Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 14/199/09. The contractual start date was in April 2017. The draft manuscript began editorial review in May 2023 and was accepted for publication in October 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Jansen et al. This work was produced by Jansen et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Jansen et al.
Chapter 1 Introduction
This chapter contains material reproduced from Jansen et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trauma is a major cause of death and disability. Trauma (physical injury) disproportionately affects the young, killing those who might otherwise have lived long and productive lives. 2 It is the leading cause of death for children and adults under the age of 46, accounting for nearly half of all deaths in this age group. 3 Taken together, traumatic injuries account for more years of potential life lost before age 75 years than any other cause, including cancer or heart disease. 2,4–6
The most common cause of preventable death after injury is haemorrhage. The natural history of uncontrolled haemorrhage is of falling cardiac output and hypotension and ultimately failure of compensatory mechanisms with consequent cerebral and myocardial hypoperfusion, leading to death. 7
Non-compressible torso haemorrhage (haemorrhage originating from within the torso) is particularly challenging, as bleeding generally cannot be controlled without surgery or angioembolisation. 8–10 In patients in whom haemorrhage is either unrecognised or torrential, exsanguination (severe loss of blood) and death occur prior to definitive hemostasis. 5 However, when haemorrhage is controlled expeditiously, patients often recover. 11
Temporary aortic occlusion can limit haemorrhage and help to maintain perfusion to the heart and brain, and is associated with improved survival. 12–14 An adjunctive intervention to temporarily control haemorrhage is thus conceptually attractive, and could potentially reduce the number of haemorrhage-related deaths, and deaths overall.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a novel technique whereby a percutaneously inserted balloon is inflated in the aorta (Figure 1), potentially providing a relatively quick means of temporarily controlling haemorrhage, by markedly reducing distal blood pressure and blood flow, and therefore blood loss, until definite control of haemorrhage (usually by means of an operation or sometime angioembolisation) can be obtained.
FIGURE 1.
Resuscitative endovascular balloon occlusion of the aorta, deployed via the right common femoral artery. Reproduced from Jansen et al. (2022). 1

Resuscitative endovascular balloon occlusion of the aorta increases cardiac afterload and proximal aortic pressure, and thus improves perfusion of the heart and brain; and large animal models of uncontrolled haemorrhage have shown REBOA to be highly effective. 15–18 However, REBOA is not without potential risks. Insertion of the device is technically challenging – arterial cannulation in patients with profound haemorrhagic shock is difficult. Failure to insert the device could waste valuable time that would potentially be better spent taking the patient directly to an operating theatre, to obtain surgical control of bleeding. Insertions may also be associated with major damage to blood vessels. Even if the balloon is successfully deployed, the (intentional) severe reduction in distal blood pressure and blood flow, unless very short, can result in impaired tissue perfusion, ischaemic damage or thromboses, which may be irreversible.
The current evidence for REBOA in injured humans is limited and conflicting. There are a number of case series;19–21 cohort studies (retrospective and prospective),22–25 with divergent results; and several scoping reviews, systematic reviews and meta-analyses. 26–30 There are also military clinical practice guidelines31 and a position statement from the American College of Emergency Physicians and the American College of Surgeons. 32 However, there are no randomised clinical trials.
The objective of the UK-REBOA trial was to establish the clinical and cost-effectiveness of REBOA in addition to standard care (SC), as compared with SC alone, for the management of uncontrolled torso haemorrhage, in specialist major trauma centres (MTCs).
In Chapter 2, we describe the trial design and methodology. Chapters 3 and 4 describe the elicitation exercise (undertaken to inform the Bayesian analysis) and the mixed-methods trial process evaluation, respectively. In Chapters 5 and 6, we present the baseline characteristics of the study population and the clinical results. In Chapters 7 and 8, we describe the health economic evaluation and the health economic decision modelling, respectively. Finally, in Chapter 9, we discuss the results of the trial and consider implications for practice and recommendations for research.
Chapter 2 Trial design and methods
This chapter contains material reproduced from Jansen et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Overview
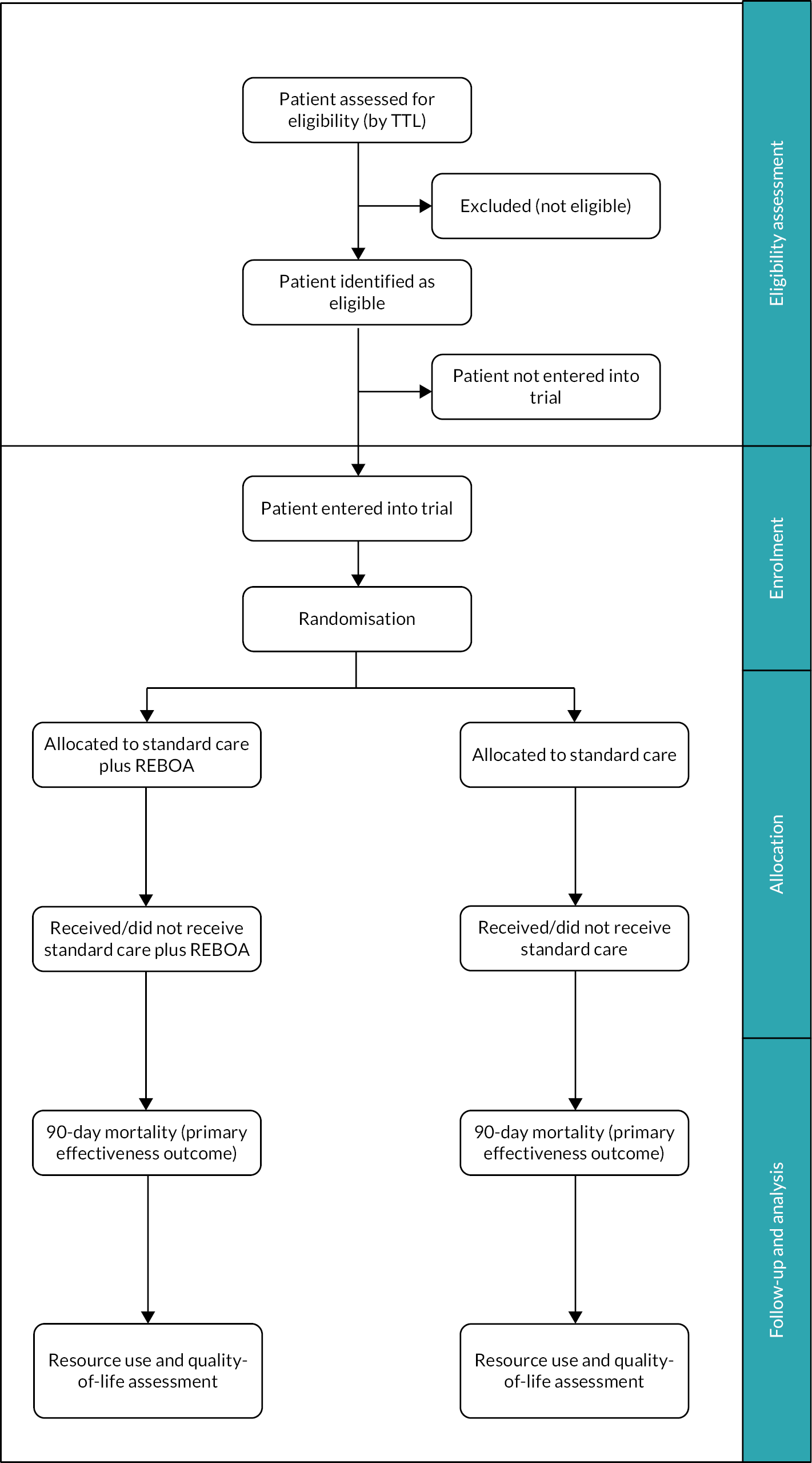
The UK-REBOA trial was a pragmatic, multicentre, Bayesian, group-sequential, open-label parallel-group, two-arm randomised controlled trial (RCT) comparing SC plus REBOA and SC. The aim was to recruit 120 adult patients with exsanguinating torso haemorrhage. The primary outcome was 90-day mortality.
We adopted a Bayesian approach to maximise the information that could be gathered with the relatively small sample size that was available. A Bayesian design is fundamentally different to the more traditional frequentist design in that it gives the probability of a specific treatment effect, given data from however many cases are available, rather than a p-value approach and a judgement that an effect is statistically significant (or not). It can also incorporate prior information about an intervention and effectively uses data from the trial patients to update what is known about an intervention. The Bayesian paradigm also fits well with clinical decision-making as it gives an estimate of the direct probability of a specific treatment effect given the data, rather than a more statistical p-value which can be harder to interpret directly. Additionally, the Bayesian framework is well suited to allowing interim analyses (it does not require the same level of inflation of the overall Type I error as in the frequentist approach). The trial design is summarised in Figure 2. Participants were recruited to the trial and were followed up for 6 months post randomisation.
FIGURE 2.
Trial design TTL, trauma team leader.
The trial protocol has been published in an open access journal1 and is available on the project web page at https://fundingawards.nihr.ac.uk/award/14/199/09.
The University of Aberdeen and NHS Grampian co-sponsored the trial. The trial was approved by the Greater Manchester South Ethics Committee (REC), reference 17/NW/0352, Integrated Research Application System (IRAS) 226135. The study was prospectively registered on the International Standard Randomised Controlled Trial Number (ISRCTN) website (www.isrctn.com) on 23 August 2017 as ISRCTN16184981.
The study included an elicitation, described in Chapter 3, an embedded mixed-methods process evaluation described in Chapter 4, and a health economic evaluation described in Chapters 7 and 8. The dedicated methods for these individual components are described in the respective chapters.
Eligibility criteria
The trial sought to enrol trauma patients with exsanguinating haemorrhage, in the emergency department (ED).
Inclusion criteria
Patients were eligible if they met the following criteria:
-
aged, or believed to be aged, 16 years or older
-
with confirmed or suspected life-threatening torso haemorrhage
-
which was thought to be amenable to adjunctive treatment with REBOA.
These criteria were chosen because they reflect the global assessment that expert clinicians intuitively perform when evaluating severely injured patients, and the pressured clinical setting in which this research has to be conducted.
Exclusion criteria
Women known or thought to be pregnant at presentation and patients with who that were deemed clinically unsurvivable were excluded.
Study setting
The trial was conducted in 16 MTCs in England (see Appendix 2). MTCs are specialist, tertiary centres designated to provide definitive care for seriously injured patients. Screening, recruitment and baseline data collection took place in the EDs of these MTCs. Patients were followed up to discharge from acute care, and by using data linkage after discharge.
Recruitment
Patients were deemed eligible for recruitment and appropriate for randomisation based on the assessment of the trauma team leader (TTL) – typically a consultant in emergency medicine, surgery or anaesthesia identified as the designated TTL – in the ED.
Assignment of interventions: sequence generation
Patients were enrolled by the TTL, or delegate, using a dedicated and secure website, accessible from handheld devices such as smartphones, tablets (one of which was provided to each centre) or a desktop computer in the resuscitation area. This mechanism took cognisance of the extreme acuity with which eligible patients would present and minimised distraction of the medical team. The website linked directly to the 24-hour randomisation system at the Centre for Healthcare Randomised Trials (CHaRT), based in the Health Services Research Unit (HSRU), University of Aberdeen. All TTLs and research staff were issued usernames and passwords for the randomisation website. The website was designed to require minimal data entry, so as not to distract clinicians from delivering life-saving care. Randomisation to SC or SC plus REBOA was in a 1 : 1 allocation ratio, by permuted blocks (in randomly generated blocks of two and four), in order to reduce predictability and selection bias.
Once a user had logged in, they saw image (a) shown in Figure 3, with the ‘Test Centre’ field auto-populated with the user’s hospital name. Users who worked in more than one MTC were able to select which site they were in.
FIGURE 3.
The randomisation system. (a) Randomisation screen; (b) Output of randomisation – randomised to SC + REBOA; and (c) Output of randomisation – randomised to SC.

In order to randomise a patient, a simple ID (such as the patient’s ‘trauma name’ or record number) had to be entered. These data were used to subsequently link to patients’ records, but was not included in the case report form (CRF).
The user then had to press ‘Randomise’. The system then returned the patient’s allocation status [images (b) and (c) in Figure 3].
Concealment
This was an open-label study. However, the allocation sequence was concealed from the TTL until they logged into the dedicated website and randomised the patient. They were then notified which intervention arm the patient had been randomised to (see Figure 3).
Consent
Patients who were eligible for inclusion in the trial were incapacitated and unable to give consent at the time of eligibility assessment and randomisation. Similarly, there was not sufficient time to consult a surrogate decision-maker or even an independent medical practitioner for advice about including the patient. Enrolment therefore took place without prior consent following appropriate ethics approval for this approach. There is a legal provision and precedent33 for conducting research in these circumstances, in England, in the form of the Mental Capacity Act (2005) (for trials that are not Clinical Trials of Investigational Medicinal Products).
Consent for continuing participation (i.e. data collection) was sought from the patient by a member of the UK-REBOA trial team taking care of the patient at the hospital site once they were no longer in a critical condition or from a personal (or nominated professional) consultee. This was defined as being cared for in a ward area [rather than an intensive care unit (ICU) or high-dependency unit (HDU)]. We did not seek consent when patients died before consultees could be approached.
Intervention and comparator description
Patients were randomised to one of two treatment arms:
Standard care: Patients allocated to the control group received ‘SC’, as expected in a specialist MTC. Such treatment typically included intubation, blood transfusion including blood products in a 1 : 1 : 1 ratio, and early operative or endovascular haemorrhage control. Treatment could also have included open aortic occlusion of the thoracic or abdominal aorta.
Standard care plus REBOA: Patients allocated to this arm would also receive the technique of endovascular aortic occlusion, in the ED for the purpose of resuscitation, as part of an overall treatment strategy. The addition of REBOA to current standard treatment was intended to provide earlier, temporary haemorrhage control to facilitate transfer to an operating theatre or interventional radiology suite for definitive haemostasis. The trial sought to evaluate the technique of REBOA rather than a specific brand of device, and therefore permitted the use of any licensed occlusion balloon, and did not prescribe or mandate a particular product.
In line with observed changes in the clinical condition of the patient following randomisation, in patients who had been randomised to the SC plus REBOA arm of the trial, clinicians were at liberty to not insert the balloon occlusion device if: the patient’s haemodynamic status improved (either spontaneously or as a result of ongoing blood transfusions) as they were deemed to no longer have life-threatening torso haemorrhage amenable to adjunctive treatment with REBOA; they deteriorated (to the point of imminent death); or there was technical difficulty in obtaining arterial access, and it was felt that operative control of haemorrhage could be obtained more quickly. Patients were also free to withdraw from the study.
The duration of balloon inflation is important, as prolonged occlusion of the aorta leads to profound distal ischaemia and (if the balloon is deflated) reperfusion injury, which can be fatal. Balloon inflation and deflation times are often inaccurately recorded in clinical practice, and these data points were therefore included on the website used to enrol and randomise patients. Once a patient had been randomised to SC plus REBOA, a new screen appeared on which the time of balloon inflation and final deflation (if there were multiple attempts) could be recorded ( Figure 4). In addition, if partial occlusion was used (to allow some blood flow to the lower part of the body), this could also be recorded.
FIGURE 4.
Data collection tool within the study website.
Outcome
Primary outcome
The primary clinical outcome was 90-day mortality (defined as death within 90 days of injury, before or after discharge from hospital). This outcome was intended to capture any potential late harmful effects of REBOA.
The primary economic outcome was lifetime incremental cost per quality-adjusted life-year (QALY) gained, modelled over a lifetime horizon, from a health and personal social services perspective.
Secondary outcome
Secondary clinical outcomes included 3-, 6- and 24-hour mortality, in-hospital mortality, 6- month mortality, length of stay (in hospital and ICU), 24-hour blood product use, need for haemorrhage control procedure (operation or angioembolisation), time to commencement of haemorrhage control procedure, complications/safety data and functional outcome [measured using the extended Glasgow Outcome Scale (GOS-E) at discharge].
Secondary economic outcomes included 6-month costs from a health service and personal social services perspective, as well as quality of life [measured using EuroQol Group’s 5-dimension health status 5-level questionnaire (EQ-5D-5L)] at 6 months; and incremental cost per QALY gained at 6 months.
To note, the clinical outcomes were chosen prior to the publication of a core outcome set for patients undergoing REBOA,34 and prior to the publication of recommendations regarding the choice of outcomes for haemorrhage control trials. 35
Sample size
The concept of an effect size and an associated sample size calculation does not figure per se in a Bayesian framework. Instead, a Bayesian trial gives the probability of a specific treatment effect, given data from a set number of cases. Therefore, we designed the trial around the available number of patients, rather than calculating a minimum sample size required, based on a retrospective study of national Trauma Audit and Research Network (TARN) data. 16 We estimated that 10 high-volume MTCs would admit approximately 80 patients who might benefit from REBOA per year, approximately half of whom would be enrolled into the trial, and further estimated that we would be able to enrol 120 patients over a period of 3 years, with a staggered start to recruitment across the sites. Actual enrolment rates in early sites were lower than our original estimates, and we therefore added a further six MTCs. (Trauma is less common in the UK than in, for example, the USA, and ballistic injuries caused by gunshot wounds in particular are rare.)
Data collection and management
The data collection strategy for the UK-REBOA trial was designed to minimise the burden on participants and clinicians and the avoidance of duplication. The trial drew on routinely collected data (all major trauma patients are audited), primarily from the TARN registry, and was effectively a registry-enabled RCT, although the case identification was not based on the registry, and the linkage occurred later. A summary of the within-trial data collection is shown in Table 1.
| Up to 24 hours | ICU discharge | Hospital discharge | 90 days | 6 months | |
|---|---|---|---|---|---|
| Mortality | ✓ | ✓ | ✓ | ✓ | |
| Length of stay | ✓ | ✓ | |||
| Blood product use | ✓ | ||||
| Need for haemorrhage control procedure | ✓ | ||||
| Time to commencement of haemorrhage control procedure | ✓ | ||||
| EQ-5D-5L | ✓ | ✓ | |||
| GOS-E | ✓ | ||||
| Resource use and costs | ✓ | ✓ | ✓ | ✓ | |
| Complications | ✓ |
Trauma Audit and Research Network National Trauma Registry data
Data on the treatment of trauma patients are routinely collected by TARN, the national trauma registry for England, to which all MTCs are required to submit data. TARN collects demographic, injury, treatment and outcome data [including the GOS-E, and – through a third-party provider – patient-reported outcome measures, including EQ-5D-5L]. Data collected by TARN directly are reported to be very complete and of high quality. 36
NHS digital data
In addition to drawing on TARN data, the trial also linked to NHS England’s Hospital Episode Statistics (HES) data to obtain information on hospital resource use and to Office of National Statistics (ONS) data for medium-term (6-month) mortality.
Mortality
Survival status and, where applicable, date and time of death were recorded in both the TARN and ONS data. However, in order to minimise delays in reporting, we also obtained death data directly from sites.
EuroQol Group’s 5-dimension health status 5-level questionnaire
EuroQol Group’s 5-dimension health status 5-level questionnaire data were also collected. These were initially to be collected directly from the TARN registry. Following the first TARN linkage, it became clear that the EQ-5D-5L results collected by the third-party provider contracted by TARN were incomplete. We therefore asked sites to collect EQ-5D-5L data prior to discharge, and subsequently, at approximately 6 months after randomisation, by telephone.
Data management
Data were entered directly into electronic case report forms (eCRFs) on the UK-REBOA trial website.
Confidentiality
Data collected during the course of the research were kept strictly confidential and only accessed by members of the UK-REBOA trial team (or individuals from the sponsor organisation or recruitment sites where relevant to the trial). Participants were allocated an individual study number upon randomisation. Participants’ details were stored on a password-protected database and only accessible to the study team. Participant’s data were fully anonymised for analysis and reporting.
Statistical methods
The statistical methods for the clinical outcomes are described below. The methods for the health economics analysis are described in Chapters 7 and 8.
General rules for statistical analysis
The trial analysis followed a statistical analysis plan (see additional files www.fundingawards.nihr.ac.uk/award/14/199/09; accessed June 2024), which was agreed in advance by the Trial Steering Committee (TSC). The main analysis was based on the intention-to-treat (ITT) principle (i.e. analysed as randomised). There were two planned interim analyses for survival (see Interim analyses) and a final analysis on all outcomes after follow-up was complete. The interim analyses were timed to occur when one-third and then two-thirds of the expected number of patients had been recruited (in line with the recruitment projections) and completed the 90-day follow-up. We wanted to ensure that, should the intervention be deemed beneficial or indeed harmful at an early stage, the number of patients unnecessarily exposed in the trial would be minimised (especially given the concerns raised in one of the previous studies of REBOA of potential for harm). Baseline and follow-up data were summarised using appropriate descriptive statistics and graphical summaries. Treatment effects are presented with 95% credible intervals (CrIs) for the primary and secondary outcomes. Unless stated, all analyses were carried out using Stata 17. 37
Analysis of primary clinical outcome
The number of eligible patients was known to be small, and we therefore adopted a Bayesian inferential framework for this trial, which has been described in detail in another publication. 38 The primary end point was the log odds ratio (OR) of 90-day mortality after MTC treatment with REBOA, compared to MTC treatment alone:
where pR and ps are the proportions of patients who died, to 90 days, after SC plus REBOA and SC, respectively.
Bayesian designs permit the inclusion of prior information about δ. The final analysis of the trial used a Bayesian logistic regression with 200,000 iterations allowing for 10,000 iteration burn-in and checking for convergence using autocorrelation and trace plots. We used a range of prior probability distributions, to contextualise the trial’s findings. This approach has been used in a number of recent studies. 39,40 A minimally informative prior was on the log OR of N(0, 1.282) which rules out extreme ORs, and a non-informative prior on the intercept of N(0, 102). The enthusiastic priors were obtained through elicitation and are described in Chapter 3. We also present a Kaplan–Meier survival curve.
Analysis of secondary outcomes
Secondary outcomes were also analysed using a Bayesian approach with 200,000 iterations allowing for 10,000 iteration burn-in and checking for convergence using autocorrelation and trace plots. For 3-, 6- and 24-hour mortality, in-hospital mortality, 6-month mortality, need for haemorrhage control procedure and complications/safety, logistic regression was used using the same minimally informative prior as the primary outcome on the log OR and a non-informative prior on the intercept. For length of stay and time to commencement of haemorrhage control procedure, linear regression using non-informative priors was used. GOS-E was analysed using ordered logistic regression and 24-hour blood product use was analysed using negative binomial regression both with non-informative priors.
Sensitivity analysis
Adjusted analysis
The primary outcome, 90-day mortality and 3, 6, 24 hours, in-hospital, and 6 months mortality were unadjusted for any covariates; however, we also pre-specified covariates that might be important to adjust for. These were age, gender, Injury Severity Score (ISS), Abbreviated Injury Scales (AIS), pre-hospital cardiopulmonary resuscitation (CPR), systolic blood pressure (SBP) on arrival in the ED, CPR on arrival in ED and time from arrival to randomisation. We also did a post hoc analysis adjusting for centre as a random effect.
Learning curve effect
There is the possibility that there could have been a learning curve effect at the site level. The learning curve was undertaken at site level as the management of major trauma cases involves the whole team (and not just the REBOA operator). As such, the whole team were learning how to integrate REBOA into their management pathway. Therefore, a sensitivity analysis removing the first participant randomised to SC plus REBOA from each site was done with the same analysis as for the primary outcome analysis.
Competing risk
For length of stay, death is a competing event, therefore a competing risks analysis was done.
Interim analyses
We had planned two interim analyses, after 40 and 80 randomised participants, and a final analysis after the expected maximum of 120 randomised participants. This analysis was based on survival and not mortality.
The stopping rules included:
Harm: Defined by the probability that the 90-day survival OR fell below 1 (i.e. REBOA is harmful) at the first or second interim analysis, was 90% or greater. More formally, our Bayesian futility criterion at each stage was P (δ < 0 | y) ≥ 0.9, where δ is the log OR and y is the observed data.
Success: REBOA would be declared ‘successful’ if the probability that the 90-day survival OR exceeded 1 at the final analysis was 95% or greater, so our Bayesian success criterion was defined as P (δ > 0 | y) ≥ 0.95. Our calculations are based on an estimated control group (standard MTC treatment alone) with a 90-day survival rate of 66.5%. 16
In short, the trial would stop if the posterior probability for harm was 90% or greater, or the posterior probability for benefit was 95% or greater at either interim analysis.
Methods in analysis to handle classifying and analysing protocol non-adherence
We recognised that a number of patients who were randomised to REBOA might not proceed to have full balloon occlusion, for a variety of clinical reasons. These patients are not ‘cross-overs’, but sit on a spectrum of how far a patient progresses down a REBOA-strategy pathway, depending on intercurrent events. There are three main types of intercurrent events:
-
technical failure (inability to achieve arterial access/insert the device)
-
patients improved as a result of other resuscitative measures, and REBOA no longer indicated
-
patients deteriorated, and REBOA no longer possible.
We classified patients, in line with clinical scenarios encountered, as follows:
-
R0 REBOA deemed inappropriate, decided against.
-
R1/C1 Arterial access not attempted (patient improved).
-
R1/C2 Arterial access not attempted (patient deteriorated).
-
R2 Arterial access attempted, but unsuccessful.
-
R3/C1 Arterial access achieved, no balloon insertion (patient improved).
-
R4/C1 Catheter inserted, but balloon not inflated (patient improved).
-
R5 Catheter inserted, balloon inflated.
Classifying patients in this way allowed us to consider the impact of these intercurrent events. 41
We conducted three analyses to accommodate for the intercurrent events, which answered slightly different questions. The first (the main analysis) relates to effectiveness, whereas the second and third relate to efficacy and safety.
QUESTION 1: ‘Does a strategy that includes REBOA (in addition to standard MTC care) reduce the mortality of exsanguinating trauma patients, ignoring all intercurrent events (such as REBOA not being deployed due to clinical improvement, deterioration, or technical failure)?’
This is the ITT analysis, and is relatively straightforward. It is the ‘policy question’ (that healthcare policy-makers want answered) and evaluated the effectiveness (the principal aim of the trial) in a pragmatic fashion. The problem is that, with many patients who were randomised to REBOA not progressing to full occlusion given clinical changes in the patients, the estimate of the treatment effect was conservative (due to potential dilution of treatment effect).
The totality of the REBOA arm tells us what happens in real-life clinical practice, but the interpretation of the results is complex. In order to address the issue of these intercurrent events, we conducted two principal stratum/complier average causal effect (CACE) analyses. These analyses are preferable to a traditional per-protocol analysis, which wastes data and is subject to selection bias. 42 The analysis used a two-staged residual inclusion estimator approach with non-informative priors. For safety, we also did an as-treated analysis using non-informative priors. CACE assumes that the patients in the SC arm, had they been offered REBOA, would have had the same proportion of patients who would not have received REBOA (because of intercurrent events). This is a reasonable assumption, since an equal number of patients in the SC arm would be expected to improve/deteriorate or be difficult to cannulate.
We debated the use of the term ‘compliance’. Although widely established in the statistical/methodological literature, it does not translate well to the circumstances observed in REBOA. Firstly, patients in the UK-REBOA trial were not ‘non-compliant’. Decisions regarding whether REBOA was still indicated, or not, were made by doctors. However, doctors were also not ‘non-compliant’ since the decision not to proceed with insertion was not arbitrary but forced on the provider by intercurrent events.
We believe that better terms to indicate the extent of REBOA treatment received are ‘strategy’ or ‘pathway’. However, since the term ‘compliance’ is established in the CACE analysis literature, we have retained it for the presentation of the CACE analyses.
QUESTION 2: ‘Does a strategy that includes REBOA (in addition to standard MTC care) reduce the mortality of exsanguinating trauma patients; when there is no technical failure, and when patients’ clinical condition did not change (improve or deteriorate)?’
Patients in the non-R5 categories were not excluded from the analysis. CACE analysis simply assumes that there would have been an equal proportion of these patients in the SC arm.
QUESTION 3: ‘Does a strategy that includes REBOA (in addition to standard MTC care) reduce the mortality of exsanguinating trauma patients; when there is no technical failure?’
For the purpose of this analysis, we defined ‘compliance’ (with the caveats regarding the terminology noted above) as patients who were classified as anything other than R2 and ‘non-compliance’ as all patients classified as R2 (arterial access attempted but unsuccessful).
Intervention implementation and training
Most of the participating sites had not used REBOA previously. The implementation strategy had three components.
Initial training
We designed a custom intervention implementation and training package, which was delivered as part of the trial site set-up, to facilitate the introduction of REBOA. The aim of the training package was two fold: firstly, to teach REBOA, and secondly, to introduce clinicians to the trial. The instruction was largely based on experience at the Royal London Hospital, as well as the Basic Endovascular Skills for Trauma and Endovascular Skills for Trauma and Resuscitative Surgery courses. Training was initially spread out over 2 days, but after delivering four of the courses, and following feedback from hospitals, we decided to compress the training into a single day. The training was delivered by two senior clinicians, and comprised a small number of didactic tutorials (indications, team organisation, imaging, ethics, post-REBOA management), followed by small group work, focusing on equipment familiarisation, individual skills training and team training. The tutorials were intended to provide background, recognising the diverse clinical backgrounds of the participants. Scenario-based team training in a simulated resuscitation room was utilised to develop decision-making regarding the incorporation of REBOA into standard resuscitative care, as well as the practical process of trial randomisation.
Development of a local service delivery and training framework, for ongoing skill development and training of new staff
Recognising the importance of ongoing and reminder training, we worked with sites to develop a sustainable, local service delivery and training framework. This involved the designation of ‘super-users’ and ‘training leads’ who organised regular refresher training, and initial training for new staff.
Reminder training sessions
The nature of the reminder training session was left to sites, but typically included discussion regarding clinical decision-making, application of the inclusion criteria, ethical considerations, post-REBOA management of patients, as well as simulations using a mannequin (provided by the trial).
Oversight and monitoring
Project Management Group
The study was led by CHaRT, a UK Clinical Research Collaboration registered Clinical Trials Unit in HSRU at the University of Aberdeen. The Project Management Group (PMG) consisted of the two co-Chief Investigators (co-CIs), a Senior Trial Manager, a Trial Manager and a Data Coordinator.
Trial Steering Committee
The trial was overseen by an independent TSC, which included a chairperson, a clinician, a statistician and two patient/public representatives. The TSC met at least annually. The TSC adhered to a charter that they agreed and signed at the start of the trial.
Data Monitoring Committee
The trial was monitored by an independent Data Monitoring Committee (DMC) who also oversaw the interim analyses. The DMC met at least annually, and reported to the TSC. The DMC adhered to a charter that they agreed and signed at the start of the trial.
Adverse event reporting and harms
As this study was recruiting trauma patients with life-threatening injuries and a high chance of dying, it was expected that many of the patients would experience events that are the consequence of the patient’s life-threatening injuries, resulting critical illness and treatment. All adverse events (AEs)/device effects occurring between randomisation and discharge were recorded in the appropriate eCRF and closely monitored by the oversight committees.
Expected complications
Death and a number of expected complications (including some which result in life-threatening illness, permanent impairment of structure or function, additional medical or surgical intervention, or prolonged hospital stay) were pre-specified outcomes and therefore not reported as serious adverse events (SAEs) or serious adverse device effects (SADEs). Only unexpected SAEs/SADEs were to be reported to the sponsor.
Adverse events related to REBOA
The following AEs could be expected to occur as a result of using REBOA.
-
Access-related adverse device effects (ADEs): External haemorrhage at insertion site requiring treatment other than simple pressure, pseudoaneurysm, arteriovenous fistula, dissection of artery, extremity ischaemia, stenosis of artery, distal embolism, air embolism, infection requiring surgical intervention, need for patch angioplasty (surgical repair), need for arterial bypass, need for amputation.
-
Other ADEs: Balloon rupture, aortic rupture, side branch cannulation.
Adverse events related to standard treatment
The following AEs could be expected to occur as a result of standard aortic occlusion by means of a thoracotomy or laparotomy:
-
AEs related to external thoracic aortic occlusion: Descending thoracic aortic injury, lung injury/bronchopleural fistula, cardiac injury, oesophageal injury, empyema, wound infection requiring surgical intervention, sternal non-union, rib fractures, extremity ischaemia, distal embolism, infection requiring antibiotics only, infection requiring surgical intervention.
-
AEs related to external abdominal aortic occlusion: Abdominal aortic injury, wound infection requiring surgical intervention, extremity ischaemia, distal embolism, infection requiring antibiotics only, infection requiring surgical intervention.
Adverse events common to both treatments
-
AEs related to impaired organ perfusion: Acute kidney injury requiring renal replacement therapy, mesenteric ischaemia requiring surgical intervention, paraplegia (permanent), paraplegia (temporary), acute respiratory distress syndrome, stroke (embolic or hypoperfusion-related), multiorgan failure.
Adverse event/device effect reporting
The principal investigator (PI) at each site, or their delegated investigator, was responsible for recording and reporting of AEs/ADEs observed during the study period on a trial-specific AE and SAE/SADE eCRF. The PI attempted, if possible, to establish a diagnosis based on the participant’s signs and symptoms. When a diagnosis for the reported signs or symptoms was known, the PI reported the diagnosis as an AE/ADE, rather than reporting the individual symptoms.
Serious adverse event/device effect reporting
All events meeting the study definition of a SAE or SADE were to be entered onto the SAE/SADE eCRF and submitted to the central trial office within 24 hours of the PI becoming aware of the event. The PI at the site was instructed not to wait until all information about the event was available before notifying the trial office of an SAE/SADE. Information not available at the time of the initial report was documented on a follow-up SAE/SADE eCRF. Follow-up information was sought and submitted as it became available. The follow-up information described whether the event had resolved or persisted, if and how it was treated and whether the patient continued on the study or had been withdrawn from treatment. Once received, seriousness, causality and expectedness were confirmed by the Cheif Investigator (CI or delegated clinical lead).
Unanticipated serious adverse device effects
Unanticipated serious adverse device effects (USADEs) were defined as SAEs that were deemed to be related to the study device or any of the research procedures and were unanticipated. USADEs were to be notified to the sponsor and Research Ethics Committee (REC) within 15 days of the trial office becoming aware of the event.
Assessment of seriousness
The PI or designee made an assessment of seriousness. As stated above, death and a number of expected complications (including some that result in life-threatening illness, permanent impairment of structure or function, additional medical or surgical intervention, or prolonged hospital stay) were pre-specified outcomes and were therefore not reported as SAEs/SADEs.
Assessment of causality
The PI or designee was instructed to make an assessment of the causality (i.e. relationship to trial device). Events that were possibly, probably or definitely related to the device were defined and reported as related to the device. Events that were assessed as possibly related or unrelated were defined as not being related. This was determined as follows: (1) Definitely: There was clear evidence to suggest a causal relationship, and other possible contributing factors could be ruled out. (2) Probably: There was evidence to suggest a causal relationship, and the influence of other factors is unlikely. (3) Possibly: There was some evidence to suggest a causal relationship (e.g. the event occurred within a reasonable time after using the device). However, the influence of other factors may have contributed to the event (e.g. the patient’s clinical condition, other concomitant events). (4) Unlikely: There was little evidence to suggest there is a causal relationship (e.g. the event did not occur within a reasonable time after administration of the trial intervention). There was another reasonable explanation for the event (e.g. the patient’s clinical condition, other concomitant treatments). (5) Not related: There was no evidence of any causal relationship. (6) Not assessable: Unable to assess the information available.
