Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 17/17/02. The contractual start date was in September 2018. The draft manuscript began editorial review in April 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Brown et al. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Brown et al.
Chapter 1 Introduction
Some sections of this report have been reproduced from the study protocol (www.fundingawards.nihr.ac.uk/award/17/17/02). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Pilonidal disease is a common condition that affects around 26/100,000 of the population – predominantly young, working people. 1 The term ‘pilonidal’ derives from the Latin words for hair (pilus) and nest (nidus). It is an acquired disease resulting in obstruction of hair follicles in the natal cleft (the anatomical groove between the buttocks). Subsequent rupture of the follicles leads to abscess and sinus formation. Risk factors for development of the condition include male gender, extensive body hair, young adulthood, family history, local trauma, sedentary lifestyle, poor hygiene, an anatomically deep natal cleft and obesity. 1–3 Once established, the condition persists and progresses through insertion of ingrown or loose hairs into the sinuses. 2,3 The term pilonidal sinus disease (PSD) encapsulates a wide spectrum of abnormalities ranging from relatively asymptomatic simple sinuses to complex abscess cavities with multiple sinus tracks that persist despite repeated surgical intervention. Individuals present either as an emergency with a painful abscess between the buttocks or electively with a chronic cycle of pain and discharge from the sinuses, causing significant disruption to employment, relationships and quality of life (QoL). 4
The ideal management of PSD should be simple, safe, cost-effective, easy to perform and lead to a rapid return to normal activities, with low rates of acute wound complications (including infection, seroma, haematoma), recurrence and rapid wound healing. These aims are not reliably delivered by current surgical practice and there is no consensus on how to manage based on disease characteristics.
Pattern of disease and management options
Patients often present with acute infection and abscess formation. Abscesses usually require hospitalisation with incision and drainage of the abscess cavity. One in five patients present with recurrent symptoms following emergency surgery. 5 This picture of relapsing and remitting infections is typical of chronic PSD.
Treatment of chronic PSD is surgical, usually using two essential components: excision and closure. The exceptions are phenol injection and fistuloscope/diathermy as stand-alone treatments (which aim to induce fibrosis and obliterate the tracks) and seton insertion which may induce fibrosis, allowing the possibility of a simpler subsequent surgery. There is no clear consensus as to which approach for each component is superior. For those procedures that involve excision, the tissue removed may be minimal (e.g. curettage or excision of the ‘pit’) or there may be substantial excision of the affected area and surrounding tissue to ensure complete removal of disease. The resultant wound may be left open to heal slowly by secondary intention, or it is closed with glue6–8 or sutures. The skin closure technique may be midline or off-midline. In the off-midline technique, the wound is positioned adjacent to the natal cleft, rather than in the natal cleft itself, in order to theoretically aid healing. 8,9 Examples include: the Karydakis flap, Bascom cleft closure (Bascom II), rhomboid and Limberg flaps.
Monetary and humanistic burden
Pilonidal disease is relatively common and represents a significant burden to primary and secondary care in the NHS. The 2012 hospital episode statistics (HES) data reported 13,239 hospital admissions for PSD. 10 At present, both emergency and the most common elective excisional surgical treatments leave large open wounds that may take months to heal. 6,7 Patients consequently require prolonged wound care from community healthcare services. As the disease tends to affect young otherwise healthy adults, this prolonged need for dressings and general wound care impacts on education, work, intimacy and social life, pain, recurrent infection and fear of wound deterioration, all severely affecting QoL. 11,12 Alternative techniques including minimally invasive interventions that aim to close the wound away from the midline may reduce the burden to the patient, but their efficacy outside the care of dedicated enthusiasts is not clear.
Current evidence base
The optimum treatment that is both easy to perform and results in rapid healing and minimal complications is not clear. This is reflected in varied practice throughout the UK with a perceived random selection of the procedure techniques detailed above. Some of these procedures result in lengthy healing times and long periods of incapacity. The literature on PSD is large but mainly consists of single-centre cohort studies looking at individually favoured techniques. Many of these have reported very low recurrence and infection rates for almost all procedures. 13 It has proven difficult to replicate these results in ‘real life’. In addition to the literature being mainly from single-centre cohorts, most studies make no attempt to stratify patients or detail the extent of disease or the adjuvant management (antibiotics, anaesthetic, postoperative care). There have been numerous randomised controlled trials (RCTs), and nearly 40 systematic reviews that focus on management – including two Cochrane Reviews. Most of these systematic reviews include meta-analyses of cohort studies only or analyse comparative RCTs and non-RCTs (often combining these data) for numerous interventions with varied controls. The methodological flaws of many individual studies and systematic reviews, coupled with the uncertainty of front-running interventions and an absence of a universally accepted control, make the value and interpretation of the data difficult. 14
The first Cochrane Review demonstrated that healing through secondary intention had lower overall recurrence rates compared to primary closure but at the expense of longer healing times. 14 Another systematic review reached the same conclusion but also compared two types of closure, suggesting off-midline to be preferable to midline. 15 The authors also concluded that outcome measures, such as time to healing, were poorly analysed, and health economic data were lacking. They proposed that future trials should be adequately powered, multicentric and include valid methods of assessing surgical outcomes. A systematic review of wound care after excision found no best practice guidelines and only one clinical pathway. 16
The second Cochrane Review focused on fibrin glue (FG) in the treatment of PSD. 17 The authors concluded this was a promising and appealing option as monotherapy given the non-invasive nature and that it could be performed as a day-case procedure, under local anaesthesia. These conclusions echo the conclusions of a previous meta-analysis, both suggesting a need for further research. 18 Nevertheless, the research to date has largely considered FG as an adjunct to surgery and although small, single-centre observational studies6,7,19–21 have been published, there is no RCT of FG as monotherapy in treatment of PSD.
Rationale
Currently, there is a lack of evidence regarding classification of disease, what are the front-running interventions, whether there is clinical equipoise for these interventions and whether comparative studies for these interventions are feasible in terms of recruitment, and finally what outcome measures are relevant to patients, can be easily and reliably measured and are sufficiently sensitive to change. Given the efficacy uncertainty surrounding a multitude of operative techniques, compounded by the reported negative implications for recovery, there is a need to improve the evidence base to guide future pilonidal management. 14
Research objectives
The aim of the PITSTOP study is to answer the following research questions:
-
What are the different subtypes of pilonidal disease for which the various treatment options are indicated?
-
What combinations of excision and closure techniques are used?
-
Which outcomes do patients value and which interventions do they prefer?
-
What further research is needed?
To answer the research questions posed, we aimed to complete the following:
-
Conduct a survey of clinicians to assess management preferences.
-
Follow patients with symptomatic pilonidal sinus referred to each collaborating site, prospectively record details of their pit/track anatomy, surgical management, medical events and health-related QoL until 6 months after their operation.
-
Describe the combination of interventions currently in use and quantify clinical and patient-reported outcomes (PROs) associated with each.
-
Identify patient-specific disease features that might predict poor outcome in each treatment group by risk-modelling methods.
-
Derive a case mix-adjusted estimate of the risks associated with common treatment options, using causal inference methods to provisionally rank the optimal management strategies among patients for whom more than one treatment is considered appropriate.
-
Provide an overview of patient views and experiences.
-
Collect the views of patients on which interventions they would rather avoid and which outcomes they most value.
-
Validate a classification system.
-
Reach a surgeon-based consensus on which subtypes of pilonidal disease may benefit from which treatment options.
-
Reach a surgeon and patient-based consensus on research priorities.
Chapter 2 Consultant surgeon survey
Methods
Survey design and development
A survey was developed to identify the most frequently used interventions for specific clinical scenarios in current PSD practice. As this was a novel survey, it was designed by study collaborators and followed the CHERRIES statement checklist of recommendations. 22 The survey included questions on the following: the mean number of primary elective procedures performed annually, factors affecting choice of procedure, treatment choice for recurrent disease presentation and the factors affecting treatment choice for recurrent disease treatment. The survey was piloted to determine the clinical sensibility.
Data collection
To maximise completion rates, the survey could be completed online or on paper. The online survey was hosted on the Research Electronic Data CaptureTM (REDCap) system managed centrally by the University of Sheffield Clinical Trials Research Unit (CTRU). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources. 23,24 Paper surveys were returned by post or via e-mail. The questionnaires were anonymised at the respondent level.
Sampling
The survey was disseminated via the UK surgical trainee research collaboratives, led jointly by the South Yorkshire Surgical Research Group and the North-West Research Collaborative. Collaborators were asked to deliver the questionnaire to consultant colorectal surgeons in their units. The first point of contact was made through the National Research Collaborative e-mail lists, and electronic contact to local collaborative leads was cascaded locally. The collaborators were asked to circulate the survey locally to three consultants and thereafter return the completed questionnaires to the REDCap system.
Data analysis
All aspects of data management were provided by the CTRU in accordance with their standard operating procedures. The data emanating from this survey were captured and stored in the REDCap software.
Results
The link was followed by 200 surgeons and completed by 113 participants. Of these, 109 routinely cared for patients with PSD. These 109 were entered into the final analysis, giving a final response rate of 54.5%.
Respondent practice overview
Respondents reported a median caseload of 15 patients per year [interquartile range (IQR) 10–20 patients] and indicated that recurrent disease accounted for 20% of overall workload (IQR 10–30%). Of those estimating their recurrence rates (n = 97), 19 (19.5%) were unaware of their recurrence rate, 14 (14.4%) estimated their rate to be <5%, 36 (37.1%) to be in the 6–15% range, and 28 (28.8%) in the 16–30% range.
With regards to hair management, depilation was recommended by 54 (49.5%), laser hair removal by 32 (29.4%), salt baths by 14 (12.8%), shaving by 52 (47.7%) and waxing by 32 (29.4%).
Operative strategies employed
A wide range of treatment strategies were employed by responding surgeons, summarised in Table 1. Excision of disease with wound left open was the most frequently used strategy (71 responses; 65.1%), followed by Karydakis flap (62 responses; 58.1%). Curettage with phenol injection (1 response; 0.9%) and endoscopic pilonidal sinus treatment (EPSiT) (2 responses; 1.8%) were the least frequently performed interventions.
| Operation | Yes, N = 109 |
|---|---|
| Excise and leave open | 71 (65.1%) |
| Karydakis | 62 (56.8%) |
| Excise and midline closure | 48 (44.0%) |
| Bascom’s cleft lift | 47 (43.3%) |
| Rhomboid flap | 30 (27.5%) |
| Bascom’s I | 27 (24.7%) |
| Curettage and glue | 17 (15.5%) |
| Pit picking alone | 10 (9.2%) |
| Other flap | 7 (6.4%) |
| EPSiT | 2 (1.8%) |
| Curettage and phenol | 1 (0.9%) |
Participants were asked to provide a first-, second, and third-choice preference for their interventions. Karydakis was the first-preference treatment for 24/96 respondents (25.0%), followed by Bascom’s II (n = 18; 18.7%), and curettage and glue (n = 15; 15.5%). For second-preference treatments, local excision with wound left open was the most popular with 21/85 participants (24.7%), followed by local excision with midline closure for 15 (17.6%) and Karydakis procedure for 14 (16.4%). The most popular third-preference treatment was local excision with wound left open (27/32; 84.4%), followed by local excision with midline closure for 12 (37.5%), and Bascom’s II for 7 (21.9%) respondents (see Appendix 3, Figure 12).
Case vignettes
Case vignettes demonstrated heterogeneity across respondents. Case one (recurrent disease) showed a preference for rhomboid flap or ‘other’ procedures (22.6% and 25.5%, respectively). For case two (female with primary disease and cosmesis concerns), preferences turned to favour conservative management (21.6%), followed by excision and primary closure (16.0%) and cleaning/curettage of tracts (14.1%). Case three assessed recurrent disease and requirement for minimal time off work. For this scenario, most respondents opted for conservative management with hair removal (25.4%), followed by curettage of tracts (16.0%). Of note, 15.1% would offer a Karydakis procedure in this setting. Responses are summarised in Table 2.
| Operation | 16-year-old male, six previous surgeries with other surgeons, has recurrent disease and partially open wound/sinus 1 cm long in natal cleft that has been like that for 9 months. Wants to play contact sport. Parents not happy (N = 107); n (%) | 19-year-old female, fair skin, dark hair, previous abscess drainage, swelling and discomfort in natal cleft, very worried about cosmesis and what the scar will look like if you operate, N = 106; n (%) | 30-year-old male plumber who has had previous surgery, no details available, and now present with recurrent disease. Single discharging pit around scar, and can’t afford much time off work, N = 106; n (%) |
|---|---|---|---|
| Bascom’s cleft lift procedure | 12 (11.3%) | 7 (6.6%) | 12 (11.3%) |
| Bascom’s I procedure | 2 (1.9%) | 13 (12.2%) | 6 (5.7%) |
| Cleaning/curettage tracts | 7 (6.5%) | 15 (14.1%) | 17 (16.0%) |
| Conservative/hair removal | 14 (13.2%) | 23 (21.6%) | 27 (25.4%) |
| Excision and primary closure | 0 (0%) | 17 (16.0%) | 9 (8.5%) |
| Karydakis procedure | 11 (10.3%) | 13 (12.2%) | 16 (15.1%) |
| Lay open ± marsupialisation | 9 (8.4%) | 6 (5.7%) | 8 (7.5%) |
| Other | 27 (25.5%) | 10 (9.4%) | 10 (9.4%) |
| Rhomboid flap | 24 (22.6%) | 1 (0.9%) | 1 (0.9%) |
| Z-Plasty flap | 1 (0.9%) | 1 (0.9%) | 0 (0%) |
Training
Surgical training programmes were the key training setting for commonly offered procedures. These included training in wide local excision with wound left open or closed for 59/71 (83.1%) and 36/48 (75.0%) of those offering the procedures, respectively. Similar numbers were seen for Bascom’s I (21/27; 77.7%) and Karydakis procedure (49/62; 79.0%). For some procedures, no formal training was reported by 5–10% of respondents. Courses, observation of colleagues and reference material such as text or video was also variably used. A summary of training experiences is presented in Table 3.
| Number offering | No formal training | Course/workshop | Observed colleagues | Training in registrar programme/fellowship | Videos/text | |
|---|---|---|---|---|---|---|
| Wide local excision, leave open | 71 | 4/68 (5.8%) | 1/68 (1.5%) | 4/68 (5.9%) | 59/68 (85.2%) | 0/68 (0%) |
| Wide local excision with closure | 48 | 4/48 (8.3%) | 1/48 (2.1%) | 6/48 (12.5%) | 36/48 (75.0%) | 1/48 (2.1%) |
| Bascom’s cleft lift | 42 | 2/26 (7.7%) | 4/26 (15.3%) | 9/26 (34.6%) | 9/26 (34.6%) | 2/26 (7.7%) |
| Pit picking/Bascom’s I | 27 | 3/34 (8.8%) | 4/34 (11.7%) | 4/34 (11.7%) | 21/34 (61.8%) | 2/34 (5.9%) |
| Karydakis | 62 | 2/75 (2.7%) | 4/75 (5.3%) | 13/75 (17.3%) | 49/75 (65.3%) | 7/75 (9.3%) |
| Rhomboid flap | 30 | 2/29 (6.9%) | 2/29 (6.9%) | 5/29 (17.2%) | 15/29 (51.7%) | 5/29 (17.2%) |
| Other flap | 7 | 1/5 (20.0%) | 0/5 (0%) | 2/5 (40.0%) | 2/5 (40.0%) | 0/5 (0%) |
| Curettage and glue | 17 | 0/22 (0%) | 5/22 (22.7%) | 7/22 (31.8%) | 5/22 (22.7%) | 7/22 (31.8%) |
| Curettage and phenol | 1 | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| EPSiT | 2 | 0/2 (0%) | 0/2 (0%) | ½ (50%) | 0/2 (0%) | 0/2 (0%) |
Discussion
Overview
The key finding of this survey is the heterogeneity and variation in practice of consultant colorectal surgeons who treat PSD. It demonstrates a relatively low annual volume of operative procedures (around 15) when compared to other conditions such as colorectal cancer surgery. This number is slightly higher than the median of four cases per year identified through HES.
One in four surgeons perceived they had treatment failure rates approaching 30% in this study. This is somewhat at odds with the published literature, which often claims cure in > 90% of cases. 13 This demonstrates dissonance between published reports and real-world experience of clinicians. Conversely, 1 in 10 respondents reported recurrence rates of < 5%, which is concordant with the literature. This gap in outcomes may arise from issues with the quality of the literature, where the often surprisingly high quality of outcomes has been challenged. 14 Alternatively, it may reflect a small group of clinicians with a high volume of practice and associated good outcomes. 25 This poses three key questions. First, should complex pilonidal disease, or even all pilonidal disease, be managed by a group of high-volume surgeons? This is an approach that is advocated in other aspects of surgery such as rectal cancer and inflammatory bowel disease. 26,27 The second question to ask is whether we should improve training opportunities (highlighted here as limited) for colorectal surgeons to improve their skill set. Finally, we should ask whether there is a need for better monitoring of outcomes in PSD, as with registries established for other conditions. The findings of this study largely match those from a previous survey conducted in 2011,28 suggesting little has changed in a decade, making these questions more important to improve care.
Surgeons expressed preferences for some treatments such as excise and leave open. The literature suggests these should be considered outdated as they are associated with significant wound morbidity. 15 Sixty-five per cent of surgeons used the leave open technique with healing occurring by secondary intention, and 44% used a midline closure technique. Surgeons expressed a stronger preference for asymmetric closure than when previously assessed,28 in keeping with global trends. 29 In contrast, minimally invasive techniques such as EPSiT and pit picking appear to be relatively unpopular treatments. This suggests that surgeons are focused on cure rather than symptomatic relief. This may contrast with stated patient preferences where they are willing to trade a less major procedure in exchange for a higher risk of recurrence. 30
The survey does have limitations. Surveys of this nature can present artificial choices and do not permit qualification of answers. The use of vignettes does, however, allow some direct comparison of preferences. The survey may have drawn in experts and enthusiastic practitioners in pilonidal disease. However, the heterogeneity of responses does not reflect unity of thought, and responses are in keeping with published surveys. The response rate of 54.5% should reassure us as to representativeness of the survey.
Implications for policy-makers
This study presents three key actions for policy-makers. First, there is a need to agree a general framework for interventions to standardise pathways of care. This should be supported by best available evidence. Where such evidence is not available, funding should be secured to inform such guidance. Secondly, this is an area with no clear registry or oversight. Policy-makers should consider whether collection of granular data on practice and outcomes might aid initiatives to improve care, or even justify specialisation or centralisation of practice. Finally, surgeons’ practice is driven by their postgraduate training and persists into their independence. Therefore, it is important to offer opportunities for further training in new techniques. This would allow implementation of new techniques and may support the decommissioning of outdated procedures.
Implications for researchers
The level of heterogeneity likely speaks in part to uncertainty. Researchers should consider whether the findings presented here would support the delivery of specific procedures in a head-to-head RCT, or whether a ‘bucket’ approach would be more pragmatic.
Conclusion
This survey demonstrates significant heterogeneity in surgeon practice preference. It suggests that limited access to training opportunities may impede efforts to improve practice in the area.
Chapter 3 Cohort study
Methods
Aims
A prospective, multicentre observational cohort study was conducted to:
-
describe the disease characteristics of participants undergoing treatment for PSD
-
describe procedures currently in use and quantify clinical outcomes and PROs associated with each
-
identify patient-specific disease features that might predict poor outcome in each treatment group
-
derive a case mix-adjusted estimate of the risks associated with common treatment options.
Participants
Eligible patients were undergoing definitive elective treatment for PSD at study recruiting centres.
Inclusion criteria
-
Consenting patients aged 16 years or older and with PSD.
Exclusion criteria
-
Asymptomatic disease.
-
Currently pregnant.
-
Unable to give consent.
-
Acute abscess.
-
Hypersensitivity to the sealants.
Study procedures
Recruitment and consent
Patients considered suitable for surgery were identified from general practitioner (GP) secondary care referrals, surgery waiting lists or clinics. Once identified, participants were given an approved participant information sheet detailing the study – sent in the post or provided in person. Patients were invited to attend a recruiting clinic. At the clinic, a member of the research team explained the study to the participant and offered them an opportunity for them to ask any questions. The principal investigator (PI) or delegated research team member confirmed eligibility and ensured written informed consent was obtained prior to any patient data being collected. Participants were advised that they were able to withdraw from the study at any point without any impact on their routine NHS care. As is standard practice, the surgeon would discuss the condition, possible interventions and their advantages and disadvantages. Patients were given a minimum of 24 hours between receiving the participant information sheet and consenting to the study.
In response to the COVID-19 pandemic, adjustments were made to allow the continuation of recruitment and consent procedures. Consent could be obtained by post. An invitation letter, participant information sheet and a postal consent form were posted to the patient. The research team were able to contact the patient to provide an overview of the study and answer questions. The patient was instructed to complete two consent forms: one to be returned to the research team, one for their own records. Once received, the research team contacted the patient to complete the postal consent review form.
Intervention
The study was observational, and surgeons were not asked to change their usual practice. Surveys suggested that around six procedures were in common use,28,31–33 which can broadly be described as:
-
major excision with asymmetric closure (‘Bascom II cleft lift’)
-
major excision with lateral closure (‘Karydakis’)
-
major excision with lateral closure with rhombic flap
-
major excision with midline closure
-
major excision and leave open
-
minimal excision (‘Bascom I’ or ‘pit picking’).
Other approaches include curettage (‘scraping out’) or phenol injection with glue closure. For the purpose of analysis, procedures were classified as either ‘minor’ or ‘major’ procedures, with the latter further subdivided as ‘asymmetric/lateral closure’, ‘midline closure’ or ‘leave open’.
Data collection
Data were collected by trained research personnel. All patient data were recorded on the case record form. Copies of the consent and patient information sheets were kept in the participant’s hospital case notes. A copy of the consent form was uploaded onto the REDCap data capture system for monitoring purposes. All data were recorded on the REDCap data capture system.
Assessment schedule
Participants completed baseline questionnaires after eligibility and consent were confirmed. Details of the procedure were collected on the day of procedure. Outcome data were collected on days 1 and 7 after procedure and then at an in-person clinic visit and a further follow-up 6 months after the procedure. Participant data could also be collected opportunistically at a final ‘study completion’ visit. The outcomes collected are listed in Recruitment and participant flow.
Safety assessments
Participants were asked to report complications at days 1 and 7 post procedure, and again at the clinic visit and 6 months. Participants were prompted specifically for incidence of bleeding, seroma, haematoma, infection, dehiscence, maceration, flap necrosis or discharge. Other adverse events (AEs) were collected only if considered related to the study treatment.
Statistical methods
Sample size
The study aimed to recruit approximately 800 patients, with at least 100 within each of the front-running management strategies. Doing so allows proportions to be estimated within each management strategy to a standard error of ≤ 5% and pain numeric rating scale to within a standard error of 0.2 points, assuming that the standard deviation (SD) of a 10-point scale would not exceed two units.
Outcomes
The outcomes and their timing and description are listed in Table 4. All outcomes were self-reported aside from the clinician-assessed scarring question. No single primary outcome was prespecified in this study; methods to elicit outcomes of most importance to study participants are described in subsequent chapters.
| Name/timing | Description |
|---|---|
| Pain [baseline, day 1 (current pain only), day 7, clinic visit and 6 months] |
|
| Health status (baseline, day 7, clinic visit and 6 months) |
|
| Impression of shared decision-making (baseline) |
|
| Return to normal activities (days 7, clinic visit and 6 months) | Time from procedure to return to normal activities (censored if not returned at last contact). |
| Length of time to healing (clinic visit and 6 months) | Time from procedure to wound healing (censored if not returned at last contact). |
| Recurrencea Treatment failureb |
|
| Wound impact (clinic visit and 6 months) | Cardiff Wound Impact Questionnaire (CWIQ):
|
| Decision regret (6 months) | Decision regret scale based on five questions, ranging from 0 (least regret) to 100 (greatest regret). |
| Scarring (6 months) |
|
| Complications (days 1 and 7, clinic visit and 6 months) | Presence of complications (bleeding, seroma, infection, flap necrosis, haematoma, maceration, dehiscence, discharge other related to procedure). |
Comparisons
The sample size precluded reliable comparisons between specific subtypes, with only one procedure (Karydakis) providing at least 100 participants with non-missing outcome data. Instead, we undertook the following risk-adjusted treatment comparisons on broader categories of procedure types:
-
any major excisional procedure versus any minor procedure
-
any major excisional procedure with asymmetric closure versus any minor procedure (minimal excision).
Outcomes were summarised descriptively in relation to the treatment received for the less broad treatment groupings whose number did not permit risk-adjusted modelling.
Methods for obtaining risk-adjusted comparisons
Procedures were compared using risk-adjusted methods to reduce bias due to treatment selection, since the extent of disease is likely associated with both the type of procedure and the response.
Statistical methods for risk-adjusted outcomes
Three broad approaches were taken to risk-adjust these comparisons.
-
Regression modelling.
Risk adjustment was undertaken separately to attempt to adjust for imbalance in prognostic features across the procedure groups. Each outcome was modelled separately since features do not affect all outcomes equally. For each outcome, three models were fitted:
-
all features
-
features associated with the outcome
-
the Wysocki disease classification alone.
Model 2 is a ‘compromise’ model which trades off missing potentially important features against model parsimony (overfitting) and the impact of missing covariate data. Covariates were removed on the basis of Akaike’s information criteria (AIC) and the size of the c-statistic of the model. All models were discussed and agreed with a core study clinical team prior to revealing comparative data.
Continuous outcomes were modelled using linear regression, and differences with 95% confidence intervals (CIs) between treatment groups were estimated from the regression coefficient for the procedure group. Binary outcomes were modelled using logistic regression and absolute differences in proportions were assessed using the difference in marginal probabilities. Time to wound healing and time to return to normal activities were modelled using either Cox regression or parametric accelerated survival time, the choice of which depended on which fitted best to the distribution. Proportional hazards were assessed using scaled Schoenfeld residuals and the Grambsch–Therneau test, and the fit of parametric survival distributions was assessed using Q-Q plots. 34 The parametric model was chosen as the lowest AIC among four different approaches (Weibull, log-normal, log-logistic and generalised gamma).
-
Propensity score approaches.
The second approach used a different approach which attempts to balance treatment groups based on features affecting the choice of procedure they received, rather than features associated with outcome. Two approaches were taken.
-
inverse probability weighting (IPW)
-
nearest neighbour matching.
Features were assessed using logistic regression in which treatment choice was the outcome. Covariates were identified analogously to model 1(ii) above. The same propensity score adjustments were used for each outcome. The propensity score-adjusted models were then used to calculate predicted outcomes in both arms, following which their difference and 95% CI were estimated.
Linear and logistic regression models were used for continuous and binary outcomes, respectively. Time-to-event outcomes were fitted within the propensity score framework only if the assumption of accelerated failure time distributions was met based as outlined above.
-
Augmented IPW/IPW with regression adjustment.
The final approach is a combination of approaches 1 and 2 which simultaneously models both treatment selection and outcome using the same covariates used in 1(ii) and 2. The differences in predicted outcomes and their 95% Cis were estimated for binary or continuous outcomes. Time-to-event outcomes (wound healing and return to normal activities) were compared using IPW with regression adjustment. In this, treatment selection was balanced using IPW, and the outcome was modelled adjusting for covariates in 1(ii). The AIC was used to select the best-fitting distribution (Weibull, log-normal, log-logistic or generalised gamma).
Factors affecting outcome and choice of treatment
Previous publications have suggested several possible risk factors which may affect outcomes. The following factors were considered as potential risk factors for poor outcome:
-
Demographic features: sex, body mass index (BMI), depth of natal cleft, presence and type of gluteal hair (none, mild, dense) and smoking status.
-
Disease characteristics: pit density (number of pits divided by spread of pits), presence of unilateral or bilateral disease, distance from furthest lateral opening to the nearest pit, presence of pus and Wysocki disease classification.
The same features were also assessed for their association with choice of procedure.
Statistical assumptions
All modelling approaches make assumptions (some of which are untestable), and no single method is clearly optimal. Approaches 1 (regression) and 2 (propensity score) are unbiased only if the models incorporate all relevant features and are correctly specified. Approach 3 is termed a ‘doubly robust’ method and is unbiased if either of the two-component models is correctly specified, but is more complex and more susceptible to overfitting. In view of this, no single method was identified as the primary risk adjustment. Instead, the findings from models were assessed for their consistency and, where they provided conflicting estimates, the plausibility of each model was considered.
A preliminary assessment of overlap was undertaken prior to any modelling in order to ensure that different treatments had non-zero probability of uptake in different subgroups. 35
All participants undergoing a procedure were included in the analyses.
Patient and public involvement
Two patient and public involvement (PPI) representatives joined the study. The PPI representatives reviewed all patient-facing documents to ensure readability, understanding and format. One PPI representative sat on the steering committee panel and provided an instrumental patient voice in the management of the study. This PPI representative suggested the inclusion of a supplementary participant information sheet to be available for patients who were waiting for their clinic appointment. The patient representatives were also consulted when writing the plain English summaries and dissemination materials.
Results
Recruitment and participant flow
Thirty-one UK sites recruited participants over a 46-month period from May 2019 to March 2022 (see Appendix 2). Figure 1 shows the flow of participants through the cohort: in total, 729 participants consented to be part of the cohort study. Participants were excluded from analyses if they did not have a procedure during the study (n = 45), if they were ineligible due to an incorrect diagnosis (n = 7), or if there was not enough information provided in order to categorise their procedure (n = 10). A total of 667 participants were included in the analysis cohort, of whom 476 (71%) provided follow-up data at 6 months. The number of participants who consented was lower than our anticipated sample size of 800, with 100 patients in each of the front-running management strategies.
FIGURE 1.
Participant flow for the PITSTOP study. PIS, patient information sheet.
