Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 17/135/02. The contractual start date was in May 2019. The draft manuscript began editorial review in January 2023 and was accepted for publication in May 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Gurusamy et al. This work was produced by Gurusamy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Gurusamy et al.
Chapter 1 Background and rationale
Sections of this chapter have been reproduced from Gurusamy et al. ,1 under licence CC-BY-4.0.
What is the problem being addressed?
Approximately 7 million people worldwide and 160,000 people in the UK develop colorectal, ovarian or gastric cancer each year,2 of whom 8–50% develop peritoneal metastases. The peritoneum is one of the commonest sites of metastases in these cancers3–9 and is often the only site of metastases. 8–10 In general, people with peritoneal metastases have poorer prognosis than those with other sites of metastases (liver or lung),11 with median reported survival ranging from 6 to 24 months, depending on the primary cancer and treatment received. 12–14
Treatment of peritoneal metastases from colorectal, ovarian or gastric cancer
The current standard of care (SoC) for people with peritoneal metastases from these cancers is systemic chemotherapy, either alone or in combination with either cytoreductive surgery (CRS) or palliative surgery. 8,9,13–16 Hyperthermic intraoperative peritoneal chemotherapy (HIPEC) + CRS ± systemic chemotherapy is an alternative treatment for these patients. The main principle of HIPEC + CRS is to remove all visible (macroscopic) peritoneal metastases, followed by HIPEC to treat any remaining microscopic peritoneal metastases. 17 HIPEC involves peritoneal circulation of chemotherapy drugs (usually mitomycin C, 5-Fluorouracil and oxaliplatin or cisplatin)18 heated to temperatures of 42 °C, at which the chemotherapy drugs are potentiated. 19 Until only a decade ago, < 5% of patients with peritoneal metastases underwent HIPEC + CRS; however, this has progressively increased to about 10% of patients by 2012. 9,10,15 HIPEC + CRS has been commissioned by the NHS England for patients with peritoneal metastases from appendiceal tumours and colorectal adenocarcinoma.
Why is this research important to patients and health and care services?
Although HIPEC + CRS has the potential to improve survival and health-related quality of life (HRQoL) in people with peritoneal metastases,15,20,21 there have been concerns raised about its safety. Reports have shown a 30-day mortality after HIPEC + CRS of 1–3%7 and a major complication rate of 32%,7,22 albeit that it might be possible to achieve 30-day mortality of < 1% and major complication rate around 10–15% in high-volume centres. The average cost of HIPEC + CRS per patient varies from about USD 20,000 to 80,000. 23–29 Because of these reasons, this research is important to address the significant uncertainty about the benefits of an intervention that carries significant risk of harm to patients and major costs to the NHS. Patients and the public were involved in the design, conduct and interpretation of data of this research as part of steering committee to ensure that this research remains relevant and considers the views of the patients. They are also involved in dissemination of the findings.
Review of existing evidence
There have been several overviews, systematic reviews and Health Technology Assessment (HTA) investigating this area. Prior to starting this research, 16 systematic reviews of comparative studies had been undertaken, comparing HIPEC + CRS to other treatment modalities in peritoneal metastases from colorectal, ovarian or gastric cancer. 7,18,21,30–42 Ten of these included at least one randomised controlled trial (RCT), but the conclusions were largely based on non-randomised studies. 7,18,21,30,32–34,36,40,42 Although most of these systematic reviews concluded that HIPEC + CRS can improve survival in people with peritoneal metastases, all had limitations and deficiencies. Firstly, all were at high risk of bias according to ROBIS (risk of bias in systematic reviews) tool,43 with concerns about bias across all domains. Secondly, the systematic reviews included only a single RCT14 and/or based their evidence predominantly on non-randomised studies, without any adjustment for baseline differences in disease-related or patient-related prognostic characteristics. 7,18,21,30,32–34,36,40,42 Finally, meta-analyses could only include a small proportion of the results from the studies because of the way these results had been reported (e.g. proportion survived vs. median survival). 18,21,30,36,38 Therefore, there is still considerable uncertainty about the benefits of HIPEC + CRS and which patient groups will benefit from it.
Prior to the start of this research, there had also been two formal HTAs on this issue. 27,44 The first HTA reviewing patients with peritoneal disease from colorectal cancer concluded that there was moderate-quality evidence that HIPEC + CRS prolonged survival based on a single RCT, but the costs were high. 27 The second HTA on ovarian cancer did not include any RCTs and concluded there was no clear benefit of HIPEC + CRS for ovarian peritoneal metastases. 44
Chapter 2 Aims and objectives
The overarching aim of this project is to answer the following research questions:
Does HIPEC + CRS improve survival and/or quality of life (QoL) compared to CRS ± systemic chemotherapy or systemic chemotherapy alone in people with peritoneal metastases (from colorectal, gastric or stage III or greater epithelial ovarian cancers) who can withstand major surgery, and is it cost-effective in the NHS setting?
Primary objectives
To compare the relative benefits and harms of HIPEC + CRS ± systemic chemotherapy versus CRS ± systemic chemotherapy or systemic chemotherapy alone in people with peritoneal metastases from colorectal, gastric or ovarian gastric cancers eligible to undergo HIPEC + CRS by a systematic review and meta-analysis.
Secondary objectives
To compare the cost-effectiveness of HIPEC + CRS ± systemic chemotherapy versus CRS ± systemic chemotherapy or systemic chemotherapy alone from an NHS and personal social services (PSS) perspective using a model-based cost–utility analysis.
Chapter 3 Systematic review methods
Eligibility criteria
Types of studies
All RCTs, regardless of the publication status, year of publication and language of publication, were included.
Setting
Secondary or tertiary care with expertise to perform HIPEC + CRS.
Types of participants
Inclusion criteria
People with synchronous or metachronous peritoneal metastases from colorectal cancer, gastric cancer or ovarian cancer eligible to undergo HIPEC + CRS regardless of the involvement of other organs and whether the primary cancer was resected completely [i.e. resected completely (R0 resection)].
Exclusion criteria
Studies on pseudomyxoma peritonei (PMP) were excluded.
Intervention
HIPEC + CRS ± systemic chemotherapy.
Control
CRS ± systemic chemotherapy or systemic chemotherapy alone.
Outcomes
Primary outcomes
Secondary outcomes
-
Time to disease progression: defined as time from randomisation to death in people who died of treatment or disease-related causes, time from randomisation to recurrence in people in whom complete CRS was achieved and time from randomisation to disease progression as defined by RECIST (Response Evaluation Criteria in Solid Tumors) criteria of 20% increase in size of the tumour or appearance of new lesions47 or similar criteria used by authors. This equates to recurrence-free survival or disease-free survival when complete CRS is achieved.
-
Non-serious adverse events or Clavien–Dindo grade I or II. 45,46
-
Patient-reported outcome measures.
Search strategy
Electronic searches
We searched MEDLINE, EMBASE, Cochrane library and the Science Citation Index for published trials, as well as ClinicalTrials.gov and WHO ICTRP trial registers for ongoing or unreported studies. The search strategies, which combine the Cochrane sensitivity maximising RCT filter48 with a combination of subject headings and free text terms relating to the interventions and diseases of interest, are provided in Appendix 1. Searches were updated periodically until 14 April 2022.
Other resources
We also searched the reference lists of all identified studies for additional studies eligible for inclusion and contacted experts in the field for further studies.
Data collection and management
Selection of studies
Two review authors independently screened the titles and abstracts of all records retrieved and made the final selection based on full text (after translation if required, i.e. there were no language restrictions). We documented the selection process to enable the completion of the preferred reporting items for systematic review and meta-analysis (PRISMA) flowchart. We resolved discrepancies through discussion.
Data collection
We collected the following data:
-
contact details of the study author and the study contact
-
information required to assess the risk of bias
-
patient demographics: age, gender, comorbidities, performance index
-
cancer details (including severity)
-
intervention details
-
control details
-
follow-up details
-
outcome data
-
resource utilisation data (to guide health economic analysis)
-
operating time
-
quantity of blood and blood products transfused
-
length of hospital stay (including readmissions)
-
length of intensive care unit stay
-
chemotherapy regimen used in HIPEC and in control group, if applicable
-
proportion in whom surgery was performed and the nature of surgery in the control group
-
additional surgery and other palliative treatments.
-
We were unable to perform an individual participant data (IPD) meta-analysis as planned because of unforeseen circumstances related to COVID-19. This led to trialists who were also clinical researchers being unable to engage for transfer of IPD. We did not foresee that study authors (surgeons) would be sufficiently engaged with providing IPD soon because of the backlog with surgeries and the fatigue induced by COVID-19. Therefore, we performed a meta-analysis based on aggregate data.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool version 2 to assess the risk of bias in RCTs. 49
Meta-analysis of clinical effectiveness
Measures of treatment effect and data synthesis
We used risk ratio (RR) for binary outcomes (proportion of people with serious adverse events), mean difference (MD) for continuous outcomes (HRQoL as only trial reported this information in analysable format), rate ratios for count outcomes (number of serious adverse events) and hazard ratio (HR) for time-to-event outcomes (overall all-cause mortality and time to progression) with their respective 95% confidence intervals (CIs).
When meta-analysis was possible (at least two studies having similar participants, intervention, control and outcomes), we performed a random-effects model meta-analysis using the DerSimonian and Laird method50 for binary outcomes and the inverse variance method for other types of outcomes.
Dealing with missing data
We performed an intention-to-treat analysis. 51 All the trials provided outcomes on participants randomised or at least on participants who were eligible for this study, that is, people with resectable peritoneal metastases. Therefore, there was no requirement for imputation of data.
Assessment and investigation of heterogeneity
We assessed the clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. Clinical heterogeneity could be due to the types of participants included in the studies (performance index, stage of cancer, extent of peritoneal involvement, other organ involvement), different interventions (complete CRS or not, chemotherapy agents used), whether complete CRS was achieved (if the control group was CRS) or different follow-up methods (routine imaging vs. clinical examination). Different study designs and risk of bias may contribute to methodological heterogeneity. When we performed the meta-analysis, we calculated and reported the between-trial standard deviation and I2 as measures of heterogeneity.
Because of the paucity of trials and lack of information from the trials on subgroup data from the reports or by contacting the trial authors, we did not perform subgroup analysis or metaregression to investigate the effect of potential effect modifiers.
Sensitivity analysis
We performed panoramic meta-analysis as post hoc sensitivity analysis. Panoramic meta-analysis may be appropriate when the same treatment comparisons have to be compared across a range of disease conditions. 52 We used the random-effects metaregression with the cancer type as the covariate. Further details about the model used and technical details are available in Appendix 2.
Reporting bias
We assessed reporting bias by the completeness of search.
Confidence in results
The uncertainty in results was evaluated using the GRADE methodology. 53
Chapter 4 Cost-effectiveness analysis methods
We followed the National Institute for Health and Care Excellence (NICE) methodological standards for conducting our cost-effectiveness analysis. 54
Model
We performed a model-based cost–utility analysis, estimating mean costs and quality-adjusted life-years (QALYs) per patient. We performed separate cost-effectiveness analysis for each of the treatment comparisons stratified by the type of cancer in the systematic review. The time horizon was lifetime time horizon. We calculated the costs from the NHS and PSS perspectives. We discounted the costs and utilities at the rate of 3.5% per annum. 54 We had chosen the discounted rate based on the guidance set by the UK government. 55
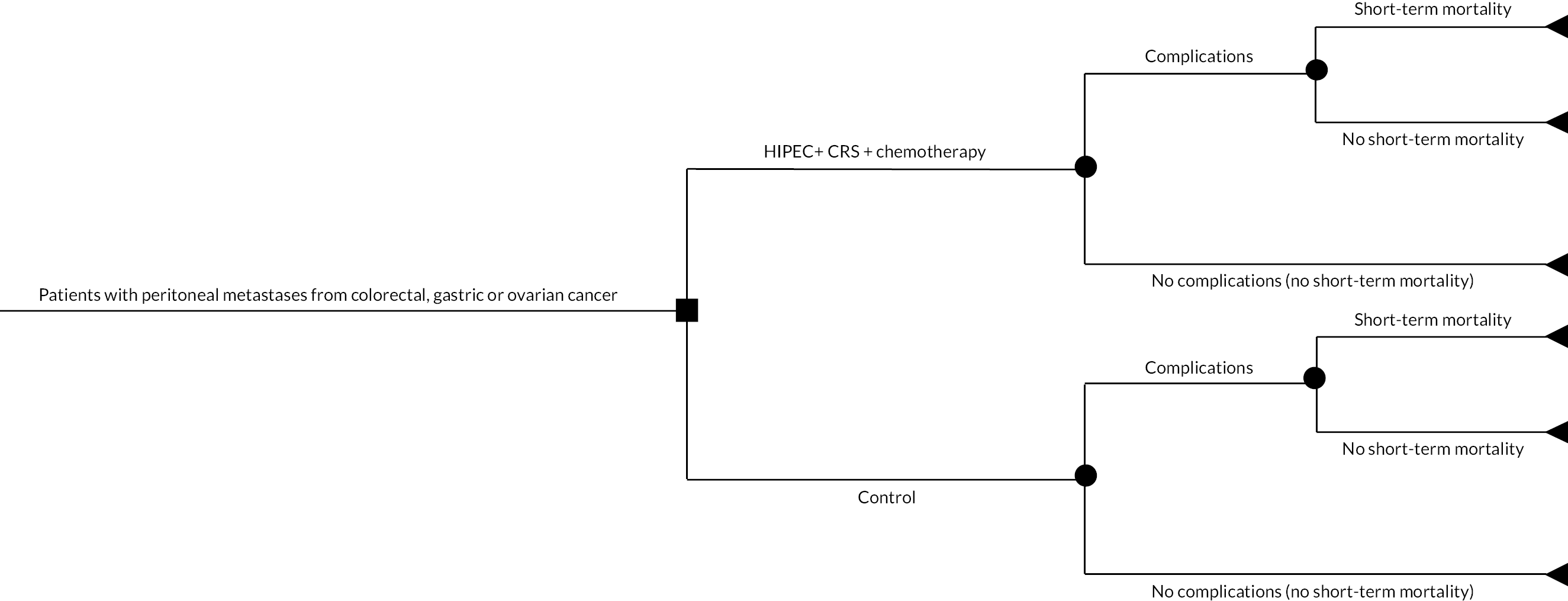
We created a decision tree model (one for each cancer) along the lines of the model that we used to compare two types of surgeries in pancreatic cancer56 and that we reported in the published protocol. 1 Briefly, a patient with peritoneal metastases from one of the three cancers (colorectal cancer, stage III or greater epithelial ovarian cancer or gastric cancer) and eligible for CRS + HIPEC can either undergo HIPEC + CRS + systemic chemotherapy or control (either CRS + systemic chemotherapy or systemic chemotherapy alone for colorectal cancer and gastric cancer and CRS + systemic chemotherapy for ovarian cancer). A proportion of patients in whom HIPEC + CRS developed complications, a proportion of whom might die within 30 days. Those who are alive at 30 days may die subsequently (a Markov model was used to model this). The decision tree pathways in the people who had control treatment were identical: some had complications, some died within 30 days and some died after 30 days.
When resource utilisation data were available from the systematic review, we used that information. For information not available from the systematic review, we performed literature searches of the NHS Economic Evaluation Database (NHS EED), the Health Economic Evaluations Database (HEED), MEDLINE and EMBASE (for MEDLINE and EMBASE, we combined the search strategy from Appendix 1 with a sensitivity maximising ‘economics’ filter developed as a part of The Hedges Project of the Health Information Research Unit of McMaster University). We also reviewed the cost-effectiveness analysis (CEA) registry at Tufts University for information on QoL. Currently, there is no Healthcare Resource Group (HRG) code available for HIPEC + CRS + systemic chemotherapy or control. We obtained resource utilisation data as part of the systematic review and converted these to costs on the basis of the NHS National Tariff, NHS National Schedule of Reference costs, British National Formulary and/or local estimates as required. All costs were expressed in Great British pounds (GBP) for the price year 2021 and were inflated and exchanged to GBP using data on national current price index57 and/or exchanged from US dollars ($) or Euros (€) to Great British pounds using the average conversion rate for 2021. 57
We assumed that the people who die in each period would do so at a constant rate during the period. When no data were available from the systematic review or published sources, a range of values were used in the model. For the costs, since the variability was not available, we used a 30% variation in the costs that we used.
Measuring cost-effectiveness
We measured cost-effectiveness using net monetary benefits (NMBs). For each treatment, we calculated the NMB as the mean QALYs per patient accruing to that treatment multiplied by decision-makers’ maximum willingness to pay for a QALY (also referred to as the cost-effectiveness threshold) minus the mean cost per patient for the treatment. In the UK, the upper limit of the maximum willingness to pay for a QALY is £20,000–30,000. 54 NMBs were calculated using the base-case parameter values to obtain the deterministic results, which do not depend on chance. The option with the highest NMB represented better value for money. The NMB for HIPEC + CRS + systemic chemotherapy minus the NMB for control is the incremental NMB. If the incremental NMB was positive, then HIPEC + CRS + systemic chemotherapy represented better value for money; if it was negative, the control represented better value for money.
A probabilistic sensitivity analysis (PSA) was also undertaken. 54 The PSA involved Monte Carlo simulation and took variability of all selected inputs into account simultaneously. Distributions were assigned to parameters to reflect the uncertainty for each parameter value. A random value from the corresponding distribution for each parameter was selected (by the computer). This generated an estimate of the mean cost and mean QALYs and the NMB associated with each treatment. This was repeated 10,000 times, and the results for each simulation were noted. The mean costs, QALYs and NMB for each treatment were calculated from the 10,000 simulations; these are probabilistic results because they depend on chance. Based on the stability tests, we increased the simulations to 15,000 for gastric cancer (HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone) and 90,000 for colorectal cancer and gastric cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The NMB was calculated for each of the 10,000 simulations, and the proportion of times each treatment had the highest NMB was calculated for a range of values for the maximum willingness to pay for a QALY. These are summarised graphically using cost-effectiveness acceptability curves. We derived the 95% CIs around the base-case values using the 2.5 and 97.5 percentiles calculated from the PSA. We also performed a value of information analysis and calculated the expected value of perfect information (EVPI) and the expected value of partially perfect information using methods suggested by Wilson et al. 58
For the deterministic univariate sensitivity analysis, each variable in the cost-effectiveness model was varied one at a time. The results of the sensitivity analysis were represented in the tornado diagram, which reflected the variation in the incremental NMB within the range of the lowest and highest value used for a parameter with all else equal. If the variation in the incremental NMB included zero, then there was uncertainty in the cost effectiveness due to the variation of the parameter.
We also performed a sensitivity analysis using information from ‘real-life’ prospective data from Christie NHS foundation trust.
We followed the ‘Consolidated Health Economic Evaluation Reporting Standards’ (CHEERS) reporting checklist for reporting the cost-effectiveness analysis. 59
Chapter 5 Results
Systematic review
Results of search
We identified 7938 records through electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Wiley) (n = 1169), MEDLINE Ovid (n = 3405), Embase Ovid (n = 930), Science Citation Index Expanded and Conference Proceedings Citation Index-Science (n = 1758), ClinicalTrials.gov (n = 152) and WHO Trials register (n = 524). There were 6019 records after removing duplicates. We excluded 5855 clearly irrelevant records through reading titles and abstracts. We retrieved a total of 164 full-text records for further assessment in detail. We included a total of eight trials for this review13,14,60–65 (see Table 1). We excluded 58 records for the reasons stated in Appendix 4. 12,66–122 We identified 38 records of ongoing trials123–160 (see Appendix 5). While some ongoing studies are clearly on people with peritoneal metastases, in other trials, a subset of participants would be eligible for a future review on the same topic. Additional reports of included, excluded and ongoing studies (60 records)16,62,161–218 are listed in Appendix 6. The reference flow is shown in Figure 1.
| Study name | Type of primary cancer | Other major inclusion/exclusion criteria | Number randomised | Post-randomisation exclusions | Mean or median age | Number of females (proportion) | Intervention vs. control | Follow-up in months |
|---|---|---|---|---|---|---|---|---|
| Quénet62 | Colorectal cancer |
|
265 | 0 | 60 | 133 (50.2%) | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Median: 64 |
| Verwaal14 | Colorectal cancer |
|
105 | 0 | 54 | 47 (44.8%) | HIPEC + CRS ± systemic chemotherapy vs. systemic chemotherapy | Median: 22 |
| Yang13 | Gastric cancer |
|
68 | 0 | 50 | 33 (48.5%) | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Median: 32 |
| Rau63 | Gastric cancer |
|
105 | Not stated | Not stated | Not stated | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Not stated |
| Rudloff64 | Gastric cancer |
|
17 | 0 | 48 | 7 (41.2%) | HIPEC + CRS ± systemic chemotherapy vs. systemic chemotherapy | Minimum: 24 |
| Van Driel65 | Ovarian cancer |
|
245 | 0 | 62 | 245 (100.0%) | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Median: 57 |
| Antonio60 | Ovarian cancer |
|
79 | 8 (unresectable) | 61 | 79 (100.0%) | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Median: 32 |
| Lim61 | Ovarian cancer |
|
184 | 0 | 53 | 184 (100.0%) | HIPEC + CRS ± systemic chemotherapy vs. CRS ± systemic chemotherapy | Median: 70 |
FIGURE 1.
Study flow diagram.

Because of the nature of the comparisons involved, we did not identify any non-randomised studies at low or moderate risk of bias, as such studies compare outcomes in completely different groups of individuals: participants likely to withstand major surgery and had limited metastases received HIPEC + CRS + systemic chemotherapy, while participants unlikely to withstand major surgery or had more extensive metastases did not receive HIPEC.
We did not identify any trial which compared CRS + systemic chemotherapy versus systemic chemotherapy alone in addition to supportive care for people with peritoneal metastases from colorectal or gastric cancer. While there were trials comparing CRS + systemic chemotherapy versus systemic chemotherapy alone in addition to supportive care in women with ovarian cancer, it was not clear whether any of these participants had peritoneal metastases, or even when it was clear that some people would have had peritoneal metastases, no separate outcome data were available for such participants. Therefore, such studies were excluded.
Characteristics of included studies
The characteristics of included studies are summarised in Table 1. Further details of HIPEC and systemic chemotherapy in these studies are summarised in Appendix 3, Tables 15 and 16. We included a total of eight trials (1068 participants) in this review. 13,14,60–65 Of the participants included in the studies, eight were excluded after randomisation as they were unresectable, leaving a total of 1060 participants included in this review. Of these, 955 participants from seven trials were included in quantitative analysis. 13,14,60–62,64,65 Two trials (370 participants) were conducted in people with peritoneal metastases from colorectal cancer,14,62 three trials (190 participants; 85 participants from two trials were included in quantitative analysis) were conducted in people with peritoneal metastases from gastric cancer13,63,64 and three trials (508 participants; 500 participants included in analysis) were conducted in people with stage III or greater epithelial ovarian cancer. 60,61,65 The follow-up period in the trials ranged from 22 months to 70 months in the seven trials that reported this information. 13,14,60–62,64,65
Participants
All trials included only adults. The mean or median age of the trial participants was between 48 and 62 years in the seven trials that reported this information. 13,14,60–62,64,65 The proportion of trial participants who were females was between 41.2% and 50.2% in the four trials on colorectal or gastric cancers that reported the number of female trial participants. 13,14,62,64
Most trials excluded participants who had extraperitoneal metastases, and because of the nature of the comparisons in this systematic review, they included only participants who were eligible to undergo major surgery and chemotherapy.
Comparisons
The comparisons in the trials were: HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy in six trials13,60–63,65 and HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy in the remaining two trials. 14,64
Outcomes
All-cause mortality was reported in an analysable format in seven trials. 13,14,60–62,64,65 Overall HRQoL was reported in one trial. 60 Serious adverse events were reported in analysable format in five trials. 13,60–62,65 Progression-free survival was reported in analysable format in four trials. 60–62,65 None of the trials reported non-serious adverse events or patient-reported outcome measures in analysable format.
Risk of bias in the trials
The overall risk of bias in the trials was low in six trials for all-cause mortality. 14,60–62,64,65 Of the remaining two trials, one was based on a conference abstract,63 and it is quite probable that this trial would also be at low risk of bias when fully published. The risk of bias in the different domains for mortality is shown in Table 2. It should be noted that most trials did not have a published protocol or a protocol that predated recruitment available from the trial register. Nevertheless, all-cause mortality was reported in most of the trials in the way it is expected. Therefore, we have considered that the risk of bias in the trials was low for all-cause mortality in most trials. Subjective outcomes such as HRQoL and serious adverse events would have been rated as some concerns as none of the trials used outcome assessor blinding. Only two trials reported participant blinding. 60,61 In the remaining trials, participants were aware of the treatment groups.
| Study name | Bias arising from the randomisation process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|
| Quénet62 | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Verwaal14 | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Yang13 | Some concerns | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Rudloff64 | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Rau63 | Some concerns | Some concerns | Low risk | Low risk | Some concerns | Some concerns |
| Antonio60 | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Van Driel65 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lim61 | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
Effect estimates
Colorectal cancer
Of the two trials in colorectal cancer, one trial compared HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy,62 and another compared HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy. 14 So, a meta-analysis was not performed. We did not calculate the indirect effect estimates because of the differences in the types of participants included in the two trials. In one trial, only participants who had macroscopically complete R1 surgical tumour reduction or residual thickness not exceeding 1 mm were included,62 but in the other trial there was no such criterion for selection. 14 In the absence of IPD analysis or effect estimates in a subset of participants who were similar in the two trials, it may be inappropriate to calculate the indirect effect estimates of CRS + systemic chemotherapy versus systemic chemotherapy in addition to supportive treatment because of possible violation of transitivity assumption (i.e. the participants in the trials were reasonably similar to allow randomisation to any of the arms being evaluated). Therefore, we have presented only the effect estimates of the direct comparisons.
HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy forcolorectal peritoneal metastases
One trial (265 participants) was included in the analysis. 62 The outcomes of interest reported by the trial included all-cause mortality, serious adverse events and time to disease progression. The forest plots are available in Figures 2–4.
FIGURE 2.
Colorectal cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): all-cause mortality. SE, standard error.
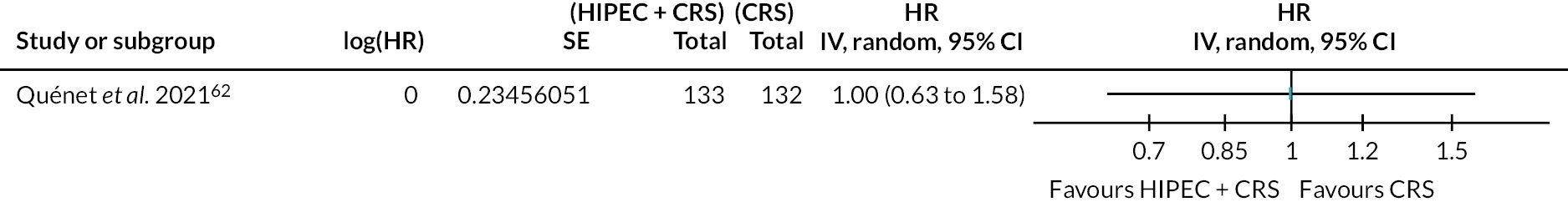
FIGURE 3.
Colorectal cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): serious adverse events.
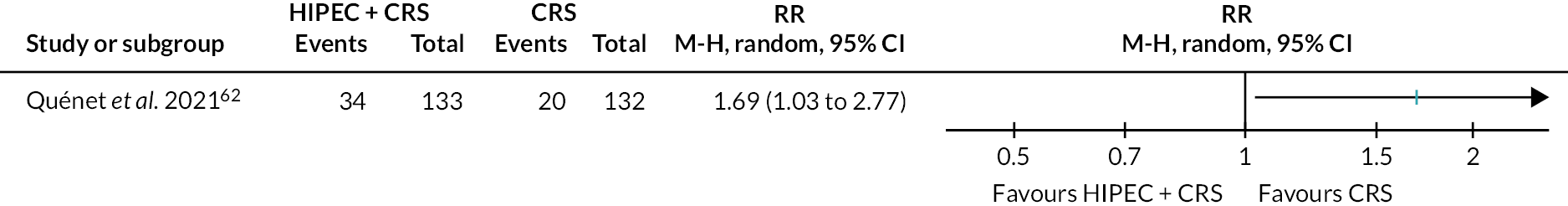
FIGURE 4.
Colorectal cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): time to disease progression. SE, standard error.
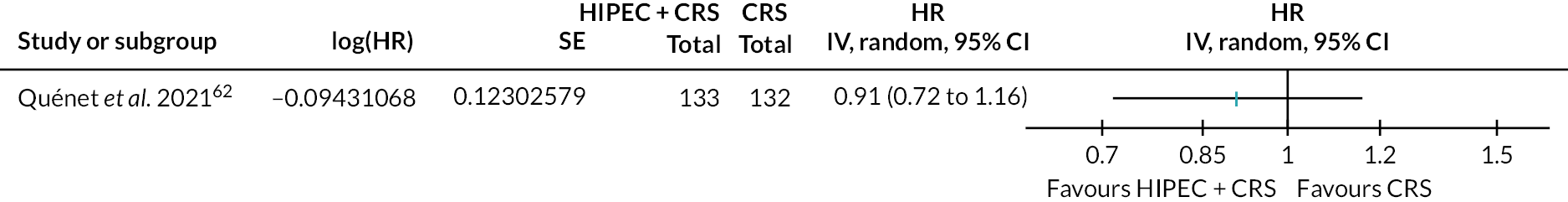
The figure shows that there is probably little or no difference in all-cause mortality between HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy.
The figure shows that HIPEC + CRS + systemic chemotherapy may increase the serious adverse events compared to CRS + systemic chemotherapy.
The figure shows that there is probably little or no difference in time to disease progression between HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy.
All-cause mortality
The evidence suggests that HIPEC + CRS + systemic chemotherapy results in little to no difference in all-cause mortality compared to CRS + systemic chemotherapy [60.6% in HIPEC + CRS + systemic chemotherapy vs. 60.6% in CRS + systemic chemotherapy; median follow-up 64 months; HR 1.00, 95% confidence interval (CI) 0.63 to 1.58; one trial; 265 participants; moderate-certainty evidence].
Serious adverse events
The evidence suggests HIPEC + CRS + systemic chemotherapy may increase the number of people who developed serious adverse events compared to CRS + systemic chemotherapy (25.6% in HIPEC + CRS + systemic chemotherapy vs. 15.2% in CRS + systemic chemotherapy; RR 1.69, 95% CI 1.03 to 2.77; one trial; 265 participants; low-certainty evidence).
Disease progression
The evidence suggests that HIPEC + CRS + systemic chemotherapy may result in little to no difference in overall disease progression compared to CRS + systemic chemotherapy (81.2% in HIPEC + CRS + systemic chemotherapy vs. 84.1% in CRS + systemic chemotherapy; median follow-up 64 months; HR 0.91, 95% CI 0.72 to 1.16; one trial; 265 participants; low-certainty evidence).
One trial (105 participants) was included in the analysis. 14 The outcomes of interest reported by the trial included all-cause mortality. The forest plot is available in Figure 5.
FIGURE 5.
Colorectal cancer (HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy): all-cause mortality. Verwaal et al. 14 SE, standard error.

The figure shows that HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy alone.
All-cause mortality
The evidence suggests that HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy (40.8% in HIPEC + CRS + systemic chemotherapy vs. 60.8% in systemic chemotherapy alone; median follow-up 22 months; HR 0.55, 95% CI 0.32 to 0.95; one trial; 105 participants; moderate-certainty evidence).
Gastric cancer
Of the three trials in gastric cancer, two trials compared HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy,13,63 and one trial compared HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy. 64 Of the two trials that compared HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy, one trial did not provide the outcomes of interest in an analysable format. 63 So, a meta-analysis was not performed. We did not calculate the indirect effect estimates because of the differences in the types of participants included in the two trials that provided quantitative data. In one trial, there was no restriction based on metastases to lung or liver,64 while in the other trial, people with metastases to lung or liver were excluded. 13 As for colorectal cancer, in the absence of IPD analysis or effect estimates in a subset of participants who were similar in the two trials, it may be inappropriate to calculate the indirect effect estimates of CRS + systemic chemotherapy versus systemic chemotherapy in addition to supportive treatment because of possible violation of transitivity assumption. Therefore, we have presented only the effect estimates of the direct comparisons.
HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy forgastric peritoneal metastases
The effect estimates for one trial (68 participants) that provided data in analysable format13 are presented below. The outcomes of interest reported by the trial included all-cause mortality and serious adverse events. The forest plots are available in Figures 6 and 7.
FIGURE 6.
Gastric cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): all-cause mortality. SE, standard error.

FIGURE 7.
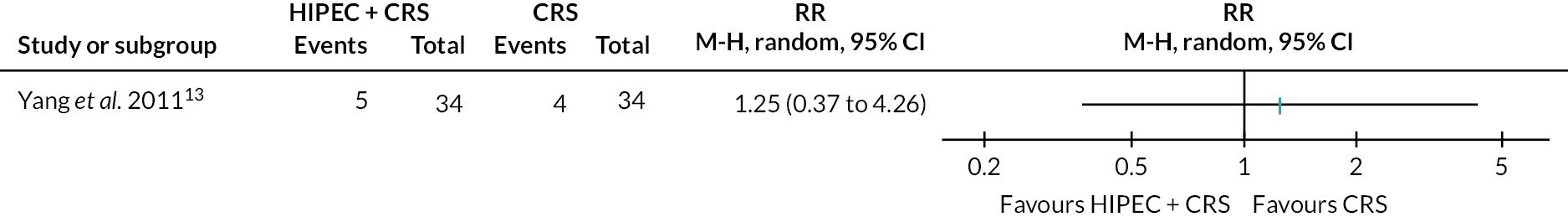
Gastric cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): serious adverse events.
The trial that did not provide data in analysable format provided a narrative statement about all-cause mortality,63 which is also included below.
The figure shows that HIPEC + CRS + systemic chemotherapy may result in lower mortality than CRS + systemic chemotherapy. However, another trial which reported mortality data in a format that could not be used for analysis showed that there is little or no difference in all-cause mortality between the groups. Therefore, the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on all-cause mortality is highly uncertain.
The figure shows that there is little or no difference in serious adverse events between the groups. Combined with the risk of bias in the trial and small size of the trial, there is high uncertainty about the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on serious adverse events.
All-cause mortality
There is high uncertainty about the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on all-cause mortality [73.8% in HIPEC + CRS + systemic chemotherapy vs. 97.1% in CRS + systemic chemotherapy; median follow-up 32 months; HR 0.38, 95% CI 0.21 to 0.70 based on the one trial (68 participants) that reported data in analysable format;13 very low-certainty evidence]. In the trial (105 participants) that did not report the data on all-cause mortality in an analysable way reported that there was no difference in all-cause mortality between HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy. 63
Serious adverse events
There is high uncertainty about the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on serious adverse events (14.7% in HIPEC + CRS + systemic chemotherapy vs. 11.8% in CRS + systemic chemotherapy; RR 1.25, 95% CI 0.37 to 4.26; one trial; 68 participants; very low-certainty evidence).
One trial (17 participants) was included in the analysis. 64 The outcomes of interest reported by the trial included all-cause mortality. The forest plot is available in Figure 8.
FIGURE 8.
