Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR128996. The contractual start date was in February 2020. The draft manuscript began editorial review in January 2023 and was accepted for publication in August 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Beese et al. This work was produced by Beese et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Beese et al.
Chapter 1 Background
Introduction
Heart failure (HF) is a condition in which the heart does not pump blood properly around the body, limiting an individual’s quality of life (QoL) and reducing length of life. This chapter describes the definition, epidemiology, causes, classification and management of HF.
Definition and classification of heart failure
HF has been defined both as a ‘syndrome recognised clinically by a constellation of symptoms and signs produced by complex circulatory and neurohormonal responses to cardiac dysfunction’ and as ‘a disease characterised by a decline in the heart’s ability to pump blood around a person’s body at normal filling pressures to meet its metabolic needs.’1 These definitions describe the clinical presentation as well as the pathophysiological process. 2 Symptoms of HF typically include shortness of breath during exertion and/or fatigue, signs of fluid retention, such as ankle swelling, and fluid in the lungs. Some patients with HF also suffer from heart rhythm abnormalities that can result in sudden death. Over time, most patients with HF experience deterioration in symptoms and hence require hospital treatment, despite medications.
In advanced stages, patients may suffer from shortness of breath at rest or minimal exertion, cachexia and muscular deconditioning, refractory fluid overload and even kidney and liver failure, a condition sometimes known as end-stage or advanced HF (AHF). 3 For consistency in this report, we will use the term AHF to describe this condition of severe HF symptoms despite conventional HF medications. The New York Heart Association (NYHA) classification system is widely used to classify the severity of symptoms related to HF. The NYHA classification has four levels of increasing severity from Class I to IV (Table 1). Patients with AHF suffer from NYHA Class III or IV symptoms.
| NYHA class | Description |
|---|---|
| I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation or dyspnoea. |
| II | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in fatigue, palpitation or dyspnoea. |
| III | Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity results in fatigue, palpitation or dyspnoea. |
| IV | Unable to carry on any physical activity without discomfort. Symptoms at rest. If any physical activity is undertaken, discomfort is increased. |
Patients with HF have severely reduced QoL, especially AHF based on a number of questionnaires that measure QoL [EuroQol-5 Dimensions (EQ-5D), Short Form questionnaire-12 items (SF-12), Short Form questionnaire-36 items (SF-36), Minnesota Living with Heart Failure Questionnaire (MLHFQ), Kansas City Cardiomyopathy Questionnaire (KCCQ)]. 4,5 QoL can be improved by medical therapy that improves HF. 6–16
Epidemiology of heart failure
Heart failure prevalence varies widely depending on definitions from an estimated 23 million people worldwide (USA 6 million, Europe 15 million). 17,18 It affects between 1% and 2% of adults in industrialised populations. 19 In the UK, as many as 920,000 people are living with HF with an incidence of 37.5 and 23 per 100,000 person-years for men and women, respectively. 20 The prevalence increases with age (Table 2), almost doubling with each decade after 65 years. The calculated lifetime risk of developing HF is 20%. 21 HF-related hospital admission rates in England have increased by 5% over the last 10 years and are estimated to increase by about 50% in the next 25 years. Nearly half of the patients admitted with HF had severe symptoms (NYHA Class III or IV). Despite advances in medical therapy, 1-year mortality remains high at about 32% in patients admitted with HF. 22
| Age bracket (years) | Prevalence |
|---|---|
| 65–74 | 1 in 35 |
| 75–84 | 1 in 15 |
| > 85 | 1 in 7 |
Data on the prevalence of AHF in the UK are lacking. A survey of European countries suggested that about 10% of all patients with HF may meet the criteria for AHF. 23 The Olmsted County (MN, USA) cohort study showed that about 14% of patients with HF met the European Society of Cardiology (ESC) criteria for AHF with an annual rate of about 33 per 100,000 and 420 per 100,000 for the under 65 and 65–79 age groups, respectively. 24
Aetiology and pathophysiology of heart failure
Any structural or physiological conditions that affect the ventricular function can cause HF. In the UK, ischaemic heart disease is the major cause of HF, but other causes include dilated cardiomyopathy, which may be familial (genetic) or caused by myocarditis, cardiotoxic drugs or hypertension and valvular heart disease. 25
Historically, descriptions of HF pathophysiology have centred on the left ventricle (LV) as this heart chamber is the most commonly affected, particularly in ischaemic heart disease. Myocardial injury results in a drop in LV function and activation of the neurohormonal system. The latter contributes to salt and water retention and progressive remodelling of the heart. Fibrosis, muscle wall thinning and increased sphericity associated with LV remodelling, often accompanied by functional mitral regurgitation, further compromise myocardial efficiency and drive the downward spiral towards end-stage or advanced AHF. Myocardial fibrosis and remodelling provide the substrate for both atrial and ventricular arrhythmias, which may worsen HF symptoms and result in sudden death.
Diagnosis of heart failure and advanced heart failure
Heart failure is a clinical diagnosis, based on patient history, physical examination and investigations, such as electrocardiography, measurement of B-type natriuretic peptide (BNP) and echocardiography. The echocardiogram is used to assess heart function [by measuring ejection fraction (EF)] and also to identify possible causes or associated features, such as mitral regurgitation. Other investigations, such as chest radiography, may also detect features to support the diagnosis of cardiomegaly, pulmonary congestion and pleural fluid accumulation, but may also exclude other differential diagnoses. Cardiac magnetic resonance imaging is increasingly used to assess the heart and identify the cause of HF. In general, BNP and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) are raised in patients with HF and the concentrations increases with the severity of symptoms. 26
The ESC first defined AHF in a statement in 2007. 27 The position statement was updated in 2018 and the criteria for AHF were defined. The American College of Cardiology and Heart Failure Society of America have also defined AHF (Table 3). These definitions of AHF are conceptually very similar – severe symptoms in association with signs of congestion, poor perfusion and hospitalisations attributable to severe cardiac dysfunction despite medical therapy. One-year mortality in patients with AHF may be close to 50% in patients with all the characteristics of AHF. 24
| All the following criteria must be present despite optimal guideline-directed treatment |
|---|
|
Recognising the significant heterogeneity in patients with AHF, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles were introduced to better describe the clinical characteristics in patients with NYHA Class III and IV HF (Table 4). The INTERMACS profiles have been widely adopted to describe the characteristics of patients with AHF undergoing assessment of heart transplantation or left ventricular assist device (LVAD) therapy.
| NYHA class III/IV | INTERMACS class | Description |
|---|---|---|
| IV | 1 | Crash and burn (cardiogenic shock) |
| IV | 2 | Deteriorating on inotropes |
| IV | 3 | Stable IVI inotropes dependent |
| IV | 4 | At home resting symptoms on oral therapy |
| IV | 5 | Comfortable at rest but symptoms with minimal activities of daily living (housebound) |
| III | 6 | Walking wounded with activities of daily living possible but meaningful activity hampered |
| III | 7 | Advanced class III |
Health economics
Currency and inflation adjusted cost for hospitalisation is highest in the USA ($125,000/patient/year), while in Europe HF inpatient costs vary from $5000 to $18,000 (2016 prices) with most costs (50–90%) derived from hospitalisations. 28,29 It usually accounts for 1–2% of a nation’s health budget. 29 Health economics indicate that the cost to the NHS is £0.75B annually (approximately 4% of the NHS budget) and continues to rise, largely related to the high prevalence of cardiovascular diseases in older age groups coupled with ageing of the population. Newer medications (angiotensin receptor blocker-neprilysin inhibitors and sodium-glucose cotransporter inhibitors) and interventions (ablations, mitral valve interventions and implantable pulmonary artery pressure monitors) add to the increasing costs of HF therapy.
Medical and electrical device therapy of heart failure
Left ventricular ejection fraction has been a central inclusion criterion over the many decades of clinical trials in HF and has shaped clinical guidelines to this day. Largely based on the LVEF thresholds used in the trials, HF has been categorised into three categories: HF with reduced EF (HfrEF), HF with mid-range EF (HfmrEF) and HF with preserved EF (HfpEF) (Table 5). This review will focus on HfrEF, as LVADs are generally not recommended in patients with HfmrEF or HfpEF.
| HfrEF | HfmrEF | HfpEF |
|---|---|---|
| Symptoms ± signs LVEF ≤ 40% |
Symptoms ± signs LVEF 41–49% |
Symptoms ± signs LVEF ≥ 50% Objective evidence of cardiac abnormalities |
Neurohormonal antagonists, beta-blockers and mineralocorticoid antagonists have well-established benefits in patients with HfrEF and remain the first-line therapy in this group of patients. More recently, the sodium-glucose cotransporter inhibitors have also proven to benefit patients with HfrEF and are likely to form another pillar of HF therapy. Other drugs of benefit in some patients with HfrEF are ivabradine and hydralazine-nitrate combination (Figure 1). Loop and thiazide diuretics are routinely used to control congestive symptoms. Treatment options in HfpEF are limited.
FIGURE 1.
European Society of Cardiology guidelines for the management of patients with HfrEF. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitor; BB, Beta blockers; BTC, bridge to candidacy; BTT, bridge to transplantation; CABG, coronary artery bypass graft; CRT-P/D, cardiac resynchronization therapy with pacemaker/defibrillator; LBBB, left bundle branch block; MCS, mechanical circulatory support; MRA, mineralocorticoid receptor antagonists; MV, mitral valve; PVI, pulmonary vein isolation; SAVR, surgical aortic valve replacement; SGLT2i, sodium-glucose co-transporter 2 inhibitor; SR, sinus rhythm; TAVI, transcatheter aortic valve implantation; TEE, transesophageal echocardiography.

Progression in HF is associated with deterioration in kidney function, low blood pressure and fluid overload, often necessitating a dose reduction in HF medications and an escalation in diuretic doses. Low cardiac output is also common at this stage, and inotropes such as dobutamine are used. These are features of AHF that herald HF prioritisation and death.
Implantable electrical devices such as implantable cardioverter defibrillators (ICDs) and biventricular pacemakers [also known as cardiac resynchronisation therapy (CRT)] are commonly used in patients with HfrEF. The latter benefit a specific subset of patients with HfrEF and left bundle branch block, with no benefit or even detrimental effect in patients with narrower QRS complexes. ICDs reduce the risk of sudden arrhythmic deaths, but do not prevent deterioration in cardiac function and death from pump failure in AHF. Shocks from ICDs are recognised indicators of poor prognosis in patients with HF. Heart transplantation or a LVAD may be considered in selected patients in whom these therapies fail.
Heart transplantation
Access to heart transplantation is limited by the shortage of suitable organ donors. In the last 10 years, the number of heart transplants (HTs) performed in the UK has dropped from a peak of almost 200 per year in 2016–8 to about 160 per year in the 2020–1 financial year, even with the adoption of donation after circulatory death heart transplantation. 30 This shortage of suitable donor organs has led to the selection of potential recipients who are most likely to benefit from transplantation based on a range of criteria including age and comorbidities. In selected patients, heart transplantation is a very effective treatment. In the UK, the median survival from heart transplantation now exceeds 10 years. However, the rigorous selection process effectively excludes the majority of patients with AHF.
Mechanical circulatory support devices
Mechanical circulatory support devices (MCSDs) have increasingly been used in the last decade to support patients with worsening HF. These MCSDs may be categorised into temporary (or short-term) and durable (or long-term) devices. The former are largely extracorporeal devices and patients are managed in hospital (often in a high-dependency or intensive care environment), while patients with the latter may be discharged home on the device. Most of the durable MCSDs used in the UK are LVADs. Total artificial hearts and biventricular assist devices will not be discussed in this review.
The terminology in left ventricular assist device therapy
Historically, the nomenclature of LVAD therapy is closely linked to candidacy or eligibility for heart transplantation and treatment intent:
-
Bridge to candidacy (BTC) refers to LVAD therapy in patients with a contraindication to heart transplantation that is potentially reversible with LVAD therapy, such as renal dysfunction or pulmonary hypertension due to left heart disease. Patients would be expected to become candidates for heart transplantation following reversal of the contraindication by LVAD therapy. Thus, the intention of LVAD therapy is to reverse the contraindication to allow heart transplantation.
-
Bridge to transplantation (BTT) refers to LVAD therapy in patients who are eligible candidates for heart transplantation but may be deteriorating on medical therapy on the waiting lists. Progression of HF while on the waiting list may result in multiorgan failure to the extent that they may no longer be suitable candidates for heart transplantation. This may result in death. The treatment objectives of BTT are to stabilise and prevent death in patients on the waiting list for heart transplantation and optimise the outcome of heart transplantation.
-
Destination therapy (DT) refers to LVAD therapy in patients who are not eligible for heart transplantation due to established contraindication(s) that are not amenable to correction by LVAD. The objective of the LVAD as DT is to provide symptomatic and prognostic benefits to patients with AHF who are at high risk of mortality on medical therapy and not suitable for heart transplantation. In an INTERMACS report, contraindications to heart transplantation included advanced age, renal dysfunction, chronic lung disease or high body mass index (BMI). Despite the initial treatment intent, approximately 10% of patients originally considered unsuitable for heart transplantation and selected for DT subsequently improved sufficiently (e.g. improvement in frailty) to undergo transplantation after 2 years of LVAD therapy. 31
Evolution of left ventricular assist devices
Left ventricular assist devices have evolved considerably over the last few decades. The first generation of LVADs were pulsatile devices. These pulsatile devices were large devices due to the need for pumping chambers. The poor durability of first-generation pulsatile devices limited longer-term outcomes, with survival limited to < 2 years in the majority of patients. The high device failure rates led to the development of non-pulsatile continuous flow LVADs.
The second-generation LVADs are non-pulsatile axial flow devices. These axial flow LVADs are significantly smaller than the first-generation pulsatile pumps, which simplified device implantation considerably. In addition to the reduction in implant-related morbidity, axial flow LVADs were also associated with improved durability and significantly improved longer-term outcomes. One of the most commonly used axial flow LVADs was the HeartMate II™ LVAD (Abbott, Chicago, IL, USA). These improvements led to greater adoption and acceptance of LVAD therapy. In the USA, LVAD implant rates increased exponentially with the introduction of the second-generation axial flow LVADs. Despite the improved durability, pump thrombosis and bleeding complicated longer-term support with second-generation LVADs.
The third-generation LVADs are centrifugal flow devices. The HeartWare™ ventricular assist device (HVAD™, Medtronic, Dublin, Republic of Ireland) is a small intrapericardial centrifugal flow pump. Promising early results led to approval for clinical use, although pump thrombosis and neurological complications were concerning. The risks of neurological complications and device failure became increasingly evident with widespread use and the device was withdrawn worldwide in June 2021. 32 At present, there are no new implants of the HeartWare HVAD, although some patients continue to be supported in the UK.
The HeartMate 3™ (HM3) LVAD (Abbott, Chicago, IL, USA) was introduced in 2015 in the UK. The HM3 LVAD is a centrifugal flow device with a number of design features to improve ‘haemocompatibility’ and reduce the risk of complications such as pump thrombosis. Clinical studies confirmed a significantly lower risk of pump thrombosis compared to HeartMate II, and an ongoing randomised trial is evaluating reduced antithrombotic therapy with HM3. 33 HM3 is now the only LVAD in use in the UK following the withdrawal of the HeartWare HVAD.
Description of continuous flow left ventricular assist devices
Continuous flow devices are so called because they generate flow throughout the cardiac cycle. In centrifugal devices, blood is drawn via the inflow cannula in the LV by an impeller within the pump and delivers the blood into the aorta via the outflow graft. The outflow of the pump is arranged perpendicularly to the inflow cannula. Flow can be changed by adjusting the pump speed (revolutions per minute) via the system controller. Pump speed must be carefully balanced as excessive pump speed could compromise right ventricular function. 34 The device is connected to a power source or a pair of batteries via an externalised drive line.
Complications of left ventricular assist device therapy
Various complications can be attributed to the abnormal interaction between the LVAD and the biological circulation, so-called haemocompatibility-related adverse events (HRAEs). Device-related haemolysis, pump thrombosis and systemic embolism, stroke, intracranial bleeding and gastrointestinal (GI) bleeding (GIB) are major HRAEs that compromise long-term outcomes of LVAD therapy. These HRAEs occur in both axial and centrifugal flow devices, but the HM3 LVAD has been associated with a lower burden of HRAEs compared to HeartMate II. 35
The LVAD supports the LV but often at the expense of the right. Right heart failure (RHF) is a major cause of morbidity and mortality. Studies have identified several risk factors for RHF post-LVAD implant, including pre-implant measures of right ventricular function and severity of HF and organ dysfunction; however, the ability to predict post-LVAD RHF remains challenging. 36 Severe RHF is associated with higher mortality. Timely deployment of a temporary right ventricular assist device (RVAD) may mitigate this risk. 37 Late RHF is increasingly recognised and may limit long-term QoL.
The continuous emptying of the LV and delivery of blood into the aorta pressurises the aorta and reduces left ventricular stroke volume in patients with LVADs. The aortic valve may not open if the LV fails to generate sufficient pressure to overcome the aortic pressure, with consequent loss of arterial pulsatility. Over time, the reduction in aortic valve opening may lead to degenerative changes of the valve and aortic regurgitation (AR). A competent aortic valve is a prerequisite of LVAD function. Severe AR results in the recurrence of HF symptoms and adversely affects long-term survival in patients with LVADs. 38
As with any implantable devices, LVADs are susceptible to infection. Infection in patients with LVADs may not be attributable to the device. Infections related to the device may be localised, related to the driveline (most common) or more severe bloodstream infection related to the pump or endocarditis. The latter may be associated with neurological complications and increased mortality. 39
Current service provision and patient pathway
In the UK, LVADs are currently commissioned in the six HT centres for the purpose of BTC and BTT. Referrals to these centres follow existing pathways in the HT service. As a BTC and BTT service, LVADs are only offered to patients who are eligible for heart transplantation. Despite the intention to bridge patients to heart transplantation, the heart allocation policy does not prioritise candidates with LVADs for transplantation.
In the most recent iteration of the heart allocation policy in the UK, patients with LVADs without complications are offered listing on the ‘non-urgent’ waiting list, the lowest priority of the three tiers (the other two tiers are ‘urgent’ and ‘super urgent’). Patients with LVAD-related complications may be upgraded to the ‘urgent’ list following approval by an adjudication panel, consisting of representatives from each of the HT centres. Paradoxically, prioritisation and transplantation only when patients develop LVAD-related complications is associated with poorer outcomes, which is inconsistent with the original concept of BTT – to optimise the outcome of heart transplantation. In effect, most patients without LVAD-related complications would continue on long-term LVAD support, simultaneously BTT (by intent) and DT (in practice).
In the most recent iteration of the ESC Guidelines, a LVAD has been recommended in patients with INTERMACS 3 or 4 AHF with contraindications for heart transplantation (Figure 2). Available solely as a bridging therapy, the rate of LVAD implantation in the UK is low compared to other European countries, especially in countries where DT is established. According to a recent study, the UK has one of the lowest LVAD implant per million population at 0.6, compared to a number of European countries (e.g. Hungary 1.0, Portugal 2.4, Spain 3.3, Belgium 4.1 and Germany 13.9) (Figure 3). 23
FIGURE 2.
European Society of Cardiology guidelines for the management of patients with AHF. BTB, bridge to bridge; BTD, bridge to decision; BTR, bridge to recovery.
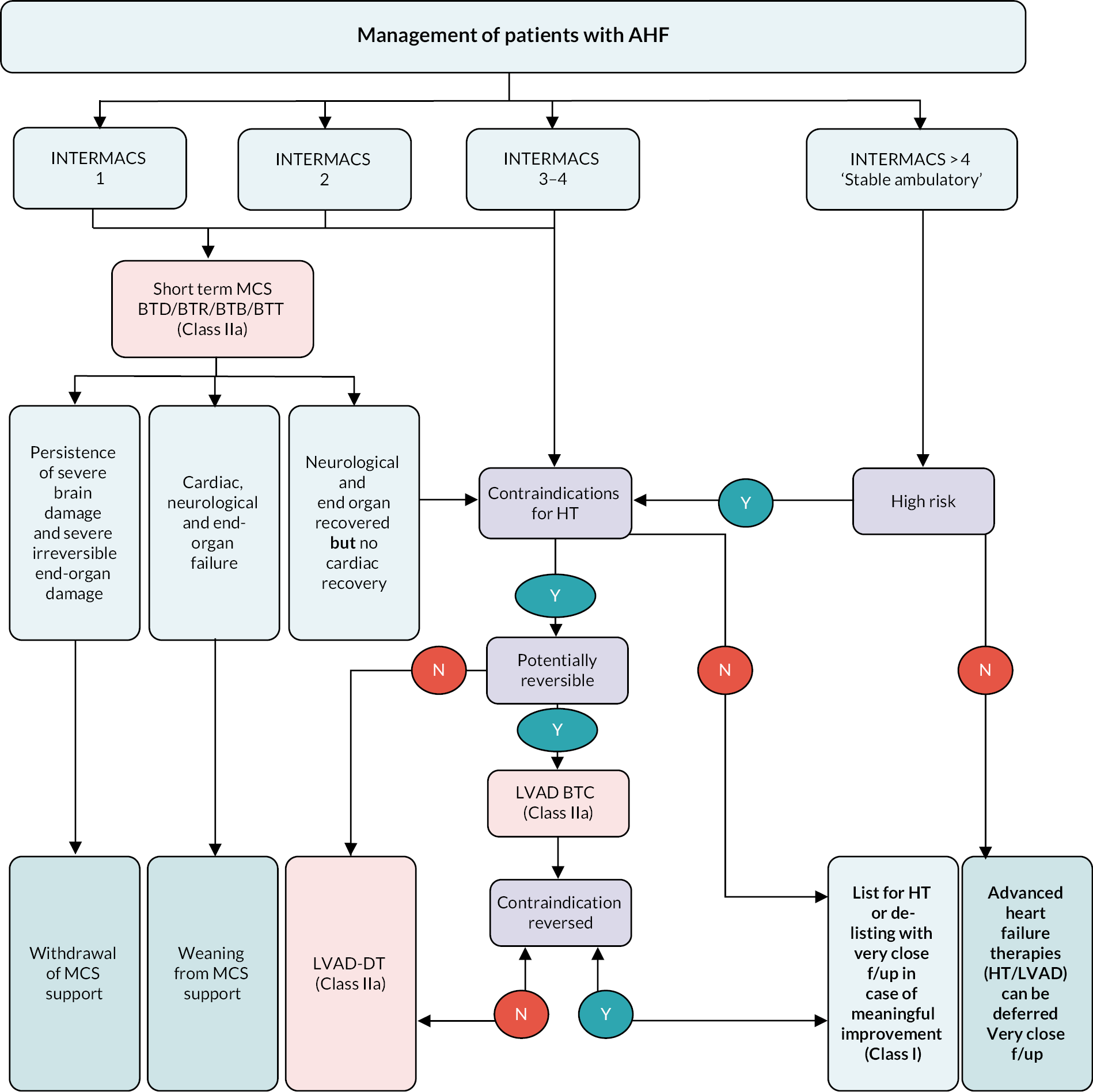
FIGURE 3.
Hospitals implanting LVAD per million people (left) and number of LVAD implantations per million people (right). Reproduced from Serefovic et al. 23 with permission from John Wiley & Sons.

In summary
Heart failure is an increasingly common problem with significant impact on individual patient’s QoL and longevity as well as population health economics. Advanced heart failure that fails to respond to medical management (MM) may be treated by LVAD implantation and/or heart transplantation. In the UK, LVAD implantation has been commissioned as a bridging therapy to transplantation. In other countries with different healthcare delivery systems, the majority of LVADs are implanted as DT in patients ineligible for heart transplantation. In the UK, a LVAD for such patients is not currently commissioned. The lack of economic evidence is a key reason that NHS England has not recommended a LVAD for DT.
Chapter 2 Aims
To make an informed decision on the use of LVADs for patients with AHF that fail to respond adequately to MM and who are ineligible for a HT, robust evidence on clinical and cost-effectiveness is required.
The aim of the research documented in this report was to address the question: What is the clinical and cost-effectiveness of a LVAD compared to MM for AHF patients ineligible for heart transplantation (DT)?
The specific objectives to address this aim were to undertake:
-
– a systematic review of available evidence on the clinical effectiveness of a LVAD as DT, including a network meta-analysis (NMA) to provide an indirect estimate of the relative effectiveness of currently available LVADs compared to MM (see Chapter 3);
-
– a systematic review of available economic evidence on the use of a LVAD as DT (see Chapter 4); and
-
– the development of an economic model to estimate the cost-effectiveness of a LVAD compared to MM from the UK NHS/personal social services (PSS) perspective (see Chapter 5).
Sources of information used in undertaking this research include trials, observational studies, economic evaluations, reports from registries, guidance from LVAD recipients and their families, clinical experts, those commissioning healthcare services and companies supplying LVADs.
An exploration of the ability of accessible data sets to provide further data relevant to LVADs as DT was also undertaken (see Appendix 1).
When this research was commissioned and begun, there were two predominantly available LVADs in the UK used for AHF patients. As outlined in ‘Evolution of left ventricular assist devices’, the HeartWare device was withdrawn in 2021. Therefore, this report, while still considering evidence from research on all LVADs for DT in part and where relevant, focuses on the remaining device, HM3. The HM3 is the only device used for AHF patients in the UK at this time.
Chapter 3 Clinical effectiveness of left ventricular assist devices compared to medical management as destination therapy in advanced heart failure patients
Introduction
Left ventricular assist device DT strategy is not currently common practice in the UK. This chapter aimed to systematically review all of the available evidence on the clinical effectiveness of LVADs as DT; including a NMA to provide an indirect estimate of the relative effectiveness of currently available LVADs compared to MM. This was also used to inform the development of the economic model in Chapter 5.
Methods
This systematic review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. 40 The review is registered on PROSPERO (CRD42020158987). 41
Eligibility criteria
Population
Patients (age > 16 years) with AHF who received a LVAD as DT and were ineligible for a HT or potential candidacy at the time of the LVAD implantation. Studies with mixed LVAD populations were also included where DT data were reported separately or could be easily acquired. Eligibility for heart transplantation is defined by individual centres based on international guidelines, but there may be variations in practice.
Intervention
There were no restrictions placed on the type of LVAD, either by flow design or by generational evolution (e.g. first-generation pulsatile pump, second-generation continuous axial flow or third-generation continuous centrifugal flow). All devices were included, regardless of current availability, for completeness of information and for use in the NMA. Studies of participants with biventricular assist devices, or RVADs were not eligible for inclusion.
On 3 June 2021, midway through conducting this project, one of the current third-generation continuous flow centrifugal LVADs, the HeartWare HVAD (used extensively throughout North America, UK and Europe) was withdrawn from the market. While studies on HVAD were included, the analysis focuses on the currently available device (HM3), which reflects the availability to patients as it is the only device currently available in the UK.
Comparator
Medical management or different generation or type of devices or no comparator.
Outcomes
All relevant key outcomes were considered. Outcomes were categorised in accordance with categories established for parameters in the economic model. These were survival, hospitalisations, major events (e.g. stroke, RHF), complications [e.g. GIB, driveline infection (DI), arrhythmias], any report of QoL and functional status (e.g. six-minute walk test). The outcomes are further defined in the data extraction details.
Types of study
Any clinical trial whether randomised, non-randomised or single-arm was included, as well as all observational studies including cohort, case-controls and case series designs. This also included any reports from patient registries of MCSDs (such as INTERMACS, etc.). Studies were eligible only where they included ≥ 50 or more DT patients. A threshold was required, given the large volume of small studies with likely limited value overall to the review. The threshold was based on calculations to determine the likely volume of missed evidence when excluding studies based on various sample size cut-offs. This was carried out by taking a sample of 200 relevant full-text articles and calculating what proportion of patients we would miss by excluding studies based on different sample size cut-offs. Excluding studies with a sample size of < 50 DT patients resulted in an estimated 4.8% of patients excluded across the evidence base. As a result, it was decided to only include studies with at least 50 participants. Systematic reviews were included to identify any additional potentially relevant primary studies.
Searches
The following databases were searched initially from inception until 20 May 2020: Cochrane Library (CENTRAL), MEDLINE and EMBASE via Ovid. For any relevant systematic reviews Epistemonikos, the Cochrane Library of Systematic Reviews and MEDLINE and EMBASE were searched. Searches incorporated free text and index terms related to population and intervention, with no restriction by study design. All database search strategies are available (see Appendix 2). The search term combinations in the example search strategy applied to the bibliographic databases were formulated in the standard way for a review and then augmented to ensure the strategy was sensitive to capturing studies known to the reviewers while keeping the yield to manageable numbers of records.
There was no restriction by date or language of publication on searches. Reference lists of relevant systematic reviews and included primary studies were checked for additional primary studies. Grey literature (e.g. institutional reports) was sought from key organisations. Conference abstracts were included if published within the previous 3 years of the search date. Ongoing and recently completed trials were searched using the World Health Organization (WHO) clinical trials portal.
Data and reports published from relevant registries were also identified from our searches. Further targeted searching was performed to identify publications that were not found during the searches.
Search updates were carried out from April 2020 until 11 January 2022. Searches for registry reports via relevant website lists (e.g. www.uab.edu/medicine/intermacs/research/publications) were undertaken at the same time as the database searches.
Study selection
All records received from the literature searches were initially entered into EndNote X9 (Clarivate Analytics) to facilitate removal of duplicates. 42 Records were entered into Covidence for screening and selection. 43 Title and abstracts were screened for potential relevance using the study eligibility criteria.
Where it was not clear if DT patients were included in the study or if any DT data were reported from the abstract alone, the full text of the study was sought. Full texts were retrieved for any potentially relevant records and checked for eligibility.
All stages of the study selection were undertaken by two reviewers independently and disagreements were resolved by third reviewer or consensus. Reasons for exclusions were recorded via Covidence and within an Excel spreadsheet.
Search results for both the cost and clinical effectiveness reviews were combined within the same EndNote and Covidence databases. During screening and selection, appropriate tags were assigned to potentially relevant records to identify them as either relevant for the clinical or cost-effectiveness review, or both.
Data extraction
Intervention studies
Data extraction of intervention studies was carried out using a predefined data extraction form, which was piloted on two included trials. Extraction was carried out by one reviewer and checked by a second with any discrepancies discussed to reach consensus.
The following data were extracted:
-
Study characteristics: including study design, setting, start and end dates, follow-up length, inclusion/exclusion criteria, number of participants who accepted, were randomised (where applicable) and completed the study, drop out and reasons.
-
Participant characteristics: including summary statistics for age, sex, ethnicity, INTERMACS score, NYHA class, comorbidities, cause of HF, current RVAD and any medications and BMI.
-
Intervention and comparator characteristics: including device type and name, number with each device, implantation details, MM dose and frequency.
-
Statistical analysis information such as methods of analysis.
-
Outcome data: survival, hospitalisation (initial length of stay, number of re-admissions), QoL (any assessment tool), major clinical events [stroke, transient ischaemic attack (TIA), RHF, RHF managed with a RVAD, myocardial infarction, pump exchange (PE)], complications (bleeding, infections, device-related infections, arrhythmias, pump thrombosis, device malfunction, hepatic dysfunction, haemolysis, hypertensions, sepsis), and functional status (any assessment tool). Outcome data were extracted at all time points reported in all measures.
Registries
Key data on all LVAD outcomes were extracted from registry reports to use alongside the trial data as trial populations were not included in the INTERMACS registry database. The following data were extracted:
-
Basic cohort characteristics: including population, age, INTERMACS scores (where reported), any subgroups analysed and device data (understanding that this information was often limited in registry reports).
-
Outcome data: survival, hospitalisation (initial length of stay, number of re-admissions), QoL (any assessment tool), major clinical events (stroke, TIA, RHF, RHF managed with a RVAD, myocardial infarction, PE), complications (bleeding, infections, device-related infections, arrhythmias, pump thrombosis, device malfunction, hepatic dysfunction, haemolysis, hypertensions, sepsis) and functional status (any assessment tool). Outcome data were extracted at all time points reported for all measures.
Single/multicentre observational studies
Data were extracted (as above) from all single/multicentre observational studies recruiting participants not included in any registries to supplement the data from trials and registries. Some of these studies were important in providing data from outside the USA. Single/multicentre observational studies that also contributed patient data to the INTERMACS database [and therefore International Registry for Mechanically Assisted Circulation (IMACs)] were only used if they reported key data missing from the previous evidence (such as survival, QoL and major events).
Risk-of-bias assessment
Risk-of-bias assessment was carried out by one reviewer and checked by a second. Tools appropriate for study design were used to assess risk of bias. For RCTs and non-RCTs, version 2 of the Cochrane risk-of-bias tool was used. 44 The randomisation domain was not applicable for non-randomised trials, and it was acknowledged that blinding is not possible in most surgery trials. Risk-of-bias assessment was carried out for the key outcomes of survival and QoL. These were considered as key outcomes as they were consistently reported across trials of this type and were important outcomes for the economic evaluation. Results of the risk of bias were presented in tabular format.
Data synthesis
Consideration, data extraction and reporting of the evidence were based on a hierarchical approach. RCTs and controlled non-randomised trials were considered in the first instance. Registry reports and uncontrolled observational studies were used to supplement findings where gaps were evident. Inclusion of studies with overlapping patient data was avoided where possible. However, all studies, regardless of design, were included and reported in the review.
Clinical trial participants are not eligible to be included in LVAD registries (such as INTERMACS and IMACS). To assess whether patients in trials differed from those in registries, exploratory analyses were undertaken comparing key population differences between the two. This was carried out where the data were available for key outcomes including survival and QoL. Additionally, changes over time in each population were assessed.
Overlap of participants between single, multicentre observational studies and registries was also considered. Many of these centres in the USA also contribute data to registries, including INTERMACS and by default, IMACS. To avoid overlap of participant data reported, data were only considered from studies that clearly did not contribute to the INTERMACS registry (as defined by the list of participating centres on the INTERMACS website www.uab.edu/medicine/intermacs/pedimacs/participating-centers-pedimacs).
Data were tabulated and analysed in a narrative approach in the first instance, firstly with comparative data stratified by device (e.g. HM3) and INTERMACS scores (where available), and then by outcome within this. Following this, non-comparative data were presented stratified by device type and INTERMACS scores (where available), and by outcome within this.
Due to large clinical and methodological heterogeneity expected in the data, no meta-analyses were performed.
Forest plots without pooled estimates were created for all data stratified by device type for each outcome category (survival, QoL, hospitalisations, major events and complications).
While all recent devices (e.g. HeartMate XVE™, HeartMate II, HeartWare HVAD, HM3) were included and analysed, priority was given to the HM3 device given the availability of devices in the UK and the recent withdrawal of the HeartWare HVAD device in June 2021.
Statistical analysis
Outcomes of mortality, hospitalisation, major events and complications
Potentially relevant data for these outcomes were reported in a variety of different forms. These included:
-
rate per participant, rate per participant-year, rate per 100 participant-years;
-
total number of events, mean number of events per participant, number of participants who had at least one event, proportion of participants who had at least one event, proportion of participants who were event free; and
-
total follow-up time in person-years, average study follow-up time, overall study follow-up time.
Where possible, these data were used to estimate (1) the proportion of participants who had an event, and (2) the event rate per 100 person-years. For each of these statistics a hierarchical approach was used to calculate the sufficient statistics for each study with preference given to those approaches that made the fewest assumptions:
Where possible, these proportions and their 95% CIs were estimated using exact binomial distribution and the results are presented in forest plots. Estimation of the number of participants with the event was as follows:
-
The number of participants with an event is reported by the study report and this number is extracted.
-
The number of participants in the study group together with the proportion of participants with the event are reported. Multiplication of these two quantities rounded to the nearest integer is used.
Where calculable, event rates per 100 participant-years follow-up were reported together with 95% confidence intervals (CIs) and these are displayed on forest plots. To estimate the standard errors and CIs it was assumed that patient events over the whole participant group followed a Poisson process and that the sampling distribution on the log-rate scale was normal.
Estimation of total study follow-up time in each group:
-
The total follow-up time was reported for each group and these numbers were extracted.
-
If the total number of events and the rate of events per patient-year (or 100 patient-years, etc.) were reported, these were multiplied together (after a suitable linear transformation if required).
-
If the follow-up time for the study and the number of participants in the study were reported, then these were multiplied together.
Estimating event rate per 100 patient-years follow-up:
-
The number of events per patient-year (or 100 patient-years etc.) was reported, then an appropriate linear transformation was used if required.
-
The total number of admissions divided by the total person-years follow for the patient group if these were both either reported or calculable as described above.
Estimating the number of events occurring in each study.
-
The total number of events for the patient group is reported by the study.
-
If the number of events per participant-year and the total follow-up time were reported or could be estimated as described above, then these were multiplied.
-
If the mean number of events per participant and the total number of participants was reported for each group these were multiplied together and rounded to the nearest integer.
Network meta-analysis
Indirect comparisons between all nodes in the network together with standard errors were calculated using the Butcher method (chained over indirect comparisons as required) on natural log relative risk (RR) scale. 45 As there was no closed loop evidence, no checks for evidence consistency were possible. As there was only one trial for each comparison a fixed effects model was used, and no estimates of heterogeneity were calculable.
Subgroup analysis
Key subgroups were considered following input from the steering group. This included reporting of data from participants with different INTERMACS profiles (indicating severity of HF) as well as different age categories. Subgroup analysis was considered where relevant data were available. While meta-analysis of subgroups was not possible due to a lack of data or differences in definitions, subgroup data were presented in forest plots without summary points.
Results
Selection
Searches for both clinical and cost-effectiveness studies identified 12,153 articles. Following removal of duplicates, 9006 articles remained. There were 982 articles found to be potentially relevant following title and abstract screening, and full texts were sought and checked against the eligibility criteria. Following this, 240 articles from 134 studies met the criteria for the clinical effectiveness review and were included.
There were 6 trials (1 non-randomised, 5 RCTs), 86 observational studies, reports from 5 registries, 5 ongoing studies and 32 systematic reviews (included for citation checking). It should be noted that some of these studies were from the same centres and reports from the same registries were considered as one single study. There were 24 relevant articles included following citation checking, 21 of which were relevant to the clinical effectiveness review. Seven full-text articles could not be retrieved. A summary of the selection process is given in Figure 4. A list of full-text articles excluded with reasons is available in Appendix 3.
FIGURE 4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram showing selection of studies. a, Only studies considered for the clinical effectivenessreview were excluded based on sample size.

Types of included studies
Studies included in the review were categorised as comparative trials, registry reports or single and multicentre observational studies.
Devices
Several LVADs have been used in the care of end-stage HF patients and these are detailed in Table 6. The pulsatile HeartMate XVE is no longer in clinical use and was replaced by the HeartMate II device. The HeartWare HVAD was withdrawn from the market in June 2021 due to concerns over increased stroke risk and pump thrombosis compared to alternative devices. 32 The HM3 device is currently the most implanted device in the USA, having been approved for clinical use by the Food and Drug Administration (FDA) for DT in 2019. 46
| Device | Type | Weight | Size | Circulatory support (RPM, flow l/min) |
Manufacturer |
|---|---|---|---|---|---|
| HeartMate XVE | Vented electric device, pulsatile flow | 1255 g | Diameter: 11.2 cm Nominal height (excluding ports) 5.8 cm (The size of the device requires patients to have a body surface area of more than 1.5 m2) |
4–10 l/minute | Thoratec Inc., Ann Arbor, MI, USA |
| HeartMate II | Axial-flow pump (continuous flow) |
350 g | Diameter: 4 cm Length: 7 cm |
10 l/minute at RPM ranging from 8000 to 15,000 | Thoratec Inc., Ann Arbor, MI, USA |
| HeartMate 3 | Fully magnetically levitated centrifugal-flow pump (continuous flow) |
200 g | Diameter: 50.3 mm Height: 55.8 mm (includes inflow cannula), 33.8 mm (excludes inflow cannula) |
10 l/minute RPM ranging from 3000 to 9000 |
Abbott Laboratories, Chicago, IL, USA |
| HeartWare HVAD | Centrifugal-flow pump (non-magnetic) (continuous flow) |
145 g | Diameter: 4 cm Length: < 2 cm |
10 l/minute at RPM ranging from 1800 to 3000 | Medtronic Inc., Minneapolis, MN, USA (formerly HeartWare Inc., Framingham, MA, USA) |
| EVAHEART 2 (EVA2) | Centrifugal-flow pump, hydraulically levitated impeller for retained pulsatility | NR | NR | 7–8 l/minute up to 2200 RPM | Evaheart Inc., Houston, TX, USA |
Table 6 also details the EVAHEART 2 (Evaheart Inc., Houston, TX, USA) LVAD, described as a centrifugal hydraulically levitated ‘open vane’ impeller that supports blood circulation and high peak flows for retained native pulsatility. The device is currently being trialled in a large RCT in the USA under a FDA-approved investigational device exemption and being compared to the HM3 device with completion estimated for 2024. 47 At the time of the report’s writing, no results or data have been published pertaining to the EVAHEART 2.
HeartMate 3 data
The HM3 is currently the only option for LVAD-eligible patients in the UK following the withdrawal of the HeartWare HVAD. This section will describe the available data pertaining to the HM3 device.
Trial data
There was one RCT (MOMENTUM 3) undertaken in the USA that compared the HM3 device to the older HeartMate II device. There were 1028 participants included in the study: 515 randomised to the HM3 (317 of these DT) and 505 randomised to the HeartMate II (307 DT). The mean age for all included DT patients was 63 (SD 12) and the majority of included participants were INTERMACS level 3 (Table 1). The following section reports data by outcome from this RCT.
Survival
Survival in DT patients implanted with the HM3 device was 84% at 12 months and 77% at 24 months of follow-up (Figure 5). This is higher than any previously reported survival data in earlier generation devices at 24 months (see Appendix 5). Survival in HM3 DT patients was higher than the HeartMate II patients in the MOMENTUM study at 24 months (77% vs. 59%, respectively) with a hazard ratio (HR) of 0.87 (95% CI 0.63 to 1.2), though this was not considered a statistically significant difference. However, survival free of disabling stroke or reoperation to replace or remove a malfunctioning device at 24 months was significantly higher in patients in the HM3 group compared to the HeartMate II group (73% vs. 57%), with a HR of 0.61 (95% CI 0.46 to 0.81).
FIGURE 5.
Survival data in the HeartMate 3 at all reported follow-up time points.

Quality of life
While clear improvements in QoL were reported in DT patients with the HM3 device in both the KCCQ and the EQ-5D at 12 and 24 months compared to baseline, similar QoL improvements were also seen in the HeartMate II group. The mean visual analogue scale (VAS) summary score for the KCCQ improved from 40 at baseline to 69 at both 12 and 24 months in the HM3 group (Figure 6), compared to 39 at baseline increasing to 70 at 12 months and 68 at 24 months in the HeartMate II group. Furthermore, the EQ-5D VAS score improved from 48 at baseline in the HeartMate II group to 74 at both 12 and 24 months (compared to an increase from 51 at baseline to 77 at 24 months in HM3). Improvements in QoL appeared to peak at 12 months and remain stable at 24 months. Scores remained similar to those at 24 months at the end of study follow-up.
FIGURE 6.
Mean QoL scores in the HeartMate 3 at all reported follow-up time points.

Hospitalisations
Rehospitalisation days were reported to be fewer in HM3 patients compared to those with the HeartMate II device (median duration 15 vs. 22 days) over 24 months. The event rate of rehospitalisation per 100 patient-years was found to be significantly lower in the HM3 group versus the HeartMate II group (212 vs. 243, HR 0.88 95% CI 0.81 to 0.96, Figure 7 Hospitalisation rate per person-year in the HeartMate 3 at 24 months follow-up).
FIGURE 7.
Hospitalisation rate per person-year in the HeartMate 3 at 24 months follow-up.

Major events
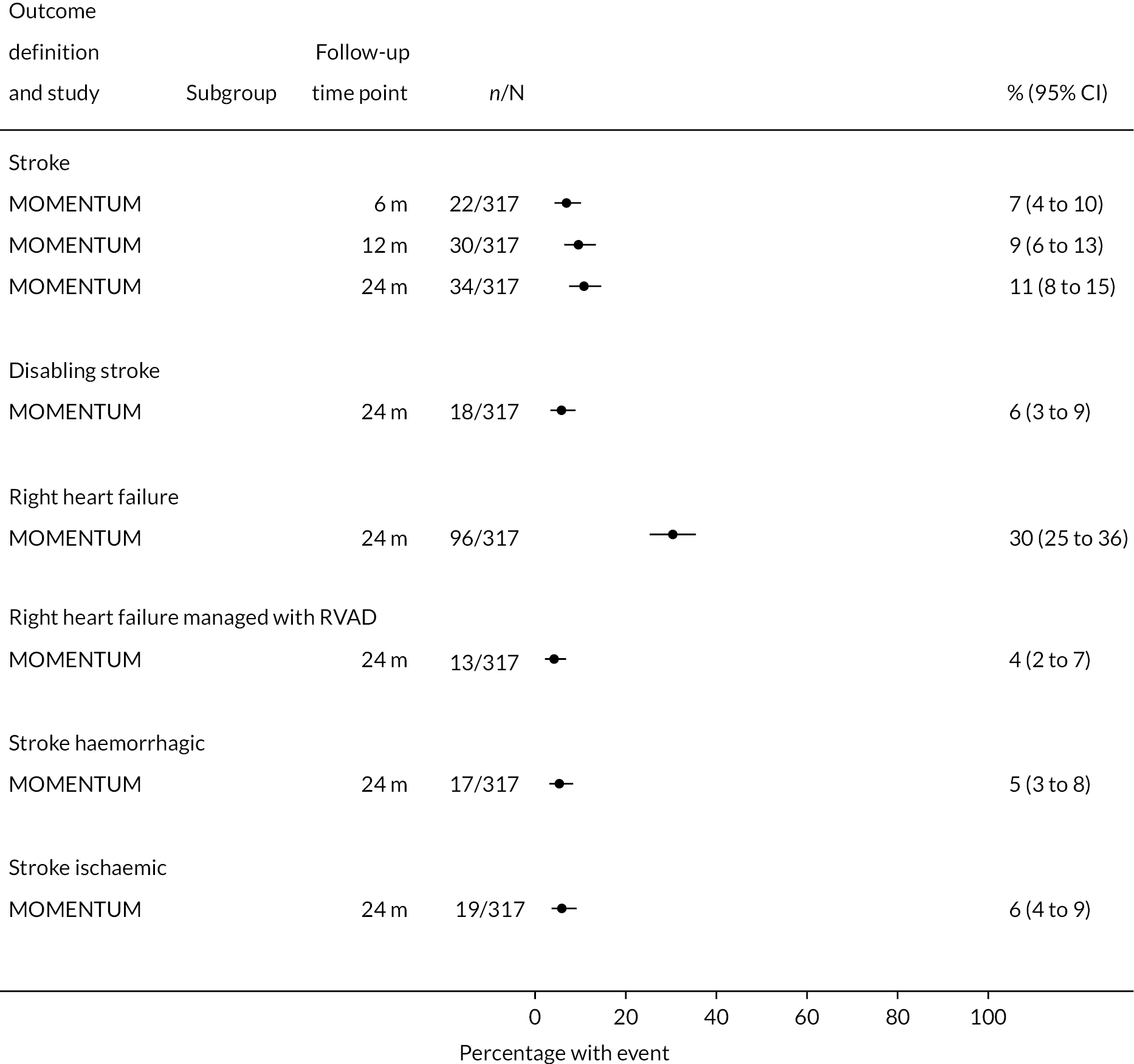
Stroke rates were reported to be lower than seen in the literature previously in the HM3 and lower than the HeartMate II group over 24 months. Regarding any stroke, there were 8 events per 100 patient-years in the HM3 group (Figure 8) (11% had a stroke at 24 months, Figure 9) compared to 19 events in the HeartMate II group (RR 0.42 95% CI 0.29 to 0.62). This was similar for disabling stroke: 4 events per 100 patient-years for HM3 versus 7 events per 100 patient-years for HeartMate II with a RR of 0.59 (95% CI 0.34 to 1.03). There were no significant differences in rates of RHF or RHF requiring RVAD between the device groups.
FIGURE 8.
Event rate per 100 person-years in the HeartMate 3 at 24 months follow-up.
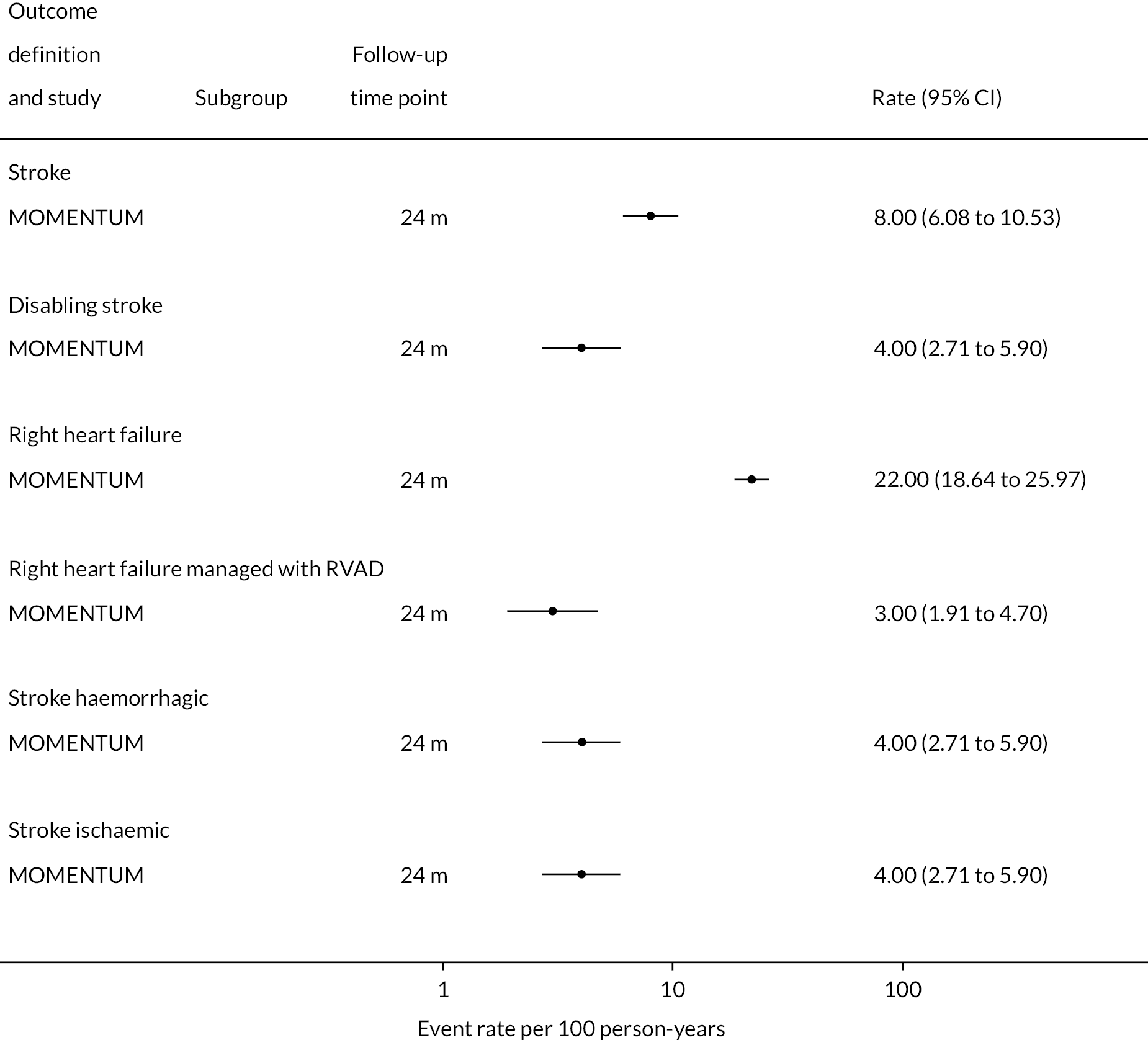
FIGURE 9.
Proportion of patients with major events in the HeartMate 3 at all reported follow-up time points.
Complications
Rates of pump thrombosis were found to be significantly lower in the HM3 group compared to the HeartMate II group with 1 event versus 12 events per 100 patient-years, respectively, and a RR of 0.1 (95% CI 0.04 to 0.24). On the other hand, DIs were common and not found to be different between the two arms.
While bleeding events were still an issue in MOMENTUM 3, they occurred less frequently in patients implanted with the HM3 compared to the HeartMate II (70 events vs. 103 events per 100 patient-years) with a RR of 0.68 (95% CI 0.59 to 0.78). More specifically, bleeding requiring surgery and GIB rates were both significantly lower in the HM3 group. The proportion of patients with complications and the complication rate are shown in Figures 10 and 11.
FIGURE 10.
Complication rate per 100 person-years in the HeartMate 3 at all reported follow-up time points.
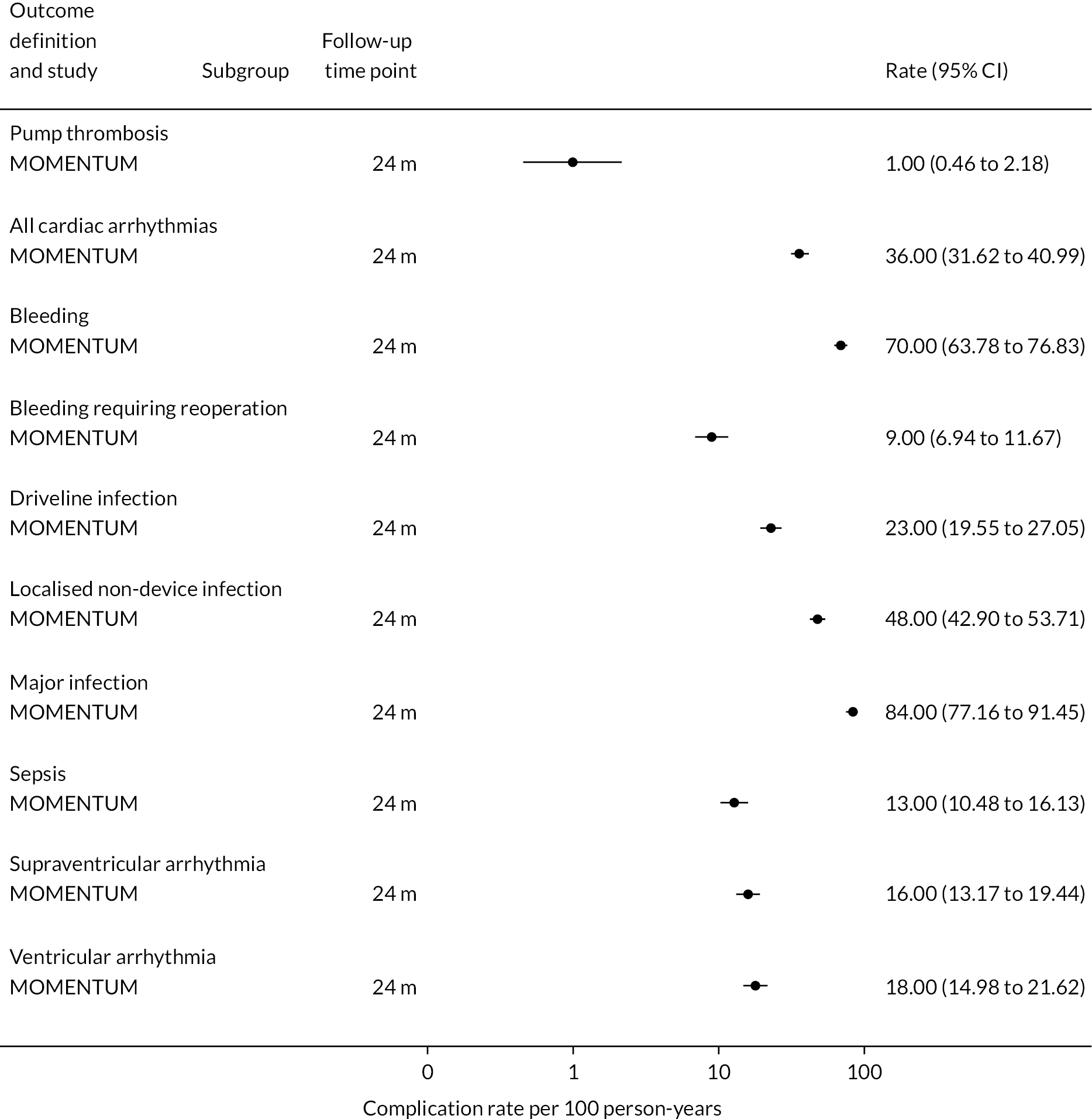
FIGURE 11.
Proportion of patients with complications in the HeartMate 3 at all reported follow-up time points.

Functional status
The mean six-minute walking distance increased in HM3 patients in MOMENTUM from 137 m at baseline to 320 m at 24 months. However, the proportion of patients with NYHA Class I or II remained similar at baseline (78%) and 24 months (78%).
Ongoing trials
Currently, there are no completed trials comparing the HM3 to any other current LVADs. In addition to the ongoing North American trial of the HM3 versus the EVAHEART device, there is an ongoing Swedish RCT comparing the HM3 to MM, and this trial is expected to be completed in 2023. 47,48 Information on this study and other ongoing trials can be found in Table 7. The Jarvik 2000® is an older-generation device of which data have not been included in this report as it is considered out of date. However, a long-standing trial record established in 2012, which did not appear to have progressed for a long period of time, was updated during the later stages of this report to indicate that the trial of the Jarvik 2000 versus HeartMate II was ongoing, with completion due in December 2023. Therefore, this should be considered in the future.
| Study | Study design | Population | No. participants (no. DT) | Intervention | Comparator | Primary outcome | Length of follow-up (months) | Estimated completion date | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Swedish evaluation of LVAD as permanent treatment in end-stage HF (SweVAD)49 | RCT | End-stage HF population ineligible for cardiac transplantation | Estimated enrolment 80 participants | HeartMate 3 | Optimal medical management | Survival at 2 years | 24 months minimum (up to 5 years) | December 2023 | Sweden NCT02592499 |
| Sustaining QoL of the aged: heart transplant or mechanical support?50 | Prospective observational | Older (60–80 years) AHF patients undergoing heart transplant or LVAD as permanent therapy | Estimated enrolment: 800 participants | Mechanical circulatory support | Heart transplant | Non-inferior change in patient HRQoL at 2 years | 24 months | March 2022 | USA NCT02568930 |
| Prospective multicentre randomised study for evaluating the EVAHEART®2 left ventricular assist system (COMPETENCE)47 |
RCT | Adult (> 18 years old), AHF NYHA Class IV patients who are refractory to AHF management and meet study inclusion/exclusion criteria will be enrolled | Estimated enrolment: 399 participants, no. DT unclear | EVA2 | HeartMate 3 | Survival to cardiac transplant or device explant for recovery free from disabling stroke (Modified Rankin score > 3) or predefined severe RHF at 6 months after implantation of the originally implanted device | 24 months | March 2024 | USA NCT01187368 |
| LVAD vs. GDMT in ambulatory AHF patients (AMBU-VAD)51 | RCT | Ambulatory AHF patients ≥ 18 years | Estimated enrolment: 92 participants, no. DT unclear | HeartMate 3 | Guideline directed medical therapy | All-cause mortality rate | 24 months | February 2025 | France, NCT04768322 |
| Evaluation of the Jarvik 2000 left ventricular assist system with post-auricular connector--DT Study52 | RCT | End-stage HF patients who are ineligible for transplant | Estimated enrolment: 350 participants, all DT | Jarvik 2000 VAS | HeartMate II | Non-inferiority to control group | 24 months | December 2023 | USA, NCT01627821 |
Additional registry data
Available registries and data
Data from INTERMACS and other registry reports (including IMACS and ITAMACS) were analysed and compared to trial data. Participants enrolled in trials were not eligible for registry inclusion in North America, which means that the populations of these two categories may differ.
Of the 37 registry reports that were included in the review, 29 were from the INTERMACS registry, 2 from IMACS, 2 from ITAMACS, 2 from ELEVATE and 2 from the Thoratec® (Ann Arbor, MI, USA) DT registry (now redundant). DT-specific patient data were limited and were mostly not reported by device type, meaning most of the data were reported across multiple LVADs or sometimes by continuous flow only or pulsatile flow types only. However, INTERMACS and IMACS reports also held the longest follow-up data, reporting up to 60 months for outcomes such as survival, major events and complications in DT patients.
INTERMACS reports contain data from sites in the USA that have agreed to supply anonymous data on registered LVAD patients at their centre. Data are entered at implant where possible, and are then entered over time for events, complications and QoL scores until death or removal/transplant. Only devices that are FDA approved are eligible for INTERMACS inclusion.
IMACS is an international registry that includes all countries and hospitals willing to participate. Currently, this also includes data from INTERMACS, European Registry for Patients with Mechanical Circulatory Support (EUROMACS), J-MACS and the UK registry. Not all of the contributing countries have a DT programme, therefore contributing DT data are limited to countries such as the USA, France and Kazakhstan.
ITAMACS is an Italian registry reporting the vast majority of LVADs and mechanical assist devices implanted in Italy.
ELEVATE is a registry that studies and reports the long-term outcomes of patients on the HM3 device following CE-mark approval in Europe and the Middle East. To date, few DT-specific data have been reported from this registry, though this may be important in the future.
HeartMate 3 data
No usable data specifically for the HM3 device from any registries were available. Patients who are involved in clinical trials are not able to register for INTERMACS, meaning that patients with the HM3 were not likely to enter the registry until after the trial had finished. While some of the later registry reports may include data from HM3 patients, these were not reported separately from other device data. Data were often reported by device type (e.g. centrifugal flow, axial flow), meaning that different devices could not be further distinguished.
Non-INTERMACS observational studies
To supplement findings from trials and registry reports, single and multicentre observational studies that were judged unlikely to overlap with any registry data (i.e. centres did not contribute data to a registry that was known) were analysed. These studies are detailed in Table 8.
| Study ID | Centre | Implant years | Total no. patients (no. DT) | INTERMACS profiles (n, %) | Mean age (SD) | Male (n, %) | Device types (n, %) | Subgroups analysed | Outcomes reported | DT data reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed 2018 | University of Florida | 1 January 2008–31 December 2015 | 111 (61) | 1–2 (52, 46.8); 3–7 (59, 53.2) |
57.6 (range 19–80) | 92 (82.8) | NR | Low/high socioeconomic status | 1-year survival, re-admission within 30 days, length of stay, aggregate VAD complications | Implant strategy was not found to significantly impact the primary outcomes |
| Aissaoui 2013 | Clinic for Thoracic and Cardiovascular Surgery of Bad Oeynhausen (Germany) | 2001–April 2011 | 488 (LVAD with temporary RVAD 45, DT 10; LVAD alone 443, DT 115) | NR | LVAD with temporary RVAD 53 (82) LVAD alone 56 (13) |
LVAD with temporary RVAD 37 (82) LVAD alone 289 (65) |
LVAD with temporary RVAD: 9 HeartMate XVE, 9 HeartMate II, 13 HeartWare, 5 VentrAssist, 5 DuraHeart, 4 Novacor. LVAD alone: 50 HeartMate XVE, 111 HeartMate II, 75 HeartWare, 47 VentrAssist, 74 DuraHeart, 53 Novacor, 18 CorAide, 9 LionHeart, 4 Incor, 2 DeBakey VADs |
LVAD with temporary RVAD/LVAD alone | Complications: renal failure, sepsis, adverse cerebral events, reoperation for bleeding, pump malfunction, and arrhythmia Cerebral complications included cerebral haemorrhage, transient ischemia, and cerebral vascular accident |
DT was univariate risk factor for death: odds ratio 7.39 (95% CI 4.09 to 13.4) |
| Akay 2019 | Unclear | May 2012–July 2016 | 222 (144) | 1–2 (124, 56) 3–4 (98, 44) |
54 (12) | 178 (80) | HeartMate II 164 (74), HeartWare HVAD 52 (23), HeartMate 3 6 (3) | Patients who developed DI/patients with no DI | Associations with DI | No. DT patients who had DI 25 |
| Aldbrecht 2015 | Medical University of Vienna | 1997–2012 | 118 (All DT) | Conservative treatment median 3 (IQR 3–3) Pulsatile flow VAD median 3 (IQR 2–3) Continuous flow VAD 3 (IQR 2–4) Patients with INTERMACS 1 were excluded |
Conservative treatment 57 (9) Pulsatile flow VAD 57 (10) Continuous flow VAD 57 (10) |
Conservative treatment 46 (92) Pulsatile flow VAD 22 (88) Continuous flow VAD 38 (88) |
Pulsatile flow VAD 25 (21) Continuous flow VAD 43 (36) |
Conservative treatment (medial therapy) Pulsatile flow VAD Continuous flow VAD |
Survival, cause of death, hospitalisations, heart transplants | All outcomes |
| Baudry 2021 | 19 French Centres | February 2006–December 2016 | 652 (303 of which are INTERMACS 4–7 and focus of analysis, 132 DT) | All patients were INTERMACS 4–7 | 61 (9.9) | 263 (86.8) | HeartMate II 224 (73.9), HeartWare HVAD 52 (17.2), Jarvik 2000 27 (8.9) | N/A | Operative and postoperative outcomes, survival, risk factors for mortality | DT as a risk factor for mortality |
| Bugetti 2016 (CA) | Italy | June 2008–December 2015 | 178 (All DT) | At implant average level was 3 (1.2) | NR | NR | Jarvik 2000 Flowmaker | N/A | Survival, QoL | 12 m survival 82%, 24 m 60%, 36 m 54% |
| Chen 2021 | 19 French centres | 2006–16 | 652 (247 DT, 38) | NR | LVAD implanted < 30 days after cardiomyopathy median 55.2 (IQR 46.9–61.4) LVAD implanted > 30 days after cardiomyopathy median 60.7 (IQR 53.3–66.9) |
561 (86) | HMII 475 (73) HVAD 127 (19) Jarvik 2000 50 (8) |
LVAD implanted < 30 days after cardiomyopathy vs. > 30 days after cardiomyopathy | All-cause mortality, cardiovascular/non-cardiac cause of death, heart transplant, complications (thrombosis, stroke, bleeding, LVAD malfunction) | No. alive at 30 days post implant, DT as a predictor of mortality |
| Consolo 2018 | San Raffaele Scientific Institute in Milan, Italy | March 2015–June 2017 | 68 (All DT) | 1 (20, 30) 2 (17, 25) 3 (15, 22) 4 (15, 22) |
64.7 (7.8) | 64 (94) | HeartMate II 15 (22), HeartWare HVAD 38 (56), HeartMate 3 15 (22) | N/A | Association between platelet activation and the development of thromboembolic events | Incidence of thromboembolic events (patients with events: stroke 4, pump thrombosis 2) |
| Cruz Rodriguez 2020 | NR | January 2008–February 2017 | 204 (77) | Inotrope use < 14 days: 1 (32, 37.2) 2 (44, 51.2) 3 (9, 10.5) 4 (1, 1.2) Inotrope use ≥ 14 days: 1 (49, 41.9) 2 (56, 47.9) 3 (10, 8.5) 4 (2, 1.7) |
Inotrope use < 14 days 51.8 (25th–75th percentile, 38.9–63) Inotrope use ≥ 14 days 56.4 (25th–75th percentile, 48.4–62.7) |
Inotrope use < 14 days 70 (81.4) Inotrope use ≥ 14 days 90 (76.9) |
Only HeartMate II and HeartWare HVAD, numbers not reported | Those on inotropes for < 14 days after implant and those on inotropes for ≥ 14 days after implant | Mortality of LVAD patient on prolonged inotropes, risk factors for early inotrope use, association of prolonged inotrope use and clinical events | Survival for DT compared to BTT HR 1.23 (95% CI 0.72 to 2.11) in a multivariate model |
| Drakos 2010 | NR | 1993–2008 | 175 (74) | NR | RVF 58.2 (12.9) No RVF 56.5 (14.4) |
RVF 61 (79) No RVF 85 (87) |
HeartMate XVE 82 (47), HeartMate VE 42 (24), HeartMate 1000 IP 17 (10), HeartMate II 25 (14), Novacor 9 (5) | RVF vs. no RVF | Survival (not by DT), predictors of RVF | DT as a predictor of RVF in a multivariate model: odds ratio 3.31 (p = 0.005) |
| Galand 2016 (CA) | Multicentre, France | 2008–16 | 223 (160 ICM, 59 DT; 63 DCM, 24 DT) | NR | ICM 60.8 (9.3) DCM 61 (13.3) |
ICM 145 (90.5) DCM 55 (86.9) |
ICM: HeartMate II 107 (66.9), HeartWare HVAD 40 (25), Jarvik 2000 12 (7.5), Ventrassist 1 (0.6) DCM: HerrtMate II |
ICM or idiopathic DCM | Survival, adverse events | 24 m DT survival: ICM 52% DCM 50% No difference between ICM/DCM or by indication |
| Galand 2020 (Same sample of patients as Chen 2021) |
19 French centres | 2006–16 | 652 (247 DT, 38) | NR | Patients aged ≥ 70 years median 71.7 (IQR 70.7–72.8) Patients aged < 70 years median 58.2 (IQR 50.0–64.7) |
561 (86) | HMII 475 (73) HVAD 127 (19) Jarvik 2000 50 (8) |
Patients aged ≥ 70 and patients aged < 70 years | All-cause mortality, cardiovascular/non-cardiac cause of death, heart transplant, complications (thrombosis, stroke, bleeding, LVAD malfunction) | Survival in DT patients ≥ 70 and < 70 presented in survival curve |
| Jaganathan 2019 (CA) | Multicentre USA | NR | 186 (53) | NR | NR | NR | NR | NR | QoL in a new LVAD QoL tool as well as already established tools | DT vs. BTT: there was no statistical difference in emotional domain (p = 0.11) Social functioning was higher in DT vs. BTT (p = 0.04) No significant difference in PHQ-9 scores between DT and BTT (p = 0.43) |
| Janssen 2021 (CA) | Single centre, the Netherlands | 2010–20 | 63 | NR | Median 63 (range 29–72) | 50 (79) | All HeartWare HVAD | Time in therapeutic range INR < and ≥ 60% | Death, and thromboembolic, neurologic and haemorrhagic events | 13 thromboembolic, 19 haemorrhagic, 19 neurologic events and 34 deaths occurred |
| Kalampokas 2021 (CA) | German centre | January 2010–May 2020 | 227 (27 Age ≥ 70, 200 < 70) | Age ≥ 70 11 (40.7) INTERMACS 4 Age < 70 45 (22.5) INTERMCAS 4 |
Age ≥ 70 73.1 (2.55) Age < 70 55.3 (10.59) |
Age ≥ 70 22 (81.5) Age < 70 174 (87) |
NR | Age ≥ 70 and age < 70 | Peri-procedural complications, mortality | All outcomes, 30 day mortality Age ≥ 70 14.8%, Age < 70 12%. Mid-term mortality (mean 2.5 years) Age ≥ 70 55.6%, Age < 70 32.5% |
| Kapuria 2016 (CA) | NR | 2010–4 | 79 (DT NR) | NR | NR | 58 (73) | NR | N/A | Incidence of GIB, predictors of GIB | DT recipients 6 times more likely to bleed as compared to BTT recipients (OR 6.32, p = 0.032) |
| Loforte 2018 | S. Orsola University Hospital in Bologna and S. Camillo Hospital in Rome | January 2006–December 2017 | Isolated LVADs 170 (30 in derivation cohort, 9 in validation cohort) Unplanned BVAD 88 (32 in derivation cohort, 7 in validation cohort) |
Isolated LVAD: Derivation cohort: 1 (4, 2.9) 2–3 (102, 75.5) 4 (29, 21.4) Validation cohort: 1 (2, 5.7) 2–3 (25, 71.4) 4 (8, 22.8) Unplanned BVAD: derivation cohort: 2–3 (58, 81.6) 4 (13, 18.3) Validation cohort: 2–3 (11, 64.7) 4 (4, 23.5) |
Isolated LVAD: Derivation cohort 54.1 (1.6) Validation cohort 53.1 (1.7) Unplanned BVAD: Derivation cohort 57.3 (2.6)Validation cohort 56.1 (1.4) |
Isolated LVAD: Derivation cohort 120 (88.9) Validation cohort 26 (74.2) Unplanned BVAD: Derivation cohort 51 (69.9) Validation cohort 11 (64.8) |
Isolated LVAD HeartMate II 56 (32.9), HeartWare HVAD 51 (30), CentriMag 35 (20.6), HeartMate 3 19 (11.2), Jarvik 2000 6 (3.5), Heart Assist 5 1 (0.59), Berlin heart Incor 1 (0.59) Unplanned BVAD CentriMag 44 (50); HeartMate II 27 (30.6) HeartWare HVAD 15 (17) HeartMate 3 2 (2.3) |
Include both isolated LVADs and unplanned BVADs | Severe RVF within 30 days of LVAD implantation, all-cause mortality | DT as a predictor of BVAD requirement in multivariate model: HR 2.0 (95% CI 1.7 to 3.9) |
| Loforte 2018 (× 2 CAs reporting results of the same study) | Italy | 2006–16 | 215 LVAD 143, BVAD 72 (DT NR) | NR | NR | NR | NR | NR | Predictors of BVAD need | DT was a major predictor for the need for BVAD (HR 2.0, 95% CI 1.7 to 3.9) |
| Medressova 2019 (CA) | Khazakstan | 2011–8 | 207 (all listed as DT though unclear as they include BTT on long-term support) | NR | 49 (13) | 188 (88) | HeartMate II, HeartWare HVAD or HeartMate 3 | NR | Survival, survival by distance from hospital | Kaplan-Meier survival 12 m 87.3%, 24 m 68.8%, 36 m 60.6%, 48 m 47.2% |
| Papathanasiou 2017 | West German Heart and Vascular Center | December 2010–June 2016 | 112 (77) | 1 (31, 27.7) 2 (18, 16.1) 3 (25, 22.3) 4 (35, 31.3) 5 (3, 2.7) |
58.4 (10.9) | 91 (81.3) | All HeartWare HVAD | Those who underwent resternotomy and those who did not have resternotomy | Prognostic significance of resternotomy, hospitalisations, infection rates, survival | DT as predictor of mortality compared to BTT HR 2.83 (95% CI 1.207 to 6.649) |
Of the 86 included observational studies, 21 were judged unlikely to overlap with registry data. Of these, 2 were carried out in the USA, 13 in Europe, 1 in Kazakhstan and 4 were unclear.
HeartMate 3 data
As with data from registry reports, there were few studies that included patients implanted with HM3 and only one of these reported device-specific data for DT patients. One trial53 reported zero pump thrombosis events in the 15 HM3 patients included in their centre over 24 months of follow-up. No further observational studies reported HM3 data specifically. Often these studies had limited numbers of HM3 patients, likely because the majority of these patients would have been in the MOMENTUM trial when the device was first approved for DT.
HeartMate 3 versus medical management
The key aim of this review is to determine the effectiveness of LVADs compared to MM in DT patients (though the HM3 is currently the only available device on the UK market). However, there are no published studies, randomised or observational in nature, comparing HM3 to MM. However, one ongoing RCT will be important in addressing this question in more detail in the future. This section of the report will detail the available HM3 versus MM data and the methods explored to indirectly compare these interventions in the absence of direct comparisons.
SweVAD study
The SweVAD study (‘Swedish evaluation of left ventricular assist device as permanent treatment in end-stage HF’) is an ongoing RCT comparing the HM3 to ‘optimal medical management’ in those ineligible for a HT. 48 The study aims to recruit 80 participants and follow them up for up to 5 years. The estimated completion date is December 2023 and it will report outcomes for survival, functional capacity, QoL and adverse events. The study is being carried out in seven University Hospitals with implantations being performed at five sites. Given that data are not expected from this study in the immediate future, methods to indirectly compare the HM3 to MM were considered.
Indirect comparison of HeartMate 3 versus medical management through randomised controlled trials
As described in the methods, NMA was considered, where possible, to allow for the indirect comparison of HM3 and MM. This involved sequential indirect comparisons of data through previous RCTs (HM3 vs. HeartMate II, HeartMate II vs. HeartMate XVE and finally HeartMate XVE vs. MM) to ultimately compare the HM3 and MM. To achieve this, data from all previous LVAD trials were extracted and analysed as required. These data are summarised below, in the first instance, to allow for background understanding of the trials and what they assessed and found.
Other trial data
There were five RCTs included in the review (including the HM3 MOMENTUM trial) as well as one non-randomised intervention study (ROADMAP). Generally, the studies compared to either MM (REMATCH and ROADMAP) or an alternative device. Table 2 details the characteristics of the included trials. The most recent and relevant device, the HM3, was compared only to the HeartMate II device. No trials have been completed that compare the HM3 to MM, though ongoing studies are currently exploring this as previously described.
INTERMACS profiles were reported at baseline in all but two of the trials. Most patients were INTERMACS level 3 in ENDURANCE studies across both study groups. Conversely, the ROADMAP study included only patients who were INTERMACS level 4–7.
The mean age did not appear to differ greatly amongst the trials and the intervention groups within each trial, ranging from 62 (HeartMate II DT) to 68 (REMATCH). However, it should be noted that age was reported for different groups of participants in each study. For example, MOMENTUM 3 reported the mean age for all DT patients included, regardless of assigned intervention, whereas most other trials reported the mean age for each arm. The ROADMAP study only reported median age, though this was similar to the other studies, even with the inclusion of participants with INTERMACS profiles 4–7 only.
It is important to note that all of these trials took place in the USA, and that currently no trial data are available in the UK or Europe, though SweVAD may be useful once completed.
HeartWare ventricular assist device
Two RCTs assessed the HeartWare HVAD device. The HVAD was compared to the HeartMate II device in both trials: ENDURANCE DT and ENDURANCE DT Supplemental Trial (an extended study of the HVAD looking at stroke outcomes). The HVAD has now been withdrawn from the market and reporting of results from this device will be of limited value.
Outcomes
Event-free survival (free of death, disabling stroke and device malfunction and/or failure requiring exchange, explantation or urgent transplantation) was higher in the HVAD compared to the HeartMate II arm, reaching 76% at 12 months in the ENDURANCE Supplemental Trial (compared to 67% in the HeartMate II arm). In the original ENDURANCE trial, the same outcome was 55% at 24 months in the HVAD group and 57% in the HeartMate II group. However, stroke rates were higher in the HVAD device arm, often occurring in the first 6 months. Rates of other events and complications were similar between the HVAD and HeartMate II arms, including major bleeding, cardiac arrhythmias and DIs. In both trials, QoL improved significantly in the HVAD group and was maintained at 12 and 24 months; however, this was also evident in the HeartMate II group.
HeartMate II
The HeartMate II device has been studied in four trials in total. Firstly, as the intervention in the HeartMate II DT RCT (vs. the HeartMate XVE) and the ROADMAP study (a non-randomised comparative trial vs. MM). It was also compared to newer devices in MOMENTUM 3 and ENDURANCE DT. The HeartMate II is currently the most widely studied LVAD.
Outcomes
In the HeartMate II DT trial survival was reported to be 68% at 12 months and 55% at 24 months in the HeartMate II group. This was superior to the HeartMate XVE (survival 58% and 24% at 12 and 24 months, respectively). The HeartMate II event-free survival was 82% at 12 months in the more recent ROADMAP study and 70% at 24 months. Events such as haemorrhagic stroke, bleeding and DIs were significantly reduced in the HeartMate II patients compared to the HeartMate XVE patients in the HeartMate II DT trial. Strokes occurred at a rate of 12 events per 100 patient-years, pump replacements 6 events per 100 patient-years and RHF requiring RVAD 2 events per 100 patient-years over 24 months. Strokes in the HeartMate II group at 24 months in the ROADMAP trial were reported at a rate of 9 events per 100 patient-years. Improvements in HeartMate II device outcomes were seen over time in different trials. QoL improved from baseline at 12 and 24 months after the HeartMate II implant in both the ROADMAP and HeartMate II DT trials using the KCCQ and EQ-5D. However, similar improvements were also seen in the control groups in these trials (MM and HeartMate XVE, respectively).
HeartMate XVE
The pulsatile-flow HeartMate XVE was the first LVAD developed in the series of HeartMate devices. It was phased out of clinical use in 2010 in favour of the continuous flow HeartMate II. Outcome data for the HeartMate XVE device can be seen in Appendix 5.
Indirect comparison of HeartMate 3 and medical management – survival
This section discusses the results of the NMA carried out to indirectly compare HM3 and MM for survival. Direct comparison data were taken from several of the RCTs described above (MOMENTUM, HeartMate II DT and REMATCH). The network diagram Figure 12 illustrates the comparisons available to enable the NMA.
FIGURE 12.
Network diagram.

