Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/167/57. The contractual start date was in October 2018. The draft report began editorial review in June 2022 and was accepted for publication in November 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Cook et al. This work was produced by Cook et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Cook et al.
Chapter 1 Introduction
Background and rationale
Pelvic fractures
Pelvic fractures can be life-threatening and people can experience significant problems with mobility following the injury. 1 They are associated with a total inpatient mortality rate of 9% and an all-cause mortality rate within 3 months of fracture of 13%. 2 All-cause mortality following pelvic fracture is approximately 50% at 3 years. 3 Mortality for fragility fractures of the pelvis is reported to be around a third after 1 year. 4
The incidence of pelvic fractures in the UK between 2008 and 2012 in men and women over 50 was 2.0 and 7.4/10,000 person-years (based on a retrospective observational study using data from the Clinical Practice Research Datalink, which is shown to be broadly representative of the UK population). 5 People can experience this injury as a result of a high-energy impact such as a road traffic accident or, in the case of older adults (generally 65 years of age and older), as a result of a low-energy fall from a standing height. 1 The UK age-specific incidence of pelvic fractures (based on a single centre) has increased from 39.6/100,000 (95% confidence interval: 31.8 to 48.1) in 1997 to 71.6/100,000 (58.4 to 81.0) in 2007–8 amongst people 65 years and older; 84% of these had pubic rami fractures. 6
Lateral compression type-1 (LC-1) fractures are a common fragility fracture of the pelvis in older adults and are associated with low bone density. 7 They typically involve a fracture of the pubic ramus, which patients experience as groin pain when they mobilise. There is usually also a ‘buckle’ fracture to the sacrum posteriorly, which is felt as low-back or buttock pain when moving the legs. The likelihood of an LC-1 fragility fracture increases with age and is more common in women. 3,8,9
Lateral compression type-1 fractures are often painful, particularly when the patient moves. This leads to reduced mobility, usually over a 1- to 2-month period, although it is estimated that 25% of patients experience pain for up to 5 years following the injury. 10 The ability of patients to mobilise following an LC-1 fragility fracture varies: some can mobilise and walk, albeit with some degree of pain, whereas others struggle to walk due to the pain. Patients who do not manage to get back walking due to ongoing pain are at greater risk of immobility-related complications. 11 These can include respiratory tract infections, urinary tract infections, pressure sores and venous thromboembolic events such as deep vein thrombosis or pulmonary embolism. 11,12 These individuals are also at risk of irreversible muscle wasting (systemic sarcopenia), loss of confidence and permanently decreased levels of independence, often leading to increased care requirements. Inability to return to independent living can result in utilisation of intermediate care or residential facilities. 13,14 Some patients do not regain their pre-injury level of walking or their prior independence with activities of daily living due to their loss of confidence and muscle strength/conditioning. 8,9,15,16 Additionally, individuals with LC-1 fractures have reported emotional stress, family strain, employment and financial difficulty, sleep disturbance and anxiety. 17
The current standard care for lateral compression type-1 fragility fractures
In the UK, standard treatment for LC-1 fractures is to ‘mobilise as pain allows’, prescribing pain relief and getting patients up within a few days of injury with physiotherapy input and getting them to mobilise with an assistive device until the fracture heals. 11,18,19 This is unlike fragility neck of femur fractures (hip fractures), which are usually treated surgically, and there is evidence that early surgery (within 1–2 days) is associated with reduced risk of death and pressure sores. 20 Despite LC-1 fractures being similarly disabling for some patients in terms of pain and immobility, while also occurring in the same patient group as hip fractures, to date, it has not been shown whether or not patients with LC-1 fragility fractures would have a better recovery with surgery than with non-surgical management.
Surgical fixation
Traditional pelvic implants carry poor ‘bite’ or ‘purchase’ in low-quality osteoporotic bone around the pelvis and surgeons have been reluctant to offer surgery to patients with LC-1 fragility fractures. External fixators, consisting of pins inside the pelvis connected to bars and clamps outside of the skin, are cumbersome, poorly tolerated and carry a high incidence of pin-site infections and soft-tissue problems. 21 The alternative is surgical fixation of the back of the pelvis with ilio-sacral screws;9 however, in the majority of elderly patients, these screws carry poor ‘purchase’ in osteoporotic bone, leading to ineffective fracture stabilisation and persistence of pain. 11
A more recent approach is use of an anterior pelvic fixation device that resembles a traditional external fixator, in that it has screws that are secured into the pelvic bone, and these are connected by a metal bar across the front of the patient. This is an internal fixation device (INFIX). Unlike traditional external fixation devices, it is fitted internally, sitting entirely underneath the patient’s skin, with no external metalwork visible. INFIX has two potential benefits over external fixation: it is less cumbersome and less inconvenient to patients, compared with pins, clamps and bars protruding out of the skin. It also does not have pin sites (where the bone pins exit through the skin), which make traditional external fixation very susceptible to local infection. The INFIX technique involves percutaneous placement of screws in the pelvic bone and connects them with a bar under the skin. 22 The pelvic bone where the screws are placed is generally strong and easy to visualise intraoperatively, even in highly osteoporotic bone. Although a proportion of implants need to be removed, this is usually done as a day case procedure. INFIX is used in younger patients with high-energy LC-1 fractures. It is now a well-described technique with a number of peer-reviewed series confirming its safety. 23 It is therefore a widely practiced, rather than ‘novel’, technique and is technically straightforward to carry out.
Existing evidence
A systematic review found no robust evaluations of the effectiveness of internal fixation with INFIX in patients with osteoporotic LC-1 fractures. 24 The review identified five case series, with four being retrospective. Participants were aged 64 or over and most had sustained their injury from a low-energy fall. A variety of fixation techniques were used. Of the 225 patients in the five studies, most had internal devices, with 25 having external fixation; most patients had more than one type of fixation.
In the single series evaluating INFIX alone, 19 of the 29 patients had LC-1 fractures. 25 Six patients had anterior fixation with INFIX alone and the remaining 23 had INFIX with additional internal fixation. Postoperatively, 22 of the 29 (76%) returned to their pre-injury walking ability, and a further 6 patients had some deterioration but could still walk. Complications included chronic pain (n = 3, 10.3%) and painful lateral femoral cutaneous nerve hyperaesthesia (n = 8, 27.5%) and, less commonly, infections, implant loosening, pneumonia and thrombosis.
Our search of clinicaltrials.gov in advance of our study identified an ongoing trial in the USA of surgical versus non-surgical management of patients aged between 18 and 80 with LC-1, LC-2 and LC-3 pelvic fractures in 130 participants. The aim of this trial is to determine which patients would benefit from early surgical stabilisation. 26 There is also a Research for Patient Benefit funded feasibility study of surgical versus non-surgical management of LC-1 pelvic fractures in patients with non-fragility fractures. 27
The existing evidence on the effectiveness of using an INFIX device to stabilise LC-1 fractures is limited to one case study with 29 participants. Surgeons are already carrying out INFIX surgeries in the absence of extensive evidence. There is a need to collect more evidence and there are currently no other clinical trials focusing on the effectiveness of surgical fixation with INFIX in adults with fragility fractures.
Aim and objectives
The aim was to assess the clinical and cost effectiveness of surgical fixation with INFIX compared to non-surgical management of LC-1 fragility fractures in older adults (L1FE trial).
The objectives of the L1FE trial were to:
-
Undertake a 12-month internal pilot to obtain robust estimates of recruitment and confirm trial feasibility.
-
Undertake a parallel group multicentre randomised controlled trial (RCT) to assess the effectiveness of surgical fixation with INFIX versus non-surgical management of LC-1 fragility fractures in older adults. The primary outcome is average patient health-related quality of life, over 6 months, assessed by the patient-reported EuroQol-5 Dimensions, five-level version (EQ-5D-5L) utility index score measured at baseline, 2 weeks, 6 weeks, 12 weeks and 6 months.
-
Undertake an economic evaluation to compare the cost effectiveness of surgical fixation compared to non-surgical management, to determine the most efficient provision of future care and to describe the resource impact on the NHS for the two treatment options.
-
Undertake a long-term review of patient well-being (EQ-5D-5L and mortality) 12 months after entering the trial.
The trial closed early due to poor recruitment. This report contains descriptive data from the trial only and focuses on the challenges faced and lessons learnt.
Chapter 2 Methods
Trial design
This study was a multicentre, pragmatic, randomised controlled superiority trial, with a 12-month internal pilot to assess assumptions about recruitment and provide guidance on optimising the trial processes before proceeding to the main trial phase. Participants were randomised between surgical and non-surgical management of LC-1 pelvic fracture with a 1 : 1 allocation ratio. The study also included an economic evaluation.
The internal pilot addressed the question of whether a sufficient number of eligible patients could be identified and recruited in 12 months to make the target sample size for the trial of 600 participants viable within the proposed 36-month recruitment period. We intended to set up a minimum of 19 sites and randomise 148 patients during the pilot. We planned to set up six sites in each of the first two quarters and the final seven sites in the third quarter. Based on data from four centres, we estimated that each centre would see on average 50–60 potentially eligible patients each year. Applying a conversion rate of 50% for potentially eligible patients to be recruited, we aimed to recruit 1 patient per site per month and a total of 148 patients across the 19 sites that would be gradually opened during the 12-month pilot phase. The recruitment progression criterion was set as a minimum average rate of one patient per site per month. A rate of 0.80–0.99 patients per centre per month was set to signify that a decision to progress may be supportable depending on other supplementary information available (e.g. number and characteristics of potential participants not approached, proportion not meeting eligibility criteria and reasons, proportion declining participation and reasons why) and whether any of the factors impeding recruitment could be remedied.
Population
Patients who met all the inclusion criteria and none of the exclusion criteria were eligible for the trial. Eligibility was assessed by research nurses/associates and was required to be confirmed by a surgeon or clinician authorised in the trial delegation log prior to recruitment.
Inclusion criteria
-
Patients aged 60 years or older.
-
An LC-1 pelvic fracture, arising from a low-energy fall from standing height or less.
-
Patients unable to mobilise independently to a distance of around 3 m and back due to pelvic pain (or perceived pelvic pain) 72 hours after injury. Use of a walking aid and verbal guidance were permitted; however, physical assistance was not.
Exclusion criteria
-
Unable to perform surgery within 10 days of injury.
-
Surgery was contraindicated due to soft-tissue concerns, or because the patient was not fit for anaesthetic (spinal or general).
-
Patients who were non-ambulatory or required physical assistance to walk, prior to their injury (use of a walking aid was permitted).
-
Concomitant injury or polytrauma that impedes mobilisation.
-
Fracture configurations not amenable to internal fixation using INFIX, with or without ilio-sacral screws.
Due to the coronavirus disease 2019 (COVID-19) pandemic, and emerging evidence that infection with COVID-19 may increase the risk of mortality in patients undergoing surgery,28 the following exclusion criterion was added when the study reopened following a pause to recruitment: patients who tested positive for COVID-19 within 72 hours of admission (applicable only where testing was standard of care).
Study setting
We intended to undertake the study at 21 NHS Major Trauma Centres (MTCs) across England, Scotland, Wales and Northern Ireland.
In order to participate in the study, sites needed to have surgeons experienced in the INFIX operation or who had the capacity to be trained to perform it. Participating surgeons were required to be familiar with the surgical procedure [have previously conducted 10 or more INFIX procedures or undergo training until the chief investigator (CI) confirmed that they were sufficiently experienced]. Level of experience was recorded, and no grade of surgeon was excluded from performing the procedure. In addition, all surgeons were required to watch a training video and read a summary guidance document.
There were no specific requirements in place on who could deliver the non-surgical rehabilitation. This was delivered in line with routine practice at each participating site.
Recruitment pathway
Patient pathways for LC-1 fractures were diverse, and therefore research staff at sites had to work across departments and teams in order to maximise patient identification. Patients were identified through collaboration with teams such as Orthopaedics, Trauma, Accident and Emergency, Care of the Elderly, as well as through consultation with orthogeriatricians, inpatient therapists and ward lists.
In order to facilitate raising and maintaining awareness of the study in various disciplines, and thus support patient identification and recruitment, the L1FE trial was registered with the National Institute for Health and Care Research (NIHR) associate principal investigator (API) scheme from the onset of the study. This scheme provides opportunities for healthcare professionals starting their career, who would not normally have the chance to be involved in clinical research, to gain practical experience with this and be formally recognised for their involvement. Due to difficulties with recruitment, we obtained agreement from NIHR to include more than one API per site, as well as to include consultant-level staff without research experience as APIs.
Informed consent
Once eligibility was confirmed, hospital research staff sought written informed consent from patients who had mental capacity to provide it. This study also included recruitment of patients who lacked capacity, and in this instance, consultee agreement or consent was sought in line with legal requirements. 29,30 Consent or consultee agreement was sought for follow-up beyond the duration of the trial to allow the possibility of future long-term follow-up including the use of routinely collected Hospital Episode Statistics (HES) and Office of National Statistics (ONS) data.
Interventions
Participants were randomised to:
-
Non-surgical management: This is the standard care for LC-1 fragility fractures in this patient population in the UK. Patients were routinely administered pain relief and seen by a physiotherapy team who worked to mobilise the patients as pain allowed.
-
Surgery: INFIX is a type of anterior INFIX; it is fitted underneath the patient’s skin. The technique involves percutaneous placement of long pedicle screws within the pelvic bone. The screws are connected by a metal rod across the front of the patient under the skin. As this was a pragmatic study, surgeons could use their preferred INFIX device. The primary fixation for every patient was INFIX. If the surgeon felt that the fracture configuration in a patient warranted supplementary ilio-sacral screw fixation, this was permissible under the trial protocol, provided adequate intraoperative pelvic imaging could be achieved. Surgery had to be undertaken within 10 days of injury.
Participants in both the non-surgical management and surgery groups received pain relief and physiotherapy as per standard care at the participating site and they were also provided with a trial rehabilitation leaflet. This leaflet detailed suggested exercises to perform and was intended to supplement and not replace advice given by the site physiotherapy team. Instructions stated ‘immediate weight bearing, as pain allows’. For both groups, the goals of physiotherapy were to improve function, strength and range of movement in both legs, while aiming to get patients back to independent mobility as soon as possible.
Participants were followed up for 6 months and completed patient questionnaires at baseline (for the day of baseline data collection and 1 week prior to injury), 2 weeks, 6 weeks, 12 weeks and 6 months post-randomisation time points. There was an additional, 12-month follow-up point for those participants who opted it on recruitment and who were recruited early enough in the study to reach this time point within the planned follow-up period.
Outcomes
Table 1 provides a summary of the participant timeline and when measures were collected.
| Time point | Study period | ||||||
|---|---|---|---|---|---|---|---|
| Post allocation | |||||||
| Enrolment/baseline | Randomisation | 2-week | 6-week | 12-weeka | 6-month | 12-monthb | |
| Eligibility screen | ✗ | ||||||
| Informed consent | ✗ | ||||||
| Demographic datac | ✗ | ||||||
| Randomisation | ✗ | ||||||
| Surgical fixation | ✗ | ||||||
| Non-surgical management | ✗ | ||||||
| EQ-5D-5L | ✗d | ✗ | ✗ | ✗ | ✗ | ✗ | |
| PROMIS (LEF and GMH) | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| TUG | ✗ | ||||||
| Pain VAS | ✗e | ✗ | ✗ | ✗ | ✗ | ✗ | |
| AMTS | ✗ | ✗ | ✗ | ||||
| 4AT | ✗ | ✗ | ✗ | ||||
| Mortality | ✗ | ✗ | |||||
| Resource use data | ✗f | ✗ | ✗g | ✗g | |||
| Imaging | ✗ | ||||||
| Complications | ✗ | ✗ | |||||
| COVID-19 statush | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| AE reporting | ✗ | ✗ | ✗ | ||||
| Change in status form | ✗ | ✗ | |||||
Primary outcome
The primary outcome measure was originally intended to be the average health-related quality of life (HRQoL), over 6 months, assessed by the patient-reported outcome measure, EQ-5D-5L utility score. However, due to the poor recruitment and the resulting low sample size, the average EQ-5D-5L utility score over the follow-up period was not feasible. Instead, the EQ-5D-5L utility scores were reported descriptively at each follow-up time point.
EQ-5D-5L was collected at baseline [for the day of baseline data collection and 1 week prior to injury (adapted with permission)], 2 weeks, 6 weeks, 12 weeks and 6 months post-randomisation time points, as well as at the optional 12-month follow-up point where relevant.
The EQ-D-5L is a validated generic patient-reported outcome measure (www.euroqol.org), including validation in patients with hip fractures and orthopaedic patients with cognitive impairment. The descriptive system has five health domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with five response options for each domain (no problems, slight problems, moderate problems, severe problems and extreme problems/unable to do). In addition, it has a health status visual analogue scale (VAS) which measures self-rated health with endpoints ranging from ‘the best health you can imagine’ to ‘the worst health you can imagine’. The EQ-5D-5L was scored according to the User Guide. 31 The measure is easily completed and can be completed by proxy (which was important for our clinical population), and it can also be scored for those who die during follow-up. EQ-5D-5L data were collected either in patient-completed questionnaires (for participants with capacity) or in proxy questionnaires (for those who lacked capacity). EQ-5D-5L proxy version 2 was used where the caregiver (the proxy) is asked to rate how he or she (the proxy) thinks the patient would rate his or her own HRQoL, if the patient were able to communicate it.
Secondary outcomes
-
Self-rated health using the EQ-5D-5L VAS
The second component of the EQ-5D-5L instrument is scored as a number ranging from 0 to 100 where 0 indicates the worse imaginable health and 100 indicates the best imaginable health. The EQ-5D-5L VAS score was collected from patients at baseline (for the day of baseline data collection and 1 week prior to injury), 2 weeks, 6 weeks, 12 weeks, 6 months and (if the patient was followed up at this time) 12 months post randomisation.
