Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 14/226/07. The contractual start date was in May 2017. The draft report began editorial review in November 2021 and was accepted for publication in April 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Carrie et al. This work was produced by Carrie et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Carrie et al.
Chapter 1 Introduction
Scientific background
The Nasal AIRway Obstruction Study (NAIROS) addresses the clinical effectiveness and cost-effectiveness of the operative procedure septoplasty in patients aged > 18 years who have symptoms of nasal obstruction in the presence of a deviated nasal septum. Septoplasty is a commonly performed operation to straighten the mid-line partition between the two nostrils. Within the bony nasal cavities, the lateral aspects (internal sidewalls) each have three nasal turbinates. These are bony structures rich in vascular and glandular tissue projecting into the nasal cavity, which are affected by variable swelling and glandular oversecretion among those with either allergic or non-allergic rhinitis (swelling of nasal cavity lining). The largest and most accessible of those is the inferior turbinate (which is lowest on the sidewall). In addition, when there is a septal deviation, a space is created in the wider nostril, and the inferior turbinate can expand into this space. 1
Many septal operations are combined with reduction of one or both of the inferior turbinates. 2 Turbinate reduction is performed on the assumption that an increase in nasal airway volume will facilitate better functional nasal airflow, which will in turn improve patient symptoms. 3 However, the evidence base for this is limited. In 2007, there were around 95,000 septoplasties performed in Germany4 and around 132,000 turbinate reductions, equating to 1% of all operations in Germany that year. In the USA, > 250,000 septal operations are performed annually. 5 Over a 12-month period in 2019/20, approximately 16,700 septoplasty procedures were performed in England,6 at a cost of £15.9M.
Nasal septal deviation can be either congenital or acquired. 7 Septal deformities may arise in early fetal development. Ruano-Gil et al. 8 noted septal deformities in 4% of 50 fetuses before any intrauterine compressive forces could act, suggesting a possible underlying hereditary factor. Overall, the most common cause of septal deviation is trauma, which can occur at any stage of life. The index injury may not be recollected; for example, in childhood, a fall as a toddler can lead to unrecognised septal trauma. In adulthood, sporting injuries or assault are commonly reported causes of septal deviation. Increasing age and male sex are also associated with a higher prevalence of septal deviation. 9
Septal deflection may cause cosmetic nasal abnormalities. However, the main, functional problems are those related to nasal obstruction: that is, snoring and sleep-disordered breathing. Nasal obstruction is reported in up to 80% of patients and is the nasal symptom most commonly presenting to otolaryngologists. 10 Nasal obstruction was also found to affect one in four of the Swedish population. 11 A significant proportion of nasal obstruction is due to rhinitis, which affects almost 20% of the European population. 12 It is both a poorly understood and poorly characterised symptom: in reality, many patients with a grossly deviated septum do not report symptoms of obstruction, whereas others, with minimal deviation, do. Nasal obstruction has been shown to increase resistance in the upper airways, thus contributing to snoring and sleep apnoea, for which patients, often prompted by their partner, seek treatment. 13 Despite the relationship between nasal obstruction and obstructive sleep apnoea, the impact of surgery in improving nasal patency remains uncertain. 14 In a small randomised controlled trial (RCT), Koutsourelakis et al. 15 found that septoplasty rarely treats obstructive sleep apnoea effectively. In contrast, another small study showed that septoplasty did improve patient-related outcomes concerning difficulty falling asleep and awakening at night. 16
The anterior nasal septum is a cartilaginous structure, never geometrically straight. Researchers have varyingly estimated the prevalence of deviation from 22% to > 70%, reflecting the lack of accepted definition or measurement of what actually constitutes a septal deviation. 7,17 Even estimates from radiological studies range from 20 to > 80%. 18,19 In addition, many nasal conditions other than deviated nasal septum, such as allergic rhinitis and chronic rhinosinusitis, present with a blocked nose. As part of routine clinical assessment of patients with nasal obstruction, these conditions must be excluded. In practice, in the absence of clinical guidelines in the UK, a clinician’s subjective naked-eye assessment of the degree of deviation underpins the decision-making for septoplasty. As a result, decision-making for surgery is subject to great variability and bias.
Studies suggest that anterior septal deviations are more likely to be associated with nasal obstruction than posterior nasal deviations10 (Figure 1). A combination of clinical assessment and fluid dynamic modelling in a 10 year retrospective study showed anterior deflections benefit most from septal surgery. 20 Location is ideally measured with cross-sectional imaging,21 but cost and radiation exposure preclude its routine use. In one cohort, however, computerised tomography scanning was found to alter the surgical plan in 8% of patients. 22 Evidence confirms that the level of the inferior turbinate is the site that determines the sensation of nasal resistance. 23,24 Cole et al. ,25 simulated nasal septal deviations in healthy adults and found that in the majority of adults, the mid and posterior nasal cavities are unresponsive to nasal septal deviation and mucosal changes, but in contrast, that the anterior part of the nose is sensitive to induced septal deviation of as little as 1 mm. The addition of what is called Cottle’s manoeuvre, where the patient pulls the cheek away from the obstructed nostril and derives symptomatic benefit, does not appear to add to the specificity of clinical examination. 26
FIGURE 1.
Typical appearance associated with a deviated nasal septum nasal obstruction, with compensatory enlargement of the inferior turbinate causing nasal obstruction. ©Frances Grierson, 2021.

Headlight illumination allows the first 1–2 cm of septum to be visualised. Septal deviation behind this point requires a nasal endoscopy to assess the mid- and posterior septum and to rule out other pathologies, such as nasal polyps associated with rhinosinusitis. 27 There are fundamental challenges in assessing symptoms of nasal obstruction. In all (except aquatic) mammals under normal circumstances, nasal airflow is greater in one nostril than the other owing to cyclical swelling in the nasal lining (this is known as the ‘nasal cycle’). As a result, over a period of 1–4 hours, each nostril exhibits a periodicity of reducing and then increasing resistance, producing a sensation of alternating blockage in each nostril. Therefore, the sensation of nasal obstruction within each nostril is not a ‘fixed’ phenomenon. 28
Role of the turbinate
Turbinate size has an impact on the volume of the nasal cavity and is influenced by factors such as environmental temperature and humidity, airborne allergens, and respiratory infections such as the common cold. All cyclical swelling within the turbinate contributes to the ‘nasal cycle’. In their classification of turbinate enlargement, Hol and Huizing29 noted compensatory turbinate enlargement in the nostril opposite the side into which the septum is deviated. This so-called compensatory enlargement tends to develop in turbinate tissue exposed to the wider of the two nasal passages30 (see Figure 1). The diagnosis of an enlarged turbinate requires a subjective clinical assessment, and it may simply serve as a diagnosis of exclusion when no other factors are contributing to a blocked nose.
In the UK, patients with nasal obstruction symptoms typically initially self-medicate with nasal decongestants or oral antihistamines. Discussions with pharmacists may lead to the patient commencing a nasal steroid spray. Failure of initial treatments may encourage the patient to seek further options from primary care. Most UK clinical commissioning guidelines will guide general practitioners (GPs) to recommend a trial of intranasal steroid or an alternative steroid medical therapy before considering referral to secondary care for treatment failures, even for those in whom an obviously deviated septum is noted. 31 There is limited scope to undertake visual assessment of the nasal cavity outside secondary care, and there may be alternative or coexistent pathologies, such as allergic rhinitis or chronic rhinosinusitis, contributing to the symptoms of nasal obstruction. Objective assessments of nasal airflow and radiological imaging are rarely undertaken in the UK setting for patients with a deviated nasal septum, although some authorities have long regarded them as useful adjuncts in clinical decision-making. 32,33
Subjective assessment
The NAIROS used the most commonly employed patient-reported outcome measures (PROMs) in rhinological practice: the Nasal Obstruction Symptom Evaluation (NOSE) scale and the Sino-nasal Outcome Test-22 items (SNOT-22). The NOSE scale was specifically developed as an outcome tool for septoplasty. 34 The five standard NOSE scale items are scored 0–4, that is the total score equals 20. Conventionally, the score is multiplied by five, such that the maximum possible score is converted to 100. A systematic review of postoperative NOSE scale data of 643 patients undergoing a variety of surgical procedures showed an overall weighted mean change of 42 of 100 scaled points. 35 Lipan and Most36 performed a receiver operating characteristic analysis of NOSE scores obtained in a heterogeneous population of 345 patients undergoing nasal surgery. They defined a NOSE score of < 30 (0–25) as ‘mild’, 30–50 as ‘moderate’, 55–75 as ‘severe’ and 80–100 as ‘extreme’. Only 6% of the study population had mild symptoms. For the NAIROS, we predicted that, as with most interventions, baseline severity is the most important determinant of outcome, that is the effect demonstrated would depend on the severity of disease in the sample studied. On the basis of Lipan and Most’s36 receiver operating characteristic findings, those with a NOSE score of < 30 were considered too mildly affected for inclusion in the NAIROS, as similar NOSE scores are reported by patients who do not have nasal obstruction.
The NAIROS randomisation stratified participants according to baseline severity and gender. The decision to reduce the size of the turbinate in the wider of the two nasal passages (unilateral reduction) in the NAIROS was recorded at baseline and was based on the individual ear, nose and throat (ENT) surgeon’s assessment of the contribution it made, in combination with the septal deviation, to the symptom of obstruction in any given patient. The NAIROS tested, as a secondary statistical analysis, the contribution of turbinate reduction to any improvement in the nasal airway, an outcome on which published results are inconclusive. Indeed, one study concluded that turbinate reduction alone could be superior to operations involving septoplasty. 2 Gender influences patient responses on the SNOT-22. 37 There are more septoplasties among males,38 in part at least because of the circumstances surrounding nasal injury. There are anatomical differences in the size and strength of nasal tissue during growth, which may affect response to surgery. 39 An early study of septoplasty outcome found that only female gender and history of previous nasal surgery were significant predictors of (worse) outcome,40 but the total evidence base is ambiguous. 41
The NAIROS measured the SNOT-22 score at baseline (before treatment), at 6 months and at 12 months, with the 6-month SNOT-22 score reported as the primary outcome measure. The SNOT-22 was first applied to septoplasty in 2003,42 with a mean drop of 16 points from baseline (mean 32 points) 3 months after septal surgery. The SNOT-22 subscale of nasal symptoms reduced from 14 to 7 points, and general health symptoms reduced from 22 to 12 points. A larger study of 126 patients found a smaller reduction of just 4 points at 6 months, perhaps because of a lower starting baseline (mean 22 points). 43
The mean SNOT-22 score in a UK study of > 2000 patients undergoing surgery for chronic rhinosinusitis was (predictably, given its origins) measured > 44 points preoperatively, with a drop of 30 points, on average, postoperatively. 44 The minimal clinically important difference (MCID) for the SNOT-22 among patients undergoing surgery for nasal polyposis and chronic rhinosinusitis was 8.9 points. In other words, a change of < 9 points on the SNOT-22 was not judged to be a meaningful improvement by the patient. The NAIROS uses a reduction of 9 points as the MCID for the SNOT-22 primary outcome. 43,45–47
Potentially the most straightforward way of measuring nasal obstruction secondary to a deviated septum is to request that the patient complete a visual analogue scale to quantify the degree of blockage. However, this is a potentially flawed approach, as patients with long-standing septal deviation may become accustomed to breathing with limited nasal airflow. 48 Boyce and Eccles49 developed the Double Ordinal Airway Subjective Scale (DOASS) as an improvement in the subjective assessment of nasal obstruction. The patient rates the nasal airflow through each nostril independently (with the opposite closed) and characterises the amount of flow on a scale of 1–10, on which 1 is complete blockage and 10 is air flowing freely through the nostril. The DOASS was noted to have a sensitivity of 81% and specificity of 61%, the latter being considerably better than an assessment by visual analogue scale of nasal obstruction.
Objective assessment of the nasal airway
A number of tools have been developed to assess nasal airflow. Rhinomanometry, which measures nasal airway resistance as a function of nasal airflow and the pressure required to create that flow, has been described as the gold standard of assessment. 50 However, it is both cumbersome and time-consuming, and thus impractical from a routine clinical perspective. It may have an advantage over subjective sensation, however, when assessing subtle differences between the two nostrils. 51 Acoustic rhinometry calculates the cross-sectional area of the nasal cavity by measuring the reflection of acoustic pulses introduced into the nostril. Although straightforward to use, it has significant limitations related to the inherent challenges of assessing the physical properties of sound transmission of air in a complex chamber such as the nasal cavity. 52
The NAIROS used two objective assessments of nasal airflow: rhinospirometry and peak nasal inspiratory flow (PNIF). Rhinospirometry measures the flow and volume of air through each nostril independently. 53 In the NAIROS, it was used to measure both maximal inhalation volume (MIV) and tidal breathing. Asymmetry of nasal airflow is expressed as a nasal partitioning ratio (NPR), calculated as follows: (VL – VR) / (VL + VR), where VL is left-sided volume and VR is right-sided volume. NPR scores range from −1 (complete left nasal cavity obstruction) to 1 (complete right nasal cavity obstruction), with 0 indicating symmetry of airflow. 54 Cuddihy and Eccles55 measured the NPR of 31 patients before and after corrective surgery for nasal septal deviation. Those patients who had a NPR beyond the normal range had a greater improvement in subjective nasal obstruction. In addition, and as quoted previously, Boyce and Eccles56 identified that the DOASS score correlated well with the NPR values obtained in rhinospirometry. PNIF measures the peak flow rate of air through both nostrils during forced inhalation. PNIF has been shown to respond to septoplasty/turbinectomy and can therefore be used for an overall assessment of nasal airflow impairment. 57
It was expected that, for the NAIROS, a combination of the subjective and objective airway assessments would enable us to construct a robust algorithm for use at baseline to predict which patients were most likely to benefit from septoplasty.
Rationale
The NHS currently purchases 17,000 surgical interventions on the nasal septum across the UK annually, yet the procedure is almost entirely lacking in a suitable evidence base or even adequate guidance. The NHS and personal costs of this practice are considerable and there is an urgent need for evaluation in a substantive study, which, with sufficient sample size and power, has the potential to influence clinical practice, patient choice and NHS commissioning.
Evidence review of septoplasty effectiveness
To our knowledge, van Egmond et al. ,58 published the only RCT on the clinical effectiveness of septoplasty in 2019. This study was based on analysis of septal surgery, compared with non-surgical management, across 206 patients in the Netherlands. They concluded that septoplasty was more effective than non-surgical management for nasal obstruction. Those patients randomised to non-surgical management did not receive a standardised treatment, and they received a variety of medical interventions considered appropriate by the treating clinician. Therefore, it is difficult to conclude whether or not a positive trial is the result of an effective intervention or low-efficacy standard care. 59 Furthermore, the primary outcome measure of the trial, the Glasgow Health Status Inventory, a health-related quality-of-life measure, demonstrated an improved score among those patients undergoing surgery. However, the Glasgow Health Status Inventory is known to have limited sensitivity to septal surgery outcomes,60 and, when compared with the pragmatic medical treatment comparator undertaken by van Egmond et al. ,58 this effect may have been exacerbated. Over 75% of the surgical cohort underwent concomitant inferior turbinate surgery, bilateral in 67% of patients, even though inferior turbinate enlargement was noted in < 50% of patients at baseline. This additional surgery is likely to exaggerate the beneficial impact of turbinate surgery over septoplasty alone. 2 In addition, there was a 30% crossover from the non-surgical to the surgical arm, introducing a further potential element of bias or dilution of the full effect of septoplasty.
In a 2018 systematic review of the evidence for septoplasty with or without inferior turbinate reduction as a treatment for nasal obstruction, van Egmond et al. 61 first compared septoplasty (with or without concurrent turbinate surgery) with non-surgical management and, second, compared septoplasty alone with septoplasty with turbinate surgery. In their review, there were no RCTs comparing septoplasty with non-surgical management, and five RCTs and six controlled trials comparing septoplasty alone with septoplasty with turbinate surgery. Included studies demonstrated substantial heterogeneity in study population, outcomes measured and time points of outcome assessment. The risk of bias was considered high in most reports. However, although subjective and objective assessment improvements did not necessarily mirror one another, studies demonstrated an overall improvement in nasal obstruction symptom. No additional benefit of turbinate surgery was reported in 8 out of 9 studies using subjective assessments, and 5 out of 7 studies using objective measures. Complications were rare, and were reported in only three studies. There were significant limitations related to short follow-up periods, which in some cases were only 9 months.
In a second systematic review of septoplasty alone, Tsang et al. 62 found six studies assessing patient satisfaction; rates varied from 69% to 100% in three studies in which patients were asked if they were satisfied or dissatisfied with outcomes at 6 months. Two studies assessed the degree of patient satisfaction, with one study indicating that 88% of patients were moderately satisfied or better at 1 year post operation, and the other reporting that 50% of patients were satisfied at 5 years post operation. There was significant heterogeneity in the method of assessment of nasal obstruction, which may have led to the finding that, in general, patients with more severe symptoms of nasal obstruction had a better outcome. The authors noted that there were high risks of bias as studies were only observational, with significant variation across multiple categories including patient population, outcome measures and follow-up duration. Overall, the authors concluded that there was insufficient evidence that septoplasty alone had good long-term patient-reported outcomes in the management of septal deviation, based on the heterogeneity of existing data and lack of RCTs. They recommended further research to define which preoperative characteristics are predictive of both subjectively and objectively positive septoplasty outcomes. Neither systematic review undertook a meta-analysis because of the substantial heterogeneity across the studies included.
Benefits and harms of interventions
Surgical
Septoplasty has level III evidence of effectiveness, and observational studies confirm good levels of patient satisfaction. The operation, if successful, requires a single procedure without the requirement of ongoing medical therapy. However, there is variation in criteria for surgery, surgical technique and postoperative follow-up. Selection criteria for surgery variance was discussed previously, but there is also variance in operative techniques among surgeons and the need for patient follow-up postoperatively. In addition, there is no good-quality evidence on either the clinical effectiveness or the cost-effectiveness of such surgery. Septal surgery also carries an economic cost, in terms of time off work or normal duties, and can be associated with side effects or complications that delay recovery and potentially necessitate additional treatment.
Following surgery, it is normal to have some symptoms, lasting between 48 and 72 hours, of minor bleeding, congestion and nasal discomfort. In our own group’s early publication63 of the outcomes among 121 septoplasty patients at 6 weeks, two postoperative complications were noted: septal perforation (1.7%) and nasal septal adhesions (3.3%). A Chinese study of 54 patients reported nasal septal adhesions in > 7% of patients. 64 Adhesions and septal haematoma may necessitate re-admission for corrective surgery. In addition, minor cosmetic change occurs in up to 30% of patients and more major change in > 4% of patients. 65 Dabrowska et al. ,66 in a retrospective series of 5639 patients undergoing septoplasty with or without turbinate reduction, reported excessive bleeding in 3.3%, septal perforation in 2.3%, infection (prolonged healing) in 3.1%, reduced smell acuity in 3.1% and dental anaesthesia in 0.1% of patients.
Septoplasty is routinely performed under general anaesthesia as a day-case procedure. An initial incision is made on one side of the septal lining allowing for the straightening or removal of areas of twisted cartilage and bone. It may not be possible to fully straighten the septum without risking the cosmetic appearance of the nose; therefore, the surgeon must use careful judgement to minimise this risk. The procedure typically takes between 45 and 60 minutes to complete. Nasal packing can be used following surgery; in the NAIROS it was not to be undertaken, if possible, because of the associated discomfort to the patient. Instead, suture repair of the septum was to be performed at the end of the procedure, as this seems to have equal efficacy and fewer disadvantages. 67–69
Medical management
Nasal steroid sprays have potent anti-inflammatory and antiallergic properties, inhibiting the release of inflammatory mediators produced by the nasal lining, thereby reducing swelling and nasal mucus in the nasal passages. Two sprays of 100 μg of mometasone furoate in each nostril twice daily for 6 weeks, followed by 100 μg per day in each nostril, for the remainder of the 6-month treatment period, was chosen based on the maximum recommended standard nasal steroid dose. This spray was identified in patient and public involvement (PPI) discussions with GPs as being restricted by formulary protocols in primary care, and therefore unlikely to have been prescribed previously. Mometasone is licensed for use among patients with reduced nasal airway and has marketing authorisation in the UK.
Medical therapy has the obvious advantage of the avoidance of general anaesthesia and surgery and their associated risks. Nasal steroid sprays are standard treatment for nasal obstruction symptoms and are deliverable in primary care. However, they may not be an effective treatment for patients with a deviated nasal septum70 and, even when they are, patients may require indefinite medical therapy, with its associated costs. Intranasal steroid sprays also have potential risks to the patient: they can cause nasal bleeding (odds ratio 1.56 in a 2020 systematic review, compared with placebo71), crusting, pharyngitis and headache, all of which may affect patient compliance, and there are reports which associate increased intraocular pressure and adrenal suppression with such sprays. Both hypersensitivity and anaphylaxis are rare complications. 72 There are few data comparing different intranasal steroid preparations. 72
Proprietary saline irrigation of the nasal cavity may be performed with either isotonic or hypertonic solutions. 73 These may either be low-positive pressure (from a spray or pump), or gravity-based pressure (using a container with a nasal spout). Saline nasal irrigations have been recommended in the management of various nasal and sinus mucosal disorders such as chronic sinusitis74 and allergic rhinitis,75 although this recommendation is based on mostly low-level evidence. Similarly, saline irrigations have been recommended following endoscopic sinus surgery, but uncertainty remains in the literature around the optimum method of delivery and composition of the saline solution. 73–79 Saline sprays have not been trialled specifically in nasal septal deviation. In consultation with GPs and the PPI group, the Stérimar isotonic spray (Sofibel SAS, Paris, France) was chosen for the NAIROS. Mometasone and Stérimar sprays, in combination with the option of deferred surgery for those in the medical management arm, was noted at initial PPI discussions to be an acceptable alternative to surgery by patients.
Aims and objectives
Primary objectives
The primary objective was to compare the clinical effectiveness of nasal septoplasty (with or without unilateral turbinate reduction) with medical management over a duration of 6 months among adults with a nasal septal deviation who have been referred to otolaryngology outpatient clinics with nasal airway obstruction.
Secondary objectives
The secondary objectives are split into three different aspects: clinical effectiveness, an economic evaluation and a mixed-methods process evaluation.
Clinical effectiveness
We aimed to measure clinical effectiveness according to:
-
subjective self-report rating of nasal airway obstruction
-
heterogeneity of estimated treatment effect, specifically according to severity of obstruction and gender
-
objective measures of nasal patency
-
safety profile recording the number of adverse events (AEs) and additional interventions required.
Measuring clinical effectiveness according to the above standards would enable us to:
-
adjust the estimate of effectiveness in the light of other baseline covariates: severity of self-reported nasal airway obstruction, gender and concomitant turbinate reduction
-
use the results in the surgical arm to explore a possible definition of technical failure in experienced hands, that is experienced surgeons (i.e. consultants or non-consultant career clinicians, but not trainee otolaryngologists)
-
assess to what extent trial participants are representative of the total population of participants referred to ENT clinics with nasal obstruction due to a septal deviation.
Economic evaluation
We conducted our economic evaluation by:
-
assessing cost-effectiveness measured in terms of the incremental cost per improvement (of ≥ 9 points) in SNOT-22 score and AEs avoided over 12 months
-
assessing cost-effectiveness measured in terms of incremental cost per quality-adjusted life-year (QALY) gained [derived from responses to the Short Form questionnaire-36 items (SF-36) converted to Short Form questionnaire-6 Dimensions (SF-6D) scores] over 12 months
-
designing a longer-term economic model to assess the costs and QALYs beyond the 12-month follow-up period.
Mixed-methods process evaluation of the trial and interventions
Our mixed-methods process evaluation was to identify, describe, understand and address:
-
barriers to optimal recruitment, and potential solutions to address these, through integration of the Qualitative research integrated within Trials (QuinteT) Recruitment Intervention (QRI)
-
participants’ and healthcare professionals’ experiences of trial participation and the interventions under evaluation
-
factors likely to influence wider implementation of trial findings.
Chapter 2 Methods
Parts of this chapter are reproduced from Rennie et al. 80 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary of trial design
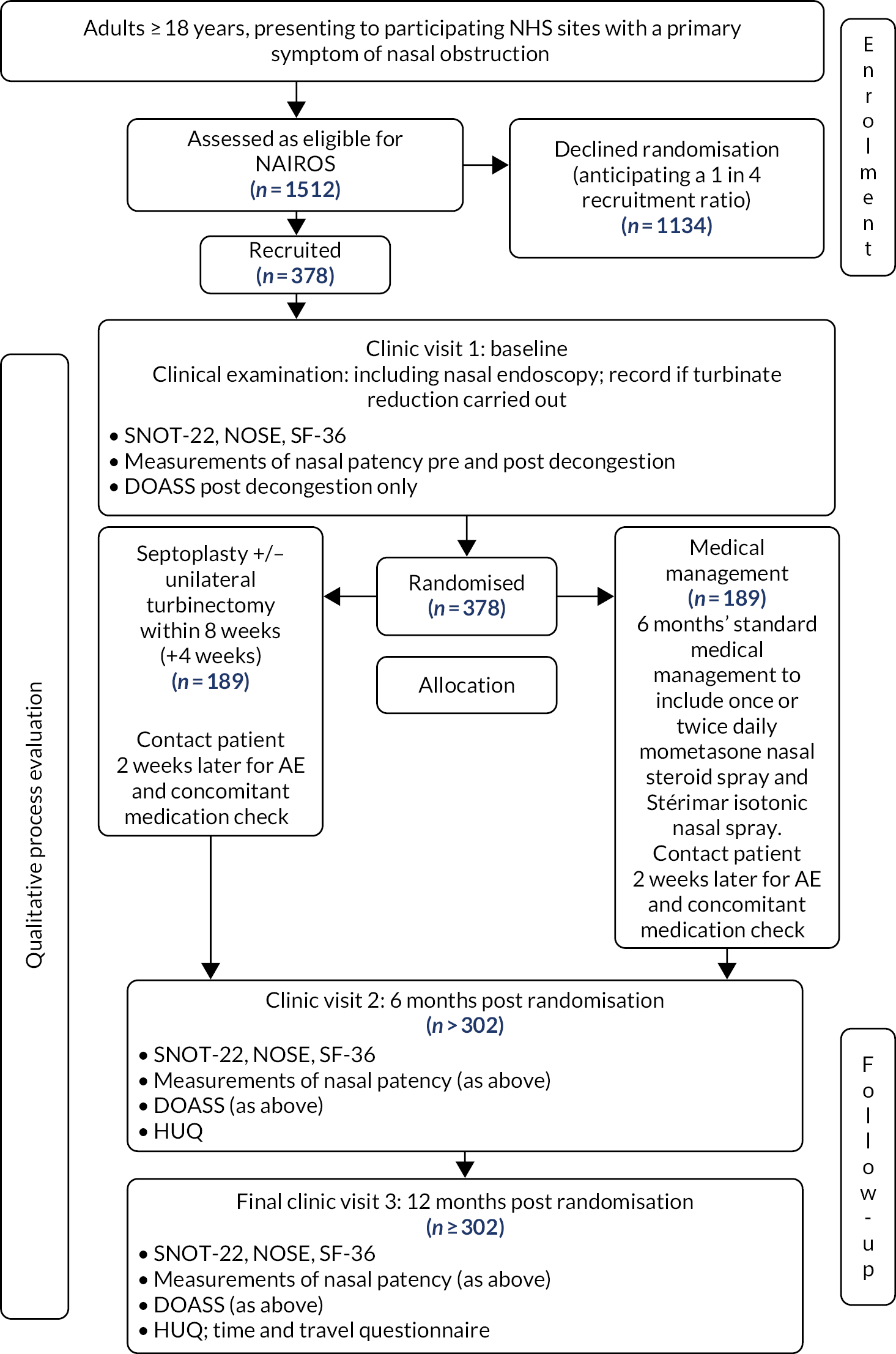
The NAIROS aimed to establish and inform guidance for the best management strategy for patients with nasal obstruction associated with a deviated septum by comparing a RCT of surgery with medical management.
Adult patients aged ≥ 18 years were identified from referrals to secondary care. Eligible patients who had septal deflection and a NOSE score of ≥ 30 were randomised to the trial. Randomisation occurred on a 1 : 1 basis, and was stratified by gender and severity (i.e. NOSE score) between septoplasty (with or without turbinate reduction), and medical management of combined isotonic saline nasal spray (Stérimar) and mometasone nasal spray.
The target recruitment figure of 378 participants from 17 NHS hospitals was achieved, with recruitment from sites across England, Scotland and Wales. Participants were followed up for 12 months post randomisation. The primary outcome was the total SNOT-22 score measured at 6 months post randomisation.
The trial included a qualitative mixed-methods process evaluation, which included a QRI to optimise recruitment during trial recruitment (see Chapter 5), and a qualitative process evaluation about staff and patient participants’ experiences of the study (see Chapter 6). It also included a full statistical evaluation (see Chapter 3) and an economic evaluation (see Chapter 4). Further details of the study design, clinical outcomes, economic outcomes and recruitment have been described previously. 80
Changes to trial design
Feasibility phase
The NAIROS researchers undertook an extensive assessment of the feasibility of the study by triangulating the views of potential patients, GPs and consultant ENT surgeons. This work assessed the willingness of patients to participate in randomisation, clinicians’ willingness to refer patients and randomly allocate to trial groups and the acceptability of the deferred surgery treatment arm.
Internal pilot
We originally intended for the NAIROS to include a 5-month internal pilot involving 10 sites. However, delays in study set-up expedited an agreement with the funder to remove the internal pilot and open all 17 sites simultaneously.
The pilot objectives of identifying, understanding and addressing any barriers to recruitment, retention or compliance with protocol were incorporated into the main trial, but extended to the full first year of recruitment. Areas for particular scrutiny during this period included patient recruitment, patient discontinuation of allocated treatment and compliance with surgery window. Monitoring of recruitment, retention and compliance with the protocol continued throughout the whole trial.
Collecting primary and secondary outcome measures
All trial interventions had been administered before the onset of the COVID-19 pandemic in March 2020. Participants in the trial follow-up were invited to complete the PROMs remotely via e-mail or post [i.e. SNOT-22, NOSE, healthcare utilisation questionnaire (HUQ), time and travel questionnaire and SF-36; see Report Supplementary Material 2, 4, 5 and 6]. The SNOT-22 (primary and secondary outcome measure) could also be completed by participants using a validated online platform hosted by Castor (Castor EDC, Amsterdam, the Netherlands); the method of completion of the SNOT-22 was noted in the database. Owing to suspension of all face-to-face clinic visits from 30 March 2020, no other clinical outcome measure scheduled after this date was collected [i.e. DOASS (see Report Supplementary Material 3), clinical examination, symptoms review, measures of nasal patency]. AEs and concomitant medications were collected remotely over telephone at the 6- and 12-month visits from 30 March 2020 until the end of the trial.
Trial registration and protocol availability
The NAIROS was included in the National Institute for Health and Care Research (NIHR) Clinical Research Network portfolio (study number 35368) and was registered on 24 March 2017 [European Union Drug Regulating Authorities Clinical Trials (EudraCT) number 2017-000893-12, International Standard Randomised Controlled Trial Number 16168569]. The protocol has been peer reviewed80 (version 5.0, dated 16 January 2019), and the final protocol version (version 8.0, dated 17 December 2020) is available on the project web page [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/ 1422607#/ (accessed 30 July 2021)].
Ethics approval and research governance
The trial sponsor was the Newcastle upon Tyne Hospitals NHS Foundation Trust (reference number 08302). Favourable ethics opinion was provided by the North East – Newcastle and North Tyneside 2 UK Health Research Authority Research Ethics Committee (REC) on 31 August 2017 (study reference number 17/NE/0239). The trial received approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) (study EudraCT number 2017-000893-12) on 17 August 2017.
Setting
The NAIROS was conducted in the following 17 secondary care hospital trusts in England, Scotland and Wales:
-
NHS Grampian (Aberdeen Royal Infirmary).
-
University Hospitals Birmingham NHS Foundation Trust (Queen Elizabeth Hospital Birmingham).
-
Bradford Teaching Hospitals NHS Foundation Trust (Bradford Royal Infirmary).
-
North Cumbria Integrated Care NHS Foundation Trust (Cumberland Infirmary).
-
County Durham and Darlington NHS Foundation Trust (Darlington Memorial Hospital/University Hospital of North Durham).
-
NHS Tayside (Ninewells Hospital).
-
James Paget University Hospitals NHS Foundation Trust (James Paget University Hospital).
-
NHS Lanarkshire (University Hospital Monklands).
-
Leeds Teaching Hospitals NHS Trust (Leeds General Infirmary).
-
Aintree University Hospitals NHS Foundation Trust (Aintree University Hospital).
-
Guy’s and St Thomas’ NHS Foundation Trust (Guy’s Hospital).
-
Newcastle upon Tyne Hospitals NHS Foundation Trust (Freeman Hospital).
-
Aneurin Bevan University Health Board (Royal Gwent Hospital).
-
University Hospitals Plymouth NHS Trust (Derriford Hospital).
-
Salisbury NHS Foundation Trust (Salisbury District Hospital).
-
Stockport NHS Foundation Trust (Stepping Hill Hospital).
-
Wrightington, Wigan and Leigh NHS Foundation Trust (Royal Albert Edward Infirmary).
Participants
We recruited 378 patients who had a deviated septum and reduced nasal airway (as indicated by a NOSE score of ≥ 30) and had been referred by their GP to ENT secondary care outpatient clinics. They were randomised in all 17 participating centres via dedicated research and standard NHS clinics. We also recruited ENT staff to participate in the process evaluation.
Eligibility criteria
Inclusion criteria
-
Adults aged ≥ 18 years.
-
Baseline NOSE score of ≥ 30.
-
Septal deflection at baseline visible via nasoendoscopy.
-
Capacity to provide informed written consent and to complete trial questionnaires.
Exclusion criteria
-
Any prior septal surgery.
-
Systemic inflammatory disease or the use of oral steroid treatment within the previous 2 weeks.
-
Granulomatosis with polyangiitis.
-
Nasoendoscopic evidence of unrelated associated pathology, for example adenoid pad, septal perforation, chronic rhinosinusitis indicated by polyposis or pus.
-
Any history of intranasal recreational drug use within the previous 6 months.
-
Breastfeeding, pregnancy or intended pregnancy for duration of involvement in the trial.
-
Bleeding diathesis.
-
Therapeutic anticoagulation (warfarin/novel oral anticoagulant therapy).
-
Clinically significant contraindication to general anaesthesia.
-
Known to be immunocompromised.
-
Presence detected of an external bony deformity likely to make a substantial contribution to the nasal obstruction (determined by clinical opinion).
The NOSE score is a validated five-item, unifactorial self-report of nasal-block severity that has been applied in previous research and audit studies. 36,81 The three recognised NOSE-derived categories of baseline severity used were 30–50 (moderate), 55–75 (severe) and 80–100 (extreme). The NAIROS aimed to recruit participants with at least moderate nasal obstruction symptoms.
Intervention
The interventions compared in the RCT were as follows:
-
surgical correction (septoplasty) of the nasal septal deviation, with or without unilateral reduction of the contralateral inferior nasal turbinate
-
medical management consisting of combined use of a nasal steroid spray and an isotonic saline spray for 6 months.
Delivery of the intervention
Septoplasty
Participants randomised to septoplasty received surgery up to 8 weeks (+ 4 weeks) after randomisation. The additional 4-week window was to allow for extenuating circumstances such as pressure on surgical waiting lists or patient cancellation, with reasons for delays to surgery collected and reported. The NAIROS stipulated that experienced surgeons who were not in training should perform the procedure. They had the option to carry out unilateral turbinate surgery on the wider side, according to their assessment of the individual patient airway. As a pragmatic study, the NAIROS did not ask surgeons to change their usual practice in this regard, reflecting the considerable variation in current UK surgical practice. Both the intention to reduce one turbinate prior to randomisation and details of the actual surgery performed were collected.
Clinical Trial of an Investigational Medicinal Product intervention: medical management
Participants randomised to medical management were supplied with 6 months of Stérimar isotonic nasal saline spray and mometasone furoate nasal steroid spray at the time of randomisation. Use of mometasone, an investigational medicinal product, was as indicated by the marketing authorisation. Twice per day for 6 weeks, each participant sprayed a daily metered Stérimar isotonic nasal saline dose into each nostril, followed by a twice-daily dose of mometasone furoate steroid spray. For the remainder of the 6-month period, the same dose of saline spray applied, and the steroid dose was reduced to 100 μg per day; participants could administer this either by spraying either two 50-μg doses into each nostril once daily or one 50-μg dose into each nostril twice daily.
Discontinuation of allocated treatment
Formal crossovers between trial arms were not permitted, but the NAIROS was designed as a pragmatic RCT; therefore, participants could discontinue allocated treatment and explore other options in standard NHS care while remaining in the trial. Medical management arm participants opting for surgical treatment were invited to defer surgery until after the 12-month follow-up visit and received standard non-trial septoplasty in line with current local waiting times (taking into account the time that they had already spent in the medical management arm). Discontinuation of allocated treatment did not constitute withdrawal from the trial.
Participants randomised to the surgical arm, or medical management participants who wished to continue using nasal sprays beyond the initial 6-month intervention period, could be prescribed mometasone furoate nasal spray and Stérimar isotonic spray (or an alternative at the discretion of the clinician) as per standard practice of the local NHS team and without the need for a NAIROS trial prescription.
Participants who wished to discontinue their allocated treatment but remain in the trial were followed up as scheduled for their allocated arm, with data analysed on an intention-to-treat (ITT) basis.
Funding of the trial intervention
As the surgical intervention was part of the standard pathway of NHS care for this cohort of patients, it was funded from the standard NHS tariff. The 6-month medical management intervention was categorised as an excess treatment cost and was funded by NHS England, Scotland and Wales.
Outcome measurements
Primary outcome
The primary outcome was defined as patient-reported assessment of nasal and general symptoms assessed using the SNOT-22 at 6 months (−2 weeks/+ 4 weeks). The SNOT-22 is scored from 22 questions with each item scored from 0 to 5. The final total score can range from 0 to 110, with a higher score indicating worse symptoms. The SNOT-22 was also assessed at baseline and 12 months.
Secondary outcomes
-
Objective assessment: nasal airflow was measured using a PNIF meter and an NV1 rhinospirometer (GM Instruments Ltd, Irvine, UK); both rhinospirometry (i.e. tidal volume and MIV) and PNIF measurements were taken at baseline and at 6 and 12 months. Additional exploratory analysis was undertaken using the rhinospirometer.
-
Quality of life was measured using the SF-36 quality-of-life questionnaire (acute/1-week recall) at baseline and at 6 and 12 months.
-
Healthcare resource use (primary and secondary care) was measured using a HUQ at baseline and at 6 and 12 months.
-
Patient-reported outcome measures: subjective – SNOT-22 subscales (rhinologic, sleep, ear/facial pain, psychological) at 12 months, NOSE scale at baseline and at 6 and 12 months, and DOASS at baseline and at 6 and 12 months were used to measure longer-term outcomes.
-
Safety measures: number and characteristics of any AEs and surgical complication/failure and reintervention within 12 months.
-
The economic evaluation compared the following between the two intervention arms:
-
costs [the average total cost per participant from the perspective of the NHS and Personal Social Services (PSS)]; sensitivity analyses included participant costs
-
QALYs, based on responses to the SF-36 converted to SF-6D scores, were derived using the area under the curve method82,83
-
improvement (of ≥ 9 points) in SNOT-22 scores
-
number of AEs
-
incremental cost per improvement (of ≥ 9 points) in SNOT-22 score
-
incremental cost in number of AEs avoided
-
incremental cost per QALY gained.
-
-
As part of the economic evaluation, costs and effects were extrapolated beyond 12 months using an economic model.
Economic analysis
The NAIROS economic analysis followed a prespecified health economics analysis plan, which outlined the analysis of the NAIROS trial data and aimed to determine the cost-effectiveness of septoplasty, compared with medical management, over 12 months and over a longer time horizon. Chapter 4 provides detail on the within-trial and longer-term economic models.
Overview of mixed-methods process evaluation
The NAIROS qualitative analysis incorporated the QRI, which aimed to support recruitment and mixed qualitative methods to understand participants’ and healthcare professionals’ experiences of septoplasty and medical management. The QRI took place throughout recruitment to the NAIROS, using qualitative and novel methods to investigate and address recruitment barriers (see Chapter 5). Qualitative interviews and focus groups were conducted throughout the trial to investigate participants’ and site staff members’ experiences of trial procedures, interventions and barriers to implementing findings into practice (see Chapter 6).
Overview of objective outcome measures and analysis
Nasal patency measurement and data collection protocols were devised through review of the scientific literature and the equipment manufacturer’s user information (GM Instruments) (see Report Supplementary Material 1), consultation with the NAIROS Trial Management Group (TMG), and consultation with a representative of the manufacturer.
Site staff were trained in these protocols in person during site initiation visits, were provided with a training video84 and had access to ad hoc support from Northern Medical Physics and Clinical Engineering and GM Instruments.
The NAIROS participants performed two types of objective measures of nasal patency both before and after decongestant: PNIF using a standard device [https://gm-instruments.com/products/nasal-measurements/pnif-meter (accessed 27 August 2021)], and rhinospirometry using an electromechanical/ software rhinospirometer device that measured airflow through each nostril independently [https://gm-instruments.com/products/nasal-measurements/nv1-rhinospirometer (accessed 27 August 2021)]. Rhinospirometry was performed during both MIV and tidal breathing. Measurements of nasal patency took place at all three trial visits (baseline and 6 and 12 months post randomisation). End volume (for the rhinospirometer) and flow (for the PNIF meter) values were read from the devices, recorded onto a case report form and then recorded onto the NAIROS database; these parameters are presented and discussed in Chapter 3.
Four baseline nasal patency parameters were assessed for potential inclusion in the sensitivity analyses (model 3; see Sensitivity analyses).
The four model-3 candidate baseline nasal patency parameters were derived from (1) post-decongestant PNIF and (2) flow rate time series data files saved using the rhinospirometry software, as follows:
-
absolute, post-decongestant, tidal breathing (NPR)
-
the change in absolute NPR following decongestant
-
post-decongestant, tidal breathing tidal volume (both sides combined)
-
post-decongestant, tidal breathing maximum flow rate (both sides combined).
MATLAB® software (version R2019; The MathWorks, Inc., Natick, MA, USA) was used to analyse rhinospirometry data files and extract the four model-3 candidate parameters. Calculations were validated by comparison to values in the manufacturer’s rhinospirometer software.
The nasal patency protocols and analyses of rhinospirometry data files to produce the four parameters are described in full in a NAIROS nasal patency measurements report (see Report Supplementary Material 1).
Participant timeline
Identification, screening and recruitment of participants
Although primary care clinicians were encouraged to refer patients with a deviated nasal septum directly to nasal research clinics, the majority of eligible patients were proactively identified by researchers from general ENT primary care referrals. Triage of paper referrals and scrutiny of ‘choose and book’ referrals were used to populate research clinics, or alternatively, research slots in general rhinology/ENT clinics. At the majority of sites, clinicians did not have the resources for dedicated research clinics. Nasal septal deviation can be challenging to diagnose in primary care; therefore, the participants selected to attend research clinics were chosen at the clinicians’ judgement.
Screening for study eligibility was maximised by training all staff involved. Potential participants, whenever possible, were sent the participant information sheet (PIS) with their clinic appointment details and were directed to the patient information video available on the trial website. 85 All patients were given a minimum of 24 hours after receiving the PIS to decide whether or not they wished to participate.
Patients were invited to provide written informed consent for the study in three stages, after they had been given sufficient time to consider the trial and had the opportunity to have any questions addressed by the local clinical team.
First, patients were invited to provide consent to undergo screening assessments to determine eligibility for inclusion in the trial.
Eligibility assessments
-
Pre-randomisation NOSE scale.
-
Age.
-
Baseline recording of four core features including endoscopy (without decongestion):
-
the side of the convexity
-
the site of deflection (whether anterior/posterior/upper/lower or all)
-
nasal endoscopy findings to look for evidence of exclusion criteria (e.g. pus/polyps)
-
whether the extent of the observer-rated airway obstruction by the septum was < or > 50% at endoscopy.
-
Second, patients were invited to provide consent for their discussion about the NAIROS trial to be audio-recorded and for their contact details to be shared with qualitative researchers for a potential telephone interview.
Third, eligible patients were invited to provide consent for the main trial. The following assessments were undertaken only once thereafter.
Assessments pre randomisation
-
The SF-36.
-
The SNOT-22.
-
Measurements of nasal patency pre and post decongestion:
-
PNIF (measured by the PNIF meter)
-
rhinospirometry, allowing calculation of NPR
-
the DOASS (post decongestion only).
-
Sites were instructed to use xylometazoline hydrochloride (Otrivine Congestion Relief 0.1% Nasal Spray®; GlaxoSmithKline plc, Brentford, UK) nasal spray as the decongestant.
Eligible patients who declined the main trial
To facilitate baseline analysis of the NAIROS trial participants with eligible patients who declined to participate, those in the latter group were invited to provide written consent to collect the following baseline data:
-
SNOT-22 score
-
NOSE score
-
intention to reduce turbinate
-
baseline recording of four core features including endoscopy (without decongestion) –
-
the side of the convexity (laterality)
-
the site of deflection (whether anterior/posterior/upper/lower or all)
-
endoscopy findings to look for evidence of exclusion criteria (e.g. pus/polyps)
-
whether the extent of the observer-rated airway blockage by the septum was < or > 50% at endoscopy.
-
-
age
-
gender
-
reasons for declining.
Screening data, including the number of participants approached, reasons for ineligibility, those interested in taking part and reasons for declining participation, were collected via a log completed by site staff conducting screening. The intention was to compare the NAIROS trial participants to the total pool of those referred at each participating site.
Randomisation
Participant allocation
At the baseline visit, consenting, eligible patients were randomised on a 1: 1 basis using the centrally administered Newcastle Clinical Trials Unit (NCTU) web-based system. Randomisation was by random permuted blocks of variable length, stratified by gender and three recognised NOSE-derived categories of baseline severity as defined previously in Eligibility criteria. The treatment allocation was open-label, with the randomisation system providing a unique trial identifier for each participant.
Withdrawal
Participants had the right to withdraw from any element of the RCT at any time without having to give a reason. Participants who withdrew their consent or were withdrawn by the investigator from the trial were not replaced. All data collected until withdrawal were retained for data analysis.
Schedule of events
Participants recruited to the main trial were followed up for 12 months from the point of randomisation (Figure 2).
FIGURE 2.
The NAIROS participant flow diagram. Reproduced from version 8.0 of the NAIROS protocol (17 December 2020) [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1422607#/ (accessed 30 July 2021)].
Septoplasty participants
The surgeon’s intention to reduce the inferior turbinate was recorded prior to septoplasty and the following information was recorded at the time of surgery:
-
the date of surgery
-
duration in theatre
-
grade of senior surgeon and senior anaesthetist present
-
whether septoplasty, with or without unilateral inferior turbinate reduction, was carried out
-
technical aspects of the surgery
-
discharge medication
-
whether or not any complications occurred
-
whether or not there was any overnight hospital admission.
Medical management participants
As the NAIROS was a pragmatic trial of standard treatment, formal participant compliance with the medical management intervention was not formally assessed. However, quantities of bottles used per participant and reasons for ceasing treatment were recorded.
Follow-up
Participants were contacted by either telephone, e-mail or text 2 weeks after randomisation (medical management) or 2 weeks after their septoplasty, to record any AEs and concomitant medication. Medical management participants were reminded to reduce their dose of mometasone nasal spray at 6 weeks.
Six months after randomisation (− 2 weeks/+ 4 weeks)
Assessments performed are detailed in the trial schedule of events (Table 1). The 6-month follow-up visit was scheduled to allow a minimum of 12 weeks’ recovery from septoplasty surgery.
| Procedures | Pre screening | Screening/consent/pre randomisation (visit 1) | Contact patient 2 weeks after randomisation (± 14 days) | Septoplasty [must occur any time up to 8 weeks (+ 4 weeks) after randomisation] | Contact patient 2 weeks after surgery (± 14 days) | 6 months (− 2 weeks/+ 4 weeks) (visit 2) | 12 months (± 2 weeks) (visit 3) |
|---|---|---|---|---|---|---|---|
| PIS given to patients referred to NAIROS clinic when appointment made | ✓ | ||||||
| Eligibility assessment | ✓ Pre randomisation | ||||||
| Demographics (sex and age) | ✓ Pre randomisation | ||||||
| Medical history | ✓ Pre randomisation | ||||||
| Informed consent (must take place prior to any study-specific activities) | ✓ Pre randomisation | ||||||
| Eligibility confirmed | ✓ Post consent and pre randomisation | ||||||
| Clinical examination [includes nasal endoscopy (without decongestion) and baseline recording of four core featuresa] | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| SNOT-22 | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| NOSE | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| DOASS (post decongestion); only for patients consenting to the main trial | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| Measurements of nasal patency (see Report Supplementary Material 1 for further information) (only for patients consenting to the main trial) | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| SF-36 (only for patients consenting to the main trial) | ✓ Post consent and pre randomisation | ✓ | ✓ | ||||
| HUQ | ✓ | ✓ | |||||
| Randomisation (following complete assessments) | ✓ | ||||||
| Medical management arm: dispensing of trial drugs (only if randomised to medical management arm); 6-month supply of Stérimar isotonic spray and mometasone given | ✓ | ||||||
| IMP and Stérimar usage (number of bottles used) | ✓ | ||||||
| Septoplasty arm (must occur any time up to 8 weeks (+ 4 weeksb) after randomisation) | ✓ | ||||||
| Post-surgery CRF | ✓ | ||||||
| Feedback on patient well-being. Contact can be made via telephone, text or e-mail | ✓ | ✓ | |||||
| Record technical failures from those operations in which widening of the nasal airway has been achieved, yet the patient’s symptoms persist | ✓ | ✓ | |||||
| If a participant did not attend the follow-up visit, telephone to remind them to complete/post SNOT-22c | ✓ | ✓ | |||||
| Time and travel questionnaire | ✓ | ||||||
| AE assessments | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Concomitant medications | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
For participants allocated to the surgical arm, any complications from the septoplasty were recorded.
Twelve months after randomisation (± 2 weeks)
Assessments at the 12-month follow-up visit are detailed in the schedule of events (see Table 1).
At both the 6- and 12-month visits, participants were given the option to discontinue allocated treatment and explore treatment options available within standard local NHS care.
Serious adverse event reporting
Adverse events were recorded from date of randomisation until the end of trial participation (at visit 3, 12 months post randomisation), at every trial visit and during the safety telephone calls described previously. AE severity was assessed by the investigator as mild/moderate/severe and assessed for causality and expectedness by reference to the Reference Safety Information. The Reference Safety Information for surgery (expected AEs) was documented in the protocol. The Reference Safety Information for the medical management arm intervention was section 4.8 of the approved summary of product characteristics for NASONEX® 50 μg/actuation (mometasone furoate) nasal spray (Merck Sharp & Dohme Corp., Rahway, NJ, USA). There were no known drug interactions listed in the approved summary of product characteristics.
Serious adverse events (SAEs) were reported to the NCTU and to the sponsor on a trial-specific report within 24 hours of the site becoming aware of the event and followed up until resolution. SAEs were reported until the end of the trial. All SAEs were summarised in the annual development safety update report to the MHRA and in the annual Clinical Trial of an Investigational Medicinal Product (CTIMP) safety report to the relevant REC.
Suspected unexpected serious adverse reactions (SUSARs) among participants in both arms underwent expedited reporting to the REC. Only SUSARs in the medical management arm required expedited reporting to the MHRA.
Definition of the end of the trial
The trial end was defined as the collection date of the final participant’s 12-month follow-up data.
Patient and public involvement
Patient and public involvement was integrated into the design, conduct and outcome stages of the study. Substantial PPI input was sought in the design of the study. Initially, 21 patients were consulted shortly before undergoing septoplasty and asked about their symptoms, about their willingness to be randomised, and for feedback on the NOSE and SNOT-22. Two-thirds of patients preferred the SNOT-22, which better matched their symptoms (in particular, the NOSE omitted snoring and headache, which were felt to be important).
Of key concern to the patients were details of the treatment received in the medical arm and whether or not randomisation to this arm precluded them from future surgery. This first phase of PPI was used to design a trial outline, which was discussed with a further 18 outpatients with nasal obstruction. During the second phase of the PPI, we were able to adjust the time for which surgery would be deferred in the control arm, and the acceptability of the nature and timing of the outcome measures. Additional input was obtained during the development of patient experience.
On receipt of funding, a PPI panel was convened, with participants recruited via ENT clinics and VOICE (URL: www.voice-global.org). Recruitment materials for the panel outlined details of the study, the expected time commitment and reimbursement for time and expenses. A member of the panel presented a patient perspective on septoplasty and the NAIROS trial, at the NAIROS launch event. Two PPI meetings with five panel members were held during study set-up to obtain input into the development of the recruitment strategy and other study processes (e.g. arrangements for participants who wished to discontinue their allocated treatment); feedback was also obtained on drafts of the PIS, consent form and recruitment video.
Subsequently, PPI panel input regarding specific patient-facing trial materials, including the trial website and the thank-you letter for trial participants, was obtained via e-mail on a more ad hoc basis. At each subsequent contact, before requesting any further input, a short update on trial progress was provided.
The Trial Steering Committee (TSC) included an independent PPI member. The TSC met regularly to review study documentation, including patient-facing documents and lay language text, and to ensure that the trial was conducted in a way that was considerate of the needs and wishes of participants.
Statistical considerations
The trial analysis followed a statistical analysis plan (SAP) (version 2.0, dated 25 March 2021). There was no formal interim analysis, only a single analysis after the database was locked on 29 January 2021. Decisions regarding the continuation of the trial were made at Data Monitoring Committee (DMC) meetings every 6 months.
Analyses are reported according to the Consolidated Standards of Reporting Trials (CONSORT) recommendations and were conducted in the validated statistical software package Stata®, version 16 (StataCorp LP, College Station, TX, USA).
Sample size calculation
The sample size calculation was based on a t-test for superiority assuming equal variance across groups, a conservative approach given the primary analysis was based on adjustment for the baseline values of SNOT-22, which generally increases statistical power. The target recruitment number of 378 participants allowed for a 20% drop-out rate (based on experience from two prior septal surgery audits). 86,87 The remaining 302 participants (151 per arm at completion) would be sufficient to deliver 90% power to detect a 9-point difference in the overall SNOT-22 score between arms. This assumed a 5% type I error rate and a standard deviation (SD) of 24 points. The MCID of 9 units was informed by relevant literature; it was felt to be the most relevant and a change of 8.9 units had been identified as being clinically meaningful. 43,45,46,88,89 The same literature was used to guide assumptions about what would be a reasonable value for the SD for the design parameter. We took a conservative approach as we assumed that the SD would be 24 points, which was the largest value reported. 43,45,46,88,89
Statistical analysis plan
Primary outcome measure
The primary outcome measure was the SNOT-22 score at 6 months. SNOT-22 scores were recorded at baseline and at 6 and 12 months post randomisation. Baseline and follow-up data were summarised using appropriate statistics and graphical summaries. Box plots (e.g. Figure 6) show summary statistics of the measurement they represent. The box represents the middle 50% of the data (lower quartile to upper quartile), the line within the box shows the median (50th percentile), the whiskers show data that fall within 1.5 × the interquartile range (IQR) and the points show data that fall outside these limits. SNOT-22 questionnaires with up to 20% of items missing were imputed, with the average of the completed questions used for missing items.
Summary statistics of overall scores, including means with associated 95% confidence intervals (CIs), are presented by treatment group and overall in Table 6.
Defining the populations for analysis
The following analyses by population were undertaken:
-
Intention-to-treat group – all ineligible and protocol-violator participants included in the analysis on an ITT basis, with participants kept in their randomised treatment group. This included outcome measures completed at any time.
-
Compliant ITT group – all participants in the ITT group, but complying with questionnaires completed within the 6-month (− 2 weeks/+ 4 weeks) return window with no consideration given to septoplasty status.
-
Per-protocol group – all participants who received the treatment they were randomised to and complied with protocol in terms of timings and compliance windows for the surgery and primary end point (6-month visit/SNOT-22 completion). This excluded participants randomised to septoplasty but who did not receive septoplasty within 12 weeks of randomisation, participants randomised to medical management who had actually received standard care septoplasty before the 6-month + 4-week primary end point, and participants whose primary end point was completed outside the compliance window.
-
Per-treatment group – this was similar to the per-protocol group, with some additional participants included. The medical management group was exactly the same as in the per-protocol group. Any participant who was randomised to septoplasty and received their septoplasty at least 10 weeks before the primary end point was included in the septoplasty group. In addition, any participant randomised to medical management but who received septoplasty at least 10 weeks before the primary end point was completed was included in the septoplasty group.
-
Non-randomised group – those eligible to be included in the NAIROS trial but who declined to take part. We planned to compare the non-randomised group with those consenting to take part in the trial; 45 patients agreed to join the non-randomised group and allow their data to be collected and analysed. However, owing to the small number of patients who agreed to join the non-randomised group (only 19 of whom provided NOSE data), a meaningful comparison between this group and those consenting to the trial could not be made and these data were not included in this report.
Analysis of the primary outcome
Primary analysis
The primary analysis was conducted by comparing scores of the two randomised treatment arms (immediate septoplasty and medical management) at 6 months. This analysis used multivariable linear regression.
The associated magnitude and significance of any between-arm differences were calculated in a multivariate regression model (referred to as model 1), adjusting for baseline severity SNOT-22 score as a continuous covariate and stratification factors at randomisation [(1) gender and (2) severity at baseline assessed by the NOSE].
Residual analysis was conducted to assess the goodness of fit of model 1. Model 1 is reported fully (see Chapter 3).
Sensitivity analyses
A number of sensitivity analyses of the primary analyses were conducted. The models for these analyses are outlined below:
-
model 2 – adjusting for continuous baseline NOSE score, rather than the three categories used at baseline, to utilise the full information from the continuous measure
-
model 3 – a series of multivariable analyses to further allow consideration of other important baseline factors in the regression model. This included age, ethnicity, site (as a random effect), smoking history, baseline levels of trial questionnaires (DOASS, endoscopy findings) and the four selected nasal patency variables.
Within the model 3 frame, non-linear relationships with continuous baseline covariates were explored for simple and suitable first order, and more complex fractional polynomial transformations, which were applied when appropriate. Building the optimal model for model 3 was based on a forward selection method (change in −2log likelihood, compared against a chi-squared distribution to assess variable inclusion). Variables were initially assessed using univariable regression against the primary outcome measure before they were included in the forward selection process; any variable with p > 0.1 was included in the forward selection process. The results of this first assessment, of all considered variables along with identified transforms, are presented in Appendix 1, Table 48. Significant variables at 5% level were retained in the final model (p < 0.05). At the end of the forward selection procedures, if any of the included covariates became non-significant (p > 0.05), the impact of removing them from the final model was assessed. Improved model suitability was assessed using Akaike information criterion, which estimates the quality of each model relative to the other models, thereby providing a means for model selection. The aim was to derive the most parsimonious model. The details of the full final model 3 can be found in Appendix 1, Table 48.
Secondary analyses
Secondary analyses of the primary outcome were performed by limiting the analysis to the specific populations: compliant ITT, per protocol and per treatment. Multiple imputation was used to include all patients who consented and were randomised, including those with missing SNOT-22 scores at the primary end point. Missing data were imputed using multiple imputation with the proportion of missing data in each group reported and compared descriptively (see Report Supplementary Material 6). Descriptive statistics of baseline variables are presented by treatment group and missing data status (with and without primary end point data, i.e. SNOT-22 score at 6 months). Baseline variables found to be predictive of missing data status are included in multiple imputation equations.
We used multiple imputation to minimise bias, to maximise use of available information and to obtain appropriate estimates of uncertainty. One thousand multiple imputation data sets were created in Stata 16 using chained equations. The multiple imputation equation includes baseline data on gender; NOSE categories and baseline SNOT-22 score; and predictors of missing data to make the missing-at-random assumption as plausible as possible. A conservative approach was adopted, and treatment group was included in the imputation model.
Secondary outcome measures
The analysis of secondary outcomes followed a broadly similar strategy to the primary outcome measure. Secondary outcomes included data at the 6-month follow-up from the other outcomes [i.e. NOSE, DOASS, PNIF (maximum of three measurements), NPR from MIV (using mean volumes from three measurements) and tidal breathing] and data for all outcomes at the 12-month follow-up. SNOT-22 subscales (rhinologic, sleep, ear/facial pain, psychological) at the three time points are presented.
Summary statistics and graphical representation of subjective scales were tabulated at randomisation and at 6 and 12 months’ follow-up, both by intervention arm and collectively. Multiple regression was used to compare the outcome scores between treatment groups at follow-up time points. Variation between sites was included as a random effect with an assumed normal distribution, with analysis including the stratification factors of baseline severity and gender. Further adjusted analyses included terms for baseline values of the scores and key demographic and clinical covariates.
Adverse events were tabulated according to the World Health Organization Common Terminology Criteria for Adverse Events, version 4.03, with the number of severe AEs (Common Terminology Criteria for Adverse Events grade 3, 4 or 5) reported as a proportion of all AEs. The number of participants experiencing at least one severe AE according to the Common Terminology Criteria for Adverse Events was reported as a proportion of all participants. Surgical complication/failure and reintervention were described and not subjected to statistical testing. Technical failures (defined as occasions when the participant self-reported symptoms remaining the same or worsening, along with surgeon opinion on whether revision surgery was required) from operations in which widening of the nasal airway was achieved were reported. Complications experienced as a result of the septoplasties are also presented in the results chapter.
We carried out a subgroup analysis for participants who were recommended to receive the inferior turbinate reduction. This led to four groups: as randomised, recommended for turbinate reduction or not. This broadly followed the primary analysis, but analysis was carried out separately for each subgroup. Subgroups by gender are also reported.
Subpopulation analyses
The subpopulation treatment effect pattern plot (STEPP) analysis90–93 approach was developed to allow researchers to investigate the heterogeneity of treatment effects on outcomes across values of a (continuously measured) covariate. STEPP is a graphical tool which allows one to visualise treatment differences, and will be useful for guiding patients and clinicians when making decisions regarding treatment choice.
The importance of baseline severity as a continuous distribution of NOSE score at randomisation was further explored graphically by STEPP analysis to display the predicted point estimates of any treatment effect (with 95% CIs) over the range of NOSE values (which ranged from 30–100 among NAIROS participants), with the aim of further informing any patient selection guidance and recommendations. The STEPP analysis was also carried out for overlapping ranges of NOSE score separately by gender.
Data monitoring, quality control and assurance
The NCTU was delegated by the sponsor to monitor trial conduct and data integrity to ensure that the trial was conducted in accordance with the protocol and the latest directive on good clinical practice (2005/28/EC). 94 All final statistical and health economics analyses were reviewed for quality assurance by independent researchers.
Trial management and oversight
The sponsor delegated day-to-day management of the trial to the NCTU and the TMG, which met approximately monthly. External, independent oversight of the trial was provided by an independent DMC and a TSC who reviewed the SAP. Details of these committees and trial monitoring have previously been described in the published protocol paper. 80 Terms of reference and trial oversight charters described roles and responsibilities of individual committees. Members were required to sign the relevant terms of reference or trial oversight charter, declaring any conflict of interest. The TSC met at least annually after the DMC meeting.
Chapter 3 Results
The analysis presented here is reported according to the CONSORT flow diagram (see Figure 3) and is based on the SAP version 2.0 (25 March 2021). SAP version 1.0 (1 February 2021) was approved immediately before data download. A further minor clarification on the definitions of ‘per-protocol’ and ‘per-treated’ analyses populations was required in the SAP following data download; hence, version 2.0 was used for the analysis. The SAP provided guidelines for the analysis of the NAIROS trial data. Any analyses that were not prespecified in the SAP are denoted as ‘unplanned’.
Recruitment
-
Number of sites: 17.
-
Date first site opened: 18 January 2018.
-
Date first participant randomised: 26 January 2018.
-
Total number of participants randomised: 378.
-
Date last participant recruited (consented): 5 December 2019.
-
Date last participant randomised: 5 December 2019.
-
Date of last participant follow-up: 17 December 2020.
-
Date of final data set download: 3 February 2021.
Randomisation and stratification factors
The trial recruited to target. Individual randomisation to the two trial arms was stratified by gender and NOSE category (see Chapter 2). The numbers of participants randomised by strata are presented in Table 2.
| Stratification factor | Trial arm, n (%) | Total (N = 378), n (%) | |
|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | ||
| Moderate/male | 21 (11) | 22 (12) | 43 (11) |
| Moderate/female | 9 (5) | 10 (5) | 19 (5) |
| Severe/male | 60 (32) | 61 (32) | 121 (32) |
| Severe/female | 29 (15) | 28 (15) | 57 (15) |
| Extreme/male | 45 (24) | 44 (23) | 89 (24) |
| Extreme/female | 24 (13) | 25 (13) | 49 (13) |
| Total | 188 (100) | 190 (100) | 378 (100) |
Two-thirds of randomised participants were males. The relative frequencies of NOSE severity levels were 16% for moderate, 47% for severe and 37% for extreme.
Treatment allocation by stratification factors for the ITT population is presented in Appendix 1, Table 25.
Site recruitment activity is presented in Appendix 1, Table 26.
Participant flow: Consolidated Standards of Reporting Trials flow diagram
Recruitment and participant flow through the trial is reported in the CONSORT diagram (Figure 3).
FIGURE 3.
The CONSORT flow diagram. a, Discontinued, no surgery (n = 8); b, discontinued, had surgery (n = 1); c, discontinued, no surgery (n = 1); d, discontinued, had surgery (n = 5).
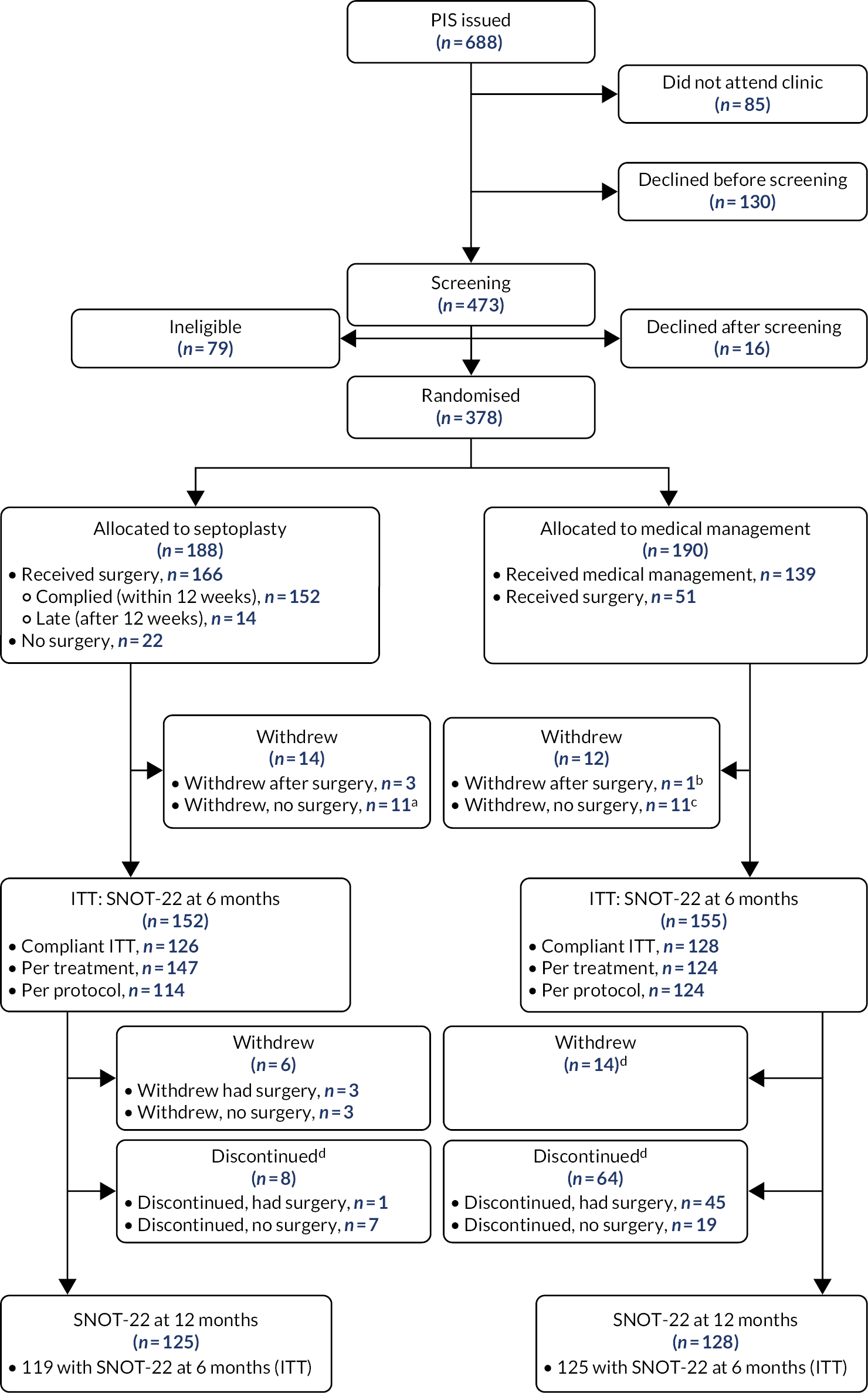
Appendix 1, Table 27, summarises weeks from randomisation to withdrawal from trial. Appendix 1, Table 28, presents a line listing of reasons for withdrawing.
As can be seen from the CONSORT diagram (see Figure 3), 16 participants randomised to septoplasty and 71 participants randomised to medical management discontinued their allocated treatment. Details of the reasons for treatment discontinuation can be found in Appendix 1, Tables 29 and 30 (septoplasty arm) and Tables 31 and 32 (medical management arm). The most common reasons given by medical management participants for discontinuing were ‘not happy with the sprays’/‘side effects of sprays’ [reported by 76 out of 98 (78%) participants] and worsening symptoms [reported by 15 out of 98 (15%) participants].
Baseline demographic data
Demographic and baseline characteristics (Table 3) show that, overall, 67% of participants were male, 88% were white and the average participant age was 39.8 years.
| Demographic and NOSE score | Trial arm | Total (N = 378) | |
|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | ||
| Gender, n (%) | |||
| Male | 126 (67) | 127 (67) | 253 (67) |
| Female | 62 (33) | 63 (33) | 125 (33) |
| Age (years) | |||
| Median (IQR) | 38 (27.5–51) | 37 (28–50) | 38 (28–50) |
| Mean (SD) | 40.3 (14.9) | 39.4 (13.9) | 39.8 (14.4) |
| Minimum, maximum | 18, 79 | 18, 80 | 18, 80 |
| Ethnic group, n (%) | |||
| White | 169 (90) | 165 (87) | 334 (88) |
| Asian (Indian/Pakistani/Bangladeshi ancestry) | 13 (7) | 14 (7) | 27 (7) |
| Other Asian | 1 (< 1) | 2 (1) | 3 (< 1) |
| Other ethnic origin | 3 (2) | 9 (5) | 12 (3) |
| Missing | 2 (1) | 0 (0) | 2 (< 1) |
| Baseline NOSE score (continuous) | |||
| Median (IQR) | 70 (60–82.5) | 70 (60–85) | 70 (60–85) |
| Mean (SD) | 69.9 (17.4) | 71.3 (17.3) | 70.6 (17.4) |
| Minimum, maximum | 30, 100 | 30, 100 | 30, 100 |
Summary tables of baseline medical history (see Table 33), clinical examination (see Table 34), and endoscopy findings (see Table 35) are presented in Appendix 1. Appendix 1, Table 36, shows the range of timings for decongestant in relation to when the nasal patency measurements were taken (there are validity requirements for ‘post-decongestant measurement start time’ to be at least 5 minutes after ‘time decongestant spray given’, and for ‘post-decongestant measurement end time’ to be within 60 minutes of ‘time decongestant spray given’).
Outcome data quality and completeness
A small number of returned questionnaires were only partially completed. Four SNOT-22 questionnaires had missing items at baseline, five had missing items at the primary end point of 6 months and four had missing items at the final 12-month data collection point. All of these were imputed by using the mean of the completed items as each had < 20% missing. One participant had one item missing from their NOSE questionnaire (20%) at the 6-month follow-up visit. This was also imputed using the mean of the other four responses.
Appendix 1, Table 37, shows data completeness in terms of the number of questionnaires returned at baseline and at the 6- and 12-month follow-up time points for the analysis groups, with an indication of numbers with partial or completely missing questionnaires.
Trial analysis populations
The full details of the populations used for the statistical analysis are defined in Chapter 2.
Table 4 displays analysis populations, based on compliance with the allocated intervention and the primary end point.
| Randomised arm | Complied with allocated interventiona | Primary end point statusb | Protocol compliance | Number of participants (N = 378) | Withdrew (n) | ITT (n) | Compliant ITT (n) | Per protocol (n) | Per treatment (n) |
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty | Complied | Complied | Complied | 114 | 1 | 114 | 114 | 114 | 114 |
| Septoplasty | Complied | Did not comply | Did not comply | 23 | 2 | 23 | 0 | 0 | 23 |
| Septoplasty | Complied | No primary end point received | Did not comply | 15 | 3 | 0 | 0 | 0 | 0 |
| Septoplasty | Did not comply | Complied | Did not comply | 8 | 0 | 8 | 8 | 0 | 8 |
| Septoplasty | Did not comply | Did not comply | Did not comply | 3 | 0 | 3 | 0 | 0 | 0 |
| Septoplasty | Did not comply | No primary end point received | Did not comply | 3 | 0 | 0 | 0 | 0 | 0 |
| Septoplasty | No surgery | Complied | Did not comply | 4 | 1 | 4 | 4 | 0 | 0 |
| Septoplasty | No surgery | No primary end point received | Did not comply | 18 | 13 | 0 | 0 | 0 | 0 |
| Total septoplasty | 188 | 20 | 152 | 126 | 114 | 145 | |||
| Medical management | Complied | Complied | Complied | 89 | 3 | 89 | 89 | 89 | 89 |
| Medical management | Complied | Did not comply | Did not comply | 19 | 3 | 19 | 0 | 0 | 0 |
| Medical management | Complied | No primary end point received | Did not comply | 31 | 14 | 0 | 0 | 0 | 0 |
| Medical management | Did not comply (received surgery) | Complied | Did not comply | 39 | 4 | 39 | 39 | 35 | 35 + 2c |
| Medical management | Did not comply (received surgery) | Did not comply | Did not comply | 8 | 1 | 8 | 0 | 0 | 0 |
| Medical management | Did not comply (received surgery) | No primary end point received | Did not comply | 4 | 1 | 0 | 0 | 0 | 0 |
| Total medical management | 190 | 26 | 155 | 128 | 124 | 124 + 2* | |||
| Trial total | 378 | 46 | 307 | 254 | 238 | 271 |
The primary analysis for this trial is on the ITT population with the primary outcome, SNOT-22, collected at the 6-month follow-up visit. The randomised participants who completed the SNOT-22 at 6 months [n = 307 (81%)] (see Table 4) comprise the ITT population.
Compliance, treatment received and numbers analysed
Appendix 1, Table 38, shows when the SNOT-22 questionnaires were completed in relation to the visit window of −2/+4 weeks stated in the protocol. Compliance with SNOT-22 completion is balanced between the two arms, with 83% of the ITT population complying with the primary end point visit window at 6 months, and 66% of those attending the 12-month visit completing the SNOT-22 questionnaires within the compliance window. Table 4 summarises key features of participants’ trial pathways and provides numbers for each of the specified analysis populations.
Consultant surgeons carried out 128 out of the 166 (77%) septoplasties undertaken among participants randomised to the septoplasty arm. Seventeen (10%) septoplasties were carried out by associate specialists and 16 (10%) septoplasties were carried out by surgeons of other grades. The records of five (3%) participants did not include the grade of the most senior operative surgeon. Further operative details can be found in Appendix 1, Tables 39 and 40.
The average septoplasty duration was 56 minutes. Data were available for 159 of the 166 septoplasty participants. Summary statistics are presented in Appendix 1, Table 41.
Details of participant compliance with the medication of the medical management arm (nasal spray usage) are provided in Appendix 1, Tables 42 and 43. When asked ‘Over the last month have you used the NAIROS medication (nasal steroid and/or saline sprays)?’, 122 (64%) participants confirmed that they had. The quantities of bottles used are also presented in Appendix 1, Tables 42 and 43. Sixty-nine (36%) participants provided this information for saline and 65 (34%) participants provided this information for steroids. The median number of saline spray bottles used was 3.5 (IQR 2.5–5) (n = 69). The median number of steroid spray bottles used was 4 (IQR 3–5.5) (n = 65). The reasons for participants ceasing use of the sprays are summarised in Appendix 1, Tables 42–44.
Time from randomisation to septoplasty
Figure 4 displays time to surgery for participants randomised to the septoplasty arm and for whom primary end point data were collected (ITT population). The red solid line shows the 8-week compliance window for septoplasty. The red hatched line shows the additional 4-week window that allowed for delays in delivering septoplasty in extenuating circumstances.
FIGURE 4.
Distribution of time from randomisation to septoplasty.