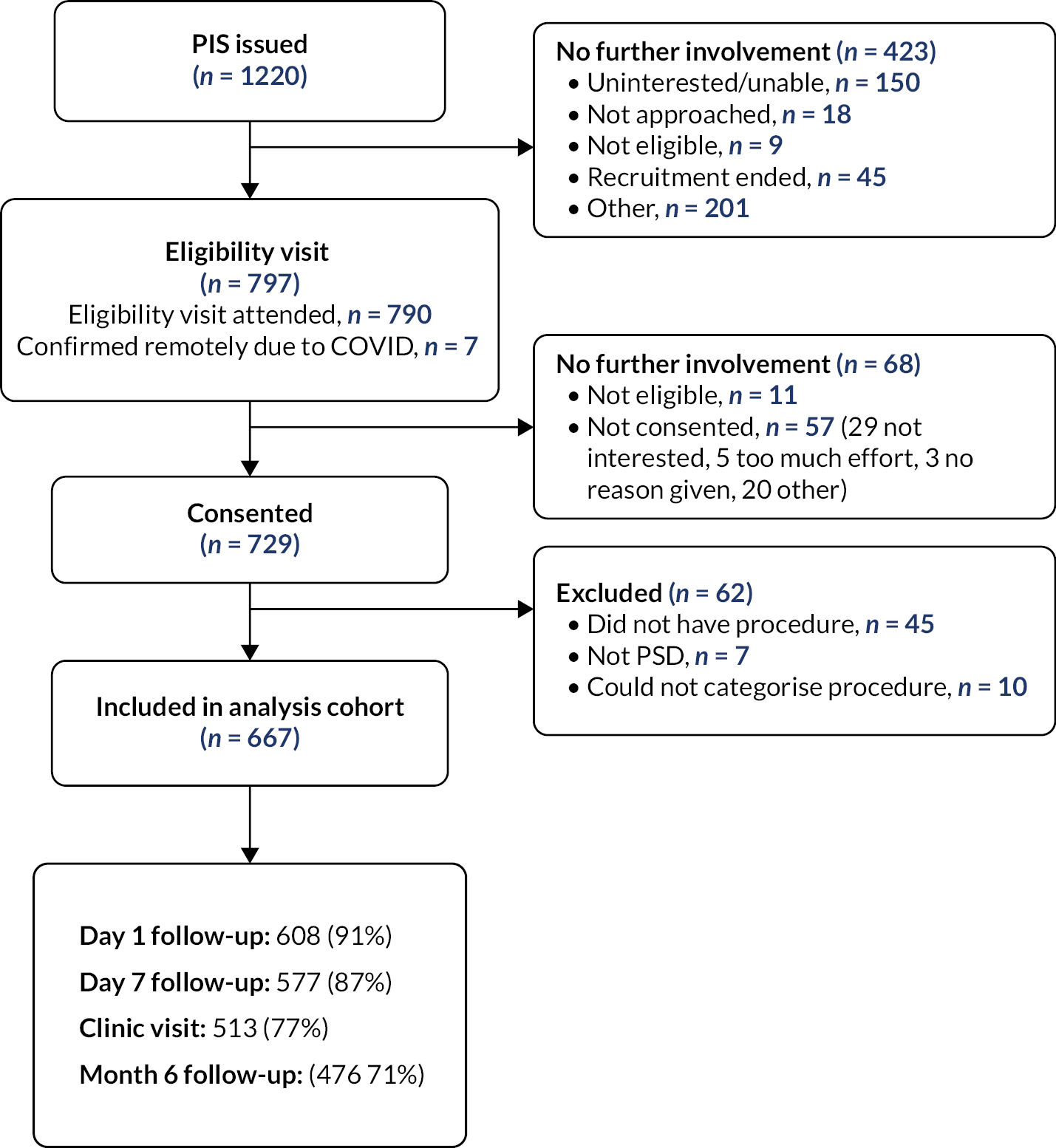
In response to COVID-19, participants were able to be followed up at the end of the study; this was completed for 574 participants and used to update complications and recurrence data.
Baseline characteristics
The characteristics of the cohort participants are included in Tables 5 and 6. There were more males (73%), and 85% of participants were white; the average age of participants was 29 years. Just over half (54%) of participants reported no previous procedures, and 22% reported a previous elective procedure for PSD.
| Characteristic | Asymmetric closure | Leave open | Midline closure | Minimal excision | All |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Age | |||||
| N (%) | 272 (100%) | 49 (100%) | 76 (100%) | 270 (100%) | 667 (100%) |
| Mean (SD) | 28.5 (9.0) | 28.1 (10.9) | 28.1 (7.7) | 29.7 (9.9) | 28.9 (9.4) |
| Median (IQR) | 27.0 (22.0–32.0) | 25.0 (20.0, 33.0) | 27.5 (22.5–31.5) | 28.0 (23.0–34.0) | 27.0 (22.0– 33.0) |
| Min, max | 16.0, 73.0 | 16.0, 64.0 | 18.0, 58.0 | 16.0, 69.0 | 16.0, 73.0 |
| BMI (kg/m2) | |||||
| N (%) | 253 (93%) | 47 (96%) | 71 (93%) | 241 (89%) | 612 (92%) |
| Mean (SD) | 29.5 (5.5) | 28.9 (6.9) | 28.9 (5.0) | 28.7 (6.1) | 29.1 (5.8) |
| Median (IQR) | 28.8 (25.5–32.8) | 28.1 (23.0– 32.7) | 28.0 (25.1–32.7) | 27.8 (24.2–32.1) | 28.3 (24.9– 32.7) |
| Min, max | 17.6, 59.5 | 17.7, 49.2 | 17.0, 39.7 | 13.1, 47.6 | 13.1, 59.5 |
| Number of baths and/or showers in a typical week | |||||
| N (%) | 260 (96%) | 49 (100%) | 74 (97%) | 261 (97%) | 644 (97%) |
| Mean (SD) | 6.9 (2.9) | 7.8 (3.8) | 7.6 (2.7) | 6.8 (2.4) | 7.0 (2.8) |
| Median (IQR) | 7.0 (5.5–7.0) | 7.0 (7.0–7.0) | 7.0 (7.0–7.0) | 7.0 (6.0–7.0) | 7.0 (6.0– 7.0) |
| Min, max | 1.0, 27.0 | 4.0, 21.0 | 3.0, 14.0 | 1.0, 14.0 | 1.0, 27.0 |
| Sex | |||||
| Male | 183 (67%) | 36 (73%) | 60 (79%) | 206 (76%) | 485 (73%) |
| Female | 89 (33%) | 13 (27%) | 16 (21%) | 64 (24%) | 182 (27%) |
| Ethnicity | |||||
| White | 228 (84%) | 41 (84%) | 72 (95%) | 229 (85%) | 570 (85%) |
| Asian/Asian British | 23 (8%) | 4 (8%) | 4 (5%) | 27 (10%) | 58 (9%) |
| Mixed/multiple ethnic groups | 9 (3%) | 1 (2%) | 0 (0%) | 3 (1%) | 13 (2%) |
| Black/African/Caribbean/Black British | 3 (1%) | 2 (4%) | 0 (0%) | 3 (1%) | 8 (1%) |
| Other ethnic group | 4 (1%) | 0 (0%) | 0 (0%) | 3 (1%) | 7 (1%) |
| Prefer not to say | 3 (1%) | 1 (2%) | 0 (0%) | 1 (0%) | 5 (1%) |
| Seated for more than 6 hours in a working day | 142 (52%) | 19 (39%) | 36 (47%) | 135 (50%) | 332 (50%) |
| Smoking status | |||||
| Non-smoker | 148 (54%) | 31 (63%) | 43 (57%) | 152 (56%) | 374 (56%) |
| Current smoker | 86 (32%) | 13 (27%) | 26 (34%) | 71 (26%) | 196 (29%) |
| Current e-cigarette smoker | 13 (5%) | 0 (0%) | 4 (5%) | 20 (7%) | 37 (6%) |
| Employment status | |||||
| Employed | 198 (73%) | 35 (71%) | 64 (84%) | 201 (74%) | 498 (75%) |
| House-partner or full-time parent/carer | 4 (1%) | 0 (0%) | 0 (0%) | 7 (3%) | 11 (2%) |
| Volunteer or between jobs | 12 (4%) | 1 (2%) | 1 (1%) | 9 (3%) | 23 (3%) |
| Student or trainee | 36 (13%) | 8 (16%) | 9 (12%) | 33 (12%) | 86 (13%) |
| Retired | 1 (0%) | 0 (0%) | 0 (0%) | 3 (1%) | 4 (1%) |
| Unemployed/not working | 18 (7%) | 4 (8%) | 2 (3%) | 13 (5%) | 37 (6%) |
| Other | 3 (1%) | 0 (0%) | 0 (0%) | 3 (1%) | 6 (1%) |
| Hair type | |||||
| 0 Bald | 5 (2%) | 4 (8%) | 2 (3%) | 7 (3%) | 18 (3%) |
| 1a Straight (fine/thin) | 57 (21%) | 9 (18%) | 20 (26%) | 71 (26%) | 157 (24%) |
| 1b Straight (medium) | 104 (38%) | 12 (24%) | 30 (39%) | 87 (32%) | 233 (35%) |
| 1c Straight (coarse) | 8 (3%) | 2 (4%) | 4 (5%) | 14 (5%) | 28 (4%) |
| 2a Wavy (fine/thin) | 24 (9%) | 4 (8%) | 4 (5%) | 14 (5%) | 46 (7%) |
| 2b Wavy (medium) | 31 (11%) | 2 (4%) | 10 (13%) | 42 (16%) | 85 (13%) |
| 2c Wavy (coarse) | 11 (4%) | 8 (16%) | 4 (5%) | 17 (6%) | 40 (6%) |
| 3a Curly (loose) | 20 (7%) | 5 (10%) | 1 (1%) | 11 (4%) | 37 (6%) |
| 3b Curly (tight) | 7 (3%) | 2 (4%) | 0 (0%) | 3 (1%) | 12 (2%) |
| 4a Kinky (soft) | 2 (1%) | 0 (0%) | 1 (1%) | 2 (1%) | 5 (1%) |
| 4b Kinky (wiry) | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| 4c Kinky (wiry) | 2 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | 3 (0%) |
| Hair cut frequency | |||||
| More than once every 4 weeks | 80 (29%) | 14 (29%) | 20 (26%) | 84 (31%) | 198 (30%) |
| Once every 4–8 weeks | 98 (36%) | 20 (41%) | 33 (43%) | 109 (40%) | 260 (39%) |
| Less than once every 8 weeks | 94 (35%) | 15 (31%) | 23 (30%) | 76 (28%) | 208 (31%) |
| Characteristic | Asymmetric closure | Leave open | Midline closure | Minimal excision | All |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Natal cleft depth (mm) | |||||
| N (%) | 253 (93%) | 40 (82%) | 53 (70%) | 236 (87%) | 582 (87%) |
| Mean (SD) | 19.1 (11.8) | 23.6 (18.4) | 20.8 (13.1) | 19.9 (11.8) | 19.9 (12.5) |
| Median (IQR) | 16.0 (10.0–25.0) | 18.5 (10.0– 33.5) | 20.0 (10.0– 30.0) | 20.0 (11.0– 25.0) | 19.0 (10.0– 25.0) |
| Min, max | 0.0, 80.0 | 5.0, 100.0 | 2.0, 63.0 | 0.0, 110.0 | 0.0, 110.0 |
| Number of pits | |||||
| N (%) | 267 (98%) | 47 (96%) | 62 (82%) | 264 (98%) | 640 (96%) |
| Mean (SD) | 2.6 (2.0) | 1.7 (1.2) | 2.3 (2.6) | 2.4 (1.7) | 2.4 (1.9) |
| Median (IQR) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Min, max | 0.0, 16.0 | 0.0, 6.0 | 0.0, 20.0 | 0.0, 17.0 | 0.0, 20.0 |
| Length of pits (spread, mm) | |||||
| N (%) | 176 (65%) | 17 (35%) | 34 (45%) | 179 (66%) | 406 (61%) |
| Mean (SD) | 36.2 (37.1) | 44.9 (36.9) | 25.5 (20.3) | 26.2 (21.9) | 31.3 (30.4) |
| Median (IQR) | 30.0 (10.0–50.0) | 30.0 (21.0–70.0) | 20.0 (10.0–35.0) | 20.0 (10.0–40.0) | 23.0 (10.0–41.0) |
| Min, max | 0.0, 320.0 | 4.0, 150.0 | 0.0, 85.0 | 2.0, 140.0 | 0.0, 320.0 |
| Pit density (pits per mm) | |||||
| N (%) | 253 (93%) | 42 (86%) | 61 (80%) | 256 (95%) | 612 (92%) |
| Mean (SD) | 0.1 (0.2) | 0.0 (0.1) | 0.2 (0.5) | 0.1 (0.2) | 0.1 (0.3) |
| Median (IQR) | 0.1 (0.0–0.2) | 0.0 (0.0–0.1) | 0.0 (0.0–0.2) | 0.1 (0.0–0.2) | 0.1 (0.0–0.2) |
| Min, max | 0.0, 2.0 | 0.0, 0.5 | 0.0, 3.0 | 0.0, 1.5 | 0.0, 3.0 |
| Number of previous procedures | |||||
| 0 | 129 (47%) | 21 (43%) | 48 (63%) | 159 (59%) | 357 (54%) |
| 1 | 73 (27%) | 14 (29%) | 15 (20%) | 72 (27%) | 174 (26%) |
| 2 | 37 (14%) | 6 (12%) | 9 (12%) | 26 (10%) | 78 (12%) |
| 3 or more | 33 (12%) | 8 (16%) | 4 (5%) | 13 (5%) | 58 (9%) |
| Previous procedures for PSD | |||||
| Elective procedure | 57 (21%) | 14 (29%) | 9 (12%) | 68 (25%) | 148 (22%) |
| Acute drainage | 101 (37%) | 19 (39%) | 20 (26%) | 55 (20%) | 195 (29%) |
| Emergency procedure | 4 (1%) | 0 (0%) | 0 (0%) | 1 (0%) | 5 (1%) |
| Months from last procedure to current procedure | |||||
| N (%) | 56 (21%) | 14 (29%) | 9 (12%) | 68 (25%) | 147 (22%) |
| Mean (SD) | 35.4 (43.3) | 28.4 (29.1) | 10.3 (8.5) | 39.5 (61.3) | 35.1 (50.6) |
| Median (IQR) | 20.5 (8.0–47.0) | 17.0 (9.0–41.0) | 11.0 (2.0–16.0) | 16.0 (8.0–41.0) | 17.0 (8.0–41.0) |
| Min, max | 0.0, 207.0 | 3.0, 100.0 | 0.0, 22.0 | 0.0, 329.0 | 0.0, 329.0 |
| Wysocki classification | |||||
| Type 1 | 46 (17%) | 7 (14%) | 27 (36%) | 102 (38%) | 182 (27%) |
| Type 2 | 148 (54%) | 19 (39%) | 41 (54%) | 116 (43%) | 324 (49%) |
| Type 3 | 20 (7%) | 8 (16%) | 3 (4%) | 19 (7%) | 50 (7%) |
| Type 4 | 54 (20%) | 13 (27%) | 3 (4%) | 31 (11%) | 101 (15%) |
| None of the above | 3 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | 4 (1%) |
| Distribution of lateral openings | |||||
| No lateral openings | 99 (36.4%) | 20 (40.8%) | 36 (47.4%) | 140 (51.9%) | 295 (44.2%) |
| Unilateral | 150 (55.1%) | 15 (30.6%) | 17 (22.4%) | 105 (38.9%) | 287 (43.0%) |
| Bilateral | 8 (2.9%) | 4 (8.2%) | 1 (1.3%) | 7 (2.6%) | 20 (3.0%) |
| Gluteal hair | |||||
| None | 49 (18%) | 7 (14%) | 10 (13%) | 31 (11%) | 97 (15%) |
| Mild | 137 (50%) | 26 (53%) | 32 (42%) | 134 (50%) | 329 (49%) |
| Dense | 84 (31%) | 13 (27%) | 23 (30%) | 99 (37%) | 219 (33%) |
| Natal cleft skin | |||||
| Maceration | 39 (14%) | 8 (16%) | 8 (11%) | 23 (9%) | 78 (12%) |
| Erosions | 29 (11%) | 4 (8%) | 4 (5%) | 14 (5%) | 51 (8%) |
| Splits | 15 (6%) | 6 (12%) | 8 (11%) | 17 (6%) | 46 (7%) |
| Wide pores | 52 (19%) | 16 (33%) | 20 (26%) | 53 (20%) | 141 (21%) |
| First-degree relatives with history of PSD | 51 (19%) | 9 (18%) | 16 (21%) | 46 (17%) | 122 (18%) |
| Relative with history of PSD | |||||
| Mother | 10 (4%) | 1 (2%) | 4 (5%) | 9 (3%) | 24 (4%) |
| Father | 19 (7%) | 7 (14%) | 6 (8%) | 18 (7%) | 50 (7%) |
| Sibling | 14 (5%) | 1 (2%) | 3 (4%) | 13 (5%) | 31 (5%) |
| Child | 2 (1%) | 0 (0%) | 0 (0%) | 1 (0%) | 3 (0%) |
| Multiple | 6 (2%) | 0 (0%) | 3 (4%) | 5 (2%) | 14 (2%) |
Data completion
Data completion rates for the cohort outcomes are presented in Appendix 3, Table 25. Return to normal activities, recurrence and wound healing were considered complete if a patient contributed those data at either clinic visit, 6-month follow-up or study close (or in the case of recurrence, it was apparent from AE reporting). Complication data were considered complete if the participant contributed data to at least one follow-up time point. Data for recurrence (94%), complication (96%) and return to normal activities (94%) were collected for most participants. The characteristics of participants that attended 6-month follow-up were compared to those that did not attend 6-month follow-up (see Appendix 3, Table 37), and the distribution of characteristics was similar between the groups; there were marginally more participants that had lateral openings in the attenders (50%) compared to the non-attenders (44%). All analyses were conducted on available data.
Treatment decisions
The breakdown of treatments received is presented in Table 7. Recorded procedure details were categorised into four categories, which were further combined into major or minor procedure categories. Over half (60%) of the participants received a major treatment, most commonly asymmetric closure (41%). Of the participants that received minimal excision, the most common treatment options were glue (n = 106, 16% of the cohort) and pit picking (n = 60, 9% of the cohort).
| N = 667 | n (%) | Procedure category | n (%) | Procedure | n (%) |
|---|---|---|---|---|---|
| Procedure type | |||||
| Major | 397 (60%) | Asymmetric closure | 272 (41%) | Bascom’s cleft lift | 86 (13%) |
| Flap | 22 (3%) | ||||
| Karydakis | 164 (25%) | ||||
| Leave open | 49 (7%) | Leave open | 43 (6%) | ||
| Leave open (marsupialisation) | 6 (1%) | ||||
| Midline closure | 76 (11%) | Midline closure | 76 (11%) | ||
| Minor | 270 (41%) | Minimal excision | 270 (41%) | Bascom’s I | 39 (6%) |
| EPSiT | 44 (7%) | ||||
| Glue | 106 (16%) | ||||
| Laser | 11 (2%) | ||||
| Pit picking | 60 (9%) | ||||
| Seton | 10 (2%) |
Further treatment details are presented in Appendix 3, Table 26. Median length of surgery was 30 minutes, and 95% were performed as day cases. Procedures were typically performed by consultant surgeons (68%).
Figure 2 shows the treatment received by disease characteristics. Participants with recurrent disease (defined as reporting any previous procedure, including acute drainage) were more likely to be given asymmetric closure; participants that were not recurrent were more likely to receive minimal excision. Over half (56%) of Wysocki type 1 (only midline pit or sinuses) participants underwent minimal excision, whereas over half (53%) of Wysocki type 4 (disease after treatment with definitive intent) were given asymmetric closure. The extent of overlap is noteworthy; for all disease characteristic categories there were a number of participants that received each treatment type, suggesting there is variety in the types of procedures considered appropriate for patients with different disease characteristics. The distribution of the number of pits was similar across procedure types (see Appendix 3, Figure 13). The proportion of patients with a minor procedure varied substantially across the sites (see Appendix 3, Figure 19), although this may be due to differing case mix across centres.
FIGURE 2.
Treatment choice by disease characteristic. Note: ‘recurrent’ defined as any reported previous procedure; type 1: only midline pit or sinuses; type 2: any midline disease with secondary sinus/es or abscess scar/s; type 3: any midline or secondary disease extending below tip of coccyx; type 4, any disease after treatment with definitive intent.

Shared decision-making and decision regret
Participant ratings of their pre-op consultation were high (Table 8), with the median (IQR) of the CollaboRATE mean score response being 3 (3–4), where 3 represents ‘a lot of effort was made’. The CollaboRATE top score was given in 36% of cases, reflecting that ‘every effort’ was made to help the patient understand their health issue, listen to the things that matter most and include what matters most to the patient in choosing what to do next. The decision regret (DR) scale, completed at month 6 follow-up, was low (median 8, IQR 0–20), and was broadly similar across the treatment categories.
| Asymmetric closure | Leave open | Midline closure | Minimal excision | All | |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| CollaboRATE mean scorea | |||||
| N (%) | 270 (99%) | 49 (100%) | 75 (99%) | 265 (98%) | 659 (99%) |
| Median (IQR) | 3 (3–4) | 4 (3–4) | 3 (3–4) | 3 (3–4) | 3 (3–4) |
| CollaboRATE top score given | |||||
| No | 182 (67%) | 28 (57%) | 51 (67%) | 155 (57%) | 416 (62%) |
| Yes | 88 (32%) | 21 (43%) | 24 (32%) | 110 (41%) | 243 (36%) |
| DR scaleb | |||||
| N (%) | 198 (73%) | 35 (71%) | 51 (67%) | 173 (64%) | 457 (69%) |
| Median (IQR) | 10 (0–20) | 8 (4–20) | 8 (0–24) | 8 (0–20) | 8 (0–20) |
| Satisfaction with effect of treatment or care | |||||
| Very satisfied | 113 (42%) | 19 (39%) | 21 (28%) | 89 (33%) | 242 (36%) |
| Satisfied | 61 (22%) | 9 (18%) | 18 (24%) | 54 (20%) | 142 (21%) |
| Neither satisfied nor dissatisfied | 15 (6%) | 0 (0%) | 6 (8%) | 19 (7%) | 40 (6%) |
| Dissatisfied | 3 (1%) | 6 (12%) | 6 (8%) | 9 (3%) | 24 (4%) |
| Very dissatisfied | 9 (3%) | 1 (2%) | 0 (0%) | 6 (2%) | 16 (2%) |
The relationship between CollaboRATE mean score and DR is shown in Appendix 3, Figure 14. There is little clear evidence of a correlation between shared decision-making (SDM) and DR, with the majority of patients being in the top left corner of the graph (representing participants that were happy with their collaboration and had few regrets about their procedure).
Decision regret was low among patients [mean (SD) 14.5 (16.7)] and was broadly similar across the procedure types. The majority of patients reported being either satisfied or very satisfied (83%) with their procedure. Seven (21%) of the participants that received a leave-open procedure reported being either dissatisfied or very dissatisfied. The majority of patients returned to normal activity by the end of follow-up (n = 550, 88%) and 75% reported the wound as having healed during the study follow-up. Almost half (47%) of participants experienced a complication during follow-up.
Outcome summaries
The continuous outcome measures, recorded over time, are presented in Appendix 3, Table 27. Self-reported pain related to pilonidal sinus was at its highest on average on day 1 after procedure compared to baseline and reduced to its lowest at the 6-month visit. The highest average pain was reported by participants that received asymmetric closure. Patient-reported EQ-5D-5L health utility reduced from baseline to day 7 (overall means 0.80 and 0.69, respectively) but had recovered at both clinic and 6-month visits (overall means 0.83 and 0.89, respectively). Participants that received minimal excision reported the least change at day 7 and the highest health utility and QoL satisfaction at clinic visit and 6 months, although the scores were more similar among treatment groups at 6 months.
Other outcome measures are presented in Appendix 3, Tables 28–30. Repacking procedures were reported by 68 participants (12%) by day 7, while 87 participants (17%) reported repacking at clinic visit. At day 7, 226 (39%) patients reported a re-dressing procedure; re-dressing by day 7 was most common in asymmetric closure (49%) and midline closure (52%). Nearly half of participants experienced a complication during follow-up (n = 301, 45%), the most common of which were infection (26%) and discharge (18%). The numbers of complications were broadly similar across the three major surgery groups, and were lower for patients who received minimal excision, particularly for bleeding, dehiscence and infection.
Risk-adjusted treatment comparisons
The primary comparison between treatments was made between major procedures (asymmetric closure, leave open, midline closure, n = 396) and minor procedures (minimal excision, n = 270). No factors were found to be collinear and so all were included in the risk adjustment. Non-linearity of continuous features (BMI, natal cleft depth, pit spread and pit distance) was investigated and all features were deemed to be sufficiently modelled using linear terms. For the propensity score modelling, sufficient overlap in risk score was observed for all outcomes, and thus risk-adjusted analysis was deemed appropriate for major versus minor procedures.
Pain
The propensity score model identified sex, presence of pus and Wysocki classification as the most important features in treatment choice. Patients were more likely to undergo major procedure if they were female, had pus, or were classified as Wysocki type 4 (disease after treatment with definitive intent) and least likely to have major procedure if type 1 (only midline pit or sinuses). These factors were used in the propensity-adjusted models for all outcome comparisons.
Pain on day 1, adjusted for factors predictive of treatment choice and outcome via augmented IPW, was higher for patients who received major procedures compared to minor procedures by 1.58 points (95% CI 1.14 to 2.01) (see Appendix 3, Table 31). This was very similar to the unadjusted difference (mean difference 1.62, 95% CI 1.23 to 2.02). The number of participants included in each analysis varied according to the factors included to adjust the models. A similar difference in pain at day 7 was observed (augmented IPW-adjusted mean difference 1.53, 95% CI 1.12 to 1.95). The difference in pain between treatment groups was similar regardless of the risk adjustment method. A post hoc sensitivity analysis that included baseline pain as a covariate yielded mean differences and 95% CIs that were within 0.1 points of these estimates.
Pain over time for major and minor procedures is shown in Figure 3 (pain at clinic visit and month 6 were not prespecified as outcomes with formal comparisons); the raw difference in means was closer at clinic visit than at day 7, and there was no difference in mean pain reported at 6 months between the procedure types.
FIGURE 3.
Pain with major and minor procedures, baseline to 6 months after surgery.

Complications
Just over half (54%) of participants receiving major procedures reported a complication, compared to 36% of participants who had a minor procedure. After augmented IPW risk adjustment, participants who received major procedures reported a 17.5% (95% CI 9.1 to 25.9%) higher absolute incidence of complications during follow-up (Table 9). The estimate of the difference was relatively consistent regardless of risk adjustment method.
| Complications | Major procedures | Minor procedures | n | Risk difference (95% CI)a |
|---|---|---|---|---|
| Raw difference | 207/385 (54%) | 94/258 (36%) | 643 | 17.3% (9.6 to 25.0) |
| Risk-adjusted – Wysocki | 638 | 16.7% (8.8 to 24.6) | ||
| Risk-adjusted – chosen model (BMI, Wysocki) | 590 | 17.3% (9.1 to 25.6) | ||
| Risk-adjusted – full model | 424 | 20.0% (10.4 to 29.6) | ||
| Propensity-adjusted – IPW | 627 | 16.5% (8.1 to 24.8) | ||
| Propensity matching | 627 | 15.9% (7.1 to 24.7) | ||
| Augmented IPW | 579 | 17.5% (9.1 to 25.9) |
Disease recurrence
Recurrence was less likely for major procedures compared to minor procedures (Table 10). Among participants who had a major procedure, 15% reported recurrence by 6 months after surgery, rising to 23% when the full follow-up period was included, compared to 27% and 34%, respectively, for participants who had minor procedures. The risk-adjusted absolute difference in 6-month recurrence was 10.1% (95% CI 2.1 to 18.1%) in favour of major procedure using augmented IPW and was of similar magnitude in other risk adjustments.
| Recurrence | Recurrence | Recurrence (within 6 months) | Treatment failure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major procedures | Minor procedures | n | Risk difference (95% CI)a | Major procedures | Minor procedures | n | Risk difference (95% CI)a | Major procedure | Minor procedure | n | Risk difference (95% CI)a | |
| Raw difference | 86/373 (23%) | 87/256 (34%) | 629 | −10.9% (−18.1 to −3.7%) | 51/337 (15%) | 61/229 (27%) | 566 | −11.5% (−18.4 to −4.6%) | 169/373 (45%) | 121/257 (47%) | 630 | −1.8% (−9.7 to 6.1%) |
| Risk-adjusted – Wysocki | 624 | −11.1% (−18.5 to −3.7%) | 561 | −11.3% (−18.4 to −4.2%) | 625 | −2.2% (−10.4 to 5.9%) | ||||||
| Risk-adjusted – chosen model (Wysocki, pit density) | 577 | −9.0% (−16.6 to −1.3%) | 516 | −9.4% (−16.7 to −2.0%) | 578 | −1.7% (−10.1 to 6.7%) | ||||||
| Risk-adjusted – full model | 409 | −8.4% (−17.7 to 0.8%) | 366 | −7.4% (−16.4 to 1.6%) | 410 | 1.2% (−8.7 to 11.1%) | ||||||
| Propensity-adjusted – inverse weighting | 613 | −13.8% (−22.0 to −5.7%) | 550 | −12.9% (−20.7 to −5.1%) | 614 | −5.2% (−13.8 to 3.4%) | ||||||
| Propensity matching | 613 | −12.0% (−20.5 to −3.5%) | 550 | −12.5% (−21.0 to −4.1%) | 614 | −3.5% (−12.5 to 5.6%) | ||||||
| Augmented IPW | 575 | −10.1% (−18.1 to −2.1%) | 514 | −9.6% (−17.3 to −1.9%) | 576 | −2.3% (−10.9 to 6.2%) | ||||||
Treatment failures (defined as the composite of recurred, not returned to normal activity, or not healed by the time of the last follow-up) were compared between treatment groups. The proportion of participants for whom treatment failed was more similar across treatment groups once healing and return to normal activities were introduced. In total, 47% of minor procedures failed at 6 months compared to 45% of major procedures (adjusted difference 2.3%, 95% CI −6.2 to +10.9%).
