The methodological quality of included diagnostic accuracy studies was assessed using the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool. 23 The methodological quality of observational studies, which reported treatment outcome only for those participants with a positive SeHCAT result, was assessed using a topic-specific adaptation of the quality assessment checklist by Wedlake et al. ,24 as used in our previous DAR,18 conducted to support the development of DG7;2 the use of this tool was carried forward to the current assessment to provide consistency. The results of the quality assessment are used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to inform recommendations for the design of future studies. Quality assessment was undertaken by one reviewer and checked by a second reviewer (MW and ER); any disagreements were resolved by consensus or discussion with a third reviewer (NA).
The results of the quality assessments are summarised and presented in tables (see Study quality) and, for QUADAS-2 assessments, are presented in full, by study, in Appendix 3.
Methods of analysis/synthesis
Meta-analysis was considered inappropriate, owing to the small number of test accuracy studies, with varying diagnostic thresholds, and between-study heterogeneity with respect to population (prior investigations), treatment regimen, definition of response, follow-up period and SeHCAT administration; therefore, we employed a narrative synthesis. The clinical effectiveness results section of this report is structured by clinical application (diagnosis of primary BAD and diagnosis of secondary BAD in people with Crohn’s disease who have not undergone ileal resection). A detailed commentary on the major methodological problems or biases that affected the studies is also provided, together with a description of how this may have affected the individual study results.
For predictive accuracy studies (studies that reported sufficient data to support the calculation of the sensitivity and specificity of SeHCAT to predict response to treatment with BASs), the absolute numbers of true positive, false negative, false positive and true negative test results of SeHCAT, compared with the reference standard of treatment response, as well as sensitivity and specificity values, with 95% confidence intervals (CIs), are presented in Table 4. The results of individual studies were plotted in the receiver operating characteristic plane, with the diagnostic threshold used for the SeHCAT test indicated (see Figure 2).
The results of studies that reported treatment outcome only for those participants with a positive SeHCAT result (i.e. sufficient data to calculate PPV only) are presented in Table 5.
Results of the assessment of clinical effectiveness
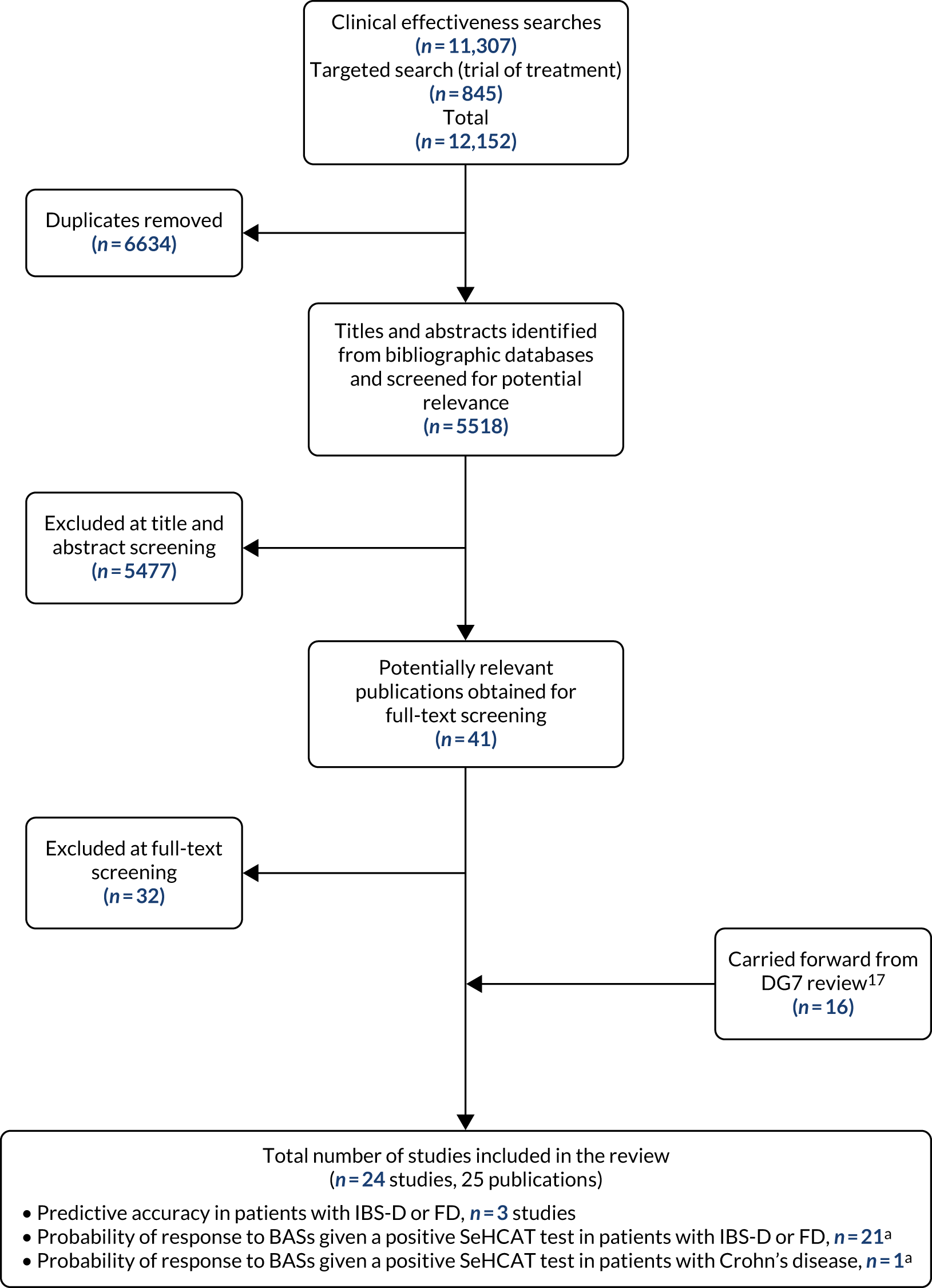
The literature searches of bibliographic databases conducted for this update identified 5518 new references. After initial screening of titles and abstracts, 41 references were considered to be potentially relevant and ordered for full-text screening; of these, nine publications were included in the review. 25–33 In addition, 16 publications, taken from the assessment report conducted for DG7,18 were carried forward and included in this review. 7,34–48 All potentially relevant studies cited in documents supplied by the test manufacturer, GE Healthcare Ltd, had already been identified by bibliographic database searches. Figure 1 shows the flow of studies through the review process. Appendix 4, Table 56, provides details, with reasons for exclusion, of all publications excluded at the full-text screening stage. Six publications49–54 that were included in our previous systematic review18 did not meet the inclusion criteria for this systematic review; these are listed in Appendix 4, Table 57. In all cases, this was because studies included participants with a variety of clinical presentations and did not report separate data for either of the two populations specified in the inclusion criteria for this assessment (see Inclusion and exclusion criteria).
FIGURE 1.
Flow of studies through the review process. a, One study provided data for both populations.
Overview of included studies
Based on the updated searches and inclusion screening described previously, and information taken from the assessment report conducted for DG7,18 a total of 24 studies,7,25–35,37–48 reported in 25 publications,7,25–48 were included in this review; the results section of this report cites studies using the primary publication only.
Fifteen of the included studies were published, in full, in peer-reviewed journals;7,30,34,35,37–45,47,48 eight were published as conference abstracts only;25–29,31–33 and one was an unpublished dissertation. 46 It should be noted that all eight studies that were published as conference abstracts only were new studies, identified during this assessment, that is the majority of the new evidence identified (eight out of nine studies) was not published, in full, in peer-reviewed journals.
No RCTs, CCTs or observational comparative studies were identified that met the inclusion criteria for this review (see Inclusion and exclusion criteria). Similarly, no observational studies were identified that reported the results of multivariable regression modelling with response to treatment with BAS as the dependent variable and index test (SeHCAT) result (continuous or categorical) as one of the independent variables. Finally, no new predictive accuracy studies (studies that reported sufficient data to support the calculation of the sensitivity and specificity of SeHCAT to predict response to treatment with BAS) were identified. All of the nine new studies included in this review25–33 were of the lowest level of evidence eligible for inclusion; these are observational studies that report some outcome data for patients treated with BASs, whereby only those patients with a positive SeHCAT test were offered treatment with BAS.
All 24 included studies provided some data about population 1: adults presenting with chronic diarrhoea with unknown cause, or FD, or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD). Three of these studies,40,43,44 all of which were previously included in the assessment report conducted for DG7,18 provided limited predictive accuracy data for this population. The remaining 21 studies reported only information about the outcome of treatment with BASs for some or all of those participants who had a positive SeHCAT test result. 7,25–35,37–39,41–43,46–48
One study7 also provided data on population 2: adults presenting with chronic diarrhoea and a diagnosis of Crohn’s disease who have not undergone ileal resection (i.e. people with suspected secondary BAD). This study reported only information about the outcome of treatment with BASs for people with Crohn’s disease who had a positive SeHCAT test result, and was previously included in the assessment report conducted for DG7. 18 No new studies meeting the inclusion criteria for population 2 were identified for this assessment report.
All 21 studies for which information on geographic location was reported were conducted in Europe; 10 were conducted in the UK,7,27–30,40,45,46,48 five in Italy,25,33,39,43,44 three in Spain,37,38,41 two in Denmark34,47 and one each in Sweden42 and France. 35
Only three of the included studies provided any information about funding, and only one UK study40 reported receipt of any industry funding [SeHCAT test supplies were provided by Amersham International Ltd (Amersham, UK), which is now part of GE Healthcare]; details of all reported funding sources are provided in Table 1.
| Study | Study details | Objective | FD | IBS-D | Crohn’s disease | Study design and outcome extracted |
|---|---|---|---|---|---|---|
| Bellini 202025 |
|
To determine the prevalence of BAM among IBS-D and FD patients referred to a tertiary gastroenterological centre in Italy, to explore the possible correlation between BAM severity, symptom severity and quality of life, and to explore whether or not the response to colestyramine could be related to BAM severity | ✓ | ✓ |
|
|
| aBorghede 201134 |
|
To investigate the frequency of BAM and treatment responses to colestyramine with SeHCAT scanning among patients experiencing chronic watery diarrhoea | ✓ |
|
||
| Farmer 201726 |
|
To compare rates of BAM in Rome III- and Rome IV-defined patients with IBS-D | ✓ |
|
||
| aFellous 199435 |
|
To determine the performance and the clinical significance of a simplified version of the SeHCAT test that measures ileal absorption of bile salt | ✓ |
|
||
| aFernandez-Bañares 200137 and a related publication36 |
|
|
✓ |
|
||
| aFernández-Bañares 200738 |
|
|
✓ | ✓ |
|
|
| aGalatola 199239 |
|
To assess the prevalence of BAM and the efficacy of colestyramine therapy in improving symptoms associated with this condition among patients with IBS-D | ✓ |
|
||
| Holmes 201227 |
|
Unclear | ✓ |
|
||
| Kumar 201329 |
|
To audit sequential patients referred for SeHCAT testing, in order to assess diagnostic value | ✓ | ✓ |
|
|
| Kumar 202028 |
|
To investigate whether or not quality of life improves with use of BAS among patients diagnosed with BAD | ✓ | ✓ |
|
|
| Lin 201630 |
|
To evaluate the natural history of BAD by examining individuals diagnosed with BAD and determining the use of and response to BASs | ✓ | ✓ |
|
|
| aMerrick 198540 |
|
To assess the value of measuring absorption of SeHCAT as a test for the presence of BAM | ✓ |
|
||
| a,cNotta 201141 |
|
To evaluate the utility of the quantification of abdominal retention of SeHCAT as a first-line diagnostic test in the early pathophysiological diagnosis of patients with chronic diarrhoea | ✓ |
|
||
| cNotta 201431 |
|
To evaluate the utility of SeHCAT testing to diagnose BAM and to assess the prevalence of BAM among patients with chronic FD | ✓ |
|
||
| cNotta 201732 |
|
To evaluate the utility of SeHCAT testing to diagnose BAM and to assess the prevalence of BAM in patients with chronic FD | ✓ |
|
||
| aRudberg 199642 |
|
To investigate the usefulness of SeHCAT testing among patients experiencing FD and to document earlier radiological investigations performed in the course of the disease | ✓ |
|
||
| aSciarretta 198643 |
|
To evaluate the diagnostic accuracy, sensitivity and specificity of the SeHCAT test | ✓ |
|
||
| aSciarretta 198744 |
|
To evaluate whether or not BAM, assessed by the SeHCAT test, had a pathogenetic role in functional chronic diarrhoea and to ascertain whether or not the small bowel transit time could be correlated with the SeHCAT test results | ✓ |
|
||
| aSinha 199845 |
|
To identify patients with idiopathic BAM, to describe their clinical features, both qualitatively and quantitatively, and to assess their response to colestyramine treatment | ✓ |
|
||
| aSmith 20007 |
|
To investigate BAM and its response to treatment among patients with chronic continuous or recurrent diarrhoea seen in a district general hospital | ✓ | ✓ |
|
|
| aTunney 201146 |
|
To assess the utility of the BSG guidelines for the investigation of chronic diarrhoea, focusing on whether or not SeHCAT should be prioritised in the investigation of chronic diarrhoea, rather than considered as a second-line option | ✓ |
|
||
| aWildt 200347 |
|
To evaluate the usefulness of SeHCAT testing by assessing the extent of BAM and describing the clinical characteristics in a group of patients with chronic diarrhoea. Clinical outcome after treatment with colestyramine was also evaluated | ✓ |
|
||
| aWilliams 199148 |
|
To determine the clinical characteristics of patients with idiopathic BAM and to identify their response to treatment | ✓ |
|
||
| Zanoni 201833 |
|
To present preliminary experience with the use of SeHCAT test | ✓ | ✓ |
|
Further details of the characteristics of study participants and the technical details of the conduct of the index test (SeHCAT) and reference standard (BAS treatment regimen) are provided in Appendix 2.
Study quality
The three studies40,43,44 that provided predictive accuracy data (information on the ability of the SeHCAT test to predict response to treatment with BAS), all of which were previously included in the assessment report conducted for DG7,18 were assessed using the QUADAS-2 tool.
The included predictive accuracy studies were all published > 30 years ago and were generally poorly reported; all three studies were rated as having an ‘unclear’ risk of bias with respect to patient selection and reference standard (no study provided details of whether or not the assessment of response to treatment was conducted blind to the results of SeHCAT testing), and two of the three studies43,44 were also rated as having an ‘unclear’ risk of bias with respect to flow and timing because the duration of follow-up over which response to treatment was assessed was not reported. Merrick et al. 40 was rated as having a ‘high’ risk of bias for the ‘flow and timing’ domain of the QUADAS-2 tool because only patients with positive or equivocal SeHCAT test results received the reference standard (treatment with BAS); patients with a negative SeHCAT test result were managed with unspecified ‘simple conservative treatment’. Sciarretta et al. 43 was rated as having a ‘high’ risk of bias for the ‘index test’ domain of the QUADAS-2 tool because the threshold used to define a positive SeHCAT test result was not prespecified.
All three studies had at least one item of ‘high’ concern regarding applicability to this assessment. In some instances, the applicability issues identified are a consequence of the age of the studies. All three studies were rated as having ‘high’ or ‘unclear’ concerns regarding the applicability of the study population to that specified in the inclusion criteria for this review; all three studies included some participants with prior cholecystectomy and no study reported previous investigations equivalent to those specified in current BSG guidelines for the investigation of chronic diarrhoea. 3 All three studies were also rated as having ‘high’ concerns regarding the applicability of the index test; the age of the studies meant that no study used the current version of the SeHCAT test, manufactured by GE Healthcare, specified in the inclusion criteria for this assessment. Merrick et al. 40 was also rated as having ‘high’ concerns regarding the applicability of the reference standard, because the management of patients with a negative SeHCAT test was not considered likely to provide a reliable indication of whether or not these patients would have responded to treatment with BAS.
The results of the QUADAS-2 assessment are summarised in Table 2 and the full assessments are provided in Appendix 3.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Study population | Index test | Reference standard | |
| Merrick 198540 | ? | ✓ | ? | ✗ | ? | ✗ | ✗ |
| Sciarretta 198643 | ? | ✗ | ? | ? | ✗ | ✗ | ✓ |
| Sciarretta 198744 | ? | ✓ | ? | ? | ✗ | ✗ | ✓ |
The methodological quality of studies that reported treatment outcome only for those participants with a positive SeHCAT result (i.e. sufficient data to calculate PPV only) was assessed using a topic-specific adaptation of the quality assessment checklist by Wedlake et al. ,24 as used in our previous DAR. 18 The results of this assessment are summarised in Table 3. These studies represent the lowest level of evidence specified in the inclusion criteria for this assessment (see Inclusion and exclusion criteria) and were generally of poor methodological quality. No study in this group provided full outcome data for patients with a negative SeHCAT test result. Ten7,27,29,30,33,34,45–48 of the 21 studies7,25–35,37–39,41,42,45–48 of this type used a retrospective study design. Eleven studies provided no clear definition of chronic diarrhoea. 7,25,27–29,31–34,41,46 Ten studies did not provide sufficient information about the SeHCAT test used to allow the testing procedure to be reproduced. 25–29,31–33,45,47 Eight studies did not clearly describe how the decision to treat patients with BASs was made. 27,29,33–35,42,46,48 Nine studies provided no or an incomplete description of the BAS treatment provided to patients with a positive SeHCAT test result. 27–29,33–35,41,46,48 Finally, six studies did not report an objective measure of response to treatment. 25,27,29,30,33,46
| Study | Question | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Study design | 2. Diarrhoea | 3. Known cause (n) | 4. SeHCAT test | 5. Cut-off values | 6. Reason for treatment | 7. Negative test | 8. Treatment | 9. Response | |
| Bellini 202025 | Prospective | No | No | No | Yes | Yes | No | Yes | No |
| aBorghede 201134 | Retrospective | No |
|
Yes | Yes | No | No | No | Yes |
| Farmer 201726 | Prospective | Yes | No | No | Yes | Yes | No | No | Yes |
| aFellous 199435 | Prospective | Yes |
|
Yes | Yes | No | Yes – some | Yes | Yes |
| aFernandez-Bañares 200137 | Prospective | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| aFernández-Bañares 200738 | Prospective | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| aGalatola 199239 | Prospective | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Holmes 201227 | Retrospective | No |
|
No | Yes | No | No | No | No |
| Kumar 201329 | Retrospective | No |
|
No | Yes | No | Yes – some | No | No |
| Kumar 202028 | Prospective | No |
|
No | No | Yes | No | No | Yes |
| Lin 201630 | Retrospective | Yes |
|
Yes | Yes | Yes | No | Yes | No |
| aNotta 201141 | Prospective | No | Unclear | Yes | Yes | Yes | No | No | Yes |
| Notta 201431 | Prospective | No | No | No | Yes | Yes | No | Yes | Yes |
| Notta 201732 | Prospective | No | No | No | Yes | Yes | No | Yes | Yes |
| aRudberg 199642 | Prospective | Yes | No | Yes | Yes | No | No | Yes | Yes |
| aSinha 199845 | Retrospective | Yes | No | No | Yes | Yes | No | Yes | Yes |
| aSmith 20007 | Retrospective | No |
|
Yes | Yes | Yes | No | Yes | Yes |
| aTunney 201146 | Retrospective | No |
|
Yes | Yes | No | No | No | No |
| aWildt 200347 | Retrospective | Yes |
|
No | Yes | Yes | No | Yes | Yes |
| aWilliams 199148 | Retrospective | Yes | No | Yes | Yes | No | No | No | Yes |
| Zanoni 201833 | Retrospective | No |
|
No | Yes | No | Unclear | No | No |
Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with diarrhoea-predominant irritable bowel syndrome or functional diarrhoea
All 24 included studies provided some data about population 1: adults presenting with chronic diarrhoea with unknown cause, suspected or diagnosed IBS-D, or FD (i.e. people with suspected primary BAD). 7,25–35,37–48
Three of these studies,40,43,44 all of which were previously included in the assessment report conducted for DG7,18 provided limited data on the accuracy of the SeHCAT test for predicting response to treatment with BAS in this population. The results of these studies are summarised in Table 4. All three studies assessed the relationship between the SeHCAT test result and response to treatment with colestyramine.
| Study | Number of participants | Index test (definition of a positive test result) | Reference standard | TP (n) | FN (n) | FP (n) | TN (n) | Sensitivity (95% CI) | Specificity (95% CI) | Tested/treated (n patients) |
|---|---|---|---|---|---|---|---|---|---|---|
| aMerrick 198540 | 43 (IBS-D) | SeHCAT < 8% | Responseb | 4 | 2 | 1 | 33c | 0.667 (0.223 to 0.957) | 0.971 (0.847 to 0.999) | 3 patients not treated |
| 43 (IBS-D) | SeHCAT ≤ 15% | Responseb | 6 | 0 | 3 | 31c | 1.000 (0.541 to 1.000) | 0.912 (0.763 to 0.981) | 3 patients not treated | |
| aSciarretta 198643 | 13 (group D only, IBS-D and 3 who had a previous cholecystectomy) | SeHCAT < 5%d | Responsee | 6 | 1 | 0 | 6 | 0.857 (0.421 to 0.996) | 1.000 (0.541 to 1.000) | All treated |
| aSciarretta 198744 | 46 (38 with IBS-D and 8 post cholecystectomy) | SeHCAT 8% cut-off value | Responsef | 19 | 1 | 1 | 25 | 0.950 (0.751 to 0.999) | 0.962 (0.804 to 0.999) | All treated |
Merrick et al. 40 reported sufficient data to allow the calculation of the performance of SeHCAT, for predicting treatment response, at two 7-day retention thresholds (< 8% and ≤ 15%). The estimated sensitivity of SeHCAT in predicting a positive response to treatment with colestyramine was 66.7% (95% CI 22.3% to 95.7%) using the < 8% threshold, and 100% (95% CI 54.1% to 100%) using the ≤ 15% threshold. The specificity estimates were 97.1% (95% CI 84.7% to 99.9%) and 91.2% (95% CI 76.3% to 98.1%) using the < 8% and ≤ 15% thresholds, respectively. 40 These results would appear to indicate that the use of the SeHCAT test with a threshold for 7-day retention of ≤ 15% (commonly used in UK clinical practice) could identify patients with IBS-D who may benefit from treatment with BASs. However, it should be noted that, although all 31 patients with a negative SeHCAT test result were classified as true negatives, this assessment was based on long-term follow-up: ‘None of the 31 patients with irritable bowel disease who retained more than 15% at seven days showed any evidence of small bowel disease, and none appeared during a follow up of at least 12, and in some up to 24 months. Simple conservative treatment resolved or eased most symptoms.’40 None of these 31 patients received treatment with colestyramine; therefore, it remains uncertain whether or not any of these patients could have benefited from treatment with BAS. One patient with a SeHCAT test result of < 8% and two with an equivocal result (8–15%) did not receive treatment with colestyramine; these patients were excluded from the analysis. 40 The remaining nine patients were treated with colestyramine; five of these had a SeHCAT test result of < 8%, one of whom did not respond to treatment, and four had an equivocal result (8–15%), two of whom responded to colestyramine and two of whom did not. 40
Sciarretta et al. 43 reported sufficient data to allow the calculation of the performance of SeHCAT, for predicting treatment response, at a threshold reported to be equivalent to a 7-day retention threshold of < 5%. The estimated sensitivity of SeHCAT in predicting a positive response to treatment with colestyramine was 85.7% (95% CI 42.1% to 99.6%) and the specificity was 100% (95% CI 54.1% to 100%). However, only 13 patients were included in this analysis. A subsequent study by Sciarretta et al. 44 estimated the sensitivity of SeHCAT in predicting a positive response to colestyramine as 95.0% (95% CI 75.1% to 99.9%) and the specificity as 96.2% (95% CI 80.4% to 99.9%), using a 7-day retention threshold of < 8% to define a positive SeHCAT test. It should be noted that there may have been overlap between the populations included in these two studies.
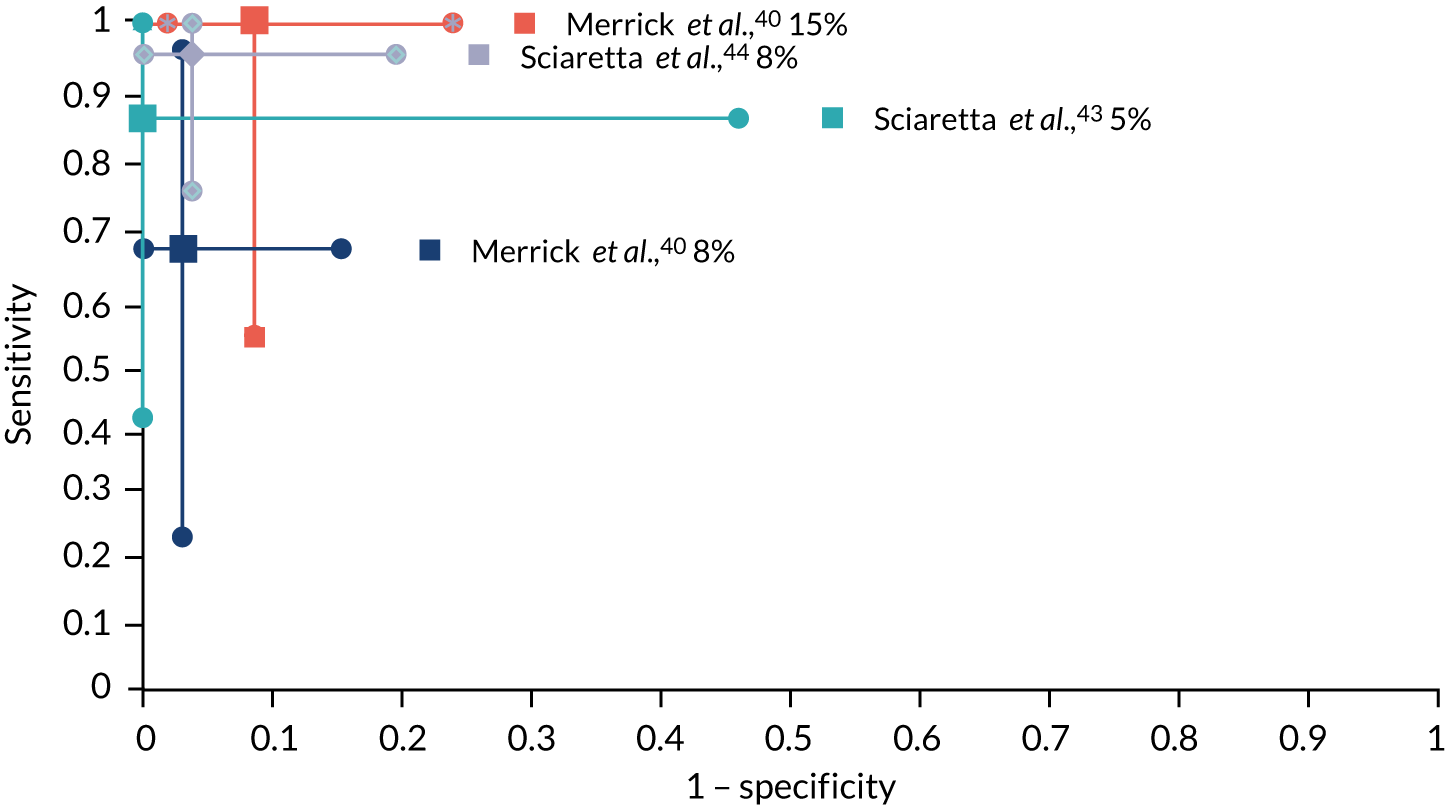
Figure 2 illustrates the variation in sensitivity and specificity with SeHCAT threshold, as reported in these three studies. 40,43,44
FIGURE 2.
Accuracy of the SeHCAT test to predict a response to treatment with colestyramine at different thresholds among patients with IBS-D. The centre dots represent the point estimates for sensitivity and specificity of SeHCAT in predicting response to treatment in the three studies at different cut-off values (5%, 8% and 15%). The vertical and horizontal lines represent the 95% CIs for sensitivity and specificity, respectively.
The between-study heterogeneity in these three studies was considerable. The principal diagnosis, method of SeHCAT administration, BAS treatment dose, definition of response to treatment and follow-up period were different between studies. Appendix 2 provides full details of study inclusion and exclusion criteria, participant characteristics, SeHCAT test methods, BAS treatment and definition of treatment response.
The remaining 21 studies7,25–35,37–39,41–43,46–48 reported information about the outcome of treatment with BAS for some or all of those participants who had a positive SeHCAT result only (i.e. sufficient information to estimate PPV), or other descriptive results.
As was the case for the predictive accuracy studies described previously, between-study heterogeneity for these studies was considerable. The principal diagnosis, threshold used to define a positive SeHCAT test, BAS treatment regimen, definition of response to treatment and follow-up period varied between studies. Appendix 2 provides full details of study inclusion and exclusion criteria, participant characteristics, SeHCAT test methods, BAS treatment and definition of treatment response, for the studies that reported this information.
Study design
When information about the BAS treatment was provided, most (13/16) studies reported the use of colestyramine alone. 25,31,32,34,35,37–39,41,42,45,47,48 Four studies reported more than one option for BAS treatment: colestyramine or colesevelam,28 colestyramine or colestipol,7 and colestyramine or colesevelam or colestipol. 30 None of these studies reported either the numbers of patients treated with each drug or the criteria used to select treatment. Eight studies reported the proportion of treated patients who were intolerant of BAS or discontinued treatment for unspecified reasons;25,29–31,34,39,45,46 rates of intolerance/discontinuation were generally high (median 15%, range 4–27%). There was insufficient information to determine whether or not levels of intolerance varied between colestyramine, colestipol and colesevelam. Only three studies reported the proportion of treated patients who were lost to follow-up: 14 out of 56 (25%),39 8 out of 32 (25%)46 and one out of six (17%). 27
Study sizes were generally small; the median number of patients with a positive SeHCAT test (across all thresholds) who received treatment with BAS was 26 (range 6–57), and the proportion of patients who experienced a positive response to treatment varied widely within a given SeHCAT test threshold (Table 5). Most of the included studies evaluated one or more of three 7-day retention thresholds (5%, 10% and 15%) for the SeHCAT test. Table 5 summarises the results for studies in this group.
| Study | Participant details (n) | Positive SeHCAT test threshold | Reference standard | Number with positive/negative test | Number (%) of patients with a positive test treated with BAS | Number (%) of patients with a negative test treated with BAS | Number (%) of patients with a positive SeHCAT test who responded to treatment with BAS (PPV) | Number (%) of responders given a negative SeHCAT test | Number (%) discontinued/intolerant of BAS |
|---|---|---|---|---|---|---|---|---|---|
| Bellini 202025 |
All 70 patients with IBS-D and FD |
≤ 5% | Response | 12/58 | NR | NR | NR | NR | NR |
| ≤ 10% | 15/55 | NR | NR | NR | NR | NR | |||
| ≤ 15% | 31/39 | 22/31 (71%) | 0/39 (0%) | NR | No patients treated | 6/22 (27%) | |||
| aBorghede 201134 |
Subgroup 114 patients with type II BAM |
< 5% | Response | 41/73 | 39/41 (95%) | 18/73 (25%) | 29b/39 (74%) | 14b/18 (78%) | 6/39 (15%) |
| < 10% | 55/59 | 53/55 (96%) | 4/59 (7%) | 41b/53 (77%) | 2b/4 (50%) | 7/53 (13%) | |||
| ≤ 15% | 68/46 | 57/68 (84%) | 0/46 (0%) | 43b/57 (75%) | No patients treated | 8/57 (14%) | |||
| Farmer 210726 | All
|
< 10% | Response | 48/159 | 48/48 (100%) | 0/159 (0%) | 36c/48 (75%) | No patients treated | NR |
| aFellous 199435 |
Subgroup 53 patients with FD |
< 10% | Response | 20/33 | NR | NR | 8d/11 (73%) | 2d/5 (40%) | NR |
| aFernandez-Bañares 200137 |
Subgroup 32 patients with FD |
< 11% | Response | 24/8 | 20/24 (83%) | 0/8 (0%) | 20e/20 (100%) | No patients treated |
|
| aFernández-Bañares 200738 |
All 62 patients with FD or IBS-D |
< 11% | Response | 37/25 | 37/37 (100%) | 0/25 (0%) | 28f,g/37 (76%) | No patients treated | NR |
| aGalatola 199239 |
All 98 patients with IBS-D |
< 11.7% | Response | 56/42 | 56/56 (100%) | 0/42 (0%) | 39h/56 (70%) | No patients treated |
|
| Holmes 201227 |
Subgroup (post test) 8 patients with type 2 BAM |
< 15% | Response | 8/0 | 6/8 (75%) | NA | 3i/6 (50%) | NA | 1/6 (17%) lost to follow-up |
| Kumar 201329 |
Subgroup 57 patients with unexplained symptoms |
< 15% | Response | 24/33 | 23/24 (96%) | Unclear
|
11j/23 (48%) | 1/39 (3%) | 6/23 (26%) intolerant of BAS |
| Kumar 202028 |
Subgroup 20 patients with idiopathic BAD |
NR | Response | 20/0 | 20/20 (100%) | NA | 9k/20 (45%) | NA | NR |
| lLin 201630 |
Subgroup (post test) 29 patients with type 2 BAM, who were contactable at follow-up |
< 10% | Response | 29/0 | 29/29 (100%) | NA | NR | NA |
|
| a,mNotta 201141 |
All 37 patients with chronic diarrhoea |
≤ 10% | Response | 16/21 | 16/16 (100%) | 0/21 (0%) | No patients treated | NR | |
| mNotta 201431 |
All 78 patients with chronic FD |
< 10% | Response | 34/44 | 34/34 (100%) | 0/44 (0%) | No patients treated | 3/34 (9%) discontinued BAS | |
| mNotta 201732 |
All 92 patients with chronic FD |
< 10% | Response | 42/50 | 42/42 (100%) | 0/50 (0%) | No patients treated | NR | |
| aRudberg 199642 |
All (excluding 3 patients who had had a previous cholecystectomy or gastric resection) 17 patients with FD |
≤ 10% | Response | 3/14 | 3/3 (100%) | 8/14 (57%) | 2p/3 (67%) | 4p/8 (50%) | NR |
| ≤ 15% | 8/9 | 7/8 (88%) | 4/9 (44%) | 6p/7 (86%) | 0p/4 (0%) | ||||
| aSinha 199845 |
All 17 patients with a history suggestive of IBS-D |
< 15% | Response | 9/8 | 9/9 (100%) | 0/8 (0%) | 6q/9 (67%) | No patients treated | 2/9 (22%) intolerant of BAS |
| aSmith 20007 |
Subgroup 197 patients with IBS-D |
< 10% | Response | 65/132 | 34/65 (52%) | 0/132 (0%) | 28r/34 (82%) | No patients treated | NR |
| aTunney 201146 |
Subgroup 86 patients with chronic diarrhoea and no known risk factors, who had no endoscopic or histological abnormalities and negative coeliac serology |
< 8 | Response | 20/66 | 20/20 (100%) | 12/66 | 10s/20 (50%) | 2s/12 (17%) |
|
| ≤ 15% | 36/50 | 32/36 (89%) | 0/50 (0%) | 12s/32 (38%) | No patients treated |
|
|||
| aWildt 200347 |
Subgroup 56 patients with possible type 2 BAM |
< 5% | Response | 13/43 | NR | NR | NR | NR | NR |
| < 10% | 21/35 | NR | NR | NR | NR | ||||
| < 15% | 24/32 | 17/24 (71%) | 0/32 (0%) | 14t,u/17 (82%) | No patients treated | ||||
| aWilliams 199148 | 181 patients | < 5% | Responsev | 23/158 | 23/23 (100%) | 21/158 (13%) | 23w/23 (100%) | 6/21 (29%) |
|
| < 10% | 39/142 | 36/39 (92%) | 8/142 (6%) | 29w/36 (81%) | 0/8 (0%) | ||||
| < 15% | 60/121 | 42/60 (70%) | 0/121 (0%) | 29w/42 (69%) | No patients treated | ||||
| Zanoni 201833 | 12 patients | < 5% | Response | 2/10 | 2/2 (100%) | 6/10 (60%) | NR | NR | NR |
| ≤ 10% | 6/6 | 6/6 (100%) | 2/6 (33%) | NR | NR | ||||
| ≤ 15% | 7/5 | 7/7 (100%) | 1/5 (20%) | NR | NR | ||||
| ≤ 20% | 8/4 | 8/8 (100%) | 0/4 (0%) | 6x/8 (75%) | No patients treated |
Response to treatment in studies evaluating a single threshold for a positive SeHCAT test
Using a 7-day retention threshold of < 5% to define a positive SeHCAT test, the proportion of test-positive patients who responded positively to treatment with BAS was reported as 74%34 and 100%48 by two studies; the proportion of SeHCAT test-positive patients in these studies who received treatment with BAS was 95%34 and 100%. 48 The equivalent data from the predictive accuracy study by Sciarretta et al. 43 indicated a treatment response rate of 100% among patients with 7-day retention values of < 5%; in this study,43 all patients with SeHCAT test results below the 5% threshold received treatment with colestyramine.
Eleven studies reported information about the rate of positive response to treatment with BAS using a 7-day retention threshold of < 10% or ≤ 10%. 7,26,31,32,34,35,37,38,41,42,48 The median proportion of SeHCAT test-positive patients who received treatment with BAS was 100% (range 52–100%) and the median response rate was 85% (range 67–100%). It should be noted that three studies from the same group31,32,41 may have had overlapping populations. All three of these studies31,32,41 classified response to treatment as complete (normalisation of stool rhythm and consistency) or partial (decrease in stool frequency and/or improvement in stool consistency); the proportion of patients in these studies who achieved a complete response ranged from 50% to 76%, and the proportion that achieved a partial response ranged from 15% to 50%.
Eight studies reported information about the rate of positive response to treatment with BAS using a 7-day retention threshold of < 15% or ≤ 15%. 27,29,34,42,45–48 The median proportion of SeHCAT test-positive patients who received treatment with BAS was 86% (range 70–100%) and the median response rate was 68% (range 38–86%). The equivalent data from the predictive accuracy study by Merrick et al. 40 indicated a treatment response rate of 67% among patients with 7-day retention values of ≤ 15%; in this study, 9 out of 12 (75%) patients with SeHCAT test results below the 15% threshold received treatment with colestyramine.
The results of studies that used other thresholds to define a positive SeHCAT test are summarised in Table 5.
Response to treatment in studies comparing multiple thresholds for a positive SeHCAT test
Four studies reported information about treatment response rates for multiple 7-day SeHCAT retention thresholds. 34,42,46,48 Two studies reported information about treatment response rates for all of the three main thresholds (15%, 10% and 5%). 34,48 In one study,34 there was little variation in the rate of response to treatment across the three thresholds (response rates of 75%, 77% and 74% for threshold values of 15%, 10% and 5%, respectively). By contrast, the second study48 reported increasing response rates as the threshold for a positive SeHCAT test was lowered (response rates of 69%, 81% and 100% for threshold values of 15%, 10% and 5%, respectively). Not all patients with a positive SeHCAT test received treatment with BAS, and the reasons for treatment decisions were not reported. The results of both studies indicated that, if a 5% or 10% threshold were applied, some patients with a negative SeHCAT result (i.e. 7-day retention values of between 5% and 15% or between 10% and 15%), who could be considered to be ‘borderline’ or ‘equivocal’ with respect to a diagnosis of BAM, and who may benefit from treatment with BAS, would be missed. The response rates for patients with 7-day SeHCAT retention values of between 5% and 15% were 14 out of 18 (78%)34 and 6 out of 21 (29%),48 and the response rates for patients with 7-day SeHCAT retention values of between 10% and 15% were two out of four (50%)34 and zero out of eight (0%). 48 Data sets were incomplete (i.e. not all patients received treatment with BAS) for all of these groups. An unpublished dissertation report46 provided information about treatment response rates for patients with a positive SeHCAT result, using two 7-day retention thresholds: 8% and 15%. All patients with 7-day SeHCAT retention values of < 8% received treatment with BAS and 32 out of 36 (89%) patients with 7-day SeHCAT retention values of ≤ 15% received treatment with BAS; response rates were 10 out of 20 (50%) and 12 out of 32 (38%), respectively. 46 The results from this study46 also indicated that, if the lower threshold were applied, some patients with a ‘borderline’ or ‘equivocal’ test result (7-day SeHCAT retention values of between 8% and 15%), who may have benefited from treatment with BAS, would be missed; 12 out of 16 (75%) patients in this group received treatment with BAS and 2 out of 12 (17%) responded positively. 46 It should be noted that no patients in any of these studies34,46,48 who had 7-day SeHCAT retention values of > 15% received treatment with BAS; estimates for the treatment response rate among SeHCAT test-negative patients do not, therefore, represent the complete spectrum of test-negative patients. One further, very small (n = 17), study42 reported results for individual patients, which allowed the calculation of proportions treated and response rates for 7-day retention thresholds of 10% and 15%. In this study,42 all three patients with a 7-day retention value of ≤ 10% received treatment with colestyramine and two out of three (67%) responded positively; seven out of eight (88%) patients with a SeHCAT 7-day retention value of ≤ 15% received treatment with colestyramine, six (86%) of whom responded positively. 42 As with the other studies that assessed multiple SeHCAT test thresholds, the results of this study42 also indicated that, if a 10% threshold were applied, some patients with a negative SeHCAT test result, who may have benefited from treatment with BAS, would be missed: four out of eight (50%) patients with a 7-day SeHCAT retention value of > 10%, who were treated with colestyramine, responded positively to treatment, and zero out of four (0%) patients with a 7-day SeHCAT retention value of > 15%, who were treated with colestyramine, responded positively to treatment. 42 It should be noted that data from Rudberg et al. 42 were incomplete; only 57% of patients who were SeHCAT test negative at the 10% threshold and 44% of patients who were SeHCAT test negative at the 15% threshold received treatment with colestyramine. In summary, few studies reported treatment response rates for multiple SeHCAT test thresholds and data were generally incomplete; hence, the extent to which patients with ‘borderline’ or ‘equivocal’ 7-day SeHCAT retention values could benefit from treatment with BAS remains unclear. The extent to which patients with 7-day retention values of > 15% may benefit from treatment with BAS is unknown.
Bowel symptoms
Three studies reported further results for bowel symptoms, in addition to rates of response to treatment with BAS. 30,37,45 Fernandez-Bañares et al. 37 reported that, among the 20 patients with FD and a 7-day SeHCAT retention value of ≥ 10% who were treated with colestyramine, the median number of daily bowel movements changed from 5 [interquartile range (IQR) 4–8] at baseline to 1 (IQR 1–2) post treatment. A change in stool consistency was also observed across all 20 treated patients; before treatment, all 20 patients had liquid/semi-liquid stools, and after treatment stools were formed/semi-formed across all 20 patients. 37 Urgency disappeared for 13 patients who had this symptom pre treatment. 37 Lin et al. 30 reported that, among 29 patients with type 2 BAM (7-day SeHCAT retention values of < 10%) who were available for follow-up after treatment with BAS, the daily frequency of bowel movements was reduced from a median of 6 (range 3–16) at diagnosis to 3.5 (range 1–16) at follow-up (median time since diagnosis 82 months). Finally, Sinha et al. 45 reported a reduction in stool frequency across all nine patients with 7-day SeHCAT retention values of ≤ 15% who were treated with colestyramine; the median stool frequency pre treatment was five per day, compared with two per day post treatment. One patient did not experience a reduction in stool frequency on treatment, although bowel motion consistency improved and the patient was reported to be happy with this outcome. 45
Health-related quality of life
Two studies also reported very limited results for changes in HRQoL among patients with a positive SeHCAT test result following treatment with BAS. 25,28 Bellini et al. 25 reported that, after 8 weeks of treatment with colestyramine, patients with mild BAM (7-day SeHCAT retention values of between 11% and 15%) showed a significant improvement on the pain domain of the Short Form questionnaire-36 items (SF-36) (p < 0.05), and patients with severe BAM (7-day SeHCAT retention values of ≤ 5%) showed significant improvements on multiple domains of the SF-36 (emotional problems, energy/fatigue, emotional well-being, social functioning, pain, general health, health change) (p < 0.05). Kumar et al. 28 reported that patients with idiopathic BAD (SeHCAT threshold not reported) showed significant improvements in the activity levels subscore (p = 0.00998) of the EuroQol-5 Dimensions (EQ-5D) questionnaire, following treatment with Questran or colesevelam; the duration of follow-up was not reported.
Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with Crohn’s disease, who have not undergone ileal resection
One study7 (results are summarised in Table 6) provided data on population 2: adults presenting with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection (i.e. people with suspected secondary BAD). This study reported only information about the outcome of treatment with BAS for people who had a positive SeHCAT result, and was included in our previous assessment report, conducted for DG7. 18 No new studies meeting the inclusion criteria for population 2 were identified for this assessment report. The single study that reported information about response to treatment with BAS among patients with Crohn’s disease provided only very limited information about response rates among patients with a positive SeHCAT test result (7-day retention value of < 10%) who were treated with colestyramine or colestipol. 7 Fewer than half (9/24) of the patients with a positive SeHCAT test result received treatment with BAS; the criteria used to decide whether or not to offer BAS were not reported. Most [8/9 (89%)] of the patients treated with BAS responded positively;7 however, the numbers treated with each BAS (colestyramine or colestipol) were not reported.
| Study | Participant details (n) | Positive SeHCAT test threshold | Reference standard | Number with positive/negative test | Number (%) of patients with a positive test treated with BAS | Number (%) of patients with a negative test treated with BAS | Number (%) of responders given a positive SeHCAT test | Number (%) of responders given a negative SeHCAT test | Number (%) discontinued/intolerant of BAS |
|---|---|---|---|---|---|---|---|---|---|
| aSmith 20007 |
Subgroup 44 patients with Crohn’s disease and no prior surgery |
< 10% | Response | 24/20 | 9/24 (38%) | 0/20 (0%) | 8b/9 (89%) | No patients treated | NR |
Appendix 2 provides all reported details of the inclusion and exclusion criteria, participant characteristics, SeHCAT test methods, BAS treatment and definition of treatment response, for this study. 7
Pooled estimates of treatment response rates for inclusion in cost-effectiveness modelling
Meta-analysis of test accuracy estimates (i.e. sensitivity and specificity) was considered inappropriate in this assessment, owing to the small number of test accuracy studies, with varying diagnostic thresholds, and between-study heterogeneity with respect to population (prior investigations), treatment regimen, definition of response, follow-up period and SeHCAT administration. However, to provide input parameters for cost-effectiveness modelling, some pooled estimates were calculated using the inverse-variance method on the logit scale, for the probability of testing positive at the 15% threshold (Table 7) and the probability of achieving a positive response to treatment, given a positive test at the 15% threshold (Table 8). The random-effects analysis was chosen because of the high heterogeneity, qualitatively assessed, in accordance with the Cochrane Handbook, section 10.10.4.1. 20
| Study | Number with a positive test | Number tested | Proportion |
|---|---|---|---|
| Borghede 201134 | 68 | 114 | 0.60 |
| Holmes 201227 | 8 | 8 | 0.99 |
| Kumar 201329 | 24 | 57 | 0.42 |
| Rudberg 199642 | 8 | 17 | 0.47 |
| Sinha 199845 | 9 | 17 | 0.53 |
| Tunney 201146 | 36 | 86 | 0.42 |
| Wildt 200347 | 24 | 56 | 0.43 |
| Williams 199148 | 60 | 181 | 0.33 |
| Fixed effect, pooled estimate (95% CI) | 0.416 (0.424 to 0.407) | ||
| Random effects, pooled estimate (95% CI) | 0.454 (0.357 to 0.555) | ||
| Study | Number who responded to treatment with BAS | Number with a positive test who received BAS | Proportion |
|---|---|---|---|
| Borghede 201134 | 43 | 57 | 0.75 |
| Holmes 201227 | 3 | 6 | 0.50 |
| Kumar 201329 | 11 | 23 | 0.48 |
| Rudberg 199642 | 6 | 7 | 0.86 |
| Sinha 199845 | 6 | 9 | 0.67 |
| Tunney 201146 | 12 | 32 | 0.38 |
| Wildt 200347 | 14 | 17 | 0.82 |
| Williams 199148 | 29 | 42 | 0.69 |
| Fixed effect, pooled estimate (95% CI) | 0.642 (0.615 to 0.668) | ||
| Random effects, pooled estimate (95% CI) | 0.638 (0.495 to 0.760) | ||
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of including SeHCAT testing in the diagnostic pathway for investigation of diarrhoea due to BAM among adults with IBS-D or FD and among adults with Crohn’s disease without ileal resection.
Identifying and reviewing published cost-effectiveness studies
A series of literature searches were performed to identify published economic evaluations, cost data and utility studies for diagnostic techniques and procedures used in the investigation of patients with chronic diarrhoea that were not included within the scope of the clinical effectiveness searches. The searches aimed to identify studies that could be used to support the development of a health economic model, to estimate the model input parameters and to answer the research questions of the assessment; the aim was not to perform a systematic review. Searches were therefore pragmatic in design, and date limits were applied when appropriate.
Methodological study design filters were included in the search strategy where relevant. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. The main EMBASE strategy for each search was independently peer-reviewed by a second information specialist, using the Canadian Agency for Drugs and Technologies in Health Peer Review Checklist. 21,22 Identified references were downloaded to EndNote X20 software for further assessment and handling. References in retrieved articles were checked for additional studies. In addition, the EndNote library created for the clinical effectiveness section (see Chapter 3, Search strategy) was also screened to identify potentially relevant economic studies.
Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies, with no date limits:
-
MEDLINE (Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily: 1946 to 21 December 2020
-
EMBASE (Ovid): 1974 to 17 January 2021
-
NHS Economic Evaluation Database (NHS EED) (CRD): up to March 2015 (note that, since March 2015, NHS EED has been an archival resource only, and the Wiley Health Economic Evaluations Database searched as part of the original 2011 study is no longer available)
-
EconLit (EBSCOhost): up to 2020/12/22
-
SCI (Web of Science): 1988 to 5 January 2021
-
Research Papers in Economics (RePEc) (http://repec.org/): up to 23 February 2021.
Supplementary searches on SeHCAT, BAD, IBS, Crohn’s disease and chronic diarrhoea were undertaken on the following resources to identify guidelines and guidance (the search was conducted from 2011):
-
Guidelines International Network (www.g-i-n.net): up to 15 December 2020
-
NHS Evidence (www.evidence.nhs.uk): up to 16 December 2020
-
ECRI Guidelines Trust (https://guidelines.ecri.org/): up to 16 December 2020
-
NICE (www.nice.org.uk): up to 15 December 2020
-
Trip database (www.tripdatabase.com/): up to 10 December 2020
-
HTA database (CRD): up to 31 March 2018
-
NIHR HTA programme: up to 16 December 2020.
Note that the National Guidelines Clearinghouse resource included in the 2011 searches is no longer available.
As described by the NICE methods guide, the information process that supports the development of a model is ‘a process of assembling evidence and this reflects an iterative, emergent process of information gathering’. 55 The following additional searches were requested by the health economists as part of this process.
Searches for utility weights for BAD, IBS, Crohn’s disease and chronic diarrhoea were conducted on the following resources:
-
Cost-Effectiveness Analysis Registry (https://research.tufts-nemc.org/cear4/Home.aspx): up to 14 January 2021
-
School of Health and Related Research Health Utilities Database (www.scharrhud.org/): up to 23 February 2021.
Additional searches were also requested for HRQoL and cost-effectiveness for both Crohn’s disease and IBS on the following resources:
-
NHS EED (CRD): up to March 2015
-
MEDLINE (Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily: 1946 to 15 December 2020.
Model structure and methodology
Model structure
Population
The cost-effectiveness of SeHCAT for the assessment of possible BAD was estimated in the two patient populations defined in Chapter 3, Inclusion and exclusion criteria.
-
Adults with chronic diarrhoea with an unknown cause, suspected or diagnosed IBS-D, or FD (i.e. people with suspected primary BAD). This group is referred to as population 1.
-
Adults with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection. This group is referred to as population 2.
Using the study by Summers et al. ,4 we assumed that the average age in both populations was 50 years, and the ratio of males to females was 35 : 75.
Conceptual model description
The structure of the health economic model is in line with that developed for the previous assessment of SeHCAT. 18 Thus, the model consists of two parts:
-
a short-term decision-analytic model reflecting the diagnostic pathway and initial response to treatment (assumed to be the first 6 months)
-
a long-term (Markov) model that estimates the lifetime costs and effects for patients receiving subsequent treatment.
An outline of the short-term model structure for the population of adults with suspected primary BAD (population 1) is presented in Figure 3. The main difference with respect to the model developed for the previous assessment of SeHCAT18 is the potential inclusion of the colonoscopy investigation in the model, based on discussions during the scoping phase suggesting that SeHCAT could be used to avoid unnecessary colonoscopies. Thus, our base-case scenario for population 1 places colonoscopy after SeHCAT testing, in accordance with most clearly expressed clinical expert opinion and BSG guidelines whereby colonoscopy is required for investigation of cancer and not for ruling out IBD. As a secondary scenario for population 1, we assumed that no colonoscopy would occur after SeHCAT testing, as this would have already occurred in the clinical pathway. Note that, in practice, colonoscopy can be excluded from the model by setting this probability equal to zero (i.e. at the colonoscopy branch all patients will follow the ‘no colonoscopy’ path and, subsequently, will be treated as IBS-D patients).
FIGURE 3.
Decision-analytic model, population 1. TOT, trial of treatment; Tx, treatment.
In the SeHCAT strategy, patients may have a positive or a negative test result. If the test is positive (i.e. the percentage of whole-body retention of bile acids is below a certain cut-off point), patients are treated with BASs and they may or may not respond to that treatment. Patients with a positive SeHCAT result and an initial response to BASs are at risk of treatment discontinuation because of BAS intolerance. In this case, patients do not go through further testing, because, given the positive SeHCAT result, it is assumed that these patients will be treated as having BAD. If the result of the SeHCAT test is negative, a proportion of patients are investigated for IBD with a colonoscopy. If, after the colonoscopy, patients are diagnosed as having IBD, then they are treated accordingly. Otherwise, patients are treated as having IBS-D. Patients testing SeHCAT negative and not undergoing colonoscopy are diagnosed as having IBS-D and are treated accordingly. All end points of the SeHCAT-negative branch are thus determined depending on whether or not patients respond to IBS-D or IBD treatment. The no-SeHCAT strategy assumes that all patients follow the same paths as for the SeHCAT-negative test. Thus, patients may be investigated for IBD with a colonoscopy, may be treated for IBD or IBS-D and may or may not respond to treatment. Finally, in the trial-of-treatment strategy, all patients receive BASs at the beginning. If patients do not respond to BASs, they follow the same paths as for the SeHCAT-negative and the no-SeHCAT strategies. Patients with an initial response to BASs are also at risk of treatment discontinuation, as in the SeHCAT-positive branch of the model. Treatment discontinuation may vary between patients with a positive SeHCAT result and those not tested.
The short-term model for population 2 (adults with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection) is shown in Figure 4. The main difference with respect to the short-term model in Figure 4 is that Crohn’s disease patients are not expected to undergo colonoscopy, because it is assumed that these patients would already have had colonoscopy to diagnose their Crohn’s disease. Therefore, all end points of the decision-analytic model are determined depending on whether or not patients respond to treatment (BAS or diarrhoea treatments for Crohn’s disease patients). This is the same structure as assumed in the previous assessment of SeHCAT. 18
FIGURE 4.
Decision-analytic model, population 2. TOT, trial of treatment; Tx, treatment; w/o, without.
To assess the long-term costs and effects of the various strategies across both populations, patients are assumed to enter a simple three-state Markov model, as shown in Figure 5.
FIGURE 5.
Markov model, populations 1 and 2.
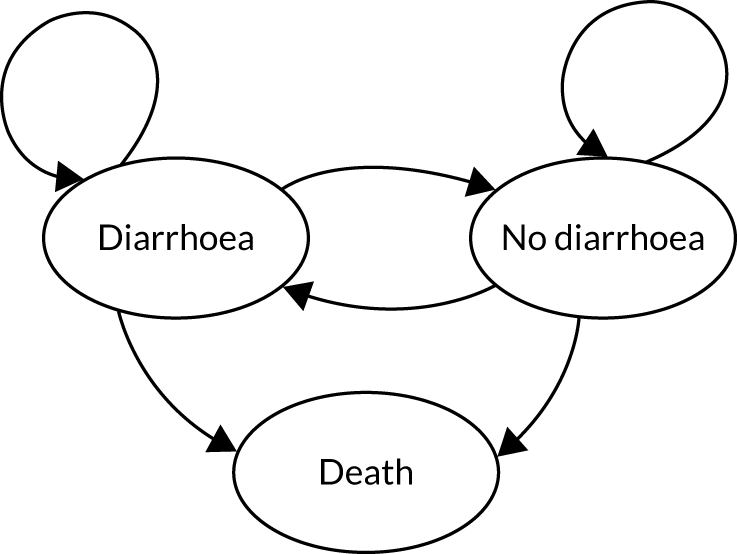
Patients who had a treatment response in the short-term model start in the ‘no-diarrhoea’ health state and patients who did not respond to treatment in the short-term model start in the ‘diarrhoea’ health state. Because the model has a lifetime time horizon, the third state included is ‘death’. In the previous assessment of SeHCAT, no link with increased mortality was found. 56 Therefore, because there is no new evidence to suggest that this has changed, only background mortality was considered in the economic model. Transitions between the ‘diarrhoea’ and ‘no-diarrhoea’ health states were informed by clinical expert opinion, as clinical data regarding the long-term effectiveness of BASs and IBD and IBS-D treatments were not identified. The cycle length is 6 months, as in the previous assessment of SeHCAT. 18 In consultation with clinical experts, it was agreed that a period of 6 months, in general, would be sufficient to capture initial response to treatment (i.e. in the decision-analytic model). Six months was also deemed as an appropriate cycle length for the Markov model as this would represent a plausible time to reassess response to treatment in the long term. Long-term adverse events, such as constipation, and treatment discontinuation were not included in the Markov model owing to lack of data. The Markov model is then parameterised according to treatment.
Strategies
Various strategies could be defined for the SeHCAT treatment option based on the test cut-off points used to classify patients (see Chapter 3, Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with diarrhoea-predominant irritable bowel syndrome or functional diarrhoea, for additional details). In the previous assessment of SeHCAT,18 cut-off points of 5%, 10% and 15% were used. However, because, in the previous assessment of SeHCAT, it was not possible to obtain evidence to estimate all model input parameters for these three SeHCAT cut-off points, many assumptions were made to populate the model for each SeHCAT cut-off value. Testing these assumptions resulted in an enormous number of scenarios, whereby almost every different cost-effectiveness outcome was possible, without knowing the actual plausibility of such scenarios. For the current assessment of SeHCAT, in the clinical expert elicitation exercise to inform parameters for which data are lacking, all clinical experts consulted provided estimates for the 15% cut-off value only. Therefore, for both populations, we compared the SeHCAT strategy at a 15% cut-off point with (1) no SeHCAT testing and no treatment with BASs, and (2) no SeHCAT testing and trial of treatment with BASs. The systematic review revealed that most studies that reported data to inform the model used the 10% and 15% cut-off points. Those data included the proportion who tested positive at the given cut-off point and response to treatment of those who tested positive. Therefore, clinical expert opinion was sought to inform treatment response of those testing negative, as well as other parameters further downstream, such as probability of colonoscopy.
Perspective, time horizon and discounting
All costs and effects were discounted by 3.5%, as per the NICE reference case. 57 The models incorporated a lifetime (50 years) time horizon to estimate outcomes in terms of quality-adjusted life-years (QALYs) and costs from the perspective of the NHS and the Personal Social Services. 58 Costs were sourced from year 2020 when possible; otherwise, costs were inflated using the NHS Cost Inflation Index (NHSCII). 58
Model parameters and implementation
This section describes the parameters used in the decision-analytic and the Markov models and how their values were estimated. When possible, input for the models was based on our systematic review (described in Chapter 3), other published literature and UK databases. When such evidence was not available, expert opinion was used. We sent out a questionnaire to the specialist committee members of this assessment and their answers were used to inform the input parameters for which data were lacking. In the absence of better evidence, it was assumed that the experts’ responses would adequately reflect the uncertainty in the elicited evidence, and the case mix observed by each expert would reflect the relevant patient population in UK clinical practice. The evidence elicitation process did not follow any specific guidance or methodology. It was considered that this approach would better reflect the current uncertainty than, for example, seeking consensus answers. The same approach was followed in the previous assessment of SeHCAT. 18 However, given the limited number of answers obtained, translating the experts’ responses into model parameters was often challenging. How inconsistent evidence was dealt with is described throughout the report, mostly as footnotes in tables, but also in the main text. When experts were unable to provide estimates, modelling assumptions were made. The model was implemented in R (the most recent version used was 4.1.0) using RStudio (the most recent version used was 2021.09.1 Build 372) (The R Foundation for Statistical Computing, Vienna, Austria). At the time of writing this report, the model is not publicly available, but it can be requested through NICE.
Diagnostic model, suspected population 1
Probabilities
As shown in Figure 3, five different probabilities (represented by the circles in the ‘no SeHCAT test’ branch) need to be estimated when SeHCAT and BAS trial of treatment are not available.
When SeHCAT and BAS trial of treatment are not available, patients in population 1 may undergo colonoscopy to detect IBD. Clinical experts’ responses to our questionnaire were used to estimate the proportion of patients who currently undergo colonoscopy. Their responses are summarised in Table 9. Experts’ answers were used to derive probabilities following the same approach as in the previous assessment of SeHCAT. 18 Thus, we assumed that the proportion of patients undergoing colonoscopy follows a triangular distribution, with the point estimate given by the experts representing the mode of the distribution. In this case, we simulated three triangular distributions (one per expert response) to estimate the pooled mean and standard deviation of the probability of undergoing colonoscopy, which is further assumed to have a beta distribution. We found a mean of 74% and a standard deviation of 1.42%. Note that knowing the mean and standard deviation of a beta random variable, say X, its parameters α and β can be calculated by solving the equations mean(X) = α/(α + β) and var(X) = αβ/[(α + β)2(α + β + 1)] for α and β. Thus, the probability of undergoing colonoscopy when SeHCAT and BAS trial of treatment are not available was parameterised as a beta(α = 706, β = 242) distribution. Note that the low standard deviation might be due to the lack of uncertainty ranges in two of the answers in Table 9 (both equal to 100%). This might underestimate the uncertainty associated with this parameter, which will be further explored in scenario analyses. Additional details on the calculations of this and the other probabilities calculated following the same approach can be found in the file ‘input parameter estimation.r’, which is part of the economic model.
| Expert | Percentage undergoing colonoscopy | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1a | 20 | 20 | 30 |
| 2 | 100 | NR | NR |
| 3b | NR | NR | NR |
| 4b | 100 | NR | NR |
As explained previously, our model was built under the assumption that colonoscopy is placed at the beginning of the no-SeHCAT strategy to detect IBD patients. Experts indicated that the proportion of IBD patients at this point of the treatment pathway is expected to be small. This is in line with the findings by Patel et al. ,59 in which table II reports that 11 patients were diagnosed as having IBD from a total of 209 patients presented with IBS-D-compatible symptoms. Thus, in our model, the probability of having IBD after colonoscopy was assumed to follow a beta(α = 11, β = 198) distribution.
Response to IBD treatment was also estimated from the experts’ answers to the questionnaire, as presented in Table 10. The approach described previously of simulating triangular distributions to derive the parameters of a beta distribution was also followed in this case. We found a mean of 72% and a standard deviation of 5%, which was parameterised as a beta(α = 49, β = 19) distribution. The uncertainty associated with this parameter was not explored in scenario analyses. The main reason was that this is a small proportion of patients, as confirmed by the experts, and, even though IBD medication is costly compared with IBS-D medication, the impact of this parameter on the model results is expected to be minor.
| Expert | Percentage of patients treated successfully | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 70 | 50 | 90 |
| 2 | 75 | 70 | 80 |
| 3 | 70 | 60 | 80 |
| 4 | NR | NR | NR |
There is uncertainty regarding the initial response to IBD treatment and the duration of this response. We assumed that response is achieved within 6 months of start of treatment, but this is variable, as acknowledged by the clinical experts consulted. Regarding the duration of the treatment effect, experts indicated that the main difference with respect to IBS-D patients is that a lifetime effect should not be assumed, because relapses are expected after initial response.
The estimated probabilities of undergoing colonoscopy (74%) and of being diagnosed as having IBD (5.3% of those undergoing colonoscopy) imply that the majority of patients (approximately 96% of all patients) would be treated as IBS-D patients. In line with the previous assessment of SeHCAT, it is assumed that IBS-D patients may receive a variety of drugs, diet advice and psychological treatment. 18 Owing to the large array of treatment options and the various orders in which they are attempted, we did not find clear data from the literature regarding how many IBS-D patients will eventually, after trying various options, respond to treatment. Therefore, response to IBS-D treatment was estimated from the answers to the questionnaire obtained from the experts, as summarised in Table 11. Furthermore, in this case, we followed the approach described previously of simulating triangular distributions to derive the parameters of a beta distribution. We found a mean of 46% and a standard deviation of 8%, parameterised as a beta(α = 17, β = 20) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
| Expert | Percentage of patients treated successfully | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 60 | 30 | 70 |
| 2 | 30 | 20 | 50 |
| 3 | 50 | 25 | 75 |
| 4 | NR | NR | NR |
There is also uncertainty regarding the initial response to IBS-D treatment and the duration of this response. We also assumed that response is achieved within 6 months of start of treatment, but this is variable, as acknowledged by the experts consulted. Unlike for IBD patients, we assumed a lifetime effect in the base-case analysis; thus, in the Markov model there is no transition to the diarrhoea health state for patients initially responding to treatment. This assumption was based on responses from clinical experts, who indicated that IBS-D is not a relapsing condition in general. In any case, scenarios in which long-term relapses were allowed were also explored.
Finally, note that the answers given in Table 11 were obtained by assuming that patients had undergone a colonoscopy and IBD was ruled out. However, to complete the model, we also need to estimate the probability of responding to IBS-D treatment when patients do not undergo colonoscopy. Based on the answers in Table 9, we estimated that 26% of patients will not undergo colonoscopy. Of these, we assumed that 5.3% of them are IBD patients (per Patel et al. 59) and, therefore, they would not respond to IBS-D treatment. For the remaining patients, we assumed the same response probability as in Table 11 (46%). Thus, in total, the mean probability of responding to IBS-D treatment, without previous colonoscopy, was estimated as [(100% – 5.3%) × 46% =] 44%. Assuming the same standard deviation of 8% as in Table 11, this was parameterised according to a beta(α = 16, β = 20) distribution.
As shown in Figure 3, 13 different probabilities need to be estimated when the SeHCAT test is available. Note that three of these correspond to probabilities associated with SeHCAT testing: the probability of testing positive, the probability of responding to BAS treatment (contingent on being SeHCAT positive) and the probability of discontinuing BAS treatment. In addition, patients testing negative for SeHCAT or not responding to BAS treatment after testing positive are assumed to follow the same pathway as for the no-SeHCAT strategy. Thus, for both model branches, the same five probabilities described above for no SeHCAT have to be estimated (i.e. 10 probabilities in total). SeHCAT-related probabilities were estimated using the results from our clinical effectiveness review. The remaining probabilities were informed by clinical experts. As our questionnaire did not include questions about patients not responding to BAS treatment after a positive SeHCAT test result, we assumed the same estimates for these patients as those obtained for patients with a negative SeHCAT test result. Thus, in practice, eight probabilities were estimated for the SeHCAT 15% strategy.
We estimated the probability of a positive SeHCAT test at the 15% threshold by performing a random-effects meta-analysis on the data from the studies in Table 7. The pooled estimate (0.454) can be seen in Table 12. This probability was further parameterised as a beta(α = 2.10, β = 2.52) distribution.
| Study | Sample size (n) | SeHCAT positive | |
|---|---|---|---|
| Number | Probability | ||
| Borghede 201134 | 114 | 68 | 0.60 |
| Holmes 201227 | 8 | 7.9 | 0.99 |
| Kumar 201329 | 57 | 24 | 0.42 |
| Rudberg 199642 | 17 | 8 | 0.47 |
| Sinha 199845 | 17 | 9 | 0.53 |
| Tunney 201146 | 86 | 36 | 0.42 |
| Wildt 200347 | 56 | 24 | 0.43 |
| Williams 199148 | 181 | 60 | 0.33 |
| RE mean | 0.454 | ||
| SE | 0.21 | ||
Patients with a positive SeHCAT test result are assumed to be treated with a BAS. In our analyses, we assumed that this is either colestyramine or colesevelam. In terms of response, however, it was not possible to distinguish between the type of BAS. We estimated the response rate to BAS, in general, using the studies described in Table 8, conducting a random-effects meta-analysis. The pooled estimate (0.638) can be seen in Table 13. This probability was parameterised as a beta(α = 1, β = 0.57) distribution.
| Study | Sample size (n) | Positive response | |
|---|---|---|---|
| Number | Probability | ||
| Borghede 201134 | 57 | 43 | 0.75 |
| Holmes 201227 | 6 | 3 | 0.50 |
| Kumar 201329 | 23 | 11 | 0.48 |
| Rudberg 199642 | 7 | 6 | 0.86 |
| Sinha 199845 | 9 | 6 | 0.67 |
| Tunney 201146 | 32 | 12 | 0.38 |
| Wildt 200347 | 17 | 14 | 0.82 |
| Williams 199148 | 42 | 29 | 0.69 |
| RE mean | 0.638 | ||
| SE | 0.30 | ||
Responses to our questionnaire also suggested that initial response to BAS treatment is achieved within 6 months of the start of treatment, and that a lifetime treatment effect duration might be assumed (thus, in the Markov model there is no transition to the diarrhoea health state for patients initially responding to treatment). Scenarios with long-term relapses are explored in Model analyses.
It is known that adherence is usually not optimal when patients are treated with BAS. Four studies reported the proportion of treated patients who, after testing positive at a SeHCAT 15% cut-off value and starting treatment with BAS, were intolerant of BAS or discontinued treatment for unspecified reasons. 29,34,45,46 Borghede et al. 34 reported that 43 out of 57 patients responded to treatment. It was also reported that 49 out of 57 patients used colestyramine continuously, that is 8 out of 57 patients were intolerant or discontinued. Kumar et al. 29 reported response to BAS in 11 out of 23 patients, and intolerance in 6 out of 23. In the study by Sinha et al. ,45 six out of nine patients responded to BAS treatment and two out of nine were intolerant. Finally, Tunney46 reported response to BAS in 12 out of 32 patients and intolerance in 5 out of 32. Therefore, in all these four studies, the response reported was based on < 100% compliance. Four other studies reported the proportion of patients intolerant of BAS, but in those studies SeHCAT was used at cut-off values of < 15%. 25,30,31,39 The studies by Bellini et al. 25 and Lin et al. 30 reported that 6 out of 22 patients and 3 out of 29 patients, respectively, were intolerant to BAS, but neither of these studies reported response to BAS. In the study by Galatola et al. ,39 39 out of 56 patients responded to BAS and 2 out of 56 were intolerant. Thus, the response reported was based on less than 100% compliance. Likewise, the study by Notta et al. 41 reported that many patients used colestyramine on demand after achieving an initial response to counteract side effects. Thus, this study also reported a response rate that is based on reduced compliance (25 out of 34 patients responded to BAS and 3 out of 34 were intolerant). Therefore, it seems reasonable to assume that, in these studies, the impact of reduced compliance on the response rate was implicitly included. Overall, rates of intolerance/discontinuation in these studies were high (median 15%, range 4–27%). However, there was insufficient information to determine whether or not levels of intolerance differed between colestyramine and colesevelam.
Furthermore, based on the responses to our questionnaire, it seems that most patients present intolerance to colestyramine and, when this occurs, patients are generally switched to colesevelam, compliance with which and response to which seem to be high. Based on the responses to our questionnaire, it was assumed in the base-case scenario that 50% of patients started with colestyramine and 50% with colesevelam. It was further assumed that a proportion of those patients starting with colestyramine will switch to colesevelam. For simplicity, we assumed that these patients will effectively move to colesevelam at the beginning of the simulation. The impact of this assumption is expected to be minor because, in practice, it could be assumed that these patients would switch to colesevelam at some point within the first 6 months (e.g. at month 3 in the model). Thus, this assumption would affect BAS costs and utilities for only half of the first model cycle. The proportion of patients treated with colestyramine in the base-case analysis was implemented as a beta(α = 7700, β = 7701) distribution.
The probability of switching from colestyramine to colesevelam was then estimated based on the experts’ responses to our questionnaire, as can be seen in Table 14. Again, we followed the approach described previously of simulating triangular distributions to derive the parameters of a beta distribution. We found a mean of 50% and a standard deviation of 2%, corresponding to a beta(α = 357, β = 356) distribution.
| Expert | Percentage of patients switching | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 20 | 10 | 30 |
| 2 | 60 | NR | NR |
| 3 | NR | NR | NR |
| 4 | NR | NR | NR |
So far, we have described the modelled pathway assumed when patients respond to BAS after a positive SeHCAT test result. When patients do not respond to BAS after a positive SeHCAT test result, or when the SeHCAT result is negative, we assumed that patients follow the same pathway as for the no-SeHCAT strategy. As explained previously, this part of the model was informed by clinical experts only and the probability estimates were assumed to be the same for patients who did not respond to BAS after a positive SeHCAT result as for patients with a negative SeHCAT result.
Following the steps described previously for the no-SeHCAT strategy, we first estimated the probability of undergoing colonoscopy (contingent on a negative SeHCAT result, or a positive SeHCAT result and no response to BAS). Experts’ answers can be seen in Table 15. These were used to simulate a triangular distribution to derive the parameters of a beta distribution, as explained previously. We found a mean of 49% and a standard deviation of 2%, corresponding to a beta(α = 338, β = 351) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
| Expert | Percentage of colonoscopy | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 5 | 1 | 7.5 |
| 2 | 5 | 2 | 10 |
| 3 | 90 | 90 | 100 |
| 4 | NR | NR | NR |
The probability of having IBD after colonoscopy was also estimated based on the findings in Patel et al. 59 Thus, the probability of having IBD after colonoscopy was assumed to follow a beta(α = 11, β = 198) distribution. It was assumed that patients who had a colonoscopy that confirmed IBD would have the same response rate, regardless of the result of the SeHCAT test. Thus, IBD treatment response is assumed to be the same as the one derived from Table 10, that is a mean of 72% and a standard deviation of 5%, modelled as a beta(α = 49, β = 19) distribution.
The majority of patients with a negative SeHCAT test result receive IBS-D treatment. Because the SeHCAT test was negative for these patients, it might be assumed that most patients who have BAD are not included in the group receiving IBS-D treatment. Hence, it is expected that the response rate to IBS-D treatment in the SeHCAT-negative subpopulation will be higher than in the no-SeHCAT strategy subpopulation (see Table 11). As in the previous assessment of SeHCAT,18 no data were available to confirm whether or not this assumption is correct and, if so, how much higher the response rate should be. We used, therefore, the responses to our questionnaire to inform this probability. These can be seen in Table 16. After simulating triangular distributions, we found a mean of 56% and a standard deviation of 5%, corresponding to a beta(α = 57, β = 45) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
| Expert | Percentage of patients successfully treated | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 80 | 70 | 90 |
| 2 | 30 | 20 | 50 |
| 3 | 10 | 5 | 20 |
| 4 | NR | NR | NR |
Finally, the probability of responding to IBS-D treatment, without previous colonoscopy, was estimated as [(100% – 5.3%) × 56% =] 53%. Assuming the same standard deviation of 5% as in Table 16, this was parameterised as a beta(α = 55, β = 49) distribution.
As shown in Figure 3, seven different probabilities need to be estimated when BAS trial of treatment (without SeHCAT testing) is available. This strategy starts with the probability of responding to BAS treatment. In the case of no response, patients are assumed to follow the same pathway as for the no-SeHCAT strategy. For this strategy, probabilities were also informed by clinical experts.
In the trial-of-treatment strategy, all patients are assumed to receive a BAS at the beginning of the modelled pathway. As in the SeHCAT 15% strategy, we assumed that this was either colestyramine or colesevelam. The proportion of patients receiving each of the BAS options was estimated from the responses to our questionnaire. We found that 85% of patients started with colestyramine and 15% with colesevelam. The proportion of patients treated with colestyramine in the BAS trial-of-treatment strategy in the base-case scenario was implemented as a beta(α = 48, β = 9) distribution. Note that this is different from the 50/50 distribution estimated for the SeHCAT strategy. Although it is unclear why the BAS proportions might differ between strategies, the higher proportion of colestyramine used in the trial-of-treatment strategy might be because of its lower costs. In any case, a range of different proportions was explored in scenario analyses.
In terms of response, it was not possible to distinguish between the type of BAS, as in the SeHCAT 15% strategy. We estimated the response rate to BAS using the responses from our questionnaire, which are summarised in Table 17. Because, in the trial-of-treatment strategy, all patients (including those without BAD) are treated with BAS, the overall response rate to BAS is expected to be lower than in the SeHCAT 15% strategy. After simulating triangular distributions, we found a mean of 30% and a standard deviation of 3%, corresponding to a beta(α = 60, β = 141) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
| Expert | Percentage of response to BAS | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 50 | 40 | 60 |
| 2 | NR | NR | NR |
| 3 | 10 | 5 | 15 |
| 4 | NR | NR | NR |
Bile acid sequestrant adherence, initial response and duration of treatment effect are assumed to be the same as for the SeHCAT strategy, described previously. Thus, the probability of switching from colestyramine to colesevelam was assumed to follow a beta(α = 357, β = 356) distribution (mean of 50% and a standard deviation of 2%).
When patients do not respond to BAS treatment, we assumed that patients follow the same pathway as for the no-SeHCAT strategy. Thus, we first estimated the probability of undergoing colonoscopy, contingent on no response to BAS. Experts’ answers can be seen in Table 18. These were used to simulate a triangular distribution to derive the parameters of a beta distribution, as explained previously. We found a mean of 90% and a standard deviation of 3%, corresponding to a beta(α = 89, β = 10) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
| Expert | Percentage of colonoscopy | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 80 | 70 | 90 |
| 2 | NR | NR | NR |
| 3 | 0 | NR | NR |
| 4 | 100 | NR | NR |
The probability of having IBD after colonoscopy (contingent on no response to BAS) was assumed to follow a beta(α = 11, β = 198) distribution, as estimated from Patel et al. 59 IBD treatment response after colonoscopy was assumed to be the same as the one derived from Table 10: a mean of 72% and a standard deviation of 5%, corresponding to a beta(α = 49, β = 19) distribution.
We did not have any indication about the probability of IBS-D treatment response after no response to BAS and colonoscopy, but we assumed that this is expected to lie somewhere in between the 46% of the no-SeHCAT strategy and the 56% of the SeHCAT 15% strategy. We estimated the parameters of a beta distribution for the base-case scenario assuming a mean response of 50% (modelling choice) and a 5% standard deviation (as in the SeHCAT strategy; see Table 16), which resulted in a beta(α = 52, β = 52) distribution. The uncertainty associated with this parameter was explored in scenario analyses. Finally, the probability of responding to IBS-D treatment, without previous colonoscopy, was estimated as [(100% – 5.3%) × 50% =] 47%. Assuming the same standard deviation of 5% as in Table 16, this corresponds to a beta(α = 49, β = 55) distribution.
Health-related quality of life
A literature search was performed in an attempt to identify updated sources of utility values for both responders (no diarrhoea) and non-responders (diarrhoea) in the model. Papers presenting utility values for IBS and IBS-D patients were retrieved from the records identified using title and abstract screening. This resulted in six papers, of which three were systematic reviews. None of the empirical studies identified reported utility values for the health states required. None of the systematic reviews reported utilities measured using the EQ-5D. The previous assessment of SeHCAT identified EQ-5D utility values for the required health states for IBS patients;18 therefore, we used the same utility values as identified previously, as described below.
Spiegel et al. 60 described EQ-5D utilities for patients with IBS who showed either ‘considerable relief’ after 3 months of usual care or ‘no considerable relief’. This study found no significant difference between the subtypes of IBS. The second paper with health-state specific utilities, by Mearin et al. ,61 presented utility scores for high and low severity symptoms. These were aggregated across IBS subtypes for patients with high-frequency symptoms (present > 50% of the time), assuming that the utility gain associated with response to treatment was equivalent to an improvement in symptom severity from high to low.
The updated review also failed to identify any evidence on the impact of BAS treatment on utility. For BAS responders, two scenarios were considered: one in which BAS responders have the same utility gain as IBS-D treatment responders and one in which the utility gain is lower, because of the generally cited unpleasantness of colestyramine, which remains the most commonly selected first-line treatment based on clinical expert opinion. As there are no data available to support this smaller increment, for the base-case analysis it was assumed that colestyramine responders have 75% of the utility increment observed among responders to IBS-D treatment. Colesevelam responders were assumed to have the full utility increment as per IBS-D treatment responders. A utility decrement due to colonoscopy was not included in the model as this was expected to have a negligible impact on the cost-effectiveness results. The base-case utility values are summarised in Table 19.
| Study | Mean | SE |
|---|---|---|
| Non-responders/diarrhoea | ||
| Mearin 200461 | 0.704 | 0.026 |
| Spiegel 200960 | 0.730 | 0.037 |
| Aggregated estimate | 0.712 | 0.021 |
| IBD/IBS-D/colesevelam responders/no diarrhoea | ||
| Mearin 200461 | 0.775 | 0.014 |
| Spiegel 200960 | 0.780 | 0.037 |
| Aggregated estimate | 0.776 | 0.013 |
| Colestyramine responders/no diarrhoea | ||
| Assumption | 0.760 | 0.020 |
Resource use and costs
Five different costs groups were distinguished in the model: (1) the costs of a SeHCAT test, (2) treatment of BAD with BAS, (3) treatment of IBS-D, (4) treatment of IBD and (5) the cost of a colonoscopy.
The cost of the SeHCAT capsule was sourced from the manufacturer as £195. The tariff for administering this diagnostic test in the NHS was estimated at £282 [Healthcare Resource Group (HRG) code RN14Z]. 62 Thus, we arrived at a total cost of £477.
Patients with a positive SeHCAT test result were assumed to receive treatment with a BAS, either colestyramine or colesevelam. The prices of the medications were derived from the British National Formulary (BNF). 63 Exact details on medication use, dosage and fraction of patients receiving the treatment are presented in Appendix 5. We estimated the cost of BAS treatment, taking the average of the dosage values reported by the experts. Thus, for colestyramine, we assumed a dosage of 5 g per day, resulting in a cost of £0.35 per day; for colesevelam, we assumed a dosage of 2.5 g per day, resulting in a cost of £2.56 per day.
For the treatment of IBS-D, we distinguished three types of resource use: (1) medication, (2) diet therapy and (3) psychological therapy. All of these were estimated based on expert opinion.
Patients treated for IBS-D may use a wide variety of medication. The experts consulted listed, for example, loperamide, codeine and tricyclic antidepressants. We estimated the cost of medication for IBS-D using the average of the dosage values and the proportion of patients reported by the experts. Table 20 presents the (weighted) costs of medication per day. The prices of the medications were derived from the BNF. 63 Exact details on medication use, dosage and fraction of patients receiving the treatment are presented in Appendix 5, Tables 58–63, including the ranges suggested by the experts.
| Drug | Percentage of patients | Cost per day (£) | Weighted average (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Lowest | Highest | Mean | Lowest | Highest | ||
| Hyoscine butylbromide (Buscopan®; Sanofi, Paris, France) | 0.15a | 0.01 | 0.4 | 0.11 | 0.0161 | 0.0011 | 0.0429 |
| Loperamide | 0.57a | 0.02 | 1 | 0.05 | 0.0300 | 0.0011 | 0.0527 |
| Amitriptyline | 0.24a | 0.01 | 0.5 | 0.04 | 0.0102 | 0.0004 | 0.0212 |
| Codeine | 0.05 | 0.02 | 0.1 | 0.12 | 0.0059 | 0.0024 | 0.0118 |
| Total (£) | 0.06 | 0.01 | 0.13 | ||||
Table 21 presents the responses of the experts to the questions of how many IBS-D patients would visit a dietitian and how many visits would be involved. The cost of one visit to a dietitian was estimated at £86.38 (NHS reference costs 2018/19, inflated to 2020 prices). 12 Dietitian costs for IBS-D were assumed for 6 months.
| Percentage of patients | Number of visits | Cost per visit (£) | Weighted cost per day (£) | |||
|---|---|---|---|---|---|---|
| Lowest | Highest | Mean | Lowest | Highest | ||
| 0.05 | 1 | 2 | 86.38 | 6.48 | 4.32 | 8.64 |
| 0.1 | 2 | 2 | 86.38 | 17.28 | 17.28 | 17.28 |
| 0.1 | 1 | 2 | 86.38 | 12.96 | 8.64 | 17.28 |
| Total (£) | 12.24 | 10.08 | 14.40 | |||
Table 22 presents the response of the experts to the questions of how many patients would receive some form of psychological therapy and how many visits would be involved. The cost was estimated to be £174 per visit for cognitive–behavioural therapy (National Tariff Payment System),62 and £101.41 per visit for hypnotherapy (previous report, inflated to 2020 prices). 18 Psychological costs for IBS-D were also assumed for 6 months.
| Type of therapy | Patients (%) | Number of visits | Cost per visit (£) | Weighted cost per day (£) | ||||
|---|---|---|---|---|---|---|---|---|
| Lowest | Highest | Lowest | Highest | Mean | Lowest | Highest | ||
| CBT | 0.05 | 0.15 | 3 | 5 | 171.00a | 68.40 | 25.65 | 128.25 |
| 0.01 | 0.05 | 2 | 2 | 171.00a | 10.26 | 3.42 | 17.10 | |
| 0.09 | 0.09 | 1 | 1 | 171.00a | 15.39 | 15.39 | 15.39 | |
| Hypnotherapy | 0.01 | 0.05 | 2 | 2 | 101.41 | 6.08 | 2.03 | 10.14 |
| 0.01 | 0.05 | 2 | 2 | 101.41 | 6.08 | 2.03 | 10.14 | |
| 0.01 | 0.01 | 1 | 1 | 101.41 | 1.01 | 1.01 | 1.01 | |
| Average cost of CBT (£) | 31.35 | 14.82 | 53.58 | |||||
| Average cost of hypnotherapy (£) | 4.39 | 1.69 | 7.10 | |||||
| Total cost (£) | 35.74 | 16.51 | 60.68 | |||||
Patients treated for IBD may also use a wide variety of medication. The experts consulted listed, for example, 5-aminosalicylic acid, azathioprine and infliximab. We estimated the cost of medication for IBD, taking the average of the dosage values and proportion of patients reported by the experts. Table 23 presents the (weighted) costs of medication per day. The prices of the medications were derived from the BNF. 63 Exact details on medication use, dosage and fraction of patients receiving the treatment are presented in Appendix 5, Tables 58–63, including the ranges suggested by the experts.
| Drug | Percentage of patients | Cost per day (£) | Weighted cost per day (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Lowest | Highest | Mean | Lowest | Highest | ||
| Mesalazine/5-aminosalicylic acid | |||||||
| Asacol® (AbbVie Inc., Chicago, IL, USA) | 0.8 | 0.7 | 0.9 | 3.92 | 3.14 | 2.75 | 3.53 |
| Octasa® (Tillotts Pharma AG, Rheinfelden, Switzerland) | 0.8 | 0.7 | 0.9 | 2.69 | 2.15 | 1.88 | 2.42 |
| Pentasa® (Ferring Pharmaceuticals, Saint-Prex, Switzerland) | 0.8 | 0.7 | 0.9 | 2.46 | 1.97 | 1.72 | 2.21 |
| Azathioprinea | 0.5 | 0.4 | 0.6 | 0.20 | 0.10 | 0.08 | 0.12 |
| Infliximabb | 0.2 | 0.1 | 0.3 | 49.01 | 9.80 | 4.90 | 14.70 |
| Adalimumab | 0.2 | 0.1 | 0.3 | 22.88 | 4.58 | 2.29 | 6.86 |
| Total (£) | 21.73 | 13.62 | 29.85 | ||||
Table 24 presents the responses of the experts to the question how many IBD patients would visit a dietitian and how many visits would be involved. The cost of one visit to a dietitian was estimated at £86.38 (NHS reference costs 2018/19, inflated to 2020 prices). 12 Dietitian costs for IBD were assumed for 6 months.
| Percentage of patients | Number of visits | Cost per visit (£) | Weighted cost per day (£) | |||
|---|---|---|---|---|---|---|
| Lowest | Highest | Mean | Lowest | Highest | ||
| 0.1 | 1 | 4 | 86.38 | 21.59 | 8.64 | 34.55 |
| 0.8 | 4 | 4 | 86.38 | 276.41 | 276.41 | 276.41 |
| Total (£) | 149.00 | 142.52 | 155.48 | |||
Table 25 presents the response of the experts to the questions of how many IBD patients would receive some form of psychological therapy and how many visits would be involved. The cost was estimated to be £174 per visit for cognitive–behavioural therapy (National Tariff Payment System)62 and £69.14 per visit for counselling (previous report, inflated to 2020 prices). 18 Psychological costs for IBD were also assumed for 6 months.
| Type of therapy | % of patients | Number of visitsa | Cost per visit (£) | Weighted cost per day (£) | ||||
|---|---|---|---|---|---|---|---|---|
| Lowest | Highest | Lowest | Highest | Mean | Lowest | Highest | ||
| CBT | 1 | 1 | 3 | 5 | 171.00b | 684.00 | 513.00 | 855.00 |
| 0.5 | 0.15 | 2 | 2 | 171.00b | 111.15 | 171.00 | 51.30 | |
| 0.1 | 0.1 | 1 | 1 | 171.00b | 17.10 | 17.10 | 17.10 | |
| Counselling | 0.2 | 0.2 | 1 | 1 | 69.14 | 13.83 | 13.83 | 13.83 |
| Average CBT cost (£) | 270.75 | 233.70 | 307.80 | |||||
| Average counselling cost (£) | 13.83 | 13.83 | 13.83 | |||||
| Total cost (£) | 284.58 | 247.53 | 321.63 | |||||
The cost of colonoscopy in the model was calculated as 90% colonoscopy plus 10% computerised tomography colonoscopy (CTC), based on one expert answer to our questionnaire. For the cost of colonoscopy, we used diagnostic colonoscopy £469 (HRG code FE32Z). The cost of CTC was calculated as the average of the following elements: single-photon emission computerised tomography with computerised tomography (SPECT-CT) of one area (£96, HRG code RN04A), SPECT-CT of two or three areas (£215, HRG code RN05A) and SPECT-CT of more than three areas (£311, HRG code RN06A) for those aged ≥ 18 years. The CTC cost was estimated to be £175.75.