Most participants [166 out of 188 (88%)] randomised to septoplasty did receive the operation (see Table 4). Of these 166 participants, 148 (89%) were included in the ITT population, meaning that they had primary outcome data collected. Of these 148 participants in the ITT population, 137 (93%) received septoplasty within the compliance window.
Figure 5 shows the distribution of time elapsed from point of randomisation to septoplasty carried out for the ITT population originally randomised to the medical management arm who discontinued treatment and opted to receive non-trial septoplasty.
FIGURE 5.
Distribution of time from randomisation to surgery for the ITT population participants randomised to the medical management arm who discontinued treatment and opted to receive non-trial septoplasty [n = 46 (24%)].
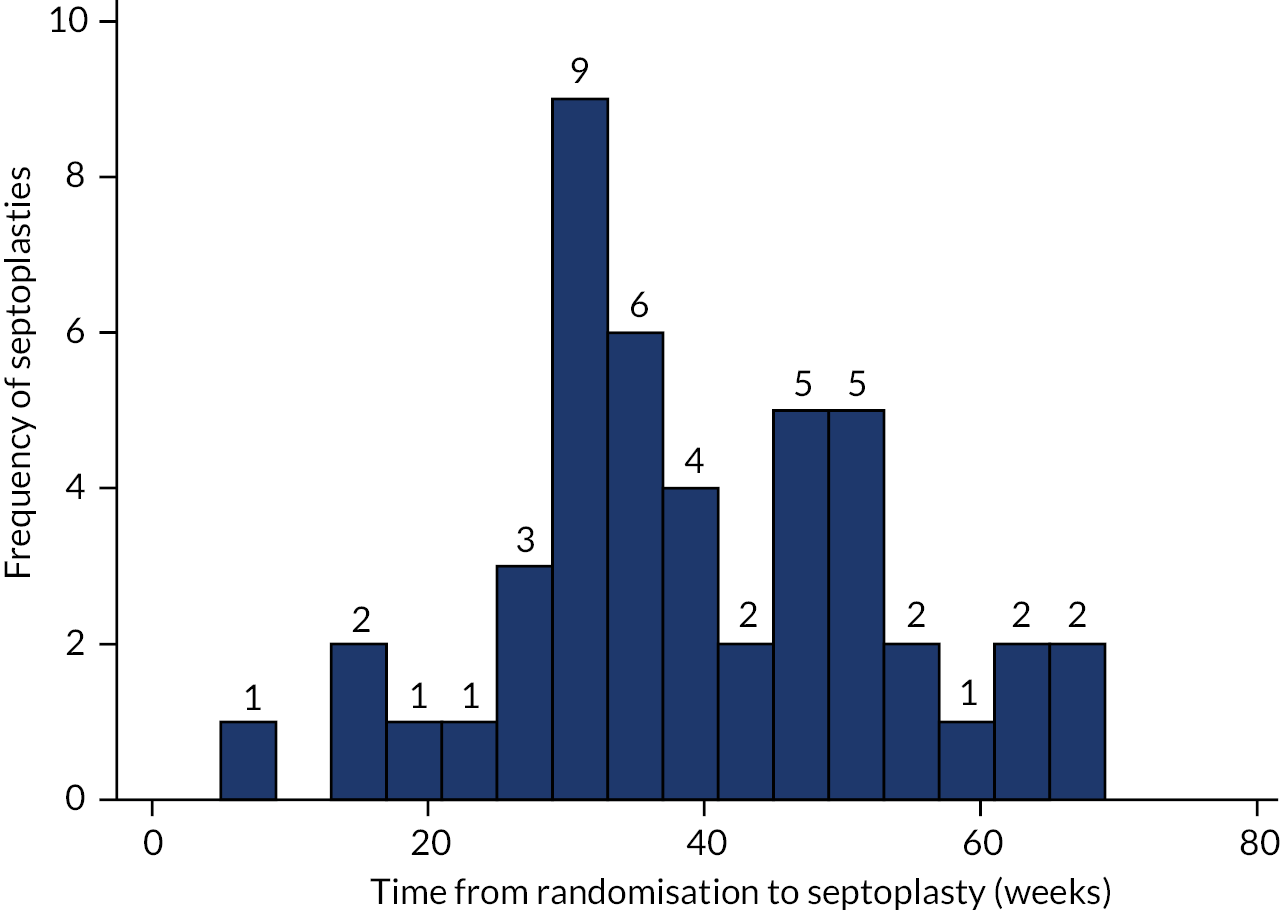
Six NHS septoplasties for participants discontinuing the medical management treatment were carried out beyond the end of the trial follow-up period (12-month visit + 2 weeks). For all presented analyses, these participants were classified as not receiving septoplasty.
Descriptive analysis of the primary outcome measure, the Sino-nasal Outcome Test-22 items
The SNOT-22 was measured at baseline and at 6 and 12 months. The distribution of scores at baseline by randomised arm can be seen in Appendix 1, Figure 23.
The SNOT-22 scores at baseline and at the primary end point (6-month visit) by allocated treatment group, and overall using descriptive statistics, are summarised in Table 5. Parametric and non-parametric variables are given as the score is integer in nature, but was treated as a continuous measure.
| ITT population | SNOT-22 scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |||||||
| All, baseline | ITT, baseline | ITT, 6 months | All, baseline | ITT, baseline | ITT, 6 months | All, baseline | ITT, baseline | ITT, 6 months | |
| Population, n (%) | 188 (100) | 152 (81) | 152 (81) | 190 (100) | 155 (82) | 155 (82) | 378 (100) | 307 (81) | 307 (81) |
| Median (IQR) | 44 (26.5–56.5) | 44 (26.5–57) | 15 (6–27) | 41.5 (27–61) | 42 (27–63) | 38 (22–54) | 42.5 (27–59) | 43 (27–59) | 25 (11–45) |
| Mean (SD) | 44.0 (20.4) | 44.5 (20.8) | 19.9 (18.0) | 44.0 (21.5) | 44.1 (21.1) | 39.5 (21.4) | 44.0 (21.0) | 44.3 (20.9) | 29.8 (22.1) |
| 95% CI about mean | 41.1 to 47.0 | 41.1 to 47.8 | 17.0 to 22.7 | 40.9 to 47.1 | 40.8 to 47.4 | 36.1 to 42.9 | 41.9 to 46.1 | 41.9 to 46.6 | 27.3 to 32.3 |
| Minimum, maximum | 6, 104 | 6, 104 | 0, 78 | 6, 96 | 6, 92 | 5, 85 | 6, 104 | 6, 104 | 0, 85 |
As expected, no major differences are observed at baseline across both arms of randomised participants in the ITT population. Table 5 shows that the ITT groups in both arms have similar scores at baseline. The SNOT-22 scores at the 6-month follow-up visit are also presented in Table 5. The raw data alone show a large difference between the arms, specifically much lower scores (i.e. an improvement in symptoms) in the septoplasty arm. The raw data for the SNOT-22 scores at all three study visits are shown in Figure 6. At 12 months, 253 (67%) participants provided SNOT-22 outcome data; 244 of these were included in the ITT group (i.e. also completed the SNOT-22 at 6 months).
FIGURE 6.
The SNOT-22 scores at each study visit (baseline, 6 months and 12 months).

Appendix 1, Table 45, shows the summary statistics for the SNOT-22 score at 12 months (secondary outcome).
The SNOT-22 scores as raw data in box plots for the ITT population (i.e. those with primary outcome data) and those with outcome data at 12 months are shown in Figure 6. It is interesting that scores tend to reduce (improve) over time in the medical management arm, but symptomatic improvement is more marked in the septoplasty arm. The improvement in scores is evident at 6 months and seems to be maintained at 12 months. Some of the improvement at 12 months in the medical management arm may have been influenced by the 37 participants who were randomised to medical management but received non-trial septoplasty after 6 months. The SNOT-22 scores for this subset of 37 participants are displayed alongside those who did not discontinue allocated treatment in Figure 7.
FIGURE 7.
The SNOT-22 scores in the medical management arm, differentiated by whether or not participants had non-trial septoplasty after 6 months (ITT population). On the left, those participants who elected to have non-trial septoplasty between 6 and 12 months. On the right, those participants who did not have non-trial septoplasty.

The participants in the medical management who did not receive non-trial septoplasty still show further improvement in symptoms at 12 months, but for the most part, this is clearly a smaller improvement than is shown in the 37 participants who received non-trial septoplasty in the second half of the trial. Those who requested septoplasty did not exhibit improvement in their 6-month SNOT-22 scores in comparison with baseline, despite the prescribed medical management. Figure 7 omits five participants who received their septoplasty before the 6-month follow-up visit (primary end point).
Appendix 1, Table 46 shows the timing of septoplasties, in relation to the primary end point, carried out on participants randomised to medical management who had non-trial septoplasty.
Efficacy analysis: primary outcome
We performed the ITT analysis on the 307 participants for whom we had SNOT-22 data at 6 months. This is the primary analysis (model 1) of the primary outcome measure of this trial, as specified in the protocol.
Model 1: the primary outcome measure is the SNOT-22 score assessed at 6 months. We analysed this score using multivariable regression models; this analysis enabled us to compare this score between the treatment groups. The associated significance of any observed difference is calculated, adjusting any treatment effect by baseline SNOT-22 score and stratification factors at randomisation [(1) gender and (2) severity at baseline (according to three NOSE categories reported in the literature95)]. The full specification of the fitted model is shown in Table 6. With the presence of baseline SNOT-22 score in the model, the stratification variables do not appear to have a major influence on the primary outcome. However, there is a suggestion that those with extreme NOSE scores at baseline will tend to have scores, on average, 5.8 units higher than those with moderate scores at baseline.
| Model 1 | Primary outcome measure: SNOT-22 score at 6 months | ||||
|---|---|---|---|---|---|
| Coefficient | SE of coefficient | Test statistic | p-value | 95% CI coefficient | |
| Arm: septoplasty (reference category: medical management) | −20.013 | 1.836 | −10.90 | < 0.0001 | −23.625 to −16.40 |
| Baseline SNOT-22 score | 0.497 | 0.053 | 9.39 | < 0.0001 | 0.393 to 0.601 |
| Gender: male (reference category: female) | −0.553 | 1.944 | −0.28 | 0.776 | −4.379 to 3.272 |
| NOSE severity: severe (reference category: moderate) | 1.981 | 2.961 | 0.67 | 0.504 | −3.846 to 7.808 |
| NOSE severity: extreme (reference category: moderate) | 5.811 | 3.459 | 1.68 | 0.094 | −0.995 to 12.617 |
| Constant | 14.954 | 3.291 | 4.54 | < 0.0001 | 8.479 to 21.430 |
Baseline SNOT-22 scores (see Table 5) are highly predictive of scores at outcome. On average, scores at 6 months tend to be 50% of the baseline score (this trend is suggested in Figure 9 for the medical management arm). Most importantly, model 1 (see Table 6) shows a statistically significant effect for randomisation to the septoplasty arm, with scores, on average, being 20 units lower (adjusted difference −20.01, 95% CI −23.63 to −16.40; p < 0.0001) than those in the medical management arm (while ensuring all other variables are the same). The lower limit of the 95% CI is −16.40 units, well below the −9 MCID units assumed for the superiority margin.
Goodness of fit for model 1 was assessed by a series of plots of residuals. The residuals appeared normally distributed with no apparent pattern in fitted values versus residuals; fewer of the standardised residuals fell outside the range (−2 to 2) (see Appendix 1, Figure 24).
Sensitivity analyses
Four sensitivity analyses for the ITT population were planned, as detailed in Chapter 2. The results are summarised in Figure 8, in which the unplanned mixed-effect model demonstrates little impact of site as a random effect. The MCID of −9 units is indicated by the vertical hatched line in Figures 8 and 9. Details of the variable transformation and selection for model 3 can be found in Appendix 1, Tables 47 and 48. Figure 8 shows that very little difference to the strength or magnitude of the signal is seen when considering the sensitivity analyses and including multiple imputation to address missing primary outcome data. Multiple imputation was used to include the full set of participants who consented and were randomised, including those with missing SNOT-22 scores at the primary end point. The proportion of missing data in each group is reported and compared descriptively. Descriptive statistics of baseline variables are presented by treatment group and missing data status (with and without primary end point data, i.e. SNOT-22 score at 6 months). All baseline variables were assessed, but none was found to be predictive of missing data status; therefore, they were not included in multiple imputation equations (see Report Supplementary Material 6). The multiple imputation model included the stratification variables that were recorded, and none was missing.
FIGURE 8.
Results of the sensitivity analyses for the ITT population (model 1) and models 2, 3 and 3a, and including multiple imputation.

Figure 9 shows the secondary analysis of the primary outcome by the three distinct analysis populations (compliant ITT, per protocol and per treatment). These results are very similar to those of the primary analysis. There is a slight reduction in the magnitude of the effect in the compliant ITT analysis to around 18 units, but the 95% CI limits are still well beyond the MCID of −9 units.
FIGURE 9.
Secondary analysis of the primary outcome by the three analysis populations (compliant ITT, per protocol and per treatment).
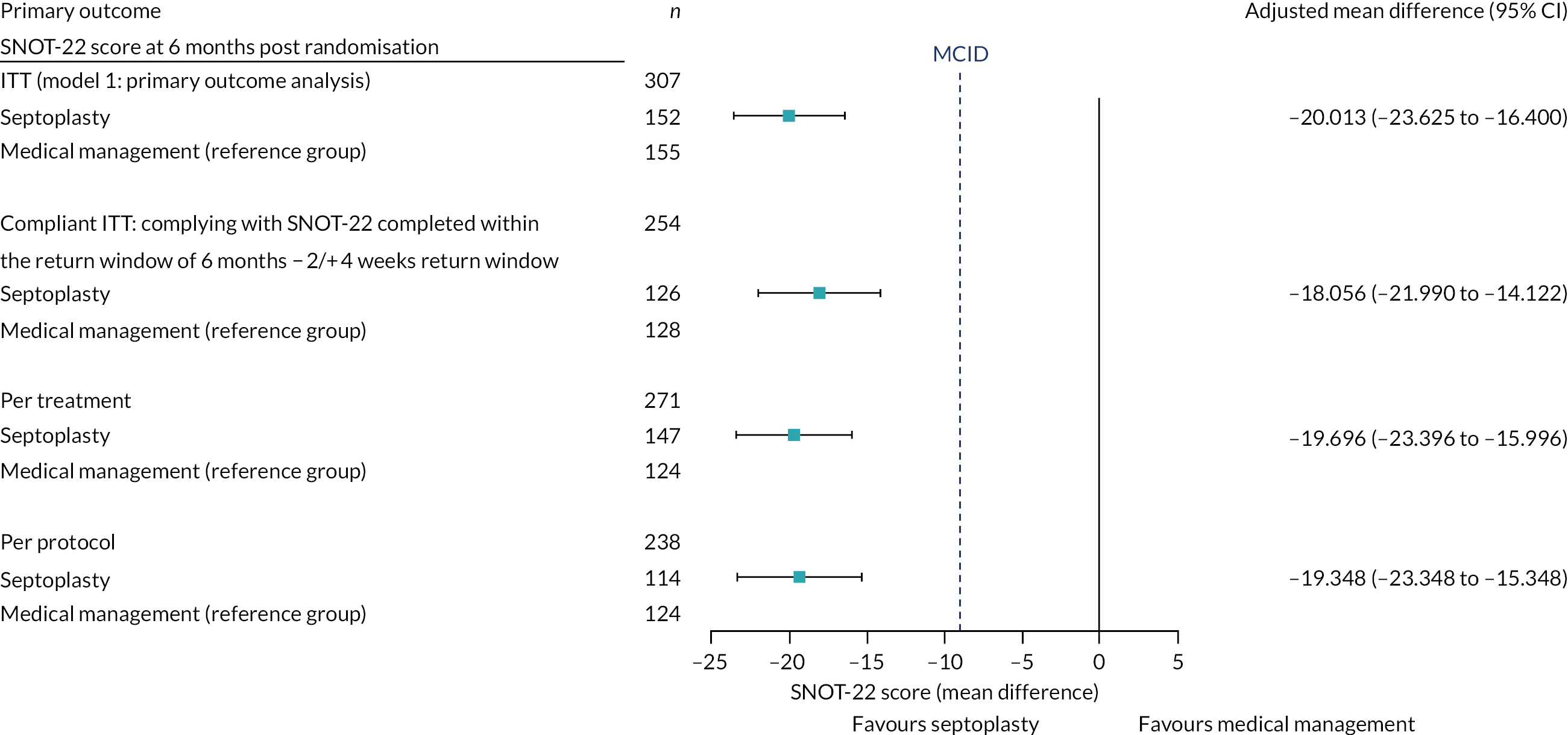
Overall summary following primary analysis and sensitivity analyses
-
Marked improvement seen in the septoplasty arm, with an average difference of −20 units (95% CI −24 to −16; p < 0.0001), compared with the medical management arm, at 6 months.
-
The lower limit of the 95% CI for SNOT-22 score improvement is −16.40 units, which is substantially greater in magnitude than the MCID of −9 units used in the sample size justification for the trial.
-
All sensitivity and secondary analyses show very similar results. There is strong pragmatic evidence that septoplasty is effective at reducing SNOT-22 score at 6 months, compared with medical management.
Descriptive analysis of secondary outcomes
This section covers the DOASS, the NOSE, rhinospirometry measurements, SNOT-22 scores at 12 months and SNOT-22 subscales. For each outcome measure, descriptive data and, when appropriate, the analysis comparing septoplasty with medical management for the available ITT population are presented.
Double Ordinal Airway Subjective Scale
The DOASS is a PROM; it collects data (a score) for each nostril. Appendix 1, Figure 25, shows the raw data collected from randomised participants at each time point, by nostril (better and worse). The summary statistics of the raw data are tabulated in Appendix 1, Table 53.
The raw data are presented in terms of worse and better nostrils. These data are paired (both nostrils measured) at each time point. The nostril with the lower score at baseline is defined as worse, and the nostril with the higher score is defined as better (range 1–10). The nostrils defined as worse and better at baseline are presented at the 6- and 12-month follow-up time points. We used the scores taken at the 6-month follow-up to define which nostril was better or worse for participants whose nostrils both had the same score at baseline.
Appendix 1, Figure 25 shows that, for participants randomised to septoplasty, the score for the worse nostril had improved by the 6-month follow-up. The difference in scores between the worse and better nostrils reduced dramatically among septoplasty patients at 6 months. The differences between the worse and better nostrils are less evident in the medical management group follow-up scores, although improvement over time is seen in both groups.
Subjective Double Ordinal Airway Subjective Scale
One method to present the DOASS as a single summary ratio is the subjective DOASS. This representation of the magnitude of the differences between the two sides is generally presented as the NPR, calculated as (left score − right score) ÷ (left score + right score). Using the subjective DOASS was advisable as this same formula is also used to combine rhinospirometry paired nostril data. As we consider that laterality is irrelevant, we present absolute subjective DOASS, which is the modulus of the subjective score (i.e. sign is ignored). Scores close to zero mean that there is little difference between the nostrils. Summary statistics for absolute subjective DOASS NPR can be found in Appendix 1, Table 50.
Figure 10 shows that scores tend to be closer to zero (symmetrical nasal passages) for the septoplasty arm, which shows a reduction in subjective severity at 6 months. There is evidence of a more modest improvement in the medical management arm at 12 months.
FIGURE 10.
Absolute subjective DOASS.
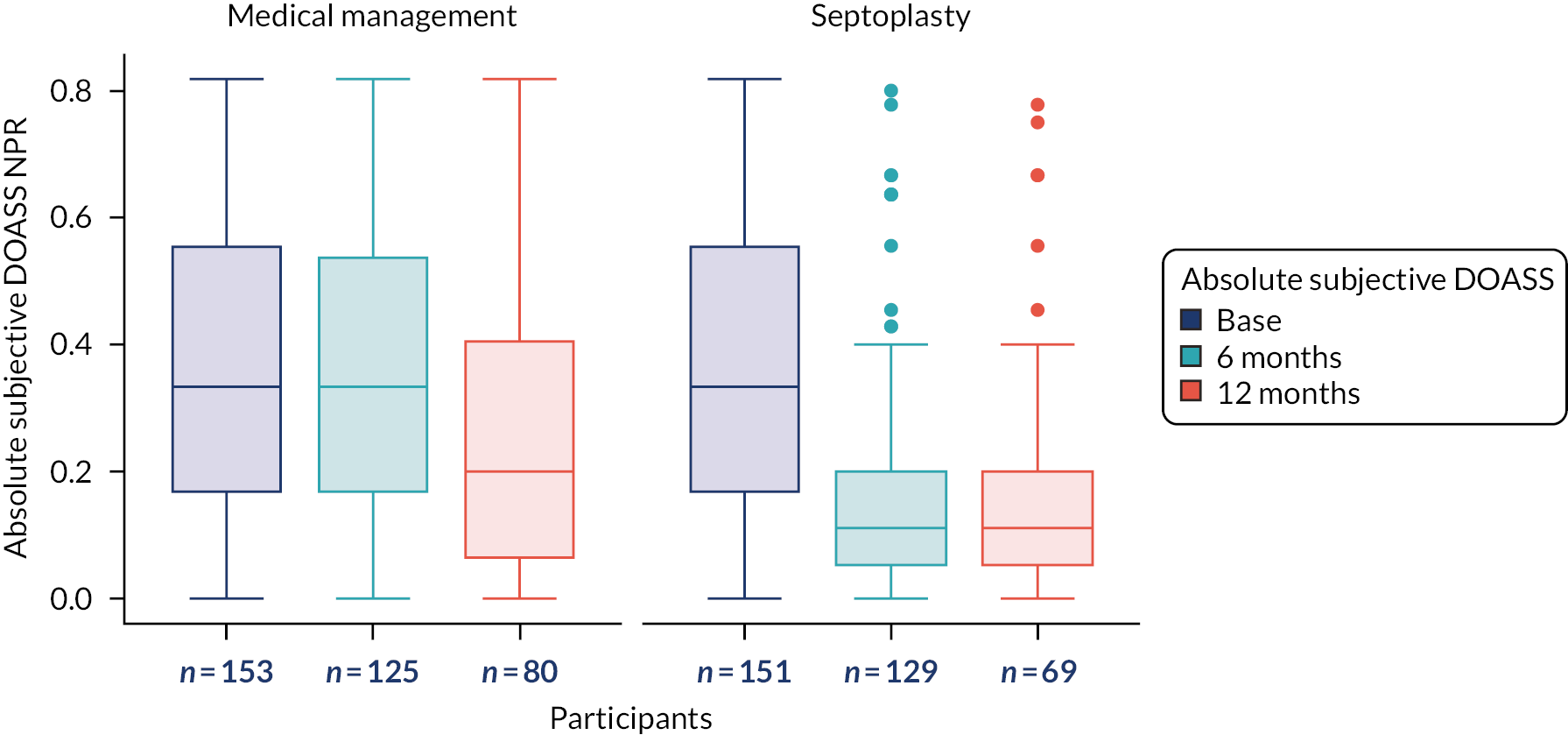
Sino-nasal Outcome Test-22 items subscales
Summary statistics for the SNOT-22 subscales at all three study visits are presented in Appendix 1, Table 51. The corresponding box plots are shown in Figure 11.
FIGURE 11.
The SNOT-22 subscales. (a) Sleep score; (b) nasal score; (c) otologic score; and (d) emotional score. (continued)
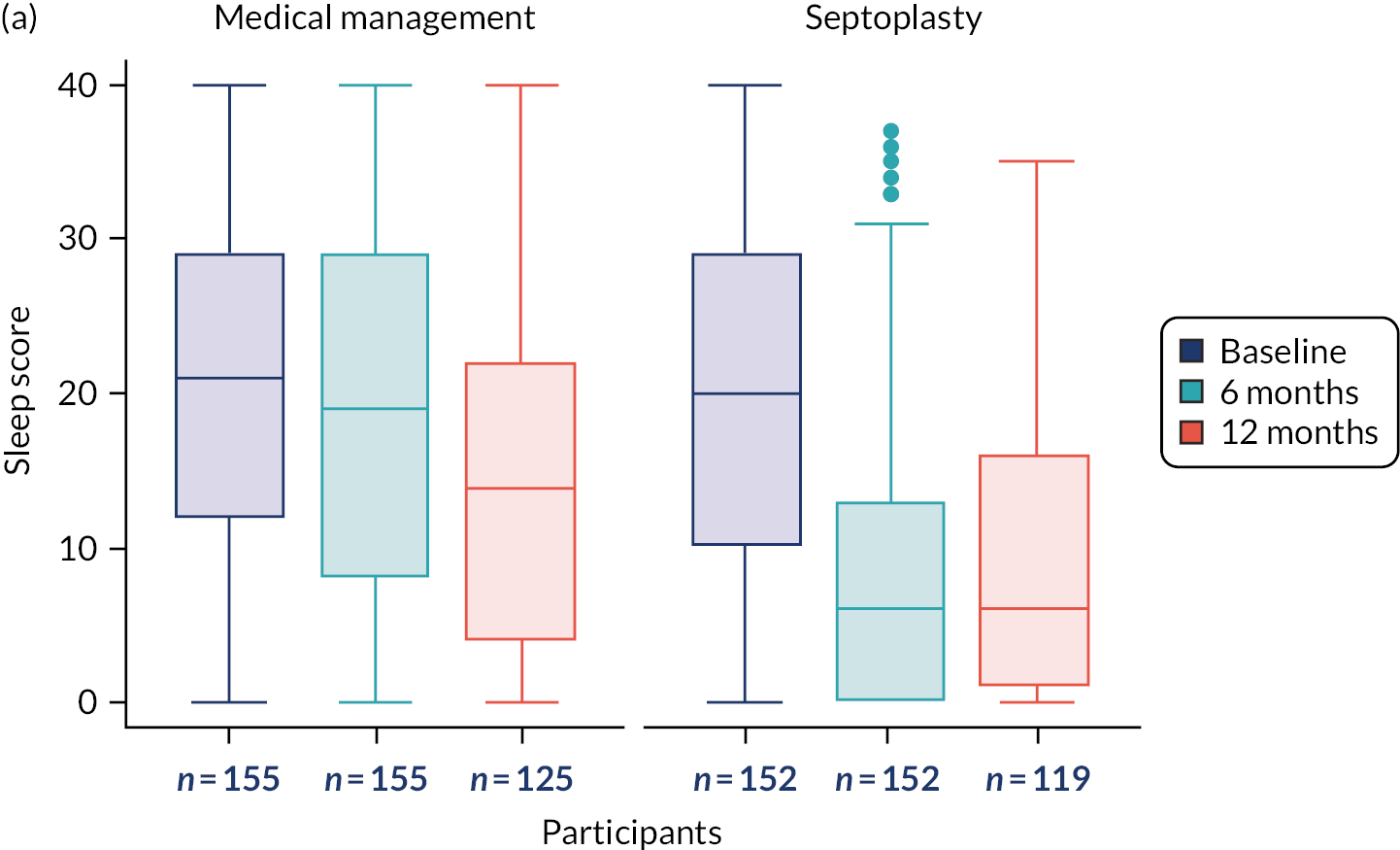
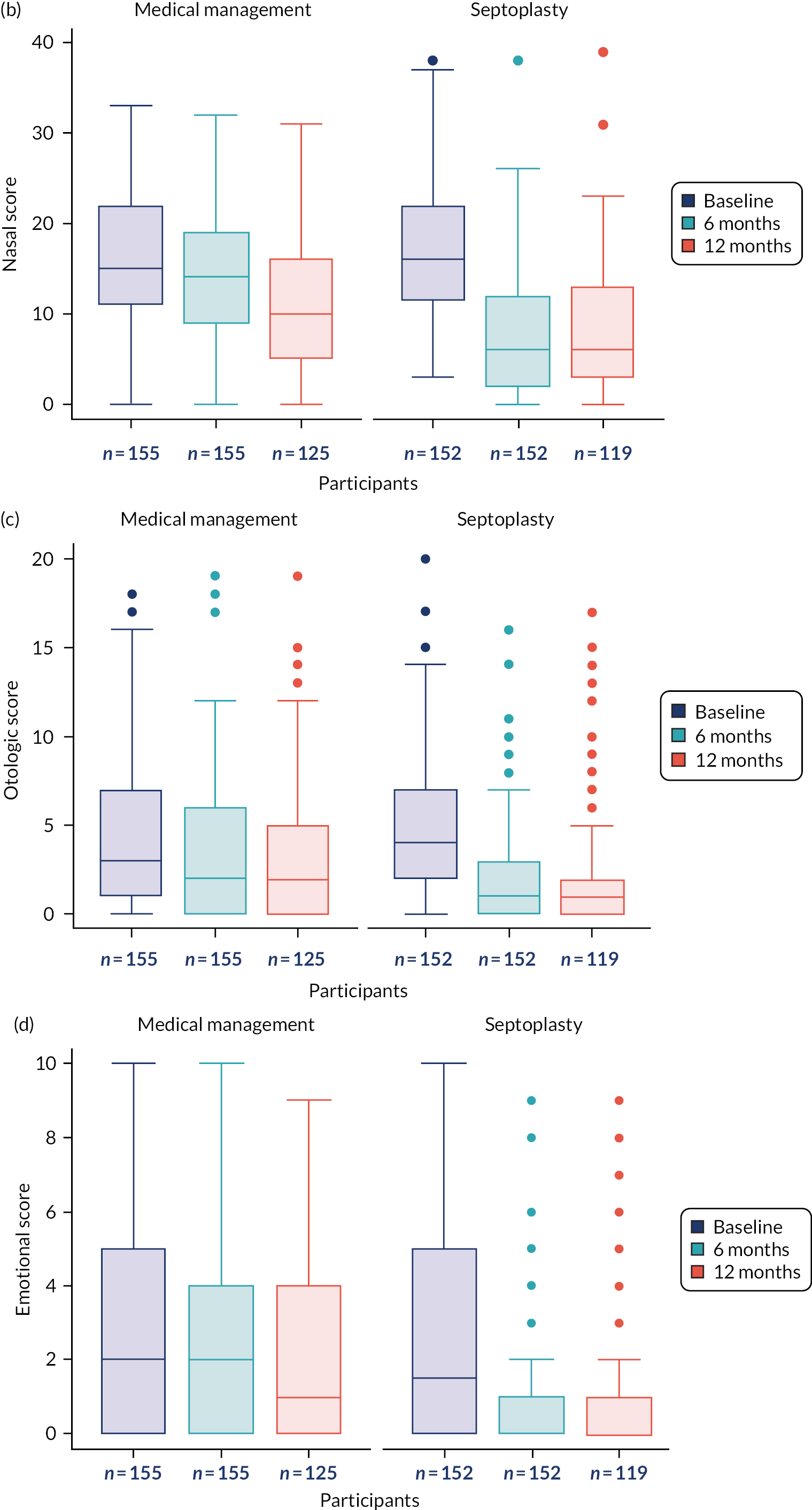
The sleep and nasal subscales comprise 8 of the 22 items in the questionnaire; the otological subscale comprises four items and the emotional subscale comprises the remaining two items.
Appendix 1, Table 51, and the associated box plots (see Figure 11) show that improved and maintained scores in the septoplasty arm, and modest improvement in the medical management arm, are found in all subscales at both 6 and 12 months. The subscale scores range from 0–40 (sleep and nasal subscales), 0–20 (otologic subscale) and 0–10 (emotional subscale).
Nasal Obstruction Symptom Evaluation
The PROM NOSE scores were collected at baseline and used as a randomisation stratification variable. NOSE scores were also collected at 6 and 12 months. Continuous NOSE scores at baseline are summarised in Table 7 by allocated treatment group and overall using descriptive statistics.
| NOSE score ITT population summary statistics | Baseline | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| ITT population, n (%) | 152 (81) | 155 (82) | 307 (81) | 145 (77) | 144 (76) | 289 (76) | 105 (56) | 118 (62) | 223 (59) |
| Median (IQR) | 70 (60–82.5) | 70 (60–85) | 70 (60–85) | 20 (10–45) | 62.5 (50–80) | 45 (20–70) | 25 (10–50) | 50 (20–70) | 35 (15–60) |
| Mean (SD) | 70.8 (16.6) | 71.7 (16.9) | 71.3 (16.7) | 29.0 (24.8) | 62.2 (23.9) | 45.5 (29.4) | 30.7 (25.9) | 47.3 (29.8) | 39.5 (29.2) |
| 95% CI about mean | 68.1 to 73 to 4 | 69.0 to 74.4 | 69.4 to 73.1 | 24.9 to 33.1 | 58.3 to 66.2 | 42.1 to 49.0 | 25.7 to 35.7 | 41.8 to 52.7 | 35.6 to 43.3 |
| Minimum, maximum | 30, 100 | 30, 100 | 30, 100 | 0, 100 | 5, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
As demonstrated by the overlapping CIs (and as expected for a randomised trial) there is no evidence of a difference in baseline scores between the two arms.
However, as evidenced by non-overlapping CIs, there are significant differences at the 6-month (primary end point) and 12-month follow-ups.
The NOSE score can range from 0 to 100, with higher scores representing worse symptoms. NOSE scores are similar at baseline across both treatment groups, as would be expected with random allocation (see Appendix 1, Figure 26). Figure 12 shows that the shift in NOSE scores at 6 and 12 months shows a pattern similar to that observed for SNOT-22 scores, that is, the scores tend to improve modestly for medical management, but more markedly for septoplasty at the 6- and 12-month time points.
FIGURE 12.
The NOSE scores at baseline, 6 months (primary end point) and 12 months, by allocated arm.
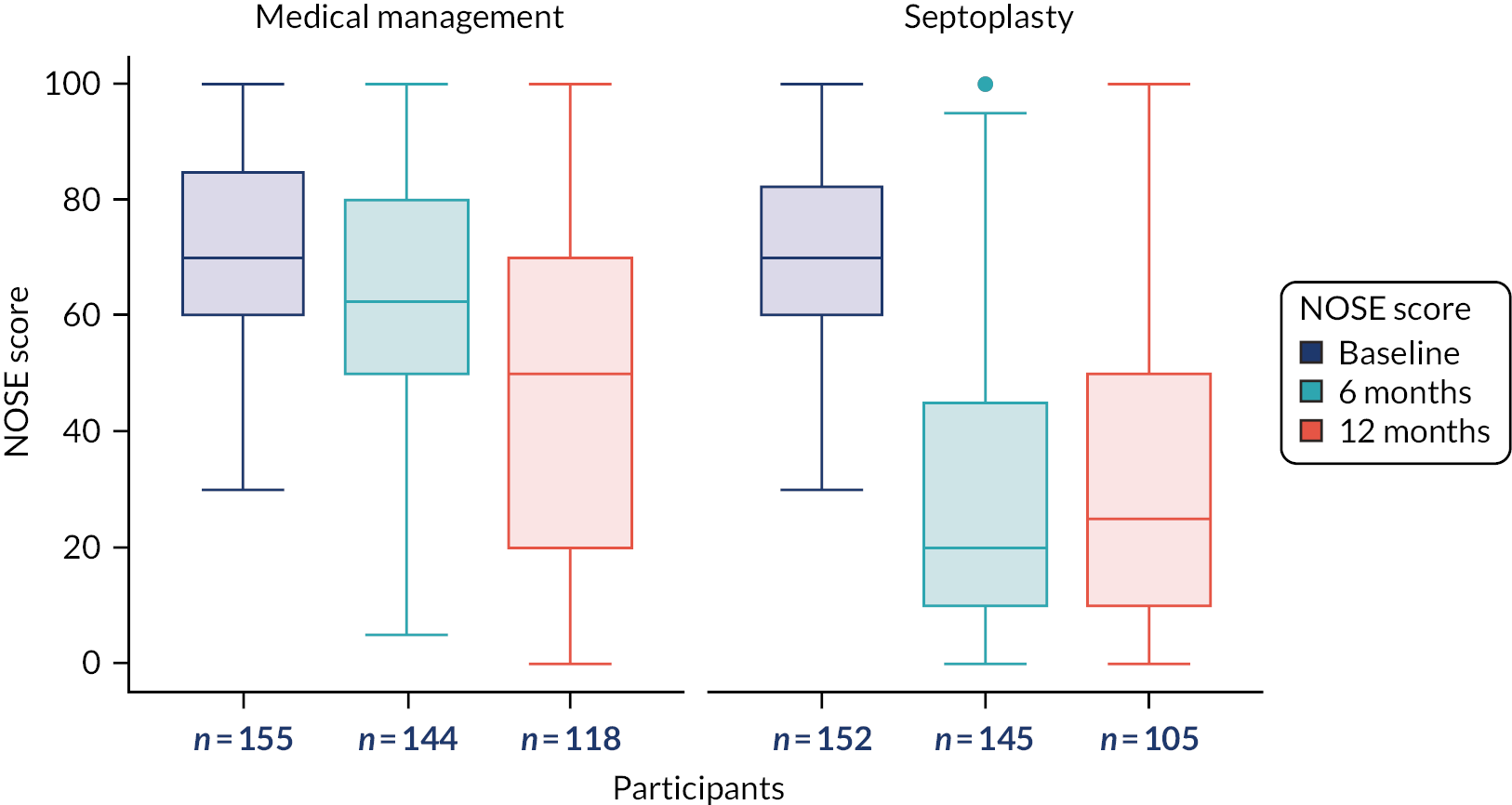
Measurements of nasal patency
Objective measurements of nasal patency were collected at all three trial visits. The measures collected were PNIF and rhinospirometry measures of MIV and tidal breathing. Measurements were taken both pre and post decongestant, with only the post-decongestant measures presented here. The absolute NPR for MIV and tidal breathing are presented in this section. Summary statistics for the following data are presented in Appendix 1:
-
PNIF (post decongestant, maximum of three measurements) (Figure 13) (see Appendix 1, Table 52)
-
inhaled volume by worse (lower-score) side and better (higher-score) side from post-decongestant MIV rhinospirometry (mean volume from three measurements) (see Appendix 1, Table 53 and Figure 27)
-
absolute NPR from post-decongestant MIV rhinospirometry (using mean volume from three measurements) (Figure 14) (see Appendix 1, Table 54)
-
inhaled volume by worse (lower) side and better (higher) side from post-decongestant, tidal breathing rhinospirometry (one measurement) (see Appendix 1, Table 55 and Figure 28).
FIGURE 13.
Post-decongestant PNIF rate (maximum of three measurements) (ITT population).

FIGURE 14.
Absolute NPR from post-decongestant MIV (using mean volume from three measurements) (ITT population).

Absolute NPR measures are defined as the modulus (ignoring the sign) of the NPR, which is calculated in the same way as for the DOASS. The COVID-19 pandemic resulted in the suspension of face-to-face visits, which had a major impact on data collection. Thus, there are limited follow-up data, particularly at 12 months. It is notable that 37 medical management participants underwent NHS surgery after 6 months, whereas only five underwent surgery before the 6-month time point.
Summary statistics of the absolute MIV NPR can be found in Appendix 1, Table 54. Summary statistics showing raw (volume) data for post-decongestant MIV by better and worse nostril can be found in Appendix 1, Table 53, with the accompanying box plot in Appendix 1, Figure 27.
Figure 15 shows the absolute tidal breathing NPR for the ITT population. Summary statistics for the absolute tidal breathing NPR can be found in Appendix 1, Table 56. Summary statistics showing raw data for post-decongestant tidal breathing by better and worse nostril can be found in Appendix 1, Table 55, with the accompanying box plot in Appendix 1, Figure 28.
FIGURE 15.
Absolute tidal breathing NPR from post decongestant (ITT population).
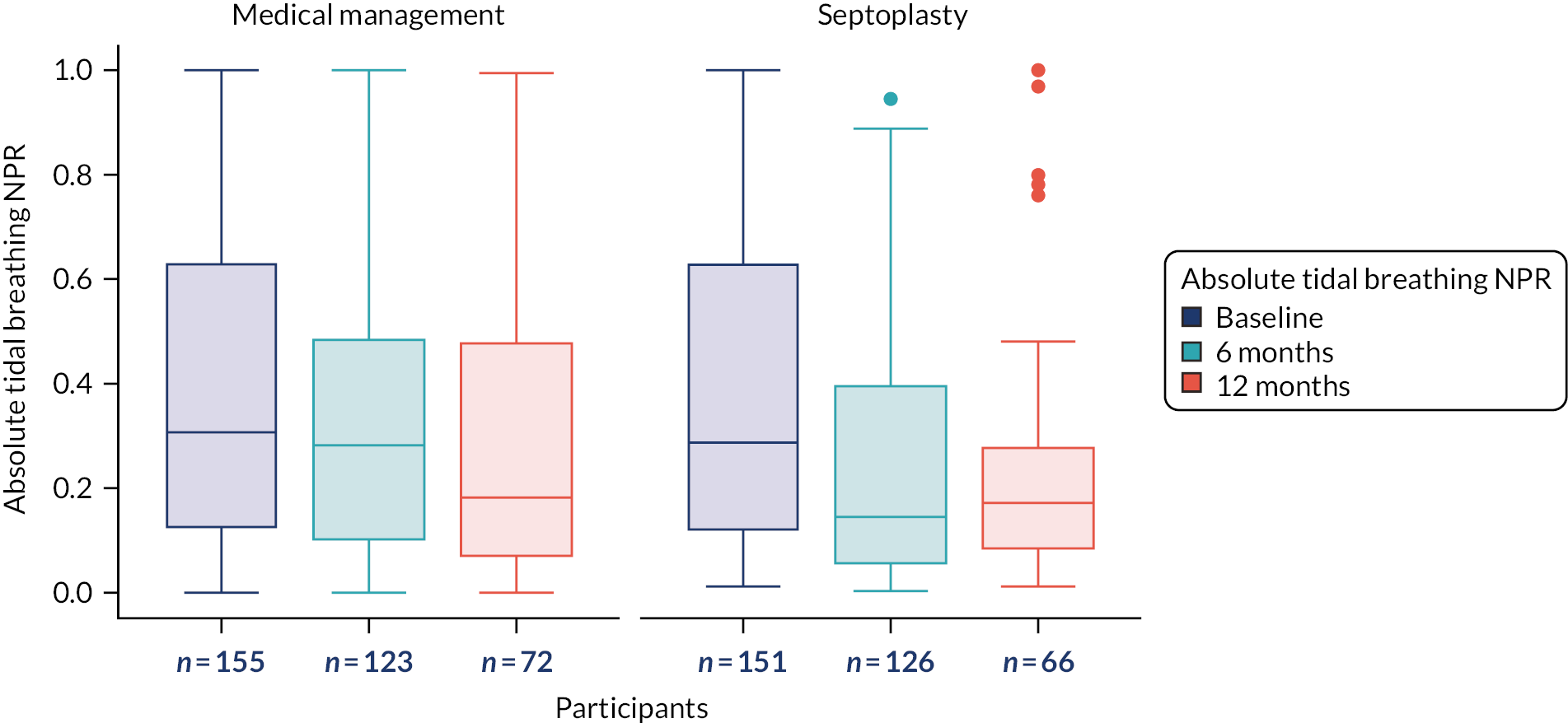
During the COVID-19 pandemic, face-to-face visits at trial sites were suspended, which meant that fewer participants completed the objective nasal patency outcome measures than had completed the PROMs. Despite the reduction in available data for the objective nasal patency measures, a marked improvement is seen by the 6-month measurement in the septoplasty arm, with a more modest improvement seen in the medical management arm.
Correlation of subjective Double Ordinal Airway Subjective Scale with rhinospirometer measures (maximal inhalation volume and tidal breathing nasal partitioning ratios)
It was in our best interests to discover if physical functional rhinospirometry measurements are necessary to assess patients’ nasal obstruction, or if PROMs alone are adequate for such assessments.
Scatterplots of subjective DOASS versus MIV NPR (see Appendix 1, Figure 29) and versus tidal breathing NPR (see Appendix 1, Figure 30) were drawn. Spearman’s correlation coefficients and 95% CIs about the fitted line are presented for each scatterplot.
The scatterplots use paired data at all time points to assess how well correlated the DOASS is with rhinospirometer measurements.
The correlation and numbers with both measures included in the analyses are as follows:
-
The correlation of subjective DOASS to MIV NPR is strong (correlation coefficient = 0.7576; p < 0.0001). The number of instances in which both measures are available is 765.
-
The correlation of subjective DOASS to tidal breathing NPR is strong (correlation coefficient = 0.7545; p < 0.0001). The number of instances in which both measures are available is 762.
Analysis of secondary outcomes
The 12-month SNOT-22 scores, and both the 6- and 12-month NOSE and DOASS scores were analysed in a way similar to that in model 1 (see Efficacy analysis: primary outcome). All analyses are for the ITT population, but numbers analysed are reduced owing to lower rates of completion for secondary outcomes.
Table 8 gives the treatment group coefficient of the regression analysis for each secondary outcome placed as the response variable, adjusted for stratification factors (gender and baseline NOSE categories) and the appropriate baseline measure for each secondary outcome, as well as a summary comment highlighting the direction of the effect.
| Secondary outcome measure | Participants (n) | Regression coefficient for treatment group | 95% CI | p-value | Direction of effect |
|---|---|---|---|---|---|
| SNOT-22 (12 months) | 244 | −10.073 | −14.537 to −5.609 | < 0.0001 | More favourable outcomes in the septoplasty arm (favours septoplasty) |
| NOSE | |||||
| 6 months | 289 | −33.965 | −39.374 to −28.557 | < 0.0001 | Favours septoplasty |
| 12 months | 223 | −16.910 | −24.200 to −9.620 | < 0.0001 | Favours septoplasty |
| Absolute subjective DOASS | |||||
| 6 months | 253 | −0.201 | −0.251 to −0.150 | < 0.0001 | Favours septoplasty |
| 12 months | 147 | −0.101 | −0.173 to −0.029 | 0.006 | Favours septoplasty |
| PNIF (post decongestant) | |||||
| 6 months | 250 | 16.461 | 6.339 to 26.533 | 0.001 | Favours septoplasty |
| 12 months | 138 | 13.086 | −0.227 to 26.400 | 0.054 | Favours septoplasty |
| Absolute MIV NPR (post decongestant) | |||||
| 6 months | 249 | −0.148 | −0.214 to −0.081 | < 0.0001 | Favours septoplasty |
| 12 months | 138 | −0.103 | −0.186 to −0.019 | 0.016 | Favours septoplasty |
| Absolute tidal breathing NPR (post decongestant) | |||||
| 6 months | 248 | −0.101 | −0.165 to −0.037 | 0.002 | Favours septoplasty |
| 12 months | 138 | −0.075 | −0.161 to 0.011 | 0.088 | Favours septoplasty |
Participants’ opinions of success of the treatment provided
One of the clinical effectiveness aims of the NAIROS trial is to use the results in the surgical arm to explore a possible definition of ‘technical failure’ in experienced hands, that is experienced surgeons, (i.e. consultants or non-consultant career clinicians, but not trainee otolaryngologists). To explore this, the investigator recorded the participant’s satisfaction with the surgery outcome in the post-surgery case report form. Responses were received from 133 out of 166 (80%) of those randomised to receive septoplasty; 116 of the 133 respondents (87%) were recorded as being satisfied and 17 (13%) were recorded as being not satisfied. In addition, for 11 of the medical management participants who requested to receive septoplasty, 10 were recorded as being satisfied and one was recorded as not being satisfied.
To explore the possible relationship between participants’ views and surgeons’ impressions as to the need for revision surgery, Table 9 combines responses to the following questions with surgeon opinions:
-
At 6 and 12 months, participants were asked by the investigator, ‘In the past 6 months do you feel your nasal symptoms have been better than/about the same as/worse than before treatment commenced?’.
-
At 6 and 12 months, investigators were asked, ‘Will septoplasty or revision septoplasty be required?’. A response of yes/no was recorded. Surgeons could recommend revision surgery if they felt that it was needed.
| Participant assessment | Frequency | Surgeon recommended revision surgery (n) | ||
|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | |
| Better at 6 months (n = 108) | Better | 47 | 0 | 0 |
| Same | 11 | 0 | 0 | |
| Worse | 3 | 0 | 0 | |
| Missing | 47 | 1 | 0 | |
| Same at 6 months (n = 19) | Better | 3 | 0 | 0 |
| Same | 9 | 2 | 0 | |
| Worse | 1 | 0 | 0 | |
| Missing | 6 | 1 | 0 | |
| Worse at 6 months (n = 7) | Better | 2 | 0 | 0 |
| Same | 0 | 0 | 0 | |
| Worse | 3 | 0 | 1 | |
| Missing | 2 | 0 | 0 | |
| Missing at 6 months (n = 32) | Better | 7 | 0 | 1 |
| Same | 2 | 0 | 0 | |
| Worse | 1 | 0 | 0 | |
| Missing | 22 | 0 | 0 | |
| Total | 166 | 4 | 2 | |
Only six revision surgeries were recommended for participants randomised to receive septoplasty, four at 6 months and two at 12 months. In one case, the recommendation for revision surgery by the investigator contradicted the participant’s view that their symptoms had improved.
Subgroup analyses
Turbinate reduction
At the baseline visit, participants were assessed to determine whether or not it would be appropriate to reduce the inferior turbinate. These assessments are summarised in Appendix 1, Table 57. The relationship between this recommendation and the occurrence of turbinate surgery is shown in Appendix 1, Table 58. A subgroup analysis for participants in the septoplasty arm only was carried out.
Of the 155 septoplasties for which information on turbinate reduction was available, 88 (57%) included a turbinate reduction.
Of the 166 septoplasties carried out in the septoplasty arm, 148 have both baseline and 6-month SNOT-22 data, and so could be included in this analysis.
We analysed the data using linear regression adjusting for baseline SNOT-22 score and the stratification factors of severity and gender (Table 10). The dependent variable was SNOT-22 score at 6 months, and the model was fitted with the additional binary variable that indicated whether or not the turbinate was reduced in surgery (see Appendix 1, Figure 31).
| Model 1 | Regression coefficient | SE of coefficient | Test statistic | p-value | 95% CI coefficient |
|---|---|---|---|---|---|
| Turbinate reduced (reference: not reduced) | 2.788 | 2.814 | 0.99 | 0.324 | −2.776 to 8.352 |
| Baseline SNOT-22 score | 0.389 | 0.076 | 5.12 | < 0.0001 | 0.239 to 0.539 |
| Gender: male (reference category: female) | −1.307 | 2.889 | −0.45 | 0.652 | −7.019 to 4.405 |
| NOSE severity: severe (reference category: moderate) | −1.773 | 4.650 | −0.38 | 0.704 | −10.968 to 7.422 |
| NOSE severity: extreme (reference category: moderate) | −1.139 | 5.212 | −0.22 | 0.827 | −11.445 to 9.168 |
| Constant | 2.819 | 5.177 | 0.54 | 0.587 | −7.419 to 13.057 |
The regression analysis shows that, on average, adjusted scores were 2.79 points higher (95% CI −2.78 to 8.35 points; p = 0.324) for those who received a turbinate reduction than for those who did not. As turbinate reduction was not a randomised intervention, but a clinical/surgical decision made within the randomised septoplasty, no conclusion can be drawn as to whether this was an effective part of the surgery.
Subpopulation treatment effect pattern plot by baseline Nasal Obstruction Symptom Evaluation score
Figure 16 shows the STEPP for the ITT population, demonstrating the impact of baseline NOSE score on the primary outcome (i.e. SNOT-22 score at 6 months).
FIGURE 16.
The STEPP of all participants in the ITT population.

The green line with shaded 95% CI limits in Figure 16 shows the average effect of being randomised to septoplasty for those with specific NOSE scores at baseline. For those with moderate NOSE scores at baseline, the average improvement in SNOT-22 score is around 5 units. Those with scores of around 60 have an average improvement of around 15 units, and those with extreme scores at baseline can improve by as much as 30 units. All of the values covered by the green line show improvements in SNOT-22 scores. However, it is clear that improvement increases as baseline severity increases.
It is important to consider the ‘floor’ effect of the SNOT-22 variable, which means that participants with SNOT-22 scores of < 20 at baseline have limited scope for improvement. 96 Baseline SNOT-22 scores ranged from 6 to 104 (see Table 5), with 75% of scores being ≥ 27 and hence well above the impact of the floor effect.
A STEPP analysis of SNOT-22 scores at 6 months by DOASS (worse side at baseline) did not show a reportable trend between the two variables (see Appendix 1, Figure 32).
Analysis by gender
A subgroup analysis of the primary outcome by gender was performed. This is reported using paired data to assess individual changes in SNOT-22 scores from baseline to 6 months, and baseline to 12 months (presented in Appendix 1, Table 59; see Figure 33 for corresponding box plots). As part of the subgroup analysis by gender, STEPPs were produced separately for males and females [see Appendix 1, Figures 34 (male) and 35 (female)]. No major differences between gender were observed.
Safety
Adverse events
A total of 227 AEs were reported across 123 unique participants. Differentiated by arm, this equates to 95 AEs reported by 62 unique participants in the medical management arm and 132 AEs reported by 61 unique participants in the septoplasty arm. The severity of reported AEs is tabulated in Appendix 1, Table 60. The action taken to address the AEs is tabulated in Appendix 1, Table 61, which shows that 61% of AEs required no action, 11% required that treatment be interrupted or discontinued and 28% were treated with concomitant medications. The status of the AEs (ongoing or resolved) at the end of the trial is tabulated in Appendix 1, Table 62. This table shows that 66% of AEs were categorised as resolved, 26% were categorised as ongoing and the other 8% were categorised as ‘resolving’. Appendix 1, Table 63, summarises causality and severity for the AEs by randomised arm.
Three participants randomised to septoplasty had an AE date after the end of follow-up in the ITT population. Two were within the compliance window for the 12-month visit and one was beyond the compliance window (see Appendix 1, Table 64).
AE start dates were not recorded for six participants; however, their AE completion dates are provided (see Appendix 1, Table 65). Line listings of the 227 AEs for all participants can be found in Appendix 1, Table 66. As the AE event terms were reported as free text in the database, these subsequently underwent Medical Dictionary for Regulatory Activities (MedDRA) coding. Table 11 presents a summary of the AEs, categorised according to approximate lowest-level MedDRA term.
| AE category by lowest-level MedDRA term | Trial arm (n) | Total (n) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| Nasal pain | 19 | 5 | 24 |
| Other pain | 12 | 7 | 19 |
| Nasal-induced infection/fever/temperature | 11 | 7 | 18 |
| Infection/fever/temperature | 11 | 8 | 19 |
| Epistaxis/bleeding/clot | 13 | 20 | 33 |
| Rhinorrhoea/mucus | 9 | 5 | 14 |
| Cough/cold/influenza | 9 | 5 | 14 |
| Headache | 2 | 6 | 8 |
| Dry nose/itching/crusting | 1 | 6 | 7 |
| Blocked nose | 6 | 0 | 6 |
| Numbness | 4 | 1 | 5 |
| Nose shape/asymmetry | 4 | 0 | 4 |
| Reflux/heartburn | 0 | 4 | 4 |
| Ear blocked/tinnitus/labyrinthitis | 3 | 0 | 3 |
| Anxiety/depression | 0 | 3 | 3 |
| Swelling | 3 | 0 | 3 |
| Dizziness | 2 | 0 | 2 |
| Nausea | 1 | 1 | 2 |
| Perforation | 2 | 0 | 2 |
| Sense of smell/taste | 2 | 0 | 2 |
| Adhesion/synechiae | 1 | 0 | 1 |
| Tiredness/fatigue | 1 | 0 | 1 |
| Other | 15 | 17 | 33 |
| Total | 132 | 95 | 227 |
Serious adverse events
There were 23 SAEs reported by 16 unique participants. Differentiated by arm, this equates to nine SAEs reported by five unique participants in the medical management arm and 14 SAEs by 11 unique participants in the septoplasty arm.
Serious AE causality and severity by randomised arm is summarised in Appendix 1, Table 67; Appendix 1, Table 68, groups the SAEs by both category and severity. Figure 17 shows the timings of SAEs; many of these correspond with the timing of septoplasty.
FIGURE 17.
Time from randomisation to SAE start date.