-
Physical function using the eight-item Patient-Reported Outcome Measures Information System (PROMIS) lower extremity function (LEF) (mobility) – Short Form. 32
Lower extremity function is an extremely important outcome domain for people with a LC-1 fracture, due to the impact of the injury on ability to mobilise. Measured at baseline, 2 weeks, 6 weeks, 12 weeks and 6 months (by proxy where required).
-
Physical function using Timed Up and Go (TUG)
This test, used routinely in clinical practice, assesses walking speed, mobility, balance and fall risk. 33,34 The TUG test involves measuring the time it takes for a patient to rise from a chair, walk 3 m, turn 180 degrees and return to sitting in the chair. The outcome of this test is the time in seconds taken to complete the test and lower times indicate better mobility. In addition, the number and type of walking aids used by the patient to complete the test was recorded. This measure was undertaken at the 12-week follow-up only in the clinic setting (there was no attempt to perform this where a remote visit was undertaken).
-
Global mental health using the PROMIS scale v1.2 – Global Health Mental 2a subscale
This is a two-question subscale on global mental health (GMH). Inclusion of this subscale was highly commended by our patient and public involvement (PPI) group. Measured at baseline, 2 weeks, 6 weeks, 12 weeks and 6 months (by proxy where required).
-
Pain experienced from their pelvis using a VAS
A unidimensional measure of pain intensity in adults. 35 This is a validated measure for acute and chronic pain in adults where scores are recorded by the patient placing a handwritten mark on a 10-cm line that represents a continuum from ‘No pain’ to ‘Worst imaginable pain’. 36 This outcome was collected from people with capacity only, at baseline, 2 weeks, 6 weeks, 12 weeks, 6 months, and an optional 12-month follow-up for those recruited early within the study.
-
Delirium using the Abbreviated Mental Test Score
Abbreviated Mental Test Score is a short, verbal test widely used in clinical practice to screen for confusion and dementia. 37,38 Recent data confirm its validity in emergency admissions in older adults within UK hospitals. 37 Measured at baseline, 2 weeks and at 12 weeks. Repeat assessment at 12 weeks was to confirm whether delirium was temporary or represented a permanent change.
-
Delirium using the 4AT Rapid Assessment Test for Delirium
4AT Rapid Assessment Test for Delirium is a short, practical instrument validated for detecting delirium, routinely used in clinical practice. 39,40 Postoperative delirium is a known complication for the elderly, particularly those with dementia. Therefore, its use as an outcome measure was to monitor this potential adverse effect of surgery. The strengths of the 4AT Rapid Assessment Test for Delirium are that it can be used on patients who are drowsy or agitated (which is common after surgery), it does not require specialist training and it takes less than 2 minutes to complete. Measured at baseline, 2 weeks and at 12 weeks. Repeat assessment at 12 weeks was to confirm whether delirium was temporary or represented a permanent change.
-
Displacement of the pelvis
A radiological assessment of the pelvis was to be performed at the 12 weeks’ visit for all participants and was to investigate whether there is clinically significant displacement of the pelvic ring; the week 12 investigator case report form (CRF) included a yes/no question asking if there has been clinically significant displacement of the pelvic ring. These X-rays would be standard care for patients undergoing surgical fixation but may be over and above what is routine practice for patients being managed non-surgically. To allow for scheduling around local restrictions and capacity within imaging departments arising from the impacts of the COVID-19 pandemic, the 12-week X-ray could be performed up to 6 months post randomisation.
-
Mortality
Collected at 6 months (and 12 months for those participants who agreed to this additional follow-up).
Complications and adverse events
Lateral cutaneous nerve injury was an adverse event of special interest (AESI), and information on this was collected on an adverse event (AE) form. Patients were also asked about this in the 2-week, 6-week, 12-week and 6-month questionnaires, as well as in the 12-month questionnaires for those who agreed to this additional follow-up.
Information on expected complications, including additional surgery, was collected in hospital at 2 weeks, at 12 weeks and at discharge (if after 2 weeks). Expected complications that were recorded included (but were not limited to): neurological complications, deep wound infection [using Centres for Disease Control (CDC) and Prevention definition],41 superficial infection (using CDC definition), rehospitalisation, re-operation (including removal of implant) and skin problems.
We collected data for the AESI and any unexpected AEs that were related to treatment for the original injury. We collected AE data from the point of randomisation up to 6 months post randomisation for all patients and up to 12 months post randomisation for patients that agreed to this additional time point. All AEs were listed on the appropriate AE or serious adverse event (SAE) CRF for routine return to York Trials Unit (YTU).
The planned process for SAEs was that they were entered onto the SAE reporting form and forwarded to YTU within 24 hours of the investigator becoming aware of them. Once received, causality and expectedness were to be confirmed by the CI. SAEs that were deemed to be unexpected and related to the trial were to be notified to the Research Ethics Committee (REC) and sponsor within 15 days and reported to the Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) at the soonest meeting.
Resource use
Information on resource use throughout patients’ hospital stays and at discharge was collected to assess the impact on the NHS as part of the economic evaluation. Data collected in CRFs included length of hospital stay, medication, surgery details and details of therapy during rehabilitation. The 2-week and late discharge CRFs also collected details on any aids or adaptations required and any change of place of residence (e.g. own home to residential care home) at discharge relative to baseline. Resource use data were also to be collected in the 12-week patient questionnaire, from patients with capacity only. This included information on any readmittance to hospital, outpatient care received, any additional medications, aids or adaptations since discharge and return to work.
Patient preference data
Patient preference data were collected for patients invited to participate in the study. Patient preference data were collected on the baseline CRF for patients who did consent to the study and on an optional, separate patient preference form for those who did not. The baseline CRF asked which treatment the patient would have preferred or if they had no preference. The patient preference form asked what the patient’s preferred study treatment was: ‘No preference’, ‘Non-operative management’ or ‘Surgical fixation’. The strength of their preference was also assessed on a scale of 1–10 (with 1 corresponding to no preference and 10 corresponding to very strong preference). Also, the patient was asked how effective they thought each treatment would be: ‘Very ineffective’, ‘Fairly ineffective’, ‘Can’t decide’, ‘Fairly effective’, ‘Very effective’. The patient preference form given to patients who did not consent to participate in the study asked them to record their reason for non-consent.
Sample size
It was estimated that 600 participants were required to address the study objectives. The primary outcome was the EQ-5D-5L over 6 months. To be conservative, we took the lowest published estimate of the minimal clinically important differences (MCID) (0.074)42 with an estimated standard deviation (SD) of 0.25 (estimated from the 0.30 reported by Adachi et al. 43 for the 3L version and adjusted down to account for the 5L version’s greater sensitivity). Based on these assumptions we would have needed to analyse 480 participants (240 per group) and, after accounting for loss to follow-up of 20%, we would have needed to recruit and randomise 600 participants for a study with 90% power (2p = 0.05).
Assignment of interventions
Intervention allocation was assigned using a secure, web-based randomisation system developed for the L1FE study by the software development team at YTU. The online L1FE Data Management System was an independent secure randomisation service for sequence allocation hosted by YTU and accessed by site research staff either by telephone or via the internet. Research staff who were delegated the responsibility to randomise patients on their site delegation log were granted access using a personal log in.
Once an eligible patient had consented and their baseline forms had been completed, research staff recorded their information on the L1FE Randomisation System. The system confirmed eligibility and then performed independent and concealed random allocation (1 : 1), using computer-generated permuted blocks of random sizes (4, 6 and 8), stratified by centre. The patient was allocated to either surgical fixation or non-surgical management.
Patients and treating clinicians were informed of the allocation. As with many surgical trials, where the surgical site is clearly visible, it was not feasible to blind patients, surgeons or outcome assessors.
Data collection and management
Paper CRFs were used to record data. Data were collected at recruiting sites by research staff on hospital CRFs and participants completed CRFs by post. For participants who lacked capacity, a proxy version was completed on the participants’ behalf; to be pragmatic, the proxy could be a family member, friend or carer who had regular contact with the participant. All CRFs were returned to YTU for scanning and processing.
To minimise attrition, we used multiple methods to keep in contact with participants. We asked participants for full contact details (including mobile phone number and e-mail address). We also collected alternative contact details of someone who could be contacted if the participant changed address. Participants (or their proxy) could complete the 2- and 12-week questionnaires in clinic when attending in person or they could be completed over the phone. The 6-week, 6-month and 12-month questionnaires were completed either by post or over the phone for the patient’s convenience. Pre-notification letters were sent out before the postal follow-up questionnaires were due, to help prime participants, and a text message reminder was also sent on the day participants were expected to receive the postal questionnaire. This has been shown to significantly reduce time to questionnaire response. 44 Where a participant did not return their follow-up CRF, there were also up to two follow-up postal reminders and a telephone reminder at each time point. The telephone reminder gave participants the option to complete an abridged questionnaire (a minimum of the EQ-5D-5L) by telephone. The study team also called the participant when there were missing data on the primary outcome (and other missing data as feasible) when a postal questionnaire was returned.
Participants were free to fully withdraw from the study at any point; however, it was also possible for them to withdraw from only one aspect of the trial if participation became a burden. For example, participants could continue with clinical visits only, postal questionnaires only or data collection from their hospital records only with no participant involvement. It was anticipated that these options would reduce the need for patients to fully withdraw from the trial and enable some useful data to still be collected.
Data management
An electronic management system was used to track participant recruitment and study status as well as CRF returns. Data from CRFs were processed by administrative personnel at YTU. Data were verified through cross-checking of the data against the hard copy of the CRF. A Validation Plan for the CRFs was written by the trial coordinators and trial statistician in consultation with the YTU Data Manager. The Plan included detailed coding for the CRFs and data query resolution rules/procedures. Quality Control was applied at each stage of data handling to ensure that all data were reliable and processed correctly.
Data were handled in accordance with the Data Protection Act 2018, GDPR legislation, the latest Directive on Good Clinical Practice, and local policy.
All personal information collected about enrolled participants was stored electronically in a secure environment at the University of York, with permissions for access in line with standard operating procedures (SOPs). All paper records containing personal information such as consent forms and consultee declaration forms were stored safely in a separate compartment of a locked cabinet.
Clinical information was only looked at by responsible individuals from the study team, the Sponsor, the NHS Trust or from regulatory authorities, where it was relevant to the patient taking part in this research as he or she agreed to at the time of consent or consultee declaration.
Once randomised, patients were assigned a participant ID number. This was used on all CRFs, and individual participants were only to be referred to by their participant ID number to maintain patient confidentiality. All study data were completely anonymised for any analyses, reports and publications.
Study Within A Trial
Improving retention of participants is important to all RCTs and there is a need to develop and test interventions to improve retention. The L1FE trial acted as a host trial for an embedded trial, referred to as a Study Within A Trial (SWAT). The objective of this SWAT was to evaluate the impact of making a courtesy introductory telephone call to newly recruited trial participants, on response rates to follow-up questionnaires compared with a written card with equivalent information, or nothing. This SWAT was registered on the Medical Research Council (MRC) SWAT Repository. SWAT Ref 114: Effects of telephone calls or postcards to trial participants following enrolment on retention in a randomised trial. Due to recruiting so few participants there are no results to report on this study.
Analysis of internal pilot
The L1FE study was designed with an internal 12-month pilot phase to test assumptions about recruitment and confirm whether the trial is feasible. The target was to set up a minimum of 19 sites during the pilot with the aim of including all interested sites by month 13. Assuming one patient per site per month, 148 patients should be randomised. An average recruitment rate of one patient per centre per month would support a decision to progress to the main trial. An average rate of 0.80–0.99 per centre per month would suggest that a decision to progress may be supportable depending on other supplementary information available (e.g. number and characteristics of potential participants not approached, proportion not meeting eligibility criteria and reasons, proportion declining participation and reasons why) and whether any of the factors impeding recruitment could be remedied.
Data on the number of sites and average number of patients recruited per site per month are presented.
Data on screening, eligibility and recruitment for the trial are presented in Table 2 as frequencies and proportions and at an overall level. The following information on screening, eligibility and recruitment into the trial are reported:
-
number of patients assessed for eligibility
-
number and proportion of patients assessed for eligibility who were found to be eligible
-
number and proportion of eligible patients who were approached for consent
-
number and proportion of patients approached for consent who consented to participation
-
number and proportion of consented patients who were randomised.
A summary of the frequencies for patient preferences and reasons for refusing consent for non-consenting patients are also reported.
A summary of how identified potential patients progress through the trial and their reasons for dropping out are shown in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Details of all participating surgeons’ prior experience with the INFIX procedure were collected as part of the trial. Equipoise is an essential concept in trials and was covered during the training delivered as part of the site set-up process. The assumption of surgeon equipoise was monitored during recruitment by scanning reasons for exclusion during screening and reasons for crossover following randomisation that may reflect surgeon preferences, as well as during research staff teleconferences where site staff were asked about recruitment progress, as well as barriers and facilitators to recruitment at their site.
Final analysis
All analyses were conducted in STATA v17 (StataCorp, College Station, TX, USA). Full analyses were detailed in a statistical analysis plan (SAP), which was finalised following patient recruitment prior to the completion of data collection and database lock.
Overview
The originally planned primary analysis was a mixed-effects linear regression model, with EQ-5D-5L scores at 2 weeks’, 6 weeks’, 12 weeks’ and 6 months’ follow-up as the dependent variable, adjusting for baseline EQ-5D-5L, randomised group and other pertinent baseline characteristics as fixed effects. The plan was to control for potential clustering at hospital site level by including it in the model as a random effect and to account for the correlation of scores within patients over time by means of an appropriate covariance structure. The secondary outcomes were to be analysed by similar mixed-effects linear regression models. Mortality was planned to be analysed using a logistic regression model.
Originally, it was intended that statistical analysis would generally be conducted using two-sided hypothesis tests with a 5% significance level. However, due to poor recruitment no formal statistical hypothesis testing has been undertaken, as the achieved sample size is too low for the conclusions of these tests to be meaningful. As a result, all analyses of the primary and secondary outcome measures are descriptive in nature. Analyses are conducted on an intention-to-treat (ITT) basis, with patients being analysed in the groups to which they were randomised.
Baseline data
Baseline data of the outcome measures and information on patient characteristics at baseline are presented descriptively by randomised group and overall in a table. For continuous measures, the mean, median, SD, first and third quartiles, minimum and maximum are reported. For categorical outcome measures, the data are presented as outcome measures and percentages.
Primary analysis
Analysis of the EQ-5D-5L utility index, the primary outcome which measures HRQoL, was descriptive in nature with no formal hypothesis testing due to the small sample size. The mean and median of the EQ-5DL-5L utility index score at each follow-up period in the study are reported. In addition to the mean and median, the following summary statistics are reported for the EQ-5D-5L utility index score at follow-up period: SD, first quartile, third quartile, minimum, maximum, number of observations and number of missing observations. All reported summary statistics are given at both a treatment group and overall level.
Sensitivity and subgroup analyses
No sensitivity analyses were undertaken. Originally there was to be a subgroup analysis exploring how patient’s knowledge and experience of their allocated treatment affected the results of their primary outcome measure; this was going to be achieved by including an interaction between patient preference and treatment group in the linear mixed-effects model for EQ-5D-5L utility index score. However, this subgroup analysis was not undertaken, as there was no statistical modelling of the primary outcome.
Secondary outcomes
A descriptive analysis of secondary outcomes was undertaken due to the low sample size. There was no formal hypothesis testing or statistical modelling. All summary statistics are reported on both a treatment arm and overall level for the secondary outcomes. These include mean, SD, median, minimum, maximum for continuous variables and counts and proportions for categorical variables.
Complications and adverse events
Expected complications (expected AEs)
A summary reporting the number and proportion of patients experiencing each expected complication and the number of patients experiencing any expected complication in both treatment arms at 2 weeks, 12 weeks and discharge (if after 2 weeks) will be created. The proportion of patients affected by each type of complication at 2 weeks, 12 weeks and discharge (if after 2 weeks) and the proportion of patients affected by any expected complication at 2 weeks, 12 weeks and discharge (if after 2 weeks) will all be secondary outcome measures.
Adverse events
The number of (all) AEs and the proportion of patients with at least one AE in each treatment arm and overall are reported for both the 6- and 12-month follow-up time points; and broken down by outcome of the AE, the relationship of the AE to the treatment, action taken, seriousness of the AE, severity of the AE and whether the AE was expected. The number of times each reason for classification was used in classifying the AE is reported.
The number of SAEs and the proportion of patients with at least one SAE in each treatment arm are reported for both the 6- and 12-month follow-up time points; and broken down by outcome of the SAE, the relationship of the SAE to the treatment, action taken and whether the SAE was expected. The number of times each reason for classification was used in classifying the SAE is reported.
The number of reported incidents of lateral cutaneous nerve injury in patients in the surgical fixation of INFIX treatment arm since the patient received surgical fixation of the INFIX device and the proportion of patients in this treatment arm who experienced lateral cutaneous nerve injury since they received the INFIX device at 2 weeks, 6 weeks, 12 weeks, 6 months and 12 months are reported.
Economic evaluation
Due to the trial stopping early, it was not possible to undertake the planned economic evaluation as outlined in the study protocol.
The planned economic evaluation was to be conducted from the recommended NHS and personal social services (PSS) perspective according to NICE guidance. 45 Data were collected on the costs and outcomes of each trial participant. Participants were asked to complete economic resource use questionnaires at 12 weeks and 6 months as well as at the optional 12-month time point. This included hospital (e.g. inpatient, outpatient, A&E) and community and social care resource used; and for the purposes of secondary analysis, costs associated with lost productivity and out-of-pocket costs. Hospital forms were designed to collect information on the cost of surgery (e.g. time in theatre, staff time, consumables and devices, nights in hospital after the procedure), complications, physiotherapy and removal of devices. Relevant UK unit costs, such as NHS Reference costs and Personal Social Services Research Unit (PSSRU) costs of health and social care, would have been applied to each resource item to value total resource use in each group.