Assessment of expectedness
The PI or designee made an assessment of expectedness for each SAE/SADE regardless of the causal relationship to the trial device.
Follow-up procedures
All AEs/ADEs assessed by the PI or designee as possibly, probably or definitely related to the device and all SAEs/SADEs that occurred during this time were to be followed until they were resolved or were clearly determined to be due to a patient’s stable or chronic condition or intercurrent illness(es). The CRF was updated with the date and time of resolution or confirmation that the event was due to the patient’s illness as soon as this information became available.
Recording and reporting of urgent safety measures
If the PI, designee or a member of study staff became aware of information that necessitated an immediate change in study procedure to protect clinical trial participants from any immediate hazard, they were instructed to report the urgent safety measure (USM) immediately to the trial office. The trial office would then report any USM immediately to the sponsor, and liaise with the sponsor and site to implement immediate procedures to eliminate any hazard. The trial office would also report immediately by telephone to the REC that had approved the study and follow this up with an e-mail written notice within 3 days of becoming aware of the USM. The e-mail notice would state the reason for the USM and the plan for further action. The PI or designee was to respond to queries from the trial office immediately to ensure the adherence to these reporting requirements.
Protocol amendments
Protocol amendments were agreed among the PMG and then categorised by sponsor before being reported for approval to the REC. There were five protocol amendments which are summarised in Table 2.
| Version number, date | Summary of amendment |
|---|---|
| Version 2, 20 July 2017 | Change of contact details for CI Clarifications within the safety section of the protocol |
Revision to text describing the length of training sessions |
|
| Version 3, 14 September 2017 | Clarification of secondary outcomes (24-hour in-hospital mortality; safety data); addition of new secondary outcome (procedural performance details) |
| Version 4, 18 April 2019 | Addition of within-study collection of EQ-5D-5L to supplement routine TARN data and confirmation of plans for imputation of missing EQ-5D-5L clarification that: |
|
|
| Version 5, 15 November 2020 | To update timelines to reflect 24 months extension to the study |
| Version 6, 25 November 2021 | Addition of 3- and 6-hour mortality as secondary clinical outcomes |
Study documentation
Documentation used in the UK-REBOA trial is available in the additional files www.fundingawards.nihr.ac.uk/award/14/199/09 (accessed June 2024).
Breaches
One non-serious breach was reported to the sponsor during the study. This is related to the use of the Clinician Topic Guide in the process evaluation before it had been approved by the REC. As part of the corrective action, this document was submitted and approved by REC.
Patient and public involvement
As noted above, the TSC included two patient/public representatives. In the early stages of study development, they had opportunity to input into the study design, and to review and comment on protocol and associated documentation.
Chapter 3 Elicitation of prior probability distributions
This chapter contains material reproduced from Jansen et al. 43 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
When a clinical trial is being planned, there is usually some existing knowledge regarding the effect of the intervention. A Bayesian approach to design and analysis of clinical trials can explicitly consider such data, which is referred to as a prior probability distribution, or ‘prior’ for short. Although the design and main analysis of the UK-REBOA trial rely on a non-informative prior, which ‘lets the data speak for itself’, additionally, we planned to elicit and use informative priors to help contextualise the interpretation of our results.
Informative priors can be derived from a number of data sources. One option is to conduct an ‘expert elicitation’, a formal data acquisition process where experts are assisted in converting their knowledge into mathematical format. 44–47 This method is particularly helpful when the evidence originates from divergent sources, which are difficult to summarise mathematically.
This chapter describes the elicitation exercise conducted as part of the UK-REBOA trial, to derive prior probability distributions to help contextualise the interpretation for the primary and secondary outcomes of the trial.
Methods
The methodology for conducting expert elicitations is well described. These studies typically require an in-person meeting, lasting hours or days, where the participants are introduced to the subject matter and Bayesian methodology. Several rounds of elicitations are conducted, with intervening analyses, presentation of results and discussion.
In-person elicitation meetings are time-consuming and expensive, and – during a pandemic – difficult to organise and justify. We therefore conducted a remote, online elicitation exercise.
Videoconferencing platform
We used the Zoom platform (Zoom Video Communications, San Jose, CA, USA). The process and the group discussions were moderated by the CIs of the UK-REBOA trial.
Framework
We used the Sheffield Elicitation Framework methodology, as described by O’Hagan. 48 We adhered to good practice recommendations for eliciting expert opinion47,48 including preparation of the participants for the elicitation workshop, use of an elicitation protocol approved by a REC, provision of feedback to experts and an opportunity to revise elicited responses. 48
Participants
We invited 20 subject matter experts to participate. All participants were from the UK, to reflect the setting of the trial. Invitees included the grant holders (with the exception of those involved with the design and conduct of the elicitation itself), as well as site PIs. We reasoned that these individuals would have both knowledge of the published evidence for using REBOA, and personal experience. Participants included emergency medicine physicians (n = 12); pre-hospital care doctors (n = 3); surgeons (n = 4) and intensivists (n = 1).
Quantities of interest
The quantities of interest chosen to inform the analysis of the UK-REBOA trial were those specified by the protocol: 90-day mortality (the primary outcome of the trial), 6-hour mortality, in-hospital mortality and 24-hour mortality (secondary outcomes). For each of these time points, we elicited experts’ opinions regarding treatment with REBOA (in addition to SC), and without REBOA (SC alone).
Information provided in preparation for the elicitation
We provided participants in advance with an overview of the elicitation process and the concept of subjective probabilities, as well as an evidence dossier which included reference to known studies of REBOA. We provided no commentary on the studies in the evidence dossier so as not to introduce any bias into the process. The list of included studies is included in the evidence dossier in Appendix 3. We also asked participants to provide us with any other published studies or abstracts of which they were aware. The dossier was distributed by e-mail the week prior to the elicitation.
Phases
On the day, the elicitation exercise was split into seven phases:43
-
Presentation of background information on the UK-REBOA trial. We did not present evidence relating to the intervention at this point to avoid bias by ‘anchoring’ the participants.
-
Introduction to Bayesian principles, focusing on the distinction between probability under frequentist and Bayesian paradigms, and emphasising that Bayesian probability represents the subjective level of uncertainty of an event happening and can vary among individuals.
-
Introduction to quantities of interest and their parameters: lower and upper bounds and most likely value of mortality, at different time points, in patients treated with REBOA (in addition to SC) or SC alone.
-
Elicitation training exercise. We worked an example with the participants, using the same online tool used for the actual elicitation, to increase familiarity with the process.
-
Elicitation, first round: Participants’ beliefs for the quantities of interest were elicited using the online elicitation tool. We calculated prior distributions for each participant’s elicited beliefs, and then graphed and presented deidentified individual responses.
-
Group discussion. Participants were then encouraged to discuss their choices. We emphasised that the purpose of the discussion was not to come to a consensus but rather to calibrate individual opinions, and to resolve any questions relating to process.
-
Elicitation, second round: The second round was designed to allow participants to revise and calibrate their beliefs, and therefore used the same questions as the first. Participants were provided with an individual code, to allow first- and second-round responses to be compared. The results were, once again, presented as deidentified individual responses.
The elicitation was supported by a biostatistician, who explained the concepts and was available throughout the day to answer questions.
Data collection
We created an interactive online graphical tool, based on previous work by Mason et al. ,49 and our own work,43 using R software and the Shiny package. 50,51 The purpose of the tool was to allow participants to use it online, while also following instructions/conversation on Zoom. It was also designed to be user-friendly and intuitive.
Participants were provided with individual log-ins, so that responses could be tracked. For each quantity of interest, participants were first asked to provide their ‘most likely’ (median) estimate for a given quantity of interest, using a slider. Participants were then asked to quantify their certainty by selecting lower and upper plausible values, again using a slider. Once participants had selected their values, they clicked a button which then displayed their selection as a probability density graph. An example screenshot is included in Appendix 4, Figure 17. The choices could then be amended, with corresponding changes to the graphical output.
Individual responses were electronically submitted, and analysed in real time. The results (individual as well as pooled) were then displayed, again using Zoom’s screen-sharing function, for discussion.
Derivation of prior probability distributions
To obtain the expert-elicited prior distribution for the model, we adopted the following strategy. We aggregated individual expert-elicited beta distributions into a single pooled distribution (at each time point for each of the intervention and control groups) considering equal weight for each expert. We then sampled 500,000 observations for each pooled distribution and calculated the OR of the sampled data of the intervention to the control group at a given time point. The log-transformed OR of the distribution was incorporated as the prior distribution in analysis models (see Chapter 2 for details).
Mathematical aggregation of experts’ judgement and parameterising the prior distribution
The following section outlines the approaches used to combine the expert knowledge into a single prior distribution, parameterise the pooled prior distribution for incorporating in a logistic regression model with Bayesian inferential framework in the context of a randomised control trial setting.
We describe here the strategy to aggregate K experts’ judgement of the intervention and control arms using equal pooling and implementation of the algorithm in an RCT setting.
-
Capture individual expert judgement as a beta distribution and obtain the parameters of individual beta distribution for a given scenario (say, 6-hour mortality), that is one each for the intervention (I) and control (C) groups at a given time point
-
Obtain the linear pool of the beta distributions of all experts (fg), considering equal weight for each expert, for each of the intervention and control group.
-
Sample (n = 500,000) from the corresponding linear pool of the distribution for each of the intervention and control arm.
-
Calculate the OR of mortality for the intervention to control arms.
-
Calculate the logarithm of OR.
-
Summarise the parameters of the distribution of log OR assuming a normal distribution with mean μ and variance (σ2). The derived distribution (with mean and variance as hyperparameters) represents the prior distribution of the regression coefficient (intervention vs. control) of the logistic regression model. The prior distribution is defined as:
Results
Process
In total, the elicitation took 6 hours to complete. We encountered no significant technical difficulties with the videoconferencing platform (such as not having or being unable to use Zoom; disconnections; or loss of video or audio feeds).
Despite a relatively large number of participants and additional observers, we found that moderating the session was straightforward. Furthermore, we found that the group discussions resulted in meaningful deliberation and interaction, without being dominated by a small number of individuals.
We also encountered no major technical difficulties (crashes, inability to submit data, inability to enter data, etc.) with the app, and all participants were able to submit their data.
Prior probability distributions
As expected, there was a convergence of elicited distributions in round two. The derived prior probability distributions are summarised in Table 3 and were used in the treatment effect estimation models in Chapter 6. The prior distributions are presented visually in Appendix 4, Figures 18–25.
| Time | Mean | Variance | SD | Lower | Upper |
|---|---|---|---|---|---|
| 6 hours | −0.3834 | 0.9282 | 0.9634 | −2.2717 | 1.5048 |
| 24 hours | −0.3329 | 0.7765 | 0.8812 | −2.0600 | 1.3943 |
| 90 days | −0.3025 | 0.6761 | 0.8223 | −1.9141 | 1.3090 |
| In-hospital | −0.3584 | 0.6454 | 0.8034 | −1.9331 | 1.2162 |
Discussion
Subject matter experts, on average, estimated in-hospital and 90-day mortality in this patient group, without the use of REBOA, to be in excess of 50%. Mortality at earlier time points (6 and 24 hours) was estimated to be closer to 25%.
The elicited data and the resulting prior probability distributions indicate that the experts, on average, had a favourable opinion of REBOA, that is they expect the addition of REBOA to SC to improve mortality at all time points.
The process of conducting the elicitation online went smoothly and resulted in the participation of 20 experts from all over the UK to participate during the COVID-19 pandemic. Traditional elicitation exercises have been delivered in-person with classic methods, such as ‘chips and bins’ or ‘roulette’. However, an online elicitation, with appropriate software and support, can help to provide more contemporary results. We found that, after some instruction, participants were able to use the online tool without difficulty.
Chapter 4 Embedded process evaluation
Parts of this chapter are reproduced with permission from Lawrie et al., Behavioural optimisation to address trial conduct challenges: case study in the UK-UK-REBOA trial. Trials 2022;23(1):398. DOI: 10.1186/s13063-022-06341-6. PMID: 35550599; PMCID: PMC9097042. 52 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
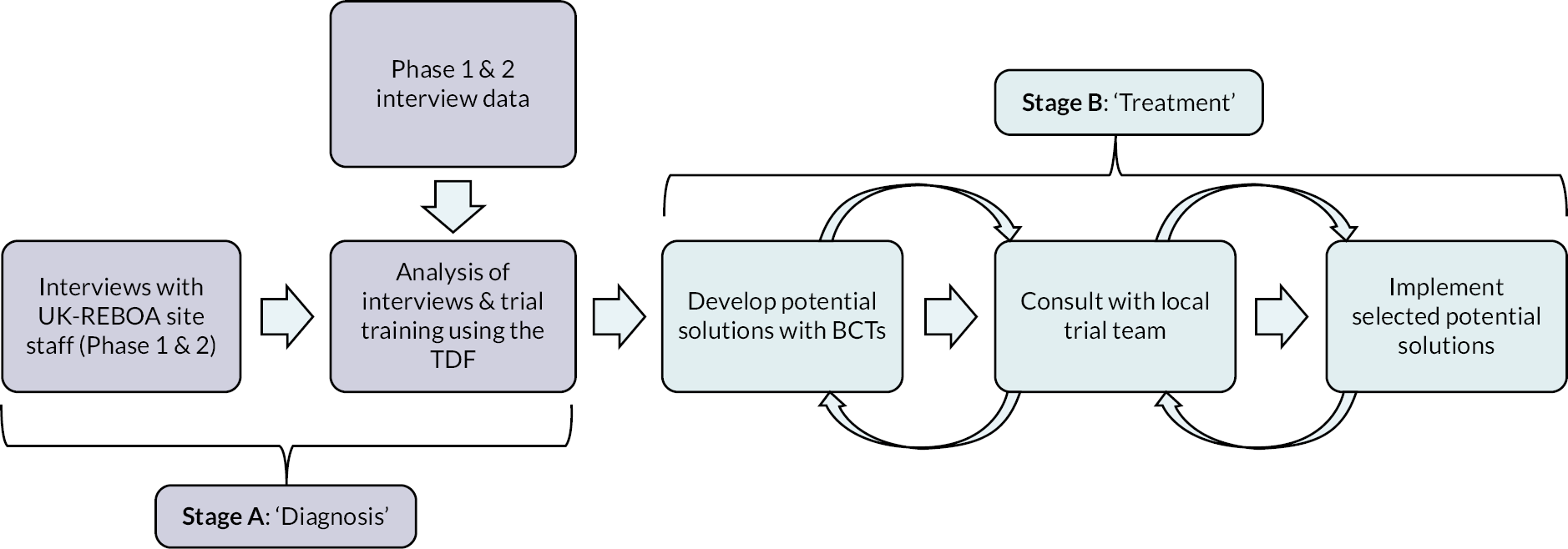
Clinical trials of complex interventions, of which REBOA would be considered one, face many challenges. 53 Understanding and intervening on challenges to the delivery of the UK-REBOA trial were deemed critical for trial success. An embedded process evaluation was incorporated at the design stage of the trial to identify challenges relating to trial design or conduct that could be addressed and modified to facilitate the delivery of the trial. The process evaluation consisted of two stages: a first stage (A) to explore and diagnose any core problems to the successful delivery of the trial and a second stage (B) to develop solutions to address the problems and identify enhancements (see Figure 5 for an overview). Stage A was further composed of two phases. Phase 1 targeted staff at sites which were the early adopters of the trial and explored any initial difficulties associated with the set-up and activation of trial processes (focussing on recruitment and intervention delivery). Phase 2 focused on activity once the trial was established and sites had more experience of the trial, randomising participants and deploying the REBOA catheter. Both phases generated recommendations to improve trial delivery.
FIGURE 5.
Steps involved in the ‘diagnosis’ (Stage A) and ‘treatment’ (Stage B) of issues related to trial recruitment and REBOA intervention delivery. BCT, behavioural change techniques; TDF, Theoretical Domains Framework.
The process evaluation was designed to be responsive to the needs of the trial. As such, the original plans to interview trial participants and/or consultees were not progressed; largely due to only very small numbers of participants/consultees declining consent for further follow up, which suggested no significant issues that required investigation. In addition, the original analysis of participant recruitment, using the adapted screened, eligible, approached, randomised framework54 was not applied within the process evaluation due to the low throughput of patients. Therefore, the process evaluation focused exclusively on key clinical site staff and included interviews as planned but also non-participant observation of site training.
Methods
This process evaluation was approved as part of the UK-REBOA trial by the Greater Manchester South Ethics Committee (17/NW/0352, IRAS project ID: 226135). Informed written consent was obtained from all participants. Documentation used in the process evaluation is available in the additional files www.fundingawards.nihr.ac.uk/award/14/199/09 (accessed June 2024).
Phase 1: Identifying initial difficulties associated with set-up and activation of trial processes
Sampling and recruitment
Recruitment in Phase 1 was purposive and targeted staff who had a role in the recruitment or randomisation of a patient in the first six sites to randomise a patient into the UK-REBOA trial. Site staff across active centres were sent an e-mail invitation (on behalf of the Clinical Co-CI) to participate in the interview study along with a participant information leaflet (PIL) and asked to contact the process evaluation team if interested. On contact with the process evaluation team, staff were provided with the opportunity to discuss the qualitative interview study further and book a mutually convenient time for a telephone interview. Two attempts were made to engage with potential participants. Sampling was informed by the key principles of information power, because the aim of the process evaluation was focused, the sample was specific (site staff involved in recruitment), rich narratives were provided during the interviews and no cross-case analysis was conducted. 55
Data collection
Qualitative data were collected through telephone interviews conducted with site staff who were recruiting patients to the UK-REBOA trial. A topic guide (developed by the process evaluation team and Co-CIs) was used to direct questions and aimed to elicit site staff’s thoughts, comments, involvement and experience with the trial. Interviews took place between April and June 2018. Interviews were conducted by two members of the research team and were audio-recorded and transcribed verbatim.
Data analysis
The approach to analysis was systematic and interpretive, applying an inductive thematic analysis using the Framework approach. 56 One researcher re-read the interview transcripts and generated codes in NVivo (QSR International, Warrington, UK) (used to facilitate data management and initial coding)57 which described relevant features of the data prior to collating into themes. Themes summarised the semantic content of interviewee responses and represented salient issues that were articulated by multiple participants. 58 Following review/refinement of themes, a thematic framework was developed by three members of the team which described the content of all themes and provided illustrative quotes to facilitate data analysis. The thematic coding framework was informed by both a priori questions and issues identified as emerging from the data. A double coder checked the themes and accurately described the content of participants’ responses in a sample of interview transcripts. Any coding discrepancies identified during this process were discussed to reach consensus.
Solution development
Themes from the analysis were tabulated and identified as barriers and/or facilitators to trial delivery. The trial PMG met with the researchers who conducted the qualitative work to discuss potential solutions to the issues identified in the interviews. Proposed solutions were considered in relation to acceptability and deliverability.
Phase 2: Exploring barriers and facilitators for recruitment and intervention delivery in established trial sites
Design overview
As the process evaluation developed, it was recognised that many of the challenges within the trial were dependent on people’s behaviour, that is clinicians performing actions (such as randomising a patient or delivering the intervention) that may not be part of their routine practice. There is now a growing body of evidence that suggests behavioural science has the potential to add value to exploring and providing solutions for challenges in the conduct of clinical trials. 59 Within Phase 2 of this process evaluation, we applied the Theoretical Domains Framework (TDF) as a method to help inform data collection and analysis. The TDF is an established framework that categorises behaviour into 14 domains that inhibit or enable behaviour (knowledge, skills, social/professional role and identity, beliefs about capabilities, beliefs about consequences, optimism, reinforcement, intentions, goals, memory/attention/decision processes, environmental context and resources, social influences, emotion and behavioural regulation).
Recent studies have highlighted the utility of the TDF to identify behavioural processes in clinical trials where the performance could be improved. 60–63 The TDF was identified as an ideal framework to support components of this process evaluation as it provides an opportunity to examine behaviours which need to change in order to improve the conduct of a trial, and represents the first step in the process of developing behaviour change interventions. 64 Interventions can be developed to address trial process challenges through mapping barriers and facilitators onto behavioural change techniques (BCTs) via established methods in the behavioural science literature. 65 BCTs are defined as the smallest active ingredient of an intervention such as feedback on behaviour or goal setting, and they can be used alone or in combination with other BCTs. 64 We aimed to develop and implement potential solutions (containing BCTs) to minimise the barriers and maximise the facilitators to trial recruitment and intervention delivery identified from interviews with site staff. 64
Sampling and recruitment
Individuals invited to participate in Phase 2 were from sites which had either recruited a number of patients into the trial, experienced notable difficulties with recruitment, had recently randomised a patient to the trial and/or reported a missed opportunity to recruit an eligible patient. Staff in various roles who were involved in recruitment were invited to take part. E-mail invites were distributed as per previous description for Phase 1.
Data collection
Qualitative interviews were conducted via Microsoft Teams. Interviews were conducted by one member of the research team in October 2020. The topic guide was informed by the TDF, focused on recruitment and intervention delivery and the issues previously identified as important in Phase 1 – that is deployment and insertion of the REBOA catheter. The topic guide was developed and refined by two members of the process evaluation team.
Non-participant observation was conducted during the on-site training for a new recruiting centre at site setup. Detailed notes considering critical conduct problems and behaviours related to trial delivery were collected during this session and considered alongside training materials delivered and provided to sites. Trial training and support materials provided to site staff were compiled and coded using the TDF and BCT Taxonomy v1. 66 These were collected to identify areas where the process evaluation team could help to improve trial processes via adaptation of existing training and support materials (see Stage B subsection under Data analysis).
Data analysis
Stage A: Identification of salient theoretical domains framework domains relevant for recruitment and intervention-related behaviours
Data from Phase 1 interviews were transferred into NVivo alongside the data from Phase 2. This facilitated exploration of the factors that influence recruitment and intervention delivery across all cases, using the TDF, as opposed to using an inductive approach to analysis. We used a TDF coding guide to aid data interpretation, which was developed and iteratively updated during the coding process. One researcher coded transcribed data into the relevant TDF domains. Three of the 18 interview transcripts were independently double-coded and exhibited a large degree of agreement across the double-coding. Any disagreements were resolved by a third researcher and updates to the coding guide were added where appropriate.
After coding data into TDF domains, belief statements (representative descriptions of utterances across participants) were generated. 67 Belief statements were designed to present details on how each domain may be influencing the behaviours of interest, namely: (1) recruitment of patients to the trial and (2) delivery of the REBOA intervention. The research team collectively discussed the belief statements to agree they were an accurate representation of the quotes coded within each domain.
Established TDF analysis methods were used to identify the domains that were most likely to influence the target behaviours. 64 This included: (1) the frequency of belief statements across all domains (statements with a frequency of > 75% were considered most ‘relevant’ as per other TDF-based studies);61 (2) evidence of strong beliefs that influence the behaviours (i.e. the strength of conviction illustrated by participants during the interviews); (3) and the presence and prevalence of conflicting beliefs. This resulted in some domains that contained frequently reported belief statements not being identified as salient as there was no evidence of strong beliefs, from interviews, that influenced the target behaviours or conflicting beliefs within the domain.
Prior to the identification of potential solutions to mitigate trial challenges, we reviewed the barriers relevant to all domains that were amenable to change within the scope of this project. We omitted those that required wider infrastructure changes as delivering large-scale system changes was unlikely to be realised short term to aid the trial delivery (e.g. such as a lack of additional personnel to support recruitment) or were not amenable to change (i.e. low number of eligible patients). All criteria were evaluated concurrently (via group consensus) to judge the relevance of each domain.
Stage B: Identification of behavioural change techniques to inform the development of potential solutions to help improve trial processes
Following identification of the salient domains, components of potential solutions were determined using a standardised process that involved mapping the relevant theoretical domains to BCTs using the Theory and Techniques Tool. 64,65 The BCTs identified as potentially relevant for selected TDF domains were collated, discussed by the research team, and adapted to the clinical context of the UK-REBOA trial. In addition, existing training and support materials provided to site staff were reviewed to examine the presence of BCTs that may already be delivered in the trial as an opportunity to enhance existing trial practices.
Behavioural change techniques proposed by the research team were presented at a meeting with the Trial Manager and Co-CIs to discuss the applicability of selected BCTs to support specific trial behaviours (recruitment and intervention delivery). We applied the APEASE criteria (acceptability, practicability, effectiveness, affordability, side-effects and equity) to support the final selection of the content and mode of delivery for the potential solutions to improve trial processes. 64
During solution development, training materials were updated in response to the findings of the behavioural investigation and implemented in follow-on training for sites. Trainers were briefed on the purpose of the behavioural approach to the review of training materials and encouraged to embed BCTs within the delivery. Training delivery with regard to BCT content was assessed by observation with feedback provided to the training team post session by the process evaluation lead. Training attendees (i.e. clinical staff tasked with trial delivery) were also asked in their feedback to consider the main message they had taken away from the training in order to determine the most salient aspects of the training content and whether updated content was received as intended.
Results
Sample characteristics
Forty-nine interview invitations were distributed to eligible site staff. Seventeen participants from eight sites were interviewed across both phases (Phase 1 n = 13, Phase 2 n = 5; one participant was interviewed in both phases), with the majority identifying their role as Trauma Consultants (n = 9, 53%) ( Table 4). One of these participants was interviewed in both Phase 1 and 2 as they provided initial perspectives on early process problems and later experiences of more established trial process problems. Taken together, the interviews lasted an average of 37 minutes, ranging between approximately 22 minutes and 1 hour.
Phase 1 findings: Identifying initial difficulties associated with set-up and activation of trial processes
Seven primary themes were identified across the interviews, which could be further organised into barriers or facilitators of trial delivery. The seven primary themes and whether they were reported as a barrier or a facilitator, or both, are summarised in Table 5. Each of these identified themes will be presented in turn with examples.
| Theme | Barrier | Facilitator |
|---|---|---|
|
✓ | ✓ |
|
✓ | ✓ |
|
✓ | ✓ |
|
✓ | ✓ |
|
✓ | |
|
✓ | |
|
✓ |
Skills and competencies related to intervention delivery
Skills and competencies were identified as both a barrier and a facilitator by site staff. Findings within this theme largely reflected the specific skills, and associated expertise, required to deliver the intervention, that is insert the catheter and deploy the balloon. When discussed as a barrier, interviewees cited reasons such as making sure an appropriately qualified person was available to deliver the intervention.
I think most people who are in the game are concerned about or have nervousness around is actually once the app says, you know use REBOA, that’s where people’s blood vessels start to go up a bit! In terms of am I going to get it in right? Am I going to do it right, that sort of thing. I think, having never done it in anger before, but only as part of the training scenario.
Consultant 1
The need for staff experienced in delivering the intervention was also cited as a facilitator to successful trial delivery.
Any surgeon that is comfortable with personally being able to open a chest and put a clamp on. If they are happy doing that, I can’t see them having a big issue with putting a REBOA balloon in. If the technical aspect of deploying a REBOA balloon is taken away from the trauma surgeon … that may make it easier to integrate it into other trauma centres.
Consultant 2
Both barriers and facilitators in this theme cited the throughput of patients as a factor in influencing competencies and a site’s ability to successfully deliver the trial.
So I think the urban centres are likely to find it easier purely because they’re going to have larger numbers you know, obviously the more haemodynamically compromised patients that come through your system then the easier it tends to be to introduce new techniques because you’re getting the numbers which people can gain technical experience in using those. So I imagine that most of the big cities will not struggle to do that.
Consultant 3
The numbers are likely to be such that I suspect most A&E [Accident and Emergency] doctors may not get enough experience to ever subsequently feel confident to do it themselves.
Radiologist 1
Resource commitment to successfully deliver the trial
With regard to resource commitment, the main facilitator cited by interviewees was dedicated staff members whose responsibility it is to deliver, or support delivery of, the trial. A lack of resource commitment in terms of monetary support to purchase the intervention, providing 24-hour cover for staff to help deliver the trial, and recognition for involvement with research projects were identified as barriers in the interviews.
Individual and community equipoise
Across the interviews, equipoise (or lack of equipoise) was evident as a complex issue that many interviewees highlighted. Many were not necessarily in individual equipoise but recognised that across the clinical community mixed views were held, and thus community equipoise rather than individual equipoise was more apparent.
At the moment they have to be really, really sick. And a little bit frustrating as a purely research side is when we go down there and say, ‘What do you think about this patient? Can we put them into REBOA and the trial?’ which happens probably once every 3 weeks I would say, probably a little more often than once a month, and the usual answer is, ‘No, they’re not sick enough and, so I don’t want to get the randomisation side that says REBOA and therefore I’m not going to do it’. And [name] really has said to us, ‘If the clinicians are thinking REBOA, if they’re thinking REBOA then randomise. But if they’re not thinking REBOA then we’re not going to randomise at all’. So yeah, I think that’s what it’s mainly makes it difficult to get them into the trial.
Registrar 1
For one of the sites involved, they already delivered in-hospital REBOA and identified this existing knowledge of application as potentially problematic for staff linked to the trial.
At our site, probably the only thing that hinders us is that we have already been delivering this procedure so there is some understanding about where it sits, and who needs it before we entered the trial. So, actually, the trial seems to muddle people up a little bit because some of our team are convinced they know when to use it and when not to, some of the team aren’t. I just wonder if that probably makes it slightly more confusing at our centre than any other centre where it’s completely new, they’ve never heard of it. They are more likely to feel that they have genuine equipoise.
Consultant 3
However, there were some who viewed community equipoise as the driver for delivering the trial. And indeed, the requirement for further evidence to support clinical decision-making was cited as a reason to promote the trial.
Well, and actually some of those other views stem from the lack of hard evidence, and, so, I mean I broadly find it slightly bizarre that you can be so polarised when the evidence is relatively weak, but some people aren’t … I think that their arguments could be won with better evidence.
Registrar 1
Responses from the interviews also highlighted that equipoise in an emergency care setting may be impacted by real-time events – staff perspectives regarding preferences for treatment (e.g. REBOA or standard major trauma care) reportedly changed often depending on the clinical presentation of the patient, and subsequent interpretations of patient eligibility.
Interpretations of patient eligibility
Closely linked to the viewpoints surrounding equipoise were interpretations of patient eligibility. In some instances, it was perceived as a barrier with interviewees stating there was ambiguity around who was eligible due to a lack of existing definition or assessment of exsanguination. This was also linked to variability in clinicians’ interpretation of eligibility but was balanced against the pragmatic nature of the trial and its applicability to real-world practice.
I think the key word is exsanguination. The indication, if you read them verbatim, talk about exsanguination, the definition of exsanguination is bleeding to death, but the question is how does each individual clinician interpret that? How do we prove that, and how do we diagnose that? How do we do all that in a very short space of time, pre-hospital or ED environment. It’s a very difficult question. I am not really sure how to answer it any better. I think [NAME] just is setting out to make a pragmatic trial. The point is, if the procedure is delivered on a broader scale, everybody will interpret it slightly differently, everybody will do it slightly differently, so actually what you are testing is probably the correct thing to be testing how clinicians across the country in different centres will perform this procedure.
Consultant 4
Working relationships
The need for teamwork, good communication and inclusion of a wide range of specialties to successfully deliver the trial was cited across interviews. For some sites, this was regarded as a facilitator, whereby staff reported that the involvement of various people occupying different roles (such as anaesthetists and vascular surgeons) could enhance recruitment processes. Having a system in place that capitalised on the expertise of individuals from multiple specialties was perceived to be particularly important given the infrequency of REBOA eligible cases.
So, we’ve set up a system here where the operators are a mixed bag of ED [Emergency Department], vascular … anaesthetic individuals involved as well … set up, and to activate us, as and when … the actual opportunities are few and far between, they are incredibly rare. So, we’ve elected to have a system whereby we get activated by the trauma team leader, a code red comes in and looks like it may or may not be suitable for REBOA.
Consultant 1
However, according to some staff, difficult working relationships or deferral to senior colleagues was raised as a potential barrier in randomising patients. This was intrinsically linked to perceptions of equipoise among the team or key members.
He’s the PI for the centre, for the [hospital], but he was clinically involved in a case, and I think some of the team felt that they needed REBOA in and he really didn’t, so …
Registrar 1
Specifics to operationalising key aspects of the trial
Key barriers or problems relating directly to specifics of the UK-REBOA trial were identified. In a fast-paced and pressurised setting, it could be easy for some staff to forget where the randomisation app was stored on the electronic device, particularly when regular access to the app was not required (due to the low throughput of patients eligible for trial recruitment). During access to the app, other difficulties could also arise – such as non-technical errors related to signing into the device.
I think there are other things, because the randomisation process is on the app, which is a good thing, the only problem to me with that is because I’m about as technical as a sack of potatoes ‘Where did I put the app?’ Trying to find the [expletive] app, and heaven forbid if they ask the password! [laughter] For something that you would maybe only access once ever month…
Consultant 1
Trial training: generic and site-specific
Training, both the specific training received as part of the trial and research/clinical training more broadly, was perceived as a facilitator. The training provided during early phases of the trial was regarded as crucial in terms of ensuring all staff understood the parameters of the trial, the technicalities of the intervention as well as the non-technical skills involved in decision-making about randomisation and intervention delivery. Overall, training was perceived to facilitate enhanced (joint) decision-making, understanding and communication among team members with different levels of seniority, as well as creating institutional awareness of the trial across hospital sites.
Well so the other thing that’s really important is having training in the technique and in the decision making. So we have a monthly training session for REBOA, so the aim is that we get all the senior nursing staff and all the senior EM [Emergency Medicine] staff and surgical staff and critical care staff trained to provide the … to understand both the parameters of the trial and the things that REBOA may help with and may not help with, so then you’ve got a better informed joint decision making actually. And it [training] allows team members who are not the team leader also to make those prompts, ‘Have we considered this patient for REBOA?’ So I think having that better group understanding of the trials and the things that may help is very helpful.
Consultant 3
the people who actually run the trial at our site are [name] and [name], very proactive in terms of creating institutional awareness both of the procedure in general and of the trial in particular. So, they are … there has been a lot of educational stuff, and all the consultants have full buy-in.