Table 12 shows the SAEs recategorised by the clinical team. A line listing of the 23 SAEs is presented in Appendix 1, Table 69. The median time from septoplasty to SAE is 0 days and the maximum time is 8 days.
| Categorised SAE | Trial arm (n) | Total (n) | SUSARa | |
|---|---|---|---|---|
| Septoplasty | Medical managementa | |||
| Anaesthetic complication | 3a | 2 | 5 | 3 |
| Infection | 2 | 2 | 4 | |
| Postoperative bleeding | 5a | 1 | 6 | 1 |
| Vasovagal episode | 2 | 0 | 2 | |
| Polypharmacy overdose | 0 | 3 | 3 | |
| Trauma unrelated to the trial | 0 | 1 | 1 | |
| Inappropriate hospital admissionb | 2 | 0 | 2 | |
| Total | 14 | 9 | 23 | |
There were four reported SUSARS: four mild events in the septoplasty arm that were related to septoplasty. These are shown in the last column in Appendix 1, Table 69.
Surgical complication/failure and reintervention within 12 months
Participants allocated to either trial arm who received trial or non-trial septoplasty at any point during the trial were asked to report AEs at 6 and 12 months; 174 participants responded. The numbers of participants who responded (yes/no) to the specific questions about complications at either or both of these time points are summarised in Table 13. Participants could also report ‘other’ complications. These are listed in Appendix 1, Table 70. Complications that could not be coded in specific categories in Table 13 were classified as ‘other’ complications.
| Complication | Frequency | Unique participants (n) | Rate (frequency ÷ n) (%) |
|---|---|---|---|
| Bleeding nose necessitating re-admission to hospital | 7 | 174 | 4 |
| Infection requiring antibiotic treatment | 20 | 172 | 12 |
| Decrease in sense of smell | 19 | 171 | 11 |
| Numbness of upper teeth | 18 | 171 | 11 |
| Change in the appearance of the nose | 17 | 171 | 10 |
| Other surgery complicationsa | 7 | 173 | 4 |
| Adhesionsa | 7 | 179 | 4 |
| Perforationsa | 6 | 179 | 3 |
The ‘other surgery complications’ were assessed by the chief investigator and a TMG clinician. Three were recategorised and are included in Table 13 in the relevant categories. Nine of these were not deemed to be complications and are not included in Table 13, leaving seven other complications which are listed in Appendix 1, Box 1. One complication is related to adhesions, and so is included in Table 13. Total unique participant numbers vary owing to incomplete data collection. Table 13 also summarises the total reports of adhesions and perforations that occurred by randomised arm. One adhesion and two perforations were reported as AEs, but were also listed in the clinical exam electronic case report form (eCRF).
Summary of key findings
-
The trial recruited to target; primary outcome data are available for 81% of participants.
-
There is strong evidence that randomisation to the septoplasty arm reduces a participant’s SNOT-22 score by approximately 20 units more than randomisation to the medical management arm at 6 months.
-
At 12 months, the larger reduction in SNOT-22 score in the septoplasty arm, compared with the medical management arm, is sustained but diminished to 10 units; this is still a statistically significant difference.
-
All sensitivity analyses confirm that the message of the primary outcome is a strong and consistent signal, with similar improvements seen in all secondary outcomes.
-
The STEPPs show that the more severely symptomatic a participant is at baseline, the larger a reduction in SNOT-22 scores they should expect from the septoplasty.
-
Nineteen out of 23 SAEs were related to septoplasty undertaken in either arm of the trial. No participants required return to theatre for bleeding or infective reasons in the short term. NAIROS-reported complications were those recognised in the septoplasty/turbinate reduction literature.
Chapter 4 Economic evaluation
This chapter reports the economic evaluation conducted as part of the NAIROS trial, which included both a within-trial analysis and a longer-term model-based analysis.
Within-trial analysis
Introduction
The question addressed by the within-trial analysis was as follows: for adults experiencing nasal obstruction associated with a deviated septum, what is the cost-effectiveness of surgical management compared with medical management at 12 months?
The within-trial analysis included a cost-effectiveness analysis (CEA) and a cost–utility analysis (CUA). The CEA estimated the incremental cost per improvement in SNOT-22 score (defined as a change of ≥ 9 points, as outlined in Chapter 2, Sample size calculation) and the incremental cost per AE avoided. The CUA estimated the incremental cost per QALY gained. For the CUA, responses to the SF-36 were converted into SF-6D utility scores using standard algorithms; these were then used to estimate QALYs82 using the area under the curve approach. 83 As costs and effects were reported for 12 months, they were not discounted in the within-trial analysis.
For the within-trial analysis, the following outcomes are reported:
-
NHS/PSS costs of managing individuals with nasal airway obstruction associated with a deviated septum
-
direct and indirect costs to participants from nasal airway obstruction associated with a deviated septum
-
changes in the SNOT-22 score at 6 and 12 months post randomisation l total number of AEs at 12 months post randomisation
-
QALYs based on responses to the SF-36, administered at baseline and at 6 and 12 months post randomisation
-
incremental cost-effectiveness ratios (ICERs) –
-
incremental cost per improvement (of ≥ 9 points) in SNOT-22 score
-
incremental cost per AE avoided
-
incremental cost per QALY gained.
-
Methods
This analysis was designed and conducted according to best practice, conforming to the Consolidated Health Economic Evaluation Reporting Standards. 97 All analyses were undertaken using Stata version 16. All economic analyses were based on an ITT principle.
Cost data collection
The economic evaluation was conducted from the perspectives of the UK NHS and PSS. Costs were in 2020 Great British pounds. Data on resource use, use of services, and time away from usual activities were combined with trial-specific estimates and nationally available data to produce a total cost for each trial participant. 98,99
Details of surgical treatment (septoplasty) were recorded on eCRFs and follow-up healthcare resource use data were collected via a specifically designed HUQ (see Report Supplementary Material 4), administered at 6 and 12 months post randomisation. The HUQ is a bespoke questionnaire used in previous studies that was adapted for this study100,101 based on input from the TMG and PPI. Medications received were collected in the concomitant medication eCRF, which was completed 2 weeks post randomisation or 2 weeks post surgery, depending on the randomised allocation, and at 6 and 12 months post randomisation. The collection of these healthcare costs allowed us to determine the average total cost of managing an individual with nasal airway obstruction associated with a deviated septum to the NHS.
Sensitivity analyses took a broader perspective that considered individual participant costs. Participant costs were collected in the HUQs and the time and travel questionnaire (see Report Supplementary Material 4 and 5). Similar to the HUQ, the time and travel questionnaire is a bespoke questionnaire that was adapted for this study. 100,101 The time and travel questionnaire was administered 12 months post randomisation. Both questionnaires identified direct (e.g. out-of-pocket purchase of pain medication) and indirect (e.g. time off paid work/usual activities) costs to participants.
Intervention costs
Participants were randomised to receive either surgery (septoplasty) or medical management (two different nasal sprays used concomitantly for 6 months) for their nasal airway obstruction.
Septoplasty with or without turbinate reduction
The NHS day-case tariff for septoplasty (CA11A) was used in the base-case analysis (see Appendix 2, Table 71). Every participant reported to have received septoplasty during the 12-month follow-up was assigned this cost. It was assumed that those who were lost to follow-up and had not yet received surgery did not receive septoplasty. In a sensitivity analysis, costs associated with surgery were estimated using microcosting. 102
For the microcosting exercise, the cost of each surgery was based on information provided in the eCRF, taking into account the mix of staffing, overheads, and consumable and reusable resources required. 102 The duration of admission was estimated using the number of nights in hospital reported in the eCRF. Reusable and consumable resources were based on resources needed to undertake a septoplasty; additional resources were assigned if turbinate reduction was performed. Reusable and consumable resources were identified using personal communication with clinical staff (Sean Carrie and Graham Stobbs, The Newcastle upon Tyne Hospitals NHS Foundation Trust, 8 April 2021, personal communication) and are detailed in Appendix 2, Table 72. Staff costs reflected the grade of the senior operating surgeon and senior anaesthetist recorded on the eCRF. In addition to the staff recorded on the eCRF, the number, type and grade of all other staff routinely present were identified based on clinical advice (Sean Carrie and Graham Stobbs, personal communication). Based on this advice, we also accounted for the presence of a scrub nurse, floor nurse, healthcare assistant, operating department practitioner and anaesthetic specialist registrar. The length of time in the operating theatre was estimated by deducting the time in the operating room from the time out of the operating room recorded on the eCRF. The name, dose, frequency and duration of any discharge medication were identified from the eCRF.
The unit costs of consumable and reusable resources identified during the microcosting process were sourced using information from the Newcastle site (Graham Stobbs, The Newcastle upon Tyne Hospitals NHS Foundation Trust, 1 September 2021, personal communication), and are detailed in Appendix 2, Table 72. Staff salaries, inpatient stays and discharge medication were costed from routine sources. 103–105
The surgery eCRF was completed for participants randomised to septoplasty who had surgery; it was not completed for participants randomised to medical management who then went on to have septoplasty. Therefore, total surgery costs based on microcosting estimates were calculated only for those randomised to septoplasty who received septoplasty. These surgery costs were aggregated to estimate an average total cost for septoplasty, which was assigned to participants in both arms who received surgical management.
Medical management
All participants randomised to medical management were provided with nasal sprays, which were to be used daily for 6 months. Regardless of compliance, the costs associated with providing these nasal sprays to participants was assumed. Participants were provided with five of the Stérimar nasal spray canisters and 11 of the mometasone nasal spray bottles. The cost of these nasal sprays was obtained from routine sources (see Appendix 2, Table 71). 106
All information on the interventions received was used to estimate the total intervention resource use and total intervention cost per participant for each randomised arm. These estimates were presented as average total resource use and average total intervention cost per participant per arm.
Healthcare costs (excluding intervention costs)
The 6-monthly HUQs captured information on the type and frequency of primary and secondary care resource use at 6 and 12 months post randomisation. Primary care resources included visits with a GP, visits with a nurse, and ‘other health professional’ consultations. Participants were asked to provide details on ‘other health professionals’. Primary care consultations could take place at the healthcare provider’s practice, at the participant’s home or over the telephone (including telephone calls to NHS call centres). Secondary care resources included visits to an accident and emergency (A&E) department, outpatient clinics and hospital admissions (day patient or overnight).
Participants could also report additional medications to manage their nasal obstruction; this information was collected in the concomitant medication eCRF.
All information on healthcare contacts and medications are presented as the average total number of participants who used each healthcare service, and the average total number of visits for each healthcare resource use at each time point per arm.
The costs associated with each of these healthcare contacts and medications were collected from routine sources98,99,102 and combined with the frequency of resource use to estimate the total healthcare cost for each participant and the average total cost per participant per arm (see Appendix 2, Table 71).
Participant costs
Participant costs were included as a sensitivity analysis. Direct and indirect costs to participants were captured via both the HUQ and the time and travel questionnaire. The HUQ collected information on out-of-pocket payments for private health care or personal care. The HUQ also collected information on time away from usual activities owing to illness to capture the opportunity cost of participants’ time.
The time and travel questionnaire collected additional participant costs. These were the direct and indirect costs associated with attending healthcare appointments. The questionnaire captured information on how participants travelled to each type of healthcare appointment (including out-of-pocket expenses), how much time they spent at each type of appointment, what they would otherwise have been doing and whether they were alone or accompanied by someone else. All information on participant costs for each type of healthcare appointment was summarised as average totals per participant.
Unit costs to derive participant costs were collected from routine sources and from the time and travel questionnaire (see Appendix 2, Table 71). The cost associated with time off paid work was estimated using the median national wage rate. 107 The unit cost for time away from usual activities was based on non-working time reported by the Department for Transport. 108 Travel costs were derived based on the mode of transportation reported. Mileage rates were estimated based on rates reported by the Automobile Association. 109 Parking costs or public transport fares were reported in the time and travel questionnaire. Time and travel costs were summarised for each type of healthcare appointment to estimate a unit cost for each face-to-face healthcare appointment. These unit costs were combined with the number of visits reported in the HUQs to estimate the average total cost per participant.
Adverse event costs
Information on AEs and SAEs was collected via the AE eCRF completed 2 weeks post randomisation, 2 weeks post surgery and throughout the follow-up period. Some AEs may have resulted in additional medications or hospitalisation. In the base-case analysis, the costs of AEs, medication and/or hospitalisation were excluded to prevent double-counting. It was assumed that any medications received were reported on the concomitant medication form. It was also assumed that any AEs resulting in hospitalisation were captured in the HUQ. The frequency of medications and hospitalisations reported in the AE eCRF was compared with responses to the concomitant medication eCRF and the HUQ. If more medications and/or hospitalisations were reported in the AE eCRF, then the cost of these resources was incorporated in a sensitivity analysis. The costs of these hospitalisations and medications were obtained from routine sources103,105 and were assigned to each AE hospitalisation and medication to estimate the average total AE cost per participant.
To summarise, data collection on resource use and costs can be split into:
-
interventions costs (surgery and nasal sprays) collected via eCRFs
-
treatment costs collected via the HUQ and the concomitant medication form
-
participant costs collected via the HUQ and the time and travel questionnaire.
Estimation of effects
Three effectiveness measures were used in this economic evaluation: nasal function measured by SNOT-22 score, number of AEs, and QALYs based on responses to the SF-36.
Estimation of health outcomes for the cost-effectiveness analysis
Two different outcome measures were used in the CEA:
-
improvement in SNOT-22 score at 6 and 12 months post randomisation
-
number of AEs.
First, we used nasal function, measured by the SNOT-22 administered at baseline and at 6 and 12 months post randomisation, as a measure of effectiveness. A clinically significant change in SNOT-22 scores was defined as a difference of ≥ 9 points (see Chapter 2, Statistical considerations). The individual SNOT-22 scores were derived by the statistical team as previously outlined (see Chapter 2, Analysis of the primary outcome). The change in SNOT-22 score between baseline and 12 months was estimated for every participant and presented as the average total proportion of participants who had an improvement in SNOT-22 scores (i.e. a difference of ≥ 9 points) per arm. This analysis was replicated to identify the difference in SNOT-22 scores between baseline and 6 months for every participant.
The second measure of effectiveness was the number of AEs reported in each arm. 110,111 The total number of AEs was aggregated for each participant and presented as the average total number of AEs per arm.
Estimation of quality-adjusted life-years for the cost–utility analysis
The SF-36 was administered at baseline and at 6 and 12 months post randomisation. The SF-36 is a practical, reliable and valid measure of physical and mental health. 112 The responses to the SF-36 were converted into the SF-6D, a preference-based utility index, using a standard algorithm to produce a health-state utility score. 82 The SF-6D comprises six multilevel dimensions: physical functioning, role limitations, social functioning, bodily pain, mental health and vitality. The area under the curve approach was used to assign time weighting to the utility scores. The time-weighted average of the scores based on the responses to the SF-36 throughout the follow-up period allowed us to generate QALY values for each participant. Equation 1 is an illustrative example of how QALYs were estimated:
Comparative incremental analyses of costs and outcomes between trial arms
Unadjusted and adjusted (regression) analyses were performed to estimate the cost-effectiveness of septoplasty compared with medical management. All results were presented as point estimates of the mean incremental costs, effects and cost-effectiveness.
The cost-effectiveness plane (Figure 18) illustrates how a decision is made based on the economic results. The difference in QALYs is shown on the x-axis and the difference in costs is shown on the y-axis. At the origin there is no difference in costs and effects between the two management strategies. If septoplasty was found to be both less costly and more effective (south-east quadrant), it would be dominant and would be considered cost-effective. If septoplasty was found to be both more costly and less effective (north-west quadrant), medical management would be dominant and septoplasty would not be considered cost-effective. If septoplasty was found to be more effective, but more costly, or less effective and less costly (north-east and south-west quadrants, respectively), then we would have to consider which management strategy is more likely to be cost-effective. In this situation, decisions were based on the ICER, which estimates the cost per additional unit of effect (i.e. the difference in costs divided by the difference in effects between the two arms). In this situation the ICER is compared with the threshold value society places on an additional unit of effect, if there is one available (the additional unit of effect is illustrated by ‘a’, the dotted line in Figure 18).
FIGURE 18.
Cost-effectiveness plane. a, Society’s threshold to pay for one more unit of effect.
Cost–effectiveness analysis
In the base-case analysis, the CEA was based on the incremental cost per additional participant who had an improvement in SNOT-22 score at 12 months post randomisation. In sensitivity analyses, the measure of effectiveness was changed to (1) incremental cost per additional participant who had an improvement in SNOT-22 score at 6 months post randomisation and (2) incremental cost per AE avoided at 12 months post randomisation (see Sensitivity analysis for further details). The average total cost and average total proportion of participants who had an improvement in SNOT-22 scores were estimated for each management strategy. These were presented as point estimates of mean incremental costs and effects and the incremental cost per additional participant who had an improvement in SNOT-22 score. If there was no dominant management strategy, the ICER would be difficult to interpret (as there is no threshold value for an improvement in SNOT-22 score with which to compare the ICER) hence the need for the CUA.
Cost–utility analysis
The CUA was based on the incremental cost per QALY gained. The average total cost and average total QALYs were estimated for each arm and presented as point estimates of mean incremental costs and effects (QALYs) and the incremental cost per QALY gained. This can be compared with a decision-makers’ threshold value. For example, a typical threshold for an additional QALY in England, according to the National Institute for Health and Care Excellence (NICE), is approximately £20,000. 113
Adjusted analysis: seemingly unrelated regression
We adjusted our analyses, using seemingly unrelated regression (SUR)114 to estimate the difference in cost-effectieveness between the two management strategies. SUR permits the simultaneous estimation of costs and effects, calculated at individual level, while accounting for unobserved individual characteristics that could affect both costs and effects and lead to potential correlation between these two variables. 115 In addition, the SUR allowed us to control for additional covariates (age, gender, ethnicity, baseline SNOT-22 score and baseline utility scores) that may have affected costs and/or effects.
Sensitivity analysis
It was anticipated that there would be missing responses to the participant questionnaires (the HUQ and the SF-36). In the base-case analysis, missing data were imputed using chained multiple imputation methods. 116 Chained multiple imputation makes multiple predictions for missing cost and effect data simultaneously. 117 Data were assumed to be missing at random. Differences in baseline characteristics, including baseline utility, between participants with missing and participants with complete data were undertaken using t-tests to validate this assumption.
Sensitivity analyses were conducted to assess the robustness of the base-case results to realistic variations in the levels of underlying data. The following deterministic sensitivity analyses were used in the base-case analysis:
-
CEA sensitivity analyses –
-
the measure of effectiveness was improvement in the SNOT-22 score from baseline to 6 months
-
the measure of effectiveness was the number of AEs.
-
-
CUA sensitivity analyses –
-
surgery costs were estimated using microcosting and compared with the NHS tariff
-
participant costs included
-
costs and QALYs were estimated for all participants with complete data only (i.e. no imputations for missing data)
-
the eligibility criteria were changed and the base-case CUA was run for participants who had a severe or extreme baseline NOSE score only
-
incremental cost per QALY at 6 months.
-
Stochastic sensitivity analysis, using the bootstrapping technique,118 explored the impact of the statistical imprecision surrounding estimates of costs, effects and cost-effectiveness. The bootstrapped results are presented on a cost-effectiveness plane (see Appendix 2, Figure 36) and used to illustrate the distribution of incremental costs and incremental effects from which we can identify the uncertainty in our results. 119
The bootstrapped results were also presented as cost-effectiveness acceptability curves (CEACs) (see Appendix 2, Figure 37). CEACs allow us to identify the management strategy that maximises net benefits at each threshold value for an additional unit of health effect (i.e. an improvement in SNOT-22 scores, AEs avoided and QALYs gained). 120
Results
Data validity and completeness
The response rates to participant questionnaires (the HUQ and the SF-36) used to inform the economic analysis are presented in Appendix 2, Table 73. There was a decline in response to the participant questionnaires over the 12-month follow-up period. Slightly fewer participants in the septoplasty arm responded to the participant questionnaires at 6 and 12 months than at baseline. The overall response rates were 66% for the HUQ and 68% for the SF-36. There was no difference in baseline characteristics or utilities between responders and non-responders [mean differences: age −3.15 (p = 0.0787), gender −0.04 (p-value = 0.5329), baseline utility −0.010 (p-value = 0.5768)], so we assumed that data were missing at random.
Resource use and costs
Over the 12-month follow-up, participants reported contacts with various healthcare providers in primary and secondary care settings. On average, participants in both arms reported similar healthcare contacts, with visits to a GP being the most frequently reported contact for both arms. Further details are provided in Appendix 2, Table 74.
Details on unit costs, which were combined with healthcare contacts, are provided in Appendix 2, Table 71. On average, participants reported similar healthcare costs at 6 and 12 months, which was expected given the resources reported in Appendix 2, Table 74. Medications prescribed during the follow-up are listed in Appendix 2, Table 75. Primary and secondary healthcare costs could be estimated for 66% of participants (n = 204). After these costs were combined with the intervention costs (septoplasty and nasal sprays) and medication costs, septoplasty was, on average, more costly at 12 months than medical management (mean difference £1277, 95% CI £1068 to £1487; p < 0.01). This difference in average total costs was maintained when missing cost data were imputed (mean difference £1189, 95% CI £1014 to £1363; p < 0.01). Table 14 presents the average total cost for each arm by cost category.
| Cost | Medical management (N = 155) | Septoplasty (N = 152) | Mean differencea (95% CI) (£) | ||
|---|---|---|---|---|---|
| Participants providing data (n) | Mean (SD) | Participants providing data (n) | Mean (SD) | ||
| Surgery costsb | 155 | 593 (902) | 152 | 1905 (314) | |
| Discharge medications | 9 | 8 (7) | 123 | 8 (7) | |
| Nasal spray costs | 155 | 91 (0) | 152 | 0 (0) | |
| HUQ costs | |||||
| At 6 months | 142 | 134 (238) | 140 | 156 (157) | |
| At 12 months | 115 | 143 (168) | 99 | 97 (128) | |
| Total | 109 | 261 (292) | 95 | 276 (234) | |
| Medication costs | 63 | 8 (15) | 84 | 16 (32) | |
| Total costs | 109 | 930 (980) | 95 | 2207 (358) | 1277 (1068 to 1487) |
| Total costs: multiple imputation | 155 | 973 (1028) | 152 | 2162 (375) | 1189 (1014 to 1363) |
Microcosting
In the septoplasty arm, 148 participants underwent the procedure and provided data on the eCRF for the microcosting exercise. Details of the resources used to undertake septoplasty for these participants are reported in Appendix 2, Table 72. On average, the length of time in theatre was 57 minutes and 99% of participants had septoplasty ± inferior turbinate reduction.
Participant costs
Participants’ time away from usual activities (including work) owing to illness is presented in Appendix 2, Table 74. On average, participants randomised to septoplasty reported more time away from usual activities at 6 months than those in the medical management arm (mean difference 1.09 days; p = 0.38). However, at 12 months, participants in the septoplasty arm reported, on average, fewer days away from usual activities (mean difference −2.39 days; p = 0.1155).
Private healthcare use is summarised in Appendix 2, Table 76. The majority of costs reported were not related to the participants’ deviated septa and were not included in further analysis.
Responses to the time and travel questionnaire are summarised in Appendix 2, Table 77.
Effectiveness outcomes
Summaries of all effectiveness measures by randomised arm are presented in Table 15.
| Outcome measure | Medical management (N = 155) | Septoplasty (N = 152) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Participants providing data (n) | Mean (SD) | Participants providing data (n) | Mean (SD) | ||
| Improvement in SNOT-22 score | |||||
| At 6 months | 155 | 0.381 (0.49) | 152 | 0.803 (0.40) | 0.422 (0.32 to 0.52) |
| At 12 months | 125 | 0.504 (0.50) | 119 | 0.756 (0.43) | 0.252 (0.13 to 0.37) |
| Number of AEs | 155 | 0.54 (0.90) | 152 | 0.84 (1.50) | 0.294 (0.02 to 0.57) |
| SF-6D utility scores | |||||
| At baseline | 152 | 0.712 (0.14) | 149 | 0.715 (0.14) | |
| At 6 months | 140 | 0.729 (0.14) | 140 | 0.789 (0.14) | |
| At 12 months | 117 | 0.742 (0.16) | 103 | 0.777 (0.14) | |
| QALYs | 111 | 0.741 (0.13) | 99 | 0.761 (0.13) | 0.021 (−0.01 to 0.06) |
| QALYs: multiple imputation | 152 | 0.727 (0.12) | 149 | 0.766 (0.12) | 0.040 (0.01 to 0.07) |
Estimation of health outcomes for the cost-effectiveness analysis
On average, a greater proportion of participants randomised to septoplasty than to medical management reported improved nasal function (measured as an improvement of ≥ 9 points in SNOT-22 scores) at 6 and 12 months, compared with baseline scores [mean difference at 6 months 0.42 (95% CI 0.32 to 0.52; p < 0.01), mean difference at 12 months 0.25 (95% CI 0.13 to 0.37; p < 0.01)].
On average, participants randomised to septoplasty reported more AEs than those randomised to medical management at 12 months (mean difference 0.29, 95% CI 0.02 to 0.57; p = 0.0382).
Estimation of quality-adjusted life-years for the cost–utility analysis
On average, participants reported their health status, measured by the SF-6D, as being less than perfect throughout the trial follow-up period. At baseline, participants in both arms reported having a similar health status and both reported improvements in their health status over the trial period. However, on average, those randomised to septoplasty reported greater improvements in their health status at 6 and 12 months post randomisation than those in the medical management arm. Participants in the septoplasty arm also reported more QALYs at 12 months (mean difference 0.02, 95% CI −0.01 to 0.06; p = 0.235) than those in the medical management arm. This difference in QALYs increased when missing SF-6D data were imputed (mean difference 0.04, 95% CI 0.01 to 0.07; p < 0.01).
Economic evaluation
Incremental cost-effectiveness analysis
Table 16 presents the unadjusted average total costs and average total proportion of participants who reported an improvement in nasal function measured using the SNOT-22 per randomised arm at 12 months. On average, septoplasty was more costly and more effective than medical management. Adjusted analyses were used to estimate incremental costs and effects; these estimates were used to estimate the ICER. The incremental cost for an improvement of ≥ 9 points in SNOT-22 score per participant was £4855. As the threshold value placed on an improvement in SNOT-22 score increases, so does the probability of septoplasty being considered cost-effective.
| Investigation strategy | Cost (95% CI) (£)a | Incremental cost (95% CI) (£)b | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an improvement in SNOT-22 scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £500 | £1000 | £3000 | £5000 | ||||||
| Outcome: SNOT-22 score – results | ||||||||||
| Medical management (costs, n = 155; outcomes, n = 125) | 973 (810 to 1137) | 1306 (1124 to 1489) | 0.504 (0.42 to 0.59) | 0.269 (0.16 to 0.38) | 4855 | 1.00 | 1.00 | 1.00 | 1.00 | 0.45 |
| Septoplasty (costs, n = 152; outcomes, n = 119) | 2162 (2102 to 2222) | 0.756 (0.68 to 0.83) | 0.00 | 0.00 | 0.00 | 0.00 | 0.55 | |||
The results of the stochastic sensitivity analysis are presented in Figures 19 and 20. Figure 19 is the cost-effectiveness plane; for all of the bootstrapped iterations, septoplasty was more costly and more effective than medical management. Figure 20 is the CEAC and shows that, as the value placed on an additional participant’s SNOT-22 score improving by ≥ 9 points in SNOT-22 score increases, so does the probability of septoplasty being considered cost-effective.
FIGURE 19.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CEA SNOT-22 score at 12 months (multiple imputation results).

FIGURE 20.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CEA SNOT-22 score at 12 months (multiple imputation results).
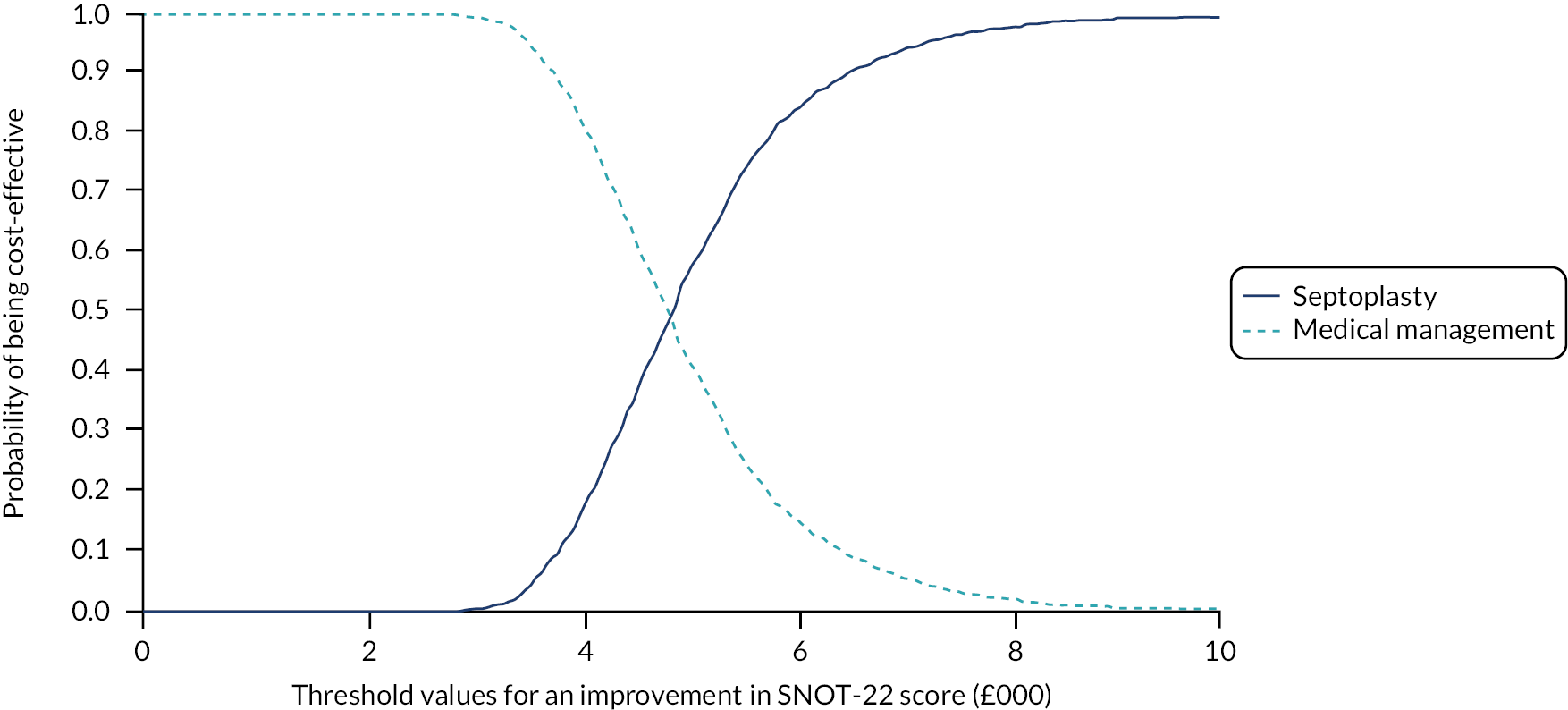
Incremental cost–utility analysis
Table 17 presents the unadjusted average total costs and average total QALYs per randomised arm at 12 months. On average, septoplasty was more costly and more effective than medical management. Adjusted analyses were used to estimate incremental costs and effects; these estimates were used to estimate the ICER. The incremental cost per QALY gained was £27,114. Similar to the CEA results, as the value placed on the additional benefit of septoplasty increased, so did the probability of septoplasty being considered cost-effective. Assuming a £20,000 threshold, septoplasty had a 15% probability of being considered cost-effective, which increased to 68% as the threshold increased to £30,000 at 12 months.
| Investigation strategy | Costa (95% CI) (£) | Incremental costb (95% CI) (£) | Effecta (95% CI) | Incremental effectb (95% CI) | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: QALYs – results | ||||||||||
| Medical management (costs, n = 155; outcomes, n = 152) | 973 (810 to 1137) | 1193 (1018 to 1368) | 0.728 (0.71 to 0.75) | 0.044 (0.03 to 0.06) | 27,114 | 1.00 | 1.00 | 0.85 | 0.32 | 0.01 |
| Septoplasty (costs, n = 152; outcomes, n = 149) | 2162 (2102 to 2222) | 0.767 (0.75 to 0.79) | 0.00 | 0.00 | 0.15 | 0.68 | 0.99 | |||
The results of the stochastic sensitivity analysis are presented in Appendix 2, Figures 36 and 37. In all of the bootstrapped iterations, septoplasty was more costly and more effective than medical management. The majority of the bootstrapped iterations are above the £20,000 NICE threshold. 113 The CEAC illustrates that, as the value placed on an additional QALY increases, so does the probability of septoplasty becoming cost-effective.
Sensitivity analysis
Cost-effectiveness sensitivity analyses
The measure of effectiveness was improvement in the Sino-nasal Outcome Test-22 items score from baseline to 6 months When costs and improvement in SNOT-22 scores were estimated at 6 months, septoplasty was, on average, more costly and more effective than medical management (see Appendix 2, Table 78, and Figures 38 and 39). The incremental cost per additional participant to have an improvement of ≥ 9 points in SNOT-22 score was £4303, compared with £4855 at 12 months. The difference in the ICER was driven by the larger proportion of participants in the septoplasty arm experiencing an improvement of ≥ 9 points in SNOT-22 score at 6 months than at 12 months.
The measure of effectiveness was the number of adverse events When the number of AEs was used as the measure of effectiveness in the CEA, septoplasty was, on average, more costly and less effective at reducing the number of AEs experienced by participants (see Appendix 2, Table 79, and Figures 40 and 41). In this analysis, septoplasty was dominated by medical management; over the range of threshold values considered to avoid an AE, the probability of septoplasty being considered cost-effective was zero.
Cost–utility sensitivity analyses
Surgery costs were estimated using microcosting and compared with the NHS tariff. When surgery costs were estimated using microcosting, the average total cost was £1276, which is lower than the NHS tariff of £1956. On average, septoplasty was more costly and more effective than medical management at 12 months (see Appendix 2, Table 80, and Figures 42 and 43), with an incremental cost per QALY of £16,682. The probability of septoplasty being considered cost-effective was 79% at a £20,000 threshold for an additional QALY.
Participant costs
When costs incurred by participants were included in the analysis, septoplasty was, on average, more costly and more effective at 12 months (see Appendix 2, Table 81, and Figures 44 and 45). The incremental cost per QALY was £24,136, and septoplasty had a 29% probability of being considered cost-effective at a £20,000 threshold.
Costs and quality-adjusted life-years were estimated for all participants with complete data only (i.e. no imputations for missing data)
When costs and QALYs were estimated for those with complete cost data (n = 204) and QALY data (n = 210), septoplasty was, on average, more costly and more effective at 12 months (see Appendix 2, Table 82, and Figures 46 and 47). The incremental cost per QALY was £37,371, and septoplasty had a 0% probability of being considered cost-effective at a £20,000 threshold, which increased to 23% and 83% at £30,000 and £50,000 thresholds, respectively.
The eligibility criteria were changed
When costs and QALYs were estimated for those who were classified as severe or extreme at baseline based on their NOSE scores (N = 267: medical management, n = 133; septoplasty, n = 134), septoplasty was, on average, more costly and more effective at 12 months (see Appendix 2, Table 83, and Figures 48 and 49). The incremental cost per QALY was £22,980, and septoplasty had a 24% probability of being considered cost-effective at a £20,000 threshold, which increased to 89% at a £30,000 threshold.
Incremental cost per quality-adjusted life-year at 6 months
When costs and QALYs were estimated at 6 months, septoplasty was, on average, more costly and more effective than medical management (see Appendix 2, Table 84, and Figures 50 and 51). The incremental cost per QALY was > £100,000, and septoplasty had a 0% probability of being cost-effective at the different threshold values for an additional QALY. This result is expected, as the shorter follow-up is effectively a bias against the more effective surgery because it allows for less time for gains in QALYs to offset the initial higher costs of surgery.
Model-based
Introduction
The within-trial results are useful to inform decisions about the cost-effectiveness of septoplasty in the short term (12 months). However, they provide limited information on the costs and benefits associated with septoplasty over the longer term. This is an important limitation of the within-trial analysis. We anticipated that the surgical arm could be more costly and potentially more effective, but the time horizon of the trial would not be sufficient for the additional benefit to offset the additional costs. This is illustrated in the analyses presented above when we compare the incremental cost per QALY for septoplasty versus medical management at 6 months’ follow-up (>£100,000) with that at 12 months (£27,114). To address this, we conducted a further model-based analysis. The question being addressed by the model-based analysis was as follows:
For adults suffering with nasal obstruction associated with deviated septum, if costs and effects were extrapolated beyond the 12-month follow-up period, at what point, if any, does surgical management have the higher probability of being considered cost-effective compared with medical management?
For the model-based analysis, the following outcomes are reported:
-
NHS/PSS costs of managing individuals with nasal airway obstruction associated with a deviated septum (including septoplasty) over a longer time horizon
-
QALYs over a longer time horizon
-
incremental cost per QALY gained.
Methods
An economic model was used to extrapolate the trial findings and estimate the cost-effectiveness of septoplasty, compared with medical management, at 24 and 36 months post randomisation. The model design and parameters used are described in this section.
Economic model
Model structure
A simple decision tree model was developed using TreeAge (TreeAge Software, Inc., Williamstown, MA, USA) to extrapolate costs and QALYs beyond the 12-month trial follow-up period. The model was designed to replicate the pathway of the trial participants. Figure 21 is an illustration of the model pathway. Data were obtained from the trial to estimate the relative costs and utilities of septoplasty, compared with medical management (see Incremental cost–utility analysis). Costs, QALYs and cost-effectiveness were estimated at 24 and 36 months.
FIGURE 21.
Model structure.

Model assumptions
The model was designed to incorporate the relative differences between septoplasty and medical management identified from the within-trial analysis.
The following assumptions were made for the economic model:
-
The model took the perspectives of the UK NHS and PSS.
-
Costs and utility data used in the model were based on the multiple imputation data.
-
Both arms of the model (medical management and septoplasty) were equivalent at the start of the model (i.e. it was assumed that costs and utilities reported by the medical management arm throughout the 12-month trial follow-up were the base for both arms).
-
The utility and healthcare resource costs for those who went on to have surgery were assumed to be equivalent to the medical management arm plus an additional adjustment was made based on the trial data (see Costs and Utilities for further information).
-
For extrapolation, the cost and utility values reported after 12 months were assumed to be the same as those reported in the last 6 months of the trial (i.e. between 6 and 12 months).
-
Costs and utilities at 24 and 36 months were discounted at 3.5%. 113
-
Assumptions concerning further treatments (septoplasty and nasal sprays) post 12 months were based on clinical guidance and explored in sensitivity analyses.
-
Given the short time horizon of the model, the average age of trial participants (40 years) and the trial data, it was assumed that no participants died over the 36-month time horizon.
-
It was assumed, given the limited data available, that those who had surgery would not need a revision surgery.
-
All model parameters were defined as statistical distributions in the model, and distributional assumptions were based on trial data.
-
Alternative assumptions were explored in sensitivity analysis (see Sensitivity analysis below).
Model parameters
The model parameters were informed using trial data and the distribution of each parameter using the mean, SD and shape of the distribution. Table 18 summarises all of the parameters used in the model.
| Model parameters | Mean (SD) | Distribution |
|---|---|---|
| Costs | ||
| Nasal spray | 91 (–) | No assumptions made on distribution as this was a fixed cost |
| Surgery | 1956 (–) | No assumptions made on distribution as this was a fixed cost |
| Healthcare resource use costs at 6 months (multiply imputed) | 138 (235) | Gamma |
| Healthcare resource use costs at 12 months (multiplyimputed) | 148 (156) | Gamma |
| Healthcare resource use costs adjustment at 6 months for those in the septoplasty arm who had surgery | 21 (23) | Gamma |
| Healthcare resource use costs adjustment at 12 months for those in the either arm who had surgery | −61 (18) | Gamma |
| Utilities | ||
| Baseline | 0.720 (0.14) | Beta |
| Utility at 6 months (multiply imputed) | 0.728 (0.14) | Beta |
| Utility at 12 months (multiply imputed) | 0.735 (0.15) | Beta |
| Utility adjustment at 6 months for those who had surgery in the septoplasty arm | 0.063 (0.012) | Beta |
| Utility adjustment at 12 months for those in either arm who had surgery | 0.068 (0.14) | Beta |
| Transition probabilities | ||
| Probability of having surgery (medical management) at 12 months | 0.30 | No assumptions made on distribution as based on trial data |
| Probability of having surgery (medical management arm only) at 24 months | 0.15 | No assumptions made on distribution as based on clinical advice, but explored in sensitivity analyses |
| Probability of having surgery (medical management arm only) at 36 months | 0.075 | No assumptions made on distribution as based on clinical advice, but explored in sensitivity analyses |
| Probability of having medical management at 24 and 36 months (medical management arm only) | 0.50 | No assumptions made on distribution as based on clinical advice, but explored in sensitivity analyses |
| Probability of having surgery (septoplasty arm only) at 12 months | 0.97 | No assumptions made on distribution as based on trial data |
Costs
The costs and cost adjustments for surgery used in the model are reported in Table 18. Intervention costs (surgery and/or nasal sprays) were assumed to be as reported in the trial and are assigned in the model depending on the pathway (i.e. nasal spray costs were assigned to the medical management arm only, the NHS tariff for septoplasty was assigned to those who went on to have surgery regardless of their randomised allocation). After 12 months it was assumed that there would be a demand for further treatments by those randomised to medical management. Those who went on to have surgery between 12 and 36 months were assigned the NHS tariff for septoplasty and those who continued with management received nasal spray costs for the remainder of the model.
We assumed that healthcare resource use costs would be equivalent at the start of the model and adjustments were made depending on whether or not surgery had been performed. The adjustment in costs at 6 and 12 months was based on an ordinary least squares regression of the multiply imputed healthcare resource use costs at 6 and 12 months. The same covariates used in the SUR (randomised arm, age, gender, ethnicity and baseline utility scores) were used. At 6 months, those randomised to septoplasty reported slightly higher healthcare resource use costs, but at 12 months they reported lower costs than those in the medical management arm (see Table 18). We assumed that healthcare resource use costs incurred after 12 months, assigned at 6 monthly intervals, were equivalent to those reported during the last 6 months of the trial (i.e. 12-month costs). Adjustments to healthcare resource use costs associated with surgery were assigned depending on when during the 36 months surgery was performed.
All costs (treatment and healthcare resource use) incurred after 12 months were discounted at 3.5%.
Utilities
The utility values and utility adjustments used to estimate QALYs in the model are reported in Table 19. Similar to costs, the multiply imputed utility data reported by the medical management arm throughout the 12-month trial follow-up were assumed to be the base and adjustments were made if surgery was performed. Similar to the cost adjustments, utility adjustments were estimated using an ordinary least squares regression controlling for the same covariates as the SUR (randomised arm, age, gender, ethnicity and baseline utility scores). At both 6 and 12 months, those randomised to septoplasty reported higher utility values; this adjustment was applied to the QALY equation depending on when septoplasty was performed. Similar to costs, utilities were assigned at 6-monthly intervals (18, 24, 30 and 36 months) and those incurred post 12 months were discounted at 3.5%.
| Investigation strategy | Cost (£) | Incremental cost (£) | Effect | Incremental effect | ICER (£) | Probability that septoplasty is cost- effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: QALYs at 24 months – results | ||||||||||
| Medical management | 1483 | 833 | 1.46 | 0.06 | 13,221 | 1.0 | 0.97 | 0.01 | 0.00 | 0.00 |
| Septoplasty | 2316 | 1.53 | 0.0 | 0.03 | 0.99 | 1.00 | 1.00 | |||
| Outcome: QALYs at 36 months – results | ||||||||||
| Medical management | 1785 | 703 | 2.20 | 0.10 | 7368 | 1.0 | 0.05 | 0.00 | 0.00 | 0.00 |
| Septoplasty | 2488 | 2.29 | 0.00 | 0.95 | 1.00 | 1.00 | 1.00 | |||
Transition probabilities
Transition probabilities in the model were based on the probability of those randomised to each arm undergoing septoplasty; these probabilities are detailed in Table 18. As expected, a higher proportion of those randomised to septoplasty than to medical management underwent surgery (97% vs. 30%, respectively). We assumed in the base-case analysis that no additional surgeries were undertaken at 24 or 36 months for those randomised to septoplasty. Based on clinical advice, we also assumed that there would be a steady decline in the uptake rate of septoplasty for those in the medical management arm after 12 months, and that a proportion of those randomised to medical management would recommence using nasal sprays to manage their deviated septum. These assumptions were explored in sensitivity analyses that varied the transition probabilities by ± 50%.
Model validation
We internally validated the model by checking the model structure, calculations and data parameters. 121 We undertook further validation of the model by running it for 12 months, to replicate the results of the within-trial analysis. Comparing these results allowed any potential discrepancies in the model parameters to be identified.
Sensitivity analysis
A probabilistic sensitivity analysis was undertaken to quantify any potential uncertainty in the model based on the model parameters. Each model parameter, except the intervention costs (surgery and nasal spray) and transition probabilities for surgery, which were fixed, had a measure of uncertainty surrounding it (SD) and was assigned a statistical distribution. The probabilistic sensitivity analysis facilitates the estimation of costs and effects using a set of parameters drawn from the statistical distribution using Monte Carlo simulations. Similarly to the bootstrapping of the within-trial results, 1000 Monte Carlo simulations were drawn to estimate the probability of septoplasty being considered cost-effective at a range of thresholds for an additional QALY.
Results
The model results at 24 and 36 months are presented in this section.
Costs
Similar to the within-trial results, septoplasty was, on average, more costly than medical management at both time horizons (24 and 36 months). Table 19 details the average total costs for both arms at each time horizon.
Effectiveness
Septoplasty was, on average, more effective in terms of QALYs gained at 24 and 36 months (see Table 19).
Incremental cost per quality-adjusted life-year gained
Table 19 details the model results at 24 and 36 months. The conclusions of the model remained similar to that of the within-trial results in that septoplasty was, on average, more costly but more effective than medical management. However, as we extrapolated costs and QALYs over a longer period (36 months), the incremental cost per additional QALY reduced to £13,221 (at 24 months) and £7368 (at 36 months). At 24 and 36 months, septoplasty had the highest probability of being considered cost-effective at a £20,000 threshold for an additional QALY, compared with medical management (99% at 24 months, 100% at 36 months; see Appendix 2, Figures 52–55).
Sensitivity analyses exploring variations in the probability of surgery in the medical management arm did not change conclusions: septoplasty had the higher probability of being considered cost-effective at a £20,000 threshold for an additional QALY at 24 and 36 months.
Chapter 5 Qualitative research integrated within Trials Recruitment Intervention study
Introduction
Randomised evaluations of health care are vital to promoting an evidence-based culture in surgery, but executing them to a high standard can be challenging.
The NAIROS team anticipated challenges in recruitment, based on the members’ own experiences of previous/ongoing surgical trials and awareness of the existing literature and others’ experiences of under-recruitment. Engagement with patients and members of the public in designing the NAIROS also signalled the prospect of recruitment challenges, particularly patient and clinician preferences for surgery. We anticipated significant recruitment challenges in this RCT, particularly as we assumed that many patients would have been prescribed steroid sprays prior to hospital referral. The study team also foresaw the possibility of surgeons’ habitual practices and individual experiences complicating recruitment.
Given these concerns, the NAIROS included an integrated QRI: a complex intervention that seeks to prevent and address recruitment issues in RCTs. Since conception of the QRI methods in the NIHR-funded Prostate cancer testing and Treatment (ProtecT) study,122,123 the QRI has iteratively developed through its application to many RCTs deemed challenging for recruitment, culminating in publication of the QRI protocol in 2016,124 and methodological guidance on how to implement the intervention. 125
The ethos of the QRI is to develop an empirically grounded, rapid understanding of recruitment, and then use these insights to design tailored solutions to optimising recruitment. This occurs in two cyclical phases that run contemporaneously to the trial’s recruitment period. Recruitment issues are investigated in phase 1; these inform which actions are required to optimise recruitment in phase 2.
The QRI was incorporated into the NAIROS trial partway through its competitive funding application, and thus the budget for this work was limited. The QRI was funded for 12 months of the 20-month recruitment period, to coincide with the planned internal pilot. A new ‘pre-emptive’ phase was also incorporated into the NAIROS, consisting of activities to optimise recruitment from the inception of the NAIROS (e.g. training). This stage drew on a wealth of evidence from previous RCTs that had integrated QRIs, and the latest published literature. When sites opened to recruitment, the pre-emptive phase was followed by the two-phased approach described previously.
Methods
The methods and activities spanning the pre-recruitment and recruitment phases of the NAIROS trial are outlined below.
Methods in the pre-recruitment phase: pre-emptive support
Anticipated sources of recruitment difficulty were addressed by:
-
critically reviewing patient-facing materials
-
designing and delivering training to raise awareness of common recruitment issues and solutions
-
consolidating key messages from training in a written ‘tips’ document.
Methods in recruitment phase: phase 1 and phase 2 of the QuinteT Recruitment Intervention
Methods to investigate actual (rather than anticipated) recruitment processes in the NAIROS are outlined below (phase 1), followed by a summary of how these informed responsive actions to optimise recruitment (phase 2).
Phase 1 of the QuinteT Recruitment Intervention
Interviews with the Nasal AIRway Obstruction Study recruiters
Semistructured interviews were conducted with members of the TMG and with recruiting staff across sites, to explore views on the NAIROS question/study design and experiences of recruitment to date. The purpose of the QRI was explained in a ‘healthcare professional PIS’, and all interview participants gave written informed consent prior to the interview. Interviews were conducted over the telephone or face to face and were audio-recorded with permission. Interviews were guided by an interview schedule, informed by prior research and literature on recruitment to RCTs, including the QRI team members’ experiences of working on previous RCTs. Topics covered included views about the NAIROS research question/design, organisation of recruitment processes at sites and experiences of inviting patients to consider trial participation.
Audio-recording of discussions between potential randomised controlled trial participants and recruitment staff
Recruiters across sites were encouraged to audio-record their appointments with potential RCT participants. Ten encrypted recorders were shared between 17 sites for the duration of recruitment. Both healthcare professionals and patients provided written informed consent for audio-recording appointments. Recordings were periodically securely transferred to the QRI team for analysis.
Screening log data collection and analysis
A log of each potentially eligible participant was created at site level and periodically sent to the NCTU, where the information was logged and maintained. This log was used to identify points where potential participants were lost from the recruitment pathway. We requested that sites complete the logs for all patients screened for trial participation who had a blocked nose and suspected deviated septum.
Analysis of phase 1 data
QuinteT Recruitment Intervention interviews were transcribed and analysed thematically using constant comparative approaches, adopted from grounded theory. 126 Transcripts were stored and managed using NVivo software, version 12 (QSR International, Warrington, UK) to facilitate analysis.
Audio-recorded recruitment consultations were transcribed selectively, focusing on discussion about the trial, with other parts of recordings summarised through notes. Consultations were analysed thematically, using inductive approaches, albeit with a priori interests informed by researchers’ previous experience and engagement with the literature (e.g. communication of equipoise, elicitation and management of treatment preferences). Consultations were regularly revisited, often with a new analytical lens informed by other QRI activities and emerging recruitment issues. As a result, content analysis was sometimes employed when we intended to identify discussion pertaining to a specific topic. A detailed explanation of the blend of inductive and deductive approaches used for QRI consultation analysis have been reported elsewhere. 125
The QRI analysis used several approaches to enhance rigour. Every transcript was independently coded by at least two researchers to enhance the credibility of the findings reported. Findings were discussed and refined through regular meetings between those involved in QRI data collection/analysis. We also intended to seek out ‘negative cases’ throughout, to ensure that the QRI findings were a full and accurate representation of the breadth of views/experiences (interviews) and practices (consultations) reported.
Phase 2 of the QuinteT Recruitment Intervention
The findings from phase 1 data sources were regularly shared with the TMG through reports and meetings, to inform the design and implementation of ‘actions’ to address recruitment issues. The actions implemented are described below in Results.
Results
QuinteT Recruitment Intervention activities are reported here chronologically, with empirical findings and actions to optimise recruitment reported in tandem across three sections:
-
pre-emptive strategies to optimise recruitment (i.e. before sites opened)
-
phase 1 QRI findings (understanding recruitment in practice)
-
phase 2 QRI actions to address sources of recruitment difficulty while the trial was under way.
Pre-emptive strategies to optimise recruitment
Given that the NAIROS team anticipated that patient preferences/expectations for surgery would be an obstacle to recruitment, key issues considered at the outset of the RCT centred around conveying equipoise and exploring treatment preferences, to ensure that patients were fully informed. These topics were covered in training materials and considered in patient-facing refinements to recruitment materials (e.g. PISs and videos), as summarised below.
Recruitment training
A trial launch meeting was held on 11 May 2017, prior to sites opening to recruitment. This included a recruitment training session covering the following elements:
-
encouraging consistent messaging about the NAIROS within sites, among trial personnel and colleagues who may interact with patients along the clinical pathway; the training materials included short phrases that non-recruiting colleagues may use to avoid formulation (or reinforcement) of patient expectations for surgery (e.g. ‘We do not know which treatment is better’, ‘You’ll be hearing about the NAIROS study’)
-
suggested wording for introducing the NAIROS, to convey its national scale and integration with NHS care
-
raising awareness of equipoise, including what the term means, and ways it can be lost in communication with patients
-
encouraging recruiters to explore and understand patients’ treatment preferences, with a view to safeguarding informed consent.
The points relating to communication were reinforced through a ‘tips and guidance’ document: a single sheet of bullet points to support recruiters’ explanations of the trial. This was disseminated to all sites at the trial launch. The aforementioned pillars of recruitment training were carried forward to the site initiation visits held with each centre before they opened to recruitment.
Revisions to the participant information sheet
The PIS was scrutinised in terms of its clarity and consistency and how well it captured the equipoise underpinning the NAIROS. Revisions to the PIS were informed by previous research, including evidence around ways in which equipoise can be over-ridden or undermined,127 patients’ understanding of common explanations of randomisation128 and insights from previous RCTs with integrated QRIs or qualitative process evaluations led by NAIROS team members. Iterations to the PIS were made over several reviews between April to November 2017.
Several statements throughout the original PIS (version 0.1) were identified to be potentially disruptive for conveying equipoise. For example, the nature of the clinical problem was often framed in terms of the patient’s deviated septum, rather than their symptoms of nasal obstruction. This was addressed through edits that framed the clinical problem around patients’ symptoms of blocked nose, as per the trial protocol. In alignment with this, further revisions aimed to address any subtle indications that patients’ nasal obstruction was definitely caused by the deviated septum. Statements were included to explain that nasal obstruction could be caused by inflammation and mucus (Table 20), and that people with septal deviations can experience normal breathing. The original PIS also focused on surgery a great deal, without equal mention to medical management. For example, the section on side effects covered only surgical risks, with no mention of the risks associated with medical management.
| Key concepts/issues | Details of issues | Changes implemented |
|---|---|---|
| Conveying equipoise | Presenting the twisted septum as the main problem requiring resolution:Doctors do not know whether surgical care (septoplasty) or medical management is the best method for treating a twisted septum (midline nasal partition) PIS version 0.1 | Focus on the symptoms of the blocked nose, rather than the anatomical deformity (the deviated septum):Doctors do not know whether surgery (septoplasty) or medical management is the best method for treating problems with a blocked nose (nasal airway obstruction) PIS version 0.7 |
| You have been referred to the Ear, Nose and Throat clinic as you have had problems breathing through your nose due to a suspected twisted septum PIS version 0.1 | You have been referred to the Ear, Nose and Throat clinic as you have had problems breathing through your nose (‘nasal airway obstruction’) PIS version 0.7 | |
| Presenting the twisted septum as the cause of the nasal blockage:You have had problems breathing through your nose due to a suspected twisted septum | Presenting uncertainty about the cause and best treatment for the nasal blockage:The nasal airway obstruction could be due to inflammation and mucus which affect breathing through the nose. A twisted septum can cause problems with breathing through the nose but it is not always the cause of nasal airway obstruction. Sometimes people with a very twisted septum can still breathe fine through their nose PIS version 0.7 | |
| Only risks of surgery presented under ‘risks’ and ‘side effects’ | Incorporation of two subsections: one covering risks of surgery and one covering risks of medical management | |
| Explanation of trial terminology: ‘randomisation’ | References to a computer ‘picking’ a treatment:Your group will be picked by a computer. We call this ‘randomisation’. Your doctor will not have any say on which group you are put in Version 0.1 | Presenting a full explanation of the process and purpose of randomisation:The group you are allocated to will be determined by chance, through a process called ‘randomisation’. This process will help us to achieve two groups of patients that are similar in every respect, with exception to the treatment they receive. This will help us to fairly compare these treatments at the end of the study. Neither you nor your doctor can choose the group you are assigned to, but the team involved in your care are confident that either group will be suitable for you PIS version 0.7 |
Other revisions to the PIS concerned the explanation of ‘randomisation’. References to the computer ‘deciding’ treatment were removed and replaced with a description of the process and purpose of randomisation. This change was prompted by the process evaluation team’s experience of working on a previous RCT, in which patients had assumed that the computer determined the best treatment for them. Findings from previous QRIs had also indicated that patients can interpret the computer as having agency. 128
Feedback from the PPI panel review of the draft PIS was incorporated into the updated version of the PIS included in substantial amendment 1 to the REC. This was the first version of the PIS implemented at sites.
Refinements to recruitment video script
The aforementioned principles informed the content of a recruitment video which simulated a recruitment consultation between an ENT consultant and a potential participant (a patient actor). We reviewed the script several times to ensure it was consistent with the edited PIS and recruitment training materials. Script edits focused largely on conveying equipoise, as the details around medical management were less well developed in comparison with the details for septoplasty. Trial processes were also often described in terms of surgery, rather than medical management. In summary, the revisions were centred around:
-
identifying areas of the script where surgery was described and explained without similar attention devoted to medical management
-
increasing information about medical management (e.g. period of intended use).
Phase 1: QuinteT Recruitment Intervention findings – understanding recruitment in practice
The first site opened to recruitment 5 months later than planned, in January 2018, owing to governance and regulatory approval delays. Ten sites were projected to be open by this point. With recruitment already behind schedule, rapid understanding of barriers to patient accrual was critical in the remaining 7 months of the funded QRI.
All phase 1 QRI data collection (apart from two preliminary interviews with TMG members which were conducted before began) was focused on actual recruitment interactions and experiences. QRI data collection in phase 1 comprised 19 interviews, 108 audio-recorded consultations and regular scrutiny of screening logs. Findings from these sources were triangulated to build an in-depth understanding of recruitment processes across sites. Findings from the interviews and audio-recorded consultation analysis are presented separately, with cross-references to other data sources when relevant.
Screening log analysis over first 6 months
Scrutiny of monthly screening logs provoked questions about how recruitment was organised across the NAIROS sites. Logs collected over the first 6 months indicated that most eligible patients consented to randomisation, with many sites having 100% conversion rates. The number of patients entered on the logs was, however, highly variable, prompting questions around processes for identifying and approaching patients across sites. For example, no patients had been entered onto the log over the first 2–3 months of some sites opening to recruitment. The early stages of identifying/approaching patients became a key focus for further investigation in QRI interviews.
Interview findings
Sample of interview informants and presentation of findings
A total of 19 recruiters, from 11 of the 15 sites open to recruitment at the time, participated in interviews between May 2017 and August 2018. Of these 19 informants, three were research nurses and 16 were consultant ENT surgeons. Most informants (17/19) had experience of recruiting to the NAIROS as their sites had been open for 2–5 months at the time of interview. Two interviews were conducted before recruitment began with individuals who held dual roles as recruiters and TMG members. Thirteen of the 19 interviews were semistructured and directed by the QRI topic guide. Six were structured discussions about sites’ recruitment pathways.
Findings from the interviews have been presented according to three topics: (1) perceptions of equipoise and the need for the NAIROS, (2) organisation of recruitment at the site level and (3) experiences of discussing the trial with patients. Illustrative anonymised quotations have been presented throughout, with careful attention to present negative cases when relevant. Identifiers for quotations show the following:
-
the interview participant’s number (‘R’ = ‘recruiter’)
-
site number, specific to the QRI study (note that a QRI-specific site numbering system was used that was different from the wider trial’s site numbering; this was partly because only a subset of sites took part in the QRI study. The QRI-specific numbering system also preserves sites’ anonymity)
-
role of the individual [research nurse or surgeon, with an indication of which surgeons were also the principal investigator (PI) for the site].
Perceptions of equipoise and the need for the Nasal AIRway Obstruction Study
Recruiters’ perceptions of equipoise were explored in the early stages of the QRI to provide context for understanding recruitment issues, but these were also important topics covered in the trial’s qualitative process evaluation (see Chapter 6). To avoid replication, detailed insights around recruiters’ perceptions of the trial question and eligibility criteria are reported in the context of the process evaluation, which builds on some of the data collected for the QRI. In terms of optimising recruitment, the below findings (relating to this part of the QRI topic guide) were key:
-
Surgeons and research nurses indicated support and commitment to recruiting to the NAIROS, acknowledging a need for evidence to inform policy and practice around management of nasal obstruction. Several informants (all surgeons) alluded to the NAIROS’s significance to commissioners and policy-makers, as shown through comments that there was an increasing expectation or ‘demand’ (R9, site 5) to produce evidence to justify continuation of existing practices:
I think it’s a really important subject. Politically, it’s important […], where there are increasing pressures to give good evidence that what we’re doing, optimally, for patients is the right thing and is going to be a proven benefit for them.
R15 (surgeon PI, site 7)
-
Most surgeons anticipated that the NAIROS would address clinical uncertainties around which patients would benefit the most from septoplasty. In alignment with this, most surgeons anticipated that the NAIROS would show a relationship between degree of septal deviation and benefit:
I think it will show that, in patients who have significant symptoms, and a significantly deviated nasal septum, that there are benefits to be had from surgery. So I think it will be very much based on our baseline stratification.
R11 (surgeon, site 6)
-
Most informants expressed that they were comfortable with and willing to approach the full spectrum of eligible patients for the NAIROS, despite some surgeons acknowledging that this required them to over-ride instincts about which patients would benefit from septoplasty. Two surgeons raised the prospect of not approaching patients whom they felt would benefit from a surgical procedure outside the NAIROS protocol. Based on interviews/screening log data, it was not possible to gauge the extent to which this quantitatively affected recruitment.
These findings provided insight into clinical professionals’ vantage points as they embarked on recruiting to the NAIROS (see Chapter 6 for more insights about eligibility assessment).
Organisation of recruitment at the site level
Recruiters’ interview accounts revealed variable ways in which the NAIROS had been integrated within routine practice, which had potential to support or hinder recruitment, specifically by having implications for the identification of potentially eligible patients (referred to as ‘NAIROS candidates’ hereafter).
Identifying Nasal AIRway Obstruction Study candidates and ascertaining eligibility Initially, most (and eventually all) sites relied on dedicated research clinics, to conduct eligibility assessments, consent discussions, randomisation and baseline assessments, given the difficulties of incorporating these tasks into time-limited routine clinics:
The first time we did it, it took us 2 and a half hours, and that is purely because it was the first time we were doing it. You need to cater for the patients needing to go through all that. So, it’s not really feasible in a clinic setting.
R20 (surgeon PI, site 10)
Most sites were able to accommodate research clinics into clinical schedules, but their frequency varied, ranging from once a week to once a month. Therefore, although NAIROS clinics were necessary, their frequency and the limited numbers of patients they could accommodate constrained recruitment:
… our clinics are full every month. We don’t have scope at the moment to add more patients in […], room space is a problem. We have a room and the consultant has a room, so if we’re both recruiting different patients, even if we stagger patients, we would still need an extra room.
RN7 (research nurse, site 3)
Although most sites had managed to set up research clinics, identifying patients to invite to these clinics was a commonly reported challenge across sites. Research nurses and surgeons described two approaches to identifying NAIROS candidates, as shown in Figure 22.
FIGURE 22.
Approaches to identifying patients potentially eligible for the NAIROS from routine NHS care.