For the planned economic evaluation, the raw EQ-5D-5L scores at baseline (today and 1 week prior to injury), 2 weeks, 6 weeks, 12 weeks and 6 months post randomisation according to domain would have been displayed, in order to examine the movements between levels for each domain according to group. The overall difference in EQ-5D-5L index scores between the two groups would have been examined through regression methods, consistent with the model selected in the statistical analysis. The EQ-5D-5L health states would have been valued using the mapping function developed by van Hout et al. (2012) in accordance with NICE recent recommendation (www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf). Quality-adjusted life-years (QALYs) would have been calculated by plotting the utility scores at each of the four time points and estimating the area under the curve. 46 For the analysis, regression methods would have been used to express the incremental cost per QALYs gained. Results would have been presented using incremental cost-effectiveness ratios (ICERs) and cost-effectiveness acceptability curves (CEACs) generated via non-parametric bootstrapping. The pattern of missing data would have been analysed and handled by means of multiple imputation (MI) methods. 47 A range of sensitivity analyses would have been conducted to test the robustness of the results using different scenarios, including probabilistic sensitivity analysis. We proposed to undertake longer-term modelling if this had been deemed appropriate (i.e. there was a non-dominant situation in the trial-based evaluation). To do this, we would have undertaken a secondary analysis to explore how the differences observed during the trial evolved beyond the study. For this projection, we would have used a decision-modelling approach to extrapolate the cost-effectiveness data observed in the study to a lifetime horizon. The analysis would have been based on a combination of observed in-trial cost and HRQoL and projections of life expectancy.
If the planned economic evaluation had gone ahead, full analyses would have been detailed in a pre-specified health economics analysis plan (HEAP) that would have been signed off by the trial management team and oversight committees.
From the limited data collected, we have provided a descriptive analysis of resource use. Data of resource use during the patient’s stay in hospital up to and including discharge (which encompasses length of hospital stay, medication, rehabilitation and surgery details) and since their discharge from hospital (which includes re-admission to hospital, outpatient care received, any additional medications, aids or adaptions since discharge and return to work) are analysed descriptively by comparing summary statistics from each treatment arm. Descriptive statistics include counts and proportions for categorical variables and mean, SD, median, minimum, maximum for continuous variables (or discrete variables which have a large range of values).
Regulatory approvals and protocol amendments
The L1FE study was given favourable opinion by London – Harrow REC (Ref: 19/LO/0555) on 16 July 2019. The Health Research Authority (HRA) has also given governance approval on 17 July 2019. The study was given approval by Scotland A REC (REF 21/SS/0002), specifically related to the inclusion of patients who lack capacity to consent in Scotland on 12 February 2021.
Written, informed consent to participate was obtained from all participants with capacity. Participants were informed of their right to withdraw from the study at any time and for any reason, and all participants were made aware that withdrawal would not affect their routine care. The study included patients who lack capacity, and, as appropriate, consultee declaration was sought from a personal or nominated consultee (in England, Wales and Northern Ireland) or consent from a guardian, Welfare Attorney or nearest relative (in Scotland). The process for seeking consultee declaration for patients lacking capacity in England, Wales and Northern Ireland has been approved by the REC in England and was in accordance with the Mental Capacity Act 2005 for England and Wales and in accordance with the Mental Capacity Act (NI) 2016 for Northern Ireland. The process for seeking consent for patients without capacity in Scotland was in accordance with the Adults with Incapacity Act (Scotland) 2000 and approved by the REC in Scotland. The Mental Capacity Act 2005, the Mental Capacity Act (NI) 2016 and the Adults with Incapacity Act (Scotland) 2000 establish a framework for the protection of the rights of people who lack the capacity to decide themselves. They are designed to ensure that the interests and rights of people who lack capacity are protected and that their current and previously expressed wishes are respected.
The final version of the protocol is V3.2, dated 22 December 2020, and is available from the funder website. All substantial amendments were submitted to the HRA (and the REC where required) having been agreed with: the funding body, Sponsor, TSC, DMEC and the Trial Management Group (TMG). Minor modifications to the protocol were agreed with the funding body, TMG and Sponsor before submission for approval to the HRA. All amendments are listed in Appendix 1.
Chapter 3 Results
Overview
The internal pilot took place in two separate phases. The trial first opened to recruitment on 2 August 2019 and this first phase of the pilot continued until 19 March 2020, when recruitment was paused for 1 year due to the COVID-19 pandemic. The trial re-opened for recruitment on 15 March 2021 with a revised internal pilot end date of 15 September 2021 agreed with the funder. Recruitment targets were not being met in the first pilot phase and, despite adjustments and mitigations to address the various challenges, the recruitment difficulties were exacerbated by the COVID-19 pandemic during the second phase of the pilot. It became clear before the end of the second phase that the study was not viable at that time and the team proposed to the funder that recruitment should be suspended early rather than waiting for the pilot phase to complete. It was agreed with the funder to stop recruitment on 13 August 2021. Follow-up was completed for all participants (last follow-up was completed in January 2022) and a close-down plan implemented.
Site set-up
Eleven sites in England and Wales were opened during the first phase of the internal pilot (see Appendix 2). Ten of these re-opened following the study pause and one declined to participate further. A further 10 sites, including 3 in Scotland and 1 in Northern Ireland, were interested in participating and were in various stages of set-up prior to closure. The 11 sites were open for a combined total of 92 months.
Eligibility, screening and recruitment of patients
The flow of participants through the trial is reported in the CONSORT flow diagram (Figure 1).
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.

Table 2 provides a summary of potential participants identified for the study. During the internal pilot, 316 patients were assessed for eligibility, of whom 43 were eligible (13.6%). The main reasons for ineligibility were: patient able to mobilise independently or with supervision to 3 m and back (n = 161), concomitant injury or polytrauma that impedes mobilisation (n = 57), surgery contraindicated (n = 40), patient did not have a low-energy LC-1 pelvic fracture (n = 38), unable to schedule surgery within 10 days of injury (n = 34), patient was under 60 years of age (n = 23) and/or the patient was non-ambulatory or required assistance prior to injury (n = 22).
| Number of patients assessed for eligibility, n | 316 |
| Number of eligible patients, n (%) | 43 (13.6) |
| Number of eligible patients approached for consent, n (%) | 36 (83.7) |
| Number of patients approached providing consent, n (%) | 11 (30.6) |
| Number of consenting patients randomised into study, n (%) | 11 (100.0) |
It was predicted that 50% of eligible patients would consent and be recruited to the study. Of the 43 eligible participants, 36 (83.7%) were approached for consent, of whom 11 (30.6%) provided consent. Eleven (100%) of the consenting patients were randomised into the study. The most common reason for eligible patients not consenting to take part were that they were unwilling to be randomised to a treatment (n = 10) (Table 3). Four non-consenting patients completed a patient preference form. All four patients stated they had a preference for surgical fixation with the average strength of this preference being 7.8 (SD 2), indicating a strong preference. All patients could not decide how effective they thought surgical fixation would be. Two patients thought non-surgical management would be fairly effective and two could not decide.
| Reasons for refusing consenta | n (%) |
|---|---|
| Unwilling to participate in research | 3 (12) |
| Unwilling to be randomised to a treatment | 10 (40) |
| Next of kin unavailable | 2 (8) |
| Consultee did not want to complete CRFs | 1 (4) |
| No reason given | 10 (40) |
Recruitment rate
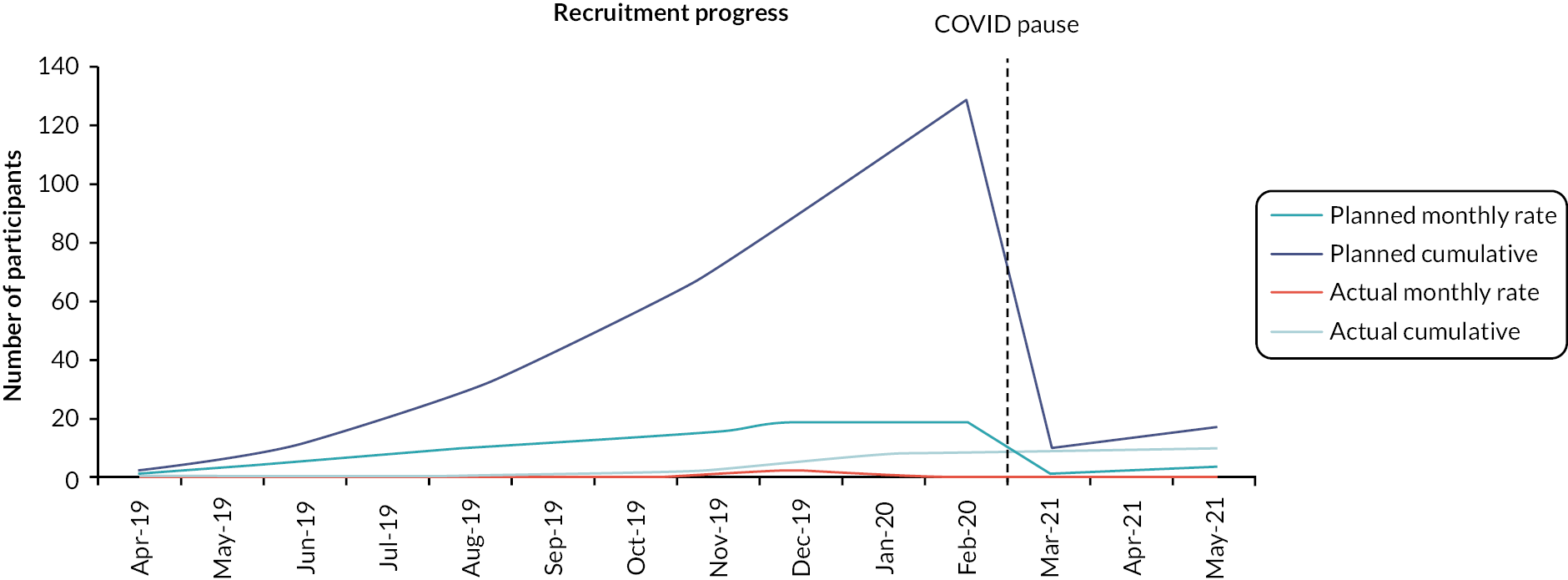
The average recruitment rate per site per month was 0.175. The average recruitment rate was well below the target rate of one patient per site per month. See Figure 2 for predicted and actual recruitment. Table 4 provides a summary of screening and recruitment data by site. The number of patients screened varied greatly between centres and there is not a clear relationship between this and the length of time each centre was open to recruitment. The number of patients randomised varied between sites, with 7 out of the 11 sites not recruiting any participants at all. Of the four sites that did recruit, the CI’s site at Bart’s Health NHS Trust recruited the most successfully, with 8 of the 11 participants randomised from this site. North Bristol NHS Trust, South Tees Hospitals NHS Foundation Trust and St George’s University Hospitals NHS Foundation Trust all recruited a single participant.
FIGURE 2.
Predicted and actual recruitment.
| Centre | Centre open (months) | Screened | Eligible | Approached | Randomised |
|---|---|---|---|---|---|
| Bart’s Health NHS Trust | 13 | 52 | 21 | 20 | 8 (15) |
| Cambridge University Hospitals NHS Foundation Trust | 8 | 17 | 1 | 1 | 0 (0) |
| North Bristol NHS Trust | 9 | 89 | 6 | 3 | 1 (1) |
| Nottingham University Hospitals NHS Trust | 9 | 38 | 0 | 0 | 0 (0) |
| South Tees Hospitals NHS Foundation Trust | 8 | 25 | 4 | 3 | 1 (4) |
| Oxford University Hospitals NHS Foundation Trust | 7 | 6 | 0 | 0 | 0 (0) |
| Cardiff and Vale University Health Board | 9 | 17 | 2 | 1 | 0 (0) |
| University Hospitals Coventry and Warwickshire NHS Trust | 3 | 5 | 0 | 0 | 0 (0) |
| Kings College Hospital NHS Foundation Trust | 8 | 23 | 1 | 0 | 0 (0) |
| St George’s University Hospitals NHS Foundation Trust | 10 | 22 | 6 | 6 | 1 (5) |
| Imperial College Healthcare NHS Trust | 8 | 22 | 2 | 2 | 0 (0) |
| Total | 92 | 316 | 43 | 36 | 11 (3) |
Randomisation and follow-up
Eleven patients were randomised, five to INFIX surgical fixation and six to non-surgical management (see Figure 1). Three participants in the non-surgical management group were withdrawn from the study: one participant died, one participant lost capacity and their family member no longer wanted them to take part in the study, and one participant had sight problems and considered themselves too frail to continue (see Figure 1). Of the five participants randomised to surgical fixation, all received surgery and no one in the non-surgical management group received surgery. All participants in the surgical fixation group completed the 6-month questionnaires (5/5) and the remaining participants in the non-surgical management group provided data at 6 months (3/6). Not all participants were followed up at 12 months.
Demographics and baseline data
Table 5 provides the demographic characteristics and baseline data as randomised. The average age of participants in the trial was 83, with the youngest 72 and oldest 97. Participants were predominantly female (82%), white (82%), non-smokers (82%) and all retired. Of those who responded, none of the participants had a previous fracture or used steroids and 18% reported having diabetes. The average EQ-5D-5L utility score and VAS score 1 week prior to the injury (collected on the day of the baseline assessment) was 0.7 (SD 0.3) and 60 (SD 25) and at baseline was −0.2 (SD 0.3) and 32 (SD 27), respectively. The average PROMIS physical function lower extremity score was 10.5 (SD 5.6) and GMH score was 36.9 (SD 9.6). The average AMTS was 7.1 (SD 3.6) and 4AT score was 1.5 (SD 2.0). The baseline VAS pain score was only completed for six participants and ranged from 9 to 99 with the average being 69 (SD 33) and median 78 (IQR 55–96).
| Control (n = 6) | Intervention (n = 5) | Overall (n = 11) | |
|---|---|---|---|
| Age (years) | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 83.3 (8.7) | 83.8 (8.1) | 83.5 (8.0) |
| Median (Q1, Q3) | 81.5 (79.0, 89.0) | 86.0 (76.0, 88.0) | 83.0 (76.0, 89.0) |
| Min, max | 72.0, 97.0 | 75.0, 94.0 | 72.0, 97.0 |
| Ethnicity, n (%) | |||
| White | 6 (100.0) | 3 (60.0) | 9 (81.8) |
| Black | 0 (0.0) | 1 (20.0) | 1 (9.1) |
| Other | 0 (0.0) | 1 (20.0) | 1 (9.1) |
| Sex, n (%) | |||
| Male | 2 (33.3) | 0 (0.0) | 2 (18.2) |
| Female | 4 (66.7) | 5 (100.0) | 9 (81.8) |
| Current smoking status, n (%) | |||
| Smoker | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Non-smoker | 5 (83.3) | 4 (80.0) | 9 (81.8) |
| Work status, n (%) | |||
| Retired | 6 (100.0) | 5 (100.0) | 11 (100.0) |
| Diabetes, n (%) | |||
| Yes | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| No | 4 (66.7) | 3 (60.0) | 7 (63.6) |
| Missing | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Use of steroids, n (%) | |||
| No | 5 (83.3) | 4 (80.0) | 9 (81.8) |
| Missing | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Previous fracture, n (%) | |||
| Yes | 0 (0.0) | 1 (20.0) | 1 (9.1) |
| No | 5 (83.3) | 3 (60.0) | 8 (72.7) |
| Missing | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| EQ-5D-5L utility score (1 week prior to fall – collected at baseline) | |||
| N (missing) | 5 (1) | 1 (4) | 6 (5) |
| Mean (SD) | 0.7 (0.4) | 0.7 (a) | 0.7 (0.3) |
| Median (Q1, Q3) | 0.8 (0.6, 0.8) | 0.7 (0.7, 0.7) | 0.7 (0.6, 0.8) |
| Min, max | 0.1, 1.0 | 0.7, 0.7 | 0.1, 1.0 |
| EQ-5D VAS (1 week prior to fall – collected at baseline) | |||
| N (missing) | 5 (1) | 2 (3) | 7 (4) |
| Mean (SD) | 66 (26) | 45 (21) | 60 (25) |
| Median (Q1, Q3) | 75 (70, 80) | 45 (30, 60) | 70 (30, 80) |
| Min, max | 20, 85 | 30, 60 | 20, 85 |
| EQ-5D-5L utility score | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | −0.1 (0.4) | −0.2 (0.2) | −0.2 (0.3) |
| Median (Q1, Q3) | −0.2 (−0.3, −0.1) | −0.3 (−0.4, −0.2) | −0.3 (−0.4, −0.1) |
| Min, max | −0.6, 0.7 | −0.4, 0.2 | −0.6, 0.7 |
| EQ-5D-5L VAS | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 31 (27) | 34 (31) | 33 (27) |
| Median (Q1, Q3) | 30 (10, 40) | 30 (9, 60) | 30 (9, 60) |
| Min, max | 0, 75 | 0, 70 | 0, 75 |
| Physical function (lower extremity) | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 8.8 (1.0) | 12.6 (8.1) | 10.5 (5.6) |
| Median (Q1, Q3) | 8.5 (8.0, 10.0) | 9.0 (8.0, 11.0) | 9.0 (8.0, 10.0) |
| Min, max | 8.0, 10.0 | 8.0, 27.0 | 8.0, 27.0 |
| GMH | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 39.6 (10.7) | 33.8 (8.0) | 36.9 (9.6) |
| Median (Q1, Q3) | 36.5 (36.5, 44.4) | 36.5 (25.8, 36.5) | 36.5 (25.8, 44.4) |
| Min, max | 25.8, 57.7 | 25.8, 44.4 | 25.8, 57.7 |
| AMTS | |||
| N (missing) | 6 (0) | 4 (1) | 10 (1) |
| Mean (SD) | 7.8 (3.9) | 6.0 (3.3) | 7.1 (3.6) |
| Median (Q1, Q3) | 9.5 (8.0, 10.0) | 6.0 (4.0, 8.0) | 8.5 (6.0, 10.0) |
| Min, max | 0.0, 10.0 | 2.0, 10.0 | 0.0, 10.0 |
| 4AT score | |||
| N (missing) | 6 (0) | 4 (1) | 10 (1) |
| Mean (SD) | 0.7 (1.2) | 2.8 (2.5) | 1.5 (2.0) |
| Median (Q1, Q3) | 0.0 (0.0, 1.0) | 2.5 (1.0, 4.5) | 0.5 (0.0, 3.0) |
| Min, max | 0.0, 3.0 | 0.0, 6.0 | 0.0, 6.0 |
Patient preference at baseline
Five participants in the non-surgical management group provided data on their preferences at baseline; two had no preference and three had a preference for INFIX surgical fixation.