Registrar 2
Phase 1: Proposed solutions based on interview diagnostics from early adopter sites
Table 6 describes potential solutions, developed in collaboration with the PMG, some of which were implemented immediately, and others were combined into solutions within Phase 2 using a behavioural approach. In addition, findings from this Phase were also shared at an Investigators Meeting of recruiting centres in June 2019. Opportunities for discussion and suggestion of solutions was encouraged.
| Phase 1 theme | Potential solution(s) |
|---|---|
|
Top-up training for sites. |
|
No direct solution due to funding but highlight opportunities to sites to draw on any existing research infrastructure or explore through Research and Development departments to support access to Research Nurses, for example. |
|
An e-mail ‘Update’ on equipoise: reinforcing the need for the trial and incorporating findings from interviews to encourage equipoise will be drafted and disseminated to sites. Use as a prompt for discussion on PI teleconferences. |
|
Develop clinical vignettes for PIs to work through on teleconferences which highlight different parameters of eligibility and aid discussion. |
|
Solutions suggested for other barriers may help to address. |
|
Intrinsically linked to equipoise – address equipoise to help address working relationships. In addition, shared learning calls, which are already implemented, could help to address skills and competencies and relational aspects through team building. |
Phase 2 findings: Using a behavioural approach to explore the barriers and facilitators of REBOA recruitment and intervention delivery
Stage A: Identification of behaviourally focused recruitment challenges
Six of the 14 TDF domains, detailed in the Design overview section above, were considered relevant to both the processes of recruitment to the UK-REBOA trial (i.e. randomisation) and the processes of delivering the trial intervention (the deployment of the REBOA catheter), specifically: Skills; Environmental context and resources; Beliefs about capabilities; Beliefs about consequences; Social influences; and Memory, attention and decision processes. Thirty-eight belief statements were identified across these six TDF domains. The themes are presented below (with dominant TDF domains specified in brackets). Notably, some of the themes presented in this section overlapped with themes identified from Phase 1 of the process evaluation. However, categorising interview content (from both Phases 1 and 2) into the TDF, an established behavioural framework, allowed us to link identified factors to theory-informed potential solutions using established methods in the field of behavioural science.
Six themes were identified:
-
Skills required for successful recruitment and intervention delivery (TDF Domain: Skills): Recognising patients who may require REBOA was regarded as an essential skill which influenced both recruitment and intervention delivery, as well as the technical skills surrounding the deployment of REBOA. Staff reported concerns around maintaining these competencies due to the low frequency of eligible patients.
-
Environment, context and resources’ impact on recruitment and intervention delivery (TDF Domain: Environmental Context and Resources). Some indicated that a lack of staff available (notably those who could deliver REBOA) on a 24/7 basis deterred recruitment and intervention delivery in some instances. Other site staff indicated that the presence of dedicated Research Nurses and Clinical Fellows facilitated recruitment. The clinical context of REBOA was also regarded as stressful and fast-paced, which could sometimes act as a barrier to both recruitment and intervention delivery.
-
Beliefs about clinicians’ capabilities to deliver REBOA (TDF Domain: Beliefs about Capabilities): A lack of confidence was acknowledged by clinicians who were (or would have been) responsible for delivering REBOA. This was often related to the limited opportunities available to deliver REBOA outside of a simulated context.
-
Beliefs about the consequences of REBOA recruitment and intervention delivery (TDF Domain: Beliefs about Consequences): Many staff recognised the potential clinical benefits associated with the REBOA intervention as well as the institutional benefits associated with their involvement in the trial. However, most staff also acknowledged that the anticipated negative side effects of REBOA could intensify apprehension and inhibit staff from performing this high-risk procedure.
-
Social influences of REBOA recruitment and intervention delivery (TDF Domain: Social Influences): Mixed perspectives related to equipoise and trial patient eligibility among the team sometimes acted as barriers to recruitment and REBOA enactment. The content within this theme is also highlighted in the ‘Working Relationships’ and ‘Individual and Community Equipoise’ themes reported in Phase 1 findings.
-
Memory, attention and decision-making processes during the conduct of UK-REBOA trial delivery (TDF Domain: Memory, Attention and Decision-making Processes): Difficulties assessing patient eligibility resulted in observable discrepancies in decision-making across sites – namely the exact timing of randomisation. Dual acts of considering randomisation and intervention delivery within a stressful fast-paced setting also demanded significant mental resources.
An extended table containing the content and frequency of all TDF domains and associated belief statements is published in full elsewhere (Lawrie et al. 2022). 52
Stage B: Development and delivery of potential solutions to improve trial processes
The findings from Stage A provided diagnostic information relating to the core behavioural conduct challenges and informed the development of potential solutions that were designed to enhance recruitment and delivery of the REBOA intervention (Stage B). We identified 24 potential BCTs that could support UK-REBOA trial recruitment and clinical intervention delivery based on the barriers and facilitators highlighted in Stage A. Detailed descriptions of the solutions developed are described elsewhere. 52 Appendix 5 provides a thorough overview of the proposed solutions, first by the mode of delivery (i.e. via Training, Environmental Restructuring and/or Enablement), followed by the content of the proposed solutions, linked BCTs, beliefs statements to illustrate how the interview findings informed the solution development, and the APEASE assessment. While many of the identified barriers were actionable through development of targeted solutions, it is important to recognise that some barriers (such as the need for dedicated research nurses or clinical research fellows, or a 24/7 service to deliver the REBOA intervention) were not amenable to change within the bounds of the trial, and talk to wider infrastructure support costs for research more generally. Therefore, these challenges were not prioritised for solution development within the UK-REBOA trial.
The prioritised evidence-based potential solutions identified included a range of strategies designed to mitigate the barriers and maximise the facilitators identified from Stage A. Some of these strategies were already active within existing trial practices, such as prompt sheets that described recruitment and intervention delivery (targeting the TDF domain Memory, Attention and Decision-making processes). Findings also suggested staff could benefit from sharing existing mannequins across sites to facilitate rehearsal of the REBOA procedure and recruitment processes: this was proposed to mitigate issues related to maintaining the competencies to conduct randomisation and deliver REBOA (targeting the TDF domain Skills).
Other strategies developed from the evidence gathered during the process evaluation were also identified. These strategies were delivered within various settings – summarised below.
Trial meetings
The fortnightly, routine, PI conference calls were often structured to encourage discussion of issues related to randomisation, experiences of trial processes enacted (or not), anonymised case details about patient eligibility and procedural descriptions of recruitment/intervention delivery. As such, these meetings already incorporated strategies to improve recruitment and intervention delivery [BCTs such as social support (practical) and social comparison, targeting the TDF domain ‘Social Influences’]. The process evaluation team emphasised the value of these meetings to the local trial teams, and suggested methods to maximise BCTs within these PI meetings. This included encouraging PIs to prompt staff to proactively plan for any events that may occur unexpectedly on the basis of their past experiences (of randomisation and REBOA intervention delivery), as well as to consider solutions to overcome challenges that may arise in the future (incorporating the BCTs Action planning and Problem solving – targeting the TDF domain ‘Beliefs about capabilities’).
Findings from Phase 2 were also disseminated across three separate online meetings: two collaborative meetings held on 18 March 2021 and 26 October 2021, and a TSC meeting on 11 October 2021. The process evaluation team used these meetings as an opportunity to raise awareness of notable challenges related to trial recruitment and REBOA intervention delivery, as well as to gain feedback about the conduct/results of the evaluation. Meeting attendees were also encouraged to reflect on any improvements that could be made, based on the findings, to enhance recruitment and intervention delivery processes in the UK-REBOA trial.
Updates to training material and delivery
Training was adapted to incorporate a greater emphasis on staff contributions and the value of the research (i.e. the UK-REBOA trial) in potentially changing clinical practice. The content from the training slides and presentation scripts were reviewed to ensure they explicitly contained BCTs that would maximise recruitment and facilitate the delivery of REBOA.
E-mail/Twitter feedback on recruitment activity
Monthly recruitment updates which contained BCTs were distributed to site staff via e-mail. The process evaluation team worked with the local trial teams to ensure that the BCTs selected would support recruitment and intervention delivery at that site. These e-mail updates included information related to the number of patients that had been recruited at their site in relation to other sites, incorporating the BCTs ‘Social comparison’ and ‘Feedback on behaviour’. The support available to trial staff was also reinforced within the updates, by providing contact details of the Trial Manager, Co-CIs, and clinical training lead (including the BCT ‘Social Support, Practical’). This solution was modelled on audit and feedback interventions, which are a foundational component of quality improvement initiatives in clinical care and have been used in other large multicentre trials as a recruitment intervention targeting healthcare professionals.
The interview findings also supported the ongoing praising of staff for their efforts in the trial (applying the BCT ‘Social Reward’). Praise was communicated via Twitter/E-mail following a randomisation.
Development and implementation of an infographic
A bespoke infographic was produced that was designed to target mixed levels of equipoise among trauma teams (see Appendix 6). This was developed in liaison with the local trial staff, using the expertise from the process evaluation team to ensure it contained strategies that were primarily designed to mitigate the barriers around individual and community equipoise, but also more general views around the consequences of recruitment and intervention delivery (i.e. using BCTs that target the TDF domains ‘Beliefs about consequences’ and ‘Social influences’).
The infographic contained information that reinforced the purpose of the trial with information about the social and environmental consequences of trial recruitment/REBOA intervention delivery (incorporating the BCT ‘Information about social and environmental consequences’), as well as contact details of the clinical Co-CI and clinical training lead to indicate the support available [incorporating the BCTs social support (practical) and credible source]. The infographic was distributed by the trial office to all site staff involved in recruitment (via email) and was requested to be shared among other site staff involved in the trial (electronic and paper copies for sharing).
Discussion
The purpose of this process evaluation was to inform the delivery of the trial by investigating the barriers and facilitators of recruitment and REBOA intervention delivery. Phase 1 was designed to identify barriers promptly during trial initiation and set-up across the first active sites, whereas PhasOf the 90 participants enrolled, 46 were randomly assignede 2 focused on exploring barriers and facilitators of REBOA recruitment and intervention delivery when sites were more established. We applied a behavioural framework in Phase 2 to direct analysis and generate solutions designed to enhance trial practices using established methods from the behavioural science literature.
Embedded process evaluations often provide an opportunity to evaluate the challenges that can threaten trial rigour during the conduct of the study. Notably, Phase 2 of this process evaluation provides an example of how a behavioural science approach can be used to proactively implement strategies to address challenges, extending previous studies that have largely focused on identifying problems for trial recruitment and retention. 60–63 Our embedded process evaluation study therefore demonstrates that the incorporation of a behavioural approach to understanding trial processes provided practical advantages: understanding the underlying determinants that affected behaviour, attitudes and beliefs in the UK-REBOA trial provided an avenue to implement theoretically informed evidence-based solutions to potentially enhance trial practices.
Although elements of the trial were redesigned with the aim of enhancing recruitment and intervention delivery using techniques from behavioural science, it would have been useful to conduct a formal evaluation of the effectiveness of these techniques. Another potential limitation of this process evaluation was that we were unable to recruit more site staff to interview using a TDF-based topic guide. This was due to site staff capacity limitations during the COVID-19 pandemic. Therefore, data from Phase 2 (which combined interviewee responses from both phases of the evaluation) represented interview responses from questions within two separate topic guides. However, during the analysis, common TDF-based themes were identified throughout all of the interviews. This demonstrates the flexibility and relevance of applying the TDF within the analysis process when the interview questions may/may not be guided by the theoretical domains. 68 In addition, our sample comprised of individuals who were largely supportive of the REBOA intervention. It may have been insightful to target recruitment towards individuals who had reservations about their sites’ participation in the UK-REBOA trial and/or the intervention.
Conclusion
Both phases of this process evaluation revealed several barriers and facilitators to trial recruitment and intervention delivery in the UK-REBOA trial. Phase 2 highlighted the value of using a behavioural approach to adapt elements of a trial to optimise processes, including theoretically informed solutions that had the potential to proactively address recruitment and intervention delivery challenges.
Chapter 5 Baseline characteristics and procedural details
Recruitment
The first recruitment site was opened to recruitment on 30 October 2017. The first participant was recruited in January 2018. In total, 16 sites opened to recruitment, and 12 of these recruited participants to the UK-REBOA trial. The second interim analysis (which commenced on 9 March 2022) and included 80 participants, triggered one of the pre-specified stopping rules and recruitment to the trial was suspended on 16 March 2022. Ninety participants had been recruited overall by this time [the final 10 had not yet reached the follow-up time for the primary outcome measure (90-day mortality) and thus were ineligible to be included in the interim analysis]. The trial had originally planned to randomise 120 patients.
Impact of COVID-19 on recruitment
Recruitment had also been previously paused by sponsor on 18 March 2020 due to COVID-19. Sites were able to reopen to recruitment from July 2020; however, not all sites were able to reopen. The number of patients recruited, by sites, is shown in Appendix 7, Table 23 and Figure 26 show recruitment over time. The three highest recruiting sites were Leeds, Royal London Hospital and Birmingham.
Group allocation
Of the 90 participants enrolled, 46 were randomly assigned to a strategy of SC plus REBOA (SC + REBOA) and 44 to SC alone. Figure 6 shows the Consolidated Standards of Reporting Trialsdiagram for the UK-REBOA trial.
FIGURE 6.
Consolidated Standards of Reporting Trials diagram.
One of the participants allocated to the SC strategy chose not to continue to participate after 4 days, but data collected up until this point could be used. Outcome data for this participant are therefore available up to this time point (3-, 6- and 24-hour mortality) only.
Patient characteristics
Demographics, comorbidities, injury severity and injury pattern are shown in Table 7. The groups were well-matched in terms of age, gender, comorbidities, mechanism of injury and injury severity. ISS scores (calculated once all injuries have been identified) ranged from 0 to 75. A score of 0 indicates no injury, and a score of 75 represents injuries which are not usually compatible with survival. A score of > 15 is accepted as indicative of severe injury and major trauma, and a score of > 25 indicates very severe injury. The median ISS for the UK-REBOA trial was 41 (25th percentile 29, 75th percentile 50); the majority of participants were very severely injured. There were some differences in injury pattern, with a high AIS for the head region in participants allocated to the SC + REBOA arm.
| SC + REBOA N = 46 |
SC N = 44 |
|
|---|---|---|
| Demographics | ||
| Median age (Q1–Q3), years | 46 (33–62) | 39 (30–56) |
| Male sex, n (%) | 28 (61) | 34 (77) |
| Comorbidity | ||
| Median Charlson Comorbidity Index (Q1–Q3); n | 0 (0–1); 33 | 0 (0–1); 40 |
| Mechanism of injury | ||
| Blunt, n (%) | 44 (96) | 43 (98) |
| Penetrating, n (%) | 2 (4) | 1 (2) |
| Injury severity | ||
| Median ISS (Q1–Q3) | 41 (29–50) | 41 (29–50) |
| ISS band | ||
| Minor, n (%) | 0 | 1 (2) |
| Moderate, n (%) | 1 (2) | 1 (2) |
| Severe, n (%) | 7 (15) | 4 (9) |
| Very severe, n (%) | 38 (83) | 38 (86) |
| Injury pattern | ||
| AIS head, median (Q1–Q3) | 3 (0–4) | 0 (0–5) |
| AIS face, median (Q1–Q3) | 0 (0–2) | 0 (0–2) |
| AIS thorax, median (Q1–Q3) | 4 (3–4) | 4 (1–4) |
| AIS abdomen, median (Q1–Q3) | 2 (0–3) | 2 (0–4) |
| AIS spine, median (Q1–Q3) | 2 (0–2) | 2 (0–2) |
| AIS pelvis, median (Q1–Q3) | 2 (0–5) | 2 (0–5) |
| AIS limbs, median (Q1–Q3) | 2 (2–3) | 3 (2–3) |
| AIS other, median (Q1–Q3) | 0 (0–1) | 0 (0–1) |
Table 8 shows pre-hospital and ED vital signs and participant transport characteristics. Patients were profoundly hypotensive, but slightly more so in the SC + REBOA strategy group. This is attributable to the extreme urgency of the clinical presentation, highlighted by the fact that 10 (22%) in SC + REBOA and 11 (25%) in SC strategy experienced a traumatic cardiac arrest in the pre-hospital setting.
| SC + REBOA N = 46 |
SC N = 44 |
|
|---|---|---|
| Pre-hospital | ||
| Vital signs | ||
| SBP, mmHg | ||
| Median (Q1–Q3); n | 85 (66–120); 34 | 97 (71–128); 37 |
| ≤ 90 mmHg, n (%) | 18 (53) | 17 (46) |
| ≤ 70 mmHg, n (%) | 11 (32) | 9 (24) |
| Heart rate, bpm, median (Q1–Q3); n | 113 (94–133); 42 | 109 (77–133); 40 |
| Respiratory rate, b.r.p.m., median (Q1–Q3); n | 21 (12–30); 38 | 22 (16–30); 42 |
| Oxygen saturation, %, median (Q1–Q3); n | 88 (90–95); 32 | 92 (81–98); 43 |
| Glasgow Coma Scale, median (Q1–Q3); n | 10 (3–14); 42 | 10 (3–14); 42 |
| CPR | ||
| Yes, n (%) | 10 (22) | 11 (25) |
| No, n (%) | 33 (72) | 33 (75) |
| Missing, n (%) | 3 (7) | – |
| Method of transport | ||
| Helicopter, n (%) | 17 (37) | 21 (48) |
| Ambulance, n (%) | 22 (48) | 19 (43) |
| Ambulance and helicopter, n (%) | 6 (13) | 3 (7) |
| Missing | 1 (2) | 1 (2) |
| Time from injury to ED arrival | ||
| Minutes, median (Q1–Q3) | 90 (70–125); 39 | 97 (78–119); 41 |
| ED | ||
| Pre-alert issued,a n (%) | ||
| Yes | 40 (87) | 39 (89) |
| No | 3 (7) | 4 (9) |
| Missing | 3 (7) | 1 (2) |
| Massive haemorrhage protocol activated, n (%) | ||
| Yes | 40 (87) | 39 (89) |
| No | 6 (13) | 4 (9) |
| Missing | – | 1 (2) |
| Consultant present in ED, n (%) | ||
| Yes | 45 (98) | 44 (100) |
| Missing | 1 (2) | – |
| ED vital signs | ||
| SBP, mmHg | ||
| Median (Q1–Q3) | 84 (58–115); 44 | 99 (72–115); 42 |
| ≤ 90 mmHg, n (%) | 26 (59) | 19 (45) |
| ≤ 70 mmHg, n (%) | 18 (41) | 9 (21) |
| Heart rate, bpm, median (Q1–Q3); n | 105 (88–123); 45 | 120 (87–135); 43 |
| Respiratory rate, b.r.p.m., median (Q1–Q3); n | 20 (17–30); 38 | 20 (18–26); 40 |
| Oxygen saturation, %, median (Q1–Q3); n | 99 (90–100); 39 | 99 (95–100); 40 |
| Glasgow Coma Scale, median (Q1–Q3); n | 3 (3–11); 39 | 3 (3–15); 39 |
| CPR on arrival, n (%) | ||
| Yes | 4 (9) | 4 (9) |
| No | 36 (78) | 39 (89) |
| Missing | 6 (13) | 1 (2) |
For the ED arrival characteristics, participants were still hypotensive on arrival, although slightly more so in the SC + REBOA group. Otherwise, the groups were well matched.
Treatment received
Of the 46 participants allocated to the SC + REBOA strategy, 19 had the device inserted and inflated (Figure 7). The remaining 27 participants progressed to different time points along this pathway. In eight participants, arterial access was attempted but could not be established (R2); in three, arterial access was not attempted, because the participant had improved with other resuscitative measures (R1/C1); in nine, arterial access was achieved, but the REBOA device was not inserted, because the participant had improved with other resuscitative measures (R3/C1); in five, arterial access was achieved, and the device inserted, but the balloon not inflated, because the participant had improved with other resuscitative measures (R4/C1); and in two, arterial access was not attempted because the participant rapidly deteriorated (R1/C2).
FIGURE 7.
Treatment received.
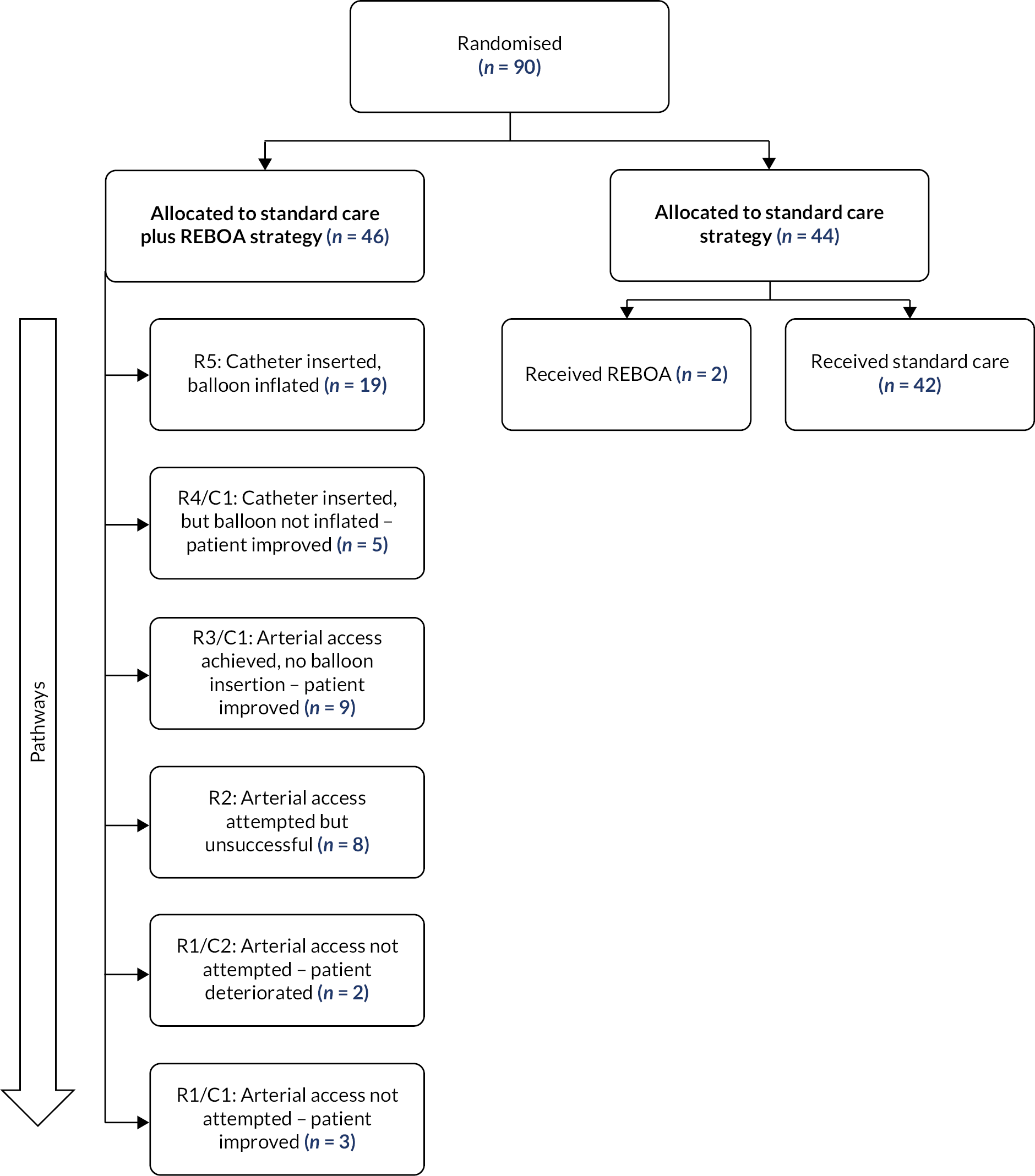
Of the 44 participants allocated to the SC strategy, 2 participants received SC + REBOA. In both participants, the device was inserted in the operating theatre.
Procedural details
Table 9 describes the technical aspects of the 21 REBOA insertions, 19 in SC + REBOA and 2 in SC. The method of arterial access was percutaneous [17 (89%) in SC + REBOA and 2 (100%) in SC] and on the right [13 (68%) in SC + REBOA and 2 (100%) in SC]. The median duration of balloon inflation was 29 minutes in SC + REBOA and 71 minutes in SC.
| SC + REBOA N = 46 |
SC N = 44 |
|
|---|---|---|
| Received REBOA, n | 19 | 2 |
| Arterial access | ||
| Prior femoral arterial line, n (%) | 5 (26) | 1 (50) |
| Method | ||
| Percutaneous, n (%) | 17 (89) | 2 (100) |
| Percutaneous followed by cutdown, n (%) | 2 (11) | – |
| Site | ||
| Left, n (%) | 5 (26) | – |
| Right, n (%) | 13 (68) | 2 (100) |
| Both, n (%) | 1 (5) | – |
| REBOA operator | ||
| Same as TTL, n (%) | 18 (95) | 2 (100) |
| Someone other than TTL, n (%) | 1 (5) | – |
| Sheath size | ||
| 7F, n (%) | 15 (79) | 2 (100) |
| 8F, n (%) | 3 (16) | – |
| 9F, n (%) | 1 (5) | – |
| Occlusion | ||
| Zone I, n (%) | 10 (53) | – |
| Zone III, n (%) | 9 (47) | 2 (100) |
| Partial REBOAa | ||
| No, n (%) | 11 (58) | 1 (50) |
| Yes, n (%) | 8 (42) | 1 (50) |
| Location of balloon deflation | ||
| ED, n (%) | 10 (53) | – |
| Operating theatre, n (%) | 5 (26) | 2 (100) |
| Died with balloon inflated, n (%) | 4 (21) | – |
| Time to balloon inflation (from ED arrival) | ||
| Minutes, median (Q1–Q3) | 32 (20–47) | 124 (32–216) |
| Duration of balloon inflation | ||
| Minutes, median (Q1–Q3) | 29 (19–64) | 71 (69–72) |
Chapter 6 Clinical results
In this chapter, we present the clinical results for the trial. Discussion of the clinical results will be presented in Chapter 9.
Primary outcome: 90-day mortality
The primary outcome of the trial was death within 90 days of injury.
Intention-to-treat-analysis
Of the 46 participants allocated to SC + REBOA strategy, 25 (54%) died within 90 days. Of the 43 SC participants for whom primary outcome data are available (1 participant decided against continued participation), 18 (42%) died (Table 10). Using the minimally informative prior, the OR for 90-day mortality was 1.58 (95% CrI 0.72 to 3.52). The posterior probability of an OR > 1 (i.e. that REBOA was harmful) was 86.9% (Figure 8).
| SC + REBOA N = 46 n (%) |
SC N = 44 n (%) |
Minimally informative prior | Elicited enthusiastic prior | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CrI | Posterior probability (%) of OR > 1 | OR | 95% (CrI) | Posterior probability (%) of OR > 1 | |||
| Primary outcome | ||||||||
| 90-day mortalitya | ||||||||
| Death within 90 days | 25 (54) | 18 (42) | 1.58 | (0.72 to 3.52) | 86.9 | 1.40 | (0.66 to 2.96) | 81.0 |
| Survived to 90 days | 21 (46) | 25 (58) | ||||||
| Secondary outcomes | ||||||||
| 3-hour mortality | ||||||||
| Death within 3 hours | 11 (24) | 2 (5) | 4.25 | (1.33 to 15.99) | 99.3 | 3.01 | (1.05 to 9.47) | 97.7 |
| Survived to 3 hours | 35 (76) | 42 (95) | ||||||
| 6-hour mortality | ||||||||
| Death within 6 hours | 13 (28) | 4 (9) | 3.14 | (1.13 to 9.76) | 98.6 | 2.48 | (0.95 to 6.82) | 96.6 |
| Survived to 6 hours | 33 (72) | 40 (91) | ||||||
| 24-hour mortality | ||||||||
| Death within 24 hours | 17 (37) | 10 (23) | 1.85 | (0.79 to 4.46) | 91.8 | 1.61 | (0.72 to 3.67) | 87.2 |
| Survived to 24 hours | 29 (63) | 34 (77) | ||||||
| In-hospital mortalitya | ||||||||
| Death while in hospital | 25 (54) | 18 (42) | 1.58 | (0.72 to 3.52) | 86.9 | 1.40 | (0.66 to 2.96) | 81.0 |
| Survived to discharge | 21 (46) | 25 (58) | ||||||
| 6-month mortalitya | ||||||||
| Death within 6 months | 25 (54) | 18 (42) | 1.58 | (0.72 to 3.52) | 86.9 | 1.40 | (0.66 to 2.96) | 81.0 |
| Survived to 6 months | 21 (46) | 25 (58) | ||||||
FIGURE 8.
Posterior probability of mortality (OR > 1).
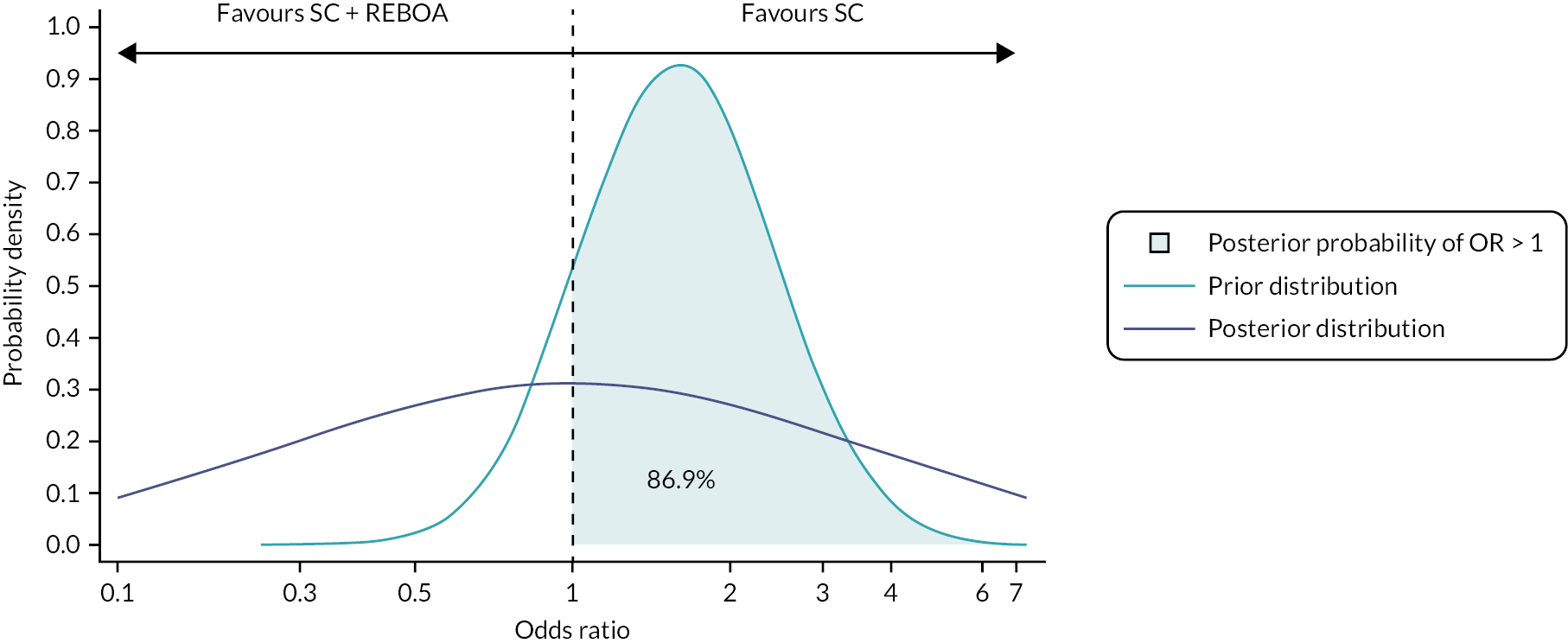
When using the elicited enthusiastic prior, the OR for 90-day mortality was 1.40 (95% CrI 0.66 to 2.96) with a posterior probability of an OR > 1 of 81.0%. The adjusted analyses for covariates (see Appendix 8, Table 24) and centre (see Appendix 8, Table 25) showed similar results.
Survival curves
Figure 9 shows the survival curves. There were more early deaths (within hours) in the SC + REBOA group, but deaths in this group also continued to 10 days.
FIGURE 9.
Survival curves.
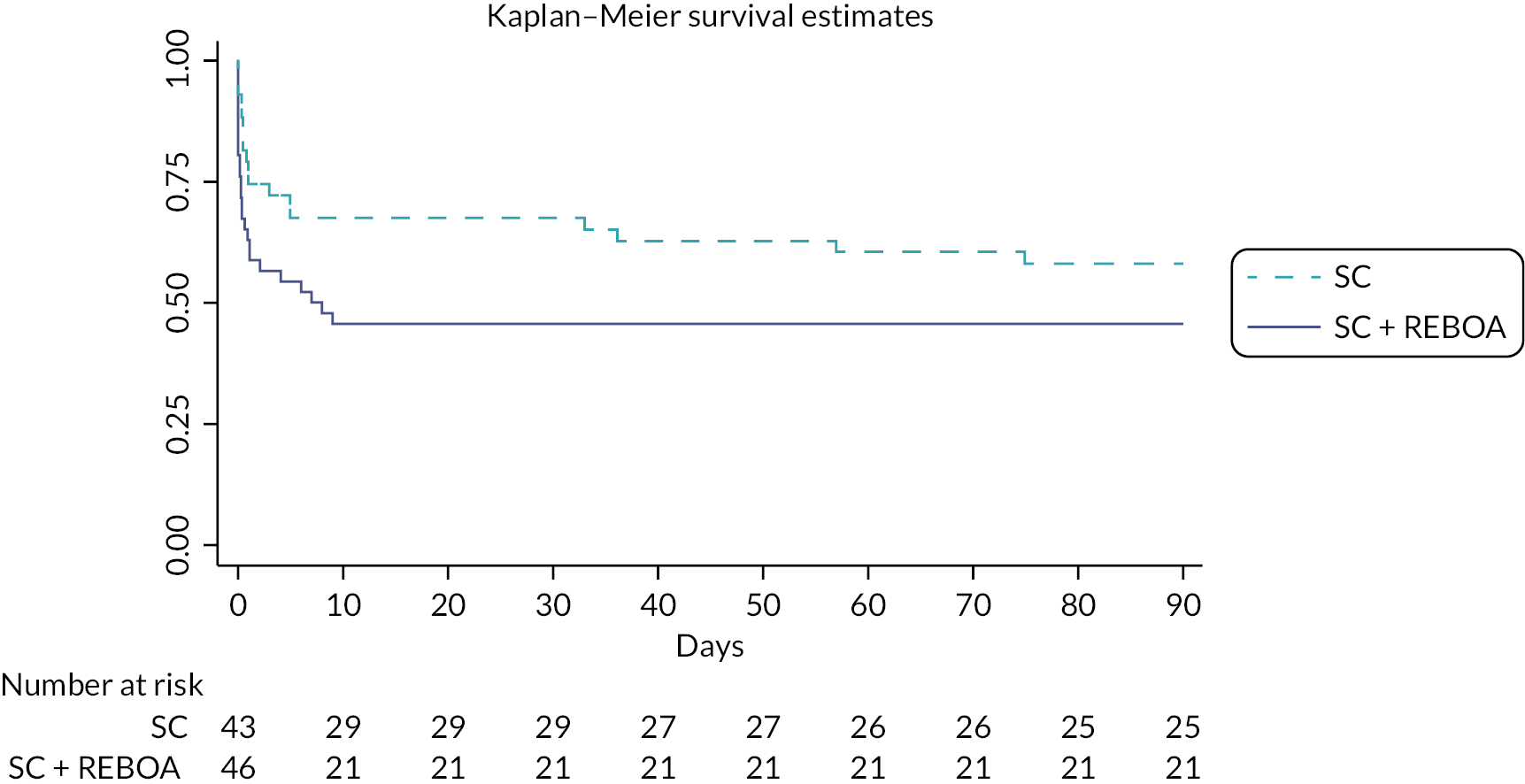
Secondary outcomes
Mortality at other time points
Mortality at 3, 6, and 24 hours, in-hospital and at 6 months is also shown in Table 10. For in-hospital and 6 months, the results were of the same order as 90-day mortality. For 3-, 6- and 24-hour mortality, however, there was an increased level of mortality in the SC + REBOA arm compared to SC with the greatest difference at 3 hours – 11 (24%) deaths in SC + REBOA compared with 2 (5%) deaths in SC, OR 4.25 95% CrI (1.33 to 15.99). For the adjusted analysis at other time points, the results were similar (see Appendix 8, Table 26–29). Appendix 8, Table 30 shows the learning curve analysis of excluding the first participant randomised to SC + REBOA from each site.