The first method involved screening referral letters accessed through ‘choose and book’ (described below) and written referral letters. Site PIs (all of whom were surgeon consultants) tended to triage patients referred to their own clinics, with further involvement from research nurses and other consultants depending on the site. Irrespective of who and how many individuals were involved in screening, identifying patients based on referral letters alone had its limitations. According to several recruiters, letters did not contain the level of detail needed to gauge NAIROS suitability, resulting in those screening erring towards a more inclusive approach. As a result, the limited capacity research clinics could be filled with patients who were not even suitable for eligibility testing:
We’ve found it quite difficult, I think, to assess patients adequately enough from the GP letters, so it’s difficult to know whether they’re even a suitable patient until you bring them into the NAIROS [clinic]. For example, the first patient that I saw in the clinic had other problems with their nose that meant that they were ineligible to meet the eligibility criteria.
R15 (surgeon PI, site 7)
Some sites were able to address these issues with support from research nurses, who were able to conduct additional investigations of patient records to better gauge suitability, but this support was not universally available.
Another limitation of identification through letters was the transition to electronic ‘choose and book’ processes in some sites, whereby patients select their own appointment in routine clinics. Informants from one site explained that as a result of these ‘choose and book’ proceses, patients had often already arranged their routine care appointment when they were invited to attend a NAIROS clinic:
Patients, when they get their information pack, are usually halfway along the journey.
R1 (surgeon PI, site 1)
Considering the above difficulties, most sites either moved towards relying on consultants to identify patients who had been referred to their routine clinics (see the pathway on the right in Figure 22), or reportedly adopted this approach from the outset. According to several informants, consultant-led identification in clinics had the benefit of greater precision in referring suitable candidates to NAIROS clinics:
… if we draw our patients from a pool that have already been already seen in the main clinics and are already deemed as potentially suitable for septal surgery or a trial of medical treatment, I think that’s probably the way forward. Otherwise, I think you end up spending a lot of time seeing patients that, probably, will never have been eligible to be included in the trial in the first place.
R15 (surgeon PI, site 7)
‘In-clinic’ identification was operationalised in different ways across sites, with each model involving consultants who did not hold formal recruitment roles. In some sites, consultants examined patients in routine clinics and referred potentially eligible candidates to research clinics. In others, consultants triaged letters and re-routed patients with a septal deviation to a research nurse or the routine clinic of the NAIROS PI, who would then invite suitable candidates to research clinics:
I’m the main recruiting clinician. […] We triage our inpatients every week. So different consultants would triage patients and then they will identify potential patients who might fit the NAIROS criteria and let me know about them.
R9 (surgeon PI, site 5)
The success of in-person identification of NAIROS candidates hinged on engagement from clinical staff who were not directly involved in the NAIROS. Informants varied in their impressions of how engaged their colleagues had been, but several emphasised the importance of regular promotion of the NAIROS, through presentations and departmental meetings:
Because I think the thing about recruitment, you’ve got to remind people all the time, yes? Putting just a poster is not enough. […] Yes, so I’m seeing them – I think it’s the [date] is our [meeting name] – and I will remind everybody. That’s the whole department there, which is good. So all the consultants, all the registrars, all the doctors.
R18 (surgeon PI, site 9)
Supporting non-Nasal AIRway Obstruction Study consultants to introduce the trial There was some indication from interviews that recruitment to the NAIROS was being constrained by patients declining invitations to attend research clinics:
So, for every one that considers it, I think there are at least two who would say, ‘No, no’. So, round about 40–50%. It is high.
R20 (surgeon PI, site 10)
The original NAIROS screening log did not capture events preceding eligibility assessments, and thus the proportion of patients lost in the pathway before hearing the ‘full explanation’ of the trial was not formally documented. Interviews did, however, highlight the ENT consultants’ pivotal role in introducing the trial, in addition to their role in identifying candidates:
… well, usually it’s not easy to find someone that is a proper candidate for the study, for different reasons. But even when you do that, sometimes it’s not easy seeing the patient in 15 minutes of an appointment to easily introduce them to the study or to have enough time to do that. I believe this is not only for me, it’s for everyone, because we try to ask the help of other clinics so we can get someone that is a candidate. I mean, it doesn’t need to be too long or too much to introduce the patient at the start, but sometimes it’s not easy to get there.
R19 (surgeon, site 9)
Taken together, these accounts reinforced the idea that NAIROS recruitment relied on events and processes that preceded the formal ‘recruitment setting’ outlined in the protocol. Engagement from non-recruiting clinicians in identifying patients and providing high-level introductions to the trial appeared to be key to recruitment success.
Recruiters’ experiences of discussing the trial with patients
Explaining the NAIROS to patients was a key element of the recruitment process. The sections that follow are based on recruiters’ interview accounts, and draw on their experiences of explaining the trial.
Informants from sites that had not yet opened to recruitment anticipated that patient expectations and preferences for surgery would be a key barrier to recruitment. Those with recruitment experience (at the time of interview) also reflected back on their belief that patients would expect or prefer septoplasty. This belief stemmed from their assumption that many patients would have already received steroid sprays in primary care:
I think the majority will probably come, or the majority who have a preference will have a preference towards surgery. I think that’s possibly culturally, and traditionally, because in the UK, unlike a lot of other healthcare systems, you have a general practice where you have a sort of gatekeeping role. So, you have quite a lot of treatment done in general practice, and they will have had treatment in general practice, and so I think quite a few of them will, when you are referred to hospital, sometimes it’s almost with an expectation you’re going to have an operation.
R11 (surgeon, site 6)
Recruiters’ descriptions of their actual patient encounters suggested that the above concerns had not materialised. Surgeons and research nurses from different sites commented that recruitment had proceeded better than anticipated, as evident through unprompted remarks about how patients’ reactions had surprised them:
Yes, I think we are meeting our targets so far. I’m certainly surprised. I thought some patients would say no, but I think just about every patient we’ve approached has said yes so far.
R4 (surgeon PI, site 2)
In contrast to concerns about patients holding strong preferences/expectations for surgery, several recruiters discussed how the uncertainty around surgery versus medical management had resonated with patients:
I feel confident and there are some patients I’ve come across who genuinely don’t know which way they would like to go – either medical therapy or surgical therapy – and they’ve been delighted that that’s been taken out of their hand with the trial.
R9 (surgeon PI, site 5)
Certainly the feedback and the impression I’m getting from the patients we talk to, unless they’ve already definitely made up their minds which they want to try, which we’ve had a couple of … otherwise they seem quite open to it, the question itself, you know? A lot of them are maybe asking themselves, ‘What is best? Should I take medication or should I have surgery?’.
R6 (research nurse, site 3)
Preferences were, nonetheless, still raised by several recruiters when asked about the main barriers to recruitment. When discussed, references to preferences were either tempered with the positive accounts of recruitment (as per the previous quotation from R6), or framed as a reason why some patients had declined participation:
Interviewer: From your perspective, what were the facilitators and the barriers to getting people engaged in the study?
R14 (Research nurse, site 6): I think it is people who are determined that they want surgery. That’s the biggest barrier. I think there has been three or four who quite clearly, from the outset, they maybe have reasonable reasons and rationale for wanting surgery. Again, that is something that, just through the way it’s gone, has changed my mind. I thought NAIROS would struggle to recruit […]. I thought that people would, once they got as far as this, would be expecting surgery and they would reject the ideas of the sprays because they would have tried that. So I thought it would be quite difficult to recruit to, but just from our first few clinics and first few patients, I can see that the questions for patients are much wider and they’re not as straightforward.
We may not have regarded preferences as a significant obstacle to recruitment as most recruiters expressed confidence in engaging with patients’ preferences. There were some exceptions to this (n = 3, all research nurses), whereby informants framed preferences as a clear-cut signal to stop discussing the trial, as further discussion would be futile or inappropriate. Notably, two of these individuals had not been involved in the NAIROS from its outset and had not attended the pre-recruitment training. By contrast, all surgeons (including site PIs) indicated that they were comfortable exploring preferences, particularly if these appeared to be based on expectations for surgery:
R1 (surgeon, site 1): Most of them come with an expectation to have surgery.
Interviewer: How do you manage that?
R1 (surgeon, site 1): I tell them what the evidence is and why we conduct trials – to help decide what the best way to manage patients with this is. I help them to understand that if it’s an anatomical obstruction, where the nasal septum is slightly off to one side and causing an obstruction that, actually, will have been there for most of their lives, but often their symptoms are only recent and so it’s something else that’s changed. That’s where equipoise comes in, in that it’s a good research question.
The one informant who framed preferences as a dominant issue discussed this in the context of inviting patients to attend research clinics. This informant estimated that around half of patients had declined the invitation [see the quotation from R20 (surgeon PI, site 10) in Supporting non-Nasal AIRway Obstruction Study consultants to introduce the trial]; however, none of the other informants discussed these challenges.
In summary, interviews with the NAIROS staff provided an opportunity for staff to reflect on their interactions with patients. This was, for most, a positive experience. Nonetheless, these positive accounts must be considered alongside the fact that discussions in the research clinic are unlikely to reflect the views of those patients less willing to consider participating in the NAIROS. There is also a limit as to what can be confidently gauged from people’s accounts of their interactions with others, especially when reflecting on past events. However, it was clear that many recruiters were surprised about how patients had engaged with the trial, despite the existence of preferences and expectations for surgery. It was also clear that most recruiters felt equipped to engage with preferences and expectations. The final section presents findings from the analysis of audio-recorded recruitment conversations, to provide insight into how the trial was conveyed by recruiters and received by patients.
Analysis of audio-recorded consultations
Recruiters had already received multiple forms of training and resources to support communication when recruitment began, although these materials were based on previous QRI insights adapted for the NAIROS. Audio-recording NAIROS consultations provided opportunities for more specific feedback and training, based on the actual (rather than anticipated) issues to emerge.
Sample of consultations and presentation of findings
A total of 108 consultations with 105 patients were audio-recorded, contributed by 16 recruiters from eight sites. Of these:
-
15 consultations did not include any discussion about the NAIROS or treatment within/outside the trial
-
the 93 remaining consultations included at least some discussion pertaining to the NAIROS.
Despite the pre-emptive recruitment training/resources, we labelled the consultations as ‘pre feedback’ or ‘post feedback’, depending on when recruiters first received NAIROS-specific feedback relating to their practices, either on an individual or on a group basis. For most recruiters, the first substantive feedback event occurred on 20 September 2018 (month 9 of the recruitment period). Two recruiters received individual feedback before this point (months 7 and 8 of recruitment). Of the 93 consultations, 57 were pre feedback and 36 were post feedback.
Most audio-recorded consultations were with patients who appeared to agree to randomisation, although the recruitment outcomes of consultations were not always captured, and recordings did not always align with screening log information. The outcome of the consultation was not a core focus when analysing the recordings; rather, the focus was on the clarity and accuracy of information provision and patients’ reactions to information.
The key findings from the audio-recordings that informed feedback to recruiters are summarised in the following sections, Conveying equipoise and Understanding patients’ expectations and preferences.
Findings are supported by illustrative extracts, labelled according to recruiter number (‘Rx’), site number and consultation number. The speaker is represented as ‘Rx’ (recruiter) or ‘P’ (patient). All recruiters were surgeons, unless otherwise specified.
Conveying equipoise
Each of the 93 recordings included examples of recruiters articulating equipoise, using a variety of techniques to express and reinforce its meaning.
Some approaches to conveying equipoise aligned with the pre-emptive training and ‘tips and guidance’ documents issued in the ‘pre-recruitment’ phase. For example, the guidance suggested conveying equipoise by expressing:
-
uncertainty around whether septoplasty or medical management was more effective for symptoms of blocked nose
-
the appropriateness of both treatments for the eligible patient
-
the advantages/disadvantages and practical/clinical considerations of both treatments.
These techniques were demonstrated in some form by all recruiters who contributed recordings. Uncertainty as to which treatment was best was most consistently articulated:
The idea of the study, as I said at the start, was that we’re not entirely sure. In fact, we’re not sure at all which type of treatment is better: whether surgery or having the spray in your nose improves things and whether one is working better than the other.
R17, site 8, consultation 2
Many recruiters routinely communicated that both treatments were standard, appropriate approaches to treating a blocked nose outside the context of the trial:
They’re all good options. We don’t know what the best option is.
R10 (site 6, consultation 4)
We could, quite happily, give you either treatment and both would be perfectly acceptable treatments.
R17 (site 8, consultation 1)
The ‘tips and guidance’ document was less prescriptive in terms of how to balance the treatment arms. It did not articulate any specific information about what advantages/disadvantages to mention. In practice, the recordings showed that descriptions of the trial arms’ risks/benefits and practical considerations varied across recruiters, in terms of the type of information covered and the level of detail expressed. However, given the protracted nature of the recruitment and treatment pathway in the NAIROS, there is a possibility that recruiters provided further information about the risks/side effects of surgery before or after the recorded consultations provided. Some of the challenges relating to imbalanced explanations of the trial arms, as apparent through the recorded consultations, are integrated throughout the consultation findings presented below.
Uncertainty around surgery effectiveness In departure from the ‘tips and guidance’ document, some recruiters tended to specifically frame uncertainty around septoplasty, pointing out its lack of an evidence base and the possibility that it may not resolve nasal congestion. This approach was evident in many consultations where patients engaged with the premise of the trial and consented to randomisation, but there were also examples where this created a challenging foundation for recruitment. The prospect that surgery may not work was not a convincing rationale for randomisation to some, particularly those who had tried medical therapy before. For example, the prospect of surgery not working was not a sufficient deterrent for the patient below, who felt they should at least try it:
I don’t think the spray’s going to do anything to be honest, I don’t. I honestly don’t think it will work, so I’ll just go straight for the operation […]. Just go straight for the operation. If the operation’s not successful, so be it.
Patient, R10, site 6, consultation 1
For this patient, medical management was not a viable option, as shown through their implicit assumption that it would not ‘do anything’. This was based on their prior experience of spray use. In this patient’s case, simply hearing that surgery may not work was insufficient to discourage them from trying it. In contrast to this extract, other consultations were foregrounded with uncertainty around whether surgery or medical management was more appropriate for addressing nasal congestion, with explicit mention of how medical management may successfully resolve symptoms. Many recruiters substantiated these points by explaining that the treatment in the trial would likely differ to what they had used before, which appeared to be an effective strategy for presenting medical management as a viable option:
Patient: I would have the operation because when I was in [place] they gave us that, the spray stuff and it’s, it didn’t seem to do much.
R11, site 6, consultation 2: Yes, do you remember which one it was that you had?
Patient: I can’t remember. I had it for about … what? The spray, I used that for about 7, 8 months or something, but it didn’t seem to do much.
R11, site 6, consultation 2: Yes, OK. The spray that we’re using is a different one, more than likely. It may have been something like Beconase [GlaxoSmithKline plc, Brentford, UK]. In general practice it’s usually (Beconase). We’re using a newer generation one and we’re using it in combination with a nasal salt spray and it’s given to you as a package.
Septal deviation and surgical mechanisms of action Some recruiters tended to frame the NAIROS discussion around the deviated septum and/or septoplasty’s aim to straighten this anatomical deformity. Both recruiters and patients often directly or indirectly associated this with resolution of nasal obstruction. There were several examples of patients expressing a desire for their septum to be ‘fixed’ or ‘go back to normal’:
R12, site 6, consultation 1: You’ve been asked to see us because you’ve got a bent partition to your nose. One of the treatments for that would be a surgical operation to try and straighten the partition of your nose.
[Later]
Patient: To be honest, I’d like to have it rectified and fixed and maybe having it straightened might be the best outcome …
R17, site 8, consultation 5: The point with all of this is clearly you’ve got a problem with a blocked nose that you’re concerned about. We’ve had a look in and we’ve seen that the dividing partition of your nose – what we call the septum – is bent over to one side.
Patient: Yes. I just want to go back to normal.
R17, site 8, consultation 5: The problem that we’ve got is that there isn’t any good evidence to show that, that being bent over, doing a procedure to straighten it up actually improves the breathing overall.
Patient: It definitely will.
R17, site 8, consultation 5: The assumption is that, ‘Yes, it does’, and that’s why we’ve been doing the operation for years, but it doesn’t work in everyone. In some people we look in, they’ve got a bent nose internally. The septum’s bent over. They’ve got a blocked-up nose. We do an operation to straighten up and they say, ‘You know what? There’s no damn difference whatsoever. I’m still feeling completely blocked up.’ Despite us looking in their nose and thinking, ‘Well everything’s straight’. What this study is aiming to do is to look at that situation and say, ‘Actually, can we predict which patients will benefit from the surgery and which ones are going to be in that group that it makes no difference to?’
Patient: I’ll definitely benefit, because I do a lot of physical exercise. That’s where the big problem is.
As illustrated above, surgery’s potential to fix the bent septum resonated with some patients. Several recruiters adopted techniques that addressed this issue, including disassociating nasal obstruction from the septal deviation, and clarifying the spray’s intended mode of action.
The following example was used to dissociate symptoms from deviation:
What we do know is that there are plenty of patients around who have a blocked nose and a straight dividing partition. The problem is more to do with the lining of their nose than it is to do with the shape of it. Clearly, we’re trying to work out, ‘How important are these two different aspects?’
R17, site 8, consultation 5
In a later example, the mechanism of action of spray was outlined:
Whether you have an operation to straighten your nose up or whether we give you some spray as a steroid to try and shrink the lining of your nose down a little bit and settle down any inflammation.
R17, site 8, consultation 5
‘Delayed’ surgery Some consultations included references to patients having surgery at the end of the trial if their symptoms had not resolved through medical management. Some patients appeared reassured by this, although there were also examples where these discussions overshadowed the notion of equipoise, as patients appeared to fixate on the timing of when they would receive surgery. In these examples, the discussion about the trial was dominated by the question of ‘when’ surgery would be performed, rather than surgery’s comparative effectiveness relative to medical management:
It’s still the 6 months with the steroid, nasal sprays and then possibly running to 12 months before the option of surgery? What I have right now, it’s not hugely debilitating but it’s a right nuisance. Another year, against 2, 4 months, I don’t know, 6, I’m not sure, yes. Let’s say I was part of the randomised group that was going to have the septoplasty, when would that happen?
Patient, R11, site 6, consultation 1
Understanding patients’ expectations and preferences
Patient preferences featured regularly in the audio-recorded consultations, but did not arise as a major barrier to recruitment.
Preferences manifested on a spectrum, ranging from no preferred treatment, to clear-cut preferences for an arm and refusal of trial participation. Between these extremes, there were many examples where patients expressed concerns or views about treatments, which were subsequently explored and discussed. Only 8 out of 93 consultations included a clear articulation of a preference that precluded trial entry; 7 out of 8 of these were from ‘pre-feedback’ consultations. The remaining 85 consultations either did not include evidence of patients holding preferences (n = 53), or included patient views/concerns/preferences that shifted through exploration and discussion (n = 32).
The infrequency of articulated preferences may have been linked to recruiters’ practices of conveying equipoise (discussed in Conveying equipoise), and/or may have reflected an already filtered sample of patients at research clinics. Nonetheless, consultation recordings included clear evidence of recruiters eliciting and exploring patients’ treatment concerns and preferences, as suggested in the pre-recruitment training/guidance document.
On discussing their preferences or treatment concerns, most patients confirmed that they were happy to be randomised to either treatment, or explained their preference was not strong enough to deter them from trial participation (R8). Preferences for both surgery and medical management decreased following recruiters’ provision of further treatment information (e.g. R17 and R12):
R21, site 11, consultation 6: OK, what about preferences at the moment? Having heard this, do you feel you would prefer one or the other or you don’t know at the moment and that’s why you’re in the trial and this?
Patient: I don’t know. I don’t know which one would be more beneficial.
Fine, yeah. I mean, naturally I’d prefer to go down the medical route, but I’m not going to rule one out because ultimately I just want it to be better than it is.
Patient, R8, site 4, consultation 2
R17, site 8, consultation 2: Do you feel particularly that you want to do one treatment more than another?
Patient: I would have said the operation because I would think the operation would sort it. I think that’s why I’m interested in doing this [NAIROS] because, as you say, it doesn’t mean that it does solve the problem.
Doctor: When you say you would have – are you still feeling that you would prefer the operation now, or are you–?
Patient: I must admit I’m not that fussed really. […] I must admit I’m not that fussed at all. I would have taken it for granted that you would probably have known which was the best.
R12, site 6, consultation 3: So for you to enter the trial you’d have to be content with the idea of being listed for surgery, and if you’re not content with that then the operation is not for you […].
Patient: Yes. It’s not to say that I wouldn’t be happy with, even, the operation if it looks like it’s the right procedure to go down. It’s just the matter of timing for me, like I was saying before, work commitments and whatnot. So that was probably. How long was the recovery?
[Surgeon discusses recovery time]
Patient: Well if that’s the case I’m happy to … If it is a shorter space of recovery than I anticipated it might be, then that was the big stumbling block for me, to be honest.
Eight of the consultations were with patients who appeared to decline trial participation on the basis of a preference. In one of these cases, the patient felt that they had not persevered with steroid sprays long enough, having heard the recruiter’s explanation of the importance of persevering and complying with steroid sprays:
Patient: I just don’t feel as if I’ve used the sprays for long periods of time. I’ve maybe used them for a few weeks and then stopped. Nobody has said, ‘Well, take them for longer’.
R17, S8, consultation 3: I think the reason that we – most patients if they’ve used them for about 6 weeks or so will be able to say whether they’ve got benefit from them. If you have only used it for a week or 2 then I’d say yes, probably you haven’t given it long enough.
In all other cases, patients had preferences for septoplasty, having tried sprays to no avail. In most cases, these appeared to be informed decisions, following recruiters’ exploration of the type of sprays used, and information provision about the sprays used in the trial. There were, however, some examples where recruiters accepted patients’ decisions without further discussion. In these cases, it was not clear if patients’ views could have changed, as per other patients whose preferences dissolved on receiving further information.
Summary of recruiters’ communication practices
Recruiters across sites were generally successful in implementing the QRI ‘tips and guidance’ issued at the start of the trial; this was evident from their generally clear articulation of equipoise and willingness to actively elicit and engage with patient preferences. Recordings did, however, identify new issues that undermined equipoise, such as foregrounding consultations with discussion about the septal deviation, and failure to explain the intended mechanisms of action for both surgical and medical management arms. There were also some examples of recruiters accepting preferences at face value, without further exploration.
In addition to highlighting potential sources of recruitment difficulty, the consultations also provided insight into examples of successful techniques for addressing the above issues, providing an ideal source for feedback and training. The final section summarises how these insights, alongside findings from interviews, were formulated into phase 2, ‘QRI actions’, to support recruitment throughout the remainder of the NAIROS.
Phase 2: actions to optimise recruitment
The findings from the interviews and audio-recorded consultations were initially fed back to the chief investigator via a report in August 2018 (month 8 of recruitment) and circulated to the TMG in September 2018. The report informed a series of actions which are summarised in the following sections.
September 2018: investigators’ meeting
Principal investigators, research nurses and consultants from all 17 sites were invited to an investigators’ meeting held on 20 September 2018. Sites that were not yet open to recruitment were also invited to attend. The meeting slides and recording of the presentations were broadcast via a live link to individuals who could not attend and recorded and disseminated to all sites for retrospective viewing. The QRI feedback slides focused on two core topics identified as priority areas for optimising recruitment: strategies for identifying eligible NAIROS participants from routine clinics and explaining the trial to patients.
Key lessons captured in the feedback highlighted the challenges of identifying patients through referral letters, as well as the importance of engaging clinical colleagues to support ‘in-clinic’ identification of NAIROS candidates. Communication feedback drew on anonymised extracts of recorded consultations to illustrate ‘good practice’ and issues to avoid, and focused primarily on:
-
providing recruiters with NAIROS-specific strategies for conveying equipoise – specifically, the importance of framing the medical problem around symptoms of blocked nose rather than septal deviation and explaining the ways in which medical management could plausibly help to address symptoms of blocked nose
-
caution around placing too much emphasis on the prospect of receiving surgery at the end of the trial if medical management had not worked, in the light of audio-recordings revealing how this could overshadow the uncertainty around the comparative effectiveness of medical management versus septoplasty
-
examples of how to address patient preferences – specifically when patients attended recruitment consultations having already tried medical management.
September 2018: updated ‘tips and guidance’ document
The QRI team, the chief investigator and the TMG collaboratively produced an updated ‘tips and guidance’ document for recruiters, informed by the evidence from the phase 1 report shared with the TMG. The document featured more bespoke strategies for explaining the NAIROS, informed by findings from the audio-recorded consultations. This was disseminated to all sites at the September 2018 investigators’ meeting, and issued to sites via e-mail. It was also provided to sites at subsequent site initiation visits for new sites that opened after the investigators’ meeting.
Consultant ‘cue card’ and video, to support non-recruiting ear, nose and throat consultants to introduce the Nasal AIRway Obstruction Study
Phase 1 findings indicated that the key obstacle to recruitment to the NAIROS was the identification and referral of patients to NAIROS clinics or NAIROS recruiters. Engagement with ENT consultants who were not directly involved in the NAIROS emerged as an important priority in efforts to optimise recruitment, and thus efforts were focused on supporting PIs to achieve this locally. Interviews had provided insight into the challenges of providing a high-level, comprehensive introduction to the trial in routine NHS clinics. In response to this, the QRI team and the TMG produced a single-page ‘cue card’, comprising a series of bullet points to support non-recruiting consultants to introduce the trial. The cue card was reinforced through a simulated video of the chief investigator articulating the points to a patient actor. The video and cue cards were disseminated to sites on 1 December 2018.
Site-specific teleconferences to address identification/approach issues
The QRI researchers, the chief investigator and the trial manager organised and conducted a series of recruitment teleconferences with sites identified to have lower than anticipated screening activities, as indicated through continued scrutiny of monthly screening logs after the investigators’ meeting. The teleconferences focused on local organisational and logistical issues, to increase the absolute numbers of NAIROS candidates identified/approached. The teleconferences were conducted at five sites between 10 December 2018 and 7 February 2019, with site PIs and research nurses. All those who attended these teleconferences were encouraged to use the resources developed (i.e. cue cards and video).
Equipoise/preferences remote training webinar
A dedicated training webinar focusing on recruitment communication – specifically, conveying equipoise and engagement with patients’ treatment preferences – was arranged in the later stages of the trial to support recruiting and non-recruiting ENT surgeons to manage patients’ expectations/preferences for surgery. Drawing on insights and examples of ‘good practice’ from the audio-recorded consultations, the QRI team, the chief investigator and clinical members of the TMG produced a script/video of a recruitment encounter illustrating examples of how to respond to patients’ expectations/preferences. This was coupled with a training presentation, delivered live to NAIROS sites in September 2019. Copies of the recordings/slides were shared with all sites following the webinar.
Nasal AIRway Obstruction Study recruitment outcomes
Overall, despite anticipated challenges, the NAIROS successfully recruited its target sample without the need for a funding extension. Although there were substantial delays in sites opening and a change to the original planned pilot time frames, the intended sample size was achieved within 22 months, 1 additional month relative to the anticipated recruitment duration. Participants were recruited from each of the 17 sites that opened to the NAIROS, with no site closures. Recruitment issues were identified as the trial progressed, but it was possible to address these through tailored actions. This chapter has outlined plausible ways in which the QRI supported recruitment, through pre-emptive and responsive actions.
Limitations of the QuinteT Recruitment Intervention
Although it was possible to discern key recruitment issues and deploy strategies to address these through the QRI, there were limitations around the reach and depth of the methods. The sample of recruiters who agreed to audio-record consultations and participate in an interview were self-selected, and not all recruiters/centres took part. Therefore, a full picture of recruitment across all sites was not possible, potentially hindering opportunities for further bespoke recruitment support. The QRI findings are also limited by snapshots of recruitment practices captured through select audio-recorded consultations; as a result, it was not possible to capture what patients had been told before or after the recorded encounter, which, in turn, limited potential to fully understand how the recruitment pathway (and interactions throughout) may have shaped decisions about trial participation. Although resources did not permit this in the NAIROS, case studies of a sample of eligible NAIROS patients’ experiences as they move through clinics and interact with different personnel would have provided more in-depth and comprehensive insights into how recruitment plays out in practice. The process evaluation, presented in the next chapter, does, however, provide insight into patients’ experiences of the trial, and provided opportunities for triangulation through regular meetings and sharing of emerging findings between the QRI and process evaluation researchers.
Chapter 6 Qualitative process evaluation
Introduction
Surgery and surgical trials are complex interventions, which have multiple components and contextual factors to be taken into consideration. 129 Recent guidance130 has reinforced the importance of understanding complex interventions as events in systems, recommending attention to context and to understanding how evidence generated might inform decision-making. Qualitative process evaluations can be valuable in this respect, guiding the optimisation of both health interventions and trial processes and supporting implementation. 62,131–133
Quantitative findings suggest that most patients (56–100%) undergoing septoplasty are satisfied with the outcome. 62,134 However, outcomes of septoplasty on symptoms and quality of life may be more mixed. 43,135 To our knowledge, no studies have investigated qualitatively patient or health professional experiences of delivering and receiving septoplasty.
The aim of the process evaluation was to describe and understand patients’ and healthcare professionals’ experiences of the NAIROS trial, including the interventions under evaluation, and to identify any factors likely to influence the wider implementation of the trial findings.
Methods
Design
The process evaluation was designed as a qualitative study comprising in-depth interviews with patients and staff (surgeons and research nurses) involved in the NAIROS trial and a thematic analysis informed by normalisation process theory (NPT). 136,137 NPT has been widely applied in understanding the implementation of complex interventions in health care. 137 In the context of the NAIROS, the trial itself (and all associated processes, including those under evaluation) was identified as the complex intervention. NPT incorporates four constructs: coherence (how people make sense of the trial), cognitive participation (whether or not people are willing and able to buy in to implementing the trial), collective action (people’s ability to take on the work needed to implement the trial) and reflexive monitoring (people’s reflection on the benefits and costs of the trial). 136
Recruitment and sampling
Participants
At trial recruitment, eligible patients who had agreed or declined to participate in the trial were invited to consent to contact from the qualitative substudy team. Participants were subsequently purposively sampled (site, gender, allocated arm) and approached with further information using their preferred method of contact (telephone, e-mail) and invited to take part in an in-depth telephone interview. A further purposive sample of participants (site, gender, allocated arm, participants discontinuing allocated arm) was recruited to a follow-up interview; this included, but was not limited to, those who had participated in an initial interview. A purposive sample (site, role) of healthcare professionals (surgeons, research nurses) who were involved in the NAIROS trial were also invited to participate in interviews.
The number of interviews conducted was guided by the principles of data saturation,138 whereby participants were recruited until additional interviews did not seem to generate substantially new information in the context of our developing preliminary analysis. Patients were mainly recruited at two time points: around the time that they were recruited to the study, and at 6-month follow-up. A small number of additional interviews were conducted at other time points to understand patients’ reasons for discontinuing their allocated trial treatment. Patient interviews were conducted between February 2018 and January 2020. NAIROS staff members were interviewed at only one time point: between November 2019 and February 2020 (at the end of trial recruitment).
Data collection
Interview processes
Topic guides were developed based on our own and other existing research (relating to trial recruitment and conduct, and on septoplasty), our theoretical framework (NPT)136,137 and discussion with the wider NAIROS team. The topic guide was updated during the study on the basis of early interviews and participation in the TMG. All interviews were conducted by an experienced qualitative researcher. Staff interviews were conducted by the researcher who had previously been involved in the QRI. This enabled the selection of participants and the content of interviews to be informed by what was already known about sites. Following an opportunity to ask further questions about participation, verbal consent, using an approved (as part of the REC review) verbal consent checklist, was obtained and recorded at the start of the interview.
Analysis of the interviews
Qualitative data management and analysis
All interviews were audio-recorded, transcribed verbatim and edited to ensure the anonymity of the participants. All transcripts were managed in the qualitative analysis software NVivo (version 12). During data collection, regular meetings were held (between NR and JM/CW) to discuss the preliminary analysis and to make decisions about further data collection. 139 When concerns about specific aspects of trial conduct emerged in the patient interviews, these which were fed back to the wider TMG (via attendance at the monthly TMG meetings or more rapidly by e-mail if necessary) and, when appropriate, to sites. For example, patients in the medical treatment arm highlighted issues with accessing the pharmacy at certain sites, and patients who were planning to travel by airplane raised concerns about the size of the saline solution bottles. In addition to this ad hoc feedback, a presentation on patients’ experiences of aspects of trial conduct was given at the investigators meeting in September 2018. This covered examples of positive feedback regarding interactions with NAIROS site staff, examples of patients’ preferences being influenced by discussion with NAIROS recruiters, misunderstandings regarding the role of eligibility checks, and positive experiences of both trial interventions. We used thematic analysis with a coding framework to analyse the data. 140,141 The coding framework was developed by two researchers (NR and KL). The framework was developed after reading through several interview transcripts and reviewing previous literature in the topic area of qualitative process evaluations of clinical trials, and with reference to our sensitising theoretical framework, NPT. 136 To refine the coding framework further, two researchers (NR and KL) coded two different interviews independently and discussed their findings. Because most codes were relevant in both patient and staff interviews (e.g. patients described their symptoms of nasal obstruction; staff described how they used these symptoms, together with other factors, when deciding on an appropriate course of treatment), we decided to use the same coding framework for both patient and staff interviews, to facilitate a comprehensive and cohesive analysis.
One researcher (KL) coded all healthcare professionals’ and patients’ interviews using the same coding framework. Kelly Lloyd and Nikki Rousseau discussed the codes and findings collaboratively, and generated themes through these discussions. After the development of the main themes from the NAIROS interview data, we considered the themes in the context of four core constructs of NPT. 136
Results
Fourteen staff members (surgeons and research nurses) working on the NAIROS trial were recruited and interviewed across 11 sites. Staff interviews lasted between 24 and 81 minutes. As shown in Table 21, 31 patients were recruited. Seven patients were interviewed at two time points, and 23 patients were interviewed at one time point only. One interview was conducted with a patient who declined to participate in the trial. In total, 39 patient interviews were conducted and interviews ranged from 6 to 33 minutes in duration. Sites and participants are labelled (e.g. site 1, surgeon 1) to give an indication of the spread of data; however, to maximise confidentiality, site and participant numbers used in this chapter do not correspond with those used elsewhere in this report.
| Description | n |
|---|---|
| Intervention | |
| Septoplasty | 13 |
| Medical management | 16 |
| N/A: dropped out of trial | 1 |
| N/A: declined to participate in the trial | 1 |
| Gender | |
| Male | 21 |
| Female | 10 |
| Ethnicity | |
| White | 28 |
| Asian | 1 |
| Other ethnicity | 2 |
| Age (years) | |
| 18–30 | 9 |
| 31–40 | 6 |
| 41–50 | 5 |
| 51–60 | 5 |
| 61–70 | 4 |
| > 70 | 2 |
| Site number | |
| 1 | 1 |
| 2 | 10 |
| 3 | 2 |
| 5 | 5 |
| 6 | 2 |
| 8 | 1 |
| 10 | 3 |
| 11 | 2 |
| 12 | 2 |
| 13 | 2 |
| 14 | 1 |
| Number of interviews | |
| Recruitment interview only | 17 |
| Post-treatment interview only | 6 |
| Recruitment and post-treatment interview | 7 |
| Declined the trial interview | 1 |
After analysing both the staff and patient interview data, four main themes were identified: anticipated impacts of the NAIROS trial, making a decision about surgery, experiences of treatment, and reflections on the trial.
Anticipated impacts of the Nasal AIRway Obstruction Study trial
Views of the NAIROS research question were relevant to both patient and staff motivation to participate in the trial. For staff, this related to how valuable they felt the evidence that a trial would generate might be, whereas patients were more interested in how the NAIROS research question resonated with their own understandings and expectations of the causes of, and treatment for, their nasal obstruction.
A lack of high-quality evidence supporting the use of septoplasty and the uncertainty of its future appeared to motivate some surgeons to take part in the trial:
Yes, if we are honest, you know, the evidence base for the septoplasty was almost non-existent … understanding what could benefit them and which patients – having some objective measures to determine suitability was high time.
Surgeon 1
Staff members felt that the research question was appropriate and long overdue in the ENT specialty. They felt it was an important question that needed to be investigated by a robust RCT:
In terms of the question it’s asking, my feeling was, ‘Thank [expletive] somebody is finally doing this properly’. There have been a couple of small studies that have shown some quite interesting results, pointing at patients with post-decongestion unilateral nasal … You know, objective nasal airflow reduction being the patients that benefit. They’re small studies, they weren’t done in a very rigorous way, so it was hard to be clear that that was a thing that needs to happen.
Surgeon 2
I think it’s a very good question, and we don’t have a formal answer for it without having done a randomised controlled trial. We do an awful lot of operations on the NHS, and that’s just from historic, and having the data behind it to say, ‘Actually, this is very effective’ or, ‘This isn’t effective’ or, ‘This treatment is better’ is obviously a great way forward. ENT, like a lot of specialties, we’re starting to get pressurised by the NHS to justify why we do something, what our action is.
Surgeon 3
There was variation as to whether some investigators felt that the aim of the NAIROS was to provide high-quality evidence for the effectiveness of septoplasty, or whether this was a trial to compare which treatment for nasal obstruction was more effective. One research nurse stated that when they originally took part in the NAIROS they felt that the aim of the trial was to confirm the effectiveness of septoplasty. However, after observing that patients returning for their NAIROS review appointment had positive and negative experiences of both treatment arms, they changed their view and instead saw it as a trial comparing two equal treatments:
Interviewer: Did you have any reservations about NAIROS before you started recruiting to it?
Research nurse 1: Yes, because with thinking that it was just to confirm what people thought I, kind of, had the reservation thinking that people who were randomised to the medical arm were not getting the optimum treatment.
Interviewer: Right. Did that change, then, in terms of …?
Research nurse 1: Yes, when people have come back and people have been quite happy with the sprays.
Several surgeons described wanting the results of the NAIROS to clearly demonstrate which patients are going to benefit from surgery and which will benefit from medical management. In turn, these results could lead to a reduction in the number of unnecessary procedures performed:
Interviewer: What would you hope NAIROS to show?
Surgeon 1: Show which patients, in these groups, do best for the surgery and which patients, perhaps, can just be managed medically.
[W]hat I’m hoping comes out of the trial is probably that septoplasty is useful, but it’s useful for certain people and not for everybody, and medical treatment is better for some others.
Surgeon 4
Surgeons also described wanting a more standardised approach to assessing which patients will benefit from septoplasty:
If we find that some of the symptoms’ scores have a good correlation with people that benefit, or the rhinospirometry measurements and stuff correlate well, then that might be quite a useful way of judging who would be a good candidate for the operation and who might not.
Surgeon 4
However, one surgeon was cautious of the idea of using objective measures for septoplasty referral:
I’m not convinced it’s [assessment tools] the only thing that you should rely on.
Surgeon 5
The results from the NAIROS trial also had the potential to demonstrate that septoplasty is no more effective than medical management. One surgeon noted that their colleagues were reluctant to be involved in the trial as they felt that the NAIROS trial could threaten the future of septoplasty:
I think some people felt threatened by the study because they felt, ‘By putting patients in the study, this is all adding to the evidence that’s going to take away septoplasty. We’re not going to be allowed to do it anymore.’
I was trying to explain to them, ‘Well, actually, this is going to produce evidence that might support septoplasty. As far as taking it away, if they’re going to take it away, they’ll take it away anyway. This is our one opportunity to contribute to potentially preserving the operation, if we can show a benefit.’.
Surgeon 6
Overall, the impacts that surgeons anticipated or desired from the NAIROS trial appeared to influence their motivation to participate.
Patients’ motivation to participate in the NAIROS trial was also influenced by their expectations of the impacts of the NAIROS treatment options. Patients’ understandings of the cause of their symptoms (e.g. that they had a clear nasal septum deviation associated with a specific injury) and their perceptions of the likelihood of a successful treatment outcome (drawing on prior experience with medical management and on wider experiences of friends and family) affected their views of the NAIROS research question:
My first reaction, to be honest, I told her that I would like to have an operation done because, in my personal opinion, if the bone is then tilted towards the side, a medicine cannot cure that.
Patient 16
Because I was put in this situation with a traumatic blow to the face, I think, probably, going under the knife was the way to fix it.
Patient 18
I was hoping I wouldn’t be [randomised to surgery], because a friend of mine, he’s just had, basically, the same surgical procedure done, and it’s made no difference to him whatsoever.
Patient 19
However, the majority of patients were uncertain as to what the best treatment for their nose symptoms was, and so regarded the NAIROS research question in a positive light:
I think people think of an operation as a straightforward thing, but nothing is straightforward, really. It’s under a general anaesthetic, it’s the dangers that go with it. And with the success rates, as well, they weren’t 100% that it would work. So, I thought well, rather than going down the route of the operation, why not try the study, using the spray, and see what sort of success I have from that first. And then, because I had the option to go back for an operation, that was always there in the background, if the steroids failed.
Patient 20
Making a decision about surgery
There was considerable complexity and heterogeneity in terms of both patients’ symptoms of nasal obstruction and how clinicians used these symptoms, together with other factors, to make a decision about whether or not to offer surgery.
Staff described a decision-making process that took into account the nature and severity of the symptoms; the length of time these had been experienced; the nature of symptom onset (whether or not it was associated with a particular injury or other event); whether or not symptoms varied seasonally; and the structure of the nose, including the degree of deviation and where the deviation occurred.
Patients presented with a range of different symptoms in clinic, including nasal blockage, trouble sleeping, snoring and frequent nosebleeds (Table 22). Several patients could recall an injury that had damaged or broken their nose, whereas others did not remember such an incident.
| Patients’ symptoms | Example from patient |
|---|---|
| Feelings of nasal blockage | My right nostril is pretty much useless, it’s blocked 99% of the timePatient 1 |
| Difficulties sleeping | More recently, it had got to the stage where it was causing me a lot of difficulty at night sleepingPatient 2 |
| Snoring | I started snoring and I’d never snored in my life, and obviously it’s waking me up in the middle of the night, you know?Patient 3 |
| Dry mouth | And my nose would just shut down, and I’d end up breathing through my mouth, and wake up in the middle of the night with a mouth that’s like sandpaperPatient 2 |
| Frequent nosebleeds | I had frequent nosebleeds throughout the day; I could just randomly have one without any trauma to the nosePatient 4 |
| Recurrent sinus infections and migraines | I was constantly getting sinus infections. I was very prone to sinus infections. I used to get a lot of headachesPatient 5 |
| Olfactory dysfunction | It is not that good because I can’t really smell food. The past year I haven’t been able to smell food properlyPatient 6 |
| Difficulties exercising | When I go running, or if I do exercise, then it’s slightly harder than what I rememberPatient 7 |
Staff members also reported a complex pattern and history of symptoms presented by patients in clinic:
I suppose there are the people that are not quite congenital, but something has happened very early in life and they have grown up with it to some extent. Quite often they don’t complain that much, because they have never known anything different. Sometimes it’s because they have developed a bit of an allergy or something, and maybe the septum is not the whole story.
Then I suppose quite a few of them are traumatic, and that can be people who play rugby or football or things like that, or the younger core who go out on a Friday night and have a fight. There are a few of them around.
Then you probably move on to older people, who I think have probably always had a bit of a septal deviation, but the cartilages have been quite firm. Then they get to 50, 60 or a bit more and everything starts to droop a little bit, and then they have a problem.
Surgeon 4
The heterogeneity across patients seen in clinic and the lack of standardised measurement tools for assessing patients’ septa and symptoms has led to a subjective approach among clinicians with regard to septoplasty referrals:
We don’t really have a good way of objectively assessing the degree of deviation of the septum. So it did come down to, pre-NAIROS, essentially just looking in the nose and seeing how twisted it was and it is a challenge there because nobody has a completely straight septum and some patients will have a septum that’s twisted into the opposite nostril rather than the affected nostril. So the decision to operate was not really based on just the degree of deflection of the septum, it was the additional symptoms as well that the patient’s complaining of.
Surgeon 7
Examining the nose, I will be looking to see if they actually do have a septal deviation and if, in my mind, I think it’s significant enough to cause the degree of symptoms that they have. If they have such a mild deviation and such a significant degree of symptoms, I wouldn’t necessarily correlate the two. I’d be very wary about offering them surgery because their expectations are that all those symptoms that they’re complaining of will improve, whereas I find that they don’t tend to. Structurally, it has to be significant enough for me to think, ‘I can make a difference’.
Surgeon 6
Clinicians also displayed subjective decision-making in relation to managing patients’ symptoms. Most surgeons reported that they often recommended medical management to patients before offering them surgery. Surgeons whose current practice more closely followed the protocol of the NAIROS may have also been more inclined to take part in the trial:
Personally, I don’t think it’s changed what I do. I’ve always maintained giving them proper medical treatment first. That’s, I think, partly why I was quite happy to buy into the study, because it followed my normal practice more or less.
Surgeon 6
However, some clinicians stated that, in routine care, they would often refer patients straight for surgery if their septum was perceived to be highly deviated, or if they had a particular incidence of a traumatic injury:
Interviewer: And in terms of the reasons why you would put people straight through to surgery?
Surgeon 8: Well might be the GP letter, it might be the patient’s history, you know, ‘I could breathe alright up my nose, then I fell on it’ … so that chronological context would be helpful. And just the severity of some people have a very twisted septum that you can see they’ve got a vertical fracture line that’s effectively presenting– the bottom end of the septum is a door across one nostril so those ones you’d be more inclined just to go for surgery.
In the trial, surgeons who felt that particular patients were more in need of having surgery straight away, rather than potentially being prescribed medical management, may not have recruited these patients to the NAIROS trial for this reason. Therefore, the trial population may not have included patients with the most severely deviated septa:
I’m aware that some colleagues have not necessarily recruited the most, well when I say– anecdotally, I’m aware that some colleagues have not necessarily recruited the most severe nasal septal deflections to the study. So I think we may not necessarily be looking at the entire population of deviated nasal septums.
Surgeon 7
Generally, despite the high heterogeneity in staff criteria for referring patients for septoplasty, surgeons felt that the eligibility criteria in the NAIROS protocol was appropriate for the trial:
Interviewer: [A]nd you are happy with the patients who were supposed to be put into NAIROS, the eligibility criteria?
Surgeon 4: Yes. There was nobody we recruited that I wasn’t happy to operate on. I think that’s the main thing.
There was also variation in other aspects of ENT routine practice, including whether or not clinicians used any outcome measures (e.g. SNOT-22, NOSE), and whether or not they followed up with patients after their operation outside the trial setting:
In general we wouldn’t have employed any objective testing, I wouldn’t have done any patient-reported outcome measures, I would have just proceeded with the operation.
Experiences of treatment for nasal obstruction
This theme relates to the experiences of delivering (staff) and receiving (patients) treatment for nasal obstruction.
Performing septoplasty: variation in surgical practice
Surgeons reported that septoplasty was an operation that they had learned to perform independently relatively early in their surgical ENT career. Septoplasty was described as a commonly occurring operation in ENT practice, but one that could be difficult to accomplish well:
It’s one of those operations which people would say is an intermediate-skilled operation, but actually to do it well is pretty skilful.
Surgeon 7
I suspect a lot of people will imagine a septoplasty is a simple procedure, and it’s one of the first operations ENT surgeons learn to do by themselves. It’s actually quite a potentially complicated operation to get perfectly right, just because there are lots of different areas that may need addressing.
Surgeon 3
Variation in practice was not only limited to the criteria for septoplasty referrals. There was also variation in how surgeons carried out septoplasty, with one surgeon describing that their surgical technique changed as they gained more experience carrying out the operation:
Anatomical knowledge of knowing where the airflow manoeuvre is the narrowest. The subtleties, as you gain more experience, you realise it’s not just the septum which causes problems with nasal airflow, there is also the soft tissue and the cartilages in particular, the lateral cartilages which can also have an effect, and it’s identifying those and addressing those subtleties which give a better outcome for the patients.
Surgeon 3
Another key point of variation among surgical practice was the surgeon’s threshold for offering turbinate reduction alongside septoplasty:
So for me I think I have a relatively low threshold for offering turbinate reduction at the time of septal surgery. Patients are clearly having a general anaesthetic anyway to correct the septum, reducing the bulk of the turbinate in my opinion is going to lead an improvement in the – an additional improvement in the airway, and therefore I would have generally offered it if a turbinate was large.
Surgeon 7
The large variation in surgeons’ practice of septoplasty and turbinate reduction led to the NAIROS protocol being designed to be more flexible in regard to surgeons’ practice. This flexibility in the protocol regarding surgical procedure was welcomed by surgeons who took part in the trial:
Turbinate reduction was optional, so it was nice to have the ability to do that.
Surgeon 1
The only one thing I had to do differently, which I was compelled to do because of study design, was … There were some patients who did have, either, allergic rhinitis or intrinsic rhinitis. Their lining, their turbinates were very congested. I would ordinarily have reduced both turbinates, on each side. The study restricted me to only doing the one. So there was a little bit of restriction to my normal practice in that. As far as surgical technique, and what I did, I didn’t do anything different and I didn’t feel curtailed.
Surgeon 6
Patients’ experiences of treatment
Patients who were randomised to medical management had differing opinions on their experiences of this treatment arm; some patients reported side effects from the sprays whereas others did not. Several people in the medical management arm mentioned nosebleeds. This is consistent with the data on AEs (see Appendix 1, Table 67), which show more nosebleeds reported in the medical management arm. One participant reported that they had been advised that their nosebleeds may have been associated with the mode of administration (inserting the spray bottle too deeply), and other people highlighted that using the spray was difficult when there was an obstruction:
Interviewer: How did you find the nasal sprays?
Patient 8, medication: To be honest, it didn’t do good for me at all … No, it sort of gave me two nosebleeds. I’ve never experienced nosebleeds before, so I didn’t get on well with them at all.
Obviously, when you’re trying to put a nasal spray up a nose that doesn’t allow anything to go up it, it’s difficult. But I persevered.
Patient 15, medication
I was fine with them. At first a bit weird, you know these sprays that went down the back of your throat, you feel them going down your nose and then down the back of your throat, you have to put your nose up in the air, but you feel it. I got used to it, and when you’re used to it, it’s normal.
Patient 3, medication
Medical treatment could be a burden to patients (albeit a usually minor one). In addition to potential discomfort and difficulties with application, several patients reported that remembering to take the medicine was burdensome, although many had established a routine to help them remember, for example placing the bottle next to their toothpaste. During the first 6 months of the NAIROS trial, medical treatment was free for patients, but this would not be the case after this period, and this was mentioned as being something that would be unwelcome over the longer term. A few people reported minor issues with obtaining their supply of NAIROS medicines; although, in routine care, patients would not need to attend hospital pharmacy, long-term use of medications would imply a periodic need to obtain new supplies. This again represents a small additional burden (particularly given that the NAIROS population was generally younger, of working age and might not otherwise be frequent users of health care):
[W]hen you’re there and they show you this stuff. It’s like, ‘Right. It’s that up your nose’, for however long. I could do without that, basically.
Patient 16
I mean, there was no cost out … no cost towards it, so that’s even better. You don’t want to pay for the medicine all the time.
Patient 3
We also explored the experiences of patients randomised to septoplasty. Typically, patients who received the operation described the experience as neutral or positive:
Yes, surgery was fine, all well-handled, everything was explained to me again, and I have no adverse comment whatsoever, everything went well.
Patient 8, septoplasty
However, several patients experienced notable painful side effects following the operation:
After the surgery it was painful. I also got sent home without any nasal wash or anything, which Dr [name], when I went for my follow-up, he said I should have had that when I left the hospital.
Patient 9, septoplasty
Well, I was in a lot of pain. Pretty much every day was just constant dripping from my nose afterwards. Then I think it was exactly a week later I ended up having to go into A&E because I woke up in, basically, a pool of blood.
Patient 10, septoplasty
Several patients stated that they would have liked further information on recovery and aftercare following surgery. Patients described feeling uninformed on these key aspects of their treatment and would have benefitted from knowing what they could or could not do during recovery (e.g. blow their nose):
To be honest with you, I got no information at all after the operation. I was told to do the nasal washouts and that was pretty much it, but I got no other information. When I rang the hospital about it, all I got was, ‘Well, yes, it’s just a water solution that you’re supposed to be using’. That was it. I don’t know. Even painkillers and stuff like that, obviously I had nothing, so it was just paracetamols and stuff in the house. To me, I feel it would have been better if I was given something a bit stronger initially.
Patient 10, septoplasty
Interviewer: Would you have liked information about anything else?
Patient 5, septoplasty: I think the follow-up more than anything. I was a bit curious, I’ve had to do a bit research myself obviously now I’m on the, on the healing side. It’s like, ‘Well, when can I blow my nose? How can I manage this? When can I start sinus flushing and stuff?’. Because it’s really, really blocked. So more on the aftercare would have been a bit better.
The interviews with patients also explored whether or not they felt that their allocated treatment was successful in reducing their symptoms. We found that patients in both arms described differing levels of success with their treatment allocation. Experiences of the medical treatment arm varied. Some people felt that there were no beneficial effects and/or that any marginal benefits were outweighed by the disadvantages of use (associated pain and/or nosebleeds); these patients typically rapidly discontinued use and sought surgical intervention:
It wasn’t long into it, I think. I’d been on it for 3 weeks or something and I decided to contact them …. They asked me if I’d want to carry on for a little bit longer with the nasal spray, so I tried for another week, but it was just too sore. I messaged them saying that I’d like to be reassessed for the surgical path …. I didn’t really see any changes at all, not for the better and not for the worse either. It was just painful taking the nasal spray.
Patient 1
Other patients felt that the spray did offer benefits, albeit small and/or short-lived. Some people reported that the beneficial effects typically lasted a few hours after application, suggesting that the benefits were more linked to the flushing effect of the saline spray, rather than an anti-inflammatory effect of the steroid. However, even these benefits might be worth having; for example, they enabled one person to obtain adequate sleep when this had not been possible prior to treatment:
[I]f I put that saline water up and put the steroid spray in, it gives me relief a little bit. For a couple of hours, it’s very good … After that, it goes.
Patient 16, medication
With the medication I’m taking now, it will give me temporary, 2 or 3 hours, relief and that’s it. Especially the medication I’m going through now, it’s helping me to have a good night’s sleep, which I was not having for a very long time.
Patient 11, medication
Other people obtained more substantial benefit from the spray. One person reported significant improvement and was informed at their clinical review that their enlarged turbinate had shrunk as a result of treatment, providing a clear mechanism to explain the improvement experienced:
Interviewer: How has your breathing been since you started on the sprays?
Patient 11, medication: Actually, I was waiting for this question to come up. Much better, but I still get a little bit of congestion.
I didn’t really have any side effects; it really helped me. I didn’t have any symptoms of hay fever throughout the summer at all, which was great. When I went to see the consultant at the end, she said that, I think it was turbinate up my nose or something had been quite swollen, but I think the steroids had shrunk them a bit. So it seemed to really have cleared up my airways.
Patient 21, medication
Septoplasty also had varying levels of success. However, those who found the operation effective typically experienced quite high levels of success, with patients feeling that they had experienced a massive reduction in their symptoms following the operation:
So, you know, ultimately, having the septoplasty, although it was uncomfortable and not particularly pleasant, it has been great. I can now breathe.
Patient 12, septoplasty
I’m still having trouble sleeping, but … I don’t think it’s down to my nose. In fact my breathing is a lot better now.
Patient 13, septoplasty
However, some patients who underwent surgery felt that the operation resulted in little to no change in their symptoms:
Well, the operation itself was fine, yes, but unfortunately it hasn’t worked.
Patient 10, septoplasty
The surgery itself was fine. The results I’m not particularly happy about because I’m feeling no change at all in the way I’m breathing.
Patient 1, medication (requested septoplasty)
Reflections on the trial
Overall, the trial recruited the target number of participants. A number of potential facilitators of the NAIROS trial that contributed to its successful recruitment were identified by participants (Table 23). Challenges in relation to the set-up and running of the trial are presented in Table 24. Participants also identified a number of ways in which they had reflected on or made changes in practice as a result of their participation in the NAIROS.
| NAIROS facilitators | Example from participant |
|---|---|
| Research nurses | So, the biggest key to success which I’m going to take away is the research nurse is absolutely essential. I naively thought I might be able to struggle through that one, and I would never have copedSurgeon 3 |
| Surgery offered after 6 months | I think it was very important, because I was willing to give the nasal sprays a go again, but I also knew within a year of it, I would get the surgery to definitely fix it anywayPatient 14, septoplasty |
| Team effort | We’ve worked really well as a team. We are a small team, and we kind of all know what everybody is doing, so we just kind of get on with it and it works. Yes, we’ve worked really well togetherResearch nurse 2 |
| Established medication | The only concern I had was, was this a trial of the medication or was this a process trial? I wasn’t happy with putting medical stuff in that hadn’t been testedPatient 15, medication |
| QRI | I think the QRI involvement has been key actually to getting us to where we are with our recruitment numbers |
| What’s been more important in that is the involvement, the ideas, the focus on recruitment that QRI have broughtSurgeon 7 | |
| Information resources | I think it was really good, because it was the right amount. I think any more, then people would probably not want to read it to the same extent. Any less, you couldn’t really have any less because it contained all the right informationPatient 7, medication |
| Effective communication of clinical equipoise | I totally took my decision on the basis of what [consultant] said to me, 50% to 70% chance of getting the normal breathing back through the operation, which can be cured, almost the same ratio, with the medicine. I thought, ‘Fine, I’ll try the medicine first’Patient 16, medication |
| NAIROS – barriers | Example from participant |
|---|---|
| Patients’ comprehension of randomisation | I assume that, depending how many points I totalled up, determined whether I would need the spray or the surgeryPatient 17, septoplasty |
| Complicated consent process | The consent process was, perhaps, a little bit cumbersome with the three separate consent points. I know, this end, that we got into a, sort of, routine for that, but I think that could be streamlined into just one single consentResearch nurse 1 |
| NAIROS database | It’s not very user friendly, I would say, and there are quite a few glitches in the databaseResearch nurse 2 |
| Challenges with surgery offered later | Then he really, really wasn’t happy about that at all, because our waiting list at the moment is about 70 weeks’ for septoplasty. It has gone up massivelyResearch nurse 3 |
| Issues with follow-up | A couple of days before the date I’ll be like contacting them, ‘Please make sure you fill in the questionnaires and send them back by this date.’ kind of thing. That’s been quite hard to encourage the patients basically, especially the surgical onesResearch nurse 3 |
| Colleagues not proactive with recruiting | Well I expected a bit more buy-in because I did get a lot more verbal support from my colleagues initiallySurgeon 4 |
| I did expect my colleagues to refer more patients to me, but it didn’t turn out as I hopedSurgeon 6 | |
| Logistical problems with NAIROS recruitment clinics | More of an issue was that there weren’t enough clinics, there were too many people who could be … too many potentials to fit in to the clinicsResearch nurse 1 |
| The NAIROS as a CTIMP | This being a CTIMP type of study, you can’t just hold the clinics anywhere you like, you have to have access to pharmacySurgeon 8 |
What worked well in the Nasal AIRway Obstruction Study
Several consultants mentioned the key role of the research nurse in delivering the trial and facilitating recruitment of patients (see Table 23). Staff members also highlighted the importance of having a research team at site that worked well together, as this benefited site set-up, the running of the trial and recruitment. Staff also cited the involvement of the QRI team as an important contributor to the successful recruitment to the NAIROS trial. Patients praised the information resources for the trial, which they felt described the trial clearly.
The treatment options provided appeared to be a contributing factor to the trial recruiting well. For example, several patients reported that they would have been reluctant to take part if the trial was testing new medication. In addition, there were benefits to the medical management arm explicitly including the possibility of deferred surgery. Patients felt that they had nothing to lose by taking part in the trial because, at some point, either within 8 weeks or after 6 months, they could still undergo septoplasty.
Challenges with the Nasal AIRway Obstruction Study
Participants noted several challenges when implementing the NAIROS, and identified aspects of the NAIROS that they would have changed on reflection. Challenges for sites included issues with using the NAIROS database, the consent forms being overly complicated, problems with collecting patients’ follow-up data, logistical issues in organising recruitment at clinics and lack of team effort from some surgeons’ colleagues at site. There were also issues in practice relating to when patients could receive septoplasty once they had completed 6 months of medical management. One staff member described how the site’s waiting list for septoplasty had increased from approximately 4 months at the beginning of trial to > 16 months at its end point, leading to frustration when one patient was not able to have surgery after the medical management arm as quickly as they had anticipated.
Across a small proportion of patient interviews, we also noted issues with patients’ comprehension of the trial randomisation process. Two patients suggested that the reason they were allocated to their treatment arm was because of the scores they obtained on eligibility or baseline assessment tools. In addition, one patient thought the trial was offering only medication as the initial means of treatment, and was unaware that they could have been randomised to the surgical arm.
Overall though, most staff members and patients were pleased with how the trial was designed, and many felt that they had little to no feedback to provide on the trial:
I don’t think anything else could’ve been done differently. I think it was well supported and I think it was designed as well as it could be for a difficult question, for something that’s a well-established operation.
Surgeon 6
Furthermore, the majority of patients demonstrated a good level of understanding of the randomisation process, and of the trial itself.
Changes in practice during and after the Nasal AIRway Obstruction Study
One surgeon noted that, during recruitment, they noticed issues with several patients’ comprehension of random allocation. In response to this, the surgeon changed their practice to check if the patient would be comfortable with both treatment options:
One of the things I did learn was the difference between saying to someone, ‘Are you happy to be randomised?’ and saying to them, ‘If you get randomised to X, are you happy to stay with X?’ They’re the same thing on a logical basis, but in reality they’re slightly different questions for patients. I think asking that question … Certainly, if I’m recruiting to something like this, I will definitely be doing that in the future because I think you got some surprising answers from that sometimes.
Surgeon 2
Following the NAIROS, several surgeons noted that there were aspects of the trial that they were considering implementing into their routine practice, such as particular outcome measures (e.g. NOSE). However, they were waiting for the trial results first before they changed their current practice:
The NOSE score is useful. Whether I would change to that, or stick to the SNOT, I don’t know. I think I’d be more keen to see how useful it is when we actually look at our post-NAIROS data to actually see, ‘How valid is it? How useful is it with these sorts of patients?’ before I definitely change practice.
Surgeon 6
Other aspects of the NAIROS trial that surgeons were planning to incorporate into their routine care included the recommendation to patients of frequent saline douching:
I didn’t tend to use the saline douching as much as the protocol did, I must admit. I tend to now, because I sort of got used to the study protocol and I think, ‘Yes, that makes some sense’. Beforehand, I tended not to give the saline douching as much unless they were complaining of a lot of post-nasal drip.
Surgeon 2
Discussion
This qualitative process evaluation of the NAIROS trial identified considerable variation and complexity in routine clinical decision-making about septoplasty, in terms of which patients should be offered surgery in the place of medical management for nasal obstruction, and in how surgery was conducted. Surgeons recognised this diversity; they hoped that the NAIROS findings would help to guide future decision-making, and they valued the flexibility that the NAIROS protocol offered regarding surgical approach to septoplasty (e.g. regarding turbinate reduction). Among patients, there was a diversity of presenting symptoms and experiences both prior to and following receipt of their allocated treatment. Trials comparing very different interventions can be challenging to recruit to,142 with 1 in 5 surgical RCTs discontinued early and 1 in 3 completed trials remaining unpublished. 143 The NAIROS trial (comparing medical with surgical management) recruited its target number of participants. Possible reasons for the success of recruitment in the NAIROS trial include the following: surgeons felt that there was value in addressing the NAIROS research questions; the type of treatments the trial was comparing, as these involved established rather than novel therapies, and a clear route to surgical treatment was communicated in the event that those randomised to medical management did not obtain symptom relief; the effective communication of clinical equipoise in the trial; and the teamwork of the site staff. Challenges to implementing the trial were also identified, including continuing confusion among some patients regarding aspects of randomisation (e.g. the role of the eligibility criteria). To understand further the key barriers to and facilitators of trial implementation, we have considered the results of the NAIROS qualitative evaluation in relation to the core constructs of NPT (coherence, cognitive participation, collective action and reflexive monitoring). 136
Coherence: how people make sense of the Nasal AIRway Obstruction Study trial
Healthcare professionals considered the research question of the NAIROS to be of great importance to the field of septoplasty, which had previously suffered from a lack of high-quality evidence. In usual practice there were variations in decision-making for septoplasty, including how septum deviation was assessed and which patient symptoms were considered important. This variation in current practice appears to be due to the range of symptoms patients present with in clinic, and the lack of valid and standardised methods for septoplasty referral. However, most surgeons felt that medical management could be a valid alternative to septoplasty for at least some patients. Overall, clinicians described the NAIROS protocol positively, owing to its consistency with current practice, its suggested criteria for assessing eligibility of patients and the flexibility with regard to the surgical technique it allowed them to employ (e.g. optional turbinate reduction).
Health professionals had subtle but important differences in terms of what they understood the aim of the NAIROS to be. Some surgeons believed that the aim of the trial was to provide high-quality evidence for the effectiveness of septoplasty to ensure its future funding. In contrast, several surgeons believed more strongly in the clinical equipoise of the trial; these surgeons hoped that the findings of the NAIROS could help to identify which patients benefit from septoplasty and which benefit from medical management. Generally, patients demonstrated a good level of understanding of the trial, and used the NAIROS information resources (e.g. leaflet, website, digital versatile disc) well to understand and make sense of the trial. However, there were instances when patients’ sense-making of the trial did not align with the trial itself. For example, as in previous studies, a small number of patients demonstrated a lack of understanding of the computer randomisation process. 144
Cognitive participation: whether or not people were willing and able to buy in to implementing the trial
Many surgeons were motivated to take part in the NAIROS because they felt that the results could provide the high-quality evidence needed to ensure the future of septoplasty, whereas other surgeons felt that the results could reduce the number of unnecessary septoplasties conducted. However, there were also difficulties with healthcare professionals buying in to the trial, with some surgeons struggling to convince their colleagues of the value of the NAIROS.
In addition, there were instances of healthcare professionals not adhering to the trial inclusion and exclusion criteria, which may have led to patients with a highly deviated septum not being approached to take part in the NAIROS.
Patients were positive about the fact that the trial was testing established medication and surgery, rather than new and experimental treatments. Previous studies have identified patients’ preference for a particular treatment arm as a barrier to trial recruitment. 131,145,146 By offering patients enrolled in the medical management arm the option of being put on the septoplasty waiting list after 6 months, this may have minimised the effect of patients’ preference on recruitment rates.
Collective action: people’s ability to take on the work needed to implement the Nasal AIRway Obstruction Study trial
Most staff members were positive about trial implementation and felt that the research team collaborated effectively together to execute the trial at site. The involvement of the QRI in the NAIROS was also cited by staff as having a positive influence on recruitment rates. As in previous studies,148,149 research nurses in particular were highlighted by surgeons as being essential to the implementation of the trial at their sites. However, participants also described several challenges in relation to implementation. For example, there were issues with retention of patients at follow-up and logistical difficulties organising NAIROS recruitment. Furthermore, colleagues’ initial enthusiasm about the NAIROS trial did not always translate into action during the trial, such as aiding with screening and recruitment of patients.
Reflexive monitoring: people’s reflection on the benefits and costs of the trial
One staff participant described how their views of the treatments changed during the trial. Originally, they believed that surgery was much more effective than medical management; however, after witnessing a number of patients experience symptom relief from the steroid sprays, they came to view the trial as comparing two more equal treatments. Although the NAIROS quantitative data show that, as a group, those randomised to septoplasty experience more improvement than those randomised to medical management, the qualitative data demonstrate nuance and variation at the level of individuals, with some participants not observing any improvement following septoplasty and others experiencing benefit with medical management. For one patient, visible changes in turbinate size were felt to be attributable to steroid use and to account for improved nasal air flow. Steroid treatment is an established treatment for enlarged turbinates,150 but further research is needed to clarify the role of medical management in the presence of a deviated septum. Patients with septal deviation could clearly understand how straightening the deviation might improve airflow, but sometimes struggled to understand a comparable mechanism for medical management. Providing a mechanism that includes observable changes in nasal structures (‘shrink’) may be more consistent with patients’ understandings of nasal obstruction in the presence of septal deviation than broader terms like ‘decongestant’.
Previous quantitative research has shown mixed findings in regard to patients’ rates of satisfaction and quality of life following septoplasty. 43,62,134,135 In the NAIROS interviews, many patients suggested that information on recovery post septoplasty was lacking, which led to them feeling unsupported following surgery. Inadequate information on aspects such as recovery has been linked to dissatisfaction with surgery and increased anxiety levels among those undergoing day surgery. 151 More comprehensive information on recovery post septoplasty might increase patients’ feelings of support and satisfaction with surgery. Medical management was generally well tolerated in the trial; however, a number of patients did experience nosebleeds and/or discomfort when using the spray. Patients incorporated the sprays into their daily routines to help them remember to use them, but some expressed concerns about the potential cost and burden of long-term medication use. In interviews no-one mentioned potential side effects of steroids, or specific concerns about the use of steroid medications long-term, but these have been a concern in other populations152 and might emerge as a greater concern in the medium to long term.
Overall, many healthcare professionals did not plan to change their current practice until the results of the NAIROS were released. Even then, many felt that the NAIROS matched their current practice quite closely and were considering implementing only a small number of changes, such as the use of particular outcome measures. One surgeon also described how taking part in the NAIROS had altered their recruitment technique for future trials.
Summary of findings and implications
-
Pre the NAIROS, clinician decision-making for septoplasty was heterogenous, with a mixture of standardised measures (e.g. NOSE, SNOT-22), history, symptoms, prior trial of medical management and anatomy being variously employed by surgeons in different centres.
-
Surgeons would value guidance to help them identify patients who might benefit from septoplasty and those who might benefit from medical management.
-
The decision by the trial team to incorporate the option for deferred surgery in the medical management arm appears to have been a key factor in the successful recruitment to the NAIROS trial.
-
Many patients were willing to try medical management once it was explained that surgery was not guaranteed to provide symptom relief, and valued the opportunity to see whether or not surgery could be avoided.
-
For some people, medical management did deliver an improvement in symptoms, with a reduction in turbinate size being provided as one possible mechanism of action.
-
Some people found the sprays difficult to use; one person was advised that their nosebleeds were associated with using their spray incorrectly. Personalised guidance on spray use, taking account of any abnormal nasal anatomy, might enable optimal use of this intervention in this patient group.
-
Long-term use of steroid sprays would be associated with an element of treatment burden, with patients mentioning cost, the need to remember to use the spray and minor inconvenience of obtaining supplies as specific burdens.
-
In spite of the strong overall effect in the NAIROS, some individuals did not experience noticeable benefit from surgery. There is a continued need to understand the varying subgroups experiencing nasal obstruction so that patients do not undergo surgery unnecessarily and obtain treatment that does improve their symptoms.
Strengths and limitations of the qualitative study
A strength of the qualitative study is that we recruited patients from multiple trial sites, from different age groups and at different time points in the trial pathway. However, the sample was less diverse in terms of ethnicity. In addition, we recruited and interviewed multiple surgeons and research nurses from a range of trial sites. An important limitation of the study is that we recruited only one patient who declined the trial. Therefore, we were unable to explore the factors that influenced patients’ decision not to participate in the NAIROS.
Conclusion
Prior to the NAIROS, decisions regarding the appropriateness of surgery for individual patients were made on the basis of a complex and largely subjective combination of symptoms, history and patient anatomy. Surgeons indicated that they would welcome clearer criteria to guide decision-making.
Although trial findings show that, as a group, participants in the surgical arm experience more improvement than those in the medical management arm, the qualitative study demonstrated that individual experiences varied. Some participants did not observe any improvement following septoplasty and others experienced benefit with medical management. Research should continue to explore and understand this variation. Patients undergoing medical management might benefit from individual advice regarding application of the sprays, taking into account distorted anatomy, to maximise effectiveness and reduce side effects. Although most patients were able to incorporate spray use into their daily routines, long-term spray use was perceived by some to be burdensome. Some participants undergoing septoplasty reported being underprepared for the immediate post-surgery period; better information and support could improve their experience.
Chapter 7 Discussion
Septoplasty is an operation carried out to correct a deviated nasal septum predominantly for functional purposes. It is used in the treatment of conditions, such as a blocked nose, snoring and sleep disruption, that fail to respond to conservative management. There is an absence of nationally or internationally accepted guidelines to inform clinicians and commissioners about this procedure, whose indications are practice based rather than evidence based. Van Egmond et al. 58 published the only prior RCT of this surgical procedure in 2019. The outputs of the NAIROS make a substantial contribution to the evidence base of the clinical effectiveness and cost-effectiveness of septoplasty among adults with a deviated nasal septum and associated nasal obstruction. The NAIROS demonstrates that septoplasty, with or without turbinate reduction, results in a significantly greater improvement in patient-reported outcomes at 6 months than a defined medical regimen of nasal steroid and saline sprays, and this improvement is sustained to 12 months.
The NAIROS RCT was a pragmatic trial reflecting current NHS pathways for patients with nasal obstruction, nasal blockage or nocturnal nasal symptoms deemed likely to be the result of a deviated nasal septum by the investigating clinician.
Concerns regarding surgical efficacy and cost-effectiveness mean that access to septoplasty surgery is subject to geographic variation across Clinical Commissioning Groups in England and Wales in particular. 153,154 The NAIROS RCT was a superiority trial designed to meet the commissioning brief set out by the NIHR Health Technology Assessment (HTA) programme (14/226). This mandated a RCT with a non-surgical arm whose outputs should include measures of nasal function, resolution of symptoms, disease-specific quality-of-life measures, AEs and cost-effectiveness over a minimum 12-month follow-up period.
The primary goals were to assess the magnitude of septoplasty risks and benefits and to stratify for baseline severity and gender, as female gender might predict worse outcome. 155 Inferior turbinate surgery is frequently performed alongside septoplasty, but, in the absence of high-quality evidence for the additional benefit of this surgery, clinicians’ turbinate practices vary. The NAIROS trial investigators reached a consensus that turbinate surgery should be performed according to the clinical judgement of individual patient requirements. We predefined the 9-point difference in overall SNOT-22 scores at 6 months to represent the MCID. Recruitment to surgical trials can be challenging; our team applied prior knowledge of running RCTs to the NAIROS. 100,156 The QRI team at the University of Bristol [URL: www.bristol.ac.uk/population-health-sciences/research/groups/social-sciences-health/quintet/ (accessed 16 February 2022)] was commissioned to understand the NAIROS recruitment processes and barriers to and facilitators of recruitment, to suggest improvements and to work with the TMG and site investigators on the implementation of those improvements. The trial aimed to complete recruitment by 31 May 2019, but delays in set-up resulted in the trial achieving full recruitment on 5 December 2019.
The onset of the COVID-19 pandemic resulted in the suspension of all face-to-face clinics from 30 March 2020; therefore, remaining trial participants were invited to complete primary and secondary outcome measures remotely. By then recruitment had been achieved and all trial interventions had been completed. Details of AEs were collected remotely by telephone at the 6- and 12-month follow-ups.
Statement and interpretation of results
Clinical effectiveness
The NAIROS demonstrates that septoplasty, with or without turbinate reduction, is a highly effective procedure with evidence that it may be considered cost-effective at 24 months.
The primary analysis was ITT based on a sample of 378 participants, comparing SNOT-22 over 6 months from randomisation to initial follow-up, adjusting for baseline severity (NOSE score), baseline SNOT-22 score and gender. A 6-month interval was chosen as a suitable compromise, allowing sufficient time for surgery to be performed and wound-healing to take place, yet ensuring a timely primary outcome assessment to minimise default from follow-up. As stipulated in the commissioning brief, clinical effectiveness and cost-effectiveness were also assessed at 12 months.
The baseline characteristics of the NAIROS recruits were balanced between the treatment arms (see Table 3). Males comprised 67%: septoplasty is known to be more commonly undertaken among males of middle age as a result of septal deviation caused by assault to the face and sporting injuries. 17 A peak age in mid-adulthood among participants is to be expected for similar reasons. There was a significant preponderance of the white ethnic group (88%), as expected from existing UK census data. 157
Overall, the improvement in SNOT-22 score in the septoplasty arm occurred notably earlier and was greater than expected; this was sustained over the trial period. A more modest improvement from 6 to 12 months was noted in the medical management arm, even after removing those participants who withdrew and had non-trial septoplasty. Those who withdrew and who underwent non-trial septoplasty had an appreciably greater reduction in SNOT-22 score at 12 months.
The primary analysis indicated a greater reduction of an average of 20 points (p < 0.0001) in SNOT-22 symptom severity score in the septoplasty arm than in the medical management arm at 6 months (see Table 6). In addition, the lower limit of the 95% CI was −16.4 points, substantially in excess of our a priori MCID of a 9-point reduction in SNOT-22 scores post treatment. Additional treatment efficacy analyses adjusted for covariates all showed similarly positive impacts of surgery over medical management (see Figure 8).
The impact of baseline severity as measured by the NOSE score was evident, demonstrating greater SNOT-22 scores at the primary outcome point with increasing baseline severity. This was accounted for by the inclusion of the baseline SNOT-22 scores as a covariate. The coefficient for this variable was highly significant and close to 0.5, meaning that, at 6 months, SNOT-22 scores were approximately half what they were at baseline (holding all other factors constant). The effect was mirrored in both genders by the subgroup analysis of paired primary outcome data to assess individual changes in SNOT-22 scores by a STEPP analysis (see Figure 16). Several retrospective studies158,159 have noted a similar impact of baseline severity on post-operative subjective nasal obstruction, but without robust quantification. This novel and important NAIROS analysis enables clinicians to quantify expected outcome improvements of patients contemplating septoplasty, with or without turbinate reduction, predicated on the baseline NOSE score. The STEPP analysis, in parallel with an understanding of the potential risks associated with both medical treatment and surgery, substantially improves the quality of information available to the clinician and patient in the clinical decision-making process around septoplasty.
Van Egmond et al.’s58 2019 study reached similar conclusions to those of the NAIROS in a trial of 203 participants randomised to receive either septoplasty, with or without turbinate surgery, or non-surgical management. Neither study found a relationship between outcomes and age or gender. The NAIROS reported a 14-point SNOT-22 surgical superiority score at 12 months, compared with 9.7 points in van Egmond’s58 study, although the latter’s data transformation and variable medical arm treatments makes direct comparison difficult. Van Egmond et al. ’s58 subjective, categorical, clinician-rated assessment of baseline severity failed to correlate with outcome. The mild category comprised 30% of participants, but there is no evidence for the comparability of the subjective assessment with quantitative NOSE scores. In contrast, the NAIROS substantially enhances understanding of the role of quantitative patient-reported data in the selection of patients for surgery by demonstrating that the degree of improvement in symptoms is closely related to baseline severity stratification.
Discontinuation of allocated treatment
The NAIROS trial design did not allow for formal crossovers from one treatment arm to the other. The ITT analysis therefore risks accepting a null hypothesis if the non-surgical arm ultimately contains too many individuals who have undergone surgery, resulting in an underestimation of the treatment effect size. 160 Approximately 20% of participants in the trial did not receive the treatment to which they were allocated. Of 190 participants allocated to medical management, 46 (24%) subsequently had surgery. At 6 months’ follow-up, only five patients had discontinued medical treatment and had undergone surgery, but, by the 12-month follow-up, a further 37 (30%) of the 125 retained participants had undergone surgery and completed SNOT-22 scores (see Figure 7). All but 12% of the septoplasty arm underwent surgery. Despite these groups discontinuing allocated treatment, the NAIROS ITT analysis found superior benefit from surgery, confirmed by per-treatment and per-protocol analyses (see Figure 9).
Secondary outcome measures
The benefits of septoplasty are also seen in the secondary outcome measures, including clinical airflow assessment, PROMs and objective measurements of PNIF and rhinospirometry.
Double Ordinal Airway Subjective Scale
The DOASS improved dramatically in the septoplasty arm at 6 months and 12 months. Absolute subjective DOASS, the subjective comparator of the worse versus the better nostril airflow, revealed a significant treatment-related shift from predominantly unilateral nasal airflow to equal airflow through both nostrils, which was more marked in magnitude in the surgical arm than in medical treatment arm at both 6 and 12 months (see Figure 10). As a simple clinical patient-reported outcome, the DOASS reflects the findings of previous studies,161,162 showing the specific aerodynamic benefit of septoplasty. Therefore, it may be a useful tool to audit surgical outcomes, or indeed future trials of nasal airway surgery.
Nasal Obstruction Symptom Evaluation score
At baseline, continuous NOSE scores had a broad distribution, with a median score of 70 (IQR 60–82.5) in the surgical arm and 70 (IQR 60–85) in the medical arm (see Table 7). In concordance with van Egmond et al. ,58 the NOSE score in the NAIROS showed a consistently large effect in favour of septoplasty at 12 months. In North America in particular,163 the NOSE is a widely used brief PROM, validated for septoplasty. 164 The NAIROS has shown the value of the NOSE as both a baseline severity measure and outcome measure, with global utility.
Subscales of the Sino-nasal Outcome Test-22 items
Results consistently show greater improvement in the surgical arm than in the medical treatment arm in scores across each of the four SNOT-22 domains (nasal, sleep, ear and emotional) (see Figure 11). Bugten et al. 165 found similar improvements in all four subscales in an uncontrolled study of septoplasty assessed at 6 and 12 months. The impact of septal surgery in improving the SNOT-22 scores is mirrored in other studies of snoring166 and eustachian tube dysfunction. 167 The impact on sleep may be equally important to the participants’ bed partners as it is to the participants themselves. Factors such as fatigue, emotional instability and mood are known to inflate SNOT-22 scores in tandem with nasal disease severity. 168 The SNOT-22 measures the spectrum of symptom improvement following septoplasty; however, the brevity of the NOSE, which also includes a sleep item, is a more practical tool in a busy clinical setting.
Objective airway assessment
UK ENT clinicians rarely offer objective airway assessment prior to surgery, which is currently predicated on their subjective clinical assessment. The NAIROS has demonstrated that an endoscopic assessment of the nasal airway, augmented by baseline NOSE score, predicts the degree of symptomatic improvement following surgery for nasal obstruction. Objective airway assessment was requested in the NIHR HTA programme commissioning brief and the NAIROS has offered a unique opportunity to assess any added value from two of the most commonly reported nasal airway assessment measures.
The COVID-19 pandemic had a considerable impact on NAIROS data acquisition, particularly clinical nasal examination, the DOASS and objective airway assessments, which were not permitted after March 2020.
Peak nasal inspiratory flow rates at 6 and 12 months demonstrated a marginally greater improvement in the septoplasty arm than in the medical management arm (see Figure 13). Similar results have also been identified in other studies. 57,58,166 PNIF has been recommended as a simple, reliable and reproducible test of nasal airway patency. 169,170 However, studies to date have not shown good correlation between PNIF and patient-reported outcomes in nasal conditions,171,172 and PNIF does not provide information on participants’ individual nostril airflow, which is of particular relevance in assessing septoplasty outcomes.
Rhinospirometric measurements of the absolute MIV results after septoplasty (see Figure 14) demonstrate a significant improvement towards equal airflow through each nostril in comparison with medical management, aligning with the patients’ DOASS scores. The tidal breathing assessment shows similar improvements (see Figure 15). Notably, there was no evidence of negative impact on the better breathing nostril as a result of surgery. The statistical direction of effect still favours septoplasty at 12 months, but neither MIV nor tidal breathing were significantly different between the two arms at 12 months (see Table 8); this is most likely owing to the much smaller sample size and the effect of 25% of medical management participants withdrawing to have non-trial surgery.
What is the relationship of subjective and objective measures?
Aziz et al.’s173 systematic review found objective assessment tools for diagnosing nasal septal deviation to be of limited use if used in isolation, lacking sensitivity and specificity compared with clinician nasal examination. However, Boyce et al. ,56 using rhinospirometry as a gold standard, reported that the patient-reported DOASS had a sensitivity of 100% and specificity of 60% when assessing 46 patients with a deviated nasal septum. Unlike some objective assessments of the nasal airway, the DOASS can provide discriminatory airflow information on both nostrils, even in the presence of complete unilateral nasal obstruction.
The NAIROS assessed the relationship between the DOASS and the NPR in all 307 participants who attended for baseline assessments. A correlation of p = 0.77 was demonstrated between the DOASS and MIV (see Appendix 1, Figure 29) and a further correlation of p = 0.78 between the DOASS and tidal breathing (see Appendix 1, Figure 30), indicating a strong correlation between subjective and objective airflow measurements. This suggests that the DOASS can provide useful airflow assessment in lieu of objective assessments such as rhinospirometry, which is expensive, cumbersome and operator dependent.
To test the relationship between the primary outcome and airflow measurements, the NAIROS analysed SNOT-22 outcomes in relation to baseline severity stratification with post-decongestant DOASS (see Appendix 1, Figure 32). The NAIROS did not find a clinically useful relationship to facilitate the use of the DOASS to predict postoperative symptom (PROM) improvement following surgery, although this was a post-decongestion measurement, thus not necessarily reflecting normal nasal airflow.
In summary, objective measures of nasal airflow show greater nasal airway improvements across the range of baseline severity in the septoplasty arm than in the medical management arm. Rhinospirometry and the DOASS appear to demonstrate greater utility than PNIF as objective assessments. However, the use of the NOSE in combination with clinical examination offers a robust, reliable and inexpensive assessment for surgical decision-making.
Reduction of the inferior turbinate
The NAIROS protocol permitted turbinate reduction, reflecting UK surgical practice. Of the 155 septoplasties for which information on turbinate reduction was available, 88 (57%) included a turbinate reduction.
There was considerable variation across trial sites on the frequency of turbinate reduction performance. The intention had been to perform a stratified analysis by turbinate reduction, but, given that there was considerable variance between the intention to reduce the turbinate at baseline and actual turbinate reduction at the time of surgery, this was not particularly informative (see Appendix 1, Table 57). At baseline, those in the surgical arm who were recommended for turbinate reduction had slightly higher SNOT-22 scores.
The outputs of the NAIROS infer that turbinate surgery added no additional improvement to septoplasty alone (see Table 10). However, the decision to perform turbinate reduction was at the discretion of the investigator and, had this additional procedure not taken place, the improvements noted may not have been evident. Van Egmond et al. 58 similarly reported no additional benefit of turbinate surgery. The evidence for turbinate surgery in the literature is conflicting. Van Egmond et al. ’s61 2018 systematic review concluded that ‘the limited number of studies comparing septoplasty with concurrent turbinate surgery to septoplasty alone generally showed postoperative improvement, but their results should be interpreted with caution due to methodological “flaws” ’. The trial does not address the question of whether or not turbinate surgery is more beneficial in improving the nasal airway than septoplasty alone.
Treatment success
The NAIROS specifically asked participants who received septoplasty about success of treatment (see Table 9). At 6 months, 134 out of 166 participants in the septoplasty ITT arm provided information on their perception of nasal airway improvement (i.e. better/same/worse): 108 out of 134 (80.5%) felt that their nasal breathing had improved, 19 (14.1%) considered their nasal breathing to be the same and seven (5.2%) reported that it had deteriorated. At 12 months, fewer data were available, with only 89 out of 166 participants providing information. Of those, 66 out of 89 (74.2%) participants felt that their nasal breathing was better and eight (8.9%) believed it was worse. Van Egmond et al. 58 reported that subjective and objective benefits of septoplasty persisted over the full 24 months of follow-up, although they did not expressly report patient satisfaction rates. In contrast, Pedersen et al. ,158 published an assessment of patient-reported satisfaction at 12 months using a subjective, categorical, patient-rated assessment of severity. They reported no improvement in 69% of patients with mild nasal obstruction and in 43% of patients with moderate nasal obstruction before surgery. In addition, 15% of all patients in the study by Pedersen et al. reported severe nasal obstruction 12 months after surgery. Neither study accurately quantified mild symptoms. Overall, the NAIROS participant perception of improvement in their nasal airway appears favourable, compared with other studies, potentially because patients with mild baseline scores were excluded. Our qualitative work reported that septoplasty had varying levels of success among the participants sampled. However, those who found the operation effective typically experienced quite high levels of success, with participants feeling that they had experienced a major reduction in their symptoms following the operation. Studies reporting success of septoplasty demonstrate considerable heterogeneity21,174 in the manner of assessment, making direct comparisons difficult.
Only 6 out of 166 surgical arm participants (3.6%) were recommended to consider revision septoplasty, although the reasons underpinning these decisions were not recorded. For four participants, the decision to offer revision surgery was reported for either ‘same’ or ‘worse’ symptoms. For the remaining two participants, symptoms were reported as ‘better’. In the only other RCT of septoplasty with or without turbinate surgery,58 1% of patients required revision septoplasty. Reported rates in the literature vary from 1% to 12%. 175–177
In all NAIROS cases, surgery was performed by experienced surgeons in both teaching and district hospital otolaryngology departments. The results of both the NAIROS and van Egmond et al. 58 provide a benchmark for colleagues and trainees in the wider surgical community to assess nasal airway surgery outcomes in their own practices.
Safety
Adverse events/serious adverse events
None of the 227 AEs or 23 SAEs related to treatment was deemed to be life-threatening. Appendix 1, Tables 63 and 67, and Table 11 highlight the categories and causes of AEs.
Overall, complication rates on initial assessment appear higher in comparison with published series, but several factors are noteworthy. First, the regular scrutiny of participants in a trial setting is greater than in standard NHS practice. Second, the trial-specific questioning regarding a potential complication may invite a positive response, in contrast to standard practice where no specific enquiry is made. Third, the mandatory postoperative endoscopic trial assessment would identify asymptomatic adhesions or perforations that typically go unnoticed in standard practice.
Some degree of mild bleeding is expected after nasal surgery. The NAIROS reports seven participants (4%) requiring re-admission to hospital with nasal bleeding, all of whom had septoplasty with inferior turbinate reduction. In a review of postoperative complications following septoplasty with or without turbinate reduction in 5639 patients, Dąbrowska-Bień et al. 66 reported a postoperative bleeding rate of 3.3% overall (without/with turbinate reduction: 2.6%/4.1%). However, the heterogeneity of inclusion criteria and treatment of the bleeding group were unclear. In anticipation of the potential for postoperative bleeding, the NAIROS protocol stipulated nasal septal suture closure, rather than the often-used alternative of nasal packing. Avoidance of nasal packing also facilitated day-care treatment. In a systematic review, Wang et al. 178 noted that postoperative pain, headache and adhesions were significantly lower in the transseptal suturing group than in the nasal-packing group. Nasal packing and transseptal suturing appear to be equivalent regarding postoperative bleeding, septal perforation and infection rates.
Postoperative infection following nasal surgery covers a broad range of conditions, from that of increasing nasal discomfort assumed to be secondary to infection, through to obvious postoperative cellulitis or inflammation that may be more severe and even require hospitalisation. Van Egmond et al. 58 reported a 7% postoperative infection rate, lower than the NAIROS (12%). In a review of 10 studies, Kullar et al. 179 noted that perioperative/postoperative antibiotics did not reduce the incidence of infection following septorhinoplasty.
We noted a higher incidence of participants reporting a reduction in sense of smell following surgery (11%) than in other studies,66 but about equal to that of the 2019 Swedish National Register study. 158 A closer analysis of the NAIROS outcomes was conducted in relation to this issue. We cross-referenced participants’ responses to loss of smell (see Appendix 1, Table 66) at 6 and 12 months with the SNOT-22 question regarding ‘loss of sense of smell or taste’. Only four participants (3%) reported a worsening of sense of smell between baseline and 6 or 12 months in response to both assessments. Smell perception is recognised as being highly subjective,180 and is difficult to characterise without using specific psychophysical tests.
In a study of 100 septoplasty patients undergoing standardised preoperative and postoperative photography, Vuyk et al. 181 noted significant postoperative nasal aesthetic changes in 1% of patients, with minor changes in > 20% of patients. Other studies report cosmetic changes in between 0.4% and 7% of patients. 66,158 The NAIROS identified 17 participants at 6 or 12 months (10%) who noted a change in the appearance of their nose. It is important to note that judging subtle changes in nasal appearance without photographic records is highly subjective and, furthermore, any postoperative cosmetic change may be considered beneficial, as opposed to detrimental. The NAIROS did not quantify this further.
Dental or palatal numbness is also a recognised AE following septal surgery. In a series of 107 septoplasty patients, Chandra et al. 182 noted a prevalence of 2.8% of such numbness. The NAIROS noted 18 participants (11%) with this complaint at 6 or 12 months.
Overall, more complications were reported at 6 months than at 12 months, although, at 12 months, COVID-19 restrictions had led to a cessation of clinical nasal assessments.
Perforations were found in the nasal septum of 6 out of 179 (3%) NAIROS participants at 6 or 12 months. Other studies quote similar rates: Dąbrowska-Bień et al. 66 found perforations in 2.3%, Pederson et al. 183 found perforations in 2% and van Egmond et al. found perforations in 2% of patients. 58 Adhesions (scarring) between the nasal septum and side walls of the nose were noted in 7 out of 179 (4%) participants at 6 or 12 months. Adhesions, many of which are asymptomatic, have been noted in up to 36% of patients in a review of retrospective studies. 184 All NAIROS participants underwent postoperative nasal endoscopy, a level of scrutiny that is not routine in clinical practice and is likely to have identified participants with small nasal septal perforations and adhesions that are commonly asymptomatic findings.
Economic evaluation
On average, septoplasty was more costly and more effective in terms of improvements in SNOT-22 scores and QALYs gained. When AEs were used as the outcome measure, septoplasty, a surgical intervention, incurred a greater number, as expected.
The incremental cost per QALY gained at 12 months was £27,114. The probability of septoplasty, with or without turbinate surgery, being cost-effective, compared with medical management, was 15% at a £20,000 threshold for an additional QALY, and 68% at a £30,000 threshold. Using strict NICE guidance, septoplasty, with or without turbinate surgery, has a low probability of being considered cost-effective at 12 months. A sensitivity analysis estimating the cost of surgery using microcosting, instead of the NHS tariff, suggested that septoplasty had a 79% probability of being considered cost-effective at 12 months at a £20,000 threshold for an additional QALY. This result provides further support that it is the cost of surgery determining cost-effectiveness at 12 months. However, these results need to be interpreted with caution.
It was anticipated that septoplasty was likely to be more costly and potentially more effective, but the 12-month duration of the trial was insufficient for the additional benefit to offset the additional costs of surgery. This is illustrated in the economic analyses in which we compare the incremental cost per QALY for septoplasty versus medical management at 6 months’ follow-up (> £100,000) with that at 12 months (£27,114). For this reason, an economic model was undertaken to extrapolate the trial results to 24 and 36 months post randomisation. The model was a simple decision-tree populated using the trial data and clinical inputs. At 24 months, septoplasty remained, on average, more costly and more effective in terms of QALYs gained; however, the ICER reduced to around £13,000, which is well within the £20,000 NICE threshold for an additional QALY. By 36 months, the septoplasty ICER had reduced to around £7000 per QALY. In these analyses, septoplasty had the highest probability of being considered cost-effective, compared with medical management, at 24 months (99%) and 36 months (100%), at a £20,000 threshold for an additional QALY.
This is the first RCT to evaluate the cost-effectiveness of septoplasty, compared with a standardised medical management regime, in the management of a deviated nasal septum.
Strengths and limitations
Study strengths
-
To our knowledge, the NAIROS is the first RCT of septoplasty, with or without turbinate surgery, incorporating two well-defined, reproducible treatment arms.
-
The trial was designed to have 90% power to detect the MCID of 9 units on the SNOT-22 scale. The trial recruited to target and the observed attrition level was no higher than allowed for in the sample size calculations.
-
The primary outcome is patient-reported, and a major strength of the trial is that we amended the protocol to collect this remotely by e-mail, post and an online platform. This helped to maximise data accrual, especially during the later period of the trial when face-to-face visits were halted owing to COVID-19.
-
We recruited participants from a wide range of sites across Great Britain, which included both large teaching hospitals and smaller district general hospitals, to represent the whole population.
-
A broad range of well-recognised, validated outcome measures, both subjective and objective, were used to determine the success of treatment among the trial participants. The trial recruited to target with no funding extension.
-
An electronic version of the primary outcome PROM was successfully used to achieve the target number of participants reporting and returning the associated data.
-
We conducted the primary statistical analysis on the complete-case data. A sensitivity analysis accounted for the small number of withdrawals from the trial and missing data using multiple imputation. The additional analyses undertaken supported the primary ITT analysis conclusions. The per-protocol and per-treatment analyses corroborate the ITT results, confirming the greater improvement in patient-reported outcomes of those participants receiving surgery than those treated medically.
-
The objective measures of nasal airflow, specified by the funder, confirmed the impact of surgery over medical management, although these did not contribute over and above PROMs in determining the best management strategy for patients with a deviated nasal septum.
-
A major strength of this trial was the embedded economic evaluation. The response rate to the data collection tools used to inform the economic analysis was relatively high, with nearly two-thirds of participants having complete data. Costs and effects were estimated at 12 months and were extrapolated so that the longer-term costs and benefits associated with septoplasty could be considered. Finally, a number of sensitivity analyses were undertaken to test the robustness of the economic conclusions. Reassuringly, these analyses did not change our conclusions, in that septoplasty was, on average, more costly but more effective than medical management. An adjusted analysis using SUR was used to estimate the difference in costs and QALYs. This is a robust method for estimating the ICER as it facilitates the simultaneous estimation of costs and effects and takes into consideration unobserved individual characteristics that may affect costs and effects. 114
-
The inclusion of a qualitative process evaluation enabled a comprehensive understanding of the benefits and burdens of both treatments and highlighted ways in which treatments could be optimised. It also ensured that learning from the trial is captured to inform future surgical trials.
-
Patients were influential voices throughout the evolution of the NAIROS RCT, from PPI at the design outset to ongoing contributions from the lay participant on the TSC.
Study limitations
-
All patients who were sent a PIS were entered on the screening log. Pre-screening patient telephone contact and dedicated NAIROS clinics were not permitted by the NHS REC. As a result, it is likely that only those motivated patients identified in GP referral letters/busy ENT clinics, and who were prepared to return for NAIROS assessments, were recruited and took part in the randomisation process.
-
The NAIROS consent process was complex and time-consuming, incorporating three stages. The first two stages of consent (eligibility and audio-recordings) could be completed by a research nurse, thereby saving time in a busy clinical environment. However, given that the trial was a CTIMP, stage 3, the consent to the main trial, required a clinician. The consent process and the trial assessments took at least 1–2 hours, limiting the number of patients recruited in each clinic.
-
Although it was recommended that recruitment take place in research clinics, many sites were unable to do so, which led to challenges in finding both time and physical space for recruitment at some sites.
-
At baseline > 80% of patients had NOSE scores in the severe/extreme category. It is likely that some patients with less severe nasal obstruction either responded to treatment provided in primary care or, once referred, did not wish to participate in the trial.
-
The qualitative substudy interviewed only one person who declined to participate in the trial. We therefore know relatively little about the preferences and experiences of those who did not wish to participate in the trial.
-
Nasal obstruction is a non-specific symptom with many underlying possibilities (e.g. allergic rhinitis, non-allergic rhinitis, nasal valve dysfunction). In addition, making a diagnosis of septal deviation is challenging in primary care because of the limitations of nasal examination without specialist equipment.
-
The NAIROS surgical interventions were performed by experienced surgeons. In NHS practice, septoplasty with or without turbinate reduction is frequently performed by junior trainee surgeons, albeit often supervised by more senior colleagues.
-
COVID-19 had a significant impact on the NAIROS trial. All forms of airway clinical assessment and objective testing of nasal airway function were suspended from March 2020 onwards. As a result of the smaller numbers of participants assessed at 12 months, this may have had an impact on the precision of the statistical outputs.
-
Twelve months is too short a time horizon for the costs associated with a surgical intervention such as septoplasty to be offset by the benefits; however, surgery becomes cost-effective within 24 months.
-
One of the main challenges of the economic evaluation was the microcosting exercise. First, the NHS tariff includes additional costs such as overheads that were not included in the microcosting exercise. Second, costs were sourced from only one site, but are presented for the reader to judge generalisability. Finally, septoplasty data used to inform the microcosting exercise were collected only for participants randomised to septoplasty, but 30% (n = 47) of participants randomised to medical management underwent septoplasty. This has meant that variations in surgical costs were not captured in the bootstrapping analysis as every participant who underwent surgery was assigned the same cost.
-
The economic model was populated using data from a single study. However, validity checks and sensitivity analyses were undertaken to ensure the robustness of these results.
Generalisability
The challenges faced in the design, implementation and analysis of surgical RCTs are well recognised. 129,185
Our qualitative work showed that the trial design was considered positively. Clinicians considered normal clinical practice as being reflected by a trial of medical therapy before offering surgery. Patients considered the option of deferred surgery, if randomised to the medical management arm, as a key factor in deciding to participate in the main trial.
Nasal obstruction is the most common nasal symptom presentation in secondary care. The NAIROS excluded those with nasal bone deviation, as stipulated in the commissioning brief. The selection criteria were unable to exclude those with concomitant allergic or non-allergic rhinitis. The NAIROS participants may have had both nasal structural and mucosal disease, for which current standard NHS care may involve both septoplasty and nasal steroid spray in combination, whereas trial participants were offered only a single treatment category.
One investigator in the qualitative study noted that there may be unwillingness of colleagues to refer the most severely deviated nasal septum patients for recruitment, in the belief that severe deviation at the front of the nostril will respond to surgical treatment only. Notably, this concern was not raised by other investigators and the NAIROS recruited patients with a broad range of NOSE score severities.
There is a dichotomy of potential treatment duration in the two trial arms as it relates to the ‘real world’. After an initial perioperative symptom exacerbation, a successful septoplasty produces a permanent change in the nasal airway and symptomatic improvement is expected to be long-lasting. In contrast, nasal steroid sprays require potentially unlimited duration of treatment, with possible side effects, as well as a willingness of patients to comply with and pay for treatment.
We had intended, during the course of the NAIROS, to compare the main trial ‘acceptors’ and ‘decliners’ to gather information on the generalisability of the trial cohort to the total population of those referred for consideration of surgery. However, this was not practical given the small number of ‘decliners’ in the cohort recorded on the screening logs. There were several reasons for this. First, the difficulty in defining eligible recruits, alluded to previously. In addition, the NHS REC stipulated that telephone pre-screening of patients was not permitted. As a result, some patients seen for assessment did not have a deviated septum. In most sites, participants who were sent a PIS, attended clinic and were found to be eligible were subsequently recruited, and only 45 participants who consented to eligibility screening, but not to the main trial, had data available for analysis.
The potential applicability of the NAIROS outcomes across different ethnic groups should be tested further.
Variations in surgical technique can affect the generalisability of surgical RCT findings. 185,186 The NAIROS minimised variability in surgical technique by standardising critical parts of the procedure (closed approach, site of incision, closure) and postoperative care undertaken. However, surgeons were allowed to adopt a variety of techniques to manipulate the cartilage skeleton into place, and so overall its results reflect the generality of current surgical practice.
Implications for practitioners and health services
There are currently no evidence-based guidelines for the definitive treatment of nasal obstruction in the presence of a deviated nasal septum. In the UK many existing Clinical Commissioning Groups require up to 6 months of nasal steroid treatment in primary care before sanctioning referral to secondary care ENT services. 153,154 A 2015 US-based clinical consensus statement187 agreed a ‘trial of medical therapy of more than 4 weeks’ duration is unnecessary to assess surgical candidacy for septoplasty. However, the panel did not reach consensus on the statement that a four-week trial of nasal steroid prior to septoplasty is sufficient to assess surgical candidacy’.
Given that approximately 16,000 septoplasties are undertaken annually in the NHS, the NAIROS results justify the research investment made, despite its hitherto limited evidence base.
The NAIROS is the first RCT that definitively demonstrates that septoplasty, with or without turbinate reduction, in comparison with a medically treated cohort, is a highly effective procedure with evidence that it may be considered cost-effective at 24 months. The NAIROS-generated evidence confirms that the use of a brief assessment tool (the NOSE) in combination with endoscopic assessment/confirmation of a deviated nasal septum as the principal pathology defines those patients who will have benefit from septoplasty, with or without turbinate reduction. Although objective measurements have corroborated the superiority of surgery over medical management, the NAIROS has not demonstrated that such measurements provide useful information beyond that of clinical and baseline PROM assessment.
Most patients will have sought treatment in community and primary care settings and will have had a trial of nasal steroid spray. We recommend that patients who do not see improvement with this first-line treatment are referred to secondary care to undergo a clinical nasal airway assessment and complete the NOSE severity scale. When weighing up the risks and benefits of different treatment strategies, those with a NOSE score of > 30 can reliably be advised that septoplasty, with or without turbinate surgery, is a highly effective, evidence-based treatment option and that the level of predicted benefit will relate to the severity of their symptom burden at presentation.
Context of trial/informing NHS guidance
The NAIROS has shown that baseline assessment using the NOSE scale can predict the degree of postoperative symptom improvement. In this regard, we have operationalised the NOSE score and would recommend its use in standard clinical practice for preoperative decision-making for nasal septal surgery.
Modest overall improvements in SNOT-22 scores in the medical management arm were evident at both follow-up intervals. The reasons for this improvement, beyond the initial 6-month trial period of steroids/saline spray, are likely to be related to several factors. First, as previously described, around 25% of the medical management arm underwent septoplasty during the trial. Second, it is recognised that rhinitis has a fluctuating impact on nasal symptoms and this may have potentially contributed to the improvement effect noted. Nasal steroids/saline improve congestion and nasal airflow among patients with allergic and non-allergic rhinitis. 75,188,189 It is expected that a proportion of participants should obtain some benefit from this treatment given the prevalence of rhinitis in the general population. 190 Existing guidelines187 indicate that there is no consensus among clinicians as to the benefit of medical management (in comparison with surgery) as a treatment for patients with a deviated nasal septum. The most common reasons cited for ceasing medical treatment in the NAIROS were ‘not happy with sprays/side effects’ (78%) and worsening symptoms (15%). Pragmatically, nasal spray delivery may be impeded or impossible in cases of a particularly deviated septum. In practice, treatment discussions with patients wishing to consider medical management should also include reference to the potential risks of side effects including minor nosebleeds, nasal dryness and irritation, and the need for long-term treatment and associated costs.
The majority of patients presenting to primary care with nasal obstruction (and without red-flag symptoms) who do not have a deviated nasal septum may be treated medically. Those whose symptoms persist despite treatment should be referred to secondary care for a thorough and careful clinical assessment, including nasal endoscopy and NOSE score assessment. The NAIROS has demonstrated from both clinical and health-effectiveness perspectives that, for those patients with a NOSE score of > 30 and a deviated nasal septum, septoplasty, with or without turbinate reduction, is superior to long-term medical therapy.
Implications for patients and the public
Ultimately, it is for the patient to decide based on the information provided whether, after weighing up risks and benefits, they wish to consider medical therapy or to proceed with surgery.
The NAIROS has developed evidence showing that septoplasty, with or without turbinate reduction, outperforms a combination of nasal steroid/saline sprays in the treatment of nasal obstruction associated with a deviated nasal septum. Given the heterogeneity of patients who present with nasal blockage, an initial trial of medical management remains appropriate for most patients. The duration of this treatment is not yet defined.
After a trial in the primary care setting of nasal steroid/saline medication, those patients referred to secondary care who have endoscopic evidence of septal deflection in the presence of moderate, severe or extreme NOSE scores, when weighing up the risks and benefits of different treatment strategies, can reliably be advised that septoplasty, with or without turbinate surgery, is a highly effective, evidence-based treatment option, and that the level of predicted benefit will relate to the severity of their symptom burden at presentation.
Implications for research
-
The most important research priority to emerge from the NAIROS trial is the requirement for a patient decision aid to explore management of a deviated nasal septum. Surgery is more clinically effective, but carries a greater risk of AEs. Medical management carries fewer risks, but results in ongoing costs. A decision support tool would allow a patient to discuss their own individual health risks, along with their values and preferences, at the time of referral, and to integrate these with the NAIROS outputs, which can also be used to enhance and update currently available patient information resources.
-
We recommend that a meta-analysis of outcomes with van Egmond et al. ’s58 RCT be conducted to analyse the pooled data and reach objective conclusions on the clinical effectiveness and cost-effectiveness of septoplasty in the short and long term based on the strengths and limitations of both RCTs.
-
Further studies are required to assess the optimum medical treatment and its duration in the management of nasal obstruction associated with a deviated septum.
-
Further work to determine the use of the DOASS as a discriminatory tool with possible utility in primary care.
Chapter 8 Conclusions
Septoplasty, with or without turbinate reduction, is a clinically effective intervention. Participants with a deviated nasal septum with a moderate, severe or extreme baseline severity of nasal obstruction symptoms had an improvement in patient-reported outcomes at 6 and 12 months. This improvement surpassed that of standardised medical management. A sensitivity subanalysis confirmed that surgery is increasingly effective with increasing baseline symptom severity. Improvements were correspondingly noted in both participants’ perception of nasal airflow and objective measures of nasal function.
The NAIROS-generated evidence confirms that a NOSE score of > 30 in association with a symptomatic deviated nasal septum determines which patients will benefit from septoplasty, with or without turbinate reduction.
Septoplasty, with or without turbinate reduction, is more costly and effective than medical management. The results suggest that surgery has a low probability of being cost-effective at 12 months but may be considered cost-effective at 24 months.
The findings of the qualitative substudy were consistent with an improved outcome in the surgical treatment arm, but demonstrated that, on an individual level, there were still patients who did not report benefit from surgery and others who did experience useful symptom improvement with medical management. Additional information might enable medical treatment to be optimised and improve the patient experience of surgery, particularly in the postoperative period. Surgeons reported that they would ideally like to be able to identify in advance which patients were most likely to benefit from surgery, but there were mixed views about the use of standardised outcome measures as part of decision-making.
Acknowledgements
The NAIROS trial was possible only because of the generous support and enthusiasm of a large number of individuals.
Clinical research staff from across the UK contributed to the trial and we express our gratitude to all staff at sites in the UK for their support of trial recruitment, intervention delivery and data collection. We would like to thank the following centres: Aneurin Bevan University Health Board; Bradford Teaching Hospitals NHS Foundation Trust; County Durham and Darlington NHS Foundation Trust; Guy’s and St Thomas’ NHS Foundation Trust; James Paget University Hospitals NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Liverpool University Hospitals NHS Foundation Trust; Newcastle upon Tyne Hospitals NHS Foundation Trust; NHS Grampian; NHS Lanarkshire; NHS Tayside; North Cumbria Integrated Care NHS Foundation Trust; University Hospitals Plymouth NHS Trust; Salisbury NHS Foundation Trust; Stockport NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; and Wrightington, Wigan and Leigh NHS Foundation Trust.
We would like to thank all the site staff who took part in the qualitative interviews.
We would also like to thank the NCTU staff, including previous team members: trial manager, Dr Ann-Marie Hynes; clinical trial administrators, Dr Eva-Maria Holstein, Mrs Wendy Banks; secretary, Ms Ruby Smith-Whelan; data manager, Dr Alaa Abouhajar; and senior trial managers, Dr Lesley Hall, Dr Jared Thornton and Dr Sonya Carnell.
Furthermore, we would like to thank Graham Stobbs for assistance with the health economics microcosting, and Dr Lyndsay Lindley and Ms Joan Mackintosh for conducting the qualitative staff and participant interviews.
We thank Ms Maria Allen and Mr Ian Campbell for providing clinical trials pharmacy guidance and review on behalf of the sponsor.
We would like to thank all the PPI advisors who have provided helpful advice and guidance when reviewing the trial and patient-facing documents.
We would like to thank the members of the NAIROS TSC: Professor Valerie Lund (chairperson), Mrs Natalie Rowland (independent statistician), Professor Helen Mason (independent health economist), Mr Russell Cathcart (independent clinician), Professor Tim Woolford (independent clinician), and Ms Andrea Anderson and Ms Irene Soulsby (independent lay members).
We thank the members of the NAIROS DMC for all their valuable guidance: Professor Steff Lewis (chairperson), Mr Stephen Gerry (independent statistician) and Professor Gerald McGarry (independent clinician).
We are indebted to Miss Frances Grierson for generating Figure 1.
Finally, but most importantly, we would like to thank every participant who took part in the NAIROS trial.
Contributions of authors
Sean Carrie (https://orcid.org/0000-0003-1722-1814) (Consultant Otolaryngologist and Head and Neck Surgeon) was the trial chief investigator; provided substantial input to the design and execution of the trial; authored the introductory, discussion and conclusion chapters of the final report; and had substantial input into review of all other sections of the final report.
Tony Fouweather (https://orcid.org/0000-0002-2292-0495) (Trial Statistician) wrote the SAP, conducted the main trial analysis, wrote the results chapter, and reviewed and contributed to the final report.
Tara Homer (https://orcid.org/0000-0002-6664-0671) (Trial Health Economist) wrote the health economics analysis plan, conducted the health economics analysis, interpreted the health economic data, wrote the economic evaluation chapter, and reviewed and contributed to the final report.
James O’Hara (https://orcid.org/0000-0002-4096-3296) (Consultant Otolaryngologist and Head and Neck Surgeon, site PI) was a co-applicant for funding, contributed to the design of the protocol, contributed to trial recruitment, aided in the analysis of the results, and reviewed and contributed to the final report.
Nikki Rousseau (https://orcid.org/0000-0001-8826-3515) (University Academic Fellow in Healthcare Technology Evaluation) led the qualitative aspects of the trial, contributed to the design of the trial, was a co-applicant for funding, interpreted qualitative data, wrote the qualitative chapter of the final report, and reviewed and contributed to the final report.
Leila Rooshenas (https://orcid.org/0000-0002-6166-6055) (Senior Lecturer in Qualitative Health Science) led the QRI, interpreted the QRI data, authored the QRI chapter, and reviewed and contributed to the final report.
Alison Bray (https://orcid.org/0000-0003-1402-804X) (Lead Healthcare Scientist) developed the nasal patency measurement protocols, provided technical support to the trial sites, contributed to the design of the trial protocol, authored the nasal patency measurements report, and reviewed and contributed to the final report.
Deborah D Stocken (https://orcid.org/0000-0001-8031-1738) (Professor of Clinical Trials Research) contributed to the trial design, protocol and SAP; was a co-applicant for funding; interpreted trial data; and reviewed and contributed to the final report.
Laura Ternent (https://orcid.org/0000-0001-7056-298X) (Trial Senior Health Economist) contributed to the design of the protocol, was a co-applicant for funding, contributed to the design of the health economics analysis plan, interpreted the health economic data, and reviewed and contributed to the final report.
Katherine Rennie (https://orcid.org/0000-0003-1703-3768) (Clinical Trials Manager) was a trial manager for the trial, provided day-to-day management of trial conduct, performed monitoring to ensure that the trial was conducted to good clinical practice requirements, and co-wrote and edited the final report.
Emma Clark (https://orcid.org/0000-0003-0065-1463) (Clinical Trials Manager) was a trial manager for the trial, provided day-to-day management of trial conduct, performed monitoring to ensure that the trial was conducted to good clinical practice requirements, and co-wrote and edited the final report.
Nichola Waugh (https://orcid.org/0000-0003-2956-7978) (Database Manager) was the database manager for the trial. Nichola designed the database; monitored data entered by sites for the duration of the trial; cleaned and prepared data in preparation for analysis, and for summarisation and presentation to the TMG as and when required; and contributed to the final report.
Alison J Steel (https://orcid.org/0000-0003-1279-1455) (Senior Trial Manager) led NCTU’s involvement in the trial, giving guidance on all aspects of governance and trial delivery, and reviewed and edited the final report for important intellectual content.
Jemima Dooley (https://orcid.org/0000-0003-3418-8112) (Qualitative Researcher, QRI) was part of the QRI team. She delivered pre-recruitment training, collected and analysed recruitment data, delivered phase 2 training, contributed to recruitment tips and guidance, and reviewed the final report.
Michael Drinnan (https://orcid.org/0000-0002-2181-8202) (Head of Clinical Engineering) contributed to the trial design, was a co-applicant for funding, reviewed the nasal patency measurements report, and reviewed and contributed to the final report.
David Hamilton (https://orcid.org/0000-0002-9653-6453) (Consultant Otolaryngologist and Head and Neck Surgeon, Co-Applicant) was a co-applicant for funding, contributed to the design of the protocol and PPI, and reviewed and contributed to the final report.
Kelly Lloyd (https://orcid.org/0000-0002-0420-2342) (Research Assistant) analysed the qualitative interview data and co-authored the qualitative chapter of the final report.
Yemi Oluboyede (https://orcid.org/0000-0002-9891-8279) (Senior Lecturer) contributed to the design of the protocol and reviewed and contributed to the final report.
Caroline Wilson (https://orcid.org/0000-0002-1678-7974) (Qualitative Researcher, QRI) was part of the NAIROS QRI team. She conducted interviews and analysed recruitment data, developed and delivered phase 2 training, contributed to recruitment tips and guidance, contributed to data collection of the qualitative process evaluation interviews and reviewed the final report.
Quentin Gardiner (https://orcid.org/0000-0002-3711-9326) (Consultant ENT Surgeon, site PI) was a co-applicant for funding, contributed to trial recruitment and reviewed the final report.
Naveed Kara (https://orcid.org/0000-0002-4492-194X) (Consultant ENT Surgeon, site PI) was a co-applicant for funding, contributed to trial recruitment and reviewed the final report.
Sadie Khwaja (https://orcid.org/0000-0001-7129-8789) (Consultant ENT Surgeon, Site PI) was a co-applicant for funding, contributed to trial recruitment and reviewed the final report.
Samuel Chee Leong (https://orcid.org/0000-0002-7213-0387) (Consultant ENT Surgeon, Site PI) was a co-applicant for funding, contributed to trial recruitment and reviewed the final report.
Sangeeta Maini (https://orcid.org/0000-0003-3015-0707) (Consultant ENT Surgeon, Site PI) was a co-applicant for funding, contributed to trial recruitment and reviewed the final report.
Jillian Morrison (https://orcid.org/0000-0003-3965-1516) (Professor of General Practice, Clerk of Senate and Vice Principal) provided advice about liaison and communication with primary care during the trial, was a co-applicant for funding, and reviewed and contributed to the final report.
Paul Nix (https://orcid.org/0000-0002-7284-710X) (Consultant ENT Surgeon, site PI) was a co-applicant for funding, contributed to trial recruitment, and reviewed and contributed to the final report.
Janet A Wilson (https://orcid.org/0000-0002-6416-5870) (Emerita Professor of Otolaryngology) was a co-applicant for funding, contributed to the design of the protocol, contributed to trial recruitment, aided in the analysis of the results, and reviewed and contributed to the final report.
M Dawn Teare (https://orcid.org/0000-0003-3994-0051) (Trial Senior Statistician) edited the SAP, led the final study analysis, and co-wrote and reviewed the final report.
Publication
Carrie S, O’Hara J, Fouweather T, Homer T, Rousseau N, Rooshenas L, et al. Clinical effectiveness of septoplasty versus medical management for nasal airways obstruction: multicentre, open label, randomised controlled trial. BMJ 2023;383:e075445. https://doi.org/10.1136/bmj-2023-075445
Rennie KJ, O’Hara J, Rousseau N, Stocken D, Howel D, Ternent L, et al. Nasal Airway Obstruction Study (NAIROS): a Phase III, open-label, mixed-methods, multicentre RCT of septoplasty versus medical management of a septal deviation with nasal obstruction. Trials 2020;21:179.
Data-sharing statement
The final anonymised data set from this trial will be available to the scientific community via an online data repository, subject to regulatory and ethics approval. Requests for data should be directed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Baumann I, Baumann H. A new classification of septal deviations. Rhinology 2007;45:220-3.
- Harrill WC, Pillsbury HC, McGuirt WF, Stewart MG. Radiofrequency turbinate reduction: a NOSE evaluation. Laryngoscope 2007;117:1912-9. https://doi.org/10.1097/MLG.0b013e3181271414.
- Clement WA, White PS. Trends in turbinate surgery literature: a 35-year review. Clin Otolaryngol Allied Sci 2001;26:124-8. https://doi.org/10.1046/j.1365-2273.2001.00450.x.
- Baumann I. Quality of life before and after septoplasty and rhinoplasty. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010;9.
- Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope 2010;120:635-8. https://doi.org/10.1002/lary.20777.
- NHS Digital. Hospital Episode Statistics . Admitted Patient Care – England 2019–20 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2019-20 (accessed 24 January 2020).
- Gray L. Deviated nasal septum. Incidence and etiology. Ann Otol Rhinol Laryngol Suppl 1978;87:3-20. https://doi.org/10.1177/00034894780873S201.
- Ruano-Gil D, Montserrat-Viladiu JM, Vilanova-Trías J, Burgés-Vila J. Deformities of the nasal septum in human foetuses. Rhinology 1980;18:105-9.
- Wojas O S-DP, Grzanka A, Krzych-Fałta E, Samoliński BK. Nasal septum deviation by age and sex in a study population of Poles. J Rhinol Otol 2019;7:1-6.
- Grymer LF, Hilberg O, Pedersen OF. Prediction of nasal obstruction based on clinical examination and acoustic rhinometry. Rhinology 1997;35:53-7.
- Jessen M, Malm L. Definition, prevalence and development of nasal obstruction. Allergy 1997;52:3-6. https://doi.org/10.1111/j.1398-9995.1997.tb04876.x.
- Radon K, Gerhardinger U, Schulze A, Zock JP, Norback D, Toren K, et al. Occupation and adult onset of rhinitis in the general population. Occup Environ Med 2008;65:38-43. https://doi.org/10.1136/oem.2006.031542.
- Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol 2005;99:2440-50. https://doi.org/10.1152/japplphysiol.00772.2005.
- Sharma S, Wormald JCR, Fishman JM, Andrews P, Kotecha BT. Rhinological interventions for obstructive sleep apnoea – a systematic review and descriptive meta-analysis. J Laryngol Otol 2019;133:168-76. https://doi.org/10.1017/S0022215119000240.
- Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J 2008;31:110-7. https://doi.org/10.1183/09031936.00087607.
- Velasco LC, Arima LM, Tiago RS. Assessment of symptom improvement following nasal septoplasty with or without turbinectomy. Braz J Otorhinolaryngol 2011;77:577-83. https://doi.org/10.1590/S1808-86942011000500007.
- Min YG, Jung HW, Kim CS. Prevalence study of nasal septal deformities in Korea: results of a nation-wide survey. Rhinology 1995;33:61-5.
- Smith KD, Edwards PC, Saini TS, Norton NS. The prevalence of concha bullosa and nasal septal deviation and their relationship to maxillary sinusitis by volumetric tomography. Int J Dent 2010;2010. https://doi.org/10.1155/2010/404982.
- Moshfeghi M, Abedian B, Ghazizadeh Ahsaie M, Tajdini F. Prevalence of nasal septum deviation using cone-beam computed tomography: a cross-sectional study. Contemp Clin Dent 2020;11:223-8. https://doi.org/10.4103/ccd.ccd_110_19.
- Dinis PB, Haider H. Septoplasty: long-term evaluation of results. Am J Otolaryngol 2002;23:85-90. https://doi.org/10.1053/ajot.2002.30987.
- Haroon Y, Saleh HA, Abouissa AH. Nasal soft tissue obstruction improvement after septoplasty without turbinectomy. Eur Arch Oto Rhino Laryngol 2012;270:2649-55. https://doi.org/10.1007/ s00405-012-2304-0.
- Günbey E, Günbey HP, Uygun S, Karabulut H, Cingi C. Is preoperative paranasal sinus computed tomography necessary for every patient undergoing septoplasty?. Int Forum Allergy Rhinol 2015;5:839-45. https://doi.org/10.1002/alr.21545.
- Haight JS, Cole P. The site and function of the nasal valve. Laryngoscope 1983;93:49-55. https://doi.org/10.1288/00005537-198301000-00009.
- Bachmann W, Legler U. Studies on the structure and function of the anterior section of the nose by means of luminal impressions. Acta Otolaryngol 1972;73:433-42. https://doi.org/10.3109/00016487209138963.
- Cole P, Chaban R, Naito K, Oprysk D. The obstructive nasal septum. Effect of simulated deviations on nasal airflow resistance. Arch Otolaryngol Head Neck Surg 1988;114:410-2. https://doi.org/10.1001/archotol.1988.01860160054020.
- Bonaparte JP, Campbell R. A prospective cohort study assessing the clinical utility of the Cottle maneuver in nasal septal surgery. J Otolaryngol Head Neck Surg 2018;47. https://doi.org/10.1186/s40463-018-0292-9.
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020;58:1-464. https://doi.org/10.4193/Rhin20.601.
- Kahana-Zweig R, Geva-Sagiv M, Weissbrod A, Secundo L, Soroker N, Sobel N. Measuring and characterizing the human nasal cycle. PLOS ONE 2016;11. https://doi.org/10.1371/ journal.pone.0162918.
- Hol MK, Huizing EH. Treatment of inferior turbinate pathology: a review and critical evaluation of the different techniques. Rhinology 2000;38:157-66.
- Berger G, Hammel I, Berger R, Avraham S, Ophir D. Histopathology of the inferior turbinate with compensatory hypertrophy in patients with deviated nasal septum. Laryngoscope 2000;110:2100-5. https://doi.org/10.1097/00005537-200012000-00024.
- ENT UK, The Royal College of Surgeons . Resources n.d. www.entuk.org/guidelines (accessed 12 November 2020).
- Sipilä J, Suonpää J, Silvoniemi P, Laippala P. Correlations between subjective sensation of nasal patency and rhinomanometry in both unilateral and total nasal assessment. ORL J Otorhinolaryngol Relat Spec 1995;57:260-3. https://doi.org/10.1159/000276754.
- Roithmann R, Cole P, Chapnik J, Barreto SM, Szalai JP, Zamel N. Acoustic rhinometry, rhinomanometry, and the sensation of nasal patency: a correlative study. J Otolaryngol 1994;23:454-8.
- Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg 2004;130:157-63. https://doi.org/10.1016/j.otohns.2003.09.016.
- Rhee JS, Sullivan CD, Frank DO, Kimbell JS, Garcia GJ. A systematic review of patient-reported nasal obstruction scores: defining normative and symptomatic ranges in surgical patients. JAMA Facial Plast Surg 2014;16:219-25. https://doi.org/10.1001/jamafacial.2013.2473.
- Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg 2013;15:358-61. https://doi.org/10.1001/jamafacial.2013.344.
- Lal D, Rounds AB, Divekar R. Gender-specific differences in chronic rhinosinusitis patients electing endoscopic sinus surgery. Int Forum Allergy Rhinol 2016;6:278-86. https://doi.org/10.1002/alr.21667.
- NHS Digital . Hospital Episode Statistics Admitted Patient Care – England, 2014–15 n.d. www.hscic.gov.uk/hes (accessed 18 January 2021).
- Park SW, Choi J, Park HO, Lim YS, Lee KS, Kim NG, et al. Are gender differences in external noses caused by differences in nasal septal growth?. J Craniomaxillofac Surg 2014;42:1140-7. https://doi.org/10.1016/j.jcms.2014.01.045.
- Siegel NS, Gliklich RE, Taghizadeh F, Chang Y. Outcomes of septoplasty. Otolaryngol Head Neck Surg 2000;122:228-32. https://doi.org/10.1016/S0194-5998(00)70244-0.
- Bezerra TF, Stewart MG, Fornazieri MA, Pilan RR, Pinna Fde R, Padua FG, et al. Quality of life assessment septoplasty in patients with nasal obstruction. Braz J Otorhinolaryngol 2012;78:57-62. https://doi.org/10.1590/S1808-86942012000300011.
- Buckland JR, Thomas S, Harries PG. Can the Sino-nasal Outcome Test (SNOT-22) be used as a reliable outcome measure for successful septal surgery?. Clin Otolaryngol Allied Sci 2003;28:43-7. https://doi.org/10.1046/j.1365-2273.2003.00663.x.
- Hytönen ML, Lilja M, Mäkitie AA, Sintonen H, Roine RP. Does septoplasty enhance the quality of life in patients?. Eur Arch Oto Rhino Laryngol 2012;269:2497-503. https://doi.org/10.1007/ s00405-012-1931-9.
- Abdalla S, Alreefy H, Hopkins C. Prevalence of Sinonasal Outcome Test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clin Otolaryngol 2012;37:276-82. https://doi.org/10.1111/j.1749-4486.2012. 02527.x.
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 2009;34:447-54. https://doi.org/10.1111/ j.1749-4486.2009.01995.x.
- Pannu KK, Chadha S, Kaur IP. Evaluation of benefits of nasal septal surgery on nasal symptoms and general health. Indian J Otolaryngol Head Neck Surg 2009;61:59-65. https://doi.org/10.1007/ s12070-009-0036-2.
- Buckland JR, Thomas S, Harries PG. Can the Sino-nasal Outcome Test (SNOT-22) be used as a reliable outcome measure for successful septal surgery?. Clin Otolaryngol 2003;28:43-7. https://doi.org/10.1046/j.1365-2273.2003.00663.x.
- Aziz T, Biron VL, Ansari K, Flores-Mir C. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg 2014;43. https://doi.org/10.1186/ 1916-0216-43-11.
- Boyce JM, Eccles R. Assessment of subjective scales for selection of patients for nasal septal surgery. Clin Otolaryngol 2006;31:297-302. https://doi.org/10.1111/j.1749-4486.2006.01243.x.
- Schumacher MJ. Nasal dyspnea: the place of rhinomanometry in its objective assessment. Am J Rhinol 2004;18:41-6. https://doi.org/10.1177/194589240401800109.
- Sipilä J, Suonpää J, Laippala P. Sensation of nasal obstruction compared to rhinomanometric results in patients referred for septoplasty. Rhinology 1994;32:141-4.
- Tomkinson A. Acoustic rhinometry: its place in rhinology. Clin Otolaryngol Allied Sci 1997;22:189-91. https://doi.org/10.1046/j.1365-2273.1997.00045.x.
- Hanif J, Eccles R, Jawad SS. Use of a portable spirometer for studies on the nasal cycle. Am J Rhinol 2001;15:303-6. https://doi.org/10.1177/194589240101500503.
- Roblin DG, Eccles R. Normal range for nasal partitioning of airflow determined by nasal spirometry in 100 healthy subjects. Am J Rhinol 2003;17:179-83. https://doi.org/10.1177/ 194589240301700401.
- Cuddihy PJ, Eccles R. The use of nasal spirometry as an objective measure of nasal septal deviation and the effectiveness of septal surgery. Clin Otolaryngol Allied Sci 2003;28:325-30. https://doi.org/10.1046/j.1365-2273.2003.00714.x.
- Boyce JM, Eccles R. Assessment of subjective scales for selection of patients for nasal septal surgery. Clin Otolaryngol 2006;31:297-302. https://doi.org/10.1111/j.1749-4486.2006.01243.x.
- Døsen LK, Kvinnesland K, TarAngen M, Shiryaeva O, Gay C, Haye R. Unilateral and bilateral PNIF in quality control of nasal septal surgery. Int J Otolaryngol 2018;2018. https://doi.org/10.1155/2018/7846843.
- van Egmond MMHT, Rovers MM, Hannink G, Hendriks CTM, van Heerbeek N. Septoplasty with or without concurrent turbinate surgery versus non-surgical management for nasal obstruction in adults with a deviated septum: a pragmatic, randomised controlled trial. Lancet 2019;394:314-21. https://doi.org/10.1016/S0140-6736(19)30354-X.
- Ayling K, Brierley S, Johnson B, Heller S, Eiser C. How standard is standard care? Exploring control group outcomes in behaviour change interventions for young people with type 1 diabetes. Psychol Health 2015;30:85-103. https://doi.org/10.1080/08870446.2014.953528.
- Calder NJ, Swan IRC. Outcomes of septal surgery. J Laryngol Otol 2007;121:1060-3. https://doi.org/10.1017/S0022215107006500.
- van Egmond MMHT, Rovers MM, Tillema AHJ, van Neerbeek N. Septoplasty for nasal obstruction due to a deviated nasal septum in adults: a systematic review. Rhinology 2018;56:195-208. https://doi.org/10.4193/Rhin18.016.
- Tsang CLN, Nguyen T, Sivesind T, Cervin A. Long-term patient-related outcome measures of septoplasty: a systematic review. Eur Arch Otorhinolaryngol 2018;275:1039-48. https://doi.org/10.1007/s00405-018-4874-y.
- Arunachalam PS, Kitcher E, Gray J, Wilson JA. Nasal septal surgery: evaluation of symptomatic and general health outcomes. Clin Otolaryngol Allied Sci 2001;26:367-70. https://doi.org/10.1046/ j.0307-7772.2001.00481.x.
- Wang T, Han D, Zhang L, Zang H, Li Y, Liu C. A modified septoplasty with three high tension lines resection. Acta Otolaryngol 2010;130:593-9. https://doi.org/10.3109/00016480903311237.
- Daudia A, Alkhaddour U, Sithole J, Mortimore S. A prospective objective study of the cosmetic sequelae of nasal septal surgery. Acta Otolaryngol 2006;126:1201-5. https://doi.org/10.1080/ 00016480600672675.
- Dąbrowska-Bień J, Skarżyński PH, Gwizdalska I, Łazęcka K, Skarżyński H. Complications in septoplasty based on a large group of 5639 patients. Eur Arch Otorhinolaryngol 2018;275:1789-94. https://doi.org/10.1007/s00405-018-4990-8.
- Wang WW, Dong BC. Comparison on effectiveness of trans-septal suturing versus nasal packing after septoplasty: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274:3915-25. https://doi.org/10.1007/s00405-017-4709-2.
- Kim JS, Kwon SH. Is nonabsorbable nasal packing after septoplasty essential? A meta-analysis. Laryngoscope 2017;127:1026-31. https://doi.org/10.1002/lary.26436.
- Quinn JG, Bonaparte JP, Kilty SJ. Postoperative management in the prevention of complications after septoplasty: a systematic review. Laryngoscope 2013;123:1328-33. https://doi.org/10.1002/ lary.23848.
- Rudy SF, Kandathil C, Spataro EA, Moubayed SP, Most SP. Effect of nasal steroids on nasal obstruction in septal deviation: a double-blind randomized controlled trial. Facial Plast Surg Aesthet Med 2020;22:243-8. https://doi.org/10.1089/fpsam.2020.0150.
- Donaldson AM, Choby G, Kim DH, Marks LA, Lal D. Intranasal corticosteroid therapy: systematic review and meta-analysis of reported safety and adverse effects in adults. Otolaryngol Head Neck Surg 2020;163:1097-108. https://doi.org/10.1177/0194599820931455.
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AG. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011993.pub2.
- Liu L, Pan M, Li Y, Tan G, Yang Y. Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis. Braz J Otorhinolaryngol 2020;86:639-46. https://doi.org/10.1016/j.bjorl.2020.03.008.
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011995.pub2.
- Head K, Snidvongs K, Glew S, Scadding G, Schilder AG, Philpott C, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev 2018;6. https://doi.org/10.1002/ 14651858.CD012597.pub2.
- Chen XZ, Feng SY, Chang LH, Lai XP, Chen XH, Li X, et al. The effects of nasal irrigation with various solutions after endoscopic sinus surgery: systematic review and meta-analysis. J Laryngol Otol 2018;132:673-9. https://doi.org/10.1017/S0022215118000919.
- Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic saline versus isotonic saline nasal irrigation: systematic review and meta-analysis. Am J Rhinol Allergy 2018;32:269-79. https://doi.org/10.1177/1945892418773566.
- Segboer C, Gevorgyan A, Avdeeva K, Chusakul S, Kanjanaumporn J, Aeumjaturapat S, et al. Intranasal corticosteroids for non-allergic rhinitis. Cochrane Database Syst Rev 2019;11. https://doi.org/10.1002/14651858.CD010592.pub2.
- Succar EF, Turner JH, Chandra RK. Nasal saline irrigation: a clinical update. Int Forum Allergy Rhinol 2019;9:S4-S8. https://doi.org/10.1002/alr.22330.
- Rennie KJ, O’Hara J, Rousseau N, Stocken D, Howel D, Ternent L, et al. Nasal Airway Obstruction Study (NAIROS): a Phase III, open-label, mixed-methods, multicentre randomised controlled trial of septoplasty versus medical management of a septal deviation with nasal obstruction. Trials 2020;21. https://doi.org/10.1186/s13063-020-4081-1.
- Stewart MG, Smith TL, Weaver EM, Witsell DL, Yueh B, Hannley MT, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg 2004;130:283-90. https://doi.org/10.1016/j.otohns.2003.12.004.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. https://doi.org/10.1136/bmj.300.6719.230.
- Bray A. NAIROS: A Guide to Measurements of Nasal Patency n.d. https://youtu.be/ZuK66Jf_iGc (accessed 27 August 2021).
- Newcastle University . NAIROS n.d. www.nairos.co.uk (accessed 2 September 2021).
- Arunachalam PS, Kitcher E, Gray J, Wilson JA. Nasal septal surgery: evaluation of symptomatic and general health outcomes. Clin Otolaryngol Allied Sci 2001;26:367-70. https://doi.org/10.1046/j.0307-7772.2001.00481.x.
- Enache A, Lieder A, Issing W. Nasal septoplasty with submucosal diathermy to inferior turbinates improves symptoms at 3 months postoperatively in a study of one hundred and one patients. Clin Otolaryngol 2014;39:57-63. https://doi.org/10.1111/coa.12219.
- Phillips PS, Stow N, Timperley DG, Sacks R, Srubiski A, Harvey RJ, et al. Functional and cosmetic outcomes of external approach septoplasty. Am J Rhinol Allergy 2011;25:351-7. https://doi.org/10.2500/ajra.2011.25.3650.
- Poirrier AL, Ahluwalia S, Goodson A, Ellis M, Bentley M, Andrews P. Is the Sino-Nasal Outcome Test-22 a suitable evaluation for septorhinoplasty?. Laryngoscope 2013;123:76-81. https://doi.org/10.1002/lary.23615.
- Bonetti M, Gelber RD. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med 2000;19:2595-609. https://doi.org/10.1002/ 1097-0258(20001015)19:19<2595::AID-SIM562>3.0.CO;2-M.
- Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics 2004;5:465-81. https://doi.org/10.1093/biostatistics/5.3.465.
- Bonetti M, Zahrieh D, Cole BF, Gelber RD. A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med 2009;28:1255-68. https://doi.org/10.1002/sim.3524.
- Yip WK, Bonetti M, Cole BF, Barcella W, Wang XV, Lazar A, et al. Subpopulation treatment effect pattern plot (STEPP) analysis for continuous, binary, and count outcomes. Clin Trials 2016;13:382-90. https://doi.org/10.1177/1740774516643297.
- Legislation.gov.uk . Commission Directive 2005 28 EC of 8 April 2005 Laying Down Principles and Detailed Guidelines for Good Clinical Practice As Regards Investigational Medicinal Products for Human Use, As Well As the Requirements for Authorisation of the Manufacturing or Importation of Such Products n.d. www.legislation.gov.uk/eudr/2005/28 (accessed 30 June 2022).
- Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg 2013;15:358-61. https://doi.org/10.1001/jamafacial. 2013.344.
- Gillett S, Hopkins C, Slack R, Browne JP. A pilot study of the SNOT 22 score in adults with no sinonasal disease. Clin Otolaryngol 2009;34:467-9. https://doi.org/10.1111/j.1749-4486.2009. 01975.x.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- NHS Improvement . 2017/18/Reference/Costs n.d. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 27 June 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Rubie I, Haighton C, O’Hara J, Rousseau N, Steen N, Stocken DD, et al. The NAtional randomised controlled Trial of Tonsillectomy IN Adults (NATTINA): a clinical and cost-effectiveness study: study protocol for a randomised control trial. Trials 2015;16. https://doi.org/10.1186/ s13063-015-0768-0.
- Pickard R, Chadwick T, Oluboyede Y, Brennand C, von Wilamowitz-Moellendorff A, McClurg D, et al. Continuous low-dose antibiotic prophylaxis to prevent urinary tract infection in adults who perform clean intermittent self-catheterisation: the AnTIC RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22240.
- Wordsworth S, Ludbrook A, Caskey F, Macleod A. Collecting unit cost data in multicentre studies. Creating comparable methods. Eur J Health Econ 2005;6:38-44. https://doi.org/10.1007/s10198-004-0259-9.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: PSSRU, University of Kent; 2020.
- NHS . Agenda for Change – Pay Rates n.d. www.healthcareers.nhs.uk/working-health/working-nhs/nhs-pay-and-benefits/agenda-change-pay-rates/agenda-change-pay-rates (accessed 21 September 2021).
- NHS . 2019/20/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication (accessed 21 September 2021).
- Boots . Sterimar Isotonic Nasal Hygiene Spray 100 Ml n.d. www.boots.com/sterimar-isotonic-nasal-hygiene-spray-100ml-10028386 (accessed 21 September 2021).
- Office for National Statistics . Annual Survey of Hours and Earnings n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2021 (accessed 21 September 2021).
- Department for Transport . Transport Analysis Guidance n.d. www.gov.uk/guidance/transport-analysis-guidance-tag (accessed 21 September 2021).
- Automobile Association . Car Running Costs n.d. www.theaa.com/driving-advice/driving-costs/running-costs (accessed 21 September 2021).
- Mäkitie A, Aaltonen LM, Hytönen M, Malmberg H. Postoperative infection following nasal septoplasty. Acta Otolaryngol Suppl 2000;543:165-6. https://doi.org/10.1080/000164800454297.
- Becker SS, Dobratz EJ, Stowell N, Barker D, Park SS. Revision septoplasty: review of sources of persistent nasal obstruction. Am J Rhinol 2008;22:440-4. https://doi.org/10.2500/ajr.2008.22.3200.
- Ware JE. SF-36 health survey update. Spine 2000;25:3130-9. https://doi.org/10.1097/00007632-200012150-00008.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013.
- Fiebig DG. A Companion to Theoretical Econometrics. Oxford: Blackwell Publishing Ltd; 2003.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing … presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258 (20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-4. https://doi.org/10.1177/0272989X9001000308.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Donovan J, Hamdy F, Neal D, Peters T, Oliver S. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7140.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (Prostate testing for cancer and Treatment) study. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the QuinteT Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/ s13063-016-1391-4.
- Rooshenas L, Paramasivan S, Jepson M, Donovan JL. Intensive triangulation of qualitative research and quantitative data to improve recruitment to randomized trials: the QuinteT approach. Qual Health Res 2019;29:672-9. https://doi.org/10.1177/1049732319828693.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. London: Aldine Transaction; 1967.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Jepson M, Elliott D, Conefrey C, Wade J, Rooshenas L, Wilson C, et al. An observational study showed that explaining randomization using gambling-related metaphors and computer-agency descriptions impeded randomized clinical trial recruitment. J Clin Epidemiol 2018;99:75-83. https://doi.org/10.1016/j.jclinepi.2018.02.018.
- Blencowe NS, Brown JM, Cook JA, Metcalfe C, Morton DG, Nicholl J, et al. Interventions in randomised controlled trials in surgery: issues to consider during trial design. Trials 2015;16. https://doi.org/10.1186/s13063-015-0918-4.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Davies G, Mills N, Holcombe C, Potter S. Perceived barriers to randomised controlled trials in breast reconstruction: obstacle to trial initiation or opportunity to resolve? A qualitative study. Trials 2020;21. https://doi.org/10.1186/s13063-020-4227-1.
- Bamford C, Poole M, Brittain K, Chew-Graham C, Fox C, Iliffe S, et al. Understanding the challenges to implementing case management for people with dementia in primary care in England: a qualitative study using normalization process theory. BMC Health Serv Res 2014;14. https://doi.org/10.1186/s12913-014-0549-6.
- Toyserkani NM, Frisch T. Are too many septal deviations operated on? A retrospective patient’s satisfaction questionnaire with 11 years follow-up. Rhinology 2012;50:185-90. https://doi.org/10.4193/Rhino11.218.
- Valsamidis K, Printza A, Constantinidis J, Triaridis S. The impact of olfactory dysfunction on the psychological status and quality of life of patients with nasal obstruction and septal deviation. Int Arch Otorhinolaryngol 2020;24:e237-e246. https://doi.org/10.1055/ s-0040-1701269.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implementation Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Grbich C. Qualitative Research in Health: An Introduction. London: SAGE Publications Ltd; 1998.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. 4 Journal of Administration and Governance 2009;72.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. https://doi.org/10.1186/ 1745-6215-12-78.
- Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ 2014;349. https://doi.org/10.1136/bmj.g6870.
- Krieger JL, Palmer-Wackerly A, Dailey PM, Krok-Schoen JL, Schoenberg NE, Paskett ED. Comprehension of randomization and uncertainty in cancer clinical trials decision making among rural, Appalachian patients. J Cancer Educ 2015;30:743-8. https://doi.org/10.1007/ s13187-015-0789-0.
- Mittal R, Harris IA, Adie S, Naylor JM. Factors affecting patient participation in orthopaedic trials comparing surgery to non-surgical interventions. Contemp Clin Trials Commun 2016;3:153-7. https://doi.org/10.1016/j.conctc.2016.05.007.
- Biggs K, Hind D, Bradburn M, Swaby L, Brown S. Design, planning and implementation lessons learnt from a surgical multi-centre randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3649-0.
- Horwood J, Johnson E, Gooberman-Hill R. Understanding involvement in surgical orthopaedic randomized controlled trials: a qualitative study of patient and health professional views and experiences. Int J Orthop Trauma Nurs 2016;20:3-12. https://doi.org/10.1016/j.ijotn.2015.05.002.
- McDermott C, Vennik J, Philpott C, le Conte S, Thomas M, Eyles C, et al. Maximising recruitment to a randomised controlled trial for chronic rhinosinusitis using qualitative research methods: the MACRO conversation study. Trials 2021;22. https://doi.org/10.1186/ s13063-020-04993-w.
- Karataş A. Pretreatment prediction of the outcomes of intranasal steroid sprays in cases with inferior turbinate hypertrophy. Turk Arch Otorhinolaryngol 2017;55:105-10. https://doi.org/10.5152/tao.2017.2443.
- Bellani ML. Psychological aspects in day-case surgery. Int J Surg 2008;6:44-6. https://doi.org/10.1016/j.ijsu.2008.12.019.
- Baggott C, Chan A, Hurford S, Fingleton J, Beasley R, Harwood M, et al. Patient preferences for asthma management: a qualitative study. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-037491.
- NHS East and North Hertfordshire Clinical Commissioning Group. Bedfordshire and Hertfordshire Priorities Forum Guidance . Subject: Septoplasty, Rhinoplasty, and Septorhinoplasty 2016. www.enhertsccg.nhs.uk/sites/default/files/documents/Feb2017/Guidance-71-Septoplasty-rhinoplasty-and-septorhinoplasty-Janaury-2017.pdf (accessed 27 June 2022).
- Bristol, North Somerset and South Gloucestershire Clinical Commissioning Group . Nasal Treatment – Non Cosmetic for All Ages Policy n.d. https://bnssgccg.nhs.uk/library/non-cosmetic-nasal-treatment-policy/ (accessed 27 June 2022).
- Siegel NS, Gliklich RE, Taghizadeh F, Chang Y. Outcomes of septoplasty. Otolaryngol Head Neck Surg 2000;122:228-32. https://doi.org/10.1016/S0194-5998(00)70244-0.
- Watson G, O’Hara J, Carding P, Lecouturier J, Stocken D, Fouweather T, et al. TOPPITS: Trial Of Proton Pump Inhibitors in Throat Symptoms. Study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1267-7.
- UK Government . UK Population by Ethnicity: Population Statistics and 2011 Census Data n.d. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity (accessed 2 September 2021).
- Pedersen L, Schiöler L, Finjan S, Davidsson Å, Sunnergren O, Holmberg K, et al. Prognostic factors for outcome after septoplasty in 888 patients from the Swedish National Septoplasty Register. Eur Arch Otorhinolaryngol 2019;276:2223-8. https://doi.org/10.1007/s00405-019-05440-6.
- Anderson K, Ritchie K, Chorney JM, Bezuhly M, Hong P. The impact of septoplasty on health-related quality of life in paediatric patients. Clin Otolaryngol 2016;41:144-8. https://doi.org/10.1111/coa.12485.
- Weinstein GS, Levin B. Effect of crossover on the statistical power of randomized studies. Ann Thorac Surg 1989;48:490-5. https://doi.org/10.1016/S0003-4975(10)66846-4.
- Boyce J, Eccles R. Assessment of subjective scales for selection of patients for nasal septal surgery – response. Clin Otolaryngol 2007;32. https://doi.org/10.1111/j.1365-2273.2007.01366.x.
- Hanif J, Jawad SS, Eccles R. A study to assess the usefulness of a portable spirometer to quantify the severity of nasal septal deviation. Rhinology 2003;41:11-5.
- Gerecci D, Casanueva FJ, Mace JC, Annen A, Barrett DM, Kim MM, et al. Nasal Obstruction Symptom Evaluation (NOSE) score outcomes after septorhinoplasty. Laryngoscope 2019;129:841-6. https://doi.org/10.1002/lary.27578.
- Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg 2004;130:157-63. https://doi.org/10.1016/j.otohns.2003.09.016.
- Bugten V, Nilsen AH, Thorstensen WM, Moxness MH, Amundsen MF, Nordgård S. Quality of life and symptoms before and after nasal septoplasty compared with healthy individuals. BMC Ear Nose Throat Disord 2016;16. https://doi.org/10.1186/s12901-016-0031-7.
- Low WK. Can snoring relief after nasal septal surgery be predicted?. Clin Otolaryngol Allied Sci 1994;19:142-4. https://doi.org/10.1111/j.1365-2273.1994.tb01199.x.
- Deron P, Clement PA, Derde MP. Septal surgery and tubal function: early and late results. Rhinology 1995;33:7-9.
- Prus-Ostaszewska M, Wysocki J, Niemczyk K, Balcerzak J. The correlation of the results of the survey SNOT-20 of objective studies of nasal obstruction and the geometry of the nasal cavities. Otolaryngol Pol 2017;71:1-7. https://doi.org/10.5604/01.3001.0009.8408.
- Holmstrom M. The use of objective measures in selecting patients for septal surgery. Rhinology 2010;48:387-93. https://doi.org/10.4193/Rhino10.072.
- Holmström M, Scadding GK, Lund VJ, Darby YC. Assessment of nasal obstruction. A comparison between rhinomanometry and nasal inspiratory peak flow. Rhinology 1990;28:191-6.
- Andrews PJ, Choudhury N, Takhar A, Poirrier AL, Jacques T, Randhawa PS. The need for an objective measure in septorhinoplasty surgery: are we any closer to finding an answer?. Clin Otolaryngol 2015;40:698-703. https://doi.org/10.1111/coa.12455.
- Volstad I, Olafsson T, Steinsvik EA, Dahl FA, Skrindo I, Bachmann-Harildstad G. Minimal unilateral peak nasal inspiratory flow correlates with patient reported nasal obstruction. Rhinology 2019;57:436-43. https://doi.org/10.4193/Rhin19.178.
- Aziz T, Biron VL, Ansari K, Flores-Mir C. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg 2014;43. https://doi.org/10.1186/1916-0216-43-11.
- Sundh C, Sunnergren O. Long-term symptom relief after septoplasty. Eur Arch Otorhinolaryngol 2015;272:2871-5. https://doi.org/10.1007/s00405-014-3406-7.
- Dinis PB, Haider H. Septoplasty: long-term evaluation of results. Am J Otolaryngol 2002;23:85-90. https://doi.org/10.1053/ajot.2002.30987.
- Bohlin L, Dahlqvist A. Nasal airway resistance and complications following functional septoplasty: a ten-year follow-up study. Rhinology 1994;32:195-7.
- Becker SS, Dobratz EJ, Stowell N, Barker D, Park SS. Revision septoplasty: review of sources of persistent nasal obstruction. Am J Rhinol 2008;22:440-4. https://doi.org/10.2500/ajr.2008.22.3200.
- Wang WW, Dong BC. Comparison on effectiveness of trans-septal suturing versus nasal packing after septoplasty: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274:3915-25. https://doi.org/10.1007/s00405-017-4709-2.
- Kullar R, Frisenda J, Nassif PS. The more the merrier? Should antibiotics be used for rhinoplasty and septorhinoplasty? A review. Plast Reconstr Surg Glob Open 2018;6. https://doi.org/10.1097/GOX.0000000000001972.
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinology 2016;56:1-30. https://doi.org/10.4193/Rhino16.248.
- Vuyk HD, Langenhuijsen KJ. Aesthetic sequelae of septoplasty. Clin Otolaryngol Allied Sci 1997;22:226-32. https://doi.org/10.1046/j.1365-2273.1997.00035.x.
- Chandra RK, Rohman GT, Walsh WE. Anterior palate sensory impairment after septal surgery. Am J Rhinol 2008;22:86-8. https://doi.org/10.2500/ajr.2008.22.3114.
- Pedersen LA, Dölvik S, Holmberg K, Emanuelsson CA, Johansson H, Schiöler L, et al. Surgery to relieve nasal obstruction: outcome for 366 patients operated on by one senior surgeon. Eur Arch Otorhinolaryngol 2021;278:3867-75. https://doi.org/10.1007/s00405-021-06696-7.
- Matthias C. [Surgery of the nasal septum and turbinates.]. Laryngorhinootologie 2007;86:1-14. https://doi.org/10.1055/s-2007-966303.
- Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-9.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. https://doi.org/10.1136/bmj.324.7351.1448.
- Han JK, Stringer SP, Rosenfeld RM, Archer SM, Baker DP, Brown SM, et al. Clinical consensus statement: septoplasty with or without inferior turbinate reduction. Otolaryngol Head Neck Surg 2015;153:708-20. https://doi.org/10.1177/0194599815606435.
- Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy 2008;38:19-42. https://doi.org/10.1111/j.1365-2222.2007.02888.x.
- Penagos M, Compalati E, Tarantini F, Baena-Cagnani CE, Passalacqua G, Canonica GW. Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta-analysis of randomized, double-blind, placebo-controlled. Clinical Trials. Allergy 2008;63:1280-91. https://doi.org/10.1111/j.1398-9995.2008.01808.x.
- Ghouri N, Hippisley-Cox J, Newton J, Sheikh A. Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 2008;101:466-72. https://doi.org/10.1258/jrsm.2008.080096.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
Appendix 1 Additional statistical analysis
| Stratification factor | Trial arm, n (%) | Total (N = 307), n (%) | |
|---|---|---|---|
| Septoplasty (N = 152) | Medical management (N = 155) | ||
| Moderate male | 11 (7) | 15 (10) | 26 (8) |
| Moderate female | 7 (5) | 7 (5) | 14 (5) |
| Severe male | 54 (36) | 53 (34) | 107 (35) |
| Severe female | 23 (15) | 27 (17) | 50 (16) |
| Extreme male | 35 (23) | 31 (20) | 66 (22) |
| Extreme female | 22 (14) | 22 (14) | 44 (14) |
| Total | 152 (100) | 155 (100) | 307 (100) |
| Site number | Site | Date opened | Date last participant recruited | Recruitment duration (weeks) | Number recruited |
|---|---|---|---|---|---|
| 01 | Newcastle | 18 January 2018 | 23 September 2019 | 87.6 | 76 |
| 08 | Aberdeen | 31 January 2018 | 16 July 2019 | 75.9 | 37 |
| 14 | Aintree | 12 June 2018 | 5 November 2019 | 73.0 | 27 |
| 15 | Leeds | 5 April 2018 | 5 December 2019 | 87.0 | 27 |
| 02 | Great Yarmouth | 14 March 2018 | 28 August 2019 | 76.0 | 22 |
| 07 | Dundee | 23 January 2018 | 15 October 2019 | 90.0 | 20 |
| 09 | Stockport | 10 April 2018 | 30 September 2019 | 76.9 | 20 |
| 16 | Lanarkshire | 5 July 2018 | 14 November 2019 | 71.0 | 20 |
| 04 | Wigan | 14 March 2018 | 5 November 2019 | 85.9 | 18 |
| 03 | Bradford | 25 May 2018 | 19 November 2019 | 77.6 | 17 |
| 06 | Plymouth | 24 April 2018 | 29 October 2019 | 79.0 | 17 |
| 17 | Salisbury | 9 April 2018 | 25 November 2019 | 85.0 | 17 |
| 10 | London | 28 February 2019 | 13 November 2019 | 36.9 | 14 |
| 11 | Newport | 17 May 2018 | 9 October 2019 | 72.9 | 13 |
| 12 | Birmingham | 23 March 2018 | 3 September 2019 | 75.6 | 13 |
| 13 | Carlisle | 1 May 2018 | 19 September 2019 | 72.3 | 11 |
| 05 | Darlington | 23 April 2018 | 24 June 2019 | 61.0 | 9 |
| Total | 18 January 2018 | 5 December 2019 | 98.0 | 378 |
| Septoplasty arm (n = 188) | Medical management arm (n = 190) | Overall (n = 378) | |
|---|---|---|---|
| Withdrew, n (%) | 20 (11) | 26 (14) | 46 (12) |
| Time in weeks from randomisation to withdrawal | |||
| Median (IQR) | 24 (14–33) | 31 (18–50) | 27 (18–48) |
| Range (minimum, maximum) | (0, 65) | (0, 68) | (0, 68) |
| Trial arm | Time from randomisation to withdrawing (weeks) | Reason did not complete the trial as randomised | More information of reasons for withdrawing from the trial |
|---|---|---|---|
| Septoplasty | 0 | Other | Changed mind |
| Septoplasty | 5 | Other | Patient unable to stay overnight |
| Septoplasty | 7 | Other | Patient no longer wanted surgery |
| Septoplasty | 8 | Other | Patient had another surgery booked so cancelled her nasal surgery |
| Septoplasty | 10 | No reason given by participant | |
| Septoplasty | 18 | No reason given by participant | |
| Septoplasty | 21 | Protocol deviation | Sponsor advised that the patient would need to be withdrawn as they missed the deadline for surgery as stated in protocol |
| Septoplasty | 21 | Participant lost interest in the trial | Patient has lots of other legal issues with another specialty and she feels she does not want to take up any more of the NHS time. Nothing to do with the NAIROS |
| Septoplasty | 23 | Other | After further reflection did not want surgery after being randomised to surgery |
| Septoplasty | 24 | Other | Trial site requested withdrawal because septoplasty was being performed after the 8-week window, as the patient cancelled the first surgery date |
| Septoplasty | 26 | Other | Symptoms had resolved after surgery, so the participant did not want to complete any more follow-up |
| Septoplasty | 27 | Other | Unable to contact patient |
| Septoplasty | 28 | Other | Does not want any more surgery and nasal symptoms have improved by themselves |
| Septoplasty | 30 | No reason given by participant | |
| Septoplasty | 32 | Participant has other duties (e.g. caring) | Patient finished university education in Plymouth and relocated to Cornwall. It was not convenient for him to come back for surgery because of his new life commitments |
| Septoplasty | 34 | Participant lost interest in the trial | |
| Septoplasty | 51 | Other | Work commitments make it difficult to meet the trial timelines |
| Septoplasty | 52 | No reason given by participant | Participant contacted clinic to cancel her 12-month appointment on 28 October 2019 stating she does not want any further appointments |
| Septoplasty | 52 | Participant has other duties (e.g. caring) | |
| Septoplasty | 66 | Other | Patient complained about all the questionnaires, and even after having surgery, she is no better off and wants nothing further to do with the trial |
| Medical Management | 0 | Other | Patient completed full-screen appointment, prescription taken to pharmacy; however, changed his mind |
| Medical Management | 0 | Other | Patient was randomised to medical management but he wanted surgery; therefore, he withdrew from the trial |
| Medical Management | 0 | Participant lost interest in the trial | |
| Medical Management | 2 | Other | Unable to wait for prescription at pharmacy on day. Not been back to collect prescription as not had time. Does not want to continue in trial |
| Medical Management | 9 | Other | Did not feel that the medical management arm was effective in treating his nasal issue |
| Medical Management | 10 | Other | Kept forgetting to take the nasal sprays and did not feel it was making any difference |
| Medical Management | 18 | Participant has other duties (e.g. caring) | Participant discovered she had other health issues unrelated to the trial and had to focus on those |
| Medical Management | 21 | Other | Patient wrote on form ‘Condition resolved through change of diet, loss of weight and increased exercise’ |
| Medical Management | 24 | Participant lost interest in the trial | Patient did not attend for 6-month follow-up appointment. Several attempts made to contact; when finally spoke on the telephone, patient stated he had stopped taking medication approximately 2 months ago, did not really feel any benefit so did not contact us |
| Medical Management | 25 | Other | Patient feels that steroid spray or nasal surgery would not improve her current symptoms |
| Medical Management | 28 | Other | Patient wanted surgery |
| Medical Management | 29 | Other | Patient is undergoing other medical investigations. Becoming quite muddled with hospital appointments and purpose of NAIROS trial |
| Medical Management | 30 | Other | Patient wanted surgery, not feeling any benefit |
| Medical Management | 32 | Participant lost interest in the trial | |
| Medical Management | 39 | Participant has other duties (e.g. caring) | |
| Medical Management | 45 | Participant lost interest in the trial | Participant only completed 6-month SNOT-22 remotely and he finally withdrew himself at 12 months |
| Medical Management | 47 | Participant lost interest in the trial | |
| Medical Management | 48 | No reason given by participant | |
| Medical Management | 48 | Participant lost interest in the trial | Was randomised to medical management, which provided no relief of symptoms. Is waiting for surgery and feels she is wasting her time coming back for another NAIROS appointment |
| Medical Management | 51 | No reason given by participant | He stated that he did not want to be involved in the trial; no reason given |
| Medical Management | 51 | Participant lost interest in the trial | Patient unhappy with surgical outcome |
| Medical Management | 53 | No reason given by participant | |
| Medical Management | 54 | Other | Participant declined any further trial activity |
| Medical Management | 54 | Participant lost interest in the trial | Declined to attend 12-month review. Agreed to complete questionnaires by post, but has failed to return them |
| Medical Management | 61 | Other | Patient did not attend several 12-month visit appointments and then contacted the PI’s secretary to say that he no longer wished to be followed up by ENT. Has also not returned 12-month questionnaires even though has had three reminders |
| Medical Management | 68 | Other | Unable to attend because of his current job circumstances |
| Reason discontinued septoplasty | Frequency |
|---|---|
| Fear of surgery | 1 |
| No time to commit to surgery | 3 |
| Advised against surgery | 1 |
| Change of mind | 9 |
| Symptoms resolved | 3 |
| Other reason | 6 |
| Total | 23 |
| Fear of surgery | No time to commit to surgery | Advised against surgery | Change of mind | Symptoms resolved | Other reason | Other reason text |
|---|---|---|---|---|---|---|
| No | No | No | Yes | No | No | NA |
| No | Yes | No | No | No | No | NA |
| No | No | No | Yes | No | No | NA |
| No | No | Yes | Yes | Yes | No | NA |
| Yes | Patient attended for operation; however, was unable to stay overnight so he did not have the operation | |||||
| No | No | No | Yes | No | No | NA |
| No | No | No | Yes | No | No | NA |
| No | No | No | Yes | Yes | Yes | Following cardiology investigations as well |
| No | No | No | Yes | No | No | NA |
| No | No | No | No | No | Yes | Wanted to postpone surgery until end of May 2019 so they could continue to play rugby |
| No | Yes | No | No | No | No | NA |
| Yes | Yes | No | Yes | Yes | No | NA |
| No | No | No | Yes | No | No | NA |
| Yes | Not happy with results of septoplasty; wants medical management | |||||
| Yes | Unable to contact patient; therefore, do not know the reason | |||||
| Yes | Owing to capacity issues (oncology and COVID-19), unable to see [participant] in study time frame |
| Reason discontinued medical management | Frequency | Recategorised frequencies |
|---|---|---|
| Concerns about time | 2 | 2 |
| Side effects of spray | 10 | 11 |
| Unhappy with spray | 36 | 65 |
| Worse symptoms | 15 | 15 |
| Other reason | 35 | 5 |
| Total | 98 | 98 |
| Concerns about time | Side effects of spray | Unhappy with spray | Worse symptoms | Other reason | Other reason text | Recategorisation of ‘other’ |
|---|---|---|---|---|---|---|
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Was not interested in taking part | |
| No | No | No | No | Yes | Steroids not felt to be effective | Unhappy with sprays |
| No | Yes | Yes | No | No | NA | |
| No | Yes | No | Yes | Yes | Did not help the symptoms, felt it was not helping | Unhappy with sprays |
| Yes | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Finished at 6-month point to request surgery | |
| No | No | Yes | Yes | No | NA | |
| No | No | Yes | Yes | No | NA | |
| No | No | No | No | Yes | Nasal blockage; requires surgery | Unhappy with sprays |
| No | Yes | Yes | Yes | No | NA | |
| No | Yes | Yes | No | Yes | Does not want to be using nasal sprays long term | Unhappy with sprays |
| No | No | No | No | Yes | Needed surgery | Unhappy with sprays |
| No | No | Yes | No | Yes | Medication did not work | Unhappy with sprays |
| No | No | No | No | Yes | Requested crossover | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | No | No | Yes | No | NA | |
| No | No | Yes | Yes | No | NA | |
| No | No | Yes | Yes | No | NA | |
| No | Yes | Yes | No | Yes | Had a nosebleed when using the spray | Side effects |
| No | No | No | No | Yes | Patient stated no improvement; requested surgery | Unhappy with sprays |
| No | No | No | No | Yes | Would like septoplasty. Will continue using nasal sprays until surgery | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Sprays not as effective as participant would like | Unhappy with sprays |
| No | No | Yes | Yes | No | NA | |
| No | No | No | No | Yes | Patient stated not much improvement | Unhappy with sprays |
| No | No | No | Yes | No | NA | |
| Yes | NA | |||||
| No | No | No | No | Yes | Did not find sprays beneficial | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| Yes | Symptoms not improving | Unhappy with sprays | ||||
| No | No | Yes | Yes | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Had septoplasty before 6 months was completed of the medication. This was a local administration error | |
| Yes | Yes | Yes | Wanted to have surgery following epistaxis | |||
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Felt little improvement | Unhappy with sprays |
| No | No | No | No | Yes | Patient requested surgery | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | No | No | Yes | No | NA | |
| Yes | Did not make a difference | Unhappy with sprays | ||||
| No | No | No | No | Yes | Patient feels their breathing is not improving | Unhappy with sprays |
| No | No | No | No | Yes | Patient requested septoplasty | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Not getting sufficient benefit from the sprays | Unhappy with sprays |
| No | No | No | No | Yes | Job position changed so was unable to continue with the trial | |
| No | No | Yes | No | No | NA | |
| No | No | Yes | Yes | No | NA | |
| Yes | Changed mind to a preference for surgery | Unhappy with sprays | ||||
| No | No | Yes | No | Yes | To have septoplasty | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | No | No | Yes | Requested surgery; still has high NOSE score | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | Yes | No | No | No | NA | |
| No | No | No | No | Yes | To have septoplasty | Unhappy with sprays |
| Yes | Would like septoplasty | Unhappy with sprays | ||||
| No | No | No | No | Yes | Ineffective | Unhappy with sprays |
| No | No | No | No | Yes | Symptoms no better with the spray | Unhappy with sprays |
| No | No | No | No | Yes | Did not think it helped | Unhappy with sprays |
| No | Yes | No | No | No | NA | |
| No | No | Yes | No | No | NA | |
| No | No | Yes | Yes | Yes | Symptoms decreased but not sleeping well: spray not working | Unhappy with sprays |
| No | No | Yes | No | No | NA | |
| No | Yes | Yes | Yes | No | NA | |
| Yes | Yes | No | No | Yes | Symptoms have remained the same | Unhappy with sprays |
| No | No | No | Yes | No | NA | |
| No | No | Yes | No | No | NA |
| Medical history | Trial arm, n (%) | Overall (N = 378), n (%) | Non-randomised (N = 45), n (%) or n | |
|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | |||
| Is overall sense of blocked nose bilateral or unilateral? | ||||
| Bilateral | 79 (42) | 77 (41) | 156 (41) | 11 (24) |
| Unilateral | 109 (58) | 113 (59) | 222 (59) | 7 (16) |
| Which side is worst? | ||||
| Left side worse | 87 (46) | 84 (44) | 171 (45) | 5 |
| Right side worse | 80 (43) | 82 (43) | 162 (43) | 7 |
| Both sides equal | 21 (11) | 24 (13) | 45 (12) | 6 |
| Does blockage change from side to side and/or is it cyclical? | ||||
| Yes | 52 (72) | 49 (26) | 101 (27) | 1 |
| No | 136 (28) | 140 (74) | No | 136 (28) |
| Missing | 0 (0) | 1 (< 1) | 1 (< 1) | 0 |
| Is there history of nasal trauma? | ||||
| Yes | 79 (42) | 95 (50) | 174 (46) | 8 |
| No | 109 (58) | 95 (50) | 204 (54) | 10 |
| If so, what was approximate age of patient (in years) | ||||
| n (% of history of nasal trauma) | 78 (99) | 95 (100) | 173 (99) | 8 (100) |
| Median (IQR) | 21 (15–37) | 17 (14–26) | 19 (15–31) | 15 (10.5–17) |
| Mean (SD) | 28.0 (17.3) | 20.7 (13.0) | 24.0 (15.5) | 15.6 (9.8) |
| Range (minimum, maximum) | (5, 78) | (1, 61) | (1, 78) | (3, 37) |
| Did this nasal trauma result in definite change in nasal obstruction? | ||||
| Yes | 48 (61) | 62 (65) | 110 (63) | 7 |
| No | 30 (38) | 32 (34) | 62 (36) | 1 |
| missing | 1 (1) | 1 (1) | 2 (1) | |
| Smoking status | ||||
| Current smoker | 24 (13) | 37 (19) | 61 (16) | 2 |
| Ex-smoker | 54 (29) | 54 (28) | 108 (29) | 1 |
| Never smoked | 110 (59) | 99 (52) | 209 (55) | 16 |
| Clinical exam | Trial arm, n (%) | Overall (N = 378), n (%) | Non-randomised (N = 45), n | |
|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | |||
| If the patient is having surgery, would it be appropriate to reduce the inferior turbinate? | ||||
| Yes | 132 (70) | 150 (79) | 282 (75) | 7 |
| No | 52 (28) | 32 (17) | 84 (22) | 2 |
| Not applicable | 4 (2) | 8 (4) | 12 (3) | 6 |
| To which side does the nasal septum deflect, so diminishing the airway? | ||||
| Right | 79 (42) | 67 (35) | 146 (39) | 9 |
| Left | 71 (38) | 86 (45) | 157 (42) | 5 |
| Both | 38 (20) | 37 (19) | 75 (20) | 1 |
| Neither | 0 (0) | 0 (0) | 0 (0) | 0 |
| Please specify the area of septum deflecting into that airway (right airway) | ||||
| (Right + both) septoplasty, n = 117; medical management, n = 104; total, N = 221 | 116 (99) | 104 (100) | 220 (> 99) | |
| Anterior | 46 (39) | 53 (51) | 99 (45) | |
| Posterior | 13 (11) | 15 (14) | 28 (13) | |
| Upper | 11 (9) | 8 (8) | 19 (9) | |
| Lower | 13 (11) | 11 (11) | 24 (11) | |
| All | 30 (26) | 16 (15) | 46 (21) | |
| None | 3 (3) | 1 (< 1) | 4 (2) | 5 |
| Missing | 1 (< 1) | 0 (0) | 1 (< 1) | |
| Please specify the area of septum deflecting into that airway (left airway) | ||||
| (Left + both) septoplasty, n = 109; medical management, n = 123; total, N = 232 | 108 (99) | 123 (100) | 231 (> 99) | |
| Anterior | 43 (39) | 50 (41) | 93 (40) | 3 |
| Posterior | 17 (16) | 19 (15) | 36 (16) | 0 |
| Upper | 8 (7) | 8 (7) | 16 (7) | 0 |
| Lower | 12 (11) | 15 (12) | 27 (12) | 1 |
| All | 26 (24) | 29 (24) | 55 (24) | 1 |
| None | 2 (2) | 2 (2) | 4 (2) | |
| Missing | 1 (< 1) | 0 (0) | 1 (< 1) | |
| Endoscopy findings | Trial arm, n (%) | Overall (N = 378), n (%) | Non-randomised (N = 45), n | |
|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | |||
| Right inferior turbinate enlarged? | ||||
| Yes | 89 (47) | 110 (42) | 199 (53) | 8 |
| No | 99 (53) | 80 (58) | 179 (47) | 7 |
| Left inferior turbinate enlarged? | ||||
| Yes | 97 (52) | 94 (49) | 191 (51) | 10 |
| No | 91 (48) | 95 (50) | 186 (49) | 5 |
| Missing | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Observer-rated airway block | ||||
| ≤50% | 53 (28) | 52 (27) | 105 (28) | 2 |
| > 50% | 135 (72) | 138 (73) | 273 (72) | 13 |
| Evidence of adhesions? | ||||
| Yes | 2 (1) | 2 (1) | 4 (1) | 0 |
| No | 186 (99) | 188 (99) | 374 (99) | 15 |
| Side of adhesions? | ||||
| Left | 0 (0) | 0 (0) | 0 (0) | |
| Right | 0 (0) | 2 (100) | 2 (50) | |
| Both | 1 (50) | 0 (0) | 1 (25) | |
| Not applicable | 1 (50) | 0 (0) | 1 (25) | |
| Evidence of perforation? | ||||
| Yes | 1 (< 1) | 0 (0) | 1 (< 1) | 0 |
| No | 187 (> 99) | 190 (100) | 377 (> 99) | 15 |
| Timing of decongestant measurement times | Septoplasty arm (n = 188) | Medical management arm (n = 190) | Overall (n = 378) |
|---|---|---|---|
| Start time: baseline | |||
| n (%) | 188 | 190 | 378 |
| Time | |||
| Median (IQR) | 10 (7–12) | 10 (7–14) | 10 (7–13) |
| Mean (SD) | 10.3 (4.2) | 11.4 (5.6) | 10.9 (5.0) |
| Range (minimum, maximum) | (5, 27) | (5, 33) | (5, 33) |
| End time: baseline | |||
| n (%) | 188 | 189 | 377 |
| Time | |||
| Median (IQR) | 15 (12.5–18) | 15 (12–20) | 15 (12–20) |
| Mean (SD) | 16.1 (5.4) | 17.2 (7.2) | 16.6 (6.4) |
| Range (minimum, maximum) | (8, 35) | (7, 50) | (7, 50) |
| Start time: 6 months | |||
| n (%) | 128 | 123 | 251 |
| Time | |||
| Median (IQR) | 10 (7–13) | 10 (6–14) | 10 (7–13) |
| Mean (SD) | 10.9 (5.6) | 10.6 (5.4) | 10.7 (5.5) |
| Range (minimum, maximum) | (5, 38) | (5, 35) | (5, 38) |
| End time: 6 months | |||
| n (%) | 127 | 124 | 251 |
| Time | |||
| Median (IQR) | 15 (12–19) | 14 (11–19) | 14 (11–19) |
| Mean (SD) | 15.5 (5.9) | 15.5 (6.1) | 15.5 (6.0) |
| Range (minimum, maximum) | (7, 43) | (3, 36) | (3, 43) |
| Start time: 12 months | |||
| n (%) | 71 | 73 | 144 |
| Time | |||
| Median (IQR) | 10 (7–16) | 10 (7–15) | 10 (7–16) |
| Mean (SD) | 12.0 (6.2) | 12.2 (6.8) | 12.1 (6.5) |
| Range (minimum, maximum) | (5, 33) | (5, 46) | (5, 46) |
| End time: 12 months | |||
| n (%) | 70 | 73 | 143 |
| Time | |||
| Median (IQR) | 14 (12–20) | 15 (11–21) | 15 (11–21) |
| Mean (SD) | 16.9 (8.0) | 16.8 (7.8) | 16.8 (7.8) |
| Range (minimum, maximum) | (8, 54) | (8, 57) | (8, 57) |
| CRF | Septoplasty arm (N = 188), n (%) | Medical management arm (N = 190), n (%) | |||||
|---|---|---|---|---|---|---|---|
| Missing | Partial | Complete | Missing | Partial | Complete | ||
| Baseline visit 1* | Baseline demographics | 0 (0) | 2 (1) | 186 (99) | 0 (0) | 0 (0) | 190 (100) |
| Clinical examination nasal endoscopy | |||||||
| 1. The side of the convexity | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| 2. The side of deflection | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| 3. Endoscopy findings | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| 4. Extent of the airway block by the septum | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| SNOT-22 | 0 (0) | 2 (1) | 186 (99) | 0 (0) | 2 (1) | 188 (99) | |
| NOSE | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| DOASS (post decongestion) | 1 (< 1) | N/A | 187 (99) | 2 (1) | N/A | 188 (99) | |
| Measurements of nasal patencya | |||||||
| 1. PNIF (forced sniff) (maximum of three repeats) | 0 (0) | 0 (0) | 188 (100) | 0 (0) | 0 (0) | 190 (100) | |
| 2. NPR (MIV) (mean of three repeats) | 0 (0) | 0 (0) | 188 (100) | 1 (< 1) | 2 (1) | 187 (98) | |
| 3. NPR (tidal breathing) | 1 (< 1) | 0 (0) | 187 (> 99) | 1 (< 1) | 2 (1) | 187 (98) | |
| Surgery | Post-surgery CRF [for those receiving surgery as randomised (n = 166)] | 42 (25) | 0 (0) | 124 (75) | N/A | N/A | N/A |
| Safety follow-up telephone call 2 weeks post surgery (n = 166) | 42 (25) | N/A | 124 (75) | N/A | N/A | N/A | |
| Medical management | Safety follow-up telephone call 2 weeks post randomisation in the medical management arm (n = 190) | N/A | N/A | N/A | 39 (21) | N/A | 151 (79) |
| 6-month follow-up visit | SNOT-22 (MACRO or Castor) | 36 (19) | 2 (1) | 150 (80) | 35 (18) | 3 (2) | 152 (80) |
| NOSE | 43 (23) | 1 (< 1) | 144 (77) | 45 (24) | 0 (0) | 145 (76) | |
| DOASS (post decongestion) | 59 (31) | N/A | 129 (69) | 64 (34) | N/A | 126 (66) | |
| Clinical examination nasal endoscopy* | |||||||
| 1. PNIF (forced sniff) (maximum of three repeats) | 61 (32) | 0 (0) | 127 (68) | 66 (35) | 0 (0) | 123 (65) | |
| 2. NPR (MIV) (mean of three repeats) | 61 (32) | 2 (1) | 125 (66) | 66 (35) | 0 (0) | 124 (65) | |
| 3. NPR (tidal breathing) | 61 (32) | 1 (< 1) | 126 (67) | 66 (35) | 2 (1) | 122 (64) | |
| 12-month follow-up visit | SNOT-22 (MACRO or Castor) | 63 (34) | 1 (< 1) | 124 (66) | 62 (33) | 3 (2) | 125 (66) |
| NOSE | 78 (41) | 0 (0) | 110 (59) | 69 (36) | 0 (0) | 121 (64) | |
| DOASS (post decongestion) | 115 (61) | N/A | 73 (39) | 109 (57) | N/A | 81 (43) | |
| Clinical examination nasal endoscopy* | |||||||
| 1. PNIF (forced sniff) (maximum of three repeats) | 118 (63) | 0 (0) | 70 (37) | 116 (61) | 1 (< 1) | 73 (38) | |
| 2. NPR (MIV) (mean of three repeats) | 117 (62) | 1 (< 1) | 70 (37) | 116 (61) | 1 (< 1) | 73 (38) | |
| 3. NPR (tidal breathing) | 117 (62) | 1 (< 1) | 70 (37) | 116 (61) | 2 (1) | 72 (38) | |
| Trial arm | 6 months (primary end point) | 12 months | ||||
|---|---|---|---|---|---|---|
| Number completed | Complied,a n (%) | Not complied,b n (%) | Number completed | Complied,c n (%) | Not complied,d n (%) | |
| Septoplasty | 152 | 126 (83) | 26 (17) | 125 | 81 (65) | 44 (35) |
| Medical management | 155 | 128 (83) | 27 (17) | 126 | 84 (67) | 42 (33) |
| Steps of septoplasty | Septoplasty (N = 166), n (%) | Medical management (N = 51), n |
|---|---|---|
| Closed approach | 155 (93) | 13 |
| Unilateral hemitransfixion incision | 134 (81) | 13 |
| Unilateral mucoperichondrial flap | 63 (38) | 6 |
| Bilateral mucoperichondrial flap | 103 (62) | 8 |
| Cartilage resection | 146 (88) | 12 |
| Cartilage scoring | 10 (6) | 3 |
| Septal cartilage grafting | 0 (0) | 0 |
| Maxillary crest medialised | 90 (54) | 4 |
| Mattress sutures to close | 133 (80) | 12 |
| Sutures to hemitransfixion incision | 141 (85) | 13 |
| Nasal splints | 36 (22) | 2 |
| Nasal packing | 45 (27) | 3 |
| Unilateral turbinate surgery | 92 (55) | 7 |
| Turbinate reduced | 91 (55) | 8 |
| Turbinate resected | 11 (7) | 0 |
| Septoplasty step | Frequency (% of 166) |
|---|---|
| Was septoplasty ± turbinate reduction carried out? | |
| No | 4 (2) |
| Yes | 156 (94) |
| Missing | 6 (4) |
| Did the patient stay overnight in hospital? | |
| No | 146 (88) |
| Yes | 14 (8) |
| Missing | 6 (4) |
| If yes (stayed overnight), how many nights? | All 14 stayed 1 night |
| Grade of most senior operative | |
| Surgeon | 128 (77) |
| Consultant | 17 (10) |
| Associate specialist | 16 (10) |
| Other | 5 (3) |
| Grade of most senior anaesthetist | |
| Consultant | 144 (87) |
| Associate specialist | 7 (4) |
| Staff grade | 3 (2) |
| Other | 7 (4) |
| Missing | 5 (3) |
| Closed approach | |
| No | 2 (1) |
| Yes | 155 (93) |
| Missing | 9 (5) |
| Unilateral hemitransfixion incision | |
| No | 25 (15) |
| Yes | 134 (81) |
| Missing | 7 (4) |
| Unilateral mucoperichondrial flap | |
| No | 95 (57) |
| Yes | 63 (38) |
| Missing | 8 (5) |
| Bilateral mucoperichondrial flap | |
| No | 55 (33) |
| Yes | 103 (62) |
| Missing | 8 (5) |
| Cartilage resection | |
| No | 12 (7) |
| Yes | 146 (88) |
| Missing | 8 (5) |
| Cartilage scoring | |
| No | 146 (88) |
| Yes | 10 (6) |
| Missing | 10 (6) |
| Maxillary crest medialised | |
| No | 64 (39) |
| Yes | 90 (54) |
| Missing | 12 (7) |
| Mattress sutures to close | |
| No | 24 (14) |
| Yes | 133 (80) |
| Missing | 9 (5) |
| Sutures to hemitransfixion incision | |
| No | 15 (9) |
| Yes | 141 (85) |
| Missing | 10 (6) |
| Nasal splints | |
| No | 123 (74) |
| Yes | 36 (22) |
| Missing | 7 (4) |
| Nasal packing | |
| No | 114 (69) |
| Yes | 45 (27) |
| Missing | 7 (4) |
| Unilateral inferior turbinate surgery | |
| No | 63 (38) |
| Yes | 92 (55) |
| Missing | 11 (7) |
| Unilateral? | |
| No | 53 (32) |
| Yes | 78 (47) |
| Missing | 35 (21) |
| Inferior turbinate reduced | |
| No | 63 (38) |
| Yes | 91 (55) |
| Missing | 12 (7) |
| Inferior turbinate resected | |
| No | 143 (86) |
| Yes | 11 (7) |
| Missing | 12 (7) |
| Septoplasty participants (N = 166) | |
|---|---|
| n (% of all surgeries) | 159 (96) |
| Duration (minutes) | |
| Median (IQR) | 56 (44–65) |
| Mean (SD) | 56.2 (17.9) |
| Range (minimum, maximum) | (17, 105) |
| Missing, n (%) | 7 (4) |
| Question and answers | 6 months only |
|---|---|
| Over the previous month, have you used the NAIROS medication (nasal steroid and/or saline sprays)?, n (%) | 122 (64) |
|
17 |
|
1 |
|
3 |
|
14 |
|
87 |
| On the days you used the sprays, has it usually been | |
|
65 |
|
48 |
| Question and answer | Saline spray (N = 69) | Steroid spray (N = 65) |
|---|---|---|
| Saline/steroid bottles used: median (IQR) for n who returned this information | 3.5 (2.5–5) | 4 (3–5.5) |
| Are you still using the spray?, n (%) | 132 (69) | 132 (69) |
| Responded ‘yes’, n | 90 | 96 |
| Responded ‘no’, n | 42 | 36 |
| If no, about how long ago did you stop using it?, n | 42 | 36 |
| Within the previous month, n | 25 | 23 |
| 2–3 months ago, n | 13 | 8 |
| > 3 months ago, n | 4 | 4 |
| Did not use it at all, n | 0 | 1 |
| Why did you stop using it? | ||
| Do not think it helped | 18 | 19 |
| Think it gave me side effectsa | 6 | 5 |
| Other reasona | 19 | 14 |
| Reasons for stopping using sprays (reason or side effect) | Saline spray, n (%) | Steroid spray, n (%) |
|---|---|---|
| End of 6 months/ran out/finished supplies (other reason) | 15 (60) | 9 (53) |
| Nasal pain and/or bleeding (side effect) | 3 (12) | 3 (18) |
| Not helpful (other reason) | 2 (8) | 0 (0) |
| Symptoms eased | 0 (0) | 1 (6) |
| Reflux/gag (side effect) | 1 (4) | 0 (0) |
| Rhinorrhoea (side effect) | 1 (4) | 1 (6) |
| Laziness (other reason) | 1 (4) | 0 (0) |
| In prison (other reason) | 1 (4) | 1 (6) |
| SAE after accident; had surgery | 0 (0) | 1 (6) |
| Occupational reasons | 0 (0) | 1 (6) |
| No reason given (side effect) | 1 (4) | 0 (0) |
| Total | 25 (100) | 17a (100) |
FIGURE 23.
Baseline SNOT-22 scores, by randomised arm.