Primary outcome
Table 6 reports the EQ-5D-5L utility score by intervention group and overall for baseline, 2 weeks, 6 weeks, 12 weeks, 6 months and 12 months post randomisation, including the number.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | −0.12 (0.43) | −0.21 (0.23) | −0.16 (0.34) |
| Median (Q1, Q3) | −0.21 (−0.27, −0.13) | −0.26 (−0.35, −0.17) | −0.26 (−0.35, −0.13) |
| Min, max | −0.59, 0.69 | −0.43, 0.16 | −0.59, 0.69 |
| Perfect health (11111), n (%) | 0, (0.0) | 0, (0.0) | 0, (0.0) |
| Week 2 | |||
| N (missing) | 5 (0) | 4 (0) | 9 (0) |
| Mean (SD) | 0.01 (0.35) | 0.12 (0.38) | 0.05 (0.34) |
| Median (Q1, Q3) | 0.07 (0.05, 0.22) | 0.14 (−0.17, 0.40) | 0.07 (0.03, 0.26) |
| Min, max | −0.59, 0.28 | −0.36, 0.53 | −0.59, 0.53 |
| Perfect health (11111), n (%) | 0, (0.0) | 0, (0.0) | 0, (0.0) |
| Week 6 | |||
| N (missing) | 3 (0) | 3 (0) | 6 (0) |
| Mean (SD) | 0.49 (0.14) | 0.34 (0.22) | 0.42 (0.19) |
| Median (Q1, Q3) | 0.44 (0.39, 0.65) | 0.34 (0.12, 0.57) | 0.42 (0.34, 0.57) |
| Min, max | 0.39, 0.65 | 0.12, 0.57 | 0.12, 0.65 |
| Perfect health (11111), n (%) | 0, (0.0) | 0, (0.0) | 0, (0.0) |
| Week 12 | |||
| N (missing) | 2 (0) | 5 (0) | 7 (0) |
| Mean (SD) | 0.04 (0.23) | 0.35 (0.24) | 0.26 (0.27) |
| Median (Q1, Q3) | 0.04 (−0.13, 0.20) | 0.34 (0.23, 0.34) | 0.23 (0.10, 0.34) |
| Min, max | −0.13, 0.20 | 0.10, 0.74 | −0.13, 0.74 |
| Perfect health (11111), n (%) | 0, (0.0) | 0, (0.0) | 0, (0.0) |
| Month 6 | |||
| N (missing) | 3 (0) | 5 (0) | 8 (0) |
| Mean (SD) | 0.39 (0.51) | 0.28 (0.33) | 0.32 (0.37) |
| Median (Q1, Q3) | 0.49 (−0.17, 0.84) | 0.27 (0.10, 0.36) | 0.32 (−0.01, 0.63) |
| Min, max | −0.17, 0.84 | −0.12, 0.77 | −0.17, 0.84 |
| Perfect health (11111), n (%) | 0, (0.0) | 0, (0.0) | 0, (0.0) |
| Month 12 | |||
| N (missing) | 1 (0) | 1 (0) | 2 (0) |
| Mean (SD) | 1.00 (a) | −0.20 (a) | 0.40 (0.85) |
| Median (Q1, Q3) | 1.00 (1.00, 1.00) | −0.20 (−0.20, −0.20) | 0.40 (−0.20, 1.00) |
| Min, max | 1.00, 1.00 | −0.20, −0.20 | −0.20, 1.00 |
| Perfect health (11111), n (%) | 1, (100.0) | 0, (0.0) | 1, (50.0) |
Secondary outcomes
EQ-5D-5L VAS
Table 7 reports the EQ-5D-5L VAS score by intervention group and overall for baseline, 2 weeks, 6 weeks, 12 weeks, 6 months and 12 months post randomisation, including the number.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 31 (27) | 34 (31) | 32 (27) |
| Median (Q1, Q3) | 30 (10, 40) | 30 (9, 60) | 30 (9, 60) |
| Min, max | 0, 75 | 0, 70 | 0, 75 |
| Week 2 | |||
| N (missing) | 5 (0) | 4 (0) | 9 (0) |
| Mean (SD) | 43 (27) | 53 (21) | 47 (23) |
| Median (Q1, Q3) | 55 (35, 60) | 55 (40, 65) | 55 (35, 60) |
| Min, max | 0, 65 | 25, 75 | 0, 75 |
| Week 6 | |||
| N (missing) | 3 (0) | 3 (0) | 6 (0) |
| Mean (SD) | 65 (27) | 53 (23) | 59 (23) |
| Median (Q1, Q3) | 55 (45, 95) | 55 (30, 75) | 55 (45, 75) |
| Min, max | 45, 95 | 30, 75 | 30, 95 |
| Week 12 | |||
| N (missing) | 2 (0) | 5 (0) | 7 (0) |
| Mean (SD) | 62 (11) | 53 (26) | 56 (22) |
| Median (Q1, Q3) | 63 (55, 70) | 60 (50, 70) | 60 (50, 70) |
| Min, max | 55, 70 | 10, 75 | 10, 75 |
| Month 6 | |||
| N (missing) | 2 (1) | 5 (0) | 7 (1) |
| Mean (SD) | 63 (25) | 53 (15) | 56 (16) |
| Median (Q1, Q3) | 63 (45, 80) | 50 (40, 60) | 50 (40, 75) |
| Min, max | 45, 80 | 40, 75 | 40, 80 |
| Month 12 | |||
| N (missing) | 1 (0) | 1 (0) | 2 (0) |
| Mean (SD) | 90 | 0 | 45 (64) |
| Median (Q1, Q3) | 90 | 0 | 45 (0, 90) |
| Min, max | 90, 90 | 0 | 0, 90 |
PROMIS lower extremity function score
Appendix 3, Table 14 provides details of the number of missing data items for this measure and Table 8 provides a summary of the scores over time.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 8.8 (1.0) | 12.6 (8.1) | 10.5 (5.6) |
| Median (Q1, Q3) | 8.5 (8.0, 10.0) | 9.0 (8.0, 11.0) | 9.0 (8.0, 10.0) |
| Min, max | 8.0, 10.0 | 8.0, 27.0 | 8.0, 27.0 |
| Week 2 | |||
| N (missing) | 4 (1) | 4 (0) | 8 (1) |
| Mean (SD) | 12.3 (4.0) | 11.8 (4.5) | 12.0 (4.0) |
| Median (Q1, Q3) | 12.0 (9.0, 15.5) | 11.0 (8.0, 15.5) | 12.0 (8.0, 15.5) |
| Min, max | 8.0, 17.0 | 8.0, 17.0 | 8.0, 17.0 |
| Week 6 | |||
| N (missing) | 3 (0) | 2 (1) | 5 (1) |
| Mean (SD) | 22.0 (9.5) | 14.0 (1.4) | 18.8 (8.1) |
| Median (Q1, Q3) | 17.0 (16.0, 33.0) | 14.0 (13.0, 15.0) | 16.0 (15.0, 17.0) |
| Min, max | 16.0, 33.0 | 13.0, 15.0 | 13.0, 33.0 |
| Week 12 | |||
| N (missing) | 2 (0) | 4 (1) | 6 (1) |
| Mean (SD) | 11.0 (4.2) | 17.8 (8.5) | 15.5 (7.7) |
| Median (Q1, Q3) | 11.0 (8.0, 14.0) | 16.5 (11.5, 24.0) | 14.0 (9.0, 19.0) |
| Min, max | 8.0, 14.0 | 9.0, 29.0 | 8.0, 29.0 |
| Month 6 | |||
| N (missing) | 3 (0) | 5 (0) | 8 (0) |
| Mean (SD) | 20.3 (14.3) | 16.4 (9.2) | 17.9 (10.5) |
| Median (Q1, Q3) | 17.0 (8.0, 36.0) | 16.0 (8.0, 20.0) | 16.5 (8.0, 25.0) |
| Min, max | 8.0, 36.0 | 8.0, 30.0 | 8.0, 36.0 |
Timed Up and Go test
One participant randomised to the surgical fixation group provided TUG data at week 12. This participant did not use any walking aids and completed the test in 18 seconds.
PROMIS global mental health
Table 9 reports the PROMIS GMH 2a score by intervention group and overall for baseline, 2 weeks, 6 weeks, 12 weeks, 6 months post randomisation, including the number.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 5 (0) | 11 (0) |
| Mean (SD) | 39.6 (10.7) | 33.8 (8.0) | 36.9 (9.6) |
| Median (Q1, Q3) | 36.5 (36.5, 44.4) | 36.5 (25.8, 36.5) | 36.5 (25.8, 44.4) |
| Min, max | 25.8, 57.7 | 25.8, 44.4 | 25.8, 57.7 |
| Week 2 | |||
| N (missing) | 4 (1) | 4 (0) | 8 (1) |
| Mean (SD) | 44.4 (14.4) | 40.3 (10.2) | 42.3 (11.8) |
| Median (Q1, Q3) | 40.5 (34.3, 54.5) | 38.2 (32.0, 48.6) | 40.5 (32.0, 48.6) |
| Min, max | 32.0, 64.6 | 32.0, 52.8 | 32.0, 64.6 |
| Week 6 | |||
| N (missing) | 3 (0) | 2 (1) | 5 (1) |
| Mean (SD) | 47.6 (9.0) | 28.9 (4.4) | 40.1 (12.2) |
| Median (Q1, Q3) | 44.4 (40.6, 57.7) | 28.9 (25.8, 32.0) | 40.6 (32.0, 44.4) |
| Min, max | 40.6, 57.7 | 25.8, 32.0 | 25.8, 57.7 |
| Week 12 | |||
| N (missing) | 2 (0) | 5 (0) | 7 (0) |
| Mean (SD) | 37.2 (16.1) | 39.6 (6.7) | 38.9 (8.7) |
| Median (Q1, Q3) | 37.2 (25.8, 48.6) | 36.5 (36.5, 44.4) | 36.5 (32.0, 48.6) |
| Min, max | 25.8, 48.6 | 32.0, 48.6 | 25.8, 48.6 |
| Month 6 | |||
| N (missing) | 2 (1) | 5 (0) | 7 (1) |
| Mean (SD) | 48.6 (0.0) | 33.2 (6.8) | 37.6 (9.3) |
| Median (Q1, Q3) | 48.6 (48.6, 48.6) | 32.0 (32.0, 32.0) | 32.0 (32.0, 48.6) |
| Min, max | 48.6, 48.6 | 25.8, 44.4 | 25.8, 48.6 |
Pain visual analogue scale
One participant in the surgical fixation group recorded the pain VAS score across all time points up to 6 months. The score was 99 (100 = worst imaginable pain) at baseline moving to 52 across weeks 2, 6 and 12 and then reducing to 5 at 6 months. In the non-surgical management group, five participants recorded the pain VAS at baseline, three participants at week 2 and week 6, none at week 12, two participants at month 6 and one participant at month 12. The average scores across time points was 63 (SD 34) at baseline, 54 (SD 10) at week 2, 26 (SD 24) at week 6, one participant rated their pain as 1 and the other 34 at 6 months and at 12 months it was rated as 1 (0 = no pain).
Abbreviated Mental Test Score
Table 10 reports the AMTS by intervention group and overall for baseline, 2 weeks and 12 weeks, including the number.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 4 (1) | 10 (1) |
| Mean (SD) | 7.8 (3.9) | 6.0 (3.3) | 7.1 (3.6) |
| Median (Q1, Q3) | 9.5 (8.0, 10.0) | 6.0 (4.0, 8.0) | 8.5 (6.0, 10.0) |
| Min, max | 0.0, 10.0 | 2.0, 10.0 | 0.0, 10.0 |
| Week 2 | |||
| N (missing) | 4 (1) | 3 (2) | 7 (3) |
| Mean (SD) | 7.5 (5.0) | 5.7 (4.5) | 6.7 (4.5) |
| Median (Q1, Q3) | 10.0 (5.0, 10.0) | 6.0 (1.0, 10.0) | 10.0 (1.0, 10.0) |
| Min, max | 0.0, 10.0 | 1.0, 10.0 | 0.0, 10.0 |
| Week 12 | |||
| N (missing) | 1 (0) | 1 (3) | 2 (3) |
| Mean (SD) | 9.0 (a) | 10.0 (a) | 9.5 (0.7) |
| Median (Q1, Q3) | 9.0 (9.0, 9.0) | 10.0 (10.0, 10.0) | 9.5 (9.0, 10.0) |
| Min, max | 9.0, 9.0 | 10.0, 10.0 | 9.0, 10.0 |
4AT rapid test for delirium
Table 11 reports the 4AT score for intervention group and overall for baseline, 2 weeks and 12 weeks post randomisation, including the number.
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Baseline | |||
| N (missing) | 6 (0) | 4 (1) | 10 (1) |
| Mean (SD) | 0.7 (1.2) | 2.8 (2.5) | 1.5 (2.0) |
| Median (Q1, Q3) | 0.0 (0.0, 1.0) | 2.5 (1.0, 4.5) | 0.5 (0.0, 3.0) |
| Min, max | 0.0, 3.0 | 0.0, 6.0 | 0.0, 6.0 |
| Week 2 | |||
| N (missing) | 4 (1) | 5 (0) | 9 (1) |
| Mean (SD) | 1.8 (3.5) | 2.2 (2.5) | 2.0 (2.8) |
| Median (Q1, Q3) | 0.0 (0.0, 3.5) | 2.0 (0.0, 3.0) | 0.0 (0.0, 3.0) |
| Min, max | 0.0, 7.0 | 0.0, 6.0 | 0.0, 7.0 |
| Week 12 | |||
| N (missing) | 1 (0) | 2 (2) | 3 (2) |
| Mean (SD) | 0.0 (a) | 1.0 (1.4) | 0.7 (1.2) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | 1.0 (0.0, 2.0) | 0.0 (0.0, 2.0) |
| Min, max | 0.0, 0.0 | 0.0, 2.0 | 0.0, 2.0 |
Complications and adverse events
There were no recorded clinically significant displacements of the pelvic ring during the trial. There was one participant who died during the trial who was randomised to the non-surgical management group. No responses were received at week 2 for expected complications but 9 of the 11 randomised patients had data at discharge (5 non-surgical management and 4 surgical fixation). Of those nine patients, one person in the surgical group had acute renal failure with no other expected complications noted. At week 12, five responses were received (one non-surgical management and four surgical fixation) and one person in the surgical group had a urinary tract infection and delirium. One participant in the surgical fixation group had a single non-SAE within 6 months of randomisation which was considered unexpected but not related to the trial intervention. Another participant in the surgical fixation group had a lateral cutaneous nerve injury which was an AESI.
Resource use
Appendix 4, Tables 15–21 provide details of resource use during hospital stay in relation to physiotherapy rehabilitation, medication use, surgery, length of hospital stay, walking aids provided on discharge, location of discharge and medication provided to take home. Of those providing data, all participants received a physiotherapy leaflet and therapy. The average number of sessions in the non-surgical management group was 9.2 (SD 5) and in the surgical fixation group was 8.6 (SD 5.4) and for the average session duration was 31.5 (SD 8.2) and 41.3 (SD 5.4) respectively. Most participants received pharmacological venous thromboembolism (VTE) prophylaxis across both groups, but no other medication was reported being used during the hospital stay. All five participants randomised to surgery received surgical fixation with INFIX. Most participants received a general anaesthetic and had a local infiltration. In theatre the lead surgeon was a consultant with a registrar/speciality trainee or associate specialist as the first assistant present. In the non-surgical management group two patients had their length of hospital stay recorded and these were 7 and 14 days. In the surgical fixation group three patients had their length of stay recorded and the median was 24 days with a minimum of 23 and maximum of 39 days. No data were returned for the number of walking aids patients were discharged with in the surgical fixation group and in the non-surgical management group one patient was discharged with a walking frame and one with a wheelchair. The discharge location was recorded for one participant in the non-surgical management group (rehabilitation unit) and three participants in the surgical fixation group (live alone, live alone but with support from carers and early supported discharge). All participants with data were discharged with antibiotics (across both groups) and of all those in the surgical fixation group received analgesia and two of the four in the non-surgical management group received analgesia.
Appendix 4, Tables 22–26 provide details of resource use in relation to participants’ pelvic fracture since discharge from hospital, at 12 weeks post randomisation for physiotherapy, outpatient care, community care, private treatment, medication use, walking aids bought or received since discharge and days off work and unpaid activities missed. One participant in the surgical fixation group received five sessions of NHS physiotherapy in the community which lasted on average 15 minutes. One participant in the non-surgical management group received five sessions of private physiotherapy which lasted on average 45 minutes. One participant in the surgical fixation group received two orthopaedic care appointments in relation to their pelvic fracture since discharge from hospital and attended the emergency department once. They also had a single GP home visit and three GP telephone contacts. One participant in the non-surgical management group had a GP telephone contact, a visit from the district nurse and received a four-wheel walker and a shower and sink seat since discharge from hospital, at 12 weeks post randomisation. One participant in the surgical fixation group reported being unable to do unpaid activities (e.g. chores, shopping) for 91 days.