Return to normal activities
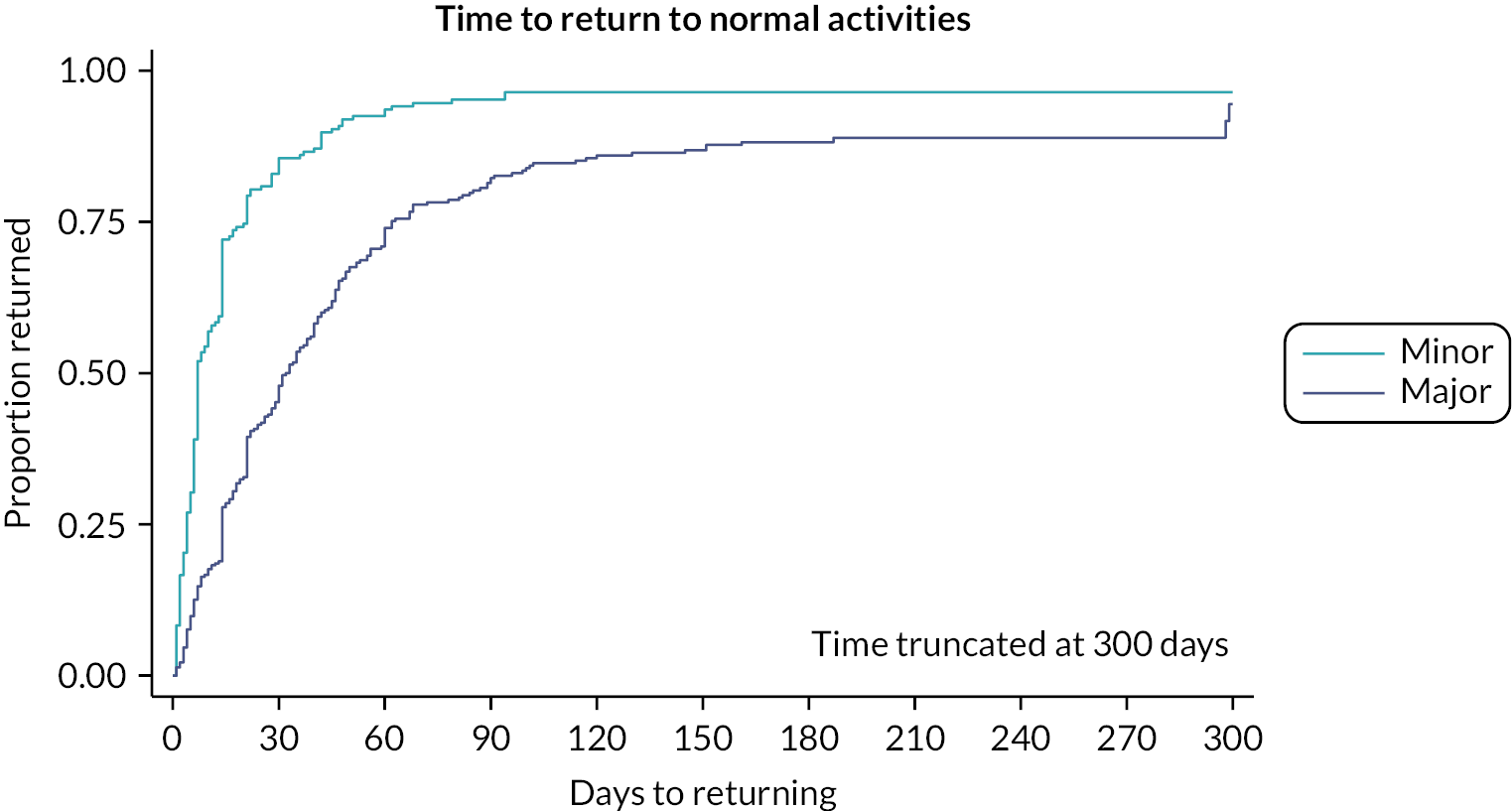
Time to return to normal activity was compared between major and minor procedures (Table 11 and Figure 4). While nearly all participants had returned to normal activity by the end of follow-up, the time taken was far quicker among those undergoing minimal excision (median 7 days) than those who had major procedures (median 32 days). At 6 months, 12% of participants who had major procedures and 4% of participants who had minor procedures were yet to return to normal activity (see Figure 4). Several participants dropped out, providing either a censored time or no data at all; in the best-case scenario where these were assumed to have recovered, the proportion of participants who returned to normal activity at 6 months would be 96% for major procedures and 98% for minor procedures. Participants who received major procedures took on average 21 days longer to return to normal activity than those receiving minor procedures, and the difference was greater using risk adjustment models. The mean difference as estimated by augmented IPW was 25.9 days (95% CI 18.4 to 33.4 days), with regression adjustment approaches providing estimates closer to the unadjusted difference. Similar findings were present when comparing asymmetric closure procedures to the minimally invasive approaches (see Appendix 3, Figure 19 and Table 40).
FIGURE 4.
Time to return to normal activities by major or minor procedure type.
| Time to return to normal activity | n | Difference (days) (95% CI)a |
|---|---|---|
| Raw difference | 607 | 21.0 (16.3 to 25.7) |
| Risk-adjusted – Wysocki | 600 | 20.3 (15.6 to 24.9) |
| Risk-adjusted – chosen model (natal cleft depth, Wysocki, lateral distribution) | 502 | 19.8 (14.7 to 24.9) |
| Risk-adjusted – full model | 403 | 19.8 (14.0 to 25.6) |
| Propensity-adjusted – inverse weighting | 589 | 35.6 (19.8 to 51.4) |
| IPW + regression | 502 | 25.9 (18.4 to 33.4) |
Wound healing
Participants having major procedures also took longer to heal than those who had a minor procedure. The median time to healing was 30 days among people undergoing minimal procedures, compared to 70 days among those undergoing a major procedure. However, as highlighted in Figure 5, around 25% of participants in both groups had wounds that had not healed. Some of the individuals lost to follow-up (LTFU) may have healed, but a best-case scenario where those censored prior to 6 months were assumed to have healed would still mean 10% of participants considered their wound unhealed at 6 months. Unadjusted and regression-based risk adjustments both estimated the difference in wound healing to be over a month greater following a major procedure, while propensity score methods estimated larger differences but with wider CIs (augmented IPW estimate 53.5 days, 95% CI 28.8 to 78.2 days; Table 12).
FIGURE 5.
Time to wound healing by major or minor procedure type.

| Major procedure | Minor procedure | |||||
|---|---|---|---|---|---|---|
| Model | n | Median (IQR) days | n | Median (IQR) days | N | Mean difference (95% CI)a |
| Raw difference | 336 | 70 (31–52) | 217 | 30 (14–54) | 553 | 39.7 (27.0 to 52.4) |
| Risk-adjusted – Wysocki | 546 | 36.7 (23.8 to 49.6) | ||||
| Risk-adjusted – chosen model (Wysocki, BMI, smoking status, pus) | 452 | 34.8 (19.9 to 49.6) | ||||
| Risk-adjusted – full model | 368 | 38.2 (22.3 to 54.1) | ||||
| Propensity-adjusted –IPW | 536 | 111.3 (−10.9 to 233.4) | ||||
| Augmented IPW | 452 | 53.5 (28.8 to 78.2) | ||||
Pairwise comparisons of asymmetric closure and minimal excision (removing participants that had the major procedures – leave open and midline closure) produced results in keeping with the comparison between major and minor procedures (see Appendix 3, Tables 37–43).
Surgeon variation
A post hoc analysis looked at recurrence rates among surgeons who operated on at least 10 participants. In total 13 surgeons undertook at least 10 procedures (range 10–55 procedures, median 14). In total 282/667 participants underwent procedure by one of these surgeons. While recurrence and treatment failure rates varied among the 13 surgeons, outcomes were more favourable among participants whose surgeons treated 10 or more cases (see Appendix 3, Table 32). Overall recurrence at 6 months was lower among participants treated by these surgeons (40%) compared with surgeons that treated fewer cases (60%), with similar differences seen for recurrence at any time (42 vs. 48%) and treatment failure (40 vs. 60%).
Adverse events
Adverse events and serious adverse events (SAEs) are presented in Table 13; 107 (16%) patients experienced at least one AE during follow-up, and the most common category of AE was wound infection (59%). Eleven participants experienced an SAE, nine of which were inpatient hospitalisation.
| Asymmetric closure | Leave open | Midline closure | Minimal excision | All | |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Number (%) of participants who experienced ≥ 1 AE | 64 (24%) | 7 (14%) | 19 (25%) | 17 (6%) | 107 (16%) |
| Number of all AEs (including repeated events) | 94 | 8 | 24 | 19 | 145 |
| Category | |||||
| Anaesthetic AE | 2 (2%) | 1 (13%) | 0 (0%) | 0 (0%) | 3 (2%) |
| Bleeding/haematoma | 7 (7%) | 0 (0%) | 4 (17%) | 0 (0%) | 11 (8%) |
| Dehiscence | 22 (23%) | 0 (0%) | 6 (25%) | 2 (11%) | 30 (21%) |
| Discharge | 2 (2%) | 1 (13%) | 3 (13%) | 3 (16%) | 9 (6%) |
| Medication AE | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (3%) |
| Seroma | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) |
| Seton break | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Wound infection | 54 (57%) | 6 (75%) | 11 (46%) | 14 (74%) | 85 (59%) |
| Number (%) of participants who experienced ≥ 1 SAE | 6 (2%) | 1 (2%) | 2 (3%) | 2 (1%) | 11 (2%) |
| Number of all SAEs (including repeated events) | 6 | 1 | 2 | 2 | 11 |
| Seriousness | |||||
| Inpatient hospitalisation | 4 (67%) | 1 (100%) | 2 (100%) | 2 (100%) | 9 (82%) |
| Considered medically significant by the investigator | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) |
| Category | |||||
| Bleeding/haematoma | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) |
| Seton break | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) |
| Wound infection | 4 (67%) | 1 (100%) | 2 (100%) | 2 (100%) | 9 (82%) |
| Expected SAE | 5 (83%) | 1 (100%) | 1 (50%) | 2 (100%) | 9 (82%) |
Discussion
The prospective cohort study was the main component of the PITSTOP study and consists of one of the largest data sets of real-world experience gathered on PSD to date. Although these are subject to the potential biases of non-randomised comparisons, the data suggest that there is more postoperative pain and failure of treatment after major excisional procedures compared to minimally invasive procedures, associated with an increased time to healing and longer time to return to normal activities. This is the case even after risk adjustment for patient demographics and severity of disease.
The demographics of this cohort are unsurprising: the disease tends to affect a young, predominantly male population who are overweight. 1 Interestingly, despite descriptions of patients as having coarse hair,36 we found the majority were assessed as having fine or medium hair. Around half of the group have had previous surgery for PSD, usually acute drainage of an abscess. Around one in five patients have had more than two procedures, with a significant minority having had three or more procedures. The disease varies in severity from simple midline disease to around half having some form of lateral extension. Around 10% have complex disease (bilateral disease or disease below the coccyx), making surgical intervention challenging and potentially limiting options for treatment. Recurrent disease – which may also, but not necessarily, be considered complex – was reported in 15%.
Twelve different types of surgical approach were utilised. This is more than in a previous survey on UK practice,28 the increase being mainly due to the expanded repertoire of minimally invasive techniques including glue, laser and endoscopic treatment over the last 10 years. By far the commonest procedures were excision and asymmetric closure techniques (Karydakis and Bascom’s II), which is consistent with previous data. While considered outdated due to the risk of failure and protracted recovery time,15,37,38 roughly one in six procedures involved excision-and-leave-open of the skin defect or primary closure in the midline. Reasons for the persistence of these procedures in UK practice have been discussed in Chapter 2. It is feasible that complex situations – for example, advanced bilateral disease, markedly infected wounds or other unusual variants of disease – meant that no other procedure was possible. However, it seems unlikely that these uncommon variants account for all such procedures carried out.
Despite the plethora of minimally invasive procedures currently practised in the UK, only 40% of patients had this approach. Given that this was among a group of surgeons interested in pilonidal disease, this approach could well be even less in the context of all UK surgeons. Minimally invasive procedures are certainly not suitable for all patients. For those with extensive disease or complex recurrence, minimally invasive approaches may not be effective. However, it would appear logical that, for most patients with non-recurrent disease confined to the midline or with simple lateral extensions, such a technique would have been feasible. One exception would be the patient with multiple pits within a small area or those with extensive underlying cavities where pit picking or Bascom’s I would result in a large midline defect. Even considering these caveats, only around 60% of patients with disease confined to the midline and only 40% of patients with lateral extensions had a minimal procedure. Many more could have been treated less invasively.
Analysis of preoperative demographic and disease characteristics revealed some factors that made a major excisional technique more likely, including recurrent disease and the presence of pus. More surprising is the association of being female with an excisional procedure unrelated to disease extent and complexity.
The relatively low utilisation of minimally invasive procedures becomes very relevant when outcomes are considered. Minimally invasive techniques lead to less pain at all time points and especially in the first week after surgery. Patients undergoing minimal intervention reported pain (on a 0–10 scale) around 1.5–1.7 units lower than those undergoing major excision on day 1 and day 7. Complications were also more common with major excision, with 15–20% excess seen after major excision. Time to wound healing and return to normal activities were significantly shorter after minimally invasive procedures, allowing patients to return to work, study and socialisation much faster. In contrast, these data confirm that major excisional techniques are around 10% more likely to cure the disease. This draws into question whether patients prefer a higher chance of cure in preference to more pain, more complications and a more protracted recovery. Such trade-offs are explored in Mixed-methods substudy and Discrete choice experiment.
It is plausible that the differences seen when comparing these two intervention groups relate to the case mix. For example, more extensive disease would be more likely to require major excision, but patients would, regardless of intervention, be more likely to have a complicated and protracted recovery. We attempted to control for case mix by correcting for risk factors with statistical modelling. We prespecified demographic and disease characteristics that previous literature had identified as influencing outcome, and assessed sensitivity to this via alternative models that were developed after discussion with the core study clinical team. Regardless of the model used, the association with postoperative outcomes remained consistent. It is also worth noting the substantial overlap in procedure types even among ostensibly similar subgroups of disease, which suggests that the choice of procedure may be driven as much by patient choice and surgical familiarity as it is by the severity of the disease.
A noteworthy result was the time to return to normal activities and the time to healing. The literature is full of reports of spectacular efficacy for several procedures. 13,39,40 Yet regardless of intervention type, at least 10% and possibly up to 25% of patients had not healed by 6 months, and up to 12% of the major excision group had not returned to normal activities. This suggests that, in the real world as compared to specialist units, these procedures may not necessarily be as effective as the literature would suggest, and there may be a significant postoperative burden for patients of which many will not have been made aware during informed consent.
It is likely that patients have different interpretations of the terms ‘recurrence’ and ‘wound healing’ and may have considered these as interchangeable when telling us about their disease during follow-up. In the true sense, recurrence means disease that has healed after surgery but which then arises again, at the same site or at a different site to the original disease. This should be distinguished from disease that fails to heal at all after surgery or indeed when surgery achieves excision of the pits, but the patient is left with an unhealed wound, often in the midline. True recurrence is probably much less common and requires a protracted length of time to detect accurately. 41 Failure to heal or persistence of disease is probably easier to define, but there remains an issue as to the time point when the intervention should be considered to have healed and not recurred. Consensus from the European Society of Coloproctology working party on pilonidal guidelines has proposed that 6 months after surgery would seem an appropriate time point (Asha Senapati, September 2023, personal communication). If a 25% failure rate was observed, regardless of technique, this would certainly be inferior to most of the reported literature. 13,39,40 This has repercussions when it comes to SDM before surgery.
The difference between this study’s observed failure rate and that reported may relate to the skill and experience of individual surgeons. It may be that experts in pilonidal surgery can achieve healing rates equivalent to those in the literature. 25,42–45 Our study partly triangulated this theory: surgeons responsible for more cases (defined here as treating 10 or more study participants) had better outcomes than surgeons who treated fewer participants, but recurrence was still higher than in previous literature. This could imply some surgeons are more skilled than others, although numbers were small, and the healing rates were not controlled for factors such as technique, case volume and disease severity. An important limitation is that this analysis was not risk-adjusted, and disease characteristics (and hence outcomes) may differ between experienced and less experienced surgeons. Nevertheless, it is unlikely that more experienced surgeons would be systematically allocated easier-to-treat patients, and so disease severity is unlikely to be the reason for these differences. This may justify referral to specialist centres for those patients with particularly complex disease.
The recognised issues with major excision in terms of protracted wound healing and the reported high failure rate with excision and midline closure mean that grouping such procedures along with asymmetric closure and flap procedures15 may have skewed the results in favour of minimal intervention. Fortunately, the number of patients in the asymmetric closure group (Karydakis and Bascom cleft closure) was sufficient to allow us to carry out a more granular analysis. Despite excluding these other procedures, the outcomes were similar for cleft closure techniques compared to minimally invasive techniques. Patients who had an asymmetric closure had more early pain, a higher complication rate and longer time to healing and return to normal activity than those treated with minimally invasive procedures. Failure was 5–8% less likely but the rate was still much higher than in most of the reported literature. 13
When one considers this potential high rate of failure, it is somewhat surprising that the DR was so low. Patients were mainly satisfied with their decision for surgery and in addition felt that the options and outcomes were explained well to them. The CollaboRATE scores were high, suggesting the SDM process was good. These findings somewhat contradict other data suggesting that many are not offered a range of operations, in particular minimally invasive procedures. Data should perhaps be interpreted with caution as they may reflect a social desirability bias. 46
There are limitations to both these data and their interpretation. An obvious limitation is the incompleteness of the data: 1 in 10 patients had missing day 1 data, and 6-month data were only available in around three-quarters of patients. This is despite rigorous governance processes and dedicated research nurses assiduously following up the patients. The study period did fall during the COVID pandemic, and this will have influenced the ability to follow up rigorously in some cases. Otherwise, the incompleteness of the data is probably a reflection of the demographic of the group, which tends to consist of young active working people, predominantly male. Such a demographic may be less likely to respond to follow-up calls. 47,48 Interestingly, this is not the case in virtually all published series with follow-up of greater than 12 months. 49–58 Most of these trials report complete data collection and all have more than 90% attending for follow-up. It is unclear how these other studies succeeded in such an astonishing rate of attendance. One study in particular reported follow-up data involving a clinic visit 5 years after surgery. 51 The authors countenance a follow-up of at least this long if recurrence rates are to be considered accurate. 41 While they may be correct, such a follow-up period is not practical and is likely to produce levels of incomplete data far exceeding 25% if carried out in the UK. While we did manage to obtain data for healing, complications and return to normal activities in at least 83% of participants, in some cases this was at only around the 6-week follow-up.
Other limitations relate to the multitude of interventions that were included in the cohort. While analysable data were available for 667 participants, there were 12 different interventions carried out. There were just not enough data to compare individual procedures in any meaningful way. If we had aimed to have 100 patients for each procedure rather than just the front-running procedures, a cohort of over 6000 patients would have been required. It was felt justified to group similar techniques by invasiveness. Minimally invasive procedures have a distinct commonality in that they do not involve major skin/subcutaneous tissue excision and instead focus on destruction/removal of the pit and underlying cavity. Major procedures involve excision of the disease and surrounding skin with or without closure of the wound created. There are clearly subtleties for the way both minimal and major interventions are carried out and advocates will proclaim the benefits and harms of each. Indeed, the literature is full of such comparisons. 40 As such, a broad categorisation may be criticised, particularly for the major excisional group where excision-and-leave-open or closure in the midline techniques is considered by many to be obsolete compared with an asymmetric closure technique. 15,37,38 Nevertheless, even when a more granular analysis of asymmetric closure versus minimally invasive techniques was carried out, similar differences in terms of pain, recovery, failure to heal and complications were seen.
The final limitation relates to the risk adjustment. Demographic and disease characteristics that may influence outcome are currently unproven. We decided on such parameters by consensus among the core study clinical team. We accommodated the uncertainty by including multiple permutations of the risk adjustment model. Overall, these permutations led to similar results, strengthening the justification for the model and the overall conclusions. Nevertheless, all risk adjustment is predicated on being able to fully quantify ‘risk’, which is both a strong and untestable assumption.
Conclusions
The real-world experience of surgery for pilonidal disease is not as good as the literature would suggest. Many patients have a protracted recovery regardless of intervention, and failure is common. The utilisation of minimally invasive techniques could be increased and would reduce the burden of postoperative recovery substantially while accepting a small reduction in cure rate. Patient QoL and health economics studies, including investigation on the cost to society of longer absences from work and education, may inform shared decisions on first-line treatment of PSD in the future.
Chapter 4 Mixed-methods substudy
Sections of this chapter have been reproduced from Strong et al. 30. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Methods
Design
A multiple-case design was employed to compare more than one data type between and within more than one person. 59 The case study was nested in the observational cohort, with two embedded units of analysis at baseline and 6 months: qualitative longitudinal semistructured interviews and quantitative cohort data.
Participants
Sampling was purposive and sought to recruit PITSTOP cohort participants with symptomatic PSD referred for elective surgical treatment. This sampling method aimed for maximum variation based on the following: Wysocki classification (an indicator of disease severity), surgical procedure (excision and closure techniques) and NHS Foundation Trust. Initial contact was made by telephone. All potential participants were e-mailed the participant information sheet and provided verbal consent prior to participation in the interview.
Data collection
At baseline, participants completed a patient-reported experience measure (PREM) of SDM. SDM is scored 0 to 9 (0 indicating poor SDM; 9 indicating good SDM). 60 At 6 months, participants completed a PREM of DR. Using a 5-point scale, healthcare DR is scored 0 (low DR) to 100 (high DR). 61 In addition, the following outcome measures were collected: pain, length of time to healing and post-surgery complications. Semistructured telephone interviews were conducted between June 2019 and September 2020. A minimum of 20 interviews was considered adequate to understand common perceptions and experiences of treatment choices, thereby achieving data saturation. 62,63 A topic guide was designed using the coping in deliberation (CODE) and Sekhon’s Acceptability framework. 64,65 Baseline interviews adapted key ‘choice’ (e.g. ‘did you let the surgeon choose your treatment?’) and ‘options’ (e.g. ‘did the surgeon talk you through the risks and benefits?’) questions from the CODE framework. 64 At 6 months, the interview guide asked CODE questions related to decision ‘consolidation’ (e.g. ‘was this the right decision?’). Throughout, probing questions covered dimensions of Sekhon’s acceptability framework,65 as well as intervention attributes, to inform the discrete choice experiment (DCE). The interviews were recorded using an encrypted digital recorder and transcribed verbatim.
Patient and public involvement
One patient representative participated in a pilot interview. The aim of the pilot interview was to assess the apprehension of the topic guide questions and review interview cohesion. No amendments were made to the topic guide.
Data analysis
Transcripts were analysed using the National Centre for Social Research ‘Framework’ analysis approach. This approach was chosen because it allows for coding of a priori and de novo themes. 66 After familiarising ourselves with the data set, we independently coded a sample of transcripts using the CODE and Acceptability frameworks64,65 on NVivo (QSR International, Warrington, UK) version 11 before conferring. During the analysis and interpretation, integration of qualitative and quantitative data occurred to understand: (1) how disease characteristics and surgeon preferences interacted with patient values in treatment choices; and (2) how participants appraised treatments given particular outcomes. We used joint display tables to look for convergences and divergences between cohort data (disease features/treatment choices/outcomes) with experiences, views and values. 67,68 Patient experts were invited to provide feedback on a lay summary of triangulated results to assess acceptability.
Results
Sample
An initial expression of interest was made by 266 cohort participants. The final sample comprised 20 patients (median age 28; range 20–64) from 13 NHS Foundation Trusts in the UK (Table 14). Only 13 participants completed baseline (median 16 minutes, range 6–47 minutes) and follow-up interviews (median 18 minutes, range 11–37 minutes).
| ID | Male/female | Age | Baseline | Surgery | 6 month | Follow-up interview? | |||
|---|---|---|---|---|---|---|---|---|---|
| Prior operations | Severity | SDM | Excision/closure | DRa | Recurrenceb | ||||
| Complete data set | |||||||||
| 1 | Male | 31 | 0 | 1 | 2.6 | LE/MC | 10 | No | Y |
| 3 | Male | 29 | 1 | 1 | 4 | PP/LO | 10 | Yes | Y |
| 5 | Male | 28 | 0 | 1 | 3 | LE/LC | 5 | No | Y |
| 6 | Female | 20 | 3 | 1 | 4 | LE/LO | 30 | No | Y |
| 8 | Male | 27 | 2 | 4 | 3 | LE/LO | 20 | No | Y |
| 9 | Male | 25 | 0 | 1 | 4 | LE/LC | 5 | No | Y |
| 10 | Male | 64 | 0 | 3 | 3.6 | Se only | 5 | No | Y |
| 11 | Male | 27 | 1 | 4 | 3 | Cu/LO | 5 | No | Y |
| 14 | Male | 23 | 0 | 2 | 2 | EPSiT/LO | 15 | No | Y |
| 16 | Female | 26 | 2 | 4 | 4 | LE/LC(K) | 0 | No | Y |
| 17 | Male | 22 | 2 | 3 | 4 | LE/LC(K) | 0 | No | Y |
| 18 | Female | 27 | 0 | 1 | 4 | PP/LO | 50 | No | Y |
| 19 | Male | 40 | 0 | 4 | 2.66 | LE/MC,M,LC | 0 | No | Y |
| Incomplete data set | |||||||||
| 2 | Female | 31 | 2 | 4 | 4 | LE/LC(K) | LTFU | LTFU | Refused |
| 7 | Female | 33 | 1 | 2 | 4 | Cu,PP/FG | 0 | No | LTFU |
| 12 | Male | 49 | 0 | 1 | 4 | Cu/FG | LTFU | LTFU | LTFU |
| 13 | Male | 28 | 0 | 1 | 4 | Cu/FG | LTFU | LTFU | LTFU |
| 15 | Male | 44 | 0 | 2 | 2.33 | LE/Se,Fl | LTFU | LTFU | Refused |
| 20 | Male | 34 | 0 | 4 | 4 | LE/Fl | LTFU | Yes | LTFU |
| 21 | Female | 25 | 1 | 2 | 2 | LE/MC | 0 | No | LTFU |
Coping in deliberation framework: health threat
Newly diagnosed participants were unfamiliar with, and expressed confusion about, the cause and prognosis of PSD. They detailed their experience of soreness, inflammation, discharge and odour which disrupted employment, physical activity and relationships. Participants discussed the impact this had on psychosocial well-being.
I was told initially, 'Oh that could be it, and then it might go away' … but once you get it once, that’s it: it’s coming back … If I was a bit more aware of that I would have probably started to look into the surgeries quicker.
18: no previous pilonidal disease
Some participants were reluctant to address their condition, deciding to tolerate distress and delay treatment. Often, an exacerbation of symptoms would cause participants to present to emergency services for treatment.
I said to [my girlfriend], 'Look, I can’t really see it properly. Is it still getting bigger?' And, she said, 'Oh bloody hell … get in the car.' So, we went straight to [hospital].
03: one previous episode of PSD
Other participants discussed barriers to secondary care treatment referrals due to their GP not taking their condition seriously.
… just gave me some antibiotics … it just kept getting more painful and worse … I went back three times … then she put me on sort of the path to go back to surgery, but she didn’t send me [as] an urgent patient … so I had to wait for maybe like five months.
14: no previous PS
Another saw their GP numerous times over 25 years and was repeatedly dissuaded from surgery.
he basically sort of said to me that it’s a very precarious operation … that the success rate wasn’t very high … that it was something that if I could live with.