All cost parameters included in the model, except the costs of a SeHCAT test, which are assumed to be fixed, were implemented as a triangular distribution with the limits calculated from the experts’ responses and shown in Tables 20–25. We acknowledge that this is not the most commonly used parameterisation and that it has certain limitations. First, we attempted to fit a gamma distribution to the cost estimates derived from the experts’ answers. However, this was not possible for all cost parameters because the method used to fit a gamma distribution did not find a solution for all cost parameters. Given that it was not possible to implement all cost input parameters as gamma distributions, we adopted a more pragmatic and simpler approach: cost input parameters were modelled as triangular distributions.
Markov model, bile acid diarrhoea, population 1
Patients enter the Markov model in the ‘no-diarrhoea’ or the ‘diarrhoea’ health state depending on whether or not they had an initial treatment response in the short-term decision-analytic model. The Markov model is then parameterised according to treatment; thus, in practice, there are four different Markov models: two BAS models (one for colestyramine and one for colesevelam), an IBD model and a IBS-D model. The cycle length used is 6 months, in line with the previous assessment of SeHCAT. 18
In the previous assessment of SeHCAT, it was concluded that ‘there are clear indications that patients may move from ND [no diarrhoea] to D [diarrhoea] and vice versa. However, from the data available, these transition probabilities are impossible to quantify’. 18 A range of (a priori) equally plausible scenarios with various values was then defined with the purpose of showing the impact of the transition probability assumptions on the model outcomes, without selecting one as a base-case scenario. As explained above, testing the impact of these assumptions required a large number of scenario analyses, resulting in very different cost-effectiveness outcomes, without knowing the actual plausibility of such scenarios. In this assessment, transitions between the ‘diarrhoea’ and ‘no-diarrhoea’ health states were thus informed by clinical expert opinion, as new clinical data regarding the long-term effectiveness of BAS and IBD and IBS-D treatments were not identified in our systematic review.
The clinical experts consulted for this assessment suggested that, in general, patients initially responding to BAS and to IBS-D treatments are expected to respond for their entire lifetime and that no relapses in the long term should be expected. Therefore, for the base-case scenario, it was assumed that BAS and IBS-D treatment responders start the Markov model in the ‘no-diarrhoea’ health state and the only possible transition is to the ‘death’ health state (i.e. transition to ‘diarrhoea’ is not possible). To account for the uncertainty regarding this base-case assumption, scenarios were conducted in which relapses were allowed to occur over the time horizon.
Regarding IBD patients, experts indicated that, unlike IBS-D patients, relapses are expected to occur after initial response to treatment. Therefore, transitions between ‘no-diarrhoea’ and ‘diarrhoea’ health states were allowed in the IBD Markov model. In particular, it was assumed in the base-case scenario that IBD treatment responders experience, on average, one relapse every 5 years, as suggested by some clinical experts’ responses to our questionnaire. Because we assumed a time horizon of 50 years, a total of 10 cycles (of 6 months) of relapse were considered (one cycle = 6 months, 60 months = 5 years). Setting the transition probability from ‘no diarrhoea’ to ‘diarrhoea’ equal to 0.0045 results in approximately 5 undiscounted life-years in the ‘diarrhoea’ health state. Therefore, this was chosen for the base-case scenario. Several scenarios were run to test the impact of this assumption on the cost-effectiveness results.
In line with the previous assessment of SeHCAT,18 we also assumed that no excess mortality is associated with BAD. 56 Age- and gender-specific mortality estimates were derived from the 2017–2019 England and Wales Interim Life Tables. 65 Using the study by Summers et al. ,4 we assumed that the average age in population 1 was 50 years, and the ratio of males to females was 35 : 75. Although these age and gender estimates are for a wider population than that specified in our inclusion criteria, looking at the patient characteristics in Summers et al. ,4 we estimated that more than half of patients would fall into our population of interest. As UK-specific demographic data were not used in the previous assessment of SeHCAT, we considered Summers et al. 4 to be the best option.
The Markov models for responders use the same resource use, costs and utility estimates as reported in previous sections for the short-term decision-analytic model. Utilities were adjusted for ageing using the equation estimated by Ara and Brazier. 66 For patients who did not respond to any treatment in the initial phase (i.e. the patients entering the Markov model in the ‘diarrhoea’ health state), we assumed that patients use loperamide to reduce the stool frequency.
Diagnostic model, population 2
The main difference with respect to the short-term model for population 1 is that Crohn’s disease patients are assumed to already have had colonoscopy to diagnose Crohn’s disease.
Probabilities
As shown in Figure 4, only the probability of responding to diarrhoea treatment for Crohn’s disease patients with suspected BAD (represented by the circle in the ‘no SeHCAT test’ branch) has to be estimated when SeHCAT and BAS trial of treatment are not available. Diarrhoea treatments for Crohn’s disease patients may vary between patients because the diarrhoea may occur as a symptom of relapse, but also when patients are in remission. In the first case, treatment may be targeted at treating the relapse, as this is expected to decrease the diarrhoea. In the second case, diarrhoea-specific treatments such as loperamide, codeine, diet or nutritional therapies may be considered. Thus, owing to the wide range of treatment options and the various orders in which they are attempted, it was not possible to find data from the literature regarding how Crohn’s disease patients without ileal resection will eventually, after trying various options, respond to their treatment for the diarrhoea. Therefore, response to diarrhoea treatment was estimated from the answers to the questionnaire obtained from the experts. These are presented in Table 26. The approach described previously of simulating triangular distributions to derive the parameters of a beta distribution was also followed in this case. We found a mean of 40% and a standard deviation of 6% and it was implemented as a beta(α = 30, β = 45) distribution.
| Expert | Percentage of patients successfully treated | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 70 | 50 | 80 |
| 2 | NR | NR | NR |
| 3 | 10 | 5 | 25 |
| 4 | NR | NR | NR |
There is uncertainty regarding the initial response to diarrhoea treatment and the duration of this response among Crohn’s disease patients without ileal resection. Based on the experts’ answers to our questionnaire, we assumed, in the base-case scenario, that response is achieved within 6 months of start of treatment, even though this is also variable, as acknowledged by the clinical experts. Despite the uncertainty regarding the duration of the treatment effect, we assumed that relapses are expected, as assumed for IBD patients in population 1. Scenarios to test this assumption were explored in the scenario analysis section of this report.
As shown in Figure 4, five different probabilities need to be estimated when SeHCAT testing is available. Note that three of these correspond to the probabilities associated with SeHCAT testing, namely the probability of testing positive, the probability of responding to BAS treatment (contingent on being SeHCAT positive) and the probability of discontinuation of BAS treatment. In addition, for patients testing negative for SeHCAT or not responding to BAS treatment after testing positive, the probability of responding to diarrhoea treatment for Crohn’s disease patients with suspected BAD (described previously for the no-SeHCAT strategy) has to be estimated. SeHCAT-related probabilities were estimated using the results from our clinical effectiveness review. The remaining probabilities were informed by clinical experts. As our questionnaire did not include questions about patients not responding to BAS treatment after a positive SeHCAT test result, we assumed for these patients the same estimates as those obtained for patients with a negative SeHCAT test result. Thus, in practice, four probabilities were estimated for the SeHCAT 15% strategy.
The probability of a positive test result in the Crohn’s disease population was estimated from the study by Smith et al. ,7 as explained in Chapter 3. This estimate can be seen in Table 27.
| Study | Sample size (n) | Number of positive SeHCAT tests | Probability of positive SeHCAT test |
|---|---|---|---|
| Smith 20007 | 44 | 24 | 0.55 |
| Mean | 0.55 | ||
| SE | 0.08 |
Patients with a positive SeHCAT test result are assumed to be treated with a BAS. Based on the responses to our questionnaire provided by the clinical experts consulted for this assessment, we assumed in the base-case scenario that 63% of patients started with colestyramine and 37% with colesevelam. Note that colestipol was not included in the cost-effectiveness analyses because none of the clinical experts consulted had experience with it (i.e. the proportion of patients treated with colestipol estimated by the experts was 0%). In the absence of a better source of evidence, it was assumed that the experts’ responses would represent UK clinical practice. In terms of response, however, it was not possible to distinguish between the type of BAS and the estimated response rate to BAS, in general, based on Smith et al. 7 This can be seen in Table 28. Note that this is based on a small sample size and the relatively high response rate does not seem to be in line with the expectations of the experts, who, in the answers to our questionnaire, estimated this probability to be, at most, 70%. The uncertainty surrounding this input parameter was explored in scenario analyses.
| Study | Sample size (n) | Positive response | |
|---|---|---|---|
| Number | Probability | ||
| Smith 20007 | 9 | 8 | 0.89 |
| Mean | 0.89 | ||
| SE | 0.11 | ||
Again, the answers provided by the experts seem to suggest that initial response to BAS treatment is achieved within 6 months of start of treatment. However, there is uncertainty regarding treatment effect duration. For consistency with the base-case scenario in population 1 and in the absence of a better evidence source, we also assumed a lifetime effect as in population 1 (thus, in the Markov model there is no transition to the diarrhoea health state for patients initially responding to treatment), but this is unclear. Scenarios with long-term relapses are also explored in Sensitivity and scenario analysis.
Regarding adherence to BAS treatment, the same approach described for population 1 was also followed for this population. Thus, the impact of reduced compliance was assumed to be included in response and only switching from colestyramine to colesevelam was permitted in the model. The probability of switching from colestyramine to colesevelam was then estimated based on the experts’ responses, shown in Table 29. Again, we followed the approach described previously of simulating triangular distributions to derive the parameters of a beta distribution. We found a mean of 44% and a standard deviation of 3%, corresponding to a beta(α = 132, β = 169) distribution.
| Expert | % of patients dropping out | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 15 | 10 | 30 |
| 2 | 70 | NR | NR |
| 3 | 2 | 2 | 5 |
| 4 | NR | NR | NR |
When patients do not respond to BAS after a positive SeHCAT result, or when the SeHCAT result is negative, we assumed that patients follow the same pathway as in the no-SeHCAT strategy. This part of the model was informed by clinical experts only. As our questionnaire did not include questions about no response to BAS after a positive SeHCAT result, the same estimates were assumed as those obtained for patients with a negative SeHCAT result.
Patients with a negative SeHCAT test receive treatment for their chronic diarrhoea. Because SeHCAT was negative for these patients, it might be assumed that most patients who have BAD are not included in the group receiving chronic diarrhoea treatment. Hence, it is expected that the response rate for chronic diarrhoea treatment in the SeHCAT-negative subpopulation will be higher than in the no-SeHCAT population. In the previous assessment of SeHCAT,18 no data were available to confirm this assumption. In fact, it was assumed that the increase was the same as for the IBS-D population. In this assessment, we used the responses to our questionnaire to inform this probability. These can be seen in Table 30. After simulating triangular distributions, we found a mean of 42% and a standard deviation of 6%, corresponding to a beta(α = 26, β = 35) distribution. Note that this estimate is higher than (as expected), but close to, the same probability estimated for no SeHCAT (40%). In population 1, this difference was larger. The uncertainty associated with this parameter was further explored in scenario analyses.
| Expert | % of patients successfully treated | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 70 | 50 | 90 |
| 2 | NR | NR | NR |
| 3 | 10 | 5 | 25 |
| 4 | NR | NR | NR |
As shown in Figure 4, three different probabilities need to be estimated when BAS trial of treatment (without SeHCAT testing) is available. This strategy starts with the probability of responding to BAS treatment. In the case of no response, patients are assumed to follow the same pathway as in the no-SeHCAT strategy. For this strategy, probabilities were informed by clinical experts.
In the trial-of-treatment strategy, all patients are assumed to receive a BAS at the beginning of the modelled pathway. We assumed that this is either colestyramine or colesevelam. The proportion of patients receiving each of the BAS options was estimated from the responses to our questionnaire; they are summarised in Table 31. It was estimated that 58% of patients started with colestyramine and 42% with colesevelam. Note that this is different from the 63/37 distribution assumed in the SeHCAT strategy. It is also unclear why the BAS proportions differed between strategies for this population. Furthermore, for population 1, it was argued that the higher proportion of colestyramine used in the trial-of-treatment strategy might be because of its lower costs. In this population, this does not happen, and it is unclear why. In any case, a range of different proportions was explored in scenario analyses.
| Expert | Percentage of patients treated with colestyramine | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 90 | 80 | 100 |
| 2 | 20 | 10 | 50 |
| 3 | 0 | 0 | 0 |
| 4 | NR | NR | NR |
In terms of response, we did not distinguish between the type of BAS. In the trial-of-treatment strategy, all patients are treated with BAS, including those with no BAD; therefore, response to BAS is expected to be lower than in the SeHCAT 15% strategy. The probability of response to BAS trial of treatment was estimated from the answers to our questionnaire; they are summarised in Table 32. After simulating triangular distributions, we found a mean of 33% and a standard deviation of 3%, corresponding to a beta(α = 71, β = 146) distribution. The uncertainty associated with this parameter was further explored in scenario analyses.
| Expert | % of response to BAS | Uncertainty range (%) | |
|---|---|---|---|
| Lowest | Highest | ||
| 1 | 50 | 40 | 60 |
| 2 | NR | NR | NR |
| 3 | 15 | 10 | 20 |
| 4 | NR | NR | NR |
Bile acid sequestrant adherence, initial response and duration of treatment effect are assumed to be the same as for the SeHCAT strategy. Thus, the probability of switching from colestyramine to colesevelam was assumed to follow a beta(α = 132, β = 169) distribution. For this population, we did not have any indication about what happens to patients after no response to BAS, but, as we did for population 1, we assumed that this lies somewhere between the 40% of the no-SeHCAT strategy and the 42% of the SeHCAT 15% strategy; thus, the only choice possible was 41% (modelling choice). Then we estimated the parameters of a beta distribution for the base-case scenario assuming a 6% standard deviation as in the SeHCAT strategy, which resulted in a beta(α = 25, β = 36) distribution. The uncertainty associated with this parameter was explored in scenario analyses.
Health-related quality of life
No studies were identified that specifically address the issue of diarrhoea among Crohn’s disease patients. It was thus assumed that the utility decrement as a result of diarrhoea in this patient population is the same as for population 1. To calculate QALYs, we used the utility estimate from Buxton et al. ,67 in which EQ-5D utilities were estimated by mapping from the Inflammatory Bowel Disease Questionnaire in a sample of 3672 patients with moderate to severe active Crohn’s disease. A mean of 0.7 was found, with a standard deviation of 0.25. It was assumed that this utility reflects the HRQoL in the diarrhoea health state; thus, the utility for the no-diarrhoea health state would be 0.764. Again, in the base-case scenario, it was assumed that BAS treatment (colestyramine) responders would have a utility gain of 75% of diarrhoea decrement, to account for the tolerability issues associated with colestyramine. This resulted in an estimated utility of 0.748 for colestyramine responders. Colesevelam responders were assumed to have the full utility increment, as per no diarrhoea. The base-case utility values are summarised in Table 33.
| Source | Mean | SE |
|---|---|---|
| Non-responders/diarrhoea | ||
| Buxton 200767 | 0.70 | 0.004 |
| Responders/no diarrhoea | ||
| Assumption | 0.764 | 0.004 |
| BAS treatment (colestyramine) responders/no diarrhoea | ||
| Assumption | 0.748 | 0.004 |
Resource use and costs
The costs considered for population 2 can be classified into three groups: (1) the costs of a SeHCAT test, (2) treatment with BAS and (3) treatment of diarrhoea among Crohn’s disease patients.
The cost of a SeHCAT test is the same as for population 1. The cost of the SeHCAT capsule was sourced from the manufacturer as £195 and the tariff for administering the test was estimated at £282; thus, we arrived at a total cost of £477.
Patients with a positive SeHCAT test result were assumed to receive treatment with a BAS, either colestyramine or colesevelam. The prices of the medications were derived from the BNF. 63 Exact details on medication use, dosage and fraction of patients receiving the treatment are presented in Appendix 5. We estimated the cost of BAS treatment, taking the average of the dosage values reported by the experts. We assumed the same dosages as in population 1; thus, for colestyramine we assumed a dosage of 5 g per day, resulting in a cost of £0.35 per day, and, for colesevelam, we assumed a dosage of 2.5 g per day, resulting in a cost of £2.56 per day.
Medical treatment of chronic diarrhoea among Crohn’s patients without ileal resection was based on the previous SeHCAT report. 18 Table 34 shows the (weighted) costs of medication per day. The prices of the medications were derived from the BNF. 63 Exact details on medication use, dosage and fraction of patients receiving the treatment are presented in Appendix 5, Tables 58–63.
| Drug | Percentage of patients | Cost per day (£) | Weighted cost per day (£) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Lowest | Highest | Mean | Lowest | Highest | ||
| Loperamide | 0.6 | 0.25 | 1 | 0.13 | 0.08 | 0.03 | 0.13 |
| Codeine | 0.4 | 0 | 0.8 | 0.12 | 0.05 | 0.00 | 0.09 |
| Corticosteroids | 0.77 | 0.5 | 1 | 1.38 | 1.06 | 0.69 | 1.38 |
| Adalimumab | 0.1 | 0 | 0.3 | 22.88 | 2.29 | 0.00 | 6.86 |
| Pentasa | 0.6 | 0.4 | 0.7 | 2.46 | 1.48 | 0.98 | 1.72 |
| Azathioprine | 0.5 | 0.2 | 0.7 | 0.17 | 0.09 | 0.03 | 0.12 |
| BAS | 0.5 | 0.05 | 0.9 | 1.46 | 0.73 | 0.07 | 1.31 |
| Total cost (£) | 5.76 | 1.81 | 11.62 | ||||
Markov model, population 2
The approach to derive transitions between the ‘diarrhoea’ and ‘no-diarrhoea’ health states is the same as the one described for population 1. Patients enter the Markov model in the ‘no-diarrhoea’ or the ‘diarrhoea’ health state, depending on whether or not they had an initial treatment response. The Markov model is then parameterised according to treatment. In practice, there are three different Markov models: two BAS models (one for colestyramine and one for colesevelam) and one model for the treatment of chronic diarrhoea among Crohn’s disease patients. The cycle length used is also 6 months.
Clinical experts consulted for this assessment suggested that, in general, patients initially responding to BASs are expected to respond for their entire lifetime and that no relapses in the long term should be expected. Therefore, for the base-case scenario, it was assumed that BAS responders start the Markov model in the ‘no-diarrhoea’ health state and the only possible transition is to the ‘death’ health state (i.e. transition to ‘diarrhoea’ is not possible). To account for the uncertainty regarding this assumption, scenarios were conducted in which relapses are allowed to occur over the time horizon. For responders to diarrhoea treatment for Crohn’s disease (without BAS), we followed the same approach as described for IBD patients in population 1, whereby a few relapses are expected to occur during a patient’s lifetime. Therefore, transitions between ‘no diarrhoea’ and ‘diarrhoea’ are allowed in the Markov model. As in population 1, it was assumed in the base-case scenario that responders to diarrhoea treatment (without BAS) experience, on average, one relapse every 5 years. As we assumed a time horizon of 50 years, a total of 10 cycles of relapse was considered (1 cycle = 6 months, 60 months = 5 years). Setting the transition probability from ‘no diarrhoea’ to ‘diarrhoea’ equal to 0.00575 results in approximately 5 undiscounted life-years in the ‘diarrhoea’ health state. Therefore, this was chosen for the base-case scenario. Several scenarios were run to test the impact of this assumption on the cost-effectiveness results.
Regarding mortality, we followed the same approach as in the previous assessment of SeHCAT,18 whereby no reports were found in the literature that chronic diarrhoea itself in Crohn’s patients would lead to excess mortality. 56 However, patients with Crohn’s disease have a shorter life expectancy than the general population. A meta-analysis by Canavan et al. 68 showed a pooled estimate of the standardised mortality ratio (SMR) of 1.52 (95% CI 1.32 to 1.74). Thus, we have applied this SMR to the overall mortality in the UK population, for which we again used the 2017–2019 England and Wales Interim Life Tables. 65 Using the study by Summers et al. ,4 we assumed that the average age in population 1 was 50 years, and the ratio of males to females was 35 : 75. We assumed the same age/gender distribution as in population 1 because the study does not distinguish between subpopulations. Nevertheless, we still considered Summers et al. 4 to also be the best option for the Crohn’s disease population.
Furthermore, for population 2, the Markov models for responders use the same resource use, costs and utility estimates as reported in previous sections for the short-term decision-analytic model. Utilities were adjusted for ageing using the equation estimated by Ara and Brazier. 66 For patients who did not respond to any treatment in the initial phase (i.e. the patients entering the Markov model in the ‘diarrhoea’ health state), we assumed that patients use loperamide to reduce the stool frequency.
Summary input parameters
The input parameters used in the health economic models for populations 1 and 2 are summarised in Tables 35 and 36, respectively.
| Category | Description | Mean value | Distribution | Distribution parameters |
|---|---|---|---|---|
| Branch probability | Probability of undergoing colonoscopy in the no-SeHCAT strategy | 0.74 | Beta |
|
| Probability of having IBD after colonoscopy | 0.053 | Beta |
|
|
| Probability of responding to IBD treatment (in IBD patients) | 0.72 | Beta |
|
|
| Probability of responding to IBS-D treatment when no SeHCAT is available after colonoscopy | 0.46 | Beta |
|
|
| Probability of responding to IBS-D treatment when no SeHCAT is available without colonoscopy | 0.44 | Beta |
|
|
| Probability of having a positive SeHCAT test at cut-off value of 15% | 0.454 | Beta |
|
|
| Probability of responding to BAS given a positive SeHCAT test at a cut-off value of 15% | 0.638 | Beta |
|
|
| Probability of being treated with colestyramine (as opposed to colesevelam) in the SeHCAT 15% strategy | 0.5 | Beta |
|
|
| Probability of switching from colestyramine to colesevelam | 0.5 | Beta |
|
|
| Probability of undergoing colonoscopy after a negative SeHCAT test | 0.49 | Beta |
|
|
| Probability of responding to IBS-D treatment after a negative SeHCAT test and colonoscopy | 0.56 | Beta |
|
|
| Probability of responding to IBS-D treatment after a negative SeHCAT test without colonoscopy | 0.53 | Beta |
|
|
| Probability of being treated with colestyramine (as opposed to colesevelam) in BAS TOT strategy | 0.85 | Beta |
|
|
| Probability of responding to BAS TOT | 0.30 | Beta |
|
|
| Probability of undergoing colonoscopy after no response to BAS TOT | 0.90 | Beta |
|
|
| Probability of responding to IBS-D treatment after no response to BAS TOT and colonoscopy | 0.50 | Beta |
|
|
| Probability of responding to IBS-D treatment after no response to BAS TOT without colonoscopy | 0.47 | Beta |
|
|
| Transition probability | Transition probability from ‘diarrhoea’ to ‘no diarrhoea’ | 0 | Fixed | NA |
| Transition probability from ‘no diarrhoea’ to ‘diarrhoea’ (IBS-D, BAS) | 0 | Fixed | NA | |
| Transition probability from ‘no diarrhoea’ to ‘diarrhoea’ (IBD) | 0.0045 | Triangular |
|
|
| Cost | Cost per day of IBS-D medication | £0.06 | Triangular |
|
| Diet costs per 6 months associated with IBS-D | £12.24 | Triangular |
|
|
| Psychological costs per 6 months associated with IBS-D | £35.74 | Triangular |
|
|
| Cost per day of IBD medication | £21.73 | Triangular |
|
|
| Diet costs per 6 months associated with IBD | £149 | Triangular |
|
|
| Psychological costs per 6 months associated with IBD | £289.33 | Triangular |
|
|
| Cost per day of BAS medication (colestyramine) | £0.35 | Triangular |
|
|
| Cost per day of BAS medication (colesevelam) | £2.56 | Triangular |
|
|
| Cost of SeHCAT capsule | £195 | Fixed | NA | |
| Cost of administering SeHCAT test | £282 | Fixed | NA | |
| Maintenance and service costs of SeHCAT test | £0 | Fixed | NA | |
| Cost of colonoscopy | £440 | Triangular |
|
|
| Cost per day of loperamide | £0.03 | Triangular |
|
|
| Utility | Utility associated with health state ‘diarrhoea’ | 0.71 | Beta |
|
| Utility associated with health state ‘no diarrhoea’ (IBS-D, IBD, colesevelam) | 0.78 | Beta |
|
|
| Utility associated with health state ‘no diarrhoea’ (colestyramine) | 0.76 | Beta |
|
| Category | Description | Mean value | Distribution | Distribution parameters |
|---|---|---|---|---|
| Branch probability | Probability of responding to diarrhoea treatment for Crohn’s disease patients when SeHCAT test is not available | 0.40 | Beta |
|
| Probability of being treated with colestyramine (as opposed to colesevelam) in the SeHCAT 15% strategy | 0.63 | Beta |
|
|
| Probability of switching from colestyramine to colesevelam | 0.44 | Beta |
|
|
| Probability of having a positive SeHCAT test at a cut-off value of 15% | 0.55 | Beta |
|
|
| Probability of responding to BAS given a positive SeHCAT test at a cut-off value of 15% | 0.89 | Beta |
|
|
| Probability of responding to diarrhoea treatment for Crohn’s disease patients after a negative SeHCAT test | 0.42 | Beta |
|
|
| Probability of being treated with colestyramine (as opposed to colesevelam) in BAS TOT strategy | 0.58 | Beta |
|
|
| Probability of responding to BAS TOT | 0.33 | Beta |
|
|
| Probability of responding to diarrhoea treatment for Crohn’s disease after no response to BAS TOT | 0.50 | Beta |
|
|
| Transition probability | Transition probability from ‘diarrhoea’ to ‘no diarrhoea’ | 0 | Fixed | NA |
| Transition probability from ‘no diarrhoea’ to ‘diarrhoea’ (BAS) | 0 | Fixed | NA | |
| Transition probability from ‘no diarrhoea’ to ‘diarrhoea’ (diarrhoea treatment for Crohn’s disease patients) | 0.00575 | Triangular |
|
|
| Cost | Cost per day of diarrhoea medication for Crohn’s disease patients | £5.76 | Triangular |
|
| Cost per day of BAS medication (colestyramine) | £0.35 | Triangular |
|
|
| Cost per day of BAS medication (colesevelam) | £2.56 | Triangular |
|
|
| Cost of SeHCAT capsule | £195 | Fixed | NA | |
| Cost of administering SeHCAT test | £282 | Fixed | NA | |
| Maintenance and service costs of SeHCAT test | £0 | Fixed | NA | |
| Utility | Utility associated with health state ‘diarrhoea’ | 0.70 | Beta |
|
| Utility associated with health state ‘no diarrhoea’ (Crohn’s disease) | 0.76 | Beta |
|
|
| Utility associated with health state ‘no diarrhoea’ | 0.75 | Beta |
|
Overview of main model assumptions
Population 1: probabilities for the no-SeHCAT strategy
-
Colonoscopy takes place at the beginning of the no-SeHCAT strategy to detect IBD patients.
-
Response to IBD treatment is achieved within 6 months of start of treatment. No lifetime treatment effect was assumed, as relapses are expected after initial response.
-
Response to IBS-D treatment is achieved within 6 months of start of treatment. We assumed a lifetime effect in the base-case scenario; thus, in the Markov model, there is no transition to the diarrhoea health state for patients initially responding to treatment.
Population 1: probabilities for the SeHCAT 15% strategy
-
Patients testing negative for SeHCAT or not responding to BAS treatment after testing positive are assumed to follow the same pathway as for the no-SeHCAT strategy.
-
Patients not responding to BAS treatment after a positive SeHCAT test result or patients with a negative SeHCAT test result were assumed to have the same estimates as those obtained for the no-SeHCAT strategy.
-
Patients with a positive SeHCAT test result are assumed to be treated with a BAS (either colestyramine or colesevelam). For treatment response, an overall response rate to BAS was estimated. Initial response to BAS treatment is achieved within 6 months of start of treatment and a lifetime treatment effect duration is assumed.
-
Patients with a colonoscopy confirming IBD would have the same response rate regardless of the result of the SeHCAT test.
-
The response rate for IBS-D treatment in the SeHCAT-negative subpopulation was assumed to be higher than in the no-SeHCAT strategy subpopulation.
-
There is no BAS discontinuation, but there is switching. BAS switching is allowed from colestyramine to colesevelam only.
-
Colesevelam seems to be well tolerated; thus, no dropout from colesevelam is modelled. Even though colesevelam dropouts might occur in practice, this seems a reasonable assumption given that this is expected to happen in a small proportion of patients and the lack of information regarding how these patients will be treated afterwards.
-
Dropouts are assumed to occur in the first 6 months only. Therefore, long-term dropout was not included in the model.
-
Dropout was assumed to have no effect on response rate. As explained previously, it is assumed that the impact of reduced compliance on the response rate is implicitly included in the studies identified in our systematic review.
-
Dropout was assumed to have an effect on HRQoL and costs. Colesevelam is assumed to be associated with a higher utility than colestyramine, but it is also more costly.
Population 1: probabilities for the no-SeHCAT and bile acid sequestrant trial-of-treatment strategy
-
In the case of no response to BAS trial of treatment, patients follow the same pathway as for the no-SeHCAT strategy.
-
All patients are assumed to receive a BAS at the beginning of the modelled pathway; 85% of patients started with colestyramine and 15% with colesevelam.
-
Bile acid sequestrant adherence, initial response and duration of treatment effect are assumed to be the same as for the SeHCAT 15% strategy.
-
The probability of having IBD after colonoscopy and IBD treatment response after colonoscopy were assumed to be the same as for the no-SeHCAT strategy.
-
The probability of IBS-D treatment response after no response to BAS and colonoscopy was assumed to lie somewhere in between the 46% of the no-SeHCAT strategy and the 56% of the SeHCAT 15% strategy. For the base-case scenario, we assumed a mean response of 50%.
Population 1: utility values
-
The same IBS utility values as identified in the previous report were used. 18
-
For the base-case scenario, it was assumed that colestyramine responders have 75% of the utility increment observed among responders to IBS-D treatment. Colesevelam responders were assumed to have the full utility increment, as per IBS-D treatment responders.
-
A utility decrement due to colonoscopy was not included in the model as this was expected to have a negligible impact on the cost-effectiveness results.
Population 1: resource use and costs
-
Resource use was based on expert opinion.
-
The costs of treatment for IBS-D (no SeHCAT and SeHCAT negative) consist of medication, diet and psychological therapy costs. Dietitian and psychological therapy costs were assumed for 6 months.
-
Inflammatory bowel disease treatment costs (no SeHCAT and SeHCAT negative) consist of medication, diet and psychological therapy costs. Dietitian and psychological therapy costs were assumed for 6 months.
-
Bile acid malabsorption treatment (SeHCAT positive) consists of medication costs only.
-
Cost of colonoscopy in the model was calculated as 90% colonoscopy plus 10% CTC, based on one expert answer to our questionnaire.
Population 2: probabilities for the no-SeHCAT strategy
Response to diarrhoea treatment in Crohn’s disease patients is achieved within 6 months of start of treatment. Relapses are expected, as assumed for IBD patients in population 1.
Population 2: Probabilities for the SeHCAT 15% strategy
-
For patients not responding to BAS treatment after a positive SeHCAT test result, the same estimates were assumed as those obtained for patients with a negative SeHCAT test result.
-
Patients with a positive SeHCAT test result are treated with a BAS (63% started with colestyramine and 37% with colesevelam). For treatment response, an overall response rate to BAS was estimated.
-
Initial response to BAS treatment is achieved within 6 months of start of treatment and a lifetime treatment effect duration is assumed.
-
When patients do not respond to BAS after a positive SeHCAT test result, or when the SeHCAT test result is negative, patients follow the same pathway as in the no-SeHCAT strategy. The same estimates as those obtained for patients with a negative SeHCAT test result were assumed.
-
Patients with a negative SeHCAT test result receive treatment for their chronic diarrhoea. Hence, it was assumed that the response rate for chronic diarrhoea treatment in the SeHCAT-negative subpopulation will be higher than in the no-SeHCAT population.
Population 2: probabilities for the no-SeHCAT and BAS trial-of-treatment strategy
-
In the case of no response to BAS trial of treatment, patients are assumed to follow the same pathway as for the no-SeHCAT strategy.
-
All patients are assumed to receive a BAS (58% colestyramine and 42% colesevelam).
-
In terms of response, we did not distinguish between the type of BAS.
-
Bile acid sequestrant adherence, initial response and duration of treatment effect are assumed to be the same as for the SeHCAT strategy.
Population 2: utility values
A mean of 0.7 was found for the HRQoL in the diarrhoea health state. The utilities for the no-diarrhoea health state and for BAS responders were calculated following the assumptions in population 1.
Population 2: resource use and costs
-
Resource use was based on expert opinion.
-
Medical treatment of Crohn’s disease was assumed to be the same as in the previous assessment of SeHCAT. 18 Treatment costs were calculated by using updated unit costs.
-
BAD treatment (SeHCAT positive) consists of medication costs only.
Markov model
-
Cycle length: 6 months.
-
Time horizon: 50 years (100 cycles).
-
Bile acid sequestrant and IBS-D treatment responders start the Markov model in the ‘no-diarrhoea’ health state and the only possible transition is to the ‘death’ health state (i.e. transition to ‘diarrhoea’ is not possible).
-
Ten cycles (of 6 months) of relapse were considered (1 cycle = 6 months, 60 months = 5 years) for IBD treatment responders.
-
The average age in populations 1 and 2 was 50 years, and the ratio of males to females was 35 : 75.
-
Utilities were adjusted for ageing.
-
For patients who did not respond to any treatment in the initial phase (i.e. the patients entering the Markov model in the ‘diarrhoea’ health state), we assumed that patients use loperamide to reduce the stool frequency.
Model analyses
Analyses were conducted as cost-effectiveness analyses for both populations of interest. Costs and effects were discounted by 3.5%. Analyses incorporated a 50-year time horizon to estimate outcomes in terms of life-years, lifetime QALYs and lifetime costs from the perspective of the NHS and the Personal Social Services. Costs were sourced from the year 2020 when possible; otherwise, costs were inflated using the NHSCII. 58 Other outcomes included in the analyses were short-term costs, response to treatment and, for population 1, colonoscopies avoided. These three outcomes were calculated in the decision-analytic model (thus, assumed to be in the first 6 months of the simulation). Parameter uncertainty was explored through probabilistic sensitivity analysis (PSA) and structural uncertainty was explored through scenario analyses. Deterministic one-way or multiway sensitivity analyses were not conducted. The main reason for this was the lack of published uncertainty data for most of the input parameters of the model. It was felt that any attempt to derive plausible and comparable uncertainty ranges for all input parameters (e.g. 95% CIs) would be unfeasible and, thus, the results of the deterministic sensitivity analyses would be at risk of being misleading. Unlike the previous assessment of SeHCAT,18 the value of information associated with the model uncertainty was not explored in this case. The main reason for this was again the lack of data; because of this, we believe that additional research is needed to reduce both parameter and structural uncertainty. Furthermore, it is not possible to assess whether or not the current model uncertainty has been properly captured because, for the majority of input parameters, uncertainty ranges were derived from clinical expert opinion (four experts at most, but in general one or two) and from modellers’ choices. Therefore, again, the results of any value-of-information analyses would be at risk of being misleading.
Secondary analysis
The base-case scenario was built under the assumption that colonoscopy was not part of the clinical pathway before patients enter the model. This analysis is, however, likely to deviate from current guidelines. As explained previously, this was nevertheless chosen as the base-case analysis following scoping discussions. Given its importance, a scenario in which colonoscopy is not included in the cost-effectiveness model (e.g. assumed to occur before SeHCAT testing or trial of treatment with BAS) was presented separately.
Scenario analyses
A series of scenario analyses were conducted to explore the most important areas of uncertainty in the model described above. Summaries of the scenario analyses conducted are given in the following sections.
Scenario analysis 1: alternative probability of undergoing colonoscopy in population 1
The number of colonoscopies avoided is one of the outcomes of interest for population 1. Thus, assumptions regarding the probability of undergoing colonoscopy are expected to be an important driver of the cost-effectiveness results in this population. In the base-case analysis, it was assumed that the probability of undergoing colonoscopy (for all patients) in the no-SeHCAT strategy was 74%. In the SeHCAT 15% strategy, colonoscopy was included in the model only after a negative SeHCAT test result or after a positive result but no response to BAS treatment. For this subgroup of patients, the probability of undergoing colonoscopy was 49%. Likewise, in the BAS trial-of-treatment strategy, colonoscopy was included in the model only after no response to BAS treatment; for this subgroup of patients, the probability of undergoing colonoscopy was 90%. Two additional scenarios were explored: one in which colonoscopy is not included in the model (note that this is the secondary analysis for population 1; results can be seen above) and one in which the probability of undergoing colonoscopy is 100%. The latter implies that (1) all patients in the no-SeHCAT strategy, (2) patients with a negative SeHCAT test result, or a positive test result but no response to BAS treatment, and (3) patients not responding to BAS trial of treatment are assumed to undergo colonoscopy.
Scenario analysis 2: alternative probability of response to diarrhoea-predominant irritable bowel syndrome treatment in population 1
In the no-SeHCAT strategy, the probability of responding to IBS-D treatment (after colonoscopy ruled out IBD) was estimated at 46%, based on clinical experts’ answers to our questionnaire. For SeHCAT-negative patients, it was assumed that most patients who have BAD are not included in the group receiving IBS-D treatment. Therefore, the response rate to IBS-D treatment in the SeHCAT-negative subpopulation was assumed to be higher than in the no-SeHCAT strategy, and a mean response of 56% was estimated. In the absence of any evidence, for the BAS trial-of-treatment strategy we assumed that the probability of IBS-D treatment response was somewhere in between the other two strategies and a mean response of 50% was assumed. In addition, within each strategy, it was assumed that the probability of IBS-D treatment response was slightly lower among patients who did not undergo colonoscopy because a larger proportion of IBD patients (who were consequently assumed not to respond to IBS-D treatment) were included in this subgroup of patients. We explored several scenarios in which we varied the percentage of response to IBS-D treatment for the no-SeHCAT, the SeHCAT-negative (or positive and no response to BAS) and the BAS trial-of-treatment (no response to BAS) strategies, as shown in Table 37.
| Scenario | Strategy (% of response) | |||||
|---|---|---|---|---|---|---|
| No SeHCAT | SeHCAT 15% | BAS TOT | ||||
| After colonoscopy | No colonoscopy | After colonoscopy | No colonoscopy | After colonoscopy | No colonoscopy | |
| Base-case scenario | 46 | 44 | 56 | 53 | 50 | 47 |
| IBS-D scenario 1 | 50 | 47 | 56 | 53 | 50 | 47 |
| IBS-D scenario 2 | 50 | 47 | 56 | 53 | 56 | 53 |
| IBS-D scenario 3 | 56 | 53 | 56 | 53 | 56 | 53 |
| IBS-D scenario 4 | 70 | 66 | 56 | 53 | 50 | 47 |
Scenario analysis 3: alternative probability of a positive SeHCAT test result and response to bile acid sequestrant treatment in population 1
The unconditional response to BAS treatment in the SeHCAT 15% strategy is obtained by multiplying the probability of testing positive by the probability of response to BAS given a positive test result. In the base-case scenario, these probabilities were estimated from our systematic literature review as 0.454, 0.638 and 0.299 for SeHCAT 15%, response to BAS given a positive SeHCAT result and response to BAS trial of treatment, respectively. We explored scenarios in which, in the SeHCAT 15% strategy, the probability of testing positive and the probability of response to BAS were changed at the same time according to the limits of their CIs, in a form of worst-case and best-case scenarios. The probability of response to BAS trial of treatment was estimated to be 30% from clinical experts’ answers. We explored scenarios in which this probability was decreased and increased by 10%. These scenarios are summarised in Table 38.
| Scenario | Probability | ||
|---|---|---|---|
| SeHCAT 15% positive | Response to BAS given a positive SeHCAT test | Response to BAS TOT | |
| Base-case scenario | 0.454 | 0.638 | 0.299 |
| Scenario 1 | 0.357 | 0.495 | 0.299 |
| Scenario 2 | 0.555 | 0.760 | 0.299 |
| Scenario 3 | 0.454 | 0.638 | 0.2 |
| Scenario 4 | 0.454 | 0.638 | 0.4 |
Scenario analysis 4: alternative distribution of bile acid sequestrant treatment in population 1
Based on clinical experts’ responses, in the base-case scenario it was assumed that, in the SeHCAT 15% strategy, 50% of patients started with colestyramine and 50% with colesevelam. In the BAS trial-of-treatment strategy, these proportions were 85% and 15% for colestyramine and colesevelam, respectively. We explored scenarios in which all patients were treated with colestyramine, in which all patients were treated with colesevelam and in which there was no difference in BAS distribution between the SeHCAT 15% and the BAS trial-of-treatment strategies (one scenario assumed a 50/50 proportion and the other one an 85/15). Note that, in terms of response, it was not possible to distinguish between the type of BAS. Therefore, these scenarios had an effect on costs and utilities only because these were different for colestyramine and colesevelam.
Scenario analysis 5: alternative health-state utilities in population 1
We explored a scenario in which it was assumed that patients who respond to colestyramine (BAS) treatment receive 100% of the utility gain associated with not experiencing diarrhoea, instead of the 75% gain in utility assumed in the base-case scenario to account for additional tolerability issues and side effects of this treatment. In an additional scenario, we assumed utility values from each individual literature source in Table 19 instead of the pooled values used in the base-case scenario. In these scenarios, the utility decrement associated with colestyramine was still calculated based on an assumed 75% gain. Finally, a scenario was explored in which no age adjustment of the utility values was carried out.
Scenario analysis 6: alternative cost inputs in population 1
Because costs included in the model were estimated as combinations of several medications, resource use and assumptions, it was unfeasible to conduct scenario analyses on the cost components separately. Therefore, a pragmatic approach was taken in this case and all costs were varied by 20%.
Scenario analysis 7: alternative transition probabilities in population 1
Transitions in the Markov model represent the probabilities of experiencing diarrhoea relapse or remission. In the base-case scenario, it was assumed that only IBD patients experienced relapse after initial response to treatment. A probability of 0.45% per model cycle (6 months) was assumed. BAS and IBS-D treatment responders were assumed to remain in the no-diarrhoea health state (or to die) for their entire time horizon. Likewise, non-responders (to any treatment) stayed in the diarrhoea health state, where the only possible transition was to the death health state. We explored several scenarios in which patients were allowed to experience relapse in all models. We increased the probability of relapse to assess how this would affect the results. In one scenario, the possibility to experience remission was also included in the analysis. The scenarios conducted are summarised in Table 39.
| Scenario | Transitions, P[D → ND], P[ND → D] | ||
|---|---|---|---|
| BAS models | IBS-D model | IBD model | |
| Base-case scenario | 0, 0 | 0, 0 | 0, 0.0045 |
| Scenario 1 | 0, 0.0045 | 0, 0.0045 | 0, 0.0045 |
| Scenario 2 | 0.0045, 0.0045 | 0.0045, 0.0045 | 0.0045, 0.0045 |
| Scenario 3 | 0, 0.0045 × 2 | 0, 0.0045 × 2 | 0, 0.0045 × 2 |
| Scenario 4 | 0, 0.0045 × 5 | 0, 0.0045 × 5 | 0, 0.0045 × 5 |
Scenario analysis 8: alternative mortality estimates in population 1
Following the advice of a clinical expert who suggested that BAD might be associated with increased mortality, compared with the general population, we ran a scenario in which excess mortality was included in the model. We used the SMR of 1.52 from Canavan et al. ,68 as in the Crohn’s disease population analyses.
Scenario analysis 9: alternative probability of response to diarrhoea-specific treatment in population 2
In the base-case scenario, the probability of responding to diarrhoea-specific treatment (without BAS) in the Crohn’s disease population was 40% for the no-SeHCAT strategy. For SeHCAT-negative patients, a mean response of 42% was estimated; for the BAS trial-of-treatment strategy, we assumed a mean response of 41% (as the only possible value between the other two). The impact of assuming different response rates to diarrhoea-specific treatment on the cost-effectiveness results was studied in the scenarios described in Table 40.
| Scenario | Response to diarrhoea treatment without BAS (%) | ||
|---|---|---|---|
| No SeHCAT | SeHCAT 15% | BAS TOT | |
| Base-case scenario | 40 | 42 | 41 |
| Scenario 1 | 42 | 42 | 42 |
| Scenario 2 | 70 | 42 | 70 |
Scenario analysis 10: alternative probability of a positive SeHCAT result and response to bile acid sequestrant treatment in population 2
The unconditional response to BAS treatment in the SeHCAT 15% strategy is obtained by multiplying the probability of testing positive by the probability of response to BAS given a positive test. In the base-case scenario, these probabilities were estimated from our systematic literature review as 0.55, 0.89 and 0.33 for SeHCAT 15%, response to BAS given a positive SeHCAT result and response to BAS trial of treatment, respectively. We explored scenarios in which, in the SeHCAT 15% strategy, the probability of testing positive and the probability of response to BAS were changed at the same time according to the limits of their CIs, in a form of worst-case and best-case scenarios. The probability of response to BAS trial of treatment was estimated to be 0.33 from clinical experts’ answers. We explored scenarios in which this probability was decreased to 0.23 and increased to 0.5. These scenarios are summarised in Table 41.
| Scenario | Probability | ||
|---|---|---|---|
| SeHCAT 15% positive | Response to BAS given a positive SeHCAT test | Response to BAS TOT | |
| Base-case scenario | 0.55 | 0.89 | 0.33 |
| Scenario 1 | 0.39 | 0.67 | 0.33 |
| Scenario 2 | 0.71 | 1.00 | 0.33 |
| Scenario 3 | 0.55 | 0.89 | 0.23 |
| Scenario 4 | 0.55 | 0.89 | 0.50 |
Scenario analysis 11: alternative distribution of bile acid sequestrant treatment in population 2
Based on clinical experts’ responses, in the base-case scenario it was assumed that, in the SeHCAT 15% strategy, 63% of patients started with colestyramine and 37% with colesevelam. In the BAS trial-of-treatment strategy, these values were 58% and 42% for colestyramine and colesevelam, respectively. We explored scenarios in which all patients were treated with colestyramine, in which all patients were treated with colesevelam and in which there was no difference in BAS distribution between the SeHCAT 15% and the BAS trial-of-treatment strategies (one scenario assumed a 63/37 proportion and the other one a 58/42). Note that, in terms of response, it was not possible to distinguish between the type of BAS. Therefore, these scenarios had an effect on costs and utilities only because these were different for colestyramine and colesevelam.