Cause of death
The causes of death, at different time points, are shown in Table 11. The cause of death was listed as ‘unknown’ when a clinician could not determine the cause of death. Typically, this occurred when a patient died very early, before any imaging or operations could be performed, and/or if the patient had injuries that resulted in bleeding, as well as other major injuries, such as a traumatic brain injury. Furthermore, for some patients, a coroner’s report (and therefore cause of death) was still pending at the time of analysis. (These reports can take months to years to be returned to hospitals.)
| SC + REBOA | SC | |
|---|---|---|
| Death within 3 hours, n | 11 | 2 |
| Bleeding | 6 (55) | – |
| Traumatic brain injury | 2 (18) | – |
| Unknown | 3 (27) | 2 (100) |
| Death within 6 hours, n | 13 | 4 |
| Bleeding | 7 (54) | 2 (50) |
| Traumatic brain injury | 3 (23) | – |
| Unknown | 3 (23) | 2 (50) |
| Death within 24 hours, n | 17 | 10 |
| Bleeding | 8 (47) | 2 (20) |
| Traumatic brain injury | 4 (24) | 5 (50) |
| Unknown | 5 (29) | 3 (30) |
| Death while in hospital, n | 25 | 18 |
| Traumatic brain injury | 9 (36) | 8 (44) |
| Bleeding | 8 (32) | 3 (17) |
| Multiorgan failure | 2 (8) | 3 (17) |
| Respiratory | – | 1 (6) |
| Spinal cord injury | 1 (4) | – |
| Unknown | 5 (20) | 3 (17) |
| Death within 90 days (primary outcome), n | 25 | 18 |
| Traumatic brain injury | 9 (36) | 8 (44) |
| Bleeding | 8 (32) | 3 (17) |
| Multiorgan failure | 2 (8) | 3 (17) |
| Respiratory | – | 1 (6) |
| Spinal cord injury | 1 (4) | – |
| Unknown | 5 (20) | 3 (17) |
| Death within 6 months, n | 25 | 18 |
| Traumatic brain injury | 9 (36) | 8 (44) |
| Bleeding | 8 (32) | 3 (17) |
| Multiorgan failure | 2 (8) | 3 (17) |
| Respiratory | – | 1 (6) |
| Spinal cord injury | 1 (4) | – |
| Unknown | 5 (20) | 3 (17) |
Table 11 shows that death due to haemorrhage was more common in the SC + REBOA strategy. This difference is apparent at all points, but is accounted for by deaths that occurred early (within 24 hours), after which time there were no further bleeding-related deaths in the SC + REBOA group. Although there were some early deaths due to traumatic brain injury, the overall number of deaths due to traumatic brain injury was similar in both groups.
Haemorrhage control procedures
Table 12 shows the proportion of participants that underwent operations, operations that involved haemorrhage control and the time from randomisation to commencement of such procedures. There were 14 (30%) participants in SC + REBOA and 19 (43%) in SC that underwent a haemorrhage control procedure (OR 0.60, 95% CrI 0.26 to 1.37). The mean time from admission to haemorrhage control procedure (minutes) was 42 [standard deviation (SD) 121] in SC + REBOA and 28 (SD 41) in SC (MD 14.41 95% CrI –22.80 to 52.20). The majority of the participants had a haemorrhage control laparotomy [7/14 (50%) in SC + REBOA and 12/19 (63%) in SC], with one participant having two haemorrhage control procedures.
| SC + REBOA n = 46 |
SC n = 44 |
Effect size | 95% CrI | |
|---|---|---|---|---|
| Haemorrhage control procedures | ||||
| Need for haemorrhage control procedure for all participants | ||||
| Yes, n (%) | 14 (30) | 19 (43) | 0.60 | (0.26 to 1.37) |
| No, n (%) | 32 (70) | 25 (57) | ||
| Time from randomisation to haemorrhage control procedure (minutes) | ||||
| Mean (SD); n | 42 (121); 44 | 28 (41); 44 | 14.41 | (−22.80 to 52.20) |
| Median (Q1–Q3) | 0 (0–42) | 0 (0–55) | ||
| Had an operation, n (%) | 31 (67) | 35 (80) | ||
| Haemorrhage control procedure for those that had operation, n (%) | 14 (45) | 19 (54) | ||
| Time from randomisation to haemorrhage control procedure for those that had a procedure (minutes) | ||||
| Mean (SD); n | 155 (197); 12 | 65 (40); 19 | ||
| Median (Q1–Q3) | 83 (56–156) | 64 (34–83) | ||
| Type of haemorrhage control procedures for those who had a haemorrhage control procedure, n (%) | ||||
| Haemorrhage control laparotomy | 7 (50) | 12 (63) | ||
| Extremity vascular ligation, shunting or repair | 2 (14) | 4 (21) | ||
| Pelvic packing | 4 (29) | 1 (5) | ||
| Angioembolisationa | 2 (14) | 2 (11) | ||
| Haemorrhage control thoracotomy | 1 (7) | – | ||
| Length of stay | ||||
| ICU stay (days) | ||||
| All patients | ||||
| Mean (SD); n | 7 (9); 45 | 15 (18); 44 | −8.58 | (−14.46 to −2.58) |
| Median (Q1–Q3) | 2 (0–9) | 5 (1–28) | ||
| Removing those with a length of stay of 1 day | ||||
| Mean (SD); n | 7 (10); 39 | 19 (18); 35 | −11.40 | (−17.88 to −4.77) |
| Median (Q1–Q3) | 4 (0–10) | 14 (3–33) | ||
| Hospital stay (days) | ||||
| All patients | ||||
| Mean (SD); n | 20 (26); 46 | 43 (54); 43 | −22.16 | (−39.53 to −4.71) |
| Median (Q1–Q3) | 8 (1–34) | 19 (1–63) | ||
| Removing those with a length of stay of 1 day | ||||
| Mean (SD); n | 34 (27); 27 | 61 (59); 33 | −26.31 | (−50.27 to −1.72) |
| Median (Q1–Q3) | 29 (9–48) | 38 (17–91) | ||
| Hospital- and ICU-free days | ||||
| ICU-free days (base 90 days) | ||||
| Mean (SD); n | 35 (40); 46 | 40 (37); 43 | −4.79 | (−20.75 to 11.31) |
| Median (Q1–Q3) | 0 (0–80) | 45 (0–78) | ||
| ICU-free days (base 6 months) | ||||
| Mean (SD); n | 78 (86); 46 | 94 (82); 43 | −14.46 | (−48.79 to 20.25) |
| Median (Q1–Q3) | 0 (0–173) | 138 (0–171) | ||
| Hospital-free days (base 90 days) | ||||
| Mean (SD); n | 22 (30); 46 | 41 (39); 43 | −18.58 | (−32.86 to −3.93) |
| Median (Q1–Q3) | 0 (0–49) | 41 (0–82) | ||
| Hospital-free days (base 6 months) | ||||
| Mean (SD); n | 64 (73); 46 | 69 (74); 43 | −3.32 | (−33.34 to 27.11) |
| Median (Q1–Q3) | 0 (0–142) | 23 (0–151) | ||
| Blood product and tranexamic acid use | ||||
| Red cell concentrate, units | ||||
| Mean (SD); n | 10 (9); 46 | 11 (9); 43 | 0.92 | (0.66 to 1.29) |
| Median (Q1–Q3) | 7 (4–12) | 9 (4–17) | ||
| Plasma, units | ||||
| Mean (SD); n | 8 (8); 46 | 11 (10); 43 | 0.73 | (0.49 to 1.08) |
| Median (Q1–Q3) | 6 (3–10) | 7 (4–18) | ||
| Platelets, pools | ||||
| Mean (SD); n | 1 (3); 46 | 2 (2); 43 | 0.87 | (0.50 to 1.52) |
| Median (Q1–Q3) | 1 (0–2) | 1 (0–2) | ||
| Cryoprecipitate, units | ||||
| Mean (SD); n | 2 (3); 46 | 2 (3); 43 | 0.79 | (0.41 to 1.53) |
| Median (Q1–Q3) | 0 (0–2) | 2 (0–3) | ||
| Tranexamic acid, grams | ||||
| Mean (SD); n | 1413 (580); 46 | 1568 (695); 44 | 0.90 | (0.70 to 1.16) |
| Median (Q1–Q3) | 1000 (1000–2000) | 2000 (1000–2000) | ||
| Extended GOS | ||||
| Mean (SD); n | 2 (2); 44 | 3 (2); 43 | 0.58 | (0.26 to 1.25) |
| Median (Q1–Q3) | 1 (1–4) | 3 (1–5) | ||
| Categories, n (%) | ||||
| One (death) | 24 (52) | 17 (39) | ||
| Two (persistent vegetative state) | – | – | ||
| Three (severe disability) | 5 (11) | 8 (18) | ||
| Four (moderate disability) | 8 (17) | 7 (16) | ||
| Five (good recovery) | 7 (15) | 11 (25) | ||
| Missing | 2 (4) | 1 (2) | ||
Length of stay
Table 12 shows length of ICU and hospital stay for all patients. The table also shows length of stay for only those patients who survived to ICU (i.e. excluding early deaths). For ICU the median time (days) spent for SC + REBOA was 2 (25th percentile 0, 75th percentile 9) and 5 (25th percentile 1, 75th percentile 28) for SC. For length of stay in hospital (days), the median time was 8 (25th percentile 1, 75th percentile 34) in SC + REBOA and 19 (25th percentile 1, 75th percentile 63) in SC. Due to death being a competing event, the competing risk analysis showed, for those in the SC + REBOA arm, a reduced length of stay (subhazard ratio of 0.75, 95% confidence interval 0.42 to 1.34) (see Appendix 8, Figure 27). In order to better account for the competing risk of death, we also calculated hospital-free and ICU-free days, as shown in Table 12.
Blood product use
Table 12 shows blood product and tranexamic acid use in the first 24 hours following injury. Overall, there was little difference between SC + REBOA and SC. For red cell concentrate, the median number of units was 7 (25th percentile 4, 75th percentile 12) in SC + REBOA and 9 (25th percentile 4, 75th percentile 17) in SC.
Functional outcome
For the functional outcome, GOS-E, the mean score was 2 (SD 2) in SC + REBOA and 3 (SD 2) in SC (proportional OR 0.58 95% CrI 0.26 to 1.25) (see Table 12). The GOS-E ranges from one (death) to five (good recovery). Table 12 also shows these categories with majority of participants being under category one [death; 24/46 (52%) in SC + REBOA and 17/43 (39%) in SC].
Complications
In the SC + REBOA group, the number of participants with a complication (excluding death) was 6 (13%) and 10 (23%) in SC group (OR 0.54 95% CrI 0.19 to 1.48) (Table 13). There were no device-related AEs.
| SC + REBOA N = 46 |
SC N = 43a |
|
|---|---|---|
| Complications | ||
| Yes | 6 (13) | 10 (23) |
| No | 40 (87) | 33 (76.7) |
| Number of complications | ||
| One | 3 (50) | 5 (50) |
| Two | 2 (33) | 4 (40) |
| Three | 1 (17) | 1 (10) |
| Details | ||
| Access-related | ||
| Pseudoaneurysm | 2 (33) | 1 (10) |
| Distal embolism | 1 (17) | 1 (10) |
| External haemorrhage at insertion site | 1 (17) | – |
| Arteriovenous fistula | – | 1 (10) |
| Extremity ischaemia | 1 (17) | – |
| Need for patch angioplasty (surgical repair) | 1 (17) | – |
| AEs related to external thoracic/abdominal aortic occlusion | ||
| Lung injury/bronchopleural fistula | – | 1 (10) |
| Infection requiring antibiotics only | – | 1 (10) |
| AEs related to impaired perfusion | ||
| Acute kidney injury requiring renal replacement therapy | 3 (50) | 5 (50) |
| Multiorgan failure | 1 (17) | 5 (50) |
| Acute respiratory distress syndrome | – | 1 (10) |
Additional analyses
As discussed in the methods (see Chapter 2), a number of patients who were randomised to REBOA did not proceed to have full balloon occlusion, for a variety of clinical reasons (intercurrent events e.g. patients improved so REBOA no longer indicated; patient deteriorated so REBOA no longer possible). These patients are not ‘cross-overs’ or true non-compliers, but reside on a spectrum of how far a patient has progressed down the REBOA-strategy pathway. As previously discussed, we had identified two additional analyses to accommodate for these intercurrent events via CACE analysis:
Complier average causal effect analysis 1
Question: ‘Does a strategy that includes REBOA (in addition to standard MTC care) reduce the mortality of exsanguinating trauma patients; when there is no technical failure, and when patients’ clinical condition did not change (improve or deteriorate)?'
As noted in Chapter 2, CACE ‘compliance’ (with the caveats regarding the terminology noted in Chapter 2) in the SC + REBOA arm was defined as patients who were classified as R5 (catheter inserted, balloon inflated) and ‘non-compliance’ as all others (i.e. any patient in whom the balloon was not inflated, whether due to technical failure or changes in the patient’s condition). In the SC group, the two patients who had REBOA were classified as ‘non-compliance’ and all other patients were regarded as ‘compliers’. Appendix 8, Table 31 shows the baseline characteristics of these groups. As expected, there are differences between ‘compliers’ and ‘non-compliers’. Table 14 shows the results of the CACE analysis, for all mortality time points. This shows that, even when inability to cannulate and changes in patients’ clinical condition are taken into consideration, the use of REBOA was associated with increased odds of mortality.
| SC + REBOA N = 46 | SC N = 44 | OR | 95% CrI | Posterior probability (%) of OR > 1 | |||
|---|---|---|---|---|---|---|---|
| Complied N = 20 | Did not comply N = 26 | Complied N = 42 | Did not comply N = 2 | ||||
| N = 19 | N = 26 | N = 41 | N = 2 | ||||
| Death within 90 days | |||||||
| Yes | 13 (68) | 12 (44) | 17 (41) | 1 (50) | 4.25 | (0.41 to 45.07) | 88.9 |
| No | 6 (32) | 15 (56) | 24 (59) | 1 (50) | |||
| Death within 6 months | |||||||
| Yes | 13 (68) | 12 (44) | 17 (41) | 1 (50) | 4.25 | (0.41 to 45.07) | 88.9 |
| No | 6 (32) | 15 (56) | 24 (59) | 1 (50) | |||
| Death while in hospital | |||||||
| Yes | 13 (68) | 12 (44) | 17 (41) | 1 (50) | 4.25 | (0.41 to 45.07) | 88.9 |
| No | 6 (32) | 15 (56) | 24 (59) | 1 (50) | |||
| N = 20 | N = 26 | N = 42 | N = 2 | ||||
| Death within 24 hours | |||||||
| Yes | 8 (42) | 9 (33) | 10 (24) | – | 6.59 | (0.53 to 91.96) | 92.8 |
| No | 11 (58) | 18 (67) | 32 (76) | 2 (100) | |||
| Death within 6 hours | |||||||
| Yes | 7 (37) | 6 (22) | 4 (10) | – | 48.28 | (1.88 to 2009.68) | 99.1 |
| No | 12 (63) | 21 (78) | 38 (90) | 2 (100) | |||
| Death within 3 hours | |||||||
| Yes | 5 (26) | 6 (22) | 2 (5) | – | 234.20 | (4.32 to 72,295.55) | 99.8 |
| No | 14 (74) | 21 (78) | 40 (95) | 2 (100) | |||
Complier average causal effect analysis 2
Question: ‘Does a strategy that includes REBOA (in addition to standard MTC care) reduce the mortality of exsanguinating trauma patients; when there is no technical failure?'
For the purpose of this analysis, in the SC + REBOA arm, we defined ‘compliance’ for the CACE analysis (with the caveats regarding the terminology noted in Chapter 2) as patients who were classified as anything other than R2 (arterial access attempted, but unsuccessful) and ‘non-compliance’ as all patients classified as R2. In the SC group, the two patients who had REBOA were classified as ‘non-compliance’ and all other patients were regarded as ‘compliers’. Appendix 8, Table 32 shows the baseline characteristics of these groups. As expected, there are differences between ‘compliers’ and ‘non-compliers’. Table 15 shows the results of the CACE analysis, for all mortality time points which showed that, even when the patients in whom cannulation was not possible is taken into account, the use of REBOA was associated with increased odds of mortality.
| SC + REBOA N = 46 | SC N = 44 | OR | 95% CrI | Posterior Probability (%) of OR > 1 | |||
|---|---|---|---|---|---|---|---|
| Complied N = 36 | Did not comply N = 10 | Complied N = 42 | Did not comply N = 2 | ||||
| N = 36 | N = 10 | N = 41 | N = 2 | ||||
| Death within 90 days | |||||||
| Yes | 18 (50.0) | 7 (70.0) | 17 (41) | 1 (50) | 2.07 | (0.64 to 6.72) | 88.9 |
| No | 18 (50.0) | 3 (30.0) | 24 (59) | 1 (50) | |||
| Death within 6 months | |||||||
| Yes | 18 (50.0) | 7 (70.0) | 17 (41) | 1 (50) | 2.07 | (0.64 to 6.72) | 88.9 |
| No | 18 (50.0) | 3 (30.0) | 24 (59) | 1 (50) | |||
| Death while in hospital | |||||||
| Yes | 17 (47.2) | 7 (70.0) | 17 (41) | 1 (50) | 2.07 | (0.64 to 6.72) | 88.9 |
| No | 19 (52.8) | 3 (30.0) | 24 (59) | 1 (50) | |||
| N = 37 | N = 9 | N = 42 | N = 2 | ||||
| Death within 24 hours | |||||||
| Yes | 24 (66.7) | 5 (50.0) | 10 (24) | - | 2.59 | (0.73 to 9.79) | 93.1 |
| No | 12 (33.3) | 5 (50.0) | 32 (76) | 2 (100) | |||
| Death within 6 hours | |||||||
| Yes | 9 (25.0) | 4 (40.0) | 4 (10) | - | 6.88 | (1.37 to 45.11) | 99.1 |
| No | 27 (75.0) | 6 (60.0) | 38 (90) | 2 (100) | |||
| Death within 3 hours | |||||||
| Yes | 7 (19.4) | 4 (40.0) | 2 (5) | - | 14.78 | (2.02 to 240.52) | 99.7 |
| No | 29 (80.6) | 6 (60.0) | 40 (95) | 2 (100) | |||
As-treated (safety) analysis
The results of the as-treated (safety) analysis are shown in Appendix 8, Tables 33 and 34.
Chapter 7 Costs and quality-adjusted life-years over 6 months’ follow-up
Background
The purpose of this chapter is to descriptively summarise the resource use, costs, life-years and QALYs for UK-REBOA trial participants. We report costs and economic outcomes for REBOA added to standard of care (SC + REBOA), compared to SC alone over a follow-up period of 6 months post randomisation from a UK NHS perspective. As with the trial clinical analyses, we adopted a Bayesian framework of analysis and the base-case results are presented from an ITT analysis.
Objectives
The primary economic objective of the UK-REBOA trial was to evaluate the lifetime incremental cost-effectiveness (cost-per-QALY) for the SC + REBOA versus SC alone from a UK NHS perspective. The secondary economic objectives were to measure the total healthcare cost, quality of life (using the EQ-5D-5L mapped to EQ-5D-3L), life-years, and incremental cost per QALY and per life-year at 6 months post randomisation. A health economics analysis plan (HEAP), v1.0, was developed prior to the analysis of the trial data (see additional files www.fundingawards.nihr.ac.uk/award/14/199/09; accessed June 2024).
Important amendments to the pre-specified health economics analysis plan
Given that the clinical results of the trial showed that REBOA leads to increased mortality (see Chapter 6), reporting incremental cost-effectiveness ratios (ICERs) as pre-specified in v1.0 of the HEAP will not provide any additional meaningful information for decision-makers. Results of cost-effectiveness conclusions or estimates of the ICER could never practically lead to the ethical adoption of REBOA in this setting, therefore presenting ratios would be non-informative regardless of the magnitude of cost savings that might be achieved through early mortality. We therefore have not reported ICERs and instead have focused reporting on the estimates of costs, life-years and QALYs separately. These estimates of resource use and utilities may be useful and informative for future economic evaluations in trauma care and are therefore reported in as much detail as possible to facilitate future use of the parameters. Other than the amendments to the analysis plan described here, the health economics analysis follows the pre-specified HEAP v1.0.
Methods
Resource use and costs – index hospitalisation
Resuscitative endovascular balloon occlusion of the aorta refers to the insertion of a balloon, usually through the femoral artery. REBOA intends to obstruct blood flow upon inflation. The pragmatic trial design allowed any REBOA device to be used. It is assumed that there are no additional staff resources required to administer REBOA and that the skills to deliver REBOA would already be available within the MTC team and would be incorporated into their workload.
Typically, in major trauma, a multidisciplinary trauma team will assemble prior to the patient’s arrival and will meet with the ambulance crew. We have therefore developed an assumed staff mix of the trauma team which was combined with Personal Social Services Research Unit (PSSRU) cost per working hour to calculate the cost per hour of trauma team staff. 69 This was added to the cost of overheads of the ED sourced from Public Health Scotland (PHS), based on data from Scottish MTCs. 70 We applied the cost of the trauma team and ED overheads from arrival (randomisation) through to the patient’s transfer to an operating theatre, death or exit from the ED, whichever happens first. The volume of blood products required for transfusion was sourced from TARN data linkage for trial participants, and unit costs were obtained from the literature and uplifted to 2020–1 prices. 71
The complexity of the treatment required for these injuries means that standard NHS reference costs may under estimate the true opportunity cost of treatment, particularly as such patients require the use of multidisciplinary trauma teams, large blood transfusions, multiple operative procedures and lengthy stays in critical care and on hospital wards. We therefore costed individual components of resource and summed these component costs to generate a total cost for the whole initial hospitalisation admission period. Total NHS resource use for the index hospitalisation was obtained from patient-level data in TARN and the key resource use variables for costing include time of arrival, time of ED departure, time of first operation, time of death/discharge, number and type of operative procedures and volume of blood transfusions that were required.
Hospital resource use was reported and costed per unit of activity (hour, minute, day) using national average unit costs reported by PHS. 70 Scottish unit costs were used because they provide a greater level of detail in costs than the published English costing sources and were therefore more appropriate for a component costing approach. These included the direct and indirect costs for the entirety of the participants’ stay in hospital (in theatre, in the ward and ICU). For operative costs, we applied national average unit costs based on the primary specialty in which the procedure falls. Duration of each operative procedure was not available from TARN. We therefore categorised each procedure as likely to be short (up to 2 hours), medium (2–4 hours) or long (4–6 hours). This categorisation was based on clinical expert judgement of the trial CI. While there is inevitably variability in the duration of each operative procedure, the approach allows an allocation of costs that broadly reflects duration and specialty of different procedures.
The cost of time in types 1 (e.g. general ward), 2 (e.g. HDU) and 3 (ICU/critical care) wards were calculated using the duration of stay in each department and the average direct and allocated cost per day sourced from PHS. 70
Details of all unit costs applied for the index hospitalisation are summarised in Table 16.
| Resource use item | Units | Unit cost (GBP) | Year | Source and notes |
|---|---|---|---|---|
| Devices and diagnostics | ||||
| REBOA device | Per participant | £1825 | 2022 | Personal communication with REBOA supplier, May 2022. |
| CT scan | Per scan | £84 | 2019–20 | Uplifted to 2020–1 prices (£87) using PSSRU inflation indices. Data and intelligence. Expenditure and activity – radiology services, hospital cost breakdown (R120). PHS.70 |
| Blood products | ||||
| Red blood cells | Per unit | £49 | 2014–5 | Uplifted to 2020–1 prices (£54) using PSSRU inflation indices.71 |
| Platelets | Per unit | £58 | 2014–5 | Uplifted to 2020–1 prices (£65) using PSSRU inflation indices.71 |
| Fresh-frozen plasma | Per unit | £38 | 2014–5 | Uplifted to 2020–1 prices (£42) using PSSRU inflation indices.71 |
| Cryoprecipitate | Per unit | £49 | 2014–5 | Uplifted to 2020–1 prices (£55) using PSSRU inflation indices.71 |
| Trauma team staff costs | ||||
| Consultant: medical | Per working hour | £123 | 2020–1 | Jones and Burns, 202169 |
| Consultant: surgical | Per working hour | £122 | 2020–1 | Jones and Burns, 202169 |
| Associate specialist | Per working hour | £120 | 2020–1 | Jones and Burns, 202169 |
| Nurse consultant (Band 8a) | Per working hour | £70 | 2020–1 | Jones and Burns, 202169 |
| Modern matron (Band 8a) | Per working hour | £70 | 2020–1 | Jones and Burns, 202169 |
| Nurse advanced–team manager (Band 7) | Per working hour | £62 | 2020–1 | Jones and Burns, 202169 |
| Nurse specialist–team leader (Band 6) | Per working hour | £51 | 2020–1 | Jones and Burns, 202169 |
| Nurse (Band 5) | Per working hour | £41 | 2020–1 | Jones and Burns, 202169 |
| Clinical support worker higher level nursing (Band 3) | Per working hour | £29 | 2020–1 | NHS. Agenda for change – payrates. 2021. Jones and Burns, 202169 |
| Total cost of trauma team per hour (13 staff) | Per working hour | £1784 | 2020–1 | 3× Consultant medical, 1× Consultant surgical, 2× Associate specialists, 1× Band 8a nurse, 1× Band 7 nurse, 3× Band 6 nurse, 1× Band 5 nurse, 1× Band 3 nurse. |
| Hospital department | ||||
| ED | Per hour | £21 | 2019–20 | Uplifted to 2020–1 prices (£22) using PSSRU inflation indices. Includes allocated and laboratory costs. Weighted average by discharges of Scottish hospitals within the major trauma network. Data and intelligence. Specialty group costs – inpatients in all specialties (excluding long stay), A&E (R040). PHS.70 |
| ICU (level 3 ward)b | Per day | £3104 | 2019–20 | Uplifted to 2020–1 prices (£3200, £1968, £1446, £1412, £966, £787 for ICU, neurosurgery, HDU, plastic surgery, general surgery and orthopaedic surgery, respectively) using PSSRU inflation indices. Includes: direct cost per case excluding theatre costs divided by the specialty average length of stay weighted by discharges of Scottish MTCs.a |
| Neurosurgery ward (level 1)b | Per day | £1909 | 2019–20 | |
| HDU (level 2 ward)b | Per day | £1403 | 2019–20 | |
| Plastic surgery ward (level 1)b | Per day | £1370 | 2019–20 | Data and intelligence. Specialty group costs – inpatients in all specialties (excluding long stay) (R040). PHS.70 |
| General surgery ward (level 1)b | Per day | £937 | 2019–20 | |
| Orthopaedic surgery ward (level 1)b | Per day | £763 | 2019–20 | |
| Theatre | ||||
| Dental | Per hour | £1114 | 2019–20 | Uplifted to 2020–1 prices (£1148, £1353, £1319, £1323, £2107, £1426, £1347, £1411, £1316, £1197, respectively) using PSSRU inflation indices. Includes: average direct cost per hour of theatre across Scotland plus allocated costs per hour of theatre (£241) weighted by activity of Scottish MTCsa |
| General surgery (excluding vascular surgery) | Per hour | £1313 | 2019–20 | |
| Gynaecology | Per hour | £1280 | 2019–20 | |
| Maxillofacial surgery | Per hour | £1283 | 2019–20 | Data and intelligence. Theatre – direct cost per hour, by specialty (R142X). PHS. |
| Neurosurgery | Per hour | £2044 | 2019–20 | |
| Orthopaedics | Per hour | £1383 | 2019–20 | Data and intelligence. Theatre services (R140). PHS.70 |
| Plastic surgery and burns | Per hour | £1307 | 2019–20 | |
| Thoracic surgery | Per hour | £1369 | 2019–20 | |
| Urology | Per hour | £1277 | 2019–20 | |
| Vascular surgery | Per hour | £1161 | 2019–20 | |
Resource use and costs – discharge to 6 months’ follow-up
Secondary care contacts and episodes of care that were commenced between the date of discharge from the index hospitalisation through 6 months post randomisation were sourced, where available, through linkage of patient records to the HES database. The HES database provides information on a variety of secondary care contacts including inpatient admitted patient care, critical care admissions, outpatient consultations and use of accident and emergency (A&E) services and includes secondary care rehabilitation service usage. The database provides the Healthcare Resource Groups (HRGs) and speciality codes that enable mapping of each secondary care contact to the appropriate NHS unit cost. Unit costs are obtained from NHS reference costs for 2020–1.
We had pre-planned a sensitivity analysis that would apply NHS reference costs to the index hospitalisation stay based on HES data from NHS Digital. However, this analysis was not able to be conducted because data linkage, where it was possible to identify the index hospitalisation, was only possible for 39/90 (43%) patients randomised to the study. NHS Digital agreed to provide data linkage only for participants who provided patient or consultee consent (56/90) and of those 56 with consent, data linkage was only available for 39 participants. This was despite the process of obtaining consent being approved by ethics and being conducted in accordance with the trial protocol. Of the remaining 51 patients who were not linked, 37 died during their index hospitalisation and so zero costs were imputed. In total, follow-up costs were available for 76 trial participants. Data availability following the NHS Digital data linkage process is summarised in Figure 10.
FIGURE 10.
NHS Digital data linkage.
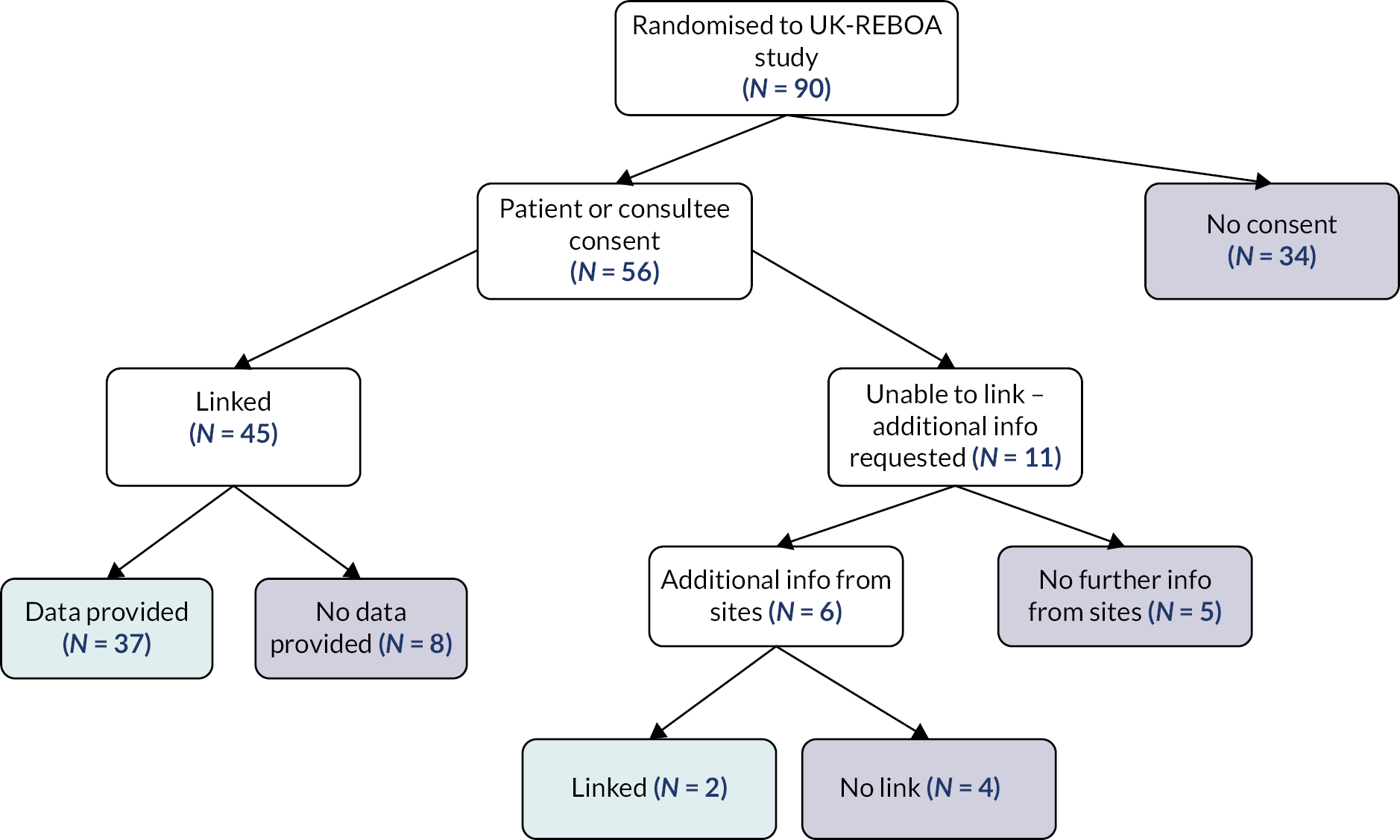
All costs are reported from a UK NHS perspective in Great British pounds (GBP; year 2020–1). Healthcare resource use is reported descriptively for each arm of the study, as n (%) for categorical data such as number of procedures and mean (SD) for continuous data (such as length of stay). Total per-participant costs (resource use × unit costs) are reported as mean (SD) for each arm of the trial from index hospitalisation to 6-month follow-up. Incremental costs for SC + REBOA compared to SC alone are estimated using Bayesian generalised linear regression models, with non-informative priors. The most appropriate distributional family and link function for cost data was determined to be a gamma family based on a Parks test, with an identity link. The gamma model accounts for the non-normality of cost data (i.e. a small proportion of participants with lengthy hospital stays and very high NHS costs). Regression models were adjusted for age and gender covariates.
Life-year and quality-adjusted life-year outcomes
EuroQol Group’s 5-dimension health status 5-level questionnaire data were available from the TARN data set at two follow-up points. The first is administered through TARN prior to the patient’s discharge from their index hospitalisation for major trauma care. The second is administered as a postal questionnaire through a third-party provider at 6 months post admission. Given that there is no generally accepted valuation set for the EQ-5D-5L, we generated utilities by first cross-walking the raw EQ-5D-5L data to the 3L version and applying UK general population tariffs to generate health state utilities. Due to the severity of injury sustained by participants in this trial, it was assumed that all patients were unconscious at the point of randomisation and were therefore assigned an EQ-5D-3L utility value of −0.402 at baseline. Participants who died during the study follow-up period were assigned a utility value of 0 from the date of death until the end of follow-up. These utility scores were then used to calculate the participant’s QALYs over the observed 6-month period using the area under the curve (AUC) approach. The AUC approach assumes a linear change in utility between the time points measured. Incremental life-years and incremental QALYs were estimated using Bayesian ordinary least squares regression models as the data appear to be normally distributed. Life-year and QALY regressions are adjusted for age and gender covariates.