| SNOT-22 scores at 12 months, ITT population | Trial arm | Overall (ITT, N = 307) | |
|---|---|---|---|
| Septoplasty (ITT, N = 152) | Medical management (ITT, N = 155) | ||
| ITT population, n (% of ITT n) | 119 (65) | 125 (81) | 244 (79) |
| Median (IQR) | 15 (8–30) | 29 (12–45) | 20.5 (10–39) |
| Mean (SD) | 21.2 (19.0) | 30.4 (21.6) | 25.9 (20.9) |
| 95% CI about mean | 17.7 to 24.6 | 26.6 to 34.3 | 23.3 to 28.5 |
| Minimum, maximum | 0, 91 | 0, 78 | 0, 91 |
| Medical management non-trial surgery | Frequency (all surgeries) | ITT (with baseline and 6-month SNOT-22 score) |
|---|---|---|
| Surgery before primary end point (6-month SNOT-22 score) | 9 | 5 |
| Surgery between 6 and 12 months | 37 | 37 |
| Surgery beyond 12 months (12-month SNOT-22 score) | 4 | 4 |
| Surgery date unknown | 1 | 0 |
| Total | 51 | 46 |
FIGURE 24.
Residual analysis. (a) Residuals vs. fitted values; (b) histogram of residuals; (c) normal probability plot of the residuals; and (d) standardised residuals vs. fitted values. (continued)
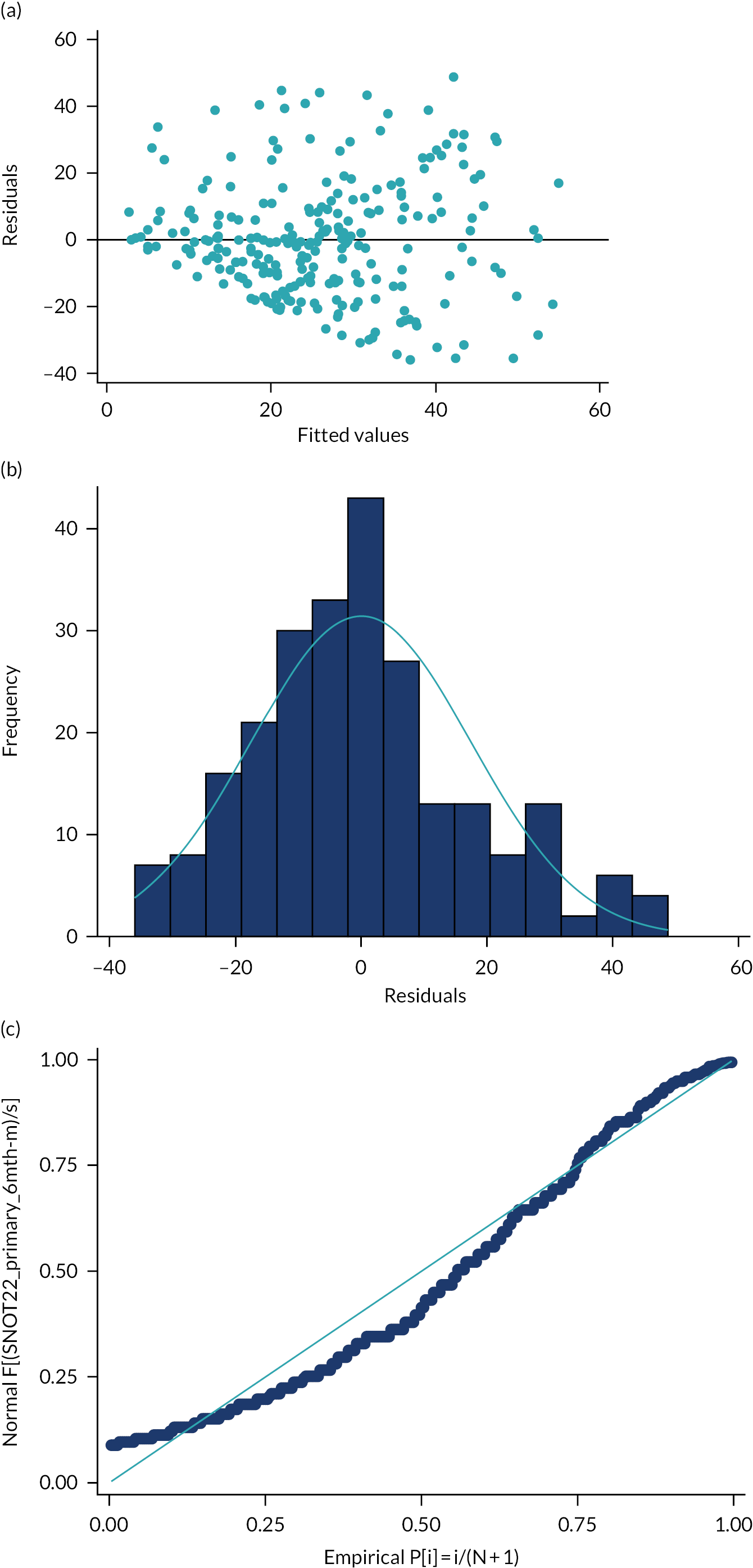