Scenario analysis 12: alternative health-state utilities in population 2
We explored the same scenarios as those defined for population 1. Thus, a scenario in which patients who respond to colestyramine (BAS) treatment receive 100% of the utility gain (instead of the 75% in the base-case scenario) and a scenario in which no age adjustment of the utility values was assumed.
Scenario analysis 13: alternative cost inputs in population 2
As explained for population 1, a pragmatic approach was taken to define cost-related scenario analyses and all costs were varied by 20%.
Scenario analysis 14: alternative transition probabilities in population 2
The same approach described for population 1 was followed for population 2. Thus, we explored several scenarios in which patients were allowed to experience relapse in all models. We increased the probability of relapse to assess how this would affect the results. In one scenario, the possibility to experience remission was also included in the analysis. The scenarios conducted are summarised in Table 42.
| Scenario | Transitions, P[D → ND], P[ND → D] | |
|---|---|---|
| BAS models | Diarrhoea-specific treatmenta model | |
| Base-case scenario | 0, 0 | 0, 0.00575 |
| Scenario 1 | 0, 0.00575 | 0, 0.00575 |
| Scenario 2 | 0.00575, 0.00575 | 0.00575, 0.00575 |
| Scenario 3 | 0, 0.00575 × 2 | 0, 0.00575 × 2 |
| Scenario 4 | 0, 0.00575 × 5 | 0, 0.00575 × 5 |
Scenario analysis 15: alternative mortality estimates in population 2
In the base-case analysis, we used the SMR 1.52, estimated from Canavan et al. 68 In this scenario analysis, we explored the impact of mortality on the cost-effectiveness results by using the limits of the CI reported by Canavan et al. 68 (i.e. 95% CI 1.32 to 1.74).
Results of the cost-effectiveness analyses
In this section, we summarise the cost-effectiveness results of the three strategies per population. Long-term results are presented as incremental cost-effectiveness ratios (ICERs) estimated as costs per additional QALY gained in a full incremental analysis fashion. Short-term results (first 6 months) focused on the percentage of response to treatment and, for population 1, also the percentage of avoided colonoscopies. Given the uncertainties in the cost-effectiveness evidence described previously, many assumptions had to be made to make it possible to perform the cost-effectiveness analyses. Assessing the impact of these assumptions on the cost-effectiveness results indicated that a large number of scenarios had to be run. In this section, we focus on the scenarios that had the largest impact on the base-case ICERs. Full results, for all scenarios, are presented in Appendix 6, Tables 64–84.
Results of the base-case analysis, population 1
The results of the base-case analysis for population 1 are shown in Table 43. It can be seen that the BAS trial-of-treatment strategy was dominated by the SeHCAT 15% strategy (the latter provided more QALYs at lower costs). Therefore, the relevant comparison for the ICER calculation was SeHCAT 15% versus no SeHCAT, for which the ICER was £9688, which is below the commonly used threshold of £20,000 per QALY gained. The SeHCAT 15% strategy was estimated to provide 0.23 additional QALYs at an incremental cost of £2236, compared with the no-SeHCAT strategy. The base-case analysis also revealed that, in the short term, the SeHCAT 15% strategy is the one with more colonoscopies avoided per patient (65%) and with the highest response rate for any type of medication (68%), but that these come at the highest initial costs (£786), because of the costs of the SeHCAT test. The cost per colonoscopy avoided was the lowest for the SeHCAT 15% strategy (£786 ÷ 0.65 = £1209) and the cost per response was the lowest for the BAS trial-of-treatment strategy (£507 ÷ 0.65 = £780). Life-years were 19.96 for all three strategies. Because no difference in mortality across strategies was assumed in the model, the same life-years were expected to be estimated for the three strategies.
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
Results of the secondary analysis, population 1
As explained previously, the secondary analysis was based on the assumption that patients had undergone colonoscopy before entering the model. Thus, in practice, this scenario was run by removing all colonoscopy branches from Figure 3, that is by setting the probability of colonoscopy equal to 0.
The results of the secondary analysis for population 1 are summarised in Table 44. The SeHCAT 15% strategy was estimated to provide the highest QALYs at the highest costs, but, unlike the base-case analysis, the BAS trial-of-treatment strategy was no longer dominated by the SeHCAT 15% strategy. We observed that the ICER of BAS trial of treatment versus no SeHCAT and the ICER of SeHCAT 15% versus BAS trial of treatment are close to (but <) the £20,000 per QALY threshold. The secondary analysis also showed that, in the short term, the SeHCAT 15% strategy was the one with the highest response rate for any type of medication (67%), but that this comes at the highest initial costs (£553), because of the costs of the SeHCAT test. The cost per response was the highest for the SeHCAT 15% strategy (£553 ÷ 0.67 = £825). For the other two strategies, this cost was nearly the same: £134 for no SeHCAT and £135 for BAS trial of treatment. Life-years (not shown in Table 44) were also 19.96 for all strategies, as in the base-case analysis, as no changes in mortality were assumed.
| Strategy | Colonoscopy avoideda | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | NA | 44 | 59 | 13.8026 | 374 | |||
| BAS TOT | NA | 63 | 85 | 13.9825 | 3767 | 0.1799 | 3393 | 18,860 |
| SeHCAT 15% | NA | 67 | 553 | 14.0408 | 4922 | 0.0583 | 1115 | 19,125 |
Results of the probabilistic sensitivity analyses, population 1
The base-case PSA cost-effectiveness results can be seen in Table 45. These aligned well with the deterministic results; the BAS trial-of-treatment strategy was dominated by the SeHCAT 15% strategy and the ICER of SeHCAT 15% versus no SeHCAT was £9661.
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | 26 | 46 | 560 | 13.8236 | 4687 | |||
| BAS TOT | 37 | 66 | 564 | 14.0151 | 7431 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 826 | 14.0623 | 6993 | 0.2387 | 2306 | 9661 |
The cost-effectiveness plane and cost-effectiveness acceptability curves (CEACs) resulting from the PSA are shown in Figures 6 and 7, respectively. Note that the cost-effectiveness plane shows the results of pairwise comparisons versus the no-SeHCAT strategy only. It can be seen that the vast majority of the simulations are in the eastern quadrants, in which both BAS trial of treatment and SeHCAT 15% are more effective than no SeHCAT. Most of the simulations are in the north-eastern quadrant of the cost-effectiveness plane, where BAS trial of treatment and SeHCAT 15% are also more costly than no SeHCAT. The CEACs show that, at lower values of the threshold ICER, no SeHCAT is the strategy with the largest probability of being cost-effective, given that its costs are the lowest. However, this probability rapidly decreases as the threshold ICER increases, and SeHCAT 15% becomes the strategy most likely to be deemed as cost-effective, with a 67% probability of being cost-effective at a threshold ICER of £20,000 per QALY gained, and a 73% probability at a threshold ICER of £30,000 per QALY gained.
FIGURE 6.
The cost-effectiveness plane from the PSA base-case results, population 1.
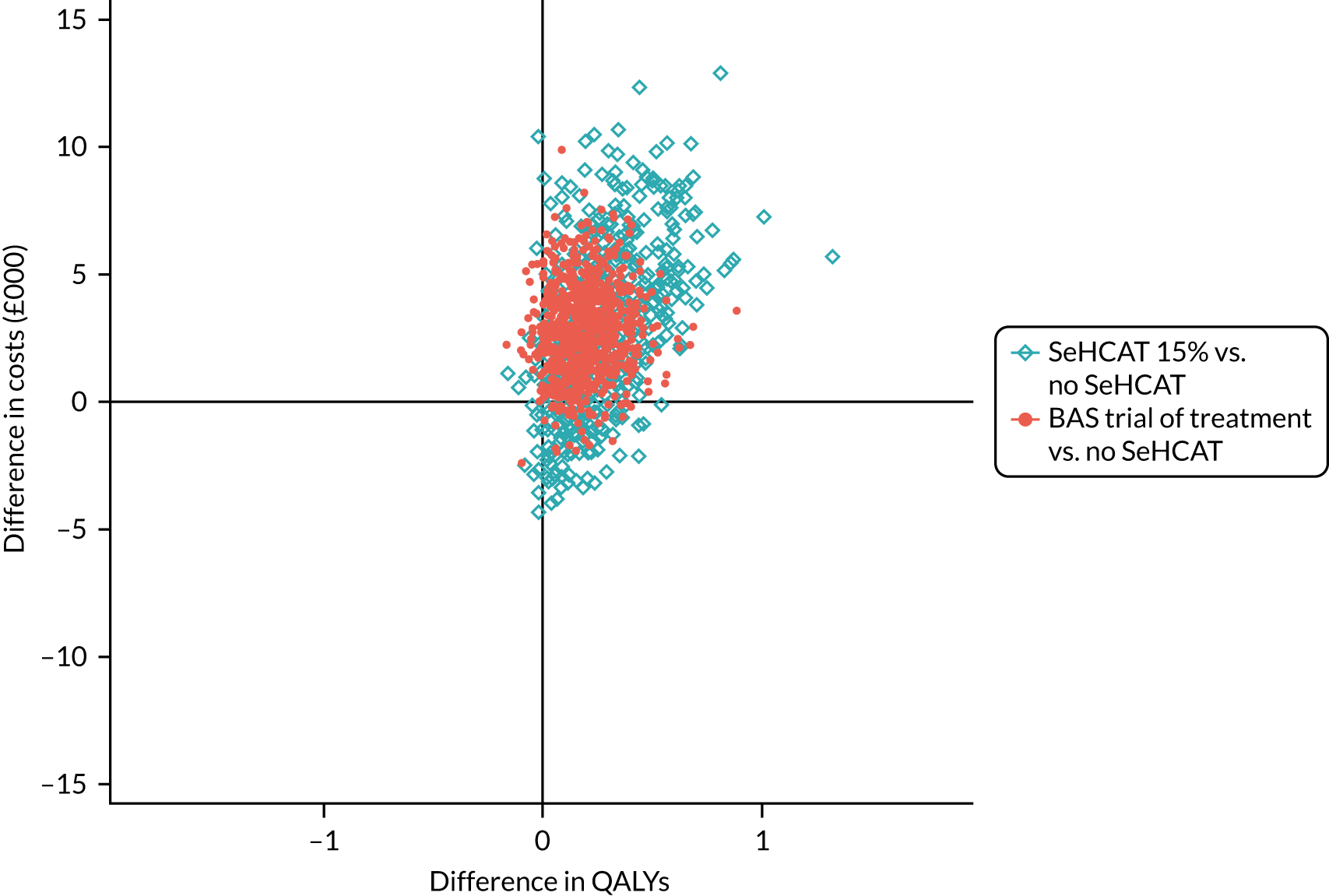
FIGURE 7.
The CEACs from the PSA base-case results, population 1.
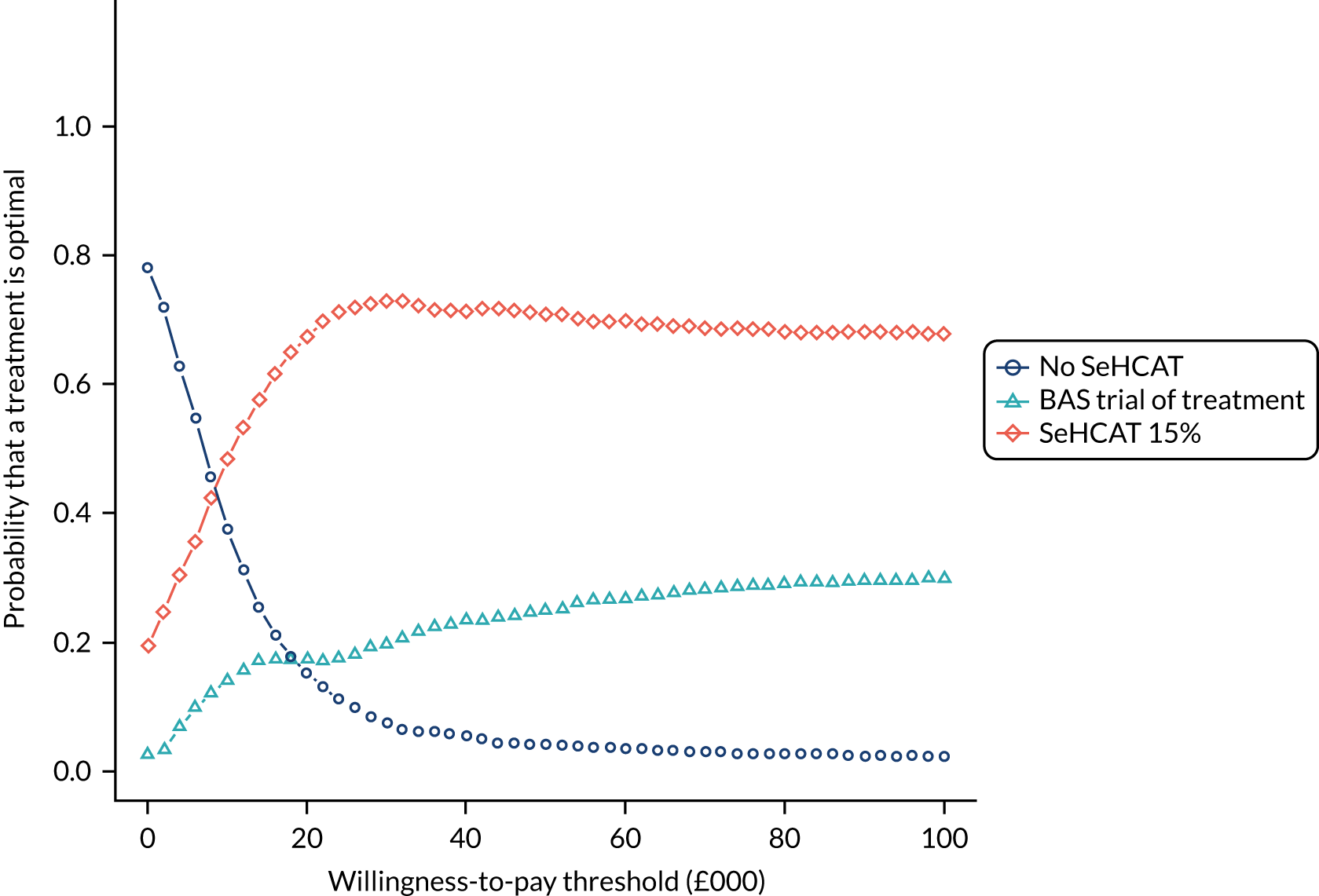
A PSA was also run under the assumptions of the secondary analysis, whereby no colonoscopy was included in the model. The PSA results can be seen in Table 46. In this case, they are also in line with the results of the deterministic analysis. The SeHCAT 15% strategy was estimated to provide the most QALYs at the highest costs, but no strategy was dominated. The ICER of BAS trial of treatment versus no SeHCAT and the ICER of SeHCAT 15% versus BAS trial of treatment were close to the £20,000 per QALY threshold, but, unlike the deterministic analysis, the ICER of SeHCAT 15% versus BAS trial of treatment was now above this threshold.
| Strategy | Colonoscopy avoideda | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | NA | 44 | 62 | 13.8021 | 374 | |||
| BAS TOT | NA | 63 | 143 | 13.9893 | 3806 | 0.1871 | 3432 | 18,343 |
| SeHCAT 15% | NA | 67 | 596 | 14.0539 | 5168 | 0.0647 | 1361 | 21,036 |
The cost-effectiveness plane and CEACs resulting from the PSA in the secondary analysis are shown in Figures 8 and 9, respectively. The cost-effectiveness plane shows, again, the results of pairwise comparisons versus no SeHCAT only. It can be seen that the vast majority of the simulations are in the north-eastern quadrant of the cost-effectiveness plane, in which both BAS trial of treatment and SeHCAT 15% are more effective and more costly than no SeHCAT. The difference in uncertainty between the two comparisons is remarkable, especially on the costs side. However, this can be explained by the distribution of costs, which is notably wider in the SeHCAT strategy. The CEACs show that, at lower values of the threshold ICER, no SeHCAT is the strategy with the largest probability of being cost-effective. This probability decreases as the threshold ICER increases; at a threshold ICER of approximately £20,000 per QALY gained, SeHCAT 15% and no SeHCAT have nearly the same probability of being cost-effective. At larger values of the threshold ICER, SeHCAT 15% is the strategy most likely to be considered cost-effective. In particular, at a threshold ICER of £30,000 per QALY gained, this probability is approximately 50%.
FIGURE 8.
The cost-effectiveness plane from PSA secondary analysis results (no colonoscopy), population 1.
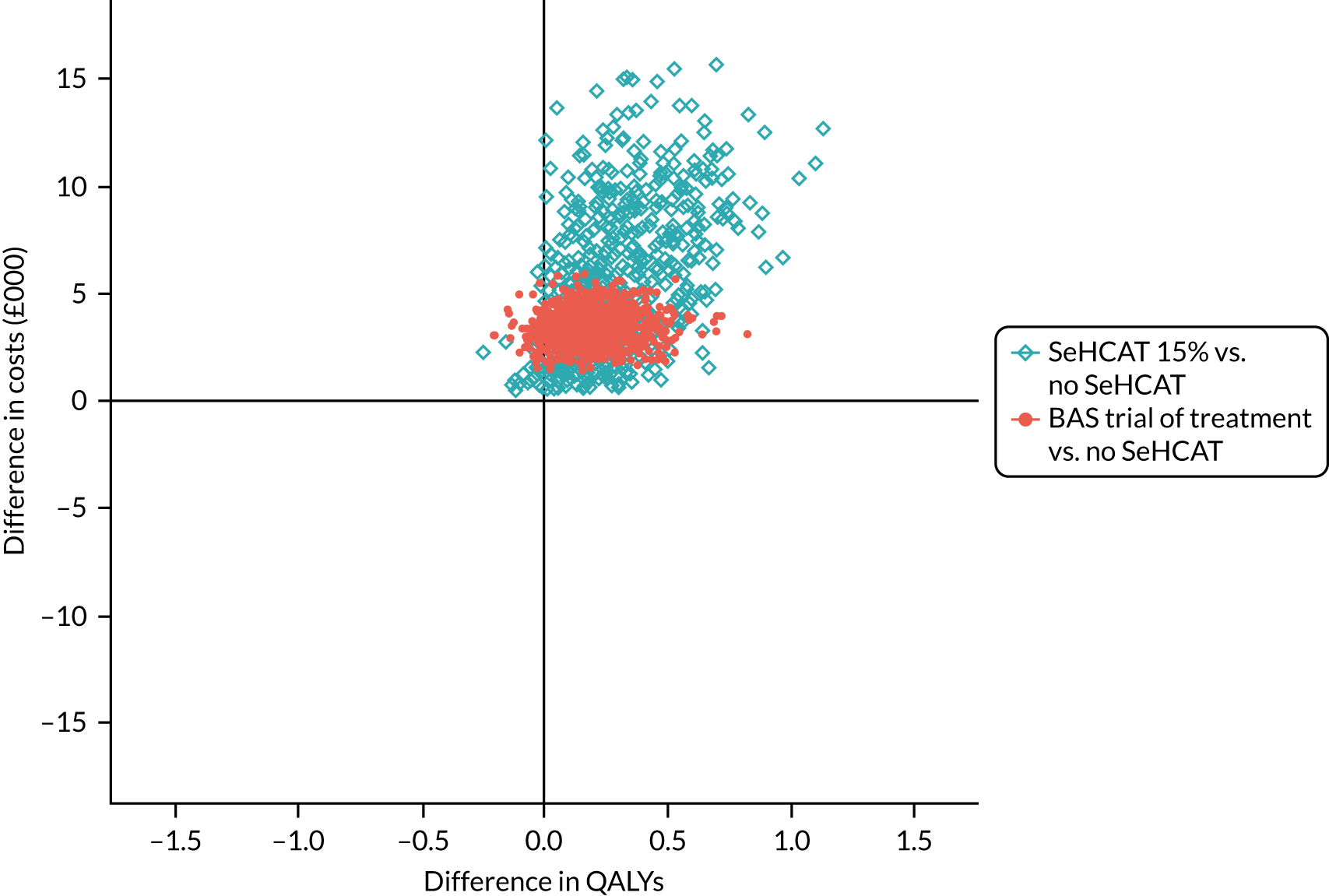
FIGURE 9.
The CEACs from PSA secondary analysis results (no colonoscopy), population 1.
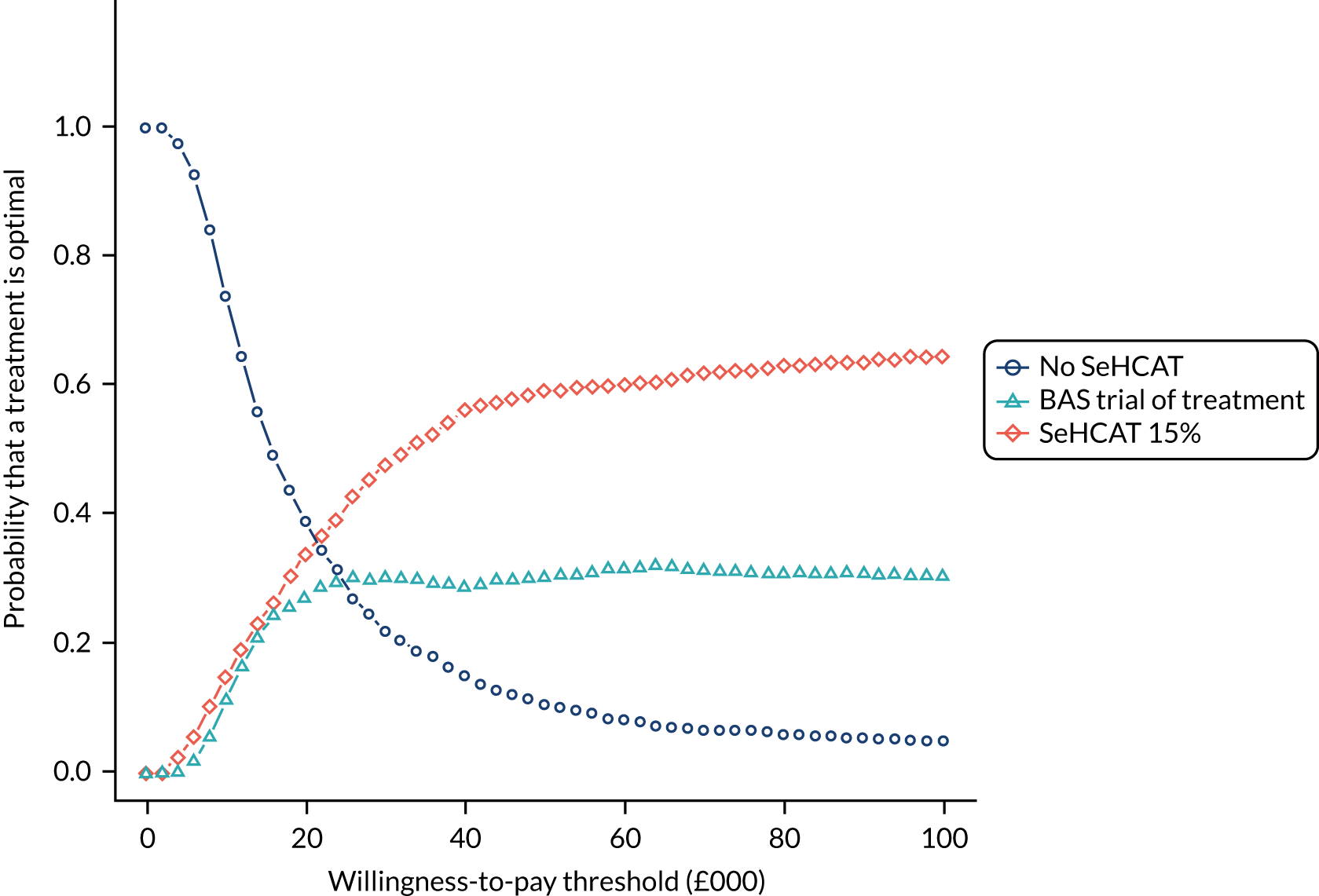
Results of the scenario analyses, population 1
Scenario analysis 1: alternative probability of undergoing colonoscopy in population 1
In the base-case analysis, the probability of undergoing colonoscopy (for all patients) in the no-SeHCAT strategy was 74%. In the SeHCAT 15% strategy, this probability was 49%, but only for patients with a negative SeHCAT result, or with a positive result but no response to BAS treatment. In the BAS trial-of-treatment strategy, the probability of undergoing colonoscopy was 90%, but only for patients not responding to BAS treatment. The impact of changing assumptions regarding colonoscopy on the cost-effectiveness results was partially investigated in the secondary analysis described previously, in which patients undergo colonoscopy before entering the model. In addition, the scenario in which the probability of undergoing colonoscopy was 100% in all strategies was explored in this section. As can be seen in Table 47, short-term costs increased, as expected, given that more patients underwent colonoscopy, but this resulted in a slightly higher response rate, mostly because IBD patients were more accurately identified with colonoscopy. The more responders, the higher the long-term QALYs and total costs. The SeHCAT 15% strategy was estimated to provide the most QALYs at the highest costs, but without dominating either of the other two strategies. The ICER of BAS trial of treatment versus no SeHCAT was £9136, and the ICER of SeHCAT 15% versus BAS trial of treatment was £21,140, which is above the £20,000 per QALY threshold. The analysis also revealed that, in the short term, the BAS trial-of-treatment strategy was the one with more colonoscopies avoided per patient (30%) and positive SeHCAT 15% was the one with the highest response rate for any type of medication (69%). The cost per colonoscopy avoided and the cost per response were the lowest for the BAS trial-of-treatment strategy (£1847 and £839, respectively).
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| Base-case scenario (no SeHCAT = 74%, SeHCAT 15% = 49%, BAS TOT = 90%) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| Colonoscopy scenario 1 (secondary analysis, no colonoscopy) | ||||||||
| No SeHCAT | NA | 44 | 59 | 13.8026 | 374 | |||
| BAS TOT | NA | 63 | 85 | 13.9825 | 3767 | 0.1799 | 3393 | 18,860 |
| SeHCAT 15% | NA | 67 | 553 | 14.0408 | 4922 | 0.0583 | 1115 | 19,125 |
| Colonoscopy scenario 2 (no SeHCAT = 100%, SeHCAT 15% = 100%, BAS TOT = 100%) | ||||||||
| No SeHCAT | 0 | 47 | 727 | 13.832 | 6210 | |||
| BAS TOT | 30 | 66 | 554 | 14.013 | 7863 | 0.181 | 1653 | 9136 |
| SeHCAT 15% | 29 | 69 | 1028 | 14.070 | 9069 | 0.057 | 1206 | 21,140 |
In summary, the scenario analyses results showed that, by changing assumptions regarding the probability of undergoing colonoscopy, the cost-effectiveness results also change. The base-case scenario illustrates a situation in which the SeHCAT 15% strategy dominates the BAS trial-of-treatment strategy and, compared with the no SeHCAT strategy, the ICER is well below the £20,000 per QALY threshold. In the secondary analysis, there was no dominance and both ICERs were slightly below the £20,000 per QALY threshold. In the scenario with 100% probability of colonoscopy, no strategy is dominated. The ICER of BAS trial of treatment versus no SeHCAT is considerably below the £20,000 per QALY threshold and the ICER of SeHCAT 15% versus BAS trial of treatment is marginally above the £20,000 per QALY threshold. Note that the probability of colonoscopy is incorporated in the model through three different parameters (one per strategy). Other combinations were not explored because the number of scenarios would become unmanageable in practice. It is obvious, but important to emphasise, that different combinations of these three parameters might result in different model outcomes. Therefore, it is important to determine in advance the plausibility of the assumptions made regarding these parameters to be able to focus on the scenarios that can be deemed as relevant in practice.
Scenario analysis 2: alternative probability of response to diarrhoea-predominant irritable bowel syndrome treatment in population 1
In the no-SeHCAT strategy, the probability of responding to IBS-D treatment (after colonoscopy ruled out IBD) was 46%. For SeHCAT-negative patients, a mean response of 56% was estimated, and for the BAS trial-of-treatment strategy, we assumed a mean response of 50%. Within each strategy, it was assumed that the probability of IBS-D treatment response was slightly lower among patients who did not undergo colonoscopy. These were estimated at 44%, 53% and 47% for the no SeHCAT, SeHCAT 15% and BAS trial-of-treatment strategies, respectively. The impact of assuming different response rates to IBS-D treatment on the cost-effectiveness results can be seen in Table 48. Note that changes in IBS-D treatment response rates do not affect the probability of colonoscopy nor the costs accrued during the first 6 months in the model (decision-analytic model). Therefore, in Table 48, the percentage of colonoscopies avoided and the initial costs were not included as these were the same as in the base-case analysis. As can be seen in Table 48, the more responders, the greater the number of long-term QALYs and the higher the total costs. The no-SeHCAT strategy is dominated or unlikely to be considered as cost-effective unless the response rate to IBS-D treatment is assumed to be larger than the overall response rate in the SeHCAT 15% or the BAS trial-of-treatment strategies. This can be seen in IBS-D scenario 4, in which the no-SeHCAT strategy became dominant, given that it is also the strategy with the lowest costs. However, this scenario is based on a IBS-D treatment response rate of 70%, which is likely to be unrealistic. The BAS trial-of-treatment strategy is more costly than the SeHCAT 15% strategy. Therefore, when the overall response to treatment is higher in the SeHCAT 15% strategy, BAS trial of treatment will be dominated. As shown in IBS-D scenarios 3 and 4, even if the overall response to treatment is higher in the BAS trial-of-treatment strategy, it does not imply that BAS trial of treatment will dominate or will be likely to be deemed as cost-effective, compared with SeHCAT 15%. In these two scenarios, the overall response rate was 1% higher for BAS trial of treatment, resulting in an ICER of £627,500. This scenario is already based on an equal response rate to IBS-D treatment for both strategies, which might be unrealistic. BAS trial of treatment might be deemed cost-effective, compared with the SeHCAT 15% strategy, only when its overall treatment response rate is much higher than in SeHCAT 15%, which again is likely to be unrealistic.
| Strategy | Responsea (%) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||
| Base-case scenariob | ||||||
| No SeHCAT | 47 | 13.8242 | 4720 | |||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| IBS-D scenario 1c | ||||||
| No SeHCAT | 50 | 13.8660 | 4728 | |||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.189 | 2228 | 11,788 |
| IBS-D scenario 2d | ||||||
| No SeHCAT | 50 | 13.8660 | 4728 | |||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.1890 | 2228 | 11,788 |
| BAS TOT | 69 | 14.0558 | 7458 | 0.0008 | 502 | 627,500 |
| IBS-D scenario 3e | ||||||
| No SeHCAT | 56 | 13.9323 | 4741 | |||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.1227 | 2215 | 18,052 |
| BAS TOT | 69 | 14.0558 | 7458 | 0.0008 | 502 | 627,500 |
| IBS-D scenario 4f | ||||||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | Dominated by no SeHCAT | ||
| No SeHCAT | 69 | 14.0892 | 4771 | |||
Scenario analysis 3: alternative probability of a positive SeHCAT result and response to bile acid sequestrant treatment in population 1
First, we considered scenarios in which, in the SeHCAT 15% strategy, the probability of testing positive and the probability of response to BAS were changed at the same time according to the limits of their CIs. Then we explored scenarios in which the probability of response to BAS trial of treatment was decreased and increased by 10%. In all scenarios, the no-SeHCAT strategy was the strategy providing fewer QALYs, but was also the least costly. This was, in general, because of its overall low response rate (47%), compared with the other two strategies (at least 61% for BAS trial of treatment in the most pessimistic scenario) (Table 49). Thus, dominance or cost-effectiveness between the SeHCAT 15% and the BAS trial-of-treatment strategies was determined basically depending on overall response to treatment. When overall response was the highest in the SeHCAT 15% strategy, it always dominated or extendedly dominated BAS trial of treatment. In the two scenarios in which BAS trial of treatment achieved the highest overall response (scenarios 1 and 4), the difference in QALYs with respect to SeHCAT 15% was small enough to result in ICERs equal to £272,969 and £919,167, respectively. These two scenarios are based on response rates to BAS treatment that are higher for the BAS trial-of-treatment strategy, which is likely to be unrealistic. In fact, in the base-case scenario, the response rate to BAS is nearly the same for the two strategies. This is in line with the assumption made in the previous assessment of SeHCAT,18 whereby, in the absence of evidence, it was assumed that response to BAS in the trial-of-treatment strategy was equivalent to the percentage of BAS responders in the SeHCAT 15% strategy.
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| Base-case scenario (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| BAS scenario 1 (SeHCAT positive = 0.357, BAS response | SeHCAT positive = 0.495, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| SeHCAT 15% | 60 | 63 | 819 | 14.0031 | 5702 | 0.1789 | 982 | 5489 |
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | 0.0064 | 1747 | 272,969 |
| BAS scenario 2 (SeHCAT positive = 0.555, BAS response | SeHCAT positive = 0.760, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Extendedly dominated by SeHCAT 15% | ||
| SeHCAT 15% | 72 | 74 | 748 | 14.1156 | 8423 | 0.2914 | 3703 | 12,708 |
| BAS scenario 3 (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.20) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 28 | 61 | 566 | 13.9644 | 6857 | Extendedly dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2307 | 2236 | 9692 |
| BAS scenario 4 (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.40) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2307 | 2236 | 9692 |
| BAS TOT | 46 | 70 | 446 | 14.0561 | 8059 | 0.0012 | 1103 | 919,167 |
Scenario analysis 4: alternative distribution of bile acid sequestrant treatment in population 1
In the base-case scenario, it was assumed that, in the SeHCAT 15% strategy, 50% of patients started with colestyramine and 50% with colesevelam; in the BAS trial-of-treatment strategy, these values were 85% and 15%, respectively. We explored scenarios in which all patients were treated with colestyramine, in which all patients were treated with colesevelam and in which there was no difference in BAS distribution between the SeHCAT 15% and the BAS trial-of-treatment strategies (one scenario assumed a 50/50 proportion and the other one an 85/15). None of these scenarios differed significantly from the base-case scenario; in all of them, BAS trial of treatment was dominated by SeHCAT 15% and the ICER of SeHCAT 15% versus no SeHCAT ranged from £5217 to £13,405. Full results are shown in Appendix 6, Table 71.
Scenario analysis 5: alternative health-state utilities in population 1
In all utility scenarios run, BAS trial of treatment was dominated by SeHCAT 15% and the ICER of SeHCAT 15%, compared with no SeHCAT, ranged from £8633 to £12,265. Assuming that patients responding to colestyramine received the full utility benefit of not experiencing diarrhoea increased the incremental QALYs gained in the SeHCAT 15% versus no SeHCAT comparison, resulting in a decrease in the ICER of approximately £800. Using different sources of utility values for IBS, selecting the values from either Mearin et al. 61 or Spiegel et al. 60 instead of the pooled values, affected not only the health-state utility values themselves, but the implied utility decrement for diarrhoea. Using the utilities from Mearin et al. 61 increased the effective decrement in utility due to diarrhoea (0.071, vs. the base-case scenario result of 0.064). This resulted in larger incremental QALYs and a drop of approximately £1000 in the ICER. Using the utilities from Spiegel et al. 60 decreased the effective decrement in utility due to diarrhoea (0.05, vs. the base-case scenario result of 0.064), which increased the ICER by approximately £2600 per QALY gained. Removing the age adjustment on utilities resulted in a small decrease in the ICER of approximately £600. Full results are shown in Appendix 6, Table 72.
Scenario analysis 6: alternative cost inputs in population 1
In all cost scenarios explored, the BAS trial-of-treatment strategy was dominated by the SeHCAT 15% strategy. In the comparison between SeHCAT 15% and no SeHCAT, the ICER ranged from £6079 to £13,297. The cost elements that had the most influence on the ICER were the cost of BAS treatment, followed by the cost of IBD medication and the cost of the SeHCAT test. All other cost elements had a fairly small impact on the ICER, with an impact of < £200. Full results are shown in Appendix 6, Table 73.
Scenario analysis 7: alternative transition probabilities in population 1
In all transition probability scenarios run, results were very similar to the base-case scenario, with the BAS trial-of-treatment strategy being dominated by the SeHCAT 15% strategy, and all ICERs for comparison between SeHCAT 15% and no SeHCAT ranging from £8658 to £9688 (the base-case ICER). Thus, even multiplying by five the probability of relapse, results did not practically change, possibly because this increase in relapse was included in all strategies. To observe a larger impact, the difference in transition probabilities should be different per strategy, which is likely to be unrealistic. Full results are shown in Appendix 6, Table 74.
Scenario analysis 8: alternative mortality estimates in population 1
We ran a scenario in which excess mortality was included in the model using a SMR equal to 1.52, estimated from Canavan et al. ,68 as in the Crohn’s disease analyses. This scenario resulted in fewer QALYs and lower costs for all strategies as a consequence of a reduced life expectancy. However, the ICER was decreased by only £70. Full results are shown in Appendix 6, Table 75.
Results of the base-case analysis, population 2
The results of the base-case analysis for population 2 are shown in Table 50, where it can be seen that the no-SeHCAT strategy was dominated by the BAS trial-of-treatment strategy. Therefore, the relevant comparison for the ICER calculation is SeHCAT 15% versus BAS trial of treatment, for which the ICER was £1127. The SeHCAT 15% strategy was estimated to provide 0.1071 additional QALYs at an incremental cost of £185, compared with BAS trial of treatment. The base-case analysis also indicated that, in the short term, the SeHCAT 15% strategy is the one with the highest response rate for any type of medication (71%), but that this comes at the highest initial costs (£1061), owing to the inclusion of the SeHCAT test. The cost per response is the lowest for the BAS trial-of-treatment strategy (£756 ÷ 0.6 = £1260). Life-years (not shown in Table 50) were 18.696 for all strategies. As no difference in mortality across strategies was assumed in the model, the same life-years were expected to be estimated for the three strategies.
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
Results of the probabilistic sensitivity analyses, population 2
The base-case PSA results for population 2 can be seen in Table 51. These aligned well with the deterministic results, but now the SeHCAT 15% strategy is dominant because the lowest total costs are estimated for this strategy. In general, PSA costs are higher than the deterministic ones. This can be explained by the skewness of the triangular distributions chosen to parameterise the cost inputs of the model.
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| No SeHCAT | 40 | 1180 | 12.6857 | 15,686 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 895 | 12.9006 | 14,880 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1172 | 13.0084 | 14,795 | |||
The cost-effectiveness plane and CEACs resulting from the PSA are shown in Figures 10 and 11, respectively. Note the cost-effectiveness plane shows the results of pairwise comparisons versus no SeHCAT. It can be seen that all simulations (except one) are in the eastern quadrants, in which both BAS trial of treatment and SeHCAT 15% are more cost-effective than no SeHCAT. Approximately half of the simulations are in the south-eastern quadrant of the cost-effectiveness plane, where BAS trial of treatment and SeHCAT 15% are dominant, compared with no SeHCAT. The CEACs show that, for any positive value of the threshold ICER, SeHCAT 15% is the strategy with the largest probability of being cost-effective. In particular, at a threshold ICER of £20,000 per QALY gained, the estimated probability of being cost-effective is 89%; at a threshold ICER of £30,000 per QALY gained, it is 92%.
FIGURE 10.
Cost-effectiveness plane from base-case PSA results, population 2.
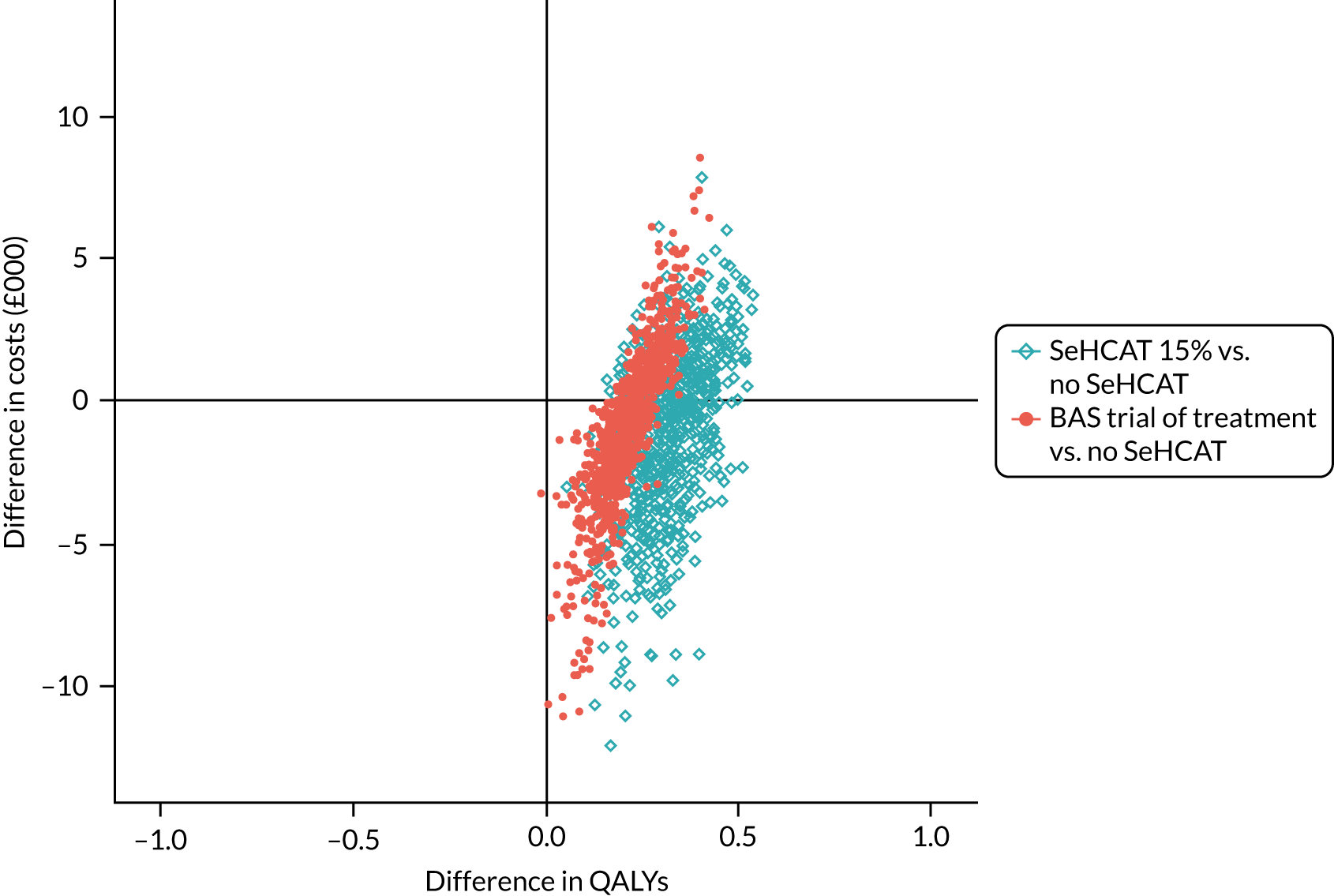
FIGURE 11.
The CEACs from the base-case PSA results, population 2.