15: no previous pilonidal disease
Coping in deliberation framework: choice
Once referred to secondary care, 9/20 participants were offered a choice of treatment. Absence of choice was rarely viewed negatively. Due to their own limited knowledge of the condition, some participants viewed healthcare professionals as best placed to make decisions about their care, especially where emergency surgery was concerned. If offered a choice, participant preference was based on one or more of the following factors: previous experience of surgery (n = 3); surgeon’s guidance (n = 3); invasiveness of the treatment (n = 3); or anticipated recovery time (n = 2). One participant rejected their consultant’s treatment advice to undergo a ‘leave open’ procedure due to the employment opportunity costs – they would require additional time off work (Table 15). Other participants utilised social networks (significant others, friends and relatives) or the internet to support decision-making, with some acquiring a sense of control from independently researching the condition and treatment options.
| Participant information | Decision-making | |||
|---|---|---|---|---|
| ID | Number of prior procedures | Key outcome | CollaboRATE score | Sample quote (coding) |
| 21 | 1 | Recurrence | 2 | ‘[The surgeon] said they’d cut like a flap out, get everything out and sort of stitch it back up … that was the only option … that or managing with medication … I was like, yeah, do what you have to do.’ (Presentation of choice) |
| 14 | 0 | ADL | 2 | ‘I only really got a say in it this time … cos it was a new surgery coming though …. They offered me to do the other one if I wanted’ (Presentation of choice) |
| 15 | 0 | Recovery time | 2.33 | ‘If you’re asking me how it felt like, it felt like I didn’t have a choice.’ (Presentation of choice) ‘at first I, [the consultant] sort of said, oh you might be back in a … couple of weeks and then when my friend said oh, 12 weeks for this open wound to heal, I thought … I can’t take that long off work. I can’t afford it.’ (Preference construction) |
| 1 | 0 | Recurrence | 2.6 | ‘[The surgeon] said you either don’t have the surgery and hope that it maybe sorts itself out … I took the decision that the chance of the surgery resolving the matter was worth the risk that it might still reoccur … with no other sort of major health issues that seemed like an easy enough choice’ (Presentation of choice) |
| 19 | 0 | Recurrence | 2.66 | ‘No, [the surgeon] did not give me any option. He just said, just, just he only mentioned the surgery. As I say, I wasn’t given any other options’ (Presentation of choice) |
| 5 | 0 | Pain | 3 | ‘[The doctor] said that they’ll operate and that was pretty much it … just leave it, or you could have the operation and I thought, well, best to try and get it sorted before it keeps getting infected, and gets worse’ (Presentation and interpretation of options) |
| 8 | 2 | Smell | 3 | ‘[The surgeon] give me options of what I wanted and I just wanted one, like obviously cos I had it packed last time, it healed better that way, so I asked for it that way.’ (Preference construction) |
| 11 | 1 | Pain and ADL | 3 | ‘[The consultant] explained to me that you know, we could try medication first and then if that doesn’t work, we could try surgery … it was a scraping out I think … that was something [the consultant] recommended’ (Presentation of choice) |
| 10 | 0 | (Not specified) | 3.6 | ‘I didn’t decide any treatment. The treatment was decided for me by the consultant … I’m not medically qualified, you know … I’m told what the problem is and how it can be rectified. We go along with that.’ (Decision) |
| 2 | 2 | ADL | 4 | ‘No, there was only one procedure left’ (Presentation and interpretation of options) |
| 3 | 1 | Recurrence and pain | 4 | ‘It’s not me fighting this battle … I’m just a battlefield. You guys are fighting it … by the time I got to A&E, they may have given me options, I can’t remember …. I’m quite happy to accept that I don’t know what I’m talking about, so even if I’m given options I will say to the man giving me options, what would you do’ (Presentation and interpretation of options) |
| 6 | 3 | Reducing anxiety of knocking the sinus (reduce symptoms) | 4 | ‘I saw my consultant and he said … depending on the MRI, I’ll give you a few options … one is that we do the same but obviously different in theatre and then the, the other option is to have it, like, lasered, removed’ (Health threat) |
| 7 | 1 | ADL | 4 | ‘They gave me two options but obviously because I have to get a mastectomy in September … I wouldn’t have been healed in time … my immune’s so low as well, we said that the glue one’d be more beneficial for me.’ (Preference construction) |
| 9 | 0 | Recurrence | 4 | ‘The wording was this is the best thing to go for … either don’t have the surgery and hope that it maybe it sorts itself out … or sort of cutting it out … I wasn’t really exploring every single option available’ (Preference construction) |
| 12 | 0 | Recurrence | 4 | ‘[The surgeon] gave me two or three different options that we could take i.e. stitching, gluing, leaving alone etc. and I thought the gluing one sounded the best and of course she agreed that she would like to do the gluing one anyway but she wanted me to make the choice really.’ (Presentation of choice) |
| 13 | 0 | Closing the wound | 4 | ‘It was either an option of having it packed, which the doctor said can take up to a month for it to be fully healed … obviously being self-employed, I need to be back in work … I just plumped for the one that sounded like the one that I thought would work the best and I think it was a newer procedure’ (Presentation of choice/presentation and interpretation of options) |
| 16 | 2 | ADL | 4 | ‘I could leave it and just live with it, which obviously for me wasn’t an option! … my other option was to get a cosmetic surgeon in … So, I was just kind of worried that I would always kind of be left with some sort of wound’ (Health threat) |
| 17 | 2 | Recurrence | 4 | ‘[The nurse] just told me I’d be having emergency surgery … someone looked at me that following morning and decided that I definitely had to have the incision and drainage. They didn’t go through the details of why that was, I’ll be honest … I didn’t know the ins and outs of what I had, and I didn’t know if there was any other options available.’ (Presentation of choice) |
| 18 | 0 | Solve the problem | 4 | ‘I wasn’t given the choice as such of which ones to do but when [the consultant] said that this is what she recommends, I completely took that on board from somebody with her kind of experience and knowledge of it’ (Presentation of choice) |
| 20 | 0 | ADL and recurrence | 4 | ‘[The GP] said you’ve got two options, I either give you some antibiotics and pain relief now or I recommend you go to hospital … I wanted to maintain as much quality of life as possible while listening to the consultant’s guidance’ (Preference construction) |
Coping in deliberation framework: key outcomes at the time of decision-making
Not all participants specified a single important outcome when undergoing surgery. The following were discussed: avoiding recurrence (n = 8), return to normal activities (n = 6) and/or the elimination of symptoms (n = 7). Six participants were not aware of procedural risks; others expressed awareness of risks presented by anaesthesia (n = 2), infection or bleeding (n = 4), the wound not healing (n = 5) and recurrence (n = 8).
Coping in deliberation framework: consolidation
After surgery, most participants were anxious of aggravating the wound and/or delaying wound healing. Many implemented adaptations, including physical (altering seating positions) and behavioural (reducing exercise duration and changing the type of exercise) changes, which negatively impacted their well-being.
It has made me reticent to engage in some activities … exercise and things like that … through the pain and discomfort, and also the chance of sort of popping the cyst...
01: no previous pilonidal disease
Many participants required daily or weekly primary care wound management support via GP appointments or district nurse visits. Due to its location, most experienced difficulties managing the wound independently. They used mirrors for physical inspection or were dependent on others to help examine and manage the wound (including cleaning, dressing and packing). Some participants cited the reliance on others as a loss of independence, while others acknowledged how emotional support alleviated distress.
I think the worst part of it is that you always have to rely on someone else to do, like, a dressing for you … you can’t drive cos you can’t sit down … you basically you can’t do anything.
06: sinus excised and left open
Acceptability framework: key outcomes at 6-month follow-up
Six months post surgery, some participants reported hoping surgery would address: pain (n = 3); recurrence (n = 5); wound healing (n = 1); the smell (n = 1); the inconvenience (n = 1); impaired ability to perform ADL (n = 1). In five cases (01, 05, 11, 14, 17; see Appendix 3, Table 33), these priorities had changed since baseline. During the recovery period, some participants accepted the recurrent nature of the condition. Others managed their own treatment expectations by considering any symptomatic improvement as an indicator of effectiveness.
I’ve still got some kind of stuff going down on there that is just a recurring thing … if I’ve had four operations, it probably won’t get rid of [it].
06
Some participants specified a single important outcome 6 months after surgery. The following were discussed: the wound healing in the expected time (n = 3); avoidance of recurrence (n = 4); return to ADL (n = 1). Three participants who had undergone PSD surgery for the first time found their treatment to be effective. When asked, they did not feel they would have done anything differently (see Appendix 3, Table 33).
Discussion
This substudy explored how patients make, and sometimes regret, treatment decisions for PSD. Patients are often reluctant to address the condition due to inadequate knowledge and embarrassment. GPs are often hesitant to refer patients to secondary care services as surgical approaches are perceived to be poorly evidenced. Once referred, patients may not be involved in the choice of surgical treatment. Typically, they are unconcerned with and uninformed about the burden post procedure (wound management, practical support, and risk of infection and/or recurrence). Therefore, they may experience unanticipated difficulties when trying to cope with these matters. Patients undergoing their first surgery are often overly optimistic about the effectiveness of treatment. In contrast, those with recurrent disease may experience higher treatment regret and psychosocial distress due to poorly informed decisions. Irrespective of prior experience of PSD, making decisions about surgical treatment is challenging. 64 Digesting new and complex treatment information can be difficult. Insufficiently informed, patients are unable to articulate what they would have done differently, with many citing a change in outcome priorities after 6 months. Patients with a previous history of PSD who had undergone an excise-and-leave-open procedure – which is associated with high levels of pain, wound management and extended healing times – demonstrated the highest levels of DR.
This substudy is limited as postsurgical wound healing can take over 6 months and recurrence of PSD may take place over many years. Therefore, the attitudes of participants may be affected due to the short follow-up period. The COVID-19 pandemic significantly impacted our ability to follow up participants. 69,70 Although remote data collection was possible, engaging patients in research was difficult as many were experiencing COVID-related barriers. 71 In PSD studies, attrition rates are poorly reported and this is thought to be due to the young, mobile, mainly male population. 15,72–74 High attrition rates may also result from an unwillingness to disclose negative experiences,75 or a loss of interest in research after the wound has healed and they have returned to work. 76
The mixed-methods approach identified divergences and inconsistencies between different data sets – particularly, how patients reflect on their treatment decisions after surgery. Even if they are involved in SDM, if patients are not fully informed about treatment and post-surgery pathways, their expectations may not be met. 77 Levels of self-reported DR in this study are in line with the 1 in 7 rate reported across 73 surgical studies, in which regret was mainly associated with type of surgery, health outcomes and absence of SDM. 78 Another systematic review has flagged decisional conflict and anxiety as predictive of DR. 79 Surgeons80,81 and patients82 may have reasons for avoiding SDM, and our findings complicate the common assumption that SDM leads to increased decisional satisfaction. 83 Systematic reviews in other contexts suggest that unmet information needs are common and distressing. 84–87 There are growing concerns that self-report measures of SDM may not capture the quality of the interaction or the multistaged nature of the process. 88,89 PREMs may be compromised by social desirability or acquiescence bias,90–93 and open-ended questions may reveal significant problems from patients who report high levels of satisfaction on survey instruments. 94,95 Triangulation of research methods is useful to identify such problems. 96,97
Clinical teams should ensure patients are provided with sufficient information about available surgical procedures. Expectations associated with postprocedural aftercare and the uncertainties surrounding clinical outcomes should be managed. Surgeons may not actively engage in wound care discussions as this is perceived as the responsibility of primary care services. 98 However, insufficient information may impact a patient’s ability to self-manage post surgery. Therefore, patients should receive tailored verbal and written information regarding treatment outcomes during relevant consultation appointments. 99 Discussing expectations with patients may provide an opportunity to address false optimism. 100,101 In other settings, patient expectations predict treatment satisfaction and functional outcomes post surgery. 102
Awareness-raising among GPs and surgeons is needed to avoid delays in treatment where PSD is poorly recognised. Where pilonidal surgery is seen as unglamorous,103 or surgeons only specialise in one technique,104 patients with recurrent disease should be referred rapidly onward to relevant specialists. Both SDM and the consent process may be compromised if patients are poorly informed about their condition, available treatments and the probability of various outcomes. 80 This is challenging when there are many available treatments supported by variable evidence. 14 There are around 20 systematic reviews and meta-analyses on surgical techniques alone, and around 15 more on medical, wound care and other topics. An overview of these reviews should be an urgent research priority to adequately inform SDM and the development of decision support tools. Until then, the review by Stauffer and colleagues remains one of the most comprehensive overviews focusing on time to recurrence with different surgical techniques. 13 Finally, discharge planning should begin at pre-assessment visits, involving the patient, day surgery nurses and district nurses. 105–107 Postoperative wound care is enhanced by continuity of care from a limited number of community-based health professionals. 108
In conclusion, ensuring people with PSD are provided with sufficient information regarding wound care management and risks of recurrence associated with different surgical approaches may facilitate decision-making and minimise treatment regret. An overview of systematic reviews is needed to inform decision support tools. Healthcare professionals should communicate the uncertainties about treatment effects in addition to the time frames, adaptations and psychosocial impact associated with recovery.
Chapter 5 Discrete choice experiment
Sections of this chapter have been reproduced from Wickramasekera et al. 109 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Methods
Design and theoretical/conceptual framework
Discrete choice experiments are an attribute-based measure of benefit; they assume that healthcare interventions, services or polices can be described by their attributes. In a DCE, respondents are required to make trade-off decisions regarding the quantity or quality of a good or service. The resulting choices are analysed to estimate the overall utility (value) and willingness to trade between services. The use of DCEs to identify patient preferences in health and healthcare has increased. Where no clear treatment decision exists, accurate quantification of patient preferences for risks and benefit is crucial.
Development of survey
The survey contained four separate sections. These were:
-
patient characteristics and disease history
-
a treatment ranking exercise
-
DCE-specific tasks
-
survey feedback questions.
The ranking exercise and DCE tasks were developed by conducting qualitative interviews with 20 patients. The patients were asked to identify the key attributes and levels that they considered when choosing a treatment (see Chapter 4 for further information). A thematic analysis of the interview data was conducted and identified a list of factors that patients considered important when making treatment decisions. PPI members and clinicians reviewed an initial list of themes and selected attributes considered the most important for inclusion in the DCE and ranking task.
Nine attributes were included. These were:
-
type of excision and closure
-
type of anaesthetic
-
length of hospital stay
-
wound care
-
pain medication requirement
-
infection risk
-
healing time
-
risk of recurrence
-
scarring.
Currently, there are 18 theoretically possible surgical options for PSD. 14,110 Clinicians developed a treatment classification of five treatment groups. This was considered important due to the potential cognitive burden of ranking 18 treatment categories. The treatment categories, related descriptions and attributes were informed by the literature, clinical input and PPI piloting (see Appendix 3, Box 1).
Two attributes were included in the DCE: risk of infection/persistence and recovery time. This selection was based on two reasons. Firstly, these two attributes were assessed as most important by patients and clinicians. Secondly, DCE attributes must be independent to avoid presenting implausible combinations of attribute and level profiles. The levels for the DCE attributes were selected based on plausible values published in the literature and additional input from clinicians.
The choice tasks were constructed based on an orthogonal design using a design catalogue. 111 The survey contained 16 hypothetical DCE tasks. Participants were asked to choose between two combinations of outcomes with varying levels (see Appendix 3, Box 1). Forced unlabelled choices were presented – ‘treatment A’ or ‘treatment B’. An ‘opt-out’ alternative was not provided for the purposes of realism. A dominant task was included – where one treatment option is logistically superior – to test participant understanding of the task (see Appendix 3, Box 2).
Patient and public involvement
Patient and public involvement representatives were heavily involved in the design and implementation of the DCE. They assisted with the following tasks:
-
Reviewed the initial list of themes to finalise the attributes.
-
Reviewed the acceptability of the treatment category descriptions.
-
Prior to the dissemination of the survey, three PPI representatives reviewed the survey to assess comprehensibility, interpretation and complexity of tasks.
Sampling
Discrete choice experiment sample sizes can vary from 100 to 1000 plus. 112 An Orme113 rule of thumb formula was adopted to estimate the minimum sample size. Using this formula – {N = 500 × [4 (maximum number of levels)]/[2 (number of alternatives) × 16 (number of tasks)]} – the estimated sample size was 63.
Recruitment
All participants aged 16 years and above with symptomatic PSD, referred for elective surgery and participating in the PITSTOP cohort study, were invited to take part in the survey. Interested participants were e-mailed a Qualtrics® (Qualtrics, Provo, UT, USA) link which included a participant information sheet, consent form and the questionnaire. Participants with symptomatic PSD and not participating in the PITSTOP cohort could also take part in the DCE by accessing a digital QR code advertised on a study leaflet. The study leaflet was displayed in NHS Foundation Trust colorectal outpatient clinics and disseminated via the PITSTOP Twitter website.
Data analysis
Descriptive statistics were calculated for patient characteristics, disease history and survey feedback variables. DCE responses were modelled using conditional logistic regression where the dependent variable was the preferred treatment choice, and the independent variables were risk of infection/persistence and recovery time. Linearity of the attributes was assessed before deciding to treat risk as a linear variable (Figure 6). Regression coefficients were used to estimate the relative importance of attributes. Maximum acceptable risk (MAR) is the rate at which patients are willing to sacrifice a benefit of one attribute in exchange for an improvement in another. MARs were calculated by dividing the ratio of recovery time coefficients by the infection/persistence coefficient and 95% CIs calculated using the delta method. 114 Latent class models were used to analyse individual heterogeneity and to identify subsets of patients with varying preferences. The optimal number of classes was selected using the Bayesian information criterion (BIC) and consistent Akaike information criterion (CAIC) and model parsimony. Data were analysed using Stata® 17 (StataCorp LP, College Station, TX, USA).
FIGURE 6.
Regression model 1 results reproduced in a diagram.
Results
Participants
One hundred and eleven participants were included in the DCE survey. The completion rate was 74% (423 unique visitors entered the survey, 150 participants consented to take part and 3 participants declined). Table 16 reports the characteristics of the 111 included participants. Of these, 75 (68%) respondents were male and 73 (66%) were between the ages of 17 and 29 years; 89 respondents (80%) were employed and 97 (87%) were white. Except for six respondents, the rest of the sample reported having had at least one surgery for PSD. The respondents had various types of surgeries, including excision of the skin and closure of the wound with stitches (26%); excision of the sinuses only and leave the wound open to heal (23%); excision of skin and leave the wound open (23%); excision of the skin and closure of the wound with a skin flap and stitches (9%); and excision of the sinuses and closure of the wound with glue (19%).
| N = 111 | No. | % |
|---|---|---|
| Sex | ||
| Male | 75 | 68 |
| Female | 36 | 32 |
| Age (years) | ||
| 17–29 | 73 | 66 |
| 30–39 | 24 | 22 |
| 40–49 | 8 | 7 |
| 50–59 | 4 | 4 |
| 60–69 | 2 | 2 |
| Median age, years (range) | 28 (17–65) | |
| Which of the following best describes your main activity? | ||
| Employed | 89 | 80 |
| Retired | 1 | 1 |
| Homemaker | 3 | 3 |
| Carer | 1 | 1 |
| Student | 17 | 15 |
| Ethnicity | ||
| White | 97 | 87 |
| Black | 2 | 2 |
| Asian | 2 | 2 |
| Mixed | 10 | 9 |
| Education | ||
| Primary | 4 | 4 |
| GCSE | 16 | 14 |
| A-level | 48 | 43 |
| Degree | 42 | 38 |
| Prefer not to say | 1 | 1 |
| Previous pilonidal sinus surgeries (including both emergency drainage and previous ‘definitive’ elective repair) | ||
| 0 | 6 | 6 |
| 1 | 67 | 64 |
| 2 | 17 | 16 |
| 3 | 6 | 6 |
| 4 | 5 | 5 |
| 5 | 2 | 2 |
| 6 | 1 | 1 |
| 10 | 1 | 1 |
| Type of previous pilonidal sinus surgeries | ||
| Excision of skin and leave the wound open (e.g. leave open/marsupialisation) | 29 | 23 |
| Excision of the skin and closure of the wound with stitches (e.g. midline closure, Bascom’s cleft closure, Karydakis) | 33 | 26 |
| Excision of the skin and closure of the wound with a skin flap and stitches (e.g. rhomboid, Limberg, Dufourmental) | 11 | 9 |
| Excision of the sinuses and closure of the wound with glue | 24 | 19 |
| Excision of the sinuses only and leave the wound open to heal (e.g. pit picking, EPSiT, laser) | 29 | 23 |
Patient preferences
Appendix 3, Table 34 presents the regression modelling results of the DCE. Patients preferred treatments with lower risk of infection/persistence and this attribute was modelled linearly (see model 2 in Appendix 3, Table 34). Risk of infection/persistence was the most important attribute when patients were choosing a treatment, with an attribute importance score of 70%. Patients also preferred treatments with shorter recovery time; for example, compared to a treatment that takes 12 weeks to recover, a treatment with a 1-, 2- or 6-week recovery period was preferred. However, their preferences were not linear, so this attribute was modelled as a categorical variable. Treatments with shorter recovery time had an attribute importance score of 30%.
Maximum acceptable risk
When choosing a treatment, patients were willing to make trade-offs between risk of infection/persistence and recovery time (Table 17). These trade-offs were measured using MAR, which is the maximum risk of infection/persistence participants are willing to accept to have a treatment with faster recovery times. The highest-risk patients were willing to accept was a 17.08 risk of infection/persistence in return for a treatment with 2-week recovery period compared to a treatment with 12 weeks recovery period. Patients were willing to accept a 10.49 increase in risk of infection/persistence to have a treatment with 6-week recovery period compared to 12-week recovery period. Patients were willing to accept a 6.59 increase in risk of infection/persistence to have a treatment with a faster recovery period (2 weeks compared to 6 weeks).
| Treatment benefit (for selected level changes) | MAR of infection/persistence | 95% CI calculated using the delta method |
|---|---|---|
| Recovery time reduction from 12 weeks to 2 weeks | 17.08a | 14.83 to 19.33 |
| Recovery time reduction from 12 weeks to 6 weeks | 10.49 | 8.76 to 12.22 |
| Recovery time reduction from 6 weeks to 2 weeks | 6.59 | 4.88 to 8.30 |
Preference heterogeneity
Differences in preferences between patients were explored using the latent class modelling approach. This identified two groups of respondents with different preferences (Table 18). The first subgroup of respondents (class 1) were risk-averse and so they were only willing to accept a small risk (1.51–2.15) in exchange for a treatment with faster recovery time (Table 19). The second subgroup of respondents (class 2) showed stronger preferences for treatments with shorter recovery time: they were willing to accept higher risks of infection/persistence (22.35–34.67) to receive treatments with quicker recovery time. Of all the demographic variables used to predict whether a respondent belonged to class 1 or 2, only age of the respondents was statistically significant (see Table 18). The results show that respondents in the 17–29 age group were more likely to belong to class 1 and respondents above the age of 30 were more likely to belong to class 2.
| Class1 | Class2 | |
|---|---|---|
| Week = 12 (reference level) | ||
| Week = 1 | 0.159 (0.405) |
2.192*** (0.209) |
| Week = 2 | 0.929 (0.488) |
2.861*** (0.214) |
| Week = 6 | 0.652 (0.363) |
1.844*** (0.171) |
| Risk (%) | −0.432*** (0.049) |
−0.083*** (0.008) |
| Class membership | ||
| Sex = female | −0.280 (0.469) |
Reference |
| Age = 17–29 years | 1.365** (0.486) |
|
| Activity = employed | 0.375 (0.564) |
|
| Number of non-emergency surgeries patients had for pilonidal sinus | −0.008 (0.163) |
|
| Education = degree | 0.450 (0.456) |
|
| Constant | −1.485* (0.692) |
|
| Observations | 3328 | |
| Log-likelihood | −569.55 | |
| BIC | 1206.51 | |
| CAIC | 1220.51 | |
| Treatment benefit (for selected level changes) | Subgroup 1 (class 1) MAR of infection/persistence (95% CI) | Subgroup 2 (class 2) MAR of infection/persistence |
|---|---|---|
| Recovery time reduction from 12 weeks to 2 weeks | 2.15 (−0.03 to 4.34) | 34.67 (28.24 to 41.10) |
| Recovery time reduction from 12 weeks to 6 weeks | 1.51 (−0.11 to 3.13) | 22.35 (17.35 to 27.35) |
Ranking of treatments
Patients ranked the treatments they preferred in order of importance, and Figure 7 shows the results of this ranking task. The best preferred treatment was complex flap (e.g. Limberg, Dufourmental) procedures (27%), followed by excision of the sinuses only (22%), glue (19%), excision of the skin and closure of the wound with stitches (18%) and lastly leave open (14%). The least preferred treatment was leave open (35%), followed by glue (23%), complex flap procedures (18%), excision of the sinuses only (17%) and excision of the skin and closure of the wound with stitches (7%).
FIGURE 7.
Ranking of treatments.

Participants’ understanding of and engagement with the survey
Table 20 reports the results of the questions used to test the internal validity of the DCE.
| Internal validity (dominance questions) passed or failed? | N | % |
|---|---|---|
| Failed | 18 | 16 |
| Passed | 93 | 84 |
| Always choose the same side (e.g. left profile) in all the DCE questions? | ||
| Yes | 0 | 0 |
| No | 111 | 100 |
| I found the ranking question made sense – please tell us how strongly you agree | ||
| Strongly disagree | 3 | 3 |
| Disagree | 2 | 2 |
| Uncertain | 10 | 9 |
| Agree | 60 | 54 |
| Strongly agree | 36 | 32 |
| I understood the questions about making choices between different treatment options | ||
| Strongly disagree | 1 | 1 |
| Disagree | 1 | 1 |
| Uncertain | 8 | 7 |
| Agree | 59 | 53 |
| Strongly agree | 42 | 38 |
| When choosing options, I needed more information than was provided | ||
| Strongly disagree | 16 | 14 |
| Disagree | 43 | 39 |
| Uncertain | 24 | 22 |
| Agree | 21 | 19 |
| Strongly agree | 7 | 6 |
| I found making a choice between different treatments confusing | ||
| Strongly disagree | 21 | 19 |
| Disagree | 56 | 50 |
| Uncertain | 15 | 14 |
| Agree | 15 | 14 |
| Strongly agree | 4 | 4 |
| Median time to complete survey, minutes (range) | 12 (4–5388) | |
Most of the respondents said that they understood the DCE tasks (91%) and ranking task (86%). Ninety-three (84%) of the respondents correctly answered the DCE question with a logically correct answer (dominance test). None of the respondents were always choosing the same side (left or right) profiles of the DCE tasks. Fewer than 25% said that the DCE task was confusing and that they needed more information.
Discussion
This substudy assessed patient treatment preferences for PSD. When choosing a surgical treatment, patients prioritised risk of infection/persistence relative to recovery time. However, patients were willing to compromise and accept treatments associated with varying degrees of greater risk of infection/persistence in favour of treatments that were associated with quicker recovery. The results provide insight into the type of treatments patients accept and value. In the overall group, patients were willing to accept up to a 17-percentage-point increase in risk of infection/persistence for treatments with a shorter recovery period. This suggests that some patients are willing to accept less invasive treatments with shorter recovery periods and greater risk of infection/persistence (e.g. glue and/or pit picking) over more invasive treatments with longer recovery periods but reduced risk of infection/persistence (e.g. an excise-and-leave-open procedure). In the ranking task, similar results were found: open surgery was ranked as the least favoured treatment option. This is understandable given the impact of prolonged wound care management on psychosocial well-being. 115
The results demonstrated preference heterogeneity, which indicates the importance of providing treatments tailored to subgroups of patients with distinct preferences. Patients aged 30 years and over were willing to accept up to a 35-percentage-point increase in risk of infection/persistence for treatments with a shorter recovery period. This suggests that patients within this age bracket would be likely to reject an excise-and-leave-open procedure in favour of a treatment associated with a faster recovery period. In our sample, this age demographic reported that they were either homemakers, retired or had caring responsibilities. Therefore, it is plausible that their personal circumstances may have influenced their preference for a treatment associated with a shorter recovery time. In contrast, patients aged 17–29 years were more risk averse and were only willing to accept a two-percentage-point increase in risk of infection/persistence for treatments with a shorter recovery period. The differences in preference heterogeneity further support the tenet that patients should be involved in making decisions about their surgical care to avoid treatment DR. 30 Literature exploring SDM in PSD is growing. 30,74,116 Such studies have highlighted the importance of providing patients with sufficient information for each available surgical procedure (including wound care management) to aid treatment decision-making. 30,74,116
This substudy is the first to conduct a DCE to assess PSD patient treatment preferences. A robust methodology was employed; qualitative interviews were conducted to inform the development of the survey; experienced clinicians and PPI representatives contributed throughout the design and implementation phases. Relevant interval validity checks were made and identified task comprehension, supporting confidence in the results.
However, this substudy has limitations. The sample size was sufficient to estimate overall modelled preferences, but a larger sample size would have allowed greater confidence in the analyses classifying members to different latent classes. During recruitment, several methods were employed to increase response rate. As PSD typically affects a young, working-age population, engagement barriers can be incurred. 117 Currently, there is no consensus on how PSD should be classified. 110 The clinicians developed five treatment categories for the ranking task based on their own clinical experience and the literature. A different team of clinicians may have made different classification decisions. In addition, the DCE included two key attributes to avoid presenting implausible combinations of attribute and level profiles. However, in a real-life context, patients may consider other factors not included in the DCE to support treatment decision-making. Finally, 16% of participants failed the internal validity test, demonstrating that the risk information presented was not well understood. In future, including a numeracy test or presenting risk information using pictorial icon ranges may support internal validity.
Chapter 6 Consensus exercise
Methods
This process was conducted over three phases:
-
Phase 1: item generation using a ‘So what, now what’ focus group
-
Phase 2: online modified Delphi over three rounds of iterative voting
-
Phase 3: consensus meeting to confirm prioritisation of items.
Stakeholders
Two stakeholder groups were defined: patients with previous experience of PSD and clinicians with an interest in PSD. Clinicians included those with certificates of completion of training in general surgery and nurse specialists with wound care practice. Participants were recruited via e-mail, national organisations and social media. Snowball sampling was also used for clinician recruitment. Participants were invited to participate in one, two or all three phases of the consensus exercise.
Patient and public involvement
Fifteen patient representatives with relevant experience were recruited to the patient stakeholder group following substudy conception, and contributed to the delivery and analysis. Of these, 6/15 PPI representatives attended the initial workshop and supported the generation of the longlist of recommendations; 4/15 PPI representatives attended the virtual consensus meeting and highlighted the importance of ensuring the final set of recommendation statements were conceivable to a patient audience.
Generation of longlist
In accordance with Delphi methodology, the study consisted of three phases. In phase 1, an online workshop was conducted. This was based on Rolfe’s critical reflection model, ‘What? So What? Now What?’. 118 In the ‘What?’ phase, data or information is presented. In this case, researchers presented findings from the cohort study, mixed-methods study and survey-based work. In the ‘So What?’ phase, participants are encouraged to reflect and discuss the information. Participants were asked to consider how the presented data reflected their experiences, and how this matched wider experiences. In the ‘Now What?’ phase, participants discuss how the information should be used to influence the next stage. Participants were asked to frame their ideas as statements related to policy or research ideas. In the workshop, the following data were presented: preliminary PITSTOP cohort data, a systematic review of classification systems,110 a mapping reviewing of PSD,119 the PITSTOP DCE survey109 and the PITSTOP mixed-methods study. 30 Participants were asked to consider two questions: ‘How can we use this data to improve and/or inform clinical practice?’ and ‘What are the key research questions generated by this data?’. A longlist of potential practice and research recommendation statements was generated. The steering committee assessed the readability of these statements. Prior to attending the workshop, all participants received an information sheet and completed an online electronic consent form.
e-Delphi consensus
In phase 2, a three-round e-Delphi consensus was conducted. The Delphi surveys were delivered using Qualtrics. In round 1, all participants were e-mailed a participant information sheet and a link to the survey. Upon accessing the survey, participants were asked to complete an online consent form. The following information was captured: age, gender, demographics, ethnicity, e-mail address and stakeholder respondent group (patient, surgeon and specialist nurse). The longlist of recommendation statements was presented in a random order, and each statement was supplemented with a written summary to aid understanding. At the end of the survey, respondents were encouraged to propose any additional statements. Additional items were reviewed at the end of round 1.
In rounds 2 and 3, the remaining longlisted items were presented in random order. Ratings of items were reviewed after the close of each round. Respondents received an e-mail copy of results which included their vote and how that compared to each stakeholder group’s votes.
During each round, participants voted on the importance of each recommendation using a 9-point Likert scale (one being ‘not important’ and nine being ‘very important’). Recommendations were shortlisted if the following was satisfied: (1) > 70% participants within both stakeholder groups rate the recommendation as 7–9; or (2) 90% participants within a single stakeholder group rate the recommendation as 7–9. Recommendations that reached consensus after three rounds were considered at the consensus meeting. All items had to be rated to complete the surveys. Only those who completed a survey round were eligible to participate in the subsequent round. At the end of round 3, all participants were asked if they were interested in participating in the virtual consensus meeting.