Gastric cancer (HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy): all-cause mortality. SE, standard error.
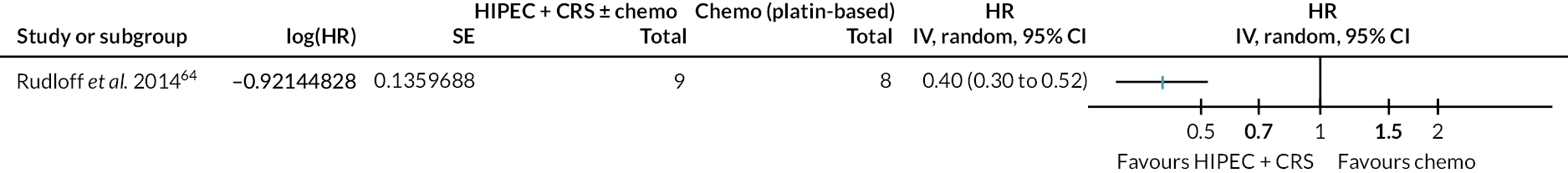
The figure shows that HIPEC + CRS + systemic chemotherapy probably results in lower mortality than systemic chemotherapy alone.
All-cause mortality
The evidence suggests that HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy (40.8% in HIPEC + CRS + systemic chemotherapy vs. 100% in systemic chemotherapy alone; minimum follow-up 24 months; HR 0.40, 95% CI 0.30 to 0.52; one trial; 17 participants; moderate-certainty evidence).
Ovarian cancer (stage III or greater epithelial ovarian cancer requiring interval cytoreductive surgery)
Three trials (500 participants) compared HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy. 60,61,65 The outcomes of interest reported by all the three trials included all-cause mortality, serious adverse events and time to disease progression. Of these three trials, two reported number of people with serious adverse events,60,65 and another trial reported number of serious adverse events. 61 Health-related quality of life was reported in analysable format in one trial60 and in a format that could not be included in the analysis in another trial. 65 Therefore, we have not presented this information. The forest plots are available in Figures 9–13.
FIGURE 9.
Ovarian cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): all-cause mortality. SE, Standard error.
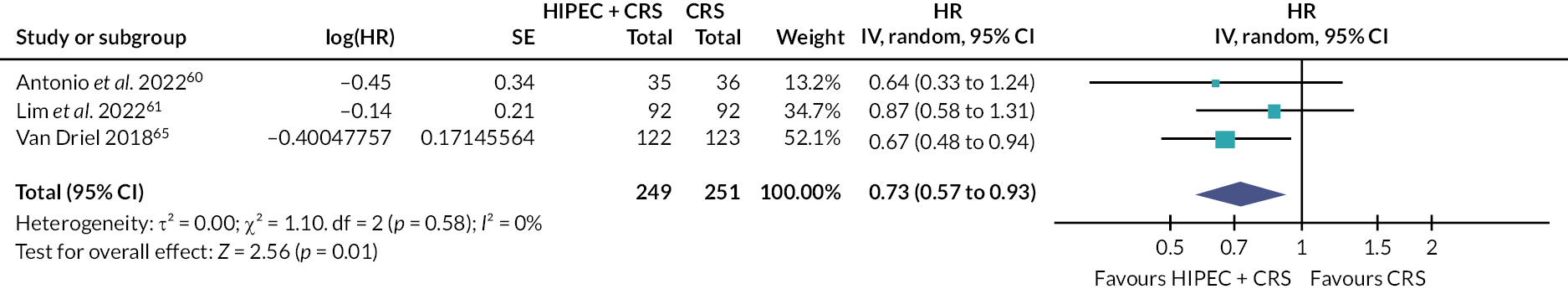
FIGURE 10.
Ovarian cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): HRQoL. SE, standard error; SD, standard deviation.
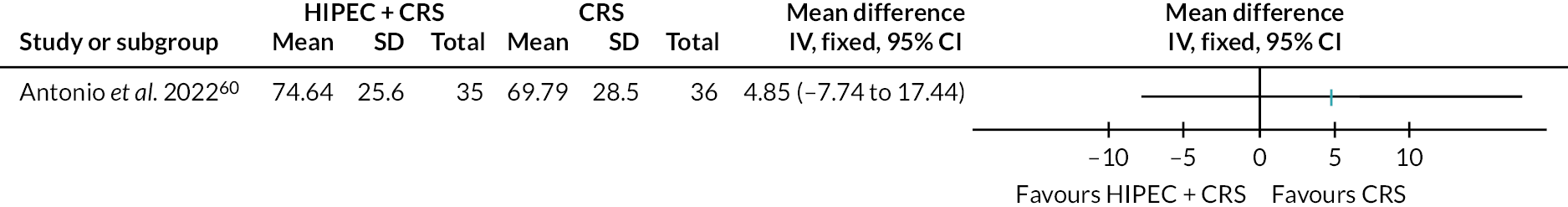
FIGURE 11.
Ovarian cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): serious adverse events (proportion).

FIGURE 12.
Ovarian cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): serious adverse events (number per participant). SE, standard error.

FIGURE 13.
Ovarian cancer (HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy): time to disease progression. SE, standard error.

The figure shows that HIPEC + CRS + systemic chemotherapy probably results in lower mortality and disease progression than CRS + systemic chemotherapy.
The figure also shows that there may be little or no difference in the HRQoL between HIPEC + CRS + systemic chemotherapy and CRS + systemic chemotherapy.
The figure also shows that there may be little or no difference in the proportion of participants who developed serious adverse events between HIPEC + CRS + systemic chemotherapy and CRS + systemic chemotherapy.
The figure also shows that the number of serious adverse events was probably higher in HIPEC + CRS + systemic chemotherapy compared to CRS + systemic chemotherapy.
The figure shows that HIPEC + CRS + systemic chemotherapy probably results in lower disease progression than CRS + systemic chemotherapy.
All-cause mortality
The evidence suggests that HIPEC + CRS + systemic chemotherapy probably results in lower all-cause mortality compared to CRS + systemic chemotherapy (46.3% in HIPEC + CRS + systemic chemotherapy vs. 57.4% in CRS + systemic chemotherapy; median follow-up 32 to 70 months; HR 0.73, 95% CI 0.57 to 0.93; three trials; 500 participants; moderate-certainty evidence).
Health-related quality of life
The evidence suggests that HIPEC + CRS + systemic chemotherapy may result in little to no difference in the HRQoL (Global Health Status at 12 months) compared to CRS + systemic chemotherapy (MD 4.85, 95% CI −7.74 to 17.44; one trial; 71 participants; low-certainty evidence). In another trial where data were not reported in analysable format,65 HIPEC + CRS + systemic chemotherapy resulted in little to no difference in the HRQoL [European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire–Core 30 (QLQ-C30)] at 24 months.
Serious adverse events
The evidence suggests that HIPEC + CRS + systemic chemotherapy may result in little to no difference in number of people who developed serious adverse events compared to CRS + systemic chemotherapy (26.7% in HIPEC + CRS + systemic chemotherapy vs. 25.2% in CRS + systemic chemotherapy; RR 1.06, 95% CI 0.73 to 1.54; two trials; 316 participants; moderate-certainty evidence). The evidence suggests HIPEC + CRS + systemic chemotherapy probably increases the number of serious adverse events compared to CRS + systemic chemotherapy (41.4 events per 100 participants in HIPEC + CRS + systemic chemotherapy vs. 32.6 events per 100 participants in CRS + systemic chemotherapy; rate ratio 1.27, 95% CI 1.09 to 1.49; one trial; 184 participants; moderate-certainty evidence).
Disease progression
The evidence suggests that HIPEC + CRS + systemic chemotherapy may result in lower disease progression compared to CRS + systemic chemotherapy (75.8% in HIPEC + CRS + systemic chemotherapy vs. 85.7% in CRS + systemic chemotherapy; median follow-up 32–70 months; HR 0.73, 95% CI 0.60 to 0.89; three trials; 500 participants; low-certainty evidence).
Heterogeneity
There was no evidence of heterogeneity in any of the meta-analyses as indicated by good overlap of CIs of effect estimates from the trials, between-study standard deviation (τ = 0), I2 = 0% and the p-value of chi-squared test for heterogeneity being not statistically significant (see Figures 9–13).
Sensitivity analysis
An exploratory panoramic meta-analysis revealed that HIPEC + CRS + systemic chemotherapy results in little to no difference in all-cause mortality compared to CRS + systemic chemotherapy, as indicated by the 95% credible intervals (CrI).
-
Colorectal cancer: HR 1.00 (95% CrI 0.15 to 6.77).
-
Gastric cancer: HR 0.38 (95% CrI 0.05 to 2.64).
-
Ovarian cancer: HR 0.73 (95% CrI 0.24 to 2.18).
There was no evidence of differences in survival by cancer types [coefficient for cancer type: gastric cancer vs. colorectal cancer −0.96 (95% CrI −3.67 to 1.78) and ovarian cancer vs. colorectal cancer −0.32 (95% CrI −2.53 to 1.90), although the between-study standard deviation was 0.29 (95% CrI 0.01 to 3.08)].
Reporting bias
We have searched all the major databases for medical publications and clinical trial registers. We did not identify any registered and completed clinical trial which has not reported the results over an extended period of time.
Certainty of evidence
The certainty of evidence and the reasons for downgrading the evidence are available in Table 3. Most of the evidence related to all-cause mortality was of moderate certainty.
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with CRS + SC (or SC alone) | Risk with HIPEC + CRS + SC | |||||
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||||
| All-cause mortality (median follow-up: 64 months) | 606 per 1000 | 606 per 1000 (444 to 771) | HR 1.00 (0.63 to 1.58) | 265 (1 RCT) | ⨁⨁⨁◯ Moderateb |
|
| Serious adverse events (short term) | 152 per 1000 | 256 per 1000 (156 to 420) | RR 1.69 (1.03 to 2.77) | 265 (1 RCT) | ⨁⨁◯◯ Lowb,c |
|
| Time to disease progression (median follow-up: 64 months) | 841 per 1000 | 812 per 1000 (734 to 881) | HR 0.91 (0.72 to 1.16) | 265 (1 RCT) | ⨁⨁◯◯ Lowb,c |
|
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||||
| All-cause mortality (median follow-up: 22 months) | 608 per 1000 | 402 per 1000 (259 to 589) | HR 0.55 (0.32 to 0.95) | 105 (1 RCT) | ⨁⨁⨁◯ Moderateb |
|
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||||
| All-cause mortality (median follow-up: 32 months) | 971 per 1000 | 738 per 1000 (523 to 915) | HR 0.38 (0.21 to 0.70) | 68 (1 RCT) | ⨁◯◯◯ Very lowb,d,e |
Another trial including 105 participants indicated that there was no difference in all-cause mortality between the two groups but could not be included in the analysis because the numbers were not reported in a format suitable for analysis |
| Serious adverse events (short term) | 118 per 1000 | 147 per 1000 (44 to 501) | RR 1.25 (0.37 to 4.26) | 68 (1 RCT) | ⨁◯◯◯ Very lowb,c,d |
|
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||||
| All-cause mortality (minimum follow-up: 24 months) | 1000 per 1000 | 1000 per 1000 (1000 to 1000) | HR 0.40 (0.30 to 0.52) | 17 (1 RCT) | ⨁⨁⨁◯ Moderateb |
|
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||||
| All-cause mortality (median follow-up: 32–70 months) | 574 per 1000 | 463 per 1000 (385 to 547) | HR 0.73 (0.57 to 0.93) | 500 (3 RCTs) | ⨁⨁⨁◯ Moderateb |
|
| HRQoL assessed with Global Health Status Scale from 0 to 100 (mean follow-up: 12 months) | The mean HRQoL was 69.79 | MD 4.85 more (7.74 fewer to 17.44 more) | - | 71 (1 RCT) | ⨁⨁◯◯ Lowb,c |
|
| Serious adverse events (proportion) (short term) | 252 per 1000 | 267 per 1000 (184 to 387) | RR 1.06 (0.73 to 1.54) | 316 (2 RCTs) | ⨁⨁◯◯ Lowb,c |
|
| Serious adverse events (number per participant) (short term) | 326 per 1000 | 414 per 1000 (355 to 486) | Rate ratio 1.27 (1.09 to 1.49) | 184 (1 RCT) | ⨁⨁⨁◯ Moderatec |
|
| Time to disease progression (median follow-up: 32–70 months) | 857 per 1000 | 758 per 1000 (688 to 822) | HR 0.73 (0.60 to 0.89) | 500 (3 RCTs) | ⨁⨁◯◯ Lowb,c |
|
Cost-effectiveness
The decision tree is available in Figure 14. The input parameters for the different comparisons are available in Tables 4–8. The cost estimates for different aspects of treatment and the sources of information for different comparisons are available in Appendix 7, Tables 17–21. The results of the analyses are available in Tables 9–13. The file that was used to perform the cost-effectiveness analysis is available as Report Supplementary Material 1. This file can be used to calculate the cost-effectiveness based on local cost estimates.
FIGURE 14.
Decision tree (HIPEC + CRS + systemic chemotherapy vs. control). Please see detailed description of this decision tree in the text.
| Parameters | Type of distribution | Mean (uniform), number with event (dichotomous) | Number without event (dichotomous) | Lower limit | Upper limit | Source/notes |
|---|---|---|---|---|---|---|
| Complications (HIPEC + CRS) | Beta | 34 | 99 | 0 | 0.5 | Systematic review (from Quénet et al. 202162) |
| Short-term mortality (HIPEC + CRS) | Beta | 2 | 131 | 0 | 0.1 | Quénet et al. 202162 |
| Survival ln (HR) | Continuous | 0 | 0.2345605 | −2 | 2 | Systematic review (from Quénet et al. 202162) |
| Complications (control) | Beta | 20 | 112 | 0 | 0.5 | Systematic review (from Quénet et al. 202162) |
| Short-term mortality (control) | Beta | 2 | 130 | 0 | 0.1 | Quénet et al. 202162 |
| 5-year mortality (control) | Beta | 80 | 53 | 0 | 1 | Systematic review (from Quénet et al. 202162) |
| Cost (HIPEC + CRS) complicated | Uniform | 16,308.92895 | 11,416.25 | 21,202 | See Appendix 7, Table 17 | |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 11,939.472 | 8357.63 | 15,521 | See Appendix 7, Table 17 | |
| Cost (control) complicated | Uniform | 10,568.45695 | 7397.92 | 13,739 | See Appendix 7, Table 17 | |
| Cost (control) uncomplicated | Uniform | 6199 | 4339.3 | 8058.7 | See Appendix 7, Table 17 | |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Leimkuhler et al. 2020;219 EORTC CLQ30 mapped to 5Q5D using Kim et al. 2012220 |
| QoL (complicated CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetically same as HIPEC group |
| QoL (uncomplicated CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Hypothetically same as HIPEC group |
| QoL (long term) HIPEC | Beta | 0.785 | 0.215 | 0.1 | 0.9 | Malcolm et al. 2021221 |
| QoL (long term) control | Beta | 0.785 | 0.215 | 0.1 | 0.9 | Malcolm et al. 2021221 |
| Parameters | Type of distribution | Mean (uniform), number with event (dichotomous) | Number without event (dichotomous) | Lower limit | Upper limit | Source/notes |
|---|---|---|---|---|---|---|
| Complications (HIPEC + CRS) | Beta | 34 | 99 | 0 | 0.5 | No information from Verwaal et al. 2003;14 therefore used details from Quénet et al. 202162 |
| Short-term mortality (HIPEC + CRS) | Beta | 4 | 50 | 0 | 0.1 | Verwaal et al. 200314 |
| Survival ln (HR) | Continuous | −0.597837001 | 0.2775921 | −2 | 2 | Systematic review (from Verwaal et al. 200314) |
| Complications (control) | Beta | 10 | 41 | 0 | 0.5 | Estimated from (Verwaal et al. 200314) |
| Short-term mortality (control) | Beta | 0 | 51 | 0 | 0.1 | Verwaal et al. 200314 |
| 5-year mortality (control) | Beta | 80 | 53 | 0 | 1 | Systematic review (Verwaal et al. 200314) |
| Cost (HIPEC + CRS) complicated | Uniform | 24,432.82095 | 17,102.97 | 31,763 | See Appendix 7, Table 18 | |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 20,063.364 | 14,044.35 | 26,082 | See Appendix 7, Table 18 | |
| Cost (control) complicated | Uniform | 13,072.25695 | 9150.58 | 16,994 | See Appendix 7, Table 18 | |
| Cost (control) uncomplicated | Uniform | 8702.8 | 6091.96 | 11,314 | See Appendix 7, Table 18 | |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Leimkuhler et al. 2020;219 EORTC CLQ30 mapped to 5Q5D using Kim et al. 2012220 |
| QoL (complicated CRS) (short term) | Beta | 0.57 | 0.43 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated CRS) (short term) | Beta | 0.67 | 0.33 | 0.1 | 0.9 | Flyum et al. 2021222 |
| QoL (long term) HIPEC | Beta | 0.785 | 0.215 | 0.1 | 0.9 | Malcolm et al. 2021221 |
| QoL (long term) control | Beta | 0.67 | 0.33 | 0.1 | 0.9 | Flyum et al. 2021222 |
| Parameters | Type of distribution | Mean (uniform), number with event (dichotomous) | Number without event (dichotomous) | Lower limit | Upper limit | Source/notes |
|---|---|---|---|---|---|---|
| Complications (HIPEC + CRS) | Beta | 5 | 29 | 0 | 0.5 | Systematic review (from Yang et al. 201113) |
| Short-term mortality (HIPEC + CRS) | Beta | 0 | 34 | 0 | 0.1 | No information |
| Survival ln (HR) | Continuous | −0.446287103 | 0.4782931 | −2 | 2 | Systematic review (from Yang et al. 201113) |
| Complications (control) | Beta | 4 | 30 | 0 | 0.5 | Systematic review (from Yang et al. 201113) |
| Short-term mortality (control) | Beta | 0 | 34 | 0 | 0.1 | No information |
| 5-year mortality (control) | Beta | 33 | 1 | 0 | 1 | Systematic review (from Yang et al. 201113) |
| Cost (HIPEC + CRS) complicated | Uniform | 20,727.35891 | 14,509.15 | 26,946 | See Appendix 7, Table 19 | |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 16,357.90196 | 11,450.53 | 21,265 | See Appendix 7, Table 19 | |
| Cost (control) complicated | Uniform | 17,397.58591 | 12,178.31 | 22,617 | See Appendix 7, Table 19 | |
| Cost (control) uncomplicated | Uniform | 13,028.12896 | 9119.69 | 16,937 | See Appendix 7, Table 19 | |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Leimkuhler et al. 2020;219 EORTC CLQ30 mapped to 5Q5D using Kim et al. 2012220 |
| QoL (complicated CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetically same as HIPEC group |
| QoL (uncomplicated CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Hypothetically same as HIPEC group |
| QoL (long term) HIPEC | Beta | 0.85 | 0.15 | 0.1 | 0.9 | van der Wielen et al. 2022223 |
| QoL (long term) control | Beta | 0.85 | 0.15 | 0.1 | 0.9 | van der Wielen et al. 2022223 |
| Parameters | Type of distribution | Mean (uniform), number with event (dichotomous) | Number without event (dichotomous) | Lower limit | Upper limit | Source/notes |
|---|---|---|---|---|---|---|
| Complications (HIPEC + CRS) | Beta | 5 | 29 | 0 | 0.5 | No information from Rudloff et al. 2014;64 therefore used details from Yang et al. 201113 |
| Short-term mortality (HIPEC + CRS) | Beta | 0 | 9 | 0 | 0.1 | Rudloff et al. 201464 |
| Survival ln (HR) | Continuous | −0.916290732 | 0.1403205 | −2 | 2 | Systematic review (from Rudloff et al. 201464) |
| Complications (control) | Beta | 0 | 8 | 0 | 0.5 | No information |
| Short-term mortality (control) | Beta | 0 | 8 | 0 | 0.1 | No information |
| 5-year mortality (control) | Beta | 8 | 0 | 0 | 1 | Systematic review (from Rudloff et al. 201464) |
| Cost (HIPEC + CRS) complicated | Uniform | 32,325.51895 | 22,627.86 | 42,023 | See Appendix 7, Table 20 | |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 27,956.062 | 19,569.24 | 36,343 | See Appendix 7, Table 20 | |
| Cost (control) complicated | Uniform | 22,785.41695 | 15,949.79 | 29,621 | See Appendix 7, Table 20 | |
| Cost (control) uncomplicated | Uniform | 18,415.96 | 12,891.17 | 23,941 | See Appendix 7, Table 20 | |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.43 | 0.57 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.53 | 0.47 | 0.1 | 0.9 | Leimkuhler et al. 2020;219 EORTC CLQ30 mapped to 5Q5D using Kim et al. 2012220 |
| QoL (complicated CRS) (short term) | Beta | 0.54 | 0.46 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated CRS) (short term) | Beta | 0.64 | 0.36 | 0.1 | 0.9 | Carter et al. 2015224 |
| QoL (long term) HIPEC | Beta | 0.85 | 0.15 | 0.1 | 0.9 | van der Wielen et al. 2022223 |
| QoL (long term) Control | Beta | 0.64 | 0.36 | 0.1 | 0.9 | Carter et al. 2015224 |
| Parameters | Type of distribution | Mean (uniform), number with event (dichotomous) | Number without event (dichotomous) | Lower limit | Upper limit | Source/notes |
|---|---|---|---|---|---|---|
| Complications (HIPEC + CRS) | Beta | 42 | 115 | 0 | 0.5 | Systematic review (from van Driel et al. 201865 and Antonio et al. 202260) |
| Short-term mortality (HIPEC + CRS) | Beta | 1 | 156 | 0 | 0.1 | From: van Driel et al. 201865 and Antonio et al. 202260 |
| Survival ln (HR) | Continuous | −0.314710745 | 0.124887 | −2 | 2 | Systematic review (from van Driel et al. 2018,65 Antonio et al. 2022,60 Lim et al. 202261) |
| Complications (control) | Beta | 40 | 119 | 0 | 0.5 | Systematic review (from van Driel et al. 201865 and Antonio et al. 202260) |
| Short-term mortality (control) | Beta | 2 | 157 | 0 | 0.1 | From: van Driel et al. 201865 and Antonio et al. 202260 |
| 5-year mortality (control) | Beta | 123 | 92 | 0 | 1 | Systematic review (from van Driel et al. 201865 and Lim et al. 202261). The number of deaths was reported only in these two trials, but the HRs were reported in the three trials |
| Cost (HIPEC + CRS) complicated | Uniform | 15,964.05095 | 11,174.84 | 20,753 | See Appendix 7, Table 21 | |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 11,594.594 | 81,16.216 | 15,073 | See Appendix 7, Table 21 | |
| Cost (control) complicated | Uniform | 12,336.65695 | 8635.66 | 16,038 | See Appendix 7, Table 21 | |
| Cost (control) uncomplicated | Uniform | 7967.2 | 5577.04 | 10,357 | See Appendix 7, Table 21 | |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.5013 | 0.4987 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.6013 | 0.3987 | 0.1 | 0.9 | Antonio et al. 2022,60 converted using Kim et al. 2012220 |
| QoL (complicated CRS) (short term) | Beta | 0.504612 | 0.495388 | 0.1 | 0.9 | Hypothetical 0.1 less in complicated |
| QoL (uncomplicated CRS) (short term) | Beta | 0.604612 | 0.395388 | 0.1 | 0.9 | Antonio et al. 2022,60 converted using Kim et al. 2012220 |
| QoL (long term) HIPEC | Beta | 0.606 | 0.394 | 0.1 | 0.9 | Antonio et al. 2022,60 converted using Kim et al. 2012220 |
| QoL (long term) control | Beta | 0.606 | 0.394 | 0.1 | 0.9 | Antonio et al. 2022,60 converted using Kim et al. 2012220 |
Colorectal cancer
HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for colorectal peritoneal metastases
The results of the cost-effectiveness analysis are presented in Tables 9–13 and Figures 15–17. The deterministic results show that HIPEC + CRS + systemic chemotherapy results in more costs and similar QALYs as CRS + systemic chemotherapy. The incremental NMBs at willingness to pay (WTP) of £20,000 and £30,000 were −£6162.83 and −£6164.19, respectively, that is, incremental NMB was < 0, indicating that HIPEC + CRS + systemic chemotherapy was not cost-effective compared to CRS + systemic chemotherapy in NHS (see Table 9).
| Treatment | Costs | QALYs | NMBa | |
|---|---|---|---|---|
| £20,000 | £30,000 | |||
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £13,021 | 6.3270 | £113,519.32 | £176,789.54 |
| CRS + chemotherapy | £6861 | 6.3272 | £119,682.14 | £182,953.73 |
| Incremental | £6160 | −0.0001 | −£6162.83 | −£6164.19 |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||
| HIPEC + CRS + chemotherapy | £21,074 | 8.9770 | £158,465.13 | £248,234.86 |
| Chemotherapy | £9560 | 3.0058 | £50,555.67 | £80,613.28 |
| Incremental | £11,515 | 5.9712 | £107,909.46 | £167,621.58 |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £16,930 | 17.1311 | £325,692.76 | £497,004.04 |
| CRS + chemotherapy | £13,542 | 16.2530 | £311,518.04 | £474,048.15 |
| Incremental | £3388 | 0.8781 | £14,174.73 | £22,955.89 |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||
| HIPEC + CRS + chemotherapy | £28,563 | 18.6927 | £345,289.89 | £532,216.48 |
| Chemotherapy | £18,416 | 14.0955 | £263,493.51 | £404,448.25 |
| Incremental | £10,147 | 4.5972 | £81,796.38 | £127,768.23 |
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £12,657 | 6.5189 | £117,721.33 | £182,910.73 |
| CRS + chemotherapy | £9066 | 4.0013 | £70,959.52 | £110,972.50 |
| Incremental | £3591 | 2.5176 | £46,761.81 | £71,938.23 |
FIGURE 15.
Scatterplot (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The scatterplot shows that the points are clustered in the north-east and north-west quadrants, indicating that HIPEC + CRS + systemic chemotherapy results in more costs and similar QALYs as CRS + systemic chemotherapy.
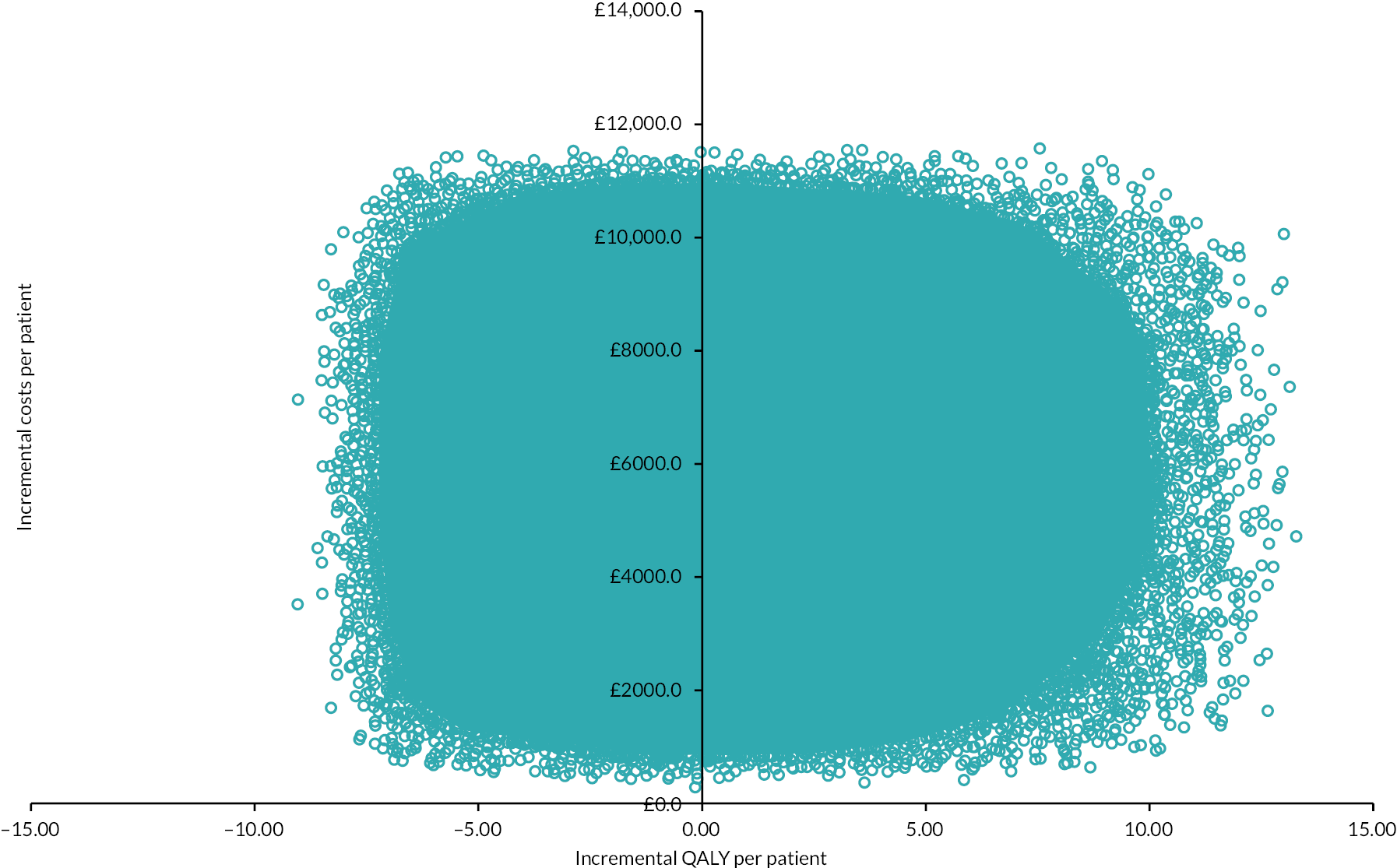
FIGURE 16.
Cost-effectiveness acceptability curve (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The cost-effectiveness acceptability curve indicates that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 50% at WTP thresholds up to £60,000.

FIGURE 17.
Tornado plot (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The univariate sensitivity analysis reveals that the major uncertainties relate to the effectiveness of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy in terms of survival and long-term HRQoL. ln, natural logarithm.

The PSA revealed that there was considerable uncertainty in the incremental NMB (see Table 10). The scatterplot revealed that the points were clustered in the north-east and north-west quadrants, confirming that HIPEC + CRS + systemic chemotherapy results in more costs and similar QALYs as CRS + systemic chemotherapy (see Figure 15).
| Treatment | Costs | QALYs | NMBa | |
|---|---|---|---|---|
| £20,000 | £30,000 | |||
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £12,463 (95% CI £9331 to £15,582) | 6.1718 (95% CI 1.1429 to 11.4222) | £110,973 (95% CI £10,397 to £215,978) | £172,691 (95% CI £21,843 to £330,246) |
| CRS + chemotherapy | £6528 (95% CI £4841 to £8204) | 5.9786 (95% CI 1.1814 to 8.4504) | £113,045 (95% CI £17,077 to £162,529) | £172,832 (95% CI £28,877 to £247,011) |
| Incremental | £5936 (95% CI £2049 to £9811) | 0.1932 (95% CI −5.8078 to 7.1457) | −£2073 (95% CI −£122,112 to £137,008) | −£141 (95% CI −£180,212 to £208,473) |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||
| HIPEC + CRS + chemotherapy | £20,596 (95% CI £15,364 to £25,723) | 8.6721 (95% CI 1.5551 to 14.0329) | £152,847 (95% CI £10,256 to £260,008) | £239,568 (95% CI £25,645 to £400,566) |
| Chemotherapy | £9130 (95% CI £6766 to £11,462) | 2.8728 (95% CI 0.5562 to 4.5809) | £48,327 (95% CI £1970 to £82,345) | £77,055 (95% CI £7555 to £128,058) |
| Incremental | £11,467 (95% CI £5171 to £17,572) | 5.7993 (95% CI −1.7495 to 11.8965) | £104,520 (95% CI −£46,759 to £227,057) | £162,513 (95% CI −£64,427 to £345,845) |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £16,927 (95% CI £12,722 to £21,131) | 15.7213 (95% CI 3.6692 to 18.1617) | £297,500 (95% CI £56,485 to £349,456) | £454,714 (95% CI £93,228 to £530,854) |
| CRS + chemotherapy | £13,543 (95% CI £10,127 to £16,957) | 14.9190 (95% CI 3.4656 to 17.2325) | £284,836 (95% CI £55,791 to £333,592) | £434,025 (95% CI £90,434 to £505,718) |
| Incremental | £3383 (95% CI −£2643 to £9419) | 0.8024 (95% CI −12.5666 to 13.6696) | £12,664 (95% CI −£254,806 to £270,104) | £20,688 (95% CI −£380,551 to £406,649) |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||||
| HIPEC + CRS + chemotherapy | £28,156 (95% CI £20,712 to £35,662) | 17.1404 (95% CI 3.9852 to 19.8173) | £314,651 (95% CI £51,404 to £374,273) | £486,055 (95% CI £91,213 to £572,129) |
| Chemotherapy | £18,427 (95% CI £13,186 to £23,673) | 13.5237 (95% CI 2.4548 to 19.8082) | £252,046 (95% CI £30,970 to £380,426) | £387,282 (95% CI £55,213 to £578,093) |
| Incremental | £9729 (95% CI −£664 to £20,215) | 3.6167 (95% CI −12.7241 to 16.9960) | £62,606 (95% CI −£263,733 to £330,185) | £98,773 (95% CI −£390,946 to £500,566) |
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||||
| HIPEC + CRS + chemotherapy | £12,054 (95% CI £9067 to £15,053) | 6.3020 (95% CI 1.1644 to 11.2114) | £113,986 (95% CI £11,005 to £211,674) | £177,006 (95% CI £22,564 to £323,785) |
| CRS + chemotherapy | £8511 (95% CI £6380 to £10,625) | 3.9277 (95% CI 0.7494 to 6.4870) | £70,044 (95% CI £6390 to £121,491) | £109,322 (95% CI £13,952 to £186,353) |
| Incremental | £3543 (95% CI −£463 to £7586) | 2.3742 (95% CI −4.3425 to 8.9759) | £43,942 (95% CI −£90,407 to £176,080) | £67,684 (95% CI −£133,694 to £265,809) |
The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was 46.5% and 47.6% at WTP of £20,000 and £30,000, respectively. The CEAC curve indicated that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 50% at even higher thresholds (see Figure 16).