Results
Costs
Tables 17 and 18 detail the results of the resource use and costs generated for the index hospitalisation using the component costing approach. The main driver of cost is the length of stay in hospital in general and particularly in critical care. Together, total hospital length of stay costs account for approximately 80% of the total index admission costs. Due to the larger number of earlier deaths in the SC + REBOA group, key cost drivers of critical care and hospital length of stay are substantially lower in the SC + REBOA group compared to SC.
| Item | SC + REBOA | SC | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Time in ED (minutes)b | 45 | 263.02 | 307.67 | 43 | 261.40 | 295.52 |
| Number of operations (n) | 45 | 3.13 | 5.43 | 43 | 5.65 | 8.16 |
| Length of stay in critical care (days) | 45 | 6.60 | 9.32 | 43 | 14.72 | 17.53 |
| Total length of stay (days) | 45 | 18.42 | 24.80 | 43 | 41.52 | 53.78 |
| SC + REBOA | SC | |||||
|---|---|---|---|---|---|---|
| Item | Mean (£) | SD (£) | N | Mean (£) | SD (£) | N |
| REBOA device costs | 852 | 921 | 45 | 85 | 389 | 43 |
| Trauma team | 4761 | 5569 | 45 | 4731 | 5349 | 43 |
| ED overheads | 96 | 113 | 45 | 96 | 108 | 43 |
| ED CT scan | 87 | 49 | 45 | 103 | 44 | 43 |
| ED Blood products | 513 | 436 | 45 | 547 | 446 | 43 |
| Operations | 14,054 | 19,852 | 45 | 23,311 | 32,058 | 43 |
| Level 3 ward (critical care) | 21,262 | 34,077 | 45 | 53,767 | 62,836 | 43 |
| Level 2 ward | 8788 | 17,573 | 45 | 25,319 | 47,296 | 43 |
| Level 1 ward | 7067 | 16,730 | 45 | 8201 | 22,212 | 43 |
| Total ward costs | 37,117 | 50,505 | 45 | 87,287 | 102,884 | 43 |
| Total index hospitalisation costs | 57,384 | 62,863 | 45 | 116,064 | 128,957 | 43 |
Table 19 shows that, over 6-month follow-up, SC + REBOA remains substantially less costly than SC alone, due to the competing risk of mortality. The overall finding was robust to whether models were adjusted for age, sex or ISS score.
| Item | SC + REBOA | SC | Unadjusted mean difference (95% CrI) |
||||
|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | N | Mean (£) | SD (£) | N | ||
| Index admissiona | 57,384 | 62,863 | 45 | 116,064 | 128,957 | 43 | −£61,205 (−£106,881 to −£22,297) |
| Hospital costs for episodes of care commenced between index admission discharge and 6 months post injury | |||||||
| Inpatient stay | 10,647 | 32,157 | 40 | 4075 | 7090 | 36 | + £6800 (+ £3379 to + £11,222) |
| Outpatient attendance | 954 | 1432 | 40 | 1309 | 1762 | 36 | −£397 (−£1020 to + £123) |
| Critical care admission | 0 | – | 40 | 0 | – | 36 | -– |
| Total costs discharge to 6 months | 11,601 | 32,417 | 40 | 5385 | 7849 | 36 | + £6566 (+ £2707 to + £11,042) |
| Total NHS costs (non-adjusted) b | 59,049 | 70,983 | 40 | 91,980 | 100,403 | 36 | −£35,470 (−£75,616 to + £424) |
| Total NHS costs (adjusted for age and gender) | – | – | – | – | – | – | −£21,997 (−£65,912 to + £13,193) |
| Total NHS costs (adjusted for age, gender and ISS) | – | – | – | – | – | – | −£20,949 (−£50,705 to + £11,206) |
Quality-adjusted life-years
EuroQol Group’s 5-dimension data collected within the study are presented descriptively in Figures 11–14, in accordance with EuroQol reporting recommendations. 72 Data were available and are reported for N = 29 (SC + REBOA = 15; SC = 14) survivors at discharge and for N = 20 survivors at 6 months (SC + REBOA = 10; SC = 10). Given imputation of 0 utilities for participants who died, it was possible to derive QALYs for N = 57/90 (63%) participants. Data completeness for EQ-5D-5L was lower than expected. This was driven in part to a large proportion of missing data from TARN’s partner provider at 6 months, but also missed TARN data collection prior to discharge. The available data from TARN were supplemented with additional efforts of the trial office to collect further EQ-5D-5L data through participating sites. Life-year gains (LYGs), utilities and QALYs calculated for each arm of the trial are reported in Table 20.
FIGURE 11.
Proportion of respondents reporting any problems at hospital discharge.

FIGURE 12.
Proportion of respondents reporting any problems at 6 months.

FIGURE 13.
Proportion of respondents reporting severe problems at hospital discharge.
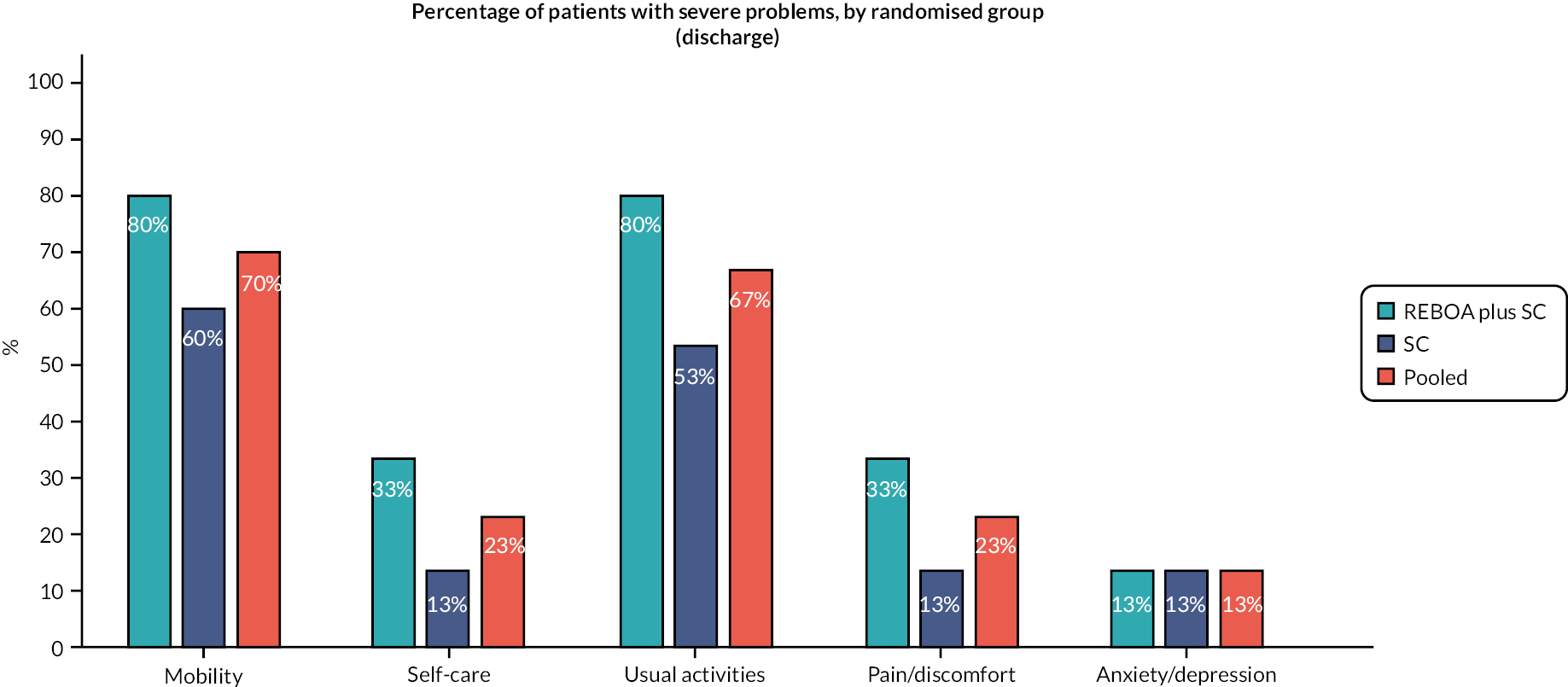
FIGURE 14.
Proportion of respondents reporting severe problems at hospital 6 months.

| Item | SC + REBOA | SC | Mean difference (95% CrI) |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||
| Life-years | |||||||
| Life-years gained | 0.232 | 0.247 | 45 | 0.305 | 0.236 | 43 | −0.074 (−0.175 to + 0.030) |
| Quality of life (EQ-5D a health state utilities) among survivors | |||||||
| EQ-5Da utility at baseline | −0.402 | – | – | −0.402 | – | – | |
| EQ-5Da utility at discharge | 0.147 | 0.339 | 15 | 0.388 | 0.349 | 14 | |
| EQ-5Da utility at 6 months | 0.188 | 0.399 | 10 | 0.538 | 0.296 | 10 | |
| Quality of life (EQ-5D a health state utilities) with 0s imputed for death | |||||||
| EQ-5Da utility at discharge | 0.057 | 0.218 | 39 | 0.181 | 0.305 | 30 | |
| EQ-5Da utility at 6 months | 0.055 | 0.226 | 34 | 0.192 | 0.313 | 28 | |
| QALYs (baseline –0.402) | |||||||
| Unadjusted | 0.014 | 0.065 | 33 | 0.042 | 0.109 | 24 | −0.029 (−0.075 to + 0.017) |
| Adjusted age and gender | −0.024 (−0.070 to + 0.025) |
||||||
| Adjusted age, gender and ISS | −0.027 (−0.071 to + 0.018) |
||||||
| QALYs (baseline 0) | |||||||
| Unadjusted | 0.022 | 0.068 | 33 | 0.056 | 0.110 | 24 | −0.035 (−0.083 to + 0.011) |
| Adjusted age and gender | −0.030 (−0.080 to + 0.017) |
||||||
| Adjusted age, gender and ISS | −0.032 (−0.075 to + 0.015) |
||||||
Discussion
In summary, care for trauma patients included in this trial was expensive, with most costs incurred during the index hospitalisation, with less intense use of hospital resource over follow-up. On average, participants in the SC + REBOA arm of the study incurred lower costs than in SC, due to the competing risk of death. Similarly, life-years accrued and QALYs over 6 months post randomisation were also lower in the SC + REBOA arm compared to SC due to a greater proportion of trial participants dying in the SC + REBOA arm and with mortality also occurring earlier in the follow-up period for the SC + REBOA arm. Results for both costs and QALYs remain robust to adjustment age, gender and ISS score and whether baseline utility is set to the unconscious (−0.402) state or set to 0.
Given that REBOA was both less costly and less effective, an analysis of cost-effectiveness would place REBOA in the southwest quadrant of the cost-effectiveness plane, where decision-makers would normally consider the cost savings achieved for each QALY lost. While this is a perfectly valid consideration for decision-making when the magnitude of QALY loss is small, the large differences in mortality between the arms for this study mean that an assessment of cost-effectiveness in the SW quadrant is not informative for decision-makers, regardless of the magnitude of cost-savings achieved. One limitation of the within-trial analyses is that the costs are all incurred up front, whereas the benefits of life-years saved and improvements in quality of life for survivors are likely to be accrued well beyond the 6-month trial follow-up. We therefore develop a simple decision analysis model to extrapolate life-year and QALY gains for survivors over a lifetime horizon (presented in Chapter 8).
Chapter 8 Decision analysis modelling
Background
In this chapter, we extrapolate the short-term costs and outcomes (mortality and utilities) from 6 months presented in Chapter 7, over a full lifetime horizon. Considering costs and outcomes over a longer time horizon is particularly important in scenarios where substantial costs of saving a life in trauma care are incurred up-front, but the benefits in terms of extended length of life and recovery leading to improvements in quality of life among survivors are not fully realised until well beyond the 6-month period of data observation in the trial. Any statements on cost-effectiveness in this setting should therefore be made only on consideration of the lifetime economic modelling presented in this chapter.
Methods
A decision analysis model was created using TreeAge Pro 2021 software73 to calculate expected costs, life-years and QALYs of SC + REBOA and standard major trauma care alone in adult patients with uncontrolled torso haemorrhage. The model extrapolated short-term (6-month) trial outcomes over a lifetime horizon from a UK NHS perspective. Development of the model structure, parameterisation and analysis methods are described in the following sections.
Model structure
A decision tree with a Markov cohort model was used to calculate expected costs, life-years and QALYs. The decision tree phase of the model captured 6-month mortality risks (and hence LYGs), costs and quality of life (utilities) among survivors as observed in the trial. The proportion of the cohort who were alive at 6 months post randomisation then entered a Markov cohort model, where costs and outcomes were accumulated over a lifetime horizon. As the primary outcome from the study was mortality, a simple two-state Markov model, with states for alive and dead is used to capture longer-term outcomes. The model was built flexibly to capture a range of different assumptions about the extent to which pre-injury quality of life is achievable among survivors, and the time taken to reach a steady state of quality of life. A simplistic model structure focussing on longer-term extrapolation of survival outcomes is consistent with other modelling work in trauma care, including the economic modelling approach taken for the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2 (CRASH-2) study. 74 The model structure is illustrated in Figure 15.
FIGURE 15.
Economic model structure.
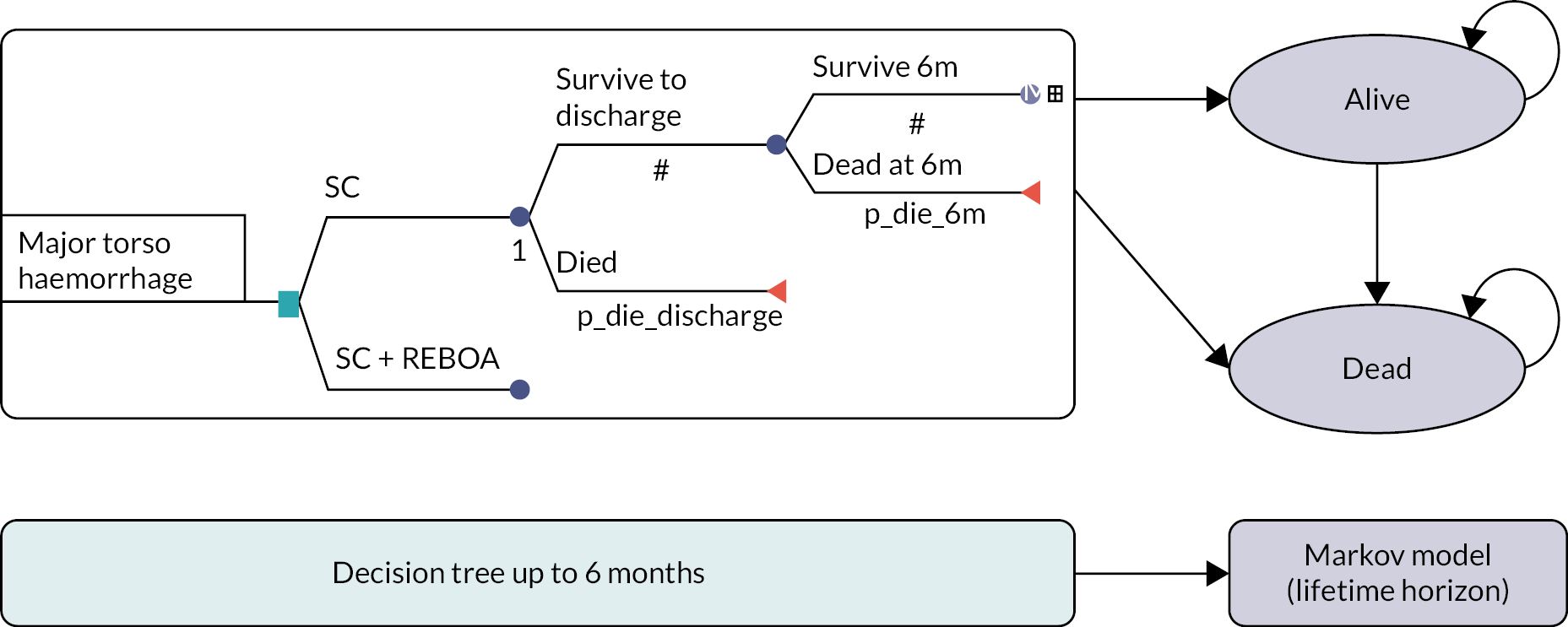
Model parameters
Patients enter the decision tree phase of the model at the point of randomisation to the trial (i.e. the point of arrival in the ED at hospital). The age and gender characteristics of the cohort were aligned with the baseline trial characteristics, where mean (SD) age was 45.35 (18.00) and the proportion of female was 28/88 (31.8%).
The trial data were used to inform the probability of survival to hospital discharge and further survival to 6 months post randomisation. Intervention costs (including the full-index hospital admission costs) were applied based on ITT costing, using the TARN data described in Chapter 7, while follow-up use of resource usage to 6 months post randomisation was based on the available linked data for survivors from NHS Digital HES. Similarly, EQ-5D utility data collected during the trial, cross-walked from the 5L to 3L and valued using UK general population tariffs, were applied at hospital discharge and 6 months. For the 6-month follow-up data, mortality risk, costs and utilities were assumed to be treatment-specific, capturing any potential effect of REBOA.
The long-term trajectory of patient recovery is uncertain, and it is uncertain whether short-term differences in quality of life would be maintained over a lifetime horizon or converge to being equal among survivors at some future time point. We have therefore taken a conservative approach to modelling the longer-term trajectory of patient recovery. We assume that return to general population quality-of-life norms would converge to the pooled mean utility from the trial in the first model cycle. An alternative assumption, to assume that differences in utilities were accrued indefinitely, albeit based on a small sample are explored in scenario analysis. Scenario analysis also explored the impact of applying treatment pooled costs and utility data for the period between hospital discharge and 6 months, on the grounds that any resource use incurred post discharge would be related to initial injury rather than REBOA.
Long-term outcomes, including mortality, long-term recovery in quality of life (utilities) and ongoing costs related to initial injury were obtained from targeted literature searches with variation across studies tested extensively in scenario analyses. Longer-term utility data were available from two studies. The first study reported utility data for N = 335 trauma survivors in the Netherlands. Mean (SD) utility, based on the Dutch value set was 0.691 (0.299) for patients with an initial ISS ≥ 16 followed up for between 12 and 18 months. 75 These utilities were applied at the end of 18 months. A second, smaller study of N = 56 patients provided 15 years of EQ-5D-3L follow-up data for a cohort of major trauma patients in the Netherlands. Average utility (assumed to be mean), applying the Dutch value set at 15 years to a subgroup initially with ISS > 16 was 0.660. 76 A measure of uncertainty such as standard error (SE) or SD was not reported, so it was assumed that the SD of the sampling distribution was equal to 43% of the mean, applying the same ratio of mean : SD as observed from the 18-month study data. 75 The longer-term data from the literature were consistent with an assumption that most trauma survivors will reach their threshold of recovery by 1–2 years post injury. Beyond 15 years, the minimum of the 15-year utility or general population norms was applied, and general population utility was modelled to reduce over time as the surviving proportion of the cohort age.
For the base-case analysis, long-term mortality risk was assumed to be equal to the UK general population age- and sex-adjusted all-cause mortality (ACM) probability. The assumption is justified on the grounds that excess mortality among trauma patients is mostly expected to occur within the initial hospitalisation period. This is consistent with data observed from other trauma studies, including CRASH-2, where the hazard of mortality reduces dramatically over time. 74 However, it is still feasible to assume that survivors may experience an increased mortality risk later in life due to the long-term sequelae of their initial injury (e.g. due to compromised mobility or related chronic illness). To explore the impact of a potential excess mortality risk on model outcomes, scenario analysis applies an excess mortality risk of 1.5.
Patients who survive major trauma events may be at risk of longer-term disability, lower quality of life and thus would also be expected to consume more healthcare resources compared to the general population average. A prospective cohort study from the Netherlands, among 174 trauma patients with an ISS ≥ 16 found that post-hospital costs, including rehabilitation, were mean (SD), €7770 (€13,640) over 24 months of follow-up, with the highest post-hospital costs incurred for spinal injuries. 77 Inflating from 2017 values to 2021 values and converting to GBP, results in a 6-monthly cycle-specific cost of £1764 [(€7770 * 1.06 * 0.86)/4] per 6-monthly cycle, up to 2 years. 78 Beyond 2 years, excess costs were assumed to be £0 for the remainder of the model time horizon. Scenario analysis explores the impact of assuming £0 excess cost for survivors beyond 6 months and applying the full cycle-specific cost for the duration of the model time horizon.
Time horizon and discounting
The Markov model was run over a lifetime horizon with a cycle length of 6 months. Costs and outcomes accruing beyond the first year of the model time frame (i.e. beyond cycle 1) were discounted at a rate of 3.5% per annum in line with NICE’s guide for the methods of technology appraisal. 79 Costs and outcomes are half-cycle corrected to accommodate the assumption that costs are incurred, and outcomes accrued mid-way through each 6-monthly model cycle.
Model analyses
The model was fully probabilistic, with each model parameter sampled probabilistically from its underlying distribution as specified in Table 21, using 10,000 Monte Carlo simulations. Results are expressed as mean expected lifetime costs, LYGs and QALYs per patient for SC + REBOA care and for SC alone. Calculations included both the 6-month costs and outcomes incurred in the decision tree phase of the model, summed with the lifetime costs and outcomes among 6-month survivors extrapolated over a lifetime from the Markov cohort model. As with the within-trial analysis, we do not report ICERs, but have sampled the probabilistic simulations of incremental costs and incremental QALYs on the cost-effectiveness plane, to illustrate the combined uncertainty surrounding costs and outcomes. QALYs are further adjusted by a multiplier in line with the NICE methods guide to account for QALY severity weightings. Using the QALY shortfall calculator, https://shiny.york.ac.uk/shortfall/, we apply the following parameters: average age of 45, proportion female = 32%, discount rate of 3.5% and remaining undiscounted QALYs = 0.01 if untreated. Remaining QALYs of 0.01 was an assumption for the calculation on the grounds that participants in the REBOA study were at immediate risk of death. Therefore, the maximum QALY weighing of 1.7 is applied.
| Utilities | Mean value | SDa | Distribution | Alpha | Beta | Source/notes |
|---|---|---|---|---|---|---|
| Utility baseline | −0.402 | – | Fixed | – | – | Assumption |
| Utility discharge (treatment pooled) | 0.263 | 0.067 | Beta | 11.09 | 31.09 | UK-REBOA trial data |
| Utility discharge (treatment specific SC) | 0.388 | 0.093 | Beta | 10.26 | 16.19 | |
| Utility discharge (diff: SC + REBOA vs. SC) | −0.208 | 0.128 | Normal | −0.208 | 0.128 | |
| Utility 6 months (treatment pooled) | 0.363 | 0.086 | Beta | 10.99 | 19.28 | |
| Utility 6 months (treatment-specific SC) | 0.538 | 0.094 | Beta | 14.60 | 12.53 | |
| Utility 6 months (diff: SC + REBOA vs. SC) | −0.311 | 0.184 | Normal | −0.311 | 0.184 | |
| Utility general population | Calculated | Calculated | Fixed | – | – | Ara and Brazier 201080 |
| Utility long-term – 2 years | 0.691 | 0.016 | Beta | 575.64 | 257.42 | Holtslag et al., 200775 |
| Utility long-term – 15 years | 0.660 | 0.038 | Beta | 101.91 | 52.50 | Wad et al., 201876 |
| Time parameters (days) | Mean value | SD a | Distribution | Alpha | Lambda | Source/notes |
| Time to death (in hospital) SC (n = 16) | 5.427 | 2.889 | Gamma | 3.53 | 0.650 | UK-REBOA trial data |
| Time to death (in hospital) SC + REBOA (n = 24) | 1.675 | 0.565 | Gamma | 8.79 | 5.247 | |
| Time to discharge (survivors) SCb (n = 27) | 62.282 | 11.120 | Gamma | 31.37 | 0.504 | |
| Time to discharge (survivors) SC + REBOAb (n = 21) | 37.556 | 5.463 | Gamma | 47.26 | 1.258 | |
| Probabilities | n | N | Distribution | Alpha ( n ) | Beta ( N – n ) | |
| p die: discharge SC | 16 | 43 | Beta | 16 | 27 | UK-REBOA trial data |
| p die: discharge SC + REBOA | 24 | 45 | Beta | 24 | 21 | |
| p die: 6 months SC | survive discharge | 2 | 27 | Beta | 2 | 25 | |
| p die: 6 months SC + REBOA | survive discharge | 1 | 21 | beta | 1 | 20 | |
| p die: ACM | Calculated | Calculated | Fixed | – | – | ONS, 202181 |
| Excess mortality | Mean value | SD a | Distribution | Alpha | Beta | |
| Excess mortality risk for survivors (base case) | 1.0 | – | Fixed | – | – | Assumption |
| Excess mortality risk for survivorsc (scenario analysis) | 1.5 | 0.30 | Normal | 1.5 | 0.30 | Assumption |
| Costs | Mean value | SD a | Distribution | Alpha | Lambda | |
| Index hospitalisation cost (SC + REBOA) | £57,384 | £9371 | Gamma | 37.50 | 0.0007 | UK-REBOA trial data |
| Index hospitalisation cost (SC) | £116,064 | £19,666 | Gamma | 34.83 | 0.0003 | |
| Index hospitalisation cost (pooled) | £80,062 | £10,766 | Gamma | 55.30 | 0.0007 | |
| Follow-up cost (SC + REBOA) | £11,601 | £5126 | Gamma | 5.12 | 0.0004 | |
| Follow-up cost (SC) | £5385 | £1308 | Gamma | 16.95 | 0.0031 | |
| Follow-up cost (pooled) | £8772 | £2809 | Gamma | 9.75 | 0.0011 | |
| Excess costs of survivors (6 months to 2 years) | £1764 | £3087 | Gamma | 0.327 | 0.0002 |
While the HEAP had specified that an expected value of perfect information (EVPI) analysis would be undertaken, this was not undertaken because regardless of the EVPI results, it would not be deemed ethically justifiable to randomise patients to REBOA in this specific setting in a future study, given the clinical results of the trial. Additional scenario analyses (all applied probabilistically) were conducted to explore the impact of key modelling assumptions around the extent to which pre-injury quality of life is achieved among survivors and assumptions about the extent of ongoing long-term costs of rehabilitation of trauma patients on results.
Subgroup analyses
Given that the magnitude of available data for key model parameters, especially given utilities were small, we did not conduct any subgroup analyses using the economic model.
Value of information analysis
Value of information analysis was not undertaken as planned again as a consequence of the clinical results of the trial. The analysis makes no statements about the value of research around the use of REBOA in other settings.
Model validation
The model was built by one health economist and independently checked against the Tappenden and Chilcott criteria by a second health economist on the study team. 79 Face validity was assessed through discussion of long-term extrapolations with the trial team, including clinical expert opinion.
Results
Base-case analysis (probabilistic)
Table 22 shows the expected value of costs, LYGs and QALYs for each treatment strategy in the base case and for several scenario analyses undertaken. QALYs are reported with and without a 1.7 multiplier for severity weighting.
| Strategy | Cost (£) | Inc. cost (£) | LY | Inc. LY | QALY | Inc. QALY | Severity weighted QALY (× 1.7) | Severity weighted inc. QALY (× 1.7) |
|---|---|---|---|---|---|---|---|---|
| Base-case analysis | ||||||||
| SC | 122,603 | 11.84 | 7.18 | 12.20 | ||||
| SC + REBOA | 65,440 | −57,163 | 9.05 | −2.79 | 5.45 | −1.72 | 9.27 | −2.93 |
| Scenario 1: Remove excess treatment costs for survivors beyond 6 months | ||||||||
| SC | 120,117 | 11.84 | 7.18 | 12.20 | ||||
| SC + REBOA | 62,880 | −57,237 | 9.04 | −2.80 | 5.45 | −1.73 | 9.26 | −2.94 |
| Scenario 2: Apply lifetime excess costs for survivors | ||||||||
| SC | 160,575 | 11.84 | 7.17 | 12.19 | ||||
| SC + REBOA | 94,144 | −66,431 | 9.04 | −2.80 | 5.44 | −1.73 | 9.25 | −2.94 |
| Scenario 3: Apply long-run SMR = 1.5 | ||||||||
| SC | 123,080 | 11.15 | 6.78 | 11.53 | ||||
| SC + REBOA | 65,499 | −57,581 | 8.53 | −2.62 | 5.15 | −1.63 | 8.76 | −2.77 |
| Scenario 4: Undiscounted results | ||||||||
| SC | 122,927 | 21.06 | 12.52 | 21.28 | ||||
| SC + REBOA | 65,629 | −57,298 | 16.15 | −4.91 | 9.56 | −2.95 | 16.26 | −5.02 |
| Scenario 5: Return everyone to general population norms by 2 years | ||||||||
| SC | 123,012 | 11.84 | 9.34 | 15.88 | ||||
| SC + REBOA | 65,543 | −57,469 | 9.07 | −2.77 | 7.12 | −2.22 | 12.10 | −3.78 |
Substantial cost savings associated with SC + REBOA are due to the higher risk of mortality, occurring earlier in the patient journey compared to SC, meaning that there would be substantial life-year and QALY losses associated with the adoption of SC + REBOA in this setting. Despite the cost savings, the magnitude of QALY loss over a lifetime shows that SC + REBOA is both harmful (lost life-years and QALYs) and would be an inefficient use of scarce healthcare resources, when considering increased valuation of a QALY accrued at the end of life. Parameter uncertainty surrounding base-case results, with QALYs weighted at 1.7, is illustrated on the cost-effectiveness plane in Figure 16.
FIGURE 16.
Scatterplot of the cost-effectiveness plane over a lifetime horizon (QALY weighting = 1.7).

Assuming a threshold value of a QALY = £30,000, but applying the maximum weighting for a QALY (multiplier = 1.7), turquoise dots indicate an inefficient use of resource (71% probability for base-case analysis), whereas dark blue dots indicate that REBOA is the most efficient use of resource (29% probability for the base-case analysis).
Iterations on the cost-effectiveness plane show that REBOA is definitively less costly (probability > 99%), due to the competing risk of mortality, but that it is also substantially less effective in terms of QALYs accrued over a lifetime horizon (probability 91%). The findings are robust to a range of scenario analyses undertaken. The results are also consistent with the findings of the clinical effectiveness.
Chapter 9 Discussion
This is the first randomised trial ever to be conducted examining the potential clinical effectiveness of the addition of REBOA to standard MTC care for the management of exsanguinating haemorrhage.
Summary of main findings
In this trial, the group that received care that included REBOA (SC + REBOA) was observed to have a high probability (above 80% in all analyses) of increased mortality at 90 days (the primary outcome) compared with the SC group.
This difference was apparent at all time points, and it was noted that the posterior probabilities of mortality increased with earlier time points. Given that more proximate mortality end points are now thought to better reflect the effect that haemorrhage control interventions – which, by definition, exert their effect early35 – the progressively higher posterior probabilities at earlier time points add weight to the likelihood that REBOA is harmful.
The survival curves provide further evidence of the likely harmful early effects of REBOA. There was a sharp, early – within the first few hours – drop in survival, which likely represents failure to control haemorrhage. However, it is noteworthy that deaths in this group continued out to 10 days. This excess of early deaths was also apparent in the analysis of length of stay where patients who were allocated to the SC + REBOA strategy had fewer hospital-free and ICU-free days than those who received SC alone, suggesting that patients who did survive to ICU admission had sustained additional physiological insults that made them less likely to survive.
The findings from two additional sensitivity analyses, which adjusted for potential baseline imbalances between the groups, were consistent with the primary analysis. A principal stratification analysis, to account for intercurrent events, did not alter the findings. Furthermore, even when enthusiastic priors – derived during a formal expert elicitation exercise – were applied to the trial’s results, the direction of the findings did not change. The addition of REBOA to SC, as delivered in MTCs in England, was observed to increase mortality, compared with the SC group.
A possible explanation for this finding of increased mortality was the observed delay in the SC + REBOA group in obtaining definitive haemorrhage control. This could be seen in the increased proportion of early deaths due to (uncontrolled) haemorrhage. Death due to haemorrhage was more common in the SC + REBOA group, and all of these deaths occurred within 24 hours, and most of them within 3 hours, of randomisation. Furthermore, in patients who survived to a definitive haemorrhage control procedure, it took, on average, an additional 26 minutes to commence these procedures.
Relevance to existing literature
The UK-REBOA trial is the only randomised clinical trial of the addition of REBOA to standard MTC care in trauma patients. Previously published observational studies, which are of variable quality, and at risk of bias, reported both positive and negative effects of REBOA. Three large, retrospective studies, from the USA and Japan, which had similar populations and similar treatment profiles to the UK-REBOA trial, reported results that would align with the findings of the UK-REBOA trial.
A retrospective study from the USA by Joseph et al. , using the 2015–6 national, multi-institutional American College of Surgeons Trauma Quality Improvement Program data set, propensity-score-matched 140 patients who received REBOA to 280 patients who did not. 82 Among the REBOA group, median ISS was 29 (Q1–Q3 18–38) – somewhat lower than in the UK-REBOA trial – and 129 patients (92.1%) had a blunt mechanism of injury. Patients were also less hypotensive than patients in our trial. The mean SBP was 108.8 millimetres of mercury (mmHg) (SD 32.7) for patients who underwent REBOA, and 106.5 mmHg (SD 28.7) for patients treated without REBOA. There was no significant difference between groups in 24-hour blood transfusion requirements. Patients in the REBOA group received a median of nine units of red blood cells (Q1–Q3 5–20), seven units of platelets (Q1–Q3 3–13) and nine units of plasma (Q1–Q3 6–20). In the no-REBOA group, patients received a median of 10 units of red blood cells (Q1–Q3 4–21), 8 units of platelets (Q1–Q3 3–12) and 10 units of plasma (Q1–Q3 7–20). These numbers are very similar to those in our trial. Median hospital length of stay was 8 days (Q1–Q3 1–20) for patients in the REBOA group and 10 days (Q1–Q3 5–22) in the no-REBOA group – shorter than in our trial, but this difference is explained by the fact that this was a study of patients in US trauma centres, which have shorter lengths of stay. Median ICU length of stay was 5 days (Q1–Q3 2–14) for REBOA patients and 6 days (Q1–Q3 3–15) for non-REBOA patients, which is more similar to the results in our trial. As in our trial, the in-hospital mortality rate was higher in the REBOA group (35.7%) than in the no-REBOA group (18.9%) (p = 0.01). Twenty-four-hour mortality was also higher in the REBOA group (26.4%) than in the non-REBOA group (11.8%) (p = 0.01). The authors concluded that placement of REBOA in severely injured trauma patients was associated with a higher mortality rate compared with a similar cohort of patients who were treated without REBOA. (The lower overall mortality rates reported may be a reflection of the lower injury severity or the less severe cardiovascular compromise.)