Assessment of the transitivity assumption
Baseline patient characteristics in the three RCTs included in the NMA are shown in Table 9. While most patient and treatment characteristics appeared similar, it shows that IV inotropic drugs (and INTERMACs 1–3) were required by 68% in REMATCH, compared to 87% in MOMENTUM with HeartMate II in the middle at 79%. If the baseline INTERMACS level is an effect modifier for any of the comparisons made in the network, then this would break the transitivity assumption and introduce bias.
| Study ID (no. publications) | Study design | No. participants (no. DT) | Intervention | Comparator | Mean age (SD) | Sex (n, % male) | INTERMACS Profile (n, %) | Length of follow-up (months) | Notes |
|---|---|---|---|---|---|---|---|---|---|
| REMATCH 200154 (10) |
RCT | 129 (All DT) | HeartMate XVE LVAD (n = 68) | Medical management (n = 61) | HeartMate 3 66 ± 9.1 Medical management 68 ± 8.2 |
HeartMate 53 (78) Medical management 50 (82) |
NR | 24 months (1 patient in LVAD group still alive at 30 months) | |
| HMII DT 200955 (10) |
RCT | 200 (All DT) | HMII LVAD (n = 134) | HeartMate XVE LVAD (n = 66) | HMII 62 ± 12 HeartMate XVE 63 ± 12 |
HMII 108 (81) HeartMate XVE 61 (92) |
NR | 24 months | Seven papers are retrospective analyses of both the HMII DT and BTT trials and include HMII single arm only |
| ENDURANCE DT 201756 (22, all ENDURANCE papers) |
RCT | 445 (All DT) | HeartWare HVAD (n = 297) | HMII LVAD (n = 148) | HeartWare 63.9 ± 11.6 HMII 66.2 ± 10.2 |
HeartWare 227 (76.4) HMII 122 (82.4) |
HeartWare 1: 10 (3.4); 2: 86 (29.0); 3: 120 (40.4); 4: 59 (19.9); 5–7: 22 (7.4). HMII 1: 5 (3.4); 2: 46 (31.1); 3: 60 (40.5); 4: 27 (18.2); 5–7: 10 (6.8) |
24 months | |
| ENDURANCE DT 2 201857 (22, all ENDURANCE papers) |
RCT | 465 (All DT) | HeartWare HVAD (n = 308) | HMII LVAD (n = 157) | HeartWare 63.3 ± 11.4 HMII 64.2 ± 11.1 |
HeartWare 252 (82) HMII 125 (80) |
HeartWare 1: 12 (3.9); 2: 101 (32.8); 3: 133 (43.4); 4–7: 62 (20) HMII 1: 4 (2.5); 2: 51 (32.5); 3: 68 (43.3); 4–7: 34 (21.7) |
12 months | |
| MOMENTUM 3 201958 (17) |
RCT | 1028 (624 DT) | HM3 LVAD (n = 317 DT) | HMII LVAD (n = 307 DT) | Reported for all DT patients only: 63 ± 12 | Reported for all DT patients only: 513 (82.2) | Reported for all DT patients only: 1: 12 (1.9); 2: 187 (30.0); 3: 325 (52.1); 4: 89 (14.3); 5–7: 7 (1.1); not provided: 4 (0.6) | 24 months | |
| ROADMAP 2015 (8) |
Multicentre, prospective observational | 200 (All DT) | HMII LVAD (n = 97) | MM (n = 103) | HMII median 64 (range: 55–70) MM median 66 (range 54–74) |
HMII 75 (77) MM 71 (69) |
HMII: 4: 63 (65); 5: 21 (22); 6: 10 (10); 7: 0 (0). MM: 4: 35 (34); 5: 29 (28); 6: 35 (34); 7: 2 (2) |
24 months |
Network meta-analysis results
The results of the NMA are shown in Table 10.
| Comparator 1 | Comparator 2 | Evidence type | RR | 95% CI |
|---|---|---|---|---|
| HM3 | HeartMate II | Direct58 | 0.88 | 0.64 to 1.22 |
| HM3 | HeartMate XVE | Indirect | 0.48 | 0.27 to 0.84 |
| HM3 | MM | Indirect | 0.25 | 0.13 to 0.47 |
| HeartMate II | HeartMate XVE | Direct56 | 0.54 | 0.34 to 0.86 |
| HeartMate II | MM | Indirect | 0.28 | 0.16 to 0.49 |
| HeartMate XVE | MM | Direct54 | 0.52 | 0.43 to 0.78 |
The direct evidence results reported by the three included trials were used to derive the indirect estimates using the methods described in Network meta-analysis. These data were used to produce a RR of death of 0.25 (95% CI 0.13 to 0.47) in the HM3 compared to MM at 24 months. This translates to a 75% reduction in the risk of death in patients with a HM3 compared to those on MM within 24 months. However, the results should be treated with caution due to the wide uncertainty levels placed around the RR and questions relating to the transitivity assumption as described above.
Data to carry out the NMA were only available for the survival outcome. There were insufficient data to allow for indirect comparisons of any other outcomes (QoL, major events, complications, etc.).
Concerning other comparative data, this was also considered when carrying out the NMA. The ROADMAP study was considered a non-randomised comparative intervention study. However, there were not sufficient relevant data to use in a NMA. No comparative observational studies compare MM to HVAD.
There were no other easily accessible data for direct comparative estimates from studies, even when considering observational studies.
This section reports the remaining non-HM3 specific data that are available from registry reports. It includes data from registries such as INTERMACS, IMACS and ITAMACS. The majority of registry data are reported for a group of LVADs (e.g. continuous flow devices) or do not differentiate by device type at all. There were 47 registry report articles; 37 from INTERMACS, 4 from IMACS, 2 from ITAMACS and the remaining reports were from ELEVATE and the older Thoratec DT registry. However, seven of the INTERMACS reports were only included for completeness, but their data were not included in the analysis due to the age of the data and the duplicate reporting of outcomes from similar reports over the same implant periods.
Survival
Survival was reported in 15 INTERMACS reports at various time points, as well as for other registries including IMACS (n = 3) and ITAMACS (n = 2). 31,38,46,59–74 Survival appeared to increase over the calendar time of the data set, as expected with the introduction of newer, more effective devices (Figure 13). This is also in line with data reported in the trials. Recent data from INTERMACS74 suggested survival was 80% in DT patients at 12 months in a cohort of patients implanted between 2014 and 2017, using either the HVAD or HeartMate II device. The longest participant follow-up time reported was at 60 months from the INTERMACS registry in a cohort of patients implanted between 2014 and 2018 (this included 14.8% HM3 patients). Survival here was reported to be 43% in DT patients. 46 Follow-up data beyond 24 months is not reported in any of the trials; therefore registry data and observational studies are currently the only sources for this longer-term data.
FIGURE 13.
Survival data of destination therapy patients from registry reports over time on any device at 12 months.

Quality of life
Quality of life was reported in a small number of registry reports. Kirklin 2012 (INTERMACS) reported QoL with the EQ-5D VAS at various points in a cohort of patients implanted with continuous flow devices between 2006 and 2011. 69 Scores improved from 44 at baseline to 72.2 at 12 months of follow-up. This is similar to scores reported in other trials. A more recent report from INTERMACS continuous flow patients (implanted 2008–13) reported improvements in the KCCQ overall score from 33.6 at baseline to 67.1 at 24 months. 75 No further QoL data have been reported.
Hospitalisations
Hospitalisations were not reported widely across registry reports. An INTERMACS analysis of US patients reported that rehospitalisation’s occurred in 75% of patients in transplant centres and 77% of patients in non-transplant centres at 12 months of follow-up (cohort implanted 2012–4). 62 Differential late re-admissions to hospital between shock (190 per 100 patient-years) and non-shock (181 per 100 patient-years) have been reported from the INERMACS registry in a more recent study. 74
Major events
Nine papers reported major events from registry reports (eight from INTERMACS, one from IMACS). 37,59,60,62,68,69,74,76,77 Acharya reported that strokes occurred in 12% of DT patients (implanted 2012–5) in 35 months of follow-up. 59 In the Brinkley report, 12% of transplant centre patients suffered a stroke (censored for death, transplant or explant) at 12 months, and 8.2% in non-transplant centre patients. 62 In both groups, 19% of patients had RHF at 12 months. In the earlier Kirklin analysis, there were 20.8 events of RHF per 100 patient-years in 24 months of follow-up. 69 Pump exchange was reported in 6% of patients at 12 months in Aleksova. 60
Complications
Seven articles reported complications including bleeding events, infections and device complications. 60,62,69,74,78–80 The Kirklin analysis reported 143 bleeding events per 100 patient-years at 24 months of follow-up. 69 There were also 13.8 device malfunction events and 97.1 infections per 100 patient-years. On the other hand, the more recent Michelis analysis reported 5.6 device malfunction events per 100 patient-years at 12 months as well as 8.7 DI events and 2.2 pump thrombosis events per 100 patient-years. 80 In the Brinkley report, 45% of transplant centre patients had bleeding events at 12 months, and 43% in the non-transplant centres. 62 It also reported device infections at 14% and 10% and device malfunctions at 16% and 14% in transplant and non-transplant centre patients, respectively, at 12 months.
Non-INTERMACS observational studies
Data from 21 observational studies thought to not overlap with patients in the INTERMACS registry are presented in this section.
Survival
Survival was reported in four of these studies but was not reported by individual device type. Adlbrecht (Germany) reported survival of 72% at 24 months follow-up in patients with continuous flow devices implanted between 1997 and 2012. 81 Medressova reported survival of 68.8% at 24 months (Kazakhstan) in patients implanted between 2011 and 2018 across multiple devices. 82 A French study (Galand) reported survival only by age in patients implanted between 2006 and 2016. 83 It reported that survival was higher in patients ≥ 70 (51%) compared to those < 70 (46%) at 24 months across multiple devices. A German study of patients implanted between 2010 and 2020 (Kalampokas), which also stratified by age, reported mortality of 55.6% and 32.5% at a mean 2.5-year follow-up in ages ≥ 70 and < 70, respectively. 76
Quality of life
No non-INTERMACS studies reported QoL as an outcome.
Hospitalisations
Only one study reported mean months out-of-hospital as 21.6 over a 24-month follow-up period. 81 This was only reported for all continuous flow devices.
Major events
Kalampokas reported the need for an intraoperative RVAD, with 11.1% of patients ≥ 70 and 26.5% patients < 70 years of age. 76 Results were not stratified by device. The study also reported the rate of periprocedural stroke incidence (though periprocedural is not defined) as 14.8% versus 10.5% in patients aged ≥ 70 and < 70, respectively. Two studies reported major events in the HVAD device. Consolo reported 10.5% patients suffered a stroke in 24 months and Janssen reported 19 haemorrhagic events in 156 patients. 53,84
Complications
Three non-INTERMACS observational studies reported various complications. 53,63,85 Akay reported that 17.4% patients had DIs in continuous flow devices. One study (Consolo) reported that 2.6% HVAD patients had a pump thrombosis, whereas HM3 patients (n = 15) had none in the 24-month follow-up period.
Interagency registry for mechanically assisted circulatory support observational studies
The final category of studies included were observational studies from centres that also contributed data to INTERMACS or other registries. There were 65 studies. These studies were not included in the full analysis to avoid double counting of patient data where possible Table 30 (see Appendix 6) summarises the characteristics of the studies. Data from this category were only considered if key data were unavailable from the previous study types. However, sufficient data were found in the trials, registries and observational studies not overlapping with registries.
Subgroup analysis
Some subgroups were considered important to explore to determine if any differences arose in outcomes in a particular group of patients, such as certain age groups or INTERMACS classes. However, there were no data specific to HM3 available for any relevant subgroups. Data from the HeartMate II device stratified by INTERMACS class were reported in the ROADMAP study (INTERMACS 4 vs. INTERMACS 5–7) but in no RCTs. Seven reports59,60,69,72,74,76,86 from registries reported some outcomes by age group including survival, major events and QoL and three reported by INTERMACS class. 63,69,74 One observational study reported survival by age group (see Appendix 6). 83
Subgroup comparisons within device type
Appendix 5 shows results from observational studies that show the comparative effects of different predictors on a range of outcomes such as mortality (usually) or stroke, or bleeding, etc. None of these studies reported results specific to HM3. Instead, they look at these predictors in participants who received any continuous flow device, any LVAD, or HeartMate XVE only. For the former two, study periods vary from 2006 to 2010 and 2013 to 2018, so it is likely that few participants would have received the HM3. It is unclear how well these reported results might generalise to contemporary implants of HM3.
One study reported HRs for the outcome of mortality by individual INTERMACS Class (1, 2, 3, 5, 6) versus Class 4. 69 This study included participants with any LVAD and included data from 2012 to 2014. We use these data to inform the subgroup analyses in the economic model.
Risk of bias and quality assessment
Trials
Risk of bias was varied across the trials and outcomes (Table 11). Three studies had low risk of bias for survival (ROADMAP, REMATCH and ENDURANCE DT). Most studies had some concerns or high risk-of-bias for QoL. A more detailed description of the risk of bias assessment is available (see Appendix 4).
| Study name | Outcome | Domain 1 (randomisation process) | Domain 2 (deviations from intended interventions) | Domain 3 (missing outcome data) | Domain 4 (measurement of the outcome) | Domain 5 (selection of reported result) | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| ROADMAP (2015) | Survival | N/A | Low | Low | Low | Low | Low |
| QoL | N/A | Low | High | Some concerns | Low | High | |
| REMATCH (2001) | Survival | Some concerns | Low | Low | Low | Low | Low |
| QoL | Some concerns | Low | High | Low | Some concerns | High | |
| MOMENTUM 3 (2019) | Survival | Low | Some concerns | Low | Low | Low | Some concerns |
| QoL | Low | Some concerns | Low | Some concerns | Low | Some concerns | |
| HeartMate II DT (2009) | Survival | Some concerns | High | Low | Low | Some concerns | High |
| QoL | Some concerns | High | High | Some concerns | Some concerns | High | |
| ENDURANCE DT (2017) | Survival | Low | Low | Low | Low | Low | Low |
| QoL | Low | Some concerns | High | Some concerns | Low | High | |
| ENDURANCE DT 2 (2018) | Survivala | Low | High | Low | Low | Some concerns | High |
| QoL | Low | Low | Low | Some concerns | High | High |
For the randomisation process, three studies were low risk for both outcomes, though the REMATCH and HeartMate II DT trial had some concerns. While the randomisation domain was not applicable to ROADMAP, it should be acknowledged that this study was non-randomised and therefore prone to more biases.
Regarding domain two, blinding was generally an issue throughout the studies, as this could not be achieved, though it was unlikely to affect survival. Also, a per-protocol analysis was used in some studies (e.g. HeartMate II DT and ENDURANCE DT), which was not always the most appropriate method. Missing outcome data were an issue, particularly when measuring QoL (four studies had high risk of bias) and there were often many participants not completing this at follow-ups. Generally, the reporting of results did not appear to be done selectively and five studies were either low risk or had some concerns. Most trials published a protocol and extensive appendices detailing pre-planned methods and analyses.
Issues were mainly with the QoL outcomes across studies, which due to its mostly self-report nature, resulted in some concerns or high risk of bias for the missing outcome data domain and deviations from the intended outcome domain. These issues lead to the overall risk of bias being high in ROADMAP, REMATCH, HeartMate II DT and both ENDURANCE studies for the QoL outcome. The overall risk of bias remained low for survival in ROADMAP, REMATCH and ENDURANCE DT, with the other studies still having some concerns. The randomisation domain was not applicable for ROADMAP.
Registries
Reports from the INTERMACS registry and other registries were not quality assessed in the same way the clinical trials were assessed. The validity and quality of the data were considered in relation to the methods of data collection, auditing and missing data. While data from registries could be considered as more reflective of real-world data, there are limitations.
Contributions to the INTERMACS registry are voluntary at US centres, though the majority of centres choose to input their data and the registry is now considered the gold standard for clinical outcomes registries. Only patients with FDA-approved devices are able to enrol in the registry. Centres who wish to participate must have at least one member of staff trained on all aspects of INTERMACS to allow for certification and training is offered for all staff who will be involved in entering data. Processes are in place to monitor and ensure the quality of the data including regular checks for compliance, completeness and accuracy by the data and clinical coordinating centre (University of Alabama, Birmingham). For example, improbable or impossible data combinations are flagged as errors to sites and any questionable data must be verified with the site. Further auditing to ensure the highest possible quality data is carried out via telephone calls or site visits where monitors review the accuracy of web-based data submissions and documents. An independent monitoring board also review the database and meet annually. 87,88
Regarding outcome data, outcomes and adverse events are defined by INTERMACS and generally used consistently across reports of INTERMACS data. However, different registry reports do report different outcomes (e.g. survival, survival as part of a composite outcome, etc.) and also exclude certain populations (e.g. those with missing QoL scores, those with early deaths), which could introduce selection bias.
Furthermore, there are potential issues with the loss to follow-up of patients. For approximately 9.6% of patients in the INTERMACS registry as a whole, follow-up data are not available due to a lack of informed consent from patients. This may affect outcome data, particularly survival. 89
One limitation of INTERMACS and other registries when compared to trials is the lack of randomisation to devices. Centres will often only use one type of LVAD, which could introduce bias (though current available device options are limited following the HeartWare HVAD withdrawal). Registries other than INTERMACS are mostly prone to the same biases and issues. IMACS is an amalgamation of various device registries (including INTERMACS, EUROMACS and ITAMACS), and therefore the validity of the data is largely based on the individual databases themselves. There is limited information available on registries such as ITAMACS and EUROMACS, so it is more difficult to ascertain the processes they have in place to maintain quality.
Discussion
Summary of findings
There is a large volume of evidence analysing and summarising the use of LVADs for DT in patients with end-stage HF, extending from RCT data to single-centre observational data. However, much of this evidence is now redundant due to devices no longer being used or having been withdrawn from the market, as well as very few comparative studies being carried out.
Findings from the NMA in this review demonstrate that LVADs are effective alternatives to transplant and may offer survival rates of nearly 77% at 24 months with the HM3 device, which is the only currently available device in the UK (based on evidence from MOMENTUM, HeartMate II DT and REMATCH). However, comparative study evidence for the HM3 is limited to one RCT, with no direct comparisons made to MM. Indirect comparisons to MM demonstrated a clear, significant benefit for HM3 when considering risk of mortality, though with a wider ranging CI and some concerns about transitivity. The SweVAD trial results and further device-specific data at longer follow-up times would increase the certainty of the findings. 48 Complications and events such as stroke and pump thrombosis may still remain an issue.
Strengths and weakness of the review
There were several strengths to this review, which include the large body of evidence included, with this review being the most comprehensive of its kind to date. This review has considered effectiveness for important subgroups including INTERMACS level and age. The review also considered the difficulties of overlapping patient populations (e.g. within registries and observational studies) and tried to address this by including data in a hierarchical approach. The trials were assessed for risk of bias and the limitations of registry data were considered in the context of the results. Furthermore, decisions on review methods were discussed extensively with clinical experts, as well as a wider steering group of independent clinicians, specialists and LVAD patients.
While every effort was taken to minimise risk of bias during the review process, some issues remain. Due to the large volume of evidence, a pragmatic approach was required to manage the review. This involved a hierarchical approach to analysis of the evidence, meaning that some studies were only considered in the analysis if they did not overlap with other patient populations. However, this also ensured there was no duplication of patient data. To further manage the large volume of evidence, studies with < 50 DT patients were excluded, which may have resulted in missed data. However, the impact of excluding these studies was assessed and found to be minimal (approximately 4.8% of patients) and should not cause bias. Finally, to limit the search hits to a manageable volume, terms for LVAD indication were included as requirements in the strategy (e.g. DT, BTT). This could potentially have resulted in missed studies, but it is likely that any important articles were found via citation checking of key publications and searches of INTERMACS publications via the Society of Thoracic Surgeons’ INTERMACS website.
Strengths and weaknesses of the evidence
One of the most significant limitations of the included evidence is the lack of direct comparisons between the HM3 and MM. No studies have been completed that compare the HM3 and MM, either randomised trials or observational studies. This means that any comparisons made between these two interventions had to be made indirectly, which requires assumptions (though the SweVAD study will address this in the near future). Much of the evidence identified is no longer relevant as devices have been withdrawn or have been superseded by newer versions.
Furthermore, there are currently no studies in DT patients in the UK due to the current guidelines for LVADs being for BTT only. Therefore, findings from studies carried out elsewhere may not be representative of the UK population.
However, there are also several strengths of the evidence that is included. The evidence is wide ranging in terms of study design, meaning that there is both clinical trial data and real-world data from comprehensive registries. This allows for consideration of the differences in these data. The registry data are extensive and INTERMACS is considered the gold standard for patient data registries in health care. However, reporting of HM3 specific data in DT patients is still limited outside of MOMENTUM.
Evidence in context
This review summarises the existing evidence on LVADs for DT. Several evidence reviews have previously explored the effectiveness of LVADs for DT from various countries. A Belgian health technology assessment (HTA) report reviewed evidence up to 2015 and included the REMATCH and ENDURANCE trials, as well as several INTERMACS reports. 90 They found that survival and QoL improved in LVAD patients, though complications (e.g. bleeding and stroke) were an issue. Another HTA report from Canada drew similar conclusions but reiterated the high costs of the devices and surgery. 91 However, these reports were not as inclusive as this review and were carried out before approval of the HM3 so do not reflect the current device availability.
There was another NMA which previously assessed LVADs for DT. 92 This NMA included HM3, HeartWare HVAD, HeartMate II, HeartMate XVE, and MM. It included four RCTs and four observational studies. The primary analysis included the trials only. There are a number of limitations identified in this NMA. It is unclear where the incidence rate ratios (IRRs) come from, for example for the outcome of death for MOMENTUM the IRR per 100 person-years was 0.61 in the paper, whereas the HR in MOMENTUM for death was 0.87. Furthermore, the NMA appeared to only include one year of follow-up from each study.
Implications for stakeholders/future research
This systematic review has demonstrated that the HM3 LVAD is an effective alternative for patients who are not eligible for HT. The current evidence indicates a clear survival benefit in LVADs compared to MM based on early trials and this is maintained with the HM3 when indirectly compared to MM via NMA. The HM3 also shows reduced stroke and other complications and events when compared to other devices. This information may be important for stakeholders in the UK who may consider recommending LVADs and, more specifically, the HM3 for DT in the future.
However, there are still missing data that may be key in determining the true effectiveness of LVADs when compared to MM. The lack of comparative studies, whether randomised or observational, directly comparing the HM3 with MM reduces the certainty of the effect, but publication of the SweVAD trial results could be significant evidence for future decision-makers. Furthermore, the cost-effectiveness of this device will be an important consideration for stakeholders, and this will be explored in the coming chapters.
Finally, research and development of LVADs continues. The new centrifugal flow EVAHEART 2 device has a pump and impeller design, which allows for gentle blood circulation while retaining pulsatility and is currently being trialled in the USA. The FDA-approved RCT is currently comparing the EVAHEART 2 device with the HM3 and aims to enrol approximately 400 patients. 47 This could lead to the possibility of an alternative to the HM3 device for both BTT and DT patients in the future.
Chapter summary
Left ventricular assist devices, and specifically the HM3, are effective treatments for end-stage HF in patients who are ineligible for HT. The HM3 demonstrates survival of 77% at 2 years, an improvement over other devices as well as MM (based on indirect comparisons only), though some adverse events are still common. Direct evidence on the HM3 versus MM from the SweVAD trial should help to cement findings, though the cost of the device, surgery and long-term patient care should be considered when determining if the device should be recommended for DT in the UK.
Chapter 4 Systematic review of economic analyses of left ventricular assist devices as destination therapy
Introduction
There has been wide-ranging debate about the cost-effectiveness of LVAD therapy for patients with AHF both in the UK and globally. 93 In particular, there is a lack of robust evidence around the cost-effectiveness of LVADs compared to MM for patients with AHF ineligible for heart transplantation (i.e. LVAD intended as DT). In the UK, a recent NHS Specialised Commissioning consultation highlighted this lack of economic evidence, and this is a key reason why NHS England has not recommended a LVAD for DT. 89 The outcomes associated with a LVAD as DT for patients with AHF thus need to be carefully considered against the resources required, and any additional costs must be evaluated in terms of any additional benefits that can be attributed to them. 94 In this chapter, a review of the existing Health Economics Literature on the use of a LVAD as DT for patients with AHF is presented. The aim of the systematic review is to identify existing economic evidence concerning a LVAD for DT in patients with AHF who are ineligible for heart transplantation and to evaluate the methodological quality of such studies. In addition, if the findings of the systematic review indicated that a de novo model-based analysis was required, the results would also be used to inform the development of the model and the associated parameters.
Methods
A review was conducted according to the guidelines of the UK’s Centre for Review and Dissemination (CRD) and reported following the PRISMA guidelines. 40,95 The review was registered on PROSPERO, the international prospective register of systematic reviews (CRD42020158987). 41 The search strategy was formulated using the population, intervention, comparator and outcomes (PICO) framework. 96
Inclusion and exclusion criteria
Papers were included if they met the following criteria:
-
Participants – AHF patients (age > 16 years) who are ineligible for HT and are receiving a LVAD intended as DT.
-
Intervention – Any LVAD irrespective of type, mechanism or generation. Studies of participants with biventricular assist devices, or RVADs were not eligible for inclusion.
-
Comparators – MM or different generation or type of devices or no comparator.
-
Outcomes – QoL, cost or incremental cost-effectiveness ratios (ICERs).
To capture as many studies as possible, no restrictions were placed on study design, year of publication or language. Any formal economic evaluations or studies of effectiveness with an assessment of costs or QoL studies were included. Conference proceedings published in the last 3 years (from the date of the searches) were included. The economic evaluations could take the form of a cost-consequence analysis, cost–benefit analysis, cost-effectiveness analysis (CEA) or cost–utility analysis (CUA). Conference proceedings, editorials, reviews or studies that reported on the use of technology for interventions unrelated to AHF were excluded.
Search strategy
A comprehensive search of six electronic databases was conducted from inception until 20 May 2020 in the first instance. Search updates were carried out on 19 May 2021 and 11 January 2022. The electronic databases included three general databases: Cochrane Library (CENTRAL), MEDLINE and EMBASE via Ovid; and three specialist economics databases: EconLit, CEA registry and the NHS Economic Evaluation Database (NHS EED).
Combinations of keywords with the Boolean logic terms ‘OR’ and ‘AND’ were used. Search terms were refined using the MESH library. Search terms for each search strategy are listed in Appendix 2.
The reference lists of key papers were hand-searched to identify additional papers.
Study screening and selection
Following the database search, removal of duplicates was facilitated with EndNote X9 and study selection was performed independently by two reviewers in two stages with the use of Covidence systematic review software. 42,43 Titles and abstracts were screened, and full texts obtained for studies that potentially met the inclusion criteria. The full texts were then checked against the inclusion criteria to assess their eligibility for inclusion in the review. All discrepancies were resolved by discussion between the two reviewers or by a third reviewer.
Quality assessment
Quality assessment of the economic evaluations was conducted with appropriate tools. For trial-based studies, the Consensus on Health Economic Criteria (CHEC) tool was applied; this tool is widely used for the appraisal of economic evaluations. 97 For model-based studies, the Philips Checklist was used. 98 The Philips Checklist is specifically designed for the assessment of modelling studies and is recommended by both the National Institute for Health and Care Excellence (NICE) and the Cochrane Collaboration. 99
Data extraction
The selected studies were read carefully to identify data important to the systematic review. A data extraction template was developed based on the study objectives and subsequent planned analysis. Data extraction was performed independently by two reviewers (TA and CO) using a standardised form. Information was extracted from each paper on the study background and the condition that was studied.
Given the review’s objectives, information on the types of models used, and especially the range of health states used in the models were extracted. Model inputs such as resource use and QoL measures were also sought.
A narrative synthesis was undertaken as is recommended when the methodologies of the included studies are heterogeneous (Centre for Reviews and Dissemination 2008). 95 The quality appraisal was undertaken to inform the analysis rather than to exclude studies. As part of the analysis, approaches to economic evaluation, model structures, time horizons, cycle lengths and parameter inputs were compared and contrasted. This included an assessment of assumptions and the validity of model inputs and the sources of the costs and utility values.
Results
Selection
There were 19 studies from 20 articles that were relevant and included in the cost-effectiveness review. Three of these studies were identified by hand searching. The identified studies included 5 cost analyses and 14 economic evaluations. The PRISMA diagram is provided in Chapter 1, Figure 4.
Included studies
The main characteristics of the included studies are summarised in Table 12. The majority of the studies were conducted in the USA (n = 9) and some in the UK (n = 4). Most of the studies aimed to compare the health and cost outcomes of LVADs with MM, except one study that used HT as the comparator. 100 The study population was patients who received LVADs intended as DT because of ineligibility for a HT.
| # | Author and year | Setting | Intervention/comparator | Study design | Perspective | Main data source | Time horizon | Currency, price year, discount rate | Main findings | Sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Adang 2006104 | NT | LVAD (not specified)/MM | CUA – model | Payer | 52 patients from University Medical Centre Utrecht | 3 years | €, 2006, 3% | Incremental cost per QALY: €112,000 |
Deterministic and PSA |
| 2 | Baras 2017101 | USA | LVAD (not specified)/MM | CUA – model | Third partya | 220 patients from Medicare database | Lifetime | US$, 2016, 3% | Incremental cost per QALY: $209,400 (low risk) 171,000 (high risk) |
|
| 3 | CETZ 2000105 | CAN | LVAD (not specified)/MM | CEA | Payer | Literature based; 1993 and 1995 | 12 years | CAN$, 1999, 5% | Incremental cost per LY: $67,883 |
Deterministic |
| 4 | Chew 2017106 | USA | HeartMate II/MM | CUA – model | Payer | Literature based | Lifetime | US$, 2015, 1.5% | Incremental cost per QALY: $230,692 |
Deterministic and PSA |
| 5 | Chimanji 2016107 | USA | LVAD (not specified)/MM | CA | Payer | 121 patients from Ohio State University hospital | 1 year | US$, 2014, N/A | Cost of LVAD pp: $314,851 Cost of MM pp: $299,000 |
Not conducted |
| 6 | Clegg 2007108 | UK | HeartMate/MM | CUA | Payer | Literature based and internal data from NHS trust for costs | 5 years | £, UN, costs 6%, QALYs 1.5% |
Incremental cost per QALY: £170,616 |
Deterministic |
| 7 | Droogne 2014100 | BG | HeartMate II/HT | CEA | Payer | 6 DT patients (BENEMACS) | 1 year | €, 2010, N/A | Incremental cost per LY: €156,100 |
Not conducted |
| 8 | Girling 2007109 | UK | HeartMate/MM | CUA | Payer | Literature based and used cost data from Clegg 2007 | 2 years | £, UN, 3.5% | Incremental cost per QALY: £76,766 |
Deterministic |
| 9 | Health Qual. On. 201691 | CAN | LVAD (not specified)/MM | CA | Payer | Literature based, 47 BTT patients and expert opinion | 1 year | CAN$, 2015, N/A | Cost of LVAD pp: $185,400 Cost of MM pp: $32,250 |
Deterministic |
| 10 | Lim 2021110 | UK | HM3/MM | CUA – model | Payer | MOMENTUM 3, REMATCH and ROADMAP trial data | 5 years | £, UN, 3.5% | Incremental cost per QALY: £47,361 |
Deterministic and PSA |
| 11 | Long 2014111 | USA | All on INTERMACS registry by 2013/MM | CUA – model | Payer | Literature-based | Lifetime | US$, 2012, 3% | Incremental cost per QALY: $201,600 |
Deterministic |
| 12 | Mehra 2018112 | USA | HM3/HeartMate II | CA | Payer | 361 patients in MOMENTUM 3 trial | 2 years | US$, 2017, 0% | Re-admission costs for HeartMate III DT: $39,773, HeartMate II DT: UN | Deterministic |
| 13 | Messori 2009103 | IT | HeartMate/MM | CUA | Not stated | 68 DT patients | Lifetime | €, 2004, 1% | Incremental cost per QALY: €66,683 |
Deterministic |
| 14 | Neyt 2013102 | NT | HeartMate II/MM | CUA – model | Societal (payer + transportation costs) | REMATCH and HeartMate II DT, 69 BTT patients from University Medical Centre Utrecht | Lifetime | €, UN, 4% and 1.5% |
Incremental cost per QALY: €107,600 |
Deterministic and PSA |
| 15 | Oz 2003113 | USA | HeartMate II/MM | CA | Payer | 52 DT patients from REMATCH | 1 year | US$, UN, N/A | Initial cost of LVAD pp: $210,187 Re-admission costs pp: $105,326 |
Not conducted |
| 16 | Rogers 2012114 | USA | HeartMate II/MM | CUA – model | Payer | 83 DT patients | 5 years | US$, 2009, 3% | Incremental cost per QALY: $198,184 |
Deterministic |
| 17 | Silvestry 2019115 | USA | HeartWare/MM | CUA – model | Payer | Medicare – number not provided | 10 years | US$, 2017, 3% | Incremental cost per QALY: $102,587 |
Deterministic and PSA |
| 18 | Schueler 2021116 | UK | HeartWare/MM | CUA – model | Payer | ENDURANCE Sup. Trial and SHFM | Lifetime | £, 2019, 3.5% | Incremental cost per QALY: £46,207 |
Deterministic and PSA |
| 19 | Slaughter 2011117 | USA | HeartMate II/MM | CA | Payer | 83 DT patients | At implantation | US$, 2009, N/A | Cost of LVAD pp: $193,812 | Not conducted |
Most economic evaluations (n = 12) used a CUA approach and only two conducted a CEA. Markov-based modelling was applied in eight studies. The perspective was stated as the service provider in most studies, just two evaluations reported adopting a societal perspective and in one study the perspective was not explicitly stated. 101–103 The cost data were generally based on studies with small sample sizes. Comparability across studies was very low due to the substantial differences in methodology. The findings and limitations of the studies are discussed below.
Health outcomes
The studies considered outcomes such as survival probability, initial hospitalisation and the probability of hospitalisation per year after the initial discharge. Economic evaluations conducting a CUA (n = 12) estimated incremental quality-adjusted life-years (QALYs) as well as survival and hospitalisations. Table 13 provides the survival and hospitalisation rates and health utilities used in the included studies.
| # | Study | 1-year survival (LVAD/comparator) |
Hospitalisation per patient per year (LVAD/comparator) |
Health utilities (LVAD/comparator) |
|---|---|---|---|---|
| 1 | Baras 2017 | 0.83/0.84 (low risk) 0.83/0.73 (high risk) |
2.00/0.84 2.00/1.80 |
0.70/0.40 |
| 2 | Chimanji 2016 | 0.88/0.78a | Not estimated | N/A |
| 3 | Chew 2017 | 0.58/0.16 | 2.64/3.15 | 0.85/0.53 depending on NYHA classesb |
| 4 | Clegg 2007 | 0.52/0.25 | Not provided | 0.56/0.40 |
| 5 | Droogne 2014 | 0.83/0.84a | Not provided | N/A |
| 6 | Girling 2007 | 0.50/0.25 | Not provided | 0.81/0.55 |
| 7 | Health Qual. On. 2016 |
0.81/N/A | 2.64/N/A | N/A |
| 8 | Lim 2021 | 0.85/0.25 | N/A | 0.55/0.74 |
| 9 | Long 2014 | 0.77/0.26 | Not provided | 0.52 (first month) and 0.72/0.53 |
| 10 | Messori 2009 | 0.52/0.28 | Not provided | 0.81/0.55 |
| 11 | Neyt 2013 | 0.68/0.28 | 2.64/3.15 | 0.81/0.55 |
| 12 | Rogers 2012 | 0.70/0.30 | 2.52/1.59 | 0.85/0.53 depending on NYHA classes |
| 13 | Silvestry 2019 | 0.76/0.67 | N/A/0.3 | 0.80/0.64 |
| 14 | Schueler 2021 |
0.79/0.36 | N/A | 0.72/0.54 |
| 15 | Slaughter 2011 | 0.51/0.25 | Not provided | N/A |
The economic evaluations mostly used clinical data from HeartMate II DT and REMATCH trials or the INTERMACS registry. However, the utilisation of data differed substantially. Only four studies that conducted model-based CUAs explicitly stated how hospitalisations after the LVAD implantation were incorporated.
Generally, very few studies included utility values that were specific to DT patients rather than BTT patients. Only three studies applied utility values that were specific to DT patients. 110,115,116 Schueler et al. converted utility values derived from a US population to UK values, using the Dolan algorithm. 116 One study used the utilities reported by Moskowitz et al., which were estimated amongst 29 bridged patients. 118 Long et al. 111 applied the values for BTT patients by Sharples et al. 102 for the first month. 119 The other utility sources used in the economic evaluations did not distinguish between DT and BTT patients. Patients’ monthly NYHA classifications were used in three studies to estimate health utilities. 106,114
Costs
Most studies reported direct medical costs, which included the device cost, initial hospitalisation, re-admissions and outpatient visits. Seven studies also considered the cost of a LVAD replacement in the case of a device failure. 101,106,110,111,114–116 In addition, the analysis by Neyt et al. 102 considered the travel and ‘social work’ costs, although no definition was provided for ‘social work’. One study reported adopting a societal perspective but only the direct costs were incorporated into the analysis. 101
The cost estimates were usually based on a small number of patients from a single centre, and this caused variability. Most studies used standard discounting rates based on setting. Four studies used cost data for BTT patients since the LVAD was not part of the standard care for DT patients in the Netherlands and the UK. 102,109,111,116,120
There were significant variations in the cost estimates, depending on the setting, device and methodology of the studies (e.g. included costs, time horizon). The largest cost component was the initial cost of the LVAD implantation, and in 2019 prices it varied from £83,567 in Belgium and £91,162 in the UK to £220,176 in the USA (Figure 14). 100,113,116 It is important to note that the device and implantation costs might have included different components, considering the differences in healthcare delivery across countries. The follow-up cost estimates ranged from £33,873 for 2 years to £402,309 over a lifetime. 101,112 The estimated cost of MM varied from £6009 for 1 year to £264,271 over 6 years. 101
FIGURE 14.
Index cost of LVAD implantation in 2019 prices.
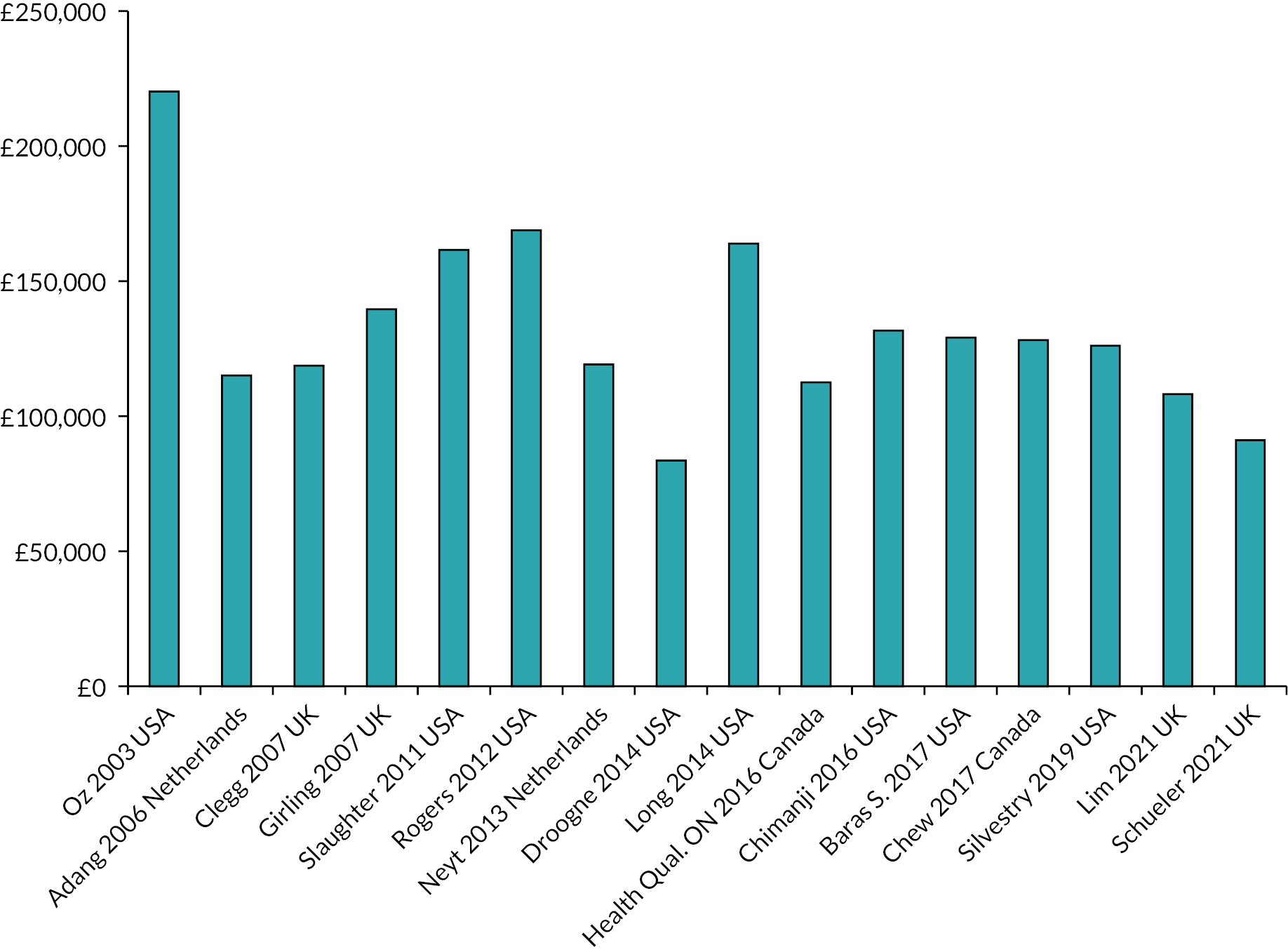
The cost-effectiveness of a left ventricular assist device as destination therapy
The review identified 14 studies that estimated the cost-effectiveness of a LVAD as DT. The majority of the economic evaluations concluded that LVADs were not cost-effective compared to MM (n = 8) or HT (n = 1) as DT, while three recent evaluations, including two from the UK, found favourable results. 110,115,116 The overall conclusion was not clear in two evaluations. 103,105
The main outcome was the incremental cost per QALY gained in 14 studies and the incremental cost per life-year gained in two studies. It is important to note that Neyt et al. and Clegg et al. applied different discount rates for costs (4% and 6%, respectively) and outcomes (1.5%), while all others used the same rate for costs and outcomes. 90,108
The cost-effectiveness was estimated over a time horizon of 5 years or longer in nine studies (Figure 15), and the incremental cost per QALY ranged between £46,207 and £238,401 in 2019 prices. 108,116 These studies were comparable because all had a payer’s perspective except one, which included transport costs. 90 Despite the differences in the cost estimates, there was a downward trend over time in the ICER per QALYs reported. Baras Shreibati et al. calculated the incremental cost per QALY to be £152,953 or £124,904, based on two different mortality risk estimates. 101 Only one study considered the impact of severity on the cost-effectiveness, based on the INTERMACS profiles. 110 In a subgroup analysis, it was found that the ICER per QALY was lower for the patients with an INTERMACS profile 1–3 (£45,616) and higher for those with an INTERMACS profile 4–7 (£65,018), compared to the base-case estimate (£47,361).
FIGURE 15.
Incremental cost per QALY estimates in economic evaluations with a time horizon of 5 years or longer.

The incremental cost of a LVAD as DT per QALY was found higher than the standard thresholds in all evaluations except two studies, which estimated an ICER that was just below NICE’s threshold of £50,000 per QALY for end-of-life treatments. Both were recently conducted studies from the UK health service perspective, with one based on the now withdrawn HeartWare HVAD device (£46,207), and the other on the HM3 device (£47,361). 110,116
Economic evaluations with Markov models
Model structure and parameters
The characteristics of the economic evaluations with Markov models are summarised in Table 12. All the models had a time horizon of 5 years or longer, except one with a 3-year horizon. 114 All studies applied monthly cycles while Lim et al. 110 and Chew et al. 106 used three-monthly cycles.
Only two studies used health states other than being ‘dead’ or ‘alive’. 102,111 Only Baras Shreibati et al. 101 considered the probability of becoming eligible for a HT after receiving a LVAD, but the probability was assumed to be the same for the LVAD and MM patients. Baras Shreibati et al. incorporated the increased mortality risk following a major stroke and Long et al. 111 modelled some of the complications as separate health states and applied specific mortality probabilities and health utilities for patients in these states.
Overall, the mortality risks for the first 2 years were obtained from the published literature, and extrapolated risks were used beyond that. One of the UK-based models used the Seattle Heart Failure Model (SHFM) to estimate the mortality risks. 116 This model estimates the survival rates at 1 and 2 years after the implantation. The second UK study extrapolated the values beyond 2 years, averaging the data available for the latest 6 months (Table 14). 110
| Author and year | Setting | Time horizon | Health states | Clinical inputs | Included costs | Price year, discount rate | QALY gains LVAD and MM | Costa of LVAD and MM | ICER/QALYa |
|---|---|---|---|---|---|---|---|---|---|
| Adang 2006104 | NT | 3 years | Alive, dead | Survival, hospitalisation, QALEs | Hospitalisation, re-admission and outpatient costs | 2006, 3% |
1.34 (QALE) 0.27 (QALE) |
£135,875 £21,200 |
£107,929 |
| Baras 2017101 | USA | 6 years | Alive, dead |
Survival, stroke, pump replacement, hospitalisation | Hospitalisation, re-admission and outpatient costs | 2016, 3% |
4.41 2.67 (low-risk MM) 1.63 (high-risk MM) |
£530,442 £183,120 (low-risk MM) £264,271 (high-risk MM) |
£124,904 (low risk) £152,953 (high-risk) |
| Chew 2017106 | CAN | Lifetime | Alive, dead |
Survival, hospitalisation, device failure, QALYs |
Hospitalisation, re-admission and outpatient costs | 2015, 1.5% |
1.48 0.39 |
£172,610 £19,420 |
£140,068 |
| Lim 2021110 | UK | 5 years | Alive, dead |
Survival, stroke, GIB, DI, pump failure, QALYs |
Hospitalisation, re-admission and outpatient costs | UN, 3.5% |
2.83 0.43 |
£141,598 £28,047 |
£47,361 |
| Long 2014111 | USA | Lifetime | Stroke, GIB, DI, pump failure, dead |
Survival, stroke, GIB, DI, pump failure, QALYs |
Hospitalisation, re-admission, outpatient and end-of-life care costs |
2013, 3% |
2.79 0.41 |
£406,497 £77,187 |
£138,195 |
| Neyt 2013102 | NT | Lifetime | No event, hospitalisation, death |
Survival, hospitalisation QALYs | Hospitalisation, re-admission and outpatient costs, travel costs | UN, 4% and 1.5% |
Incremental QALY gain: 2.83 | Incremental cost of LVAD: £281,602 | £101,305 |
| Rogers 2012114 | USA | 5 years | Alive, dead |
Survival, hospitalisation QALYs | Hospitalisation, re-admission and outpatient costs | 2009, 3% |
1.87 0.37 |
£300,370 £52,385 |
£165,170 |
| Silvestry 2019115 | USA | 10 years | Alive, dead | Survival, stroke, GIB, DI, pump failure, RHF, QALYs |
Costs of adverse events, re-admission for MM patients, outpatient costs | 2017, 3% |
3.83 0.80 |
£344,662 £79,847 |
£87,370 |
| Schueler 2020116 | UK | Lifetime | Alive, dead | Survival, stroke, GIB, DI, PE, RHF, other adverse events, QALYs | Hospitalisation, costs of adverse events for LVAD patients, inpatient and outpatient costs for MM patients | 2019, 3.5% |
3.42 0.54 |
£204,222 £77,790 |
£46,207 |
Six studies considered one or more adverse events (Table 15). However, the probability of stroke in the control group was considered only in two studies. 110,115 The impact of major events on mortality was incorporated only in one model for stroke, GIB and DI (Long et al.). All of the models assumed that after one cycle, which was 1 month in all except two that used three-monthly cycles, the impacts of the complications on morbidity and QoL were completely reversed. 106,110 However, in the model by Silvestry et al.,115 BTT patients experiencing a severe stroke [modified Rankin Scale (mRS) ≥ 4] became ineligible for transplant, although the probability of experiencing a severe stroke was not provided.
| Economic evaluation | Major events | Monthly event rate | Source |
|---|---|---|---|
| Lim et al. 2022110 (3-monthly values) | Stroke | 0.006 (1–3 months) 0.008 (4–6 months) 0.012 (7–9 months) 0.014 (10–12 months) |
MOMENTUM 3 |
| PE | 0.004 | Same as above | |
| DI | 0.023 (1–3 months) 0.030 (4–6 months) 0.031 (7–12 months) |
Same as above | |
| GIB | 0.138 (1–3 months) 0.041 (4–6 months) 0.031 (7–9 months) 0.025 (10–12 months) |
Same as above | |
| RHF hospitalisation | 0.046 (1–3 months) 0.027 (4–6 months) 0.034 (7–9 months) 0.009 (10–12 months) |
Same as above | |
| RVAD | 0.05 of all patients | Same as above | |
| Sepsis | 0.107 (1–3 months) 0.008 (4–6 months) 0.007 (7–9 months) |
Same as above | |
| Schueler 2021116 | Stroke | 0.014 (ischaemic) 0.005 (haemorrhagic) |
ENDURANCE Supplemental data, Medtronic internal data |
| PE | 0.006 | Same as above | |
| DI | 0.020 | Same as above | |
| GIB | 0.048 | Same as above | |
| RHF | 0.025 | Same as above | |
| Silvestry 2019115 | Stroke | 0.014 (ischaemic) 0.005 (haemorrhagic) |
ENDURANCE Supplemental data, Medtronic internal data |
| VAD thrombus | 0.005 | Same as above | |
| Device failure | 0.001 | Same as above | |
| DI | 0.020 | Same as above | |
| GIB | 0.048 | Same as above | |
| RHF | 0.025 | Same as above | |
| RVAD | 7% of the RHF population | Same as above | |
| Other AEs | 0.038 | Same as above | |
| Stroke MM | 0.002 | Baras 2017 | |
| Re-admission MM (apart from stroke) | 0.300 | Baras 2017 | |
| Baras 2017101 | Stroke LVAD | 0.008 | ROADMAP study, SCD-HeFT trial |
| LVAD pump replacement | 0.004 | ENDURANCE trial, INTERMACs | |
| HT received annual (LVAD and MM) | 2.4% | ROADMAP | |
| Stroke MM | 0.002 | ROADMAP study, SCD-HeFT trial | |
| Death after HT | 0.05 (1 month) 0.0135 (2–12 months) 0.0087 (13+ months) |
Healy 2016 Lund 2015 |
|
| Death after stroke | 0.4 | Holloway 2005 | |
| Chew 2017106 | Device failure | 0.005 | Cook 2014 |
| Long 2014111 | Stroke (first 12 months) | 0.004 | INTERMACS |
| Device failure (first 12 months) | 0.004 | INTERMACS | |
| DI (first 12 months) | 0.019 | Aggarwal 2012 | |
| GIB (first 12 months) | 0.011 | INTERMACS | |
| Death due to stroke | 0.40 | Not provided | |
| Death due to DI | 0.23 | Not provided |
Main findings of the modelling studies
All CUAs reported greater QALY gains for LVAD patients and significantly higher costs. The expected QALYs per patient on MM ranged from 0.27 over 3 years and 0.80 over 6 years and it was between 1.34 over 3 years and 4.41 over 6 years for LVAD recipients. Similarly, there was a wide variation in the expected costs per MM and LVAD recipients. The estimated incremental cost per QALY ranged from £87,370 to £165,170 in the USA, £88,595 in the Netherlands and £46,207 in the UK. 102,114,115
Uncertainty
Uncertainty around the findings was assessed with deterministic and probabilistic sensitivity analyses (PSAs) in all modelling studies. An overview of the analyses revealed that it was difficult to identify a particular parameter as the main source of uncertainty because one-way sensitivity analyses showed that the cost-effectiveness estimates were sensitive to different parameters in each study. For example, one study found that estimates were most sensitive to re-admission rates and costs (Baras Shreibati et al.) and another found that estimates were most sensitive to LVAD implant cost and survival expectancy (Chew et al.). 101,106
Six studies conducted PSAs. 102,104,106,110,115,116 According to the cost-effectiveness acceptability analysis, the probability of cost-effectiveness at a $100,000 threshold per QALY was 0% in one study and 33% in two analyses in the USA, while it was 50% at a €100,000 threshold per QALY in the Netherlands. 101,102,106,115 This probability was 97% at a threshold of £50,000 in one UK-based study. 110
Risk of bias
The quality of the economic evaluations was assessed using the CHEC and Philips criteria and overall judged as poor to moderate (see Appendix 7). Some important issues were identified in terms of the quality of the studies. For example, none of them justified the sources of parameters except two, which used systematic reviews to identify the mortality risk and health utilities within the first 2 years. 110,116 Additionally, some studies did not provide all the parameters used in the estimates. For instance, two studies did not provide the estimated mortality risks beyond 2 years despite using extrapolated data. 106,110 Similarly, Silvestry et al. did not provide the number of patients in different stroke groups generated based on the mRS. 115 Furthermore, studies did not fully explain how the complications were incorporated, although many included the hospitalisation costs.
Some studies did not consider all important cost components, for example, some omitted hospitalisation costs or the cost of device replacement in the case of a device failure. Similarly, some relevant variables, such as improvement in QoL, were not subjected to sensitivity analysis in some studies. Only Rogers et al. explored the alternatives to the assumptions about the estimated mortality risks beyond the available data in a sensitivity analysis. 114 The studies used data from small numbers of patients from a single centre to identify the cost inputs. Moreover, some studies used data from BTT patients in the absence of cost estimates specific to DT patients. 111
Limitations of the existing economic evaluations
The existing economic evaluations had important limitations. Firstly, only four studies adopted a lifetime perspective. 101,106,111,116 Although the average life expectancy for LVAD DT patients is low, some patients may live beyond the limited time horizons adopted in the existing studies. In addition, it is important to consider the impact of patients’ characteristics such as gender, INTERMACS profile and age.
Another limitation was that the models generally only had only two health states (dead, alive), and only two studies included other health states. 102,111 Although two studies modelled some complications as separate health states, it was assumed that after a month, the impact of the complications would be reversed. Hence, the long-term impacts of the complications on DT patients (both LVAD and MM) have not been fully considered in existing economic evaluations.
A further consideration was that only one model considered the probability of DT patients becoming eligible for a HT. 102 However, in this study, the same probability of eligibility for HT was assumed for both LVAD and MM patients, but DT patients ineligible for a transplant would not be able to become BTT if they are receiving MM.
None of the studies considered the impact of INTERMACS profiles or age on mortality, morbidity or QoL except one (Lim et al. 110), which might have an important impact on the cost-effectiveness outcomes. 101
Discussion
Summary of findings
This review aimed to identify existing economic evidence concerning the use of LVADs in patients with AHF who are ineligible for a HT. The study identified 19 economic analyses focusing on the cost implications based on the screening criteria. Among these 19 studies, 14 were full economic evaluations, assessing both the health and cost impacts of LVADs. Most economic evaluations (n = 8) concluded that LVADs were not cost-effective compared to MM for patients with AHF who are ineligible for a HT. On the other hand, two UK-based evaluations reported favourable findings. The studies had some limitations, such as limited consideration of time horizon and the omission of some clinically significant adverse events.
Strengths and weakness of the review
The review was conducted based on a preregistered protocol (CRD42020158987) and the CRD and PRISMA guidelines were followed. The review was comprehensive as there was no restriction on the database search concerning dates or languages. Two independent reviewers conducted the study selection and quality assessment.
There are some limitations to be acknowledged. In line with the study objectives, the quality assessment focused on economic evaluations only. Thus, the quality of the cost analysis studies was not evaluated.
Strengths and weaknesses of the evidence
The cost-effectiveness outcomes were consistent in the published studies, given that the majority found that a LVAD was not a cost-effective alternative to medical therapy in patients with AHF who were not eligible for heart transplantation. The only two studies reporting favourable outcomes estimated ICER per QALYs to be just below the £50,000 threshold, defined as the end-of-life criteria in the UK.
However, the data inputs and methods used in the studies varied widely. For example, the index cost of LVAD implantation differed considerably from one study to another. It is difficult to explain this variation because studies from the same countries reported considerably different figures. In contrast, the variation in the ICER per QALY estimates was low within the same country in the studies published over the last 10 years. The studies from the European countries were more likely to report lower ICER per QALY estimates compared to the US-based studies.
The evidence on the cost-effectiveness of LVADs for patients with different INTERMACS profiles was not conclusive since only one study conducted an analysis based on the INTERMACS profiles, and the model inputs used in this study were not provided. 110
The economic evaluations had some significant limitations regarding the data used. For example, very few studies used DT-specific health utility data and the others relied on utility estimates for BTT patients. The impact of LVADs on health utilities might be different in DT patients compared to BTT patients. Similarly, the cost data were usually based on a small number of patients and there was limited consideration of the ongoing costs such as outpatient costs. In most studies, these were not addressed, for example, by conducting a sensitivity analysis. There were also some methodological limitations in the economic evaluations included in the review. For instance, only three studies adopted a lifetime perspective, estimating long-term health and cost impacts. Additionally, there was a lack of data on outpatient costs and palliative care, especially for the MM patients.
Additionally, most economic evaluations used clinical data based on HeartWare HVAD. However, HeartWare HVAD has recently been withdrawn by the producing company due to safety concerns. Thus, the findings of these studies should be interpreted with caution.
Evidence in context
Only two UK-based studies found LVADs a cost-effective treatment compared to MM in patients with AHF who are ineligible for a HT, both reporting ICER per QALY estimates just below the £50,000 threshold. 110,116 However, the evidence was not conclusive since these evaluations had some limitations. Firstly, one of these studies focussed on HeartWare HVAD, which has recently been withdrawn. 116 Secondly, these studies did not consider the impact of adverse events on life expectancy, utilising two health states in the economic models. Additionally, the cost inputs used in these two models varied considerably. For example, the cost of LVAD implantation was £91,162 in the study by Schueler et al. 116 and £108,223 in the study by Lim et al.;110 however, as these are two different devices, this could account for some of the cost differences. Additionally, the latter study estimated the additional cost of a LVAD compared to MM at £113,552. Thus, the key difference between the two treatment options was the device cost, estimating only a small cost for the cost of adverse events (£5329). However, the corresponding figure in the study by Scheuler et al. was £35,070.
Implications for stakeholders/future research
There were some limitations in the economic evaluations regarding their methodology and data inputs. Additionally, discrepancies were found between two recent UK-based models, which reported ICERs just below £50,000. Thus, the existing evidence is not sufficient to make commissioning decisions in the UK.
A new economic evaluation, which considers all the important adverse events with more recent data over a lifetime horizon, is needed. Considering the scarce data available regarding the ongoing costs of both MM and a LVAD, it would be valuable to identify the key parameters that have a significant impact on the ICER per QALY estimates and demonstrate how they influence the cost-effectiveness findings. This would guide future research.
Chapter summary
The evidence identified in this systematic review suggests that the estimates of the cost-effectiveness of a LVAD as DT has improved over time. This may be explained by the increased life expectancy associated with newer generation devices and a reduction in adverse events and device costs. However, the estimated incremental cost per QALY has tended to remain higher than the accepted thresholds in most studies.
The existing evaluations have important limitations, such as not considering a number of important complications that can occur after the LVAD implantation. In addition, none of the existing studies considered the impact of INTERMACS profiles and age at the time of implantation. In terms of setting, there were only two UK-based economic models, but one study evaluated a device that has since been withdrawn due to safety concerns. Hence, the findings of this review suggest that a novel and more comprehensive economic evaluation of using LVADs as DT in patients who are not eligible for a transplant is needed.
Chapter 5 Economic evaluation
Introduction
The previous chapter presented a review of published cost-effectiveness studies and highlighted a range of limitations in the existing evidence base relating to the cost-effectiveness of a LVAD as a DT for patients who are ineligible for a transplant. The review concluded that, in light of these limitations, it was necessary to build a new model.
This chapter presents the methods and results of the model-based economic evaluation, which was undertaken to determine the cost-effectiveness of a LVAD as DT for this patient group, compared to MM. Benefits of treatment for patients need to be balanced against the resources required to achieve this outcome, and additional costs must be assessed in terms of any additional benefits that can be attributed to them. 94 Initially, there is an explanation of the methods employed in this analysis, in terms of the model structure, input parameters and the analyses undertaken, followed by a presentation and discussion of the results. The evaluation and reporting were informed by a range of relevant guidance. 121,122
Methods
Model description
Economic modelling was required to estimate the long-term implications and to be able to incorporate all the key information collected in the different trials into an economic model. A Markov model was designed to evaluate the cost-effectiveness of a LVAD as DT for patients who are ineligible for a HT, compared to MM. A Markov model was appropriate for this analysis due to the chronic nature of the condition under consideration. 123
Figure 16 presents the simplified overall model structure. In the intervention group, HT ineligible patients received a LVAD, while the comparator group received MM (representing usual care for DT patients in the UK).
FIGURE 16.
Simplified model structure. a, Major event: stroke, GIB, DI, LVAD failure as relevant.

The cycle length for the model was 1 month, and at the end of the first cycle, patients could be either alive without any major event, alive with major events, or dead. The health states for patients receiving LVADs and MM are demonstrated in Figures 17 and 18, respectively.
FIGURE 17.
Health states for LVAD recipients.

FIGURE 18.
Health states for medical management patients.

A major event was defined as any health condition that substantially increases long-term mortality risk, and these were modelled as separate health states. The major events identified for patients receiving LVADs were stroke, RHF and AR. For the MM group, the major event included was stroke. In addition, complications with no or limited impact on long-term mortality were incorporated in the model for LVAD patients to estimate their QoL impacts and the costs. These conditions are GIB, DI, PI, PE and arrhythmia.
In the base case, it was assumed that patients would not be eligible for HT at all. This was to reflect the central aim of the analysis, which focused on patients who were ineligible for transplant. However, given that some patients may become eligible for transplant at a later date, a sensitivity analysis was conducted to explore this impact on the results. For the sensitivity analysis, two additional health states were added to the model structure, BTT and HT. These states are shown in Figure 17 for completeness.
Development of the model
A decision-analytic model was developed to synthesise the most appropriate evidence identified by the systematic reviews in Chapters 3 and 4. The model development was informed by expert opinion, one patient and public involvement (PPI) meeting, and three steering committee discussions, which included clinicians, commissioners and patient representatives. All of the probabilities and utility values used in the model are provided (see Appendix 8).
Mortality risk
Mortality rates published by the Office for National Statistics were used to obtain the age-standardised mortality risks. 124 The age at the time of the implant was assumed to be 65 years in the base case. The overall mortality risks for DT and MM patients were adjusted for the probability of death due to the major events, applying the below formula where ME stands for major events. 125
As the systematic review of the clinical literature found no trial comparing contemporary LVADs to MM directly, estimating the mortality risks in the LVAD and MM arms required some assumptions. Four different potential methods were identified:
-
Non-comparative, net weight estimates: The mortality risks reported for LVAD recipients in the MOMENTUM trial and MM patients in the REMATCH trial were utilised to obtain monthly probabilities in the model. 54,126 Thus, it was assumed that the profiles of patients in the MOMENTUM trial matched perfectly to the MM patients in the REMATCH trial, and the clinical effectiveness of standard care had not changed over the last 20 years.
-
Non-comparative, weighted estimates for MM: The mortality risks for LVAD recipients in the MOMENTUM trial were used. The mortality risk in the MM arm was obtained based on a weighted average, using data from the REMATCH and MEDAMACS trials and the proportions of INTERMACS 2 and 3 and INTERMACS 4 and 5 patients in the MOMENTUM trial, respectively (Rose, Mehra, Ambardekar). 54,126,127 Thus, it was assumed that the mortality risks reported in the REMATCH trial included MM patients who could be considered to have a similar disease progression to INTERMACS 2 and 3, and that the MEDAMACS data included MM patients who could be considered to have a similar disease progression to INTERMACS 4 and 5 patients.
-
Comparative estimates mapped to MM in REMATCH: The mortality risks for MM patients in the MM arm were used. The mortality risks for the LVAD patients were obtained based on the RR estimated in the NMA reported in Chapter 3 and the mortality risks for MM patients. Thus, it was assumed that the profiles of patients in the MOMENTUM trial matched perfectly to the MM patients in the REMATCH trial, and the other trials that were used in the NMA reported in Chapter 3. It was also assumed that the mortality risk estimates for the MM patients in the REMATCH trial were more reliable than the mortality risk estimates for the LVAD recipients in the MOMENTUM trial.
-
Comparative estimates mapped to the LVAD in the MOMENTUM trial: The mortality risks for LVAD patients in the MOMENTUM trial were utilised. The mortality risks for the MM patients were obtained based on 1/RR estimated in the NMA reported in Chapter 3 and the mortality risks for MM patients. Thus, it was assumed that the profiles of patients in the MOMENTUM trial matched perfectly to the MM patients in the REMATCH trial and the other trials that were used in the NMA. It was also assumed that the mortality risk estimates for the LVAD recipients in the MOMENTUM trial were more reliable than the mortality risk estimates for the MM patients in the REMATCH trial.
It was deemed important to explore the impacts of incorporating the estimates from these four options into the evaluations, since all included different assumptions. The estimated mortality risks based on the four options are provided (see Table 33 and Appendix 8).
In the absence of mortality data beyond 2 years, it was necessary to extrapolate the limited trial data to estimate cost-effectiveness for a longer time horizon. The model assumed that the mortality risk after the 24th month would be the same as the risk reported for the months between 13th and 24th.
Major events
The major events were modelled separately, and every major event was represented in a health state (Figure 19).
FIGURE 19.
Transition between major events.