| Covariate | Number | Coefficient | SE | Test statistic | p-value |
|---|---|---|---|---|---|
| Age (continuous) | 307 | 0.085 | 0.087 | 0.98 | 0.328 |
| Age log transform | 307 | 2.674 | 3.356 | 0.80 | 0.426 |
| Age complex transform (age3) | 307 | 0.000 | 0.000 | 1.27 | 0.204 |
| Ethnicity (reference: white) | 272 | ||||
| Asian | 20 | 0.739 | 5.154 | 0.14 | 0.886 |
| Other Asian | 3 | −5.477 | 12.915 | −0.42 | 0.672 |
| Other ethnic origin | 10 | 1.695 | 7.163 | 0.24 | 0.813 |
| Missing | 2 | N/A | N/A | N/A | N/A |
| Site (reference: site 1 – Newcastle) | 62 | ||||
| 2. Great Yarmouth | 19 | 0.093 | |||
| 3. Bradford | 12 | 0.164 | |||
| 4. Wigan | 12 | 0.595 | |||
| 5. Darlington | 7 | 0.459 | |||
| 6. Plymouth | 12 | 0.948 | |||
| 7. Dundee | 17 | 0.018 | |||
| 8. Aberdeen | 34 | 0.294 | |||
| 9. Stockport | 13 | 0.272 | |||
| 10. London | 12 | 0.805 | |||
| 11. Newport | 11 | 0.070 | |||
| 12. Birmingham | 12 | 0.577 | |||
| 13. Carlisle | 10 | 0.494 | |||
| 14. Aintree | 17 | 0.072 | |||
| 15. Leeds | 25 | 0.573 | |||
| 16. Lanarkshire | 17 | 0.585 | |||
| 17. Salisbury | 15 | 0.452 | |||
| Smoking history (reference: smoker) | 46 | ||||
| Ex-smoker | 88 | −4.728 | 3.953 | −1.20 | 0.233 |
| Never smoked | 173 | −11.434 | 3.605 | −3.17 | 0.002 |
| Block (reference: bilateral) | 128 | ||||
| Unilateral | 179 | −1.506 | 2.561 | −0.59 | 0.557 |
| Nasal trauma (reference: yes) | 140 | ||||
| No | 167 | −2.316 | 2.533 | −0.91 | 0.361 |
| Reduce turbinate (reference: yes) | 230 | ||||
| No | 66 | −5.410 | 3.081 | −1.76 | 0.080 |
| Not applicable | 11 | ||||
| Airway block observer-rated scale (reference: ≤50%) | 85 | ||||
| > 50% | 222 | −1.855 | 2.822 | −0.66 | 0.511 |
| Baseline absolute subjective DOASS | 304 | 2.022 | 2.901 | 0.70 | 0.486 |
| Complex transform (baseline absolute subjective DOASS−0.5) | 304 | 0.788 | 1.449 | 0.54 | 0.587 |
| Log baseline absolute subjective DOASS | 286 | −0.238 | 1.803 | −0.13 | 0.895 |
| Baseline worst DOASS | 304 | −1.257 | 0.617 | −2.04 | 0.043 |
| Complex transform (baseline worst DOASS−0.5) | 304 | 15.108 | 6.296 | 2.40 | 0.017 |
| Log baseline worst DOASS | 304 | −4.689 | 2.003 | −2.34 | 0.020 |
| Baseline PNIF (post decongestant) | 307 | −0.048 | 0.025 | −1.94 | 0.054 |
| Log baseline PNIF (post decongestant) | 305 | −0.312 | 1.694 | −0.18 | 0.854 |
| Complex transform (best is linear−1) | N/A | N/A | N/A | N/A | N/A |
| Baseline absolute NPR (post decongestant) | 307 | 3.465 | 4.114 | 0.84 | 0.400 |
| Log baseline absolute NPR (post decongestant) | 305 | −0.122 | 1.079 | −0.01 | 0.991 |
| Complex transform (−1) | 307 | 0.034 | 0.027 | 1.22 | 0.224 |
| Baseline absolute tidal breathing ratio (post decongestant) | 306 | 0.552 | 4.082 | 0.14 | 0.893 |
| Log baseline absolute tidal breathing (post decongestant) | 305 | 0.485 | 1.164 | 0.42 | 0.677 |
| Complex transform (0) | 306 | 0.686 | 1.170 | 0.59 | 0.558 |
| Medical physics-derived endoscopy variables | |||||
| Absolute NPR | 273 | −0.889 | 4.182 | −0.21 | 0.832 |
| Log absolute NPR | 273 | 0.204 | 1.220 | 0.17 | 0.867 |
| Complex transform (3) | 273 | −4.391 | 4.316 | −1.02 | 0.310 |
| Absolute decongestant NPR | 270 | −4.021 | 5.684 | −0.71 | 0.480 |
| Log absolute decongestant NPR | 165 | −0.535 | 1.143 | −0.47 | 0.640 |
| Complex transform (−2) | 270 | 8.34 × 10−6 | 7.60 × 10−6 | 1.10 | 0.274 |
| Total maximum flow rate | 273 | −0.005 | 0.005 | −1.06 | 0.292 |
| Log total maximum flow rate | 273 | −3.407 | 2.699 | −1.26 | 0.208 |
| Complex transform (−2) | 273 | 280129.6 | 118248.3 | 2.37 | 0.019 |
| Median tidal volume | 264 | −0.003 | 0.003 | −1.17 | 0.245 |
| Log median tidal volume | 264 | −5.123 | 1.979 | −2.58 | 0.011 |
| Complex transform (−1) | 264 | 1995.859 | 627.311 | 3.18 | 0.002 |
| Primary outcome measure: SNOT-22 score at 6 months | Coefficient | SE of coefficient | Test statistic | p-value | 95% CI of coefficient |
|---|---|---|---|---|---|
| Arm: (reference category: medical management) | |||||
| Septoplasty | −20.426 | 1.918 | −10.65 | < 0.001 | −24.203 to −16.650 |
| Baseline SNOT-22 score | 0.454 | 0.063 | 7.16 | < 0.001 | 0.329 to 0.578 |
| Gender (ref category: female) | |||||
| Male | 1.158 | 2.075 | 0.56 | 0.577 | −2.929 to 5.245 |
| NOSE severity (continuous) | 0.134 | 0.075 | 1.78 | 0.076 | −0.014 to 0.283 |
| Reciprocal of median tidal volume | 1446.909 | 475.713 | 3.04 | 0.003 | 510.134 to 2383.685 |
| Constant | 6.198 | 4.630 | 1.34 | 0.182 | −2.919 to 15.315 |
FIGURE 25.
The DOASS raw data (ITT population). Graphs by arm. ITT population: medical management, n = 155; septoplasty, n = 152.