An earlier retrospective study by Norii et al. ,25 using 2004–11 data from the Japan Trauma Data Bank, propensity-score-matched 351 patients treated with REBOA to 1456 patients treated without REBOA. The median ISS in the REBOA group was 34 (Q1–Q3 22–45) and 29 (Q1–Q3 19–42) in the non-REBOA group, again slightly lower than in our trial. The probability of survival in the REBOA-treated group (26.2%) was significantly lower than the survival in the untreated (51.3%, p < 0.0001), for a crude conditional OR of survival by REBOA treatment of 0.30 (95% confidence interval, 0.23 to 0.40). The authors concluded that treatment with REBOA was associated with higher mortality compared with similarly ill trauma patients who did not receive REBOA. 25
A further retrospective study from Japan by Inoue et al. 83 also propensity-score-matched 625 patients who received REBOA, to 625 who did not. The in-hospital mortality was significantly higher in subjects who underwent REBOA (61.8% vs. 45.3%; absolute difference 16.5%; 95% confidence interval 10.9 to 22.0%). This study used the same data set as the Norii study. 25 The date range was not reported in the Inoue study, and there may thus be overlap.
In contrast, García et al. , in a propensity-score-matched study of 345 patients, 28 of whom received REBOA, from Columbia, found that patients treated with REBOA had lower risk-adjusted odds of mortality (OR 0.20, 95% confidence interval 0.05 to 0.77, p = 0.01). 84 Similarly, Yamamoto et al. ,85 in another propensity score-matched study of data from the national Japanese trauma registry, found that survival to discharge was higher among patients treated with REBOA than among those treated without REBOA (45.3% vs. 32.5%; OR 1.72; 95% CI 1.01 to 2.93; p = 0.04).
Health economic analysis
Care for trauma patients included in this trial was expensive, with most costs incurred during the index hospitalisation, with less intense use of hospital resource over follow-up. On average, participants in the SC + REBOA arm of the study incurred lower costs than in SC, due to the competing risk of death. Similarly, life-years accrued and QALYs over 6 months post randomisation were also lower in the SC + REBOA arm compared to SC due to a greater proportion of trial participants dying in the SC + REBOA arm and with mortality also occurring earlier in the follow-up period for the SC + REBOA arm. Results for both costs and QALYs remain robust to adjustment age, gender and ISS score and whether baseline utility is set to the unconscious (−0.402) state or set to 0.
Given that REBOA was both less costly and less effective, an analysis of cost-effectiveness would place REBOA in the southwest quadrant of the cost-effectiveness plane, where decision-makers would normally consider the cost savings achieved for each QALY lost. While this is a perfectly valid consideration for decision-making when the magnitude of QALY loss is small, the large differences in mortality between the arms for this study mean that an assessment of cost-effectiveness in the SW quadrant is not informative for decision-makers, regardless of the magnitude of cost-savings achieved. One limitation of the within-trial analyses is that the costs are all incurred up-front, whereas the benefits of life-years saved and improvements in quality of life for survivors are likely to be accrued well beyond the 6-month trial follow-up. We therefore developed a simple decision analysis model to extrapolate life-year and QALY gains for survivors over a lifetime horizon.
Substantial cost savings associated with SC + REBOA are due to the higher risk of mortality, occurring earlier in the patient journey compared to SC, meaning that there would be substantial life-year and QALY losses associated with the adoption of SC + REBOA in this setting. Despite the cost savings, the magnitude of QALY loss over a lifetime show that SC + REBOA is both harmful (lost life-years and QALYs) and would be an inefficient use of scarce healthcare resources, when considering increased valuation of a QALY accrued at the end of life.
Iterations on the cost-effectiveness plane show that UK-REBOA is definitively less costly (probability 99%), due to the competing risk of mortality but that it is also substantially less effective in terms of QALYs accrued over a lifetime horizon (probability 91%). The findings are robust to a range of scenario analyses undertaken, with the probability of SC being the optimal treatment strategy ranging from 66% to 81% at a threshold value of a QALY = £50,000. The results are consistent with the findings of the clinical effectiveness.
Strengths
The UK-REBOA trial has a number of strengths. Most importantly, it is the only RCT ever conducted of REBOA in trauma patients. This is in part a reflection of the difficulties inherent in evaluating a complex and technically challenging intervention in patients at imminent risk of dying, but the UK-REBOA trial showed that such a randomised trial could be done. It included an integrated training programme by design, recognising the challenges of evaluating a new technology that clinicians may not have been exposed to previously and thus mitigated against potential learning curve effects. The trial was pragmatic in design, with simple inclusion criteria that were based on the clinical judgement of experienced clinicians allowing clinicians to quickly evaluate suitability for the trial even in the somewhat chaotic environment of the ED. This study reflected the complex and dynamic situation faced by trauma teams when treating patients with severe haemorrhage with some patients responding rapidly to standard resuscitation during the time period that encompassed the decision-making, randomisation and preparation time to perform REBOA. The trial used routinely collected data extensively to minimise the burden on the clinical staff (although there were some issues with that approach – see below). It also adopted a Bayesian analytical framework and group sequential design, which allowed for robust interpretation even in a small population. The Bayesian group sequential design facilitated the interim analyses, and the interpretation of the findings.
Limitations
The UK-REBOA trial also has a number of limitations.
This was a small trial, reflecting the relative infrequency of exsanguinating traumatic haemorrhage in the UK. There were some imbalances between the groups, particularly with regards to SBP on arrival in the ED, which was found to be lower in patients allocated to the SC + REBOA strategy; and the presence of traumatic brain injuries, which were possibly more severe in patients allocated to the SC + REBOA strategy. However, analyses adjusting for these found no material effect on the results. Furthermore, the proportion of deaths attributed to traumatic brain injury was broadly similar in the two groups.
There is a possibility that the results are in part due to inexperience with the REBOA technique, despite the extensive training programme instituted as part of the trial. Most (albeit not all) of the participating centres had never used REBOA before in the trauma setting, and as such most of the insertions were performed by clinicians who had never used the technique before. The technical skills required to perform arterial cannulation in severely shocked patients, under pressure, are considerable. In addition, clinical teams would have had little previous experience in managing patients once the device had been successfully inserted and inflated. Having said that, a post hoc sensitivity analysis, which excluded the first patient randomised to the SC + REBOA strategy at each site, showed marginally increased odds of mortality and posterior probabilities of harm compared to the main analysis. This analysis, however, could only account for institutional learning effects. The number of patients in the trial as a whole, and the number of patients enrolled by individual clinicians, were too small to allow for a similar analysis of individual learning effects.
Lastly, participating trauma centres’ research infrastructure (only one had research staff available around the clock, to assist with recruitment and data collection) limited the collection of more granular procedural data, or mechanistic data, such as blood pressure readings. Our decision to rely on routinely collected audit data, for the baseline characteristics, resulted in some missing data, which then had to be queried later, although this did not impact on the results.
Context
The trial was conducted in the UK, where injury epidemiology and the trauma care delivery framework differ from other locations. The findings of the UK-REBOA trial should be interpreted in this context.
Pre-hospital care in the UK is of very high and uniform quality. Ambulance services are large organisations that adhere to nationally agreed clinical standards. Almost all trauma patients receive tranexamic acid on scene and blunt trauma patients have a pelvic binder applied before transport. In addition, ambulance services in the UK commonly provide pre-hospital transfusion support, and many patients receive treatment from a critical care paramedic or pre-hospital care doctor.
The reorganisation of in-hospital trauma care in England – which included the establishment of regional trauma networks and the designation of MTCs and trauma units; as well as the development of nationally agreed clinical standards for trauma care – has markedly improved mortality from injury over the past decade. 86 However, case volume (and operative case volume for haemorrhage control, in particular) in many MTCs is lower than in other countries, reflecting very high road safety standards and very low levels of interpersonal violence in the country. Furthermore, in most MTCs, the responsibility for the control of torso haemorrhage rests with surgeons who do not only provide trauma care. These surgeons are often on call from home, rather than resident in the hospital. The initial care of trauma patients is therefore usually the responsibility of senior emergency medicine doctors, but surgeons are called early (even before the arrival of a patient), on the basis of pre-alerts. Nevertheless, these organisational differences may have impacted on the speed with which trauma patients were treated and, in particular, operated on, if needed.
Intercurrent events
There were a number of pathways experienced by those who were allocated to the SC + REBOA strategy, due to intercurrent events. These findings demonstrated that obtaining arterial access in severely shocked patients; and that distinguishing between patients who are experiencing continuing, severe haemorrhage from those in whom bleeding has stopped and only require transfusion is challenging. All clinicians working in the ED recognise the dynamic and unpredictable nature of severe trauma with haemorrhage. We considered these issues by means of a principal stratification analysis that did not alter our conclusions. However, perhaps most importantly, our experiences reflect ‘real life’ and shed light on the reality of the management of major trauma care where a patient’s condition can change very rapidly. A trial where every patient randomised to REBOA receives full REBOA is never expected given the dynamic situation. The implementation and evaluation of any system incorporating REBOA must consider these challenges.
Methodological issues
The trial encountered a number of methodological issues, which are worth highlighting:
Registry-enabled design
We relied on data routinely collected by TARN, England’s national trauma registry, to characterise the trial population (although most outcomes were collected directly, using a UK-REBOA trial-specific eCRF). Our intent was to make the trial as simple to run as possible, particularly given that many patients presented out of hours, and to avoid duplication of effort. However, there were a number of downstream effects. Firstly, the time from discharge to submission of data to TARN, by sites, was variable, and often prolonged for patients awaiting injury details from post-mortem examinations. This made the characterisation of the trial population more difficult, especially for interim analyses. There were also more missing data than anticipated, requiring queries to sites. (Fortunately, these data had actually been recorded in patients’ health records, but not been transcribed into TARN.) Some of these issues may relate to the large proportion of patients who died, and sometimes died very early on. Lastly, we discovered that a small number of patients had opted out from all national health data collection (https://digital.nhs.uk/services/national-data-opt-out). These patients have all their TARN records deleted, without a record of such a deletion (which also comes under the opt-out). Fortunately, we were able to obtain the necessary data points directly from sites, under research-without-prior-consent rules.
Working with NHS Digital
The data obtained from NHS Digital (required for the health economic analysis) was of high quality, but it took almost the entire duration of the trial to secure the necessary permissions to use it. Data linkage was not agreed to for participants without either participant or consultee consent, limiting the usefulness of the data for the health economic analysis. This was largely related to the research-without-prior-consent framework, which NHS Digital had only limited familiarity with. Multiple changes in case workers, each requiring additional questions to be answered, further compounded the issue. Future research-without-prior-consent trials relying on NHS Digital data would benefit from early discussion.
Initial error in statistical design
Our initial design parameters contained an error in the formulation of the variance in calculations, resulting in an overestimation of the operating characteristics. Following extensive consultation with the funder, and external reviewers, we relaxed the success threshold, and added informative priors, resulting in acceptable probabilities of declaring success if REBOA had indeed been beneficial. 38 As it turns out, the design error – or the revised design – had no impact, given that REBOA turned out likely to be harmful, and the large size of the effect.
Streamlined framework with limited dataset
Executing clinical trials in patients at imminent risk of dying, particularly when they present out of hours, and with little notice, is extremely difficult. Although the UK’s research delivery framework has many advantages, like all healthcare systems, it is not well suited to support such studies. Our reliance on clinicians to enrol patients and collect some data (albeit minimal) in real-time necessitated a limited dataset, focused on answering the trial’s key question. If we had our time again, we would likely have instigated more comprehensive data collection, to capture data such as changes in blood pressure in response to balloon inflation and other physiological data. However, this would have greatly increased the cost of the trial.
Choice of primary outcome
We have already alluded to the issue of choice of primary outcome, and the change in thinking that has taken place over the past 5 years. Our choice of 90-day mortality was based partly on the critical care literature, and concern that early benefit (prevention of early death) might be associated with late harm (increased mortality as a result of acute respiratory distress syndrome, acute kidney injury, etc.).
Experience with the ‘Pragmatic, Randomized Optimal Platelet and Plasma Ratios’ (PROPPR) trial, which compared two types of transfusion strategies in trauma patients,87,88 and several subsequent trials, has resulted in a better understanding of the impact of the time point at which mortality is evaluated. Deaths from haemorrhage occur early. Later deaths are typically due to causes such as traumatic brain injury or multiple organ failure. Although the latter may occur equally in both arms of the study, and the difference thus remains the same, the baseline mortality rate increases, which makes it more difficult to detect differences. A recent consensus statement – which was the product of a conference that involved the National Institute of Health, the Food and Drug Administration, the Department of Defense and researchers – has therefore advocated for using more proximate mortality endpoints in trauma haemorrhage control trials. 35
However, the above debate did not impact on the UK-REBOA trial, because the signal (of harm) is so strong – even at 90 days post injury. However, in keeping with the assertions above, the effect size is even greater at earlier time points.
Setting
The UK-REBOA trial was a study of the use of REBOA in-hospital. The use of REBOA in-hospital may, however, be ‘too late’, especially since patients can be delayed in arrival at the hospital and, when there, they can often be taken to an operating theatre, for definitive control of haemorrhage, very quickly. Potentially the result may have been different had REBOA been considered in the pre-hospital setting, which at the time of the trial design was not being considered in the UK. The pre-hospital environment differs from the in-hospital setting conceptually; it makes sense to obtain haemorrhage control as early as possible after injury, before large volumes of blood have been lost, and inflammatory sequelae are superimposed onto haemorrhagic shock. The findings of the trial should therefore not be extrapolated to the pre-hospital setting.
Equality, diversity and inclusion
We collected data on age and sex; the distribution in the UK-REBOA trial is broadly representative of the trauma patient population in the UK. We did not collect data on ethnicity, socioeconomic status, education or health literacy. Enrolment took place without consent, which may have removed some of the barriers to research participation (e.g. those related to English language and health literacy) that studies requiring consent may face.
Patient and public involvement
We have two PPI co-applicants (AP, NW) who were involved in the development of the study protocol and associated patient-facing paperwork, and contributed to meetings of the PMG throughout the lifespan of the study. The PPI representatives were invited to attend meetings where the initial results of the study were shared and have had the opportunity to comment on the plain language summary. The TSC included two independent PPI members who contributed to discussion at these meetings and were available to comment on aspects of the trial throughout its lifespan. The trial manager supported the PPI representatives so that they were able to contribute fully.
Conclusions
The analyses of the primary and secondary endpoints in this trial of trauma patients with haemorrhagic shock show that a management strategy that includes REBOA, when used in-hospital, has a high probability of being associated with increased mortality, compared to SC alone.
Implications for practice
The continuing use of REBOA, at least in the UK in-hospital setting, should be re-evaluated.
Implications for research
This trial examined the role of in-hospital REBOA. Given the time from injury to hospital, and the aim of REBOA being to provide early temporary haemorrhage control, the role (if any) of treating patients earlier with REBOA (i.e. in the pre-hospital setting) remains unclear. Further research to clarify the potential (or not) of pre-hospital REBOA may be indicated.
This trial showed that while using routinely collected data was intrinsic to the trial and added to the streamlined nature of the data collection, use of routine data raised a number of issues (e.g. the time from discharge to submission of data to the registry; missing data; extensive permissions and delay in receiving data via NHS Digital). There is a need for further research into the development needs of registries and routine data to enable the routine support of clinical trials.
Additional information
Contributions of authors
Jan O Jansen (https://orcid.org/0000-0001-8863-4398) (Co-Chief Investigator) co-led the conception and design of the trial, the conduct of the trial, the interpretation of results and writing/editing the report.
Jemma Hudson (https://orcid.org/0000-0002-6440-6419) (Statistician) conducted the statistical analysis of clinical data and contributed to the interpretation of results and writing/editing the report.
Charlotte Kennedy (https://orcid.org/0000-0002-1974-6318) (Health Economist) conducted the health economic analysis and contributed to the interpretation of results and writing/editing the report.
Claire Cochran (https://orcid.org/0000-0001-7349-7685) (Trial Manager) was responsible for the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (CHaRT Director) contributed to the conception and design of the trial, oversaw the statistical analysis and contributed to the interpretation of results and writing/editing the report.
Katie Gillies (https://orcid.org/0000-0001-7890-2854) (Process Evaluation Lead) contributed to the design of the trial, oversaw the process evaluation and contributed to the interpretation of results and writing/editing the report.
Robbie Lendrum (https://orcid.org/0000-0003-3206-3228) (Co-Education Lead) contributed to the conception and design of the trial, designed and led the implementation and training programme and contributed to the interpretation of results and writing/editing the report.
Samy Sadek (https://orcid.org/0009-0009-6064-1102) (Co-Education Lead) designed and led the implementation and training programme and contributed to the interpretation of results and writing/editing the report.
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Health Economist) contributed to the design of the trial, oversaw the health economic analysis and contributed to the interpretation of results and writing/editing the report.
Gillian Ferry (https://orcid.org/0009-0006-9012-9271) (Assistant Trial Manager) contributed to the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Louisa Lawrie (https://orcid.org/0000-0002-9867-2184) (Research Fellow) conducted the process evaluation and contributed to the interpretation of results and writing/editing the relevant parts of the report.
Mintu Nath (https://orcid.org/0000-0002-0753-0464) (Statistician) contributed to the design and analysis and to the interpretation of results and writing/editing the report.
Seonaidh Cotton (https://orcid.org/0000-0002-7883-0608) (Senior Trial Manager) was responsible for the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Samantha Wileman (https://orcid.org/0000-0002-1031-1449) (Quality Assurance Manager) contributed to the conduct of the trial, the interpretation of results and writing/editing the report.
Mark Forrest (https://orcid.org/0000-0002-2395-8823) (Senior IT Manager) led the development of the study website and mobile app and contributed to the interpretation of results and writing/editing the report.
Karim Brohi (https://orcid.org/0000-0003-0643-8866) (Professor of Trauma Sciences) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Tim Harris (https://orcid.org/0000-0002-9146-258X) (Professor of Emergency Medicine) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Fiona Lecky (https://orcid.org/0000-0001-6806-0921) (Clinical Professor of Emergency Medicine) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Chris Moran (https://orcid.org/0009-0006-8886-8884) (Professor of Orthopaedic Trauma Surgery) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Jonathan J Morrison (https://orcid.org/0000-0001-7462-8456) (Associate Professor of Surgery) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor of Medical Statistics and Trial Methodology) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Alan Paterson (https://orcid.org/0000-0001-5885-0743) (Emeritus Professor) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Nigel Tai (https://orcid.org/0000-0001-8493-9063) (Consultant Trauma and Vascular Surgeon) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Nick Welch (https://orcid.org/0000-0003-4201-9781) contributed to the conception and the design of the trial from a Patient and Public Involvement perspective and contributed to the interpretation of results and writing/editing the report.
Marion K Campbell (https://orcid.org/0000-0001-5386-4097) (Co-Chief Investigator) co-led the conception and design of the trial, the conduct of the trial, the interpretation of results and writing/editing the report.
Acknowledgements
We extend our thanks to all the participants who took part in the study and without whom the study would not be possible. We are grateful to all the staff at recruitment sites that facilitated recruitment and data collection (listed below) and for contributing to regular REBOA Meetings.
We thank the members of the Trial Steering Committee, including our two patient representatives, for their valued contribution and oversight of the study and for their attendance at regular Trial Steering Committee meetings. The independent members of the committee are: Keith Abrams, Josie Horton (PPI independent member), Victoria Le Brec (PPI independent member), Danny McAuley (TSC chair) and Rory Rickard. We are grateful to the members of the Data Monitoring Committee: Peter Andrews, Joseph Du Bose, Simon Gates (DMC chair), Zaffer Qasim and Kathy Rowan.
We are grateful to Kirsty McCormack for her help in developing the original funding application and to other staff within CHaRT and HSRU for their contribution to the management and administration of the study: Patrycja Bromm, Annemarie Clancy, Fernanda Dias Da Silva, Andrea Fraser, Alison McDonald, Graham Scotland, Beverly Smith and Zareen Thorlu-Bangura. We thank Dan Brunsdon, Eilidh Duncan, Taylor Coffey and Zoe Skea for their support with the process evaluation. We also thank the Programming Team in CHaRT for developing and maintaining the study website and app. The statistical consultants who contributed to this trial were Thomas Jaki and Philip Pallman.
Our thanks also go to the Research Governance team (Stacey Dawson, Louise King and Lynn McKay) at the University of Aberdeen for their advice and support during the study. We are grateful for the assistance we received from Louise Cotterell, Anne Buckle and Kerry Duffus in managing the study budget. We thank Juliette Snow and Rachael West for their help with contracting.
We are grateful to the Trauma Audit and Research Network for providing data, and for Antoinette Edwards, Sophie Jones and Paul Symonds for helping to resolve data queries.
The Health Services Research Unit (HSRU) and the Health Economics Research Unit (HERU) are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Staff who facilitated recruitment and data collection
Aintree University Hospital NHS Foundation Trust: Carol Brooks, Shirley Cooper, Calum Edge, Kate Fenlon, John Fletcher, John Gilbert, Melanie Harrison, Tanya Ingram, Raimundas Lunevicius, Gemma Mullen, John Pilavakis, Olga Rutka, Abdo Sattout (PI), Sarah Stevenson, Lilian Wajero, Timothy Wharton.
Barts Health NHS Trust (Royal London Hospital): Sumira Alberali, Ben Bloom, Noemi Caponi, Richard Carden, Arun Castro, Raine Astin-Chamberlain, Michael Carver, Ben Clarke, Helen Curnoni, Anna Dobbie, Neil Durge, Derek Hicks, Shabana Issa, Tony Joy, Brian Kennedy, Charlotte Lindsay, John Mackenna, John Mackenney, Max Marsden, Somayyeh Mossadegh, Anna Morgan, Dan Nevin, Henry Nnajiuba, Helen Oliver, Nimca Omer, Andrea Rosetto, Jennifer Ross, Claire Rourke, William Rush, Samy Sadek (PI), Joanna Shepherd, Rebecca Stoner, Anthony Thaventhiran, Harriet Tucker, Grace Tunesi, Paul Vulliamy, Simon Walsh, Anne Weaver, Jared Wohlgemut.
Hull University Teaching Hospitals NHS Trust: Sina Askari-jirhandeh, Andrew Blackmore, Biju Cherian, Tom Cowlam (PI), Mark Higson, Matthew Hines, Victoria Jowett, Simon Long, Vicki Martinson, Jeremy Osman, Jack Preece, Ben Rayner, Krystyna Simpson, Augustine Smithies, Chris Srinivasan, Paul Stewart, Elizabeth Stone, Dhruba Tamuli, William Townend, Fraser Young.
Imperial College Healthcare NHS Trust (St Mary’s): Zazyneb Al-Saadi, Chris Aylwin (PI), George Bailey, Colin Bicknell, Niamh Bohnacker, Michela Cicchetti, Jo-Anna Conyngham, Andrea D’Mello, Marwa El-Zanfaly, Sarah Finlay, Dan Frith (PI), Shehan Hettiarachty, Sophie Hunter, Nadia Guidozzi, Oliver Jefferson, Rachel Jennings, Moya Kirmond, Athanasia Kountourgioti, Chris Lambert, Graham Lawton, Sonia Fernandez Lopez, Heather McLachlan, Anu Mitra, Noora Mohamoud, Abby Harper Payne, Alberto Rigoni, Alexander Rolls, Cosmo Scurr, Danny Sharpe, Rhys Thomas, Sophie Turner, Lajeesh Vettikkat, Eyston Vaughan-Huxley, Claire Webster, Isabella Wilkes, Rachel Williams, Mark Wilson, Michael Wilson, Henry Wydell, Louise Young, Karolina Zimmerman.
King’s College Hospital NHS Foundation Trust: Duncan Bew (PI), Roger Bloomer, Emma Clarey, Ellie Corcoran, Hannah Cotton, Adam Czapran, Maria Depante, Clare Donegan, Matt Edwards, Tom Hurst, Kevin O’Reilly, Sian Saha, Malcolm Tunnicliff, Umar Wali, Simone Waitt.
Leeds Teaching Hospitals NHS Trust: Hala Ahmed, Kimberley Atha, Rebecca Blythe, Camilla Boyton, Stephen Bush, Paul Curley, Peter Cutting, Rosie Darwood, Nikki Dewhirst, Sally Docherty, Joanne Fletcher, Karen Flood, Yien Fah Fong, James Forsyth, Thushan Gooneratne, Christopher Hammond, A Hassan, Sarah Higgins, Susannah Howard, Craig Irvine, Harriet Jennings, D.H. Jones, Jonathan Jones, Megan Lawrence, Christina Leddie, James Lenton, Simon McPherson, Nonica Maftei, Andrew Mavor, Sundararaj Manou, Rashmi Menon, Samantha Monkman, Jacqueline Moorhead, Frances Mulley, Ahmed Nassef, Stuart Nuttall, Jai Patel, Emily Pawson, Kate Priestly, Sapna Puppala, Sara Rahma, David Russell, Julian Scott, Laura Scott, David Shaw, Venugopal Shankar, Tim Stansfield (PI), Sally Stone, Adene Thornton, Constatinosis Tingerides, Merane Todd, Max Troxler, Venugopal Shanker, Junaid Sultan, Katie Smith, Tom Wallace, Paul Walker, Emma Watson, Alexander Wilkins, Christina Wood, Janet Woods, Amanda Yang, Shahzadi Zeb.
Newcastle upon Tyne Hospitals NHS Foundation Trust: Dion Arbid, Maite Babio-Galan, Benjamin Brown, Lauren Butler, Matt Cadamy, Tony Calder, Ryan Clark, Stephen Clark, Brian Carroll, Jim Connolly, Peter Coyne, Leigh Dunn, Peter Goode, Nigel Fox, Arti Gulati, Abigail Harrison, Carole Hays, Robert Jarman, Philip Johnstone (PI), Jacques Kerr, Sarah Kirtley, Jenny Lett, Sohom Maitra, James McCaslin, Margaret McNeil, Craig Ord, Rebecca Petini, Sarah Platt, Reuben Saharia, Julia Scott, Bas Sen, Graham Soulsby, Kirsty Tristam, Peter Truran, Jason Urron Kimberley Webster, Tessa Wilkinson, John Williams, John Wright.
North Bristol NHS Trust: Jonathan Benger, Kristina Birch, Julian Blackham, Borislava Borislavova, John Bowditch, Adam Brown, Jules Brown, James Cameron, David Campbell, Ed Carlton, Phil Cowburn, Kate Crewdson, Leilah Dare, Keith Davies, Beverley Faulkner, Emma Gendall, Marianne Gillings, Elizabeth Goff, Scott Grier, Joydeep Grover, Debbie Harris, Timothy Hooper, Vicki Hughes, Dominic Janssen, Richard Jeavons, Claire Jewkes, Kirsten Jones, Ben Jordan, Phillip Kaye, Jason Kendall, Nagaraj Kumar, Simon Laing, David Lockey, Gordon Macfarlane, Alex Manara, Aidan Marsh, Rebecca Maxwell, Stephen Meek, Nicola Morgan, Patrick Morgan, Chris Newell, Delia Parnam-Cope, Simon Odum, Caroline Oliver, Harvey Pynn, Emma Redfern, Stephen Robinson, Kerry Smith, Reston Smith, Susan Smith, Jasmeet Soar, Deanna Stephens, Julian Thompson (PI), Ian Thomas, Matthew Thomas, Rebecca Thorpe, Edward Valentine, Sian Veysey, Ben Walton, Curtis Whittle, Ruth Worner, Paul Younge.
Nottingham University Hospitals NHS Trust: Shafique Ahmad, Lauren Blackburn, Adam Brooks (PI), Kate Brown, Paul Candler, Shalini Chinna, David Clarkson, Craig Douglas, Darren Forward, Ramzi Freij, Angelo La Valle, Chris Lamb, Shane Macsweeney, Chris Mason, Alex Navarro, Rory O’Connor, Matt O’Meara, Bob Winter, Adam Wolverson, Ju Young Um.
Oxford University Hospitals NHS Foundation Trust (Oxford): Chris Acott, Nurul Ali, Susan Anthony, Elaine Armstrong, Tanya Baron, Graham Barker, Neil Barker, Steven Barker, Sally Beer, Charlotte Brown, Rebekah Caseley, Andrew Chadwick, Benjamin Clare-Grey, Rebecca Clark, Sarah Cocker, Jessica Cottrell, Barbara Crowe, Andrea Dale, Colin Davenport, Luciana De Barbosa-Dias, Alberto Sobrino Diaz, Karen Dineen, Iain Edgar, Alexis Espinosa, Aoife Fitzgerald, Maria Garcia, Domonique Georgiou, Ben Griffiths, Conrad Groenwald, Oliver Harris, Jonathan Hughes, Rudi Jarai, Aimee Jeffs, Deon Louw, Joshua Lyons, Rufino Roger Magallano, Grigoris Makris, Sachin Mandalia, Priyadarshini Marathe, Jose Martinez, Syed Masud, Alex Mattin, Maria Fernandez Mendoza, Rocio Fernandez Mendoza, Edward Norris-Cervetto, Alex Novak (PI), John McMaster, Ross Moy, Aman Paul, Elena Perez, Claire Pickering, Victoria Prabhu, Vishakha Prasad, Surabhi Ramsundar, Veronica Sanchez, Ros Simpson, Priyanka Singh, Simon Smith, Enrico Sorrentino, Tim Sparkes, Hannah Thraves, Andrew Wigham, James Winchester.
Sheffield Teaching Hospitals NHS Foundation Trust: Paul Barker, Tom Bircher, Sarah Bird, Trevor Cleveland, Chris Connolly, Ben Cooper, Andreas Crede, Michael Dennison, Ben Edwards, Gordon Fuller, Stephen Goode, John Griffiths, Sherif Hemaya, Anil Hormis, Khurram Iftikhar, Robert Jones, Avril Kuhrt, Daniel Kusuma, Tom Locker, Ben Loryman, George Lye, Edward Mills, Timothy Moll, Christopher Press, Mark Regi, Stuart Reid (PI), Steve Rowe, Karen Robinson, Neil Sambridge, Justin Squires, Neil Strawbridge, Stephen Thomas, Douglas Turner, Erica Wallis, Matthew Wiles, Anna Wilson, Samantha Wilson, Chris Yap.
South Tees Hospital NHS Foundation Trust (Middlesbrough): Kerry Colling, Tracy Ruddick, Chris Smith (PI).
St Georges University Hospitals NHS Foundation Trust: Thomas Breen, Louisa Brown, Diego Campos Ceia, Raj Das, Nadine Davison, Richard Carden, Orla Dooley, Alex Eeles, Kevin Enright, Risolvo Gianluca, Grazia Freni, Sobika Gangeswaran, Will Glazebrook, Mark Haden, Tim Hardiman, Richard Hartopp, Paul Holmes, Hannah Hornsby, Mansoor Husain, Anthony Hudson (PI), Heather Jarman, Jeyanathan Jeysankar, Sarah Jolly, Gabriel Jones, Kamila Kalka, Sarah Krishnamandan, Ana Lisboa, Alvina Lone, Catherine Loughran, William McGuiness, Marco Machado, Ahmed Mahdi, Raul Marques, Leto Malli, Gary Maytham, Phil Moss, Faheem Obaidullah, Teresa Parras, Benjamin Patterson, Alex Pickard, Naomi Pritchard, Michael Putnis, Mehrad Ramzamy, Lakshmi Ratnam, Seyed Renani, Mark Reaveley, Daniel Roberts, Oluwaseun Samuel, Guy Sanders, Ariadna Sanchez, Claire Seel, Narani Sivayoham, Audrey Tan, Lorenzo Tiraboschi, Harriet Tucker, Pallavi Walkar.
University Hospital Birmingham NHS Foundation Trust: Amy Bamford, Colin Bergin, Barry Boland, Matthew Boylan, Ronald Carrera, Amy Clark, Lauren Cooper, Liesl Depsy, Natalie Dooley, Moin Durrani, Karen Ellis, Emma Fellows, Morgan Foster, Stephanie Goundry, Mycroft Halliwell-Ewen, Jennifer Hardy, Samantha Harkett, Damian Keene, Justine Lee, Hadassah Lhlenfeldt, Christopher McGhee, Azam Majeed, Tracy Mason, Ansar Mahmmod (PI), Kalyana Murali, Aoife Neal, Paul Parker, Stephanie Porter, Emma Reeves, Saif Rehman, Angus Selby, Hazel Smith, Elaine Spruce, Jon Vendrew.
University Hospitals Coventry and Warwickshire NHS Trust: Daniel Anotike, James Ashford, Richard Atkins, Chris Bassford, Richard Bowman, Pamela Bremmer, James Chinery, Sasathorn Chutimaworaphan, Laura Conway, Tim Daves, James Davidson, Marie Fogarty, Arun George (PI), Edward Hartley, Duncan Haynes, Michael Helme, Marius Holmes, Aliraza Husain, Karen Jones, Andrew Kelly, Anna Kurek, Lucinda Lacey, Caroline Leech, Thomas Mcarthy, George Madden, Laura May, Katy Moon, Catherine Morgan, Charles Pairaudeau, Mark Pell, Mark Porter, Sathananthan Ratrani, Matt Robbins, Rachel Rose, Magdy Sakr, Robert Simpson, Carly Swann, Chris Turner, Imogen Virgo, Geraldine Ward, Laura Wild, Helen Wilkins, Nichola Williams, Matthew Wyse.
University Hospitals of North Midlands NHS Trust: Felicity Avann, John Beckett, Jon Bingham, Astrophel Castro, James Chinery (PI), David Cooper, Chris Day, Scott Farmery, Richard Fawcett, Kay Finney, Minerva Gellamucho, Richard Hall, Paul Hancock, Joanne Hiden, Ben Hockenhall, Alex James, Matt O’Meara (PI), Mary-Jane Newton, Sam Parry, Ida Ponce, Fatemah Sakinia, Jo Smith, Richard Smith, Resti Varquez.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used #datasaveslives.
You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
This trial was approved by the North West – Greater Manchester South Research Ethics Committee on 26 June 2017, REC reference: 17/NW/0352.
Information governance statement
The University of Aberdeen is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, the University of Aberdeen is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.abdn.ac.uk/about/privacy/.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/LTYV4082.