Assessing barriers to recruitment and actions taken to increase enrolment
The study did not have a formal process evaluation. The main barriers to recruitment and actions taken to address them, outlined below, are based on documentation from TMG Meetings, two cross-site meetings with recruitment/research staff from participating centres, individual communications between the trial team and research staff and other trial documentation. We also held a round-table meeting of the central trial team at the end of the study to inform the drafting of this section of the report.
Barriers to recruitment
Patient pathways
The L1FE study was embedded within orthopaedic departments, and research staff in these departments were the direct point of contact for the central trial team. However, in many centres, patients with fragility fractures of the pelvis are admitted under medical or orthogeriatric teams or to clinical assessment units, outside the immediate remit of the orthopaedic team. This contrasted with many other orthopaedic trials, where patients can be identified directly from the daily trauma meeting. We were aware of this at the outset and each research team was briefed at the site initiation visit (SIV) about the need to identify patients for the trial beyond the orthopaedic department. However, this remained an ongoing issue at several centres during the trial.
Patients often arrived through the emergency department, making it important for A and E nurses and radiographers to be aware of the study. Additionally, it was helpful if site recruitment/research staff regularly screened the picture archiving and communications system (PACS), to identify potential participants being admitted via the emergency department, as well as screening on-call admissions lists from orthopaedic and care of the elderly teams. Upon leaving the emergency department, patients could then be admitted to the Geriatric ward, Orthopaedic ward, Medical ward or an Admissions unit. Thus, it was necessary for research staff to make physiotherapists, doctors and ward nurses, in each of these departments, aware of the trial and alert to identifying patients. Permeating knowledge of the trial around multiple departments with regular repeated sessions to accommodate staff changeover (i.e. between shifts as well as staff turnover) was an ongoing challenge that many sites found resource-intense and draining.
Despite efforts made at each SIV and reminders where relevant opportunities arose, screening logs and communication with sites revealed fewer patients being screened than expected, suggestive of the potential that patients were being missed (not screened). Furthermore, it was noted that site research staff had been identifying and screening patients almost exclusively through radiology PACS requests, rather than from multiple sources.
Seventy-two-hour mobility eligibility criterion
In order to be included in the study, patients were required to be ‘unable to mobilise independently to a distance of around 3 m and back, due to pelvic pain (or perceived pelvic pain) 72 hours after injury’. Use of a walking aid and verbal guidance were permitted, but physical assistance was not. Patients were only permitted to be screened for the study 72 hours post injury and could not be approached by study staff before this.
The 72-hour mobility eligibility criterion was discussed by the TMG and TSC early in the study, with some members expressing the opinion that as most patients would be mobile by 72 hours they would no longer be in hospital to be recruited. There was discussion around whether the assessment of mobility should happen sooner. However, there was broad agreement that patients who managed to mobilise before 72 hours would likely not benefit from surgery, as they were already mobile. Patients who were still unable to mobilise at 72 hours were at risk of immobility-associated complications, and they were the ones who had the potential to benefit from surgical management. The purpose of the 72-hour criterion was to avoid unnecessary surgeries being carried out on patients who would have been able to mobilise anyway. Therefore, after further discussion, the TSC agreed that the inclusion criterion was necessary.
With regard to approaching the patients only after 72 hours post injury, a TSC member expressed concern that informing patients early that they might be considered for a surgical trial if they were unable to mobilise might impact their attitude towards their recovery. It might even lead to demoralisation if it was later decided they were ineligible for the study. The TSC agreed, and it was confirmed that patients must not be approached about the study any earlier than 72 hours post injury. At the time, it was agreed that these required timelines were still realistic in terms of recruiting patients to the study.
In practice however, fewer patients than expected met this inclusion criterion and this was a key factor in not meeting our recruitment target. At the first cross-site meeting for recruitment teams, it was noted by site staff that more patients were mobile at 72 hours post injury than initially anticipated. Most often, patients were on their feet by 72 hours following their injury.
In other cases, the inability to approach the patient until after 72 hours had passed meant that a plan had already been made for the patient by the team caring for them, and this had already been communicated to the patient and/or their next of kin. For example, some sites reported that if a patient was not mobile by day one or two post injury, plans were made to discharge the patient to a rehabilitation unit. By the time the patient was found to meet the mobility inclusion criterion at 3 days post injury, patients were resolved to non-operative care which had been discussed with them and it was challenging to change the patients’ view on the planned course of action by introducing the study as an option at this point.
There was a perception amongst recruitment/research staff at two sites that when staff treating patients were aware the patient was being considered for the trial, their determination to get them on their feet before 72 hours led to increased awareness of the patient’s pain management, leading to increased use of analgesics, and with this increased dose the patient was able to mobilise, where perhaps this would not have been the case otherwise. This suggests a lack of equipoise amongst physiotherapists at some sites. That said, the number of patients who were screened at 72 hours, who were then deemed to be independently mobile (and so were not included), has to be recognised. It may well be that general analgesic management of patients with fragility fractures of the pelvis has undergone stepwise change since the L1FE study was planned. The screening data suggest that there may have been changes in how these patients are managed within the first few days post injury.
Patient or next-of-kin equipoise and consent
Site research staff expressed that, in their experience, it is challenging to achieve equipoise with this patient population, as the preference for them tends to be for conservative management (in this case, non-surgical). Patients of this age group often have comorbidities, are frail and may have dementia. Therefore, making a decision about participating in a trial where the interventions are very different, in terms of intensity and risks, is potentially a daunting one.
Also, the recruiting window was relatively small: 3 days before the initial approach was permitted, with the surgery required to take place before day 10 (but in reality this was often shorter due to 1 day per week operating lists or no operating at weekends at some sites). From the perspective of recruiting staff, the resulting consent process was experienced as time consuming, involving difficult conversations and a lot of back-and-forth, waiting for patients to discuss the trial with their relatives and/or surgeon, or for next of kin to do so. Research staff at one site commented that it was a more efficient use of their time to recruit more patients to studies where the consent process was shorter or less complex than to spend extended periods of time recruiting one patient to this study. At the first cross-site research staff meeting, the idea of a video or script to explain the trial to the patients and guide the consent process was discussed, and sites thought this would be helpful.
Surgeon equipoise and attitudes towards the study
Opinions from site research staff on surgeon equipoise were mixed. While some reported that they did not believe this to be a problem, others reported examples which indicated it was an issue for some surgeons. We had feedback from research staff at a site that, on one occasion, after dedicating a significant amount of time speaking to a patient and their next of kin to establish equipoise and obtain consent, in their view a surgeon then dissuaded the patient and their family from participating in the study. Additionally, at the first cross-site recruiting staff meeting, a principal investigator (PI) expressed discomfort with the idea of presenting the trial and the two treatment options to the patient in a neutral way. They noted that patients expect input or recommendations from the surgeon on whether surgical or non-surgical management would be best, and surgeons feel the pressure to have an opinion or an answer to which treatment is best. At the second cross-site recruiting staff meeting, a team member from one site stated that there were surgeon capacity issues due to a lack of equipoise from some of the surgeons, who preferred not to be involved.
Furthermore, some site research staff thought that PIs could be doing more to make the trial known among staff and to ensure the trial was being considered when a patient’s care plan was being discussed, with staff at some sites reporting that there was much room for improvement here. Additionally, the trial coordination team faced difficulties opening sites to recruitment due to a lack of response by some PIs to the team’s attempts to communicate, and, in general, the trial team often found it more challenging to establish contact with PIs than with research staff. However, we do not have any information on this from the surgeon perspective.
One factor noted by the CI, in his ongoing discussions with surgeons in recruiting centres, was the choice of pelvic fixation. Some were not particularly experienced in using the INFIX device and so voiced a degree of reluctance on that basis. Others felt that they would have preferred the choice of pelvic fixation (if the patient randomised to surgery) to be pragmatic – not specifying a particular implant. In addition, a small number of PIs reported that equipoise in their centres was challenged by clinicians (surgical, medical and allied therapists) who did not feel there was a significant clinical problem in relation to fragility fractures of the pelvis and who wanted more evidence that this patient group have poor outcomes that warrant investigation. A pelvic surgeon at one site (not a PI) commented that he thought the vast majority of patients mobilise quickly.
‘Admissions avoidance’ policies
Many admitting hospitals have developed well-established pathways for discharging patients who do not need in-hospital medical care. Patients with fragility fractures of the pelvis are deemed to fall into this category because historically treatment is analgesia and physiotherapy. Particularly during the COVID-19 pandemic, these ‘admissions avoidance’ pathways have been strengthened, such that patients would be discharged well within the 72-hour period to nursing homes, residences with care packages or rehabilitation hospitals and could not be screened for the trial.
Actions taken to address recruitment issues
Additional communication with sites
Cross-site staff meetings
Screening logs submitted by site staff alerted us that recruitment was not proceeding as expected. Additionally, phone calls and correspondence from sites made the team aware of frustration among site research staff due to unfruitful recruitment efforts, and often the challenges they identified were common across sites. We arranged two cross-site meetings, with the aim of bringing together research staff at sites to discuss the challenges with recruitment that they were experiencing, share knowledge and provide support from the central trial team. Summaries from each of these meetings were produced and circulated to all recruiting sites in order to share knowledge across sites, including lessons learnt and possible solutions.
The first of these two cross-site recruitment staff meetings was held on 1 April 2020, shortly after the decision was made to pause recruitment to the study due to the COVID-19 pandemic. Inevitably this impacted on the discussions, but we tried to maintain focus on any adjustments needed to address barriers to recruitment to prepare for restarting recruitment. The 2-hour meeting was held via teleconference. All site staff were invited, and it was attended by 9 staff members from 9 out of 11 recruiting sites: 1 PI, 1 research physiotherapist, 3 research nurses, 1 research practitioner, 1 clinical research coordinator, 1 clinical research assistant and 1 API geriatrician). The agenda included an update from the central trial team and CI on recruitment so far and the decision to pause the study; a segment by the consultant orthogeriatrician from the TMG on the importance of geriatrician involvement at sites; a segment by a L1FE API on the benefits of the API scheme; and a discussion around recruitment led by the trial co-ordination team. This included sharing of experiences recruiting for TULIP [Trial of surgical vs. non-surgical treatment of lateral compression injuries of the pelvis with complete sacral fractures (LC-1) in the non-fragility fracture patient – a feasibility study], a feasibility trial of surgical versus non-surgical treatment of LC-1 fractures of the pelvis in non-fragility fracture patients,27 and a discussion of current experiences recruiting for the L1FE trial.
The first cross-site staff meeting provided valuable insight on the challenges involved in recruiting to the L1FE trial, leading to a commitment from the trial team to use the study pause to further explore and attempt to mitigate the issues identified, through the development of various new resources (detailed below) to assist site research staff with patient identification, eligibility assessment and consent.
The second cross-site staff meeting was held on 7 July 2021, after the study had reopened to recruitment and the sites had been given access to the additional resources developed by the team during the study pause. It was a similar format to the first event and was attended by eight people representing 5 out of 10 reopened sites, in addition to the central trial team. Attendees from the recruiting sites included two research physiotherapists, five research nurses and one orthopaedic research manager. The agenda included introductions, a welcome message from the CI, a study update and a discussion about the attendees’ current experiences with the following: screening patients; the 72-hour mobility criterion; equipoise, patient preference and consent; the impact of COVID-19 on the study; and the API scheme. Finally, there was an opportunity for site staff to provide feedback on the resources and support materials developed by the trial team during the study pause, which recruiting sites now had access to. This meeting informed the discussions surrounding the feasibility of continuing with the trial in the climate of the COVID-19 pandemic, which ultimately led to the decision to stop the trial.
Sponsor site trial team engagement with sites
As surgeon equipoise and surgeons’ attitudes towards the study were identified as potential barriers to recruitment, the CI contacted the PIs and other surgeons at recruiting sites and those potentially interested in joining the trial, in order to open up communication with them and address these issues. It became clear that most recruiting site PIs were earnestly invested in the trial. A small number remained unconvinced or had been unable to convince their colleagues, which led to a number of detailed discussions instigated by the CI to bring targeted individuals around. Of the sites that were open to recruitment, only one had personnel that remained recalcitrant against the trial.
The trial’s consultant orthogeriatrician engaged in similar discussions with geriatricians at sites and the research physiotherapist from the Sponsor site also contacted research physiotherapists at other recruiting sites to offer support and get a sense of how recruitment was progressing and any issues encountered after the pause in recruitment during the COVID-19 pandemic.
WhatsApp group chat
In order to provide an additional shared place where site staff could ask questions about the study and remain informed of any updates, a WhatsApp group chat was created on 9 September 2020 by the central trial team. The group description reads: ‘Designed to keep the L1FE community updated, share tips and successes. Please use to discuss L1FE recruitment/retention in general terms and in a supportive manner. No patient identifiable information should be transmitted to this group. You are responsible for the content of any messages you send. NHS information governance guidance should be adhered to’.
Members were added gradually, with the group eventually reaching 50 members, including the CI, trial coordination team, surgeons, PIs, research staff, APIs and some co-applicants. This was not restricted to recruiting sites and included PIs at proposed sites that had not yet opened to recruitment. The majority of the 50 members were added or joined via the group’s joining link at the time of reopening in March 2021. The remaining members joined throughout summer 2021.
At the time of reopening recruitment to the L1FE study, after most members were added, the CI sent a welcome message to the group, where he asked everyone to add their research teams and other relevant colleagues to the group and requested contact details of other colleagues in order to get in touch and assist with set-up of further sites. The following media were also shared with the group by the CI: the L1FE Protocol; a document summarising the inclusion and exclusion criteria; a QR code to join the group easily (for group members to distribute to colleagues); and three videos created by the trial team during the pause in recruitment due to the COVID-19 pandemic.
Clarification of 72-hour mobility inclusion criterion
Early in the study, site screening logs showed that many patients were not meeting the 72-hour mobility inclusion criterion. We were aware that this may have been simply because fewer than expected people were meeting this criterion. However, discussions with site research staff and questions raised at SIVs demonstrated that there was some confusion around interpretation of this criterion which may have been affecting the number of people meeting the criterion. In response to this feedback, the study team updated the wording in the protocol (forming part of substantial amendment 1 which was submitted to the HRA and REC on 3 February 2020, reviewed by a committee on 12 April 2020 and received favourable opinion on the 17 June 2020).
In protocol V1.1 23.05.2019 the inclusion criterion was:
After 72 hours post-injury, patients who are unable to mobilise independently or with supervision (with or without a walking aid) to a distance of around 3 metres and back due to pelvic pain or perceived pelvic pain
In protocol V2.1 14.04.2020 the inclusion criterion was updated to:
Patient still unable to mobilise to a distance of around 3 metres and back due to pelvic pain (or perceived pelvic pain) 72 hours after injury. Use of a walking aid and/or supervision are permitted.
Additional guidance on all inclusion and exclusion criteria was added to the SIV slides and the trial manual.
During the first cross-site research staff meeting, it became evident that there was still the potential for the 72-hour mobility inclusion criterion to be interpreted differently, particularly in terms of the amount of assistance provided during the assessment of mobility, and this may have contributed to the large number of patients being excluded due to this criterion at most sites. With this in mind, we clarified the wording of this criterion further in the study protocol and updated the associated site set-up and training resources. The updated protocol formed part of substantial amendment 2 and was submitted to the HRA and REC on 27 October 2020 and received favourable opinion from REC and HRA on 27 January 2021. In protocol V3.0 23.07.2020 the inclusion criterion was updated to:
Patient unable to mobilise independently to a distance of around 3 m and back, due to pelvic pain (or perceived pelvic pain) 72 hours after injury. Use of a walking aid and verbal guidance are permitted, however physical assistance is not.
To add further clarity, the central trial team developed the 4-minute video described below, titled ‘Eligibility Assessment – 72-hour Mobility Assessment’, to support research physiotherapists and recruiting staff in their assessment of this inclusion criterion.
Production of resources to support recruitment
Introduction to L1FE video
During the recruitment pause we developed a video titled ‘Introduction to L1FE’ in response to requests by sites for clarification of some of the background information to the trial. The 7-minute video consists of the CI summarising the rationale for the trial, the difference between stable and unstable pelvic fractures, patient population for the study, known complications of immobility and the two treatment options being evaluated. The intention was that the video could be used as an introduction to the study for prospective sites and would also support recruiting staff in presenting the study to patients with equipoise. The video was made available via the YTU website and on YouTube, circulated to staff at all recruiting sites and made available to site teams prior to SIVs via a hyperlink and Twitter feed.
Patient identification animation
We developed a video to mitigate against the challenge of needing to recruit patients from diverse care patient pathways. The 3-minute narrated animation, titled ‘Identifying L1FE Patients’, shows different places in the hospital where L1FE participants might be found, with advice on how to identify them. The video was made available via the YTU website and on YouTube, was circulated to staff at all recruiting sites and made available to site teams prior to SIVs via a hyperlink and twitter feed.
Seventy-two-hour mobility eligibility assessment video
Led by the trial team research physiotherapist, we developed a 4-minute video titled ‘Eligibility Assessment – 72-hour Mobility Assessment’. It consists of the CI explaining the rationale behind this inclusion criterion and a research physiotherapist demonstrating, with the help of an actress acting as a patient, how to assess whether a patient met this inclusion criterion. The aim of this video was to provide additional clarity to the implementation of this eligibility criterion by demonstrating four different scenarios: three scenarios where the patient requires some form of assistance or is unable to fully complete the mobility assessment and therefore is deemed as not mobile and meets this inclusion criterion and one scenario where the patient successfully completed the mobility assessment and therefore is deemed as mobile and not eligible for the study. The video, made available via the YTU website and on YouTube, was circulated to staff at all recruiting sites and made available to site teams prior to SIVs via a hyperlink and Twitter feed.