Stroke
Stroke was incorporated as two different health states to take account of symptoms and disability using the widely used mRS to define non-disabling stroke as a score < 4, and disabling stroke for patients with a mRS score ≥ 4. 128 The model used age-specific probabilities for stroke. Patients experiencing a stroke could die or move to the states for non-disabling stroke or disabling stroke. Among those experiencing a stroke, it was assumed that 0.25 of them died, and the mortality risk was assumed to be reversed within a month for non-disabling stroke, while with disabling stroke the risk increased to 0.035/month permanently. 57,129
Right heart failure
Right heart failure is usually defined as early and late RHF because of the greater health and cost impacts of early RHF. 130 However, there is no consensus on the definition of early RHF in the literature, varying from 14 days to 300 days. 131,132 In this study, early RHF was defined as RHF occurring during the first month after LVAD implantation, because this was the most widely used definition. 133,134 Late RHF was defined as any RHF after the first month, which required hospitalisation.
Patients experiencing RHF within the first month could either die or move back to their previous states in the next cycle, because early RHF is usually a transient state. Patients experiencing late RHF could die or stay in the RHF state, due to the long-term impacts on mortality and QoL. Early RHF was assumed to cause a postoperative mortality risk (0.035), while late RHF would increase the mortality risk to the level expected for MM patients (0.021). The probability of receiving a RVAD was applied to the patients who experienced early RHF, while it was assumed that late RHF would not generate a need for a RVAD, in line with current UK guidelines.
Aortic regurgitation
Patients experiencing AR could die, have a stroke or stay in the AR state. Similar to RHF, a postoperative mortality risk (0.035) was applied for AR. For those who survived, an increased mortality risk (0.008) was assumed. 38
Experiencing more than one major event
In the case where patients experienced more than one major event, the major event with the greatest impact on mortality and QoL was utilised. For example, patients who previously experienced a stroke could go on to experience a disabling stroke, and after that they would stay in the disabling stroke state or die. Since the impacts of RHF and AR on mortality and QoL are greater than the impacts of non-disabling stroke, patients experiencing non-disabling stroke and AR or RHF moved back to the AR or RHF states in the next cycle, unless they died due to stroke. However, patients experiencing disabling-stroke and AR or RHF stayed in the disabling-stroke state because the health impacts of disabling stroke are likely to be greater than the impacts of AR and RHF. Similarly, patients experiencing RHF and AR stayed in the RHF state.
The transitions within the model are summarised in Table 16.
| Starting state | Jump to state | Complications |
|---|---|---|
| DT (LVAD) | Death Non-disabling stroke, disabling stroke, RHF, AR, DT (LVAD) |
GIB, DF, DI, PE, arrhythmia |
| Non-disabling stroke | Death Non-disabling stroke, disabling stroke, AR, RHF |
GIB, DF, DI, PE, arrhythmia |
| Disabling stroke | Death Disabling stroke |
GIB, DF, DI, PE, arrhythmia |
| RHF | Death Disabling stroke, RHF |
GIB, DF, DI, PE, arrhythmia |
| AR | Death RHF, disabling stroke, AR |
GIB, DF, DI, PE, arrhythmia |
| MM | Death Non-disabling stroke, disabling stroke |
Re-admission for any reason |
Complications
The complications incorporated into the model could be experienced by all patients receiving LVADs.
Health utilities
To estimate QALYs, baseline health utilities were first applied to all the patients in the model. The baseline utility value reported in the MOMENTUM trial was used for the MM patients in the model (0.51). Similarly, the utility values reported in the MOMENTUM trial were utilised in the LVAD arm (0.76 and 0.77, Mehra). 126
Utility decrements
Utility decrements were applied to those who experienced a major event or complication. It was assumed that major events would cause a permanent utility loss, while complications would result in a reduction in utility during the cycle in which the complication occurs.
Utility loss due to stroke
In a recent study, the health utilities (and utility losses) after stroke based on the mRS scores were estimated in Table 17. 135
| mRS score | Utilities | Utility losses |
|---|---|---|
| mRS 0 | 1 | 0 |
| mRS 1 | 0.91 | 0.09 |
| mRS 2 | 0.76 | 0.24 |
| mRS 3 | 0.65 | 0.35 |
| mRS 4 | 0.33 | 0.67 |
| mRS 5 & 6 | 0 | 1 |
Kirklin et al., based on INTERMACS, reported proportions of patients by mRS, 3 months after stroke as follows: mRS 0 & 1: 31/115 = 0.27 and mRS 2 & 3: 10/115 = 0.09. 129 Thus, the percentage within non-disabling stroke were: mRS 0 & 1: 31/41 = 0.76 and mRS 2 & 3: 10/41 = 0.24.
The average utility loss for non-disabling stroke was calculated as:
Utility loss due to disabling stroke
The patients who experienced a disabling stroke experienced a utility decrement of 0.67. 135
Costs
The costs and resource use associated with the intervention and comparator were estimated using a range of sources. To identify the one-off cost inputs, for example the costs associated with stroke, NHS reference costs were used for the operation costs while the number of hospital days in the intensive care unit (ICU) and in a cardiac ward were estimated through discussions with two practicing heart surgeons, working in the NHS. The ongoing cost inputs, for example outpatient costs for LVAD recipients, were identified from the systematic review (see Chapter 3). All the costs are presented in 2019 prices and the future costs and benefits were discounted at 3.5% as per NICE guidelines.
For LVAD recipients experiencing early RHF, the usual practice in the UK is to make some adjustments to the existing LVAD setting (in addition to the treatment with inotropes) or insertion of a temporary RVAD rather than implanting a RVAD into the right ventricular, and thus the cost of the device is expected to be much lower.
The cost inputs used in the model are provided (see Appendix 8 and Table 35).
Modelling assumptions
A range of assumptions were required for the analysis due to the limitations associated with the data available and for computational practicalities. The assumptions were as follows:
-
Any patient could experience only one major event within a given month. Patients experiencing a major event were assumed not to experience any of the complications within the same month.
-
Disabling stroke was assumed to comprise 4.6% of all stroke cases (mRS > 4), both in LVAD and MM recipients. 57,136
-
Patients who experienced AR or RHF could experience non-disabling stroke unlimited times. This is because those patients would transition back to the AR or RHF states in the following cycle, unless they died.
-
The probability of a disabling stroke amongst patients with a prior non-disabling stroke experience was estimated based on the study by Kirklin et al. who reported that 17% of those who experienced non-disabling stroke had a second stroke within 3 years, which was 0.002 monthly. 137 The probability of death amongst patients experiencing a second stroke was assumed to be the same as for the first stroke (0.25). Similarly, it was assumed that of the patients who survived a second stroke, 4.6% would have a mRS > 4, as in the first stroke.
-
Apart from the relationship between stroke and disabling stroke, previous experience of major events and complications was assumed not to have an impact on the probability and outcomes of a major event or a complication. This was to reflect the published evidence, for example Truby et al. found that experiencing AR did not have a significant impact on the risk of stroke, bleeding and arrhythmia, conditional on survival to 1 year. 38
-
For model simplicity, it was assumed that valve replacement did not have any impact on QoL or life expectancy.
-
It was assumed that 57% of arrhythmia cases would be ventricular and a higher QALY loss (0.06) was applied for these patients. 112,138
Population
The study population was a hypothetical cohort of 1000 patients with AHF deemed ineligible for heath transplant. The mean age was 65 years and 50% of the cohort was assumed to be female, based on the findings of the systematic review.
Intervention and comparator
The patients in the intervention group received LVADs, while those in the control group received usual care. Usual care was defined as MM, which consisted of treatment with inotropes to enhance cardiac contractility and ongoing monitoring.
Outcomes and analysis
The main outcomes were expressed in terms of incremental costs per life year (LY) and QALY gained. For this, the healthcare costs, LYs and QALYs per patient were calculated for the MM and LVAD arms. The incremental cost per QALY and LY was estimated as follows:
All of the analyses were conducted from the NHS/PSS perspective over a lifetime horizon and the outcomes at shorter time periods (i.e. 2 and 5 years) were also estimated. According to NICE guidelines, health technologies with a ICER per QALY between £20,000 and £30,000 are considered cost-effective in the UK. 122 Until recently, NICE allowed using a higher threshold (£50,000 per QALY) if the treatment is indicated for patients with a short life expectancy, normally < 24 months and that there is sufficient evidence to indicate that the treatment offers an extension to life, normally of at least an additional 3 months, compared to current NHS treatment. As the clinical evidence summarised in Chapter 3 indicates, providing LVADs as DT for AHF patients who are ineligible for a HT meets these criteria.
The recently updated guidelines suggest a severity weighting based on a QALY shortfall estimate. The absolute QALY shortfall is defined as the difference between the expected QALYs for a specific age and sex group in the general population and the expected QALYs for the patient population if not treated. The proportional QALY is estimated by dividing the absolute QALY shortfall by the remaining QALYs for the same age and sex group in the general population. The QALY Shortfall Calculator developed by Sheffield University was used to estimate the QALY shortfalls in this study. 139 Weights were then applied following NICE recommendations on specific weights, based on the estimated QALY shortfalls (Table 18).
| QALY weight | Proportional QALY shortfall | Absolute QALY shortfall |
|---|---|---|
| 1 | < 0.85 | < 12 |
| × 1.2 | 0.85–0.95 | 12–18 |
| × 1.7 | At least 0.95 | At least 18 |
Sensitivity analysis
A range of deterministic and probabilistic sensitivity analyses were conducted to explore the uncertainties around the model parameters. In the first sensitivity analysis, the impact of incorporating the probability of transitioning to BTT and then HT for LVAD patients on the model outcomes was estimated. Secondly, key parameters were varied and the impacts on the model outcomes were presented on tornado diagrams. Finally, PSAs were conducted to estimate the uncertainties around the model outcomes. The details of these analyses are provided below.
Transition to bridge to transplant and heart transplant
In order to explore the impact of LVAD patients becoming eligible for transplant, a sensitivity analysis was conducted to incorporate a small proportion of LVAD recipients moving to the BTT state (0.06/month), starting 6 months after the implant and until 3 years after the implant. 130 In the next cycle, some BTT patients (0.028/month) would have the probability of receiving a HT until 3 years after the implant. 137 BTT patients could experience a major event, have a HT, or die. BTT patients experiencing a major event became ineligible for a HT and moved back to the DT state or died. The model inputs used for this analysis are provided (see Table 35 and Appendix 8).
One-way sensitivity analysis
There was limited or conflicting evidence on some of the model parameters in the literature, such as the monthly ongoing costs for MM and LVAD patients. Different values were used for these parameters to estimate the impact on the model outcomes and to identify the parameters with the greatest impact. The model inputs used for this analysis are provided in Appendix 8, Table 37.
Probabilistic sensitivity analyses
A PSA involves running thousands of different versions of the model where parameters are varied randomly alongside the prespecified distributions around parameter values. 140 The distributions were defined based on the nature of the parameters, with beta distributions used for binomial data and gamma distributions for costs. 94 Additionally, the difference in sampling method was applied for the parameters, which had different probabilities at different time points. 141 This method takes the relationship between the probabilities at different time points into account, by estimating the mean and variance of the logit distribution for each parameter to obtain the probabilistic values. The independent random sampling method was utilised for the remaining probabilities that did not require such adjustment and for the cost inputs.
Additionally, a cost-effectiveness acceptability curve was produced, to allow estimation of the cost-effectiveness outcomes at different willingness to pay (WTPs) thresholds. 142
Value of Information analysis
Value of Information (VoI) analysis is an appropriate tool to determine whether a further trial is needed, and to calculate the optimal trial size. 143 The simplest measure is the expected value of perfect information (EVPI); this represents the net benefit, expressed in monetary terms, of making the decision after all uncertainty has been resolved rather than under the current conditions of uncertainty. To express the health benefit in monetary terms requires the WTPs per QALY to be specified, for example the £20,000–30,000 per QALY specified by NICE. 144 The population level EVPI reflects the number of individuals who will benefit from the decision.
Any study costing more than the EVPI can be ruled out, but a study costing less than the EVPI may still not be worthwhile, and further types of analysis of VoI are required. Expected value of perfect parameter information (EVPPI) gives the value of certainty for a subset of the parameters in a model.
To determine whether a new primary study is worthwhile requires an assessment of the expected value of sample information (EVSI). This considers the possible outcomes from a study. The expected net benefit of sampling (ENBS) is the EVSI less than the cost of the study. The optimal sample size is the one that maximises the ENBS, except that, if no sample size can be found to give a positive ENBS, the study can be ruled out.
Exploratory subgroup analysis by Interagency Registry for Mechanically Assisted Circulatory Support profiles
An exploratory subgroup analysis was undertaken to assess the cost-effectiveness of LVADs based on the clinical characteristics of patients. The cost-effectiveness of LVADs was analysed separately for INTERMACS profiles 1, 2 & 3, 4 & 5. INTERMACS 1 was evaluated separately, but not included in the base case because DT patients in this group usually do not receive a LVAD. In the absence of trials that compared LVAD recipients to patients on MM by the INTERMACS profiles, the best available evidence and expert views were used to define the key model inputs in this analysis.
The mortality risks and health utilities used for this analysis are provided in Table 39 (see Appendix 8). Based on expert view, it was assumed that all medically managed DT patients with an INTERMACS 1 profile would die within 6 months. In the absence of evidence on the QoL amongst MM patients with an INTERMACS 1 profile, it was assumed to be 0.1 within the first month and after that QoL in ICU (0.26) was applied as a proxy. Grady et al. reported that LVAD implantation increased QoL by 0.11 units amongst patients with an INTERMACS 1 profile, and this was used to estimate the health utilities in INTERMACS 1 DT patients who received a LVAD (0.26 + 0.11 for the first year). 145
Results
Primary analysis
To be comprehensive, two base-case analyses were undertaken for the primary analysis, using two separate estimates of mortality risks and utilities. Based on the systematic review of the clinical literature, four different methods were defined to estimate mortality risks and health utilities. Economic evaluations were conducted for all four of these options, keeping all the remaining parameters constant, to identify the base case. Based on the face validity of the model outcomes and the assumptions associated with each option, it was decided to present two base cases: non-comparative, net weight estimates and comparative estimates mapped to LVAD in MOMENTUM. The justifications for not including the other two options are provided below and the model outcomes for these options are provided (see Appendix 8).
-
Non-comparative, net weight estimates: Taken forward as one of the two base cases and the outcomes are provided below.
-
Non-comparative, weighted estimates for MM: This option required combining data from three different trials and the modelling outcomes were similar to the non-comparative, net weight estimates. Thus, it was decided to present the outcomes as an appendix.
-
Comparative estimates mapped to MM in REMATCH: The model outcomes were not realistic for the LVAD recipients. It suggested that patients receiving a LVAD would have 2.86 LYs and 2.02 QALYs on average. This was deemed too low, underestimating the benefits of LVADs compared to the findings of recent trials. Thus, it was decided to present the outcomes as an appendix.
-
Comparative estimates mapped to LVAD in MOMENTUM: Taken forward as one of the two base cases and the outcomes are provided below.
Deterministic model outcomes
The deterministic lifetime outcomes provided in Table 19 showed that using the non-comparative, net weight estimates and the comparative estimates (mapped to LVAD in MOMENTUM), LVAD would be expected to produce additional QALYs of 2.86 and 2.51 per patient, respectively, compared to MM. The incremental costs were £152,735 and £146,275, respectively. Therefore, although the QALY and cost estimates in the MM arm were higher using the comparative estimates mapped to LVAD in MOMENTUM (compared with the non-comparative net weight estimates), the ICERs produced were similar at £53,496 and £58,244, respectively.
| Lifetime outcomes | Non-comparative, net weight estimates | Comparative estimates mapped to LVAD in MOMENTUM | ||||
|---|---|---|---|---|---|---|
| MM | LVAD | Incremental | MM | LVAD | Incremental | |
| Expected LYs per patient | 0.92 | 4.65 | 3.73 | 1.68 | 4.74 | 3.06 |
| Expected QALYs per patient | 0.46 | 3.32 | 2.86 | 0.85 | 3.36 | 2.51 |
| Cost per patient | £18,886 | £171,621 | £152,735 | £26,534 | £172,809 | £146,275 |
| Incremental cost per LY | £40,911 | £47,818 | ||||
| Incremental cost per QALY | £53,496 | £58,244 | ||||
Table 20 provides the model outcomes at the end of 2 years. These estimates showed that by the end of the second year, LVADs would reduce expected deaths by 66% using the non-comparative, net weight estimates and by 44% using the comparative estimates mapped to LVAD in MOMENTUM. The ICERs at 2 years were estimated to be £131,593 and £151,101, respectively.
| Two-year outcomes | Non-comparative, net weight estimates | Comparative estimates mapped to LVAD in MOMENTUM | ||||
|---|---|---|---|---|---|---|
| MM | LVAD | Incremental | MM | LVAD | Incremental | |
| % of deaths | 91 | 25 | −66 | 69 | 25 | −44 |
| Expected LYs per patient | 0.85 | 1.71 | 0.87 | 1.09 | 1.72 | 0.63 |
| Expected QALYs per patient | 0.43 | 1.23 | 0.80 | 0.55 | 1.23 | 0.68 |
| Cost per patient | £17,173 | £121,843 | £104,670 | £17,576 | £121,853 | £104,277 |
| Incremental cost per LY | £120,868 | £164,831 | ||||
| Incremental cost per QALY | £131,593 | £154,101 | ||||
Table 21 provides the model outcomes at the end of 5 years. These estimates showed that by the end of the first 5 years, LVADs would reduce expected deaths by 46% using the non-comparative, net weight estimates and by 39% using the comparative estimates mapped to LVAD in MOMENTUM. The ICERs at 5 years were estimated to be £67,997 and £77,775, respectively.
| Five-year outcomes | Non-comparative, net weight estimates | Comparative estimates mapped to LVAD in MOMENTUM | ||||
|---|---|---|---|---|---|---|
| MM | LVAD | Incremental | MM | LVAD | Incremental | |
| % of deaths | 100 | 54 | −46 | 92 | 52 | −39 |
| Expected LYs per patient | 0.92 | 3.30 | 2.38 | 1.57 | 3.34 | 1.77 |
| Expected QALYs per patient | 0.46 | 2.36 | 1.90 | 0.79 | 2.38 | 1.59 |
| Cost per patient | £18,855 | £148,002 | £129,148 | £24,815 | £148,522 | £123,707 |
| Incremental cost per LY | £54,314 | £69,936 | ||||
| Incremental cost per QALY | £67,997 | £77,775 | ||||
Severity weighted incremental cost-effectiveness ratio estimates
The updated NICE guidelines recommend using QALY weights based on the QALY shortfalls a certain population faces as a result of an illness. 122 The absolute QALY shortfalls for the study population were estimated as 10.38 using the non-comparative, net weight estimates and 9.95 using the comparative estimates mapped to LVAD in MOMENTUM, while the proportional QALY shortfalls were 0.96 and 0.92, respectively. The incremental QALY gains estimated by the model were weighted according to the proportional QALY shortfalls, as recommended.
The appropriate weighting was 1.7 in the non-comparative, net weight estimates, and 1.2 in the comparative estimates mapped to LVAD in MOMENTUM. The weighted ICER per QALY estimates were £31,468 and £48,537, respectively (Table 22). Therefore, although the QALY-weighting reduced the ICERs, they were still above the upper bound of NICE’s recommended cost-effectiveness threshold (£30,000 per QALY).
| Base-case analyses | Mean QALYs for MM | Proportional QALY shortfall | Recommended QALY weight | ICER per QALY | Weighted ICER per QALY |
|---|---|---|---|---|---|
| Non-comparative, net weight estimates | 0.46 | 0.96 | 1.7 | £53,496 | £31,468 |
| Comparative estimates mapped to LVAD in MOMENTUM | 0.85 | 0.92 | 1.2 | £58,244 | £48,537 |
Sensitivity analyses
One-way and probabilistic sensitivity analyses were conducted to address the structural and parametric uncertainties in the model estimates.
Transition to bridge to transplant and heart transplant
The impact of incorporating the probability of transition from DT to BTT states and from BTT to HT states was estimated in a sensitivity analysis. The analysis showed that the ICER was reduced slightly to £52,762 and £57,470, respectively (Table 23).
| Lifetime outcomes | Non-comparative, net weight estimates | Comparative estimates mapped to LVAD in MOMENTUM | ||||
|---|---|---|---|---|---|---|
| MM | LVAD | Incremental | MM | LVAD | Incremental | |
| Expected LYs per patient | 0.92 | 4.58 | 3.66 | 1.68 | 4.66 | 2.98 |
| Expected QALYs per patient | 0.46 | 3.26 | 2.80 | 0.84 | 3.30 | 2.45 |
| Cost per patient | £18,886 | £166,433 | £147,547 | £26,534 | £167,540 | £141,006 |
| Incremental cost per LY | £40,351 | £47,279 | ||||
| Incremental cost per QALY | £52,762 | £57,470 | ||||
One-way sensitivity analyses
Figures 20 and 21 show the results of the one-way sensitivity analyses, conducted by varying one parameter at a time to understand their impact on the ICER estimates. The input values were chosen from the studies identified in the systematic review reported in Chapter 3 and from two previous UK studies. 110,116 The analysis showed that the outpatient costs for LVAD recipients had the greatest impact on the ICER estimates. This was followed by the outpatient costs for patients on MM and the mortality risk amongst LVAD patients.
FIGURE 20.
One-way sensitivity analysis for non-comparative, net weight estimates. OMM, optimal medical management.

FIGURE 21.
One-way sensitivity analysis for comparative estimates mapped to LVAD in MOMENTUM.
Since the deterministic sensitivity analysis showed that outpatient costs had a substantial impact on the ICER estimates, further analyses were conducted to explore how the results changed when different values reported in the literature were utilised for these specific parameters. The parameters used in these analyses and the impact on results are provided in Tables 24 and 25, and the details of these values are summarised in Table 37 (see Appendix 8).
| Value (£) | ICER per QALY estimates | Source | |
|---|---|---|---|
| Non-comparative, net weight estimates (£) | Comparative estimates mapped to LVAD in MOMENTUM (£) | ||
| 72 | 36,623 | 38,714 | Lim et al. 2021110 |
| 958 | 53,946 | 58,244 | Chew 2017106 |
| 986 | 54,029 | 58,861 | Neyt 2013102 |
| 1943 | 72,253 | 79,956 | Rogers 2021114 |
| 1952 | 74,424 | 80,155 | Clegg 2007108 |
| 2139 | 75,986 | 84,277 | Long 2014111 |
| 2172 | 76,614 | 85,004 | Shreibati 2017101 |
| 2598 | 84,726 | 94,394 | Silvestry 2019115 |
| 1603 | 65,769 | 72,462 | Mean value |
| Value (£) | ICER per QALY estimates | Source | |
|---|---|---|---|
| Non-comparative, net weight estimates (£) | Comparative estimates mapped to LVAD in MOMENTUM (£) | ||
| 72 | 55,706 | 62,824 | Lim et al. 2021 |
| 336 | 54,686 | 60,710 | Adang 2006 |
| 644 | 53,496 | 58,244 | Clegg 2007 |
| 958 | 52,282 | 55,730 | Chew 2017 |
| 1187 | 51,397 | 53,897 | Neyt 2013 |
| 1943 | 48,475 | 47,843 | Shreibati 2017 |
| 2457 | 46,489 | 43,728 | Rogers 2021 |
| 2951 | 44,579 | 39,773 | Silvestry 2019 |
| 1886 | 48,695 | 48,300 | Mean value |
The findings showed that the ICER estimates ranged between £36,623 and £94,394, but remained above £50,000 except for four cases where the input values were significantly different from the others reported in the literature.
Additional analyses were also conducted to vary the mortality risk for the LVAD after 24 months to understand the impacts of incorporating the values used in the most recent economic evaluations conducted in the UK (Table 26). The estimated ICERs ranged between £43,380 and £58,844 using the non-comparative, net weight estimates, and between £45,807 and £59,846 in the comparative estimates mapped to LVAD in MOMENTUM.
| Value | ICER estimates | Source | |
|---|---|---|---|
| Non-comparative, net weight estimates (£) | Comparative estimates mapped to LVAD in MOMENTUM (£) | ||
| 0.0020 | 43,380 | 45,807 | Lim et al. 2021 |
| 0.0070 | 53,496 | 58,244 | MOMENTUM |
| 0.0110 | 58,884 | 65,376 | Scheuler 2021 |
| 0.0067 | 52,721 | 59,846 | Mean value |
When the mean of all the values identified for these three parameters (ongoing costs for the LVAD and MM and mortality risk in LVAD recipients after 24 months) were entered into the model simultaneously, the ICER values were £60,272 and £61,675 using the non-comparative, net weight estimates and the comparative estimates (mapped to LVAD in MOMENTUM).
Probabilistic sensitivity analysis
The PSA showed some uncertainty around the model outputs based on 10,000 iterations. The mean estimates and the 95% CIs are provided in Table 27 and the ICER estimates are shown in Figures 22 and 23. The uncertainty around incremental QALY gains was greater using the comparative estimates mapped to LVAD in MOMENTUM compared to the non-comparative, net weight estimates, although the uncertainty around cost differences was similar.
| Outcomes | MM | LVAD | Incremental | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| Non-comparative, net weight estimates | |||||||||
| Expected LYs per patient | 0.93 | 0.80 | 1.08 | 4.59 | 4.14 | 5.10 | 3.67 | 3.19 | 4.19 |
| Expected QALYs per patient | 0.48 | 0.41 | 0.55 | 3.26 | 2.94 | 3.61 | 2.7824 | 2.46 | 3.14 |
| Cost per patient | £18,953 | £17,107 | £21,016 | £171,281 | £144,725 | £200,692 | £152,329 | £125,665 | £181,812 |
| Incremental cost per QALY £54,748 | |||||||||
| Probability of cost-effectiveness at £30,000 0% | |||||||||
| Comparative estimates mapped to LVAD in MOMENTUM | |||||||||
| Expected LYs per patient | 1.67 | 0.70 | 2.77 | 4.71 | 4.23 | 5.27 | 3.04 | 2.04 | 3.98 |
| Expected QALYs per patient | 0.86 | 0.36 | 1.43 | 3.31 | 2.98 | 3.68 | 2.4512 | 1.91 | 2.97 |
| Cost per patient | £26,473 | £16,488 | £37,929 | £173,151 | £146,703 | £203,179 | £146,677 | £119,015 | £177,751 |
| Incremental cost per QALY £59,840 | |||||||||
| Probability of cost-effectiveness at £30,000 0% | |||||||||
FIGURE 22.
Probabilistic sensitivity analysis based on 10,000 iterations for non-comparative, net weight estimates.

FIGURE 23.
Probabilistic sensitivity analysis based on 10,000 iterations for comparative estimates mapped to LVAD in MOMENTUM.
The analysis also found that the probability of cost-effectiveness at a WTP threshold of £30,000 was 0%.
The probability of cost-effectiveness of a LVAD as DT at different WTP thresholds were explored in a cost-effectiveness acceptability analysis (Figures 24 and 25). This analysis showed that the probability of cost-effectiveness was 21% and 11% at a WTP threshold of £50,000 per QALY using the non-comparative, net weight estimates and the comparative estimates mapped to MM in REMATCH, respectively. The probability of cost-effectiveness reached 100% at a WTP threshold of £75,000 and £91,000 per QALY using the non-comparative, net weight estimates and the comparative estimates mapped to LVAD in MOMENTUM, respectively.
FIGURE 24.
Cost-effectiveness acceptability curve for non-comparative, net weight estimates.

FIGURE 25.
Cost-effectiveness acceptability curve for comparative estimates mapped to LVAD in MOMENTUM.

Value of Information analysis
The EVPI at different WTP thresholds was explored (Figures 26 and 27), and at a WTP of £30,000 per QALY, EVPI was estimated as £0 both using the non-comparative, net weight estimates and the comparative estimates mapped to LVAD in MOMENTUM. EVPI per person was highest when the WTP per QALY was £55,000 using the non-comparative, net weight estimates and £60,000 in the comparative estimates mapped to LVAD in MOMENTUM, reaching £6957 and £8436, respectively.
FIGURE 26.
Expected value of perfect information for non-comparative, net weight estimates.