The univariate sensitivity analysis revealed that CRS + systemic chemotherapy was cost-effective (compared to HIPEC + CRS + systemic chemotherapy) for most of the parameters for the entire range tested (see Table 11). The main parameters when the intervention becomes cost-effective were when HIPEC + CRS + systemic chemotherapy results in better survival and better long-term HRQoL compared to CRS + systemic chemotherapy (see Table 11; Figure 17).
| Variable | Distribution | Range tested | Step | Threshold (WTP: £20,000 per QALY) | Threshold (WTP: £30,000 per QALY) |
|---|---|---|---|---|---|
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |||||
| Complications (HIPEC + CRS) | Beta | 0–0.5 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Short-term mortality (HIPEC + CRS) | Beta | 0–0.1 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Survival ln (HR) | Continuous | −2 to 2 | 0.05 | Control becomes cost-effective when value is 0 | Control becomes cost-effective when value is 0 |
| Complications (Control) | Beta | 0–0.5 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Short-term mortality (control) | Beta | 0–0.1 | 0.05 | Intervention becomes cost-effective when value is 0.1 | Intervention becomes cost-effective when value is 0.05 |
| 5-year mortality (control) | Beta | 0–1 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Cost (HIPEC + CRS) complicated | Uniform | 11,391.51–21,155.66 | 500 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 8332.89–15,475.37 | 500 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Cost (control) complicated | Uniform | 7397.92–13,738.99 | 500 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| Cost (control) uncomplicated | Uniform | 4339.3–8058.7 | 500 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| QoL (complicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| QoL (uncomplicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Control is cost-effective for the range tested | Control is cost-effective for the range tested |
| QoL (long term) HIPEC | Beta | 0.1–0.9 | 0.05 | Intervention becomes cost-effective when value is 0.85 | Intervention becomes cost-effective when value is 0.85 |
| QoL (long term) Control | Beta | 0.1–0.9 | 0.05 | Control becomes cost-effective when value is 0.75 | Control becomes cost-effective when value is 0.8 |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | |||||
| Complications (HIPEC + CRS) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (HIPEC + CRS) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Survival ln (HR) | Continuous | −2 to 2 | 0.05 | Control becomes cost-effective when value is 0 | Control becomes cost-effective when value is 0 |
| Complications (control) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (control) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| 5-year mortality (control) | Beta | 0–1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) complicated | Uniform | 17,028.75–31,624.83 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 13,970.13–25,944.54 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) complicated | Uniform | 9150.58–16,993.93 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) uncomplicated | Uniform | 6091.96–11,313.64 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated CRS) (short term) | Beta | 0.1 to 0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (long term) HIPEC | Beta | 0.1–0.9 | 0.05 | Intervention becomes cost-effective when value is 0.35 | Intervention becomes cost-effective when value is 0.3 |
| QoL (long term) control | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |||||
| Complications (HIPEC + CRS) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (HIPEC + CRS) | Beta | 0–0.1 | 0.05 | Control becomes cost-effective when value is 0.05 | Control becomes cost-effective when value is 0.05 |
| Survival ln (HR) | Continuous | −2 to 2 | 0.05 | Control becomes cost-effective when value is −0.05 | Control becomes cost-effective when value is 0 |
| Complications (control) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (control) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| 5-year mortality (control) | Beta | 0–1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) complicated | Uniform | 14,459.67–26,853.67 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 11,401.05–21,173.38 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) complicated | Uniform | 12,178.31–22,616.86 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) uncomplicated | Uniform | 9119.69–16,936.57 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (long term) HIPEC | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (long term) control | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | |||||
| Complications (HIPEC + CRS) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (HIPEC + CRS) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Survival ln (HR) | Continuous | −2 to 2 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Complications (control) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (control) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| 5-year mortality (control) | Beta | 0–1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) complicated | Uniform | 22,603.12–41,977.23 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 19,544.5–36,296.93 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) complicated | Uniform | 15,949.79–29,621.04 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) uncomplicated | Uniform | 12,891.17–23,940.75 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (long term) HIPEC | Beta | 0.1–0.9 | 0.05 | Intervention becomes cost-effective when value is 0.7 | Intervention becomes cost-effective when value is 0.7 |
| QoL (long term) control | Beta | 0.1–0.9 | 0.05 | Control becomes cost-effective when value is 0.85 | Control becomes cost-effective when value is 0.85 |
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |||||
| Complications (HIPEC + CRS) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (HIPEC + CRS) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Survival ln (HR) | Continuous | −2 to 2 | 0.05 | Control becomes cost-effective when value is 0 | Control becomes cost-effective when value is 0 |
| Complications (control) | Beta | 0–0.5 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Short-term mortality (control) | Beta | 0–0.1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| 5-year mortality (control) | Beta | 0–1 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) complicated | Uniform | 11,100.62–20,615.43 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (HIPEC + CRS) uncomplicated | Uniform | 8042–14,935.13 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) complicated | Uniform | 8635.66–16,037.65 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| Cost (control) uncomplicated | Uniform | 5577.04–10,357.36 | 500 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated HIPEC + CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (complicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (uncomplicated CRS) (short term) | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
| QoL (long term) HIPEC | Beta | 0.1–0.9 | 0.05 | Intervention becomes cost-effective when value is 0.4 | Intervention becomes cost-effective when value is 0.4 |
| QoL (long term) control | Beta | 0.1–0.9 | 0.05 | Intervention is cost-effective for the range tested | Intervention is cost-effective for the range tested |
The EVPI between was £25 and £39 million per 1000 people (see Table 12). The expected value of perfect parameter information (EVPPI) could not be estimated because of insufficient computer memory.
| Willingness-to-pay threshold | |
|---|---|
| £20,000 | £30,000 |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |
| £25,489,715.67 | £39,621,153.71 |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | |
| £3,169,055.03 | £4,155,359.80 |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |
| £31,579,625.20 | £46,847,924.61 |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | |
| £29,220,352.71 | £41,999,823.03 |
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | |
| £12,781,413.10 | £18,668,735.51 |
HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases
The results of the cost-effectiveness analysis are presented in Tables 9–13 and Figures 18–21. The deterministic results show that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone. The incremental NMBs at WTP of £20,000 and £30,000 were £107,909.46 and £167,621.58, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy may be cost-effective compared to systemic chemotherapy alone in NHS (see Table 9).
FIGURE 18.
Scatterplot (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The scatterplot shows that the points are clustered in the north-east quadrant, indicating that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone.

FIGURE 19.
Cost-effectiveness acceptability curve (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The cost-effectiveness acceptability curve indicates that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 90% at WTP thresholds >£10,000 up to £60,000.
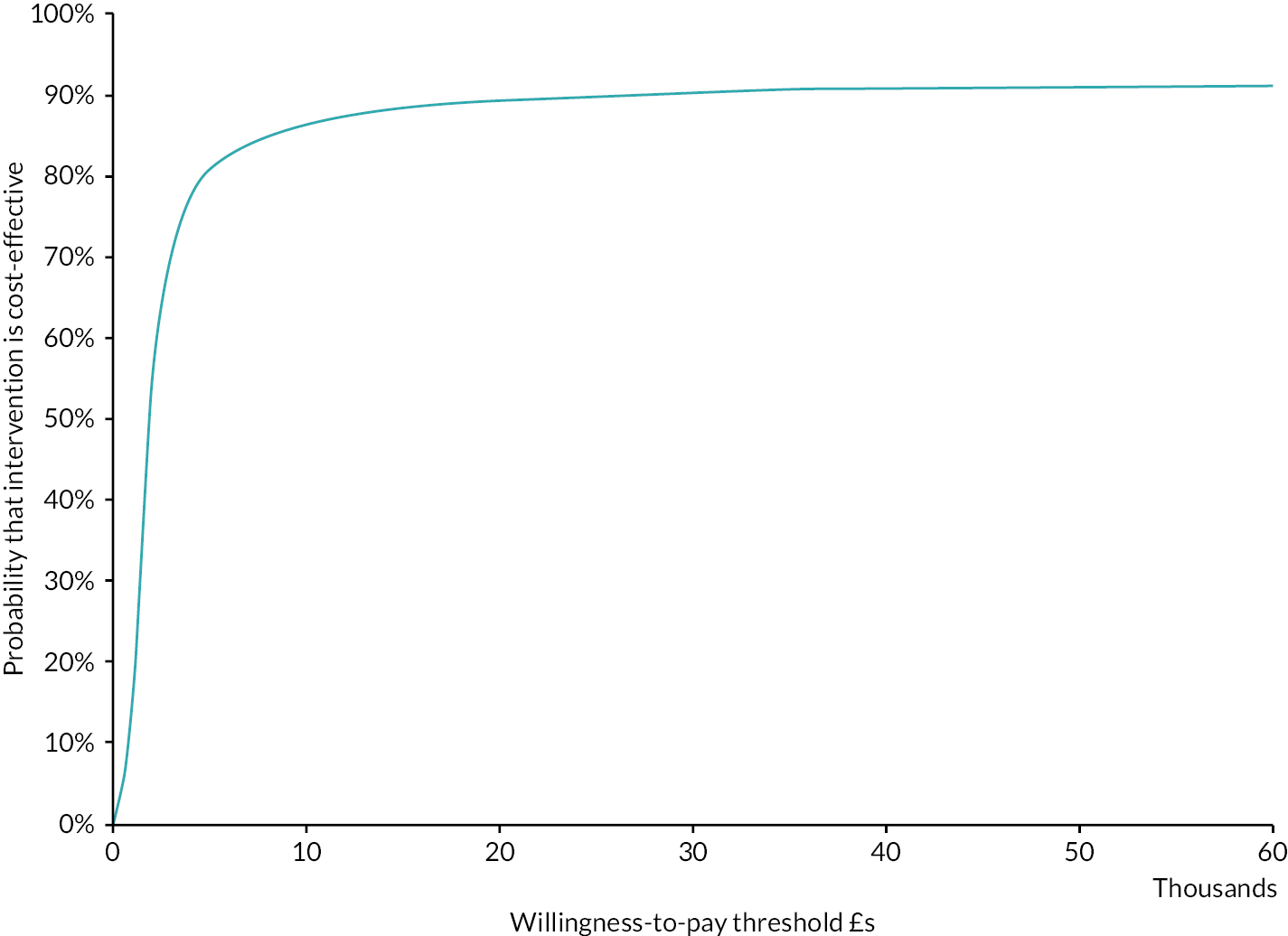
FIGURE 20.
Tornado plot (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The univariate sensitivity analysis reveals that the major uncertainties relate to the effectiveness of HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy alone in terms of survival and long-term HRQoL. ln, natural logarithm.
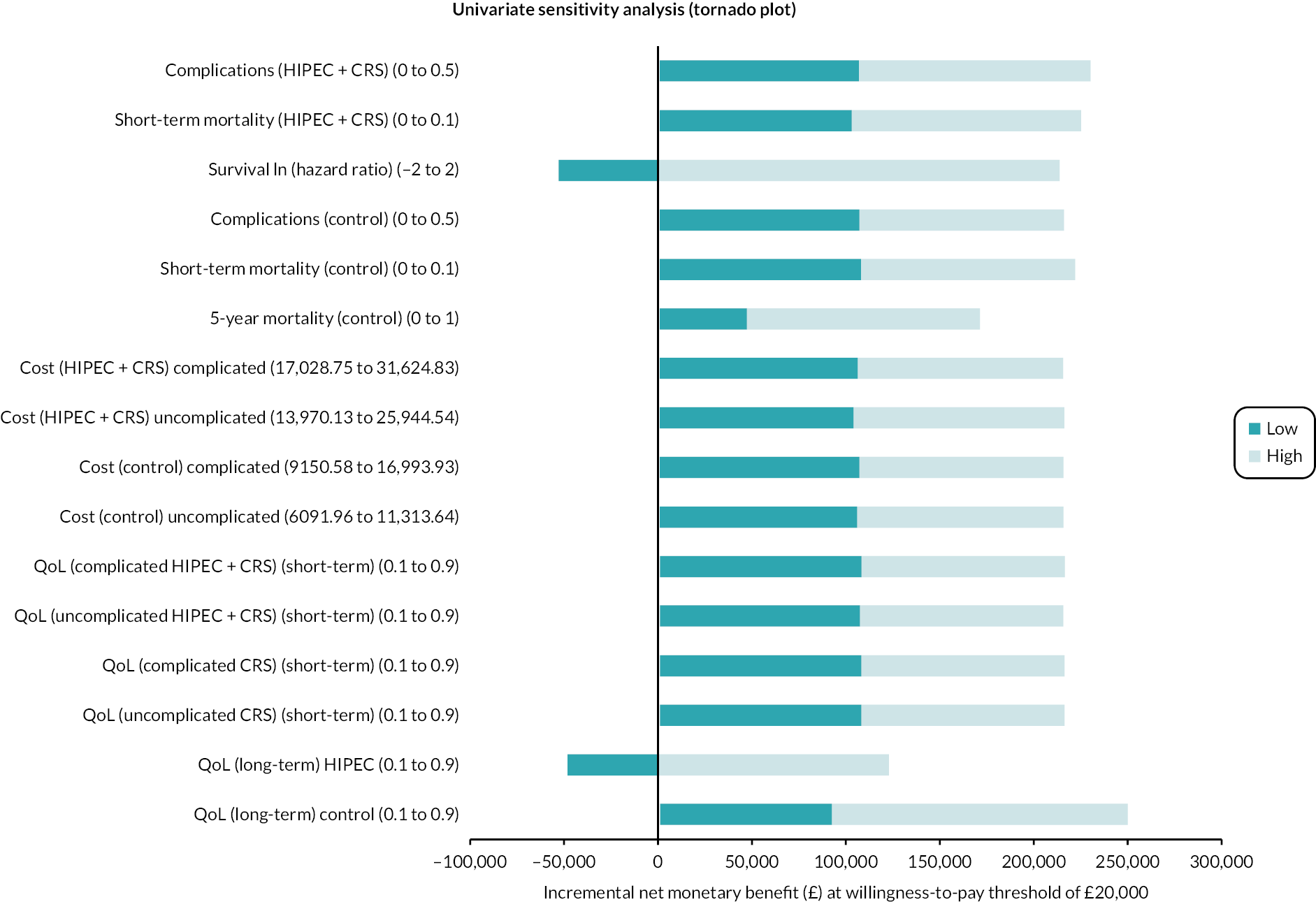
FIGURE 21.
Expected value of perfect parameter information (colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone).
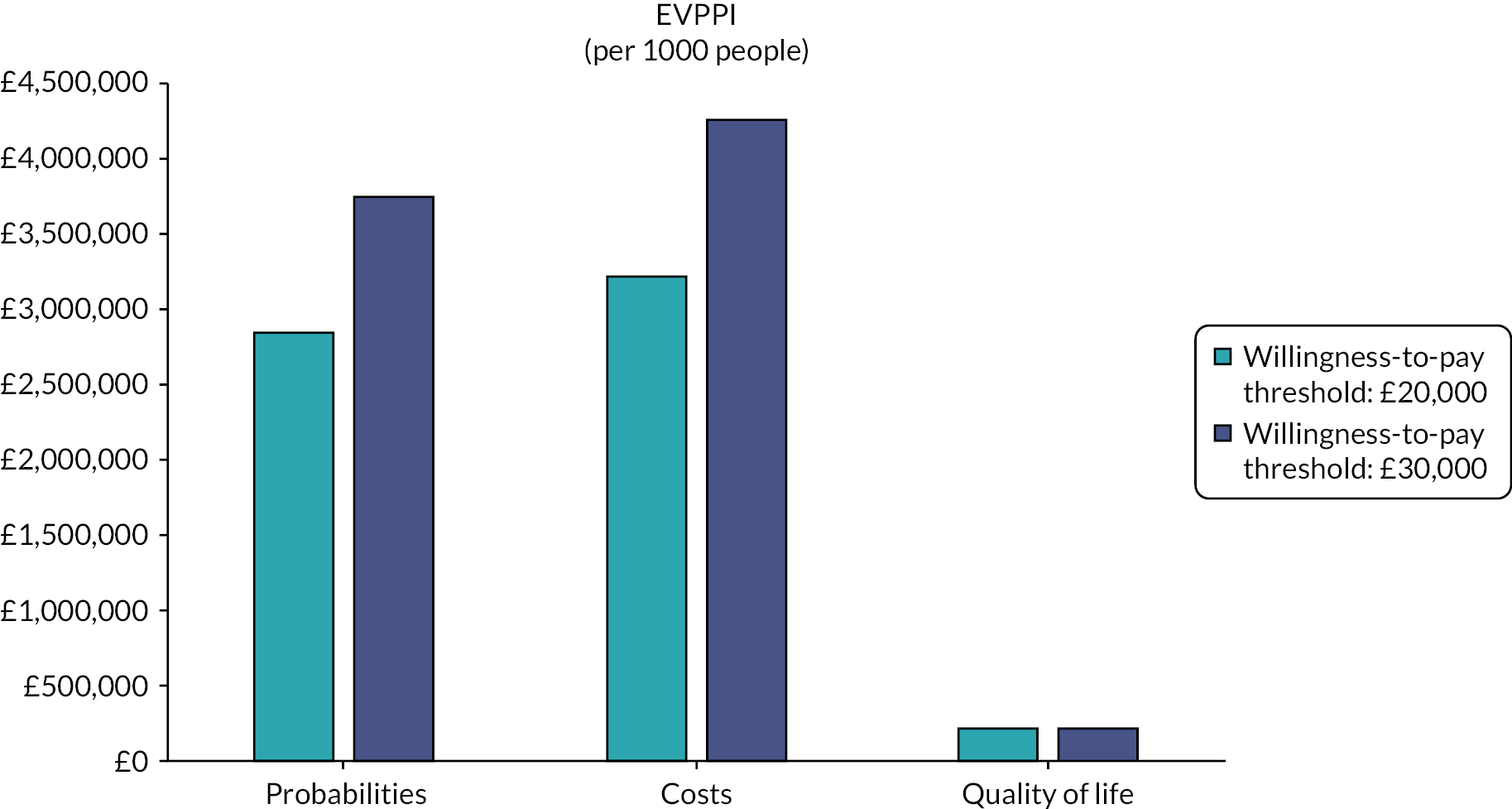
The PSA revealed that there was some uncertainty in the incremental NMB (see Table 10). The scatterplot revealed that the points were clustered in the north-east quadrant, confirming that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone (see Figure 18).
The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was 89.3% and 90.3% at WTP of £20,000 and £30,000, respectively. The CEAC curve indicated that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 90% at WTP thresholds >£10,000 up to £60,000 (see Figure 19).
The univariate sensitivity analysis revealed that HIPEC + CRS + systemic chemotherapy was cost-effective compared to systemic chemotherapy alone for most of the parameters for the entire range tested (see Table 11). The main parameters when the intervention becomes cost-effective was when HIPEC + CRS + systemic chemotherapy results in better survival and better long-term HRQoL compared systemic chemotherapy alone (see Table 11; Figure 20).
The EVPI was between £3 and £4 million per 1000 people (see Table 12). The EVPPI shows that the main uncertainties appear to be in the probabilities and costs (see Table 13; Figure 21).
| Parameters | WTP threshold: £20,000 | WTP threshold: £30,000 |
|---|---|---|
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||
| Probabilities | Not estimablea | Not estimablea |
| Costs | Not estimablea | Not estimablea |
| QoL | Not estimablea | Not estimablea |
| Colorectal cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||
| Probabilities | £2,841,100.52 | £3,744,343.32 |
| Costs | £3,217,756.26 | £4,247,070.12 |
| QoL | £214,928.69 | £219,547.80 |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||
| Probabilities | Not estimablea | Not estimablea |
| Costs | Not estimablea | Not estimablea |
| QoL | Not estimablea | Not estimablea |
| Gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone | ||
| Probabilities | £29,139,433.25 | £41,796,876.45 |
| Costs | £29,196,348.60 | £42,016,418.43 |
| QoL | – | – |
| Ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy | ||
| Probabilities | £12,325,971.11 | £17,989,799.71 |
| Costs | £13,308,599.36 | £19,451,913.17 |
| QoL | £58,459.84 | £71,190.49 |
Gastric cancer
HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for gastric peritoneal metastases
The results of the cost-effectiveness analysis are presented in Tables 9–13, Figures 22–24. The deterministic results show that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than CRS + systemic chemotherapy. The incremental NMBs at WTP of £20,000 and £30,000 were £14,174.73 and £22,955.89, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy may be cost-effective compared to CRS + systemic chemotherapy in NHS (see Table 9).
FIGURE 22.
Scatterplot (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The scatterplot shows that the points are clustered in the north quadrant, indicating that HIPEC + CRS + systemic chemotherapy results in more costs, but there is uncertainty in QALYs than CRS + systemic chemotherapy.
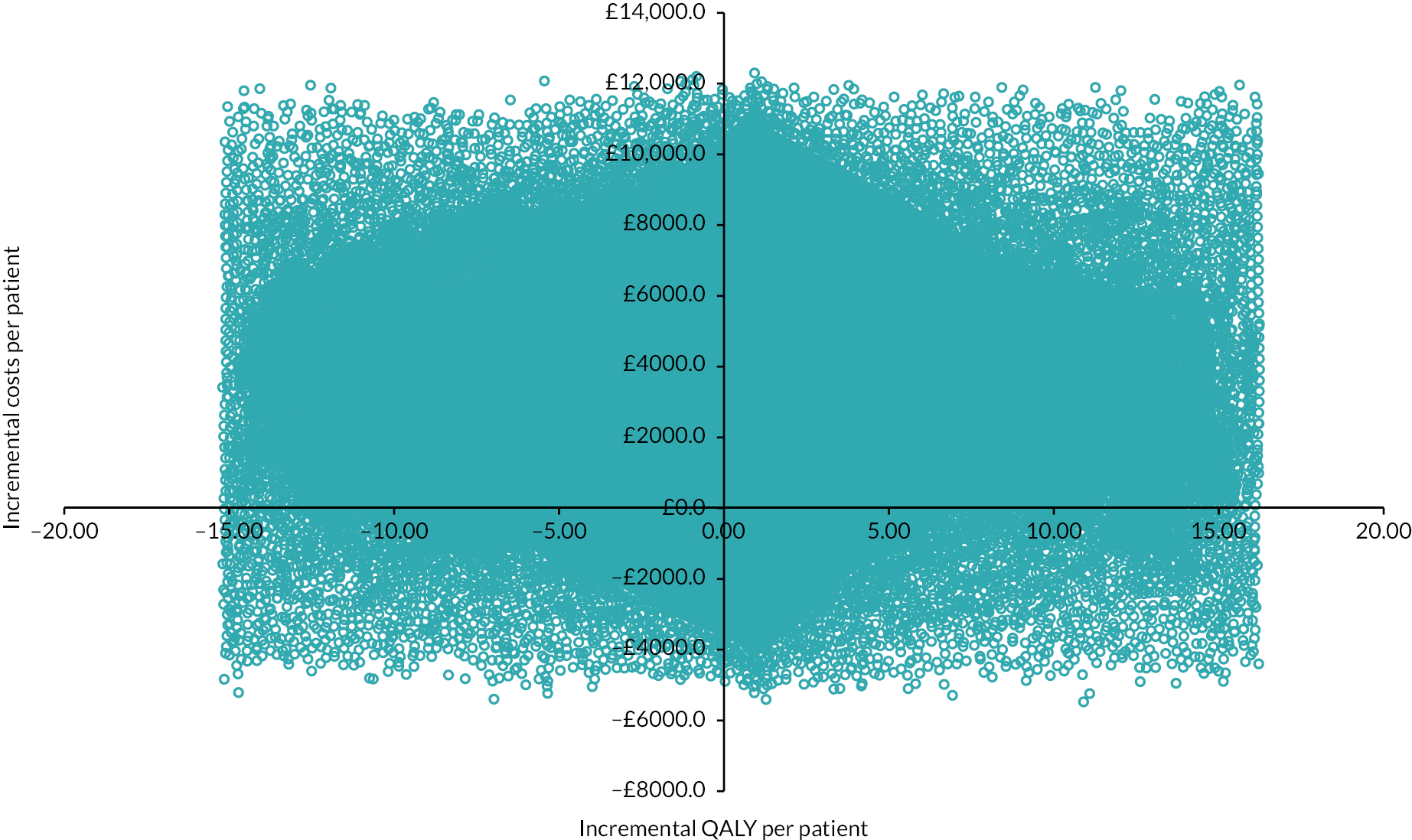
FIGURE 23.
Cost-effectiveness acceptability curve (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The cost-effectiveness acceptability curve indicates that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 70% at WTP thresholds >£10,000 up to £60,000.
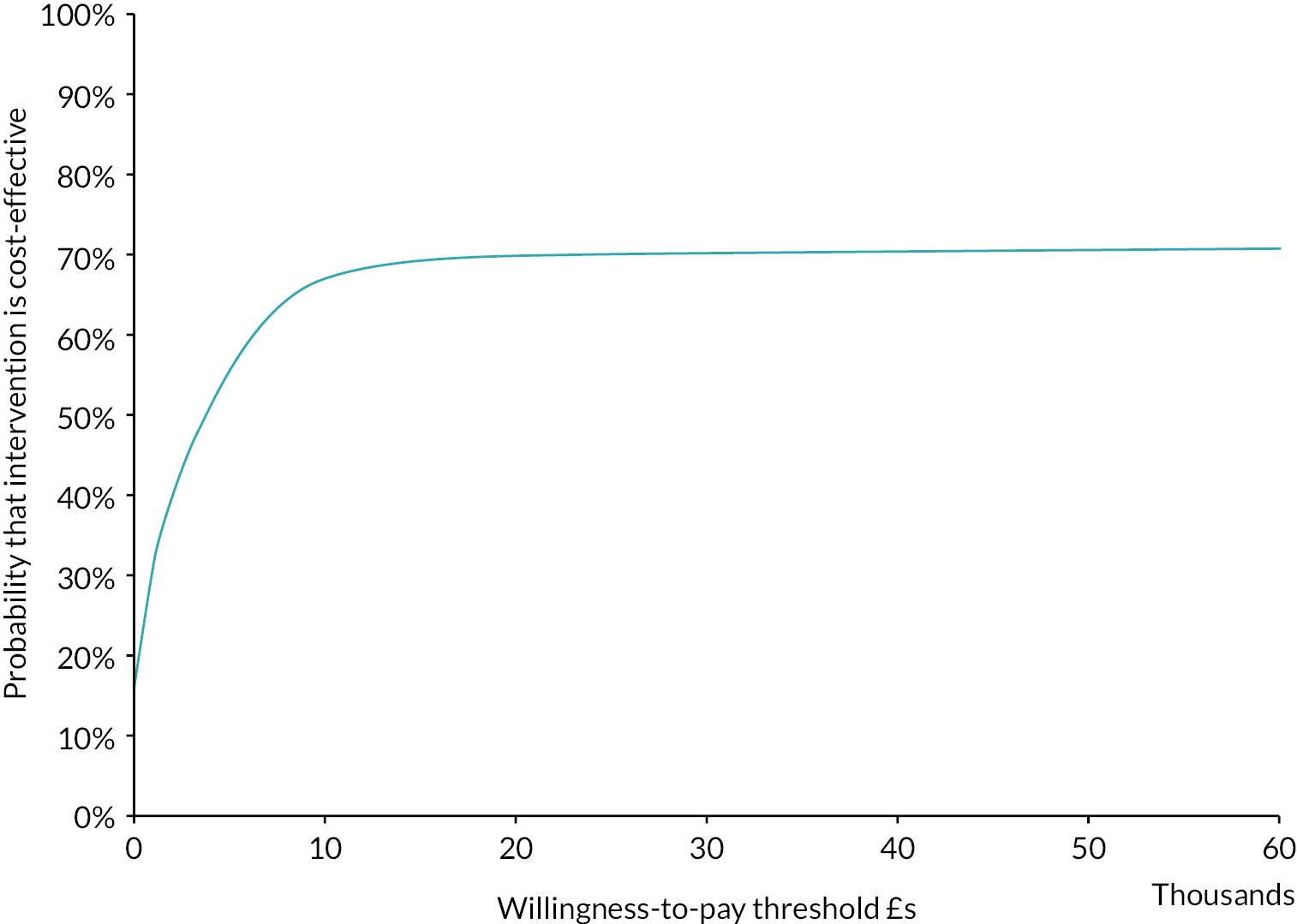
FIGURE 24.
Tornado plot (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The univariate sensitivity analysis reveals that the major uncertainties relate to the effectiveness of HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy in terms of survival and long-term HRQoL. ln, natural logarithm.

The PSA revealed that there was considerable uncertainty in the incremental NMB (see Table 10). The scatterplot revealed that the points were clustered in the north quadrants, confirming that HIPEC + CRS + systemic chemotherapy results in more costs than CRS + systemic chemotherapy, but there is uncertainty in QALYs compared to CRS + systemic chemotherapy (see Figure 22).
The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was 69.8% and 70.3% at WTP of £20,000 and £30,000, respectively. The CEAC curve indicated that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 70% at WTP thresholds >£10,000 up to £60,000 (see Figure 23).
The univariate sensitivity analysis revealed that HIPEC + CRS + systemic chemotherapy was cost-effective compared to CRS + systemic chemotherapy for most of the parameters for the entire range tested (see Table 11). The main parameters when the intervention becomes cost-effective was when HIPEC + CRS + systemic chemotherapy results in better survival and better long-term HRQoL compared to CRS + systemic chemotherapy (see Table 11; Figure 24).
The EVPI was between £32 million and £47 million per 1000 people (see Table 12). The EVPPI could not be estimated because of insufficient computer memory.
HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy for gastric peritoneal metastases
The results of the cost-effectiveness analysis are presented in Tables 9–13, Figures 25–28. The deterministic results show that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone. The incremental NMBs at WTP of £20,000 and £30,000 were £81,796.38 and £127,768.23, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy may be cost-effective compared to systemic chemotherapy alone in the NHS (see Table 9).
FIGURE 25.
Scatterplot (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The scatterplot shows that the points are clustered in the north-east quadrant, indicating that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone.

FIGURE 26.
Cost-effectiveness acceptability curve (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The cost-effectiveness acceptability curve indicates that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 55–65% at WTP thresholds >£10,000 up to £60,000.
FIGURE 27.
Tornado plot (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone). The univariate sensitivity analysis reveals that the major uncertainties relate to the effectiveness of HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone in terms of long-term HRQoL. ln, natural logarithm.

FIGURE 28.
Expected value of perfect parameter information (gastric cancer: HIPEC + CRS + systemic chemotherapy vs. systemic chemotherapy alone).
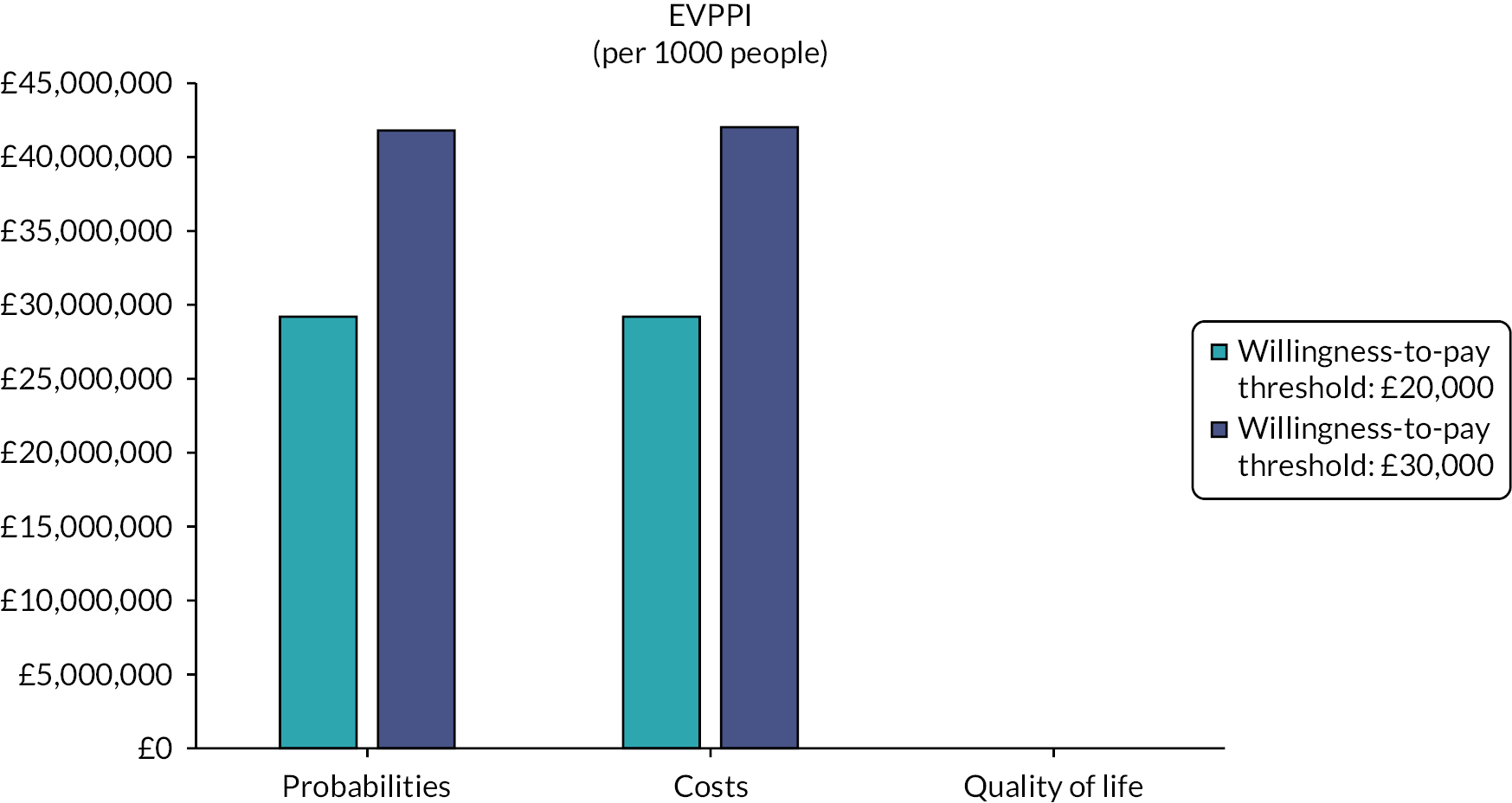
The PSA revealed that there was considerable uncertainty in the incremental NMB (see Table 10). The scatterplot revealed that the points were clustered in the north-east quadrant, confirming that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than systemic chemotherapy alone (see Figure 25).
The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was 63.0% and 64.9% at WTP of £20,000 and £30,000, respectively. The CEAC curve indicated that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 55–65% at WTP thresholds >£10,000 up to £60,000 (see Figure 26).
The univariate sensitivity analysis revealed that HIPEC + CRS + systemic chemotherapy was cost-effective compared to systemic chemotherapy alone for most of the parameters for the entire range tested (see Table 11). The main parameters when the intervention becomes cost-effective was when HIPEC + CRS + systemic chemotherapy results in better long-term HRQoL compared to systemic chemotherapy alone (see Table 11; Figure 27).
The EVPI was between £29 and £42 million per 1000 people (see Table 12). The EVPPI shows that the main uncertainties appear to be in the probabilities and costs (see Table 13; Figure 28).
Ovarian cancer (stage III or greater epithelial ovarian cancer requiring interval cytoreductive surgery)
The results of the cost-effectiveness analysis are presented in Tables 9–13 and Figures 29–32. The deterministic results show that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than CRS +systemic chemotherapy. The incremental NMBs at WTP of £20,000 and £30,000 were £46,761.81 and £71,938.23, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy was cost-effective compared to CRS +systemic chemotherapy in NHS (see Table 9).
FIGURE 29.
Scatterplot (ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The scatterplot shows that the points are clustered in the north-east quadrant, indicating that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than CRS + systemic chemotherapy.

FIGURE 30.
Cost-effectiveness acceptability curve (ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The cost-effectiveness acceptability curve indicates that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 70% at WTP thresholds >£10,000 up to £60,000.