Patient-facing consent video
In response to issues raised by recruiting staff at the second cross-site recruiters meeting, we developed a patient-facing video to support the consent process. The script was submitted to HRA and REC on 18 February 2021, and given favourable REC opinion and approved by the HRA on 7 April 2021. The video was then recorded, edited and approved by the team in August 2021. It is 12 minutes long and consists of the research physiotherapist at the Sponsor site going through the consent conversation with an actress acting as a patient, including the most frequently asked questions and how to respond to them. The video has subtitles in order to facilitate communication with patients who were hearing impaired. Unfortunately, this resource could not be implemented by sites prior to the study’s closure.
Consent guidance
From the start of the trial, a ‘Consent Guidance and FAQ’ live document was in use to provide site research staff with specific guidance on recruitment. This was updated during the recruitment pause.
Updates were based on feedback received during the first cross-site research staff meeting, the experiences of the trial team, as well as further incorporating guidance and content from the NIHR GRANULE workshop and including links to this and other free online training resources at NIHR Learn platform [e.g. ‘Informed consent with adults lacking capacity’ and ‘Receiving informed consent from adults lacking capacity (including urgent public health studies)’]. We made formatting changes to make the document easier to follow, such as text boxes and headings to divide the document into clearer, more practical sections. The guidance document started with a reminder about the required timelines around approaching and consenting patients relative to the time of their injury and how to maintain equipoise. It also covered aspects of the Mental Capacity Act 2005 and how to gain personal or professional consultee agreement for a patient who lacks capacity to consent for themselves to be able to participate. There were three main sections: ‘The Conversation’, ‘Frequently Asked Questions’ and ‘Training Materials’. ‘The Conversation’ included the following subheadings: ‘Brief introduction: Yourself and the study’; ‘Clinical information and study details’; ‘Patient preference’; ‘Research concepts and practice’; and ‘Next steps’. ‘Frequently Asked Questions’ included the five most frequently asked questions during the consent conversation and suggestions for how to respond to them.
Patient information sheet
The patient information sheet (PIS) needed to be updated in order to incorporate changes to the study resulting from the COVID-19 pandemic; however, in light of the recruitment challenges we also undertook a full review. A PPI meeting was held to obtain further views and feedback on this document. Members of the PPI group suggested the PIS could be clearer, less repetitive and more reader-friendly with shorter sentences and paragraphs. They explained why certain things, which were important to them, should be mentioned sooner within the document, such as their GP’s awareness of their participation and an explanation of the surgical procedure. The group suggested adding prompts to let them know they could request to have the document read to them, as well as including respective page numbers on the contents list, making that section bigger at the start of the document and adding the confidentiality and data protection sections to this list. They also suggested providing more reassurance about issues that were of concern to them, such as close monitoring of pain relief, different devices used for the INFIX procedure and information about removal of the metalwork in case this was necessary.
Associate principal investigator scheme
The L1FE trial was registered with the NIHR API scheme from the onset of the study. This scheme provides opportunities for healthcare professionals starting their career, who would not normally have the chance to be involved in clinical research, to gain practical experience with this and be formally recognised for their involvement.
The trial team encouraged research staff and PIs at each site to promote the scheme and to offer junior doctors and orthopaedic trainees the opportunity to be involved in the study and to be formally recognised, particularly as it became evident that greater efforts were required to raise awareness of the trial across different departments and specialties. In order to help remediate this barrier to recruitment, we obtained agreement from NIHR to include more than one API per site, as well as to include consultant-level staff without research experience as APIs. This was intended to encourage orthogeriatricians to engage with the study, and to support and enhance patient identification and maintain enthusiasm and awareness of the L1FE study; however, there was no uptake of this offer at the consultant level. Three teams appointed an API prior to the recruitment pause. Three APIs were appointed at three different sites following the recruitment pause and prior to the decision to close the study.
Impact of the COVID-19 pandemic on recruitment
The global pandemic had a very strongly destabilising effect on the L1FE trial which was already struggling, as the barriers to recruitment, mentioned above, were greatly exacerbated. Even after the trial restart, research teams were still depleted and in conjunction with research and development teams were required to prioritise which studies to open again. Admissions avoidance teams were strengthened, in terms of personnel and community resources, and orthopaedic clinicians were encouraged to take a more non-operative treatment approach to fracture management in general. This made it counterintuitive for clinicians to encourage patients into a trial that had operative treatment as one of the arms, where previously the management would have been entirely non-operative. Patients and relatives also became noticeably more reluctant to consider surgery within the confused mix of information and emotions that surrounded (and continue to surround) COVID-19.
Due to the patient population and the nature of the injury, a high proportion of participants lacked capacity to consent for themselves. From the study outset, comprehensive materials and guidance were in place for the inclusion of people who lacked capacity or had fluctuating levels of capacity due to injury-related delirium. Prior to the COVID-19 pandemic, approximately half of participants were patients without mental capacity to consent for themselves and obtaining consultee agreement was not a problematic aspect of the study. However, when the study reopened to recruitment in March 2021, feedback from research staff was that it had become difficult to obtain personal consultee agreement from the next of kin, as this had to be done remotely due to restrictions in place on family visits to hospital. All discussions with consultees were documented in the medical records of the patient, and where agreement was obtained in principle, they were to be posted a copy of the consultee agreement form and written information. Consultees were asked to return a signed copy of the consultee agreement form using a pre-paid envelope. When site staff contacted next of kin by telephone, they found that they were, understandably, wishing to use the contact with the hospital to obtain further details about the status of their hospitalised relative rather than discussing a research study. Due to their workload at the time, exacerbated by redeployment and prioritisation of more urgent studies such as vaccine trials, research staff said they found it difficult to find the time that these intensive discussions with next of kin (and preparation for the call) required. They also felt that next-of-kin were reluctant to engage in these discussions, when the person on the other side of the phone was someone they had not met or who was not part of their relative’s regular care team. Site recruitment/research staff felt that when family were allowed into hospital, it was easier to build trust, as family members were more familiar with the team. However, when this took place remotely, it felt out of context and it was difficult to build rapport. There was a perception amongst staff that patients’ next of kin were also reluctant to agree inclusion on behalf of the patient when they were unable to see their relative, have a better idea of how they were doing or get a better sense of the study.
The Admissions Avoidance schemes were much more active during the COVID-19 pandemic, with additional resources made available to them resulting in fewer elderly patients with injuries from falls being managed in hospital. At some sites, Admissions Avoidance teams supported patients through alternative community pathways such as rehabilitation or respite, to prevent hospital admission or to discharge them in as timely a manner as possible, which could happen before the evaluation of ability to mobilise 72 hours following injury, required for the trial.
Additionally, there was a perception by recruiting staff and patients when the study reopened that the surgical option would prolong the patient’s hospital stay, worsening issues with presenting the study treatments in a balanced way and adversely impacting on patient preference and willingness to participate.
Furthermore, research staff at some sites reported that the COVID-19 pandemic had a large impact on the emotional and mental state of patients. They thought that patients were more fearful, wanting to go home rather than stay in hospital, or choosing to wait at home longer before deciding to come into hospital for treatment. Staff observed that restrictions on family visits had an immense impact on delirium and the associated confusion, which increased due to a lack of familiar faces. On the whole, this had an impact on what patients were prepared to put themselves through, or what their next of kin were willing to agree to.
It was reported by site research staff that there was reduced operating theatre space, site and bed capacity, and surgeon availability, as well as team capacity issues. During the peak of the COVID-19 pandemic, many research teams had been redeployed to alternative areas such as clinical care. At the time we reopened sites for recruitment to the L1FE study, most research staff members had returned to their previous roles; however, there were still issues with capacity due to the accumulated backlog. Numerous studies that had also paused due to the COVID-19 pandemic were also restarting, there were ongoing COVID-19 vaccine studies and new studies opening, all contributing to staff capacity problems.
Chapter 4 Discussion
We aimed to assess the clinical and cost effectiveness of surgical fixation with INFIX compared to non-surgical management of LC-1 fragility fractures in older adults. We set out to undertake a multicentre, pragmatic, randomised controlled superiority trial, with a 12-month internal pilot to assess assumptions about recruitment and provide guidance on optimising the trial processes before proceeding to the main trial phase. The trial did not proceed beyond the internal pilot phase due to much lower anticipated recruitment than anticipated.
Main findings
We opened 11 sites in England during the internal pilot, below the 19 sites to be set up by the end of the pilot. Eleven patients were randomised out of the target of 148 for the pilot. The average recruitment rate was 0.175 per site per month, well below the target rate of one patient per site per month indicating that the trial was not feasible at this time.
The number of patients screened varied greatly across sites, regardless of the duration they had been open to recruitment and size. This suggests that sites were implementing the screening process in different ways or with different levels of enthusiasm/support for the trial or they were having varying levels of success in implementing the trial due to local circumstances. It was predicted that 50% of eligible patients would consent and be recruited to the study. A high proportion (36 of 43; 83.7%) of eligible participants were approached to seek consent for participation. The number of patients randomised varied between sites, with 7 out of the 11 sites not recruiting any participants at all. Eight of the 11 participants were randomised from a single site, the CI’s site. Three further sites each recruited a single participant.
The lack of feasibility of the trial was influenced by several different factors. In Chapter 3, details are provided about the various mitigations the trial team implemented to address these where possible. The key factors in being unable to recruit the target sample were as follows.
The eligibility criteria
A key factor was that more patients than expected were able to mobilise independently or with supervision to 3 m and back at 72 hours post injury. This was an important eligibility criterion to avoid unnecessary surgeries being carried out on patients who would have been able to mobilise anyway. Patients who were still unable to mobilise at 72 hours were at risk of immobility-associated complications, and they were the ones who had the potential to benefit from surgical management. Reliable epidemiological data were not available prior to the trial on the proportion of over 60-year-olds with an LC-1 pelvic fracture able to mobilise at 72 hours post injury. We found that just over half of the screened patients were able to mobilise independently to 3 m and back at this stage. Based on feedback from sites, there was a concern that the ability to mobilise independently was being assessed differently across sites and across physiotherapists, with different levels of assistance provided. The protocol was amended to make explicit that use of a walking aid and verbal guidance were permitted, but physical assistance was not. A video was also developed to support research physiotherapists in their assessment of this inclusion criterion. There was a perception amongst some recruiting staff that the focus on patient mobilisation at 72 hours and an awareness that this would be assessed increased awareness of pain management, an important component of current standard care. There was anecdotal feedback from sites that these factors were related to lack of equipoise amongst some physiotherapists and a concern to help ensure patients avoided surgery. However, we recognise that the situation may simply be that more patients are able to independently mobilise at 72 hours post injury than anticipated. Opinions from site research staff on surgeon equipoise were mixed. While some reported that they did not believe this to be a problem, others reported examples which indicated that equipoise was an issue for some surgeons.
We had physiotherapist involvement in the trial team and consulted with physiotherapists at sites. Similarly, we had orthogeriatrician involvement. However, on reflection, the majority of our work at the grant application stage focused on surgeons and their willingness to be involved in the trial. If a trial in the future was under consideration, factors affecting equipoise in a wider range of healthcare professionals involved in the patient pathway should be considered at an earlier stage.
Patient pathway
It is likely that a reasonable number of potentially eligible patients were not being screened. At the design stage of the trial, based on a review of records from two of the centres, there were 312 patients admitted with low-energy fractures over 29 months (November 2013 to March 2016) aged 60 years or older. Therefore, the numbers being screened are lower than expected. It is not surprising to find this gap, given the complex admissions pathway for this group of patients. Patients often arrived through the emergency department, making it important for A and E nurses and radiographers to be aware of the study. Additionally, it was helpful if site recruitment/research staff regularly screened the PACS, to identify potential participants being admitted via the emergency department, as well as screening on-call admissions lists from orthopaedic and care of the elderly teams. Upon leaving the emergency department, patients could then be admitted to the Geriatric ward, Orthopaedic ward, Medical ward or an Admissions unit.
We were aware of this at the outset and each research team was briefed at the SIV about the need to identify patients for the trial beyond the orthopaedic department. Other resources and ongoing support were provided during the trial. However, this remained an ongoing issue at several centres. Permeating knowledge of the trial around multiple departments with regular repeated sessions to accommodate staff changeover (i.e. between shifts as well as staff turnover) was an ongoing challenge that many sites found resource-intensive and draining, particularly following the COVID-19 pandemic.
Consent from patients
Just over 30% of eligible patients (11 of 36) who were approached consented to participate in the trial (or a consultee agreed on their behalf). This compounded the impact of the high number of patients not meeting the eligibility criteria. The most common reason for eligible patients not consenting to take part was that they were unwilling to be randomised to a treatment (n = 10). It is difficult to determine the extent to which the consent rate could have been improved from the information we have available. Making a decision about participating in a trial where the interventions are very different in intensity and risks is potentially a daunting one for anyone and this is likely to be especially so in the population for the L1FE trial where the average age of participants was 83 years and people may be frail and/or have dementia.
COVID-19 pandemic
The internal pilot had been running for 7 months when recruitment was paused due to the COVID-19 pandemic in March 2020. The recruitment challenges were apparent by this stage and we used the pause in recruitment to develop strategies to address these, as outlined in Chapter 3. Despite adjustments and mitigations to address the various challenges, and a short extension to the pilot phase agreed by the funder, we found recruitment barriers to be exacerbated by the ongoing impacts of the pandemic when we reopened to recruitment in March 2021. Even aspects that had been functioning relatively well beforehand became difficult. In particular, admissions avoidance teams were strengthened and orthopaedic clinicians were encouraged to take a more non-operative treatment approach to fracture management in general. This made it particularly difficult for the L1FE trial where the existing standard care was non-operative management. It also became more difficult for staff at sites to protect time for the consent process for L1FE which was considered time intensive. Where patients lacked capacity and consultee agreement was required, the restrictions on family visits became a barrier to building trust and rapport with next of kin. Staff observed that restrictions on family visits had an immense impact on delirium and the associated confusion, which increased due to a lack of familiar faces. On the whole, this had an impact on what patients were prepared to put themselves through, or what their next of kin were willing to agree to.
Study results
Of the 11 patients consenting to participate in the trial, 5 were randomised to INFIX surgical fixation and 6 to non-surgical management. Three participants in the non-surgical management group were withdrawn from the study: one participant died, one lost capacity and their family member no longer wanted them to take part in the study and one had sight problems and considered themselves too frail to continue. Of the five participants randomised to surgical fixation all received surgery, and no one in the non-surgical management group received surgery. Completion rates of PROMS were high (while acknowledging the limitations of the very small sample for interpretation): all participants in the surgical fixation group completed questionnaires at 6 months and all the remaining participants in the non-surgical management group provided data at 6 months (3/6).
We reported the outcome data descriptively due to the very small sample and it is not appropriate to derive any conclusions from the limited data available.
Strengths and limitations
The ability of patients to mobilise following an LC-1 fragility fracture varies: some can mobilise and walk, albeit with some degree of pain, whereas others struggle to walk due to the pain. Patients who do not manage to get back walking due to ongoing pain are at greater risk of immobility-related complications. 11 The L1FE trial was designed to robustly address an important question related to the management of LC-1 fragility fractures in people 60 years old and over. Prior to this trial, a systematic review found that no robust evaluations of the effectiveness of surgical management had been undertaken. 24 The main limitation of the study is that recruitment to the trial was well below target and this important research question remains unaddressed. The internal pilot was a robust mechanism by which to establish the feasibility of the main trial. However, although we were able to identify the key barriers to recruitment, a qualitative process evaluation would have enriched the information available, in particular capturing in more depth the extent to which lack of equipoise was an issue.
Patient and public involvement
Patient and public involvement played an important role in the L1FE study to ensure the relevance and clarity of information and keep the patient perspective at the fore front of the trial conduct. A patient and public advisory group (PAG) was set up, to provide input into and feedback on the design of the research, participant resources including the patient information sheets (PISs) and consent/assent forms and the plain language summary of findings presented in this report.
The PAG consisted of three to four members at any one time with a total of seven people contributing throughout the study. Participants were identified through the Osteoporosis Society Network, who supported this research and through involvement@York, a PPI network at the University of York. All PAG members had experience of fragility fractures that affect mobility; this could have either affected them directly or affected somebody they cared for. The contributing members changed throughout the study as their availability and personal circumstances changed, for example one member chose not to comment on the final plain language summary as they were experiencing health problems. PPI members gave their input through a combination of one-to-one feedback with a member of the team or group meetings; these took part at the grant application stage, twice during the trial set-up phase and during the pause in recruitment for COVID-19.
Consultation with PPI members at the grant application stage highlighted their concerns that older women’s health problems are neglected and the L1FE study highlights a condition affecting older women. Feedback was incorporated in the study design. For example, the group thought that it would be discriminatory not to include patients with dementia; this was very useful and people who lacked capacity to consent for themselves were included in the study. Recruiting participants 3 days post fracture was well received, as they thought it allowed time for patients to come to terms with the trauma before making decisions about taking part in the research.
During the study set-up, three PPI members took part in individual conversations between July and August 2018 to discuss the trial design. They were asked to give feedback on the lay summary and the rehabilitation handout. They thought these were clear and easy to understand; however, members thought that the images of exercises on the rehabilitation handout could be improved. Alternative images and photographs were explored by the clinical team; however, they felt that the original pictures gave a clearer demonstration of the exercises which was deemed to be important from a safety perspective. The members also discussed a series of outcome measures. The PPI members felt they could relate to the EQ-5D-5L and PROMIS functional mobility questions; however, they thought that the Disability Rating Index asked about activities that were not relevant to this patient group. This was very helpful and contributed to the trial teams’ choice of outcome measures. In some instances, not all patient feedback could be acted on, for example some patients did not like the layout of standardised outcome measures; however, the study team were unable to alter the layout of these.
In January 2019, during trial set-up, members of the PPI group were sent patient-facing documents to review and comment on. This included all PISs and consent/consultee agreement forms both for patients with capacity and for consultees. The documents were widely acceptable and well received. Members commented that some of the language around GDPR regulations was too formal; this could not be amended due to legal requirements.