FIGURE 27.
Expected value of perfect information for comparative estimates mapped to LVAD in MOMENTUM.
In additional analyses, EVPPI and EVPPI per person were found to be £0 for all the parameters. Since the EVPI estimates were too low and indicated that conducting further trials was not advisable, analysis whose principal purpose is to estimate the optimal size for a future trial (i.e. EVSI and ENBS values) was deemed unnecessary.
Exploratory subgroup analysis by Interagency Registry for Mechanically Assisted Circulatory Support profiles
The subgroup analysis by INTERMACS profiles showed that patients in the LVAD arm who had less severe HF would gain more LYs and QALYs compared to patients with more severe conditions (Table 28). ICER per QALY estimates were estimated as £84,800, £65,458 and £58,815 for the INTERMACS groups 1, 2 & 3 and 4 & 5, respectively. Thus, the ICER remained above the £30,000 threshold in all cases.
| Lifetime outcomes (INTERMACS 1) | MM | LVAD | Incremental |
|---|---|---|---|
| Expected LYs per patient | 0.37 | 3.05 | 2.68 |
| Expected QALYs per patient | 0.06 | 1.65 | 1.59 |
| Cost per patient | £13,333 | £148,214 | £134,882 |
| Incremental cost per LY | £50,336 | ||
| Incremental cost per QALY | £84,800 | ||
| Lifetime outcomes (INTERMACS 2 & 3) | |||
| Expected LYs per patient | 0.92 | 3.93 | 3.01 |
| Expected QALYs per patient | 0.36 | 2.54 | 2.17 |
| Cost per patient | £18,886 | £161,052 | £142,166 |
| Incremental cost per LY | £47,198 | ||
| Incremental cost per QALY | £65,458 | ||
| Lifetime outcomes (INTERMACS 4 & 5) | |||
| Expected LYs per patient | 1.00 | 4.14 | 3.14 |
| Expected QALYs per patient | 0.51 | 2.96 | 2.45 |
| Cost per patient | £19,714 | £163,958 | £144,244 |
| Incremental cost per LY | £45,967 | ||
| Incremental cost per QALY | £58,815 | ||
Discussion
Summary of findings
This study involved an economic evaluation of LVADs as DT for AHF patients ineligible for a HT compared to MM from the NHS/PSS perspective. A decision-analytic model with a lifetime horizon and one-month cycles was developed based on the systematic reviews in Chapters 3 and 4.
The economic evaluation found that a LVAD would increase life expectancy by 3.73 and 3.06 years, produce an additional 2.86 and 2.56 QALYs per person and the incremental costs to the NHS would be £152,735 and £146,275 per person using the non-comparative, net weight estimates and the comparative estimates mapped to LVAD in MOMENTUM, respectively. Thus, at a WTP threshold of £50,000 per QALY, LVADs were not cost-effective compared to MM for AHF patients ineligible for a HT. The deterministic sensitivity analysis showed that inclusion of the probability of becoming eligible for a HT did not change the findings, while the outpatient costs for LVAD recipients had a significant impact on ICER estimates. The PSA showed some uncertainty around the model outcomes, especially in terms of the incremental QALY gains. The probability of cost-effectiveness at a WTP threshold of £30,000 was 0% based on 10,000 iterations. According to the exploratory subgroup analysis, a LVAD was not cost-effective for any specific INTERMACS group evaluated (£84,800 for INTERMACS 1, £65,458 for INTERMACS 2 & 3 and £56,256 for INTERMACS 4 & 5).
Decision-making based on severity weighted bridge to transplant estimates
The updated NICE guidelines recommend using QALY weights defined based on the shortfall between the expected QALYs in the same age and sex group of general population and the expected QALYs in the study population in the absence of the novel intervention being evaluated. This study estimated the expected QALYs for MM patients as 0.46 using the non-comparative, net weight estimates and 0.85 using the comparative estimates mapped to LVAD in MOMENTUM, and the corresponding proportional QALY shortfalls were 0.96 and 0.92. The recommended QALY weights were 1.2 if the proportional QALY shortfall was between 0.85 and 0.95, and 1.7 if the shortfall was above 0.95. Thus, there was a substantial gap between the weights recommended for the two base-case analyses in this study, although the proportional QALY shortfalls were close.
Utilising different QALY weightings increased the difference between ICER estimates in the two base-case analyses from £4748 (£53,496 and £58,244) to £17,069 (£31,468 and £48,537). Applying the higher weight (1.7) to ICER estimates for the comparative estimates mapped to LVAD in MOMENTUM would reduce the ICER from £58,244 to £34,261, and the weighted ICER was £31,468 for the non-comparative, net weight estimates. On the other hand, applying the lower weight (1.2) to the non-comparative, net weight estimates would reduce the ICER from £53,496 to £44,580, and the weighted ICER was £48,537 for analysis using the comparative estimates mapped to LVAD in MOMENTUM. From a decision-maker’s perspective, the subgroup analysis based on clinical characteristics suggested that severity did not make a difference to the overall results, given that the ICER remained above £30,000 in all estimates.
Strengths and limitations of modelling
The economic model was developed based on the best available evidence identified by the systematic reviews of the clinical and economic evidence. The model development was also informed by discussions with clinicians, commissioners and patient representatives.
In the absence of a direct comparison between the contemporary LVADs and MM, specific attention was paid to the parameters selected for mortality risks, and four different sets of mortality risks were defined. The impacts of these four options on modelling outcomes were explored and discussed with the clinicians in the research project team. Since each of these options were based on different assumptions, it was deemed appropriate to present two base-case estimates, namely using the non-comparative, net weight estimates and the comparative estimates mapped to LVAD in MOMENTUM.
The non-comparative, net weight estimates used life expectancy data on MM dated from 2001, thus the estimates assumed that the clinical effectiveness of standard care had not changed over the last 20 years Therefore, these estimates might be overestimating the benefits of LVADs, if the life expectancy of MM patients has improved over the last 20 years. Additionally, it was assumed that the profiles of patients in the MOMENTUM trial matched perfectly to the profiles of MM patients in the REMATCH trial. This was a reasonable assumption given that the MM patients in the REMATCH trial were on inotropes and the majority of LVAD recipients in the MOMENTUM trial were classified as INTERMACS 2 & 3. However, if this assumption is incorrect and MM patients in the REMATCH trial had worse health statuses compared to the baseline health statuses of the patients in the MOMENTUM trial, the model outcomes might be overestimating the benefits of LVADs.
In the comparative estimates mapped to LVAD in MOMENTUM, considering that trials might have chosen healthier patients to implant LVADs, estimating the mortality risks in the MM arm based on the mortality risks reported in the MOMENTUM trial and the RR estimated in Chapter 3. The clinical effectiveness of LVADs compared to MM as DT in AHF patients was reasonable. However, this assumed that the profiles of patients in the MOMENTUM trial matched perfectly to the MM patients in the REMATCH trial and the other trials used for the statistical analysis reported in Chapter 3. The model outcomes provided a higher life expectancy and QoL for the MM arm and a higher ICER estimate compared to the non-comparative, net weight estimates and the previous UK studies. 110,116 Therefore, the comparative estimates mapped to LVAD in MOMENTUM might be underestimating the benefits of LVADs.
Another consideration was the lack of mortality data beyond 2 years. This required making an assumption to extrapolate the mortality data. The model used the mortality risk reported for the months between 13 and 24 in the trial for the time points beyond 2 years. The sensitivity analyses showed that this was a conservative assumption and had a modest impact on the cost-effectiveness outcome. Therefore, the model might be underestimating the cost-effectiveness of LVADs if the impact on mortality beyond 2 years is greater than the values used in the model. On the other hand, the model might be overestimating the cost-effectiveness of LVADs if the impact on mortality beyond 2 years is lower than the values used.
Additionally, data on the ongoing cost for patients on MM were limited. There was only one UK-based estimate, but the details of this estimate were not clear and the reported value was deemed too high. Thus, estimates from other studies in countries with similar healthcare systems were utilised in the base case. Specific attention was paid to these parameters, and the impact of using different value inputs on the model outcomes was explored in the deterministic sensitivity analyses. When the mean of all the values identified in the literature were utilised, the ICER estimates were similar to the base-case estimates.
Since currently only one type of LVAD is available, the key model parameters were defined from the studies that included only this type of LVAD. However, device-specific data were not available for some major events and complications and data that included previous versions of the LVAD were used for these parameter estimates. This means that the model might be underestimating or overestimating the benefits of the LVAD, depending on the parameters chosen. However, the deterministic sensitivity analysis showed that none of these parameters had a substantial impact on the model results.
Another consideration, there was no trial that compared a LVAD to MM for defined INTERMACS profiles. Hence, the subgroup analyses were based on discrete data from different sources, and for some parameters assumptions were made based on expert views. It is difficult to speculate on the impacts of these assumptions on study findings in the absence of data, and therefore, the subgroup analyses should be considered exploratory.
Findings in context
The systematic review in Chapter 4 identified two recent UK-based economic evaluations. 110,116 One of these studies focused on a type of LVAD that was withdrawn from use in the course of this project. 116 The other study estimated an ICER of £47,361 per QALY gained. 110 The key difference between the two studies related to the mortality risks assumed for LVAD recipients beyond 24 months. Lim et al. extrapolated the values, averaging the changes in the last 6 months while in this study the risk for the 24th month was used for the remaining cycles. 110 When the extrapolated values used in that study were entered into the current model, the ICER per QALY estimates reduced from £54,295 to £49,120, as shown in the deterministic sensitivity analysis. In terms of the incremental costs, for the study by Lim et al. the cost difference between the LVAD and MM arm was only £113,552, which was mainly related to the cost of the device (£109,140). 110 The corresponding figure in the current analysis were £152,735 and £146,275. Thus, the study by Lim et al. assumed very little difference between patients on MM and LVAD in terms of adverse events and complications. 110
The systematic review in Chapter 4 as DT identified only one study that considered the impact of INTERMACS profiles on the cost-effectiveness estimates. 110 That study estimated a lower ICER for patients with INTERMACS 2 & 3 profiles and a higher ICER for the INTERMACS 4–7 group. This contradicts the findings of the current evaluation, which suggests that LVADs are less cost-effective in more severe patients compared to patients who are less unwell. It is not possible to explain the reasons behind this difference since the model inputs for the INTERMACS profiles-based analyses were not provided in the previous study.
Parameters with the greatest impact on incremental cost-effectiveness ratio per quality-adjusted life-year estimates
The deterministic sensitivity analyses indicated that two parameters had substantial impacts on the model results: outpatient costs for LVAD and MM patients. Thus, additional analyses were conducted to estimate the impact of using different value inputs for the parameters with the greatest impact. The value inputs for these analyses were taken from the studies identified in the systematic review reported in Chapter 3 and their applicability to the UK setting was unclear. Thus, these analyses were only exploratory and are not intended to guide decision-making.
The outpatient costs for LVADs had a substantial impact on the ICER estimates due to the wide range of values reported in the literature, ranging from £72 to £2598. The differences between the cost estimates from the published studies can be partly explained by the differences in healthcare provision across countries, given that the highest figure was reported in a study from the USA while the lowest figure was from a UK-based study. 110,115 Another potential reason might be the costs of different procedures delivered during an outpatient appointment. Depending on the values chosen, ICER estimates in the non-comparative, net weight estimates varied between £36,623 and £84,726, while they were between £38,714 and £94,394 in the comparative estimates mapped to LVAD in MOMENTUM. Different values were also reported for the outpatient costs in MM patients, varying between £72 and £2951. The highest cost estimate (£2951/month) resulted with ICER estimates of £44,579 in the non-comparative, net weight estimates and £39,773 using the comparative estimates mapped to LVAD in MOMENTUM.
Implications for stakeholders/future research
The economic evaluations reported in this chapter show that from the NHS/PSS perspective, LVADs as DT for AHF patients are not cost-effective compared to MM at a WTP threshold of £30,000 per QALY. This finding is unaltered by applying severity weightings to the QALY estimates as recommended by the updated NICE guidelines.
The sensitivity analyses indicate that varying the outpatient costs for patients on LVADs and MM had a significant impact on the results. Thus, future research focusing on defining these cost items might be helpful for decision-makers in the UK, although the VoI analysis suggested that the current evidence is sufficient for decision-making. The exploratory subgroup analyses conducted based on the best available evidence and expert views were consistent with the base-case analysis. However, the existing evidence was insufficient to reach a full conclusion on the cost-effectiveness of LVADs for patients with different INTERMACS profiles. Further research is needed to estimate the impacts of INTERMACS profiles on the cost-effectiveness estimates. Thus, these findings should be interpreted with caution.
Another consideration is the impact of applying severity weights as recommended by NICE on the study results. Although the severity weighted estimates did not change the cost-effectiveness findings of this evaluation, the increased differences between the outcomes of the two base-case analyses warrant attention. Health conditions that result in 0.95 QALY shortfalls would require a QALY weighting of 1.2, but the weight would jump to 1.7 for a QALY shortfall of 0.96. NICE defined the weight groups based on how the end-of-life criteria were applied in previous appraisals, and there is no evidence on whether this truly reflects societal preferences. In addition, the QALY shortfall cut-offs are higher than those used in other countries, such as the Netherlands (0.70). 146 Further research is needed to understand the appropriateness and potential implications of the severity-weightings on cost-effectiveness decisions for different health technologies and interventions.
Chapter summary
This chapter presented the findings of an economic evaluation of LVADs compared to MM as DT amongst patients with AHF ineligible for a HT from the NHS/PSS perspective. The analysis in this chapter indicates that LVADs are not cost-effective compared to MM based on current WTP thresholds recommended in UK national guidelines. The economic evaluation was developed based on the best available evidence and expert views. However, it is important to take into account the limitations arising from the available data and the modelling assumptions that needed to be adopted. In particular, the value of estimates for outpatient costs for LVAD and MM patients had a substantial impact on the cost-effectiveness estimates, and thus further research is needed to obtain more accurate information.
Chapter 6 Discussion
Research question and aims
We aimed to answer the following research question:
-
What is the clinical effectiveness and cost-effectiveness of LVADs for DT in patients with AHF when compared to MM?
Objectives
The clinical effectiveness and cost-effectiveness evidence were summarised in two systematic reviews, using the same search strategy. All study designs were included, and the analysis and reporting were focused on the HM3 device. Outcomes in the clinical effectiveness review were categorised as survival, major events, complications, hospitalisations, QoL or functional status. Appropriate, validated risk-of-bias tools were applied to assess the quality of the included evidence in both reviews. A NMA was undertaken to allow an indirect comparison of the HM3 and MM to be made for the survival outcome.
Evidence from the systematic reviews and NMA, as well as guidance from clinical experts, patients and commissioners were used to inform the development of a Markov model to compare costs and effectiveness of LVADs used for DT when compared to MM. Two base cases were presented based on differing assumptions of mortality risks for both arms. Both deterministic and probabilistic sensitivity analyses were carried out, as well as subgroup analyses to explore the potential impact of different INTERMACS profiles.
Summary of findings
Systematic review of clinical effectiveness
The systematic review summarised a large volume of evidence analysing the use of LVADs for DT in patients with end-stage HF with over 130 studies included. The withdrawal of the HeartWare HVAD during the review process meant that priority of reporting was given to data from HM3, the only available device in the UK. 32 One RCT (MOMENTUM 3) assessed the HM3 in comparison to the HeartMate II and reported survival of 76.7% at 24 months compared to 59% in the HeartMate II, the highest survival noted for a device at this follow-up point. 147 The HM3 device also demonstrated fewer bleeding and stroke events than earlier devices; issues that contributed to the withdrawal of the HeartWare HVAD. Furthermore, pump thrombosis could still be considered an issue in the HM3.
Data from the 5-year extended observational follow-up of MOMENTUM 3 were recently presented at the ESC congress. 148 While this was outside of our latest search update and therefore not included in the analysis, overall survival was reported at 54.8% in DT HM3 patients at 5 years, compared to 39.4% in those with the HeartMate II. This was higher than any survival figures reported in INTERMACS analyses of multiple device types, though these analyses did not include the HM3. 31,46,72 This reiterates the survival benefit of the HM3 over other devices and may be useful in future economic models.
While the HM3 has demonstrated clinical effectiveness, there is no direct evidence comparing the device to the standard treatment of MM. To determine this, a NMA was undertaken to establish an indirect comparison using data from previously conducted RCTs. The analysis demonstrated a significant benefit for HM3 compared to MM for risk of mortality, but there were concerns regarding transitivity, and CIs around this indirect estimate were wide.
Systematic review of cost-effectiveness
There was limited evidence pertaining to the cost-effectiveness of LVADs for DT. Most of the 19 studies included were conducted in the USA and were from the perspective of the service provider. The incremental cost per QALY estimates ranged from £46,207 to £238,401 in 2019 prices. Of the studies, 14 were full economic evaluations, which looked at the health and cost impacts of LVADs with 8 of these studies reporting that LVADs were not cost-effective when compared to MM for DT patients. However, two of the UK-based evaluations did report positive findings with the ICER per QALYs reported to be just below the £50,000 threshold, defined as the end-of-life criteria in the UK. Some studies did not include adverse events and there was often limited consideration of time horizon.
Economic evaluation
Based on the two base cases employed in the model, neither found LVADs to be cost-effective at a WTP threshold of £50,000 per QALY when compared to MM in DT patients. The non-comparative, net-weight estimates approach yielded an additional 2.86 QALYs per person with an ICER of £53,496. On the other hand, the comparative estimates mapped to LVAD in MOMENTUM approach produced an additional 2.51 QALYs per person with an ICER of £58,244.
Furthermore, subgroup analysis by INTERMACS profile did not demonstrate cost-effectiveness of LVADs for DT in any specific INTERMACS groupings. LVADs were closest to cost-effectiveness at the £50,000 threshold in patients with INTERMACS profiles 4 & 5 (ICER of £58,815), using the non-comparative, net-weight estimates approach.
Strengths and weaknesses of the reviews and economic evaluation
Systematic review of clinical effectiveness
The systematic review included a large volume of evidence sourced using a comprehensive search strategy in key databases. Articles were also sought via citation checking and targeted searching of mechanical circulatory support registry reports (e.g. INTERMACS, IMACS). Input was sought from clinical experts as well as patients when considering aspects of the review such as the search strategy, outcome definitions and when planning the synthesis. Robust methods were used throughout to ensure the potential for bias was limited.
However, some limitations were evident. Due to the high volume of evidence found, studies with a sample size of < 50 DT patients were excluded in the clinical effectiveness review. While this may have resulted in the loss of some evidence, calculations (previously described) determined that this would result in < 5% loss of patients across the evidence base.
A pragmatic approach to searching was also applied to manage the huge database of hits that were produced with a less specific search strategy. Various terms for ‘DT’ as well as ‘bridge to transplant’ were used to reduce hits from over 20,000 to around 12,000 across all databases. This could potentially have resulted in the loss of some relevant evidence. Finally, approximately four single-centre observational studies could potentially have had comparative data on different devices available that were not reported. However, the authors of these papers were not contacted due to project time constraints and the likelihood of obtaining any valuable evidence.
Weaknesses were also noted within the included evidence. For example, there was very limited device-specific data reported outside of the trials, which made it difficult to make meaningful comparisons and analyses. Furthermore, subgroup data (by INTERMACS or age) were also sparse, which meant it was difficult to determine if devices were more or less effective in patients in different subgroups. No direct comparisons were made in the evidence between the HM3 and MM, which resulted in the need to carry out a NMA. However, there were concerns regarding the transitivity assumption and CIs around this indirect estimate were wide.
Systematic review of cost-effectiveness
Many of the strengths and limitations of the clinical effectiveness review are also applicable to the cost-effectiveness review, due to the use of the same search strategy, duplicate screening, selection and implementation of appropriate and valid risk-of-bias checklists and piloted data extraction. Further studies were found via systematic review citation checking and contact with clinical experts.
All modelling studies were quality assessed in duplicate. Furthermore, limitations with the evidence itself were also present. The overall quality of the included studies was not high and various issues were evident. For example, only two studies justified the sources of parameters, and many studies did not explain how complications were incorporated. Finally, all but two of the economic evaluations included were not UK-based.
Economic evaluation
Strengths of the economic model produced were clear. The model itself was comprehensive and developed based on the best available evidence produced from the systematic reviews. It considered clinical perspectives as well as device and class of INTERMACS. Clear sources for the parameters were presented and these were based on the best available evidence as well as input from clinicians, commissioners and patients. Decisions of which base cases to use were made following in-depth discussions and processes, and these were presented alongside clear justifications together with descriptions of all of the options considered.
Limitations were, however, unavoidable and mostly pertained to the costs of MM. These costs were not clear from the UK perspective and while attempts were made to acquire data, which more accurately reflected the MM costs, these were unsuccessful. Therefore, data from various sources were used, which could now be considered potentially inaccurate. Questions were also raised over other health-related costs that are not related to a LVAD and whether these should be included in the model.
While the base cases were discussed and considered at length, various assumptions had to be made for each. The non-comparative, net weight estimates approach assumed that the INTERMACS profiles across the MOMENTUM trial and the REMATCH trial were the same. This approach also used life expectancy data for MM from 2001, meaning the estimates assume that the clinical effectiveness of standard care has not changed over the last 20 years.
Finally, no direct relative effect measures were available between the HM3 and MM as there are currently no completed trials comparing these interventions. Data from patients with particular INTERMACS profiles were also limited, meaning that subgroup analysis carried uncertainties.
Findings in context
Previous systematic reviews of clinical and cost-effectiveness
Several systematic reviews and HTAs have been carried out which assessed both the clinical and cost-effectiveness of LVADs for DT. Reports from Canada, Belgium and Sweden all found LVADs to be beneficial for survival compared to MM, though there were some concerns over thromboembolic and device complications. 90,91,149 However, these reports were published before any HM3 data were available and therefore are not reflective of the current device market in the UK. The Canadian report also reiterated the high costs of devices and implant surgery, though once again these were not reflective of the current HM3 device.
A previous NMA assessed LVADs for end-stage HF, but this did not focus solely on DT patients but included patients with all LVAD indications. 92 The NMA included the HM3, HVAD, HeartMate II, HeartMate XVE and MM and focused on four RCTs and four observational studies. Results showed a RR for death of 0.62 in the HM3 when compared to HeartMate XVE; however, this only appeared to be for 12 months of follow-up. The paper also demonstrated further limitations as described earlier in Chapter 3.
Comparisons between the HeartMate 3 and medical management
Issues were highlighted in both systematic reviews due to the lack of available direct comparative evidence between the HM3 and MM. This meant using indirect data produced from the NMA (which relied upon various assumptions and resulted in uncertainty) for the economic model. While no results were published at the time of write-up of this report, an ongoing RCT is currently comparing the HM3 with MM in Sweden. The SweVAD trial aims to enrol 80 participants of DT indication who will be randomised to the HM3 or MM across seven Swedish University Hospitals. 48 The study will follow-up patients for a minimum of 2 years and is estimated to be completed in December 2023. Results of this trial will be important for updating any economic models with more robust comparative data between the HM3 device and MM.
Previous economic models
The systematic review of cost-effectiveness identified two previous UK economic models, both of which found LVADs to be cost-effective in comparison to MM in patients with AHF ineligible for transplant, in contrast with both base-case analyses presented here. 110,116 Both models reported ICER per QALY estimates at just below the NICE £50,000 threshold. However, one of these models was based on the now withdrawn HeartWare HVAD and is no longer relevant. Additionally, applying the severity weighting suggested by NICE (2022) did not change the cost-effectiveness outcomes.
The Lim et al. HM3 model found a ICER of £47,361 per QALY gained (compared to £54,748 and £59,840 in each of our base cases). 110 Differences were evident between the model presented here and the Lim model. Lim et al. extrapolated the mortality risk values, averaging the changes in the last 6 months while in this model, the risk for the 24th month was used for the remaining cycles. Differences in assumed rates of adverse events and complications were evident, with Lim et al. assuming very few differences between patients on MM and the HM3 compared to this model. The Lim model also used only two health states and did not consider the impact of adverse events on life expectancy.
Overall, while the model in this report did not find the HM3 to be cost-effective compared to MM when considering the current NICE thresholds, it does remain close to this cut-off and is not drastically different to the previously reported models.
Implications for practice and future research
Implications for practice
The systematic review of clinical effectiveness and NMA demonstrate a survival benefit for the HM3 compared to MM in end-stage HF patients ineligible for transplant. However, the economic model did not find the HM3 to be cost-effective when compared to MM at the £50,000 threshold. This would suggest that currently, though the clinical evidence supports the use of LVADs for DT, there may not be enough evidence to support the use in the UK setting in regard to cost-effectiveness. This remained the case when analyses were limited to particular INTERMACS groupings, suggesting that there is not enough evidence to support use in particular groups of patients either. However, data on survival and other outcomes in DT patients by INTERMACS profile were limited and therefore more data are required to produce more robust results. Future research may be key in refining the estimates of cost-effectiveness of the HM3 compared to MM in DT patients, allowing for clear recommendations to be made.
Recommendations for future research
The systematic reviews and economic evaluation have highlighted gaps and issues with the current available evidence. These issues may help to direct key areas for future research.
Ongoing trials
The lack of direct comparative data between the HM3 and MM remains an issue and future research needs to address this. The ongoing SweVAD trial, as mentioned earlier, will provide this comparison. This data will be useful in future economic models. Further ongoing trials may also be important in addressing outstanding questions and uncertainty. The AMBU-VAD is another ongoing RCT comparing the HM3 to MM in ambulatory HF patients (INTERMACS ≥ 4), with a proposed enrolment of 92 patients taking place in France. 51 The study is expected to be completed in February 2025 and may help to inform whether the HM3 could be particularly useful in the ambulatory population, which have only been explored minimally in previous studies.
Another ongoing trial is currently assessing a new LVAD, the EVAHEART 2, compared to the HM3 device. 47 This is a RCT with an estimated enrolment of 400 patients aiming to determine non-inferiority of the EVAHEART 2 compared to the HM3. This trial is ongoing under a FDA investigational device exemption, to allow the study of the safety and effectiveness of the new device with the aim of introducing it to the market. This may offer an alternative to the HM3 in the future, depending on the results of the trial, which is expected to be completed in March 2024. Any future economic evaluations may need to consider this new device when determining the cost-effectiveness of LVADs.
Cost of medical management
One important issue is the uncertain costs for patients with AHF on MM. There are few to no data available on the current costs of MM in the UK, and all recent models have relied upon cost data, which are either very old or from other countries that could be considered to have similar healthcare systems. Alternative value inputs were considered in the deterministic sensitivity analysis and when the mean of all the values identified in the literature were used, ICER per QALY estimates were similar to the base-case estimates. However, the true cost of MM in the UK remains unclear and therefore a major audit is recommended to establish these costs and to include them in future economic models.
Observational studies and registry reports
Observational studies (essentially case series) included in the clinical effectiveness review rarely reported data by device. It is recommended that future publications from single centres should adhere to a more consistent reporting structure, clearly reporting by indication of device and by the device implanted, allowing for more consistent analyses in future reviews.
INTERMACS and IMACS registry reports remain the largest sources of LVAD patient data in the real world and they generally do not distinguish by device to avoid bias in favour of any particular manufacturer. However, they do often report the wider device type (e.g. continuous flow). Many INTERMACS reports were not included as they did not report by indication; therefore future registry analyses should focus on reporting this where available. However, it is important to note that due to the recent changes in the heart allocation system in the USA, almost all new implantations listed in INTERMACS are now for DT indication. This means that in the future, INTERMACS reports may be more reflective of the DT population than in earlier years, which were dominated by BTT indications.
Research into subgroups
Another key area that future research should focus on is the analysis of subgroups of LVAD patients. If LVADs are found to be more cost-effective in particular subgroups, such as certain INTERMACS profiles, select groups may then be recommended to receive LVADs. Therefore, future studies should focus on measuring and reporting results by INTERMACS profiles, as well as considering any other important subgroups.
While subgroups could be important, there is also the possibility of removing the indication labels of DT, BTT and BTC altogether and focusing on making implant decisions for individual patients based on suitability. This is currently in practice in other countries, such as Poland, and could be a consideration in the UK where only approximately 120 LVADs are implanted each year.
Patient and public involvement
Patient and public involvement were included throughout the project. The PPI group included both patient representatives as well as family members and carers to ensure a range of perspectives and experiences. The PPI group met several times throughout the study with members of the research team, including both clinicians and methodologists. The first meeting was held at the beginning of the research to convey the aims of the work and to allow PPI members to share their experiences to enable the non-clinical research team members to develop an understanding of living with HF and a LVAD. This meeting also offered the opportunity for PPI to comment on the proposed research and identify outcomes of importance regarding the systematic review of clinical effectiveness and aspects to be included in the economic evaluation. Two members were also invited to attend the wider steering group meetings for the project, to offer PPI insight and perspective.
Results of the research were conveyed to the PPI group during a meeting following submission of the report. Representatives also had the opportunity to comment on plain English summaries.
Conclusions
Findings from the clinical effectiveness review demonstrate that LVADs have significantly improved over time and the currently available HM3 LVAD is considered clinically effective in patients with end-stage HF ineligible for transplant, with the available evidence suggesting it may offer survival of over 75% at 2 years of follow-up with reduced complications and major events in comparison to older devices. However, there are no studies comparing the currently available device in the UK to MM.
Findings from the review of economic evaluations show that the estimates of the cost-effectiveness of a LVAD as DT vary widely depending on factors such as device, perspective, analysis approach and when and where studies were conducted. However, cost-effectiveness has improved over time, which may be explained by the increased life expectancy associated with newer generation devices and a reduction in adverse events and device costs. However, the estimated incremental cost per QALY gained compared to MM tended to remain higher than the accepted thresholds of cost-effectiveness applied in the UK.
Findings from the economic evaluation of LVADs compared to MM as DT amongst patients with AHF ineligible for a HT from the NHS perspective indicates that LVADs may not be cost-effective compared to MM with estimates of cost-effectiveness being just higher than current WTP thresholds recommended in the UK. Better data on outpatient costs for LVAD and MM patients are required, as these have an impact on the cost-effectiveness estimates,
While currently there is no evidence from studies directly comparing the HM3 device to MM, there is an ongoing RCT being undertaken in Sweden (SweVAD trial) comparing the two. The trial is due to complete final follow-up in December 2023. Hopefully, it should allow for relative effects of current device and current MM to be determined, which will enable more robust data to be used to update the current economic evaluation and the clinical effectiveness review, rather than relying upon indirect comparisons with wide uncertainty.
In addition, an audit of MM costs in DT patients in the UK is needed to reduce uncertainties in the economic evaluation. Finally, future trials and other studies should report results by patient severity profiles (e.g. INTERMACS classification), and if registry/observational studies, then also by device implanted, as these will aid in developing reliable subgroup analyses based on severity profiles to aid identification of whether a LVAD is (more) cost-effective for some groups of DT patients.
Additional information
Contributions of authors
Sophie Beese (https://orcid.org/0000-0001-6329-0779) (Research fellow, systematic reviews) led on the systematic review of clinical effectiveness (including search strategy development), jointly led on the systematic review of cost-effectiveness and worked on all aspects of each review; wrote the first draft of clinical effectiveness chapter and discussion chapter; wrote the scientific summary and was involved in the organisation of the project.
Tuba S Avşar (https://orcid.org/0000-0002-4143-3852) (Research fellow, health economics) led on the economic evaluation and model development, jointly led on the systematic review of cost-effectiveness; worked on both clinical and cost-effectiveness systematic reviews; wrote the first draft of the cost-effectiveness systematic review and economic evaluation chapters.
Malcolm Price (https://orcid.org/0000-0002-7352-3027) (Associate professor, biostatistics) led on the network meta-analysis as part of the systematic review of clinical effectiveness, and gave statistical input and advice for all research chapters with particular focus on the clinical review and economic evaluation.
David Quinn (https://orcid.org/0000-0003-2465-305X) (Cardiothoracic surgeon) made significant clinical input on all aspects of the project, worked on screening and selection for both systematic reviews; led on all aspects of patient and public involvement.
Hoong S Lim (https://orcid.org/0000-0002-6569-1805) (Consultant cardiologist) made significant clinical input on all aspects of the project, worked on screening of articles for both systematic reviews.
Janine Dretzke (https://orcid.org/0000-0002-2591-6918) (Senior research fellow, systematic reviews) contributed to the development of the search strategy, was involved in screening of articles for both systematic reviews, development of approach to inclusion and analysis of studies for the clinical review.
Chidubem O Ogwulu (https://orcid.org/0000-0002-8133-7021) (Research fellow, health economics) was involved in screening and selection of articles for both systematic reviews, carried out data extraction for review of clinical effectiveness.
Pelham Barton (https://orcid.org/0000-0002-0519-3724) (Honorary reader in mathematical modelling) gave overall economic input and advice on the systematic review of cost-effectiveness and the economic evaluation.
Louise J Jackson (https://orcid.org/0000-0001-8492-0020) (Associate professor, health economics) gave overall economic input and significant advice on the systematic review of cost-effectiveness and economic evaluation.
David Moore (https://orcid.org/0000-0002-4163-4080) (Associate professor, evidence synthesis) led the project overall, conceptualisation of project and funding acquisition, gave input and advice on all aspects of the project.
Acknowledgements
We would like to acknowledge and thank the late Professor Domenico Pagano for his contributions to the development of this project at the grant application stage.
We would also like to thank Isobel Harris (research fellow, University of Birmingham) for her contribution to the screening of articles for the systematic reviews. We are grateful for Pawana Sharma (research fellow, University of Birmingham) for undertaking risk-of-bias checking for the included trials in the systematic review of clinical effectiveness.
We also extend our thanks and appreciation to the members of our patient and public involvement group and their close family members for offering their invaluable insight into living with a LVAD, their abilities regarding activities of daily living, QoL and perspectives before and after receiving a LVAD. We thank those members for attending and offering input during both PPI and steering group meetings.
Finally, we also thank our steering group members for their vital experience and input on many aspects of the project. We recognise the significant clinical knowledge and input from Professor Francis Pagani, Professor Mandeep Mehra and Dr Nawwar Al-Attar. We are also grateful for the invaluable input from Sarah Watson on policy insights from the NHS England perspective. Their knowledge allowed us to understand the current clinical picture of LVAD for DT, information that the trials, observational and registry studies might provide and guidance on model parameters and/or detail on the current uncertainties with regard to making a commissioning decision on LVAD for DT in the UK.
Equality, diversity and inclusion
Participant representation: This study utilises publicly available research and therefore reflects the diversity of this evidence.
Research team and wider involvement: The research team were compiled for their relevant clinical and methodological expertise. They were employed by equal opportunities employers with active inclusive staff development programmes.
PPI members were approached for participation based on having received the intervention under consideration and on potential clinical similarity to those for whom the intervention is being considered in this report.
Data-sharing statement
This is a quantitative study using published and/or publicly available data. Data generated in the study are presented within the report. If required, further information can be obtained from the corresponding author.
Ethics statement
Ethical approval was not required for this study because it involves systematic reviews and an economic analysis using publicly available evidence.
Information Governance statement
This study did not utilise any personal information or data.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/MLFA4009.
Primary conflicts of interest: Hoong Sern Lim and David Quinn have received funding from the devices industry including from Abbot and Abiomed for payment or honoraria for lectures, as well as support for travel to meetings. No other conflicts of interest were noted.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Poole-Wilson PA. Chronic heart failure: causes, pathophysiology, prognosis, clinical manifestations, investigations. Dis Heart 1989:48-57.
- Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al. The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failures: a systematic review and economic evaluation. Health Technol Assess 2005;9:1-148. https://doi.org/10.3310/hta9450.
- National Institute for Health and Care Excellence . Chronic Heart Failure in Adults: Diagnosis and Management 2018. www.nice.org.uk/guidance/ng106 (accessed 1 August 2022).
- Peters M, Crocker H, Dummett S, Jenkinson C, Doll H, Fitzpatrick R. Change in health status in long-term conditions over a one year period: a cohort survey using patient-reported outcome measures. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/s12955-014-0123-2.
- Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail 2009;15:17-23. https://doi.org/10.1016/j.cardfail.2008.09.006.
- Wolfel EE. Effects of ACE inhibitor therapy on quality of life in patients with heart failure. Pharmacotherapy 1998;18:1323-34. https://doi.org/10.1002/j.1875-9114.1998.tb03155.x.
- Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002;40:111-8. https://doi.org/10.1016/S0735-1097(02)01932-0.
- Curiati JA, Bocchi E, Freire JO, Arantes AC, Braga M, Garcia Y, et al. Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005;11:465-72. https://doi.org/10.1089/acm.2005.11.465.
- Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJS, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD003331.pub5.
- MacIver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation 2012;126:866-74. https://doi.org/10.1161/CIRCULATIONAHA.111.040279.
- Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation 2007;115:1975-81. https://doi.org/10.1161/CIRCULATIONAHA.106.670901.
- Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail 2013;15:94-102. https://doi.org/10.1093/eurjhf/hfs148.
- Chamberlain AM, McNallan SM, Dunlay SM, Spertus JA, Redfield MM, Moser DK, et al. Physical health status measures predict all-cause mortality in patients with heart failure. Circ Heart Fail 2013;6:669-75. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000291.
- Di Giulio P. Network of Nurses of GISSI-HF . Should patients perception of health status be integrated in the prognostic assessment of heart failure patients? A prospective study. Qual Life Res 2014;23:49-56. https://doi.org/10.1007/s11136-013-0468-8.
- Wu JR, Lennie TA, Frazier SK, Moser DK. Health-related quality of life, functional status, and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs 2016;31:236-44. https://doi.org/10.1097/jcn.0000000000000248.
- Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol 2013;61:1259-67. https://doi.org/10.1016/j.jacc.2012.12.038.
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7-11. https://doi.org/10.15420/cfr.2016:25:2.
- Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol 2021;28:1682-90. https://doi.org/10.1093/eurjpc/zwaa147.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/Task Force Members . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;18:891-975. https://doi.org/10.1002/ejhf.592.
- British Heart Foundation . National Audit of Cardiac Rehabilitation (NACR) Quality and Outcomes Report 2020. www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2020 (accessed 1 August 2022).
- McMurray JJV, Stewart S. The burden of heart failure. Eur Heart J Suppl 2002;4:D50-8. https://doi.org/10.1016/S1520-765X(02)90160-4.
- National Institute for Cardiovascular Outcomes Research . National Heart Failure Audit 2020 21 Summary Report 2021.
- Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, et al. National Heart Failure Societies of the ESC member countries (see Appendix) . The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906-14. https://doi.org/10.1002/ejhf.2143.
- Dunlay SM, Roger VL, Killian JM, Weston SA, Schulte PJ, Subramaniam AV, et al. Advanced heart failure epidemiology and outcomes: a population-based study. JACC Heart Fail 2021;9:722-32. https://doi.org/10.1016/j.jchf.2021.05.009.
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421-8. https://doi.org/10.1053/euhj.1998.1280.
- Mant J, Al-Mohammad A, Swain S, Laramée P. Guideline Development Group . Management of chronic heart failure in adults: synopsis of the National Institute for Health and clinical excellence guideline. Ann Intern Med 2011;155:252-9. https://doi.org/10.7326/0003-4819-155-4-201108160-00009.
- Metra M, Ponikowski P, Dickstein K, McMurray JJV, Gavazzi A, Bergh C-H, et al. Heart Failure Association of the European Society of Cardiology . Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007;9:684-94. https://doi.org/10.1016/j.ejheart.2007.04.003.
- Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes 2011;4:68-75. https://doi.org/10.1161/circoutcomes.110.957225.
- Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord 2018;18. https://doi.org/10.1186/s12872-018-0815-3.
- National Health Service Blood and Transplant . Annual Report on Cardiothoracic Organ Transplantation 2021. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25266/nhsbt-annual-report-on-cardiothoracic-organ-transplantation-202021.pdf (accessed 28 August 2023).
- Molina EJ, Shah P, Kiernan MS, Cornwell WK, Copeland H, Takeda K, et al. The society of thoracic surgeons Intermacs 2020 annual report. Ann Thorac Surg 2021;111:778-92. https://doi.org/10.1016/j.athoracsur.2020.12.038.
- Medtronic Mechanical Circulatory Support . FDA Alerts Health Care Providers to Stop New Implants of Certain Ventricular Assist Device System 2021. www.fda.gov/news-events/press-announcements/fda-alerts-health-care-providers-stop-new-implants-certain-ventricular-assist-device-system (accessed 3 June 2021).
- Mehra MR, Crandall DL, Gustafsson F, Jorde UP, Katz JN, Netuka I, et al. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo‐controlled, non-inferiority ARIES HM3 trial. Eur J Heart Fail 2021;23:1226-37. https://doi.org/10.1002/ejhf.2275.
- Lim HS, Howell N, Ranasinghe A. The physiology of continuous-flow left ventricular assist devices. J Card Fail 2017;23:169-80. https://doi.org/10.1016/j.cardfail.2016.10.015.
- Saeed O, Colombo PC, Mehra MR, Uriel N, Goldstein DJ, Cleveland J, et al. Effect of aspirin dose on hemocompatibility-related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant 2020;39:518-25. https://doi.org/10.1016/j.healun.2020.03.001.
- Frankfurter C, Molinero M, Vishram-Nielsen JK, Foroutan F, Mak S, Rao V, et al. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation: a systematic review. Circ Heart Fail 2020;13. https://doi.org/10.1161/CIRCHEARTFAILURE.120.006994.
- Kiernan MS, Grandin EW, Brinkley M, Kapur NK, Pham DT, Ruthazer R, et al. Early right ventricular assist device use in patients undergoing continuous-flow left ventricular assist device implantation: incidence and risk factors from the Interagency Registry for Mechanically Assisted Circulatory Support. Circ Heart Fail 2017;10. https://doi.org/10.1161/circheartfailure.117.003863.
- Truby LK, Garan AR, Givens RC, Wayda B, Takeda K, Yuzefpolskaya M, et al. Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS registry. JACC Heart Fail 2018;6:951-60. https://doi.org/10.1016/j.jchf.2018.07.012.
- O’Horo JC, Saleh OMA, Stulak JM, Wilhelm MP, Baddour LM, Sohail MR. Left ventricular assist device infections: a systematic review. ASAIO J 2018;64. https://doi.org/10.1097/MAT.0000000000000684.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. https://doi.org/10.1136/bmj.n71.
- Sophie Beese DMPBMPSLDQJD . Clinical and cost effectiveness of left ventricular assist devices as destination therapy for advanced heart failure n.d.
- The EndNote Team . EndNote 2013.
- Veritas Health Innovation . Covidence Systematic Review Software n.d.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. https://doi.org/10.1016/s0895-4356(97)00049-8.
- Teuteberg JJ, Cleveland JC, Cowger J, Higgins RS, Goldstein DJ, Keebler M, et al. The Society of Thoracic Surgeons Intermacs 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg 2020;109:649-60. https://doi.org/10.1016/j.athoracsur.2019.12.005.
- NCT, Evaheart Inc . Prospective Multi-Center Randomized Study for Evaluating the EVAHEART®2 Left Ventricular Assist System 2010. https://classic.clinicaltrials.gov/ct2/show/NCT01187368 (accessed September 2020).
- Karason K, Lund LH, Dalen M, Bjorklund E, Grinnemo K, Braun O, et al. SweVAD Investigators . Randomized trial of a left ventricular assist device as destination therapy versus guideline-directed medical therapy in patients with advanced heart failure. Rationale and design of the SWEdish evaluation of left Ventricular Assist Device (SweVAD) trial. Eur J Heart Fail 2020;22:739-50. https://doi.org/10.1002/ejhf.1773.
- NCT . Swedish Evaluation of Left Ventricular Assist Device As Permanent Treatment in End-Stage Heart Failure 2015. https://clinicaltrials.gov/study/NCT02592499 (accessed May 2021).
- Grady KL, Andrei A, Pollan L, Shi H, Adam H, Kao A, et al. Sustaining quality of life of the aged: heart transplant or mechanical support (SUSTAIN-IT): baseline findings. J Heart Lung Transplant 2018;37. https://doi.org/10.1016/j.healun.2018.01.085.
- NCT, Hospices Civils de Lyon Y . LVAD Versus GDMT in Ambulatory Advanced Heart Failure Patients 2021.
- NCT, Jarvik Heart IY . Evaluation of the Jarvik 2000 Left Centricular Assist System With Post-Auricular Connector–Destination Therapy Study 2012.
- Consolo F, Sferrazza G, Motolone G, Contri R, Valerio L, Lembo R, et al. Platelet activation is a preoperative risk factor for the development of thromboembolic complications in patients with continuous-flow left ventricular assist device. Eur J Heart Fail 2018;20:792-800. https://doi.org/10.1002/ejhf.1113.
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group . Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. https://doi.org/10.1056/NEJMoa012175.
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. HeartMate II Investigators . Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. https://doi.org/10.1056/NEJMoa0909938.
- Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017;376:451-60. https://doi.org/10.1056/NEJMoa1602954.
- Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. ENDURANCE Investigators . HVAD: the ENDURANCE supplemental trial. JACC Heart Fail 2018;6:792-80. https://doi.org/10.1016/j.jchf.2018.05.012.
- Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev Technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol 2019;5. https://doi.org/10.1001/jamacardio.2019.5323.
- Acharya D, Loyaga-Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, et al. INTERMACS analysis of stroke during support with continuous-flow left ventricular assist devices: risk factors and outcomes. JACC Heart Fail 2017;5:703-11. https://doi.org/10.1016/j.jchf.2017.06.014.
- Aleksova N, Alba AC, Fan CPS, Amin F, Kiamanesh O, McGuinty C, et al. The effect of age on outcomes after destination-therapy left ventricular assist device implantation: an analysis of the IMACS registry. Can J Cardiol 2021;37:467-75. https://doi.org/10.1016/j.cjca.2020.06.010.
- Arnold SV, Jones PG, Allen LA, Cohen DJ, Fendler TJ, Holtz JE, et al. Frequency of poor outcome (death or poor quality of life) after left ventricular assist device for destination therapy: results from the INTERMACS registry. Circ Heart Fail 2016;9. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002800.
- Brinkley DM, DeNofrio D, Ruthazer R, Vest AR, Kapur NK, Couper GS, et al. Outcomes after continuous-flow left ventricular assist device implantation as destination therapy at transplant versus nontransplant centers. Circ Heart Fail 2018;11. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004384.
- Feltrin G, Pappalardo F, Gerosa G, Russo CF, Rinaldi M, Maccherini M, et al. Destination therapy in a single uropean country insights from the ITAMACS registry. J Heart Lung Transplant 2016;35:S149-50.
- Goldstein DJ, Meyns B, Xie R, Cowger J, Pettit S, Nakatani T, et al. Third annual report from the ISHLT Mechanically Assisted Circulatory Support registry: a comparison of centrifugal and axial continuous-flow left ventricular assist devices. J Heart Lung Transplant 2019;38:352-63. https://doi.org/10.1016/j.healun.2019.02.004.
- Grady KL, Naftel DC, Myers S, Dew MA, Weidner G, Spertus JA, et al. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: analyses from INTERMACS. J Heart Lung Transplant 2015;34:213-21. https://doi.org/10.1016/j.healun.2014.10.001.
- Kanwar MK, McIlvennan CK, Lohmueller LC, Bailey SH, Rogers JG, Teuteberg J, et al. Defining optimal outcomes in patients with left ventricular assist devices. ASAIO J 2021;67:397-404. https://doi.org/10.1097/MAT.0000000000001228.
- Kirklin JK, Cantor R, Mohacsi P, Gummert J, De By T, Hannan MM, et al. First annual IMACS report: a global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J Heart Lung Transplant 2016;35:407-12. https://doi.org/10.1016/j.healun.2016.01.002.
- Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011;30:115-23. https://doi.org/10.1016/j.healun.2010.12.001.
- Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, Miller M, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation?. J Thorac Cardiovasc Surg 2012;144:584-603; discussion 597. https://doi.org/10.1016/j.jtcvs.2012.05.044.
- Kirklin JK, Xie R, Cowger J, de By T, Nakatani T, Schueler S, et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2018;37:685-91. https://doi.org/10.1016/j.healun.2018.01.1294.
- Lala A, Rowland J, Gelijns A, Bagiella E, Moskowitz A, Ferket B, et al. Does indication for LVAD at time of implant matter in younger patients?. J Heart Lung Transplant 2019;38. https://doi.org/10.1016/j.healun.2019.01.292.
- Lala A, Rowland JC, Ferket BS, Gelijns AC, Bagiella E, Pinney SP, et al. Strategies of wait-listing for heart transplant vs durable mechanical circulatory support alone for patients with advanced heart failure. JAMA Cardiol 2020;5. https://doi.org/10.1001/jamacardio.2020.0631.
- Song HK, Gelow JM, Mudd J, Chien C, Tibayan FA, Hollifield K, et al. Limited utility of tricuspid valve repair at the time of left ventricular assist device implantation. Ann Thorac Surg 2016;101:2168-74. https://doi.org/10.1016/j.athoracsur.2016.03.040.
- Symalla T, Peev MP, Song T, Naftel D, Myers S, Koehl D, et al. STS INTERMACS database: the key to conduct single-arm trials in advanced heart failure patients. Ann Thorac Surg 2022;113:808-15. https://doi.org/10.1016/j.athoracsur.2021.04.045.
- White-Williams C, Fazeli P, Kirklin JK, Pamboukian SV, Grady KL. Health-related quality of life differs by pre-operative implant strategy from before through mid-term after surgery: findings from INTERMACS. J Heart Lung Transplant 2020;39. https://doi.org/10.1016/j.healun.2020.01.826.
- Kalampokas N, Erbel-Khurtsidze S, Sipahi F, Rellecke P, Tudorache I, Boeken U, et al. Periprocedural outcome after left ventricular assist device implantation in septuagenarians 2021;69.
- Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, et al. Pump thrombosis in the Thoratec HeartMate II device: an update analysis of the INTERMACS registry. J Heart Lung Transplant 2015;34:1515-26. https://doi.org/10.1016/j.healun.2015.10.024.
- Goldstein DJ, Naftel D, Holman W, Bellumkonda L, Pamboukian SV, Pagani FD, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 2012;31:1151-7. https://doi.org/10.1016/j.healun.2012.05.004.
- Jaiswal A, Myers L, Topkara VK, Colombo PC, Baran D, Le Jemtel TH. Clinical outcomes of adults with elevated body mass index with destination therapy: an analysis of INTERMACS registry. J Heart Lung Transplant 2017;36. https://doi.org/10.1016/j.healun.2017.01.478.
- Michelis KC, Zhong L, Peltz M, Pandey A, Tang WHW, Rohatgi A, et al. Dynamic forecasts of survival for patients living with destination left ventricular assist devices: insights from INTERMACS. J Am Heart Assoc 2020;9. https://doi.org/10.1161/JAHA.119.016203.
- Adlbrecht C, Hüelsmann M, Wurm R, Eskandary F, Neuhold S, Zimpfer D, et al. Outcome of conservative management vs. assist device implantation in patients with advanced refractory heart failure. Eur J Clin Invest 2016;46:34-41. https://doi.org/10.1111/eci.12562.
- Medressova A, Pya Y, Bekbossynov S, Andossova S, Dzhetybayeva S, Bekbossynova M, et al. Left ventricular assist devices as destination therapy. ASAIO J 2019;65.
- Galand V, Flecher E, Chabanne C, Lelong B, Goeminne C, Vincentelli A, et al. Septuagenarian population has similar survival and outcomes to younger patients after left ventricular assist device implantation. Arch Cardiovasc Dis 2020;113:701-9. https://doi.org/10.1016/j.acvd.2020.05.018.
- Janssen E, van Rein N, van der Meer F, Beeres S, Jukema J, Tops L. Absolute risk of death is lower in left ventricular assist device patients with good anticoagulation control. J Heart Lung Transplant 2021;40. https://doi.org/10.1016/j.healun.2021.01.1087.
- Akay MH, Nathan SS, Radovancevic R, Poglajen G, Jezovnik MK, Candelaria IN, et al. Obesity is associated with driveline infection of left ventricular assist devices. ASAIO J 2019;65:678-82. https://doi.org/10.1097/MAT.0000000000000916.
- Grady KL, Sherri W, Naftel DC, Myers S, Gelijins A, Moskowitz A, et al. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: findings from Interagency Registry for Mechanically Assisted Circulatory Support. J Heart Lung Transplant 2016;35:777-88. https://doi.org/10.1016/j.healun.2016.01.1222.
- The Society of Thoracic Surgeons . STS INTERMACS Database 2022. www.uab.edu/medicine/surgery/intermacs (accessed 1 May 2021).
- National Heart Lung and Blood Institute . Interagency Registry for Mechanically Assisted Circulatory Support: Manual of Operations and Procedures 2014.
- National Health Service England Specialised Commissioning . Evidence Review: Mechanical Assist Devices for Circulatory Support (Destination Therapy) in People With Advanced Heart Failure 2017.
- Neyt M, Leroy R, Devos C, Van Brabandt H. Health Technology Assessment (HTA) No. 264. Brussels: Belgian Health Care Knowledge Centre (KCE); 2016.
- Health Quality Ontario . Left ventricular assist devices for destination therapy: a Health Technology Assessment. Ont Health Technol Assess Ser 2016;16:1-60.
- Cavarretta E, Marullo AGM, Sciarretta S, Benedetto U, Greco E, Roever L, et al. A network meta-analysis of randomized trials and observational studies on left ventricular assist devices in adult patients with end-stage heart failure. Eur J Cardiothorac Surg 2019;55:461-7. https://doi.org/10.1093/ejcts/ezy285.
- Schmier JK, Patel JD, Leonhard MJ, Midha PA. A systematic review of cost-effectiveness analyses of left ventricular assist devices: issues and challenges. Appl Health Econ Health Policy 2019;17:35-46. https://doi.org/10.1007/s40258-018-0439-x.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; 2005.
- Centre for Reviews and Dissemination . Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care 2008.
- Booth A, Fry-Smith A. Developing the Research Question. MD: National Information Center on Health Services and Health Care Technology; 2003.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:iii-v. https://doi.org/10.3310/hta8360.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011.
- Droogne W, Jacobs S, Van den Bossche K, Verhoeven J, Bostic RR, Vanhaecke J, et al. Cost of 1-year left ventricular assist device destination therapy in chronic heart failure: a comparison with heart transplantation. Acta Clin Belg 2014;69:165-70. https://doi.org/10.1179/2295333714Y.0000000017.
- Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail 2017;5:110-9. https://doi.org/10.1016/j.jchf.2016.09.008.
- Neyt M, Van den Bruel A, Smit Y, De Jonge N, Erasmus M, Van Dijk D, et al. Cost-effectiveness of continuous-flow left ventricular assist devices. Int J Technol Assess Health Care 2013;29:254-60. https://doi.org/10.1017/S0266462313000238.
- Messori A, Trippoli S, Bonacchi M, Sani G. Left ventricular assist device as destination therapy: application of the payment-by-results approach for the device reimbursement. J Thorac Cardiovasc Surg 2009;138:480-5. https://doi.org/10.1016/j.jtcvs.2009.02.016.
- Adang E, Groenewoud H, van Hees F, van der Wilt G. Introduction of Artificial and Support Heart as Destination Therapy for Patients with End-stage Heart Failure. Nijmegen: Universitair Medisch Centrum St Radboud; 2006.
- Conseil d’évaluation des technologies de la santé du Q, McGregor M . Implantable Ventricular Assist Devices: Should They Be Used in Quebec: Report Submitted to the Minister of Research, Science and Technology of Québec 2003. https://cap.banq.qc.ca/notice?id=p::usmarcdef_0003016137.
- Chew DS, Manns B, Miller RJH, Sharma N, Exner DV. Economic evaluation of left ventricular assist devices for patients with end stage heart failure who are ineligible for cardiac transplantation. Can J Cardiol 2017;33:1283-91. https://doi.org/10.1016/j.cjca.2017.07.012.
- Chimanji N, Kilic A, Hasan A, Higgins RS, Whitson BA, Kilic A. Institutional cost comparison between heart transplants and left ventricular assist device implantations. Exp Clin Transplant 2016;14:656-9. https://doi.org/10.6002/ect.2015.0213.
- Clegg AJ, Scott DA, Loveman E, Colquitt J, Royle P, Bryant J. Clinical and cost-effectiveness of left ventricular assist devices as destination therapy for people with end-stage heart failure: a systematic review and economic evaluation. Int J Technol Assess Health Care 2007;23:261-8. https://doi.org/10.1017/S0266462307070353.
- Girling AJ, Freeman G, Gordon JP, Poole-Wilson P, Scott DA, Lilford RJ. Modeling payback from research into the efficacy of left-ventricular assist devices as destination therapy. Int J Technol Assess Health Care 2007;23:269-77.
- Lim HS, Shaw S, Carter AW, Jayawardana S, Mossialos E, Mehra MR. A clinical and cost-effectiveness analysis of the HeartMate 3 left ventricular assist device for transplant-ineligible patients: a United Kingdom perspective. J Heart Lung Transplant 2021;41:174-86. https://doi.org/10.1016/j.healun.2021.11.014.
- Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail 2014;7:470-8. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000807.
- Mehra MR, Salerno C, Cleveland JC, Pinney S, Yuzefpolskaya M, Milano CA, et al. Healthcare resource use and cost implications in the MOMENTUM 3 long-term outcome study. Circulation 2018;138:1923-34. https://doi.org/10.1161/CIRCULATIONAHA.118.035722.
- Oz MC, Gelijns AC, Miller L, Wang C, Nickens P, Arons R, et al. Left ventricular assist devices as permanent heart failure therapy: the price of progress. Ann Surg 2003;238:577-83; discussion 583. https://doi.org/10.1097/01.sla.0000090447.73384.ad.
- Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail 2012;5:10-6. https://doi.org/10.1161/CIRCHEARTFAILURE.111.962951.
- Silvestry S, Mahr C, Slaughter M, Levy W, Cheng R, May D, et al. Cost-effectiveness of a small intrapericardial centrifugal LVAD versus medical management and heart transplantation. J Heart Lung Transplant 2019;38.
- Schueler S, Silvestry SC, Cotts WG, Slaughter MS, Levy WC, Cheng RK, et al. Cost-effectiveness of left ventricular assist devices as destination therapy in the United Kingdom. ESC Heart Fail 2021;8:3049-57. https://doi.org/10.1002/ehf2.13401.
- Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg 2011;26:535-41. https://doi.org/10.1111/j.1540-8191.2011.01292.x.
- Moskowitz AJ, Weinberg AA, Oz MC, Williams DL. Quality of life with an implanted left ventricular assist device. Ann Thorac Surg 1997;64:1764-9. https://doi.org/10.1016/S0003-4975(97)01000-X.
- Sharples LD, Dyer M, Cafferty F, Demiris N, Freeman C, Banner NR, et al. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). J Heart Lung Transplant 2006;25:1336-43. https://doi.org/10.1016/j.healun.2006.09.011.
- Clegg AJ, Scott DA, Loveman E, Colquitt JL, Royle P, Bryant J. Clinical and cost-effectiveness of left ventricular assist devices as a bridge to heart transplantation for people with end-stage heart failure: a systematic review and economic evaluation. Eur Heart J 2007;27:2929-38. https://doi.org/10.1093/eurheartj/ehi857.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR Health Economic Evaluation Publication Guidelines – CHEERS Good Reporting Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual 2022. www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed February 2022).
- Briggs AH, Sculpher MJ, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Office for National Statistics . National Life Tables – Life Expectancy in the UK: 2018 to 2020 2021. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2018to2020#:~:text=Across%20the%20UK%2C%20life%20expectancy,years%20for%20females%20in%20Northern (accessed May 2021).
- Flack S, Taylor M, Trueman P. Cost-effectiveness of Interventions for Smoking Cessation. York: York Health Economics Consortium; 2008.
- Mehra MR, Cleveland JC, Uriel N, Cowger JA, Hall S, Horstmanshof D, et al. MOMENTUM 3 Investigators . Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: a study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail 2021;23:1392-400. https://doi.org/10.1002/ejhf.2211.
- Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde-McLean RC, DeVore AD, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant 2019;38:408-17. https://doi.org/10.1016/j.healun.2018.09.021.
- Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin Scale and its use in future stroke trials. Stroke 2017;48:2007-12. https://doi.org/10.1161/strokeaha.117.017866.
- Kirklin JK, Naftel DC, Myers SL, Pagani FD, Colombo PC. Quantifying the impact from stroke during support with continuous flow ventricular assist devices: an STS INTERMACS analysis. J Heart Lung Transplant 2020;39:782-94. https://doi.org/10.1016/j.healun.2020.04.006.
- Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:S52-9. https://doi.org/10.3978/j.issn.2072-1439.2013.10.26.
- Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et al. HeartMate II Clinical Investigators . Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. https://doi.org/10.1016/j.jtcvs.2009.11.020.
- Baumwol J, Macdonald PS, Keogh AM, Kotlyar E, Spratt P, Jansz P, et al. Right heart failure and “failure to thrive” after left ventricular assist device: clinical predictors and outcomes. J Heart Lung Transplant 2011;30:888-95. https://doi.org/10.1016/j.healun.2011.03.006.
- Hatano M, Jimba T, Fujiwara T, Tsuji M, Bujo C, Ishida J, et al. Late-onset right ventricular failure after continuous-flow left ventricular assist device implantation: case presentation and review of the literature. J Cardiol 2022;80:110-5. https://doi.org/10.1016/j.jjcc.2021.12.009.
- Lo Coco V, De Piero ME, Massimi G, Chiarini G, Raffa GM, Kowalewski M, et al. Right ventricular failure after left ventricular assist device implantation: a review of the literature. J Thorac Dis 2021;13:1256-69. https://doi.org/10.21037/jtd-20-2228.
- Chaisinanunkul N, Adeoye O, Lewis RJ, Grotta JC, Broderick J, Jovin TG, et al. DAWN Trial and MOST Trial Steering Committees . Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utility-weighted modified Rankin Scale. Stroke 2015;46:2238-43. https://doi.org/10.1161/strokeaha.114.008547.
- Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11. https://doi.org/10.7326/0003-4819-154-1-201101040-00289.
- Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. https://doi.org/10.1016/j.healun.2017.07.005.
- Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, et al. Sudden Cardiac Death in Heart Failure Trial Investigators . Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med 2008;359:999-1008. https://doi.org/10.1056/NEJMoa0706719.
- McNamara N, Narroway H, Williams M, Brookes J, Farag J, Cistulli D, et al. Contemporary outcomes of continuous-flow left ventricular assist devices – a systematic review. Ann Cardiothorac Surg 2021;10:186-208. https://doi.org/10.21037/acs-2021-cfmcs-35.
- Baio G, Dawid AP. Probabilistic sensitivity analysis in health economics. Stat Methods Med Res 2015;24:615-34. https://doi.org/10.1177/0962280211419832.
- Ren S, Minton J, Whyte S, Latimer NR, Stevenson M. A new approach for sampling ordered parameters in probabilistic sensitivity analysis. Pharmacoeconomics 2018;36:341-7. https://doi.org/10.1007/s40273-017-0584-3.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Koffijberg H, Rothery C, Chalkidou K, Grutters J. Value of information choices that influence estimates: a systematic review of prevailing considerations. Med Decis Making 2018;38:888-900. https://doi.org/10.1177/0272989x18797948.
- Dillon A. Carrying NICE Over the Threshold. London: National Institute for Health and Care Excellence; 2015.
- Grady KL, Naftel D, Stevenson L, Dew MA, Weidner G, Pagani FD, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant 2014;33:412-21. https://doi.org/10.1016/j.healun.2013.10.017.
- Skedgel C, Henderson N, Towse A, Mott D, Green C. Considering severity in Health Technology Assessment: can we do better?. Value Health 2022;25:1399-403. https://doi.org/10.1016/j.jval.2022.02.004.
- Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, Colombo PC, et al. MOMENTUM 3 Investigators . A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440-50. https://doi.org/10.1056/NEJMoa1610426.
- Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno C, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA 2022;328:1233-42. https://doi.org/10.1001/jama.2022.16197.
- Karason K, Dellgren G, Isaksson E, Lidén H, Liljegren A, Redfors B, et al. HTA-centrum: Västra Götalandsregionen; 2014.
- NHS Blood and Transplant . Annual Report on Ventricular Assist Devices 2017.
- National Institute for Cardiovascular Outcomes Research . About NICOR 2019. www.nicor.org.uk/about-nicor/ (accessed November 2021).
- National Institute for Cardiovascular Outcomes Research . The Myocardial Ischaemia National Audit Project (MINAP) 2019. https://www.nicor.org.uk/national-cardiac-audit-programme/heart-attack-audit-minap (accessed November 2021).
- European Registry for Patients with Mechanical Circulatory Support . About EUROMACS 2019. www.euromacs.org/about (accessed November 2021).
- University of Alabama at Birmingham . The STS Intermacs Database 2018. www.uab.edu/medicine/surgery/intermacs (accessed November 2021).
- The International Society for Heart and Lung Transplantation . The International Society for Heart and Lung Transplantation Registry for Mechanically Assisted Circulatory Support (IMACS) 2019. https://ishlt.org/research-data/registries/international-registry-for-mechanically-assisted-c (accessed November 2021).
- Clarke A, Pulikottil-Jacob R, Connock M, Suri G, Kandala NB, Maheswaran H, et al. Cost-effectiveness of left ventricular assist devices (LVADs) for patients with advanced heart failure: analysis of the British NHS bridge to transplant (BTT) program. Int J Cardiol 2014;171:338-45. https://doi.org/10.1016/j.ijcard.2013.12.015.
- Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the Multicenter Study of MagLev Technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol 2020;5:411-9. https://doi.org/10.1001/jamacardio.2019.5323.
- Jalowiec A, Grady KL, White-Williams C. Predictors of rehospitalization time during the first year after heart transplant. Heart Lung 2008;37:344-55. https://doi.org/10.1016/j.hrtlng.2007.10.007.
- Starling RC, Estep JD, Horstmanshof DA, Milano CA, Stehlik J, Shah KB, et al. ROADMAP Study Investigators. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2-year results. JACC Heart Fail 2017;5:518-27. https://doi.org/10.1016/j.jchf.2017.02.016.
- Tattevin P, Flécher E, Auffret V, Leclercq C, Boulé S, Vincentelli A, et al. Risk factors and prognostic impact of left ventricular assist device-associated infections. Am Heart J 2019;214:69-76. https://doi.org/10.1016/j.ahj.2019.04.021.
- Luengo-Fernandez R, Walli-Attaei M, Gray A, Torbica A, Maggioni AP, Huculeci R, et al. Economic burden of cardiovascular diseases in the European Union: a population-based cost study. Eur Heart J 2023;44:4752-67. https://doi.org/10.1093/eurheartj/ehad583.
- Jentzer JC, Schrage B, Holmes DR, Dabboura S, Anavekar NS, Kirchhof P, et al. Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2021;10:604-12. https://doi.org/10.1093/ehjacc/zuaa035.
Appendix 1 An exploration of the data sets to provide further data relevant to left ventricular assist device as destination therapy
Several registries contain data on patients receiving mechanical circulatory support. Information on their contents and access arrangements were explored to determine their relevance and usefulness in providing additional information to this report.
The NHS Blood and Transplant service collects data in the context of LVADs as bridge to transplant only. 150
The National Institute for Cardiovascular Outcomes Research (NICOR) and the Myocardial Ischemia National Audit Project do not routinely collect data on LVADs (personal communication with NICOR). 151,152
The EUROMACS contains Europe-wide data including that on LVADs. 153 It does not contain much UK data at this time. Submission of patient data is not mandatory, and the extent of any long-term data is unclear.
There have been a number of publications reporting data and analyses from INTERMACS. 154 Some of these relate to DT. Relevant publications/data have been used in this report.
There have also been several publications reporting data and analyses from the IMACS, some of which contain DT data. 155 Relevant publications that reported DT-specific data have been used in this report.
Appendix 2 Search strategies
MEDLINE search strategy
Database: Ovid MEDLINE(R)
Search strategy:
------------ ------------- -------------- ------------- ---------- -----------
-
(left adj4 ventric* adj4 assist*).ti,ab.
-
Assisted Circulation/
-
(Assis* adj4 circulat*).ti,ab.
-
(heartware hvad or heartware vad or heartmate or ventracor ventrassist or jarvik or flowmaker or micromed debakey or debakey vad or reliantheart or heartassist or berlin incor or terumo duraheart or evaheart).ti,ab.
-
(LVAD or LVAS or HVAD or VAD).ti,ab.
-
Heart-Assist Devices/
-
(continuous-flow adj3 device?).ti,ab.
-
circulatory support device?.ti,ab.
-
(Heart* adj4 assist* adj4 (device* or system* or pump* or treat* or therap* or surg*)).ti,ab.
-
(axial-flow adj3 device?).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
-
exp Heart Failure/
-
Shock, Cardiogenic/
-
(cardiogenic* adj3 shock*).ti,ab.
-
Cardiomyopathies/
-
(cardiomyopath* or myocardit*).ti,ab.
-
exp Ventricular Dysfunction/
-
(ventricul* adj4 dysfunct*).ti,ab.
-
Myocarditis/
-
myocardit*.ti,ab.
-
((end-stage or endstage* or end stage* or advance* or acute) adj4 heart* adj4 failur*).ti,ab.
-
heart failure*.ti,ab.
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
-
((Destinat* or permanent*) adj4 (therap* or treat* or surg*)).ti,ab.
-
DT.ab,ti.
-
((long-term or longest-term) and (LVAD or HVAD or LVAS or VAD or treat? or device?)).ti,ab.
-
((ineligible or ‘not eligible’ or ‘not candidate$1’ or non-candidate$1) adj4 transplant$).ti,ab.
-
BTT.ti,ab.
-
BTC.ti,ab.
-
(bridge adj3 (decision or transplant* or recover* or candidacy)).ti,ab.
-
or 25 or 26 or 27 or 28 or 29 or 30
-
11 and 23 and 31
-
animals/ not humans/
-
32 not 33
***************************
EMBASE search strategy
Database: EMBASE
Search strategy:
------------- ---------------- -------------- ------------ ------------- ------------
-
(left adj4 ventric* adj4 assist*).ti,ab.
-
assisted circulation/
-
(Assis* adj4 circulat*).ti,ab.
-
(heartware hvad or heartware vad or heartmate or ventracor ventrassist or jarvik or flowmaker or micromed debakey or debakey vad or reliantheart or heartassist or berlin incor or terumo duraheart or evaheart).ti,ab.
-
(LVAD or LVAS or HVAD or VAD).ti,ab.
-
heart assist device/
-
(continuous-flow adj3 device?).ti,ab.
-
circulatory support device?.ti,ab.
-
(Heart* adj4 assist* adj4 (device* or system* or pump* or treat* or therap* or surg*)).ti,ab.
-
(axial-flow adj3 device?).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
-
exp heart failure/
-
cardiogenic shock/
-
(cardiogenic* adj3 shock*).ti,ab.
-
cardiomyopathy/
-
(cardiomyopath* or myocardit*).ti,ab.
-
exp heart ventricle function/
-
(ventricul* adj4 dysfunct*).ti,ab.
-
myocarditis/
-
myocardit*.ti,ab.
-
((end-stage or endstage* or end stage* or advance* or acute) adj4 heart* adj4 failur*).ti,ab.
-
heart failure*.ti,ab.
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
-
((Destinat* or permanent*) adj4 (therap* or treat* or surg*)).ti,ab.
-
DT.ab,ti.
-
((long-term or longest-term) and (LVAD or HVAD or LVAS or VAD or treat? or device?)).ti,ab.
-
((ineligible or ‘not eligible’ or ‘not candidate$1’ or non-candidate$1) adj4 transplant$).ti,ab.
-
BTT.ti,ab.
-
BTC.ti,ab.
-
(bridge adj3 (decision or transplant* or recover* or candidacy)).ti,ab.
-
24 or 25 or 26 or 27 or 28 or 29 or 30
-
11 and 23 and 31
-
exp animal/ not exp human/
-
32 not 33
***************************
Cochrane CENTRAL search strategy:
ID Search hits
-
(left NEAR/4 ventric* NEAR/4 assist*):ti,ab
-
MeSH descriptor: [Assisted Circulation] explode all trees
-
(Assis* NEAR/4 circulat*):ti,ab
-
(heartware hvad or heartware vad or heartmate or ventracor ventrassist or jarvik or flowmaker or micromed debakey or debakey vad or reliantheart or heartassist or berlin incor or terumo duraheart or evaheart):ti,ab
-
(LVAD or LVAS or HVAD or VAD):ti,ab
-
MeSH descriptor: [Heart-Assist Devices] explode all trees
-
(continuous-flow NEAR/3 device?):ti,ab
-
‘circulatory support device?’:ti,ab
-
(Heart* NEAR/4 assist* NEAR/4 (device* or system* or pump* or treat* or therap* or surg*)):ti,ab
-
(axial-flow NEAR/3 device?):ti,ab
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
-
MeSH descriptor: [Heart Failure] explode all trees
-
MeSH descriptor: [Shock, Cardiogenic] this term only
-
(cardiogenic* NEAR/3 shock*):ti,ab
-
MeSH descriptor: [Cardiomyopathies] this term only
-
(cardiomyopath* or myocardit*):ti,ab
-
MeSH descriptor: [Ventricular Dysfunction] explode all trees
-
(ventricul* NEAR/4 dysfunct*):ti,ab
-
MeSH descriptor: [Myocarditis] this term only
-
myocardit*:ti,ab
-
((end-stage or endstage* or end stage* or advance* or acute) NEAR/4 heart* NEAR/4 failur*):ti,ab
-
‘heart failure*’:ti,ab
-
#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
-
((Destinat* or permanent*) NEAR/4 (therap* or treat* or surg*)):ti,ab
-
DT:ti,ab
-
((long-term or longest-term) and (LVAD or HVAD or LVAS or VAD or treat? or device?)):ti,ab
-
((ineligible or ‘not eligible’ or ‘not candidate$1’ or non-candidate$1) NEAR/4 transplant$):ti,ab
-
BTT:ti,ab
-
BTC:ti,ab
-
(bridge NEAR/3 (decision or transplant* or recover* or candidacy)):ti,ab
-
#24 or #25 or #26 or #27 or #28 or #29 or #30
-
#11 and #23 and #31
EconLit search strategy:
TX left ventricular assist device OR TX LVAD OR TX assisted circulation OR TX ((LVAS or HVAD or VAD)) OR TX heart assist device OR TX ((heartware hvad or heatware vad or heartmate or ventracor ventrassist or jarvik or flowmaker or micromed debakey vad or reliantheart or heartassist or berlin incur or terumo duraheart or evaheart)) OR TX continuous flow device OR TX axial flow device OR TX continuous-flow LVAD
NHSEED search strategy:
-
MeSH DESCRIPTOR Heart-Assist Devices EXPLODE ALL TRESS IN NHSEED
-
((lvad or hvad or lvas or vad)) OR ((left ventric* assist device* or heart assist device* or continuous flow device or axial flow device)) OR ((heartware hvad or heartware vad or heartmate or ventracor ventrassist or jarvik or flowmaker or micromed debakey or debakey vad or reliantheart or heartassist or berlin incur or terumo duraheart or evaheart)) IN NHSEED
-
#1 OR #2
Appendix 3 Excluded studies
| Study ID | Reason for exclusion |
|---|---|
| Abdalla S, Kaan A, Nazzari H, Ignaszewski A, Virani S, Toma M. Risk factors of neurological events in patients supported with continuous flow LVADs. J Heart Lung Transplant 2020;39(4 Suppl.):S398. | Do not report DT data |
| Abdeen MS, Albert A, Maxhera B, Hoffmann T, Petrov G, Sixt S, et al. Implanting permanent left ventricular assist devices in patients on veno-arterial extracorporeal membrane oxygenation support: do we really need a cardiopulmonary bypass machine? Eur J Cardio-Thorac Surg 2016;50:542–7. | < 50 DT patients |
| Ada Ip A, Roldan J, Moss N. Differences between ischemic and non-ischemic cardiomyopathy and its relationship with long-term outcomes following ventricular assist device placement. Eur J Heart Fail 2019;21(Suppl. 1):83. | Do not report DT data |
| Adachi I, Burki S, Horne D, Jeewa A, Elias B, McKenzie E, et al. Continuous flow VAD support at a tertiary pediatric center: compared to pedimacs data. J Heart Lung Transplant 2017;36(4 Suppl. 1):S280–1. | Paediatric population |
| Adachi I. Pediatric ventricular assist device support as a permanent therapy: clinical reality. J Thorac Cardiovasc Surg 2019;158:1438–41. | Paediatric population |
| Adamo L, Tang Y, Nassif ME, Novak E, Jones PG, LaRue S, et al. The HeartMate risk score identifies patients with similar mortality risk across all INTERMACS profiles in a large multicenter analysis. JACC Heart Fail 2016;4:950–8. | Do not report DT data |
| Adamson RM, Bower BL, Sundareswaran KS, Farrar DJ, Dembitsky WP. Radiologic assessment of HeartMate II position: minimal pump migration after long-term support. J Heart Lung Transplant 2015;34:1617–23. | Do not report DT data |
| Adamson RM, Dembitsky WP, Reichman RT, Moreno-Cabral RJ, Daily PO. Mechanical support: assist or nemesis? J Thorac Cardiovasc Surg 1989;98:915–20; discussion 920. | < 50 DT patients |
| Adamson RM, Stahovich M, Chillcott S, Baradarian S, Chammas J, Jaski B, et al. Clinical strategies and outcomes in advanced heart failure patients older than 70 years of age receiving the HeartMate II left ventricular assist device: a community hospital experience. J Am Coll Cardiol 2011;57:2487–95. | Do not report DT data |
| Adatya S, Egnaczyk G, Katz JN, Brieke A, Stulak J, Nathan S, et al. The effect of pre-existing hypercoagulable disorders on outcomes in patients with LVADS. J Heart Lung Transplant 2017;36(4 Suppl. 1):S11. | Do not report DT data |
| Adesiyun TA, McLean RC, Tedford RJ, Whitman GJR, Sciortino CM, Conte JV, et al. Long-term follow-up of continuous flow left ventricular assist devices: complications and predisposing risk factors. Int J Artif Organs 2017;40:622–8. | Do not report DT data |
| Adlbrecht C, Hulsmann M, Wurm R, Eskandary F, Neuhold S, Zuckermann A, et al. Outcome of conservative management vs. assist device implantation in patients with advanced refractory heart failure. Eur J Clin Invest 2016;46:34–41. | Duplicate record |
| Adnan Yousaf A, Mihiyaddin S, Aldweik M, Ashraf S. Prolonged use of Levitronix-right ventricular assist device (RVAD) in patients with long term left ventricular assist device (LVAD). Eur J Heart Fail 2018;20(Suppl. 1):87. | Do not report DT data |
| Afzal A, Nisar T, Jamil A, Kluger A, Felius J, Gong T, et al. Impact of renal dysfunction on patients undergoing left ventricular assist device implantation. J Card Fail 2019;25(8 Suppl.):S147. | Do not report DT data |
| Agrawal S, Garg L, Nanda S, Sharma A, Bhatia N, Manda Y, et al. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices – a meta-analysis. Int J Cardiol 2016;222:379–84. | Do not report DT data |
| Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 2016;67:291–9. | < 50 DT patients |
| Ahmed N, Gandhi H, Kim Y, Saeed O, Patel S, Murthy S, et al. Neutrophil to lymphocyte ratio at the time of LVAD implant predicts 30-day readmission. J Heart Lung Transplant 2018;37(4 Suppl. 1):S319. | Do not report DT data |
| Akdemir B, Jedeon Z, Cogswell R, Schultz J, Wald LV, John R, et al. Atrial fibrillation and mortality in patients with LVAD: a single center cohort. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2019;140. | Do not report DT data |
| Akin S, Muslem R, Constantinescu AA, Manintveld OC, Birim O, Brugts JJ, et al. 18F-FDG PET/CT in the diagnosis and management of continuous flow left ventricular assist device infections: a case series and review of the literature. ASAIO J 2018;64:e11–9. | < 50 DT patients |
| Akiyama M, Kawatsu S, Yoshioka I, Adachi O, Kumagai K, Saiki Y. Comparison of renal function after implantation of continuous-flow and pulsatile left ventricular assist devices. J Card Fail 2017;23(10 Suppl. 1):S35–6. | Do not report DT data |
| Alba AC, McDonald M, Rao V, Ross HJ, Delgado DH. The effect of ventricular assist devices on long-term post-transplant outcomes: a systematic review of observational studies. Eur J Heart Fail 2011;13:785–95. | Do not report DT data |
| Aleksova N, Alba A, Fan CS, Amin F, Kiamanesh O, McGuinty C, et al. The effect of age on outcomes following destination therapy left ventricular assist device implantation: an analysis of the IMACS registry. J Heart Lung Transplant 2020;39(4 Suppl.):S37. | Do not report DT data |
| Alemany HS, Unlu O, Pabon M, Sobol I, Krishnan U, Goyal P, et al. Impact of intra-operative transfusions on post left ventricular assist device placement outcomes: a single center study. J Am Coll Cardiol 2020;75(11):980. | Do not report DT data |
| Alsara O, Reeves RK, Pyfferoen MD, Trenary TL, Engen DJ, Vitse ML, et al. Inpatient rehabilitation outcomes for patients receiving left ventricular assist device. Am J Phys Med Rehabil 2014;93:860–8. | Do not report DT data |
| Al-Sarie M, Rauf A, Kfoury AG, Catino A, Wever-Pinzon J, Bonios M, et al. Myocardial structural and functional response after long-term mechanical unloading with continuous flow left ventricular assist device: axial versus centrifugal flow. JACC Heart Fail 2016;4:570–6. | < 50 DT patients |
| Al-Sarie M, Rauf A, Wever-Pinzon J, Catino A, Stehlik J, Kfouri A, et al. Myocardial and end-organ response after long-term mechanical unloading with continuous-flow left ventricular assist device: axial-versus centrifugal-flow. J Heart Lung Transplant 2016;(1):S330–1. | Do not report DT data |
| Alvarez J, Duero Posada J, Moayedi Y, Alhussein M, Runeckles K, Ross H, et al. Clinical differences between contemporary continuous flow left ventricular assist devices: a single center comparison between heartware, heartmate II and heartmate 3. Can J Cardiol 2017;33(10 Suppl. 1):S70–1. | Do not report DT data |
| Amione-Guerra J, Bhimaraj A, Ashrith G, Bruckner B, Suarez EE, Park MH, et al. Implantation of continuous flow-left ventricular assist devices (CF-LVAD) in the extremely obese (BMI ≥ 40 kg/m2): a single center experience. J Heart Lung Transplant 2016;35(Suppl.):S374. | Do not report DT data |
| Amione-Guerra J, Cordero-Reyes AM, Bhimaraj A, Trachtenberg BH, Torre-Amione G, Park MH, et al. Elevated transpulmonary gradient is a predictor of survival in patients with WHO Group II pulmonary hypertension treated with continuous-flow left ventricular assist devices (CF-LVAD). J Heart Lung Transplant 2016;35(Suppl.):S163–4. | Do not report DT data |
| Anderson RD, Lee G, Virk S, Bennett RG, Hayward CS, Muthiah K, et al. Catheter ablation of ventricular tachycardia in patients with a ventricular assist device: a systematic review of procedural characteristics and outcomes. JACC Clin Electrophysiol 2019;5:39–51. | < 50 DT patients |
| Andrews M, Wesner S, Watkins R, Katz JN. No distance is too great: a patient’s commute to their implantation center is not associated with worse outcomes following placement of a left ventricular assist device. J Heart Lung Transplant 2016;35(Suppl.):S377. | Do not report DT data |
| Angermayr L, Velasco Garrido M, Busse R. Ventricular assist devices for heart failure. GMS Health Technol Assess 2007;3:Doc10. | Wrong study design |
| Ankersmit HJ, Tugulea S, Spanier T, Weinberg AD, Artrip JH, Burke EM, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet 1999;354:550–5. | < 50 DT patients |
| Anonymous. Corrections: short- and long-term outcomes of continuous-flow left ventricular assist device therapy in 79 patients with end-stage heart failure. Pol Arch Intern Med 2020;130:926–7. | Wrong patient population |
| Anonymous. Special report: cost-effectiveness of left-ventricular assist devices as destination therapy for end-stage heart failure. Technol Eval Cent Assess Program Exec Summ 2004;19:1. | Duplicate record |
| Anonymous. Special report: left ventricular assist devices as destination therapy for end-stage heart failure–cost-effectiveness analysis. TEC Bulletin [Electronic Resource] 2003;20:33–4. | Duplicate record |
| Ansari M, Garcia D. Intra-aortic balloon pump and peripheral LVAD for treatment of cardiogenic shock. Catheter Cardiovasc Interv 2017;89(Suppl. 2):S46. | Do not report DT data |
| Anselmi A, Galand V, Vincentelli A, Boule S, Dambrin C, Delmas C, et al. Current results of left ventricular assist device therapy in France: the ASSIST-ICD registry. Eur J Cardio Thorac Surg 2020;16. | Do not report DT data |
| Anselmi A, Galand V, Vincentelli A, Boule S, Dambrin C, Delmas C, et al. Current results of left ventricular assist device therapy in France: The ASSIST-ICD registry. Eur J Cardio-Thorac Surg 2020;58:112–20. | Do not report DT data |
| Anwer LA, Tchantchaleishvili V, Poddi S, Daly RC, Joyce LD, Kushwaha SS, et al. Atrial fibrillation should guide prophylactic tricuspid procedures during left ventricular assist device implantation. ASAIO J 2018;64:586–93. | Do not report DT data |
| Arabia FA, Smith RG, Jaffe C, Wild JC, Rose DS, Nelson RJ, et al. Cost analysis of the Novacor Left Ventricular Assist System as an outpatient bridge to heart transplantation. ASAIO J 1996;42:M546–9. | Wrong patient population |
| Araujo-Gutierrez R, Potter LM, Teigen L, Schultz J, Estep JD, John R, et al. Pre-operative pectoralis muscle quantity and attenuation by computed tomography are predictive of recurrent gastrointestinal bleeding on left ventricular assist device support: a multicenter analysis. J Heart Lung Transplant 2020;39(4 Suppl.):S396–7. | Do not report DT data |
| Asleh R, Schettle SS, Khan FW, Kushwaha SS. Left ventricular assist devices as destination therapy in stage D heart failure. J Geriatr Cardiol 2019;16:592–600. | Wrong study design |
| Asuka E, Pak S, Thiess AK, Torres A. Gastrointestinal bleeding as a complication in continuous flow ventricular assist devices: a systematic review with meta-analysis. J Clin Med Res 2020;12:543–59. | Do not report DT data |
| Atluri P, Fairman AS, MacArthur JW, Goldstone AB, Cohen JE, Howard JL, et al. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg 2013;28:770–5. | Do not report DT data |
| Atluri P, Goldstone AB, Kobrin DM, Cohen JE, MacArthur JW, Howard JL, et al. Ventricular assist device implant in the elderly is associated with increased, but respectable risk: a multi-institutional study. Ann Thorac Surg 2013;96:141–7. | Do not report DT data |
| Aurora L, Ahluwalia G, Mahan M, Williams CT. Impact of social determinants on outcomes in patients with left ventricular assist devices. J Card Fail 2019;25(8 Suppl.):S127. | Do not report DT data |
| Aurora L, Sadiq O, Nemeh H, Williams C. Left ventricular assist devices complicated by gastrointestinal bleeding and outcomes on transplant. Am J Transplant 2018;18(Suppl. 4):651. | Do not report DT data |
| Auvil B, Chung J, Ameer A, Han J, Helmers M, Birati E, et al. Asymptomatic moderate aortic insufficiency with a left ventricular assist device portends a worse long-term survival. ASAIO J 2018;64(Suppl. 1):63. | Do not report DT data |
| Avancena AL, Peng DM, Lee J, Si M, Schumacher KR, Hutton DW. Cost-effectiveness of immediate ventricular assist device implantation in children with inotrope-dependent heart failure. J Heart Lung Transplant 2020;39(4 Suppl.):S87. | Paediatric population |
| Avramovic N, Dell’Aquila AM, Weckesser M, Milankovic D, Vrachimis A, Sindermann JR, et al. Metabolic volume performs better than SUVmax in the detection of left ventricular assist device driveline infection. Eur J Nucl Med Mol Imaging 2017;44:1870–7. | < 50 DT patients |
| Axelrad JE, Pinsino A, Trinh P, Colombo P, Yuzefpolskaya M, Gonda T. Endoscopic evaluation in patients with CF-LVADS and gastrointestinal bleeding: are we ready Q for a paradigm shift to improve care? Am J Gastroenterol 2017;112(Suppl. 1):S318. | Do not report DT data |
| Ayers BC, Wood K, Lee E, Bruckel J, Ling F, Kutyifa V, et al. PROMISing new tool correlates well with Kansas City cardiomyopathy questionnaire in left ventricular assist device patients. J Heart Lung Transplant 2019;38(4 Suppl.):S438. | Do not report DT data |
| Aymami M, Donal E, Guihaire J, Le Helloco A, Federspiel M, Galli E, et al. Rest and exercise adaptation of the right ventricular function in long-term left ventricular assist device patients: a prospective, pilot study. J Card Fail 2016;22:240–1. | Do not report DT data |
| Aymami M, Haddad F, Amsallem M, Marques M, Sallam K, Wheeler M, et al. External validation of right heart failure risk scores following LVAD implantation and evaluation of emerging echocardiographic indices. Arch Cardiovasc Dis Suppl 2017;9:46. | Do not report DT data |
| Aymami M, Haddad F, Wheeler M, Amsallem M, Marques M, Adams J, et al. External validation of right heart failure risk scores following left ventricular assist device implantation and evaluation of the role of emerging echocardiographic indices. Circulation Conference: American Heart Association’s. 2016;134. | Do not report DT data |
| Baffy NJ, Horsley-Silva JL, Ramirez FC. Endoscopic management and outcomes of gastrointestinal bleeding in patients with left ventricular assist device (LVAD). Gastrointest Endosc 2018;87(6 Suppl. 1):AB422. | Do not report DT data |
| Baker WL, Radojevic J, Gluck JA. Systematic review of phosphodiesterase-5 inhibitor use in right ventricular failure following left ventricular assist device implantation. Artif Organs 2016;40:123–8. | < 50 DT patients |
| Balachandran IC, Kennedy K, Nunez JF, Kiernan M, Grandin E, Buxton AE, et al. The effect of digoxin use on outcomes in patients with durable LVADs: an intermacs analysis. J Am Coll Cardiol 2020;75(11):993. | Do not report DT data |
| Balakumaran K, Garcia RA, Schwab T, Gaznabi S, Dahm JT, Peng SL, et al. Predictive preoperative characteristics for right ventricular failure after left ventricle assist device placement. J Card Fail 2020;26(10 Suppl.):S128. | Do not report DT data |
| Bansal A, Akhtar F, Desai S. Post-approval experience with fully magnetically levitated continuous flow left ventricular assist device – single center experience. J Heart Lung Transplant 2020;39(4 Suppl.):S416. | Do not report DT data |
| Bansal A, Schexnayder D, Akhtar F, Bansal A, Velasco-Gonzalez C, Verma A, et al. Right heart failure in different left ventricular assist devices: single-center experience. Ochsner J 2019;19:194–8. | Do not report DT data |
| Bansal N, Hailpern SM, Katz R, Hall YN, Kurella Tamura M, Kreuter W, et al. Outcomes associated with left ventricular assist devices among recipients with and without end-stage renal disease. JAMA Intern Med 2018;178:204–9. | Do not report DT data |
| Bart NK, Malik S, Emmanuel S, Andresen D, Muthiah K, Hayward CS. Blood stream infection in patients with permanent mechanical circulatory support: risk factors for on-pump mortality. J Heart Lung Transplant 2019;38(4 Suppl.):S319. | Do not report DT data |
| Bedzra EKS, Dardas TF, Cheng RK, Pal JD, Mahr C, Smith JW, et al. Pulmonary function tests do not predict mortality in patients undergoing continuous-flow left ventricular assist device implantation. J Thorac Cardiovasc Surg 2017;154:1959–70.e1. | Do not report DT data |
| Bejko J, Toto F, Gregori D, Gerosa G, Bottio T. Left ventricle assist devices and driveline’s infection incidence: a single-centre experience. J Artif Organs 2018;21:52–60. | < 50 DT patients |
| Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, Kushwaha SS, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Fail 2017;19:926–46. | < 50 DT patients |
| Benedetti G, Mohite P, Smail H, Garcia Saez D, Patil NP, Husain M, et al. Long-term follow-up and predicting factors of de novo aortic regurgitation after LVAD implantation. J Heart Lung Transplant 2018;37(4 Suppl. 1):S291. | Do not report DT data |
| Benjamin MM, Garacci Z, Sundarajan S, Mohammed A. Longer duration of milrinone associated with higher risk for right ventricular failure following left ventricular assist device implantation in stage D heart failure patients. J Card Fail 2019;25(8 Suppl.):S47. | Do not report DT data |
| Beyersdorf F. Economics of ventricular assist devices: European view. Ann Thorac Surg 2001;71:S192–4; discussion S202. | Wrong study design |
| Bhat G, Kumar S, Aggarwal A, Pauwaa S, Rossell G, Kurien S, et al. Experience with noncardiac surgery in destination therapy left ventricular assist devices patients. ASAIO J 2012;58:396–401. | < 50 DT patients |
| Bielka A, Kalinowski M, Pacholewicz J, Antonczyk R, Zakliczynski M, Przybylowski P, et al. Left ventricular assist devices offer similar one-year survival to the heart transplant recipients – single center experience. Kardiologia Polska 2018;76(Suppl. 1):365–6. | Do not report DT data |
| Bielka A, Kalinowski M, Pacholewicz J, Malyszek-Tumidajewicz J, Waszak J, Copik I, et al. Short- and long-term outcomes of continuous-flow left ventricular assist device therapy in 79 patients with end-stage heart failure. Pol Arch Intern Med 2020;130:589–97. | Wrong patient population |
| Bielka A, Kalinowski M, Pacholewicz J. Erratum: short-and long-term outcomes of continuous-flow left ventricular assist device therapy in 79 patients with end-stage heart failure. Pol Arch Intern Med 2020;130:926–7. | Wrong patient population |
| Bieniarz MC, Delgado R. The financial burden of destination left ventricular assist device therapy: who and when? Curr Cardiol Rep 2007;9:194–9. | Wrong study design |
| Birati EY, Hanff TC, Maldonado D, Grandin EW, Kennel PJ, Mazurek JA, et al. Predicting long term outcome in patients treated with continuous flow left ventricular assist device: the Penn-Columbia Risk Score. J Am Heart Assoc 2018;7:e006408. | Do not report DT data |
| Birks EJ, Tansley PD, Yacoub MH, Bowles CT, Hipkin M, Hardy J, et al. Incidence and clinical management of life-threatening left ventricular assist device failure. J Heart Lung Transplant 2004;23:964–9. | Do not report DT data |
| Bishawi M, Bell S, Cai L, Landford W, Arif S, McLarty A, et al. Antibiotic prophylaxis strategies in LVAD implantation and LVAD infections: a systematic review of the literature. J Heart Lung Transplant 2017;36(4 Suppl. 1):S242. | Do not report DT data |
| Bishawi M, Joseph J, Patel C, Schroder J, Daneshmand M, Bowles D, et al. Risk factors for stroke on left ventricular assist devices. J Card Surg 2018;33:348–52. | Do not report DT data |
| Bjelic M, Ayers B, Paic F, Bernstein W, Barrus B, Chase K, et al. Study results suggest less invasive HeartMate 3 implantation is a safe and effective approach for obese patients. J Heart Lung Transplant Off Publ Int Soc Heart Transplant 2021;40:990–7. | Do not report DT data |
| Blumer V, Hernandez G, Ortiz M, Cioff J, Chaparro S. Oncologic patients with advanced heart failure: to VAD or not to VAD? Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Bobenko A, Schoenrath F, Knierim JH, Friede T, Verheyen N, Mehra MR, et al. Exercise training in patients with a left ventricular assist device (Ex-VAD): rationale and design of a multicentre, prospective, assessor-blinded, randomized, controlled trial. Eur J Heart Fail 2019;21:1152–9. | Wrong intervention |
| Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 2011;30:402–7. | Do not report DT data |
| Braun OO, Nilsson J, Gustafsson F, Dellgren G, Fiane AE, Lemstrom K, et al. Continuous-flow LVADs in the Nordic countries: complications and mortality and its predictors. Scand Cardiovasc J 2019;53:14–20. | < 50 DT patients |
| Brewer RJ, Cabrera R, El-Atrache M, Zafar A, Hrobowski TN, Nemeh HM, et al. Relationship of tricuspid repair at the time of left ventricular assist device implantation and survival. Int J Artif Organs 2014;37:834–8. | < 50 DT patients |
| Brewer RJ, Lanfear DE, Sai-Sudhakar CB, Sundareswaran KS, Ravi Y, Farrar DJ, et al. Extremes of body mass index do not impact mid-term survival after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2012;31:167–72. | Do not report DT data |
| Brinkley DM, Wang L, Yu C, Kiernan MS. The effect of renin-angiotensin-aldosterone system inhibition on morbidity and mortality during long-term continuous-flow left ventricular assist device support. J Heart Lung Transplant 2020;39(4 Suppl.):S132. | Do not report DT data |
| Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014;7:68–75. | Wrong outcomes |
| Brisco MA, Sundareswaran KS, Milano CA, Feldman D, Testani JM, Ewald GA, et al. Incidence, risk, and consequences of atrial arrhythmias in patients with continuous-flow left ventricular assist devices. J Card Surg 2014;29:572–80. | Do not report DT data |
| Brozzi NA, Cifuentes RO, Saba IC, Macon C, Ghodsizad A, Andreopoulos F, et al. Long-term outcomes of elderly patients receiving continuous flow left ventricular support. J Card Surg 2020;35:3405–8. | Do not report DT data |
| Bruce CR, Minard CG, Wilhelms LA, Abraham M, Amione-Guerra J, Pham L, et al. Caregivers of patients with left ventricular assist devices: possible impacts on patients’ mortality and interagency registry for mechanically assisted circulatory support-defined morbidity events. Circ Cardiovasc Qual Outcomes 2017;10:01. | Do not report DT data |
| Brush S, Budge D, Alharethi R, McCormick AJ, MacPherson JE, Reid BB, et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant 2010;29:1337–41. | < 50 DT patients |
| Bryce K, Pehote M, Lanfear D. Cognitive functioning and post-LVAD outcomes: influence of comorbidities and specific cognitive domains. J Card Fail 2016;22(Suppl. 8):S124. | Do not report DT data |
| Bunte MC, Blackstone EH, Thuita L, Fowler J, Joseph L, Ozaki A, et al. Major bleeding during HeartMate II support. J Am Coll Cardiol 2013;62:2188–96. | < 50 DT patients |
| Burke MA, Alexy T, Kamioka N, Shafi T, Turbyfield CT, Stowe J, et al. Outflow graft obstruction causing recurrent heart failure after left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S98. | Do not report DT data |
| Cai J, Xia W, Akhabue E, Setoguchi S, Okwuosa I, Greenberg P. Primary causes of hospitalization among patients with left ventricular assist devices. J Heart Lung Transplant 2021;40(4 Suppl.):S418. | Do not report DT data |
| Casida J, Aikens J, Pagani F, Ewald G, Craddock H, Pavol M, et al. Advancing the science of self-management in adults with long-term left ventricular assist devices. Artif Organs 2018;42:1095–103. | < 50 DT patients |
| Casida J, Wu HS, Harden J, Chern J, Carie A. Development and initial evaluation of the psychometric properties of self-efficacy and adherence scales for patients with a left ventricular assist device. Prog Transplant 2015;25:107–15. | < 50 DT patients |
| Casida JM, Abshire M, Ghosh B, Yang JJ. The relationship of anxiety, depression, and quality of life in adults with left ventricular assist devices. ASAIO J 2018;64:515–20. | < 50 DT patients |
| Casida JM, Wu HS, Abshire M, Ghosh B, Yang JJ. Cognition and adherence are self-management factors predicting the quality of life of adults living with a left ventricular assist device. J Heart Lung Transplant 2017;36:325–30. | < 50 DT patients |
| Castedo E, Martinez Cabeza P, Perez de la Sota E, Sbraga F, Polo ML, Arribas JM, et al. First ESPAMACS official report: 369 mechanical circulatory support devices (October 2014–May 2016). Cirugia Cardiovascular 2016;23:15–21. | < 50 DT patients |
| Cavarretta E, Marullo AGM, Sciarretta S, Benedetto U, Greco E, Roever L, et al. A network meta-analysis of randomized trials and observational studies on left ventricular assist devices in adult patients with end-stage heart failure. Eur J Cardio-Thorac Surg 2019;55:461–7. | Wrong study design |
| Chair SY, Cheng L. The effectiveness of cardiac rehabilitation in patients with left ventricular assist devices (LVADs): a systematic review and meta-analysis. ASAIO J 2018;64(Suppl. 1):70. | Do not report DT data |
| Chang HH, Chen PL, Chen IM, Kuo TT, Weng ZC, Huang PJ, et al. Cost–utility analysis of direct ventricular assist device vs double bridges to heart transplantation in patients with refractory heart failure. Clin Transplant 2017;31. | Wrong patient population |
| Chen S, Lin A, Liu E, Gowan M, May LJ, Doan LN, et al. Outpatient outcomes of pediatric patients with left ventricular assist devices. ASAIO J 2016;62:163–8. | Paediatric population |
| Chen S, Lin A, Liu E, May LJ, Doan LN, Maeda K, et al. Discharge outcomes in children supported with continuous flow left ventricular assist devices. J Heart Lung Transplant 2015;(1):S324. | Paediatric population |
| Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102–8. | < 50 DT patients |
| Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Database of Abstracts of Reviews of Effects (DARE). 2010. | Duplicate record |
| Chickerillo K, Andrade AA, Kukla L, Khan M, Hussain S, Krause N, et al. Chronic kidney disease and left ventricular assist device patients: a retrospective review. J Card Fail 2019;25(8 Suppl.):S152. | Do not report DT data |
| Chickerillo K, Andrade AA, Kukla L, Krause N, Aicher T, Paliga R, et al. 2-year outcomes of LVAD patients with chronic kidney disease. ASAIO J 2021;67(Suppl. 2):94. | Do not report DT data |
| ChiCtr, Teda International Cardiovascular Hospital Y. A clinical trial to evaluate the efficacy and safety of implantable magnetic fluid suspension ventricular assist device in patients with end-stage heart failure. 2019. | Do not report DT data |
| Chien CV, Gelow JM, Mudd JO, Davis J, Lee CS. Right atrial pressure is the best hemodynamic predictor of heart failure hospitalizations in patients on LVAD support. J Card Fail 2016;22(Suppl. 8):S22. | Do not report DT data |
| Choi JH, Luc JGY, Moncho Escrivá E, Phan K, Rizvi SSA, Patel S, et al. Impact of concomitant mitral valve surgery with LVAD placement: systematic review and meta-analysis. Artif Organs 2018;42:1139–47. | < 50 DT patients |
| Chou B, Lamba HK, Long G, Parikh V, Chatterjee S, George J, et al. Outcomes of LVAD implantation in ischemic versus nonischemic cardiomyopathy. J Heart Lung Transplant 2019;38(4 Suppl.):S449. | Do not report DT data |
| Chou BP, Lamba HK, Cheema FH, Civitello AB, Delgado RM, Simpson L, et al. Outcomes of repeat left ventricular assist device exchange. ASAIO J 2020;66:64–8. | < 50 DT patients |
| Christle JW, Moneghetti K, Haddad F, Myers J, Ashley EA, Wheeler M, et al. Serial echo-cardiopulmonary exercise test ramping for evaluation of patients with VAD. Eur Heart J 2018;39(Suppl. 1):386. | Do not report DT data |
| Christle JW, Moneghetti KJ, Ha R, Haddad F, Banerjee D, Wheeler MT. Combining echocardiography with cardiopulmonary exercise stress testing to evaluate recovery of systolic function in patients with left ventricular assist devices. Eur J Heart Fail 2017;19(Suppl. 1):112. | Do not report DT data |
| Chu JK, Sarda S, Falkenstrom K, Boydston W, Chern JJ. Ventricular access device infection rate: a retrospective study and review of the literature. Child’s Nerv Syst 2014;30:1663–70. | Paediatric population |
| Chu SK, McCormick Z, Hwang S, Rydberg L. Outcomes of acute inpatient rehabilitation in patients with left ventricular assist devices. PM&R 2013;(1):S141. | < 50 DT patients |
| Chung JJ, Stetson R, Chen CW, Gaffey AC, Rame J, Acker MA, et al. Doing the math: the costs of long term mechanical circulatory support. J Heart Lung Transplant 2017;36(4 Suppl. 1):S287. | Do not report DT data |
| Chung JJ, Stetson R, Gordon J, Chen CW, Gaffey AC, Rame JE, et al. Better with time: an economic assessment of long-term mechanical circulatory support in a population surviving at least 1 year with a left ventricular assist device. Semin Thorac Cardiovasc Surg 2018. | Wrong indication |
| Chung JJ, Stetson R, Gordon J, Chen CW, Gaffey AC, Rame JE, et al. Better with time: an economic assessment of long-term mechanical circulatory support in a population surviving at least 1 year with a left ventricular assist device. Semin Thorac Cardiovasc Surg 2020;32:738–46. | Do not report DT data |
| Chyou JY. Relationship of gender, baseline QRS duration, and composite of cardiovascular readmission and death in patients with left ventricular assist device. Heart Rhythm 2017;14(5 Suppl. 1):S578. | Do not report DT data |
| Clarke A, Pulikottil-Jacob R, Connock M, Suri G, Kandala NB, Maheswaran H, et al. Cost-effectiveness of left ventricular assist devices (LVADs) for patients with advanced heart failure: analysis of the British NHS bridge to transplant (BTT) program. Int J Cardiol 2014;171:338–45. | Wrong indication |
| Clarke N, Pruszynski JE, Drazner M, Huffman LC, Peltz M. Device choice does not influence outcomes after left ventricular assist device (LVAD) in patients bridged to transplant or destination therapy. J Am Coll Surg 2017;225(4 Suppl. 1):S28. | Do not report DT data |
| Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al. The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation. Health Technol Assess (Winchester, England) 2005;9:1–132, iii. | Wrong study design |
| Clegg AJ, Scott DA, Loveman E, Colquitt JL, Royle P, Bryant J. Clinical and cost-effectiveness of left ventricular assist devices as a bridge to heart transplantation for people with end-stage heart failure: a systematic review and economic evaluation. Eur Heart J 2006;27:2929–38. | Wrong indication |
| Cleveland JC, Jr, Naftel DC, Reece TB, Murray M, Antaki J, Pagani FD, et al. Survival after biventricular assist device implantation: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J Heart Lung Transplant 2011;30:862–9. | Do not report DT data |
| Cogswell R, John R, Shultz J, Martin C, Thenappan T, Kamdar F, et al. Pre-operative pectoralis muscle quantity and attenuation by computed tomography are predictive of recurrent gastrointestinal bleeding on left ventricular assist device support. J Heart Lung Transplant 2018;37(4 Suppl. 1):S73. | Do not report DT data |
| Cogswell R, Murray T, Araujo R, Teigen L, Trachtenberg B, Schultz J, et al. A novel model incorporating pectoralis muscle measures to predict mortality after ventricular assist device implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S108. | Do not report DT data |
| Cogswell R, Murray T, Araujo R, Teigen L, Trachtenberg B, Schultz JN, et al. External validation of the Minnesota pectoralis muscle risk score to predict mortality after ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S37. | Do not report DT data |
| Cogswell R, Teigen L, Allen T, Estep J, Araujo R, Schultz J, et al. Measurement of pectoralis muscle quantity and attenuation by computed tomography using routinely available software is feasible and predicts mortality after LVAD implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S445. | Do not report DT data |
| Cogswell R, Teigen L, Schultz J, Thenappan T, Lin L, Kealhofer J, et al. Pre-operative pectoralis muscle measures by computed tomography predict early right heart failure deaths after left ventricular assist device. J Heart Lung Transplant 2018;37(4 Suppl. 1):S468. | Do not report DT data |
| Connelly JH, Abrams J, Klima T, Vaughn WK, Frazier OH. Acquired commissural fusion of aortic valves in patients with left ventricular assist devices. J Heart Lung Transplant 2003;22:1291–5. | Wrong patient population |
| Cool JA, Parikh NS, Kamel H, Karas MG, Boehme AK. Stroke risk and mortality in patients with ventricular assist devices. Stroke Conference: American Heart Association/American Stroke Association. 2016;47. | Do not report DT data |
| Corral JE, Yarlagadda B, Kroner PT, Goswami R, Leoni-Moreno JC, Raimondo M, et al. Left ventricular assist device (LVAD), heart transplantation and acute pancreatitis: a retrospective cohort. Gastroenterol 2019;156(6 Suppl. 1):S-1036. | Do not report DT data |
| Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail 2010;3:668–74. | < 50 DT patients |
| Cowger JA, Aaronson KD, Romano MA, Haft J, Pagani FD. Consequences of aortic insufficiency during long-term axial continuous-flow left ventricular assist device support. J Heart Lung Transplant 2014;33:1233–40. | < 50 DT patients |
| Cowger JA, Naka Y, Aaronson K, Horstmanshoff D, Gulati S, Rinde-Hoffman D, et al. Quality of life and functional capacity assessment in the multicenter study of maglev technology in patients undergoing mechanical circulatory support therapy with Heartmate 3 (Momentum 3) pivotal trial. J Heart Lung Transplant 2017;36(4 Suppl. 1):S66–7. | Do not report DT data |
| Cowger JA, Shah P, Singh R, Pagani FD, Aaronson KD, Dardas TF, et al. Long term survivors of LVAD support: what attributes describe their survival advantage? J Heart Lung Transplant 2017;36(4 Suppl. 1):S422. | Do not report DT data |
| Coyle L, Yost G, Gallagher C, Graney N, Siemeck R, Bhat G, et al. Impatct of morbid obesity after left ventricular assist device placement. J Heart Lung Transplant 2017;36(4 Suppl. 1):S182–3. | Do not report DT data |
| Critsinelis A, Lamba H, Volkovicher N, Kurihara C, Kawabori M, Sugiura T, et al. Effects of continuous flow left ventricular assist devices on long-term kidney function. ASAIO J 2018;64(Suppl. 1):75. | Do not report DT data |
| Critsinelis A, Lamba HK, Kurihara C, Kawabori M, Sugiura T, Santiago A, et al. Comparison of HMII and HVAD outcomes in patients supported for over two years. ASAIO J 2018;64(Suppl. 1):77. | Do not report DT data |
| Critsinelis AC, Kurihara C, Kawabori M, Sugiura T, Civitello AB, Morgan JA. Preoperative prealbumin level as a predictor of outcomes in patients who underwent left ventricular assist device implantation. Am J Cardiol 2017;120:1998–2002. | Do not report DT data |
| Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009;137:208–15. | < 50 DT patients |
| Cruz RR, Teo L, Loh J, Chan L, Khoo CY, Ng CT, et al. The use of ARNI in the prevention of ventricular assist device implantation among patients with advanced heart failure: a single center experience. Eur J Heart Fail 2019;21(Suppl. 1):84–5. | Do not report DT data |
| Cushing K, Kushnir V. Gastrointestinal bleeding following LVAD placement from top to bottom. Dig Dis Sci 2016;61:1440–7. | Wrong study design |
| Dailey J, Nguyen LH, Kohli A, Ha J, Russell MB, Dhingra R, et al. 968 a Multi-center study of left ventricular assist device (LVAD)-related bleeding. Gastrointest Endosc 2020;91(6 Suppl.):AB84–5. | Do not report DT data |
| Daimee UA, Wang M, Papernov A, Sherazi S, McNitt S, Vidula H, et al. Renal function changes following left ventricular assist device implantation. Am J Cardiol 2017;120:2213–20. | Do not report DT data |
| Dang NC, Topkara VK, Leacche M, John R, Byrne JG, Naka Y. Left ventricular assist device implantation after acute anterior wall myocardial infarction and cardiogenic shock: a two-center study. J Thorac Cardiovasc Surg 2005;130:693–8. | Do not report DT data |
| de By TMMH, Castedo E, Krabatsch T, Mohacsi P, Meyns B, Netuka I, et al. The EUROMACS Registry of patients who receive mechanical circulatory support: role and perspectives. Cir Cardiovascular 2016;23:22–5. | Wrong intervention |
| Dean D, Kallel F, Ewald GA, Tatooles A, Sheridan BC, Brewer RJ, et al. Reduction in driveline infection rates: results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J Heart Lung Transplant 2015;34:781–9. | Do not report DT data |
| DeFilippis E, Haythe J, Marshall D, Lin E, Axsom K, Truby L, et al. Sex differences in characteristics and outcomes following HeartMate 3 left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S337. | Do not report DT data |
| Demirozu ZT, Critsinelis A, Cohn WE, Radovancevic R, Ho J, Hernandez R, et al. Experience with the HeartMate II left ventricular assist device in patients older than 60 years. Heart Surg Forum 2019;22:E124–30. | Do not report DT data |
| Demirozu ZT, Radovancevic R, Hochman LF, Gregoric ID, Letsou GV, Kar B, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant Offi Publ Int Soc Heart Transplant 2011;30:849–53. | Do not report DT data |
| Deng MC, Wilhelm MJ, Scheld HH. Effects of exercise during long-term support with a left ventricular assist device. Circulation 1998;97:1212–3. | Wrong study design |
| Deniz E, Hanke JS, Rojas SV, Egger C, Urribari A, Reiss N, et al. Predictors of thrombembolic events in left ventricular assist devices. Eur Heart J 2016;37(Suppl. 1):792. | Do not report DT data |
| Deo SV, Hasin T, Altarabsheh SE, McKellar SH, Shah IK, Durham L 3rd, et al. Concomitant tricuspid valve repair or replacement during left ventricular assist device implant demonstrates comparable outcomes in the long term. J Card Surg 2012;27:760–6. | < 50 DT patients |
| Deo SV, Sharma V, Altarabsheh SE, Hasin T, Dillon J, Shah IK, et al. Hepatic and renal function with successful long-term support on a continuous flow left ventricular assist device. Heart Lung Circ 2014;23:229–33. | < 50 DT patients |
| Deo SV, Sharma V, Cho YH, Shah IK, Park SJ. De novo aortic insufficiency during long-term support on a left ventricular assist device: a systematic review and meta-analysis. ASAIO J (Am Soc Artif Intern Organs: 1992) 2014;60:183–8. | < 50 DT patients |
| Deshmukh A, Anyanwu E, Uriel N, Jeevanandam V, Tung R, Ozcan C. Left atrial structural remodeling with ventricular assist device. Circulation Conference: American Heart Association’s. 2016;134. | Do not report DT data |
| Deshmukh A, Kim G, Moss JD, Nayak HM, Burke M, Jeevanandam V, et al. Atrial arrhythmias and clinical outcomes in patients with left ventricular assist devices. Heart Rhythm 2016;(1):S588. | Do not report DT data |
| Diakos N, Taleb I, Pinzon OW, Javan H, Kfoury A, Stehlik J, et al. BIUx2 × 2. J Heart Lung Transplant 2019;38(4 Suppl.):S252. | Do not report DT data |
| Dobbels F, Mauthner O, Milisen K. Frailty in left ventricular assist device destination therapy: putting a new motor in a rickety old car running out of gas? J Heart Lung Transplant 2014;33:347–9. | Wrong study design |
| Donahey EE, Polly DM, Vega JD, Lyon M, Butler J, Nguyen D, et al. Multidrug-resistant organism infections in patients with left ventricular assist devices. Tex Heart Inst J 2015;42:522–7. | < 50 DT patients |
| Dong T, Doshi N, Steinberg R, Nayak A, O’Connell C, Howser J, et al. SIPAT B score predicts mortality in both BTT and DT male LVAD patients. J Card Fail 2019;25(8 Suppl.):S157. | Do not report DT data |
| Downs EA, Johnston LE, LaPar DJ, Yarboro LT, Kern JA, Kirby JL, et al. Impact of preoperative glycemic control on long-term mechanical circulatory support device implantation. J Heart Lung Transplant 2016;(1):S377. | Do not report DT data |
| Drews T, Dandel M, Krabatsch T, Potapov E, Stepanenko A, Hennig E, et al. Long-term mechanical circulatory support in 198 patients: largest single-center experience worldwide. ASAIO J 2011;57:9–16. | Do not report DT data |
| Drews T, Jurmann M, Michael D, Miralem P, Weng Y, Hetzer R. Differences in pulsatile and non-pulsatile mechanical circulatory support in long-term use. J Heart Lung Transplant 2008;27:1096–101. | < 50 DT patients |
| Drews T, Stepanenko A, Dandel M, Buz S, Lehmkuhl HB, Hetzer R. Mechanical circulatory support in patients of advanced age. Eur J Heart Fail 2010;12:990–4. | Do not report DT data |
| Drks, Klinik für Herzchirurgie R-KCBNN. Clinical course and prognostic significance of mitral valve insufficiency under LVAD (left ventricular assist device) therapy. 2020. | Do not report DT data |
| Duran A, Guha A, Bhimaraj A, Trachtenberg B, Park MH, Estep J, et al. Outcomes associated with complete versus partial LV unloading in patients with LVAD. J Heart Lung Transplant 2019;38(4 Suppl.):S458. | Do not report DT data |
| Elkaryoni A, Khan MS, Al Badarin F, Poonia J, Potturi N, Ellakany K, et al. An implantable cardioverter defibrillator is not associated with a reduction in mortality in advanced heart failure patients with continuous flow left ventricular assist device: a systematic review and meta-analysis. Circ Conf 2018;138. | Do not report DT data |
| Elzeneini M, Mahmoud A, Elsayed AH, Mahtta D, Al-Ani M, Aranda J, et al. Preoperative bleeding and blood product transfusion association with the preoperative use of aspirin and heparin in left ventricular assist device implantation. J Card Fail 2019;25(8 Suppl.):S148. | Do not report DT data |
| Emerson D, Chikwe J, Catarino P, Hassanein M, Deng L, Cantor RS, et al. Contemporary left ventricular assist device outcomes in an aging population: an STS INTERMACS analysis. J Am Coll Cardiol 2021;78(9):883–94. | Do not report DT data |
| Ensor CR, Paciullo CA, Cahoon WD, Jr, Nolan PE, Jr. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann Pharmacother 2011;45:60–77. | Wrong study design |
| Evans RW. Costs and insurance coverage associated with permanent mechanical cardiac assist/replacement devices in the United States. J Card Surg 2001;16:280–93. | Wrong study design |
| Evans RW. Economic impact of mechanical cardiac assistance. Prog Cardiovasc Dis 2000;43:81–94. | Wrong study design |
| Exarchos TP, Rigas G, Goletsis Y, Stefanou K, Jacobs S, Trivella MG, et al. A dynamic Bayesian network approach for time-specific survival probability prediction in patients after ventricular assist device implantation. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine & Biology Society 2014;2014:3172–5. | Do not report DT data |
| Fang JC. Rise of the machines–left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med 2009;361:2282–5. | Wrong study design |
| Felix S, Oerlemans M, Ramjankhan F, Buijsrogge M, Kirkels H, De Jonge N. Further improvement of exercise capacity one year after implantation of a CF-left ventricular assist device. J Heart Lung Transplant 2018;37(4 Suppl. 1):S129. | Do not report DT data |
| Felix S, Oerlemans MIF, Asselbergs FW, Van Laake LW, De Jonge N. Predictors of late right heart failure after mechanical circulatory support. Eur Heart J 2020;41(Suppl. 2):1101. | Do not report DT data |
| Felix SE, Ramjankhan FZ, De Heer F, De Jonge N, Buijsrogge MP, Suyker WJ. Mid term results of mechanical circulatory support in the Netherlands, a single centre experience from the university medical centre Utrecht (UMCU). J Heart Lung Transplant 2016;(1):S332. | Do not report DT data |
| Felix SEA, Oerlemans MIF, Van Laake LW, Ramjankhan FZ, Buijsrogge MP, Kirkels JH, et al. Increasing exercise capacity in the first year after implantation of a CF-left ventricular assist device. Eur J Heart Fail 2018;20(Suppl. 1):259. | Do not report DT data |
| Felix SEA, Ramjankhan FZ, De Heer F, Buijsrogge MP, Suyker WJL, Hulstein N, et al. Single center long term results of mechanical circulatory support in the Netherlands. Eur J Heart Fail 2016;(1):374–5. | Do not report DT data |
| Feller ED, Sorensen EN, Haddad M, Pierson RN, 3rd, Johnson FL, Brown JM, et al. Clinical outcomes are similar in pulsatile and nonpulsatile left ventricular assist device recipients. Ann Thorac Surg 2007;83:1082–8. | < 50 DT patients |
| Fendler TJ, Nassif ME, Kennedy KF, Joseph SM, Silvestry SC, Ewald GA, et al. Global outcome in patients with left ventricular assist devices. Am J Cardiol 2017;119:1069–73. | Do not report DT data |
| Fick A, Tymkew HA, Deters ML, Martin K, Ratermann JD, Reilly A, et al. Functional status and discharge location of patients post LVAD surgery in the acute care setting. Cardiopulm Phys Ther J 2021;32(3):e8. | Do not report DT data |
| Fine NM, Park SJ, Stulak JM, Topilsky Y, Daly RC, Joyce LD, et al. Proximal thoracic aorta dimensions after continuous-flow left ventricular assist device implantation: longitudinal changes and relation to aortic valve insufficiency. J Heart Lung Transplant 2016;35:423–32. | Wrong outcomes |
| Fitzpatrick JR, 3rd, Frederick JR, Hsu VM, Kozin ED, O’Hara ML, Howell E, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286–92. | Do not report DT data |
| Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail 2012;5:286–93. | Wrong study design |
| Florisson DS, Conte SM, De Bono JA, Newcomb AE. Do patients with the centrifugal flow HeartMate 3 or HeartWare left ventricular assist device have better outcomes compared to those with axial flow HeartMate II? Interact Cardiovasc Thorac Surg 2019;29:844–51. | < 50 DT patients |
| Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg 2013;95:1276–81. | < 50 DT patients |
| Forni A, Chiominto B, Faggian G. Surgical therapy in end stage heart failure: should we change our vision? J Heart Lung Transplant 2016;35(4 Suppl.):S278. | < 50 DT patients |
| Frankfurter C, Molinero M, Vishram-Nielsen JKK, Foroutan F, Mak S, Rao V, et al. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation: a systematic review. Circ Heart Fail 2020:CIRCHEARTFAILURE120006994. | Do not report DT data |
| Friedman JA. Experiences of left ventricular assist device-destination therapy recipients: a systematic review and meta-synthesis. Heart Lung 2020. | Wrong study design |
| Fujino T, Imamura T, Nitta D, Rodgers D, Nguyen A, Chung B, et al. Longitudinal trend of tricuspid regurgitation following left ventricular assist device implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S70–1. | Do not report DT data |
| Fukuhara S, Ikegami H, Polanco AR, Song JJ, Han J, Takeda K, et al. Concomitant repair for mild aortic insufficiency and continuous-flow left ventricular assist devices. Eur J Cardio-Thorac Surg 2017;52:1062–8. | < 50 DT patients |
| Fukuhara S, Takeda K, Chiuzan C, Han J, Kurlansky PA, Takayama H, et al. Concomitant mitral repair and continuous-flow left ventricular assist devices: is it warranted? J Thorac Cardiovasc Surg 2017;154:1303–12.e4. | < 50 DT patients |
| Gaffey AC, Chen CW, Chung JJ, Han J, Bermudez CA, Wald J, et al. Is there a difference in bleeding after left ventricular assist device implant: centrifugal versus axial? J Cardiothorac Surg 2018;13:22. | Do not report DT data |
| Gaffey AC, Chen CW, Han J, Rame JE, Kiernan MS, Chung J, et al. Should aggressive implantation of a temporary RVAD be utilized as means of recovery for RV function to allow for long-term left ventricular assist device therapy. Circulation Conference: American Heart Association’s. 2016;134. | Do not report DT data |
| Galand V, Auffret V, Flecher E, Pichard C, Boule S, Vincentelli A, et al. Occurrence of early ventricular arrhythmias after left ventricular assist device implantation is the strongest predictor of post-operative mortality: new insight from the assist-Icd study. Heart Rhythm 2019;16(5 Suppl.):385–6. | Duplicate record |
| Galand V, Flecher E, Auffret V, Pichard C, Boule S, Vincentelli A, et al. Early ventricular arrhythmias after LVAD implantation is the strongest predictor of 30-day post-operative mortality. JACC Clin Electrophysiol 2019;5:944–54. | Do not report DT data |
| Galand V, Flecher E, Chabanne C, Lelong B, Goeminne C, Vincentelli A, et al. Outcomes of left ventricular assist device implantation in patients with uncommon etiology cardiomyopathy. Am J Cardiol 2020;125:1421–8. | Do not report DT data |
| Gallo M, Valadon CL, Trivedi JR, Slaughter MS. Reduced pulsatility during continuous flow left ventricular assist device does not affect kidney function. J Heart Lung Transplant 2018;37(4 Suppl. 1):S118. | Do not report DT data |
| Galvao M, Immekus J, Saeed O, Fida N, Browne A, Goldstein DJ, et al. An international survey to assess referral thresholds for destination therapy in non-inotrope dependent patients: results of the consensus-DT study. J Heart Lung Transplant 2013;(1):S131–2. | Wrong study design |
| Galvao M, Saeed O, Immekus J, Goldstein DJ, Maybaum S. An international survey to assess referral thresholds for destination therapy in non-inotrope-dependent patients: results of the CONSENSUS-DT study. J Card Fail 2014;20:492–7. | Wrong study design |
| Ganga HV, Leung A, Jantz J, Choudhary G, Stabile L, Levine DJ, et al. Supervised exercise training versus usual care in ambulatory patients with left ventricular assist devices: a systematic review. PLOS ONE 2017;12:e0174323. | < 50 DT patients |
| Garan AR, Levin AP, Topkara V, Thomas SS, Yuzefpolskaya M, Colombo PC, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:1611–6. | Do not report DT data |
| Garcia RA, Balakumaran K, Schwab T, Gaznabi S, Dahm J, Peng SL, et al. Short and long term mortality in patients with right heart failure after left ventricular assist device placement. J Card Fail 2020;26(10 Suppl.):S136. | Do not report DT data |
| Gelijns AC, Richards AF, Williams DL, Oz MC, Oliveira J, Moskowitz AJ. Evolving costs of long-term left ventricular assist device implantation. Ann Thorac Surg 1997;64:1312–9. | Wrong indication |
| Gelijns AC, Russo MJ, Hong KN, Brown LD, Ascheim DD, Moskowitz AJ. Dynamics of device innovation: implications for assessing value. Int J Technol Assess Health Care 2013;29:365–73. | Wrong study design |
| Genev I, Yost G, Gregory M, Gomez K, Pappas P, Tatooles A, et al. Improved nutrition status in patients with advanced heart failure implanted with a left ventricular assist device. Nutr Clin Pract 2019;34:444–9. | Do not report DT data |
| Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Bhama JK, Bermudez CA, et al. Early adverse events as predictors of 1-year mortality during mechanical circulatory support. J Heart Lung Transplant 2010;29:981–8. | < 50 DT patients |
| George I, Xydas S, Topkara VK, Ferdinando C, Barnwell EC, Gableman L, et al. Clinical indication for use and outcomes after inhaled nitric oxide therapy. Ann Thorac Surg 2006;82:2161–9. | Do not report DT data |
| Gibreal M, Katugaha S, Abdullah K, Stulak J, Cowger JA, Salerno C, et al. Bloodstream infection in patients with LVAD and CIED. J Heart Lung Transplant 2017;36(4 Suppl. 1):S39–40. | Do not report DT data |
| Goda A, Takayama H, Pak SW, Uriel N, Mancini D, Naka Y, et al. Aortic valve procedures at the time of ventricular assist device placement. Ann Thorac Surg 2011;91:750–4. | < 50 DT patients |
| Goldenthal I, Hickey KT, Colombo PC, Naka Y, Garan AR, Yuzefpolskaya M, et al. Atrial fibrillation is associated with recurrent ventricular arrhythmias after LVAD implant: incidence and impact in a consecutive series. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2019;140. | Do not report DT data |
| Goldstein DJ, Maybaum S, MacGillivray TE, Moore SA, Bogaev R, Farrar DJ, et al. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J Card Fail 2012;18:392–5. | Do not report DT data |
| Gosev I, Kiernan MS, Eckman P, Soleimani B, Kilic A, Uriel N, et al. Long-term survival in patients receiving a continuous-flow left ventricular assist device. Ann Thorac Surg 2018;105:696–701. | Do not report DT data |
| Grady KL, Andrei AC, Xu Y, Petty MG, Kao A, Hsich EM, et al. Change in caregiver burden from before to early after surgery: findings from the sustaining quality of life of the aged: transplant or mechanical support (SUSTAIN-IT) study. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2019;140. | Do not report DT data |
| Grady KL, Jackson K, Wortman K, Buono S, Beiser D, Murks C, et al. Self-reported physical health with a left ventricular assist device: findings from the mechanical circulatory support measures of adjustment and quality of life (MCS A-QOL) study. J Heart Lung Transplant 2019;38(4 Suppl.):S437–8. | Do not report DT data |
| Grady KL, Meyer PM, Mattea A, Dressler D, Ormaza S, White-Williams C, et al. Change in quality of life from before to after discharge following left ventricular assist device implantation. J Heart Lung Transplant 2003;22:322–33. | Do not report DT data |
| Grady KL, Okwuosa I, Petty M, Adam H, Pollan L, Andrei AC, et al. Sustaining quality of life of the aged: transplant or mechanical support: baseline caregiver quality of life. Circulation Conference. 2018;138. | Do not report DT data |
| Grady KL, Sherri W, Naftel DC, Myers S, Gelijins A, Moskowitz A, et al. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: findings from interagency registry for mechanically assisted circulatory support. J Heart Lung Transplant 2016;35:777–88. | Do not report DT data |
| Grady KL, Xu Y, Andrei A, Warzecha A, Kao A, Hsich EM, et al. Both patient and caregiver factors are related to patient health-related quality of life before surgery. J Heart Lung Transplant 2020;39(4 Suppl.):S92–3. | Do not report DT data |
| Grinstein J, Kadakkal A, Rodrigo M, Hofmeyer M, Mohammed S, Butt N, et al. Early renal recovery after left ventricular assist device implantation is associated with improved clinical outcomes in patients with kidney disease at baseline. J Heart Lung Transplant 2019;38(4 Suppl.):S359. | Do not report DT data |
| Grupper A, Park SJ, Pereira NL, Schettle SD, Gerber Y, Topilsky Y, et al. Role of ventricular assist therapy for patients with heart failure and restrictive physiology: Improving outcomes for a lethal disease. J Heart Lung Transplant 2015;34:1042–9. | < 50 DT patients |
| Guenther SP, Fong R, Abovwe N, Shad R, MacArthur JW, Teuteberg J, et al. A decade of ***single center HeartWareTM HVADTM Experience. J Heart Lung Transplant 2020;39(4 Suppl.):S339. | Do not report DT data |
| Guidi JL, Troutman G, Birati EY, Wald J, Fox A, Ortega-Legaspi J, et al. Survival in intermacs 1 & 2 patients after LVAD therapy: does chronicity of heart failure matter? J Heart Lung Transplant 2018;37(4 Suppl. 1):S128. | Do not report DT data |
| Gulati G, Grandin EW, Kennedy KF, Cabezas F, DeNofrio D, Kociol R, et al. Pre-implant phosphodiesterase-5 inhibitor use is associated with higher rates of severe early right heart failure after IVAD implantation: an INTERMACS analysis. J Heart Lung Transplant 2018;37(4 Suppl. 1):S195. | Do not report DT data |
| Gunasingam N, Williams D. Bleeding in patients with left ventricular assist devices: The experience of a single centre. J Gastroenterol Hepatol (Australia) 2016;31(Suppl. 2):34. | Do not report DT data |
| Gupta S, Roy S, Cogswell R, Thenappan T, Liao K, John R. Readmission within 30 days after left ventricular assist device implantation is associated with increased long-term mortality. J Heart Lung Transplant 2016;(1):S259–60. | Do not report DT data |
| Gupta S, Roy S, John R, Cogswell R. Sodium nadir during left ventricular assist device implantation is not associated with increased mortality. J Heart Lung Transplant 2016;(1):S339–40. | Do not report DT data |
| Halkar M, Nowacki AS, Kendall K, Efeovbokhan N, Gorodeski EZ, Moazami N, et al. Utility of the psychosocial assessment of candidates for transplantation in patients undergoing continuous-flow left ventricular assist device implantation. Prog Transplant 2018;28:220–5. | Do not report DT data |
| Hamdan R, Fakih S, Mohammad M, Charif F, Abdallah H, Safa S, et al. The Lebanese left ventricular assist device experience, a success story despite the odds. J Cardiothorac Surg 2020;15:192. | < 50 DT patients |
| Hamed S, Schmack B, Mueller F, Ehlermann P, Hittmann D, Ruhparwar A, et al. Implementation of an intensified outpatient follow-up protocol improves outcomes in patients with ventricular assist devices. Clin Res Cardiol 2019;108:1197–207. | < 50 DT patients |
| Han J, Mauro CM, Kurlansky PA, Fukuhara S, Yuzefpolskaya M, Topkara VK, et al. Impact of obesity on readmission in patients with left ventricular assist devices. Ann Thorac Surg 2018;105:1192–8. | Do not report DT data |
| Han J, Pinsino A, Royzman E, Gaudig A, Mabasa M, Takayama H, et al. Predictors of postoperative vasoplegia in patients receiving left ventricular assist devices. J Heart Lung Transplant 2018;37(4 Suppl. 1):S127–8. | Do not report DT data |
| Han J, Takayama H, Kurlansky PA, Garan AR, Yuzefpolskaya M, Topkara VK, et al. Outcomes of multiple concomitant valve procedures in patients receiving continuous-flow left ventricular assist device. J Heart Lung Transplant 2017;36(4 Suppl. 1):S30. | Do not report DT data |
| Han J, Takeda K, Takayama H, Kurlansky PA, Mauro CM, Colombo PC, et al. Durability and clinical impact of tricuspid valve procedures in patients receiving a continuous-flow left ventricular assist device. J Thorac Cardiovasc Surg 2016;151:520–7.e1. | Do not report DT data |
| Han JJ, Chung J, Chen CW, Gaffey AC, Sotolongo A, Justice C, et al. Different clinical course and complications in Interagency Registry for Mechanically Assisted Circulatory Support 1 (INTERMACS) patients managed with or without extracorporeal membrane oxygenation. ASAIO J 2018;64:318–22. | Do not report DT data |
| Han JJ, Iyengar A, Patrick WL, Goldenring J, Molina M, Ameer A, et al. Impact of socioeconomic status on outcomes post-ventricular assist device implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S128–9. | Do not report DT data |
| Han JJ, Sooppan R, Johnson AP, Chen CW, Gaffey AC, Phillips EC, et al. Higher body mass index increases risk of HeartMate II pump thrombosis but does not adversely affect long-term survival. Circ J 2017;81:213–9. | Do not report DT data |
| Hanff TC, Mazurek JA, Grandin EW, Padegimas A, Howard J, Forde-McLean R, et al. The anemic stress index – a novel index that predicts short and long term mortality of patients on continuous flow ventricular assist devices. J Heart Lung Transplant 2017;36(4 Suppl. 1):S173–4. | Do not report DT data |
| Hashim T, Sanam K, Revilla-Martinez M, Morgan CJ, Tallaj JA, Pamboukian SV, et al. Clinical characteristics and outcomes of intravenous inotropic therapy in advanced heart failure. Circ Heart Fail 2015;8:880–6. | Wrong study design |
| Hasin T, Deo S, Maleszewski JJ, Topilsky Y, Edwards BS, Pereira NL, et al. The role of medical management for acute intravascular hemolysis in patients supported on axial flow LVAD. ASAIO J 2014;60:9–14. | Do not report DT data |
| Hasin T, Grupper A, Dillon JJ, Maleszewski JJ, Li Z, Topilsky Y, et al. Early gains in renal function following implantation of HeartMate II left ventricular assist devices may not persist to one year. ASAIO J 2017;63:401–7. | < 50 DT patients |
| Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol 2012;59:26–36. | < 50 DT patients |
| Heaton J, Li M. Adverse events of third generation left ventricular assist devices: insights from the manufacturer and user facility device experience (Maude) database. J Am Coll Cardiol 2021;77(18 Suppl. 1):645. | Do not report DT data |
| Heilmann C, Kuijpers N, Beyersdorf F, Trummer G, Berchtold-Herz M, Zeh W, et al. Does listing for heart transplant for longer than 30 days before ventricular assist device implantation influence utilization of psychotherapeutic support and outcome? Eur J Cardio-Thorac Surg 2012;41:1371–6; discussion 1376. | < 50 DT patients |
| Hernandez AF, Shea AM, Milano CA, Rogers JG, Hammill BG, O’Connor CM, et al. Long-term outcomes and costs of ventricular assist devices among Medicare beneficiaries. JAMA 2008;300:2398–406. | Wrong patient population |
| Hernandez-Montfort JA, Ton VK, Xie R, Fisher A, Meyns B, Nakatani T, et al. Longitudinal impact of temporary mechanical circulatory support on durable left ventricular assist device outcomes: an IMACS registry analysis. J Heart Lung Transplant 2019;38(4 Suppl.):S33. | Do not report DT data |
| Hetzer R, Kaufmann MF, Potapov E, Krabatsch T, Delmo Walter EM. Rotary blood pumps as long-term mechanical circulatory support: a review of a 15-year Berlin experience. Semin Thorac Cardiovasc Surg 2016;28:12–23. | Do not report DT data |
| Hickey K, Garan H, Mancini DM, Colombo PC, Sciacca RR, Abrams MP, et al. Atrial fibrillation in patients with left ventricular assist devices. Heart Rhythm 2016;(1):S220. | Do not report DT data |
| Hickey KT, Garan H, Mancini DM, Colombo PC, Naka Y, Sciacca RR, et al. Atrial fibrillation in patients with left ventricular assist devices: incidence, predictors, and clinical outcomes. JACC Clin Electrophysiol 2016;2:793–8. | Do not report DT data |
| Hickey KT, Reading M, Flannery MA, Te-Frey RT, Pineda MT, Ross KA, et al. Changes in the clinical characteristics of LVAD patients. ASAIO J 2018;64(Suppl. 1):103. | Do not report DT data |
| Hirji SA, Sabatino ME, Minhas AMK, Okoh AK, Fudim M, Vaduganathan M, et al. Contemporary nationwide heart transplantation and left ventricular assist device outcomes in patients with histories of bariatric surgery. J Card Fail 2021. | Do not report DT data |
| Holley CT, Fitzpatrick M, Roy SS, Alraies MC, Cogswell R, Souslian L, et al. Aortic insufficiency in continuous-flow left ventricular assist device support patients is common but does not impact long-term mortality. J Heart Lung Transplant 2017;36:91–6. | < 50 DT patients |
| Holman WL, Naftel DC, Eckert CE, Kormos RL, Goldstein DJ, Kirklin JK. Durability of left ventricular assist devices: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg 2013;146:437–41.e1. | Do not report DT data |
| Holmberg E, Ahn H, Peterzen B. More than 20 years’ experience of left ventricular assist device implantation at a non-transplant Centre. Scand Cardiovasc J 2017;51:293–8. | < 50 DT patients |
| Holtz JE, Potter AN, Macpherson NC, Nguyen D, Kormos RL, Teuteberg JJ, et al. Worsening renal function after ventricular assist device placement is associated with twice the risk of death over 3 years. J Heart Lung Transplant 2016;(1):S263. | Do not report DT data |
| Hong Y, Dufendach K, Wang Y, Thoma F, Kilic A. Impact of hepatic steatosis on outcomes after left ventricular assist device implantation. J Card Surg 2021;36:2277–83. | Do not report DT data |
| Horsley-Silva JL, Noelting J, Love WT, Zawadowski G, Octavio P, Hardaway B, et al. Gastrointestinal bleeding in patients with left ventricular assist devices – a single center retrospective cohort study. Gastrointest Endosc 2016;(1):AB470. | Do not report DT data |
| Horton SC, Khodaverdian R, Chatelain P, McIntosh ML, Horne BD, Muhlestein JB, et al. Left ventricular assist device malfunction: an approach to diagnosis by echocardiography. J Am Coll Cardiol 2005;45:1435–40. | < 50 DT patients |
| Hulman M, Ondrusek M, de By T, Antonides CFJ, Artemiou P, Hudec V, et al. Single centre 12 year experience with durable mechanical circulatory support: comparison with the EUROMACS registry. Bratisl Lek Listy 2021;122:371–8. | Do not report DT data |
| Hutchinson OZ, Oz MC, Ascherman JA. The use of muscle flaps to treat left ventricular assist device infections. Plast Reconstr Surg 2001;107:364–73. | Do not report DT data |
| Ibrahim M, Cortesi C, Croix GS, Chaparro S. Renal dysfunction and outcomes after left ventricular assist device: a systematic review. J Card Fail 2019;25(8 Suppl.):S160–1. | Do not report DT data |
| Idris A, Al-Khadra Y, Kabach A, Darmoch F, Moussa Pacha H, Soud M, et al. The impact of peripheral arterial disease on advanced heart failure patients undergoing left ventricular assist device surgery. Eur Heart J 2018;39(Suppl. 1):386. | Do not report DT data |
| Imamura T, Kinugawa K, Nitta D, Inaba T, Maki H, Hatano M, et al. Readmission due to driveline infection can be predicted by new score by using serum albumin and body mass index during long-term left ventricular assist device support. J Artif Organs 2015;18:120–7. | < 50 DT patients |
| Imamura T, Kinugawa K, Shiga T, Endo M, Kato N, Inaba T, et al. Preoperative levels of bilirubin or creatinine adjusted by age can predict their reversibility after implantation of left ventricular assist device. Circ J 2013;77:96–104. | Do not report DT data |
| Ivak P, Pirk J, Tucanova Z, Maly J, Szarszoi O, Melenovsky V, et al. A single-center experience with magnetically levitated left ventricular assist device for treatment of end-stage heart failure. Clin Exp Surg 2020;8:7–16. | Do not report DT data |
| Ivak P, Pitha J, Wohlfahrt P, Kralova Lesna I, Stavek P, Dorazilova Z, et al. Endothelial dysfunction expressed as endothelial microparticles in patients with end-stage heart failure. Physiol Res 2014;63(Suppl. 3):S369–73. | < 50 DT patients |
| Iyengar A, Kwon OJ, Tamrat M, Salimbangon A, Satou N, Benharash P, et al. The in-hospital cost of ventricular assist device therapy: implications for patient selection. ASAIO J 2017;63:725–30. | Wrong indication |
| Izzy S, Renault S, Rubin D, Vaitkevicius H, Smallwood J, Givertz MM, et al. Neurovascular complications during long-term left ventricular assist device (LVAD) support: Single center experience. Stroke Conference: American Heart Association/American Stroke Association. 2016;47. | Do not report DT data |
| Izzy S, Rubin DB, Ahmed FS, Akbik F, Renault S, Sylvester KW, et al. Cerebrovascular accidents during mechanical circulatory support: new predictors of ischemic and hemorrhagic strokes and outcome. Stroke 2018;49:1197–203. | Do not report DT data |
| Jacobs S, Verhoeven J, Geens J, Rega F, Meyns B. One-year cost comparison between cardiac transplantation and LVAD therapy. Int J Artif Organs 2011;34(8):608. | Wrong patient population |
| Jacobs S, Verhoeven J, Meyns B. LVAD therapy becomes less expensive than transplantation after 29 months. Artif Organs 2012;36(5):A37. | Wrong patient population |
| Jaiswal A, Truby LK, Chichra A, Jain R, Myers L, Patel N, et al. Impact of obesity on ventricular assist device outcomes. J Card Fail 2020;26:287–97. | Do not report DT data |
| Jaiswal A, Truby LK, Chichra A, Jain R, Myers L, Patel N, et al. Impact of obesity on ventricular assist device outcomes: obesity and VAD outcomes. J Card Fail 2020;26:287–97. | Do not report DT data |
| Jaworska E, Wlodarczyk A, Budasz-Swiderska M. Clinical and cost-effectiveness of third-generation, implantable left ventricular assist devices for people with end-stage heart failure: a systematic review. Value Health 2012;15(7):A345. | Wrong indication |
| Jedeon Z, Cogswell R, Schultz J, John R, Roukoz H. Beta blocker and renin-angiotensin system inhibitors are associated with decreased mortality in patients with left ventricular assist devices. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2019;140. | Do not report DT data |
| Jennings DL, Wagner JL, To L, Nemerovski CW, Kalus JS, Morgan JA, et al. Epidemiology and outcomes associated with anemia during long-term support with continuous-flow left ventricular assist devices. J Card Fail 2014;20:387–91. | Wrong outcomes |
| John F, Sembrano R, Roy S, Gupta S, Nayak R, Plack D, et al. Pre-operative predictors for admission to a rehabilitation facility after LVAD implantation and its impact on long term survival. J Heart Lung Transplant 2016;(1):S165. | < 50 DT patients |
| John R, Kamdar F, Eckman P, Colvin-Adams M, Boyle A, Shumway S, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg 2011;92:1593–9; discussion 1599. | < 50 DT patients |
| John R, Naka Y, Park SJ, Sai-Sudhakar C, Salerno C, Sundareswaran KS, et al. Impact of concurrent surgical valve procedures in patients receiving continuous-flow devices. J Thorac Cardiovasc Surg 2014;147:581–9; discussion 589. | Do not report DT data |
| Johnson BV, Rao S, Kurcik KL, Mazurek JA, Tanna MS, Atluri P, et al. Aortic valve thickening as a novel risk factor for development of aortic incompetence after left ventricular assist device implantation. J Card Fail 2019;25(8 Suppl.):S161. | Do not report DT data |
| Johnson MR. The benefits and risks of left ventricular assist device implantation; where is the point of clinical equipoise? Cardiology (Switzerland) 2016;134(Suppl. 1):284. | Do not report DT data |
| Jonida Bejko J, Bottio T, Carrozzini M, Comisso M, Toto F, Tarzia V, et al. Propensity matched comparison of two different continuous-flow left ventricular assist devices. A matter of device? Eur J Heart Fail 2017;19(Suppl. 1):114. | Do not report DT data |
| Jorde U, Siddiqi N, Luke A, Sims DB, Saeed O, Patel SR, et al. Effect of a multifaceted team management approach on survival and stroke rates in heartmate 2 recipients. J Heart Lung Transplant 2017;36(4 Suppl. 1):S423. | Do not report DT data |
| Jorde UP, Shah AM, Sims DB, Madan S, Siddiqi N, Luke A, et al. Continuous-flow left ventricular assist device survival improves with multidisciplinary approach. Ann Thorac Surg 2019;108:508–16. | Do not report DT data |
| Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, Thomas SS, et al. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail 2014;7:310–9. | Do not report DT data |
| Joseph SM, Hall SA, Lima B, Robertson JO, Naftel DC, Myers S, et al. Does inotrope dependence make a difference in outcome in patients receiving left ventricular assist device? comparison between intermacs profiles 3 vs 4/5 from the intermacs registry. J Heart Lung Transplant 2017;36(4 Suppl. 1):S139–40. | Do not report DT data |
| Joshi Y, Borie M, Aissaoui N, Bel A, Latremoullie C, Jouan J. Experience with true percutaneous veno-pulmonary extracorporeal membrane oxygenation as right ventricular temporary support following left ventricular assist device insertion. Artif Organs 2020;44(3):E65–6. | Do not report DT data |
| Jprn U, Hokkaido University Hospital N, Osaka University Hospital Tokyo University H. A multicenter prospective study on prediction of right heart failure and prognosis in patients with severe heart failure after left ventricular assist device implantation. Japan: National Institute of Public Health; 2015. | Do not report DT data |
| Jprn U, Osaka University hospital Y. Clinical research of the left ventricular assist device with post-auricular connector. Japan: National Institute of Public Health; 2017. | < 50 DT patients |
| Kalathiya RJ, Houston BA, Chaisson JM, Grimm JC, Stevens GR, Sciortino CM, et al. Cardiac index declines during long-term left ventricular device support. Artif Organs 2016;40:1105–12. | < 50 DT patients |
| Kalavrouziotis D, Tong MZ, Starling RC, Massiello A, Soltesz E, Smedira NG, et al. Percutaneous lead dysfunction in the HeartMate II left ventricular assist device. Ann Thorac Surg 2014;97:1373–8. | Wrong outcomes |
| Kalinowski M, Kothari S, Kobeszko M, Josephson G, Cotts W, Pauwaa S, et al. Investigation of gastrointestinal bleeding among left ventricular assist device recipients. Am J Gastroenterol 2019;114(Suppl.):S338–9. | Do not report DT data |
| Kalinowski M, Kothari S, Kobeszko M, Josephson G, Cotts W, Pauwaa S, et al. Investigation of repeat gastrointestinal bleeding among continuous flow left ventricular assist device recipients. Gastroenterology 2020;158(6 Suppl. 1):S-248. | Do not report DT data |
| Kalinowski M, Kothari S, Kobeszko M, Josephson G, Cotts W, Pauwaa S, et al. A comparison of characteristics in left ventricular assist device patients with and without gastrointestinal bleeding. J Investig Med 2019;67(5):903–4. | Do not report DT data |
| Kalinowski M, Kothari S, Kobeszko M, Josephson G, Cotts W, Pauwaa S, et al. A comparison of characteristics in left ventricular assist device patients with and without gastrointestinal bleeding. Gastroenterology 2019;156(6 Suppl. 1):S–253. | Do not report DT data |
| Kalinowski M, Kothari S, Kobeszko M, Josephson G, Cotts W, Pauwaa S, et al. An institutional review of gastrointestinal bleeding among 563 continuous flow left ventricular assist device recipients. Gastrointest Endosc 2020;91:AB605–6. | Do not report DT data |
| Kalya AV, Tector AJ, Crouch JD, Downey FX, McDonald ML, Anderson AJ, et al. Comparison of Novacor and HeartMate vented electric left ventricular assist devices in a single institution. J Heart Lung Transplant 2005;24:1973–5. | Do not report DT data |
| Kanwar M, Khoo C, Lohmueller L, Bailey S, Murali S, Antaki J. Predicting post LVAD acute severe right heart failure using Bayesian analysis. J Heart Lung Transplant 2019;38(4 Suppl.):S357. | Do not report DT data |
| Kanwar MK, Lohmueller LC, Teuteberg J, Kormos RL, Rogers JG, Benza RL, et al. Risk assessment in patients with a left ventricular assist device across INTERMACS profiles using Bayesian analysis. ASAIO J 2019;65:436–41. | Wrong outcomes |
| Kapelios CJ, Lund LH, Selzman CH, Meyers SL, Koliopoulou A, Stehlik J, et al. Early and late right heart failure following LVAD implantation: epidemiology, natural history and outcomes. An analysis of the STS INTERMACS database. J Heart Lung Transplant 2019;38(4 Suppl.):S20. | Do not report DT data |
| Karen Booth K, Urban M, Robinson-Smith N, Woods A, Wrightson N, Scheuler S, et al. Right heart failure post LVAD therapy-timing of RVAD placement predicts survival. Eur J Heart Fail 2016;(1):480. | Do not report DT data |
| Karmpalioti M, Soyama Y, Huntjens P, Raymer D, LaRue S, Itoh A, et al. Clinical and echocardiographic prognostic markers of right ventricular failure after left ventricular assist device. J Am Coll Cardiol 2020;75(11):991. | Do not report DT data |
| Kassi M, Eshelbrenner C, Amione-Guerra J, Estep J. Utility of echocardiography in defining inflow cannula malposition in left ventricular assist device and the association with adverse outcome. J Heart Lung Transplant 2016;(1):S97. | Do not report DT data |
| Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H, et al. Pre-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant Off Publ Int Soc Heart Transplant 2012;31:1–8. | Do not report DT data |
| Katz JN, Adamson RM, John R, Tatooles A, Sundareswaran K, Kallel F, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: a one-year analysis of the US-TRACE study. J Heart Lung Transplant 2015;34:1542–8. | Do not report DT data |
| Katz JN, Jensen BC, Chang PP, Myers SL, Pagani FD, Kirklin JK. A multicenter analysis of clinical hemolysis in patients supported with durable, long-term left ventricular assist device therapy. J Heart Lung Transplant 2015;34:701–9. | Do not report DT data |
| Kawabori M, Kurihara C, Critsinelis A, Chou BP, Zhang Q, Kaku Y, et al. Effect of cardiac arrest with aortic cross-clamping during left ventricular assist device implantation. Interact Cardiovasc Thorac Surg 2020;30:47–53. | Do not report DT data |
| Kawabori M, Kurihara C, Critsinelis AC, Sugiura T, Kaku Y, Civitello AB, et al. Gastrointestinal bleeding after HeartMate II or HVAD implantation: incidence, location, etiology, and effect on survival. ASAIO J 2020;66:283–90. | Do not report DT data |
| Kawabori M, Kurihara C, Sugiura T, Civitello AB, Cohn WE, Fraizer OH, et al. Effect of preoperative small left ventricle on patients with chronic heart failure undergoing implantation of long-term continuous flow ventricular assist devices: comparative analysis of heartmate II and heartware devices. J Heart Lung Transplant 2017;36(4 Suppl. 1):S340–1. | Do not report DT data |
| Kawabori M, Kurihara C, Sugiura T, Cohn WE, Civitello AB, Frazier OH, et al. Continuous-flow left ventricular assist device implantation in patients with a small left ventricle. Ann Thorac Surg 2018;105:799–806. | Do not report DT data |
| Kawabori M, Kurihara C, Sugiura T, Delgado MR, Simpson L, Nair AP, et al. Effect of aortic cross-clamping during left ventricular assist device implantation: a single institutional 13-year experience over 500 implantations. J Heart Lung Transplant 2018;37(4 Suppl. 1):S481. | Do not report DT data |
| Kawabori M, Soffer J, Mastorianni M, Zhan Y, Warner KG, Rastegar H, et al. The effect of postoperative vasoplegia to the survival of LVAD recipients. J Heart Lung Transplant 2019;38(4 Suppl.):S360. | Do not report DT data |
| Kervan U, Kocabeyoglu SS, Aygun E, Demirkan B, Sert D, Akin Y, et al. Is tricuspid annular plane systolic excursion (TAPSE) predictor for out patients with LVAD right ventricular failure in LVAD patients with preoperative low value TAPSE on mild-term follow-up. Transplantation 2018;102(7 Suppl. 1):S836. | Do not report DT data |
| Khan A, Fan H, Sparrow C, Raymer D, Nassif M, Ewald G, et al. Cancer in patients with left ventricular assist devices. J Card Fail 2016;22(Suppl. 8):S126. | Do not report DT data |
| Khan MS, Yuzefpolskaya M, Memon MM, Usman MS, Garan AR, Demmer RT, et al. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: a meta-analysis. Circ Conf 2018;138. | Do not report DT data |
| Khan MS, Yuzefpolskaya M, Memon MM, Usman MS, Yamani N, Garan AR, et al. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. ASAIO J 2020;66:401–8. | Do not report DT data |
| Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, et al. Trends in the use and outcomes of ventricular assist devices among Medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol 2014;63:1395–404. | Wrong indication |
| Kiernan MS, Sundareswaran KS, Pham DT, Kapur NK, Pereira NL, Strueber M, et al. Preoperative determinants of quality of life and functional capacity response to left ventricular assist device therapy. J Card Fail 2016;22:797–805. | Do not report DT data |
| Kilic A, Katz JN, Joseph SM, Brisco-Bacik MA, Uriel N, Lima B, et al. Changes in pulmonary artery pressure before and after left ventricular assist device implantation in patients utilizing remote haemodynamic monitoring. ESC Heart Fail 2019;6:138–45. | Do not report DT data |
| Kilic A, Pagani FD, Likosky DS, Seese LM, Althouse AD, Kormos RL. Identifying temporal relationships between adverse events following implantation of durable left ventricular assist devices. Circ Conf 2018;138. | Do not report DT data |
| Kilic A, Phillips G, Chimanji N, Sai-Sudhakar CB, Hasan A, Higgins RSD, et al. Cost comparison between heart transplantation and left ventricular assist device implantation. J Card Fail 2014;(1):S84. | Wrong patient population |
| Kilic A, Sultan I, Yuh DD, Shah AS, Baumgartner WA, Cameron DE, et al. Ventricular assist device implantation in the elderly: nationwide outcomes in the United States. J Card Surg 2013;28:183–9. | Wrong indication |
| Kim M, Lamba HK, Chou B, Civitello AB, Delgado RM, Simpson L, et al. Outcomes and predictors of postoperative atrial fibrillation in patients with left ventricular assist devices. Stroke Conference: American Heart Association/American Stroke Association. 2019;50. | Do not report DT data |
| Kimura M, Nawata K, Kinoshita O, Yamauchi H, Itoda Y, Imamura T, et al. Cerebrovascular accident rate is different between centrifugal and axial-flow pumps, but survival and driveline infection rates are similar. Transplant Proc 2017;49:121–4. | Do not report DT data |
| Kitahara H, Najjar S, Ahmed S, Lam PH, Kadakkal A, Mohammed S, et al. One-year survival rate after thoracotomy for left ventricular assist device implantation compared with sternotomy. J Heart Lung Transplant 2020;39(4 Suppl.):S440-S1. | Do not report DT data |
| Kocabeyoglu S, Pac M, Kervan U, Karahan M, Aygun E, Beyazal O, et al. Preoperative patient optimization using low dose levosimendan improves outcomes of patients after left ventricular assist device implantation. J Heart Lung Transplant 2018;37(4 Suppl. 1):S484-S5. | Do not report DT data |
| Kociol RD. Time for MADIT-VAD?: ICDs among LVAD patients. JACC Heart Fail 2016;4:780–2. | Wrong study design |
| Koratala A, Olaoye OA, Kazory A. AKI and renal replacement therapy after implantation of left ventricular assist device. J Am Soc Nephrol 2017;28:975. | Wrong study design |
| Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, et al. The society of thoracic surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg 2019;107:341–53. | Duplicate record |
| Kugler C, Malehsa D, Schrader E, Tegtbur U, Guetzlaff E, Haverich A, et al. A multi-modal intervention in management of left ventricular assist device outpatients: dietary counselling, controlled exercise and psychosocial support. Eur J Cardio-Thorac Surg 2012;42:1026–32. | Do not report DT data |
| Kumar A, Shivamurthy P, Gluck J, Kluger J. Single center experience of ventricular arrhythmias in left ventricular assist device patients. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Kumar S, Rivas-Lasarte M, Rashid SM, Scatola A, Rochlani Y, Murthy S, et al. External validation and comparison of the EUROMACS and right ventricular failure risk score for right ventricular failure prediction after left ventricular assist device. J Heart Lung Transplant 2020;39(4 Suppl.):S38. | Do not report DT data |
| Kurihara C, Cohn WE, Kawabori M, Sugiura T, Civitello AB, Morgan JA. Long-term continuous-flow left ventricular assist device support after left ventricular outflow tract closure. ASAIO J 2019;65:558–64. | Do not report DT data |
| Kurihara C, Critsinelis A, Kawabori M, Sugiura T, Civitello AB, Morgan JA. Effect of preoperative atrial fibrillation on patients with chronic heart failure who undergo long-term continuous-flow LVAD implantation. ASAIO J 2018;64:594–600. | Do not report DT data |
| Kurihara C, Critsinelis AC, Kawabori M, Sugiura T, Loor G, Civitello AB, et al. Frequency and consequences of right-sided heart failure after continuous-flow left ventricular assist device implantation. Am J Cardiol 2018;121:336–42. | Do not report DT data |
| Kurihara C, Kawabori M, Critsinelis A, Sugiura T, Civitello AB, Simpson L, et al. Incidence and impact of severe LVAD-related infections requiring surgical treatment in obesity patients on long-term heartmate II and heartware support. J Heart Lung Transplant 2018;37(4 Suppl. 1):S361–2. | Do not report DT data |
| Kurihara C, Kawabori M, Sugiura T, Critsinelis AC, Wang S, Cohn WE, et al. Bridging to a long-term ventricular assist device with short-term mechanical circulatory support. Artif Organs 2018;42:589–96. | Do not report DT data |
| Kyo S, Nishimura T. Destination therapy by implantable LVAD: agenda for introduction of DT in Japan. J Card Fail 2016;22(9 Suppl. 1):S158. | Do not report DT data |
| Kyvernitakis A, Pappas O, Farmakiotis D, Mahale P, Horn E, Murali S, et al. Predictors and clinical implications of bloodstream infections in continuous flow left ventricular assist device recipients: a single institutional experience of 212 patients. Open Forum Infect Dis 2017;4(Suppl. 1):S548–9. | Do not report DT data |
| LaBuhn C, Uriel N, Sayer G, Kim G, Kalantari S, Raikhelkar J, et al. Surgical and nursing management in the care of patients post LVAD implant without the use of blood products. ASAIO J 2018;64(Suppl. 1):113. | < 50 DT patients |
| LaBuhn CJ, Jeevanandam V, Rodgers D, Ota T, Sayer G, Kim G, et al. Life during long term LVAD support. J Heart Lung Transplant. 2018;37(4 Suppl. 1):S476. | Do not report DT data |
| Lagrue S, Roussoulieres A, Dewachter C, Rimouche A, Cullus P, Luc Vachiery J. Pulmonary hypertension does not influence outcome following implantation of a left-ventricular assist device. Acta Cardiol 2019;74(Suppl. 1):24–5. | Do not report DT data |
| Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, et al. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardio-Thorac Surg 2010;37:357–61. | Do not report DT data |
| Lai GYY, Kesavabhotla K, Potts M, Jahromi B. Outcomes and management of intracranial hemorrhage (ICH) in patients with ventricular assist devices (VAD) Louise Eisenhardt Travel Scholarship. J Neurosurg 2018;128(4):44. | Do not report DT data |
| Lamba HK, Kim M, Hart L, Chou B, Rao C, Chatterjee S, et al. Different risk factors for ischemic and hemorrhagic stroke on continuous flow left ventricular assist device support. J Heart Lung Transplant 2019;38(4 Suppl.):S171–2. | Do not report DT data |
| Lampropulos JF, Kim N, Wang Y, Desai MM, Barreto-Filho JAS, Dodson JA, et al. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open Heart 2014;1(1) (no pagination). | Wrong patient population |
| Lampropulos JF, Kim N, Wang Y, Krumholz HM. Trends in 1-year hospitalizations and costs from 2004 to 2010 among Medicare fee-for-service beneficiaries with LVADs. Circulation: Cardiovascular Quality and Outcomes Conference: American Heart Association’s Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. 2013;6. | Wrong patient population |
| Lander BS, Patel K, Blackstone EH, Nordseth T, Starling RC, Gorodeski EZ. Post-acute care trajectories in the first year following hospital discharge after left ventricular assist device implantation. J Am Med Dir Assoc 2016;17:908–12. | Do not report DT data |
| Lander MM, Kunz N, Dunn E, Althouse AD, Lockard K, Shullo MA, et al. Substantial reduction in driveline infection rates with the modification of driveline dressing protocol. J Card Fail 2018;24:746–52. | Do not report DT data |
| Landis ZC, Soleimani B, Stephenson ER, El-Banayosy A, Pae WE. Severity of end-organ damage as a predictor of outcomes after implantation of left ventricular assist device. ASAIO J 2015;61:127–32. | < 50 DT patients |
| Lei L, Mansoor E, Cooper GS, Wong RC. Epidemiology and risk factors of upper gastrointestinal bleeding after ventricular assist devices implantation in the United States. Gastroenterology 2018;154(6 Suppl. 1):S-698. | Do not report DT data |
| Lenartova K, Vila P, Williamson S, Moravcova S, Wypych-Zych A, Stevens K, et al. LVAD, heart failure journey continues. J Cardiothorac Vasc Anesth 2020;34(Suppl. 1):S10. | Do not report DT data |
| Letsou GV, Ho JK, Musfee FI, Frazier OH, Wang N. Causes of mortality in patients undergoing continuous-flow LVAD implantation. ASAIO J 2020;66(Suppl. 2):3. | Do not report DT data |
| Letsou GV, Ho JK, Musfee FI, Frazier OH, Wang N. Post-transplant survival after continuous-flow LVAD implantation is superior to survival after pulsatile-flow LVAD implantation. ASAIO Journal 2020;66(Suppl. 2):37. | Do not report DT data |
| Leuck A, Schultz J, Mazzulla F, John R, Spratt J, Walts AN, et al. Bridge to transplant status is associated with improved survival after the development of left ventricular assist device infection. J Heart Lung Transplant 2017;36(4 Suppl. 1):S259. | Do not report DT data |
| Levin AP, Uriel N, Takayama H, Mody KP, Ota T, Yuzefpolskaya M, et al. Device exchange in HeartMate II recipients: long-term outcomes and risk of thrombosis recurrence. ASAIO J 2015;61:144–9. | < 50 DT patients |
| Levin L, Wieselthaler G, Aras M, Klein L. Malnutrition is associated with right ventricular failure at the time of left ventricular device implantation. Artif Organs 2020;44(3):E122–3. | Do not report DT data |
| Lietz K, Branch A, McGrath M, Herre J. Relationship between the cost and cause of hospital readmissions after LVAD implantation. J Heart Lung Transplant 2014;(1):S101–2. | Wrong patient population |
| Lim S, Chue CD, Ranasinghe A, Mascaro JG. Discontinuation of aspirin in HeartMate 3: long-term outcomes. J Heart Lung Transplant 2020;39(4 Suppl.):S48. | Do not report DT data |
| Lochel S, Maukel LM, Weidner G, de By TMMH, Spaderna H. Gender differences in psychosocial and clinical characteristics in the European Registry for Patients with Mechanical Circulatory Support. Heart Lung 2021;50(6):845–52. | Do not report DT data |
| Loehn T, Mierke J, Kuehns C, Schweigler T, Pfluecke C, Youssef A, et al. Long-term survival after early vs. late initiation of percutaneous mechanical support in infarct-related cardiogenic shock. Eur Heart J 2018;39(Suppl. 1):624. | Do not report DT data |
| Loforte A, Gliozzi G, Mariani C, De By T, Schonrath F, Potapov E, et al. Concomitant cardiac procedures during implantation of long-term continuous-flow LVADs: a European registry for patients with mechanical circulatory support (EUROMACS) analysis. Artif Organs 2020;44(3):E75. | Do not report DT data |
| Loforte A, Gliozzi G, Mariani C, De By T, Schonrath F, Potapov E, et al. Concomitant mitral valve surgery in left ventricular assist device recipients: a European registry for patients with mechanical circulatory support (EUROMACS) analysis. Artif Organs 2020;44(3):E125. | Do not report DT data |
| Loforte A, Montalto A, Lilla della Monica P, Lappa A, Contento C, Menichetti A, et al. Mechanical circulatory support in advanced heart failure: single-center experience. Transplant Proc 2014;46:1476–80. | < 50 DT patients |
| Loforte A, Montalto A, Musumeci F, Mariani C, Polizzi V, Lilla Della Monica P, et al. A novel risk model to predict right ventricular failure after continuous flow left ventricular assist device implantation. J Heart Lung Transplant 2018;37(4 Suppl.):S370. | Do not report DT data |
| Loforte A, Musumeci F, Montalto A, Pilato E, Lilla Della Monica P, Grigioni F, et al. Use of mechanical circulatory support devices in end-stage heart failure patients. J Card Surg 2014;29:717–22. | < 50 DT patients |
| Loghmanpour NA, Kanwar MK, Druzdzel MJ, Benza RL, Murali S, Antaki JF. A new Bayesian network-based risk stratification model for prediction of short-term and long-term LVAD mortality. ASAIO J 2015;61:313–23. | Do not report DT data |
| Logstrup BB, Nemec P, Schoenrath F, Gummert J, Pya Y, Potapov E, et al. Heart failure etiology and risk of right heart failure in adult left ventricular assist device support: the European Registry for Patients with Mechanical Circulatory Support (EUROMACS). Scand Cardiovasc J 2020;54:306–14. | Do not report DT data |
| Lolay GAK, Guglin M, George B, Kido K. The value of N. terminal pro. brain natriuretic peptide after LVAD implantation. J Am Coll Cardiol 2016;(1):1371. | Do not report DT data |
| Louis C, Gosev I, McNitt S, Prasad S, Vidula H, Alexis J, et al. Risk of adverse events in patients undergoing left ventricular assist device implantation in cardiogenic shock. ASAIO J 2019;65(Suppl. 1):46. | Do not report DT data |
| Louis C. Risk of adverse events with left ventricular assist devicein cardiogenic shock patients. Circulation Conference: American Heart Association’s. 2019;140. | Do not report DT data |
| Love WT, Zawadowski GM, Noelting J, Horsley-Silva JL, Staley LL, Amity ME, et al. Increased risk of gastrointestinal bleeding in heartmate II compared to heartware left ventricular assist devices – a single center retrospective cohort. J Heart Lung Transplant 2016;(1):S79. | Do not report DT data |
| Luc JGY, Tchantchaleishvili V, Phan K, Dunlay SM, Maltais S, Stulak JM. Medical therapy compared with surgical device exchange for left ventricular assist device thrombosis: a systematic review and meta-analysis. ASAIO J (Am Soc Artif Intern Organs: 1992) 2019;65:307–17. | Do not report DT data |
| Luigi Adamo L, Tang Y, Nassif M, Novak E, Jones PG, Larue S, et al. The heart mate risk score identifies patients with similar mortality risk across all INTERMACS classes in a large multicenter analysis: low INTERMACS class should not be a contraindication to LVAD. Eur J Heart Fail 2016;(1):307. | Do not report DT data |
| Lundgren S, High R, Poon C, Raichlin E, Zolty R, Burdorf A, et al. Psychosocial factors and outcomes with left ventricular assist device therapy. J Heart Lung Transplant 2017;36(4 Suppl. 1):S338. | Do not report DT data |
| Lundgren S, Stoller D, Lyden E, Burdorf A, Hyden M, Zolty R, et al. Outcomes in patients on digoxin following LVAD implantation. J Card Fail 2019;25(8 Suppl.):S165–6. | Do not report DT data |
| Magnetta DA, Kang J, Wearden PD, Smith KJ, Feingold B. Cost-effectiveness of ventricular assist device destination therapy for advanced heart failure in Duchenne muscular dystrophy. J Heart Lung Transplant 2016;(1):S353. | Wrong patient population |
| Magnetta DA, Kang J, Wearden PD, Smith KJ, Feingold B. Cost-effectiveness of ventricular assist device destination therapy for advanced heart failure in Duchenne Muscular Dystrophy. Pediatr Cardiol 2018;39:1242–8. | Paediatric population |
| Maharaj V, Masotti M, Schultz J, Martin CM, John R, Alexy T, et al. Trends in renal function prior to and after LVAD placement and association with post LVAD mortality. J Heart Lung Transplant 2021;40(4 Suppl.):S422. | Do not report DT data |
| Maharaj V, Schultz J, Charpentier V, Duval S, John R, Shaffer A, et al. Higher body mass index is associated with end stage renal failure after left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S410. | Do not report DT data |
| Mahfood Haddad T, Saurav A, Smer A, Azzouz MS, Akinapelli A, Williams MA, et al. Cardiac rehabilitation in patients with left ventricular assist device: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev 2017;37:390–6. | Do not report DT data |
| Mahr C, McGee E, Cheung A, Mokadam NA, Strueber M, Slaughter MS, et al. Cost-effectiveness of thoracotomy approach for the implantation of a small intrapericardial centrifugal LVAD. J Heart Lung Transplant 2020;39(4 Suppl.):S366. | Wrong patient population |
| Mahr C, McGee E, Cheung A, Mokadam NA, Strueber M, Slaughter MS, et al. Cost-effectiveness of thoracotomy approach for the implantation of a centrifugal left ventricular assist device. ASAIO J 1992. | Duplicate record |
| Mahr C, McGee E, Cheung A, Mokadam NA, Strueber M, Slaughter MS, et al. Cost-effectiveness of thoracotomy approach for the implantation of a centrifugal left ventricular assist device. ASAIO J (Am Soc Artif Intern Organs: 1992) 2020;66:855–61. | Wrong patient population |
| Mai X, Clerkin K, Topkara VK, Takeda K, Demmer R, Yuzefpolskaya M, et al. Comparison of survival and modes of death by ICD status among LVAD recipients. J Heart Lung Transplant 2017;36(4 Suppl. 1):S182. | Do not report DT data |
| Mai X, Topkara VK, Wong K, Castagna F, Trinh PN, Sreekanth S, et al. Long-term outcomes in continuous-flow left ventricular assist device patients with and without ICD. J Heart Lung Transplant 2016;(1):S24. | Do not report DT data |
| Majeed F, Kop WJ, Poston RS, Kallam S, Mehra MR. Prospective, observational study of antiplatelet and coagulation biomarkers as predictors of thromboembolic events after implantation of ventricular assist devices. Nat Clin Pract Cardiovasc Med 2009;6:147–57. | < 50 DT patients |
| Makki N, Mesubi O, Steyers C, Olshansky B, Abraham WT. Meta-analysis of the relation of ventricular arrhythmias to all-cause mortality after implantation of a left ventricular assist device. Am J Cardiol 2015;116:1385–90. | < 50 DT patients |
| Makki N, Mesubi O, Steyers C, Olshansky B. Ventricular arrhythmias and mortality after left ventricular assist devices implantation: a meta-analysis. Circulation Conference: American Heart Association’s. 2014;130. | Do not report DT data |
| Malick A, Naka Y, Sanchez J, Butler C, Ning Y, Kurlansky P, et al. Development of De Novo aortic insufficiency in patients with HeartMate 3. J Heart Lung Transplant 2020;39(4 Suppl.):S406–7. | Do not report DT data |
| Malik S, Raichlin E, Lyden E, Hewlett A. Gastrointestinal bleeding in patients with left ventricular assist devices; whereas the leak and what are the outcomes? Gastrointest Endosc 2017;85(5 Suppl. 1):AB447. | Do not report DT data |
| Maltais S, Anwer LA, Haglund NA, Cowger J, Shah P, Aaronson KD, et al. Temporal differences in outcomes during long-term mechanical circulatory support. J Card Fail 2017;23:852–8. | Do not report DT data |
| Maltais S, Anwer LA, Tchantchaleishvili V, Haglund NA, Dunlay SM, Aaronson KD, et al. Left lateral thoracotomy for centrifugal continuous-flow left ventricular assist device placement: an analysis from the mechanical circulatory support research network. ASAIO J 2018;64:715–20. | Do not report DT data |
| Maltais S, Haglund N, Shah P, Cowger J, Aaronson KA, Pagani F, et al. Lessons learned from over 250 continuous-low centrifugal left ventricular assist device implantation: growing experience and outcomes in alternative approaches era. J Heart Lung Transplant 2016;(1):S39. | Do not report DT data |
| Maltais S, Haglund NA, Davis ME, Cowger J, Shah P, Pagani FD, et al. Heart failure etiology influences outcomes after continuous-flow LVAD implantation. J Heart Lung Transplant 2016;(1):S110–1. | Do not report DT data |
| Maltais S, Tchantchaleishvili V, Daly RC, Joyce DL, Joyce LD, Kushwaha SS, et al. Should prophylactic tricuspid valve surgery be considered in patients with atrial fibrillation undergoing LVAD implantation? J Heart Lung Transplant 2017;36(4 Suppl. 1):S29–30. | Do not report DT data |
| Marasco SF, Summerhayes R, Quayle M, McGiffin D, Luthe M. Cost comparison of heart transplant vs. left ventricular assist device therapy at one year. Clin Transplant 2016;30:598–605. | Wrong indication |
| Mariani S, Dogan G, Hanke JS, Wendl RM, Chatterjee A, Deniz E, et al. Left ventricular assist device implantation via left thoracotomy and upper hemisternotomy: long-term follow-up of 111 patients. Int J Artif Organs 2019;42(8):413. | Do not report DT data |
| Mariani S, Li T, Bounader K, Boethig D, Schode A, Hanke JS, et al. Sex differences in outcomes following less-invasive left ventricular assist device implantation. Ann Cardiothorac Surg 2021;10:255–67. | Do not report DT data |
| Mariani S, Michaelis J, Dogan G, Hanke J, Chatterjee A, Bothig D, et al. Minimally invasive implatation of left ventricular assist devices: a tool to increase survival in women. Artif Organs 2020;44(3):E63. | Do not report DT data |
| Markham R, Challa A, Cafaro J, Bancroft J, Wockner L, Kyranis S, et al. Comparison of CoaguChek XS INR and laboratory inr in patients with a Heartware continuous flow left ventricular assist device. Heart Lung Circ 2016;25(Suppl. 2):S260. | Wrong indication |
| Martin SI, Wellington L, Stevenson KB, Mangino JE, Sai-Sudhakar CB, Firstenberg MS, et al. Effect of body mass index and device type on infection in left ventricular assist device support beyond 30 days. Interact Cardiovasc Thorac Surg 2010;11:20–3. | Do not report DT data |
| Masashi Kawabori M, Lofftus S, Vest A, Pramil V, Zhan Y, Warner KG, et al. Preoperative geriatric nutrition risk index scoring is predictive of survival in LVAD recipients. Eur J Heart Fail 2019;21(Suppl. 1):83–4. | Do not report DT data |
| McIlvennan CK, Bryce K, Lindenfeld J, Allen LA, Lanfear DE. Assessment of cognitive function prior to and after implantation of left ventricular assist device. J Heart Lung Transplant 2016;(1):S165–6. | Do not report DT data |
| McIlvennan CK, Thompson JS, Matlock DD, Cleveland JC, Jr, Dunlay SM, LaRue SJ, et al. A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for advanced heart failure: DECIDE-LVAD: rationale, design, and pilot data. J Cardiovasc Nurs 2016;31:E8–20. | Wrong intervention |
| McKellar SH, Deo S, Daly RC, Durham LA, 3rd, Joyce LD, Stulak JM, et al. Durability of central aortic valve closure in patients with continuous flow left ventricular assist devices. J Thorac Cardiovasc Surg 2014;147:344–8. | Do not report DT data |
| Meehan K, Graney N, Combs P, Andrade A, Macaluso G, Tatooles A, et al. Outcomes of mechanical circulatory support in women in a single center. Int J Artif Organs 2018;41(9):540. | Do not report DT data |
| Mehta P, Imamura T, Belkin MN, Rodgers D, Sarswat N, Kim G, et al. Neurohormonal blockade reduces adverse events during LVAD support. J Heart Lung Transplant 2018;37(4 Suppl. 1):S180–1. | Do not report DT data |
| Melehy A, Seres D, Mullen H, Sanchez J, Kurlanksy P, Garan R, et al. Comprehensive nutrition assessment before left ventricular assist device implantation in chronically Ill hospitalized patients. J Heart Lung Transplant 2019;38(4 Suppl.):S82. | Do not report DT data |
| Melnikov S, Abuhazira M, Golobov D, Yaari V, Jaarsma T, Ben Gal T. Depression and anxiety moderate the relationship between body image and personal well-being among patients with an implanted left ventricular assist device. J Cardiovasc Nurs 2020;35:149–55. | Wrong study design |
| Menon AK, Baranski SK, Unterkofler J, Autschbach R, Moza AK, Goetzenich A, et al. Special treatment and wound care of the driveline exit site after left ventricular assist device implantation. Thorac Cardiovasc Surgeon 2015;63:670–4. | < 50 DT patients |
| Meyer AL, Malehsa D, Bara C, Budde U, Slaughter MS, Haverich A, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail 2010;3:675–81. | < 50 DT patients |
| Meyer AL, Malehsa D, Bara C, Haverich A, Strueber M. Implantation of rotary blood pumps into 115 patients: a single-centre experience. Eur J Cardio-Thorac Surg 2013;43:1233–6. | < 50 DT patients |
| Meyns B, Jacobs S, Van Den Bossche K, Verhoeven J, Bostic RR, Vanhaecke J, et al. Cost of 1-year LVAD destination therapy in chronic heart failure: a comparison with heart transplantation. J Heart Lung Transplant 2013;(1):S98–9. | < 50 DT patients |
| Mihalj M, Heinisch PP, Schober P, Dobner S, Fuerholz M, Martinelli M, et al. Third generation continuous flow left ventricular assist devices: a comparative outcome analysis by device type. Eur Heart J 2021;42(Suppl. 1):946. | < 50 DT patients |
| Milgrom A, Collins A, Grubbs JA, Derrick C, Logan K, Edelson W, et al. Determinants of infection at a nontransplanting cardiothoracic LVAD program. Open Forum Infect Dis 2019;6(Suppl. 2):S450. | Do not report DT data |
| Miller LW, Lietz K. Candidate selection for long-term left ventricular assist device therapy for refractory heart failure. J Heart Lung Transplant 2006;25:756–64. | Wrong study design |
| Mirza KK, Xie R, Cowger J, Kirklin JK, Meyns B, Gustafsson F, et al. Comparative analysis of regional outcomes and adverse events after continuous-flow left ventricular assist device implantation: an IMACS analysis. J Heart Lung Transplant 2020;39:904–14. | Do not report DT data |
| Mishra V, Fiane AE, Winsnes BA, Geiran O, Sorensen G, Hagen TP, et al. Cardiac replacement therapies: outcomes and costs for heart transplantation versus circulatory assist. Scand Cardiovasc J 2017;51:1–7. | Wrong indication |
| Moayedifar R, Sandner S, Riebandt J, Wiedemann D, Haberl T, Schloeglhofer T, et al. Renal function after ventricular assist device implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S347–8. | Do not report DT data |
| Moazami N, Ewald GA, Pagani FD, John R, MacGillivray TE, Chen L, et al. Clinical durability and low incidence of pump replacement of a continuous-flow left ventricular assist device. J Heart Lung Transplant 2009;(1):S207. | Wrong indication |
| Moazami N, Milano CA, John R, Sun B, Adamson RM, Pagani FD, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500–5. | Do not report DT data |
| Mohamedali B, Bhat G, Yost G, Tatooles A. Changes in spirometry after left ventricular assist device implantation. Artif Organs 2015;39:1046–50. | Do not report DT data |
| Mohamedali B, Bhat G. The influence of pre-left ventricular assist device (LVAD) implantation glomerular filtration rate on long-term LVAD outcomes. Heart Lung Circ 2017;26:1216–23. | Do not report DT data |
| Mohamedali B, Yost G, Bhat G. Is diabetes mellitus a risk factor for poor outcomes after left ventricular assist device placement? Tex Heart Inst J 2017;44:115–9. | Do not report DT data |
| Mohamedali B, Yost G, Bhat G. Mechanical circulatory support improves diabetic control in patients with advanced heart failure. Eur J Heart Fail 2014;16:1120–4. | Do not report DT data |
| Mohamedali B, Yost G, Bhat G. Obesity as a risk factor for consideration for left ventricular assist devices. J Card Fail 2015;21:800–5. | Do not report DT data |
| Mohiyaddin S, Yousaf A, Hailan A, Aldweik M. Prolonged use of levitronix right ventricular assist device (RVAD) in patients with long term left ventricular assist device (LVAD). Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Molfetta A, Casadio L, Fresiello L, Iacobelli R, Filippelli S, Perri G, et al. Short-and long-term changes in ventricular loading conditions in LVAD patients: pulsatile vs continuous flow LVAD. J Cardiovasc Transl Res 2018;11(1):69–70. | Paediatric population |
| Molina EJ, Najjar SS, Ahmed S, Rodrigo ME, Hofmeyer M, Kadakkal A, et al. Real world experience with the HeartMate 3 (HM3) Left Ventricular Assist Device (LVAD): analysis of the first 125 consecutive patients at a single institution. J Heart Lung Transplant 2020;39(4 Suppl.):S338. | Do not report DT data |
| Molina EJ, Najjar SS, Ahmed S, Rodrigo ME, Hofmeyer M, Kadakkal A, et al. Real world experience with the HeartMate 3 (HM3) Left Ventricular Assist Device (LVAD): analysis of the first 125 consecutive patients at a single institution. J Heart Lung Transplant 2020;39:S338. | Do not report DT data |
| Mondellini GM, Pinsino A, Braghieri L, Javaid A, Lin EF, Cagliostro B, et al. Serum cystatin C as a predictor of early outcomes and long-term mortality in contemporary LVAD patients. J Heart Lung Transplant 2020;39(4 Suppl.):S182. | Do not report DT data |
| Moreno L, Leblanc M, Gurley M, McCarthy P, Paganini EP. Dialytic support in patients with acute renal failure with implantable left ventricular assist devices. J Intensive Care Med 1997;12:33–9. | < 50 DT patients |
| Moreno SG. Letter by moreno regarding article, ‘cost-effectiveness analysis of continuous flow left ventricular assist devices as destination therapy’. Circ Heart Fail 2012;5:e50. | Wrong study design |
| Morgan JA, Paone G, Nemeh HW, Henry SE, Gerlach B, Williams CT, et al. Non-cardiac surgery in patients on long-term left ventricular assist device support. J Heart Lung Transplant 2012;31:757–63. | < 50 DT patients |
| Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2012;31:715–8. | < 50 DT patients |
| Morgan JA, Paone G, Nemeh HW, Murthy R, Williams CT, Lanfear DE, et al. Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant 2013;32:398–403. | Do not report DT data |
| Morgan JA, Tsiouris A, Nemeh HW, Hodari A, Karam J, Brewer RJ, et al. Impact of concomitant cardiac procedures performed during implantation of long-term left ventricular assist devices. J Heart Lung Transplant 2013;32:1255–61. | Do not report DT data |
| Morreale C, Paliga R, Kothari S, Meehan K, Coyle L, Morack M, et al. Psychosocial characteristics and outcomes in patients with ventricular assist device. J Heart Lung Transplant 2019;38(4 Suppl.):S448. | Do not report DT data |
| Morris KL, Haleem A, Patel A, Garcia-Cortes R, Chaudhry S, Zanotti G, et al. Increased Right Heart Failure (RHF) in high BMI LVAD recipients. J Heart Lung Transplant 2020;39(4 Suppl.):S38. | Do not report DT data |
| Morrissey O, Xie R, Schaenman J, Husain S, Mooney M, Nakatani T, et al. Epidemiology of fungal infections (FI) in mechanical circulatory support device (MCSD) recipients: analysis of IMACS registry 2013-2015. J Heart Lung Transplant 2017;36(4 Suppl. 1):S27. | Do not report DT data |
| Morshuis M, Garbade J, Zimpfer D, Shaw S, Lavee J, Gustafsson F, et al. Clinical outcomes with heartmate 3TM left ventricular assist device as treatment for advanced heart failure: 12-month outcomes from the ELEVATE registry. J Heart Lung Transplant 2018;37(4 Suppl. 1):S84. | Do not report DT data |
| Movahedi F, Carey L, Zhang Y, Padman R, Antaki J, Kanwar M. Analysis of post-LVAD clinical pathways. J Heart Lung Transplant 2017;36(4 Suppl. 1):S352. | Do not report DT data |
| Movahedi F, Lohmueller L, Zhang Y, Padman R, Antaki J. Clinical journey of severe heart failure patients after left ventricular assistance device implant. J Card Fail 2017;23(8 Suppl. 1):S107. | Do not report DT data |
| Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Kron IL, Kern JA. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. J Thorac Cardiovasc Surg 2013;145:566–74. | Wrong indication |
| Murphy M, Foster M, Wald J, Marble J, Rao SD, Atluri P, et al. Partial recovery of ejection fraction with neurohormonal blockade improves long-term event free survival for patients with continuous flow LVAD. J Heart Lung Transplant 2020;39(4 Suppl.):S132–3. | Do not report DT data |
| Musa T, Chue C, Lim HS. Renal recovery following use of left ventricular assist device in advanced heart failure patients. Heart 2017;103(Suppl. 5):A16. | Do not report DT data |
| Muslem R, Akin S, Constantinescu AA, Manintveld O, Brugts JJ, van der Heiden CW, et al. Long-term mechanical durability of left ventricular assist devices: an urgent call for periodic assessment of technical integrity. ASAIO J 2018;64:521–8. | Do not report DT data |
| Muslem R, Caliskan K, Akin S, Yasar YE, Sharma K, Gilotra NA, et al. Effect of age and renal function on survival after left ventricular assist device implantation. Am J Cardiol 2017;120:2221–5. | Do not report DT data |
| Muslem R, Ong CS, Tomashitis B, Schultz J, Ramu B, Craig ML, et al. Pulmonary arterial elastance and INTERMACS-defined right heart failure following left ventricular assist device. Circ Heart Fail 2019;12:e005923. | Do not report DT data |
| Myers TJ, Macris MP. Clinical experience with the HeartMate left ventricular assist device. Heart Failure 1994;10:247–56 + 58. | Wrong indication |
| Nadziakiewicz P, Pacholewicz J, Zakliczynski M, Niklewski T, Borkowski J, Hrapkowicz T, et al. Comparison of mechanical circulatory support by the use of pulsatile left ventricular assist devices polvad MEV and continuous flow heart ware and Heart Mate II in a single-center experience. Transplant Proc 2016;48:1770–4. | < 50 DT patients |
| Naik A, Akhter SA, Fedson S, Jeevanandam V, Rich JD, Koyner JL. Acute kidney injury and mortality following ventricular assist device implantation. Am J Nephrol 2014;39:195–203. | Do not report DT data |
| Nakagawa S, Luna JM, Topkara VK, Yuzefpolskaya M, Vawdrey D, Garan AR, et al. Novel palliative care integration program increases days alive and out of hospital and reduces inpatient direct costs among left ventricular assist device patients. J Heart Lung Transplant 2017;36(4 Suppl. 1):S173. | Wrong patient population |
| Nakanishi K, Homma S, Han J, Takayama H, Colombo PC, Yuzefpolskaya M, et al. Prevalence, predictors, and prognostic value of residual tricuspid regurgitation in patients with left ventricular assist device. J Am Heart Assoc 2018;7:24. | Do not report DT data |
| Namn Y, Cohen-Mekelburg S, Sherman Z, Crawford CV. Predictors of gastrointestinal rebleed events in patients with a ventricular assist device (VAD). Am J Gastroenterol 2017;112(Suppl. 1):S303–4. | Do not report DT data |
| Nativi-Nicolau J, Healy A, Abdelrahman S, Jaramillo J, Elmer A, Tagge S, et al. Readmission rates in patients with left ventricular assist devices. J Heart Lung Transplant 2016;(1):S335. | Do not report DT data |
| Nayak A, Neill C, Kormos RL, Lagazzi L, Halder I, McTiernan C, et al. Chemokine receptor patterns and right heart failure in mechanical circulatory support. J Heart Lung Transplant 2017;36:657–65. | < 50 DT patients |
| NCT, Abbott Medical Devices Y, Thoratec C. PREVENtion of HeartMate II Pump Thrombosis 2014. URL: https://classic.clinicaltrials.gov/ct2/show/NCT02158403 (accessed September 2020). | Do not report DT data |
| NCT, Abbott Medical Devices Y, Thoratec Corporation Yes 29/10/ NCT. Thoratec HeartMate II Left Ventricular Assist System (LVAS) for Destination Therapy. 2005. URL: https://classic.clinicaltrials.gov/ct2/show/NCT00121485 (accessed September 2020). | Duplicate record |
| NCT, Abbott Medical Devices Y. Implantation of the HeartMate 3 in Subjects with Heart Failure Using Surgical SWIFT HM3 PMS. 2020. URL: https://classic.clinicaltrials.gov/ct2/show/NCT04548128 (accessed September 2020). | < 50 DT patients |
| NCT, Joe Elie Salem N. Medical Care versus Ventricular Assist Device for the Management of Endstage Heart Failure (MEVADE). 2017. URL: https://classic.clinicaltrials.gov/ct2/show/NCT03105726 (accessed September 2020). | Do not report DT data |
| NCT, Medtronic Cardiac R, Heart Failure Y. A Clinical Trial to Evaluate the HeartWare™ VentricularAssist System (ENDURANCE SUPPLEMENTAL TRIAL). 2013. URL: https://classic.clinicaltrials.gov/ct2/show/NCT01966458 (accessed September 2020). | Duplicate record |
| NCT, Medtronic Cardiac R, Heart Failure Y. Post-approval Study on Patients Who Received a HeartWare HVAD® During IDE Trials. 2013. URL: https://classic.clinicaltrials.gov/ct2/show/NCT01832610 | Wrong patient population |
| NCT, Medtronic Cardiac R, Heart Failure Y. The HeartWare™ Ventricular Assist System as Destination Therapy of Advanced Heart Failure: The ENDURANCE Trial. 2010. URL: https://classic.clinicaltrials.gov/ct2/show/NCT01166347 | Duplicate record |
| NCT, Thomas Jefferson University Y, Thoratec C, National Skeletal Muscle Research C, Greater New York Geriatric Cardiology C. Frailty: Prevalence and Response to Left Ventricular Assist Device Therapy in Older Heart Failure Patients. 2014. URL: https://classic.clinicaltrials.gov/ct2/show/NCT02156583 | < 50 DT patients |
| NCT, Vastra Gotaland Region Y, Karolinska University H, University Hospital L, Skane University H, Uppsala University H, et al. Swedish Evaluation of Left Ventricular Assist Device as Permanent Treatment in End-stage Heart Failure. 2015. URL: https://classic.clinicaltrials.gov/ct2/show/NCT02592499 | Duplicate record |
| NCT, Ventracor Y, International Center for Health O, Innovation R. VentrAssistTM LVAD for the Treatment of Advanced Heart Failure – Destination Therapy. 2007. URL: https://classic.clinicaltrials.gov/ct2/show/NCT00490321 | Do not report DT data |
| NCT. Dabigatran as an Alternative Anticoagulant in Patients With Left Ventricular Assist Device (LVAD). 2016. URL: https://classic.clinicaltrials.gov/ct2/show/NCT02872649. | Wrong intervention |
| NCT. Evaluation of the Jarvik 2000 Left Ventricular Assist System with Post-auricular Connector–Destination Therapy Study. 2012. URL: https://clinicaltrialsgov/show/NCT01627821. | Duplicate record |
| NCT. LVAD in Non Cardiac Transplant Candidates and Non Responders to Resynchronization. 2010. URL: https://clinicaltrialsgov/show/NCT01126944. | < 50 DT patients |
| NCT. LVAD versus GDMT in Ambulatory Advanced Heart Failure Patients. 2021. URL: https://clinicaltrialsgov/show/NCT04768322. | Duplicate record |
| NCT. Self-management App for Patients with Left-ventricular Assist Devices. 2017. URL: https://clinicaltrialsgov/show/NCT03049748. | < 50 DT patients |
| NCT. VentrAssistTM LVAD for the Treatment of Advanced Heart Failure – Destination Therapy. 2007. URL: https://clinicaltrialsgov/show/NCT00490321. | < 50 DT patients |
| Nelson T, George S, Phancao A, El Banayosy A, Long JW. Effects of residential distance from LVAD implanting center on survival. J Heart Lung Transplant 2019;38(4 Suppl.):S345. | Do not report DT data |
| Neo CL, Kerk KL, Tay JH, Tan JL, Leam JL, Tan TE, et al. Functional recovery post left ventricular assist device implantation in INTERMACS profile 1 population. J Heart Lung Transplant 2019;38(4 Suppl.):S352. | Do not report DT data |
| Nitta D, Kinugawa K, Imamura T, Amiya E, Hatano M, Kinoshita O, et al. A Useful scoring system for predicting right ventricular assist device requirement among patients with a paracorporeal left ventricular assist device. Int Heart J 2018;59:983–90. | Do not report DT data |
| Nowacka A, Hullin R, Tozzi P, Barras N, Regamey J, Yerly P, et al. Short-term single-centre experience with the HeartMate 3 left ventricular assist device for advanced heart failure. Eur J Cardio Thorac Surg Off J Eur Assoc Cardio Thorac Surg. 2020;01. | < 50 DT patients |
| Numan L, Ramjankhan FZ, Oberski DL, Oerlemans MIFJ, Aarts E, Gianoli M, et al. Propensity score-based analysis of long-term outcome of patients on HeartWare and HeartMate 3 left ventricular assist device support. ESC Heart Fail 2021;8:1596–603. | Do not report DT data |
| Numan L, Ramjankhan FZ, Oberski DL, Oerlemans MIFJ, Aarts E, Gianoli M, et al. Propensity score-based analysis of long-term outcome of patients on HeartWare and HeartMate 3 left ventricular assist device support. ESC Heart Fail 2021;8(2):1596–603. | Do not report DT data |
| Numan L, Ramjankhan FZ, Oberski DL, Oerlemans MIFJ, Aarts E, Gianoli M, et al. Long-term outcome of patients on HeartWare and HeartMate 3 support in a single centre: a propensity score-based analysis. Eur J Heart Fail 2021;23(Suppl. 2):148–9. | Do not report DT data |
| Nunes AJ, MacArthur RG, Klarenbach SW. Cost of ventricular assist device therapy at a large canadian transplantation centre. Can J Cardiol 2012;(1):S267. | < 50 DT patients |
| Nunes AJ, Wiebe N, Chatterley P, MacArthur RG, Klarenbach SW. Systematic review of the cost-effectiveness of mechanical circulatory support. Can J Cardiol 2012;(1):S176–7. | Wrong indication |
| O’Horo JC, Abu Saleh OM, Stulak JM, Wilhelm MP, Baddour LM, Rizwan Sohail M. Left ventricular assist device infections: a systematic review. ASAIO J 2018;64:287–94. | Do not report DT data |
| Okoh AK, Chan O, Schultheis M, Fugar S, Kang N, Kaplon S, et al. Racial disparities and outcomes after left ventricular assist device implantation as bridge to transplantation or destination therapy. Innov Technol Tech Cardiothorac Vasc Surg 2019;14:236–42. | Do not report DT data |
| Okwuosa I, Anderson AS, Petty M, Hubert A, Pollan L, Andrei AC, et al. Sustaining quality of life of the aged: transplant or mechanical support (sustain-it): baseline caregiver burden. Circ Conf 2018;138. | Do not report DT data |
| Okwuosa IS, Xu Y, Andrei A, Warzecha A, Kao A, Hsich E, et al. Sustaining quality of life of the aged: transplant or mechanical support (Sustain-It): caregiver perceived burden. J Heart Lung Transplant 2020;39(4 Suppl.):S34–5. | Do not report DT data |
| Olagoke OO, Ezegwu O, Olagoke AA, Golzar Y. Incidence, trends and predictors of palliative care consultation among patients admitted for LVAD implantation in the United States. J Card Fail 2019;25(8 Suppl.):S59. | Do not report DT data |
| Oliveira GH, Dupont M, Naftel D, Myers SL, Yuan Y, Tang WH, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2014;63:240–8. | Do not report DT data |
| Olmsted RZ, Critsinelis A, Kurihara C, Kawabori M, Sugiura T, Civitello AB, et al. Severe LVAD-related infections requiring surgical treatment: incidence, predictors, effect on survival, and impact of device selection. J Card Surg 2019;34(2):82–91. | Duplicate record |
| Olsen C, Mandawat A, Triana T, Samsky MD, Chiswell K, Ravi K. Recovery of left ventricular function on LVAD support is associated with improved outcomes. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2020;142. | Do not report DT data |
| Osaki S, Edwards NM, Velez M, Johnson MR, Murray MA, Hoffmann JA, et al. Improved survival in patients with ventricular assist device therapy: the University of Wisconsin experience. Eur J Cardio-Thorac Surg 2008;34:281–8. | < 50 DT patients |
| Oswald H, Schultz-Wildelau C, Gardiwal A, Lüsebrink U, König T, Meyer A, et al. Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients. Eur J Heart Fail 2010;12:593–9. | Do not report DT data |
| Otten A, Kurz S, Anwar S, Potapov E, Krall C, O’Brien B, et al. Prognostic value of 3-dimensional echocardiographical heart volume assessment in patients scheduled for left ventricular assist device implantation. Eur J Cardio-Thorac Surg 2018;54:169–75. | Do not report DT data |
| Oz MC, Argenziano M, Catanese KA, Gardocki MT, Goldstein DJ, Ashton RC, et al. Bridge experience with long-term implantable left ventricular assist devices. Are they an alternative to transplantation? Circulation 1997;95:1844–52. | Do not report DT data |
| Oz MC, Goldstein DJ, Pepino P, Weinberg AD, Thompson SM, Catanese KA, et al. Screening scale predicts patients successfully receiving long-term implantable left ventricular assist devices. Circulation 1995;92:II169–73. | Do not report DT data |
| Pagani FD, Long JW, Dembitsky WP, Joyce LD, Miller LW. Improved mechanical reliability of the HeartMate XVE left ventricular assist system. Ann Thorac Surg 2006;82:1413–8. | < 50 DT patients |
| Parikh U, Lamba H, Vincent J, Civitello AB, Nair A, Taimeh Z, et al. Pre-operative hyponatremia as a risk factor for mortality in patients after left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S430–1. | Do not report DT data |
| Park CJ, Murray M, Kohmoto T, Lushaj E. Impact of distance from implant center on mechanical circulatory device patient outcomes. J Heart Lung Transplant 2019;38(4 Suppl.):S458–9. | Do not report DT data |
| Park JR, Brady PA, Clavell A, Maleszewski J, Nkomo V, Pislaru S, et al. Predictors and impact of de novo aortic regurgitation in patients with continuous flow left ventricular assist devices. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Patel N, Bandyopadhyay D, Chakraborty S, Amgai B, Kumari A, Patel Z, et al. National gender-based in-hospital mortality and outcome in patients with left ventricular assist device: 2008-2014 National Inpatient Sample trend analysis. J Am Coll Cardiol 2020;75(11):1507. | Do not report DT data |
| Patel N, Gluck JA, Radojevic J, Coleman CI, Baker WL. Left ventricular assist device implantation improves glycaemic control: a systematic review and meta-analysis. ESC Heart Fail 2018;5:1141–9. | Do not report DT data |
| Patel N, Kalra R, Doshi R, Joly J, Bajaj NS, Arora G, et al. Costs and in-hospital mortality associated with orthotopic heart transplants and left ventricular assist devices: national inpatient sample 2009–2014. Circulation Conference: Resuscitation Science Symposium, ReSS. 2017;136. | Wrong patient population |
| Patel S, Choi JH, Moncho Escrivá E, Rizvi SSA, Maynes EJ, Samuels LE, et al. Single versus multi-drug antimicrobial surgical infection prophylaxis for left ventricular assist devices: a systematic review and meta-analysis. Artif Organs 2019;43:E124–38. | Do not report DT data |
| Pelletier D, Radio SJ. Characterization of LVAD ventricular and aortic interfaces: potential role in device thrombosis. Lab Invest 2016;(1):83A. | Do not report DT data |
| Pendyal A, Gelow JM. Hepatitis c virus infection does not impact survival following continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2017;36(4 Suppl. 1):S28. | Do not report DT data |
| Pennings K, Van De Vosse F, De Mol B, Rutten M. Estimation of left ventricular pressure with the pump as ‘pressure sensor’ in patients with a continuous flow LVAD. Int J Artif Organs 2015;38(7):378. | Do not report DT data |
| Pennington DG, Oaks TE, Lohmann DP. Permanent ventricular assist device support versus cardiac transplantation. Ann Thorac Surg 1999;68:729–33. | Wrong patient population |
| Peters A, Smith L, Kennedy J, Abuannadi M, Bergin J, Mazimba S. Comparative analysis of established risk scores and novel hemodynamic metrics in predicting right ventricular failure in left ventricular assist device patients. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Peters AE, Smith LA, Ababio P, Breathett K, McMurry TL, Kennedy JLW, et al. Comparative analysis of established risk scores and novel hemodynamic metrics in predicting right ventricular failure in left ventricular assist device patients. J Card Fail 2019;25:620–8. | < 50 DT patients |
| Petty MG, Yao X, Andrei A, Warzecha A, Kao A, Hsich E, et al. Caregiver comorbidities and anxiety are related to caregiver quality of life: findings from the sustaining qualIty of life of the aged: HT or MCS study. J Heart Lung Transplant 2020;39(4 Suppl.):S93. | Do not report DT data |
| Phan K, Haswell JM, Xu J, Assem Y, Mick SL, Kapadia SR, et al. Percutaneous transcatheter interventions for aortic insufficiency in continuous-flow left ventricular assist device patients: a systematic review and meta-analysis. ASAIO J 2017;63:117–22. | Do not report DT data |
| Phan K, Huo YR, Zhao DF, Yan TD, Tchantchaleishvili V. Ventricular recovery and pump explantation in patients supported by left ventricular assist devices: a systematic review. ASAIO J 2016;62:219–31. | Do not report DT data |
| Philip S, Chapnick E, Ghitan M, Lin YS, Kuhn-Basti M. Blood stream infection in LVAD recipients: more than meets the eye. Open Forum Infect Dis 2017;4(Suppl. 1):S546–7. | Do not report DT data |
| Pilarczyk K, Carstens H, Heckmann J, Kamler M, Koch A, Jakob H, et al. Left ventricular assist device implantation in patients with cardiogenic shock: is prior stabilization with Extracorporeal Life Support beneficial? Intensive Care Medicine Experimental Conference: 31st European Society of Intensive Care Medicine Annual Congress, ESICM. 2018;6. | Do not report DT data |
| Pitha J, Dorazilova Z, Melenovsky V, Kralova Lesna I, Stavek P, Stepankova J, et al. The impact of left ventricle assist device on circulating endothelial microparticles – pilot study. Neuroendocrinol Lett 2012;33(Suppl. 2):68–72. | < 50 DT patients |
| Poddi S, Maltais S, Asleh R, Schettle SD, Rosenbaum AN, Behfar A, et al. Analyzing the value of the pulmonary artery pulsatility index beyond predicting right heart failure. ASAIO J 2018;64(Suppl. 1):89. | Do not report DT data |
| Poddi S, Tchantchaleishvili V, Daly RC, Dunlay SM, Maltais S, Stulak JM. Age-related risk of adverse events after left ventricular assist device implantation: a device-specific comparison. J Heart Lung Transplant 2018;37(4 Suppl. 1):S367–8. | Do not report DT data |
| Popov AF, Hosseini MT, Zych B, Mohite P, Hards R, Krueger H, et al. Clinical experience with HeartWare left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg 2012;93:810–5. | < 50 DT patients |
| Potapov EV, Loforte A, Weng Y, Jurmann M, Pasic M, Drews T, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg 2008;23:185–94. | Wrong outcomes |
| Potapov EV, Nersesian G, Lewin D, Ozbaran M, De By TMMH, Stein J, et al. Propensity score-based analysis of long-term follow-up in patients supported with durable centrifugal left ventricular assist devices: the EUROMACS analysis. Eur J Cardio-Thorac Surg 2021;60(3):579–87 | Do not report DT data |
| Prichard RA, Kershaw L, Goodall S, Davidson PM, Macdonald P, Newton P, et al. Bottom up costing ventricular assist device therapy and optimal medical management-first steps to establishing cost-effectiveness. Circulation: Cardiovascular Quality and Outcomes Conference: American Heart Association’s Quality of Care and Outcomes Research. 2014;7. | Wrong patient population |
| Pulikottil-Jacob R, Suri G, Connock M, Kandala NB, Sutcliffe P, Maheswaran H, et al. Comparative cost-effectiveness of the HeartWare versus HeartMate II left ventricular assist devices used in the United Kingdom National Health Service bridge-to-transplant program for patients with heart failure. J Heart Lung Transplant 2014;33:350–8. | Wrong indication |
| Pya Y, Bekbossynova M, Jetybayeva S, Bekbossynov S, Andossova S, Salov R, et al. Initial 3-year outcomes with left ventricular assist devices in a country with a nascent heart transplantation program. ESC Heart Fail 2016;3:26–34. | Do not report DT data |
| Raikhelkar J, Fried J, Sumzin N, Clerkin K, Griffin J, Sanchez J, et al. Heartmate 3 implantation in cancer survivors with advanced heart failure. J Heart Lung Transplant 2020;39(4 Suppl.):S183. | Do not report DT data |
| Raitz G, Maning J, Macedo G, Blume V, Chaparro S. Device differences in LVAD pump thrombosis: a systematic review. ASAIO J 2019;65(Suppl. 1):70. | Do not report DT data |
| Rajagopal K, Daneshmand MA, Patel CB, Ganapathi AM, Schechter MA, Rogers JG, et al. Natural history and clinical effect of aortic valve regurgitation after left ventricular assist device implantation. J Thorac Cardiovasc Surg 2013;145:1373–9. | Do not report DT data |
| Rajapreyar I, Rame JE. Cost-effectiveness of long-term left ventricular assist device support: is the extra-welfarist model suitable for advanced heart failure? ASAIO J 2020;66:871–4. | Wrong study design |
| Rajaratnam A, El-Swais A, McTiernan C, Al Ghouleh I. Persistence of pulmonary hypertension in patients with ventricular assist devices. J Am Coll Cardiol 2021;77(18 Suppl. 1):759. | Do not report DT data |
| Rampa S, Nalliah R, Kyeong Lee M, Allareddy V, Auslender M. The rise of the machines: infections are the achilles’ heel! Crit Care Med 2016;44(12 Suppl. 1):121. | Do not report DT data |
| Randhawa VK, Lin W, Sabe MA, Bullen JA, Soltesz EG, Tang WHW, et al. Mitral regurgitation severity after continuous-flow LVAD implantation as ot associated with adverse long-term clinical outcomes. J Card Fail 2020;26(10 Suppl.):S147. | Do not report DT data |
| Randhawa VK, Soltesz EG, Faulkenberg KD, Wang Q, Wolski KE, Tong MZ, et al. Safety and impact of direct left atrial pressure monitoring in patients undergoing continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S443–4. | Do not report DT data |
| Rao V, Oz MC, Flannery MA, Idrissi KA, Argenziano M, Edwards NM, et al. Changing trends in mechanical circulatory assistance. J Card Surg 2004;19:361–6. | Wrong patient population |
| Ravichandran AK, Shah P, Singh R, Aaronson KD, Pagani FD, Stulak J, et al. Impact of patient distance from ventricular assist device-implanting center on short- and long-term outcomes. ASAIO J 2018;64:721–6. | Do not report DT data |
| Raymer D, Vader J, Platts A, Nassif M, Silvestry S, Ewald G, et al. Health literacy as a predictor of adverse outcomes after implantation of left ventricular assist device. J Card Fail 2015;(1):S98. | Wrong setting |
| Refaat M, Chemaly E, Lebeche D, Gwathmey JK, Hajjar RJ. Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol 2008;31:1246–52. | < 50 DT patients |
| Rehman AU, Birks EJ, Winters SJ. Amiodarone-induced hyperthyroidism in patients with heart failure with and without a left ventricular assist device. Endocrine Reviews Conference: 99th Annual Meeting of the Endocrine Society, ENDO. 2017;38. | Do not report DT data |
| Reynard AK, Butler RS, McKee MG, Starling RC, Gorodeski EZ. Impact of continuous flow left ventricular assist device therapy on depression and anxiety. J Am Coll Cardiol 2013;(1):E771. | Do not report DT data |
| Riebandt J, Moayedifar R, Wiedemann D, Schloglhofer T, Dimitrov K, Rajek A, et al. Less invasive left ventricular assist device implantation-5-year survival and post transplant outcomes. J Heart Lung Transplant 2018;37(4 Suppl. 1):S480. | Do not report DT data |
| Rivas-Lasarte M, Kumar S, Derbala MH, Ferrall J, Cefalu M, Rashid SM, et al. Prediction of right heart failure after left ventricular assist implantation: external validation of the EUROMACS right-sided heart failure risk score. J Heart Lung Transplant 2021;40(4 Suppl.):S168–9. | Do not report DT data |
| Roberts SC, Rich JD, Pham DT, Harap R, Stosor V. A spectrum of infectious complications in continuous-flow ventricular assist devices: a single-center longitudinal cohort. Open Forum Infect Dis 2019;6(Suppl. 2):S421–2. | Do not report DT data |
| Rockman HA, Adamson RM, Dembitsky WP, Bonar JW, Jaski BE. Acute fulminant myocarditis: long-term follow-up after circulatory support with left ventricular assist device. Am Heart J 1991;121:922–6. | Do not report DT data |
| Rodriguez DC, Algodi M, Berardi C, Makkiya M, Cyrille N, Saeed O, et al. Persistent hyponatremia early after LVAD implantation does not result in poor long-term outcomes. J Heart Lung Transplant 2016;(1):S332. | Do not report DT data |
| Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol 2007;50:741–7. | < 50 DT patients |
| Rosenbaum AN, John R, Liao KK, Adatya S, Colvin-Adams MM, Pritzker M, et al. Survival in elderly patients supported with continuous flow left ventricular assist device as bridge to transplantation or destination therapy. J Card Fail 2014;20:161–7. | < 50 DT patients |
| Rosenbaum AN, Ternus BW, Pahwa S, Stulak JM, Clavell AL, Schettle SD, et al. Risk of liver dysfunction after left ventricular assist device implantation. Ann Thorac Surg. 2021. | Wrong outcomes |
| Rossi M, Serraino GF, Jiritano F, Renzulli A. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012;15:733–40. | < 50 DT patients |
| Roukoz H, Bhan A, Ravichandran A, Ahmed MM, Bhat G, Cowger J, et al. Continued versus suspended cardiac resynchronization therapy after left ventricular assist device implantation. Sci Rep 2020;10:2573. | Do not report DT data |
| Roukoz H, Jedeon ZA, Wald LV, John R, Cogswell R. The effect of ventricular arrhythmias and Vt ablation on thrombotic events in patients with left ventricular assist device. Heart Rhythm 2019;16(5 Suppl.):485. | Do not report DT data |
| Roukoz H, Sathnur N, Bhan AK, Ravichandran A, Ahmed MM, Bhat G, et al. Continued CRT versus turning off LV lead after left ventricular assist device implant: a multicenter experience. Heart Rhythm 2018;15(5 Suppl. 1):S48. | Do not report DT data |
| Russo MARK, Akhter S, Pisarski R, Iribarne A, Raman J, Anderson A, et al. Intermacs score predicts post-implant survival and resource utilization. Eur Heart J 2011;(1):81. | Wrong patient population |
| Sadigov A, Demir E, Nalbantgil S, Demirci C, Engin C, Yagdi T, et al. The influence of left ventricular assist device implantation on short-term and long-term renal functions in end-stage heart failure patients. J Am Soc Nephrol 2019;30:441. | Do not report DT data |
| Saeed D, Garbade J, Gustafsson F, Lavee J, Morshuis M, Zimpfer D, et al. Two-year outcomes in real world patients treated with Heartmate 3TM left ventricular assist device for advanced heart failure: data from the ELEVATE Registry. J Heart Lung Transplant 2019;38(4 Suppl.):S67. | Do not report DT data |
| Saeed D, Kidambi T, Shalli S, Lapin B, Malaisrie SC, Lee R, et al. Tricuspid valve repair with left ventricular assist device implantation: is it warranted? J Heart Lung Transplant 2011;30:530–5. | Do not report DT data |
| Saeed O, Colombo PC, Mehra MR, Uriel N, Goldstein DJ, Cleveland J, et al. Effect of aspirin dose on hemocompatibility-related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant 2020;39:518–25. | Do not report DT data |
| Saing S, van der Linden N, Hayward CS, Goodall S. Cost-effectiveness of left ventricular assist devices in end stage heart failure using state transition modelling based on registry data. Value Health 2018;21(Suppl. 3):S261. | Wrong indication |
| Saito S, Nishinaka T, Yamazaki K. Long-term circulatory support with a left ventricular assist device therapy in Japan. Circ J 2010;74:624–5. | Wrong study design |
| Sajgalik P, Kim CH, Stulak JM, Kushwaha SS, Maltais S, Joyce DL, et al. Pulmonary function assessment post-left ventricular assist device implantation. ESC Heart Fail 2019;6:53–61. | Do not report DT data |
| Salih M, Ayan M, Ogunbayo G, Elghezewi A, Guglin M. Heartmate II versus heartware for heart failure: a meta-analysis. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Santiago A, Lamba H, Hart L, Nguyen M, Alnajar A, Nair A, et al. Significant decrease in HgA1c levels in diabetics after continuous-flow left ventricular assist device implantation. ASAIO J 2018;64(Suppl. 1):65. | Do not report DT data |
| Sas G, Chakor H, Bogaty P, Boothroyd L, Guertin J, Lambert L, et al. Évaluation des données probantes sur les dispositifs d’assistance ventriculaire gauche HeartMate II® et HeartWare® pour le traitement de l’insuffisance cardiaque chronique terminale [Implantable ventricular assist devices: assessment of evidence and required elements to establish a clinical registry in Québec]. HTA Database. 2012. | Duplicate record |
| Sato T, Seguchi O, Iwashima Y, Yanase M, Nakajima S, Hieda M, et al. Serum vrain natriuretic peptide concentration 60 days after surgery as a predictor of long-term prognosis in patients implanted with a left ventricular assist device. ASAIO J 2015;61:373–8. | Do not report DT data |
| Schaffer JM, Allen JG, Weiss ES, Arnaoutakis GJ, Patel ND, Russell SD, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant 2011;30:164–74. | < 50 DT patients |
| Schaffer JM, Allen JG, Weiss ES, Patel ND, Russell SD, Shah AS, et al. Evaluation of risk indices in continuous-flow left ventricular assist device patients. Ann Thorac Surg 2009;88:1889–96. | < 50 DT patients |
| Schaffer JM, Arnaoutakis GJ, Allen JG, Weiss ES, Patel ND, Russell SD, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg 2011;91:740–7; discussion 747. | < 50 DT patients |
| Scheiderer R, Belden C, Schwab D, Haney C, Paz J. Exercise guidelines for inpatients following ventricular assist device placement: a systematic review of the literature. Cardiopulm Phys Ther J 2013;24:35–42. | Do not report DT data |
| Schenk S, McCarthy PM, Blackstone EH, Feng J, Starling RC, Navia JL, et al. Duration of inotropic support after left ventricular assist device implantation: risk factors and impact on outcome. J Thorac Cardiovasc Surg 2006;131:447–54. | Do not report DT data |
| Schettle S, Alnsasra H, Clavell A, Daly R, Glasgow A, Habermann E, et al. Opioid usage in patients undergoing left ventricular assist device implantation: correlation to patient characteristics and outcomes? ASAIO J 2019;65(Suppl. 1):99. | Do not report DT data |
| Schettle S, Pereira N, Kushwaha S, Daly R, Joyce D, Joyce L, et al. Morbidity risks with long term left ventricular assist device (LVAD) support. J Heart Lung Transplant 2017;36(4 Suppl. 1):S419. | Do not report DT data |
| Schettle S, Shahin Y, Rosenbaum A, Schirger J, Weber M, Tchantchaleishvili V, et al. Heart failure duration association with gastrointestinal bleeding post left ventricular assist device. J Heart Lung Transplant 2020;39(4 Suppl.):S394–5. | Do not report DT data |
| Schettle S, Shanin Y, Schirger J, Pahwa S, Weber M, Tchantchaleishvili V, et al. Impact of gastrointestinal bleeding burden with subsequent outcomes after left ventricular assist device implant. J Heart Lung Transplant 2020;39(4 Suppl.):S399–S400. | Do not report DT data |
| Schlensak C, Benk C, Siepe M, Heilmann C, Beyersdorf F. Clinical experience with the VentrAssist left ventricular assist device. Thorac Cardiovasc Surg 2010;58(Suppl. 2):S198–201. | Do not report DT data |
| Schloglhofer T, Blood M, Pietropaolo J, Lantz J. Alleviation of VAD coordinator time burden and empowerment of HVAD patients in order to improve outcomes. J Heart Lung Transplant 2018;37(4 Suppl. 1):S294–5. | Do not report DT data |
| Schmidt S, Reichart D, Brand C, Wagner F, Bernhardt A, Blankenberg S, et al. 2-year follow-up after minimally-invasive left ventricular assist device implantation – a single center experience. Transpl Int 2017;30(Suppl. 4):43. | Do not report DT data |
| Schmidt T, Bjarnason-Wehrens B, Bartsch P, Deniz E, Schmitto J, Schulte-Eistrup S, et al. Exercise capacity and functional performance in heart failure patients supported by a left ventricular assist device at discharge from inpatient rehabilitation. Artif Organs 2018;42:22–30. | < 50 DT patients |
| Schmidt T, Bjarnason-Wehrens B, Mommertz S, Hannig M, Schulte-Eistrup S, Willemsen D, et al. Changes in total cardiac output and oxygen extraction during exercise in patients supported with an HVAD left ventricular assist device. Artif Organs 2018;42:686–94. | < 50 DT patients |
| Schmitto J, Dogan G, Hanke SJ, Riebandt J, Ozbaran M, Engin C, et al. A multicenter analysis of implantation via a thoracotomy approach of a left ventricular assist system for the treatment of advanced heart failure. Thoracic and Cardiovascular Surgeon Conference: 48th Annual Meeting German Society for Thoracic, Cardiac, and Vascular Surgery Germany. 2019;67. | Do not report DT data |
| Schrage B, Rubsamen N, Magnussen C, Gummert J, Schonrath F, de By T, et al. Derivation and validation of the EUROMACS left ventricular assist device score for long-term outcome - the EUROMACS-LVAD-score. J Heart Lung Transplant 2019;38(4 Suppl.):S107–8. | Do not report DT data |
| Schramm R, Zittermann A, Morshuis M, Schoenbrodt M, Rossing Freifrau EV, Hakim-Meibodi K, et al. Short-term outcome after centrifugal continuous flow left ventricular assist device implantation comparing the HeartWare, HVAD, and Abbot HeartMate III. Thoracic and Cardiovascular Surgeon Conference: 48th Annual Meeting German Society for Thoracic, Cardiac, and Vascular Surgery Germany. 2019;67. | Do not report DT data |
| Schramm R, Zittermann A, Morshuis M, Schoenbrodt M, von Roessing E, von Dossow V, et al. Comparing short-term outcome after implantation of the HeartWare HVAD and the Abbott HeartMate 3. ESC Heart Fail 2020. | Do not report DT data |
| Schultz J, Masotti M, Maharaj V, El Rafei A, Shaffer A, John R, et al. Impact of acute liver injury prior to left ventricular assist device therapy. J Heart Lung Transplant 2021;40(4 Suppl.):S437. | Do not report DT data |
| Schultz JN, Goodwin K, John R, Alexy T, Kamdar F, Martin C, et al. Association between angiotensin II receptor blockade and recurrent gastrointestinal bleeding on left ventricular assist device support. J Heart Lung Transplant 2018;37(4 Suppl. 1):S163. | Do not report DT data |
| Schultz JN, John R, Martin CM, Pritzker M, Missov E, Thenappan T, et al. Impact of evaluation of left ventricular apical core pathology obtained at time of LVAD implantation. J Heart Lung Transplant 2017;36(4 Suppl. 1):S82–3. | Do not report DT data |
| Seguchi O, Fujita T, Nakajima S, Sato T, Sunami H, Yanase M, et al. Left ventricular assist device therapy for burned-out phase of hypertrophic cardiomyopathy. Eur Heart J 2016;37(Suppl. 1):537. | Do not report DT data |
| Sharples LD, Dyer M, Cafferty F, Demiris N, Freeman C, Banner NR, et al. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the Evaluation of Ventricular Assist Device Programme in the UK (EVAD-UK). J Heart Lung Transplant 2006;25:1336–43. | Wrong patient population |
| Shatla I, Abumoawad A, Cheng AL, Lopez Candales A. Impact of kidney disease on left ventricular assisted device outcomes: insight from National Readmission Database. Eur Heart J 2021;42(Suppl. 1):944. | Do not report DT data |
| Sheikh FH, Ahmed S, Rodrigo ME, Hofmeyer M, Kadakkal A, Grinstein J, et al. Long term outcomes of African-American LVAD recipients. J Heart Lung Transplant 2020;39(4 Suppl.):S338–9. | Do not report DT data |
| Sheikh FH, Majure DT, Ahmed S, Rodrigo ME, Jani SM, Hofmeyer M, et al. Obesity does not impact 1 year survival after LVAD implantation. J Card Fail 2016;22 Suppl. 8):S110. | Do not report DT data |
| Sheikh FH, Majure DT, Rodrigo ME, Jani SM, Hofmeyer M, Boyce SW, et al. Characteristics and outcomes of African American LVAD recipients: a single center experience. J Heart Lung Transplant 2016;(1):S125. | Do not report DT data |
| Sherazi S, Ayers B, Polonsky B, McNitt S, Kutyifa V, Alexis J, et al. Association of cardiac rehabilitation with improved gealthcare utilization and long-term survival after left ventricular assist device implantation. J Heart Lung Transplant 2020;39(4 Suppl.):S110. | Do not report DT data |
| Shurrab M, Pettit S, Park S, Atturman S, Sbaih A, Khaleel G, et al. Is there a role for ICDs in LVAD patients? A meta-analysis. Eur Heart J 2016:103. | Do not report DT data |
| Siegenthaler MP, Frazier OH, Beyersdorf F, Martin J, Laks H, Elefteriades J, et al. Mechanical reliability of the Jarvik 2000 Heart. Ann Thorac Surg 2006;81:1752–8; discussion 1758. | < 50 DT patients |
| Silver SA, Long J, Zheng Y, Chertow GM. Outcomes after left ventricular assist device implantation in patients with AKI. J Am Soc Nephrol 2018;29:32. | Do not report DT data |
| Silvestry SC, Mahr C, Slaughter MS, Levy WC, Cheng RK, May DM, et al. Cost-effectiveness of a small intrapericardial centrifugal left ventricular assist device. ASAIO J 2020;66:862–70. | Duplicate record |
| Sims DB, Luke A, Rangasamy S, Borukhov E, Saeed O, Murthy S, et al. Reducing 30-day hospital readmission rate in left ventricular assist device patients with a structured readmission improvement plan. J Heart Lung Transplant 2017;36(4 Suppl. 1):S197–8. | Do not report DT data |
| Sladen RN, Shulman MA, Javaid A, Hodgson C, Myles PS, McGiffin D, et al. Postdischarge functional capacity, health-related quality of life, depression, anxiety, and post-traumatic stress disorder in patients receiving a long-term left ventricular assist device. J Card Fail. 2021. | Do not report DT data |
| Slaughter MS. Destination therapy: the future is arriving. Congest Heart Fail 2005;11:155–6. | Wrong study design |
| Slivnick J, Lampert B, Xu Y, Andrei A, Warzecha A, Kao A, et al. Association of patient health-related quality of life and caregiver burden in older heart failure patients receiving advanced therapies: findings from the SustainIng QualItY of Life of the Aged: Transplant or Mechanical Support (SustAIn-it) study. J Heart Lung Transplant 2020;39(4 Suppl.):S438. | Do not report DT data |
| Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L, et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail 2013;1:31–9. | < 50 DT patients |
| Solanki M, Dobson L, Alwair H, Ghafghazi S, Wysoczynski M, Slaughter MS, et al. Association of temporal trends in neutrophil lymphocyte ratio on left ventricular assist device patient outcomes. Artif Organs 2021;45:742–7. | Do not report DT data |
| Soleimani B, Haouzi A, Manoskey A, Stephenson ER, El-Banayosy A, Pae WE. Development of aortic insufficiency in patients supported with continuous flow left ventricular assist devices. ASAIO J 2012;58:326–9. | < 50 DT patients |
| Soliman OII, Akin S, Muslem R, Boersma E, Manintveld OC, Krabatsch T, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) right-sided heart failure risk score. Circulation 2018;137:891–906. | Do not report DT data |
| Soni M, Birati EY, Marble J, Eckman P, Garberich R, Weaver C, et al. Gender and spirituality influence patient care decisions after LVAD. J Heart Lung Transplant 2019;38(4 Suppl.):S441. | Do not report DT data |
| Sotolongo A. High grade mitral regurgitation after a left ventricular assist device may adversely affect survival. J Heart Lung Transplant 2017;36(4 Suppl. 1):S344. | Do not report DT data |
| Sparrow CT, Raymer DS, Radhakrishnan SL, Nassif ME, Vader JM, LaRue SJ, et al. The effect of pump speed settings on suspected pump thrombosis in patients supported with continuous-flow left ventricular assist devices. J Card Fail 2016;22(Suppl. 8):S108–9. | Do not report DT data |
| Spratt JR, Roy S, Plack D, John R, Liao K, Cogswell RJ. Survival and driveline infection rates in patients on chronic immunosuppressive therapy who undergo left ventricular assist device implantation. J Heart Lung Transplant 2016;(1):S265. | Do not report DT data |
| Stawiarski K, Agboola O, Jacoby D, Bellumkonda L, Ahmad T, Sugeng L, et al. Chloride homeostasis in end stage heart failure and LVAD recipients. J Heart Lung Transplant 2019;38(4 Suppl.):S382–3. | Do not report DT data |
| Stawiarski K, Zogg C, Park J, Jacoby D, Bellumkonda L, Chen M, et al. Gender and diastolic dysfunction may be the driver of failure of myocardial recovery following LVAD implantation. J Heart Lung Transplant 2018;37(4 Suppl. 1):S311. | Do not report DT data |
| Stern B, Maheshwari P, Gorrepati VS, Chintanaboina J, Bethards D, Boehmer J, et al. Predictors of index gastrointestinal bleed in left ventricular assist device (LVAD) patients. Gastrointest Endosc 2020;91(6 Suppl.):AB569–70. | Do not report DT data |
| Strout S, Veasey T, Rieger K, Floroff C, Wray D, Brisco M, et al. Suppressive antibiotics for LVAD-associated infections: are they helpful or harmful? J Heart Lung Transplant 2016;(1):S75–6. | Do not report DT data |
| Stulak JM, Davis ME, Haglund N, Dunlay S, Cowger J, Shah P, et al. Adverse events in contemporary continuous-flow left ventricular assist devices: a multi-institutional comparison shows significant differences. J Thorac Cardiovasc Surg 2016;151:177–89. | Do not report DT data |
| Stulak JM, Tchantchaleishvili V, Dunlay S, Sharma S, Joyce LD, Joyce DL, et al. Association of late aortic and tricuspid valve regurgitation and outcomes while on left ventricular assist device therapy. J Heart Lung Transplant 2016;(1):S127. | Do not report DT data |
| Suboc TMB, Ahmed K, Kabir C, Graney N, Paliga R, Meehan K, et al. Elderly heart failure patients with left ventricular assist device: mortality, hospital readmission, length of stay, and adverse events. Circulation Conference: Resuscitation Science Symposium, ReSS. 2017;136. | Do not report DT data |
| Sugiura T, Kurihara C, Kawabori M, Cohn WE, Civitello AB, Frazier OH, et al. Concomitant valve procedures are not associated with higher perioperative mortality. J Heart Lung Transplant 2017;36(4 Suppl. 1):S171–2. | Do not report DT data |
| Sugiura T, Kurihara C, Kawabori M, Cohn WE, Civitello AB, Frazier OH, et al. Readmission within 30 days after continuous flow ventricular assist devices implantation-comparative analysis of heartmate II and heartware devices. J Heart Lung Transplant 2017;36 4 Suppl. 1):S425. | Do not report DT data |
| Sugiura T, Kurihara C, Kawabori M, Critsinelis AC, Wang S, Civitello AB, et al. Concomitant valve procedures in patients undergoing continuous-flow left ventricular assist device implantation: a single-center experience. J Thorac Cardiovasc Surg 2019;158:1083–9.e1. | Do not report DT data |
| Sultan M, Flores E, Verma D, Argarwal S, Rayyan E, Loli A, et al. Incidence and current management of left ventricular assist device thrombus. Eur Heart J 2017;38(Suppl. 1):1046–7. | Do not report DT data |
| Sundararajan S, Kiernan MS, DeNofrio D, Vest AR. Cachexia is common in ventricular assist device recipients but not predictive of mortality. J Card Fail 2016;22(Suppl. 8):S57–8. | Do not report DT data |
| Suzuki K, Yoshioka D, Toda K, Miyagawa S, Yoshikawa Y, Hata H, et al. Outcomes of left ventricular assist device for patients with hypertrophic cardiomyopathy. J Heart Lung Transplant 2020;39(4 Suppl.):S153. | Do not report DT data |
| Szentmihalyi I, Barabas JI, Bali A, Kapus G, Tamas C, Sax B, et al. [Heart transplantation and long-term LVAD support cost-effectiveness model]. Magyar Sebeszet 2016;69:186–93. | Wrong study design |
| Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, Jorde UP, et al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant 2014;33:141–8. | Do not report DT data |
| Takeda K, Takayama H, Garan RA, Topkara VK, Han J, Fukuhara S, et al. Contemporary outcome of unplanned right ventricular assist device for severe right heart failure after continuous flow left ventricular assist device insertion. J Heart Lung Transplant 2016;(1):S55. | Do not report DT data |
| Takeda K, Takayama H, Kalesan B, Uriel N, Colombo PC, Jorde UP, et al. Long-term outcome of patients on continuous-flow left ventricular assist device support. J Thorac Cardiovasc Surg 2014;148:1606–14. | < 50 DT patients |
| Taleb I, Wever-Pinzon O, Alharethi R, Overton S, Nativi-Nicolau J, Dranow E, et al. Predicting right ventricular failure in chronic heart failure patients receiving left ventricular assist device. J Heart Lung Transplant 2020;39(4 Suppl.):S426. | Do not report DT data |
| Taleb I, Yin M, Koliopoulou A, Taleb M, Dranow E, Kemeyou L, et al. Cardiac reverse remodeling and recovery in dilated cardiomyopathy medication-naive patients requiring durable left ventricular assist device support. J Heart Lung Transplant 2019;38(4 Suppl.):S125. | Do not report DT data |
| Taleb I, Yin MY, Koliopoulou AG, Taleb M, Dranow E, Kemeyou L, et al. Cardiac reverse remodeling and recovery in dilated cardiomyopathy medication-naive patients requiring durable left ventricular assist device support. Eur Heart J 2019;40(Suppl. 1):3305. | Do not report DT data |
| Tam MC, Patel VN, Weinberg RL, Hulten EA, Aaronson KD, Pagani FD, et al. Diagnostic Accuracy of FDG PET/CT in suspected LVAD infections: a case series, systematic review, and meta-analysis. JACC Cardiovasc Imaging 2019;13:1191–202. | Wrong study design |
| Tantrachoti P, Klomjit S, Vutthikraivit W, Prieto S, Gongora E, Nair N. Impact of preoperative atrial fibrillation in patients with left ventricular assist device: a systematic review and meta-analysis. Artif Organs 2019;43:1135–43. | Do not report DT data |
| Tarzia V, Di Giammarco G, Bagozzi L, Bortolussi G, Maccherini M, Marinelli D, et al. From bench to bedside: impact of left ventricular assist device outflow conduit anastomosis position on outcome. Artif Organs 2021;45:236–43. | Do not report DT data |
| Tarzia V, Di Giammarco G, Di Mauro M, Bortolussi G, Maccherini M, Tursi V, et al. From bench to bedside: can the improvements in left ventricular assist device design mitigate adverse events and increase survival? J Thorac Cardiovasc Surg 2016;151:213–7. | Do not report DT data |
| Tarzia V, Di Giammarco G, Maccherini M, Maiani M, Agostoni P, Bagozzi L, et al. Technology and techniques: tools to mitigate adverse events and improve survival in left ventricular assist device patients. J Heart Lung Transplant 2017;36(4 Suppl. 1):S441–2. | Do not report DT data |
| Tarzia V, Di Mauro M, Bortolussi G, Bejko J, Marinelli D, Foschi M, et al. Access matters: survival advantage with minimally invasive implantation of LVAD as destination therapy. J Heart Lung Transplant 2016;(1):S53. | Do not report DT data |
| Tarzia V, Di Mauro M, Bortolussi G, Bejko J, Marinelli D, Foschi M, et al. Access matters: survival advantage with minimally invasive implantation of LVAD as destination therapy. J Heart Lung Transplant 2016;35(4 Suppl.):S53. | Do not report DT data |
| Tchantchaleishvili V, Luc JGY, Haswell J, Hallian W, Massey HT. Subxiphoid exchange of HeartMate II left ventricular assist device. ASAIO J 2017;63:414–8. | Do not report DT data |
| Teigen LM, Earthman CP, Hodges J, Shultz J, Martin C, John R, et al. A risk model incorporating pectoralis muscle measures more accurately risk predicts mortality after left ventricular assist device implantation. J Heart Lung Transplant 2018;37(4 Suppl. 1):S463. | Do not report DT data |
| Ternus B, Behfar A, Schirger J, Maltais S, Barsness G, Stulak J, et al. Pressure adjusted heart rate as a predictor of adverse outcomes after left ventricular assist device implantation. Circulation Conference. 2018;138. | Do not report DT data |
| Teuteberg J, Kormos RL, Pagani FD, Kiernan MS, Naftel DC, Myers SL, et al. New definition, same old problem: characterizing the condition of right heart failure in INTERMACS. J Heart Lung Transplant 2016;(1):S266–7. | Do not report DT data |
| Teuteberg J, Studdard G, Pagani F, Kiernan M, Oliveria G, Rame E, et al. The ebb and flow of right heart failure in INTERMACS: does right heart failure get better or worse over time? J Heart Lung Transplant 2018;37(4 Suppl. 1):S377–8. | Do not report DT data |
| Teuteberg JJ, Studdard G, Pagani F, Kiernan M, Oliveria G, Rame E, et al. The incidence of early and late clinical right heart failure and the impact on survival after continuous flow mechanical support: insights from the new intermacs definition of right heart failure. J Heart Lung Transplant 2017;36(4 Suppl. 1):S141. | Do not report DT data |
| Tigges-Limmer K, Kugler C, Brocks Y, Winkler Y, Rehn E, Morshuis M, et al. Psychosocial and sexual functioning in patients on ventricular assist device support – a crosssectional pilot study. Transpl Int 2016;29(Suppl. 3):49–50. | Do not report DT data |
| Toda K, Fujita T, Domae K, Shimahara Y, Kobayashi J, Nakatani T. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg 2011;92:929–34. | Do not report DT data |
| Topilsky Y, Hasin T, Oh JK, Borgeson DD, Boilson BA, Schirger JA, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging 2011;4:648–61. | < 50 DT patients |
| Topilsky Y, Oh JK, Shah DK, Boilson BA, Schirger JA, Kushwaha SS, et al. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. Jacc: Cardiovasc Imaging 2011;4:211–22. | Do not report DT data |
| Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail 2011;4:266–75. | Do not report DT data |
| Topkara VK, Dang NC, Barili F, Cheema FH, Martens TP, George I, et al. Predictors and outcomes of continuous veno-venous hemodialysis use after implantation of a left ventricular assist device. J Heart Lung Transplant 2006;25:404–8. | Do not report DT data |
| Topkara VK, Kondareddy S, Malik F, Wang IW, Mann DL, Ewald GA, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg 2010;90:1270–7. | < 50 DT patients |
| Tremblay PL, Herman CR, Baskett RJ. Reversibility of pulmonary hypertension with LVAD support; a meta-analysis of published reports. J Heart Lung Transplant 2018;37(4 Suppl. 1):S492. | Do not report DT data |
| Tremblay PL, Stewart SA, Baskett RJ. The effect of Left Ventricular Assist Devices (LVAD) on pulmonary hypertension: a single center review. J Heart Lung Transplant 2019;38(4 Suppl.):S491–2. | Do not report DT data |
| Tripathi B, Schneider M, Rizwan T, Arora S, Dave M, Shah H, et al. National trends in the utilization, cost burden and outcomes associated with use of long-term mechanical circulatory support: 10-year experience. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Truss W, Welden C, Edwards AL, Pamboukian SV, Peter S. Clinical predictors for repeat hospitalizations in LVAD patients with gastrointestinal bleeding. Gastroenterology 2016;(1):S238–9. | Do not report DT data |
| Tsay J, Lampert B, Whitson B, Hasson R, Emani R, Hasan A, et al. Worsening renal function in patients with low output decompensated heart failure is associated with 1-year mortality. J Heart Lung Transplant 2017;36(4 Suppl. 1):S216. | Do not report DT data |
| Tsiouris A, Brewer RJ, Borgi J, Hodari A, Nemeh HW, Cogan CM, et al. Is resternotomy a risk for continuous-flow left ventricular assist device outcomes? J Card Surg 2013;28:82–7. | < 50 DT patients |
| Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013;32:299–304. | < 50 DT patients |
| Tsiouris A, Morgan JA, Nemeh HW, Hodari A, Brewer RJ, Paone G. Sex-specific outcomes in patients receiving continuous-flow left ventricular devices as a bridge to transplantation or destination therapy. ASAIO J 2014;60:199–206. | Do not report DT data |
| Tsiouris A, Paone G, Nemeh HW, Brewer RJ, Morgan JA. Factors determining post-operative readmissions after left ventricular assist device implantation. J Heart Lung Transplant 2014;33:1041–7. | Do not report DT data |
| Tsui SSL. Updates on cardiac transplant and LVAD implants across the UK and Europe. Heart Asia 2019;11(Suppl. 1):A2. | Do not report DT data |
| Tsyganenko D, Gromann TW, Schoenrath F, Mueller M, Mulzer J, Starck C, et al. Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support. Eur J Cardio-Thorac Surg 2019;55:773–9. | Do not report DT data |
| Tsyganenko D, Hennig F, Kaufmann F, Starck C, Schonrath F, Falk V, et al. Predictors for early and midterm outcome after bridge to left ventricular assist device by extracorporeal life support. Thoracic and Cardiovascular Surgeon Conference: 47th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery, DGTHG. 2018;66. | Do not report DT data |
| Ullah W, Sattar Y, Darmoch F, Al-Khadra Y, Mir T, Ajmal R, et al. The impact of peripheral arterial disease on patients with mechanical circulatory support. Int J Cardiol Heart Vasc 2020;28:100509. | Do not report DT data |
| Unlu O, Alemany HS, Pabon M, Sobol I, Krishnan U, Goyal P, et al. Renal outcomes following left ventricular assist device placement: a single center experience. J Card Fail 2020;26(10 Suppl.):S160–1. | Do not report DT data |
| Uribarri A, Rojas SV, Hanke JS, Avsar M, Dogan G, Deniz E, et al. Is ICD implantation necessary in patients with left ventricular assist device therapy? J Heart Lung Transplant 2017;36(4 Suppl. 1):S14–5. | Do not report DT data |
| Uriel N, Colombo PC, Cleveland J, Long J, Salerno CT, Goldstein D, et al. Hemocompatibility-related outcomes in the multicenter study of maglev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) pivotal trial. J Heart Lung Transplant 2017;36(4 Suppl. 1):S65. | Do not report DT data |
| Uriel N, Mehra M. Long-term burden of hemocompatibility related adverse events in the MOMENTUM 3 trial: final analysis of the 1028 patient cohort. J Heart Lung Transplant 2019;38(4 Suppl.):S67. | Do not report DT data |
| Usoh C, Sherazi S, Szepietowska B, Kutyifa V, McNitt S, Papernov A, et al. Diabetes increases risk of mortality in heart failure patients who undergo left ventricular assist device implantation. Endocrine Reviews Conference: 99th Annual Meeting of the Endocrine Society, ENDO. 2017;38. | Do not report DT data |
| Usoh CO, Sherazi S, Szepietowska B, Kutyifa V, McNitt S, Papernov A, et al. Influence of diabetes mellitus on outcomes in patients after left ventricular assist device implantation. Ann Thorac Surg 2018;106:555–60. | Do not report DT data |
| van den Bergh WM, Lansink-Hartgring AO, van Duijn AL, Engstrom AE, Lahpor JR, Slooter AJ. Thromboembolic stroke in patients with a HeartMate-II left ventricular assist device – the role of anticoagulation. J Cardiothorac Surg 2015;10:128. | Do not report DT data |
| VanderPluym CJ, Cedars A, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, et al. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant 2018;37:89–99. | Wrong outcomes |
| VanderPluym CJ, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, Maltais S, et al. Utilization and outcomes of ventricular assist device support in adult congenital heart disease: an analysis of the interagency registry for mechanically assisted circulatory support (INTERMACS). J Heart Lung Transplant 2016;(1):S151–2. | Do not report DT data |
| Veasey TM, Floroff CK, Strout SE, McElray KL, Brisco-Bacik MA, Cook JL, et al. Evaluation of anticoagulation and nonsurgical major bleeding in recipients of continuous-flow left ventricular assist devices. Artif Organs 2019;43:736–44. | Wrong outcomes |
| Veen KM, Muslem R, Soliman OI, Caliskan K, Kolff MEA, Dousma D, et al. Left ventricular assist device implantation with and without concomitant tricuspid valve surgery: a systematic review and meta-analysis. Eur J Cardio-Thorac Surg 2018;54:644–51. | Wrong outcomes |
| Vellanki NS, Kennedy K, Grandin EW, Garan AR, Motiwala SR, Quintero P, et al. Women have more early right heart failure but no increase in later right heart failure after LVAD implantation: an INTERMACS analysis. J Card Fail 2020;26 10 Suppl.):S145. | Do not report DT data |
| Vellipuram AR, Chaudary Chaudary MR, Maud A, Rodriguez Rodriguez G, Piriyawat P, Cruz-Flores S, et al. Cerebrovascular events as complication of left ventricular assist device: analysis of nationwide inpatient sample (NIS) database (2005-2014). Eur Stroke J 2019;4(Suppl. 1):715. | Do not report DT data |
| Verma S, Bassily E, Leighton S, Mhaskar R, Sunjic I, Martin A, et al. Renal function and outcomes with use of left ventricular assist device implantation and inotropes in end-stage heart failure: a retrospective single center study. J Clin Med Res 2017;9:596–604. | Do not report DT data |
| Vest AR, Kennel PJ, Maldonado D, Young JB, Mountis MM, Naka Y, et al. Recovery of serum cholesterol predicts survival after left ventricular assist device implantation. Circ Heart Fail 2016;9:09. | Do not report DT data |
| Vidula H, Chen A, Tankut S, Yoruk A, Alexis J, Gosev I, et al. Arrhythmia burden from implantable device interrogation during long-term follow-up in LVAD patients. J Heart Lung Transplant 2021;40(4 Suppl.):S395. | Do not report DT data |
| Vidula H, Kutyifa V, Johnson BA, Strawderman RL, Harrington D, Polonsky B, et al. Readmission patterns during long-term follow-up after left ventricular assist device implantation. Am J Cardiol 2018;122:1021–7. | Wrong outcomes |
| Vidula H, McNitt S, Papernov A, Wang M, Kutyifa V, Alexis JD. Clinical relevance of late and very late right heart failure after LVAD implantation. J Heart Lung Transplant 2017;36(4 Suppl. 1):S423. | Do not report DT data |
| Vidula H, McNitt S, Wang M, Polonsky B, Sherazi S, Ayers B, et al. Long-term survival of patients requiring early temporary RVAD support following LVAD implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S358. | Do not report DT data |
| Vidula H, McNitt S, Wang M, Polonsky S, Sherazi S, Gosev I, et al. Time-dependent association of renal function with long-term survival following LVAD implantation. J Heart Lung Transplant 2019;38(4 Suppl.):S233–4. | Do not report DT data |
| Vidula H, Wang M, Antaki J, Polonsky B, Sherazi S, Alexis J, et al. Risk score for mortality prediction after one-year on left ventricular assist device support. J Heart Lung Transplant 2020;39(4 Suppl.):S181. | Do not report DT data |
| Vierecke J, Gahl B, de By T, Antretter H, Beyersdorf F, Caliskan K, et al. Results of primary biventricular support: an analysis of data from the EUROMACS registry. Eur J Cardio-Thorac Surg 2019;56:1037–45. | Do not report DT data |
| Vierecke J, Gahl B, De By TM, Loforte A, Mohacsi P. The purpose of this study was to analyze pre-and postoperative bvad euromacs-registry patient data. Artif Organs 2020;44(3):E75–6. | Do not report DT data |
| Vinholo TF, Mullan CW, Mori M, Caraballo C, Ravindra NG, Miller E, et al. Outcomes of left ventricular assist device implantation with mitral regurgitation with and without concomitant mitral operation. J Heart Lung Transplant 2020;39(4 Suppl.):S441–2. | Do not report DT data |
| Volkovicher N, Kurihara C, Critsinelis A, Kawabori M, Sugiura T, Manon M, 2nd, et al. Outcomes in patients with advanced heart failure and small body size undergoing continuous-flow left ventricular assist device implantation. J Artif Organs 2018;21:31–8. | Duplicate record |
| Vora TA, Afari-Armah N, Hofmeyer M, Sheikh FH, Rodrigo M, Molina E, et al. Heart failure hospitalizations in a contemporary LVAD population. J Card Fail 2017;23(8 Suppl. 1):S59–60. | Do not report DT data |
| Vrtovec B, Radovancevic R, Delgado RM, Radovancevic B, Bracey AW, Gregoric ID, et al. Significance of anaemia in patients with advanced heart failure receiving long-term mechanical circulatory support. Eur J Heart Fail 2009;11:1000–4. | Do not report DT data |
| Wagner T, Schrage B, Bernhardt A, Reichenspurner H, Blankenberg S, Grahn H. Right heart failure before predicts right heart failure after LVAD implantation. Eur Heart J 2018;39(Suppl. 1):385. | Do not report DT data |
| Ward ST, Liang Q, Pagani FD, Zhang M, Kormos RL, Aaronson KD, et al. A roadmap for evaluating the use and value of durable ventricular assist device therapy. J Heart Lung Transplant 2018;37:146–50. | Wrong study design |
| Warraich HJ, Allen LA, Blue LJ, Chaussee EL, Thompson JS, McIlvennan CK, et al. Comorbidities and the decision to undergo or forego destination therapy left ventricular assist device implantation: an analysis from the Trial of a Shared Decision Support Intervention for Patients and their Caregivers Offered Destination Therapy for End-Stage Heart Failure (DECIDE-LVAD) study. Am Heart J 2019;213:91–6. | Wrong intervention |
| Wasson LT, Yuzefpolskaya M, Wakabayashi M, Takayama H, Naka Y, Uriel N, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev. 2014;05. | Wrong study design |
| Waters SB, Sheridan BC, Watkins R, Duva M, Chang PP, Katz JN. Survival and thrombotic events in left ventricular assist device patients are not influenced by socioeconomic status. J Heart Lung Transplant 2016;(1):S166. | Do not report DT data |
| Wavell C, Sokolowski A, Klingel ML, Yin C, Nagpal AD. Clinical effectiveness of therapy with continuous-flow left ventricular assist devices in nonischemic versus ischemic cardiomyopathy: a systematic review and meta-analysis. Can J Surg 2021;64:E39–47. | Do not report DT data |
| Welp H, Dell’Aquila A, Hoffmeier A, Scherer M. Medical and financial considerations regarding long-term mechanical left ventricular support. Thoracic and Cardiovascular Surgeon Conference: 49th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery Germany. 2020;68. | Duplicate record |
| Welp HA, Dell’Aquila AM, Hoffmeier A, Martens S, Scherer M. Medical and economic consideration regarding long term mechanical left ventricular support. J Heart Lung Transplant 2020;39(4 Suppl.):S366. | Do not report DT data |
| Westaby S, Siegenthaler M, Beyersdorf F, Massetti M, Pepper J, Khayat A, et al. Destination therapy with a rotary blood pump and novel power delivery. Eur J Cardio-Thorac Surg 2010;37:350–6. | < 50 DT patients |
| Westhofen S, Bernhardt A, Reichenspurner H, Barten M. Gender differences in cardiac reverse remodeling in mechanically unloaded hearts. J Heart Lung Transplant 2018;37(4 Suppl. 1):S385. | Do not report DT data |
| Westhofen S, Bernhardt A, Reichenspurner H, Barten M. Gender differences in cardiac reverse remodeling in mechanically unloaded hearts. Thoracic and Cardiovascular Surgeon Conference: 48th Annual Meeting German Society for Thoracic, Cardiac, and Vascular Surgery Germany. 2019;67. | Do not report DT data |
| Westhofen S, Bernhardt A, Sadeq A, Reichenspurner H, Barten M. Cardiac reverse remodeling in mechanically unloaded hearts: analysis of gender-specific differences. Thoracic and Cardiovascular Surgeon Conference: 49th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery Germany. 2020;68. | Do not report DT data |
| Wever Pinzon JR, Wang W, Hu N, Larsen R, Yu T, Yin L, et al. Outcomes of Asian-Americans undergoing left ventricular assist device implantations as a bridge to transplant or destination therapy: an intermacs analysis. J Heart Lung Transplant 2017;36(4 Suppl. 1):S253–4. | Do not report DT data |
| White-Williams C, Fazeli-Wheeler P, Myers S, Kirklin J, Pamboukian S, Naftel D, et al. HRQOL improves from before to 2 years after MCS, regardless of implant strategy: analyses from INTERMACS. J Heart Lung Transplant 2016;(1):S25. | Do not report DT data |
| Whitson BA, Eckman P, Kamdar F, Lacey A, Shumway SJ, Liao KK, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97:2097–103. | Do not report DT data |
| Wilcox J, Kao AC, Hsich E, Dew MA, Kormos R, Andrei AC, et al. Change in caregiver health-related quality of life from before to early after surgery: findings from the Sustaining Quality of Life of the Aged: Transplant or Mechanical Support (SUSTAIN-IT) study. J Card Fail 2019;25(8 Suppl.):S15–6. | Do not report DT data |
| Wilhelms LA, Blumenthal-Barby JS, Kostick KM, Estep JD, Bruce CR. Patients’ perspectives on transplantation while undergoing left ventricular assist device support. ASAIO J 2017;63:740–4. | < 50 DT patients |
| Witman MA, Garten RS, Gifford JR, Groot HJ, Trinity JD, Stehlik J, et al. Further peripheral vascular dysfunction in heart failure patients with a continuous-flow left ventricular assist device: the role of pulsatility. JACC Heart Fail 2015;3:703–11. | Do not report DT data |
| Wong JK, Forrest A, Sherazi S, Chen L, Alexis J, Friedman SM, et al. Recurrent falls in patients with CF-LVAD’s are associated with major morbidity and mortality. J Heart Lung Transplant 2017;36(4 Suppl. 1):S100–1. | Do not report DT data |
| Wood CT, O’Malley TJ, Maynes EJ, Vishnevsky A, Morris RJ, Samuels LE, et al. Survival outcomes of stenting outflow graft stenosis in continuous-flow left ventricular assist devices: a systematic review. Heart Fail Rev. 2019. | < 50 DT patients |
| Worku B, Gambardella I, Rahouma M, Demetres M, Gaudino M, Girardi L. Thoracotomy versus sternotomy? The effect of surgical approach on outcomes after left ventricular assist device implantation: a review of the literature and meta-analysis. J Card Surg 2021. | Wrong intervention |
| Wu L, Weng YG, Dong NG, Krabatsch T, Stepanenko A, Hennig E, et al. Outcomes of HeartWare ventricular assist system support in 141 patients: a single-centre experience. Eur J Cardio-Thorac Surg 2013;44:139–45. | < 50 DT patients |
| Wu S, Xu P, Lee A, Fong M. Coronary artery disease and right ventricular function predict outcomes after ventricular assist device placement. Journal of the American College of Cardiology Conference: 67th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC. 2018;71. | Do not report DT data |
| Xu PZ, Wu S, Tun H, Adenuga G, Fong M. Impact of contemporary ventricular assist device therapy on renal outcomes in end stage heart failure. J Heart Lung Transplant 2018;37 (4 Suppl. 1):S473–4. | Do not report DT data |
| Xuereb L, Go PH, Kaur B, Akrawe S, Nemeh HW, Borgi J, et al. Impact of preoperative atrial fibrillation on postoperative thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2016;102:1543–9. | Do not report DT data |
| Xuereb L, Kaur B, Akrawe S, Rashty J, Nemeh HW, Borgi J, et al. Reoperation for bleeding does not adversely impact long-term outcomes in LVAD recipients. J Heart Lung Transplant 2016;(1):S249. | Do not report DT data |
| Yager JE, Felker GM. Left ventricular assist devices as destination therapy for end-stage heart failure. Am Heart J 2004;148:252–3. | Wrong study design |
| Yalcin YC, Rasheed M, Muslem R, Brugts JJ, Constantinescu AA, Manintveld OC, et al. Outcomes over one and a half decade following HeartMate II versus HeartMate 3 left ventricular assist device therapy: the Rotterdam experience. J Heart Lung Transplant 2021;40(4 Suppl.):S422–3. | Do not report DT data |
| Yang JA, Kato TS, Shulman BP, Takayama H, Farr M, Jorde UP, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant 2012;31:601–10. | < 50 DT patients |
| Yap S, Muslem R, Ramjankhan F, De Jonge N, Constantinescu AA, Manintveld OC, et al. Incidence and impact of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2016;(1):S10–1. | Do not report DT data |
| Yassin AS, Subahi A, Adegbala O, Abubakar H, Akintoye E, Ahmed A, et al. Clinical impact of diabetes mellitus on short-term outcomes and in-hospital mortality of cardiac mechanical support with Left Ventricular Assist Device (LVAD): a retrospective study from a National Database. Cardiovasc Revasc Med 2019;20:883–6. | Do not report DT data |
| Yin C, Wavell C, Sokolowski A, Klingel M, Nagpal D. Clinical effectiveness of continuous-flow LVAD therapy in non-ischemic versus ischemic cardiomyopathy: a systematic review and meta-analysis. Can J Cardiol 2020;36(10 Suppl.):S61–2. | Do not report DT data |
| Yin C, Wavell C, Sokolowski A, Klingel M, Nagpal D. Clinical effectiveness of continuous-flow LVAD therapy in non-ischemic versus ischemic cardiomyopathy: a systematic review and meta-analysis. Can J Cardiol 2020;36:S61–2. | Do not report DT data |
| Yoshioka D, Takayama H, Colombo PC, Yuzefpolskaya M, Garan AR, Topkara VK, et al. Changes in end-organ function in patients with prolonged continuous-flow left ventricular assist device support. Ann Thorac Surg 2017;103:717–24. | < 50 DT patients |
| Yost G, Coyle L, Gallagher C, Graney N, Siemeck R, Tatooles A, et al. The impact of extreme obesity on outcomes after left ventricular assist device implantation. J Thorac Dis 2017;9:4441–6. | < 50 DT patients |
| Yousefzai R, Baykaner T, Rappelt M, Ghashghaei R, Baeza C, Al Khayyat A, et al. Neurohormonal therapy in patients with left ventricular assist devices. J Card Fail 2016;22(Suppl. 8):S63–4. | Do not report DT data |
| Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg 2012;27:630–8. | < 50 DT patients |
| Yuzefpolskaya M, Nasiri M, Onat D, Royzman EA, Nwokocha J, Pinsino A, et al. Gut microbiome-generated metabolite trimethylamine-n-oxide is reduced after heart transplantation and continuous flow left ventricular assist device therapy in advanced heart failure patients. J Heart Lung Transplant 2018;37(4 Suppl. 1):S235. | Do not report DT data |
| Zalawadiya S, Shah A, Keebler M, John R, Gregoric I, Kilic A, et al. Impact of anemia on survival among patients with durable ventricular assist device: an analysis of the prevent study. J Heart Lung Transplant 2018;37(4 Suppl. 1):S161. | Do not report DT data |
| Zhalbinova MR, Rakhimova SE, Bekbosynova MS, Andosova SA, Abdirova BU, Akilzhanova AR. The cause of the bleeding and thrombosis in patients with implanted left ventricular assist devices. J Heart Lung Transplant 2020;39(4 Suppl.):S399. | Do not report DT data |
| Zhang L, Purohit M, Hassett C, Cho S, Buletko A. Neurologic complications of heartware and heartmate II. Neurology Conference: 71st Annual Meeting of the American Academy of Neurology, AAN. 2019;92. | Do not report DT data |
| Zhigalov K, Sa MPBO, Arjomandi Rad A, Vardanyan R, Goerdt L, Chrosch T, et al. The impact of obesity on left ventricular assist device outcomes. Medicina. 2020;56. | Do not report DT data |
| Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold-Herz M, et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE Registry. Eur Heart J 2020;41:3801–9. | Do not report DT data |
| Zimpfer D, Netuka I, Schmitto JD, Pya Y, Garbade J, Morshuis M, et al. Multicentre clinical trial experience with the HeartMate 3 left ventricular assist device: 30-day outcomes. Eur J Cardio-Thorac Surg 2016;50:548–54. | < 50 DT patients |
| Ziv O, Dizon J, Thosani A, Naka Y, Magnano AR, Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol 2005;45:1428–34. | Do not report DT data |
| Zubarevich A, Szczechowicz M, Osswald A, Arjomandi Rad A, Vardanyan R, Pompeu BOSM, et al. Impact of gender in patients with continuous-flow left ventricular assist device therapy in end-stage heart failure. Int J Artif Organs. 2021. | Do not report DT data |
Appendix 4 Risk of bias for clinical effectiveness review
| Study name | Outcome | Domain 1 (randomisation process) | Domain 2 (deviations from intended interventions) | Domain 3 (missing outcome data) | Domain 4 (measurement of the outcome) | Domain 5 (selection of reported result) | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| ROADMAP (2015) | Survival | N/A | Low | Low | Low | Low | Low |
| No deviations from intended intervention due to trial context, both ITT and as treated analyses carried out | No missing data | Survival appropriate outcome, survival outcome objective | Protocol available, analyses implemented as planned | ||||
| QoL | N/A | Low | High | Some concerns | Low | High | |
| Blinding cannot be avoided, investigators aware of treatments, no deviations arose due to trial context, as treated analysis but only similar small number of patients missing from each arm | Both intervention and control group had < 95% patient data at 12 months, no methods to correct for bias, ability to carry out QoL questionnaire may have been impeded by QoL | Valid appropriate QoL tool, self-report, outcome assessors likely aware of treatments | Protocol available and analyses align with this, QoL measured at 0, 6, 12, 24 months Reported at 0, 12, 24 – not reported at 6 months but change from baseline analysis was conducted |
||||
| REMATCH (2001) | Survival | Some concerns | Low | Low | Low | Low | Low |
| No information given on allocation concealment or full randomisation process though groups were similar | Blinding cannot be avoided. Investigators except statisticians were unaware of outcome data, ITT analysis | Data were available for all participants | Survival appropriate outcome, study personnel except statisticians blind to treatment |
Protocol available though some outcomes not mentioned
Multiple results reported for each outcome, not selected |
|||
| QoL | Some concerns | Low | High | Low | Some concerns | High | |
| No information given on allocation concealment or full randomisation process though groups were similar |
Blinding cannot be avoided
Investigators except statisticians were unaware of outcome data, ITT analysis |
Only 50% of patients had QoL data in MM group, ability to carry out QoL questionnaire may have been impeded by QoL | Valid appropriate QoL tool, study personnel except statisticians blind to treatment | Not all outcomes in protocol reported in results | |||
| MOMENTUM 3 (2019) | Survival | Low | Some concerns | Low | Low | Low | Some concerns |
| Randomisation permuted blocks and with stratification according to trial centre and was implemented through an electronic data-capture system, similar groups | Blinding cannot be avoided, event adjudicators blinded to treatments, per protocol analysis, eight patients did not receive LVAD but unlikely to impact results and reflective of real life | No missing participant data | Survival appropriate outcome, adjudicators unlikely to be blinded to survival outcome but survival is objective | Protocol available, analyses implemented as planned | |||
| QoL | Low | Some concerns | Low | Some concerns | Low | Some concerns | |
| Randomisation permuted blocks and with stratification according to trial centre and was implemented through an electronic data-capture system, similar groups | Blinding cannot be avoided, event adjudicators blinded to treatments, per protocol analysis, eight patients did not receive LVAD but unlikely to impact results and reflective of real life | Missing data at each timepoint, sensitivity analysis carried out suggesting this did not affect QoL outcome | QoL tools valid and appropriate, patients aware of treatment which, could influence self-report of QoL, but unlikely strong beliefs in any beneficial effects | Protocol available, analyses implemented as planned | |||
| HMII DT (2009) | Survival | Some concerns | High | Low | Low | Some concerns | High |
| Randomisation was stratified according to study centre and with the use of permuted blocks, no information on allocation concealment |
Blinding cannot be avoided; carers and clinicians likely knew allocation
As treated – up to 17% not analysed in intervention group and > 50% in the control, which could have a significant effect |
Some participants swapped device after randomisation due to insurance coverage, not included in analysis
However missingness in outcome unlikely to depend on true value |
Survival appropriate outcome, objective |
No protocol available though outcomes defined in trial registry page
However no clear analysis plan described |
|||
| QoL | Some concerns | High | High | Some concerns | Some concerns | High | |
| Randomisation was stratified according to study centre and with the use of permuted blocks, no information on allocation concealment |
Blinding cannot be avoided; carers and clinicians likely knew allocation
As treated analysis Some drop out, crossover and some transplanted, which could have impacted results |
Greater than half of patients did not report QoL at 24 months and at other time points (after considering those still alive) No reasons for missing data, ability to carry out QoL questionnaire may have been impeded by QoL |
QoL tools valid and appropriate, patients aware of treatment, which could influence self-report of QoL, but unlikely strong beliefs in any beneficial effects | No protocol so difficult to tell if any other analyses were intended that are not described in the results paper | |||
| ENDURANCE DT (2017) | Survival | Low | Low | Low | Low | Low | Low |
|
Randomisation was performed with the use of a permuted block, central randomisation scheme and was implemented with a web-based interactive response system
Groups were similar |
Blinding cannot be avoided, no deviations from intended intervention due to trial context, ITT and as treated analyses carried out | Only two participants randomised dropped out at the start of the study (1 from each group) and f/up was 99.7% and 99.3% for each group | Survival appropriate outcome, objective, all safety data were adjudicated by blinded assessors | Protocol and full statistical plan given, all results reported as per the analysis plan | |||
| QoL | Low | Some concerns | High | Some concerns | Low | High | |
|
Randomisation was performed with the use of a permuted block, central randomisation scheme and was implemented with a web-based interactive response system
Groups were similar |
Blinding cannot be avoided, some participants switched after randomisation due to insurance coverage (though very small number)
As treated analysis |
Around 90% of available pts completed the outcome at 24 months in both groups, unclear why QoL was not measured for 10% of patients at 24 months f/up, ability to carry out QoL questionnaire may have been impeded by QoL | QoL tools valid and appropriate, patients aware of treatment, which could influence self-report of QoL, but unlikely strong beliefs in any beneficial effects | Protocol and full statistical plan given, all results reported as per the analysis plan | |||
| ENDURANCE DT 2 (2018) | Survival a | Low | High | Low | Low | Some concerns | High |
| No information given on randomisation and allocation concealment; groups similar | Blinding cannot be avoided, modified ITT analysis that excluded 10 participants from the analysis implying more like an as treated analysis | Only 10 missing from analyses in total from both arms, unlikely to have an effect | Survival objective, definition of disabling stroke more subjective but based on established criteria, outcome unlikely to be influenced by knowledge of intervention | No protocol, little information on analysis plan | |||
| QoL | Low | Low | Low | Some concerns | High | High | |
| No information given on randomisation and allocation concealment; groups similar | Blinding cannot be avoided, no deviations from intended intervention due to trial context, ITT analysis | ITT population, no missing data at 12 months | QoL tools valid and appropriate, patients aware of treatment, which could influence self-report of QoL, but unlikely strong beliefs in any beneficial effects | No protocol, little information on analysis plan, reported EQ-5D VAS only and overall summaries only, unclear if this was intended plan |
Appendix 5 Forest plots of results of non-HeartMate3 devices by outcome
Survival
FIGURE 28.
Survival data in HeartMate XVE at 1-month follow-up.
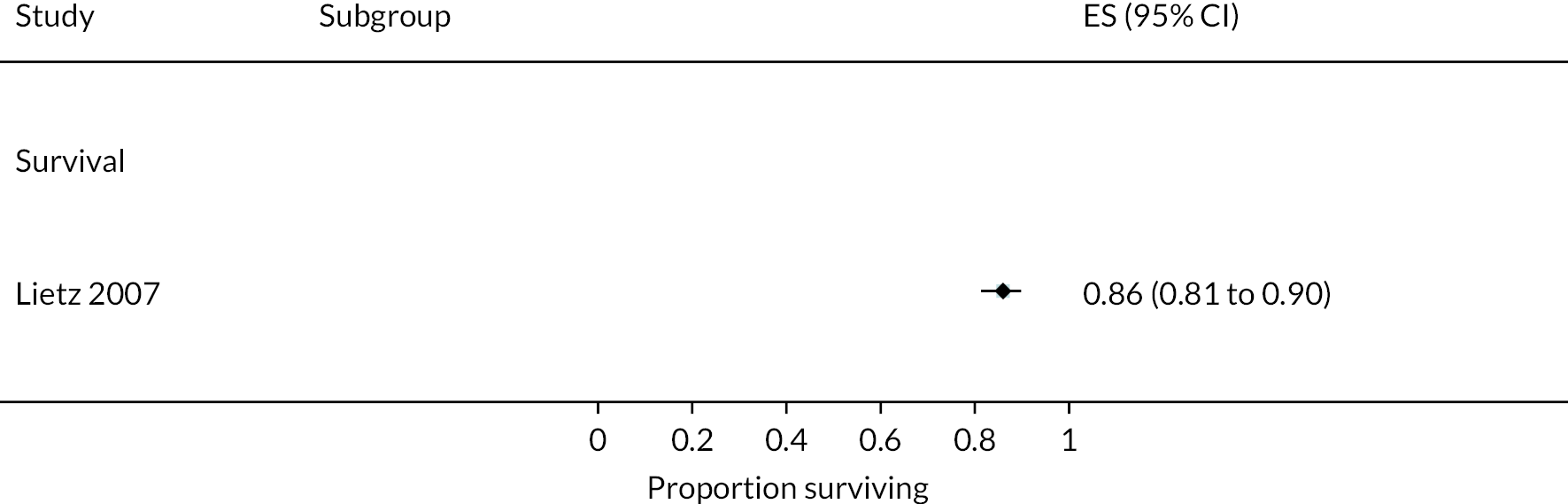
FIGURE 29.
Survival data in HeartMate XVE at 3 months follow-up. a, Data from a registry report. Events 1, censored at transplant and recovery; PP, per protocol analysis.
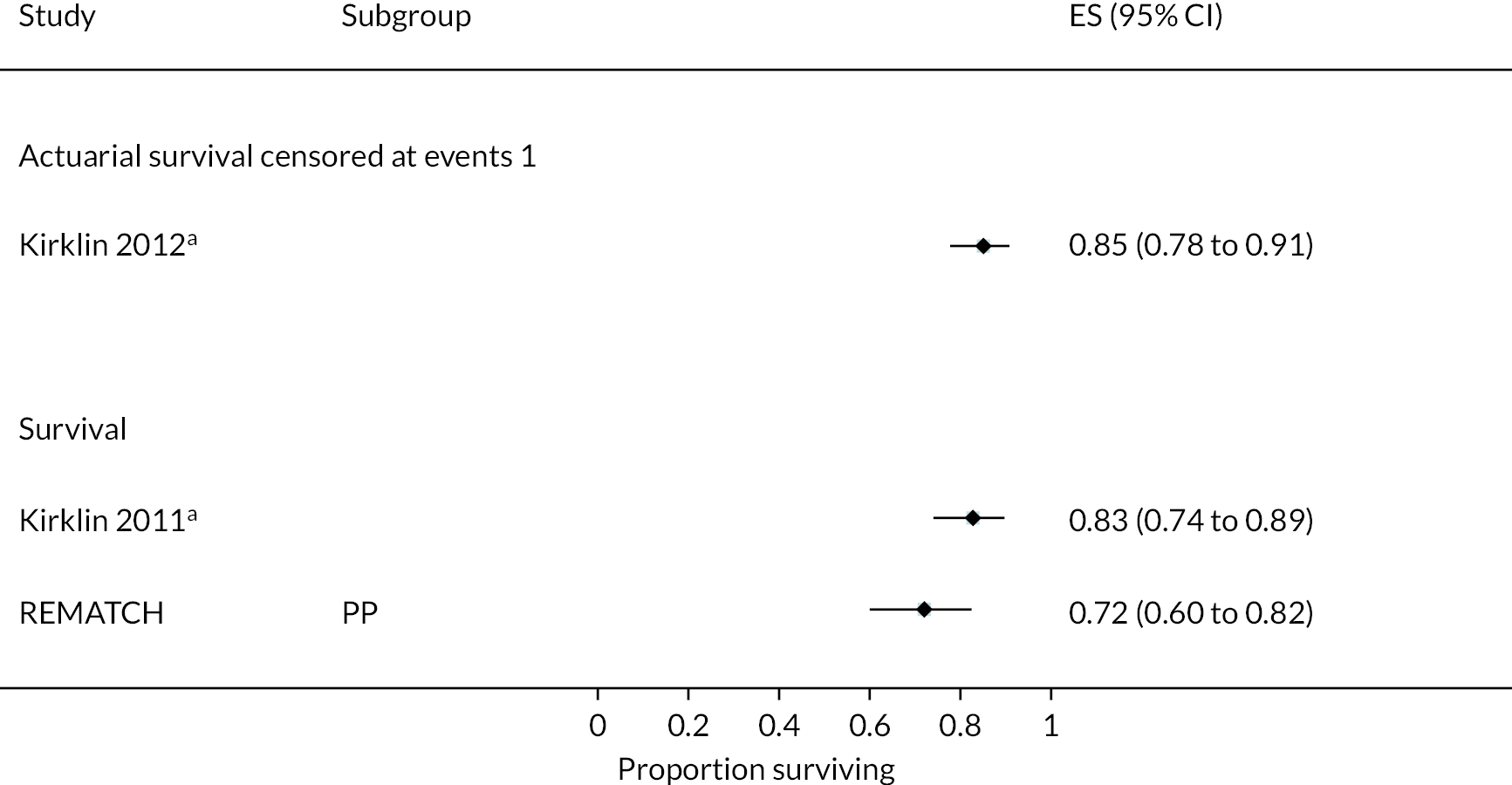
FIGURE 30.
Survival data in HeartMate XVE at 6 months follow-up. a, Data from a registry report. Survival censored at events 1, censored at transplant and recovery; event-free actuarial survival 9, free from device exchange or death secondary to device malfunction or device complication; PP, per protocol analysis.