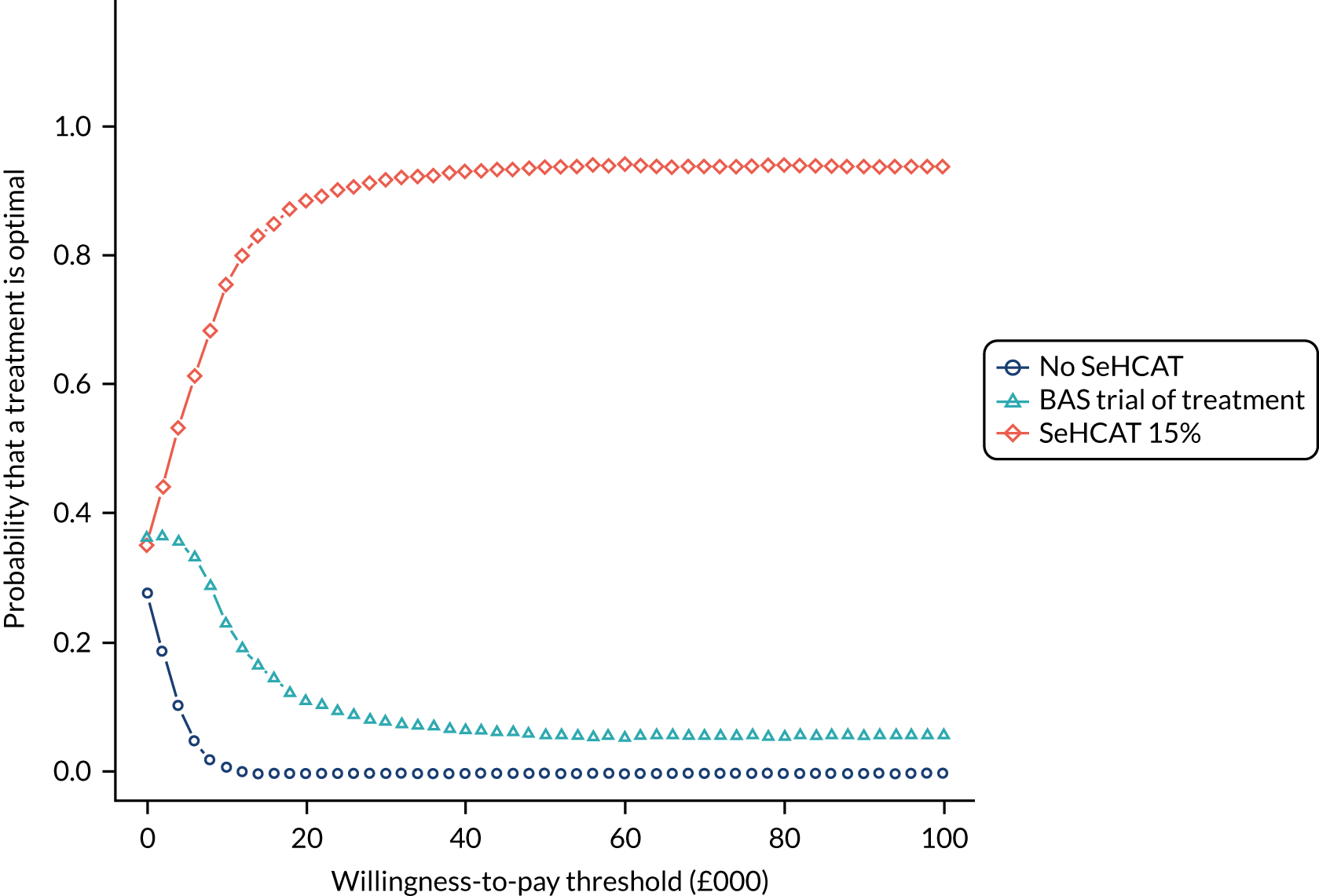
Results of the scenario analyses, population 2
Scenario analysis 9: alternative probability of response to diarrhoea-specific treatment in population 2
In the no-SeHCAT strategy the probability of responding to diarrhoea-specific treatment was 40%. For patients with a negative SeHCAT test result, a mean response of 42% was estimated, and for the BAS trial-of-treatment strategy, we assumed a mean response of 41% (as the only possible value between the other two). The impact on the cost-effectiveness results of assuming different response rates to diarrhoea-specific treatment can be seen in Table 52. The more responders, the greater the number of long-term QALYs and the higher the total costs. The no-SeHCAT strategy was dominated by either the SeHCAT 15% or the BAS trial-of-treatment strategy. The no-SeHCAT strategy was also more costly than the other two strategies because BAS treatments are less costly than the diarrhoea-specific treatment for patients responding to treatment in this population. Therefore, even when an unrealistically high response rate for no SeHCAT was assumed in scenario 2, the no-SeHCAT strategy was still dominated because of its higher costs. Scenario 3 shows an interesting situation. The overall response to treatment is 9% higher in the BAS trial-of-treatment strategy (possibly unrealistic), but the ICER is £73,684. This high ICER is mostly caused by the difference in costs: in the BAS trial-of-treatment strategy there are more responders to diarrhoea-specific treatment than in the SeHCAT 15% strategy, and such treatment is more costly than BAS. Furthermore, because in the SeHCAT 15% strategy there are more non-responders, and medication for these patients is assumed to be loperamide only, the costs for non-responders are very low.
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| Base-case scenario (no SeHCAT = 40%, SeHCAT 15% = 42%, BAS TOT = 41%) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Diarrhoea treatment without BAS scenario 1 (no SeHCAT = 42%, SeHCAT 15% = 42%, BAS TOT = 42%) | |||||||
| No SeHCAT | 42 | 1052 | 12.7059 | 15,078 | Dominated by BAS TOT | ||
| BAS TOT | 61 | 756 | 12.9075 | 14,171 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| Diarrhoea treatment without BAS scenario 2 (no SeHCAT = 70%, SeHCAT 15% = 42%, BAS TOT = 70%) | |||||||
| No SeHCAT | 70 | 1052 | 12.9809 | 24,295 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| BAS TOT | 80 | 756 | 13.0925 | 20,373 | 0.0847 | 6241 | 73,684 |
Scenario analysis 10: alternative probability of a positive SeHCAT result and response to bile acid sequestrant treatment in population 2
As with population 1, we considered scenarios in which, in the SeHCAT 15% strategy, the probability of testing positive and the probability of response to BAS were changed at the same time according to limits of their CIs. Then we explored scenarios in which the probability of response to BAS trial of treatment was decreased and increased. In all scenarios, the no-SeHCAT strategy was always dominated by one of the other two strategies, which was dominating overall, as can be seen in Table 53. Thus, dominance between SeHCAT 15% and BAS trial-of-treatment strategies was determined basically depending on overall response to treatment, with the strategy with the highest response dominating the other one. An exception to this was observed in scenario 4, in which the response rate was 71% for SeHCAT 15% and 70% for BAS trial of treatment, but BAS trial of treatment was the dominant strategy. This was because, in the base-case scenario, more patients are treated with colesevelam in the BAS trial-of-treatment strategy, and are assumed to get the full utility associated with not having diarrhoea. Thus, in the long term, this resulted in more QALYs than the SeHCAT 15% strategy, in which more patients are treated with colestyramine and, therefore, are not getting the full utility of not having diarrhoea. Note, however, that, as mentioned for population 1, this scenario is based on a response rate to BAS treatment that is higher for the BAS trial-of-treatment strategy, which is likely to be unrealistic.
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| Base-case scenario (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.339) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| BAS scenario 1 (SeHCAT positive = 0.39, BAS response | SeHCAT positive = 0.67, BAS TOT response = 0.33) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| SeHCAT 15% | 58 | 1282 | 12.8700 | 14,893 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| BAS scenario 2 (SeHCAT positive = 0.71, BAS response | SeHCAT positive = 1, BAS TOT response = 0.33) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 83 | 848 | 13.1411 | 13,396 | |||
| BAS scenario 3 (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.23) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 55 | 852 | 12.8399 | 14,190 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| BAS scenario 4 (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.5) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | Dominated by BAS TOT | ||
| BAS TOT | 70 | 586 | 13.0090 | 13,511 | |||
Scenario analysis 11: alternative distribution of bile acid sequestrant treatment in population 2
In the base-case scenario it was assumed that, in the SeHCAT 15% strategy, 67% of patients started with colestyramine and 37% with colesevelam; in the BAS trial-of-treatment strategy, these values were 58% and 42%, respectively. As with population 1, we explored scenarios in which all patients were treated with colestyramine, in which all patients were treated with colesevelam and in which there was no difference in BAS distribution between the SeHCAT 15% and the BAS trial-of-treatment strategies (one scenario assumed a 67/37 proportion and the other one a 58/42). When all patients were treated with colestyramine, SeHCAT 15% was dominant. When all patients were treated with colesevelam, no strategy dominated, but the relevant ICERs were both below the £20,000 threshold (BAS trial of treatment vs. no SeHCAT = £4581; SeHCAT 15% vs. BAS trial of treatment = £9009). When the BAS distribution was mixed and equal in both strategies, the no-SeHCAT strategy was dominated and the ICERs of SeHCAT 15% versus BAS trial of treatment were £2608 and £3030 for scenarios 3 and 4, respectively. Full results are shown in Appendix 6, Table 80.
Scenario analysis 12: alternative health-state utilities for population 2
In all utility scenarios tested, the no-SeHCAT strategy was dominated by both BAS trial of treatment and SeHCAT 15%. When comparing SeHCAT 15% with BAS trial of treatment, the ICER was always < £3000 per QALY gained. Full results are shown in Appendix 6, Table 81.
Scenario analysis 13: alternative cost inputs for population 2
Increasing the cost of BAS treatment by 20% increased the costs of the BAS trial-of-treatment and SeHCAT 15% strategies, such that they no longer dominated the no-SeHCAT strategy. However, the largest ICER obtained for the comparison of SeHCAT 15% versus BAS trial of treatment was £5143, thus well below the commonly used threshold of £20,000 per QALY gained. Decreasing the cost of Crohn’s disease antidiarrhoea medication by 20% also prevented the no-SeHCAT strategy from being dominated by either alternative strategy, but again the largest ICER, for the comparison of SeHCAT 15% versus BAS trial of treatment, was £5647. However, increasing the cost of Crohn’s disease antidiarrhoea medication by 20% resulted in the highest costs being observed again for the no-SeHCAT strategy, and SeHCAT 15% was the dominant strategy in this scenario. SeHCAT 15% was also the dominant strategy in the scenario in which the cost of BAS treatment was decreased by 20%. ICERs were < £6000 per QALY gained in all scenarios. Full results are shown in Appendix 6, Table 82.
Scenario analysis 14: alternative transition probabilities in population 2
In all transition probability scenarios run, relapse was included in the BAS Markov models (in the base-case scenario, relapse was possible only in the non-BAS model).
Including a probability of relapsing in the BAS strategies resulted in lower costs, because the costs associated with the diarrhoea health state are very low (loperamide only). Thus, despite the loss in QALYs, SeHCAT 15% was dominant in all scenarios except the last one, in which the probability of relapse was the highest explored (five times higher than in the base-case scenario). In this scenario, all strategies resulted in lower costs and fewer QALYs than in the base-case scenario, but the ICER was nearly equal (£1459). Full results are shown in Appendix 6, Table 83.
Scenario analysis 15: alternative mortality estimates in population 2
Replacing the SMR (1.52) from Canavan et al. 68 with the limits of its CI (1.32 to 1.74) had a minimal impact on the cost-effectiveness. Using the lower limit of the SMR increased life-years to 19.13 (18.70 in the base-case scenario) and increased costs and QALYs for all strategies. Likewise, using the upper limit of the SMR increased life-years to 18.25 and decreased costs and QALYs for all strategies. In both scenarios, no SeHCAT was still dominated and the ICER remained practically unchanged. Full results are shown in Appendix 6, Table 84.
Validation
Validation of the health economic models was undertaken by one of the model developers. Validation was guided by the health economic model validation-specific tools: Assessment of the Validation Status of Health-Economic decision models (AdViSHE)69 and the TECHnical VERification (TECH-VER) checklist. 70 A filled-in version of both tools can be found in the files included in the health economic model developed for this project.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
The evidence base relating to the use of SeHCAT testing among adults presenting with chronic diarrhoea with unknown cause (FD), or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD), and among adults presenting with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection (i.e. people with suspected secondary BAD) has not changed substantively since our previous systematic review,18 which was conducted to support the development of DG7. 2 The search yield increased considerably in this update assessment; searches of bibliographic databases (from inception to November 2020) identified a total of 5518 unique references, after deduplication against the EndNote library from our previous systematic review,18 compared with the total of 4240 unique references identified for the period from inception to April 2012 covered by the searches conducted for our previous systematic review. 18 However, despite the large number of records retrieved, only nine new studies were identified25–33 that met the inclusion criteria for this assessment. Most (eight out of nine) of these studies were published as conference abstracts only. 25–29,31–33 In addition, six publications,50–54,71 which were included in our previous systematic review,18 did not meet the inclusion criteria for this assessment. This current assessment includes a total of 25 publications relating to 24 studies, compared with the 24 publications relating to 21 studies included in our previous systematic review. 18
No RCTs, CCTs or observational comparative studies that met the inclusion criteria for this assessment (see Chapter 3, Inclusion and exclusion criteria) were identified. Similarly, no multivariable regression models were identified with response to treatment with BAS as the dependent variable and index test (SeHCAT) result (continuous or categorical) considered as one of the independent variables. Finally, no new predictive accuracy studies (studies that reported sufficient data to support the calculation of the sensitivity and specificity of the SeHCAT test to predict response to treatment with BAS) were identified. All of the nine new studies included in this review25–33 were of the lowest level of evidence eligible for inclusion; these were observational studies that reported some outcome data for patients treated with BAS, whereby only those patients with a positive SeHCAT test were offered treatment with BAS.
All 247,25–35,37–48 of the studies included in this assessment provided some data for population 1 and one study7 also provided data on population 2.
Three studies,40,43,44 all of which were included in our previous assessment report,18 conducted for DG7,7 provided limited data on the accuracy of the SeHCAT test for predicting response to treatment with BAS for population 1. Merrick et al. 40 reported sufficient data to allow the calculation of the performance of the SeHCAT test for predicting treatment response at two 7-day retention thresholds (< 8% and ≤ 15%). The estimated sensitivity of the SeHCAT test in predicting a positive response to treatment with colestyramine, using the < 15% threshold (commonly used in UK clinical practice),4,72 was 100% (95% CI 54.1% to 100%) and the corresponding specificity estimate was 91.2% (95% CI 76.3% to 98.1%). 40 These results would appear to indicate that the use of the SeHCAT test, with a 15% threshold, could identify patients with IBS-D who may benefit from treatment with BAS. However, it should be noted that the CIs around the sensitivity estimate were wide and, although all 31 patients with a negative SeHCAT test result were classified as true negatives, this assessment was based on long-term follow-up; none of the patients with a negative SeHCAT test result, at the > 15% retention threshold, exhibited evidence of small bowel disease and none of these patients developed evidence of small bowel disease during follow-up. Simple conservative treatment improved or resolved most symptoms. 40 None of these 31 patients received treatment with colestyramine; therefore, it remains uncertain whether or not any of these patients could have benefited from treatment with BAS. The remaining two studies43,44 provided data for 7-day SeHCAT retention thresholds of 5% and 8%.
Eight studies reported information about the rate of positive response to treatment with BAS, using a threshold of < 15% or ≤ 15%, for population 1. 27,29,34,42,45–48 The median proportion of patients with a positive SeHCAT test result who received treatment with BAS was 86% (range 70–100%) and the median response rate was 68% (range 38–86%). The equivalent data from the predictive accuracy study by Merrick et al. 40 indicated a treatment response rate of 67% among patients with 7-day SeHCAT retention values of ≤ 15%; in this study, 9 out of 12 (75%) patients with SeHCAT retention values of ≤ 15% received treatment with colestyramine. Eleven studies reported information about the rate of positive response to treatment with BAS, using a threshold of < 10% or ≤ 10%. 7,26,31,32,34,35,37,38,41,42,48 The median proportion of patients with a positive SeHCAT test result who received treatment with BAS was 100% (range 52–100%) and the median response rate was 85% (range 67–100%).
The single study that reported information about response to treatment with BAS for population 2 provided only limited information about response rates among patients with a positive SeHCAT test result (7-day retention value of < 10%) who were treated with colestyramine or colestipol. 7 Only 9 out of 24 patients with a positive SeHCAT test result received treatment with BAS and the numbers receiving each drug were not reported; eight out of nine (89%) patients treated with BAS responded positively. 7
Cost-effectiveness
We have assessed the cost-effectiveness of SeHCAT testing in the two populations described in the previous section. For both populations, the cost-effectiveness of SeHCAT compared with no SeHCAT and compared with trial of treatment with BAS was assessed. For the SeHCAT option, only the strategy based on the 15% cut-off point was included in the cost-effectiveness analyses for both populations. The main reason for this was that, in the clinical expert elicitation exercise to inform parameters for which data are lacking (the majority of parameters included in the model), all clinical experts consulted provided estimates for the 15% cut-off value only.
For each population, the following two models were combined:
-
A short-term decision-analytic model reflecting the diagnostic pathway and initial response to treatment (assumed to be the first 6 months).
-
A long-term (Markov) model that estimates the lifetime costs and effects for patients receiving subsequent treatment. The Markov model is parameterised according to treatment; thus, in practice, there is one Markov model for each type of medication included in the analyses [i.e. BAS (colestyramine and colesevelam), and IBS-D, IBD and diarrhoea medication for Crohn’s disease patients].
The main difference with respect to the model developed for the previous assessment of SeHCAT18 is that, for population 1, our model places colonoscopy after the SeHCAT test, in accordance with most clearly expressed clinical expert opinion and BSG guidelines in which colonoscopy is required for investigation of cancer and not for ruling out IBD. In practice, colonoscopy can be excluded from the model by setting this probability equal to zero. In the decision-analytic model, the number of responders, the expected costs and the number of colonoscopies avoided (when applicable) were calculated for each comparator. In the Markov models, lifetime expected (quality-adjusted) life-years and expected costs per patient were calculated for each comparator.
When possible, input parameters for the model were estimated based on our systematic review, other published literature and UK databases. When such evidence was not available, expert opinion was sought. The impact of parameter uncertainty was explored through PSAs and scenario analyses. ICERs were estimated as additional cost per additional QALY. Other outcomes included in the analyses were short-term costs, response to treatment and, for population 1, the percentage of colonoscopies avoided. These three outcomes were calculated in the decision-analytic model (thus, assumed to be in the first 6 months of the simulation).
For both populations, the SeHCAT 15% strategy dominated the other two strategies or resulted in ICERs below the common thresholds of £20,000 and £30,000 per QALY gained. Dominance or cost-effectiveness was led, in general, by treatment response as the SeHCAT 15% was the strategy with the highest response rate in the majority of the scenarios explored, including the base-case scenario for both populations. In scenarios in which the other two strategies were estimated to provide higher response rates than SeHCAT, the scenarios are likely to be based on unrealistic assumptions regarding response to no SeHCAT or BAS trial of treatment. Even in those scenarios in which overall response in the BAS trial-of-treatment strategy was higher than in the SeHCAT 15% strategy, the ICERs for the comparison of BAS trial of treatment versus SeHCAT 15% were well above the thresholds of £20,000 or £30,000 per QALY gained. SeHCAT 15% was also the strategy in which more colonoscopies were avoided.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,73 no study design filters were used, to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened; however, the search yield (proportion of studies identified that met the inclusion criteria for this assessment) was very low.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups that favours treatment. This is not the case for test accuracy studies, which measure agreement between an index test and a reference standard. It would seem likely that studies finding greater agreement (i.e. high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. It would seem likely that similar considerations would apply to the type of observational study [studies in which only participants with a positive index test (SeHCAT) result receive the reference standard (treatment with BAS)] that comprises most of the evidence in this assessment. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 74 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 74 We did not undertake a statistical assessment of publication bias in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Clear inclusion criteria were specified in the protocol for this review; the review has been registered on PROSPERO (CRD42020223877) and the protocol is available online. 1 The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for exclusion for all of the studies that were considered potentially relevant at initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 4). The review process followed recommended methods to minimise the potential for error and/or bias;15 studies were independently screened for inclusion by two reviewers, and data extraction and quality assessment were undertaken by one reviewer and checked by a second (MW and ER). Any disagreements were resolved by consensus.
There is no accepted reference standard method for diagnosing BAD, although the SeHCAT test is widely used in UK clinical practice. This being the case, the only options for evaluating the clinical effectiveness of the SeHCAT test involve methods of evaluating the impact of the test on clinical outcomes (e.g. RCTs, CCTs or observational comparative studies in which outcomes among patients who received SeHCAT testing are compared with outcomes among those who did not), or study designs (e.g. multivariable prediction modelling or predictive accuracy studies) that assess the ability of the SeHCAT test to predict patients’ response to standard treatments (BAS) for BAD. It can be argued that RCTs, CCTs and multivariable models (in which treatment response is the dependent variable) represent a higher level of evidence than conventional test accuracy studies, in that they provide a direct link between the test and the clinical outcome of interest and can incorporate controls for factors (other than the test) that may affect clinical outcome. A predictive accuracy study, in which response to treatment or a longer-term outcome is treated as the reference standard, also makes a direct link between the test and clinical outcome, but has the weakness that it does not account for factors other than the test that may affect outcome.
Three studies40,43,44 included in the review were classified as predictive accuracy studies (studies that provided data on the sensitivity and specificity of the SeHCAT test for predicting response to treatment with BAS). The methodological quality of these studies was assessed using a modification of the QUADAS-2 tool,23 which is recommended by the Cochrane Collaboration. 17 The QUADAS-2 tool is structured into four key domains covering participant selection, index test, reference standard and the flow of participants through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear); the participant selection, index test and reference standard domains are also separately rated for concerns regarding the applicability (low, high or unclear) of the study to the review question. For continuity, the methodological quality of studies that reported treatment outcome only for those participants with a positive SeHCAT result (i.e. sufficient data to calculate PPV only) was assessed using a topic-specific adaptation of the quality assessment checklist by Wedlake et al. ,24 as used in our previous DAR. 18
As was the case for our previous systematic review on this topic,18 the main limitations for this assessment are the paucity of data (only nine new studies were identified for this update assessment), the level of evidence (21/24 of the included studies were of the lowest level of evidence specified in the inclusion criteria) and the generally poor quality of the included studies (see Chapter 3, Study quality). Studies that reported information about the rate of response to treatment with BAS among participants who had a positive SeHCAT test appeared not to be using the SeHCAT test result alone to determine treatment decisions, as not all participants with a positive SeHCAT test received BAS; other reasons for deciding whether or not to offer BAS were not reported. There were substantial differences between studies included in the review (studies were generally poorly reported and there was variation in the SeHCAT test methods and thresholds, BAS treatment regimens and definition of response to treatment). The applicability of the included studies to the review question was unclear; previous investigations were generally poorly reported and not equivalent to those specified in current BSG guidelines for the investigation of chronic diarrhoea. 3
Cost-effectiveness
The main objective of this assessment was to update the previous assessment of SeHCAT conducted in 2012/2013. 18 As mentioned in the previous section, the evidence base relevant for this assessment has not changed substantively since the previous one. Therefore, current strengths and limitations are similar to those discussed in the previous report.
This report presents a full economic evaluation study in two populations of interest: (1) adult patients presenting with chronic diarrhoea with unknown cause (FD), or suspected or diagnosed IBS-D (i.e. people with suspected primary BAD); and (2) adults with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection (i.e. people with suspected secondary BAD). Short- and long-term consequences were assessed both in costs and effects of using the SeHCAT test at a 15% cut-off threshold, compared with no SeHCAT and trial of treatment with a BAS (colestyramine or colesevelam). For both patient populations, a linked evidence approach was used to model cost and consequences, to combine outcomes of the SeHCAT test and the related changes in treatment decisions and final health outcomes. Our model and analyses distinguish between the initial diagnostic phase (treatment responder vs. non-responder, and colonoscopies avoided, at 6 months) and the long-term projection of treatment response into final health outcomes (lifetime costs and consequences, the latter expressed in QALYs).
There are several differences with respect to the analyses in the previous assessment of SeHCAT. 18 As mentioned in the previous section, for the SeHCAT option, only the strategy based on the 15% cut-off point was included in the cost-effectiveness analyses because of the lack of data to inform other relevant SeHCAT strategies identified in the literature (e.g. 5% or 10%). Although including just one SeHCAT strategy could be seen as a limitation, it could be argued that it is an advantage because including other SeHCAT strategies for which data are also lacking would only increase the uncertainty around the plausibility of the cost-effectiveness results.
Another important difference in this assessment is that the health economic model extends the model previously developed by placing colonoscopy after the SeHCAT test for population 1. This was assumed in accordance with most clearly expressed clinical expert opinion and BSG guidelines in which colonoscopy is required for the investigation of cancer and not for ruling out IBD. In practice, colonoscopy can be excluded from the model by setting this probability equal to zero; in that case, the updated model can be seen as equivalent to the model in the previous assessment of SeHCAT. 18
The impact of using SeHCAT was included in the analyses in terms of BAS treatment response as reported in peer-reviewed papers. For this purpose, we selected only those papers that fulfilled our quality criteria as presented in Chapter 3. In all models developed, we have used the best available evidence to inform input parameters that were relevant for the UK. When evidence was not available through published studies or databases, we used the most likely and plausible values as reported by clinical experts. For this purpose, we sent out an updated questionnaire in which new questions were used to target the evidence gaps from the previous models. The lack of evidence was handled by performing PSAs and a wide range of scenario analyses. Unlike in the previous assessment of SeHCAT,18 this time it was preferred to have a base-case scenario for each population that was defined based on the assumptions that were deemed more plausible by the modelling team based on the available evidence and clinical experts’ feedback.
In the updated assessment we were able to incorporate patients switching from treatment with colestyramine to colesevelam using clinical experts’ inputs. Unfortunately, it was not possible to translate it into changes in response rates, but at least changes in costs and HRQoL due to treatment-switching were included in the new analyses.
A strength of the HRQoL evidence is that EQ-5D utility values were identified for patients with IBS with and without diarrhoea. However, assumptions had to be made to estimate these utilities among patients with Crohn’s disease and to estimate the utility of patients who respond to treatment with BAS in both populations, all of which represent important limitations in the HRQoL evidence.
Unit costs were retrieved from appropriate sources and were based on the 2020 costing year. Most of the information needed to calculate costs (e.g. medication use, dosage, proportion of patients requiring each type of medication) was based on experts’ opinion. The costs estimated might be considered uncertain, as questions were filled in by a maximum of four experts, and some questions were answered by just one or two. For example, if, for one medication, different dosages were given by different experts, the average of the experts’ answers was used. It is uncertain whether or not these averages would approach the true value, given the small sample size. When the full information of a certain medication was not available from the questionnaire, this medication was excluded from the model, for example vedolizumab for IBD patients. As these costs are expected to be high, the current estimated IBD costs might be underestimated. Similar issues were encountered for infliximab and for psychological treatment in the IBD population. Most notably, when clinical experts were asked about treatment of diarrhoea among Crohn’s disease patients, their answers suggested that this was similar to treatment of BAD with BAS, as colestyramine and colesevelam were mentioned. However, assuming these as treatment of diarrhoea among Crohn’s disease patients would result in no distinction between the no-SeHCAT and the BAS trial-of-treatment strategies. Therefore, diarrhoea treatment for patients with Crohn’s disease was assumed to be the same as in the previous SeHCAT report. 18
One of the main limitations of this study is still that the studies used to estimate the probability of a positive SeHCAT test result and the probability of BAS response, after a positive SeHCAT test, were based on populations other than the ones defined in this evaluation. Most IBS-D studies included patients for whom various tests had been performed and no organic cause of the diarrhoea was found. This is in contrast with the population defined in this assessment, which is patients with symptoms suggestive of functional disease for whom only basic blood tests have been performed. It is therefore likely that, in our population, the prevalence of BAD is lower than the prevalence observed in the published studies.
Another limitation that was already present in the previous assessment of SeHCAT concerns the modelling of non-responders. It is assumed in the model that non-responders would only use loperamide for some symptomatic relief. It might be likely that, for example in the IBS-D population (i.e. patients for whom no diagnostic testing other than initial blood work has been performed), some non-responders will be referred for diagnostic testing to check for organic causes of the chronic diarrhoea.
The most important limitation is still the lack of data on various important parameters of the model. This is most notably the case for patients after testing negative for SeHCAT and for the BAS trial-of-treatment strategy. The necessity to rely on expert opinion was still high because the majority of parameters included in the model were informed by the answers provided to our questionnaire.
Uncertainties
Clinical effectiveness
Two systematic reviews, published since the publication of NICE DG7,2 have provided estimates of the prevalence of BAM (as defined by a 7-day SeHCAT retention value of < 10%) among adults with IBS-D (defined by the Rome I, II or III criteria)75 or adults with IBS-D or FD with no organic explanation. 76 The pooled prevalence estimates from these two systematic reviews were 28.1% (95% CI 22.6% to 34.0%), based on data from six studies (n = 908),75 and 30.8% (95% CI 24.7% to 37.7%), based on data from 24 studies (total number of participants unclear). 76 These data support the idea that BAM may be a significant underlying pathology in a substantial proportion of patients with IBS-D or FD and, by extension, that ‘underdiagnosis’ of BAM in this population could result in patients not receiving potentially beneficial treatment with BAS, or experiencing delays in treatment. A web-based survey of 227 UK nuclear medicine departments, published in 2013 shortly after NICE DG7,2 reported that 73 out of 129 (57%) responding centres were using SeHCAT, of which 51 out of 73 (70%) reported an increase in workload over the preceding 3 years. 72 Although this study is approximately 8 years old, and hence cannot be taken as a reliable representation of current service provision, it may be worth noting that responding centres reported a very wide range of annual patient workloads, median 30 studies per year (range 1–300), indicating substantial geographic variation in service provision. 72 A subsequent prospective survey of 38 UK centres providing SeHCAT testing, published in 2016, reported that the total number of SeHCAT tests conducted by participating centres over a 6-month period was 1070;4 this study did not provide a breakdown of test numbers by centre.
Despite the apparent significance of BAM in the adult IBS-D/FD population, and the expansion of provision of SeHCAT testing in the UK, there remains a surprising lack of evidence linking the use of SeHCAT testing to patient-perceived outcomes. As described in Chapter 3, Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with diarrhoea-predominant irritable bowel syndrome or functional diarrhoea, and Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with Crohn’s disease, who have not undergone ileal resection, the available evidence is largely limited to studies that describe the proportion of patients with a positive SeHCAT test result who respond positively to treatment with BAS. The thresholds used by these studies to define a positive SeHCAT test varied and, although some studies did evaluate multiple thresholds, data were sparse and the optimal SeHCAT decision threshold, to define presence of BAM and select patients for treatment with BAS, remains unclear. For example, two studies reported information about treatment response rates for three 7-day SeHCAT retention thresholds (5%, 10% and 15%). 34,48 The results of both studies indicated that, if a 5% or 10% threshold were applied, some patients with a negative SeHCAT result that might be considered to be ‘borderline’ or ‘equivocal’ (i.e. 7-day retention values of between 5% and 15% or between 10% and 15%), who may benefit from treatment with BAS, would be missed (see Chapter 3, Performance of the SeHCAT test for predicting response to treatment with bile acid sequestrant among patients with diarrhoea-predominant irritable bowel syndrome or functional diarrhoea). Furthermore, there is apparent variation in UK practice with respect to the threshold used to define a positive SeHCAT test result; the 2013 survey of UK practice found that 42 out of 72 (58%) centres providing SeHCAT tests reported using a 7-day retention value of > 15% to define an ‘unequivocally normal’ test result, with 19% using a lower threshold and 22% using a higher threshold. 72 The 2016 survey of UK centres found that the majority [22/32 (69%)] of reporting centres used a ‘normal’ threshold of ≥ 15%. 4 However, variation in practice remained, with ‘normal’ threshold values ranging from ≥ 10% to ≥ 20%; the key findings of this study included the statement that ‘there was a high level of heterogeneity in practice, with no standardised protocol, and no consistently defined diagnostic threshold values of SeHCAT retention. 4 In summary, UK practice varies with respect to the threshold used to define a ‘normal’ SeHCAT test result, and the extent to which patients with ‘borderline’ or ‘equivocal’ 7-day SeHCAT retention values (e.g. between 10% and 15%) could benefit from treatment with BAS remains unclear, and the extent to which patients with 7-day retention values of > 15% may benefit from treatment with BAS is unknown.
Given the uncertainty regarding the optimal SeHCAT decision threshold to define presence of BAM and select patients for treatment with BAS, the potential for intra-individual variation in SeHCAT retention values (e.g. arising from variation in dietary intake before the test or concomitant medication use) may be an important consideration for the implementation of SeHCAT testing in clinical practice. The 2013 survey of UK practice found that 45 out of 72 (62%) responding centres providing SeHCAT tests reported issuing no specific instructions to patients regarding pre-test fasting and 31 out of 72 (42%) gave no specific instructions regarding medication. 72 This information was not reported in the 2016 survey. 4
‘Trial of treatment’ with BAS without testing is sometimes advocated as an alternative approach to investigating BAM as a potential undiagnosed cause of symptoms among patients with IBS-D,77,78 and ‘trial of treatment’ is a comparator for the cost-effectiveness modelling included in this assessment. However, it should be noted that a positive response to treatment with BAS cannot be considered to be 100% specific for a diagnosis of BAM, as these drugs can slow gut transit irrespective of any effect on bile acid metabolism. We identified a German-language study that reported the author’s experience (1991–2017) of using a ‘trial of treatment’ with colestyramine among patients with chronic diarrhoea for whom organic causes had been excluded. 78 This study did not meet the inclusion criteria for our systematic review, as only patients with a positive response to colestyramine were offered SeHCAT testing, and it did not provide data to inform cost-effectiveness modelling, as the total number of patients who received a ‘trial of treatment’ (and hence the proportion who responded) was not reported. 78 However, this study did report the proportion of people [8/60 (13%)] who responded positively to colestyramine and received a SeHCAT test, for whom that test was negative for BAM (7-day retention value of ≥ 20%); this may be considered indicative of the proportion of patients with unexplained chronic diarrhoea who respond positively to BAS, for whom there is no evidence of BAM. 78 In support of SeHCAT testing, it has been suggested (scoping discussions for this assessment) that SeHCAT testing and the assignment of a diagnosis of BAM may improve adherence to treatment with BASs, which are generally considered to be poorly tolerated; when reported, rates of intolerance/discontinuation in the studies included in this assessment were generally high (median 15%, range 4–27%), and the 2016 survey of SeHCAT provision and practice in the UK found that 20 out of 101 (20%) patients who were prescribed BAS reported side effects, including bloating, diarrhoea, constipation, nausea/vomiting, urticarial rash, pain and intolerance to tablets. 4 When information about the BAS treatment was provided, most (16/20) studies included in this assessment reported the use of colestyramine alone. 25,31,32,34,35,37–45,47,48 Three studies reported more than one option for treatment with BAS: colestyramine or colesevelam,28 colestyramine or colestipol,7 and colestyramine or colesevelam or colestipol;30 none of these studies reported either the numbers of patients treated with each drug or the criteria used to select treatment. Similarly, the 2016 survey of UK practice did not provide a breakdown of side effects/intolerance by type of BAS received. 4 There was insufficient information to determine whether or not levels of intolerance varied between colestyramine, colestipol and colesevelam, and no study reported information about patient preferences. We did not identify any studies that reported information about patient preferences with respect to SeHCAT testing versus ‘trial of treatment’ without testing.
Finally, there is a lack of evidence about the efficacy and safety of BAS for the treatment of patients diagnosed with BAM; the clinical effectiveness searches conducted for this assessment identified only three treatment RCTs,79–81 of which only one used a positive SeHCAT test (7-day retention value of < 10%) to define BAM and select patients for inclusion. 81 This was a very small (n = 19) placebo-controlled RCT,81 evaluating two doses (250 mg and 1 g twice daily) of a colonic release formulation of colestyramine, which found no significant effect on the primary outcome (mean daily bowel movement at week 2 of treatment), but reported reductions in instances of diarrhoea and improvements in stool consistency in the treated groups. Although outside the scope of this assessment, it should be noted that our searches identified an ongoing systematic review on the effectiveness of non-pharmacological therapies in the management of BAD among adults. 82
Cost-effectiveness
The main uncertainties in the cost-effectiveness analyses are still caused by a lack of essential data. The majority of the input parameters of the model were informed by clinical experts. In particular, evidence is especially limited as to what occurs after a negative SeHCAT result, for the BAS trial-of-treatment strategy and for the Crohn’s disease population in general. Therefore, a substantial number of assumptions had to be made to make it possible to perform the cost-effectiveness analyses.
As in the previous assessment of SeHCAT,18 the lack of evidence on the accuracy of the SeHCAT test based on a reference test implied that, in the diagnostic decision-analytic models, the most common way of modelling test accuracy, using sensitivity and specificity of the test, was not feasible. Thus, it was not possible to indicate false-positive and false-negative probabilities of testing. The accuracy of SeHCAT testing was thus based on the test result in combination with response to BAS treatment. It might occur that patients responding to BAS are true positive (patients with a true response), but they may also be false-positive patients with a placebo response.
Another unresolved uncertainty regarding the trial-of-treatment strategy relates to the placebo response that may be expected in the true IBS-D patients receiving BAS. It is well known that patients with IBS-D are likely to show high placebo responses to treatment. 83 Clinical experts pointed out that long-term inappropriate treatment with BAS could have implications for absorption of other drugs and vitamins. These long-term undesired consequences were not included in the modelled trial-of-treatment strategy. Clinical experts also indicated that a response to BAS is not helpful diagnostically because BASs are constipating drugs in any event, as known from when they were used for lowering cholesterol levels in people with no bowel problems. Therefore, using BAS as a diagnostic would be no better than using loperamide as a diagnostic test for any form of diarrhoea. In addition, transitions between the ‘diarrhoea’ and ‘no-diarrhoea’ health states might not be the same for BAS patients having a positive SeHCAT result and for patients responding to a BAS trial of treatment, as patients without a positive diagnosis may be less inclined to accept the side effects of BAS (colestyramine).
The uncertainties in the Markov model are still also unresolved. The diarrhoea health state was valued by cost and utilities irrespective of the cause of the symptom. However, there is no evidence to confirm whether or not this is true. For the increase in utility when patients become responders, we made the same previous assumption that patients responding to BAS (colestyramine) would get only 75% of the utility benefit of becoming a responder. It is unknown to what extent this assumption of 75% is realistic. However, scenario analyses showed that the impact of this assumption on the model results is minor.
Transition probabilities in the Markov model remain uncertain as well. Patients can, in theory, transition between the ‘diarrhoea’ and ‘no-diarrhoea’ health states. Being in the latter health state can be understood as an improvement of the symptoms, but relapse (i.e. transition to the ‘diarrhoea’ health state) is also possible. In the base-case analysis, it is assumed that patients responding to BAS and IBS-D treatment remain in the ‘no-diarrhoea’ health state, which can be seen as a ‘cure’ assumption. Although this can be a simplistic assumption, it was based on the opinion of the clinical experts, who indicated that, in general, patients initially responding to treatment are expected to continue responding in the long term. Making more realistic assumptions would have required more data, which were not available. Scenario analyses were conducted to test the impact of transition assumptions on the results (see, e.g., scenario analyses 7 and 14). These analyses showed that the impact on the model results was minor. This time it was also not possible to include a health state of ‘constipation’ or other adverse events in the long-term Markov model, given the lack of data. Threshold or any other type of exploratory analyses on adverse events were not considered for pragmatic reasons. The rationale for this was the same as explained previously regarding the selection of only the SeHCAT 15% cut-off point for the cost-effectiveness analyses. Scenario analyses on adverse events would not be evidence based; therefore, the relevance of such scenarios could be questionable. Thus, it was preferred to focus on other (of the many) uncertainty areas around the cost-effectiveness analyses that were deemed more important at this stage than the modelling of adverse events.
Finally, it is uncertain how the cost-effectiveness results would change should other SeHCAT strategies be included in the analyses. The available clinical evidence regarding the cut-off values defining a positive SeHCAT test shows that the various cut-off values influence test-accuracy estimates expressed in BAS treatment response. The cost-effectiveness analyses included in this report have shown that response to treatment is a key driver of the cost-effectiveness results. The strategy with the highest response rate is likely to be the preferred one in terms of health benefits, but it remains uncertain whether or not this will be translated into cost-effectiveness.
Chapter 6 Conclusions
Implications for service provision
Despite the apparent significance of BAM in the adult IBS-D/FD population, and the expansion of provision of SeHCAT testing in the UK, there remains a surprising lack of evidence linking the use of SeHCAT testing to patient-perceived outcomes. The available evidence is largely limited to studies that describe the proportion of patients with a positive SeHCAT result who respond positively to treatment with BAS. The optimal SeHCAT decision threshold, to define presence of BAM and select patients for treatment with BAS, is uncertain. The extent to which patients with ‘borderline’ or ‘equivocal’ 7-day SeHCAT retention values (e.g. between 10% and 15%) could benefit from treatment with BAS remains unclear, and the extent to which patients with 7-day retention values of > 15% may benefit from treatment with BAS is unknown. It has been suggested that SeHCAT testing and the assignment of a diagnosis of BAM may improve adherence to treatment with BAS. However, despite some evidence indicating that these treatments are generally poorly tolerated, there is a lack of information about the relative rates of adherence for different BASs and about the acceptability, to patients, of SeHCAT testing. Finally, there is a paucity of evidence about the efficacy and safety of BAS for the treatment of patients who have been diagnosed with BAM.
The evidence base has not advanced substantively since our previous assessment,18 conducted to inform the development of NICE DG7. 2
The results of the economic evaluation conducted for both populations indicated that the SeHCAT 15% strategy dominated the other two strategies or resulted in ICERs below the common thresholds of £20,000 and £30,000 per QALY gained. However, given the paucity of evidence, there is great uncertainty surrounding these analyses. Therefore, the implications for service provision of SeHCAT are still uncertain and the main reason for this uncertainty is the lack of good-quality evidence.
Suggested research priorities
Given the deficiencies in the evidence base, outlined in Implications for service provision, the optimum study design for maximum information gain would be a multiarm RCT, in which participants meeting the inclusion criteria are randomised to receive colestyramine, colestipol, colesevelam or placebo, and all participants receive SeHCAT testing. Included participants should be adults (aged ≥ 18 years) presenting with chronic diarrhoea with unknown cause, or suspected or diagnosed IBS-D, or adults (aged ≥ 18 years) presenting with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection. Participants should have undergone primary clinical assessment/investigations (as recommended in the BSG guidelines3) to exclude coeliac disease (coeliac serology and upper gastrointestinal endoscopy and biopsy among people with suspected coeliac disease), common infections (stool examination for Clostridium difficile, ova, cysts and parasites) and colorectal cancer (colonoscopy among people with altered bowel habit and rectal bleeding, and faecal immunochemical testing to guide priority investigations among people with lower gastrointestinal symptoms and no rectal bleeding), prior to inclusion in the RCT. SeHCAT testing should be undertaken in all participants, irrespective of treatment group. A study of this type could potentially allow estimation of the comparative efficacy, safety and tolerability of colestyramine, colestipol, colesevelam and placebo among all participants (equivalent to the ‘trial-of-treatment’ option described in this assessment). In addition, stratified analyses based on different 7-day SeHCAT retention values could be used to investigate variation in the comparative efficacy, safety and tolerability of colestyramine, colestipol, colesevelam and placebo with SeHCAT retention, and hence to inform the optimal SeHCAT threshold to guide treatment decisions. A further option would be stratified randomisation to disclosure or non-disclosure of SeHCAT test results prior to treatment; this option could allow assessment of the effects of testing and diagnosis on adherence to treatment.
An alternative, pragmatic option would be a prospective cohort study in which all participants (inclusion criteria as described previously) receive both treatment with a BAS and SeHCAT testing. Data from such a study could be analysed to determine the predictive accuracy (sensitivity and specificity) of one or more predefined SeHCAT thresholds for response to treatment with BAS. Alternatively, a receiver operating characteristic analysis could be used to determine the clinically optimal SeHCAT threshold.
From the cost-effectiveness perspective, it is important to emphasise that data on SeHCAT accuracy and response to BAS are not sufficient to conduct a full economic evaluation, as this would require data on all possible pathways, including treatment of patients with a negative SeHCAT result and patients not responding to BAS. Thus, the recommended research described previously should also include data collection on patients with a negative SeHCAT result and patients not responding to BAS. Because cost-effectiveness studies usually adopt a lifetime time horizon, data on long-term effects are also required. Given the gaps in the HRQoL evidence already discussed, a priority in future research should be to provide diarrhoea-specific utilities for patients with Crohn’s disease in general, as well as patients taking BAS, preferably using the EQ-5D. Because costs estimates were highly uncertain, priority should also be given to the research of costs of treatment of BAD, IBS-D, IBD and diarrhoea among Crohn’s disease patients.
Acknowledgements
Gill Worthy, statistician and systematic reviewer at Kleijnen Systematic Reviews Ltd, contributed to the title and abstract screening stage of the systematic review.
The authors would like to thank all of the clinical specialist members of the NICE Diagnostic Appraisal Committee for this topic for their assistance and expert input throughout this project. We would also would like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Subgroup for providing input on the patients’ perspective at key stages of the assessment process.
Contributions of authors
Marie Westwood (https://orcid.org/0000-0002-6257-0653) and Edyta Ryczek (https://orcid.org/0000-0001-8394-5066) planned and performed the systematic review and interpretation of evidence.
Isaac Corro Ramos (https://orcid.org/0000-0002-1294-8187), Hannah Penton (https://orcid.org/0000-0001-9492-7875) and Marscha Holleman (https://orcid.org/0000-0002-4290-8469) planned and performed the cost-effectiveness analyses and interpreted the results.
Nigel Armstrong (https://orcid.org/0000-0002-7443-4798) contributed to planning and interpretation of the systematic review and the cost-effectiveness analyses, contributed to acquisition of input data and conducted model peer review.
Caro Noake (https://orcid.org/0000-0003-0329-4772) devised and performed the literature searches and provided information support to the project.