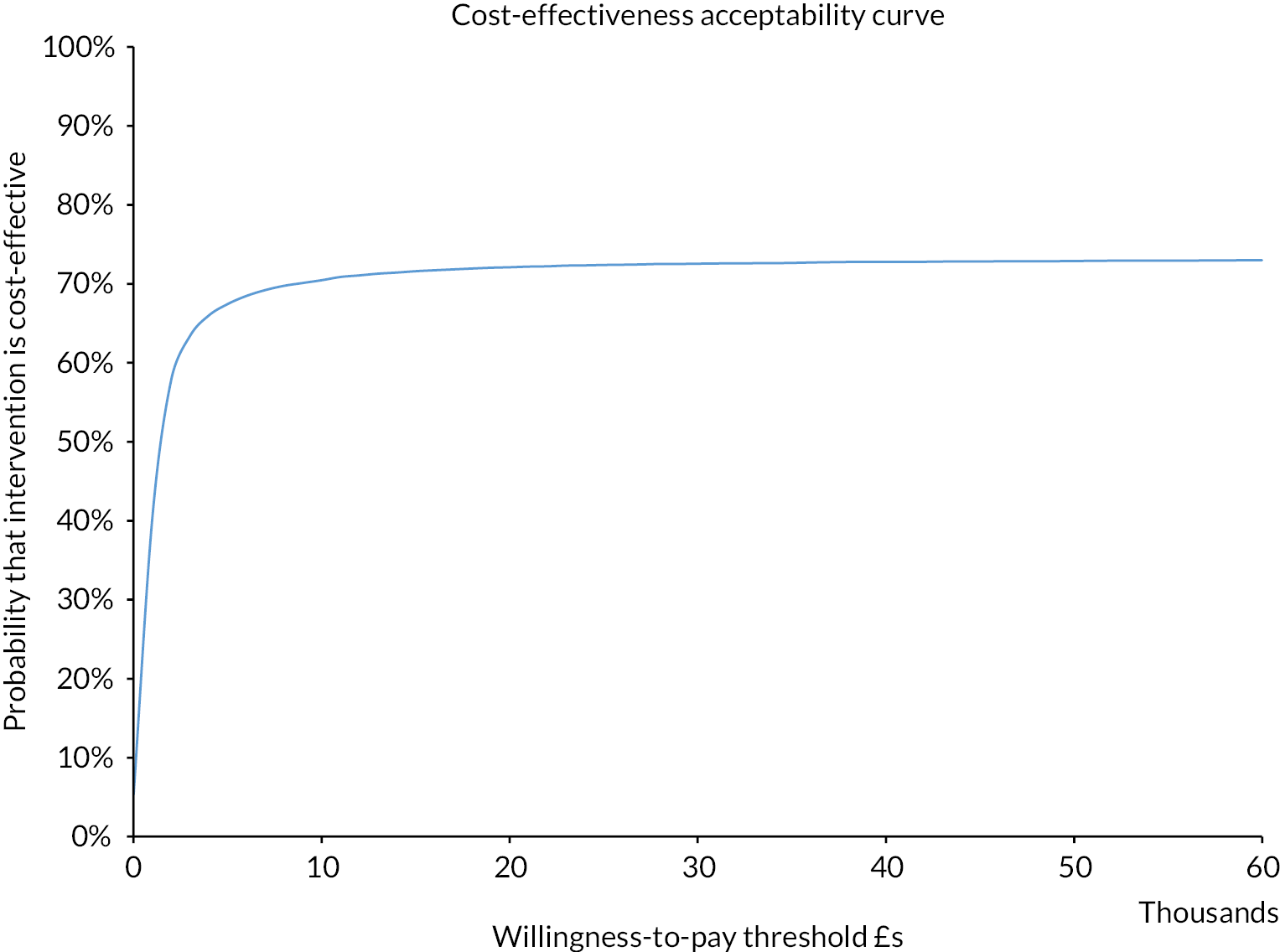
FIGURE 31.
Tornado plot (ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy). The univariate sensitivity analysis reveals that the major uncertainties relate to the effectiveness of HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy in terms of survival and long-term HRQoL. ln, natural logarithm.
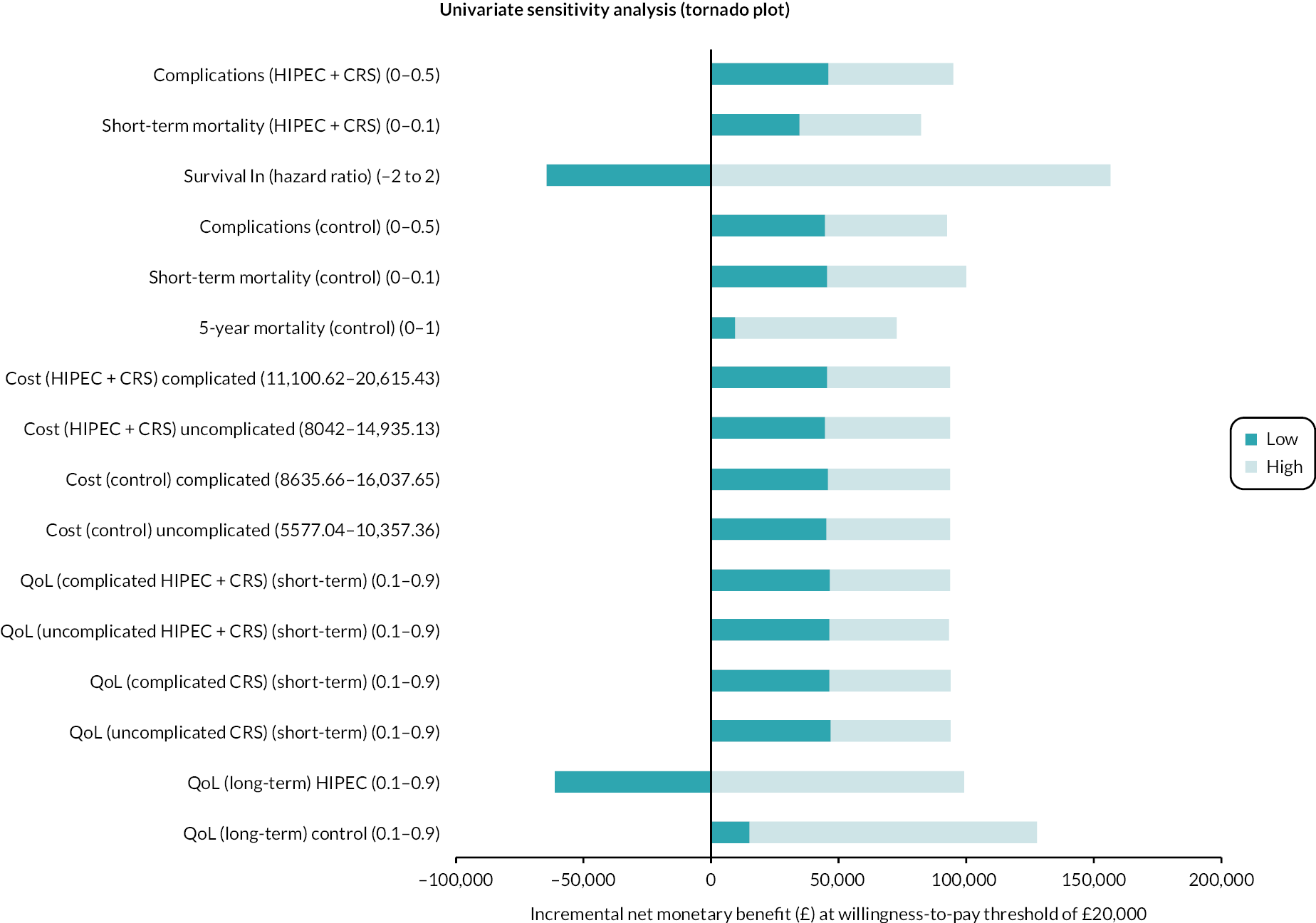
FIGURE 32.
Expected value of perfect parameter information (ovarian cancer: HIPEC + CRS + systemic chemotherapy vs. CRS + systemic chemotherapy).
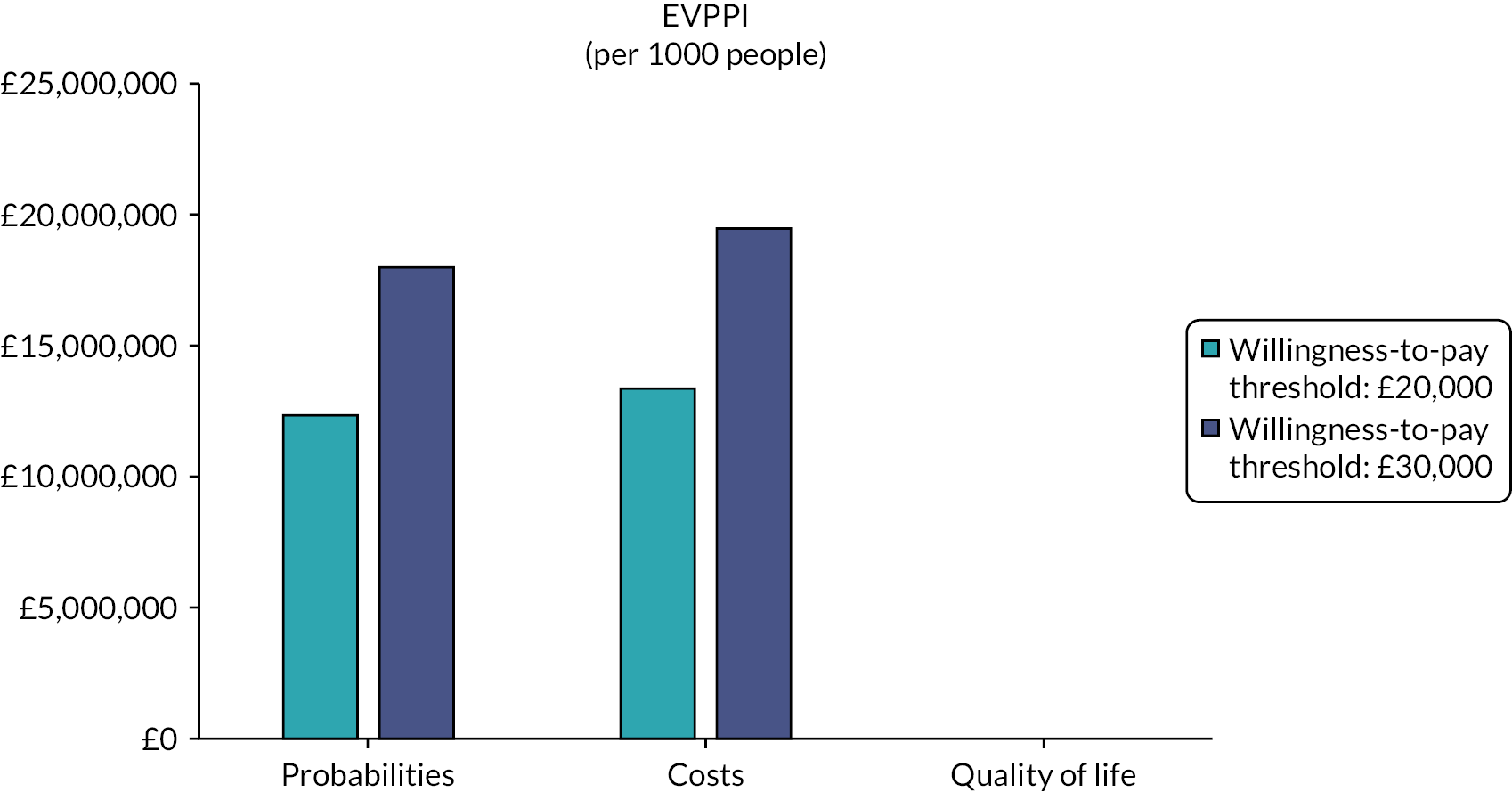
The PSA revealed that there was considerable uncertainty in the incremental NMB (see Table 10). The scatterplot revealed that the points were clustered in the north-east quadrant, confirming that HIPEC + CRS + systemic chemotherapy results in more costs and more QALYs than CRS + systemic chemotherapy (see Figure 29).
The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was 71.9% and 72.4% at WTP of £20,000 and £30,000, respectively. The CEAC curve indicated that the likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was around 70% at WTP thresholds >£10,000 up to £60,000 (see Figure 30).
The univariate sensitivity analysis revealed that HIPEC + CRS + systemic chemotherapy was cost-effective compared to CRS + systemic chemotherapy for most of the parameters for the entire range tested (see Table 11). The main parameters when the intervention becomes cost-effective were when HIPEC + CRS + systemic chemotherapy results in better survival and better long-term HRQoL compared to CRS + systemic chemotherapy (see Table 11; Figure 31).
The EVPI was between £13 and £19 million per 1000 people (see Table 12). The EVPPI shows that the main uncertainties appear to be in the probabilities and costs (see Table 13; Figure 32).
Sensitivity analysis
Sensitivity analysis using real-life data did not make major changes to the conclusions of the cost-effectiveness analysis.
Summary of cost-effectiveness analysis
The summary of cost-effectiveness analysis is available in Table 14.
| Cancer type | Controla | Probability of being cost-effective (%) | Incremental net benefits (deterministic analysis) | Incremental net benefits (PSA) |
|---|---|---|---|---|
| WTP threshold: £20,000 | ||||
| Colorectal cancer | CRS + systemic chemotherapy | 46.5 | −£6162.83 | −£2073 (95% CI −£122,112 to £137,008) |
| Colorectal cancer | Systemic chemotherapy alone | 89.3 | £107,909.46 | £104,520 (95% CI −£46,759 to £227,057) |
| Gastric cancer | CRS + systemic chemotherapy | 69.8 | £14,174.73 | £12,664 (95% CI −£254,806 to £270,104) |
| Gastric cancer | Systemic chemotherapy alone | 63.0 | £81,796.38 | £62,606 (95% CI −£263,733 to £330,185) |
| Ovarian cancer | CRS + systemic chemotherapy | 71.9 | £46,761.81 | £43,942 (95% CI −£90,407 to £176,080) |
| WTP threshold: £30,000 | ||||
| Colorectal cancer | CRS + systemic chemotherapy | 47.6 | −£6164.19 | −£141 (95% CI −180,212 to £208,473) |
| Colorectal cancer | Systemic chemotherapy alone | 90.3 | £167,621.58 | £162,513 (95% CI −£64,427 to £345,845) |
| Gastric cancer | CRS + systemic chemotherapy | 70.3 | £22,955.89 | £20,688 (95% CI −£380,551 to £406,649) |
| Gastric cancer | Systemic chemotherapy alone | 64.9 | £127,768.23 | £98,773 (95% CI −£390,946 to £500,566) |
| Ovarian cancer | CRS + systemic chemotherapy | 72.4 | £71,938.23 | £67,684 (95% CI −£133,694 to £265,809) |
Chapter 6 Discussion
Systematic review
Summary of main results
This systematic review included a total of eight RCTs. A total of 955 participants in seven RCTs were included in quantitative analysis. All comparisons other than those for ovarian cancer contained only one trial.
In people with peritoneal metastases from colorectal cancer, HIPEC + CRS + systemic chemotherapy probably results in little to no difference in all-cause mortality or progression-free survival and results in increased complications compared to CRS + systemic chemotherapy. In the same patient group, HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy alone.
In people with gastric cancer and peritoneal metastases, there is very low certainty about the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on all-cause mortality. In the same patient group, HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy.
In women with stage III or greater epithelial ovarian cancer requiring interval CRS after chemotherapy, HIPEC + CRS + systemic chemotherapy probably results in lower all-cause mortality compared to CRS + systemic chemotherapy.
Although the exploratory panoramic meta-analysis showed that there is little or no difference in the all-cause mortality in any of the cancer types, it should be noted that clinically, it is probably inappropriate to combine the different cancer types. Therefore, the results from the analysis where the different cancer types are analysed separately should be used for clinical decisions.
The overall HRQoL was assessed only in ovarian cancer. HIPEC + CRS + systemic chemotherapy may result in little to no difference in overall HRQoL compared to CRS + systemic chemotherapy.
Controversies in interpretation of data
Clinical experts in treatment of peritoneal metastases have raised concerns about the PRODIGE-7 trial. 225 In addition, when we presented our results and interpretation to clinicians and the research steering group of this project, concerns were raised about our recommendations based on PRODIGE-7.
The major concerns about the PRODIGE-7 trial were as follows.
-
The first concern was about the control group used in PRODIGE-7 trial: the control group used was CRS + systemic chemotherapy62 rather than systemic chemotherapy alone, which was the main alternative to HIPEC + CRS + systemic chemotherapy at the time when PRODIGE-7 trial began. The trial by Verwaal et al. ,14 which resulted in wider adoption of HIPEC + CRS + systemic chemotherapy, found that HIPEC + CRS + systemic chemotherapy resulted in a 45% relative reduction in the hazard rate of deaths compared to systemic chemotherapy alone. This trial by Verwaal et al. was designed to answer whether HIPEC + CRS + systemic chemotherapy decreased mortality than fluorouracil-based systemic chemotherapy commonly used at that time, and the trial answered that question with the least amount of bias. The trial by Verwaal et al. was not intended or designed to find which component of the complex treatment was responsible for the survival benefit and whether there was synergy (positive interaction) between the different components. When a complex intervention such as HIPEC + CRS is given in addition to systemic chemotherapy, the effect observed in the trial by Verwaal et al. could have been because of the surgery (CRS), the prolonged wash of the abdomen, the heat, the additional chemotherapy agent (mitomycin) given intraperitoneally or a combination of these. In the PRODIGE-7 trial, the investigators tested the added value of HIPEC (which requires special equipment, additional drugs and technicians with expertise in running the machine) to CRS, which requires mainly surgical expertise. This was an excellent and clinically relevant comparison, as it would have been unethical to use systemic chemotherapy alone as the comparator group in PRODIGE-7 when the trial by Verwaal et al. 14 showed a large survival benefit.
-
The second concern was about the sample size of the study. The trial was designed to measure a reasonably large benefit with HIPEC (a relative reduction of hazard rate of 37.5%), but this benefit was less than that observed in Verwaal et al. (45%). 14 There were no concerns raised about the expected benefit of HIPEC used to determine the sample size in PRODIGE-7 trial until the results of the PRODIGE-7 trial became available, indicating that clinicians and researchers assumed that the effect observed in the trial by Verwaal et al. was mostly due to HIPEC.
-
The third concern was about the use of HIPEC in patients in the CRS arm who developed recurrent peritoneal metastases [16/132 (12%)]. The PRODIGE-7 trial authors performed an intention-to-treat analysis and calculated the effect of whether a patient with colorectal peritoneal metastases should receive HIPEC + CRS + systemic chemotherapy or CRS + systemic chemotherapy when they present with colorectal peritoneal metastases. They also performed a per-protocol analysis which excluded these participants: the effect of HIPEC + CRS + systemic chemotherapy compared to CRS + systemic chemotherapy on survival was not changed by this per-protocol analysis.
-
The fourth concern was about the agent used and its dose and duration. A proportion of participants in both groups in the PRODIGE-7 trial received preoperative oxaliplatin-based systemic chemotherapy, which could make the cancer cells resistant to the drug used in HIPEC (and therefore reduce its effectiveness). The median survival of the participants in the HIPEC + CRS + systemic chemotherapy group in PRODIGE-7 trial was 41.8 months which was much higher than that observed in the participants in the HIPEC + CRS + systemic chemotherapy group in the trial by Verwaal et al. , which was 22.4 months. The differences are probably due to the improvements in the systemic chemotherapy, the perioperative care and the general improvement in health care over time. However, this makes the hypothesis that the ‘lack of effect of HIPEC in PRODIGE-7 was because of inadequate HIPEC’ unlikely and the hypothesis that ‘the major effect observed in the HIPEC + CRS + systemic chemotherapy in the trial by Verwaal et al. was because of CRS and/or the additional chemotherapy agent used’ more likely.
-
Another concern was the conclusion that HIPEC + CRS + systemic chemotherapy was not better than CRS + systemic chemotherapy when the survival was better in a subgroup of patients with Peritoneal Cancer Index (PCI) of 11–15 in PRODIGE-7. As the PRODIGE-7 authors correctly point out, the promising results of HIPEC + CRS + systemic chemotherapy versus CRS + systematic chemotherapy based on an exploratory post hoc analysis can be used to guide further research. The main reasons why these cannot be used to guide clinical practice are that the participant randomisation was not stratified by PCI, the analysis was post hoc and the classification of patients into PCI groups of < 11, 11–15 and > 15 was based on the data observed rather than a pre-existing classification into these subgroups. Furthermore, there is considerable uncertainty on how to measure PCI before or during surgery. 226 The PCI measured during open surgery is a considerable overestimation of PCI compared to that based on pathology. 227 It is unlikely that the PCI measured by diagnostic laparoscopy is better than that during open surgery. This uncertainty makes PCI measured preoperatively or during operation an unreliable tool to select patients for HIPEC + CRS + systemic chemotherapy.
-
Another major concern raised was whether it was appropriate to change existing clinical practice based on the few RCTs included in this project and whether we should rely on non-randomised studies to guide the treatment; after all, one does not need a RCT to find the effectiveness of a parachute while jumping from height. There are several reasons why this analogy of comparing the effectiveness of HIPEC + CRS + systemic chemotherapy based on non-randomised studies to the effectiveness of parachute (based on non-randomised studies) is inappropriate.
-
The first reason is the reliability of evidence from non-randomised studies. We did not find any non-randomised study in which similar participants with colorectal peritoneal metastases underwent HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy or systemic chemotherapy alone. Therefore, any non-randomised studies comparing HIPEC + CRS + systemic chemotherapy and systemic chemotherapy alone are likely to be heavily biased in favour of HIPEC + CRS + systemic chemotherapy, as patients who have limited cancer spread and likely to withstand major surgery would have received HIPEC + CRS + systemic chemotherapy, while those with more extensive cancer spread or unlikely to withstand major surgery would have received the control intervention of systemic chemotherapy alone. The evidence from such non-randomised studies with confounding bias is likely to be low- or very low-certainty evidence. The GRADE handbook provides some guidance on the scenarios when strong recommendations can be made based on low-certainty evidence. The scenario that is closest to the argument of parachute analogy is when low-quality evidence suggests benefit in a life-threatening situation. CRS + systemic chemotherapy provides equivalent median survival of 41 months as HIPEC + CRS + systemic chemotherapy. When there is an existing, less invasive treatment that provides equivalent survival, it can hardly be considered life-threatening to warrant recommendations based on low or very low-certainty evidence.
-
The second reason is that treatments should be based on best available evidence. By best available evidence, we mean evidence from RCTs and well-designed and delivered observational studies at low risk of bias rather than based on healthcare professional’s memory of the results from clinical practice. This is because of confirmation bias. Confirmation bias is a form of bias that gives preferential treatment to evidence supporting existing beliefs over those that counter the belief. 228 This can happen unwittingly and therefore might be an unconscious bias. 228 Because of this confirmation bias, the healthcare professionals may remember their successes more than their failures with HIPEC + CRS + systemic chemotherapy if they believe that HIPEC + CRS + systemic chemotherapy is beneficial to patients (and it is reasonable to say that it is because of this belief that they recommend HIPEC + CRS + systemic chemotherapy to the patient). One way to overcome this bias is to have a prospective, independently verifiable register in which all people with peritoneal metastases are enrolled prior to making treatment decisions. If such a prospective register shows that the median survival in people who have limited peritoneal involvement and likely to withstand major surgery have better survival with HIPEC + CRS + systemic chemotherapy than CRS + systemic chemotherapy, then one can make a case for using HIPEC + CRS + systemic chemotherapy over CRS + systemic chemotherapy. But until such time, the treatment decisions should be based on currently available moderate- or high-certainty evidence.
-
It should also be noted that HIPEC + CRS + systemic chemotherapy costs considerably more than CRS + systemic chemotherapy. Even if HIPEC + CRS + systemic chemotherapy is performed at expert centres, there is no clinical reasoning to expect lower complication rates and length of hospital stay with HIPEC + CRS + systemic chemotherapy compared to CRS + systemic chemotherapy. In a state-funded healthcare system with limited resources, the resources should be spent on maximising the health of the whole population. Therefore, HIPEC + CRS + systemic chemotherapy using any regimen cannot be recommended over CRS + systemic chemotherapy in a state-funded healthcare system such as NHS until new evidence emerges.
-
There were other recommendations which warrant further explanation. We have made a strong recommendation for CRS + systemic chemotherapy versus systemic chemotherapy alone for colorectal cancers. Moderate-certainty evidence indicated that HIPEC + CRS + systemic chemotherapy improved survival compared to systemic chemotherapy alone. While we acknowledge that the systemic chemotherapy used in the comparison of HIPEC + CRS + systemic chemotherapy is not the current treatment regimen used for disseminated colorectal cancers and the comparison was between HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy alone (rather than CRS + systemic chemotherapy vs. systemic chemotherapy alone), the survival in the control arm of PRODIGE-7 suggests that using CRS + systemic chemotherapy can result in median survival of 41 months; the median survival of disseminated colorectal cancers in England between 2013 and 2017 was < 1 year. 229 This is indirect evidence for the survival benefit of CRS + systemic chemotherapy compared to systemic chemotherapy alone. However, because of the indirectness in evidence, the certainty of evidence will be downgraded to low. As mentioned previously, there are some situations that strong recommendations can be made using GRADE system despite low-certainty evidence. As low-certainty evidence suggests considerable survival benefits with CRS + systemic chemotherapy in a situation with very poor survival in the absence of CRS, we have made a strong recommendation for CRS + systemic chemotherapy when adequate expertise is available.
For gastric cancer, there is high uncertainty about the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on all-cause mortality. The evidence suggests that HIPEC + CRS + systemic chemotherapy probably decreases all-cause mortality compared to systemic chemotherapy. However, the results are based on a single trial of 17 participants. Furthermore, the effects were estimated from Kaplan–Meier curves, which might potentially have resulted in errors. Because of the very small number of participants included in a single trial, estimation of effect estimates from Kaplan–Meier curves and the very low certainty related to whether HIPEC + CRS + systemic chemotherapy offers any benefit over CRS + systemic chemotherapy in patients with gastric cancer and peritoneal metastases, we have indicated no recommendation as to whether HIPEC + CRS + systemic chemotherapy or systemic chemotherapy alone should be used in people with gastric cancer and peritoneal metastases.
For stage III or greater epithelial ovarian cancer undergoing interval CRS, HIPEC + CRS + systemic chemotherapy probably results in lower all-cause mortality compared to CRS + systemic chemotherapy. It may result in little to no difference in HRQoL or number of people who developed serious adverse events compared to CRS + systemic chemotherapy. Although the number of serious adverse events per participant is probably higher with HIPEC + CRS + systemic chemotherapy than with CRS + systemic chemotherapy, this has to be put into context of lower mortality compared to CRS + systemic chemotherapy. Therefore, HIPEC + CRS + systemic chemotherapy should be routinely offered to women with ovarian cancer and peritoneal metastases.
Certainty of evidence
The certainty of evidence was moderate for most comparisons. Most trials were at low risk of bias for all-cause mortality. Because of the nature of the comparison, it is not possible to blind the healthcare providers to the treatment groups. However, as per the RoB 2.0 tool, this does not result in bias because all-cause mortality is an objective outcome. The main reason for downgrading the evidence related to imprecision is because of the small sample sizes in the trials and the overall comparisons.
Overall, the balance of benefits and harms appears to be favourable for HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy in stage III or greater epithelial ovarian cancer requiring interval CRS because of improvement in survival with HIPEC + CRS + systemic chemotherapy but not for other cancers. The balance of benefits and harms appears to be against HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for colorectal cancer, as the HIPEC group had more serious complications than CRS + systemic chemotherapy. It is highly likely that longevity of life and HRQoL are the two major outcomes that determine the treatment choices of people with cancer, and there is nothing to suggest that improvement in one is associated with worsening of the other. Therefore, we have made strong recommendations for clinical practice for HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for colorectal and ovarian cancers.
Overall completeness and applicability of evidence
We included only colorectal cancer, gastric cancer and stage III or greater epithelial ovarian cancer with peritoneal metastases. The participants included in the trials were adults who were likely to withstand major surgery. Most trials excluded people with extraperitoneal metastases. Therefore, these results are applicable in only people with metastases confined to the peritoneum.
It should be noted that all trials included in this review included systemic chemotherapy in both arms. Therefore, the evidence applies to people with peritoneal metastases receiving systemic chemotherapy.
It should also be noted that the findings of this review are not applicable to patients without peritoneal metastases; therefore, this review does not answer the question whether HIPEC is useful in people without peritoneal metastases who undergo CRS + systemic chemotherapy and cannot be used to guide clinical practice in people without peritoneal metastases. This review also does not answer the question whether peritoneal recurrence after treatment of peritoneal metastases (with or without HIPEC) should be treated with HIPEC.
The clinical recommendations related to CRS + systemic chemotherapy in colorectal peritoneal metastases are only applicable in centres with adequate expertise to assess the patients and perform CRS + chemotherapy, as all the evidence supporting this treatment was performed in centres that were performing this as part of HIPEC + CRS + systemic chemotherapy.
After having reviewed the dates of completion and the sample sizes in the ongoing trials, it is unlikely that our recommendations will changed in the next 5 years. However, any availability of trial results should be evaluated for their potential to change recommendations. Therefore, the results of this research and recommendations are applicable until the availability of the results of major new trials.
Potential biases in the review process
We performed a thorough search of literature. Two reviewers independently identified studies and extracted data. We followed the standard methodology for analysing the data. These are the strengths of the review process.
We were unable to obtain IPD as planned. IPD would have allowed us to refine our effect estimates for subgroups of people with peritoneal metastases from colorectal, gastric or ovarian cancer. It is difficult to estimate whether our conclusions would have changed if we had IPD; however, our systematic review and meta-analysis support similar conclusions as the trial authors, suggesting that the impact of IPD may not be major enough to warrant an IPD once the health services have recovered from the impact of COVID-19.
We estimated the HR for survival for gastric cancer trials13,64 from Kaplan–Meier curves using methods described by Parmar et al. for extracting survival data for meta-analysis. 230 We also used a survival probability of 1% in the systemic chemotherapy alone group for 24 months to allow calculations beyond 12 months, as none of the participants in the systemic chemotherapy alone survived at 12 months. 64 This was to take the survival benefit for HIPEC + CRS + systemic chemotherapy at 12 months and beyond into account. This might have introduced bias in the calculation of CIs. However, because of the small number of participants and the estimations that we have performed to calculate the effect estimates, we have concluded that there is uncertainty in the benefit of HIPEC + CRS + systemic chemotherapy in gastric cancers.
A major limitation of this research is the paucity of RCTs that could be included, which can influence the certainty of the evidence and recommendations. Our recommendations are based on existing best level of evidence on the topic and may change when new evidence from low risk of bias trials becomes available.
Agreements and disagreements with other studies or reviews
This is the first systematic review of RCTs evaluating the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy or systemic chemotherapy alone in people with peritoneal metastases from colorectal, gastric or stage III or greater epithelial ovarian cancers. We agree with the study authors for all the comparisons. We also agree with the recent ESMO (European Society for Medical Oncology) Clinical Practice Guideline on metastatic colorectal cancer which suggested that HIPEC for colorectal peritoneal metastases should only be considered in the experimental setting and CRS + systemic chemotherapy should be considered as the treatment of choice. 231 We also agree with the recent ASCO (American Society of Clinical Oncology) guidelines on the treatment of metastatic colorectal cancer, which recommended against the routine use of HIPEC + CRS + systemic chemotherapy in people with colorectal peritoneal metastases. 232 The ASCO guidelines provided a weak recommendation in favour of CRS + systemic chemotherapy for this group of patients, while we have provided a strong recommendation in favour of CRS + systemic chemotherapy. The differences in the strength of recommendation are probably due to our team applying the special circumstances (explained previously) for making strong recommendations in the presence of low-certainty evidence.
For gastric cancer, we have indicated no recommendation as compared to the Italian Association of Medical Oncology guidelines of strong recommendation against the use of HIPEC + CRS + systemic chemotherapy. 233 Some potential reasons for the differences in recommendations may be differences in methodology. There were some differences in the estimation of HRs for survival. However, even if we used the effect estimates used by methodologists involved in Italian Association of Medical Oncology guidelines, our conclusions about uncertainty in evidence with gastric cancer would not have changed. The difference is likely to be due to the consideration of information from non-randomised studies in the recommendation by the Italian Association of Medical Oncology guidelines. In practical terms, though, in a state-funded healthcare system, our recommendations and those recommended by Italian Association of Medical Oncology guidelines lead to the same result, that is patients are not offered HIPEC + CRS + systemic chemotherapy routinely.
Cost-effectiveness analysis
Summary of main results
In people with colorectal peritoneal metastases, the incremental NMBs at WTP of £20,000 and £30,000 were −£6162.83 and −£6164.19, respectively, that is, incremental NMB was < 0, indicating that HIPEC + CRS + systemic chemotherapy was not cost-effective compared to CRS + systemic chemotherapy in NHS. The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to CRS + systemic chemotherapy was < 50% for WTP thresholds of £20,000 and £30,000. In the same group of people, the incremental NMBs at WTP of £20,000 and £30,000 were £107,909.46 and £167,621.58, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy may be cost-effective compared to systemic chemotherapy alone in NHS. The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 90% for WTP thresholds of £20,000 and £30,000. This was driven by the improved survival in people with colorectal peritoneal metastases undergoing HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy alone, but it should be noted that HIPEC + CRS + systemic chemotherapy was not cost-effective compared to CRS + systemic chemotherapy.
In people with gastric peritoneal metastases, the incremental NMB was positive at WTP thresholds of £20,000 and £30,000, that is, HIPEC + CRS + systemic chemotherapy may be cost-effective compared to CRS + systemic chemotherapy and systemic chemotherapy alone. However, we used the parameters available from the systematic review. Since there is considerable uncertainty around the reliability of those parameters, we cannot conclude that HIPEC + CRS + systemic chemotherapy is cost-effective compared to CRS + systemic chemotherapy and systemic chemotherapy alone.
In women with stage III or greater epithelial ovarian cancer requiring interval CRS, the incremental NMBs at WTP of £20,000 and £30,000 were £81,796.38 and £127,768.23, respectively, that is, incremental NMB was more than zero, indicating that HIPEC + CRS + systemic chemotherapy may be cost-effective compared to systemic chemotherapy alone in NHS. The likelihood of HIPEC + CRS + systemic chemotherapy being cost-effective compared to systemic chemotherapy alone was around 70% for WTP thresholds of £20,000 and £30,000.
Strengths of the study
We followed the published protocol for the key aspects of the cost-effectiveness analysis. Any deviations from the protocol were because of the deviations related to the systematic review. We performed PSA and extensive univariate analysis to test the robustness of the findings. We have also shared the cost-effective calculations and model as supplementary file to allow healthcare decision-makers use parameter estimates from their setting and apply the results of such analysis in their local setting.
Limitations of the study
We obtained the probabilities from the systematic review. While most trials were at low risk of bias, the number of participants included was small: this introduces uncertainty. The median follow-up in the comparisons ranged between 2 and 5 years for the various comparisons. Since we used lifetime time horizon, we extrapolated that the annual rate of deaths was similar to the annual rate of deaths in the trials. It is unlikely that patients can be considered cured of cancer after a median follow-up of 2–5 years. However, we do not have any information to test our assumption since studies in this field do not have median follow-up data beyond 5 years. Future clinical trials on this topic should factor in long-term follow-up by record linkage to test this assumption.