In light of the low recruitment rate, the trial team requested PPI input to further improve the Patient Information Sheet during the pause in recruitment for the COVID-19 pandemic. Three PPI members met as a group in July 2020 and a fourth member took part in an individual meeting to discuss how the information sheet could be improved. The group felt that the information sheet was too long, especially given that the trial population were in hospital due to a fracture and may be experiencing concentration problems, and sections that could be shortened were discussed in depth. The PPI members also highlighted areas where they would like to have more reassurance for the patients such as access to pain relief medication and how the INFIX device has been used previously. The trial team were able to respond to this feedback and reorganised the information sheet to remove any repetition or unnecessary detail, sentences were shortened and the contents list and layout improved for ease of reading. Further details were also added on pain relief and the INFIX devices used.
Finally, PPI members were invited to give feedback on the plain language summary in this report and to reflect on their experience as a PPI member. Due to poor health and busy schedules, only one PPI member was able to comment and the summary was well received. PPI members discussed their involvement in the study either by e-mail or by telephone call and commented that it was well organised, they found it interesting and they enjoyed being able to have a meaningful impact on the participants’ experience, they were happy to be able to have helped, felt appreciated by the study team. One PPI member would like to have more continuous involvement throughout the trial and all three would welcome being able to contribute to research again.
As a trial team we learnt to adapt to our PPI members individual needs, enabling them to fully engage with the tasks, and this included holding a mixture of group and individual meetings at their convenience and providing the relevant materials sufficiently in advance of meetings in different formats. For example, a partially sighted PPI member preferred a hard copy of any documents to read with their magnifying glass. During the COVID-19 pandemic, the face-to-face meetings had to be changed to videoconference calls; it was important to offer PPI members technical support to make these meetings as accessible as possible and to also offer telephone calls as an alternative. Remote PPI meetings removed some barriers to participation for people with mobility difficulties, caring responsibilities or busy schedules that restricted travel. With the right technical support our PPI members were happy to meet remotely, and the trial team found the videoconference very constructive. A key learning point was the need to be responsive to the changing situation of some members and be active in involving new members in the group.
Equality, diversity and inclusion
The eligibility criteria for the study were broad. To be included, patients had to be 60 years or older and have an LC-1 pelvic fracture, arising from a low-energy fall from standing height or less. The issues are different for older people with a fragility fracture and there is a separate study investigating high-energy fractures, more commonly experienced by younger people. 27 There was no upper age limit for inclusion in our study, provided the patient was considered to be fit for surgery using spinal or general anaesthetic and there were no concerns about soft tissue. However, it was necessary to exclude people who were already not able to walk either independently or with a walking aid prior to injury. The average age of participants in the trial was 83, with the youngest 72 and oldest 97. Patients who lacked capacity to consent for themselves were eligible for inclusion in the trial, provided there was agreement from a consultee, and 5 of the 11 patients who participated in the trial lacked capacity. A single patient was not approached about the trial because they had dementia (see Figure 1). Participants were predominantly female (82%) and white (82%). This broadly reflects some evidence that the incidence rates of fragility fractures are higher in women than in men and in white women compared to black women. 48 The majority of recruiting sites were in London and the south of England, and the majority of included studies were from sites in London. However, the balance of sites would have been different if our site set-up had progressed beyond the internal pilot. We did not collect data on socioeconomic status.
Lessons for the future and future research
For some of the barriers to recruitment, it is difficult to see how we could have addressed them differently. Experience from a previous feasibility study is that where there are multiple pathways for fracture patients, recruitment can be challenging, as it was with the L1FE trial. 49 This is an area where further research on best strategies to use would be helpful.
In advance of any future trial, early feasibility work on the equipoise of key healthcare professionals involved in the care of people with LC-1 fractures would be important including physiotherapists, orthopaedic surgeons, A and E nurses/doctors and healthcare professionals specialising in care of the elderly, including orthogeriatricians. Our findings suggest that equipoise may be an issue for some of these groups.
A process evaluation alongside any future trial would be beneficial to understand and evaluate any equipoise issues across different healthcare specialities. A qualitative element to any future study would also be helpful to gain an insight into why some people did not want to be randomised and to explore the acceptability of a surgical intervention in this group of patients.
Regardless of the equipoise issues and the influences of COVID-19 on the trial, it is reasonable to conclude from our screening data and the feedback from sites that the prolonged inpatient admission times following LC-1 fragility fractures, previously reported in the literature, are no longer the norm in the UK. This may be due to a combination of early pathway changes (admissions avoidance policies, early discharge with care packages at home and community placements), along with implementation of immediate in-hospital rehabilitation (analgesia, physio and managing patient expectations). That is not to say that the research question is no longer valid, as our results show nothing of the clinical outcomes in these patients. It may be that many patients are still experiencing the same morbidities and mortality risk in the community, rather than in hospital. Therefore, a clear understanding of patient outcomes after pelvic fragility fractures is required, prior to another RCT in this field since non-operative treatment (the standard of care) appears to have changed in recent years.
Although the L1FE trial is not feasible in the current climate, other research to understand the treatment and recovery pathways of this group of patients, along with their outcomes, would be informative for future research.
Chapter 5 Conclusion
At the current time, recruitment to a trial assessing the clinical and cost effectiveness of surgical fixation with INFIX compared to non-surgical management of LC-1 fragility fractures in people aged 60 years or older is not feasible.
Further research to understand the treatment and recovery pathways of this group of patients, along with their outcomes, would be needed prior to undertaking a future trial.
Acknowledgements
We are grateful to the many people who contributed to the study and the completion of the pilot:
-
Most importantly, the patients who participated in this trial.
-
The research teams at the participating sites.
-
Members of the TSC (Professor Deborah Stocken, Chair; Professor Alan Johnstone; Dr Kika Konstantinou; Dr Katherine Davies; Professor Alan Ereira) and Data Monitoring and Ethics Committee (Professor Graeme MacLennan, Chair; Dr Cherry Kilbride; Professor Ian Pallister).
-
Members of our PPI Group.
-
Members of the TMG and the trial team at YTU and Bart’s who contributed at various stages of the study – Belen Corbacho (Health Economist), Jacob Losh (trainee statistician), Jenny Roche (trainee statistician), Michael Backhouse (Trial co-ordinator), Mehool Acharya, Peter Hull, Daren Forward, Jamilla Kassam (clinicians), Peter May, Mani Mathuri and Jessica Pawson (orthopaedic research physiotherapists involved in recruitment and data collection at Bart’s).
Contributions of authors
Elizabeth Cook (https://orcid.org/0000-0001-6902-0235) (Research Fellow, Trial Manager) contributed to protocol development, led on conduct/delivery of the trial, setting up of sites and data acquisition, report editing and commented on all drafts of the report.
Joanne Laycock (https://orcid.org/0000-0003-3005-1442) (Trial Co-ordinator) contributed to protocol development, trial conduct, setting up of sites and data acquisition, PPI engagement and reporting, report editing, and commented on all drafts of report.
Dhanupriya Sivapathasuntharam (https://orcid.org/0000-0003-0410-9153) (Consultant Geriatrician, Trauma and Perioperative Older Person’s Services) contributed to protocol development, trial conduct, report writing and editing.
Camila Maturana (https://orcid.org/0000-0002-4946-8515) (Trial Co-ordinator) contributed to trial conduct, setting up of sites and data acquisition, and report writing.
Catherine Hilton (https://orcid.org/0000-0003-0410-6788) (Specialist Research Physiotherapist) contributed to recruitment, trial conduct and data acquisition, PPI, and commented on all drafts of report.
Laura Doherty (https://orcid.org/0000-0001-5310-5808) (Trial Support Officer) contributed to project administration, data acquisition, and report editing.
Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor of Trials and Statistics) contributed to conceptualisation and design, funding acquisition, protocol development, trial conduct, statistical analyses, report writing and editing.
Catriona McDaid (https://orcid.org/0000-0002-3751-7260) (Reader in Trials) contributed to conceptualisation and design, funding acquisition, protocol development, trial conduct, report writing and editing.
David Torgerson (https://orcid.org/0000-0002-1667-4275) (Director of YTU) contributed to conceptualisation and design, protocol development, funding acquisition, trial conduct, and commented on all drafts of the report.
Peter Bates (https://orcid.org/0000-0002-9776-3930) (Consultant orthopaedic trauma surgeon), Chief Investigator, contributed to conceptualisation and design, funding acquisition, protocol development, trial conduct, data acquisition, report writing and editing.
Ethics statement
The L1FE study was approved by London – Harrow REC (Ref: 19/LO/0555) on 16 July 2019.
The study was also given approval by Scotland A REC (REF 21/SS/0002) on 12 February 2021, specifically in relation to the inclusion of patients who lack capacity to consent in Scotland.
Data-sharing statement
The data sets generated during the study (fully anonymised) are available on request. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review by the CI.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that the data are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Mostafa A, Kyriacou H, Chimutengwende-Gordon M, Khan WS. An overview of the key principles and guidelines in the management of pelvic fractures. J Perioper Pract 2021;31:341-8. https://doi.org/10.1177/1750458920947358.
- Marrinan S, Pearce MS, Jiang XY, Waters S, Shanshal Y. Admission for osteoporotic pelvic fractures and predictors of length of hospital stay, mortality and loss of independence. Age Ageing 2015;44:258-61. https://doi.org/10.1093/ageing/afu123.
- Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami: epidemiology and five-year survival. J Bone Joint Surg Br 2001;83:1141-4. https://doi.org/10.1302/0301-620x.83b8.11709.
- Kugelman DN, Fisher N, Konda SR, Egol KA. Loss of ambulatory independence following low-energy pelvic ring fractures. Geriatr Orthop Surg Rehabil 2019;25. https://doi.org/10.1177/2151459319878101.
- van der Velde RY, Wyers CE, Curtis EM, Geusens PPMM, van den Bergh JPW, de Vries F, et al. Secular trends in fracture incidence in the UK between 1990 and 2012. Osteoporos Int 2016;27:3197-206. https://doi.org/10.1007/s00198-016-3650-3.
- Clement ND, Court-Brown CM. Elderly pelvic fractures: the incidence is increasing and patient demographics can be used to predict the outcome. Eur J Orthop Surg Traumatol 2014;24:1431-7. https://doi.org/10.1007/s00590-014-1439-7.
- Morris RO, Sonibare A, Green DJ, Masud T. Closed pelvic fractures: characteristics and outcomes in older patients admitted to medical and geriatric wards. Postgrad Med J 2000;76:646-50. https://doi.org/10.1136/pmj.76.900.646.
- Andrich S, Haastert B, Neuhaus E, Neidert K, Arend W, Ohmann C, et al. Epidemiology of pelvic fractures in Germany: considerably high incidence rates among older people. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0139078.
- Studer P, Suhm N, Zappe B, Bless N, Jakob M. Pubic rami fractures in the elderly: a neglected injury?. Swiss Med Wkly 2013;143. https://doi.org/10.4414/smw.2013.13859.
- Farouk O, El-Adly W, Khalifa YE. Erratum to: late fixation of vertically unstable type-C pelvic fractures: difficulties and surgical solutions. Eur Orthop Traumatol 2015;6. https://doi.org/10.1007/s12570-014-0272-0.
- Soles GL, Ferguson TA. Fragility fractures of the pelvis. Curr Rev Musculoskelet Med 2012;5:222-8. https://doi.org/10.1007/s12178-012-9128-9.
- Greenleaf JE, Kozlowski S. Physiological consequences of reduced physical activity during bed rest. Exerc Sport Sci Rev 1982;10:84-119. https://www.ncbi.nlm.nih.gov/pubmed/6749519.
- Koval KJ, Aharonoff GB, Schwartz MC, Alpert S, Cohen G, McShinawy A, et al. Pubic rami fracture: a benign pelvic injury?. J Orthop Trauma 1997;11:7-9. https://doi.org/10.1097/00005131-199701000-00003.
- Taillandier J, Langue F, Alemanni M, Taillandier-Heriche E. Mortality and functional outcomes of pelvic insufficiency fractures in older patients. Joint Bone Spine 2003;70:287-9. https://doi.org/10.1016/s1297-319x(03)00015-0.
- Breuil V, Roux CH, Testa J, Albert C, Chassang M, Brocq O, et al. Outcome of osteoporotic pelvic fractures: an underestimated severity. Survey of 60 cases. Joint Bone Spine 2008;75:585-8. https://doi.org/10.1016/j.jbspin.2008.01.024.
- Uchida K, Kokubo Y, Yayama T, Nakajima H, Miyazaki T, Negoro K, et al. Fracture of the pelvic ring: a retrospective review of 224 patients treated at a single institution. Eur J Orthop Surg Traumatol 2011;21:251-7.
- Lefaivre KA, Slobogean GP, Ngai JT, Broekhuyse HM, O’Brien PJ. What outcomes are important for patients after pelvic trauma? Subjective responses and psychometric analysis of three published pelvic-specific outcome instruments. J Orthop Trauma 2014;28:23-7. https://doi.org/10.1097/BOT.0b013e3182945fe9.
- Krappinger D, Struve P, Schmid R, Kroesslhuber J, Blauth M. Fractures of the pubic rami: a retrospective review of 534 cases. Arch Orthop Trauma Surg 2009;129:1685-90. https://doi.org/10.1007/s00402-009-0942-5.
- Scheyerer MJ, Osterhoff G, Wehrle S, Wanner GA, Simmen HP, Werner CM. Detection of posterior pelvic injuries in fractures of the pubic rami. Injury 2012;43:1326-9. https://doi.org/10.1016/j.injury.2012.05.016.
- Moja L, Piatti A, Pecoraro V, Ricci C, Virgili G, Salanti G, et al. Timing matters in hip fracture surgery: patients operated within 48 hours have better outcomes. A meta-analysis and meta-regression of over 190,000 patients. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0046175.
- Vaidya R, Colen R, Vigdorchik J, Tonnos F, Sethi A. Treatment of unstable pelvic ring injuries with an internal anterior fixator and posterior fixation: initial clinical series. J Orthop Trauma 2012;26:1-8. https://doi.org/10.1097/BOT.0b013e318233b8a7.
- Vaidya R, Martin AJ, Roth M, Nasr K, Gheraibeh P, Tonnos F. INFIX versus plating for pelvic fractures with disruption of the symphysis pubis. Int Orthop 2017;41:1671-8. https://doi.org/10.1007/s00264-016-3387-9.
- Dahill M, McArthur J, Roberts GL, Acharya MR, Ward AJ, Chesser TJS. The use of an anterior pelvic internal fixator to treat disruptions of the anterior pelvic ring: a report of technique, indications and complications. Bone Joint J 2017;99-B:1232-6. https://doi.org/10.1302/0301-620X.99B9.BJJ-2016-1025.R2.
- Booth A, Ingoe HMA, Northgraves M, Coleman E, Harden M, Kassam J, et al. Effectiveness of surgical fixation for lateral compression type one (LC-1) fragility fractures of the pelvis: a systematic review. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-024737.
- Fang C, Alabdulrahman H, Pape HC. Complications after percutaneous internal fixator for anterior pelvic ring injuries. Int Orthop 2017;41:1785-90. https://doi.org/10.1007/s00264-017-3415-4.
- O’Toole R. Pelvis RCT: Impact of Surgery on Pain in Lateral Compression Type Pelvic Fractures n.d. https://ClinicalTrials.gov/show/NCT02625766:https://clinicaltrials.gov/show/NCT02625766 (accessed 23 May 2022).
- Barnfield S, Ingram J, Halliday R, Griffin X, Greenwood R, Kandiyali R, et al. TULIP: a randomised controlled trial of surgical versus non-surgical treatment of lateral compression injuries of the pelvis with complete sacral fractures (LC1) in the non-fragility fracture patient – a feasibility study protocol. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-036588.
- COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020;396:27-38. https://doi.org/10.1016/S0140-6736(20)31182-X.
- Great Britain . Mental Capacity Act 2005 2005.
- Scottish Parliament . Adults With Incapacity (Scotland) Act 2000 2000.
- EuroQol Research Foundation . EQ-5D-5L User Guide 2015. https://euroqol.org/ (accessed 23 May 2022).
- PROMIS Health Organization . PROMIS (Patient Reported Outcome Measurement Information System) n.d. www.promishealth.org/healthmeasures/ (accessed 23 May 2022).
- Brooks D, Davis AM, Naglie G. Validity of 3 physical performance measures in inpatient geriatric rehabilitation. Arch Phys Med Rehabil 2006;87:105-10. https://doi.org/10.1016/j.apmr.2005.08.109.
- Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 2002;82:128-37. https://doi.org/10.1093/ptj/82.2.128.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63:S240-52. https://doi.org/10.1002/acr.20543.
- Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev 2018;2. https://doi.org/10.5435/JAAOSGlobal-D-17-00088.
- Pendlebury ST, Klaus SP, Mather M, de Brito M, Wharton RM. Routine cognitive screening in older patients admitted to acute medicine: abbreviated mental test score (AMTS) and subjective memory complaint versus Montreal Cognitive Assessment and IQCODE. Age Ageing 2015;44:1000-5. https://doi.org/10.1093/ageing/afv134.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. https://doi.org/10.1093/ageing/1.4.233.
- Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43:496-502. https://doi.org/10.1093/ageing/afu021.
- Hendry K, Quinn TJ, Evans J, Scortichini V, Miller H, Burns J, et al. Evaluation of delirium screening tools in geriatric medical inpatients: a diagnostic test accuracy study. Age Ageing 2016;45:832-7. https://doi.org/10.1093/ageing/afw130.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. https://doi.org/10.1016/j.ajic.2008.03.002.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Adachi JD, Adami S, Gehlbach S, Anderson FA, Boonen S, Chapurlat RD, et al. GLOW Investigators . Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin Proc 2010;85:806-13. https://doi.org/10.4065/mcp.2010.0082.