Primary conflicts of interest: Jemma Hudson, Graeme MacLennan, Dwayne Boyers and Seonaidh Cotton report NIHR grant funding to their institution. Marion K Campbell reports NIHR grant funding to her institution, and membership of the following: NIHR CTU Standing Advisory Committee (2014–18); MRC Better Methods Better Health Panel (2023–ongoing); NIHR Clinical Trials Fellowships Panel (2017–18) and NIHR HSDR Board (2012–15). John Norrie reports NIHR grant funding to his institution and membership of the following: EME Funding Committee Sub-Group Remit and Comp Check (August 2019–current), HTA General Committee (2016–19); HTA Post-Funding Committee teleconference (POC members to attend; 2016–19); HTA Funding Committee Policy Group (formerly CSG; 2016–19); COVID-19 Reviewing (2020); HTA Commissioning Committee (2010–16). Katie Gillies reports NIHR grant funding to her institution; MRC funding to her institution; Intuitive funding to her institution; and membership NIHR HTA Clinical Evaluation and Trials Committee (2020–ongoing). Fiona Lecky reports NIHR grant funding to her institution and membership of the following: HTA EESC Methods Group (2010–18); HTA EESC Panel (2010–18) and HTA Post-Funding Committee teleconference (POC members to attend; 2010–16). Robbie Lendrum reports NIHR grant funding to his institution; and stock or stock options – Advisory Board Certus Critical Care. Chris Moran reports paid Chair of European Masters Fracture Forum (Smith and Nephew); National Clinical Director for Trauma 2013–20 (NHS England); and National Strategic Incident Director 2020–present (NHS England). Jonathan J Morrison reports stock or stock options in Prytime Medical Inc. Anthony Hudson reports travel costs from NIHR Grant funding to travel to PI meeting. Ansar Mahmood reports payment or honoraria from Stryker Orthopaedics for medical education; payment or honoraria from Arthrex for medical education; payment or honoraria from Depuy Synthes for medical education; and is President of the British Trauma Society. Alex Novak reports RCEM Grant funding to his institution; SBRI Grant funding to his institution; NIHR Grant funding to his institution; consulting fees from GE Healthcare paid to his institution and self; support for attending meetings from GE Healthcare; and support for attending meetings from National Consortium of Intelligent Medical Imaging. Julian Thompson reports contracts as Clinical Director of Severn Major Trauma Network; payment or honoraria from University of Stavanger (Associate Professor, education and research). Other authors report no conflicts of interest.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Jansen JO, Cochran C, Boyers D, Gillies K, Lendrum R, Sadek S, et al. UK-REBOA Trial grantholders . The effectiveness and cost-effectiveness of resuscitative endovascular balloon occlusion of the aorta (REBOA) for trauma patients with uncontrolled torso haemorrhage: study protocol for a randomised clinical trial (the UK-REBOA trial). Trials 2022;23. https://doi.org/10.1186/s13063-022-06346-1.
- National Academies of Sciences, Engineering, and Medicine . A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury 2016. https://doi.org/10.17226/23511.
- Centre of Disease Control and Prevention . 10 Leading Causes of Death by Age Group, United States: 2017 n.d. www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2017-508.pdf (accessed 4 May 2023).
- Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma 2007;62:142-6.
- Davis JS, Satahoo SS, Butler FK, Dermer H, Naranjo D, Julien K, et al. An analysis of prehospital deaths: who can we save?. J Trauma Acute Care Surg 2014;77:213-8.
- Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 2012;73:S431-7.
- Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg 1995;32:925-1002.
- Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am 2012;92:843-58. https://doi.org/10.1016/j.suc.2012.05.002.
- Morrison JJ, Stannard A, Rasmussen TE, Jansen JO, Tai NRM, Midwinter MJ. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J Trauma Acute Care Surg 2013;75:263-8. https://doi.org/10.1097/TA.0b013e318299da0a.
- Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res 2013;184:414-21. https://doi.org/10.1016/j.jss.2013.05.099.
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60:S3-11.
- Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma 1976;16:610-5.
- Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J Trauma 1984;24:387-92.
- Sankaran S, Lucas C, Walt AJ. Thoracic aortic clamping for prophylaxis against sudden cardiac arrest during laparotomy for acute massive hemoperitoneum. J Trauma 1975;15:290-6.
- Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery 2013;153:848-56.
- Barnard EB, Morrison JJ, Madureira RM, Lendrum R, Fragoso-Iñiguez M, Edwards A, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a population based gap analysis of trauma patients in England and Wales. Emerg Med J 2015;32:926-32.
- White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery 2011;150:400-9.
- Morrison JJ, Ross JD, Markov NP, Scott DJ, Spencer JR, Rasmussen TE. The inflammatory sequelae of aortic balloon occlusion in hemorrhagic shock. J Surg Res 2014;191:423-31.
- Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg 2013;75:506-11. https://doi.org/10.1097/TA.0b013e31829e5416.
- Martinelli T, Thony F, Decléty P, Sengel C, Broux C, Tonetti J, et al. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma 2010;68:942-8.
- Saito N, Matsumoto H, Yagi T, Hara Y, Hayashida K, Motomura T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78:897-903; discussion 904.
- Brenner M, Teeter W, Hoehn M, Pasley J, Hu P, Yang S, et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg 2018;153:130-5.
- DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg 2016;81:409-19.
- Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg 2015;79:523-30; discussion 530.
- Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg 2015;78:721-8.
- Bekdache O, Paradis T, Shen YBH, Elbahrawy A, Grushka J, Deckelbaum DL, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a scoping review protocol concerning indications—advantages and challenges of implementation in traumatic non-compressible torso haemorrhage. BMJ Open 2019;9.
- Borger van der Burg BLS, Dongen TTCF, Morrison JJ, Hedeman Joosten PPA, DuBose JJ, Hörer TM. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg 2018;44:535-50.
- Castellini G, Gianola S, Biffi A, Porcu G, Fabbri A, Ruggieri MP, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in patients with major trauma and uncontrolled haemorrhagic shock: a systematic review with meta-analysis. World J Emerg Surg 2021;16.
- Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 2016;80:324-34. https://doi.org/10.1097/TA.0000000000000913.
- Manzano Nunez R, Naranjo MP, Foianini E, Ferrada P, Rincon E, García-Perdomo HA, et al. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J Emerg Surg 2017;12.
- Cannon J, Morrison J, Lauer C, Grabo D, Polk T, Blackbourne L, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhagic shock. Mil Med 2018;183:55-9.
- Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open 2018;3.
- Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. PARAMEDIC trial collaborators . Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. https://doi.org/10.1016/S0140-6736(14)61886-9.
- Nahmias J, Byerly S, Stein D, Haut ER, Smith JW, Gelbard R, et al. A core outcome set for resuscitative endovascular balloon occlusion of the aorta: a consensus based approach using a modified Delphi method. J Trauma Acute Care Surg 2022;92:144-51. https://doi.org/10.1097/TA.0000000000003405.
- Holcomb JB, Moore EE, Sperry JL, Jansen JO, Schreiber MA, Del Junco DJ, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg 2021;273:395-401. https://doi.org/10.1097/SLA.0000000000004563.
- Marincowitz C, Bouamra O, Coats T, Surendra Kumar D, Lockey D, Mason L, et al. Major trauma presentations and patient outcomes in English hospitals during the COVID-19 pandemic: An observational cohort study. PLoS Med 2023;20. https://doi.org/10.1371/journal.pmed.1004243.
- College Station TSL . Stata Statistical Software: Release 17 2021. https://blog.stata.com/2021/04/20/stata-17-released/ (accessed 4 May 2023).
- Jansen JO, Pallmann P, MacLennan G, Campbell MK. ; UK-REBOA Trial Investigators . Bayesian clinical trial designs: another option for trauma trials?. J Trauma Acute Care Surg 2017;83:736-41. https://doi.org/10.1097/TA.0000000000001638.
- Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA 2018;320:2251-9. https://doi.org/10.1001/jama.2018.14276.
- Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017;318:1550-60. https://doi.org/10.1001/jama.2017.14972.
- Clark TP, Kahan BC, Phillips A, White I, Carpenter JR. Estimands: bringing clarity and focus to research questions in clinical trials. BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2021-052953.
- Cook JA, MacLennan GS, Palmer T, Lois N, Emsley R. Instrumental variable methods for a binary outcome were used to informatively address noncompliance in a randomized trial in surgery. J Clin Epidemiol 2018;96:126-32. https://doi.org/10.1016/j.jclinepi.2017.11.011.
- Jansen J, Wang H, Holcomb J, . Elicitation of prior probability distributions for a proposed Bayesian randomized clinical trial of whole blood for trauma resuscitation. Transfusion 2020;60:498-506. https://doi.org/10.1111/trf.15675.
- Colson AR, Cooke RM. Expert elicitation: using the classical model to validate experts’ judgments. Rev Environ Econ Policy 2018;12:113-32. https://doi.org/10.1093/reep/rex022.
- Hassall KL, Dailey G, Zawadzka J, Milne AE, Harris JA, Corstanje R, et al. Facilitating the elicitation of beliefs for use in Bayesian belief modelling. Environ Model Softw 2019;122. https://doi.org/10.1016/j.envsoft.2019.104539.
- Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Methods to elicit beliefs for Bayesian priors: a systematic review. J Clin Epidemiol 2009;63:355-69. https://doi.org/10.1016/j.jclinepi.2009.06.003.
- O’Hagan A. Expert knowledge elicitation: subjective but scientific. Ame Stat 2019;73:69-81. https://doi.org/10.1080/00031305.2018.1518265.
- O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain judgements: eliciting expert probabilities 2006. https://doi.org/10.1002/0470033312.
- Mason AJ, Gomes M, Grieve R, Ulug P, Powell JT, Carpenter J. Development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes: application to the IMPROVE trial. Clin Trials 2017;14:357-67. https://doi.org/10.1177/1740774517711442.
- R Foundation for Statistical Computing, Vienna, Austria . A Language and Environment for Statistical Computing 2016. www.R-project.org/ (accessed 4 May 2023).
- Chang W, Cheng J, Allaire JJ, Sievert C, Schloerke B, Xie Y, et al. Shiny: Web Application. Framework for R. R Package Version 1.2.0. Updated 2019. https://CRAN.R-project.org/package=shiny (accessed 4 May 2023).
- Lawrie L, Duncan EM, Jansen JO, Campbell MK, Brunsdon D, Skea Z, et al. Behavioural optimisation to address trial conduct challenges: case study in the UK-REBOA trial. Trials 2022;23:398-6. https://doi.org/10.1186/s13063-022-06341-6.
- Foster N, Little P. Methodological issues in pragmatic trials of complex interventions in primary care. Br J Gen Pract 2012;62:10-1. https://doi.org/10.3399/bjgp12X616238.
- Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19:50-6. https://doi.org/10.1186/s13063-017-2413-6.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60. https://doi.org/10.1177/1049732315617444.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Jackson K, Bazeley P. Qualitative Data Analysis with NVivo. Los Angeles, CA: SAGE Publications Ltd; 2019.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Gillies K, Brehaut J, Coffey T, Duncan EM, Francis JJ, Hey SP, et al. How can behavioural science help us design better trials?. Trials 2021;22:8-82. https://doi.org/10.1186/s13063-021-05853-x.
- Brehaut JC, Lavin Venegas C, Hudek N, Presseau J, Carroll K, Rodger M. Using behavioral theory and shared decision-making to understand clinical trial recruitment: interviews with trial recruiters. Trials 2021;22:2-98. https://doi.org/10.1186/s13063-021-05257-x.
- Newlands R, Duncan EM, Presseau J, Treweek S, Lawrie L, Bower P, et al. Why trials lose participants: a multitrial investigation of participants’ perspectives using the theoretical domains framework. J Clin Epidemiol 2021;137:1-13. https://doi.org/10.1016/j.jclinepi.2021.03.007.
- Lawrie L, Duncan EM, Dunsmore J, Newlands R, Gillies K. Using a behavioural approach to explore the factors that affect questionnaire return within a clinical trial: a qualitative study based on the theoretical domains framework. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-048128.
- Presseau J, Ivers NM, Newham JJ, Knittle K, Danko KJ, Grimshaw JM. Using a behaviour change techniques taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implement Sci 2015;10:55-7. https://doi.org/10.1186/s13012-015-0248-7.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. London: Silverback Publishing; 2014.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660-80.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77-9. https://doi.org/10.1186/s13012-017-0605-9.
- McGowan LJ, Powell R, French DP. How can use of the theoretical domains framework be optimized in qualitative research? A rapid systematic review. Br J Health Psychol 2020;25:677-94. https://doi.org/10.1111/bjhp.12437.
- Jones K, Burns A. Unit Costs of Health and Social Care 2021 2021. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-of-health-and-social-care-2021/ (accessed 4 May 2023).
- Public Health Scotland . Scottish Health Service Costs. Costs Book 2020 (April 2019 to March 2020) 2021. https://publichealthscotland.scot/publications/scottish-health-service-costs/scottish-health-service-costs-costsbook-2020-april-2019-to-march-2020/ (accessed 4 May 2023).
- Stokes EA, Wordsworth S, Staves J, Mundy N, Skelly J, Radford K, et al. Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom national health service. Transfusion 2018;58:846-53. https://doi.org/10.1111/trf.14493.
- Devlin N, Parkin D, Janssen B. Cham: Springer; 2020.
- Williamstown MTSL, 2021. TreeAge Software. Treeage Pro; 2021.
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1-79. https://doi.org/10.3310/hta17100.
- Holtslag HR, van Beeck EF, Lindeman E, Leenen LP. Determinants of long-term functional consequences after major trauma. J Trauma 2007;62:919-27. https://doi.org/10.1097/01.ta.0000224124.47646.62.
- Wad MS, Laursen T, Fruergaard S, Morgen SS, Dahl B. Survival and health related quality of life after severe trauma − a 15 years follow up study. Injury 2018;49:191-4. www.sciencedirect.com/science/article/pii/S0020138317306642.
- van der Vlegel M, Haagsma JA, Havermans RJM, de Munter L, de Jongh MAC, Polinder S. Long-term medical and productivity costs of severe trauma: results from a prospective cohort study. PLOS ONE 2021;16. https://doi.org/10.1371/journal.pone.0252673.
- CCEMG – EPPI-Centre Cost Converter v.1.4 n.d. https://eppi.ioe.ac.uk/costconversion/ (accessed 4 May 2023).
- Tappenden P, Chilcott JB. Avoiding and identifying errors and other threats to the credibility of health economic models. PharmacoEconomics 2014;32:967-79. https://doi.org/10.1007/s40273-014-0186-2.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- National Life UK, 1980–1982 to 2018–2020 2021. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 20 January 2023).
- Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg 2019;154:500-8. https://doi.org/10.1001/jamasurg.2019.0096.
- Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J Trauma Acute Care Surg 2016;80:559-66; discussion 566. https://doi.org/10.1097/TA.0000000000000968.
- García AF, Manzano-Nunez R, Orlas CP, Ruiz-Yucuma J, Londoño A, Salazar C, et al. Association of resuscitative endovascular balloon occlusion of the aorta (REBOA) and mortality in penetrating trauma patients. Eur J Trauma Emerg Surg 2021;47:1779-85. https://doi.org/10.1007/s00068-020-01370-9.
- Yamamoto R, Cestero RF, Suzuki M, Funabiki T, Sasaki J. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: a propensity score matching analysis. Am J Surg 2019;218:1162-8. https://doi.org/10.1016/j.amjsurg.2019.09.007.
- Moran CG, Lecky F, Bouamra O, Lawrence T, Edwards A, Woodford M, et al. Changing the system – major trauma patients and their outcomes in the NHS (England) 2008–17. EClinicalMedicine 2018;2–3:13-21. https://doi.org/10.1016/j.eclinm.2018.07.001.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. PROPPR Study Group . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471-82. https://doi.org/10.1001/jama.2015.12.
- Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC. PROPPR Study Group . Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock 2017;47:567-73. https://doi.org/10.1097/SHK.0000000000000788.
Appendix 1 Members of UK-REBOA Study Group
Chris Aylwin (https://orcid.org/0000-0002-3212-1689) (Consultant Vascular and Trauma Surgeon, St Mary’s Hospital, London, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Duncan Bew (https://orcid.org/0000-0003-2849-3567) (Trauma and Acute Care Surgeon, King’s College Hospital, London, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Adam Brooks (https://orcid.org/0000-0002-9673-0248) (Consultant Hepatobiliary and Trauma Surgeon, Queen’s Medical Centre, Nottingham, UK) was principal investigator at a UK-REBOA recruitment site and contributed to the interpretation of results and writing/editing the report.
James Chinery (https://orcid.org/0000-0001-7938-3291) (Consultant in Trauma Anaesthesia and Pre-hospital Emergency Medicine, University Hospitals of North Midlands, Stoke, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Tom Cowlam (https://orcid.org/0009-0005-1907-1639) (Consultant Anaesthetist, Hull University Teaching Hospitals, Hull, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Dan Frith (https://orcid.org/0000-0002-9719-0327) (Consultant Trauma Surgeon, St Mary’s Hospital, London, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Arun George (https://orcid.org/0000-0003-4104-693X) (Consultant in Emergency Medicine, University Hospital, Coventry, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Anthony Hudson (https://orcid.org/0009-0005-3231-8077) (Consultant in Emergency Medicine, St George’s University Hospital, London, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Phillip Johnstone (https://orcid.org/0000-0002-9955-6425) (Consultant in Emergency Medicine, Royal Victoria Infirmary, Newcastle, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Ansar Mahmood (https://orcid.org/0000-0003-1359-6651) (Consultant in Trauma and Orthopaedic Surgery, Queen Elizabeth Hospital, Birmingham, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Alex Novak (https://orcid.org/0000-0002-5880-8235) (Consultant in Emergency Medicine and Ambulatory Care, John Radcliffe Hospital, Oxford, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Matt O’Meara (https://orcid.org/0009-0000-4388-9338) (Consultant Anaesthetist, University Hospitals of North Midlands, Stoke, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Stuart Reid (https://orcid.org/0000-0002-3129-7711) (Consultant in Emergency Medicine and Major Trauma, Sheffield Teaching Hospital, Sheffield, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Abdo Sattout (https://orcid.org/0000-0003-1848-9396) (Consultant in Emergency Medicine, Aintree University Hospital, Liverpool, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Chris Smith (https://orcid.org/0000-0002-7646-4122) (Consultant in Emergency Medicine and Pre-hospital Emergency Medicine, James Cook University Hospital, Liverpool, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Tim Stansfield (https://orcid.org/0000-0002-5875-6279) (Consultant Vascular and Trauma Surgeon, Leeds General Infirmary, Leeds, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Julian Thompson (https://orcid.org/0009-0003-8949-1220) (Consultant in Intensive Care Medicine and Anaesthesia, Southmead Hospital, Bristol, UK) was principal investigator at a UK-REBOA trial recruitment site and contributed to the interpretation of results and writing/editing the report.
Appendix 2 United Kingdom-resuscitative endovascular balloon occlusion of the aorta trial recruitment sites
Aintree University Hospital, Liverpool
James Cook University Hospital, Middlesbrough
John Radcliffe Hospital, Oxford
King’s College Hospital, London
Leeds General Infirmary, Leeds
Queen Elizabeth Hospital, Birmingham
Queen’s Medical Centre, Nottingham
Royal Hull Infirmary, Hull
Royal Stoke University Hospital, Stoke
Royal Victoria Infirmary, Newcastle
Sheffield Teaching Hospital, Sheffield
Southmead Hospital Bristol, Bristol
St George’s University Hospital, London
St Mary’s Hospital, London
The Royal London Hospital, London
University Hospital, Coventry
Appendix 3 Evidence dossier used for elicitation exercise

Evidence for consideration
We have identified the following references as potentially relevant to the elicitation meeting. You may wish to review these or keep them for reference.
We do not expect you to read them all – this list is purely for your information. Please read as many or as few as you feel able to (although it would likely be helpful for you to dip into at least one or two of them if you have time).
We have only included studies that relate to the impact of REBOA on mortality, in humans, with a comparison group. The hyperlinks are clickable so that you can review the abstracts and/or full papers. There are two sections: Systematic reviews and meta-analyses, and comparative studies. Lastly, we have also included our epidemiological paper. The hyperlinks are clickable and will take you to abstracts in PubMed.
This is not a systematic review. If there are other studies that you are aware of, which we have failed to include, please let us know and we will add them.
Systematic reviews and meta-analyses
-
A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients.
Manzano Nunez R, Naranjo MP, Foianini E, Ferrada P, Rincon E, García-Perdomo HA, et al. World J Emerg Surg 2017;12:30. https://doi.org/10.1186/s13017-017-0142-5
-
Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of trauma patients: a systematic literature review.
Petrone P, Pérez-Jiménez A, Rodríguez-Perdomo M, Brathwaite CEM, Joseph DK. Am Surg 2019;85(6):654–62. PMID: 31267908.
-
Resuscitative endovascular balloon occlusion of the aorta in trauma: a systematic review of the literature.
Gamberini E, Coccolini F, Tamagnini B, Martino C, Albarello V, Benni M, et al. World J Emerg Surg 2017;12:42. https://doi.org/10.1186/s13017-017-0153-2. eCollection 2017. PMID: 28855960.
-
Resuscitative Endovascular Balloon Occlusion of the Aorta for Control of Non-Compressible Truncal Hemorrhage: A Review of Clinical Effectiveness and Guidelines [Internet].
-
Richardson R, Adcock L. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 7 March 2018. PMID: 30325621.
-
A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination.
-
Borger van der Burg BLS, van Dongen TTCF, Morrison JJ, Hedeman Joosten PPA, DuBose JJ, Hörer TM, Hoencamp R. Eur J Trauma Emerg Surg 2018;44:535–50. https://doi.org/10.1007/s00068-018-0959-y. Epub May 21 2018. PMID: 29785654.
-
A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock.
Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. J Trauma Acute Care Surg 2016;80:324–34. https://doi.org/10.1097/TA.0000000000000913. PMID: 26816219.
-
Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of trauma patients: a systematic literature review.
Petrone P, Pérez-Jiménez A, Rodríguez-Perdomo M, Brathwaite CEM, Joseph DK. Am Surg 2019;85:654–62. PMID: 31267908.
Comparative studies
-
1. Resuscitative endovascular balloon occlusion of the aorta (REBOA) for severe torso trauma in Japan: a descriptive study.
Matsumoto S, Hayashida K, Akashi T, Jung K, Sekine K, Funabiki T, Moriya T. World J Surg 2019;43:1700–7. https://doi.org/10.1007/s00268-019-04968-2. PMID: 30824958.
-
9. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: A propensity score matching analysis.
-
Yamamoto R, Cestero RF, Suzuki M, Funabiki T, Sasaki J. Am J Surg 2019;218:1162–8. https://doi.org/10.1016/j.amjsurg.2019.09.007. Epub Sep 13 2019. PMID: 31540683.
-
10. Resuscitative endovascular balloon occlusion of the aorta or resuscitative thoracotomy with aortic clamping for noncompressible torso hemorrhage: a retrospective nationwide study.
-
Aso S, Matsui H, Fushimi K, Yasunaga H. J Trauma Acute Care Surg 2017;82:910–4. https://doi.org/10.1097/TA.0000000000001345. PMID: 28430760.
-
11. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma.
-
Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. JAMA Surg. 2019;154:500–8. https://doi.org/10.1001/jamasurg.2019.0096. PMID: 30892574.
-
12. Association of resuscitative endovascular balloon occlusion of the aorta (REBOA) and mortality in penetrating trauma patients.
-
García AF, Manzano-Nunez R, Orlas CP, Ruiz-Yucuma J, Londoño A, Salazar C, et al. Eur J Trauma Emerg Surg 2020. https://doi.org/10.1007/s00068-020-01370-9. Online ahead of print. PMID: 32300850.
-
13. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients.
-
Norii T, Crandall C, Terasaka Y. J Trauma Acute Care Surg 2015;78:721–8. https://doi.org/10.1097/TA.0000000000000578. PMID: 25742248.
-
14. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan.
-
Abe T, Uchida M, Nagata I, Saitoh D, Tamiya N. Crit Care 2016;20:400. https://doi.org/10.1186/s13054-016-1577-x. PMID: 27978846.
-
1. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis.
-
Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. J Trauma Acute Care Surg 2016;80:559–66; discussion 566–7. https://doi.org/10.1097/TA.0000000000000968. PMID: 26808039.
-
16. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA).
-
DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, et al. ; AAST AORTA Study Group. J Trauma Acute Care Surg 2016;81:409–19. https://doi.org/10.1097/TA.0000000000001079. PMID: 27050883.
-
17. Temporal changes in REBOA utilization practices are associated with increased survival: an analysis of the aorta registry.
-
Bukur M, Gorman E, DiMaggio C, Frangos S, Morrison JJ, Scalea TM, et al. ; and the AAST AORTA Study Group. Shock 2020. https://doi.org/10.1097/SHK.0000000000001586. Online ahead of print. PMID: 32842023.
Epidemiological studies
-
18. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a population based gap analysis of trauma patients in England and Wales.
-
Barnard EB, Morrison JJ, Madureira RM, Lendrum R, Fragoso-Iñiguez M, Edwards A, et al. Emerg Med J 2015;32:926–32. https://doi.org/10.1136/emermed-2015-205217. PMID: 26598631.
Appendix 4 Elicitation exercise
FIGURE 17.
Screenshot showing the ‘sliders’ used to set the median and lower and upper plausible values, and the resulting graphical output as a probability density distribution.
FIGURE 18.
Plots of beta distribution of each individual expert (dashed line) and the plot of linear pool of the beta distributions of all experts (solid line) for (a) REBOA and (b) standard care for 6-hour mortality.

FIGURE 19.
The prior distribution of logarithm of OR for REBOA for standard care based on 10,000 samples for 6-hour mortality.

FIGURE 20.
Plots of beta distribution of each individual expert (dashed line) and the plot of linear pool of the beta distributions of all experts (solid line) for (a) REBOA and (b) standard care for 24-hour mortality.

FIGURE 21.
The prior distribution of logarithm of OR for REBOA to standard care based on 10,000 samples for 24-hour mortality.
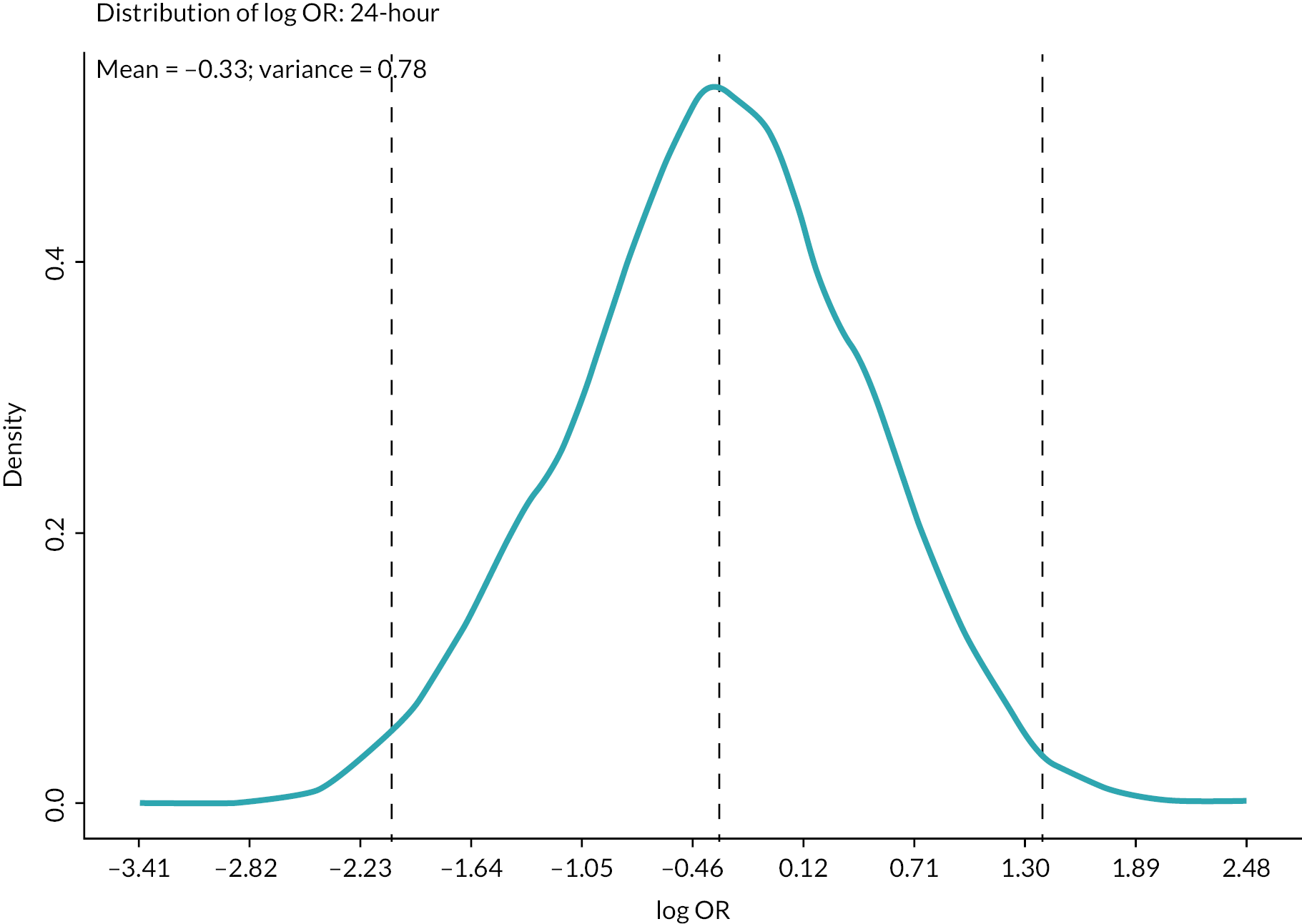
FIGURE 22.
Plots of beta distribution of each individual expert (dashed line) and the plot of linear pool of the beta distributions of all experts (solid line) for (a) REBOA and (b) standard care for 90-day mortality.
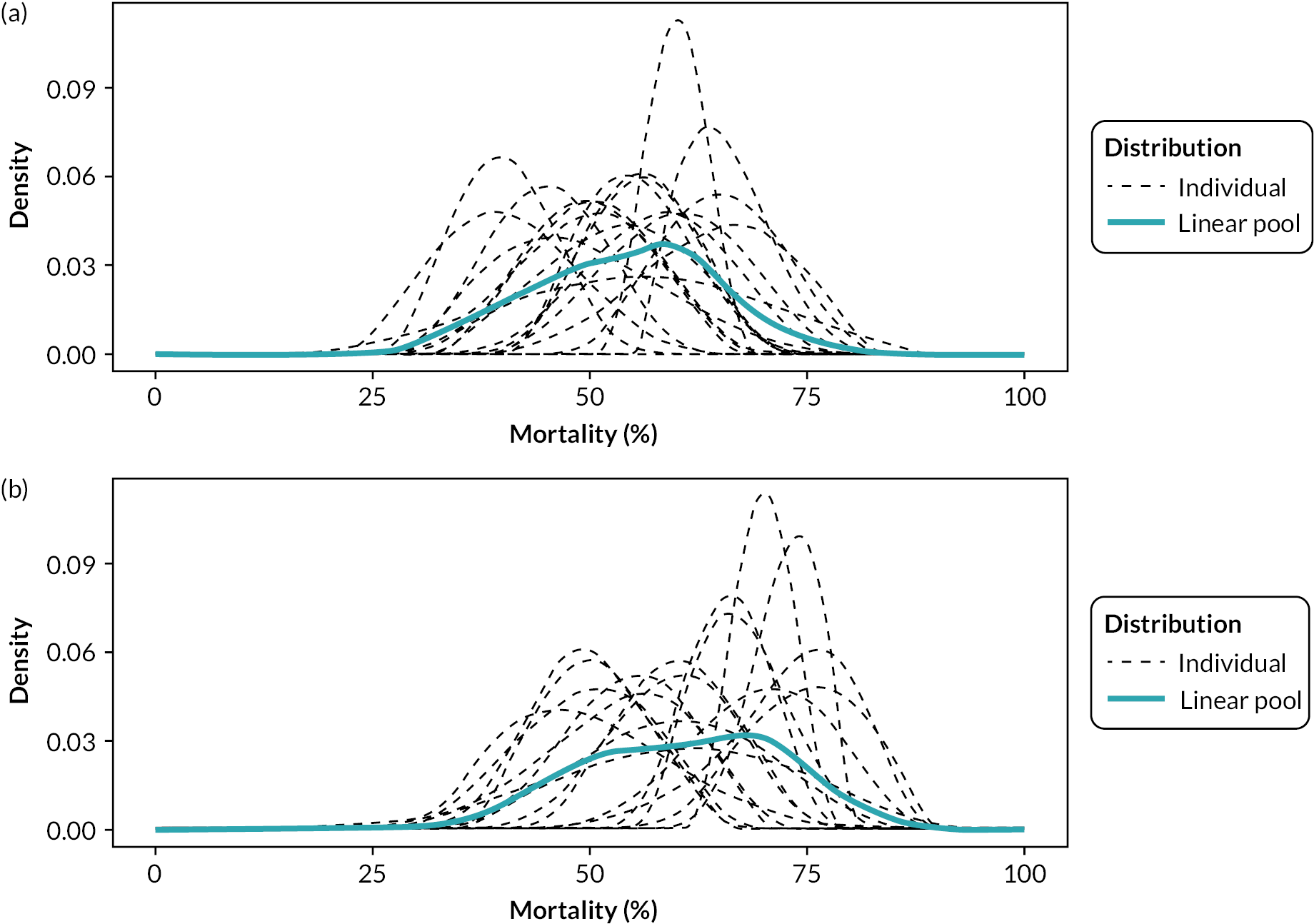
FIGURE 23.
The prior distribution of logarithm of OR for REBOA to standard care based on 10,000 samples with 90-day mortality.
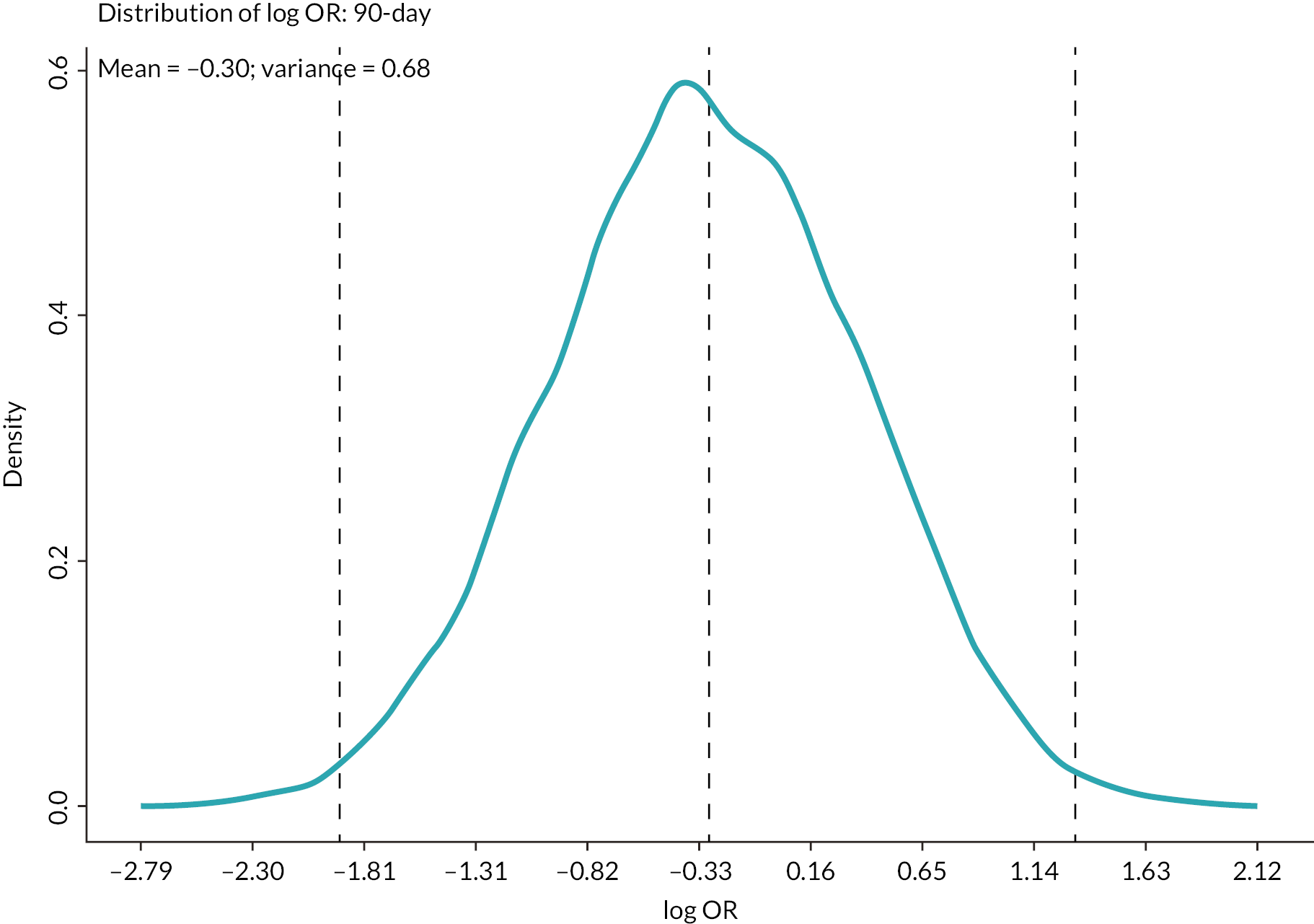
FIGURE 24.