FIGURE 31.
Survival data in HeartMate XVE at 12 months follow-up. a, Data from a registry report. Survival censored at events 1, censored at transplant and recovery; event-free actuarial survival 9, free from device exchange or death secondary to device malfunction or device complication.
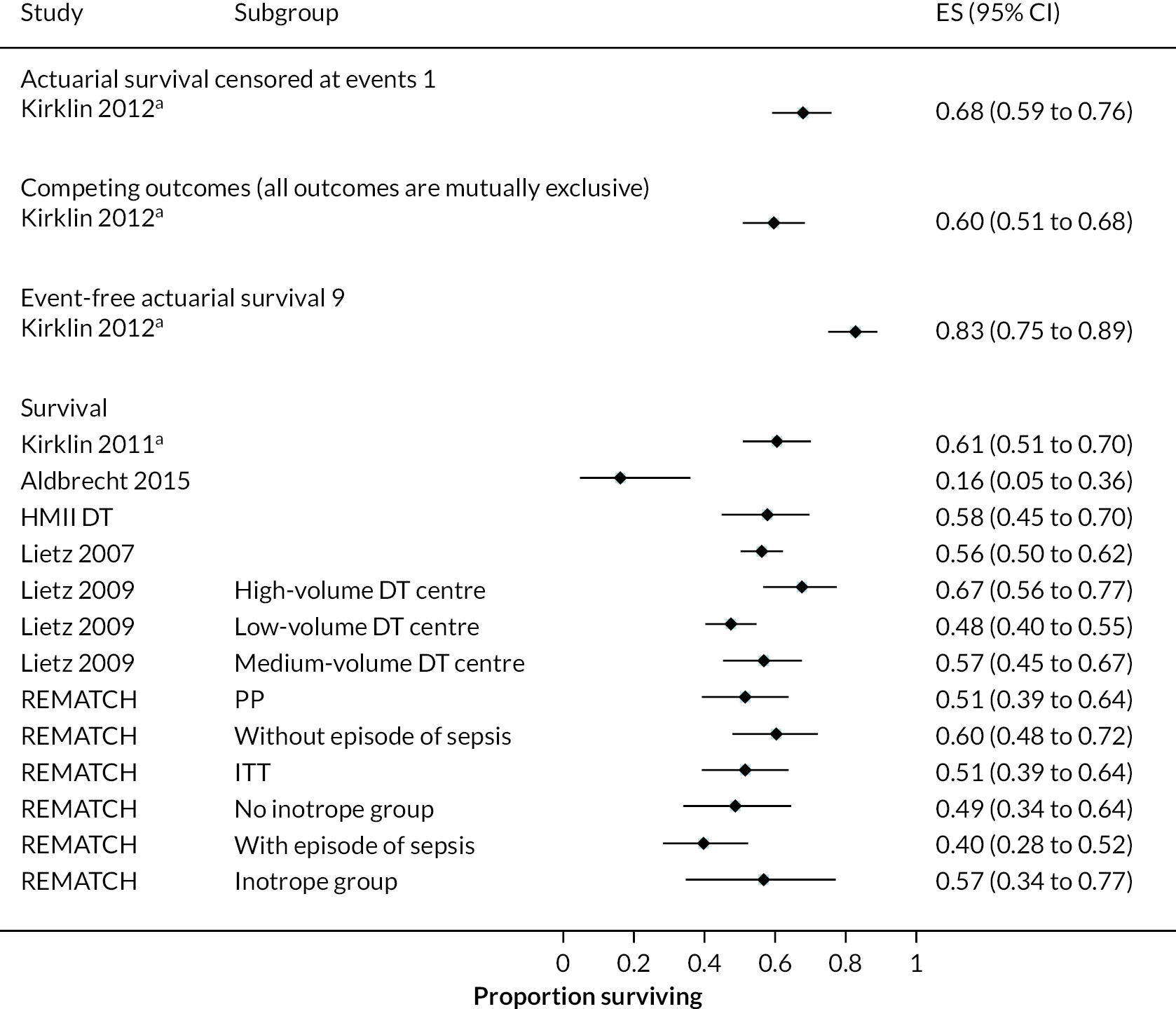
FIGURE 32.
Survival in HeartMate XVE at 18 months follow-up. PP, per protocol analysis.