Maiwenn Al (https://orcid.org/0000-0001-9763-0436) provided senior advice and support to the cost-effectiveness analyses.
All authors were involved in drafting and/or commenting on the report.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence . A Systematic Review and Economic Evaluation of SeHCAT (Tauroselcholic [75Selenium] Acid) for the Investigation of Bile Acid Diarrhoea. Diagnostic Assessment Report Commissioned by the NIHR HTA Programme on Behalf of the National Institute for Health and Care Excellence – Protocol n.d. www.nice.org.uk/guidance/dg44/documents/final-protocol (accessed 13 April 2022).
- National Institute for Health and Care Excellence . SeHCAT (Tauroselcholic [75 Selenium] Acid) for the Investigation of Diarrhoea Due to Bile Acid Malabsorption in People With Diarrhoea-Predominant Irritable Bowel Syndrome (IBS-D) or Crohn’s Disease Without Ileal Resection. Diagnostics Guidance [DG7] n.d. www.nice.org.uk/guidance/dg7 (accessed 19 December 2011).
- Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018;67:1380-99. https://doi.org/10.1136/gutjnl-2017-315909.
- Summers JA, Peacock J, Coker B, McMillan V, Ofuya M, Lewis C, et al. Multicentre prospective survey of SeHCAT provision and practice in the UK. BMJ Open Gastroenterol 2016;3. https://doi.org/10.1136/bmjgast-2016-000091.
- National Institute for Health and Care Excellence . Suspected Cancer: Recognition and Referral. NICE Guideline [NG12] n.d. www.nice.org.uk/guidance/ng12 (accessed 27 October 2020).
- National Institute for Health and Care Excellence . Irritable Bowel Syndrome in Adults: Diagnosis and Management. Clinical Guideline [CG61] n.d. www.nice.org.uk/guidance/CG61 (accessed 19 December 2011).
- Smith MJ, Cherian P, Raju GS, Dawson BF, Mahon S, Bardhan KD. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond 2000;34:448-51.
- Fernandes DCR, Poon D, White LL, Andreyev HJN. What is the cost of delayed diagnosis of bile acid malabsorption and bile acid diarrhoea?. Frontline Gastroenterol 2019;10:72-6.
- Turner JM, Pattni SS, Appleby RN, Walters JR. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol 2017;8:279-83. https://doi.org/10.1136/flgastro-2017-100826.
- Bannaga A, Kelman L, O’Connor M, Pitchford C, Walters JR, Arasaradnam RP. How bad is bile acid diarrhoea: an online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol 2017;4. https://doi.org/10.1136/bmjgast-2016-000116.
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme: SeHCAT (Tauroselcholic [75 Selenium] Acid) for the Investigation of Bile Acid Diarrhoea: Final Scope n.d. www.nice.org.uk/guidance/dg44/documents/final-scope (accessed 17 November 2020).
- NHS England . 2018/19/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 11 July 2022).
- National Institute for Health and Care Excellence . Bile Acid Malabsorption: Colesevelam. Evidence Summary [ESUOM22] n.d. www.nice.org.uk/advice/esuom22/ (accessed 27 October 2020).
- Sadowski DC, Camilleri M, Chey WD, Leontiadis GI, Marshall JK, Shaffer EA, et al. Canadian Association of Gastroenterology clinical practice guideline on the management of bile acid diarrhea. J Can Assoc Gastroenterol 2020;3:e10-e27. https://doi.org/10.1093/jcag/gwz038.
- Centre for Reviews and Dissemination . Systematic Reviews – CRD’s Guidance for Undertaking Reviews in Healthcare 2009.
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme: Interim Methods Statement (Version 2) n.d. www.nice.org.uk/media/164/3C/DAPInterimMethodsStatementProgramme.pdf (accessed 12 January 2011).
- Cochrane Diagnostic Test Accuracy Working Group . Handbook for DTA Reviews n.d. http://srdta.cochrane.org/handbook-dta-reviews (accessed 23 March 2011).
- Riemsma R, Al M, Corro Ramos I, Deshpande SN, Armstrong N, Lee YC, et al. SeHCAT [tauroselcholic (selenium-75) acid] for the investigation of bile acid malabsorption and measurement of bile acid pool loss: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17610.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] n.d. www.cochrane-handbook.org/ (accessed 23 March 2011).
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0 (Updated July 2019) n.d. https://training.cochrane.org/handbook (accessed 6 July 2020).
- McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies (PRESS EBC). Evid Based Libr Inf Pract 2010;5:1-6. https://doi.org/10.18438/B8SG8R.
- Canadian Agency for Drugs and Technologies in Health . CADTH Peer Review Checklist for Search Strategies n.d. www.cadth.ca/en/resources/finding-evidence-is (accessed 17 July 2013).
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707-17. https://doi.org/10.1111/j.1365-2036.2009.04081.x.
- Bellini M, Pancetti A, Ciranni F, Bertani L, Paglianiti I, Berti G, et al. Bile acid malabsorption: an overlooked diagnosis? Digestive Disease Week 2020; accepted abstracts published online May 2020. Gastroenterology 2020;158.
- Farmer AD, Hoole S, Slattery S, Frost J, Aziz Q. Bile acid malabsorption is numerically less prevalent in patients with Rome IV defined irritable bowel syndrome with diarrhea compared to ROME III. Presented at Digestive Disease Week 2017, Chicago, IL, USA, 6–9 May 2017. Gastroenterology 2017;152.
- Holmes R, Hayat JO, Irwin A, Heenan S, Kang JY, Poullis A. Review of SeHCAT use at St. George’s 2005–2010: an underutilised investigation? Presented at Digestive Disorders Federation Meeting 2012, Liverpool, UK, 17–20 June 2012. Gut 2012;61.
- Kumar A, Hughes L, Phipps O, Simmons SJ, Green M, Steed H, et al. The impact of bile salt sequestrants on the quality of life of patients with bile acid diarrhoea (BAD). Digestive Disease Week 2020; accepted abstracts published online May 2020. Gastroenterology 2020;158.
- Kumar L, Jamshed B, Emmanuel A. An audit of clinical outcomes of SeHCAT study in patients with chronic diarrhoea. Presented at British Society of Gastroenterology Annual Meeting 2013, Glasgow, UK, 24–27 June 2013. Gut 2013;62.
- Lin S, Sanders DS, Gleeson JT, Osborne C, Messham L, Kurien M. Long-term outcomes in patients diagnosed with bile-acid diarrhoea. Eur J Gastroenterol Hepatol 2016;28:240-5. https://doi.org/10.1097/MEG.0000000000000541.
- Notta P, Martinez Pimienta G, Martin-Comin J, Bajen M, Rodriguez-Rubio J, Puig O, et al. Abdominal retention index of 75SeHCAT according to response to treatment with resincolestiramina. Presented at the 27th Annual Congress of the European Association of Nuclear Medicine, Gothenburg, Sweden, 18–22 October 2014. Eur J Nucl Med Mol Imaging 2014;41.
- Notta PC, Suils Ramón J, Rubio-Alvarez L, Rodriguez-Gasen A, Maisterra-Santos S, Guardiola-Capo J, et al. Abdominal retention index of 75SeHCAT according to response to treatment with resincolestiramine. Presented at 30th Annual Congress of the European Association of Nuclear Medicine, Vienna, Austria, 21–25 October 2017. Eur J Nucl Med Mol Imaging 2017;44:S832-3.
- Zanoni L, Tabacchi E, Barbara G, Stanghellini V, Bortolotti M, Cogliandro R, et al. 75SeHCAT scan in bile acid malabsorption in chronic diarrhea: a preliminary Italian single center experience. Presented at 31st Annual Congress of the European Association of Nuclear Medicine, Dusseldorf, Germany, 13–17 October 2018. Eur J Nucl Med Mol Imaging 2018;45.
- Borghede MK, Schlütter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF. Bile acid malabsorption investigated by selenium-75-homocholic acid taurine ((75)SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med 2011;22:e137-40. https://doi.org/10.1016/j.ejim.2011.08.013.
- Fellous K, Jian R, Haniche M, Marteau P, Messing B, Rain JD, et al. Measurement of ileal absorption of bile salts with the selenium 75 labelled homotaurocholic acid test. Validation and clinical significance. Gastroenterol Clin Biol 1994;18:865-72.
- Fernandez-Banares F, Esteve M, Espinos JC, Forne M, Martin-Comin J, Viver JM. Bile acid malabsorption (BAM) in patients with functional chronic diarrhea: response to cholestyramine. Gastroenterology 2000;118.
- Fernandez-Bañares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46:2231-8. https://doi.org/10.1023/a:1011927302076.
- Fernández-Bañares F, Esteve M, Salas A, Alsina M, Farré C, González C, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol 2007;102:2520-8. https://doi.org/10.1111/j.1572-0241.2007.01438.x.
- Galatola G, Ferraris R, Pellerito R, Barlotta A, Umberto OM, Sciarretta TG, et al. The prevalence of bile acid malabsorption in irritable bowel syndrome and the effect of cholestyramine: an uncontrolled open multicentre study. Eur J Gastroenterol Hepatol 1992;4:533-7.
- Merrick MV, Eastwood MA, Ford MJ. Is bile acid malabsorption underdiagnosed? An evaluation of accuracy of diagnosis by measurement of SeHCAT retention. Br Med J 1985;290:665-8. https://doi.org/10.1136/bmj.290.6469.665.
- Notta PC, Ramal D, Maisterra S, Rodríguez Gasen A, Maymó S, Sabaté A, et al. Measurement of 75Se-SeHCAT abdominal retention in the initial diagnosis of bile acid absorption (BAM). Rev Esp Med Nucl 2011;30:297-300. https://doi.org/10.1016/j.remn.2011.03.007.
- Rudberg U, Nylander B. Radiological bile acid absorption test 75SeHCAT in patients with diarrhoea of unknown cause. Acta Radiol 1996;37:672-5. https://doi.org/10.1177/02841851960373P250.
- Sciarretta G, Vicini G, Fagioli G. Use of 23-selena-25-homocholyltaurine to detect bile acid malabsorption in patients with ileal dysfunction or diarrhea. Gastroenterology 1986;91:1-9. https://doi.org/10.1016/0016-5085(86)90431-2.
- Sciarretta G, Fagioli G, Furno A, Vicini G, Cecchetti L, Grigolo B, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut 1987;28:970-5. https://doi.org/10.1136/gut.28.8.970.
- Sinha L, Liston R, Testa HJ, Moriarty KJ. Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramine. Aliment Pharmacol Ther 1998;12:839-44. https://doi.org/10.1046/j.1365-2036.1998.00388.x.
- Tunney R. The Clinical Value of SeHCAT in the Diagnosis of Bile Acid Malabsorption: An Evaluation of the British Society of Gastroenterology Guidelines for the Investigation of Chronic Diarrhoea [Report] 2011.
- Wildt S, Nørby Rasmussen S, Lysgård Madsen J, Rumessen JJ. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand J Gastroenterol 2003;38:826-30. https://doi.org/10.1080/00365520310004461.
- Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption – a review of clinical presentation, diagnosis, and response to treatment. Gut 1991;32:1004-6. https://doi.org/10.1136/gut.32.9.1004.
- Dyson J, Barbour J. Use of SeHCAT Testing: Investigation of Diarrhoea and Patient Satisfaction [Abstract] n.d. http://uegw.congress-online.com/uegw2011/guest/AbstractView?ABSID=12003 (accessed 7 February 2012).
- Eusufzai S. Bile acid malabsorption in patients with chronic diarrhoea. Scand J Gastroenterol 1993;28:865-8. https://doi.org/10.3109/00365529309103126.
- Eusufzai S, Axelson M, Angelin B, Einarsson K. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut 1993;34:698-701. https://doi.org/10.1136/gut.34.5.698.
- Ford GA, Preece JD, Davies IH, Wilkinson SP. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J 1992;68:272-6. https://doi.org/10.1136/pgmj.68.798.272.
- Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn’s disease and indications for its assessment using SeHCAT. Gut 1994;35:90-3. https://doi.org/10.1136/gut.35.1.90.
- Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 2010;8:159-65. https://doi.org/10.1016/j.cgh.2009.10.020.
- Kaltenthaler E, Tappenden P, Paisley S, Squires H. NICE DSU Technical Support Document 13: Identifying and Reviewing Evidence to Inform the Conceptualisation and Population of Cost-Effectiveness Models n.d. www.nicedsu.org.uk (accessed 8 December 2016).
- Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 1995;122:107-12. https://doi.org/10.7326/0003-4819-122-2-199501150-00005.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 (2018 Revision). Process and Methods [PMG9] n.d. www.nice.org.uk/process/pmg9/ (accessed 30 July 2019).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- Patel P, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P, et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross-sectional survey. Scand J Gastroenterol 2015;50:816-23. https://doi.org/10.3109/00365521.2015.1007079.
- Spiegel B, Harris L, Lucak S, Mayer E, Naliboff B, Bolus R, et al. Developing valid and reliable health utilities in irritable bowel syndrome: results from the IBS PROOF Cohort. Am J Gastroenterol 2009;104:1984-91. https://doi.org/10.1038/ajg.2009.232.
- Mearin F, Baró E, Roset M, Badía X, Zárate N, Pérez I. Clinical patterns over time in irritable bowel syndrome: symptom instability and severity variability. Am J Gastroenterol 2004;99:113-21. https://doi.org/10.1046/j.1572-0241.2003.04023.x.
- NHS . Consultation on 2021–22 National Tariff Payment System: Consultation Notice, Annexes and Supporting Documents n.d. www.england.nhs.uk/publication/2021-22-tariff-consultation/ (accessed 11 May 2021).
- Joint Formulary Committee . British National Formulary n.d. www.bnf.org (accessed 10 April 2012).
- Catt H, Bodger K, Kirkham JJ, Hughes DA. Value assessment and quantitative benefit–risk modelling of biosimilar infliximab for Crohn’s disease. PharmacoEconomics 2019;37:1509-23. https://doi.org/10.1007/s40273-019-00826-0.
- Office for National Statistics . National Life Tables 2017–2019: UK n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 11 July 2022).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Buxton MJ, Lacey LA, Feagan BG, Niecko T, Miller DW, Townsend RJ. Mapping from disease-specific measures to utility: an analysis of the relationships between the Inflammatory Bowel Disease Questionnaire and Crohn’s Disease Activity Index in Crohn’s disease and measures of utility. Value Health 2007;10:214-20. https://doi.org/10.1111/j.1524-4733.2007.00171.x.
- Canavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn’s disease. Aliment Pharmacol Ther 2007;25:861-70. https://doi.org/10.1111/j.1365-2036.2007.03276.x.
- Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics 2016;34:349-61. https://doi.org/10.1007/s40273-015-0327-2.
- Büyükkaramikli NC, Rutten-van Mölken MPMH, Severens JL, Al M. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. PharmacoEconomics 2019;37:1391-408. https://doi.org/10.1007/s40273-019-00844-y.
- Dyson J, Bartholomew P, Barbour J. Use of SeHCAT Testing: Investigation of Diarrhoea and Patient Satisfaction n.d.
- Smith MJ, Perkins AC. A survey of the clinical use of SeHCAT in the UK. Nucl Med Commun 2013;34:306-13. https://doi.org/10.1097/MNM.0b013e32835e8989.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. https://doi.org/10.1016/j.jclinepi.2010.07.006.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
- Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2015;42:3-11. https://doi.org/10.1111/apt.13227.
- Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 2016;65:1951-9. https://doi.org/10.1136/gutjnl-2015-309889.
- Hendy P, Florin T. Letter: therapeutic trial is more informative than SeHCAT to diagnose bile acid malabsorption. Aliment Pharmacol Ther 2015;42. https://doi.org/10.1111/apt.13320.
- Wenzel H. Primary bile acid diarrhea in a community gastroenterology practice. Z Gastroenterol 2019;57:734-9. https://doi.org/10.1055/a-0825-2437.
- Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, et al. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn’s disease: a randomized, double-blind, placebo-controlled study. J Crohns Colitis 2014;8:1471-9. https://doi.org/10.1016/j.crohns.2014.05.009.
- Vijayvargiya P, Camilleri M, Carlson P, Nair A, Nord SL, Ryks M, et al. Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Clin Gastroenterol Hepatol 2020;18:2962-70.e6.
- Albireo A. A Double-Blind, Randomized, Placebo-Controlled, Study to Demonstrate the Efficacy and Safety of 250 mg or 1 g A3384 Administered Orally Twice Daily for Two Weeks to Patients With Bile Acid Malabsorption (BAM) Bile Acid Diarrhoea (BAD) n.d. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number: 2013– 002924-17 (accessed 2 February 2021).
- McKenzie Y, Burden S, Todd C, Sremanakova J. Effectiveness of Non-Pharmacological Therapies in the Management of Bile Acid Diarrhoea in Adults: A Protocol for a Systematic Review n.d. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020188328 (accessed 14 April 2021).
- Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770-98. https://doi.org/10.1136/gut.2007.119446.
- Papaioannou D, Brazier J, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature n.d. www.sheffield.ac.uk/sites/default/files/2022-02/TSD9-HSUV-values_FINAL.pdf (accessed 18 August 2011).
- Centre for Reviews and Dissemination . Search Strategies: NHS EED EMBASE Using OvidSP (Economics Filter) n.d. www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase (accessed 2 June 2014).
- Centre for Reviews and Dissemination . Search Strategies: NHS EED MEDLINE Using OvidSP (Economics Filter). n.d. www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
- Thaysen EH, Orholm M, Arnfred T, Carl J, Rødbro P. Assessment of ileal function by abdominal counting of the retention of a gamma emitting bile acid analogue. Gut 1982;23:862-5. https://doi.org/10.1136/gut.23.10.862.
- Nyhlin H, Merrick MV, Eastwood MA, Brydon WG. Evaluation of ileal function using 23-selena-25-homotaurocholate, a-gamma-labeled conjugated bile acid. Initial clinical assessment. Gastroenterology 1983;84:63-8. https://doi.org/10.1016/S0016-5085(83)80167-X.
- Ludgate SM, Merrick MV. The pathogenesis of post-irradiation chronic diarrhoea: measurement of SeHCAT and B12 absorption for differential diagnosis determines treatment. Clin Radiol 1985;36:275-8. https://doi.org/10.1016/S0009-9260(85)80059-3.
- Monks R, Boyd GS. Biologic stability of tauro-23-[75Se] selena-25-homocholic acid. J Nucl Med 1988;29:1411-18.
- Appleby RN, Nolan JD, Johnston IM, Pattni SS, Fox J, Walters JR. Novel associations of bile acid diarrhoea with fatty liver disease and gallstones: a cohort retrospective analysis. BMJ Open Gastroenterol 2017;4. https://doi.org/10.1136/bmjgast-2017-000178.
- Arms-Williams B, Mylan P, Woodward C, Green J, Jones E, Khan M. Is SeHCAT scanning necessary? An analysis of SeHCAT scans and risk factors for bile acid diarrhoea in a series of 262 patients with diarrhoea. Presented at the British Society of Gastroenterology Annual General Meeting, Liverpool, UK, 20–23 June 2016. Gut 2016;65.
- Aujla UI, Arfan R, Nimba AN, Mahmood A, Zar S. Role of SeHCAT scanning in diagnosis of bile salt malabsorption: a university hospital experience. Presented at Digestive Disease Week 2014, Chicago, IL, USA, 3–6 May 2014. Gastroenterology 2014;146.
- Baena Garcia A, Partida Palma F, Garcia Martinez S, de Bonilla Candau M, Pajares Vinardell M. 75Se-Homocholic acid taurine scintigraphy (75SeHCAT R), a standard benchmark test in bile acid malabsorption?. Rev Esp Med Nucl Imagen Mol 2019;38:305-11.
- Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84-92. https://doi.org/10.1136/gutjnl-2013-305965.
- Barber Caselles C, Lobo Alvarez B, Mosquera JL, Aguade-Bruix S, Azpiroz F, Santos J. Retrospective study: role of SeHCAT test in the diagnosis of bile acid malabsorption as a cause of chronic diarrhoea and potential risk factors associated. Presented at the 25th United European Gastroenterology Week, Barcelona, Spain, 28 October–1 November 2017. United European Gastroenterol J 2017;5:A772-3.
- Bronte A, Bastidas J, Rosales J, Grisanti F, Zuaznabar J, Herraiz M, et al. 75Se-SeHCAT test in bile acids malabsorption. Methodological contributions. Presented at the European Association of Nuclear Medicine Annual Congress, online, 22–30 October 2020. Eur J Nucl Med Mol Imaging 2020;47:S394-5.
- Carrasco-Labra A, Lytvyn L, Falck-Ytter Y, Surawicz CM, Chey WD. AGA Technical review on the evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology 2019;157:859-80. https://doi.org/10.1053/j.gastro.2019.06.014.
- Damsgaard B, Dalby HR, Krogh K, Jørgensen SMD, Arveschough AK, Agnholt J, et al. Long-term effect of medical treatment of diarrhoea in 377 patients with SeHCAT scan diagnosed bile acid malabsorption from 2003 to 2016; a retrospective study. Aliment Pharmacol Ther 2018;47:951-7. https://doi.org/10.1111/apt.14533.
- Fernandes D, Poon D, White L, Andreyev J. PWE-096: what is the cost of delayed diagnosis of bile acid malabsorption?. Gut 2018;67.
- Fraccascia N, Tabacchi E, Zanoni L, Barbara G, Stanghellini V, Bortolotti M, et al. 75SeHCAT scan in chronic diarrhea for bile acid malabsorption: update of an Italian single center experience (OP-803). Presented at the European Association of Nuclear Medicine Annual Congress, online, 22–30 October 2020. Eur J Nucl Med Mol Imaging 2020;47:S1-753.
- Fullard K, Clingan E, Cusick P, Valiozis I, Danta M, Moffitt M, et al. 75-seleno-homocholic acid-taurine (SEHCAT) test: a single-centre audit of study indications, and the influence of results on management. Presented at the 49th Annual Scientific Meeting of the Australian and New Zealand Society of Nuclear Medicine, Adelaide, SA, Australia, 26–28 April 2019. Intern Med J 2019;49.
- Kok B, Mistry A, Malhotra R, Burdsall J, Hall M, Ransford R, et al. SeHCAT – cast a wider net. Presented at the British Society of Gastroenterology Annual General Meeting, Glasgow, UK, 24–27 June 2013. Gut 2013;62:A123-4.
- Kurien M, Gleeson JT, Osborne C, Messham L, Sanders DS. Factors predictive of bile acid diarrhoea and long term treatment outcomes. Presented at the British Society of Gastroenterology Annual General Meeting, Manchester, UK, 16–19 June 2014. Gut 2014;63:A259-60.
- ClinicalTrials.gov . Trial to Understand Efficacy of Colesevelam in Diarrhea Predominant IBS Patients With Bile Acid Malabsorption n.d. https://ClinicalTrials.gov/show/NCT03270085 (accessed 15 April 2021).
- Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, et al. Canadian Association of Gastroenterology clinical practice guideline for the management of irritable bowel syndrome (IBS). J Can Assoc Gastroenterol 2019;2:6-29. https://doi.org/10.1093/jcag/gwy071.
- Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med 2015;15:252-7. https://doi.org/10.7861/clinmedicine.15-3-252.
- Pierry I, Cheesewright J, Doyle S, Redman S, Graham R, Little D. Patient outcomes in abnormal and equivocal SeHCAT studies. Presented at the 47th Annual Spring Meeting of the British Nuclear Medicine Society, Oxford, UK, 1–3 April 2019. Nucl Med Commun 2019;40.
- Reid F, Peacock J, Coker B, McMillan V, Lewis C, Keevil S, et al. A multicenter prospective study to investigate the diagnostic accuracy of the SeHCAT test in measuring bile acid malabsorption: research protocol. JMIR Res Protoc 2016;5. https://doi.org/10.2196/resprot.4467.
- Sanchez MM, Rodriguez AR, Buso MN, Davesa IT, Garcia MS, Sureda NF, et al. Utility of the SeHCAT procedure: may improve the quality of life of patients affected of bile acid malabsorption?. Eur J Nucl Med Mol Imaging 2016;43.
- Siu W, Ko I, McKiddle F, Clegg F, Bain G, McKinlay A. Is there a correlation between severity of bile acid malabsorption (BAM) and response to treatment? Presented at the British Society of Gastroenterology Annual General Meeting, Liverpool, UK, 5–7 June 2018. Gut 2018;67.
- Talavera Rubio MP, Casillas Sagrado E, Bellon Guardia M, Garcia Vicente A, Jimenez Londono GA, Olivencia Palomar P, et al. Prevalence of bile acid diarrhea as diagnosis by SeHCAT scanning and its response to treatment. Presented at the 31st Annual Congress of the European Association of Nuclear Medicine, Dusseldorf, Germany, 13–17 October 2018. Eur J Nucl Med Mol Imaging 2018;45:S329-30.
- Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156:1233-8.
- Woolson KL, Sherfi H, Sulkin T, Palmer J, Murray IA. SeHCAT: nice or not nice? Presented at the British Society of Gastroenterology Annual General Meeting, Manchester, UK, 16–19 June 2014. Gut 2014;63:A258-9.
Appendix 1 Literature search strategies
The following search strategies were based on those reported in the 2011 review; strategies were amended in line with the agreed final scope and updated to include any new terminology for the condition and interventions, and to compensate for any changes to search interfaces. Some resources, such as the Wiley Health Economic Evaluations Database and the National Guidelines Clearinghouse, are no longer available, and additional resources, such as Northern Light’s conference proceedings and ECRI Guidelines Trust, have been added to maintain the breadth of resources searched. To ensure completeness, all searches in both the clinical effectiveness and cost-effectiveness sections were screened for all areas of interest. For full details of strategies used in the 2011 review, see appendix 1 of Riemsma et al. 18
Clinical effectiveness
| Database | Date range searched | Hits (n) |
|---|---|---|
| EMBASE | 1974 to 25 November 2020 | 4797 |
| MEDLINE and PreMEDLINE | 1946 to 30 November 2020 | 2282 |
| CDSR and CDSR Protocols | Up to November 2020, issue 11 | 134 |
| CENTRAL | Up to November 2020, issue 11 | 404 |
| DARE | Up to March 2015 | 13 |
| HTA database (CRD) | Up to March 2018 | 3 |
| SCI | 1970 to 27 November 2020 | 1714 |
| KSR Evidence | Up to 1 December 2020 | 141 |
| LILACS | Up to 27 November 2020 | 246 |
| NIHR HTA | Up to 26 November 2020 | 3 |
| PROSPERO | Up to 26 November 2020 | 77 |
| ClinicalTrials.gov | Up to 26 November 2020 | 388 |
| WHO ICTRP | Up to 2 December 2020 | 301 |
| EUCTR | Up to 2 December 2020 | 70 |
| Northern Light | 2010 to December 2020, week 46 | 341 |
| CPCI-S | 1990 to 30 November 2020 | 390 |
| UEG Week 2020 (11–13 October, virtual) | 2020 | 3 |
| Total | 11,307 |
EMBASE (Ovid)
Date range searched: 1974 to 25 November 2020.
Date searched: 26 November 2020.
(SeHCAT OR BAS) + BAD (No A)
-
tauroselcholic acid/ (233)
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2).ti,ab,ot,hw,rn,tn. (397)
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75).ti,ab,ot,hw,tn. (1596)
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate).ti,ab,ot,hw,tn. (52)
-
(selenium adj3 “75”).ti,ab,ot,hw,tn. (860)
-
or/1-5 (2179)
-
bile acid sequestrant/ (1459)
-
((bile adj3 (acid or salt) adj3 sequest$) or BAS).ti,ab,ot,hw,rn. (19,061)
-
Colestipol/ or (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6).ti,ab,ot,hw,rn,tn. (2940)
-
Colestyramine/ or (colestyramine or chol-less or choles or cholesthexal or cholestyramin or cholestyramine or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid or lipocol-merz or lismol or locholest or prevalite or quantalan or questran or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0).ti,ab,ot,hw,rn,tn. (11,381)
-
Colesevelam/ or (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7).ti,ab,ot,hw,rn,tn. (1406)
-
aluminum hydroxide/ or (aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or alu-cap or alu-tab or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxyde or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or ulcerin-p or vanogel or 21645-51-2 or 1330-44-5 or 80206-84-4 or brasivil or rocgel or alugel or hydrated alumina or basalgel or dialume or nephrox).ti,ab,ot,hw,rn,tn. (10,894)
-
or/7-12 (41,822)
-
6 or 13 (43,803)
-
(BAM or I-BAM or IBAM or PBAM or BSM or BAD).ti,ab,ot,hw. (60,921)
-
bile acid diarrh?ea$.ti,ab,ot,hw. (227)
-
chronic diarrhea/ or bile acid/ or bile salt/ (36,292)
-
((chronic or explosive or smelly or watery or recur$ or persist$ or protract$ or continual$ or continuous$ or sustain$ or constant$ or relentless$ or unrelent$ or functional or aggressive) adj2 (diarrh?e$ or diarrea$ or f?eces)).ti,ab,ot,hw. (16,726)
-
(malabsorb$ or mal-absorb$ or malabsorp$ or mal-absorp$).ti,ab,ot,hw. (24,005)
-
((bile or biliary) adj3 (acid$ or salt$)).ti,ab,ot,hw. (50,197)
-
or/15-20 (148,024)
-
14 and 21 (5860)
-
animal/ (1,492,379)
-
animal experiment/ (2,624,468)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).mp. (6,912,383)
-
or/23-25 (6,912,383)
-
exp human/ (21,744,415)
-
human experiment/ (528,150)
-
or/27-28 (21,746,205)
-
26 not (26 and 29) (5,288,236)
-
22 not 30 (4797)
MEDLINE(Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily
Date range searched: 1946 to 30 November 2020.
Date searched: 1 December 2020.
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2).ti,ab,ot,hw,rn. (10)
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75).ti,ab,ot,hw,rn. (375)
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate).ti,ab,ot,hw,rn. (373)
-
(selenium adj3 “75”).ti,ab,ot,hw. (185)
-
or/1-4 (788)
-
((bile adj3 (acid or salt) adj3 sequest$) or BAS).ti,ab,ot,hw,rn. (5844)
-
Colestipol/ or (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6).ti,ab,ot,hw,rn. (551)
-
Cholestyramine Resin/ or (colestyramine$ or chol-less or choles or cholesthexal or cholestyramin or cholestyramine$ or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid$ or lipocol-merz or lismol or locholest or prevalite or quantalan or questran$ or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 or mk 135 or mk135).ti,ab,ot,hw,rn. (3644)
-
Colesevelam Hydrochloride/ or (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7).ti,ab,ot,hw,rn. (302)
-
Aluminum Hydroxide/ or (aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or alu-cap or alu-tab or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxyde or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or ulcerin-p or vanogel or 21645-51-2 or 1330-44-5 or 80206-84-4).ti,ab,ot,hw,rn. (6299)
-
or/6-10 (15,925)
-
5 or 11 (16,640)
-
(BAM or I-BAM or IBAM or PBAM or BSM or BAD).ti,ab,ot,hw. (40,139)
-
bile acid diarrh?ea$.ti,ab,ot,hw. (111)
-
diarrhea/ (48,230)
-
((chronic or explosive or smelly or watery or recur$ or persist$ or protracted or continual$ or continuous$ or sustain$ or constant$ or relentless$ or unrelent$ or functional or aggressive) adj2 (diarrh?e$ or diarrea$)).ti,ab,ot,hw. (10,235)
-
"Bile Acids and Salts"/ (22,496)
-
(malabsorb$ or mal-absorb$ or malabsorp$ or mal-absorp$).ti,ab,ot,hw. (17,246)
-
((bile or biliary) adj3 (acid$ or salt$)).ti,ab,ot,hw. (40,041)
-
or/13-19 (147,664)
-
12 and 20 (2978)
-
animals/ not (animals/ and humans/) (4,727,656)
-
21 not 22 (2282)
Cochrane Central Register of Controlled Trials (Wiley) and Cochrane Database of Systematic Reviews (Wiley)
Searched up to November 2020, issue 11.
Date searched: 26 November 2020.
#1 (tauroselcholic or selenohomocholyltaurine or “75018-71-2”) 5
#2 SeHCAT or “Se-HCAT” or 75SeHCAT or “Se-75” or “75-SeHCAT” or SE75 3000
#3 “ 23-seleno-25-homo-tauro-cholic acid” or selenium homocholic acid taurine or “23-selena-25-homocholyltaurine” or “23-selena-25-homotaurocholate” or “23- selena-25-homotaurocholic-acid” or selenium radioisotopes or tauroselenocholic acid or “75Se-homotaurocholate” 6
#4 selenium near “75” 33
#5 #1 OR #2 OR #3 OR #4 3033
#6 ((bile near (acid or salt) near sequest*) or BAS) 4488
#7 MeSH descriptor: [Colestipol] explode all trees 90
#8 Colestipol or cholestabyl or cholestipol or colestid or “diethylenetriamine-epichlorohydrin-copolymer” or “diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane” or “epichlorohydrin-copolymer-with-diethylenetriamine” or “flavored-colestid” or lestid or “u-26,597a” or “u-26597-a” or “u-26597a” or “u-26,597a” or “25085-17-0” or “37296-80-3” or “50925-79-6” 177
#9 MeSH descriptor: [Cholestyramine Resin] explode all trees 275
#10 (colestyramine or “chol-less” or choles or cholesthexal or cholestyramin or cholestyramine or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid or “lipocol-merz” or lismol or locholest or prevalite or quantalan or questran or “resincoles-tiramina” or resincolestiramina or “vasosan-p-granulat” or “vasosan-s-granulat” or “11041-12-6” or “58391-37-0”) 556
#11 MeSH descriptor: [Colesevelam Hydrochloride] explode all trees 107
#12 (Colesevelam or cholestagel or “gt-31-104” or “gt-31-104hb” or “gt-31-104” or “gt-31-104hb” or “gt31-104” or “gt31-104hb” or “gt31-104” or “gt31-104hb” or welchol or lodalis or “182815-43-6” or “182815-44-7”) 177
#13 MeSH descriptor: [Aluminum Hydroxide] explode all trees 579
#14 (aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or “alu-cap” or “alu-tab” or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxide or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or “ulcerin-p” or vanogel or “21645-51-2” or “1330-44-5” or “80206-84-4” or brasivil or rocgel or alugel or hydrated alumina or basalgel or dialume or nephrox) 7070
#15 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 11,461
#16 #5 or #15 12,601
#17 (bile acid near (diarrhoe* or diarrhe* or diarrea*)):ti,ab,kw 39
#18 (chronic or explosive or smelly or watery or recur* or persist* or protract* or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) near (diarrhoe* or diarrhe* or diarrea*):ti,ab,kw 1364
#19 (malabsorb* or “mal-absorb*” or malabsorp* or “mal-absorp*”):ti,ab,kw 1045
#20 (BAM or “I-BAM” or IBAM or PBAM or BSM or BAD):ti,ab,kw 2863
#21 ((bile or biliary) near (acid* or salt*)):ti,ab,kw 2196
#22 MeSH descriptor: [Bile Acids and Salts] explode all trees 1193
#23 MeSH descriptor: [Diarrhea] this term only 3119
#24 #17 or #18 or #19 or #20 or #21 or #22 or #23 10,503
#25 #16 and #24 539.
CDSR retrieved 131 records.
CDSR Protocols retrieved three records.
CENTRAL retrieved 404 records.
Database of Abstracts of Reviews of Effects (Centre for Reviews and Dissemination) and Health Technology Assessment database (Centre for Reviews and Dissemination) (www.crd.york.ac.uk/CRDWeb/)
Searched up to March 2015 and March 2018, respectively.
Date searched: 26 November 2020.
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2) 3
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75) 3
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid opr 75Se-homotaurocholate) 0
-
(selenium near “75”) 5
-
#1 OR #2 OR #3 OR #4 5
-
(((bile near (acid or salt) near sequest*) or BAS)) 30
-
MeSH DESCRIPTOR Colestipol EXPLODE ALL TREES 3
-
((Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6)) 21
-
MeSH DESCRIPTOR Cholestyramine Resin EXPLODE ALL TREES 6
-
((colestyramine or chol-less or choles or cholesthexal or cholestyramin or cholestyramine or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid or lipocol-merz or lismol or locholest or prevalite or quantalan or questran or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0)) 37
-
MeSH DESCRIPTOR Colesevelam Hydrochloride EXPLODE ALL TREES 1
-
(Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7) 4
-
MeSH DESCRIPTOR Aluminum Hydroxide EXPLODE ALL TREES 4
-
((aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or alu-cap or alu-tab or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxide or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or ulcerin-p or vanogel or 21645-51-2 or 1330-44-5 or 80206-84-4 or brasivil or rocgel or alugel or hydrated alumina or basalgel or dialume or nephrox)) 10
-
#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 86
-
((chronic or explosive or smelly or watery or recur* or persist* or protract* or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) near (diarrhoe* or diarrhe* or diarrea*)) 50
-
((malabsorb* or mal-absorb* or malabsorp* or mal-absorp*)) 44
-
((bile acid near (diarrhoe* or diarrhe* or diarrea*))) 0
-
((BAM or I-BAM or IBAM or PBAM or BSM or BAD)) 76
-
((bile or biliary) near (acid* or salt*)) 38
-
MeSH DESCRIPTOR Diarrhea EXPLODE ALL TREES 228
-
MeSH DESCRIPTOR Bile Acids and Salts EXPLODE ALL TREES 49
-
#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 442
-
#1 OR #15 88
-
#23 AND #24 17
-
(#25) IN DARE 13
-
(#25) IN HTA 3
Science Citation Index (Web of Science)
Date range searched: 1970 to 27 November 2020.
Date searched: 27 November 2020.
#21 1714 #19 not #20
#20 3,873,007 TS=(cat or cats or dog or dogs or animal or animals or rat or rats or hamster or hamster or feline or ovine or canine or bovine or sheep or mice)
#19 2659 #18 AND #12
#18 326,297 #17 OR #16 OR #15 OR #14 OR #13
#17 46,505 TS= ((bile or biliary) SAME (acid* or salt*))
#16 9898 TS= (malabsorb* or mal-absorb* or malabsorp* or mal-absorp*)
#15 5873 TS= ((chronic or explosive or smelly or watery or recur* or persist* or protracted or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) SAME (diarrh?e* or diarrea*))
#14 308 TS=bile acid diarrh?ea*
#13 265,782 TS= (BAM or I-BAM or IBAM or PBAM or BSM or BAD)
#12 43,961 #11 OR #5
#11 41,620 #10 OR #9 OR #8 OR #7 OR #6
#10 31,554 TS= (aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or alu-cap or alu-tab or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxyide or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or ulcerin-p or vanogel or 21645-51-2 or 1330-44-5 or 80206-84-4 or brasivil or rocgel or alugel or hydrated alumina or basalgel or dialume or nephrox)
#9 459 TS= (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7)
#8 2051 TS= (colestyramine* or chol-less or choles or cholesthexal or cholestyramin or cholestyramine* or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid* or lipocol-merz or lismol or locholest or prevalite or quantalan or questran* or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 or mk 135 or mk135)
#7 528 TS= (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6)
#6 9187 TS=((bile SAME (acid or salt) SAME sequest*) or BAS)
#5 2542 #4 OR #3 OR #2 OR #1
#4 1942 TS= (selenium SAME “75”)
#3 85 TS= (23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate)
#2 964 TS= (SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75)
#1 9 TS=(tauroselcholic or selenohomocholyltaurine or 75018-71-2)
KSR Evidence (https://ksrevidence.com/)
Searched up to 1 December 2020.
Date searched: 1 December 2020.
-
SeHCAT OR “Se-HCAT” OR 75SeHCAT OR “Se-75” OR “75-SeHCAT” OR SE75 in All text 6 results
-
tauroselcholic OR selenohomocholyltaurine OR “selenium homocholic acid taurine” OR “tauroselenocholic acid” OR “75Se-homotaurocholate” in All text 2 results
-
(“bile acid sequest*” or “bile salt sequest*”) in All text 14 results
-
Colestipol OR cholestabyl OR colestid in All text 4 results
-
colestyramine or Questra* or Cholybar or Olestyr in All text 3 results
-
Colesevelam or cholestagel or welchol or lodalis in All text 9 results
-
"aluminum hydroxide” or Ageldrate in All text 2 results
-
BAM or “I-BAM” or IBAM or PBAM or BSM in All text 16 results
-
(bile or biliary) AND (acid* or salt*) in All text 116 results
-
#9 or #8 or #7 or #6 or #5 or #4 or #1 or #2 or #3 in All text 141 results
Literature in the Health Sciences in Latin America and the Caribbean (LILACS) (https://lilacs.bvsalud.org/en/)
Searched up to 27 November 2020.
Date searched: 27 November 2020.
(SeHCAT OR “Se-HCAT” ) OR (tauroselcholic OR selenohomocholyltaurine OR “selenium homocholic acid taurine” OR “tauroselenocholic acid” OR “bile acid sequest*”) OR (Colestipol OR cholestabyl OR cholestipol OR colestid OR flavored-colestid OR lestid ) OR (colestyramine OR chol-less OR choles OR cholesthexal OR cholestyramin OR cholestyramine OR cholybar OR cholytar OR colestepril OR colestiramina OR colestran OR colestrol OR colestyramin OR cuemid OR lipocol-merz OR lismol OR locholest OR prevalite OR quantalan OR questran OR resincoles-tiramina OR resincolestiramina OR vasosan-p-granulat OR vasosan-s-granulat ) OR (Colesevelam OR cholestagel) OR (“aluminum hydroxide” OR Ageldrate OR “al u creme” OR alcid OR aldrox OR algeldraat OR algeldrate OR algelox OR alhydrogel OR alkagel OR alocol OR alokreem OR “alterna gel” OR “alu cap” OR alu-cap OR alu-tab OR alucol OR aludrox OR alugelibys OR alumigel OR “alumina gel” OR “alumina trihydrate” OR “aluminium hydroxide” OR “aluminium hydroxide” OR aluminoid OR aluminox OR “aluminum hydrate” OR “aluminum hydroxide gel” OR “aluminum oxide trihydrate” OR “aluminum trihydrate” OR alutab OR amphogel OR amphojel OR amphotabs OR antiphos OR bayerite OR chefarox OR collumina OR collumol OR colodral OR colugel OR creamalin OR cremORin OR diplogel OR luagel OR gastracol OR gastrosetarderm OR gelumina OR hoemigel OR hycolal OR hydracoll OR “hydrated alumina” OR hydrolum OR hydronal OR hydroxal OR lactalumina OR neutroxide OR palliacol OR pepsamar OR ulcerin-p OR vanogel OR brasivil OR rocgel OR alugel OR “hydrated alumina” OR basalgel OR dialume OR nephrox) OR (BAM OR I-BAM OR IBAM OR PBAM OR BSM) OR (((bile OR biliary) AND (acid* OR salt*) AND (diarrhoe* OR diarrhe* OR diarrea* OR malabsorb* OR mal-absorb* OR malabsorp* OR mal-absorp*)))
246 results (filtered to LILACS).
National Institute for Health and Care Research Health Technology Assessment (www.nihr.ac.uk)
Searched up to 26 November 2020.
Date searched: 26 November 2020.
Browsed by relevant terms; found 3 records.
PROSPERO (International Prospective Register of Systematic Reviews) (Centre for Reviews and Dissemination) (www.crd.york.ac.uk/PROSPERO/)
Searched up to 26 November 2020.
Date searched: 26 November 2020.
#1 SeHCAT OR Se-HCAT OR 75SeHCAT OR Se-75 OR 75-SeHCAT OR SE75 3
#2 tauroselcholic OR selenohomocholyltaurine OR selenium homocholic acid taurine OR tauroselenocholic acid OR 75Se-homotaurocholate 1
#3 bile acid sequest* 25
#4 MeSH DESCRIPTOR Colestipol EXPLODE ALL TREES 0
#5 Colestipol OR cholestabyl OR cholestipol OR colestid OR flavored-colestid OR lestid OR u-26,597a OR u-26597-a OR u-26597a OR u-26,597a OR 25085-17-0 OR 37296-80-3 OR 50925-79-6 10
#6 colestyramine or chol-less or choles or cholesthexal or cholestyramin or cholestyramine or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid or lipocol-merz or lismol or locholest or prevalite or quantalan or questran or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 13
#7 Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7 6
#8 MeSH DESCRIPTOR Aluminum Hydroxide EXPLODE ALL TREES 0
#9 aluminum hydroxide or Ageldrate or al u creme or alcid or aldrox or algeldraat or algeldrate or algelox or alhydrogel or alkagel or alocol or alokreem or alterna gel or alu cap or alu-cap or alu-tab or alucol or aludrox or alugelibys or alumigel or alumina gel or alumina trihydrate or aluminium hydroxide or aluminium hydroxide or aluminoid or aluminox or aluminum hydrate or aluminum hydroxide gel or aluminum oxide trihydrate or aluminum trihydrate or alutab or amphogel or amphojel or amphotabs or antiphos or bayerite or chefarox or collumina or collumol or colodral or colugel or creamalin or cremorin or diplogel or f 1000 or f1000 or fluagel or gastracol or gastrosetarderm or gelumina or hoemigel or hycolal or hydracoll or hydrated alumina or hydrolum or hydronal or hydroxal or lactalumina or neutroxide or palliacol or pepsamar or ulcerin-p or vanogel or 21645-51-2 or 1330-44-5 or 80206-84-4 or brasivil or rocgel or alugel or hydrated alumina or basalgel or dialume or nephrox 22
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 59
#11 BAM or I-BAM or IBAM or PBAM or BSM 18
#12 ((bile or biliary) near (acid* or salt*) near (diarrhoe* or diarrhe* or diarrea* or malabsorb* or mal-absorb* or malabsorp* or mal-absorp*)) 5
#13 #10 OR #11 OR #12 77
Clinical trials resources
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/search/advanced)
Searched up to 26 November 2020.