None of the trials used EQ-5D, the preferred utility value for cost-effectiveness using NICE guidance. We were able to obtain the HRQoL from only one trial (in ovarian cancer); this trial used EORTC CLQ-C30, which we mapped to EQ-5D. We used HRQoL from observational studies in patients with advanced cancers to estimate the HRQoL in people who underwent potentially curative or palliative treatments. This introduces additional uncertainty in the results.
There is no HRG code for CRS. We used the costs for complex general abdominal procedures to estimate the costs for CRS. For information on the variability in the costs, we have included 30% variability in the costs, a practice widely followed in health economic analysis. Better estimation of costs and their variability can decrease the uncertainty in costs. This can be performed as standalone research or as part of clinical trials. However, probabilities were important sources of uncertainty in the EVPPI of all comparisons, and HRQoL were important sources of uncertainty in the EVPPI of many of the comparisons. Therefore, standalone cost estimation studies are unlikely to result in better information for decision-making.
While the small sample size in trials is taken into account during calculations of the uncertainty, there is currently no method of integrating the risk of bias in the trials, errors in calculation of effect estimates and reproducibility of results in the cost-effectiveness analyses. Therefore, the cost-effectiveness results may not be accurate when the studies are at high risk of bias, when the results are based on a single study or when the calculations of estimates are subject to error. This is a limitation of the studies included in the systematic review rather than the methods used in the cost-effectiveness analysis per se.
We did not perform a cost-effectiveness analysis that considers the three interventions HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy versus systemic chemotherapy alone in a single analysis because of the same reasons for not performing the network analysis comparing these treatments simultaneously because of concerns about transitivity assumption in the included trials. Therefore, we have presented the cost-effectiveness analyses from HIPEC + CRS +/– systemic chemotherapy versus CRS +/– systemic chemotherapy and HIPEC + CRS +/– systemic chemotherapy versus systemic chemotherapy alone as separate analyses.
While the stability tests (see Appendix 8) indicated that the coefficient of variation was < 2% for HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy for colorectal cancer and gastric cancer and HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for ovarian cancer, the coefficient of variation was around 2.5% for HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for gastric cancer and 4% and 30% for HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy for WTP thresholds of £20,000 and £30,000 for colorectal cancer. One needs a computer with high memory to complete the analysis with more simulations than we were able to and much longer processing times. This is only of academic interest as all the analyses showed that the incremental NMBs were in the same direction in all the 30 instances of analysis. Therefore, although the probability of being cost-effective and value of information analyses based on the number of iterations that produce stable results may vary from our report, our conclusions and recommendations would not have been different.
Agreements and disagreements with other cost-effectiveness analyses
Prior to the start of this research, there had also been two formal HTAs on this issue. 27,44 The first HTA reviewing patients with peritoneal disease from colorectal cancer concluded that there was moderate-quality evidence that HIPEC + CRS prolonged survival based on a single RCT, but the costs were high. 27 The second HTA on ovarian cancer did not include any RCTs and concluded there was no clear benefit of HIPEC + CRS for ovarian peritoneal metastases. 44 The major reason for this disagreement with these two studies is because of the availability of additional trials. Our results are broadly in agreement with a cost-effectiveness analysis based on one of the trials included in the systematic review,65 which concluded that HIPEC + CRS + systemic chemotherapy resulted in higher costs and higher QALYs compared to CRS + systemic chemotherapy in women with stage III or greater epithelial ovarian cancer who required interval CRS. 177
Chapter 7 Conclusions
Implications for practice
In people with peritoneal metastases from colorectal cancer, based on the results of PRODIGE-7 trial, HIPEC + CRS + systemic chemotherapy probably results in little to no difference in all-cause mortality or progression-free survival and results in increased complications compared to CRS + systemic chemotherapy. Therefore, HIPEC based on Oxaliplatin regimen used in PRODIGE-7 trial + CRS + systemic chemotherapy should not be used (strong recommendation). Because of the lack of reliability of preoperative or per-operative PCI, the lack of pre-PRODIGE-7 trial standard classification of PCI into PCI < 10, 11–15 and > 15 and pre-defined subgroup analysis based on the PCI classification, HIPEC based on oxaliplatin regimen used in PRODIGE-7 trial + CRS + systemic chemotherapy cannot be recommended for any subgroups.
Because of the median survival observed in the CRS + systemic chemotherapy arm of PRODIGE-7 trial (41 months) and the poor survival observed in people with disseminated colorectal peritoneal metastases (<12 months in England), CRS + systemic chemotherapy should be offered to people with peritoneal metastases from colorectal cancer when the metastases are confined to the peritoneum and when the patient is likely to withstand major surgery in centres that have experience in performing CRS + systemic chemotherapy (strong recommendation).
Because of variability in the results of trials comparing HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy, small number of participants in the trial comparing HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy alone and the methods used to estimate survival in two trials, there is considerable uncertainty as to whether HIPEC + CRS + systemic chemotherapy or CRS + systemic chemotherapy should be offered to patients with gastric cancer and peritoneal metastases (no recommendation).
Based on three trials showing similar survival benefits in women with stage III or greater epithelial ovarian cancer and metastases confined to the abdomen requiring and likely to withstand interval CRS after chemotherapy, HIPEC + CRS + systemic chemotherapy should be offered routinely to such women in centres with experience in performing HIPEC + CRS + systemic chemotherapy (strong recommendation).
Implications for research
The value of information analyses shows that the value of perfect information is more than £10 million for all comparisons other than for the comparison of HIPEC + CRS + systemic chemotherapy versus systemic chemotherapy alone for colorectal peritoneal metastases.
Colorectal cancer
Type of study
Randomised controlled trial.
Participants
People with peritoneal metastases from colorectal cancer but without extraperitoneal metastases eligible to undergo major surgery.
Intervention: HIPEC + CRS + systemic chemotherapy
The pharmacological agent used and the duration of treatment are of considerable debate. A 90-minute HIPEC (temperature 42–43 °C) of mitomycin C (30 mg/m2 body surface area) and a sensitising dose of 400 mg/m2 of 5-FU have been proposed. 225,234
Control: CRS + systemic chemotherapy
CRS and systemic chemotherapy as in the intervention arm.
There is uncertainty in whether preoperative systemic chemotherapy should be given in people with colorectal peritoneal metastases. 235 It is unlikely that a consensus can be reached regarding the timing of systemic chemotherapy in the absence of moderate- or high-certainty evidence. A RCT is unlikely to be possible to answer the question of when the systemic chemotherapy in the intervention and control arms should be given alongside HIPEC, as we do not recommend HIPEC + CRS + systemic chemotherapy as the SoC. Therefore, we recommend that the trial participants be stratified by whether they received preoperative chemotherapy at the time of randomisation.
Outcomes
The primary outcome could be all-cause mortality or progression-free survival. Concerns have been raised about the PRODIGE 7 trial since 14/132 (10.6% of participants in the CRS + systemic chemotherapy group) received HIPEC after peritoneal recurrence. 225 However, by using an intention-to-treat analysis as used by Quénet et al. ,62 one would obtain an unbiased estimate of the treatment decision whether HIPEC should be used routinely along with CRS + systemic chemotherapy. Therefore, all-cause mortality can be used as a primary outcome. The advantage of using all-cause mortality is that we are using a direct clinical objective measure that is important to patients. However, to conduct a trial based on the mortality is likely to require a very long follow-up, as the median survival of participants in the HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy arms was 41.7 months and 41.2 months, respectively: it is difficult to conduct studies to detect differences in all-cause mortality because of the small difference between the groups and duration of follow-up required in such trials.
It is unlikely that the studies can be powered to detect differences in HRQoL. HIPEC + CRS + systemic chemotherapy is more invasive than CRS + systemic chemotherapy; in the best-case scenario for HIPEC + CRS + systemic chemotherapy, the short-term HRQoL can be expected to be similar in the two groups.
Time-to-disease progression is a surrogate subjective outcome for all-cause mortality and has been proposed as the primary outcome for assessing the effectiveness of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy. It is reasonable to use this measure since the effect of HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy on time-to-disease progression appears to be consistent with that on all-cause mortality in the few trials that reported both time-to-disease progression and all-cause mortality. In the PRODIGE-7 trial, the time to disease progression in the HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy was 13.1 months and 11.1 months, respectively. Based on an alpha error of 0.05, power of 0.8, accrual time of 36 months and additional follow-up after recruitment of last patient of 24 months, 641 participants are required in each group (1282 participants in total before loss to follow-up). Allowing a 5% loss to follow-up, 1350 participants will be required. If a power of 0.9 is used, 858 participants are required in each group (1716 participants in total) before loss to follow-up, and 1807 participants are required after a 5% loss to follow-up.
There are no core outcome measures for this patient group. However, we suggest including patient-reported outcome measures such as pain, nausea, vomiting, constipation, diarrhoea, dyspnoea, insomnia, depression and physical function236 as trial outcomes to help with shared decision-making. We also recommend that long-term follow-up by health records be considered to ensure that the time-to-disease progression is a good surrogate outcome for all-cause mortality.
The presence of incomplete CRS may introduce heterogeneity in the treatment effects. Future trials should investigate the effect of completeness of CRS as a potential effect modifier.
Gastric cancer
Type of study
Randomised controlled trial.
Participants
People with gastric cancer and peritoneal metastases but without extraperitoneal metastases eligible to undergo major surgery.
Intervention and control
Similar considerations as for colorectal cancer.
Outcomes
Similar considerations as for colorectal cancer. However, the sample size calculations are different, as the prognosis of patients with gastric cancer and peritoneal metastases appears to be less than that of patients with colorectal cancer and peritoneal metastases. When Rau et al. report their study63 fully, the sample size calculations can be based on that.
Ovarian cancer
Types of studies
-
Implementation research: barriers/facilitators for implementing routine HIPEC + CRS + systemic chemotherapy in women with stage III or greater epithelial ovarian cancer who require interval CRS.
-
Service delivery research: regional versus local services for treatment of women with stage III epithelial ovarian cancer who require and are suitable for interval CRS.
-
RCT: HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy in women with stage III or greater epithelial ovarian cancer who undergo primary CRS. This is already being evaluated in OVHIPEC-2.
People with extraperitoneal metastases localised to lung or liver
Feasibility of a trial of HIPEC + CRS + treatment of lung or liver metastases by ablation (or surgery) + systemic chemotherapy versus CRS + treatment of lung or liver metastases by ablation (or surgery) + systemic chemotherapy versus palliative treatment.
Equality, diversity and inclusion
Other than the ovarian cancer trials, the proportion of women and men in the trials was similar, with women consisting of 40–50% of the overall sample size. There was no report in the trials about inclusion of people with disabilities. There was also no report of ethnicity in the trials. Therefore, we are unable to comment whether the proportion of trial participants who belonged to different ethnic groups was similar to that in the population.
Our research team was diverse, with representation from many ethnic groups and genders.
Additional information
Contributions of authors
Kurinchi Gurusamy (https://orcid.org/0000-0002-0313-9134) was involved in data collection, wrote the manuscript and is the guarantor of this manuscript.
Jeffrey Leung (https://orcid.org/0000-0003-2348-9702) was involved in data collection and drafting of the article.
Claire Vale (https://orcid.org/0000-0001-5157-0634) critically revised the manuscript.
Danielle Roberts (https://orcid.org/0000-0003-1515-5702) was involved in data collection and critically revised the manuscript.
Audrey Linden (https://orcid.org/0000-0002-2255-4958) was involved in data collection and critically revised the manuscript.
Xiao Wei Tan (https://orcid.org/0000-0003-4752-4074) was involved in data collection and critically revised the manuscript.
Priyal Taribagil (https://orcid.org/0000-0002-6516-3615) was involved in data collection and critically revised the manuscript.
Sonam Patel (https://orcid.org/0000-0003-3313-2600) was involved in data collection and critically revised the manuscript.
Elena Pizzo (https://orcid.org/0000-0003-0790-7505) critically revised the manuscript.
Brian Davidson (https://orcid.org/0000-0002-9152-5907) critically revised the manuscript.
Tim Mould (https://orcid.org/0009-0003-1558-6382) critically revised the manuscript.
Mark Saunders (https://orcid.org/0000-0002-7195-4712) critically revised the manuscript.
Omer Aziz (https://orcid.org/0000-0002-3765-2702) critically revised the manuscript.
Sarah O’Dwyer (https://orcid.org/0000-0002-0726-3220) critically revised the manuscript.
All authors approved this manuscript for publication.
Acknowledgements
We acknowledge the advice provided by Prof Catrin Tudur Smith, University of Liverpool, UK; Ms Lindy Berkman, Bowel Cancer Research UK and Prof Edward Wilson, University of Exeter, UK. Their invaluable advice has resulted in improvement in the methods and interpretation of data. Ms Lindy Berkman also helped with drafting the plain language summary.
Sponsor
University College London
Deviations from the original protocol
Systematic review
-
We were unable to perform an IPD meta-analysis as planned because of unforeseen circumstances related to COVID-19. This led to trialists who were also clinical researchers being unable to engage for transfer of IPD. We do not foresee that study authors (surgeons) will be sufficiently engaged with providing IPD in the near future because of the backlog with surgeries and the fatigue induced by COVID-19. Therefore, we performed a meta-analysis based on aggregate data.
-
We did not combine CRS and palliative systemic chemotherapy together as control group. This was because of the results of PRODIGE-7 trial in which participants who received CRS and systemic chemotherapy which was the control group had considerably longer survival than trials in which palliative systemic chemotherapy was used as the control group. Therefore, we planned to perform network meta-analysis comparing HIPEC + CRS +/– systemic chemotherapy, CRS +/– systemic chemotherapy and systemic chemotherapy alone to account for the considerable differences in the survival between CRS and systemic chemotherapy versus palliative systemic chemotherapy. However, even this was not possible because of concerns about transitivity assumption in the included trials. Therefore, we have presented the evidence from HIPEC + CRS +/– systemic chemotherapy versus CRS +/– systemic chemotherapy and HIPEC + CRS +/– systemic chemotherapy versus systemic chemotherapy alone as separate analyses.
-
We planned to include people with appendiceal adenocarcinomas under colorectal cancer as they behave in a similar way to colorectal adenocarcinomas. However, we did not find any trials that included appendiceal adenocarcinomas.
-
We resolved all differences through discussion. There was no need for arbitration or sensitivity analysis.
-
As all the trials provided outcomes on participants randomised or at least on participants who were eligible for this study, that is, people with resectable peritoneal metastases, we did not perform the best-case and worst-case scenario analysis.
-
We were unable to perform various planned subgroup analyses because the analysis was based on aggregate and because of the paucity of data.
-
We did not perform the planned sensitivity analyses because of the lack of the IPD data and because of the few trials included under each analysis.
-
We performed additional sensitivity analysis which we have clearly highlighted as post hoc analysis.
Cost-effectiveness analysis
-
For the same reasons discussed above, we performed separate cost-effectiveness analysis for HIPEC + CRS + systemic chemotherapy versus CRS + systemic chemotherapy and
-
For the same reasons discussed above, the summary data rather than IPD data were used for the analysis. As a result, we were unable to perform any of the planned subgroup analyses.
-
As separate data were not available for most aspects in people who underwent complete CRS, this was not included in the decision tree.
-
Short-term mortality was considered at 30 days (rather than 90 days), as reported by most trials.
Patient and public involvement
Patients and public were involved in the design, conduct and interpretation of data of this research as part of steering committee. There were no difficulties or obstacles in the process of involving patients and public in this research. Ms Lindy Berkman, Bowel Cancer Research UK was involved in the research steering committee and suggested addition of patient-reported outcome measures to the outcomes. They were also involved in the interpretation of data and the implications for clinical practice. They were also involved in drafting the plain language summary.
While they were disappointed by the paucity of patient-reported outcome measures in clinical trials on this topic, their comments allowed this research to be relevant not only to clinicians but also ensures that it has taken into consideration the concerns and needs of the patients and public. The extent of patient involvement in this research will help promote more patient and public involvement in future research and will empower patients to continue provide valuable advice.
Data-sharing statement
All summary data have been shared as online supplements. All data used for health economics data are available as online supplement. Any other requests can be directed to the corresponding author.
Ethics statement
This project was approved by the UCL Research Ethics Committee (Ethics number: 16023/001) on 24th September 2019.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/KWDG6338.
Primary conflicts of interest: The promotions and salary of Kurinchi Gurusamy depend on high-quality research and publications. The clinical practice of the clinicians in the project: Tim Mould, Mark Saunders, Omer Aziz and Sarah O’Dwyer may be altered by the findings of the review.
Kurinchi Gurusamy declares support for the present manuscript from NIHR and receipt of equipment, materials, drugs, medical writing, gifts or other services from NIHR. Jeffrey Leung, Danielle Roberts and Sarah O’Dwyer declare support for the present manuscript: NIHR. Elena Pizzo was a member of the HTA Clinical Evaluation and Trials Committee 2019–23, and declares support from NIHR ARC. Claire Vale, Audrey Linden, Xiao Wei Tan, Priyal Taribagil, Sonam Patel, Brian Davidson, Tim Mould, Mark Saunders and Omer Aziz declare no conflict or disclosure of interest.
Information governance statement
Not applicable as we used summary data and IPD was not performed.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publication
Gurusamy K, Leung J, Vale C, Roberts D, Linden A, Tan XW, et al. Cytoreductive surgery plus hyperthermic intraoperative peritoneal chemotherapy for people with peritoneal metastases from colorectal, ovarian or gastric origin: A systematic review of randomized controlled trials [published online ahead of print Apr 24 2024] World J Surg 2024. https://doi.org/10.1002/wjs.12186. PMID: 38658171.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Gurusamy K, Vale CL, Pizzo E, Bhanot R, Davidson BR, Mould T, et al. Cytoreductive surgery (CRS) with hyperthermic intraoperative peritoneal chemotherapy (HIPEC) versus standard of care (SoC) in people with peritoneal metastases from colorectal, ovarian or gastric origin: protocol for a systematic review and individual participant data (IPD) meta-analyses of effectiveness and cost-effectiveness. BMJ Open 2020;10.
- International Agency for Research on Cancer (World Health Organization) . Estimated Number of Incident Cases, Both Sexes, All Cancers Excluding Non-Melanoma Skin Cancer, Worldwide in 2012 2017. https://gco.iarc.fr/today/online-analysis-table?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=0&statistic=0&prevalence=0&color_palette=default (accessed 3 December 2017).
- Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011;128:2717-25.
- Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6.
- Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012;99:699-705.
- van Gestel YRBM, Thomassen I, Lemmens VEPP, Pruijt JFM, van Herk-Sukel MPP, Rutten HJT, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol 2014;40:963-9.
- Baratti D, Kusamura S, Pietrantonio F, Guaglio M, Niger M, Deraco M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit Rev Oncol Hematol 2016;100:209-22.
- Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622-8.
- Quere P, Facy O, Manfredi S, Jooste V, Faivre J, Lepage C, et al. Epidemiology, management, and survival of peritoneal carcinomatosis from colorectal cancer: a population-based study. Dis Colon Rectum 2015;58:743-52.
- Klaver YL, Lemmens VE, Creemers GJ, Rutten HJ, Nienhuijs SW, de Hingh IH. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann Oncol 2011;22:2250-6.
- Hwang M, Jayakrishnan TT, Green DE, George B, Thomas JP, Groeschl RT, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer 2014;50:1747-57.
- Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570-5.
- Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81.
- Verwaal VJ, Van Ruth S, De Bree E, Van Slooten GW, Van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43.
- Razenberg LG, van Gestel YR, Creemers GJ, Verwaal VJ, Lemmens VE, de Hingh IH. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol 2015;41:466-71.
- Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32.
- Elias D, Goere D, Dumont F, Honore C, Dartigues P, Stoclin A, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332-40.
- Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, et al. The 30-year experience – a meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer 2017;79:1-14.
- Schaaf L, van der Kuip H, Zopf W, Winter S, Munch M, Murdter TE, et al. A Temperature of 40 °C appears to be a critical threshold for potentiating cytotoxic chemotherapy in vitro and in peritoneal carcinomatosis patients undergoing HIPEC. Ann Surg Oncol 2015;22:S758-65.
- Seretis C, Youssef H. Quality of life after cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: a systematic review. Eur J Surg Oncol 2014;40:1605-13.
- Huang CQ, Min Y, Wang SY, Yang XJ, Liu Y, Xiong B, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget 2017;8:55657-83.
- Cavaliere D, Cirocchi R, Coccolini F, Fagotti A, Fambrini M, Federici O, et al. Italian Society of Surgical Oncology (SICO) . 1st evidence-based Italian consensus conference on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinosis from ovarian cancer. Tumori 2017;103:525-36.
- Baratti D, Scivales A, Balestra MR, Ponzi P, Di Stasi F, Kusamura S, et al. Cost analysis of the combined procedure of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol 2010;36:463-9.
- Bonastre J, Chevalier J, Elias D, Classe JM, Ferron G, Guilloit JM, et al. Cost-effectiveness of intraperitoneal chemohyperthermia in the treatment of peritoneal carcinomatosis from colorectal cancer. Value Health 2008;11:347-53.
- Chua TC, Martin S, Saxena A, Liauw W, Yan TD, Zhao J, et al. Evaluation of the cost-effectiveness of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (peritonectomy) at the St George hospital peritoneal surface malignancy program. Ann Surg 2010;251:323-9.
- Lee Z, Chia C, Teo M. Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy cost-effective for metastatic colorectal cancer?. Ann Surg Oncol 2016;23.
- Ludwigs K, Breimer ME, Brorson F, Carlsson G, Daxberg EL, Hjalmarsson Y, et al. Cytoreductive surgery and intraperitoneal chemotherapy (HIPEC or EPIC) in patients with colorectal adenocarcinoma and peritoneal carcinomatosis (Structured abstract). Health Technol Assess Database 2013;4.
- Naffouje SA, O’Donoghue C, Salti GI. Evaluation of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a community setting: a cost–utility analysis of a hospital’s initial experience and reflections on the health care system. J Surg Oncol 2016;113:544-7.
- Tentes AAK, Pallas N, Korakianitis O, Mavroudis C, Spiridonidou A, Zorbas G, et al. The cost of cytoreductive surgery and perioperative intraperitoneal chemotherapy in the treatment of peritoneal malignancy in one Greek institute. J BUON 2012;17:776-80.
- Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009;16:2152-65.
- Ceelen WP, Hesse U, de Hemptinne B, Pattyn P. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg 2000;87:1006-15.
- Chia CS, Seshadri RA, Kepenekian V, Vaudoyer D, Passot G, Glehen O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: a systematic review. Pleura Peritoneum 2016;1:67-7.
- Chua TC, Esquivel J, Pelz JO, Morris DL. Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol 2013;107:566-73.
- Di Vita M, Cappellani A, Piccolo G, Zanghi A, Cavallaro A, Bertola G, et al. The role of HIPEC in the treatment of peritoneal carcinomatosis from gastric cancer: between lights and shadows. Anticancer Drugs 2015;26:123-38.
- Eveno C, Pocard M. Randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: a systematic review. Pleura Peritoneum 2016;1:169-82.
- He TF, Chen ZR, Xing CG. Cytoreductive surgery combined with intraperitoneal chemotherapy in the treatment of colorectal peritoneal metastasis: a meta-analysis. Int J Clin Exp Med 2016;9:20562-70.
- Hotouras A, Desai D, Bhan C, Murphy J, Lampe B, Sugarbaker PH. Heated IntraPEritoneal Chemotherapy (HIPEC) for patients with recurrent ovarian cancer: a systematic literature review. Int J Gynecol Cancer 2016;26:661-70.
- Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2015;41:1578-89.
- Lopez-Lopez V, Cascales-Campos PA, Schneider MA, Gil J, Gil E, Gomez-Hidalgo NR, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in elderly patients. A systematic literature review. Surg Oncol 2016;25:378-84.
- Mirnezami R, Moran BJ, Harvey K, Cecil T, Chandrakumaran K, Carr N, et al. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J Gastroenterol 2014;20:14018-32.
- Kireeva GS, Gafton GI, Guseynov KD, Senchik KY, Belyaeva OA, Bespalov VG, et al. HIPEC in patients with primary advanced ovarian cancer: is there a role? A systematic review of short- and long-term outcomes. Surg Oncol 2018;27:251-8.
- Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J. Clinical studies in CRS and HIPEC: trials, tribulations, and future directions – a systematic review. J Surg Oncol 2018;117:245-59.
- Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34.
- Ubago-Pérez R, Matas-Hoces A, Beltrán-Calvo C, Romero-Tabares A. Hyperthermic intraperitoneal chemotherapy. Efficacy and safety in the treatment of ovarian cancer peritoneal carcinomatosis (Structured abstract). Health Technol Assess Database 2013. https://database.inahta.org/article/14082 (accessed 27 February 2023).
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21:837-41.
- Hemming K, Pinkney T, Futaba K, Pennant M, Morton DG, Lilford RJ. A systematic review of systematic reviews and panoramic meta-analysis: staples versus sutures for surgical procedures. PLOS ONE 2013;8.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 4 June 2018).
- Treasury H. The Green Book- Central Government Guidance on Appraisal and Evaluation. HM Treasury; 2022.
- Gurusamy KS, Riviere D, van Laarhoven CJH, Besselink M, Abu-Hilal M, Davidson BR, et al. Cost-effectiveness of laparoscopic versus open distal pancreatectomy for pancreatic cancer. PLOS ONE 2017;12.
- ONS . Euro to GBP Exchange 2022. www.ons.gov.uk/economy/nationalaccounts/balanceofpayments/timeseries/thap/mret (accessed 27 February 2023).
- Wilson ECF. A practical guide to value of information analysis. PharmacoEcon 2015;33:105-21.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50.
- Antonio CCP, Alida GG, Elena GG, Rocio GS, Jeronimo MG, Luis ARJ, et al. Cytoreductive surgery with or without HIPEC after neoadjuvant chemotherapy in ovarian cancer: a phase 3 clinical trial. Ann Surg Oncol 2022;29:2617-25.
- Lim MC, Chang SJ, Park B, Yoo HJ, Yoo CW, Nam BH, et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg 2022;157:374-83.
- Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. UNICANCER-GI Group and BIG Renape Group . Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:256-66.
- Rau B, Lang H, Konigsrainer A, Gockel I, Rau HG, Seeliger H, et al. 1376O The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): a randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann Oncol 2021;32.
- Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84.
- van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230-40.
- Anthuber M, Strasmuller A. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for colorectal cancer?. Chirurg 2021;92.
- Arjona-Sanchez A. Hyperthermic intraperitoneal chemotherapy as adjuvant therapy in locally advanced colon cancer. Tech Coloproctol 2021;25:147-8.
- Asero S, Caruso M, Vallone N, Luciani AG, Lombardo V, Terranova G, et al. Cytoreductive surgery (CS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in treatment of peritoneal surface malignances: report of a phase II clinical study. In Vivo 2009;23:645-7.
- Bartlett DL, Ryan DP. The role of HIPEC in patients with advanced colorectal cancer. Clin Adv Hematol Oncol: H&Amp;O 2020;18:103-5.
- Batista TP. Comment on: surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2017;24:630-.
- Batista TP, Carneiro VCG, Tancredi R, Teles ALB, Badiglian L, Leao CS. Neoadjuvant chemotherapy followed by fast-track cytoreductive surgery plus short-course hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: preliminary results of a promising all-in-one approach. Cancer Manag Res 2017;9:869-78.
- Behbakht K, Cohn DE, Straughn JM. Hyperthermic intraperitoneal chemotherapy (HIPEC) is cost-effective in the management of primary ovarian cancer. Gynecol Oncol 2018;151:4-5.
- Cascales Campos PA, Gonzalez-Gil A, Gomez-Ruiz AJ, Gil-Gomez E, Alconchel-Gago F, Navarro-Barrios A, et al. Risk factors and management of incisional hernia after cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal surface malignancies. Hernia 2020;24:257-63.
- Cascales-Campos PA, Gil J, Feliciangeli E, Gil E, Gonzalez-Gil A, Lopez V, et al. The role of hyperthermic intraperitoneal chemotherapy using paclitaxel in platinum-sensitive recurrent epithelial ovarian cancer patients with microscopic residual disease after cytoreduction. Ann Surg Oncol 2015;22:987-93.
- Cashin PH, Mahteme H, Spang N, Syk I, Frodin JE, Torkzad M, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur J Cancer 2016;53:155-62.
- ChiCTR-IIR . A Bidirectional Conversion Treatment for Gastric Cancer With Peritoneal Metastasis: A Prospective Phase II Clinical Research 2017. www.chictr.org.cn/showproj.aspx?proj=20551 (accessed 27 February 2023).
- ChiCTR-INR . Cytoreductive Surgery With Intraperitoneal Hyperthermic Perfusion Chemotherapy Clinical Study on Treatment of Peritoneal Carcinoma and Application 2016. www.chictr.org.cn/showproj.aspx?proj=16497 (accessed 27 February 2023).
- ChiCTR2100043156 . A Prospective, Randomized, Controlled Clinical Trial to Evaluate the Efficacy and Safety of Interval Debulking Surgery Combined With Cisplatin and Docetaxel Hyperthermic Intraperitoneal Chemotherapy in Patients With Advanced Ovarian Cancer 2021. www.chictr.org.cn/showproj.aspx?proj=66616 (accessed 27 February 2023).
- Chua TC, Liauw W, Robertson G, Chia WKJ, Soo KC, AlObaid A, et al. Towards randomized trials of cytoreductive surgery using peritonectomy and hyperthermic intraperitoneal chemotherapy for ovarian cancer peritoneal carcinomatosis. Gynecol Oncol 2009;114:137-9; author reply 139.
- Chua TC, Liauw W, Robertson G, Morris DL. Establishing evidence for change in ovarian cancer surgery – proposing clinical trials of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer peritoneal carcinomatosis. Gynecol Oncol 2009;115:166-8.
- Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med 2019;381:1929-39.
- Cui HB, Ge HE, Bai XY, Zhang W, Zhang YY, Wang J, et al. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp Ther Med 2014;7:1083-8.
- Dellinger TH, Han ES. Cancer Regional Therapy. Cham: Springer International Publishing Ag; 2020.
- Demuytere J, Carlier C, Willaert W, Tummers P, Denys H, Van Kerschaver O, et al. Debulking and HIPEC for ovarian cancer: effects of perfusion temperature on morbidity and cisplatin pharmacokinetics. Eur J Surg Oncol 2021;47.
- Elias D, Pocard M, Goere D. HIPEC with oxaliplatin in the treatment of peritoneal carcinomatosis of colorectal origin. Cancer Treat Res 2007;134:303-18.
- EUCTR-000138-37-Nl . Treatment of PERItoneal Dissemination in Stomach Cancer Patients With CytOreductive Surgery and Hyperthermic IntraPEritoneal Chemotherapy 2013. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2013-000138-37 (accessed 27 February 2023).
- EUCTR-004058-27-It . Randomized Study Comparing Surgery and Local Chemotherapy Versus Standard Chemotherapy in the Treatment of Colorectal Carcinomatosis 2013. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2012-004058-27 (accessed 27 February 2023).
- EUCTR-009467-59-Be . Feasibility Study of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Patients With Stage III or Only Pleural Stage IV Ovarian Carcinoma in First Line Therapy – HIPEC-Ovarian Carcinoma 2010. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2009-009467-59 (accessed 27 February 2023).
- EUCTR2020-005210-18-SE . EFFIPEC – Efficacy of Heated Chemotherapy Administered into the Abdomen 2021. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-005210-18 (accessed 27 February 2023).
- Evrard S, Maziere C, Desolneux G. HIPEC: standard of care or an experimental approach?. Lancet Oncol 2012;13:e462-3.
- Gustave Roussy CCGP . Treatment of a Cancerous Disease of the Peritoneum With Complete Cytoreductive Surgery and Intraperitoneal Chemohyperthermia 2003.
- Harter P, Reuss A, Sehouli J, Chiva L, du Bois A. Brief report about the role of hyperthermic intraperitoneal chemotherapy in a prospective randomized phase 3 study in recurrent ovarian cancer from Spiliotis et al. Int J Gynecol Cancer 2017;27:246-7.
- Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. DESKTOP III Investigators . Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Eng J Med 2021;385:2123-31.
- Hilpert F, Harter P, Pujade-Lauraine E, Reuss A, Pfisterer J, Roy-Coquard I, et al. Complete surgical debulking in advanced ovarian carcinoma improves prognosis in any FIGO stage – analysis of 3126 prospectively randomized patients in AGO-OVAR/GINECO Phase 3 Trials. Onkologie 2010;33.
- Iavazzo C, Spiliotis J. Is there a promising role of HIPEC in patients with advanced mucinous ovarian cancer?. Arch Gynecol Obstet 2021;303:597-8.
- Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol 2018;36:1922-9.
- Jones IHW. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival: An exploratory analysis of a prospectively randomized phase III study of the arbeitsgemeinschaft gynaekologische onkologie ovarian cancer study group (AGO-OVAR). Obstet Gynecol Surv 2007;62:719-20.
- Klempner SJ, Ryan DP. HIPEC for colorectal peritoneal metastases. Lancet Oncol 2021;22:162-4.
- Knodler M, Korfer J, Kunzmann V, Trojan J, Daum S, Schenk M, et al. Randomised phase II trial to investigate catumaxomab (anti-EpCAM x anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer. Br J Cancer 2018;119:296-302.
- Lim SL, Havrilesky LJ, Habib AS, Secord AA. Cost-effectiveness of hyperthermic intraperitoneal chemotherapy (HIPEC) at interval debulking of epithelial ovarian cancer following neoadjuvant chemotherapy. Gynecol Oncol 2019;153:376-80.
- NCT . Hyperthermic Intraperitoneal Chemotherapy for Advanced Gastric Cancer With Peritoneal Metastatis 2017. https://classic.clinicaltrials.gov/ct2/show/NCT03604614 (accessed 27 February 2023).
- NCT . Chemotherapy With or Without Surgery in Treating Patients With Recurrent Ovarian Cancer 2000. http://clinicaltrials.gov/show/NCT00006356 (accessed 27 February 2023).
- NCT . Debulking and Chemotherapy With or Without Intraperitoneal Chemotherapy to Treat Peritoneal Carcinomatosis 2003. https://clinicaltrials.gov/show/NCT00052962 (accessed 27 February 2023).
- NCT . Perioperative Systemic Therapy for Isolated Resectable Colorectal Peritoneal Metastases 2016. https://clinicaltrials.gov/show/NCT02758951 (accessed 27 February 2023).
- NCT . Secondary Cytoreductive Surgery in Platinum-Resistant Recurrent Ovarian Cancers 2021. https://ClinicalTrials.gov/show/NCT04795596 (accessed 27 February 2023).
- NCT04847063 . Individualized Response Assessment to Heated Intraperitoneal Chemotherapy (HIPEC) for the Treatment of Peritoneal Carcinomatosis from Ovarian, Colorectal, Appendiceal, or Peritoneal Mesothelioma Histologies 2021. https://clinicaltrials.gov/show/NCT04847063 (accessed 27 February 2023).
- Petrillo M, Costantini B, Cianci S, Ronsini C, Cosentino F, Shallayeva E, et al. Comparison of quality of life after secondary cytoreductive surgery (SCS) ± HIPEC in recurrent ovarian cancer. Gynecol Oncol 2015;137.
- Pocard M, Boige V. [Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal colorectal carcinomatosis: a newly validated standard whose contribution remains to be assessed]. Bull Cancer 2005;92:151-4.
- Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et al. Gynecologic Oncology Group . Secondary surgical cytoreduction for advanced ovarian carcinoma. N Eng J Med 2004;351:2489-97.
- Rovers KP, Bakkers C, Nienhuijs SW, Burger JWA, Creemers GJM, Thijs AMJ, et al. Perioperative systemic therapy vs. cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases: a phase 2 randomized clinical trial. JAMA Surgery 2021;156:710-20.
- Rovers KP, Bakkers C, Simkens G, Burger JWA, Nienhuijs SW, Creemers GM, et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019;19.
- Rubiales AS, del Valle ML. Survival analysis in a randomized trial of HIPEC in ovarian cancer. Ann Surg Oncol 2017;24:s6-31.
- Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, Greggi S, et al. Randomized controlled phase III study to evaluate secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer-ago desktop III/ENGOT OV20. Int J Gynecol Cancer 2017;27.
- Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021;22:439-49.
- Strohlein MA, Bulian DR, Heiss MM. Clinical efficacy of cytoreductive surgery and hyperthermic chemotherapy in peritoneal carcinomatosis from gastric cancer. Expert Rev Anticancer Ther 2011;11:1505-8.
- van de Laar R, Kruitwagen R, Zusterzeel PLM, Van Gorp T, Massuger L. Correspondence: premature stop of the SOCceR trial, a multicenter randomized controlled trial on secondary cytoreductive surgery netherlands trial register number: NTR3337. Int J Gynecol Cancer 2017;27.
- Vanderburg MEL, Vanlent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian-cancer. N Eng J Med 1995;332:629-34.
- Wang P, Zhou ZX. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: trial results of a phase 2, randomized, clinical trial. Ann Surg Oncol 2017;24:s6-23.
- Wisselink D, Klaver C, Brandt A, Bremers A, Burger J, Van Grevenstein W, et al. Adhesion formation after resection of locally advanced colon cancer within the COLOPEC trial based on standardized reexploration. Colorectal Dis 2019;21:61-2.
- Yonemura Y, Canbay E, Ishibashi H, Hirano M, Mizumoto A, Takao N, et al. Cancer Regional Therapy. Cham: Springer International Publishing Ag; 2020.
- Zhang GY, Chen XC, Pan K, Xia LG, Zuo M, Zheng T. Application of hyperthermic intraoperative intraperitoneal chemotherapy in patients with gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2007;10:362-4.
- Cisplatin uptake in ovarian cancer peritoneal metastases after HIPEC. Pleura Peritoneum 2021;6:eA42-eA3.
- Zivanovic O, Chi DS, Zhou Q, Iasonos A, Konner JA, Makker V, et al. Secondary cytoreduction and carboplatin hyperthermic intraperitoneal chemotherapy for platinum-sensitive recurrent ovarian cancer: an MSK team ovary phase II study. J Clin Oncol 2021;39:2594-604.
- Center MM . HOT: HIPEC in Ovarian Cancer As Initial Treatment 2014. https://classic.clinicaltrials.gov/ct2/show/NCT02124421 (accessed 27 February 2023).
- Center U . Comparing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) Using Mitomycin-C Versus Melphalan for Colorectal Peritoneal Carcinomatosis 2017. https://classic.clinicaltrials.gov/ct2/show/NCT03073694 (accessed 27 February 2023).
- Center WRAM, Institute NC . Standard Therapy With or Without Surgery and Mitomycin C in Treating Patients With Advanced Limited Peritoneal Dissemination of Colon Cancer 2010. https://classic.clinicaltrials.gov/ct2/show/NCT01167725 (accessed 27 February 2023).
- ChiCTR . A Randomized Controlled Study of Modified Cytoreductive Surgery Combined With Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastasis of Gastric Cancer 2020. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000036567 (accessed 27 February 2023).
- Cui SZ, Institute TMUC, Hospital, Hospital CPG, University HM, Southern Medical University C . Efficacy of HIPEC Combined With Systemic Chemotherapy and CRS on Peritoneal Metastases From Gastric Cancer 2017. https://classic.clinicaltrials.gov/ct2/show/NCT03179579 (accessed 27 February 2023).
- Drks . Prospective Multicenter Phase III Clinical Trial Using Cytoreductive Surgery With Hyperthermic Intraoperative Chemotherapy (HIPEC) After Preoperative Chemotherapy in Patients With Peritoneal Carcinomatosis of Gastric Cancer Incl. Adenocarcinoma of the Esophagogastreal Junction 2011. http://www.drks.de/DRKS00003078 (accessed 27 February 2023).
- EUCTR . Assessment of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in First or Secondary Platinum-Resistant Recurrent Ovarian Epithelial Cancer. HIPOVA-01 2019. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-002480-94-FR (accessed 27 February 2023).
- Koemans WJ, van der Kaaij RT, Boot H, Buffart T, Veenhof A, Hartemink KJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 2019;19.
- Koole S, van Stein R, Sikorska K, Barton D, Perrin L, Brennan D, et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int J Gynecol Cancer 2020;30:888-92.
- Liu H, Li G, Su X, Sun Y, Hu J, Huang L, et al. CLASS-05 trial: a randomized controlled phase III trial of cytoreductive surgery 1 hyperthermic intraperitoneal chemotherapy (HIPEC) 1 systemic chemotherapy vesus systemic chemotherapy alone for patients with limited peritoneal carcinomatosis of gastric cancer. Ann Oncol 2017;28.
- NCT . Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC) in Relapse Ovarian Cancer Treatment 2011. https://clinicaltrials.gov/show/NCT01376752 (accessed 27 February 2023).
- NCT05250648 . Clinical Trial on HIPEC With Mitomycin C in Colon Cancer Peritoneal Metastases (GECOP-MMC) 2022. https://clinicaltrials.gov/show/NCT05250648 (accessed 27 February 2023).
- Cui SZ, University SYS-S, Third Affiliated Hospital SYSU, Hospital H, University X, University T . Efficacy of HIPEC As NACT and Postoperative Chemotherapy in the Treatment of Advanced-Stage Epithelial Ovarian Cancer 2018. https://classic.clinicaltrials.gov/ct2/show/NCT03180177 (accessed 27 February 2023).
- Diaz-Montes T, Sittig M, Ryu H, Gushchin V, Sardi A. A phase II randomized study: outcomes after cytoreductive surgery (CRS) with or without carboplatin hyperthermic intraperitoneal chemotherapy (HIPEC) followed by adjuvant chemotherapy as initial treatment of advanced stage (stage III/IV) ovarian, fallopian tube, and primary peritoneal cancer. J Clin Oncol Conf 2016;34.
- NCT . Efficacy of HIPEC in the Treatment of Advanced-Stage Epithelial Ovarian Cancer After Cytoreductive Surgery 2019. https://classic.clinicaltrials.gov/ct2/show/NCT03373058 (accessed 27 February 2023).
- Lambret CO . Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer (CHIPPI) 2019.
- Mexico I . HIPEC in Ovarian Carcinoma Clinical Stage IIIC and IV During Interval Laparotomy 2017.
- NCT . Hyperthermic Intraperitoneal Chemotherapy With Paclitaxel in Advanced Ovarian Cancer 2016.
- NCT . Primary Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) 2018.
- NCT . HIPEC in the Treatment of Stage IIc-IV Epithelial Ovarian Cancer After CRS (HIPECOC) 2019. https://clinicaltrials.gov/show/NCT04280185 (accessed 27 February 2023).
- Salcedo-Hernandez R, Cetina L, Cantu-De-Leon D, Cordoba V, Tiznado R, Isla-Ortiz D, et al. Hyperthermic intraperitoneal chemotherapy in stage IIIC and IV clinical stage ovarian carcinoma during interval laparotomy. Phase II study. Interim analysis of morbility and perioperative mortalityy. Pleura Peritoneum 2018;3:sa326-sa7.
- Euctr-001715-31-Es . Cytoreduction With or Without Intraoperative Intraperitoneal Hyperthermic Chemotherapy (HIPEC) in Patients With Peritoneal Carcinomatosis from Ovarian Cancer, Fallopian Tube or Primary Peritoneal Carcinoma: Randomized Clinical Trial 2011. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2011-001715-31 (accessed 27 February 2023).
- Grosso G, Lotti M, Rossetti D, Ansaloni L, Frigerio L. Cytoreduction and HIPEC vs. only cytoreduction surgery after neoajuvant chemotherapy for treatment of ovarian cancer naive patients: a phase III multi center randomized ongoing trial. Int J Gynecol Cancer 2013;23.
- Hospital Z . Cytoreductive Surgery(CRS) Plus Hyperthermic Intraperitoneal Chemotherapy(HIPEC) With Lobaplatin in Advanced and Recurrent Epithelial Ovarian Cancer 2017. https://clinicaltrials.gov/study/NCT03371693 (accessed 27 February 2023).
- Lyon H. Cytoreductive Surgery and HIPEC in First or Secondary Platinum-resistant Recurrent Ovarian Epithelial Cancer. 2019.
- Classe JM, Campion L, Meeus P, Quenet F, Leblanc E, Werner R, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) a promising treatment for relapsed intraperitoneal ovarian cancer. Chipor an ongoing phase III, European multicentric randomized trial. Int J Gynecol Cancer 2017;27:1467-8.
- Du Bois A, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: final analysis of AGO DESKTOP III/ENGOT-ov20. J Clin Oncol 2020;38.
- Hongbing C, Hospital Z . A Randomized Prospective Trail of HIPEC in Recurrent Ovarian Cancer Patients With HRR Mutation 2020. https://classic.clinicaltrials.gov/ct2/show/NCT04473339 (accessed 27 February 2023).
- NCT . Cytoreductive Surgery and HIPEC in First or Secondary Platinum-Resistant Recurrent Ovarian Epithelial Cancer 2017. https://clinicaltrials.gov/show/NCT03220932 (accessed 27 February 2023).
- NTR . LOROCSON Study: Late Onset Recurrent Ovarian Cancer: Surgery or Not 2005. https://trialregister.nl/trial/306 (accessed 27 February 2023).
- ChiCTR . A Phase III Multicenter Prospective Randomized Controlled Clinical Trial of Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Epithelial Ovarian Cancer After Comprehensive Staging Laparotomy 2019. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900024452 (accessed 27 February 2023).
- El Hajj H, Vanseymortier M, Hudry D, Bogart E, Abdeddaim C, Leblanc E, et al. Rationale and study design of the CHIPPI-1808 trial: a phase III randomized clinical trial evaluating hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer patients treated with primary or interval cytoreductive surgery. ESMO Open 2021;6.
- ChiCTR . A Randomized Controlled Study for Comparing the Efficacy of Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) Added in Primary Epithelial Ovarian Fallopian Tube Carcinoma in Primary Exploration and Interval Debulking Surgery (IDS) With Conventional Therapy 2020. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000028894 (accessed 27 February 2023).
- ChiCTR . A Randomized Controlled Trial for Comparing the Efficacy of Intraperitoneal Hyperthermic Chemotherapy (HIPEC) With Conventional Treatment During Primary Subtractive Surgery (PDS) for Newly Diagnosed Ovarian Fallopian Tube Epithelial Cancer in Stage II–IV 2020. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000034192 (accessed 27 February 2023).
- National Cancer Center, Korea . HIPEC for Platinum-Resistant Recurrent Ovarian Cancer 2022. https://ClinicalTrials.gov/show/NCT05316181 (accessed 27 February 2023).
- NCT . A Randomized Prospective Trail of HIPEC in Recurrent Ovarian Cancer Patients With HRR Mutation 2020. https://ClinicalTrials.gov/show/NCT04473339 (accessed 27 February 2023).
- Wu MF, Wang LJ, Ye YF, Liu CH, Lu HW, Yao TT, et al. Efficacy of neoadjuvant hyperthermic intraperitoneal chemotherapy in advanced high-grade serous ovarian cancer (the NHIPEC trial): study protocol for a randomised controlled trial. BMJ Open 2021;11.
- Anonymous . Cost effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in stage III ovarian cancer on the basis of a randomized phase III trial. Obstet Gynecol Surv 2019;74:592-3.
- Cascales Campos PA, Gonzalez Gil A, Gil Gomez E, Gonzalez Sanchez R, Martinez Garcia J, Alonso Romero JL, et al. ASO visual abstract: cytoreductive surgery with or without HIPEC after Neoadjuvant chemotherapy in ovarian cancer: a phase III clinical trial. Ann Surg Oncol 2022;29:2628-9.
- Charite University BG, Aid GC . Cytoreductive Surgery (CRS) With Without HIPEC in Gastric Cancer With Peritoneal Carcinomatosis 2014. https://classic.clinicaltrials.gov/ct2/show/NCT02158988 (accessed 27 February 2023).
- Chekman C, Layoune R, Hocine O, Raissi N, Hamida AF, Khodja HA, et al. An open prospective randomized trial comparing primary complete cytoreduction surgery to debulking surgery after chemotherapy in advanced stage (FIGO's IIIC) ovarian carcinoma. Int J Gynecol Cancer 2015;25.
- Euctr-003466-34-Nl . Phase III Randomised Clinical Trial for Stage III Ovarian Carcinoma Randomising Between Secondary Debulking Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy (OVHIPEC-I) – OVHIPEC-I 2006. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2006-003466-34 (accessed 27 February 2023).
- Euctr-006175-20-Fr . Essai De Phase III Evaluant La Place De La ChimioHyperthermie IntraPeritoneale Per Operatoire (CHIP) Apres Resection Maximale d’une Carcinose Peritoneale d’origine Colorectale Associee a Une Chimiotherapie Systemique – CHIP 2007. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2006-006175-20 (accessed 27 February 2023).
- Gonzalez Gil A, Gomez Ruiz AJ, Gil Gomez E, Gil Martinez J, Cascales Campos P. Morbidity and mortality after a first cytoreduction with or without HIPEC in ovarian cancer IIIC–IV. Preliminary results of the prospective and randomized clinical trial. Pleura Peritoneum 2018;3.
- Gonzalez Gil A, Gomez Ruiz AJ, Gil Gomez E, Gil Martinez J, Cascales Campos P. Quality of life after a first cytoreduction with or without HIPEC in ovarian cancer IIIC–IV. Preliminary results of the prospective and randomized clinical trial. Pleura Peritoneum 2018;3.
- Institute NC, Center N . Prospective Randomized Trial Comparing Gastrectomy, Metastasectomy Plus Systemic Therapy Versus Systemic Therapy Alone: GYMSSA Trial 2009. https://classic.clinicaltrials.gov/ct2/show/NCT00941655 (accessed 27 February 2023).
- Kerkar SP, Kemp CD, Duffy A, Kammula US, Schrump DS, Kwong KF, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials 2009;10.
- Koole S, Bruijs L, Lahaye M, Fabris C, Sikorska K, Schagen Van Leeuwen J, et al. Location of recurrent disease after cytoreduction with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer: results of the phase III ovhipec study. Int J Gynecol Cancer 2018;28:611-2.
- Koole S, Van Driel W, Kieffer J, Sikorska K, Schagen Van Leeuwen J, Schreuder H, et al. Health-related quality of life after hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer: results of the phase III OVHIPEC study. Ann Oncol 2017;28.
- Koole S, Van Driel W, Sikorska K, Schagen Van Leeuwen J, Schreuder H, Hermans R, et al. Adverse events after hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer: phase III OVHIPEC study. Int J Gynecol Cancer 2017;27:38-9.
- Koole SN, Bruijs L, Fabris C, Sikorska K, Engbersen M, Schagen van Leeuwen JH, et al. Central radiology assessment of the randomized phase III open-label OVHIPEC-1 trial in ovarian cancer. Int J Gynecol Cancer 2020;30:1928-34.
- Koole SN, Kieffer JM, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RH, et al. Health-related quality of life after interval cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with stage III ovarian cancer. Eur J Surg Oncol 2021;47:101-7.
- Koole SN, van Driel WJ, Sonke GS. Hyperthermic intraperitoneal chemotherapy for ovarian cancer: The heat is on. Cancer 2019;125:4587-93.
- Koole SN, van Lieshout C, van Driel WJ, van Schagen E, Sikorska K, Kieffer JM, et al. Cost effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in stage III ovarian cancer on the basis of a randomized phase III trial. J Clin Oncol 2019;37:2041-50.
- Koole SN, Van Lieshout C, Van Driel WJ, Van Schagen E, Sikorska K, Kieffer JM, et al. Cost effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in stage III ovarian cancer on the basis of a randomized phase III trial. Obstet Gynecol Surv 2019;74:592-3.
- Li Y, Yang X, Yang G, Zhou Y, Yonrmura Y. An evaluation of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy on patients with peritoneal carcinomatosis: final results of a phase II prospective and randomized clinical trial. J Clin Oncol Conference: ASCO Annual Meeting 2011;29.
- Li Y, Zhou Y, Xie C, Peng C, Huang C, Yang X, et al. Cytoreductive surgery plus hyperthermic intra-peritoneal chemotherapy for peritoneal carcinomatosis from gastric cancer. Chinese J Clin Oncol 2012;39:1734-40.
- Lim MC, Chang SJ, Yoo HJ, Nam BH, Bristow R, Park SY. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J Clin Oncol Conf 2017;35.
- Murcia F . Cytoreduction With or Without Intraoperative Intraperitoneal Hyperthermic Chemotherapy (HIPEC) in Patients With Peritoneal Carcinomatosis from Ovarian Cancer, Fallopian Tube or Primary Peritoneal Carcinoma 2012. https://classic.clinicaltrials.gov/ct2/show/NCT02328716 (accessed 27 February 2023).
- NCT . Surgery Plus Intraoperative Peritoneal Hyperthermic Chemotherapy (IPHC) to Treat Peritoneal Carcinomatosis 2007. http://clinicaltrials.gov/show/NCT00454519 (accessed 27 February 2023).
- Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. Perioperative outcomes of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal carcinomatosis: PRODIGE 7 randomized trial. Eur J Surg Oncol 2016;42.
- Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of hyperthermic intra-Peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol Conf 2018;36.
- Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis. PRODIGE 7. Eur J Surg Oncol 2019;45.
- Rau B, Loeffler M, Rau HG, Sulkowski U, Kuhlmann J, Weimann A, et al. Perioperative chemotherapy and cytoreductive surgery with versus without HIPEC in gastric cancer with limited peritoneal metastases: a randomized phase III study (GASTRIPEC). J Clin Oncol 2015;33.
- Ubachs J, Koole S, Bruijs L, Lahaye M, Fabris C, Schagen Van Leeuwen J, et al. Loss of skeletal muscle mass during neoadjuvant chemotherapy and the relation to survival in patients with ovarian cancer; a prospective analysis of the ovhipec-1 cohort. Int J Gynecol Cancer 2019;29.
- Ubachs J, Koole S, Bruijs L, Lahaye M, Fabris C, Van Leeuwen JS, et al. Loss of skeletal muscle mass during neoadjuvant chemotherapy and the relation to survival in patients with ovarian cancer: a prospective analysis of the OVHIPEC-1 cohort. J Cachexia, Sarcopenia and Muscle 2020;11:302-3.
- Unicancer . Systemic Chemotherapy With or Without Intraperitoneal Chemohyperthermia in Treating Patients Undergoing Surgery for Peritoneal Carcinomatosis from Colorectal Cancer 2008. https://classic.clinicaltrials.gov/ct2/show/NCT00769405 (accessed 27 February 2023).
- Van Driel W, Sikorska K, Van Leeuwen JS, Schreuder H, Hermans R, De Hingh I, et al. A phase 3 trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer. J Clin Oncol Conf 2017;35.
- Van Driel WJ, Koole S, Sikorska K, Schagen Van Leeuwen J, Schreuder HW, Hermans R, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer. Int J Gynecol Cancer 2017;27.
- Cashin PH, Mahteme H, Syk I, Frodin JE, Glimelius B, Graf W. Quality of life and cost effectiveness in a randomized trial of patients with colorectal cancer and peritoneal metastases. Eur J Surg Oncol 2018;44:983-90.
- Coleman RL, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, Kim B, et al. A phase III randomized controlled trial of secondary cytoreductive surgery (SCS) followed by platinum-based chemotherapy (PC) platinum-sensitive, recurrent ovarian cancer (PSOC)-surgical parameters. Int J Gynecol Cancer 2018;28.
- Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. Obstet Gynecol Surv 2020;75:165-6.
- Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer (vol 385, pg 2123, 2021). N Eng J Med 2022;386.
- Lim S, Havrilesky L, Cohn D, Habib A, Secord A. Cost-effectiveness of hyperthermic intraperitoneal chemotherapy (HIPEC) at interval debulking of epithelial ovarian cancer following neoadjuvant chemotherapy. Int J Gynecol Cancer 2018;28.
- NCT . Cytoreduction and Intraperitoneal Chemotherapy Versus Systemic Chemotherapy in Colorectal Peritoneal Carcinomatosis 2012. http://clinicaltrials.gov/show/NCT01524094 (accessed 27 February 2023).
- NCT . Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC) in Ovarian Cancer Recurrence 2012. http://clinicaltrials.gov/show/NCT01539785 (accessed 27 February 2023).
- Petrillo M, Costantini B, Cianci S, Ronsini C, Scambia G, Fagotti A. Comparison of quality of life after secondary cytoreductive surgery (SCS) ± hipec in recurrent ovarian cancer. Int J Gynecol Cancer 2014;24.
- Rovers K, Burger J, Wiezer R, Aalbers A, Tuynman J, Radema S, et al. Safety and efficacy of perioperative systemic therapy with cytoreductive surgery and HIPEC versus upfront surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: update of a multicentre, openlabel, parallel-group, phase II–III, randomised superiority study (CAIRO6). Pleura Peritoneum 2018;3:sa299-300.
- van de Laar R, Zusterzeel PL, Van Gorp T, Buist MR, van Driel WJ, Gaarenstroom KN, et al. Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): a multicenter randomised controlled study. BMC Cancer 2014;14.
- Zang R, Zhu J, Shi T, Liu J, Tu D, Yin S, et al. A randomized phase III trial of secondary cytoreductive surgery in later recurrent ovarian cancer: SOC1/SGOG-OV2. J Clin Oncol Conf 2020;38.
- Center MSKC, Clinic M, Florida BHS, HealthCare H, Pittsburgh U, Chicago U . Outcomes After Secondary Cytoreductive Surgery With or Without Carboplatin Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Followed by Systemic Combination Chemotherapy for Recurrent Platinum-Sensitive Ovarian, Fallopian Tube, or Primary Peritoneal Cancer 2013. https://classic.clinicaltrials.gov/ct2/show/NCT01767675 (accessed 27 February 2023).
- ChiCTR . A Phase III Multicenter Prospective Randomized Controlled Clinical Trial of Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Epithelial Ovarian Cancer After Comprehensive Staging Laparotomy 2019. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900024452 (accessed 27 February 2023).
- ChiCTR . A Randomized Controlled Study of Modified Cytoreductive Surgery Combined With Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastasis of Gastric Cancer 2020. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000036567 (accessed 27 February 2023).
- Classe JM, Campion L, Ray coquard I, Leblanc E, Quenet F, Meeus P, et al. Chipor (hyperthermic intraperitoneal chemotherapy for ovarian cancer relapse): ongoing randomized phase III study evaluating hyperthermic intraperitoneal chemotherapy in the treatment of ovarian cancer relapse. Int J Gynecol Cancer 2013;23.
- Classe JM, Jaffre I, Ioic C, Meeus P, Leblanc E, Houvenaeghel G, et al. CHIPOR (Hyperthermic Intraperitoneal Chemotherapy [HIPEC]) – A promising treatment for relapsed intraperitoneal ovarian cancer. Chipor an ongoing phase III, European multicentric randomized trial. Unicancer-FEDEGYN 02. Strahlenther Onkol 2018;194.
- Classe JM, Meeus P, Ray-Coquard I, Quenet F, Berton-Rigaud D, Leblanc E, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) a promising treatment for relapsed intraperitoneal ovarian cancer. An ongoing phase III, european multicentric randomized trial. Int J Gynecol Cancer 2015;25:1319-20.
- Du Bois A, Vergote I, Ferron G, Reuss A, Meier W, Greggi S, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. J Clin Oncol Conf 2017;35.
- Euctr-002616-22-It . Efficacy of Intraperitoneal Chemohyperthermia Associated With Cytoreductive Surgery in the Peritoneal Tube Epithelial Neoplasia Advanced-Stage Ovarian 2012. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2012-002616-22 (accessed 27 February 202).
- Institute TNC . Primary Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) 2019. https://classic.clinicaltrials.gov/ct2/show/NCT03772028 (accessed 27 February 2023).
- The Netherlands Cancer Institute . Primary Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) 2020. https://ClinicalTrials.gov/show/NCT03772028 (accessed 27 February 2023).
- Institute TNC, Center EM, Hospital SA, Groningen UMC, Eindhoven CZ . Gastrectomy + Cytoreductive Surgery + HIPEC for Gastric Cancer With Peritoneal Dissemination 2017. https://classic.clinicaltrials.gov/ct2/show/NCT03348150 (accessed 27 February 2023).
- NCT . HIPEC in the Treatment of Stage IIc–IV Epithelial Ovarian Cancer After CRS (HIPECOC) 2020. https://ClinicalTrials.gov/show/NCT04280185 (accessed 27 February 2023).
- NCT05246020 . Efficacy of Neoadjuvant Hyperthermic Intraperitoneal Chemotherapy in Advanced High-Grade Serous Ovarian Cancer (the NHIPEC Trial) 2022. https://clinicaltrials.gov/show/NCT05246020 (accessed 27 February 2023).
- Van Stein RM, Koole SN, Sikorska K, Barton DP, Perrin L, Brennan D, et al. Primary cytoreductive surgery with or without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: the OVHIPEC-2 trial in progress. J Clin Oncol Conf 2020;38.
- Zivanovic O, Chi D, Zhou Q, Iasonos A, Makker V, Grisham RN, et al. A randomized phase II trial of secondary cytoreductive surgery (SCS) ± carboplatin hyperthermic intraperitoneal chemotherapy (HIPEC) in patients (pts) with recurrent platinumsensitive ovarian cancer (EOC). J Clin Oncol Conf 2020;38.
- Leimkuhler M, Hentzen J, Hemmer PHJ, Been LB, van Ginkel RJ, Kruijff S, et al. Systematic review of factors affecting quality of life after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2020;27:3973-83.
- Kim SH, Jo MW, Kim HJ, Ahn JH. Mapping EORTC QLQ-C30 onto EQ-5D for the assessment of cancer patients. Health Qual Life Outcomes 2012;10.
- Malcolm FL, Adiamah A, Banerjea A, Whitehead D, Gupta A, West J, et al. Nottingham Colorectal Service . Long-term health-related quality of life following colorectal cancer surgery: patient-reported outcomes in a remote follow-up population. Colorectal Dis 2021;23:213-25.
- Flyum IR, Mahic S, Grov EK, Joranger P. Health-related quality of life in patients with colorectal cancer in the palliative phase: a systematic review and meta-analysis. BMC Palliat Care 2021;20.
- van der Wielen N, Daams F, Rosati R, Parise P, Weitz J, Reissfelder C, et al. Health related quality of life following open versus minimally invasive total gastrectomy for cancer: results from a randomized clinical trial. Eur J Surg Oncol 2022;48:553-60.
- Carter GC, King DT, Hess LM, Mitchell SA, Taipale KL, Kiiskinen U, et al. Health state utility values associated with advanced gastric, oesophageal, or gastro-oesophageal junction adenocarcinoma: a systematic review. J Med Econ 2015;18:954-66.
- Cashin P, Sugarbaker PH. Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: lessons learned from PRODIGE 7. J Gastrointest Oncol 2021;12:S120-8.
- Simkens GA, Wintjens A, Rovers KP, Nienhuijs SW, de Hingh IH. Effective strategies to predict survival of colorectal peritoneal metastases patients eligible for cytoreductive surgery and HIPEC. Cancer Manag Res 2021;13:5239-49.
- de Boer NL, Brandt-Kerkhof ARM, Madsen EVE, Doukas M, Verhoef C, Burger JWA. The accuracy of the surgical Peritoneal Cancer Index in patients with peritoneal metastases of colorectal cancer. Dig Surg 2021;38:205-11.
- Nickerson RS. Confirmation bias: a ubiquitous phenomenon in many guises. Rev Gen Psychol 1998;2:175-220.
- Cancer Research UK . Bowel Cancer Survival Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival (accessed 27 February 2023).
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34.
- Cervantes A, Adam R, Rosello S, Arnold D, Normanno N, Taieb J, et al. ESMO Guidelines Committee. Electronic address: . Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10-32.
- Morris VK, Kennedy EB, Baxter NN, Benson AB, Cercek A, Cho M, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol 2022;41:678-700.
- Associazione Italiana di Oncologia Medica . Tumori Peritoneali Primitivi Esecondari n.d. https://snlg.iss.it/wp-content/uploads/2022/01/LG-457-AIOM_Peritoneo.pdf (accessed 27 February 2023).
- Arjona-Sánchez A, Barrios P, Boldo-Roda E, Camps B, Carrasco-Campos J, Concepción Martín V, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018;18.
- Flood MP, Kong JCH, Wilson K, Mohan H, Waters PS, McCormick JJ, et al. The Impact of neoadjuvant chemotherapy on the surgical management of colorectal peritoneal metastases: a systematic review and meta-analysis. Ann Surg Oncol 2022;29:6619-31.
- Di Maio M, Basch E, Denis F, Fallowfield LJ, Ganz PA, Howell D, et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline. Ann Oncol 2022;33:878-92.
- Drummond M. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK; New York, NY, USA: Oxford University Press; 2015.
- Foundation Trust Network . Operating Theatres – Maximising a Valuable Resource 2022. https://nhsproviders.org/media/1128/operating-theatres-final.pdf (accessed 27 February 2023).
- Office for National Statistics . Consumer Price Inflation Tables 2022. www.ons.gov.uk/economy/inflationandpriceindices/datasets/consumerpriceinflation (accessed 27 February 2023).
- NHS England . NHS Tariffs November 2022. www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/ (accessed 27 February 2023).
- Joint Formulary Committee . British National Formulary 83 2022.
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLOS ONE 2010;5.
- NHS Reference Costs n.d. www.england.nhs.uk/publication/2020-21-national-cost-collection-data-publication/ (accessed February 2023).
- Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes – a systematic review. Int J Cancer 2012;130:2845-56.
Appendix 1 Search strategies
MEDLINE
-
Hyperthermia, Induced/
-
((hyperthermic or heated) adj3 (intraperitoneal or intra-peritoneal) adj3 (chemotherapy or chemotherapies)).ti,ab.
-
(intraperitoneal adj3 chemohyperthermia).ti,ab.
-
(HIPEC or IPHC or HIIC).ti,ab.
-
1 or 2 or 3 or 4
-
Cytoreduction Surgical Procedures/
-
((cytoreductive or cytoreduction or debulking) adj3 (surgery or surgeries or surgical or procedure or procedures)).ti,ab.
-
6 or 7
-
5 or 8
-
exp Colorectal Neoplasms/
-
exp Ovarian Neoplasms/
-
Stomach Neoplasms/
-
((colorectal or bowel or colon or colonic or rectum or rectal or ovary or ovaries or ovarian or gastric or stomach) adj3 (cancer or cancers or carcinoma or carcinomas or tumour or tumours or tumor or tumors or neoplasm or neoplasms)).ti,ab.
-
10 or 11 or 12 or 13
-
9 and 14
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
-
exp animals/not humans.sh.
-
24 not 25
-
15 and 26
-
(cost: or cost benefit analys: or health care costs).mp.
-
15 and 28
-
27 or 29
EMBASE
-
hyperthermic intraperitoneal chemotherapy/
-
((hyperthermic or heated) adj3 (intraperitoneal or intra-peritoneal) adj3 (chemotherapy or chemotherapies)).ti,ab.
-
(intraperitoneal adj3 chemohyperthermia).ti,ab.
-
(HIPEC or IPHC or HIIC).ti,ab.
-
1 or 2 or 3 or 4
-
cytoreductive surgery/
-
((cytoreductive or cytoreduction or debulking) adj3 (surgery or surgeries or surgical or procedure or procedures)).ti,ab.
-
6 or 7
-
5 or 8
-
exp colon cancer/
-
exp rectum cancer/
-
exp ovary cancer/
-
exp stomach cancer/
-
((colorectal or bowel or colon or colonic or rectum or rectal or ovary or ovaries or ovarian or gastric or stomach) adj3 (cancer or cancers or carcinoma or carcinomas or tumour or tumours or tumor or tumors or neoplasm or neoplasms)).ti,ab.
-
10 or 11 or 12 or 13 or 14
-
9 and 15
-
exp crossover-procedure/ or exp double-blind procedure/ or exp randomized controlled trial/ or single-blind procedure/
-
(((((random* or factorial* or crossover* or cross over* or cross-over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or ervice* or volunteer*).af.
-
17 or 18
-
16 and 19
-
(cost or costs).tw.