- Ashby R, Turner G, Cross B, Mitchell N, Torgerson D. A randomized trial of electronic reminders showed a reduction in the time to respond to postal questionnaires. J Clin Epidemiol 2011;64:208-12. https://doi.org/10.1016/j.jclinepi.2010.01.020.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3:1-152.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7.
- Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, et al. Epidemiology of fractures in the United Kingdom 1988–2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone 2016;87:19-26. https://doi.org/10.1016/j.bone.2016.03.006.
- Cook E, Scantlebury A, Booth A, Turner E, Ranganathan A, Khan A, et al. Surgery versus conservative management of stable thoracolumbar fracture: the PRESTO feasibility RCT. Health Technol Assess 2021;25:1-126. https://doi.org/10.3310/hta25620.
Appendix 1 Summary of amendments submitted to the HRA and REC
| Amendment reference | Brief description of amendment | Date of submission to HRA | HRA categorisation/date | Date of favourable ethical opinion |
|---|---|---|---|---|
| Non-substantial Amendment 1 | Change in PI at Cardiff, Salford, Cambridge, South Tees, Aintree, Oxford. Addition of new site – Preston | 9 August 2019 | B 05 September 2019 |
NA |
| Non-substantial Amendment 2 | Suspension of recruitment in response to COVID-19. Temporary change to method of participant follow-up – all remote until further notice | NA | NA | NA |
| Substantial Amendment 1 | Added clarity to the 72-hour mobility criteria. Allows the inclusion of recruiting sites within Northern Ireland. Clarified the information for recruitment of adults without capacity in Scotland. Protocol updated |
4 February 2021 | A (not categorised by HRA) | 10 May 2020 |
| Substantial Amendment 2 | Restart following COVID-19 pause. Protocol updated |
27 October 2021 | A | 13 January 2021 |
| Substantial Amendment 3 | Participant consent video script | 18 February 2021 | C 19 February 2021 |
07 April 2021 |
| Non-substantial Amendment 3 | Change in PI at Southampton, Liverpool. Addition of new site – Aberdeen. Site name changes – Salford and Liverpool | 19 April 2021 | B 19 April 2021 |
NA |
| Scotland Substantial Amendment 1a | All PISs and consent forms updated with Scotland specific terminology, including the collection of the CHI number | 14 April 2021 | C (no study-wide review required) | NA |
| Scotland Non-substantial Amendment 2 | All consent forms updated to correct Clinical to Community in the abbreviation CHI | 30 April 2021 | C (no study-wide review required) | NA |
| Non-substantial Amendment 4 | Study closure | 08 November 2021 | C (no study-wide review required) | NA |
Appendix 2 Study centres and PIs
| Centre | Hospital trust | PI |
|---|---|---|
| Bart’s Health NHS Trust | Bart’s Health NHS Trust | Peter Bates (CI) |
| Addenbrookes Hospital | Cambridge University Hospital NHS Foundation Trust | Daud Chou |
| Southmead Hospital | North Bristol NHS Trust | Mehool Acharya |
| Queens Medical Centre | Nottingham University Hospitals NHS Trust | Daren Forward |
| The James Cook University Hospital | South Tees Hospitals NHS Foundation Trust | Craig White |
| John Radcliffe Hospital | Oxford University Hospitals NHS Foundation Trust | David Noyes |
| University Hospital of Wales | University Hospital of Wales | Gareth Roberts |
| Walsgrave General Hospital | University Hospital Coventry and Warwickshire NHS Trust | Sunit Patil |
| Kings College Hospital | Kings College Hospital NHS Foundation Trust | Paul Harnett |
| St George’s Hospital | St George’s University Hospitals NHS Foundation Trust | Omar Sabri |
| St Marys Hospital | Imperial College Healthcare NHS Trust | Jasvinder Daurka |
Appendix 3 Additional tables from statistical analyses
| Control (N = 6) | Intervention (N = 5) | Overall (N = 11) | |
|---|---|---|---|
| Missing items at baseline, n (%) | |||
| 0 | 6 (100) | 5 (100) | 11 (100) |
| Missing items at 2 weeks, n (%) | |||
| 0 | 4 (80.0) | 4 (100.0) | 8 (88.9) |
| 1 | 1 (20.0) | 0 (0.0) | 1 (11.1) |
| Missing items at 6 weeks, n (%) | |||
| 0 | 3 (100.0) | 2 (66.7) | 5 (83.3) |
| 1 | 0 (0.0) | 1 (33.3) | 1 (16.7) |
| Missing items at 12 weeks, n (%) | |||
| 0 | 2 (100.0) | 4 (80.0) | 6 (85.7) |
| 1 | 0 (0.0) | 1 (20.0) | 1 (14.3) |
| Missing items at 6 months, n (%) | |||
| 0 | 3 (100.0) | 5 (100.0) | 8 (100.0) |
| Missing items at 12 months, n (%) | |||
| 3+ | 1 (100.0) | 1 (100.0) | 2 (100.0) |
Appendix 4 Resource use
| Control (N = 5) | Intervention (N = 5) | Overall (N = 10) | |
|---|---|---|---|
| Leaflet received, n (%) | |||
| Yes | 5 (100.0) | 4 (80.0) | 9 (90.0) |
| Missing | 0 (0.0) | 1 (20.0) | 1 (10.0) |
| Therapy received, n (%) | |||
| Yes | 5 (100.0) | 4 (80.0) | 9 (90.0) |
| Missing | 0 (0.0) | 1 (20.0) | 1 (10.0) |
| Number of sessions | |||
| N (missing) | 5 (0) | 5 (0) | 10 (0) |
| Mean (SD) | 9.2 (5.0) | 8.6 (5.4) | 8.9 (4.9) |
| Median (Q1, Q3) | 9.0 (5.0, 12.0) | 11.0 (7.0, 11.0) | 10.0 (5.0, 12.0) |
| Min, max | 4.0, 16.0 | 0.0, 14.0 | 0.0, 16.0 |
| Patient average session duration | |||
| N (missing) | 5 (0) | 4 (1) | 9 (1) |
| Mean (SD) | 31.5 (8.2) | 41.3 (5.4) | 35.9 (8.4) |
| Median (Q1, Q3) | 31.7 (26.3, 36.9) | 43.0 (37.6, 45.1) | 36.9 (31.7, 41.7) |
| Min, max | 21.1, 41.7 | 33.8, 45.7 | 21.1, 45.7 |
| Control (N = 5) | Intervention (N = 5) | Overall (N = 10) | |
|---|---|---|---|
| Pharmacological venous thromboembolism prophylaxis use, n (%) | |||
| Yes | 4 (80.0) | 4 (80.0) | 8 (80.0) |
| No | 1 (20.0) | 0 (0.0) | 1 (10.0) |
| Missing | 0 (0.0) | 1 (20.0) | 1 (10.0) |
| General antibiotic use, n (%) | |||
| No | 5 (100.0) | 4 (80.0) | 9 (90.0) |
| Missing | 0 (0.0) | 1 (20.0) | 1 (10.0) |
| Patient-controlled analgesia use, n (%) | |||
| No | 5 (100.0) | 4 (80.0) | 9 (90.0) |
| Missing | 0 (0.0) | 1 (20.0) | 1 (10.0) |
| Patient-controlled analgesia use, n (%) | |||
| Missing | 5 (100.0) | 5 (100.0) | 10 (100.0) |
| Patient-controlled analgesia use, n (%) | |||
| Missing | 5 (100.0) | 5 (100.0) | 10 (100.0) |
| Number of patients receiving surgery, n | 5a |
|---|---|
| Anaesthesia | |
| Type of anaesthesia used, n (%) | |
| General only | 3 (60) |
| Regional only | 0 (0) |
| General and regional | 1 (20) |
| Type of perioperative analgesia used, n (%) | |
| Regional nerve block | 0 (0) |
| Intravenous | 1 (20) |
| Local infiltration | 3 (60) |
| Other | 0 (0) |
| Theatre | |
| Lead surgeon grade, n (%) | |
| Consultant | 4 (80) |
| Associate specialist | 0 (0) |
| Registrar/speciality trainee | 0 (0) |
| Fellow | 0 (0) |
| Other | 0 (0) |
| First assistant present, n (%) | 4 (80) |
| Grade of first assistant, n (%) | |
| Consultant | 0 (0) |
| Associate specialist | 1 (20) |
| Registrar/speciality trainee | 3 (60) |
| Fellow | 0 (0) |
| Other | 0 (0) |
| Staff members present | |
| Operating department practitioner | |
| Mean (SD) | 0.5 (0.7) |
| Median (min, max) | 0.5 (0, 1) |
| Nurse | |
| Mean (SD) | 2.5 (0.6) |
| Median (min, max) | 2.5 (2, 3) |
| Nurse specialist/PA | |
| Mean (SD) | 0.5 (0.7) |
| Median (min, max) | 0.5 (0, 1) |
| Clinical support worker | |
| Mean (SD) | 0.5 (0.7) |
| Median (min, max) | 0.5 (0, 1) |
| Consultant anaesthetist | |
| Mean (SD) | 1 (0) |
| Median (min, max) | 1 (1, 1) |
| Associate specialist anaesthetist | |
| Mean (SD) | 0.5 (0.7) |
| Median (min, max) | 0.5 (0, 1) |
| Fellow | |
| Mean (SD) | 0 (0) |
| Median (min, max) | 0 (0, 0) |
| Registrar/speciality trainee anaesthetist | |
| Mean (SD) | 1 (0) |
| Median (min, max) | 1 (1, 1) |
| Core trainee | |
| Mean (SD) | 1 (1.4) |
| Median (min, max) | 1 (0, 2) |
| Foundation doctor | |
| Mean (SD) | 0 (0) |
| Median (min, max) | 0 (0, 0) |
| Surgery details | |
| Type of surgery completed, n (%) | |
| INFIX | 4 (80) |
| Other | 0 (0) |
| INFIX iliac bolt diameter (mm) | |
| Mean (SD) | 8.3 (0.3) |
| Median (min, max) | 8.5 (8, 8.5) |
| INFIX iliac bolt length (mm) | |
| Mean (SD) | 87.5 (9.6) |
| Median (min, max) | 85 (80, 100) |
| Supplementary sacro-iliac screws | |
| Used, n (%) | 3 (60) |
| Type of screw used | |
| Trans-sacral | 3 |
| Unilateral | 0 |
| Number of screws used | |
| Mean (SD) | 1 (0) |
| Median (min, max) | 1 (1, 1) |
| Other surgical fixation required, n (%) | |
| Yes | 0 (0) |
| No | 1 (20) |
| Loan set/kit used, n (%) | |
| Yes | 1 (20) |
| No | 3 (60) |
| Unplanned procedures undertaken, n (%) | |
| Yes | 0 (0) |
| No | 4 (80) |
| Procedures not related to the pelvis completed, n (%) | |
| Yes | 0 (0) |
| No | 4 (80) |
| Planned pelvic procedures in the future, n (%) | |
| Yes | 0 (0) |
| No | 4 (80) |
| Perioperative prophylactic antibiotics | |
| Used, n (%) | 3 (60) |
| Control (N = 5) | Intervention (N = 5) | Overall (N = 10) | |
|---|---|---|---|
| Hospital stay (days) | |||
| N (missing) | 2 (3) | 3 (2) | 5 (6) |
| Mean (SD) | 10.5 (4.9) | 28.7 (9.0) | 21.4 (12.1) |
| Median (Q1, Q3) | 10.5 (7.0, 14.0) | 24.0 (23.0, 39.0) | 23.0 (14.0, 24.0) |
| Min, max | 7.0, 14.0 | 23.0, 39.0 | 7.0, 39.0 |
| Control (N = 5) | Intervention (N = 5) | Overall (N = 10) |
|
|---|---|---|---|
| Crutch | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Stick | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Zimmer frame | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.5 (0.7) | a | 0.5 (0.7) |
| Median (Q1, Q3) | 0.5 (0.0, 1.0) | a | 0.5 (0.0, 1.0) |
| Min, max | 0.0, 1.0 | a | 0.0, 1.0 |
| Stair lift | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Wheelchair | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.5 (0.7) | a | 0.5 (0.7) |
| Median (Q1, Q3) | 0.5 (0.0, 1.0) | a | 0.5 (0.0, 1.0) |
| Min, max | 0.0, 1.0 | a | 0.0, 1.0 |
| Commode | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Rails | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Hospital bed | |||
| N (missing) | 2 (3) | 0 (5) | 2 (9) |
| Mean (SD) | 0.0 (0.0) | a | 0.0 (0.0) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Ramp/doorstep | |||
| N (missing) | 1 (4) | 0 (5) | 1 (10) |
| Mean (SD) | 0.0 (a) | a | 0.0 (a) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Other | |||
| N (missing) | 1 (4) | 0 (5) | 1 (10) |
| Mean (SD) | 0.0 (a) | a | 0.0 (a) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | a | 0.0 (0.0, 0.0) |
| Min, max | 0.0, 0.0 | a | 0.0, 0.0 |
| Characteristic | Control (N = 1) | Intervention (N = 3) | Overall (N = 4) |
|---|---|---|---|
| Location, n (%) | |||
| Live alone | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Live with wife/husband/partner | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Live alone but with support from relatives or friends | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Live alone but with support from carers | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Live with friends | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Live with relatives | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Early supported discharge | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Rehabilitation unit | 1 (100.0) | 0 (0.0) | 1 (25.0) |
| Residential care home without care support | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Residential care home with care support | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nursing home | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Control (N = 5) | Intervention (N = 5) | Overall (N = 10) | |
|---|---|---|---|
| Antibiotics discharge, n (%) | |||
| No | 4 (80.0) | 4 (80.0) | 8 (80.0) |
| Missing | 1 (20.0) | 1 (20.0) | 2 (20.0) |
| Analgesia discharge, n (%) | |||
| Yes | 2 (40.0) | 4 (80.0) | 6 (60.0) |
| No | 2 (40.0) | 0 (0.0) | 2 (20.0) |
| Missing | 1 (20.0) | 1 (20.0) | 2 (20.0) |
| Control (N = 1) | Intervention (N = 1) | Overall (N = 2) | |
|---|---|---|---|
| NHS physiotherapy (hospital) | |||
| Missing, n (%) | 1 (100) | 0 (0) | 1 (50) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of sessions | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Average duration (minutes) | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| NHS physiotherapy (community) | |||
| Missing, n (%) | 1 (100) | 0 (0) | 1 (50) |
| Received, n (%) | 0 (0) | 1 (100) | 1 (50) |
| Number of sessions | |||
| Mean (SD) | a | 5 (a) | 5 (a) |
| Median (min, max) | a | 5 (5, 5) | 5 (5, 5) |
| Average duration (minutes) | |||
| Mean (SD) | a | 15 (a) | 15 (a) |
| Median (min, max) | a | 15 (15, 15) | 15 (15, 15) |
| NHS physiotherapy (private) | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 1 (100) | 0 (0) | 1 (50) |
| Number of sessions | |||
| Mean (SD) | 5 (a) | a | 5 (a) |
| Median (min, max) | 5 (5, 5) | a | 5 (5, 5) |
| Average duration (minutes) | |||
| Mean (SD) | 45 (a) | a | 45 (a) |
| Median (min, max) | 45 (45, 45) | a | 45 (45, 45) |
| Control (N = 1) | Intervention (N = 1) | Overall (N = 2) | |
|---|---|---|---|
| Orthopaedics | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 1 (100) | 1 (50) |
| Number of visits | |||
| Mean (SD) | a | 2 (a) | 2 (a) |
| Median (min, max) | a | 2 (2, 2) | 2 (2, 2) |
| Pathology | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Radiology | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Emergency department | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 1 (100) | 1 (50) |
| Number of visits | |||
| Mean (SD) | a | 1 (a) | 1 (a) |
| Median (min, max) | a | 1 (1, 1) | 1 (1, 1) |
| Pain clinic | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Rehabilitation | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Mental health services | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Control (N = 1) | Intervention (N = 1) | Overall (N = 2) | |
|---|---|---|---|
| GP practice visit | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| GP home visit | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 1 (100) | 1 (50) |
| Number of visits | |||
| Mean (SD) | a | 1 (a) | 1 (a) |
| Median (min, max) | a | 1 (1, 1) | 1 (1, 1) |
| GP telephone contact | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 1 (100) | 1 (100) | 2 (100) |
| Number of visits | |||
| Mean (SD) | 1 (a) | 3 (a) | 2 (1.4) |
| Median (min, max) | 1 (1, 1) | 3 (3, 3) | 2 (1, 3) |
| Practice nurse at GP practice | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Occupational therapist | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| District nurse | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 1 (100) | 0 (0) | 1 (50) |
| Number of visits | |||
| Mean (SD) | 1 (a) | a | 1 (a) |
| Median (min, max) | 1 (1, 1) | a | 1 (1, 1) |
| Mental health service | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Control (N = 1) | Intervention (N = 1) | Overall (N = 2) | |
|---|---|---|---|
| Private specialist for clinical assessment | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
| Private specialist for surgical treatment | |||
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Received, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Number of visits | |||
| Mean (SD) | a | a | a |
| Median (min, max) | a | a | a |
List of abbreviations
- AE
- adverse event
- AESI
- adverse event of special interest
- AMTS
- Abbreviated Mental Test Score
- API
- associate principal investigator
- CI
- chief investigator
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- GMH
- global mental health
- HRA
- Health Research Authority
- HRQoL
- health-related quality of life
- INFIX
- internal fixation device
- LC-1
- lateral compression type-1
- LEF
- lower extremity function
- NIHR
- National Institute for Health and Care Research
- PACS
- picture archiving and communications system
- PI
- principal investigator
- PIS
- patient information sheet
- PPI
- patient and public involvement
- PROMIS
- Patient-Reported Outcome Measures Information System
- QALYs
- quality-adjusted life-years
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SIV
- site initiation visit
- SWAT
- Study Within A Trial
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TUG
- Timed Up and Go test
- VAS
- visual analogue scale
- YTU
- York Trials Unit