| DOASS measurements, ITT population | Worst side | Better side | ||||
|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| Baseline | ||||||
| ITT population, n (% of N) | 151 (80) | 153 (81) | 304 (80) | 151 (80) | 153 (81) | 304 (80) |
| Median (IQR) | 4 (2–5) | 3 (2–5) | 4 (2–5) | 8 (7–9) | 8 (7–9) | 8 (7–9) |
| Mean (SD) | 3.9 (2.0) | 3.7 (2.1) | 3.8 (2.0) | 7.7 (1.8) | 7.7 (1.9) | 7.7 (1.8) |
| 95% CI about mean | 3.6 to 4.2 | 3.3 to 4.0 | 3.6 to 4.0 | 7.4 to 8.0 | 7.4 to 8.0 | 7.5 to 7.9 |
| Minimum, maximum | 1, 10 | 1, 9 | 1, 10 | 3, 10 | 1, 10 | 1, 10 |
| 6 months (primary end point) | ||||||
| ITT population, n (% of N) | 129 (69) | 125 (66) | 254 (67) | 129 (69) | 125 (66) | 254 (67) |
| Median (IQR) | 8 (6–9) | 4 (2–6) | 6 (4–8) | 8 (7–9) | 8 (6–9) | 8 (7–9) |
| Mean (SD) | 7.2 (2.4) | 4.6 (2.4) | 5.9 (2.7) | 8.1 (1.8) | 7.4 (2.2) | 7.8 (2.0) |
| 95% CI about mean | 7.8 to 8.4 | 7.1 to 7.8 | 5.6 to 6.2 | 7.8 to 8.4 | 7.1 to 7.8 | 7.5 to 8.0 |
| Minimum, maximum | 1, 10 | 1, 10 | 1, 10 | 3, 10 | 1, 10 | 1, 10 |
| 12 months | ||||||
| ITT population, n (% of N) | 69 (37) | 80 (42) | 149 (39) | 69 (37) | 80 (42) | 149 (39) |
| Median (IQR) | 8 (6–9) | 6 (4–8) | 7 (5–9) | 9 (7–10) | 8 (7–10) | 9 (7–10) |
| Mean (SD) | 7.3 (2.3) | 6.0 (2.8) | 6.6 (2.6) | 8.1 (2.2) | 8.0 (2.0) | 8.0 (2.1) |
| 95% CI about mean | 6.8 to 7.9 | 5.4 to 6.7 | 6.2 to 7.1 | 7.6 to 8.6 | 7.5 to 8.4 | 7.7 to 8.4 |
| Minimum, maximum | 2, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 |
| Absolute subjective DOASS, ITT population | Baseline | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| ITT population, n (% of N) | 151 (80) | 153 (81) | 304 (80) | 129 (69) | 125 (66) | 254 (67) | 69 (37) | 80 (42) | 149 (39) |
| Median (IQR) | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.1 (0.1–0.2) | 0.3 (0.2–0.5) | 0.2 (0.1–0.4) | 0.1 (0.1–0.2) | 0.2 (0.1–0.4) | 0.1 (0.1–0.3) |
| Mean (SD) | 0.4 (0.2) | 0.4 (0.2) | 0.4 (0.2) | 0.2 (0.2) | 0.4 (0.2) | 0.3 (0.2) | 0.2 (0.2) | 0.3 (0.2) | 0.2 (0.2) |
| 95% CI about mean | 0.3 to 0.4 | 0.3 to 0.4 | 0.3 to 0.4 | 0.1 to 0.2 | 0.3 to 0.4 | 0.2 to 0.3 | 0.1 to 0.2 | 0.2 to 0.3 | 0.2 to 0.3 |
| Minimum, maximum | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 | 0, 0.8 |
| SNOT-22 subscale | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Nasal (n) | 152 | 152 | 119 | 155 | 155 | 125 | 307 | 307 | 244 |
| Median (IQR) | 16 (11.5–22) | 6 (2–12) | 6 (3–13) | 15 (11–22) | 14 (9–19) | 10 (5–16) | 16 (11–22) | 10 (5–17) | 8 (4–15) |
| Mean (SD) | 17.0 (7.4) | 7.8 (6.7) | 8.2 (7.3) | 16.3 (7.4) | 14.5 (7.2) | 11.3 (8.0) | 16.6 (7.4) | 11.2 (7.7) | 9.8 (7.8) |
| 95% CI | 15.8 to 18.2 | 6.7 to 8.9 | 6.9 to 9.5 | 15.1 to 17.5 | 13.4 to 15.6 | 9.9 to 12.7 | 15.8 to 17.5 | 10.3 to 12.0 | 98.8 to 10.8 |
| Minimum, maximum | 3, 38 | 0, 38 | 0, 39 | 0, 33 | 0, 32 | 0, 31 | 0, 38 | 0, 38 | 0, 39 |
| Sleep (n) | 152 | 152 | 119 | 155 | 155 | 125 | 307 | 307 | 244 |
| Median (IQR) | 20 (10–29) | 6 (0–13) | 6 (1–16) | 21 (12–29) | 19 (8–29) | 14 (4–22) | 20 (11–29) | 11 (3–23) | 9 (2–20) |
| Mean (SD) | 19.8 (11.1) | 8.8 (9.8) | 9.5 (10.0) | 20.5 (11.0) | 18.8 (11.7) | 14.0 (11.0) | 20.1 (11.0) | 13.8 (11.9) | 11.8 (10.7) |
| 95% CI | 18.0 to 21.6 | 7.2 to 10.4 | 7.7 to 11.3 | 18.7 to 22.2 | 16.9 to 20.6 | 12.0 to 16.0 | 18.9 to 21.4 | 12.5 to 15.2 | 10.5 to 13.2 |
| Minimum, maximum | 0, 40 | 0, 37 | 0, 35 | 0, 40 | 0, 40 | 0, 40 | 0, 40 | 0, 40 | 0, 40 |
| Otological (n) | 152 | 152 | 119 | 155 | 155 | 125 | 307 | 307 | 244 |
| Median (IQR) | 4 (2–7) | 1 (0–3) | 1 (0–2) | 3 (1–7) | 2 (0–6) | 2 (0–5) | 4 (1–7) | 2 (0–5) | 1 (0–4) |
| Mean (SD) | 5.1 (4.5) | 2.3 (3.2) | 2.3 (3.7) | 4.5 (4.3) | 3.8 (4.1) | 3.3 (3.9) | 4.8 (4.4) | 3.1 (3.7) | 2.8 (3.8) |
| 95% CI | 4.3 to 5.8 | 1.8 to 2.8 | 1.6 to 2.9 | 3.8 to 5.2 | 3.2 to 4.5 | 2.6 to 4.0 | 4.3 to 5.3 | 2.6 to 3.5 | 2.3 to 3.3 |
| Minimum, maximum | 0, 20 | 0, 16 | 0, 17 | 0, 18 | 0, 19 | 0, 19 | 0, 20 | 0, 19 | 0, 19 |
| Emotional (n) | 152 | 152 | 119 | 155 | 155 | 125 | 307 | 307 | 244 |
| Median (IQR) | 1.5 (0–5) | 0 (0–1) | 0 (0–1) | 2 (0–5) | 2 (0–4) | 1 (0–4) | 2 (0–5) | 0 (0–3) | 0 (0–3) |
| Mean (SD) | 2.7 (2.9) | 1.0 (1.9) | 1.2 (2.2) | 2.8 (3.0) | 2.4 (2.7) | 1.8 (2.3) | 2.7 (2.9) | 1.7 (2.4) | 1.5 (2.3) |
| 95% CI | 2.2 to 3.1 | 0.7 to 1.3 | 0.8 to 1.6 | 2.3 to 3.3 | 2.0 to 2.8 | 1.4 to 2.2 | 2.4 to 3.1 | 1.4 to 2.0 | 1.2 to 1.8 |
| Minimum, maximum | 0, 10 | 0, 9 | 0, 9 | 0, 10 | 0, 10 | 0, 9 | 0, 10 | 0, 10 | 0, 9 |
FIGURE 26.
Distribution of baseline continuous NOSE scores, by allocated arm.
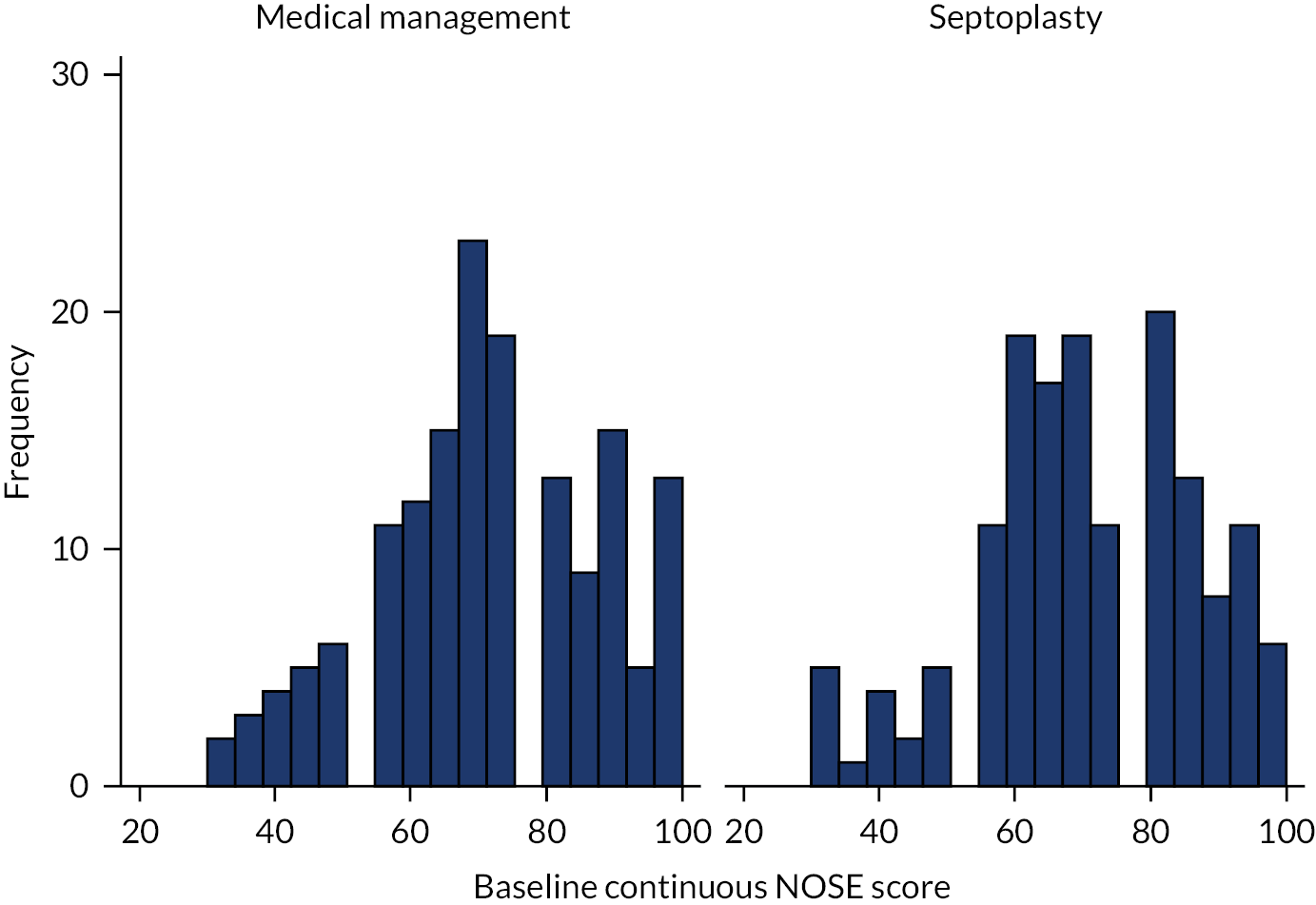
| PNIF post-decongestant measurements, ITT population | Baseline | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| ITT population, n (% of N) | 152 (81) | 155 (82) | 307 (81) | 127 (68) | 123 (65) | 250 (66) | 66 (35) | 72 (38) | 138 (37) |
| Median (IQR) | 100 (60–130) | 95 (69–138) | 100 (65–130) | 120 (80–160) | 100 (70–140) | 110 (75–150) | 112.5 (80–150) | 110 (70–160) | 110 (80–160) |
| Mean (SD) | 102.0 (51.2) | 102.0 (49.4) | 102.0 (50.2) | 125.1 (61.7) | 107.6 (48.4) | 116.5 (56.2) | 121.2 (62.1) | 116.0 (50.7) | 118.5 (56.3) |
| 95% CI about mean | 93.8 to 110.2 | 94.1 to 109.8 | 96.4 to 107.6 | 114.3 to 136.0 | 99.0 to 116.3 | 109.5 to 123.5 | 106.0 to 136.5 | 104.1 to 127.9 | 109.0 to 128.0 |
| Minimum, maximum | 0, 265 | 1, 270 | 0, 270 | 0, 320 | 1, 270 | 0, 320 | 1, 290 | 30, 230 | 1, 290 |
| Time point | Worse side | Better side | ||||
|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| Baseline | ||||||
| ITT population, n (% of N) | 152 (81) | 155 (82) | 307 (81) | 152 (81) | 155 (82) | 307 (81) |
| Median (IQR) | 0.5 (0.2–0.9) | 0.4 (0.2–0.9) | 0.5 (0.2–0.9) | 1.4 (0.9–2.0) | 1.4 (0.9–1.9) | 1.4 (0.9–2.0) |
| Mean (SD) | 0.6 (0.6) | 0.6 (0.6) | 0.6 (0.6) | 1.5 (1.0) | 1.5 (0.8) | 1.5 (0.9) |
| 95% CI about mean | 0.5 to 0.7 | 0.5 to 0.7 | 0.5 to 0.7 | 1.4 to 1.7 | 1.3 to 1.6 | 1.4 to 1.6 |
| Minimum, maximum | 0, 5 | 0, 5 | 0, 5 | 0, 5 | 0, 5 | 0, 5 |
| 6 months (primary end point) | ||||||
| ITT population, n (% of N) | 126 (67) | 123 (65) | 249 (66) | 126 (67) | 123 (65) | 249 (66) |
| Median (IQR) | 1.0 (0.6–1.3) | 0.7 (0.3–1.1) | 0.8 (0.5–1.2) | 1.4 (0.8–1.8) | 1.4 (0.9–1.9) | 1.4 (0.9–1.8) |
| Mean (SD) | 1.0 (0.6) | 0.7 (0.5) | 0.9 (0.6) | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.7) |
| 95% CI about mean | 0.9 to 1.1 | 0.6 to 0.8 | 0.8 to 0.9 | 1.3 to 1.6 | 1.3 to 1.6 | 1.3 to 1.5 |
| Minimum, maximum | 0, 2.7 | 0, 2.9 | 0, 2.9 | 0, 4.5 | 0, 3.5 | 0, 4.5 |
| 12 months | ||||||
| ITT population, n (% of N) | 66 (35) | 72 (38) | 138 (37) | 66 (35) | 72 (38) | 138 (37) |
| Median (IQR) | 1.1 (0.6–1.5) | 0.9 (0.5–1.4) | 1.0 (0.5–1.4) | 1.3 (0.9–1.8) | 1.4 (1.0–2.0) | 1.3 (0.9–1.9) |
| Mean (SD) | 1.1 (0.6) | 0.9 (0.6) | 1.0 (0.6) | 1.3 (0.7) | 1.5 (0.8) | 1.4 (0.7) |
| 95% CI about mean | 1.0 to 1.3 | 0.8 to 1.1 | 0.9 to 1.1 | 1.2 to 1.5 | 1.3 to 1.7 | 1.3 to 1.6 |
| Minimum, maximum | 0.2, 3.0 | 0, 3.2 | 0, 3.2 | 0, 3.7 | 0.2, 3.8 | 0, 3.8 |
FIGURE 27.
Worse-side (lower) and better-side (higher) inhaled volumes from post-decongestant MIV rhinospirometry (using mean volumes from three measurements), ITT population.
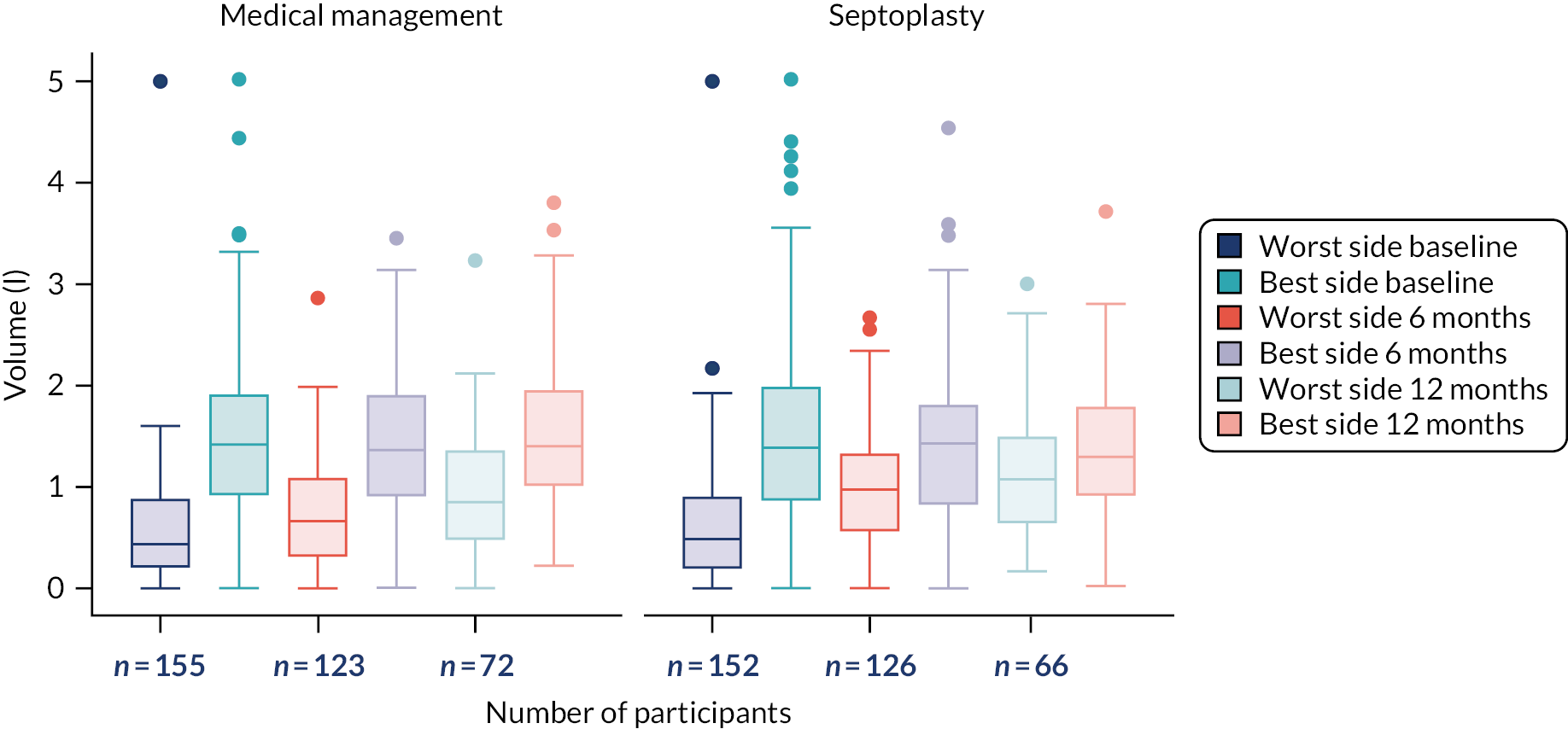
| Absolute NPR post-decongestant measurements, ITT population | Baseline | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| ITT population, n (% of N) | 152 (81) | 155 (82) | 307 (81) | 126 (67) | 123 (65) | 249 (66) | 66 (35) | 72 (38) | 138 (37) |
| Median (IQR) | 0.3 (0.2–0.7) | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.2 (0.1–0.4) | 0.4 (0.1–0.6) | 0.3 (0.1–0.5) | 0.1 (0.1–0.3) | 0.3 (0.1–0.5) | 0.2 (0.1–0.4) |
| Mean (SD) | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.3) | 0.3 (0.2) | 0.4 (0.3) | 0.3 (0.3) | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) |
| 95% CI about mean | 0.4 to 0.5 | 0.4 to 0.5 | 0.4 to 0.5 | 0.2 to 0.3 | 0.4 to 0.5 | 0.3 to 0.4 | 0.2 to 0.3 | 0.3 to 0.4 | 0.2 to 0.3 |
| Minimum, maximum | 0, 1 | 0, 1 | 0, 1 | 0, 1 | 0, 1 | 0, 1 | 0, 0.9 | 0, 1 | 0, 1 |
| MIV (L) | Worst side | Better side | ||||
|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| Baseline | ||||||
| ITT population, n (% of N) | 151 (80) | 155 (82) | 306 (81) | 151 (80) | 155 (82) | 306 (81) |
| Median (IQR) | 1.5 (0.6–2.5) | 1.5 (0.7–2.4) | 1.5 (0.6–2.5) | 3.2 (3.0–3.4) | 3.2 (3.0–3.4) | 3.2 (3.0–3.4) |
| Mean (SD) | 1.5 (1.0) | 1.5 (1.0) | 1.5 (1.0) | 3.0 (0.8) | 3.1 (0.7) | 3.1 (0.7) |
| 95% CI about mean | 1.4 to 1.7 | 1.4 to 1.7 | 1.4 to 1.6 | 2.9 to 3.2 | 3.0 to 3.2 | 3.0 to 3.1 |
| Minimum, maximum | 0, 3.6 | 0, 3.3 | 0, 3.6 | 0.1, 5 | 0.4, 4.6 | 0.1, 5 |
| 6 months (primary end point) | ||||||
| ITT population, n (% of N) | 126 (67) | 123 (65) | 249 (66) | 126 (67) | 123 (65) | 249 (66) |
| Median (IQR) | 2.5 (1.5–3.1) | 1.9 (1.0–3.0) | 2.2 (1.2–3.1) | 3.1 (2.6–3.3) | 3.1 (2.8–3.3) | 3.1 (2.7–3.3) |
| Mean (SD) | 2.3 (1.0) | 1.9 (1.1) | 2.1 (1.1) | 2.8 (0.9) | 3.0 (0.7) | 2.9 (0.8) |
| 95% CI about mean | 2.1 to 2.5 | 1.7 to 2.1 | 2.0 to 2.2 | 2.6 to 3.0 | 2.8 to 3.1 | 2.8 to 3.0 |
| Minimum, maximum | 0.1, 4.5 | 0, 4.6 | 0, 4.6 | 0.2, 5.0 | 0.6, 4.6 | 0.2, 5 |
| 12 months | ||||||
| ITT population, n (% of N) | 66 (35) | 72 (38) | 138 (37) | 66 (35) | 72 (38) | 138 (37) |
| Median (IQR) | 2.6 (2.0–3.2) | 2.2 (1.1–3.1) | 2.4 (1.4–3.1) | 3.1 (2.5–3.4) | 3.1 (2.7–3.3) | 3.1 (2.6–3.3) |
| Mean (SD) | 2.4 (1.0) | 2.0 (1.1) | 2.2 (1.1) | 2.8 (0.9) | 2.7 (1.0) | 2.8 (0.9) |
| 95% CI about mean | 2.2 to 2.7 | 1.7 to 2.3 | 2.0 to 2.4 | 2.6 to 3.0 | 2.5 to 3.0 | 2.6 to 2.9 |
| Minimum, maximum | 0, 4.5 | 0, 3.7 | 0, 4.5 | 0.1, 3.8 | 0, 4.2 | 0, 4.2 |
FIGURE 28.
Worse-side (lower) and better-side (higher) inhaled volumes from post-decongestant, tidal breathing rhinospirometry, ITT population. Graphs by arm. ITT population: medical management, n = 155; septoplasty, n = 152.

| Absolute tidal breathing NPR post-decongestant measurements, ITT population | Baseline | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | Septoplasty (N = 188) | Medical management (N = 190) | Overall (N = 378) | |
| ITT population, n (% of N) | 151 (80) | 155 (82) | 306 (81) | 126 (67) | 123 (65) | 249 (66) | 66 (35) | 72 (38) | 138 (37) |
| Median (IQR) | 0.3 (0.1–0.6) | 0.3 (0.1–0.6) | 0.3 (0.1–0.6) | 0.1 (0.1–0.4) | 0.3 (0.1–0.5) | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) | 0.2 (0.1–0.5) | 0.2 (0.1–0.4) |
| Mean (SD) | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.3) | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) |
| 95% CI about mean | 0.3 to 0.4 | 0.3 to 0.4 | 0.36 to 0.43 | 0.2 to 0.3 | 0.3 to 0.4 | 0.25 to 0.32 | 0.2 to 0.3 | 0.2 to 0.4 | 0.23 to 0.31 |
| Minimum, maximum | 0, 1 | 0, 1 | 0, 1 | 0, 0.9 | 0, 1 | 0, 1 | 0, 1 | 0, 1 | 0, 1 |
FIGURE 29.
Subjective DOASS against MIV NPR (post decongestant, using mean volumes from three measurements).

FIGURE 30.
Subjective DOASS against tidal breathing NPR, post decongestant.

| Recommendation | Turbinate, n (%) | Total, n (%) | |
|---|---|---|---|
| Not reduced | Reduced | ||
| Turbinate reduction recommended | 30 (45) | 78 (89) | 108 (70) |
| Turbinate reduction not recommended | 33 (49) | 10 (11) | 43 (28) |
| Not applicable | 4 (6) | 0 (0) | 4 (3) |
| Total | 67 (100) | 88 (100) | 155 (100) |
| Turbinate reduction and baseline assessment | Septoplasty arm (n) |
|---|---|
| Turbinate not reduced in line with baseline assessment | 30 |
| Turbinate not reduced, opposing baseline assessment | 33 |
| Turbinate reduced in line with baseline assessment | 78 |
| Turbinate reduced, opposing baseline assessment | 10 |
| Turbinate not reduced; baseline assessment ‘not applicable’ | 4 |
| Turbinate reduced; baseline assessment ‘not applicable’ | 0 |
| Turbinate reduction information missing | 11 |
| Total | 166 |
FIGURE 31.
Relationship of turbinate reduction with SNOT-22 scores.

FIGURE 32.
The STEPP of the SNOT-22 vs. the DOASS.

| Category | Change from baseline to | |
|---|---|---|
| 6 months | 12 months | |
| Septoplasty, male | ||
| N | 100 | 76 |
| Median (IQR) | −24.5 (−40.5 to −10) | −22.5 (−38.5 to −9) |
| Mean (SD) | −24.2 (20.7) | −23.7 (21.1) |
| 95% CI | −28.3 to −20.1 | −28.5 to −18.9 |
| Minimum, maximum | −67, 28 | −76, 28 |
| Septoplasty, female | ||
| n | 52 | 43 |
| Median (IQR) | −25 (−42 to −10) | −22 (−38 to −5) |
| Mean (SD) | −25.4 (20.0) | −22.0 (21.2) |
| 95% CI | −31.0 to −19.8 | −28.5 to −15.5 |
| Minimum, maximum | −64, 12 | −70, 27 |
| Medical management, male | ||
| n | 99 | 79 |
| Median (IQR) | −3 (−14 to 9) | −10 (−24 to 0) |
| Mean (SD) | −3.3 (17.6) | −11.0 (18.0) |
| 95% CI | −6.8 to 0.2 | −15.0 to −67.0 |
| Minimum, maximum | −55, 38 | −57, 36 |
| Medical management, female | ||
| n | 56 | 46 |
| Median (IQR) | −5.5 (−15.5 to −0.5) | −9 (−27 to −2) |
| Mean (SD) | −6.8 (14.7) | −14.3 (22.5) |
| 95% CI | −10.7 to −2.8 | −21.0 to −7.6 |
| Minimum, maximum | −52, 27 | −63, 40 |
FIGURE 33.
Changes in SNOT-22 scores by gender.

FIGURE 34.
The STEPP of changes in SNOT-22 score by gender (male). N = 199: septoplasty, n = 100; medical management, n = 99.
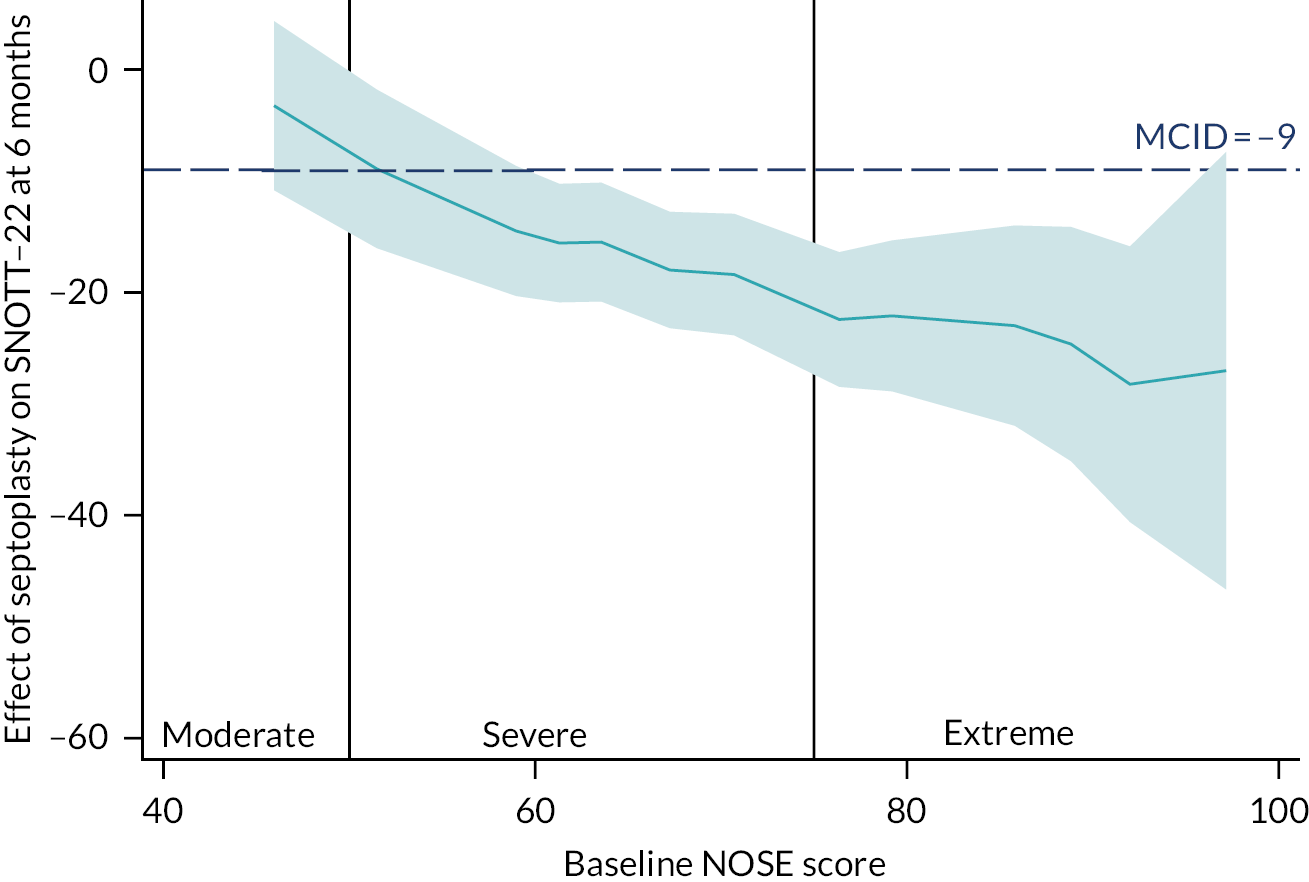
FIGURE 35.
The STEPP of changes in SNOT-22 score by gender (female). N = 108: septoplasty, n = 52; medical management, n = 56.

| AE | Trial arm (n) | Total (N) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| Life-threatening | 0 | 0 | 0 |
| Severe | 5 | 1 | 6 |
| Moderate | 39 | 22 | 61 |
| Mild | 88 | 72 | 160 |
| Total | 132 | 95 | 227 |
| Action taken | Trial arm, n (%) | Total, N (%) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| No action taken | 82 (63) | 57 (59) | 139 (61) |
| Treatment adjusted/interrupted | 1 (< 1) | 14 (14) | 15 (7) |
| Treatment discontinued | 0 (0) | 8 (8) | 8 (4) |
| Concomitant medications | 45 (35) | 18 (19) | 63 (28) |
| Non-drug therapy given | 2 (1) | 0 (0) | 2 (< 1) |
| Hospitalisation | 0 (0) | 0 (0) | 0 (0) |
| Total | 130 (100) | 97 (100) | 227 (100) |
| Action taken | Trial arm, n (%) | Total, N (%) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| Resolved | 95 (72) | 55 (58) | 150 (66) |
| Resolving | 14 (11) | 5 (5) | 19 (8) |
| Ongoing | 23 (17) | 35 (37) | 58 (26) |
| Total | 132 (100) | 95 (100) | 227 (100) |
| Related to treatment | Severity | Trial arm (n) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| Definitely | Mild | 19 | 5 |
| Moderate | 6 | 0 | |
| Severe | 5 | 0 | |
| Life-threatening | 0 | 0 | |
| Probable | Mild | 12 | 21 |
| Moderate | 8 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Possible | Mild | 24 | 9 |
| Moderate | 11 | 8 | |
| Severe | 0 | 1 | |
| Life-threatening | 0 | 0 | |
| Unlikely | Mild | 9 | 6 |
| Moderate | 3 | 3 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Unrelated | Mild | 23 | 31 |
| Moderate | 10 | 11 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Not assessable | Mild | 1 | 0 |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Total | 131a | 95 | |
| Study ID | Randomised date | AE date | Start AE date | Stop AE date | Completion | Time randomised to AE start (weeks) |
|---|---|---|---|---|---|---|
| 20020 | 8 May 2019 | 9 May 2020 | 9 May 2020 | 22 December 2020 | 52.42857 | |
| 80003 | 23 April 2018 | 28 April 2019 | 28 April 2019 | 2 May 2019 | 52.85714 | |
| 60002 | 29 June 2018 | 8 August 2019 | 8 August 2019 | 8 August 2019 | 13 February 2020 | 57.85714 |
| Study ID | Randomised date | AE date | Start AE date | Stop AE date | Completion |
|---|---|---|---|---|---|
| 10008 | 29 March 2018 | 20 May 2019 | |||
| 80003 | 23 April 2018 | 20 November 2020 | |||
| 80007 | 24 May 2018 | 20 November 2020 | |||
| 80032 | 29 May 2019 | 20 November 2020 | |||
| 120004 | 2 November 2018 | 30 April 2019 | |||
| 170015 | 10 May 2019 | 20 November 2019 |
| Trial arm | AE | Related to treatment | Severity | Time from randomisation to AE (weeks) | Action taken | Outcome |
|---|---|---|---|---|---|---|
| Septoplasty | Nasal pain | Definitely | Severe | 1 | Concomitant medications | Resolved |
| Septoplasty | Uncontrollable minor haemorrhage | Definitely | Severe | 5 | Concomitant medications | Resolved |
| Septoplasty | Pain (nasal) | Definitely | Severe | 6 | Concomitant medications | Resolved |
| Septoplasty | Pain | Definitely | Severe | 6 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Severe | 9 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Moderate | 2 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Moderate | 4 | Concomitant medications | Resolved |
| Septoplasty | Blocked nose – bilateral | Definitely | Moderate | 6 | No action taken | Resolving |
| Septoplasty | Nasal pain | Definitely | Moderate | 6 | Concomitant medications | Resolved |
| Septoplasty | Pain | Definitely | Moderate | 7 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Moderate | 9 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Mild | 2 | No action taken | Resolved |
| Septoplasty | Postoperative pain | Definitely | Mild | 3 | No action taken | Resolved |
| Septoplasty | Nasal pain | Definitely | Mild | 4 | Concomitant medications | Resolved |
| Septoplasty | Postoperative facial pain | Definitely | Mild | 5 | No action taken | Resolved |
| Septoplasty | Nasal pain | Definitely | Mild | 5 | Concomitant medications | Resolved |
| Septoplasty | Nasal pain | Definitely | Mild | 5 | Concomitant medications | Resolved |
| Septoplasty | Epistaxis (right nostril) | Definitely | Mild | 6 | No action taken | Resolved |
| Septoplasty | Minor nasal bleeding post operation | Definitely | Mild | 6 | No action taken | Resolved |
| Septoplasty | Numbness of upper teeth | Definitely | Mild | 6 | No action taken | Resolved |
| Septoplasty | Nasal pain | Definitely | Mild | 6 | Concomitant medications | Resolved |
| Septoplasty | Numbness in upper teeth | Definitely | Mild | 7 | No action taken | Resolving |
| Septoplasty | Pain (nasal) | Definitely | Mild | 7 | Concomitant medications | Resolved |
| Septoplasty | Pain | Definitely | Mild | 8 | Concomitant medications | Resolved |
| Septoplasty | Bleeding from right nostril | Definitely | Mild | 9 | No action taken | Resolved |
| Septoplasty | Bleeding from left nostril | Definitely | Mild | 9 | No action taken | Resolved |
| Septoplasty | Blood clot in right nostril | Definitely | Mild | 9 | Non-drug therapy given | Resolved |
| Septoplasty | Adhesion | Definitely | Mild | 28 | No action taken | Ongoing |
| Septoplasty | Perforation | Definitely | Mild | 28 | No action taken | Ongoing |
| Septoplasty | Septal perforation | Definitely | Mild | Missing | No action taken | Ongoing |
| Medical management | Nosebleeds | Definitely | Mild | 0 | No action taken | Resolving |
| Medical management | Pain and bleeding after 2 weeks | Definitely | Mild | 2 | Treatment discontinued | Resolved |
| Medical management | Nosebleeds | Definitely | Mild | 2 | No action taken | Resolved |
| Medical management | Intermittent nosebleeds | Definitely | Mild | 18 | Treatment discontinued | Resolved |
| Medical management | Postoperative nasal infection | Definitely | Mild | 28 | Concomitant medications | Resolved |
| Septoplasty | Nasal congestion | Probable | Moderate | 4 | No action taken | Resolved |
| Septoplasty | Nose infection | Probable | Moderate | 5 | No action taken | Resolved |
| Septoplasty | Sinusitis | Probable | Moderate | 6 | Concomitant medications | Resolved |
| Septoplasty | Dizziness on movement | Probable | Moderate | 6 | No action taken | Resolved |
| Septoplasty | Excessive nasal mucus | Probable | Moderate | 6 | No action taken | Resolved |
| Septoplasty | Painful nose | Probable | Moderate | 6 | Concomitant medications | Resolved |
| Septoplasty | Sinus infection | Probable | Moderate | 10 | Concomitant medications | Resolved |
| Septoplasty | Infection in both nostrils | Probable | Moderate | 14 | Concomitant medications | Resolved |
| Septoplasty | Discomfort at tip of nose when touching it | Probable | Mild | 3 | No action taken | Resolving |
| Septoplasty | Nasal congestion | Probable | Mild | 6 | No action taken | Ongoing |
| Septoplasty | Raw area on left septal wall | Probable | Mild | 6 | No action taken | Resolved |
| Septoplasty | Numbness of teeth | Probable | Mild | 7 | No action taken | Resolved |
| Septoplasty | Headaches | Probable | Mild | 8 | No action taken | Resolved |
| Septoplasty | 2–3 days’ postoperative pyrexia (38.0 °C) | Probable | Mild | 8 | No action taken | Resolved |
| Septoplasty | Nasal pain | Probable | Mild | 8 | No action taken | Resolved |
| Septoplasty | One episode of epistaxis lasting 30 minutes | Probable | Mild | 8 | No action taken | Resolved |
| Septoplasty | Nasal infection | Probable | Mild | 9 | No action taken | Resolved |
| Septoplasty | Infection requiring antibiotics | Probable | Mild | 13 | No action taken | Resolved |
| Septoplasty | Epistaxis (small amount) | Probable | Mild | 14 | No action taken | Resolved |
| Septoplasty | Pain | Probable | Mild | 14 | Concomitant medications | Resolved |
| Medical management | Patient c/o pain/irritation when administering medication. Goes away soon after | Probable | Mild | 0 | No action taken | Ongoing |
| Medical management | Patient experiencing congestion and a runny nose for approximately 30 minutes following administration of spray | Probable | Mild | 0 | No action taken | Resolved |
| Medical management | Runny nose for 5 minutes following administration | Probable | Mild | 0 | No action taken | Ongoing |
| Medical management | Nasal crusting | Probable | Mild | 0 | No action taken | Ongoing |
| Medical management | Patient experiencing a runny nose for approximately 5 minutes following administration of sprays | Probable | Mild | 0 | No action taken | Resolved |
| Medical management | Sore throat | Probable | Mild | 0 | No action taken | Resolved |
| Medical management | Epistaxis | Probable | Mild | 0 | No action taken | Ongoing |
| Medical management | Dryness to nasal passages | Probable | Mild | 1 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Nasal pain | Probable | Mild | 1 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Epistaxis | Probable | Mild | 1 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Nasal bleeding | Probable | Mild | 1 | No action taken | Resolved |
| Medical management | Bleed from left nostril when douching | Probable | Mild | 1 | No action taken | Ongoing |
| Medical management | Pain | Probable | Mild | 2 | Treatment adjusted/ interrupted | Resolving |
| Medical management | Small amount of bleeding when blowing nose | Probable | Mild | 2 | Treatment adjusted/ interrupted | Resolving |
| Medical management | Bleed from both nostrils when douching | Probable | Mild | 2 | No action taken | Ongoing |
| Medical management | Dry nose | Probable | Mild | 2 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Nasal itching | Probable | Mild | 4 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Pain in nostrils | Probable | Mild | 4 | Treatment adjusted/ interrupted | Resolved |
| Medical management | Rash inside nose | Probable | Mild | 4 | Treatment adjusted/ interrupted | Ongoing |
| Medical management | Epistaxis | Probable | Mild | 6 | Treatment discontinued | Ongoing |
| Medical management | Rhinorrhoea | Probable | Mild | Missing | No action taken | Ongoing |
| Medical management | Fever | Possible | Severe | 40 | Concomitant medications | Resolved |
| Septoplasty | New burning sensation; feels like sinusitis | Possible | Moderate | 5 | No action taken | Ongoing |
| Septoplasty | Postoperative infection | Possible | Moderate | 6 | Concomitant medications | Resolving |
| Septoplasty | Loss of taste | Possible | Moderate | 6 | No action taken | Resolved |
| Septoplasty | Postoperative nasal infection | Possible | Moderate | 7 | No action taken | Resolved |
| Septoplasty | Runny nose | Possible | Moderate | 8 | Concomitant medications | Resolved |
| Septoplasty | Ear blocked | Possible | Moderate | 8 | No action taken | Resolved |
| Septoplasty | Postoperative nasal wound infection | Possible | Moderate | 9 | Concomitant medications | Resolved |
| Septoplasty | Right hypoglossal nerve palsy | Possible | Moderate | 11 | No action taken | Ongoing |
| Septoplasty | Postoperative infection | Possible | Moderate | 12 | Concomitant medications | Resolved |
| Septoplasty | Infection | Possible | Moderate | 25 | Concomitant medications | Resolved |
| Septoplasty | Revision septoplasty | Possible | Moderate | 47 | Non-drug therapy given | Ongoing |
| Medical management | Rhinorrhoea | Possible | Moderate | 0 | No action taken | Ongoing |
| Medical management | Sore throat | Possible | Moderate | 3 | Treatment adjusted/ interrupted | Resolved |
| Medical management | Nosebleeds associated with medication | Possible | Moderate | 8 | Treatment adjusted/ interrupted then discontinued | Resolved |
| Medical management | Epistaxis from left nostril | Possible | Moderate | 14 | No action taken | Resolved |
| Medical management | Headaches | Possible | Moderate | 19 | No action taken | Ongoing |
| Medical management | Bleeding from right nasal passage | Possible | Moderate | 20 | Treatment discontinued | Resolved |
| Medical management | Pain from right nasal passage | Possible | Moderate | 20 | Treatment discontinued | Resolved |
| Medical management | Infection requiring antibiotic treatment | Possible | Moderate | 21 | Concomitant medications | Resolved |
| Septoplasty | Mouth sores | Possible | Mild | 4 | No action taken | Resolved |
| Septoplasty | Headaches | Possible | Mild | 4 | Concomitant medications | Ongoing |
| Septoplasty | Expelling yellow mucus from both nostrils | Possible | Mild | 4 | No action taken | Resolved |
| Septoplasty | Sore throat | Possible | Mild | 4 | No action taken | Resolved |
| Septoplasty | Painful upper front teeth | Possible | Mild | 5 | No action taken | Resolving |
| Septoplasty | Brief drop in SATs | Possible | Mild | 5 | Unknown | Resolved |
| Septoplasty | Numb upper lip | Possible | Mild | 5 | No action taken | Resolving |
| Septoplasty | Patient states burning sensation to left nostril | Possible | Mild | 5 | No action taken | Resolved |
| Septoplasty | Clear discharge from left nostril | Possible | Mild | 6 | No action taken | Resolved |
| Septoplasty | Insomnia | Possible | Mild | 6 | Concomitant medications | Resolved |
| Septoplasty | Epistaxis | Possible | Mild | 6 | No action taken | Resolved |
| Septoplasty | Loss of sense of smell | Possible | Mild | 6 | No action taken | Resolved |
| Septoplasty | Infection | Possible | Mild | 7 | No action taken | Resolved |
| Septoplasty | Patient felt had infection as was producing large amount of mucus in nose | Possible | Mild | 7 | Concomitant medications | Resolved |
| Septoplasty | Raised temperature for 48 hours | Possible | Mild | 8 | Concomitant medications | Resolved |
| Septoplasty | Subjective asymmetry to nostrils | Possible | Mild | 8 | Unknown | Ongoing |
| Septoplasty | Nosebleeds | Possible | Mild | 8 | No action taken | Ongoing |
| Septoplasty | Nasal hypersensitivity to cold air | Possible | Mild | 10 | No action taken | Ongoing |
| Septoplasty | Swelling to the left side of the nose | Possible | Mild | 13 | No action taken | Resolved |
| Septoplasty | Right nosebleed | Possible | Mild | 15 | Concomitant medications | Resolving |
| Septoplasty | Right nosebleed | Possible | Mild | 24 | Concomitant medications | Resolved |
| Septoplasty | Change of shape of nose | Possible | Mild | 52 | No action taken | Ongoing |
| Septoplasty | Bilateral alar battens with auricular cartilage graft | Possible | Mild | 58 | No action taken | Resolved |
| Septoplasty | Nasal congestion | Possible | Mild | Concomitant medications | Ongoing | |
| Medical management | Slight blood on tissue after blowing nose | Possible | Mild | 0 | No action taken | Resolved |
| Medical management | Nasal pain | Possible | Mild | 0 | Concomitant medications | Resolved |
| Medical management | Irritation in top of mouth | Possible | Mild | 0 | No action taken | Resolving |
| Medical management | Nasal bleeding when blowing nose | Possible | Mild | 0 | No action taken | Ongoing |
| Medical management | Minor nosebleed (spotting) on a couple of occasions | Possible | Mild | 0 | No action taken | Resolved |
| Medical management | Headache | Possible | Mild | 4 | Concomitant medications | Resolved |
| Medical management | Nausea | Possible | Mild | 6 | Treatment discontinued | Ongoing |
| Medical management | Epistaxis | Possible | Mild | 10 | Treatment adjusted/ interrupted | Resolved |
| Medical management | Notices some dried blood on tissues when blowing nose. Advice given regarding application of nasal sprays | Possible | Mild | 13 | No action taken | Resolved |
| Septoplasty | Sore throat | Unlikely | Moderate | 4 | No action taken | Resolved |
| Septoplasty | Nausea | Unlikely | Moderate | 11 | No action taken | Ongoing |
| Septoplasty | Swelling in left side of nose and face, patient reported | Unlikely | Moderate | 48 | No action taken | Resolved |
| Medical management | Heartburn | Unlikely | Moderate | 1 | Treatment adjusted/ interrupted | Resolved |
| Medical management | Possible sinus infection and cough | Unlikely | Moderate | 26 | No action taken | Ongoing |
| Medical management | Complaining of heartburn | Unlikely | Moderate | 32 | No action taken | Ongoing |
| Septoplasty | Feels bones in fingers are sore | Unlikely | Mild | 1 | No action taken | Resolved |
| Septoplasty | Feels tired | Unlikely | Mild | 1 | No action taken | Resolved |
| Septoplasty | Tinnitus | Unlikely | Mild | 5 | No action taken | Resolving |
| Septoplasty | Increased mucus production | Unlikely | Mild | 7 | Concomitant medications | Resolved |
| Septoplasty | Sinus infection | Unlikely | Mild | 9 | Concomitant medications | Resolved |
| Septoplasty | Sinusitis | Unlikely | Mild | 20 | No action taken | Resolved |
| Septoplasty | Nasal congestion | Unlikely | Mild | 20 | No action taken | Ongoing |
| Septoplasty | Rhinitis | Unlikely | Mild | 26 | Concomitant medications | Ongoing |
| Septoplasty | Rhinitis | Unlikely | Mild | 51 | Concomitant medications | Ongoing |
| Medical management | Headache | Unlikely | Mild | 0 | Concomitant medications | Resolved |
| Medical management | Throat infection | Unlikely | Mild | 1 | Concomitant medications | Resolved |
| Medical management | Gastric reflux | Unlikely | Mild | 2 | Concomitant medications | Ongoing |
| Medical management | Urinary tract infection | Unlikely | Mild | 5 | Concomitant medications | Resolved |
| Medical management | Sinus infection | Unlikely | Mild | 45 | No action taken | Resolved |
| Medical management | Reduced liver enzymes | Unlikely | Mild | 47 | No action taken | Resolved |
| Septoplasty | Nasal pain – bridge of nose | Unrelated | Moderate | 6 | No action taken | Resolved |
| Septoplasty | Post-surgery nasal pain | Unrelated | Moderate | 6 | No action taken | Resolved |
| Septoplasty | Urinary tract infection | Unrelated | Moderate | 7 | No action taken | Resolved |
| Septoplasty | Cold symptoms | Unrelated | Moderate | 7 | No action taken | Resolved |
| Septoplasty | Wisdom tooth infection | Unrelated | Moderate | 7 | Concomitant medications | Resolved |
| Septoplasty | ECG changes post induction of anaesthesia. ST depression and T-wave inversion | Unrelated | Moderate | 8 | Treatment adjusted/ interrupted | Ongoing |
| Septoplasty | Nasal obstruction | Unrelated | Moderate | 10 | No action taken | Ongoing |
| Septoplasty | Sciatica | Unrelated | Moderate | 17 | Concomitant medications | Ongoing |
| Septoplasty | Influenza | Unrelated | Moderate | 39 | No action taken | Resolved |
| Septoplasty | Fractured wrist | Unrelated | Moderate | 44 | No action taken | Resolving |
| Medical management | Headache | Unrelated | Moderate | 2 | No action taken | Resolved |
| Medical management | Sore shoulder | Unrelated | Moderate | 2 | No action taken | Resolved |
| Medical management | Toothache due to a gum infection | Unrelated | Moderate | 3 | No action taken | Resolved |
| Medical management | Ablation for varicose veins | Unrelated | Moderate | 6 | No action taken | Resolved |
| Medical management | Exacerbation of anxiety | Unrelated | Moderate | 16 | No action taken | Ongoing |
| Medical management | Diagnosis of fatty liver (non-acute) | Unrelated | Moderate | 18 | Treatment discontinued | Ongoing |
| Medical management | Asthma exacerbation | Unrelated | Moderate | 19 | Concomitant medications | Resolved |
| Medical management | Anxiety | Unrelated | Moderate | 22 | No action taken | Resolved |
| Medical management | Sinusitis | Unrelated | Moderate | 24 | No action taken | Resolved |
| Medical management | Subluxation of left shoulder | Unrelated | Moderate | 29 | No action taken | Ongoing |
| Medical management | Back pain | Unrelated | Moderate | 43 | No action taken | Resolving |
| Septoplasty | Post anaesthesia antibiotics given prophylactically because of splenectomy | Unrelated | Mild | 6 | No action taken | Resolved |
| Septoplasty | Kept in hospital overnight because of pre-existing condition; arranged at pre assessment | Unrelated | Mild | 7 | No action taken | Resolved |
| Septoplasty | Pulled muscle in hip | Unrelated | Mild | 8 | No action taken | Resolving |
| Septoplasty | Chest pain – sinus tachycardia on ECG | Unrelated | Mild | 9 | No action taken | Resolved |
| Septoplasty | Swollen lower lip | Unrelated | Mild | 10 | No action taken | Resolved |
| Septoplasty | Viral cold | Unrelated | Mild | 14 | Concomitant medications | Resolved |
| Septoplasty | Facial pain | Unrelated | Mild | 14 | No action taken | Ongoing |
| Septoplasty | Small fracture distal fibula | Unrelated | Mild | 16 | No action taken | Resolved |
| Septoplasty | Viral cold | Unrelated | Mild | 19 | Concomitant medications | Resolved |
| Septoplasty | Dental extraction | Unrelated | Mild | 21 | Concomitant medications | Resolved |
| Septoplasty | Dizziness – when moving head | Unrelated | Mild | 22 | No action taken | Ongoing |
| Septoplasty | Fungal ear infection | Unrelated | Mild | 22 | Concomitant medications | Resolved |
| Septoplasty | Mechanical fall causing bruising to nose. No nasal or facial fracture | Unrelated | Mild | 23 | No action taken | Resolved |
| Septoplasty | Viral cold | Unrelated | Mild | 23 | Concomitant medications | Resolving |
| Septoplasty | Rhinitis | Unrelated | Mild | 24 | No action taken | Resolved |
| Septoplasty | COVID-19 | Unrelated | Mild | 28 | No action taken | Resolved |
| Septoplasty | Muscular chest pains | Unrelated | Mild | 33 | No action taken | Resolving |
| Septoplasty | Cough | Unrelated | Mild | 39 | No action taken | Resolved |
| Septoplasty | Type 2 diabetes | Unrelated | Mild | 49 | No action taken | Ongoing |
| Septoplasty | Labyrinthitis | Unrelated | Mild | 49 | Concomitant medications | Resolved |
| Septoplasty | Head collision with a cow. Bleeding nose, which is now swollen, and two black eyes | Unrelated | Mild | 53 | No action taken | Resolving |
| Septoplasty | Influenza-like symptoms and nasal congestion | Unrelated | Mild | Missing | No action taken | Resolved |
| Septoplasty | Cold | Unrelated | Mild | Missing | No action taken | Resolved |
| Medical management | Sinus infection | Unrelated | Mild | 0 | Concomitant medications | Resolved |
| Medical management | Common cold | Unrelated | Mild | 1 | No action taken | Resolved |
| Medical management | Depression | Unrelated | Mild | 1 | No action taken | Ongoing |
| Medical management | Viral head cold | Unrelated | Mild | 1 | No action taken | Resolved |
| Medical management | Influenza, as described by participant | Unrelated | Mild | 1 | Treatment adjusted/ interrupted and con medications | Resolved |
| Medical management | Headache | Unrelated | Mild | 2 | No action taken | Resolved |
| Medical management | Cold/influenza | Unrelated | Mild | 3 | Concomitant medications | Resolved |
| Medical management | Sinusitis | Unrelated | Mild | 5 | Concomitant medications | Ongoing |
| Medical management | Headaches | Unrelated | Mild | 6 | Concomitant medications | Ongoing |
| Medical management | Viral head cold | Unrelated | Mild | 7 | No action taken | Resolved |
| Medical management | Hip pain | Unrelated | Mild | 11 | No action taken | Resolved |
| Medical management | Bites to ankles | Unrelated | Mild | 12 | No action taken | Resolved |
| Medical management | Perianal infection | Unrelated | Mild | 13 | No action taken | Resolved |
| Medical management | Bleeding from the nose | Unrelated | Mild | 17 | No action taken | Resolved |
| Medical management | Arthritis in left hip | Unrelated | Mild | 21 | No action taken | Ongoing |
| Medical management | Diagnosis of type 2 diabetes | Unrelated | Mild | 21 | No action taken | Ongoing |
| Medical management | Epigastric pain | Unrelated | Mild | 23 | No action taken | Ongoing |
| Medical management | Back injury | Unrelated | Mild | 25 | No action taken | Ongoing |
| Medical management | Sinusitis | Unrelated | Mild | 26 | Concomitant medications | Resolved |
| Medical management | Vasovagal episode | Unrelated | Mild | 27 | No action taken | Resolved |
| Medical management | Hit in face by ball | Unrelated | Mild | 31 | No action taken | Resolved |
| Medical management | Muscular strain, right shoulder, following MVA | Unrelated | Mild | 35 | No action taken | Resolved |
| Medical management | Hypertension | Unrelated | Mild | 36 | Concomitant medications | Ongoing |
| Medical management | Vitamin D deficiency | Unrelated | Mild | 37 | No action taken | Resolved |
| Medical management | Heartburn | Unrelated | Mild | 38 | No action taken | Resolved |
| Medical management | Diagnosis of osteopenia | Unrelated | Mild | 42 | No action taken | Ongoing |
| Medical management | Chest infection | Unrelated | Mild | 42 | No action taken | Resolved |
| Medical management | Bladder infection | Unrelated | Mild | 43 | Concomitant medications | Resolved |
| Medical management | Nasal tip numbness | Unrelated | Mild | 43 | No action taken | Resolved |
| Medical management | Developed dermatitis | Unrelated | Mild | 45 | No action taken | Ongoing |
| Medical management | Right lower quadrant abdominal pain | Unrelated | Mild | Missing | No action taken | Ongoing |
| Septoplasty | Nasal infection | Missing | Moderate | 10 | No action taken | Resolved |
| Septoplasty | Rhinitis | Not assessable | Mild | 50 | No action taken | Resolving |
| Related to treatment | Severity | Trial arm (n) | |
|---|---|---|---|
| Septoplasty | Medical management | ||
| Definitely | Mild | 5 | 0 |
| Moderate | 1 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Probable | Mild | 2 | 0 |
| Moderate | 1 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Possible | Mild | 0 | 0 |
| Moderate | 1 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Unlikely | Mild | 1 | 0 |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 | |
| Life-threatening | 0 | 0 | |
| Unrelated | Mild | 2 | 1 |
| Moderate | 1 | 5 | |
| Severe | 0 | 1 | |
| Life-threatening | 0 | 2 | |
| Total | 14 | 9 | |
| SAE coded | Grade | Trial arm (n) | Total (n) | |
|---|---|---|---|---|
| Septoplasty | Medical management | |||
| Unexpected events occurring during surgical intervention (e.g. excessive bleeding) (A) | Mild | 0 | 0 | 0 |
| Moderate | 0 | 1 | 1 | |
| Severe | 0 | 0 | 0 | |
| Life-threatening | 0 | 0 | 0 | |
| Significant postoperative bleeding, above that normally expected following the surgical intervention (B) | Mild | 3 | 0 | 3 |
| Moderate | 2 | 1 | 3 | |
| Severe | 0 | 0 | 0 | |
| Life-threatening | 0 | 0 | 0 | |
| Complications related to the administration of the general anaesthetic (C) | Mild | 5 | 1 | 6 |
| Moderate | 0 | 1 | 1 | |
| Severe | 0 | 1 | 1 | |
| Life-threatening | 0 | 0 | 0 | |
| Unexpected events related to septoplasty (D) | Mild | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | |
| Life-threatening | 0 | 0 | 0 | |
| Other (E) | Mild | 2 | 0 | 2 |
| Moderate | 2 | 2 | 4 | |
| Severe | 0 | 0 | 0 | |
| Life-threatening | 0 | 2 | 2 | |
| Total | 14 | 9 | 23 | |
| Trial arm | SAE (codes in parentheses are listed Table 68) | Related to treatment | Severity | Time in weeks from randomisation to SAE | Action taken | Outcome | SUSAR | |
|---|---|---|---|---|---|---|---|---|
| Septoplasty | Postoperative epistaxis with possible infection (B) | Definitely | Moderate | 7 | Hospitalisation | Resolved | ||
| Septoplasty | Postoperative epistaxis (B) | Definitely | Mild | 5 | Hospitalisation | Resolved | Yes | |
| Septoplasty | Vasovagal (C) | Definitely | Mild | 5 | Hospitalisation | Resolved | ||
| Septoplasty | Shortness of breath, anxiety (C) | Definitely | Mild | 6 | Hospitalisation | Resolved | Yes | |
| Septoplasty | Nasal bleeding post operation (B) | Definitely | Mild | 9 | Hospitalisation | Resolved | ||
| Septoplasty | Postoperative oxygen desaturation (C) | Definitely | Mild | 10 | Hospitalisation | Resolved | Yes | |
| Medical management | Unwell post operatively (C) | Definitely | Mild | 29 | Hospitalisation | Resolved | ||
| Septoplasty | Re-admission owing to infection (E) | Probable | Moderate | 7 | Hospitalisation | Resolved | ||
| Septoplasty | Chest pain (C) | Probable | Mild | 4 | Hospitalisation | Resolved | Yes | |
| Septoplasty | Admitted with epistaxis from septal perforation (B) | Probable | Mild | 32 | Hospitalisation | Resolved | ||
| Septoplasty | Post-septoplasty haemorrhage (B) | Possible | Moderate | 7 | Hospitalisation | Resolved | ||
| Septoplasty | Drop in blood pressure necessitating overnight stay (C) | Unlikely | Mild | 6 | Hospitalisation | Resolved | ||
| Medical management | Traumatic abdominal injury (E) | Unrelated | Life-threatening | 3 | Hospitalisation | Resolving | ||
| Medical management | Polypharmacy overdose (E) | Unrelated | Life-threatening | 48 | Hospitalisation | Resolved | ||
| Medical management | Respiratory, thoracic and mediastinal disorders (pneumonia) (C) | Unrelated | Severe | 16 | Hospitalisation | Resolved | ||
| Medical management | Polypharmacy overdose (E) | Unrelated | Moderate | 14 | Hospitalisation | Resolved | ||
| Septoplasty | Viral infection (E) | Unrelated | Moderate | 22 | Concomitant medications | Resolved | ||
| Medical management | Polypharmacy overdose (E) | Unrelated | Moderate | 23 | Hospitalisation | Resolved | ||
| Medical management | Chest tightness (C) | Unrelated | Moderate | 26 | Hospitalisation | Resolved | ||
| Medical management | Postoperative bleed (B) | Unrelated | Moderate | 34 | Hospitalisation | Resolved | ||
| Medical management | Postoperative infection with drifting of nasal septum (A) | Unrelated | Moderate | 36 | Hospitalisation | Ongoing | ||
| Septoplasty | Inappropriate admission (E) | Unrelated | Mild | 6 | Hospitalisation | Resolved | ||
| Septoplasty | Did not meet criteria for day-case surgery (E) | Unrelated | Mild | 10 | Hospitalisation | Resolved | ||
| Original text | Recategorised by clinical team |
|---|---|
| Had overnight stay in hospital as he was given a hot cup of tea to drink post op[eration] and then had nasal bleeding. Discharged home the next day | Bleeding |
| Severe nasal bleeding and blocked nose, attended A[&]E – nose packed | Bleeding |
| Was given antibiotics by GP but no symptoms or findings suggestive of infection so NO infection confirmed | Infection |
| Chest pain | Other |
| Clinic 9 April 2019 with concern regarding nasal support, but feels this has settled now and is happy | Other |
| Inflammation secondary to splint – Naseptin[®, Alliance Pharmaceuticals plc, Chippenham, UK] given | Other |
| Upper front teeth painful and upper lip numb | Other |
| Rhinitis more noticeable | Other |
| Left nasal valve collapse. Improvement of left anterior septal deviation | Other |
| Nasal tip numbness | Other |
| Small polyp arising right middle turbinate. Had polyps many years ago. Will review at 12 months | Remove not a complication |
| Sense of smell improved | Remove not a complication |
| Patient still feels a little bit of pain and is uncomfortable; however, symptoms decreased and feel much better | Remove not a complication |
| Questionnaires by post. No info[rmation] on numbness, teeth, change in appearance of nose. SNOT-22 ticked to say sense of smell mild pre, and moderate post, surgery | Remove not a complication |
| Unable to complete at this time as surgery carried out after 6-month assessment. Will assess at 2-week follow-up phone call | Remove not a complication |
| P[atien]t asymptomatic, has L[eft] mild anterior septal deviation | Remove not a complication |
| Two stitches have come out | Remove not a complication |
| Unable to contact; patient not answering telephone | Remove not a complication |
| Nasal pain requiring tramadol | Remove not a complication |
Chest pain.
Clinic 9 April 2019 with concern regarding nasal support, but feels this has settled now and is happy.
Inflammation secondary to splint – Naseptin given.
Upper front teeth painful and upper lip numb.
Rhinitis more noticeable.
Left nasal valve collapse. Improvement of left anterior septal deviation.
Nasal tip numbness.
Line listings not covered in Table 70. Edited by clinical team to remove non-complication text (n = 7).
Appendix 2 Additional health economic analysis
| Resource use | Unit cost (£) | Source | Notes |
|---|---|---|---|
| GP consultation | 34.00 | PSSRU 2020103 | |
| Practice nurse consultation | 10.50 | PSSRU 2020,103 PSSRU 2015190 | Assumed to be a 15-minute consultation |
| Nurse telephone consultation | 4.20 | PSSRU 2020,103 PSSRU 2015190 | Assumed to be a 6-minute consultation |
| GP telephone consultation | 8.00 | PSSRU 2020103 | |
| NHS 111/NHS 24 | 15.05 | PSSRU 2020103 | GP-led triage |
| GP home visit | 49.02 | PSSRU 2020,103 PSSRU 2015190 | Assumed to be an 11.4-minute consultation |
| Nurse home visit | 17.50 | PSSRU 2020,103 PSSRU 2015190 | Assumed to be a 25-minute consultation |
| A&E visit | 182 | NHS reference costs 2019/20105 | |
| Outpatient visit | 147 | NHS reference costs 2019/20105 | |
| ENT outpatient visit | 112 | NHS reference costs 2019/20105 | |
| Hospital admission (inpatient or day patient) | 378 | NHS reference costs 2019/20105 | Assumed to be regular day/night admission |
| Septoplasty | 1956 | NHS reference costs 2019/20105 | Day-case procedure |
| Time away from work | |||
| Paid work day rate | 134.40 | ONS | |
| Time and travel costs | |||
| Total inpatient time and travel cost | 52.62 | Time and travel questionnaire | |
| Total outpatient time and travel cost | 44.65 | Time and travel questionnaire | |
| Total GP time and travel cost | 15.81 | Time and travel questionnaire | |
| Resource use | Unit cost (£) | Source |
|---|---|---|
| Theatre cost (per minute) | 13.75 | |
| Ward cost (total recovery time) | 60.00 | |
| Pre-assessment cost | 9.50 | |
| Consultant (per minute) | 1.90 | |
| Registrar (per minute) | 0.83 | |
| Scrub nurse (per minute) | 0.83 | |
| Operating department practitioner (per minute) | 0.67 | |
| Healthcare assistant (per minute) | 0.23 | |
| Anaesthetist assistant (per min) | 0.67 | |
| Septoplasty tray (cost per use) | 5.37 | Assumed 10-year lifespan, 3.5% discount for equivalent annual cost and used on average once per week |
| Septoplasty tray (autoclave) | 40.00 | |
| Anaesthetic consumables | 46.15 | |
| Surgical consumable | 7.14 | |
| Gowns – TSSU | 2.00 |
| Costs | Trial arm, n (%) | |
|---|---|---|
| Medical management (N = 155) | Septoplasty (N = 152) | |
| HUQ | ||
| 6 months | 142 (92) | 140 (92) |
| 12 months | 115 (74) | 99 (65) |
| 6 and 12 months | 109 (70) | 95 (63) |
| QALYs: SF-36 | ||
| Baseline | 152 (98) | 149 (98) |
| 6 months | 140 (90) | 140 (92) |
| 12 months | 117 (75) | 103 (68) |
| Baseline and 6 and 12 months | 111 (72) | 99 (65) |
| Resource | Medical management (N = 155) | Septoplasty (N = 152) | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | ||||
| 6 months | |||||||
| Hospital admission | 0.06 (0.23) | 142 | 0.08 (0.27) | 140 | |||
| Number of days admitted | 0.21 (1.28) | 142 | 0.09 (0.31) | 140 | |||
| A&E visit | 0.12 (0.33) | 142 | 0.11 (0.31) | 140 | |||
| Number of A&E visits | 0.17 (0.55) | 142 | 0.12 (0.37) | 140 | |||
| ENT outpatient visit | 0.18 (0.38) | 142 | 0.38 (0.49) | 140 | |||
| Number of ENT outpatient visits | 0.25 (0.65) | 141 | 0.63 (1.01) | 140 | |||
| Outpatient visit | 0.18 (0.39) | 142 | 0.18 (0.38) | 140 | |||
| Number of outpatient visits | 0.55 (2.72) | 142 | 0.36 (0.98) | 140 | |||
| Face-to-face consultations | 0.46 (0.50) | 142 | 0.53 (0.50) | 140 | |||
| GP consultation | 0.41 (0.49) | 142 | 0.44 (0.50) | 140 | |||
| Number of GP consultations | 0.88 (1.72) | 142 | 0.92 (1.40) | 140 | |||
| Nurse consultation | 0.19 (0.39) | 142 | 0.17 (0.38) | 140 | |||
| Number of nurse consultations | 0.25 (0.62) | 142 | 0.29 (0.72) | 140 | |||
| Other consultations | 0.09 (0.29) | 142 | 0.14 (0.34) | 140 | |||
| Number of other consultations | 0.33 (1.87) | 142 | 0.23 (0.63) | 140 | |||
| Home consultations | 0.01 (0.12) | 142 | 0.00 (0.00) | 140 | |||
| GP consultation – home | 0.01 (0.08) | 142 | 0.00 (0.00) | 140 | |||
| Number of GP consultations – home | 0.02 (0.25) | 142 | 0.00 (0.00) | 140 | |||
| Nurse consultation – home | 0.00 (0.00) | 142 | 0.00 (0.00) | 140 | |||
| Number of nurse consultations – home | 0.00 (0.00) | 142 | 0.00 (0.00) | 140 | |||
| Other consultations – home | 0.01 (0.08) | 142 | 0.00 (0.00) | 140 | |||
| Number of other consultations – home | 0.01 (0.17) | 142 | 0.00 (0.00) | 140 | |||
| Telephone consultations | 0.19 (0.39) | 142 | 0.15 (0.36) | 139 | |||
| GP consultation – telephone | 0.11 (0.31) | 142 | 0.06 (0.25) | 139 | |||
| Number of GP consultations – telephone | 0.16 (0.53) | 142 | 0.12 (0.51) | 139 | |||
| Nurse consultation – telephone | 0.04 (0.18) | 142 | 0.03 (0.17) | 139 | |||
| Number of nurse consultations – telephone | 0.04 (0.23) | 142 | 0.06 (0.38) | 139 | |||
| NHS 111 consultation | 0.04 (0.20) | 142 | 0.02 (0.15) | 139 | |||
| Number of NHS 111 consultations | 0.04 (0.20) | 142 | 0.03 (0.21) | 139 | |||
| Other consultations – telephone | 0.02 (0.14) | 142 | 0.04 (0.19) | 139 | |||
| Number of other consultations – telephone | 0.02 (0.15) | 130 | 0.05 (0.25) | 139 | |||
| Private health care | 0.04 (0.19) | 140 | 0.01 (0.12) | 138 | |||
| Days off work | 4.37 (11.55) | 137 | 5.46 (8.77) | 133 | |||
| 12 months | |||||||
| Hospital admission | 0.05 (0.22) | 115 | 0.00 (0.00) | 99 | |||
| Number of days admitted | 0.09 (0.51) | 115 | 0.00 (0.00) | 99 | |||
| A&E visit | 0.11 (0.31) | 114 | 0.09 (0.29) | 99 | |||
| Number of A&E visits | 0.13 (0.43) | 114 | 0.09 (0.29) | 99 | |||
| ENT outpatient visit | 0.38 (0.49) | 114 | 0.18 (0.39) | 99 | |||
| Number of ENT outpatient visits | 0.69 (1.11) | 113 | 0.25 (0.64) | 99 | |||
| Outpatient visit | 0.21 (0.41) | 114 | 0.19 (0.40) | 99 | |||
| Number of outpatient visits | 0.32 (0.79) | 114 | 0.32 (073) | 99 | |||
| Face-to-face consultations | 0.50 (0.50) | 115 | 0.45 (0.50) | 99 | |||
| GP consultation | 0.34 (0.48) | 115 | 0.35 (0.48) | 99 | |||
| Number of GP consultations | 0.61 (1.18) | 114 | 0.80 (1.48) | 99 | |||
| Nurse consultation | 0.18 (0.39) | 115 | 0.18 (0.39) | 99 | |||
| Number of nurse consultations | 0.25 (0.59) | 115 | 0.25 (0.59) | 99 | |||
| Other consultations | 0.15 (0.36) | 115 | 0.08 (0.27) | 99 | |||
| Number of other consultations | 0.60 (3.32) | 114 | 0.15 (0.61) | 99 | |||
| Home consultations | 0.03 (0.16) | 115 | 0.02 (0.14) | 99 | |||
| GP consultation – home | 0.01 (0.09) | 115 | 0.01 (0.10) | 99 | |||
| Number of GP consultations – home | 0.01 (0.09) | 115 | 0.01 (0.10) | 99 | |||
| Nurse consultation – home | 0.01 (0.09) | 115 | 0.00 (0.00) | 99 | |||
| Number of nurse consultations – home | 0.01 (0.09) | 115 | 0.00 (0.00) | 99 | |||
| Other consultations – home | 0.00 (0.00) | 115 | 0.01 (0.10) | 99 | |||
| Number of other consultations – home | 0.00 (0.00) | 115 | 0.05 (0.50) | 99 | |||
| Telephone consultations | 0.28 (0.45) | 115 | 0.20 (0.40) | 99 | |||
| GP consultation – telephone | 0.19 (0.40) | 115 | 0.10 (0.30) | 99 | |||
| Number of GP consultations – telephone | 0.22 (0.55) | 112 | 0.17 (0.62) | 99 | |||
| Nurse consultation – telephone | 0.07 (0.26) | 115 | 0.02 (0.14) | 99 | |||
| Number of nurse consultations – telephone | 0.09 (0.39) | 114 | 0.04 (0.28) | 99 | |||
| NHS 111 consultation | 0.02 (0.13) | 115 | 0.02 (0.14) | 99 | |||
| Number of NHS 111 consultations | 0.01 (0.09) | 114 | 0.02 (0.14) | 99 | |||
| Other consultations – telephone | 0.06 (0.24) | 115 | 0.09 (0.29) | 99 | |||
| Number of other consultations – telephone | 0.08 (0.34) | 110 | 0.12 (0.38) | 95 | |||
| Private health care | 0.02 (0.13) | 113 | 0.03 (0.17) | 98 | |||
| Days off work | 4.62 (11.84) | 111 | 2.23 (9.42) | 94 | |||
| Medication name | Frequency |
|---|---|
| Aciclovir | 2 |
| Adcal | 1 |
| Alendronic acid | 1 |
| Amiloride hydrochloride | 1 |
| Amitriptyline | 8 |
| Amlodipine | 5 |
| Amoxicillin | 16 |
| Aspirin | 2 |
| Atenolol | 1 |
| Atorvastatin | 7 |
| Augmentin | 1 |
| Avamys nasal spray | 3 |
| Azithromycin | 1 |
| Baclofen | 1 |
| Bactroban nasal cream | 6 |
| Beclometasone | 6 |
| Beconase nasal spray | 1 |
| Becotide | 1 |
| Benadryl | 1 |
| Betamethasone | 5 |
| Bisoprolol | 1 |
| Candesartan | 2 |
| Cannabis oil | 2 |
| Carbamazepine | 1 |
| Carbocisteine | 1 |
| Cephalexin | 2 |
| Cerelle | 1 |
| Cetirizine | 6 |
| Charcoal | 1 |
| Chlorhexidine hydrochloride | 1 |
| Chlorphenamine | 1 |
| Citalopram | 4 |
| Clarithromycin | 6 |
| Clindamycin | 1 |
| Clopidogrel | 2 |
| Co-amoxiclav | 21 |
| Co-codamol | 11 |
| Codeine | 5 |
| Contraceptive pill | 1 |
| Creon | 1 |
| Cyclizine | 2 |
| Dermovate | 1 |
| Diazepam | 2 |
| Dihydrocodeine | 5 |
| Doxycycline | 9 |
| Duloxetine | 3 |
| Dymista® nasal spray (Meda AB, Solna, Sweden) | 5 |
| ECG | 2 |
| Elleste Solo™ patches (Meda AB) | 2 |
| EpiPen® (Mylan UK Healthcare Limited, Potters Bar, UK) | 1 |
| Erythromycin | 1 |
| Escitalopram | 2 |
| Esomeprazole | 2 |
| Estradiol | 1 |
| Fexofenadine | 5 |
| Flixonase | 1 |
| Flucloxacillin | 6 |
| Fluoxetine | 4 |
| Fluticasone | 5 |
| Folic acid | 1 |
| Furosemide | 2 |
| Gabapentin | 4 |
| Gaviscon (Reckitt Benckiser Group plc, Slough, UK) | 1 |
| Hydrocortisone | 1 |
| Ibuleve gel | 1 |
| Ibuprofen | 20 |
| Imigran | 1 |
| Ipratropium bromide | 3 |
| Irbesartan | 1 |
| Iv fluids | 1 |
| Lamotrigine | 4 |
| Lansoprazole | 7 |
| Lantus pen | 1 |
| Laxido | 1 |
| Lemsip | 2 |
| Levothyroxine | 4 |
| Lisinopril | 2 |
| Lithium | 1 |
| Loestrin | 1 |
| Loperamide | 1 |
| Loratadine | 5 |
| Lorazepam | 2 |
| Macrobid | 1 |
| Mebeverine | 1 |
| Metformin | 1 |
| Methotrexate | 1 |
| Microgynon | 1 |
| Migraleve | 1 |
| Mirabegron | 2 |
| Mirtazapine | 3 |
| Mometasone nasal spray | 13 |
| Montelukast | 1 |
| Multivitamin | 1 |
| Naproxen | 5 |
| Nasal douche | 8 |
| Naseptin cream | 15 |
| Nasonex nasal spray | 13 |
| Neilmed sinus rinse | 12 |
| Neomycin sulfate | 1 |
| Nitrofurantoin | 2 |
| Nova pen | 1 |
| Novorapid | 1 |
| Oestrogen gel | 1 |
| Olanzapine | 1 |
| Omega 3 | 1 |
| Omeprazole | 13 |
| Otomize spray | 1 |
| Otrivine nasal spray | 2 |
| Oxygen | 1 |
| Oxytetracycline | 1 |
| Pantoprazole | 1 |
| Paracetamol | 35 |
| Paroxetine | 1 |
| Penicillin | 3 |
| Pirinase | 1 |
| Piriton | 1 |
| Pramipexole | 1 |
| Prednisolone | 2 |
| Pregabalin | 4 |
| Progesterone | 1 |
| Propranolol | 5 |
| Quetiapine | 3 |
| Radiography | 2 |
| Ramipril | 5 |
| Ranitidine | 1 |
| Rigevidon | 1 |
| Roaccutane | 1 |
| Rosuvastatin | 1 |
| Salbutamol | 8 |
| Saline nasal spray | 1 |
| Senna | 1 |
| Seretide | 1 |
| Sertraline | 7 |
| Sildenafil | 1 |
| Silver nitrate | 1 |
| Sodium chloride | 2 |
| Stérimar nasal spray | 14 |
| Sudafed® nasal spray (Johnson & Johnson, Brunswick, NJ, USA) | 1 |
| Symbicort | 1 |
| Tadalafil | 1 |
| Tamsulosin | 3 |
| Tapentadol | 1 |
| Thyroxine | 1 |
| Tiotropium | 1 |
| Tizanidine hydrochloride | 1 |
| Tolterodine | 1 |
| Topiramate | 1 |
| Tramadol | 4 |
| Tranexamic acid | 5 |
| Troponin | 2 |
| Uniphyllin | 1 |
| Varenicline | 2 |
| Venlafaxine | 2 |
| Ventolin | 3 |
| Vitamin B | 1 |
| Vitamin D | 5 |
| Ventilation/perfusion lung scan | 1 |
| Xylometazoline hydrochloride | 1 |
| Zimovane | 1 |
| Zolpidem | 1 |
| Zopiclone | 1 |
| Zydol | 1 |
| Resource used | Frequency |
|---|---|
| 6 months | |
| Bupa (London, UK) | 2 |
| GP in Tunisia | 1 |
| Microsuction | 1 |
| Therapy | 1 |
| Physiotherapy to knee | 1 |
| Ealing Luxmedica | 1 |
| 12 months | |
| ENT consultation | 1 |
| Osteopath | 2 |
| Physiotherapy | 2 |
| Therapist | 1 |
| Resource use | n | Mean (SD) |
|---|---|---|
| Hospital admissions | ||
| Travel | ||
| Car | 116 | 0.72 (0.45) |
| Car miles | 78 | 15.00 (16.15) |
| Car time (minutes) | 78 | 31.05 (23.66) |
| Car parking costs (£) | 68 | 2.20 (2.49) |
| Taxi | 116 | 0.09 (0.28) |
| Taxi time (minutes) | 10 | 15.80 (8.74) |
| Taxi fare (£) | 10 | 7.71 (2.64) |
| Public transport | 116 | 0.07 (0.25) |
| Public transport time (minutes) | 8 | 22.63 (10.88) |
| Public transport fare (£) | 8 | 1.65 (1.53) |
| Walking | 116 | 0.02 (0.13) |
| Walking time (minutes) | 2 | 10 (7.07) |
| Cost (£) | 2 | 0.00 (0.00) |
| Total participant travel cost (£) | 116 | 12.01 (13.61) |
| Total participant travel time (minutes) | 116 | 24.23 (22.73) |
| Activity if not at appointment | ||
| Paid work | 110 | 0.70 (0.46) |
| Other | 110 | 0.30 (0.46) |
| Total participant travel time cost (£) | 116 | 10.88 (12.09) |
| Carer | 116 | 0.53 (0.50) |
| Time waiting at hospital (minutes) | 62 | 223.31 (227.65) |
| Activity if not at appointment | ||
| Paid work | 66 | 0.61 (0.49) |
| Other | 66 | 0.39 (0.49) |
| Time cost travel and waiting (£) | 56 | 60.94 (61.61) |
| Travel cost (£) | 61 | 0.60 (1.96) |
| Total time and travel cost for a hospital admission | 116 | 52.62 (63.41) |
| Hospital appointments | ||
| Travel | ||
| Car | 185 | 0.86 (0.34) |
| Car miles | 154 | 11.49 (12.50) |
| Car time (minutes) | 153 | 24.73 (18.25) |
| Car parking costs (£) | 145 | 1.29 (1.66) |
| Taxi | 185 | 0.01 (0.10) |
| Taxi time (minutes) | 2 | 20.00 (0.00) |
| Taxi fare (£) | 2 | 9.50 (0.71) |
| Public transport | 185 | 0.08 (0.27) |
| Public transport time (minutes) | 13 | 39.46 (29.60) |
| Public transport fare (£) | 11 | 2.89 (3.86) |
| Walking | 185 | 0.02 (0.15) |
| Walking time (minutes) | 4 | 18.75 (13.77) |
| Cost (£) | 4 | 0.00 (0.00) |
| Total participant travel cost (£) | 185 | 10.79 (11.20) |
| Total participant travel time (mins) | 185 | 25.13 (19.14) |
| Activity if not at appointment | ||
| Paid work | 159 | 0.77 (0.42) |
| Other | 159 | 0.23 (0.42) |
| Total participant travel time cost (£) | 185 | 10.37 (0.69) |
| Time waiting (minutes) | 171 | 84.47 (93.45) |
| Total participant waiting time cost (£) | 155 | 20.28 (27.66) |
| Carer | 180 | 0.22 (0.41) |
| Activity if not at appointment | ||
| Paid work | 40 | 0.53 (0.51) |
| Other | 40 | 0.47 (0.51) |
| Time cost travel and waiting (£) | 32 | 33.29 (40.05) |
| Travel cost (£) | 17 | 1.28 (3.38) |
| Total time and travel cost for a hospital appointment | 185 | 44.65 (50.49) |
| GP appointments | ||
| Travel | ||
| Car | 142 | 0.59 (0.49) |
| Car miles | 82 | 5.19 (8.69) |
| Car time (minutes) | 83 | 12.90 (12.80) |
| Car parking costs (£) | 78 | 0.45 (1.32) |
| Taxi | 142 | 0.04 (0.18) |
| Taxi time (minutes) | 4 | 11.25 (6.29) |
| Taxi fare (£) | 4 | 9.13 (6.41) |
| Public transport | 142 | 0.08 (0.27) |
| Public transport time (minutes) | 11 | 16.82 (9.56) |
| Public transport fare (£) | 9 | 1.61 (1.81) |
| Walking | 142 | 0.15 (0.36) |
| Walking time (minutes) | 18 | 11.39 (6.55) |
| Cost (£) | 16 | 0.00 (0.00) |
| Total participant travel cost (£) | 142 | 3.66 (7.43) |
| Total participant travel time (mins) | 142 | 10.62 (11.59) |
| Activity if not at appointment | ||
| Paid work | 112 | 0.72 (0.45) |
| Other | 112 | 0.28 (0.45) |
| Total participant travel time cost (£) | 142 | 4.29 (6.42) |
| Time waiting (minutes) | 135 | 36.33 (35.12) |
| Total participant waiting time cost (£) | 110 | 8.74 (10.07) |
| Carer | 139 | 0.09 (0.29) |
| Activity if not at appointment | ||
| Paid work | 15 | 0.40 (0.51) |
| Other | 15 | 0.60 (0.51) |
| Time cost travel and waiting (£) | 9 | 9.70 (5.64) |
| Travel cost (£) | 6 | 0.00 (0.00) |
| Total time and travel cost for a GP visit | 142 | 15.81 (19.36) |
FIGURE 36.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA multiple imputation results.