Virtual consensus meeting
In phase 3, an online consensus meeting was held to finalise the set of recommendations. Invitations were issued to interested participants, with efforts made to encourage participation from members of the public/patients. A target of > 15 participants with at least three patient representatives was felt to be reasonable for this prioritisation exercise as it reflected proportions recruited to round 1 of the consensus. The meeting was held using the Google Meet™ videoconferencing platform (Google Inc., Mountain View, CA, USA). Electronic consent was taken prior to the meeting. Participants were presented with a total of 34 statements: 15 policy and 19 research. After the presentation, participants were instructed to complete two separate Qualtrics surveys. A constant sum question type was used to calculate the sum of scores for each statement. In the first survey, participants were asked to distribute 100 points between the 15 policy statements dependent on priority. This could be all points to a single item, an even division, or however the respondent felt appropriate, as long as 100 points were distributed. The total points allocated to each item were then calculated, and the five highest-scoring items were considered as top priorities. In the second survey, participants distributed 100 points between the 19 policy statements dependent on priority. The same approach to summing points was conducted.
Results
Longlisting of potential outcomes
Following the initial ‘So What, Now What’ workshop, 33 items were generated for the longlist by clinicians and patients. The flow of items is presented in Figure 8.
FIGURE 8.
Flow of items through the e-Delphi.

Delphi round 1
Consent forms were completed by 57 potential candidates from both stakeholder groups, and 57 completed round 1. Characteristics of respondents’ participation among stakeholder groups are presented in Table 21. This included 15 patients, 40 surgeons and 1 nurse specialist. A total of 33 items were considered for level of priority; 15 items reached a priori consensus for inclusion for the final consensus meeting. Respondents proposed a further 12 items for review. Outcomes voted on in each round, along with the proportion of each panel rating them 7–9, are presented in Appendix 3, Tables 35 and 36.
| Round 1 | Round 2 | Round 3 | ||
|---|---|---|---|---|
| Participant type | Patient | 15 | 14 | 14 |
| Surgeon | 40 | 38 | 36 | |
| Nurse specialist | 1 | 1 | 1 | |
| Retention rate | – | – | 95% | 91% |
Delphi round 2
In round 2, 53 participants completed the survey. This included 14 patients, 38 surgeons and 1 nurse specialist, 95% of those who completed round 1. Respondents were sent a summary of voting patterns from the first round and were asked to vote on 30 items: 18 for reconsideration and an additional 12 statements proposed. Of these, 18 items met the a priori criteria for inclusion in the consensus meeting.
Delphi round 3
In round 3, 51 participants completed the survey. This included 14 patients, 36 surgeons and 1 nurse specialist. This reflected 96% of those who completed round 2 and 91% who completed round 1. One further item was carried to the consensus meeting.
Consensus meeting
The consensus meeting was attended by three patient representatives and 15 clinicians. One clinician withdrew during the meeting due to work commitments. The top five policy statements and research recommendations are presented in Table 22.
| Statement number | Policy statement | Sum |
|---|---|---|
| 1 | Any treatment of pilonidal disease should aim to be less disruptive than the disease itself. | 270 |
| 2 | Minimally invasive techniques should be considered as the first-line intervention, as these are associated with low operative morbidity and comparable recurrence and healing rates to more extensive interventions. | 174 |
| 3 | Surgeons should have access to opportunities to learn new techniques for the treatment of pilonidal sinus disease. | 140 |
| 4 | A classification tool for pilonidal sinus should help to inform treatment options. | 140 |
| 5 | Delayed return to work is an important outcome following treatment. | 134 |
| Statement number | Research statement | Sum |
| 1 | A future randomised trial (RCT) should include two broad groups of interventions – major (i.e. asymmetric closure, leave open and midline closure) versus minor (i.e. minimal excision). | 189 |
| 2 | A core outcome set for pilonidal disease might help us understand what outcomes are important to clinicians and patients following treatment of pilonidal disease. It may also improve future evaluations of treatments. | 179 |
| 3 | Future research should compare major procedures (e.g. flaps) against minor procedures (e.g. pit picking, glue) stratified by disease severity. | 148 |
| 4 | There is a need for a patient-reported outcome to be used in future pilonidal sinus research. | 119 |
| 5 | Future research should aim to define an algorithm or decision tree to aid surgeon decision-making. | 100 |
Discussion
Overview
Research in surgery has been much maligned over the years,120 and pilonidal disease is no exception. 14 This is reflected in relatively weak or vague guidance to support practice. The Delphi we conducted has identified high-priority practice and research topics to guide the further development of the field. Consensus statements on practice topics strongly reflect the findings from previous sections. The top practice recommendation reflects the need to avoid harm related to interventions. This is supported by findings from the mixed-methods study which showed high levels of regret associated with poor wound healing. The cohort study supports the top two policy recommendations as it demonstrates the ongoing morbidity from poor wound healing after major procedures. In contrast, the third policy recommendation does not relate to clinical outcomes, but to the need to train surgeons in new techniques as highlighted by the clinician survey. This is particularly important if the top two priorities are to be achieved, as upskilling of surgeons will be required to facilitate techniques beyond ‘excise and leave open’. The need for a classification tool with clinical reference is clear. In other areas of colorectal surgery, classification tools and systems facilitate decision-making around treatment pathways. 121,122 This inconsistency in PSD means that it can be difficult to compare outcomes between patients and surgeons due to an inconsistent baseline description and treatment selection. Finally, mixed-methods and cohort studies highlighted the importance of return to work in this typically young and economically active patient group.
The recommended research priorities provide direction on next steps. Priorities one and three discuss the potential design of a future RCT. These suggest that a pragmatic approach to design, using an umbrella-type approach with interventions grouped into severity or grade of procedure, would be a appropriate. 123 We have seen similar approaches in other proctology studies. 124,125 The fifth priority also demonstrates the need to understand interventions which work across the treatment pathway. These are not limited to surgical interventions, as adjuvant treatment such as hair removal, use of antibiotics and wound dressings may also play a role in this pathway. 119 With this in mind, it may be more appropriate and efficient for a funder to commission a multiarm, multistage trial, with rerandomisation of patients who develop recurrence of PSD. The importance of measuring relevant outcomes in a consistent manner is emphasised, with the need for a core outcome set identified as a priority. 126 In addition, participants highlighted the need for the development of patient-reported outcome measures (PROMs). PROMs are important in any core outcome set. 127 A multidimensional PROM may include an assessment of return to work, wound healing and recurrent symptoms, all of which have been identified as essential in prior work packages.
Patient and public involvement
The consensus was based on the engagement of patients and members of the public. Initial findings were discussed with patient representatives, and these ideas were used when delivering the initial workshop. This was also attended by patients who were able to express their priorities. This engagement continued at all stages. During the final consensus meeting, patient representatives were invited to comment regularly to ensure their voice was considered in the final ranking.
Impact for policy-makers
This exercise sets out five clear policy statements which could form the basis for the development of future guidelines by informing PICO (population, intervention, control/comparison, outcome) development. The findings of limited training opportunities following qualification as a consultant surgeon may not be limited to this area, and policy-makers should be cognisant of this when commissioning services.
Impact for researchers
Researchers can use the findings of this study to direct future research. The directives here are for pragmatic trials established to address questions along the treatment pathway. Studies should also have a clear patient focus.
Conclusion
This consensus exercise has involved patients and clinicians to identify five key policy and five key research priorities. These should form the foundation of future work in the field.
Chapter 7 Wysocki classification validation exercise
Background
Clinically, classification systems may have a prognostic function and could ultimately allow stratified treatment. Such systems also ensure that more precise research comparisons can be carried out. While there are some existing classification systems for PSD,128–135 they are not used in routine practice or for research comparisons. Few studies evaluate their use to inform choice of treatment130,133–135 and no study has analysed the reliability or predictive validity of such a tool. 110 Given the huge variation in treatment that we have shown both with the PITSTOP surgeon survey and the cohort study, and the existing issues with the current comparative literature, there is a need for a classification tool that is both reliable and valid. A suitably pragmatic classification system could be integrated into clinical practice to support treatment decisions and the counselling of patients on likely outcomes. Such a system should be simple to use, reflect clinical practice and be meaningful in terms of prognostication.
Rationale
There is no commonly used classification tool to characterise PSD. The absence of such a tool represents an important knowledge gap, since surgeons faced with PSD typically have little exposure to the disease and there exists little guidance on how to treat it.
At the inaugural meeting of the Pilonidal Society in Berlin in 2017, a panel of 13 surgeons gathered to establish a simple classification system for PSD. 136 Each member of the panel had a special interest in PSD and had either written other classifications132,134 or had published widely on PSD. Components of a classification system were longlisted and refined by online consensus involving a large group of PSD surgeons137 to form a final configuration that was felt to be easy to use, clinically meaningful and had potential statistical validity.
The tool classifies PSD into one of four categories:
-
type 1: only midline pit or sinuses
-
type 2: any midline disease with secondary sinus/es or abscess scar/s
-
type 3: any midline or secondary disease extending below tip of coccyx
-
type 4: any disease after treatment with definitive intent.
While there may be agreement among the panel for the eventual classification (referred to as the ‘Wysocki classification’), none of the parameters of ease of use, reliability and validity have been tested. We aimed to do so by assessing the level of agreement between surgeons when used in clinical practice.
Aims
The aims of this substudy were to:
-
Quantify how well different assessors agree in their classification.
-
Quantify how classification relates to surgeon’s experience.
Additional exploratory aims were to:
-
Identify specific patients with low agreement, which may in turn help clarify or even modify the classification system.
-
To present, within each subtype, the frequency with which each treatment option is chosen.
Methods
Participating surgeons
Sampling was purposive and sought to recruit 15 colorectal surgeons. This sampling method aimed for maximum variation based on experience: five expert surgeons who registered an interest in pilonidal disease and who offered a specialised service, five surgeons who carried out pilonidal sinus surgery as part of a general surgical service and five final-year colorectal trainees. Initial contact with surgeons was made by e-mail, and interested surgeons were e-mailed the participant information sheet.
Participating patients
Stimuli required for the validation exercise were obtained from the main cohort study.
All participants referred for elective surgery and participating in the PITSTOP cohort study were asked if they agreed to the surgical site being photographed before surgery (an optional item on the consent form).
Participant photographs of the PSD surgical site were usable for the exercise if they satisfied the following criteria:
-
The participant was eligible for the main cohort study (aged 16 years and above with symptomatic PSD, referred for elective surgery).
-
The participant consented to a preoperative photograph to be taken of the surgical site.
-
The photograph was usable (i.e. unblurred).
-
The participant associated with the photograph had been classified using the Wysocki classification system at the time of procedure.
Assessment schedule
Each participant was asked to independently rate 36 cases using the Wysocki classification, with allocation of surgeons to cases selected to ensure overlap with other assessors (Figure 9). A total of 90 photographs were each assessed by two specialist surgeons, two general surgeons and two trainee surgeons. Surgeons were sent photographs accompanied by the medical history (previous PSD history, including number of elective procedures and emergency drains) electronically. Surgeons were not told the classification as recorded by the original surgeon at the time of procedure.
FIGURE 9.
Allocation of patients with PSD to surgical assessors.
The substudy was also used to provide an exploratory assessment of surgical opinion. Participating surgeons were asked to record their preferred treatment for each patient they assessed, with the aim being to quantify variation in practice among practitioners. Surgeons recorded their assessments in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheets which were returned to CTRU and combined into an analysis data set.
Statistical methods
Agreement was quantified as both raw and chance-corrected agreement. Raw agreement is the percentage of patients for whom the assessments agree, while chance-corrected agreement is the ratio of observed to expected agreement. Both raw and chance-corrected agreement are essentially proportions in which one means complete agreement while zero reflects complete disagreement. As the four categories are not ordinal, agreement is a simple yes/no construct in which any difference is considered ‘disagreement’.
Raw agreement was defined as (100 × number in which all raters agree / number rated) and was accompanied by a 95% Wilson score interval. Chance-corrected agreement was defined using the unweighted kappa statistic and the unweighted Gwet AC1 statistics. 138 Agreement among surgeons was reported overall and within for expert, general and trainee surgeons. Finally, the agreement was calculated for each surgeon in relation to the original assessment made at the time of procedure. Analyses were conducted using Stata version 17. 139
Sample size
The sample size was based on: (1) a hypothesis test to rule out a minimal kappa statistic;140 (2) the standard error of raw agreement; and (3) the number of patients expected to consent and provide usable photographs. For (1), an internal pilot was undertaken in which study surgeons were asked to assess photographs obtained either online or via published articles. A total of 41 pictures were assessed by seven surgeons (five specialists and two trainees) and yielded an overall kappa statistic of 0.42 (0.55 if trainees were excluded). Since the assessments were based on pictures alone and did not include prior history, these may be an underestimate. Based on this, a target of κ = 0.45 was used. The expected lowest limit for chance-corrected agreement was set at κ = 0.3, which represents the lowest acceptable agreement if this classification were to be introduced into practice. The sample size calculation also depends on the expected prevalence of each class, which was estimated from the cohort study (approximately 25% type 1, 50% type 2, 10% type 3 and 15% type 4 at the point of data review). A sample size of 90 was adequate to rule out differences of 15% between expected and minimum kappa with 90% power and 5% significance (1); to estimate raw agreement to within a CI half-width of ±10% (2); and to be accommodated by the number of photographs available (expected around 150) (3).
Results
Participants
Fifteen surgeons participated in the classification exercise as described in Chapter 7, Assessment schedule. A total of 166 patients consented to and provided a photograph of the diseased area, of which three were too unclear to use and were removed. Ninety participants were randomly selected from these for assessment, all of whom had been assigned a classification of 1–4 by the original treating clinician. The majority were classified as type 2 disease at the time of procedure, and 60% underwent a major procedure, with the most common treatments being a Karydakis asymmetric closure (n = 42) or glue (n = 29) (Table 23).
| Characteristic | No (%) |
|---|---|
| Classification at procedure | |
| Type 1 | 14 (16%) |
| Type 2 | 52 (58%) |
| Type 3 | 10 (11%) |
| Type 4 | 14 (16%) |
| Procedure | |
| Major excision | 54 (60%) |
| Karydakis | 42 (47%) |
| Bascom’s cleft lift | 2 (2%) |
| Leave open | 5 (6%) |
| Leave open (marsupialisation) | 1 (1%) |
| Midline closure | 4 (4%) |
| Minimal excision | 36 (40%) |
| Glue | 29 (32%) |
| EPSiT | 6 (7%) |
| Pit picking | 1 (1%) |
Agreement among participating surgeons
The agreement among surgeons is summarised in Table 24. Of the 540 assessments (90 patient photographs and case histories each having 6 assessments), 14 (3%) of assessments were classified ‘none of the above’, affecting 12 (13%) of the patients.
| Surgeon | No. agreea | Percentage (95% CI) | Kappa (95% CI) | Gwet AC1 (95% CI) |
|---|---|---|---|---|
| Expert | 65 | 72% (62 to 80%) | 0.54 (0.38 to 0.69) | 0.67 (0.56 to 0.79) |
| General | 63 | 70% (60 to 78%) | 0.54 (0.40 to 0.69) | 0.64 (0.52 to 0.76) |
| Trainee | 64 | 71% (61 to 79%) | 0.58 (0.43 to 0.72) | 0.65 (0.54 to 0.77) |
| Overall | 34 | 38% (28 to 48%) | 0.52 (0.42 to 0.61) | 0.63 (0.56 to 0.69) |
Overall, the six assessors all reached the same consensus in 38% of participants, with a chance-corrected kappa statistic of 0.52 (95% CI 0.42 to 0.61) and a Gwet AC1 statistic of 0.63 (95% CI 0.56 to 0.69). Agreement between pairs of surgeons was higher, with specialist surgeons agreeing in 72% of patients, general surgeons agreeing in 70% of patients and trainee surgeons agreeing in 71% of patients. The overall agreement is lower since this measure required all six assessors to agree. All six surgeons agreed in 34 (38%) of patients, and five of the six surgeons agreed for 19 (21%). The chance-corrected kappa agreement was above 0.5 (conventionally considered ‘moderate’) and the Gwet AC1 measure over 0.6 for all subgroups of surgical expertise.
Agreement between participating surgeons and original classification
Each assessor’s agreement with the original classification is described in Figure 10 and 11. Surgeons were less likely to agree with the original classification than with other surgeons given the same photograph. Raw agreement ranged between 47% (17/36) and 75% (27/36) with chance-corrected kappa statistics ranging from 0.11 to 0.59, and chance-corrected Gwet AC1 agreement statistics from 0.35 to 0.71.
FIGURE 10.
Raw agreement between assessment and original.

FIGURE 11.
Kappa chance-corrected agreement between assessment and original.

Treatment choice
Surgeons differed markedly when asked how they would treat the individual participants. Overall, surgeons in the substudy recommended a minimally invasive procedure in 46% of cases, but the figure ranged from 0% to 94% (34/36) of patients. There was substantial variation in practice among all levels of expertise, and the variation in recommendation did not appear related to the actual patients assessed (Appendix 3, Figure 16).
The surgeons surveyed were slightly more likely to recommend minimally invasive surgery (46%) than those that actually received this approach (40%). Although the percentage favouring asymmetric closure (45%) was similar to the treatment actually received (49%), the specific procedure types differed: the surgeons surveyed were more likely to use a Bascom’s cleft lift (22%) than a Karydakis (16%). The use of midline closure (5%) and leave open (4%) approaches were uncommon, most notably among the specialist surgeons surveyed.
Discussion
There are three potential roles of a classification system. Two are clinical – predicting prognosis and guiding treatment – and the third is primarily for research purposes, allowing more precise comparative studies to be carried out and reducing the potential for selection bias. While there have been eight previously proposed classification systems for PSD,110 each used judgemental methodology to develop their systems and identified homogeneous categories based on the experience of the investigators. The classifications were mainly used to select patients for different procedures. However, none provided analyses to demonstrate ease of use, reliability or predictive criterion validity. We have shown that the Wysocki classification, developed from components of these other systems, demonstrates moderate but acceptable agreement among the surgeons participating in this classification exercise. Agreement was similar among specialist, general and trainee colorectal surgeons, which offers reassurance that this system could be used across a range of surgeons.
While the level of agreement between substudy assessors and the original classification was lower, this is likely to be attributable to a mixture of picture quality and other features that were not available to the assessor in this exercise. A minority of cases (2.5%) were considered not to fall into any of the four categories, which was similar to the incidence seen in the cohort study (Chapter 3). This itself is relevant and indicates that this classification system incorporates reliably definable disease characteristics for almost all presentations of disease.
The findings from this substudy inform the main study, and vice versa. The general agreement seen in this substudy among surgeons lends weight to the Wysocki classification as an objective measure when conducting the risk adjustment. In turn, the cohort study found this classification to be an important feature in predicting response to treatment, with class 1 disease, in particular, being associated with more favourable outcomes. If the Wysocki classification is used to prognosticate and to inform the appropriate treatment, it is therefore important to demonstrate its reproducibility. While this substudy demonstrated only moderate agreement, its magnitude exceeded previously reported inter-rater reliability of other surgical classifications such as grading of haemorrhoids (κ = 0.38141) and dysplastic colorectal adenomas (κ = 0.38142). These findings support the use of the Wysocki classification in accurately defining subtypes of PSD.
Finally, the opportunistic question ‘what treatment would you recommend’ – while not central to the validation exercise – triangulates the findings of the consultant surgeon survey (see Chapter 2) in demonstrating that the choice of procedure is highly surgeon-dependent rather than evidence-based. This is, perhaps surprisingly, even the case in the specialist group where, for instance, the variation in minimally invasive procedures is immense. This reiterates that the disease characteristics are not the sole driver of treatment choice. This matters for the risk-adjusted comparisons in the cohort study, since non-randomised comparisons are known to be biased in situations when (1) treatment is defined by severity and (2) severity cannot easily be quantified and modelled.
Chapter 8 Discussion
Overview
The Idea, Development, Exploration, Assessment, Long-term follow-up (IDEAL) framework143 was designed to improve the quality of surgical research. Several large, multicentre, prospective longitudinal cohorts have followed this framework, allowing some understanding of variations in practice and their effects on outcomes of other surgical techniques. 144–146 PITSTOP also followed this framework, with the aim of answering some key questions in the surgical field of pilonidal disease. The need for this approach to pilonidal disease is clear. While the literature on this subject is vast, the quality of this literature is poor. An initial mapping exercise found that only 12% of the 983 identified primary research articles were randomised trials, and our current understanding of treatment relies mainly on poorly designed cohort studies which cannot provide us with reliable and reproducible estimates of treatment effects. 119 There is an absence of clear front-running surgical interventions. 13 Interventions are numerous and there are issues with heterogeneity of definitions and measurements of outcome. 13
Given the multitude of management options available, the initial work stream focused on surgeon preferences. A survey of over 100 UK surgeons was considered representative of real-world UK practice. Even with evidence to the contrary, a substantial proportion of surgeons who answered the survey perceived very high failure rates regardless of intervention. Again, regardless of the evidence, many practised non-surgical interventions and carried out procedures that experts in the field would consider obsolete (namely excision and leave open or midline closure). Even when minimally invasive procedures were perhaps appropriate, they were not considered as options by a substantial proportion.
This apparent disregard for the evidence could relate to the recognition of a low-quality evidence base and dismissal of the literature. 13 It could also relate to the unglamorous nature of pilonidal surgery. The specialist colorectal surgeon who is often tasked with managing the condition in the UK may tend to focus on more challenging conditions. ‘Pilonidal sinus cases are often just fillers on all day lists’ is a quote from an involved member of the core clinical team. The apprentice style of UK surgical training for pilonidal disease may also offer an explanation for the perpetuation of outdated techniques or even newer techniques done incorrectly. A mentor surgeon who has ‘always done it this way’ and a training syllabus that fails to keep pace with modern developments may be contributors.
Although a survey hints at the real-world experience of pilonidal sinus surgery in the UK, a more robust confirmation of this experience is required. The main work package of the PITSTOP trial was therefore a prospective cohort study. Involving over 30 centres throughout the UK, this again was considered reflective of current practice. The multitude of procedures utilised, and the most performed procedures, were consistent with the survey findings. Indeed, the continued use of potentially obsolete procedures (excise and leave open and midline closure) was also confirmed.
While 40% of procedures were classed as minimally invasive, the disease characteristics of the cohort suggest that more patients would have been eligible for such techniques. This is pertinent because such procedures were shown to result in less pain, less complication risk and more rapid return to normal activities, even after accounting for case mix. While the chance of cure was increased with the more major excisions, it may be that some patients would prefer to trade more rapid and less complex recovery for a moderate decrease in efficacy.
Perhaps the most surprising result was the protracted time a significant proportion of patients took to heal and return to normal activities. It is possible that one-quarter of patients had not healed or had persistent disease at 6 months regardless of type of intervention. In an essentially active working population, possibly one in eight patients had not resumed normal activities. This has important implications in terms of the impact on health resource as well as the ability to counsel patients accurately before surgery. It contradicts most of the literature on the subject, with many studies reporting extremely low failure rates. 39,40,147
One explanation for this difference between our findings and the reported literature is the definition of ‘failure’ compared with ‘recurrence’. This is a major issue with the existing evidence. Very few studies attempt to define what is meant by ‘recurrence’. A review of the relevant literature taken from the most recent guidelines148 indicates that over 85% of studies that investigate recurrence as an outcome fail to define it at all.
In the true sense of the word, ‘recurrence’ refers to disease that has resolved after an intervention but then recurs after a sufficient period to indicate it is not simply the original disease. It should be differentiated from disease that never resolves, or symptoms that remain unresolved due to an unhealed wound. Therefore, if previous studies report on true recurrence, the incidence may be very low as this is likely to be rare. The most clinically valid outcome is a combination of true recurrence and failure of healing after a reasonable time point. We reported on this combination as being relevant clinically. However, the incomplete data on ‘recurrence’ specifically after at least 6 months and the fact that patients themselves reported on ‘recurrence’, adding an element of subjectivity, may limit our interpretation of the results, and further in-depth analysis is required to allow robust comparisons with other studies.
Another explanation of the difference between our data and the literature is the skill of the surgeon. The cohort study included multiple surgeons of varied expertise. It may be that certain experts can achieve the success portrayed in the literature. 11 Analysis of individual surgeon data did show a difference in treatment failure rates between surgeons. Although numbers were small, this may justify that for optimal care patients, particularly those with complex disease, should be referred to specialist units. Alternatively, the skills of the more general surgeon should be enhanced.
Data from the consultant survey and the cohort study revealed a preference in favour of more aggressive interventions rather than minimally invasive procedures. This suggests that some surgeons may focus on cure rather than symptomatic improvement and believe that more major procedures result in a higher chance of cure even if minimally invasive procedures are possible. Our cohort data confirm a higher cure rate. However, patients may wish to trade this 10–15% increased chance of cure for significantly less pain, fewer complications and a more rapid return to normal activities. We explored this hypothesis utilising two qualitative methodologies: a mixed-methods substudy and a DCE.
The mixed-methods study suggested a lack of SDM for some patients, with many not being given a choice of procedure or informed fully about postoperative burden of care. This led to high levels of DR when procedures were not completely successful and protracted periods of recovery became necessary. Even when patients were involved in the decision-making, if not fully informed about postsurgical pathways, their expectations were often not met. Sufficient and accurate information about the risks of protracted postprocedural aftercare should be highlighted to address the false optimism many patients may have. 99,101 If given a choice, some patients may elect for alternative procedures where outcomes may differ.
The element of patient choice was explored further in the DCE. While cure of the disease was considered a priority by many, some were prepared to trade the chance of complete cure for a less protracted recovery. This was particularly the case for older patients (> 30 years) where an acceptance of up to 35% increased risk of persistent disease was tolerated in exchange for a shorter recovery period. This again emphasises the need for SDM and tailoring treatment according to the individual patient and their treatment goals.
While these two workstreams suggested a need for improved decision-making and the potential for DR after surgery, the data from the cohort study looking at these parameters revealed conflicting results. The median CollaboRATE score, a tool for assessing the quality of SDM, was very high. In addition, 84% of those who were assessed for DR were either very satisfied or satisfied with the surgery. This is despite around 45% having complications of surgery and 25% having persistent symptoms 6 months after surgery. These contrasting data could be explained by social desirability bias – the tendency to report higher scores out of gratitude or deference. 46
Of course, SDM becomes difficult if the surgeon only specialises in one technique. 30 Such surgeons should consider expanding their armamentarium to provide an individualised recommendation and choice for the patient, or consider referring to a specialist who may be able to offer such a service.
The literature on pilonidal sinus surgery is confusing and misleading due not only to multiple interventions and no obvious gold standard comparator, the lack of definitions (particularly of recurrence), but also to the heterogeneity of disease severity. Many researchers make no attempt to classify or stratify disease. As such, it is difficult to draw meaningful conclusions about comparative studies. Attempts to classify disease for the purposes of improving the quality of research have been made, and these have been reviewed as part of the PITSTOP study. 110 The Wysocki classification demonstrated moderate but acceptable agreement among the surgeons participating in this classification exercise. While there was only moderate agreement, the kappa exceeded previously reported inter-rater reliability of other surgical classifications such as grading of haemorrhoids120 and dysplastic colorectal adenomas. 142 Agreement was similar among specialist, general and trainee colorectal surgeons, which offers reassurance that this system could be used across a range of surgeons. Only 2.5% of cases were considered not to fall in the four categories, indicating that the classification system incorporates reliably definable disease characteristics for almost all presentations of disease. Finally, there was a suggestion that the classification could be prognostically valid, with class 1 disease, in particular, being associated with more favourable outcomes.
We concluded the PITSTOP study with a consensus exercise. This utilised a ‘so what, now what’ workshop incorporating data from the cohort study, an e-Delphi exercise and Qualtrics survey technology to consolidate patient and survey views as to the front-running policy and research statements. The policy statements highlighted some key outcomes from the other work packages and are included in the implications for practice and research discussed below.
Implications for practice
While minimally invasive procedures may not be suitable for all, they should form part of the armamentarium of each pilonidal sinus surgeon. Such interventions fit with the philosophy of not making the surgery worse than the disease itself.
The perceived high failure rate for pilonidal disease by many UK surgeons is a concern. Perpetuation of obsolete techniques by a substantial proportion, combined with newer techniques potentially done badly, emphasises the need for better guidance and training. National associations should take on this challenge by providing up-to-date guidance and influencing training through workshops and mentorship programmes.
Shared decision-making is essential, with patients offered an array of interventions allowing them to choose based on preferred outcomes. Many patients would be happy to trade a shorter recovery period for less chance of cure. They value the time to return to normal activities as an outcome, and this should be included in the decision-making process, aiding selection of interventions. If surgeons practise a ‘one fit for all’ intervention, they should consider learning a broader range of techniques or referring patients to a surgeon who can offer this service. An individualised approach based on the severity of disease and the wishes of the patient, combined with detailed information about interventions and potential aftercare, will improve patient expectations and reduce DR.
The Wysocki classification seems to provide a reliable tool and has some validity when it comes to prognostication. Further work is required to develop the tool to include some form of stratification of disease. This will help the surgeon in the choice of which interventions are appropriate.
Implications for research
The grouping of procedures into those that involve major excision and those that are minimally invasive could simplify both the process and the interpretation of future comparative trials. The impression from the core clinical team involved in PITSTOP was that this grouping was fair, although perhaps with the exclusion of excise-and-leave-open and midline closure techniques in the major excision group.
A classification system involving relevant disease characteristics is essential if future comparative trials are to be interpreted and meta-analysed in a meaningful way. Such a system should strive to include some form of stratification of disease severity. The Wysocki classification goes some way to meeting these requirements. Further application may allow development of a treatment algorithm or decision tree to aid surgical decision-making.
Future trials should include a robustly developed core outcome set which includes important PROs.