FIGURE 33.
Survival in HeartMate XVE at 24 months follow-up. a, Data from a registry report. Survival censored at events 1, censored at transplant and recovery; event-free actuarial survival 9, free from device exchange or death secondary to device malfunction or device complication; event-free survival 4, free from disabling stroke or reoperation to repair or replace LVAD; ITT, intention to treat analysis; PP, per protocol analysis.
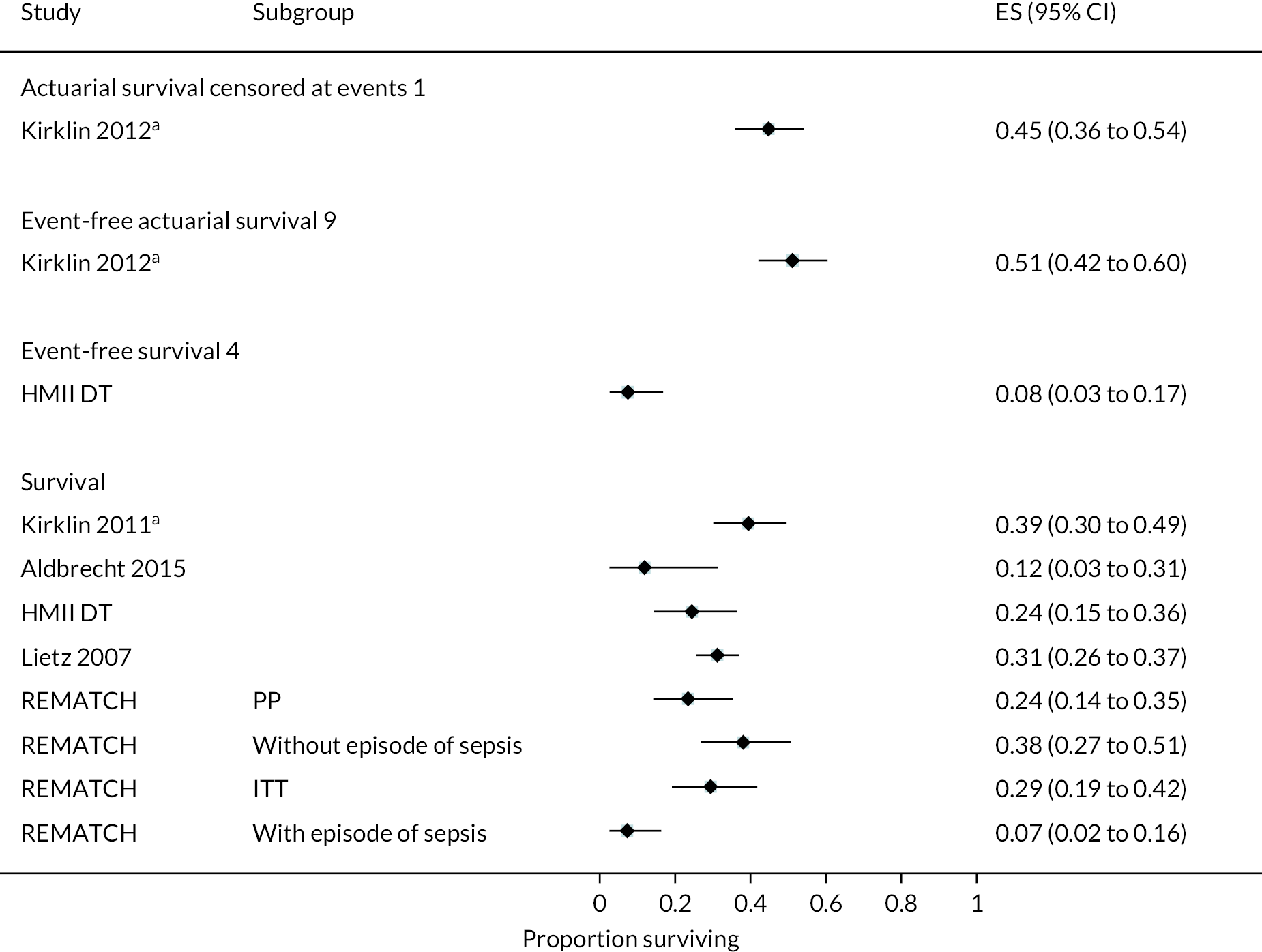
FIGURE 34.
Survival in HeartWare HVAD at 6 months follow-up. Survival censored at events 3, free from disabling stroke or need for device replacement.
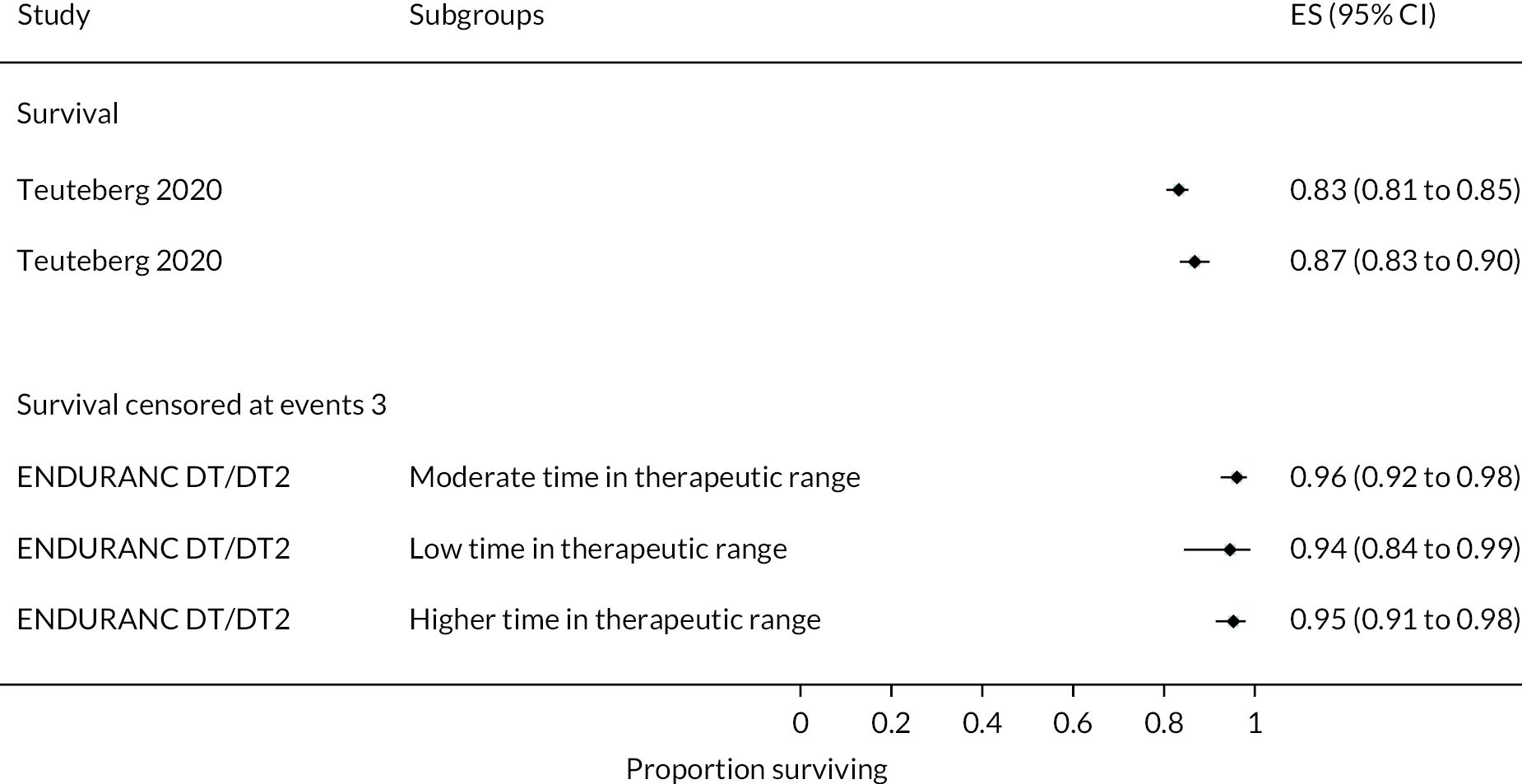
FIGURE 35.
Survival in HeartWare HVAD at 12 months follow-up. Event-free survival 5, free from disabling stroke and device malfunction or failure requiring exchange, explantation, or urgent transplantation; survival censored at events 3, censored either at time of device explant due to PE or removal for recovery, heart transplant, or at loss to follow-up 2 years post implant.
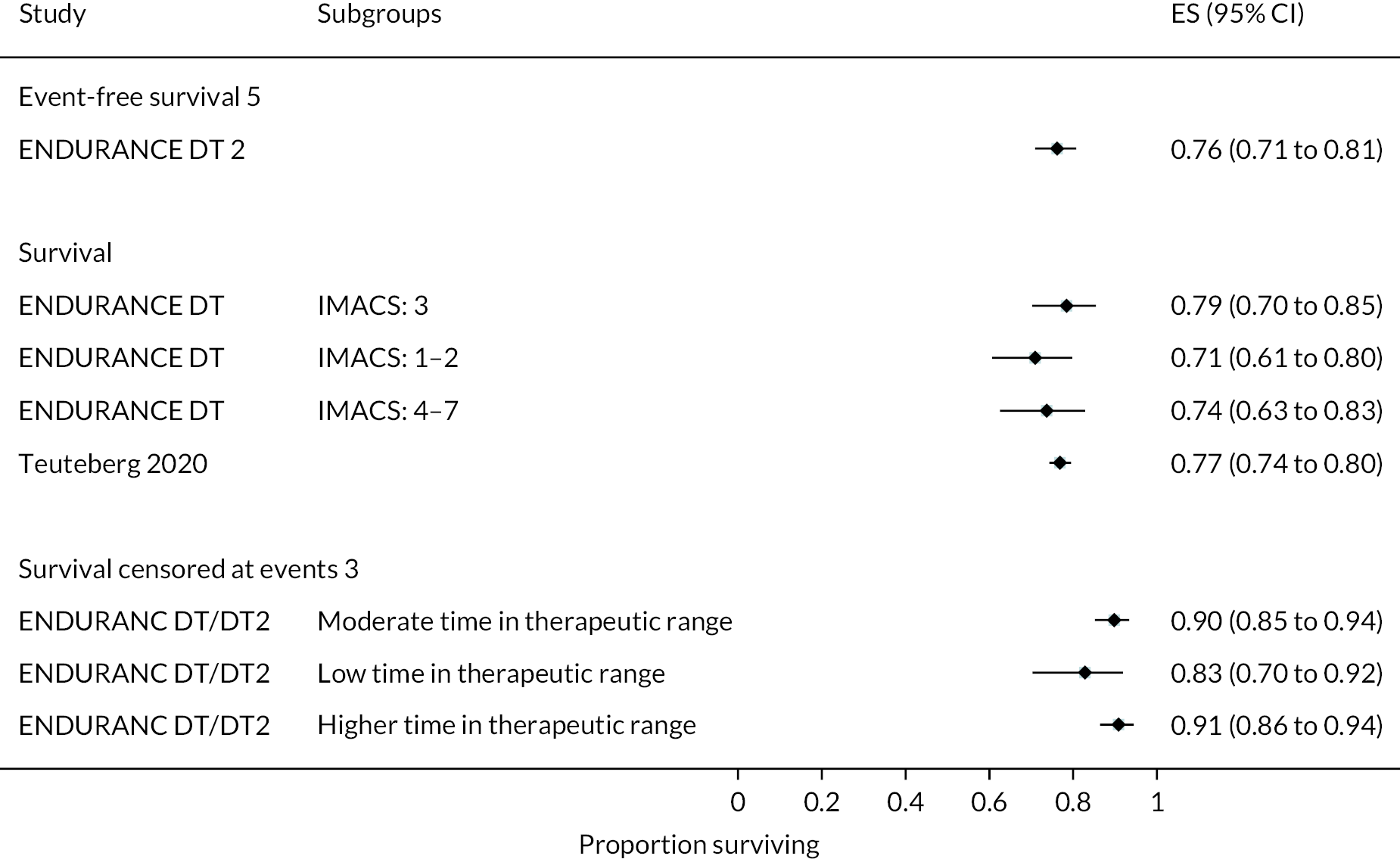
FIGURE 36.
Survival in HeartWare HVAD at 18 months follow-up. Survival censored at events 3, censored either at time of device explant due to pump exchange or removal for recovery, heart transplant, or at loss to follow-up 2 years post implant.