Plots of beta distribution of each individual expert (dashed line) and the plot of linear pool of the beta distributions of all experts (solid line) for (a) REBOA and (b) standard care for in-hospital mortality.

FIGURE 25.
The prior distribution of logarithm of OR for REBOA to standard care based on 10,000 samples for in-hospital mortality.

Appendix 5 Process evaluation: overview of proposed solutions
| Proposed solution (s) | Proposed content | Selected BCT(s) (domain-relevant/supplementary) | Belief statements (salient barriers/enablers, linked to TDF domains) | Inclusion record (including APEASE criteria) |
|---|---|---|---|---|
| Training | Target altruistic emotions – express satisfaction of being part of a trial which will influence clinical practice | 5.6. Information about emotional consequences | ‘Reputational benefit for the institute associated with being able to recruit patients and deploy REBOA’ (TDF Beliefs about consequences) | Include BCTs 5.6., 9.2., 3.2., 5.1., 5.2.: All APEASE criteria met |
| Encourage reflection of the pros/cons to recruitment in the trial generally. Including advantages of knowing which clinical method is most effective. Highlight how the research will influence clinical practice | 9.2. Pros and cons | ‘REBOA may be beneficial’ (TDF Beliefs about consequences) | Exclude BCT 5.5: May not be acceptable. Many valid reasons for not recruiting eligible patients, external, out-with control. APEASE Acceptability, Equity and Side-Effects criteria not met | |
| Remind staff about the potential benefits of REBOA to patients with traumatic injury, despite the associated risks. Also benefits of not doing REBOA – SC. Purpose of the trial is to find out which method is best | 5.1. Information about health consequences | ‘REBOA may cause complications’ (TDF Beliefs about consequences) | ||
| Highlight that staff are contributing to valuable research which will also benefit the reputation of each institute | 5.3. Information about social and environmental consequences |
‘It can be difficult to define exsanguinating haemorrhage’ (TDF Beliefs about consequences) | ||
| Present case studies of real-life examples where patients have been treated with REBOA and SC, and highlight the valuable contribution of the trial | 5.2. Salience of consequences | |||
| Link the benefit of taking part in the trial to anticipated regrets of failing to recruit eligible patients. Remind staff of the scarcity of cases. Highlight the requirement to address trial research question | 5.5. Anticipated regret | |||
| Training | Include step-by-step instructions on how to recognise eligibility and perform REBOA: provide a demonstration by presenting video clips. All sites have to agree on eligibility criteria. Provide case study examples | 6.1. Demonstration of the behaviour | ‘Recognising an eligible patient requires expertise’ (TDF Skills) ‘Insertion of REBOA can be technical’ (TDF Skills) ‘Concerns about competency due to low throughput of cases’ (TDF Skills) |
Include all BCTs (already delivered during on-site training): APEASE criteria met |
| Set easy-to-achieve tasks (e.g. the areas which site staff find simple to complete, such as navigating the randomisation app) and progress to more complex steps, such as monitoring eligibility and performing REBOA | 8.7. Graded tasks 8.1. Behavioural practice/rehearsal |
|||
| Training | Incorporate advice on how to reduce the cognitive load of performing REBOA and randomising a patient. This can include assigning other tasks completed simultaneously to different members of the team | 11.3. Conserving mental resources | ‘You need to remember technical aspects of REBOA’ (TDF Memory Attention and Decision Processes) ‘Our team is inclined to wait to see if our patient requires REBOA’ (TDF Memory Attention and Decision Processes) |
|
| Environmental restructuring | Social prompt: Assign an individual to prompt REBOA randomisation/delivery when a potentially eligible patient is flagged. This could include prompting eligibility assessment or technical aspects of REBOA. Remind healthcare professionals of protocol | 7.1. Prompts/cues | The clinical context for REBOA is inherently stressful and fast-paced (TDF Environmental Context and Resources) | Include all BCTs: APEASE criteria met. While some BCTs were already incorporated in trial practices, it was recommended that delivery of all BCTs should be monitored to ensure continuous implementation |
| Encourage the use of memory aid sheets to facilitate memory of REBOA recruitment and the procedure. Can include provision of cue cards to be slotted into staff lanyards. Sites could purchase a mannequin/or recycle use of existing mannequin to practice REBOA on a weekly basis |
12.5. Adding objects to the environment | ‘There are so few patients who require REBOA’ (TDF Environmental Context and Resources) | ||
| Arrange for colleagues to provide practical help to recruitment and delivery of REBOA in each shift. This may include providing contact details of those who can help during out-of-hours | 3.2. Social support (practical) | ‘The ability to recruit depends on staff availability’ (TDF Environmental Context and Resources) | ||
| Assign REBOA champion roles at each site, highlight support available during team meetings | 12.2. Restructuring the social environment | |||
| Ensure staff have a device with the app readily accessible for randomisation and gather essential equipment or prepare a REBOA trolley to assist in the delivery of the intervention | 12.5. Adding objects to the environment | |||
| This could also include a diagram of the ideal positioning of staff during a code red call | 12.6. Body changes 12.1. Restructuring the physical environment |
|||
| Enablement | Encourage staff to praise local efforts of recruitment and REBOA delivery when applicable. Praise can also be communicated via e-mail, as well as during local PI meetings | 10.4. Social reward | ‘Our team is enthusiastic about the UK-REBOA trial’ (TDF Social influences) | Include all APEASE criteria met |
| Encourage sites to provide monthly updates on the progress of UK-REBOA trial recruitment and intervention delivery during trial meetings. Facilitate detailed discussion about recruitment procedures: ask staff to provide a description of latest recruitment cases including ‘near misses’ (when applicable). CIs to provide information about whether they approve of the procedures/decisions adopted | 6.3. Information about others’ approval | ‘People can hold different views about patient eligibility’ (TDF Social influences) ‘Our team has mixed levels of individual equipoise’ (TDF Social influences, TDF Beliefs about Consequences) |
While some BCTs were already incorporated in trial practices, it was recommended that delivery of all BCTs should be monitored to ensure continuous implementation | |
| Prompt discussion of what went well and what might have been done differently. Include action plans to tackle similar situations in the future | 3.2. Social Support (Practical) 6.2. Social comparison 1.2. Problem solving 1.4. Action planning |
|||
| Maintain the enthusiasm of REBOA by advising staff to encourage others to recruit and randomise eligible participants | 3.1. Social support (unspecified, practical) | |||
| See examples listed above 5.3.: Designed to target mixed levels of team equipoise (beliefs about the consequences of REBOA intervention delivery). Delivered as bespoke infographic to be distributed to all site staff | 5.3. Information about social and environmental consequences |
|||
| Provide contact details of Clinical CI and Clinical Lead: Highlight support available | 3.2. Social support (practical) | |||
|
Persuasion
enablement |
Remind staff that they have successfully performed REBOA and recruited participants in simulation and/or in real life. Enabled by PIs |
15.3. Focus on past success Can also be incorporated into training |
‘Clinicians have to be confident to deliver REBOA; this can influence recruitment’ (TDF Beliefs about capabilities) ‘There is lots of nervousness around delivering REBOA related to personal abilities’ (TDF Beliefs about capabilities) |
Exclude: Difficult to implement. Depends on factors less amenable to change – for example PI personality and workplace culture. BCTs 15.3. and 15.1. can instead be incorporated via trial Training practices. APEASE Effectiveness criteria not met. APEASE Practicability criteria not met for BCT 15.4. Difficult to implement in a trauma care setting |
| Local PIs can actively persuade relevant staff members that they are capable of performing the REBOA intervention during conversations/meetings. Highlight transferable skills of trial recruitment – include successful past experience of trial involvement | 15.1. Verbal persuasion about capability | |||
| Encourage staff to practice positive self-talk as a team: this could include discussing one’s own achievements/successes in a group setting. PIs to deliver | 15.4. Self-talk |
Appendix 6 Process evaluation: infographic

Appendix 7 Recruitment
| Site | SC + REBOA N = 46 | SC N = 44 |
Total N = 90 |
|---|---|---|---|
| Leeds General Infirmary | 9 (20) | 10 (23) | 19 (21) |
| The Royal London Hospital | 7 (15) | 7 (16) | 14 (16) |
| Queen Elizabeth Hospital, Birmingham | 5 (11) | 6 (14) | 11 (12) |
| John Radcliffe Hospital, Oxford | 4 (9) | 3 (7) | 7 (8) |
| Southmead Hospital Bristol | 4 (9) | 3 (7) | 7 (8) |
| Queen’s Medical Centre, Nottingham | 3 (7) | 4 (9) | 7 (8) |
| University Hospital, Coventry | 4 (9) | 3 (7) | 7 (8) |
| St George’s University Hospital | 3 (7) | 3 (7) | 6 (7) |
| Royal Victoria Infirmary, Newcastle | 2 (4) | 2 (5) | 4 (4) |
| Aintree University Hospital | 2 (4) | 1 (2) | 3 (3) |
| Sheffield Teaching Hospital | 2 (4) | 1 (2) | 3 (3) |
| St Mary’s Hospital, London | 1 (2) | 1 (2) | 2 (2) |
FIGURE 26.
Recruitment over time.

Appendix 8 Additional clinical results
| OR | 95% CrI | Posterior probability (%) of OR > 1a | |
|---|---|---|---|
| Age | 1.39 | (0.59 to 3.28) | 77.3 |
| Gender | 1.53 | (0.69 to 3.48) | 85.1 |
| ISS | 1.63 | (0.73 to 3.77) | 88.1 |
| AIS head | 1.61 | (0.72 to 3.79) | 87.2 |
| AIS face | 1.65 | (0.73 to 3.75) | 88.5 |
| AIS chest | 1.68 | (0.74 to 3.90) | 89.5 |
| AIS abdomen | 1.50 | (0.67 to 3.44) | 83.6 |
| AIS spine | 1.72 | (0.76 to 4.05) | 89.9 |
| AIS pelvic | 1.61 | (0.71 to 3.64) | 87.2 |
| AIS limbs | 1.69 | (0.73 to 3.99) | 89.0 |
| AIS other | 1.60 | (0.72 to 3.59) | 87.4 |
| Pre-hospital CPRb | 1.69 | (0.69 to 4.20) | 87.4 |
| ED SBPc | 1.53 | (0.69 to 3.52) | 84.9 |
| CPR on arrivalb | 1.62 | (0.72 to 3.71) | 87.9 |
| Time from arrival to randomisationd | 1.59 | (0.71 to 3.61) | 87.4 |
| Alle | 1.80 | (0.59 to 5.58) | 84.9 |
| All (removing ISS)f | 1.67 | (0.55 to 5.30) | 81.6 |
| AIS and ED SBP | 1.53 | (0.65 to 3.61) | 84.0 |
| SC + REBOA | SC | Minimally informative prior | Elicitation prior | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CrI | Posterior probability (%) of OR > 1a | OR | 95% CrI | Posterior probability (%) of OR > 1a | |||
| N = 46 | N = 43 | |||||||
| Death within 90 days | ||||||||
| Yes | 25 (54) | 18 (42) | 1.60 | (0.71 to 3.66) | 87.1 | 1.41 | (0.67 to 2.98) | 82.0 |
| No | 21 (46) | 25 (58) | ||||||
| Death within 6 months | ||||||||
| Yes | 25 (54) | 18 (42) | 1.60 | (0.71 to 3.66) | 87.1 | 1.41 | (0.67 to 2.98) | 82.0 |
| No | 21 (46) | 25 (58) | ||||||
| Death while in hospital | ||||||||
| Yes | 25 (54)Mortality at other time points | 18 (42) | 1.60 | (0.71 to 3.66) | 87.1 | 1.41 | (0.67 to 2.98) | 82.0 |
| No | 21 (46) | 25 (58) | ||||||
| N = 46 | N = 44 | |||||||
| Death within 24 hours | ||||||||
| Yes | 17 (37) | 10 (23) | 1.93 | (0.80 to 4.84) | 92.7 | 1.65 | (0.73 to 3.79) | 88.7 |
| No | 29 (63) | 34 (77) | ||||||
| Death within 6 hours | ||||||||
| Yes | 13 (28) | 4 (9) | 3.24 | (1.15 to 10.19) | 98.7 | 2.52 | (0.95 to 6.93) | 96.7 |
| No | 33 (72) | 40 (91) | ||||||
| Death within 3 hours | ||||||||
| Yes | 11 (24) | 2 (5) | 4.32 | (1.34 to 16.39) | 99.3 | 3.05 | (1.05 to 9.43) | 97.9 |
| No | 35 (76) | 42 (95) | ||||||
| OR | 95% CrI | Posterior probability (%) of OR > 1a | |
|---|---|---|---|
| Age | 3.89 | (1.16 to 15.52) | 98.6 |
| Gender | 4.24 | (1.29 to 16.33) | 99.2 |
| ISS | 5.20 | (1.50 to 21.78) | 99.6 |
| AIS head | 4.36 | (1.36 to 16.85) | 99.4 |
| AIS face | 4.32 | (1.34 to 17.03) | 99.3 |
| AIS chest | 5.80 | (1.68 to 23.96) | 99.8 |
| AIS abdomen | 4.12 | (1.24 to 16.10) | 99.0 |
| AIS spine | 5.80 | (1.73 to 23.04) | 99.8 |
| AIS pelvic | 4.40 | (1.36 to 17.16) | 99.4 |
| AIS limbs | 4.40 | (1.36 to 17.04) | 99.4 |
| AIS other | 4.48 | (1.39 to 17.08) | 99.4 |
| Pre-hospital CPRb | 4.63 | (1.35 to 18.58) | 99.3 |
| ED SBPc | 4.20 | (1.31 to 16.71) | 99.2 |
| CPR on arrivalb | 6.20 | (1.66 to 29.38) | 99.7 |
| Time from arrival to randomisationd | 4.35 | (1.36 to 16.82) | 99.4 |
| Alle | 9.80 | (1.80 to 57.91) | 99.6 |
| All (removing ISS)f | 8.77 | (1.80 to 49.74) | 99.6 |
| AIS and ED SBP | 4.25 | (1.28 to 15.96) | 99.1 |
| OR | 95% CrI | Posterior probability (%) of OR > 1a | |
|---|---|---|---|
| Age | 2.88 | (0.97 to 9.47) | 97.1 |
| Gender | 3.05 | (1.07 to 9.63) | 98.1 |
| ISS | 3.41 | (1.17 to 11.32) | 98.8 |
| AIS head | 3.22 | (1.14 to 10.18) | 98.7 |
| AIS face | 3.20 | (1.15 to 10.10) | 98.7 |
| AIS chest | 4.32 | (1.44 to 15.14) | 99.6 |
| AIS abdomen | 3.03 | (1.08 to 9.65) | 98.2 |
| AIS spine | 4.18 | (1.46 to 13.59) | 99.6 |
| AIS pelvic | 3.22 | (1.15 to 10.26) | 98.8 |
| AIS limbs | 3.64 | (1.25 to 11.79) | 99.2 |
| AIS other | 3.20 | (1.15 to 10.07) | 98.7 |
| Pre-hospital CPRb | 3.60 | (1.17 to 12.10) | 98.8 |
| ED SBPc | 3.08 | (1.09 to 9.83) | 98.3 |
| CPR on arrivalb | 4.42 | (1.36 to 17.18) | 99.3 |
| Time from arrival to randomisationd | 3.25 | (1.16 to 10.11) | 98.8 |
| Alle | 9.75 | (2.12 to 50.39) | 99.9 |
| All (removing ISS)f | 10.44 | (2.27 to 52.24) | 99.8 |
| AIS and ED SBP | 3.08 | (1.08 to 9.51) | 98.2 |
| OR | 95% CrI | Posterior probability (%) of OR > 1a | |
|---|---|---|---|
| Age | 1.66 | (0.69 to 4.20) | 86.7 |
| Gender | 1.80 | (0.75 to 4.49) | 90.4 |
| ISS | 1.88 | (0.78 to 4.66) | 91.9 |
| AIS head | 1.88 | (0.77 to 4.75) | 91.8 |
| AIS face | 1.92 | (0.81 to 4.75) | 93.1 |
| AIS chest | 2.10 | (0.88 to 5.43) | 95.1 |
| AIS abdomen | 1.72 | (0.70 to 4.29) | 87.9 |
| AIS spine | 2.34 | (0.95 to 6.12) | 96.7 |
| AIS pelvic | 1.87 | (0.78 to 4.71) | 92.1 |
| AIS limbs | 2.12 | (0.86 to 5.46) | 94.8 |
| AIS other | 1.94 | (0.80 to 4.86) | 93.0 |
| Pre-hospital CPRb | 2.20 | (0.78 to 6.86) | 93.1 |
| ED SBPc | 1.82 | (0.75 to 4.47) | 91.0 |
| CPR on arrivalb | 2.01 | (0.82 to 5.19) | 93.5 |
| Time from arrival to randomisationd | 1.87 | (0.79 to 4.65) | 92.4 |
| Alle | 4.22 | (1.15 to 15.21) | 98.5 |
| All (removing ISS)f | 4.53 | (1.25 to 17.59) | 98.9 |
| AIS and ED SBP | 1.81 | (0.74 to 4.56) | 90.4 |
| OR | 95% CrI | Posterior probability (%) of OR > 1a | |
|---|---|---|---|
| Age | 1.39 | (0.59 to 3.28) | 77.3 |
| Gender | 1.53 | (0.69 to 3.48) | 85.1 |
| ISS | 1.63 | (0.73 to 3.77) | 88.1 |
| AIS head | 1.61 | (0.72 to 3.79) | 87.2 |
| AIS face | 1.65 | (0.73 to 3.75) | 88.5 |
| AIS chest | 1.68 | (0.74 to 3.90) | 89.5 |
| AIS abdomen | 1.50 | (0.67 to 3.44) | 83.6 |
| AIS spine | 1.72 | (0.76 to 4.05) | 89.9 |
| AIS pelvic | 1.61 | (0.71 to 3.64) | 87.2 |
| AIS limbs | 1.69 | (0.73 to 3.99) | 89.0 |
| AIS other | 1.60 | (0.72 to 3.59) | 87.4 |
| Pre-hospital CPRb | 1.69 | (0.69 to 4.20) | 87.4 |
| ED SBPc | 1.53 | (0.69 to 3.52) | 84.9 |
| CPR on arrivalb | 1.62 | (0.72 to 3.71) | 87.9 |
| Time from arrival to randomisationd | 1.59 | (0.71 to 3.61) | 87.4 |
| Alle | 1.80 | (0.59 to 5.58) | 84.9 |
| All (removing ISS)f | 1.67 | (0.55 to 5.30) | 81.6 |
| AIS and ED SBP | 1.53 | (0.65 to 3.61) | 84.0 |
| SC + REBOA N = 34 | SC N = 44 | OR | 95% CrI | Posterior probability of OR > 1 (%) | |
|---|---|---|---|---|---|
| N = 34 | N = 43 | ||||
| Death within 90 days | |||||
| Yes | 21 (62) | 18 (42) | 2.06 | (0.87 to 5.01) | 95.1 |
| No | 13 (38) | 25 (58) | |||
| Death within 6 months | |||||
| Yes | 21 (62) | 18 (42) | 2.06 | (0.87 to 5.01) | 95.1 |
| No | 13 (38) | 25 (58) | |||
| Death while in hospital | |||||
| Yes | 21 (62) | 18 (42) | 2.06 | (0.87 to 5.01) | 95.1 |
| No | 13 (38) | 25 (58) | |||
| N = 34 | N = 44 | ||||
| Death within 24 hours | |||||
| Yes | 13 (38) | 10 (23) | 1.92 | (0.76 to 4.93) | 91.5 |
| No | 21 (62) | 34 (77) | |||
| Death within 6 hours | |||||
| Yes | 9 (26) | 4 (9) | 2.86 | (0.94 to 9.25) | 96.8 |
| No | 25 (74) | 40 (91) | |||
| Death within 3 hours | |||||
| Yes | 9 (26) | 2 (5) | 4.58 | (1.38 to 17.64) | 99.4 |
| No | 25 (74) | 42 (95) | |||
FIGURE 27.
Competing risk regression.
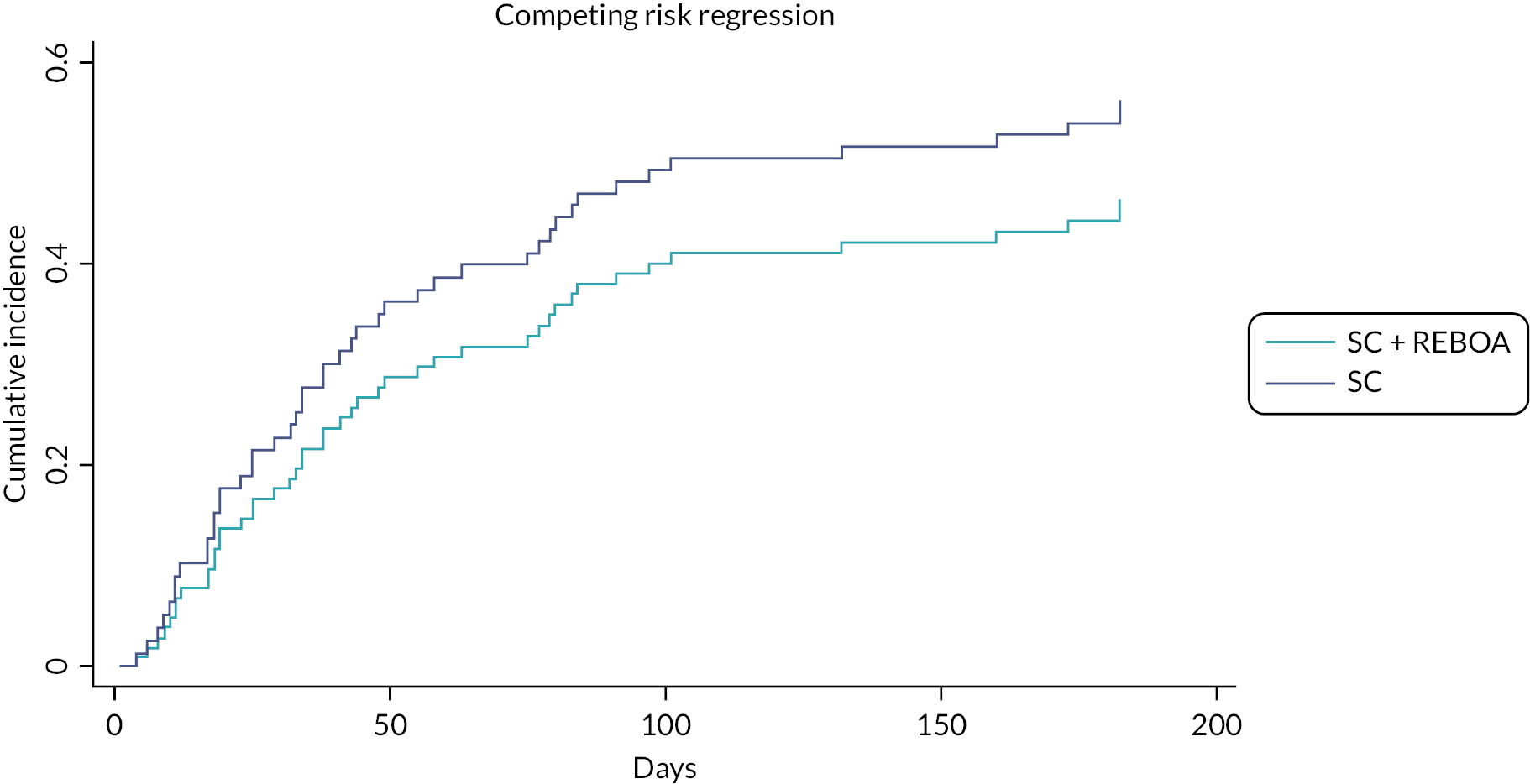
| SC + REBOA | SC | |||
|---|---|---|---|---|
| Complied | Did not comply | Complied | Did not comply | |
| n = 19 | n = 27 | n = 42 | N = 2 | |
| Demographics | ||||
| Median age (Q1–Q3) (years) | 57 (41–70) | 38 (30–54) | 38 (29–57) | 52 (49–55) |
| Male sex, n (%) | 10 (53) | 18 (67) | 33 (79) | 1 (50) |
| Comorbidity | ||||
| Median CCI (Q1–Q3); n | 1 (0–5); 14 | 0 (0–1); 19 | 0 (0–1); 38 | 2 (0–3); 2 |
| Mechanism of injury | ||||
| Blunt, n (%) | 19 (100) | 25 (93) | 41 (98) | 2 (100) |
| Penetrating, n (%) | – | 2 (7) | 1 (2) | – |
| Injury severity | ||||
| Median ISS (Q1–Q3) | 41 (35–54) | 41 (25–48) | 41 (29–50) | 39 (27–50) |
| ISS band | ||||
| Minor | – | – | 1 (2) | – |
| Moderate | – | 1 (4) | 1 (2) | – |
| Severe | 2 (10) | 5 (19) | 4 (10) | – |
| Very severe | 17 (90) | 21 (78) | 36 (86) | 2 (100) |
| Injury pattern | ||||
| AIS head [median (Q1–Q3)] | 3 (0–5) | 0 (0–4) | 2 (0–5) | 0 (0–0) |
| AIS face [median (Q1–Q3)] | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–0) |
| AIS thorax [median (Q1–Q3)] | 3 (3–4) | 4 (3–4) | 4 (1–4) | 4 (3–4) |
| AIS abdomen [median (Q1–Q3)] | 2 (0–3) | 2 (0–3) | 2 (0–4) | 4 (3–5) |
| AIS spine [median (Q1–Q3)] | 2 (0–3) | 2 (0–2) | 0 (0–2) | 0 (0–0) |
| AIS pelvis [median (Q1–Q3)] | 4 (0–5) | 2 (0–4) | 2 (0–5) | 1 (0–2) |
| AIS limbs [median (Q1–Q3)] | 2 (2–3) | 2 (2–3) | 3 (1–3) | 3 (3–3) |
| AIS other [median (Q1–Q3)] | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| Vital signs | ||||
| SBP (mmHg) | 72 (50–109); 18 | 91 (68–123); 26 | 97 (72–114); 40 | 120 (115–124); 2 |
| ≤ 90 mmHg, n (%) | 13 (72) | 13 (50) | 19 (48) | – |
| ≤ 70 mmHg, n (%) | 8 (44) | 10 (39) | 9 (23) | – |
| CPR on arrival | ||||
| Yes | 3 (16) | 1 (4) | 4 (10) | – |
| No | 14 (74) | 22 (82) | 37 (88) | 2 (100) |
| Missing | 2 (11) | 4 (15) | 1 (2) | – |
| SC + REBOA | SC | |||
|---|---|---|---|---|
| Complied | Did not comply | Complied | Did not comply | |
| n = 36 | n = 10 | n = 42 | N = 2 | |
| Demographics | ||||
| Median age (Q1–Q3) (years) | 46 (33–63) | 47 (31–62) | 38 (29, 57) | 52 (49, 55) |
| Male sex, n (%) | 21 (58) | 7 (70) | 33 (79) | 1 (50) |
| Comorbidity | ||||
| Median CCI (Q1–Q3); n | 0 (0–2); 27 | 1 (0–1); 6 | 0 (0, 1) | 2 (0, 3) |
| Mechanism of injury | ||||
| Blunt, n (%) | 36 (100) | 8 (80) | 41 (98) | 2 (100) |
| Penetrating, n (%) | – | 2 (20) | 1 (2) | – |
| Injury severity | ||||
| Median ISS (Q1–Q3) | 41 (36–50) | 33 (21–43) | 41 (29–50) | 39 (27–50) |
| ISS band | ||||
| Minor | – | – | 1 (2) | |
| Moderate | – | 1 (10) | 1 (2) | |
| Severe | 4 (11) | 3 (30) | 4 (10) | |
| Very severe | 32 (89) | 6 (60) | 36 (86) | 2 (100) |
| Injury pattern | ||||
| AIS head [median (Q1–Q3)] | 3 (0–5) | 0 (0–3) | 2 (0–5) | 0 (0–0) |
| AIS face [median (Q1–Q3)] | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–0) |
| AIS thorax [median (Q1–Q3)] | 4 (3–4) | 3 (1–4) | 4 (1–4) | 4 (3–4) |
| AIS abdomen [median (Q1–Q3)] | 2 (0–3) | 0 (0–2) | 2 (0–4) | 4 (3–5) |
| AIS spine [median (Q1–Q3)] | 2 (0–2) | 1 (0–2) | 0 (0–2) | 0 (0–0) |
| AIS pelvis [median (Q1–Q3)] | 2 (0–5) | 1 (0–4) | 2 (0–5) | 1 (0–2) |
| AIS limbs [median (Q1–Q3)] | 2 (2–3) | 3 (2–3) | 3 (1–3) | 3 (3–3) |
| AIS other [median (Q1–Q3)] | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| Vital signs | ||||
| SBP (mmHg) | 88 (58–115); 35 | 69 (50–83); 9 | 97 (72–114); 40 | 120 (115–124); 2 |
| ≤ 90 mmHg, n (%) | 19 (54) | 7 (78) | 19 (48) | – |
| ≤ 70 mmHg, n (%) | 13 (37) | 5 (56) | 9 (23) | – |
| CPR on arrival | ||||
| Yes | 4 (11) | – | 4 (10) | – |
| No | 29 (81) | 7 (70) | 37 (88) | 2 (100) |
| Missing | 3 (8) | 3 (30) | 1 (2) | – |
| SC + REBOA N = 21 | SC N = 69 | OR | 95% CrI | |
|---|---|---|---|---|
| Death within 90 days | ||||
| Yes | 14 (67) | 29 (43) | 2.77 | (1.01 to 8.20) |
| No | 7 (33) | 39 (57) | ||
| Death within 6 months | ||||
| Yes | 14 (67) | 29 (43) | 2.77 | (1.01 to 8.20) |
| No | 7 (33) | 39 (57) | ||
| Death while in hospital | ||||
| Yes | 14 (67) | 29 (43) | 2.77 | (1.01 to 8.20) |
| No | 7 (33) | 39 (57) | ||
| Death within 24 hours | ||||
| Yes | 8 (38) | 19 (28) | 1.60 | (0.56 to 4.50) |
| No | 13 (62) | 50 (72) | ||
| Death within 6 hours | ||||
| Yes | 7 (33) | 10 (14) | 2.96 | (0.91 to 9.37) |
| No | 14 (67) | 59 (86) | ||
| Death within 3 hours | ||||
| Yes | 5 (24) | 8 (12) | 2.33 | (0.62 to 8.21) |
| No | 16 (76) | 61 (88) | ||
| SC + REBOA N = 38 | SC N = 52 | OR | 95% CrI | |
|---|---|---|---|---|
| Death within 90 days | ||||
| Yes | 19 (50) | 24 (47) | 1.13 | (0.49 to 2.63) |
| No | 19 (50) | 27 (53) | ||
| Death within 6 months | ||||
| Yes | 19 (50) | 24 (47) | 1.13 | (0.49 to 2.63) |
| No | 19 (50) | 27 (53) | ||
| Death while in hospital | ||||
| Yes | 19 (50) | 24 (47) | 1.13 | (0.49 to 2.63) |
| No | 19 (50) | 27 (53) | ||
| Death within 24 hours | ||||
| Yes | 12 (32) | 15 (29) | 1.13 | (0.45 to 2.82) |
| No | 26 (68) | 37 (71) | ||
| Death within 6 hours | ||||
| Yes | 9 (24) | 8 (15) | 1.72 | (0.58 to 5.14) |
| No | 29 (76) | 44 (85) | ||
| Death within 3 hours | ||||
| Yes | 7 (18) | 6 (12) | 1.75 | (0.52 to 5.95) |
| No | 31 (82) | 46 (88) | ||
List of abbreviations
- A&E
- accident and emergency
- ACM
- all-cause mortality
- ADE
- adverse device effect
- AE
- adverse event
- AIS
- Abbreviated Injury Scale
- APEASE
- acceptability, practicability, effectiveness, affordability, side effects and equity
- AUC
- area under the curve
- BCT
- behavioural change techniques
- CACE
- complier average causal effect
- CHaRT
- Centre for Healthcare Randomised Trials
- CI/Co-CI
- Chief Investigator/Co-Chief Investigator
- COVID-19
- coronavirus disease discovered in 2019
- CPR
- cardiopulmonary resuscitation
- CRF
- case report form
- CrI
- credible interval
- DMC
- Data Monitoring Committee
- eCRF
- electronic case report form
- ED
- emergency department
- EQ-5D-5L
- EuroQol Group’s 5-dimension health status 5-level questionnaire
- EVPI
- expected value of perfect information
- GBP
- Great British pounds
- GOS-E
- Glasgow Outcome Scale (Extended)
- HDU
- high-dependency unit
- HEAP
- health economics analysis plan
- HES
- hospital Episode Statistics
- HSRU
- Health Services Research Unit
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IRAS
- integrated Research Application System
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ISS
- Injury Severity Score
- ITT
- intention to treat
- LYGs
- life-year gains
- mmHg
- millimetres of mercury
- MTC
- major trauma centre
- NIHR
- National Institute for Health and Care Research
- ONS
- Office of National Statistics
- PHS
- Public Health Scotland
- PI
- principal investigator
- PIL
- participant information leaflet
- PMG
- Project Management Group
- PSSRU
- Personal Social Services Research Unit
- Q
- quartile
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REBOA
- resuscitative endovascular balloon occlusion of the aorta
- REC
- Research Ethics Committee
- SADE
- serious adverse device effect
- SAE
- serious adverse event
- SBP
- systolic blood pressure
- TARN
- Trauma Audit and Research Network
- TDF
- Theoretical Domains Framework
- TSC
- Trial Steering Committee
- TTL
- trauma team leader
- USADE
- unanticipated serious adverse device effect
- USM
- urgent safety measure