Date searched: 26 November 2020.
Expert search option:
(SeHCAT OR Se-HCAT OR 75SeHCAT OR Se-75 OR 75-SeHCAT OR SE75) OR (tauroselcholic OR selenohomocholyltaurine OR selenium homocholic acid taurine OR tauroselenocholic acid OR 75Se-homotaurocholate) OR (Colestipol OR cholestabyl OR cholestipol OR colestid OR flavored-colestid OR lestid OR u-26,597a OR u-26597-a OR u-26597a OR u-26,597a OR 25085-17-0 OR 37296-80-3 OR 50925-79-6) OR (colestyramine OR chol-less OR choles OR cholesthexal OR cholestyramin OR cholestyramine OR cholybar OR cholytar OR colestepril OR colestiramina OR colestran OR colestrol OR colestyramin OR cuemid OR lipocol-merz OR questran) OR (Colesevelam OR cholestagel OR gt-31-104 OR gt-31-104hb OR gt-31-104 OR gt-31-104hb OR gt31-104 OR gt31-104hb OR gt31-104 OR gt31-104hb OR welchol OR lodalis OR 182815-43-6 OR 182815-44-7) OR (aluminum hydroxide OR Ageldrate OR al u creme OR alcid OR aldrox OR algeldraat OR algeldrate OR algelox OR alhydrogel OR alkagel OR alocol OR alokreem OR alterna gel OR alu cap OR hycolal OR hydracoll OR hydrated alumina OR hydrolum OR hydronal OR hydroxal OR lactalumina OR neutroxide)
ClinicalTrials.gov retrieved 388 records.
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/)
Searched up to 2 December 2020.
Date searched: 2 December 2020.
Basic search option: search terms box.
| Search terms | Results (n) |
|---|---|
| SeHCAT OR Se-HCAT OR 75SeHCAT OR Se-75 OR 75-SeHCAT OR SE75 | 2 |
| tauroselcholic OR selenohomocholyltaurine OR selenium homocholic acid taurine OR tauroselenocholic acid OR 75Se-homotaurocholate | 0 |
| (Colestipol OR cholestabyl OR cholestipol OR colestid OR flavored-colestid OR lestid OR u-26,597a OR u-26597-a OR u-26597a OR u-26,597a OR 25085-17-0 OR 37296-80-3 OR 50925-79-6) | 7/9 |
| (colestyramine OR chol-less OR choles OR cholesthexal OR cholestyramin OR cholestyramine OR cholybar OR cholytar OR colestepril OR colestiramina OR colestran OR colestrol OR colestyramin OR cuemid OR lipocol-merz OR questran) | 39/64 |
| (Colesevelam OR cholestagel OR gt-31-104 OR gt-31-104hb OR gt-31-104 OR gt-31-104hb OR gt31-104 OR gt31-104hb OR gt31-104 OR gt31-104hb OR welchol OR 182815-43-6 OR 182815-44-7) | 66/76 |
| aluminum hydroxide OR Ageldrate OR al u creme OR alcid OR aldrox OR algeldraat OR algeldrate OR algelox OR alhydrogel OR alkagel OR alocol OR alokreem OR alterna gel OR alu cap OR hycolal OR hydracoll OR hydrated alumina OR hydrolum OR hydronal OR hydroxal OR lactalumina OR neutroxide | 189/268 |
| Total | 303 |
| Total without duplicates | 301 |
European Union Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/)
Searched up to 2 December 2020.
Date searched: 2 December 2020.
Advanced search option: search terms box.
| Search terms | Results (n) |
|---|---|
| SeHCAT OR Se-HCAT OR 75SeHCAT OR Se-75 OR 75-SeHCAT OR SE75 | 11 |
| tauroselcholic OR selenohomocholyltaurine OR selenium homocholic acid taurine OR tauroselenocholic acid OR 75Se-homotaurocholate | 1 |
| (Colestipol OR cholestabyl OR cholestipol OR colestid OR flavored-colestid OR lestid OR u-26,597a OR u-26597-a OR u-26597a OR u-26,597a OR 25085-17-0 OR 37296-80-3 OR 50925-79-6) | 2 |
| (colestyramine OR chol-less OR choles OR cholesthexal OR cholestyramin OR cholestyramine OR cholybar OR cholytar OR colestepril OR colestiramina OR colestran OR colestrol OR colestyramin OR cuemid OR lipocol-merz OR questran) | 58 |
| (Colesevelam OR cholestagel OR gt-31-104 OR gt-31-104hb OR gt-31-104 OR gt-31-104hb OR gt31-104 OR gt31-104hb OR gt31-104 OR gt31-104hb OR welchol OR 182815-43-6 OR 182815-44-7) | 10 |
| aluminum hydroxide OR Ageldrate OR al u creme OR alcid OR aldrox OR algeldraat OR algeldrate OR algelox OR alhydrogel OR alkagel OR alocol OR alokreem OR alterna gel OR alu cap OR hycolal OR hydracoll OR hydrated alumina OR hydrolum OR hydronal OR hydroxal OR lactalumina OR neutroxide | 1 |
| Total | 83 |
| Total without duplicates | 70 |
Conference searches
Northern Light Life Sciences Conference Abstracts (Ovid)
Date range searched: 2010 to December 2020, week 46.
Date searched: 1 December 2020.
SeHCAT OR (BAS + BAD)
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2).af. (1)
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75).af. (84)
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate).af. (0)
-
(selenium adj3 “75”).af. (0)
-
or/1-4 (84)
-
((bile adj3 (acid or salt) adj3 sequest$) or BAS).af. (1813)
-
Colestipol/ or (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6).af. (30)
-
Cholestyramine Resin/ or (colestyramine$ or chol-less or choles or cholesthexal or cholestyramin or cholestyramine$ or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid$ or lipocol-merz or lismol or locholest or prevalite or quantalan or questran$ or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 or mk 135 or mk135).af. (83)
-
Colesevelam Hydrochloride/ or (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7).af. (117)
-
or/6-9 (2000)
-
diarrhea/ (28,402)
-
“Bile Acids and Salts”/ (0)
-
((bile or biliary) adj3 (acid* or salt*)).af. (2490)
-
(malabsorb$ or mal-absorb$ or malabsorp$ or mal-absorp$).af. (922)
-
((chronic or explosive or smelly or watery or recur$ or persist$ or protracted or continual$ or continuous$ or sustain$ or constant$ or relentless$ or unrelent$ or functional or aggressive) adj3 (diarrh?e$ or diarrea$)).af. (746)
-
bile acid diarrh?ea$.af. (53)
-
(BAM or I-BAM or IBAM or PBAM or BSM or BAD).ti,ab. (3287)
-
or/11-17 (34,584)
-
10 and 18 (277)
-
5 or 19 (341)
Conference Proceedings Citation Index – Science (Web of Science)
Date range searched: 1990 to 30 November 2020.
Date searched: 1 December 2020.
Indexes = CPCI-S Timespan = All years.
# 18 390 #5 or #17
# 17 137 #10 and #16
# 16 63,296 #11 or #12 or #13 or #14 or #15
# 15 4517 TS= ((bile or biliary) SAME (acid* or salt*) )
# 14 761 TS= (malabsorb* or mal-absorb* or malabsorp* or mal-absorp*)
# 13 52 TS=bile acid diarrh?ea*
# 12 284 TS= ((chronic or explosive or smelly or watery or recur* or persist* or protracted or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) SAME (diarrh?e* or diarrea*))
# 11 57,908 TS= (BAM or I-BAM or IBAM or PBAM or BSM or BAD)
# 10 1306 #6 or #7 or #8 or #9
# 9 67 TS= (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7)
# 8 143 TS= (colestyramine* or chol-less or choles or cholesthexal or cholestyramin or cholestyramine* or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid* or lipocol-merz or lismol or locholest or prevalite or quantalan or questran* or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 or mk 135 or mk135)
# 7 48 TS= (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6)
# 6 1088 TS=((bile SAME (acid or salt) SAME sequest*) or BAS)
# 5 257 #1 or #2 or #3 or #4
# 4 151 TS= (selenium SAME “75”)
# 3 6 TS= (23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate)
# 2 148 TS= (SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75)
# 1 0 TS=(tauroselcholic or selenohomocholyltaurine or 75018-71-2)
Named conferences previously individually searched in 2011 review
| Conference | On EMBASE | On Northern Light | Web search |
|---|---|---|---|
| BSG Annual Meetings | 2013–2018 |
Annual Meeting 2013–2019 No 2012 meeting found |
2020 postponed until February 2021 |
|
Advances in Clinical Oesophageal Investigation Conference (Ascona Essentials 2011) Online Learning in Gastroenterology (OLGa) (https://olga.uegf.org/portal/documents-explore.html#solr0)a |
NA | NA | NA |
|
8th Summer School of Gastroenterology (ASNEMGE-SS-PRAGUE 2011) Online Learning in Gastroenterology (OLGa) (http://olga.uegf.org/portal/documents-explore.html#solr0)a |
NA | NA | NA |
|
GASTRO2009 Online Learning in Gastroenterology (OLGa) (http://olga.uegf.org/portal/documents-explore.html#solr0)a |
NA | NA | NA |
| United European Gastroenterology Week Online Learning in Gastroenterology (OLGa) (http://olga.uegf.org/portal/documents-explore.html#solr0)a | United European Gastroenterology Week 2012–2019 | 2020 online (see below) |
United European Gastroenterology Week 2020 (https://ueg.eu/library)
Search limited to 2020.
Date searched: 4 February 2021.
| Keyword | Results (n) |
|---|---|
| “SeHCAT” | 3 |
| “Se-HCAT” | 0/3 (duplicate) |
| “75SeHCAT” | 0/1 |
| “75-SeHCAT” | 0/1 |
| Total | 3 |
Targeted search: trial of treatment
| Database | Date range searched | Hits (n) |
|---|---|---|
| EMBASE | 1974 to 17 February 2021 | 707 |
| MEDLINE + PreMEDLINE | 1946 to 17 February 2021 | 138 |
| Total | 845 | |
EMBASE (Ovid)
Date range searched: 1974 to 17 February 2021.
Date searched: 18 February 2021.
IBS/Crohns + BAS
-
irritable colon/ (27,190)
-
(Irritable bowel syndrome$ or IBS or IBS-D).ti,ab,ot,hw. (25,851)
-
((spastic or irritable or spasm or unstable) adj2 colon).ti,ab,ot,hw. (27,358)
-
((Colitis or colitides) adj2 (spastic or mucous or mucomembraneous or mucomembranous)).ti,ab,ot,hw. (33)
-
colonospasm.ti,ab,ot,hw. (0)
-
or/1-5 (33,265)
-
((cleron or Crohn$) adj3 disease).ti,ab,ot,hw. (103,028)
-
exp Crohn disease/ (94,904)
-
((regional or regionalis or granulomatous) adj3 (enteritis or enterocolitis)).ti,ab,ot,hw. (686)
-
morbus crohn.ti,ab,ot,hw. (1247)
-
Ileocolitis.ti,ab,ot,hw. (626)
-
(ileitis adj3 (terminal or regional)).ti,ab,ot,hw. (601)
-
colitis granulomatous.ti,ab,ot,hw. (8)
-
or/7-13 (103,673)
-
6 or 14 (134,788)
-
bile acid sequestrant/ (1478)
-
((bile adj3 (acid or salt) adj3 sequest$) or BAS).ti,ab,ot,hw,rn. (19,064)
-
Colestipol/ or (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6).ti,ab,ot,hw,rn,tn. (2965)
-
Colestyramine/ or (colestyramine or chol-less or choles or cholesthexal or cholestyramin or cholestyramine or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid or lipocol-merz or lismol or locholest or prevalite or quantalan or questran or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0).ti,ab,ot,hw,rn,tn. (11,483)
-
Colesevelam/ or (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7).ti,ab,ot,hw,rn,tn. (1427)
-
or/16-20 (31,271)
-
15 and 21 (707)
MEDLINE(Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily
Date range searched: 1946 to 17 February 2021.
Date searched: 18 February 2021.
-
Irritable bowel syndrome/ (7599)
-
(Irritable bowel syndrome$ or IBS or IBS-D).ti,ab,ot,hw. (16,494)
-
((spastic or irritable or spasm or unstable) adj2 colon).ti,ab,ot,hw. (583)
-
((Colitis or colitides) adj2 (spastic or mucous or mucomembraneous or mucomembranous)).ti,ab,ot,hw. (48)
-
colonospasm.ti,ab,ot,hw. (0)
-
or/1-5 (16,927)
-
((cleron or Crohn$) adj3 disease).ti,ab,ot,hw. (56,412)
-
Crohn Disease/ (39,573)
-
((regional or regionalis or granulomatous) adj3 (enteritis or enterocolitis)).ti,ab,ot,hw. (1214)
-
morbus crohn.ti,ab,ot,hw. (869)
-
Ileocolitis.ti,ab,ot,hw. (430)
-
(ileitis adj3 (terminal or regional)).ti,ab,ot,hw. (757)
-
colitis granulomatous.ti,ab,ot,hw. (7)
-
or/7-13 (56,906)
-
6 or 14 (73,191)
-
((bile adj3 (acid or salt) adj3 sequest$) or BAS).ti,ab,ot,hw,rn. (6014)
-
Colestipol/ or (Colestipol or cholestabyl or cholestipol or colestid or diethylenetriamine-epichlorohydrin-copolymer or diethylenetriamine-polymer-with-1-chloro-2,3-epoxypropane or epichlorohydrin-copolymer-with-diethylenetriamine or flavored-colestid or lestid or u-26,597a or u-26597-a or u-26597a or u-26,597a or 25085-17-0 or 37296-80-3 or 50925-79-6).ti,ab,ot,hw,rn. (550)
-
Cholestyramine Resin/ or (colestyramine$ or chol-less or choles or cholesthexal or cholestyramin or cholestyramine$ or cholybar or cholytar or colestepril or colestiramina or colestran or colestrol or colestyramin or cuemid$ or lipocol-merz or lismol or locholest or prevalite or quantalan or questran$ or resincoles-tiramina or resincolestiramina or vasosan-p-granulat or vasosan-s-granulat or 11041-12-6 or 58391-37-0 or mk 135 or mk135).ti,ab,ot,hw,rn. (3666)
-
Colesevelam Hydrochloride/ or (Colesevelam or cholestagel or gt-31-104 or gt-31-104hb or gt-31-104 or gt-31-104hb or gt31-104 or gt31-104hb or gt31-104 or gt31-104hb or welchol or lodalis or 182815-43-6 or 182815-44-7).ti,ab,ot,hw,rn. (307)
-
or/16-19 (9845)
-
15 and 20 (138)
Guidelines
| Database | Date range searched | Hits (n) |
|---|---|---|
| Trip | 2011 to 10 December 2020 | 1022 |
| GIN | 2011 to 15 December 2020 | 11 |
| HTA database | Up to 31 March 2018 | 117 |
| NICE | Up to 15 December 2020 | 13 |
| NIHR HTA | Up to 16 December 2020 | 42 |
| ECRI | Up to 16 December 2020 | 31 |
| NHS Evidence | Up to 16 December 2020 | 355 |
| Total | 1591 | |
Trip database (www.tripdatabase.com/)
Date range searched: 2011 to 10 December 2020.
Date searched: 10 December 2020.
The search was conducted from 2011 to present to provide a year’s overlap with the original searches.
| Terms searched (guidelines only, 2011 to present) | Hits |
|---|---|
| BAM or I-BAM or IBAM or PBAM or “Bile acid malabsorption” |
|
| SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75 |
|
| "chronic diarrhea” or “chronic diarrhoea” or “functional diarrhea” or “functional diarrhoea" |
|
| “Irritable bowel syndrome” or “Irritable bowel syndromes” or IBS or IBS-D or “spastic colon” |
|
| “Crohns disease” or “Crohn disease” or “Crohn’s disease” |
|
| Total | 1022 |
Guidelines International Network (www.g-i-n.net)
Date range searched: 2011 to 15 December 2020.
Date searched: 15 December 2020.
| Terms searched | Hits (n) |
|---|---|
| SeHCAT | 0 |
| Se-HCAT | 0 |
| 75SeHCAT | 0 |
| Bile acid* | 0 |
| Bile salt* | 0 |
| BAM | 0 |
| BAD | 0/1 (not relevant) |
| Irritable bowel syndrome* | 3 |
| IBS* | 0 |
| spastic colon | 0 |
| Crohn* | 3 |
| diarrhea* | 5 |
| diarrhoea* | 0/2 (dupes) |
| Total (after deduplication) | 11 |
Health Technology Assessment database (Centre for Reviews and Dissemination) (www.crd.york.ac.uk/CRDWeb/)
Searched up to 31 March 2018.
Date searched: 16 December 2020.
-
MeSH DESCRIPTOR Irritable Bowel Syndrome EXPLODE ALL TREES 103
-
(((Irritable bowel syndrome* or IBS or IBS-D or spastic colon))) 189
-
((BAM or I-BAM or IBAM or PBAM )) 1
-
(((Bile near acid*) OR (Biliary near acid*) OR (Bile near salt*) OR (Biliary near salt*)) ) 38
-
MeSH DESCRIPTOR Crohn Disease EXPLODE ALL TREES 220
-
(((Crohn* near disease)) ) 356
-
((((chronic near diarrhoea*) or (chronic near diarrhea*))) ) 22
-
MeSH DESCRIPTOR Diarrhea EXPLODE ALL TREES 228
-
((SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75) ) 3
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 792
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) IN HTA 117
National Institute for Health and Care Excellence guidance (https://guidance.nice.org.uk/)
Searched up to 15 December 2020.
Date searched: 15 December 2020.
Limited to published guidelines only.
| Terms searched | Hits (n) |
|---|---|
| SeHCAT | 1 |
| Bile acid | 0/1 |
| Bile salt | 0 |
| diarrhoea | 2/3 (duplicate) |
| diarrhea | 0 |
| Irritable bowel syndrome | 2/4 (dupes) |
| IBS | 0/1 |
| Crohn | 8/9 |
| Crohn’s | 0/5 |
| Total (prior to deduplication) | 13/24 |
National Institute for Health and Care Research Health Technology Assessment (www.nihr.ac.uk)
Searched up to 16 December 2020.
Date searched: 16 December 2020.
| Terms searched | Hits (n) |
|---|---|
| SeHCAT | 3 |
| Bile acid | 1/4 (dupe) |
| Bile salt | 0/1 |
| diarrhoea | 18/20 |
| diarrhea | 0/1 |
| Irritable bowel syndrome | 11/13 |
| IBS | 2/13 |
| Crohn | 3 |
| Crohn’s | 4 |
| Total (prior to deduplication) | 42/62 |
ECRI (www.ecri.org/)
Searched up to 16 December 2020.
Date searched: 16 December 2020.
| Terms searched | Hits (n) |
|---|---|
| BAM OR “I BAM” OR IBAM OR PBAM OR “Bile acid malabsorption” OR “Bile acid diarrhoea” | 0 |
| SeHCAT OR “Se HCAT” OR 75SeHCAT OR “Se 75” OR “75 SeHCAT” OR SE75 | 0 |
| “chronic diarrhea” OR “chronic diarrhoea” OR “functional diarrhea” OR “functional diarrhoea” | 3 |
| “Irritable bowel syndrome” OR “Irritable bowel syndromes” OR IBS OR “IBS D” OR “spastic colon” | 27/28 (dupe) |
| “Crohns disease” OR “Crohn disease” OR “Crohn’s disease” | 1 |
| Total | 31/32 |
NHS Evidence (www.evidence.nhs.uk/)
Searched up to 16 December 2020.
Date searched: 16 December 2020.
Limited to guidance and health technology assessments.
| Terms searched | Hits (n) |
|---|---|
| BAM OR “I BAM” OR IBAM OR PBAM OR “Bile acid malabsorption” OR “Bile acid diarrhoea” | 33 |
| SeHCAT OR “Se HCAT” OR 75SeHCAT OR “Se 75” OR “75 SeHCAT” OR SE75 | 4/13 (dupes) |
| “chronic diarrhea” OR “chronic diarrhoea” OR “functional diarrhea” OR “functional diarrhoea” | 68/77 |
| “Irritable bowel syndrome” OR “Irritable bowel syndromes” OR IBS OR “IBS D” OR “spastic colon” | 181/220 |
| “Crohns disease” OR “Crohn disease” OR “Crohn’s disease” | 69/87 |
| Total | 355/430 |
Cost-effectiveness searches
| Database | Date range searched | Hits (n) |
|---|---|---|
| EMBASE | 1974 to 7 January 2021 | 908 |
| MEDLINE + PreMEDLINE | 1946 to 7 January 2021 | 571 |
| SCI | 1988 to 5 January 2021 | 1036 |
| NHS EED | Up to March 2015 | 92 |
| EconLit | Up to 22 December 2020 | 87 |
| IDEAS (RePEc) | Up to 23 February 2021 | 94 |
| CEA Registry | 2012 to 14 January 2021 | 270 |
| ScHARRHUD | Up to 23 February 2021 | 6 |
| Total | 3064 | |
EMBASE (Ovid)
Date range searched: 1974 to 7 January 2021.
Date searched: 8 January 2021.
(SeHCAT or BAD) + (Costs or HRQoL)
-
tauroselcholic acid/ (236)
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2).ti,ab,ot,hw,rn,tn. (401)
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75).ti,ab,ot,hw,tn. (1604)
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate).ti,ab,ot,hw,tn. (52)
-
(selenium adj3 “75”).ti,ab,ot,hw,tn. (865)
-
or/1-5 (2192)
-
(BAM or I-BAM or IBAM or PBAM or BSM).ti,ab,ot,hw. (5123)
-
bile acid diarrh?ea$.ti,ab,ot,hw. (234)
-
chronic diarrhea/ (6082)
-
((chronic or explosive or smelly or watery or recur$ or persist$ or protract$ or continual$ or continuous$ or sustain$ or constant$ or relentless$ or unrelent$ or functional or aggressive) adj2 (diarrh?e$ or diarrea$ or f?eces)).ti,ab,ot,hw. (16,950)
-
bile acid/ or bile salt/ (30,709)
-
((bile or biliary) adj3 (acid$ or salt$)).ti,ab,ot,hw. (50,633)
-
or/11-12 (50,633)
-
(malabsorb$ or mal-absorb$ or malabsorp$ or mal-absorp$ or diarrh?e$ or diarrea$ or f?eces).ti,ab,ot,hw. (456,414)
-
13 and 14 (6257)
-
7 or 8 or 9 or 10 or 15 (27,573)
-
6 or 16 (29,353)
-
health-economics/ (33,339)
-
exp economic-evaluation/ (314,387)
-
exp health-care-cost/ (298,733)
-
exp pharmacoeconomics/ (206,492)
-
or/18-21 (663,912)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (1,123,509)
-
(expenditure$ not energy).ti,ab. (42,225)
-
(value adj2 money).ti,ab. (2528)
-
budget$.ti,ab. (40,216)
-
or/23-26 (1,161,371)
-
22 or 27 (1,493,189)
-
letter.pt. (1,161,283)
-
editorial.pt. (682,769)
-
note.pt. (835,840)
-
or/29-31 (2,679,892)
-
28 not 32 (1,371,483)
-
(metabolic adj cost).ti,ab. (1586)
-
((energy or oxygen) adj cost).ti,ab. (4490)
-
((energy or oxygen) adj expenditure).ti,ab. (32,838)
-
or/34-36 (37,782)
-
33 not 37 (1,363,739)
-
exp animal/ (26,642,890)
-
exp animal-experiment/ (2,658,841)
-
nonhuman/ (6,445,151)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,871,157)
-
or/39-42 (28,677,854)
-
exp human/ (21,887,724)
-
exp human-experiment/ (531,547)
-
44 or 45 (21,889,585)
-
43 not (43 and 46) (6,789,265)
-
38 not 47 (1,240,250)
-
17 and 48 (805)
-
quality adjusted life year/ or quality of life index/ (30,846)
-
Short Form 12/ or Short Form 20/ or Short Form 36/ or Short Form 8/ (37,865)
-
“International Classification of Functioning, Disability and Health"/ or “ferrans and powers quality of life index”/ or “gastrointestinal quality of life index"/ (3639)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (43,041)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (2536)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (9907)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (1590)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (466)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (995)
-
“health related quality of life”.ti,ab,ot. (66,854)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (20,857)
-
“assessment of quality of life”.ti,ab,ot. (3015)
-
(euroqol or euro qol or eq5d$ or eq 5d$).ti,ab,ot. (22,328)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (35,847)
-
(hye or hyes).ti,ab,ot. (140)
-
health$ year$ equivalent$.ti,ab,ot. (41)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (3255)
-
(quality time or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or index of well being).ti,ab,ot,hw. (1248)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (5242)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (26,726)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (12,758)
-
15d.ti,ab,ot. (2629)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (652)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (20,906)
-
(utilities or disutili$).ti,ab,ot. (12,817)
-
or/50-74 (201,789)
-
animal/ or animal experiment/ (4,123,202)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,931,026)
-
or/76-77 (6,931,026)
-
exp human/ or human experiment/ (21,889,542)
-
78 not (78 and 79) (5,299,870)
-
75 not 80 (198,713)
-
letter.pt. (1,161,283)
-
editorial.pt. (682,769)
-
note.pt. (835,840)
-
or/82-84 (2,679,892)
-
81 not 85 (193,533)
-
17 and 86 (117)
-
49 or 87 (908)
Health-related quality-of-life free-text terms based on figure 4 in Papaioannou et al. 84
Economics terms based on costs filter. 85
MEDLINE(Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily
Date range searched: 1946 to 7 January 2021.
Date searched: 8 January 2021.
-
(tauroselcholic or selenohomocholyltaurine or 75018-71-2).ti,ab,ot,hw,rn. (11)
-
(SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75).ti,ab,ot,hw,rn. (379)
-
(23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate).ti,ab,ot,hw,rn. (375)
-
(selenium adj3 “75”).ti,ab,ot,hw. (185)
-
or/1-4 (794)
-
(BAM or I-BAM or IBAM or PBAM or BSM).ti,ab,ot,hw. (4250)
-
bile acid diarrh?ea$.ti,ab,ot,hw. (114)
-
((chronic or explosive or smelly or watery or recur$ or persist$ or protracted or continual$ or continuous$ or sustain$ or constant$ or relentless$ or unrelent$ or functional or aggressive) adj2 (diarrh?e$ or diarrea$)).ti,ab,ot,hw. (10,304)
-
“Bile Acids and Salts”/ (22,567)
-
((bile or biliary) adj3 (acid$ or salt$)).ti,ab,ot,hw. (40,304)
-
9 or 10 (40,304)
-
(malabsorb$ or mal-absorb$ or malabsorp$ or mal-absorp$ or diarrh?e$ or diarrea$ or f?eces).ti,ab,ot,hw. (239,246)
-
11 and 12 (4702)
-
6 or 7 or 8 or 13 (18,913)
-
5 or 14 (19,523)
-
economics/ (27,278)
-
exp “costs and cost analysis"/ (241,445)
-
economics, dental/ (1915)
-
exp “economics, hospital"/ (24,882)
-
economics, medical/ (9116)
-
economics, nursing/ (4002)
-
economics, pharmaceutical/ (2965)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (834,449)
-
(expenditure$ not energy).ti,ab. (31,015)
-
(value adj1 money).ti,ab. (36)
-
budget$.ti,ab. (30,332)
-
or/16-26 (988,351)
-
((energy or oxygen) adj cost).ti,ab. (4195)
-
(metabolic adj cost).ti,ab. (1467)
-
((energy or oxygen) adj expenditure).ti,ab. (25,724)
-
or/28-30 (30,394)
-
27 not 31 (981,379)
-
letter.pt. (1,116,589)
-
editorial.pt. (553,178)
-
historical article.pt. (361,613)
-
or/33-35 (2,011,424)
-
32 not 36 (944,275)
-
15 and 37 (460)
-
quality-adjusted life years/ or quality of life/ (212,806)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (26,438)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (2228)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (6146)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (869)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (411)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (624)
-
“health related quality of life”.ti,ab,ot. (45,881)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (13,582)
-
"assessment of quality of life".ti,ab,ot. (1885)
-
(euroqol or euro qol or eq5d$ or eq 5d$).ti,ab,ot. (11,979)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (21,793)
-
(hye or hyes).ti,ab,ot. (73)
-
health$ year$ equivalent$.ti,ab,ot. (40)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (1593)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (928)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (4351)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (15,549)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (8299)
-
15d.ti,ab,ot. (1754)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (427)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (12,970)
-
(utilities or disutili$).ti,ab,ot. (7771)
-
or/39-61 (271,557)
-
animals/ not (animals/ and humans/) (4,741,294)
-
62 not 63 (269,197)
-
letter.pt. (1,116,589)
-
editorial.pt. (553,178)
-
historical article.pt. (361,613)
-
or/65-67 (2,011,424)
-
64 not 68 (259,509)
-
15 and 69 (130)
-
38 or 70 (571)
Health-related quality-of-life free-text terms based on figure 4 in Papaioannou et al. 84
Economics terms based on costs filter. 86
Science Citation Index Expanded
Date range searched: 1988 to 5 January 2021.
Date searched: 5 January 2021.
Indexes=SCI-EXPANDED Timespan=All years.
# 48 1036 #47 OR #23
# 47 522 #46 AND #12
# 46 946,570 #45 OR #44 OR #43 OR #42 OR #41 OR #40 OR #39 OR #38 OR #37 OR #36 OR #35 OR #34 OR #33 OR #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24
# 45 291,280 TS=(utilities or disutili*)
# 44 161,090 TS=(utilit* SAME (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*) )
# 43 27,032 TS=(HSUV* or health state* value* or health state* preference* or HSPV*)
# 42 2073 TS=15d
# 41 41,585 TS=(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble* or “willingness to pay”)
# 40 15,178 TS=(QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL*)
# 39 35,560 TS=(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”)
# 38 444,159 TS=(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”)
# 37 1915 TS=(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)
# 36 8182 TS=(health* year* equivalent)
# 35 82 TS=(hye or hyes)
# 34 20,238 TS=(hql or hrql or hqol or “h qol” or hrqol or “hr qol”)
# 33 12,058 TS=(euroqol or euro qol or eq5d* or “eq 5d*”)
# 32 1445 TS=(“assessment of quality of life”)
# 31 29,569 TS=(Quality adjusted life or Quality-adjusted-life)
# 30 47,359 TS=(“health related quality of life”)
# 29 37,605 TS=(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight)
# 28 22,310 TS=(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty)
# 27 1757 TS=(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D)
# 26 27,957 TS=(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve)
# 25 59,806 TS=(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six)
# 24 32,632 TS=(sf36 or sf 36 Or sf-36 or short form 36 or shortform 36 or sf thirtysix or
sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six)
# 23 576 #22 AND #12
# 22 1,458,042 #17 NOT #21
# 21 214,593 #20 OR #19 OR #18
# 20 42,199 TS=((energy or oxygen) SAME expenditure)
# 19 14,878 TS=(metabolic SAME cost)
# 18 168,956 TS=((energy or oxygen) SAME cost)
# 17 1,637,264 #16 OR #15 OR #14 OR #13
# 16 85,538 TS=(budget*)
# 15 1818 TS=(value NEAR/1 money)
# 14 30,032 TS=(expenditure* not energy)
# 13 1,557,509 TS=(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*)
# 12 15,074 #11 OR #5
# 11 12,835 #10 OR #7 OR #6
# 10 1180 #9 AND #8
# 9 32,130 TS= (malabsorb* or mal-absorb* or malabsorp* or mal-absorp* or diarrh?e* or diarrea*)
# 8 46,799 TS= ((bile or biliary) SAME (acid* or salt*))
# 7 5833 TS= ((chronic or explosive or smelly or watery or recur* or persist* or protracted or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) SAME (diarrh?e* or diarrea*))
# 6 6045 TS= (BAM or I-BAM or IBAM or PBAM)
# 5 2434 #4 OR #3 OR #2 OR #1
# 4 1858 TS= (selenium SAME “75”)
# 3 79 TS= (23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate)
# 2 926 TS= (SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75)
# 1 5 TS= (tauroselcholic or selenohomocholyltaurine or 75018-71-2)
Health-related quality-of-life free-text terms based on figure 4 in Papaioannou et al. 84
Economics terms based on costs filter. 86
NHS Economic Evaluation Database (Centre for Reviews and Dissemination) (www.crd.york.ac.uk/CRDWeb/)
Searched up to March 2015.
Date searched: 22 December 2020.
-
((tauroselcholic or selenohomocholyltaurine or 75018-71-2)) 3
-
((SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75)) 3
-
((23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate)) 0
-
((selenium near “75”)) 5
-
#1 OR #2 OR #3 OR #4 5
-
MeSH DESCRIPTOR Diarrhea EXPLODE ALL TREES 228
-
MeSH DESCRIPTOR Bile Acids and Salts EXPLODE ALL TREES 49
-
(((BAM or I-BAM or IBAM or PBAM or BSM or BAD))) 76
-
(((bile or biliary) near (acid* or salt*))) 38
-
(((chronic or explosive or smelly or watery or recur* or persist* or protract* or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) near (diarrhoe* or diarrhe* or diarrea*))) 50
-
#6 OR #7 OR #8 OR #9 OR #10 404
-
#5 OR #11 406
-
(#12) IN NHSEED 92
EconLit (EBSCO)
Searched up to 22 December 2020.
Date searched: 22 December 2020.
Search modes: Boolean/Phrase
S10 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9) (87)
S9 (bile N4 acid*) or (biliary N4 acid*) or (bile N4 salt*) (1)
S8 (bile N4 acid*) or (biliary N4 acid*) or (bile N4 salt*) (0)
S7 (BAM or I-BAM or IBAM or PBAM) (57)
S6 (diarrhoe* or diarrhe* or diarrea*) N4 (chronic or explosive or smelly or watery or recur* or persist* or protracted or continual* or continuous* or sustain* or constant* or relentless* or unrelent* or functional or aggressive) (5)
S5 (selenium N4 “75”) (0)
S4 (selenium N4 “75”) (0)
S3 (23-seleno-25-homo-tauro-cholic acid or selenium homocholic acid taurine or 23-selena-25-homocholyltaurine or 23-selena-25-homotaurocholate or 23- selena-25-homotaurocholic-acid or selenium radioisotopes or tauroselenocholic acid or 75Se-homotaurocholate) (0)
S2 (SeHCAT or Se-HCAT or 75SeHCAT or Se-75 or 75-SeHCAT or SE75) (0)
S1 TX (tauroselcholic or selenohomocholyltaurine or 75018-71-2) (0)
Internet Documents in Economics Access Service (Research Papers in Economics) (https://ideas.repec.org/)
Searched up to 23 February 2021.
Date searched: 23 February 2021.
Date limit: 2010–2021.
| Search terms in title | Hits (n) |
|---|---|
| 'SeHCAT | “Se-HCAT” | 75SeHCAT | “75-SeHCAT”' | 0 |
| “bile acid diarrhea” | 0 |
| “bile acid diarrhea” | 0 |
| “chronic diarrhea” | 6 |
| “chronic diarrhoea” | 0 |
| “Irritable bowel syndrome” | IBS | 48 |
| crohn | crohns | 40 |
| Total | 94 |
Cost-Effectiveness Analysis Registry (https://healtheconomicsdev.tuftsmedicalcenter.org/cear2/search/search.aspx)
Date range searched: 1976 to 14 January 2021.
Date searched: 14 January 2021.
Results were limited to 2012 to present to follow on from the original search run on 6 February 2012.
| Terms searched | Ratios 2012 to present | Utility weights 2012 to present | Total |
|---|---|---|---|
| #1 Bile acid | 1 | 0 | 1 |
| #2 chronic diarrhea | 0 | 1 | 1 |
| #3 chronic diarrhoea | 0 | 0 | 0 |
| #4 IBS | 34 | 28 | 62 |
| #5 Irritable bowel syndrome | 1 | 5 | 6 |
| #6 Crohn | 100 (of 270 results, will only display first 100) | 100 (of 230) | 200 |
| Total | 136 | 134 | 270 |
School of Health and Related Research Health Utilities Database (www.scharrhud.org/)
Searched up to 23 February 2021.
Date searched: 23 February 2021.
| Terms searched | Total (n) |
|---|---|
| (Bile acid* or bile salt* or chronic) AND (diarrhea or diarrhoea or malabsorption) | 0 |
| (IBS or Irritable bowel syndrome) | 4 |
| Crohn* | 2 |
| Total | 6 |
Additional search for IBS/Crohns + Economic evaluations/Costs/HRQoL
Note that these searches are based on Search B: IBS + Cost/QoL [quality of life] and Search E: Crohns + Cost/QoL from the 2011 review; these were combined for efficiency.
| Database | Date range searched | Hits (n) |
|---|---|---|
| MEDLINE + PreMEDLINE | 1946 to 15 December 2020 | 1869 |
| NHS EED | Up to March 2015 | 95 |
| Total | 1964 | |
MEDLINE(Ovid) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily
Date range searched: 1946 to 15 December 2020.
Date searched: 17 December 2020.
IBS/Cohn’s + Cost/QoL
-
Irritable bowel syndrome/ (7531)
-
(Irritable bowel syndrome$ or IBS or IBS-D).ti,ab,ot,hw. (16,275)
-
((spastic or irritable or spasm or unstable) adj2 colon).ti,ab,ot,hw. (580)
-
((Colitis or colitides) adj2 (spastic or mucous or mucomembraneous or mucomembranous)).ti,ab,ot,hw. (46)
-
colonospasm.ti,ab,ot,hw. (0)
-
or/1-5 (16,708)
-
((cleron or Crohn*) adj3 disease).ti,ab,ot,hw. (55,778)
-
Crohn Disease/ (39,374)
-
((regional or regionalis or granulomatous) adj3 (enteritis or enterocolitis)).ti,ab,ot,hw. (1211)
-
morbus crohn.ti,ab,ot,hw. (863)
-
Ileocolitis.ti,ab,ot,hw. (428)
-
(ileitis adj3 (terminal or regional)).ti,ab,ot,hw. (751)
-
colitis granulomatous.ti,ab,ot,hw. (7)
-
or/7-13 (56,265)
-
6 or 14 (72,337)
-
economics/ (27,278)
-
exp “costs and cost analysis"/ (241,055)
-
economics, dental/ (1915)
-
exp “economics, hospital"/ (24,854)
-
economics, medical/ (9115)
-
economics, nursing/ (4002)
-
economics, pharmaceutical/ (2962)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (830,091)
-
(expenditure$ not energy).ti,ab. (30,889)
-
(value adj1 money).ti,ab. (36)
-
budget$.ti,ab. (30,225)
-
or/16-26 (983,786)
-
((energy or oxygen) adj cost).ti,ab. (4187)
-
(metabolic adj cost).ti,ab. (1463)
-
((energy or oxygen) adj expenditure).ti,ab. (25,656)
-
or/28-30 (30,314)
-
27 not 31 (976,823)
-
letter.pt. (1,114,970)
-
editorial.pt. (551,460)
-
historical article.pt. (361,434)
-
or/33-35 (2,007,937)
-
32 not 36 (939,757)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (26,334)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (2)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (2216)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (11,866)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab. (21,684)
-
(hye or hyes).ti,ab. (72)
-
health$ year$ equivalent$.ti,ab. (40)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (1578)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (484)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (17,231)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL).ti,ab. (35,444)
-
(FDDQL or GSRS-self or GSRS or GSRS-IBS or IBS-36 or IBS-QOL or IBS-SSS or IBS-D or WPAI:IBS* or IBSQoL).ti,ot. (95)
-
(GIQLI or DHSI or PDAI or HBI or “Harvey Bradshaw Index” or WPAI:CD* or “UC-CD Health Status” or SPACE-Q or PCDAI or CDEIS or CDAI or CLIQ or SES-CD).ti,ot. (135)
-
((Irritable Bowel Syndrome or Crohn$) adj Quality Of Life).ti,ab,ot,hw. (55)
-
(Quality of Life Questionnaire for Functional Digestive Disorders or Gastrointestinal Symptom Rating Scale).ti,ab,ot,hw. (443)
-
(Gastrointestinal Quality of Life index or Digestive Health Status Instrument).ti,ab,ot,hw. (442)
-
or/38-53 (74,796)
-
37 or 54 (992,674)
-
animals/ not (animals/ and humans/) (4,734,778)
-
55 not 56 (932,744)
-
15 and 57 (2793)
-
limit 58 to yr="2010 -Current” (1869)
NHS Economic Evaluation Database (Centre for Reviews and Dissemination)
Searched up to March 2015.
Date searched: 22 December 2020.