FIGURE 37.
Survival in HeartWare HVAD at 24 months follow-up. Event-free survival 3, free from disabling stroke or need for device replacement; ITT, intention to treat analysis; survival censored at events 3, censored either at time of device explant due to PE or removal for recovery, heart transplant, or at loss to follow-up 2 years post implant.

FIGURE 38.
Survival in HeartWare HVAD at 36 months follow-up.
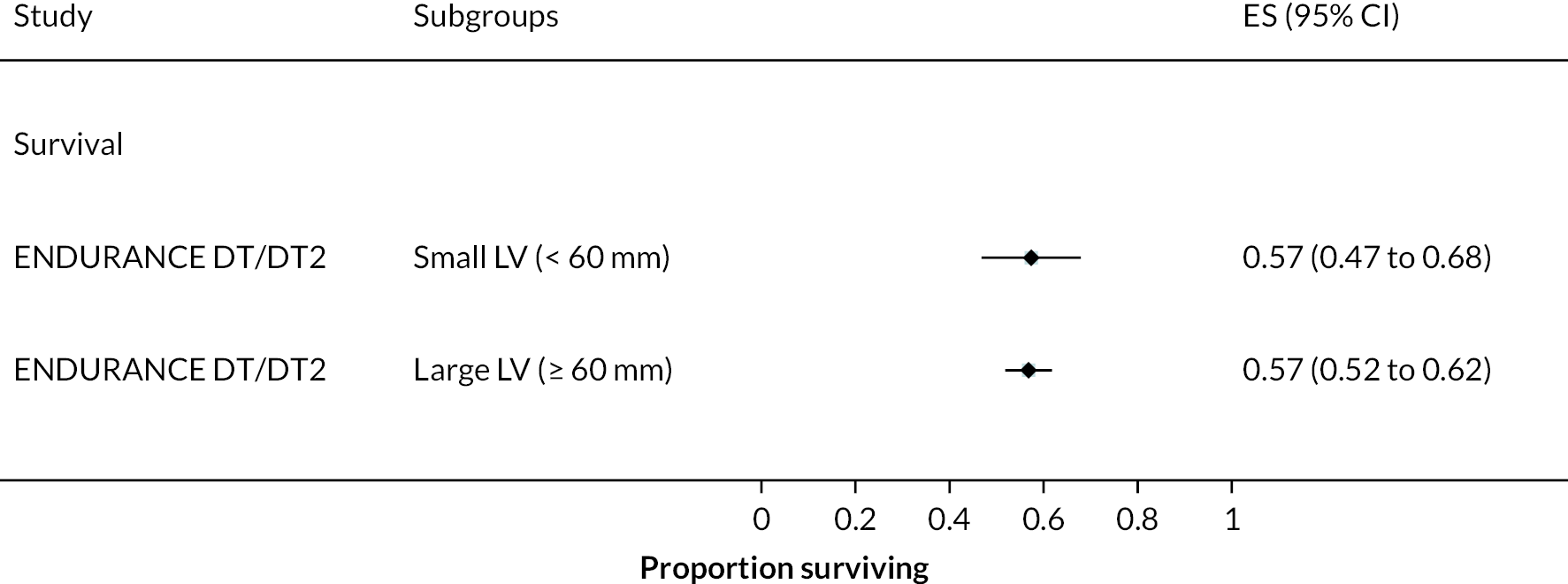
FIGURE 39.
Survival in HeartMate II at 1-month follow-up.
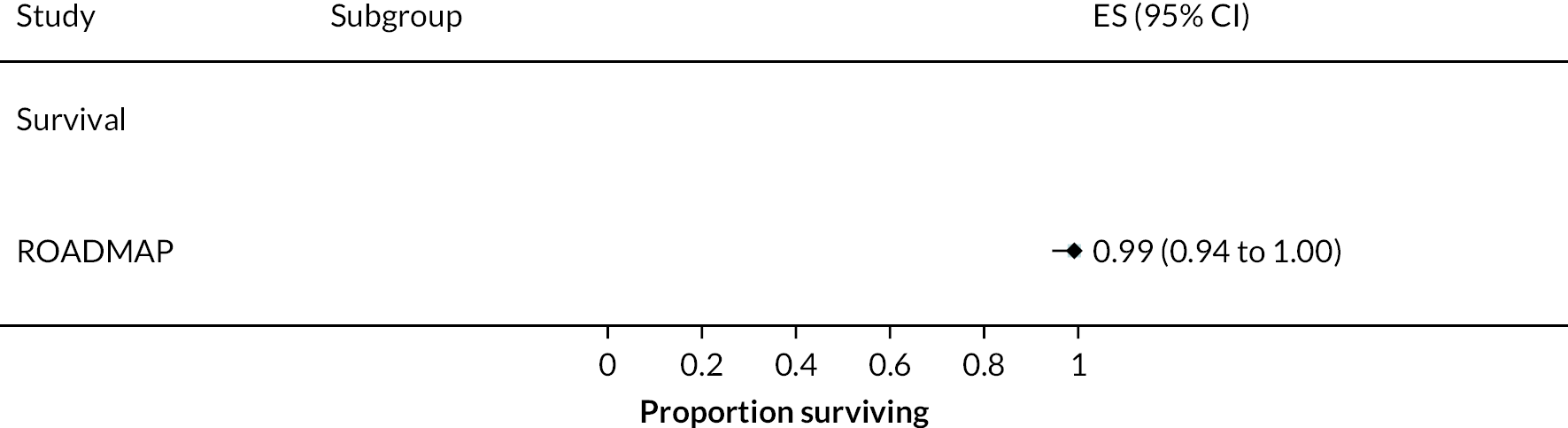
FIGURE 40.
Survival in HeartMate II at 6 months follow-up. Event-free survival 2, free from disabling stroke.

FIGURE 41.
Survival in HeartMate II at 12 months follow-up. Event-free survival 2, free from disabling stroke; event-free survival 5, free from disabling stroke, and device malfunction or failure requiring exchange, explantation, or urgent transplantation; event-free survival 7, free from urgent heart transplant or delayed LVAD; ITT, intention to treat analysis; PP, per protocol analysis.

FIGURE 42.
Survival in HeartMate II at 24 months follow-up. Event-free survival 2, free from disabling stroke; event-free survival 3, free from disabling stroke or need for device replacement; event-free survival 4, free from disabling stroke or reoperation to repair or replace LVAD; event-free survival 6, free from urgent heart transplant; event-free survival 7, free from urgent heart transplant or delayed LVAD; ITT-intention to treat analysis; PP, per protocol analysis.
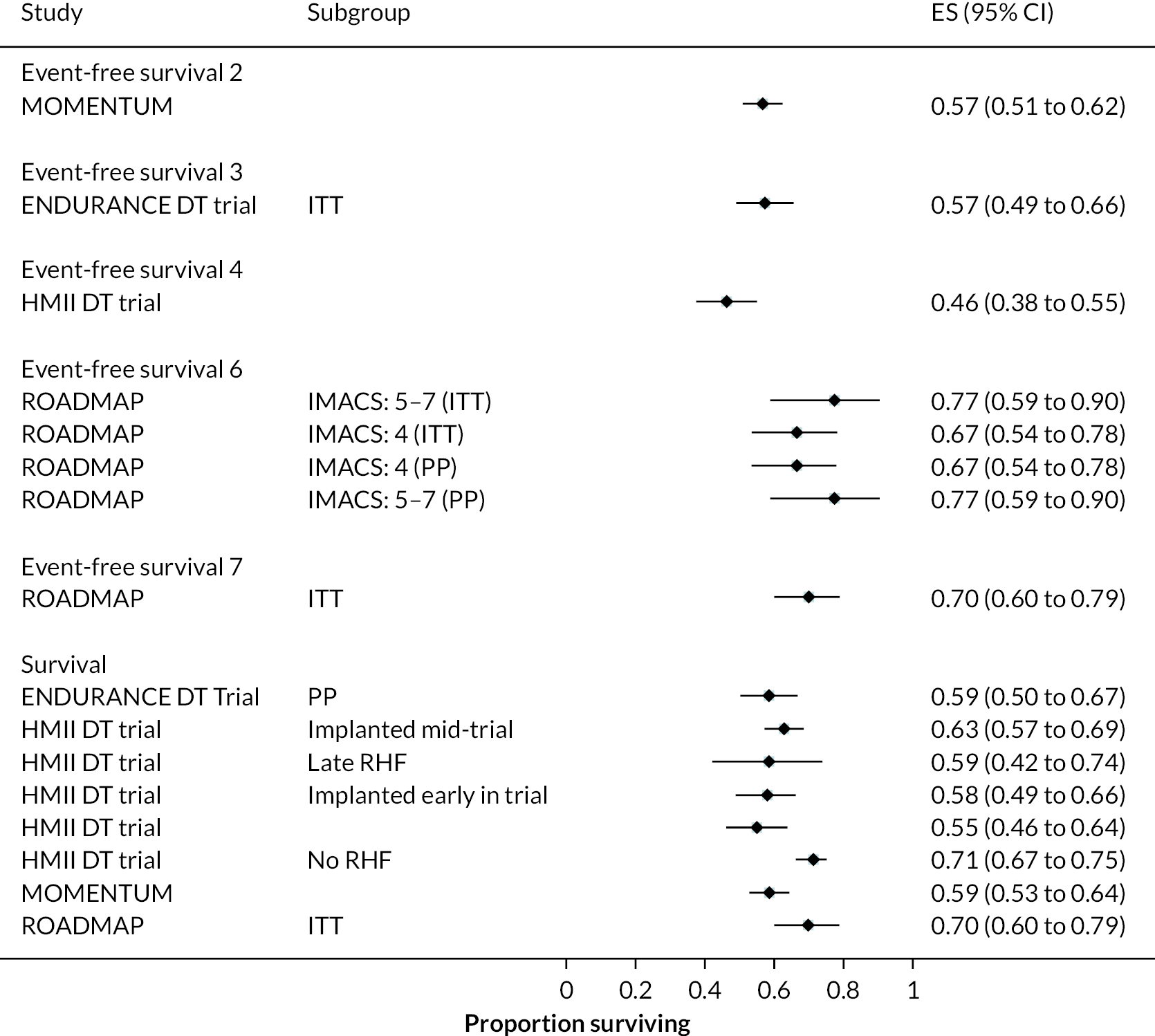
FIGURE 43.
Survival in HeartMate II at 36 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery.
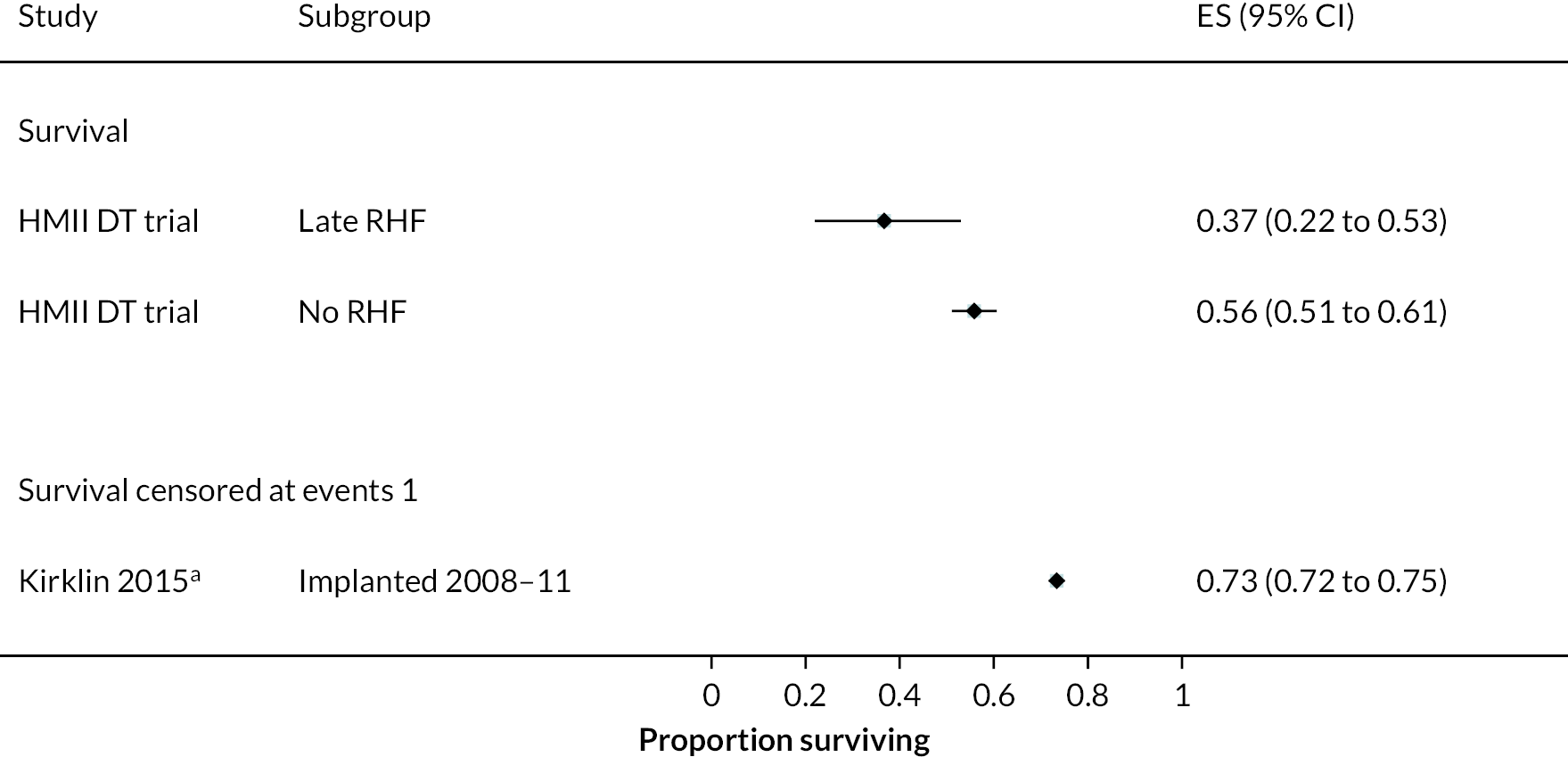
FIGURE 44.
Survival in HeartMate II at 48 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery.
FIGURE 45.
Survival in studies with multiple devices at 1-month follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery.

FIGURE 46.
Survival in studies with multiple devices at 3 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery.

FIGURE 47.
Survival in studies with multiple devices at 6 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery; event-free survival 7, free from urgent heart transplant or delayed LVAD.
FIGURE 48.
Survival in studies with multiple devices at 12 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery; event-free survival 7, free from urgent heart transplant or delayed LVAD; event-free survival 1, free from stroke; survival censored at events 2, censored at transplant or cessation of support.
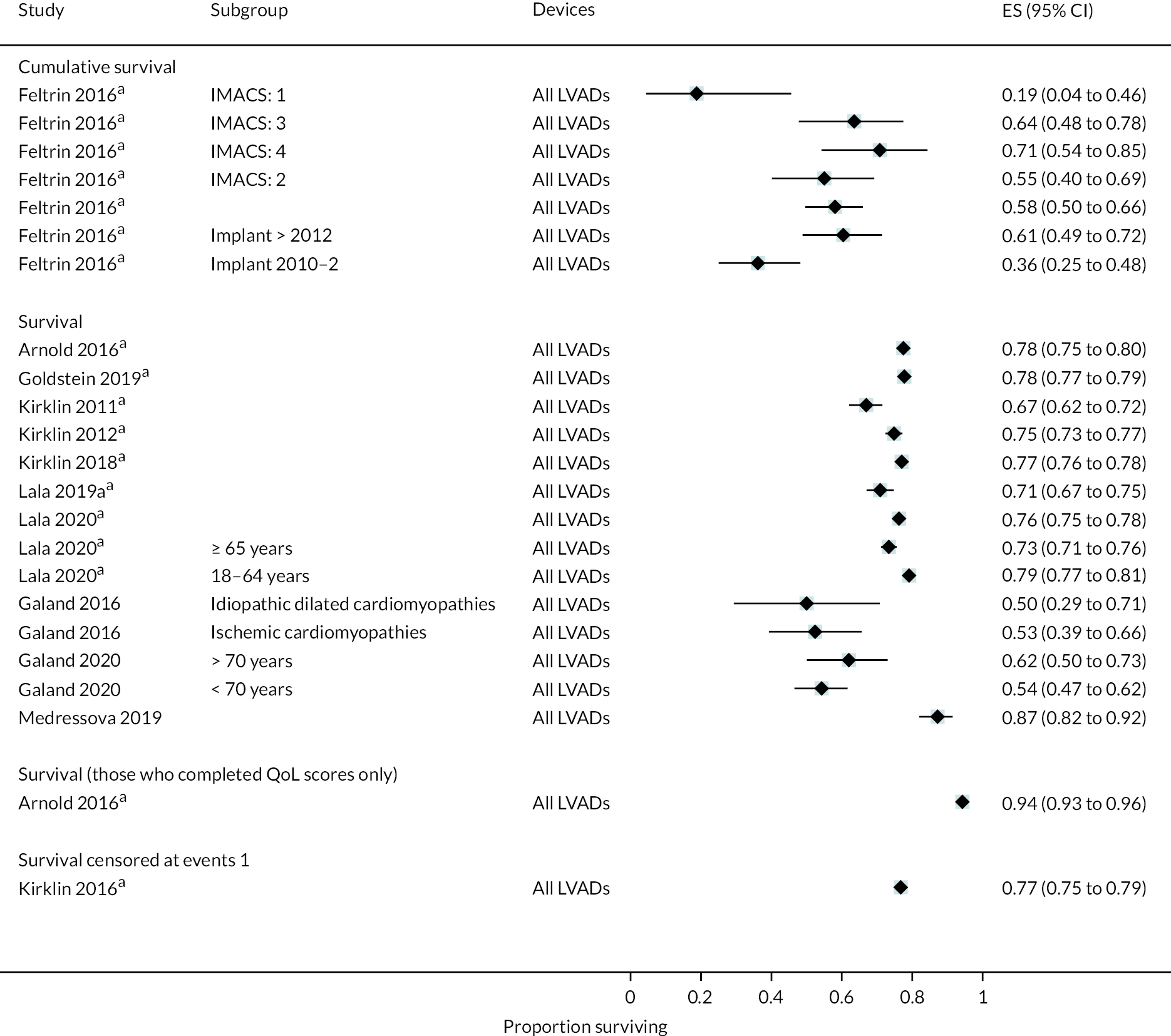
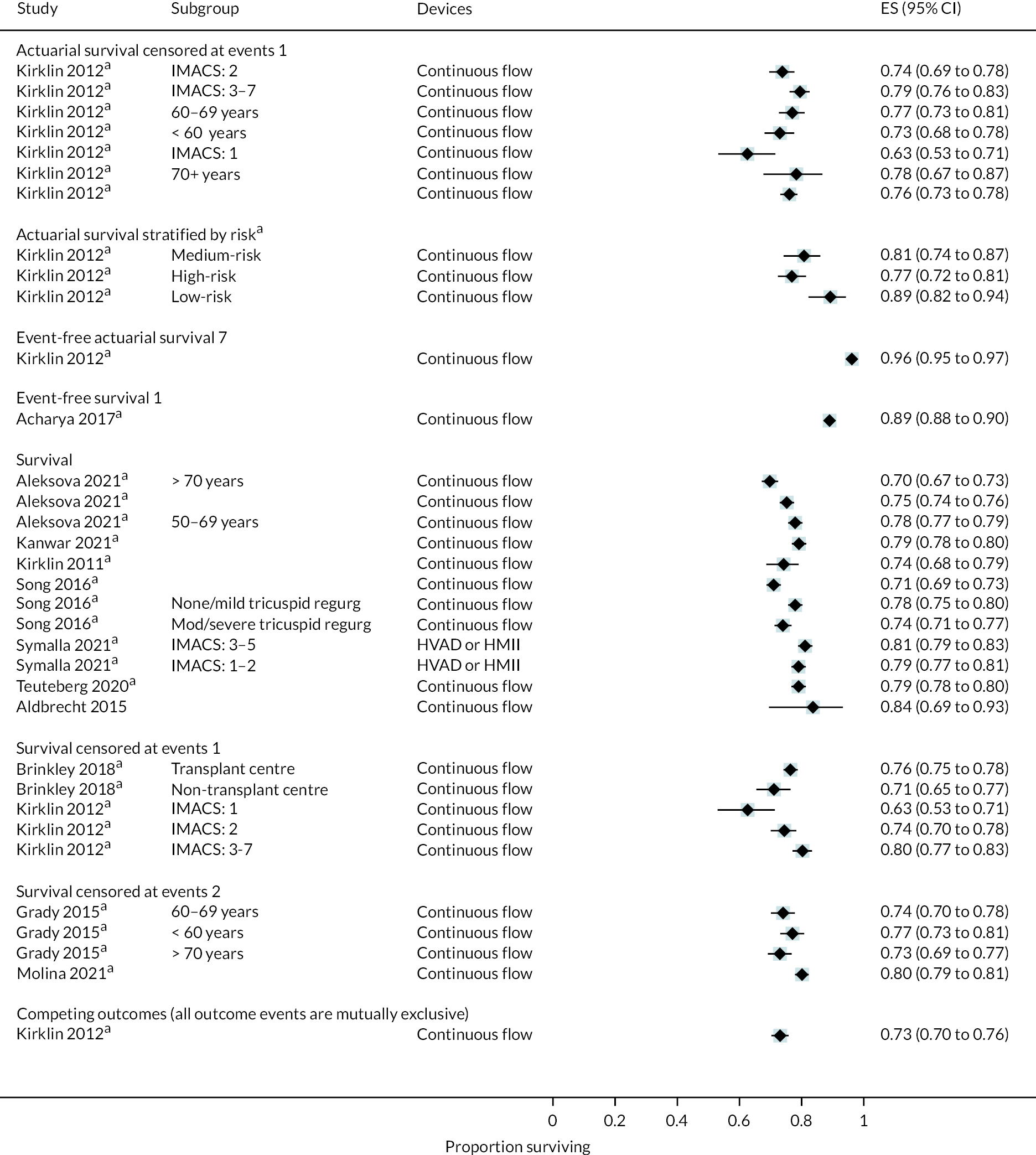
FIGURE 49.
Survival in studies with multiple devices at 18 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery.
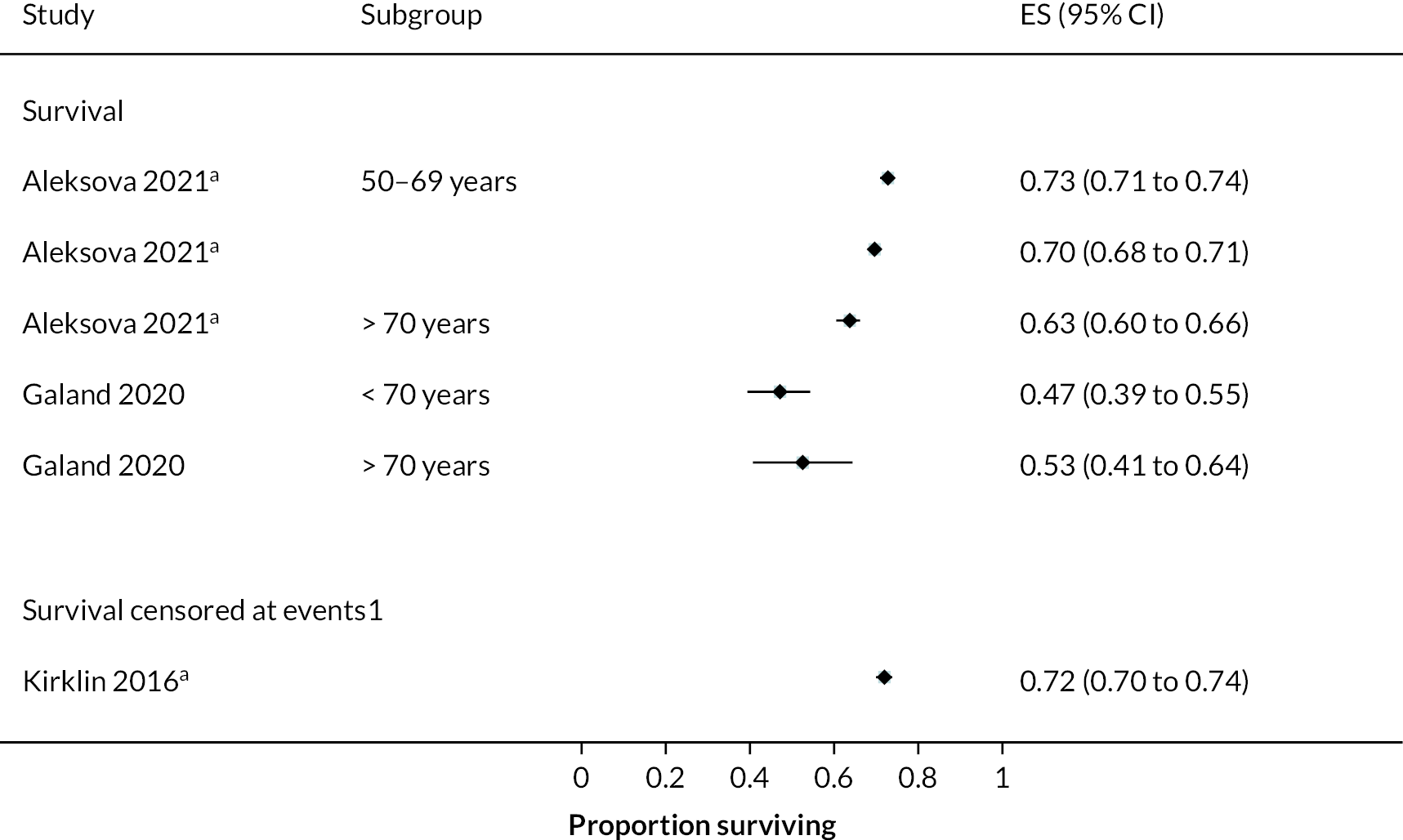
FIGURE 50.
Survival in studies with multiple devices at 24 months follow-up. a, Data from a registry report; survival censored at events 1, censored at transplant and recovery; event-free survival 7, free from urgent heart transplant or delayed LVAD; survival censored at events 2, censored at transplant or cessation of support.
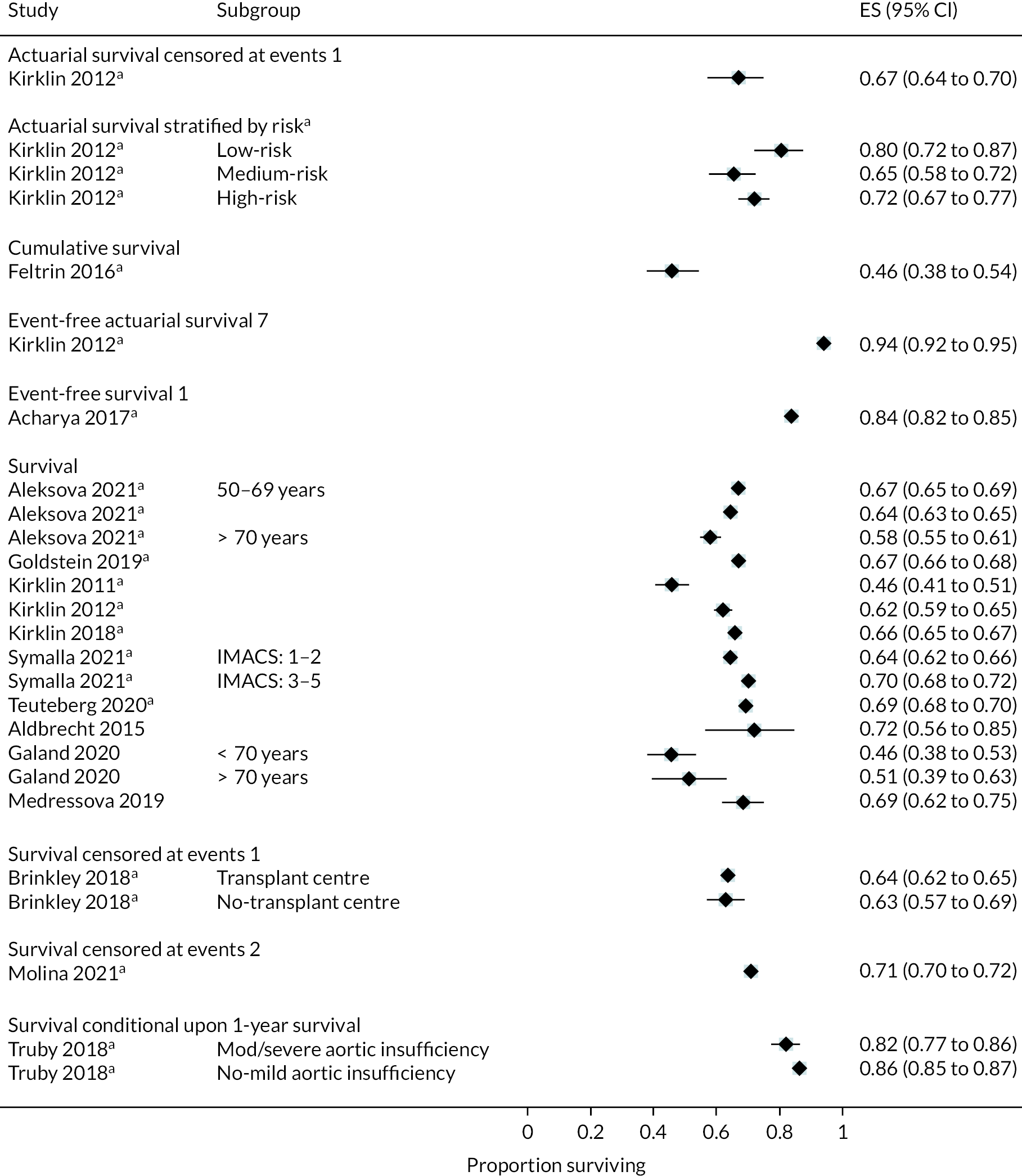
FIGURE 51.
Survival in studies with multiple devices at 36 months follow-up. a, Data from a registry report; survival censored at events 2, censored at transplant or cessation of support.
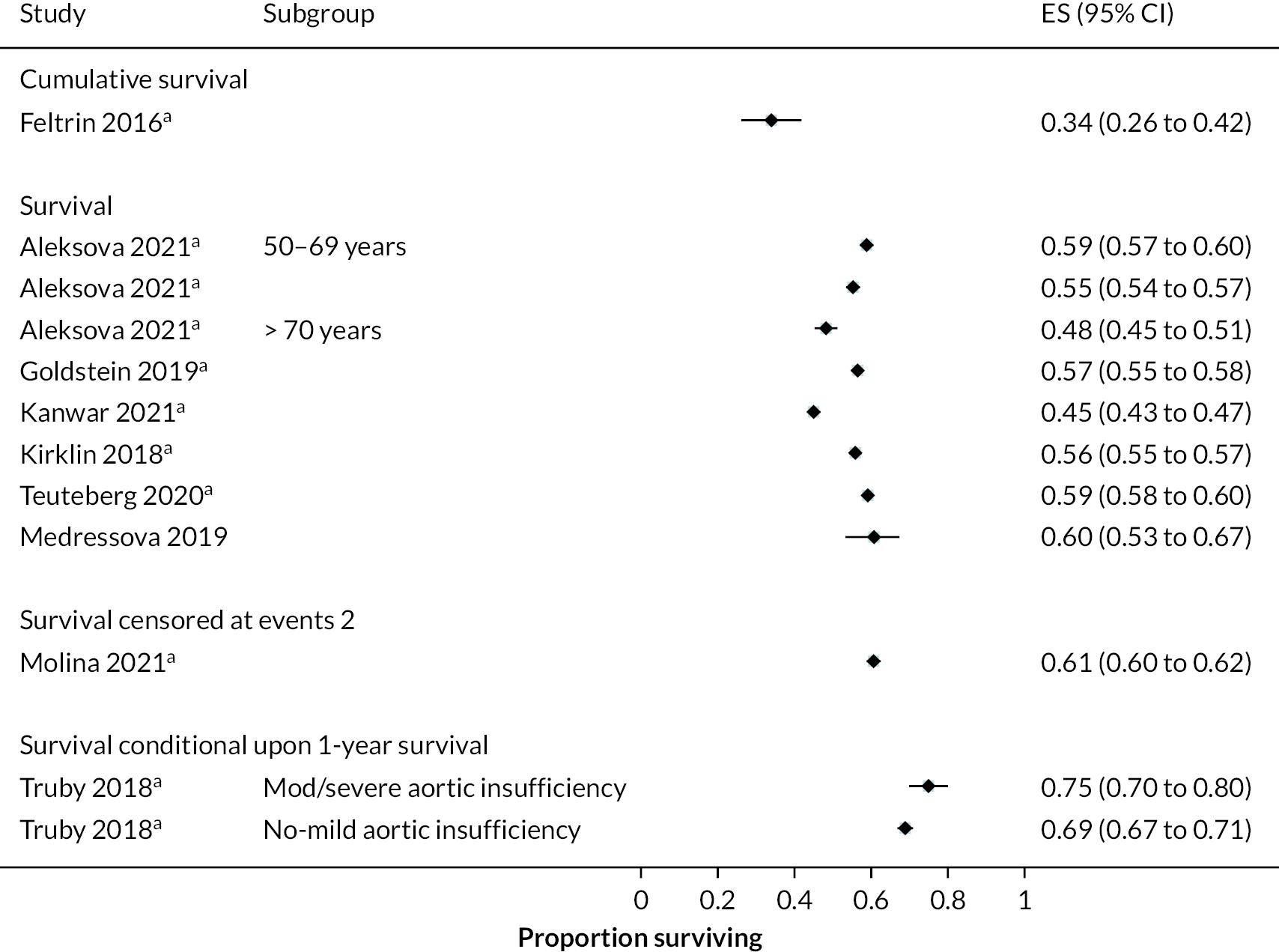
FIGURE 52.
Survival in studies with multiple devices at 48 months follow-up. a, Data from a registry report; survival censored at events 2, censored at transplant or cessation of support.
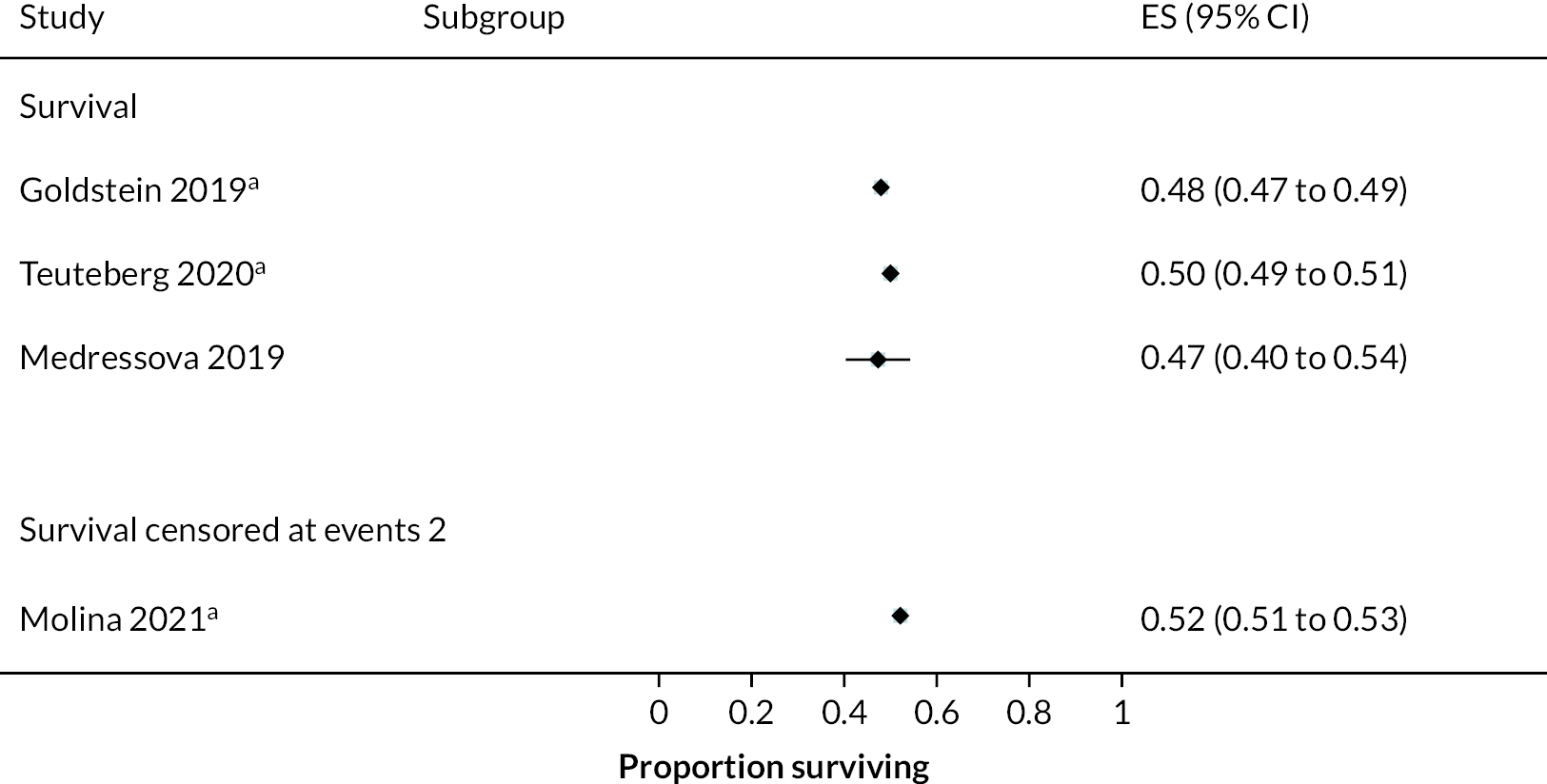
FIGURE 53.
Survival in studies with multiple devices at 60 months follow-up. a, Data from a registry report; survival censored at events 2, censored at transplant or cessation of support.

Quality of life
FIGURE 54.
Mean QoL scores from different QoL tools in the HeartMate XVE at all reported time points.

FIGURE 55.
Mean change from baseline in QoL score in the HeartWare HVAD at 12 months.

FIGURE 56.
Mean QoL scores from different QoL tools in the HeartMate II at all reported time points.
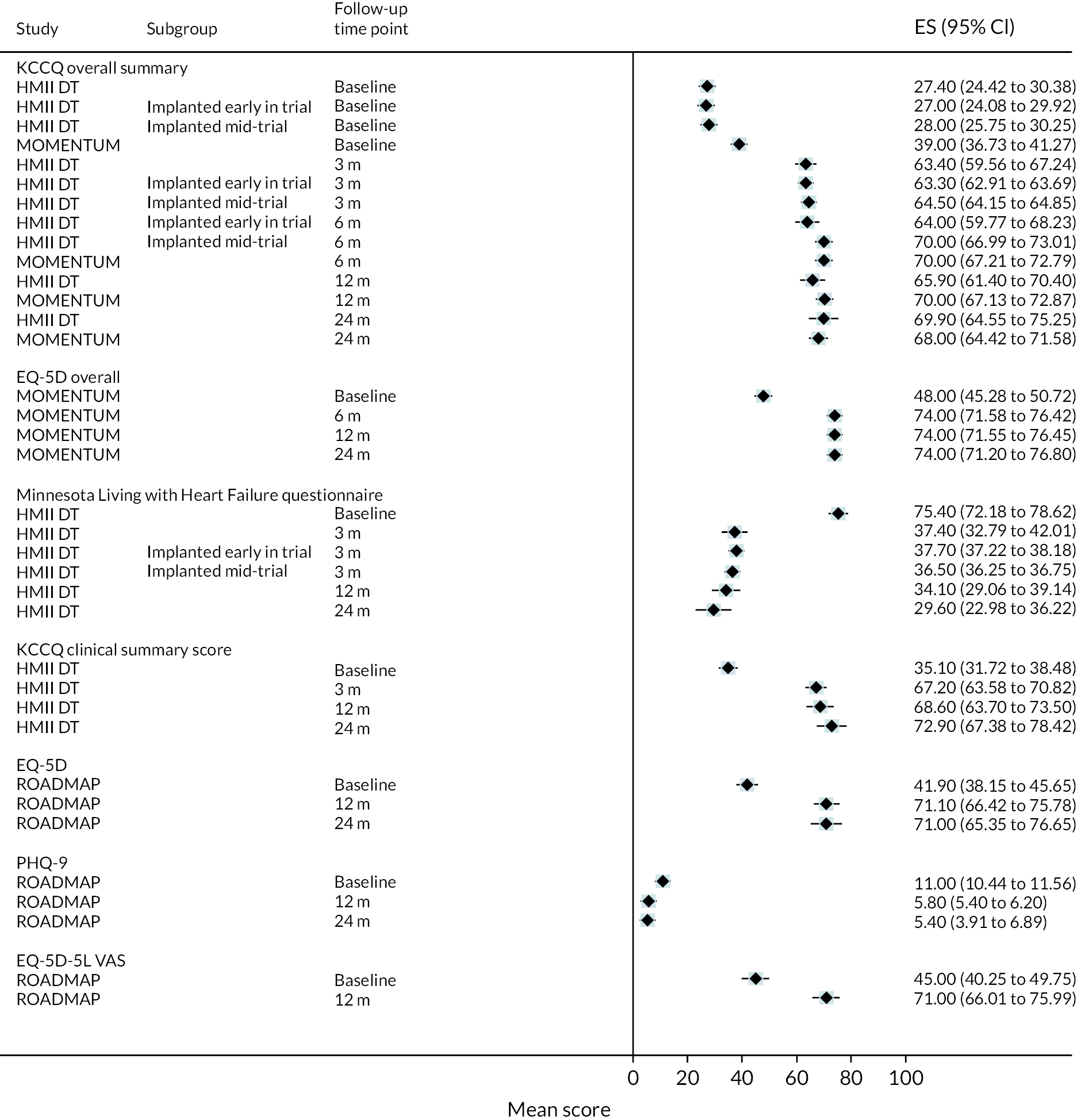
FIGURE 57.
Mean change from baseline in QoL score in the HeartMate II at all reported time points.
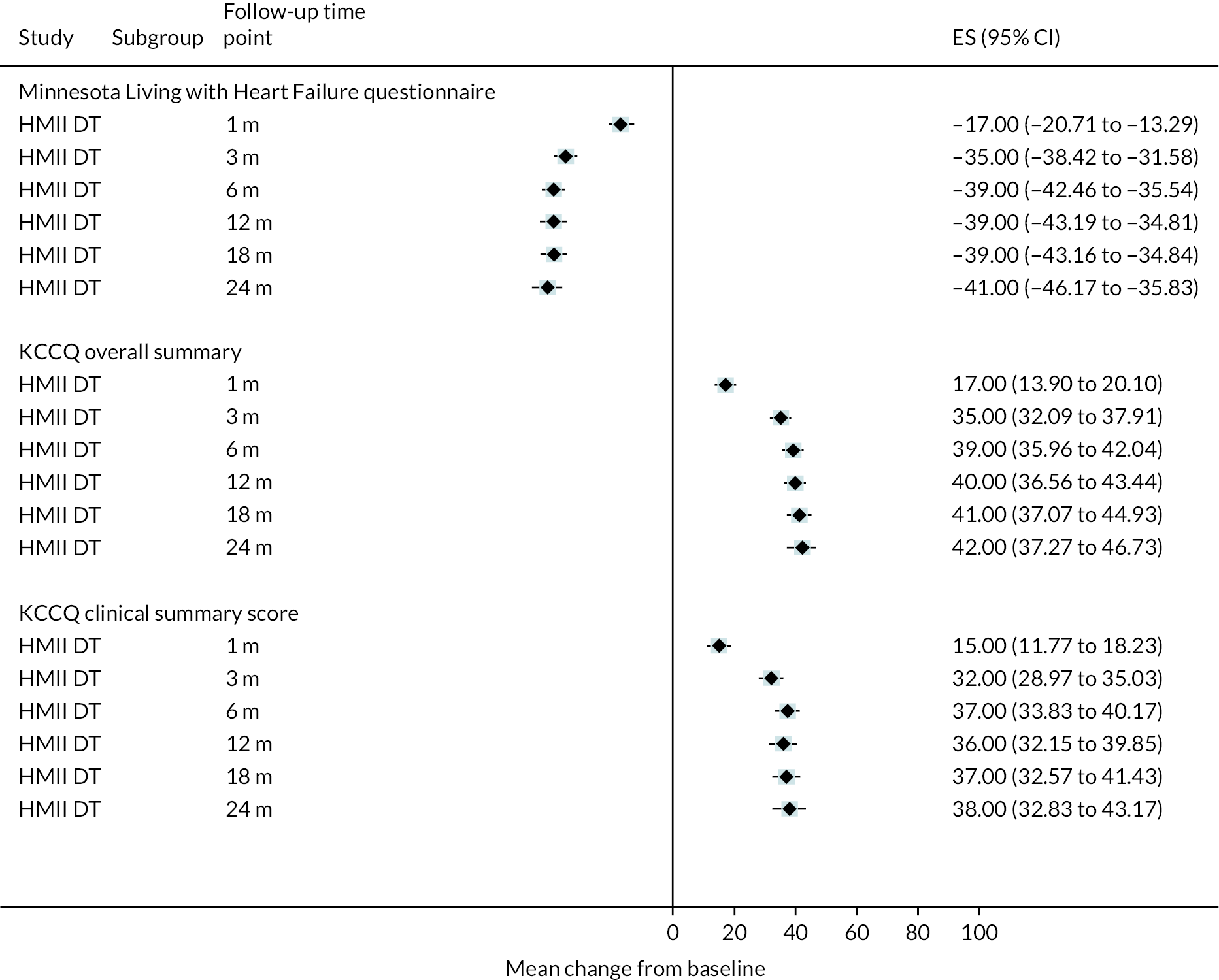
FIGURE 58.
Mean QoL scores from different QoL tools in studies with multiple devices at all reported time points. *Report from The National Heart, Lung, and Blood Institute Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).

Hospitalisations
FIGURE 59.
Hospitalisation rate per person-years with the HeartMate XVE at all reported time points.
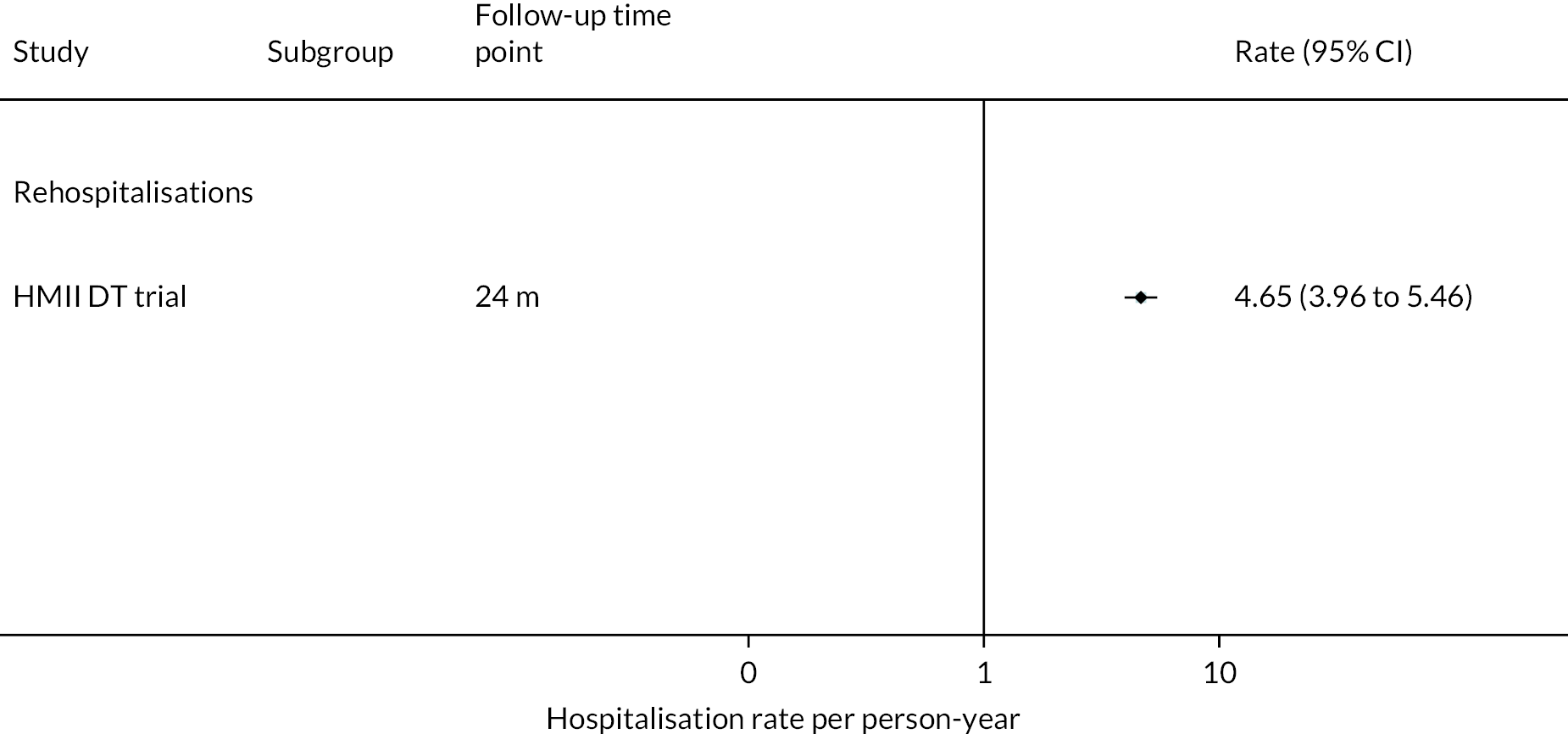
FIGURE 60.
Proportion of people hospitalised with the HeartMate XVE at all reported time points.

FIGURE 61.
Hospitalisation rate per person-years with the HeartWare HVAD at all reported time points.

FIGURE 62.
Mean duration of hospital stay in days with the HeartWare HVAD at all reported time points.

FIGURE 63.
Hospitalisation rate per person-years with the HeartMate II at all reported time points.
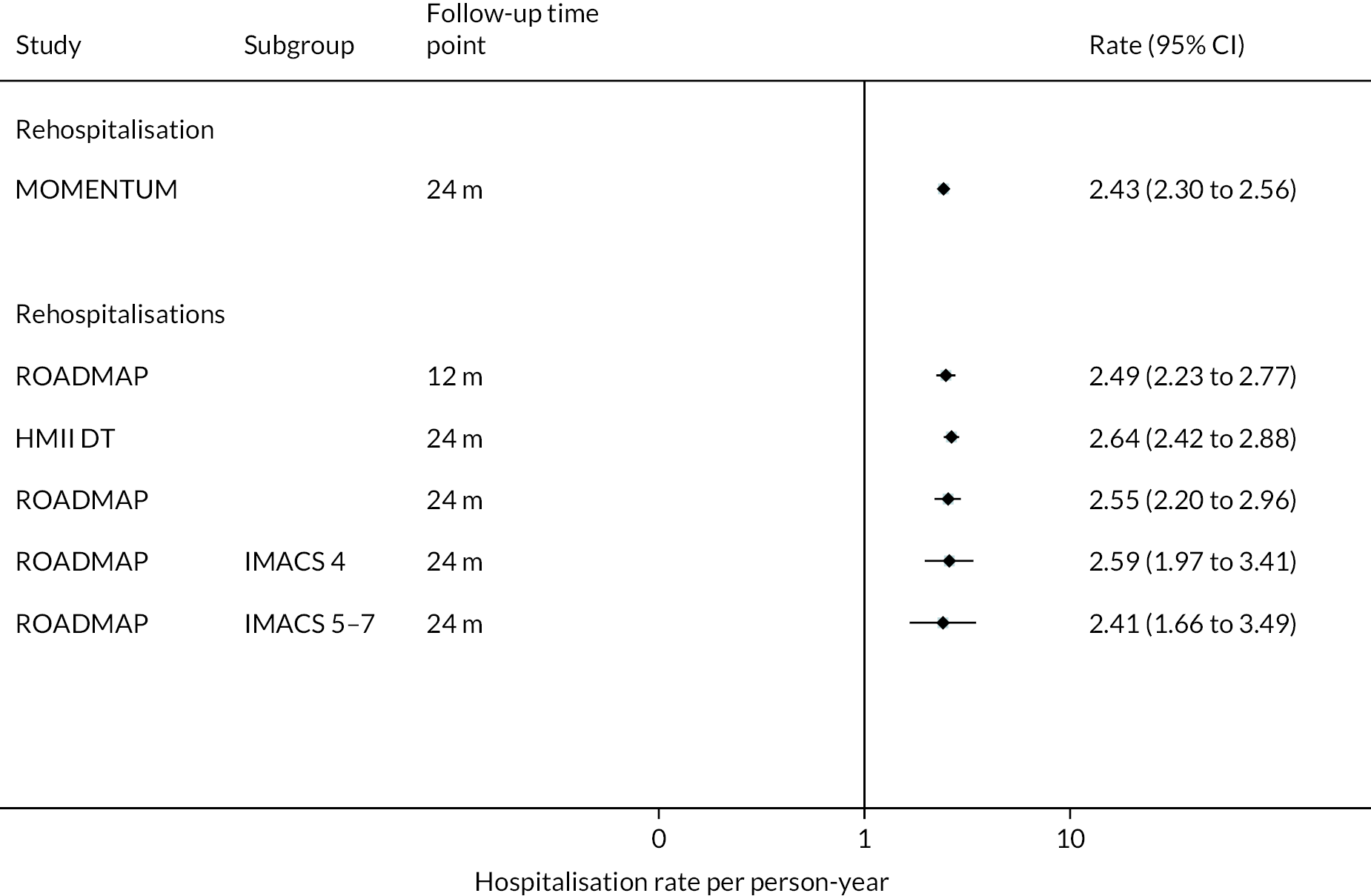
FIGURE 64.
Duration of initial hospital stay in days with the HeartMate II.
FIGURE 65.
Proportion of people hospitalised with the HeartMate II at all reported time points.

FIGURE 66.
Hospitalisation rate per person-years in studies with multiple devices at all reported time points. a, Data are from a registry report.

FIGURE 67.
Proportion of people hospitalised in studies with multiple devices at all reported time points. a, Data are from a registry report.

Major events
FIGURE 68.
Event rate for major events per 100 person-years with the HeartMate XVE at all reported time points.

FIGURE 69.
Proportion of people with a major event with the HeartMate XVE at all reported time points.

FIGURE 70.
Major event rate per 100 person-years with the HeartWare HVAD at all reported time points.
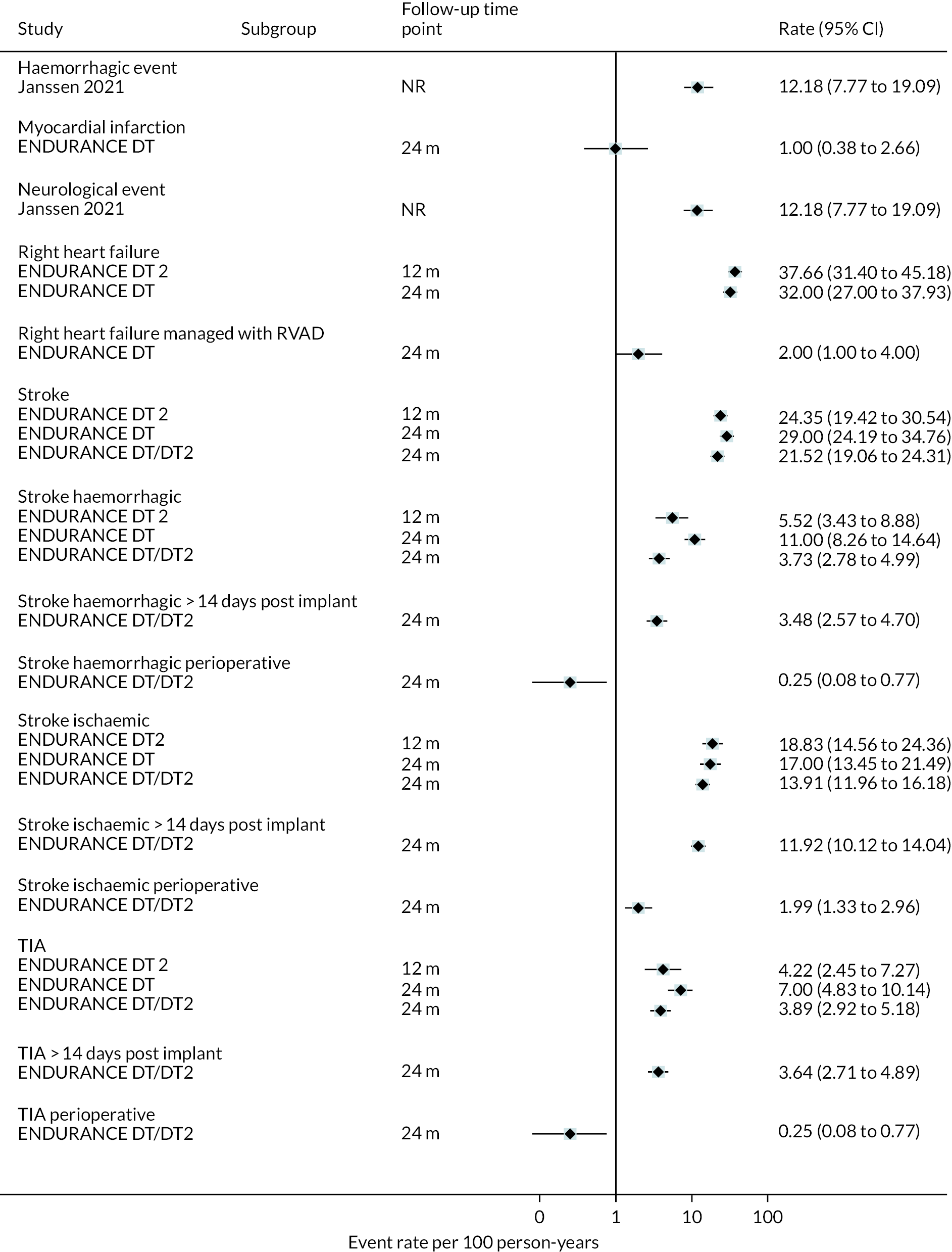
FIGURE 71.
Mean number of major events with the HeartWare HVAD at all reported time points.

FIGURE 72.
Proportion of people with events with the HeartWare HVAD at all reported time points.

FIGURE 73.
A and B major event rate per 100 person-years with the HeartMate II at all reported time points.


FIGURE 74.
A and B proportion of patients with major events with the HeartMate II at all reported time points.
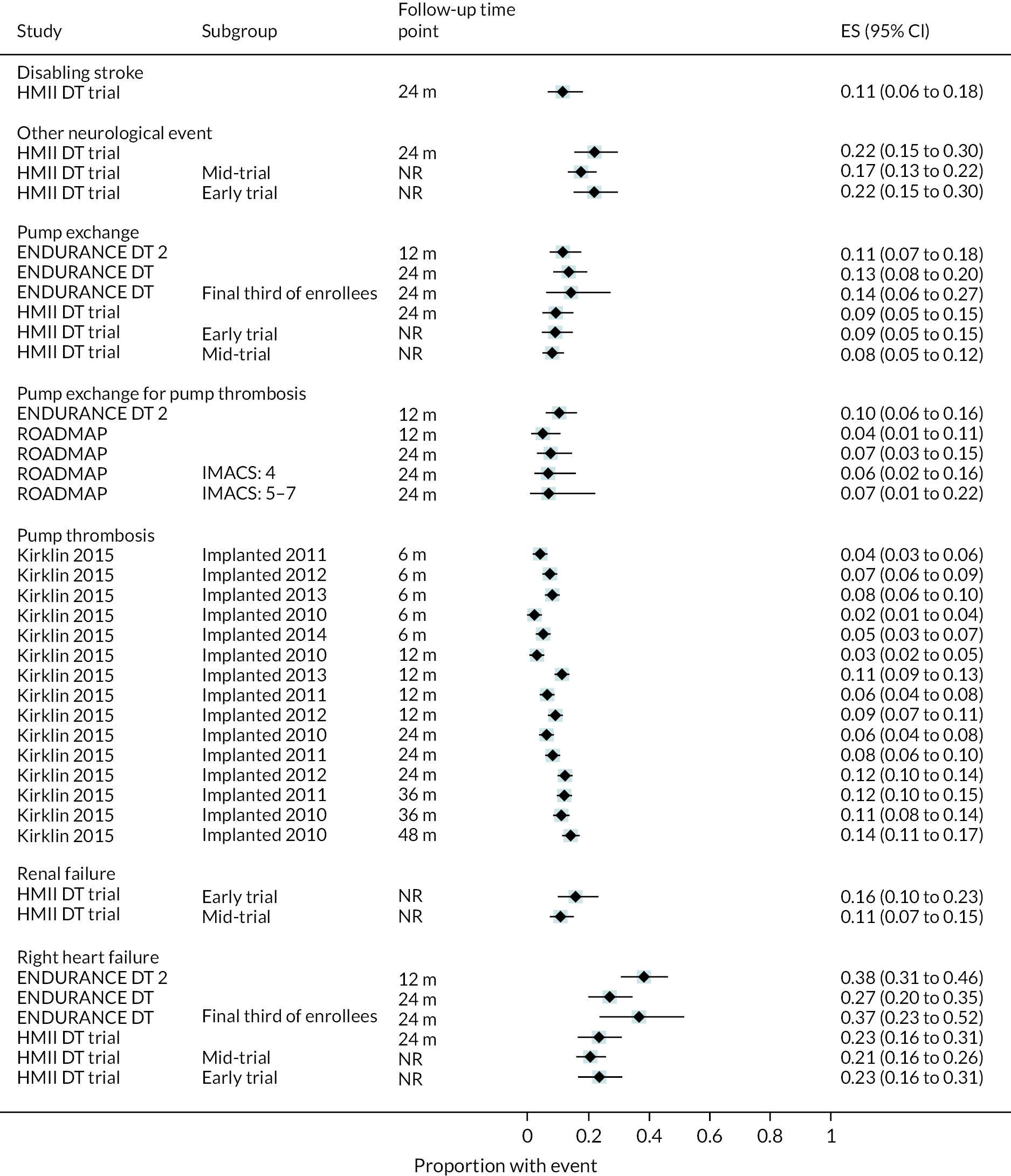

FIGURE 75.
Major event rate per 100 person-years in studies with multiple devices at all reported time points. a, Data are from a registry report.
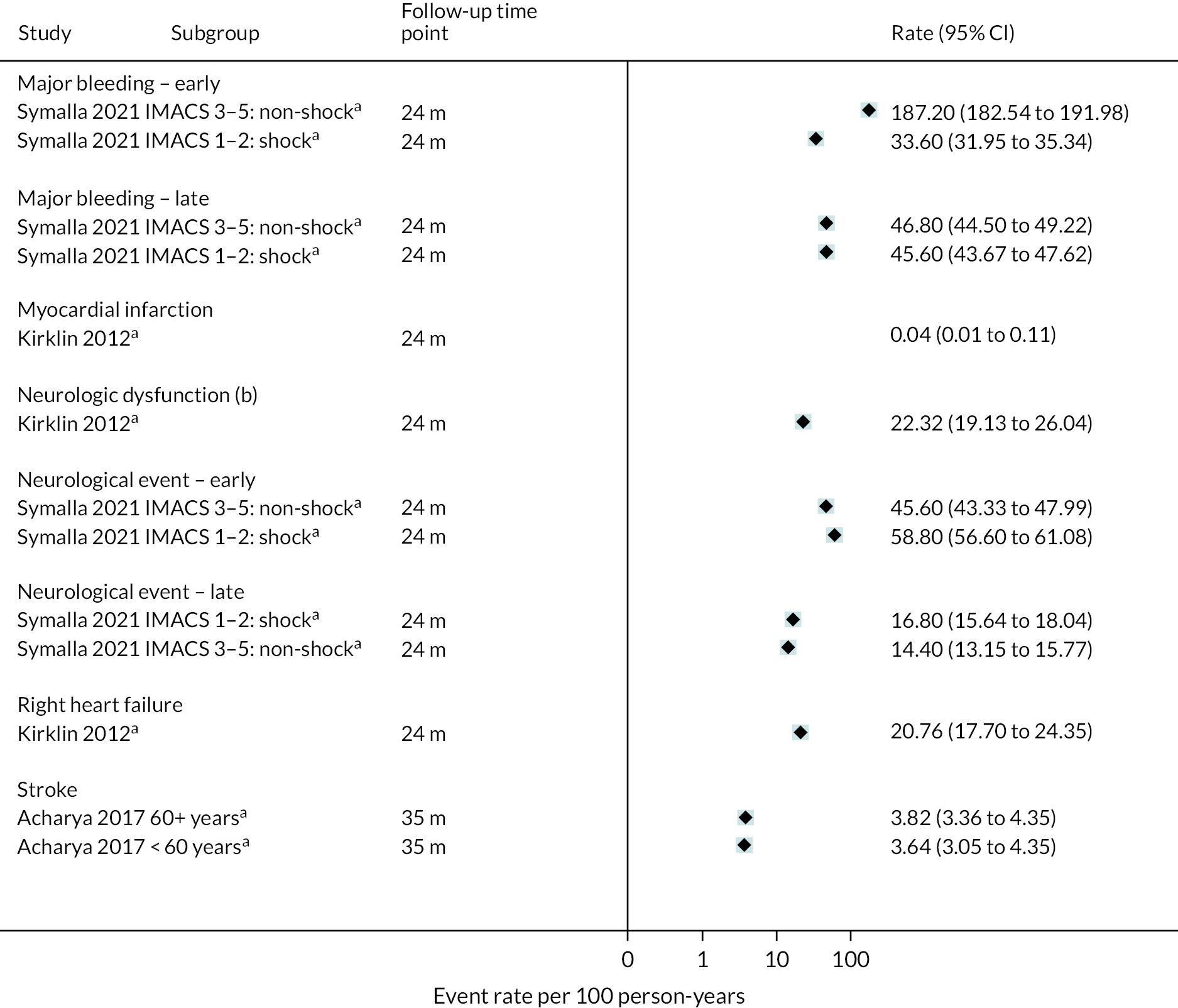
FIGURE 76.
Proportion of people with major events in studies with multiple devices at all reported time points. a, Data are from a registry report.
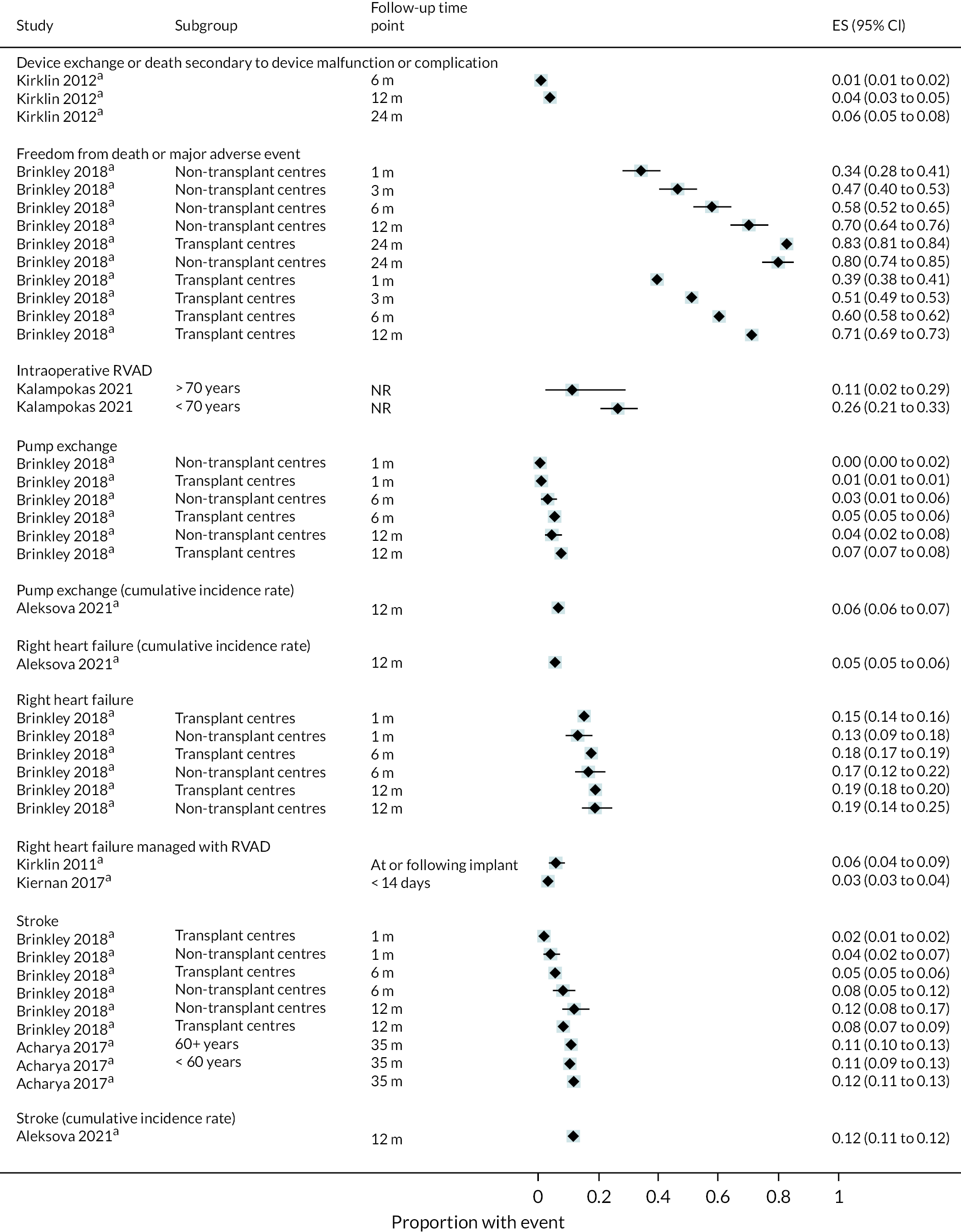
Complications
FIGURE 77.
A and B rate of complications per 100 person-years in the HeartMate XVE at all reported time points. a, Data are from a registry report.
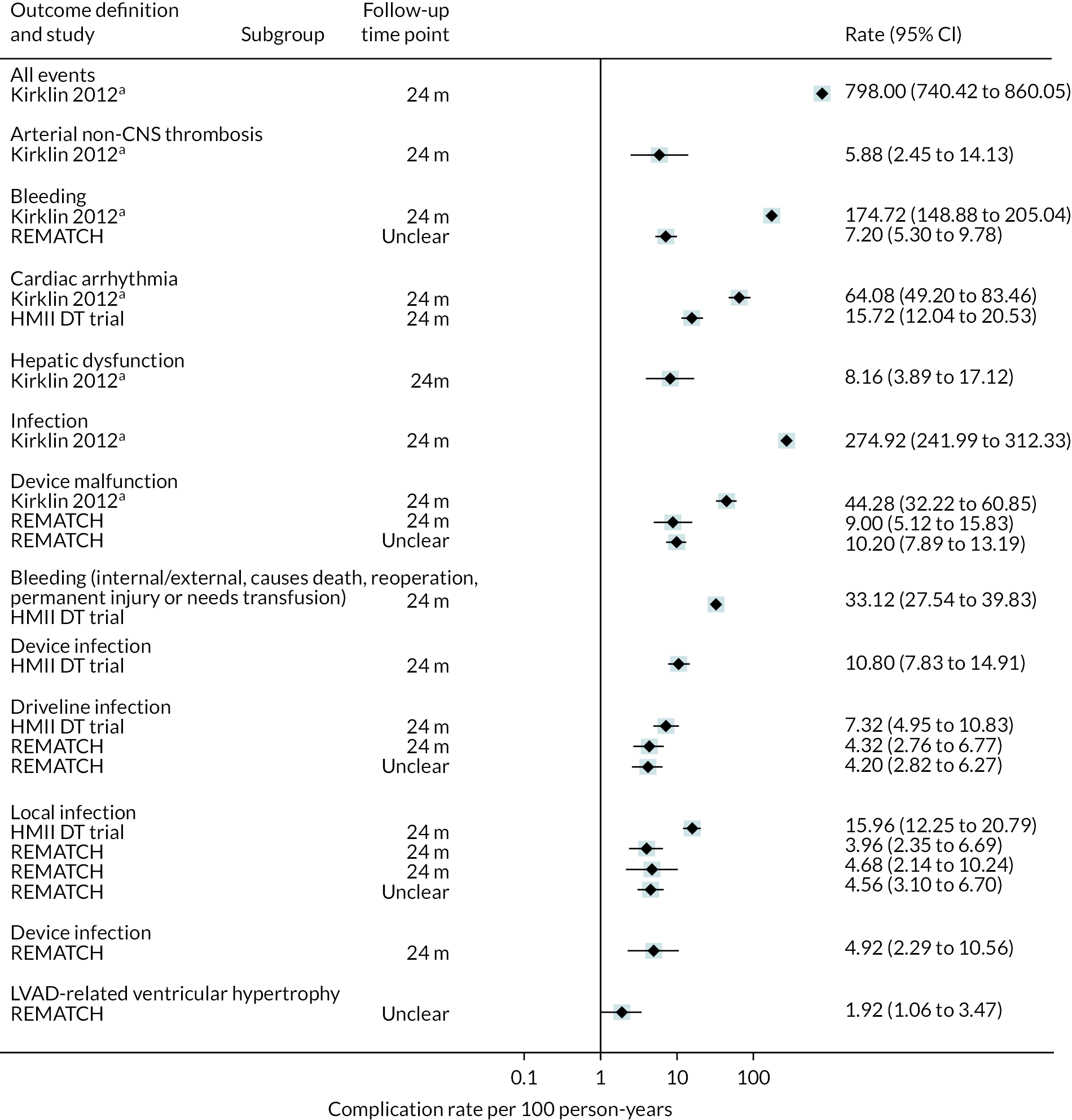

FIGURE 78.
Proportion of people with complications in the HeartMate XVE at all reported time points.

FIGURE 79.
Complication rate per 100 person-years in the HeartWare HVAD device at all reported time points.