Equality, diversity and inclusion
No active steps were taken to make participation representative. Participants were representative of the disease population, with people of different disease severity included. White people were marginally overrepresented (see Table 5): 85% in PITSTOP versus 82% in the UK. We achieved a representative sample of Asian/Asian British people (9% in PITSTOP vs. 9% in the UK). We somewhat under-represented mixed/multiple ethnic groups (2% in PITSTOP vs. 3% in the UK) and black/African/Caribbean/Black British (1% in PITSTOP vs. 4% in the UK). This deficit could be addressed in future studies by opening more sites in London and the West Midlands and developing materials that are inclusive, accessible and encouraging to under-represented groups. Our core research team includes non-white members and a range of clinical and methodological expertise. Development opportunities were provided for entry-level researchers to present at conferences149 and act as first/corresponding authors30,110 on papers in peer-reviewed journals. The Associate PI scheme gave five junior clinicians, two of them non-white, the opportunity to contribute to the study.
Patient and public involvement
Patients informed the design of this study, ensured the methods selected were appropriate for patients, and reviewed and commented on questionnaires and other data collection methods. They advised on the appropriateness of the plain English summary and were named as co-applicants. Patient representatives steered the project through the research process, attending management group meetings. They assisted in the design of the protocol, patient information and consent forms. In the mixed-methods substudy, they assisted the analysts in developing themes from the data and contributed to the interpretation of data, with one person with lived experience acting as a co-author on the resulting publication. Expert patients were participants in the Delphi survey. Patients have helped us to design plain English summaries of findings.
Additional information
Contributions of authors
Steven Brown (https://orcid.org/0000-0002-0980-2793) (Professor of Surgery, Chief Investigator) conceived of or designed the work, was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Daniel Hind (https://orcid.org/0000-0002-6409-4793) (Professor of Evaluation, Assistant Director) conceived of or designed the work, was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Emily Strong (https://orcid.org/0000-0002-2381-4088) (Study Manager) was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mike Bradburn (https://orcid.org/0000-0002-3783-9761) (Senior Statistician) conceived of or designed the work, was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Farhat Vanessa Nasim Din (https://orcid.org/0000-0001-5466-8282) (Professor of Surgery, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ellen Lee (https://orcid.org/0000-0003-4529-7410) (Statistician Research Fellow) conceived of or designed the work, was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew J Lee (https://orcid.org/0000-0001-9971-1635) (Clinical Research Fellow, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, drafted the monograph, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jonathan Lund (https://orcid.org/0000-0001-5195-2181) (Professor of Surgery, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christine Moffatt (https://orcid.org/0000-0002-2436-0129) (Wound Management Nurse, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jonathan Morton (https://orcid.org/0000-0003-0544-5474) (Consultant Surgeon, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Asha Senapati (https://orcid.org/0000-0003-2597-9967) (Consultant Surgeon, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Philip Shackley (https://orcid.org/0000-0002-1862-0596) (Reader in Health Economics, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Peter Vaughan-Shaw (https://orcid.org/0000-0002-9790-6882) (Surgical Trainee, Co-applicant) conceived of or designed the work, was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Arkadiusz Peter Wysocki (https://orcid.org/0000-0001-6880-9285) (Consultant Surgeon, Co-applicant) conceived of or designed the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tia Callaghan (https://orcid.org/0000-0003-3255-2849) (Study Manager) was involved in the acquisition of data for the work, was involved in the interpretation of data for the work, produced the first draft of the report (with Nyantara Wickramasekera), revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Helen Jones (https://orcid.org/0000-0001-8238-7779) (Consultant Surgeon) was involved in the acquisition of data for the work, revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nyantara Wickramasekera (https://orcid.org/0000-0002-6552-5153) (Health Economist Research Fellow) was involved in the interpretation of data for the work, produced the first draft of the report (with Tia Callaghan), revised the work critically for important intellectual content, was involved in the final approval of the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following: research nurses and support staff in the 33 participating NHS Trusts for participant screening, consent, data collection and patient follow-up. We thank all participants who took part in the study.
We acknowledge advice and oversight from members of the project steering committee: Dr Dermot Burke (Chair, Consultant Colorectal Surgeon), Mr Stephen Chapman (NIHR Academic Clinical Fellow), Professor Catrin Tudor-Smith (Statistician) and Mr Ryan Edridge (Patient representative).
The following were also involved in the acquisition of data for the work:
Dale Vimalachandran (consultant surgeon), Jennie Grainger (consultant surgeon), Jeremy Wilson (consultant surgeon), Jared Torkington (consultant surgeon), Sandeep Kapur (consultant surgeon), Richard Stevenson (consultant surgeon), Najam Husain (consultant surgeon), Karim Muhammad (consultant surgeon), Richard Brady (consultant surgeon), David Donnelly (consultant surgeon), Michael Lim (consultant surgeon), Raj Rajaganeshan (consultant surgeon), Martyn Evans (consultant surgeon), Lyndsay Pearce (consultant surgeon), Godwin Dennison (consultant surgeon), Paul Mackay (consultant surgeon), Kenneth Keogh (consultant surgeon), Sudhaker Mangam (consultant surgeon), Mohan Harilingham (consultant surgeon), James Pitt (consultant surgeon), Sanjay Chaudri (consultant surgeon), Franceso De Fabio (consultant surgeon), Graham Branagan (consultant surgeon), Alex Hardy (consultant surgeon), Ali Khalafalla (consultant surgeon), Feliz Mazarelo (consultant surgeon), Yasuko Maeda (consultant surgeon), Nikhill Pawa (consultant surgeon).
Special thanks to Jennie Grainger (consultant surgeon), Khalafalla Ali (colorectal surgeon), Sunanda Mahapatra (general surgeon) and Peter Vaughan-Shaw for proofreading draft chapters of the monograph.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The study received approval from East of England – Cambridge South Research Ethics Committee (REC reference 18/EE/0370) on 26 November 2018.
Information governance statement
NIHR and The University of Sheffield are committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation The University of Sheffield is the Data Processor; Sheffield Teaching Hospitals NHS Foundation Trust is the Data Controller, and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for University of Sheffield’s Data Protection Officer, at (www.sheffieldclinicalresearch.org/).
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/KFDQ2017.
Primary conflicts of interest: All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no non-financial interests that may be relevant to the submitted work. Mike Bradburn is a current member of the HTA Commissioning Committee. Steven Brown was a member of the HTA Commissioning Committee from October 2017 to September 2019. Daniel Hind was a member of the HTA Clinical Evaluation and Trials Committee and HTA Fast Track Committee – June 2021.
Publications
Beal EM, Lee MJ, Hind D, Wysocki AP, Yang F, Brown SR. A systematic review of classification systems for pilonidal sinus. Tech Coloproctol 2019;23(5):435–43.Kumar M, Clay WH, Lee MJ, Brown SR, Hind D. A mapping review of sacrococcygeal pilonidal sinus disease. Tech Coloproctol 2021;25(6):675–82.
Strong E, Callaghan T, Beal E, Moffatt C, Wickramasekera N, Brown S, et al. , PITSTOP Project Management Group, PITSTOP Collaborators. Patient decision-making and regret in pilonidal sinus surgery: a mixed-methods study. Colorectal Dis 2021;23(6):1487–98.
Wickramasekera N, Strong E, Shackley P, Callaghan T, Lee M, Hind D, Brown S, PITSTOP Project Management Group and PITSTOP Collaborators. Patient preferences for pilonidal sinus treatments: a discrete choice experiment survey. Colorectal Dis 2023;25:984–994. https://doi.org/10.1111/codi.16482
Brown SR, Hind D, Strong E, Bradburn M, Din F, Lee E, et al. PITSTOP Management Group. Real-world practice and outcomes in pilonidal surgery: Pilonidal Sinus Treatment Studying The Options (PITSTOP) cohort. Br J Surg 2024;111(3):znae009. https://doi.org/10.1093/bjs/znae009
Lee MJ, Lee E, Bradburn M, Hind D, Strong EB, Din F, et al. PITSTOP Management Group and the PITSTOP Validators. Classification and stratification in pilonidal sinus disease: findings from the PITSTOP cohort [published online ahead of print 21 April 2024]. Colorectal Dis 2024. https://doi.org/10.1111/codi.16989.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Søndenaa K, Andersen E, Nesvik I, Søreide JA, Sondenaa K, Andersen E, et al. Patient characteristics and symptoms in chronic pilonidal sinus disease. Int J Colorectal Dis 1995;10:39-42.
- Hull TL, Wu J. Pilonidal disease. Surg Clin North Am 2002;82:1169-85.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg 1992;62:385-9.
- Ertan T, Koc M, Gocmen E, Aslar AK, Keskek M, Kilic M, et al. Does technique alter quality of life after pilonidal sinus surgery?. Am J Surg 2005;190:388-92.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg 1988;75:60-1.
- Elsey E, Lund JN. Fibrin glue in the treatment for pilonidal sinus: high patient satisfaction and rapid return to normal activities. Tech Coloproctol 2013;17:101-4.
- Patti R, Angileri M, Migliore G, Sparancello M, Termine S, Crivello F, et al. Use of fibrin glue in the treatment of pilonidal sinus disease: a pilot study. G Chir 2006;27:331-4.
- Thompson MR, Senapati A, Kitchen P. Simple day-case surgery for pilonidal sinus disease. Br J Surg 2011;98:198-209.
- Enriquez-Navascues JM, Emparanza JI, Alkorta M, Placer C. Meta-analysis of randomized controlled trials comparing different techniques with primary closure for chronic pilonidal sinus. Tech Coloproctol 2014;18:863-72.
- Hospital Episode Statistics . Admitted Patient Care – England 2012-13 2013.
- Vermeulen H, Ubbink D, Goossens A, de Vos R, Legemate D. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2004;2004.
- Price PE, Butterworth RJ, Bale S, Harding KG. Measuring quality of life in patients with granulating wounds. J Wound Care 1994;3:49-50.
- Stauffer VK, Luedi MM, Kauf P, Schmid M, Diekmann M, Wieferich K, et al. Common surgical procedures in pilonidal sinus disease: a meta-analysis, merged data analysis, and comprehensive study on recurrence. Sci Rep 2018;8.
- Brown SR, Lund JN. The evidence base for pilonidal sinus surgery is the pits. Tech Coloproctol 2019;23:1173-5.
- Al-Khamis A, McCallum I, King PM, Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev 2010;2010.
- McCallum IJD, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ 2008;336:868-71.
- Harris CL, Holloway S. Development of an evidence-based protocol for care of pilonidal sinus wounds healing by secondary intent using a modified Reactive Delphi procedure Part 2: methodology, analysis and results. Int Wound J 2012;9:173-88.
- Lund J, Tou S, Doleman B, Williams JP. Fibrin glue for pilonidal sinus disease. Cochrane Database Syst Rev 2017;1.
- Handmer M. Sticking to the facts: a systematic review of fibrin glue for pilonidal disease. ANZ J Surg 2012;82:221-4.
- Lund JN, Leveson SH. Fibrin glue in the treatment of pilonidal sinus: results of a pilot study. Dis Colon Rectum 2005;48:1094-6.
- Isik A, Eryılmaz R, Okan I, Dasiran F, Firat D, Idiz O, et al. The use of fibrin glue without surgery in the treatment of pilonidal sinus disease. Int J Clin Exp Med 2014;7:1047-51.
- Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES). J Med Internet Res 2004;6.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2008;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95. https://doi.org/10.1016/j.jbi.2019.
- Ojo D, Flashman K, Thomas G, Tozer P, Senapati A. Cleft closure (Bascom’s cleft lift) for 714 patients – treatment of choice for complex and recurrent pilonidal disease (a cohort study). Colorect Dis 2023;25:1839-43.
- Fearnhead NS, Lee MJ, Acheson AG, Worley G, Faiz OD, Brown SR. Variation in practice of pouch surgery in England – using SWORD data to cut to the chase and justify centralization. Colorect Dis 2018;20:597-605.
- O’Connell E, McDevitt J, Hill ADK, McNamara DA, Burke JP. Centralisation of rectal cancer care has improved patient survival in the republic of Ireland. Eur J Surg Oncol 2022;48:890-5.
- Shabbir J, Chaudhary BN, Britton DC. Management of sacrococcygeal pilonidal sinus disease: a snapshot of current practice. Int J Colorectal Dis 2011;26:1619-20.
- Burnett D, Smith SR, Young CJ. The surgical management of pilonidal disease is uncertain because of high recurrence rates. Cureus 2018;10.
- Strong E, Callaghan T, Beal E, Moffatt C, Wickramasekera N, Brown S, et al. Patient decision‐making and regret in pilonidal sinus surgery: a mixed‐methods study. Color Dis 2021;23:1487-98.
- Murphy S, Wysocki AP. Pilonidal sinus disease surveys. Pilonidal Sinus J 2017;3:19-27.
- Schein M. Treating pilonidal disease: you do not need to detonate a naval mine to catch a fish. World J Surg 2017;41:1303-4.
- Fabricius R, Petersen LW, Bertelsen CA. Treatment of pilonidal sinuses in Denmark is not optimal. Dan Med Bull 2010;57.
- Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part III: multivariate data analysis – choosing a model and assessing its adequacy and fit. Br J Cancer 2003;89:605-11.
- Busso M, DiNardo J, McCrary J. New evidence on the finite sample properties of propensity score reweighting and matching estimators. Rev Econ Stat 2014;96:885-97.
- Harlak A, Mentes O, Kilic S, Coskun K, Duman K, Yilmaz F. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics 2010;65:125-31.
- Iesalnieks I, Ommer A, Petersen S, Doll D, Herold A. German national guideline on the management of pilonidal disease. Langenbecks Arch Surg 2016;401:599-60.
- Steele SR, Perry WB, Mills S, Buie WD. Surgeons SPTF of the AS of C and R . Practice parameters for the management of pilonidal disease. Dis Colon Rectum 2013;56:1021-7.
- Baur T, Stauffer VK, Vogt AP, Kauf P, Schmid M, Luedi MM, et al. Recurrence rates after uncommon surgical procedures for pilonidal sinus disease. Coloproctology 2019;41:96-100.
- Bi S, Sun K, Chen S, Gu J. Surgical procedures in the pilonidal sinus disease: a systematic review and network meta-analysis. Sci Rep 2020;10.
- Milone M, Velotti N, Manigrasso M, Anoldo P, Milone F, De Palma GD. Long-term follow-up for pilonidal sinus surgery: a review of literature with metanalysis. Surgeon 2018;16:315-20.
- Gallo G, Carpino A, De Paola G, Fulginiti S, Novelli E, Ferrari F, et al. Endoscopic pilonidal sinus treatment: a tertiary care academic center experience. Front Surg 2021;8.
- Yildiz MK, Ozkan E, Odabasi HM, Kaya B, Eris C, Abuoglu HH, et al. Karydakis flap procedure in patients with sacrococcygeal pilonidal sinus disease: experience of a single centre in Istanbul. ScientificWorldJournal 2013;2013.
- Sinnott CJ, Glickman LT. Limberg flap reconstruction for sacrococcygeal pilonidal sinus disease with and without acute abscess: our experience and a review of the literature. Arch Plast Surg 2019;46:235-40.
- Immerman SC. The Bascom cleft lift as a solution for all presentations of pilonidal disease. Cureus 2021;13.
- Badejo MA, Ramtin S, Rossano A, Ring D, Koenig K, Crijns TJ. Does adjusting for social desirability reduce ceiling effects and increase variation of patient-reported experience measures?. J Patient Exp 2022;9.
- Tsai T, Shih L-C, Lee IT, Ng T-Y, Wang J-Y, Hsu C-L, et al. Older age is associated with better compliance with follow-up in Taiwan after functional endoscopic sinus surgery. In Vivo 2020;34:2571-6.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations. Med Care 2004;42:200-9.
- Dag A, Colak T, Turkmenoglu O, Sozutek A, Gundogdu R. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery 2012;151:113-7.
- Boztug CY, Karaagac Akyol T, Benlice C, Koc MA, Doganay Erdogan B, Ozcebe OI, et al. Platelet-rich plasma treatment improves postoperative recovery in patients with pilonidal sinus disease: a randomized controlled clinical trial. BMC Surg 2021;21.
- Milone M, Velotti N, Manigrasso M, Vertaldi S, Di Lauro K, De Simone G, et al. Long-term results of a randomized clinical trial comparing endoscopic versus conventional treatment of pilonidal sinus. Int J Surg 2020;74:81-5.
- Elbanna HG, Emile SH, Youssef M, Thabet W, El-Hamed TMA, Ghnnam WM. Novel approach of treatment of pilonidal sinus disease with thrombin gelatin matrix as a sealant. Dis Colon Rectum 2016;59:775-80.
- Milone M, Fernandez LMS, Musella M, Milone F. Safety and efficacy of minimally invasive video-assisted ablation of pilonidal sinus: a randomized clinical trial. JAMA Surg 2016;151:547-53.
- Yang Y, Yu L, Wang Y, Shi J, Li J, Shang F, et al. Comparative analysis on the effect of Z‐plasty versus conventional simple excision for the treatment of sacrococcygeal pilonidal sinus: A retrospective randomised clinical study. Int Wound J 2020;17:555-61.
- Yildiz A. Partial primary closure in sacrococcygeal pilonidal sinus: Modified with suture technique. Asian J Surg 2022;45:381-5.
- Shabbir F, Ayyaz M, Farooka MW, Toor AA, Sarwar H, Malik AA. Modified Limberg’s flap versus primary closure for treatment of pilonidal sinus disease: a comparative study. J Pak Med Assoc 2014;64:1270-3.
- Arslan K, Said Kokcam S, Koksal H, Turan E, Atay A, Dogru O. Which flap method should be preferred for the treatment of pilonidal sinus? A prospective randomized study. Tech Coloproctol 2014;18:29-37.
- Saydam M, Ozturk B, Sinan H, Balta AZ, Demir P, Ozer MT, et al. Comparison of modified Limberg flap transposition and lateral advancement flap transposition with Burow’s triangle in the treatment of pilonidal sinus disease. Am J Surg 2015;210:772-7.
- Yin R. Case Study Research: Identifying Your Case(s) and Establishing the Logic of Your Case Study. London: Sage Publications; 2014.
- Elwyn G, James P, Grande SW, Thompson R, Walsh T, Ozanne EM. Patient Education and Counseling Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns 2013;93:102-7.
- Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281-92.
- Guest G, Bunce A, Johnson L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods 2006;18:59-82.
- Morse JM. Data were saturated. Qual Health Res 2015;25:587-8.
- Witt J, Elwyn G, Wood F, Brain K. Decision making and coping in healthcare: the coping in deliberation (CODE) framework. Patient Educ Couns 2012;88:256-61.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17.
- Ritchie J, Cartwright MFJ. Analyzing Qualitative Data. London: Routledge; 1994.
- Yin RK. Mixed methods research: are the methods genuinely integrated or merely parallel?. Res Sch 2006;13:41-8.
- Guetterman TC, Fetters MD, Creswell JW. Integrating quantitative and qualitative results in health science mixed methods research through joint displays. Ann Fam Med 2015;13:554-61.
- Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 2020;19:376-7.
- Patel SS, Webster RK, Greenberg N, Weston D, Brooks SK. Research fatigue in COVID-19 pandemic and post-disaster research: causes, consequences and recommendations. Disaster Prev Manag 2020;29:445-5.
- Bradt J. Impact of COVID-19 on clinical research. Nord J Music Ther 2020;29:297-9.
- Slauson-Blevins K, Johnson KM. Doing gender, doing surveys? Women’s gatekeeping and men’s non-participation in multi-actor reproductive surveys. Sociol Inq 2016;86:427-49.
- Rourke DO, Lakner E. Gender bias: analysis of factors causing male underrepresentation in surveys. Int J Public Opin Res 1989;1:164-76.
- Segre D. What you should remember in managing pilonidal disease. Front Surg 2021;8.
- Graham K, Grewal I, Lewis J. Ethics in Social Research: The Views of Research Participants. London; 2007.
- Carson P, Hong CJ, Otero-Vinas M, Arsenault EF, Falanga V. Liver enzymes and lipid levels in patients with lipodermatosclerosis and venous ulcers treated with a prototypic anabolic steroid (stanozolol). Int J Low Extrem Wounds 2015;14:11-8.
- Yamauchi K, Nakao M, Nakashima M. Correlates of regret with treatment decision-making among Japanese women with breast cancer: results of an internet-based cross-sectional survey. BMC Womens Health 2019;19.
- Wilson A, Ronnekleiv-Kelly SM, Pawlik TM. Regret in surgical decision making: a systematic review of patient and physician perspectives. World J Surg 2017;41:1454-65.
- Becerra Pérez MM, Menear M, Brehaut JC, Légaré F. Extent and predictors of decision regret about health care decisions. Med Decis Mak 2016;36:777-90.
- Kannan S, Seo J, Riggs KR, Geller G, Boss ZDB EF. Surgeons’ views on shared decision-making. J Patient-Centered Res Rev 2020;7:8-18.
- Shelton RC, Brotzman LE, Crookes DM, Robles P, Neugut AI. Decision-making under clinical uncertainty: An in-depth examination of provider perspectives on adjuvant chemotherapy for stage II colon cancer. Patient Educ Couns 2019;102:284-90.
- Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: A systematic review. Soc Sci Med 2005;61:2252-64.
- Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, Poenaru D. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13:667-81.
- Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009;17:1117-28.
- Raybould G, Babatunde O, Evans AL, Jordan JL, Paskins Z. Expressed information needs of patients with osteoporosis and/or fragility fractures: a systematic review. Arch Osteoporos 2018;13.
- Adams E, Boulton M, Watson E. The information needs of partners and family members of cancer patients: a systematic literature review. Patient Educ Couns 2009;77:179-86.
- Wang T, Molassiotis A, Chung BPM, Tan J-Y. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care 2018;17.
- Williams D, Edwards A, Wood F, Lloyd A, Brain K, Thomas N, et al. Ability of observer and self-report measures to capture shared decision-making in clinical practice in the UK: a mixed-methods study. BMJ Open 2019;9.
- Heen AF, Vandvik PO, Brandt L, Montori VM, Lytvyn L, Guyatt G, et al. A framework for practical issues was developed to inform shared decision-making tools and clinical guidelines. J Clin Epidemiol 2021;129:104-13.
- Lagha E, Noble A, Smith A, Denvir M, Leslie S. Patient reported experience measures (PREMs) in chronic heart failure. J R Coll Physicians Edinb 2012;42:301-5.
- Saunders CL, Elliott MN, Lyratzopoulos G, Abel GA. Do differential response rates to patient surveys between organizations lead to unfair performance comparisons?. Med Care 2016;54:45-54.
- Ahmed F, Burt J, Roland M, Ahmed F, Burt J, Roland AM, et al. Measuring patient experience: concepts and methods. Patient 2014;7:235-41.
- Cabitza F, Dui LG, Banfi G. PROs in the wild: assessing the validity of patient reported outcomes in an electronic registry. Comput Methods Programs Biomed 2019;181.
- Jenkinson C. The picker patient experience questionnaire: development and validation using data from in-patient surveys in five countries. Int J Qual Heal Care 2002;14:353-8.
- Trujols J, Iraurgi I, Oviedo-Joekes E, Guàrdia J. A critical analysis of user satisfaction surveys in addiction services: opioid maintenance treatment as a representative case study. Patient Prefer Adherence 2014;8:107-17.
- Moffatt S, White M, Mackintosh J, Howel D. Using quantitative and qualitative data in health services research – what happens when mixed method findings conflict?. BMC Health Serv Res 2006;6.
- Munafò MR, Davey Smith G. Robust research needs many lines of evidence. Nature 2018;553:399-401.
- McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I. Nurses’ and surgeons’ views and experiences of surgical wounds healing by secondary intention: a qualitative study. J Clin Nurs 2020;29:2557-71.
- Bekker CL, Mohsenian Naghani S, Natsch S, Wartenberg NS, van den Bemt BJF. Information needs and patient perceptions of the quality of medication information available in hospitals: a mixed method study. Int J Clin Pharm 2020;42:1396-404.
- McHugh GA, Luker KA. Individuals’ expectations and challenges following total hip replacement: a qualitative study. Disabil Rehabil 2012;34:1351-7.
- Baumeister RF, Vohs KD, Oettingen G. Pragmatic prospection: how and why people think about the future. Rev Gen Psychol 2016;20:3-16.
- Mahomed NN, Liang MH, Cook EF, Daltroy LH, Fortin PR, Fossel AH, et al. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J Rheumatol 2002;29:1273-9.
- Purkiss SF. Decision making in surgery: a pilonidal sinus. Br J Hosp Med 1993;50:554-6.
- Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ 2005;330.
- Mottram A. ‘They are marvellous with you whilst you are in but the aftercare is rubbish’: a grounded theory study of patients’ and their carers’ experiences after discharge following day surgery. J Clin Nurs 2011;20:3143-51.
- Aune E, Struksnes S. Home care nurses’ experience of providing health-care to patients with hard-to-heal wounds. J Wound Care 2019;28:178-87.
- Friman A, Klang B, Ebbeskog B. Wound care by district nurses at primary healthcare centres: a challenging task without authority or resources. Scand J Caring Sci 2011;25:426-34.
- Maybin J, Charles A, Honeyman M. Understanding Quality in District Nursing Services: Learning from Patients, Carers and Staff. The Kings Fund; 2016.
- Wickramasekera N, Strong E, Shackley P, Callaghan T, Lee M, Hind D, et al. Patient preferences for pilonidal sinus treatments: a discrete choice experiment survey. Colorect Dis 2023;25:984-9. https://doi.org/10.1111/codi.16482.
- Beal EM, Lee MJ, Hind D, Wysocki AP, Yang F, Brown SR. A systematic review of classification systems for pilonidal sinus. Tech Coloproctol 2019;23:435-43.
- Hahn G, Shapiro S. A Catalogue and Computer Program for the Design and Analysis of Orthogonal Symmetric and Asymmetric Fractional Factorial Designs. Schenectady, New York: General Electric Research and Development Center; 1966.
- de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient 2015;8:373-84.
- Orme B. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison: Research Publishers; 2010.
- Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ 2007;16:827-40.
- Stewart AM, Baker JD, Elliott D. The effects of a sacrococcygeal pilonidal sinus wound on activities of living: thematic analysis of participant interviews. J Clin Nurs 2011;20:3174-82.
- Harris C, Sibbald RG, Mufti A, Somayaji R. Pilonidal sinus disease: 10 steps to optimize care. Adv Skin Wound Care 2016;29:469-78.
- Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. Digit Med 2022;5.
- Rolfe G, Freshwater D, Jasper M. Critical Reflection for Nursing and the Helping Professions: A User’s Guide. London: Palgrave; 2001.
- Kumar M, Clay WH, Lee MJ, Brown SR, Hind D. A mapping review of sacrococcygeal pilonidal sinus disease. Tech Coloproctol 2021;25:675-82.
- Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996;347:984-5.
- Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg 1978;12:85-109.
- Kozar RA, Crandall M, Shanmuganathan K, Zarzaur BL, Coburn M, Cribari C, et al. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg 2018;85:1119-22.
- Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 2019;20.
- Jayne DG, Scholefield J, Tolan D, Gray R, Senapati A, Hulme CT, et al. A multicenter randomized controlled trial comparing safety, efficacy, and cost-effectiveness of the Surgisis anal fistula plug versus surgeon’s preference for transsphincteric fistula-in-ano: the FIAT trial. Ann Surg 2021;273:433-41.
- Girling C, Lee MJ, Vimalchandran D, Jayne DJ, Stancliffe S, Wailoo A, et al. Protocol for the ORION trial (RadiO fRequency ablatION for haemorrhoids): a randomised controlled trial. Tech Coloproctol 2023;27:117-24.
- Webbe J, Sinha I, Gale C. Core outcome sets. Arch Dis Child Educ Pract Ed 2018;103:163-6.
- Tripartite Gastrointestinal Recovery SBO Group . A core outcome set for clinical studies of adhesive small bowel obstruction. Colorectal Dis 2022;24:1204-10.
- Lapsekill E, Coskun M, Oztas M, Urkan M, Can MF. A classification proposal for the sacrococcygeal pilonidal sinus disease (SPSD). Eur Surg Res 2013;50.
- Irkörücü O. Management for pilonidal disease: before you compare, use a classification system. Asian J Surg 2016;39:260-1.
- Irkorucu O, Erdem H, Reyhan E. The best therapy for pilonidal disease: which management for which type?. World J Surg 2012;36:691-2.
- Quinodoz PD, Chilcott M, Grolleau JL, Chavoin JP, Costagliola M. Surgical treatment of sacrococcygeal pilonidal sinus disease by excision and skin flaps: the Toulouse experience. Eur J Surg 1999;165:1061-5.
- Doll D, Vassiliu P. Another pilonidal classification—PLLATIN. Pilonidal Sinus J 2018;4:1-3.
- Guner A, Cekic AB, Boz A, Turkyilmaz S, Kucuktulu U. A proposed staging system for chronic symptomatic pilonidal sinus disease and results in patients treated with stage-based approach. BMC Surg 2016;16.
- Tezel E. A new classification according to navicular area concept for sacrococcygeal pilonidal disease. Color Dis 2007;9:575-6.
- Awad M, Elbaset A, Ebraheem S, Tantawy E, Elhafez M, Elsayed A. A scoring system as a method to evaluate pilonidal sinus disease to make an easy decision for its management. Indian J Plast Surg 2009;42.
- Wysocki AP, Andersson RE, Gips M, Girgin M, Guner A, Immerman S, et al. Towards a classification for sacrococcygeal pilonidal disease – Berlin 2017. Pilonidal Sinus J 2018;4:5-12.
- International Pilonidal Society . Survey towards pilonidal classification. Pilonidal Sinus J 2018;4:5-12.
- Flight L, Julious SA. The disagreeable behaviour of the kappa statistic. Pharm Stat 2015;14:74-8.
- StataCorp . Stata Statistical Software: Release 17 2021.
- Rotondi MA, Donner A. A confidence interval approach to sample size estimation for interobserver agreement studies with multiple raters and outcomes. J Clin Epidemiol 2012;65:778-84.
- Dekker L, Han-Geurts IJM, Grossi U, Gallo G, Veldkamp R. Is the Goligher classification a valid tool in clinical practice and research for hemorrhoidal disease?. Tech Coloproctol 2022;26:387-92.
- Jensen P, Krogsgaard MR, Christiansen J, Braendstrup O, Johansen A, Olsen J. Observer variability in the assessment of type and dysplasia of colorectal adenomas, analyzed using kappa statistics. Dis Colon Rectum 1995;38:195-8.
- Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG. IDEAL Group IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ 2013;346.