FIGURE 37.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA multiple imputation results.

| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an improvement in SNOT-22 scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £500 | £1000 | £3000 | £5000 | ||||||
| Outcome: SNOT-22 score at 6 months – results | ||||||||||
| Medical management (costs, n = 142; outcomes, n = 155) | 294 (216 to 372) | 1790 (1698 to 1882) | 0.381 (0.30 to 0.46) | 0.416 (0.32 to 0.51) | 4303 | 1.00 | 1.00 | 1.00 | 1.00 | 0.13 |
| Septoplasty (costs, n = 140; outcomes, n = 152) | 2071 (2016 to 2125) | 0.803 (0.74 to 0.87) | 0.00 | 0.00 | 0.00 | 0.00 | 0.87 | |||
FIGURE 38.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CEA sensitivity analysis (costs and SNOT-22 scores estimated at 6 months).
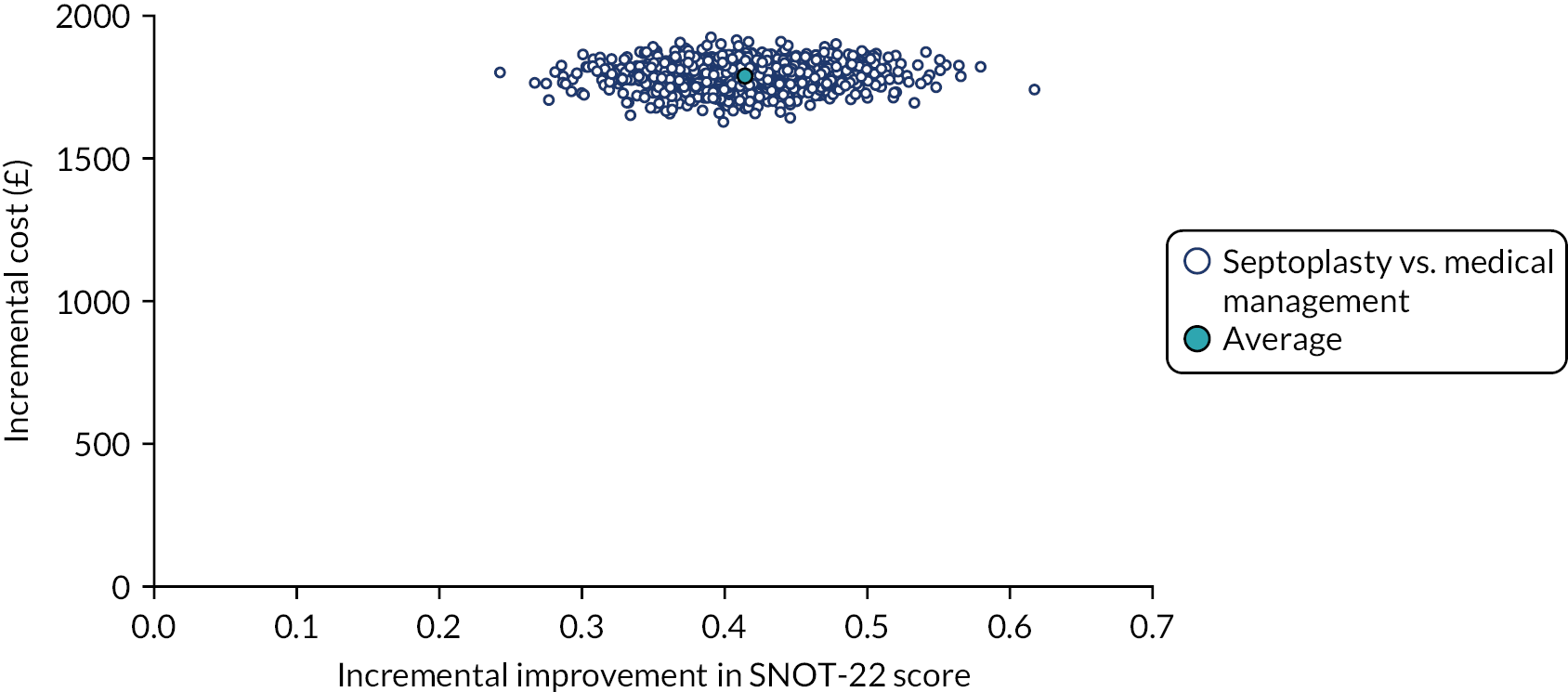
FIGURE 39.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CEA sensitivity analysis (costs and SNOT-22 score estimated at 6 months).
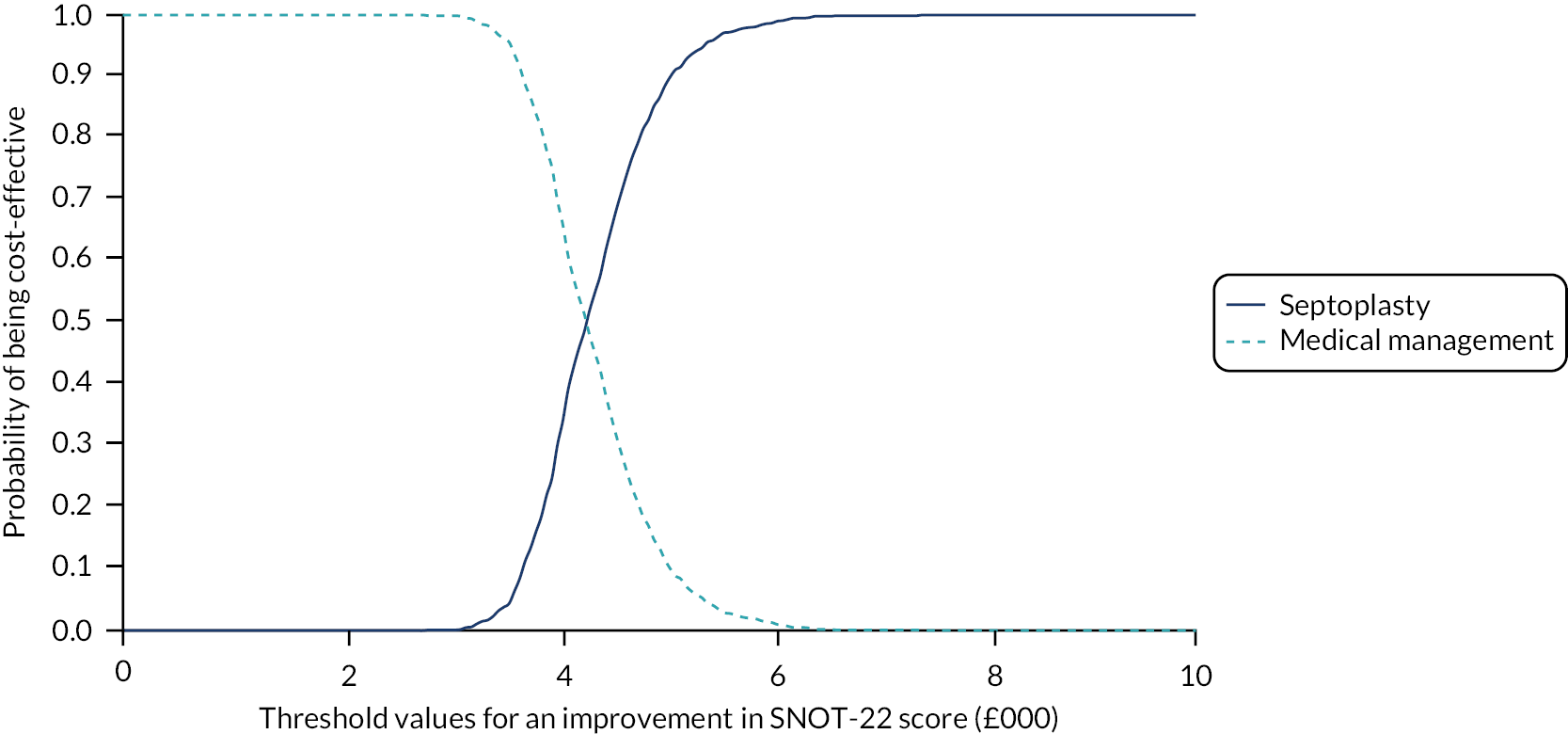
| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay to avoid an AE | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £500 | £1000 | £3000 | £5000 | ||||||
| Outcome: AEs – results | ||||||||||
| Medical management (costs, n = 155; outcomes, n = 155) | 973 (810 to 1137) | 1193 (1018 to 1368) | 0.542 (0.40 to 0.69) | 0.302 (0.03 to 0.58) | Septoplasty is dominated | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Septoplasty (costs, n = 152; outcomes, n = 152) | 2162 (2102 to 2222) | 0.836 (0.59 to 1.08) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
FIGURE 40.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CEA sensitivity analysis multiple imputation results (AEs as the measure of effectiveness).
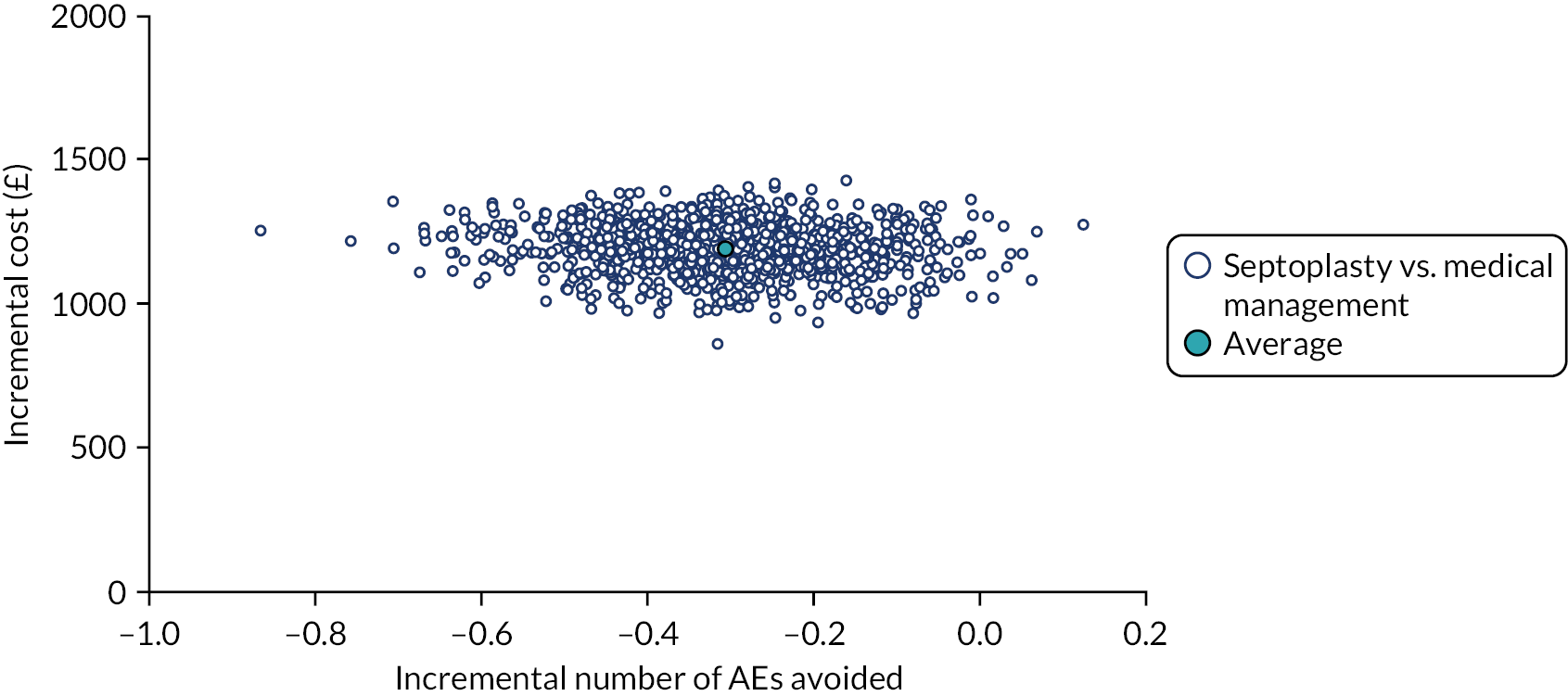
FIGURE 41.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CEA sensitivity analysis multiple imputation results (AEs as the measure of effectiveness).

| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: surgery costs estimated using microcosting – results | ||||||||||
| Medical management (costs, n = 155; outcomes, n = 152) | 797 (650 to 884) | 734 (608 to 860) | 0.728 (0.71 to 0.75) | 0.044 (0.03 to 0.06) | 16,682 | 1.00 | 0.99 | 0.21 | 0.01 | 0.00 |
| Septoplasty (costs, n = 152; outcomes, n = 149) | 1500 (1453 to 1547) | 0.767 (0.75 to 0.79) | 0.00 | 0.01 | 0.79 | 0.99 | 1.00 | |||
FIGURE 42.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (septoplasty costs estimated using microcosting).

FIGURE 43.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (septoplasty costs estimated using microcosting).
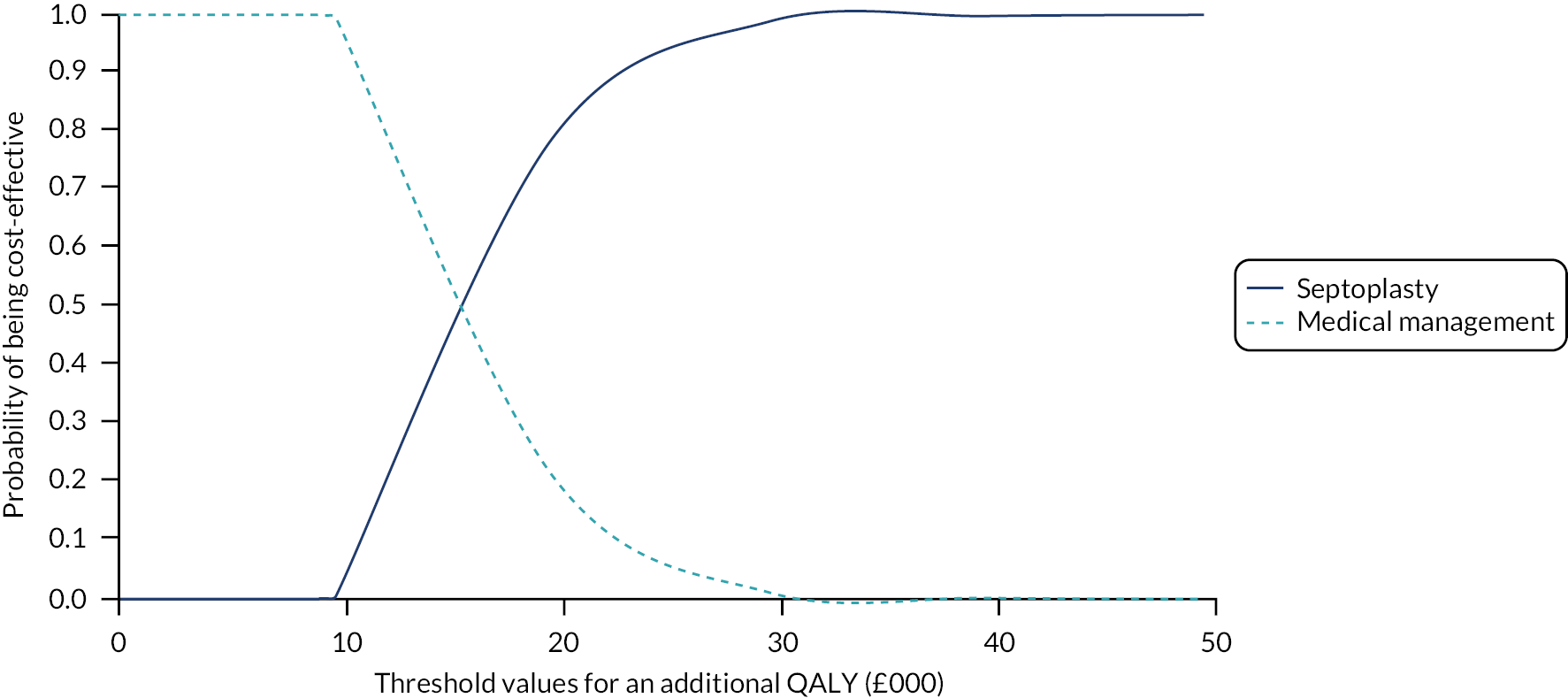
| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: participant costs included – results | ||||||||||
| Medical management (costs, n = 155; outcomes, n = 152) | 2054 (1644 to 2465) | 1062 (532 to 1593) | 0.728 (0.71 to 0.75) | 0.044 (0.03 to 0.06) | 24,136 | 1.00 | 0.98 | 0.71 | 0.27 | 0.02 |
| Septoplasty (costs, n = 152; outcomes, n = 149) | 3087 (2753 to 3421) | 0.767 (0.75 to 0.79) | 0.00 | 0.02 | 0.29 | 0.73 | 0.98 | |||
FIGURE 44.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (participant costs included in total costs).

FIGURE 45.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (participant costs included in total costs).
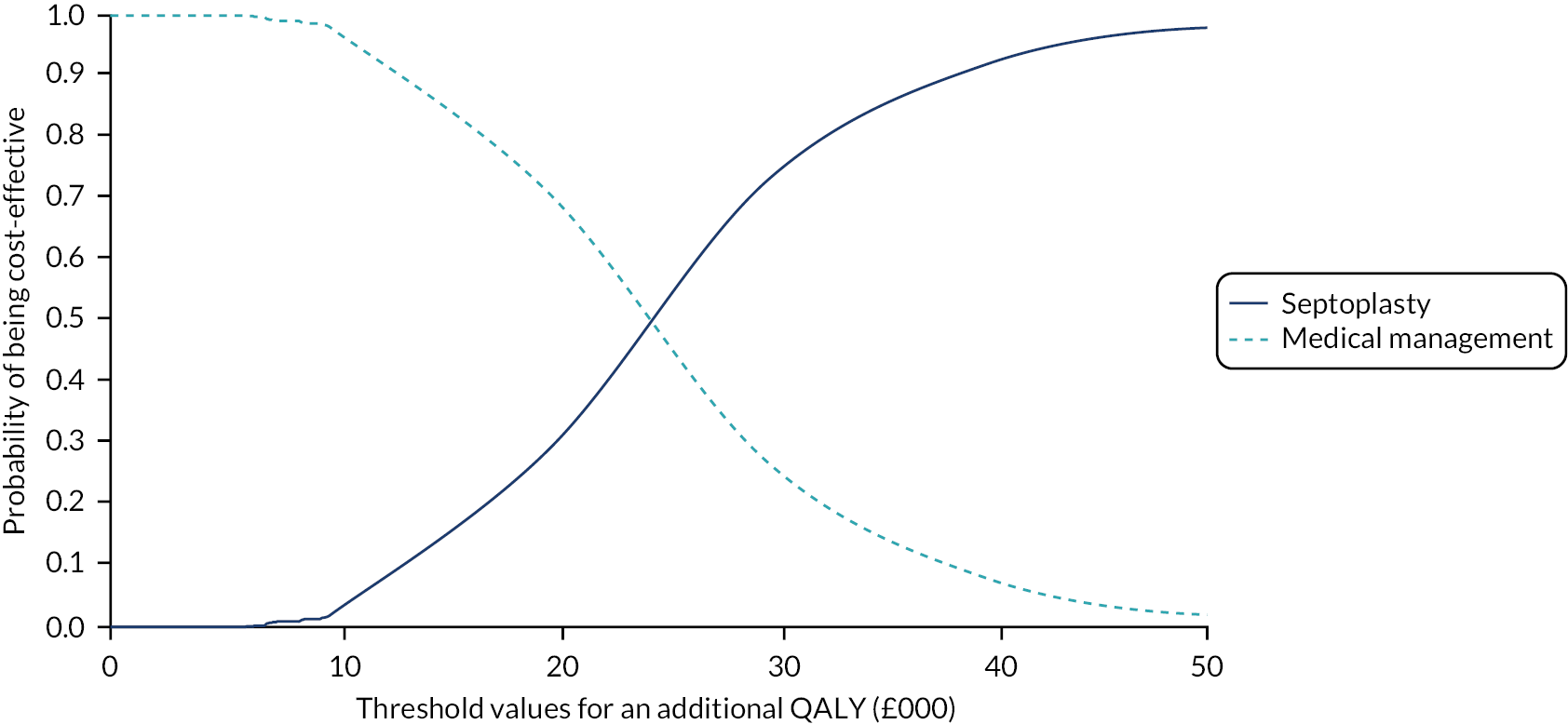
| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: complete case – results | ||||||||||
| Medical management (costs, n = 109; outcomes, n = 111) | 930 (744 to 1116) | 1308 (1100 to 1515) | 0.741 (0.72 to 0.76) | 0.035 (0.02 to 0.05) | 37,371 | 1.00 | 1.00 | 1.00 | 0.77 | 0.17 |
| Septoplasty (costs, n = 95; outcomes, n = 99) | 2207 (2134 to 2280) | 0.761 (0.74 to 0.79) | 0.00 | 0.00 | 0.00 | 0.23 | 0.83 | |||
FIGURE 46.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis (costs and QALYs estimated for those with complete data – no imputation).
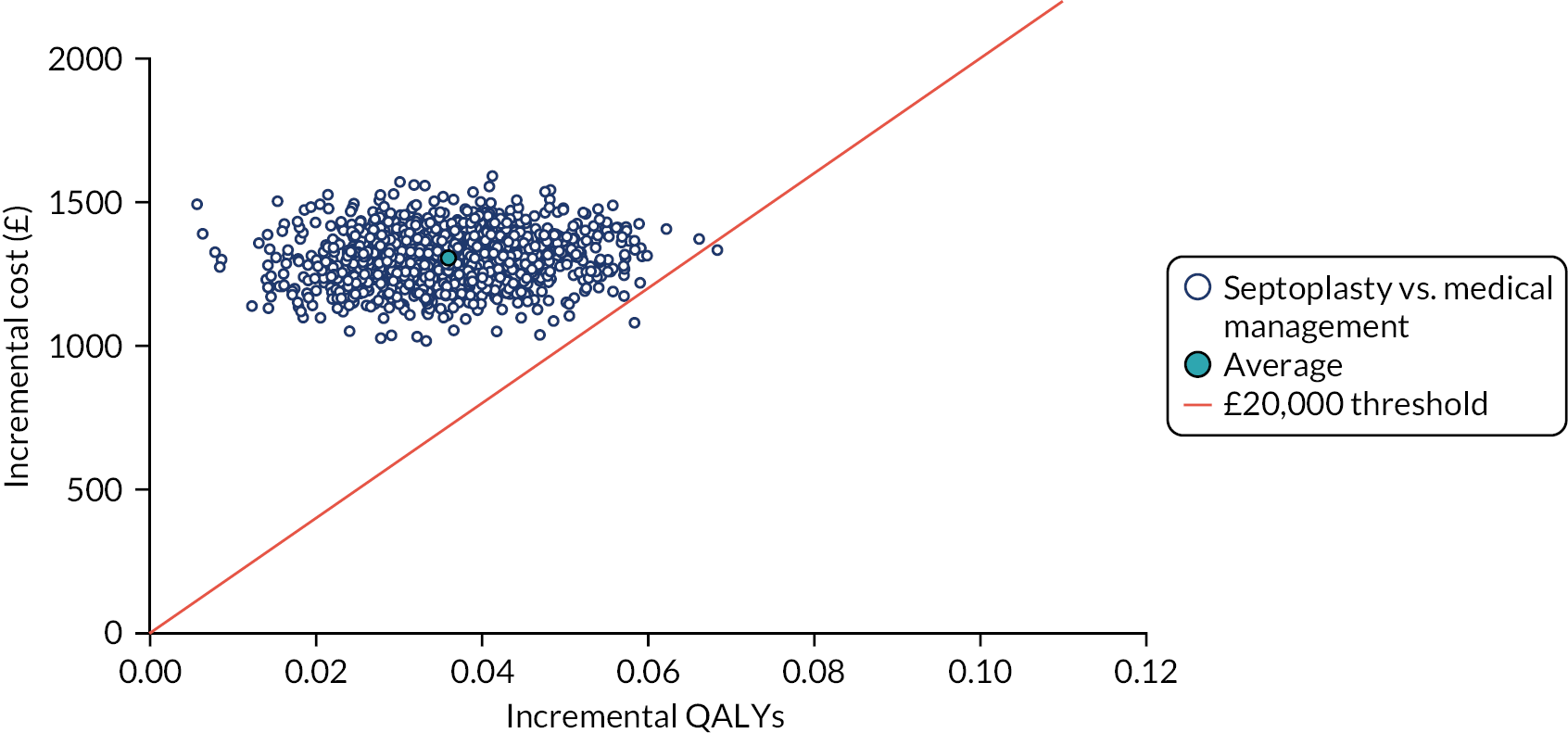
FIGURE 47.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis (costs and QALYs estimated for those with complete data – no imputation).
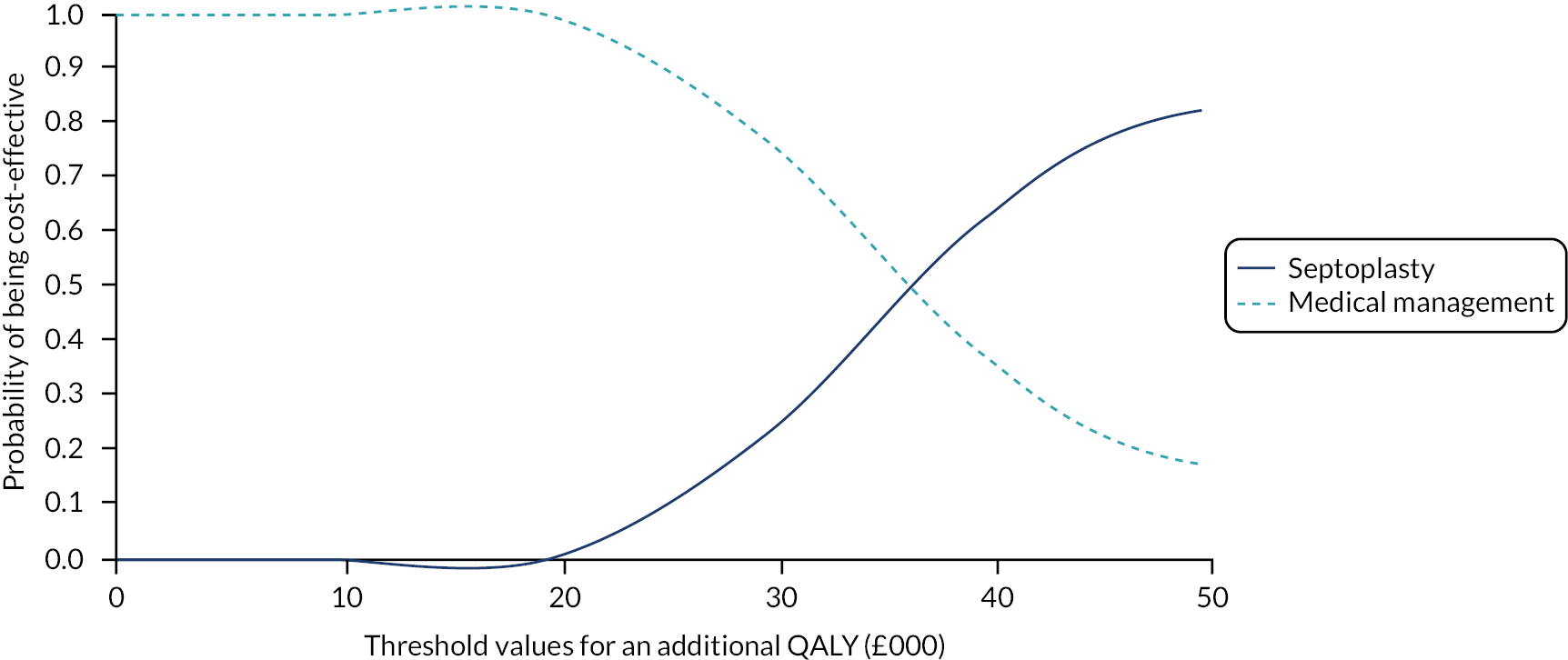
| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: QALYs at 12 months – results | ||||||||||
| Medical management (costs, n = 133; outcomes, n = 130) | 1053 (872 to 1234) | 1126 (936 to 1315) | 0.715 (0.69 to 0.74) | 0.049 (0.03 to 0.07) | 22,980 | 1.00 | 1.00 | 0.76 | 0.11 | 0.00 |
| Septoplasty (costs, n = 134; outcomes, n = 131) | 2166 (2107 to 2225) | 0.769 (0.75 to 0.79) | 0.00 | 0.00 | 0.24 | 0.89 | 1.00 | |||
FIGURE 48.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (changing eligibility criteria).

FIGURE 49.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis multiple imputation results (changing eligibility criteria).
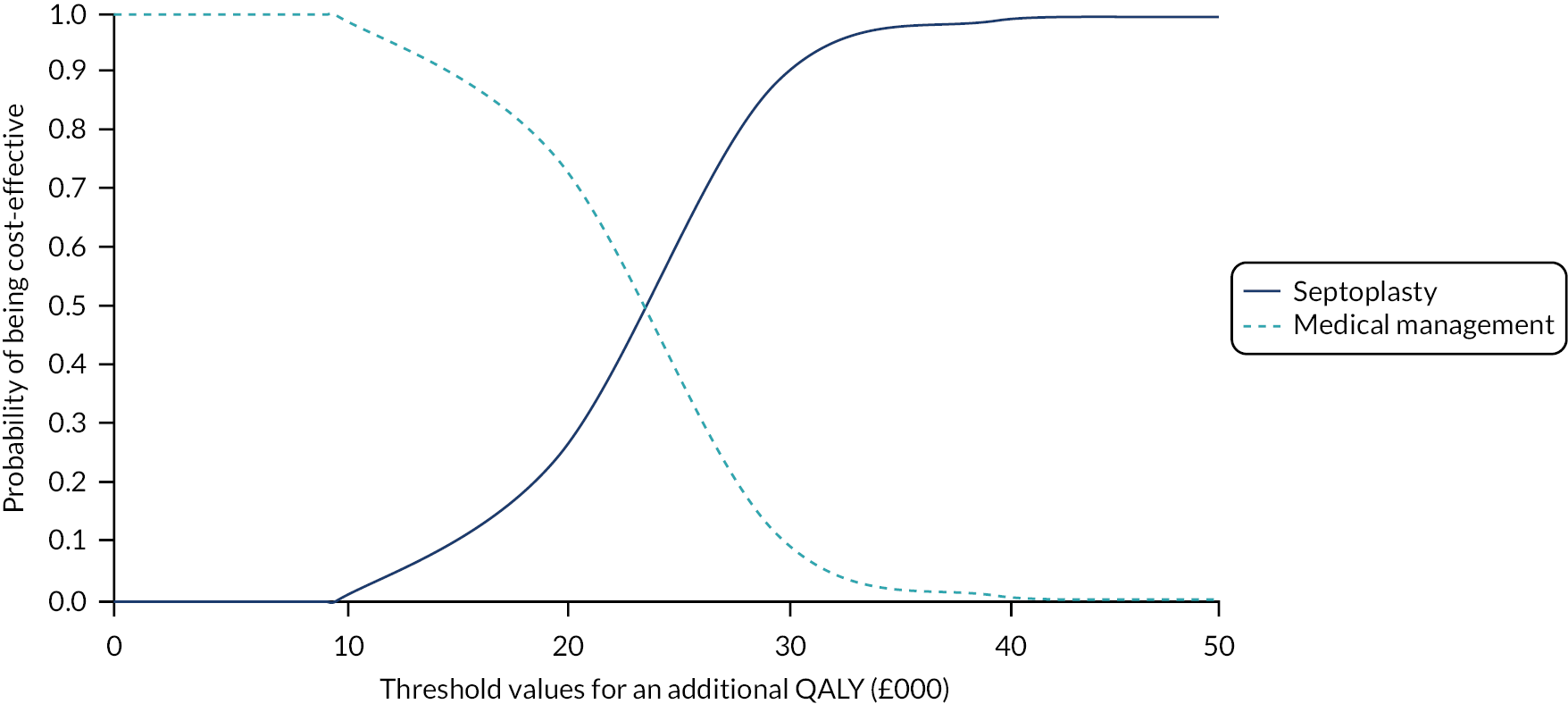
| Investigation strategy | Cost (95% CI)a (£) | Incremental cost (95% CI)b (£) | Effect (95% CI)a | Incremental effect (95% CI)b | ICER (£) | Probability that septoplasty is cost-effective for different threshold values for society’s willingness to pay for an additional QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Outcome: QALYs at 6 months – results | ||||||||||
| Medical management (costs, n = 142;outcomes, n = 138) | 294 (216 to 372) | 1787 (1693 to 1881) | 0.363 (0.35 to 0.37) | 0.015 (0.01 to 0.02) | 119133 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Septoplasty (costs, n = 140; outcomes, n = 137) | 2071 (2016 to 2125) | 0.373 (0.36 to 0.39) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
FIGURE 50.
Cost-effectiveness plane for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis (incremental cost per QALY at 6 months).

FIGURE 51.
The CEAC for septoplasty vs. medical management using the adjusted bootstrapped CUA sensitivity analysis (incremental cost per QALY at 6 months).

FIGURE 52.
Cost-effectiveness plane for septoplasty vs. medical management based on the economic model (incremental cost per QALY at 24 months).
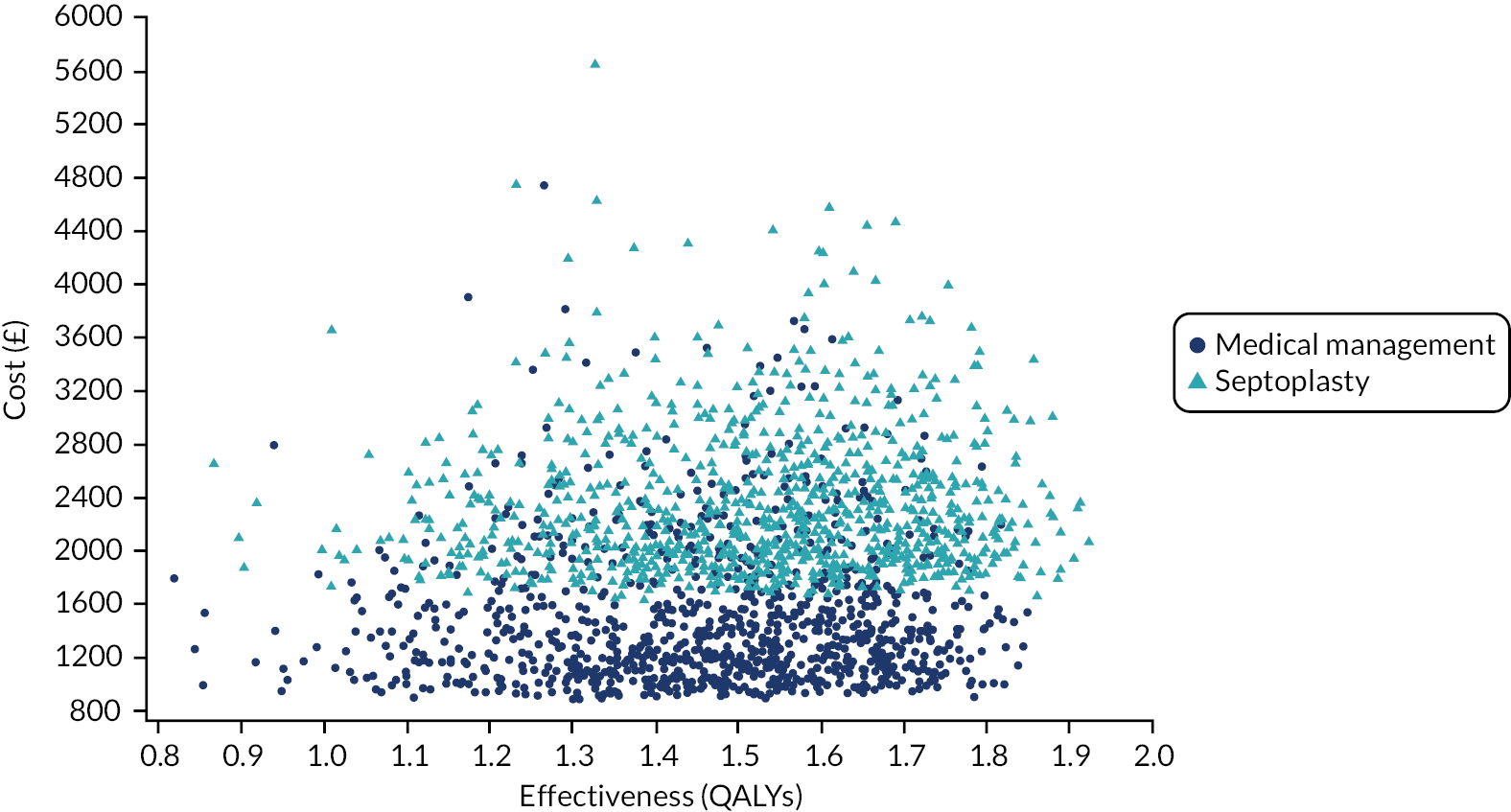
FIGURE 53.
The CEAC for septoplasty vs. medical management based on the economic model (incremental cost per QALY at 24 months).

FIGURE 54.
Cost-effectiveness plane for septoplasty vs. medical management based on the economic model (incremental cost per QALY at 36 months).
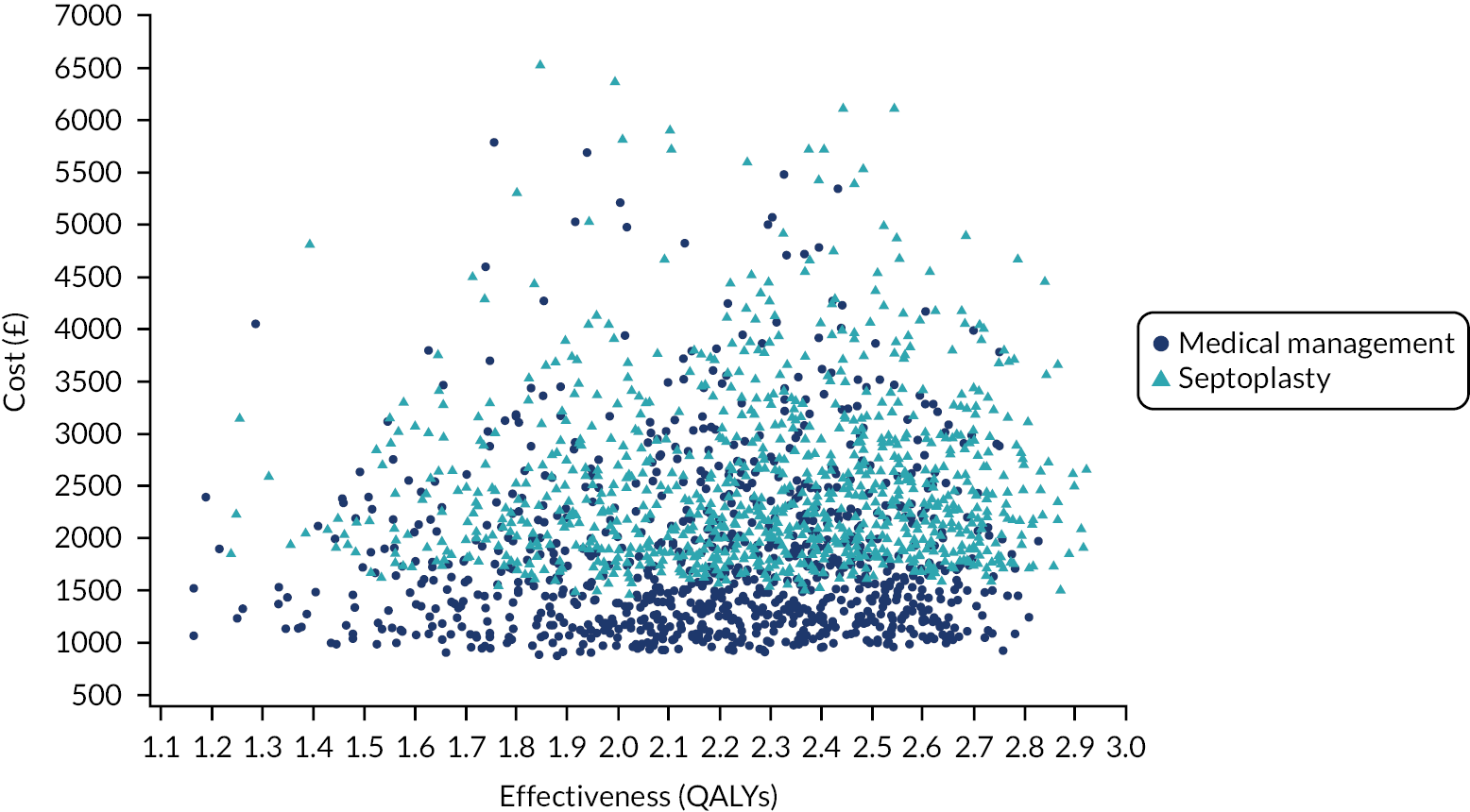
FIGURE 55.
The CEAC for septoplasty vs. medical management based on the economic model (incremental cost per QALY at 36 months).
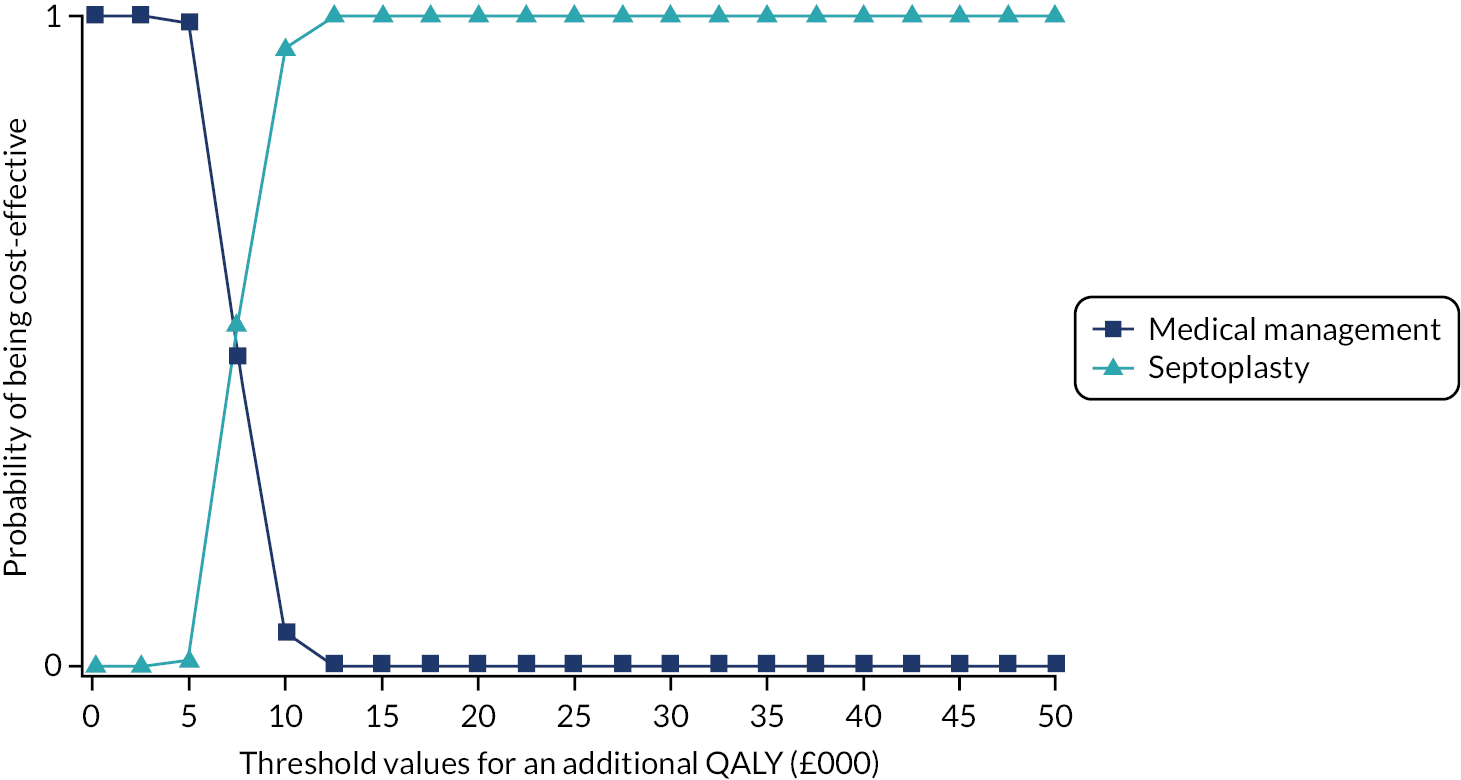
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTIMP
- Clinical Trial of an Investigational Medicinal Product
- CUA
- cost–utility analysis
- DMC
- Data Monitoring Committee
- DOASS
- Double Ordinal Airway Subjective Scale
- eCRF
- electronic case report form
- ENT
- ear, nose and throat
- EudraCT
- European Union Drug Regulating Authorities Clinical Trials
- GP
- general practitioner
- HTA
- Health Technology Assessment
- HUQ
- healthcare utilisation questionnaire
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MCID
- minimal clinically important difference
- MedDRA
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MIV
- maximal inhalation volume
- NAIROS
- Nasal AIRway Obstruction Study
- NCTU
- Newcastle Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NOSE
- Nasal Obstruction Symptom Evaluation
- NPR
- nasal partitioning ratio
- NPT
- normalisation process theory
- PI
- principal investigator
- PIS
- participant information sheet
- PNIF
- peak nasal inspiratory flow
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QRI
- Qualitative research integrated within Trials Recruitment Intervention
- QuinteT
- Qualitative research integrated within Trials
- R
- recruiter
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SNOT-22
- Sino-nasal Outcome Test-22 items
- STEPP
- subpopulation treatment effect pattern plot
- SUR
- seemingly unrelated regression
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/MVFR4028).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