-
16 and 21
-
20 or 22
Cochrane
-
MeSH descriptor: (Hyperthermia, Induced) this term only
-
((hyperthermic or heated) near/3 (intraperitoneal or intra-peritoneal) near/3 (chemotherapy or chemotherapies))
-
(intraperitoneal near/3 chemohyperthermia)
-
(HIPEC or IPHC or HIIC)
-
#1 or #2 or #3 or #4
-
MeSH descriptor: [Cytoreduction Surgical Procedures] this term only
-
((cytoreductive or cytoreduction or debulking) near/3 (surgery or surgeries or surgical or procedure or procedures))
-
#6 or #7
-
#5 or #8
-
MeSH descriptor: (Colorectal Neoplasms) explode all trees
-
MeSH descriptor: (Ovarian Neoplasms) explode all trees
-
MeSH descriptor: (Stomach Neoplasms) this term only
-
((colorectal or bowel or colon or colonic or rectum or rectal or ovary or ovaries or ovarian or gastric or stomach) near/3 (cancer or cancers or carcinoma or carcinomas or tumour or tumours or tumor or tumors or neoplasm or neoplasms))
-
#10 or #11 or #12 or #13
-
#9 and #14
Science Citation Index
-
TS=((hyperthermic or heated) near/3 (intraperitoneal or intra-peritoneal) near/3 (chemotherapy or chemotherapies))
-
TS=(intraperitoneal near/3 chemohyperthermia)
-
TS=(HIPEC or IPHC or HIIC)
-
#3 OR #2 OR #1
-
TS=((cytoreductive or cytoreduction or debulking) near/3 (surgery or surger-ies or surgical or procedure or procedures))
-
#5 or #4
-
TS=((colorectal or bowel or colon or colonic or rectum or rectal or ovary or ovaries or ovarian or gastric or stomach) near/3 (cancer or cancers or carci-noma or carcinomas or tumour or tumours or tumor or tumors or neoplasm or neoplasms))
-
TS=(random* or placebo* or blind* or meta-analysis or cost or costs)
-
#8 AND #7 AND #6
WHO trials register
Condition: colorectal OR bowel OR colon OR colonic OR rectum OR rectal OR ovary OR ovaries OR ovarian OR gastric OR stomach
Intervention: HIPEC OR hyperthermic intraperitoneal chemotherapy OR IPHC OR intraperitoneal chemohyperthermia OR HIIC OR heated intraoperative intraperitoneal chemotherapy OR CRS OR CRS
ClinicalTrials.gov
Condition: colorectal OR bowel OR colon OR colonic OR rectum OR rectal OR ovary OR ovaries OR ovarian OR gastric OR stomach
Study Type: Interventional Studies (Clinical Trials)
Intervention/treatment: HIPEC OR hyperthermic intraperitoneal chemotherapy OR IPHC OR intraperitoneal chemohyperthermia OR HIIC OR heated intraoperative intraperitoneal chemotherapy OR CRS OR CRS
Interventional studies, phase 2, 3, 4
Interventional Studies | colorectal OR bowel OR colon OR colonic OR rectum OR rectal OR ovary OR ovaries OR ovarian OR gastric OR stomach | HIPEC OR hyperthermic intraperitoneal chemotherapy OR IPHC OR intraperitoneal chemohyperthermia OR HIIC OR heated intraoperative intraperitoneal chemotherapy OR CRS OR CRS | Phase 2, 3, 4
Cost-effectiveness analysis (CEA) registry
The following terms were searched:
Hyperthermic
Cytoreduction
Cytoreductive
Appendix 2 Methods for panoramic meta-analyses
Model used
(Modified from Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: Heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011; last updated April 2012. www.nicedsu.org.uk)
#Summary, random, subgroup – categorical covariate
# Trial-level data given as treatment differences
# Random effects model for multi-arm trials
# Works for up to 4 arms in a trial
model{ #*** PROGRAM STARTS
for(i in 1:ns2) { #LOOP THROUGH 2-ARM STUDIES
y[i,2] ~ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2-arm trials
#Deviance contribution for trial i
resdev[i] <- (y[i,2]-delta[i,2])*(y[i,2]-delta[i,2])*prec[i,2]
# in case 3-arm and 4-arm studies = 0
Sigma[i,2,2] <- 0
Sigma2[i,2,2] <- 0
Omega[i,2,2] <- 0
Omega2[i,2,2] <- 0
ydiff[i,2]<- 0
z.t[i,2]<- 0
}
for(i in (ns2 + 1):(ns2+ns3)) {# LOOP THROUGH THREE-ARM STUDIES
for (k in 1:(na[i]-1)) {# set variance-covariance matrix
for (j in 1:(na[i]-1)) {
Sigma[i,j,k] <- V[i]*(1-equals(j,k)) + var[i,k+1]*equals(j,k)
# in case 4-arm studies = 0
Sigma2[i,j,k] <- V[i]*(1-equals(j,k)) + var[i,k+1]*equals(j,k)
}
}
Omega[i,1:(na[i]-1),1:(na[i]-1)] <- inverse(Sigma[i,,]) #Precision matrix
Omega2[i,1:(na[i]-1),1:(na[i]-1)] <- inverse(Sigma[i,,]) #Precision matrix
# multivariate normal likelihood for 3-arm trials
y[i,2:na[i]] ~ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]-1),1:(na[i]-1)])
#Deviance contribution for trial i
for (k in 1:(na[i]-1)){# multiply vector & matrix
ydiff[i,k]<- y[i,(k+1)] - delta[i,(k+1)]
z.t[i,k]<- inprod(Omega[i,k,1:(na[i]-1)], ydiff[i,1:(na[i]-1)])
}
resdev[i]<- inprod(ydiff[i,1:(na[i]-1)], z.t[i,1:(na[i]-1)])
}
for(i in (ns2+ns3 + 1):(ns2+ns3+ns4)) {# LOOP THROUGH 4-ARM STUDIES
for (k in 1:(na[i]-1)) {# set variance-covariance matrix
for (j in 1:(na[i]-1)) {
Sigma2[i,j,k] <- V[i]*(1-equals(j,k)) + var[i,k+1]*equals(j,k)}
}
Omega2[i,1:(na[i]-1),1:(na[i]-1)] <- inverse(Sigma2[i,,]) #Precision matrix
# multivariate normal likelihood for 4-arm trials
y[i,2:na[i]] ~ dmnorm(delta[i,2:na[i]],Omega2[i,1:(na[i]-1),1:(na[i]-1)])
#Deviance contribution for trial i
for (k in 1:(na[i]-1)){# multiply vector & matrix
ydiff[i,k]<- y[i,(k+1)] - delta[i,(k+1)]
z.t[i,k]<- inprod(Omega2[i,k,1:(na[i]-1)], ydiff[i,1:(na[i]-1)])
}
resdev[i]<- inprod(ydiff[i,1:(na[i]-1)], z.t[i,1:(na[i]-1)])
}
for(i in 1:(ns2+ns3+ns4)){# LOOP THROUGH ALL STUDIES
w[i,1] <- 0 # adjustment for multi-arm trials is zero for control arm
delta[i,1] <- 0 # treatment effect is zero for control arm
for (k in 2:na[i]) {# LOOP THROUGH ARMS
var[i,k] <- pow(se[i,k],2) # calculate variances
prec[i,k] <- 1/var[i,k] # set precisions
}
for (k in 2:na[i]) {# LOOP THROUGH ARMS
# trial-specific LOR distributions
#categorical covariate – covariate 1 (×1) refers to gastric cancer versus colorectal cancer and covariate 2 (×2) refers to ovarian cancer versus colorectal cancer
delta[i,k] <- temp.delta[i,k] + (beta1[t[i,k]]-beta1[t[i,1]]) * ×1[i] + (beta2[t[i,k]]-beta2[t[i,1]]) * ×2[i]
temp.delta[i,k] ~ dnorm(md[i,k],taud[i,k])
# mean of random effects distributions, with multi-arm trial correction
md[i,k] <- d[t[i,k]] - d[t[i,1]] + sw[i,k]
# precision of random effects distributions (with multi-arm trial correction)
taud[i,k] <- tau *2*(k-1)/k
# adjustment, multi-arm RCTs
w[i,k] <- (delta[i,k] - d[t[i,k]] + d[t[i,1]])
# cumulative adjustment for multi-arm trials
sw[i,k] <- sum(w[i,1:k-1])/(k-1)
}
}
totresdev <- sum(resdev[]) #Total Residual Deviance
d[1]<-0 # treatment effect is zero for reference treatment
beta1[1] <- 0 # covariate 1 effect is zero for reference treatment
beta2[1] <- 0 # covariate 2 effect is zero for reference treatment
# vague priors for treatment effects
for (k in 2:nt){
d[k] ~ dnorm(0,.0001)
beta1[k] <- B1 # common covariate effect (covariate 1)
beta2[k] <- B2 # common covariate effect (covariate 2)
}
B1 ~ dnorm(0,.0001) # vague prior for covariate effect (covariate 1)
B2 ~ dnorm(0,.0001) # vague prior for covariate effect (covariate 2)
sd ~ dunif(0,5) # vague prior for between-trial SD
tau <- pow(sd, −2) # between-trial precision = (1/between-trial variance)
# treatment effect when covariate: ×1, ×2 = 0,0 (colorectal cancer); ×1, ×2 = 1,0 (gastric cancer); ×1, ×2 = 0,1 (ovarian cancer)
d2.colorectal <- d[2]
d2.gastric <- d[2] + (beta1[2]-beta1[1])
d2.ovarian <- d[2] + (beta2[2]-beta2[1])
}
}
} # *** PROGRAM ENDS
Data formatted for analysis in OpenBugs
#Mortality; 1 = CRS; 2 = HIPEC + CRS
# nt = number of treatments, ns2 = 2-arm trials, ns3 = 3-arm trials, ns4 = 4-arm trials
# ×1, ×2 = covariates (×1, ×2: 0, 0 = colorectal cancer; 1, 0 = gastric cancer; 0, 1 = ovarian cancer)
# t[, 1], t[,2] etc: indicate treatment 1, treatment 2, y[,2], y[,3]: treatment difference of treatment 2 vs. treatment 1, treatment 3 vs. treatment 1 etc, se[,2], se[,3]: standard errors of treatment difference of treatment 2 vs. treatment 1, treatment 3 vs. treatment 1 etc, na: number of arms, V: variance in treatment 1 (not required for 2-arm trials)
list(nt = 2,ns2 = 5,ns3 = 0,ns4 = 0, ×1 = c(0,1,0,0,0), ×2 = c(0,0,1,1,1))
| t[,1] | t[,2] | t[,3] | t[,4] | y[,2] | y[,3] | y[,4] | se[,2] | se[,3] | se[,4] | na[] | V[] | #study |
| 1 | 2 | NA | NA | 0 | NA | NA | 0.234561 | NA | NA | 2 | NA | #Quénet 2021 |
| 1 | 2 | NA | NA | −0.96203 | NA | NA | 0.306198 | NA | NA | 2 | NA | #Yang 2011 |
| 1 | 2 | NA | NA | −0.45 | NA | NA | 0.34 | NA | NA | 2 | NA | #Antonio 2022 |
| 1 | 2 | NA | NA | −0.14 | NA | NA | 0.21 | NA | NA | 2 | NA | #Lim 2022 |
| 1 | 2 | NA | NA | −0.40048 | NA | NA | 0.171456 | NA | NA | 2 | NA | #Van Driel 2018 |
| END |
Technical details
A random-effects model was used with common between-study variance for the different cancer types. Three chains were used. The initial values provided were:
list(d=c(NA,0), sd=1,B1=0, B2=0)
list(d=c(NA,-1), sd=4,B1=-1, B2=-1)
list(d=c(NA,2), sd=2,B1=1.5, B2=1.5)
The model was initialised and run for a burn-in of 300,000 simulations to ensure convergence and ran for a further 300,000 simulations. Thin (at 30) and over-relax were used to ensure convergence.
Convergence
Unprocessed results
| Mean | SD | MC_error | val2.5pc | Median | val97.5pc | Start | Sample | |
|---|---|---|---|---|---|---|---|---|
| B1 | −0.9584 | 1.409 | 0.002573 | −3.669 | −0.9612 | 1.778 | 300,001 | 900,000 |
| B2 | −0.3165 | 1.139 | 0.002579 | −2.527 | −0.3176 | 1.897 | 300,001 | 900,000 |
| d2.colorectal | −0.00277 | 0.9864 | 0.002052 | −1.918 | −1.95E-04 | 1.912 | 300,001 | 900,000 |
| d2.gastric | −0.9611 | 1.008 | 0.001175 | −2.906 | −0.9611 | 0.9706 | 300,001 | 900,000 |
| d2.ovarian | −0.3193 | 0.5703 | 7.91E-04 | −1.431 | −0.3175 | 0.7797 | 300,001 | 900,000 |
| SD | 0.5709 | 0.7729 | 0.00142 | 0.01214 | 0.2935 | 3.083 | 300,001 | 900,000 |
| Dbar | Dhat | DIC | pD | |||||
| y | −0.4598 | −4.684 | 3.765 | 4.224 | ||||
| total | −0.4598 | −4.684 | 3.765 | 4.224 |
FIGURE 33.
Convergence
Appendix 3 Additional characteristics of included studies
| Study name | Type of primary cancer | HIPEC | Systemic chemotherapy | Was systemic chemotherapy given preoperatively |
|---|---|---|---|---|
| Quénet et al. 202162 | Colorectal cancer | HIPEC was administered with either the closed or open abdomen techniques according to each centre’s standard approach. In both approaches, systemic chemotherapy (400 mg/m² fluorouracil and 20 mg/m² folinic acid) was delivered intravenously 20 minutes before intraperitoneal infusion of oxaliplatin (460 mg/m² if the open technique was used and 360 mg/m² if the closed technique was used) in 2 l/m² of dextrose at 43 °C over 30 minutes. | The chemotherapy and targeted therapy regimens used were at investigators’ discretion. 110 patients in CRS plus HIPEC group and 109 in the CRS alone group were treated with preoperative chemotherapy. Patients in both groups received a median of 6 cycles of preoperative chemotherapy. 48 (44%) of 133 patients in the HIPEC group and 46 (42%) of patients in the surgery only group received preoperative oxaliplatinbased treatment. | 219/265 (82.6%) received preoperative chemotherapy |
| Verwaal et al. 200314 | Colorectal cancer | To increase the volume of the abdominal cavity and to prevent spillage of lavage fluid, the skin of the laparotomy wound was pulled up against a retractor. A plastic sheet covered the laparotomy opening to reduce heat loss and to avoid drug spilling. A central aperture was made to allow manipulation to achieve optimal drug and heat distribution. The perfusion circuit consisted of a centrally placed inflow catheter, outflow catheters, placement in the pelvis below left and right diaphragm, a roller pump, and a heat exchanger. Temperature probes were attached to inflow and outflow catheters. Perfusion was started with a minimum of 3 l of isotonic dialysis fluid, at 1–2 l/min, and an inflow temperature of 41–42 °C. As soon as the temperature in the abdomen was stable above 40 °C, MMC (mitomycin) was added to the perfusate at a dose of 17.5 mg/m2 followed by 8.8 mg/m2 every 30 minutes. The total dose was limited to 70 mg at maximum. If the core temperature exceeded 39 °C, the inflow temperature was reduced. After 90 minutes, the perfusion fluid was drained from the abdomen, and bowel continuity was restored. | Chemotherapy was given in the local setting, usually by the patients’ own medical oncologist, and consisted of fluorouracil (intravenous [IV] push-dose of 400 mg/m2) and leucovorin (IV 80 mg/m2) on an outpatient basis (modified Laufman regimen). Treatment was given weekly for 26 weeks, or until progression, death or unacceptable toxicity. | No |
| Yang et al. 201113 | Gastric cancer | After surgery, HIPEC was performed before closure of abdominal cavity, as this open technique is believed to provide optimal thermal homogeneity and spatial diffusion, with 120 mg of cisplatin and 30 mg of mitomycin C each dissolved 6 l of heated saline (drug concentration cisplatin 20 lg/ml, mitomycin C 5 lg/ml). An outflow tube for perfusion was placed in Douglas’ pouch just before HIPEC. The heated perfusion solution was infused into the peritoneal cavity at a rate of 500 ml/minute through the inflow tube introduced from an automatic hyperthermia chemotherapy perfusion device (ES-6001, Wuhan E-sea Digital Engineering, Wuhan, China). The skin of the abdomen is attached to a retractor ring and a plastic sheet covered the open wound to keep the temperature stable. The perfusion in the peritoneal cavity was stirred manually with care not to infuse directly on the bowel surface. The temperature of the perfusion solution in peritoneal space was kept at 43.0 ± 0.5 °C and monitored with a thermometer on real time. The total HIPEC time was 60–90 minutes, after which the perfusion solution in the abdominal cavity was removed through the suction tube, and drainage tubes were placed at appropriate sites depending on the type of primary operation. | Not stated | Not stated |
| Rau et al. 202163 | Gastric cancer | CRS + The HIPEC treatment consisted of mitomycin C 15 mg/m2 and cisplatin 75 mg/m2, in 5 l of saline (60 minutes, 42 °C) | Preoperative chemotherapy 3 cycles, each cycle 21 days. Patients with negative or unknown HER-2 status receive epirubicin 50 mg/m² infusion (maximum 100 mg/day). Oxaliplatin 130 mg/m² infusion (maximum 260 mg/day) and capecitabine oral 625 mg/m² two times a day (maximum 2500 mg/day). Patients with positive HER-2 status received Cisplatin: 80 mg/m² infusion (maximum of 160 mg/day). Capecitabine: oral 1000 mg/m2 (two times a day maximum of 4000 mg/day), on day 1–14. Trastuzumab: 8 mg/kg infusion (on cycle 1 and 6 mg/kg on cycle 2 and 3). 4–12 weeks after surgery, 3 cycles of postoperative chemotherapy were applied. |
Yes |
| Rudloff et al. 201464 | Gastric cancer | Hyperthermic intraperitoneal chemotherapy (HIPEC) was administered using a closed circuit of oxaliplatin solution at 460 mg/m2 in 5% dextrose in water (D5W) at 41 °C for 30 minutes. Prior to perfusion a single dose each of fluorouracil (5-FU) 400 mg/m2 IV in 50 ml D5W and leucovorin 20 mg/m2 IV in 50 ml D5W were administered over 5 minutes to enhance the effect of regional oxaliplatin delivered IP. The perfusion flow rate was then maintained at ~2.0 l/min and a perfusate volume, which moderately distends the abdominal cavity, correlating with intra-abdominal pressures of 5–15 mm Hg (2.0 l/m2). | Within 14 days of study randomization patients began FOLFIXIRI treatment (in the systemic chemotherapy arm; in the HIPEC + CRS arm, systemic chemotherapy was started within 8 weeks of surgical resection). Systemic chemotherapy was administered once every 14 days and repeated for 12 cycles (approximately 6 months). On treatment day #1 irinotecan was administered IV over 90 minutes followed by leucovorin and oxaliplatin, given concomitantly over 2 hours, followed by 5-FU given via continuous infusion (CIV) over 48 hours. | No |
| Van Driel et al. 201865 | Ovarian cancer | HIPEC was administered at the end of the cytoreductive surgical procedure with the use of the open technique. In brief, the abdomen was filled with saline that circulated continuously with the use of a roller pump through a heat exchanger. By circulation of the heated saline, an intra-abdominal temperature of 40 °C (104 °F) was maintained. Perfusion with cisplatin at a dose of 100 mg/m2 and at a flow rate of 1 l/minute was then initiated (with 50% of the dose perfused initially, 25% at 30 minutes, and 25% at 60 minutes). The perfusion volume was adjusted such that the entire abdomen was exposed to the perfusate. The HIPEC procedure took 120 minutes in total, including the 90-minute perfusion period. At the end of the perfusion, drains were used to empty the abdominal cavity as completely as possible. To prevent nephrotoxicity, sodium thiosulphate was administered at the start of perfusion as an intravenous bolus (9 g/m2 in 200 ml), followed by a continuous infusion (12 g/m2 in 1000 ml) over 6 hours. | Patients received 3 cycles of neoadjuvant chemotherapy with carboplatin (area under the curve of 5–6 mg/ml/ minute) and paclitaxel (175 mg/m2 of body-surface area). Patients received an additional 3 cycles of carboplatin and paclitaxel after surgery. | Yes |
| Antonio et al. 202260 | Ovarian cancer | At the end of the surgery, HIPEC was administered by the open technique (Coliseum) to the patients of the experimental arm according to the following scheme: cisplatin 75 mg/m2 diluted for perfusion in 3 l of dialysis fluid (Dialisan, Shanghai Plop Medical Technology Co., Ltd, China), with circulation maintained in a constant flow of 0.5–0.7 l/minute longer than 60 minutes. Two intra-abdominal thermometers positioned in the pelvis and diaphragmatic area were used to monitor the temperature during perfusion, with maintenance of a constant temperature between 42 and 43.8 °C. During the intervention, the temperature was strictly controlled through an oesophageal thermometer, with the objective of keeping the patient normothermic (37.8 °C), using physical measures and serotherapy | All the patients were treated with a minimum of 3 cycles of systemic NACT with carboplatin (AUC 5) and paclitaxel (175 mg/m2) before surgery. After recovery and hospital discharge, up to 6 cycles of systemic adjuvant chemotherapy were completed per patient with the same carboplatin and paclitaxel scheme. | Yes |
| Lim et al. 202261 | Ovarian cancer | Intraoperative HIPEC (75 mg/m2 of cisplatin) was perfused through a closed technique with a target temperature of 41.5 °C for 90 minutes using the Belmont Hyperthermia Pump system (Belmont Instrument Corporation). Women randomized to the HIPEC group received blanket cooling, intravenous cold fluid hydration, and ice pack application over the head before and during HIPEC procedures. After the cytoreductive and reconstructive surgical procedures, two inflow and two outflow tubes were placed in the pelvic cavity and in the subdiaphragmatic space, respectively. The abdominal wall was closed in layers with a water-tight fit, and 0.9% normal saline was injected into the closed abdominal cavity. After smooth circulation to and from the HIPEC pump was confirmed, the chemotherapeutic agent was mixed with the circulating fluid. During the 90-minute HIPEC perfusion procedure, the patients were gently shaken from side to side to ensure even distribution of the chemotherapeutic agent within the peritoneal cavity. Sodium thiosulfate was not used in the initial 71 cases, given the low incidence of serum creatinine elevation in the phase 2 study. However, in the remaining 21 patients, 4 g/m2 of sodium thiosulfate was administered as a bolus infusion immediately before HIPEC, and 12 g/m2 was administered over 6 hours during and after the HIPEC procedures. | During postoperative recovery, if the patients could tolerate a general diet without evidence of active infection and with an acceptable clinical condition to sustain chemotherapy, we administered 6 cycles of intravenous paclitaxel and carboplatin in both groups. | 77/184 (41.8%) received preoperative chemotherapy |
| Study name | Type of primary cancer | Drugs | Temperature (°C) | Duration (minutes) | Technique (open or closed) |
|---|---|---|---|---|---|
| Quénet et al. 202162 | Colorectal cancer | Oxaliplatin (IP) + IV fluorouracil + IV folinic acid | 43 | 30 | Either |
| Verwaal et al. 200314 | Colorectal cancer | Mitomycin | 41–42 | 90 | Open |
| Rudloff et al. 201464 | Gastric cancer | Oxaliplatin (IP) + IV fluorouracil + IV folinic acid | 41 | 30 | Closed |
| Yang et al. 201113 | Gastric cancer | Cisplatin + mitomycin | 43 | 60–90 | Open |
| Rau et al. 202163 | Gastric cancer | Cisplatin + mitomycin | 42 | 60 | Not stated |
| Van Driel et al. 201865 | Ovarian cancer | Cisplatin | 40 | 90 | Open |
| Antonio et al. 202260 | Ovarian cancer | Cisplatin | 42–43.8 | 60 | Open |
| Lim et al. 202261 | Ovarian cancer | Cisplatin | 41.5 | 90 | Closed |
Appendix 4 Excluded studies: reasons for exclusion
| Reason for exclusion | Excluded study references |
|---|---|
| Not a RCT | 66–74, 79, 80, 83, 85, 86, 88, 90–92, 95, 98, 100, 108, 112, 115, 118, 120 |
| Not in people with peritoneal metastases or no separate data on people with peritoneal metastases | 12, 81, 82, 87, 93, 102, 107, 109, 113, 114, 116, 119, 121 |
| Primary cancer type not clear | 103, 117 |
| Not HIPEC + CRS | 75, 76, 84, 96, 97, 101, 122 |
| Not investigating the effect of HIPEC | 77, 78, 89, 99, 104, 106, 110, 111 |
| Withdrawn due to poor accrual | 105 |
| Incorrect reference | 94 |
Appendix 5 Ongoing studies
| Reference | Participants | Intervention | Control | Outcome measures | Planned duration of follow-up | Start date | Anticipated end date |
|---|---|---|---|---|---|---|---|
| Mercy Medical Center124 | Stage III or IV ovarian cancer (n = 48) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: postoperative complication rates Secondary: QoL, overall survival, progression-free survival |
5 years | April 14 | April 28 |
| University of Kansas Medical Center125 | Peritoneal surface disease (PSD) due to colorectal cancer or high-grade appendiceal cancer (n = 100) | HIPEC (melphalan) + CRS | HIPEC (mitomycin C) + CRS | Primary: comprehensive complication index (CCI) score | 2 years | July 17 | July 25 |
| Walter Reed Army Medical Center, National Care institute126 | Stage III or IV colon cancer (n = 340) |
HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: overall survival Secondary: progression-free survival, QoL |
1 year | July 10 | Current status unknown (estimated completion in May 14) |
| ChiCTR154 | Stage ICI-IIIB ovarian cancer (n = 300) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: survival rate Secondary: disease-free survival, side effects of the program |
5 years | July 19 | Not stated |
| ChiCTR127 | Gastric adenoma with limited peritoneum implantation (involving peritoneum <2 areas) (n = 42) | HIPEC + CRS | CRS | Primary: overall survival Secondary: progression-free survival |
2 years | August 20 | March 23 |
| Classe et al.149 | Intraperitoneal first epithelial ovarian cancer relapse (more than 6 months after the end of the initial treatment), resectable without distant metastasis (n = 415) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: overall survival Secondary: progression-free survival |
4 years | April 11 | December 22 |
| Cui et al.128 | Gastric adenocarcinoma with peritoneal carcinomatosis (n = 88) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: overall survival Secondary: risk factors for morbidity and mortality |
3 years | August 17 | August 22 |
| Cui et al.136 | Stage III–IV primary epithelial ovarian cancer, tubal cancer and primary peritoneal cancer (n = 263) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: disease-free survival Secondary: overall survival, risk factors for morbidity and mortality, QoL |
3 years | March 18 | July 22 |
| Diaz-Montes et al.137 | Stage III–IV ovarian, fallopian tube and primary peritoneal cancer (n = 48) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: postoperative complication rates Secondary: overall survival, progression-free survival, QoL |
5 years | April 14 | April 28 |
| Drks et al.129 | Gastric cancer with peritoneal carcinomatosis and Krukenberg tumours (n = 180) | HIPEC + CRS+ systemic chemotherapy | CRS + systemic chemotherapy | Primary: overall survival Secondary: safety (30 days complication rate), progression-free survival, QoL, adverse events, length of hospitalisation |
2.5 years | March 14 | Not stated |
| Grosso et al.146 | Stage III ovarian cancer (n = 94) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: disease-free survival Secondary: overall survival, morbidity, length of hospital stay, return to normal activities, QoL |
5 years | October 13 | Not stated |
| Hongbing and Hospital151 | Primary or recurrence ovarian, peritoneal or fallopian tube epithelial cancer; first intra-abdominal recurrence without distant metastasis (including unique resectable pleural metastasis which are platinum-sensitive; resectable single lymphatic metastasis retroperitoneal or inguinal) (n = 280) | HIPEC + CRS+ systemic chemotherapy | CRS + systemic chemotherapy | Primary: progression-free survival Secondary: overall survival, serious adverse events |
3 years | August 20 | December 23 |
| Affiliated Cancer Hospital & Institute of Guangzhou Medical University138 | Stage III primary epithelial ovarian cancer, tubal cancer, and primary peritoneal cancer (n = 310) | HIPEC + CRS+ systemic chemotherapy | CRS + systemic chemotherapy | Primary: recurrence-free survival Secondary: overall survival, progression-free survival, QoL, risk factors for morbidity and mortality |
3 years | October 19 | July 23 |
| Zhongnan Hospital147 | Stage III primary or recurrence ovarian, peritoneal or fallopian tube epithelial cancer (n = 112) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: overall survival Secondary: progression-free survival, QoL, postoperative complications |
5 years | December 17 | March 23 |
| Koemans et al.131 | Primary cT3–cT4 gastric tumour including regional lymph nodes that is considered to be resectable, with limited peritoneal dissemination (PCI < 7) and/or tumour positive peritoneal cytology are confirmed by laparoscopy or laparotomy (n = 182) | HIPEC + CRS + gastrectomy | Palliative systemic chemotherapy | Primary: overall survival Secondary: progression-free survival |
5 years | October 17 | October 29 |
| Koole et al.132 | Stage III primary epithelial ovarian, fallopian tube or primary peritoneal cancer (n = 538) | HIPEC + CRS | CRS | Primary: overall survival Secondary: recurrence-free survival, adverse events |
5 years | January 20 | April 26 |
| Lambret139 | Stage III primary epithelial ovarian carcinoma or fallopian tube carcinoma or peritoneal carcinoma (including serous papillary adenocarcinoma, clear-cell carcinoma, mucinous adenocarcinoma and endometrioid carcinoma) (n = 362) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: disease-free survival Secondary: overall survival, adverse events, QoL |
5 years | April 19 | August 28 |
| Lyon148 | Platinum-resistant epithelial ovarian carcinoma (n = 132) | HIPEC + CRS + systemic chemotherapy | Systemic chemotherapy | Primary: progression-free survival Secondary: overall survival, mortality, morbidity, adverse events, QoL |
3 years | July 17 | November 22 |
| Instituto Nacional de Cancerologia de Mexico140 | Stage IIIC–IVA ovarian cancer (n = 100) | HIPEC + CRS | CRS | Primary: mortality, morbidity, QoL
Secondary: disease-free survival, overall survival |
5 years | September 17 | December 25 |
| NCT134 | Epithelial ovarian carcinoma with only peritoneal relapse occurred at least 6 months from the initial treatment, resectable without distant metastasis (with the exception of communicating pleura effusion, sensitive to platine-based second-line chemotherapy and resectable lymph nodes in the groin or retro peritoneal) (n = 415) | HIPEC + CRS | CRS | Primary: overall survival Secondary: relapse-free survival |
4 years | April 11 | December 22 |
| NCT 141 | Stage II–IV ovarian cancer (n = 60) | HIPEC + CRS | CRS | Primary: overall survival Secondary: progression-free survival, postoperative complications |
3 years | January 12 | Current status: still recruiting (estimated completion date: December 19) |
| NCT152 | Platinum-resistant epithelial ovarian carcinoma (n = 132) | HIPEC + CRS | Systemic chemotherapy + bevacizumab | Primary: progression-free survival Secondary: overall survival, QoL, potential treatment-related mortality and morbidity |
3 years | November 19 | November 22 |
| NCT142 | Stage III primary epithelial ovarian, fallopian tube or extra-ovarian cancer (n = 538) | HIPEC + CRS | CRS | Primary: overall survival Secondary: recurrence-free survival, adverse events |
5 years | January 20 | April 26 |
| NCT143 | Primary epithelial ovarian cancer, fallopian tube cancer and primary peritoneal cancer (n = 202) | HIPEC + CRS + systemic chemotherapy | Systemic chemotherapy | Primary: progression-free survival | 3 years | June 20 | March 24 |
| NTR153 | Recurrence of epithelial ovarian cancer, after first-line chemotherapy with a disease-free interval of at least 6 months (n = 700) | CRS + systemic chemotherapy | Systemic chemotherapy | Primary: progression-free survival Secondary: overall survival, surgical treatment related complications, QoL |
Not stated | October 5 | Not stated |
| Salcedo-Hernandez et al. 144 | Stage IIIC and IV ovarian cancer (n = 100) | HIPEC + CRS+ systemic chemotherapy | CRS + systemic chemotherapy | Primary: mortality, morbidity, QoL
Secondary: overall survival, disease-free survival |
1 year | January 18 | December 25 |
| ChiCTR156 | Stage III–IV primary epithelial ovarian/fallopian tube cancer (n = 135) | HIPEC + CRS | Conventional therapy | Primary: overall survival Secondary: disease-free survival, grade 3–4 adverse events, QoL |
5 years | January 20 | December 27 |
| ChiCTR157 | Stage II–IV primary epithelial, fallopian tube cancer (n = 300) | HIPEC + CRS | CRS + systemic chemotherapy | Primary: progression-free survival Secondary: overall survival, grade 3–4 adverse reactions, QoL |
5 years | July 20 | July 27 |
| El Hajj et al. 155 | Stage III epithelial ovarian carcinoma, fallopian tube carcinoma or primary peritoneal cancer (n = 362) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: disease-free survival Secondary: overall survival, trade-off between efficacy and morbidity, QoL |
5 years | March 19 | August 28 |
| Euctr FR130 | First or second recurrence of platin-resistant epithelial ovarian cancer (n = 220) | HIPEC + CRS + systemic chemotherapy | Monochemotherapy ± bevacizumab | Primary: progression-free survival Secondary: overall survival |
3 years | July 21 | Not stated |
| National Cancer Center, Korea158 | Epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer (n = 140) | HIPEC + systemic chemotherapy | Systemic chemotherapy | Primary: progression-free survival Secondary: overall survival, cancer-specific survival, adverse events, QoL |
5 years | April 22 | December 29 |
| NCT05250648135 | Stage IV colon adenocarcinoma, except signet ring cell carcinomas (n = 216) | HIPEC + CRS | CRS | Primary: peritoneal recurrence-free survival Secondary: disease-free survival, postoperative complications, QoL, overall survival |
3 years | February 22 | January 27 |
| Wu et al.160 | Stage IIIC–IVA, high-grade serous ovarian cancer (n = 80) | NHIPEC + systemic chemotherapy | Systemic chemotherapy | Primary: the proportion of service who achieve a CRS of 3 following NACT Secondary: progression-free survival, overall survival, adverse events |
2 years | September 20 | Not stated |
| Zivanovic et al.123 | Epithelial ovarian carcinoma, primary peritoneal carcinoma or fallopian tube carcinoma that has recurred >6 months since platinum-based chemotherapy (first recurrence) and who are scheduled for secondary surgical evaluation/cytoreduction (n = 99) | HIPEC + CRS + systemic chemotherapy | CRS + systemic chemotherapy | Primary: proportion of service who are without evidence of disease progression Secondary: toxicity and postoperative complication rate |