- Vohra RS, Spreadborough P, Johnstone M, Marriott P, Bhangu A, Alderson D, et al. Protocol for a multicentre, prospective, population-based cohort study of variation in practice of cholecystectomy and surgical outcomes (the CholeS study). BMJ Open 2015;5.
- Lee MJ, Sayers AE, Drake TM, Marriott PJ, Anderson ID, Bach SP, et al. National prospective cohort study of the burden of acute small bowel obstruction. BJS Open 2019;3:354-66.
- Glancz LJ, Poon MTC, Coulter IC, Hutchinson PJ, Kolias AG, Brennan PM. Does drain position and duration influence outcomes in patients undergoing burr-hole evacuation of chronic subdural hematoma? Lessons from a UK multicenter prospective cohort study. Neurosurgery 2019;85:486-93.
- Milone M, Basso L, Manigrasso M, Pietroletti R, Bondurri A, La Torre M, et al. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of pilonidal disease. Tech Coloproctol 2021;25:1269-80.
- Segre D, Pozzo M, Perinotti R, Roche B. Italian Society of Colorectal Surgery. The treatment of pilonidal disease: guidelines of the Italian Society of Colorectal Surgery (SICCR). Tech Coloproctol 2015;19:607-13.
- Beal E, Hind D, Bradburn M, Lee E, Howard A, Shackley P, et al. Design and rationale of the PIlonidal sinus Treatment – STudying the OPtions (PITSTOP) study: a multicentre cohort, nested mixed-methods case study and discrete choice experiment (poster presentation). Int J Surg 2018;59.
Appendix 1 PITSTOP Project Management Group
Khalafalla Ali, Mike Bradburn, Richard Brady, Graham Branagan, Steven Brown, Sanjay Chaudri, Francesco Di Fabio, Godwin Dennison, Farhat Din, David Donnelly, Martyn Evans, Francois Gerald, Sarah Gonzalez, Jennie Grainger, Alex Hardy, Mohan Harilingam, Daniel Hind, Philip Hopley, Najam Husain, Helen Jones, Sandeep Kapur, Kenneth Keogh, Ellen Lee, Matt Lee, Michael Lim, Jon Lund, Paul Mackey, Yasuko Maeda, Sanjay Mahaptra, Sudhaker Mangam, Felix Mazarelo, Christine Moffatt, Jon Morton, Karim Muhammad, Nikhill Pawa, Lyndsay Pearce, James Pitt, Raj Rajaganeshan, Asha Senapati, Phil Shackley, Richard Simmonds, Richard Stevenon, Jared Torkington, Peter Vaughan-Shaw, Dale Vimalachandran, Jeremy Wilson, Peter Wysocki.
Appendix 2 Cohort study participating sites
Countess of Chester Hospital
Sheffield Teaching Hospitals
Wirral University Teaching Hospital
University Hospital of Wales – Cardiff
Norfolk and Norwich University Hospitals
Oxford University Hospital
St Mark’s Hospital London
Glasgow Royal Infirmary
Queen Alexandra Hospital – Portsmouth
Addenbrookes Hospital – Cambridge
Royal Derby Hospital
Western General Hospital – Edinburgh
Burton Hospital
Tameside and Glossop Integrated Care NHS Foundation Trust
Newcastle Upon Tyne Hospitals NHS Foundation Trust
Manchester Royal Infirmary
York Teaching Hospital NHS Foundation Trust
St Helens and Knowsley Teaching Hospitals NHS Trust
Swansea Bay University Health Board – Morriston Hospital
Salford Royal NHS Foundation Trust
Yeovil District Hospital NHS Foundation Trust
Queen Elizabeth The Queen Mother Hospital – East Kent
Royal Devon and Exeter NHS Foundation Trust
East Suffolk and North Essex NHS Foundation Trust – Ipswich
Musgrove Park Hospital – Taunton
Salisbury NHS Foundation Trust
Leicester General Hospital
Trafford General Hospital
Peterborough City Hospital
Hinchingbrooke Hospital
Chelsea and Westminster Hospital NHS Foundation Trust
Appendix 3 Additional figures and tables
FIGURE 12.
Procedure preferences of responding surgeons.
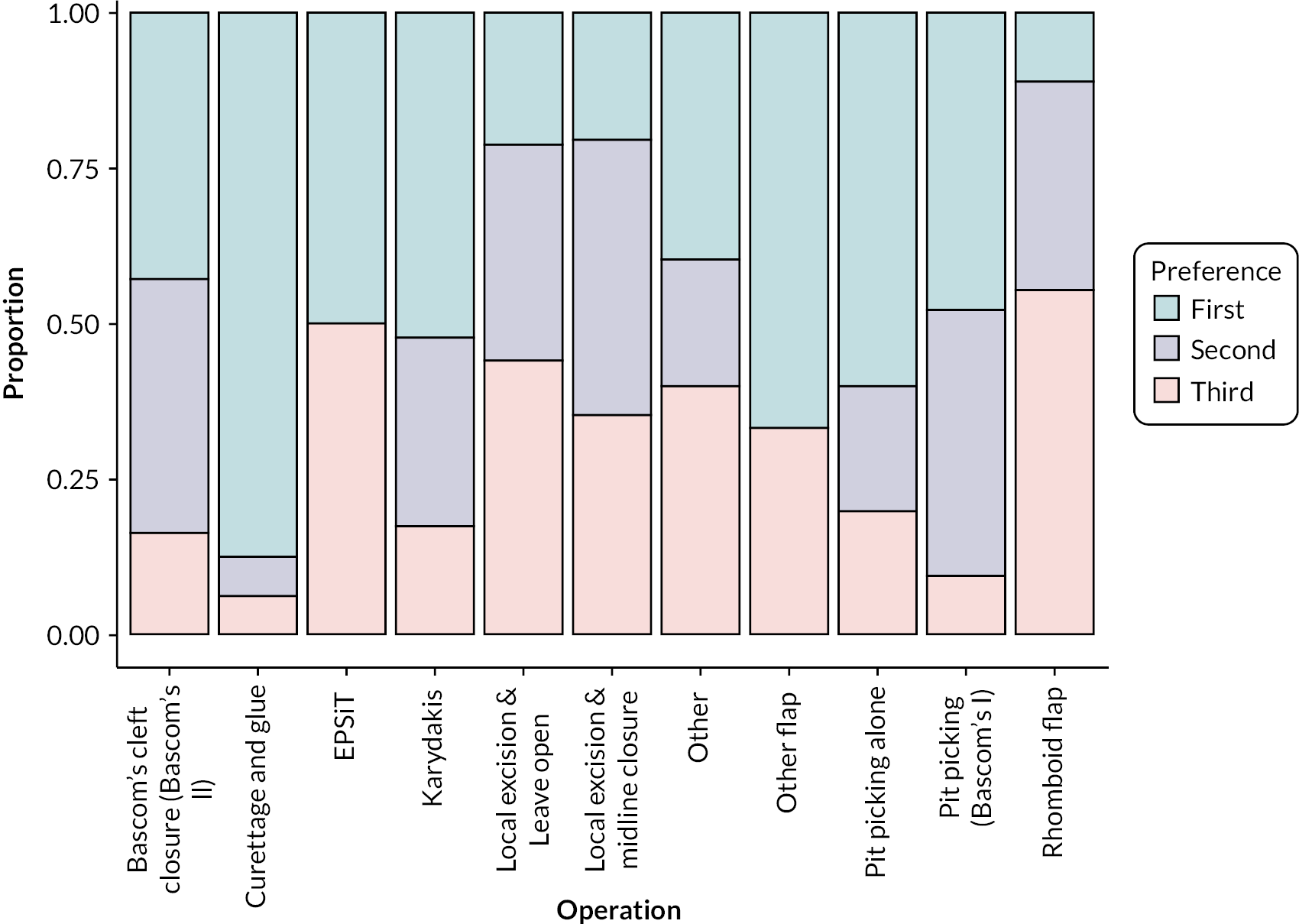
| Outcome, n (%) with available data | Time point | |||||
|---|---|---|---|---|---|---|
| All | Baseline | Day 1 | Day 7 | Clinic visit | 6 months | |
| Any outcome data | 667 (100%) | 608 (91%) | 577 (87%) | 513 (77%) | 476 (71%) | |
| Complications | 608 (91%) | 576 (86%) | 510 (76%) | 474 (71%) | ||
| Pain (today) | 666 (100%) | 606 (91%) | 574 (86%) | 501 (75%) | 470 (70%) | |
| Pain (worst in last week) | 665 (100%) | 574 (86%) | 502 (75%) | 470 (70%) | ||
| EQ-5D | ||||||
| EQ-5D-5L health utility (crosswalk) | 654 (98%) | 572 (86%) | 494 (74%) | 466 (70%) | ||
| EQ-5D – your health today | 658 (99%) | 572 (86%) | 493 (74%) | 466 (70%) | ||
| CWIQ | ||||||
| Physical symptoms and daily living experience | 497 (75%) | 467 (70%) | ||||
| Physical symptoms and daily living stress | 496 (74%) | 461 (69%) | ||||
| Well-being | 495 (74%) | 460 (69%) | ||||
| QoL | 495 (74%) | 465 (70%) | ||||
| QoL satisfaction | 495 (74%) | 464 (70%) | ||||
| Repacking procedures | 563 (84%) | 496 (74%) | 458 (69%) | |||
| Replacement/removal of dressing | 561 (84%) | 486 (73%) | 447 (67%) | |||
| Service interactions | 572 (86%) | 501 (75%) | 471 (71%) | |||
| Returned to normal activities | 607 (91%) | |||||
| Wound healed | 553 (83%) | |||||
| Any reported recurrence | 629 (94%) | |||||
| Any complication during follow-up | 643 (96%) | |||||
| DR | 456 (68%) | |||||
| Scar spread | 246 (37%) | |||||
| Scar overall impression | 241 (36%) | |||||
| Scar itch (in past 24 hours) | 412 (62%) | |||||
| Scar pain (in past 24 hours) | 412 (62%) | |||||
| Procedure information | Asymmetric closure | Leave open | Midline closure | Minimal excision | All |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Length of surgery (minutes) | |||||
| N (%) | 271 (100%) | 47 (96%) | 74 (97%) | 261 (97%) | 653 (98%) |
| Mean (SD) | 47.0 (22.3) | 25.2 (14.7) | 40.5 (18.8) | 19.0 (15.9) | 33.5 (23.1) |
| Median (IQR) | 45 (30, 60) | 20 (15, 33) | 36 (27, 50) | 15 (9, 24) | 30 (16, 45) |
| Min, max | 10, 171 | 5, 67 | 13, 105 | 2, 136 | 2, 171 |
| Category of hospital stay | |||||
| Day case | 251 (92%) | 45 (92%) | 73 (96%) | 266 (99%) | 635 (95%) |
| Inpatient | 20 (7%) | 4 (8%) | 3 (4%) | 2 (1%) | 29 (4%) |
| Grade of operating surgeon | |||||
| Consultant | 172 (63%) | 38 (78%) | 46 (61%) | 199 (74%) | 455 (68%) |
| Non-consultant | 100 (37%) | 11 (22%) | 30 (39%) | 71 (26%) | 212 (32%) |
| Type of anaesthetic during operation | |||||
| General | 227 (83%) | 41 (84%) | 74 (97%) | 179 (66%) | 521 (78%) |
| Spinal | 11 (4%) | 4 (8%) | 0 (0%) | 5 (2%) | 20 (3%) |
| Local | 34 (13%) | 3 (6%) | 2 (3%) | 85 (31%) | 124 (19%) |
| Sedation (for those using local anaesthetic) | |||||
| No | 7 (3%) | 1 (2%) | 1 (1%) | 56 (21%) | 65 (10%) |
| Yes | 27 (10%) | 1 (2%) | 1 (1%) | 26 (10%) | 55 (8%) |
| Antibiotics used at induction | 232 (85%) | 21 (43%) | 42 (55%) | 108 (40%) | 403 (60%) |
| Antibiotics used post surgery | 88 (32%) | 1 (2%) | 17 (22%) | 17 (6%) | 123 (18%) |
FIGURE 13.
Treatment choice by number of pits (N = 640).
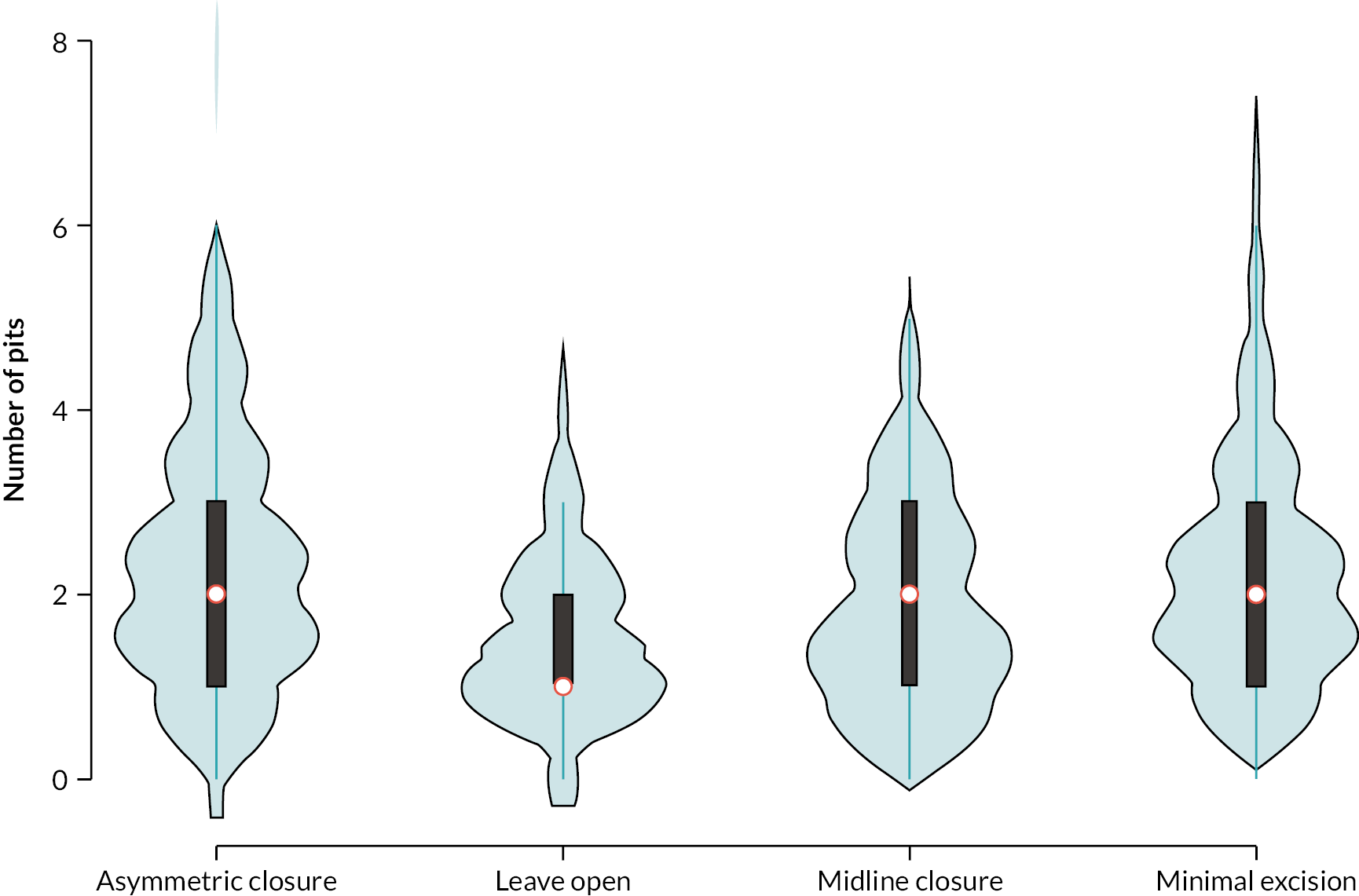
| Outcome | Asymmetric closure | Leave open | Midline closure | Minimal excision | All | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Pain (today)a | ||||||||||
| Baseline | 271 | 1.9 (2.2) | 49 | 2.9 (2.6) | 76 | 1.6 (2.0) | 270 | 1.8 (2.3) | 666 | 1.9 (2.3) |
| Day 1 | 253 | 4.5 (2.4) | 43 | 2.9 (2.6) | 68 | 3.9 (2.8) | 242 | 2.6 (2.2) | 606 | 3.6 (2.5) |
| Day 7 | 246 | 3.3 (2.3) | 41 | 4.1 (2.8) | 66 | 3.7 (2.8) | 221 | 1.9 (2.2) | 574 | 2.8 (2.5) |
| Clinic visit | 218 | 1.6 (2.1) | 34 | 1.8 (2.4) | 51 | 2.3 (2.5) | 198 | 0.8 (1.7) | 501 | 1.4 (2.1) |
| 6-month visit | 202 | 0.6 (1.5) | 34 | 1.4 (2.2) | 54 | 0.7 (1.9) | 180 | 0.8 (1.6) | 470 | 0.7 (1.6) |
| Pain (worst in last week)a | ||||||||||
| Baseline | 270 | 3.6 (3.0) | 49 | 4.5 (3.2) | 76 | 3.4 (3.2) | 270 | 3.3 (3.0) | 665 | 3.5 (3.0) |
| Day 7 | 246 | 5.4 (2.5) | 41 | 5.9 (2.9) | 66 | 5.5 (3.1) | 221 | 3.0 (2.8) | 574 | 4.5 (3.0) |
| Clinic visit | 218 | 2.7 (2.8) | 34 | 3.0 (2.6) | 51 | 3.4 (2.9) | 199 | 1.5 (2.5) | 502 | 2.3 (2.8) |
| 6-month visit | 202 | 1.0 (2.0) | 34 | 2.2 (2.7) | 54 | 1.1 (2.3) | 180 | 1.4 (2.5) | 470 | 1.3 (2.3) |
| EQ-5D-5L health utility (crosswalk)b | ||||||||||
| Baseline | 267 | 0.79 (0.20) | 48 | 0.76 (0.19) | 74 | 0.81 (0.20) | 265 | 0.82 (0.19) | 654 | 0.80 (0.20) |
| Day 7 | 246 | 0.65 (0.21) | 41 | 0.60 (0.22) | 66 | 0.61 (0.27) | 219 | 0.79 (0.20) | 572 | 0.69 (0.23) |
| Clinic visit | 214 | 0.80 (0.21) | 34 | 0.75 (0.22) | 49 | 0.75 (0.20) | 197 | 0.89 (0.17) | 494 | 0.83 (0.20) |
| 6-month visit | 201 | 0.90 (0.18) | 34 | 0.82 (0.19) | 53 | 0.89 (0.19) | 178 | 0.89 (0.16) | 466 | 0.89 (0.17) |
| EQ-5D – your health todayc | ||||||||||
| Baseline | 268 | 76.6 (16.1) | 48 | 74.4 (18.2) | 73 | 76.1 (12.9) | 269 | 77.4 (16.8) | 658 | 76.7 (16.2) |
| Day 7 | 247 | 73.5 (16.1) | 41 | 71.1 (19.6) | 64 | 71.5 (19.4) | 220 | 79.4 (17.7) | 572 | 75.4 (17.6) |
| Clinic visit | 214 | 78.7 (16.9) | 34 | 81.1 (15.0) | 49 | 80.4 (14.3) | 196 | 85.3 (13.7) | 493 | 81.6 (15.6) |
| 6-month visit | 202 | 81.0 (19.8) | 34 | 79.4 (19.6) | 53 | 82.5 (12.9) | 177 | 84.0 (16.2) | 466 | 82.2 (17.8) |
| CWIQ QoLd | ||||||||||
| Clinic visit | 216 | 7.5 (2.1) | 34 | 7.6 (1.7) | 48 | 7.5 (1.8) | 197 | 8.6 (1.7) | 495 | 7.9 (1.9) |
| 6-month visit | 201 | 8.4 (1.7) | 34 | 7.6 (2.4) | 53 | 8.0 (2.0) | 177 | 8.6 (1.5) | 465 | 8.4 (1.7) |
| CWIQ QoL satisfactiond | ||||||||||
| Clinic visit | 216 | 7.4 (2.2) | 34 | 7.5 (2.0) | 48 | 7.8 (1.7) | 197 | 8.6 (1.7) | 495 | 7.9 (2.0) |
| 6-month visit | 201 | 8.3 (1.9) | 34 | 7.5 (2.6) | 53 | 8.1 (2.1) | 176 | 8.5 (1.9) | 464 | 8.3 (2.0) |
| CWIQ Physical symptoms and daily living experiencee | ||||||||||
| Clinic visit | 216 | 81.2 (22.0) | 34 | 76.5 (21.4) | 49 | 75.1 (22.1) | 198 | 91.8 (15.0) | 497 | 84.5 (20.4) |
| 6-month visit | 202 | 92.7 (14.8) | 34 | 85.2 (22.0) | 54 | 92.5 (14.3) | 177 | 92.7 (12.3) | 467 | 92.1 (14.6) |
| CWIQ Physical symptoms and daily living stresse | ||||||||||
| Clinic visit | 216 | 85.0 (22.2) | 34 | 82.1 (21.9) | 49 | 81.2 (20.8) | 197 | 94.9 (12.9) | 496 | 88.4 (19.6) |
| 6-month visit | 197 | 94.8 (14.6) | 34 | 88.3 (18.2) | 53 | 94.8 (12.3) | 177 | 95.7 (9.5) | 461 | 94.7 (13.0) |
| CWIQ Well-beinge | ||||||||||
| Clinic visit | 216 | 57.9 (23.3) | 34 | 54.1 (24.2) | 49 | 58.6 (22.6) | 196 | 68.7 (22.5) | 495 | 62.0 (23.6) |
| 6-month visit | 199 | 68.5 (22.4) | 34 | 56.5 (22.0) | 52 | 66.7 (24.3) | 175 | 68.1 (22.8) | 460 | 67.3 (22.9) |
| Outcome | Asymmetric closure | Leave open | Midline closure | Minimal excision | All |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Repacking procedure since last follow-up | |||||
| Day 1 | 0/254 (0%) | 0/43 (0%) | 0/69(0%) | 0/242 (0%) | 0/608 (0%) |
| Day 7 | 16/248 (6%) | 25/41 (61%) | 5/67(7%) | 22/221 (10%) | 68/577 (12%) |
| Clinic visit | 37/222 (17%) | 21/35 (60%) | 14/53(26%) | 15/203 (7%) | 87/513 (17%) |
| 6-month visit | 22/205 (11%) | 11/35 (31%) | 6/56(11%) | 6/180 (3%) | 45/476 (9%) |
| Re-dressing procedure since last follow-up | |||||
| Day 1 | 0/254 (0%) | 0/43 (0%) | 0/69(0%) | 0/242 (0%) | 0/608 (0%) |
| Day 7 | 122/248 (49%) | 18/41 (44%) | 35/67(52%) | 51/221 (23%) | 226/577 (39%) |
| Clinic visit | 105/222 (47%) | 16/35 (46%) | 21/53(40%) | 37/203 (18%) | 179/513 (35%) |
| 6-month visit | 36/205 (18%) | 8/35 (23%) | 12/56(21%) | 11/180 (6%) | 67/476 (14%) |
| Time point | Asymmetric closure | Leave open | Midline closure | Minimal excision | All | |
|---|---|---|---|---|---|---|
| Complication | (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| During follow-up | Any complication | 135/272 (50%) | 26/49 (53%) | 46/76 (61%) | 94/270 (35%) | 301/667 (45%) |
| Bleeding | 49/272 (18%) | 14/49 (29%) | 16/76 (21%) | 15/270 (6%) | 94/667 (14%) | |
| Dehiscence | 46/272 (17%) | 2/49 (4%) | 17/76 (22%) | 8/270 (3%) | 73/667 (11%) | |
| Discharge | 44/272 (16%) | 12/49 (24%) | 17/76 (22%) | 46/270 (17%) | 119/667 (18%) | |
| Seroma | 11/272 (4%) | 0/49 (0%) | 5/76 (7%) | 3/270 (1%) | 19/667 (3%) | |
| Infection | 83/272 (31%) | 15/49 (31%) | 26/76 (34%) | 51/270 (19%) | 175/667 (26%) | |
| Day 1 | Any complication | 17/254 (7%) | 8/44 (18%) | 10/69 (14%) | 7/242 (3%) | 42/609 (7%) |
| Bleeding | 8/254 (3%) | 5/44 (11%) | 5/69 (7%) | 3/242 (1%) | 21/609 (3%) | |
| Dehiscence | 1/254 (0%) | 0/44 (0%) | 1/69 (1%) | 0/242 (0%) | 2/609 (0%) | |
| Discharge | 3/254 (1%) | 3/44 (7%) | 1/69 (1%) | 4/242 (2%) | 11/609 (2%) | |
| Seroma | 1/254 (0%) | 0/44 (0%) | 2/69 (3%) | 0/242 (0%) | 3/609 (0%) | |
| Infection | 3/254 (1%) | 0/44 (0%) | 0/69 (0%) | 0/242 (0%) | 3/609 (0%) | |
| Day 7 | Any complication | 47/248 (19%) | 9/42 (21%) | 21/67 (31%) | 30/220 (14%) | 107/577 (19%) |
| Bleeding | 15/248 (6%) | 4/42 (10%) | 5/67 (7%) | 4/220 (2%) | 28/577 (5%) | |
| Dehiscence | 9/248 (4%) | 0/42 (0%) | 4/67 (6%) | 2/220 (1%) | 15/577 (3%) | |
| Discharge | 10/248 (4%) | 2/42 (5%) | 5/67 (7%) | 11/220 (5%) | 28/577 (5%) | |
| Seroma | 0/248 (0%) | 0/42 (0%) | 2/67 (3%) | 0/220 (0%) | 2/577 (0%) | |
| Infection | 20/248 (8%) | 4/42 (10%) | 9/67 (13%) | 16/220 (7%) | 49/577 (8%) | |
| Clinic visit | Any complication | 100/221 (45%) | 12/36 (33%) | 30/54 (56%) | 48/202 (24%) | 190/513 (37%) |
| Bleeding | 29/221 (13%) | 3/36 (8%) | 12/54 (22%) | 6/202 (3%) | 50/513 (10%) | |
| Dehiscence | 35/221 (16%) | 1/36 (3%) | 11/54 (20%) | 3/202 (1%) | 50/513 (10%) | |
| Discharge | 27/221 (12%) | 6/36 (17%) | 8/54 (15%) | 20/202 (10%) | 61/513 (12%) | |
| Seroma | 9/221 (4%) | 0/36 (0%) | 3/54 (6%) | 2/202 (1%) | 14/513 (3%) | |
| Infection | 61/221 (28%) | 10/36 (28%) | 15/54 (28%) | 26/202 (13%) | 112/513 (22%) | |
| 6-month visit | Any complication | 61/204 (30%) | 13/36 (36%) | 20/56 (36%) | 42/179 (23%) | 136/475 (29%) |
| Bleeding | 16/204 (8%) | 4/36 (11%) | 6/56 (11%) | 4/179 (2%) | 30/475 (6%) | |
| Dehiscence | 27/204 (13%) | 1/36 (3%) | 5/56 (9%) | 4/179 (2%) | 37/475 (8%) | |
| Discharge | 12/204 (6%) | 6/36 (17%) | 8/56 (14%) | 25/179 (14%) | 51/475 (11%) | |
| Seroma | 4/204 (2%) | 0/36 (0%) | 2/56 (4%) | 1/179 (1%) | 7/475 (1%) | |
| Infection | 26/204 (13%) | 4/36 (11%) | 7/56 (13%) | 16/179 (9%) | 53/475 (11%) |
| Characteristic | Asymmetric closure | Leave open | Midline closure | Minimal excision | All |
|---|---|---|---|---|---|
| (n = 272) | (n = 49) | (n = 76) | (n = 270) | (n = 667) | |
| Scar spread, N | 130 | 14 | 25 | 77 | 246 |
| None to near-invisible | 13 (10%) | 3 (21%) | 7 (28%) | 28 (36%) | 51 (21%) |
| Pencil-thin line | 40 (31%) | 0 (0%) | 5 (20%) | 20 (26%) | 65 (26%) |
| Mild spread, noticeable on close inspection | 49 (38%) | 6 (43%) | 6 (24%) | 18 (23%) | 79 (32%) |
| Moderate spread, obvious scarring | 24 (18%) | 4 (29%) | 5 (20%) | 11 (14%) | 44 (18%) |
| Severe spread | 4 (3%) | 1 (7%) | 2 (8%) | 0 (0%) | 7 (3%) |
| Participant satisfaction, N | 201 | 34 | 51 | 177 | 463 |
| Very satisfied | 113 (56%) | 18 (53%) | 21 (41%) | 89 (50%) | 241 (52%) |
| Satisfied | 61 (30%) | 9 (26%) | 18 (35%) | 54 (31%) | 142 (31%) |
| Neither satisfied nor dissatisfied | 15 (7%) | 0 (0%) | 6 (12%) | 19 (11%) | 40 (9%) |
| Dissatisfied | 3 (1%) | 6 (18%) | 6 (12%) | 9 (5%) | 24 (5%) |
| Very dissatisfied | 9 (4%) | 1 (3%) | 0 (0%) | 6 (3%) | 16 (3%) |
| Scar impression – desirable scar | 107/128 (84%) | 9/12 (75%) | 16/23 (70%) | 67/78 (86%) | 199/241 (83%) |
| Scar itch (in past 24 hours) | 71/195 (36%) | 9/25 (36%) | 14/39 (36%) | 32/153 (21%) | 126/412 (31%) |
| Scar pain (in past 24 hours) | 67/195 (34%) | 8/25 (32%) | 21/38 (55%) | 24/154 (16%) | 120/412 (29%) |
| Returned to normal activities | 195/260 (75.0%) | 27/44 (61.4%) | 48/69 (69.6%) | 211/241 (87.6%) | 481/614 (78.3%) |
| Wound healed | 176/243 (72.4%) | 23/39 (59.0%) | 44/64 (68.8%) | 167/224 (74.6%) | 410/570 (71.9%) |
| Any complication during follow-up | 135/265 (51%) | 26/46 (57%) | 46/74 (62%) | 94/258 (36%) | 301/643 (47%) |
| Any reported recurrence | 55/257 (21%) | 13/46 (28%) | 18/70 (26%) | 87/256 (34%) | 173/629 (28%) |
| Recurrence within 6 months | 28/226 (12%) | 10/44 (23%) | 13/67 (19%) | 61/229 (27%) | 112/566 (20%) |
| Treatment failureb | 109/257 (42%) | 23/46 (50%) | 37/70 (53%) | 121/257 (47%) | 290/630 (46%) |
| Recurrence apparent from AE report | 12 | 3 | 2 | 16 | 33 |
| Model | Major procedure | Minor procedure | N | Mean difference (95% CI)a | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Pain (day 1) | ||||||
| Raw difference | 364 | 4.22 (2.53) | 242 | 2.60 (2.24) | 606 | 1.62 (1.23 to 2.02) |
| Risk-adjusted – Wysocki | 601 | 1.64 (1.24 to 2.05) | ||||
| Risk-adjusted – chosen model (sex, smoking, Wysocki) | 544 | 1.56 (1.14 to 1.98) | ||||
| Risk-adjusted – full model | 404 | 1.70 (1.20 to 2.20) | ||||
| Propensity-adjusted – IPW | 591 | 1.54 (1.08 to 2.00) | ||||
| Propensity matching | 591 | 1.64 (1.17 to 2.10) | ||||
| Augmented IPW | 536 | 1.58 (1.14 to 2.01) | ||||
| Pain (day 7) | ||||||
| Raw difference | 353 | 3.44 (2.50) | 221 | 1.86 (2.18) | 574 | 1.58 (1.18 to 1.98) |
| Risk-adjusted – Wysocki | 569 | 1.56 (1.15 to 1.97) | ||||
| Risk-adjusted – chosen model (lateral distribution, sex, Wysocki) | 514 | 1.45 (1.03 to 1.87) | ||||
| Risk-adjusted – full model | 382 | 1.47 (0.98 to 1.95) | ||||
| Propensity-adjusted –IPW | 559 | 1.57 (1.14 to 2.00) | ||||
| Propensity matching | 559 | 1.65 (1.23 to 2.07) | ||||
| Augmented IPW | 512 | 1.53 (1.12 to 1.95) | ||||
| N per surgeon with outcome data | Recurrence | Recurrence within 6 months | Treatment failure |
|---|---|---|---|
| ≥ 9 | ≥ 7 | ≥ 9 | |
| Recurrence, % | |||
| Min, max | 0, 61 | 0, 55 | 18, 78 |
| Median (IQR) | 25 (18–31) | 17 (0–27) | 39 (34–53) |
| Recurrence within 6 months (N = 566) | Any reported recurrence (N = 629) | Treatment failure (N = 630) | |
| Recurrence among surgeons with ≥ 10 procedures [n (%)] | 45 (40.2%) | 73 (42.2%) | 116 (40.0%) |
| Range among 13 surgeons | 0–55% | 0–61% | 18–78% |
| Recurrence among surgeons with < 10 procedures [n (%)] | 67 (59.8%) | 100 (57.8%) | 174 (60.0%) |
| Participant information | Decision regret | |||||
|---|---|---|---|---|---|---|
| ID | Excision | Closure | Time to healing (days) | Pain/post-surgery complications | Score | Sample quote (coding) |
| 16 | Local excision | Lateral closure and Karydakis | 62 | 0 | 0 | ‘Everything was great from that first consultation at the doctors to all the way through my recovery. So yeah, I’ve not really got anything to change about it.’ (CODE: consolidation) |
| 19 | Local excision | Primary midline closure, marsupialisation and lateral closure | 28 | 0 | 0 | ‘I would’ve done it much earlier. As I say, I waited a very long time, probably 12, 13 years, possibly more!’ (CODE: consolidation) |
| 21 | Local excision | Midline closure | 78 | 0 | 0 | Follow-up interview not complete |
| 7 | Curettage and pit picking | FG | 51 | 0 | 0 | Follow-up interview not complete |
| 17 | Local excision | Lateral closure and Karydakis | 54 | 0 | 0 | ‘I think the first surgery was so quick that I wasn’t really able to almost consider what I was getting done … I didn’t have any time to think about what was happening so it meant afterwards I didn’t really take it seriously enough’ (Intervention coherence) |
| 5 | Local excision | Lateral closure | 60 | 0 | 5 | ‘As I say it all, all went well. You know there’s, there’s no reason for me to want to do anything differently’ (Acceptability: perceived effectiveness; CODE: consolidation) |
| 9 | Local excision | Lateral closure and Karydakis | Length of time not specified | 0 | 5 | ‘Tried to get it [treatment] sooner’ (CODE: consolidation) |