-
MeSH DESCRIPTOR Irritable Bowel Syndrome EXPLODE ALL TREES 103
-
((Irritable bowel syndrome* or IBS or IBS-D) ) 189
-
(((spastic or irritable or spasm or unstable) NEAR colon)) 0
-
(((Colitis or colitides) NEAR (spastic or mucous or mucomembraneous or mucomembranous)) ) 0
-
((colonospasm) ) 0
-
#1 OR #2 OR #3 OR #4 OR #5 189
-
MeSH DESCRIPTOR Crohn Disease EXPLODE ALL TREES 220
-
(((cleron or Crohn*) NEAR disease)) 356
-
(morbus crohn) 0
-
(((regional or regionalis or granulomatous) NEAR (enteritis or enterocolitis))) 0
-
((Ileocolitis) ) 1
-
((ileitis NEAR (terminal or regional))) 0
-
((colitis granulomatous) ) 0
-
#8 OR #9 OR #10 OR #11 OR #12 OR #13 356
-
#6 OR #14 537
-
(#15) IN NHSEED 95
Appendix 2 Data extraction tables
| Study | Participants (n) | Inclusion criteria | Exclusion criteria | Previous diagnostic investigations | Participant characteristics |
|---|---|---|---|---|---|
| Bellini 202025 | Total patients (n = 70)
|
Consecutive IBS-D and FD patients referred to a tertiary gastroenterology centre | None reported | NR |
|
| aBorghede 201134 | Total patients (n = 298)
|
All patients who received a SeHCAT scan during a 5-year period (2004–2009) | None reported | NR |
|
| Farmer 201726 | Total patients (n = 207)
|
Consecutive patients, with IBS-D, from a secondary care centre. IBS-D was defined according to the Rome III criteria (November 2014 to May 2016) or the Rome IV criteria (May 2016 to November 2016) | Serological/histological features of coeliac disease or a prior history of cholecystectomy or small bowel resection | NR | IBS-D (Rome III):
|
IBS-D (Rome IV):
|
|||||
| aFellous 199435 | Total patients (n = 106)
|
Patients with chronic diarrhoea referred to the hospital between 1990 and 1992 for a SeHCAT test to explore the cause of diarrhoea. Diarrhoea was defined as at least three soft stools or liquid diarrhoea per day for > 6 months. Normal hepatic balance | Insufficient clinical/biological information (n = 63) |
All patients were without clinical or biological abnormalities, and all had normal colonoscopy with biopsy and ileostomy When the clinical context and the examinations listed above did not allow the functional character of the diarrhoea to be confirmed, other investigations were carried out (duodenal biopsies, ileal biopsies, hail transit hormonal assays, Schilling test, D-xylose test, respiratory tests) |
Group 1:
|
Group 2:
|
|||||
Group 3:
|
|||||
| aFernandez-Bañares 200137 | Total patients (n = 83)
|
Consecutive patients, recruited between 1996 and 1999, with the following:
|
None reported |
All patients underwent the same diagnostic workup for chronic diarrhoea: bacterial cultures and faecal examination for ova and parasites; routine blood biochemistry and haematology (C-reactive protein, serum Ta and TSH, IgA anti-gliadin and anti-endomysium antibodies); small bowel follow-through; colonoscopy with multiple biopsies Additional investigations performed for some patients: biopsies of the second and/or third part of the duodenum, lactose hydrogen breath test, anorectal manometry, retrograde ileoscopy with biopsy of the terminal ileum |
Group 1:
|
Group 2:
|
|||||
| Fernández-Bañares 200738 |
Total patients (n = 62) Chronic watery diarrhoea and fulfilling the Rome II criteria for FD or IBS-D |
Consecutive adult (> 18 years) patients with non-bloody chronic watery diarrhoea, defined as more than three loose or liquid bowel movements a day for at least 4 weeks and a stool weight of > 200 g per day. Participants were required to fulfil the Rome II criteria for either FD or IBS-D | Previous cholecystectomy or vagotomy | Normal physical examination and blood analysis, including routine blood biochemistry and haematological counts, C-reactive protein, serum T4-TSH, and serum IgA-anti-endomysial and IgA-human anti-tissue transglutaminase antibodies. Negative faecal bacterial cultures and exam for ova and parasites. Normal full colonoscopy with multiple biopsies |
|
| aGalatola 199239 |
Total patients (n = 98) IBS-D |
Patients referred for a gastroenterological consultation, by their GP, because of abdominal pain or distress, who gave a history of increased bowel frequency (more than three per day) lasting for at least 3 months | Previous major abdominal surgical procedures (except cholecystectomy), liver disease, or an identified organic cause of symptoms | Negative results for routine biochemical, haematological, endoscopic, radiological and histological examinations implemented according to the clinical indications to search for an organic cause of their symptoms |
|
| Holmes 201227 |
Total patients (n = 55) Patients for whom notes were available (n = 44) SeHCAT-positive patients, with notes available (n = 28) |
Patients who had undergone SeHCAT testing, between 1 January 2005 and 31 December 2010 | None reported | NR |
|
| Kumar 201329 | Total patients (n = 88)
|
Consecutive patients referred for SeHCAT testing over a 1-year period | None reported | NR | None reported |
| Kumar 202028 | Total patients (n = 51)
|
Patients who had undergone a SeHCAT test for the investigation of chronic diarrhoea | None reported | NR | None reported |
| Lin 201630 |
Total patients (n = 515) SeHCAT-positive patients, commenced on BAS following diagnosis, who were contactable at follow-up: |
Patients who had undergone a SeHCAT test for the investigation of chronic diarrhoea, between 2001 and 2012 | None reported |
Previous colonic investigation (colonoscopy/barium enema/colon capsule), 434/515 (84%) Oesophagogastroduodenoscopy, 305/515 (59%) Small bowel investigations, 233/515 (45%) Coeliac serology, 433/515 (84%) |
|
| aMerrick 198540 | Patients (n = 106), normal controls (n = 63)
|
Normal controls: people who did not have gastrointestinal symptoms Patients: NR |
None reported | Diagnoses were based on a combination of clinical history, haematological findings, biochemistry and, when appropriate, barium follow-through, barium enema, and biopsy of the colon or small bowel. A hydrogen breath test was performed for patients who had undergone vagotomy. All diagnoses were verified by follow-up of at least 1 year | Group 1:
|
Group 2:
|
|||||
Group 3:
|
|||||
| Group 4: no details reported | |||||
| aNotta 201141 | Total patients (n = 37) | Patients with chronic diarrhoea for a duration of > 1 month and no previous treatment | Patients who were aged < 18 years, pregnant or breastfeeding | NR |
|
| Notta 201431 | Total patients (n = 78) | Patients with chronic FD | None reported | NR |
|
| Notta 201732 | Total patients (n = 92) | Patients with chronic FD | None reported | NR |
|
| aRudberg 199642 | Total patients (n = 20)
|
Patients with chronic or recurrent diarrhoea of unknown cause. Lactose-restricted diet, loperamide or anticholinergic agents had not relieved their symptoms | Patients with periods of constipation, dominating abdominal pain or fragmented mucous stools | Clinical, endoscopic and radiological examinations were performed, as well as laboratory tests, to exclude IBD, lactose intolerance, coeliac disease, abuse of laxative or other forms of diarrhoea |
|
| aSciarretta 198643 | Total participants (n = 89)
|
None reported | None reported | Group D: no evidence of organic pathology of the digestive tract, intestinal parasites, food allergies, or endocrine or metabolic diseases | Group D:
|
| aSciarretta 198744 | Total participants (n = 69), 23 healthy volunteers and 46 patients with IBS-D (n = 38) or prior cholecystectomy (n = 8) | Patients suffering from chronic or recurrent diarrhoea, which was thought to be functional | None reported | Chemical and microbiological faecal analyses were normal. Radiographic examinations of the large and small bowels, carried out using two contrast media, were negative. Diabetes and other endocrine disorders, and food allergies, were excluded | Patients:
|
| aSinha 199845 | Total patients (n = 17), patients with a positive SeHCAT test (n = 9) | Patients with chronic diarrhoea referred to the department and selected to undergo the SeHCAT test, based on a history suggestive of IBS-D (Manning criteria) and no other obvious cause of diarrhoea, who had a positive SeHCAT test result | None reported | Possible secondary causes of BAM were excluded by performing the following investigations for all patients: routine blood tests, random glucose, haematinic screen, stool microscopy and culture, small bowel enema to exclude structural ileal disease, gastroscopy and duodenal biopsy to exclude coeliac disease, para-aminobenzoic acid test to exclude pancreatic insufficiency, hydrogen and 14C-glycocholate breath tests to exclude bacterial overgrowth, barium enema and colonoscopy (six out of nine patients) to exclude large bowel disease | Patients with a positive SeHCAT test:
|
| aSmith 20007 | Total patients (n = 304)
|
Patients with chronic continuous or recurrent diarrhoea | None reported | NR | None reported |
| aTunney 201146 | Total patients (n = 276) with chronic diarrhoea, of whom 136 had no known risk factors | Patients who underwent SeHCAT scanning, for the investigation of chronic diarrhoea, between April 2005 and January 2011 | Patients referred from and managed by other hospital trust and patients seen on a private basis. Patients who did not have a SeHCAT scan at 7 days or who had technically void results. Patients taking a trial of BAS during investigation. Patients with no information in their electronic records | Over 80% of the patients with no known risk factors or diarrhoea post cholecystectomy had had documented coeliac screening, and 80% of the patients with no known risk factors for chronic diarrhoea had some form of bowel endoscopy | All patients:
|
| aWildt 200347 |
Total patients (n = 135) Groups, excluding two patients who were lost to follow-up (n = 133): |
Patients with chronic diarrhoea (defined by subjective reports of > 3 weeks’ change in stool frequency and/or consistency) who were investigated for BAM using the SeHCAT test | None reported | The SeHCAT test was generally carried out as a second-line investigation. First-line diagnostic evaluation, at minimum, included sigmoidoscopy or colonoscopy with mucosal biopsies, faecal examination for parasites and bacteria, biochemistry (haemoglobin, white blood cell count, C-reactive protein, electrolytes, renal parameters, liver function tests and thyroid-stimulating hormone). First-line evaluation also frequently included tests for coeliac disease and lactose malabsorption, and stool volume and stool lipid concentration |
|
| aWilliams 199148 | Patients (n = 181) | Patients referred for measurement of SeHCAT retention because of unexplained diarrhoea between 1982 and 1989 | Patients with IBD who had undergone previous radiotherapy to the abdomen; any form of bowel resection or other abdominal surgery were excluded | Stool culture, rigid sigmoidoscopy, barium enema, barium follow-through, jejunal biopsy and vitamin B12 absorption studies were performed in all patients | Patients with severe BAM (< 5%) (n = 23):
|
Patients with moderate BAM (≥ 5% to < 10%), who were treated with BAS (n = 13):
|
|||||
Patients with mild BAM (≥ 10 to < 15%) (n = 21b):
|
|||||
| Zanoni 201833 | Total patients (n = 12) with chronic diarrhoea without a known cause (n = 3) or IBS-D not responding to standard medication (n = 9) | Patients referred for SeHCAT with chronic diarrhoea without a known cause or IBS-D not responding to standard medication between November 2017 and April 2018 | NR | NR |
|
| Study | SeHCAT (index test) details | Treatment and response (reference standard) details |
|---|---|---|
| Bellini 202025 |
|
|
| aBorghede 201134 |
|
|
| Farmer 201726 |
|
|
| aFellous 199435 |
|
|
| aFernandez-Bañares 200137 |
|
|
| aFernández-Bañares 200738 |
|
|
| aGalatola 199239 |
|
|
| Holmes 201227 |
|
|
| Kumar 201329 |
|
|
| Kumar 202028 |
|
|
| Lin 201630 |
|
|
| aMerrick 198540 |
|
|
| aNotta 201141 |
|
|
| Notta 201431 |
|
|
| Notta 201732 |
|
|
| aRudberg 199642 |
|
|
| aSciarretta 198643 |
|
|
| aSciarretta 198744 |
|
|
| aSinha 199845 |
|
|
| aSmith 20007 |
|
|
| aTunney 201146 |
|
|
| aWildt 200347 |
|
|
| aWilliams 199148 |
|
|
| Zanoni 201833 |
|
|
Appendix 3 The quality assessment of diagnostic accuracy studies-2 assessments
Study: Merrick et al.40
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
Prospective study, but not clear if a consecutive or random sample of patients was enrolled. The study included four groups of patients:
|
|
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| The previous assessments/investigations undergone by patients in group 4 were not clear | |
| Do the included patients match the question? | Concerns: unclear |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| A tracer dose of < 100 µg of SeHCAT was administered labelled with 40 kBq (1 µCi) of selenium-75 (Amersham International). Seven days later, the patients reattended and a further whole-body count was obtained. Results were reported for prespecified 7-day retention thresholds and the index test was conducted before the reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: high |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Treatment: simple conservative treatment (colestyramine) in test-positive and ‘equivocal’ patients. Three patients (< 10%) were not treated. Test-negative patients were followed up for 12 to 24 months and received ‘simple conservative treatment’, which was reported to have ‘eased most or all symptoms’ | |
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Patients with SeHCAT 7-day retention of > 15% did not receive treatment with BAS; these patients were managed with ‘simple conservative treatment’. Patients were followed up for 12 to 24 months | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | No |
| Was response to treatment assessed over an adequate period? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: high |
Study: Sciarretta et al.43
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
Prospective study, but not clear if a consecutive or random sample of patients was enrolled. The study included four groups of patients:
|
|
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Group 4 included three patients with previous cholecystectomy. Patients in group 4 had no evidence of organic pathology of the digestive tract, intestinal parasites, food allergies, or endocrine or metabolic diseases; however, details of specific previous assessments/investigations were not provided | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| 370 kBq (10 µCi) of SeHCAT (provided by Amersham International Ltd) in capsule form, containing < 100 µg of active ingredient absorbed on inert carrier, was administered orally. A positive test was described as ‘SeHCAT values below the norm’. The lower limit of normal was reported as 34% for data obtained from the exponential abdominal activity retention curve for healthy controls on day 3; this was described by the authors as equivalent to a 7-day retention cut-off value of 5%. The index test was conducted before the reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: high |
| B. Applicability | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: high |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| All patients in group 4 were treated with colestyramine | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients in group 4 received the index test and were treated with colestyramine. The follow-up period was not reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Was response to treatment assessed over an adequate period? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: unclear |
Study: Sciarretta et al.44
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Not clear if the study was prospective or retrospective | |
| The study included healthy volunteers and patients with IBS or cholecystectomy. Data were extracted only for IBS/cholecystectomy patients | |
| There may be some overlap in populations in the two Sciarretta papers43,44 | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: unclear |
| B. Applicability | |
| Eight of the included patients had prior cholecystectomy. It was not clear that all assessments/investigations specified in current BSG guidelines3 had been carried out: chemical and microbiological faecal analyses were normal. Radiographic examinations of the large and small bowels, carried out using two contrast media, were negative. Diabetes and other endocrine disorders and food allergies were excluded | |
| Do the included patients match the question? | Concerns: high |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| 370 kBq (10 µCi) of SeHCAT (provided by Amersham International Ltd) in capsule form, containing < 100 µg of active ingredient absorbed on inert carrier, was administered orally. The threshold was prespecified as a 7-day retention value of 8%. The index test was conducted before the reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: high |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| All patients received treatment with 2–8 g of colestyramine, twice daily for at least 10 days. When colestyramine was not effective in relieving symptoms, therapy was discontinued. When colestyramine was effective, therapy was stopped for 7 days and started again if symptoms returned. A positive test was defined as symptom resolution on treatment and return of symptoms when treatment was discontinued. Stool frequency was taken as the average number of bowel actions per day over a 1-week period and was recorded before and after colestyramine administration | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | Risk: unclear |
| B. Applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| All patients received the index test and were treated with colestyramine. The follow-up period was not reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Was response to treatment assessed over an adequate period? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | Risk: unclear |
Appendix 4 Details of excluded studies with rationale
To be included in the review, studies had to fulfil the following criteria.
Population
Adults presenting with chronic diarrhoea with unknown cause, suspected or diagnosed IBS-D or FD (i.e. people with suspected primary BAD)
OR
Adults presenting with chronic diarrhoea and a diagnosis of Crohn’s disease, who have not undergone ileal resection (i.e. people with suspected secondary BAD).
Setting
Secondary care.
Index test
SeHCAT test (GE Healthcare UK Limited, Chalfont St Giles, UK).
Comparator
No SeHCAT test. (RCTs, CCTs and comparative observational studies.)
Reference standard
Response to treatment with BAS (predictive accuracy and response rate studies).
Outcome
-
Effect of testing on treatment plan (e.g. surgical or medical management, or further testing).
-
Effect of testing on clinical outcome (e.g. morbidity and adverse events).
-
Effect of testing on adherence to treatment.
-
Prognosis: the ability of test result to predict clinical outcome (i.e. response to treatment).
-
Predictive accuracy: sensitivity and specificity of SeHCAT for the prediction of treatment response.
-
Treatment outcome among patients with a positive SeHCAT result (i.e. sufficient data to calculate PPV).
-
Acceptability of tests to patients or surrogate measures of acceptability (e.g. waiting time and associated anxiety).
-
Adverse events associated with testing (e.g. pain/discomfort experienced during the procedure and waiting times before results).
-
Health-related quality of life.
Study design
Randomised controlled trials, CCTs, comparative observational studies, multivariable regression modelling studies, predictive accuracy studies, observational studies reporting response to treatments among patients with a positive SeHCAT test.
Table 56 summarises studies that were screened for inclusion based on full-text publication, but did not fulfil one or more of the above criteria. Table 57 summarises studies that were included in our previous systematic review, but that did not meet the inclusion criteria for this assessment. Studies were assessed sequentially against criteria; as soon as a study had failed based on one of the criteria, it was not assessed against subsequent criteria. The table shows which of the criteria each study fulfilled (‘yes’) and on which item it failed (‘no’) or was unclear.
| Study | Study design | Setting | Population | Intervention/index test | Comparator | Reference standard | Outcome |
|---|---|---|---|---|---|---|---|
| Albireo 201481 | Yes | Yes | Unclear | No | |||
| Appleby 201791 | Yes | Yes | Yes | Yes | NA | No | |
| Arms-Williams 201692 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Aujla 201493 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Baena Garcia 201994 | No | ||||||
| Bajor 201595 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Barber Caselles 201796 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Beigel 201479 | No | ||||||
| Bronte 202097 | Yes | Yes | No | ||||
| Carrasco-Labra 201998 | No | ||||||
| Damsgaard 201899 | Yes | Yes | Yes | Yes | NA | Unclearb | Unclearb |
| Fernandes 2018100 | No | ||||||
| Fraccascia 2020101 | Yes | Yes | Yes | Yes | NA | Unclearc | Unclearc |
| Fullard 2019102 | Yes | Yes | No | ||||
| Hendy 201577 | No | ||||||
| Kok 2013103 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Kurien 2014104 | No | ||||||
| ClinicalTrials.gov 2019105 | No | ||||||
| Moayyedi 2019106 | No | ||||||
| Orekoya 2015107 | Yes | Yes | Noa,b | Yes | NA | Yes | Yes |
| Pierry 2019108 | Yes | Yes | Noa,c | Yes | NA | Yes | Unclearc |
| Reid 2016109 | Yes | Yes | Yes | Yes | NA | Yes | No |
| Sanchez 2016110 | Yes | Yes | Noa,c | Yes | NA | Yes | Yes |
| Siu 2018111 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Slattery 201575 | No | ||||||
| Smith 201372 | No | ||||||
| Talavera Rubio 2018112 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
| Valentin 201676 | No | ||||||
| Vijayvargiya 2019113 | No | ||||||
| Wenzel 201978 | No | ||||||
| Woolson 2014114 | Yes | Yes | Noa,b | Yes | NA | Yes | Unclearb |
Appendix 5 Details on the estimation of medication costs
Details on the estimation of the medication costs used in this report are summarised in Tables 58–63.
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| A | Colestyramine | 50 | 2–8 g per day | 2–8 g | 5 g | £0.28 per 4 g | £0.35 |
| A | Colesevelam | 50 | 625 mg twice a day or four doses a day | 1250–2500 mg | 2500 mg | £0.64 per 625 mg | £2.56 |
| B | Colestyramine | 50 (40–60) | 2–8 g daily | 2–8 g | 5 g | £0.28 per 4 g | £0.35 |
| B | Colesevelam | 50 (40–60) | 625 mg up to six times per day | 3750 mg | 2500 mg | £0.64 per 625 mg | £2.56 |
| C | Colesevelam | 100 | 625 mg t.d.s. | 1875 mg | 2500 mg | £0.64 per 625 mg | £2.56 |
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| D | Colestyramine | 85 (70–95) | 2–8 g per day | 5 g | 5 g | £0.28 per 4 g | £0.35 |
| Colesevelam | 10 (10–20) | 1250–2500 mg | 2500 mg | 2500 mg | £0.64 per 625 mg | £2.56 | |
| F | Colesevelam | 100 | 1875 mgb | 2500 mg | 2500 mg | £0.64 per 625 mg | £2.56 |
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| G | Buscopan | 10 (1–15) | 10 b.i.d. p.r.n. | 20 mg | 20 mg | £0.05 per 10 mg | £0.11 |
| G | Loperamide | 20 (2–25) | 1 or 2 times per day | 1 or 2 times per day | 2 mg | £0.05 per 2 mg | £0.05 |
| G | Amitriptyline | 20 (15–35) | 5–10 mg | 5–10 mg | 11 mg | £0.04 per 10 mg | £0.04 |
| H | Loperamide | 50 (20–75) | 2 mg p.r.n. | 2 mg | 2 mg | £0.05 per 2 mg | £0.05 |
| H | Codeine | 5 (2–10) | 30 mg p.r.n. | 30 mg | 75 mg | £0.05 per 30 mg | £0.12 |
| H | Amitriptyline | 3 (1–5) | 10 mg+ | 10 mg | 11 mg | £0.21 per 4 mg | £0.04 |
| H | Buscopan | 20 (10–40) | NR | 20 mg | £0.05 per 10 mg | £0.11 | |
| I | Loperamide | 100 | 2 mg | 2 mg | 2 mg | £0.05 per 2 mg | £0.05 |
| I | TCA, assume amitriptyline | 50 | NR | 8 mg | 11 mg | £0.04 per 10 mg | £0.04 |
| J | Amitriptyline | NR | 10–20 mg | 10–20 mg | 11 mg | £0.04 per 10 mg | £0.04 |
| J | Loperamide | NR | 2 mg p.r.n. Maximum 16 mg per day | 2 mg | £0.05 per 2 mg | £0.05 | |
| J | Codeine | NR | 30 mg q.d.s. p.r.n. | 120 mg | 75 mg | £0.05 per 30 mg | £0.12 |
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| K | Asacol | 80 (70–90) | 4.8 g | 4.8 g | 4.8 g | £0.65 per 0.8 g | £3.92 |
| K | Octasa | 80 (70–90) | 4.8 g | 4.8 g | 4.8 g | £0.45 per 0.8 g | £2.69 |
| K | Pentasa | 80 (70–90) | 4 g | 4 g | 4 g | £0.61 per 1 g | £2.46 |
| K | Azathioprine | 50 (40–60) | 2.5 mg/kg weight adjusted | 2.5 mg/kg weight adjusted | 2.3 mg/kg | £0.06 per 50 mg | £0.20 |
| K | Infliximabb | 20 (10–30) | 10 mg/kg every 8 weeks | 14 mg | 14 mg | £377 per 100 mg | £49.01 |
| K | Adalimumabc | 20 (10–30) | 40 mg e.o.w. | 3 mg | 3 mg | £316.80 per 40 mg | £22.88 |
| L | Azathioprine | 50 (40–60) | 2 mg/kg | 2 mg/kg | 2.3 mg/kg | £0.06 per 50 mg | £0.20 |
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| M | Colestyramine | 30 (10–50) | 2–4 g | 2–8 g | 5 g | £0.28 per 4g | £0.35 |
| M | Colesevelam | 50 | 625 mg two to four times a day | 1250–2500 mg | 2500 mg | £0.64 per 625mg | £2.56 |
| N | Colestyramine | 70 (50–80) | 2–8 g daily | 2–8 g | 5 g | £0.28 per 4g | £0.35 |
| N | Colesevelam | 30 (20–50) | 625 mg up to six times per day | 3750 mg | 2500 mg | £0.64 per 625mg | £2.56 |
| O | Colesevelam | 10 | 625 mg t.d.s. | 1875 mg | 2500 mg | £0.64 per 625mg | £2.56 |
| Expert | Drug | % of patients (lowest–highest) | Dosage/frequency | Total dosage per day (expert) | Dosage per day (model)a | Price per unit | Cost per day |
|---|---|---|---|---|---|---|---|
| P | Loperamide | 80 (50–100) | 2–16 mg | 2–16 mg | 5 mg | £0.05 per 2 mg | £0.13 |
| P | Codeine | 20 (0–50) | 30–120 mg | 30–120 mg | 75 mg | £0.05 per 30 mg | £0.12 |
| P | Corticosteroids | 70 (50–100) | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | £0.13 per 20 mg of predsnisolone | £1.38 |
| £2.50 per 9 mg of budesonide | |||||||
| P | Anti-TNF-α adalimumab | 10 (0–30) | 40 mg e.o.w.b | 3 mg | 3 mg | £316.80 per 40 mg | £22.88 |
| Q | Pentasa® (Ferring Pharmaceuticals, Saint-Prex, Switzerland) | 0.6 (0.4–0.7) | 4 g per day | 4 g | 4 g | £0.61 per 1 g | £2.46 |
| Q | Azathioprine | 0.5 (0.2–0.7) | 2 mg/kg per day | 156 mg (assume average body weight of 78 kg) | 156 mg (assume average body weight of 78 kg) | £0.06 per 50 mg | £0.17 |
| Q | Corticosteroids | 0.8 (0.6–1) | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | £0.13 per 20 mg of predsnisolone | £1.38 |
| £2.50 per 9 mg of budesonide | |||||||
| Q | Anti-TNF-α adalimumab | 0.1 (0–0.15) | 40 mg e.o.w.b | 3 mg | 3 mg | £316.80 per 40 mg | £22.88 |
| Q | BAS | 0.2 (0.05–0.4) | 5 g of colestyramine/2.5 g of colesevelam | £0.28 per 4 g of colestyramine | £1.46 | ||
| £0.64 per 625 mg of colesevelam | |||||||
| R | Codeine | 0.5 (0.4–0.8) | 30–120 mg | 30–120 mg | 75 mg | £0.05 per 30 mg | £0.12 |
| R | Loperamide | 0.5 (0.4–0.8) | 2–8 mg | 5 mg | 5 mg | £0.05 per 2 mg | £0.13 |
| S | BAS | 0.8 (0.7–0.9) | 5 g of colestyramine/2.5 g of colesevelam | £0.28 per 4 g of colestyramine | £1.46 | ||
| £0.64 per 625 mg of colesevelam | |||||||
| S | Loperamide | 0.3 (0.25–0.35) | 2–4 mg o.d. to t.i.d. | 2–12 mg | 5 mg | £0.05 per 2 mg | £0.13 |
| T | Codeine | 0.5 (0.4–0.8) | 30–120 mg | 30–120 mg | 75 mg | £0.05 per 30 mg | £0.12 |
| T | Loperamide | 0.8 (0.5–1) | 2–4 mg o.d. to t.i.d. | 2–12 mg | 5 mg | £0.05 per 2 mg | £0.13 |
| T | Corticosteroids | 0.8 (0.6–1) | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | 40 mg of prednisolone/9 mg of budesonide | £0.13 per 20 mg of predsnisolone | £1.38 |
| £2.50 per 9 mg of budesonide | |||||||
| T | BAS | 0.8 (0.7–0.9) | 5 g of colestyramine/2.5 g of colesevelam | £0.28 per 4 g of colestyramine | £1.46 | ||
| £0.64 per 625 mg of colesevelam |
Appendix 6 Full cost-effectiveness results
Cost-effectiveness results for all scenarios in both populations are summarised in Tables 64–84.
Population 1
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | NA | 44 | 59 | 13.8026 | 374 | |||
| BAS TOT | NA | 63 | 85 | 13.9825 | 3767 | 0.1799 | 3393 | 18,860 |
| SeHCAT 15% | NA | 67 | 553 | 14.0408 | 4922 | 0.0583 | 1115 | 19,125 |
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | 26 | 46 | 560 | 13.8236 | 4687 | |||
| BAS TOT | 37 | 66 | 564 | 14.0151 | 7431 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 826 | 14.0623 | 6993 | 0.2387 | 2306 | 9661 |
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| No SeHCAT | NA | 44 | 62 | 13.8021 | 374 | |||
| BAS TOT | NA | 63 | 143 | 13.9893 | 3806 | 0.1871 | 3432 | 18,343 |
| SeHCAT 15% | NA | 67 | 596 | 14.0539 | 5168 | 0.0647 | 1361 | 21,036 |
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| Base-case scenario (no SeHCAT = 74%, SeHCAT 15% = 49%, BAS TOT = 90%) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| Colonoscopy scenario 1 (secondary analysis, no colonoscopy) | ||||||||
| No SeHCAT | NA | 44 | 59 | 13.8026 | 374 | |||
| BAS TOT | NA | 63 | 85 | 13.9825 | 3767 | 0.1799 | 3393 | 18,860 |
| SeHCAT 15% | NA | 67 | 553 | 14.0408 | 4922 | 0.0583 | 1115 | 19,125 |
| Colonoscopy scenario 2 (no SeHCAT = 100%, SeHCAT 15% = 100%, BAS TOT = 100%) | ||||||||
| No SeHCAT | 0 | 47 | 727 | 13.832 | 6210 | |||
| BAS TOT | 30 | 66 | 554 | 14.013 | 7863 | 0.181 | 1653 | 9136 |
| SeHCAT 15% | 29 | 69 | 1028 | 14.070 | 9069 | 0.057 | 1206 | 21,140 |
| Strategy | Responsea (%) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||
| Base-case scenario | ||||||
| No SeHCAT | 47 | 13.8242 | 4720 | |||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| IBS-D scenario 1 (response no SeHCAT increased = response BAS TOT) | ||||||
| No SeHCAT | 50 | 13.8660 | 4728 | |||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.189 | 2228 | 11,788 |
| IBS-D scenario 2 (response BAS TOT increased = response no SeHCAT, response no SeHCAT as in scenario 1) | ||||||
| No SeHCAT | 50 | 13.8660 | 4728 | |||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.1890 | 2228 | 11,788 |
| BAS TOT | 69 | 14.0558 | 7458 | 0.0008 | 502 | 627,500 |
| IBS-D scenario 3 (equal response in the three strategies, equal to SeHCAT 15%) | ||||||
| No SeHCAT | 56 | 13.9323 | 4741 | |||
| SeHCAT 15% | 68 | 14.0550 | 6956 | 0.1227 | 2215 | 18,052 |
| BAS TOT | 69 | 14.0558 | 7458 | 0.0008 | 502 | 627,500 |
| IBS-D scenario 4 (no SeHCAT = 70%, SeHCAT and BAS TOT per base-case scenario) | ||||||
| BAS TOT | 65 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 68 | 14.0550 | 6956 | Dominated by No SeHCAT | ||
| No SeHCAT | 69 | 14.0892 | 4771 | |||
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| Base-case scenario (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| BAS scenario 1 (SeHCAT positive = 0.357, BAS response | SeHCAT positive = 0.495, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| SeHCAT 15% | 60 | 63 | 819 | 14.0031 | 5702 | 0.1789 | 982 | 5489 |
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | 0.0064 | 1747 | 272,969 |
| BAS scenario 2 (SeHCAT positive = 0.555, BAS response | SeHCAT positive = 0.760, BAS TOT response = 0.299) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Ext. dominated by SeHCAT 15% | ||
| SeHCAT 15% | 72 | 74 | 748 | 14.1156 | 8423 | 0.2914 | 3703 | 12,708 |
| BAS scenario 3 (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.20) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 28 | 61 | 566 | 13.9644 | 6857 | Ext. dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2307 | 2236 | 9692 |
| BAS scenario 4 (SeHCAT positive = 0.454, BAS response | SeHCAT positive = 0.638, BAS TOT response = 0.40) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2307 | 2236 | 9692 |
| BAS TOT | 46 | 70 | 446 | 14.0561 | 8059 | 0.0012 | 1103 | 919,167 |
| Strategy | Colonoscopy avoideda (%) | Responseb (%) | Initial costsc (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | |||||||
| Base-case scenario (SeHCAT = 50/50, BAS TOT = 85/15) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 507 | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| BAS scenario 1 (colestyramine only, SeHCAT = 100/0, BAS TOT = 100/0) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 504 | 14.0027 | 7077 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 779 | 14.0339 | 5813 | 0.2097 | 1094 | 5217 |
| BAS scenario 2 (colesevelam only, SeHCAT = 0/100, BAS TOT = 0/100) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 520 | 14.0461 | 9432 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 794 | 14.0760 | 8098 | 0.252 | 3378 | 13,405 |
| BAS scenario 3 (SeHCAT = 50/50, BAS TOT = 50/50) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 512 | 14.0244 | 8255 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 786 | 14.0550 | 6956 | 0.2307 | 2236 | 9692 |
| BAS scenario 4 (SeHCAT = 85/15, BAS TOT = 85/15) | ||||||||
| No SeHCAT | 26 | 47 | 557 | 13.8242 | 4720 | |||
| BAS TOT | 37 | 65 | 506 | 14.0092 | 7430 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 65 | 68 | 781 | 14.0402 | 6156 | 0.2160 | 1436 | 6648 |
| Strategy | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| Colestyramine BAS response equal to full diarrhoea decrement | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0472 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.0771 | 6956 | 0.2529 | 2236 | 8841 |
| Utility values based on Mearin et al.61 | |||||
| No SeHCAT | 13.7360 | 4720 | |||
| BAS TOT | 13.9440 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.9950 | 6956 | 0.259 | 2236 | 8633 |
| Utility values based on Spiegel et al.60 | |||||
| No SeHCAT | 14.0395 | 4720 | |||
| BAS TOT | 14.1860 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.2218 | 6956 | 0.1823 | 2236 | 12,265 |
| No age adjustment | |||||
| No SeHCAT | 14.7992 | 4720 | |||
| BAS TOT | 14.9977 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 15.0464 | 6956 | 0.2472 | 2236 | 9045 |
| Strategy | QALYs | Costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6956 | 0.2308 | 2236 | 9688 |
| Decrease cost of BAS treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 6749 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6123 | 0.2308 | 1403 | 6079 |
| Increase cost of BAS treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 8149 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 7789 | 0.2308 | 3069 | 13,297 |
| Decrease cost of colonoscopy by 20% | |||||
| No SeHCAT | 13.8242 | 4654 | |||
| BAS TOT | 14.0096 | 7394 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6925 | 0.2308 | 2271 | 9840 |
| Increase cost of colonoscopy by 20% | |||||
| No SeHCAT | 13.8242 | 4785 | |||
| BAS TOT | 14.0096 | 7504 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6987 | 0.2308 | 2202 | 9541 |
| Decrease cost of IBD dietitian by 20% | |||||
| No SeHCAT | 13.8242 | 4719 | |||
| BAS TOT | 14.0096 | 7448 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6955 | 0.2308 | 2236 | 9688 |
| Increase cost of IBD dietitian by 20% | |||||
| No SeHCAT | 13.8242 | 4721 | |||
| BAS TOT | 14.0096 | 7450 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6957 | 0.2308 | 2236 | 9688 |
| Decrease cost of IBD medication by 20% | |||||
| No SeHCAT | 13.8242 | 3918 | |||
| BAS TOT | 14.0096 | 6770 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6581 | 0.2308 | 2663 | 11,538 |
| Increase cost of IBD medication by 20% | |||||
| No SeHCAT | 13.8242 | 5522 | |||
| BAS TOT | 14.0096 | 8128 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 7331 | 0.2308 | 1809 | 7838 |
| Decrease cost of IBD psychological treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4718 | |||
| BAS TOT | 14.0096 | 7447 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6955 | 0.2308 | 2237 | 9692 |
| Increase cost of IBD psychological treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4722 | |||
| BAS TOT | 14.0096 | 7451 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6957 | 0.2308 | 2235 | 9684 |
| Decrease cost of IBS-D dietitian by 20% | |||||
| No SeHCAT | 13.8242 | 4718 | |||
| BAS TOT | 14.0096 | 7447 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6954 | 0.2308 | 2236 | 9688 |
| Increase cost of IBS-D dietitian by 20% | |||||
| No SeHCAT | 13.8242 | 4722 | |||
| BAS TOT | 14.0096 | 7451 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6958 | 0.2308 | 2236 | 9688 |
| Decrease cost of IBS-D medication by 20% | |||||
| No SeHCAT | 13.8242 | 4679 | |||
| BAS TOT | 14.0096 | 7418 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6921 | 0.2308 | 2242 | 9714 |
| Increase cost of IBS-D medication by 20% | |||||
| No SeHCAT | 13.8242 | 4761 | |||
| BAS TOT | 14.0096 | 7480 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6991 | 0.2308 | 2230 | 9662 |
| Decrease cost of IBS-D psychological treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4713 | |||
| BAS TOT | 14.0096 | 7444 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6951 | 0.2308 | 2238 | 9697 |
| Increase cost of IBS-D psychological treatment by 20% | |||||
| No SeHCAT | 13.8242 | 4727 | |||
| BAS TOT | 14.0096 | 7454 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6961 | 0.2308 | 2234 | 9679 |
| Decrease SeHCAT cost by 20% | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 6861 | 0.2308 | 2141 | 9276 |
| Increase SeHCAT cost by 20% | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.055 | 7051 | 0.2308 | 2331 | 10,100 |
| Strategy | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario (relapse in IBD model only; p = 0.0045) | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| BAS scenario 1 (relapse in IBD, IBS-D and BAS models; p = 0.0045) | |||||
| No SeHCAT | 13.7687 | 4708 | |||
| BAS TOT | 13.9335 | 7048 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.9728 | 6475 | 0.2041 | 1767 | 8658 |
| BAS scenario 2 (relapse and remission in IBD, IBS-D and BAS models; p = 0.0045) | |||||
| No SeHCAT | 13.8358 | 4944 | |||
| BAS TOT | 13.9803 | 7278 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.0171 | 6628 | 0.1813 | 1684 | 9288 |
| BAS scenario 3 (relapse in IBD, IBS-D and BAS models; p = 0.0045 × 2) | |||||
| No SeHCAT | 13.7193 | 4281 | |||
| BAS TOT | 13.8674 | 6361 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.9028 | 5881 | 0.1834 | 1600 | 8724 |
| BAS scenario 4 (relapse in IBD, IBS-D and BAS models; p = 0.0045 × 5) | |||||
| No SeHCAT | 13.6124 | 3366 | |||
| BAS TOT | 13.7244 | 4890 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.7511 | 4608 | 0.1387 | 1243 | 8962 |
| Strategy | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario (no excess mortality) | |||||
| No SeHCAT | 13.8242 | 4720 | |||
| BAS TOT | 14.0096 | 7449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 14.0550 | 6956 | 0.2308 | 2236 | 9688 |
| Scenario 1 (SMR = 1.52, per Crohn’s disease) | |||||
| No SeHCAT | 13.0241 | 4478 | |||
| BAS TOT | 13.1986 | 7021 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.2413 | 6567 | 0.2172 | 2089 | 9618 |
Population 2
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| No SeHCAT | 40 | 1180 | 12.6857 | 15,686 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 895 | 12.9006 | 14,880 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1172 | 13.0084 | 14,795 | |||
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| Base-case scenario (no SeHCAT = 40%, SeHCAT 15% = 42%, BAS TOT = 41%) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Diarrhoea treatment without BAS scenario 1 (no SeHCAT = 42%, SeHCAT 15% = 42%, BAS TOT = 42%) | |||||||
| No SeHCAT | 42 | 1052 | 12.7059 | 15,078 | Dominated by BAS TOT | ||
| BAS TOT | 61 | 756 | 12.9075 | 14,171 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| Diarrhoea treatment without BAS scenario 2 (no SeHCAT = 70%, SeHCAT 15% = 42%, BAS TOT = 70%) | |||||||
| No SeHCAT | 70 | 1052 | 12.9809 | 24,295 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| BAS TOT | 80 | 756 | 13.0925 | 20,373 | 0.0847 | 6241 | 73,684 |
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| Base-case scenario (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.339) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| BAS scenario 1 (SeHCAT positive = 0.39, BAS response | SeHCAT positive = 0.67, BAS TOT response = 0.33) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| SeHCAT 15% | 58 | 1282 | 12.8700 | 14,893 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| BAS scenario 2 (SeHCAT positive = 0.71, BAS response | SeHCAT positive = 1, BAS TOT response = 0.33) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 83 | 848 | 13.1411 | 13,396 | |||
| BAS scenario 3 (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.23) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 55 | 852 | 12.8399 | 14,190 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | |||
| BAS scenario 4 (SeHCAT positive = 0.55, BAS response | SeHCAT positive = 0.89, BAS TOT response = 0.5) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | Dominated by BAS TOT | ||
| BAS TOT | 70 | 586 | 13.0090 | 13,511 | |||
| Strategy | Responsea (%) | Initial costsb (£) | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||||
| Base-case scenario (SeHCAT = 63/37, BAS TOT = 58/42) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| BAS scenario 1 (colestyramine only, SeHCAT = 100/0, BAS TOT = 100/0) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 748 | 12.8798 | 12,825 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 71 | 1050 | 12.9792 | 12,601 | |||
| BAS scenario 2 (colesevelam only, SeHCAT = 0/100, BAS TOT = 0/100) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | |||
| BAS TOT | 60 | 767 | 12.9307 | 15,539 | 0.2444 | 1120 | 4581 |
| SeHCAT 15% | 71 | 1079 | 13.0553 | 16,662 | 0.1247 | 1123 | 9009 |
| BAS scenario 3 (SeHCAT = 63/37, BAS TOT = 63/37) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 755 | 12.8989 | 13,847 | |||
| SeHCAT 15% | 71 | 1061 | 13.0079 | 14,131 | 0.189 | 284 | 2608 |
| BAS scenario 4 (SeHCAT = 58/42, BAS TOT = 58/42) | |||||||
| No SeHCAT | 40 | 1052 | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 60 | 756 | 12.9008 | 13,946 | |||
| SeHCAT 15% | 71 | 1062 | 13.0106 | 14,279 | 0.1098 | 333 | 3030 |
| Strategy | QALYs | Costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Colestyramine BAS response equal to full diarrhoea utility decrement | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.932 | 13,946 | |||
| SeHCAT 15% | 13.0573 | 14,131 | 0.1253 | 185 | 1476 |
| Utility values based on Mearin et al.61 | |||||
| No SeHCAT | 12.7292 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9672 | 13,946 | |||
| SeHCAT 15% | 13.086 | 14,131 | 0.1188 | 185 | 1557 |
| Utility values based on Spiegel et al.60 | |||||
| No SeHCAT | 12.6003 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.7679 | 13,946 | |||
| SeHCAT 15% | 12.8516 | 14,131 | 0.0837 | 185 | 2210 |
| No age adjustment | |||||
| No SeHCAT | 13.4956 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 13.7247 | 13,946 | |||
| SeHCAT 15% | 13.839 | 14,131 | 0.1143 | 185 | 1619 |
| Strategy | QALYs | Costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Decrease cost of BAS treatment by 20% | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,140 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.0079 | 12,961 | |||
| Increase cost of BAS treatment by 20% | |||||
| No SeHCAT | 12.6863 | 14,419 | |||
| BAS TOT | 12.9008 | 14,751 | 0.2145 | 332 | 1548 |
| SeHCAT 15% | 13.0079 | 15,302 | 0.1071 | 551 | 5143 |
| Decrease SeHCAT cost by 20% | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,036 | 0.1071 | 90 | 840 |
| Increase SeHCAT cost by 20% | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,227 | 0.1071 | 281 | 2623 |
| Decrease cost of Crohn’s disease medication by 20% | |||||
| No SeHCAT | 12.6863 | 11,516 | |||
| BAS TOT | 12.9008 | 11,979 | 0.2145 | 463 | 2159 |
| SeHCAT 15% | 13.0079 | 12,584 | 0.1071 | 605 | 5647 |
| Increase cost of Crohn’s disease medication by 20% | |||||
| No SeHCAT | 12.6863 | 17,277 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 15,912 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 13.0079 | 15,679 | |||
| Strategy | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario (relapse in non-BAS model only; p = 0.00575) | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| BAS scenario 1 (relapse in non-BAS and BAS models; p = 0.00575) | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.8582 | 13,417 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 12.9446 | 13,360 | |||
| BAS scenario 2 (relapse and remission in non-BAS and BAS models; p = 0.00575) | |||||
| No SeHCAT | 12.7686 | 17,403 | Dominated by BAS TOT | ||
| BAS TOT | 12.9169 | 15,449 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 12.9911 | 14,904 | |||
| BAS scenario 3 (relapse in non-BAS and BAS models; p = 0.00575 × 2) | |||||
| No SeHCAT | 12.6404 | 12,768 | Dominated by BAS TOT | ||
| BAS TOT | 12.7922 | 11,854 | Dominated by SeHCAT 15% | ||
| SeHCAT 15% | 12.8684 | 11,843 | |||
| BAS scenario 4 (relapse in non-BAS and BAS models; p = 0.00575 × 5) | |||||
| No SeHCAT | 12.5462 | 9412 | Dominated by BAS TOT | ||
| No SeHCAT and BAS TOT | 12.6565 | 8678 | |||
| SeHCAT 15% | 12.7119 | 8759 | 0.0054 | 81 | 1459 |
| Strategy | QALYs | Total costs (£) | Incremental | ICER (£) | |
|---|---|---|---|---|---|
| QALYs | Costs (£) | ||||
| Base-case scenario (SMR = 1.52) | |||||
| No SeHCAT | 12.6863 | 14,419 | Dominated by BAS TOT | ||
| BAS TOT | 12.9008 | 13,946 | |||
| SeHCAT 15% | 13.0079 | 14,131 | 0.1071 | 185 | 1727 |
| Scenario 1 (SMR = 1.32) | |||||
| No SeHCAT | 12.9594 | 14,697 | Dominated by BAS TOT | ||
| BAS TOT | 13.1790 | 14,234 | |||
| SeHCAT 15% | 13.2886 | 14,424 | 0.1096 | 190 | 1732 |
| Scenario 2 (SMR = 1.74) | |||||
| No SeHCAT | 12.4134 | 14,141 | Dominated by BAS TOT | ||
| BAS TOT | 12.6229 | 13,658 | |||
| SeHCAT 15% | 12.7275 | 13,840 | 0.1046 | 182 | 1740 |
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False negative
- Incorrect negative test result: number of diseased persons with a negative test result. In the context of this assessment, it is the number of responders to bile acid sequestrants with a negative test result.
- False positive
- Incorrect positive test result: number of non-diseased persons with a positive test result. In the context of this assessment, it is the number of non-responders to bile acid sequestrants with a positive test result.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Likelihood ratio
- Likelihood ratios describe how many times more likely it is that a person with the target condition will receive a particular test result than a person without the target condition.
- Meta-analysis
- A statistical technique used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Opportunity costs
- The costs of forgone outcomes that could have been achieved through alternative investments.
- Positive predictive value
- The probability that people with a positive test have the disease. In the context of this assessment, it is the probability that people with a positive test will respond positively to treatment with bile acid sequestrants.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by a patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity, which result from varying the diagnostic threshold.
- Reference standard
- The best currently available method for diagnosing the target condition. The index test is compared against this to allow calculation of estimates of accuracy.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result. In the context of this assessment, it is the proportion of people with a positive test result who respond positively to treatment with bile acid sequestrants.
- Specificity
- Proportion of people without the target disorder who have a negative test result. In the context of this assessment, it is the proportion of people with a negative test result who do not respond positively to treatment with bile acid sequestrants.
- State-transition model
- A model in which individuals move (transition) between disease states as their condition changes over time. Time spent in each disease state for a single model cycle (and transitions between states) is associated with a cost and a health outcome.
- True negative
- Correct negative test result: number of non-diseased persons with a negative test result. In the context of this assessment, it is the number of non-responders to bile acid sequestrants with a negative test result.
- True positive
- Correct positive test result: number of diseased persons with a positive test result. In the context of this assessment, it is the number of responders to bile acid sequestrants with a positive test result.
List of abbreviations
- BAD
- bile acid diarrhoea
- BAM
- bile acid malabsorption
- BAS
- bile acid sequestrant
- BNF
- British National Formulary
- BSG
- British Society of Gastroenterology
- CCT
- clinical controlled trial
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CPCI-S
- Conference Proceedings Citation Index – Science
- CRD
- Centre for Reviews and Dissemination
- CTC
- computerised tomography colonoscopy
- DAR
- Diagnostic Assessment Report
- DARE
- Database of Abstracts of Reviews of Effects
- DG
- diagnostics guidance
- EQ-5D
- EuroQol-5 Dimensions
- FD
- functional diarrhoea
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBD
- inflammatory bowel disease
- IBS
- irritable bowel syndrome
- IBS-D
- diarrhoea-predominant irritable bowel syndrome
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LILACS
- Latin American and Caribbean Health Sciences Literature
- NHSCII
- NHS Cost Inflation Index
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS-2
- quality assessment of diagnostic accuracy studies-2
- RCT
- randomised controlled trial
- SCI
- Science Citation Index
- SeHCAT
- tauroselcholic [75selenium] acid
- SF-36
- Short Form questionnaire-36 items
- SMR
- standardised mortality ratio
- SPECT-CT
- single-photon emission computerised tomography with computerised tomography
- WHO
- World Health Organization