5 years | January 13 | January 23 |
Appendix 6 Additional records
| Additional reports | Main report |
|---|---|
| Included studies | |
| 162, 167, 168, 182 | 60 |
| 181 | 61 |
| 62, 166, 184–186, 190 | 62 |
| 163, 164, 187 | 63 |
| 169, 170 | 64 |
| 161 , 165 , 171 – 178 , 188 , 189 , 191 , 192 | 65 |
| 16 | 14 |
| 179 , 180 , 183 | 13 |
| Excluded studies | |
| 197 | 100 |
| 193 , 198 | 75 |
| 194 , 195 | 81 |
| 196 | 93 |
| 199 , 200 | 107 |
| 201 | 111 |
| 203 | 114 |
| 202 | 116 |
| Ongoing studies | |
| 205 | 154 |
| 206 | 127 |
| 207–209 | 149 |
| 210 | 150 |
| 211 | 146 |
| 214 | 131 |
| 212 , 213 , 217 | 132 |
| 215 | 143 |
| 216 | 160 |
| 204 , 218 | 123 |
Appendix 7 Calculation of costs used in the cost-effectiveness analysis
| Unit cost | Notes/description | Quantity per patient | Exchange rate to GBP | Total mg/m2 | Surface area (m2) | Body weight (kg) | Total drug (mg per patient) | Total cost (GBP 2022) | Source | |
|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC costs | ||||||||||
| HIPEC machine | ||||||||||
| HIPEC machine (Performer HT) | €45,000.00 | Original cost: €45,000 + VAT. Lifetime of the machine is 10 years. Annuity factor at 5% rate. | 1 | 1.19 | £53,550.00 | Hospital costs; Euro to GBP exchange rate57 | ||||
| HIPEC machine service costs per year | €5500.00 | Original cost: €5500. Service cost per year. This is the same regardless of volume | 1 | 1.19 | £6545.00 | Hospital costs | ||||
| Lifetime for machine (years) | 10 | Local estimate | Local estimate | |||||||
| Interest rate | 5% | |||||||||
| Annuity factor | 7.72 | This is calculated using the formula 1–[1/(1 + interest rate)]^years / interest rate | Drummond 2015237 | |||||||
| Cost of HIPEC machine per year | £13,479.97 | This is calculated as: Cost of HIPEC machine/annuity factor +HIPEC machine service costs per year (in GBP) | 1 | 1 | £13,479.97 | |||||
| Cost of HIPEC machine per patient | £192.57 | The machine volume per year can differ according to the number of procedures. On average, 1 machine is required for every 70 patients per year. The costs per patient are calculated as total machine costs per year/number of patients per year | 1 | 1 | £192.57 | |||||
| HIPEC disposables | ||||||||||
| Hang and Go Set per case | £1020.00 | Cost per patient of consumables | 1 | 1 | £1020.00 | Hospital costs | ||||
| Cytotoxic disposal per case | £1.85 | Cost per patient of disposable material | 1 | 1 | £1.85 | Hospital costs | ||||
| Baxter cost oxaliplatin | £47.50 | 1 | 1 | £47.50 | Hospital costs | |||||
| Baxter cost 5FU | £26.00 | 1 | 1 | £26.00 | Hospital costs | |||||
| Total cost of disposables | £1095.35 | 1 | 1 | £1095.35 | ||||||
| HIPEC practitioner | ||||||||||
| HIPEC practitioner salary (p.a) | £65.00 | This is a practitioner that runs and maintains the machine. The salary range here is £40,057–45,839. Average 5.5 hours per case. Assuming 47.5 hours per week, 42 weeks a year, the cost per hour is £65. Time per case is 5.5*£65 | 5.5 | £357.50 | ||||||
| Additional operating time | ||||||||||
| Additional operating time (NHS costs) | £637.30 | Costs per hour of operating time | 0.5 | 1 | £318.65 | NHS providers238 (adjusted for inflation using 2015 as base index and October 2021 index of 113.6);239 extra operating time = duration of HIPEC | ||||
| Additional hospital stay | ||||||||||
| Additional hospital stay | £292.00 | Per night extra hospital stay | 5 | 1 | £1460.00 | Quénet et al. 2021,62 NHS tariffs November 2022240 | ||||
| HIPEC (drugs) | This is based on non-reusable vials. The vial denominations were chosen to minimise the costs for the base-case scenario. | |||||||||
| Oxaliplatin | £2248.26 | 460 mg/m² in open technique and 360 mg/m² in closed technique (we assume an average of 410 mg/m²) = 738 mg; 3 vials of 200 mg (price 1 vial £591.26) + 1 vial 100 mg (price 1 vial £295.63) + 1 vial 50 mg (price 1 vial £178.85) | 1 | 1 | 410 | 1.8 | 738 | £2248.26 | Quénet et al. 2021,62 BNF 2022,241 Sacco et al. 2010242 | |
| 5FU fluorouracil | £6.40 | 400 mg/m² per patient = 720 mg; 2 vials of 500 mg (price per 1 vial £6.40) | 2 | 1 | 400 | 1.8 | 720 | £12.80 | Quénet et al. 2021,62 BNF 2022,241 Sacco et al. 2010242 | |
| Folinic acid (calcium folinate) | £20.00 | 20 mg/m² per patient = 36 mg; 1 vial of 50 mg (price per 1 vial £20) | 1 | 1 | 20 | 1.8 | 36 | £20.00 | Quénet et al. 2021,62 BNF 2022,241 Sacco et al. 2010242 | |
| Total cost of HIPEC drugs | £2281.06 | Total costs of HIPEC drugs (sum of HIPEC drug costs) | 1 | £2281.06 | ||||||
| Total costs of HIPEC | £5740.47 | |||||||||
| CRS costs | ||||||||||
| Uncomplicated CRS costs | £6199.00 | FF50C: Complex general abdominal procedures with CC Score 0–2 (elective) | 1 | 1 | £6199.00 | NHS tariffs November 2022240 | ||||
| Complicated CRS costs | £10,568.46 | Average of FF50A: complex general abdominal procedures with CC Score 6+ and FF50B: complex general abdominal procedures with CC Score 3–5 (weighted by the number of procedures done based on NHS reference costs) | 1 | 1 | £10,568.46 | NHS tariffs November 2022240 and NHS reference costs243 | ||||
| Systemic chemotherapy | ||||||||||
| Unit cost | Notes/description | Quantity per patient | Exchange rate to GBP | Total mg/m2 | Surface area (m2) | Body weight (kg) | Total drug (mg per patient) | Total cost (GBP 2022) | Source | |
|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC costs | ||||||||||
| HIPEC machine | ||||||||||
| HIPEC machine (Performer HT) | €45,000.00 | Original cost: €45,000 + VAT. Lifetime of the machine is 10 years. Annuity factor at 5% rate. | 1 | 1.19 | £53,550.00 | Hospital costs; Euro to GBP exchange rate57 | ||||
| HIPEC machine service costs per year | €5500.00 | Original cost: €5500. Service cost per year. This is the same regardless of volume | 1 | 1.19 | £6545.00 | Hospital costs | ||||
| Lifetime for machine (years) | 10 | Local estimate | Local estimate | |||||||
| Interest rate | 5% | |||||||||
| Annuity factor | 7.72 | This is calculated using the formula 1–[1/(1 + interest rate)]^years / interest rate | Drummond 2015237 | |||||||
| Cost of HIPEC machine per year | £13,479.97 | This is calculated as: Cost of HIPEC machine/annuity factor + HIPEC machine service costs per year (in GBP) | 1 | 1 | £13,479.97 | |||||
| Cost of HIPEC machine per patient | £192.57 | The machine volume per year can differ according to the number of procedures. On average, 1 machine is required for every 70 patients per year. The costs per patient are calculated as total machine costs per year/number of patients per year | 1 | 1 | £192.57 | |||||
| HIPEC disposables | ||||||||||
| Hang and Go Set per case | £1020.00 | Cost per patient of consumables | 1 | 1 | £1020.00 | Hospital costs | ||||
| Cytotoxic disposal per case | £1.85 | Cost per patient of disposable material | 1 | 1 | £1.85 | Hospital costs | ||||
| Baxter mitomycin | £47.00 | 1 | 1 | £47.00 | Hospital costs | |||||
| Other mitomycin consumables | £74.37 | 1 | 1 | £74.37 | Hospital costs | |||||
| Total cost of disposables | £1143.22 | 1 | 1 | £1143.22 | ||||||
| HIPEC practitioner | ||||||||||
| HIPEC practitioner salary (p.a) | £65.00 | This is a practitioner who runs and maintains the machine. The salary range here is £40,057–45,839. Average 5.5 hours per case. Assuming 47.5 hours per week, 42 weeks a year, the cost per hour is £65. Time per case is 5.5*£65 | 5.5 | £357.50 | ||||||
| Additional operating time | ||||||||||
| Additional operating time (NHS costs) | £637.30 | Costs per hour of operating time | 1.5 | 1 | £955.94 | NHS providers238 (adjusted for inflation using 2015 as base index and October 2021 index of 113.6);239 extra operating time = duration of HIPEC | ||||
| Additional hospital stay | ||||||||||
| Additional hospital stay | £292.00 | Per night extra hospital stay | 29 | 1 | £8468.00 | Verwaal et al. 2003,14 NHS tariffs November 2022240 | ||||
| HIPEC (drugs) | This is based on non-reusable vials. The vial denominations were chosen to minimise the costs for the base-case scenario. | |||||||||
| Mitomycin | £137.30 | 17.5 mg/m² followed by 8.8 mg/m² every 30 minutes (for 90 minutes). The total dose was limited to 70 mg at maximum. Three vials 20 mg at price £39 each and one 10 mg at £20.30 | 1 | 1 | 43.9 | 1.8 | 79.02 | £137.30 | Verwaal et al. 2003,14 BNF 2022,241 Sacco et al. 2010242 | |
| Total cost of HIPEC drugs | £137.30 | Total costs of HIPEC drugs (sum of HIPEC drug costs) | 1 | £137.30 | ||||||
| Total costs of HIPEC | £11,360.56 | |||||||||
| CRS costs | ||||||||||
| Uncomplicated CRS costs | £6199.00 | FF50C: Complex general abdominal procedures with CC Score 0–2 (elective) | 1 | 1 | £6199.00 | NHS tariffs November 2022240 | ||||
| Complicated CRS costs | £10,568.46 | Average of FF50A: Complex general abdominal procedures with CC Score 6+ and FF50B: Complex general abdominal procedures with CC Score 3–5 (weighted by the number of procedures done based on NHS reference costs) | 1 | 1 | £10,568.46 | NHS tariffs November 2022240 | ||||
| Systemic chemotherapy | ||||||||||
| 5FU fluorouracil (systemic) | £12.80 | 400 mg/m² per patient = 720 mg; 2 vials of 500 mg (price per 1 vial £6.40) | 26 | 1 | 400 | 1.8 | 720 | £332.80 | Verwaal 2003,14 BNF 2022,241 Sacco et al. 2010242 | |
| Folinic acid (leucovorin) systemic | £57.50 | 80 mg/m² per patient = 144 mg; 1 vial 100 mg at price £37.50 + 1 vial 50 mg at £20 each | 26 | 1 | 80 | 1.8 | 144 | £1495.00 | Verwaal et al. 2003,14 BNF 2022,241 Sacco et al. 2010242 | |
| Baxter cost 5FU | £26.00 | 26 | 1 | £676.00 | Hospital costs | |||||
| Total costs of systemic chemotherapy | £2503.80 | |||||||||
| Unit cost | Notes/description | Quantity per patient | Exchange rate to GBP | Total mg/m2 | Surface area (m2) | Body weight (kg) | Total drug (mg per patient) | Total cost (GBP 2022) | Source | |
|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC costs | ||||||||||
| HIPEC machine | ||||||||||
| HIPEC machine (performer HT) | €45,000.00 | Original cost: €45,000 + VAT. Lifetime of the machine is 10 years. Annuity factor at 5% rate. | 1 | 1.19 | £53,550.00 | Hospital costs; Euro to GBP exchange rate57 | ||||
| HIPEC machine service costs per year | €5500.00 | Original cost: €5500. Service cost per year. This is the same regardless of volume | 1 | 1.19 | £6545.00 | Hospital costs | ||||
| Lifetime for machine (years) | 10 | Local estimate | Local estimate | |||||||
| Interest rate | 5% | |||||||||
| Annuity factor | 7.72 | This is calculated using the formula 1–[1/(1 + interest rate)]^years / interest rate | Drummond et al. 2015237 | |||||||
| Cost of HIPEC machine per year | £13,479.97 | This is calculated as: cost of HIPEC machine/annuity factor + HIPEC machine service costs per year (in GBP) | 1 | 1 | £13,479.97 | |||||
| Cost of HIPEC machine per patient | £192.57 | The machine volume per year can differ according to the number of procedures. On average, 1 machine is required for every 70 patients per year. The costs per patient are calculated as total machine costs per year/number of patients per year | 1 | 1 | £192.57 | |||||
| HIPEC disposables | ||||||||||
| Hang and Go Set per case | £1020.00 | Cost per patient of consumables | 1 | 1 | £1020.00 | Hospital costs | ||||
| Cytotoxic disposal per case | £1.85 | Cost per patient of disposable material | 1 | 1 | £1.85 | Hospital costs | ||||
| Baxter mitomycin | £47.00 | 1 | 1 | £47.00 | Hospital costs | |||||
| Baxter cisplatin | £47.00 | 1 | 1 | £47.00 | Hospital costs | |||||
| Other mitomycin consumables | £74.37 | 1 | 1 | £74.37 | Hospital costs | |||||
| Total cost of disposables | £1190.22 | 1 | 1 | £1190.22 | ||||||
| HIPEC practitioner | ||||||||||
| HIPEC practitioner salary (p.a) | £65.00 | This is a practitioner who runs and maintains the machine. The salary range here is £40,057–45,839. Average 5.5 hours per case. Assuming 47.5 hours per week, 42 weeks a year, the cost per hour is £65. Time per case is 5.5*£65 | 5.5 | £357.50 | ||||||
| Additional operating time | ||||||||||
| Additional operating time (NHS costs) | £637.30 | Costs per hour of operating time | 1 | 1 | £637.30 | NHS providers238 (adjusted for inflation using 2015 as base index and October 2021 index of 113.6);239 extra operating time = duration of HIPEC | ||||
| Additional hospital stay | ||||||||||
| Additional hospital stay | £292.00 | Per night extra hospital stay | 2.5 | 1 | £730.00 | There is no information on length of hospital stay. For colorectal cancer, it was 5 days more; for ovarian cancer, it was 2 and 3 days more – a value of 2.5 was used for this analysis; NHS tariffs November 2022240 | ||||
| HIPEC (drugs) | This is based on non-reusable vials. The vial denominations were chosen to minimise the costs for the base-case scenario. | |||||||||
| Mitomycin | £78.00 | 15 mg/m² = 75 mg (2 vials 20 mg at price £39 each) | 1 | 1 | 15 | 1.8 | 27 | £78.00 | Quénet 2021,62 BNF 2022,241 Sacco et al. 2010242 | |
| Cisplatin | £73.50 | 75 mg/m² = 135 mg (3 vials 50 mg at price £24.50 each) | 1 | 1 | 75 | 1.8 | 135 | £73.50 | Quénet et al. 2021,62 BNF 2022,241 Sacco et al. 2010242 | |
| Total cost of HIPEC drugs | £151.50 | Total costs of HIPEC drugs (sum of HIPEC drug costs) | 1 | £151.50 | ||||||
| Total costs of HIPEC | £3329.77 | |||||||||
| CRS costs | ||||||||||
| Uncomplicated CRS costs | £6199.00 | FF50C: complex general abdominal procedures with CC Score 0–2 (elective) | 1 | 1 | £6199.00 | NHS tariffs November 2022240 | ||||
| Complicated CRS costs | £10,568.46 | Average of FF50A: complex general abdominal procedures with CC Score 6+ and FF50B: complex general abdominal procedures with CC Score 3–5 (weighted by the number of procedures done based on NHS reference costs) | 1 | 1 | £10,568.46 | NHS tariffs November 2022240 and NHS reference costs243 | ||||
| Systemic chemotherapy | ||||||||||
| HER-2 negative cancers | ||||||||||
| Epirubicin | £210.76 | 50 mg/m2; maximum 100 mg/day (1 vial 100 mg at £201.76) | 6 | 1 | 50 | 1.8 | 90 | £1264.56 | Rau et al. 2021,63 BNF 2022,241 Sacco et al. 2010242 | |
| Oxaliplatin | £738.06 | 130 mg/m2; maximum 260 mg/day (2 vials 100 mg at £295.63; 1 vial 50 mg £146.80) | 6 | 1 | 130 | 1.8 | 234 | £4428.36 | Rau et al. 2021,63 BNF 2022,241 Sacco et al. 2010242 | |
| Baxter oxaliplatin | £47.50 | 6 | 1 | £285.00 | Hospital costs | |||||
| Capecitabine tablets (days 1–14) | £119.00 | 625 mg/m2 two times a day for 14 days; max 2500 mg/day; price for 500 mg £225 120 tablets; price for 150 mg £30 60 tablets; total requirement: 4*500 mg + 2 *150 mg per day*14 days*6 cycles, that is (4*225/120 + 2*30/60)*14 per cycle | 6 | 1 | 625 | 1.8 | 1125 | £714.00 | Hospital costs | |
| Total cost of systemic chemotherapy (HER-2 negative) | £6691.92 | 1 | 1 | £6691.92 | ||||||
| HER-2 positive cancers | ||||||||||
| Trastuzumab | £1759.98 | 8 mg/kg (cycles 1 and 4); (1 vial 420 at £1026.66 + 2 vials 150 at £366.66) | 2 | 1 | 8 | 81 | 648 | £3519.96 | Rau et al. 2021,63 BNF 2022,241 Sacco et al. 2010242 | |
| Trastuzumab | £1393.32 | 6 mg/kg cycle 2, 3, 4 and 6; (1 vial 420 at £1026.66 + 1 vial 150 at £366.66) | 4 | 1 | 6 | 81 | 486 | £5573.28 | Rau et al. 2021,63 BNF 2022,241 Sacco et al. 2010242 | |
| Cisplatin | £73.50 | 80 mg/m2; maximum 160 mg/day; (3 vials 50 mg at price £24.50 each) | 6 | 1 | 80 | 1.8 | 144 | £441.00 | Rau et al. 2021,63 BNF 2022,241 Sacco et al. 2010242 | |
| Baxter cisplatin | £47.00 | 6 | 1 | £282.00 | Hospital costs | |||||
| Capecitabine tablets (days 1–14) | £192.99 | 1000 mg/m2 two times a day for 14 days; max 4000 mg/day; price for 500 mg £225 120 tablets; price for 300 mg £76.04 60 tablets; total requirement: 6*500 mg + 2 *300 mg per day*14 days*6 cycles, that is (6*225/120 + 2*76.04/60)*14 per cycle | 6 | 1 | 1000 | 1.8 | 1800 | £1157.91 | Hospital costs | |
| Total cost of systemic chemotherapy (HER-2 positive) | £7454.19 | 1 | 1 | £7454.19 | ||||||
| Total costs of systemic chemotherapy | Assumption of 18% HER-2 positive gastric cancers | £6829.13 | Chua and Merrett 2012244 | |||||||
| Unit cost | Notes/description | Quantity per patient | Exchange rate to GBP | Total mg/m2 | Surface area (m2) | Body weight (kg) | Total drug (mg per patient) | Total cost (GBP 2022) | Source | |
|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC costs | ||||||||||
| HIPEC machine | ||||||||||
| HIPEC machine (performer HT) | €45,000.00 | Original cost: €45,000 + VAT. Lifetime of the machine is 10 years. Annuity factor at 5% rate. | 1 | 1.19 | £53,550.00 | Hospital costs; Euro to GBP exchange rate57 | ||||
| HIPEC machine service costs per year | €5500.00 | Original cost: €5500. Service cost per year. This is the same regardless of volume | 1 | 1.19 | £6545.00 | Hospital costs | ||||
| Lifetime for machine (years) | 10 | Local estimate | Local estimate | |||||||
| Interest rate | 5% | |||||||||
| Annuity factor | 7.72 | This is calculated using the formula 1–[1/(1 + interest rate)]^years / interest rate | Drummond et al. 2015237 | |||||||
| Cost of HIPEC machine per year | £13,479.97 | This is calculated as: cost of HIPEC machine/annuity factor + HIPEC machine service costs per year (in GBP) | 1 | 1 | £13,479.97 | |||||
| Cost of HIPEC machine per patient | £192.57 | The machine volume per year can differ according to the number of procedures. On average, 1 machine is required for every 70 patients per year. The costs per patient are calculated as total machine costs per year/number of patients per year | 1 | 1 | £192.57 | |||||
| HIPEC disposables | ||||||||||
| Hang and Go Set per case | £1020.00 | Cost per patient of consumables | 1 | 1 | £1020.00 | Hospital costs | ||||
| Cytotoxic disposal per case | £1.85 | Cost per patient of disposable material | 1 | 1 | £1.85 | Hospital costs | ||||
| Baxter cost oxaliplatin | £47.50 | 1 | 1 | £47.50 | Hospital costs | |||||
| Baxter cost 5FU | £26.00 | 1 | 1 | £26.00 | Hospital costs | |||||
| Total cost of disposables | £1095.35 | 1 | 1 | £1095.35 | ||||||
| HIPEC practitioner | ||||||||||
| HIPEC practitioner salary (p.a) | £65.00 | This is a practitioner who runs and maintains the machine. The salary range here is £40,057–45,839. Average 5.5 hours per case. Assuming 47.5 hours per week, 42 weeks a year, the cost per hour is £65. Time per case is 5.5*£65 | 5.5 | £357.50 | ||||||
| Additional operating time | ||||||||||
| Additional operating time (NHS costs) | £637.30 | Costs per hour of operating time | 0.5 | 1 | £318.65 | NHS providers238(adjusted for inflation using 2015 as base index and October 2021 index of 113.6);239 extra operating time = duration of HIPEC | ||||
| Additional hospital stay | ||||||||||
| Additional hospital stay | £292.00 | Per night extra hospital stay | 17 | 1 | £4964.00 | Rudloff et al. 2014;64 NHS tariffs November 2022240 | ||||
| HIPEC (drugs) | This is based on non-reusable vials. The vial denominations were chosen to minimise the costs for the base-case scenario | |||||||||
| Oxaliplatin | £2543.89 | 460 mg/m² in closed circuit = 828 mg; 4 vials of 200 mg (price 1 vial £591.26) + 1 vial 50 mg (price 1 vial £178.85) | 1 | 1 | 460 | 1.8 | 828 | £2543.89 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| 5FU fluorouracil | £12.80 | 400 mg/m² per patient = 720 mg; 2 vials of 500 mg (price per 1 vial £6.40) | 1 | 1 | 400 | 1.8 | 720 | £12.80 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| Folinic acid leucovorin | £20.00 | 20 mg/m² per patient = 36 mg; 1 vial of 50 mg (price per 1 vial £20) | 1 | 1 | 20 | 1.8 | 36 | £20.00 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| Total cost of HIPEC drugs | £2576.69 | Total costs of HIPEC drugs (sum of HIPEC drug costs) | 1 | £2576.69 | ||||||
| Total costs of HIPEC | £9540.10 | |||||||||
| CRS costs | ||||||||||
| Uncomplicated CRS costs | £6199.00 | FF50C: complex general abdominal procedures with CC Score 0–2 (elective) | 1 | 1 | £6199.00 | NHS tariffs November 2022240 | ||||
| Complicated CRS costs | £10,568.46 | Average of FF50A: complex general abdominal procedures with CC Score 6+ and FF50B: complex general abdominal procedures with CC Score 3–5 (weighted by the number of procedures done based on NHS Reference costs) | 1 | 1 | £10,568.46 | NHS tariffs November 2022240 and NHS reference costs243 | ||||
| Systemic chemotherapy | ||||||||||
| Irinotecan | £110.00 | 165 mg/m2 administer 90 minutes = 297 mg (1 vial 300 mg at £110) | 12 | 1 | 165 | 1.8 | 297 | £1320.00 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| Oxaliplatin | £591.26 | Over 2 hours 85 mg/m² =1 53 mg; 1 vials of 200 mg (price 1 vial £591.26) | 12 | 1 | 85 | 1.8 | 153 | £7095.12 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| Baxter oxaliplatin | £47.50 | 12 | 1 | £570.00 | Hospital costs | |||||
| Folinic acid leucovorin | £147.32 | Over hours 200 mg/m² per patient = 60 mg; 1 vial of 350 mg (price per 1 vial £139.52) + 1 vial 15 mg (£39 for 5 vials) | 12 | 1 | 200 | 1.8 | 360 | £1767.84 | Hospital costs | |
| 5FU fluorouracil | £96.00 | Over 48 hours 3200 mg/m² per patient = 5760 mg; 1 vials of 5 g (price per 1 vial £64) + 1 vial 2.5 g at £32 | 12 | 1 | 3200 | 1.8 | 5760 | £1152.00 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |
| Baxter cost 5FU | £26.00 | 12 | 1 | £312.00 | Rudloff et al. 2014;64 BNF 2022;241 Sacco et al. 2010242 | |||||
| Total cost of systemic chemotherapy | £12,216.96 | |||||||||
| Unit cost | Notes/description | Quantity per patient | Exchange rate to GBP | Total mg/m2 | Surface area (m2) | Body weight (kg) | Total drug (mg per patient) | Total cost (GBP 2022) | Source | |
|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC costs | ||||||||||
| HIPEC machine | ||||||||||
| HIPEC machine (performer HT) | €45,000.00 | Original cost: €45,000 + VAT. Lifetime of the machine is 10 years. Annuity factor at 5% rate. | 1 | 1.19 | £53,550.00 | Hospital costs; Euro to GBP exchange rate57 | ||||
| HIPEC machine service costs per year | €5500.00 | Original cost: €5500. Service cost per year. This is the same regardless of volume | 1 | 1.19 | £6545.00 | Hospital costs | ||||
| Lifetime for machine (years) | 10 | Local estimate | Local estimate | |||||||
| Interest rate | 5% | |||||||||
| Annuity factor | 7.72 | This is calculated using the formula 1–[1/(1 + interest rate)]^years / interest rate | Drummond et al. 2015237 | |||||||
| Cost of HIPEC machine per year | £13,479.97 | This is calculated as: cost of HIPEC machine/annuity factor + HIPEC machine service costs per year (in GBP) | 1 | 1 | £13,479.97 | |||||
| Cost of HIPEC machine per patient | £192.57 | The machine volume per year can differ according to the number of procedures. On average, 1 machine is required for every 70 patients per year. The costs per patient are calculated as total machine costs per year/number of patients per year | 1 | 1 | £192.57 | |||||
| HIPEC disposables | ||||||||||
| Hang and Go Set per case | £1020.00 | Cost per patient of consumables | 1 | 1 | £1020.00 | Hospital costs | ||||
| Cytotoxic disposal per case | £1.85 | Cost per patient of disposable material | 1 | 1 | £1.85 | Hospital costs | ||||
| Baxter cost cisplatin | £47.00 | 1 | 1 | £47.00 | Hospital costs | |||||
| Total cost of disposables | £1068.85 | 1 | 1 | £1068.85 | ||||||
| HIPEC practitioner | ||||||||||
| HIPEC practitioner salary (p.a) | £65.00 | This is a practitioner who runs and maintains the machine. The salary range here is £40,057–45,839. Average 5.5 hours per case. Assuming 47.5 hours per week, 42 weeks a year, the cost per hour is £65. Time per case is 5.5*£65 | 5.5 | £357.50 | ||||||
| Additional operating time | ||||||||||
| Additional operating time (NHS costs) | £637.30 | Costs per hour of operating time | 1.5 | 1 | £955.94 | NHS providers238 (adjusted for inflation using 2015 as base index and October 2021 index of 113.6),239 extra operating time = duration of HIPEC | ||||
| Additional hospital stay | ||||||||||
| Additional hospital stay | £292.00 | Per night extra hospital stay | 2.5 | 1 | £730.00 | van Driel et al. 201865 and Lim et al. 2022,61 NHS tariffs November 2022240 | ||||
| HIPEC (drugs) | This is based on non-reusable vials. The vial denominations were chosen to minimise the costs for the base-case scenario. | |||||||||
| Cisplatin | £73.50 | 100 mg/m² = 180 mg (3 vials 50 mg at price £24.50 each) | 1 | 1 | 100 | 1.8 | 180 | £73.50 | van Driel et al. 2018,65 BNF 2022,241 Sacco et al. 2010242 | |
| Sodium thiosulphate | £143.00 | 9 mg/m2+12 g/m2=21 g/m2 (3 vials of 12.5 mg at £143 each) | 1 | 1 | 21 | 1.8 | 37.8 | £143.00 | Hospital costs | |
| Total cost of HIPEC drugs | £216.50 | Total costs of HIPEC drugs (sum of HIPEC drug costs) | 1 | £216.50 | ||||||
| Total costs of HIPEC | £3627.39 | |||||||||
| CRS costs | ||||||||||
| Uncomplicated CRS costs | £6199.00 | FF50C: complex general abdominal procedures with CC Score 0–2 (elective) | 1 | 1 | £6199.00 | NHS tariffs November 2022240 | ||||
| Complicated CRS costs | £10,568.46 | Average of FF50A: complex general abdominal procedures with CC Score 6+ and FF50B: complex general abdominal procedures with CC Score 3–5 (weighted by the number of procedures done based on NHS reference costs) | 1 | 1 | £10,568.46 | NHS tariffs November 2022240 and NHS reference costs243 | ||||
| Systemic chemotherapy | ||||||||||
| Carboplatin | £20.20 | (5–6 mg/minute); we assume a total of 5 mg/m2 in total; 9 mg (1 vial 50 mg at £20.20) | 6 | 1 | 5 | 1.8 | 9 | £121.20 | van Driel et al. 2018,65 BNF 2022,241 Sacco et al. 2010242 | |
| Paclitaxel | £227.50 | 175 mg/m2 = 315 mg (1 vial 300 mg at £192.50 + 1 vial 30 mg at £35) | 6 | 1 | 175 | 1.8 | 315 | £1365.00 | van Driel et al. 2018,65 BNF 2022,241 Sacco et al. 2010242 | |
| Baxter carboplatin | £47.00 | 6 | 1 | £282.00 | Hospital costs | |||||
| Total cost of systemic chemotherapy | 1 | 1 | £1768.20 | |||||||
Appendix 8 Stability tests
| Runs | Colorectal cancer CRS + systemic chemotherapy WTP: £20,000 |
Colorectal cancer CRS + systemic chemotherapy WTP: £30,000 |
Colorectal cancer systemic chemotherapy WTP: £20,000 |
Colorectal cancer systemic chemotherapy WTP: £30,000 |
Gastric cancer CRS + systemic chemotherapy WTP: £20,000 |
Gastric cancer CRS + systemic chemotherapy WTP: £30,000 |
Gastric cancer systemic chemotherapy WTP: £20,000 |
Gastric cancer systemic chemotherapy WTP: £30,000 |
Ovarian cancer CRS + systemic chemotherapy WTP: £20,000 |
Ovarian cancer CRS + systemic chemotherapy WTP: £30,000 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −£2,594,662 | −£927,336 | £104,588,977 | £162,563,866 | £8,671,402 | £14,676,274 | £63,524,459 | £100,189,895 | £43,740,841 | £67,393,389 |
| 2 | −£1,895,069 | £124,870 | £104,491,274 | £162,427,829 | £8,623,877 | £14,609,159 | £63,183,580 | £99,723,480 | £42,853,265 | £66,059,926 |
| 3 | −£2,108,338 | −£196,556 | £105,120,604 | £163,367,701 | £8,585,394 | £14,547,987 | £63,362,399 | £99,931,742 | £43,485,294 | £67,000,168 |
| 4 | −£2,018,111 | −£61,452 | £103,935,895 | £161,589,837 | £8,047,463 | £13,730,906 | £65,095,759 | £102,604,243 | £44,157,980 | £67,992,754 |
| 5 | −£2,488,646 | −£770,443 | £104,906,203 | £163,049,772 | £8,564,863 | £14,508,234 | £64,049,489 | £100,978,904 | £42,985,734 | £66,263,993 |
| 6 | −£2,382,053 | −£607,300 | £105,528,468 | £164,001,199 | £8,360,169 | £14,195,502 | £64,834,551 | £102,139,111 | £43,312,132 | £66,756,338 |
| 7 | −£2,091,864 | −£170,219 | £105,353,364 | £163,710,821 | £8,145,168 | £13,875,853 | £64,018,531 | £100,950,376 | £43,527,315 | £67,084,265 |
| 8 | −£2,680,719 | −£1,053,337 | £104,386,829 | £162,281,972 | £8,420,881 | £14,296,141 | £64,709,498 | £101,972,296 | £44,114,742 | £67,925,835 |
| 9 | −£2,526,849 | −£826,995 | £103,759,734 | £161,335,699 | £9,059,035 | £15,253,432 | £63,571,534 | £100,287,597 | £43,735,343 | £67,383,969 |
| 10 | −£2,521,454 | −£818,759 | £104,200,504 | £161,992,860 | £8,224,014 | £13,995,376 | £64,732,437 | £102,002,777 | £42,874,971 | £66,099,476 |
| 11 | −£2,297,236 | −£479,426 | £103,939,681 | £161,592,242 | £8,318,442 | £14,144,896 | £62,631,228 | £98,851,828 | £43,121,518 | £66,466,506 |
| 12 | −£2,606,487 | −£945,439 | £103,880,460 | £161,531,826 | £8,240,400 | £14,028,550 | £64,674,830 | £101,952,625 | £44,258,884 | £68,165,230 |
| 13 | −£2,523,437 | −£816,963 | £105,030,294 | £163,243,184 | £8,889,078 | £14,989,816 | £62,966,779 | £99,348,203 | £43,474,958 | £67,000,153 |
| 14 | −£2,191,001 | −£319,036 | £104,235,518 | £162,044,587 | £7,882,761 | £13,494,994 | £62,602,533 | £98,824,216 | £44,326,788 | £68,269,538 |
| 15 | −£2,732,779 | −£1,136,992 | £104,159,791 | £161,952,524 | £8,499,976 | £14,407,785 | £62,131,147 | £98,136,132 | £46,045,504 | £70,833,985 |
| 16 | −£2,464,286 | −£734,761 | £103,541,190 | £160,998,023 | £8,723,469 | £14,757,336 | £63,207,763 | £99,713,202 | £43,481,037 | £67,013,588 |
| 17 | −£2,125,950 | −£220,766 | £103,773,002 | £161,375,352 | £8,407,605 | £14,272,205 | £64,621,032 | £101,889,076 | £45,077,136 | £69,394,928 |
| 18 | −£2,577,813 | −£901,801 | £105,188,553 | £163,450,045 | £8,927,834 | £15,045,153 | £64,098,906 | £101,029,659 | £45,490,483 | £69,995,286 |
| 19 | −£2,597,124 | −£930,837 | £104,537,320 | £162,455,787 | £9,416,063 | £15,793,132 | £63,577,530 | £100,264,332 | £44,048,036 | £67,843,602 |
| 20 | −£2,548,047 | −£850,228 | £104,141,435 | £161,908,700 | £7,984,885 | £13,632,396 | £65,163,653 | £102,706,899 | £43,871,691 | £67,574,715 |
| 21 | −£2,088,322 | −£168,536 | £104,180,898 | £161,958,005 | £8,649,765 | £14,636,081 | £65,099,148 | £102,584,744 | £43,831,206 | £67,520,144 |
| 22 | −£2,348,809 | −£558,191 | £103,504,377 | £160,963,833 | £8,931,222 | £15,058,670 | £62,347,958 | £98,380,309 | £43,364,398 | £66,814,537 |
| 23 | −£1,909,802 | £103,435 | £103,969,518 | £161,653,881 | £8,490,569 | £14,394,498 | £63,382,597 | £99,989,954 | £44,226,679 | £68,121,822 |
| 24 | −£2,285,181 | −£459,093 | £104,068,705 | £161,785,317 | £8,025,067 | £13,695,906 | £64,428,363 | £101,539,766 | £43,194,954 | £66,584,529 |
| 25 | −£2,193,440 | −£324,774 | £104,239,463 | £162,072,688 | £8,229,151 | £14,006,398 | £62,515,023 | £98,656,887 | £43,343,282 | £66,782,061 |
| 26 | −£2,033,215 | −£85,046 | £105,734,783 | £164,296,038 | £8,478,399 | £14,384,267 | £63,172,906 | £99,691,217 | £43,293,320 | £66,733,465 |
| 27 | −£2,355,576 | −£571,228 | £103,791,825 | £161,387,016 | £8,629,304 | £14,596,632 | £61,733,352 | £97,509,764 | £41,948,256 | £64,692,012 |
| 28 | −£2,370,116 | −£583,028 | £104,584,330 | £162,558,866 | £8,700,297 | £14,719,083 | £64,435,004 | £101,537,730 | £43,742,206 | £67,391,670 |
| 29 | −£2,215,435 | −£356,210 | £105,080,684 | £163,301,722 | £8,163,467 | £13,903,129 | £64,634,157 | £101,891,150 | £43,548,768 | £67,083,175 |
| 30 | −£2,019,498 | −£62,354 | £104,074,238 | £161,797,254 | £8,785,862 | £14,838,341 | £64,405,813 | £101,516,954 | £43,052,803 | £66,362,902 |
| Number of iterations | 90,000 | 90,000 | 10,000 | 10,000 | 90,000 | 90,000 | 15,000 | 15,000 | 10,000 | 10,000 |
| CoV | 10.3% | 69.1% | 0.6% | 0.6% | 4.1% | 3.6% | 1.5% | 1.5% | 1.8% | 1.8% |
List of abbreviations
- ASCO
- American Society of Clinical Oncology
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CrI
- credible intervals
- CRS
- cytoreductive surgery
- EORTC
- European Organisation for Research and Treatment of Cancer
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- GBP
- Great British pounds
- HIPEC
- hyperthermic intraoperative peritoneal chemotherapy
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IPD
- individual participant data
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- PCI
- Peritoneal Cancer Index
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- QALYs
- quality-adjusted life-years
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SoC
- standard of care
- WTP
- willingness to pay
Notes
-
HIPEC decision tree
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/KWDG6338).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