FIGURE 80.
Proportion of people with complications in the HeartWare HVAD at all reported time points.
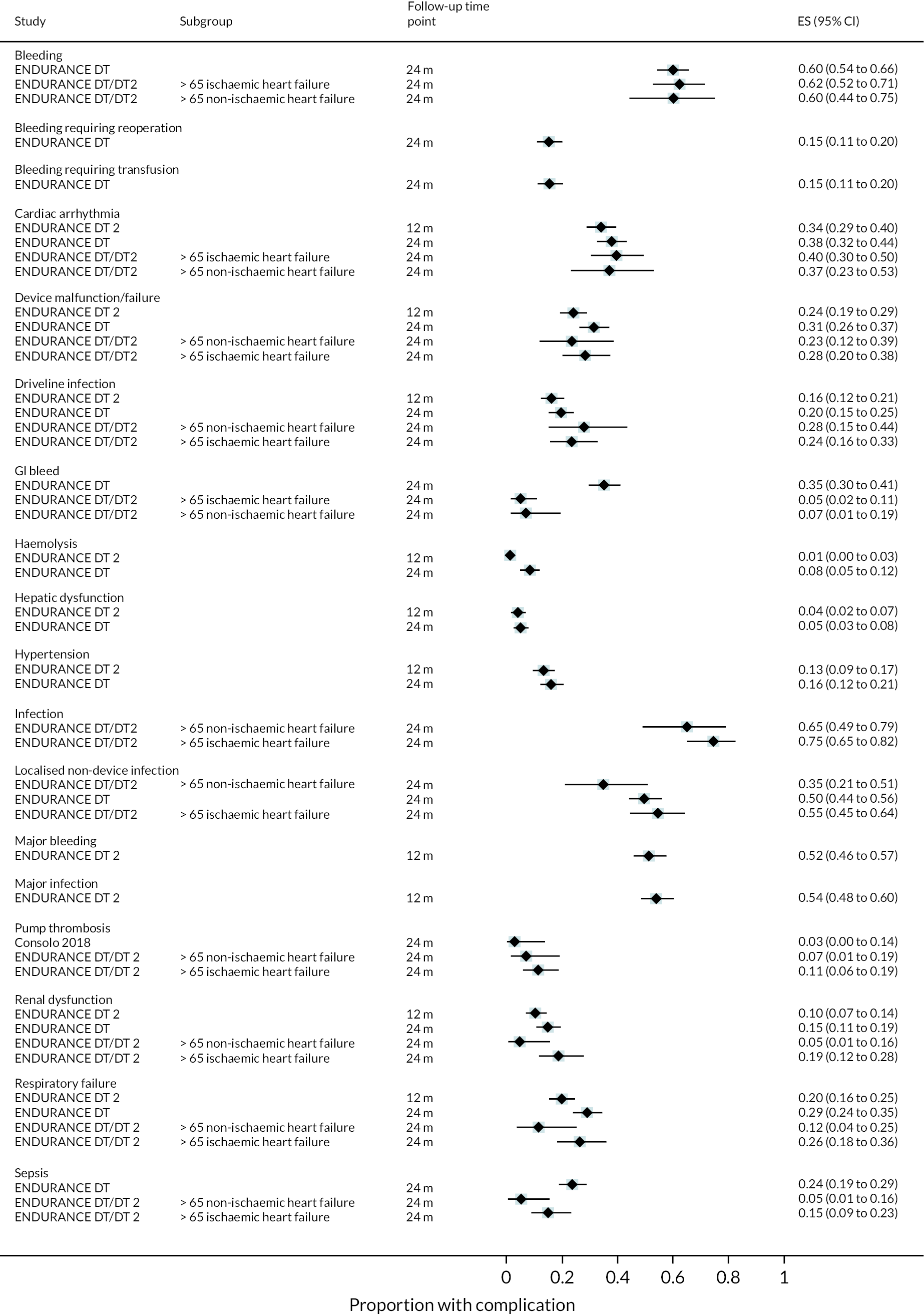
FIGURE 81.
A, B and C complication rate per 100 person-years in the HeartMate II at all reported time points.
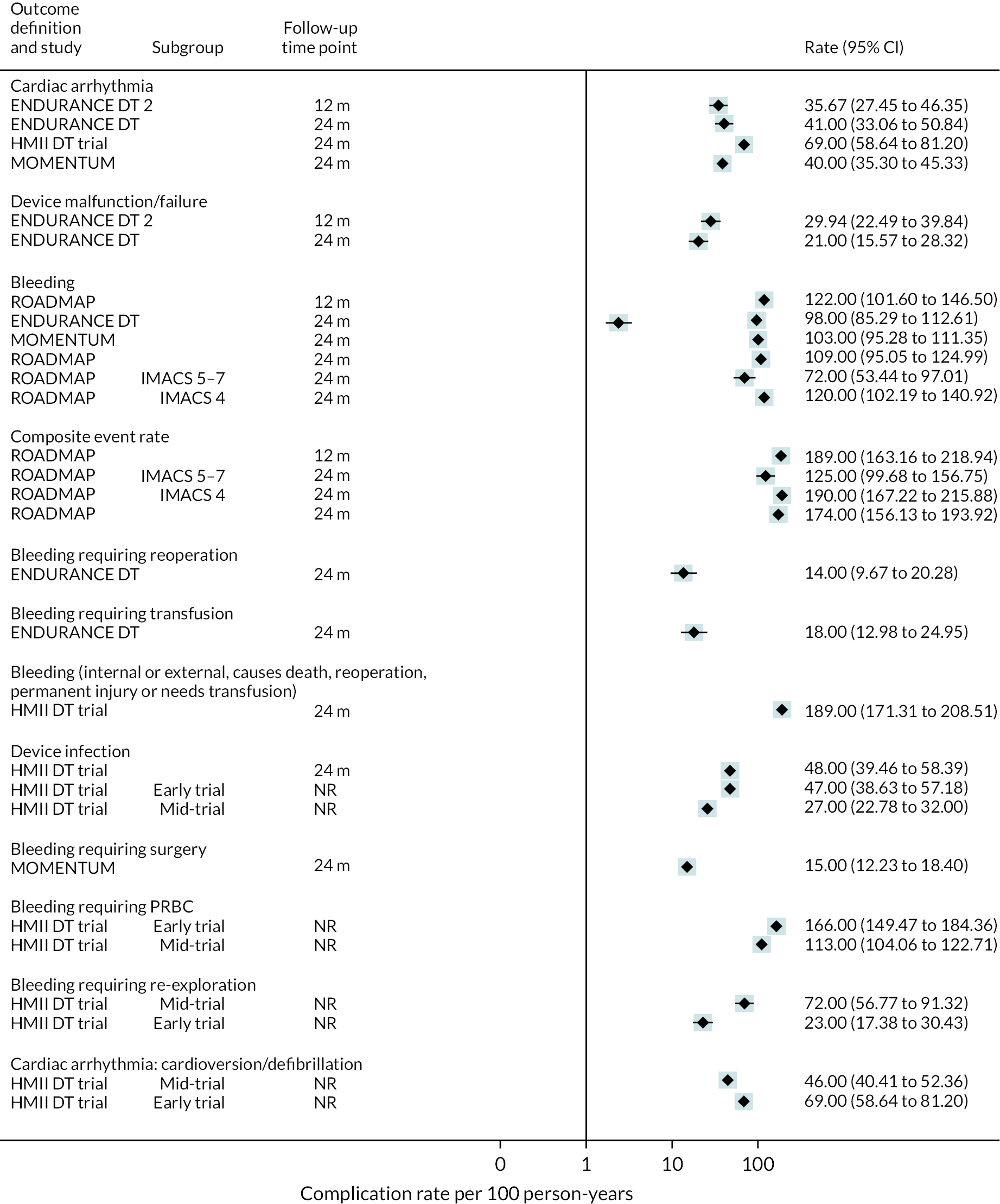
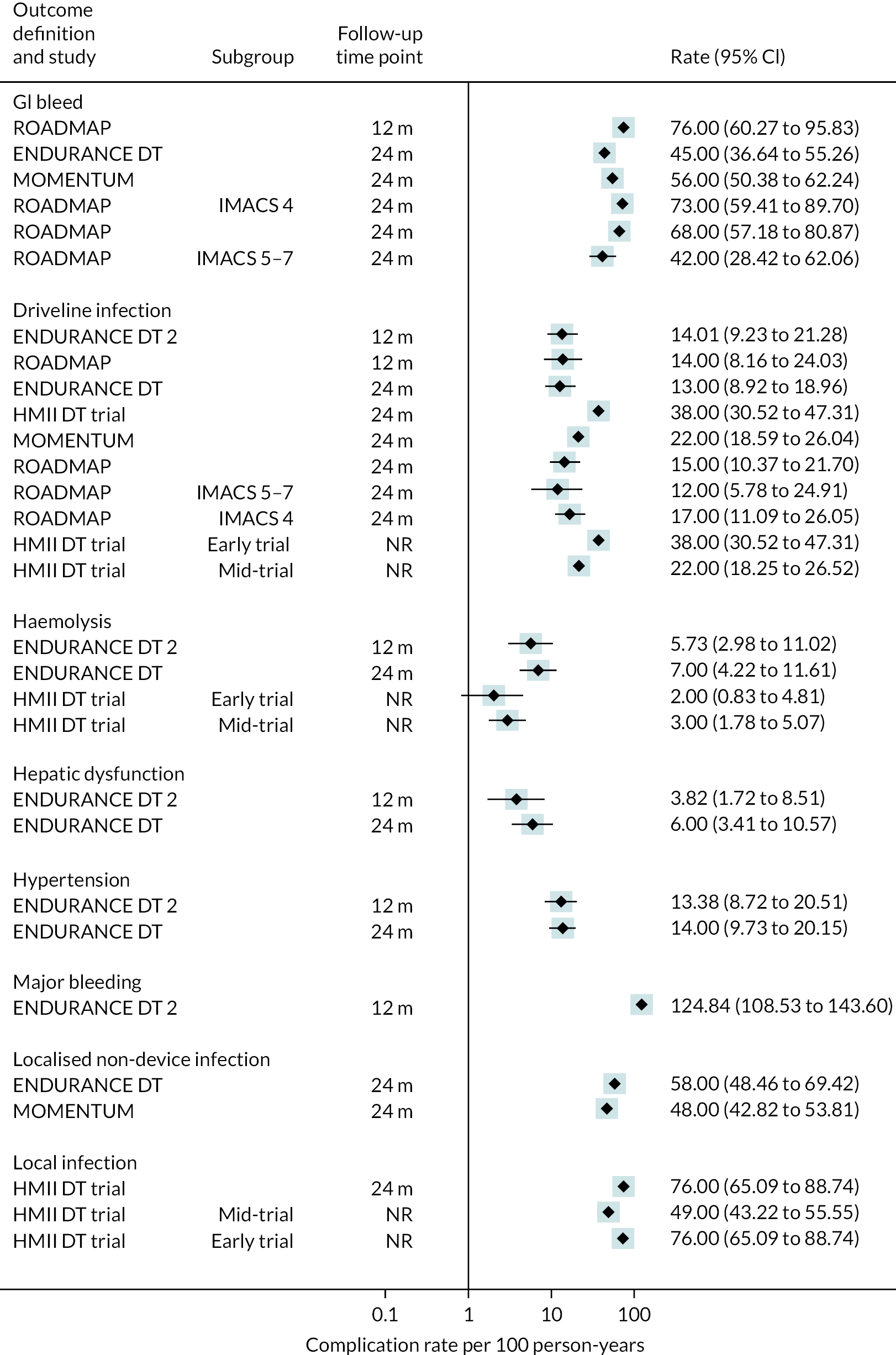
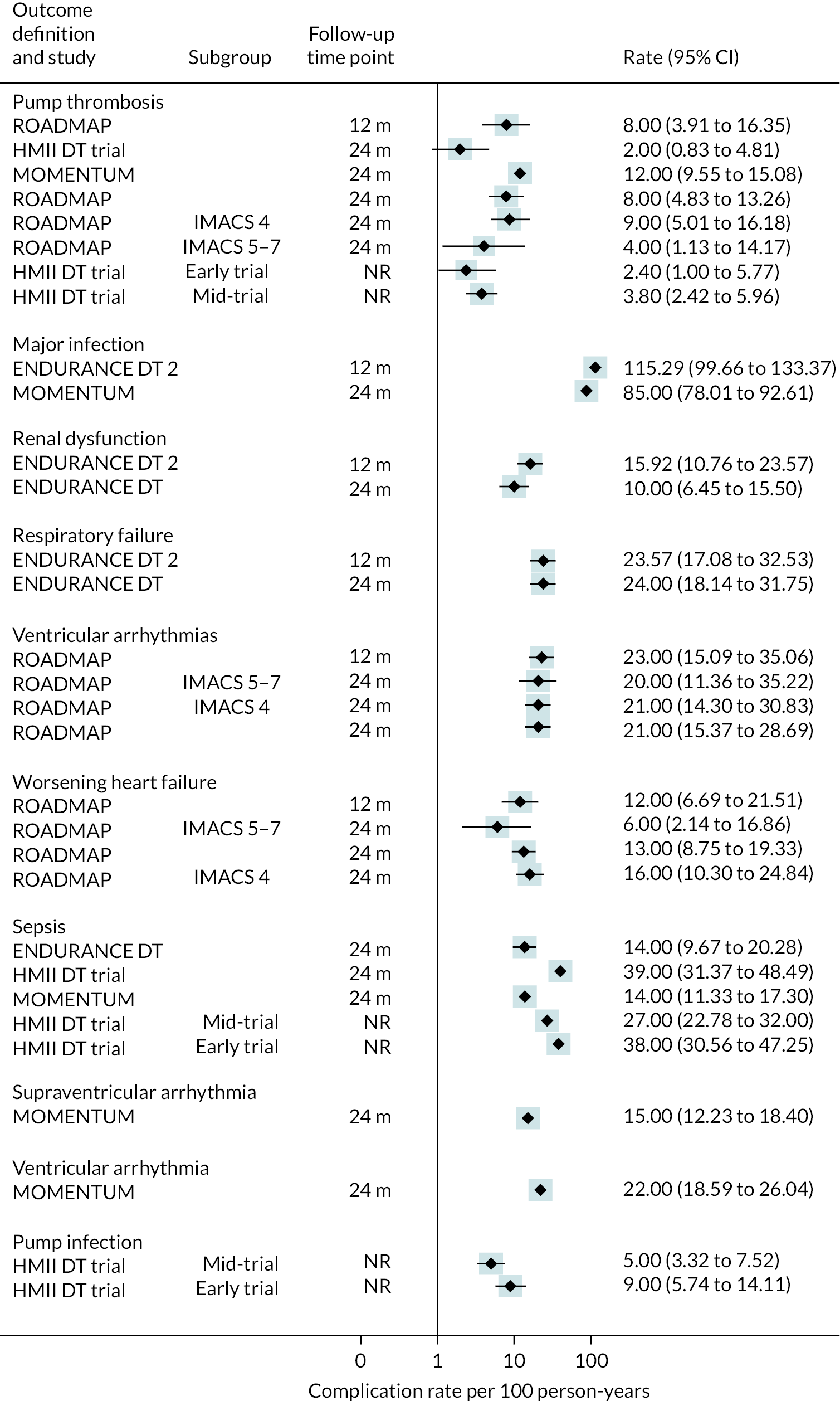
FIGURE 82.
A, B and C proportion of people with complications in the HeartMate II at all reported time points.
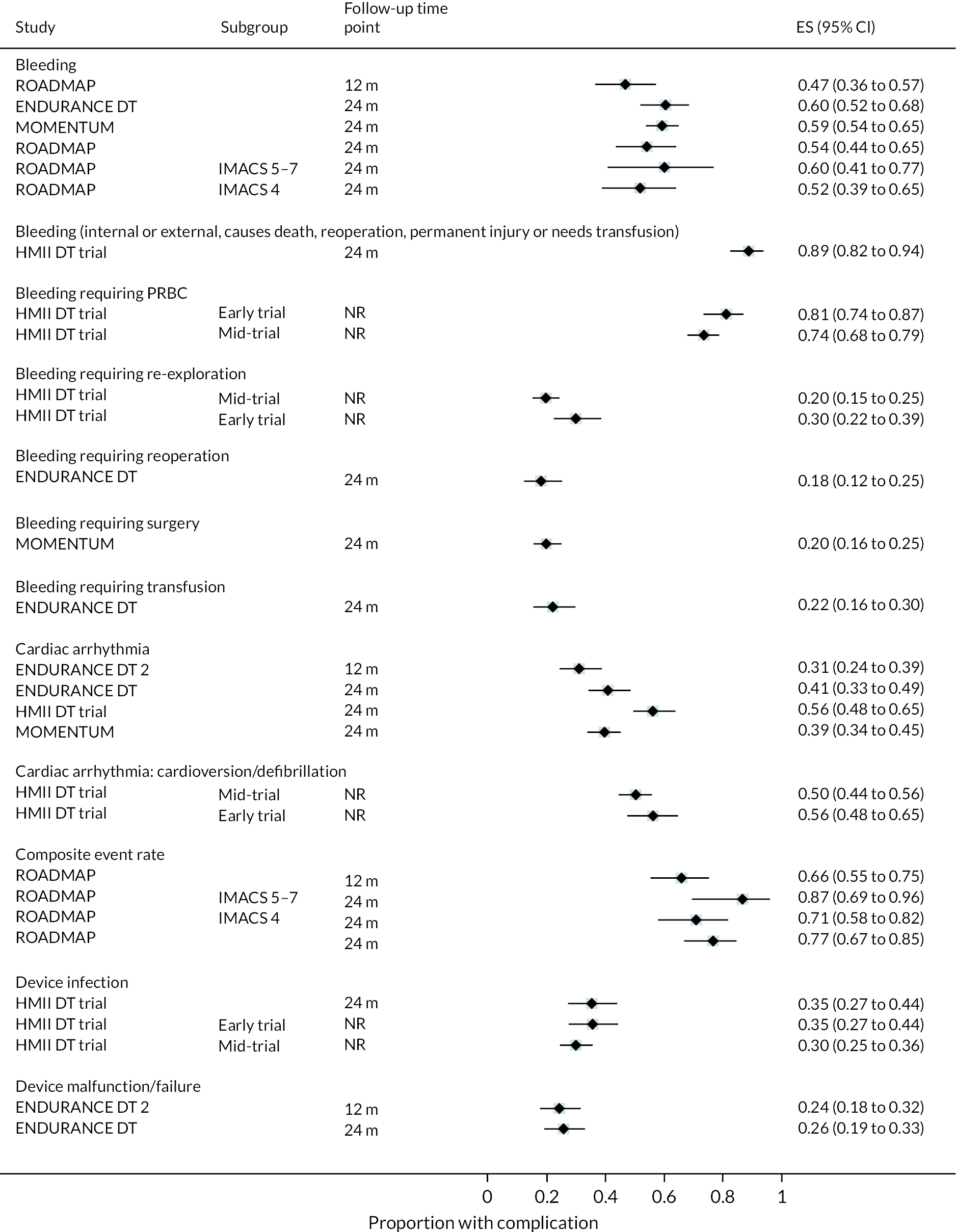
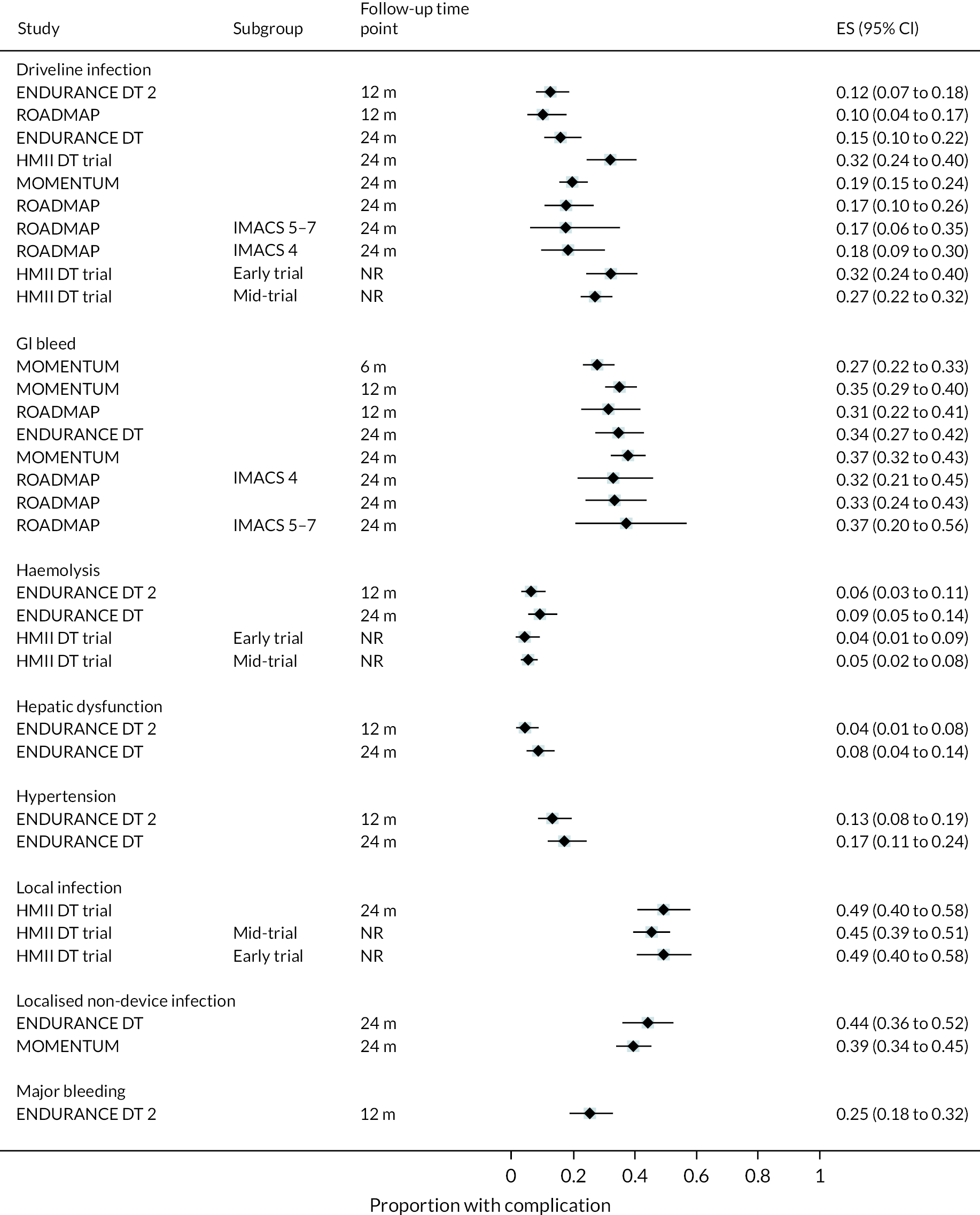

FIGURE 83.
Complication rate per 100 person-years in studies with multiple devices at all reported time points. a, Data are from a registry report.

FIGURE 84.
Proportion of people with complications in studies with multiple devices at all reported time points. a, Data are from a registry report.
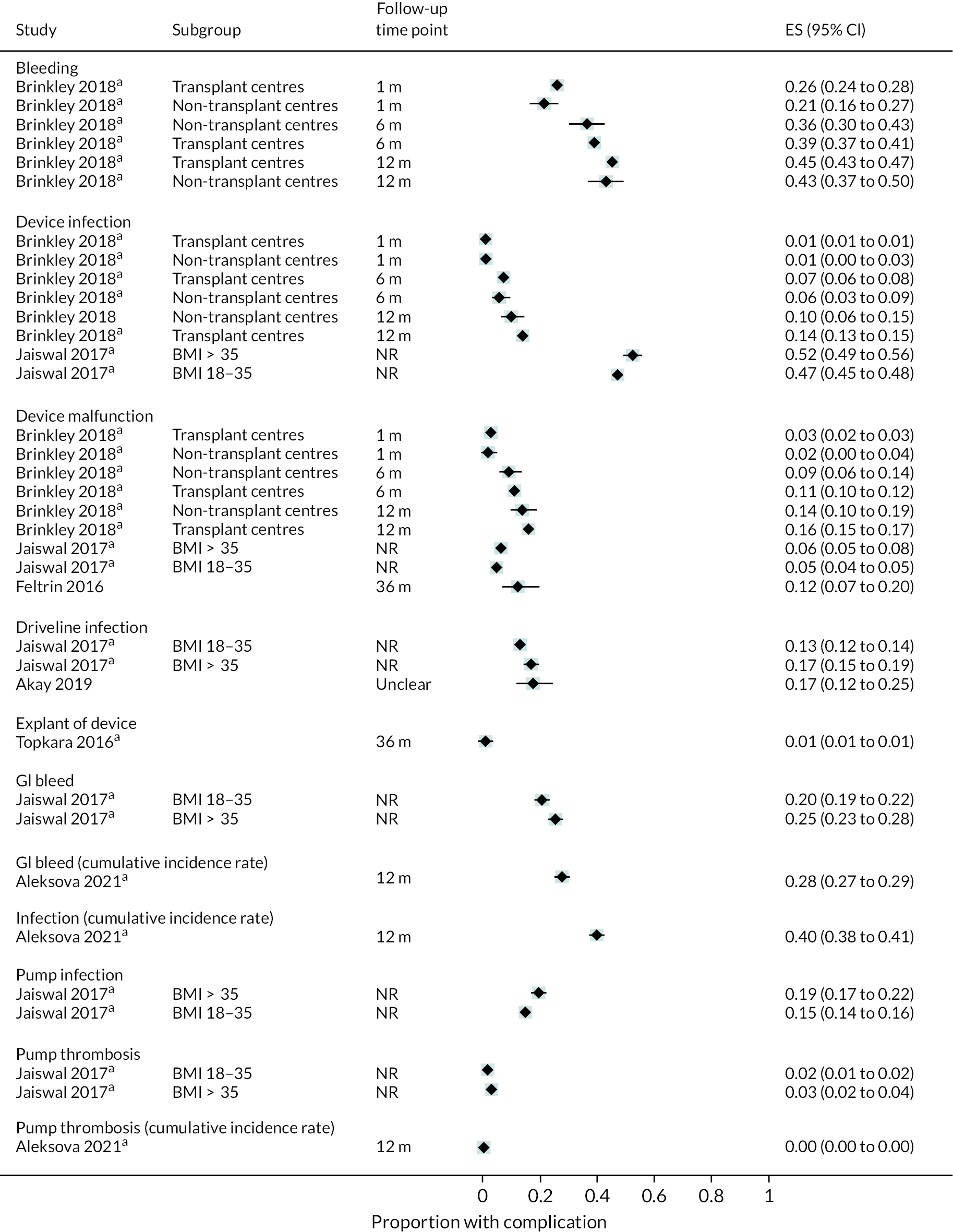
Appendix 6 Observational studies overlapping with INTERMACS
| Study ID | Centre | Implant years | Total no. patients (No. DT) | INTERMACS profiles (n, %) | Mean age (SD) | Male (n, %) | Device types (n, %) | Subgroups analysed | Outcomes reported | DT data reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Adamo 2015 | Barnes-Jewish Hospital in St Louis, Missouri | 2005–13 | 269 (86) | 1: 89 (33) 2: 146 (54) 3: 17 (6) 4 or more: 17 (6) |
55.9 (12.1) | 217 (81) | All HMII | Validation and derivation cohort of HMRS | Survival, validity of HMRS for predicting mortality, morbidity (stroke, thrombosis, GI bleed, etc.) | Survival |
| Aggarwal 2012 | Advocate Christ Medical Centre, Illinois | 2005–11 | 101 (94) | NR | GI bleed absent 61.17 (12.63) GI bleed present 64.96 (9.9) |
81 (80) | All HMII | GI bleed present or absent | GI bleed, location of GI bleed, survival | All patients are DT so for all outcomes |
| Aggarwal 2013 | Advocate Christ Medical Centre, Illinois | 2005–11 | 79 (69) | NR | Aortic insufficiency 67.67 (8.45) No aortic insufficiency 58.46 (13.2) |
67 (85) | All HMII | Aortic insufficiency present or absent | Survival, cause of death, hospitalisations predictors of aortic insufficiency | No. DT with aortic insufficiency |
| Anwer 2019 | Mayo Clinic College of Medicine, Rochester | 2007–15 | 278 (178 DT, 64) | INTERMACS 1–2 70 (39) overall | Median 62 (IQR 52.5–69) overall | 226 (81) | HMII 223 (84) HVAD 28 (10) Jarvik 2000 9 (3) VentrAssist 6 (2) DuraHeart 2 (1) | Successful implant vs. ‘failure’ | Successful or failure implant, survival, re-admissions, adverse events | DT as predictor of failure, success rate in DT, survival |
| Asleh 2017 | Mayo Clinic College of Medicine, Rochester | 2007–16 | 341 (216) | Median 3 (IQR 2–4) | 62 (IQR 52–68.9) | 272 (80) | HMII 269 (78.8) HVAD 51 (15) HM3 4 (1.2) Jarvik 2000 9 (2.6) VentrAssist 6 (1.8) DuraHeart 2 (0.6) |
Diabetes vs. non-diabetes | All-cause mortality, LVAD-related complications (stroke, pump thrombosis, DI/PI) | Mortality, device-related infection, composite nonfatal events, composite nonfatal and fatal events |
| Assouline-Dayan 2018 (conference abstract) |
University of Iowa | 2010–15 | 104 (DT NR) | NR | Median 55.8 overall | 80 (76.9) | NR | N/A | GI bleed, survival | GI bleed |
| Auvil 2019 | Perelman School of Medicine, University of Pennsylvania, Philadelphia. | 2008–18 | 221 (122 DT, 55) | NR | Median 57 overall | 190 (85) | HMII and HVAD | No, mild and moderate aortic insufficiency | Mortality, RHF, functional exercise capacity | DT as a predictor of 2-year mortality, 6-minute walking distance and RHF |
| Bryce 2016 (conference abstract) |
Unclear | 2011–4 | 100 (69 DT 69) | NR | 55.6 (12.29) overall | 78 (78) | NR | N/A | Cognitive function, survival, rehospitalisation | Cognitive function |
| Bryce 2018 (conference abstract) |
Unclear | 2011–4 | 100 (69 DT 69) | NR | 55.6 (12.29) overall | 78 (78) | NR | N/A | Cognitive function, survival, rehospitalisation | Cognitive function |
| Cagliostro 2015 | Columbia University Medical Centre | 2009–13 | 266 (DT 89) | NR | Group A 58.71 (13.24) Group B 57.81 (13.78) |
214 (80) overall | HMII 238 (89) HVAD 28 (11) |
Implanted before June 1, 2011 (Group A) Implanted after that time point (Group B) |
DI rates, freedom from infection, re-admissions | Number of DI events |
| Corral 2020 | Mayo Clinic and National Inpatient Sample (hospitalisations) | 2012–8 | 1344 (DT 407, 30.3) 55 LVAD patients from the general National inpatient sample |
NR | DT patients 63.1 (0.7) | DT patients 320 (78.6) | NR | HF controls, DT, LVAD then heart transplant, heart transplant and inpatients from NIS | Acute pancreatitis, all-cause mortality | Incidence of acute pancreatitis |
| Corral 2020 (conference abstract) | Mayo Clinic | 2012–8 | 1344 (407 DT) | NR | 60.9 (14.3) overall | 987 (73.4) overall | NR | LVAD DT, transplanted or LVAD BTT and controls with no therapy | Deaths, incidence of acute pancreatitis, predictors of acute pancreatitis | LVAD DT as predictor of acute pancreatitis |
| Coyle 2010 | Advocate Christ Medical Centre, Illinois | 2004–7 | 58 (all DT) | NR | Normal BMI 66 (11) Obese 55 (13) |
Normal BMI 33 (87) Obese 14 (70) |
HMII 36 (62) HeartMate XVE 22 (38) |
Normal BMI vs. obese | In-hospital mortality, survival, postoperative complications | All patients are DT so for all outcomes |
| Critsinelis 2018 | Texas Heart Institute/Baylor College of Medicine | 2003–16 | 526 (243 DT, 46) | INTERMACS 1 75 (14) 2 173 (33) 3 200 (38) 4 55 (21) 5 14 (3) 6 2 (0.5) 7 7 (1.5) |
NR for whole cohort | 411 (78) | HMII 403 (77) HVAD 124 (23) |
Normal, moderate or severe hypoalbuminemia | Survival, re-admission, neurological dysfunction, GI bleed, RHF | DT as predictor of mortality |
| Daneshmand 2010 | Duke University Medical Center | 2000–8 | 60 DT LVAD 93 heart transplant |
NR | DT median 60 (IQR 52–69) | DT 47 (78) | ‘Most’ were HeartMate XVE | No- DT LVAD vs. extended criteria-alternate list heart transplant | Survival, post-op length of stay, post-op wound infections, driveline/PI, renal insufficiency | All outcomes |
| Daneshmand 2015 | Duke University Medical Center | 2005–12 | 146 DT LVAD 62 heart transplant |
DT: not determined – 1 (0.1) 1–13 (9) 2–67 (46) 3–39 (27) 4–18 (12) 5–8 (6) |
DT: median 67 (IQR 59–73) | DT: 108 (74) | All HMII | No. DT LVAD vs. extended criteria-alternate list heart transplant | Overall survival, eGFR, index hospitalisations length of stay and mortality, stroke, stroke-free survival, re-admission rate per year of support | All outcomes |
| Dunlay 2014 | Mayo Clinic College of Medicine, Rochester | 2007–12 | 99 (all DT) | Median 4 (IQR 3–5) | 65.1 (9.4) | 81 (81.8) | HMII 94 (95) HVAD 5 (5) | By frailty index | Mortality, cause of death, rehospitalisations | All patients are DT so for all outcomes |
| Dunlay 2016 | Mayo Clinic College of Medicine, Rochester | 2007–14 | 89 (all DT) | NR | 64.5 (10.7) | 71 (80.7) | HMII 84 (94.4) HVAD 3 (3.4) HeartMate XVE 2 (2.2) | N/A | Patients who died on LVADs Cause of death, clinical course before death |
All patients are DT so for all outcomes |
| Fukuhura 2016 | Columbia University | 2004–14 | 340 (89 DT, 26.2) | 1–2 253 (74.4) 3–4 87 (25.6) overall |
56.6 (13.9) overall | 274 (80.6) overall | HeartMate II 281 (82.6) HeartWare 36 (10.6) Ventrassist 9 (2.6) DuraHeart 8 (2.4) DeBakey VAD 6 (1.8) |
Those with aortic valve repair vs. those without | Survival, predictors of and freedom from aortic insufficiency, adverse events (bleeding, infection, device malfunction, pump thrombosis, RHF) | Freedom from aortic insufficiency |
| Grady 2018 (conference abstract) | 13 USA sites | 2015–8 | 71 DT | NR | 67.6 (4.5) | NR | NR | N/A | HRQoL | HRQoL |
| Grady 2019 (conference abstract) | 13 USA sites | 2015–8 | 137 DT | NR | 68.6 (5.1) | NR | NR | N/A | HRQoL | HRQoL |
| Grady 2020 (conference abstract) | 13 USA sites | 2015–8 | 154 DT | NR | 68.6 (5.2) | NR | NR | N/A | HRQoL | HRQoL |
| Grady 2021 | Multiple INTERMACS sites | 2008–13 | 1620 (862 DT) | INTERMACS 1 69 (8), 2 293 (34), 3 302 (35), 4–7 198 (23) | ≥ 50,750 (82) | 707 (82) | NR | DT is long-term group ineligible for transplant, also analyse short-term and uncertain groups | HRQoL, factors associated with HRQoL | All outcomes |
| Grady 2021 (conference abstract) | 13 USA sites | 2015–8 | 154 DT | NR | 68.6 (5.2) | NR | NR | N/A | HRQoL | HRQoL |
| Han 2016 (conference abstract) |
Columbia University | 2004–13 | 341 (85 DT, 25) | NR | 56 (14) Overall | 283 (83) | NR | N/A | Re-admissions and predictors of re-admissions | Rate of admission compared to BTT |
| Hernandez 2015 | Texas Heart Institute | 2008–12 | 148 (83 DT, 56.1) | INTERMACS 1 or 2 50 (60.2) | DT 54.2 (12.8) | DT 69 (83.1) | All HMII | DT vs. BTT | Hospital re-admissions (planned or unplanned), reasons for re-admissions, predictors of re-admissions, survival | Re-admissions, unplanned re-admissions compared to BTT |
| Jedeon 2019 (conference abstract) |
University of Minnesota Heart Institute | 2010–8 | 341 (153 DT, 45) | ≤ 2 in 65.5 patients overall | 58 (14) overall | 278 (81.5) | NR | Early vs. late ventricular arrhythmias | Ventricular arrhythmias and association with mortality | Early ventricular arrhythmias in DT associated with mortality |
| Jedeon 2021 | University of Minnesota Medical School | 2010–8 | 344 (155 DT) | INTERMACS ≤ 2 100 (64.9) | 64.4 (12.9) | 125 (80.6) | HeartMate II 110 (71.9) HeartMate III 25 (16.3) HeartWare HVAD 18 (11.8) |
DT/BTT, early ventricular arrythmia vs. no early ventricular arrythmia | Predictors of ventricular arrythmias, mortality | All outcomes |
| John 2016 | University of Minnesota | 2005–14 | 267 (DT 58, 21.1) | Mean score 3.8 (1.6) overall | 57.2 (14.2) overall | 214 (81.4) overall | All HMII | Compared by time period of implantation | Survival, serious complications (including GIB, pump thrombus, haemolysis, neurological dysfunction, DI) | Survival, DT as predictor of haemolysis, stroke, GIB, DI, PE |
| Katz 2015 | 27 open heart centers that contribute to INTERMACS | 2009–12 | 276 (DT 176, 64) | DT: 1 17 (10), 2 52 (30), 3 72 (41), 4–7 35 (20) | DT ≤ 59 49 (28), 60–69 65 (37), ≥ 70 62 (35) | DT 145 (82) | All HMII | DT vs. BTT, INTERMACS score (survival only) | Survival, operative mortality, major adverse events, length of stay in hospital, rehospitalisations, physical status, QoL | Survival (and by INTERMACS score), adverse events, length of hospital stay, rehospitalisations, QoL |
| Kilic 2018 | University of Pittsburgh Medical Center | 2006–15 | 238 (DT 142, 60%) | DT 1 7 (5), 2 52 (37), 3 41 (29), 4 19 (14), 5 1 (1) |
DT normal glomerular filtration rate: 57 (16) Reduced glomerular filtration rate: 64 (1) |
DT 118 (85) | HMII 187 (79) overall No details on other devices included |
Normal glomerular filtration rate and reduced glomerular filtration rate | Survival, major postoperative complications (e.g. bleeding, stroke, sepsis, arrythmias, etc.), recovery of renal function | Survival, postoperative complications |
| Kyvernitakis 2019 | Allegheny General Hospital, Pittsburg | 2006–16 | 212 (DT 86, 41) | Overall 1 53 (25) 2 104 (49) 3 26 (12) 4 + 29 (14) |
Median 60 (range 25–80) overall | 170 (80) | HMII 170 (80), HVAD 42 (20) overall | N/A | Bloodstream infections, all-cause mortality | Bloodstream infections, DT as predictor of infection, survival |
| Lamba 2018 (conference abstract) |
Baylor College of Medicine, Texas | 1999–2017 | 615 (unclear but > 50 DT) | NR | 54.1 (13.7) overall | 485 (78.9) | HMII 493 HVAD 140 Jarvik 81 HM3 9 DuraHeart 2 From a wider cohort |
By device | Survival, neurological deficit, acute kidney injury, GI bleed, infections, RVAD | Survival |
| Maltais 2016 | University of Michigan, Mayo Clinic College of Medicine, and Vanderbilt Heart and Vascular Institute | 2004–13 | 614 (250 DT) | LVAD alone mean INTERMACS score 2.9 (1.1) LVAD with concomitant procedure 2.7 (1) overall |
LVAD alone 56 (12) LVAD with concomitant procedure 59 (13) |
497 (81) | HeartMate II 492 (80) HeartWare 122 (20) |
LVAD alone vs. LVAD with concomitant procedure at time of implant | Survival, time to first device-related event, complications (haemolysis, suspected or confirmed pump thrombus, right ventricular failure, stroke, and GIB) | DT as predictor of survival or adverse events vs. BTT |
| Maltais 2017 | 24 centres many included in INTERMACS for example Mayo clinic, Vanderbilt Medical, University of Colorado hospital | 2014–015 | 300 (234 DT) | Profile 1 38 (13) 2 90 (30) 3 121 (40) 4–7 51 (17) |
57 (13) | 248 (83) | All HMII | N/A | Adverse events, rehospitalisations, pump thrombosis, survival | No. with pump thrombosis, survival |
| Mohamedali 2017 | Advocate Christ Medical Centre | NR | 212 (all DT) | INTERMACS 1 14 (7) 2 77 (36) 3 99 (47) ≥ 4 22 (10) |
Mean arterial pressure CVP ≥ 7.5 63.2 (11) Mean arterial pressure CVP < 7.5 62.3 (11.6) |
168 (79) | All HMII | Mean arterial pressure CVP ≥ 7.5 vs. mean arterial pressure CVP < 7.5 | Post-LVAD RVF and major adverse outcomes, including death, HF hospitalisation, GIB, stroke/transient ischemic attack, intracranial haemorrhage, haemolysis, thrombosis, and infections, all-cause mortality |
All patients are DT so for all outcomes |
| Morgan 2014 | Henry Ford Hospital | 2006–12 | 126 (52) | INTERMACS 1 8 (6) 2 55 (43) 3 30 (24) 4 26 (21) 5 6 (5) 6 1 (1%) |
< 70 years 52.8 (11.4) ≥ 70 years 72.2 (2.3) |
34 (27) | HMII 113 (90) HVAD 13 (10) |
Age < 70 or ≥ 70 | Perioperative mortality, postoperative Survival, overall hospital length of stay, postoperative complication rates for bleeding requiring re-exploration, infection, stroke, respiratory failure, renal failure, RV failure, GIB, AI, re-admission rates, and causes of death |
Survival |
| Morgan 2016 | Henry Ford Hospital | 2006–15 | 231 (DT 113, 47.1) | NR | 58.2 (11.4) DT | 86 (76.1) | HMII 205 (89) HVAD 35 (11) |
BTT vs. DT | Survival, complications (bleeding, drivelines infections, pneumonia, RHF, stroke, aortic insufficiency, pump thrombosis) | Survival, complications |
| Nakagawa 2018 | Columbia University Medical Center |
2010–6 | 89 (59 DT, 66) | NR | DT 64.3 (12.4) | DT 49 (83.1) | HMII 49 (83) HVAD 10 (17) | DT vs. BTT patients who died on LVAD | Indicators of good-quality palliative care, cause of death, time on LVAD, renal replacement therapy, LVAD deactivation | All outcomes |
| Okoh 2018 (2 conference abstracts) | Beth Israel Hospital, Newark | NR | 91 (all DT) | NR | Males 59 (9) Females 52 (6) |
70 (77) | NR | By sex AF vs. normal sinus rhythm |
In-hospital death, all-cause mortality, post-LVAD implant morbidity Survival, post-LVAD adverse events (e.g. infection, thrombosis, rehospitalisation), AF as predictor of events |
All outcomes |
| Okoh 2019 | Unclear | 2008–17 | 91 (all DT) | NR | 55 (3) | 70 (77) | All HMII | ≥ Moderate mitral regurgitation vs. < moderate mitral regurgitation | Mitral regurgitation, survival, hospitalisation, complications (including device malfunction, thrombosis, major infection, stroke) | All outcomes |
| Olmstead 2019 | Baylor College of Medicine and Texas Heart Institute | 2009–16 | 437 (236, 54) | Overall 1 60 (13.7) 2 140 (32.0) 3 174 (39.8) 4 40 (9.2) 5 14 (3.2) 6 2 (0.5) 7 7 (1.6) |
55.6 (12.8) overall | 342 (78.3) overall | HMII 314 (71.9) HVAD 123 (28.1) |
Severe infection vs. no severe infection | Infection rates, survival | DT as a predictor of mortality |
| Schechter 2014 | Duke University Medical Center | 2003–12 | 342 (201 DT 58.8) | NR | Median 65 (IQR 53–71) | DT 148 (73.6) | HMII and HeartMate XVE | Primary LVAD implant vs. replacement procedures | Survival, adverse events (renal failure, right ventricular function), length of hospital stay, stroke, DI | Survival |
| Schultz 2018 (conference abstract) | University of Minnesota | NR | 366 (146 DT, 40) | NR | NR | NR | NR | By indication, DT vs. BTT | Cause of death, survival | Survival, cause of death |
| Sharma 2012 | Mayo Clinic College of Medicine, Rochester | 2007–11 | 143 (all DT) | NR | 61.3 (12.2) | 123 (86) | All HMII | DI vs. no DI | No. with drivelines infection, no. with associated pocket infection, microbiological profile, postoperative morbidities | All patients are DT so for all outcomes |
| Singh 2015 | Cleveland Clinic | 2000–12 | 391 (DT 110, 28.1) | NR | 53.9 (14.2) overall | 317 (81.1) | HeartMate XVE 132 (33.8) HMII 236 (60.4) Also included total artificial heart, which is not relevant to this review |
GI bleed vs. no GI bleed | GI bleed and causes | No. of DT patients with GI bleed |
| Slivnick 2020 (conference abstract) | 13 US sites | 2015–8 | 109 DT | NR | NR | NR | NR | N/A | HRQoL | HRQoL |
| Snipelisky 2015 | Mayo Clinic College of Medicine, Rochester | 2007–13 | 136 (all DT) | Median 3 (IQR 2–4) | 63.6 (11.8) | 113 (83.1) | NR | N/A | Mortality, hospital re-admissions Associations between psychosocial factors characteristics and all-cause re-admissions and death |
All patients are DT so for all outcomes |
| Steinberg 2020 | Emory University Hospital | 2012–6 | 569 (DT 81, 14.2) | NR | 51.7 (12.7) overall | 178 (31.3) overall | NR | Male vs. female | Eligibility for heart transplant or DT LVAD, Stanford Integrated Psychosocial Assessment for Transplant scores, survival | Stanford Integrated Psychosocial Assessment for Transplant scores, survival |
| Stulak 2015 | Mayo Clinic College of Medicine, Rochester | 2004–13 | 493 (192 DT) | NR | Median age overall 60 (range 18–79) | 395 (80) | NR | Causes of death by follow-up interval and indication | Cause of death | Cause of death |
| Stulak 2017 | University of Michigan Health System, Mayo Clinic College of Medicine, and Vanderbilt Heart |
2004–14 | 560 (264 DT) | INTERMACS 1–3 387 (69) | Median age overall 59 (range 18–82) | 465 (83) | HMII | Percutaneous driveline fracture vs. those without | Repair of driveline fracture, survival | No. with percutaneous driveline fracture |
| Suarez 2020 | Mayo Clinic College of Medicine, Rochester | 2007–17 | 203 (122 DT) | NR | 63 DT | 166 DT (82) | NR | By indication | Association of depressive symptoms (PHQ-9) with outcomes including mortality, rehospitalisation, major bleeding, neurological events | Severity of depressive symptoms |
| Takeda 2015 | Columbia Presbyterian Medical Center | 2004–13 | 293 (DT 78, 27) | NR | No RHF overall 56.6 (13.9) RHF overall 57.4 (13.3) |
243 (83%) Overall | HMII 252 (86), VentrAssist 6 (2) DuraHeart 7 (2) DeBakey 4 (1) HVAD 24 (8) |
RHF vs. no RHF | Incidence and significance of late RHF, freedom from RHF, requirement of RVAD, survival, major adverse events requiring hospitalisation (e.g. bleeding, device-related events, cerebral events, infections) | Incidence of RHF, survival by RHF or no RHF |
| Tsiouris 2015a | Henry Ford Hospital | 2006–14 | 200 (DT 102, 51) | NR | 58.4 (10.7) DT | 78 (76.5) | HMII 179 (89.5) HVAD 21 (10.5) |
BTT vs. DT | Complications (bleeding, stroke, RHF, renal failure, pneumonia, pump thrombosis), no. transplanted, survival, cause of death | Complications, survival |
| Tsiouris 2015b | Henry Ford Hospital | 2006–13 | 149 (DT 68, 45.6) | NR | 57.6 ± 10.4 DT | 47 (71) | HMII 136 (91) HVAD 13 (9) |
BTT vs. DT | Survival, complications (bleeding, DI, pneumonia, RHF respiratory failure, renal failure stroke aortic insufficiency, pump thrombosis) | Survival, complications |
| Uppalapati 2019 (conference abstract) | 13 US sites | 2015–8 | 137 DT | NR | 68.6 (5.1) | NR | NR | Stratify results by age and gender | HRQoL | HRQoL |
| Vaddiparti 2018 (conference abstract) | Hartford Hospital | 2012–6 | 78 (51 DT, 65) | NR | Median 65 overall | 66 (84) | HMII 63 (81) HVAD 15 (19) |
N/A | Stroke incidence, predictors of stroke, time to stroke, mortality from stroke | Incidence of stroke |
| Verdoorn 2017 | Mayo Clinic College of Medicine, Rochester | 2009–13 | 107 (all DT) | NR | 64.3 (10.7) | 90 (84) | HMII 102 (95) other devices not reported | N/A | Number of deaths | Number of deaths |
| Vidula 2018 (conference abstract) | University of Rochester | 2008–16 | 197 (65 DT, 33) | INTERMACS profile 1 or 2 (59) overall |
56 (12) overall | 160 (81) | All HMII | N/A | Late RHF, predictors of late RHF | DT as risk factor for late RHF |
| Vorovich 2019 (conference abstract) | 13 US sites | 2015–8 | 155 DT | NR | NR | NR | NR | N/A | Neurocognitive outcomes | Neurocognitive outcomes |
| Welden 2018 | University of Alabama | 2009–13 | 102 (DT 50, 49.02) | NR | 53.6 overall | 83 (81.37) overall | HMII 76 (74.5) HVAD 25 (24.5) |
Patients with GI bleeds vs. those without GI bleeds | GI bleeds, re-admissions for bleeding | Re-admission for bleeding compared to BTT |
| Willey 2016 | Columbia University Medical Centre | 2008–15 | 301 (DT 101 33.6) | NR | Stroke 54.7 (14.1) No stroke 58 (13.7) |
238 (79) overall | HMII 266 (88) HVAD 35 (12) |
Those with and without stroke | Mortality, number of strokes, transplantation, cause of death | Number of patients with stroke, number transplanted |
| Yalcin 2020 | University Medical Centre Rotterdam, the Netherlands Johns Hopkins Hospital, Baltimore, USA; and the Medical University Hospital, Charleston, South Carolina |
2004–17 | 400 (DT 154, 39) | 1 67 (17) 2 120 (30) 3 135 (34) ≥ 4 62 (16) Overall (only 384 had available score) |
53 (14) | 298 (75) | HMII 339 (92) HM3 22 (6) HVAD 48 (12) |
By severity of chronic kidney disease | Chronic kidney disease, kidney function, survival | No. DT with chronic kidney disease |
| Yost 2021 | Advocate Christ Medical Centre/University of Michigan Hospitals |
2005–16 | 677 (DT 602, 89) | NR | Primary LVAD Implantation 59.07 (12.99) All pts with LVAD exchange 58.52 (13.12) |
513 (76) | HMII 527 (78) HVAD 142 (22) |
Patients with LVAD exchange, patients exchanged with infection and without infection | Postoperative length of stay, in-hospital mortality, and 30- and 365-day mortality |
No. DT patients exchanged and with/without infections |
Appendix 7 Risk-of-bias assessment for cost-effectiveness review
| CHEC criteria | Cost-analysis and cost-effectiveness studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CETS 2000 | Clegg 2007 | Droogne 2014 | Girling 2007 | Messori 2009 | Chimanji 2016 | Mehra 2018 | Oz 2003 | Slaughter 2011 | Health Qual. On. 2016 | ||
| 1 | Is the study population clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | Are competing alternatives clearly described? | Y | Y | Y | Y | N | Y | Y | N/A | Y | Y |
| 3 | Is a well-defined research question posed in answerable form? | N | P | Y | P | N | Y | Y | Y | Y | Y |
| 4 | Is the economic study design appropriate to the stated objective? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5 | Is the chosen time horizon appropriate to include relevant costs and consequences? | Y | N | N | N | Y | N | N | N | N | N |
| 6 | Is the actual perspective chosen appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Are all important and relevant costs for each alternative identified? | N | N | N | N | N | Y | Y | Y | Y | N |
| 8 | Are all costs measured appropriately in physical units? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 9 | Are costs valued appropriately? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10 | Are all important and relevant outcomes for each alternative identified? | N | N | N | N | N | Y | Y | N/A | Y | Y |
| 11 | Are all outcomes measured appropriately? | Y | Y | Y | Y | Y | Y | Y | N/A | Y | Y |
| 12 | Are outcomes valued appropriately? | Y | Y | Y | Y | Y | Y | Y | N/A | Y | Y |
| 13 | Is an incremental analysis of costs and outcomes of alternatives performed? | Y | Y | Y | Y | Y | N | Y | N/A | N | N |
| 14 | Are all future costs and outcomes discounted appropriately? | Y | N/A | N/A | N/A | Y | N/A | N/A | N/A | N/A | N |
| 15 | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | N | Y | N | N | Y | N | N | N | Y | N |
| 16 | Do the conclusions follow from the data reported? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 17 | Does the study discuss the generalisability of the results to other settings and patient groups? | N | N | N | N | P | N | N | N | N | N |
| 18 | Does the article indicate that there is no potential conflict of interest of study researchers and funders? | N | N | N | N | N | Y | N | N | N | N |
| 19 | Are ethical and distributional issues discussed appropriately? | N | N | N | N | N | N | N | N | N | N |
| Overall score | 12 | 9 | 11 | 11 | 12 | 13 | 13 | 8 | 13 | 11 | |
| Modelling studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Philips criteria (for modelling studies) | Baras 2017 | Chew 2017 | Long 2014 | Neyt 2013 | Rogers 2012 | Silvestry 2019 | Adang 2006 | Schueler 2021 | Lim 2021 | |
| S1 | Is there a clear statement of the decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is the primary decision-maker specified? | N | N | N | Y | N | N | N | Y | Y | |
| Is the perspective of the model stated clearly? | Y | Y | Y | Y | Y | Y | N | Y | Y | |
| S2 | Are the model inputs consistent with the stated perspective? | N | Y | Y | Y | Y | Y | Can’t tell | Y | Y |
| Has the scope of the model been stated and justified? (I.e. have any choices or assumptions been explained sufficiently, in the context of available evidence?) | N | N | N | N | N | N | N | N | N | |
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | N | Y | Y | Y | Y | Y | Can’t tell | Y | Y | |
| S3 | Has the evidence regarding the model structure been described? | N | N | N | N | N | N | Y | Y | Y |
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are the sources of data used to develop the structure of the model specified? | N | N | N | N | N | N | N | Y | Y | |
| Are the causal relationships described by the model structure justified appropriately? | N | N | N | N | N | N | N | N | N | |
| S4 | Are the structural assumptions transparent and justified? | N | N | N | N | N | N | N | Y | Y |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | N | N | N | N | N | N | N | Y | Y | |
| S5 | Is there a clear definition of the options under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Have all feasible and practical options been evaluated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is there justification for the exclusion of feasible options? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| S6 | Is the chosen model type appropriate, given the decision problem and specified causal relationships within the model? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| S7 | Is the time horizon of the model sufficient to reflect all important differences between options? | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Is the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y | Y | Y | Y | Y | Y | N | N/A | Y | |
| S8 | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | N | N | N | N | N | N | N | Y | Y |
| S9 | Is the cycle length defined and justified in terms of the natural history of the disease? | N | N | Y | Y | Y | Y | N | Y | N |
| D1 | Are the data identification methods transparent and appropriate, given the objectives of the model? | N | N | N | N | N | N | N | Y | Y |
| Where choices were made between data sources, are these justified appropriately? | N | N | N | N | N | N | N | N | N | |
| Was particular attention paid to identifying data for the important parameters in the model? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | N | N | N | N | N | N | N | N | N | |
| Has the quality of the data been assessed appropriately? | N | N | N | N | N | N | N | N | N | |
| Where expert opinion was used, are the methods described and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| D2 | Is the pre-model data analysis methodology based on justifiable statistical and epidemiological techniques? | Y | Can’t tell | Y | Can’t tell | Y | Y | Can’t tell | Y | Y |
| Is the choice of baseline data described and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are transition probabilities calculated appropriately? | Y | Can’t tell | Y | Can’t tell | Y | Y | Can’t tell | Can’t tell | Y | |
| Has a half-cycle correction been applied to both cost and outcome? If not, has this omission been justified? | N | N | N | Y | N | N | N | N | N | |
| If relative treatment effects were derived from trial data, have they been synthesised using appropriate techniques? | Y | Y | Y | Y | Y | Y | N/A | Y | Y | |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Y | Y | Y | Y | Y | N/A | Y | Y | |
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | N | N | N | Y | N | N/A | N | N | |
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Are the utilities incorporated into the model appropriate? | N | N | N | N | N | Y | Y | Y | Y | |
| Is the source for the utility weights referenced? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are the methods of derivation for the utility weights justified? | N | Y | Y | Y | Y | Y | Y | Y | Y | |
| D3 | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | N | N | N | Y | Y | Y | Y | Y |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Is the process of data incorporation transparent? | Y | N | Y | N | Y | Y | N | Y | Y | |
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | N | N | N/A | N | N/A | N | Y | N/A | N | |
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | Y | Y | N/A | Y | N/A | Y | N | N/A | N | |
| D4 | Have the four principal types of uncertainty been addressed? If not, has the omission of particular forms of uncertainty been justified? | N | N | N | N | N | N | N | N | N |
| Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | Y | Y | Y | Y | Y | N | N | N | |
| Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | N | N | N | N | N | N | N | N | N | |
| Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | N | N | N | N | N | N | N | Y | |
| Are the methods of assessment of parameter uncertainty appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | N | N | N | N | N | N | N | N | N | |
| C1 | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | N | N | N | N | N | N | N | N |
| C2 | Are the conclusions valid given the data presented? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are any counterintuitive results from the model explained and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| If the model has been calibrated against independent data, have any differences been explained and justified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
Appendix 8 Supplemental data used to inform the economic evaluation
| Monthly mortality risks | Non-comparative, net weight estimates | Non-comparative, weighted estimates for MM | Comparative estimates mapped to MM in REMATCH | Comparative estimates mapped to LVAD in MOMENTUM |
|---|---|---|---|---|
| DT (LVAD) mortality risk | Me an | Me an | Me an | Me an |
| 1–3 months | 0.021 | 0.021 | 0.021 | 0.021 |
| 4–6 months | 0.021 | 0.021 | 0.021 | 0.021 |
| 7–12 months | 0.008 | 0.008 | 0.008 | 0.021 |
| 13+ months | 0.007 | 0.007 | 0.007 | 0.021 |
| RR of mortality in LVAD compared to MM | N/A | N/A | 0.247 | 0.247 |
| MM mortality risk | Mean | Mean | Mean | Mean |
| 1st month | 0.085 | 0.0770 | 0.084 | 0.085 |
| 2–6 months | 0.085 | 0.0770 | 0.084 | 0.085 |
| 7–12 months | 0.085 | 0.0789 | 0.031 | 0.085 |
| 13+ months | 0.085 | 0.0793 | 0.029 | 0.085 |
| Parameters | Mean/month | SE | Trial/database |
|---|---|---|---|
| Mortality | |||
| Mortality risk in LVAD in the non-comparative, net weight estimates (Mehra 2021)126 | |||
| 1–6 months | 0.021 | 0.0009 | MOMENTUM |
| 7–12 months | 0.008 | 0.0002 | |
| 13–24 months | 0.007 | 0.0004 | |
| 25+ months | 0.007 | 0.0004 | |
| Mortality risk in LVAD in the comparative estimates mapped to MM in REMATCH (Mehra 2021) | Same as above | MOMENTUM | |
| Mortality risk in MM in the non-comparative, net weight estimates (Rose 2001) | 0.085 | 0.0085 | REMATCH |
| RR of mortality used in the comparative estimates mapped to MM in REMATCH | 0.247 | 0.0861 | Our estimate (see Chapter 3) |
| Mortality risk in MM in the comparative estimates mapped to MM in REMATCH | 0.084 | 0.0084 | Our estimate |
| 0.084 | 0.0084 | ||
| 0.031 | 0.0031 | ||
| 0.029 | 0.0029 | ||
| Morbidity | |||
| First stroke in LVAD patients (Kirklin 2020) | |||
| 1–3 months | 0.017 | 0.0059 | INTERMACS |
| 4+ months | 0.003 | 0.0022 | |
| Probability of a second stroke after a non-disabling stroke (Kirklin 2020) | 0.002 | 0.0016 | Same as above |
| Proportion of disabling stroke in patients experiencing stroke (Milano 2018) | 0.28 | 0.028 | ENDURANCE DT |
| RHF in LVAD patients | |||
| 1 month (Teuteberg 2020) – early RHF | 0.140 | 0.0067 | INTERMACS 9th |
| 2+ months (Teuteberg 2020) – hospitalisation due to late RHF | 0.002 | 0.0010 | |
| Proportion requiring RVAD placement in patients with LVAD (Rogers 2017) | 0.125 | 0.0523 | ENDURANCE DT |
| Severe AR in LVAD patients (Jorde 2014) | 0.004 | 0.0048 | Columbia Uni Med. Centre |
| Proportion of AR patients requiring operation for valve replacement (Jorde 2014) | 0.33 | 0.0355 | Same as above |
| Stroke in MM patients (Homma 2012) | 0.001 | 0.0007 | WARCEF trial |
| GIB in LVAD patients – 12-month follow-up | |||
| 1–3 months (includes surgical bleeding) | 0.032 | 0.0043 | (Meta-analysis) |
| 4+ months | 0.010 | 0.0003 | |
| DI in LVAD patients | 0.011 | 0.0001 | (Meta-analysis) |
| PI in LVAD patients (Tattevin 2014) | |||
| 1st month | 0.008 | 0.0035 | ASSIST-ICD 19 centres |
| 2+ months | 0.003 | 0.0021 | |
| PE in LVAD patients (any reason) (Kirklin 2017) – device malfunctions over 72 months | 0.002 | 0.0003 | INTERMACS |
| Arrhythmia in LVAD patients – 12-month follow-up | 0.018 | 0.0015 | (Meta-analysis) |
| Probability of re-admission apart from stroke in MM patients (Ambardekar 2019) | 0.068 | 0.0356 | MEDAMACS |
| Mortality within 30 days of major events | |||
| Death due to stroke in LVAD patients (Milano 2018) – 12-month follow-up | 0.25 | 0.0475 | ENDURANCE |
| Death due to stroke in MM patientsa (Freeman 2011) | 0.085 | 0.013 | N/A |
| Death due to early RHFb | 0.021 | 0.0012 | Expert view |
| Death due to ARb | 0.021 | 0.0012 | Expert view |
| Long-term mortality in patients with major events (monthly) | |||
| Mortality risk in disabling stroke survivors (Kirklin 2020) | 0.024 | 0.0119 | INTERMACS |
| Mortality risk in AR survivors (Truby 2018) | 0.021 | 0.0112 | INTERMACS |
| Mortality risk in RHF survivors | 0.085 | 0.013 | Expert view |
| QoL (health utility) | |||
| Utility in DT (LVAD) | |||
| 1 month | 0.51 | 0.014 | (Meta-analysis) |
| 2–6 months | 0.76 | 0.011 | |
| 7–12 months | 0.77 | 0.010 | |
| 13+ months | 0.77 | 0.014 | |
| Utility in MM | 0.51 | 0.014 | (Meta-analysis) |
| Utility loss after stroke (Post piet 2001, Chaisinanunkul 2015) | 0.11 | 0.0255 | N/A |
| Utility loss after disabling stroke (Post piet 2001, Chaisinanunkul 2015) | 0.67 | 0.067 | Same as above |
| Utility in patients experiencing RHFc | 0.405 | 0.0120 | Expert view |
| Utility in patients experiencing ARb | 0.405 | 0.0120 | Expert view |
| Utility loss after GIB (Silvestry 2019) | 0.048 | 0.0048 | Expert view and previous model |
| Utility loss after DI and PI (Long 2014) | 0.156 | 0.0156 | Expert view and previous model |
| Utility loss after PE (Silvestry 2019) | 0.24 | 0.024 | Expert view and previous model |
| Utility loss after arrythmia | |||
| Utility loss after AF (Witassek 2019) | 0.012 | 0.001 | MOMENTUM |
| Utility loss after VF (Mark 2008) (0.58 of all arrythmia cases were assumed to be VF) (Mehra 2019) |
0.063 | 0.006 | |
| One-off cost items | Currency codes | Cost (2019) | SE |
|---|---|---|---|
| Complex LVAD implant cost (applied for 10% of the LVAD patients) |
ED08Z | £130,914 | £13,091 |
| Standard LVAD implant (applied for 90% of the LVAD patients) |
ED09Z | £90,484 | £9048 |
| Average LVAD implant cost | ED08Z/9Z | £94,527 | £9453 |
| Complex heart transplantation | ED04Z | £61,070 | £6011 |
| Stroke | AA35A-F | £3417 | £341 |
| RHF | EB03A-E | £1972 | £197 |
| Aortic valve replacement for AR | ED24A-ED25C | £12,928 | £1292 |
| GIB | FD03A-H | £1235 | £124 |
| DI and PI | HE81A-C | £3478 | £348 |
| PE for any reason (assumed to be same as average LVAD implantation) | ED08Z | £94,527 | £9453 |
| RVAD placement (operation cost assumed to be same as DI) | N/A | £13,740 | £1374 |
| Arrhythmia | EB07A-E | £952 | £95 |
| Death36 | N/A | £9775 | £255 |
| Monthly ongoing costs | |||
| Monthly cost for LVAD patients (outpatient) (Chew et al. 2017)106 | N/A | £958 | £244 |
| Monthly outpatient costs for MM patients (Clegg et al. 2007)108 | N/A | £644 | £64 |
| Cost per re-admission apart from stroke in MM patients (Clegg et al. 2007;108 Girling et al. 2007)109 | N/A | £3389 | £339 |
| Parameters | Mean |
|---|---|
| Overall mortality risk in BTT (LVAD) (Kirklin 2017)137 | |
| 1–12 months | 0.013 |
| 13–24 months | 0.008 |
| 25–36 months | 0.008 |
| 37–48 months | 0.010 |
| Monthly mortality risk in HT (Clarke 2014)156 | |
| 1st month | 0.050 |
| 2–12 months | 0.014 |
| 13+ months | 0.009 |
| Transition from DT to BTT (LVAD) (Goldstein 2020)157 | 0.006 |
| Transition from BTT (LVAD) to HT (Kirklin 2017)137 | |
| 1–12 months | 0.028 |
| Stroke in HT patients (Kirklin 2017)137 | |
| 1–12 months | 0.012 |
| Monthly re-admission in HT patients apart from stroke (Jalowiec 2008)158 | 0.088 |
| Utility in BTT (LVAD) (Clarke 2014)156 | 0.74 |
| Utility in HT (Clarke 2014)156 | 0.83 |
| Parameter | Value 1 | Source | Base-case | Value 2 | Source |
|---|---|---|---|---|---|
| LVAD implantation cost | £91,162 | Schueler 2020116 | £94,527 | £109,140 | Lim 2021110 |
| End-of-life care cost is doubled for MM | – | – | £9775 | £19,550 | Steering committee |
| Monthly outpatient costs in MM patients (outpatient) | £72 | Lim 2021 | £644 | £2951 | Silvestry 2019115 |
| Outpatient costs for LVAD patients | £72 | Lim 2021 | £958 | £1952 | Clegg 2007108 |
| Cost per re-admission per MM patient | £2711 | 0.80 × £3389 | £3389 | £9041 | Baras Shreibati 2017101 |
| Proportion of RHF patients receiving RVAD after a LVAD | 0.116 | Kirklin 2017 | 0.125 | 0.138 | Chapter 3 |
| Probability of RHF hospitalisation after the 2nd month | 0.001 | 0.002/2 | 0.002 | 0.004 | 0.002 × 2 |
| Probability of severe AR | 0.002 | 0.004/2 | 0.004 | 0.008 | 0.004 × 2 |
| Probability of stroke in LVAD patients | 0.008 and 0.003 | Starling 2017159 | 0.017 and 0.003 | 0.017 and 0.008 | Starling 2017 |
| Probability of stroke in MM patients | – | – | 0.001 | 0.002 | Baras Shreibati 2017 |
| GIB in LVAD recipients | 0.016 and 0.40 | Kirklin 2017 | 0.032 and 0.010 | 0.04 and 0.06 | Kirklin 2017137 |
| DI in LVAD recipients | 0.006 | 0.011/2 | 0.011 | 0.024 | Tattenvin 2019160 |
| Utility loss after disabling stroke | 0.450 | Scheuler 2020 | 0.670 | 0.7 | 0.67 × 1.05 |
| Utility loss after non-disabling stroke | 0.09 | (0.11/0.14) × 0.11 | 0.11 | 0.14 | Luengo-Fernandez 2013161 |
| Utility in MM patients | 0.40 | Baras Shreibati 2017 | 0.51 | 0.64 | Silvestry 2019 |
| Utility in LVAD recipients (12 months) | 0.70 | Baras Shreibati 2017 | 0.77 | 0.85 | Chew 2017106 |
| MM mortality risk | 0.070 | Scheuler 2020 | 0.085 | 0.09 | 0.085 × 1.05 |
| Reduced LVAD mortality risk after 12 months | 0.005 | Lim 2021 | 0.021 and 0.012 | 0.011 | Mehra 2021126 |
| Cost description | Estimated cost (2019 £) | Sources | What’s included | Study and country |
|---|---|---|---|---|
| Monthly outpatient costs per LVAD patient | £1952 £7529 (first 12 months) £2139 (13+ months) £957 £958 £986 £2172 £1943 £2598 £72 |
Papworth Hospital NHS Trust, unpublished BTT data Literature-based details are not provided Literature-based details are not provided National hospital costing data 69 BTT patients 220 patients’ Medicare claims after LVAD 83 LVAD patients in COMPANION trial Medicare data details not provided Abbott internal data |
All costs other than hospital visits All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission |
Clegg 2007120 – UK Long 2014111 – USA HQ Ontario 201691 – Can. Chew 2017106 – Can. Neyt 2013102 – NL B. Shreibati 2017101 – USA Rogers 2012114 – USA Silvestry 2019115 – USA Lim 2021110 |
| Monthly outpatient costs per MM patient | £644 £958 £2457 £1943 £2951 £1187 £336 £14,036 (1 month) £6854 (3+ months)a £6430 £72 |
Papworth Hospital NHS Trust, unpublished BTT data National hospital costing data 220 patients’ Medicare claims before LVAD 83 LVAD patients in COMPANION trial Medicare data details not provided Not provided Clegg 2007 Sharples 2006119 – BTT patients’ unpublished data Medtronic internal data Abbott internal data |
All costs other than hospital visits All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission All costs other than hospital re-admission Outpatient costs and hospital admissions (incl. stroke)b Outpatient and hospital admission costsb Outpatient costs only |
Clegg 2007 – UK Chew 2017 – Can. Rogers 2012 – USA B. Shreibati 2017 – USA Silvestry 2019 – USA Neyt 2013 – NL Adang 2006104 – NL Clarke et al. 2014 Scheuler 2021116 Lim 2021 |
| Model inputs | INTERMACS 1 | INTERMACS 2 & 3 | INTERMACS 4 & 5 |
|---|---|---|---|
| Mortality in LVAD (Teuteberg 2020)46 | |||
| 1–3 months | 0.037 | 0.021 | 0.019 |
| 4–12 months | 0.015 | 0.021 | 0.010 |
| 13–24 months | 0.015 | 0.012 | 0.010 |
| 25+ months | 0.014 | 0.012 | 0.010 |
| (INTERMACS) | (INTERMACS) | (INTERMACS) | |
| Mortality in MM | |||
| 1 month | 0.70162 | 0.085 | 0.061 |
| 2–6 months | 0.08a | (REMATCH) 54 | 0.077 |
| 7–11 months | 0.080 | ||
| 12+ months | (MedaMACS) 127 | ||
| QoL in LVAD | |||
| 1 month | 0.37 (0.26 + 0.11)a | 0.40 – 1st month | 0.51 |
| 2–6 months | 0.37 | 0.562 | 0.77 |
| 7–12 months | 0.76 | 0.76 | 0.77 |
| 13+ months | 0.76 | 0.77 | 0.77 |
| (INTERMACS) | (REMATCH) | (MOMENTUM) 157 | |
| QoL in MM | |||
| 1 month | 0.1a (first month) | 0.400 | 0.51 |
| 2+ months | 0.26a | (REMATCH) | (MOMENTUM BASELINE) |
| Lifetime outcomes | Non-comparative, weighted estimates for MM | Comparative estimates mapped to LVAD | ||||
|---|---|---|---|---|---|---|
| MM | LVAD | Incremental | MM | LVAD | Incremental | |
| Expected LYs per patient | 0.98 | 4.66 | 3.67 | 0.92 | 2.86 | 1.94 |
| Expected QALYs per patient | 0.50 | 3.32 | 2.82 | 0.46 | 2.02 | 1.56 |
| Cost per patient | £19,528 | £171,647 | £152,119 | £18,886 | £145,434 | £126,548 |
| Incremental cost per LY | £41,429 | £65,200 | ||||
| Incremental cost per QALY | £53,876 | £81,298 | ||||
Glossary
- Bridge to candidacy
- A patient too unwell to be a candidate for a therapy, but the bridge carries them to a state of being eligible.
- Bridge to transplant
- Such therapy preserves someone’s health well enough and long enough that they are able to receive a transplant after spending time waiting for an organ to become available.
- Cost-effectiveness acceptability curve
- The cost-effectiveness acceptability curve is a graph summarising the impact of uncertainty on the result of an economic evaluation, frequently expressed as an incremental cost-effectiveness ratio in relation to possible values of the cost-effectiveness threshold.
- Destination therapy
- When recovery from heart failure is not possible and patients are ineligible for a heart transplant, the therapies used are considered as destination therapy. Left ventricular assist devices can be given as a destination therapy as can medical management alone. As such, destination therapy is not an alternative to a heart transplant or therapy while awaiting a heart transplant because the patient being ineligible for a heart transplant defines it.
- Heart failure
- Heart failure is a chronic, progressive condition in which the heart muscle is unable to pump enough blood to meet the body’s needs for blood and oxygen.
- Incremental cost-effectiveness ratio
- An incremental cost-effectiveness ratio is a summary measure representing the economic value of an intervention, compared with an alternative (comparator).
- Left ventricular assist device
- A left ventricular assist device is a mechanical pump that is implanted in patients with heart failure. It helps the bottom left chamber of the heart (left ventricle) pump blood out of the heart to the aorta and the rest of the body.
- Medical management
- In this report, medical management refers to the range of medical therapies employed to treat patients with heart failure before, or in the absence of, a surgical intervention, such as a left ventricular assist device or heart transplant.
- New York Heart Association Functional Classification
- The New York Heart Association Functional Classification provides a simple way of classifying the extent of heart failure.
- Quality-adjusted life-year
- A measure of the state of health of a person or group in which the benefits, in terms of length of life, are adjusted to reflect the quality of life. One quality-adjusted life-year is equal to 1 year of life in perfect health.
List of abbreviations
- AHF
- advanced heart failure
- AR
- aortic regurgitation
- BMI
- body mass index
- BNP
- B-type natriuretic peptide
- BTC
- bridge to candidacy
- BTT
- bridge to transplant
- CE
- Conformité Européenne
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CUA
- cost–utility analysis
- DT
- destination therapy
- EF
- ejection fraction
- ESC
- European Society of Cardiology
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EUROMACS
- European Registry for Patients with Mechanical Circulatory Support
- EVPI
- expected value of perfect information
- FDA
- Food and Drug Administration
- GI
- gastrointestinal
- GIB
- gastrointestinal bleeding
- HM3
- HeartMate 3
- HR
- hazard ratio
- HRAEs
- haemocompatibility-related adverse events
- HT
- heart transplant
- HTA
- Health Technology Assessment
- HVAD
- HeartWare ventricular assist device
- ICD
- implantable cardioverter defibrillator
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IMACS
- International Registry for Mechanically Assisted Circulation
- INTERMACS
- Interagency Registry for Mechanically Assisted Circulatory Support
- IRR
- incidence rate ratio
- KCCQ
- Kansas City cardiomyopathy questionnaire
- LV
- left ventricle
- LVAD
- left ventricular assist device
- LVEF
- left ventricular ejection fraction
- LY
- life years
- MCSDs
- mechanical circulatory support devices
- MLHFQ
- Minnesota living with heart failure questionnaire
- MM
- medical management
- mRS
- modified Rankin Scale
- NHS EED
- National Health Service Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NICOR
- National Institute for Cardiovascular Outcomes Research
- NMA
- network meta-analysis
- NYHA
- New York Heart Association
- PE
- pump exchange
- PI
- pump infection
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REMATCH
- Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure
- RHF
- right heart failure
- RR
- relative risk
- RVAD
- right ventricular assist device
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- TIA
- transient ischaemic attack
- VAD
- ventricular assist device
- VAS
- visual analogue scale
- VoI
- Value of Information
- WTP
- willingness to pay
- XVE
- extended vented electric