| 10 | Seton (no excision) | 38 | 0 | 5 | ‘[So is there anything that you would have done differently?] No’ (Acceptability: perceived effectiveness/ethicality; CODE: consolidation) | |
| 11 | Curettage | No closure/leave open | 112 | 0 | 5 | ‘I think surgery was the way to go. I don’t think I could have done it differently.’ (Acceptability: perceived effectiveness; CODE: consolidation) |
| 1 | Local excision | Midline closure | Not healed | 2 | 10 | ‘The end result has been a positive one … I think that I would’ve rather had been in a position in which the wound had just been left open to be packed … that would’ve actually caused less pain and discomfort overall as well as avoiding the need to sort of visit the hospital for a follow-up’ (Acceptability: perceived effectiveness/opportunity costs) |
| 3 | Pit picking | No closure/leave open | 84 | 1 | 10 | [Is there anything that you would’ve done differently?] Not really because … it’s not a condition that you have knowledge of … if you have tingling in your left hand and you have shortness of breath, you know you’re having a heart attack … whereas this is not something you have any knowledge of so (mm) I suppose … you sort of do learn on the job with this sort of condition because it’s not that common.’ (CODE: consolidation) |
| 14 | EPSiT | No closure/leave open | Not healed | 3/Discharge | 15 | ‘The only thing I could have done is … asked for a different doctor, or…. said it was more urgent, so I could have been got in sooner … I’m pretty convinced that months of waiting around, and getting worse and splitting open my skin is the first problem with why it hasn’t healed as well as …’ (CODE: consolidation) |
| 8 | Local excision | No closure/leave open | 49 | 0 | 20 | ‘… I did everything like as soon as I could like’ (CODE: consolidation) |
| 6 | Local excision | No closure/leave open | Not healed | 5/Discharge and infection | 40 | ‘I don’t know what I would do differently but I think the, that is what I did differently to change going from [hospital name] to [hospital name].’ (self-efficacy) |
| 18 | Pit picking | Pit picking – closed and lateral wound – left open | 18 | 0 | 50 | ‘I’m glad I waited for the right person and the right procedure.’ (CODE: consolidation) |
| 2 | Local excision | Lateral closure and Karydakis | LTFU | LTFU | LTFU | Follow-up interview not complete |
| 12 | Curettage | FG | 14 | LTFU | LTFU | Follow-up interview not complete |
| 13 | Curettage | FG | LTFU | LTFU | LTFU | Follow-up interview not complete |
| 15 | Local excision | Seton and flap (type: fascial) | 8 | LTFU | LTFU | Follow-up interview not complete |
| 20 | Local excision | Flap (type: rhomboid) | LTFU | LTFU | LTFU | Follow-up interview not complete |
| Attributes | Model 1: all attributes categorical | Model 2: risk attribute linear |
|---|---|---|
| Coefficient (SE) | Coefficient (SE) | |
| Constant | 0.356*** (0.068) |
0.368*** (0.067) |
| Recovery time | ||
| Week = 12 (reference level) | 0.000 (.) |
0.000 (.) |
| Week = 1 | 1.583*** (0.155) |
1.556*** (0.151) |
| Week = 2 | 2.054*** (0.154) |
2.035*** (0.152) |
| Week = 6 | 1.256*** (0.109) |
1.250*** (0.109) |
| Risk of infection/persistence | ||
| Risk (%) = 30 (reference level) | 0.000 (.) |
– |
| Risk (%) = 20 | 1.173*** (0.113) |
– |
| Risk (%) = 10 | 2.217*** (0.145) |
– |
| Risk (%) = 5 | 3.042*** (0.160) |
– |
| Risk of infection/persistence as a linear variable | ||
| Risk (%) | - | −0.119*** (0.006) |
| Observations | 3552 | 3552 |
| Log-likelihood | −768.95 | −771.78 |
| BIC | 1605.24 | 1588.45 |
| Attribute importance score: a relative measure of the impact that an attribute has on a respondent’s choices within the DCE exercise. | ||
| Risk of infection/persistence | 70.10% | |
| Recovery time | 29.90% | |
| Statement | Round 1 | Round 2 | Round 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Policy statement | Clinicians (%) | Patients (%) | Both (%) | Clinicians (%) | Patients (%) | Both (%) | Clinicians (%) | Patients (%) | Both (%) |
| Any treatment of pilonidal disease should aim to be less disruptive than the disease itself. | 82.5 | 80.0 | 81.8 | To consensus | |||||
| Surgeons should have access to opportunities to learn new techniques for the treatment of PSD. | 97.5 | 73.3 | 90.9 | To consensus | |||||
| Lay open is associated with slow healing and delayed return to normal activities. It should rarely be considered as the first treatment option. | 60.0 | 60.0 | 60.0 | 60.5 | 76.9 | 64.7 | 62.2 | 78.6 | 66.7 |
| Minimally invasive techniques should be considered as the first-line intervention, as these are associated with low operative morbidity and comparable recurrence and healing rates to more extensive interventions. | 65 | 86.7 | 70.9 | 68.4 | 84.6 | 72.5 | To consensus | ||
| There is a need for a standard classification system/tool for PSD. | 82.5 | 53.3 | 74.5 | 81.6 | 84.6 | 82.4 | To consensus | ||
| Any classification tool should be easy to use. | 92.5 | 46.7 | 80.0 | To consensus | |||||
| A classification tool for pilonidal sinus should help to inform treatment options. | 82.5 | 66.7 | 78.2 | 76.3 | 92.3 | 80.4 | To consensus | ||
| Patients should be counselled about the risk of recurrence. | 97.5 | 80.0 | 92.7 | To consensus | |||||
| Patients should be counselled about the impact of treatments on return to normal activities. | 95 | 80.0 | 90.9 | To consensus | |||||
| Patients may wish for symptomatic improvement rather than cure, and this should be explored in early discussions. | 80.0 | 53.3 | 72.7 | 84.2 | 69.2 | 80.4 | To consensus | ||
| Clinicians and researchers need to clearly define failure of healing vs. recurrence as the two may present similarly. | 57.5 | 80.0 | 63.6 | 73.7 | 92.3 | 78.4 | To consensus | ||
| Delayed return to work is an important outcome following treatment. | 90 | 73.3 | 85.5 | To consensus | |||||
| A tool is needed to measure the impact of treatments/disease on QoL (e.g. a disease-specific PRO measure). | 82.5 | 60.0 | 76.4 | 84.2 | 69.2 | 80.4 | To consensus | ||
| We need to determine how long we should wait before deciding wound healing is delayed or failed. | 45 | 60.0 | 49.1 | 60.5 | 76.9 | 64.7 | 62.3 | 71.4 | 64.7 |
| Follow-up should continue until there is evidence of complete wound healing. | 60.5 | 92.3 | 68.6 | To consensus | |||||
| Patients with symptomatic pilonidal disease always require a secondary care referral. | 55.3 | 69.2 | 58.8 | 59.5 | 57.1 | 58.8 | |||
| Novel minimally invasive procedures (e.g. laser) should be thoroughly appraised in randomised trials before general adoption. | 73.7 | 53.8 | 70.6 | To consensus | |||||
| Imaging is rarely useful in pilonidal disease. | 42.1 | 30.8 | 39.2 | 32.4 | 42.9 | 35.3 | |||
| SDM should be employed when discussing treatment options. | 86.8 | 92.3 | 88.2 | To consensus | |||||
| Statement | Round 1 | Round 2 | Round 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Research statement | Clinicians (%) | Patients (%) | Both (%) | Clinicians (%) | Patients (%) | Both (%) | Clinicians (%) | Patients (%) | Both (%) |
| A future randomised trial (RCT) in the treatment of pilonidal sinus should compare widely used techniques. | 90 | 53.3 | 80.0 | To consensus | |||||
| Postsurgical care (e.g. wound care, follow-up etc.) is an important part of treatment strategy. Further work is required to establish the optimum way to deliver this. | 87.5 | 73.3 | 83.6 | To consensus | |||||
| Future research should aim to define an algorithm or decision tree to aid surgeon decision-making. | 77.5 | 80.0 | 78.2 | To consensus | |||||
| A future randomised trial (RCT) should include two broad groups of interventions – major (i.e. asymmetric closure, leave open and midline closure) vs. minor (i.e. minimal excision). | 67.5 | 60.0 | 65.5 | 71.1 | 92.3 | 76.5 | To consensus | ||
| A decision aid targeted at patients to help understand treatment options might improve patient satisfaction with treatment. | 80.0 | 80.0 | 80.0 | To consensus | |||||
| Classification should include an assessment of symptoms. | 82.5 | 60.0 | 76.4 | 89.5 | 92.3 | 90.2 | To consensus | ||
| Classification systems should include data related to hair type and distribution. | 32.5 | 40.0 | 34.5 | 50.0 | 76.9 | 56.9 | 51.4 | 57.1 | 52.9 |
| Classification systems should include data on recurrent skin infections in non-pilonidal areas. | 45.0 | 66.7 | 50.9 | 44.7 | 30.8 | 41.2 | 54.1 | 42.9 | 51.0 |
| Classification systems should include data on extent of disease beyond the natal cleft. | 85.0 | 46.7 | 74.5 | 81.6 | 46.2 | 72.5 | To consensus | ||
| Consistency in reporting patient and disease factors would help us better understand what characteristics are associated with good or bad outcomes. | 85.0 | 66.7 | 80.0 | 84.2 | 92.3 | 86.3 | To consensus | ||
| A core outcome set for pilonidal disease might help us understand what outcomes are important to clinicians and patients following treatment of pilonidal disease. It may also improve future evaluations of treatments. | 95.0 | 53.3 | 83.6 | To consensus | |||||
| There is a need for a PRO to be used in future pilonidal sinus research. | 90.0 | 86.7 | 89.1 | To consensus | |||||
| Future research should explore whether hair removal reduces the risk of wound complications or recurrence of pilonidal disease. | 90.0 | 86.7 | 89.1 | To consensus | |||||
| Future research should explore whether weight loss reduces the risk of wound complications or recurrence of pilonidal disease. | 52.5 | 53.3 | 52.7 | 57.9 | 53.8 | 56.9 | 56.8 | 42.9 | 52.9 |
| Future research should explore whether smoking behaviours reduce the risk of wound complications and/or recurrence of pilonidal disease. | 92.5 | 46.7 | 80.0 | To consensus | |||||
| Future research should assess the role of postoperative antibiotic treatment in wound healing and/or recurrence. | 70.0 | 66.7 | 69.1 | 60.5 | 92.3 | 68.6 | To consensus | ||
| Future research should explore the role wound dressings play in wound healing and/or recurrence. | 75 | 60.0 | 70.9 | 60.5 | 76.9 | 64.7 | 73.0 | 78.6 | 74.5 |
| A future randomised trial (RCT) should compare procedures in mild or minimal disease where the wound is left open (e.g. pit picking and EPSiT) vs. closure of the wound (e.g. glue). | 75 | 80.0 | 76.4 | To consensus | |||||
| A future randomised trial (RCT) should compare non-excisional therapies. | 77.5 | 60.0 | 72.7 | 81.6 | 76.9 | 80.4 | To consensus | ||
| Future research should explore the role of patient characteristics including genetics and microbiome on the pilonidal disease process. | 34.2 | 76.9 | 45.1 | 48.6 | 50.0 | 49.0 | |||
| Wide excision and leave open procedures should not be included in any future trial. | 44.7 | 38.5 | 43.1 | 45.9 | 42.9 | 45.1 | |||
| Future research should compare major procedures (e.g. flaps) against minor procedures (e.g. pit picking, glue) stratified by disease severity. | 76.3 | 92.3 | 80.4 | To consensus | |||||
| Characteristic | Measure | Attended 6-month follow-up | Did not attend 6-month follow-up |
|---|---|---|---|
| (n = 476) | (n = 191) | ||
| Age | N (%) | 476 (100%) | 191 (100%) |
| Median (IQR) | 27.0 (22.0–31.5) | 28.0 (23.0–35.0) | |
| Sex | Male | 338 (71%) | 147 (77%) |
| Female | 138 (29%) | 44 (23%) | |
| Ethnicity | White | 406 (85%) | 164 (86%) |
| Asian/Asian British | 45 (9%) | 13 (7%) | |
| Mixed/multiple ethnic groups | 12 (3%) | 1 (1%) | |
| Black/African/Caribbean/Black British | 4 (1%) | 4 (2%) | |
| Other ethnic group | 3 (1%) | 4 (2%) | |
| Prefer not to say | 4 (1%) | 1 (1%) | |
| BMI (kg/m2) | N (%) | 443 (93%) | 169 (88%) |
| Median (IQR) | 28.4 (24.9–32.8) | 28.1 (25.1–31.9) | |
| Number of baths and/or showers in a typical week | N (%) | 462 (97%) | 182 (95%) |
| Median (IQR) | 7.0 (6.0–7.0) | 7.0 (6.0–7.0) | |
| Seated for more than 6 hours in a working day | No | 224 (47%) | 94 (49%) |
| Yes | 241 (51%) | 91 (48%) | |
| First-degree relatives with history of PSD | No | 386 (81%) | 156 (82%) |
| Yes | 88 (18%) | 34 (18%) | |
| Smoking status | Non-smoker | 283 (59%) | 91 (48%) |
| Current smoker | 123 (26%) | 73 (38%) | |
| Current e-cigarette smoker | 26 (5%) | 11 (6%) | |
| Number of pits | N (%) | 461 (97%) | 179 (94%) |
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | |
| Length of pits (spread) | N (%) | 296 (62%) | 110 (58%) |
| Median (IQR) | 21.0 (10.0–40.0) | 25.0 (11.0–45.0) | |
| Number of previous procedures | 0 | 255 (54%) | 102 (53%) |
| 1 | 124 (26%) | 50 (26%) | |
| 2 | 54 (11%) | 24 (13%) | |
| 3 or more | 43 (9%) | 15 (8%) | |
| Previous procedure | Elective procedure for PSD | 98 (21%) | 50 (26%) |
| Acute drainage for PSD | 146 (31%) | 49 (26%) | |
| Emergency procedure for PSD | 3 (1%) | 2 (1%) | |
| Wysocki classification | Type 1 | 127 (27%) | 55 (29%) |
| Type 2 | 240 (50%) | 84 (44%) | |
| Type 3 | 38 (8%) | 12 (6%) | |
| Type 4 | 64 (13%) | 37 (19%) | |
| None of the above | 2 (0%) | 2 (1%) | |
| Distribution of lateral openings | No lateral openings | 203 (43%) | 92 (48%) |
| Unilateral | 219 (46%) | 68 (36%) | |
| Bilateral | 13 (3%) | 7 (4%) |
| Model | Asymmetric closure | Minimal excision | N | Mean difference (95% CI)a | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Pain (day 1) | ||||||
| Raw difference | 253 | 4.55 (2.37) | 242 | 2.60 (2.24) | 495 | 1.95 (1.54 to 2.36) |
| Risk-adjusted – Wysocki | 493 | 1.96 (1.54 to 2.38) | ||||
| Risk-adjusted – chosen model (sex, smoking, Wysocki) | 444 | 1.91 (1.46 to 2.35) | ||||
| Risk-adjusted – full model | 339 | 1.98 (1.46 to 2.51) | ||||
| Propensity-adjusted – IPW | 490 | 1.88 (1.38 to 2.38) | ||||
| Propensity matching | 490 | 1.99 (1.49 to 2.49) | ||||
| Augmented IPW | 441 | 1.97 (1.50 to 2.43) | ||||
| Pain (day 7) | ||||||
| Raw difference | 246 | 3.26 (2.35) | 221 | 1.86 (2.18) | 467 | 1.40 (0.99 to 1.81) |
| Risk-adjusted – Wysocki | 465 | 1.35 (0.92 to 1.78) | ||||
| Risk-adjusted – chosen model (lateral distribution, sex, Wysocki) | 437 | 1.22 (0.78 to 1.66) | ||||
| Risk-adjusted – full model | 321 | 1.21 (0.69 to 1.72) | ||||
| Propensity-adjusted – IPW | 462 | 1.39 (0.93 to 1.85) | ||||
| Propensity matching | 462 | 1.45 (1.00 to 1.91) | ||||
| Augmented IPW | 436 | 1.33 (0.89 to 1.76) | ||||
| Complications | Asymmetric closure | Minimal excision | n | Risk difference (95% CI)a |
|---|---|---|---|---|
| Raw difference | 135/265 (51%) | 94/258 (36%) | 523 | 14.5 (6.1 to 22.9) |
| Risk-adjusted – Wysocki | 521 | 13.7 (5.0 to 22.4) | ||
| Risk-adjusted – chosen model (BMI, Wysocki) | 476 | 14.4 (5.2 to 23.5) | ||
| Risk-adjusted – full model | 354 | 14.0 (3.5 to 24.5) | ||
| Propensity-adjusted – IPW | 518 | 13.9 (4.8 to 23.1) | ||
| Propensity matching | 518 | 12.8 (3.1 to 22.6) | ||
| Augmented IPW | 473 | 15.0 (5.8 to 24.3) |
| Recurrence | Recurrence | Recurrence (within 6 months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Asymmetric closure | Minimal excision | n | Risk difference (95% CI)a | Asymmetric closure | Minimal excision | n | Risk difference (95% CI)a | |
| Raw difference | 55/257 (21%) | 87/256 (34%) | 513 | −12.6 (−20.3 to −4.9) | 28/226 (12%) | 61/229 (27%) | 455 | −14.2 (−21.4 to −7.1) |
| Risk-adjusted – Wysocki | 511 | −13.1 (−21.0 to −5.2) | 453 | −14.5 (−22.0 to −7.1) | ||||
| Risk-adjusted – Chosen model (Wysocki, pit density) | 484 | −11.5 (−19.7 to −3.4) | 428 | −12.8 (−20.5 to −5.1) | ||||
| Risk-adjusted – full model | 343 | −10.1 (−20.0 to −0.2) | 304 | −9.0 (−18.5 to 0.5) | ||||
| Propensity-adjusted – inverse weighting | 508 | −16.2 (−25.1 to −7.3) | 450 | −15.7 (−24.1 to −7.2) | ||||
| Propensity matching | 508 | −13.4 (−22.6 to −4.2) | 450 | −15.2 (−24.2 to −6.2) | ||||
| Augmented IPW | 483 | −11.9 (−20.5 to −3.2) | 427 | −12.3 (−20.6 to −4.0) | ||||
| Recurrence | Asymmetric closure | Minimal excision | n | Risk difference (95% CI)a |
|---|---|---|---|---|
| Raw difference | 109/257 (42%) | 121/257 (47%) | 514 | −4.7 (−13.3 to 3.9) |
| Risk-adjusted – Wysocki | 512 | −5.4 (−14.3 to 3.4) | ||
| Risk-adjusted – chosen model (Wysocki, pit density) | 485 | −5.7 (−14.8 to 3.4) | ||
| Risk-adjusted – full model | 344 | −3.7 (−14.5 to 7.1) | ||
| Propensity-adjusted – inverse weighting | 509 | −8.4 (−18.0 to 1.2) | ||
| Propensity matching | 509 | −5.8 (−15.7 to 4.2) | ||
| Augmented IPW | 484 | −4.8 (−14.1 to 4.6) |
| Model | Asymmetric closure | Minor procedure | N | Difference | ||
|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | Mean difference (95% CI) | ||
| Raw difference | 255 | 30 (14–60) | 241 | 7 (4–21) | 496 | 18.3 (13.6 to 23.1) |
| Risk-adjusted – Wysocki | 492 | 17.7 (12.8 to 22.5) | ||||
| Risk-adjusted – chosen model (Wysocki, lateral distance, natal cleft depth | 431 | 16.6 (11.7 to 21.5) | ||||
| Risk-adjusted – full model | 340 | 15.5 (10.1 to 20.8) | ||||
| Propensity-adjusted – IPW | 489 | 25.8 (16.6 to 35.0) | ||||
| Augmented IPW | (Not estimable) | |||||
| Model | Asymmetric closure | Minor procedure | N | Difference | ||
|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | Mean difference (95% CI) | ||
| Raw difference | 239 | 57 (30–134) | 217 | 30 (14–154) | 456 | 31.3 (18.2 to 44.3) |
| Risk-adjusted – Wysocki | 452 | 28.1 (14.6 to 41.5) | ||||
| Risk-adjusted – chosen model (Wysocki, BMI, smoking status, pus) | 371 | 26.3 (10.9 to 41.7) | ||||
| Risk-adjusted – full model | 311 | 26.6 (10.9 to 42.3) | ||||
| Propensity-adjusted – IPW | 449 | 30.1 (14.5 to 45.7) | ||||
| Augmented IPW | 371 | 21.2 (3.1 to 39.3) | ||||
FIGURE 14.
CollaboRATE score at baseline by 6-month DR score.

FIGURE 15.
Gwet AC1 chance-corrected agreement between assessment and original.

FIGURE 16.
Preferred treatment by surgeon and actual treatment within the study.

FIGURE 17.
Time to return to normal activities between asymmetric closure and minimal excision.

FIGURE 18.
Time to wound healing between asymmetric closure and minimal excision.
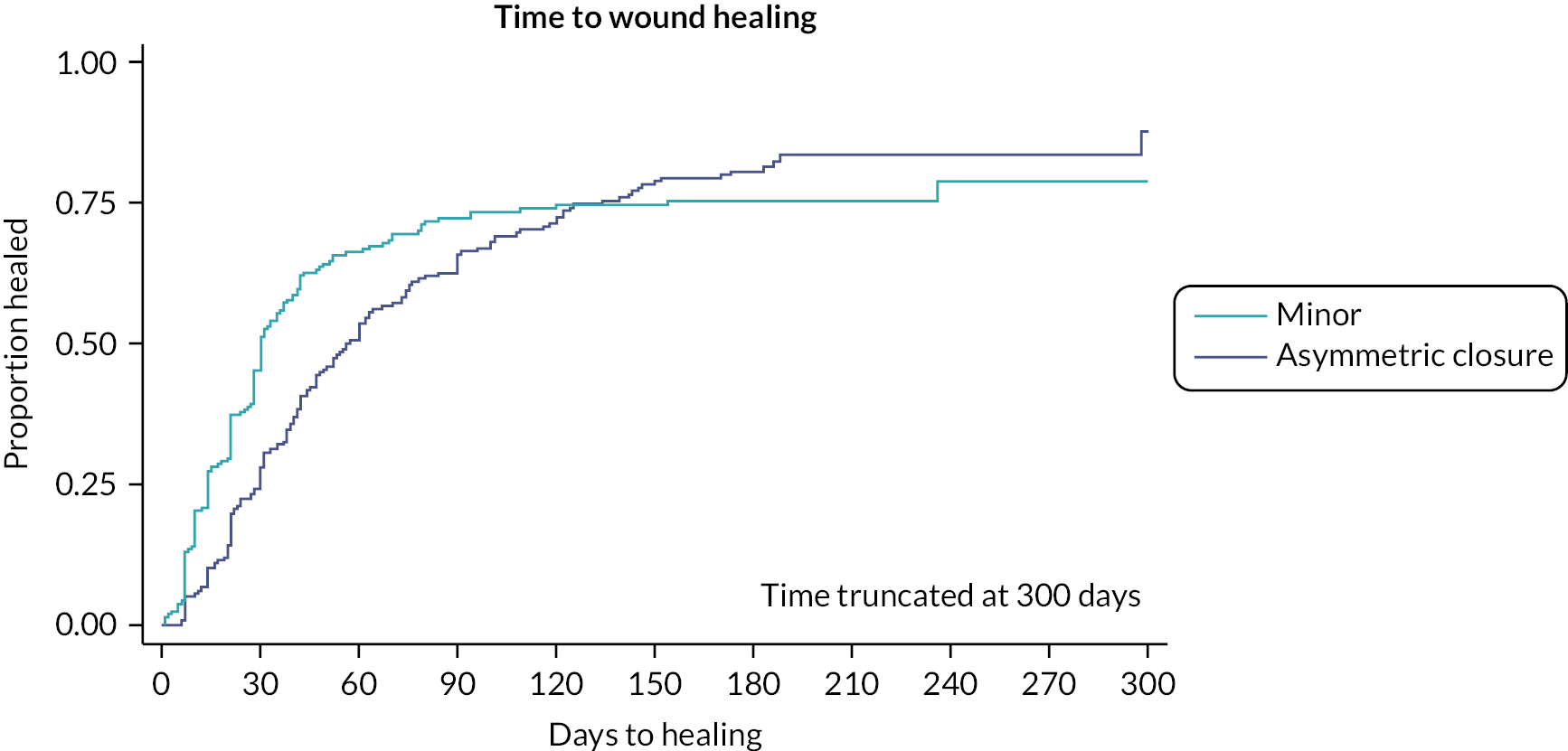
FIGURE 19.
Funnel plot of minor procedure use by site.

List of abbreviations
- ADL
- activities of daily living
- AE
- adverse event
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- BMI
- body mass index
- CAIC
- consistent Akaike information criterion
- CI
- confidence interval
- CODE
- coping in deliberation
- CTRU
- Clinical Trials Research Unit
- CWIQ
- Cardiff Wound Impact Questionnaire
- DCE
- discrete choice experiment
- DR
- decision regret
- EPSiT
- endoscopic pilonidal sinus treatment
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FG
- fibrin glue
- GP
- general practitioner
- HES
- hospital episode statistics
- IPW
- inverse probability weighting
- IQR
- interquartile range
- LTFU
- lost to follow-up
- MAR
- maximum acceptable risk
- PI
- principal investigator
- PITSTOP
- PIlonidal sinus Treatment - STudying the OPtions
- PPI
- patient and public involvement
- PREM
- patient-reported experience measure
- PRO
- patient-reported outcome
- PROM
- patient-reported outcome measure
- PSD
- pilonidal sinus disease
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SDM
- shared decision-making
